Document
Exhibit 99.1
C4 Therapeutics Reports First Quarter 2025 Financial Results and Recent Business Highlights
Updated Cemsidomide Multiple Myeloma Data Further Demonstrate Compelling Response Rates at Multiple Doses and Potential for Best-in-Class Profile; 50% ORR Observed at the Highest Dose Level of 100 µg, Including One Patient With a Minimal Residual Disease Negative Complete Response; 40% ORR Achieved at the 75 µg Dose Level
Cemsidomide Multiple Myeloma Dose Escalation is Complete; FDA Feedback Expected by Mid-Year 2025 to Support Initiation of Next Phase of Development in Early 2026
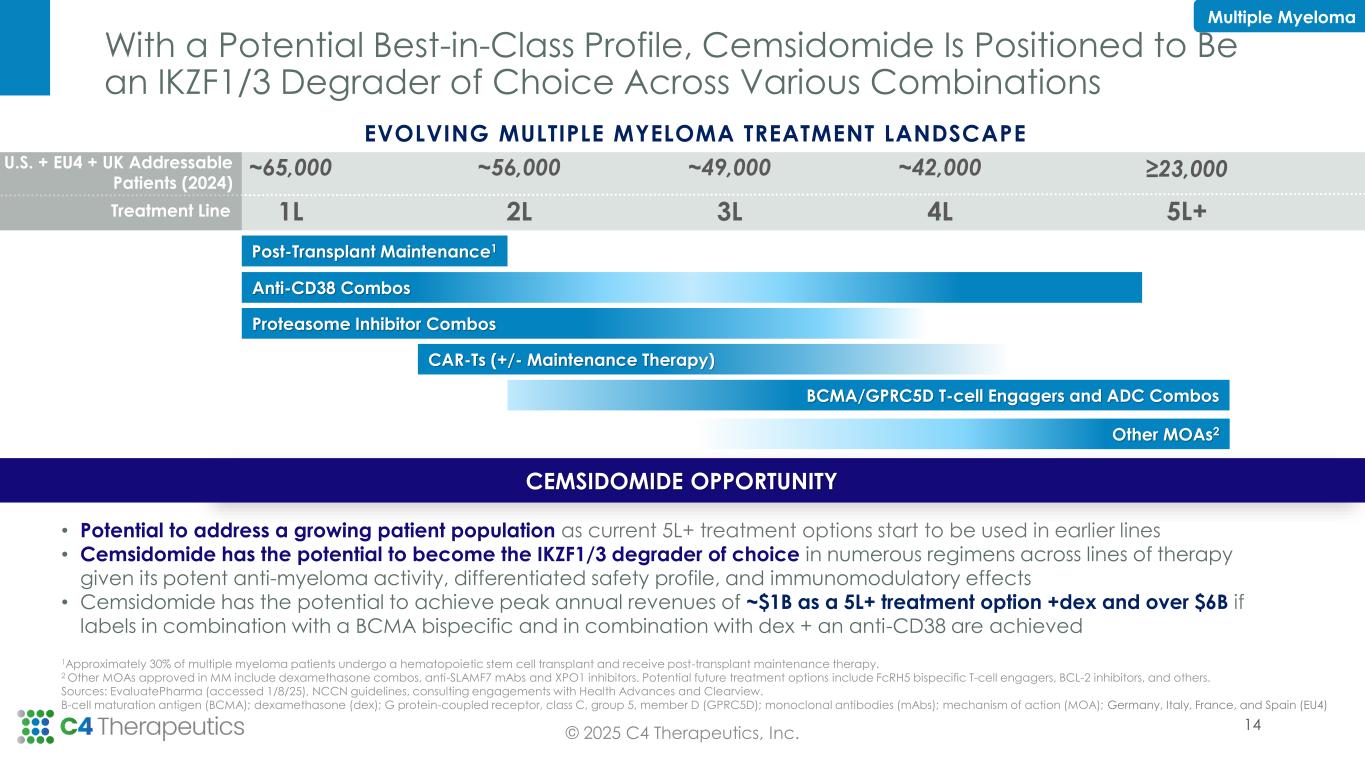
Portfolio Decision to Prioritize Cemsidomide Development; C4T to Seek Partnership Opportunities to Advance BRAF Program
CFT8919 Phase 1 Trial Continues to Advance in Partnership with Betta Pharmaceuticals; Discovery Platform Achieves Two Preclinical Milestones Under the Roche Collaboration
Cash, Cash Equivalents and Marketable Securities of $234.7 Million as of March 31, 2025 Expected to Provide Runway Into 2027
WATERTOWN, Mass., May 7, 2025 (GLOBE NEWSWIRE) -- C4 Therapeutics, Inc. (C4T) (Nasdaq: CCCC), a clinical-stage biopharmaceutical company dedicated to advancing targeted protein degradation science, today reported financial results for the first quarter ended March 31, 2025, as well as business updates.
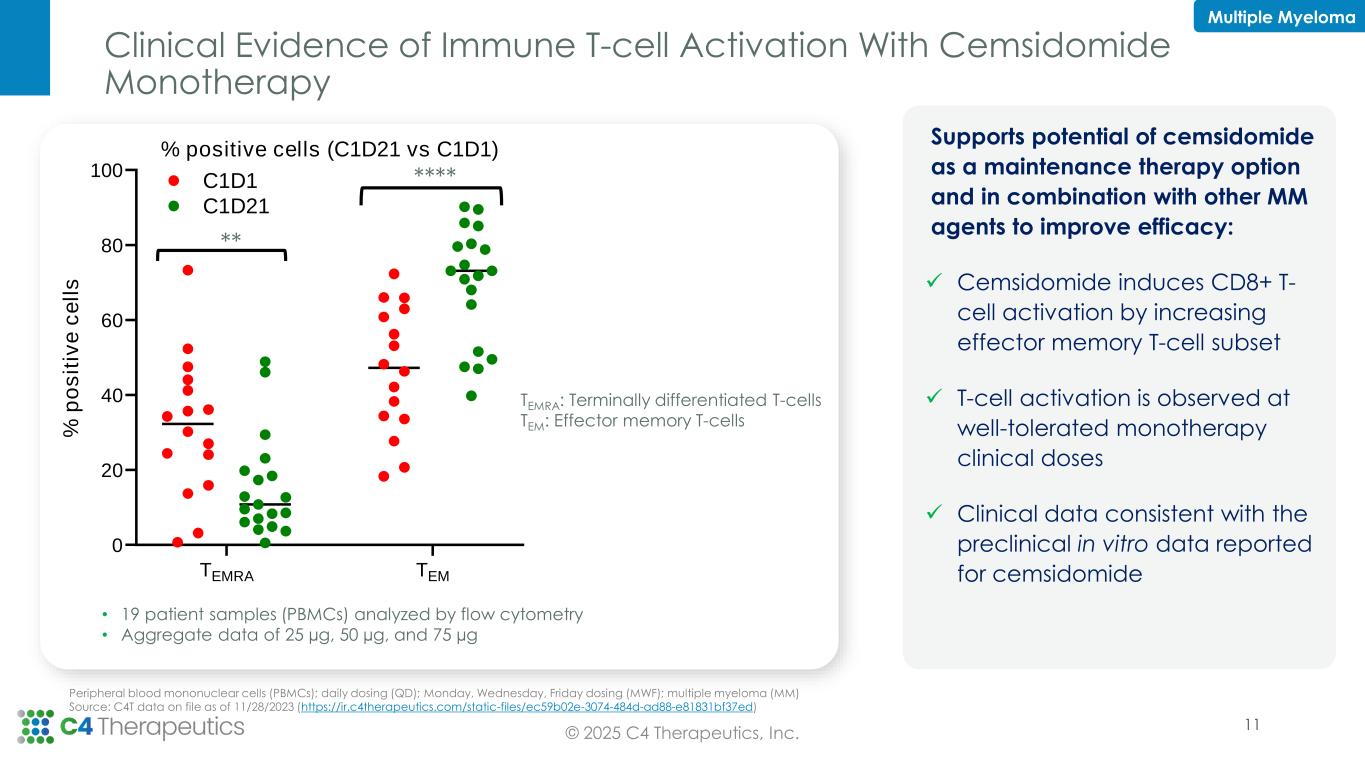
“2025 has been marked by focused execution across C4T to generate key data to optimize development plans across our clinical portfolio. With cemsidomide demonstrating compelling overall response rates at multiple dose levels, including one multiple myeloma patient at 100 µg who achieved a minimal residual disease negative complete response, we are prioritizing progressing cemsidomide to the next phase of development to realize its potential to be a best-in-class IKZF1/3 degrader,” said Andrew Hirsch, president and chief executive officer of C4 Therapeutics. “With the achievement of two preclinical milestones in our Roche collaboration, we continue to demonstrate the productivity of our TORPEDO platform to discover highly catalytic, orally bioavailable, and brain penetrant degraders. We remain focused on maximizing our cash runway, which includes advancing cemsidomide and pursuing our internal discovery pipeline focused on targets with a clear degrader rationale and compelling biology applicable to a broad range of therapeutic areas.”
