UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (date of earliest event reported): August 10, 2023
TONIX PHARMACEUTICALS HOLDING CORP.
(Exact name of registrant as specified in its charter)
| Nevada | 001-36019 | 26-1434750 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
26 Main Street, Chatham, New Jersey 07928
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (862) 904-8182
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock | TNXP | The NASDAQ Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 | Results of Operations and Financial Condition |
On August 10, 2023, Tonix Pharmaceuticals Holding Corp. (the “Company”) announced its operating results for the quarter ended June 30, 2023. A copy of the press release that discusses these matters is filed as Exhibit 99.01 to, and incorporated by reference in, this report.
| Item 7.01 | Regulation FD Disclosure. |
The Company updated its investor presentation, which is used to conduct meetings with investors, stockholders and analysts and at investor conferences, and which the Company intends to place on its website, which may contain nonpublic information. A copy of the presentation is filed as Exhibit 99.02 hereto and incorporated herein by reference.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.01 and 99.02 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the United States Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the United States Securities Act of 1933 or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 9.01 | Financial Statements and Exhibits. |
| (d) |
Exhibit No. |
Description. | ||
|
104 |
Press release of the Company, dated August 10, 2023 Corporate Presentation by the Company for August 2023 Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirement of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| TONIX PHARMACEUTICALS HOLDING CORP. | |||
| Date: August 10, 2023 | By: | /s/ Bradley Saenger | |
| Bradley Saenger | |||
| Chief Financial Officer | |||
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.01
Tonix Pharmaceuticals Reports Second Quarter 2023 Financial Results and Operational Highlights
Topline Results from Four CNS Clinical Trials Expected in the Third and Fourth Quarters of 2023
Fibromyalgia Topline Results Expected in the Fourth Quarter of 2023 for Phase 3 Potentially NDA-Enabling Study of the Centrally-Acting Non-Opioid Analgesic TNX-102 SL
Completed Strategic Acquisition of Two Marketed Acute Migraine Products: Zembrace® SymTouch® (sumatriptan injection) and Tosymra® (sumatriptan nasal spray)
CHATHAM, N.J., August 10, 2023 (GLOBE NEWSWIRE) – Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix or the Company), a biopharmaceutical company, today announced financial results for the second quarter ended June 30, 2023, and provided an overview of recent operational highlights.
“We have recently completed enrollment in four clinical trials for central nervous system (CNS) indications, representing our continuing progress towards bringing forth new treatments for serious CNS conditions that affect large patient populations,” said Seth Lederman, M.D., Chief Executive Officer of Tonix. “We look forward to data readouts before year-end 2023 for fibromyalgia, fibromyalgia-type Long COVID, chronic migraine, and depression. The topline results from the Phase 3 fibromyalgia trial are of particular importance because we believe the RESILIENT trial, if successful, will be the final efficacy trial required for submitting a New Drug Application (NDA) for approval by the U.S. Food and Drug Administration (FDA).”
On June 30, 2023, Tonix completed the acquisition of two marketed products from Upsher-Smith Laboratories, LLC (Upsher-Smith): Zembrace® SymTouch® (sumatriptan injection) 3 mg and Tosymra® (sumatriptan nasal spray) 10 mg. Zembrace SymTouch and Tosymra are both non-oral proprietary products indicated for the treatment of acute migraine with or without aura in adults. Zembrace SymTouch is the only branded sumatriptan autoinjector professionally promoted in the United States and is designed for ease of use and favorable tolerability with a low 3 mg dose. Tosymra is a novel intranasal sumatriptan product formulated with a permeation enhancer that provides rapid and efficient absorption of sumatriptan. Collectively, these products generated gross factory sales of approximately $30 million for the twelve months ended March 31, 2023. Zembrace SymTouch and Tosymra each may provide onset of migraine pain relief in as few as 10 minutes for some patients and currently have patent protection to 2036 and 2031, respectively.
Seth Lederman continued, “The acquisition of these two marketed products is transformative for Tonix as we believe that we are on track to become a fully integrated pharmaceutical company and to expand our expertise in CNS disorders by interacting with health care providers and payers. The build out of our commercial capabilities is timely, given the potential 2025 launch of TNX-102 SL(cyclobenzaprine HCl sublingual tablets) for fibromyalgia, if results from the RESILIENT trial are positive. The two marketed acute migraine products also align strongly with our clinical product candidate TNX-1900 (intranasal potentiated oxytocin), in development as a preventive medication in chronic migraineurs.”
“We plan to submit an IND to study TNX-2900 (intranasal potentiated oxytocin) in Prader-Willi syndrome, for which Orphan Drug Designation has already been granted by the FDA,” said Gregory Sullivan, M.D., Chief Medical Officer of Tonix Pharmaceuticals. “Academic collaborators have initiated enrollment in three Phase 2 investigator-initiated trials studying TNX-1900 (intranasal potentiated oxytocin). The trials in adolescent obesity and binge eating disorder at Massachusetts General Hospital (MGH) have the potential to expand the use of TNX-1900 to the treatment of disorders of appetite, eating behaviors and weight. The trial in social anxiety at University of Washington has the potential to expand the use of TNX-1900 to the treatment of disorders of social functioning.”
Dr. Lederman continued, “During the second quarter of 2023, we also announced the prioritization of our clinical stage CNS programs and de-prioritization of our COVID-19 vaccine and related therapeutic programs, as well as certain other preclinical programs in order to reallocate cash and resources. We also streamlined several CNS studies by eliminating interim analyses and reducing enrollment targets to ensure data readouts this year. We incurred a one-time cash outlay related to our acquisition of Zembrace SymTouch and Tosymra in the second quarter.”
Recent Highlights—Marketed Products
On June 30, 2023, Tonix completed the acquisition of two currently marketed products from Upsher-Smith Laboratories, LLC (Upsher-Smith): Zembrace SymTouch (sumatriptan injection) 3 mg and Tosymra (sumatriptan nasal spray) 10 mg. Zembrace SymTouch and Tosymra are both indicated for the treatment of acute migraine with or without aura in adults.
Recent Highlights—Key Product Candidates*
Upcoming Data Readouts in Central Nervous System (CNS) Pipeline During 2023
Fibromyalgia (4Q): Phase 3 Potentially NDA-Enabling Study of TNX-102 SL
Fibromyalgia-Type Long COVID (3Q): Phase 2 Proof-of-Concept Study of TNX-102 SL
Chronic Migraine (4Q): Phase 2 Proof-of-Concept Study of TNX-1900
Depression (4Q): Phase 2 Proof-of-Concept Study of TNX-601 ER
Central Nervous System (CNS) Pipeline
TNX-102 SL (cyclobenzaprine HCl sublingual tablets): once-daily at bedtime small molecule for the management of fibromyalgia (FM) – centrally-acting, non-opioid analgesic.
| · | In August 2023, the Company announced that it completed enrollment of its potentially confirmatory Phase 3 RESILIENT trial of TNX-102 SL (cyclobenzaprine HCl sublingual tablets) 5.6 mg in fibromyalgia. A total of 457 participants were randomized in the trial, which, if successful, may serve as the final, well-controlled efficacy trial required for submission of an NDA for approval by the FDA. RESILIENT is a registration-quality, double-blind, placebo-controlled study. |
| · | Topline results from the RESILIENT trial are expected in the fourth quarter of 2023 |
TNX-102 SL for the treatment of Fibromyalgia-Type Long COVID, also known as Post-Acute Sequelae of COVID-19 (PASC)
| · | In August 2023, the Company announced it completed the clinical phase of the PREVAIL study, a Phase 2 proof-of-concept study of TNX-102 SL for fibromyalgia-type Long COVID, as the last patient completed their final study visit. PREVAIL is a registration-quality, double-blind, placebo-controlled study. |
| · | Topline results from the PREVAIL Phase 2 trial are expected in the third quarter of 2023. |
TNX-1900 (intranasal potentiated oxytocin): small peptide for migraine, craniofacial pain, social anxiety disorder, insulin resistance and related disorders, and adolescent obesity and binge eating disorder
| · | Tonix has developed a formulation of intranasal oxytocin that contains magnesium (Mg2+) which is believed to potentiate the effects of oxytocin and may address the “inverted U” dose response of oxytocin. The company believes that if Mg2+ reduces the high dose inhibition of oxytocin alone, then the new formulation may improve consistency in clinical trials. |
| · | Enrollment has been completed in the proof-of-concept Phase 2 PREVENTION study of TNX-1900 for the prevention of migraine headache in chronic migraineurs with a total of 88 patients enrolled. PREVENTION is a registration-quality, double-blind, placebo-controlled study. |
| · | Topline results from the PREVENTION Phase 3 trial are expected in the fourth quarter of 2023. |
| · | In July 2023, Tonix announced that the first participant was enrolled in the investigator-initiated Phase 2 STROBE Study of TNX-1900 for the treatment of binge-eating disorder at the Massachusetts General Hospital (MGH) under the direction of Principal Investigator Elizabeth Lawson. Tonix is supporting the STROBE study through a clinical trial agreement with MGH. |
| · | In July 2023, Tonix announced that the first participant was enrolled in a Phase 2 investigator-initiated, proof-of-concept study of TNX-1900 for enhancing social safety learning in social anxiety disorder (SAD) under the direction of Principal Investigator Angela Fang. Tonix entered into an agreement with the University of Washington to examine the potential role of TNX-1900 in enhancing vicarious extinction learning in SAD, compared to healthy controls. |
| · | In July 2023, Tonix announced that the first participant was enrolled in the Phase 2 POWER study of TNX-1900 for the treatment of pediatric obesity with MGH under the direction of Principal Investigator Elizabeth Lawson. MGH is the sponsor of the National Institutes of Health-funded trial, being conducted under an investigator-initiated IND. |
| · | In May 2023, Tonix entered into a research collaboration agreement with the principal investigator of the study, Dr. Antoinette Maassen van den Brink, Professor of Neurovascular Pharmacology, Erasmus University Medical Center, to evaluate the effect of TNX-1900 on capsaicin- or electrical stimulation-induced forehead dermal blood flow in health female volunteers. |
TNX-601 ER (tianeptine hemioxalate extended-release tablets): a once-daily orally administered small molecule for the treatment of major depressive disorder (MDD), posttraumatic stress disorder (PTSD), neurocognitive dysfunction associated with corticosteroid use, and potentially Alzheimer’s disease
| · | Enrollment has been completed in the proof-of-concept Phase 2 UPLIFT study for the treatment of MDD, with 116 patients enrolled. UPLIFT is a registration-quality, double-blind, placebo-controlled study. |
| · | Topline results for the UPLIFT Phase 2 trial are expected in the fourth quarter of 2023. |
| · | Scientists at Tonix’s Research and Development Center (RDC) in Frederick, Md. established the molecular mechanism of action of tianeptine, the active ingredient of TNX-601 ER. The research supports a direct role for restoring neuroplasticity and neurogenesis, and upsets previously held beliefs about the significance of neurotransmitters in treating depression. It also provides clarity on why tianeptine does not cause sexual dysfunction, weight gain or several other treatment-limiting toxicities associated with traditional antidepressants. |
TNX-4300 (estianeptine): small molecule oral therapeutic for MDD, bipolar disorder, Alzheimer’s disease and Parkinson’s disease
| · | In July 2023, the Company announced that it would focus resources on the development of single isomer TNX-4300 as a first-in-class oral therapy for mood disorders, Alzheimer’s disease and other psychiatric and neurodegenerative conditions with memory deficits. The decision follows the Company’s announcement in May 2023 of the isolation and characterization of the (S)-isomer of tianeptine, which activates PPAR-β/δ, restores neuroplasticity in neuronal tissue culture and is free of µ-opioid receptor activity. |
TNX-1300 (recombinant double mutant cocaine esterase): biologic for life-threatening cocaine intoxication
| · | Tonix expects to initiate a potentially pivotal Phase 2 clinical study of TNX-1300 for the treatment of cocaine intoxication in the third quarter of 2023. In 2022, Tonix was awarded a Cooperative Agreement grant from the National Institute on Drug Abuse (NIDA), part of the National Institutes of Health (NIH), to support development of TNX-1300. |
| · | TNX-1300 has been granted Breakthrough Therapy designation by the FDA. |
Rare Disease Pipeline
TNX-2900 (intranasal potentiated oxytocin): small peptide for the treatment of Prader-Willi syndrome (PWS)
| · | TNX-2900 has been granted Orphan Drug designation from the FDA for the treatment of PWS and the preparation of the IND is in process. |
| · | TNX-2900 is in development to treat PWS and the planned clinical study will study its effects on hyperphagia, or pathological over-eating, in children and young adult patients with PWS. |
Immunology Pipeline
TNX-1500 (anti-CD40L monoclonal antibody): third generation anti-CD40L monoclonal antibody for prophylaxis of organ transplant rejection and treatment of autoimmune disorders.
| · | In May 2023, the IND for prevention of organ rejection in patients receiving a kidney transplant was cleared by FDA. A first-in-human Phase 1 study is expected to start in the third quarter of 2023. The first indication for TNX-1500 will be prophylaxis of organ rejection in adult patients receiving a kidney transplant, but multiple additional indications are possible, including autoimmune diseases. Two peer reviewed publications described the work at the Massachusetts General Hospital on allogeneic transplants in animals.1,2 |
*All of Tonix’s product candidates are investigational new drugs or biologics and none have been approved for any indication.
1Lassiter, G., et al. (2023). TNX-1500, a crystallizable fragment–modified anti-CD154 antibody, prolongs non-human primate renal allograft survival. American Journal of Transplantation. https://doi.org/10.1016/j.ajt.2023.03.022
2Miura, S., et al. (2023). TNX-1500, a crystallizable fragment–modified anti-CD154 antibody, prolongs non-human primate cardiac allograft survival. American Journal of Transplantation. https://doi.org/10.1016/j.ajt.2023.03.025 As of June 30, 2023, Tonix had approximately $25.6 million of cash and cash equivalents, compared to $120.2 million as of December 31, 2022.
Recent Highlights—Financial
Subsequent to the end of the second quarter of 2023, Tonix raised $7.0 million in gross proceeds through a public offering of its common stock. Cash used in operations was approximately $56.3 million for the six months ended June 30, 2023, compared to $52.2 million for the same period in 2022. Cash used by investing activities for the six months ended June 30, 2023 was approximately $27.8 million, primarily driven by the purchase of certain assets from Upsher-Smith Laboratories, LLC.
The acquisition cost of Zembrace SymTouch and Tosymra was $15 million, consisting of $12 million up front and an additional deferred payment of $3.0 million. We also purchased inventory for $10.0 million and expect to pay an additional $1.3 million related to an inventory adjustment.
On April 8, 2020, the Company entered into a sales agreement with AGP of up to $320.0 million in at-the-market offerings sales. During the quarter ended June 30, 2023, the Company sold approximately 0.4 million shares of common stock under the sales agreement, for net proceeds of approximately $1.0 million.
On August 1, 2023, the Company sold in a public offering securities consisting of 2,530,000 shares of common stock, pre-funded warrants to purchase up to 4,470,000 shares of common stock, and accompanying common warrants to purchase 7,000,000 shares of common stock, at $1.00 per unit of common stock and common warrant, and $0.99 per pre-funded warrant and common warrant. The Company received net proceeds of approximately $6.2 million, after deducting underwriting discount and other offering expenses.
Second Quarter 2023 Financial Results
R&D expenses for the second quarter 2023 were approximately $22.0 million, compared to $16.6 million for the same period in 2022. As expected, R&D expenses have increased during 2023 due to progressing clinical development programs forward and investing in our preclinical development pipeline.
G&A expenses for the second quarter 2023 were $7.0 million, compared to $6.8 million for the same period in 2022.
Net loss available to common stockholders was $28.4 million, or $2.68 per share, basic and diluted, for the second quarter 2023, compared to net loss available to common stockholders of $27.4 million, or $7.64 per share, basic and diluted, for the same period in 2022. The basic and diluted weighted average common shares outstanding for the second quarter 2023 was 10,587,096 compared to 3,584,699 shares for the same period in 2022.
Tonix Pharmaceuticals Holding Corp.*
Tonix is a biopharmaceutical company focused on commercializing, developing, discovering and licensing therapeutics to treat and prevent human disease and alleviate suffering. Tonix markets Zembrace® SymTouch® (sumatriptan injection) 3 mg and Tosymra® (sumatriptan nasal spray) 10 mg. Zembrace SymTouch and Tosymra are each indicated for the treatment of acute migraine with or without aura in adults. Tonix’s development portfolio is composed of central nervous system (CNS), rare disease, immunology and infectious disease product candidates. Tonix’s CNS development portfolio includes both small molecules and biologics to treat pain, neurologic, psychiatric and addiction conditions. Tonix’s lead CNS candidate, TNX-102 SL (cyclobenzaprine HCl sublingual tablet), is in mid-Phase 3 development for the management of fibromyalgia, having completed enrollment in a potentially registration-enabling study, and with topline data expected in the fourth quarter of 2023. TNX-102 SL is also being developed to treat Long COVID, a chronic post-acute COVID-19 condition. Enrollment in a Phase 2 study has been completed, and topline results are expected in the third quarter of 2023. TNX-601 ER (tianeptine hemioxalate extended-release tablets), is a once-daily formulation being developed as a treatment for major depressive disorder (MDD). Enrollment is now complete in the UPLIFT trial of TNX-601 ER in MDD and topline results are expected in the fourth quarter of 2023. TNX-4300 (estianeptine) is a small molecule oral therapeutic in preclinical development to treat MDD, Alzheimer’s disease and Parkinson’s disease. TNX-1900 (intranasal potentiated oxytocin), is in development for chronic migraine, and the PREVENTION study has completed enrollment with topline data expected in the fourth quarter of 2023.
TNX-1300 (cocaine esterase) is a biologic designed to treat cocaine intoxication and has been granted Breakthrough Therapy designation by the FDA. A Phase 2 study of TNX-1300 is expected to be initiated in the third quarter of 2023. Tonix’s rare disease development portfolio includes TNX-2900 (intranasal potentiated oxytocin) for the treatment of Prader-Willi syndrome. TNX-2900 has been granted Orphan Drug designation by the FDA. Tonix’s immunology development portfolio includes biologics to address organ transplant rejection, autoimmunity and cancer, including TNX-1500, which is a humanized monoclonal antibody targeting CD40-ligand (CD40L or CD154) being developed for the prevention of allograft rejection and for the treatment of autoimmune diseases. A Phase 1 study of TNX-1500 is expected to be initiated in the third quarter of 2023. Tonix’s infectious disease pipeline includes TNX-801, a vaccine in development to prevent smallpox and mpox. TNX-801 also serves as the live virus vaccine platform or recombinant pox vaccine platform for other infectious diseases. The infectious disease development portfolio also includes TNX-3900 and TNX-4000, classes of broad-spectrum small molecule antivirals.
*Tonix’s product development candidates are investigational new drugs or biologics and have not been approved for any indication.
Tonix Medicines has contracted to acquire the Zembrace SymTouch and Tosymra registered trademarks. Intravail is a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis, Inc.
This press release and further information about Tonix can be found at www.tonixpharma.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others. These forward-looking statements are based on Tonix's current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, risks related to the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Tonix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in the Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports filed with the SEC on or after the date thereof. All of Tonix's forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date thereof.
TONIX PHARMACEUTICALS HOLDING CORP.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(In Thousands, Except Share and Per Share Amounts)
(unaudited)
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| COSTS AND EXPENSES: | ||||||||||||||||
| Research and development | $ | 21,976 | $ | 16,579 | $ | 48,487 | $ | 35,001 | ||||||||
| General and administrative | 7,026 | 6,757 | 14,417 | 14,771 | ||||||||||||
| 29,002 | 23,336 | 62,904 | 49,772 | |||||||||||||
| Operating loss | (29,002 | ) | (23,336 | ) | (62,904 | ) | (49,772 | ) | ||||||||
| Interest and other income, net | 646 | 196 | 1,543 | 215 | ||||||||||||
| Net loss | (28,356 | ) | (23,140 | ) | (61,361 | ) | (49,557 | ) | ||||||||
| Preferred stock deemed dividend | — | 4,255 | — | 4,255 | ||||||||||||
| Net loss available to common stockholders | $ | (28,356 | ) | $ | (27,395 | ) | $ | (61,361 | ) | $ | (53,812 | ) | ||||
| Net loss per common share, basic and diluted | $ | (2.68 | ) | $ | (7.64 | ) | $ | (5.88 | ) | $ | (17.28 | ) | ||||
| Weighted average common shares outstanding, basic and diluted | 10,587,096 | 3,584,699 | 10,428,678 | 3,113,965 | ||||||||||||
See the accompanying notes to the condensed consolidated financial statements
TONIX PHARMACEUTICALS HOLDING CORP.
CONDENSED CONSOLIDATED BALANCE SHEETS
(In Thousands)
(Unaudited)
| June 30, 2023 | December 31, 20221 | |||||||
| Assets | ||||||||
| Cash and cash equivalents | $ | 25,617 | $ | 120,229 | ||||
| Inventory | 13,700 | — | ||||||
| Prepaid expenses and other | 12,012 | 10,548 | ||||||
| Total current assets | 51,329 | 130,777 | ||||||
| Other non-current assets | 108,407 | 94,913 | ||||||
| Total assets | $ | 159,736 | $ | 225,690 | ||||
| Liabilities and stockholders' equity | ||||||||
| Total liabilities | $ | 19,273 | $ | 18,508 | ||||
| Stockholders' equity | 140,463 | 207,182 | ||||||
| Total liabilities and stockholders' equity | $ | 159,736 | $ | 225,690 | ||||
1The condensed consolidated balance sheet for the year ended December 31, 2022 has been derived from the audited financial statements but do not include all of the information and footnotes required by accounting principles generally accepted in the United States for complete financial statements.
Investor Contact
Jessica Morris
Tonix Pharmaceuticals
investor.relations@tonixpharma.com
(862) 904-8182
Peter Vozzo
ICR Westwicke
peter.vozzo@westwicke.com
(443) 213-0505
Media Contact
Ben Shannon
ICR Westwicke
ben.shannon@westwicke.com
(919) 360-3039
Zembrace® SymTouch® (sumatriptan Injection): IMPORTANT SAFETY INFORMATION
Zembrace SymTouch (Zembrace) can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop use and get emergency help if you have any signs of a heart attack:
| · | discomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back |
| · | severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw |
| · | pain or discomfort in your arms, back, neck, jaw or stomach |
| · | shortness of breath with or without chest discomfort |
| · | breaking out in a cold sweat |
| · | nausea or vomiting |
| · | feeling lightheaded |
Zembrace is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam shows no problem.
Do not use Zembrace if you have:
| · | history of heart problems |
| · | narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) |
| · | uncontrolled high blood pressure |
| · | hemiplegic or basilar migraines. If you are not sure if you have these, ask your provider. |
| · | had a stroke, transient ischemic attacks (TIAs), or problems with blood circulation |
| · | severe liver problems |
| · | taken any of the following medicines in the last 24 hours: almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, ergotamines, dihydroergotamine. |
| · | are taking certain antidepressants, known as monoamine oxidase (MAO)-A inhibitors or it has been 2 weeks or less since you stopped taking a MAO-A inhibitor. Ask your provider for a list of these medicines if you are not sure. |
| · | an allergy to sumatriptan or any of the components of Zembrace |
Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements.
Zembrace can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.
Zembrace may cause serious side effects including:
| · | changes in color or sensation in your fingers and toes |
| · | sudden or severe stomach pain, stomach pain after meals, weight loss, nausea or vomiting, constipation or diarrhea, bloody diarrhea, fever |
| · | cramping and pain in your legs or hips; feeling of heaviness or tightness in your leg muscles; burning or aching pain in your feet or toes while resting; numbness, tingling, or weakness in your legs; cold feeling or color changes in one or both legs or feet |
| · | increased blood pressure including a sudden severe increase even if you have no history of high blood pressure |
| · | medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches get worse, call your provider. |
| · | serotonin syndrome, a rare but serious problem that can happen in people using Zembrace, especially when used with anti-depressant medicines called SSRIs or SNRIs. Call your provider right away if you have: mental changes such as seeing things that are not there (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or trouble walking. |
| · | hives (itchy bumps); swelling of your tongue, mouth, or throat |
| · | seizures even in people who have never had seizures before |
The most common side effects of Zembrace include: pain and redness at injection site; tingling or numbness in your fingers or toes; dizziness; warm, hot, burning feeling to your face (flushing); discomfort or stiffness in your neck; feeling weak, drowsy, or tired.
Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of Zembrace. For more information, ask your provider.
This is the most important information to know about Zembrace but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use. You can also visit www.upsher-smith.com or call 1-888-650-3789.
You are encouraged to report adverse effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
INDICATION AND USAGE
Zembrace is a prescription medicine used to treat acute migraine headaches with or without aura in adults who have been diagnosed with
migraine.
Zembrace is not used to prevent migraines. It is not known if it is safe and effective in children under 18 years of age.
Tosymra® (sumatriptan nasal spray): IMPORTANT SAFETY INFORMATION
Tosymra can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop Tosymra and get emergency medical help if you have any signs of heart attack:
| · | discomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back |
| · | severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw |
| · | pain or discomfort in your arms, back, neck, jaw, or stomach |
| · | shortness of breath with or without chest discomfort |
| · | breaking out in a cold sweat |
| · | nausea or vomiting |
| · | feeling lightheaded |
Tosymra is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam is done and shows no problem.
Do not use Tosymra if you have:
| · | history of heart problems |
| · | narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) |
| · | uncontrolled high blood pressure |
| · | severe liver problems |
| · | hemiplegic or basilar migraines. If you are not sure if you have these, ask your healthcare provider. |
| · | had a stroke, transient ischemic attacks (TIAs), or problems with blood circulation |
| · | taken any of the following medicines in the last 24 hours: almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, ergotamines, or dihydroergotamine. Ask your provider if you are not sure if your medicine is listed above. |
| · | are taking certain antidepressants, known as monoamine oxidase (MAO)-A inhibitors or it has been 2 weeks or less since you stopped taking a MAO-A inhibitor. Ask your provider for a list of these medicines if you are not sure. |
| · | an allergy to sumatriptan or any ingredient in Tosymra |
Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements.
Tosymra can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.
Tosymra may cause serious side effects including:
| · | changes in color or sensation in your fingers and toes |
| · | sudden or severe stomach pain, stomach pain after meals, weight loss, nausea or vomiting, constipation or diarrhea, bloody diarrhea, fever |
| · | cramping and pain in your legs or hips, feeling of heaviness or tightness in your leg muscles, burning or aching pain in your feet or toes while resting, numbness, tingling, or weakness in your legs, cold feeling or color changes in one or both legs or feet |
| · | increased blood pressure including a sudden severe increase even if you have no history of high blood pressure |
| · | medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches get worse, call your provider. |
| · | serotonin syndrome, a rare but serious problem that can happen in people using Tosymra, especially when used with anti-depressant medicines called SSRIs or SNRIs. Call your provider right away if you have: mental changes such as seeing things that are not there (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or trouble walking. |
| · | hives (itchy bumps); swelling of your tongue, mouth, or throat |
| · | seizures even in people who have never had seizures before |
The most common side effects of Tosymra include: tingling, dizziness, feeling warm or hot, burning feeling, feeling of heaviness, feeling of pressure, flushing, feeling of tightness, numbness, application site (nasal) reactions, abnormal taste, and throat irritation.
Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of Tosymra. For more information, ask your provider.
This is the most important information to know about Tosymra but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use. You can also visit www.upsher-smith.com or call 1-888-650-3789.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
INDICATION AND USAGE
Tosymra is a prescription medicine used to treat acute migraine headaches with or without aura in adults.
Tosymra is not used to treat other types of headaches such as hemiplegic or basilar migraines or cluster headaches.
Tosymra is not used to prevent migraines. It is not known if Tosymra is safe and effective in children under 18 years of age.
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.02

