UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): February 18, 2025
Solid Biosciences Inc.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 001-38360 | 90-0943402 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 500 Rutherford Avenue, Third Floor |
| Charlestown, Massachusetts 02129 |
| (Address of Principal Executive Offices) (Zip Code) |
Registrant’s telephone number, including area code: (617) 337-4680
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common Stock $0.001 par value per share | SLDB | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 | Results of Operations and Financial Condition. |
The information disclosed under the heading “Cash, Cash Equivalents and Available-for-Sale Securities as of December 31, 2024” under Item 8.01 of this Current Report on Form 8-K is herein incorporated by reference.
| Item 8.01 | Other Events. |
Initial Clinical Data from INSPIRE DUCHENNE Trial of SGT-003
On February 18, 2025, Solid Biosciences Inc. (the “Company”) announced positive initial data from the Phase 1/2 INSPIRE DUCHENNE trial evaluating SGT-003, a next-generation gene therapy product candidate intended for the treatment of Duchenne muscular dystrophy (“Duchenne”). The INSPIRE DUCHENNE trial is a Phase 1/2 first-in-human, open-label, single-dose, multicenter trial designed to evaluate the safety, tolerability and efficacy of SGT-003 in pediatric patients with Duchenne at a dose of 1E14vg/kg. SGT-003 is administered as a one-time intravenous infusion. Interim 90-day biopsy data reported in the first three participants showed an average microdystrophin expression of 110%, as measured by western blot, and improvements in multiple biomarkers that are indicators of muscle health and resilience.
The 90-day data reported of the data cutoff date of February 11, 2025 includes: microdystrophin expression, measures of restoration and activation of key elements of the dystrophin-associated protein complex, key muscle integrity biomarker evaluation, in each case, from the first three participants dosed in the INSPIRE DUCHENNE trial, and interim safety findings from the first six participants dosed in the INSPIRE DUCHENNE trial. The first three participants are two 5-year-old boys and one 7-year-old boy at the time of dosing. The second three participants are a 6-year-old boy and two 7-year-old boys at the time of dosing.
Microdystrophin Expression and Other Measures at Day 90 (N=3)
| Mean (N=3) | Participant 1 | Participant 2 | Participant 3 | |||||||||||||
| Microdystrophin Expression % Normal |
110 | % | 135 | % | 112 | % | 84 | % | ||||||||
| Microdystrophin Expression % Normal |
108 | % | 119 | % | 152 | % | 53 | % | ||||||||
| % Dystrophin Positive Fibers |
78 | % | 77 | % | 88 | % | 70 | % | ||||||||
| Vector Copies/Nucleus |
18.7 | 19.8 | 28.6 | 7.6 | ||||||||||||
| nNOS (neuronal nitric oxide synthase) % Positive Fibers |
42 | % | 48 | % | 53 | % | 25 | % | ||||||||
| Beta Sarcoglycan % Positive Fibers |
70 | % | 60 | % | 88 | % | 63 | % | ||||||||
Muscle Integrity Biomarker Evaluation at Day 90 (N=3)
| • | Mean reductions observed in markers of muscle injury and stress: |
| • | Serum creatine kinase (CK) (IU/L): -57% |
| • | Serum aspartate aminotransferase (AST) (IU/L): -45% |
| • | Serum alanine transaminase (ALT) (IU/L): -54% |
| • | Serum lactate dehydrogenase (LDH) (IU/L): -60% |
| • | Mean reductions observed in markers of muscle breakdown and dystrophic regeneration: |
| • | Serum titin (pmol/L): -42% |
| • | Embryonic myosin heavy chain (eMHC) positive fibers: -59% |
Measure of Potential Cardiac Benefit:
| • | At Day 180, the Company observed a mean cardiac function increase of 8% (N=2) from baseline as measured by left ventricular ejection fraction. The third participant had not reached Day 180 follow up as of the data cutoff date of February 11, 2025. |
| • | The Company observed a reduction in serum cardiac hs-troponin I (hs-cTnI) of -36% at Day 90 in one participant who entered the trial with elevated hs-cTnI levels. Two of the first three participants entered the trial with normal baseline cTnI levels. |
| • | Two participants in total (N=6) had elevated troponin at baseline that reduced below initial baseline values post-dose. |
Safety Update for the First Six Participants Dosed
SGT-003 was well-tolerated in the first six participants dosed as of the data cutoff date of February 11, 2025. As of the cutoff date, all six participants have reached at least 20 days post SGT-003 treatment. Adverse events observed after SGT-003 treatment were consistent with those observed in AAV gene therapy. No serious adverse events and no suspected unexpected serious adverse reactions were observed, and there was no evidence of thrombotic microangiopathy, atypical hemolytic uremic syndrome, or hemolysis. No AEs of hepatic transaminitis were observed, including no elevated gamma-glutamyl transferase levels. All treatment-related AEs resolved with no sequelae and none of the AEs that were observed required the use of additional immunomodulatory agents such as eculizumab, sirolimus or rituximab. The most common AEs observed were nausea and vomiting; transient thrombocytopenia (including one Grade 3 episode that resolved within days without intervention and with no evidence of hemolysis observed); infusion related hypersensitivity reaction (including one Grade 3 episode of prolonged fever that resolved within days without intervention); and fever. One adverse event of special interest was observed, which was a Grade 1, mild, transient hs-troponin I elevation that resolved without intervention. There was no clinical evidence of myocarditis and no EKG or echocardiographic changes observed in such participant.
The Company plans to present the information in the presentation attached hereto as Exhibit 99.1 (the “Presentation”) at conference call at 8:00 a.m. Eastern time on February 18, 2025. A copy of the Presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Anticipated Milestones in INSPIRE DUCHENNE Trial
Enrollment in the INSPIRE DUCHENNE trial is ongoing, with at least 10 total participants in the trial anticipated to be dosed by early in the second quarter of 2025 and approximately 20 total participants anticipated to be dosed by the fourth quarter of 2025. The seventh participant in the trial was dosed on February 17, 2025.
INSPIRE DUCHENNE currently has a total of six active clinical sites in the United States and Canada and approved clinical trial applications in the United Kingdom and Italy. The Company expects to activate additional trial sites by the end of 2025. In mid-2025, the Company plans to request a meeting with the U.S. Food and Drug Administration (the “FDA”) to discuss the potential for an accelerated approval regulatory pathway for SGT-003.
Planned Clinical Trial of SGT-212
In January 2025, the Company announced that the FDA cleared its investigational new drug application (“IND”) for SGT-212 for the treatment of Friedreich’s ataxia (“FA”). The Company anticipates initiating an open-label, multi-center Phase 1b clinical trial of SGT-212 in non-ambulatory and ambulatory adult patients living with FA in the second half of 2025. In the trial, SGT-212 will be administered by dual route of administration: via bilateral infusion to the dentate nuclei using a magnetic resonance imaging-guided device followed by an intravenous infusion. The planned Phase 1b clinical trial is designed to evaluate the safety and tolerability of SGT-212. Exploratory objectives include assessment of preliminary efficacy and pharmacodynamic activity. A minimum of six participants are targeted for enrollment in three cohorts. Cohort 1 is expected to include three non-ambulatory participants, cohort 2 is expected to include three ambulatory participants and cohort 3 is expected to include two non-ambulatory and two ambulatory participants. In total, the participants will be followed for five years after the date of infusion of SGT-212.
The FDA has recently granted Fast Track designation to SGT-212 for the treatment of FA.
SGT-501
SGT-501 is the Company’s gene therapy candidate for the treatment of catecholaminergic polymorphic ventricular tachycardia (“CPVT”), which is caused by a gain of function mutation in the ryanodine receptor 2 (coded for by the RYR2 gene), referred to as CPVT-1. The Company is conducting IND-enabling GLP toxicology studies of SGT-501 in non-human primates, with the in-life portion of the six-month toxicology study expected to be completed in the first quarter of 2025. The Company expects to submit an IND to the FDA for SGT-501 for the treatment of patients with CPVT-1 in the first half of 2025.
The FDA has recently granted Rare Pediatric Disease designation for SGT-501.
SGT-601
The Company anticipates submitting an IND to the FDA for SGT-601 for the treatment of TNNT2-mediated dilated cardiomyopathy in the second half of 2026.
Cash, Cash Equivalents and Available-for-Sale Securities as of December 31, 2024
The Company expects to report that it had cash, cash equivalents and available-for-sale securities of approximately $148.9 million as of December 31, 2024.
The estimated cash, cash equivalents and available-for-sale securities figure is preliminary and unaudited, represents a management estimate as of the date of this report and is subject to completion of the Company’s financial closing procedures for the year ended December 31, 2024 and does not present all necessary information for a complete understanding of the Company’s financial condition as of December 31, 2024, or the Company’s results of operations for the year ended December 31, 2024. The Company’s independent registered public accounting firm has not conducted an audit or review of, and does not express an opinion or any other form of assurance with respect to, the estimated cash figure.
