FALSE000202365800020236582025-12-062025-12-06
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
___________________________________
FORM 8-K
___________________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (date of earliest event reported): December 6, 2025
___________________________________
Bicara Therapeutics Inc.
(Exact name of registrant as specified in its charter)
___________________________________
|
|
|
|
|
|
|
|
|
|
Delaware
(State or other jurisdiction of
incorporation or organization)
|
001-42271
(Commission File Number)
|
83-2903745
(I.R.S. Employer Identification Number)
|
|
116 Huntington Avenue,
Suite 703 Boston, MA 02116
|
(Address of principal executive offices and zip code) |
(617) 468-4219 |
(Registrant's telephone number, including area code) |
___________________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
|
|
|
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
|
|
|
|
|
|
|
|
|
Securities registered pursuant to Section 12(b) of the Act: |
Title of each class |
Trading Symbol |
Name of each exchange on which registered |
| Common Stock, $0.0001 par value |
BCAX |
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 12b-2 of the Exchange Act.
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o Item 7.01 - Regulation FD Disclosure.
On December 6, 2025, Bicara Therapeutics Inc. (the “Company”) issued a press release titled “Bicara Therapeutics’ Preliminary Phase 1b Expansion Cohort Data Evaluating 750mg of Ficerafusp Alfa Weekly Plus Pembrolizumab Advances Pivotal Study Dose Selection on Track for First Quarter 2026” in connection with an oral presentation at the European Society for Medical Oncology Asia Congress (“ESMO Asia”). A copy of this press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
Also on December 6, 2025, the Company hosted a clinical update webcast. A copy of the clinical update presentation is available in the investor relations section of the Company’s website at https://ir.bicara.com/ and is being furnished as Exhibit 99.2 to this Current Report on Form 8-K.
The information set forth under Item 7.01, including Exhibits 99.1 and 99.2, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 8.01 – Other Events.
On December 6, 2025, the Company announced preliminary data from a Phase 1b expansion cohort evaluating 750mg of ficerafusp alfa weekly in combination with pembrolizumab in first-line human papillomavirus-negative recurrent/metastatic (“R/M”) head and neck squamous cell carcinoma (“HNSCC”).
Phase 1/1b expansion cohort data presented at ESMO Asia show that 750mg ficerafusp alfa in combination with pembrolizumab was generally well-tolerated, with a safety profile consistent with the known safety profile of ficerafusp alfa plus pembrolizumab in R/M HNSCC. At a preliminary duration of follow-up, 750mg of ficerafusp alfa demonstrated a 57% (17/30) confirmed overall response rate, with 10% (3/30) of patients achieving a complete response, and 29% of (5/17) responders demonstrating deep responses of at least 80% tumor shrinkage.
New biomarker data presented during the Company’s clinical update webcast show that 1500mg of ficerafusp alfa (n=11) yielded a greater increase TGF-β inhibition within the tumor microenvironment and greater immune activation, compared to 750mg of ficerafusp alfa (n=15). The increased TGF-β inhibition in the tumor translated to greater depth of clinical responses at 24 weeks. The median depth of response was 82% at the 1500mg dose versus 63% at the 750mg dose, and 64% of responders at 1500mg achieved a deep response, compared to 27% of responders at the 750mg dose.
The totality of the data suggests that a higher dose of ficerafusp alfa with greater TGF-β inhibition and immune activation drives deeper tumor responses that translate to more durable outcomes for patients. The Company plans to declare the optimal biologic dose for use in the pivotal FORTIFI-HN01 study in the first quarter of 2026.
Item 9.01 - Financial Statements and Exhibits
(d) The following exhibits are being filed herewith:
|
|
|
|
|
|
|
|
|
| Exhibit No. |
|
Description |
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized on this 8th day of December, 2025.
|
|
|
|
|
|
| Bicara Therapeutics Inc. |
|
|
By: |
/s/ Claire Mazumdar |
Name: |
Claire Mazumdar, Ph.D. |
Title: |
Chief Executive Officer |
EX-99.1
2
esmoasia2025presentation.htm
EX-99.1
esmoasia2025presentation

Bicara Therapeutics’ Preliminary Phase 1b Expansion Cohort Data Evaluating 750mg of Ficerafusp Alfa Weekly Plus Pembrolizumab Advances Pivotal Study Dose Selection on Track for First Quarter 2026 Ficerafusp alfa 750mg QW in combination with pembrolizumab demonstrates consistent overall response rate and safety profile comparable to 1500mg QW dose, further derisking pivotal FORTIFI-HN01 study interim analysis Totality of data demonstrates that greater TGF-β inhibition, observed at 1500mg of ficerafusp alfa, drives deeper tumor responses that translate to more durable outcomes for patients Pivotal FORTIFI-HN01 optimal dose declaration expected in first quarter 2026 Company to host conference call and webcast today at 9:00 a.m. ET BOSTON, December 6, 2025 – Bicara Therapeutics Inc. (Nasdaq: BCAX), a clinical-stage biopharmaceutical company committed to bringing transformative bifunctional therapies to patients with solid tumors, today presented preliminary data from a Phase 1b expansion cohort evaluating 750 mg of ficerafusp alfa weekly (QW) in combination with pembrolizumab in first-line (1L) human papillomavirus (HPV)-negative recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). The data were highlighted in an oral presentation by Deborah Wong, MD, PhD of UCLA Medical Center at the European Society for Medical Oncology (ESMO) Asia Congress and will be discussed on a company conference call and webcast today, December 6, at 9:00 a.m. ET. “Inadequate tumor penetration remains a major barrier in treating solid tumors such as R/M HNSCC,” said Claire Mazumdar, PhD, MBA, Chief Executive Officer of Bicara Therapeutics. “Ficerafusp alfa, the first and only bifunctional EGFR-directed antibody x TGF-β ligand trap, was purposefully designed to deliver deep and durable responses with the potential to meaningfully extend overall survival for patients. The data presented today mark an important advancement in our dose-optimization strategy, reinforce our confidence in the interim overall response rate analysis as the foundation for pursuing accelerated approval in the FORTIFI-HN01 pivotal trial, and further elucidate the relative contribution of TGF- β in driving deep and durable tumor responses. We have made significant progress in the FORTIFI-HN01 trial this year and are on track to declare an optimal dose in the first quarter of 2026.” Phase 1/1b expansion cohort data presented at ESMO Asia show that 750mg ficerafusp alfa in combination with pembrolizumab was generally well-tolerated, with a safety profile consistent with the known safety profile of ficerafusp alfa plus pembrolizumab in R/M HNSCC. At a preliminary duration of follow-up, 750 mg of ficerafusp alfa demonstrated a 57% confirmed overall response rate, with 10% of patients achieving a completed response, and 29% of responders demonstrating deep responses of at least 80% tumor shrinkage.

