UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 20, 2025
MONTE ROSA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
Delaware |
|
001-40522 |
|
84-3766197 |
(State or other jurisdiction |
|
(Commission |
|
(I.R.S. Employer |
321 Harrison Avenue, Suite 900
Boston, MA 02118
(Address of principal executive offices, including zip code)
(617) 949-2643
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading |
|
Name of each exchange |
Common Stock, $0.0001 par value per share |
|
GLUE |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02. Results of Operations and Financial Condition
On March 20, 2025, Monte Rosa Therapeutics, Inc. (the "Company") announced its financial results for the quarter and year ended December 31, 2024. The full text of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
Item 7.01. Regulation FD Disclosure
On March 20, 2025, the Company posted a revised presentation to the "Presentations" section of the Company's website at https://www.monterosatx.com/. A copy of the presentation is furnished as Exhibit 99.2 to this Current Report on Form 8-K.
The information under Item 2.02 and Item 7.01 in this Current Report on Form 8-K (including Exhibit 99.1 and Exhibit 99.2) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01. Financial Statements and Exhibits
(d) Exhibits
99.1 |
|
Press Release issued by Monte Rosa Therapeutics, Inc. dated March 20, 2025. |
99.2 |
|
Presentation furnished by Monte Rosa Therapeutics, Inc. on March 20, 2025. |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Monte Rosa Therapeutics, Inc. |
||
|
|
|
|
|||
Date: March 20, 2025 |
|
|
|
By: |
|
/s/ Markus Warmuth |
|
|
|
|
|
|
Markus Warmuth |
|
|
|
|
|
|
President and Chief Executive Officer |

Monte Rosa Therapeutics Announces Fourth Quarter 2024 Financial Results and Provides Corporate Update Including New Clinical Results from MRT-6160 and MRT-2359 Programs
Results of the MRT-6160 Phase 1 SAD/MAD study demonstrate deep VAV1 degradation of greater than 90%, significant T and B cell functional inhibition as well as significant inhibition of cytokine release from T and B cells following ex-vivo stimulation, and favorable safety/tolerability profile; data support clear path to Phase 2 studies and broad potential applications in immune-mediated diseases
Phase 1/2 study of MRT-2359 demonstrates encouraging signals of clinical response in castration-resistant prostate cancer (CRPC) patients resistant to AR therapy, including confirmed RECIST response; CRPC cohort will be focus moving forward with additional Phase 1/2 results expected in H2 2025; deprioritizing further expansion arms in SCLC, NSCLC and NE tumors
MRT-8102, a NEK7-directed molecular glue degrader targeting diseases driven by IL-1β and the NLRP3 inflammasome, on track for IND filing in H1 2025
Strong cash position expected to fund operations into 2028 through multiple anticipated proof-of-concept clinical readouts
Company to host conference call and webcast today at 8:00 a.m. ET
BOSTON, Mass., March 20, 2025 – Monte Rosa Therapeutics, Inc. (Nasdaq: GLUE), a clinical-stage biotechnology company developing novel molecular glue degrader (MGD)-based medicines, today reported a clinical update, business highlights, and financial results for the fourth quarter ended December 31, 2024.
“We continue to make excellent progress with our clinical and preclinical molecular glue degrader programs, targeting areas poorly addressed by conventional pharmaceutical approaches and with expansive therapeutic potential,” said Markus Warmuth, M.D., Chief Executive Officer of Monte Rosa Therapeutics. “Today, we are pleased to report new clinical results from our two clinical programs. We believe the current results from our Phase 1 study of MRT-6160 are highly encouraging and demonstrate deep VAV1 degradation, biologically meaningful levels of ex vivo T and B cell functional inhibition, including inhibition of secretion of various cytokines following ex-vivo stimulation, and a highly favorable safety/tolerability profile. These data strengthen our conviction in the broad potential application of MRT-6160 as a novel treatment approach for immune-mediated diseases, and we look forward to rapidly advancing this program alongside our collaborators at Novartis.”
Dr. Warmuth continued: “Our updated MRT-2359 results deepen our understanding of the drug’s clinical profile across several challenging-to-treat solid tumors. We have observed encouraging early signals of clinical response in heavily pretreated castration-resistant prostate cancer (CRPC), including a confirmed partial response and two patients with stable disease within the first three patients treated with MRT-2359/enzalutamide combination therapy. In light of these data, we plan to focus our ongoing MRT-2359 development efforts in CRPC, with the potential to expand this cohort to 20-30 patients, while deprioritizing other expansion arms except ER-positive breast cancer. We see CRPC as a hugely exciting opportunity for MRT-2359 with the added potential advantage for us of not having to identify biomarker positive patients, which would simplify our further clinical development. We expect to present further data from this program in the second half of the year.

Furthermore, our early-stage pipeline is also making significant strides. Our NEK7 program is on track for an IND submission for MRT-8102 in the first half of this year, supported by GLP toxicology findings that demonstrate a considerable safety margin, with a more than 200-fold exposure margin over human efficacious doses in rats and non-human primates. We’re progressing our second generation, CNS-penetrant NEK7 program towards a 2026 IND submission and have released preclinical data demonstrating impressive levels of CNS activity in multiple species. Our ‘only-in-class’ CCNE1-directed MGD program represents a unique opportunity to directly target a previously undruggable but highly validated driver oncogene. Our AI/ML-powered QuEEN™ discovery engine has been highly productive, and we are advancing several programs as we seek to expand our portfolio of oral I&I drugs targeting pathways currently served only by injectable biologics or cell therapies.”
RECENT HIGHLIGHTS
MRT-6160, VAV1-directed MGD for immune-mediated conditions

MRT-2359, GSPT1-directed MGD for MYC-driven solid tumors

NEK7-directed MGDs for inflammatory and CNS diseases driven by IL-1β and the NLRP3 inflammasome
Cyclin E1 and CDK2-directed MGD programs for treatment of solid tumors
QuEEN™ (Quantitative and Engineered Elimination of Neosubstrates) discovery engine
ANTICIPATED UPCOMING MILESTONES AND DEVELOPMENT PRIORITIES

FOURTH QUARTER AND FULL YEAR 2024 FINANCIAL RESULTS
Collaboration revenue: Collaboration revenue for the fourth quarter of 2024 was $60.6 million and $75.6 million for the year ended December 31, 2024. The Company did not record collaboration revenue in 2023. Collaboration revenue represents amounts earned from our collaboration and license agreements with Roche and Novartis.
Research and Development (R&D) Expenses: R&D expenses for the fourth quarter of 2024 were $38.9 million, compared to $27.1 million for the fourth quarter of 2023, and $121.6 million for the year ended December 31, 2024, compared to $111.3 million for the year ended December 31, 2023. These increases were driven by the successful achievement of key milestones in our R&D organization, including the continuation of the MRT-2359 clinical study, the progression and growth of our preclinical pipeline, including research performed in connection with our collaboration with Roche, the advancement of MRT-6160 to enter the clinic, and the continued development of the Company’s QuEEN™ discovery engine. Non-cash stock-based compensation constituted $2.7 million of R&D expenses for Q4 2024, compared to $2.2 million in the same period in 2023, and $10.6 million and $8.9 million for the years ended December 31, 2024 and 2023, respectively.
General and Administrative (G&A) Expenses: G&A expenses for the fourth quarter of 2024 were $8.8 million compared to $7.7 million for the fourth quarter of 2023, and $35.2 million for the year ended December 31, 2024, compared to $32.0 million for the year ended December 31, 2023. The increase in G&A expenses was a result of increased headcount and expenses in support of the Company’s growth and operations. G&A expenses included non-cash stock-based compensation of $1.8 million for the fourth quarter of 2024 and 2023, and $7.5 million and $7.7 million for the years ended December 31, 2024 and 2023, respectively.
Net Income (Loss): Net income for the fourth quarter of 2024 was $13.4 million, compared to a net loss of $33.3 million for the fourth quarter of 2023, and net losses of $72.7 million for the year ended December 31, 2024, compared to $135.4 million for the year ended December 31, 2023.
Cash Position and Financial Guidance: Cash, cash equivalents, restricted cash, and marketable securities as of December 31, 2024, were $377 million, compared to cash, cash equivalents, restricted cash, and marketable securities of $247.1 million as of September 30, 2024. The increase of $129.9 million was primarily related to upfront payment received from Novartis in connection with our license agreement.
Based on current cash, cash equivalents, restricted cash, marketable securities, the Company expects its cash and cash equivalents to be sufficient to fund planned operations and capital expenditures into 2028.
Investor Conference Call
Monte Rosa will host a conference call and webcast presentation today, March 20, 2025, at 8:00 a.m. ET. A webcast of the presentation will be accessible via the “Events & Presentations” section of Monte Rosa’s website at ir.monterosatx.com.

Registration for the conference call is available at the following link. An archived version of the webcast will be made available for 30 days following the presentation.
About MRT-2359
MRT-2359 is a potent, highly selective, and orally bioavailable investigational molecular glue degrader (MGD) that induces the interaction between the E3 ubiquitin ligase component cereblon and the translation termination factor GSPT1, leading to the targeted degradation of GSPT1 protein. The MYC transcription factors (c‑MYC, L-MYC and N-MYC) are well-established drivers of human cancers that maintain high levels of protein translation, which is critical for uncontrolled cell proliferation and tumor growth. Preclinical studies have shown this addiction to MYC-induced protein translation creates a dependency on GSPT1. By inducing degradation of GSPT1, MRT-2359 is designed to exploit this vulnerability, disrupting the protein synthesis machinery, leading to anti-tumor activity in MYC-driven tumors.
About MRT-6160
MRT-6160 is a potent, highly selective, and orally bioavailable investigational molecular glue degrader of VAV1, which in preclinical studies has shown deep degradation of its target with no detectable effects on other proteins. VAV1, a Rho-family guanine nucleotide exchange factor, is a key signaling protein downstream of both the T- and B-cell receptors. VAV1 expression is restricted to immune cells, including T and B cells. Preclinical studies have shown that targeted degradation of VAV1 protein via an MGD modulates both T- and B-cell receptor-mediated activity. This modulation is evident both in vitro and in vivo, demonstrated by a significant decrease in cytokine secretion, proteins vital for maintaining autoimmune diseases. MRT-6160 has shown promising activity in preclinical models of multiple immune-mediated conditions. Under the terms of an agreement announced in October 2024, Novartis has exclusive worldwide rights to develop, manufacture and commercialize MRT-6160 and other VAV1 MGDs.
About MRT-8102
MRT-8102 is a potent, highly selective, and orally bioavailable investigational molecular glue degrader (MGD) that targets NEK7 for the treatment of inflammatory diseases driven by IL-1β and the NLRP3 inflammasome. NEK7 has been shown to be required for NLRP3 inflammasome assembly, activation and IL-1β release both in vitro and in vivo. Aberrant NLRP3 inflammasome activation and the subsequent release of active IL-1β and interleukin-18 (IL-18) has been implicated in multiple inflammatory disorders, including gout, cardiovascular disease, neurologic disorders including Parkinson’s disease and Alzheimer’s disease, ocular disease, diabetes, obesity, and liver disease. In a non-human primate model, MRT-8102 was shown to potently, selectively, and durably degrade NEK7, and resulted in near-complete reductions of IL-1β models following ex vivo stimulation of whole blood. MRT-8102 has shown a favorable profile in non-GLP toxicology studies.
About Monte Rosa
Monte Rosa Therapeutics is a clinical-stage biotechnology company developing highly selective molecular glue degrader (MGD) medicines for patients living with serious diseases in the areas of oncology, autoimmune and inflammatory diseases, and more. MGDs are small molecule protein degraders that have the potential to treat many diseases that other modalities, including other degraders, cannot. Monte Rosa’s QuEEN™ (Quantitative and Engineered Elimination of Neosubstrates) discovery engine combines AI-guided chemistry, diverse chemical libraries, structural biology, and proteomics to identify degradable protein targets and rationally design MGDs with unprecedented selectivity. The QuEEN discovery engine enables access to a wide-ranging and differentiated target space of well-validated biology across multiple therapeutic areas.

Monte Rosa has developed the industry’s leading pipeline of MGDs, which spans oncology, autoimmune and inflammatory disease and beyond. Monte Rosa has a global license agreement with Novartis to advance VAV1-directed molecular glue degraders and a strategic collaboration with Roche to discover and develop MGDs against targets in cancer and neurological diseases previously considered impossible to drug. For more information, visit www.monterosatx.com.
Forward-Looking Statements
This communication includes express and implied “forward-looking statements,” including forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include all statements that are not historical facts and in some cases, can be identified by terms such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward-looking statements contained herein include, but are not limited to, statements about our ability to grow our product pipeline, our ability to successfully complete research and further development and commercialization of our drug candidates in current or future indications, including the timing and results of our clinical trials and our ability to conduct and complete clinical trials, statements regarding our belief that the current results from our Phase 1 study of MRT-6160 are highly encouraging and our conviction in the broad potential application of MRT-6160 as a novel treatment approach for immune-mediated diseases, statements about our ongoing MRT-2359 development plans and efforts and our belief regarding encouraging early signals of clinical responses in CRPC, statements regarding the opportunities for MRT-2359 in the CRPC population and advantages in connection thereto for the company’s clinical development plan, statements related to the potential of our ‘only-in-class’ CCNE1-directed MGD program to directly target a previously undruggable but highly validated driver oncogene, statements around the Company’s QuEENTM discovery engine and the Company’s view of its potential to identify degradable protein targets and rationally design MGDs with unprecedented selectivity, statements about the advancement and timeline of our preclinical and clinical programs, pipeline and the various products therein, including (i) our ongoing clinical development of our GSPT1 degrader referred to as MRT-2359, statements relating to our disclosure of data from our Phase 1/2 study, including safety, biomarker data and clinical activity, our ability to continue and expand the enrollment of patients and statements regarding the timing for data readouts in the second half of 2025 and beyond, statements related to prioritization of certain indications , (ii) the ongoing development of our VAV1-directed degrader, referred to as MRT-6160, statements related to the Phase 1 clinical data, including statements related to deep degradation and significance of results, as well as the safety profile, and its clear path into anticipated Phase 2 studies in collaboration with Novartis and our expectations regarding the broad potential applications in multiple immune-mediated diseases, (iii) the ongoing development and progress of our NEK7-directed MGD, referred to as MRT-8102, including our expectations to submit an IND to the FDA in the first half of 2025, and our statements around multiple anticipated preclinical and/or clinical studies, readouts and their expected timing, including results from proof-of-concept patient studies, candidates and their applicability to various indications, (iv) the ongoing development of a second-generation NEK7-directed MGD optimized for CNS penetration and our statements around expected IND submission in 2026, (v) statements around the progress of both our CDK2 and cyclin E1-directed MGD programs, including statements around timing of submission of IND applications for such programs, the expected potential clinical benefit of any of our candidates, statements around the advancement and application of our platform, statements around our ability to capitalize on and potential benefits resulting from our research and translational insights, including announcements related to preclinical programs, as well as our the ability to optimize collaborations with industry partners on our development programs, statements about obligations under our collaboration agreements, expectations around the receipt of any payments under such agreements and the future development and commercialization of various products, statements regarding regulatory filings for our development programs, including the planned timing of such regulatory filings, such as IND applications, and potential review by regulatory authorities, our use of capital, expenses and other financial results in the future, availability of funding for existing programs, ability to fund operations into 2028, as well as our expectations of success for our programs, strength of collaboration relationships and the strength of our financial position, among others.

By their nature, these statements are subject to numerous risks and uncertainties, including those risks and uncertainties set forth in our most recent Annual Report on Form 10-K for the year ended December 31, 2024, filed with the U.S. Securities and Exchange Commission on March 20, 2025, and any subsequent filings, that could cause actual results, performance or achievement to differ materially and adversely from those anticipated or implied in the statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our statements are reasonable, we cannot guarantee that the future results, performance, or events and circumstances described in the forward-looking statements will be achieved or occur. Recipients are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date such statements are made and should not be construed as statements of fact. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, any future presentations, or otherwise, except as required by applicable law. Certain information contained in these materials and any statements made orally during any presentation of these materials that relate to the materials or are based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of these materials, we have not independently verified, and make no representations as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in these materials relating to or based on such internal estimates and research.