© 2023 Tonix Pharmaceuticals Holding Corp. INVESTOR PRESENTATION NASDAQ: TNXP Version P04 75 August 10, 2023 (Doc 1300 )

2 © 2023 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 © 2023 Tonix Pharmaceuticals Holding Corp. Investment Highlights RICH PIPELINE OF THERAPEUTICS CANDIDATES IN DEVELOPMENT Tonix’s core focus is on central nervous system disorders, but we also target unmet needs across multiple therapeutic areas including immunology, infectious disease and rare disease. STRATEGIC PARTNERSHIPS Partnering strategically with other biotech companies , world - class academic and non - profit research organizations to bring innovative therapeutics to market faster. IN - HOUSE CAPABILITIES I nternal capabilities in R&D and biologics process development and GMP manufacturing to accelerate development timelines. MARKETED PRODUCTS Tonix Medicines markets two FDA - approved products Zembrace ® SymTouch ® (sumatriptan injection) and Tosymra ® (sumatriptan nasal spray) for the treatment of acute migraine in adults with or without aura 1 Zembrace SymTouch [package insert] . Maple Grove, MN : Upsher - Smith Laboratories, LLC : February 2021 - For more information, talk to your provider and read the Patient Information and Instructions for Use . – Important Safety Information is provided in the appendix 2 Tosymra [package insert]. Maple Grove, MN: Upsher - Smith Laboratories, LLC: Feb 2021 . For more information, talk to your provider and read the Patient Information and Instructions for Use . – Important Safety Information is provided in the appendix Tonix has contracted to acquire the Zembrace , SymTouch and Tosymra trademarks. Intravail is a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis , Inc.

4 © 2023 Tonix Pharmaceuticals Holding Corp. Pipeline: Key Clinical Development Programs Candidates* Indication Status/Next Milestone TNX - 102 SL 1 Fibromyalgia (FM) Long COVID (PASC 2 ) Mid - Phase 3 – enrollment complete Phase 2 – enrollment complete TNX - 1300 3 Cocaine Intoxication - FDA Breakthrough Designation Mid - Phase 2, Targeted 3Q 2023 Start TNX - 1900 4 Prevention of Chronic Migraine Phase 2 – enrollment complete 5 TNX - 601 ER Depression Phase 2 – enrollment complete 6 TNX - 2900 7 Prader - Willi Syndrome - FDA Orphan Drug Designation Phase 2 ready TNX - 1500 8 Organ Transplant Rejection/ Autoimmune Conditions Phase 1, Targeted 3Q 2023 Start *All of Tonix’s product candidates are investigational new drugs or biologics and none has been approved for any indication. 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) also has active INDs for Agitation in Alzheimer’s Disease (AAD), Alcohol Use Disorder (AUD), and Posttraumatic Stress Disorder (PTSD). All indications are Phase 2 ready. 2 Post - Acute Sequelae of COVID - 19. 3 TNX - 1300 (double - mutant cocaine esterase) is licensed from Columbia University . 4 Acquired from Trigemina ; license agreement with Stanford University; Planned investigator - initiated Binge Eating Disorder (BED) study is expected start 2Q 2023. 5 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 6 Phase 1 trial for formulation development was completed outside of the U.S.; Other potential indications include PTSD and neu roc ognitive d ysfunction from steroids 7 Co - exclusive license agreement with French National Institute of Health and Medical Research ( Inserm ) 8 anti - CD40L humanized monoclonal antibody – IND cleared 5 © 2023 Tonix Pharmaceuticals Holding Corp.
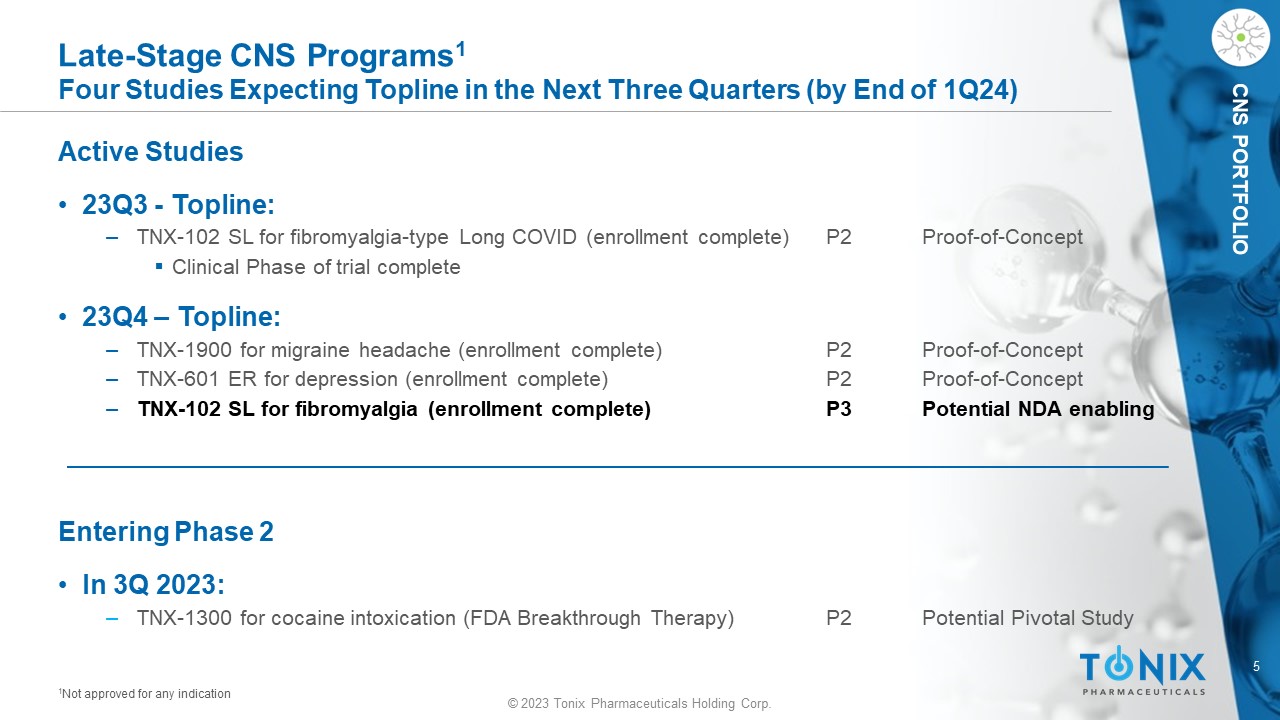
CNS PORTFOLIO Late - Stage CNS Programs 1 Four Studies Expecting Topline in the Next Three Quarters (by End of 1Q24) Active Studies • 23Q3 - Topline: ‒ TNX - 102 SL for fibromyalgia - type Long COVID (enrollment complete) P2 Proof - of - Concept ▪ Clinical Phase of trial complete • 23Q4 – Topline: ‒ TNX - 1900 for migraine headache (enrollment complete) P2 Proof - of - Concept ‒ TNX - 601 ER for depression (enrollment complete) P2 Proof - of - Concept ‒ TNX - 102 SL for fibromyalgia (enrollment complete) P3 Potential NDA enabling Entering Phase 2 • In 3Q 2023: ‒ TNX - 1300 for cocaine intoxication (FDA Breakthrough Therapy) P2 Potential Pivotal Study 1 Not approved for any indication © 2023 Tonix Pharmaceuticals Holding Corp.