New biomarker data to be presented during Bicara’s corporate call and webcast show that 1500mg of ficerafusp alfa yielded a greater increase TGF-β inhibition within the tumor microenvironment and greater immune activation, compared to 750mg of ficerafusp alfa. The increased TGF-β inhibition in the tumor translated to greater depth of clinical responses at 24 weeks. The median depth of response was 82% at the 1500mg dose vs. 63% at the 750mg dose, and 64% of responders at 1500mg achieved a deep response, compared to 27% of responders at the 750mg dose. The totality of the data suggests that a higher dose of ficerafusp alfa with greater TGF-β inhibition and immune activation drives deeper tumor responses that translate to more durable outcomes for patients. Bicara plans to declare the optimal biologic dose for use in the pivotal FORTIFI-HN01 study in the first quarter of 2026. Conference Call and Webcast Details Bicara Therapeutics will host a conference call and webcast today December 6, 2025 at 9:00 a.m. ET. Individuals may register for the conference call by clicking the link here. Once registered, participants will receive dial-in details and a unique PIN which will allow them to access the call. An audio webcast will be accessible through the Investor Relations section of Bicara’s website under Events and Presentations. An archived replay will also be available for 30 days following the webcast. About Head and Neck Squamous Cell Carcinoma Head and neck squamous cell carcinomas (HNSCCs) develop from the mucosal epithelium in the oral cavity, pharynx and larynx and are the most common malignancies that arise in the head and neck. HNSCC is one of the most common cancers in the United States and globally with a rising incidence anticipated to reach one million new global cases annually by 2030. Ten percent of HNSCC patients are diagnosed with metastatic disease and up to 30% develop a recurrence or metastases over time after receiving initial treatment for advanced HNSCC. Most cases of HNSCC are thought to result from accumulated mutations caused by carcinogenic exposures such as tobacco smoke or HPV infection. Approximately 80% of patients with R/M HNSCC are HPV-negative. These HPV-negative tumors often exhibit a recurrence pattern that is primarily local and are associated with severe morbidities, including fatal tumor bleeding, intense pain, difficulty swallowing, significant weight loss, and cachexia. This highlights a critical unmet need for therapies that have the potential to deliver durable anti-tumor responses, ultimately leading to meaningful improvements in patients' quality of life. About Ficerafusp Alfa Ficerafusp alfa is a first-in-class bifunctional antibody designed to drive tumor penetration by breaking barriers in the tumor microenvironment that have challenged the treatment of multiple solid tumor cancers. Specifically, ficerafusp alfa combines two clinically validated targets: an epidermal growth factor receptor (EGFR) directed monoclonal antibody with a domain that binds to human transforming growth factor beta (TGF-β). Through this targeted mechanism, ficerafusp alfa reverses the fibrotic and immune- excluded tumor microenvironment driven by TGF-β signaling to enable tumor penetration that drives deep and durable responses. The U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation to ficerafusp alfa in combination with pembrolizumab for the first line (1L) treatment of patients with metastatic or with unresectable, recurrent (R/M) head and neck squamous

cell carcinoma (HNSCC) whose tumors express programmed death-ligand 1 with combined positive score (CPS) ≥1, excluding human papillomavirus (HPV)-positive oropharyngeal squamous cell carcinoma. Ficerafusp alfa is currently being evaluated in FORTIFI-HN01, a pivotal Phase 2/3 clinical trial in patients with 1L R/M HNSCC. About Bicara Therapeutics Bicara Therapeutics is a clinical-stage biopharmaceutical company committed to bringing transformative bifunctional therapies to patients with solid tumors. Bicara’s lead program, ficerafusp alfa, is a first-in- class bifunctional antibody designed to drive tumor penetration by breaking barriers in the tumor microenvironment that have challenged the treatment of multiple solid tumor cancers. Specifically, ficerafusp alfa combines two clinically validated targets: an epidermal growth factor receptor (EGFR) directed monoclonal antibody with a domain that binds to human transforming growth factor beta (TGF- β). Through this targeted mechanism, ficerafusp alfa reverses the fibrotic and immune-excluded tumor microenvironment driven by TGF-β signaling to enable tumor penetration that drives deep and durable responses. Ficerafusp alfa is being developed in head and neck squamous cell carcinoma, where there remains a significant unmet need, as well as other solid tumor types. For more information, please visit www.bicara.com or follow us on LinkedIn and X. Forward-Looking Statements This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. These statements may be identified by words such as “may,” “might,” “will,” “could,” “would,” “should,” “plan,” “anticipate,” “intend,” “believe,” “expect,” “estimate,” “seek,” “predict,” “future,” “project,” “potential,” “continue,” “target” and similar words or expressions, or the negative thereof, are intended to identify forward-looking statements, although not all contain identifying words. Any statements in this press release that are not statements of historical fact may be deemed to be forward-looking statements. These forward-looking statements include, without limitation, express or implied statements regarding Bicara’s clinical development of ficerafusp alfa in combination with pembrolizumab and presentation of early data from a Phase 1b expansion cohort evaluating 750 mg of ficerafusp alfa weekly (QW) in combination with pembrolizumab in first-line (1L) human papillomavirus (HPV)-negative recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC), the expected therapeutic potential and clinical benefits of ficerafusp alfa, including potential efficacy and tolerability, and Bicara’s optimal biological dose selection plans. Any forward- looking statements in this press release are based on management's current expectations and beliefs and are subject to a number of risks and uncertainties that are difficult to predict. Factors that could cause actual results to differ include, but are not limited to, risks and uncertainties related to uncertainties inherent in the development of product candidates, including the conduct of research activities and the conduct of clinical trials; uncertainties as to the availability and timing of results and data from clinical trials; whether results from prior preclinical studies, preliminary or interim data from earlier stage clinical trials will be predictive of the results of subsequent preclinical studies and clinical trials; regulatory developments in the United States and foreign countries; whether Bicara’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements; as well as the risks and uncertainties identified in Bicara’s filings with the Securities and Exchange Commission (SEC), including its Annual Report on Form 10-K for the year ended

December 31, 2024, its Quarterly Report on Form 10-Q for the quarter ended September 30, 2025 and any subsequent filings Bicara makes with the SEC. In addition, any forward-looking statements represent Bicara’s views only as of today and should not be relied upon as representing its views as of any subsequent date. Bicara explicitly disclaims any obligation to update any forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward- looking statements. Contacts Investors Jenna Cohen IR@bicara.com Media Amanda Lazaro 1AB Amanda@1abmedia.com
EX-99.2
3
bicaraesmoasia2025clinic.htm
EX-99.2
bicaraesmoasia2025clinic
ESMO Asia 2025 Clinical Update December 6, 2025 FICERA: enabling tumor penetration to drive deep and durable responses in HPV-neg HNSCC