FIRST QUARTER 2025 HIGHLIGHTS AND RECENT ACHIEVEMENTS
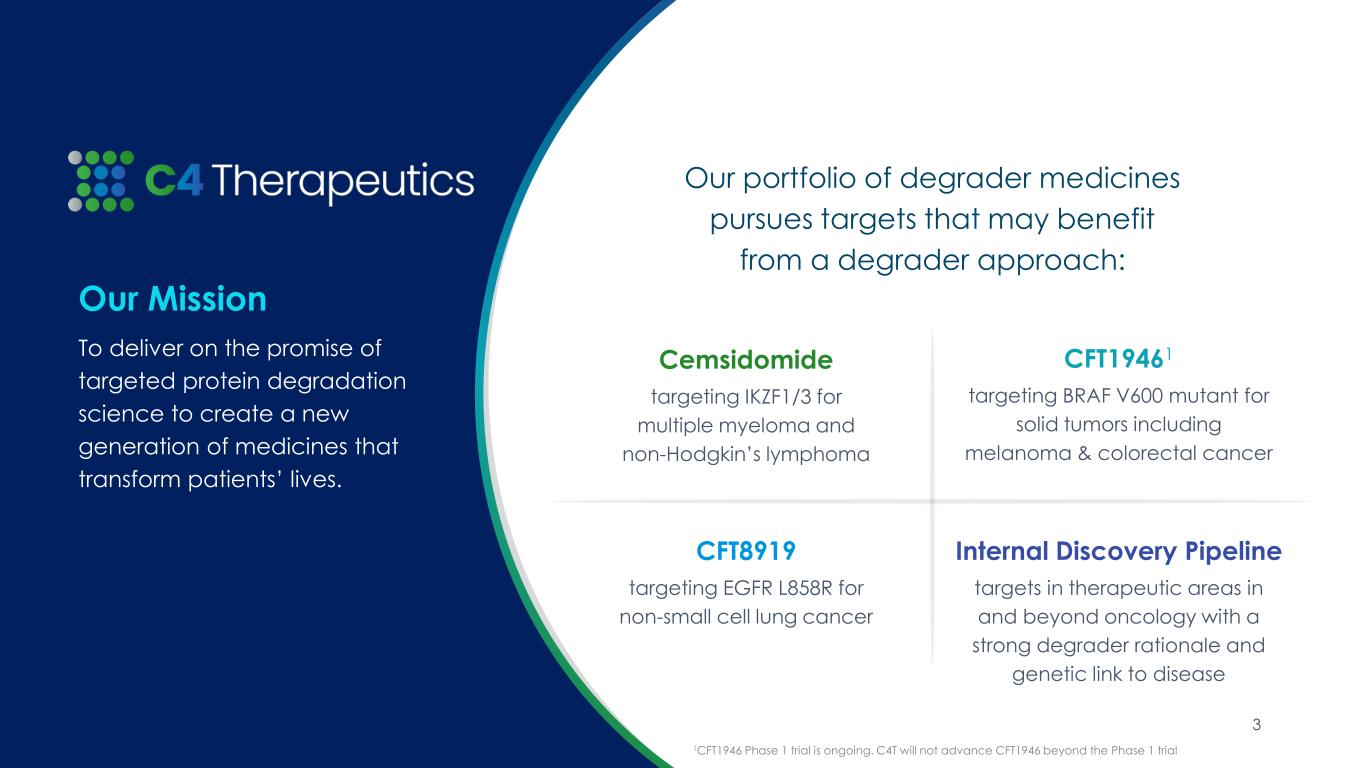
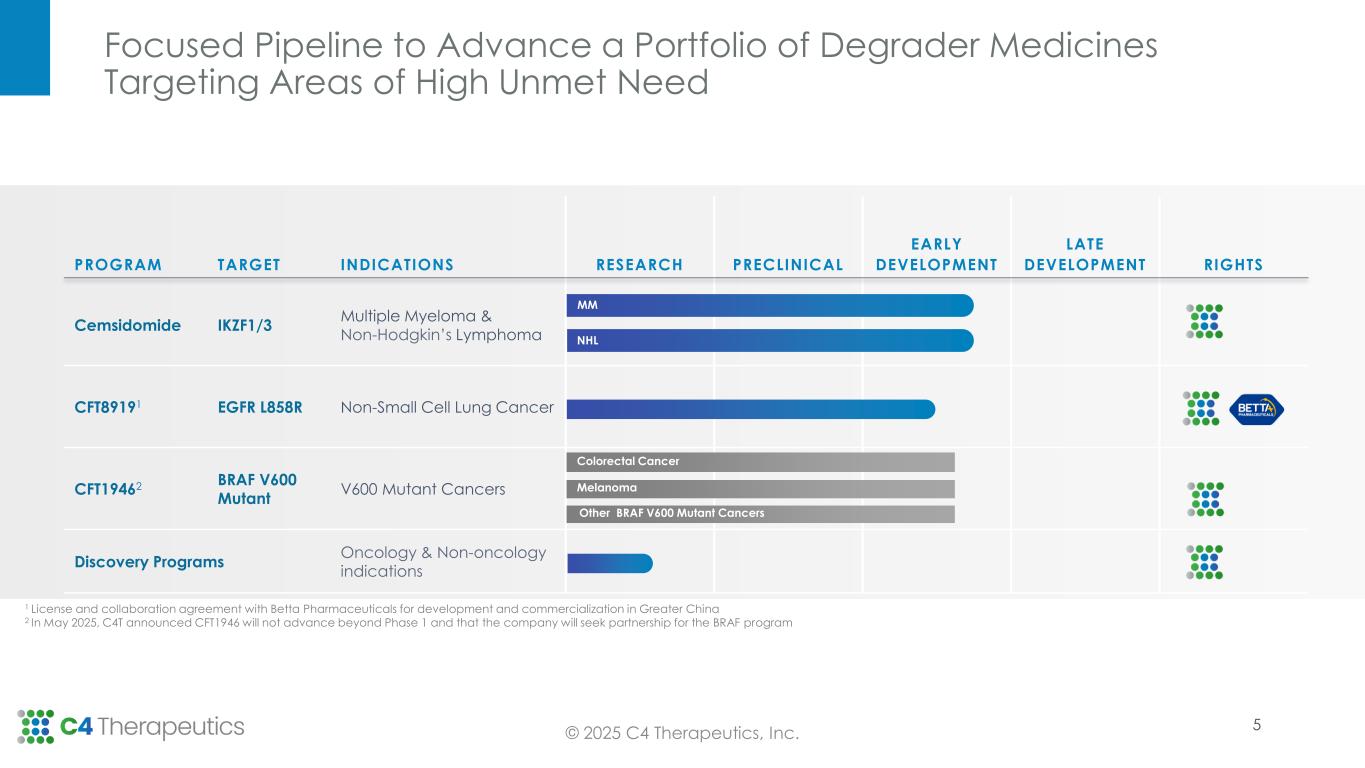
Cemsidomide:
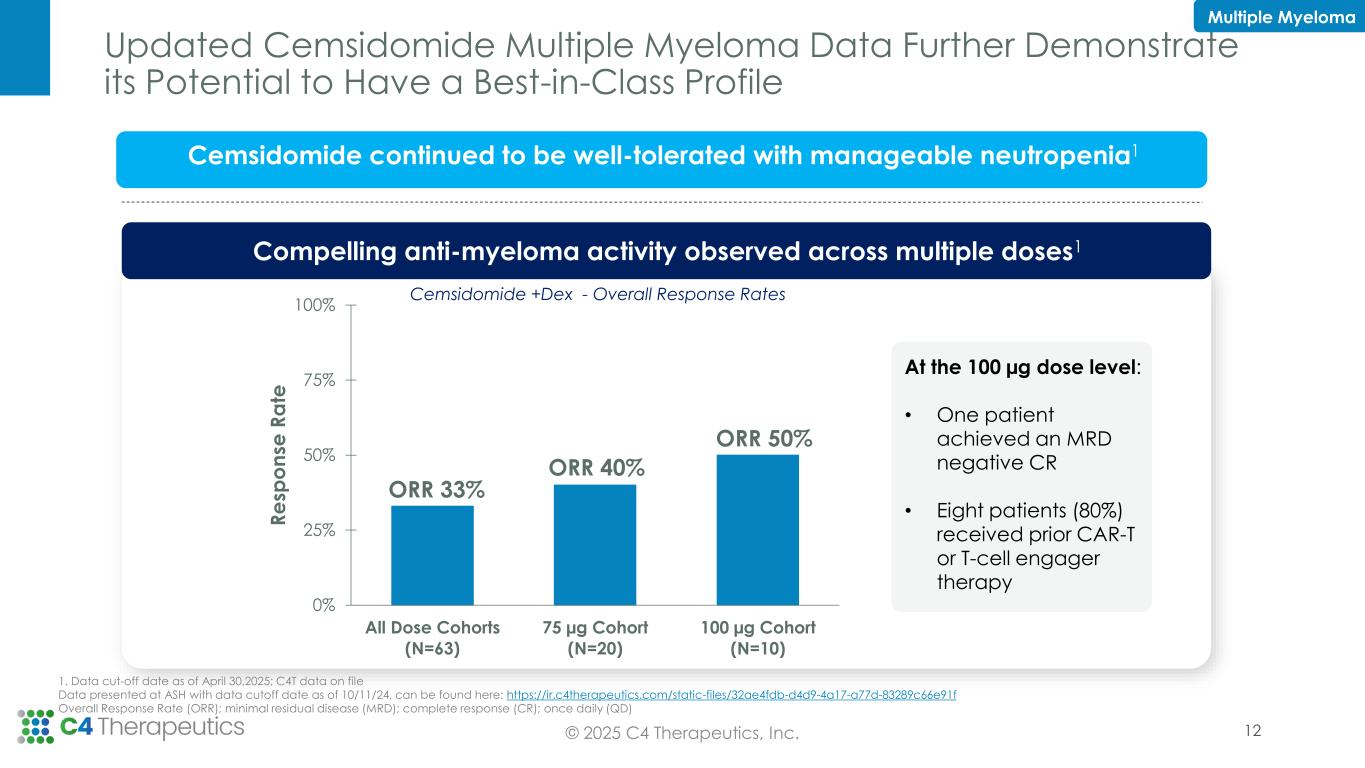
•Phase 1 dose escalation is complete in multiple myeloma (MM) with the 100 µg once daily (QD) dose level declared safe for expansion; 10 additional patients will be treated in a 100 µg QD expansion cohort to further characterize cemsidomide’s safety and efficacy profile at this dose level.
