Document
Foghorn Therapeutics Provides First Quarter 2025 Financial and Corporate Update
FHD-909 (LY4050784) advancing in Phase 1 dose escalation trial in SMARCA4 (BRG1) mutated cancers, with non-small cell lung cancer (NSCLC) as the primary target population
Data presented at AACR show synergistic activity with FHD-909 in combination with pembrolizumab and KRAS inhibitors and support clinical exploration
Selective CBP degrader on track for IND-enabling studies, targeting IND in 2026
Continued progress on Selective EP300 degrader and Selective ARID1B degrader with program updates expected in H2 2025
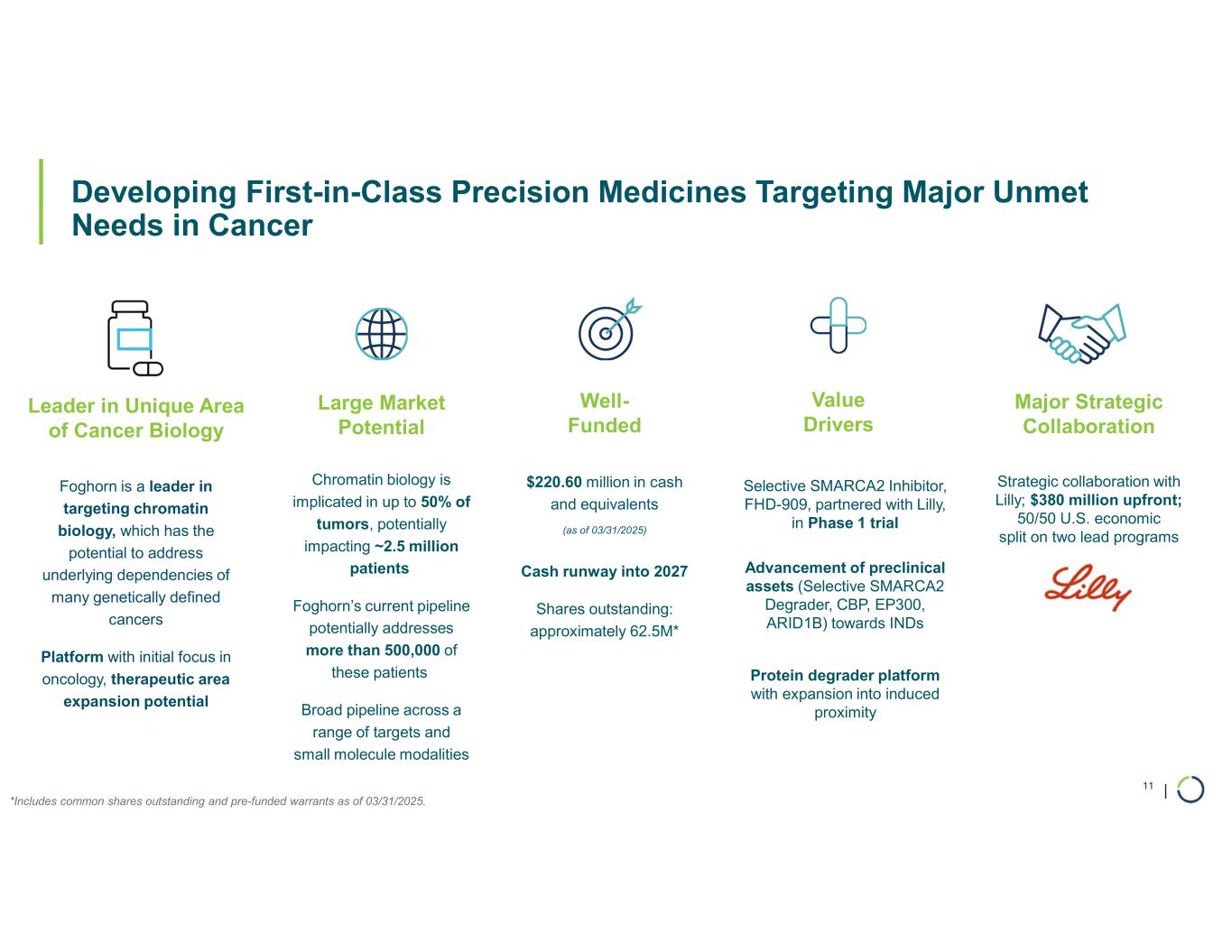
Strong balance sheet with cash, cash equivalents, and marketable securities of
$220.6 million as of March 31, 2025, provides cash runway into 2027
CAMBRIDGE, Mass. -- (GLOBE NEWSWIRE) – May 14, 2025 -- Foghorn® Therapeutics Inc. (Nasdaq: FHTX), a clinical-stage biotechnology company pioneering a new class of medicines that treat serious diseases by correcting abnormal gene expression, today provided a financial and corporate update in conjunction with the Company’s 10-Q filing for the quarter ended March 31, 2025. With an initial focus in oncology, Foghorn’s Gene Traffic Control® Platform and resulting broad pipeline have the potential to transform the lives of people suffering from a wide spectrum of diseases.
“In the first quarter, we continued to progress our novel partnered and proprietary programs, and we were pleased to share more details on the pipeline progress at this year’s AACR annual meeting. The FHD-909 Phase 1 dose escalation trial is proceeding apace. Importantly, the FHD-909 combination data with chemotherapies, KRAS inhibitors and pembrolizumab, that was shared at AACR, reinforces the expansive potential of the selective SMARCA2 inhibitor program in non-small cell lung cancer,” said Adrian Gottschalk, President and Chief Executive Officer of Foghorn. “Initial data defining potential combination opportunities in ER+ breast cancer for our Selective CBP