Forward-looking statements 2 This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements other than historical factual information are forward-looking statements, including without limitation statements regarding our clinical development of ficerafusp alfa in combination with pembrolizumab and presentation of updated results from an open-label, multicenter Phase 1/1b trial of ficerafusp alfa with pembrolizumab in patients with recurrent or metastatic head and neck squamous cell carcinoma, and the expected therapeutic potential and ability, profile and clinical benefits of ficerafusp alfa, including potential and anticipated efficacy, depth, durability, tolerability, and success, and the potential clinical results from the Phase 2/3 pivotal trial of ficerafusp alfa. In some cases, you can identify forward-looking statements because they contain words such as “may,” “might,” “will,” “would,” “shall,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “looks,” “seeks,” “predicts,” “potential,” “ongoing,” or “continue” or the negative of these words or other similar terms or expressions that concern our expectations, strategy, plans or intentions, although not all forward-looking statements are accompanied by such words. Forward-looking statements are based on assumptions and assessments made by our management in light of their experience and perceptions of historical trends, current conditions, expected future developments and other factors they believe to be appropriate, and speak only as of the date of this presentation. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or other events to be materially different from any future results, performance or other events expressed or implied by the forward-looking statements. Given these uncertainties, you should not place undue reliance on forward-looking statements. Our actual future results, performance or other events may be materially different from what we expect. Except as required by law, we assume no obligation to update these forward-looking statements, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future. Factors that could cause actual results to differ from those predicted in our forward-looking statements include, among others, risks and uncertainties related to product development, including delays or challenges that may arise in the development and regulatory approval of our current and future product candidates or programs; uncertainties as to the availability and timing of results and data from preclinical and clinical studies; the timing of and our ability to submit and obtain regulatory clearance for investigational new drug applications, initiate additional clinical trials, and submit new drug applications or biologics license applications; our ability to initiate and complete our current and expected clinical trials; our ability to establish and maintain collaborations, strategic relationships and supply arrangements, or that we will not realize the intended benefits from such relationships or arrangements; whether our cash resources will be sufficient to fund our foreseeable and unforeseeable operating expenses and capital expenditure requirements; our ability to raise additional funding on favorable terms, or at all; the rate and degree of market acceptance and clinical utility of our product candidates; the ability and willingness of our third-party collaborators to continue research and, development and manufacturing activities relating to our product candidates; the accuracy of our data analyses or estimates for the potential and market for our products; our ability, and the ability of our collaborators, to protect our intellectual property and to conduct activities for the development and commercialization of our candidates in view of third party intellectual property positions; our financial performance; our ability to retain and recruit key personnel, as well as the potential contribution of our employees and board to our growth and success as a Company; developments and projections relating to our competitors or our industry; changes in general economic conditions and global instability, in particular economic conditions in the markets on which we or our suppliers operate; changes in laws and regulations; and those risks and uncertainties identified in our filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in our most-recently filed Quarterly Report on Form 10-Q, and such other risks and uncertainties that may be described in subsequent filings we may make with the SEC. You should not rely upon forward-looking statements as predictions of future events or performance, or as a representation or warranty (express or implied) by us or any other person that we will achieve our objectives and plans in any specified time frame, on such specified terms, or at all. Although our management believes that the expectations reflected in our statements are reasonable, we cannot guarantee that the future results, performance or events and circumstances described in the forward-looking statements will be achieved or occur. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein. Market data and industry information used throughout this presentation are based on management’s knowledge of the industry and the good faith estimates of management. We also relied, to the extent available, upon management’s review of independent industry surveys and publications and other publicly available information prepared by a number of third-party sources. All of the market data and industry information used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Although we believe that these sources are reliable as of their respective dates, we cannot guarantee the accuracy or completeness of this information, and we have not independently verified this information. Projections, assumptions and estimates of our future performance and the future performance of the industry in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors. These and other factors could cause results to differ materially from those expressed in our estimates and beliefs and in the estimates prepared by independent parties. This presentation discusses potential future product candidates that are investigational only and have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these potential future product candidates for the use for which such potential future product candidates are being studied.

Agenda & today’s presenters 3 Claire Mazumdar, Ph.D., MBA Chief Executive Officer Ryan Cohlhepp, Pharm.D. President & Chief Operating Officer Tanya Green, MS Chief Development Officer ESMO Asia 2025 Clinical Update2 Preliminary safety & efficacy data for 750mg QW FICERA + pembro Looking Ahead 3 Contextualizing 750mg QW dose in FORTIFI-HN01 Trial 1 Ficerafusp alfa (FICERA) in 1L R/M HPV-Negative HNSCC First and only EGFR x TGF-β bifunctional antibody HNSCC = head and neck squamous cell carcinoma; EGFR = epidermal growth factor receptor; TGF = transforming growth factor; QW = dosed weekly

