|
Nevada
|
|
001-37603
|
|
30-1341024
|
|
(State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
|
40 Marcus Drive
Melville, New York
|
|
11747
|
|
(Address of principal executive offices)
|
|
(Zip code)
|
|
Title of each class
|
Trading Symbol(s)
|
Name of each exchange on which registered
|
| Common Stock, $0.0001 par value |
BRTX |
NASDAQ Capital Market |
|
Item 2.02.
|
Result of Operations and Financial Condition.
|
|
Item 7.01
|
Regulation FD Disclosure.
|
|
Item 9.01
|
Financial Statements and Exhibits.
|
|
Number
|
Description
|
|
99.1
|
Press release, dated November 12, 2024, issued by BioRestorative Therapies, Inc.
|
|
99.2
|
Press release, dated November 13, 2024, issued by BioRestorative Therapies, Inc.
|
|
99.3
|
Presentation Materials
|
|
104
|
Cover Page Interactive Data File (embedded within the Inline XBRL document)
|
| Company Name | |||
|
Dated: November 13, 2024
|
By:
|
/s/ Robert Kristal |
|
| Robert Kristal |
|||
| Chief Financial Officer |
|||

|
•
|
Last week, BioRestorative announced that it received a provisional license from the
New York State Department of Health (“NYSDOH”) for the processing of allogeneic (non-autologous) donor tissue material for the isolation, expansion and cryopreservation of various cell types, including stem cells, for medical research.
Previously, the Company was licensed by the NYSDOH to act as a tissue bank for the processing of mesenchymal stem cells derived from autologous donors only.
|
|
•
|
In a podium
presentation scheduled for tomorrow morning, new preliminary 26–52 week blinded data from the ongoing Phase 2 clinical trial of BRTX-100
in subjects with chronic lumbar disc disease will be presented by Francisco Silva, Vice President of Research and Development, at the Orthopaedic Research Society (ORS) Philadelphia Spine Research Society (PSRS) 7th International Spine
Research Symposium. BioRestorative will also make the data available through a public announcement.
|
|
•
|
BioRestorative continues to work toward achieving completion of patient enrollment in
the Phase 2 BRTX-100 study before the end of 2024.
|
|
•
|
In September, the
Company shared the details of its allogeneic, off-the-shelf ThermoStem®
metabolic disease platform at IFATS 2024. BioRestorative believes that cell-based therapy candidates generated from its ThermoStem® program may allow for lower dosing, and while current GLP-1 based obesity drugs result in a loss of 20-40% lean muscle mass of total weight loss, pre-clinical studies have
demonstrated that brown fat activation leads to positive effects on several organs, including heart, liver and muscle.
|
|
•
|
In October,
BioRestorative announced that the Israel Patent Office had issued a Notice of Allowance for a new patent application (Israeli Patent Appl. No. 287557) covering several fundamental aspects of the ThermoStem® platform. This, the 14th international patent to issue outside of the U.S. for the
technology, covers non-naturally occurring three-dimensional brown adipose derived stem cell (BADSC) aggregates; an encapsulation system comprising the non-naturally occurring three-dimensional brown adipose derived stem cell aggregates; a
method of making a non-naturally occurring three-dimensional brown adipose derived stem cell aggregate; and a method of treating a patient with a disorder.
|
|
•
|
The Company’s
previously reported substantive discussions with an undisclosed commercial stage regenerative medicine company with regard to a potential license of BioRestorative’s ThermoStem® metabolic intellectual property are continuing; however, no assurances can be given that a license agreement will be entered into whether on
commercially reasonable terms or otherwise.
|
|
•
|
BioRestorative derived $230,700 in product revenue from its exclusive supply
agreement with Cartessa Aesthetics, LLC in the third quarter.
