UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (date of earliest event reported): December 20, 2023
TONIX PHARMACEUTICALS HOLDING CORP.
(Exact name of registrant as specified in its charter)
| Nevada | 001-36019 | 26-1434750 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
26 Main Street, Chatham, New Jersey 07928
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (862) 904-8182
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock | TNXP | The NASDAQ Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On December 20, 2023, Tonix Pharmaceuticals Holding Corp. (the “Company”) announced that the Phase 3 RESILIENT study evaluating its TNX-102 SL (cyclobenzaprine HCl sublingual tablets) product candidate for the management of fibromyalgia met its pre-specified primary endpoint in the second of two positive Phase 3 clinical trials. A copy of the press release that discusses this matter is filed as Exhibit 99.01 and hereto and incorporated herein by reference.
The Company presented certain information regarding the Company and its product candidates on December 19, 2023. A copy of the presentation, which may contain nonpublic information, is filed as Exhibit 99.02 hereto and incorporated herein by reference.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.01 and 99.02 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the United States Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the United States Securities Act of 1933 or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 8.01 | Other Events. |
On December 20, 2023, the Company announced that the Phase 3 RESILIENT study evaluating TNX-102 SL met its pre-specified primary endpoint in the second of two positive Phase 3 clinical trials, significantly reducing daily pain compared to placebo (p=0.00005) in participants with fibromyalgia. Statistically significant and clinically meaningful results were also seen in all key secondary endpoints related to improving sleep quality, reducing fatigue, and improving overall fibromyalgia symptoms and function. Additionally, as it relates to improving daily pain, treatment with TNX-102 SL showed a clinically meaningful analgesic effect size of 0.38, with rapid onset of action, separating from placebo for each week of the study. TNX-102 SL was well tolerated with an adverse event profile comparable to prior studies, and no new safety signals were observed. The Company plans to submit a new drug application (“NDA”) to the U.S. Food and Drug Administration (“FDA”) in the second half of 2024 for TNX-102 SL for the management of fibromyalgia. The Company believes it is on track to supply TNX-102 in the U.S. upon FDA approval. The Company’s cash spend will be prioritized for the advancement this program toward FDA approval.
Table 1. Results of Primary and Secondary Endpoints for the Phase 3 RESILIENT Study of TNX-102 SL
| Outcome Measure at Week 14 | Intent-to-Treat Analysis1 | P-value | ||||||
| Primary Endpoint | ||||||||
| Daily Pain Diary, NRS | Mean Change from Baseline2 | 0.00005* | ||||||
| Key Secondary Endpoints | ||||||||
| Non-specific | ||||||||
| Patient Global Impression of Change | Proportion "Much" or "Very Much Improved"3 | <0.001* | ||||||
| Fibromyalgia Syndrome-Related | ||||||||
| FIQ-R Symptom Domain | Mean Change from Baseline | <0.001* | ||||||
| FIQ-R Function Domain | Mean Change from Baseline | 0.001* | ||||||
| PROMIS Sleep Disturbance | Mean Change from Baseline | <0.001* | ||||||
| PROMIS Fatigue | Mean Change from Baseline | <0.001* | ||||||
| Daily Sleep Quality Diary, NRS | Mean Change from Baseline | <0.001* | ||||||
| Abbreviations: FIQ-R = Fibromyalgia Impact Questionnaire - Revised; NRS = Numeric Rating Scale; PROMIS = Patient-Reported Outcomes Measurement Information System | ||||||||
| * statistically significant; to control for overall type 1 error, a pre-specified, serial gatekeeping procedure was utilized. | ||||||||
| 1Analysis by mixed model repeated measures with multiple imputation unless otherwise indicated. | ||||||||
|
2Primary endpoint analysis for FDA approvals of Cymbalta® and Lyrica® in fibromyalgia. 3Pearson’s chi-squared test, with missing data considered non-responders
|
||||||||
Summary of Topline Results of the RESILIENT Study
The RESILIENT study achieved statistical significance on the pre-specified primary efficacy endpoint: change from baseline in the weekly average of daily diary pain severity numerical rating scale (NRS) scores for TNX-102 SL 5.6 mg (LS mean [SE]: -1.8 [0.12] units) versus placebo (-1.2 [0.12] units), analyzed by mixed model repeated measures with multiple imputation (LS mean [SE] difference: -0.7 [0.16] units, p=0.00005, Table 1). All pre-specified sensitivity analyses of the primary endpoint were also statistically significant (p<0.001).
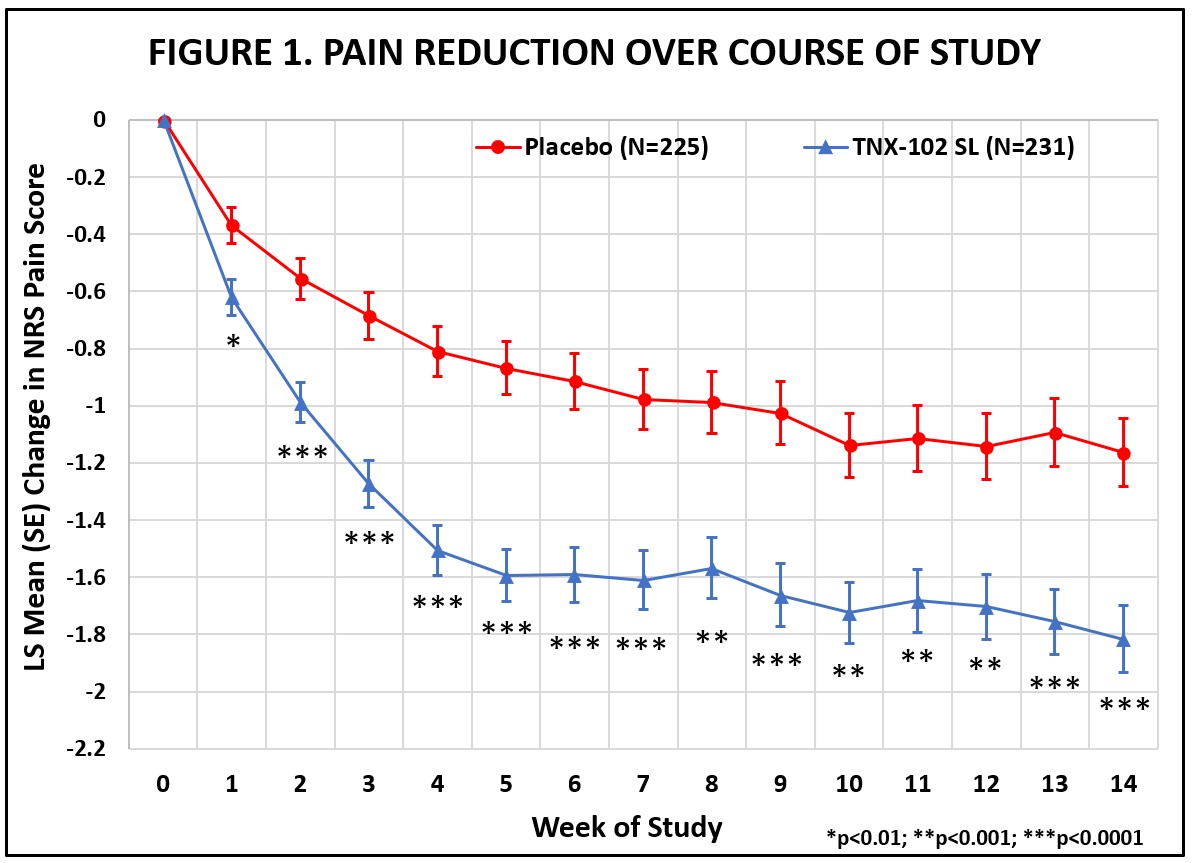
Abbreviations: LS = least squares; NRS = numerical rating scale; SE = standard error
The statistically significant improvement in pain is further substantiated when diary pain was analyzed by another standard statistical approach, a 30 percent responder analysis, with 45.9% on active and 27.1% on placebo having a 30 percent or greater reduction in pain (Pearson Chi-Squared Test; difference in proportions [95% CI]: 18.8% [10.1%, 27.4%]; nominal p<0.001).
TNX-102 SL showed statistical significance (≤0.001) on all six pre-specified key secondary efficacy outcome measures (Table 1).
Consistent with the proposed mechanism that TNX-102 SL acts in fibromyalgia through improving sleep quality, TNX-102 SL showed statistically significant improvement of sleep by two main measures. For the daily diary sleep quality ratings, improvement in sleep quality for TNX-102 SL (-1.8 [0.12] units) was greater than that of placebo (-1.2 [0.12] units; LS mean [SE] difference from placebo: -0.6 [0.17] units; p<0.001). For the PROMIS Sleep Disturbance instrument, TNX-102 SL also demonstrated greater improvement over placebo on T-scores (LS mean [SE] difference from placebo: -4.2 [0.79] units; p<0.001). TNX-102 SL showed improvement over placebo on the PROMIS Fatigue instrument T-scores (-3.0 [0.77] units; p<0.001).
At week 14 on the Fibromyalgia Impact Questionnaire – Revised (FIQ-R), there was greater improvement with TNX-102 SL than with placebo (LS mean [SE] difference from placebo: -7.7 (1.62), p<0.001). TNX-102 SL resulted in greater improvement on FIQ-R Function (LS mean [SE] difference from placebo: -5.4 [1.66], p=0.001). TNX-102 SL also separated from placebo on the FIQ-R Impact domain (nominal p=0.001).
Safety Results of the Phase 3 RESILIENT Study
In the RESILIENT study, TNX-102 SL was well tolerated, with no new safety signals observed. Among participants randomized to the TNX-102 SL and placebo arms, 81.0% and 79.2%, respectively, completed the 14-week dosing period. Administration site reactions were the most commonly reported adverse events and were higher in the TNX-102 SL treatment group. Hypoaesthesia oral and paraesthesia oral, product taste abnormal, and tongue discomfort were local effects nearly always temporally related to dose administration and transiently expressed (<60 minutes) in most occurrences. The treatment-emergent adverse events that occurred at a rate of 3.0% or greater in either arm were these four oral adverse events, along with COVID-19, somnolence, and headache. Adverse events resulted in premature study discontinuation in 6.1% of those who received TNX-102 SL compared with 3.5% of placebo recipients. There were a total of seven serious adverse events in five patients, five of which were experienced by three patients in the placebo arm, and two of which were in the TNX-102 SL arm. Of the two in the TNX-102 SL arm, one was renal cancer, deemed unrelated to study drug, and the other was acute pancreatitis with onset 14 days after dosing was completed and reported as possibly related to study drug.
Table 2. Treatment-emergent adverse events at a rate of 3% or greater in either treatment arm
| TNX-102 SL (N=231) | Placebo (N=226) | Total (N=457)* | ||||||||||
| Administration Site Reactions | N | % | N | % | N | % | ||||||
| Hypoaethesia oral | 55 | 23.8% | 1 | 0.4% | 56 | 12.3% | ||||||
| Product taste abnormal | 27 | 11.7% | 2 | 0.9% | 29 | 6.3% | ||||||
| Paraesthesia oral | 16 | 6.9% | 2 | 0.9% | 18 | 3.9% | ||||||
| Tongue discomfort | 16 | 6.9% | 0 | 0.0% | 16 | 3.5% | ||||||
| Systemic | ||||||||||||
| Adverse Events | N | % | N | % | N | % | ||||||
| COVID-19 | 10 | 4.3% | 7 | 3.1% | 17 | 3.7% | ||||||
| Somnolence | 7 | 3.0% | 3 | 1.3% | 10 | 2.2% | ||||||
| Headache | 7 | 3.0% | 4 | 1.8% | 11 | 2.4% | ||||||
*Safety Population
In females, the total score on the Changes in Sexual Functioning Questionnaire short form (CSFQ-14) at Week 14 improved (indicating better sexual functioning) in the TNX-102 SL group compared with placebo (nominal p=0.010 by analysis of covariance). This potentially indicates an important tolerability advantage over pharmacotherapeutics which potently inhibit reuptake of serotonin. The low percentage of males in the safety population (<5%) did not allow meaningful analysis of the CSFQ-14 data.
Separately, the Company completed the analysis of the Phase 2 PREVENTION study of its TNX-1900 (intranasal potentiated oxytocin) product candidate for the prevention of migraine headaches in chronic migraineurs. The trial did not meet the primary endpoint as measured by a reduction from 28-day run-in baseline in the mean number of migraine headache days during the last 28 days of the treatment phase. PREVENTION was a small proof-of-concept study, and with 88 patients enrolled across three arms (TNX-1900 30 IU QD, TNX-1900 30 IU BID and placebo), was not powered to result in a statistically significant outcome. For the primary outcome of monthly migraine days during the last 28 days of treatment, the placebo group saw a least squares mean (LSmean) change from baseline of -8.2 (1.4) [mean (standard error)]; the 30 IU group reported a LSmean change of -7.8 (1.2) and the 60 IU group had -5.8 (1.3). The comparison between placebo and TNX-1900 30 IU favored placebo with a difference of 0.4 (1.5) migraine days and an effect size of 0.07; the comparison between placebo and TNX-1900 60 IU favored placebo with a difference of 2.4 (1.5) migraine days and an effect size of 0.42. In the trial, TNX-1900 was generally well-tolerated with no treatment-emergent serious or severe adverse events.
| Item 9.01 | Financial Statements and Exhibits. |
| (d) |
Exhibit No. |
Description. | ||
|
104 |
Press Release, dated December 20, 2023 Investor Presentation by the Company Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirement of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| TONIX PHARMACEUTICALS HOLDING CORP. | |||
| Date: December 20, 2023 | By: | /s/ Bradley Saenger | |
| Bradley Saenger | |||
| Chief Financial Officer | |||
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.01
Tonix Pharmaceuticals Announces Highly Statistically Significant and Clinically Meaningful Topline Results in Second Positive Phase 3 Clinical Trial of TNX-102 SL for the Management of Fibromyalgia
Phase 3 RESILIENT study of TNX-102 SL successfully demonstrated daily pain reduction over placebo (primary endpoint, p = 0.00005)
All six key secondary endpoints, including patient global impression, fibromyalgia-specific symptoms and dysfunction, fatigue and sleep measures were significantly improved (all p ≤ 0.001)
Positive results support planned New Drug Application (NDA) submission to the FDA in the second half of 2024
An estimated 6 million to 12 million adults in the U.S. are living with fibromyalgia, the majority of whom are women
CHATHAM, N.J., December 20, 2023 (GLOBE NEWSWIRE) – Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix or the Company), a biopharmaceutical company with marketed products and a pipeline of development candidates, today announced that the Phase 3 RESILIENT study evaluating TNX-102 SL (cyclobenzaprine HCl sublingual tablets) met its pre-specified primary endpoint in the second of two positive Phase 3 clinical trials, significantly reducing daily pain compared to placebo (p=0.00005) in participants with fibromyalgia (Table 1). Statistically significant and clinically meaningful results were also seen in all key secondary endpoints related to improving sleep quality, reducing fatigue, and improving overall fibromyalgia symptoms and function. Additionally, as it relates to improving daily pain, treatment with TNX-102 SL showed a robust and clinically meaningful analgesic effect size of 0.38, with rapid onset of action, separating from placebo for each week of the study. TNX-102 SL was well tolerated with an adverse event profile comparable to prior studies, and no new safety signals were observed. Tonix plans to submit a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) in the second half of 2024 for TNX-102 SL for the management of fibromyalgia. An estimated 6 million to 12 million U.S. adults are living with fibromyalgia, the majority of whom are women.
TNX-102 SL is a tablet formulation containing 2.8 mg cyclobenzaprine HCl and is a novel, centrally-acting, non-opioid analgesic, designed to be taken once daily at bedtime for the management of fibromyalgia. RESILIENT was a 14-week randomized, double-blind, placebo-controlled trial of TNX-102 SL 5.6 mg, in which 457 participants with fibromyalgia were randomized in a 1:1 ratio to TNX-102 SL or placebo across 33 sites in the U.S. All participants received one 2.8 mg tablet of TNX-102 SL (2.8 mg) or placebo for the first 2 weeks, which was increased to two 2.8 mg tablets of TNX-102 SL (5.6 mg) or placebo for the remaining 12 weeks.
In December 2020, Tonix reported positive results from the first Phase 3 RELIEF study of TNX-102 SL 5.6 mg for the management of fibromyalgia. The RELIEF study met its pre-specified primary endpoint, significantly reducing daily pain compared to placebo (p=0.010) in participants with fibromyalgia, and showing activity in key secondary endpoints.
“We believe that the positive results of RESILIENT and RELIEF show that fibromyalgia can be successfully treated by TNX-102 SL 5.6 mg and may provide the opportunity for Tonix to have the first FDA-approved drug for fibromyalgia in more than a decade,” said Seth Lederman, M.D., President and Chief Executive Officer of Tonix Pharmaceuticals. “We are now an important step closer to bringing a new, first-line treatment to fibromyalgia patients that offers broad symptom relief and favorable tolerability for chronic use and adherence. We believe that we are well positioned to submit an NDA to the FDA under the 505(b)(2) regulatory approval pathway in the second half of 2024, and are on track to supply the U.S. market upon FDA approval.”