Ficerafusp Alfa (FICERA) in 1L R/M HPV-Negative HNSCC First and only EGFR x TGF-β bifunctional antibody 4 Granted Breakthrough Therapy Designation¥ ¥ U.S. FDA Breakthrough Therapy Designation or ficerafusp alfa in combination with pembrolizumab for the first-line (1L) treatment of patients with metastatic or with unresectable, recurrent (R/M) head and neck squamous cell carcinoma (HNSCC) whose tumors express programmed death-ligand 1 with combined positive score (CPS) ≥1, excluding human papillomavirus (HPV)-positive oropharyngeal squamous cell carcinoma.
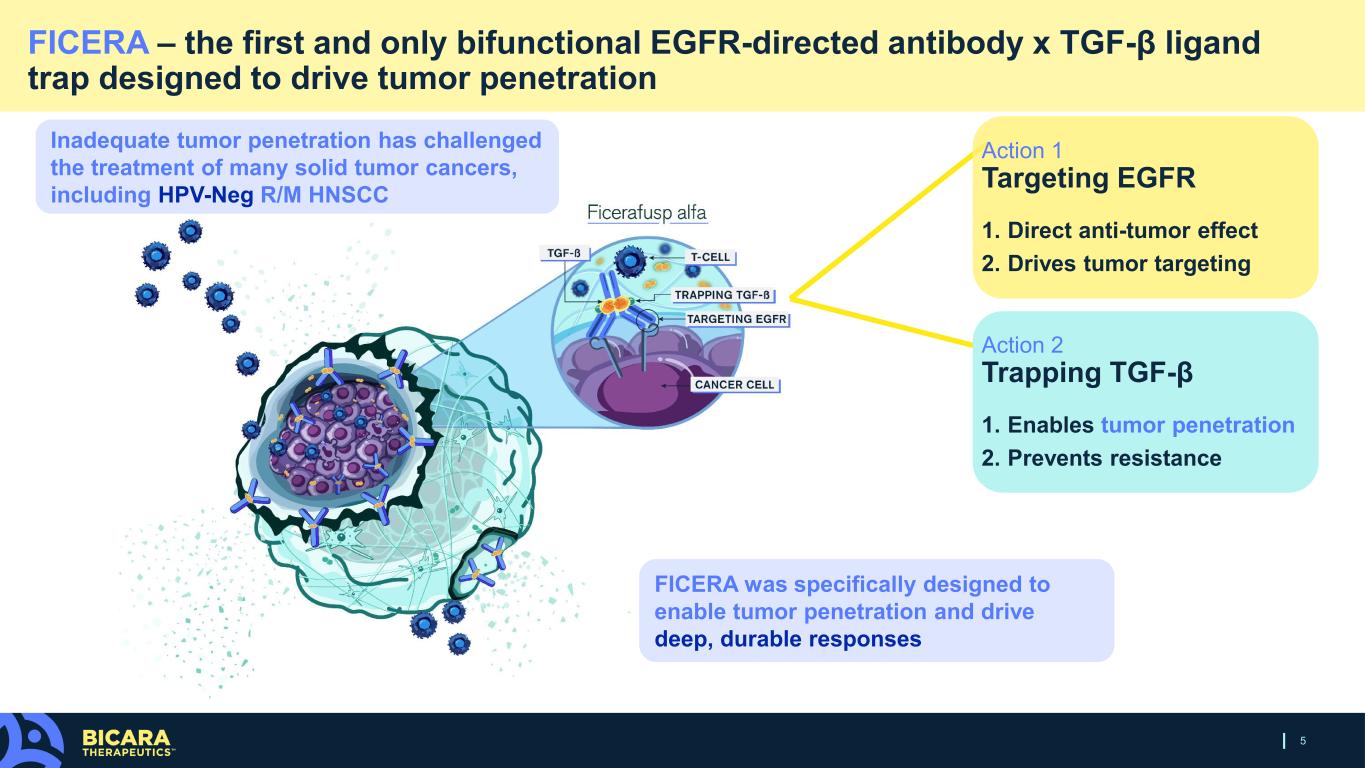
5 FICERA – the first and only bifunctional EGFR-directed antibody x TGF-β ligand trap designed to drive tumor penetration Inadequate tumor penetration has challenged the treatment of many solid tumor cancers, including HPV-Neg R/M HNSCC FICERA was specifically designed to enable tumor penetration and drive deep, durable responses Action 1 Targeting EGFR 1. Direct anti-tumor effect 2. Drives tumor targeting Action 2 Trapping TGF-β 1. Enables tumor penetration 2. Prevents resistance

6 1L HPV-negative R/M HNSCC is a sizable and growing market • Head and neck cancer accounts for ~4% of all cancers in the U.S. • Squamous cell carcinomas represent ~90% of H&N • Oropharyngeal lesions are typically tested for HPV HPV-positive caused by HPV infection HPV-negative typically caused by smoking and chewing tobacco represents 80% of HNSCC in the R/M setting and carries a worse prognosis vs. HPV-positive • Treatment decisions are guided by CPS or PD-L1 expression and options are limited to cetuximab, anti-PD1, chemotherapy Sources: Cancer.net, Cleveland Clinic (2022); SEER 2012-2018 data; Cerner (2022); Bedi et al. Mol Cancer Ther. 2012; Acta Otorhinolaryngol Ital. 2020, KeyNote-048 ph.3 trial; ASCO (2022); DRG HNSCC (2019) CPS = combined positive score. Significant and growing population Annual cases of HNSCC in U.S. High rates of recurrence/metastases Annual cases of R/M HNSCC in U.S. Most patients are HPV-negative ~80% of cases have worse outcomes ~23,000 ~67,000 ~18,400 Annual Cases in U.S.
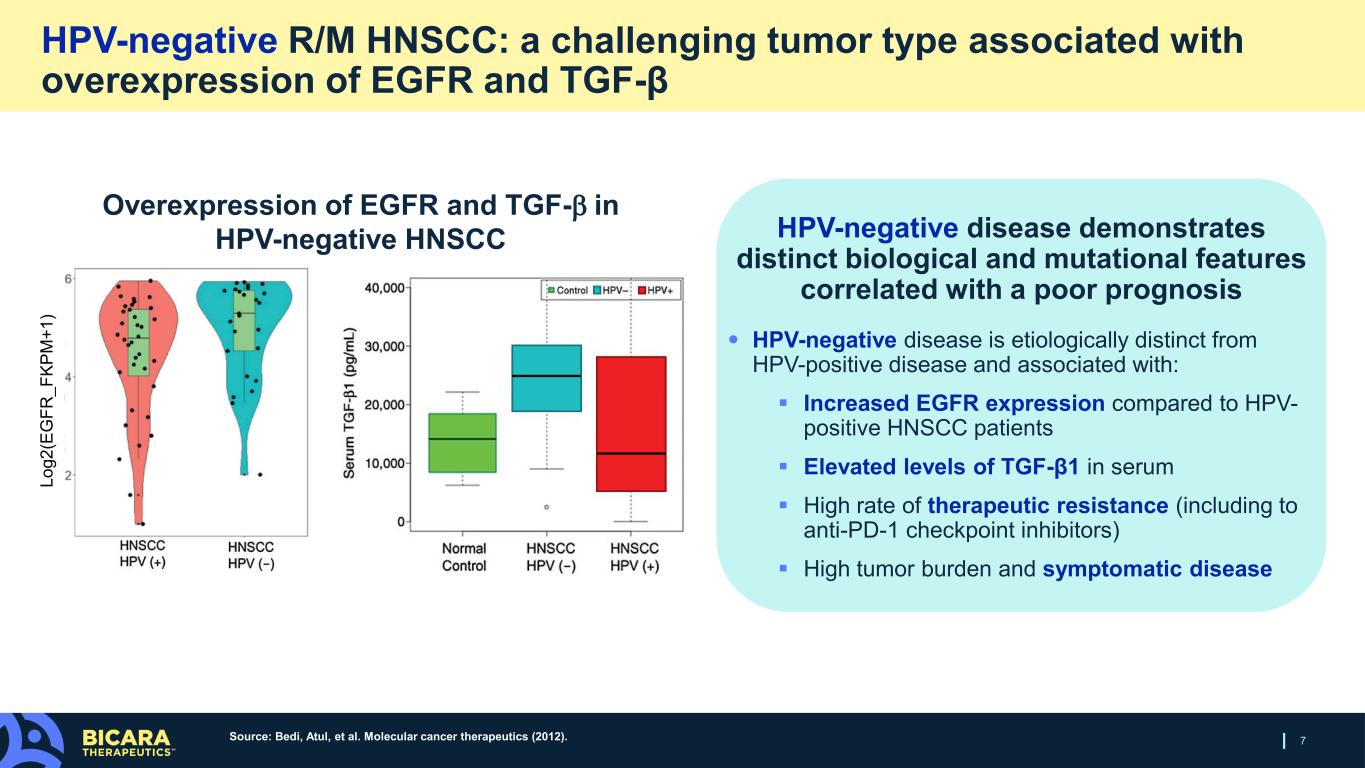
HPV-negative disease demonstrates distinct biological and mutational features correlated with a poor prognosis • HPV-negative disease is etiologically distinct from HPV-positive disease and associated with: Increased EGFR expression compared to HPV- positive HNSCC patients Elevated levels of TGF-β1 in serum High rate of therapeutic resistance (including to anti-PD-1 checkpoint inhibitors) High tumor burden and symptomatic disease 7 HPV-negative R/M HNSCC: a challenging tumor type associated with overexpression of EGFR and TGF-β Overexpression of EGFR and TGF-β in HPV-negative HNSCC Lo g2 (E G FR _F KP M +1 ) Source: Bedi, Atul, et al. Molecular cancer therapeutics (2012).

Significant unmet need for better treatment options that improve outcomes Pembrolizumab Pembrolizumab + chemotherapy Pembrolizumab + FICERA (1500mg QW)* ORR ~19% ~36% ~54%* mDOR ~23.4 months ~6.7 months 21.7 months* Median OS HPV-all ~12.3 months ~13.6 months NA HPV-negative ~9 months ~7 months 21.3 months* ~3X increase in ORR vs. pembro alone >3X increase in mDOR vs. pembro + chemo >2X increase in mOS vs. pembro +/- chemo Based on historical data. *HPV-Neg R/M HNSCC patients only (CPS ≥ 1). Sources: Burtness, Barbara, et al. The Lancet 394.10212 (2019): 1915-1928. Vasiliadou, Ifigenia, et al. International Journal of Cancer 155.5 (2024): 883-893. 3. Black, Christopher M., et al. Frontiers in Oncology 13 (2023): 1160144. Current standard of care 8
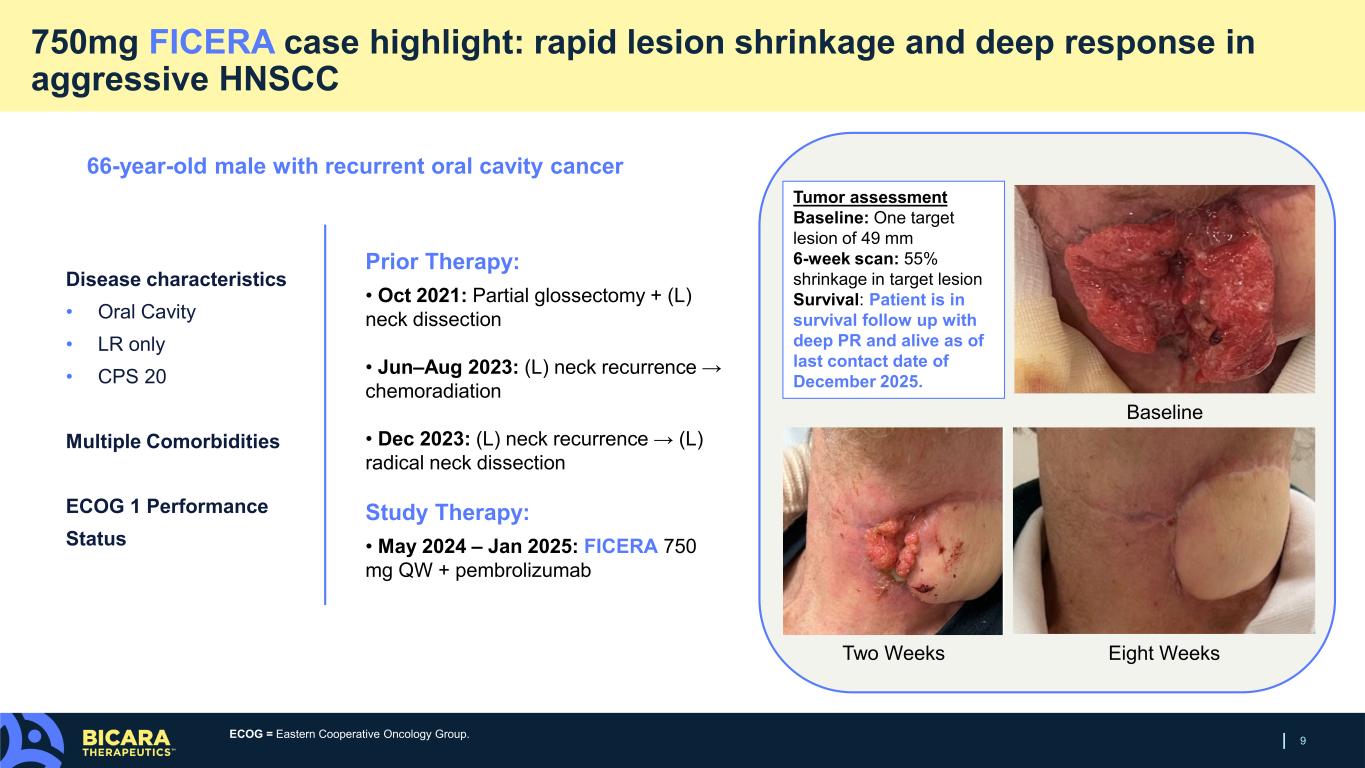
9 750mg FICERA case highlight: rapid lesion shrinkage and deep response in aggressive HNSCC Disease characteristics • Oral Cavity • LR only • CPS 20 Multiple Comorbidities ECOG 1 Performance Status Baseline Two Weeks Eight Weeks Tumor assessment Baseline: One target lesion of 49 mm 6-week scan: 55% shrinkage in target lesion Survival: Patient is in survival follow up with deep PR and alive as of last contact date of December 2025. 66-year-old male with recurrent oral cavity cancer Prior Therapy: • Oct 2021: Partial glossectomy + (L) neck dissection • Jun–Aug 2023: (L) neck recurrence → chemoradiation • Dec 2023: (L) neck recurrence → (L) radical neck dissection Study Therapy: • May 2024 – Jan 2025: FICERA 750 mg QW + pembrolizumab ECOG = Eastern Cooperative Oncology Group.