Consolidated Balance Sheets |
||||||||
(in thousands, except share amounts) |
||||||||
|
|
December 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Assets |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
224,254 |
|
|
$ |
128,101 |
|
Marketable securities |
|
|
147,895 |
|
|
|
104,312 |
|
Other receivables |
|
|
173 |
|
|
|
505 |
|
Prepaid expenses and other current assets |
|
|
5,118 |
|
|
|
3,294 |
|
Total current assets |
|
|
377,440 |
|
|
|
236,212 |
|
Property and equipment, net |
|
|
29,483 |
|
|
|
33,803 |
|
Operating lease right-of-use assets |
|
|
26,831 |
|
|
|
28,808 |
|
Restricted cash |
|
|
4,863 |
|
|
|
4,580 |
|
Other long-term assets |
|
|
115 |
|
|
|
352 |
|
Total assets |
|
$ |
438,732 |
|
|
$ |
303,755 |
|
Liabilities and stockholders’ equity |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
17,215 |
|
|
$ |
11,152 |
|
Accrued expenses and other current liabilities |
|
|
18,785 |
|
|
|
14,600 |
|
Current deferred revenue |
|
|
117,232 |
|
|
|
17,678 |
|
Current portion of operating lease liability |
|
|
3,714 |
|
|
|
3,162 |
|
Total current liabilities |
|
|
156,946 |
|
|
|
46,592 |
|
Deferred revenue, net of current |
|
|
16,147 |
|
|
|
32,323 |
|
Defined benefit plan liability |
|
|
3,702 |
|
|
|
2,713 |
|
Operating lease liability |
|
|
39,001 |
|
|
|
42,877 |
|
Total liabilities |
|
|
215,796 |
|
|
|
124,505 |
|
Commitments and contingencies |
|
|
|
|
|
|
||
Stockholders’ equity |
|
|
|
|
|
|
||
Common stock, $0.0001 par value; 500,000,000 shares authorized, 61,507,446 shares issued and outstanding as of December 31, 2024; and 50,154,929 shares issued and 50,140,233 shares outstanding as of December 31, 2023 |
|
|
6 |
|
|
|
5 |
|
Additional paid-in capital |
|
|
664,874 |
|
|
|
547,857 |
|
Accumulated other comprehensive loss |
|
|
(3,356 |
) |
|
|
(2,724 |
) |
Accumulated deficit |
|
|
(438,588 |
) |
|
|
(365,888 |
) |
Total stockholders’ equity |
|
|
222,936 |
|
|
|
179,250 |
|
Total liabilities and stockholders’ equity |
|
$ |
438,732 |
|
|
$ |
303,755 |
|

Consolidated Statements of Operations and Comprehensive Income (Loss) |
||||||||||||||||
(In thousands) |
||||||||||||||||
|
|
Three months ended |
|
|
Year ended |
|
||||||||||
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
||||
Collaboration revenue |
|
$ |
60,647 |
|
|
$ |
— |
|
|
$ |
75,622 |
|
|
$ |
— |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
|
38,866 |
|
|
|
27,135 |
|
|
|
121,563 |
|
|
|
111,272 |
|
General and administrative |
|
|
8,777 |
|
|
|
7,728 |
|
|
|
35,171 |
|
|
|
32,039 |
|
Total operating expenses |
|
|
47,643 |
|
|
|
34,863 |
|
|
|
156,734 |
|
|
|
143,311 |
|
Income (loss) from operations |
|
|
13,004 |
|
|
|
(34,863 |
) |
|
|
(81,112 |
) |
|
|
(143,311 |
) |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Interest income, net |
|
|
2,595 |
|
|
|
2,368 |
|
|
|
10,566 |
|
|
|
9,334 |
|
Foreign currency exchange gain (loss), net |
|
|
2 |
|
|
|
(779 |
) |
|
|
416 |
|
|
|
(930 |
) |
Gain on disposal of fixed assets |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
24 |
|
Loss on sale of marketable securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(131 |
) |
Total other income |
|
|
2,597 |
|
|
|
1,589 |
|
|
|
10,982 |
|
|
|
8,297 |
|
Net income (loss) before income taxes |
|
$ |
15,601 |
|
|
$ |
(33,274 |
) |
|
$ |
(70,130 |
) |
|
$ |
(135,014 |
) |
Provision for income taxes |
|
|
(2,164 |
) |
|
|
22 |
|
|
|
(2,570 |
) |
|
|
(338 |
) |
Net income (loss) |
|
$ |
13,437 |
|
|
$ |
(33,252 |
) |
|
$ |
(72,700 |
) |
|
$ |
(135,352 |
) |
Investors
Andrew Funderburk
ir@monterosatx.com
Media
Cory Tromblee, Scient PR
media@monterosatx.com
###

Proteome Editing Through Molecular Glue Degraders Innovating Beyond New Heights | March 2025

Forward-Looking Statements This communication includes express and implied “forward-looking statements,” including forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward-looking statements contained herein include, but are not limited to, statements about our ability to grow our product pipeline, statements around the Company's QuEENTM discovery engine and the Company's view of its potential to identify degradable protein targets and rationally design MGDs with unprecedented selectivity, statements related to the Company's strategic agreements, goals of such agreements, including the ability to accelerate and broaden scope of clinical development of MRT-6160 while retaining substantial value for the Company, as well as to expand platform reach to discover and develop MGDs against previously undruggable targets in cancer and neurological diseases, statements related to any milestone provided under the strategic agreements, royalty or other payments related thereto and the ability of such payments to extend our runway, statements around the productivity of the QuEEN discovery engine and the potential of the Company's MGDs against a broad spectrum of targets and potential applications thereof, statements about the advancement and timeline of its preclinical and clinical programs, including beliefs and/or statements related to next steps for specific programs and plans for such programs, statements about our ability to successfully complete research and further development and commercialization of our drug candidates in current or future indications, including the timing and results of our clinical trials and our ability to conduct and complete clinical trials, statements around multiple anticipated preclinical and/or clinical readouts and their expected timing, statements related to regulatory submissions, including timing thereof, and interactions with regulatory authorities, the applicability of candidates to various indications, the expected potential clinical benefit of any of our candidates, statements related to safety profiles and the implications thereof, statements around advancement and application of our pipeline and application of our platform, statements concerning our expectations regarding our ability to identify, nominate and the timing of our nominations of additional targets, product candidates, and development candidates, statements around our ability to capitalize on and potential benefits resulting from our research and translational insights as well as our the ability to optimize collaborations with industry partners on our development programs, obligations under our collaboration agreements, expectations around the receipt of any payments under such agreements and the future development and commercialization of various products, our use of capital, expenses and other financial results in the future, availability of funding for existing programs, ability to fund operations into 2028 through multiple anticipated proof-of-concept clinical readouts, as well as our expectations of success for our programs, strength of collaboration relationships and the strength of our financial position, among others. By their nature, these statements are subject to numerous risks and uncertainties, including those risks and uncertainties set forth in our most recent Annual Report on Form 10-K for the year ended December 31, 2024 filed with the U.S. Securities and Exchange Commission on March 20, 2025, and any subsequent filings, that could cause actual results, performance or achievement to differ materially and adversely from those anticipated or implied in the statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our statements are reasonable, we cannot guarantee that the future results, performance, or events and circumstances described in the forward-looking statements will be achieved or occur. Recipients are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date such statements are made and should not be construed as statements of fact. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, any future presentations, or otherwise, except as required by applicable law. Certain information contained in these materials and any statements made orally during any presentation of these materials that relate to the materials or are based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of these materials, we have not independently verified, and make no representations as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in these materials relating to or based on such internal estimates and research. These materials remain the proprietary intellectual property of Monte Rosa Therapeutics and should not be distributed or reproduced in whole or in part without the prior written consent of Monte Rosa Therapeutics.
Monte Rosa Therapeutics – Company Overview Proteome editing with molecular glue degraders Unique and differentiated “only in class” portfolio Arsenal of rationally designed MGDs to edit the proteome by degrading proteins with unprecedented precision Industry-leading discovery engine combining use of AI with experimental platform to enable rational design of novel MGDs Strong financial position providing cash runway into 2028 through multiple anticipated proof-of-concept clinical readouts

Monte Rosa’s rationally designed MGDs have potential applications in oncology, immunology, neuroscience and other therapeutic areas Our Molecular Glue Degraders (MGDs) Edit the Proteome Ternary complex Ubiquitination Proteasome-mediated degradation of neosubstrate Ubiquitin chain Neosubstrate Ligase Neosubstrate MGD MGD Neosubstrate (target protein) Ligase

Interrogating surfaces using geometric deep learning informs reprogrammable ligase and matching target space… Key Insights into Surface Interactions Drive Only-in-Class MGD Designs E3 ligase Neosubstrate footprint MGD footprint E3 ligase neosurface

...and creates broad opportunity to eliminate undruggable, disease-driving proteins through “only-in-class” MGDs Key Insights into Surface Interactions Drive Only-in-Class MGD Designs Grow Target Space (e.g., I&I, neurology) Activate New E3 Ligases Expand Chemical Space (MGD Library) CRBN MGDs Ligases
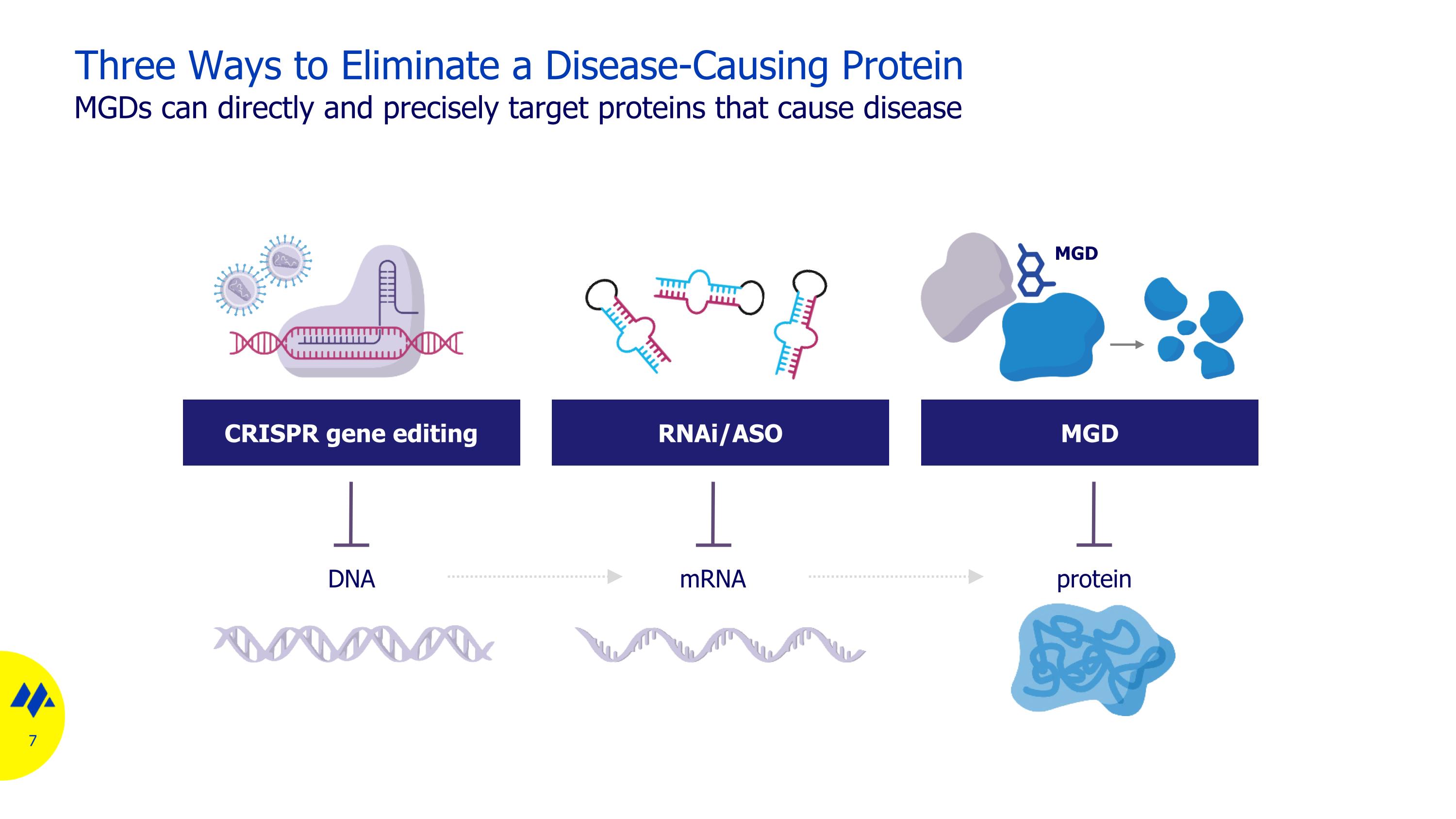
Three Ways to Eliminate a Disease-Causing Protein MGDs can directly and precisely target proteins that cause disease DNA mRNA protein CRISPR gene editing RNAi/ASO MGD MGD

Three Ways to Eliminate a Disease-Causing Protein MGDs provide advantages of large molecule modalities with orally dosed small molecules DNA mRNA protein CRISPR RNAi/ASO MGD Address undruggable space Properties Highly selective Orally bioavailable Scalable manufacturing nucleus MGD Reversible CRISPR RNAi/ASO MGD Systemic distribution
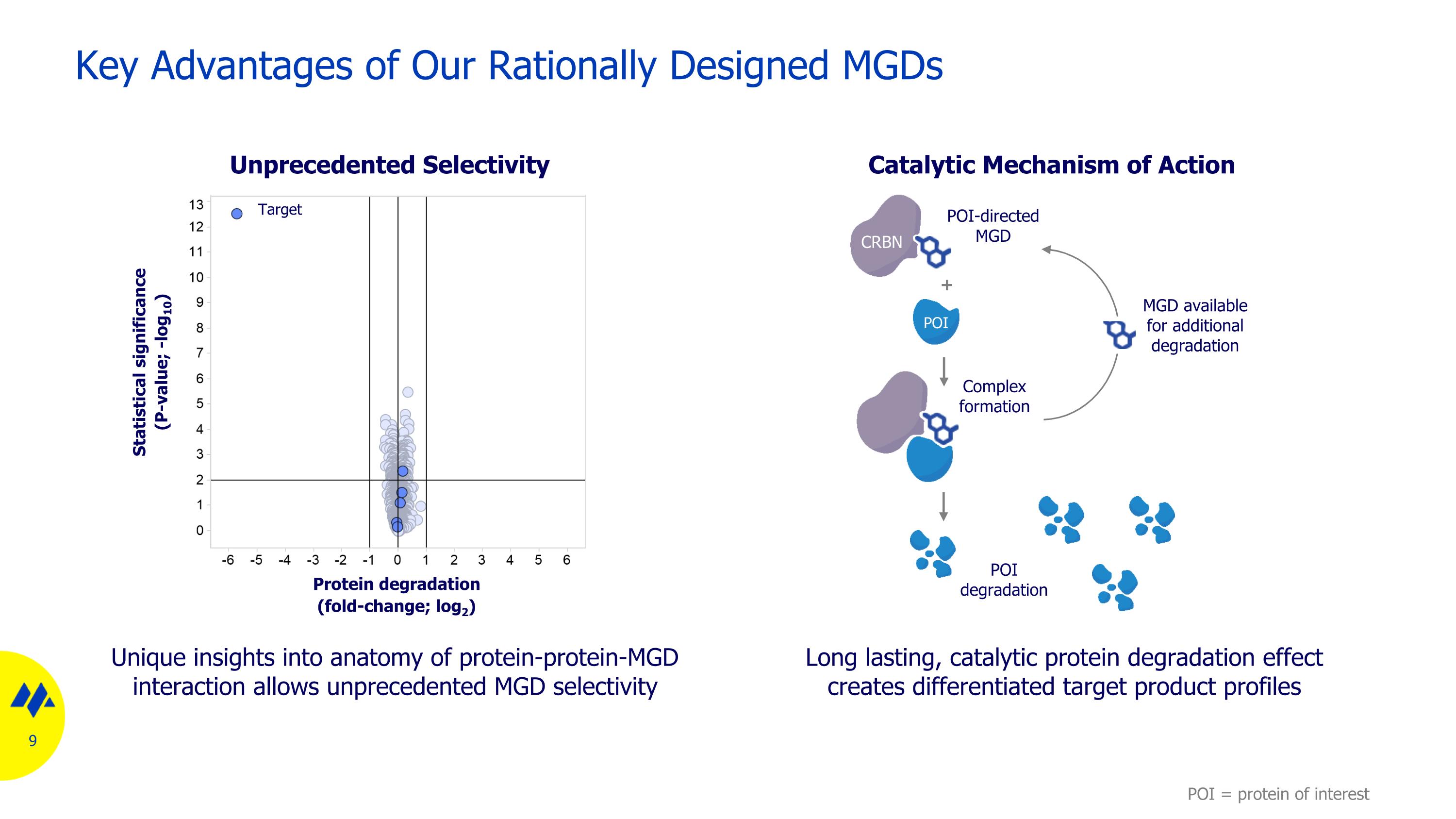
Key Advantages of Our Rationally Designed MGDs Unique insights into anatomy of protein-protein-MGD interaction allows unprecedented MGD selectivity Unprecedented Selectivity Long lasting, catalytic protein degradation effect creates differentiated target product profiles Catalytic Mechanism of Action Protein degradation (fold-change; log2) Statistical significance (P-value; -log10) Target CRBN POI POI-directed MGD + Complex formation POI degradation MGD available for additional degradation POI = protein of interest