Table 1. Results of Primary and Secondary Endpoints for the Phase 3 RESILIENT Study of TNX-102 SL
| Outcome Measure at Week 14 | Intent-to-Treat Analysis1 | P-value | ||
| Primary Endpoint | ||||
| Daily Pain Diary, NRS | Mean Change from Baseline2 | 0.00005* | ||
| Key Secondary Endpoints | ||||
| Non-specific | ||||
| Patient Global Impression of Change | Proportion "Much" or "Very Much Improved"3 | <0.001* | ||
| Fibromyalgia Syndrome-Related | ||||
| FIQ-R Symptom Domain | Mean Change from Baseline | <0.001* | ||
| FIQ-R Function Domain | Mean Change from Baseline | 0.001* | ||
| PROMIS Sleep Disturbance | Mean Change from Baseline | <0.001* | ||
| PROMIS Fatigue | Mean Change from Baseline | <0.001* | ||
| Daily Sleep Quality Diary, NRS | Mean Change from Baseline | <0.001* | ||
Abbreviations: FIQ-R = Fibromyalgia Impact Questionnaire - Revised; NRS = Numeric Rating Scale; PROMIS = Patient-Reported Outcomes Measurement Information System
*Statistically significant; to control for overall type 1 error, a pre-specified, serial gatekeeping procedure was utilized.
1Analysis by mixed model repeated measures with multiple imputation unless otherwise indicated.
2Primary endpoint analysis for FDA approvals of Cymbalta® and Lyrica® in fibromyalgia.
3Pearson’s chi-squared test responder analysis, with missing data considered non-responders
“These data are terrific news for patients with fibromyalgia,” said Daniel J. Clauw, M.D., Professor of Anesthesiology, Medicine and Psychiatry at the University of Michigan. “Despite approved medications, there remains a need for new treatment options to better address the quality of life impacts many fibromyalgia patients experience on a chronic basis. TNX-102 SL is a non-opioid, centrally-acting analgesic, the active ingredient of which has a known, favorable safety profile from decades of use. The fact that cyclobenzaprine was also beneficial in many other key symptom domains, including sleep quality, sleep disturbance and fatigue, will be appreciated by fibromyalgia patients that struggle with not just pain but multiple other symptoms.”
“These positive data from RESILIENT and previously with RELIEF, with remarkable separation from placebo on pain, sleep, and fatigue, add support to TNX-102 SL’s proposed mechanism of improving sleep quality to improve the syndromal effects of fibromyalgia,” commented Gregory Sullivan, M.D., Chief Medical Officer of Tonix Pharmaceuticals. “The sublingual formulation of TNX-102 SL, which uses our proprietary Protectic® and Angstro® technologies, is integral to our treatment paradigm. These technologies enable transmucosal delivery of cyclobenzaprine with distinctive pharmacokinetic properties that include rapid absorption after dosing and bypass of first-pass hepatic metabolism. I would like to thank the RESILIENT study participants and their families and caregivers, as well as the investigators and their hard-working staff who all made this a highly successful trial.”
Summary of Topline Results of the RESILIENT Study
The RESILIENT study achieved statistical significance on the pre-specified primary efficacy endpoint: change from baseline in the weekly average of daily diary pain severity numerical rating scale (NRS) scores for TNX-102 SL 5.6 mg (LS mean [SE]: -1.8 [0.12] units) versus placebo (-1.2 [0.12] units), analyzed by mixed model repeated measures with multiple imputation (LS mean [SE] difference: -0.7 [0.16] units, p=0.00005, Table 1). In addition, all pre-specified sensitivity analyses of the primary endpoint were statistically significant (p<0.001). Figure 1 shows reduction in pain across all weeks of the 14-week study, with nominal p<0.01 for every week. Note the rapid onset of action with separation from placebo at Week 1 was sustained throughout all weeks of dosing.
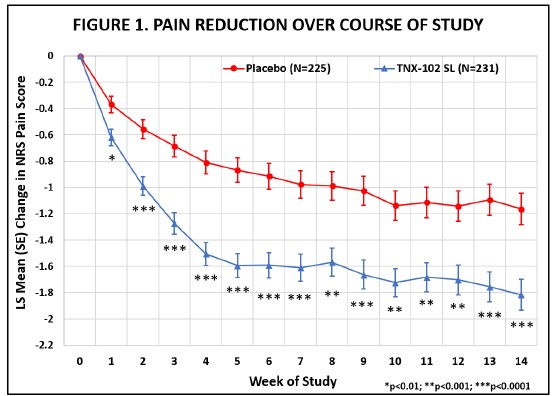
Abbreviations: LS = least squares; NRS = numerical rating scale; SE = standard error
The statistically significant improvement in pain is further substantiated when diary pain was analyzed by another standard statistical approach, a 30 percent responder analysis, with 45.9% on active and 27.1% on placebo having a 30 percent or greater reduction in pain (Pearson Chi-Squared Test; difference in proportions [95% CI]: 18.8% [10.1%, 27.4%]; nominal p<0.001).
TNX-102 SL showed statistical significance (p≤0.001) on all six pre-specified key secondary efficacy outcome measures (Table 1).
Consistent with the proposed mechanism that TNX-102 SL acts in fibromyalgia through improving sleep quality, TNX-102 SL showed statistically significant improvement of sleep by two main measures. For the daily diary sleep quality ratings, improvement in sleep quality for TNX-102 SL (-1.8 [0.12] units) was significantly greater than that of placebo (-1.2 [0.12] units; LS mean [SE] difference from placebo: -0.6 [0.17] units; p<0.001). For the PROMIS Sleep Disturbance instrument, TNX-102 SL also demonstrated significantly greater improvement over placebo on T-scores (LS mean [SE] difference from placebo: -4.2 [0.79] units; p<0.001). Fatigue is another cardinal symptom of fibromyalgia and has a major impact on quality of life. TNX-102 SL showed significant improvement over placebo on the PROMIS Fatigue instrument T-scores (-3.0 [0.77] units; p<0.001).
The Fibromyalgia Impact Questionnaire – Revised (FIQ-R) is a 21-item self-rated instrument that assesses level of function, overall impact, and symptoms due to fibromyalgia, and the sympoms and function domains were key secondary endpoints in RESILENT. At Week 14 on the FIQ-R Symptoms domain, there was significantly greater improvement with TNX-102 SL than with placebo (LS mean [SE] difference from placebo: -7.7 [1.62], p<0.001). Similarly, TNX-102 SL resulted in greater improvement on FIQ-R Function (LS mean [SE] difference from placebo: -5.4 [1.66], p=0.001). Although not a key secondary efficacy endpoint, TNX-102 SL also separated from placebo on the FIQ-R Impact domain (nominal p=0.001). These results, along with the robust effects on improving sleep and fatigue, suggests broad symptomatic coverage of the syndrome of fibromyalgia.
Safety Results of the Phase 3 RESILIENT Study
In the RESILIENT study, TNX-102 SL was well tolerated and consistent with prior trials, with no new safety signals observed. Among participants randomized to the TNX-102 SL and placebo arms, 81.0% and 79.2%, respectively, completed the 14-week dosing period. As expected based on prior TNX-102 SL studies, administration site reactions were the most commonly reported adverse events and were higher in the TNX-102 SL treatment group (Table 2). Hypoaesthesia oral and paraesthesia oral, or tongue and mouth numbness or tingling, product taste abnormal (typically a bitter aftertaste upon dosing), and tongue discomfort were local effects nearly always temporally related to dose administration and transiently expressed (<60 minutes) in most occurrences. The only treatment-emergent adverse events that occurred at a rate of 3.0% or greater in either arm were these four oral adverse events, along with COVID-19, somnolence, and headache (Table 2). Adverse events resulted in premature study discontinuation in 6.1% of those who received TNX-102 SL compared with 3.5% of placebo recipients. There were a total of seven serious adverse events in five patients, five of which were experienced by three patients in the placebo arm, and two of which were in the TNX-102 SL arm. Of the two in the TNX-102 SL arm, one was renal cancer, deemed unrelated to study drug, and the other was acute pancreatitis with onset 14 days after dosing was completed and reported as possibly related to study drug.
Table 2. Treatment-Emergent Adverse Events at a Rate of 3% or Greater in Either Treatment Arm
| TNX-102 SL (N=231) | Placebo (N=226) | Total (N=457)* | ||||||||||
| Administration Site Reactions | N | % | N | % | N | % | ||||||
| Hypoaethesia oral | 55 | 23.8% | 1 | 0.4% | 56 | 12.3% | ||||||
| Product taste abnormal | 27 | 11.7% | 2 | 0.9% | 29 | 6.3% | ||||||
| Paraesthesia oral | 16 | 6.9% | 2 | 0.9% | 18 | 3.9% | ||||||
| Tongue discomfort | 16 | 6.9% | 0 | 0.0% | 16 | 3.5% | ||||||
| Systemic | ||||||||||||
| Adverse Events | N | % | N | % | N | % | ||||||
| COVID-19 | 10 | 4.3% | 7 | 3.1% | 17 | 3.7% | ||||||
| Somnolence | 7 | 3.0% | 3 | 1.3% | 10 | 2.2% | ||||||
| Headache | 7 | 3.0% | 4 | 1.8% | 11 | 2.4% | ||||||
*Safety Population
The Changes in Sexual Functioning Questionnaire short form (CSFQ-14) served as a safety measure for assessing potential adverse effects on sexual functioning. In females, the total score on the CSFQ-14 at Week 14 improved (indicating better sexual functioning) in the TNX-102 SL group compared with placebo (nominal p=0.010 by analysis of covariance). This potentially indicates an important tolerability advantage over pharmacotherapeutics which potently inhibit reuptake of serotonin. The low percentage of males in the safety population (<5%) did not allow meaningful analysis of the CSFQ-14 data.
About the Phase 3 RESILIENT Study
The RESILIENT study is a double-blind, randomized, placebo-controlled trial designed to evaluate the efficacy and safety of TNX-102 SL (cyclobenzaprine HCl sublingual tablets) in the management of fibromyalgia. The two-arm trial randomized 457 participants in the U.S. across 33 sites. The first two weeks of treatment consist of a run-in period in which participants start on TNX-102 SL 2.8 mg (1 tablet) or placebo. Thereafter, all participants increase their dose to TNX-102 SL 5.6 mg (2 x 2.8 mg tablets) or two placebo tablets for the remaining 12 weeks. The primary endpoint is the daily diary pain severity score change (TNX-102 SL 5.6 mg vs. placebo) from baseline to Week 14 (using the weekly averages of the daily numerical rating scale scores), analyzed by mixed model repeated measures with multiple imputation.
For more information, see ClinicalTrials.gov Identifier: NCT05273749.
About Fibromyalgia
Fibromyalgia is a chronic pain disorder that is understood to result from amplified sensory and pain signaling within the central nervous system. Fibromyalgia afflicts an estimated 6 million to 12 million adults in the U.S., the majority of whom are women. Symptoms of fibromyalgia include chronic widespread pain, nonrestorative sleep, fatigue, and morning stiffness. Other associated symptoms include cognitive dysfunction and mood disturbances, including anxiety and depression. Individuals suffering from fibromyalgia struggle with their daily activities, have impaired quality of life, and frequently are disabled. Physicians and patients report common dissatisfaction with currently marketed products.
About TNX-102 SL
TNX-102 SL is a patented sublingual tablet formulation of cyclobenzaprine hydrochloride which provides rapid transmucosal absorption and reduced production of a long half-life active metabolite, norcyclobenzaprine, due to bypass of first-pass hepatic metabolism. As a multifunctional agent with potent binding and antagonist activities at the 5-HT2A-serotonergic, α1-adrenergic, H1-histaminergic, and M1-muscarinic cholinergic receptors, TNX-102 SL is in development as a daily bedtime treatment for fibromyalgia, fibromyalgia-type Long COVID (formally known as post-acute sequelae of COVID-19 [PASC]), alcohol use disorder and agitation in Alzheimer’s disease. Dr. Harvey Moldofsky, Professor Emeritus of Psychiatry and Medicine at the University of Toronto, founding Director of the University of Toronto Center for Sleep and Chronobiology, first recognized the central role of non-restorative sleep in the pathogenesis of fibromyalgia1,2. Our program is based on the subsequent pioneering work of Dr. Iredell W. Iglehart III, who recognized that a sleep-focused cyclobenzaprine treatment protocol had the potential to target non-restorative sleep and lead to improvement of fibromyalgia at the syndromal level3. Teams led by Giorgio Reiner at APR Applied Pharma Research S.A., a wholly-owned subsidiary of Relief Therapeutics Holding AG, and Professor Marino Nebuloni and Patrizia Colombo at Redox Analytical Science Srl invented and developed these underlying technologies in collaboration with Tonix. The United States Patent and Trademark Office (USPTO) issued United States Patent No. 9636408 in May 2017, Patent No. 9956188 in May 2018, Patent No. 10117936 in November 2018, Patent No. 10,357,465 in July 2019, and Patent No. 10736859 in August 2020. The Protectic™ protective eutectic and Angstro-Technology™ formulation claimed in the patent are important elements of Tonix’s proprietary TNX-102 SL composition. These patents are expected to provide TNX-102 SL, upon NDA approval, with U.S. market exclusivity until 2034/2035. In addition, Tonix has pending but not issued U.S. patent applications directed to the transmucosal absorption of CBP-HCl, with U.S. market exclusivity expected until 2033, for treating major depressive disorder in fibromyalgia, with U.S. market exclusivity expected until 2032, and for treating pain in fibromyalgia with U.S. market exclusivity expected until 2041.
1Moldofsky
H et al, Psychosom Med 1975;37:341-51.
2Moldofsky H and Scarisbrick P. Psychosom Med 1976;38:35-44.
3Iglehart IW. 2003; US Patent 6,541,523.
Tonix Pharmaceuticals Holding Corp.*
Tonix is a biopharmaceutical company focused on commercializing, developing, discovering and licensing therapeutics to treat and prevent human disease and alleviate suffering. Tonix Medicines, our commercial subsidiary, markets Zembrace® SymTouch® (sumatriptan injection) 3 mg and Tosymra® (sumatriptan nasal spray) 10 mg under a transition services agreement with Upsher-Smith Laboratories, LLC from whom the products were acquired on June 30, 2023. Zembrace SymTouch and Tosymra are each indicated for the treatment of acute migraine with or without aura in adults. Tonix’s development portfolio is composed of central nervous system (CNS), rare disease, immunology and infectious disease product candidates. Tonix’s CNS development portfolio includes both small molecules and biologics to treat pain, neurologic, psychiatric and addiction conditions. Tonix’s lead development CNS candidate, TNX-102 SL (cyclobenzaprine HCl sublingual tablet), has completed two positive Phase 3 studies for the management of fibromyalgia. Tonix intends to meet with the FDA and submit an NDA for the approval of TNX-102 SL for the management of fibromyalgia in the second half of 2024. TNX-102 SL is also being developed to treat fibromyalgia-type Long COVID, a chronic post-acute COVID-19 condition, and topline results were reported in the third quarter of 2023. TNX-1900 (intranasal potentiated oxytocin) is being studied in binge eating disorder, pediatric obesity, bone health in autism and social anxiety disorder by academic collaborators under investigator-initiated INDs. TNX-1300 (cocaine esterase) is a biologic designed to treat cocaine intoxication and has been granted Breakthrough Therapy designation by the FDA. A Phase 2 study of TNX-1300 is expected to be initiated in the first quarter of 2024 Tonix’s rare disease development portfolio includes TNX-2900 (intranasal potentiated oxytocin) for the treatment of Prader-Willi syndrome. TNX-2900 has been granted Orphan Drug designation by the FDA. Tonix’s immunology development portfolio includes biologics to address organ transplant rejection, autoimmunity and cancer, including TNX-1500, which is a humanized monoclonal antibody targeting CD40-ligand (CD40L or CD154) being developed for the prevention of allograft rejection and for the treatment of autoimmune diseases. A Phase 1 study of TNX-1500 was initiated in the third quarter of 2023. Tonix’s infectious disease pipeline includes TNX-801, a vaccine in development to prevent smallpox and mpox. TNX-801 also serves as the live virus vaccine platform or recombinant pox vaccine platform for other infectious diseases, including TNX-1800, in development as a vaccine to protect against COVID-19. During the fourth quarter of 2023, TNX-1800 was selected by the U.S. National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) Project NextGen for inclusion in Phase 1 clinical trials. The infectious disease development portfolio also includes TNX-3900 and TNX-4000, which are classes of broad-spectrum small molecule oral antivirals.
*Tonix’s product development candidates are investigational new drugs or biologics and have not been approved for any indication.
Zembrace SymTouch and Tosymra are registered trademarks of Tonix Medicines. All other marks are property of their respective owners.
This press release and further information about Tonix can be found at www.tonixpharma.com.
About Redox - Analytical Science Srl
Redox is an independent CRO company headquartered in Monza- Italy with R&D activities and customer analytical support to pharmaceutical companies for more than 30 years. From more than 25 years the analytical activities have been certified by national and international agencies (European Medicines Agency, the Italian Medicines Agency (AIFA), FDA, and etc). One of the main activitie is the development of new drug products in order to improve the pharmaceutical actions and at the same time improve the stability and reducing the cost of the new drug substance. Several unique and sophisticated analytical techniques and equipment are used in support to research and development strategies with the focus to reach the best and effective pharmaceutical formulation in a short time frame. More than 30 professional people are dedicated to our efforts and many projects are ongoing in collaboration with the pharmaceutical industry as well as with Italian and international Universities.
Further information about Redox can be found at www.labredox.com.
About APR Applied Pharma Research S.A., a wholly-owned subsidiary of Relief Therapeutics Holding AG
Relief Therapeutics is a commercial-stage biopharmaceutical company committed to advancing treatment paradigms and delivering improvements in efficacy, safety, and convenience to benefit the lives of patients living with select specialty and rare diseases. Relief Therapeutics' portfolio offers a balanced mix of marketed, revenue-generating products, our proprietary, globally patented Physiomimic™ and TEHCLO™ platform technologies and a targeted clinical development pipeline consisting of risk-mitigated assets focused in three core therapeutic areas: rare metabolic disorders, rare skin diseases and rare respiratory diseases. In addition, Relief Therapeutics is commercializing several legacy products via licensing and distribution partners. Relief Therapeutics' mission is to provide therapeutic relief to those suffering from rare diseases and is being advanced by an international team of well-established, experienced biopharma industry leaders with extensive research, development and rare disease expertise. Relief Therapeutics is headquartered in Geneva, with additional offices in Balerna, Switzerland, Offenbach am Main, Germany and Monza, Italy. Relief Therapeutics is listed on the SIX Swiss Exchange under the symbol RLF.
