Document
EMERGENT BIOSOLUTIONS REPORTS FIRST QUARTER 2024 FINANCIAL RESULTS
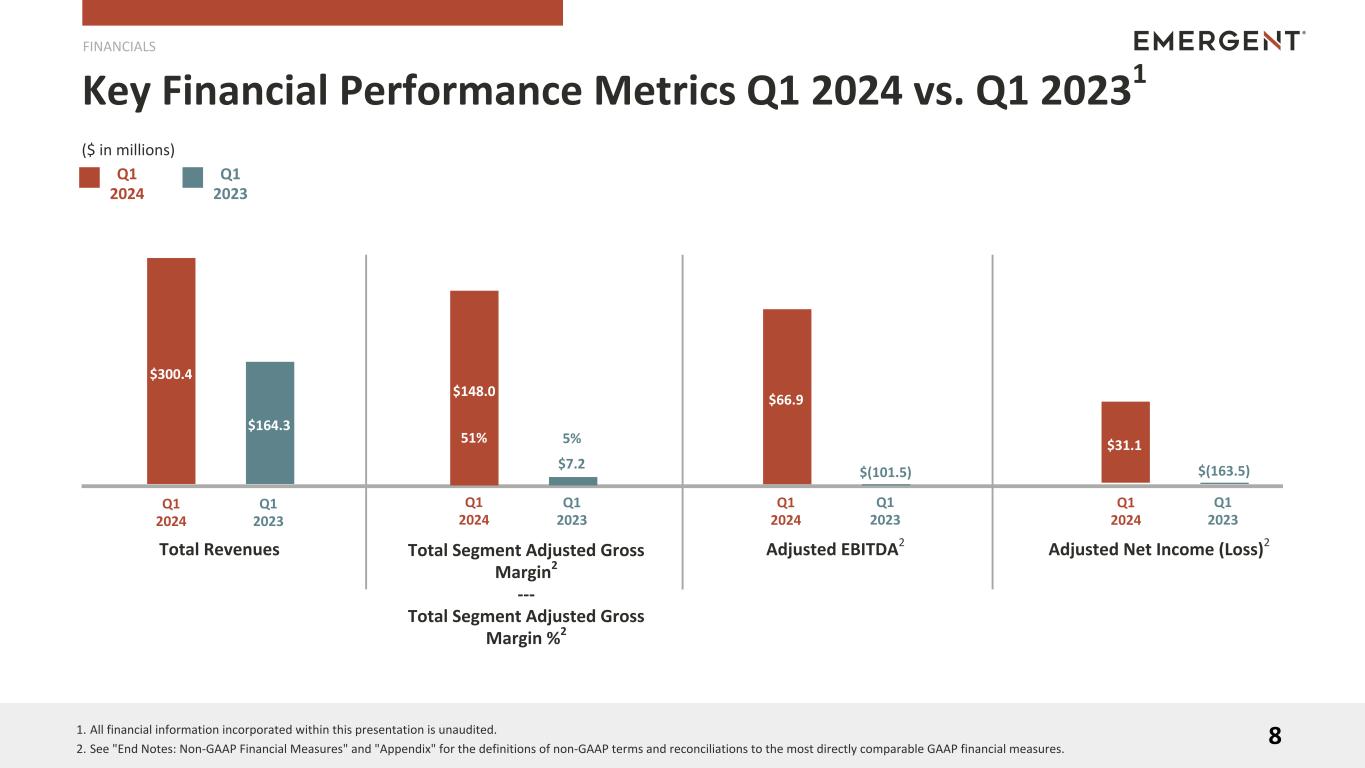
•First Quarter 2024 Total Revenues of $300.4 million, above the prior guidance range
•First Quarter 2024 Net Income of $9.0 million and Adjusted EBITDA of $66.9 million
•Updates FY 2024 guidance
GAITHERSBURG, Md., May 1, 2024—Emergent BioSolutions Inc. (NYSE: EBS) today reported financial results for the first quarter ended March 31, 2024.
"We delivered a strong quarter with growth across all our key products,” said Joe Papa, President and CEO at Emergent. “We also took significant actions to improve our debt position, reduce operating expenses and strengthen our financial flexibility. Emergent's transformation will not happen overnight. The actions we are implementing today, combined with the assets Emergent possesses, will enable us to move faster, reach farther and be more nimble. The public health threats we collectively face are changing, and so is Emergent.”
FINANCIAL HIGHLIGHTS (1)
Q1 2024 vs. Q1 2023
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in millions, except per share amounts) |
Q1 2024 |
Q1 2023 |
% Change |
| Total Revenues |
$ |
300.4 |
|
$ |
164.3 |
|
83 |
% |
| Net Income (Loss) |
$ |
9.0 |
|
$ |
(186.2) |
|
105 |
% |
| Net Income (Loss) per Diluted Share |
$ |
0.17 |
|
$ |
(3.71) |
|
105 |
% |
Adjusted Net Income (Loss) (2) |
$ |
31.1 |
|
$ |
(163.5) |
|
119 |
% |
Adjusted Net Income (Loss) per Diluted Share (2) |
$ |
0.59 |
|
$ |
(3.26) |
|
118 |
% |
Adjusted EBITDA (2) |
$ |
66.9 |
|
$ |
(101.5) |
|
166 |
% |
Total Segment Gross Margin % (2) |
51 |
% |
3 |
% |
|
Total Segment Adjusted Gross Margin % (2) |
51 |
% |
5 |
% |
|
|
|
|
|
|
|
|
|
SELECT Q1 2024 AND OTHER RECENT BUSINESS UPDATES
•Appointed industry leader Joseph C. Papa as new President, CEO and Director
•Was awarded procurement contract valued up to $235.8 Million to supply BioThrax® (Anthrax Vaccine Adsorbed) to the U.S. Department of Defense
•Received "no action indicated" (NAI) status for Baltimore Bayview Manufacturing Facility
•Continued progress on strengthening our fundamentals with key focus on our Medical Countermeasure (“MCM”) and NARCAN® products
•Amended our senior secured credit facility
FIRST QUARTER 2024 FINANCIAL PERFORMANCE (1)
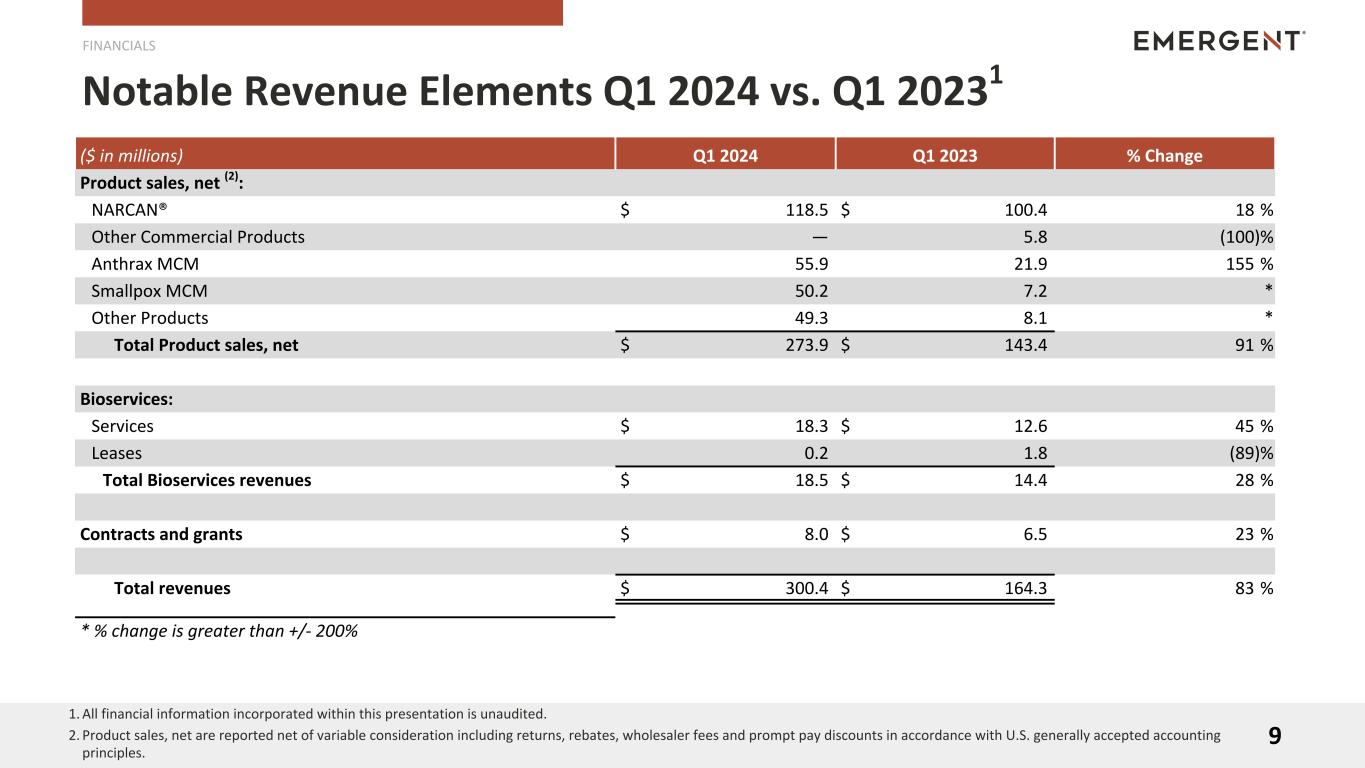
Revenues
The Company uses the following categories in discussing product/service level revenues:
•NARCAN® — comprises contributions from NARCAN® Nasal Spray
•Other Commercial Products - comprises contributions from Vaxchora® and Vivotif®, which we sold to Bavarian Nordic as part of our travel health business in May 2023
•Anthrax MCM — comprises potential contributions from CYFENDUS®, previously known as AV7909, BioThrax®, Anthrasil® and Raxibacumab
•Smallpox MCM — comprises potential contributions from ACAM2000®, VIGIV and TEMBEXA®
•Other Products — comprises potential contributions from BAT®, RSDL® and Trobigard®
•Bioservices — comprises service and lease revenues from the Bioservices business
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in millions) |
Q1 2024 |
Q1 2023 |
% Change |
Product sales, net (3): |
|
|
|
NARCAN® |
$ |
118.5 |
|
$ |
100.4 |
|
18 |
% |
| Other Commercial Products |
— |
|
5.8 |
|
(100) |
% |
| Anthrax MCM |
55.9 |
|
21.9 |
|
155 |
% |
| Smallpox MCM |
50.2 |
|
7.2 |
|
* |
| Other Products |
49.3 |
|
8.1 |
|
* |
| Total Product sales, net |
$ |
273.9 |
|
$ |
143.4 |
|
91 |
% |
|
|
|
|
| Bioservices: |
|
|
|
| Services |
$ |
18.3 |
|
$ |
12.6 |
|
45 |
% |
| Leases |
0.2 |
|
1.8 |
|
(89) |
% |
| Total Bioservices revenues |
$ |
18.5 |
|
$ |
14.4 |
|
28 |
% |
|
|
|
|
| Contracts and grants |
$ |
8.0 |
|
$ |
6.5 |
|
23 |
% |
|
|
|
|
| Total revenues |
$ |
300.4 |
|
$ |
164.3 |
|
83 |
% |
|
|
|
|
| * % change is greater than +/- 200% |
|
|
|
|
|
Products Revenue, net
NARCAN®
For Q1 2024, revenues from NARCAN® (naloxone HCl) Nasal Spray increased $18.1 million, or 18%, as compared with Q1 2023. The increase was primarily driven by higher branded NARCAN® sales to U.S. public interest channels and sales of OTC NARCAN®, partially offset by lower Canadian retail sales of branded NARCAN®.
