Document
Exhibit 99.1
Lisata Therapeutics Reports Full Year 2024 Financial Results and Provides Business Update
Promising preliminary Phase 2b (ASCEND) pancreatic cancer data (Cohort A) reported with Cohort B data anticipated in the coming months
Enrollment completed in Qilu’s Phase 2 trial evaluating certepetide for the treatment of first-line mPDAC
Advancing development portfolio with multiple milestones projected over the next 12+ months
Cash runway extending into the second quarter of 2026 with no debt
Conference call scheduled for today at 4:30 p.m. Eastern Time
BASKING RIDGE, NJ (February 27, 2025) – Lisata Therapeutics, Inc. (Nasdaq: LSTA) (“Lisata” or the “Company”), a clinical-stage pharmaceutical company developing innovative therapies for the treatment of advanced solid tumors and other serious diseases, provides a business update and reports financial results for the twelve months ended December 31, 2024.
“Throughout 2024 and now into early 2025, we continue to advance our development portfolio centered around our novel product candidate, certepetide,” stated David J. Mazzo, Ph.D., President and Chief Executive Officer of Lisata. “Following the encouraging preliminary results from the ASCEND and iLSTA trials reported at this year’s ASCO-GI Symposium, we remain committed to exploring the broad application of certepetide’s unique mechanism of action. Our development portfolio encompasses multiple clinical and preclinical trials evaluating certepetide for the treatment of various solid tumors, including pancreatic cancer, cholangiocarcinoma, glioblastoma, colon cancer, appendiceal cancer, and melanoma. In addition, we are exploring certepetide’s versatility in non-cancerous settings such as endometriosis. For Lisata, we expect 2025 to be a data-rich year and we look forward to sharing key developments as they become available.”
Development Portfolio Highlights
Certepetide as a treatment for solid tumors in combination with other anti-cancer agents
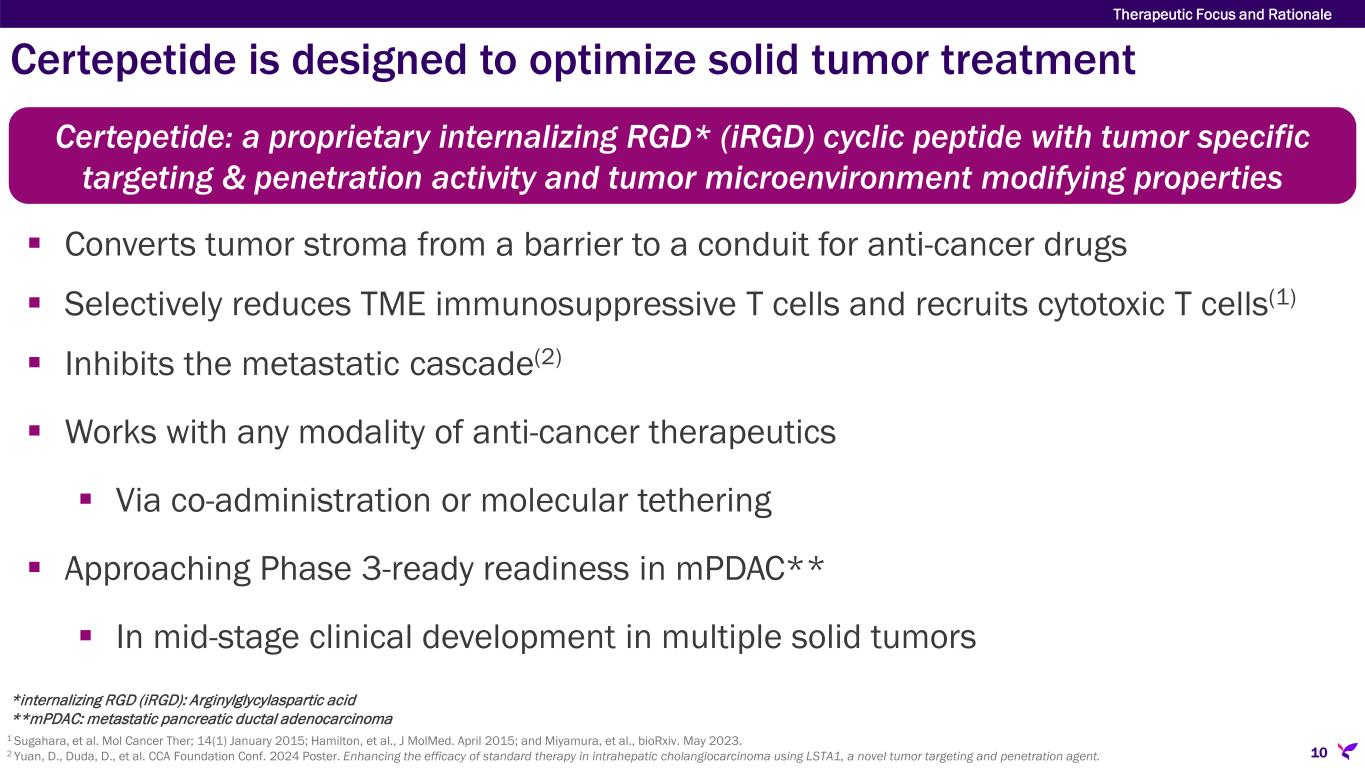