Further information about APR can be found at www.relieftherapeutics.com or by following Relief Therapeutics on LinkedIn and Twitter.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others. These forward-looking statements are based on Tonix's current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, risks related to the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. Tonix does not undertake an obligation to update or revise any forward-looking statement. Investors should read the risk factors set forth in the Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports filed with the SEC on or after the date thereof. All of Tonix's forward-looking statements are expressly qualified by all such risk factors and other cautionary statements. The information set forth herein speaks only as of the date thereof.
Investor Contact
Jessica Morris
Tonix Pharmaceuticals
investor.relations@tonixpharma.com
(862) 904-8182
Peter Vozzo
ICR Westwicke
peter.vozzo@westwicke.com
(443) 213-0505
Media Contact
Ben Shannon
ICR Westwicke
ben.shannon@westwicke.com
443-213-0495
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

© 2023 Tonix Pharmaceuticals Holding Corp. Tonix Pharmaceuticals TNX - 102 SL (sublingual cyclobenzaprine) Fibromyalgia Phase 3 RESILIENT Study ( TNX - CY - F307 ) Topline December 20, 2023 Version P0512 December 18 , 2023 (Doc 1357 )

2 © 2023 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.
© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL* Cyclobenzaprine HCl ( Protectic ® ) Fibromyalgia Status: Two potential pivotal Phase 3 studies completed • P ositive Phase 3 study (RELIEF) completed • Second Phase 3 study (RALLY) missed primary endpoint • Positive confirmatory Phase 3 study (RESILIENT) completed Next Steps: Pre - NDA Meeting with FDA Fibromyalgia - Type Long COVID Status: Phase 2 • Phase 2 study (PREVAIL) completed • Topline results reported 3Q 2023 Next Steps: Meeting with FDA regarding primary endpoint Internally developed - Patents Issued – No royalties owed *TNX - 102 SL has not been approved for any indication. Non - opiate analgesic A unique, sublingual formulation of cyclobenzaprine designed for bedtime dosing with sublingual delivery and transmucosal absorption, bypassing 1 st pass metabolism Potent binding and antagonist activities at the serotonergic - 5 - HT 2A , adrenergic - α 1 , histaminergic - H 1 , and muscarinic - M 1 cholinergic receptors to facilitate restorative sleep Innovative and proprietary PROTECTIC ® Rapid drug exposure following once nightly sublingual administration Differentiators: Relative to Oral Cyclobenzaprine • Lower daytime exposure • Avoids first - pass metabolism • Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care • Potential for better tolerability while maintaining efficacy • Not scheduled, without recognized abuse potential Indications Most Recently Pursued Acute Stress Reaction/ Acute Stress Disorder • Phase 2 ready investigator - initiated study • Department of Defense funded/ UNC will perform study Next Steps: Expect to start Phase 2 in 1Q 2024 4 © 2023 Tonix Pharmaceuticals Holding Corp.

TNX - 102 SL: Phase 3 RESILIENT Study Design General s tudy c haracteristics: • Randomized, double - blind, multicenter, placebo - controlle d study in fibromyalgia • 33 U.S. sites enrolled 457 participants with fibromyalgia as defined by 2016 Revisions to the 2010/2011 FM Diagnostic C riteria ^ Primary Endpoint: • Change from baseline to Week 14 (TNX - 102 SL vs. placebo) in weekly averages of daily diary average pain severity score • Primary Endpoint, p - value = 0.00005 Placebo once - daily at bedtime TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two - week run - in at 2.8 mg dose at bedtime followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05273749 Study Title: A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT) Trial ID: TNY - CY - F307 (‘F307’) 14 weeks ^ Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016; 46(3):319 - 329.
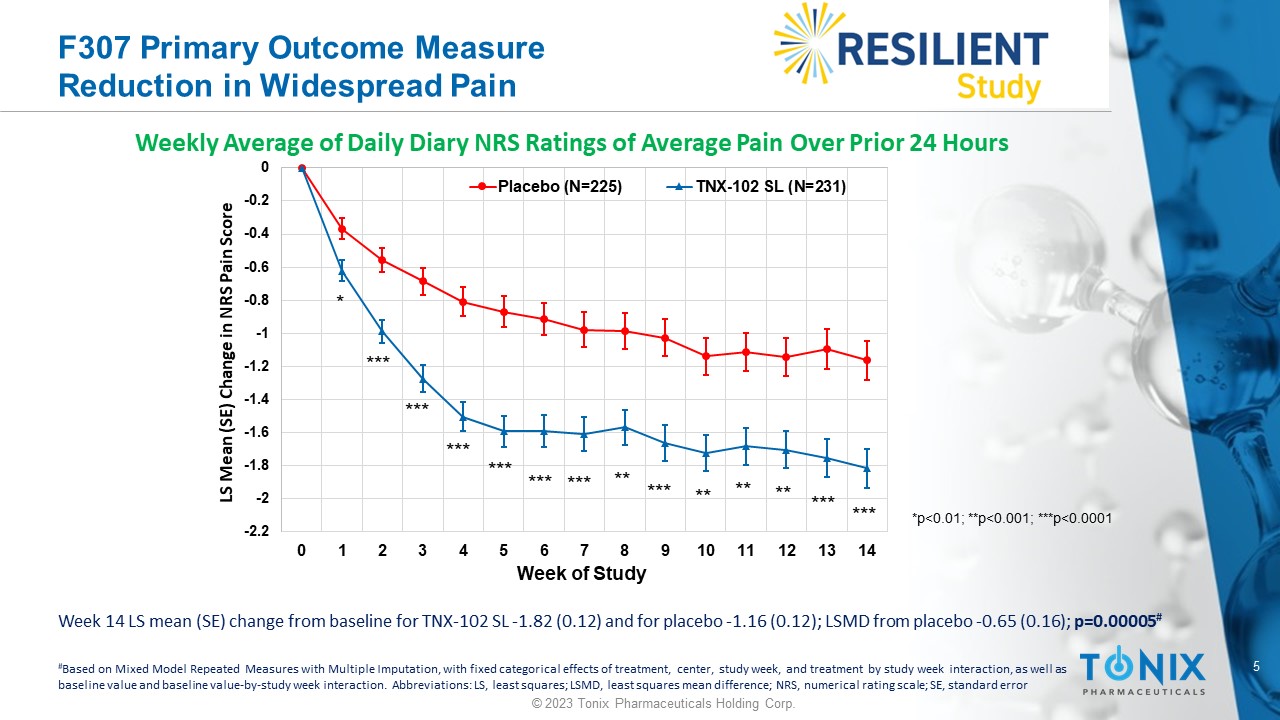
5 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Primary Outcome Measure Reduction in Widespread Pain Weekly Average of Daily Diary NRS Ratings of Average Pain Over Prior 24 Hours -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Pain Score Week of Study Placebo (N=225) TNX-102 SL (N=231) * *** *** ** ** ** *** *** *** *** *** *** *** ** *p<0.01; **p<0.001; ***p<0.0001 Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.82 (0.12) and for placebo - 1.16 (0.12); LSMD from placebo - 0.65 (0.1 6); p=0.00005 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. Abbreviations: LS, least squares; LSMD, least squares mean differe nce; NRS, numerical rating scale; SE, standard error 6 © 2023 Tonix Pharmaceuticals Holding Corp.
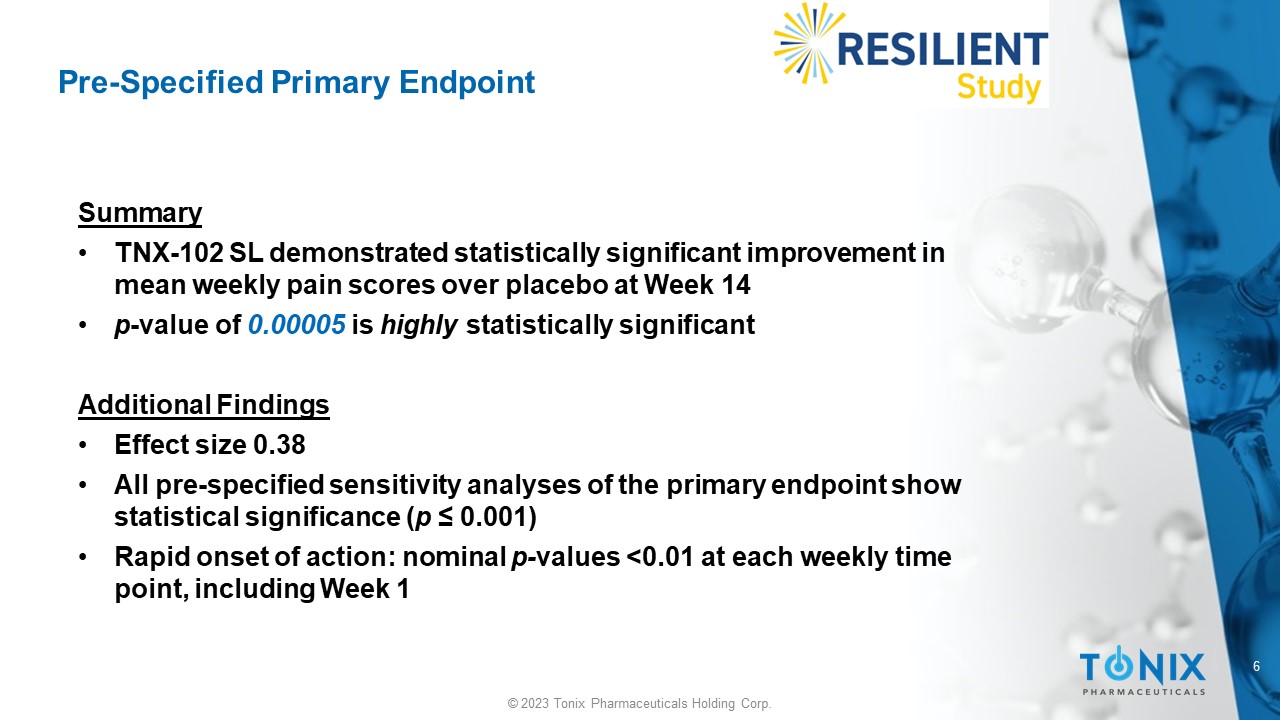
Pre - Specified Primary Endpoint Summary • TNX - 102 SL demonstrated statistically significant improvement in mean weekly pain scores over placebo at Week 14 • p - value of 0.00005 is highly statistically significant Additional Findings • Effect size 0.38 • All pre - specified sensitivity analyses of the primary endpoint show statistical significance ( p ≤ 0.001) • Rapid onset of action: nominal p - values <0.01 at each weekly time point, including Week 1 7 © 2023 Tonix Pharmaceuticals Holding Corp.
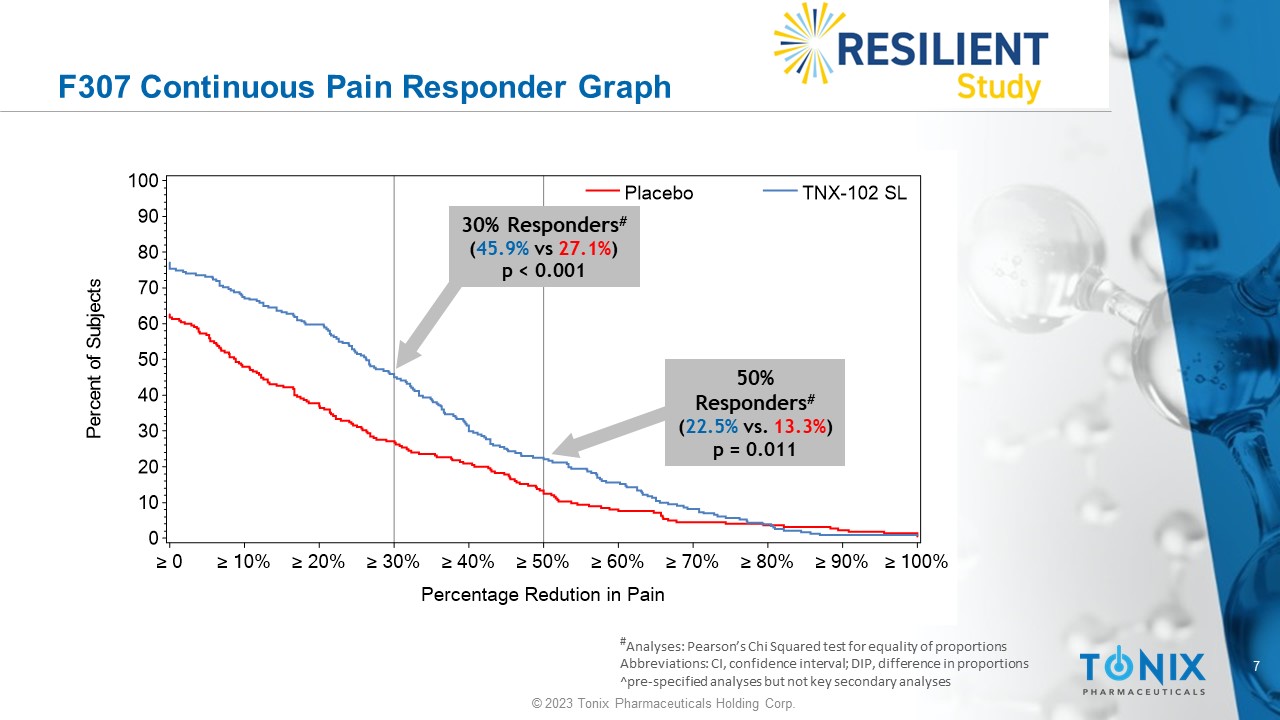
F307 Continuous Pain Responder Graph # Analyses: Pearson’s Chi Squared test for equality of proportions Abbreviations: CI, confidence interval; DIP, difference in proportions ^pre - specified analyses but not key secondary analyses P e r c e n t o f S u b j e c t s 0 10 20 30 40 50 60 70 80 90 100 Percentage Redution in Pain ≥ 0 ≥ 10% ≥ 20% ≥ 30% ≥ 40% ≥ 50% ≥ 60% ≥ 70% ≥ 80% ≥ 90% ≥ 100% Placebo TNX-102 SL 30% Responders # ( 45.9% vs 27.1% ) p < 0.001 50% Responders # ( 22.5% vs. 13.3% ) p = 0.011 8 © 2023 Tonix Pharmaceuticals Holding Corp.
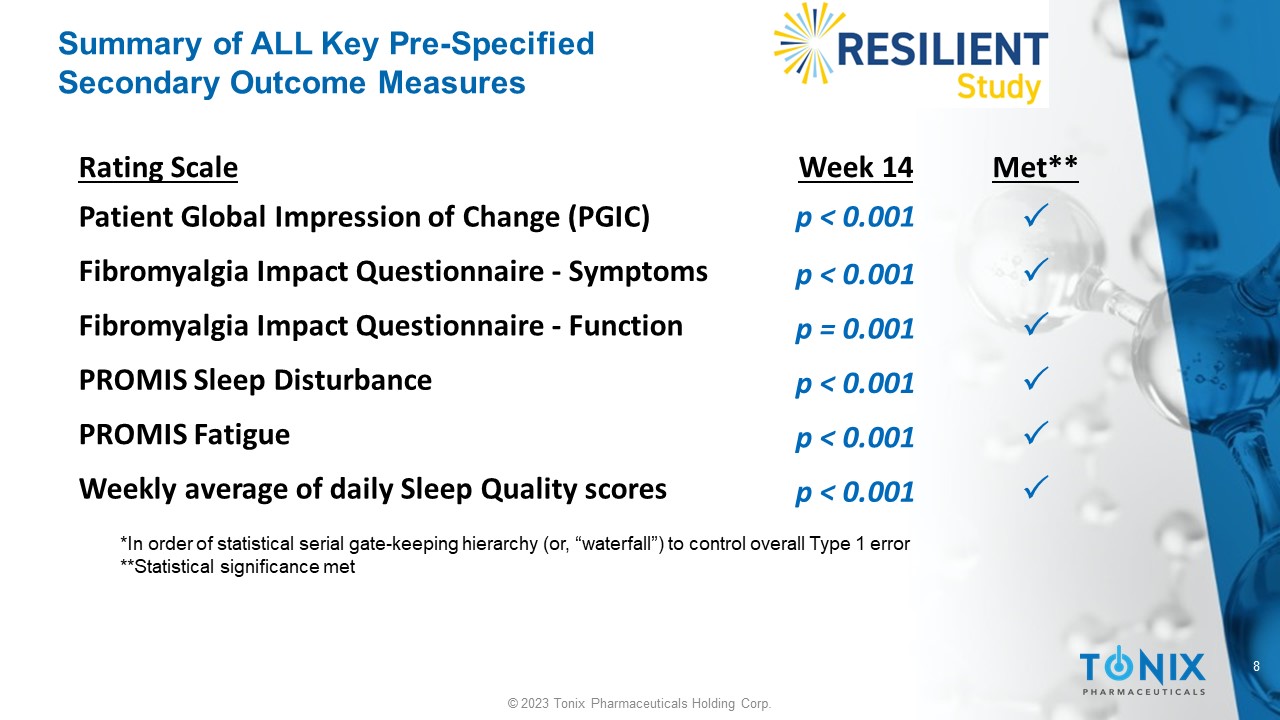
Summary of ALL Key Pre - Specified Secondary Outcome Measures *In order of statistical serial gate - keeping hierarchy (or, “waterfall”) to control overall Type 1 error **Statistical significance met Met** Week 14 Rating Scale p < 0.001 Patient Global Impression of Change (PGIC) p < 0.001 Fibromyalgia Impact Questionnaire - Symptoms p = 0.001 Fibromyalgia Impact Questionnaire - Function p < 0.001 PROMIS Sleep Disturbance p < 0.001 PROMIS Fatigue p < 0.001 Weekly average of daily Sleep Quality scores 9 © 2023 Tonix Pharmaceuticals Holding Corp.