10 Ongoing FORTIFI-HN01 trial design allows for efficient path-to-market R/M HNSCC 1L Setting CPS ≥ 1 excl. HPV-positive OPSCC R FICERA 1500mg QW + Pembro 200mg Q3W FICERA 750mg QW + Pembro 200mg Q3W Pembro 200mg Q3W FICERA optimal dose + Pembro 200mg Q3W Dose Selection Endpoint: ORR (primary) Endpoint: OS (primary) 1Q 2026 Interim Analysis & Potential Accelerated Approval Potential Full Approval Q3W = every 3 weeks; ORR = objective response rate; OS = overall survival Anticipate optimal dose selection in 1Q 2026
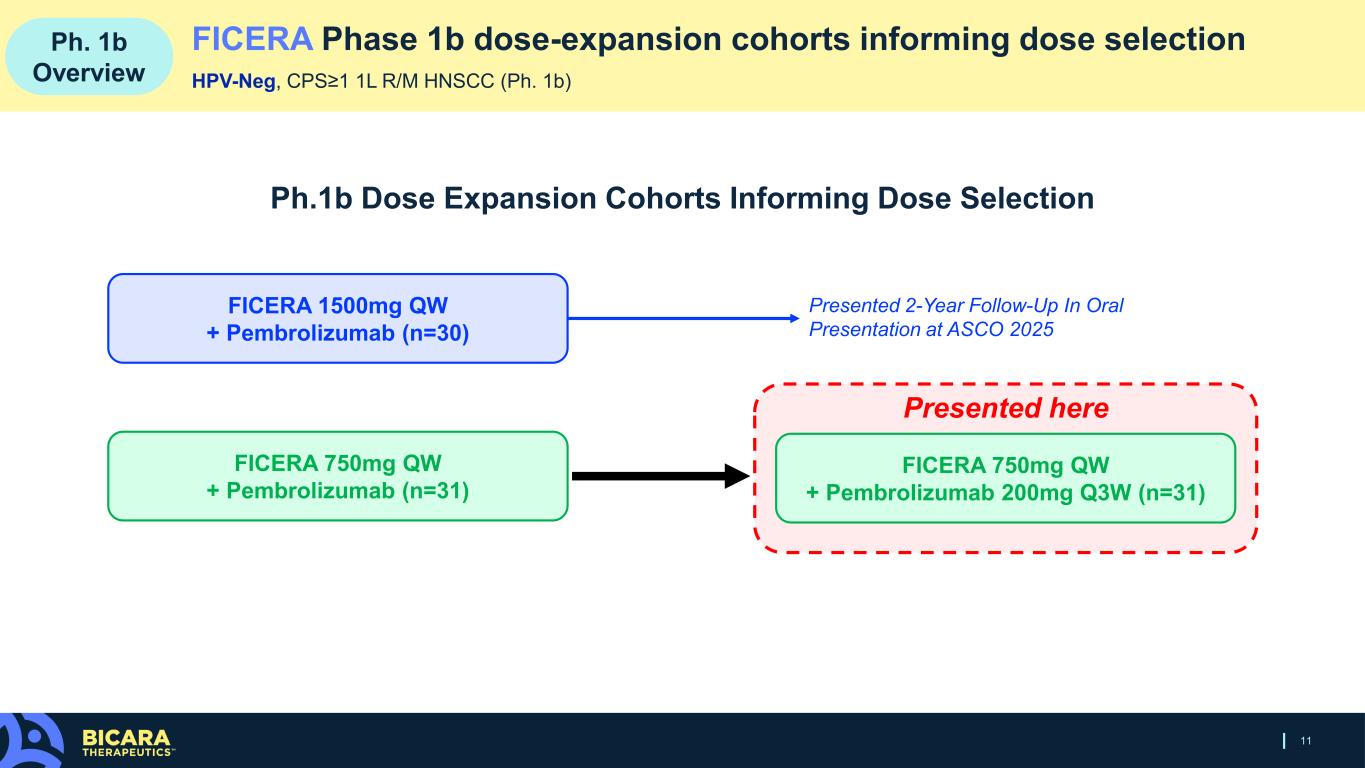
11 Ph. 1b Overview FICERA Phase 1b dose-expansion cohorts informing dose selection HPV-Neg, CPS≥1 1L R/M HNSCC (Ph. 1b) Ph.1b Dose Expansion Cohorts Informing Dose Selection Presented here FICERA 1500mg QW + Pembrolizumab (n=30) FICERA 750mg QW + Pembrolizumab (n=31) FICERA 750mg QW + Pembrolizumab 200mg Q3W (n=31) Presented 2-Year Follow-Up In Oral Presentation at ASCO 2025

ESMO Asia 2025 Clinical Update Preliminary safety & efficacy data for 750mg QW FICERA + pembro 12

13 Baseline Characteristics Data snapshot: July 9th, 2025. . Characteristic Safety set (N=31) Age Median (range) 64 (28-78) Sex – n (%) Male/Female 20/11 (65%/35%) Primary disease site – n (%) Oropharynx 5 (16%) Oral cavity 19 (61%) Hypopharynx 5 (16%) Larynx 2 (6%) CPS – n (%) 1-19 12 (39%) ≥20 19 (61%) Locoregional (LR) vs distant metastatic (DM) disease – n (%) LR only 16 (52%) LR + DM 9 (29%) DM only 6 (19%) Sum (mm) of target lesion diameters – n (%) Median, mm 41 >50 10 (32%) >70 4 (13%) ECOG performance status – n (%) 0 vs. 1 11 vs. 20 (35% vs. 65%) Population • 1L R/M HNSCC, HPV-Negative • Oral cavity, oropharynx, larynx, and hypopharynx • CPS ≥1 • ECOG performance status 0-1 Patient demographics and baseline characteristics FICERA 750mg QW + Pembrolizumab in HPV-neg, CPS≥1 1L R/M HNSCC