Other Commercial Products
For Q1 2024, revenues from Other Commercial Products decreased $5.8 million, or 100%, as compared with Q1 2023. The decrease was due to no sales of our Vaxchora® and Vivotif® products during the current quarter, which we sold to Bavarian Nordic as part of our travel health business in May 2023.
Anthrax MCM
For Q1 2024, revenues from Anthrax MCM increased $34.0 million, or 155%, as compared with Q1 2023. The increase reflects the impact of timing of sales related to CYFENDUS® and BioThrax®. Anthrax vaccine product sales are primarily made under annual purchase options exercised by the USG. Fluctuations in revenues result from the timing of the exercise of annual purchase options, the timing of USG purchases, the availability of governmental funding and Company delivery of orders that follow.
Smallpox MCM
For Q1 2024, revenues from Smallpox MCM increased $43.0 million as compared with Q1 2023. The increase was primarily due to higher VIGIV and ACAM2000® sales due to timing. Fluctuations in revenues from Smallpox MCM result from the timing of the exercise of annual purchase options in the existing procurement contracts, the timing of USG purchases, the availability of governmental funding and Company delivery of orders that follow.
Other Products
For Q1 2024, revenues from other product sales increased $41.2 million as compared with Q1 2023. The increase was primarily due to higher BAT and RSDL® product sales due to timing.
Bioservices Revenues
Services
For Q1 2024, revenues from Bioservices services increased $5.7 million, or 45%, as compared with Q1 2023. The increase was driven by an increase in production at the Company's Camden facility, partially offset by decreases in production at the Company’s Canton and Winnipeg facilities.
Leases
For Q1 2024, revenues from Bioservices leases decreased $1.6 million, or 89%, as compared with Q1 2023. The decrease was related to the completion of a lease for a Bioservices customer at our Canton facility.
Contracts and Grants
For Q1 2024, revenues from contracts and grants increased $1.5 million, or 23%, as compared with Q1 2023. The increase was due to development work in connection with EbangaTM, partially offset by the close out of other development initiatives.
Operating Expenses
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in millions) |
Q1 2024 |
Q1 2023 |
% Change |
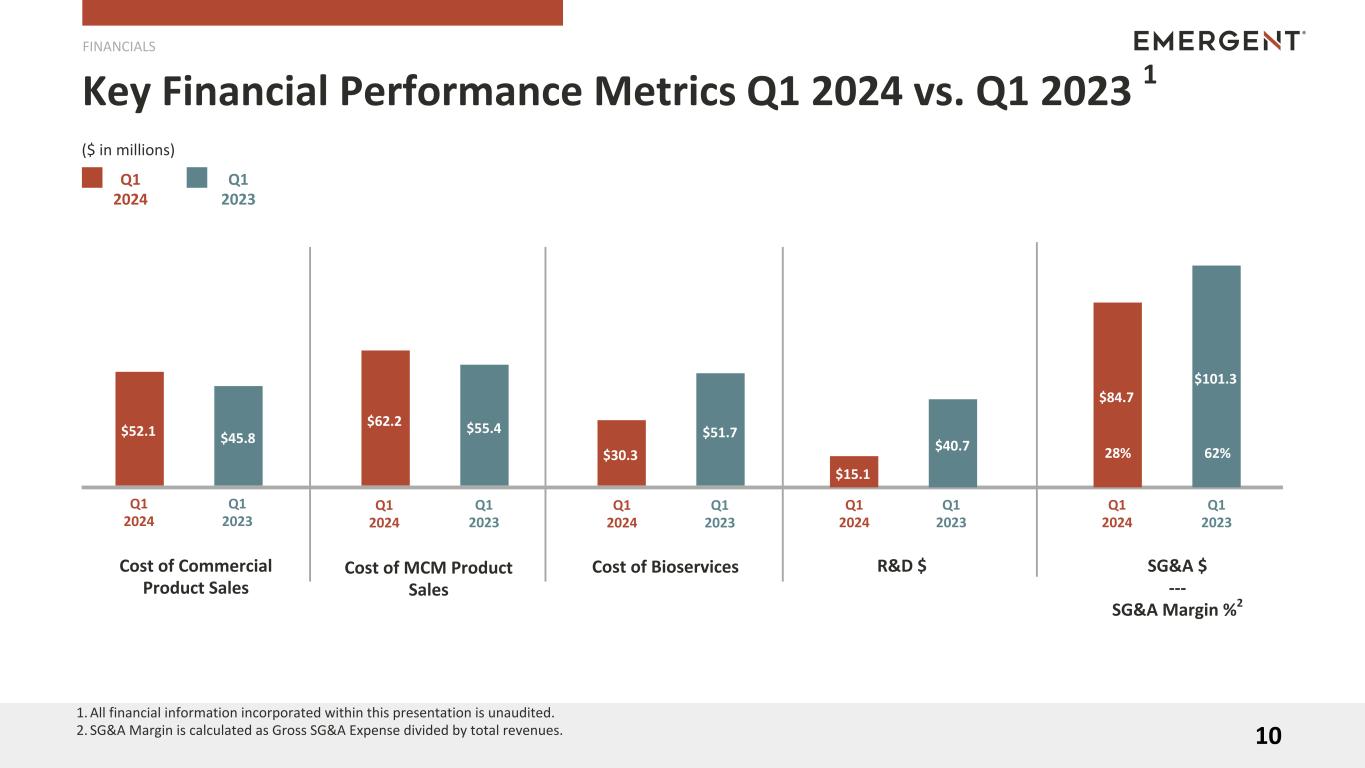
| Cost of Commercial product sales |
$ |
52.1 |
|
$ |
45.8 |
|
14 |
% |
Cost of MCM product sales |
62.2 |
|
55.4 |
|
12 |
% |
| Cost of Bioservices |
30.3 |
|
51.7 |
|
(41) |
% |
|
|
|
|
|
|
|
|
| Research and development (“R&D”) |
15.1 |
|
40.7 |
|
(63) |
% |
| Selling, general and administrative (“SG&A”) |
84.7 |
|
101.3 |
|
(16) |
% |
| Amortization of intangible assets |
16.2 |
|
17.0 |
|
(5) |
% |
| Total operating expenses |
$ |
260.6 |
|
$ |
311.9 |
|
(16) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
Cost of Commercial Product Sales
For Q1 2024, cost of Commercial product sales increased $6.3 million, or 14%, as compared with Q1 2023. The increase was primarily due to higher sales of OTC NARCAN®, which launched during the third quarter of 2023, partially offset by lower Canadian retail sales of branded NARCAN®.
Cost of MCM Product Sales
For Q1 2024, cost of MCM product sales increased $6.8 million, or 12%, as compared with Q1 2023. The increase was primarily due to higher sales of BAT®, VIGIV, BioThrax® and CYFENDUS®, partially offset by a decrease in shutdown costs.
Cost of Bioservices
For Q1 2024, cost of Bioservices decreased $21.4 million, or 41%, as compared with Q1 2023. The decrease was primarily due to a reduction in overhead costs at the Company’s Camden and Bayview facilities, coupled with no production activities at the Company’s Canton facility. The decrease in costs at the Camden facility was partially offset by increases in production activity following the remediation of matters related to the FDA's warning letter, which was closed out in October 2023.
Research and Development Expenses
For Q1 2024, R&D expenses decreased $25.6 million, or 63%, as compared with Q1 2023. The decrease was primarily due to the sale of our development program for CHIKV VLP to Bavarian Nordic and reduction in related overhead costs, as well as a reduction in overhead costs driven by the headcount reductions as a result of restructuring. The decrease was partially offset by an increase in expense related to development activities for EbangaTM.
Selling, General and Administrative Expenses
For Q1 2024, SG&A expenses decreased $16.6 million, or 16%, as compared with Q1 2023. The decrease was primarily due to decreases in compensation and other employee costs as a result of the restructuring initiatives that began during the first quarter of 2023 and a reduction in professional services fees related to general corporate initiatives in the prior year, including organizational transformation consulting fees. These decreases were partially offset by an increase in marketing expenses related to the launch of OTC NARCAN® and higher legal services fees.
ADDITIONAL FINANCIAL INFORMATION (1)
Capital Expenditures
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in millions) |
Q1 2024 |
Q1 2023 |
% Change |
| Capital expenditures |
$ |
10.8 |
|
$ |
15.1 |
|
(28) |
% |
|
|
|
|
|
|
|
|
| Capital expenditures as a % of total revenues |
4 |
% |
9 |
% |
|
|
|
|
|
|
|
|
|
|
For Q1 2024, capital expenditures decreased largely due to lower product development activities across the Company’s facilities.
SEGMENT INFORMATION
In the fourth quarter of 2023, we realigned our reportable operating segments to reflect recent changes in our internal operating and reporting process. The Company now manages the business with a focus on three reportable segments: (1) a Commercial Products segment consisting of our NARCAN® and other commercial products which were sold as part of our travel health business in the second quarter of 2023; (2) a MCM Products segment consisting of the Anthrax - MCM, Smallpox - MCM and Other products and (3) a services segment (“Services”) consisting of our Bioservices. The Company evaluates the performance of these reportable segments based on revenue and segment adjusted gross margin, which is a non-GAAP financial measure. Segment revenue includes external customer sales, but does not include inter-segment services. The Company does not allocate contracts and grants, R&D, SG&A, amortization of intangible assets, interest and other income (expense) or taxes to its evaluation of the performance of these segments.
