
from absci import de_novo_model model = de_novo_model.load_latest() antigen = model.load_pdb("7olz.pdb", chain="A") antibodies = model.predict(antigen, N=300000) from absci_library import codon_optimizer library = codon_optimizer.reverse_translate(library) library.to_csv("covid-antibody-designs.csv") library.to_wet_lab(assay="ACE") from absci import lead_opt_model lead_optimizer = lead_opt_model.load_latest() library.naturalness = lead_optimizer.naturalness(library) lead_optimizer.optimize(library).to_wet_lab(as say="SPR") from absci import genetic_algorithm; parameters=["maximize|binding_affinity:pH=7.5", "minimize|binding_affinity:pH=6.0", "maximize|human_naturalness"]; library = genetic_algorithm.multiparametric_optimization(library, parameters, evolutions=100); library.to_wet_lab(assays=["ACE", "SPR", "Bioassays"]) C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . CORPORATE PRESENTATION FALL 2025

2C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Disclaimers Forward-Looking Statements Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding any or all of the following: (i) Absci’s preclinical studies, clinical trials, as well as partnered and internally developed programs, including, without limitation, manufacturing capabilities, status of such studies and trials and expectations regarding data, safety and efficacy generally; (ii) data included in the above-described oral presentation, as well as the ability to use data from ongoing and planned clinical trials for the design and initiation of further clinical trials; (iii) Absci’s strategy, goals, anticipated financial performance and the sufficiency of its cash resources; (iv) regulatory submissions and authorizations, including timelines for and expectations regarding any anticipated regulatory agency decisions; (v) the expected benefits of its collaborations with partners; and (vi) the therapeutic value, development, and commercial potential of antibody therapies, as well as other technologies. Risks that contribute to the uncertain nature of the forward-looking statements include, without limitation, the risks and uncertainties discussed under the heading “Risk Factors” in Absci Corporation’s most recent annual report on Form 10-K and in any other subsequent filings made by Absci Corporation with the U.S. Securities and Exchange Commission. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date they are made. We disclaim any obligation or undertaking to update or revise any forward-looking statements contained in this press release, other than to the extent required by law. Market and Statistical Information This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other industry data. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. We have not independently verified the data generated by independent parties and cannot guarantee their accuracy or completeness. Trademark usage This presentation/document/webpage contains references to our trademarks and service marks and to those belonging to third parties. Absci®, ®, SoluPro®, Bionic SoluPro® and SoluPure® are Absci registered trademarks with the U.S. Patent and Trademark Office. We also use various other trademarks, service marks and trade names in our business, including the Absci AI logo mark ( ), the Unlimit with us mark ( ), Denovium, Integrated Drug Creation, HiPrBind, and IgDesign. All other trademarks, service marks or trade names referred to in this presentation/document/webpage are the property of their respective owners. Solely for convenience, the trademarks and trade names in this presentation/document/webpage may be referred to with or without the trademark symbols, but references which omit the symbols should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.

3N O N - C O N F I D E N T I A L C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D .C P Y R I G H T © 2 0 2 5 A B S I C O R P O R A T I O N . A L L R I G H T S E S E R V E D . Generative AI Drug Creation™ D I F F E R E N T I A T E D P I P E L I N E A P R O V E N A I × B I O P L A T F O R M LEADING AI MODELS • De novo AI models now unlock first-in-class biology by cracking tough epitopes and difficult-to-drug disease targets PURPOSE BUILT TEAM • 10+ approved drugs by our scientists + AI talent from OpenAI, Google, Tesla, NVIDIA INTEGRATED DATA FLYWHEEL • 77,000+ ft² automated lab generating hundreds of millions of sequence-function datapoints since 2020 A B S - 2 0 1 (anti-prolactin receptor) • Androgenetic Alopecia (AGA): Accelerated Ph1/2a trial on track to initiate December 2025, with interim efficacy readout 2H2026 • Endometriosis (Endo): Indication expansion into endometriosis with anticipated Ph2 initiation in 4Q2026 with PoC readout as early as 2H2027 A B S - 1 0 1 (anti-TL1A) • Phase 1 interim results reported with extended half-life vs 1st gen TL1A competitors; ongoing partnership discussions. E A R L Y P I P E L I N E • Advancing early-stage oncology and I&I programs including ABS- 301, ABS-501,
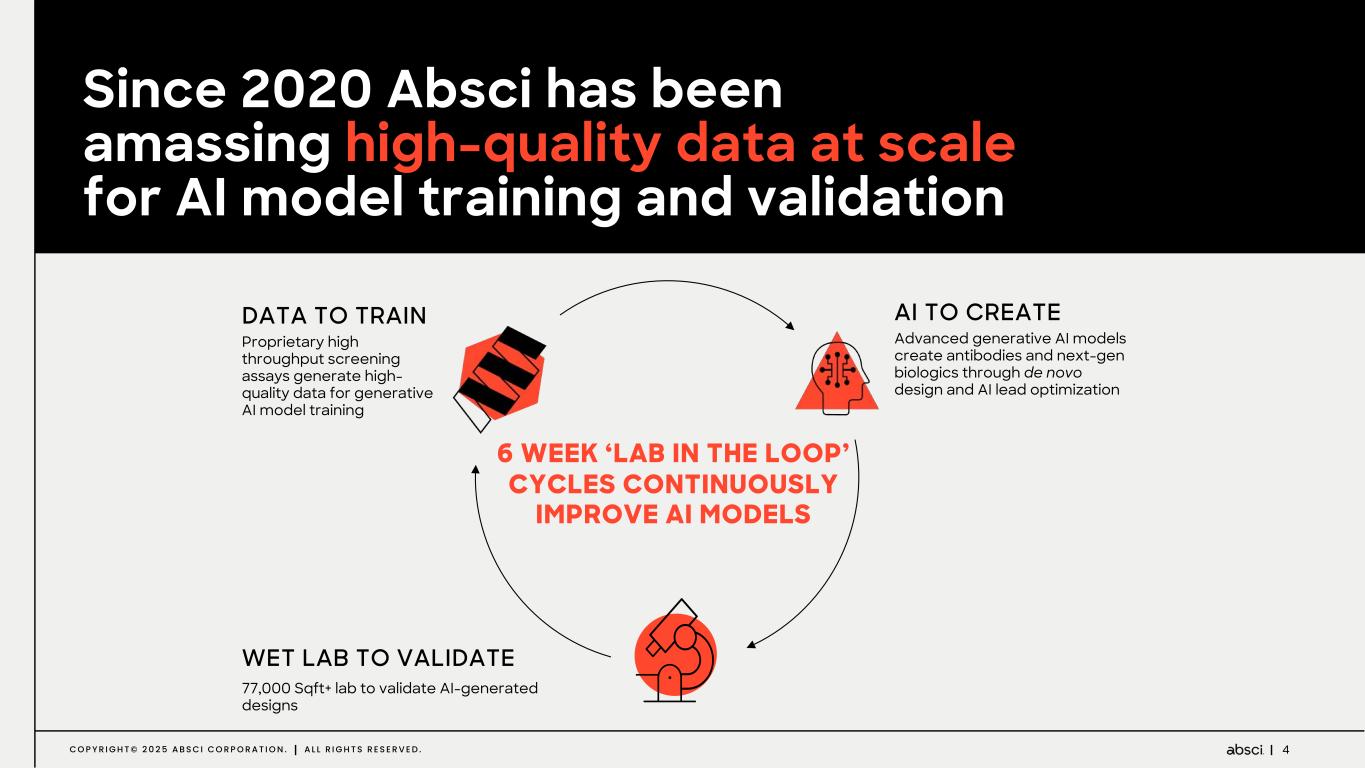
4C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Since 2020 Absci has been amassing high-quality data at scale for AI model training and validation DATA TO TRAIN Proprietary high throughput screening assays generate high- quality data for generative AI model training WET LAB TO VALIDATE 77,000 Sqft+ lab to validate AI-generated designs AI TO CREATE Advanced generative AI models create antibodies and next-gen biologics through de novo design and AI lead optimization 6 WEEK ‘LAB IN THE LOOP’ CYCLES CONTINUOUSLY IMPROVE AI MODELS

5C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Leadership in AI de novo design of antibody-based therapeutics EVQLSEVGA . . . De novo antibody design model creates epitope-specific binders given a target structure Designed in framework of choice or multiple frameworks INPUT EMBEDDING STRUCTURE DESIGN (DIFFUSION) . . . ARCPSIWKFPDEEGACQPC . . . Antigen Structure/Sequence (Epitope) PROTEIN LANGUAGE MODELS Co-optimization enables improvement of antibody attributes such as affinity and developability Precise engineering of molecule pharmacology AI LEAD OPTIMIZATIONDE NOVO ANTIBODY DESIGN

6C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . ENABLING FEATURES: MULTI-VALENCY, pH-DEPENDENT BINDING ABILITY TO ADDRESS DIFFICULT TARGET CLASSES, E.G. GPCRS EPITOPE-SPECIFIC DESIGN + EPITOPE INTERFACE OPTIMIZATION POTENTIAL TO CREATE MEANINGFUL IP: 100S TO 10,000S OF FUNCTIONALLY VALIDATED SEQUENCES ENABLED BY PROPRIETARY WET-LAB VALIDATION ENHANCED POTENCY AND MOA We use AI to create novel & differentiated therapeutics

7C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Leveraging AI throughout the end-to-end drug discovery process I N T E G R A T E D D R U G C R E A T I O N P L A T F O R M TARGET SELECTION AI reverse immunology target discovery OR Additional Target Selection approaches AI-GUIDED DRUG CREATION De novo antibody design AI-GUIDED LEAD OPTIMIZATION Affinity & developability Pharmacology engineering TM
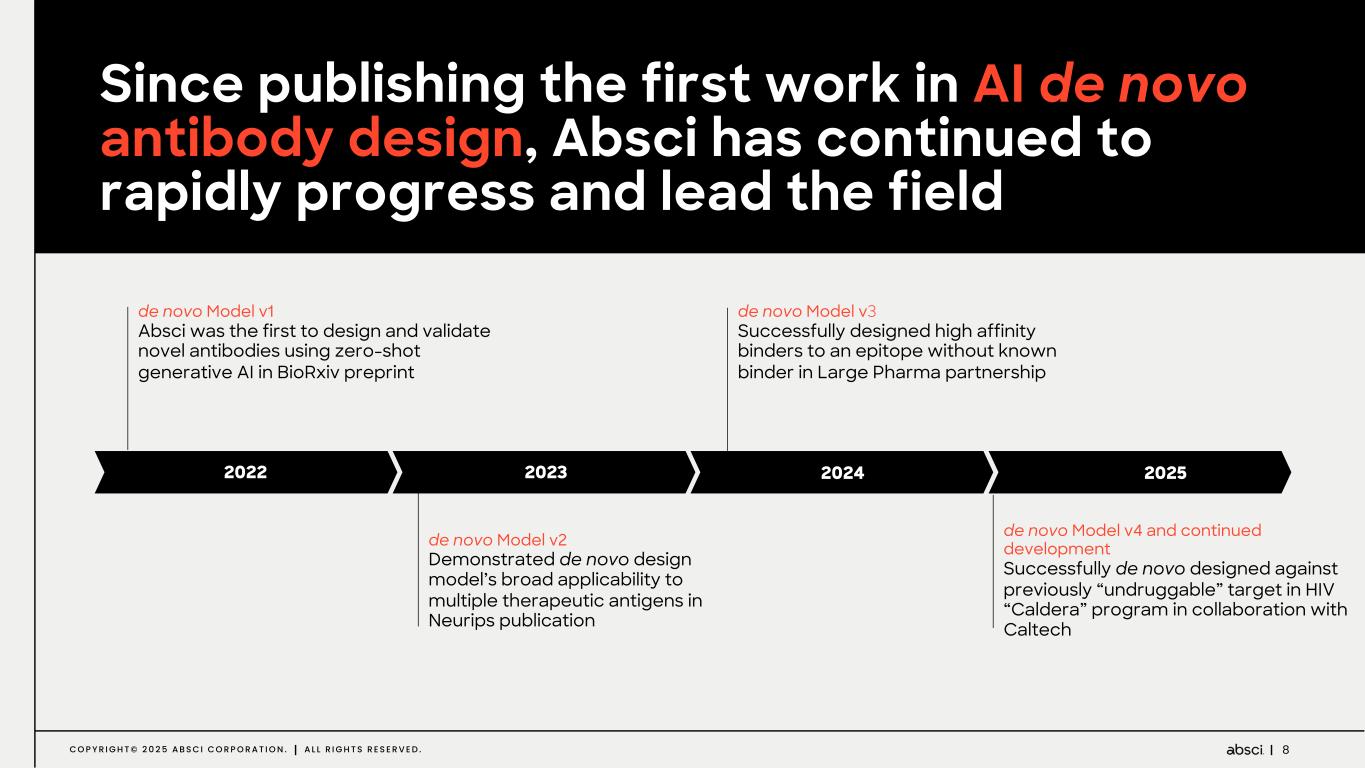
8C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . de novo Model v1 Absci was the first to design and validate novel antibodies using zero-shot generative AI in BioRxiv preprint de novo Model v2 Demonstrated de novo design model’s broad applicability to multiple therapeutic antigens in Neurips publication de novo Model v3 Successfully designed high affinity binders to an epitope without known binder in Large Pharma partnership de novo Model v4 and continued development Successfully de novo designed against previously “undruggable” target in HIV “Caldera” program in collaboration with Caltech 2022 2023 2024 2025 Since publishing the first work in AI de novo antibody design, Absci has continued to rapidly progress and lead the field
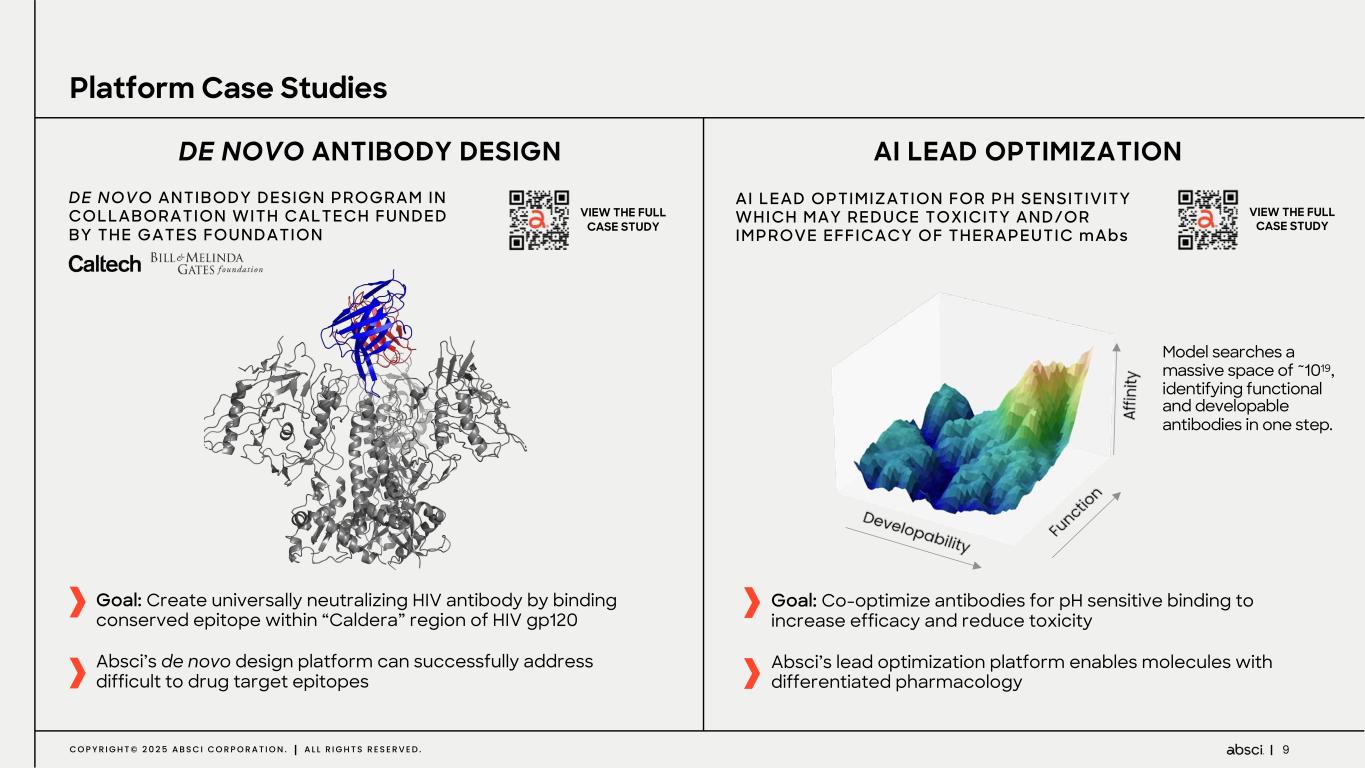
9C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Platform Case Studies AI LEAD OPTIMIZATIONDE NOVO ANTIBODY DESIGN Goal: Create universally neutralizing HIV antibody by binding conserved epitope within “Caldera” region of HIV gp120 Absci’s de novo design platform can successfully address difficult to drug target epitopes Goal: Co-optimize antibodies for pH sensitive binding to increase efficacy and reduce toxicity Absci’s lead optimization platform enables molecules with differentiated pharmacology AI LEAD OPTIMIZATION FOR PH SENSITIVITY WHICH MAY REDUCE TOXICITY AND/OR IMPROVE EFFICACY OF THERAPEUTIC mAbs VIEW THE FULL CASE STUDY DE NOVO ANTIBODY DESIGN PROGRAM IN COLLABORATION WITH CALTECH FUNDED BY THE GATES FOUNDATION VIEW THE FULL CASE STUDY Model searches a massive space of ~1019, identifying functional and developable antibodies in one step.

10C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . ”Multilingual” team with expertise in AI and drug creation Sean McClain Founder, CEO & Director Andreas Busch, PHD Chief Innovation Officer Zach Jonasson, PHD Chief Financial Officer & Chief Business Officer Amir Shanehsazzadeh SVP, Chief AI Officer Karin Wierinck Chief People Officer Christian Stegmann, PHD SVP, Drug Creation Christine Lemke, DVM SVP, Portfolio & Growth Strategy Shelby Walker, JD Chief Legal Officer Karen Mcginnis, CPA Former Chief Accounting Officer, Illumina Joseph Sirosh, PHD Former CTO, Compass VP, Amazon & Microsoft Dan Rabinovitsj VP Hardware Engineering, Meta Frans Van Houten Chairman of the Board Former CEO, Royal Phillips Sir Mene Pangalos, PHD Former EVP R&D AstraZeneca Sean McClain Founder, CEO & Board Director Ian McInnes, PHD Vice Principal and Head of College University of Glasgow Hubert Truebel, MD, PHD, MBA Chief Medical Officer AiCuris Luis Diaz, MD Head, Division of Solid Tumor Oncology Memorial Sloan Kettering Cancer Center John Wherry, PHD Director, Institute for Immunology & Immune Health, University of Pennsylvania Victor Greiff, PHD Associate Professor University of Oslo Sir Mene Pangalos, PHD Co-Chair SAB Former EVP R&D AstraZeneca Andreas Busch, PHD Co-Chair SAB Chief Innovation Officer L E A D E R S H I P T E A M B O A R D O F D I R E C T O R S S C I E N T I F I C A D V I S O R Y B O A R D E X P E R T I S E & B A C K G R O U N D F R O M Penelope Chief Morale Officer O U R P E O P L E Trademarks, service marks or trade names referred to herein are the intellectual property of their respective owners. Use of this IP does not imply affiliation, endorsement or sponsorship of any kind Mary Szela CEO & President TriSalus Life Sciences

11C O P Y R I G H T © 2 0 2 4 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . ~140 Employees 77,000+ Square Feet >$600M Trademarks, service marks or trade names referred to herein are the intellectual property of their respective owners. Use of this IP does not imply affiliation, endorsement or sponsorship of any kind W E L L - P O S I T I O N E D T O D E L I V E R “Multi-lingual” AI + Drug Discovery expertise AI team drawing on experience from tech leaders: Biologics drug discovery expertise from : Absci’s Talent and Infrastructure for Better Biologics Faster Capital raised to date, runway into 1H 2028 State-of-the-art drug creation and wet lab space in Vancouver WA, Absci AI Research (AAIR) lab in NYC, and the Innovation Centre in Zug Switzerland

12C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Advancing and expanding our pipeline of novel & differentiated assets designed using AI KEY HIGHLIGHTS A I P I P E L I N E A B S - 2 0 1 ( A G A ) Ph1/2a study for androgenetic alopecia anticipated to initiate Dec 2025 with interim PoC readout expected 2H2026 A B S - 1 0 1 Completing Ph1 study with interim data reported. Ongoing partnership and outlicensing discussions A B S - 3 0 1 Potential first-in-class asset discovered through Reverse Immunology Platform A B S - 5 0 1 Candidate ID phase for novel HER2 program designed using de novo AI A B S - 2 0 1 ( E N D O ) Indication expansion for endometriosis announced Nov 2025. Anticipate initiating Ph2 PoC study 4Q26 IBD Androgenetic Alopecia Immuno-oncology Early Discovery Programs Target ID Lead ID Candidate ID IND-Enabling Phase 1 Oncology IND* Therapeutic area DCLead *or equivalent ex-US filing Endometriosis ABS- 101 ABS- 201 ABS- 301 ABS- 501 TL1A PRLR PRLR Undisclosed Target HER2
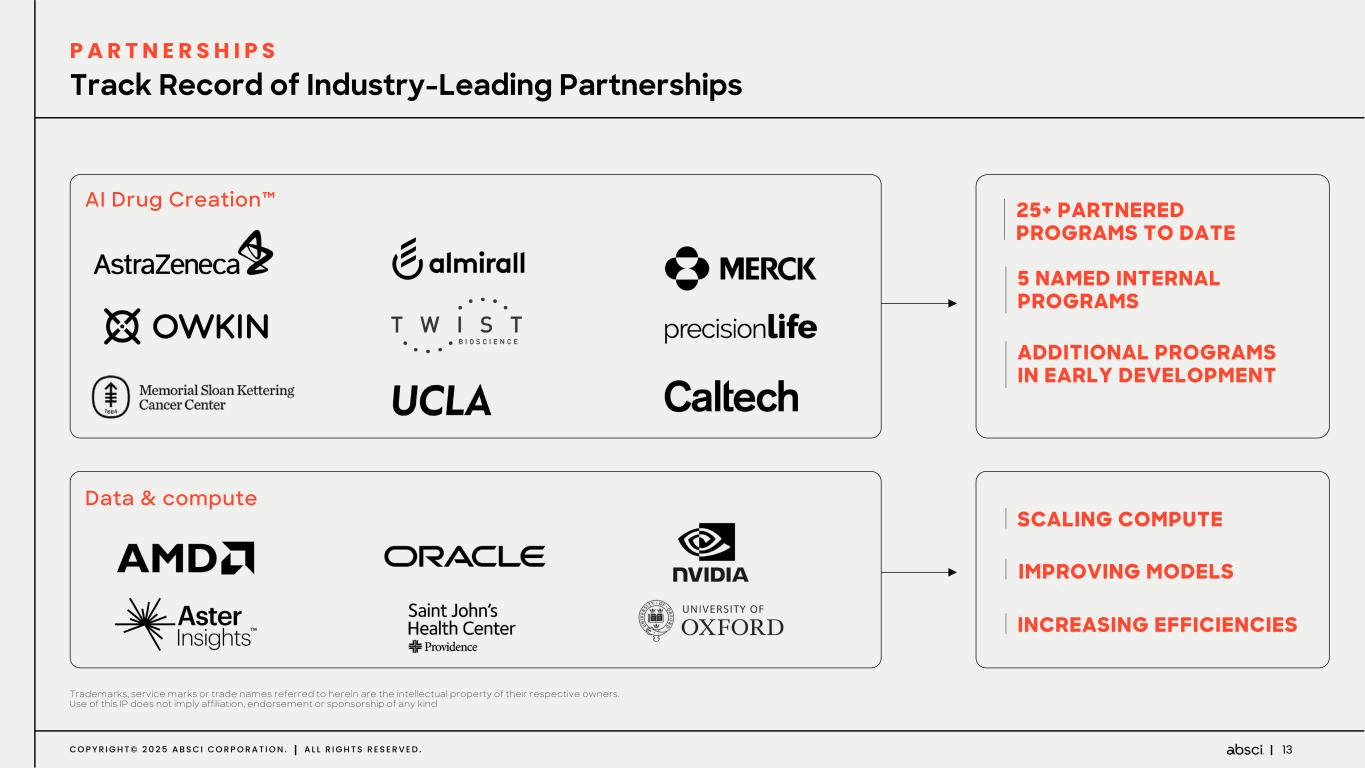
13C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . AI Drug Creation™ Data & compute 25+ PARTNERED PROGRAMS TO DATE SCALING COMPUTE 5 NAMED INTERNAL PROGRAMS ADDITIONAL PROGRAMS IN EARLY DEVELOPMENT IMPROVING MODELS INCREASING EFFICIENCIES Trademarks, service marks or trade names referred to herein are the intellectual property of their respective owners. Use of this IP does not imply affiliation, endorsement or sponsorship of any kind Track Record of Industry-Leading Partnerships P A R T N E R S H I P S