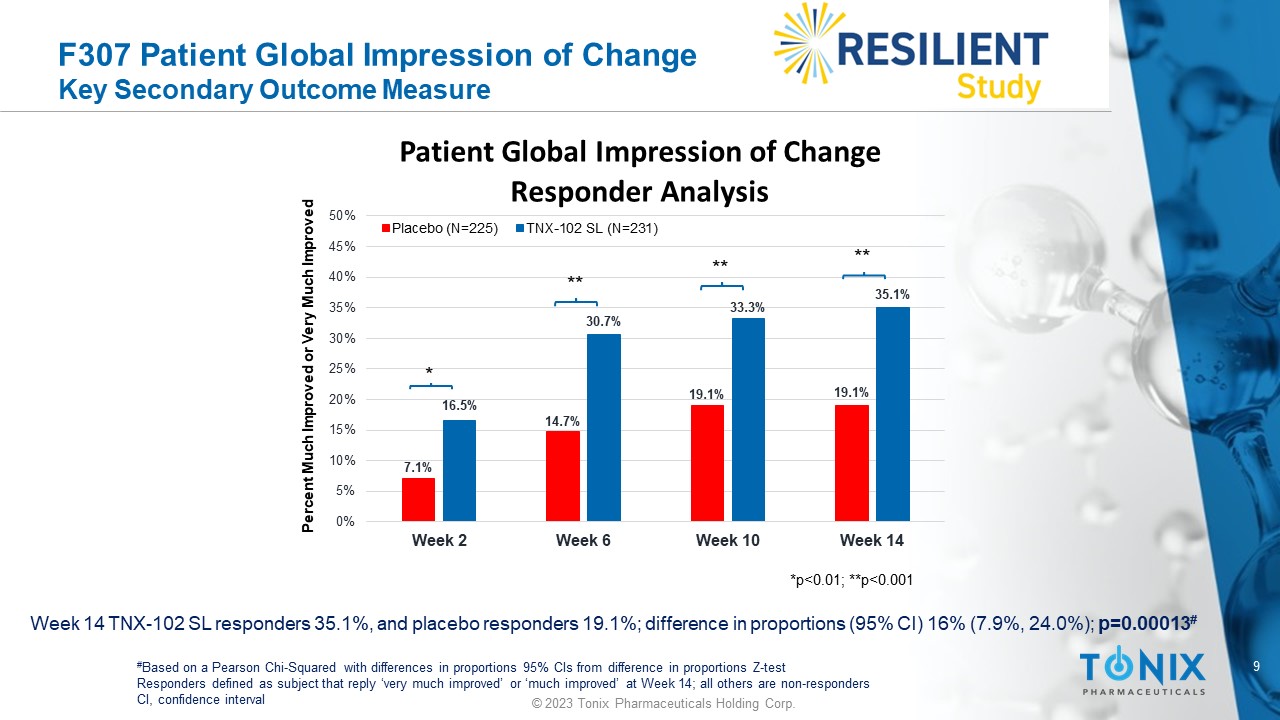
F307 Patient Global Impression of Change Key Secondary Outcome Measure Week 14 TNX - 102 SL responders 35.1%, and placebo responders 19.1%; difference in proportions (95% CI) 16% (7.9%, 24.0%); p=0.00013 # # Based on a Pearson Chi - Squared with differences in proportions 95% CIs from difference in proportions Z - test Responders defined as subject that reply ‘very much improved’ or ‘much improved’ at Week 14; all others are non - responders CI, confidence interval 7.1% 14.7% 19.1% 19.1% 16.5% 30.7% 33.3% 35.1% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% Week 2 Week 6 Week 10 Week 14 Patient Global Impression of Change Responder Analysis Placebo (N=225) TNX-102 SL (N=231) Percent Much Improved or Very Much Improved *p<0.01; **p<0.001 * ** ** ** 10 © 2023 Tonix Pharmaceuticals Holding Corp.
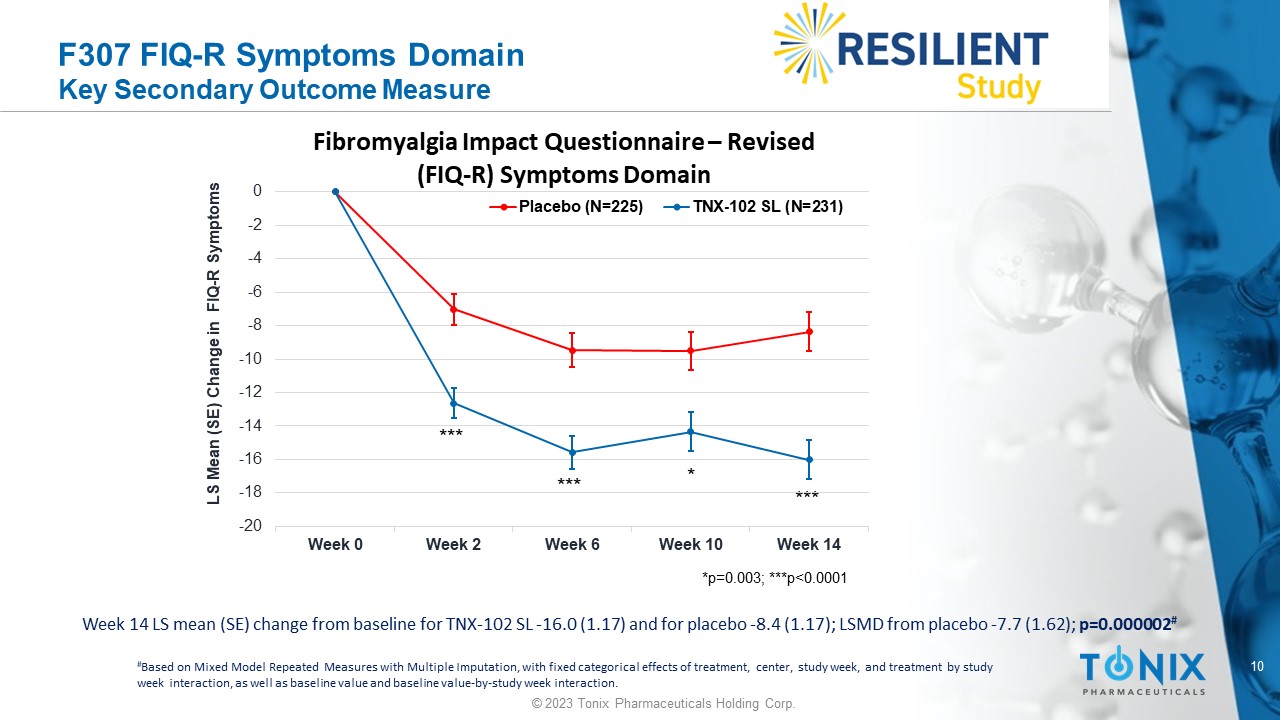
F307 FIQ - R Symptoms Domain Key Secondary Outcome Measure * *** *** *** Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 16.0 (1.17) and for placebo - 8.4 (1.17); LSMD from placebo - 7.7 (1.62) ; p=0.000002 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -20 -18 -16 -14 -12 -10 -8 -6 -4 -2 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in FIQ - R Symptoms Fibromyalgia Impact Questionnaire – Revised (FIQ - R) Symptoms Domain Placebo (N=225) TNX-102 SL (N=231) *p=0.003; ***p<0.0001 11 © 2023 Tonix Pharmaceuticals Holding Corp.
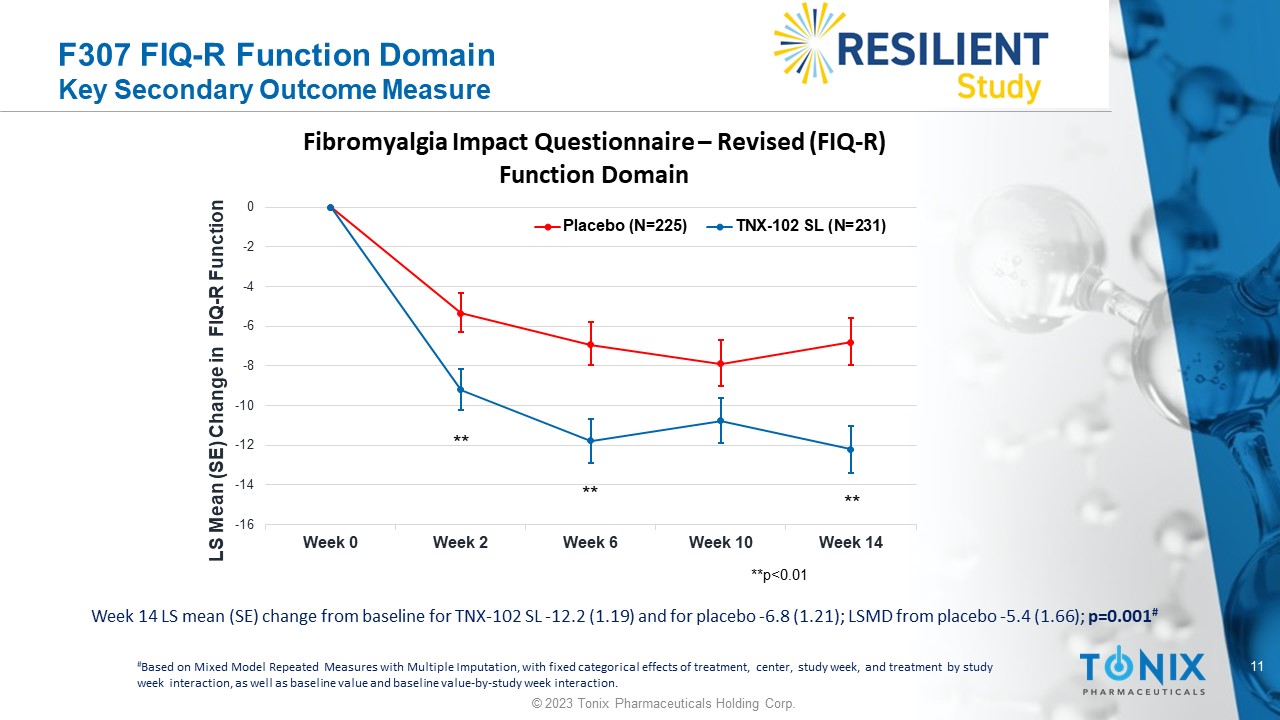
F307 FIQ - R Function Domain Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 12.2 (1.19) and for placebo - 6.8 (1.21); LSMD from placebo - 5.4 (1.66) ; p=0.001 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -16 -14 -12 -10 -8 -6 -4 -2 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in FIQ - R Function Fibromyalgia Impact Questionnaire – Revised (FIQ - R) Function Domain Placebo (N=225) TNX-102 SL (N=231) **p<0.01 ** ** ** 12 © 2023 Tonix Pharmaceuticals Holding Corp.
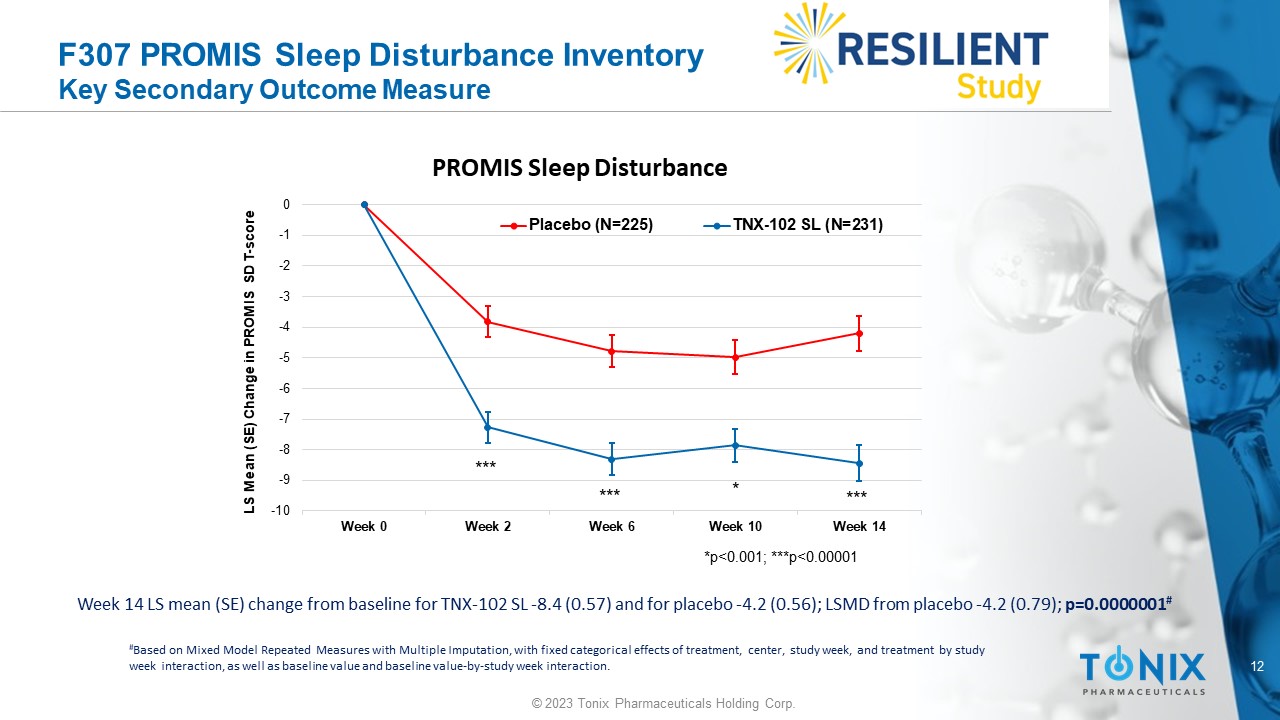
F307 PROMIS Sleep Disturbance Inventory Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 8.4 (0.57) and for placebo - 4.2 (0.56); LSMD from placebo - 4.2 (0.79); p=0.0000001 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in PROMIS SD T - score PROMIS Sleep Disturbance Placebo (N=225) TNX-102 SL (N=231) *p<0.001; ***p<0.00001 * *** *** *** 13 © 2023 Tonix Pharmaceuticals Holding Corp.
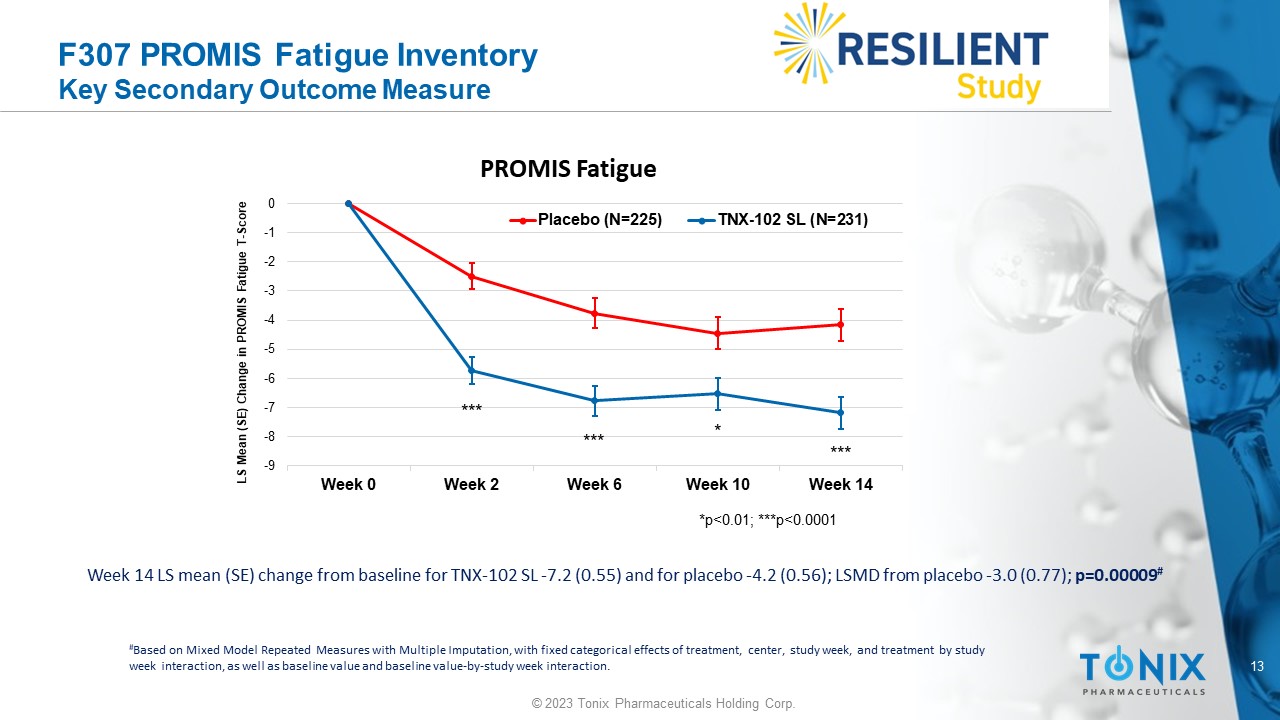
F307 PROMIS Fatigue Inventory Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 7.2 (0.55) and for placebo - 4.2 (0.56); LSMD from placebo - 3.0 (0.77); p=0.00009 # # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction. -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean (SE) Change in PROMIS Fatigue T - Score PROMIS Fatigue Placebo (N=225) TNX-102 SL (N=231) *p<0.01; ***p<0.0001 * *** *** *** 14 © 2023 Tonix Pharmaceuticals Holding Corp.