TONIX MEDICINES: MARKETED PRODUCTS

7 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Two Marketed Proprietary Migraine Drugs Autoinjector and Nasal Spray (Non - oral) Formulations of Sumatriptan Zembrace ® SymTouch ® (sumatriptan injection) 3 mg 1 Tosymra ® (sumatriptan nasal spray) 10 mg 2 ‒ Each indicated for the treatment of acute migraine with or without aura in adults ‒ Sumatriptan remains the acute migraine ‘gold standard’ treatment for many patients and continues to represent the largest seg men t of the market in terms of unit sales 3 ‒ Each may provide migraine pain relief in as few as 10 minutes for some patients 1,2,4,5 ‒ Each bypasses GI tract – provides c onvenient administration ‒ Patents to 2036 ( Zembrace ) and 2031 ( Tosymra ) Upsher - Smith Laboratories Providing Certain Commercial Operations ‒ Product acquisition closed on June 30, 2023 ‒ To support the transition of the products, Upsher - Smith is providing certain commercial operations, regulatory and other transition services to Tonix Medicines for up to nine months after closing, in exchange for agreed upon service fees. 1 Zembrace SymTouch [package insert] . Maple Grove, MN : Upsher - Smith Laboratories, LLC : February 2021 - For more information, talk to your provider and read the Patient Information and Instructions for Use . – Important Safety Information is provided in the appendix 2 Tosymra [package insert]. Maple Grove, MN: Upsher - Smith Laboratories, LLC: Feb 2021 . For more information, talk to your provider and read the Patient Information and Instructions for Use. – Important Safety Information is provided in the appendix 3 Upsher - Smith Laboratories, LLC; Data On File, 2023 4 Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatri pta n Research Group. Arch Neurol. 1992;49(12):1271 - 1276. 5 Wendt J, et al. A randomized, double - blind, placebo - controlled trial of the efficacy and tolerability of a 4 - mg dose of subcutan eous sumatriptan for the treatment of acute migraine attacks in adults. Clinical Therapeutics. 2006;28(4):517 - 526. Tonix has contracted to acquire the Zembrace , SymTouch and Tosymra trademarks. Intravail is a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis , Inc.

8 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tonix Medicines is our Commercial Subsidiary Marketed products for the treatment of acute migraine in adults with or without aura Zembrace ® SymTouch ® (sumatriptan injection) 3 mg 1 Tosymra ® (sumatriptan nasal spray) 10 mg 2 ‒ Both are proprietary non - oral formulations of sumatriptan that bypass the gastrointestinal tract Headed by President Jim Hunter ‒ Industry veteran – experience in CNS products ‒ Built Validus Pharmaceuticals Consolidated Product Sales for the 12 months ended March 31 st 2023 ‒ Factory sales: $30.4M 3 ‒ Net sales: $16.4M 3 Retail Product Sales for the 12 months ended December 31 st 2022 ‒ Retail sales: ~$23 M ( Zembrace ~$19.6 M and Tosymra ~$3.5 M) 4 Acquired from Upsher - Smith Laboratories which has managed care contracts covering ~200 M lives ‒ Contract includes a transition period during which Tonix expects to secure its own contracts 1 Zembrace SymTouch [package insert] . Maple Grove, MN : Upsher - Smith Laboratories, LLC : February 2021 - For more information, talk to your provider and read the Patient Information and Instructions for Use . – Important Safety Information is provided in the appendix 2 Tosymra [package insert]. Maple Grove, MN: Upsher - Smith Laboratories, LLC: Feb 2021 . For more information, talk to your provider and read the Patient Information and Instructions for Use . – Important Safety Information is provided in the appendix 3 Audited abbreviated financial statements of assets acquired from Upsher - Smith Laboratories, LLC as filed in the 8 - K/A dated July 18, 2023 https://ir.tonixpharma.com/sec - filings/all - sec - filings/content/0001387131 - 23 - 008497/0001387131 - 23 - 008497.pdf 4 IQVIA, 2022 sales from the National Sales Perspectives (NSP) audit within the SMART database estimates Zembrace sales of ~$19.6 M and Tosymra sales of ~$3.5 M 9 © 2023 Tonix Pharmaceuticals Holding Corp.
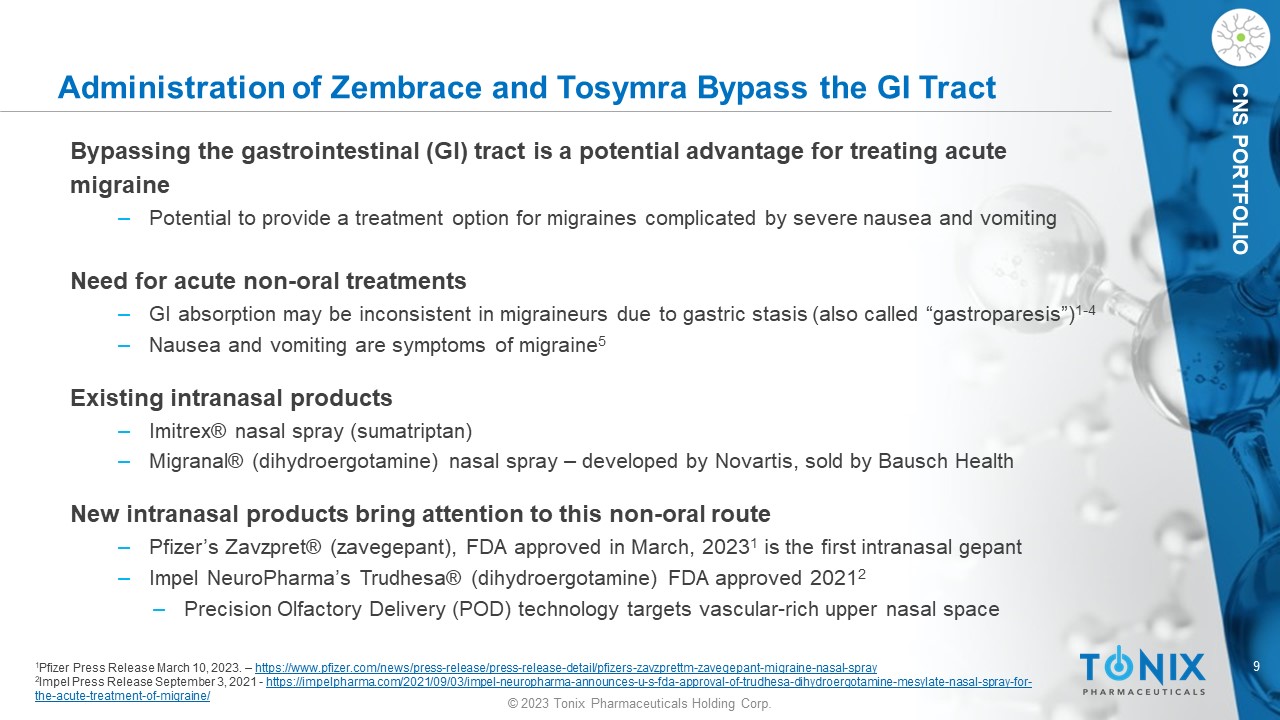
CNS PORTFOLIO Administration of Zembrace and Tosymra Bypass the GI Tract Bypassing the gastrointestinal (GI) tract is a potential advantage for treating acute migraine ‒ P otential to provide a treatment option for migraines complicated by severe nausea and vomiting Need for acute non - oral treatments ‒ GI absorption may be inconsistent in migraineurs due to gastric stasis (also called “gastroparesis”) 1 - 4 ‒ Nausea and vomiting are symptoms of migraine 5 Existing intranasal products ‒ Imitrex® nasal spray (sumatriptan) ‒ Migranal ® (dihydroergotamine) nasal spray – developed by Novartis, sold by Bausch Health New intranasal products bring attention to this non - oral route ‒ Pfizer’s Zavzpret ® ( zavegepant ), FDA approved in March, 2023 1 is the first intranasal gepant ‒ Impel NeuroPharma’s Trudhesa ® (dihydroergotamine) FDA approved 2021 2 ‒ Precision Olfactory Delivery (POD) technology targets vascular - rich upper nasal space 1 Pfizer Press Release March 10, 2023. – https://www.pfizer.com/news/press - release/press - release - detail/pfizers - zavzprettm - zavegepant - migraine - nasal - spray 2 Impel Press Release September 3, 2021 - https://impelpharma.com/2021/09/03/impel - neuropharma - announces - u - s - fda - approval - of - trudhesa - dihydroergotamine - mesylate - nasal - spr ay - for - the - acute - treatment - of - migraine/ 10 © 2023 Tonix Pharmaceuticals Holding Corp.

CNS PORTFOLIO Potential for Zembrace and Tosymra in Evolving Migraine Market Documented efficacy of Zembrace 1,2 and Tosymra 3 - 5 as abortive treatments for acute migraine ‒ M igraine pain relief possible in as few as 10 minutes for some patients and convenient administration ‒ Potential to address the unmet needs of patients using traditional or emerging oral acute migraine medications, particularly for rapid - onset treatment Zembrace and Tosymra have potential as first - line or rescue medications ‒ Depending on patient need, prescribers may utilize to treat acute migraine either first - line or in the rescue position in a comprehensive migraine toolbox ‒ Fast onset of action, high pain - relief rates ‒ For certain patients who present to ERs, Zembrace and Tosymra also provide ER staff a straightforward treatment option 1 Zembrace SymTouch [package insert] . Maple Grove, MN : Upsher - Smith Laboratories, LLC : 2019 . 2 Landy, S. et al. Efficacy and safety of DFN - 11 (sumatriptan injection, 3 mg) in adults with episodic migraine: a multicenter, ra ndomized, double - blind, placebo - controlled study. J Headache Pain. 19, 69 (2018). 3 Tosymra [package insert]. Maple Grove, MN: Upsher - Smith Laboratories, LLC: Feb 2021. 4 Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatri pta n Research Group. Arch Neurol. 1992;49(12):1271 - 1276. 5 Wendt J, et al. A randomized, double - blind, placebo - controlled trial of the efficacy and tolerability of a 4 - mg dose of subcutan eous sumatriptan for the treatment of acute migraine attacks in adults. Clinical Therapeutics. 2006;28(4):517 - 526. Intravail is a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis , Inc.

© 2023 Tonix Pharmaceuticals Holding Corp. CNS: KEY DEVELOPMENT CANDIDATES © 2023 Tonix Pharmaceuticals Holding Corp.
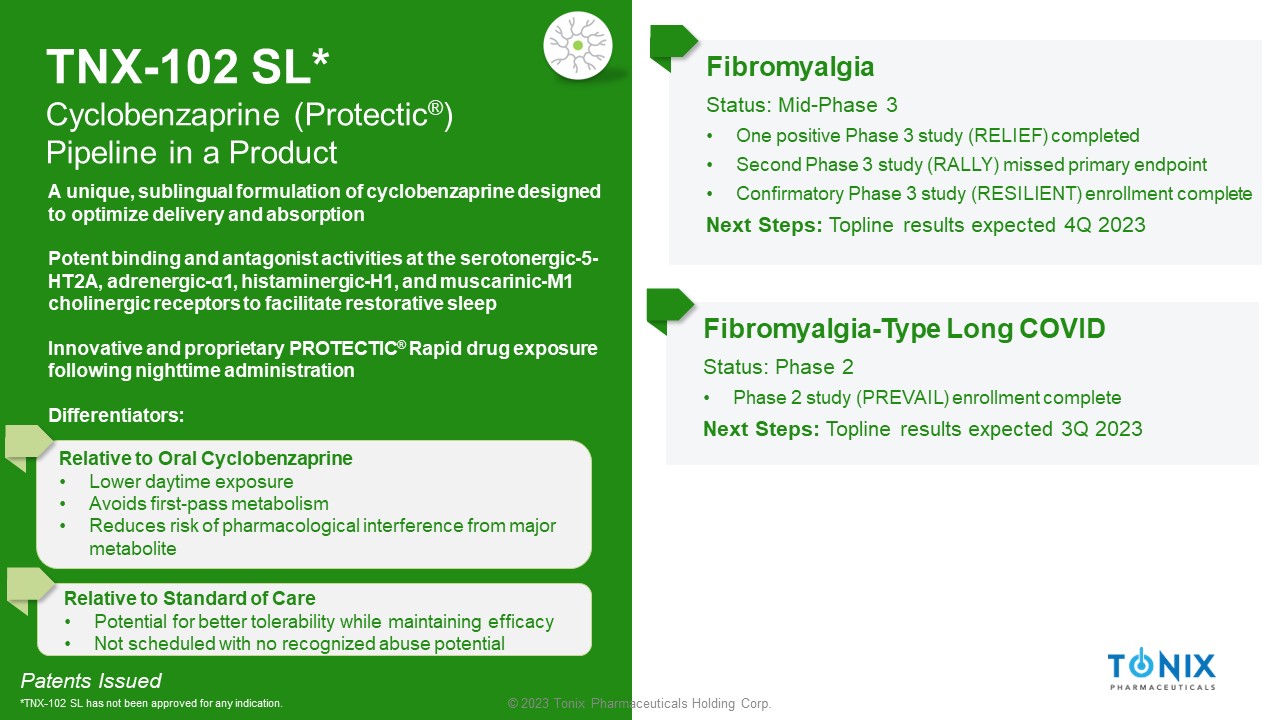
TNX - 102 SL* Cyclobenzaprine ( Protectic ® ) Pipeline in a Product Fibromyalgia Status: Mid - Phase 3 • One positive Phase 3 study (RELIEF) completed • Second Phase 3 study (RALLY) missed primary endpoint • Confirmatory Phase 3 study (RESILIENT) enrollment complete Next Steps: Topline results expected 4Q 2023 Fibromyalgia - Type Long COVID Status: Phase 2 • Phase 2 study (PREVAIL) enrollment complete Next Steps: Topline results expected 3Q 2023 Patents Issued *TNX - 102 SL has not been approved for any indication. A unique, sublingual formulation of cyclobenzaprine designed to optimize delivery and absorption Potent binding and antagonist activities at the serotonergic - 5 - HT2A, adrenergic - α1, histaminergic - H1, and muscarinic - M1 cholinergic receptors to facilitate restorative sleep Innovative and proprietary PROTECTIC ® Rapid drug exposure following nighttime administration Differentiators: Relative to Oral Cyclobenzaprine • Lower daytime exposure • Avoids first - pass metabolism • Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care • Potential for better tolerability while maintaining efficacy • Not scheduled with no recognized abuse potential 13 © 2023 Tonix Pharmaceuticals Holding Corp.

CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia Cyclobenzaprine Protectic ® Sublingual Tablets CNS PORTFOLIO Fibromyalgia (FM) is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS • A fflicts an estimated 6 - 12 million adults in the U.S., approximately 90% of whom are women 1 • Symptoms include chronic widespread pain, nonrestorative sleep, fatigue, and cognitive dysfunction • Patients struggle with daily activities, have impaired quality of life, and frequently are disabled • Physicians and patients report common dissatisfaction with currently marketed products Market Entry: Fibromyalgia Additional Indications: Long COVID, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Positive Phase 3 study RELIEF completed 2 Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT enrollment complete Next Steps: Topline results expected 4Q 2023 *TNX - 102 SL has not been approved for any indication. 1 American Chronic Pain Association (www.theacpa.org, 2019) 2 Lederman et al., (2023) Arthritis Care & Research "Efficacy and Safety of TNX - 102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia: Results From the RELIEF Trial ", doi : 10.1002/acr.25142. Epub ahead of print. PMID: 37165930. When the check engine light malfunctions, the light is on even though the car is not malfunctioning 14 © 2023 Tonix Pharmaceuticals Holding Corp.
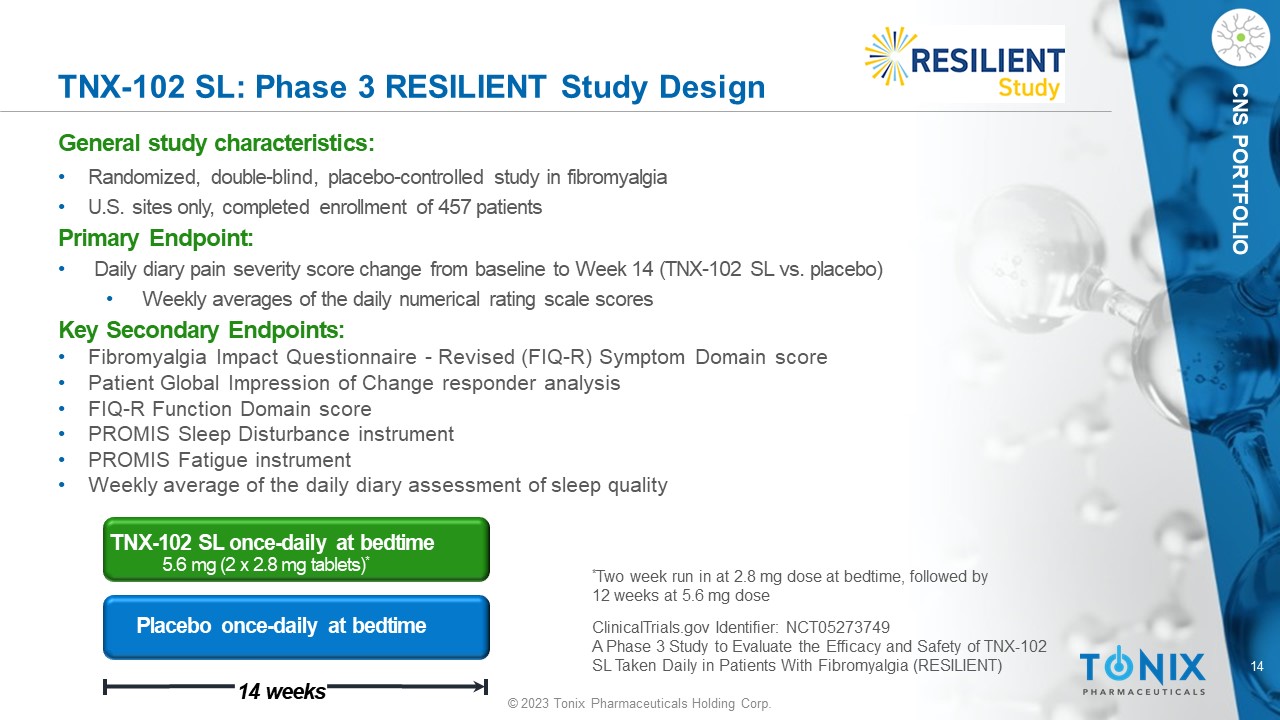
CNS PORTFOLIO TNX - 102 SL: Phase 3 R ESILIENT Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in fibromyalgia • U.S. sites only, completed enrollment of 457 patients Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) • Weekly averages of the daily numerical rating scale scores Key Secondary Endpoints: • Fibromyalgia Impact Questionnaire - Revised (FIQ - R) Symptom Domain score • Patient Global Impression of Change responder analysis • FIQ - R Function Domain score • PROMIS Sleep Disturbance instrument • PROMIS Fatigue instrument • Weekly average of the daily diary assessment of sleep quality Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05273749 A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT)
15 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia - Type Long COVID (PASC) Cyclobenzaprine Protectic ® Sublingual Tablets • Occurs in approximately 19% of recovered COVID - 19 patients 1 • As many as 40% of Long COVID patients experience multi - site pain, a hallmark of fibromyalgia 2,3 • Symptoms of Long COVID, like multi - site pain, fatigue and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) • In August 2022, the HHS released the National Research Action Plan on Long COVID 4 which endorses the connection between Long COVID and ME/CFS Market Entry : Fibromyalgia - Type Long COVID (PASC) Status: Phase 2 study PREVAIL enrollment complete Next Steps: Topline results expected 3Q 2023 1 J une 22, 2022 - CDC - https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/20220622.htm 2 Harris, H, et al. Tonix data on file. 2022 3 TriNetX Analytics *TNX - 102 SL has not been approved for any indication. CNS PORTFOLIO Additional Indications: Fibromyalgia, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder 4 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Long COVID.
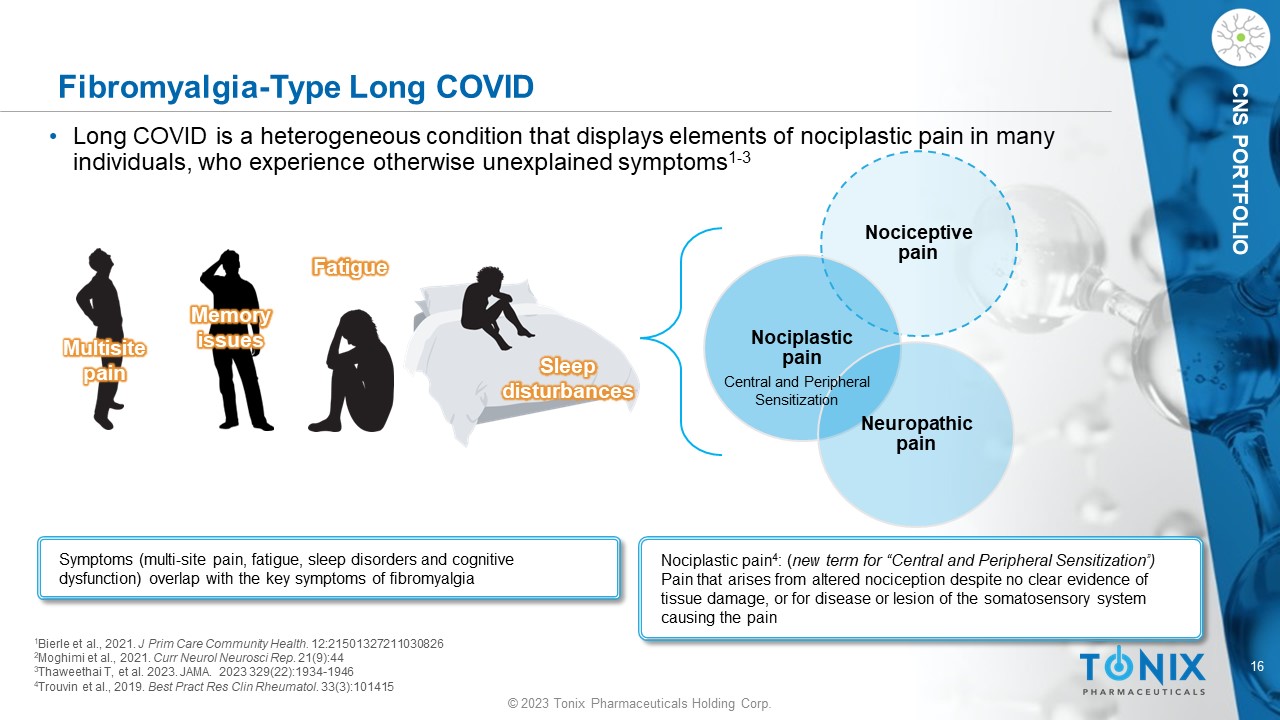
16 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia - Type Long COVID • Long COVID is a heterogeneous condition that displays elements of nociplastic pain in many individuals, who experience otherwise unexplained symptoms 1 - 3 Symptoms (multi - site pain, fatigue, sleep disorders and cognitive dysfunction) overlap with the key symptoms of fibromyalgia Multisite pain Memory issues Fatigue Sleep disturbances 1 Bierle et al., 2021. J Prim Care Community Health. 12:21501327211030826 2 Moghimi et al., 2021. Curr Neurol Neurosci Rep . 21(9):44 3 Thaweethai T, et al. 2023. JAMA. 2023 329(22):1934 - 1946 4 Trouvin et al., 2019. Best Pract Res Clin Rheumatol . 33(3):101415 Nociceptive pain Nociplastic pain Neuropathic pain Nociplastic pain 4 : ( new term for “Central and Peripheral Sensitization”) Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or lesion of the somatosensory system causing the pain Central and Peripheral Sensitization 17 © 2023 Tonix Pharmaceuticals Holding Corp.
CNS PORTFOLIO TNX - 102 SL: Phase 2 PREVAIL Study Design Study c haracteristics: • Randomized, double - blind, placebo - controlle d study of TNX - 102 SL in fibromyalgia - type Long COVID • U.S. sites only, completed enrollment of 63 patients Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) − Weekly averages of the daily numerical rating scale scores Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05472090 “A Phase 2 Study to Evaluate the Efficacy and Safety of TNX - 102 SL in Patients With Multi - Site Pain Associated With Post - Acute Sequelae of SARS - CoV - 2 Infection (PREVAIL)”
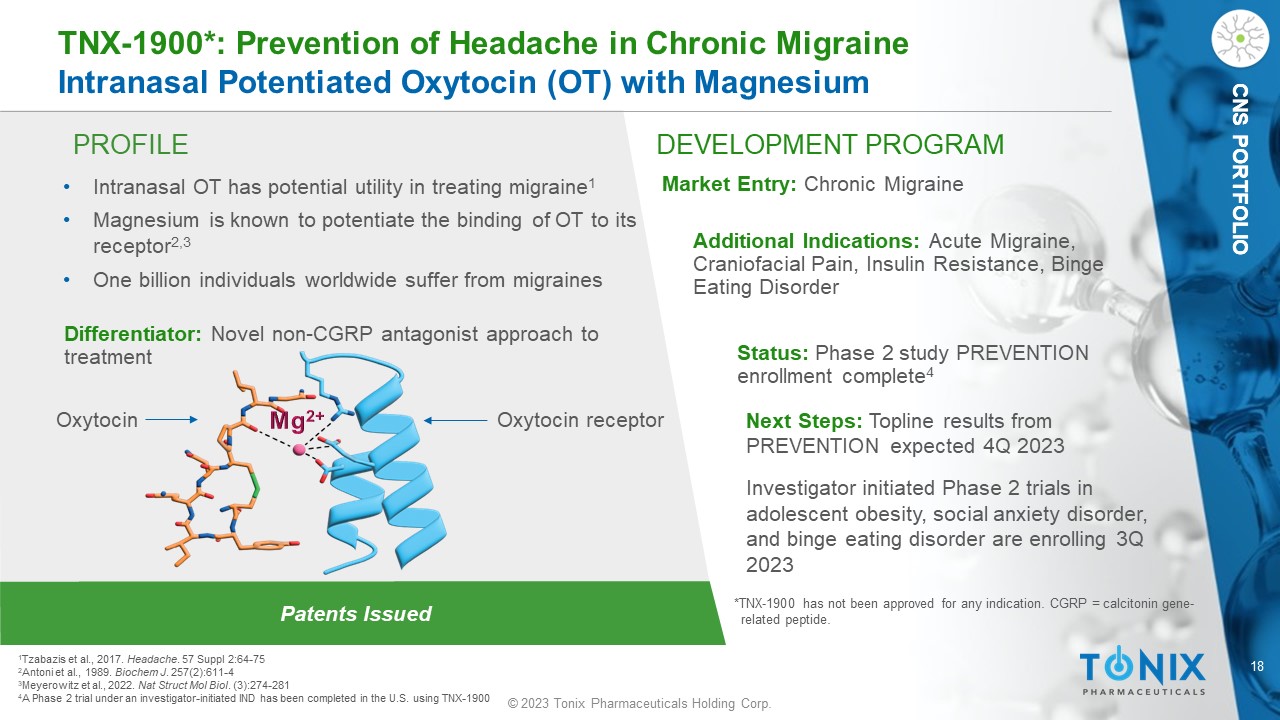
18 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 1900*: Prevention of Headache in Chronic Migraine Intranasal Potentiated Oxytocin (OT) with Magnesium CNS PORTFOLIO • Intranasal OT has potential utility in treating migraine 1 • Magnesium is known to potentiate the binding of OT to its receptor 2,3 • One billion individuals worldwide suffer from migraines Differentiator: Novel non - CGRP antagonist approach to treatment Market Entry: Chronic Migraine Additional Indications: Acute Migraine, Craniofacial Pain, Insulin Resistance, Binge Eating Disorder Status: Phase 2 study PREVENTION enrollment complete 4 Next Steps: Topline results from PREVENTION expected 4Q 2023 Investigator initiated Phase 2 t rials in adolescent obesity, social anxiety disorder, and binge eating disorder are enrolling 3Q 2023 1 Tzabazis et al., 2017. Headache . 57 Suppl 2:64 - 75 2 Antoni et al., 1989. Biochem J . 257(2):611 - 4 3 Meyerowitz et al., 2022. Nat Struct Mol Biol . (3):274 - 281 4 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 *TNX - 1900 has not been approved for any indication. CGRP = calcitonin gene - related peptide. Oxytocin receptor Oxytocin 19 © 2023 Tonix Pharmaceuticals Holding Corp.
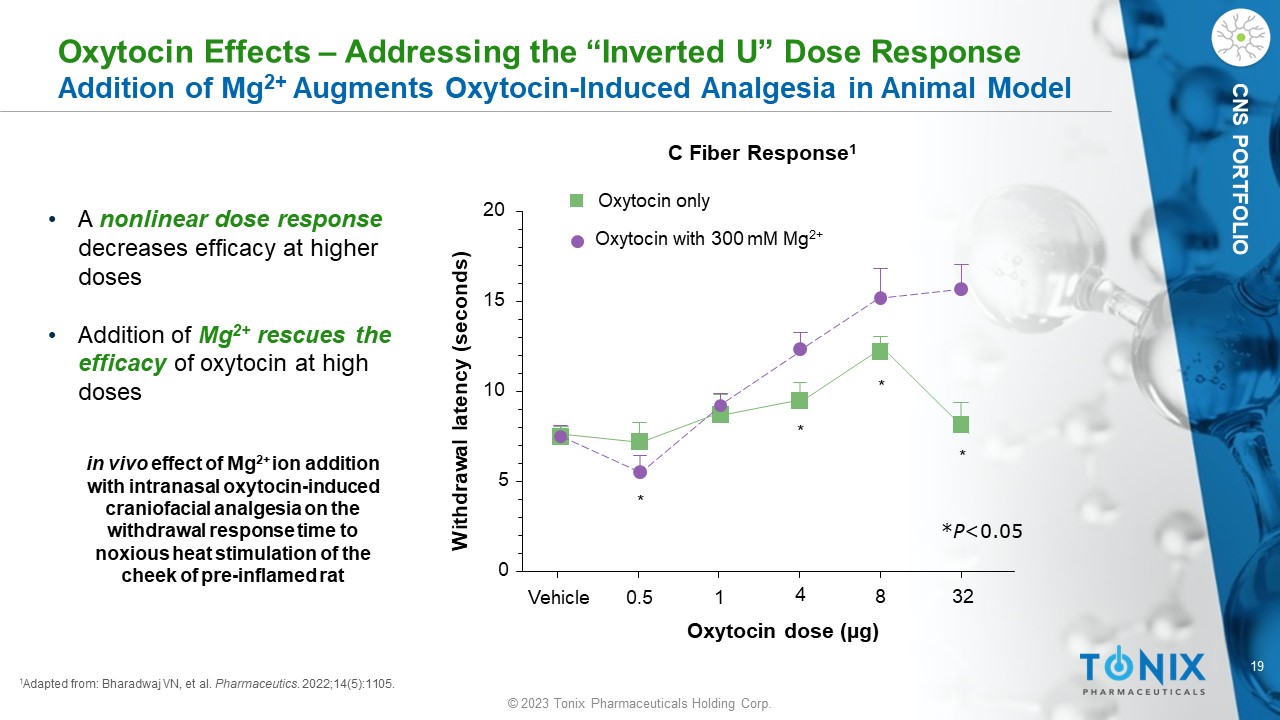
CNS PORTFOLIO 1 Adapted from: Bharadwaj VN, et al. Pharmaceutics . 2022;14(5):1105. in vivo effect of Mg 2+ ion addition with intranasal oxytocin - induced craniofacial analgesia on the withdrawal response time to noxious heat stimulation of the cheek of pre - inflamed rat Vehicle 0.5 1 4 8 32 0 5 10 15 20 Oxytocin only Oxytocin with 300 mM Mg 2+ * * * * Oxytocin dose (µg) Withdrawal latency (seconds) C Fiber Response 1 * P <0.05 Oxytocin Effects – Addressing the “Inverted U” Dose Response Addition of Mg 2+ Augments Oxytocin - Induced Analgesia in Animal Model • A nonlinear dose response decreases efficacy at higher doses • Addition of Mg 2+ rescues the efficacy of oxytocin at high doses 20 © 2023 Tonix Pharmaceuticals Holding Corp.
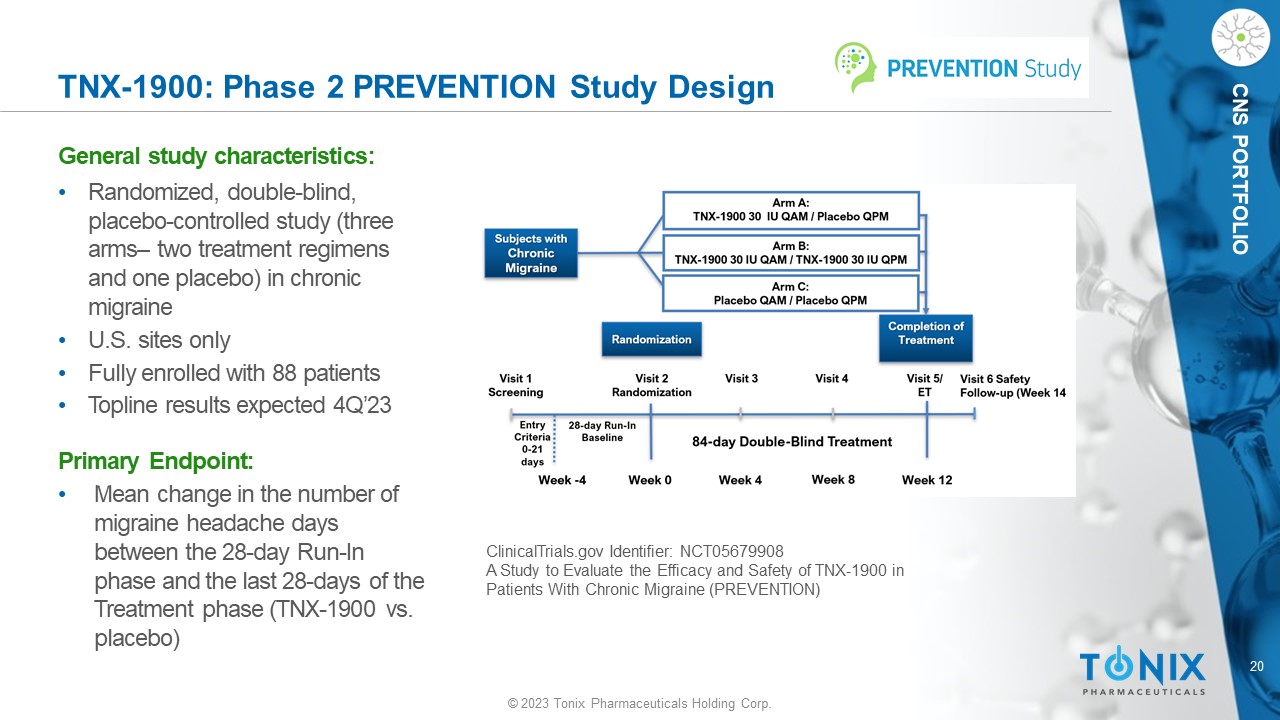
CNS PORTFOLIO TNX - 1900: Phase 2 PREVENTION Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study (three arms – two treatment regimens and one placebo) in chronic migraine • U.S. sites only • Fully enrolled with 88 patients • Topline results expected 4Q’23 Primary Endpoint: • M ean change in the number of migraine headache days between the 28 - day Run - In phase and the last 28 - days of the Treatment phase (TNX - 1900 vs. placebo) ClinicalTrials.gov Identifier: NCT05679908 A Study to Evaluate the Efficacy and Safety of TNX - 1900 in Patients With Chronic Migraine (PREVENTION) N = 50 N = 50 N = 50 21 © 2023 Tonix Pharmaceuticals Holding Corp.
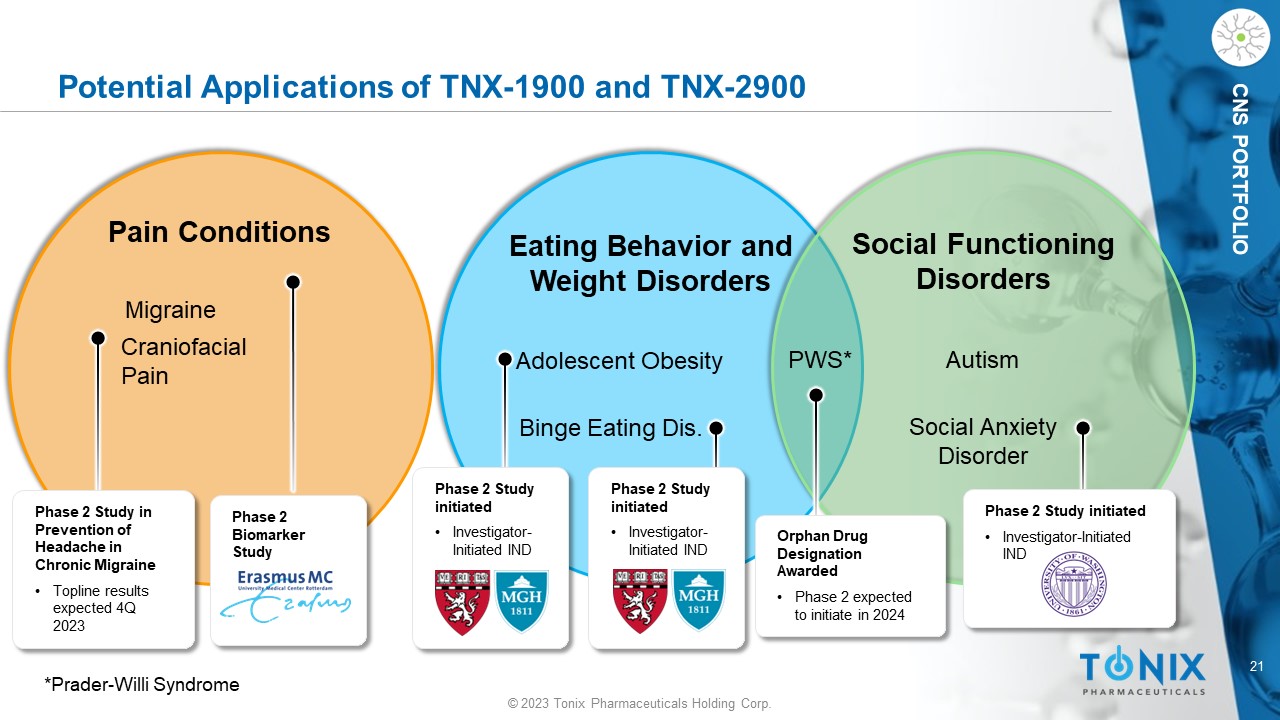
CNS PORTFOLIO Potential Applications of TNX - 1900 and TNX - 2900 Adolescent Obesity Binge Eating Dis. Eating Behavior and Weight Disorders Autism Social Functioning Disorders Social Anxiety Disorder Migraine Craniofacial Pain Pain Conditions Phase 2 Study in Prevention of Headache in Chronic Migraine • Topline results expected 4Q 2023 Phase 2 Biomarker Study Phase 2 Study initiated • Investigator - Initiated IND Phase 2 Study initiated • Investigator - Initiated IND Orphan Drug Designation Awarded • Phase 2 expected to initiate in 2024 Phase 2 Study initiated • Investigator - Initiated IND PWS* *Prader - Willi Syndrome 22 © 2023 Tonix Pharmaceuticals Holding Corp.
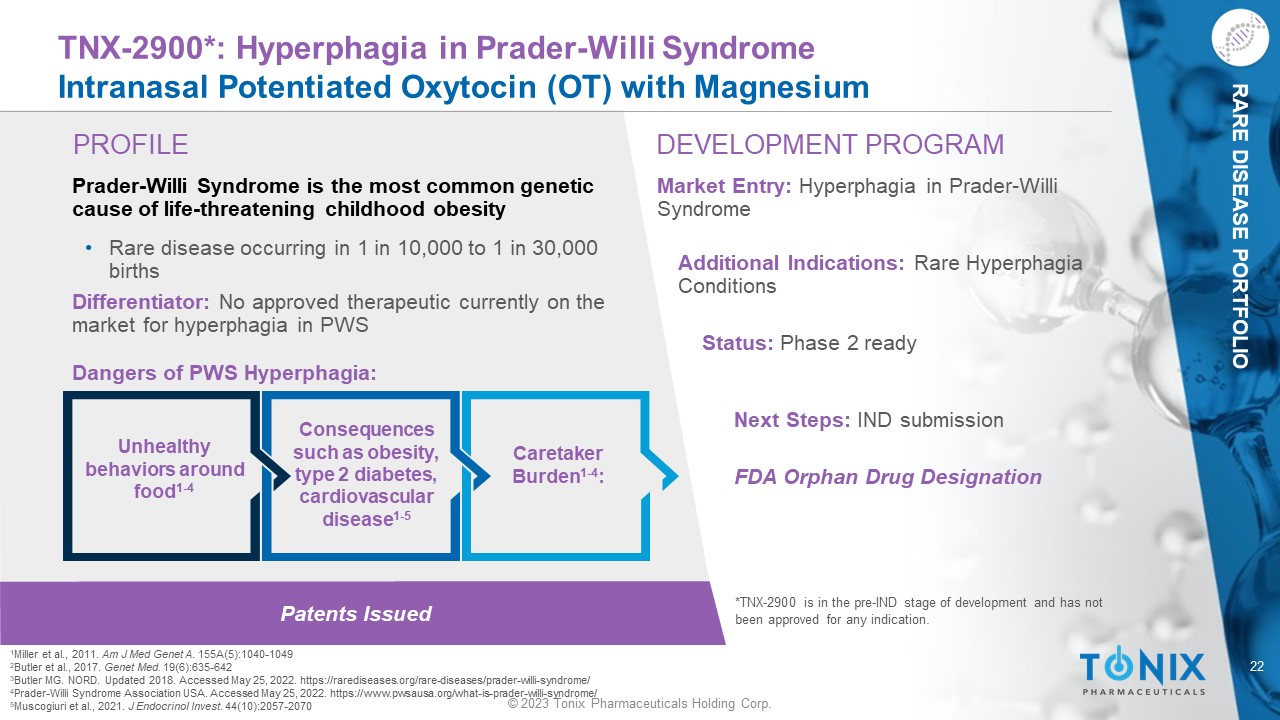
RARE DISEASE PORTFOLIO Patents Issued PROFILE DEVELOPMENT PROGRAM TNX - 2900*: Hyperphagia in Prader - Willi Syndrome Intranasal Potentiated Oxytocin (OT) with Magnesium Prader - Willi Syndrome is the most common genetic cause of life - threatening childhood obesity • Rare disease occurring in 1 in 10,000 to 1 in 30,000 births Differentiator: No approved therapeutic currently on the market for hyperphagia in PWS Dangers of PWS Hyperphagia: Market Entry: Hyperphagia in Prader - Willi Syndrome Additional Indications: Rare Hyperphagia Conditions Status: Phase 2 ready Next Steps: IND submission *TNX - 2900 is in the pre - IND stage of development and has not been approved for any indication. Caretaker Burden 1 - 4 : Unhealthy behaviors around food 1 - 4 Consequences such as obesity, type 2 diabetes, cardiovascular disease 1 - 5 1 Miller et al., 2011. Am J Med Genet A . 1 55A(5):1040 - 1049 2 Butler et al., 2017. Genet Med. 19(6):635 - 642 3 Butler MG. NORD. Updated 2018. Accessed May 25, 2022. https://rarediseases.org/rare - diseases/prader - willi - syndrome/ 4 Prader - Willi Syndrome Association USA. Accessed May 25, 2022. https://www.pwsausa.org/what - is - prader - willi - syndrome/ 5 Muscogiuri et al., 2021. J Endocrinol Invest . 44(10):2057 - 2070 FDA Orphan Drug Designation 23 © 2023 Tonix Pharmaceuticals Holding Corp.
CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 601 E R*: Depression Tianeptine Hemioxalate Extended - Release Tablets (39.4 mg) CNS PORTFOLIO • A novel, oral, extended - release once - daily tablet • Treatment effect of tianeptine sodium immediate release t.i.d. in depression is well - established • Tianeptine restores neuroplasticity in animal models • PPAR - β / δ and PPAR - γ agonist 1 Differentiators: Relative to tianeptine IR available ex - US: • Once daily dosing Relative to traditional antidepressants: • Unique mechanism of action – beyond neurotransmitter modulation • Tianeptine sodium IR has similar efficacy but less weight gain or sexual side effects than traditional antidepressants • Tianeptine’s side effects are described in labeling in countries in which it is marketed 2 Market Entry: Major Depressive Disorder (MDD) Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids, Alzheimer’s Disease 3 Status: Phase 2 MDD study UPLIFT enrollment complete Next Steps: Topline results expected 4Q 2023 1 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023. https://bit.ly/42o3jnV 2 Su mmary of product characteristics ( SmPC), European Medicines Agency, Stablon ®, www.servier.ci/sites/default/files/spc - pil/SPC_Stablon_1.pdf accessed 7 - 16 - 23. 3 García - Alberca et al., 2022. J Alzheimers Dis . 88(2):707 - 720 *TNX - 601 ER has not been approved for any indication.
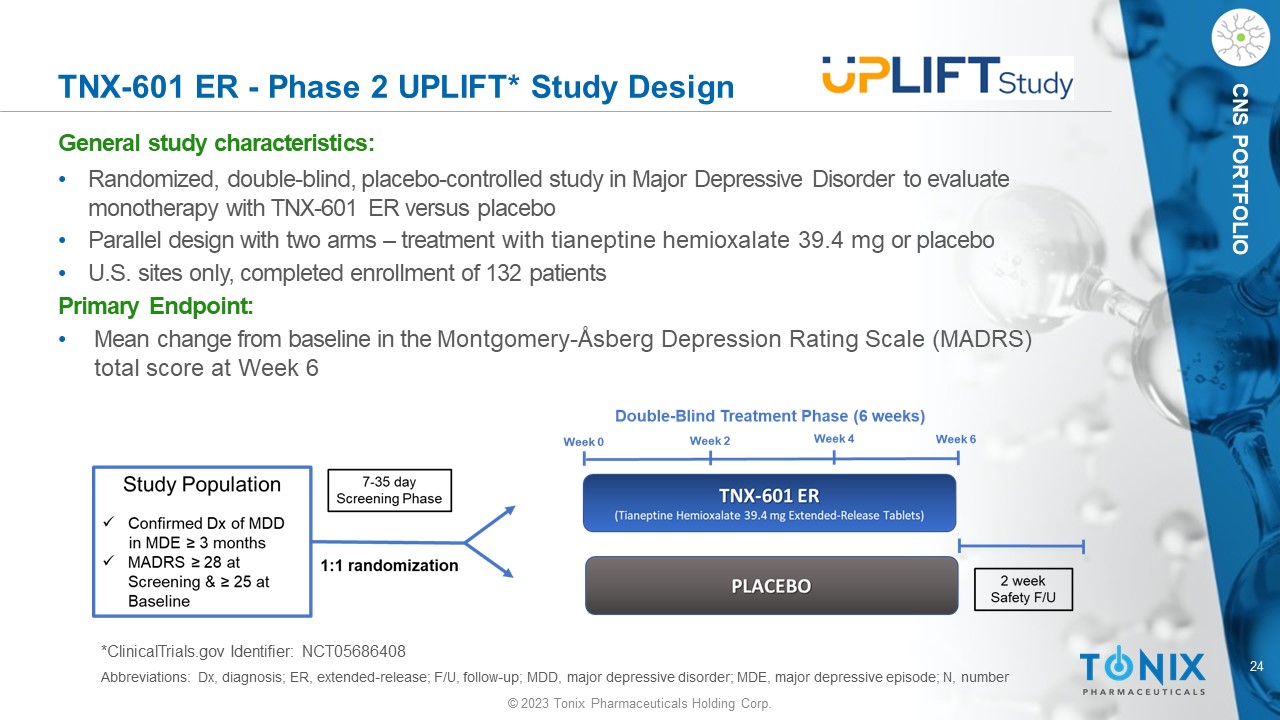
24 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 601 ER - Phase 2 UPLIFT* Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in Major Depressive Disorder to evaluate monotherapy with TNX - 601 ER versus placebo • Parallel design with two arms – treatment with tianeptine hemioxalate 39.4 mg or placebo • U.S. sites only, completed enrollment of 132 patients Primary Endpoint: • Mean change from baseline in the Montgomery - Åsberg Depression Rating Scale (MADRS) total score at Week 6 *ClinicalTrials.gov Identifier: NCT05686408 Abbreviations: Dx, diagnosis; ER, extended - release; F/U, follow - up; MDD, major depressive disorder; MDE, major depressive episo de; N, number 25 © 2023 Tonix Pharmaceuticals Holding Corp.
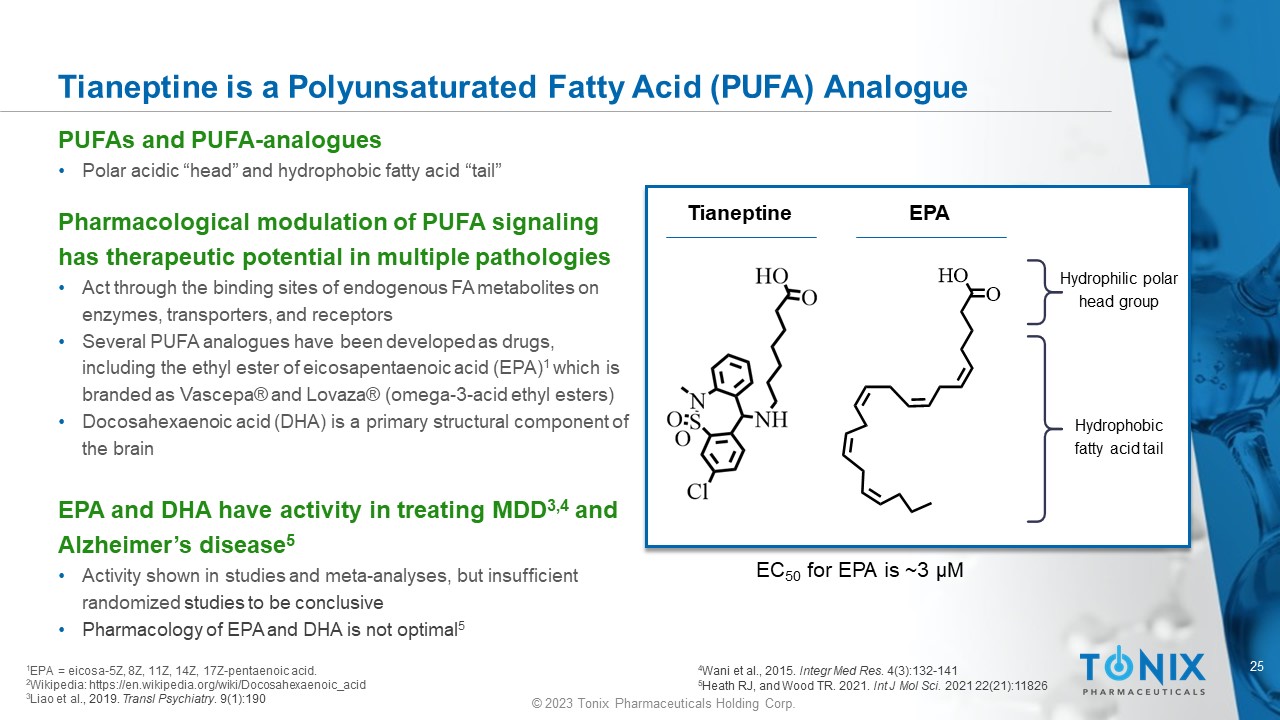
Tianeptine is a Polyunsaturated Fatty Acid (PUFA) Analogue PUFAs and PUFA - analogues • Polar acidic “head” and hydrophobic fatty acid “tail” Pharmacological modulation of PUFA signaling has therapeutic potential in multiple pathologies • Act through the binding sites of endogenous FA metabolites on enzymes, transporters, and receptors • Several PUFA analogues have been developed as drugs, including the ethyl ester of eicosapentaenoic acid (EPA) 1 which is branded as Vascepa ® and Lovaza ® (omega - 3 - acid ethyl esters) • Docosahexaenoic acid (DHA) is a primary structural component of the brain EPA and DHA have activity in treating MDD 3,4 and Alzheimer’s disease 5 • Activity shown in studies and meta - analyses, but insufficient randomized studies to be conclusive • Pharmacology of EPA and DHA is not optimal 5 EPA Hydrophilic polar head group Hydrophobic fatty acid tail 1 EPA = e icosa - 5Z, 8Z, 11Z, 14Z, 17Z - pentaenoic acid. 2 Wikipedia: https://en.wikipedia.org/wiki/Docosahexaenoic_acid 3 Liao et al ., 2019. Transl Psychiatry . 9(1):190 Tianeptine EC 50 for EPA is ~3 µM 4 Wani et al., 2015. Integr Med Res . 4(3):132 - 141 5 Heath RJ, and Wood TR. 2021. Int J Mol Sci. 2021 22(21):11826 26 © 2023 Tonix Pharmaceuticals Holding Corp.
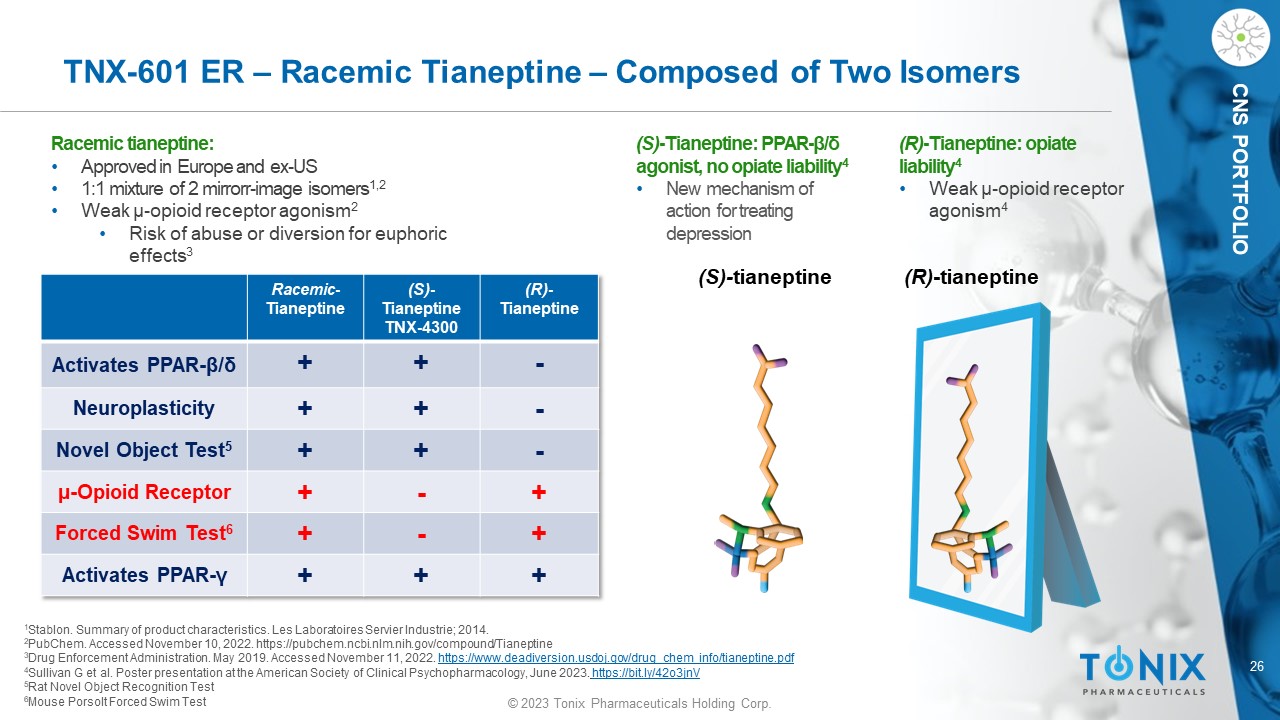
CNS PORTFOLIO TNX - 601 ER – Racemic Tianeptine – Composed of Two Isomers Racemic tianeptine : • Approved in Europe and ex - US • 1:1 mixture of 2 mirror r - image isomers 1,2 • Weak µ - o pioid receptor agonism 2 • Risk of abuse or diversion for euphoric effects 3 (S) - t ianeptine (R) - tianeptine 1 Stablon. Summary of product characteristics. Les Laboratoires Servier Industrie ; 2014. 2 PubChem. Accessed November 10, 2022. https://pubchem.ncbi.nlm.nih.gov/compound/Tianeptine 3 Drug Enforcement Administration. May 2019. Accessed November 11, 2022 . https://www.deadiversion.usdoj.gov/drug_chem_info/tianeptine.pdf 4 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023 . https://bit.ly/42o3jnV 5 Rat Novel Object Recognition Test 6 Mouse Porsolt Forced Swim Test Racemic - Tianeptine (S) - Tianeptine TNX - 4300 (R) - Tianeptine Activates PPAR - β / δ + + - Neuroplasticity + + - Novel Object Test 5 + + - µ - Opioid Receptor + - + Forced Swim Test 6 + - + Activates PPAR - γ + + + (S) - Tianeptine: PPAR - β / δ agonist, no opiate liability 4 • New mechanism of action for treating depression (R) - Tianeptine: opiate liability 4 • Weak µ - o pioid receptor agonism 4 27 © 2023 Tonix Pharmaceuticals Holding Corp.

CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 4300*: Depression, Alzheimer’s & Parkinson’s diseases Estianeptine (Single (S) - isomer of Tianeptine) CNS PORTFOLIO • Single isomer, oral treatment • Proposed mechanism of action from lab studies indicates estianeptine is the active ingredient of TNX - 601 ER 1 • PPAR - β / δ and PPAR - γ agonist • Free of µ - opioid receptor activity • Estianeptine restores neuroplasticity in tissue culture Differentiators: Relative to racemic tianeptine IR or TNX - 601 ER: • Lack of opioid liability Relative to traditional antidepressants: • Unique mechanism of action – beyond neurotransmitter modulation • Racemic tianeptine sodium IR has similar efficacy but fewer side effects than traditional antidepressants Market Entry: Major Depressive Disorder (MDD) Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids, Alzheimer’s Disease 2 Status: Pre - clinical Next Steps: Expect IND can be supported by pre - clinical and clinical data from TNX - 601 (racemic tianeptine) development 1 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023 . https://bit.ly/42o3jnV 2 García - Alberca et al., 2022. J Alzheimers Dis . 88(2):707 - 720 *TNX - 4300 is in the pre - IND stage of development and has not been approved for any indication 28 © 2023 Tonix Pharmaceuticals Holding Corp.
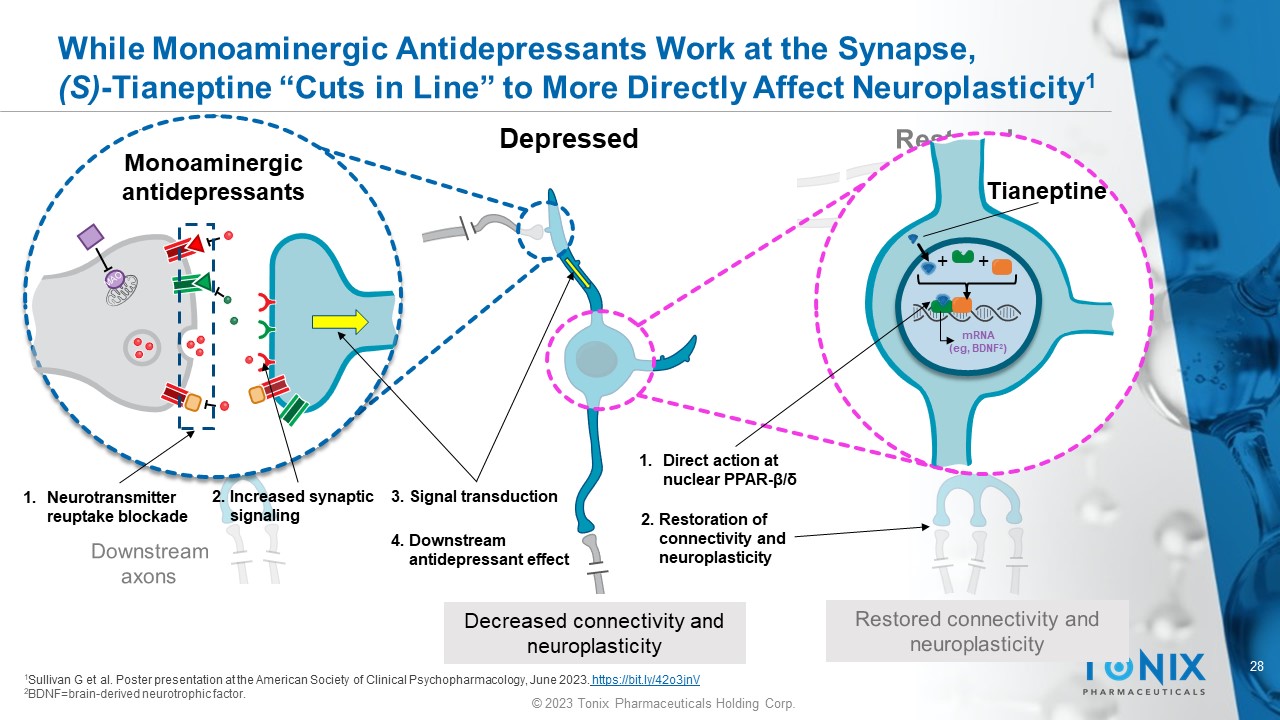
While Monoaminergic Antidepressants Work at the Synapse, (S) - Tianeptine “Cuts in Line” to More Directly Affect Neuroplasticity 1 Depressed Decreased connectivity and neuroplasticity Tianeptine + + mRNA ( eg , BDNF 2 ) 1 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023. https://bit.ly/42o3jnV 2 BDNF=brain - derived neurotrophic factor. Monoaminergic antidepressants 1. Neurotransmitter reuptake blockade 1. Direct action at nuclear PPAR - β/δ 2. Increased synaptic signaling 3. Signal transduction 4. Downstream antidepressant effect 2. Restoration of connectivity and neuroplasticity 29 © 2023 Tonix Pharmaceuticals Holding Corp.
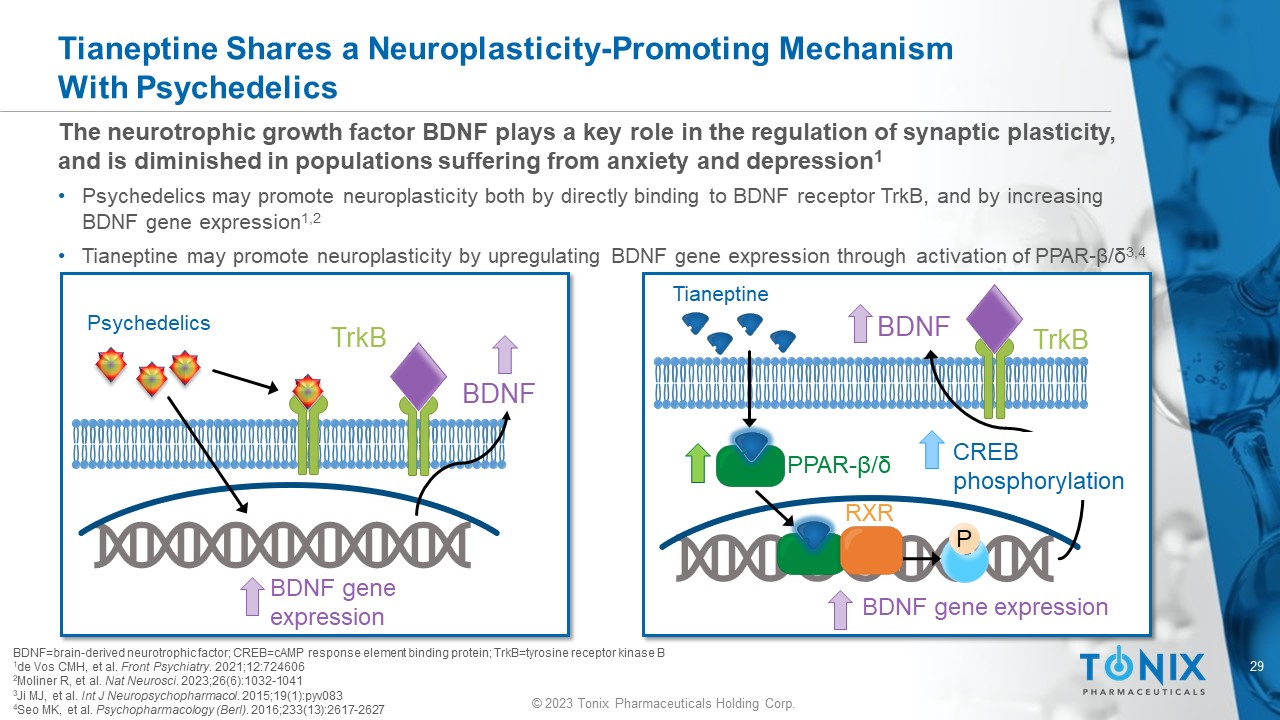
The n eurotrophic growth factor BDNF plays a key role in the regulation of synaptic plasticity, and is diminished in populations suffering from anxiety and depression 1 • Psychedelics may promote neuroplasticity both by directly binding to BDNF receptor TrkB , and by increasing BDNF gene expression 1,2 • Tianeptine may promote neuroplasticity by upregulating BDNF gene expression through activation of PPAR - β/δ 3,4 Tianeptine Shares a Neuroplasticity - Promoting Mechanism With Psychedelics BDNF=brain - derived neurotrophic factor; CREB=cAMP response element binding protein; TrkB =tyrosine receptor kinase B 1 de Vos CMH, et al. Front Psychiatry . 2021;12:724606 2 Moliner R, et al. Nat Neurosci . 2023;26(6):1032 - 1041 3 Ji MJ, et al. Int J Neuropsychopharmacol . 2015;19(1):pyv083 4 Seo MK, et al. Psychopharmacology ( Berl ) . 2016;233(13):2617 - 2627 Tianeptine PPAR - β/δ RXR P BDNF BDNF gene expression CREB phosphorylation TrkB Psychedelics BDNF BDNF gene expression TrkB 30 © 2023 Tonix Pharmaceuticals Holding Corp.
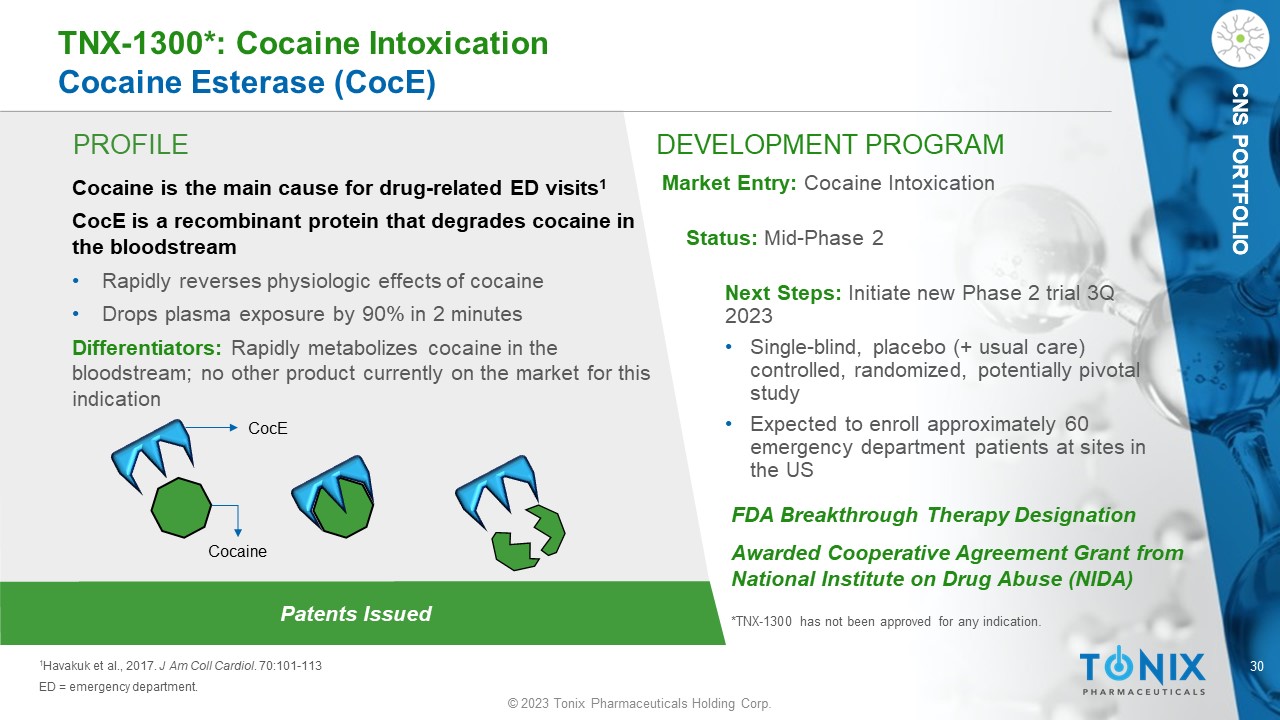
CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 1300*: Cocaine Intoxication Cocaine Esterase ( CocE ) Cocaine is the main cause for drug - related ED visits 1 CocE is a recombinant protein that degrades cocaine in the bloodstream • Rapidly reverses physiologic effects of cocaine • Drops plasma exposure by 90% in 2 minutes Differentiators: Rapidly metabolizes cocaine in the bloodstream; no other product currently on the market for this indication Market Entry: Cocaine Intoxication Status: Mid - Phase 2 Next Steps: Initiate new Phase 2 trial 3Q 2023 • S ingle - blind, placebo (+ usual care) controlled, randomized, potentially pivotal study • Expected to enroll approximately 60 emergency department patients at sites in the US 1 Havakuk et al., 2017. J Am Coll Cardiol . 70:101 - 113 ED = emergency department. FDA Breakthrough Therapy Designation Awarded Cooperative Agreement Grant from National Institute on Drug Abuse (NIDA) *TNX - 1300 has not been approved for any indication. CNS PORTFOLIO CocE Cocaine © 2023 Tonix Pharmaceuticals Holding Corp.

IMMUNOLOGY: KEY CANDIDATES
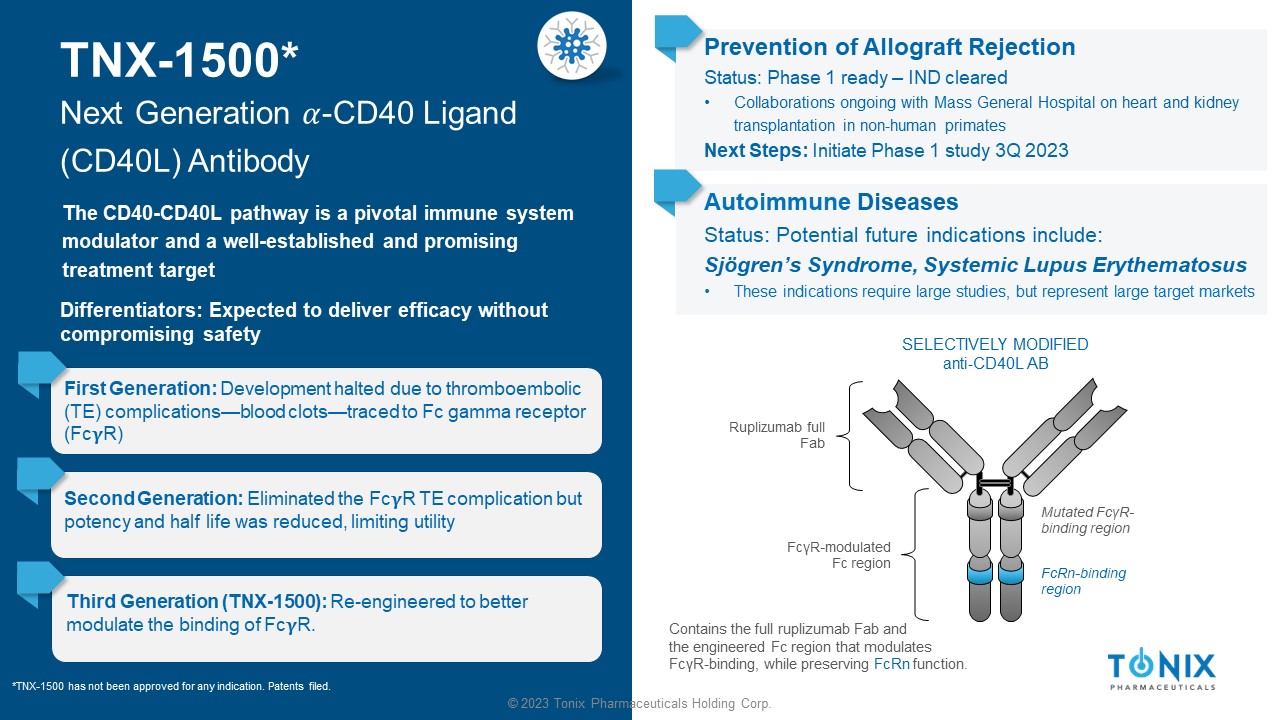
© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 1500* Next Generation ߙ - CD40 Ligand (CD40L) Antibody The CD40 - CD40L pathway is a pivotal immune system modulator and a well - established and promising treatment target First Generation: Development halted due to thromboembolic (TE) complications — blood clots — traced to Fc gamma receptor (Fc R) Prevention of Allograft Rejection Status: Phase 1 ready – IND cleared • Collaborations ongoing with Mass General Hospital on heart and kidney transplantation in non - human primates Next Steps: Initiate Phase 1 study 3 Q 2023 SELECTIVELY MODIFIED anti - CD40L AB Ruplizumab full Fab Contains the full ruplizumab Fab and the engineered Fc region that modulates Fc γ R - binding, while preserving FcRn function. Mutated Fc γ R - binding region FcRn - binding region Fc γ R - modulated Fc region Second Generation: Eliminated the Fc R TE complication but potency and half life was reduced, limiting utility Third Generation (TNX - 1500): Re - engineered to better modulate the binding of Fc R. *TNX - 1500 has not been approved for any indication. Patents filed. Differentiators: Expected to deliver efficacy without compromising safety Autoimmune Diseases Status: Potential future indications include: Sjögren’s Syndrome, Systemic Lupus Erythematosus • These indications require large studies, but represent large target markets 33 © 2023 Tonix Pharmaceuticals Holding Corp.
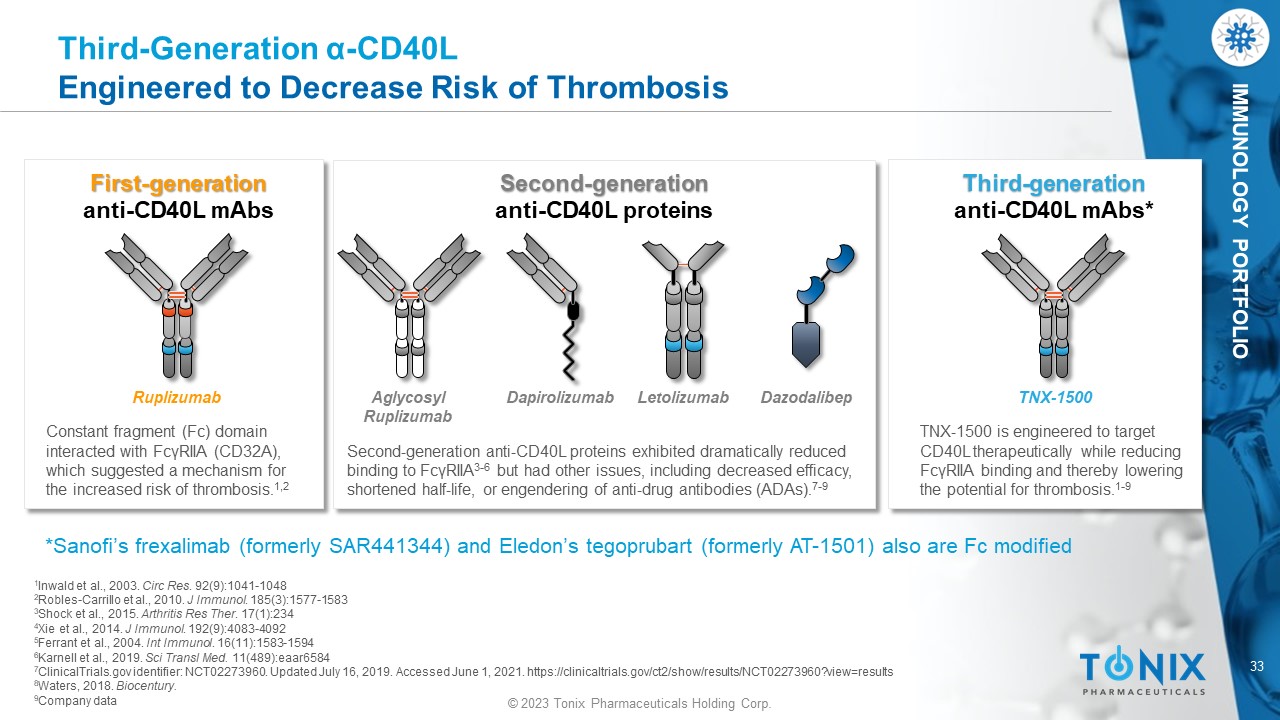
IMMUNOLOGY PORTFOLIO Third - Generation α - CD40L Engineered to Decrease Risk of Thrombosis First - generation anti - CD40L mAbs Constant fragment (Fc) domain interacted with FcγRIIA (CD32A), which suggested a mechanism for the increased risk of thrombosis. 1,2 Ruplizumab Second - generation anti - CD40L proteins Second - generation anti - CD40L proteins exhibited dramatically reduced binding to FcγRIIA 3 - 6 but had other issues, including decreased efficacy, shortened half - life, or engendering of anti - drug antibodies (ADAs). 7 - 9 Dapirolizumab Letolizumab Aglycosyl Ruplizumab Third - generation anti - CD40L mAbs * TNX - 1500 is engineered to target CD40L therapeutically while reducing FcγRIIA binding and thereby lowering the potential for thrombosis. 1 - 9 TNX - 1500 *Sanofi’s frexalimab (formerly SAR441344) and Eledon’s tegoprubart (formerly AT - 1501) also are Fc modified 1 Inwald et al., 2003. Circ Res . 92(9):1041 - 1048 2 Robles - Carrillo et al., 2010. J Immunol . 185(3):1577 - 1583 3 Shock et al., 2015. Arthritis Res Ther . 17(1):234 4 Xie et al., 2014. J Immunol . 192(9):4083 - 4092 5 Ferrant et al., 2004. Int Immunol . 16(11):1583 - 1594 6 Karnell et al., 2019. Sci Transl Med. 11(489):eaar6584 7 ClinicalTrials.gov identifier: NCT02273960. Updated July 16, 2019. Accessed June 1, 2021. https://clinicaltrials.gov/ct2/show /re sults/NCT02273960?view=results 8 Waters, 2018. Biocentury . 9 Company data Dazodalibep 34 © 2023 Tonix Pharmaceuticals Holding Corp.