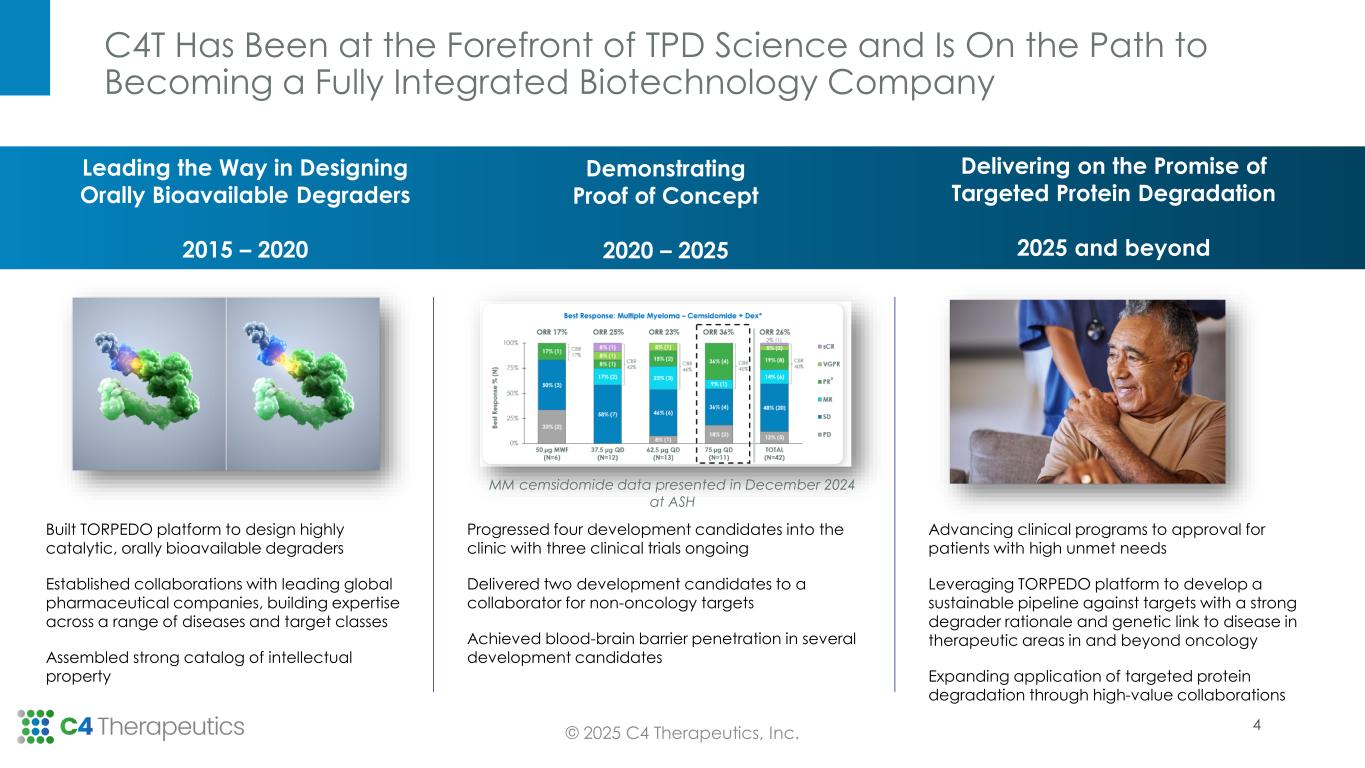
•Cemsidomide MM topline data demonstrate compelling responses rates at multiple doses:
◦As of the data cutoff date of April 30, 2025, 10 patients have been treated at the 100 µg QD dose level, achieving an overall response rate (ORR) of 50 percent. Notably, one patient who previously progressed on two prior T-cell engager therapies achieved a minimal residual disease (MRD) negative complete response (measured by flow cytometry).