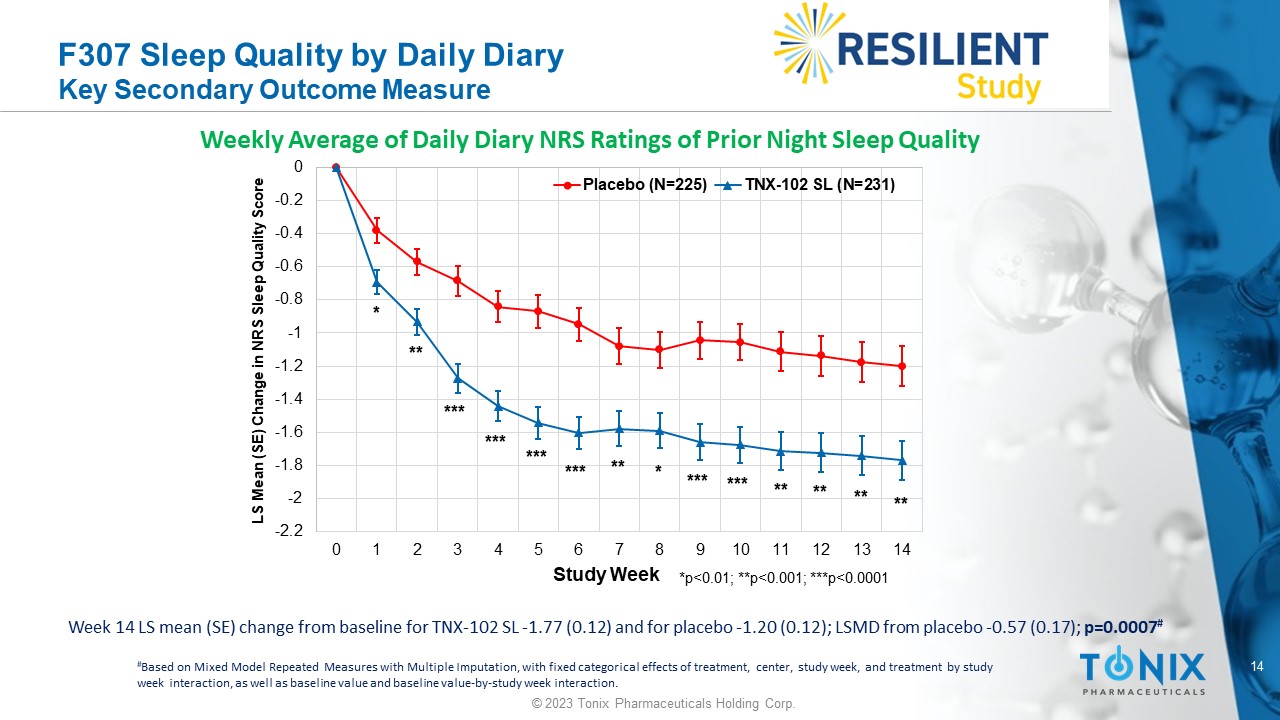
F307 Sleep Quality by Daily Diary Key Secondary Outcome Measure Week 14 LS mean (SE) change from baseline for TNX - 102 SL - 1.77 (0.12) and for placebo - 1.20 (0.12); LSMD from placebo - 0.57 (0.1 7); p=0.0007 # Weekly Average of Daily Diary NRS Ratings of Prior Night Sleep Quality -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean (SE) Change in NRS Sleep Quality Score Study Week Placebo (N=225) TNX-102 SL (N=231) *p<0.01; **p<0.001; ***p<0.0001 *** * ** ** * ** *** ** ** ** *** *** *** *** # Based on Mixed Model Repeated Measures with Multiple Imputation, with fixed categorical effects of treatment, center, study w eek , and treatment by study week interaction, as well as baseline value and baseline value - by - study week interaction.
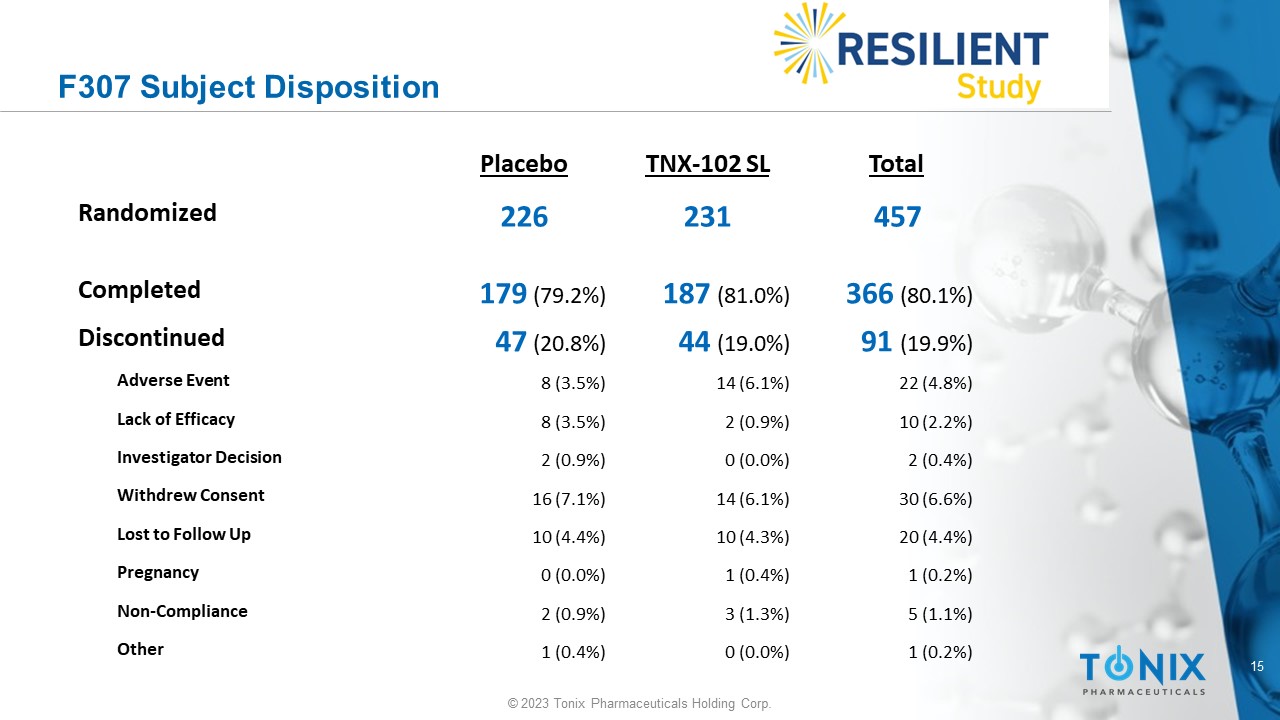
15 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Subject Disposition Total TNX - 102 SL Placebo 457 231 226 Randomized 366 (80.1%) 187 (81.0%) 179 (79.2%) Completed 91 (19.9%) 44 (19.0%) 47 (20.8%) Discontinued 22 (4.8%) 14 (6.1%) 8 (3.5%) Adverse Event 10 (2.2%) 2 (0.9%) 8 (3.5%) Lack of Efficacy 2 (0.4%) 0 (0.0%) 2 (0.9%) Investigator Decision 30 (6.6%) 14 (6.1%) 16 (7.1%) Withdrew Consent 20 (4.4%) 10 (4.3%) 10 (4.4%) Lost to Follow Up 1 (0.2%) 1 (0.4%) 0 (0.0%) Pregnancy 5 (1.1%) 3 (1.3%) 2 (0.9%) Non - Compliance 1 (0.2%) 0 (0.0%) 1 (0.4%) Other 16 © 2023 Tonix Pharmaceuticals Holding Corp.

F307 Safety Summary Among participants randomized to TNX - 102 SL and to placebo, 81.0% and 79.6%, respectively, completed the study TNX - 102 SL was generally well tolerated with an adverse event (AE) profile comparable to prior fibromyalgia studies • No new safety signals were observed • AE - related study discontinuations occurred in 6.1% and 3.6% of patients in the TNX - 102 SL and placebo groups, respectively • Events rated as mild or moderate made up 97.2% of AEs on placebo and 99.1% on TNX - 102 SL • As observed in prior studies with TNX - 102 SL, oral administration site AEs were higher in TNX - 102 SL than placebo, 42.9% and 10. 2%, respectively ‒ Most common oral AEs were oral hypoaesthesia, product taste abnormal, oral paraesthesia, and tongue discomfort (see table on nex t slide) ‒ Nearly all of these common oral AEs were temporally related to dosing and lasted <60 minutes • Serious Adverse Events (SAEs) ‒ Three placebo participants experienced an SAE: ▪ 1. Pneumonia, 2. Muscular weakness, and 3. Hypertension/Angina/Coronary Artery Disease ‒ Two TNX - 102 SL participants experienced an SAE ▪ 1. Renal carcinoma deemed not related to study drug ▪ 2. Acute pancreatitis with onset 14 days after completion of treatment phase, deemed ‘possibly related’* to study drug ⁃ Outcome: ‘Recovered/Resolved’ ⁃ *Note: participant was non - compliant with end of treatment study visits, and the last dose before onset of SAE was not known at the time that relationship with study drug was assessed by Investigator and Sponsor 17 © 2023 Tonix Pharmaceuticals Holding Corp.
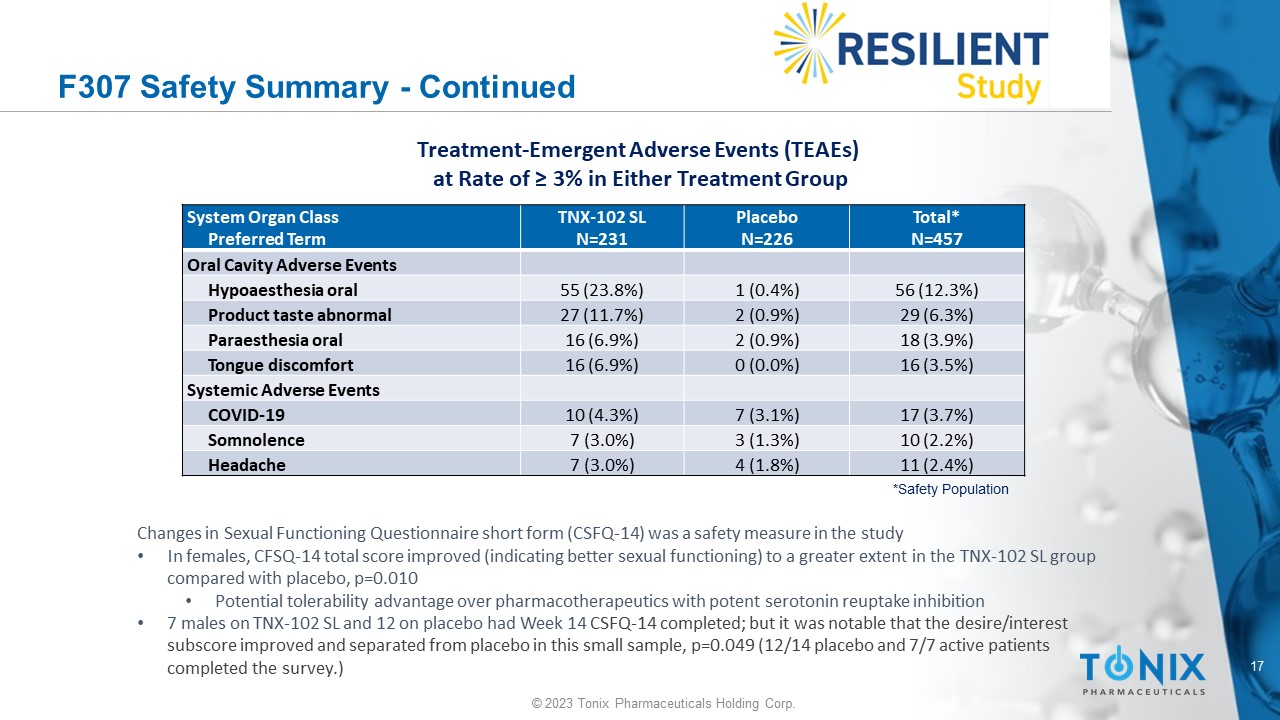
F307 Safety Summary - Continued Total* N=457 Placebo N=226 TNX - 102 SL N=231 System Organ Class Preferred Term Oral Cavity Adverse Events 56 (12.3%) 1 (0.4%) 55 (23.8%) Hypoaesthesia oral 29 (6.3%) 2 (0.9%) 27 (11.7%) Product taste abnormal 18 (3.9%) 2 (0.9%) 16 (6.9%) Paraesthesia oral 16 (3.5%) 0 (0.0%) 16 (6.9%) Tongue discomfort Systemic Adverse Events 17 (3.7%) 7 (3.1%) 10 (4.3%) COVID - 19 10 (2.2%) 3 (1.3%) 7 (3.0%) Somnolence 11 (2.4%) 4 (1.8%) 7 (3.0%) Headache *Safety Population Treatment - Emergent Adverse Events (TEAEs) at Rate of ≥ 3% in Either Treatment Group Changes in Sexual Functioning Questionnaire short form (CSFQ - 14) was a safety measure in the study • In females, CFSQ - 14 total score improved (indicating better sexual functioning) to a greater extent in the TNX - 102 SL group compared with placebo, p=0.010 • Potential tolerability advantage over pharmacotherapeutics with potent serotonin reuptake inhibition • 7 males on TNX - 102 SL and 12 on placebo had Week 14 CSFQ - 14 completed; but it was notable that the desire/interest subscore improved and separated from placebo in this small sample, p=0.049 (12/14 placebo and 7/7 active patients completed the survey.)
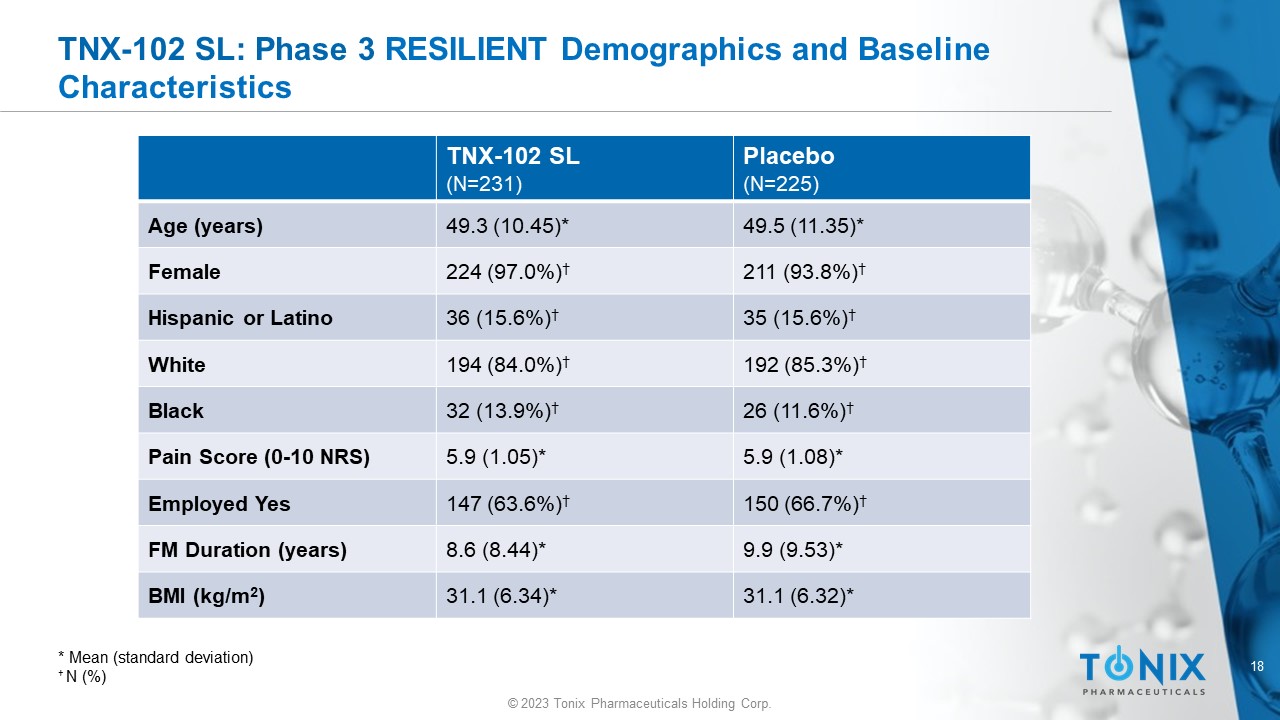
18 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL: Phase 3 RESILIENT Demographics and Baseline Characteristics Placebo (N=225) TNX - 102 SL (N=231) 49.5 (11.35)* 49.3 (10.45)* Age (years) 211 (93.8%) † 224 (97.0%) † Female 35 (15.6%) † 36 (15.6%) † Hispanic or Latino 192 (85.3%) † 194 (84.0%) † White 26 (11.6%) † 32 (13.9%) † Black 5.9 (1.08)* 5.9 (1.05)* Pain Score (0 - 10 NRS) 150 (66.7%) † 147 (63.6%) † Employed Yes 9.9 (9.53)* 8.6 (8.44)* FM Duration (years) 31.1 (6.32)* 31.1 (6.34)* BMI (kg/m 2 ) * Mean ( s tandard deviation) † N (%)
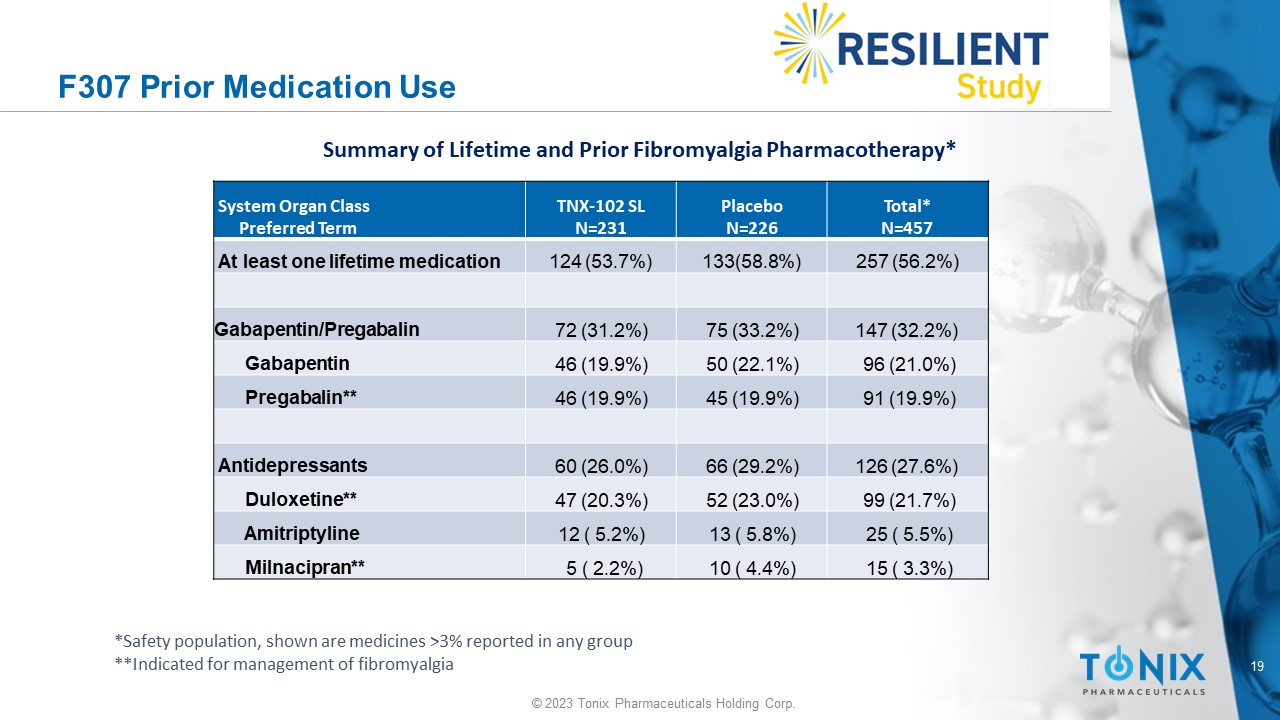
19 © 2023 Tonix Pharmaceuticals Holding Corp. F307 Prior Medication Use Total* N=457 Placebo N=226 TNX - 102 SL N=231 System Organ Class Preferred Term 257 (56.2%) 133(58.8%) 124 (53.7%) At least one lifetime medication 147 (32.2%) 75 (33.2%) 72 (31.2%) Gabapentin/Pregabalin 96 (21.0%) 50 (22.1%) 46 (19.9%) Gabapentin 91 (19.9%) 45 (19.9%) 46 (19.9%) Pregabalin** 126 (27.6%) 66 (29.2%) 60 (26.0%) Antidepressants 99 (21.7%) 52 (23.0%) 47 (20.3%) Duloxetine** 25 ( 5.5%) 13 ( 5.8%) 12 ( 5.2%) Amitriptyline 15 ( 3.3%) 10 ( 4.4%) 5 ( 2.2%) Milnacipran** Summary of Lifetime and Prior Fibromyalgia Pharmacotherapy* *Safety population, shown are medicines >3% reported in any group **Indicated for management of fibromyalgia 20 © 2023 Tonix Pharmaceuticals Holding Corp.