14 Safety Data snapshot: July 9th, 2025. *TRAEs includes TEAEs possibly, probably, or definitely related to ficerafusp alfa; also includes TEAEs with missing drug relationships. †n=1 each for TEAEs leading to dose reduction and discontinuation. AE = adverse event; TEAE = treatment-emergent adverse event; TRAE = treatment related adverse event. FICERA continues to exhibit a generally well-tolerated safety profile FICERA 750mg QW + Pembrolizumab in HPV-neg, CPS≥1 1L R/M HNSCC 750mg QW FICERA + Pembrolizumab safety profile: • The combination was tolerable with a manageable safety profile • No treatment-related deaths were reported • Safety profile at 750 mg was consistent with established safety profile of 1500mg FICERA + pembrolizumab in R/M HNSCC Preferred term, n (%) Safety set (N=31) Any grade Grade 3 Grade 4/5 Any TRAE 31 (100) 13 (42) 0 Dermatitis acneiform 26 (84) 1 (3) 0 Pruritus 12 (39) 1 (3) 0 Fatigue 12 (39) 0 0 Stomatitis 10 (32) 3 (10) 0 Epistaxis 10 (32) 1 (3) 0 Dry skin 10 (32) 0 0 Skin fissures 9 (29) 0 0 Hypophosphatemia 9 (29) 0 0 Anemia 8 (26) 3 (10) 0 Hypomagnesemia 7 (23) 0 0 Hypokalemia 7 (23) 2 (6) 0 Lipase increased 7 (23) 0 0 Amylase increased 7 (23) 0 0 TRAE leading to ficerafusp alfa discontinuation† 2 (6%) Most common (>20%) adverse events related to FICERA*
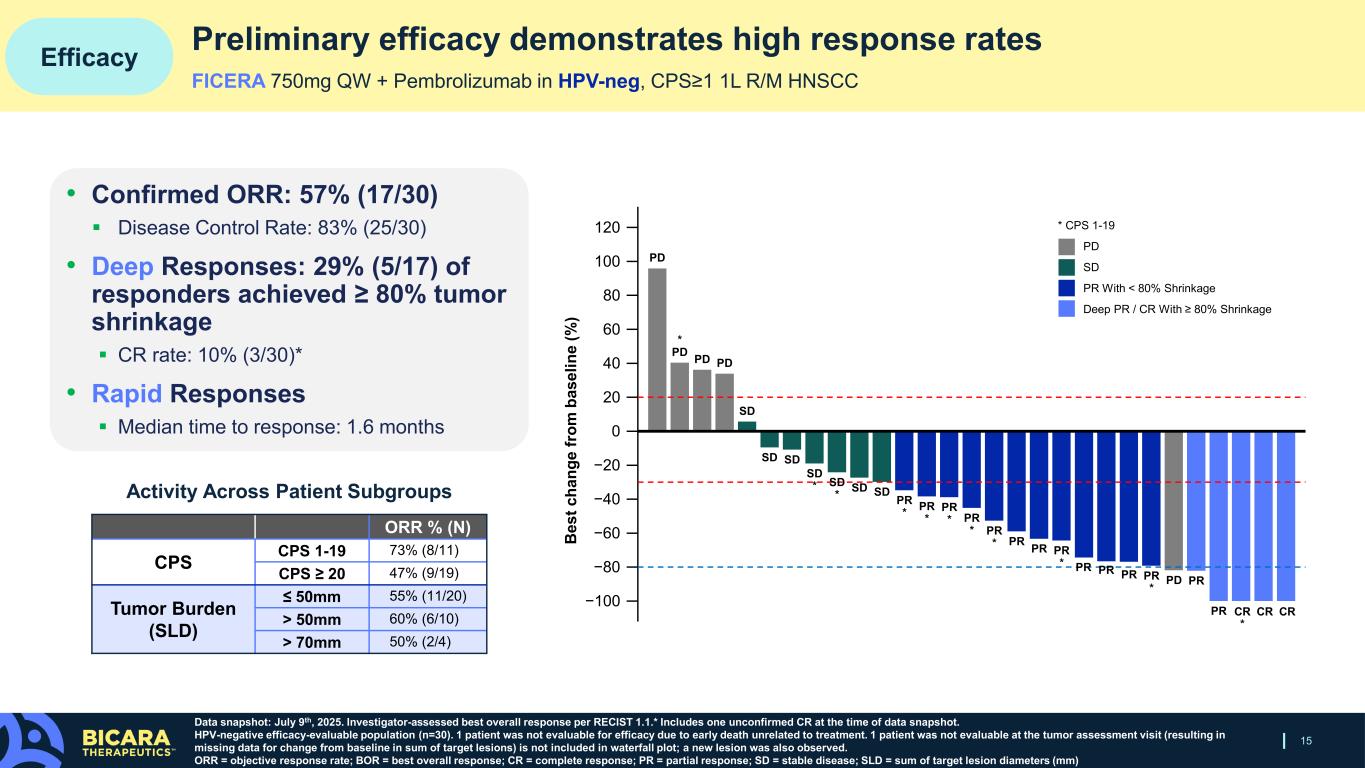
15 Efficacy Data snapshot: July 9th, 2025. Investigator-assessed best overall response per RECIST 1.1.* Includes one unconfirmed CR at the time of data snapshot. HPV-negative efficacy-evaluable population (n=30). 1 patient was not evaluable for efficacy due to early death unrelated to treatment. 1 patient was not evaluable at the tumor assessment visit (resulting in missing data for change from baseline in sum of target lesions) is not included in waterfall plot; a new lesion was also observed. ORR = objective response rate; BOR = best overall response; CR = complete response; PR = partial response; SD = stable disease; SLD = sum of target lesion diameters (mm) • Confirmed ORR: 57% (17/30) Disease Control Rate: 83% (25/30) • Deep Responses: 29% (5/17) of responders achieved ≥ 80% tumor shrinkage CR rate: 10% (3/30)* • Rapid Responses Median time to response: 1.6 months ORR % (N) CPS CPS 1-19 73% (8/11) CPS ≥ 20 47% (9/19) Tumor Burden (SLD) ≤ 50mm 55% (11/20) > 50mm 60% (6/10) > 70mm 50% (2/4) Activity Across Patient Subgroups Preliminary efficacy demonstrates high response rates 120 −100 −80 −60 −40 −20 0 40 80 100 20 B es t c ha ng e fr om b as el in e (% ) 60 PRPDPR * PRPR PR PR * PR * PR * PR * PR * SDSDSD * SD * SDSD SD PDPD * PD PD PR * PR PR CRCRCR * PR Deep PR / CR With ≥ 80% Shrinkage PR With < 80% Shrinkage SD PD * CPS 1-19 FICERA 750mg QW + Pembrolizumab in HPV-neg, CPS≥1 1L R/M HNSCC

16 Ph. 1b Summary Data snapshot: July 9th, 2025. *Breakthrough Therapy Designation (BTD) granted for the first line treatment of patients with metastatic or with unresectable, recurrent (R/M) HNSCC whose tumors express PD-L1 with CPS ≥1, excluding HPV-positive oropharyngeal squamous cell carcinoma (OPSCC). Summary and key takeaways 750mg QW FICERA demonstrates preliminary safety and efficacy profile consistent with 1500mg QW in 1L HPV-neg R/M HNSCC Breakthrough Therapy Designation granted for 1L HPV-Neg* R/M HNSCC, CPS≥1 • Continues to demonstrate a manageable safety profile • High ORR: 57% ORR (n=17/30) • Deep responses: 29% of responders had ≥80% tumor shrinkage; 10% CR rate • Rapid time to response: median 1.6 months • Data inform dose selection in the FORTIFI-HN01 study, expected Q1 2026 • Longer follow-up time will help assess durability and other longer-term efficacy parameters FICERA 750mg QW + Pembrolizumab in HPV-neg, CPS≥1 1L R/M HNSCC