Certepetide (formerly LSTA1) is an internalizing RGD, or iRGD, (arginylglycylaspartic acid) cyclic peptide designed to selectively activate the C-end rule active transport mechanism in a tumor specific manner, resulting in systemically co-administered anti-cancer agents more efficiently penetrating and accumulating in the tumor. Additionally, certepetide has been shown to modify the tumor microenvironment, diminishing its immunosuppressive nature, enhancing cytotoxic T cell concentration and inhibiting the metastatic cascade. Lisata and its collaborators have amassed significant non-clinical data demonstrating enhanced delivery of various existing and emerging anti-cancer therapies, including chemotherapies, immunotherapies, and RNA-based therapeutics. To date, certepetide has also demonstrated favorable safety, tolerability, and clinical activity in completed and ongoing clinical trials designed to demonstrate its ability to enhance the effectiveness of standard-of-care (SoC) chemotherapy for pancreatic cancer as well as the combination of chemotherapy and immunotherapy in a variety of solid tumors. Certepetide has been awarded Fast Track designation (U.S.) and Orphan Drug Designation for pancreatic cancer (U.S. and E.U.) as well as Orphan Drug Designation for glioma, osteosarcoma, and cholangiocarcinoma (U.S.). Additionally, certepetide has received Rare Pediatric Disease Designation for osteosarcoma (U.S.). Currently, certepetide is the subject of multiple ongoing or planned clinical studies being conducted globally across several solid tumor types in combination with a variety of anti-cancer regimens, including:
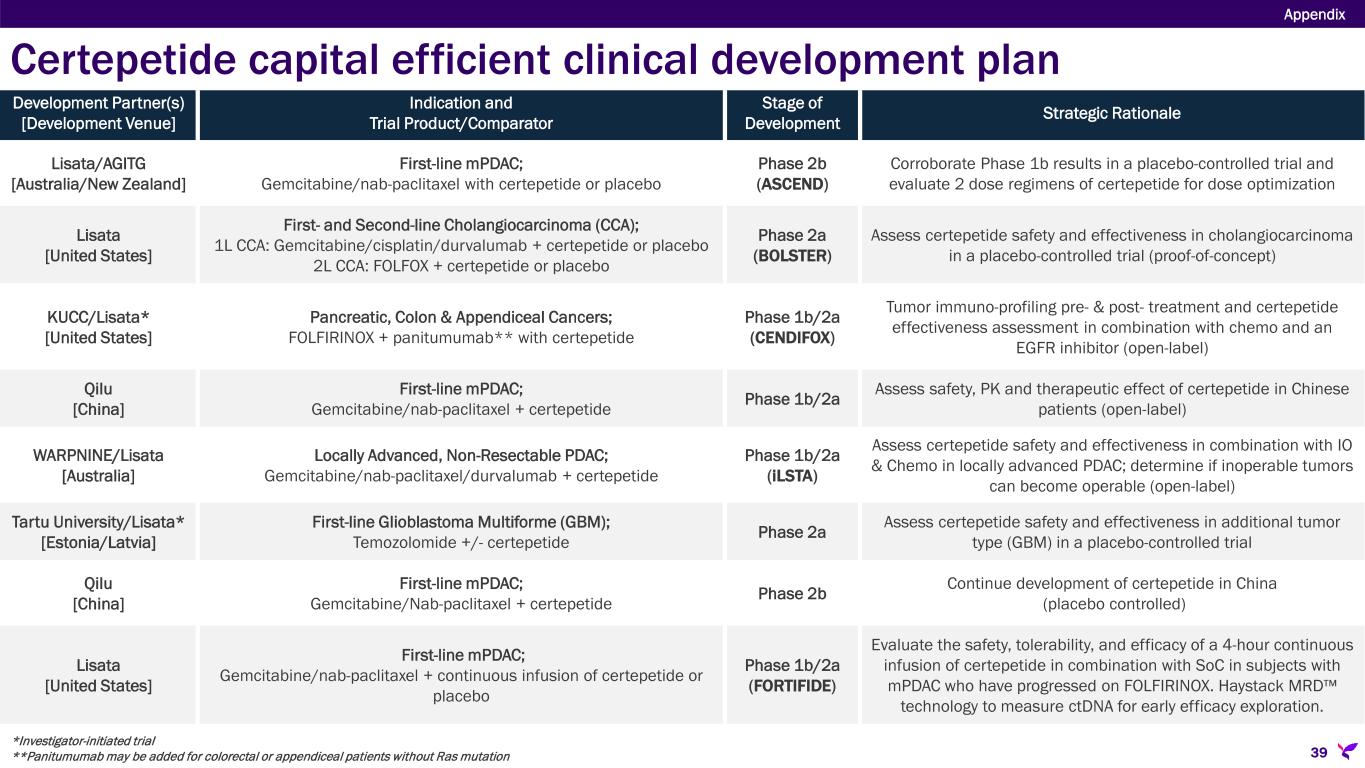
•ASCEND: Phase 2b double-blind, randomized (2:1 ratio), placebo-controlled trial evaluating two dosing regimens of certepetide in combination with SoC chemotherapy (gemcitabine/nab-paclitaxel) in patients with previously untreated metastatic pancreatic ductal adenocarcinoma (mPDAC). The trial is being conducted across 25 sites in Australia and New Zealand led by the Australasian Gastro-Intestinal Trials Group (AGITG) and coordinated by the National Health and Medical Research Council Clinical Trial Centre at the University of Sydney. Cohort A, with 95 patients receiving a single intravenous (IV) dose of certepetide 3.2 mg/kg or placebo in combination with SoC, completed enrollment in the third quarter of 2023. Preliminary Cohort A data presented at the 2025 ASCO-GI Symposium showed a positive trend in overall survival, including four complete responses in the certepetide-treated group compared to none in the placebo treated group. Data from Cohort