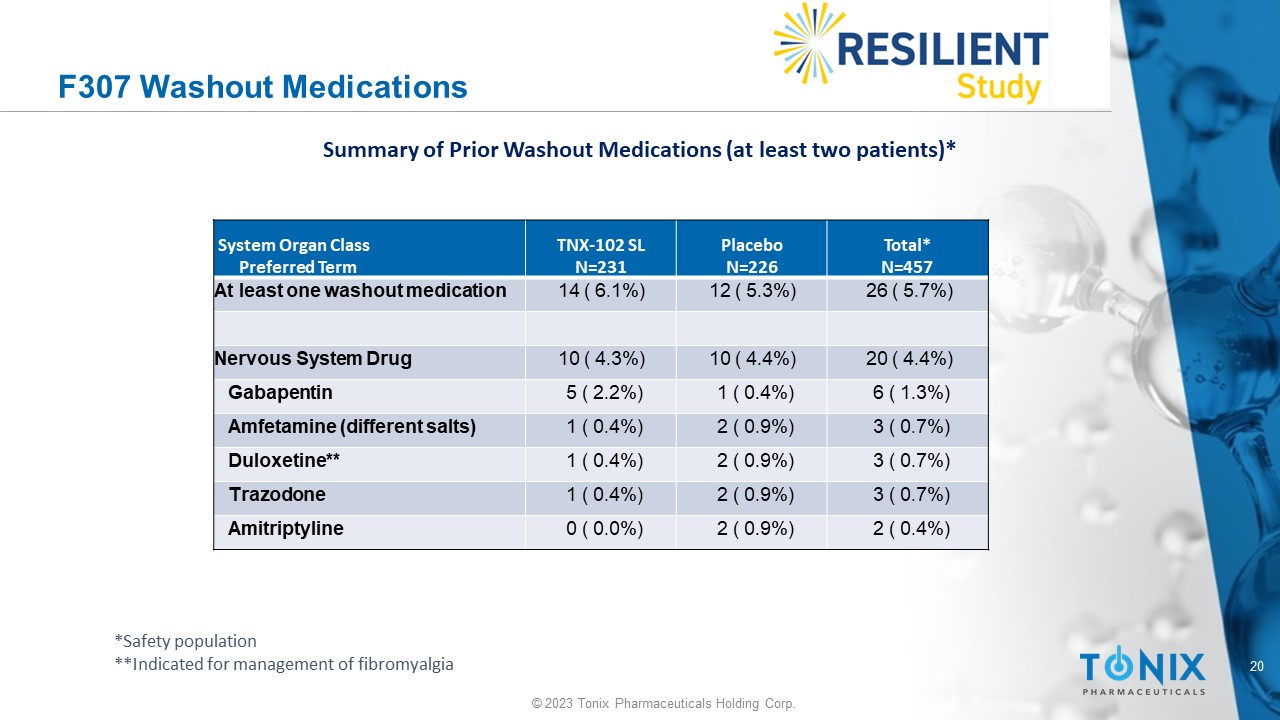
F307 Washout Medications Total* N=457 Placebo N=226 TNX - 102 SL N=231 System Organ Class Preferred Term 26 ( 5.7%) 12 ( 5.3%) 14 ( 6.1%) At least one washout medication 20 ( 4.4%) 10 ( 4.4%) 10 ( 4.3%) Nervous System Drug 6 ( 1.3%) 1 ( 0.4%) 5 ( 2.2%) Gabapentin 3 ( 0.7%) 2 ( 0.9%) 1 ( 0.4%) Amfetamine (different salts) 3 ( 0.7%) 2 ( 0.9%) 1 ( 0.4%) Duloxetine** 3 ( 0.7%) 2 ( 0.9%) 1 ( 0.4%) Trazodone 2 ( 0.4%) 2 ( 0.9%) 0 ( 0.0%) Amitriptyline Summary of Prior Washout Medications (at least two patients)* *Safety population **Indicated for management of fibromyalgia 21 © 2023 Tonix Pharmaceuticals Holding Corp.

F307 Characteristics of Study Population Pain Scores • Patients are asked to record “their average pain” for each day ‒ ‘Average’ pain for the day will almost always be lower than ‘worst’ pain for a patient’s day • Baseline pain for randomization a) A mean pain intensity score ≥4 and ≤9 on the 11 - point (0 - 10) NRS scale for the 7 days immediately preceding Visit 2, and b) No more than 2 individual days with a score <4 on the 7 days immediately preceding Visit 2, and c) No score of 10 on any of the 7 days immediately preceding Visit 2, and d) Pain scores recorded on at least 5 out of the 7 days immediately preceding Visit 2 • Mean Pain score for Baseline (BL) for the RESILIENT study was 5.9 ‒ Using the same method, BL for F304 (RELIEF) was 6.1 and BL for F306 (RALLY) was 6.0 • Breakthrough pain ‒ No explicit rescue algorithm ‒ 10 participants took an opiate during the study (6 on TNX - 102 SL and 4 on placebo)

22 © 2023 Tonix Pharmaceuticals Holding Corp. PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia Cyclobenzaprine Protectic ® Sublingual Tablets Fibromyalgia (FM) is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS • Afflicts an estimated 6 - 12 million adults in the U.S., the majority of whom are women 1 • Symptoms include chronic widespread pain, nonrestorative sleep, fatigue, and cognitive dysfunction • Patients struggle with daily activities, have impaired quality of life, and frequently are disabled • Physicians and patients report common dissatisfaction with currently marketed products Market Entry: Fibromyalgia Additional Indications: Long COVID, Acute Stress Disorder (ASD), PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Positive Phase 3 study RELIEF completed 2 Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT positive, p - value = 0.00005 Next Steps: Pre - NDA meeting with FDA *TNX - 102 SL has not been approved for any indication. 1 American Chronic Pain Association (www.theacpa.org, 2019) 2 Lederman et al., (2023) Arthritis Care & Research "Efficacy and Safety of TNX - 102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia: Results From the RELIEF Trial ", doi : 10.1002/acr.25142. Epub ahead of print. PMID: 37165930. When the check engine light malfunctions, the light is on even though the car is not malfunctioning © 2023 Tonix Pharmaceuticals Holding Corp.

THANK YOU Confidential

24 © 2023 Tonix Pharmaceuticals Holding Corp. Overview of TNX - 102 SL Scientific Rationale for Protectic ® Formulation • Engenders unique pharmacokinetic and pharmacodynamic properties that emphasize sleep properties of cyclobenzaprine while minimizing undesirable properties • Potential therapeutic value in a constellation of disorders where sleep disturbances are: • Co - morbid • Involved in the onset, progression and severity of the disease Protectic ® proprietary formulation of cyclobenzaprine that supports sublingual administration 25 © 2023 Tonix Pharmaceuticals Holding Corp.
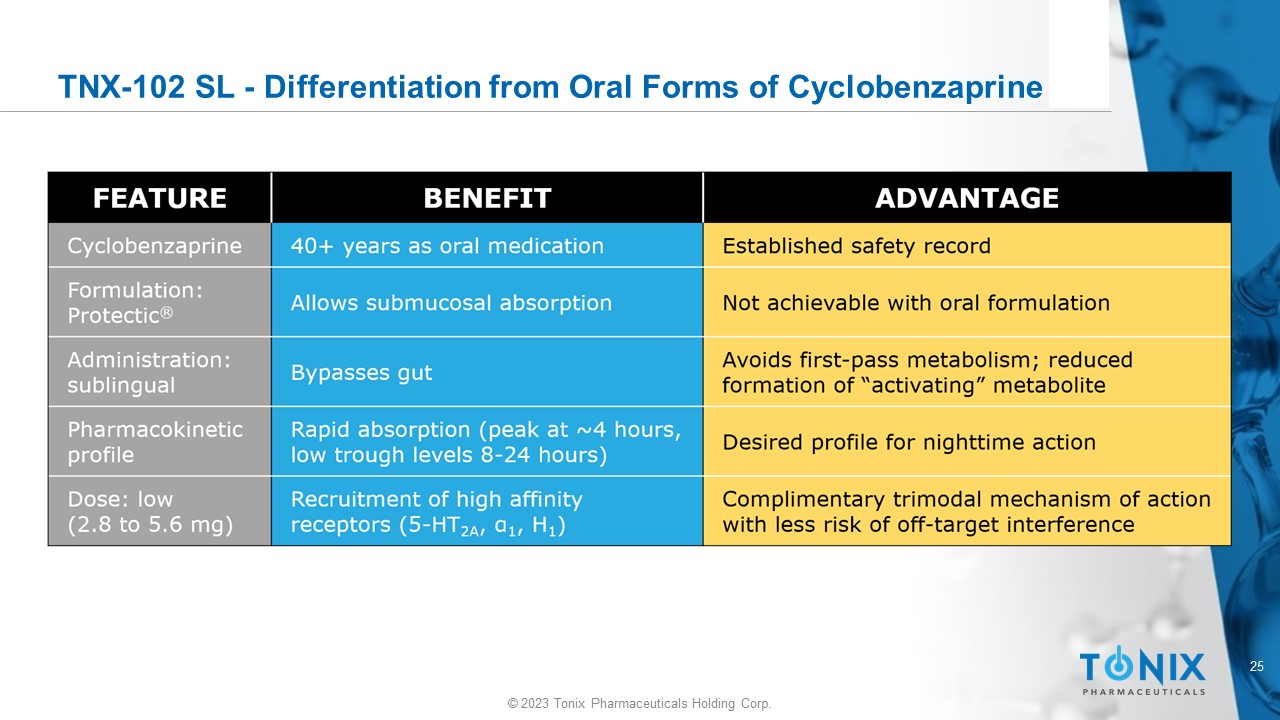
TNX - 102 SL - Differentiation from Oral Forms of Cyclobenzaprine 26 © 2023 Tonix Pharmaceuticals Holding Corp.

TNX - 102 SL – Experience in Muscle Spasm • Flexeril ® approval in 1977 received by Merck for the treatment of muscle spasm ‒ 10 mg T.I.D. for acute use (2 - 3 weeks) ‒ 1999 OTC AdCom Briefing Package noted original NDA included “ 8 long term safety studies in which patients with various neurologic disorders received cyclobenzaprine up to 80 mg per day for 1 month up to 3 years .” • 6 published studies in fibromyalgia prior to Tonix program ‒ N=246, placebo controlled, 4 - 24 week treatment periods ‒ Generally well tolerated, although equivocal efficacy across trials ‒ Relatively high doses of the immediate - release (IR) oral formulation may have clouded clear efficacy signal with typical adverse effects of IR such as high rates of drowsiness and dry mouth • Over 45 years of post - marketing safety data ‒ In recent years, ~20 million prescriptions and ~ 1 billion tablets dispensed per year ‒ Chronic cyclobenzaprine use is common ( ~12% of users) ‒ Post - marketing surveillance program following 7,607 patients (including 297 treated with 10 mg for > 30 days) found incidence of most common AEs much lower than in controlled studies • Extensive Post - Marketing Experience Including Long - Term Utilization 27 © 2023 Tonix Pharmaceuticals Holding Corp.
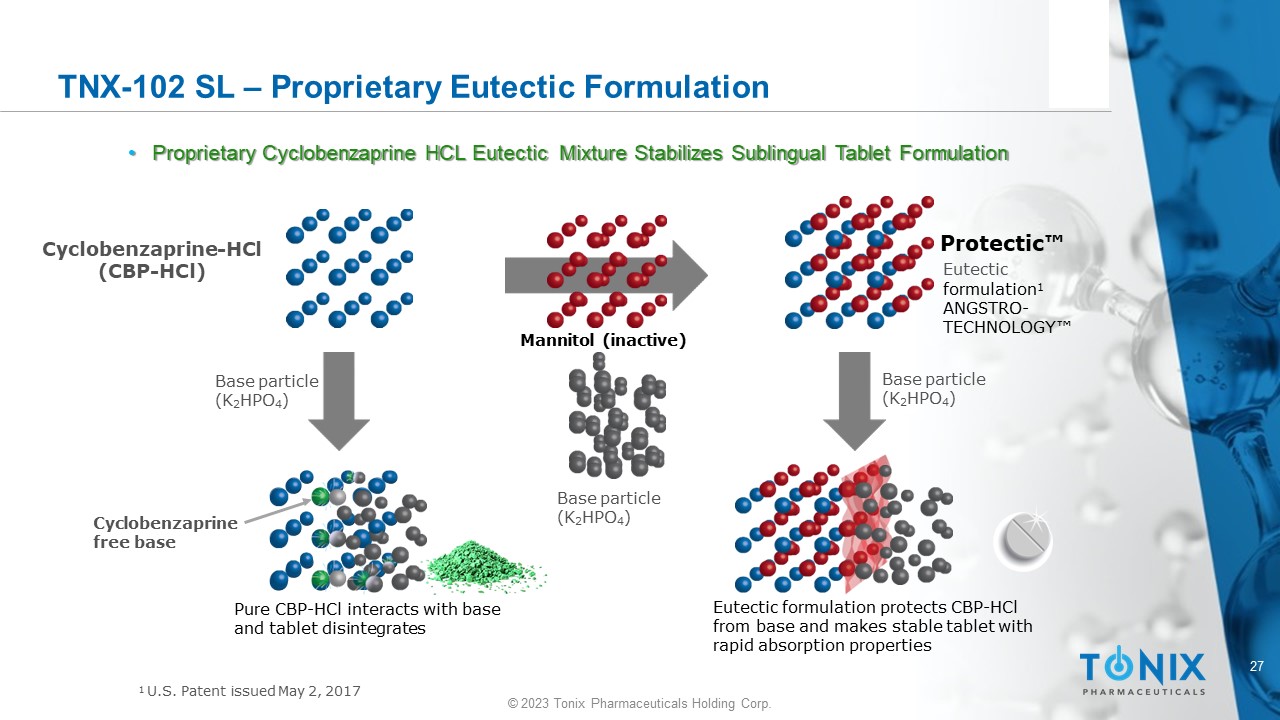
TNX - 102 SL – Proprietary Eutectic Formulation • Proprietary Cyclobenzaprine HCL Eutectic Mixture Stabilizes Sublingual Tablet Formulation Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) C y cl o be n z a p r i n e - HCl (CBP - HCl) Eutectic formulation protects CBP - HCl from base and makes stable tablet with rapid absorption properties Pure CBP - HCl interacts with base and tablet disintegrates Cy c l ob en zapr ine free base Protectic Œ Eutectic formulation 1 ANGSTRO - T E C H NO L O GY Œ Mannitol (inactive) 1 U.S. Patent issued May 2, 2017 28 © 2023 Tonix Pharmaceuticals Holding Corp.