Eight patients (80 percent) treated at this dose level received prior CAR-T or T-cell engager therapy.
◦Since October 11, 2024, six additional patients have been treated for a total of 20 patients treated at the 75 µg QD dose level. As of the data cutoff date of April 30, 2025, the 75 µg QD dose level achieved an ORR of 40 percent.
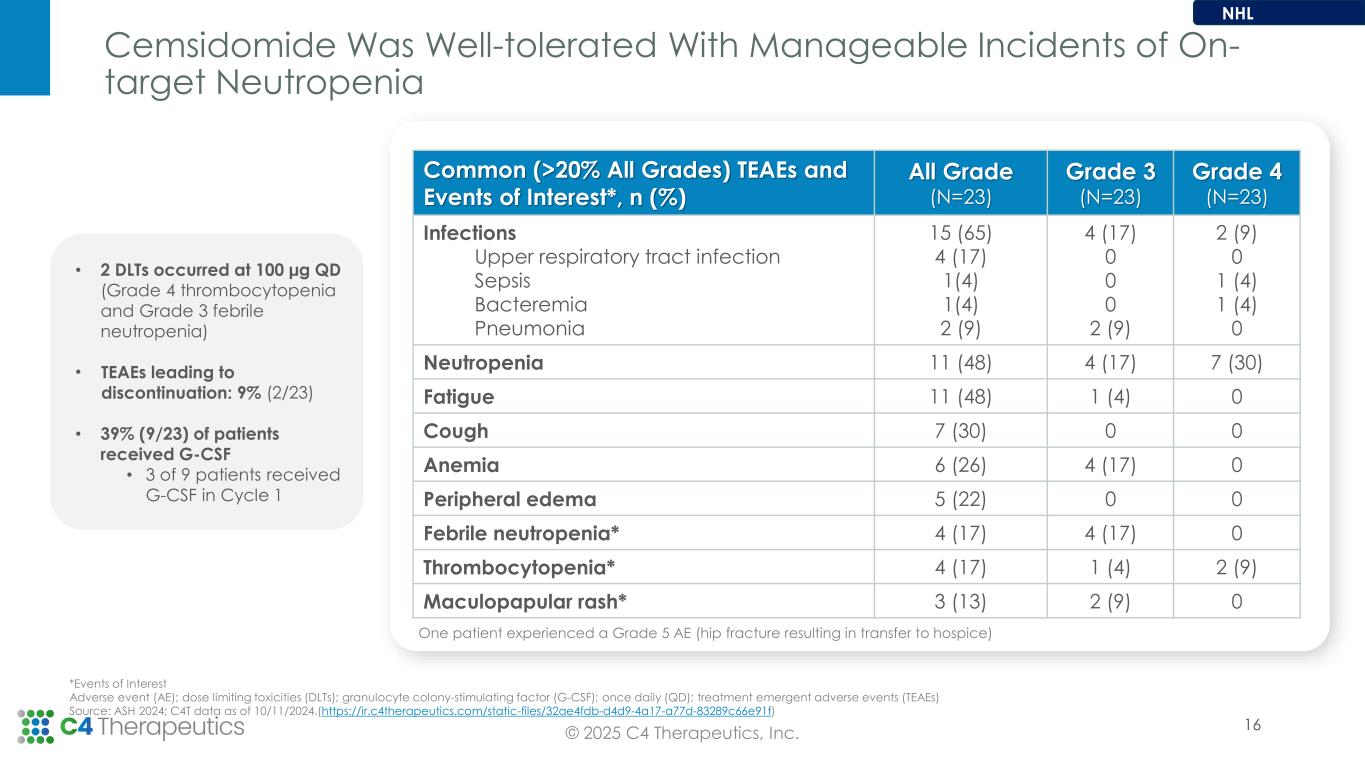
◦Cemsidomide remains well-tolerated with manageable neutropenia.
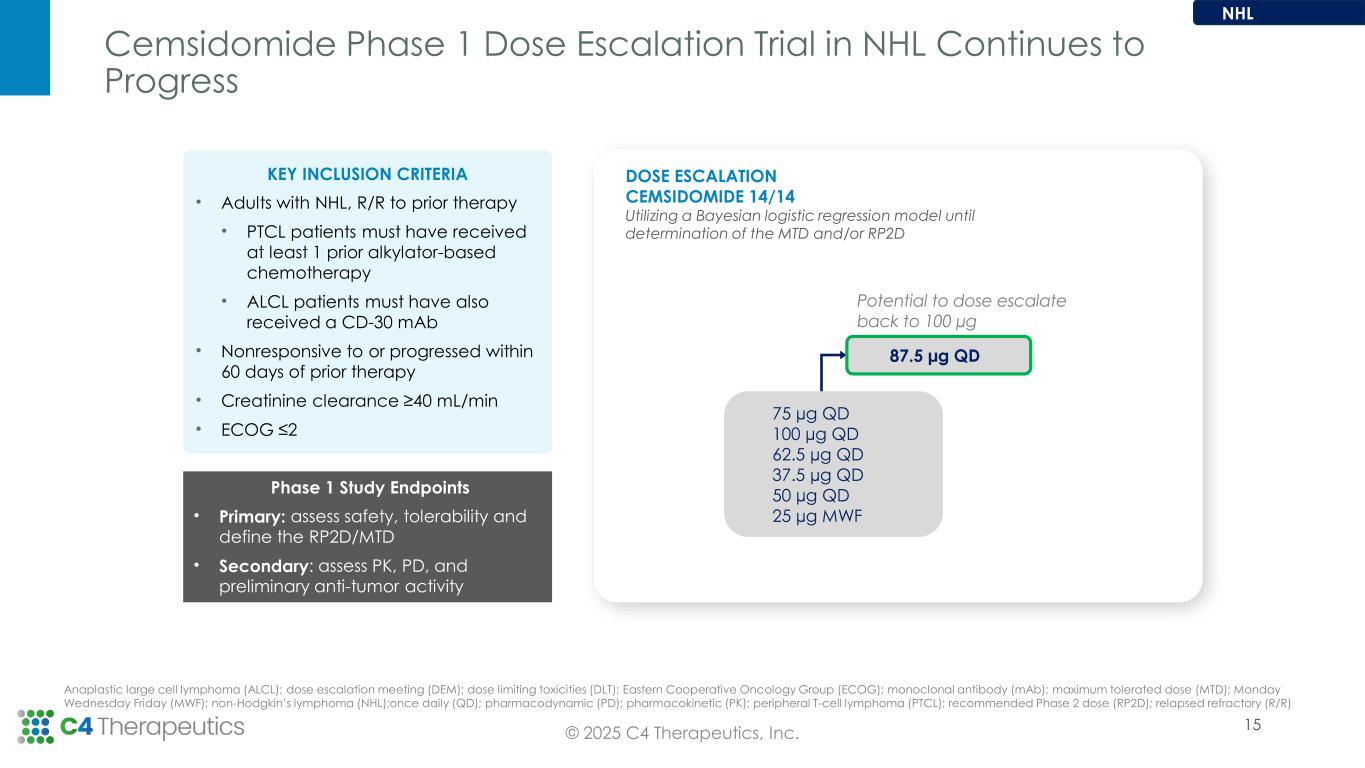
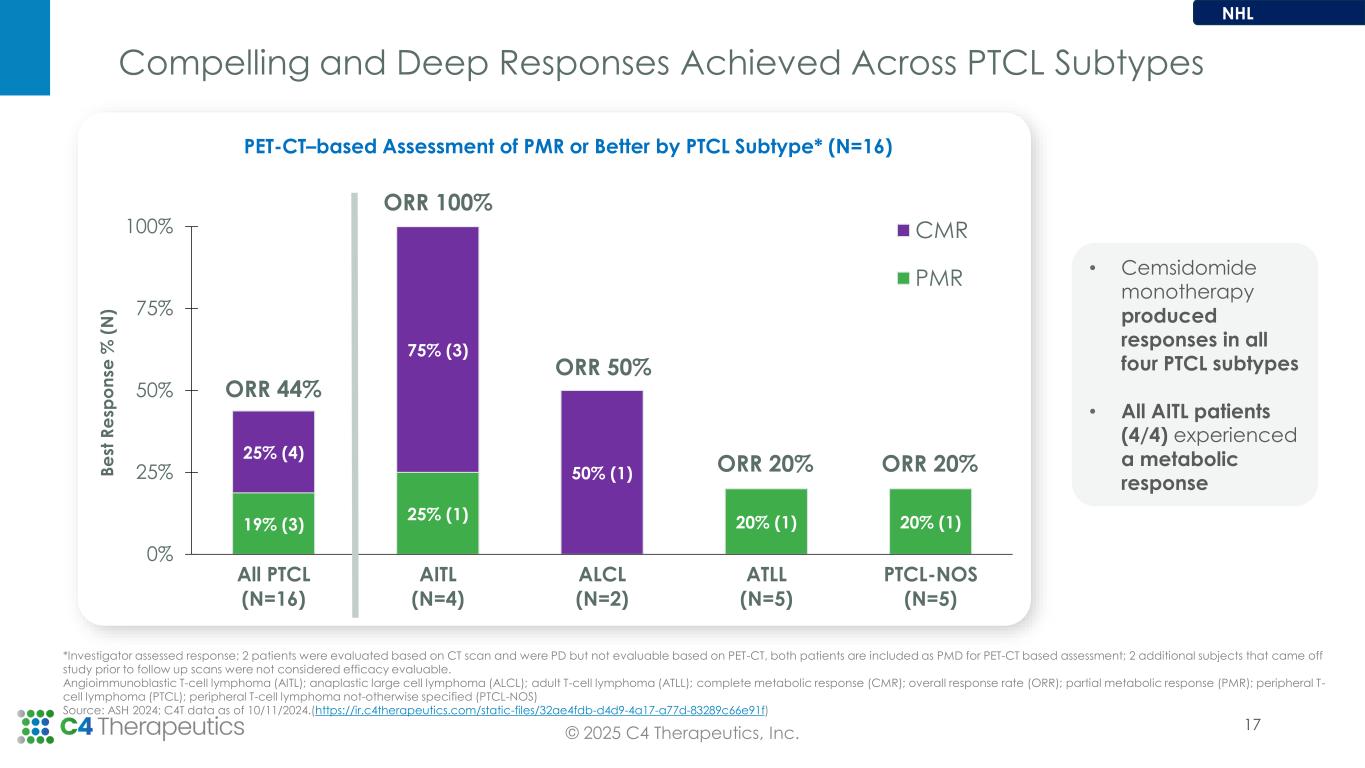
•For the non-Hodgkin’s lymphoma (NHL) arm, the Phase 1 dose escalation is ongoing at the 87.5 µg QD dose level and the maximum tolerated dose has not yet been reached.