degrader program are encouraging and support potential beyond EP300-mutant cancers. Our Selective EP300 degrader program continues to show anti-proliferative activity in a broad range of hematological malignancies. And lastly, as previously reported, we have achieved selective degradation for our Selective ARID1B program and look forward to providing a program update in 2025.”
Mr. Gottschalk continued, “These advancements with FHD-909 and our pipeline programs underscore our track record and leadership in engineering promising selective therapeutics. With our robust balance sheet and cash runway into 2027, we are in a strong position to deliver significant value with differentiated, high-impact therapeutics in 2025 and beyond.”
Recent Corporate Updates
Data presented at American Association for Cancer Research (AACR) Annual Meeting. In April 2025, Foghorn presented new preclinical data highlighting pipeline progress for potential first-in-class medicines including FHD-909, a SMARCA2 (BRM) selective inhibitor, and the Selective CBP degrader program and Selective EP300 degrader program.
Two Appointments to Board of Directors. In May, Foghorn announced the appointments of Neil Gallagher, M.D., Ph.D. and Stuart Duty, to its Board of Directors.
•Dr. Gallagher brings over 20 years of executive experience at pharmaceutical and biopharmaceutical organizations leading drug development programs across several therapeutic areas, including oncology. Currently he serves as the President, Head of Research and Development at Syndax Pharmaceuticals and held prior clinical development leadership roles at AbbVie, Amgen, Novartis, and AstraZeneca.
•Mr. Duty has over 30 years of executive experience in finance and investment banking in biotechnology and specialty pharmaceuticals. Most recently, he served as a Senior Managing Director at Guggenheim Securities, LLC where he advised senior executives and boards on a range of financing activities and strategic transactions. Previously, he held senior roles at Piper Jaffray and Montgomery Securities and operating roles at Oracle Partners and Curative Technologies.
