UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): August 14, 2024 |
Lyra Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
Delaware |
001-39273 |
84-1700838 |
||
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
480 Arsenal Way |
|
|||
Watertown, Massachusetts |
|
02472 |
||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: 617 393-4600 |
|
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
Common Stock, $0.001 par value per share |
|
LYRA |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On August 14, 2024, Lyra Therapeutics, Inc. (the “Company”) announced its financial results for the quarter ended June 30, 2024. The full text of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information contained in Item 2.02 of this Current Report on Form 8-K (including Exhibit 99.1 attached hereto) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, except as expressly provided by specific reference in such a filing.
Item 7.01 Regulation FD.
On August 14, 2024, the Company posted a corporate presentation on its website at www.lyratherapeutics.com that representatives of the Company may use from time to time in presentations or discussions with investors, analysts or other parties. A copy of the presentation is furnished as Exhibit 99.2 to this Current Report on Form 8-K. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.2.
The information contained in Item 7.01 of this Current Report on Form 8-K (including Exhibit 99.2 attached hereto) shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, except as expressly provided by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
The following Exhibits 99.1 and 99.2 shall be deemed to be furnished, and not filed:
|
Exhibit No. |
|
Description |
|
|
|
99.1 |
|
|
99.2 |
|
Corporate Presentation of Lyra Therapeutics, Inc. dated August 14, 2024 |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
Lyra Therapeutics, Inc. |
|
|
|
|
Date: |
August 14, 2024 |
By: |
/s/ Jason Cavalier |
|
|
|
Jason Cavalier, Chief Financial Officer |
Lyra Therapeutics Reports Second Quarter 2024 Financial Results and Provides Corporate Update
– Primary focus continues to be on upcoming results from ENLIGHTEN 1 Phase 3 extension study in 4Q 2024 and ENLIGHTEN 2 pivotal Phase 3 trial in CRS patients in 1H 2025, as planned –
– In parallel, the company continues to analyze data from ENLIGHTEN 1; further analysis of the ENLIGHTEN 1 data has revealed that LYR-210 demonstrated improvement over control in symptomatic endpoints in the CRS patient cohort with nasal polyps –
WATERTOWN, Mass., August 14, 2024 – Lyra Therapeutics, Inc. (Nasdaq: LYRA) (“Lyra” or the “Company”), a clinical-stage biotechnology company developing long-acting, anti-inflammatory sinonasal implants for the treatment of chronic rhinosinusitis (CRS), today reported its financial results for the second quarter ended June 30, 2024 and provided a corporate update.
“While we clearly recognize the disappointment of not meeting the primary endpoint in the previously-announced ENLIGHTEN 1 Phase 3 trial, our potential pathway to approval for LYR-210 in CRS without nasal polyps can only be determined once we unblind and analyze the full data set from the ENLIGHTEN pivotal program,” said Maria Palasis, Ph.D., President and CEO of Lyra Therapeutics. “Today we are disclosing that our further analysis of the ENLIGHTEN 1 data has revealed that LYR-210 demonstrated improvement over control in symptomatic endpoints in the CRS patient cohort with nasal polyps, which we believe reinforces the therapeutic potential of our product candidates.”
Dr. Palasis continued, “While we intend to remain opportunistic about strategic options, our primary focus remains on the two ongoing ENLIGHTEN Phase 3 trials evaluating LYR-210 in CRS patients with and without nasal polyps: the ENLIGHTEN 1 52-week extension study with results expected in Q4 2024 and the ENLIGHTEN 2 pivotal trial with enrollment on track and results expected in the first half of 2025. We plan to be pragmatic and data‑driven as we determine our path forward for CRS patients, investors, and other stakeholders.”
Highlights from May 2024 ENLIGHTEN 1 Pivotal Results and Subsequent Cost-cutting Measures
Additional Analysis from ENLIGHTEN 1 for CRS Patient Subgroup with Nasal Polyps
Milestones for Ongoing ENLIGHTEN Pivotal Program of LYR-210 in CRS
Second Quarter 2024 Financial Highlights
Cash, cash equivalents and short-term investments as of June 30, 2024 were $67.5 million, compared with $102.8 million at December 31, 2023. Based on our current business plan, we anticipate that our cash, cash equivalents and short-term investment balance is sufficient to fund our operating expenses and capital expenditures into the first quarter of 2026. Please see our Quarterly Report filed on Form 10-Q for the three and six months ended June 30, 2024 for further information regarding our cash runway guidance and other financial results.
Research and development expenses for the quarter ended June 30, 2024 were $13.3 million, an increase of $2.5 million compared to $10.8 million for the same period in 2023.
The increase in research and development expenses for the three months ended June 30, 2024 was primarily attributable to an increase of $1.7 million in allocated and support costs for shared activities within the organization driven by headcount allocation and rent increases which occurred prior to the reduction in force, an increase of $0.5 million in professional and consulting fees as we moved good manufacturing practices (“GMP”), manufacturing in house prior to the reduction in force and increased clinical and product manufacturing costs of $0.9 million as we continued to progress on our clinical trials and internal manufacturing efforts prior to the reduction in force.
These costs were offset by $0.8 million in headcount related costs period over period due to the recent restructuring.
General and administrative expenses for the quarter ended June 30, 2024 were $5.1 million, an increase of $0.6 million compared to $4.5 million for the same period in 2023.
The increase in general and administrative expenses for the three months ended June 30, 2024 was primarily driven by an increase of $0.4 million for consulting costs, as well as an increase of $0.2 million in costs shared between the General & Administrative and Research & Development functions including headcount and rent. These costs were partially offset by a decrease in the amount of $0.1 million for employee related costs due to the recent restructuring.
The Company incurred impairment costs related to property and equipment of $1.9 million for the three months ended June 30, 2024 compared to $1.6 million for the same period in 2023.
The Company incurred impairment costs related to our right-of-use asset of $22.8 million for the three months ended June 30, 2024 and there were no such charges for the same period in 2023.
The Company incurred a restructuring charge in the amount of $6.5 million primarily related to severance and retention costs for the three months ended June 30, 2024 and there were no such charges for the same period in 2023.
Net loss for the second quarter 2024 was $48.1 million compared to $15.6 million for the same period in 2023.
About Lyra Therapeutics
Lyra Therapeutics, Inc. is a clinical-stage biotechnology company developing long‑acting, anti-inflammatory sinonasal implants for the treatment of chronic rhinosinusitis (CRS). Lyra Therapeutics has two product candidates, LYR‑210 and LYR‑220, in late‑stage development for CRS, a highly prevalent inflammatory disease of the paranasal sinuses which leads to debilitating symptoms and significant morbidities. LYR-210 and LYR-220 are bioabsorbable nasal implants designed to be administered in a simple, in‑office procedure and are intended to deliver six months of continuous anti‑inflammatory drug therapy (7500µg mometasone furoate) to the sinonasal passages for the treatment of CRS with a single administration. LYR-210, being evaluated in the ENLIGHTEN Phase 3 clinical program, has a smaller dimension and is intended for patients with standard anatomy, primarily patients who have not undergone ethmoid sinus surgery. LYR-220 is a larger implant designed for CRS patients whose nasal cavity is enlarged due to previous ethmoid sinus surgery. These two product candidates are designed to treat the estimated four million CRS patients in the United States who fail medical management each year. For more information, please visit www.lyratx.com and follow us on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward-looking statements. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements regarding our focus on the two ongoing ENLIGHTEN Phase 3 trials evaluating LYR-210, our ongoing ENLIGHTEN 1 extension study and expectation for data in Q4 2024, our ongoing ENLIGHTEN 2 trial and our expectation for data in 1H 2025, our cash runway into 2026 and plans to update investors regarding our cash runway, and our plans to evaluate potential strategic options to maximize shareholder value. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause the Company's actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: any potential financial or strategic option we pursue in order to maximize shareholder value may not result in the identification of a suitable transaction, or if one is identified and pursued, may not be completed on attractive terms, or at all; our ability to sublease or assign our three leaseholds, which represent significant operating costs; our incurrence of significant losses since inception and expectation to incur significant additional losses for the foreseeable future; our recurring losses from operations raise substantial doubt regarding our ability to continue as a going concern; our need for significant additional funding in order to complete development of and obtain regulatory approval for our product candidates and commercialize our products, if approved; the failure of our ENLIGHTEN 1 Phase 3 trial to meet its primary endpoint has made it more difficult for the Company to raise capital; we could be forced to delay, reduce, or eliminate our product development programs or commercialization efforts; following the failure of our ENLIGHTEN 1 Phase 3 trial evaluating LYR-210 for the treatment of CRS to meet its primary endpoint, which was announced in May 2024, there is significant uncertainty about the Company’s ability to complete development of LYR-210 and our ability to obtain regulatory approval for LYR-210 is at least significantly delayed and may not be possible; our common stock may be delisted from The Nasdaq Global Market if we cannot regain compliance with Nasdaq’s continued listing requirements; our loss of key personnel significantly and adversely affects our ability to manufacture our product candidates, among other activities; we are no longer engaged in the manufacturing of our product candidates in-house; our business is highly dependent on the success of our most advanced product candidate, LYR-210; clinical trials required for our current product candidate and any future product candidates are expensive and time-consuming, their outcome is uncertain, and if our clinical trials do not meet safety or efficacy endpoints in these evaluations, or if we experience significant delays in these trials, our ability to commercialize our product candidates and our financial position will be impaired; any failure by a third party to conduct our pre-clinical or clinical trials according to good clinical practices and in a timely manner may delay or prevent our ability to seek or obtain regulatory approval for or commercialize our product candidates; even if LYR-210 receives marketing approval, it may fail to achieve market acceptance by physicians, patients,
third-party payors or others in the medical community necessary for commercial success; if our collaborations are not successful, including with LianBio our product candidates may not reach their full market potential; our ability to manage our obligations under our license and other strategic agreements may divert management time and our limited resources, causing delays or disruptions to our business; our operating activities may be restricted by certain covenants in our license and strategic agreements, which could limit our development and commercial opportunities; failure to obtain marketing approval in international jurisdictions would prevent our products from being marketed in such jurisdictions; developments by competitors may render our products or technologies obsolete or non-competitive or may reduce the size of our markets; the successful commercialization of our product candidates will depend in part on the extent to which governmental authorities and health insurers establish coverage, adequate reimbursement levels and pricing policies; failure to obtain or maintain coverage and adequate reimbursement for our product candidates, if approved, could limit our ability to market those products and decrease our ability to generate revenue; if we are unable to obtain, maintain, or adequately protect our intellectual property rights, we may not be able to compete effectively in our market; the impact of international terrorism, political unrest and wars on our business; and the impact of other events such as the COVID-19 pandemic may adversely impact our business and operations, including our clinical trials. These and other important factors discussed under the caption "Risk Factors" in the Company's Quarterly Report on Form 10-Q filed with the SEC on August 14, 2024 and its other filings with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While the Company may elect to update such forward-looking statements at some point in the future, it disclaims any obligation to do so, even if subsequent events cause its views to change.