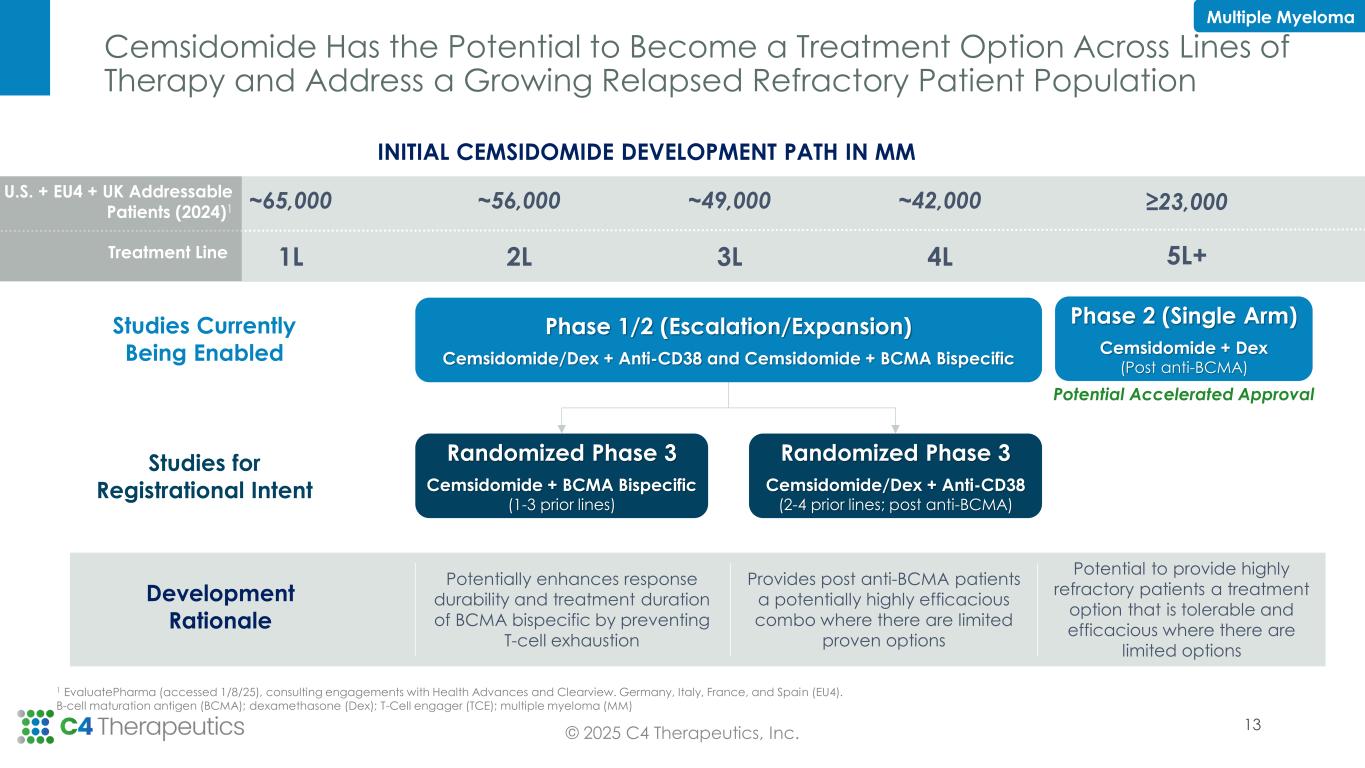
•C4T expects to receive regulatory feedback on registrational development by mid-year 2025.
CFT1946:
•Phase 1 dose escalation is complete with 640 mg BID declared as the maximum administered dose. Across the trial, which includes the dose escalation, melanoma and colorectal cancer cohorts, 89 patients were treated.
•The pharmacodynamic and safety data, including the data presented at the European Society for Medical Oncology (ESMO) Congress 2024, supports proof of mechanism and the therapeutic potential of degrading the BRAF V600 mutant protein.