Cautionary Note Regarding Forward-Looking Statements
This Current Report on Form 8-K contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements regarding future expectations, plans and prospects for the Company; the ability to successfully achieve and execute on the Company’s goals, priorities and achieve key preclinical and clinical milestones; the anticipated benefits of the Company’s development programs; the Company’s programs, including expectations for clinical development, trial design, site activations, expanded clinical development, initiation and enrollment in clinical trials, dosing, and availability of clinical trial data; planned regulatory interactions and the potential accelerated approval pathway for SGT-003; the Company’s expectations for submission of INDs; and the Company’s estimated cash, cash equivalents and available-for-sale securities as of December 31, 2024; and other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements.
These risks and uncertainties include, but are not limited to, risks associated with the Company’s ability to advance SGT-003, SGT-212, SGT-501, SGT-601, SGT-401 and other preclinical programs and capsid libraries on the timelines expected or at all; obtain and maintain necessary approvals and designations from the FDA and other regulatory authorities; replicate in clinical trials positive results found in preclinical studies and early-stage clinical trials of the Company’s product candidates; replicate preliminary or interim data from early-stage clinicals trials in the final data of such trials; obtain, maintain or protect intellectual property rights related to its product candidates; compete successfully with other companies that are seeking to develop Duchenne, Friedreich’s ataxia and other neuromuscular and cardiac treatments and gene therapies; manage expenses; and raise the substantial additional capital needed, on the timeline necessary, to continue development of SGT-003, SGT-212, SGT-501, SGT-601, SGT-401 and other candidates, achieve its other business objectives and continue as a going concern. For a discussion of other risks and uncertainties, and other important factors, any of which could cause the Company’s actual results to differ from those contained in the forward-looking statements, see the “Risk Factors” section, as well as discussions of potential risks, uncertainties and other important factors, in the Company’s most recent filings with the Securities and Exchange Commission, and other filings that the Company may make with the Securities and Exchange Commission in the future. In addition, the forward-looking statements included in this Current Report on Form 8-K represent the Company’s views as of the date hereof and should not be relied upon as representing the Company’s views as of any date subsequent to the date hereof. The Company anticipates that subsequent events and developments will cause the Company’s views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits:
| Exhibit No. |
Description | |
| 99.1 | Solid Biosciences Inc. Presentation February 2025. | |
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| SOLID BIOSCIENCES INC. | ||||||
| Date: February 18, 2025 | By: | /s/ Alexander Cumbo |
||||
| Name: | Alexander Cumbo | |||||
| Title: | Chief Executive Officer | |||||

Exhibit 99.1 February 2025 SGT-003 INSPIRE DUCHENNE DATA UPDATE © 2025 Solid Biosciences

Cautionary Note Regarding Forward Looking Statements This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements regarding future expectations, plans and prospects for the Company; the ability to successfully achieve and execute on the Company’s goals, priorities and achieve key clinical milestones; the anticipated benefits of SGT-003; the Company’s SGT-003 clinical program, including planned enrollment and site activations in the INSPIRE DUCHENNE trial, planned regulatory interactions and the potential accelerated approval pathway; and other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” “working” and similar expressions. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. These risks and uncertainties include, but are not limited to, risks associated with the Company’s ability to advance SGT-003, SGT-212, SGT-501, SGT-601, SGT-401 and other preclinical programs and capsid libraries on the timelines expected or at all; obtain and maintain necessary approvals and designations from the FDA and other regulatory authorities; replicate in clinical trials positive results found in preclinical studies and early-stage clinical trials of the Company’s product candidates; replicate preliminary or interim data from early-stage clinicals trials in the final data of such trials; obtain, maintain or protect intellectual property rights related to its product candidates; compete successfully with other companies that are seeking to develop Duchenne, Friedreich’s ataxia and other neuromuscular and cardiac treatments and gene therapies; manage expenses; and raise the substantial additional capital needed, on the timeline necessary, to continue development of SGT-003, SGT-212, SGT- 501, SGT-601, SGT-401 and other candidates, achieve its other business objectives and continue as a going concern. For a discussion of other risks and uncertainties, and other important factors, any of which could cause the Company’s actual results to differ from those contained in the forward-looking statements, see the “Risk Factors” section, as well as discussions of potential risks, uncertainties and other important factors, in the Company’s most recent filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this presentation represent the Company’s views as of the date hereof and should not be relied upon as representing the Company’s views as of any date subsequent to the date hereof. The Company anticipates that subsequent events and developments will cause the Company's views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so. This presentation contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data and estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. 2 © 2025 Solid Biosciences
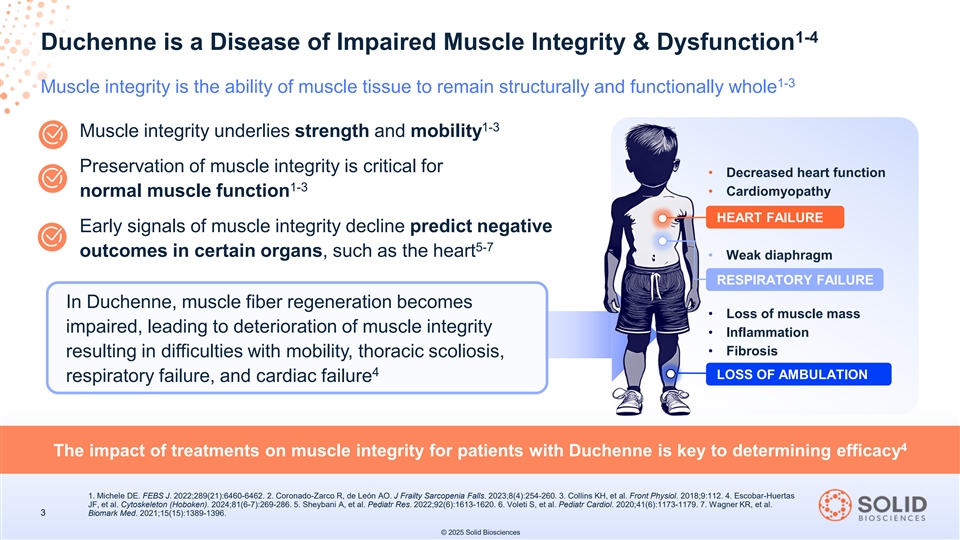
1-4 Duchenne is a Disease of Impaired Muscle Integrity & Dysfunction 1-3 Muscle integrity is the ability of muscle tissue to remain structurally and functionally whole 1-3 Muscle integrity underlies strength and mobility Preservation of muscle integrity is critical for • Decreased heart function 1-3 • Cardiomyopathy normal muscle function HEART FAILURE Early signals of muscle integrity decline predict negative 5-7 outcomes in certain organs, such as the heart • Weak diaphragm RESPIRATORY FAILURE In Duchenne, muscle fiber regeneration becomes • Loss of muscle mass impaired, leading to deterioration of muscle integrity • Inflammation • Fibrosis resulting in difficulties with mobility, thoracic scoliosis, 4 LOSS OF AMBULATION respiratory failure, and cardiac failure 4 The impact of treatments on muscle integrity for patients with Duchenne is key to determining efficacy 1. Michele DE. FEBS J. 2022;289(21):6460-6462. 2. Coronado-Zarco R, de León AO. J Frailty Sarcopenia Falls. 2023;8(4):254-260. 3. Collins KH, et al. Front Physiol. 2018;9:112. 4. Escobar-Huertas JF, et al. Cytoskeleton (Hoboken). 2024;81(6-7):269-286. 5. Sheybani A, et al. Pediatr Res. 2022;92(6):1613-1620. 6. Voleti S, et al. Pediatr Cardiol. 2020;41(6):1173-1179. 7. Wagner KR, et al. 3 Biomark Med. 2021;15(15):1389-1396. © 2025 Solid Biosciences
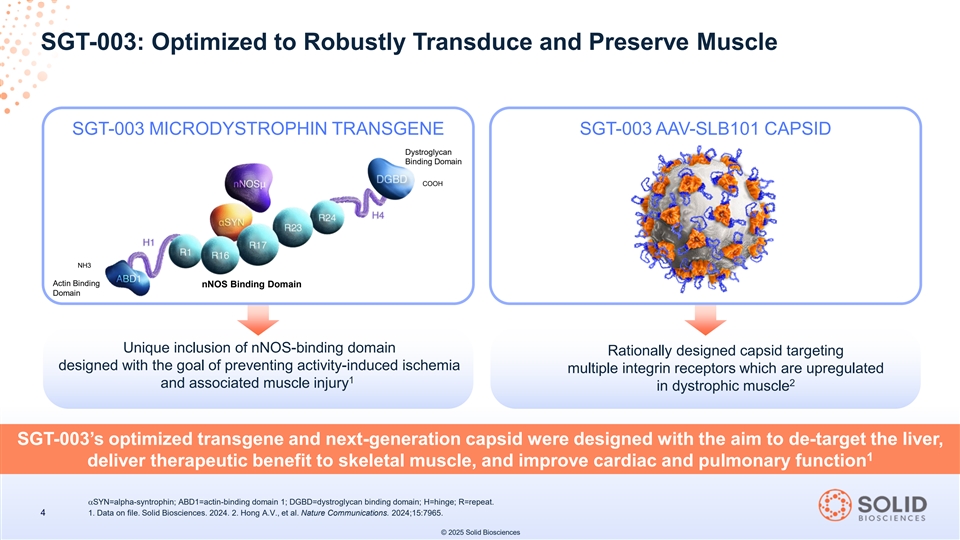
SGT-003: Optimized to Robustly Transduce and Preserve Muscle SGT-003 MICRODYSTROPHIN TRANSGENE SGT-003 AAV-SLB101 CAPSID Dystroglycan Binding Domain COOH NH3 Actin Binding nNOS Binding Domain Domain Unique inclusion of nNOS-binding domain Rationally designed capsid targeting designed with the goal of preventing activity-induced ischemia multiple integrin receptors which are upregulated 1 2 and associated muscle injury in dystrophic muscle SGT-003’s optimized transgene and next-generation capsid were designed with the aim to de-target the liver, 1 deliver therapeutic benefit to skeletal muscle, and improve cardiac and pulmonary function αSYN=alpha-syntrophin; ABD1=actin-binding domain 1; DGBD=dystroglycan binding domain; H=hinge; R=repeat. 4 1. Data on file. Solid Biosciences. 2024. 2. Hong A.V., et al. Nature Communications. 2024;15:7965. © 2025 Solid Biosciences
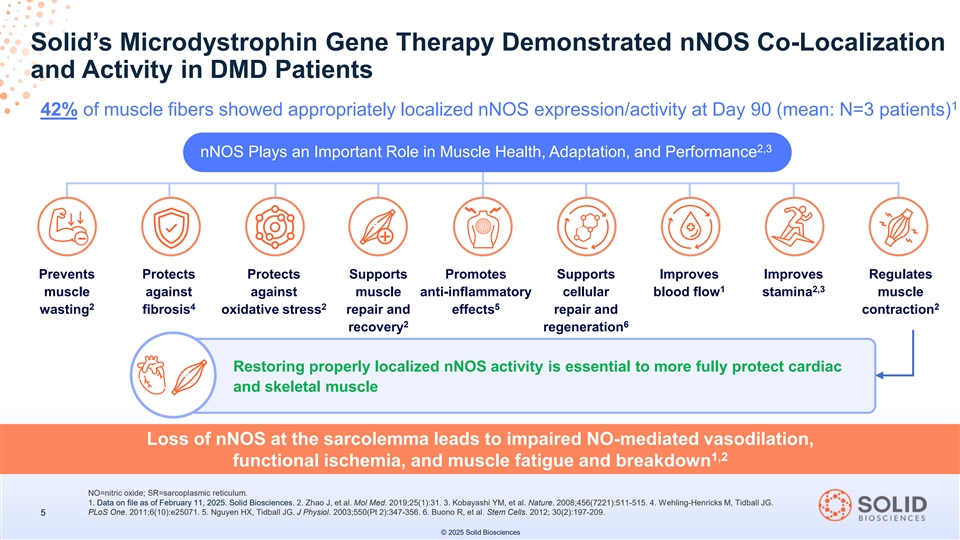