IMMUNOLOGY PORTFOLIO Other anti - CD40L Monoclonal Antibodies in Development UCB (Co - developed with Biogen) – Systemic Lupus Erythematosus (SLE) • Phase 3 Trial Currently Enrolling (NCT04294667) − Topline results expected 1H 2024 1 • Dapirolizumab pegol (pegylated Fab) Horizon (being acquired by Amgen) – Sjögren's Syndrome ( SjS ) • Two Positive Phase 2 studies reported 2,3 • Dazodalibep (tn03 fusion protein) Sanofi – Sjögren's Syndrome ( SjS ), Multiple Sclerosis (MS), Systemic Lupus Erythematosus (SLE) • Phase 2 Trial Currently Enrolling in SjS (NCT04572841) and SLE (NCT05039840) • Active Phase 2 Trial in Relapsing MS (NCT04879628) • Frexalimab , f.k.a . SAR441344 (Fc - modified) Eledon – Amyotrophic Lateral Sclerosis (ALS) and Kidney Transplant • Phase 2 Trial Completed in ALS (NCT04322149) • Phase 1/2 Trial Currently Enrolling in Kidney Transplant (NCT05027906) • T egoprubart , f.k.a . AT - 1501 (Fc - modified) Lundbeck and AprilBio – Neurology • Phase 1 Trial Currently Enrolling in Healthy Adults (NCT05136053) • APB - A1 or Lu AG22515 (HAS fusion protein) 1 https://www.ucb.com/our - science/pipeline 2 https://ir.horizontherapeutics.com/news - releases/news - release - details/horizon - therapeutics - plc - announces - phase - 2 - trial - evaluatin g 3 https://ir.horizontherapeutics.com/news - releases/news - release - details/horizon - therapeutics - plc - announces - phase - 2 - trial - evaluatin g - 0 35 © 2023 Tonix Pharmaceuticals Holding Corp.
IMMUNOLOGY PORTFOLIO Patents Filed TNX - 1700*: Gastric and Colorectal Cancers Recombinant Trefoil Factor 2 (rTFF2 - HSA) Fusion Protein Potential New Cancer Treatment • TNX - 1700 (rTFF2 - HSA) has effects on cancer by altering the tumor micro - environment • Mechanism of action: suppresses myeloid - derived suppressor cells and activates anti - cancer CD8+ T cells • Potential synergy with anti - PD1 or anti - PD - L1 monoclonal antibodies ( mAbs ) Preclinical Evidence for Inhibiting Growth of Cancer Cells • In an MC38 mouse model of colorectal cancer, mTNX - 1700 (murine TNX - 1700) alone inhibited tumor growth by 50%, and combination therapy with anti - PD1 inhibited tumor growth by 87% 1 • In an advanced mouse model of gastric cancer, mTNX - 1700 combination therapy with anti - PD1 inhibited tumor growth by 78% 2 • Mechanistically, the combination therapy reduced intratumoral MDSCs, profoundly increased tumor - infiltrating CD8+ T cells, and significantly reduced spontaneous metastasis 2 Market Entry: Immuno - oncology, combination therapy with PD1 blockers for gastric and colorectal cancer Status: Preclinical Next Steps: Animal studies ongoing *TNX - 1700 is in the pre - IND stage of development and has not been approved for any indication. Differentiator: No product yet identified consistently augments PD1 effects on cold tumors Licensed from Columbia University • Developing in partnership under sponsored research agreement 1 Daugherty et al., AACR Poster. 2023. MDSC - targeted TFF2 - MSA suppresses tumor growth and increases survival in anti - PD - 1 treated MC38 and CT26.wt murine colorectal cancer models. https://bit.ly/45XbGK9 2 Qian et al., AACR Poster. 2023. MDSC - targeted TFF2 - MSA synergizes with PD - 1 blockade therapy in diffuse - type gastric cancer. https://bit.ly/3qCQsku © 2023 Tonix Pharmaceuticals Holding Corp.