LYRA THERAPEUTICS, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(unaudited)
(in thousands, except share data)
|
|
June 30, |
|
|
December 31, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Assets |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
31,905 |
|
|
$ |
22,353 |
|
Short-term investments |
|
|
35,593 |
|
|
|
80,400 |
|
Prepaid expenses and other current assets |
|
|
1,937 |
|
|
|
2,068 |
|
Total current assets |
|
|
69,435 |
|
|
|
104,821 |
|
Property and equipment, net |
|
|
1,665 |
|
|
|
2,043 |
|
Operating lease right-of-use assets |
|
|
21,490 |
|
|
|
33,233 |
|
Restricted cash |
|
|
1,992 |
|
|
|
1,392 |
|
Other assets |
|
|
— |
|
|
|
1,111 |
|
Total assets |
|
$ |
94,582 |
|
|
$ |
142,600 |
|
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
4,971 |
|
|
$ |
3,131 |
|
Restructuring liability |
|
|
3,127 |
|
|
|
— |
|
Accrued expenses and other current liabilities |
|
|
6,095 |
|
|
|
9,374 |
|
Operating lease liabilities |
|
|
4,269 |
|
|
|
5,434 |
|
Deferred revenue |
|
|
814 |
|
|
|
1,658 |
|
Total current liabilities |
|
|
19,276 |
|
|
|
19,597 |
|
Operating lease liabilities, net of current portion |
|
|
32,479 |
|
|
|
21,447 |
|
Deferred revenue, net of current portion |
|
|
11,850 |
|
|
|
12,136 |
|
Total liabilities |
|
|
63,605 |
|
|
|
53,180 |
|
Commitments and contingencies |
|
|
|
|
|
|
||
Stockholders’ equity: |
|
|
|
|
|
|
||
Preferred stock, $0.001 par value, 10,000,000 shares authorized at June 30, 2024 |
|
|
— |
|
|
|
— |
|
Common stock, $0.001 par value; 200,000,000 shares authorized at |
|
|
65 |
|
|
|
57 |
|
Additional paid-in capital |
|
|
412,854 |
|
|
|
400,685 |
|
Accumulated other comprehensive income (loss), net of tax |
|
|
(4 |
) |
|
|
33 |
|
Accumulated deficit |
|
|
(381,938 |
) |
|
|
(311,355 |
) |
Total stockholders’ equity |
|
|
30,977 |
|
|
|
89,420 |
|
Total liabilities and stockholders’ equity |
|
$ |
94,582 |
|
|
$ |
142,600 |
|
LYRA THERAPEUTICS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONSAND COMPREHENSIVE LOSS
(unaudited)
(in thousands, except share and per share data)
|
|
Three Months Ended |
|
|
Six Months Ended June 30, |
|
|
||||||||||
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
|
||||
Collaboration revenue |
|
$ |
598 |
|
|
$ |
458 |
|
|
$ |
1,130 |
|
|
$ |
868 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
|
13,264 |
|
|
|
10,799 |
|
|
|
31,502 |
|
|
|
23,395 |
|
|
General and administrative |
|
|
5,139 |
|
|
|
4,570 |
|
|
|
10,957 |
|
|
|
9,697 |
|
|
Impairment of property and equipment |
|
|
1,883 |
|
|
|
1,592 |
|
|
|
1,883 |
|
|
|
1,592 |
|
|
Impairment of right-of-use asset |
|
|
22,836 |
|
|
|
— |
|
|
|
22,836 |
|
|
|
— |
|
|
Restructuring and other related charges |
|
|
6,450 |
|
|
|
— |
|
|
|
6,450 |
|
|
|
— |
|
|
Total operating expenses |
|
|
49,572 |
|
|
|
16,961 |
|
|
|
73,628 |
|
|
|
34,684 |
|
|
Loss from operations |
|
|
(48,974 |
) |
|
|
(16,503 |
) |
|
|
(72,498 |
) |
|
|
(33,816 |
) |
|
Other income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Interest income |
|
|
855 |
|
|
|
897 |
|
|
|
1,941 |
|
|
|
1,969 |
|
|
Total other income |
|
|
855 |
|
|
|
897 |
|
|
|
1,941 |
|
|
|
1,969 |
|
|
Loss before income tax expense |
|
|
(48,119 |
) |
|
|
(15,606 |
) |
|
|
(70,557 |
) |
|
|
(31,847 |
) |
|
Income tax expense |
|
|
(12 |
) |
|
|
(12 |
) |
|
|
(26 |
) |
|
|
(26 |
) |
|
Net loss |
|
|
(48,131 |
) |
|
|
(15,618 |
) |
|
|
(70,583 |
) |
|
|
(31,873 |
) |
|
Other comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Unrealized holding loss on short-term investments, net of tax |
|
|
(29 |
) |
|
|
(15 |
) |
|
|
(37 |
) |
|
|
(37 |
) |
|
Comprehensive loss |
|
$ |
(48,160 |
) |
|
$ |
(15,633 |
) |
|
$ |
(70,620 |
) |
|
$ |
(31,910 |
) |
|
Net loss per share attributable to common stockholders— basic and diluted |
|
$ |
(0.74 |
) |
|
$ |
(0.36 |
) |
|
$ |
(1.09 |
) |
|
$ |
(0.79 |
) |
|
Weighted-average common shares outstanding— |
|
|
65,459,678 |
|
|
|
43,676,387 |
|
|
|
64,739,520 |
|
|
|
40,273,472 |
|
|
Contact Information:
Jason Cavalier, Chief Financial Officer
917.584.7668
jcavalier@lyratx.com
Media Contact:
Kathryn Morris, The Yates Network LLC
914.204.6412
kathryn@theyatesnetwork.com

Corporate Presentation August 2024

Forward Looking Statement This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward-looking statements. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including statements regarding our focus on the two ongoing ENLIGHTEN Phase 3 trials evaluating LYR-210, our ongoing ENLIGHTEN 1 extension study and expectation for data in Q4 2024, our ongoing ENLIGHTEN 2 trial and our expectation for data in 1H 2025, whether LYR-210 if advanced would be positioned to align with current ENT practices, our cash runway into 2026 and plans to update investors regarding our cash runway, and our plans to evaluate potential strategic options to maximize shareholder value. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause the Company's actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: any potential financial or strategic option we pursue in order to maximize shareholder value may not result in the identification of a suitable transaction, or if one is identified and pursued, may not be completed on attractive terms, or at all; our ability to sublease or assign our three leaseholds, which represent significant operating costs; our incurrence of significant losses since inception and expectation to incur significant additional losses for the foreseeable future; our recurring losses from operations raise substantial doubt regarding our ability to continue as a going concern; our need for significant additional funding in order to complete development of and obtain regulatory approval for our product candidates and commercialize our products, if approved; the failure of our ENLIGHTEN 1 Phase 3 trial to meet its primary endpoint has made it more difficult for the Company to raise capital; we could be forced to delay, reduce, or eliminate our product development programs or commercialization efforts; following the failure of our ENLIGHTEN 1 Phase 3 trial evaluating LYR-210 for the treatment of chronic rhinosinusitis (CRS) to meet its primary endpoint, which was announced in May 2024, there is significant uncertainty about the Company’s ability to complete development of LYR-210 and our ability to obtain regulatory approval for LYR-210 is at least significantly delayed and may not be possible; our common stock may be delisted from The Nasdaq Global Market if we cannot regain compliance with Nasdaq’s continued listing requirements; our loss of key personnel significantly and adversely affects our ability to manufacture our product candidates, among other activities; we are no longer engaged in the manufacturing of our product candidates in-house; our business