FIRST QUARTER 2024 SEGMENT RESULTS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in millions) |
Commercial Products |
| Quarter Ended March 31, |
| 2024 |
2023 |
$ Change |
% Change |
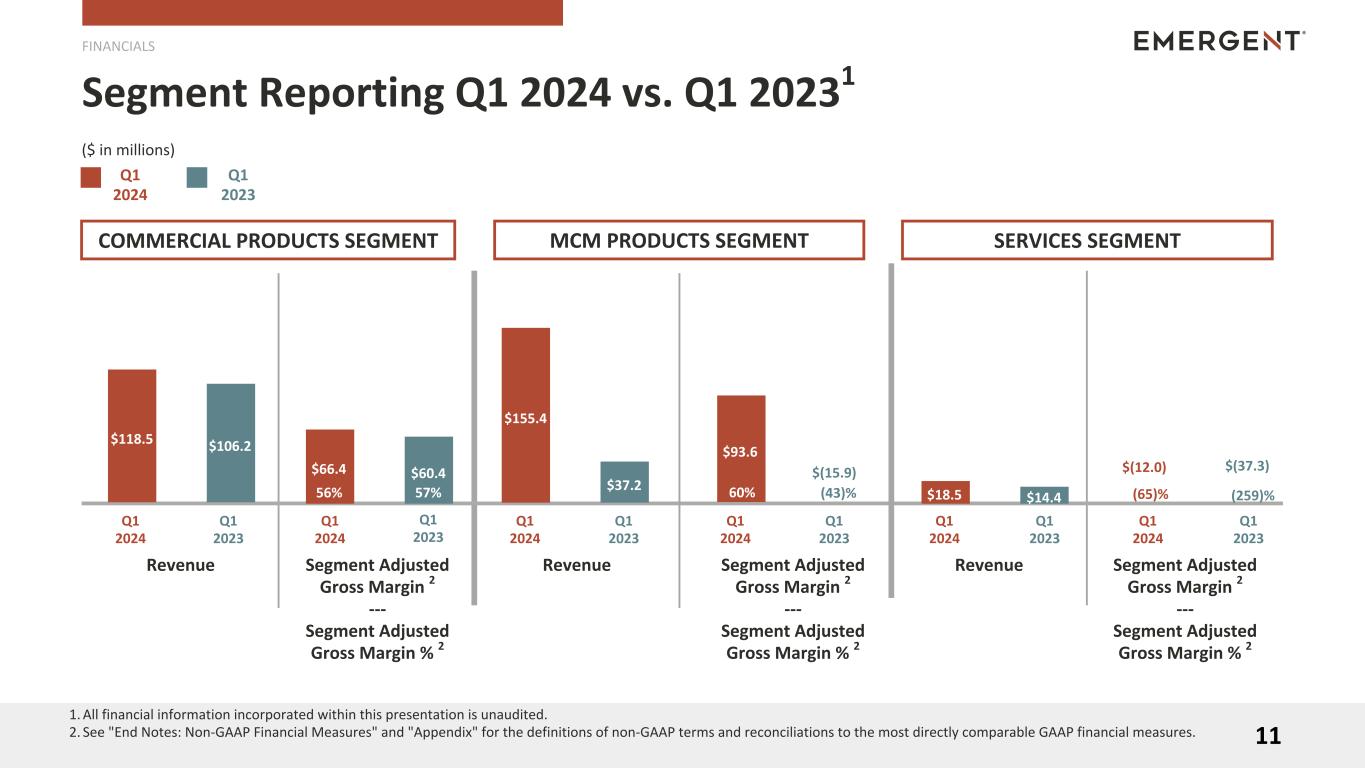
| Revenues |
$ |
118.5 |
|
$ |
106.2 |
|
$ |
12.3 |
|
12 |
% |
| Cost of sales |
52.1 |
|
45.8 |
|
6.3 |
|
14 |
% |
| Gross margin ** |
$ |
66.4 |
|
$ |
60.4 |
|
$ |
6.0 |
|
10 |
% |
| Gross margin % ** |
56 |
% |
57 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
Segment adjusted gross margin (2) |
$ |
66.4 |
|
$ |
60.4 |
|
$ |
6.0 |
|
10 |
% |
Segment adjusted gross margin % (2) |
56 |
% |
57 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
| ** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues. |
|
|
Commercial Products gross margin increased $6.0 million, or 10%, to $66.4 million in the quarter, as compared with $60.4 million in the prior year quarter. Commercial Products gross margin percentage decreased 1 percentage point to 56% for the quarter ended March 31, 2024. The decrease was largely due to the higher sales of OTC NARCAN® and lower branded NARCAN® sales. Commercial Products segment adjusted gross margin is consistent with gross margin.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in millions) |
MCM Products |
| Quarter Ended March 31, |
| 2024 |
2023 |
$ Change |
% Change |
| Revenues |
$ |
155.4 |
|
$ |
37.2 |
|
$ |
118.2 |
|
* |
| Cost of sales |
62.2 |
|
55.4 |
|
6.8 |
|
12 |
% |
| Gross margin ** |
$ |
93.2 |
|
$ |
(18.2) |
|
$ |
111.4 |
|
* |
| Gross margin % ** |
60 |
% |
(49) |
% |
|
|
| Add back: |
|
|
|
|
| Changes in fair value of contingent consideration |
$ |
0.5 |
|
$ |
0.3 |
|
$ |
0.2 |
|
67 |
% |
|
|
|
|
|
| Restructuring costs |
(0.1) |
|
2.0 |
|
(2.1) |
|
(105) |
% |
Segment adjusted gross margin (2) |
$ |
93.6 |
|
$ |
(15.9) |
|
$ |
109.5 |
|
* |
Segment adjusted gross margin % (2) |
60 |
% |
(43) |
% |
|
|
|
|
|
|
|
| * % change is greater than +/- 200% |
|
|
|
|
| ** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues. |
|
|
MCM Products gross margin increased $111.4 million to $93.2 million in the quarter, as compared with $(18.2) million in the prior year quarter. MCM Products gross margin percentage increased 109 percentage points to 60% for the quarter ended March 31, 2024. The increase was primarily due to a favorable sales mix which was weighted more heavily towards higher margin products and a decrease in shutdown costs compared to the prior quarter. MCM Product segment adjusted gross margin in the current year period excludes the impact of non-cash items related to the changes in the fair value of contingent consideration of $0.5 million and the impact of restructuring costs of $(0.1) million.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in millions) |
Services |
| Quarter Ended March 31, |
| 2024 |
2023 |
$ Change |
% Change |
| Revenues |
$ |
18.5 |
|
$ |
14.4 |
|
$ |
4.1 |
|
28 |
% |
| Cost of services |
30.3 |
|
51.7 |
|
(21.4) |
|
(41) |
% |
| Gross margin ** |
$ |
(11.8) |
|
$ |
(37.3) |
|
$ |
25.5 |
|
68 |
% |
| Gross margin % ** |
(64) |
% |
(259) |
% |
|
|
| Add back: |
|
|
|
|
| Restructuring costs |
$ |
(0.2) |
|
$ |
— |
|
$ |
(0.2) |
|
NM |
|
|
|
|
|
Segment adjusted gross margin (2) |
$ |
(12.0) |
|
$ |
(37.3) |
|
$ |
25.3 |
|
68 |
% |
Segment adjusted gross margin % (2) |
(65) |
% |
(259) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
| ** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues. |
|
| NM - Not Meaningful |
|
|
|
|
Services gross margin increased $25.5 million, or 68%, to $(11.8) million in the quarter, as compared with $(37.3) million in the prior year quarter. Services gross margin percentage increased 195 percentage points to (64)% for the quarter ended March 31, 2024. The increase was primarily due to a reduction in overhead costs at the Company’s Camden and Bayview facilities, coupled with an increase in production at our Camden facility due to the remediation of matters related to the FDA's warning letter, which was closed out in October 2023. Services segment adjusted gross margin in the current year period excludes the impact of restructuring costs of $(0.2) million.
2024 FINANCIAL FORECAST
The Company provides the following updated financial forecast for full year 2024 and initial forecast for Q2 2024, in both instances reflecting management's expectations based on the most current information available.
Full Year 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
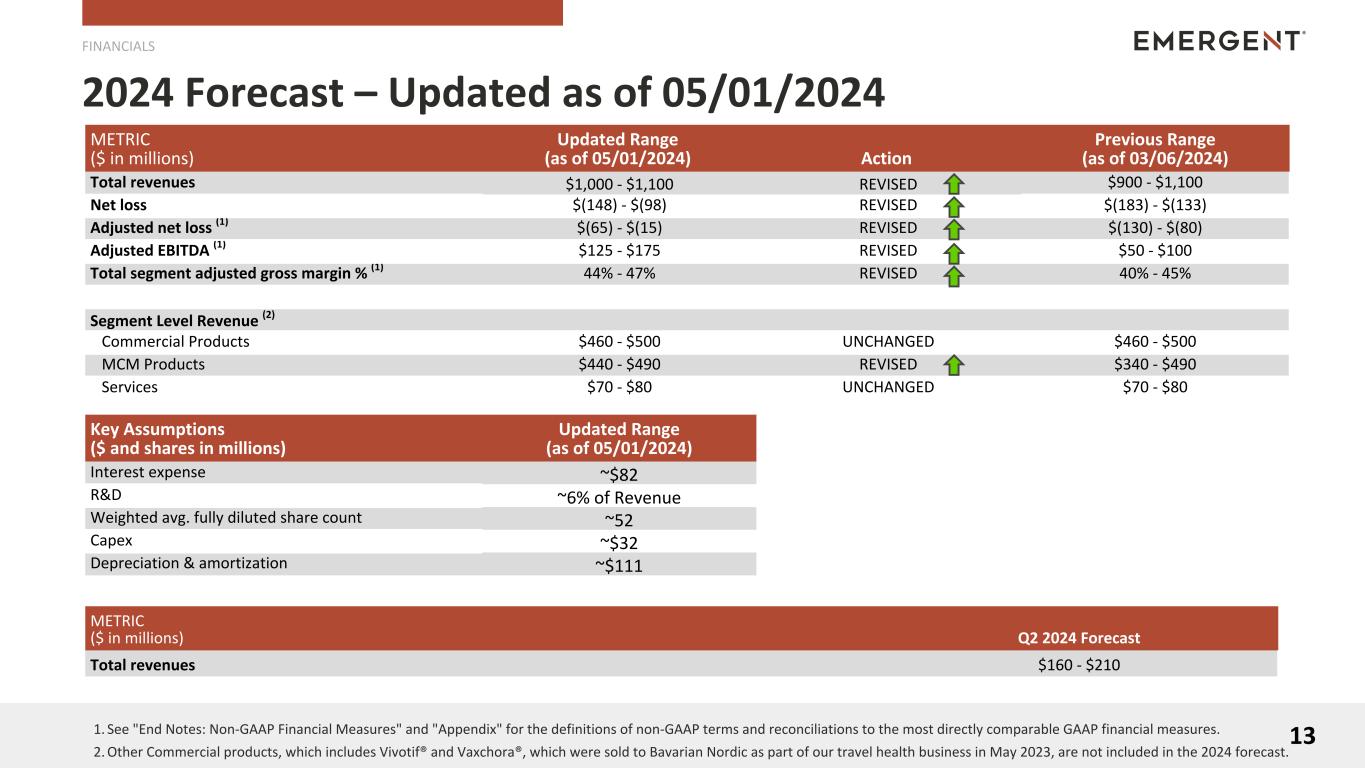
METRIC
($ in millions)
|
Updated Range
(as of 05/01/2024) |
Action |
Previous Range
(as of 03/06/2024) |
| Total revenues |
$1,000 - $1,100 |
REVISED |
$900 - $1,100 |
| Net loss |
$(148) - $(98) |
REVISED |
$(183) - $(133) |
Adjusted net loss (2) |
$(65) - $(15) |
REVISED |
$(130) - $(80) |
Adjusted EBITDA (2) |
$125 - $175 |
REVISED |
$50 - $100 |
Total segment adjusted gross margin % (2) |
44% - 47% |
REVISED |
40% - 45% |
|
|
|
|
Segment Level Revenue (4) |
|
|
|
| Commercial Products |
$460 - $500 |
UNCHANGED |
$460 - $500 |
| MCM Products |
$440 - $490 |
REVISED |
$340 - $490 |
| Services |
$70 - $80 |
UNCHANGED |
$70 - $80 |
|
|
|
|
|
|
|
Key Assumptions
($ and shares in millions)
|
Updated Range
(as of 05/01/2024) |
| Interest expense |
~$82 |
| R&D |
~6% of Revenue |
| Weighted avg. fully diluted share count |
~52 |
| Capex |
~$32 |
| Depreciation & amortization |
~$111 |
Q2 2024
|
|
|
|
|
|
|
METRIC
($ in millions)
|
Q2 2024 Forecast |
| Total revenues |
$160 - $210 |
FOOTNOTES
(1) All financial information included in this release is unaudited.