INFECTIOUS DISEASE: KEY CANDIDATES
© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 801* Recombinant Pox Vaccine (RPV) Platform Using Live Virus Technology Mpox and Smallpox Vaccine Status: Preclinical • TNX - 801 is a cloned version of horsepox 1 (without any insert) purified from cell culture Next Steps: File IND Vaccine for Future Emerging Infectious Diseases Example: TNX - 1850 for COVID - 19 Status: Model System *TNX - 801 is in the pre - IND stage of development and has not been approved for any indication. Patents filed. • Live virus vaccines are the most established vaccine technology ‒ Starting with Edward Jenner’s smallpox vaccine, the first vaccine, which eradicated smallpox ‒ Prevents forward transmission ‒ Effective in eliciting durable or long - term immunity • Economical to manufacture at scale ‒ Low dose because replication amplifies dose in vivo ‒ Single shot administration • Standard refrigeration required for shipping and storage 1 Noyce et al., 2018. PLoS One . 13(1):e0188453. Differentiators: TNX - 801* scHPXV (Horsepox) 212,811 bp 38 © 2023 Tonix Pharmaceuticals Holding Corp.
INFECTIOUS DISEASE PORTFOLIO Live Virus Vaccine Platform: Recombinant Pox Vaccine (RPV) Technology for Emerging Infectious Diseases and Oncolytics Mpox and Smallpox Future Pandemics & New Infectious Diseases COVID - 19 Biodefense Using Proven Science To Address Challenging Disease States, We Have Created A Programmable Technology Platform Aimed At Combating Future Threats To Public Health Vaccinia Horsepox ANTIGEN CODING Oncology RPV VECTOR BELIEVED SIMILAR TO EDWARD JENNER’S VACCINE 1 - 3 1 Shrick, 2017. N Engl J Med 377:1491 - 1492 2 Esparza, 2020. Vaccine . 38(30): 4773 – 4779 3 Brinkmann, 2020. Genome Biol . 21: 286 © 2023 Tonix Pharmaceuticals Holding Corp.

TEAM, CAPABILITIES, NETWORK, & UPCOMING MILESTONES 40 © 2023 Tonix Pharmaceuticals Holding Corp.

INFECTIOUS DISEASE PORTFOLIO Internal Development & Manufacturing Capabilities R&D Center (RDC) – Frederick, MD • Functions: ‒ Research advancing CNS and immunology drugs ‒ Accelerated development of vaccines and antiviral drugs against COVID - 19, its variants and other infectious diseases • Description: ~48,000 square feet, BSL - 2 with some areas designated BSL - 3 • Status: Operational Advanced Development Center (ADC) – North Dartmouth, MA • Function: Development and clinical scale manufacturing of biologics • Description: ~45,000 square feet, BSL - 2 • Status: Operational 41 © 2023 Tonix Pharmaceuticals Holding Corp.
TNX - 1300: COCAINE INTOXICATION TNX - 1700: GASTRIC AND COLORECTAL CANCERS Key Development Partners TNX - 1500: ALLOGRAFT REJECTION TNX - 1900: MIGRAINE & OTHER INDICATIONS TNX - 801: SMALLPOX AND MONKEYPOX VACCINE TNX - 1850: COVID - 19 VACCINE TNX - 2900: PRADER - WILLI SYNDROME TNX - 3700 : COVID - 19 VACCINE (ZINC NANOPARTICLE mRNA TECHNOLOGY ) TNX - 2300 : BOVINE PARAINFLUEZNA VIRUS 42 © 2023 Tonix Pharmaceuticals Holding Corp.

Milestones: Recently Completed and Upcoming Expected Clinical Trial Initiations □ 3 rd Quarter 2023 Phase 1 study start of TNX - 1500 for prevention of allograft rejection □ 3 rd Quarter 2023 Phase 2 study start of TNX - 1300 for the treatment of cocaine intoxication □ 2 nd Quarter 2022 Phase 3 RESILIENT study start of TNX - 102 SL for the management of fibromyalgia □ 3rd Quarter 2022 Phase 2 PREVAIL study start of TNX - 102 SL for the treatment of fibromyalgia - type Long COVID □ 1 st Quarter 2023 Phase 2 PREVENTION study start of TNX - 1900 for the treatment of migraine □ 1st Quarter 2023 Phase 2 UPLIFT study start of TNX - 601 ER for major depressive disorder □ 2 nd Quarter 2023 Acquisition of marketed migraine products x x Expected Data □ 3 rd Quarter 2023 Topline results of Phase 2 PREVAIL study of TNX - 102 SL for fibromyalgia - type Long COVID □ 4 th Quarter 2023 Topline results of Phase 2 PREVENTION study of TNX - 1900 for chronic migraine □ 4 th Quarter 2023 Topline results of Phase 2 UPLIFT study of TNX - 601 ER for major depressive disorder □ 4 th Quarter 2023 Topline results of Phase 3 RESILIENT study of TNX - 102 SL for fibromyalgia x x x © 2023 Tonix Pharmaceuticals Holding Corp.

THANK YOU

44 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace ® IMPORTANT SAFETY INFORMATION (1 of 2) Zembrace SymTouch ( Zembrace ) can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop use and get emergency help if you have any signs of a heart attack: D iscomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back; severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw; pain or discomfort in your arms, back, neck, jaw or stomach ; shortness of breath with or without chest discomfort ; breaking out in a cold sweat ; nausea or vomiting ; feeling lightheaded Zembrace is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam shows no problem. Do not use Zembrace if you have: H istory of heart problems ; narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) ; uncontrolled high blood pressure ; hemiplegic or basilar migraines. If you are not sure if you have these, ask your provider. H ad a stroke, transient ischemic attacks (TIAs), or problems with blood circulation ; severe liver problems ; taken any of the following medicines in the last 24 hours: almotriptan , eletriptan , frovatriptan , naratriptan, rizatriptan, ergotamines , dihydroergotamine ; are taking certain antidepressants, known as monoamine oxidase (MAO) - A inhibitors or it has been 2 weeks or less since you stopped taking a MAO - A inhibitor. Ask your provider for a list of these medicines if you are not sure. A n allergy to sumatriptan or any of the components of Zembrace Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements. Zembrace can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.

45 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Zembrace ® IMPORTANT SAFETY INFORMATION (2 of 2) Zembrace may cause serious side effects including: • Changes in color or sensation in your fingers and toes; sudden or severe stomach pain, stomach pain after meals, weight loss, na usea or vomiting, constipation or diarrhea, bloody diarrhea, fever; cramping and pain in your legs or hips; feeling of heaviness or t igh tness in your leg muscles; burning or aching pain in your feet or toes while resting; numbness, tingling, or weakness in your legs; cold fe eli ng or color changes in one or both legs or feet; increased blood pressure including a sudden severe increase even if you have no history of high blood pressure; medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches g et worse, call your provider. • Serotonin syndrome, a rare but serious problem that can happen in people using Zembrace , especially when used with anti - depressant medicines called SSRIs or SNRIs. Call your provider right away if you have: mental changes such as seeing things that are not th ere (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or tro ubl e walking. • Hives (itchy bumps); swelling of your tongue, mouth, or throat • Seizures even in people who have never had seizures before The most common side effects of Zembrace include: pain and redness at injection site; tingling or numbness in your fingers or toes; dizziness; warm, hot, burning feeling to your face (flushing); discomfort or stiffness in your neck; feeling weak, drowsy, or ti red. Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effe cts of Zembrace . For more information, ask your provider. This is the most important information to know about Zembrace but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use . You can also visit www.upsher - smith.com or call 1 - 888 - 650 - 3789. For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6e5b104f - 2b9e - 416e - 92fb - ef1bdaea867d You are encouraged to report adverse effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1 - 800 - FDA - 1088. Zembrace is a prescription medicine used to treat acute migraine headaches with or without aura in adults who have been diagnosed with migraine. Zembrace is not used to prevent migraines. It is not known if it is safe and effective in children under 18 years of age.

46 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tosymra® IMPORTANT SAFETY INFORMATION (1 of 2) Tosymra® can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop Tosymra and get emergency medical help if you have any signs of heart attack: • D iscomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back ; severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw ; pain or discomfort in your arms, back, neck, jaw, or stomach ; shortness of breath with or without chest discomfort; breaking out in a cold sweat ; nausea or vomiting ; feeling lightheaded Tosymra is not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam is done and shows no problem. Do not use Tosymra if you have: • H istory of heart problems ; narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease) ; uncontrolled high blood pressure ; severe liver problems ; hemiplegic or basilar migraines. If you are not sure if you have these, ask your healthcare provider. • H ad a stroke, transient ischemic attacks (TIAs), or problems with blood circulation ; taken any of the following medicines in the last 24 hours: almotriptan , eletriptan , frovatriptan , naratriptan, rizatriptan, ergotamines , or dihydroergotamine. Ask your provider if you are not sure if your medicine is listed above • are taking certain antidepressants, known as monoamine oxidase (MAO) - A inhibitors or it has been 2 weeks or less since you stopped taking a MAO - A inhibitor . A sk your provider for a list of these medicines if you are not sure • A n allergy to sumatriptan or any ingredient in Tosymra Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements. Tosymra can cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.

47 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tosymra may cause serious side effects including: • Changes in color or sensation in your fingers and toes; sudden or severe stomach pain, stomach pain after meals, weight loss, nausea or vomiting, constipation or diarrhea, bloody diarrhea, fever; cramping and pain in your legs or hips, feeling of heaviness or tightness in your leg muscles, burning or aching pain in your feet or toes while resting, numbness, tingling, or weakness in your legs, cold feeling or color changes in one or both legs or feet; increased blood pressure including a sudden severe increase even if you have no history of high blood pressure; medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches get worse, call your provider . • Serotonin syndrome, a rare but serious problem that can happen in people using Tosymra, especially when used with anti - depressant medicines called SSRIs or SNRIs. Call your provider right away if you have : mental changes such as seeing things that are not there (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or trouble walking. • Seizures even in people who have never had seizures before The most common side effects of Tosymra include : tingling, dizziness, feeling warm or hot, burning feeling, feeling of heaviness, feeling of pressure, flushing, feeling of tightness, numbness, application site (nasal) reactions, abnormal taste, and throat irritation. Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of Tosymra. For more information, ask your provider. This is the most important information to know about Tosymra but is not comprehensive. For more information, talk to your provider and read the Patient Information and Instructions for Use . You can also visit www.upsher - smith.com or call 1 - 888 - 650 - 3789. For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=015a5cf9 - f246 - 48bc - b91e - cd730a53d8aa You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch , or call 1 - 800 - FDA - 1088. Tosymra is a prescription medicine used to treat acute migraine headaches with or without aura in adults. Tosymra is not used to treat other types of headaches such as hemiplegic or basilar migraines or cluster headaches. Tosymra is not used to prevent migraines. It is not known if Tosymra is safe and effective in children under 18 years of age. Tosymra ® IMPORTANT SAFETY INFORMATION (2 of 2)