is highly dependent on the success of our most advanced product candidate, LYR-210; clinical trials required for our current product candidate and any future product candidates are expensive and time-consuming, their outcome is uncertain, and if our clinical trials do not meet safety or efficacy endpoints in these evaluations, or if we experience significant delays in these trials, our ability to commercialize our product candidates and our financial position will be impaired; any failure by a third party to conduct our pre-clinical or clinical trials according to good clinical practices and in a timely manner may delay or prevent our ability to seek or obtain regulatory approval for or commercialize our product candidates; even if LYR-210 receives marketing approval, it may fail to achieve market acceptance by physicians, patients, third-party payors or others in the medical community necessary for commercial success; if our collaborations are not successful, including with LianBio our product candidates may not reach their full market potential; our ability to manage our obligations under our license and other strategic agreements may divert management time and our limited resources, causing delays or disruptions to our business; our operating activities may be restricted by certain covenants in our license and strategic agreements, which could limit our development and commercial opportunities; failure to obtain marketing approval in international jurisdictions would prevent our products from being marketed in such jurisdictions; developments by competitors may render our products or technologies obsolete or non-competitive or may reduce the size of our markets; the successful commercialization of our product candidates will depend in part on the extent to which governmental authorities and health insurers establish coverage, adequate reimbursement levels and pricing policies; failure to obtain or maintain coverage and adequate reimbursement for our product candidates, if approved, could limit our ability to market those products and decrease our ability to generate revenue; if we are unable to obtain, maintain, or adequately protect our intellectual property rights, we may not be able to compete effectively in our market; the impact of international terrorism, political unrest and wars on our business; and the impact of other events such as the COVID-19 pandemic may adversely impact our business and operations, including our clinical trials. These and other important factors discussed under the caption "Risk Factors" in the Company's Quarterly Report on Form 10-Q filed with the SEC on August 14, 2024 and its other filings with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While the Company may elect to update such forward-looking statements at some point in the future, it disclaims any obligation to do so, even if subsequent events cause its views to change. This presentation also includes statistical and market data that we obtained from industry, publications and research, surveys and studies conducted by third parties or us. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. All of the market data used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. While we believe these industry publications and third-party research, surveys and studies are reliable, we have not independently verified such data. The industry in which we operate is subject to a high degree of uncertainty, change and risk due to a variety of factors, which could cause results to differ materially from those expressed in the estimates made by the independent partners and by us. Lyra’s product candidates, LYR-210 and LYR-220, have not been approved by FDA. This presentation is intended for the investor community only. Nothing herein is intended to promote the Company’s product candidates. In connection with its previously announced reduction in workforce Lyra stopped manufacturing and commercialization efforts for LYR-210, as well as development efforts for LYR-220 in an effort to reduce operating expenses. Nevertheless, we anticipate that we will continue to incur expenses as we continue the two ongoing ENLIGHTEN Phase 3 clinical trials of LYR-210.

1) Summary Health Statistics Tables for U.S. Adults: National Health Interview Survey, 2018, Tables A-2b, A-2c; 2) Baguley et al. Int Forum Allergy Rhinol, 2014;4(7):525-3; Bioabsorbable sinonasal implant designed to deliver 6 months of continuous anti-inflammatory therapy Indication: Chronic rhinosinusitis (CRS) ~12% of the US population1 ~50% of patients fail medical therapy2 Pivotal Phase 3 trials ongoing Over 100 global patents issued and pending Clinical-stage biotechnology company developing long-acting, anti-inflammatory sinonasal implants for the treatment of chronic rhinosinusitis Company Overview
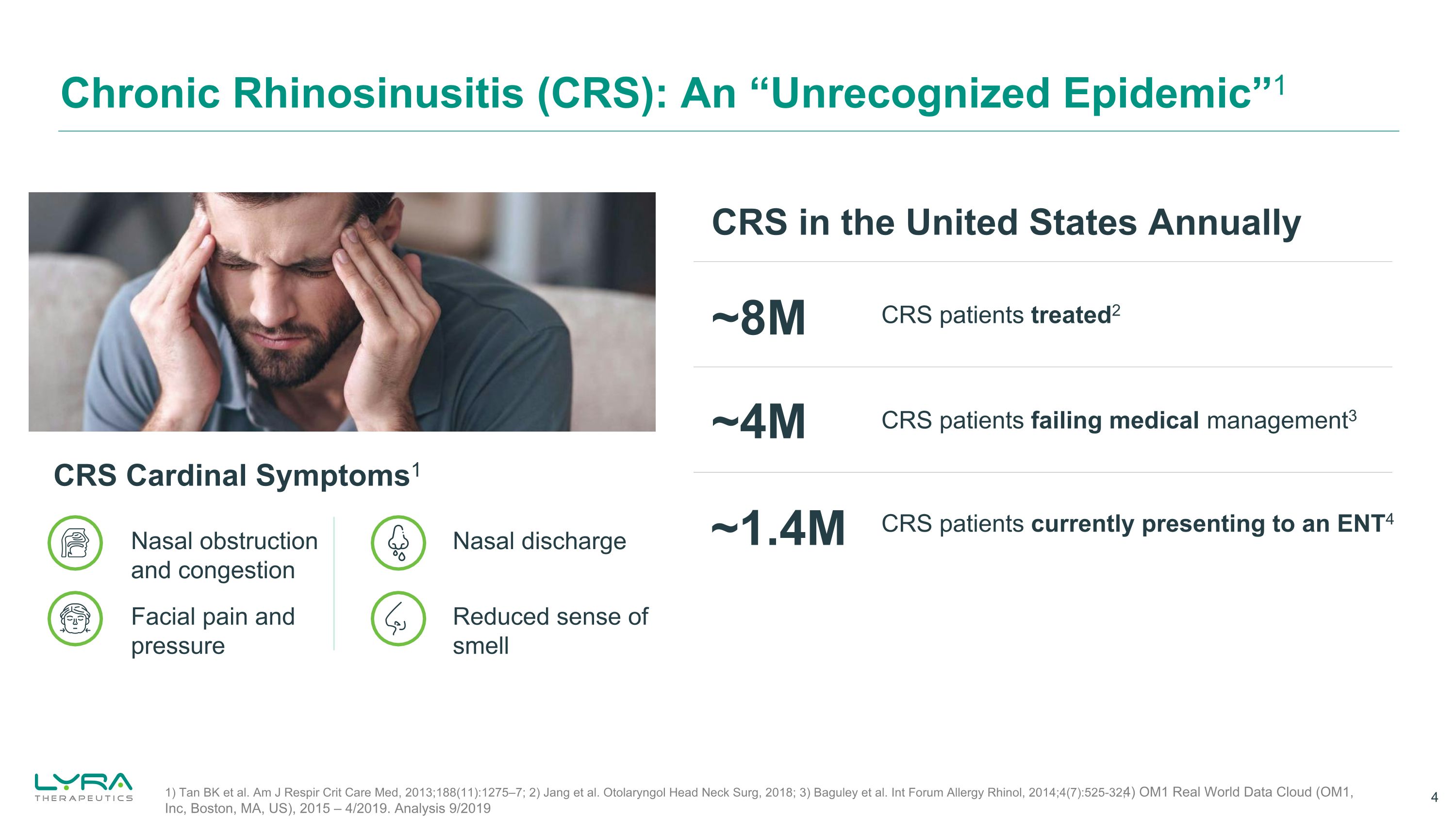
Chronic Rhinosinusitis (CRS): An “Unrecognized Epidemic”1 CRS Cardinal Symptoms1 Nasal obstruction and congestion Facial pain and pressure Nasal discharge Reduced sense of smell CRS in the United States Annually CRS patients treated2 CRS patients failing medical management3 ~8M ~4M CRS patients currently presenting to an ENT4 ~1.4M 1) Tan BK et al. Am J Respir Crit Care Med, 2013;188(11):1275–7; 2) Jang et al. Otolaryngol Head Neck Surg, 2018; 3) Baguley et al. Int Forum Allergy Rhinol, 2014;4(7):525-32; 4) OM1 Real World Data Cloud (OM1, Inc, Boston, MA, US), 2015 – 4/2019. Analysis 9/2019
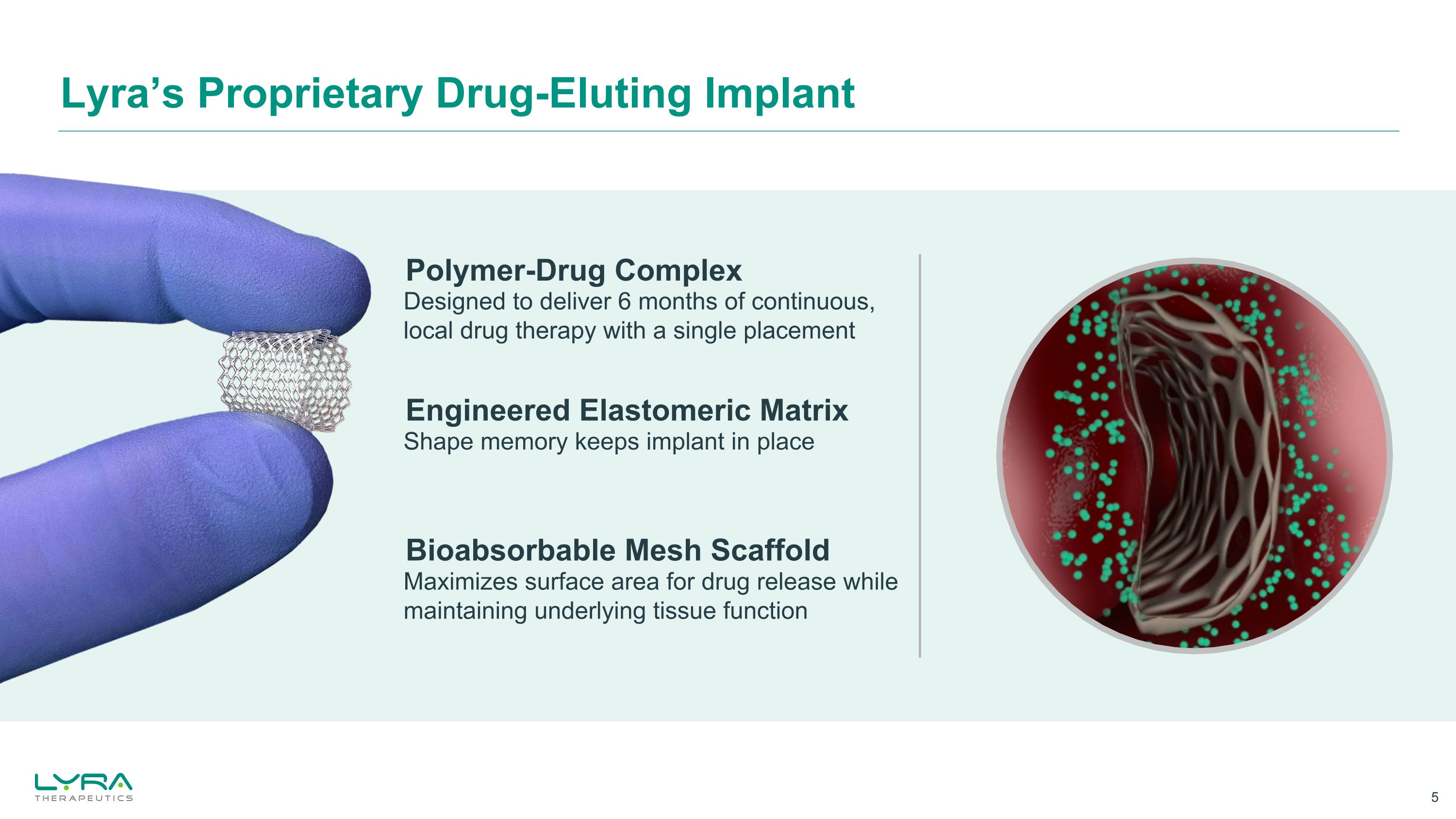
Lyra’s Proprietary Drug-Eluting Implant Engineered Elastomeric Matrix Shape memory keeps implant in place Polymer-Drug Complex Designed to deliver 6 months of continuous, local drug therapy with a single placement Bioabsorbable Mesh Scaffold Maximizes surface area for drug release while maintaining underlying tissue function
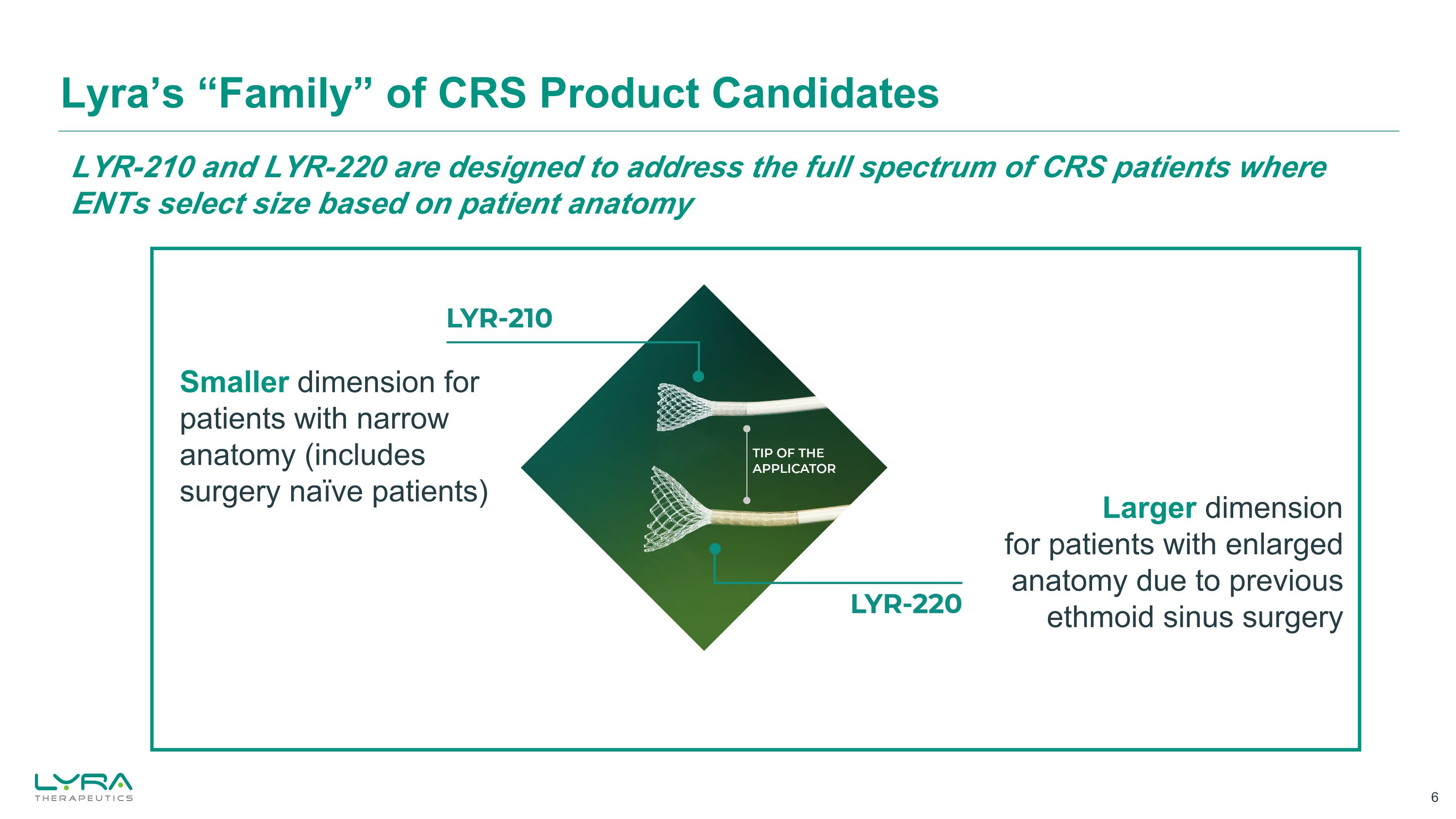
Lyra’s “Family” of CRS Product Candidates Larger dimension for patients with enlarged anatomy due to previous ethmoid sinus surgery Smaller dimension for patients with narrow anatomy (includes surgery naïve patients) LYR-210 and LYR-220 are designed to address the full spectrum of CRS patients where ENTs select size based on patient anatomy
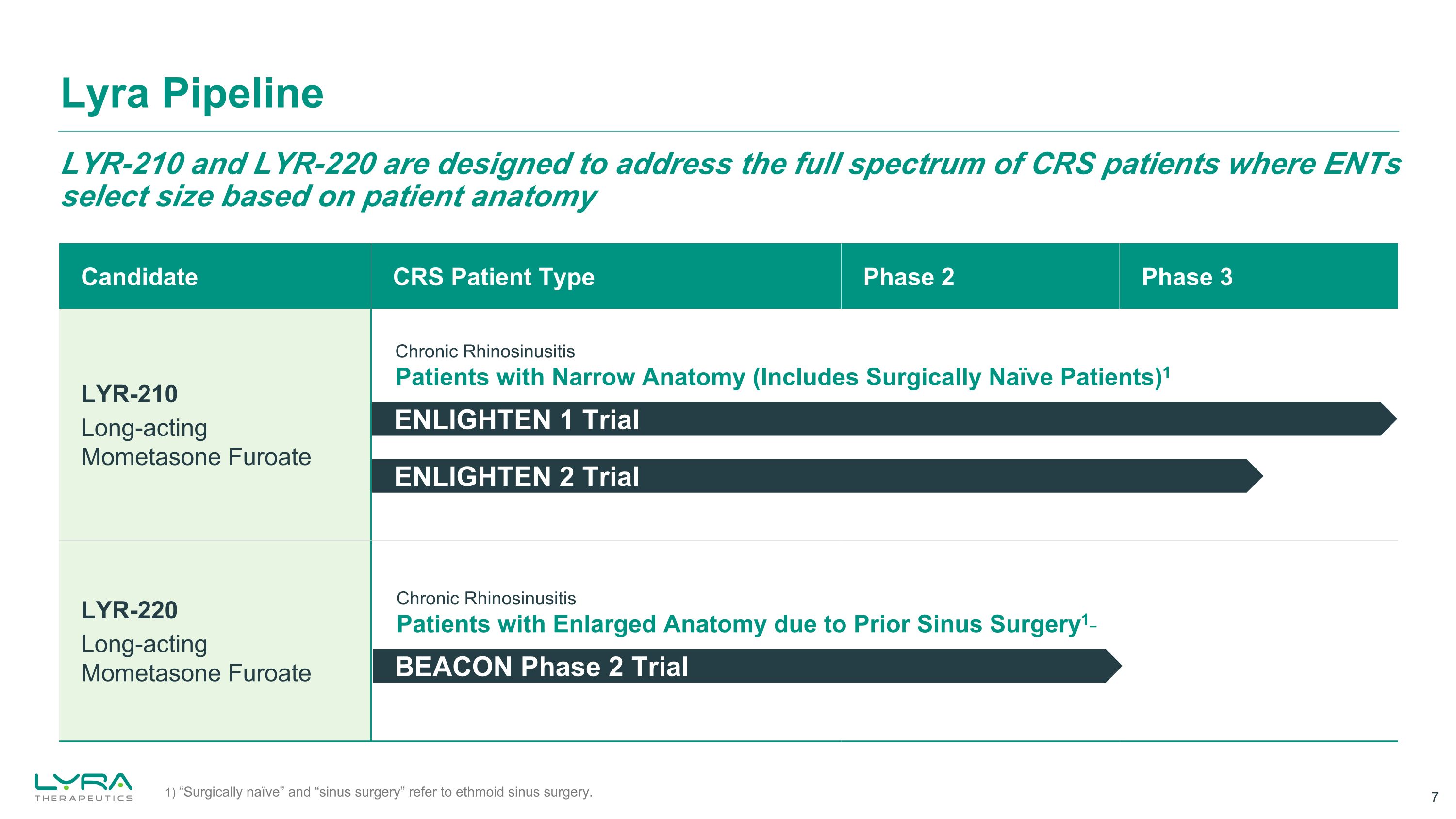
LYR-210 and LYR-220 are designed to address the full spectrum of CRS patients where ENTs select size based on patient anatomy Lyra Pipeline Candidate CRS Patient Type Phase 2 Phase 3 LYR-210 Long-acting Mometasone Furoate LYR-220 Long-acting Mometasone Furoate ENLIGHTEN 1 Trial Chronic Rhinosinusitis Patients with Narrow Anatomy (Includes Surgically Naïve Patients)1 Chronic Rhinosinusitis Patients with Enlarged Anatomy due to Prior Sinus Surgery1 BEACON Phase 2 Trial ENLIGHTEN 2 Trial 1) “Surgically naïve” and “sinus surgery” refer to ethmoid sinus surgery.