B, with 63 patients receiving two IV doses of certepetide 3.2 mg/kg or placebo administered 4 hours apart in combination with SoC, is expected in the coming months with a final analysis of both cohorts available thereafter. The exact timing is dependent on accumulating the requisite number of endpoint events in Cohort B and is not something that can be accurately predicted.
•BOLSTER: Phase 2a double-blind, placebo-controlled, multi-center, randomized trial in the U.S. evaluating certepetide in combination with SoC chemotherapy in first- and second-line cholangiocarcinoma (CCA). The Company achieved complete enrollment in first-line CCA nearly six months ahead of plan, accelerating anticipated topline data readout to mid-2025. Based on this rapid enrollment rate and the pressing need to improve treatment outcomes in patients that have progressed after first-line CCA treatment, a second cohort has been added to the BOLSTER trial evaluating certepetide in combination with SoC in subjects with second-line CCA. In September 2024, Lisata announced first patient treated in the second-line CCA cohort, with enrollment completion targeted for later this year.
•CENDIFOX: Phase 1b/2a open-label trial in the U.S. evaluating certepetide in combination with neoadjuvant FOLFIRINOX based therapies in pancreatic, colon and appendiceal cancers. In December 2024, the Company announced enrollment completion in all three cohorts. The single-center study, conducted solely at the University of Kansas Cancer Center, was designed with a 3-cycle run-in period to ensure patients met specific criteria before receiving treatment. Of the 66 patients enrolled, 50 patients met the criteria and were treated with certepetide across three cohorts, including 24 with resectable or borderline resectable pancreatic cancer, 15 with high-grade colon or appendiceal cancer and peritoneal metastasis, and 11 with oligometastatic colon cancer. The trial will provide Lisata with valuable pre- and post-treatment tumor tissue data for immune profiling, along with long-term patient outcome information. CENDIFOX data are expected in the coming months; however, given this is an investigator-initiated study, the exact timing is not in Lisata’s control. The trial is funded by the University of Kansas Cancer Center and Lisata is supplying certepetide.