•Given emerging clinical data and the company’s focus on strategic capital allocation, C4T will not advance CFT1946 beyond the ongoing Phase 1 trial. C4T has made the decision to seek partnership opportunities to advance the BRAF program given the high unmet need and strong degrader rationale for treating BRAF V600 mutant solid tumors.
•The CFT1946 Phase 1 data will be presented at a future medical meeting.
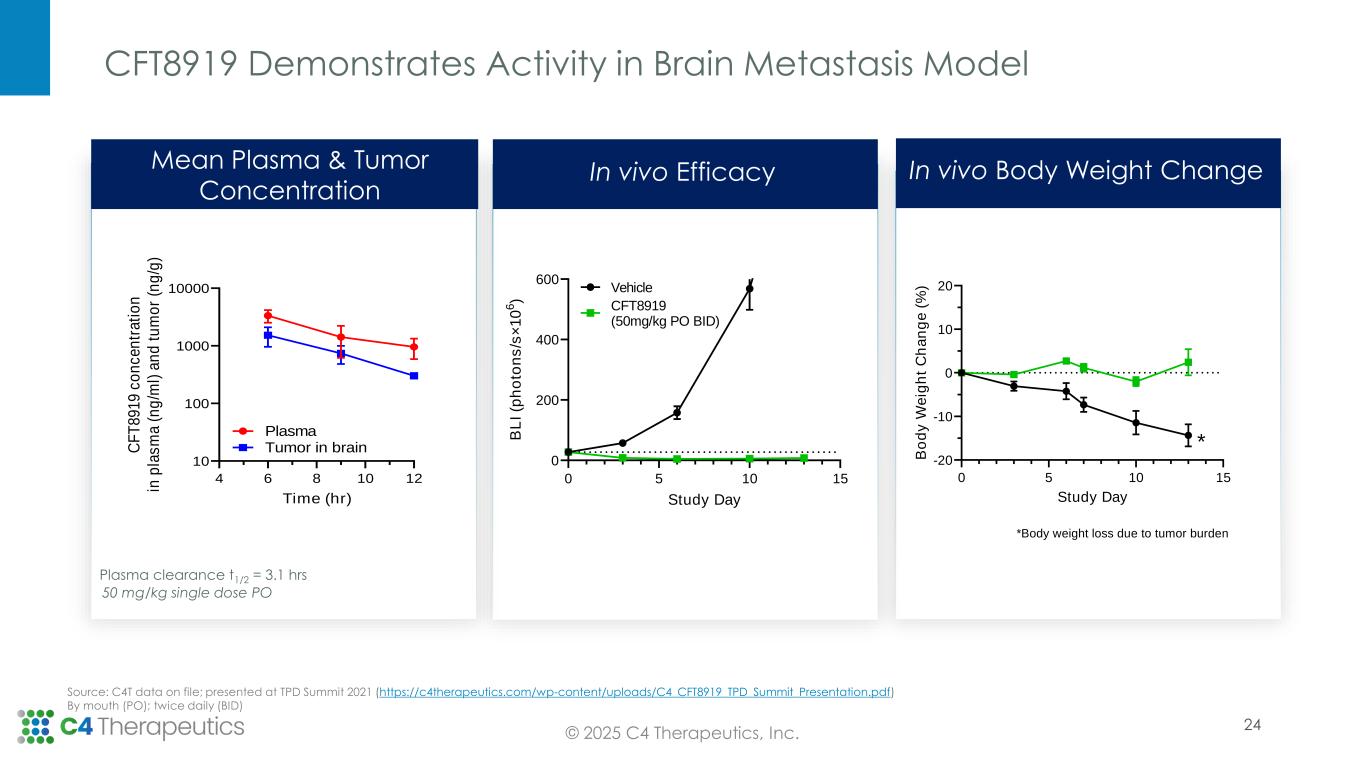
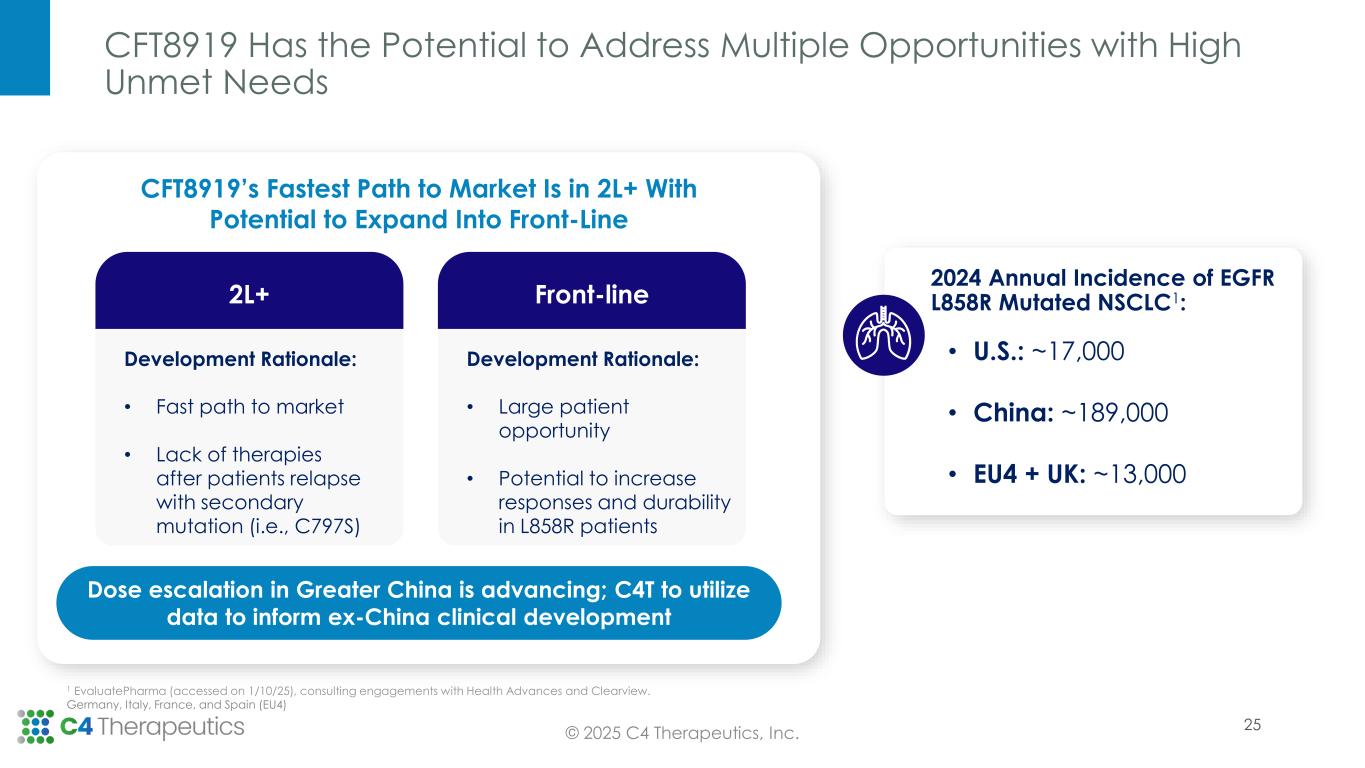
CFT8919:
•Partner Betta Pharmaceuticals continues to advance the CFT8919 Phase 1 dose escalation trial in Greater China.
Research and Discovery Collaborations:
•Advanced Roche collaboration to preclinical milestones. In March 2025, C4T earned a total of $4 million in payments upon achieving certain preclinical milestones for two programs. C4T and Roche continue to advance these programs.
•C4T continues to advance its internal research pipeline focused on targets in therapeutic areas in and beyond oncology with a strong degrader rationale and genetic link to disease.