Solid’s Microdystrophin Gene Therapy Demonstrated nNOS Co-Localization and Activity in DMD Patients 1 42% of muscle fibers showed appropriately localized nNOS expression/activity at Day 90 (mean: N=3 patients) 2,3 nNOS Plays an Important Role in Muscle Health, Adaptation, and Performance + Prevents Protects Protects Supports Promotes Supports Improves Improves Regulates 1 2,3 muscle against against muscle anti-inflammatory cellular blood flow stamina muscle 2 4 2 5 2 wasting fibrosis oxidative stress repair and effects repair and contraction 2 6 recovery regeneration Restoring properly localized nNOS activity is essential to more fully protect cardiac 4 and skeletal muscle Loss of nNOS at the sarcolemma leads to impaired NO-mediated vasodilation, 1,2 functional ischemia, and muscle fatigue and breakdown NO=nitric oxide; SR=sarcoplasmic reticulum. 1. Data on file as of February 11, 2025. Solid Biosciences. 2. Zhao J, et al. Mol Med. 2019;25(1):31. 3. Kobayashi YM, et al. Nature. 2008;456(7221):511-515. 4. Wehling-Henricks M, Tidball JG. PLoS One. 2011;6(10):e25071. 5. Nguyen HX, Tidball JG. J Physiol. 2003;550(Pt 2):347-356. 6. Buono R, et al. Stem Cells. 2012; 30(2):197-209. 5 © 2025 Solid Biosciences
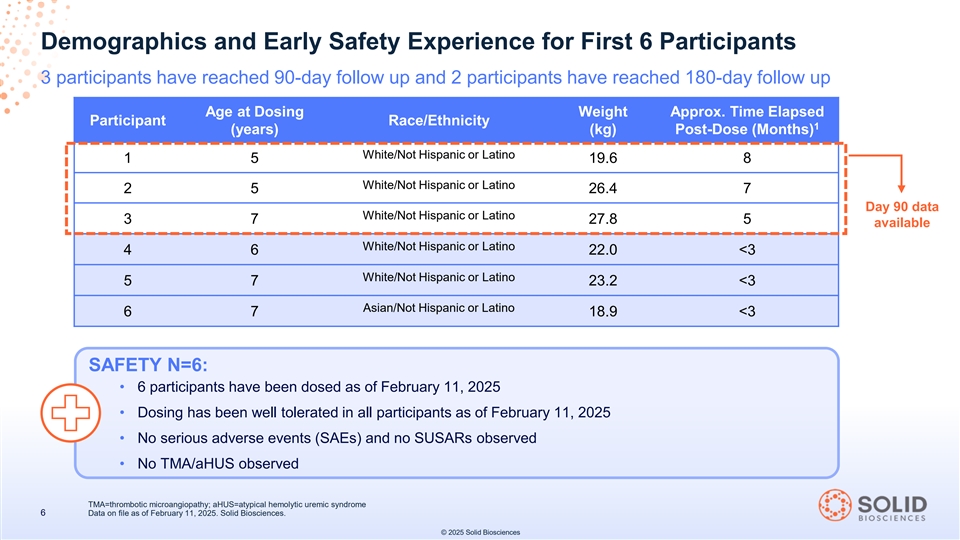
Demographics and Early Safety Experience for First 6 Participants 3 participants have reached 90-day follow up and 2 participants have reached 180-day follow up Age at Dosing Weight Approx. Time Elapsed Participant Race/Ethnicity 1 (years) (kg) Post-Dose (Months) White/Not Hispanic or Latino 1 5 19.6 8 White/Not Hispanic or Latino 2 5 26.4 7 Day 90 data White/Not Hispanic or Latino 3 7 27.8 5 available White/Not Hispanic or Latino 4 6 22.0 <3 White/Not Hispanic or Latino 5 7 23.2 <3 Asian/Not Hispanic or Latino 6 7 18.9 <3 SAFETY N=6: • 6 participants have been dosed as of February 11, 2025 • Dosing has been well tolerated in all participants as of February 11, 2025 • No serious adverse events (SAEs) and no SUSARs observed • No TMA/aHUS observed TMA=thrombotic microangiopathy; aHUS=atypical hemolytic uremic syndrome 6 Data on file as of February 11, 2025. Solid Biosciences. © 2025 Solid Biosciences
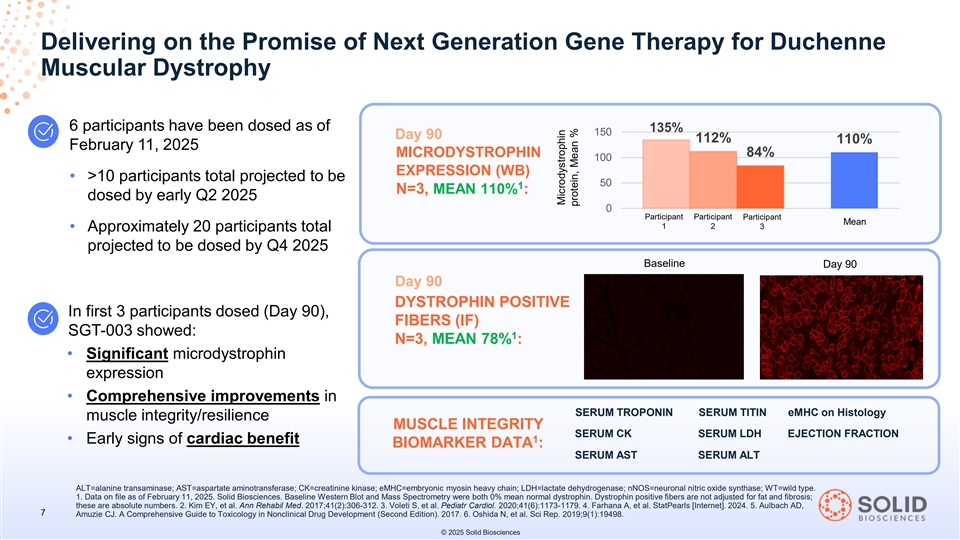
Delivering on the Promise of Next Generation Gene Therapy for Duchenne Muscular Dystrophy 6 participants have been dosed as of 135% 150 Day 90 112% 110% February 11, 2025 MICRODYSTROPHIN 84% 100 EXPRESSION (WB) • >10 participants total projected to be 50 1 N=3, MEAN 110% : dosed by early Q2 2025 0 Participant Participant Participant Mean 1 2 3 • Approximately 20 participants total projected to be dosed by Q4 2025 Baseline Day 90 Day 90 DYSTROPHIN POSITIVE In first 3 participants dosed (Day 90), FIBERS (IF) SGT-003 showed: 1 N=3, MEAN 78% : • Significant microdystrophin expression • Comprehensive improvements in SERUM TROPONIN SERUM TITIN eMHC on Histology muscle integrity/resilience MUSCLE INTEGRITY SERUM CK SERUM LDH EJECTION FRACTION 1 • Early signs of cardiac benefit BIOMARKER DATA : SERUM AST SERUM ALT ALT=alanine transaminase; AST=aspartate aminotransferase; CK=creatinine kinase; eMHC=embryonic myosin heavy chain; LDH=lactate dehydrogenase; nNOS=neuronal nitric oxide synthase; WT=wild type. 1. Data on file as of February 11, 2025. Solid Biosciences. Baseline Western Blot and Mass Spectrometry were both 0% mean normal dystrophin. Dystrophin positive fibers are not adjusted for fat and fibrosis; these are absolute numbers. 2. Kim EY, et al. Ann Rehabil Med. 2017;41(2):306-312. 3. Voleti S, et al. Pediatr Cardiol. 2020;41(6):1173-1179. 4. Farhana A, et al. StatPearls [Internet]. 2024. 5. Aulbach AD, 7 Amuzie CJ. A Comprehensive Guide to Toxicology in Nonclinical Drug Development (Second Edition). 2017. 6. Oshida N, et al. Sci Rep. 2019;9(1):19498. © 2025 Solid Biosciences Microdystrophin protein, Mean %

Significant Next Generation Microdystrophin Expression at Day 90 1 Comprehensive orthogonal measurements showed significant microdystrophin expression 1 1 Microdystrophin Expression Measured by Western Blot Microdystrophin Expression Measured by MS 200 135% 150 152% 112% 110% 150 119% 108% 84% 100 100 53% 50 50 0 0 Participant 1 Participant 2 Participant 3 Participant 1 Participant 2 Participant 3 Mean Mean 1 Dystrophin Positive Fibers Measured by IF 88% 100 78% 77% 70% 75 50 25 0 Participant 1 Participant 2 Participant 3 Mean 1 Mean microdystrophin expression (n=3) measured by western blot was observed to be >2x greater for SGT-003 (Day 2-4 90) than approved first-generation Duchenne gene therapy (Weeks 12 & 64) 1. Data on file as of February 11, 2025. Solid Biosciences. Baseline Western Blot and Mass Spectrometry were both 0% mean normal dystrophin. Baseline mean dystrophin positive fibers were 1.5% measured by IF. Dystrophin positive fibers are not adjusted for fat and fibrosis; these are absolute numbers. 2. Mendell JR, et al. Nat Med. 2025 Jan;31(1):332-341. 3. Sarepta Therapeutics EMBARK 8 Part 2 conference call, January 27, 2025. 4. The comparison presented represent cross-trial comparisons and do not involve data from a head-to-head clinical trial. © 2025 Solid Biosciences Microdystrophin Microdystrophin protein, Mean % protein, Mean % Microdystrophin protein, Mean %