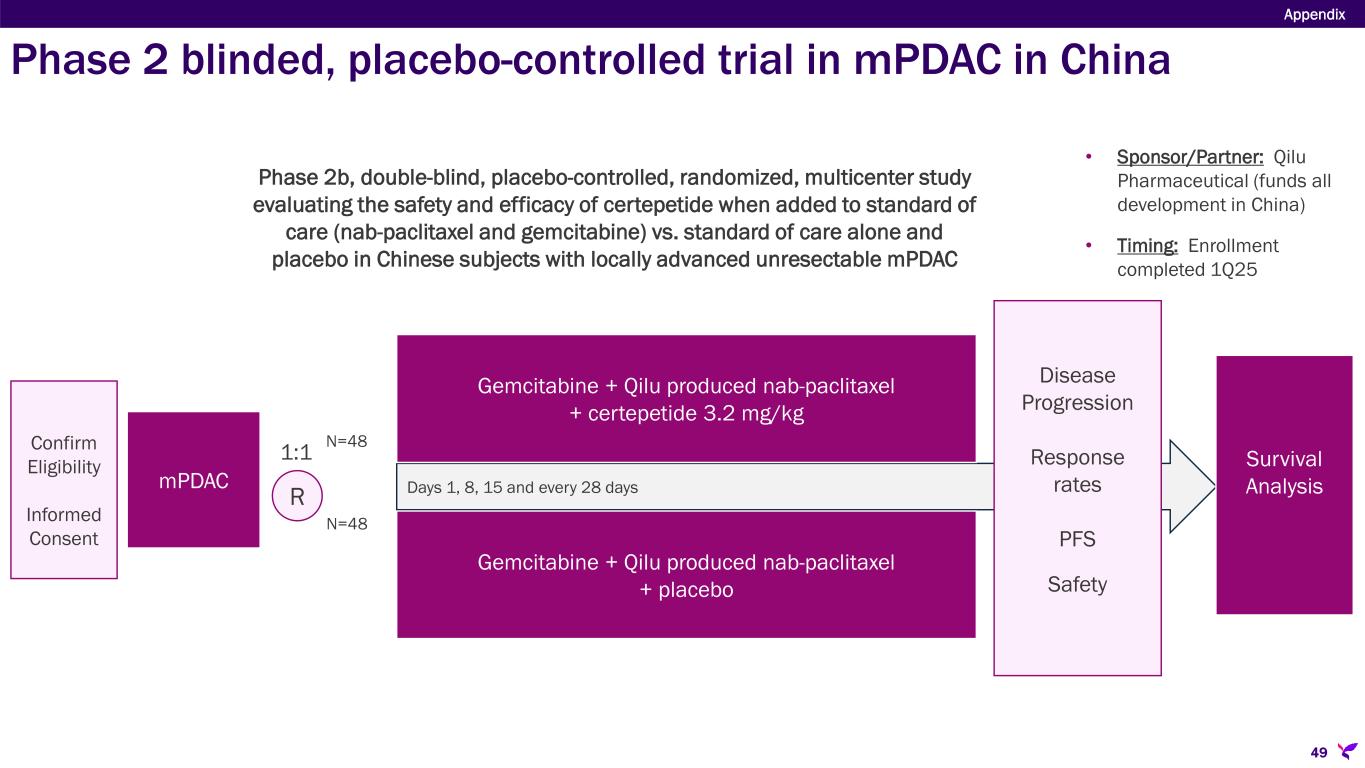
•Qilu Pharmaceutical, the licensee of certepetide in the Greater China territory, is currently evaluating certepetide in combination with gemcitabine and nab-paclitaxel as a treatment for first-line mPDAC. During the 2023 ASCO Annual Meeting, Qilu Pharmaceutical presented an abstract sharing preliminary data from the study which corroborated previously reported findings from the Phase 1b/2a trial of certepetide plus gemcitabine and nab-paclitaxel conducted in Australia in patients with first-line mPDAC. Qilu has completed enrollment in its Phase 2 trial and data are expected in the coming months.
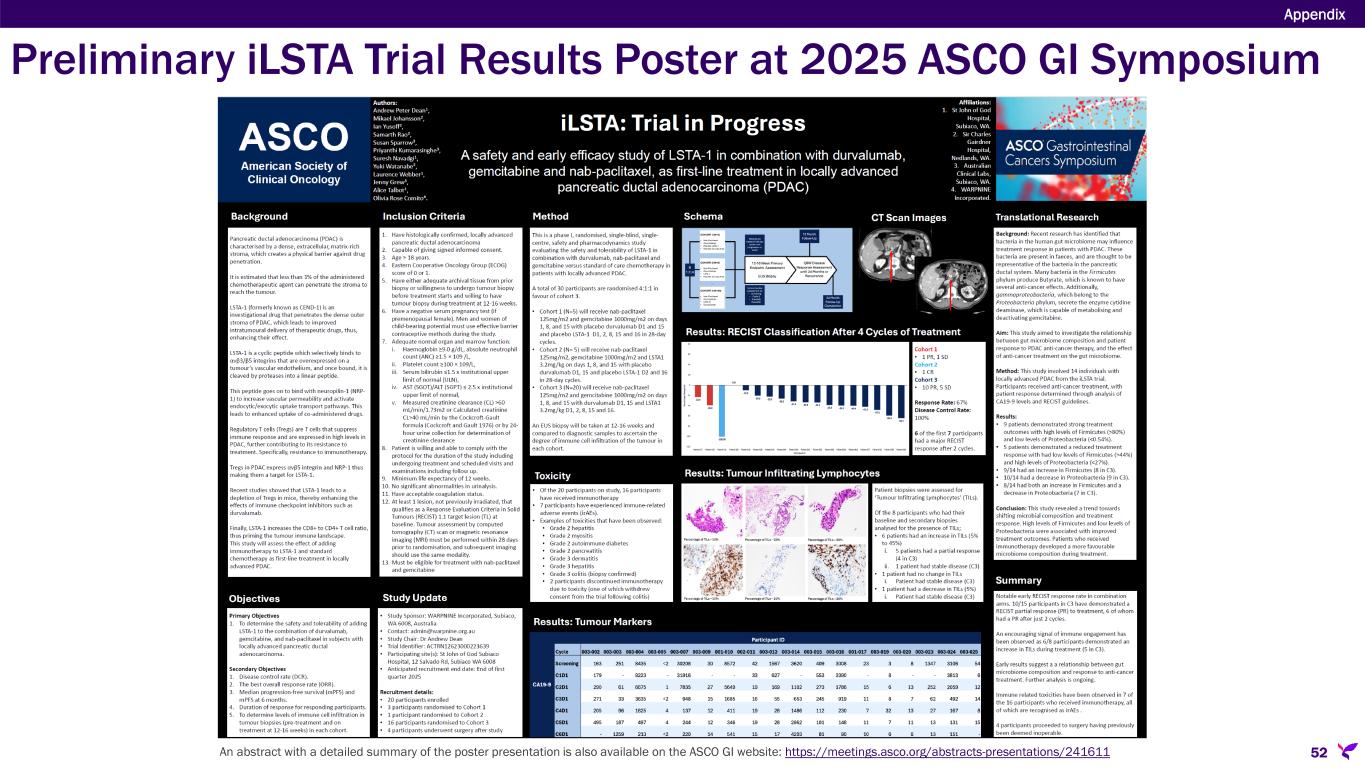
•iLSTA: Phase 1b/2a randomized, single-blind, single-center, safety and pharmacodynamic trial in Australia, funded by WARPNINE Inc., is evaluating certepetide in combination with SoC chemotherapy (nab-paclitaxel and gemcitabine) plus SoC immunotherapy (durvalumab) versus SoC alone in patients with locally advanced non-resectable PDAC. An interim analysis of the iLSTA trial, presented at the 2025 ASCO GI Symposium, showed preliminary results from the first 17 of the 30 targeted patients, corroborating preclinical data that certepetide enhances the effectiveness of immunotherapy. With 27 of the 30 patients enrolled, enrollment remains on track to be completed by the first half of 2025.
•A Lisata-funded Phase 2a, double-blind, placebo-controlled, randomized, proof-of-concept study evaluating certepetide in combination with SoC temozolomide versus temozolomide alone in patients with newly diagnosed glioblastoma multiforme (GBM) is being conducted across multiple sites in Estonia and Latvia and is planned to also include a site in Lithuania. The study is targeted to enroll 30 patients with a randomization of 2:1 in favor of the certepetide treatment group. Enrollment completion is targeted for the second half of 2025.
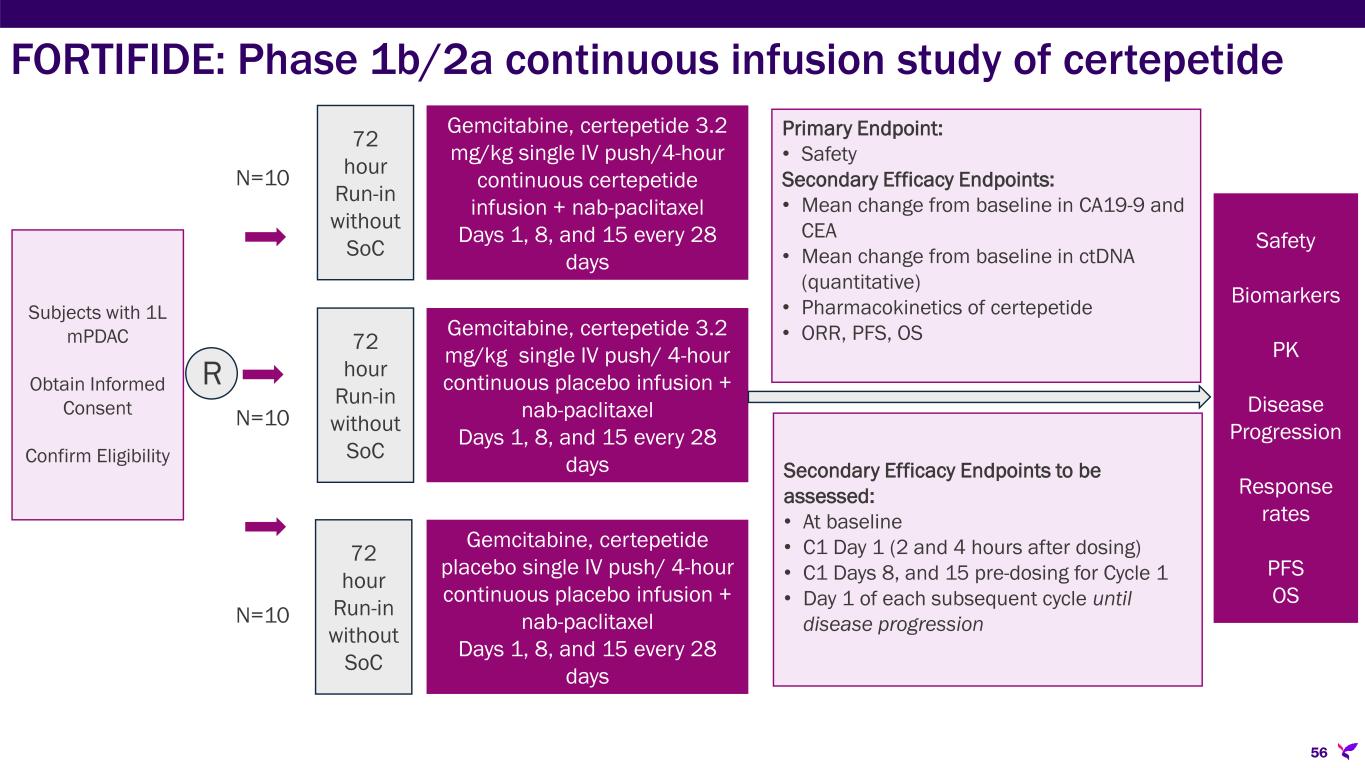
•FORTIFIDE: Phase 1b/2a, double-blind, placebo-controlled, three-arm, randomized study in the U.S. evaluating the safety, tolerability, and efficacy of a 4-hour continuous infusion of certepetide in combination with SoC in subjects with first-line mPDAC. As part of this study, Lisata has engaged Haystack Oncology to use its MRD™ technology to measure circulating tumor DNA levels at multiple timepoints in patients throughout the study as an exploratory endpoint for analyzing the early therapeutic effect of certepetide. The Company expects to enroll the first patient in the study in the first half of 2025. However, in parallel, management is investigating a potentially faster and more cost-effective approach to achieving the study objective, which may become the preferred strategy.
Lisata has entered into multiple research collaborations, including a sponsored research agreement with the University of Cincinnati to assess certepetide in combination with bevacizumab (a VEGF inhibitor) in a preclinical murine model for the treatment of endometriosis. Lisata is also partnering with Valo Therapeutics (ValoTx) to investigate the benefits of combining certepetide with ValoTx's platform technology, PeptiCRAd, an oncolytic virus, and a checkpoint inhibitor in a preclinical murine model for the treatment of melanoma.
In November 2024, Lisata entered into an Exclusive License and Collaboration Agreement with Kuva Labs, Inc. (“Kuva”), in which Lisata granted Kuva an exclusive license to explore the synergistic potential of certepetide as a targeting and delivery agent for Kuva’s NanoMark™ imaging technology in solid tumors. Under the agreement, Kuva will assume full responsibility for research, development, and commercialization costs, while Lisata will be responsible for supplying certepetide pursuant to a Clinical Supply Agreement.