Second Annual Chromatin Regulation Summit. In May 2025, Foghorn hosted its “Chromatin Regulation Summit: Targeted Protein Degradation and Induced Proximity,” at their corporate headquarters in Cambridge, Massachusetts. The live event featured presentations and panel discussions with world-renowned industry and academic key opinion leaders on current and future applications of targeted protein degradation and induced proximity modalities for the treatment of disease.
Program Overview and Upcoming Milestones
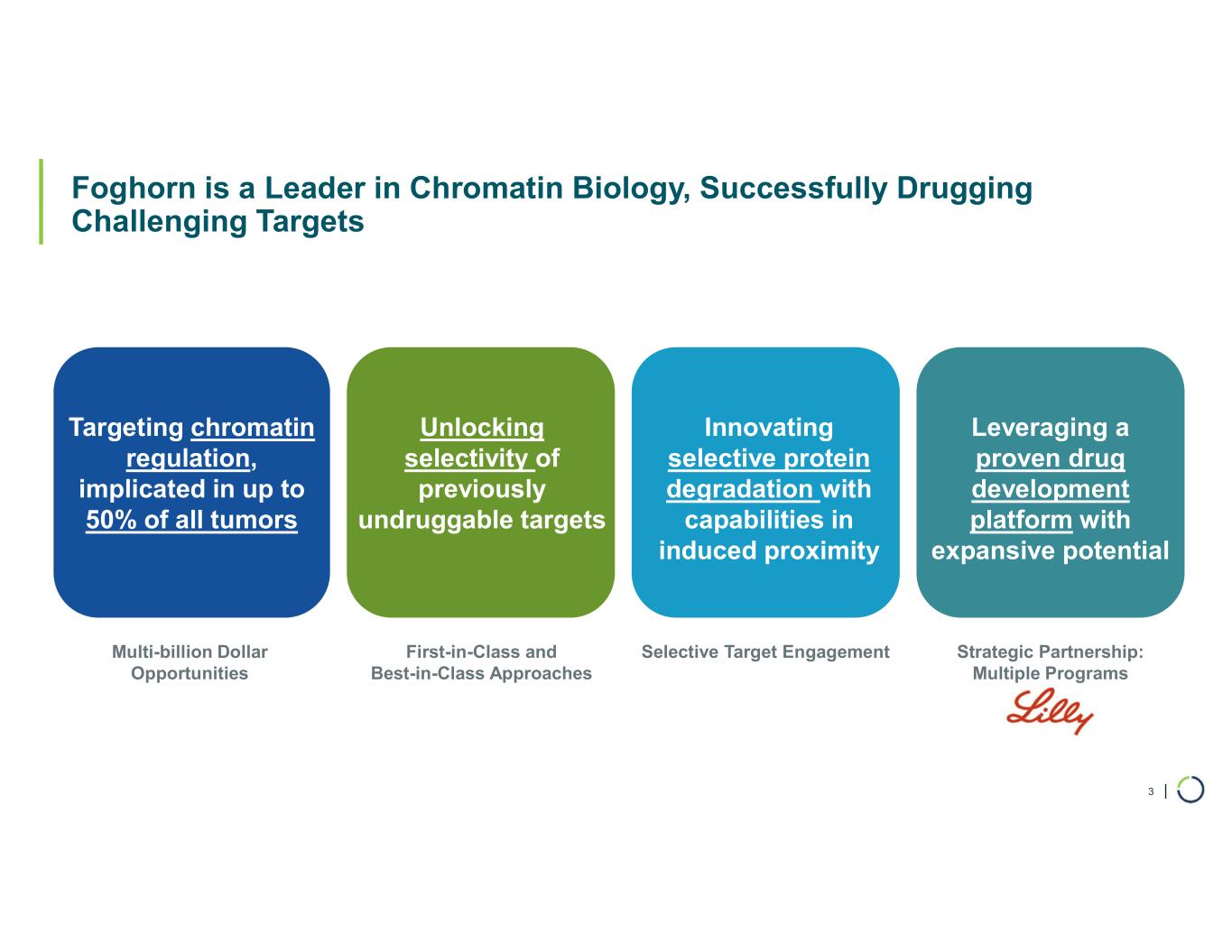
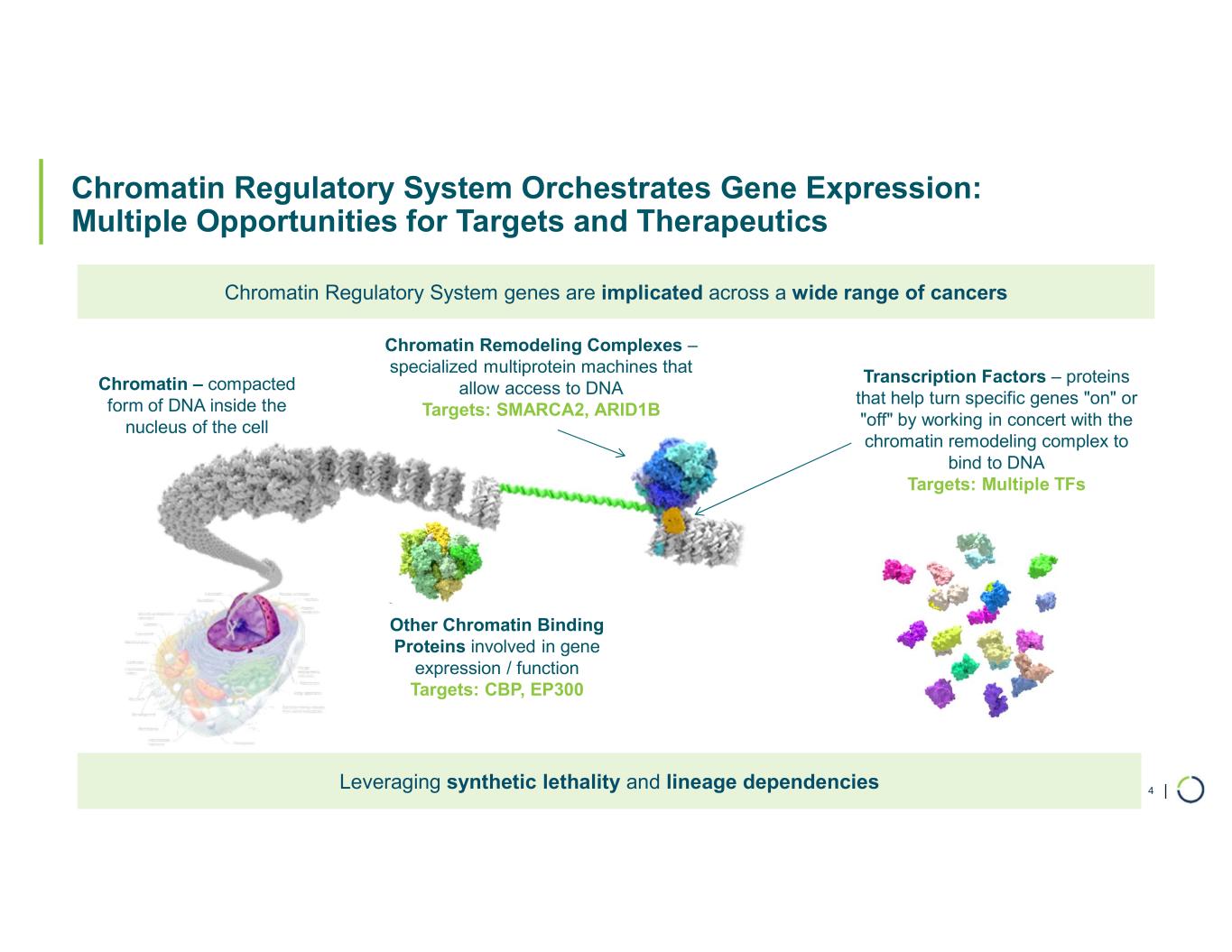
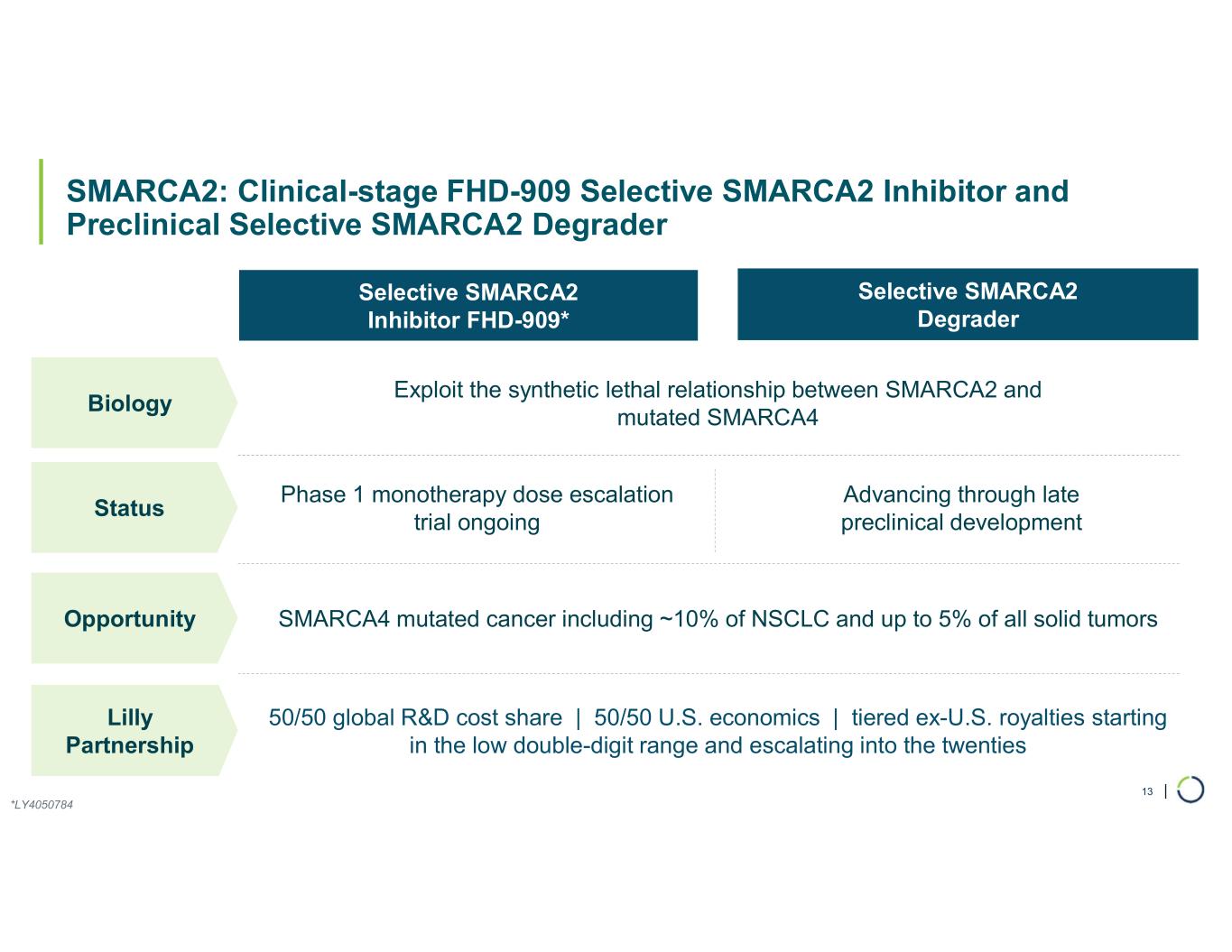
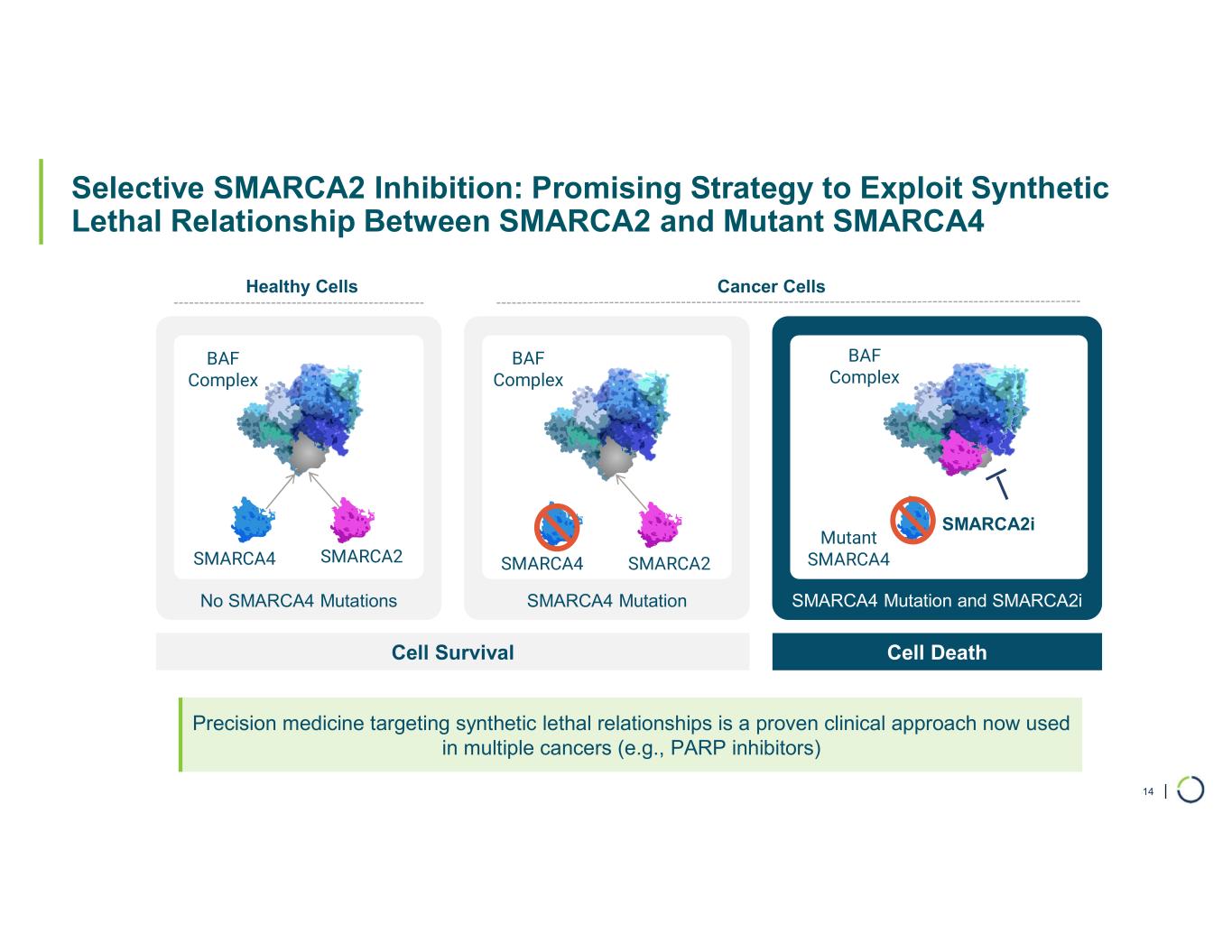
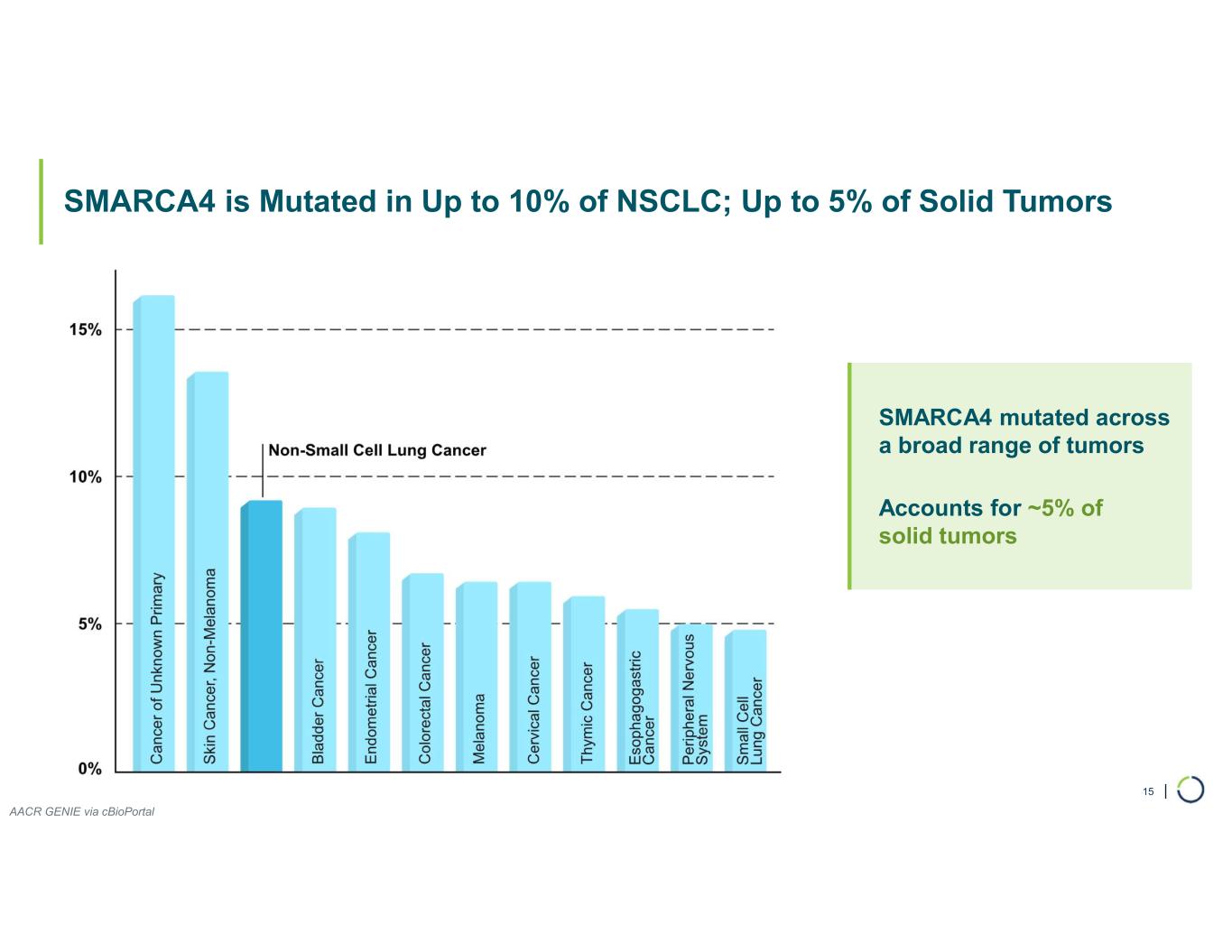
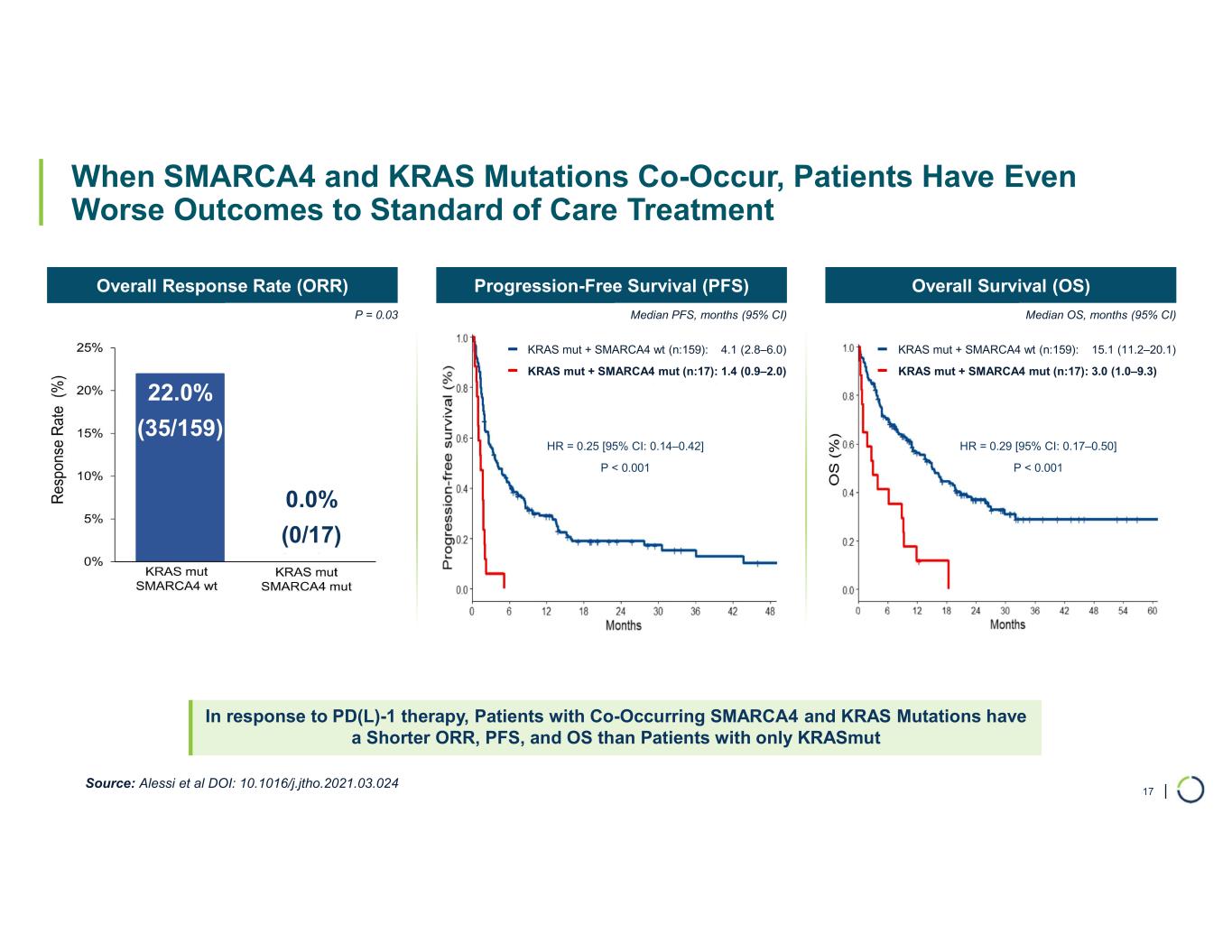
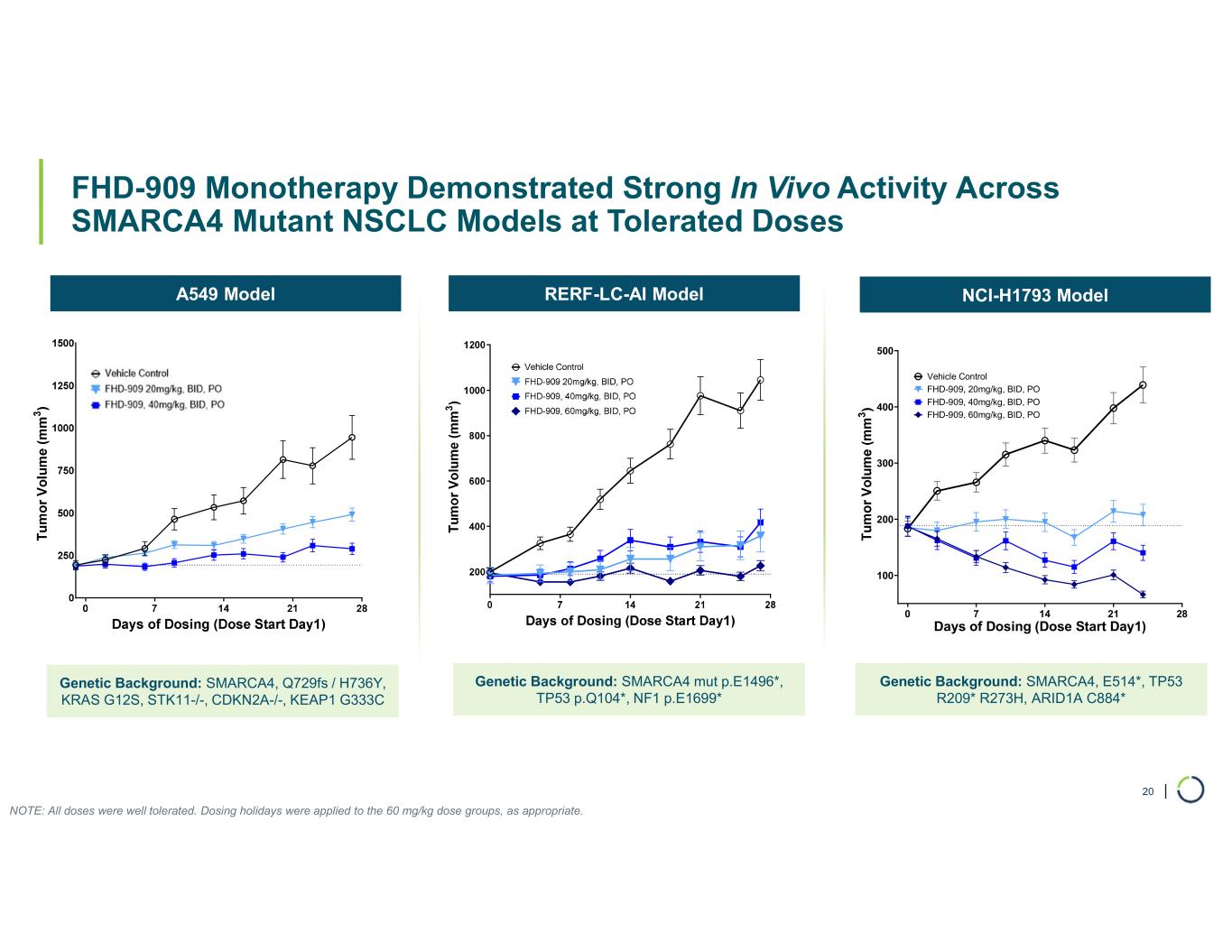
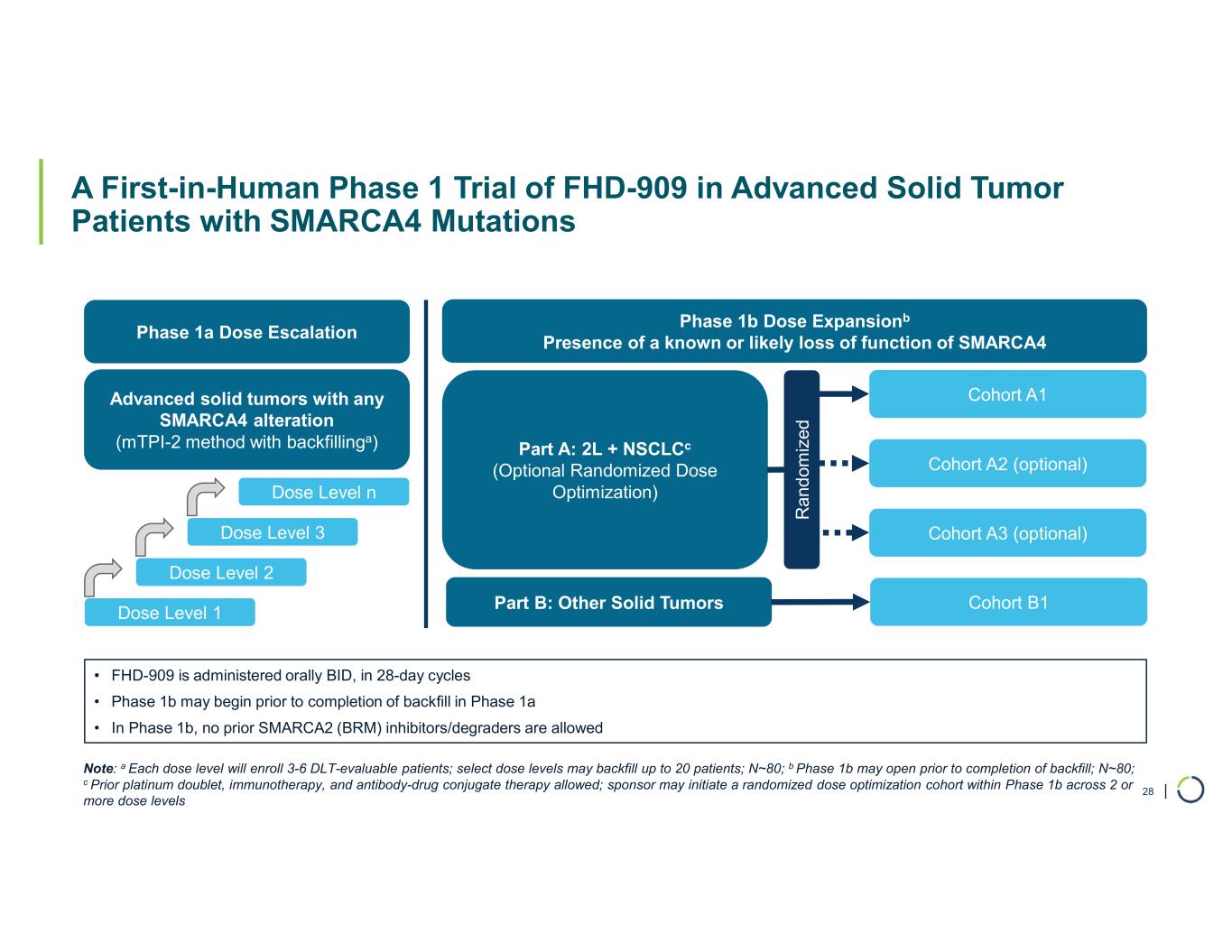
FHD-909 (LY4050784). FHD-909 is a first-in-class oral SMARCA2 selective inhibitor that has demonstrated in preclinical studies to have high selectivity over its closely related paralog SMARCA4, two proteins that are the catalytic engines across all forms of the BAF complex. Selectively blocking SMARCA2 activity is a promising synthetic lethal strategy intended to induce tumor death while sparing healthy cells. SMARCA4 is mutated in up to 10% of NSCLC alone and implicated in a significant number of solid tumors.
•Advancing Phase 1 trial. Ongoing enrollment and dose escalation in the first-in-human Phase 1, multicenter trial for FHD-909 in SMARCA4 mutated cancers, with NSCLC as the primary target population following the dosing of the first patient in October 2024.
◦At the AACR Annual Meeting in April 2025, Lilly presented, on behalf of the collaboration, the clinical study design poster for the Phase 1 trial evaluating FHD-909 in patients with SMARCA4 mutated locally advanced or metastatic solid tumors who have exhausted standard treatment options. The primary target population is NSCLC.