KEY UPCOMING MILESTONES
•Present data from completed cemsidomide Phase 1 dose escalation in MM in Q3 2025.
•Complete cemsidomide Phase 1 dose escalation in NHL and present data in Q4 2025.
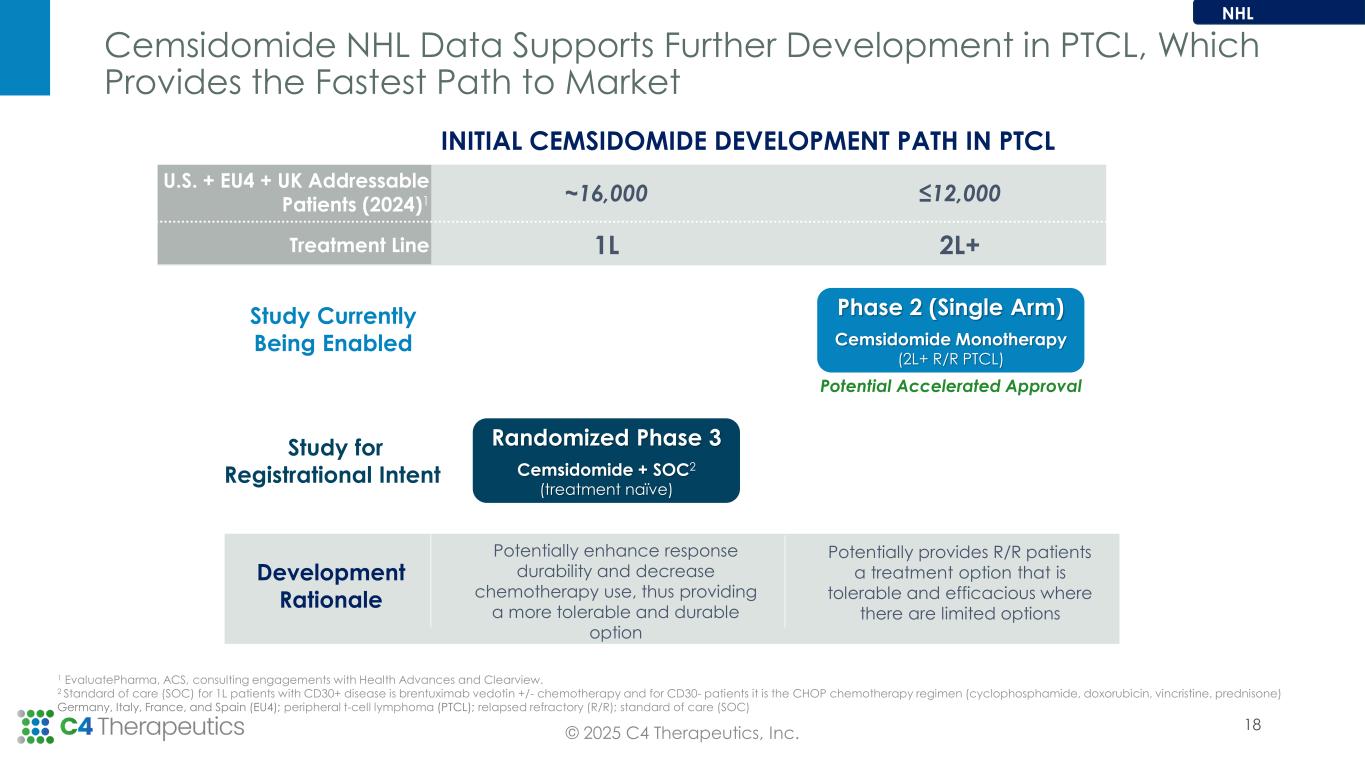
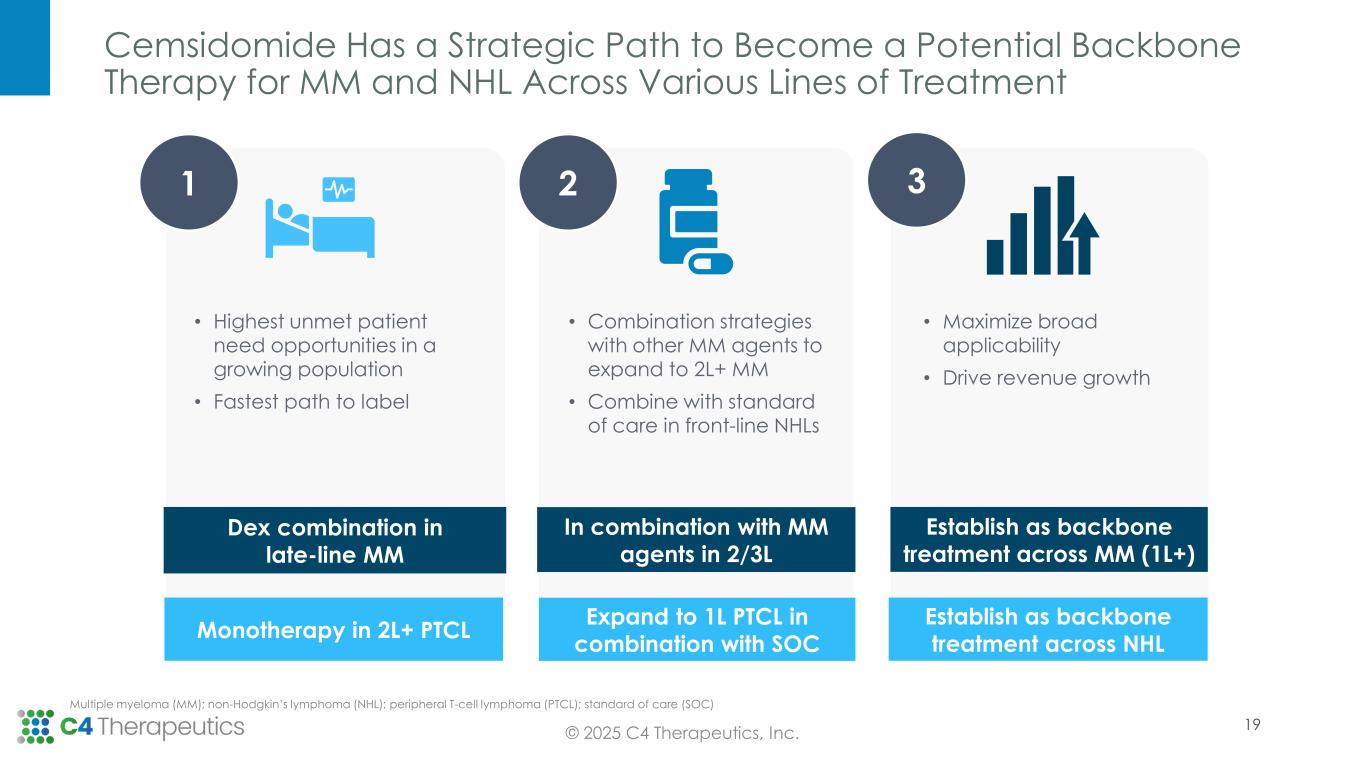
•Open expansion cohort(s) in PTCL as part of the current cemsidomide Phase 1/2 trial in the second half of 2025.
•Enable initiation of the next phase of clinical development for cemsidomide with new studies expected to initiate in early 2026.
UPCOMING INVESTOR EVENTS
•June 4, 2025: Management will participate in the Jefferies Global Healthcare Conference taking place from June 3 – June 5, 2025 in New York, New York.