Portfolio and Partnerships
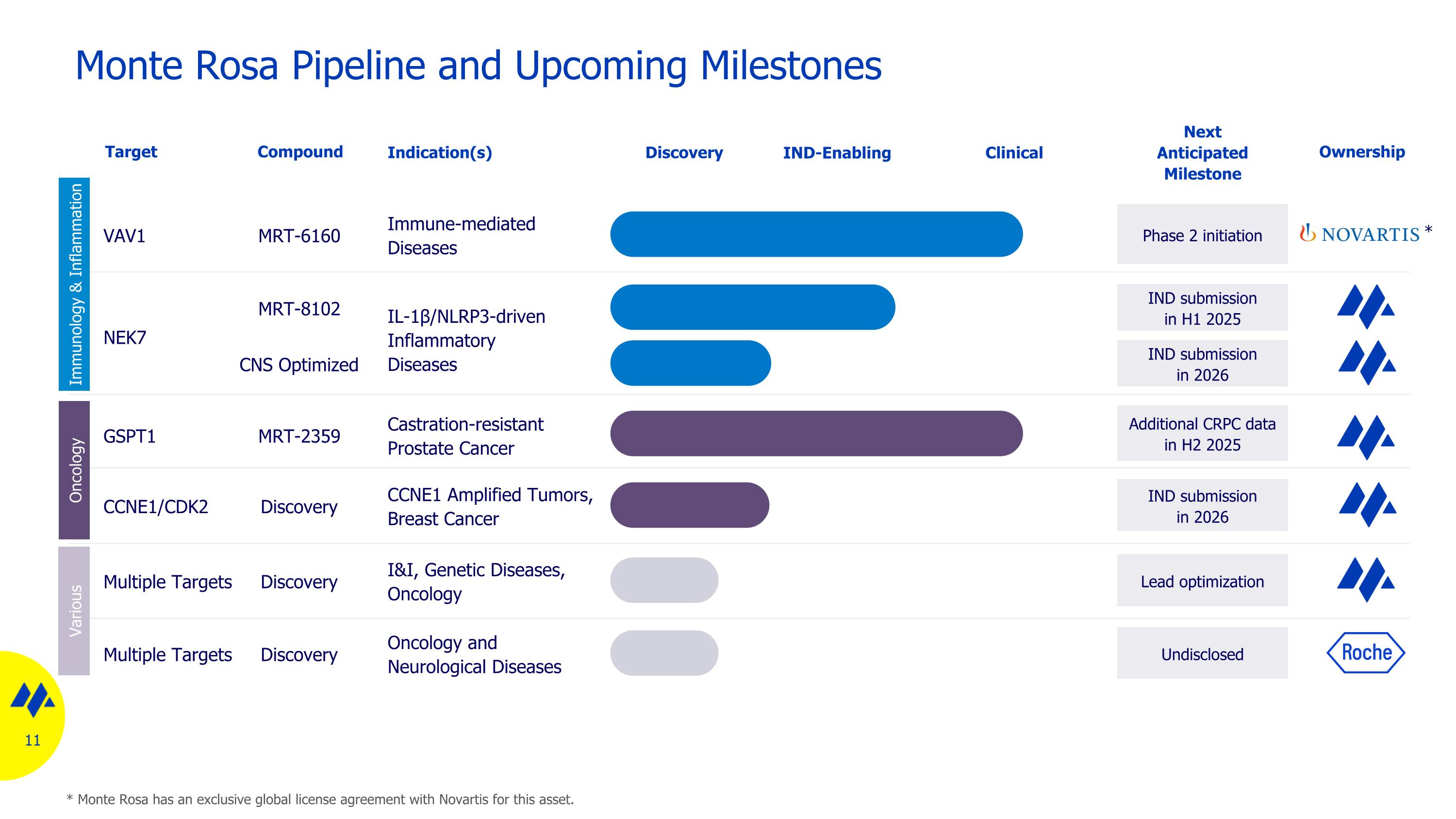
Monte Rosa Pipeline and Upcoming Milestones GSPT1 Castration-resistant Prostate Cancer IL-1β/NLRP3-driven Inflammatory Diseases VAV1 Immune-mediated Diseases Discovery Target Indication(s) Additional CRPC data in H2 2025 Next Anticipated Milestone Ownership Multiple Targets I&I, Genetic Diseases, Oncology IND-Enabling Clinical Lead optimization IND submissionin H1 2025 Phase 2 initiation Multiple Targets Oncology and Neurological Diseases Undisclosed NEK7 Compound MRT-2359 MRT-6160 MRT-8102 Discovery Discovery IND submission in 2026 CNS Optimized CCNE1/CDK2 CCNE1 Amplified Tumors, Breast Cancer IND submission in 2026 Discovery * * Monte Rosa has an exclusive global license agreement with Novartis for this asset. Immunology & Inflammation Oncology Various
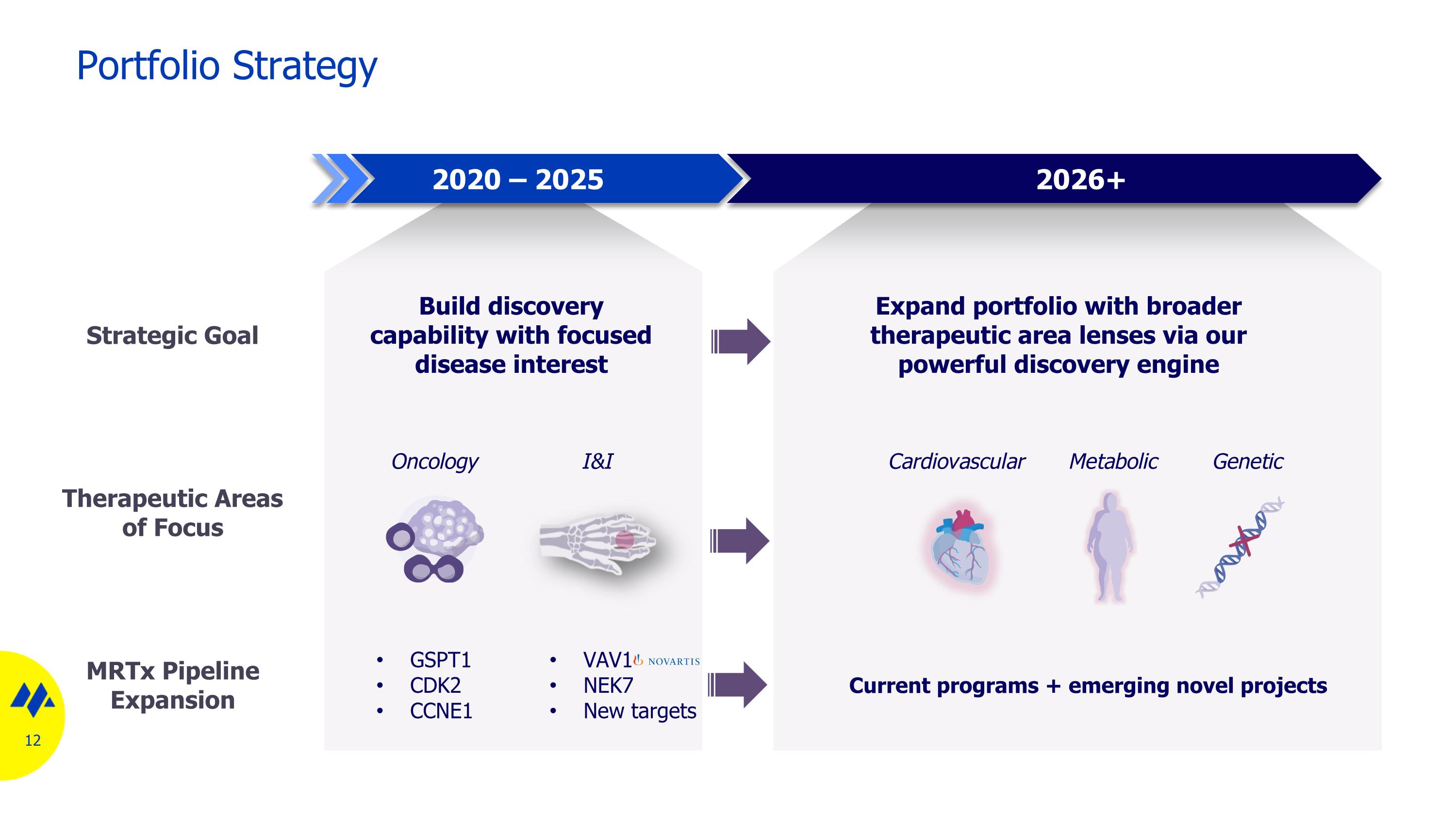
Portfolio Strategy Build discovery capability with focused disease interest Expand portfolio with broader therapeutic area lenses via our powerful discovery engine Cardiovascular Metabolic Current programs + emerging novel projects Genetic 2020 – 2025 2026+ Strategic Goal Therapeutic Areas of Focus MRTx Pipeline Expansion I&I GSPT1 CDK2 CCNE1 VAV1 NEK7 New targets Oncology
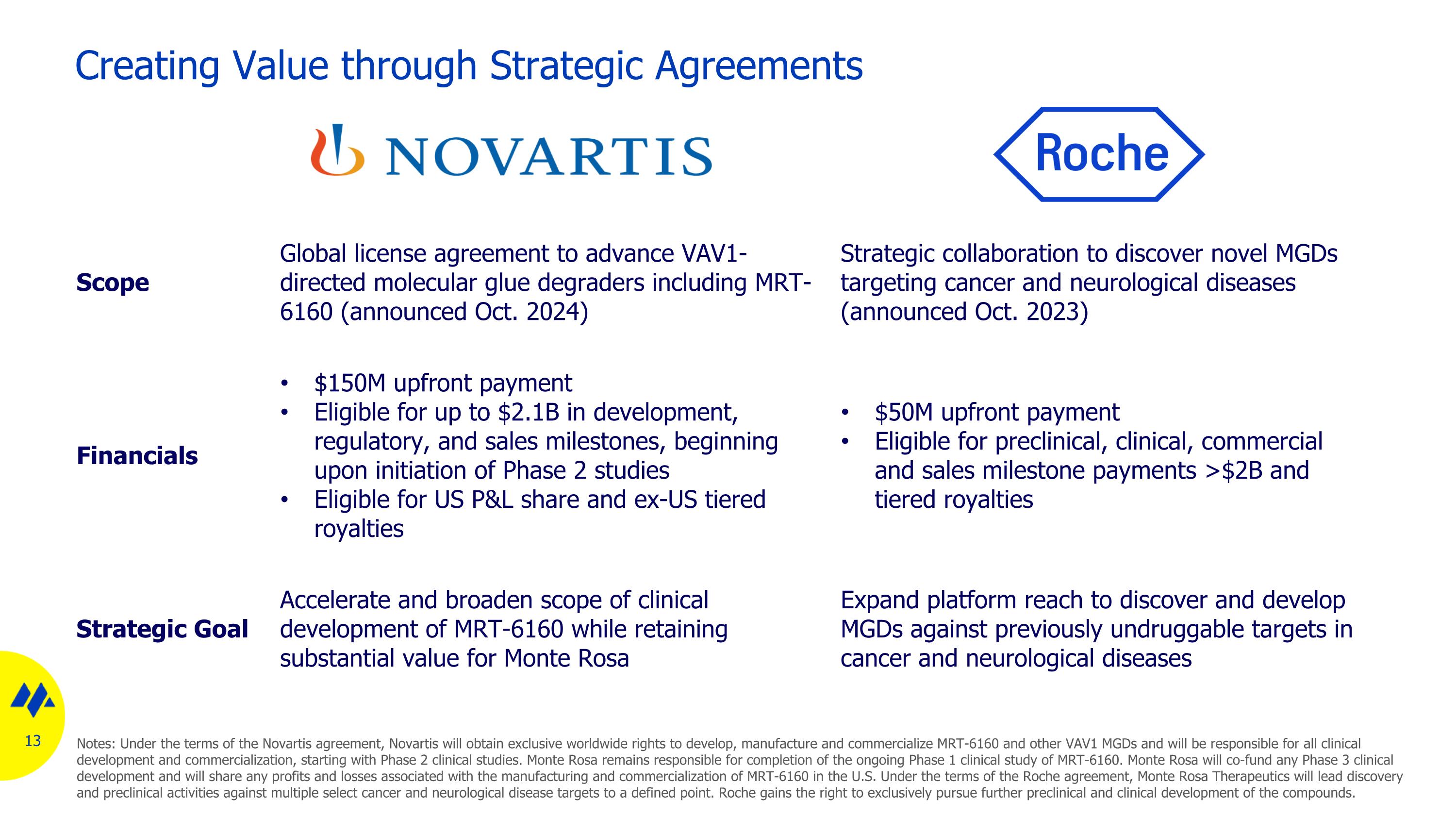
Creating Value through Strategic Agreements Scope Global license agreement to advance VAV1-directed molecular glue degraders including MRT-6160 (announced Oct. 2024) Strategic collaboration to discover novel MGDs targeting cancer and neurological diseases (announced Oct. 2023) Financials $150M upfront payment Eligible for up to $2.1B in development, regulatory, and sales milestones, beginning upon initiation of Phase 2 studies Eligible for US P&L share and ex-US tiered royalties $50M upfront payment Eligible for preclinical, clinical, commercial and sales milestone payments >$2B and tiered royalties Strategic Goal Accelerate and broaden scope of clinical development of MRT-6160 while retaining substantial value for Monte Rosa Expand platform reach to discover and develop MGDs against previously undruggable targets in cancer and neurological diseases Notes: Under the terms of the Novartis agreement, Novartis will obtain exclusive worldwide rights to develop, manufacture and commercialize MRT-6160 and other VAV1 MGDs and will be responsible for all clinical development and commercialization, starting with Phase 2 clinical studies. Monte Rosa remains responsible for completion of the ongoing Phase 1 clinical study of MRT-6160. Monte Rosa will co-fund any Phase 3 clinical development and will share any profits and losses associated with the manufacturing and commercialization of MRT-6160 in the U.S. Under the terms of the Roche agreement, Monte Rosa Therapeutics will lead discovery and preclinical activities against multiple select cancer and neurological disease targets to a defined point. Roche gains the right to exclusively pursue further preclinical and clinical development of the compounds.

VAV1 Program (MRT-6160)
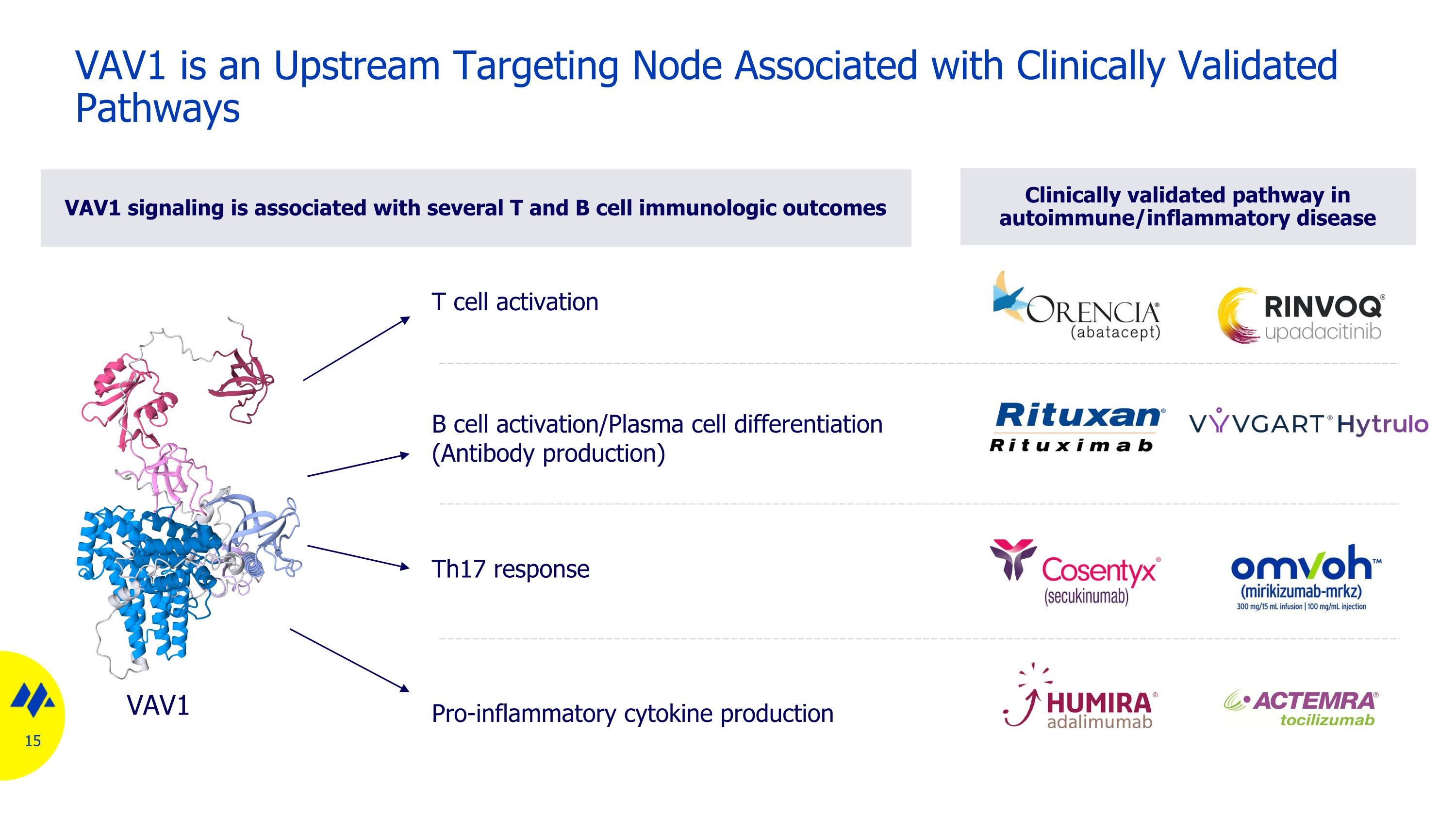
Clinically validated pathway in autoimmune/inflammatory disease VAV1 is an Upstream Targeting Node Associated with Clinically Validated Pathways T cell activation B cell activation/Plasma cell differentiation (Antibody production) Pro-inflammatory cytokine production Th17 response VAV1 signaling is associated with several T and B cell immunologic outcomes VAV1

MRT-6160 is a Potent and Highly Selective VAV1-directed MGD in vitro data CRBN binding, IC50 670 nM Ternary complex, EC50 11 nM Degradation, DC50 /Dmax (Jurkat) 7 nM / 97 % MRT-6160 induces highly selective VAV1 degradation and has a favorable ADME/DMPK profile MRT-6160 is a potent VAV1-directed MGD ADMET profile CYP DDIs IC50 > 30 µM hERG inhibition patch clamp EC50 > 30 µM Oral bioavailability all species > 50% p-value (-log10) Protein fold-change (log2) No degradation of other known cereblon neosubstrates
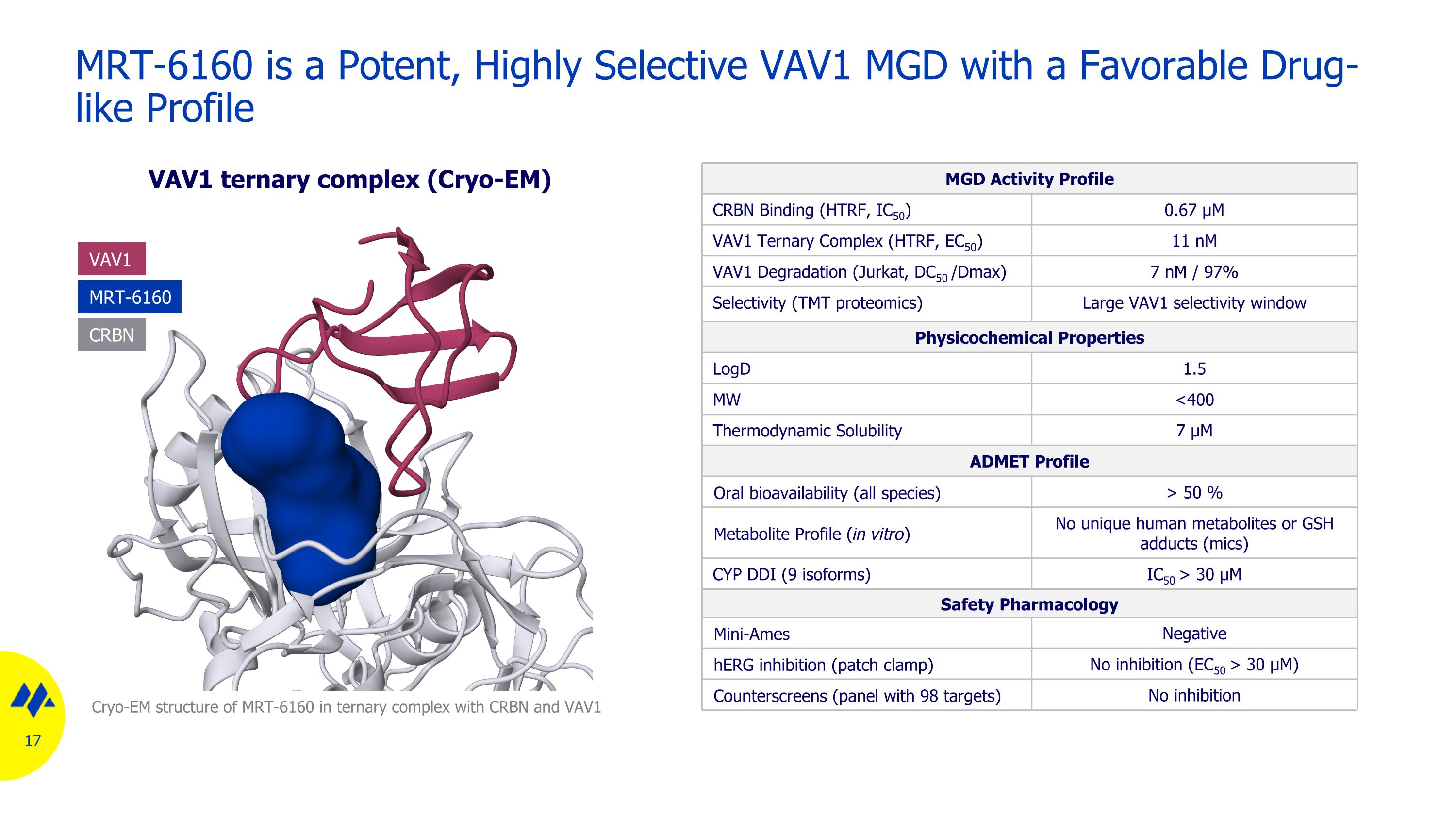
MRT-6160 is a Potent, Highly Selective VAV1 MGD with a Favorable Drug-like Profile MGD Activity Profile CRBN Binding (HTRF, IC50) 0.67 µM VAV1 Ternary Complex (HTRF, EC50) 11 nM VAV1 Degradation (Jurkat, DC50 /Dmax) 7 nM / 97% Selectivity (TMT proteomics) Large VAV1 selectivity window Physicochemical Properties LogD 1.5 MW <400 Thermodynamic Solubility 7 µM ADMET Profile Oral bioavailability (all species) > 50 % Metabolite Profile (in vitro) No unique human metabolites or GSH adducts (mics) CYP DDI (9 isoforms) IC50 > 30 μM Safety Pharmacology Mini-Ames Negative hERG inhibition (patch clamp) No inhibition (EC50 > 30 µM) Counterscreens (panel with 98 targets) No inhibition Cryo-EM structure of MRT-6160 in ternary complex with CRBN and VAV1 MRT-6160 VAV1 CRBN VAV1 ternary complex (Cryo-EM)

MRT-6160 Blocks T-cell-mediated B-cell Activity in BioMAP® Profile Upadacitinib, 1000 nM Deucravacitinib, 400 nM Ibrutinib, 1100 nM MRT-6160, 1000 nM Azathioprine, 100 μM BT coculture assay: T-cell-mediated B-cell activity T-cell independent JAKi TYK2i BTKi VAV1 MGD Azathioprine Relative protein expression levels Decreased T / B-Effector Function: IL-17A, IL-17F, IL-6, IL-2, TNF, sIgG BioMAP® Diversity Plus Platform (Eurofins). Shark tooth plots show relative expression levels of indicated proteins in Drug treated vs. DMSO controls. 3C/4H, Venular endothelial cells; LPS/SAg, Venular endothelial cells + PBMC; BT, PBMC + B cells; BF4T, Bronchial epithelial cells + dermal fibroblasts; BE3C. Bronchial epithelial cells; CASM3C, Coronary artery smooth muscle cells; HDF5CGF, Dermal fibroblasts; KF3CT, keratinocytes + dermal fibroblasts; MyoF, lung fibroblasts; IMphg, macrophages + venular epithelial cells 18
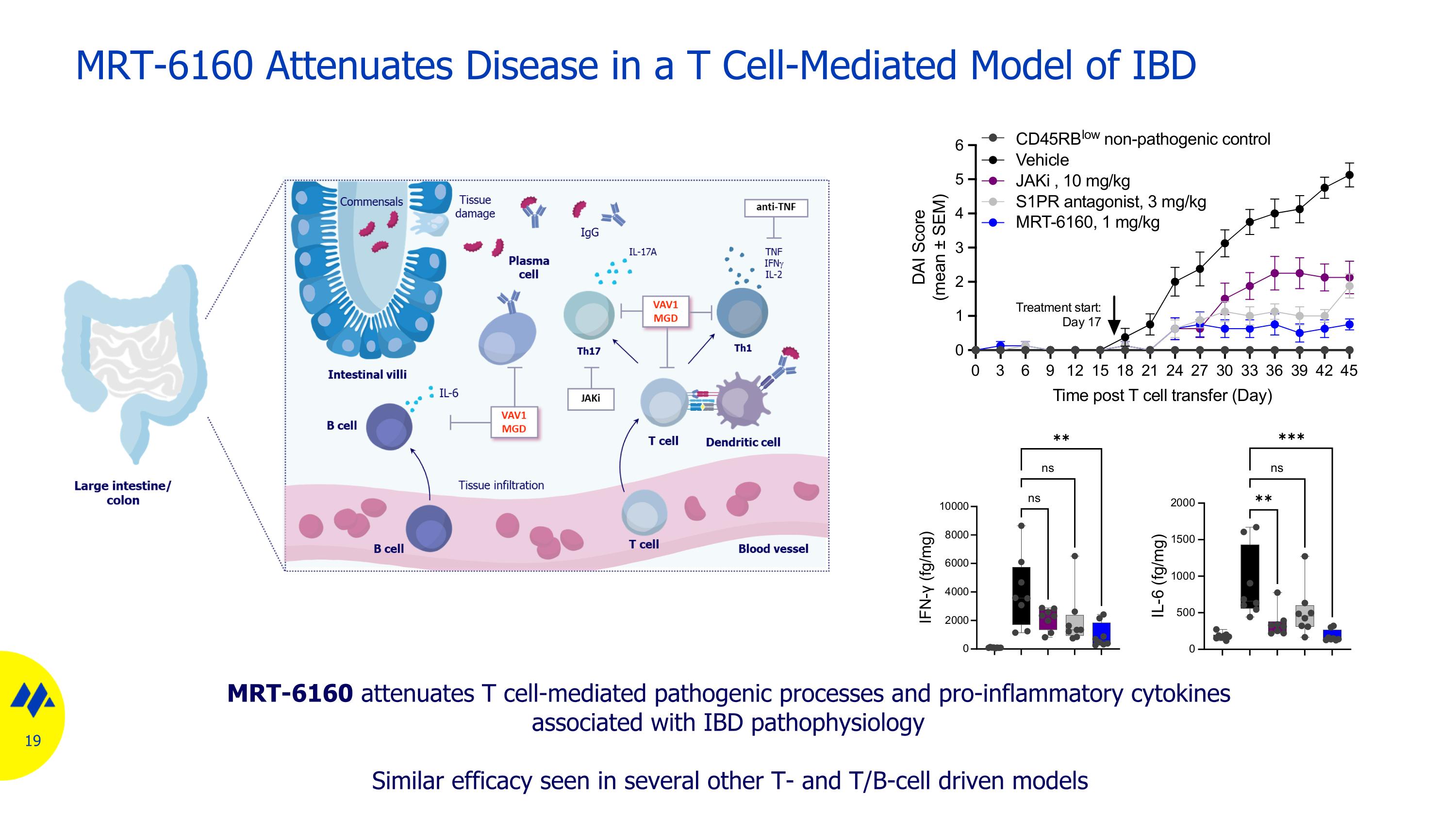
MRT-6160 Attenuates Disease in a T Cell-Mediated Model of IBD MRT-6160 attenuates T cell-mediated pathogenic processes and pro-inflammatory cytokines associated with IBD pathophysiology Similar efficacy seen in several other T- and T/B-cell driven models

Humira, Enbrel Taltz, Cosentyx Actemra, Kevzara Vyvgart Ocrevus, Rituxan Rinvoq, Xeljanz, Olumiant Sotyktu VAV1: Unique Mechanism with Broad Potential Applications Potential to address multiple autoimmune diseases with safe, oral therapy Note: Chart adapted from Hosack et al., Nat Rev Immunol 2023. Drug class sales from Evaluate Pharma. 2030E sales may include sales from anticipated future approvals. Psoriasis Ulcerative colitis Crohn’s disease Psoriatic arthritis Rheumatoid arthritis Multiple Sclerosis SLE Example Drugs TNF FcRN 2030E Drug Class Sales (I&I indications only) VAV1 Overlap Evidence of VAV1 mechanistic overlap T-cell mediated T/B-cell mediated IL17A IL6 Myasthenia gravis CD20 JAK TYK2 Approved in indication Investigational $9B $16B $3B $14B $14B $14B $3B

Safety, PK and PD Data from SAD and MAD Healthy Volunteers Phase 1 Trial of VAV1 MGD MRT-6160

MRT-6160 Phase 1 Healthy Volunteers Study: Design and Objectives All cohorts randomized & placebo controlled SAD cohorts MAD cohorts One oral dose 7 daily oral doses SAD DL5 SAD DL4 SAD DL3 SAD DL2 SAD DL1 MAD DL3 MAD DL2 MAD DL1 Study Endpoints Primary Safety and tolerability Secondary & exploratory Pharmacokinetics Pharmacodynamics VAV1 degradation in T & B cells Ex vivo response to TCR- and BCR-stimulation Enrolled > 70 subjects