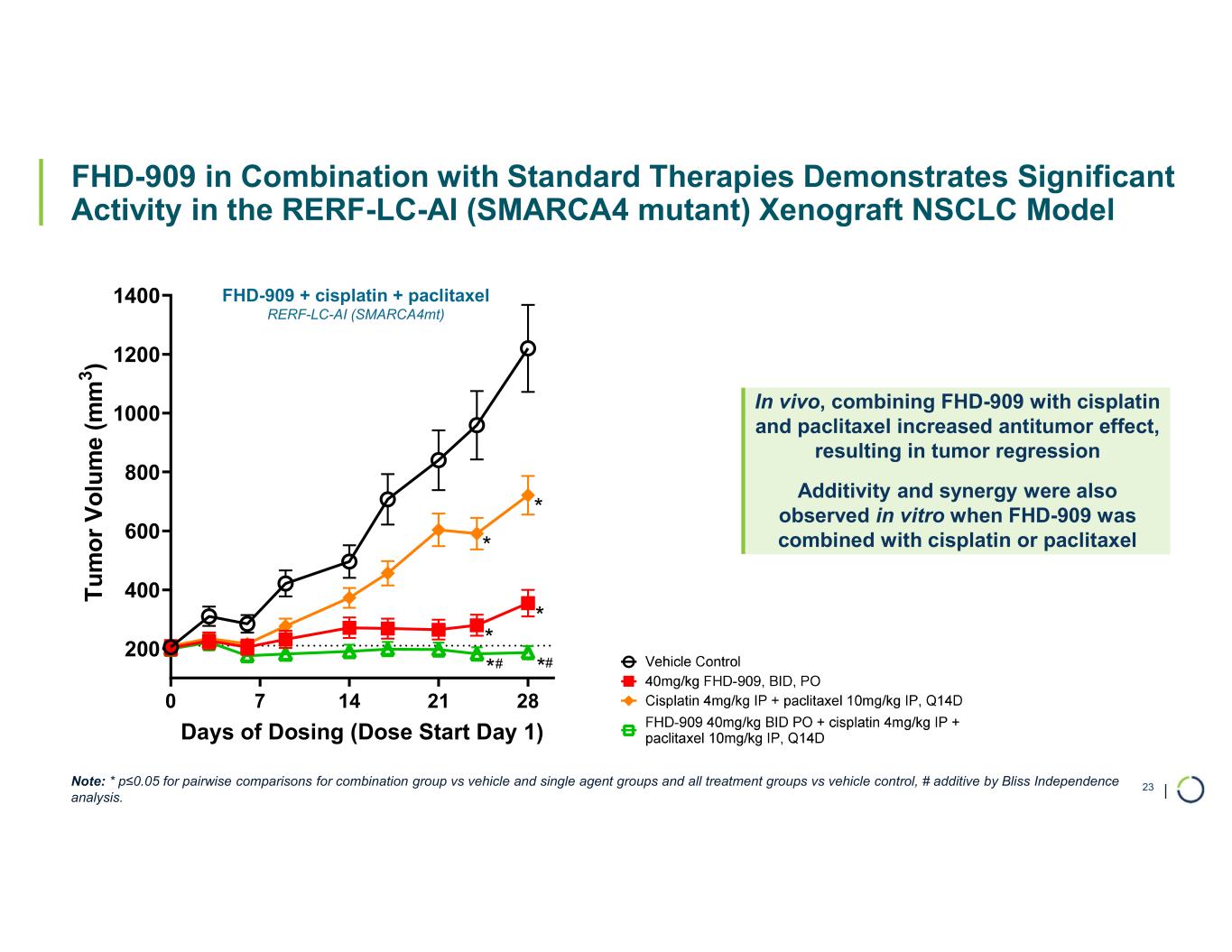
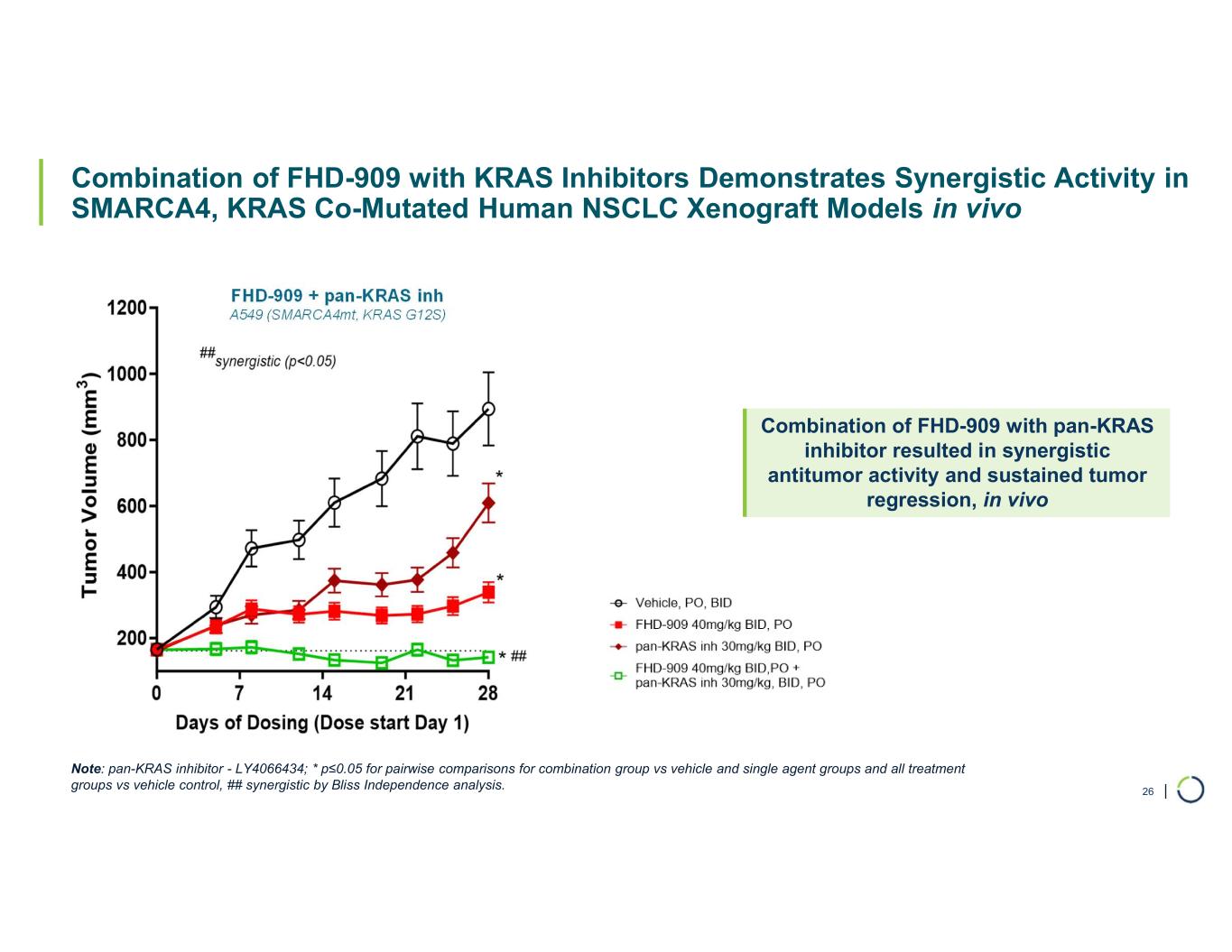
•Presented synergistic preclinical data of FHD-909 in combination with pembrolizumab and KRAS inhibitors. Also at the AACR Annual Meeting in April 2025, Lilly, on behalf of the collaboration, made an oral presentation of new preclinical data demonstrating enhanced anti-tumor activity of FHD-909 in combination with standard-of-care chemotherapies, anti-PD-1 pembrolizumab and several novel KRAS inhibitors in NSCLC animal models. The combination data will inform further development plans of FHD-909.
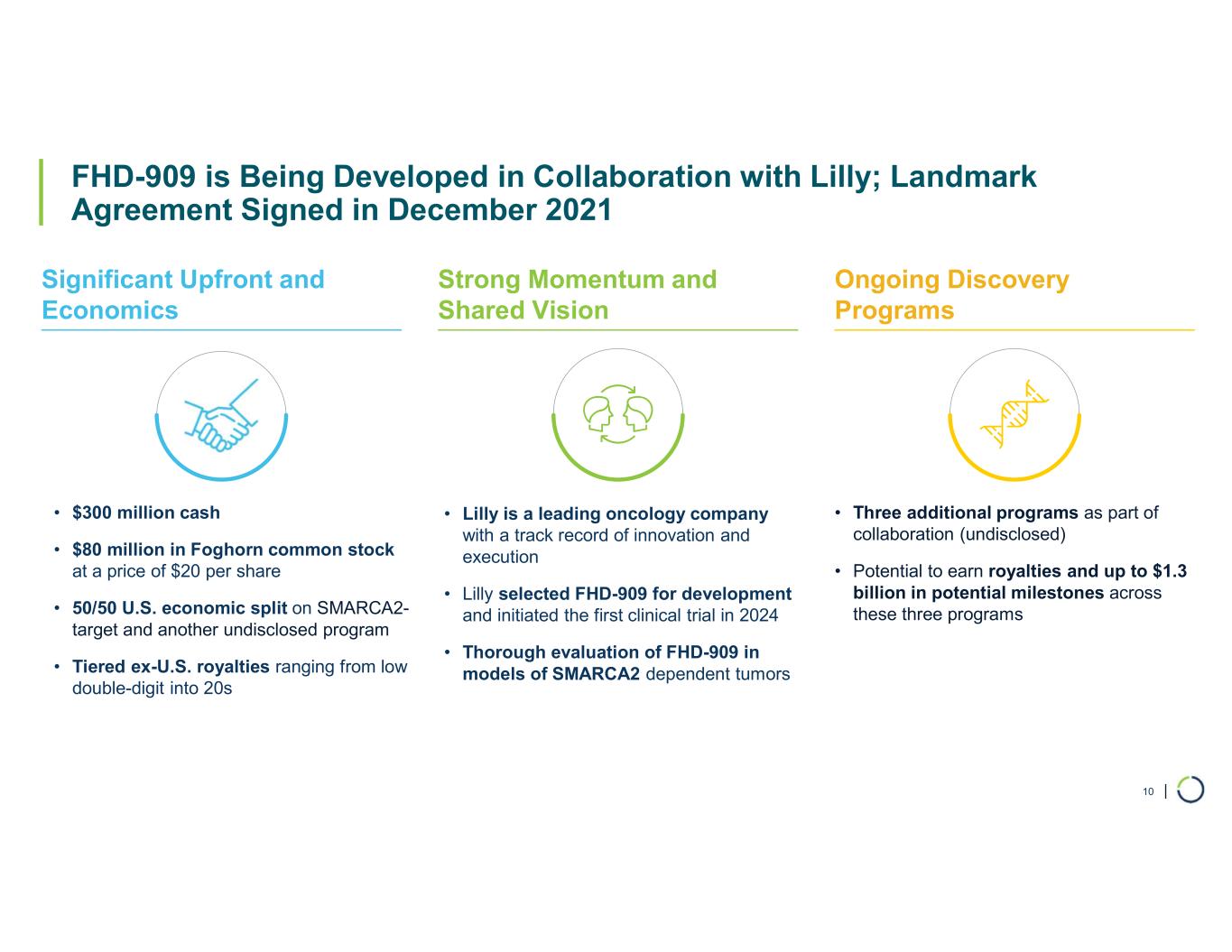
Ongoing strategic collaboration with Lilly. Collaborating with Lilly to create novel oncology medicines that includes a U.S. 50/50 co-development and co-commercialization agreement for Foghorn’s selective SMARCA2 oncology program, including a selective inhibitor and a selective degrader, and an additional undisclosed oncology target. The collaboration also includes three discovery programs from Foghorn’s proprietary Gene Traffic Control® platform.