As consideration for the license, the Company is to receive a $1.0 million upfront license fee and is eligible for certain development and commercial milestone payments of up to $19.0 million, as well as a single-digit percentage royalty on net sales.
Full Year 2024 Financial Highlights
For the year ended December 31, 2024, revenue totaled $1.0 million in connection with an upfront license fee related to the Exclusive License and Collaboration Agreement with Kuva Labs, Inc. The Company did not have any revenue for the year ended December 31, 2023.
For the year ended December 31, 2024, operating expenses totaled $23.4 million compared to $25.7 million for the year ended December 31, 2023, representing a decrease of $2.3 million or 8.9%.
Research and development expenses were approximately $11.3 million for the year ended December 31, 2024, compared to $12.7 million for the year ended December 31, 2023, representing a decrease of approximately $1.4 million, or 11.0%. This was primarily due to a reduction in expenses associated with the Phase 2b ASCEND trial which completed enrollment in the prior year, lower spend on chemistry, manufacturing and controls, and lower equity expense.
General and administrative expenses were approximately $12.1 million for the year ended December 31, 2024, compared to $13.0 for the year ended December 31, 2023, representing a decrease of approximately $0.9 million or 6.9%. This was primarily due to one-off related severance costs in the prior year associated with the elimination of the Chief Business Officer position on May 1, 2023, a reduction in equity expenses, a decrease in directors’ and officers’ insurance premiums, and a reduction in spend on legal fees partially offset by one-off settlement related costs and an increase in consulting expenses.
Overall, net losses were $20.0 million and $20.8 million for the years ended December 31, 2024 and 2023, respectively.