(2) See “Non-GAAP Financial Measures” and the "Reconciliation of Non-GAAP Financial Measures" tables for the definitions and reconciliations of these non-GAAP financial measures to the most closely related GAAP financial measures.
(3) Product sales, net are reported net of variable consideration including returns, rebates, wholesaler fees and prompt pay discounts in accordance with U.S. generally accepted accounting principles.
(4) Other Commercial products, which includes Vivotif® and Vaxchora®, which were sold to Bavarian Nordic as part of our travel health business in May 2023, are not included in the 2024 forecast.
CONFERENCE CALL, PRESENTATION SUPPLEMENT AND WEBCAST INFORMATION
Company management will host a conference call at 5:00 pm eastern time today, May 1, 2024, to discuss these financial results. The conference call and presentation supplement can be accessed from the Company's website or through the following:
By phone
Advance registration is required.
Visit https://register.vevent.com/register/BI79219c258a3a4d65a99bbd1228238ad7 to register and receive an email with the dial-in number, passcode and registrant ID.
By webcast
Visit https://edge.media-server.com/mmc/p/rxbgqwyu/.
A replay of the call can be accessed from the Emergent website.
ABOUT EMERGENT BIOSOLUTIONS INC.
At Emergent, our mission is to protect and enhance life. We develop, manufacture, and deliver protections against public health threats through a pipeline of innovative vaccines and therapeutics. For over 20 years, we have been at work defending people from things we hope will never happen—so that we are prepared just in case they ever do. We do what we do because we see the opportunity to create a better, more secure world. One where preparedness empowers protection from the threats we face. And peace of mind prevails. In working together, we envision protecting or enhancing 1 billion lives by 2030. For more information, visit our website and follow us on LinkedIn, Twitter, and Instagram.
NON-GAAP FINANCIAL MEASURES
In the accompanying analysis of financial information, we sometimes use information derived from consolidated and segment financial information that may not be presented in our financial statements or prepared in accordance with generally accepted accounting principles in the United States (“GAAP”). Certain of these financial measures are considered not in conformity with GAAP (“non-GAAP financial measures”) under the United States Securities and Exchange Commission (“SEC”) rules. Specifically, we have referred to the following non-GAAP financial measures:
•Adjusted Net Income (Loss)
•Adjusted Net Income (Loss) per Diluted Share
•Adjusted EBITDA
•Total Segment Revenues
•Total Segment Gross Margin
•Total Segment Gross Margin %
•Total Segment Adjusted Gross Margin
•Total Segment Adjusted Gross Margin %
•Segment Adjusted Gross Margin
•Segment Adjusted Gross Margin %
We define Adjusted Net Income (Loss) and Adjusted Net Income (Loss) per Diluted Share, which are non-GAAP financial measures, as net income (loss) and net income (loss) per diluted share, respectively, excluding the impact of changes in fair value of contingent consideration, acquisition and divestiture-related costs, severance and restructuring costs, other income (expense) items and non-cash amortization charges. We use Adjusted Net Income (Loss) for the purpose of calculating Adjusted Net Income (Loss) per Diluted Share. Management uses Adjusted Net Income (Loss) per Diluted Share to assess total Company operating performance on a consistent basis. We believe that these non-GAAP financial measures, when considered together with our GAAP financial results and GAAP financial measures, provide management and investors with an additional understanding of our business operating results, including underlying trends.
We define Adjusted EBITDA, which is a non-GAAP financial measure, as consolidated net income (loss) before income tax provision (benefit), interest expense, net, depreciation, amortization of intangible assets, changes in fair value of contingent consideration, severance and restructuring costs, other income (expense) items and acquisition and divestiture-related costs. We believe that this non-GAAP financial measure, when considered together with our GAAP financial results and GAAP financial measures, provides management and investors with a more complete understanding of our operating results, including underlying trends. In addition, EBITDA is a common alternative measure of operating performance used by many of our competitors. It is used by investors, financial analysts, rating agencies and others to value and compare the financial performance of companies in our industry, although it may be defined differently by different companies. Therefore, we also believe that this non-GAAP financial measure, considered along with corresponding GAAP financial measures, provides management and investors with additional information for comparison of our operating results with the operating results of other companies.
We have included the definitions of Segment Gross Margin and Segment Gross Margin %, which are GAAP financial measures, below in order to more fully define the components of certain non-GAAP financial measures presented in this press release. We define Segment Gross Margin, as a segment's revenues, less a segment's cost of sales or services. We define Segment Gross Margin %, as Segment Gross Margin as a percentage of a segments revenues. We define Segment Adjusted Gross Margin, which is a non-GAAP financial measure as Segment Gross Margin excluding the impact of restructuring costs and non-cash items related to changes in the fair value of contingent consideration. We define Segment Adjusted Gross Margin %, which is a non-GAAP financial measure, as Segment Adjusted Gross Margin as a percentage of a segment's revenues.
We define Total Segment Revenues, which is a non-GAAP financial measure, as our Total Revenues, less contracts and grants revenue, which is also equal to the sum of the revenues of our reportable operating segments. We define Total Segment Gross Margin, which is a non-GAAP financial measure, as Total Segment Revenues less our aggregate cost of sales or services. We define Total Segment Gross Margin %, which is a non-GAAP financial measure, as Total Segment Gross Margin as a percentage of Total Segment Revenues. We define Total Segment Adjusted Gross Margin, which is a non-GAAP financial measure, as Total Segment Gross Margin, excluding the impact of restructuring costs and changes in the fair value of contingent consideration. We define Total Segment Adjusted Gross Margin %, which is a non-GAAP financial measure, as Total Segment Adjusted Gross Margin as a percentage of Total Segment Revenues.
Non-GAAP financial measures are not defined in the same manner by all companies and may not be comparable with other similarly titled measures of other companies. The determination of the amounts that are excluded from these non-GAAP financial measures are a matter of management judgment and depend upon, among other factors, the nature of the underlying expense or income amounts. Non-GAAP financial measures should be considered in addition to, but not as a substitute for or superior to, the information contained in our Consolidated Statements of Operations and Consolidated Statements of Cash Flows. Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures are included in the financial tables accompanying this press release.
SAFE HARBOR STATEMENT
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including statements regarding the future performance of the Company or any of our businesses, our business strategy, future operations, future financial position, future revenues and earnings, our ability to achieve the objectives of our restructuring initiatives, including our future results, projected costs, prospects, plans and objectives of management, are forward-looking statements. We generally identify forward-looking statements by using words like “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “future,” “goal,” “intend,” “may,” “plan,” “position,” “possible,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” and similar expressions or variations thereof, or the negative thereof, but these terms are not the exclusive means of identifying such statements. Forward-looking statements are based on our current intentions, beliefs and expectations regarding future events based on information that is currently available. We cannot guarantee that any forward-looking statement will be accurate. Readers should realize that if underlying assumptions prove inaccurate or if known or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Readers are, therefore, cautioned not to place undue reliance on any forward-looking statement contained herein. Any such forward-looking statement speaks only as of the date of this press release, and, except as required by law, we do not undertake any obligation to update any forward-looking statement to reflect new information, events or circumstances.
There are a number of important factors that could cause our actual results to differ materially from those indicated by such forward-looking statements, including, among others, the availability of USG funding for contracts related to procurement of our medical countermeasure ("MCM") products, including CYFENDUS® (Anthrax Vaccine Adsorbed (AVA), Adjuvanted), BioThrax® (Anthrax Vaccine Adsorbed), and ACAM2000®, (Smallpox (Vaccinia) Vaccine, Live), among others, as well as contracts related to development of medical countermeasures; the availability of government funding for our other commercialized products, including EbangaTM (ansuvimab-zykl), BAT® (Botulism Antitoxin Heptavalent (A,B,C,D,E,F,G)-(Equine)) and RSDL® (Reactive Skin Decontamination Lotion Kit); our ability to meet our commitments to quality and compliance in all of our manufacturing operations; our ability to negotiate additional USG procurement or follow-on contracts for our MCM products that have expired or will be expiring; the commercial availability and acceptance of over-the-counter NARCAN® (naloxone HCl) Nasal Spray; the impact of the generic marketplace on NARCAN® (naloxone HCI) Nasal Spray and future NARCAN® sales; our ability to perform under our contracts with the USG, including the timing of and specifications relating to deliveries; our ability to provide Bioservices for the development and/or manufacture of product and/or product candidates of our customers at required levels and on required timelines; the ability of our contractors and suppliers to maintain compliance with current good manufacturing practices and other regulatory obligations; our ability to negotiate further commitments related to the collaboration and deployment of capacity toward future commercial manufacturing under our existing Bioservices contracts; our ability to collect reimbursement for raw materials and payment of services fees from our Bioservices customers; the results of pending stockholder litigation and government investigations and their potential impact on our business; our ability to comply with the operating and financial covenants required by our senior secured credit facilities and the amended and restated credit agreement relating to such facilities, as amended from time to time, as well as our 3.875% Senior Unsecured Notes due 2028; our ability to maintain adequate internal control over financial reporting and to prepare accurate financial statements in a timely manner; our ability to resolve the going concern qualification in our consolidated financial statements and otherwise successfully manage our liquidity in order to continue as a going concern; the procurement of our product candidates by USG entities under regulatory authorities that permit government procurement of certain medical products prior to FDA marketing authorization, and corresponding procurement by government entities outside of the United States; our ability to realize the expected benefits of the sale of our travel health business to Bavarian Nordic; the impact of the organizational changes we announced in January 2023 and August 2023; our ability to identify and acquire companies, businesses, products or product candidates that satisfy our selection criteria; the impact of cyber security incidents, including the risks from the unauthorized access, interruption, failure or compromise of our information systems or those of our business partners, collaborators or other third parties; the success of our commercialization, marketing and manufacturing capabilities and strategy; and the accuracy of our estimates regarding future revenues, expenses, capital requirements and needs for additional financing. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Readers should consider this cautionary statement, as well as the risks identified in our periodic reports filed with the Securities and Exchange Commission, when evaluating our forward-looking statements.
Trademarks
Emergent®, BioThrax®, BaciThrax®, RSDL®, BAT®, Trobigard®, Anthrasil®, CNJ-016®, ACAM2000®, NARCAN®, CYFENDUS®, TEMBEXA® and any and all Emergent BioSolutions Inc. brands, products, services and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries. All other brands, products, services and feature names or trademarks are the property of their respective owners.
|
|
|
|
|
|
|
Investor Contact
Rich Lindahl
Executive Vice President, Chief Financial Officer
lindahlr@ebsi.com
|
Media Contact
Assal Hellmer
Vice President, Communications
mediarelations@ebsi.com
|
Emergent BioSolutions Inc.
Consolidated Balance Sheets
(unaudited, in millions, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
December 31, |
| 2024 |
|
2023 |
| ASSETS |
|
|
|
| Current assets: |
|
|
|
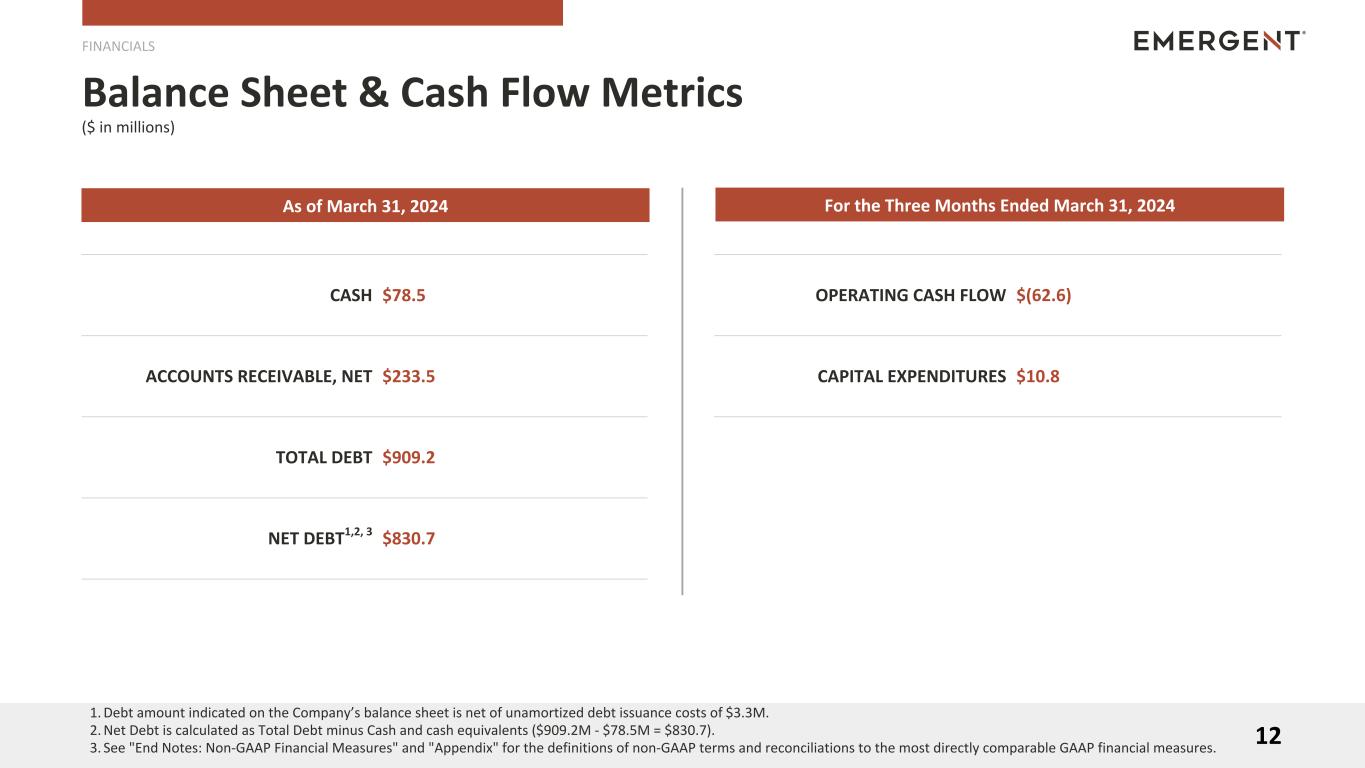
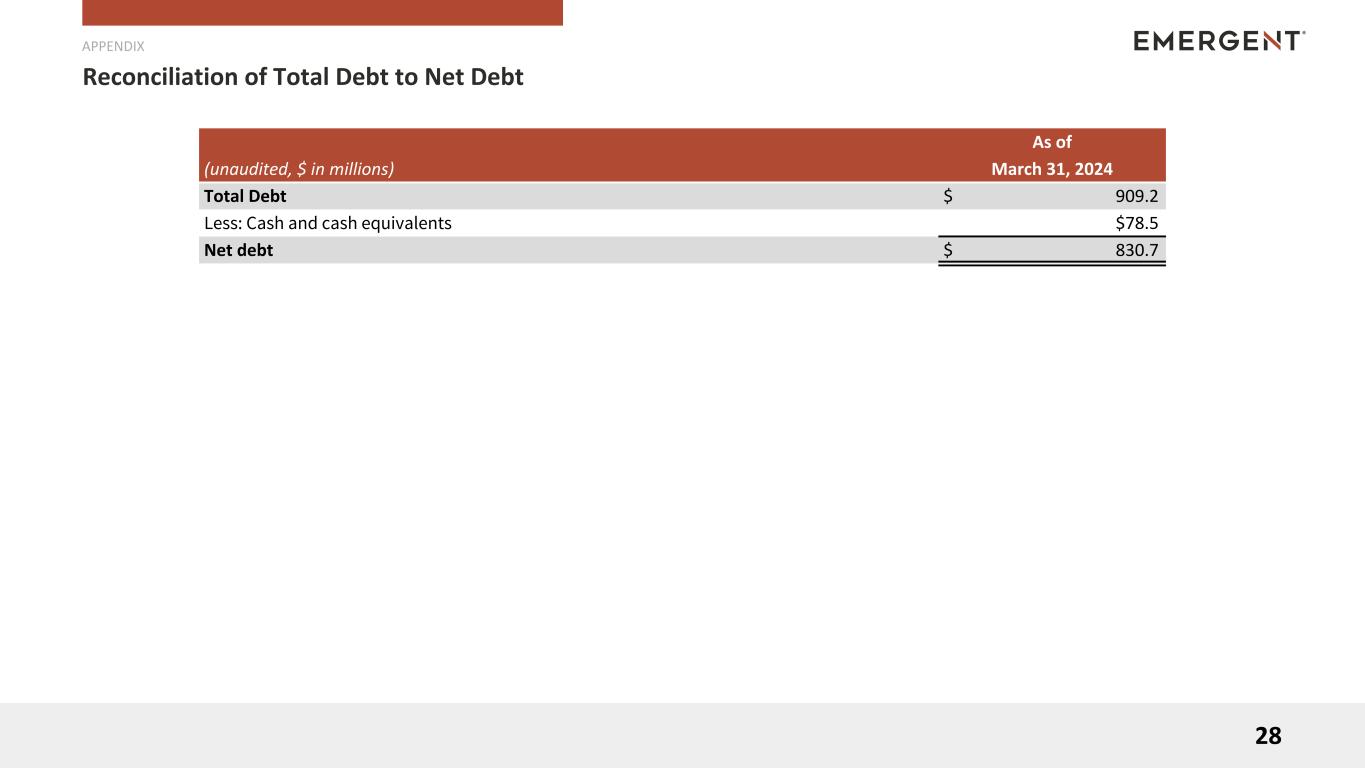
| Cash and cash equivalents |
$ |
78.5 |
|
|
$ |
111.7 |
|
| Restricted cash |
0.5 |
|
|
— |
|
| Accounts receivable, net |
233.5 |
|
|
191.0 |
|
| Inventories, net |
333.4 |
|
|
328.9 |
|
| Prepaid expenses and other current assets |
36.5 |
|
|
47.9 |
|
| Total current assets |
682.4 |
|
|
679.5 |
|
|
|
|
|
| Property, plant and equipment, net |
379.4 |
|
|
382.8 |
|
| Intangible assets, net |
550.4 |
|
|
566.6 |
|
|
|
|
|
| Other assets |
191.4 |
|
|
194.3 |
|
| Total assets |
$ |
1,803.6 |
|
|
$ |
1,823.2 |
|
|
|
|
|
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
| Current liabilities: |
|
|
|
| Accounts payable |
$ |
100.2 |
|
|
$ |
112.2 |
|
| Accrued expenses |
14.3 |
|
|
18.6 |
|
| Accrued compensation |
42.0 |
|
|
74.1 |
|
| Debt, current portion |
459.2 |
|
|
413.7 |
|
| Other current liabilities |
14.5 |
|
|
32.7 |
|
| Total current liabilities |
630.2 |
|
|
651.3 |
|
|
|
|
|
| Debt, net of current portion |
446.7 |
|
|
446.5 |
|
| Deferred tax liability |
34.8 |
|
|
47.2 |
|
| Other liabilities |
28.0 |
|
|
28.9 |
|
| Total liabilities |
$ |
1,139.7 |
|
|
$ |
1,173.9 |
|
|
|
|
|
| Stockholders’ equity: |
|
|
|
Preferred stock, $0.001 par value per share; 15.0 shares authorized, no shares issued and outstanding |
— |
|
|
— |
|
Common stock, $0.001 par value per share; 200.0 shares authorized, 58.0 and 57.8 shares issued; 52.4 and 52.2 shares outstanding, respectively. |
0.1 |
|
|
0.1 |
|
Treasury stock, at cost, 5.6 and 5.6 common shares, respectively |
(227.7) |
|
|
(227.7) |
|
| Additional paid-in capital |
909.8 |
|
|
904.4 |
|
| Accumulated other comprehensive loss, net |
(5.5) |
|
|
(5.7) |
|
| Accumulated deficit |
(12.8) |
|
|
(21.8) |
|
| Total stockholders’ equity |
$ |
663.9 |
|
|
$ |
649.3 |
|
| Total liabilities and stockholders’ equity |
$ |
1,803.6 |
|
|
$ |
1,823.2 |
|
Emergent BioSolutions Inc.