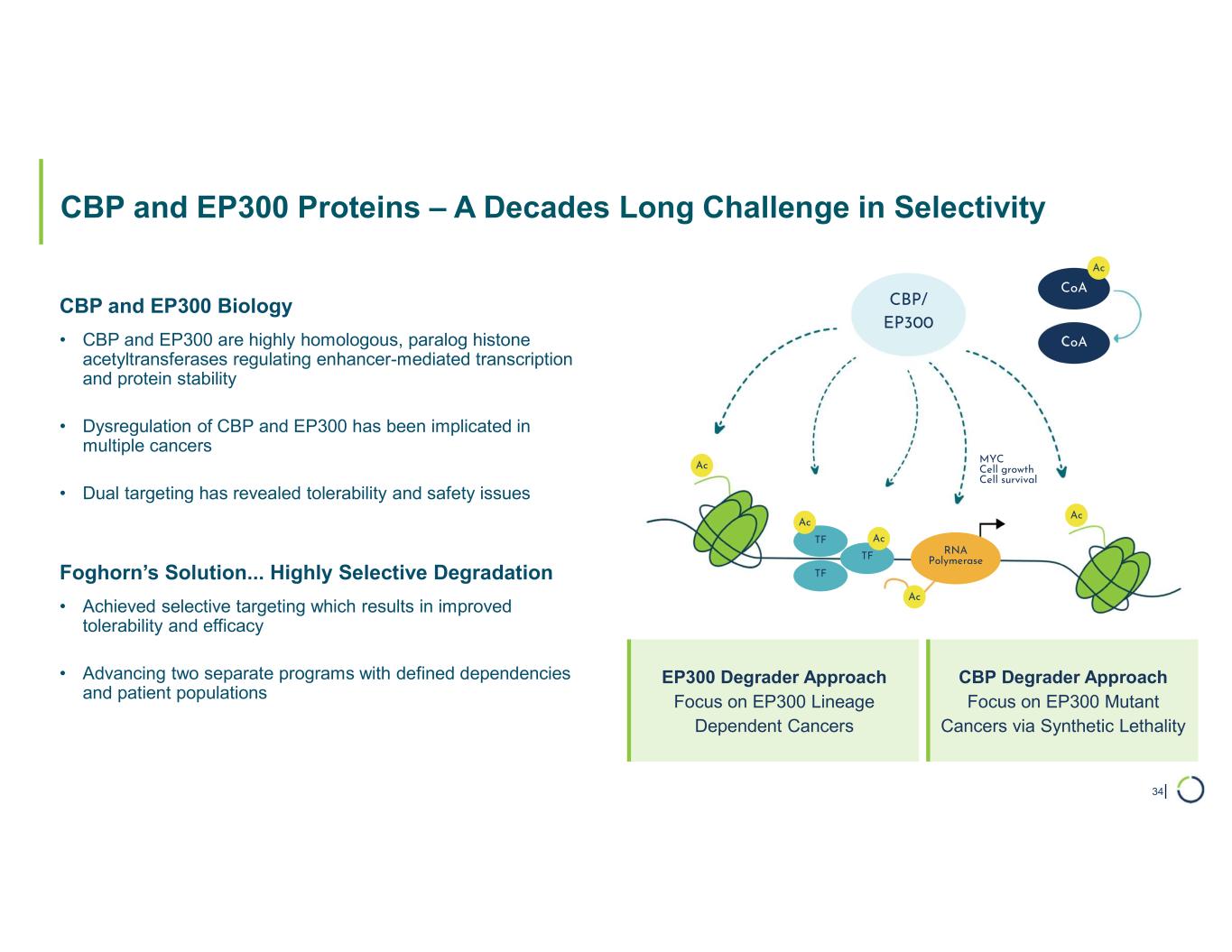
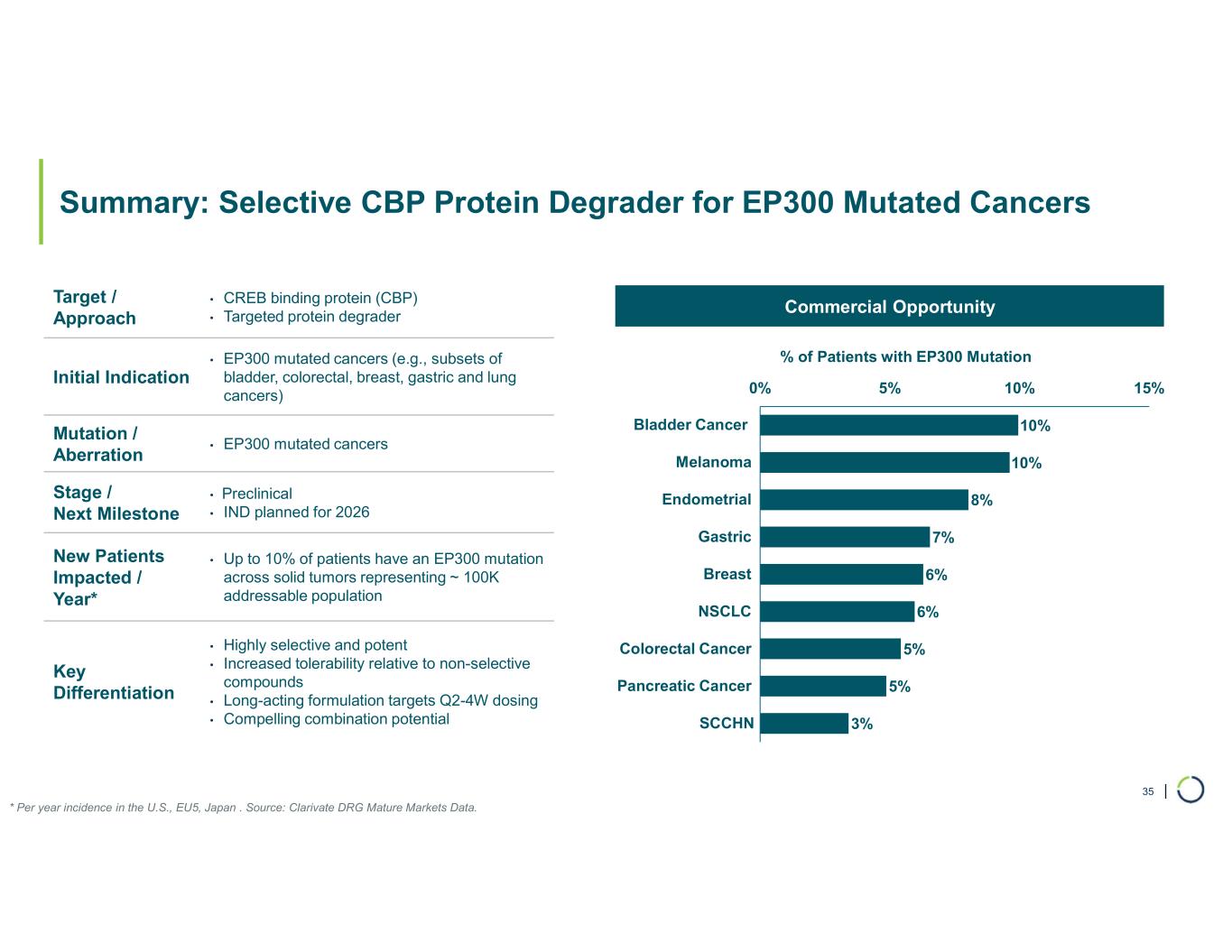
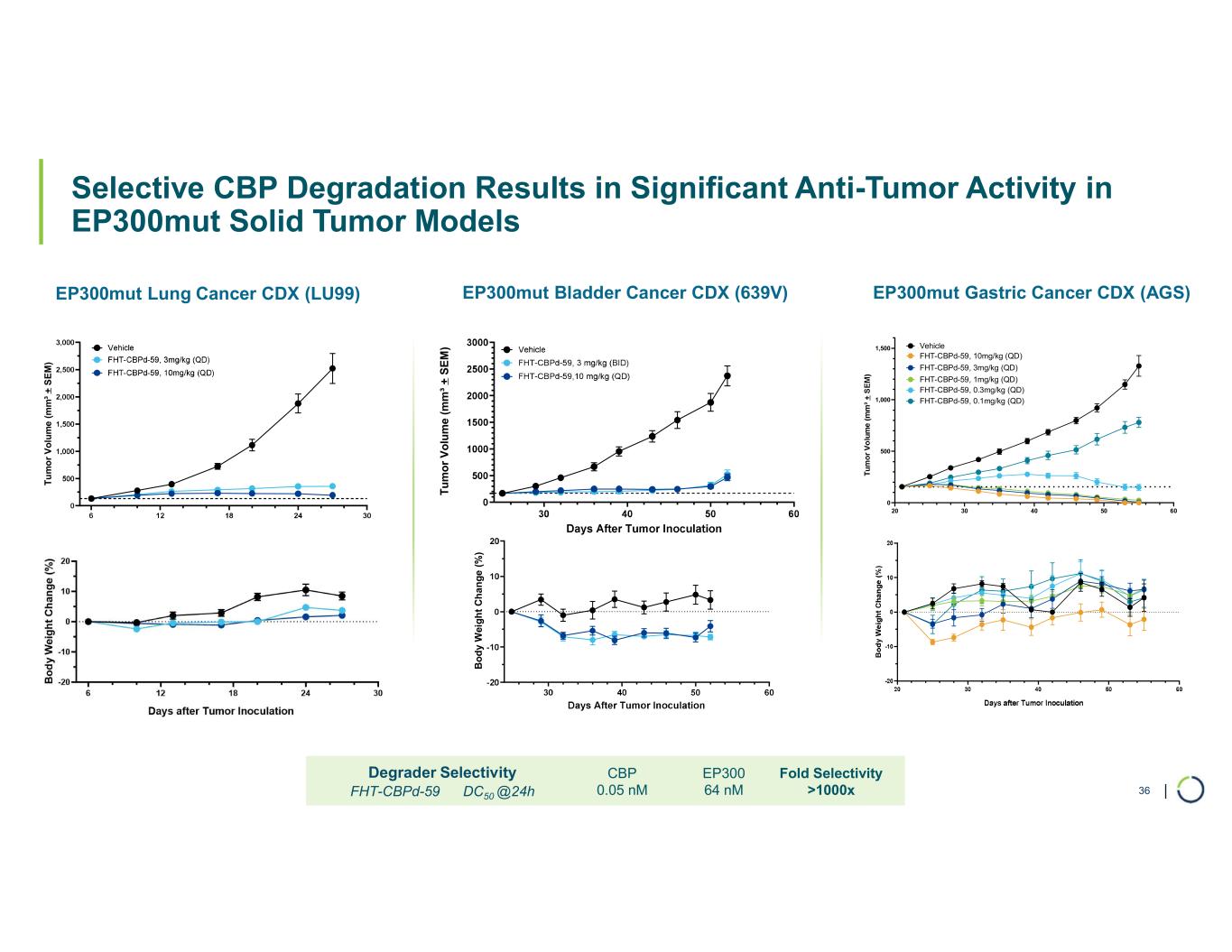
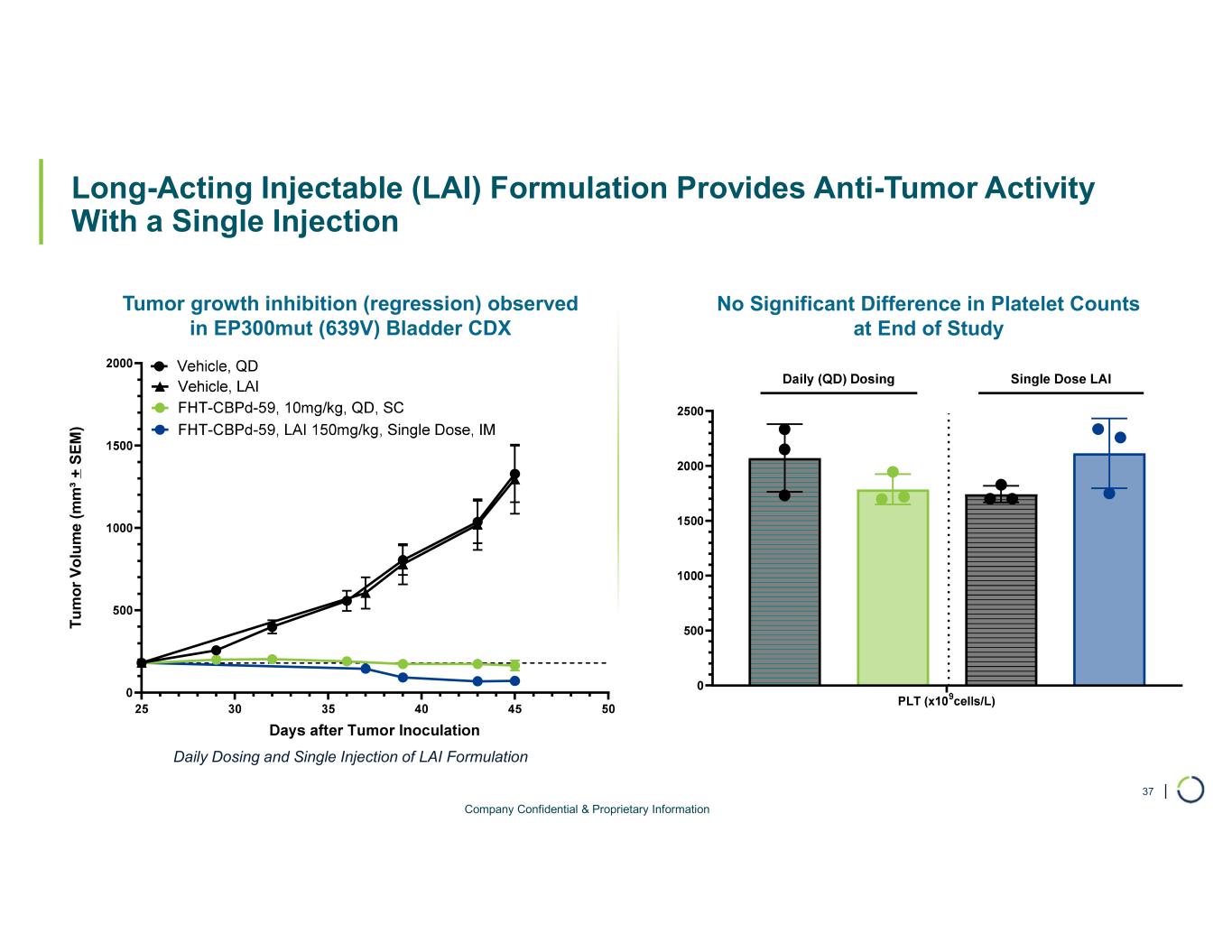
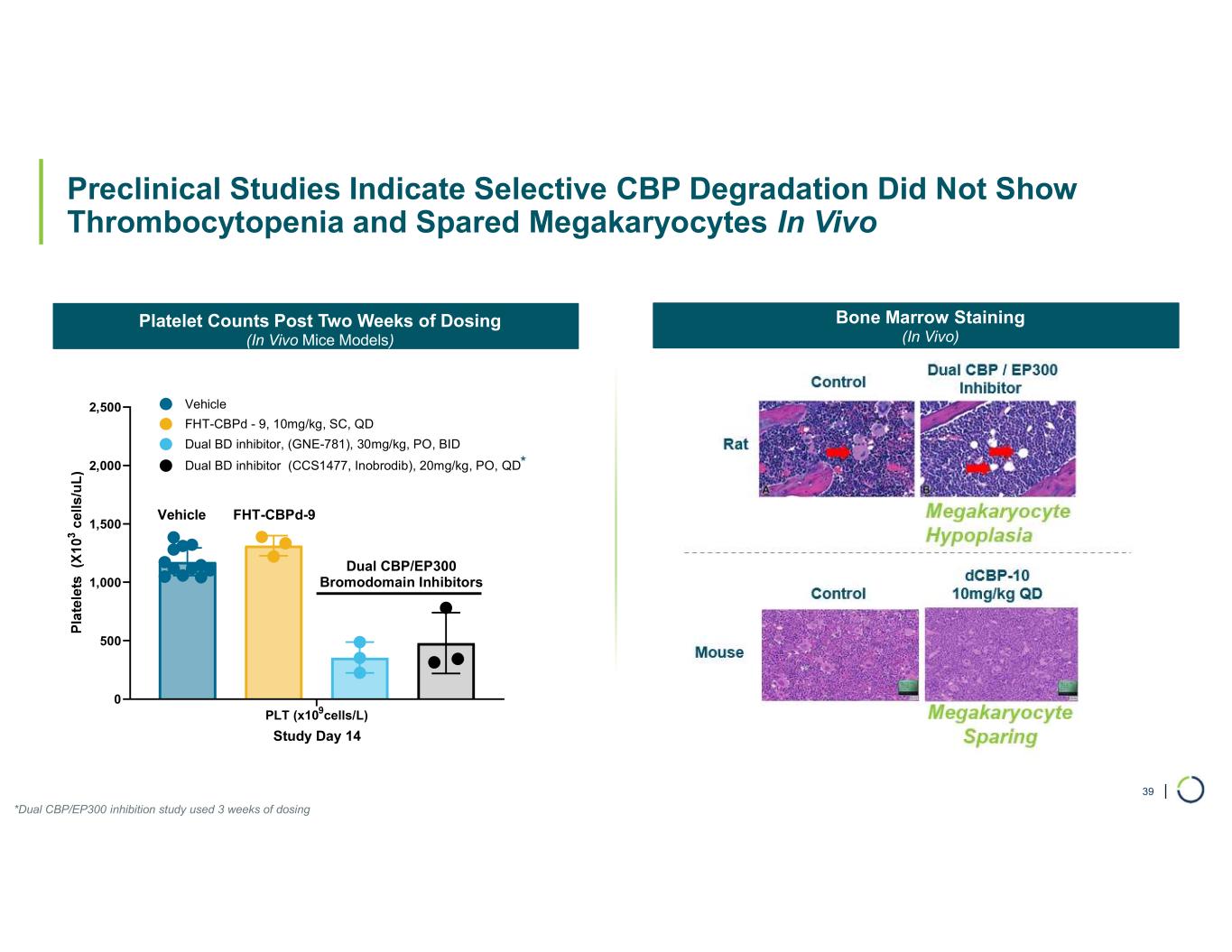
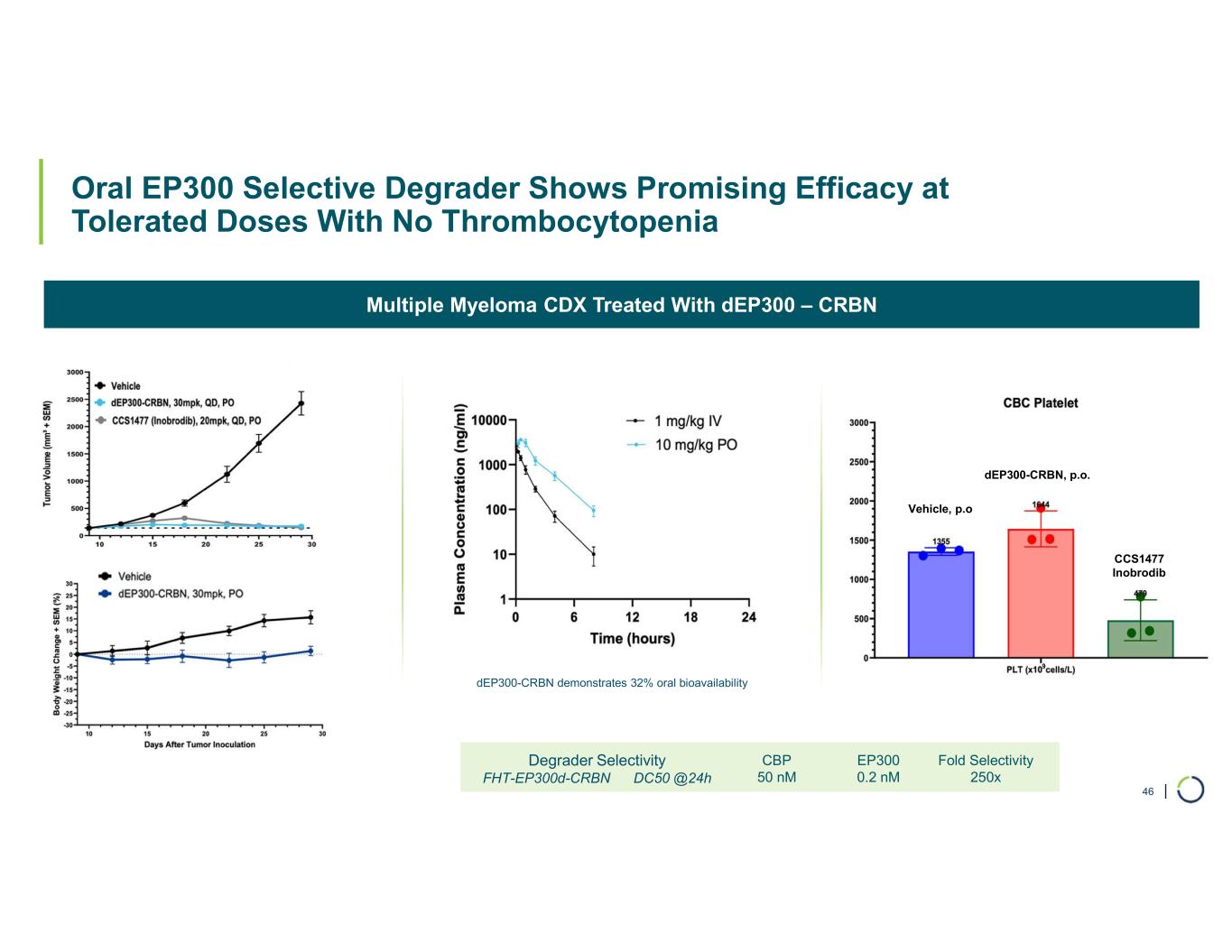
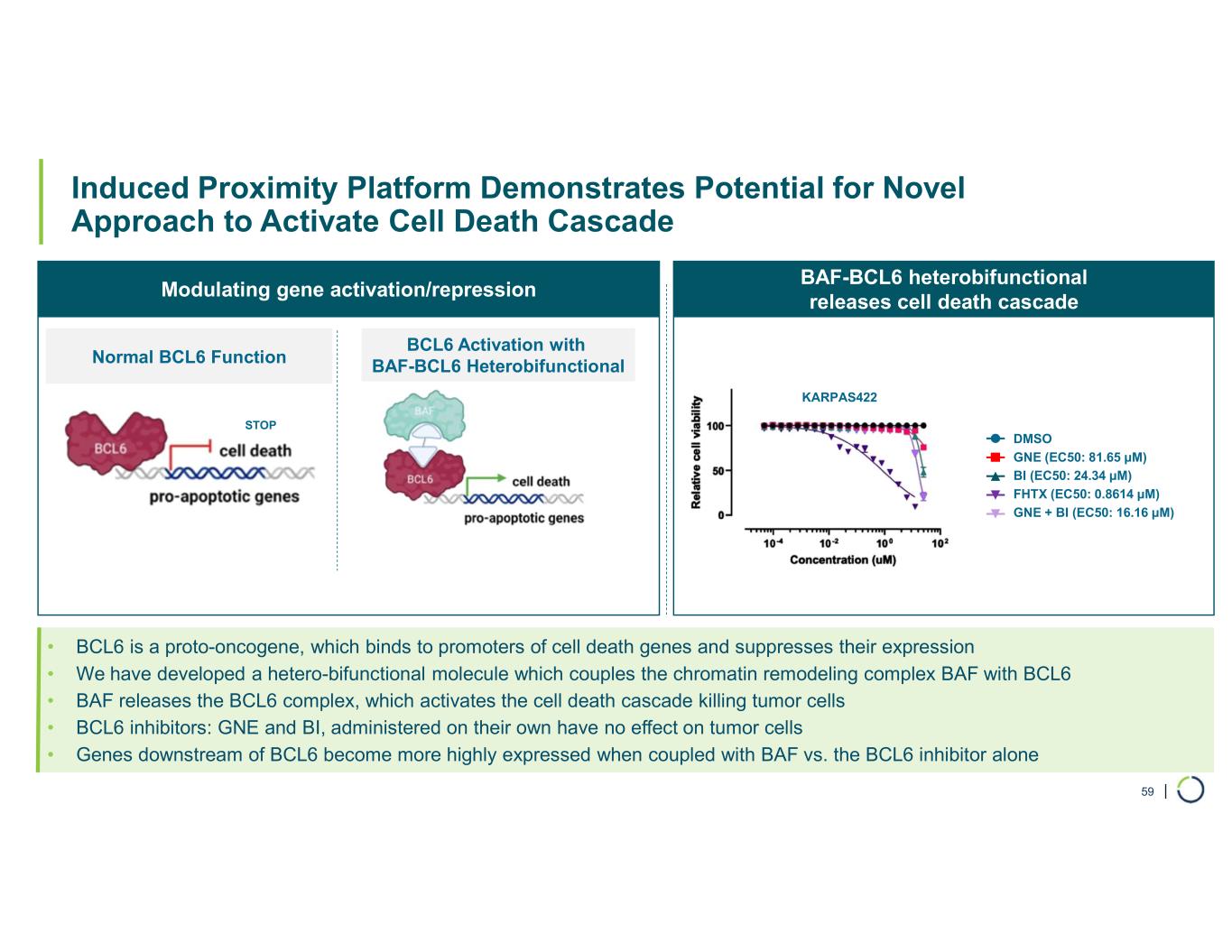
Selective CBP degrader program. Selectively targets CBP in EP300-mutated cancer cells found in many types of cancer, including bladder, gastric, and endometrial cancers. CBP and EP300 are highly similar acetyltransferases that create a synthetic lethal relationship when EP300 is mutated. Attempts to selectively drug CBP have been challenging due to the high level of similarity between the two proteins, while dual inhibition of CBP/EP300 has been limited by dose-limiting toxicities.
•Presented preclinical combination data with Selective degrader CBP in ER+ breast cancer. In April 2025, preclinical data showing Selective CBP degraders have combinatorial benefit with approved chemotherapies and targeted agents in solid tumors beyond EP300-mutant cancers was presented as a poster at the AACR Annual Meeting
•Synergistic combination activity demonstrated including with paclitaxel and CDK4/6 inhibitor abemaciclib in ER+ breast cancer
•Findings support combination opportunities for selective CBP degraders in solid tumors beyond EP300-mutant cancers
•On track for IND-enabling studies, targeting IND in 2026.
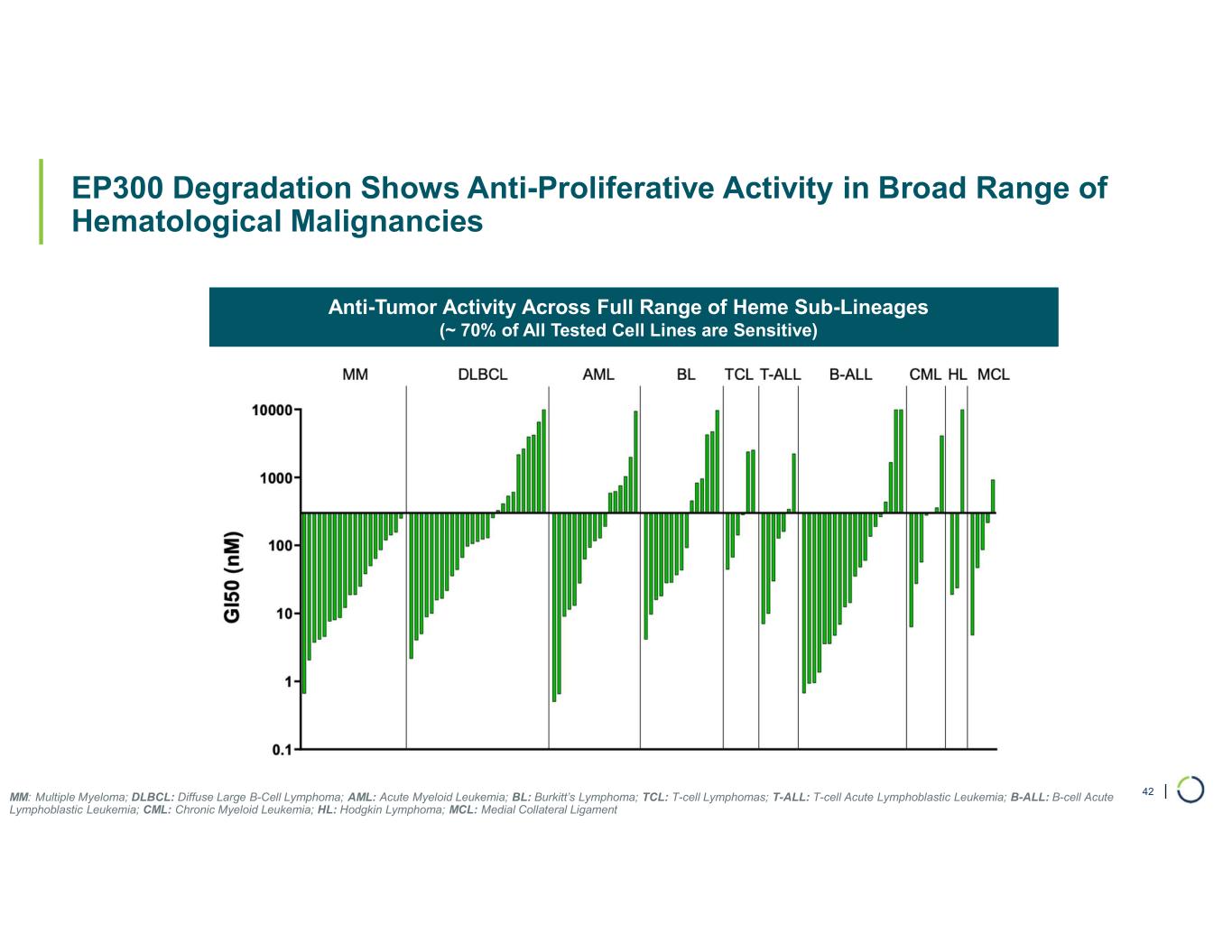
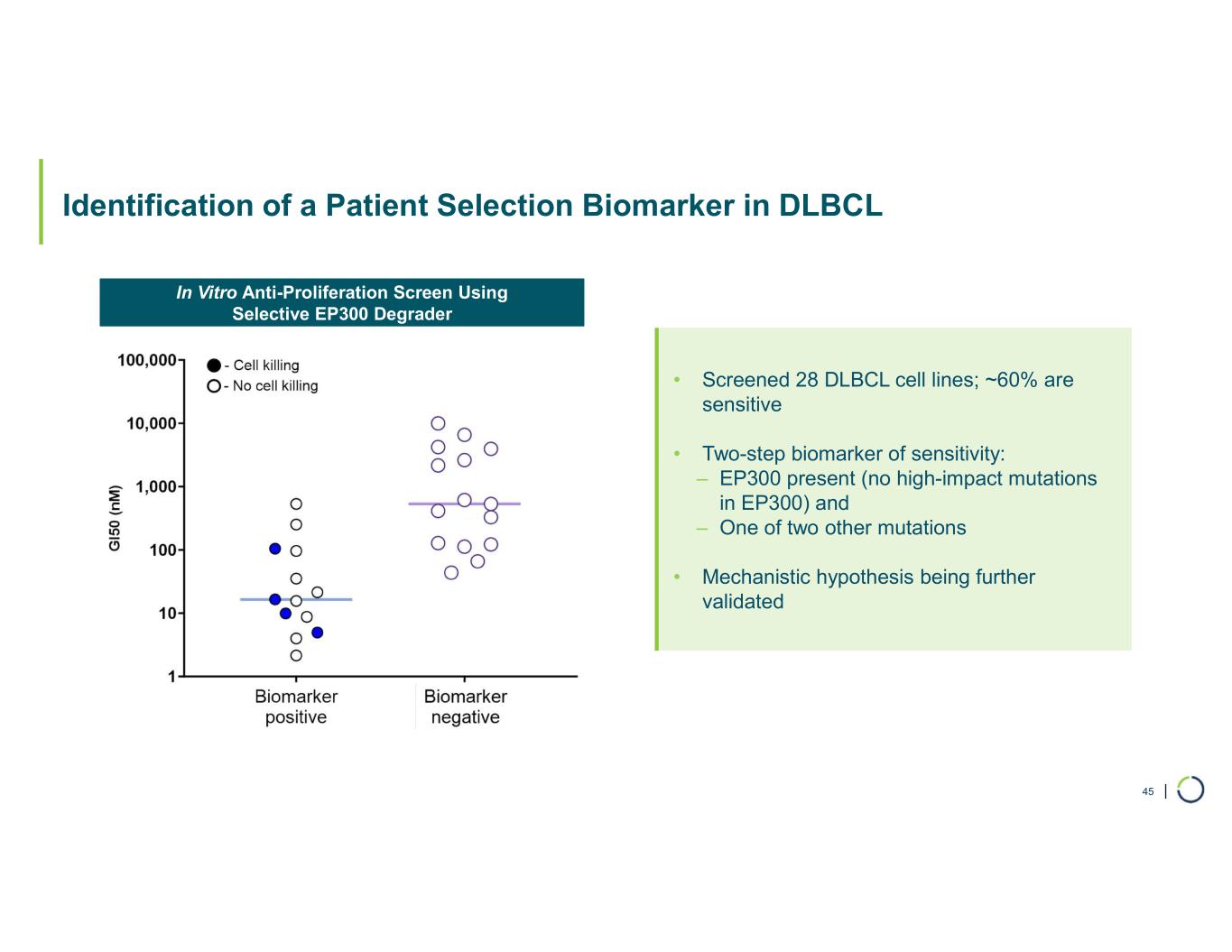
Selective EP300 degrader program. Selective degradation of EP300 for the treatment of hematopoietic malignancies and prostate cancer. Attempts to selectively drug EP300 have been challenging due to the high level of similarity between EP300 and CBP, while dual inhibition of CBP/EP300 has been limited by dose limiting toxicities. EP300 lineage dependencies are established in multiple myeloma and diffuse large B cell lymphoma.
•Presented preclinical data showing combination with diffuse large b-cell lymphoma (DLBCL) and multiple myeloma (MM) Standard-of-Care Treatment (SoC). In April 2025, preclinical data for the Selective EP300 degrader program demonstrating biological activity in hematological malignancies was presented at the AACR Annual Meeting.
•Anti-proliferative activity in a broad range of hematological malignancies in vitro, including DLBCL, MM, and follicular lymphoma
•Combination of EP300 degrader with SoC in both DLBCL and MM are highly synergistic in vitro.
•Selective EP300 degradation is effective in IMiD-resistant MM cell lines.
•Program update expected in H2 2025.
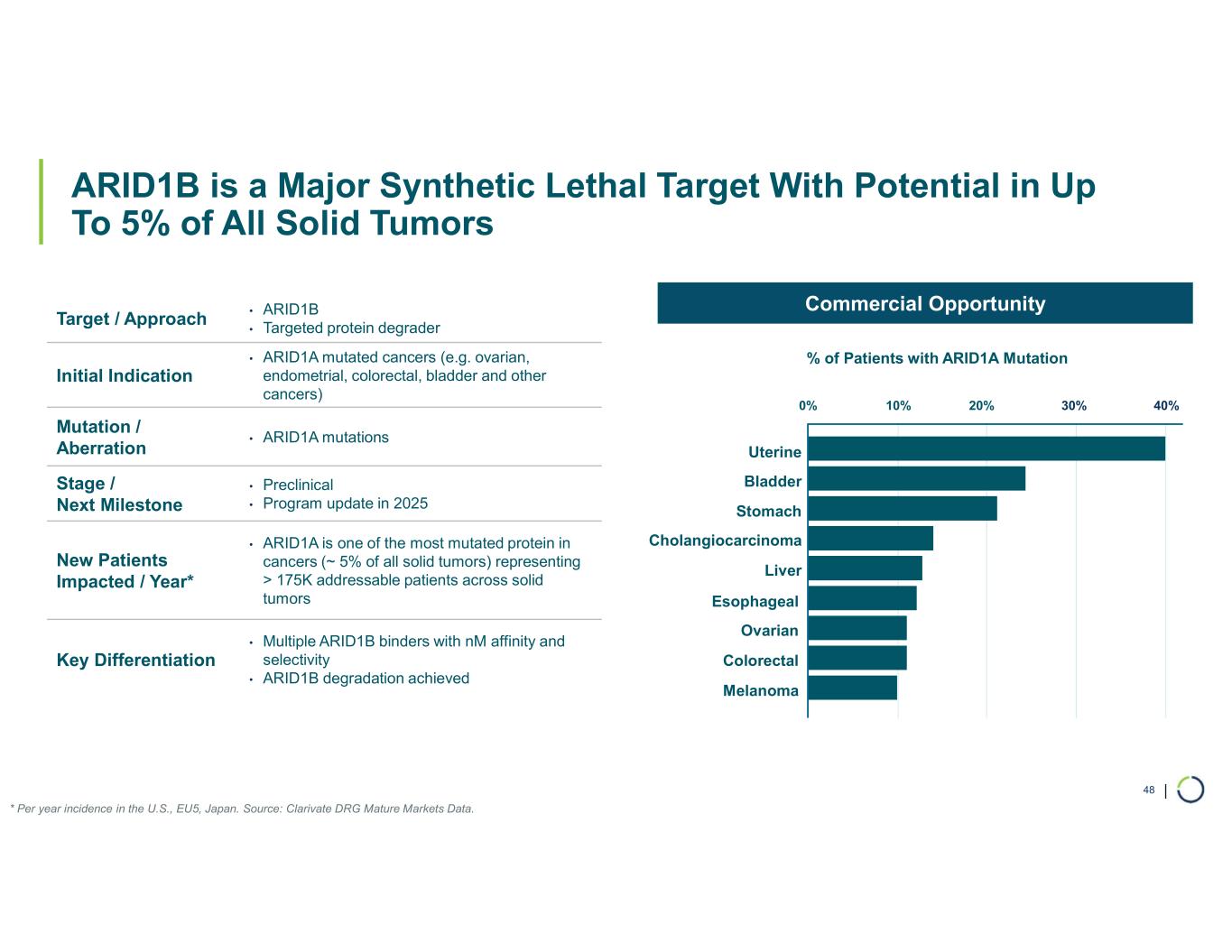
Selective ARID1B degrader program. Selectively targets and degrades ARID1B in ARID1A-mutated cancers. ARID1A is the most mutated subunit in the BAF complex and amongst the most mutated proteins in cancer. These mutations lead to a dependency on ARID1B in several types of cancer, including ovarian, endometrial, colorectal and bladder. Attempts to selectively drug ARID1B have been challenging because of the high degree of similarity between ARID1A and ARID1B and the fact that ARID1B has no enzymatic activity to target.
•ARID1B is a major synthetic lethal target implicated in up to 5% of all solid tumors.
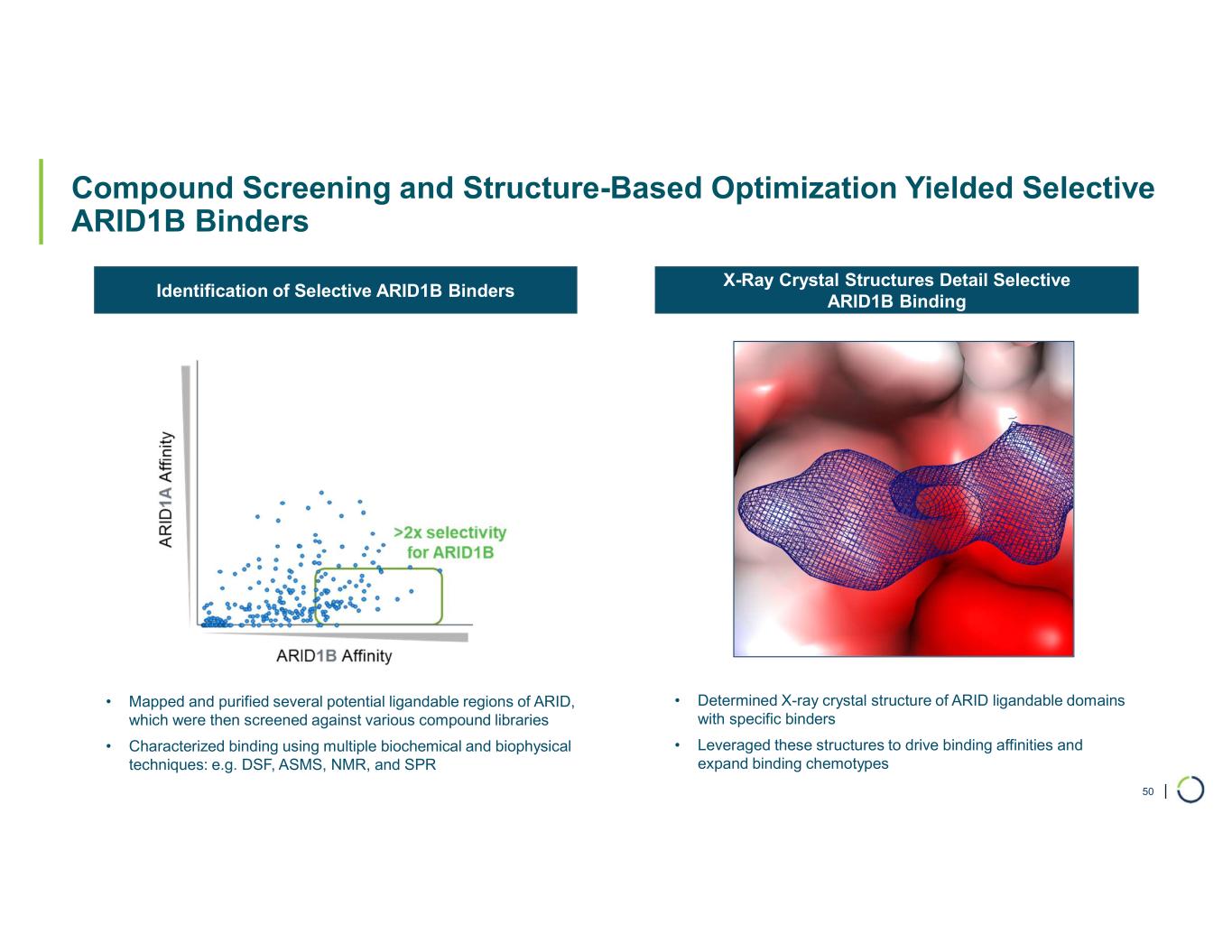
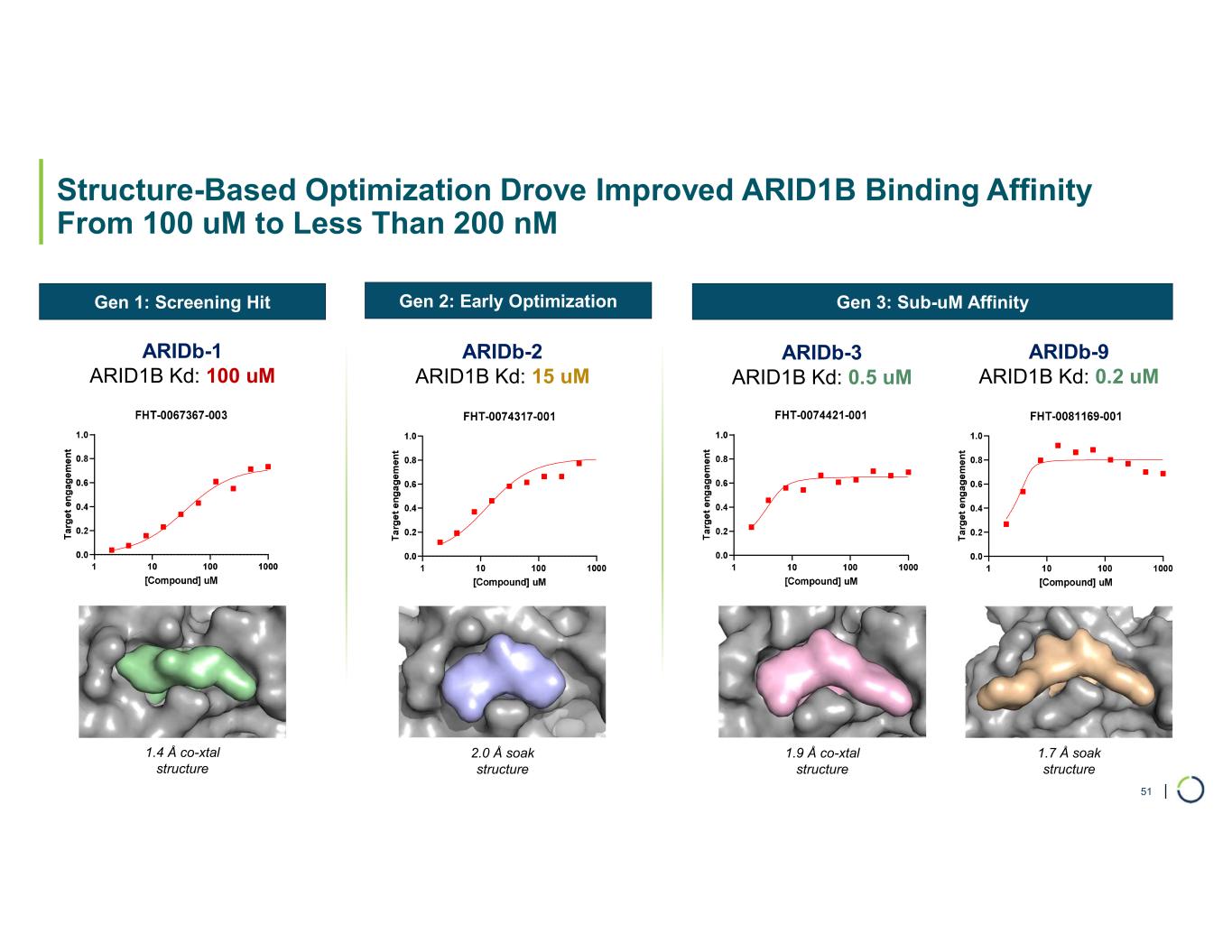
•Developed highly potent and selective binders. Preclinical data demonstrated potent and selective small molecule binders to ARID1B.
•Selective degradation of ARID1B achieved. Foghorn has successfully selectively degraded ARID1B and expects to provide an update on the Selective ARID1B degrader program in H2 2025.
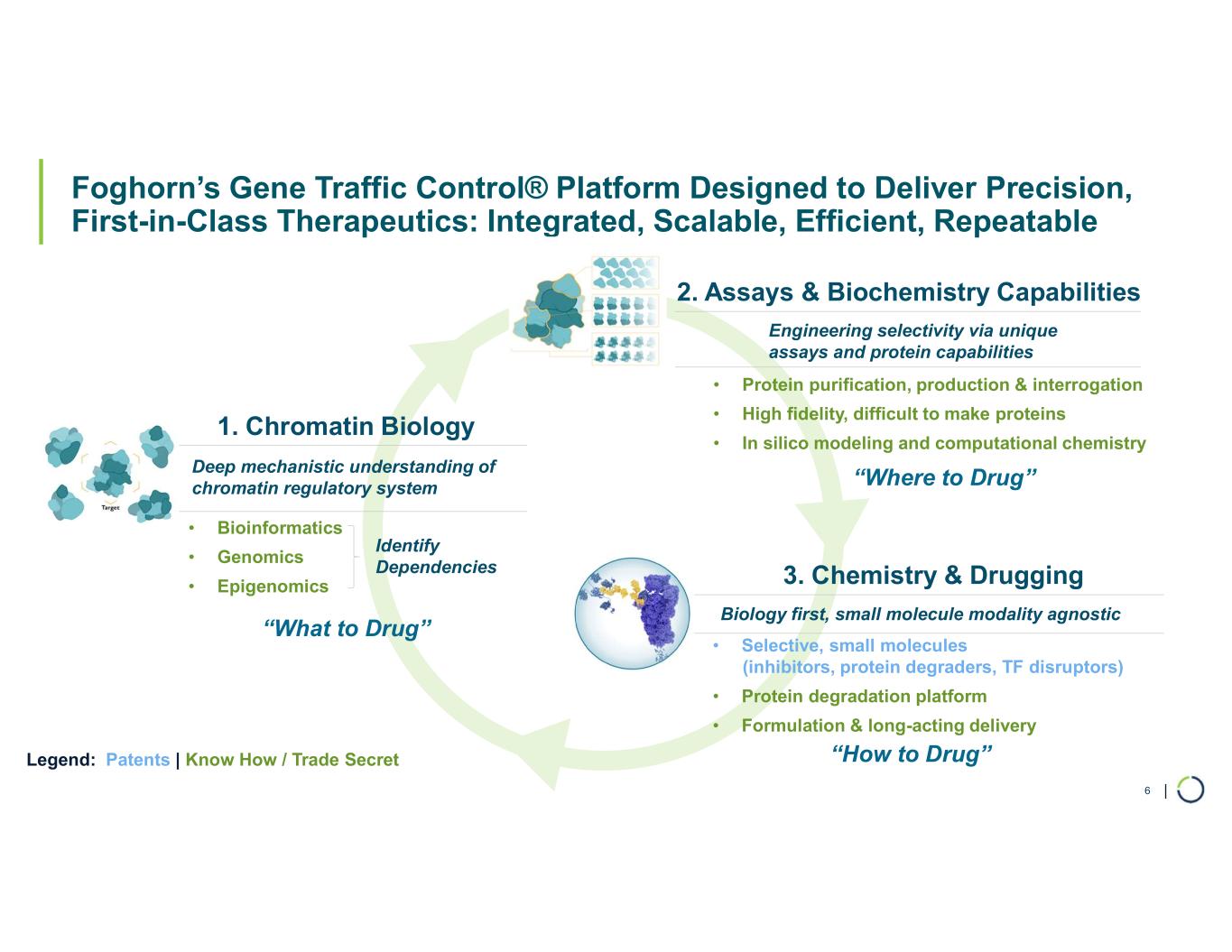
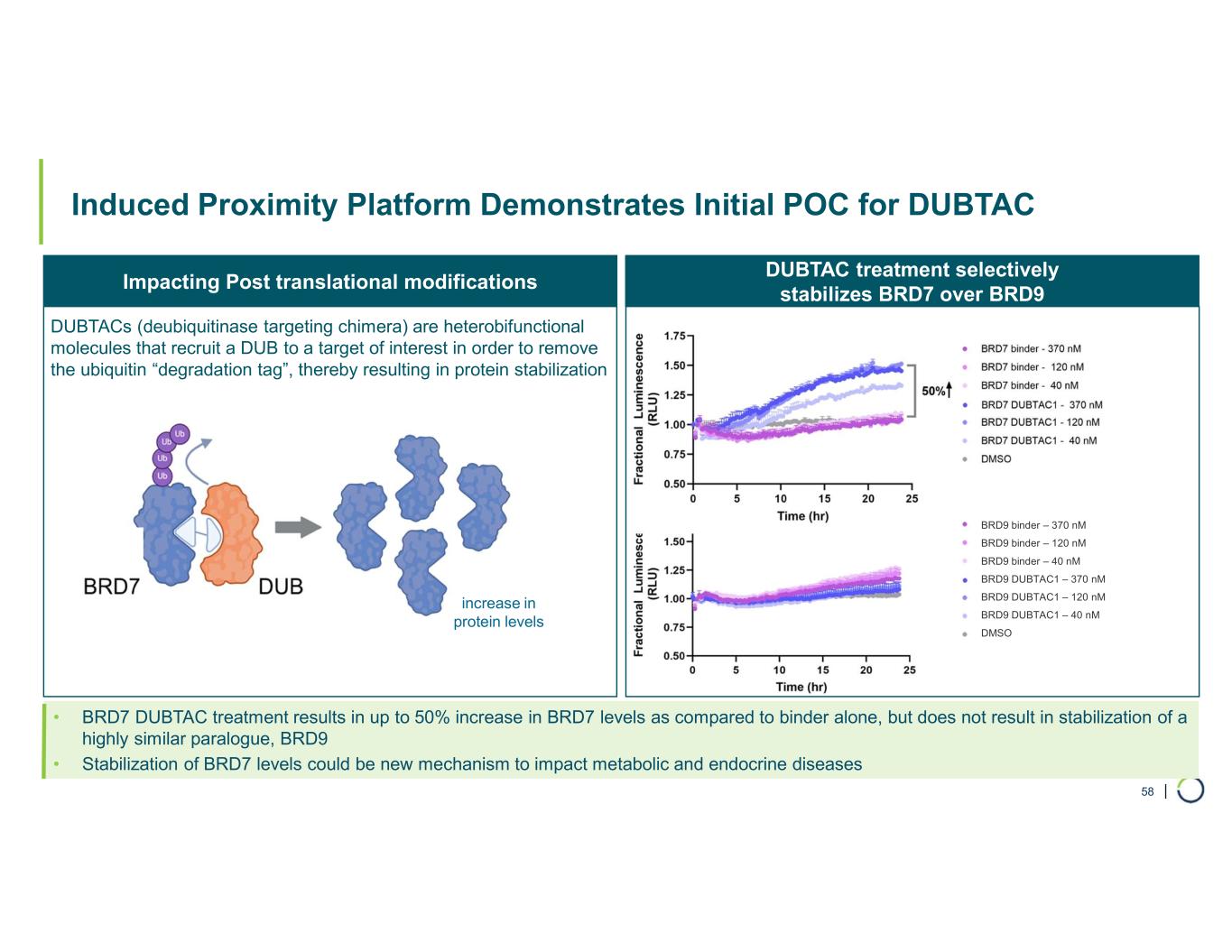
Chromatin Biology and Degrader Platform. Foghorn continues to advance its chromatin biology and degrader platform with investments in novel ligases, long-acting injectables, oral delivery, and induced proximity.
First Quarter 2025 Financial Highlights
•Collaboration Revenue. Collaboration revenue was $6.0 million for the three months ended March 31, 2025, compared to $5.1 million for the three months ended March 31, 2024. The increase was driven by the continued advancement of programs under the Lilly Collaboration Agreement.
•Research and Development Expenses. Research and development expenses were $21.6 million for the three months ended March 31, 2025, compared to $25.5 million for the three months ended March 31, 2024. The decrease is attributed to a decrease in FHD-286 costs of $2.5 million, decreases in personnel-related costs, early development and other research external costs and facilities and IT-related expenses of $2.1 million, partially offset by an increase in Lilly-partnered programs of $0.7 million.
•General and Administrative Expenses. General and administrative expenses were $7.2 million for the three months ended March 31, 2025, compared to $7.7 million for the three months ended March 31, 2024. This decrease was primarily due to lower consulting costs.
•Net Loss. Net loss was $18.8 million for the three months ended March 31, 2025, compared to a net loss of $25.0 million for the three months ended March 31, 2024.
•Cash, Cash Equivalents, and Marketable Securities. As of March 31, 2025, the Company had $220.6 million in cash, cash equivalents, and marketable securities, providing expected cash runway into 2027.
About FHD-909
FHD-909 (LY4050784) is a potent, first-in-class, allosteric, and orally available small molecule that selectively inhibits the ATPase activity of SMARCA2 (BRM) over its closely related paralog SMARCA4 (BRG1), two proteins that are the catalytic engines across all forms of the BAF complex, one of the key regulators of the chromatin regulatory system. In preclinical studies, tumors with mutations in SMARCA4 rely on SMARCA2 for their survival. FHD-909 has shown significant anti-tumor activity across multiple SMARCA4 mutant lung tumor models.
About Foghorn Therapeutics
Foghorn® Therapeutics is discovering and developing a novel class of medicines targeting genetically determined dependencies within the chromatin regulatory system. Through its proprietary scalable Gene Traffic Control® platform, Foghorn is systematically studying, identifying, and validating potential drug targets within the chromatin regulatory system. The Company is developing multiple product candidates in oncology. Visit our website at www.foghorntx.com for more information on the Company, and follow us on X (formerly Twitter) and LinkedIn.
Forward-Looking Statements
This press release contains “forward-looking statements.” Forward-looking statements include statements regarding the Company’s ongoing Phase 1 trial of FHD-909 in SMARCA4-mutated cancers, pre-clinical product candidates, expected timing of clinical data, expected cash runway, expected timing of regulatory filings, and research efforts and other statements identified by words such as “could,” “may,” “might,” “will,” “likely,” “anticipates,” “intends,” “plans,” “seeks,” “believes,” “estimates,” “expects,” “continues,” “projects” and similar references to future periods. Forward-looking statements are based on our current expectations and assumptions regarding capital market conditions, our business, the economy and other future conditions. Because forward-looking statements relate to the future, by their nature, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. As a result, actual results may differ materially from those contemplated by the forward-looking statements. Important factors that could cause actual results to differ materially from those in the forward-looking statements include regional, national or global political, economic, business, competitive, market and regulatory conditions, including risks relating to our clinical trials and other factors set forth under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, as filed with the Securities and Exchange Commission. Any forward-looking statement made in this press release speaks only as of the date on which it is made.
Condensed Consolidated Balance Sheets
(In thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2025 |
|
December 31, 2024 |
| Cash, cash equivalents and marketable securities |
$ |
220,587 |
|
|
$ |
243,747 |
|
All other assets |
38,104 |
|
|
40,235 |
|
Total assets |
$ |
258,691 |
|
|
$ |
283,982 |
|
Deferred revenue, total |
$ |
274,112 |
|
|
$ |
280,063 |
|
All other liabilities |
46,231 |
|
|
49,447 |
|
Total liabilities |
$ |
320,343 |
|
|
$ |
329,510 |
|
Total stockholders’ deficit |
$ |
(61,652) |
|
|
$ |
(45,528) |
|
Total liabilities and stockholders’ deficit |
$ |
258,691 |
|
|
$ |
283,982 |
|
Condensed Consolidated Statements of Operations
(In thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
2025 |
|
2024 |
| Collaboration revenue |
$ |
5,952 |
|
|
$ |
5,050 |
|
| Operating expenses: |
|
|
|
| Research and development |
21,626 |
|
|
25,534 |
|
| General and administrative |
7,239 |
|
|
7,710 |
|
|
|
|
|
| Total operating expenses |
$ |
28,865 |
|
|
$ |
33,244 |
|
| Loss from operations |
$ |
(22,913) |
|
|
$ |
(28,194) |
|
| Total other income, net |
$ |
4,079 |
|
|
$ |
3,178 |
|
|
|
|
|
| Net loss |
$ |
(18,834) |
|
|
$ |
(25,016) |
|
| Net loss per share attributable to common stockholders—basic and diluted |
(0.30) |
|
|
(0.59) |
|
| Weighted average common shares outstanding—basic and diluted |
62,848,673 |
|
|
42,428,813 |
|
Contact:
Karin Hellsvik, Foghorn Therapeutics Inc.
khellsvik@foghorntx.com