Balance Sheet Highlights
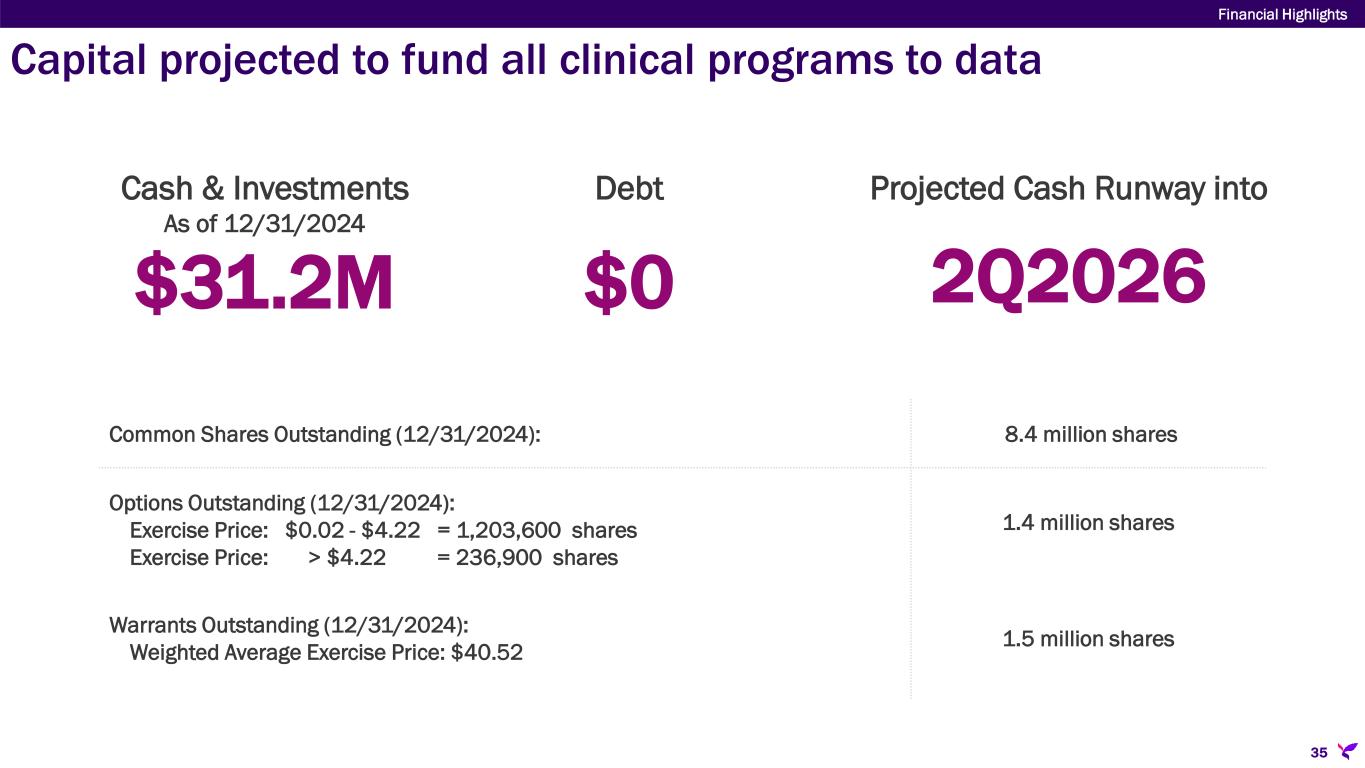
As of December 31, 2024, Lisata had cash, cash equivalents, and marketable securities of approximately $31.2 million. Based on its existing and planned activities, the Company believes available funds will support current operations into the second quarter of 2026.
Net Operating Loss Sale
Earlier this year Lisata received $0.9 million in non-dilutive funding as an approved participant of the Technology Business Tax Certificate Transfer Program (the “Program”) sponsored by the New Jersey Economic Development Authority (NJEDA). The Program enables qualifying New Jersey-based biotechnology or technology companies to sell a percentage of their New Jersey net operating losses and research and development tax credits to unrelated qualifying corporations with a lifetime cap on the tax benefit sales of $20.0 million. To date, under the Program, the Company has sold $19.6 million in tax benefits for net proceeds of $18.4 million.
Conference Call Information
Lisata will hold a live conference call today, February 27, 2025, at 4:30 p.m. Eastern Time to discuss financial results, provide a business update, and answer questions.
Those wishing to participate must register for the conference call by way of the following link: CLICK HERE TO REGISTER. Registered participants will receive an email containing conference call details with dial-in options. To avoid delays, the Company encourages participants to dial into the conference call 15 minutes ahead of the scheduled start time.
A live webcast of the call will also be accessible under the Investors & News section of Lisata’s website and will be available for replay beginning two hours after the conclusion of the call for 12 months.
About Lisata Therapeutics
Lisata Therapeutics is a clinical-stage pharmaceutical company dedicated to the discovery, development and commercialization of innovative therapies for the treatment of advanced solid tumors and other major diseases. Lisata’s cyclic peptide product candidate, certepetide, is an investigational drug designed to activate a novel uptake pathway that allows co-administered or tethered anti-cancer drugs to selectively target and penetrate solid tumors more effectively. Lisata has already established noteworthy commercial and R&D partnerships based on its CendR Platform® technology. The Company expects to announce numerous milestones over the next 1.5 years and believes that its projected capital will fund operations into the second quarter of 2026, encompassing anticipated data milestones from its ongoing and planned clinical trials. Learn more about certepetide’s mechanism of action in our short film. For more information on the Company, please visit www.lisata.com.