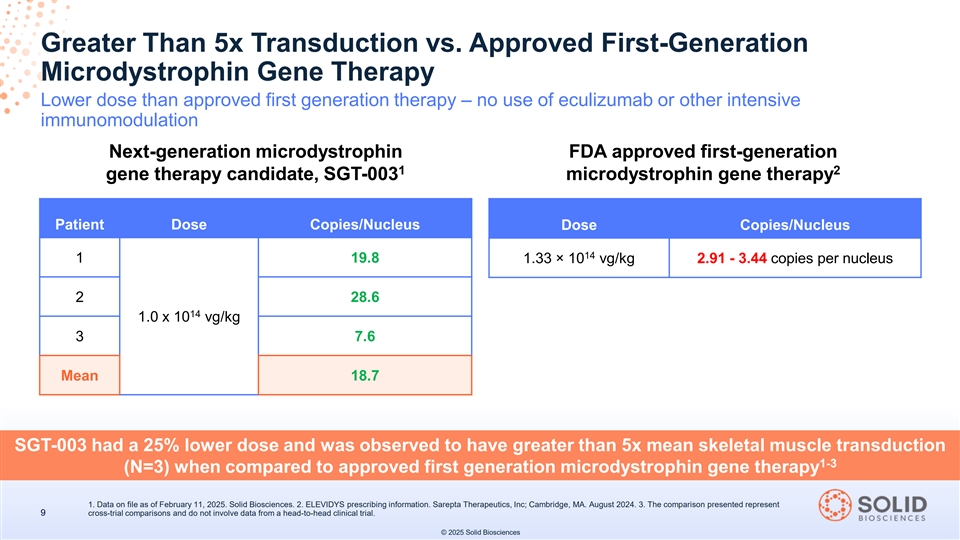
Greater Than 5x Transduction vs. Approved First-Generation Microdystrophin Gene Therapy Lower dose than approved first generation therapy – no use of eculizumab or other intensive immunomodulation Next-generation microdystrophin FDA approved first-generation 1 2 gene therapy candidate, SGT-003 microdystrophin gene therapy Patient Dose Copies/Nucleus Dose Copies/Nucleus 14 1 19.8 1.33 × 10 vg/kg 2.91 - 3.44 copies per nucleus 2 28.6 14 1.0 x 10 vg/kg 3 7.6 Mean 18.7 SGT-003 had a 25% lower dose and was observed to have greater than 5x mean skeletal muscle transduction 1-3 (N=3) when compared to approved first generation microdystrophin gene therapy 1. Data on file as of February 11, 2025. Solid Biosciences. 2. ELEVIDYS prescribing information. Sarepta Therapeutics, Inc; Cambridge, MA. August 2024. 3. The comparison presented represent 9 cross-trial comparisons and do not involve data from a head-to-head clinical trial. © 2025 Solid Biosciences
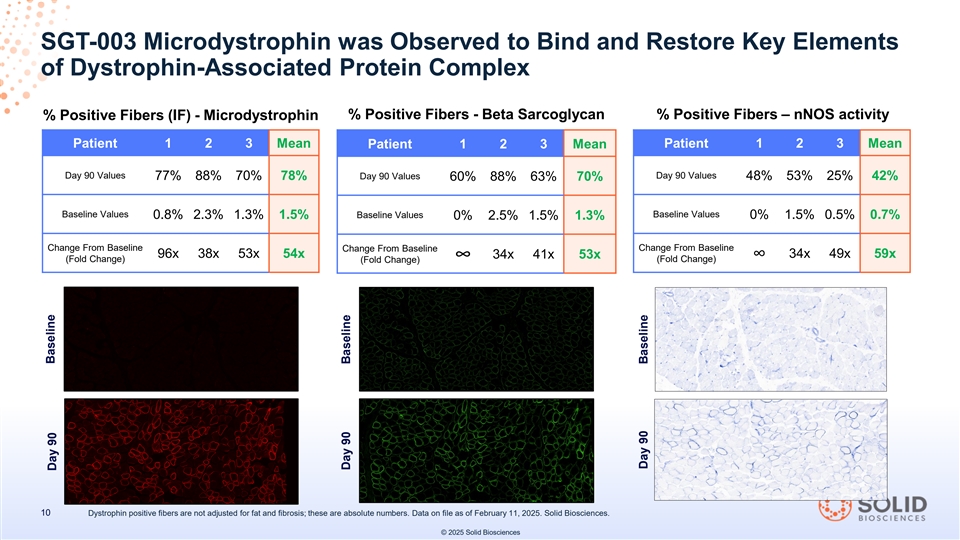
SGT-003 Microdystrophin was Observed to Bind and Restore Key Elements of Dystrophin-Associated Protein Complex % Positive Fibers (IF) - Microdystrophin % Positive Fibers - Beta Sarcoglycan % Positive Fibers – nNOS activity Patient 1 2 3 Mean Patient 1 2 3 Mean Patient 1 2 3 Mean Day 90 Values Day 90 Values 77% 88% 70% 78% Day 90 Values 48% 53% 25% 42% 60% 88% 63% 70% Baseline Values Baseline Values 0.8% 2.3% 1.3% 1.5% Baseline Values 0% 1.5% 0.5% 0.7% 0% 2.5% 1.5% 1.3% Change From Baseline Change From Baseline Change From Baseline 96x 38x 53x 54x 34x 41x 53x 34x 49x 59x ∞∞ (Fold Change) (Fold Change) (Fold Change) 10 Dystrophin positive fibers are not adjusted for fat and fibrosis; these are absolute numbers. Data on file as of February 11, 2025. Solid Biosciences. © 2025 Solid Biosciences Baseline Day 90 Day 90 Baseline Day 90 Baseline
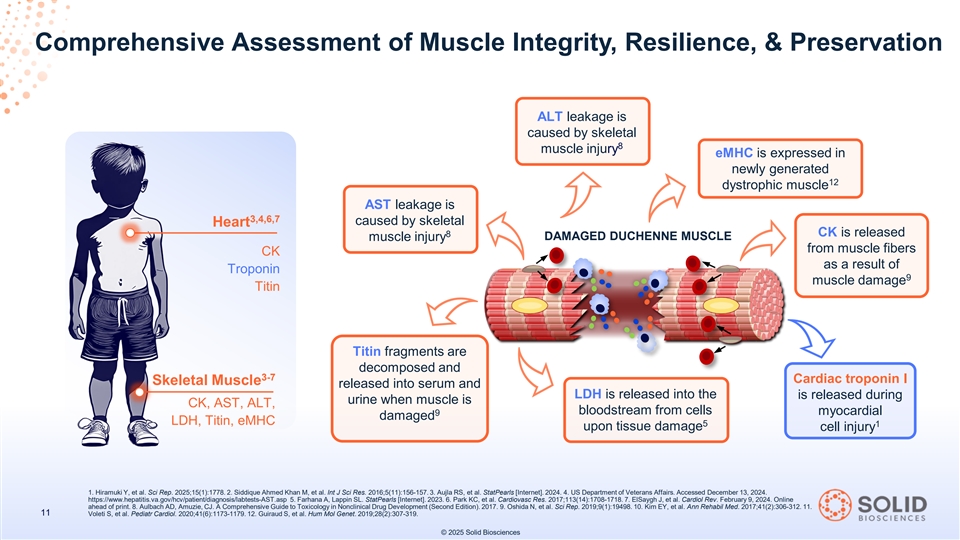
Comprehensive Assessment of Muscle Integrity, Resilience, & Preservation ALT leakage is caused by skeletal 8 muscle injury eMHC is expressed in newly generated 12 dystrophic muscle AST leakage is 3,4,6,7 caused by skeletal Heart 8 CK is released DAMAGED DUCHENNE MUSCLE muscle injury from muscle fibers CK as a result of Troponin 9 muscle damage Titin Titin fragments are decomposed and 3-7 Cardiac troponin I Skeletal Muscle released into serum and LDH is released into the is released during urine when muscle is CK, AST, ALT, bloodstream from cells myocardial 9 damaged LDH, Titin, eMHC 5 1 upon tissue damage cell injury 1. Hiramuki Y, et al. Sci Rep. 2025;15(1):1778. 2. Siddique Ahmed Khan M, et al. Int J Sci Res. 2016;5(11):156-157. 3. Aujla RS, et al. StatPearls [Internet]. 2024. 4. US Department of Veterans Affairs. Accessed December 13, 2024. https://www.hepatitis.va.gov/hcv/patient/diagnosis/labtests-AST.asp 5. Farhana A, Lappin SL. StatPearls [Internet]. 2023. 6. Park KC, et al. Cardiovasc Res. 2017;113(14):1708-1718. 7. ElSaygh J, et al. Cardiol Rev. February 9, 2024. Online ahead of print. 8. Aulbach AD, Amuzie, CJ. A Comprehensive Guide to Toxicology in Nonclinical Drug Development (Second Edition). 2017. 9. Oshida N, et al. Sci Rep. 2019;9(1):19498. 10. Kim EY, et al. Ann Rehabil Med. 2017;41(2):306-312. 11. 11 Voleti S, et al. Pediatr Cardiol. 2020;41(6):1173-1179. 12. Guiraud S, et al. Hum Mol Genet. 2019;28(2):307-319. © 2025 Solid Biosciences
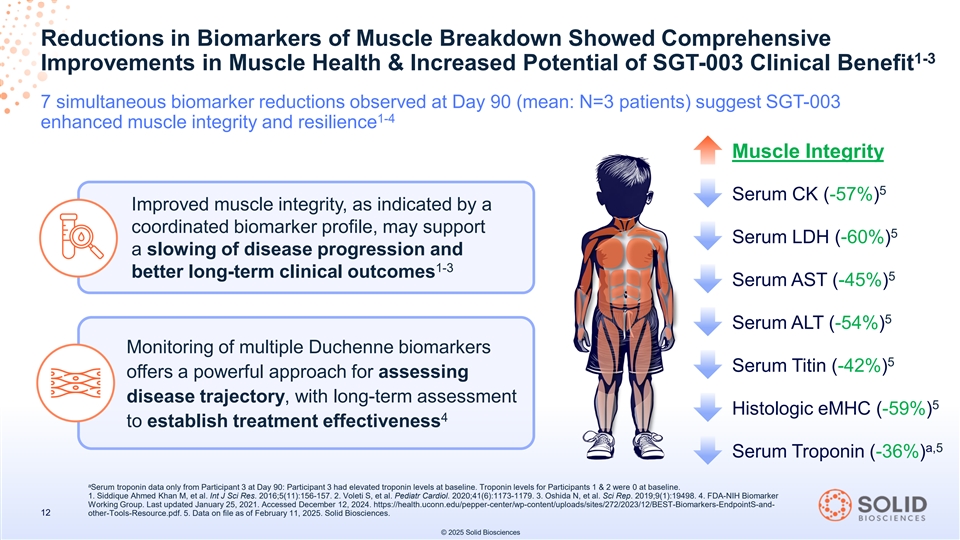
Reductions in Biomarkers of Muscle Breakdown Showed Comprehensive 1-3 Improvements in Muscle Health & Increased Potential of SGT-003 Clinical Benefit 7 simultaneous biomarker reductions observed at Day 90 (mean: N=3 patients) suggest SGT-003 1-4 enhanced muscle integrity and resilience Muscle Integrity 5 Serum CK (-57%) Improved muscle integrity, as indicated by a coordinated biomarker profile, may support 5 Serum LDH (-60%) a slowing of disease progression and 1-3 better long-term clinical outcomes 5 Serum AST (-45%) 5 Serum ALT (-54%) Monitoring of multiple Duchenne biomarkers 5 Serum Titin (-42%) offers a powerful approach for assessing disease trajectory, with long-term assessment 5 Histologic eMHC (-59%) 4 to establish treatment effectiveness a,5 Serum Troponin (-36%) a Serum troponin data only from Participant 3 at Day 90: Participant 3 had elevated troponin levels at baseline. Troponin levels for Participants 1 & 2 were 0 at baseline. 1. Siddique Ahmed Khan M, et al. Int J Sci Res. 2016;5(11):156-157. 2. Voleti S, et al. Pediatr Cardiol. 2020;41(6):1173-1179. 3. Oshida N, et al. Sci Rep. 2019;9(1):19498. 4. FDA-NIH Biomarker Working Group. Last updated January 25, 2021. Accessed December 12, 2024. https://health.uconn.edu/pepper-center/wp-content/uploads/sites/272/2023/12/BEST-Biomarkers-EndpointS-and- 12 other-Tools-Resource.pdf. 5. Data on file as of February 11, 2025. Solid Biosciences. © 2025 Solid Biosciences
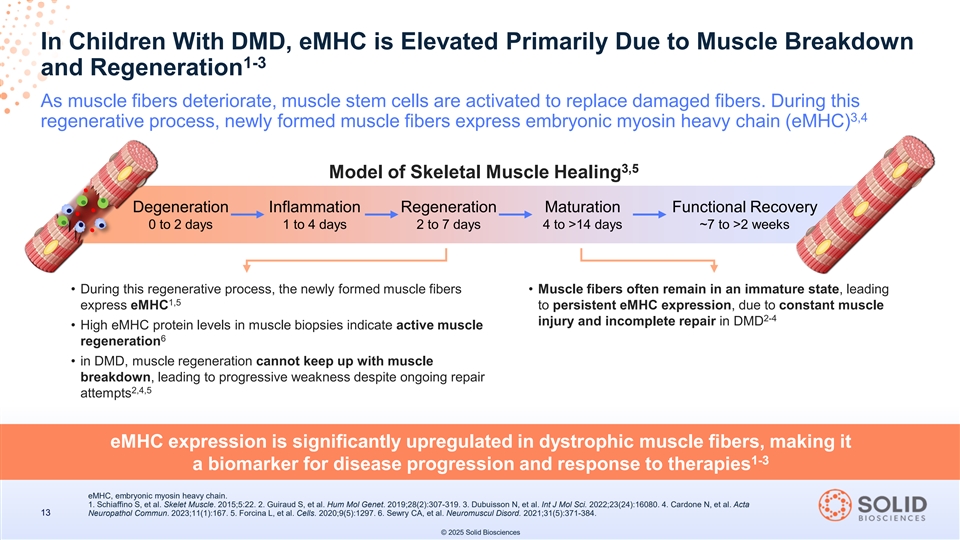
In Children With DMD, eMHC is Elevated Primarily Due to Muscle Breakdown 1-3 and Regeneration As muscle fibers deteriorate, muscle stem cells are activated to replace damaged fibers. During this 3,4 regenerative process, newly formed muscle fibers express embryonic myosin heavy chain (eMHC) 3,5 Model of Skeletal Muscle Healing Degeneration Inflammation Regeneration Maturation Functional Recovery 0 to 2 days 1 to 4 days 2 to 7 days 4 to >14 days ~7 to >2 weeks • During this regenerative process, the newly formed muscle fibers • Muscle fibers often remain in an immature state, leading 1,5 express eMHC to persistent eMHC expression, due to constant muscle 2-4 injury and incomplete repair in DMD • High eMHC protein levels in muscle biopsies indicate active muscle 6 regeneration • in DMD, muscle regeneration cannot keep up with muscle breakdown, leading to progressive weakness despite ongoing repair 2,4,5 attempts eMHC expression is significantly upregulated in dystrophic muscle fibers, making it 1-3 a biomarker for disease progression and response to therapies eMHC, embryonic myosin heavy chain. 1. Schiaffino S, et al. Skelet Muscle. 2015;5:22. 2. Guiraud S, et al. Hum Mol Genet. 2019;28(2):307-319. 3. Dubuisson N, et al. Int J Mol Sci. 2022;23(24):16080. 4. Cardone N, et al. Acta 13 Neuropathol Commun. 2023;11(1):167. 5. Forcina L, et al. Cells. 2020;9(5):1297. 6. Sewry CA, et al. Neuromuscul Disord. 2021;31(5):371-384. © 2025 Solid Biosciences
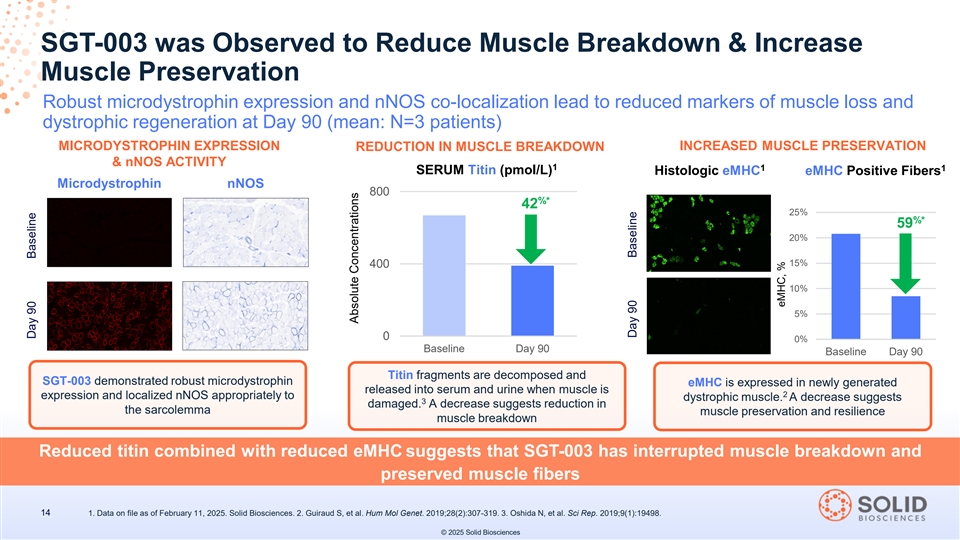
SGT-003 was Observed to Reduce Muscle Breakdown & Increase Muscle Preservation Robust microdystrophin expression and nNOS co-localization lead to reduced markers of muscle loss and dystrophic regeneration at Day 90 (mean: N=3 patients) MICRODYSTROPHIN EXPRESSION REDUCTION IN MUSCLE BREAKDOWN INCREASED MUSCLE PRESERVATION & nNOS ACTIVITY 1 1 1 SERUM Titin (pmol/L) Histologic eMHC eMHC Positive Fibers Microdystrophin nNOS 800 %* 42 25% %* 59 20% 15% 400 10% 5% 0 0% Baseline Day 90 Baseline Day 90 Titin fragments are decomposed and SGT-003 demonstrated robust microdystrophin eMHC is expressed in newly generated released into serum and urine when muscle is 2 expression and localized nNOS appropriately to dystrophic muscle. A decrease suggests 3 damaged. A decrease suggests reduction in the sarcolemma muscle preservation and resilience muscle breakdown Reduced titin combined with reduced eMHC suggests that SGT-003 has interrupted muscle breakdown and preserved muscle fibers 14 1. Data on file as of February 11, 2025. Solid Biosciences. 2. Guiraud S, et al. Hum Mol Genet. 2019;28(2):307-319. 3. Oshida N, et al. Sci Rep. 2019;9(1):19498. © 2025 Solid Biosciences Day 90 Baseline Absolute Concentrations Day 90 Baseline eMHC, %

1 Loss of Dystrophin Leads to Progressive Degeneration of Cardiac Muscle 2 Cardiomyopathy is a leading cause of death in Duchenne muscular dystrophy 3 INCIDENCE OF DUCHENNE-RELATED CARDIOMYOPATHY OCCURS EARLY IN LIFE Cardiac tissue has limited regenerative AGE 0 AGE 10 AGE 18 AGE 6 capacity: by age 25, only ~1% of cardiomyocytes will 5 turn over annually Cardiac disease 25% 59% 98% 40% is underway long evidence of evidence of evidence of of these patients before overt cardiac 4 cardiomyopathy cardiomyopathy cardiomyopathy will progress to dysfunction appears 3 3 3 3 by age 6 years by age 10 years by age 18 years heart failure Early troponin elevation is predictive of severe 6-9 Early detection of changes in the heart using troponin inform cardiac disease in neuromuscular diseases interventions to slow disease progression, improve quality of life, A hs-cTnI level >7.6 ng/L is correlated with 11 and lower the risk of severe cardiomyopathy 10 a 3-fold increased risk of cardiac disease hs-cTnI=high-sensitivity cardiac troponin. 1. Schultz TI, et al. JACC Basic Transl Sci. 2022;7(6):608-625. 2. Meyers TA, et al. Int J Mol Sci. 2019;20(17):4098. 3. Gandhi S, et al. Cells. 2024;13(14):1168. 4. James J, et al. Neuromuscul Disord. 2011;21(7):462-467. 5. Parmacek MS, Epstein JA. N Engl J Med. 2009;361(1):86-88. 6. Sheybani A, et al. Pediatr Res. 2022;92(6):1613-1620. 7. Voleti S, et al. Pediatr Cardiol. 2020;41(6):1173-1179. 8. Wagner KR, et al. Biomark Med. 2021;15(15):1389-1396. 9. Saunders JT, et al. 15 Circulation. 2011;123(13):1367-1376. 10. Spurney CF, et al. Open Heart. 2021;8(1):e001592. 11. D’Amario D, et al. Heart. 2017;103(22):1770-1779. © 2025 Solid Biosciences
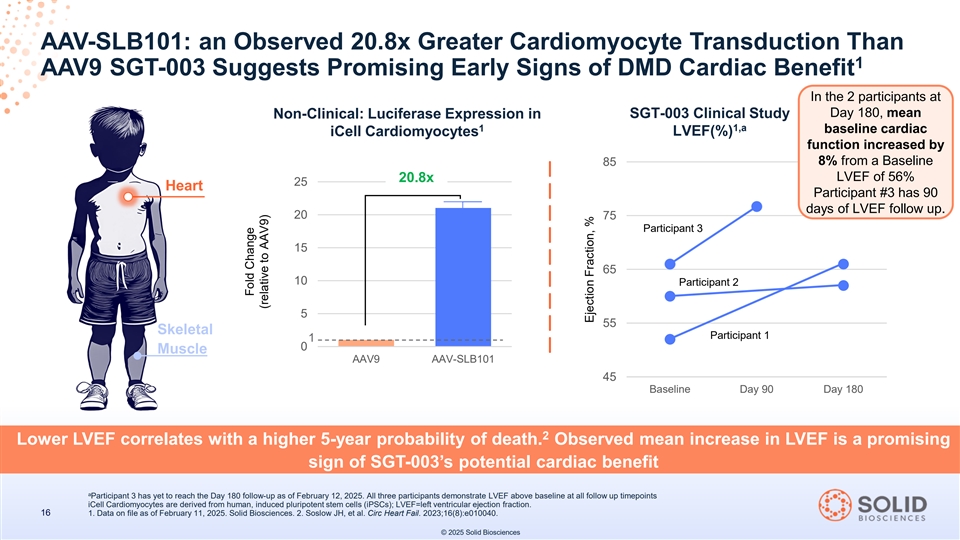
AAV-SLB101: an Observed 20.8x Greater Cardiomyocyte Transduction Than 1 AAV9 SGT-003 Suggests Promising Early Signs of DMD Cardiac Benefit In the 2 participants at Day 180, mean Non-Clinical: Luciferase Expression in SGT-003 Clinical Study 1 1,a baseline cardiac iCell Cardiomyocytes LVEF(%) function increased by 85 8% from a Baseline LVEF of 56% 20.8x 25 Heart Participant #3 has 90 days of LVEF follow up. 20 75 Participant 3 15 65 10 Participant 2 5 55 Skeletal Participant 1 1 0 Muscle AAV9 AAV-SLB101 45 Baseline Day 90 Day 180 2 Lower LVEF correlates with a higher 5-year probability of death. Observed mean increase in LVEF is a promising sign of SGT-003’s potential cardiac benefit a Participant 3 has yet to reach the Day 180 follow-up as of February 12, 2025. All three participants demonstrate LVEF above baseline at all follow up timepoints iCell Cardiomyocytes are derived from human, induced pluripotent stem cells (iPSCs); LVEF=left ventricular ejection fraction. 16 1. Data on file as of February 11, 2025. Solid Biosciences. 2. Soslow JH, et al. Circ Heart Fail. 2023;16(8):e010040. © 2025 Solid Biosciences Fold Change (relative to AAV9) Ejection Fraction, %
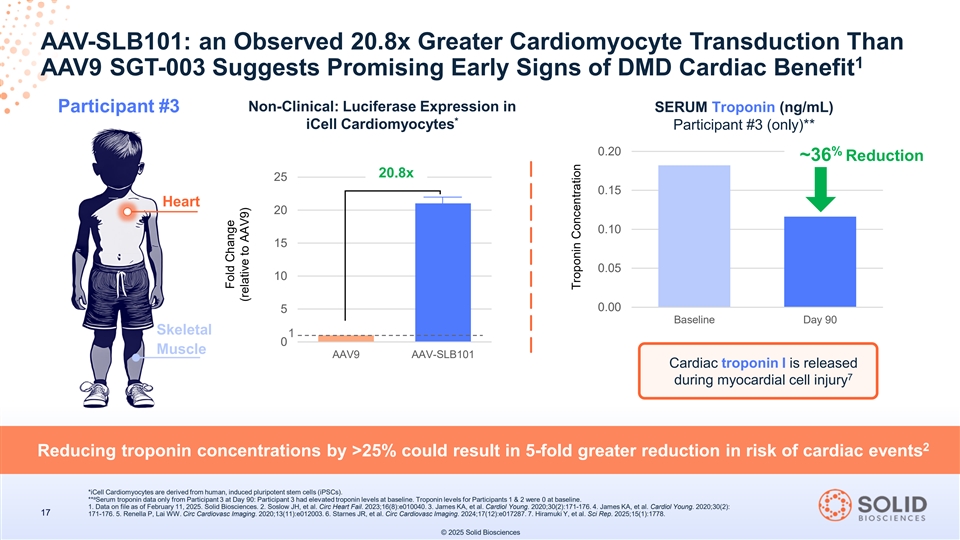
AAV-SLB101: an Observed 20.8x Greater Cardiomyocyte Transduction Than 1 AAV9 SGT-003 Suggests Promising Early Signs of DMD Cardiac Benefit Non-Clinical: Luciferase Expression in SERUM Troponin (ng/mL) Participant #3 * iCell Cardiomyocytes Participant #3 (only)** 0.20 % ~36 Reduction 20.8x 25 0.15 Heart 20 0.10 15 0.05 10 0.00 5 Baseline Day 90 Skeletal 1 0 Muscle AAV9 AAV-SLB101 Cardiac troponin I is released 7 during myocardial cell injury 2 Reducing troponin concentrations by >25% could result in 5-fold greater reduction in risk of cardiac events *iCell Cardiomyocytes are derived from human, induced pluripotent stem cells (iPSCs). a ** Serum troponin data only from Participant 3 at Day 90: Participant 3 had elevated troponin levels at baseline. Troponin levels for Participants 1 & 2 were 0 at baseline. 1. Data on file as of February 11, 2025. Solid Biosciences. 2. Soslow JH, et al. Circ Heart Fail. 2023;16(8):e010040. 3. James KA, et al. Cardiol Young. 2020;30(2):171-176. 4. James KA, et al. Cardiol Young. 2020;30(2): 17 171-176. 5. Renella P, Lai WW. Circ Cardiovasc Imaging. 2020;13(11):e012003. 6. Starnes JR, et al. Circ Cardiovasc Imaging. 2024;17(12):e017287. 7. Hiramuki Y, et al. Sci Rep. 2025;15(1):1778. © 2025 Solid Biosciences Fold Change (relative to AAV9) Troponin Concentration
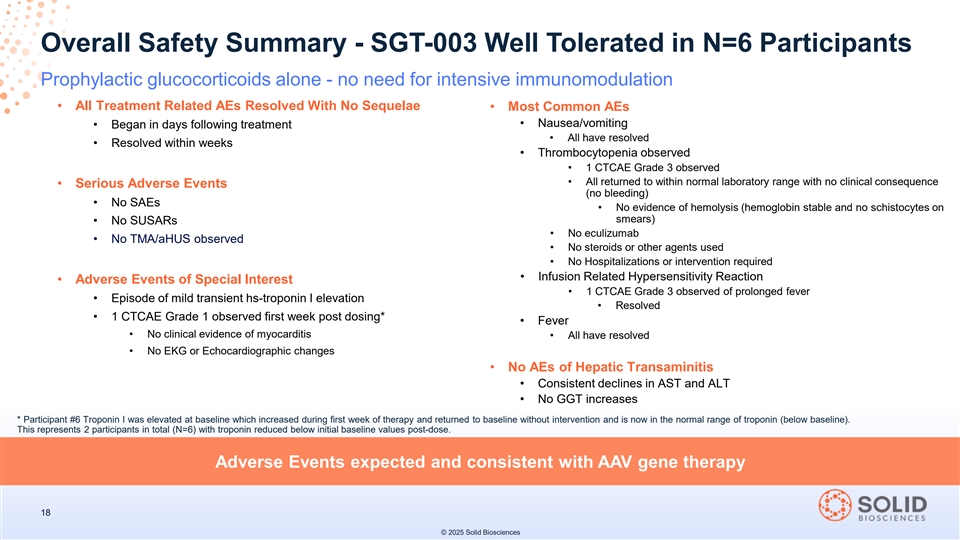