FIRST QUARTER 2025 FINANCIAL RESULTS
Revenue: Total revenue for the first quarter of 2025 was $7.2 million, compared to $3.0 million for the first quarter of 2024. The increase in revenue was primarily due to our collaborations with Merck KGaA, Darmstadt, Germany (MKDG), which commenced in March 2024, as well as our achievement of two preclinical milestones under our Roche collaboration.
Research and Development (R&D) Expense: R&D expense for the first quarter of 2025 was $27.1 million compared to $22.5 million for the first quarter of 2024. The increase in R&D expense was primarily related to clinical trial expenses for cemsidomide and CFT1946, in addition to increased preclinical spend as our research collaborations continue to advance.
General and Administrative (G&A) Expense: G&A expense for the first quarter of 2025 was $9.3 million compared to $10.3 million for the first quarter of 2024. The decrease was primarily a result of reduced personnel costs related to our 2024 restructuring activities.
Net Loss and Net Loss per Share: Net loss for the first quarter of 2025 was $26.3 million, compared to $28.4 million for the first quarter of 2024. Net loss per share for the first quarter of 2025 was $0.37 compared to $0.41 for the first quarter of 2024.
Cash Position and Financial Guidance: Cash, cash equivalents and marketable securities as of March 31, 2025 were $234.7 million, compared to $267.3 million as of December 31, 2024. The decrease was primarily the result of cash used in operating activities. The balance as of March 31, 2025 is exclusive of the $4.0 million in milestones earned under our Roche collaboration, which the company expects to receive in the second quarter of 2025. The company expects that its cash, cash equivalents and marketable securities as of March 31, 2025 will enable the company to fund its operating plan into 2027.
About C4 Therapeutics
C4 Therapeutics (C4T) (Nasdaq: CCCC) is a clinical-stage biopharmaceutical company dedicated to delivering on the promise of targeted protein degradation science to create a new generation of medicines that transforms patients’ lives. C4T is progressing targeted oncology programs through clinical studies and leveraging its TORPEDO® platform to efficiently design and optimize small-molecule medicines to address difficult-to-treat diseases. C4T’s degrader medicines are designed to harness the body’s natural protein recycling system to rapidly degrade disease-causing proteins, offering the potential to overcome drug resistance, drug undruggable targets and improve patient outcomes. For more information, please visit www.c4therapeutics.com.
About Cemsidomide
Cemsidomide is an investigational, orally bioavailable small-molecule degrader designed to be a more potent and selective degrader of IKZF1/3, transcription factors that drive multiple myeloma (MM) and non-Hodgkin’s lymphomas (NHL), with unique pharmacokinetic properties. Clinical data has shown that cemsidomide is well-tolerated. In MM, cemsidomide displays evidence of anti-myeloma activity and immunomodulatory effects. In NHL, cemsidomide displays evidence of anti-lymphoma activity. More information may be accessed at www.clinicaltrials.gov (identifier: NCT04756726).
About CFT1946
C4T has advanced preclinical and clinical research to explore how targeted protein degradation may offer improvement over approved therapies that inhibit mutant BRAF V600. C4T advanced CFT1946, an investigational, orally bioavailable brain penetrant small molecule degrader of BRAF V600 mutations in solid tumors into a Phase 1/2 global clinical trial in patients refractory to BRAF inhibitors. CFT1946 is designed to be potent and selective against the BRAF V600 mutant form. In May 2025, C4T announced the decision to not advance its BRAF program beyond the current Phase 1 trial of CFT1946 and seek partnership opportunities to maximize its potential given the high unmet need and strong degrader rationale for treating BRAF V600 mutant solid tumors.
About CFT8919
CFT8919 is an orally bioavailable allosteric degrader that is designed to be potent and selective against EGFR bearing an oncogenic L858R mutation. In preclinical studies, CFT8919 is active in in vitro and in vivo models of L858R driven non-small cell lung cancer. Importantly, CFT8919 retains full activity against additional EGFR mutations that confer resistance against approved EGFR inhibitors including L858R-C797S, L858R-T790M and L858R-T790M-C797S. C4T and Betta Pharmaceuticals have established a strategic partnership to develop CFT8919 in Greater China, where the Phase 1 clinical trial is underway. C4T retains development and commercialization rights for CFT8919 in the United States, European Union and rest of the world.
Forward-Looking Statements
This press release contains “forward-looking statements” of C4 Therapeutics, Inc. within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements may include, but may not be limited to, express or implied statements regarding our ability to develop potential therapies for patients; the design and potential efficacy of our therapeutic approaches; the predictive capability of our TORPEDO® platform in the development of novel, selective, orally bioavailable BiDAC™ and MonoDAC® degraders; the potential timing, design and advancement of our preclinical studies and clinical trials, including the potential timing for and receipt of regulatory advice or authorization related to clinical trials and other clinical development activities including clinical trial commencement or cohort initiation; our ability and the potential to successfully manufacture and supply our product candidates for clinical trials; our ability to replicate results achieved in our preclinical studies or clinical trials in any future studies or trials; our ability to replicate interim or early-stage results from our clinical trials in the results obtained when those clinical trials are completed or when those therapies complete later-stage clinical trials; regulatory developments in the United States and foreign countries; the anticipated timing and content of presentations of data from our clinical trials; and our ability to fund our future operations. Any forward-looking statements in this press release are based on management’s current expectations and beliefs of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: uncertainties related to the initiation, timing, advancement and conduct of preclinical and clinical studies and other development requirements for our product candidates; the risk that any one or more of our product candidates will cost more to develop or may not be successfully developed and commercialized; and the risk that sufficient capital to fund our future operations will be available to us on acceptable terms or at the times required. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in C4 Therapeutics’ most recent Annual Report on Form 10-K and/or Quarterly Report on Form 10-Q, as filed with the Securities and Exchange Commission. All information in this press release is as of the date of the release, and C4 Therapeutics undertakes no duty to update this information unless required by law.
Contacts:
Investors:
Courtney Solberg
Associate Director, Investor Relations
CSolberg@c4therapeutics.com
Media:
Loraine Spreen
Senior Director, Corporate Communications & Patient Advocacy
LSpreen@c4therapeutics.com
Condensed Consolidated Balance Sheet Data
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2025 |
|
December 31, 2024 |
| Cash, cash equivalents and marketable securities |
$ |
234,706 |
|
|
$ |
267,263 |
|
| Total assets |
319,524 |
|
|
349,602 |
|
| Deferred revenue |
46,702 |
|
|
47,169 |
|
| Total stockholders' equity |
195,140 |
|
|
215,986 |
|
Condensed Consolidated Statements of Operations
(in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
|
|
2025 |
|
2024 |
|
|
|
|
|
|
| Revenue from collaboration agreements |
$ |
7,238 |
|
|
$ |
3,039 |
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
| Research and development |
27,072 |
|
|
22,533 |
|
|
|
|
|
|
|
| General and administrative |
9,330 |
|
|
10,288 |
|
|
|
|
|
|
|
| Restructuring |
— |
|
|
2,437 |
|
|
|
|
|
|
|
| Total operating expenses |
36,402 |
|
|
35,258 |
|
|
|
|
|
|
|
| Loss from operations |
(29,164) |
|
|
(32,219) |
|
|
|
|
|
|
|
| Other income, net |
|
|
|
|
|
|
|
|
|
| Interest and other income, net |
2,842 |
|
|
3,858 |
|
|
|
|
|
|
|
| Total other income, net |
2,842 |
|
|
3,858 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
$ |
(26,322) |
|
|
$ |
(28,361) |
|
|
|
|
|
|
|
| Net loss per share - basic and diluted |
$ |
(0.37) |
|
|
$ |
(0.41) |
|
|
|
|
|
|
|
| Weighted-average number of shares - basic and diluted |
70,833,044 |
|
|
68,432,168 |
|
|
|
|
|
|
|