MRT-6160 Displayed a Dose-Dependent Human Pharmacokinetic Profile Dose Level T1/2 (hr) Cmax (ng/mL) AUC0-24 (hr*ng/mL) 1 24.1± 2.6 18.3 ± 1.0 300.3 ± 16.4 2 26.8 ± 1.9 32.6 ± 2.2 527.0 ± 30.3 3 33.3 ± 2.9 100.9 ± 4.6 1736.3 ± 67.1 4 28.3 ± 1.6 216.2 ± 7.0 3615.2 ± 190.8 5 27.2± 1.7 399.0 ± 31.1 6593.7 ± 369.8 Plasma concentration vs time Pharmacokinetic data (AVE ± SEM) MAD ~2-fold increase in exposure at steady state No food effect
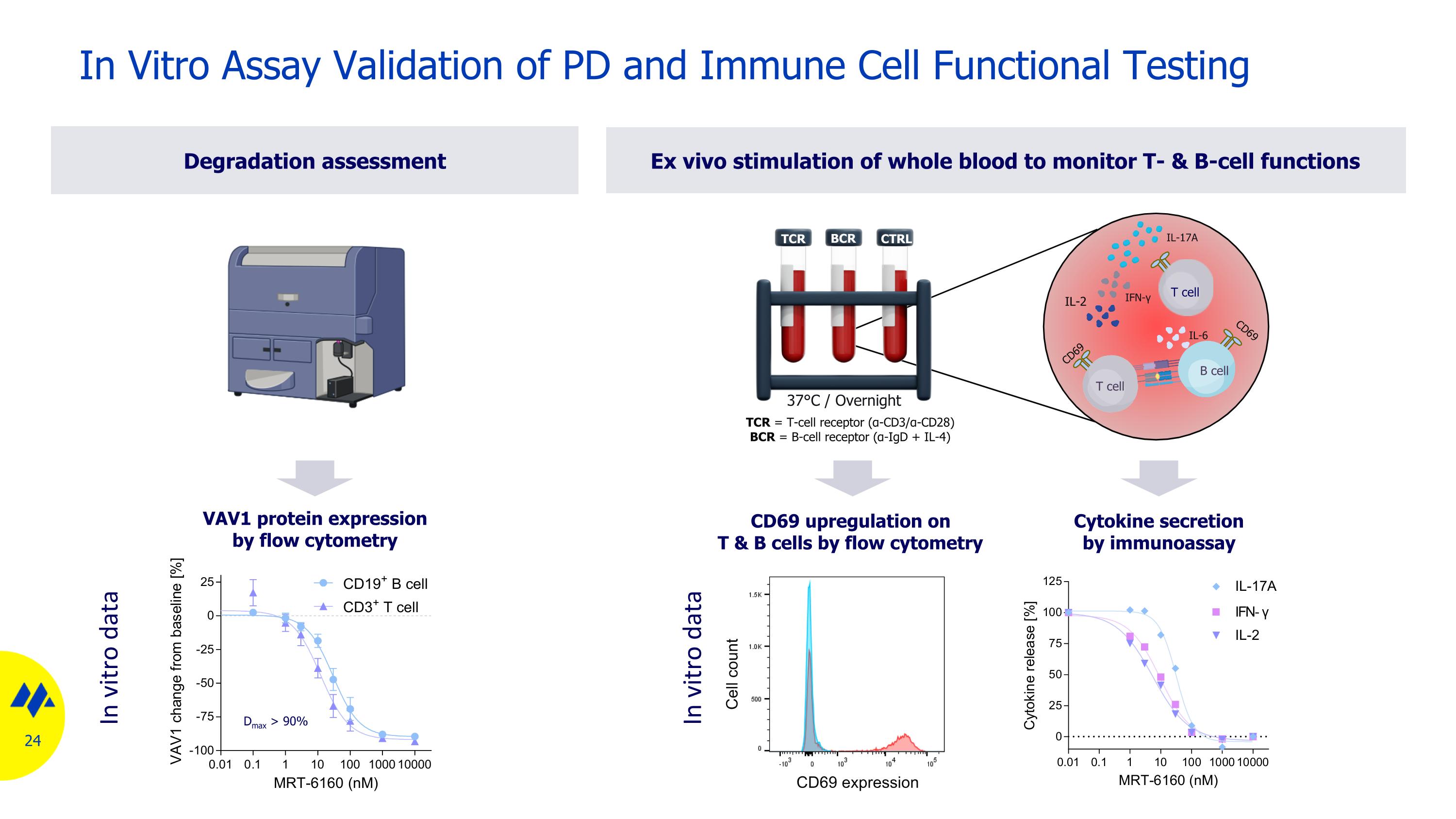
In Vitro Assay Validation of PD and Immune Cell Functional Testing Degradation assessment Ex vivo stimulation of whole blood to monitor T- & B-cell functions CD69 upregulation on T & B cells by flow cytometry VAV1 protein expression by flow cytometry CD69 IL-2 T cell B cell T cell IL-17A CD69 TCR = T-cell receptor (α-CD3/α-CD28) BCR = B-cell receptor (α-IgD + IL-4) IL-6 IFN-γ 37°C / Overnight TCR BCR CTRL Cytokine secretion by immunoassay CD69 expression Cell count Dmax > 90% In vitro data In vitro data

MRT-6160 Achieved Dose-Dependent VAV1 Degradation >90% in Peripheral Blood T Cells After Single and Multiple Dose Administration Dose-dependent, marked degradation of VAV1 in peripheral blood T cells (> 90%; except DL1) Similar results observed in peripheral blood B cells VAV1 protein reduction is sustained, with dose-dependent recovery post treatment SAD MAD Mean ± SEM

VAV1 Degradation by MRT-6160 Resulted in Significant Functional Inhibition of T and B Cells following a Single Dose Administration MRT-6160 treatment: Significantly attenuated CD69 upregulation on T and B cells following TCR stimulation, reflecting functional inhibition Significantly (up to 99%) inhibited IL-2, IFN-γ and IL-17A secretion from whole blood derived T cells following ex vivo TCR stimulation Attenuated IL-6 production by 60-90% across dose levels following B-cell stimulation Pharmacodynamic studies suggest robust functional effects on cytokine production can be achieved with >80% degradation of VAV1 Mean ± SEM SAD

MRT-6160 Resulted in Sustained Suppression of TCR-mediated CD69 Activation following Single or Multiple Dose Administration MAD Marked suppression of CD69 upregulation (>90%) in peripheral blood T and B cells following TCR stimulation (data for selected SAD and MAD dose shown as example) Similar results observed in peripheral blood B cells following BCR stimulation SAD Mean ± SEM
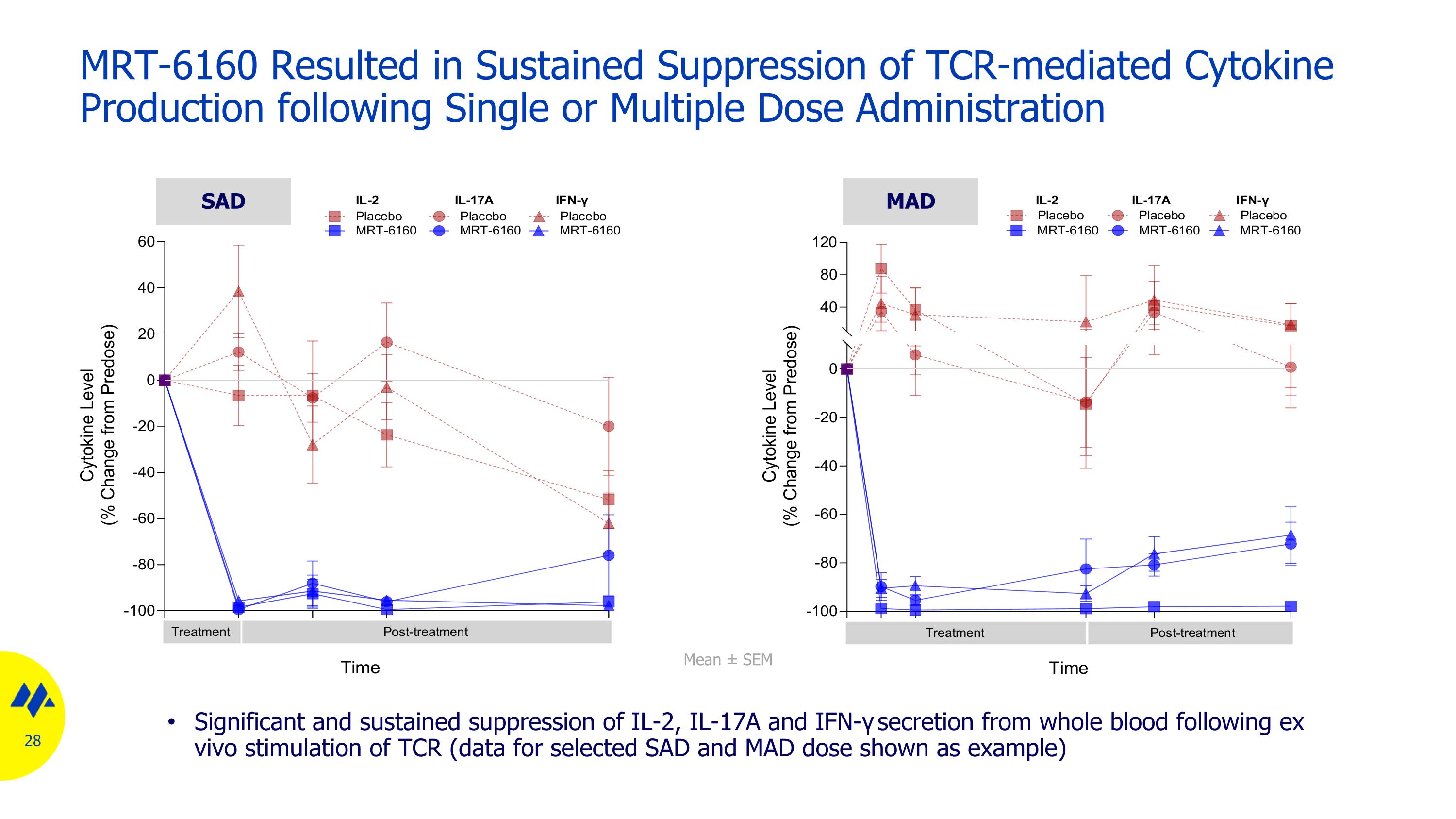
MRT-6160 Resulted in Sustained Suppression of TCR-mediated Cytokine Production following Single or Multiple Dose Administration SAD MAD Significant and sustained suppression of IL-2, IL-17A and IFN-γ secretion from whole blood following ex vivo stimulation of TCR (data for selected SAD and MAD dose shown as example) Mean ± SEM

MRT-6160 was well tolerated with no serious adverse events (SAE) Observed treatment-emergent adverse events (TEAE) were mild (82%) or moderate (18%) and self-limiting Overall TEAE frequency was similar between MRT-6160 and placebo TEAE observed in 2 or more subjects treated with MRT-6160: SAD: pain from vessel puncture (2) MAD: cough (2), diarrhea (3), feeling hot (4), headache (5), nasal congestion (2), oropharyngeal pain (3) and pyrexia (2) Safety Summary

Pharmacodynamic and functional ex vivo studies suggest significant effects on cytokine production can be achieved following marked and sustained degradation of VAV1 Demonstrated levels of VAV1 degradation consistent with levels of degradation required to induce efficacy in preclinical models Functional impact on cytokine production consistent with levels predicted to be required to achieve efficacy in humans (based on benchmark clinical data) Highly favorable safety profile in humans Presented Phase 1 data as well as chronic toxicology package support clear path into Phase 2 studies and broad potential applications in multiple immune-mediated diseases Conclusions

NEK7 Programs (MRT-8102 and CNS optimized)
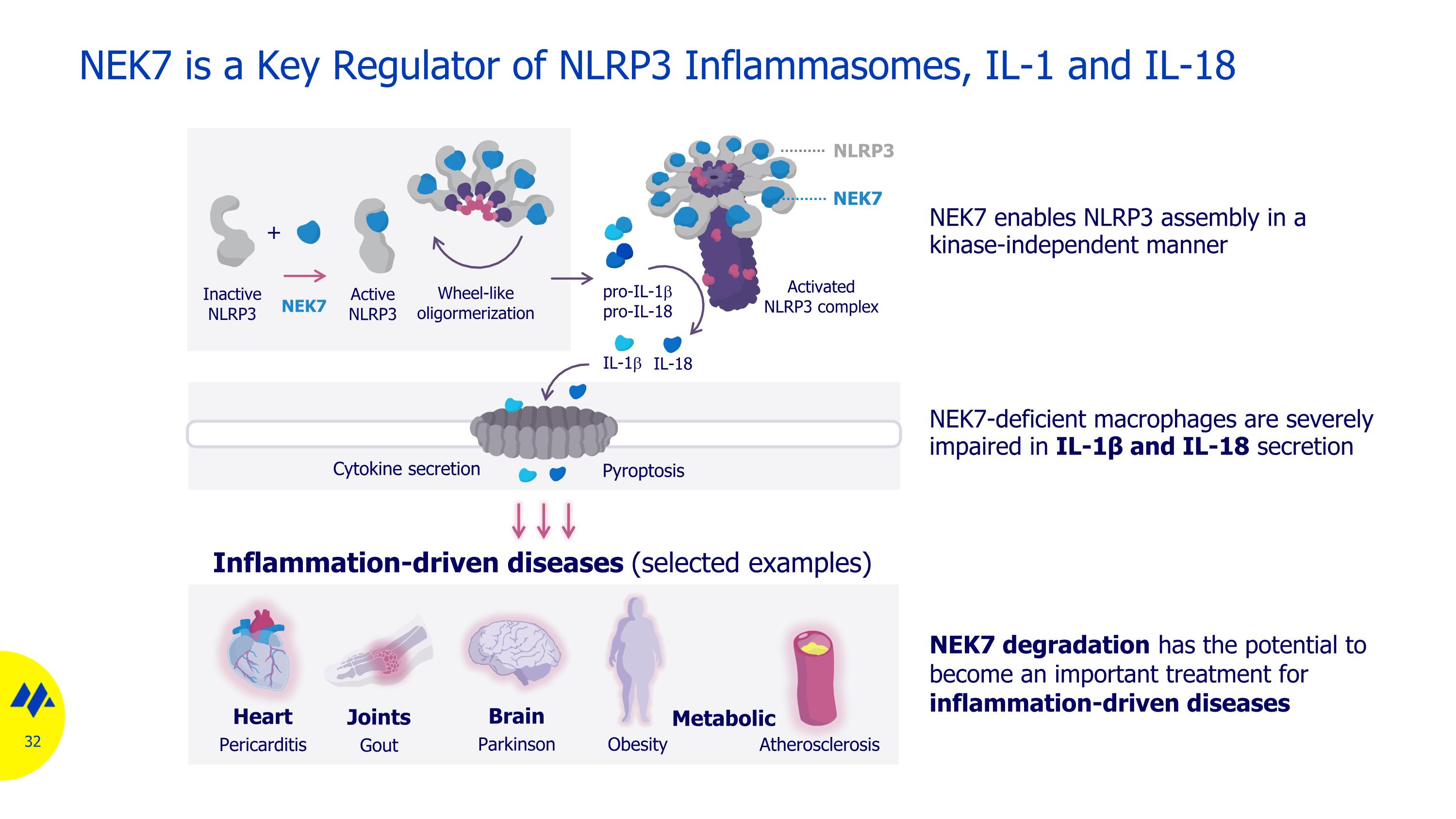
Inflammation-driven diseases (selected examples) NEK7 is a Key Regulator of NLRP3 Inflammasomes, IL-1 and IL-18 Joints Gout Brain Parkinson Heart Pericarditis Obesity Atherosclerosis Cytokine secretion Pyroptosis pro-IL-1b pro-IL-18 IL-1b IL-18 Metabolic Inactive NLRP3 NEK7 Active NLRP3 Wheel-like oligormerization + Activated NLRP3 complex NEK7 NLRP3 NEK7 enables NLRP3 assembly in a kinase-independent manner NEK7-deficient macrophages are severely impaired in IL-1β and IL-18 secretion NEK7 degradation has the potential to become an important treatment for inflammation-driven diseases

MRT-8102 is a Potent, Selective Development Candidate NEK7 MGD with a Favorable Drug-Like Profile MGD Activity Profile CRBN Binding (HTRF, IC50) 0.2 µM NEK7 Degradation (CAL51, DC50 /Dmax) 10 nM / 89% Selectivity (TMT proteomics) Excellent selectivity profile in different cell lines Physicochemical Properties LogD 1.47 MW <450 Thermodynamic Solubility 166 µM ADMET Profile Oral Bioavailability Yes Metabolite Profile (in vitro) No unique human metabolites or GSH adducts (mics) Safety Pharmacology Mini-Ames Negative hERG (patch clamp) No inhibition (IC50> 30 µM) CEREP (panel with 44 proteins) No inhibition NEK7 Ternary Complex (Crystal Structure) MRT-8102 NEK7 CRBN

MRT-8102 potently degrades NEK7 MRT-8102 induces highly selective NEK7 degradation MRT-8102, a Potent and Highly Selective NEK7-directed MGD, Induced Durable Pharmacodynamic Modulation In Vivo MRT-8102 exposure results in prolonged PD effect Cyno @ 10 mg/kg single dose MRT-8102 exposure prolonged PD effect Human PBMC @ 24h treatment Potency, selectivity, and long-lasting pharmacodynamics create potential differentiation from competitive approaches DC50 = 2.5 nM No degradation of other known CRBN neosubstrates

Preclinical GLP Tox Study Suggests Considerable Safety Margin In vivo NEK7 degradation leads to inhibition of Caspase-1 activity and IL-1β release in ex vivo stimulation assay Toxicology summary in rats and cynos (28d GLP tox) No MRT-8102 related clinical signs, no changes in immunophenotyping, no gross or clinical pathology findings at any dose level The NOAEL was the highest dose tested, 150 mg/kg/day (rat) and 100 mg/kg/day (cyno) >200-fold exposure margin over projected human efficacious dose in both species No concerns related to in vitro off-targets, mutagenicity, phototoxicity, hERG or in vivo respiratory or CNS safety pharmacology (rat) or CV safety pharmacology (cyno) NOAEL = no observed adverse effect level IL-1β and Caspase-1 in plasma after ex vivo stimulation with LPS + nigericin in cynomolgus monkeys

MRT-8102 Reduced MSU Crystal-driven Effects in Rabbit Gout Model Daily dosing from day -1 Intra-articular injection of MSU on day 0 MSKUS = musculoskeletal ultrasound *** denotes p < 0.0005 compared to MSU + Vehicle condition Joint swelling MSKUS pathologic findings Histopathology score

IL-1/NLRP3 Signaling is a Clinically Validated Pathway for Inflammatory Diseases Recurrent pericarditis Rilonacept (IL-1α/β) – approved for recurrent pericarditis Atherosclerotic cardiovascular disease Canakinumab (IL-1β) – reduction in cardiac events Cardio-immunology Rheumatology Neurology Metabolic Weight loss Multiple NLRP3 agents being studied for weight loss Neuroinflammation Epilepsy Belnacasan (CASP1) – reduction in seizures Gout Canakinumab (IL-1β) – approved for gout flares Osteoarthritis Canakinumab (IL-1β) – decreased rates of knee and hip replacement Autoinflammation CAPS Anakinra (IL-1R), canakinumab (IL-1β), rilonacept (IL-1 α/β) – approved for CAPS
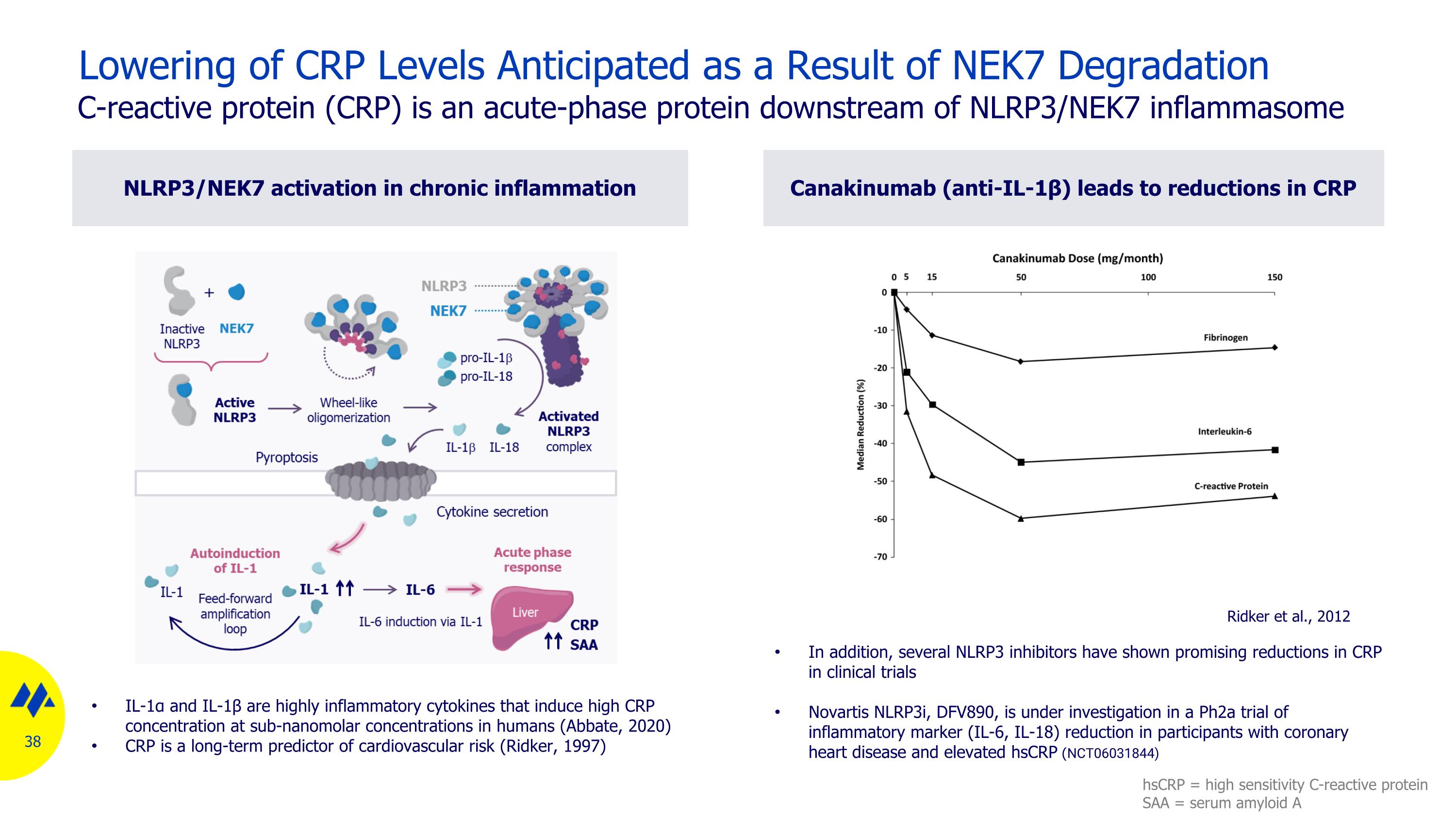
NLRP3/NEK7 activation in chronic inflammation Canakinumab (anti-IL-1β) leads to reductions in CRP Lowering of CRP Levels Anticipated as a Result of NEK7 Degradation C-reactive protein (CRP) is an acute-phase protein downstream of NLRP3/NEK7 inflammasome IL-1α and IL-1β are highly inflammatory cytokines that induce high CRP concentration at sub-nanomolar concentrations in humans (Abbate, 2020) CRP is a long-term predictor of cardiovascular risk (Ridker, 1997) Ridker et al., 2012 In addition, several NLRP3 inhibitors have shown promising reductions in CRP in clinical trials Novartis NLRP3i, DFV890, is under investigation in a Ph2a trial of inflammatory marker (IL-6, IL-18) reduction in participants with coronary heart disease and elevated hsCRP (NCT06031844) hsCRP = high sensitivity C-reactive protein SAA = serum amyloid A

MRT-8102: Efficient Development Path in Peripheral Inflammatory Diseases Phase 1 HV Planned Phase 1 (PoC) in High CRP, Cardio-immunology Indications Further Development in Gout/Pseudogout, Osteoarthritis Goal/Objective Safety, tolerability, and pharmacokinetics in healthy volunteers NEK7 degradation in blood after single and multiple daily doses Changes in levels of inflammatory cytokines Safety, and tolerability of 28-day dosing Changes in CRP levels in subjects with high CRP Generate data in cardio-immunology indications to support a path to registration Changes in gout/pseudogout flare pain or osteoarthritic pain Reduction in frequency of gout/pseudogout flares Generate data to support registration studies Anticipated Studies

CNS-Optimized NEK7 Program

Neurodegeneration Obesity Disease Stimulus CNS Inflammation CNS Saturated fatty acids Ab Monomer Oligomer Fibrils Periphery Fat-associated macrophages Activated microglia Activated microglia Activated microglia/ Fat-associated macrophage pro-IL-1b pro-IL-18 IL-1b IL-18 Activated NLRP3 complex NEK7 41 Brain-Penetrant NEK7 MGDs Have Potential to Impact Neurodegeneration, Obesity and Other Diseases

CNS-Optimized NEK7 MGD Substantially Reduced Inflammatory Cytokines in an LPS-induced Murine Model of Neuroinflammation CereblonI391V mice; n=5 per group MRT-51126: 30 mg/kg, BIDx5, p.o. Selnoflast: 50 mg/kg, QDx5, p.o. LPS: 1 mg/kg, QDx3, i.p. Sampling of left hemisphere of brain 4 hr post-final LPS dose Brain NEK7 Brain IL-1β Brain IL-6

CNS-Optimized NEK7 MGD Demonstrated Profound NEK7 Degradation and Pathway Inhibition In Vivo Over Several Days in Cynomolgus Monkey NEK7 in cyno PBMC NEK7 in cyno CSF IL-1β post-ex vivo stimulation Excellent exposure in cyno blood and CSF, correlating with deep NEK7 degradation and functional inhibition of NLRP3 pathway following ex vivo stimulation 43

Building Future Opportunities in I&I
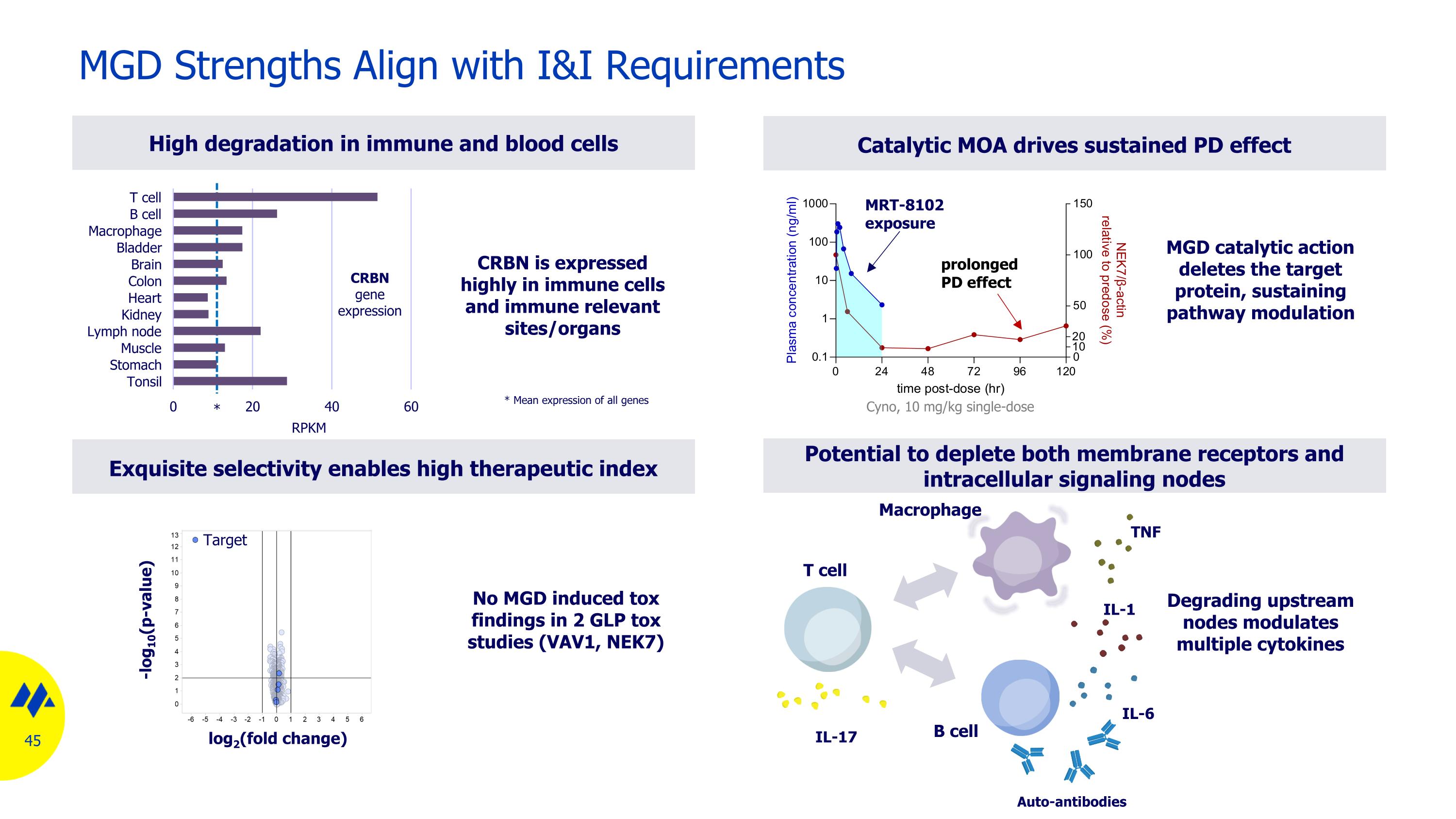
High degradation in immune and blood cells MGD Strengths Align with I&I Requirements Catalytic MOA drives sustained PD effect Degrading upstream nodes modulates multiple cytokines MRT-8102 exposure prolonged PD effect Macrophage T cell B cell Auto-antibodies IL-17 IL-6 TNF IL-1 Cyno, 10 mg/kg single-dose MGD catalytic action deletes the target protein, sustaining pathway modulation CRBN is expressed highly in immune cells and immune relevant sites/organs RPKM CRBN gene expression * * Mean expression of all genes Potential to deplete both membrane receptors and intracellular signaling nodes Exquisite selectivity enables high therapeutic index log2(fold change) -log10(p-value) Target No MGD induced tox findings in 2 GLP tox studies (VAV1, NEK7)

“CAR-T in a Pill” Degrading Undruggable Targets in Critical I&I Disease Pathways B-cell modulation Inflammatory & auto-inflammatory mDC Neutrophil NK cell pDC Complement T cell B cell Immune complex Autoantibodies Apoptotic cell Monocyte Type I IFN INNATE ADAPTIVE Neutrophil Asthma & allergies Near term focus on building a portfolio of additional oral I&I drugs “FcRn in a Pill” Immune complex Oral IL-1 drugs Increased BAFF, TNF and pro-inflammatory cytokines