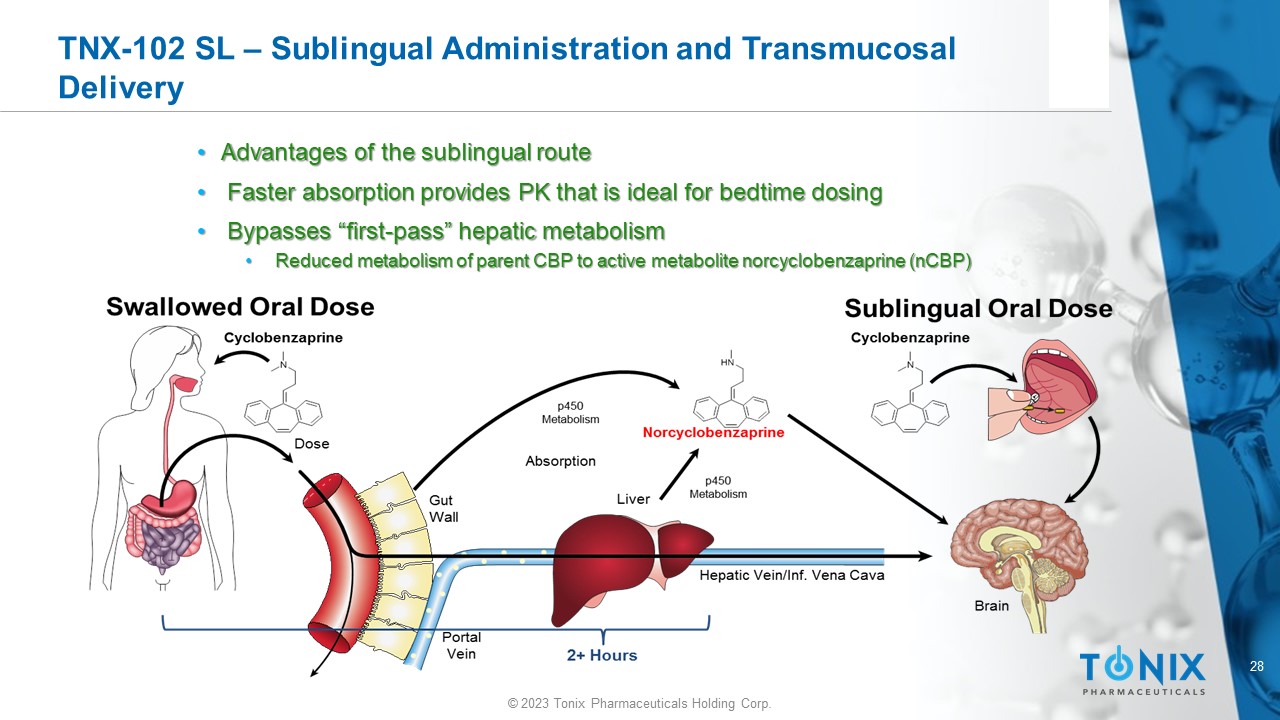
TNX - 102 SL – Sublingual Administration and Transmucosal Delivery • Advantages of the sublingual route • Faster absorption provides PK that is ideal for bedtime dosing • Bypasses “first - pass” hepatic metabolism • Reduced metabolism of parent CBP to active metabolite norcyclobenzaprine (nCBP)

29 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL 5.6 mg: Fibromyalgia Three Phase 3 Trials Completed Phase 3 Study, RESILIENT, compared TNX - 102 SL 5.6 mg and placebo • First patient enrolled in April 2022, completed p - value = 0.00005 • Parallel design, double - blind, randomized placebo - controlled study, all US sites • Primary endpoint is pain at Week 14 analyzed by MMRM with MI • All continuous key secondaries by MMRM with MI • Same key secondary efficacy endpoints as F304 RELIEF and F306 RALLY studies Phase 3 Study, RALLY, comparison of TNX - 102 SL 5.6 mg and placebo • As expected from interim analysis results published in July 2021, RALLY Study missed primary endpoint • Unexpected ~80% increase in adverse event - related discontinuations in both drug and placebo arms • Multiple imputation approach on 'Missing Data' attenuated statistical significance of efficacy endpoints’ • TNX - 102 SL was generally well tolerated with overall adverse event profile comparable to prior studies; no new safety signals observed 30 © 2023 Tonix Pharmaceuticals Holding Corp.

TNX - 102 SL: Fibromyalgia Program Update Phase 3 Study, RELIEF, compared TNX - 102 SL 5.6 mg and placebo • First patient enrolled in December 2019, completed p - value = 0.010 • Parallel design, double - blind, randomized placebo - controlled study, all US sites • Primary endpoint is pain at Week 14 analyzed by MMRM with MI 31 © 2023 Tonix Pharmaceuticals Holding Corp.
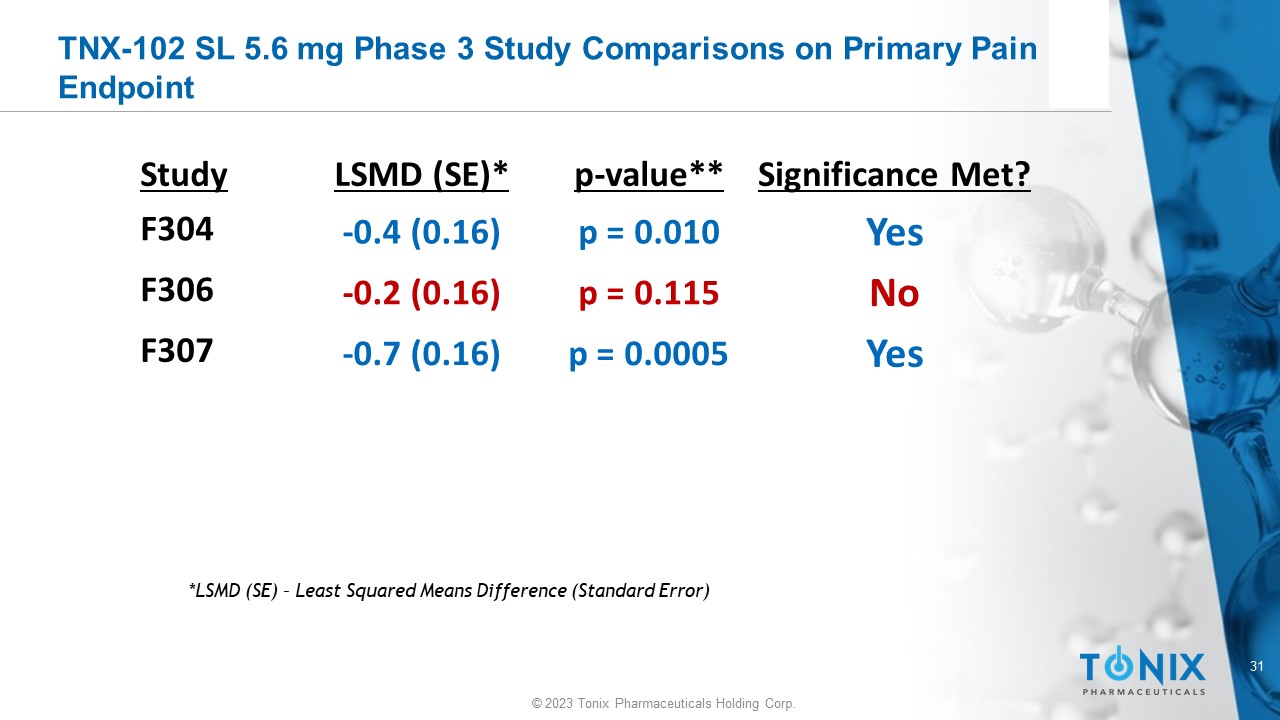
TNX - 102 SL 5.6 mg Phase 3 Study Comparisons on Primary Pain Endpoint Significance Met? p - value** LSMD (SE)* Study Yes p = 0.010 - 0.4 (0.16) F304 No p = 0.115 - 0.2 (0.16) F306 Yes p = 0.0005 - 0.7 (0.16) F307 *LSMD (SE) – Least Squared Means Difference (Standard Error)
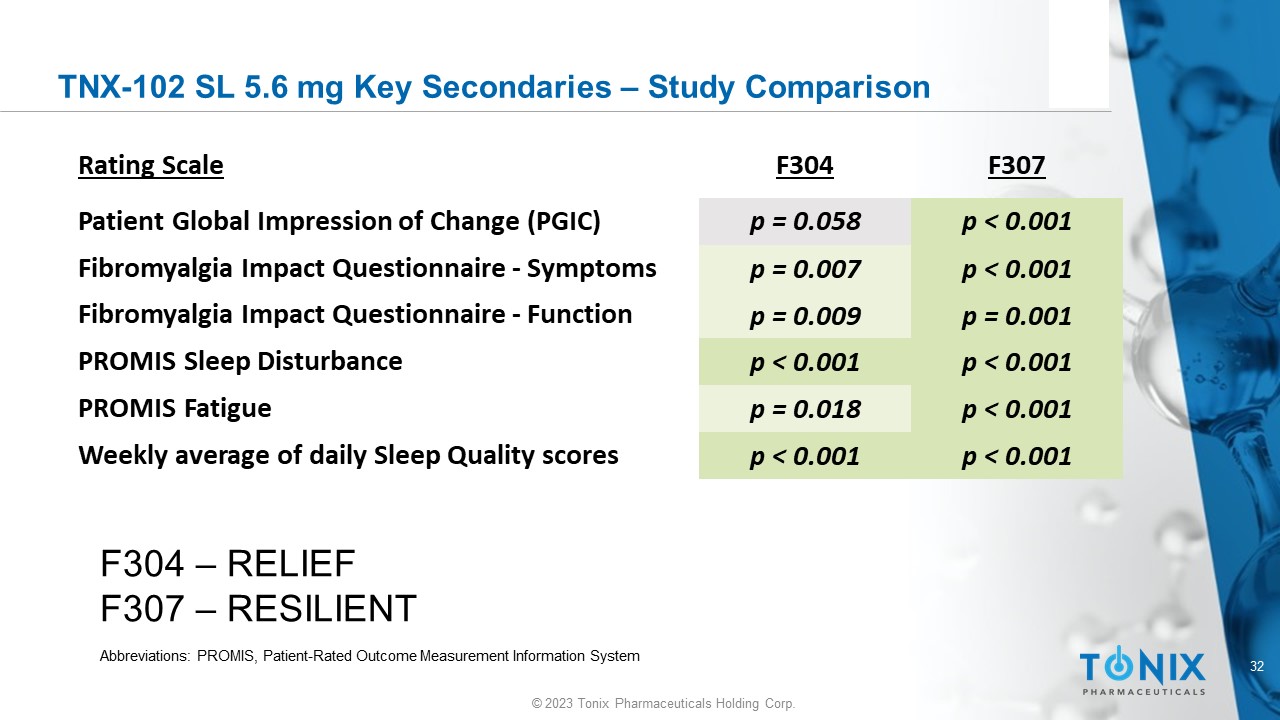
32 © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL 5.6 mg Key Secondaries – Study Comparison F307 F304 Rating Scale p < 0.001 p = 0.058 Patient Global Impression of Change (PGIC) p < 0.001 p = 0.007 Fibromyalgia Impact Questionnaire - Symptoms p = 0.001 p = 0.009 Fibromyalgia Impact Questionnaire - Function p < 0.001 p < 0.001 PROMIS Sleep Disturbance p < 0.001 p = 0.018 PROMIS Fatigue p < 0.001 p < 0.001 Weekly average of daily Sleep Quality scores F304 – RELIEF F307 – RESILIENT Abbreviations: PROMIS, Patient - Rated Outcome Measurement Information System 33 © 2023 Tonix Pharmaceuticals Holding Corp.
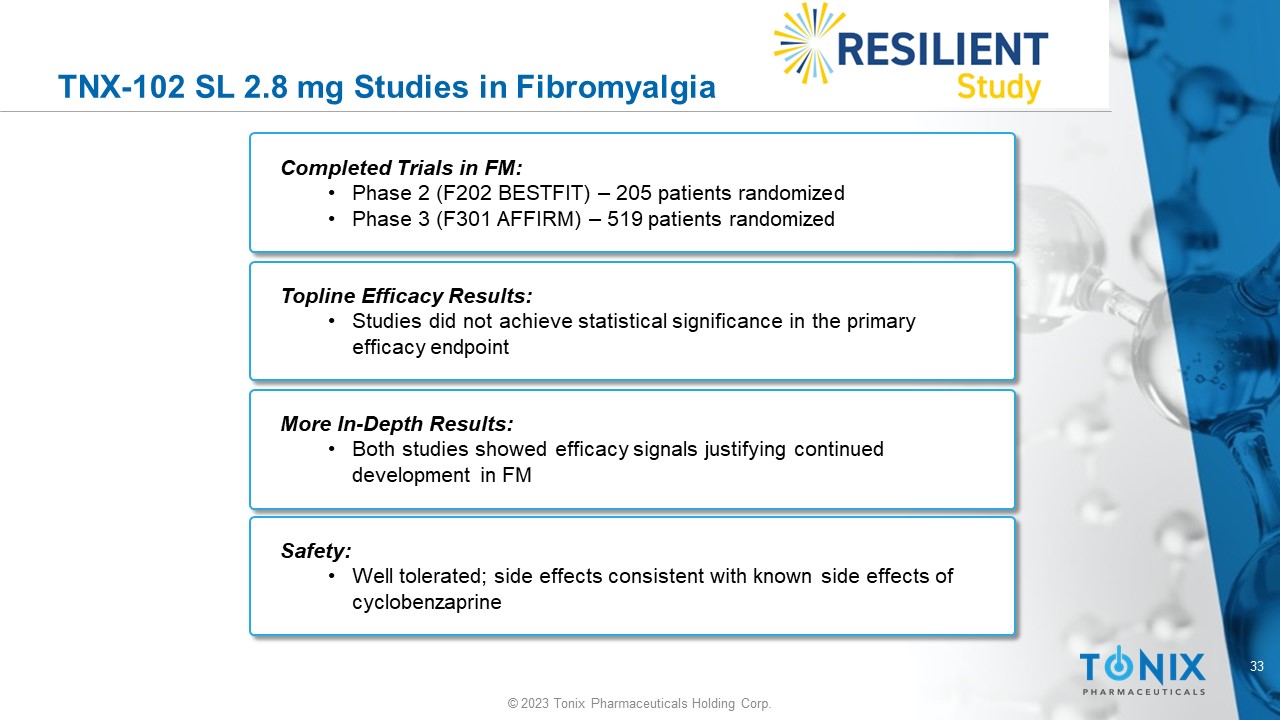
TNX - 102 SL 2.8 mg Studies in Fibromyalgia Completed Trials in FM: • Phase 2 (F202 BESTFIT) – 205 patients randomized • Phase 3 (F301 AFFIRM) – 519 patients randomized Topline Efficacy Results: • Studies did not achieve statistical significance in the primary efficacy endpoint More In - Depth Results: • Both studies showed efficacy signals justifying continued development in FM Safety: • Well tolerated; side effects consistent with known side effects of cyclobenzaprine 34 © 2023 Tonix Pharmaceuticals Holding Corp.