|

|
•
|
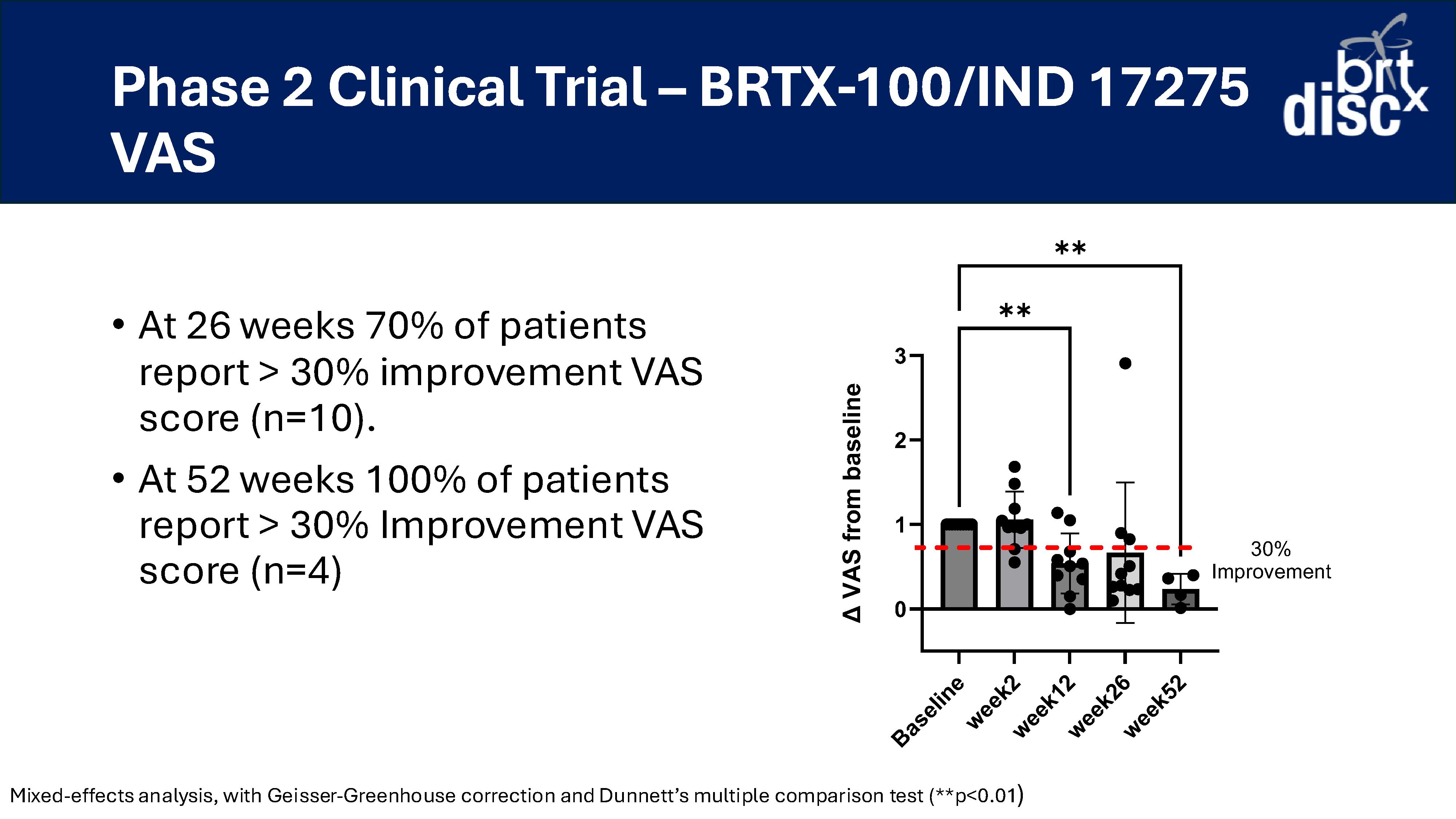
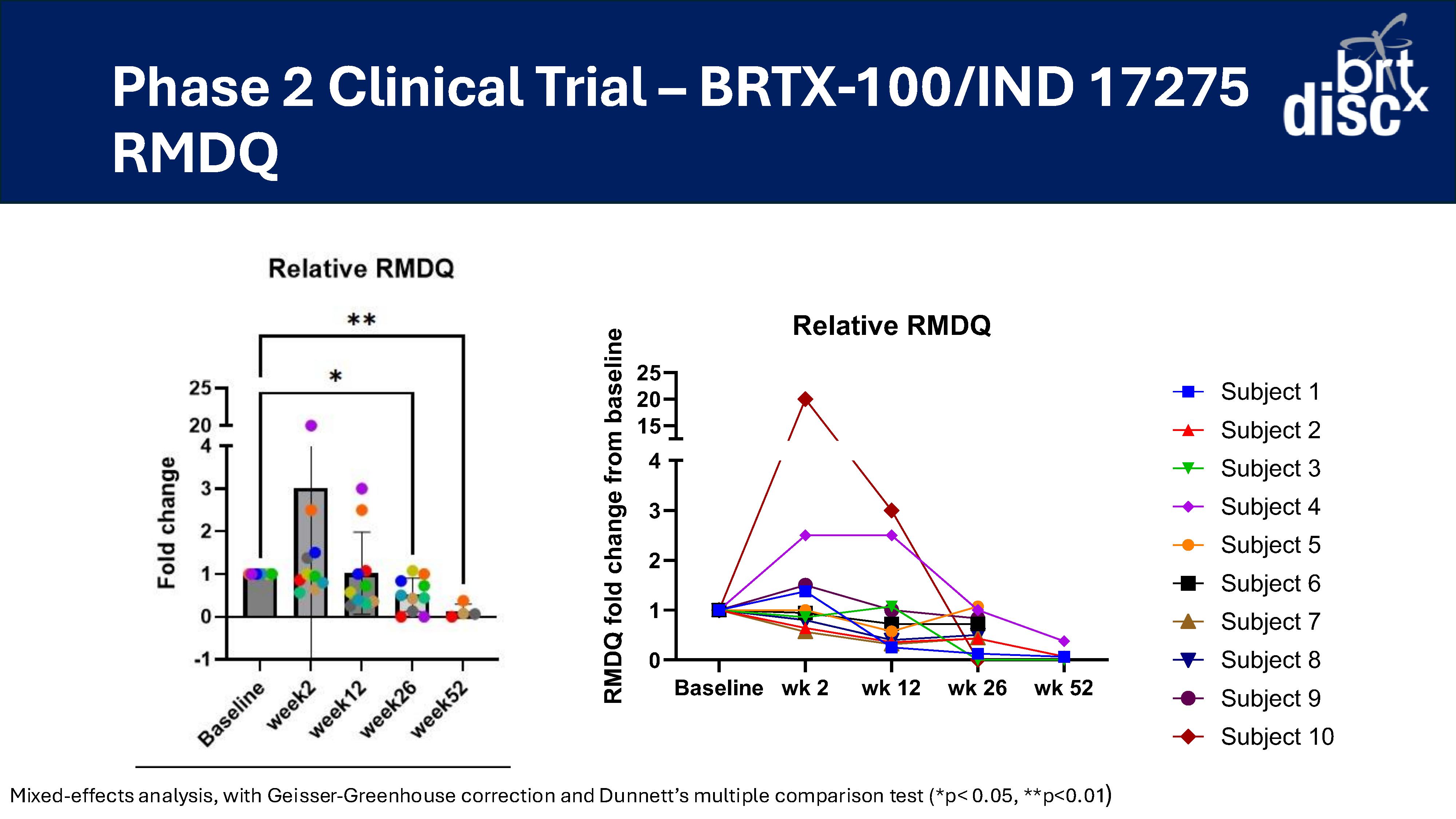
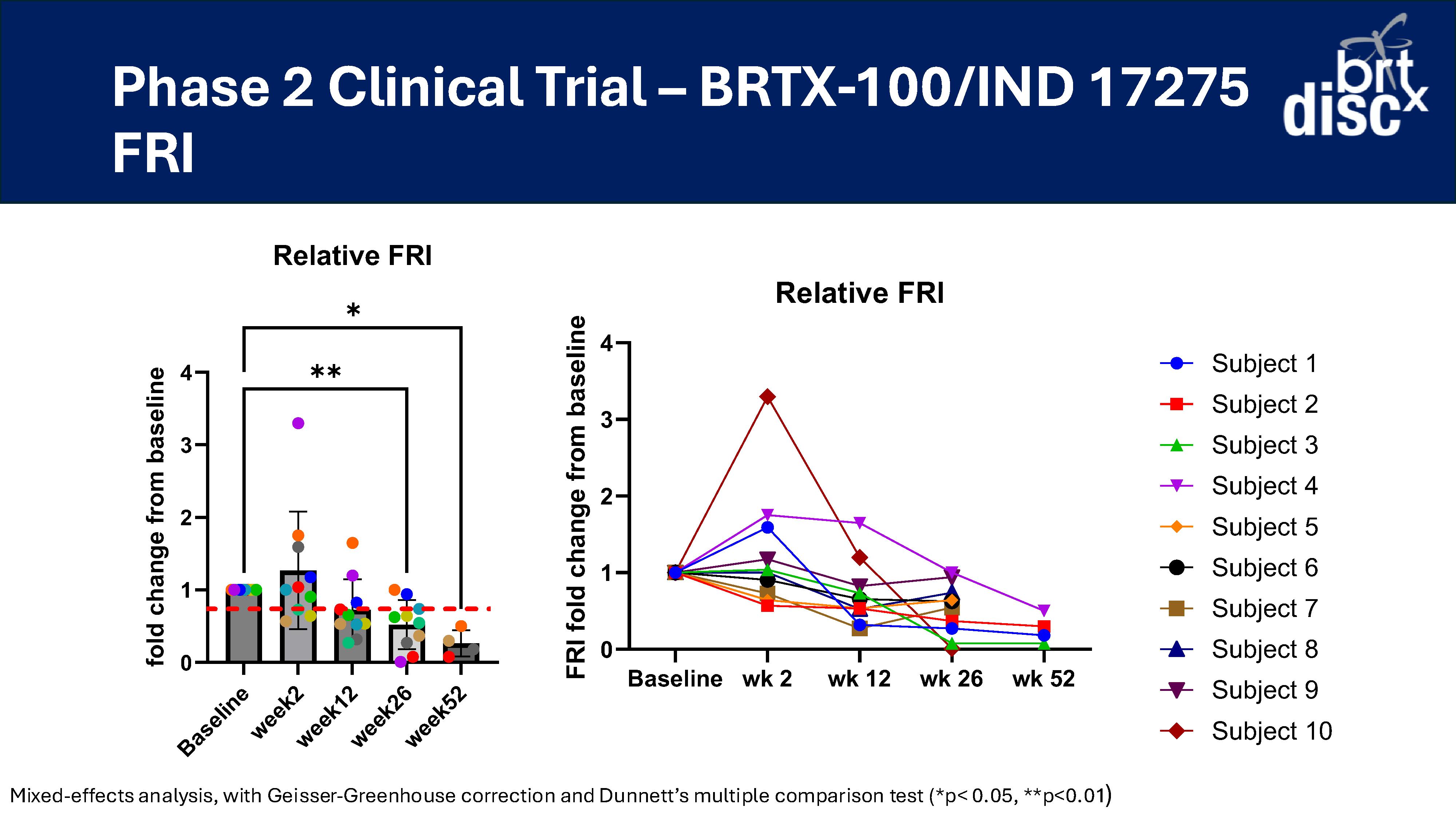
At 26 weeks, 70% of subjects (n=10) reported a >30% improvement in VAS versus baseline;
|
|
•
|
At 52 weeks, 100% of subjects (n=4) reported a >30% improvement in VAS versus baseline (n=4);
|
|
•
|
At 12 and 26 weeks, 70% of subjects (n=10) had a >30% improvement in ODI versus baseline;
|
|
•
|
At 52 weeks, 100% of subjects (n=4) had a >30% improvement in ODI versus baseline; and
|
|
•
|
At 26 weeks, 70% of subjects (n=10) reported a >30% decrease in pain (VAS) and a >30% increase in function (ODI).
|