Consolidated Statements of Operations
(unaudited, in millions, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
|
| 2024 |
|
2023 |
|
|
|
|
| Revenues: |
|
|
|
|
|
|
|
| Commercial Product sales |
$ |
118.5 |
|
|
$ |
106.2 |
|
|
|
|
|
| MCM Product sales |
155.4 |
|
|
37.2 |
|
|
|
|
|
| Total Product sales, net |
273.9 |
|
|
143.4 |
|
|
|
|
|
| Bioservices: |
|
|
|
|
|
|
|
| Services |
18.3 |
|
|
12.6 |
|
|
|
|
|
| Leases |
0.2 |
|
|
1.8 |
|
|
|
|
|
| Total Bioservices revenues |
18.5 |
|
|
14.4 |
|
|
|
|
|
| Contracts and grants |
8.0 |
|
|
6.5 |
|
|
|
|
|
| Total revenues |
300.4 |
|
|
164.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
| Cost of Commercial Product sales |
52.1 |
|
|
45.8 |
|
|
|
|
|
| Cost of MCM Product sales |
62.2 |
|
|
55.4 |
|
|
|
|
|
| Cost of Bioservices |
30.3 |
|
|
51.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
15.1 |
|
|
40.7 |
|
|
|
|
|
| Selling, general and administrative |
84.7 |
|
|
101.3 |
|
|
|
|
|
| Amortization of intangible assets |
16.2 |
|
|
17.0 |
|
|
|
|
|
| Total operating expenses |
260.6 |
|
|
311.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Income (loss) from operations |
39.8 |
|
(147.6) |
|
|
|
|
|
|
|
|
|
|
|
|
| Other income (expense): |
|
|
|
|
|
|
|
| Interest expense |
(24.3) |
|
|
(17.9) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other, net |
(3.4) |
|
|
4.9 |
|
|
|
|
|
| Total other income (expense), net |
(27.7) |
|
|
(13.0) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Income (loss) before income taxes |
12.1 |
|
|
(160.6) |
|
|
|
|
|
| Income tax provision |
3.1 |
|
|
25.6 |
|
|
|
|
|
| Net income (loss) |
$ |
9.0 |
|
|
$ |
(186.2) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net income (loss) per common share |
|
|
|
|
|
|
|
| Basic |
$ |
0.17 |
|
|
$ |
(3.71) |
|
|
|
|
|
| Diluted |
$ |
0.17 |
|
|
$ |
(3.71) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares used in computing net income (loss) per common share |
|
|
|
|
|
|
|
| Basic |
52.2 |
|
50.2 |
|
|
|
|
| Diluted |
52.2 |
|
50.2 |
|
|
|
|
|
|
|
|
|
|
|
|
Emergent BioSolutions Inc.
Consolidated Statements of Cash Flows
(unaudited, in millions)
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
| 2024 |
|
2023 |
| Operating Activities |
|
|
|
| Net income (loss) |
$ |
9.0 |
|
|
$ |
(186.2) |
|
| Adjustments to reconcile net income (loss) to net cash used in operating activities: |
|
|
|
| Share-based compensation expense |
5.9 |
|
|
6.8 |
|
| Depreciation and amortization |
27.9 |
|
|
34.6 |
|
| Change in fair value of contingent obligations, net |
0.5 |
|
|
0.3 |
|
| Amortization of deferred financing costs |
6.9 |
|
|
1.0 |
|
| Deferred income taxes |
(12.2) |
|
|
(4.7) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other |
(3.1) |
|
|
0.3 |
|
| Changes in operating assets and liabilities: |
|
|
|
| Accounts receivable |
(50.0) |
|
|
2.6 |
|
| Inventories |
(4.5) |
|
|
(30.7) |
|
| Prepaid expenses and other assets |
(6.0) |
|
|
(4.5) |
|
| Accounts payable |
(2.4) |
|
|
31.0 |
|
| Accrued expenses and other liabilities |
1.1 |
|
|
(14.7) |
|
| Long-term incentive plan accrual |
1.2 |
|
|
— |
|
| Accrued compensation |
(33.3) |
|
|
(24.3) |
|
| Income taxes receivable and payable, net |
16.1 |
|
|
12.9 |
|
| Contract liabilities |
(19.7) |
|
|
(8.4) |
|
| Net cash used in operating activities |
(62.6) |
|
|
(184.0) |
|
| Investing Activities |
|
|
|
| Purchases of property, plant and equipment |
(10.8) |
|
|
(15.1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash used in investing activities |
(10.8) |
|
|
(15.1) |
|
| Financing Activities |
|
|
|
|
|
|
|
| Proceeds from revolving credit facility |
50.0 |
|
|
— |
|
| Principal payments on revolving credit facility |
(5.0) |
|
|
— |
|
|
|
|
|
|
|
|
|
| Principal payments on term loan facility |
(3.9) |
|
|
(8.4) |
|
|
|
|
|
| Taxes paid for share-based compensation activity |
(0.4) |
|
|
(2.1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash provided by (used in) financing activities: |
40.7 |
|
|
(10.5) |
|
| Effect of exchange rate changes on cash, cash equivalents and restricted cash |
— |
|
|
(0.2) |
|
| Net change in cash, cash equivalents and restricted cash |
(32.7) |
|
|
(209.8) |
|
| Add: Net change in cash, classified within current assets held for sale |
— |
|
|
(2.6) |
|
| Cash, cash equivalents and restricted cash, beginning of period |
111.7 |
|
|
642.6 |
|
| Cash, cash equivalents and restricted cash, end of period |
$ |
79.0 |
|
|
$ |
430.2 |
|
| Supplemental cash flow disclosures: |
|
|
|
| Cash paid for interest |
$ |
21.5 |
|
|
$ |
21.6 |
|
| Cash paid for income taxes |
$ |
12.4 |
|
|
$ |
16.7 |
|
| Non-cash investing and financing activities: |
|
|
|
| Purchases of property, plant and equipment unpaid at period end |
$ |
3.3 |
|
|
$ |
7.8 |
|
|
|
|
|
| Gain on extinguishment of debt |
$ |
0.3 |
|
|
$ |
— |
|
| Reconciliation of cash and cash equivalents and restricted cash: |
|
|
|
| Cash and cash equivalents |
$ |
78.5 |
|
|
$ |
430.2 |
|
|
|
|
|
| Restricted cash |
0.5 |
|
|
— |
|
| Total |
$ |
79.0 |
|
|
$ |
430.2 |
|
Emergent BioSolutions, Inc.
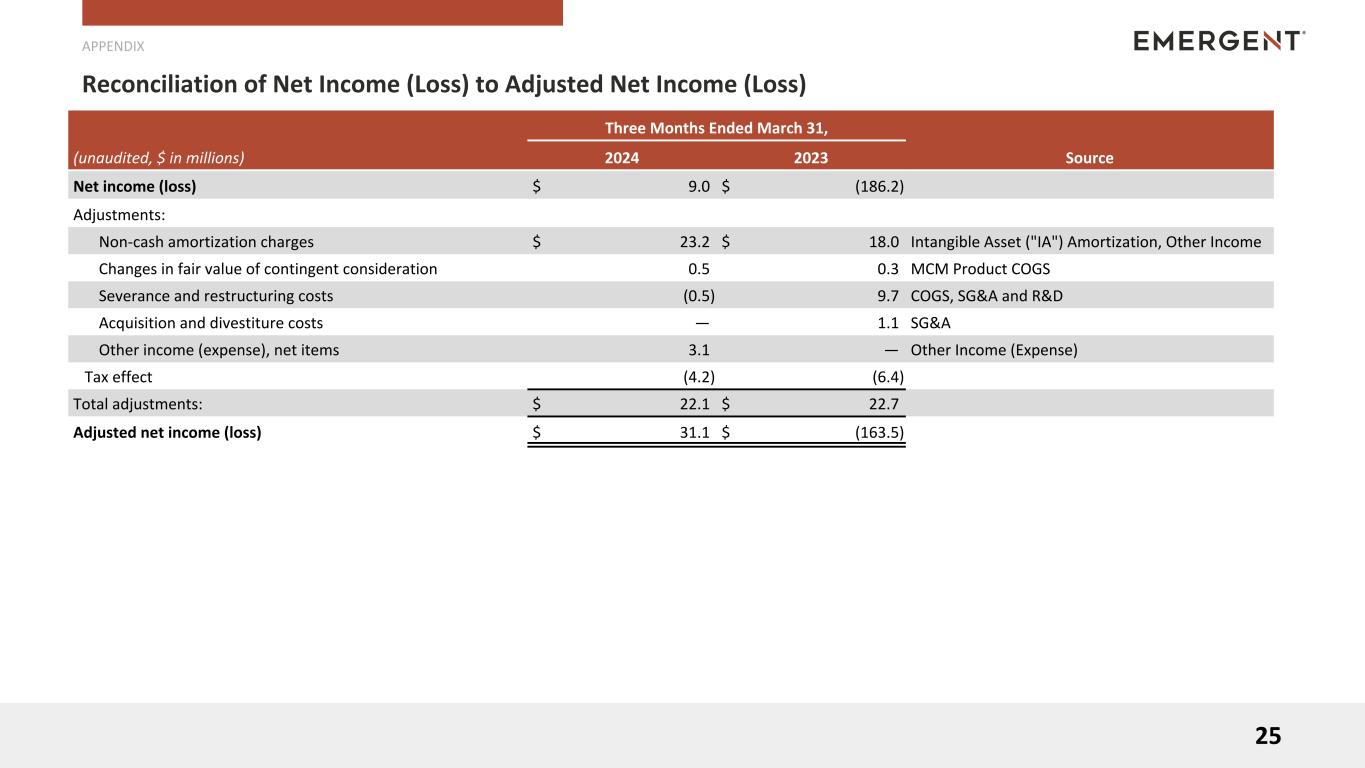
Reconciliation of Non-GAAP Financial Measures
Reconciliation of Net Income (Loss) and Net Income (Loss) per Diluted Share to Adjusted Net Income (Loss)
and Adjusted Net Income (Loss) per Diluted Share(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in millions, except per share value) |
Three Months Ended March 31, |
|
|
|
| 2024 |
2023 |
|
|
|
Source |
| Net income (loss) |
$ |
9.0 |
|
$ |
(186.2) |
|
|
|
|
|
| Adjustments: |
|
|
|
|
|
|
| Non-cash amortization charges |
$ |
23.2 |
|
$ |
18.0 |
|
|
|
|
Intangible Asset ("IA") Amortization, Other Income |
| Changes in fair value of contingent consideration |
0.5 |
|
0.3 |
|
|
|
|
MCM Product COGS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Severance and restructuring costs |
(0.5) |
|
9.7 |
|
|
|
|
COGS, SG&A and R&D |
|
|
|
|
|
|
|
| Acquisition and divestiture costs |
— |
|
1.1 |
|
|
|
|
SG&A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other income (expense), net items |
3.1 |
|
— |
|
|
|
|
Other Income (Expense) |
| Tax effect |
(4.2) |
|
(6.4) |
|
|
|
|
|
| Total adjustments: |
$ |
22.1 |
|
$ |
22.7 |
|
|
|
|
|
| Adjusted net income (loss) |
$ |
31.1 |
|
$ |
(163.5) |
|
|
|
|
|
| Net income (loss) per diluted share |
$ |
0.17 |
|
$ |
(3.71) |
|
|
|
|
|
| Adjustments: |
|
|
|
|
|
|
| Non-cash amortization charges |
$ |
0.44 |
|
$ |
0.36 |
|
|
|
|
IA Amortization, Other Income |
| Changes in fair value of contingent consideration |
0.01 |
|
0.01 |
|
|
|
|
MCM Product COGS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Severance and restructuring costs |
(0.01) |
|
0.19 |
|
|
|
|
COGS, SG&A and R&D |
|
|
|
|
|
|
|
| Acquisition and divestiture costs |
— |
|
0.02 |
|
|
|
|
SG&A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other income (expense), net items |
0.06 |
|
— |
|
|
|
|
Other Income (Expense) |
| Tax effect |
(0.08) |
|
(0.13) |
|
|
|
|
|
| Total adjustments: |
$ |
0.42 |
|
$ |
0.45 |
|
|
|
|
|
| Adjusted net income (loss) per diluted share |
$ |
0.59 |
|
$ |
(3.26) |
|
|
|
|
|
| Diluted shares used in computing Adjusted net income (loss) per diluted share |
52.2 |
|
50.2 |
|
|
|
|
|
Emergent BioSolutions, Inc.