14C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Leading AI platform driving numerous near-term value inflection points A B S - 1 0 1 Phase 1 Interim Data reported Advancing partnering and out-licensing opportunities A B S - 2 0 1 i n A G A Accelerated Ph1/2a Study Initiation December 2025 Interim PoC Readout – anticipated 2H 2026 A B S - 2 0 1 i n E N D O M E T R I O S I S Indication expansion into Endometriosis Ph2 POC anticipated to initiate 4Q2026 with PoC readout as early as 2H2027 P A R T N E R S H I P S Anticipate signing one or more partnerships, including with a Large Pharma in 2025
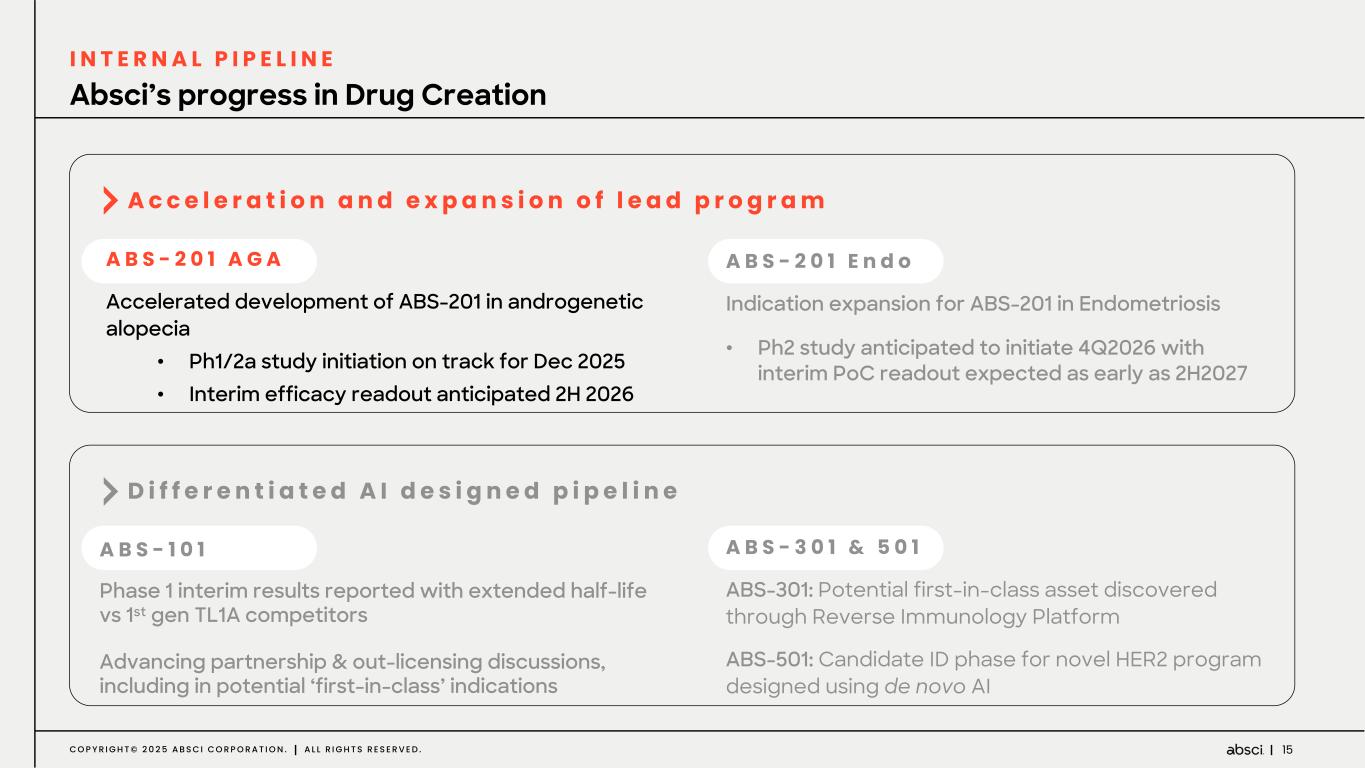
15C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . A B S - 2 0 1 A G A Accelerated development of ABS-201 in androgenetic alopecia • Ph1/2a study initiation on track for Dec 2025 • Interim efficacy readout anticipated 2H 2026 A B S - 2 0 1 E n d o Indication expansion for ABS-201 in Endometriosis • Ph2 study anticipated to initiate 4Q2026 with interim PoC readout expected as early as 2H2027 A B S - 1 0 1 Phase 1 interim results reported with extended half-life vs 1st gen TL1A competitors Advancing partnership & out-licensing discussions, including in potential ‘first-in-class’ indications A B S - 3 0 1 & 5 0 1 ABS-301: Potential first-in-class asset discovered through Reverse Immunology Platform ABS-501: Candidate ID phase for novel HER2 program designed using de novo AI A c c e l e r a t i o n a n d e x p a n s i o n o f l e a d p r o g r a m D i f f e r e n t i a t e d A I d e s i g n e d p i p e l i n e Absci’s progress in Drug Creation I N T E R N A L P I P E L I N E

16C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . ABS-201 has the potential to unlock a wholly new category of therapy in hair “re-growth” Significant unmet clinical need for androgenetic alopecia Large market: approximately 80 million patients in U.S.; highly motivated patient population CLINICAL AND COMMERCIAL UNMET NEED Straightforward clinical development path with potential for early Proof of Concept Low competition, potentially first to U.S. market DEVELOPMENT PATH Strong target validation (efficacy & safety) for treatment of androgenetic alopecia Mode of action conserved across many species Supportive pharmacological profile of ABS-201 SCIENTIFIC RATIONALE
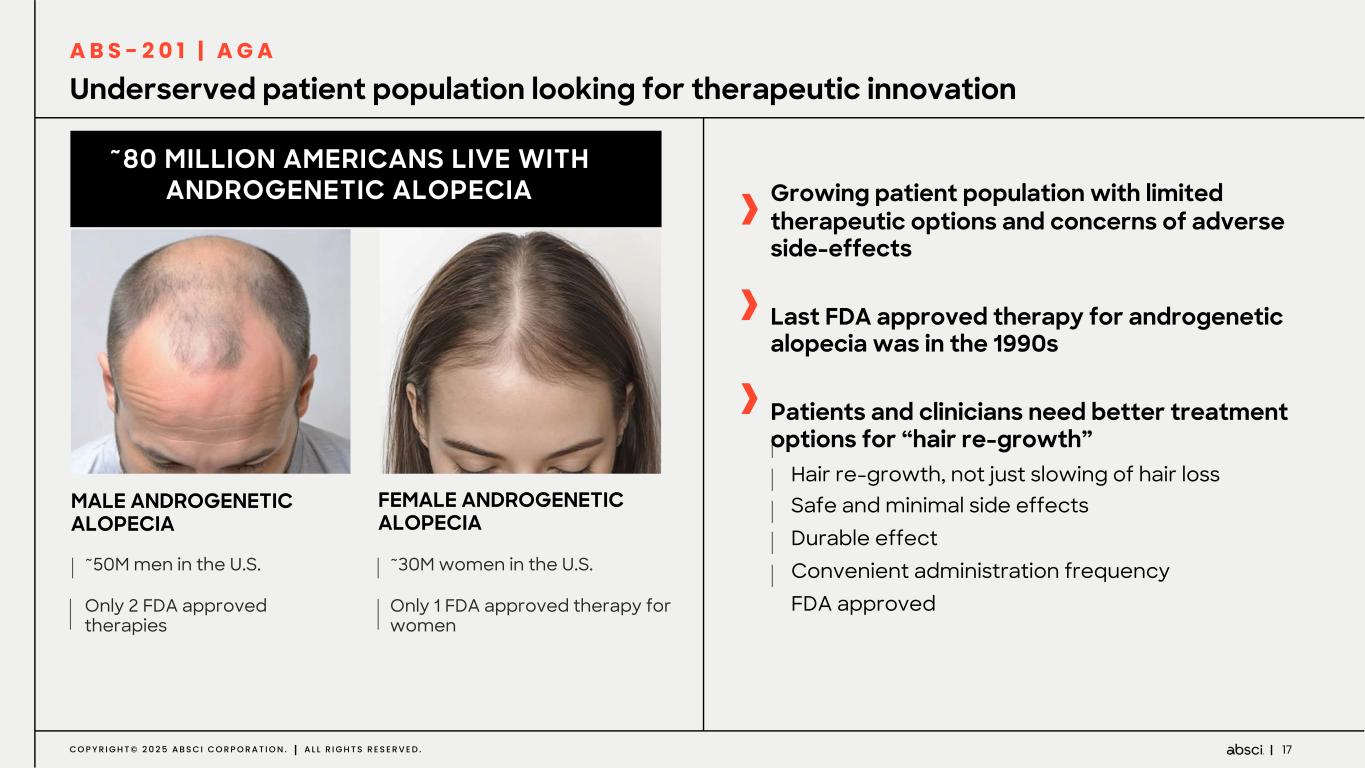
17C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Underserved patient population looking for therapeutic innovation FEMALE ANDROGENETIC ALOPECIA ~30M women in the U.S. Only 1 FDA approved therapy for women ~50M men in the U.S. Only 2 FDA approved therapies ~80 MILLION AMERICANS LIVE WITH ANDROGENETIC ALOPECIA MALE ANDROGENETIC ALOPECIA Growing patient population with limited therapeutic options and concerns of adverse side-effects Last FDA approved therapy for androgenetic alopecia was in the 1990s Patients and clinicians need better treatment options for “hair re-growth” Hair re-growth, not just slowing of hair loss Safe and minimal side effects Durable effect Convenient administration frequency FDA approved A B S - 2 0 1 | A G A

18C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . PRLR inhibition as a safe innovative alternative to current treatment options A B S - 2 0 1 | A G A P r o p o s e d d i r e c t i m p a c t o f A B S - 2 0 1 o n H a i r C y c l e S t a g e s A B S - 2 0 1 h a s t h e p o t e n t i a l t o : Catagen ↑↑ Apoptosis & Regression 2-4 weeks Telogen Resting Phase Hair falls out 3-5 months PRLR Anagen Active Growth & New Hair 2-6 years ABS-201 Anagen ↑↑ Active Growth & New Hair 2-6 years Telogen Resting Phase Hair falls out 3-5 months PRLR Catagen Apoptosis & Regression 2-4 weeks Shift the balance in hair cycle stage towards anagen phase1,2 with: • active and new hair growth • prevention of telogen effluvium Block cessation of pigmentation, which may lead to the restoration of hair pigmentation2 Promote a long-lasting effect after treatment cessation 1 doi: 10.1016/S0002-9440(10)64295-2 2 doi: 10.2353/ajpath.2006.050468

19C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Prolactin impacts on organ-cultured human hair follicles 1doi: 10.2353/ajpath.2006.050468 P r o l a c t i n - d r i v e s h a i r f o l l i c l e r e g r e s s i o n i n h u m a n e x v i v o c u l t u r e Prolactin prematurely induces a catagen- like stage in organ-cultured human hair follicles1 characterized by: Apparent cessation of pigmentation Condensed shape of the dermal papilla (DP) Diminishment of the hair matrix volume Vehicle 400 ng/ml prolactin Catagen IIIAnagen VI HS M DP IRS IRS HS ORS DP MK Inhibition of hair shaft elongation A B S - 2 0 1 | A G A
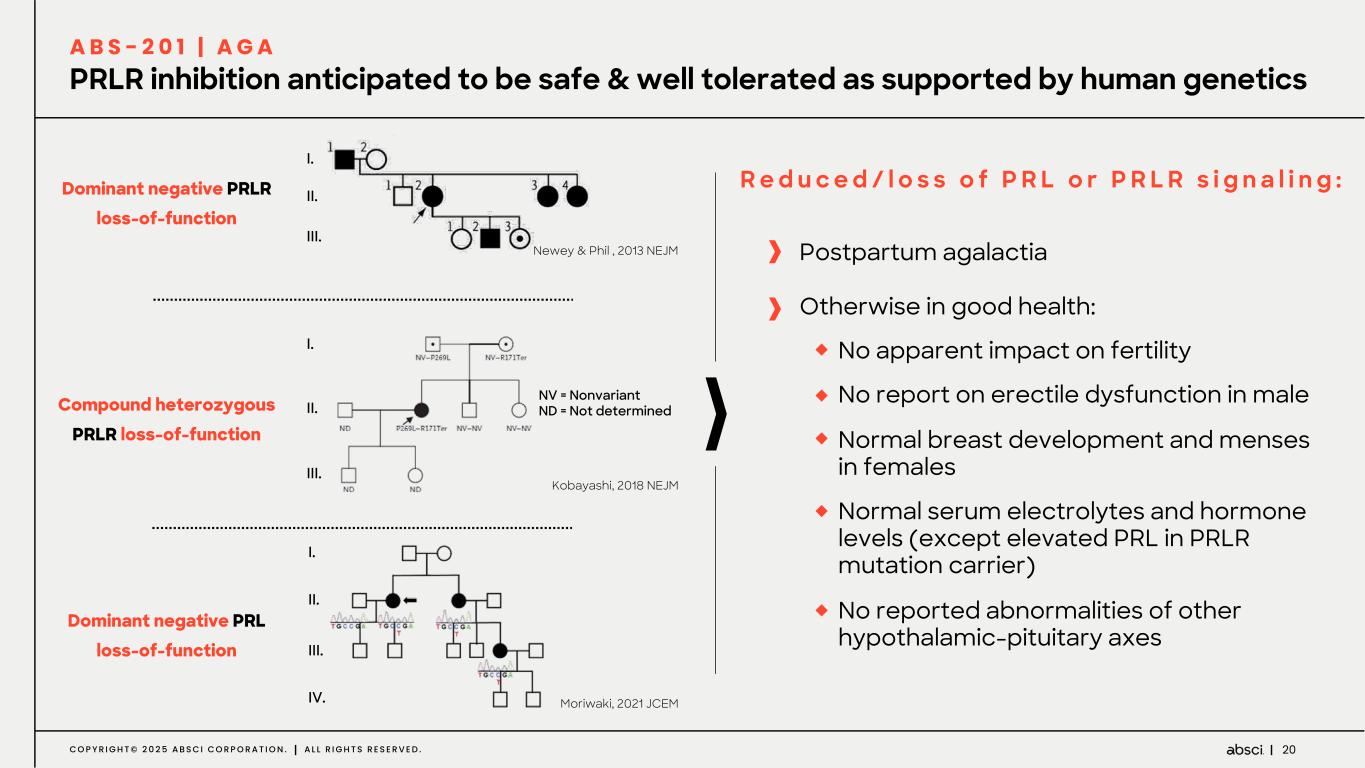
20C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . PRLR inhibition anticipated to be safe & well tolerated as supported by human genetics A B S - 2 0 1 | A G A I. II. III. NV = Nonvariant ND = Not determined I. II. III. I. II. III. IV. Kobayashi, 2018 NEJM Moriwaki, 2021 JCEM Newey & Phil , 2013 NEJM Compound heterozygous PRLR loss-of-function Dominant negative PRL loss-of-function Dominant negative PRLR loss-of-function R e d u c e d / l o s s o f P R L o r P R L R s i g n a l i n g : Postpartum agalactia Otherwise in good health: No apparent impact on fertility No report on erectile dysfunction in male Normal breast development and menses in females Normal serum electrolytes and hormone levels (except elevated PRL in PRLR mutation carrier) No reported abnormalities of other hypothalamic-pituitary axes

21C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Treatment with an anti-PRLR mAb promotes and sustains long-term hair growth in NHP TOP HEAD VIEW OF STUMPTAILED MACAQUE’S SHOWING PHENOTYPIC CHANGE OVER TIME 40mg/kg s.c. Q2W for 28 weeks Disclosure from competitor Hair density & thickness improved with short treatment duration in primate model of androgenetic alopecia Hair growth remains several years post cessation Hair regrowth observed for both male and female animals M al e Fe m al e Baseline 12 weeks 28 weeks 6 months 2 years 4 years Post-treatmentTreatment Translational Model validates PRLR Target A B S - 2 0 1 | A G A

22C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . ABS-201 shows superior efficacy vs 5% topical minoxidil in 21d hair regrowth model Administration: mAbs i.p. biweekly; Minoxidil topical daily Untreated (n=11) Isotype (n=11) Minoxidil 5% (n=11) ABS-201 30mg/kg (n=11) ABS-201 60mg/kg (n=10) ABS-201 vs minoxidil/untreated/isotype **p<0.05; ***p<0.0001 - 2way ANOVA Error bars= SEM *** *** *** * A B S - 2 0 1 | A G A

23C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . 56 day NHP PK data confirms extended half-life profile and high SC bioavailability A B S - 2 0 1 | A G A >3x extended half-life in NHPs compared to HMI-115 High subcutaneous bioavailability in NHPs at >90% In silico prediction of Q8W–Q12W dosing intervals anticipated in humans Manufacturability & developability profile believed to enable future high concentration formulation targeting >150mg/mL N H P - P K 5 6 D A Y R E S U L T S 0 20 40 60 10 2 10 3 10 4 10 5 10 6 10 7 Single Dose Comparative PK Profile in NHPs Time (d) m Ab s er um c on c (n g/ m l) ABS-201 100mg/ kg SC ABS- 201 100mg/ kg IV ABS- 201 300mg/ kg IV HMI-115 300mg/ kg IV Datapoints of animals with positive ADA rates impacting PK were excluded at corresponding timepoints onwards S I N G L E D O S E C O M P A R A T I V E P K P R O F I L E I N N H P s *assumption on HMI-115: 60mg/mL formulation and Q2W or Q4W dosing interval Based on PK/PD modeling, ABS-201 is anticipated to likely require only 2-3 doses over a 6-month treatment period, compared to HMI-115, which would likely require 6-12+* doses in the same period, assuming the AGA indication is pursued.
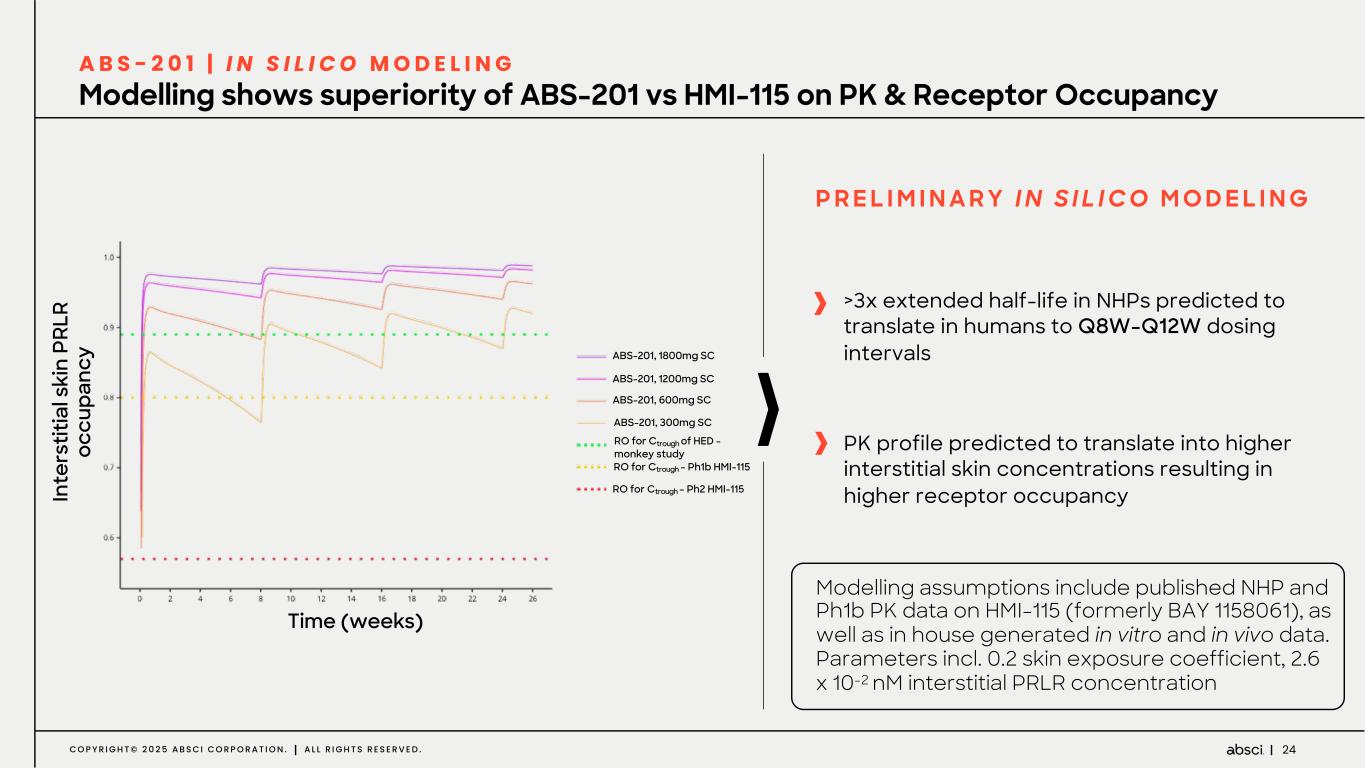
24C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . ABS-201, 1800mg SC ABS-201, 1200mg SC ABS-201, 600mg SC ABS-201, 300mg SC RO for Ctrough of HED - monkey study RO for Ctrough – Ph1b HMI-115 RO for Ctrough – Ph2 HMI-115 Time (weeks) In te rs tit ia l s ki n PR LR oc cu pa nc y Modelling shows superiority of ABS-201 vs HMI-115 on PK & Receptor Occupancy A B S - 2 0 1 | I N S I L I C O M O D E L I N G >3x extended half-life in NHPs predicted to translate in humans to Q8W-Q12W dosing intervals PK profile predicted to translate into higher interstitial skin concentrations resulting in higher receptor occupancy P R E L I M I N A R Y I N S I L I C O M O D E L I N G Modelling assumptions include published NHP and Ph1b PK data on HMI-115 (formerly BAY 1158061), as well as in house generated in vitro and in vivo data. Parameters incl. 0.2 skin exposure coefficient, 2.6 x 10-2 nM interstitial PRLR concentration

25C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Potential best-in-class PRLR antibody for treating androgenic alopecia (AGA) A B S - 2 0 1 | A G A AI-designed PRLR program for durable hair regrowth therapy addressing patient population of >80M in the US alone High affinity and potency Excellent developability profile à high- concentration formulation and great stability Anticipated low immunogenicity Extended half-life and expected longer dosing intervals Clinical development strategy expected to enable PoC in H2-2026 Potential to be first to market in the U.S.