Looking Ahead Contextualizing 750mg QW dose in FORTIFI-HN01 Trial 17

18 Efficacy By Dose 1. Data snapshot: July 9th, 2025. 2. Presented at ASCO 2025. 3. Data from KEYNOTE-048 represents CPS≥1, HPV-all population. HPV-negative efficacy-evaluable population. Investigator-assessed best overall response per RECIST 1.1. Deep response refers ≥ 80% tumor shrinkage from baseline. EE = efficacy evaluable; ORR = objective response rate; CR = complete response. ND = not disclosed. FICERA demonstrates high response rates across doses FICERA 750mg QW & 1500mg QW in HPV-neg, CPS≥1 1L R/M HNSCC 750mg QW1 1500mg QW2 Pembro Mono3 Metric EE set (N=30) EE set (N=28) KN-048 (N=257) Confirmed ORR % (N) 57% (17/30) 54% (15/28) 19% CPS 1-19 73% (8/11) 54% (7/13) 15% CPS ≥ 20 47% (9/19) 53% (8/15) 23% Disease Control Rate % (N) 83% (25/30) 89% (25/28) 61% Deep Responses % (N) 29% (5/17) 80% (12/15) ND CR Rate % (N) 10% (3/30) 21% (6/28) 5% Median Time to Response 1.6 months 1.4 months 2.1 months Consistently high ORR across multiple dose levels increases confidence in the ORR interim analysis

19 Biomarkers By Dose Bicara Therapeutics data on file. Plots show mean with SEM. **p ≤ 0.01 pSMAD2 Analysis: N=5 samples at 750mg, N=7 samples at 1500mg. Immune-activation cytokine analysis: N=24 samples for 750mg (except N=25 for CXCL10), N=27 for 1500mg. Higher doses of FICERA demonstrate increased TGF-β inhibition FICERA 750mg QW vs. 1500mg QW In HPV-neg, CPS≥1 1L R/M HNSCC pSMAD2 Tumor tissue 750mg 1500mg -50 -40 -30 -20 -10 0 % C ha ng e fr om B as el in e ns IFNGTNF-a CXCL9 CXCL10 Blood 1500mg of FICERA shows a trend for increased TGF-β inhibition in the tumor and increased pro-inflammatory cytokines in the blood vs. 750mg of FICERA Increased TGF-β Inhibition at 1500mg vs. 750mg Increased Immune-Activation at 1500mg vs. 750mg
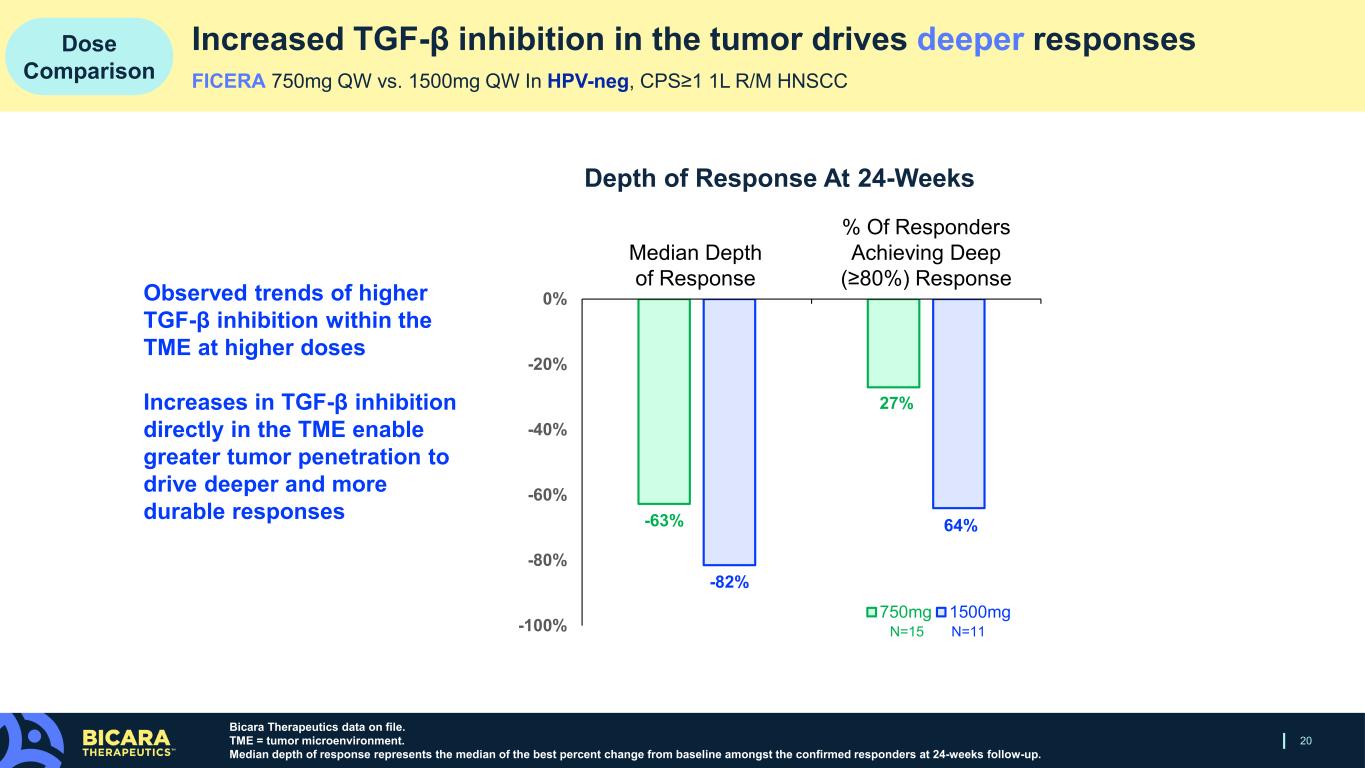
20 Dose Comparison Bicara Therapeutics data on file. TME = tumor microenvironment. Median depth of response represents the median of the best percent change from baseline amongst the confirmed responders at 24-weeks follow-up. Increased TGF-β inhibition in the tumor drives deeper responses FICERA 750mg QW vs. 1500mg QW In HPV-neg, CPS≥1 1L R/M HNSCC Median Depth of Response % Of Responders Achieving Deep (≥80%) Response Depth of Response At 24-Weeks Observed trends of higher TGF-β inhibition within the TME at higher doses Increases in TGF-β inhibition directly in the TME enable greater tumor penetration to drive deeper and more durable responses N=15 N=11 -63% -27% -82% -64% -100% -80% -60% -40% -20% 0% 750mg 1500mg

21 750 mg data further derisk pivotal FORTIFI-HN01 study 1 4 3 2 Consistently high ORR across multiple dose levels increases confidence in the ORR interim analysis Consistent safety and early efficacy profiles observed across the 750mg and 1500mg FICERA dose regimens, and generally well-tolerated Dose-dependent response in TGF-β inhibition and immune activation, which we believe drives depth and durability of response 750mg data set informs and advances FICERA dose selection Anticipate dose selection in 1Q 2026

Thank You

