GSPT1 program (MRT-2359)
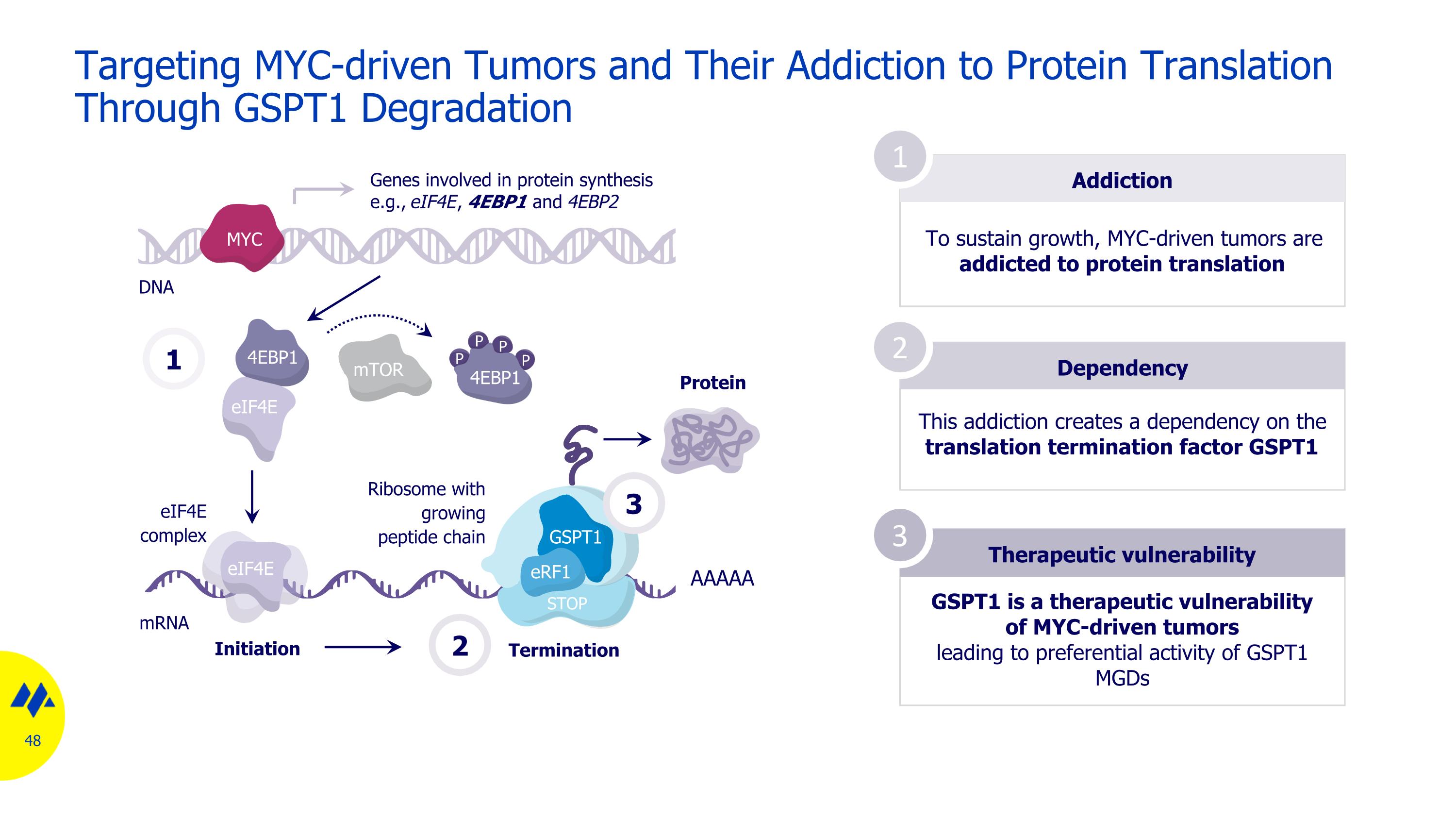
Targeting MYC-driven Tumors and Their Addiction to Protein Translation Through GSPT1 Degradation Addiction To sustain growth, MYC-driven tumors are addicted to protein translation Dependency Therapeutic vulnerability 1 2 3 This addiction creates a dependency on the translation termination factor GSPT1 GSPT1 is a therapeutic vulnerability of MYC-driven tumors leading to preferential activity of GSPT1 MGDs mRNA DNA 1 mTOR eIF4E 4EBP1 P P P P 4EBP1 eIF4E eIF4E complex Genes involved in protein synthesis e.g., eIF4E, 4EBP1 and 4EBP2 Initiation Termination AAAAA Protein 2 MYC STOP GSPT1 eRF1 Ribosome with growing peptide chain 3

MRT-2359 is a Potent and Highly Selective GSPT1-directed MGD in vitro data CRBN binding, Ki 113 nM Ternary complex, EC50 < 7 nM Degradation, DC50 (in disease relevant cell lines) 1 - 20 nM MRT-2359 induces selective GSPT1 degradation and shows favorable ADME/DMPK profile MRT-2359 is a potent GSPT1-directed MGD ADMET profile CYP DDIs > 30 µM hERG inhibition patch clamp EC50 > 30 µM Oral bioavailability all species ~50% Ternary complex modelling GSPT1 CRBN MGD No degradation of other known cereblon neosubstrates Protein fold-change (log2) p-value (-log10)

MRT-2359 Has Optimized Depth of Degradation To Achieve Preferential Activity in MYC High Cancer Cells %GSPT1 degraded (Dmax) determined by Western blot Differential Effect (MYC vs non-MYC-driven) less degradation Preferential activity in MYC high cells MRT-2359 MRT-2136 MRT-2359 displays preferential activity in MYC driven NSCLC cells Non-optimal GSPT1 MGD (MRT-2136) shows limited preferential activity Circle size corresponds to bioavailability with oral dosing

MYC-Driven Pathologies Explored in MRT-2359 Phase 1/2 trial Neuroendocrine tumors L-/N-MYC amplified tumors Heme Breast cancer ER positive metastatic SCLC L/N-MYC high NSCLC N-MYC high SCLC/NE transformation Neuroendocrine lung cancer Prostate cancer AR positive including AR-V7 N-MYC High and/or L-MYC High c-MYC High c-MYC N-MYC L-MYC Indication Size

MRT-2359 Clinical Study Results

0.5mg 5/9 Phase 2: Possible Expansion Cohorts Phase 1: Dose Escalation 1.5mg 5/9 1mg 5/9 MRT-2359-001 Phase 1/2 Clinical Study Design Lung cancer, high-grade neuroendocrine tumors and solid tumors with N-/L-MYC amplification 2mg 5/9 5/9 = 5 days on drug, 9 days off drug; 21/7 = 21 days on drug, 7 days off drug; RP2D = recommended Phase 2 dose 0.5mg 21/7 0.75mg 21/7 * Efficacy guided stratification per N-/L-MYC expression ** Retrospective stratification per N-/L-MYC expression NSCLC* SCLC** HR+/Her2- Breast Cancer (+Fulv) Prostate cancer (+Enza) N-MYC/L-MYC amplified tumors RP2D Safe dose level RP2D 0.5mg 21/7 identified as RP2D Safety assessments initiated

Patient Characteristic Total (N=59)* Age, median (range), years 63 (30-80) Female, N (%) 30 (51) Race, N (%) White 48 (81) Black 4 (7) Asian 3 (5) Other 4 (7) ECOG performance status, N (%) 0 or 1 58 (98) 2 1 (2) Cancer type, N (%) Non-small cell lung cancer 23 (39) Small cell lung cancer 13 (22) High-grade neuroendocrine cancer 16 (27) N-MYC or L-MYC amplified cancer 7 (12) Number of prior lines of therapy, median (range) 3 (1-8) Patient Demographics and Clinical Characteristics in Dose Escalation Data cut off: 10 March 2025 * 32 patients treated on the 5/9 schedule and 27 patients on the 21/7 schedule

MRT-2359 Treatment-Related AEs in > 10% patients (N=59) Dose Level 0.5 mg 5/9 (N=9) 1 mg 5/9 (N=13) 1.5 mg 5/9 (N=5) 2 mg 5/9 (N=5) 0.5 mg 21/7 (N=18) 0.75 mg 21/7 (N=9) CTC AE V5 Grade G1-2 (%) G3 (%) G4 (%) G1-2 (%) G3 (%) G4 (%) G1-2 (%) G3 (%) G4 (%) G1-2 (%) G3 (%) G4 (%) G1-2 (%) G3 (%) G4 (%) G1-2 (%) G3 (%) G4 (%) Thrombocytopenia 0 0 0 0 0 1 (8) 2 (40) 1 (20) 1 (20) 2 (40) 1 (20) 2 (40) 2 (11) 0 1 (6) 3 (33) 0 1 (11) Neutropenia 0 0 0 0 0 1 (8) 0 1 (20) 2 (40) 0 1 (20) 1 (20) 0 1 (6) 0 0 1 (11) 2 (22) Leukopenia# 0 0 0 0 0 0 0 2 (40) 1 (20) 0 1 (20) 1 (20) 0 0 0 0 2 (22) 0 Anemia 0 0 0 0 1 (8) 0 1 (20) 1 (20) 0 1 (20) 1 (20) 0 1 (6) 1 (6) 0 1 (11) 1 (11) 0 Nausea 3 (33) 0 0 4 (31) 0 0 4 (80) 0 0 2 (40) 0 0 4 (22) 0 0 4 (44) 0 0 Vomiting 1 (11) 0 0 3 (23) 0 0 5 (100) 0 0 2 (40) 0 0 4 (22) 0 0 4 (44) 0 0 Diarrhea 1 (11) 0 0 3 (23) 0 0 1 (20) 1 (20) 0 1 (20) 0 0 1 (6) 0 0 3 (33) 0 0 Hypokalemia 0 0 0 2 (15) 0 0 0 0 0 1 (20) 0 0 3 (17) 0 0 1 (11) 0 0 Fatigue 0 0 0 3 (23) 0 0 0 0 0 0 0 0 2 (11) 0 0 4 (44) 0 0 # Data combined for ‘leukopenia’ and ‘white blood cell count decreased’ Dose limiting toxicities were: thrombocytopenia (N=6), with or w/o neutropenia/leukopenia G3 ALT/AST (N=1) Doses 0.5 mg, 1 mg 5/9 and 0.5 mg, 0.75 mg 21/7 were well tolerated with mostly low-grade AEs Data cut off: 10 March 2025 Importantly, dose-limiting toxicities reported with non-selective competitor GSPT1 degraders such as hypocalcemia, hypotension and cytokine release syndrome were not reported

Biomarker Status and PD modulation in L-/N-MYC Amplified Tumors, NSCLC, SCLC and High-Grade NE Tumors L-MYC and N-MYC expression in tumor tissue obtained at baseline was assessed in 46 patients Overall, as compared to preclinical data, lower than expected frequency of tumors with high L-MYC or N-MYC in NCSLC (0%), SCLC including transformed (31%) and HG NE tumors (17%) Paired, tumor containing biopsies meeting QC criteria were obtained from 17 patients GSPT1 degradation assessed by targeted Mass Spec Optimal degradation of approximately 60% was achieved in biomarker high tumors, in line with preclinical data (see corporate deck) Cut-off: N-MYC≤3; L-MYC≤-1

Summary of Activity in SCLC, NSCLC, high-grade Neuroendocrine Tumors and L- and N-MYC Amplified tumors L-MYC and N-MYC expression in tumor tissue obtained at baseline or known L-MYC or N-MYC amplification in the absence of tissue was available in 48 patients and 37 were evaluable for response assessment per RECIST 1.1 Activity in biomarker positive patients: High L-MYC or N-MYC expression (13 patients): 1 x PR, 4 x SD (DCR 38%) Includes patients with L-MYC or N-MYC amplification without tumor tissue for expression analysis Activity in biomarker negative patients: Low L-MYC and N-MYC expression (24 patients): 1 unconfirmed PR and 3 SD (DCR 17%),
Current Data for MRT-2359 and Enzalutamide in Castrate-Resistant Prostate Cancer As of 10 March 2025, RECIST 1.1 is available for 3 patients showing: 1 PR (confirmed, -57%; heavily pretreated CRPC with AR LBD mutation H875Y) 2 SDs (both CRPC with AR-V7 transcripts, multiple prior treatments including abiraterone and enzalutamide) PSA response is available for 2 patients showing 1 PSA response (-90%) in the patient with a confirmed PR per RECIST 1.1 Safety profile has been favorable Safety and efficacy assessment continues following Simon 2-stage design Potential to expand to 20 - 30 patients if positive efficacy signal continues to be observed Further data from combination cohorts – including ER-positive breast patients – expected in H2 2025

Confirmed PR in Refractory CRPC with AR H875Y Mutation Baseline After 2 cycles After 4 cycles -46% -57% Patient continues on study (C5)
Encouraging early data in CRPC: 1 PR and 2 SD in first 3 evaluable, heavily pretreated patients with either AR mutations or AR-V7 expression Opportunity to enroll 20 - 30 patients if positive efficacy signal continues to be observed Large, high unmet need indication not requiring biomarker-based patient selection Strategic decision made not to open expansion cohorts in lung cancer and NE tumors Despite signals of clinical activity, low biomarker positivity in these indications does not support additional studies Summary

CDK2 Program

CDK2 is a Key Driver of Cell Cycle Progression in Cancer CDK2: a key cell cycle regulator Patient diagnosed incidence #s, major markets (US, EU and JP): Decision Resources Group (DRG) Therapeutic hypothesis: CDK2 is a key driver of cancers with cyclin dependent kinase pathway alterations MGDs will achieve greater selectivity against other CDKs and kinases in general, as well as more sustained pathway inhibition compared to inhibitors Clinical Opportunity: ER positive breast cancer pre and post treatment with CDK4/6 inhibitors (~474K patients) Ovarian cancer (~64K patients), endometrial cancer (~124K patients) and other tumors with CCNE1 amplification
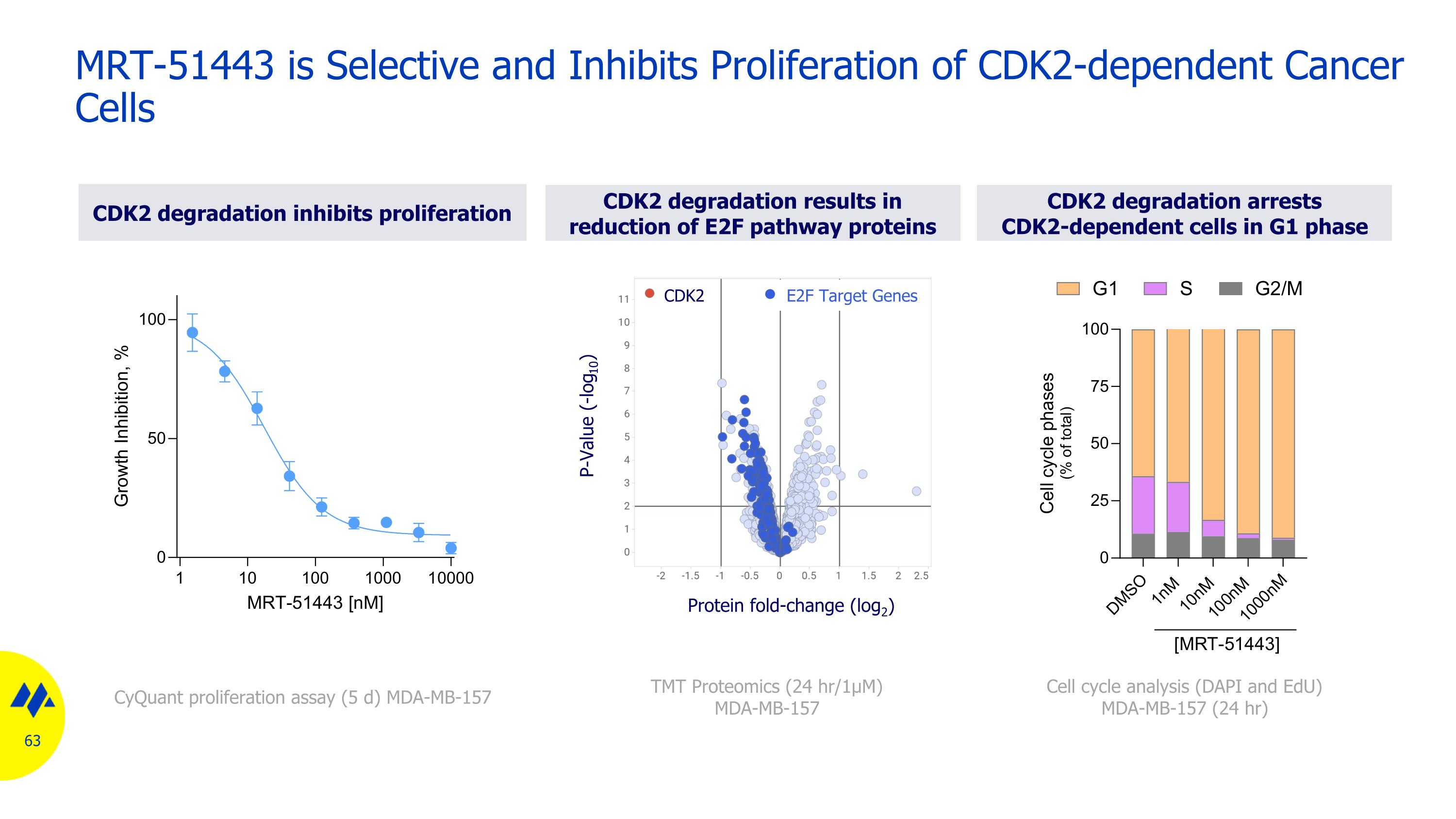
MRT-51443 is Selective and Inhibits Proliferation of CDK2-dependent Cancer Cells Cell cycle analysis (DAPI and EdU) MDA-MB-157 (24 hr) CDK2 degradation inhibits proliferation CyQuant proliferation assay (5 d) MDA-MB-157 CDK2 degradation results in reduction of E2F pathway proteins TMT Proteomics (24 hr/1μM) MDA-MB-157 Protein fold-change (log2) CDK2 E2F Target Genes P-Value (-log10) CDK2 degradation arrests CDK2-dependent cells in G1 phase