Overall Safety Summary - SGT-003 Well Tolerated in N=6 Participants Prophylactic glucocorticoids alone - no need for intensive immunomodulation • All Treatment Related AEs Resolved With No Sequelae • Most Common AEs • Nausea/vomiting • Began in days following treatment • All have resolved • Resolved within weeks • Thrombocytopenia observed • 1 CTCAE Grade 3 observed • All returned to within normal laboratory range with no clinical consequence • Serious Adverse Events (no bleeding) • No SAEs • No evidence of hemolysis (hemoglobin stable and no schistocytes on smears) • No SUSARs • No eculizumab • No TMA/aHUS observed • No steroids or other agents used • No Hospitalizations or intervention required • Infusion Related Hypersensitivity Reaction • Adverse Events of Special Interest • 1 CTCAE Grade 3 observed of prolonged fever • Episode of mild transient hs-troponin I elevation • Resolved • 1 CTCAE Grade 1 observed first week post dosing* • Fever • No clinical evidence of myocarditis • All have resolved • No EKG or Echocardiographic changes • No AEs of Hepatic Transaminitis • Consistent declines in AST and ALT • No GGT increases * Participant #6 Troponin I was elevated at baseline which increased during first week of therapy and returned to baseline without intervention and is now in the normal range of troponin (below baseline). This represents 2 participants in total (N=6) with troponin reduced below initial baseline values post-dose. Adverse Events expected and consistent with AAV gene therapy 18 © 2025 Solid Biosciences

Craig McDonald, M.D. Chair, Department of Physical Medicine & Rehabilitation, Distinguished Professor, Physical Medicine & Rehabilitation and Pediatrics, Director, Neuromuscular Disease Clinics, Director, Neuromuscular Research Laboratory, University of California Davis Health, Study Chair, CINRG Duchenne Natural History Study & Investigator, INSPIRE DUCHENNE Clinical Trial © 2025 Solid Biosciences
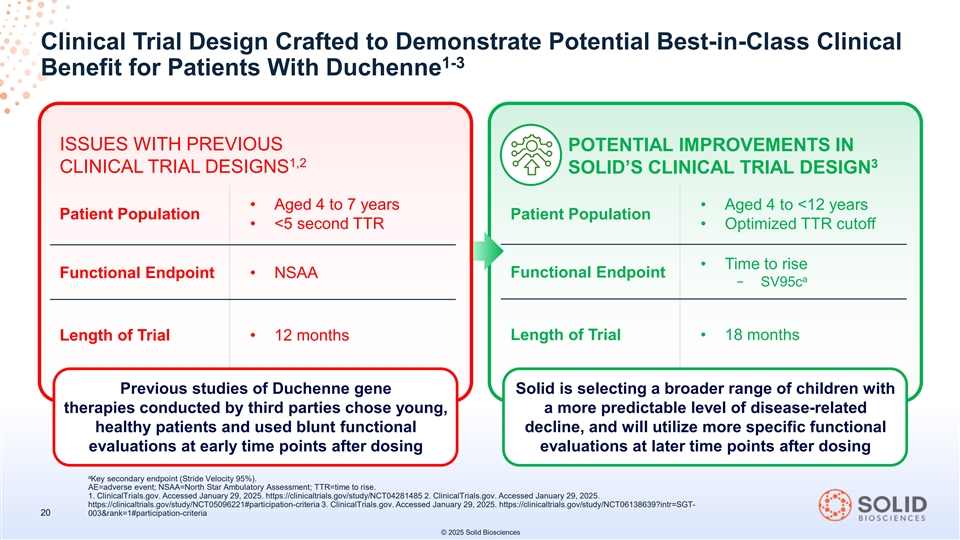
Clinical Trial Design Crafted to Demonstrate Potential Best-in-Class Clinical 1-3 Benefit for Patients With Duchenne ISSUES WITH PREVIOUS POTENTIAL IMPROVEMENTS IN 1,2 3 CLINICAL TRIAL DESIGNS SOLID’S CLINICAL TRIAL DESIGN • Aged 4 to 7 years • Aged 4 to <12 years Patient Population Patient Population • <5 second TTR • Optimized TTR cutoff • Time to rise Functional Endpoint Functional Endpoint • NSAA a − SV95c Length of Trial • 18 months Length of Trial • 12 months Previous studies of Duchenne gene Solid is selecting a broader range of children with therapies conducted by third parties chose young, a more predictable level of disease-related healthy patients and used blunt functional decline, and will utilize more specific functional evaluations at early time points after dosing evaluations at later time points after dosing a Key secondary endpoint (Stride Velocity 95%). AE=adverse event; NSAA=North Star Ambulatory Assessment; TTR=time to rise. 1. ClinicalTrials.gov. Accessed January 29, 2025. https://clinicaltrials.gov/study/NCT04281485 2. ClinicalTrials.gov. Accessed January 29, 2025. https://clinicaltrials.gov/study/NCT05096221#participation-criteria 3. ClinicalTrials.gov. Accessed January 29, 2025. https://clinicaltrials.gov/study/NCT06138639?intr=SGT- 20 003&rank=1#participation-criteria © 2025 Solid Biosciences
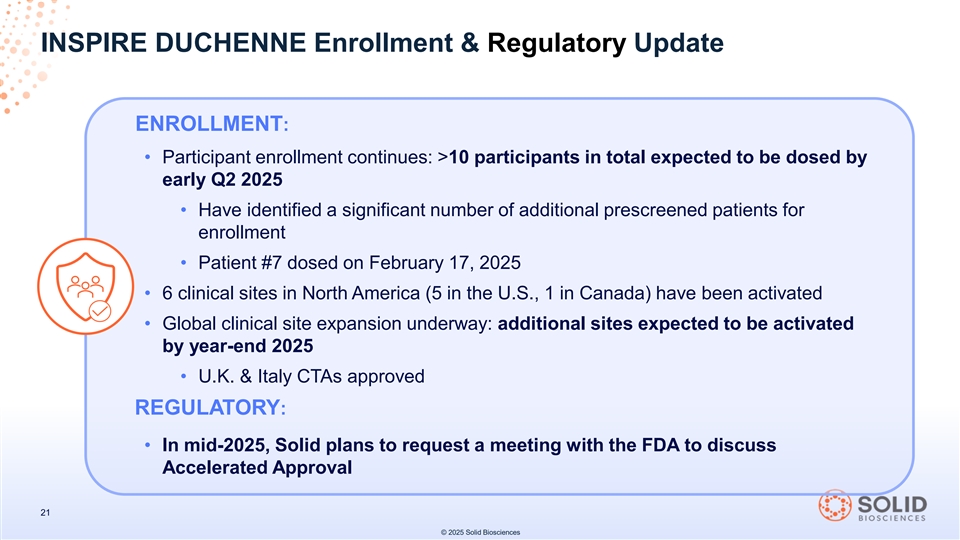
INSPIRE DUCHENNE Enrollment & Regulatory Update ENROLLMENT: • Participant enrollment continues: >10 participants in total expected to be dosed by early Q2 2025 • Have identified a significant number of additional prescreened patients for enrollment • Patient #7 dosed on February 17, 2025 • 6 clinical sites in North America (5 in the U.S., 1 in Canada) have been activated • Global clinical site expansion underway: additional sites expected to be activated by year-end 2025 • U.K. & Italy CTAs approved REGULATORY: • In mid-2025, Solid plans to request a meeting with the FDA to discuss Accelerated Approval 21 © 2025 Solid Biosciences
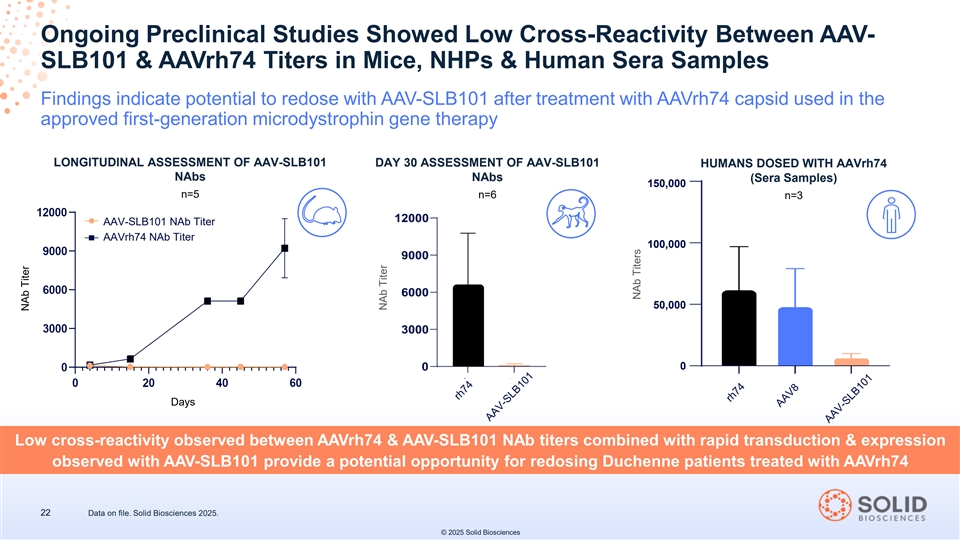
Ongoing Preclinical Studies Showed Low Cross-Reactivity Between AAV- SLB101 & AAVrh74 Titers in Mice, NHPs & Human Sera Samples Findings indicate potential to redose with AAV-SLB101 after treatment with AAVrh74 capsid used in the approved first-generation microdystrophin gene therapy LONGITUDINAL ASSESSMENT OF AAV-SLB101 DAY 30 ASSESSMENT OF AAV-SLB101 HUMANS DOSED WITH AAVrh74 NAbs NAbs (Sera Samples) 150,000 n=5 n=6 n=3 AAV-SLB101 NAb Titer AAVrh74 NAb Titer 100,000 50,000 0 Days Low cross-reactivity observed between AAVrh74 & AAV-SLB101 NAb titers combined with rapid transduction & expression observed with AAV-SLB101 provide a potential opportunity for redosing Duchenne patients treated with AAVrh74 22 Data on file. Solid Biosciences 2025. © 2025 Solid Biosciences NAb Titer NAb Titer NAb Titers

February 2025 SGT-003 INSPIRE DUCHENNE DATA UPDATE © 2025 Solid Biosciences