26C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . D E S I R E D A T T R I B U T E HMI-115 ABS-201 A F F I N I T Y + ++ I N V I T R O P O T E N C Y ++ ++ H I G H S O L U B I L I T Y - ++ S T A B I L I T Y - + E X T E N D E D ½ - L I F E - ++ B I O A V A I L A B I L I T Y - ++ P A T E N T L I F E - ++ Superior profile of ABS-201 A B S - 2 0 1 | A G A Excellent Manufacturability & Developability enables future high concentration formulation targeting 200mg/ml (ABS-201) vs. ~60mg/ml (HMI-115) E x p e c t e d i m p r o v e d e f f i c a c y a n d p a t i e n t c o n v e n i e n c e b a s e d o n : Excellent absolute bioavailability profile in NHPs (>90%) to enable subcutaneous (SC) injection > 3x extended half-life vs HMI-115 in NHPs to enable longer dosing intervals - Q8W/Q12W vs. Q2W/Q4W

27C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Straightforward path for ABS-201 clinical development C L I N I C A L T R I A L S F O R H A I R T R E A T M E N T S A R E E X P E C T E D T O B E S T R A I G H T F O R W A R D • Ease of patient recruitment • High level of KOL Interest • Ability to conduct multi-center trials • Non-invasive trial conduct W E L L D E F I N E D E N D P O I N T S W I T H V A L I D A T E D M E A S U R E S Endpoints: Quantitative measurements with follicular dermatoscope (trichoscopy) • Target area hair count (TAHC – per cm2) • Target area hair width (TAHW) • Target area hair darkness/pigmentation Investigator and participant-rated global improvement: • Reported outcomes as measured by validated scales accepted by the FDA (SSA & IGA score) • Exploratory - Canfield Male Pattern Hair Loss Patient Reported Outcome (CMPHL-PRO) A B S - 2 0 1 | A G A

28C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Leading Scientific Advisory Board of Hair Experts DR. ANTHONY ROSSI Memorial Sloan Kettering Cancer Center DR. CHESAHNA KINDRED Kindred Hair & Skin Center DR. MATT L. LEAVITT Advanced Dermatology and Cosmetic Surgery DR. MEENA SINGH Skin and Hair Center DR. MARIA K. HORDINSKY Univ. of Minnesota DR. SUZANNE KILMER Laser & Skin Surgery Center of Northern California DR. KEN WASHENIK Bosley Medical Group DR. GLYNIS ABLON Ablon Skin Institute DR. NEIL S. SADICK Sadick Dermatology DR. DORIS DAY Day Dermatology & Aesthetics Over Half a Million alopecia patients treated each year by these KOL practice networks DR. DAVID GOLDBERG Schweiger Dermatology DR. RODNEY SINCLAIR Sinclair Dermatology

29C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Accelerated development with Ph1/2a trial initiation expected in December 2025 AI-designed development candidates ü High affinity ü High potency ü Favorable manufacturability ü Long half-life A I D E S I G N o f D C C M C / P R E C L I N 2 H 2 0 2 6 IND-enabling studies ongoing Interim Proof-of- concept readout expected D E C 2 0 2 5 FiH clinical development • First participants expected to be dosed ü Favorable PK and long half-life ü High Bioavailability in NHPs • GMP manufacture of sub-Q formulation at high concentration • Expected low ADA • GLP tox studies ongoing A B S - 2 0 1 | A G A • Safety & Tolerability • Hair regrowth efficacy - Target Area Hair Count - Target Area Hair Width - Target Area Hair Darkness

30C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . A B S - 2 0 1 A G A Accelerated development of ABS-201 in androgenetic alopecia • Ph1/2ª study initiation anticipated Dec 2025 • Interim efficacy readout anticipated 2H 2026 A B S - 2 0 1 E n d o Indication expansion for ABS-201 in Endometriosis • Ph2 study anticipated to initiate 4Q2026 with interim PoC readout as early as 2H2027 A B S - 1 0 1 Phase 1 interim results reported with extended half-life vs 1st gen TL1A competitors Advancing partnership & out-licensing discussions, including in ‘first-in-class’ indications A B S - 3 0 1 & 5 0 1 ABS-301: Potential first-in-class asset discovered through Reverse Immunology Platform ABS-501: Candidate ID phase for novel HER2 program designed using de novo AI A c c e l e r a t i o n a n d e x p a n s i o n o f l e a d p r o g r a m D i f f e r e n t i a t e d A I d e s i g n e d p i p e l i n e Absci’s progress in Drug Creation I N T E R N A L P I P E L I N E

31C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Expanded development of ABS-201 in Endometriosis ADDRESSES LONG-STANDING UNMET MEDICAL NEED • Current therapies are palliative with poor tolerability • No disease-modifying options available • Affects ~190M women globally; only ~10% receive treatment today • Disease-modifying therapy has the potential to increase diagnosis and treatment rates • Peak sales potential >$4.5B with adjacent indication upside LARGE, UNTAPPED MARKET OFFERS SIGNIFICANT UPSIDE POTENTIAL • PRLR biology well supported by internal and external data • Positive read-out from HMI-115 validates mechanism • Phase 2 PoC study could start as early as Q4 2026 with interim efficacy readout anticipated 2H 2027 STRONG BIOLOGICAL AND CLINICAL RATIONALE

32C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Endometriosis significantly impacts patients Quality of Life Sources: PMID 40323608, PMID 18367178, ARTEMIS 2024 Report Endo: Endometriosis; QOL: Quality of life Endometriosis is a chronic, estrogen- dependent, inflammatory disease defined by endometrial-like lesions found outside the uterus Prevalent in up to 10% of women worldwide, and an estimated 9M women in the US suffer from endometriosis Symptoms include pelvic pain (~80-90%), heavy bleeding (~60%), infertility (~30%) and ovarian cysts (20%) Indirect disease burdens include anemia, fatigue, sleep disturbances and mood disorders A B S - 2 0 1 | E N D O M E T R I O S I S Subcategorization is typically based on location of the lesions

33C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Endometriosis (Endo) – a chronic and painful condition A B S - 2 0 1 | E N D O M E T R I O S I S Endometrial tissue Pathogenesis involves seeding of ectopic endometrial cell lesions within the abdominal-pelvic cavity • Sex hormones and inflammatory processes contribute to the chronic disease state • Nociceptive pain is closest to the source and usually caused by local inflammation. Neuropathic and nociplastic pain are further removed from the source and are often a result of nerve damage or a sensitized nervous system, respectively Diagnosis of Endo remains an evolving field, with historical standard being confirmation of lesions via laparoscopy • Guidelines now support treatment of ‘Clinically Suspected’ Endo based on a physical examination, symptomology, and non-invasive imaging • Often, Endo diagnoses (and treatment) are delayed by several years

34C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Hormonal therapies and surgical intervention make up the treatment paradigm for Endo • Endo is a chronic condition with currently no medical or surgical cure • Roughly, 75% of patients are estimated to use opioids and/or NSIADs throughout their disease course • Up to 33% of patients do not respond to hormonal treatment alone • Additionally, patients will pause treatment when seeking pregnancy • GnRH therapies are typically prescribed by Gynecologist and often require formal Dx (surgical confirmation) • Due in part to higher cost, and AE profile which limit long- term use • Notably, aromatase inhibitors are not FDA approved for Endo, but are used off-label • Up to 40% of Endo patients undergo a laparoscopy and 12% receive hysterectomies • Even after hysterectomy ~15% of patients still report pain symptoms Sources: PMID 40323608, PMID 37391793, ARTEMIS 2024 Report, GlobalData, US Census Data A B S - 2 0 1 | E N D O M E T R I O S I S Current Treatment Paradigm (US) Treated Endo patients First-line Options Combined Hormonal Contraception NSAIDs Progestins Second-line Options GnRH agonists Oral GnRH antagonist Oral GnRH antagonist + hormonal Total Estimated Endo patients Opioids NSAIDs Third-line Options Aromatase Inhibitors Laparoscopy +/- Hysterectomy

35C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . • Patients with Endo report ineffective pain management as a number one unmet need • Additionally, patients are seeking the ability to have children • The condition itself carries a higher risk for infertility • Current therapeutic interventions prevent pregnancy or mimic a post-menopausal state • There has been little advancement in novel targets for endometriosis • Contraceptive hormonal therapy has remained unchanged for decades • GnRH therapy, while effective for some needs to be stopped after 2 years due to AE risks (e.g., loss in bone density) • Endo remains underdiagnosed, which is predicted to improve with a disease-modifying therapy • Mean age of symptom onset is typically early 20s, while mean age of diagnosis is typically mid-30s Pain remains a persistent, chronic issue for women with endometriosis A B S - 2 0 1 | E N D O M E T R I O S I S Sources: PMID 40323608, PMID 17498711, ARTEMIS 2024 Report Illustrative Endometrial Patient Journeys First Period Desire to Conceive Perimenopause
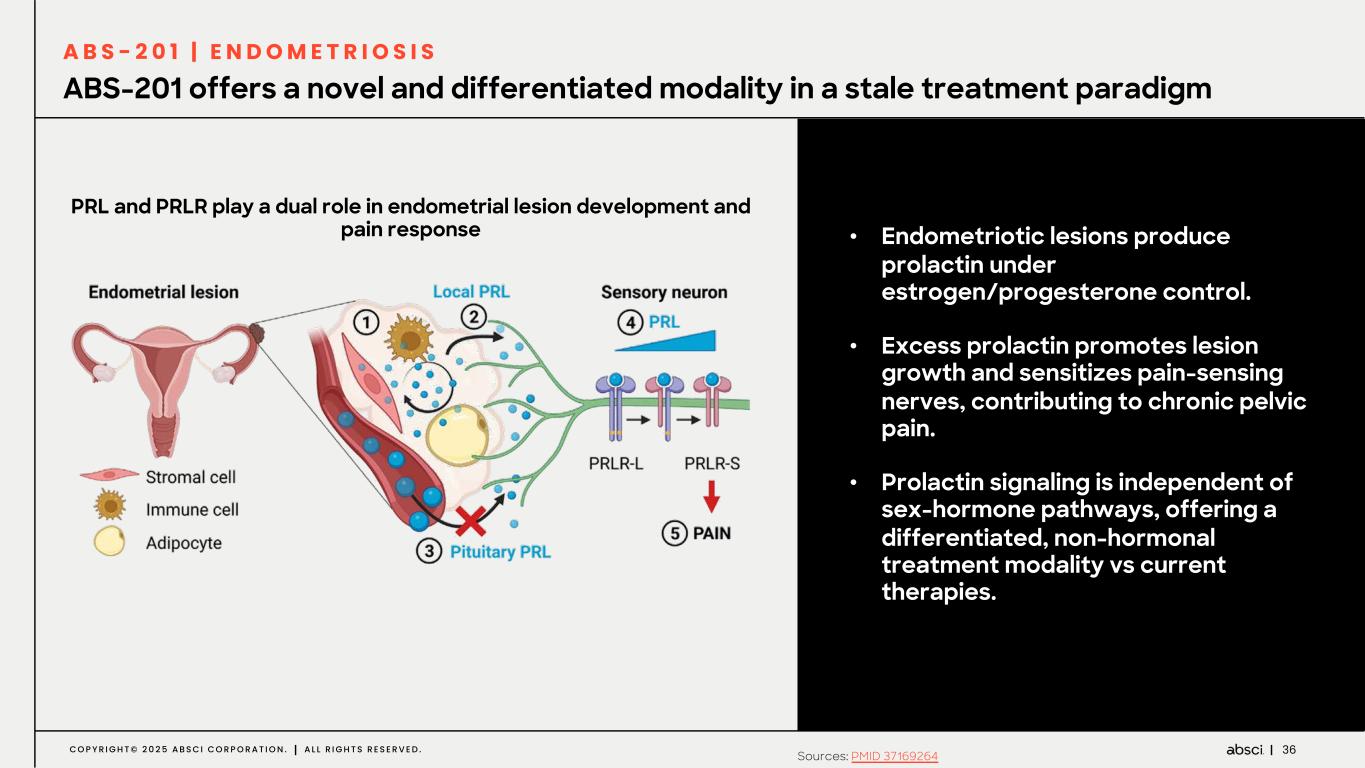
36C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . ABS-201 offers a novel and differentiated modality in a stale treatment paradigm A B S - 2 0 1 | E N D O M E T R I O S I S PRL and PRLR play a dual role in endometrial lesion development and pain response Sources: PMID 37169264 • Endometriotic lesions produce prolactin under estrogen/progesterone control. • Excess prolactin promotes lesion growth and sensitizes pain-sensing nerves, contributing to chronic pelvic pain. • Prolactin signaling is independent of sex-hormone pathways, offering a differentiated, non-hormonal treatment modality vs current therapies.
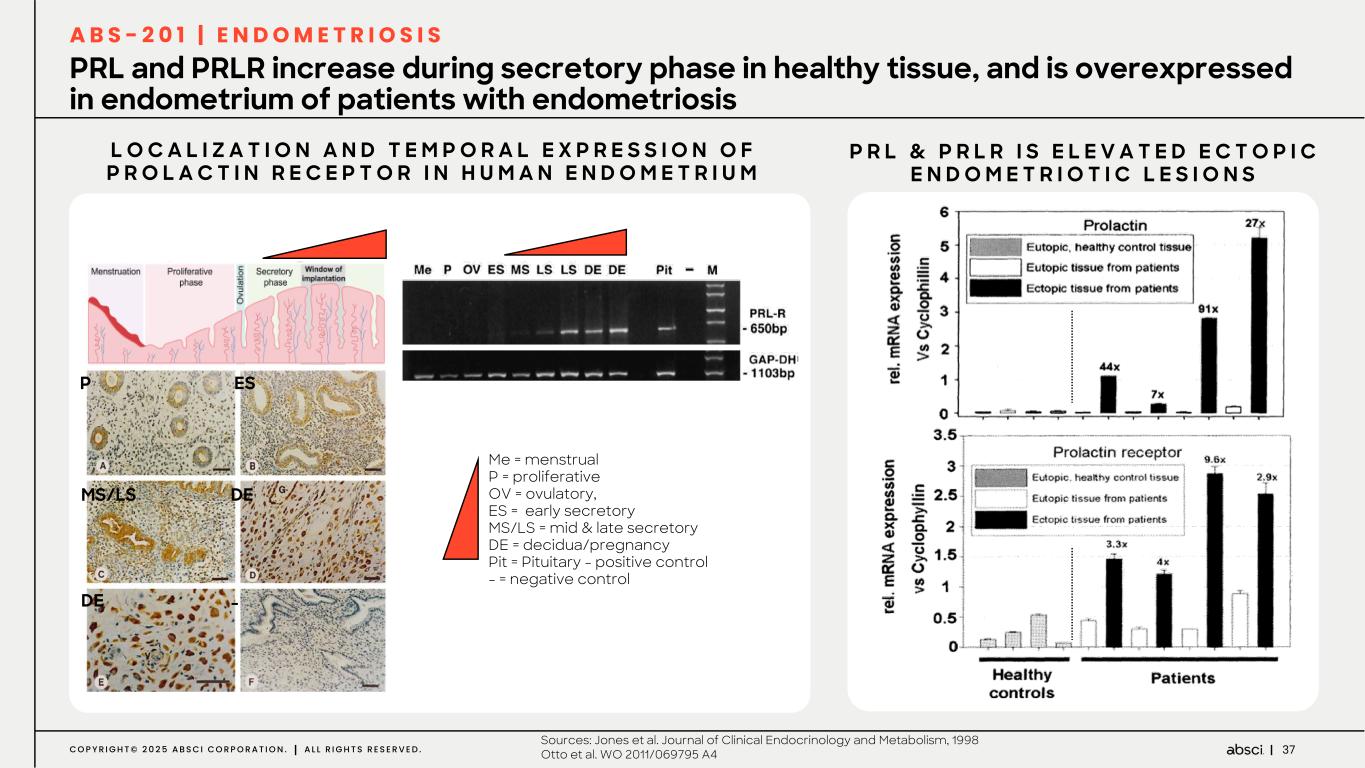
37C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . PRL and PRLR increase during secretory phase in healthy tissue, and is overexpressed in endometrium of patients with endometriosis L O C A L I Z A T I O N A N D T E M P O R A L E X P R E S S I O N O F P R O L A C T I N R E C E P T O R I N H U M A N E N D O M E T R I U M Me = menstrual P = proliferative OV = ovulatory, ES = early secretory MS/LS = mid & late secretory DE = decidua/pregnancy Pit = Pituitary - positive control - = negative control P R L & P R L R I S E L E V A T E D E C T O P I C E N D O M E T R I O T I C L E S I O N S P ES MS/LS DE DE - A B S - 2 0 1 | E N D O M E T R I O S I S Sources: Jones et al. Journal of Clinical Endocrinology and Metabolism, 1998 Otto et al. WO 2011/069795 A4
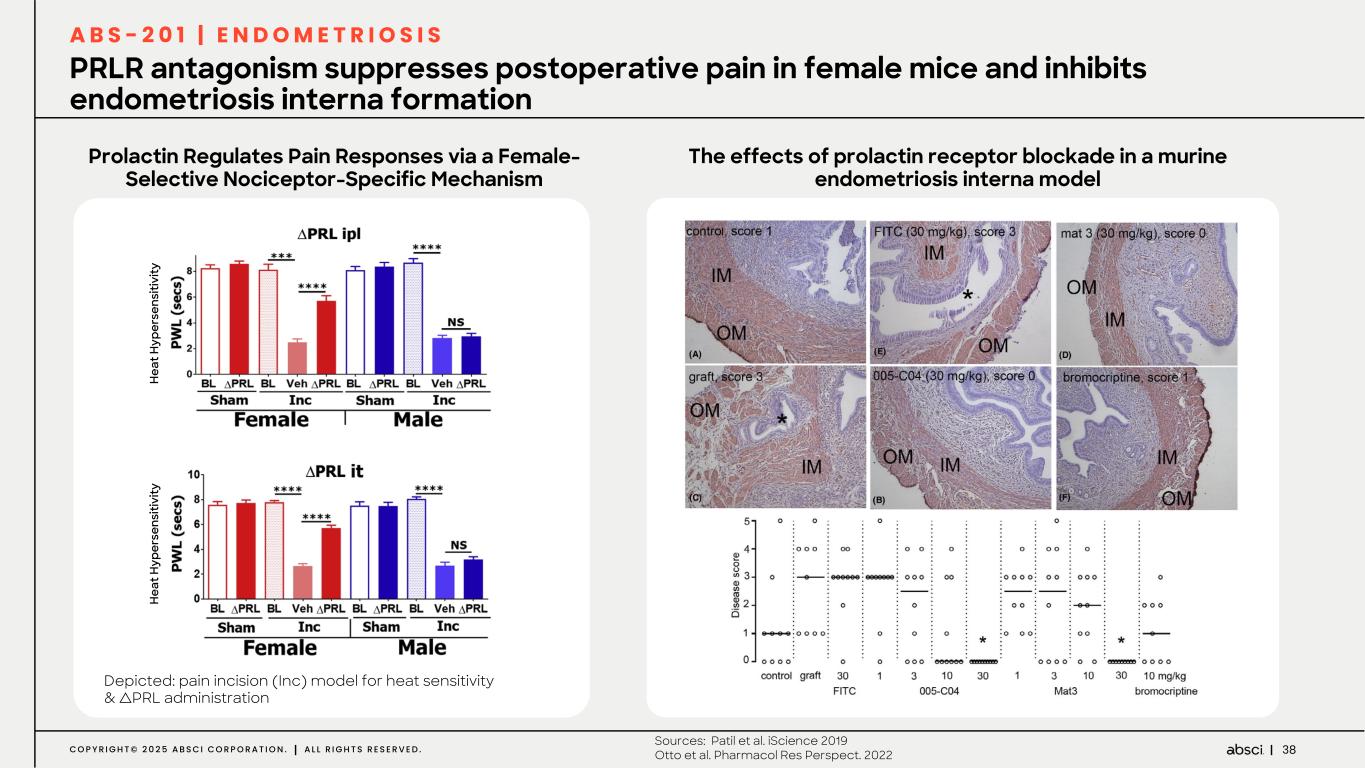
38C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . PRLR antagonism suppresses postoperative pain in female mice and inhibits endometriosis interna formation Prolactin Regulates Pain Responses via a Female- Selective Nociceptor-Specific Mechanism The effects of prolactin receptor blockade in a murine endometriosis interna model Depicted: pain incision (Inc) model for heat sensitivity & ∆PRL administration A B S - 2 0 1 | E N D O M E T R I O S I S Sources: Patil et al. iScience 2019 Otto et al. Pharmacol Res Perspect. 2022 H ea t H yp er se ns iti vi ty H ea t H yp er se ns iti vi ty
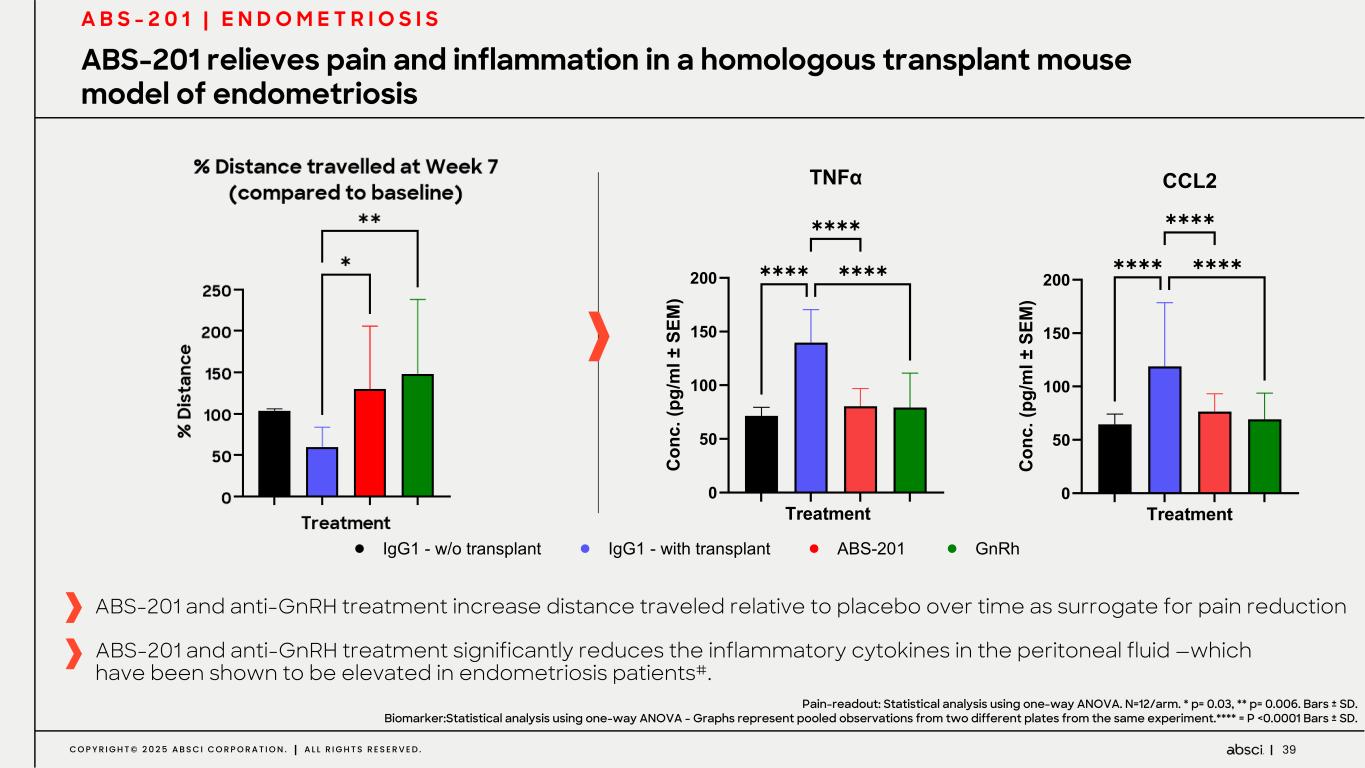
39C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . ABS-201 relieves pain and inflammation in a homologous transplant mouse model of endometriosis A B S - 2 0 1 | E N D O M E T R I O S I S ABS-201 and anti-GnRH treatment increase distance traveled relative to placebo over time as surrogate for pain reduction ! "! #!! #"! $!! %%C$ %' E) *+, -. LM 1+2 +3 45 S !!!! !!!! !!!! T89:;M9E; ! "! #!! #"! $!! %C'E F *+ ,- ./M N2 3 4.5 .S T8 9 !!!! !!!! !!!! %:;<=3;+= Pain-readout: Statistical analysis using one-way ANOVA. N=12/arm. * p= 0.03, ** p= 0.006. Bars ± SD. Biomarker:Statistical analysis using one-way ANOVA - Graphs represent pooled observations from two different plates from the same experiment.**** = P <0.0001 Bars ± SD. ABS-201 and anti-GnRH treatment significantly reduces the inflammatory cytokines in the peritoneal fluid —which have been shown to be elevated in endometriosis patients#. 2 72 322 372 422 472 522 572 ' "Fkuvcpeg"vtcxgnngf "cv"Y ggm"9 *eqo r ctgf "vq"dcugnkpg+ Vtgcvo gpv ' "F ku vc pe g !"#AB&B'()BG+I-./0I-G !"#AB&B'1G2BG+I-./0I-G 34R&S7A #-82

40C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Effects of hyperprolactinemia treatment with the dopamine agonist quinagolide on endometriotic lesions in patients with endometriosis-associated hyperprolactinemia A B S - 2 0 1 | E N D O M E T R I O S I S Symbol P value vs. control Fold regulation vs. control SERPINE1 .03254a −19.8491 AGGF1 .0376a −3.1954 CCL2 .04389a −6.1199 VEGF .04428a −5.8199 CCL10 .04770a 6.7693 RUNX1 .04901a −4.3878 CXCL12 .08206 −3.3952 BTG1 .086207 −3.3776 FST .10483 −3.0136 RHOB .12902 −1.7303 FGFBP1 .15187 −3.0283 FGF1 .165346 −2.5955 SERPINF1 .183201 1.9339 STAB .19273 −5.4869 Quinagolide treatment reduces CCL2 and VEGF mRNA level Gómez, Raul et al. Fertility and Sterility, Volume 95, Issue 3, 882 - 888.e1 Quinagolide treatment lowers prolactin serum levels Fibrosis Inflammatory/ angiogenic Quinagolide treatment reduces lesion size

41C O P Y R I G H T © 2 0 2 4 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . ABS-201 offers a novel treatment option for patients with Endometriosis • Non–sex-steroid hormonal MOA • Potential for improved AE profile, longer duration of treatment over GnRH therapies • Potential for best-in-class anti-PRLR given enhanced developability and expected half-life • Potential for dual action on pain pathway and lesion proliferation • Ability for disease-modification may increase diagnosis and treatment rates Potential to generate >$4.5B at peak sales ABS-201 offers a differentiated profile with potential for blockbuster peak sales A B S - 2 0 1 | E N D O M E T R I O S I S Treated Endo patients: 1.4 - 2.4M 1L Contraception NSAIDs Progestins 2L GnRH anti/agonists ABS-201 GnRH + add-back ABS-201 Total Addressable Endo Pts: 7.8 - 9M 3L Aromatase Inhibitors Laparoscopy +/- Hyster. ABS-201
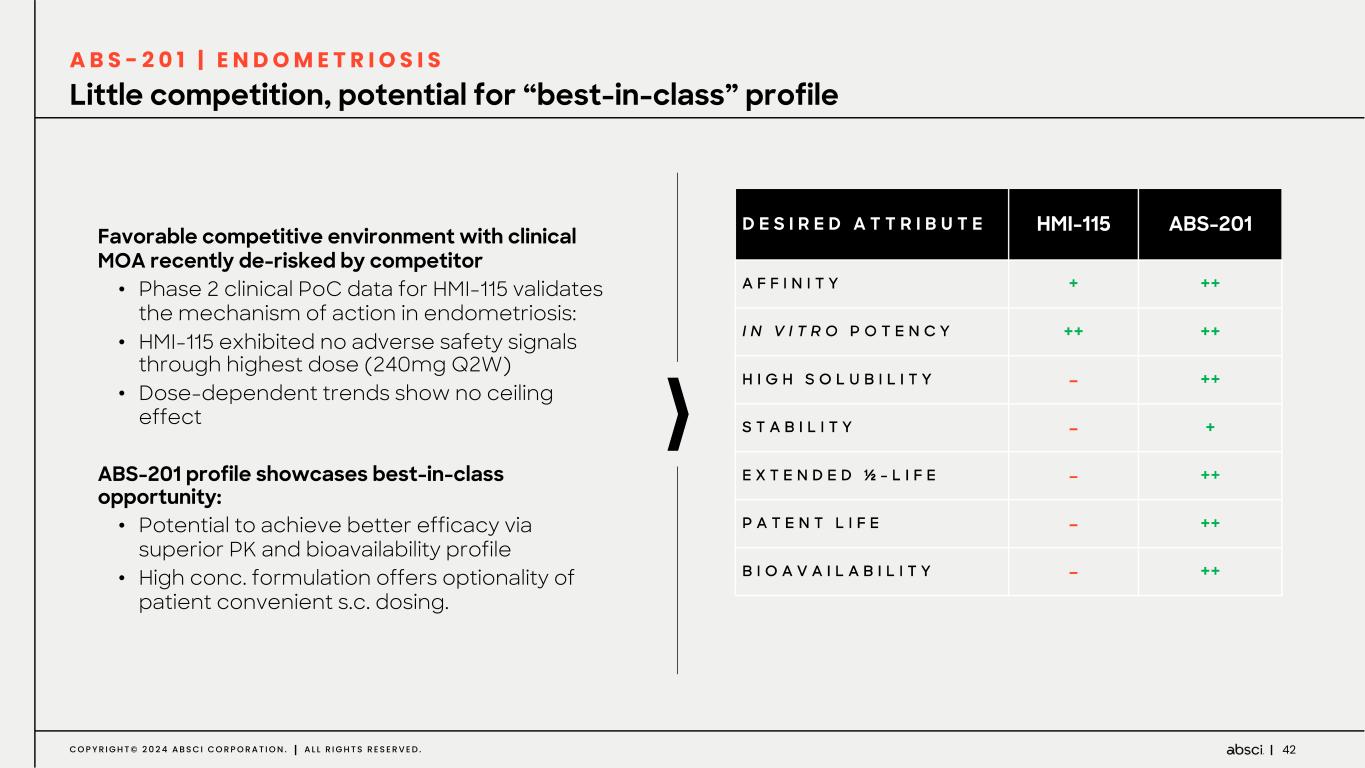
42C O P Y R I G H T © 2 0 2 4 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Little competition, potential for “best-in-class” profile A B S - 2 0 1 | E N D O M E T R I O S I S Favorable competitive environment with clinical MOA recently de-risked by competitor • Phase 2 clinical PoC data for HMI-115 validates the mechanism of action in endometriosis: • HMI-115 exhibited no adverse safety signals through highest dose (240mg Q2W) • Dose-dependent trends show no ceiling effect ABS-201 profile showcases best-in-class opportunity: • Potential to achieve better efficacy via superior PK and bioavailability profile • High conc. formulation offers optionality of patient convenient s.c. dosing. D E S I R E D A T T R I B U T E HMI-115 ABS-201 A F F I N I T Y + ++ I N V I T R O P O T E N C Y ++ ++ H I G H S O L U B I L I T Y - ++ S T A B I L I T Y - + E X T E N D E D ½ - L I F E - ++ P A T E N T L I F E - ++ B I O A V A I L A B I L I T Y - ++
43C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . A B S - 2 0 1 A G A Accelerated development of ABS-201 in androgenetic alopecia • Ph1/2ª study initiation anticipated Dec 2025 • Interim efficacy readout anticipated 2H 2026 A B S - 2 0 1 E n d o Indication expansion for ABS-201 in Endometriosis • Ph2 study anticipated to initiate 4Q2026 with interim PoC readout as early as 2H2027 A B S - 1 0 1 Phase 1 interim results reported with extended half life vs 1st gen TL1A competitors Advancing partnership & out-licensing discussions, including in ‘first-in-class’ indications A B S - 3 0 1 & 5 0 1 ABS-301: Potential first-in-class asset discovered through Reverse Immunology Platform ABS-501: Candidate ID phase for novel HER2 program designed using de novo AI A c c e l e r a t i o n a n d e x p a n s i o n o f l e a d p r o g r a m D i f f e r e n t i a t e d A I d e s i g n e d p i p e l i n e Absci’s progress in Drug Creation I N T E R N A L P I P E L I N E

44N O N - C O N F I D E N T I A L C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . AI Designed TL1A antibody in Ph1 Clinical development; ready for partnering ABS-101 Summary A B S - 1 0 1 T L 1 A No apparent impact of ADA on PK and the overall safety profile was favorable with no serious adverse events reported to date. Advancing partnership discussions, some of which leverage unique properties of ABS-101 and are focused on first-in-class indications outside of IBD Interim results from the first cohorts of Phase 1 trial demonstrated extended half- life as compared to 1st-generation anti-TL1A competitor programs. Trial on track to complete treatment period in Q1 2026 and we will no longer pursue additional internal clinical development of this asset following completion of the Phase 1 trial

45C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Validated mechanism of action in large underserved market A B S - 1 0 1 T L 1 A P O T E N T I A L R E L E V A N C E I N W I D E R A N G E O F A U T O I M M U N E I N D I C A T I O N S T L 1 A : D R 3 S I G N A L I N G C L I N I C A L L Y S H O W N T O I N D U C E P R O - I N F L A M M A T O R Y R E S P O N S E S 1 Significant market opportunities beyond IBD $22B+ Global IBD Market4 $4.5B for TL1A 0.8- 3M U.S IBD prevalence3 5M Global IBD prevalence2 1 Adapted from Takedatsu 2008 doi: 10.1053/j.gastro.2008.04.037 2 Wang 2023 http://dx.doi.org/10.1136/bmjopen-2022-065186 3 Dahlhamer, James M., et al. "Prevalence of inflammatory bowel disease among adults aged≥ 18 years— United States, 2015." Morbidity and mortality weekly report 65.42 (2016): 1166-1169. 4 Evaluate Pharma Oct 2023. T-CELL Fibroblast SIGNALING SIGNALING FIBROSIS SOLUBLE TL1A DR3 DR3 SOLUBLE TL1A INFLAMMATION
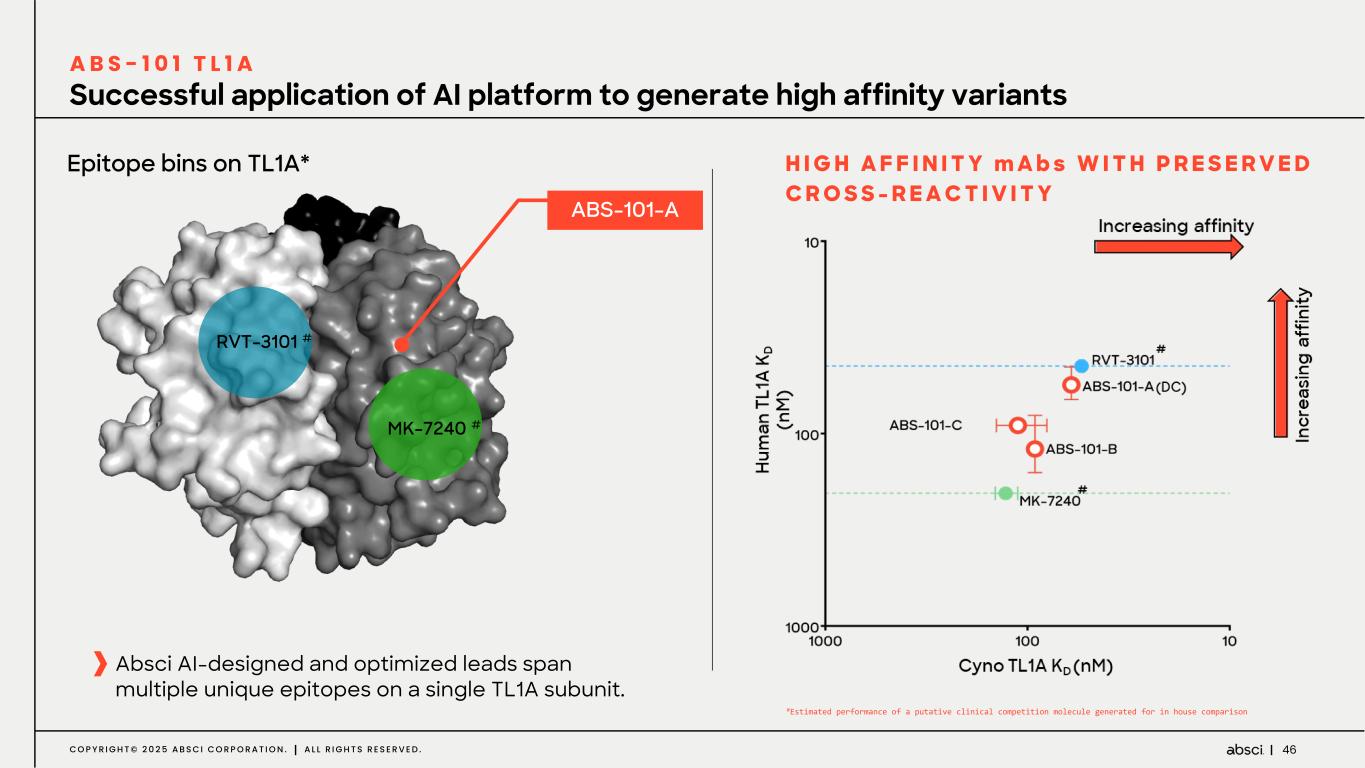
46C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Successful application of AI platform to generate high affinity variants A B S - 1 0 1 T L 1 A H I G H A F F I N I T Y m A b s W I T H P R E S E R V E D C R O S S - R E A C T I V I T Y MK-7240 # RVT-3101 # Epitope bins on TL1A* ABS-101-A Absci AI-designed and optimized leads span multiple unique epitopes on a single TL1A subunit. #Estimated performance of a putative clinical competition molecule generated for in house comparison

47C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . 1101001000 1 10 100 1000 Monom er Bind ing (pM) Tr im er B in di ng (p M ) No M onomer Bind ing H I G H A F F I N I T Y m A b s W I T H B I N D I N G T O B O T H T H E T L 1 A M O N O M E R A N D T R I M E R A I - O P T I M I Z E D L O W p M A F F I N I T Y T R A N S L A T E S T O S U P E R I O R O R E Q U I V A L E N T P O T E N C Y In cr ea si ng p ot en cy IC 50 ( nM ) 0 2 4 6 8 10 12 ABS-10 1 RVT-3 10 1# (R oche) MK-7 240 # (M erc k) APOPTOSIS INHIBITION ASSAY IN TF-1 CELLSAFFINITY BY BIOLAYER INTERFEROMETRY (BLI) AI-designed candidate with high affinity and potential for superior potency A B S - 1 0 1 T L 1 A Increasing affinity In cr ea si ng a ff in ity Weak monomer binding ABS-101 RVT-3101# (Roche) MK-7240# (Merck) TEV-48574# (Sanofi) #Estimated performance of a putative clinical competition molecule generated for in house comparison
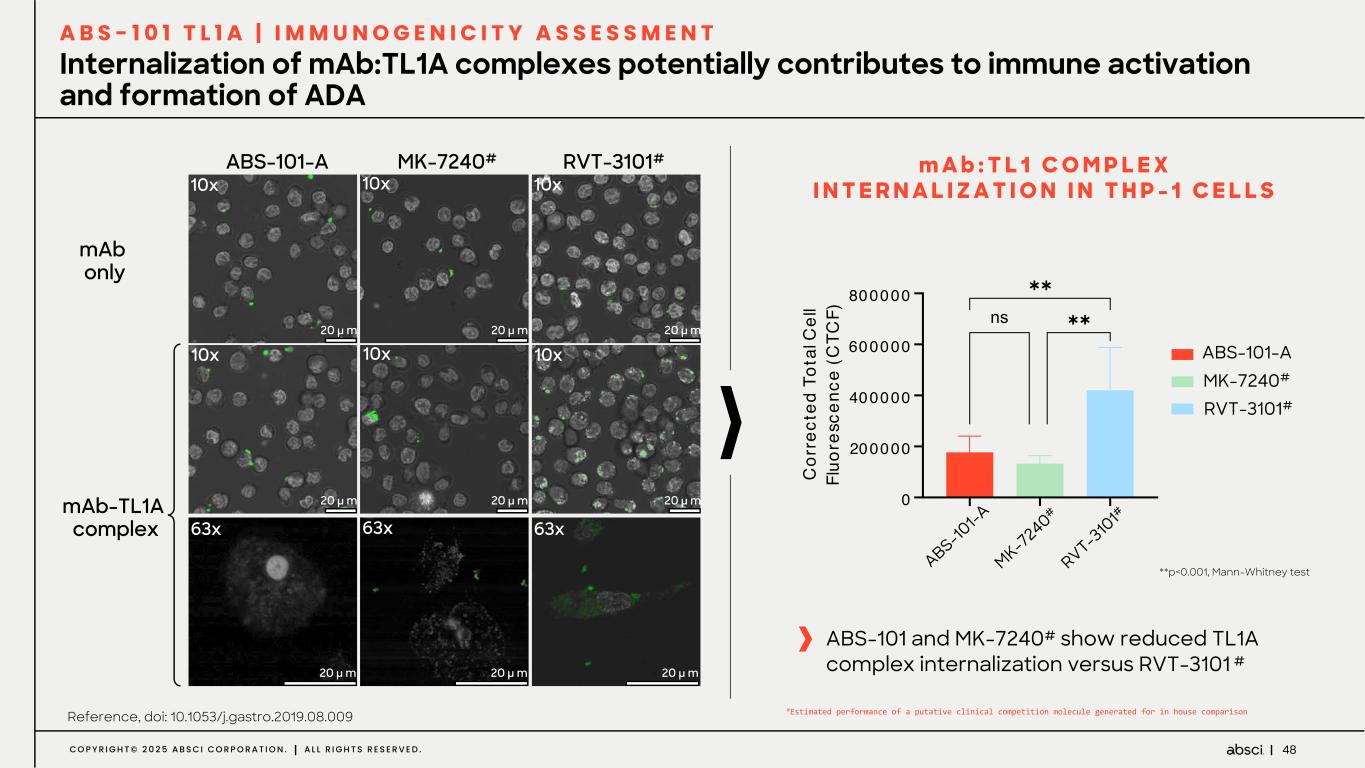
48C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Internalization of mAb:TL1A complexes potentially contributes to immune activation and formation of ADA A B S - 1 0 1 T L 1 A | I M M U N O G E N I C I T Y A S S E S S M E N T mAb only ABS-101-A RVT-3101#MK-7240# 10x 10x 10x 10x 10x 10x 63x 63x 63x 20 µ m 20 µ m 20 µ m 20 µ m 20 µ m 20 µ m 20 µ m 20 µ m 20 µ m mAb-TL1A complex m A b : T L 1 C O M P L E X I N T E R N A L I Z A T I O N I N T H P - 1 C E L L S **p<0.001, Mann-Whitney test Reference, doi: 10.1053/j.gastro.2019.08.009 ABS-101 and MK-7240# show reduced TL1A complex internalization versus RVT-3101 # AB S- 10 1 MK - 7 24 0 RV T- 310 1 0 200000 400000 600000 800000 C or re ct ed T ot al C el l Fl uo re sc en ce (C TC F) !"#AB&B '(A)*+& ,-KA/B&B M1 !! !! ABS-101-A MK-7240# RVT-3101# S-10 1-A MK-7 240# RVT-3 10 1# #Estimated performance of a putative clinical competition molecule generated for in house comparison
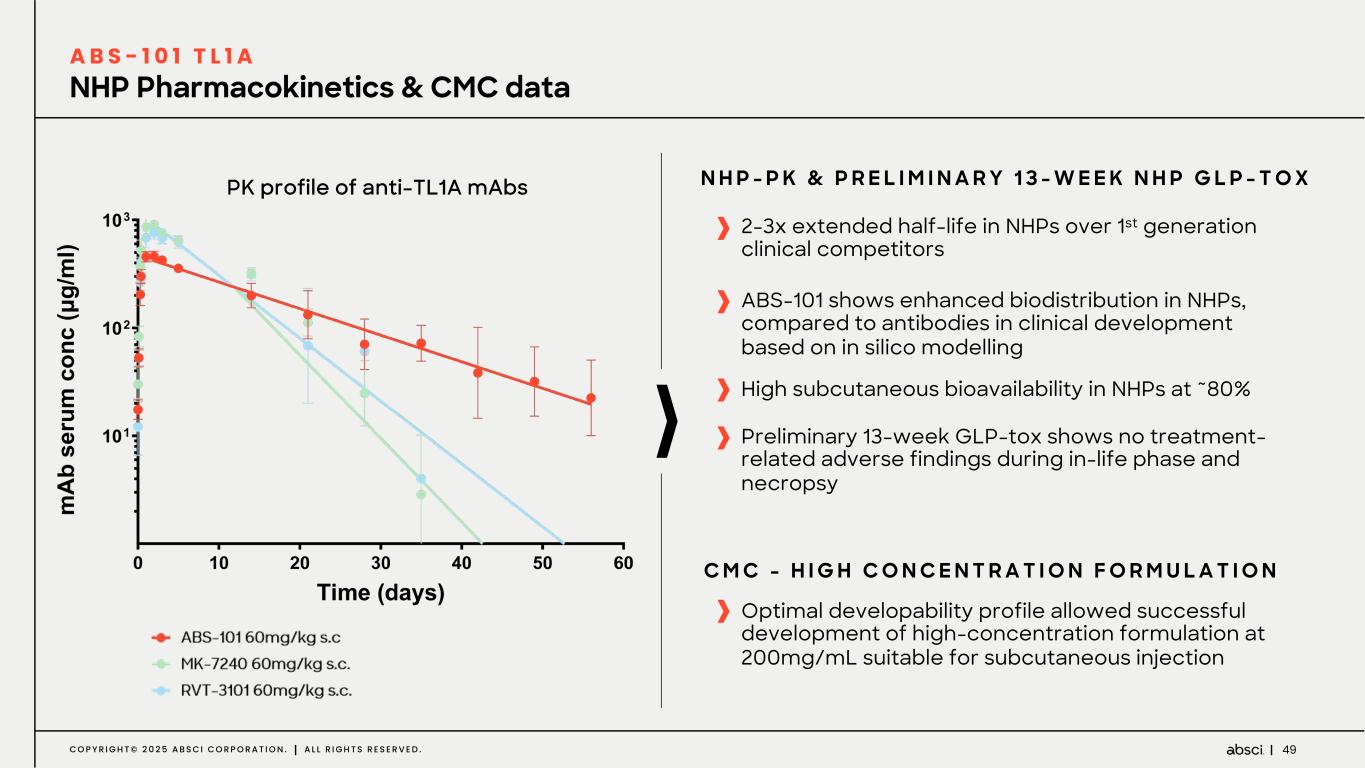
49C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . NHP Pharmacokinetics & CMC data A B S - 1 0 1 T L 1 A 2-3x extended half-life in NHPs over 1st generation clinical competitors ABS-101 shows enhanced biodistribution in NHPs, compared to antibodies in clinical development based on in silico modelling Optimal developability profile allowed successful development of high-concentration formulation at 200mg/mL suitable for subcutaneous injection C M C - H I G H C O N C E N T R A T I O N F O R M U L A T I O N Preliminary 13-week GLP-tox shows no treatment- related adverse findings during in-life phase and necropsy High subcutaneous bioavailability in NHPs at ~80% N H P - P K & P R E L I M I N A R Y 1 3 - W E E K N H P G L P - T O XPK profile of anti-TL1A mAbs

50C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . ABS-101 Non-Human Primate (NHP) data A B S - 1 0 1 T L 1 A ! "! #! A! %! &! '! !(" " "! "!! "!!! )*+, ,- ." L 01 23 4 5 !"#AB&B'B()*+,*'-K/K !"#AB&B'M&)*+,*'-K/K !"#AB&B'M&)*+,*'1K2K !"#AB&B'3&&)*+,*'-K/K 4RSA3B&B'M&')*+,*'-K/K T8AV(:&'M&')*+,*'-K/K Data confirm engagement of soluble TL1A (sTL1A) in non-human primates. A B S - 1 0 1 S H O W S D O S E - D E P E N D E N T A N D S U S T A I N E D T A R G E T E N G A G E M E N T ABS-101's extended half-life translates into sustained target engagement compared to first generation TL1A antibodies at comparable dose and route of administration. Target engagement is dose-dependent with a ceiling effect. Total sTL1A after single dose
51C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Phase 1 Clinical trial interim data reported; advancing partnership discussions A B S - 1 0 1 T L 1 A AI-designed Development Candidate ü High affinity ü High potency ü Long half-life ü Favorable manufacturability D I S CO V E R Y C M C / P R E C L I N I C A L M A Y 2 0 2 5 IND-enabling studies to evaluate: ü GMP manufacture of sub-Q formulation at high concentration ü Favorable PK and long half-life ü High Bioavailability in NHPs • Low ADA ü 13-week GLP tox: No treatment- related adverse findings during in-life phase and necropsy observed Phase 1 double-blind, placebo-controlled trial initiated in Australia Phase 1 interim data readout demonstrated: ü Extended half-life compared to 1st generation competitors ü No apparent impact of ADA on PK ü Favorable overall safety profile; no serious AEs N O V 2 0 2 5
52C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . A B S - 2 0 1 A G A Accelerated development of ABS-201 in androgenetic alopecia • Ph1/2ª study initiation anticipated Dec 2025 • Interim efficacy readout anticipated 2H 2026 A B S - 2 0 1 E n d o Indication expansion for ABS-201 in Endometriosis • Ph2 study anticipated to initiate 4Q2026 with interim PoC readout as early as 2H2027 A B S - 1 0 1 Phase 1 interim results reported with extended half life vs 1st gen TL1A competitors Advancing partnering & out-licensing discussions, including in ‘first-in-class’ indications A B S - 3 0 1 & 5 0 1 ABS-301: Potential first-in-class asset discovered through Reverse Immunology Platform ABS-501: Candidate ID phase for novel HER2 program designed using de novo AI A c c e l e r a t i o n a n d e x p a n s i o n o f l e a d p r o g r a m D i f f e r e n t i a t e d A I d e s i g n e d p i p e l i n e Absci’s progress in Drug Creation I N T E R N A L P I P E L I N E
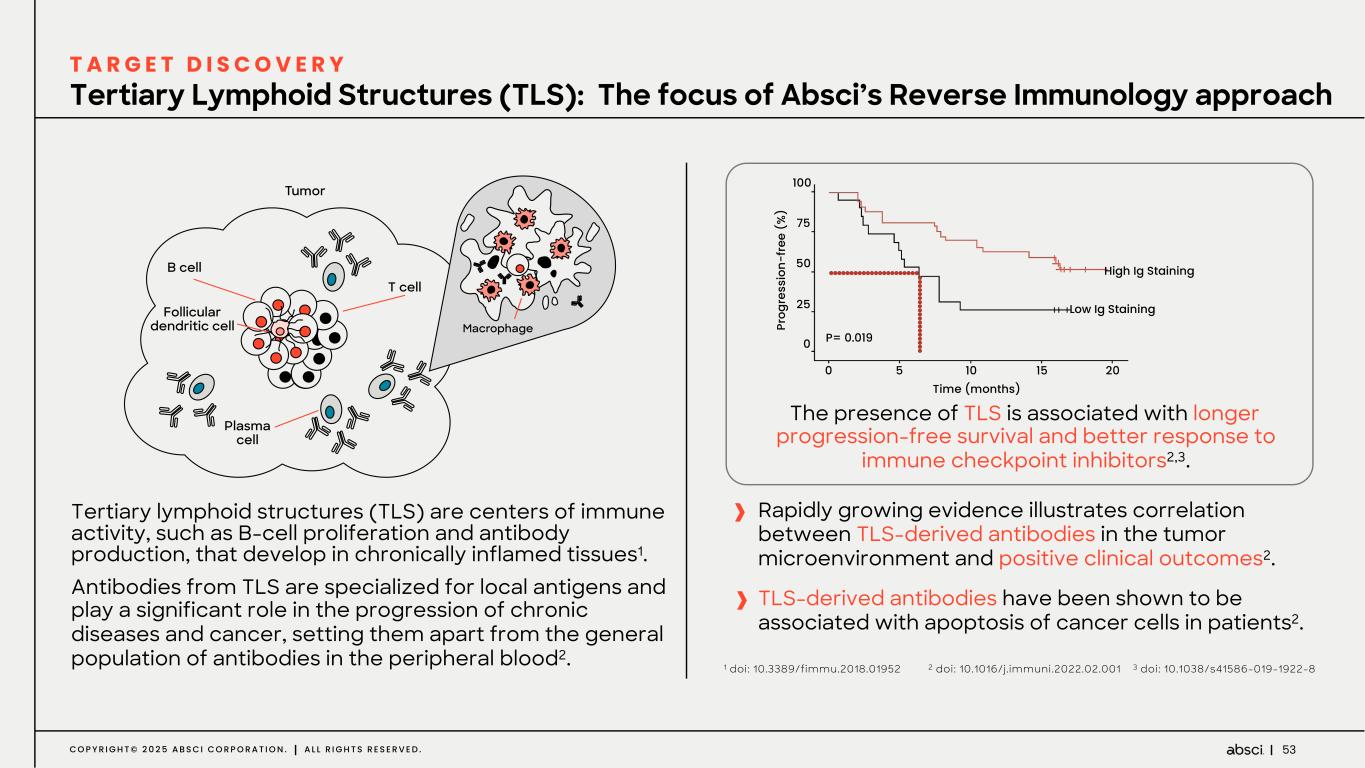
53C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . The presence of TLS is associated with longer progression-free survival and better response to immune checkpoint inhibitors2,3. Rapidly growing evidence illustrates correlation between TLS-derived antibodies in the tumor microenvironment and positive clinical outcomes2. TLS-derived antibodies have been shown to be associated with apoptosis of cancer cells in patients2. Tertiary lymphoid structures (TLS) are centers of immune activity, such as B-cell proliferation and antibody production, that develop in chronically inflamed tissues1. Antibodies from TLS are specialized for local antigens and play a significant role in the progression of chronic diseases and cancer, setting them apart from the general population of antibodies in the peripheral blood2. 100 75 50 25 10 15 20 0 0 5 High Ig Staining Low Ig Staining Pr og re ss io n- fr ee ( % ) Time (months) P= 0.019 Tertiary Lymphoid Structures (TLS): The focus of Absci’s Reverse Immunology approach T A R G E T D I S C O V E R Y 1 doi: 10.3389/fimmu.2018.01952 2 doi: 10.1016/j.immuni.2022.02.001 3 doi: 10.1038/s41586-019-1922-8
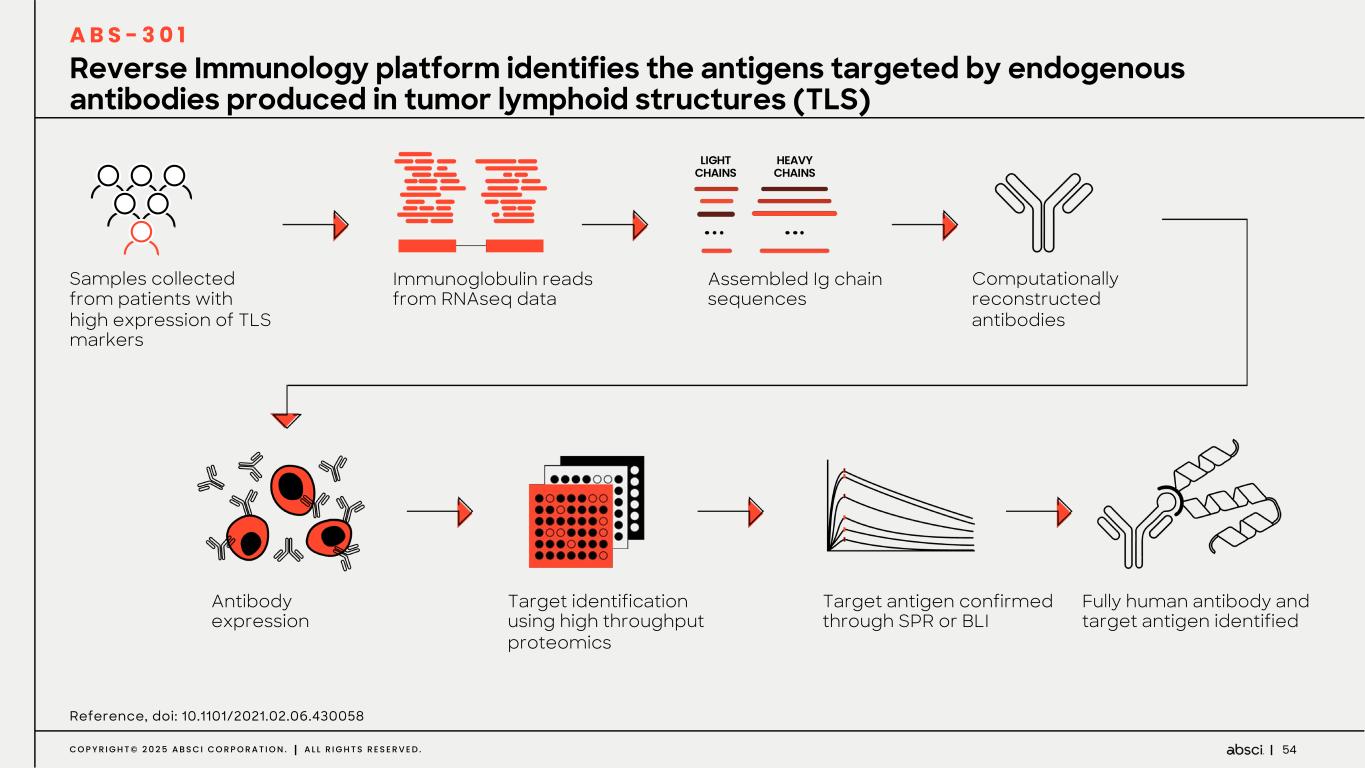
54C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . LIGHT CHAINS HEAVY CHAINS Samples collected from patients with high expression of TLS markers Antibody expression Immunoglobulin reads from RNAseq data Target identification using high throughput proteomics Assembled Ig chain sequences Target antigen confirmed through SPR or BLI Computationally reconstructed antibodies Fully human antibody and target antigen identified Reference, doi: 10.1101/2021.02.06.430058 Reverse Immunology platform identifies the antigens targeted by endogenous antibodies produced in tumor lymphoid structures (TLS) A B S - 3 0 1
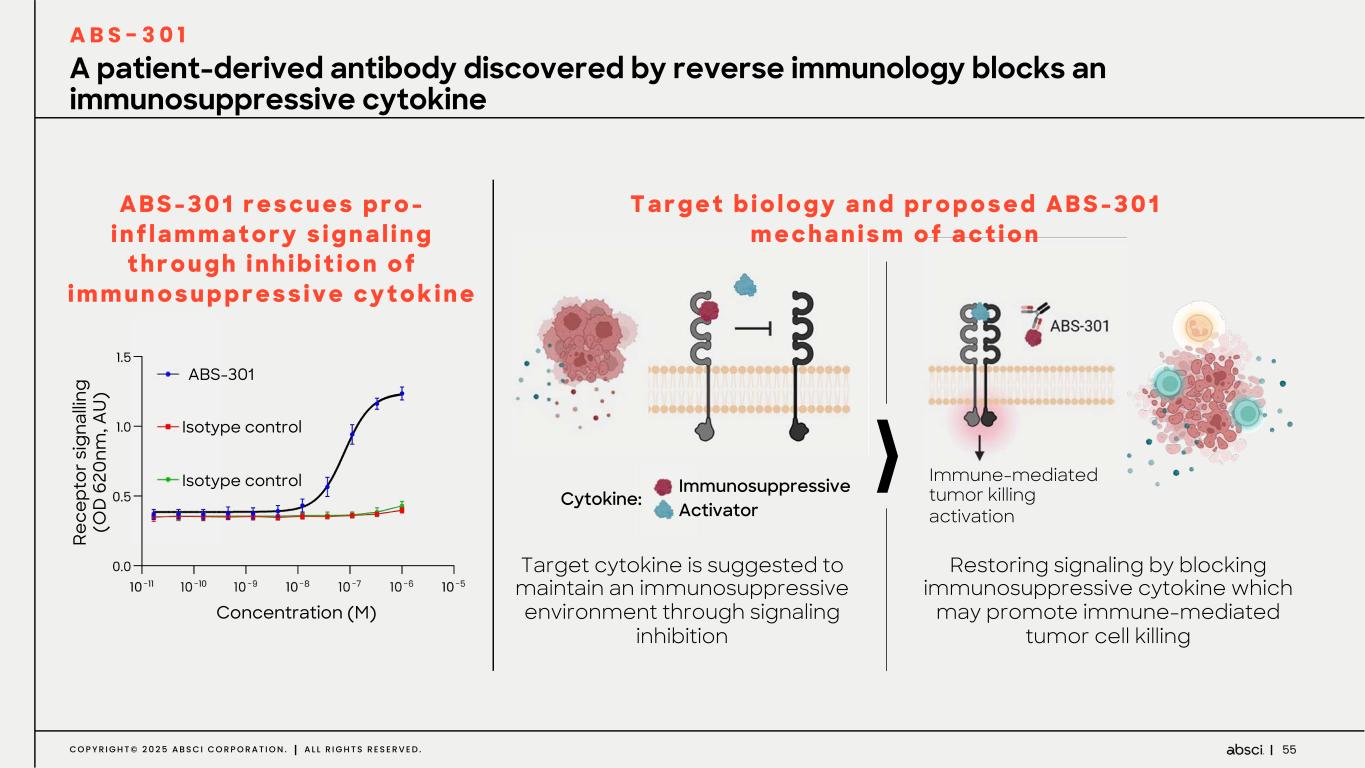
55C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . A B S - 3 0 1 r e s c u e s p r o - i n f l a m m a t o r y s i g n a l i n g t h r o u g h i n h i b i t i o n o f i m m u n o s u p p r e s s i v e c y t o k i n e A patient-derived antibody discovered by reverse immunology blocks an immunosuppressive cytokine A B S - 3 0 1 Restoring signaling by blocking immunosuppressive cytokine which may promote immune-mediated tumor cell killing Target cytokine is suggested to maintain an immunosuppressive environment through signaling inhibition Re ce pt or s ig na llin g (O D 6 20 nm , A U ) ABS-301 Isotype control Isotype control Concentration (M) Immunosuppressive Activator Immune-mediated tumor killing activation Cytokine: T a r g e t b i o l o g y a n d p r o p o s e d A B S - 3 0 1 m e c h a n i s m o f a c t i o n
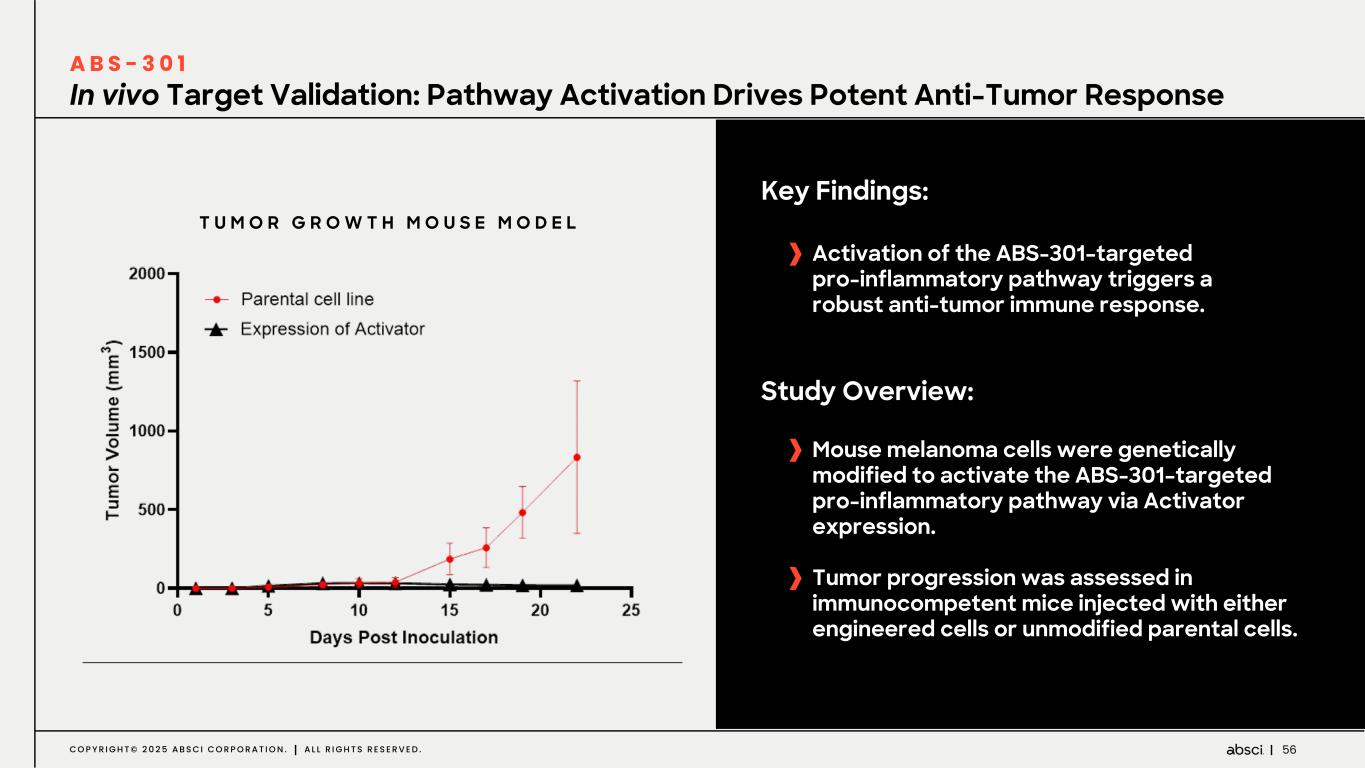
56C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . In vivo Target Validation: Pathway Activation Drives Potent Anti-Tumor Response A B S - 3 0 1 Key Findings: Activation of the ABS-301–targeted pro-inflammatory pathway triggers a robust anti-tumor immune response. Study Overview: Mouse melanoma cells were genetically modified to activate the ABS-301–targeted pro-inflammatory pathway via Activator expression. Tumor progression was assessed in immunocompetent mice injected with either engineered cells or unmodified parental cells. T U M O R G R O W T H M O U S E M O D E L

57C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Expression of ABS-301’s target suggests broad potential in squamous cell carcinomas A B S - 3 0 1 Distribution of ABS-301 target expression across squamous cell carcinoma cohorts. ABS-301 target expression log2(TPM+1) Values shown are log2(TPM+1) normalized. Multiple biopsies from a patient are included in the analysis. Source: Tempus

58C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Expression in Lung Squamous Cell Carcinoma (LUSC): no change with treatment and strong negative correlation with CD8+ T cell infiltration A B S - 3 0 1 In LUSC, univariate analysis of ABS-301 expression indicate only a minor change in expression between pre- and post-treatment suggesting opportunity for combination therapy. S u s t a i n e d t a r g e t e x p r e s s i o n i n L U S C ABS-301 target expression shows a strong negative correlation with CD8+ T cell infiltration with a minimal effect on Treg infiltration supporting immunosuppressive activity of target in vivo. C D 8 + I n f i l t r a t i o n n e g a t i v e l y c o r r e l a t e d w i t h t a r g e t e x p r e s s i o n i n L U S C Source: Tempus Source: TCGA Pre-treatment Post-treatment A BS -3 01 ta rg et e xp re ss io n (lo g2 (T PM +1 )) A BS -3 01 e xp re ss io n le ve l (lo g2 T PM )
59C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Ongoing preclinical studies exploring broad application in immuno-oncology A B S - 3 0 1 Based on literature and potential competitive molecules, the following indications could be of interest: *dependent on stage of diagnosis References provided in appendix Indication US Prevalence Estimated 5-year survival rate* US Sales in 2030 NSCLC Calculated: ~202K in 2023 28% $27B SCC 30% of NSCLC cases Calculated: ~61K 24% Calculated Sales: $8.1B Head and Neck SCC ~54K in 2022 68.5% Calculated Sales: $2.3B Esophageal Cancer ~21K in 2022 20% $1.5B SCC ~20% of cases Calculated: ~4.2K Calculated Sales: $0.3B Cervical Cancer ~14K in 2023 $0.6B SCC 90% of cases Calculated: ~13K 67% Calculated Sales: $0.6B Skin Cancer, non-melanoma Incidence = ~3,300K 95-100% $1.0B SSC Incidence = ~700K 95% Calculated Sales: $0.2B
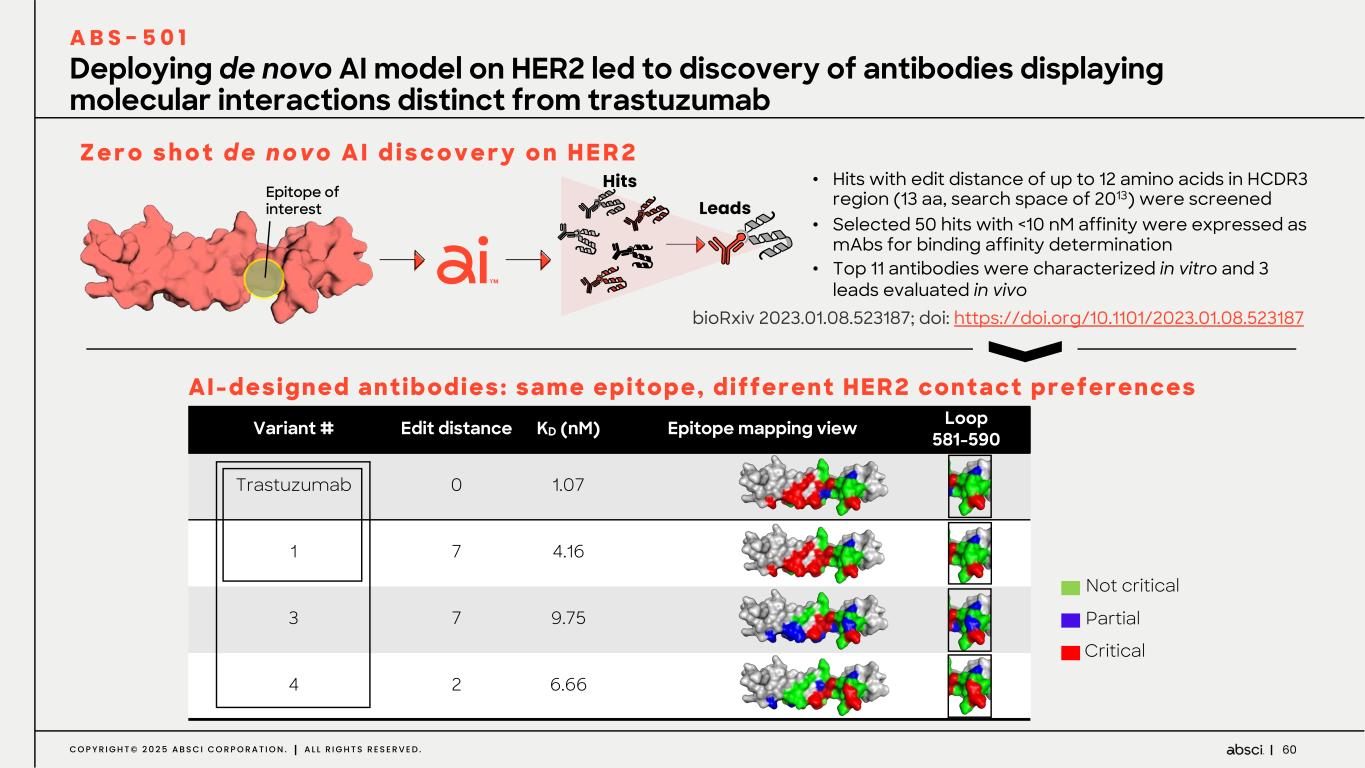
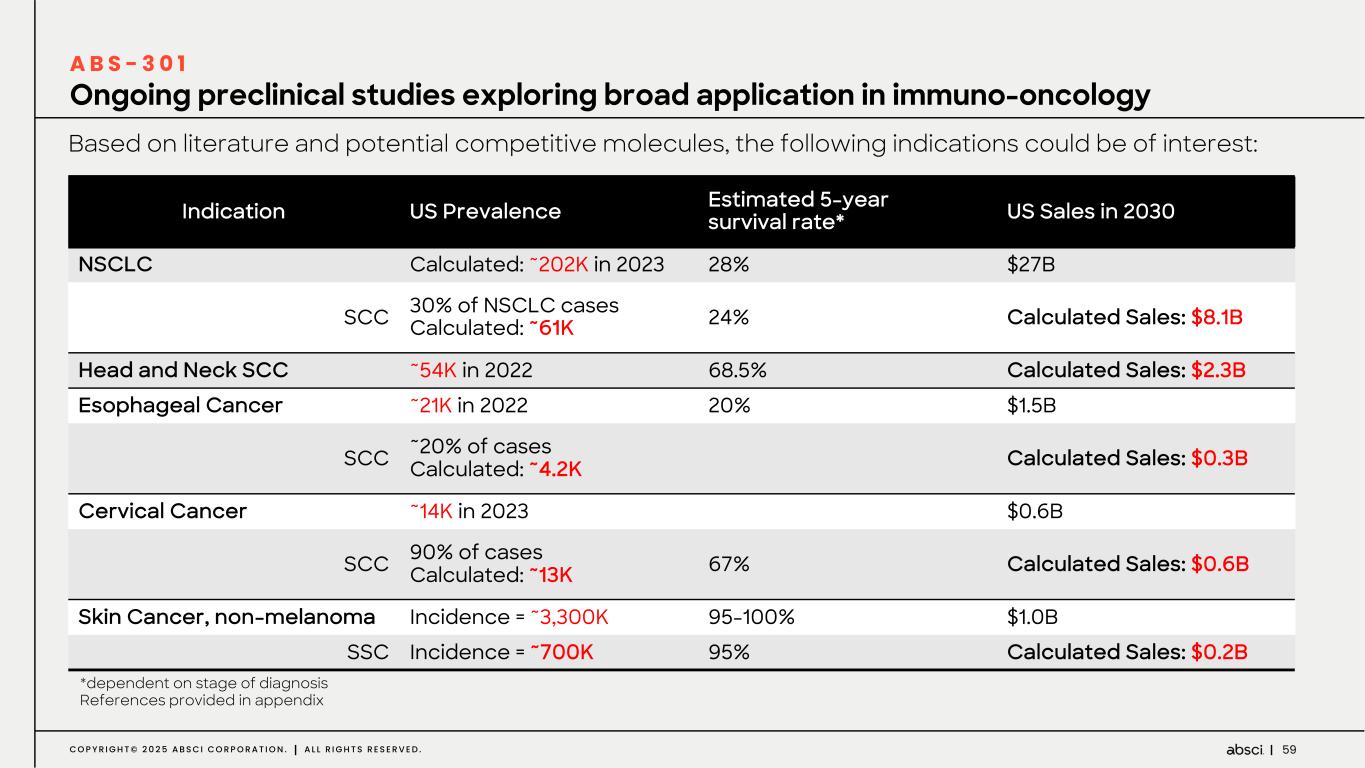
60C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . • Hits with edit distance of up to 12 amino acids in HCDR3 region (13 aa, search space of 2013) were screened • Selected 50 hits with <10 nM affinity were expressed as mAbs for binding affinity determination • Top 11 antibodies were characterized in vitro and 3 leads evaluated in vivo Deploying de novo AI model on HER2 led to discovery of antibodies displaying molecular interactions distinct from trastuzumab A B S - 5 0 1 Variant # Edit distance KD (nM) Epitope mapping view Loop 581-590 Trastuzumab 0 1.07 1 7 4.16 3 7 9.75 4 2 6.66 Partial Critical Not critical Z e r o s h o t d e n o v o A I d i s c o v e r y o n H E R 2 AI-designed antibodies: same epitope, different HER2 contact preferences Epitope of interest Hits Leads bioRxiv 2023.01.08.523187; doi: https://doi.org/10.1101/2023.01.08.523187
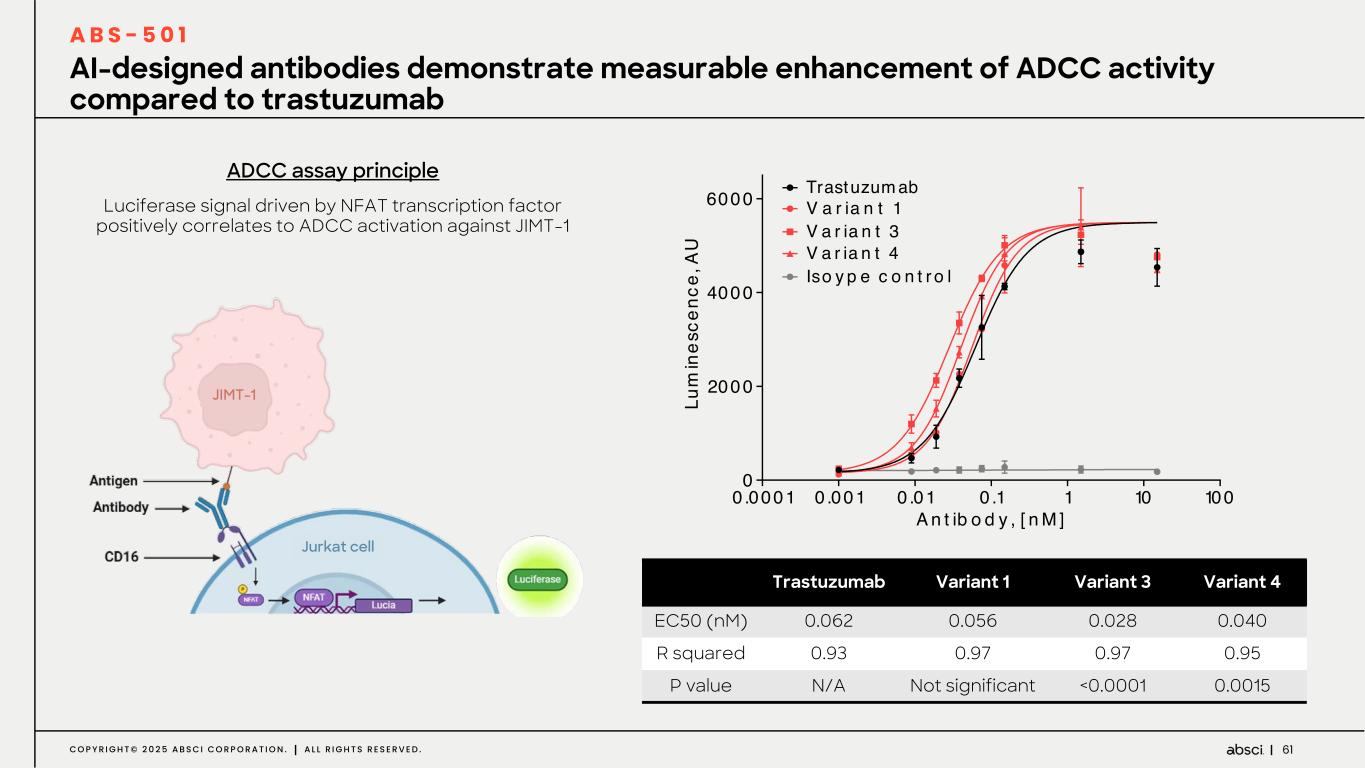
61C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Luciferase signal driven by NFAT transcription factor positively correlates to ADCC activation against JIMT-1 AI-designed antibodies demonstrate measurable enhancement of ADCC activity compared to trastuzumab A B S - 5 0 1 ADCC assay principle Trastuzumab Variant 1 Variant 3 Variant 4 EC50 (nM) 0.062 0.056 0.028 0.040 R squared 0.93 0.97 0.97 0.95 P value N/A Not significant <0.0001 0.0015 0 .0001 0 .001 0 .01 0 .1 1 10 100 0 2000 4000 6000 A n t ib o d y , [ n M ] Lu m in es ce nc e, A U Trastuzum ab V a r ia n t 1 V a r ia n t 3 V a r ia n t 4 Iso y p e c o n t r o l JIMT-1 Jurkat cell
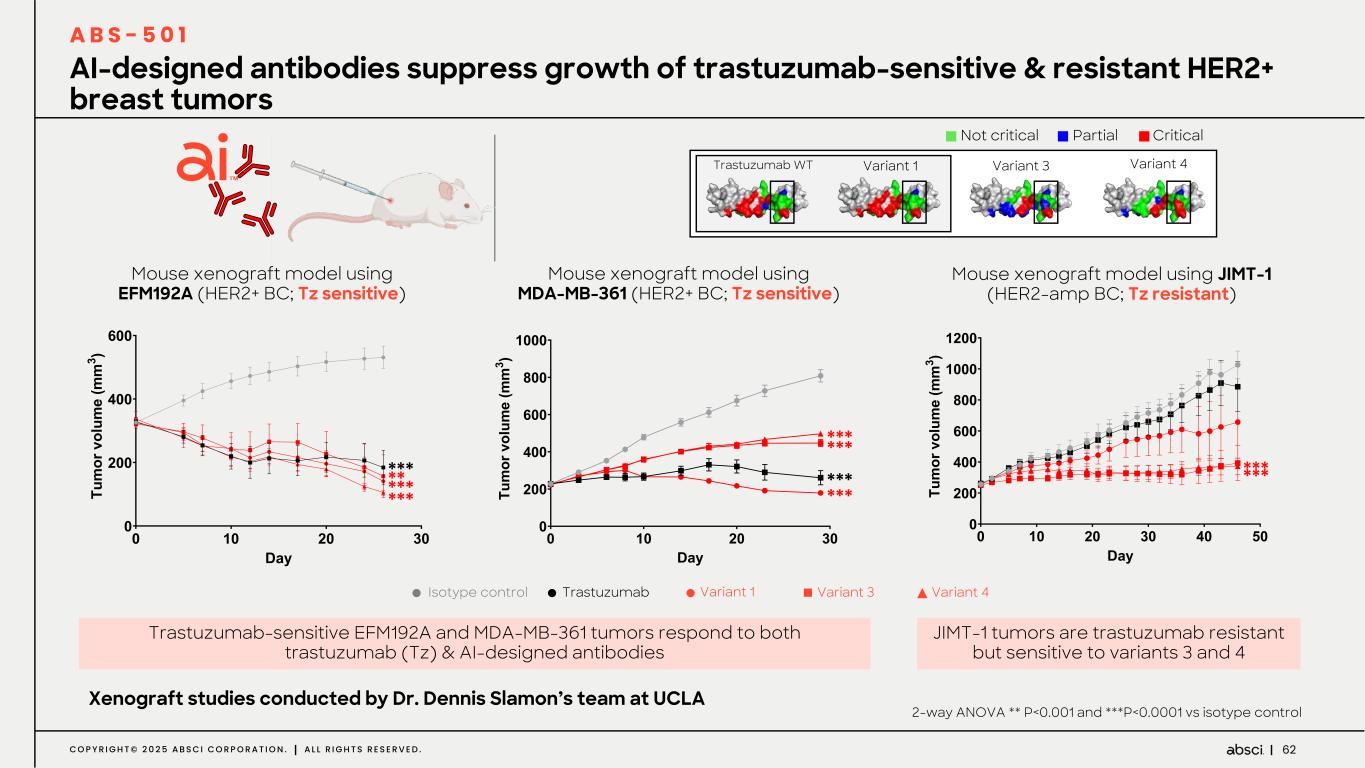
62C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . AI-designed antibodies suppress growth of trastuzumab-sensitive & resistant HER2+ breast tumors A B S - 5 0 1 Partial Critical Not critical Trastuzumab WT Variant 1 Variant 3 Variant 4 Mouse xenograft model using EFM192A (HER2+ BC; Tz sensitive) Mouse xenograft model using JIMT-1 (HER2-amp BC; Tz resistant) Trastuzumab-sensitive EFM192A and MDA-MB-361 tumors respond to both trastuzumab (Tz) & AI-designed antibodies Xenograft studies conducted by Dr. Dennis Slamon’s team at UCLA Isotype control Trastuzumab Variant 1 Variant 3 Variant 4 ! "! #! $! ! #!! %!! &!! D() *+ , -. /0 -1 +, 2/ 3, , $ 4 ! "! #! $! %! &! ! #!! %!! D!! (!! "!!! "#!! )*+ ,- . /0 12 /3 -. 41 5. . $ 6 *** ****** *** *** Mouse xenograft model using MDA-MB-361 (HER2+ BC; Tz sensitive) ! "! #! $! ! #!! %!! &!! D!! "!!! ()* +, - ./ 01 .2 ,- 30 4- - $ 5 *** *** ****** 2-way ANOVA ** P<0.001 and ***P<0.0001 vs isotype control JIMT-1 tumors are trastuzumab resistant but sensitive to variants 3 and 4 **
63C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . AI-designed antibodies create opportunities to address unmet medical need A B S - 5 0 1 Modality switch or combination opportunities under consideration to address unmet medical needs Later-line treatment regimens for HER2-positive cancer: • Monotherapy • Combination therapy with targeted small molecules M u l t i p l e p a t h s p o s s i b l e f o r t h e r a p e u t i c d e v e l o p m e n t : C u r r e n t l y e x p l o r i n g b r e a s t c a n c e r a s o p p o r t u n i t y : a l t e r n a t i v e t o o r p o s t E n h e r t u® Despite Enhertu’s good efficacy, leading oncologists are only moderately satisfied due to toxicity (e.g. interstitial lung disease); less toxic therapy and effective treatment post-Enhertu are key unmet needs. “Post-Enhertu is really where the action is right now in the field. I think the first company that comes up with something that has significant benefit in Enhertu progressive disease is going to win.” – KOL Enhancing efficacy and expanding indications (e.g. Enhertu resistance): • Antibody-drug conjugates (ADCs) • Multi-specific antibodies +

64C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Leading AI models to create novel & differentiated therapeutics “Smart” biologics Enhanced Potency & MOA Engineer selectivity, minimizing off target toxicity Agonism vs. Antagonism Bind Specific extracellular domains Target Specific conformations Address difficult target classes e.g. GPCRs ADDRESS COMPLEX AND PREVIOUSLY “HARD TO DRUG” TARGETS INTRODUCE PRECISE CONTROL OVER ANTIBODY DESIGN

65C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Leadership in AI de novo design of antibody-based therapeutics EVQLSEVGA . . . De novo antibody design model creates epitope-specific binders given a target structure Designed in framework of choice or multiple frameworks INPUT EMBEDDING STRUCTURE DESIGN (DIFFUSION) . . . ARCPSIWKFPDEEGACQPC . . . Antigen Structure/Sequence (Epitope) PROTEIN LANGUAGE MODELS Co-optimization enables improvement of antibody attributes such as affinity and developability Precise engineering of molecule pharmacology AI LEAD OPTIMIZATIONDE NOVO ANTIBODY DESIGN
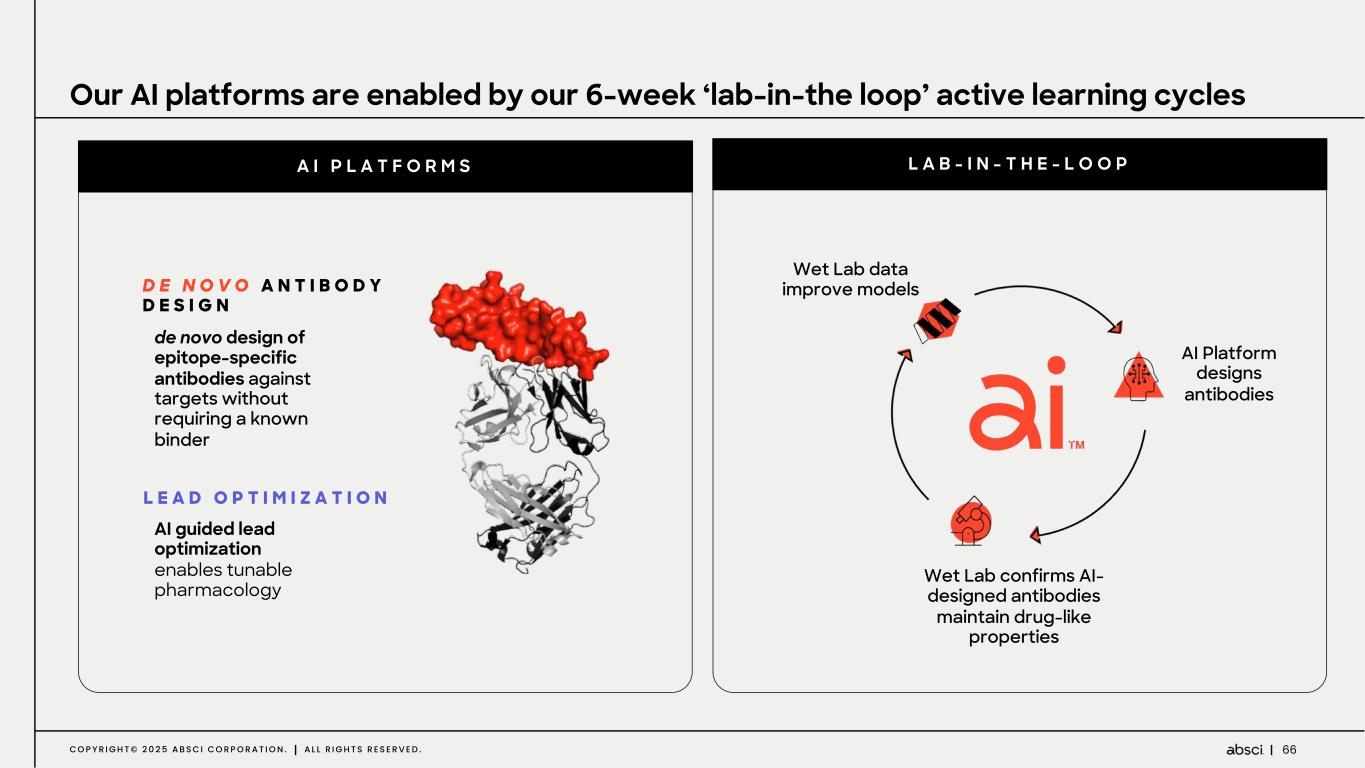
66C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . AI Platform designs antibodies Wet Lab confirms AI- designed antibodies maintain drug-like properties Wet Lab data improve models D E N O V O A N T I B O D Y D E S I G N Our AI platforms are enabled by our 6-week ‘lab-in-the loop’ active learning cycles A I P L A T F O R M S L A B - I N - T H E - L O O P L E A D O P T I M I Z A T I O N AI guided lead optimization enables tunable pharmacology de novo design of epitope-specific antibodies against targets without requiring a known binder
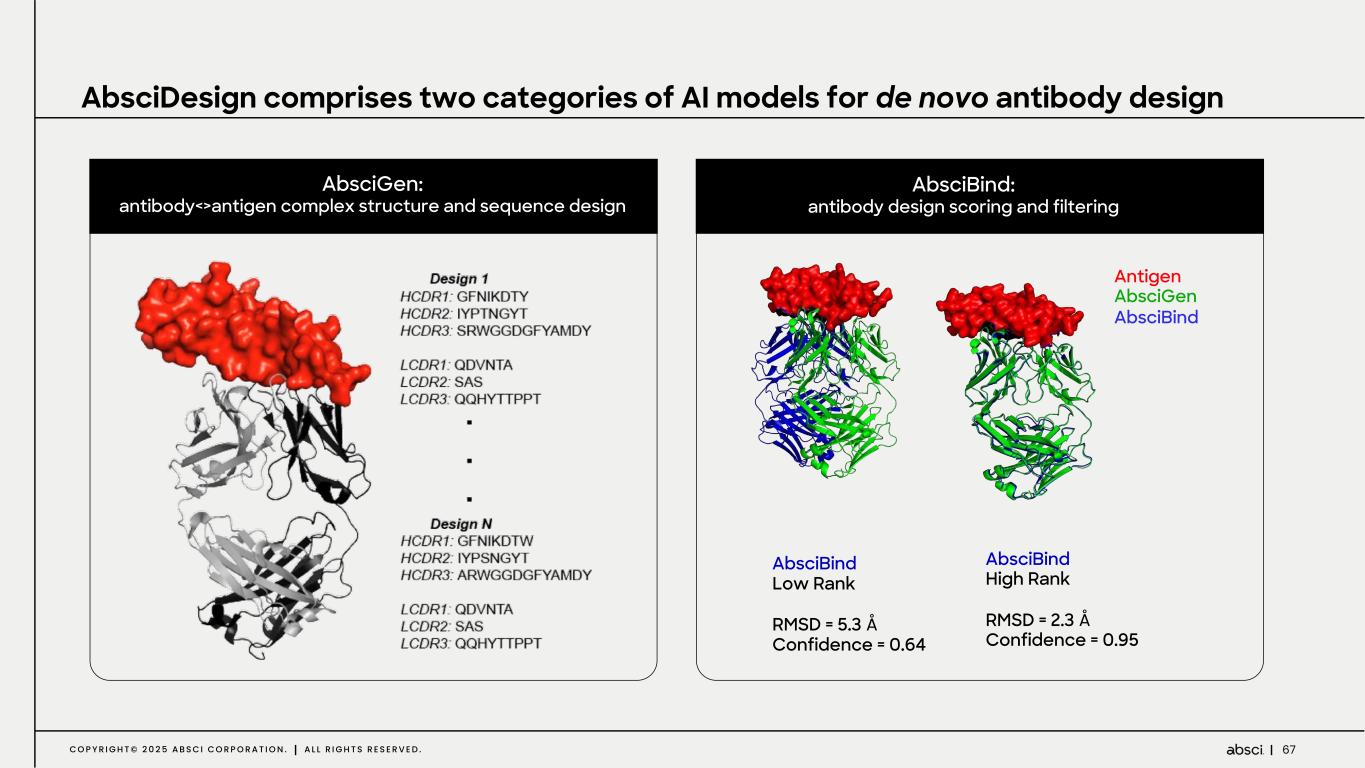
67C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . AbsciGen: antibody<>antigen complex structure and sequence design AbsciBind: antibody design scoring and filtering Antigen AbsciGen AbsciBind AbsciBind High Rank RMSD = 2.3 Å Confidence = 0.95 AbsciBind Low Rank RMSD = 5.3 Å Confidence = 0.64 AbsciDesign comprises two categories of AI models for de novo antibody design
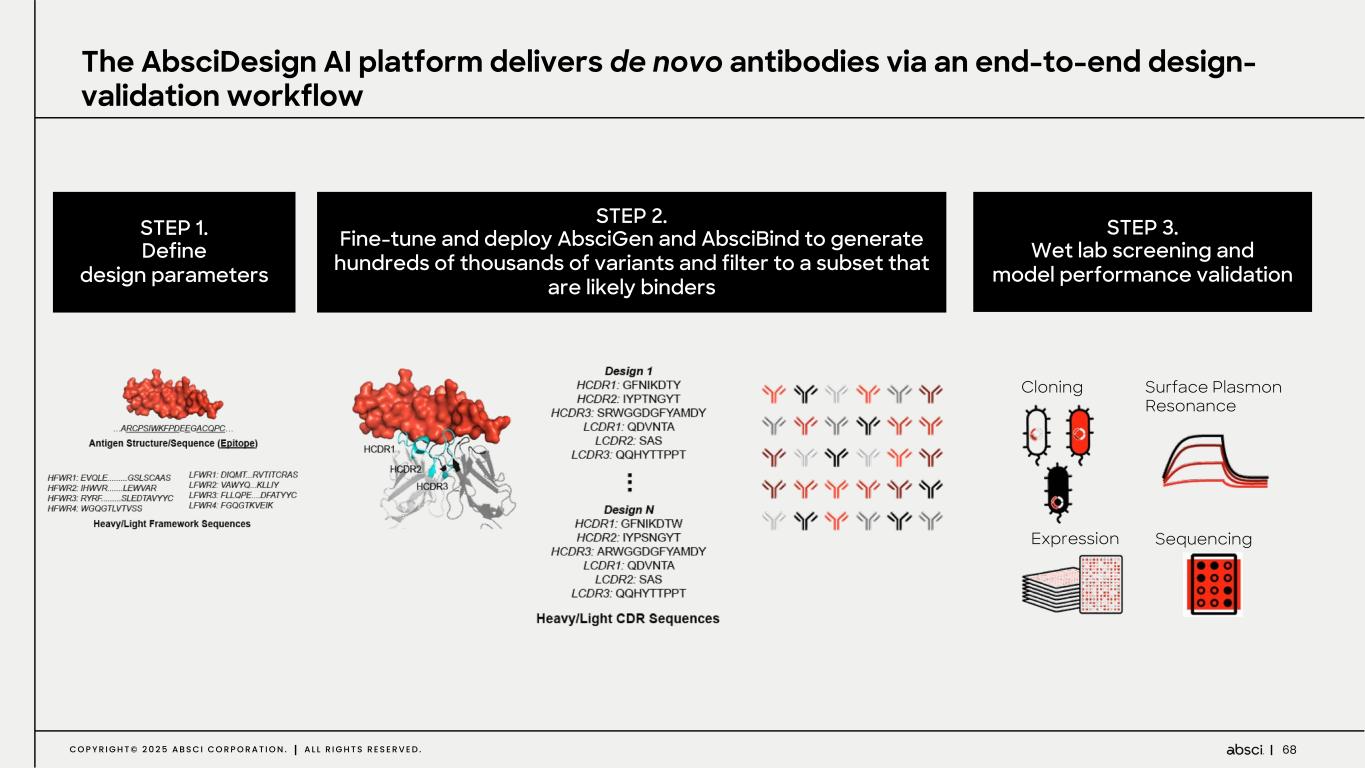
68C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . STEP 1. Define design parameters STEP 2. Fine-tune and deploy AbsciGen and AbsciBind to generate hundreds of thousands of variants and filter to a subset that are likely binders STEP 3. Wet lab screening and model performance validation The AbsciDesign AI platform delivers de novo antibodies via an end-to-end design- validation workflow Cloning Expression Surface Plasmon Resonance Sequencing

69C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . C A S E S T U D Y de novo design of an antibody that binds the Caldera region of HIV-1 trimer
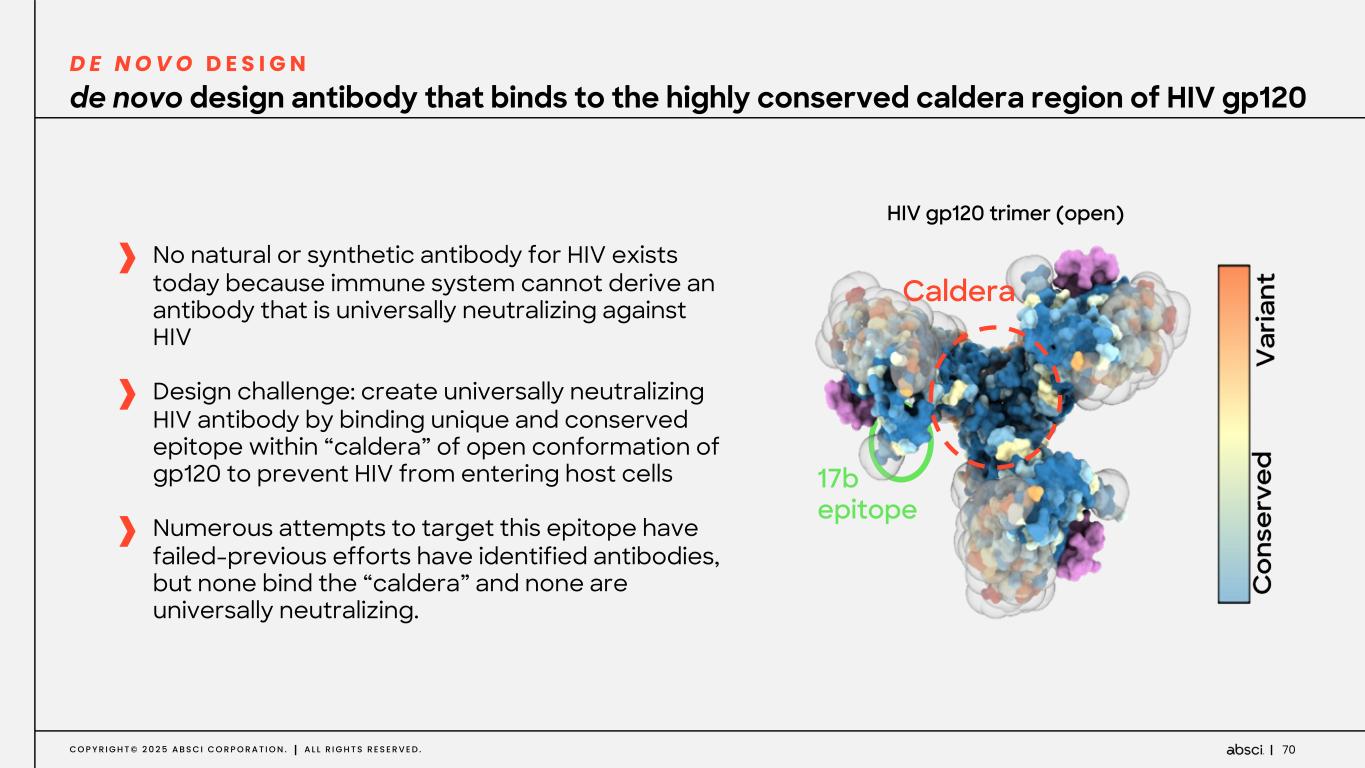
70C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . No natural or synthetic antibody for HIV exists today because immune system cannot derive an antibody that is universally neutralizing against HIV Design challenge: create universally neutralizing HIV antibody by binding unique and conserved epitope within “caldera” of open conformation of gp120 to prevent HIV from entering host cells Numerous attempts to target this epitope have failed-previous efforts have identified antibodies, but none bind the “caldera” and none are universally neutralizing. de novo design antibody that binds to the highly conserved caldera region of HIV gp120 D E N O V O D E S I G N 17b epitope Caldera HIV gp120 trimer (open)
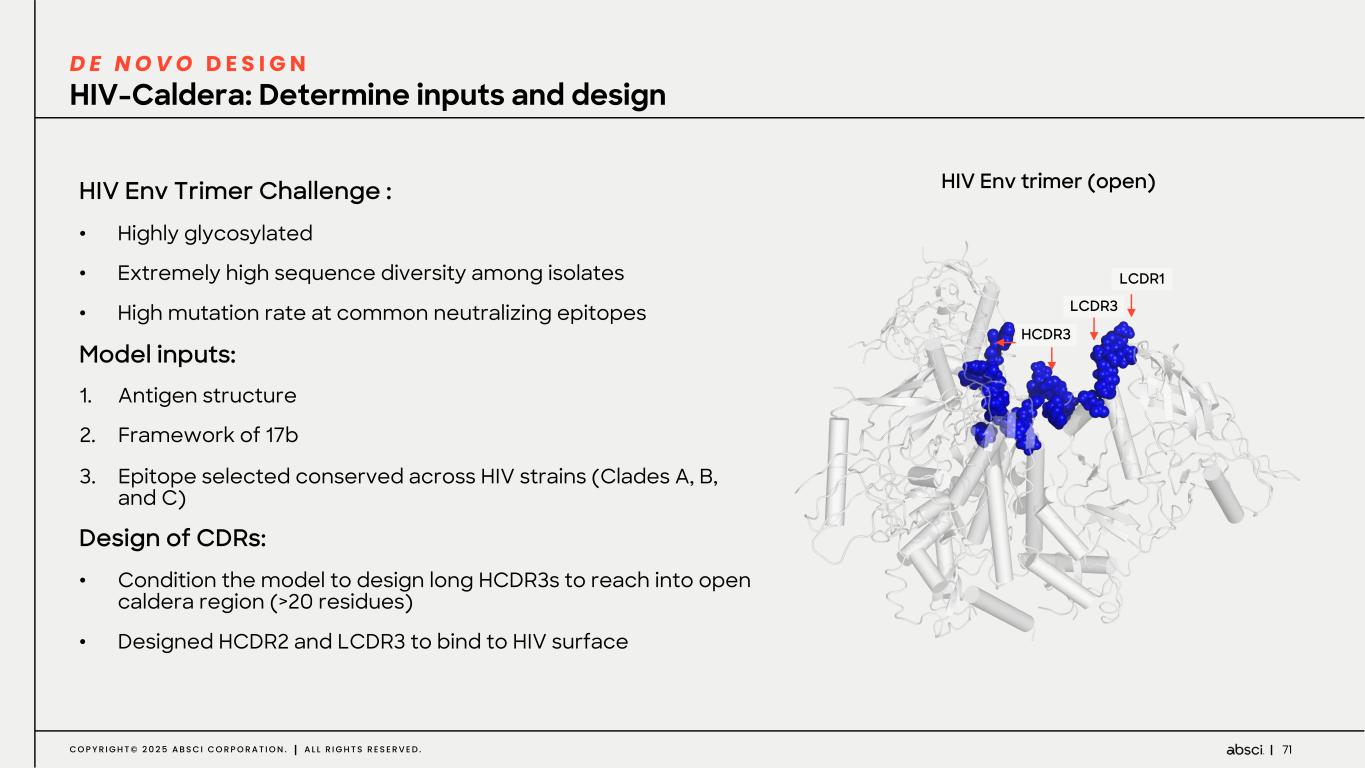
71C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . HIV-Caldera: Determine inputs and design D E N O V O D E S I G N HIV Env Trimer Challenge : • Highly glycosylated • Extremely high sequence diversity among isolates • High mutation rate at common neutralizing epitopes Model inputs: 1. Antigen structure 2. Framework of 17b 3. Epitope selected conserved across HIV strains (Clades A, B, and C) Design of CDRs: • Condition the model to design long HCDR3s to reach into open caldera region (>20 residues) • Designed HCDR2 and LCDR3 to bind to HIV surface HIV Env trimer (open) HCDR3 LCDR1 LCDR3
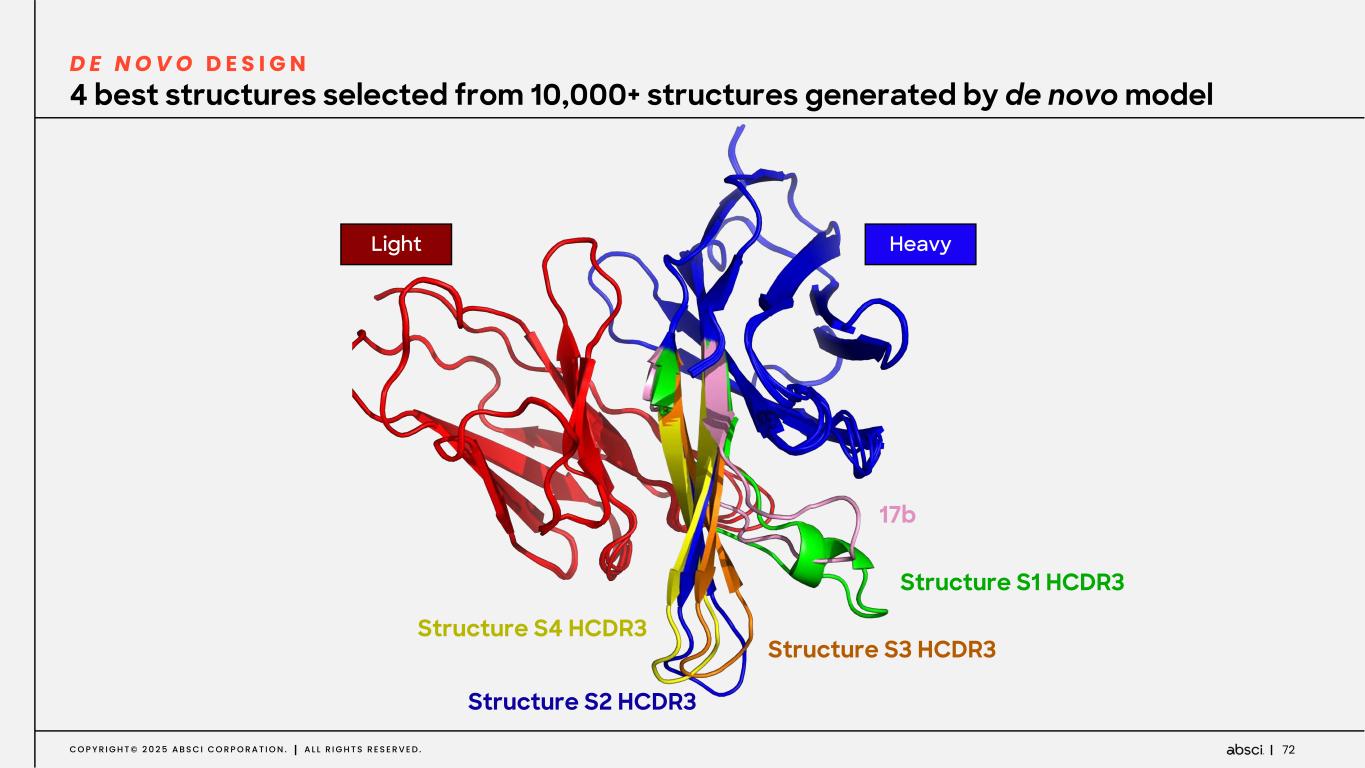
72C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . 4 best structures selected from 10,000+ structures generated by de novo model D E N O V O D E S I G N 17b Structure S1 HCDR3 Structure S3 HCDR3 Structure S2 HCDR3 Structure S4 HCDR3 HeavyLight

73C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Applied molecular dynamics simulation to de novo designed antibodies D E N O V O D E S I G N

74C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . H A ta g Antigen Clade A Env trimer Closed Open Enriched de novo library binds open, not closed, Env trimer conformation in YSD D E N O V O D E S I G N Closed Open Clade B Env trimer

75C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . SPR data demonstrate binding characteristics consistent with binding of caldera D E N O V O D E S I G N
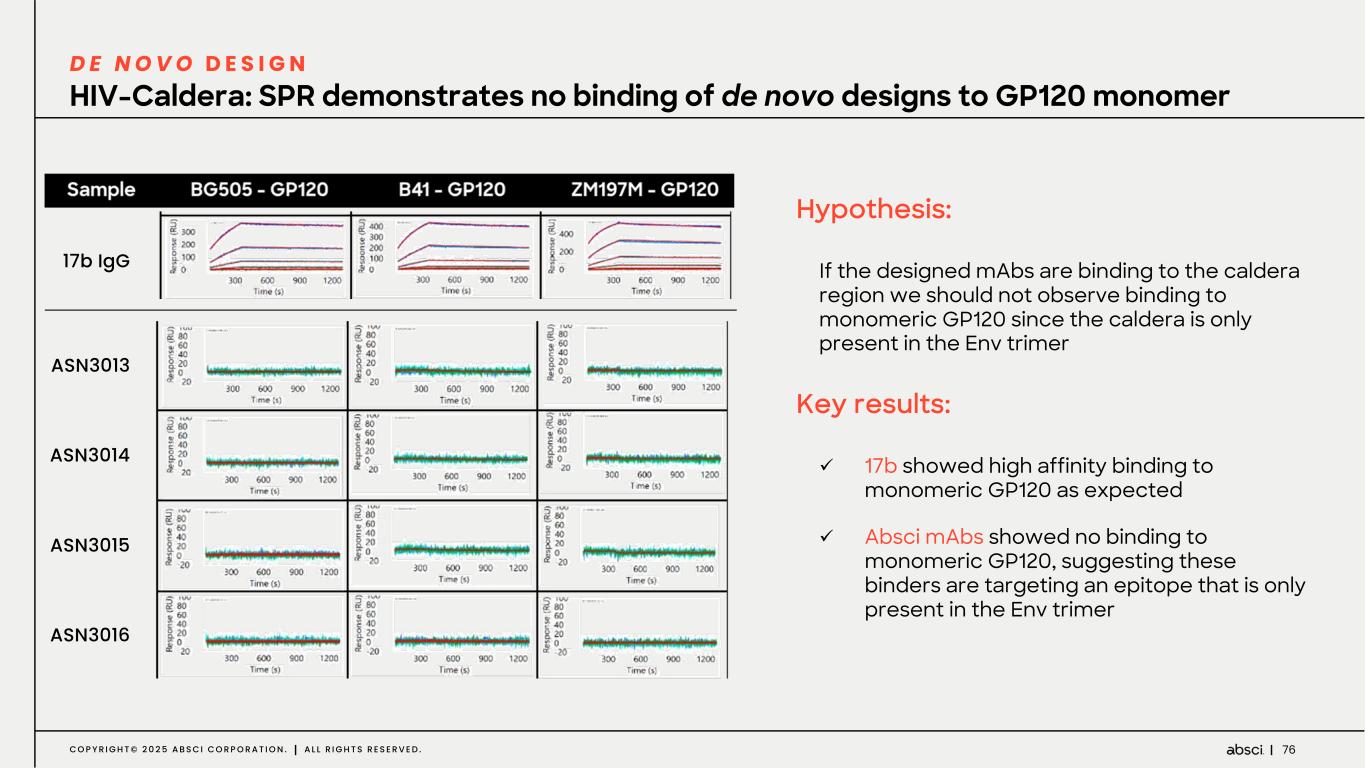
76C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . HIV-Caldera: SPR demonstrates no binding of de novo designs to GP120 monomer D E N O V O D E S I G N Hypothesis: If the designed mAbs are binding to the caldera region we should not observe binding to monomeric GP120 since the caldera is only present in the Env trimer Key results: ü 17b showed high affinity binding to monomeric GP120 as expected ü Absci mAbs showed no binding to monomeric GP120, suggesting these binders are targeting an epitope that is only present in the Env trimer ASN3013 ASN3014 ASN3015 ASN3016 17b IgG
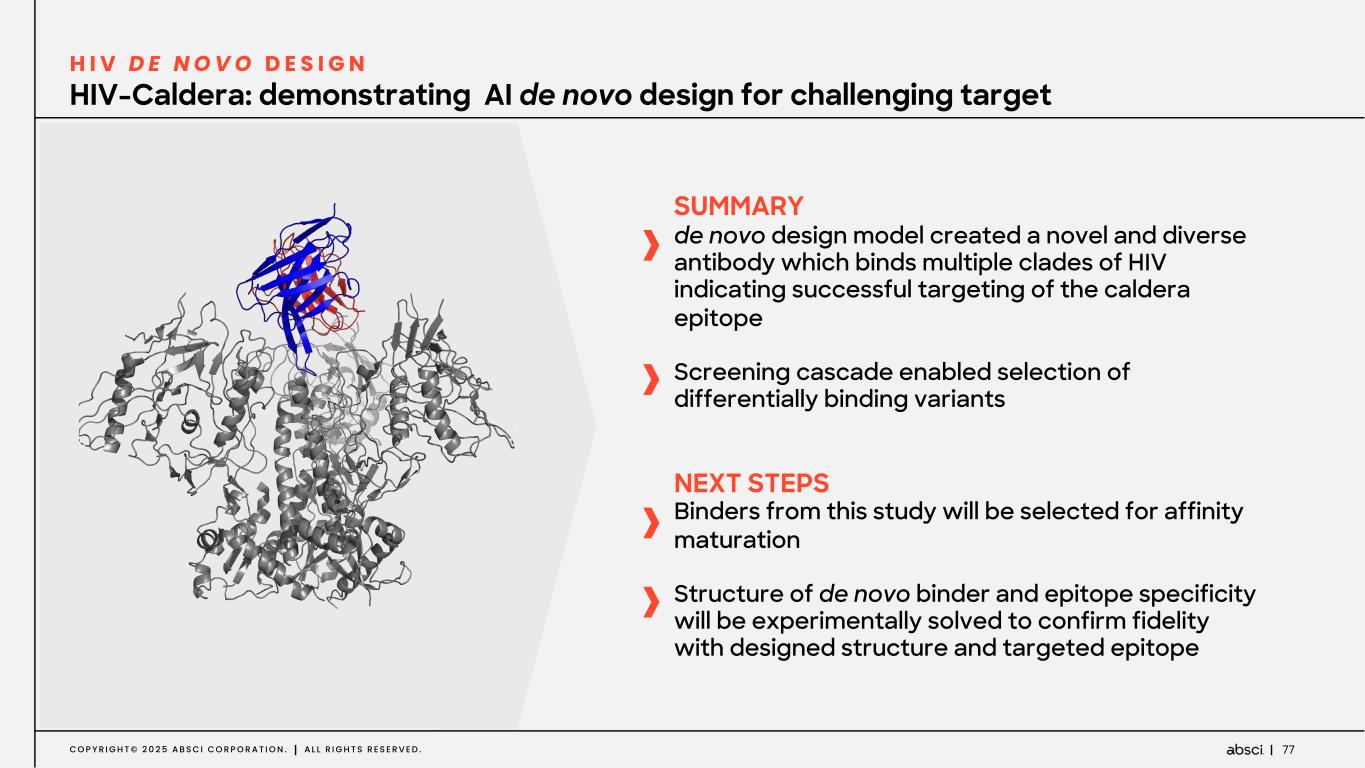
77C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . HIV-Caldera: demonstrating AI de novo design for challenging target H I V D E N O V O D E S I G N SUMMARY de novo design model created a novel and diverse antibody which binds multiple clades of HIV indicating successful targeting of the caldera epitope Screening cascade enabled selection of differentially binding variants NEXT STEPS Binders from this study will be selected for affinity maturation Structure of de novo binder and epitope specificity will be experimentally solved to confirm fidelity with designed structure and targeted epitope

78C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . CASE STUDY A I O p t i m i z a t i o n f o r p H s e n s i t i v i t y
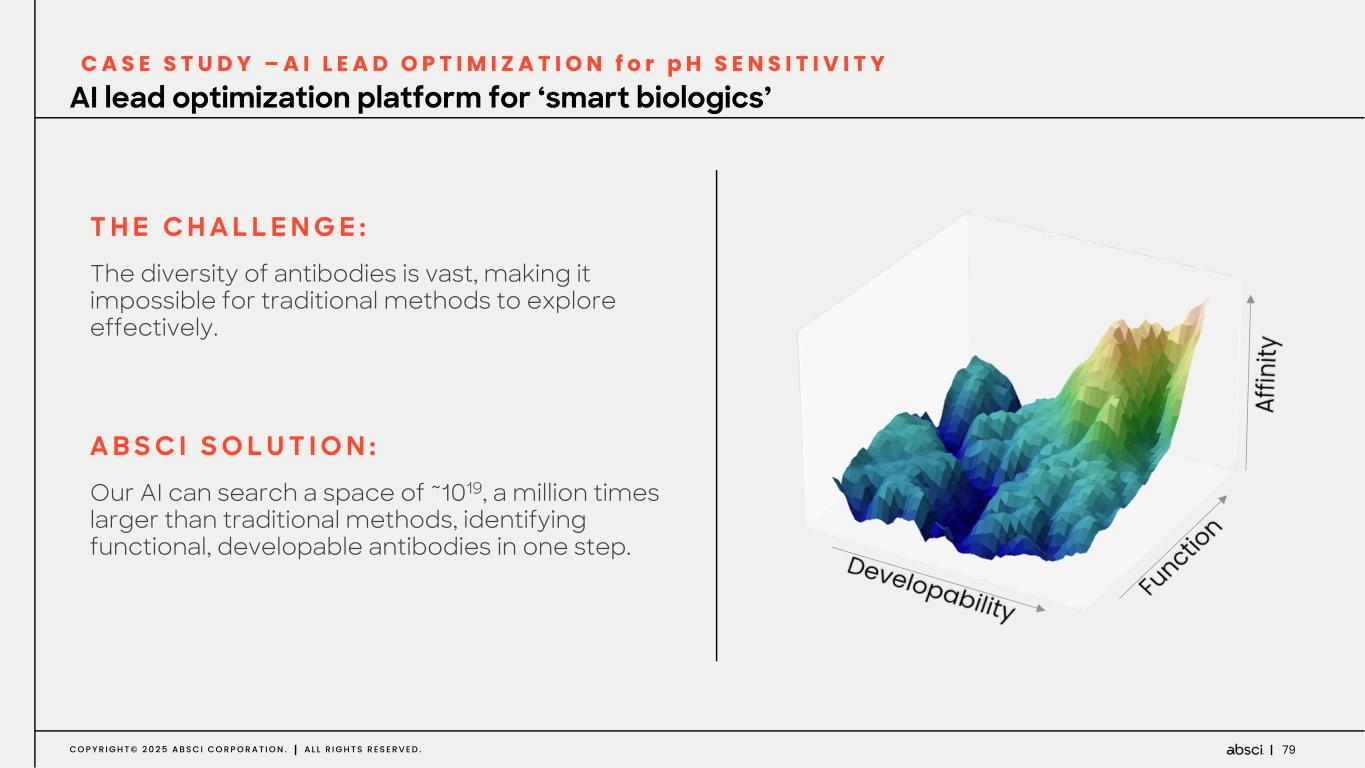
79C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . C A S E S T U D Y – A I L E A D O P T I M I Z A T I O N f o r p H S E N S I T I V I T Y AI lead optimization platform for ‘smart biologics’ T H E C H A L L E N G E : The diversity of antibodies is vast, making it impossible for traditional methods to explore effectively. A B S C I S O L U T I O N : Our AI can search a space of ~1019, a million times larger than traditional methods, identifying functional, developable antibodies in one step.
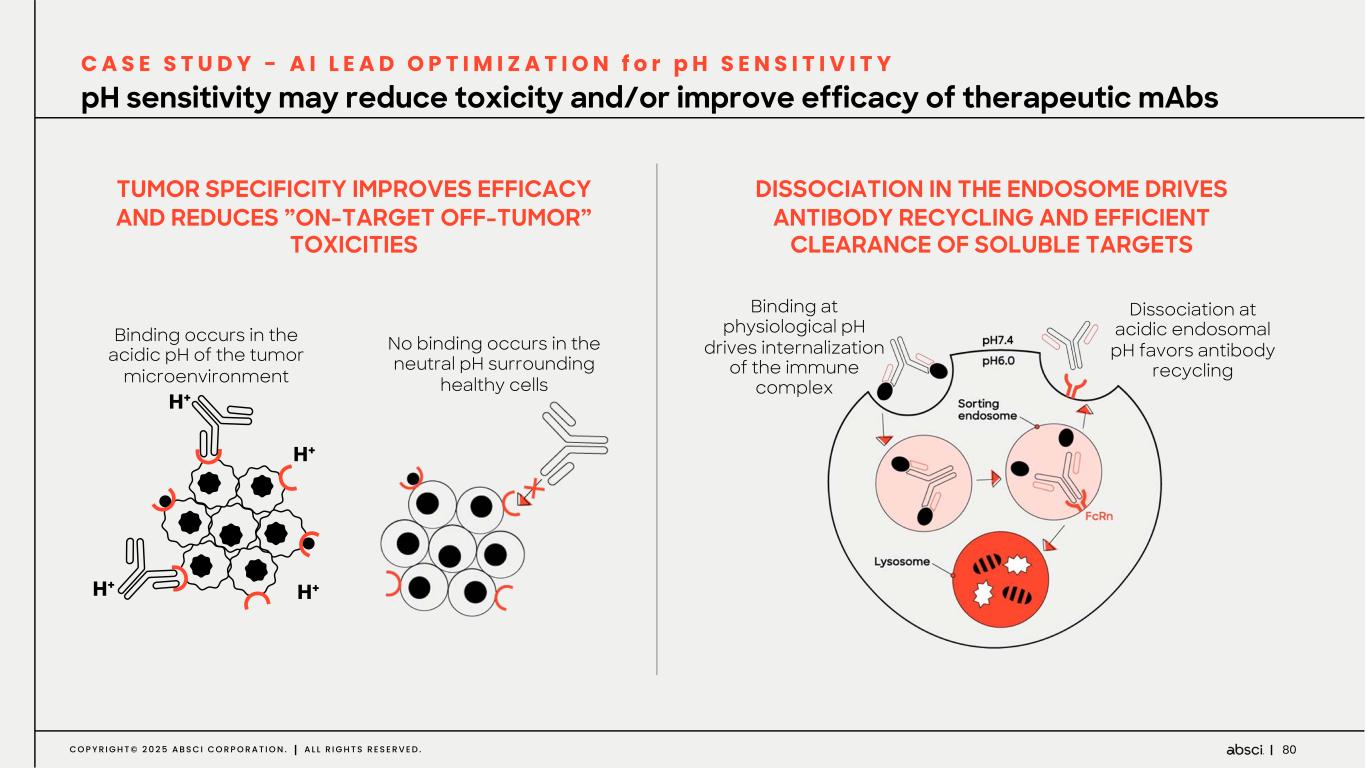
80C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . TUMOR SPECIFICITY IMPROVES EFFICACY AND REDUCES ”ON-TARGET OFF-TUMOR” TOXICITIES pH sensitivity may reduce toxicity and/or improve efficacy of therapeutic mAbs C A S E S T U D Y - A I L E A D O P T I M I Z A T I O N f o r p H S E N S I T I V I T Y Binding occurs in the acidic pH of the tumor microenvironment No binding occurs in the neutral pH surrounding healthy cells DISSOCIATION IN THE ENDOSOME DRIVES ANTIBODY RECYCLING AND EFFICIENT CLEARANCE OF SOLUBLE TARGETS Dissociation at acidic endosomal pH favors antibody recycling Binding at physiological pH drives internalization of the immune complex
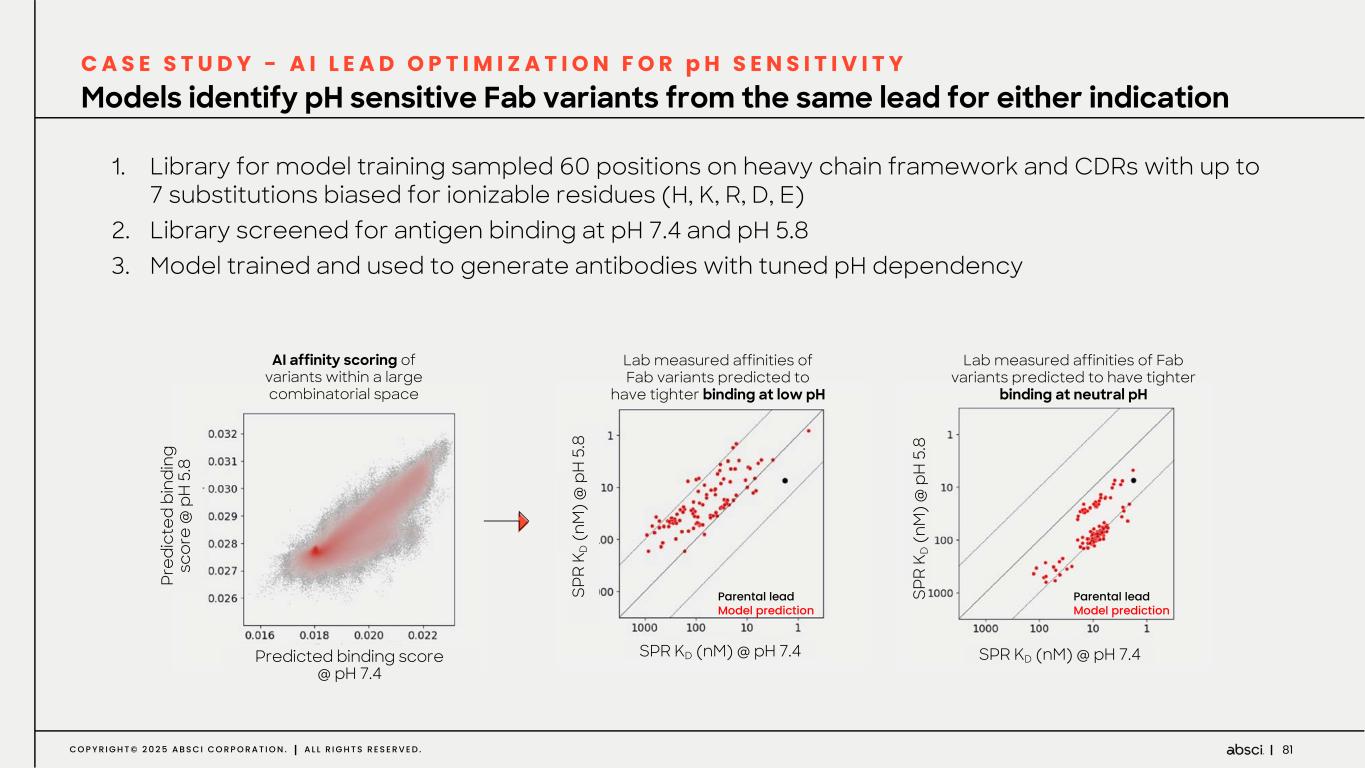
81C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Models identify pH sensitive Fab variants from the same lead for either indication C A S E S T U D Y - A I L E A D O P T I M I Z A T I O N F O R p H S E N S I T I V I T Y 1. Library for model training sampled 60 positions on heavy chain framework and CDRs with up to 7 substitutions biased for ionizable residues (H, K, R, D, E) 2. Library screened for antigen binding at pH 7.4 and pH 5.8 3. Model trained and used to generate antibodies with tuned pH dependency Pr ed ic te d bi nd in g sc or e @ p H 5 .8 Predicted binding score @ pH 7.4 AI affinity scoring of variants within a large combinatorial space SPR KD (nM) @ pH 7.4 SP R K D (n M ) @ p H 5 .8 Lab measured affinities of Fab variants predicted to have tighter binding at neutral pH Parental lead Model predictionSP R K D ( nM ) @ p H 5. 8 Lab measured affinities of Fab variants predicted to have tighter binding at low pH Parental lead Model prediction SPR KD (nM) @ pH 7.4 SP R K D (n M ) @ p H 5 .8
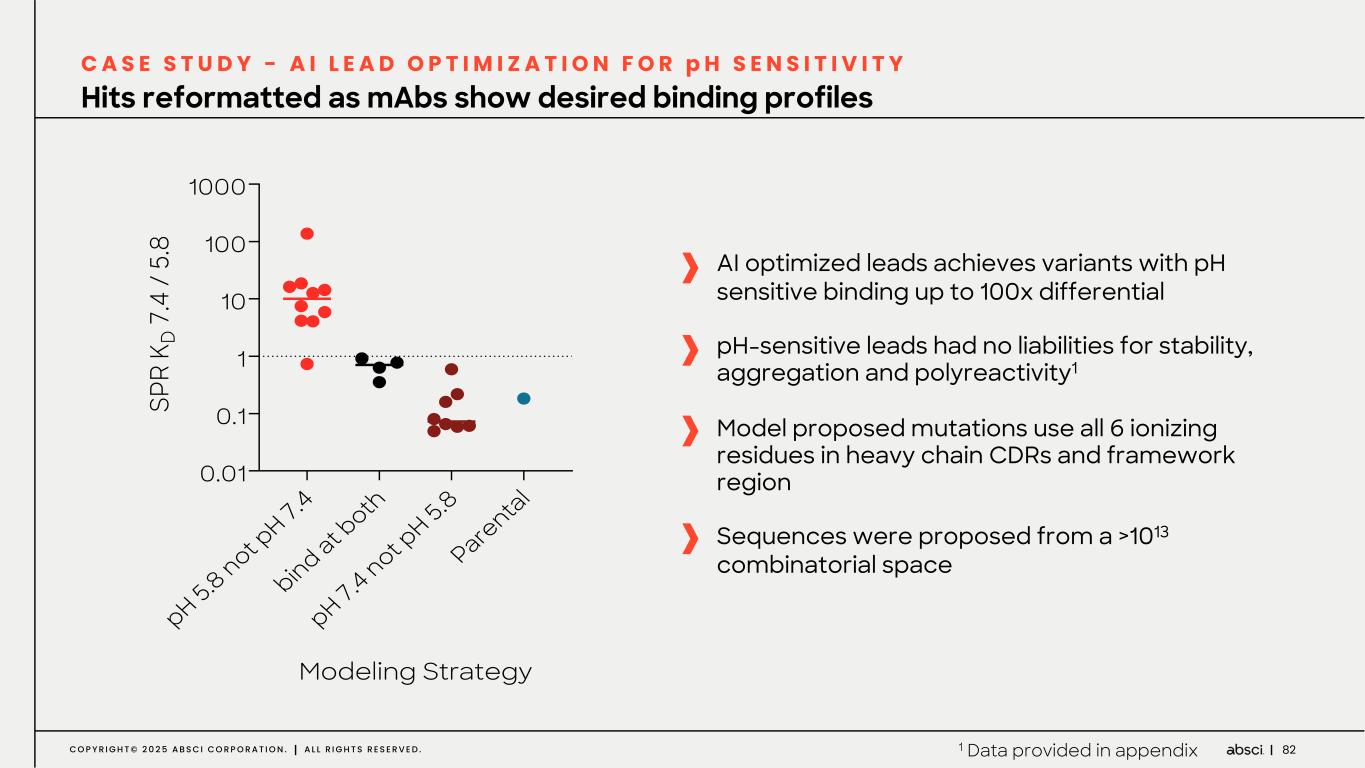
82C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . Hits reformatted as mAbs show desired binding profiles C A S E S T U D Y - A I L E A D O P T I M I Z A T I O N F O R p H S E N S I T I V I T Y AI optimized leads achieves variants with pH sensitive binding up to 100x differential pH-sensitive leads had no liabilities for stability, aggregation and polyreactivity1 Model proposed mutations use all 6 ionizing residues in heavy chain CDRs and framework region Sequences were proposed from a >1013 combinatorial space pH 5.8 not p H 7.4 bind at b oth pH 7.4 not p H 5.8 Pare ntal 0.01 0.1 1 10 100 1000 Modeling Strategy SP R K D 7 .4 / 5. 8 1 Data provided in appendix

83C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D . D E N O V O D E S I G N de novo design model created molecule binds multiple clades of HIV suggesting successful targeting of the caldera epitope Represents second disclosed target success for our de novo platform in the 2nd half of this year Absci’s de novo design platform can successfully address difficult to drug target epitopes A I O P T I M I Z A T I O N Models identify unseen variants with 10x-20x pH sensitivity in both directions, and up to 100x differential compared to parental molecule after only one round Designed leads had no liabilities indicating the ability to successfully search a fitness landscape Absci’s lead optimization platform enables molecules with differentiated pharmacology Summarized platform case studies

Better biologics for patients, faster 84C O P Y R I G H T © 2 0 2 5 A B S C I C O R P O R A T I O N . A L L R I G H T S R E S E R V E D .




















































