MRT-51443 Displays Superior Selectivity Versus Clinical CDK2 Inhibitors Clinical CDK2 inhibitors demonstrate off target activity in biochemical kinome profiling 7-day CyQuant Assay CDK2 inhibitors but not MGDs inhibit proliferation in part through CDK2-independent mechanisms Carna Mobility Shift Assay; 1 μM CDK2i or CDK2 MGD, across 323 human kinases CDK2 Inhibitor CDK2 MGD CDK2 CDK3 CDK5 CDK4 CDK6 CDK7 CDK9 CDK1 CDK2 CDK3 CDK5 CDK4 CDK6 CDK7 CDK9 CDK1
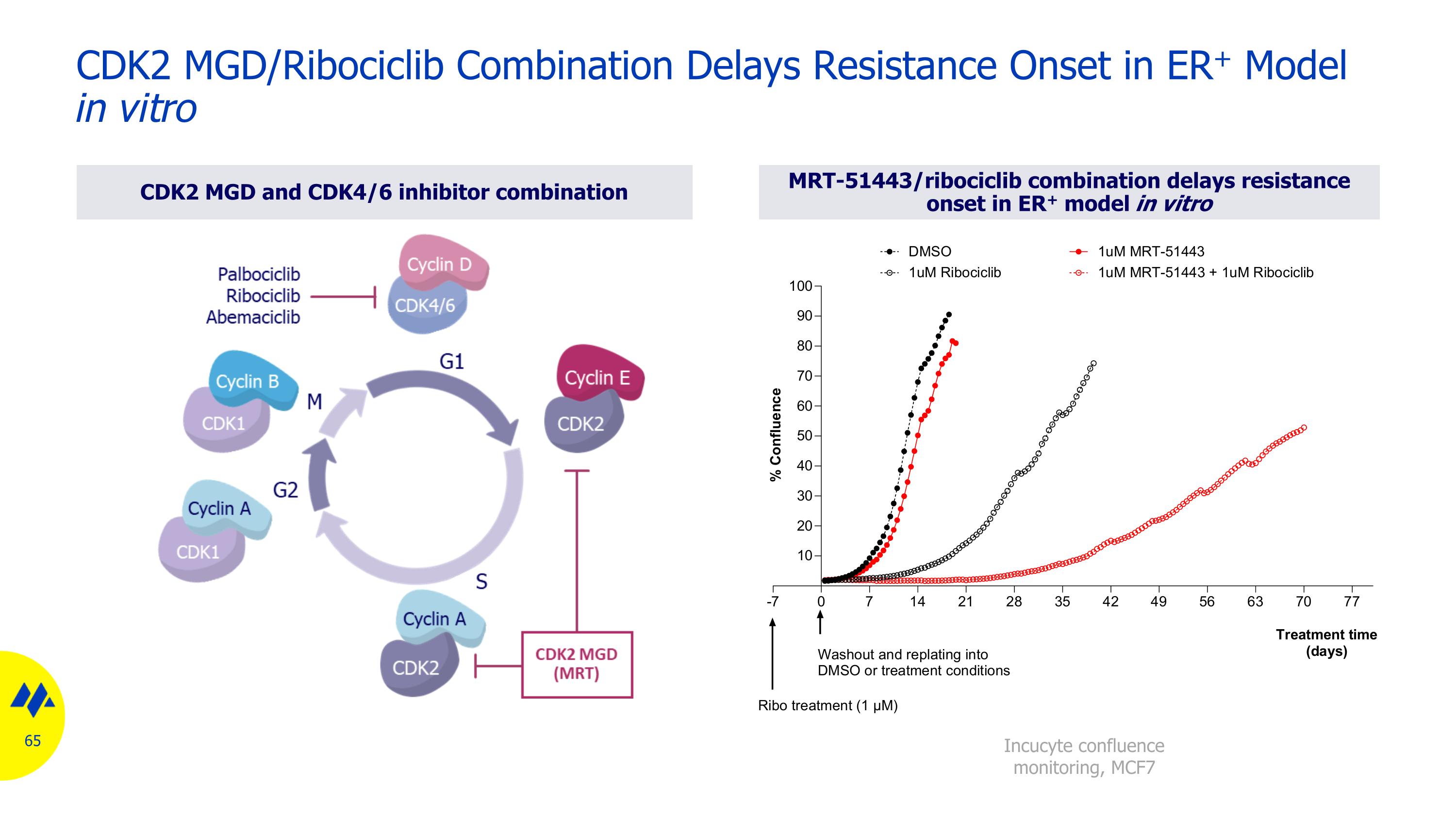
CDK2 MGD and CDK4/6 inhibitor combination MRT-51443/ribociclib combination delays resistance onset in ER+ model in vitro CDK2 MGD/Ribociclib Combination Delays Resistance Onset in ER+ Model in vitro Incucyte confluence monitoring, MCF7

MRT-51443 induces robust tumor regression in combination with CDK4/6 inhibition and fulvestrant MRT-51443 triple combination substantially reduces tumor growth vs. ribo + fulv MCF7 T47D Median: -10% Median: -61% Median: -3% Median: -77% Ribociclib + Fulvestrant MRT-51443 + Ribo + Fulv Ribociclib + Fulvestrant MRT-51443 + Ribo + Fulv 28-day efficacy; MRT-51443 30 mpk PO BID, ribociclib 75 mpk PO QD, fulvestrant 5 mg/mouse s.c. QW MRT-51443 Demonstrates Activity in Combination with CDK4/6 Inhibitor and Fulvestrant in ER+ Breast Cancer Model 66

CCNE1 Program

CCNE1 (Cyclin E1) is a Target for Solid Tumors with Deregulated Cyclin E1 Therapeutic hypothesis: CCNE1 (Cyclin E1) is a well-recognized human oncogene that drives multiple hallmarks of cancer, and has been considered undruggable Selective degradation of cyclin E1 can target tumors with deregulated cyclin E1 (amplification or overexpression) Clinical opportunity: First-in-class Cyclin E1 degraders for Cyclin E1 amplified cancers Ovarian (~19%), endometrial (~10%), and gastric (~10%) cancer Breast cancer and others Cyclin E drives multiple hallmark cancer mechanisms Cell death and differentiation Cell cycle progression/proliferation S G2 M G1 Drug resistance MCMs CDT1 Cyclin E CDK2 Cyclin E G0 – S progression MCM5 Cyclin E Centrosome duplication Cyclin E

69 CCNE1 degradation leads to downstream pathway suppression MRT-50969 induces robust G1/S cell cycle arrest Western blot, OVISE, 24h TMT Proteomics, MDA-MB-157 Rb K/O 1μM, 24h P-value (-log10) Protein fold-change (log2) CCNE1 MRT-50969 is highly selective for CCNE1 FACS, EdU incorporation, 48h In vitro data CRBN binding, IC50 0.15 mM Ternary complex, EC50 3 nM Degradation, DC50/Dmax 3 nM / 94 % MRT-50969 is a Potent and Highly Selective CCNE1-directed MGD

GI50 = growth inhibition 50%, the concentration of drug required to inhibit the growth of cancer cells in vitro by 50% MRT-50969 Shows Superior Differential Activity in CCNE1 Dependent Cell Lines Compared to Clinical-Stage CDK2 Inhibitors OVCAR3 vs A2780 MDA-157 vs T47D 5 Day CyQuant assay, bars indicate median GI50
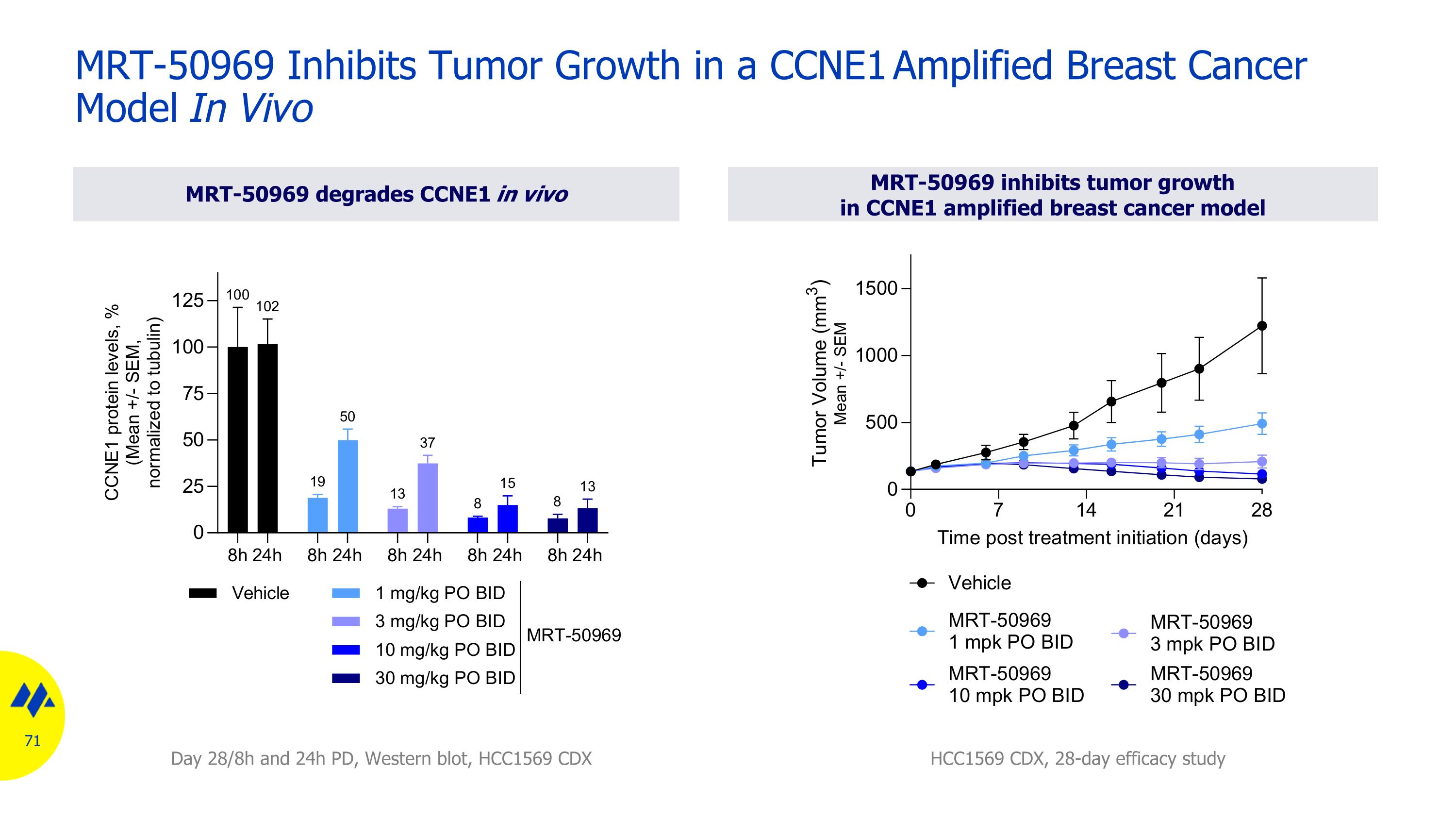
MRT-50969 inhibits tumor growthin CCNE1 amplified breast cancer model MRT-50969 Inhibits Tumor Growth in a CCNE1 Amplified Breast Cancer Model In Vivo MRT-50969 degrades CCNE1 in vivo HCC1569 CDX, 28-day efficacy study Day 28/8h and 24h PD, Western blot, HCC1569 CDX

21-day efficacy study in MKN1 CDX model MRT-50969 Inhibits Tumor Growth in a CCNE1 Amplified Gastric Cancer Model In Vivo Day 21/8h and 24h PD, Western blot, MKN1 CDX MRT-50969 inhibits tumor growth in CCNE1 amplified gastric cancer model MRT-50969 degrades CCNE1 in vivo

QuEEN™ Discovery Engine

Overcoming Past Limitations of Molecular Glue Degraders Traditional thinking Monte Rosa Therapeutics approach ‘Target space is limited’ QuEENTM has vastly expanded the degradable target space across a broad range of undruggable protein classes ‘MGDs are identified by serendipity’ QuEENTM enables target centric and systematic discovery of MGDs ‘MGDs are not selective’ High selectivity achievable even within the same protein class, family and isoforms, mitigating off-target safety concerns ‘Med Chem rules don’t apply to MGDs’ AI-driven and structure-based design enable rational med chem optimization of MGDs

Rationally-designed MGDs create diverse E3 ligase neosurfaces, enabling recruitment of new targets Our geometric deep learning algorithms use surfaces to predict targets. Our surface-based algorithms design MGDs to recruit targets. Our platforms generate actionable data-at-scale to test & train (“data moat”) Our Critical Insight: Surfaces are Critical for MGD Discovery Surfaces, not structures, mediate PPIs and targeted protein degradation E3 ligase Neosubstrate footprint MGD footprint E3 ligase neosurface

AF, Rosetta, PDB GlueShot: de novo MGD Design for Novel Targets E3 ligase structures Protein structures AF, Rosetta, PDB Surface fingerprint fAIceit™ Virtual library FLASH™ & GlueAID™ Surface of MGD + ligase COSMOS™ Fingerprint matching Headlong™ & fAIceit™ Nobel prize MGDs that induce PPIs with drug-like properties Neosubstrate footprint MGD + ligase footprint Geometric and chemical surface characterization

QuEEN™ Unique Capabilities AI/ML In silico discovery using proprietary AI-powered algorithms Proximity Screening Specialized suite of biochemical, cellular and proteomics assays to assess proximity and degradation in high throughput Structure-based Design Proprietary database of protein structures to enable rapid optimization of MGD chemistry Proteomics Integrated proteomics engine and database to identify novel targets and explore cellular complex formation and protein degradation MGD Library Growing >50K compound library for novel degron and target space exploration Breakthroughs enabling rapid discovery of potent, selective, and oral MGDs

Our Proprietary and Unique MGD Library Creates Future High Value Programs Rational library design Enabled by proprietary chemistry Deep understanding of CRBN surfaces Optimised drug-like properties Continued diversification Each sublibrary captures a unique design concept Cover novel MGD chemical-space Library expansion Current screening deck >50K MGDs Expansion reaches new target space

Target ID Guided by surface mimicry In silico screening Screen for activity in ternary complexes Proprietary AI/ML Engines Enable the Discovery of Reprogrammable Ligases, Neosubstrates, and Selective MGDs Proprietary AI/ML engines Ligase matching PPI propensity & surface complementarity MGD discovery Generate MGDs with drug-like properties

QuEENTM: How it Works Target and ligase ID Surface-centric discovery process Actionable data-at-scale Proteomics Virtual screens Structural biology High throughput screens Predict Design Test & Train AI-powered chemistry Surface-aware MGD generation & optimization

QuEEN™ Toolbox to Rapid Discovery of Oral MGDs Predict Target & ligase ID fAIceit™ Ultra-fast fingerprint search for surface-based matchmaking E3 ligase reprogrammability fAIceit mimicry target ID Structural biology X-ray & cryo-EM Headlong™ virtual screens Proteomics mass-spec farm HT library screening Design AI-powered chemistry Test & Train Actionable data-at-scale Rhapsody™ ternary complexes FLASH™ virtual library GlueAID™ ADMET & synthesis HitMan™ diverse library

in silico experimentation Algorithms Use MGD-focused, Moated Data to Identify Targets and Design MGDs FLASH™ virtual library Proteomics mass-spec farm HT library screening MILLION protein measurements fAIceit mimicry target ID Structural biology X-ray & cryo-EM Headlong™ virtual screens 37 6.5 >125 MILLION MGD activity measurements TOTAL Structures 250 BILLION Protein surface matchings 37 BILLION Virtual MGDs 651 MILLION Compounds screened Lab experimentation Scalable Data Lake with purpose-built data services for seamless data movement and unified governance Cloud First and Cloud Native

Team

World-Class Leadership Deep expertise in molecular glue discovery, drug development and precision medicine Filip Janku, M.D., Ph.D. Chief Medical Officer Markus Warmuth, M.D. Chief Executive Officer John Castle, Ph.D. Chief Data and Information Officer Sharon Townson, Ph.D. Chief Scientific Officer Phil Nickson, Ph.D., J.D. Chief Business and Legal Officer Jennifer Champoux Chief Operating Officer Magnus Walter, DPhil SVP, Drug Discovery Andrew Funderburk SVP, Investor Relations and Strategic Finance

Thank You