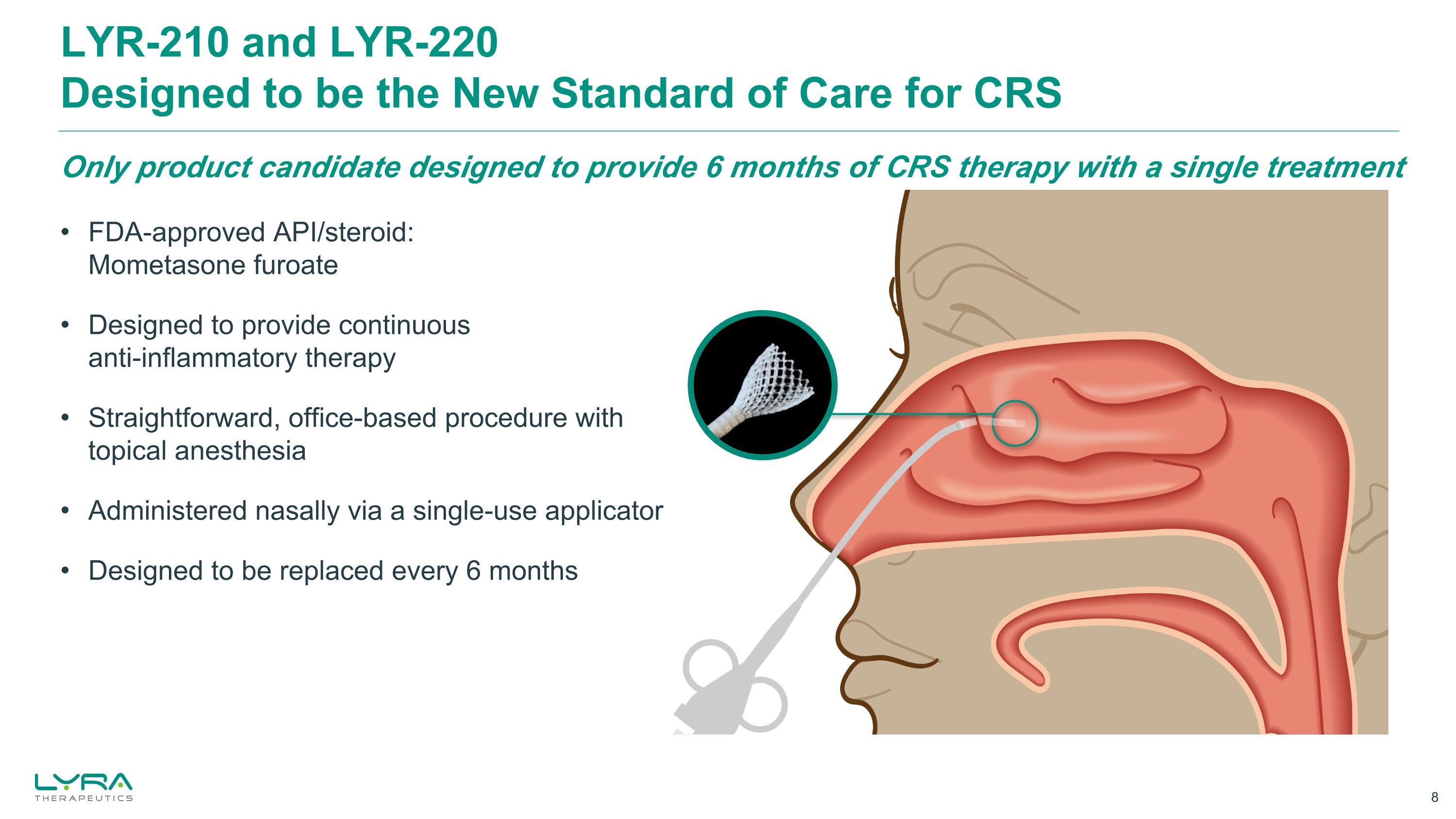
FDA-approved API/steroid:Mometasone furoate Designed to provide continuous anti-inflammatory therapy Straightforward, office-based procedure with topical anesthesia Administered nasally via a single-use applicator Designed to be replaced every 6 months Only product candidate designed to provide 6 months of CRS therapy with a single treatment LYR-210 and LYR-220Designed to be the New Standard of Care for CRS
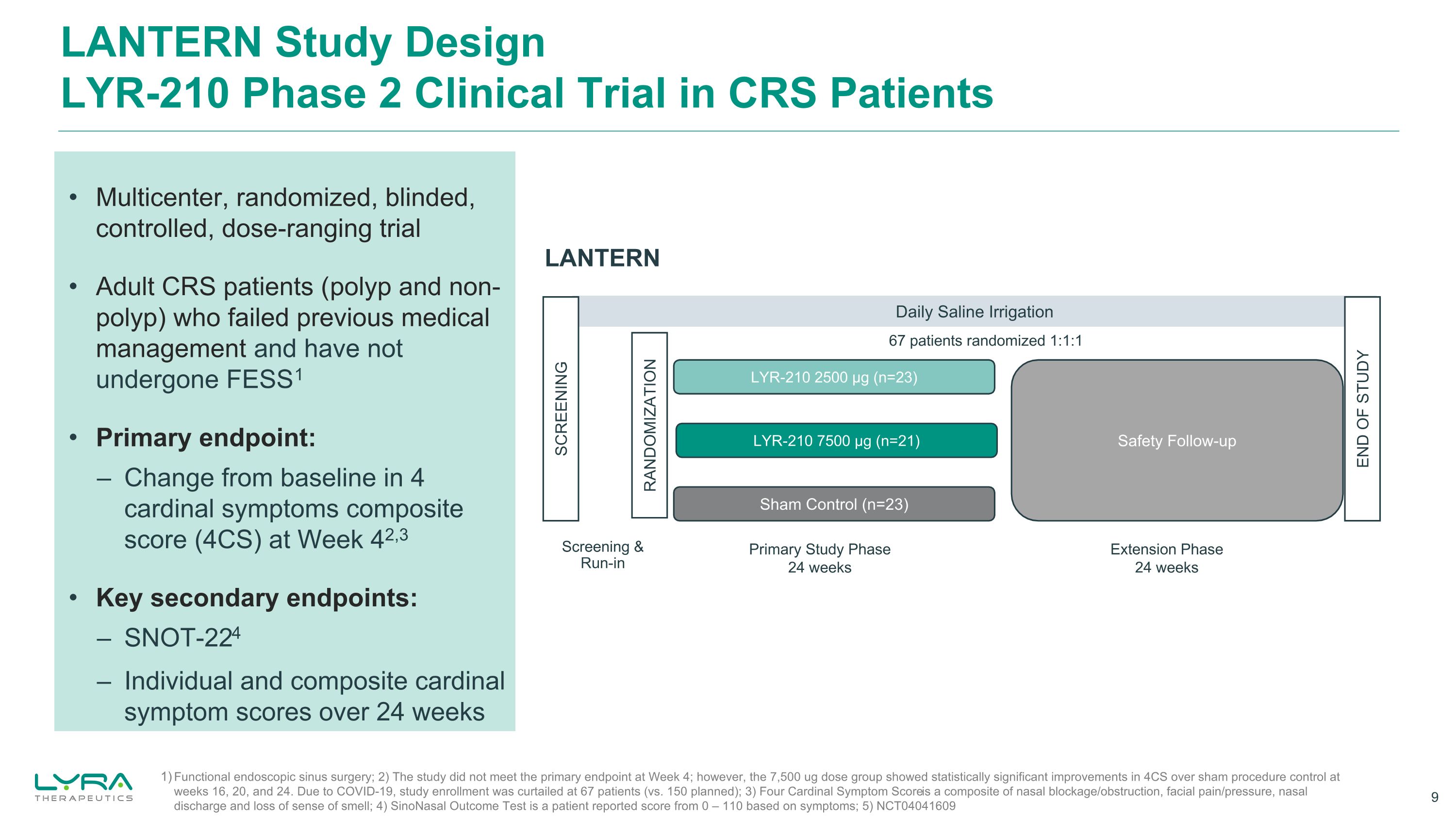
LANTERN Study DesignLYR-210 Phase 2 Clinical Trial in CRS Patients LANTERN Daily Saline Irrigation Sham Control (n=23) LYR-210 2500 μg (n=23) SCREENING RANDOMIZATION END OF STUDY LYR-210 7500 μg (n=21) Primary Study Phase 24 weeks Extension Phase 24 weeks Screening &Run-in Safety Follow-up Functional endoscopic sinus surgery; 2) The study did not meet the primary endpoint at Week 4; however, the 7,500 ug dose group showed statistically significant improvements in 4CS over sham procedure control at weeks 16, 20, and 24. Due to COVID-19, study enrollment was curtailed at 67 patients (vs. 150 planned); 3) Four Cardinal Symptom Score is a composite of nasal blockage/obstruction, facial pain/pressure, nasal discharge and loss of sense of smell; 4) SinoNasal Outcome Test is a patient reported score from 0 – 110 based on symptoms; 5) NCT04041609 67 patients randomized 1:1:1 Multicenter, randomized, blinded, controlled, dose-ranging trial Adult CRS patients (polyp and non-polyp) who failed previous medical management and have not undergone FESS1 Primary endpoint: – Change from baseline in 4 cardinal symptoms composite score (4CS) at Week 42,3 Key secondary endpoints: – SNOT-224 – Individual and composite cardinal symptom scores over 24 weeks
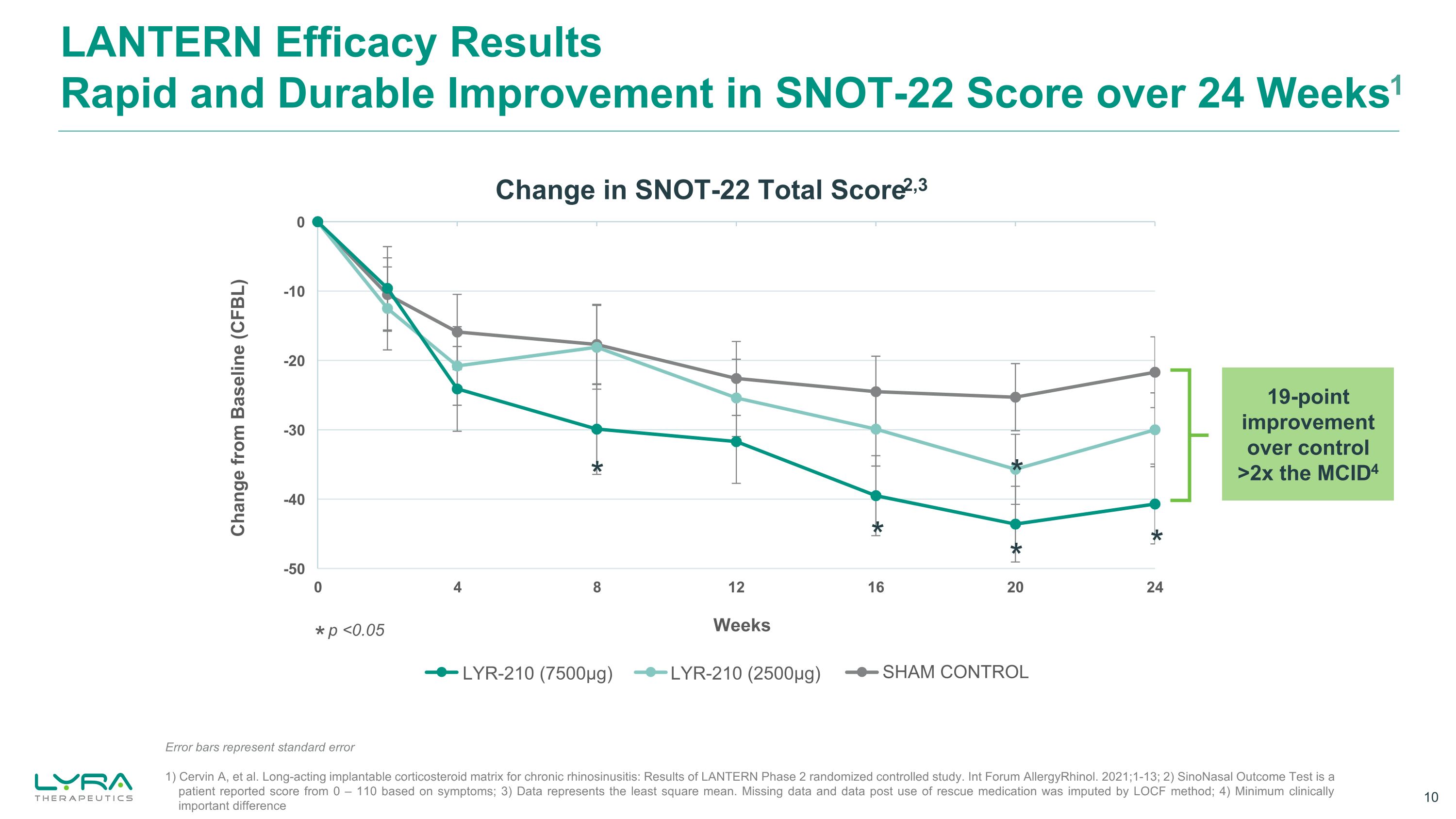
LANTERN Efficacy ResultsRapid and Durable Improvement in SNOT-22 Score over 24 Weeks1 1) Cervin A, et al. Long-acting implantable corticosteroid matrix for chronic rhinosinusitis: Results of LANTERN Phase 2 randomized controlled study. Int Forum AllergyRhinol. 2021;1-13; 2) SinoNasal Outcome Test is a patient reported score from 0 – 110 based on symptoms; 3) Data represents the least square mean. Missing data and data post use of rescue medication was imputed by LOCF method; 4) Minimum clinically important difference * p <0.05 * * * * * Change in SNOT-22 Total Score2,3 19-point improvement over control >2x the MCID4 LYR-210 (2500μg) LYR-210 (7500μg) SHAM CONTROL Error bars represent standard error