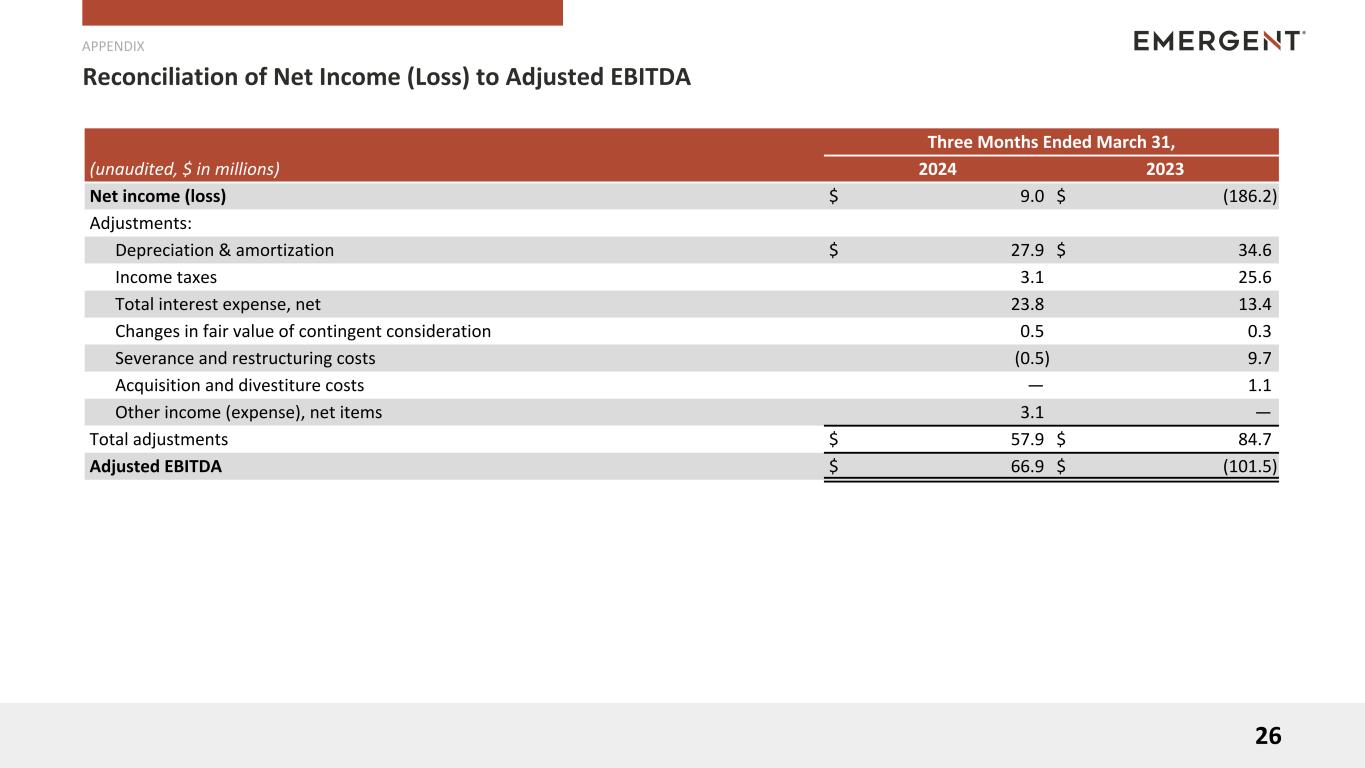
Reconciliation of Net Income (Loss) to Adjusted EBITDA (1)
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in millions) |
Three Months Ended March 31, |
|
|
| 2024 |
2023 |
|
|
|
| Net income (loss) |
$ |
9.0 |
|
$ |
(186.2) |
|
|
|
|
| Adjustments: |
|
|
|
|
|
| Depreciation & amortization |
$ |
27.9 |
|
$ |
34.6 |
|
|
|
|
| Income taxes |
3.1 |
|
25.6 |
|
|
|
|
| Total interest expense, net |
23.8 |
|
13.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Changes in fair value of contingent consideration |
0.5 |
|
0.3 |
|
|
|
|
| Severance and restructuring costs |
(0.5) |
|
9.7 |
|
|
|
|
|
|
|
|
|
|
| Acquisition and divestiture costs |
— |
|
1.1 |
|
|
|
|
|
|
|
|
|
|
| Other income (expense), net items |
3.1 |
|
— |
|
|
|
|
| Total adjustments |
$ |
57.9 |
|
$ |
84.7 |
|
|
|
|
| Adjusted EBITDA |
$ |
66.9 |
|
$ |
(101.5) |
|
|
|
|
Emergent BioSolutions, Inc.
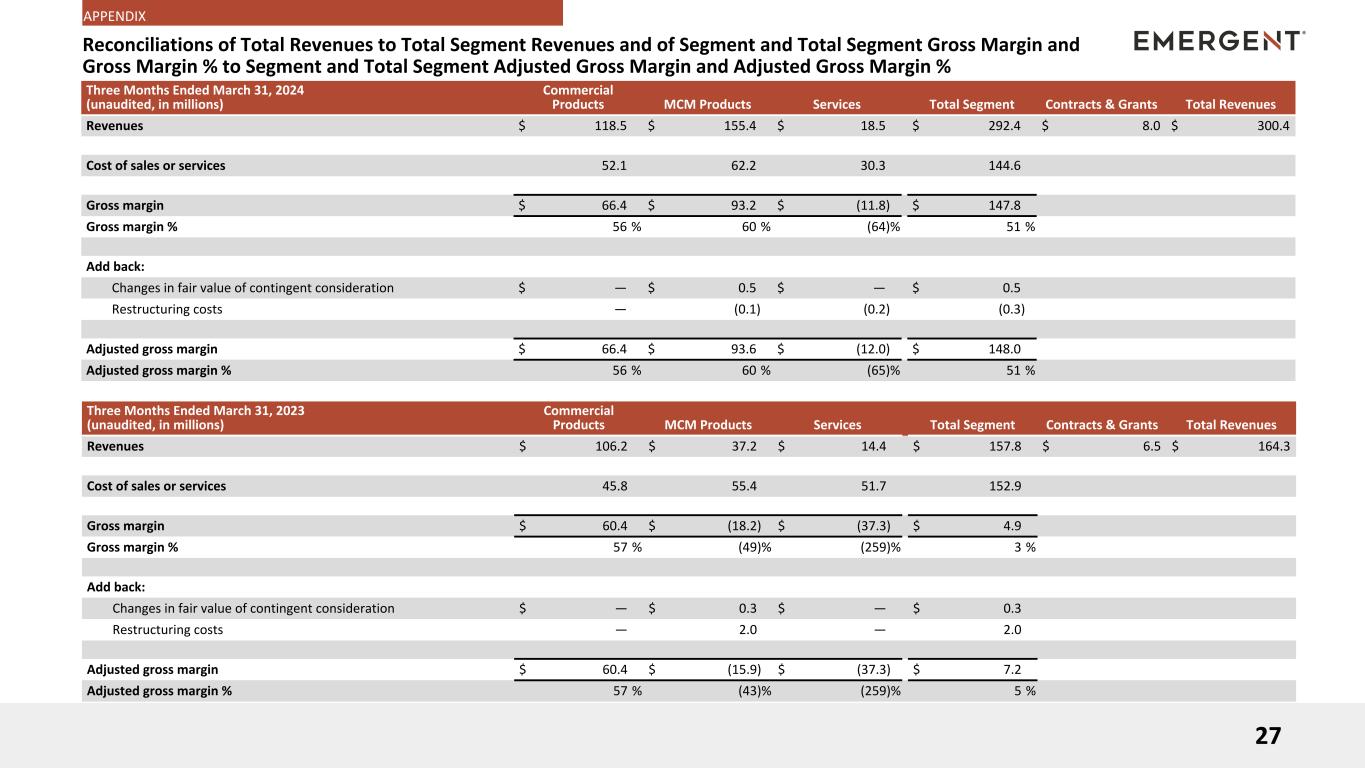
Reconciliations of Total Revenues to Total Segment Revenues and of Segment and Total Segment Gross Margin and Gross Margin %
to Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin % (1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, 2024
(unaudited, in millions)
|
Commercial Products |
MCM Products |
Services |
|
Total Segment |
Contracts & Grants |
Total Revenues |
| Revenues |
$ |
118.5 |
|
$ |
155.4 |
|
$ |
18.5 |
|
|
$ |
292.4 |
|
$ |
8.0 |
|
$ |
300.4 |
|
|
|
|
|
|
|
|
|
| Cost of sales or services |
52.1 |
|
62.2 |
|
30.3 |
|
|
144.6 |
|
|
|
|
|
|
|
|
|
|
|
| Gross margin |
$ |
66.4 |
|
$ |
93.2 |
|
$ |
(11.8) |
|
|
$ |
147.8 |
|
|
|
| Gross margin % |
56 |
% |
60 |
% |
(64) |
% |
|
51 |
% |
|
|
|
|
|
|
|
|
|
|
| Add back: |
|
|
|
|
|
|
|
| Changes in fair value of contingent consideration |
$ |
— |
|
$ |
0.5 |
|
$ |
— |
|
|
$ |
0.5 |
|
|
|
|
|
|
|
|
|
|
|
| Restructuring costs |
— |
|
(0.1) |
|
(0.2) |
|
|
(0.3) |
|
|
|
|
|
|
|
|
|
|
|
| Adjusted gross margin |
$ |
66.4 |
|
$ |
93.6 |
|
$ |
(12.0) |
|
|
$ |
148.0 |
|
|
|
| Adjusted gross margin % |
56 |
% |
60 |
% |
(65) |
% |
|
51 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, 2023
(unaudited, in millions)
|
Commercial Products |
MCM Products |
Services |
|
Total Segment |
Contracts & Grants |
Total Revenues |
| Revenues |
$ |
106.2 |
|
$ |
37.2 |
|
$ |
14.4 |
|
|
$ |
157.8 |
|
$ |
6.5 |
|
$ |
164.3 |
|
|
|
|
|
|
|
|
|
| Cost of sales or services |
45.8 |
|
55.4 |
|
51.7 |
|
|
152.9 |
|
|
|
|
|
|
|
|
|
|
|
| Gross margin |
$ |
60.4 |
|
$ |
(18.2) |
|
$ |
(37.3) |
|
|
$ |
4.9 |
|
|
|
| Gross margin % |
57 |
% |
(49) |
% |
(259) |
% |
|
3 |
% |
|
|
|
|
|
|
|
|
|
|
| Add back: |
|
|
|
|
|
|
|
| Changes in fair value of contingent consideration |
$ |
— |
|
$ |
0.3 |
|
$ |
— |
|
|
$ |
0.3 |
|
|
|
|
|
|
|
|
|
|
|
| Restructuring costs |
— |
|
2.0 |
|
— |
|
|
2.0 |
|
|
|
|
|
|
|
|
|
|
|
| Adjusted gross margin |
$ |
60.4 |
|
$ |
(15.9) |
|
$ |
(37.3) |
|
|
$ |
7.2 |
|
|
|
| Adjusted gross margin % |
57 |
% |
(43) |
% |
(259) |
% |
|
5 |
% |
|
|
Emergent BioSolutions, Inc.
Reconciliation of Net Loss Forecast to Adjusted Net Loss Forecast
|
|
|
|
|
|
|
|
|
| ($ in millions) |
2024 Full Year Forecast |
Source |
| Net loss |
$(148) - $(98) |
|
| Adjustments: |
|
|
| Non-cash amortization charges |
$65 |
IA Amortization Other Income |
| Changes in fair value of contingent consideration |
2 |
MCM Product COGS |
|
|
|
| Severance and restructuring costs |
21 |
COGS, SG&A and R&D |
|
|
|
| All Other |
5 |
Acquisition/divestiture costs and non operating investment loss |
| Tax effect |
(10) |
|
| Total adjustments: |
$83 |
|
| Adjusted net loss |
$(65) - $(15) |
|
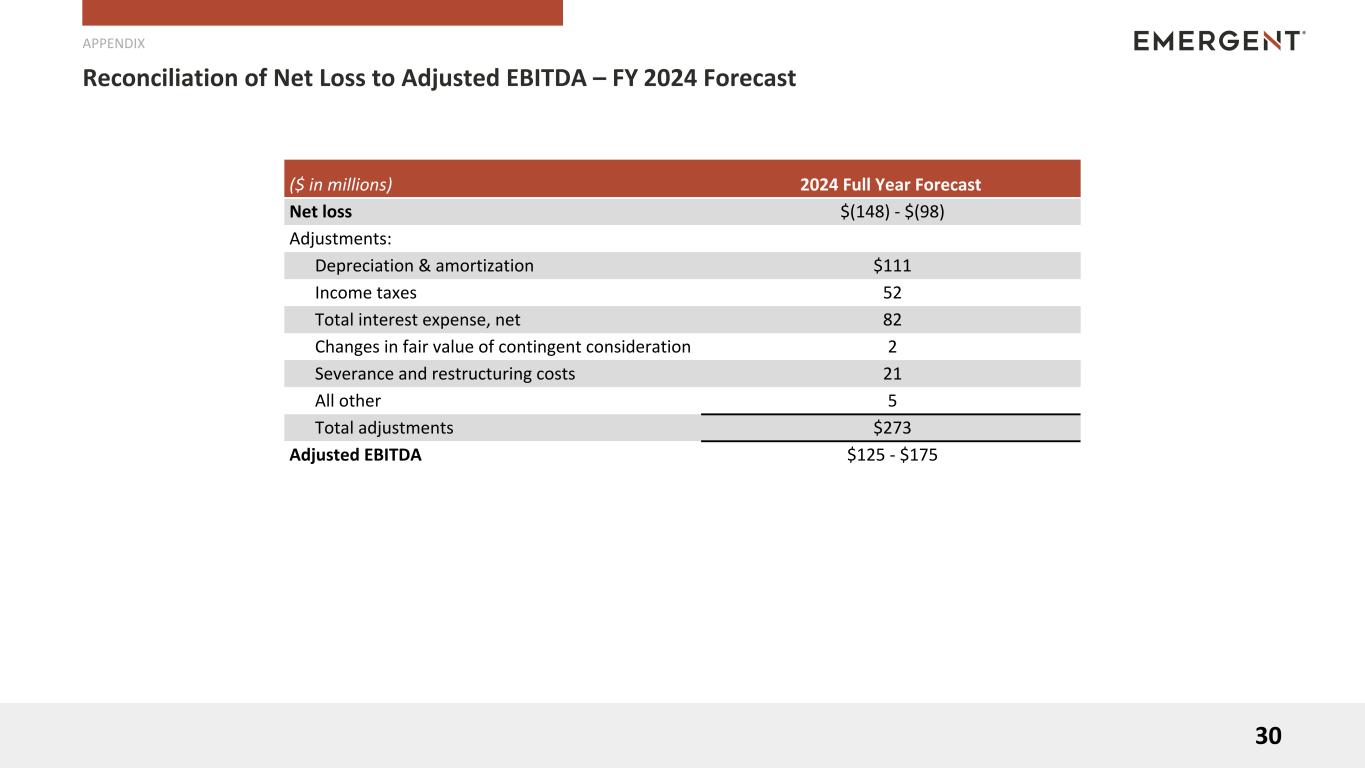
Reconciliation of Net Loss Forecast to Adjusted EBITDA Forecast
|
|
|
|
|
|
| ($ in millions) |
2024 Full Year Forecast |
| Net loss |
$(148) - $(98) |
| Adjustments: |
|
| Depreciation & amortization |
$111 |
| Income taxes |
52 |
| Total interest expense, net |
82 |
|
|
|
|
| Changes in fair value of contingent consideration |
2 |
| Severance and restructuring costs |
21 |
| All other |
5 |
| Total adjustments |
$273 |
| Adjusted EBITDA |
$125 - $175 |
Emergent BioSolutions, Inc.
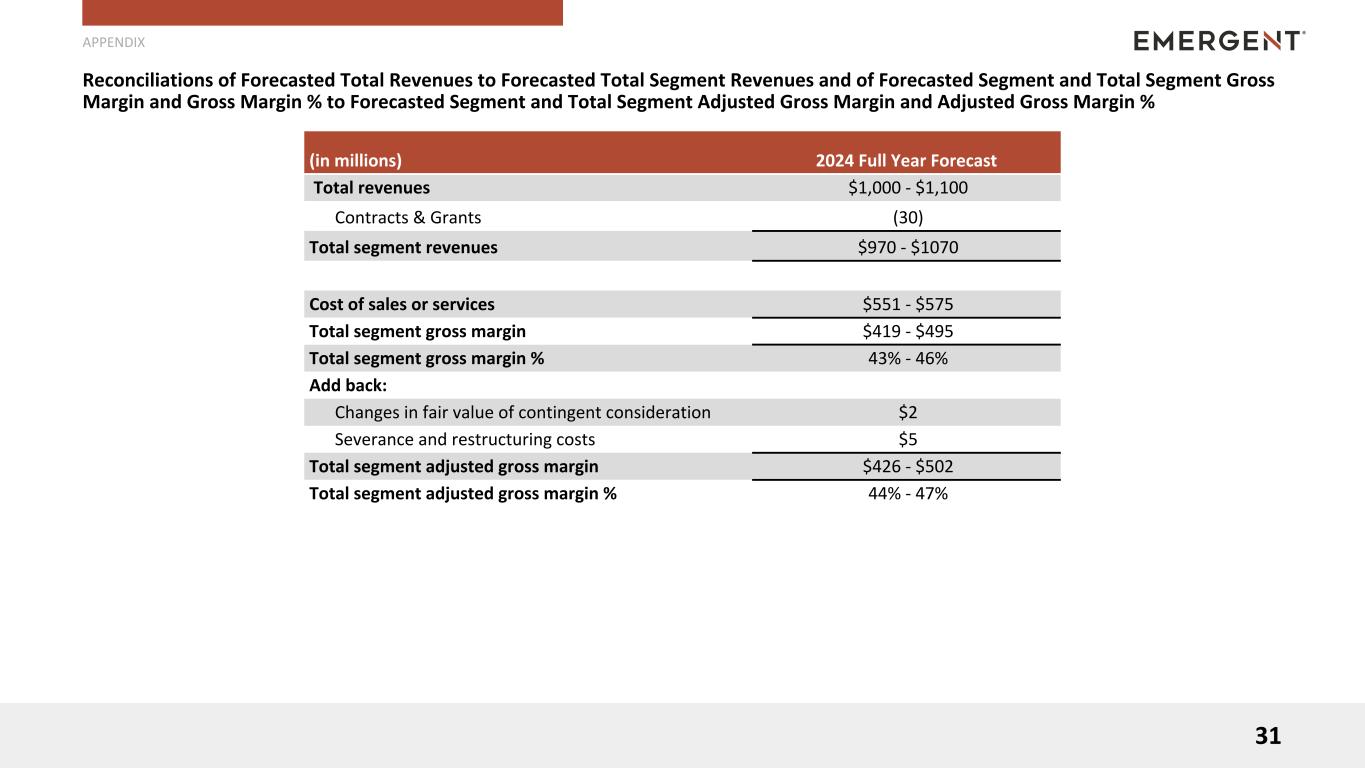
Reconciliations of Forecasted Total Revenues to Forecasted Total Segment Revenues and of Forecasted Segment and Total Segment Gross Margin and Gross Margin % to Forecasted Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin % (1)
|
|
|
|
|
|
|
(in millions)
|
2024 Full Year Forecast |
| Total revenues |
$1,000 - $1,100 |
| Contracts & Grants |
(30) |
| Total segment revenues |
$970 - $1070 |
|
|
| Cost of sales or services |
$551 - $575 |
| Total segment gross margin |
$419 - $495 |
| Total segment gross margin % |
43% - 46% |
| Add back: |
|
| Changes in fair value of contingent consideration |
$2 |
|
|
| Severance and restructuring costs |
$5 |
| Total segment adjusted gross margin |
$426 - $502 |
| Total segment adjusted gross margin % |
44% - 47% |