Forward-Looking Statements
This communication contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this communication regarding the Company’s clinical development programs are forward-looking statements. In addition, when or if used in this communication, the words “may,” “could,” “should,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “predict” and similar expressions and their variants, as they relate to Lisata or its management, may identify forward-looking statements. Examples of forward-looking statements include, but are not limited to, the potential efficacy of certepetide as a treatment for patients with metastatic pancreatic ductal adenocarcinoma and other solid tumors; our beliefs about the potential uses and benefits of certepetide; statements relating to Lisata’s continued listing on the Nasdaq Capital Market; expectations regarding the capitalization, resources and ownership structure of Lisata; the approach Lisata is taking to discover and develop novel therapeutics; the adequacy of Lisata’s capital to support its future operations and its ability to successfully initiate and complete clinical trials; and the difficulty in predicting the time and cost of development of Lisata’s product candidates. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: results observed from a single patient case study are not necessarily indicative of final results and one or more of the clinical outcomes may materially change following more comprehensive reviews of the data and as more patient data becomes available, including the risk that unconfirmed responses may not ultimately result in confirmed responses to treatment after follow-up evaluations; the risk that product candidates that appeared promising in early research and clinical trials do not demonstrate safety and/or efficacy in larger-scale or later clinical trials; the safety and efficacy of Lisata’s product candidates, decisions of regulatory authorities and the timing thereof, the duration and impact of regulatory delays in Lisata’s clinical programs, Lisata’s ability to finance its operations, the likelihood and timing of the receipt of future milestone and licensing fees, the future success of Lisata’s scientific studies, Lisata’s ability to successfully develop and commercialize drug candidates, the timing for starting and completing clinical trials, rapid technological change in Lisata’s markets, the ability of Lisata to protect its intellectual property rights; and legislative, regulatory, political and economic developments. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors included in Lisata’s Annual Report on Form 10-K filed with the SEC on February 27, 2025, and in other documents filed by Lisata with the Securities and Exchange Commission. Except as required by applicable law, Lisata undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events, or otherwise.
Lisata Therapeutics Contact:
Investors:
Lisata Therapeutics
John Menditto
Vice President, Investor Relations and Corporate Communications
Phone: 908-842-0084
Email: jmenditto@lisata.com
Media:
ICR Healthcare
Elizabeth Coleman
Account Supervisor
Phone: 203-682-4783
Email: elizabeth.coleman@icrhealthcare.com
– Tables to follow –
Lisata Therapeutics, Inc.
Selected Financial Data
(in thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Twelve Months Ended December 31, |
|
|
|
|
|
|
2024 |
|
2023 |
|
| (in thousands, except per share data) |
|
|
|
|
|
|
|
|
| Statement of Operations Data: |
|
|
|
|
|
|
|
|
| Revenue |
|
|
|
|
$ |
1,000 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
|
|
|
|
11,334 |
|
|
12,734 |
|
|
| General and administrative |
|
|
|
|
12,075 |
|
|
12,974 |
|
|
| Total operating expenses |
|
|
|
|
23,409 |
|
|
25,708 |
|
|
| Operating loss |
|
|
|
|
(22,409) |
|
|
(25,708) |
|
|
| Investment income, net |
|
|
|
|
1,883 |
|
|
2,724 |
|
|
| Other expense, net |
|
|
|
|
(257) |
|
|
(186) |
|
|
| Net loss before benefit from income taxes and noncontrolling interests |
|
|
|
|
(20,783) |
|
|
(23,170) |
|
|
| Benefit from income taxes |
|
|
|
|
(798) |
|
|
(2,330) |
|
|
| Net loss |
|
|
|
|
(19,985) |
|
|
(20,840) |
|
|
| Less - net income (loss) attributable to noncontrolling interests |
|
|
|
|
— |
|
|
— |
|
|
| Net loss attributable to Lisata Therapeutics, Inc. common stockholders |
|
|
|
|
$ |
(19,985) |
|
|
$ |
(20,840) |
|
|
|
|
|
|
|
|
|
|
|
| Basic and diluted loss per share attributable to Lisata Therapeutics, Inc. common stockholders |
|
|
|
|
$ |
(2.40) |
|
|
$ |
(2.58) |
|
|
| Weighted average common shares outstanding |
|
|
|
|
8,329 |
|
|
8,073 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
| Balance Sheet Data: |
|
|
|
|
|
|
|
|
| Cash, cash equivalents and marketable securities |
|
|
|
$ |
31,245 |
|
|
$ |
50,535 |
|
|
| Total assets |
|
|
|
|
35,002 |
|
|
54,694 |
|
|
| Total liabilities |
|
|
|
|
5,685 |
|
|
6,800 |
|
|
| Total equity |
|
|
|
|
29,317 |
|
|
47,894 |
|
|
# # #