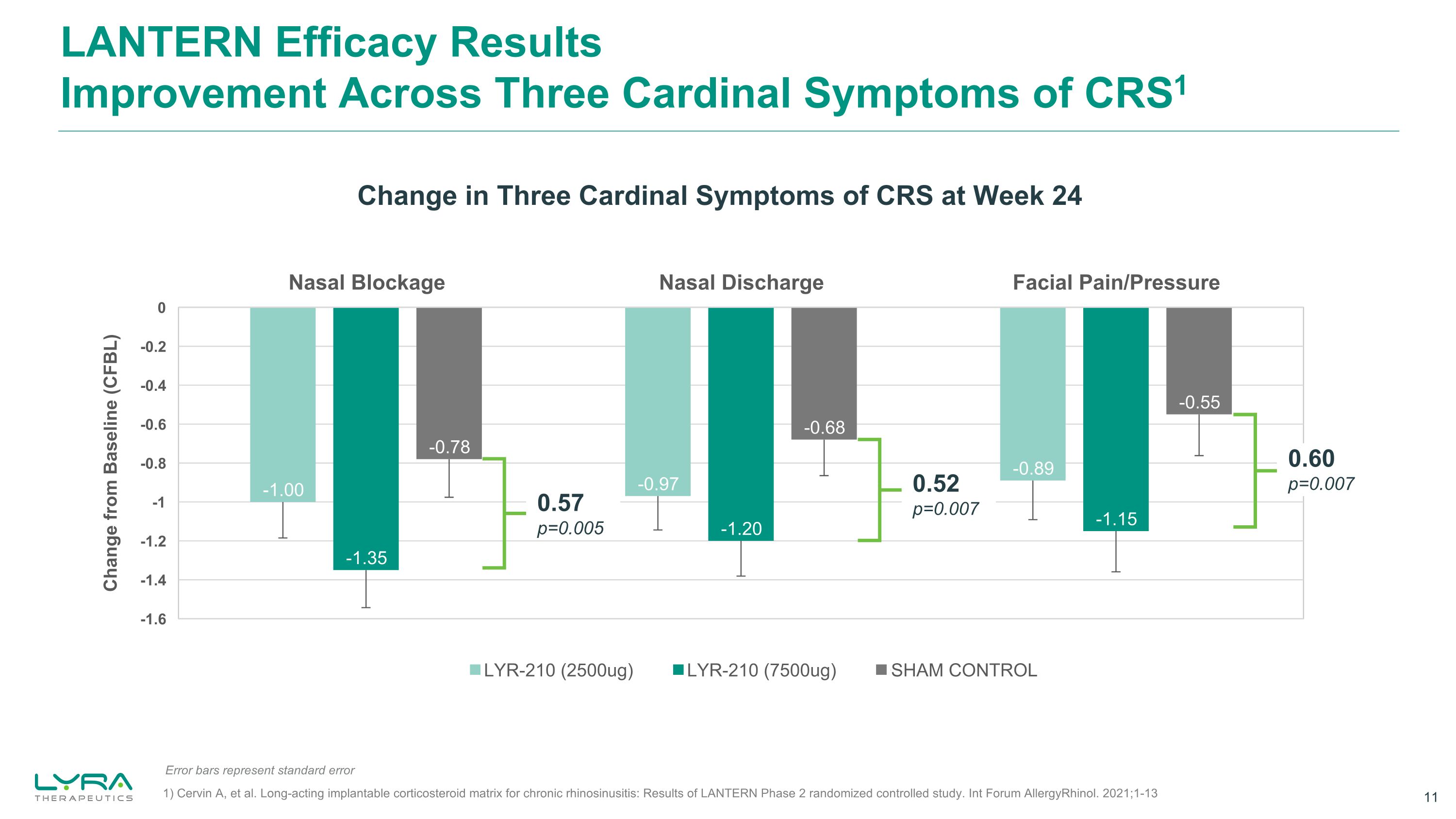
LANTERN Efficacy ResultsImprovement Across Three Cardinal Symptoms of CRS1 Change in Three Cardinal Symptoms of CRS at Week 24 0.57 p=0.005 0.52 p=0.007 0.60 p=0.007 SHAM CONTROL Error bars represent standard error 1) Cervin A, et al. Long-acting implantable corticosteroid matrix for chronic rhinosinusitis: Results of LANTERN Phase 2 randomized controlled study. Int Forum AllergyRhinol. 2021;1-13
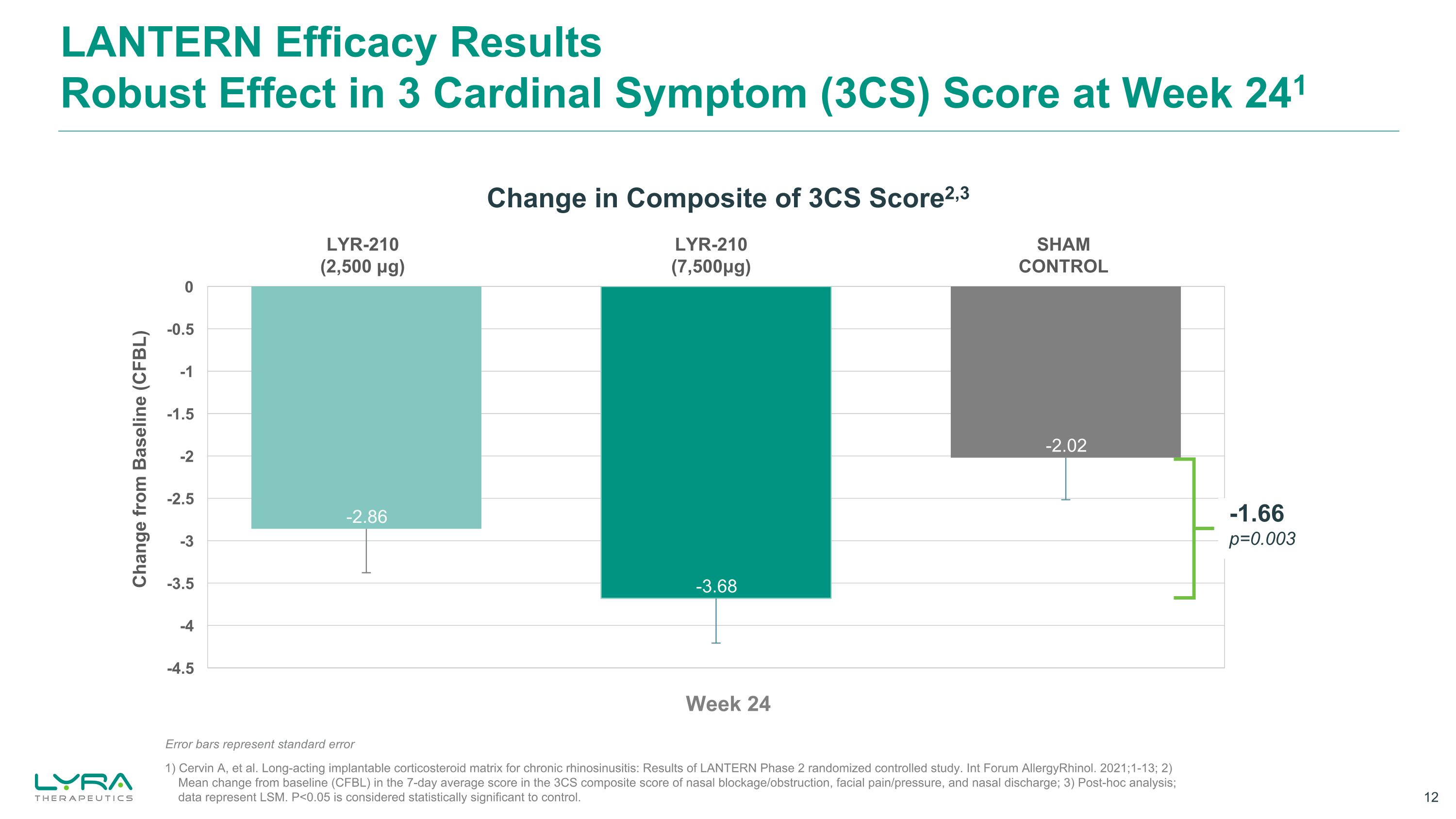
LANTERN Efficacy ResultsRobust Effect in 3 Cardinal Symptom (3CS) Score at Week 241 1) Cervin A, et al. Long-acting implantable corticosteroid matrix for chronic rhinosinusitis: Results of LANTERN Phase 2 randomized controlled study. Int Forum AllergyRhinol. 2021;1-13; 2) Mean change from baseline (CFBL) in the 7-day average score in the 3CS composite score of nasal blockage/obstruction, facial pain/pressure, and nasal discharge; 3) Post-hoc analysis; data represent LSM. P<0.05 is considered statistically significant to control. Change from Baseline (CFBL) Week 24 Change in Composite of 3CS Score2,3 Error bars represent standard error LYR-210 (7,500μg) LYR-210 (2,500 μg) SHAMCONTROL -1.66 p=0.003
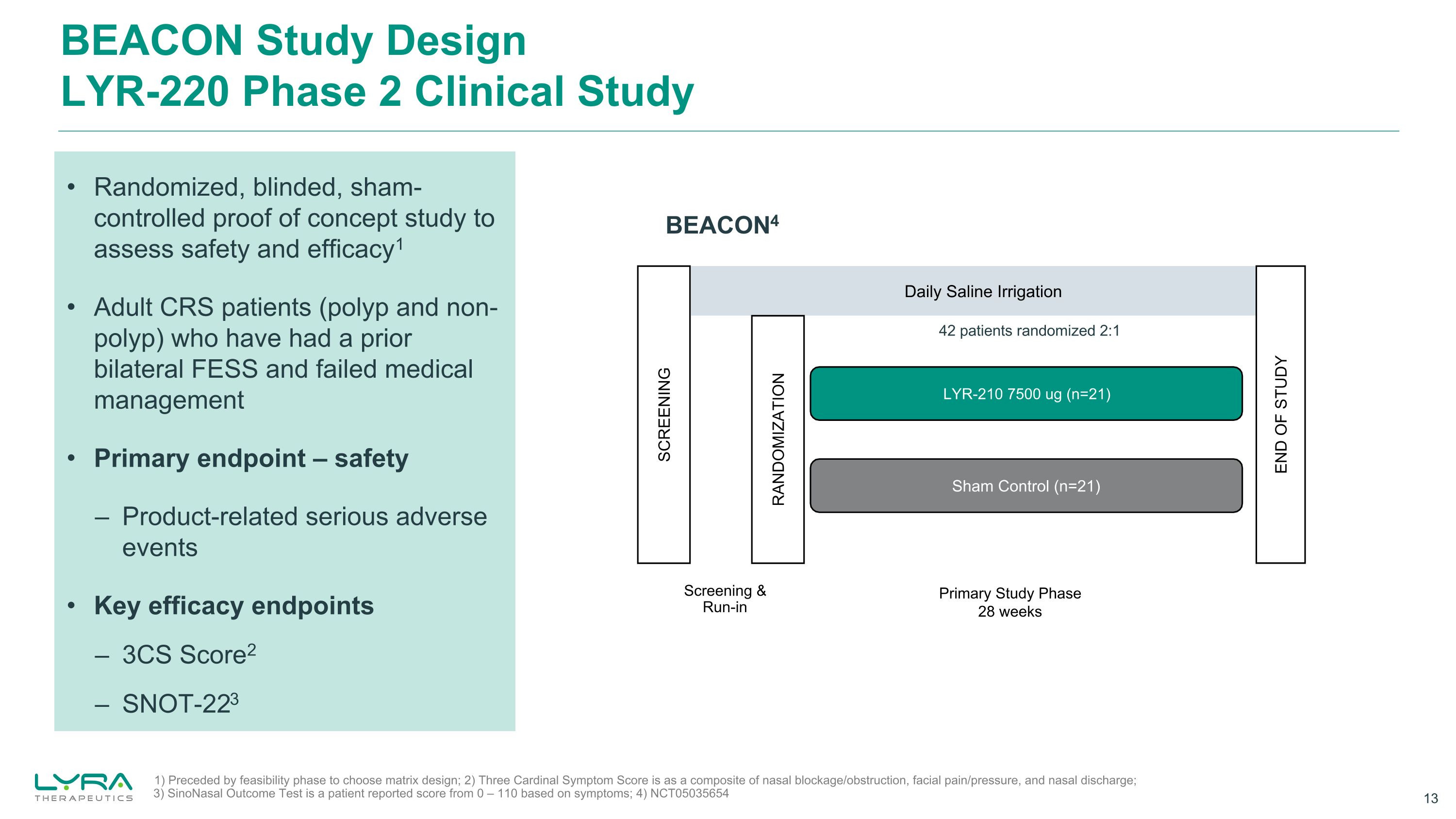
BEACON Study DesignLYR-220 Phase 2 Clinical Study Randomized, blinded, sham-controlled proof of concept study to assess safety and efficacy1 Adult CRS patients (polyp and non-polyp) who have had a prior bilateral FESS and failed medical management Primary endpoint – safety – Product-related serious adverse events Key efficacy endpoints – 3CS Score2 – SNOT-223 1) Preceded by feasibility phase to choose matrix design; 2) Three Cardinal Symptom Score is as a composite of nasal blockage/obstruction, facial pain/pressure, and nasal discharge; 3) SinoNasal Outcome Test is a patient reported score from 0 – 110 based on symptoms; 4) NCT05035654 BEACON4 Daily Saline Irrigation Sham Control (n=21) LYR-210 7500 ug (n=21) SCREENING RANDOMIZATION END OF STUDY Primary Study Phase 28 weeks Screening &Run-in 42 patients randomized 2:1
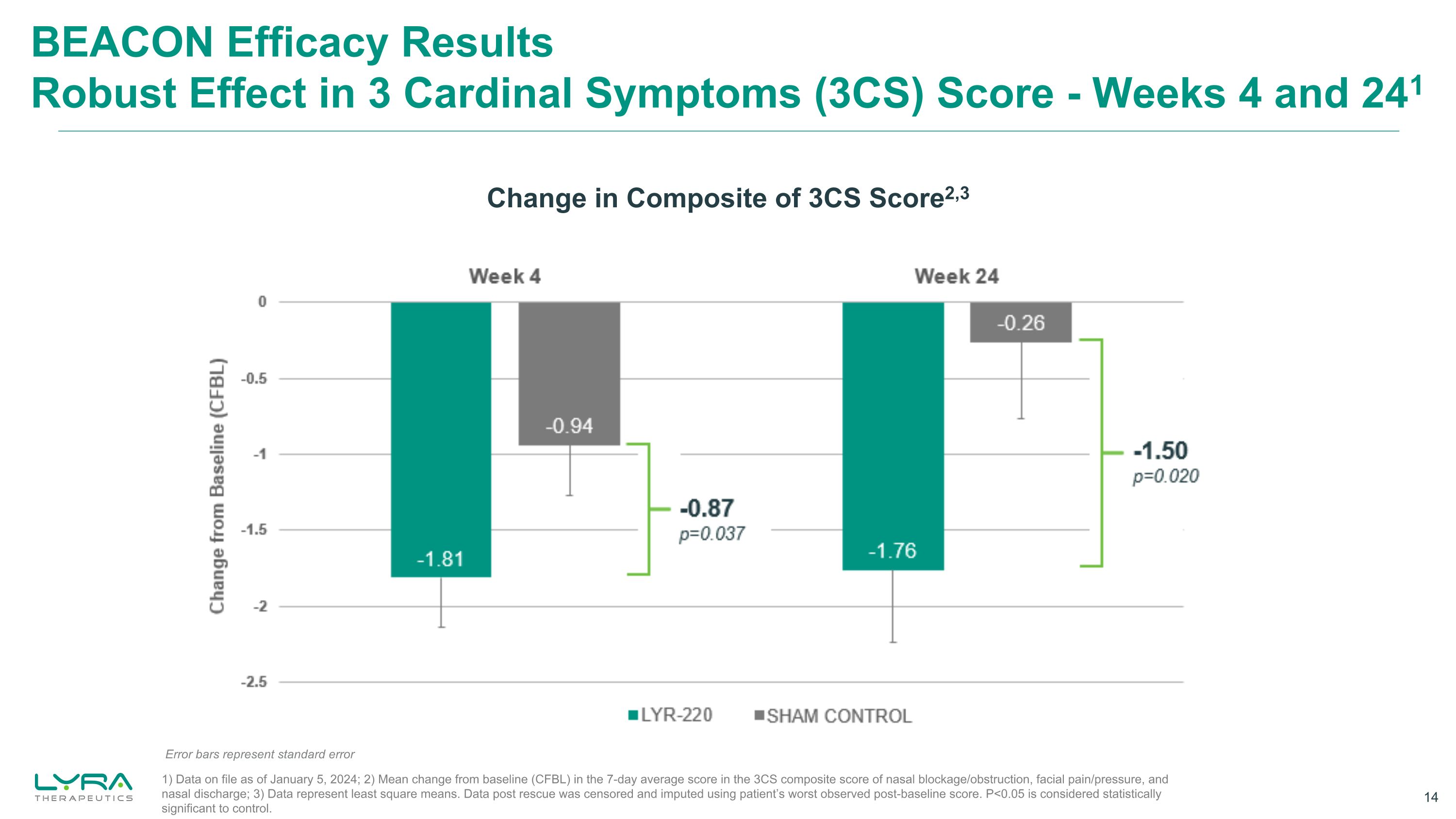
BEACON Efficacy Results Robust Effect in 3 Cardinal Symptoms (3CS) Score - Weeks 4 and 241 Change in Composite of 3CS Score2,3 Error bars represent standard error 1) Data on file as of January 5, 2024; 2) Mean change from baseline (CFBL) in the 7-day average score in the 3CS composite score of nasal blockage/obstruction, facial pain/pressure, and nasal discharge; 3) Data represent least square means. Data post rescue was censored and imputed using patient’s worst observed post-baseline score. P<0.05 is considered statistically significant to control. Bar Chart
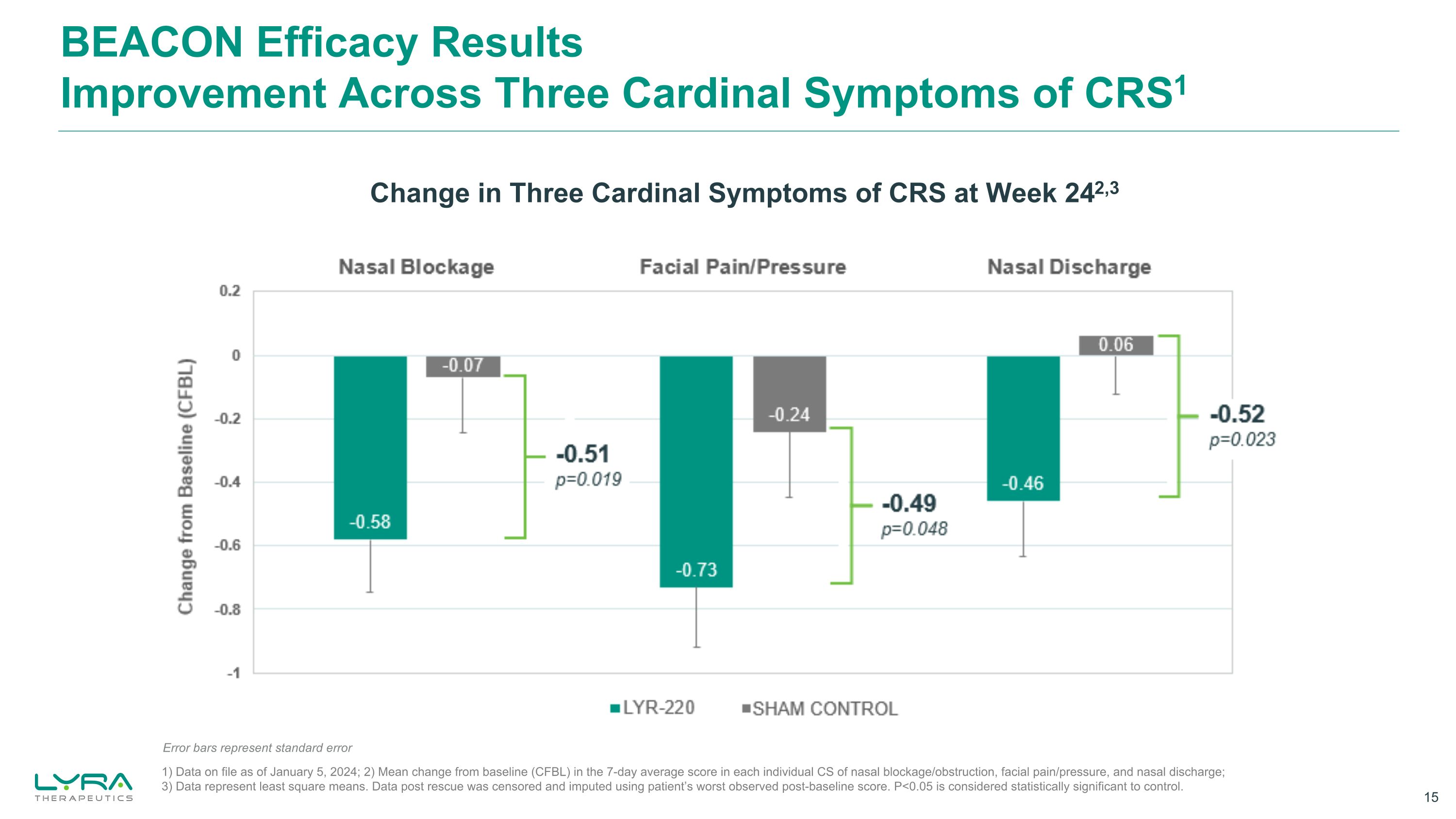
BEACON Efficacy Results Improvement Across Three Cardinal Symptoms of CRS1 Change in Three Cardinal Symptoms of CRS at Week 242,3 1) Data on file as of January 5, 2024; 2) Mean change from baseline (CFBL) in the 7-day average score in each individual CS of nasal blockage/obstruction, facial pain/pressure, and nasal discharge; 3) Data represent least square means. Data post rescue was censored and imputed using patient’s worst observed post-baseline score. P<0.05 is considered statistically significant to control. Error bars represent standard error Bar Chart
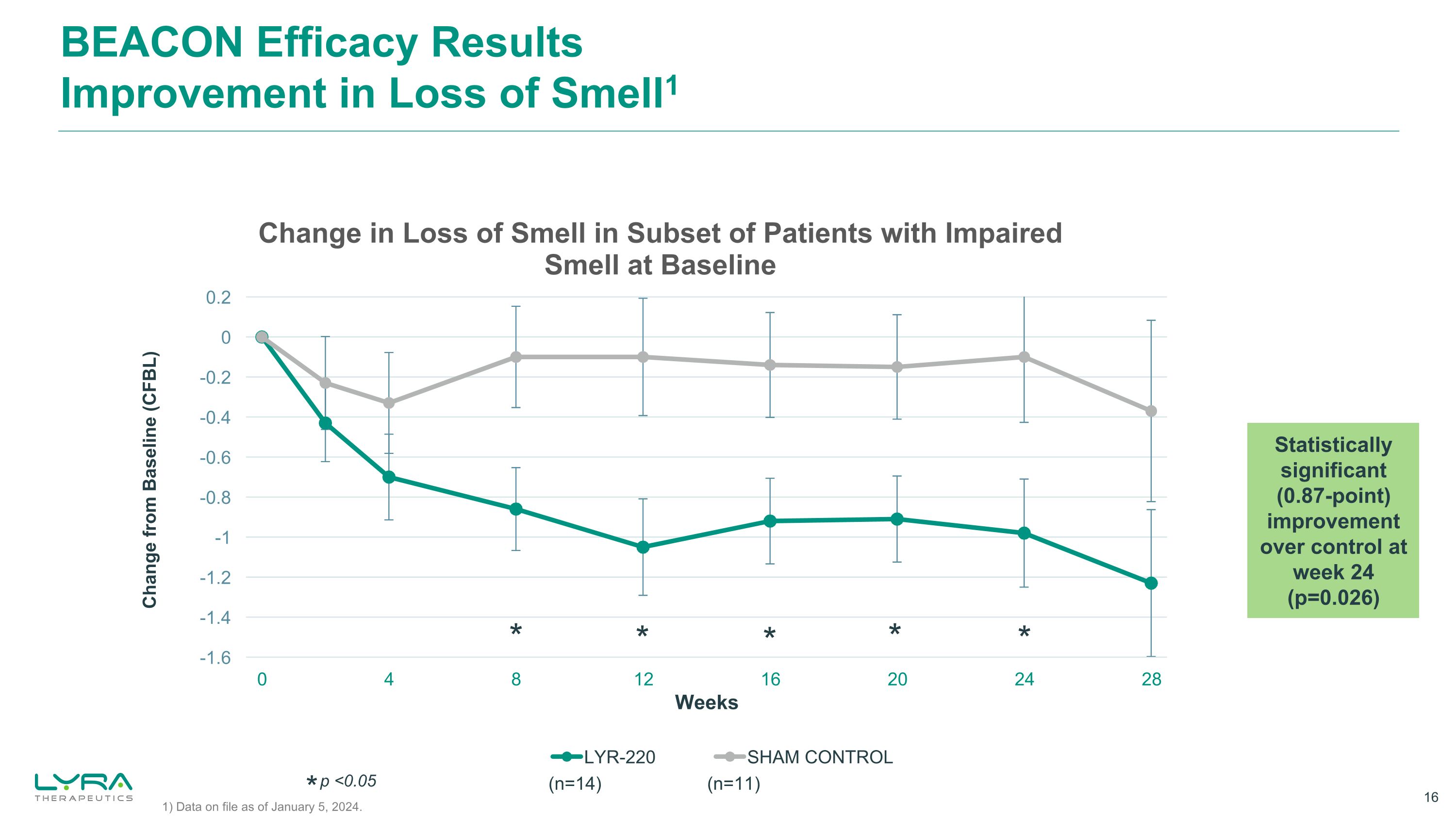
BEACON Efficacy Results Improvement in Loss of Smell1 Change from Baseline (CFBL) * * * * * Statistically significant (0.87-point) improvement over control at week 24 (p=0.026) * p <0.05 1) Data on file as of January 5, 2024. (n=14) (n=11)
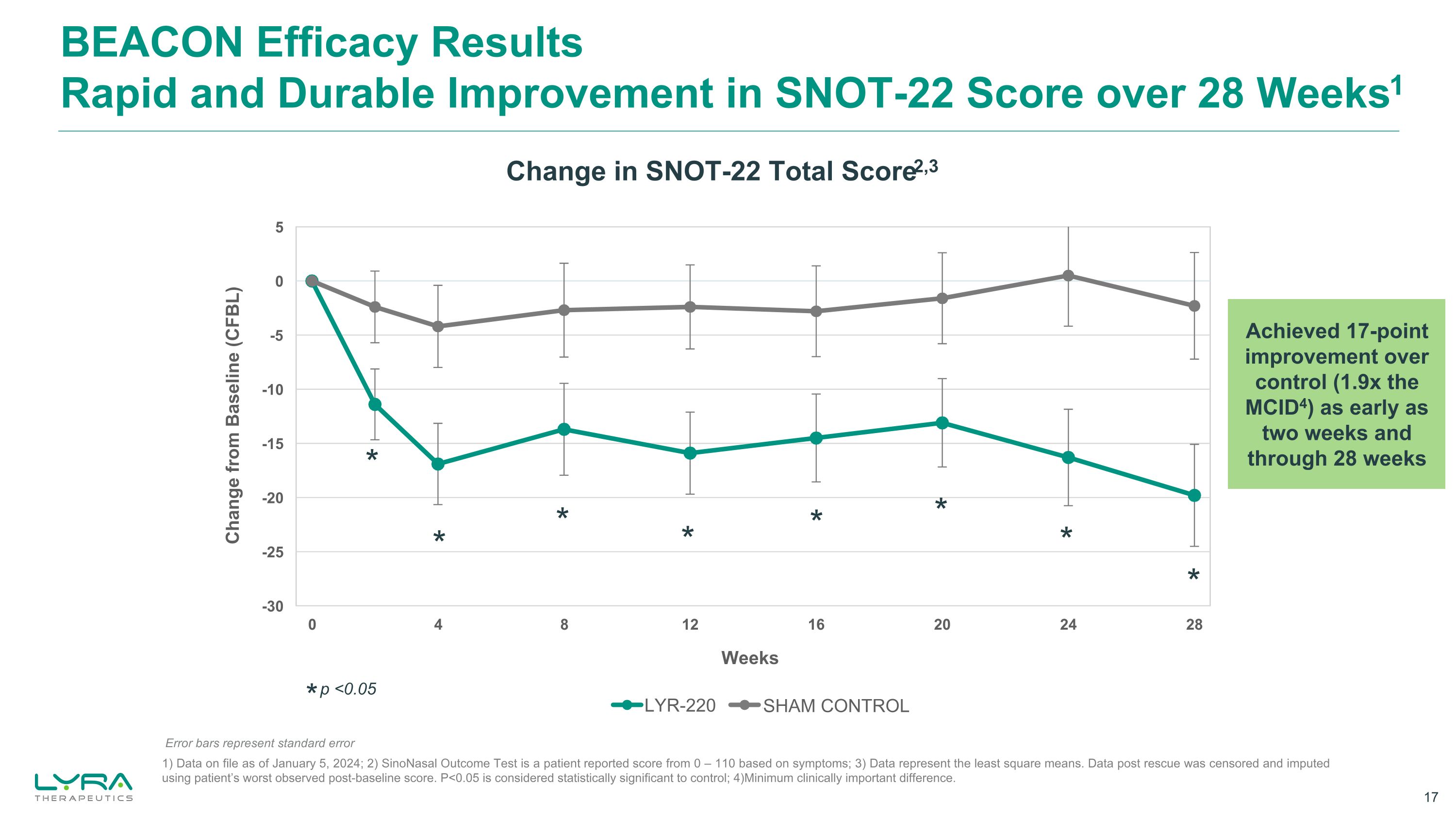
BEACON Efficacy Results Rapid and Durable Improvement in SNOT-22 Score over 28 Weeks1 * p <0.05 * * * * * * * Change in SNOT-22 Total Score2,3 Weeks Change from Baseline (CFBL) Achieved 17-point improvement over control (1.9x the MCID4) as early as two weeks and through 28 weeks SHAM CONTROL Error bars represent standard error 1) Data on file as of January 5, 2024; 2) SinoNasal Outcome Test is a patient reported score from 0 – 110 based on symptoms; 3) Data represent the least square means. Data post rescue was censored and imputed using patient’s worst observed post-baseline score. P<0.05 is considered statistically significant to control; 4)Minimum clinically important difference. *
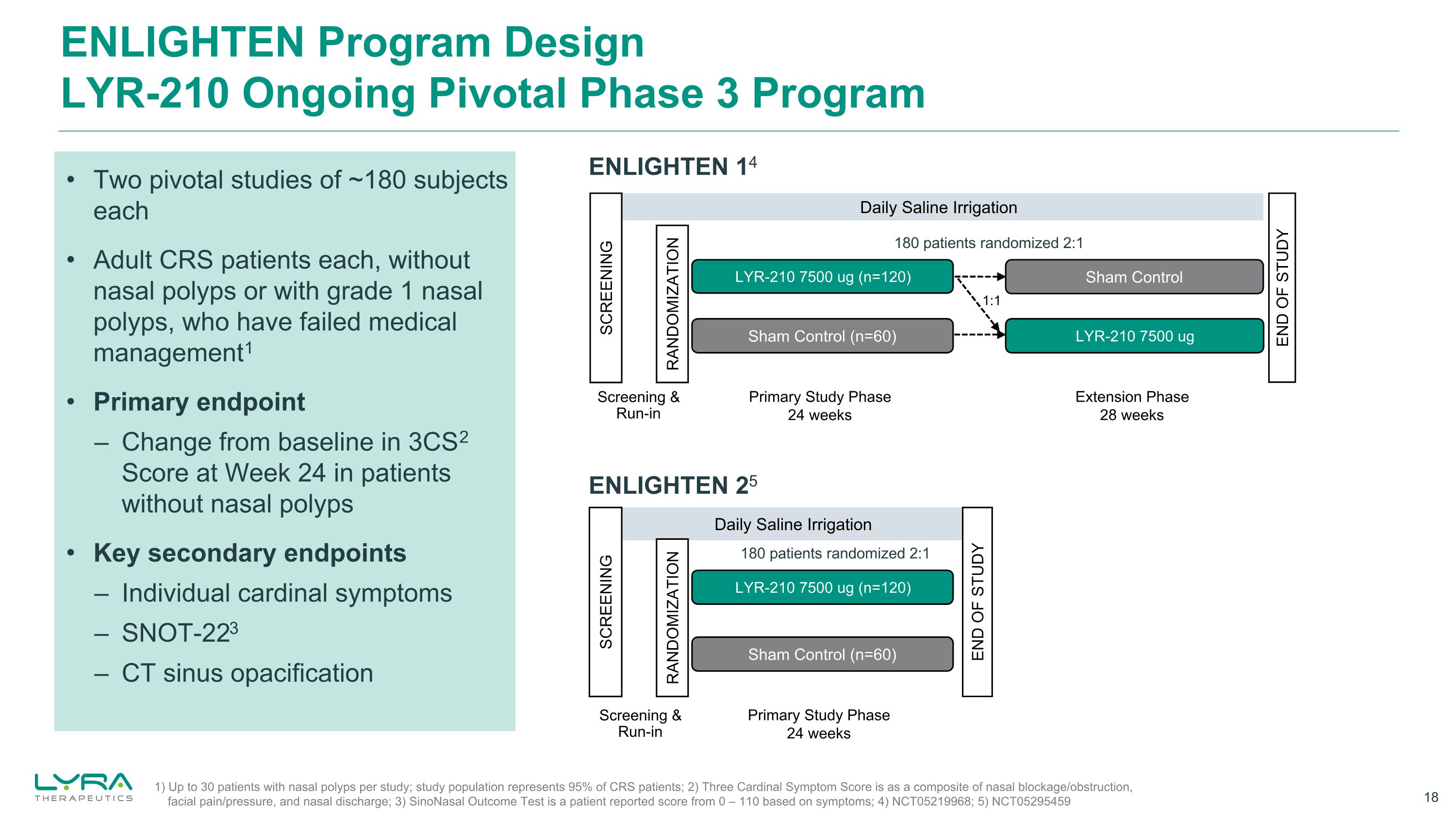
ENLIGHTEN Program DesignLYR-210 Ongoing Pivotal Phase 3 Program ENLIGHTEN 14 Daily Saline Irrigation Sham Control Sham Control (n=60) LYR-210 7500 ug (n=120) LYR-210 7500 ug SCREENING RANDOMIZATION Primary Study Phase 24 weeks Extension Phase 28 weeks Screening &Run-in 1:1 END OF STUDY 1) Up to 30 patients with nasal polyps per study; study population represents 95% of CRS patients; 2) Three Cardinal Symptom Score is as a composite of nasal blockage/obstruction, facial pain/pressure, and nasal discharge; 3) SinoNasal Outcome Test is a patient reported score from 0 – 110 based on symptoms; 4) NCT05219968; 5) NCT05295459 ENLIGHTEN 25 Daily Saline Irrigation Sham Control (n=60) LYR-210 7500 ug (n=120) SCREENING RANDOMIZATION END OF STUDY Primary Study Phase 24 weeks Screening &Run-in Two pivotal studies of ~180 subjects each Adult CRS patients each, without nasal polyps or with grade 1 nasal polyps, who have failed medical management1 Primary endpoint – Change from baseline in 3CS2 Score at Week 24 in patients without nasal polyps Key secondary endpoints – Individual cardinal symptoms – SNOT-223 – CT sinus opacification 180 patients randomized 2:1 180 patients randomized 2:1
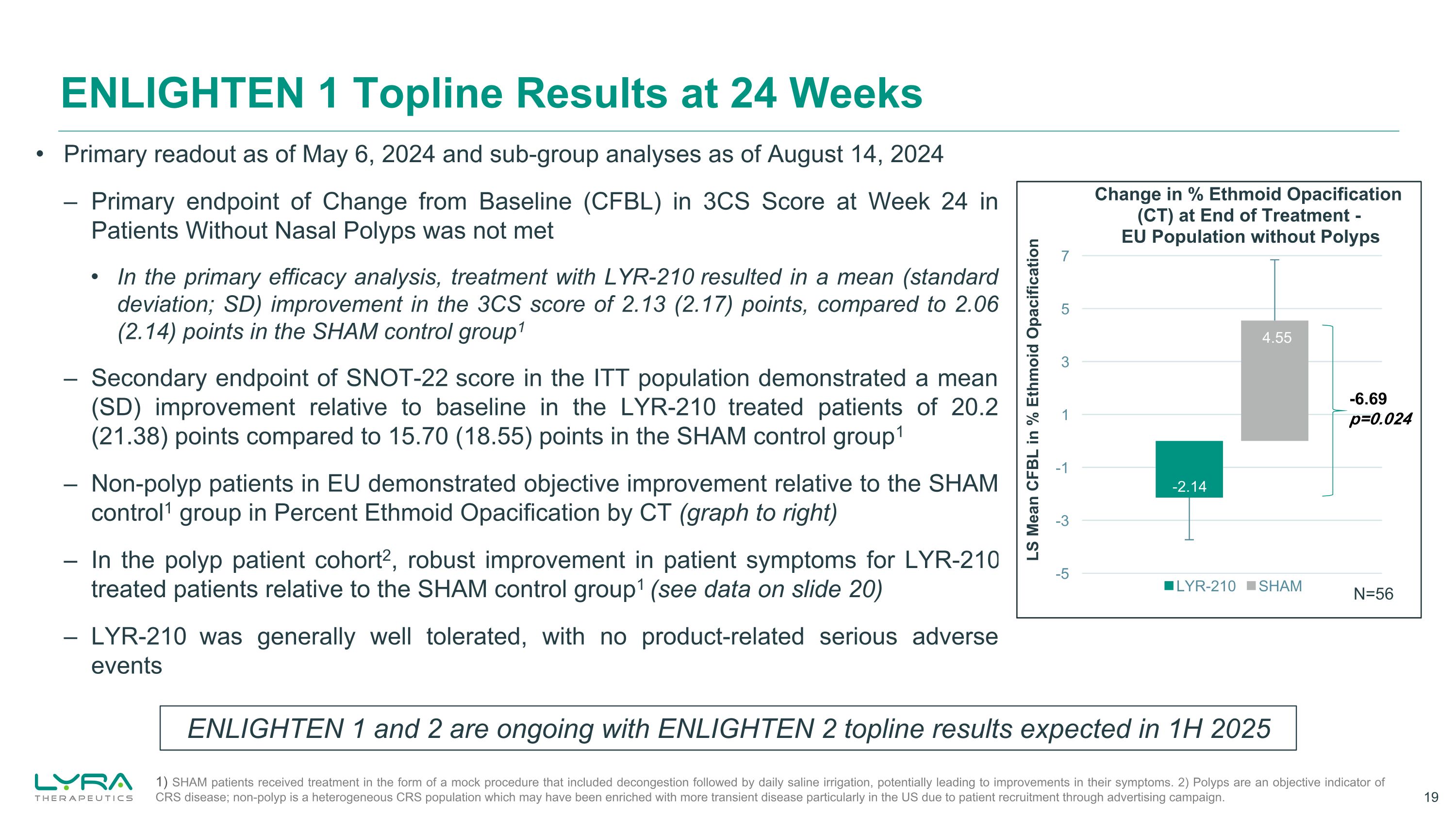
Primary readout as of May 6, 2024 and sub-group analyses as of August 14, 2024 – Primary endpoint of Change from Baseline (CFBL) in 3CS Score at Week 24 in Patients Without Nasal Polyps was not met In the primary efficacy analysis, treatment with LYR-210 resulted in a mean (standard deviation; SD) improvement in the 3CS score of 2.13 (2.17) points, compared to 2.06 (2.14) points in the SHAM control group1 – Secondary endpoint of SNOT-22 score in the ITT population demonstrated a mean (SD) improvement relative to baseline in the LYR-210 treated patients of 20.2 (21.38) points compared to 15.70 (18.55) points in the SHAM control group1 – Non-polyp patients in EU demonstrated objective improvement relative to the SHAM control1 group in Percent Ethmoid Opacification by CT (graph to right) – In the polyp patient cohort2, robust improvement in patient symptoms for LYR-210 treated patients relative to the SHAM control group1 (see data on slide 20) – LYR-210 was generally well tolerated, with no product-related serious adverse events ENLIGHTEN 1 Topline Results at 24 Weeks ENLIGHTEN 1 and 2 are ongoing with ENLIGHTEN 2 topline results expected in 1H 2025 1) SHAM patients received treatment in the form of a mock procedure that included decongestion followed by daily saline irrigation, potentially leading to improvements in their symptoms. 2) Polyps are an objective indicator of CRS disease; non-polyp is a heterogeneous CRS population which may have been enriched with more transient disease particularly in the US due to patient recruitment through advertising campaign. -6.69 p=0.024 N=56
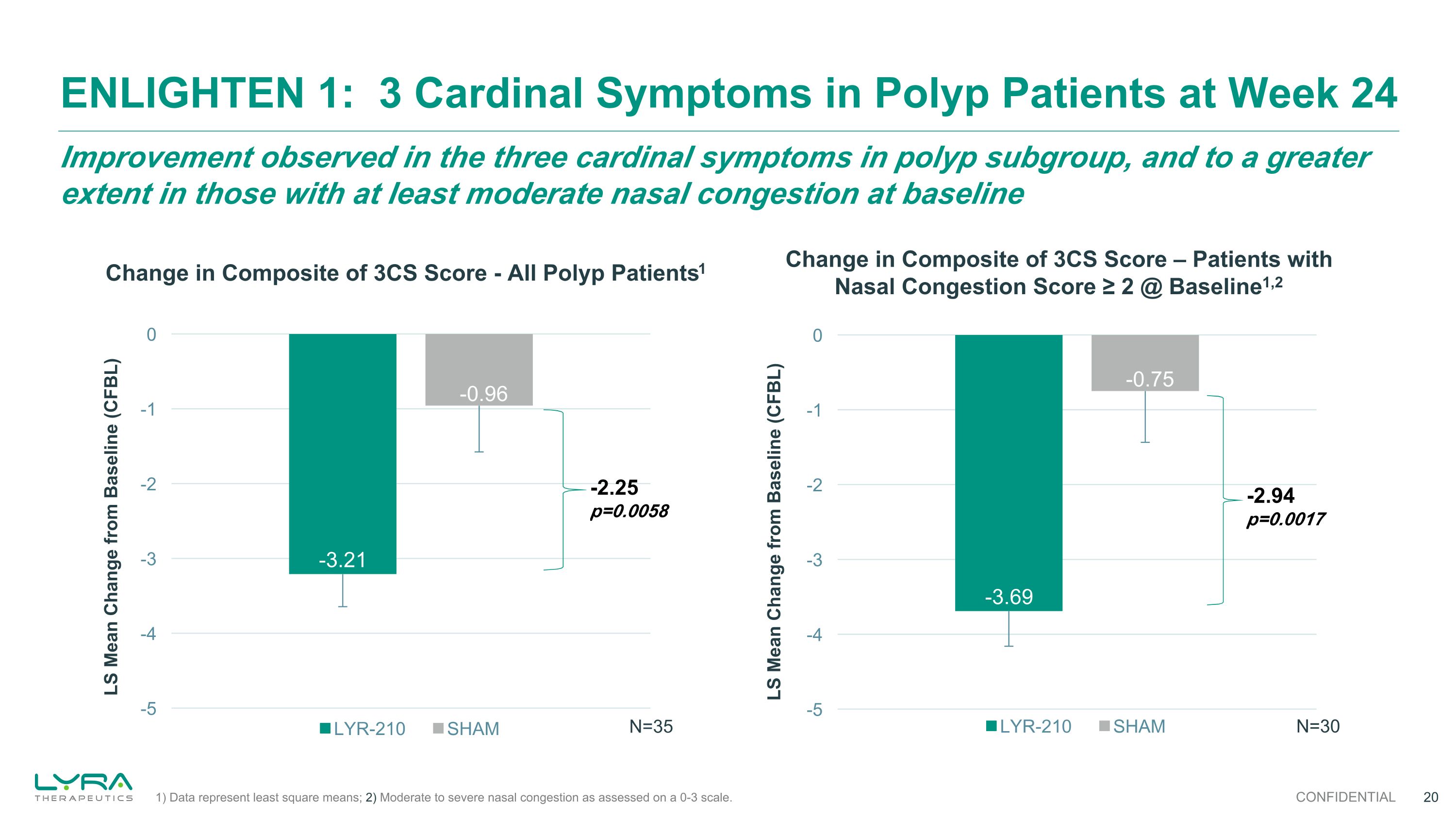
-2.25 p=0.0058 Improvement observed in the three cardinal symptoms in polyp subgroup, and to a greater extent in those with at least moderate nasal congestion at baseline ENLIGHTEN 1: 3 Cardinal Symptoms in Polyp Patients at Week 24 CONFIDENTIAL Change in Composite of 3CS Score - All Polyp Patients1 N=35 -2.94 p=0.0017 Change in Composite of 3CS Score – Patients with Nasal Congestion Score ≥ 2 @ Baseline1,2 N=30 1) Data represent least square means; 2) Moderate to severe nasal congestion as assessed on a 0-3 scale.
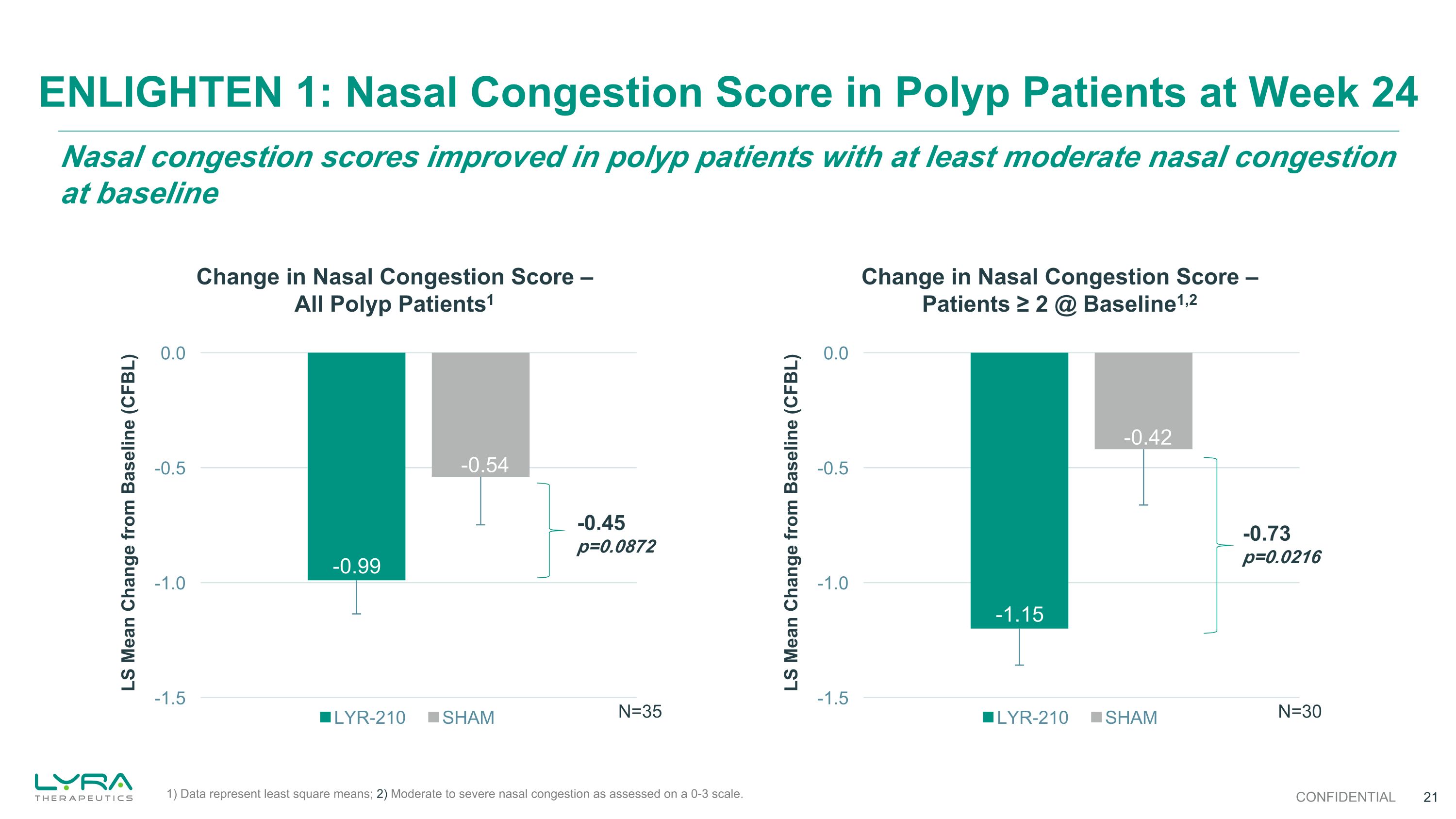
-0.73 p=0.0216 Nasal congestion scores improved in polyp patients with at least moderate nasal congestion at baseline ENLIGHTEN 1: Nasal Congestion Score in Polyp Patients at Week 24 CONFIDENTIAL Change in Nasal Congestion Score – All Polyp Patients1 Change in Nasal Congestion Score – Patients ≥ 2 @ Baseline1,2 -0.45 p=0.0872 N=35 N=30 1) Data represent least square means; 2) Moderate to severe nasal congestion as assessed on a 0-3 scale.
LYR-210, if Advanced, Would Be Positioned To Align With Current ENT Practices Office-based procedure that ENTs are accustomed to performing Treatment option for patients who are unwilling to undergo surgery, allowing ENTs to serve more patients in their care Expected to fit into ENT practice reimbursement models

Anticipated Milestones LYR-210: ENLIGHTEN Phase 3 Program May 2024: Topline data from ENLIGHTEN 1 Q4 2024: Extension study data from ENLIGHTEN 1 2H 2024: Complete enrollment in ENLIGHTEN 2 1H 2025: Topline data from ENLIGHTEN 1

Financial Profile Cash, cash equivalents and short-term investments of $67.5 million as of June 30, 2024 65.5 million common shares outstanding as of August 1, 2024