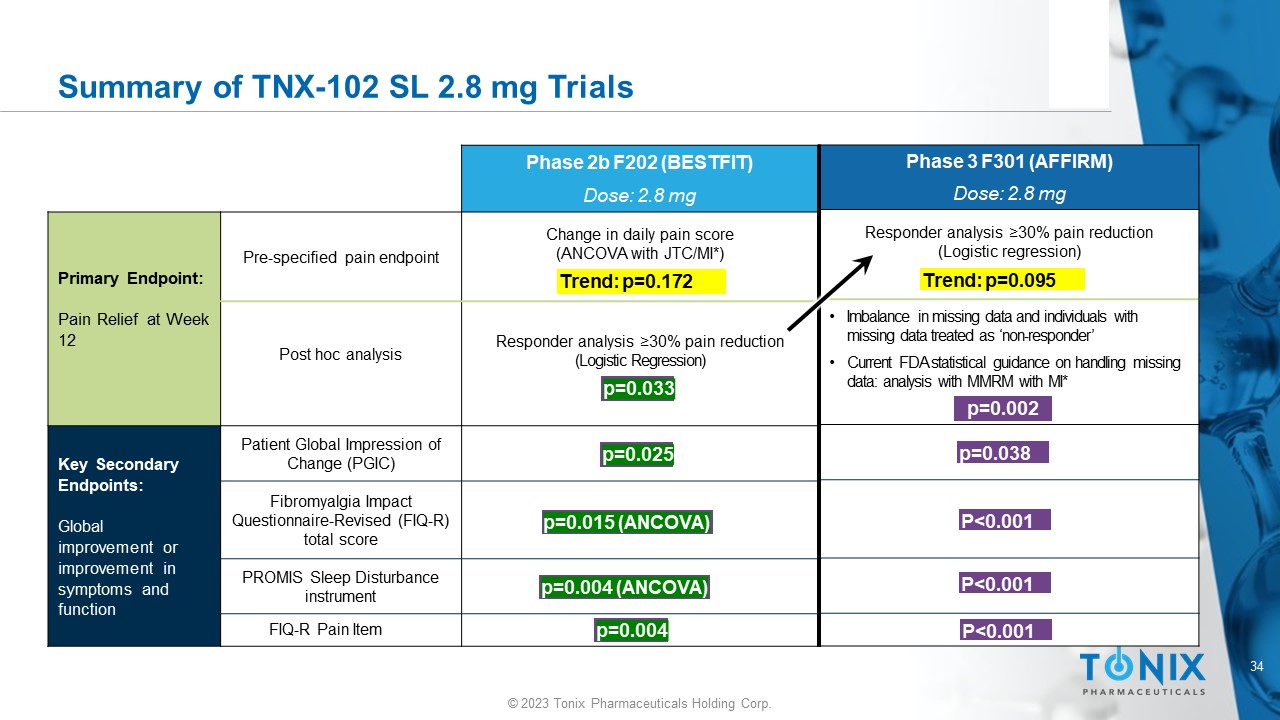
Summary of TNX - 102 SL 2.8 mg Trials p=0.005 p=0.038 P<0.001 P<0.001 P<0.001 Phase 3 F301 (AFFIRM) Dose: 2.8 mg Responder analysis ≥ 30% pain reduction (Logistic regression) • Imbalance in missing data and individuals with missing data treated as ‘non - responder’ • Current FDA statistical guidance on handling missing data: analysis with MMRM with MI* p=0.002 p=0.038 P<0.001 P<0.001 P<0.001 Trend: p=0.095 Phase 2b F202 (BESTFIT) Dose: 2.8 mg Change in daily pain score (ANCOVA with JTC/MI*) Pre - specified pain endpoint Primary Endpoint: Pain Relief at Week 12 Responder analysis ≥30% pain reduction (Logistic Regression) Post hoc analysis Patient Global Impression of Change (PGIC) Key Secondary Endpoints: Global improvement or improvement in symptoms and function Fibromyalgia Impact Questionnaire - Revised (FIQ - R) total score PROMIS Sleep Disturbance instrument FIQ - R Pain Item Trend: p=0.172 p=0.033 p=0.025 p=0.015 (ANCOVA) p=0.004 (ANCOVA) p=0.004 35 © 2023 Tonix Pharmaceuticals Holding Corp.
TNX - 102 SL Dose Response Response Dosage Dose - Response Inconsistent response No clear response Consistent response Consistent but trade - offs* (AEs) Basic Pharmacology • Dose can make the difference in the strength of the response 36 © 2023 Tonix Pharmaceuticals Holding Corp.
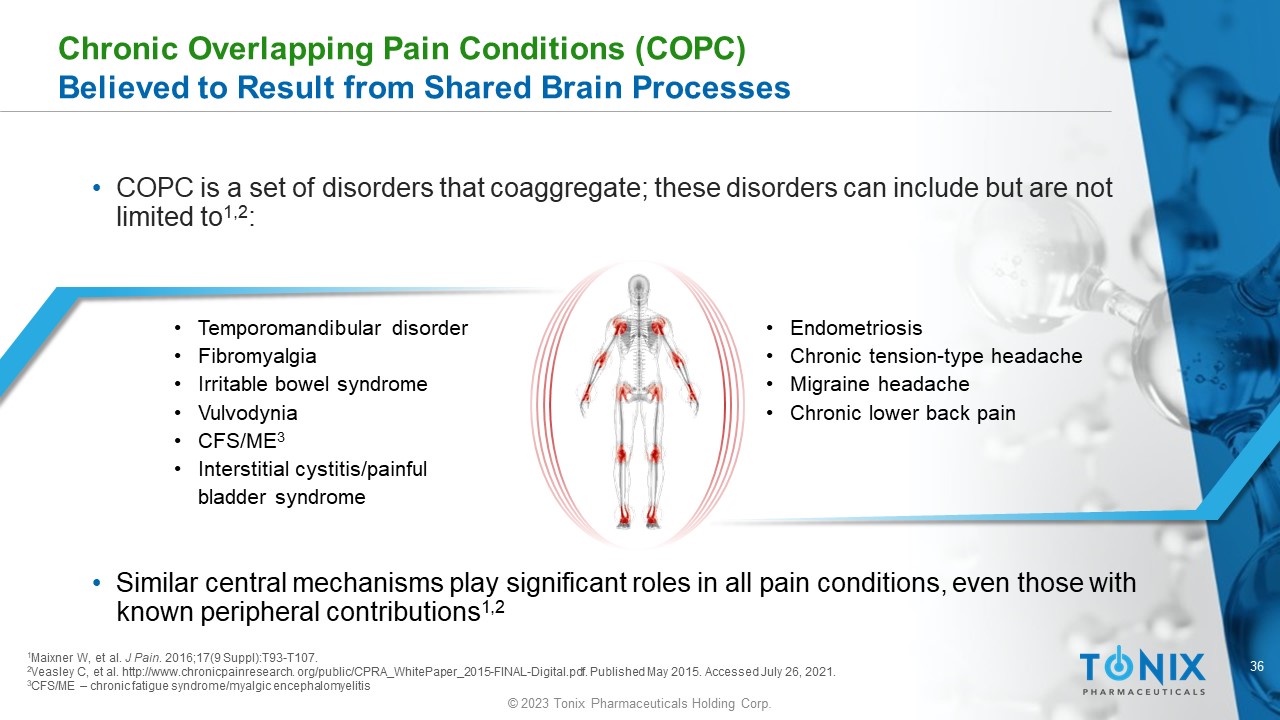
Chronic Overlapping Pain Conditions (COPC) Believed to Result from Shared Brain Processes • COPC is a set of disorders that coaggregate ; these disorders can include but are not limited to 1,2 : • Temporomandibular disorder • Fibromyalgia • Irritable bowel syndrome • Vulvodynia • CFS/ME 3 • Interstitial cystitis/painful bladder syndrome • Endometriosis • Chronic tension - type headache • Migraine headache • Chronic lower back pain 1 Maixner W, et al. J Pain . 2016;17(9 Suppl):T93 - T107. 2 Veasley C, et al. http://www.chronicpainresearch. org/public/CPRA_WhitePaper_2015 - FINAL - Digital.pdf. Published May 2015. Accesse d July 26, 2021. 3 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis • Similar central mechanisms play significant roles in all pain conditions, even those with known peripheral contributions 1,2 37 © 2023 Tonix Pharmaceuticals Holding Corp.
Role of Infections in Triggering Fibromyalgia or Chronic fatigue (CFS) - Like Illnesses Infection initiates an autoreactive process, which affects several functions, including brain and energy metabolism 2 - 7 • Infections can trigger any of these conditions in approximately 10% of exposed individuals • The initial location of the infection determines the subsequent pain syndrome • Any type of infectious diarrhea will trigger irritable bowel syndrome (IBS) in 10% to 20% of those exposed 1 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Lo ng COVID, 200 Independence Ave SW, Washington, DC 20201. 2 Blomberg J, et al. Front Immunol. 2018;9:229. Published 2018 Feb 15. 3 Warren JW, et al. Urology. 2008;71(6):1085 - 1090. 4 Buskila D, et al. Autoimmun Rev. 2008;8(1):41 - 43. 5 Hickie I, et al. BMJ. 2006;333(7568):575. 6 Parry SD, et al. Am J Gastroenterol. 2003;98(9):1970 - 1975. 7 Halvorson HA, et al. Am J Gastroenterol. 2006;101(8):1894 - 1942. • Symptoms of Long COVID, like multi - site pain, fatigue and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). • In August 2022, the HHS released the National Research Action Plan on Long COVID 1 which endorses the connection between Long COVID and chronic fatigue syndrome.
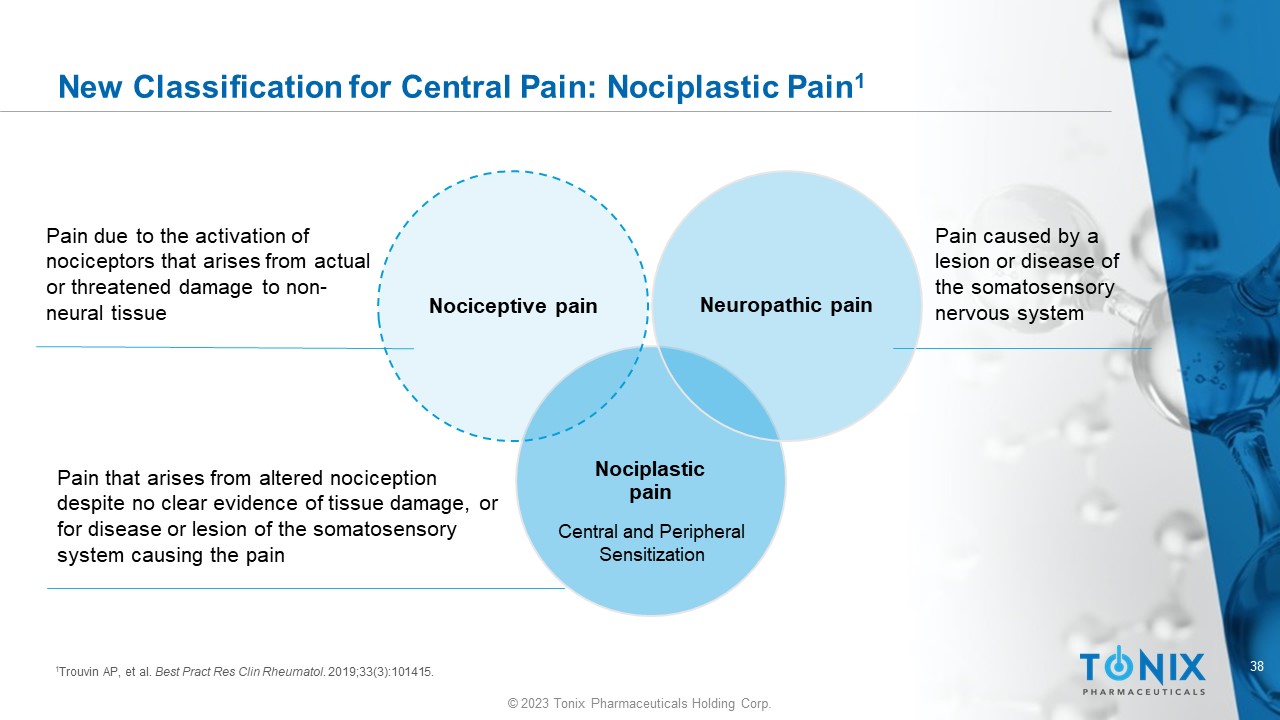
38 © 2023 Tonix Pharmaceuticals Holding Corp. New Classification for Central Pain: Nociplastic Pain 1 Nociplastic pain Nociceptive pain Neuropathic pain Pain due to the activation of nociceptors that arises from actual or threatened damage to non - neural tissue Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or lesion of the somatosensory system causing the pain Pain caused by a lesion or disease of the somatosensory nervous system 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. Central and Peripheral Sensitization 39 © 2023 Tonix Pharmaceuticals Holding Corp.
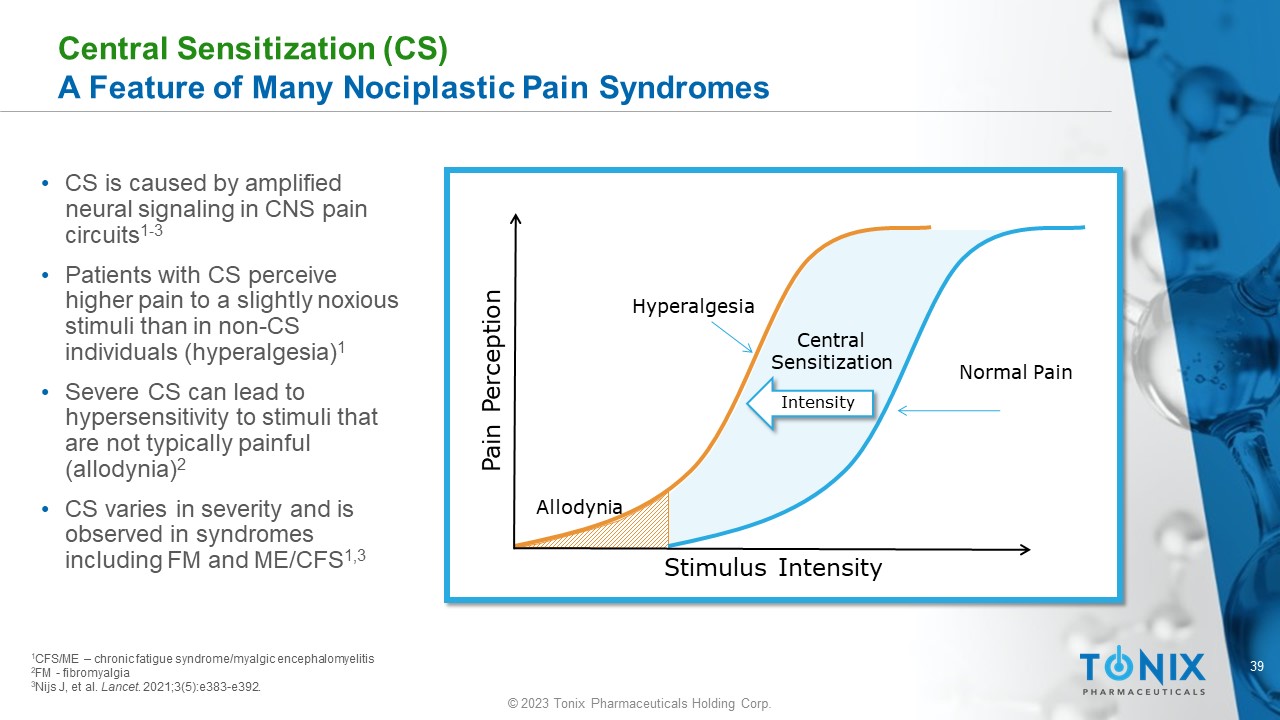
Central Sensitization (CS) A Feature of Many Nociplastic Pain Syndromes • CS is caused by amplified neural signaling in CNS pain circuits 1 - 3 • Patients with CS perceive higher pain to a slightly noxious stimuli than in non - CS individuals (hyperalgesia) 1 • Severe CS can lead to hypersensitivity to stimuli that are not typically painful (allodynia) 2 • CS varies in severity and is observed in syndromes including FM and ME/CFS 1,3 Stimulus Intensity Pain Perception Normal Pain Hyperalgesia Allodynia Central Sensitization Intensity 1 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis 2 FM - fibromyalgia 3 Nijs J, et al. Lancet . 2021;3(5):e383 - e392.
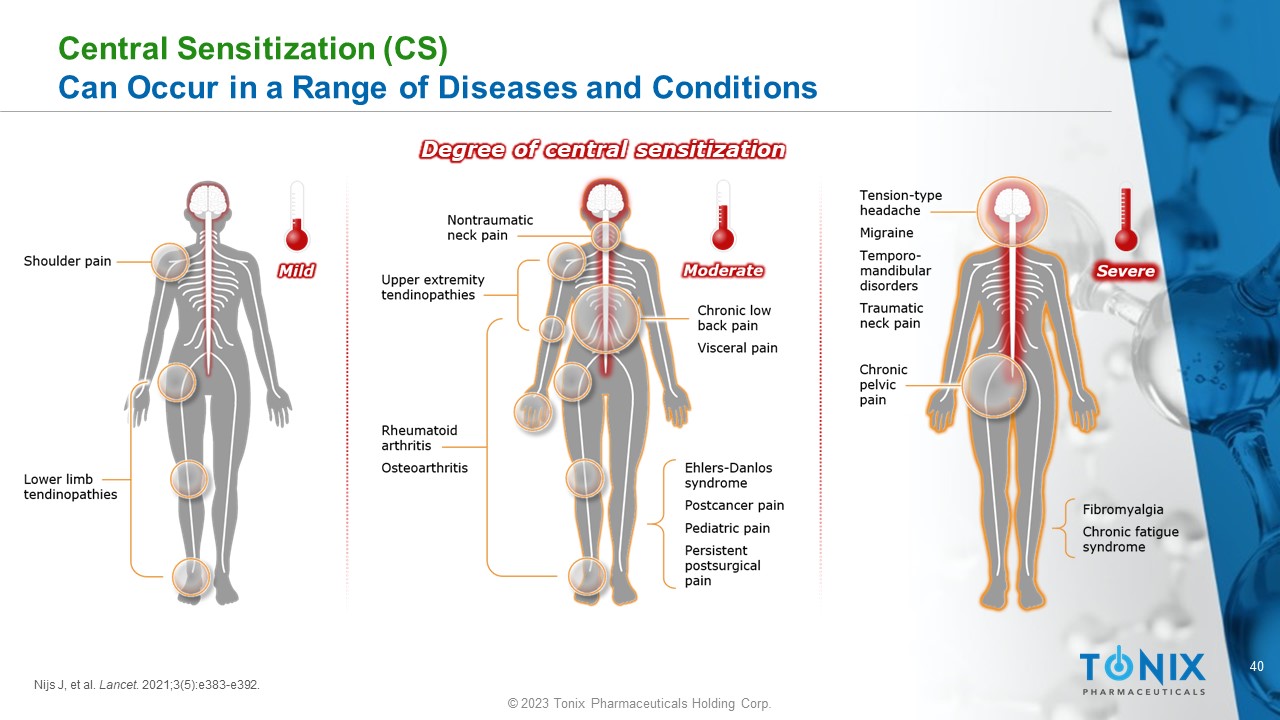
40 © 2023 Tonix Pharmaceuticals Holding Corp. Central Sensitization (CS) Can Occur in a Range of Diseases and Conditions Degree of central sensitization Nijs J, et al. Lancet . 2021;3(5):e383 - e392.

© 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU