UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
Current Report Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
December 5, 2025
NEXALIN TECHNOLOGY, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-41507 | 27-5566468 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
1776 Yorktown, Suite 550
Houston, TX 77056
(Address of principal executive offices and zip code)
Registrant’s telephone number, including area code: (832) 260-0222
Check the appropriate box below if the Form 8-K is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, par value $0.001 per share | NXL | The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 8.01. | Other Events. |
Attached is an updated Investor Presentation of Nexalin Technology, Inc., a Delaware corporation, as of December 5, 2025.
| Item 9.01. | Financial Statements and Exhibits. |
| (d) | Exhibits |
| Exhibits | Description of Exhibit | |
| 99.1 | Investor Presentation of Nexalin Technology, Inc. as of December 5, 2025. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL Document). |
|
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: December 5, 2025 | Nexalin Technology, Inc. | |
| By | /s/ Mark White | |
| Mark White, | ||
| Chief Executive Officer | ||
|
|
Exhibit 99.1


HARNESSING THE POWER OF ADVANCED FREQUENCY NEUROSTIMULATION TO IMPROVE MENTAL HEALTH INVESTOR PRESENTATION | DECEMBER 2025 NASDAQ: NXL This presentation (this "Presentation") has been prepared by Nexalin Technology, Inc . (“Nexalin” or the “Company”) exclusively for informational purposes only, and is not, and may not be relied on in any manner as, legal, tax, regulatory, or investment advice, and shall not constitute an offer to sell or a solicitation of an offer to buy securities . The information contained herein is qualified in its entirety by reference to information contained or incorporated by reference into our reports and other filings filed with the Securities and Exchange Commission, including the risk factors contained therein . Except where otherwise indicated herein, the information provided herein is based on matters as they exist as of the date of preparation and not as of any future date, and will not be updated or otherwise revised to reflect information that subsequently becomes available, or circumstances existing or changes occurring after the date of preparation . The industry and market data and other statistical information contained in this presentation are based on management’s own estimates, independent publications, government publications, reports by market research firms or other published independent sources, and, in each case, management believes are reasonable. Although we believe these sources are reliable, we have not independently verified the information. None of the independent industry publications used in this presentation were prepared on our behalf or on behalf of any of our affiliates and none of the sources cited by us consented to the inclusion of any data from its reports, and we have not sought their consent. Certain information contained in this Presentation constitutes "forward - looking statements," which can be identified by the use of forward - looking terminology such as "may," "will," "should," "expect," "anticipate;" "target," "project," "estimate," "intend," "continue" or "believe," or the negatives thereof or other variations thereon or comparable terminology. Due to various risks and uncertainties, actual events or results or the actual performance of the Company may differ materially from those reflected or contemplated in such forward - looking statements. Readers should not to place undue reliance on any forward - looking statements. All opinions, estimates, forecasts of future performance and other future - looking statements as to performance (collectively, “projections”) are presented for illustrative purposes only and are not necessarily, and do not purport to be, indicative, or a guarantee, of future results. The projections are based on certain underlying assumptions made by the Company based upon information available to it. The projections assume, among other matters, the Company will be able to raise sufficient additional capital consistent with, and sufficient to timely fund, its business plan. The primary assumptions which the Company's projections, aside from the timing and amount of investment in the Company, are based on the following: the nature of and rate of release of products, some of which are in clinical development but have not completed advanced clinical trials; success in preclinical studies or clinical trials; our products and product candidates not being subject to reclassification by the FDA; our ability to obtain necessary regulatory approvals; not experiencing delays or difficulties in the enrollment of patients in clinical trials; the ability of collaborators to manufacture sufficient quantity of product for development, clinical trials or potential commercialization; obtaining and maintaining patent, trademark and trade secret protection, and regulatory exclusivity for our products and preclinical program; making satisfactory arrangements with third parties for manufacturing capabilities, launching commercial sales of products, if and when approved; acceptance of the therapies, if and when approved, by healthcare providers, physicians, clinicians, patients and third - party payors; and being able to obtain and maintain healthcare coverage and adequate reimbursement. While the Company believes that such assumptions are reasonable, competitive and regulatory factors, as well as general economic factors, could result in the Company’s failure to meet its objectives. The selection and weighting of assumptions requires the exercise of judgment and is subject to uncertainty due to effects that economic, regulatory, legislative or other changes may have on future events. Variations in such assumptions, particularly investment and patient growth, could significantly affect the projections. Some assumptions on which the projections are based inevitably will not materialize or may differ from that projected, and unanticipated events and circumstances will occur. Therefore, the actual results achieved during the projection period will vary from the projections, and the variations may be material. The projections have not been audited, reviewed or compiled by any independent public accountants. Accordingly, no opinion is expressed nor any form of assurance given with respect to the projections. The projections were not prepared with a view to compliance with published guidelines of the Securities and Exchange Commission or any other securities commission, or the guidelines established by the American Institute of Certificate Public Accountants or other administrative body. 2 Alzheimer’s and Dementia 2 60M * Anxiety 2 360M * TBI/PTSD (Military) 2,3 350M * Depression 2 330M * Mental Health is a Global Epidemic A Market full of Drugs, a Mind Full of Problems 3 INVESTOR PRESENTATION | DECEMBER 2025 Insomnia 1 850M * * Number of Patients

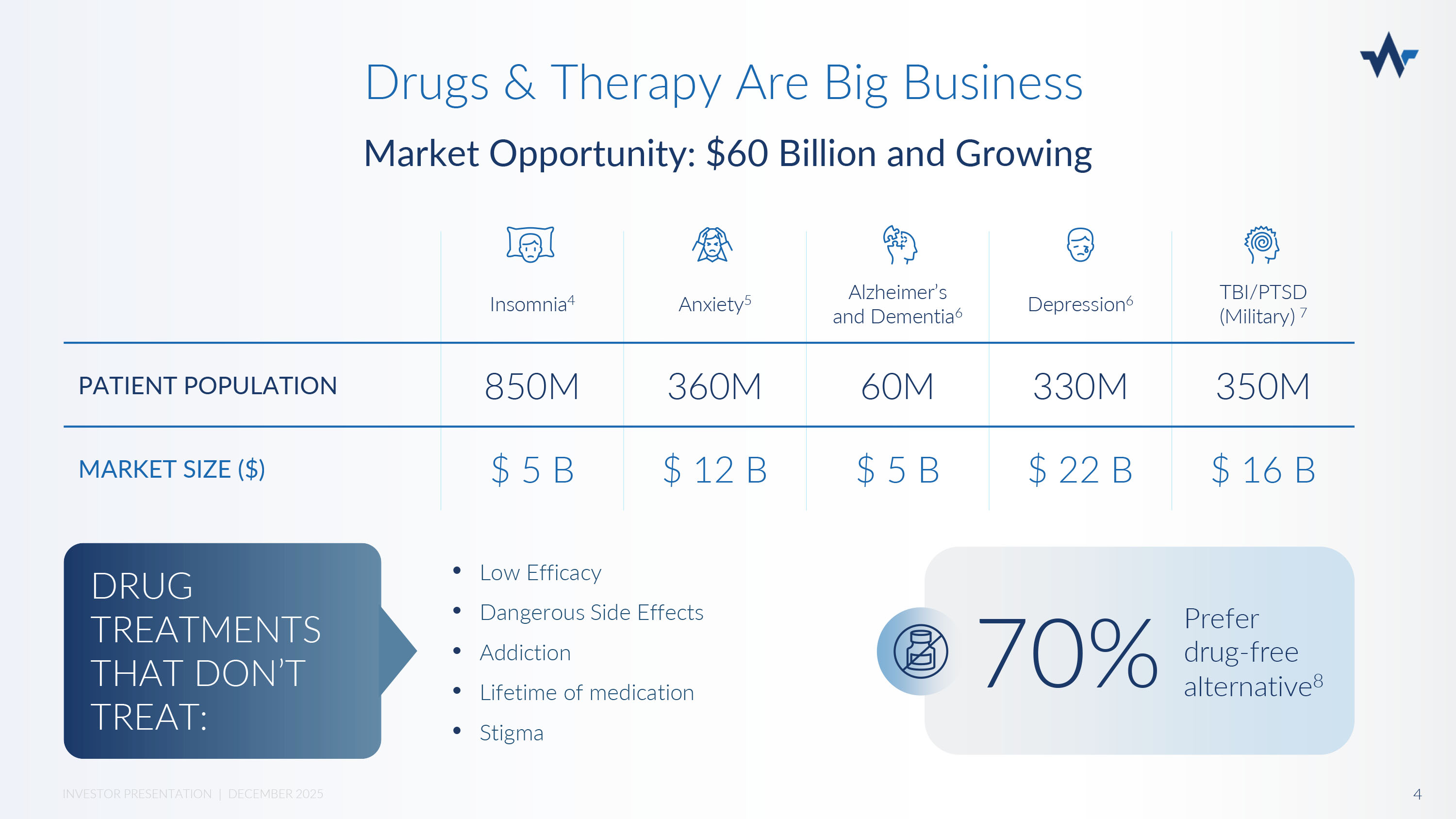
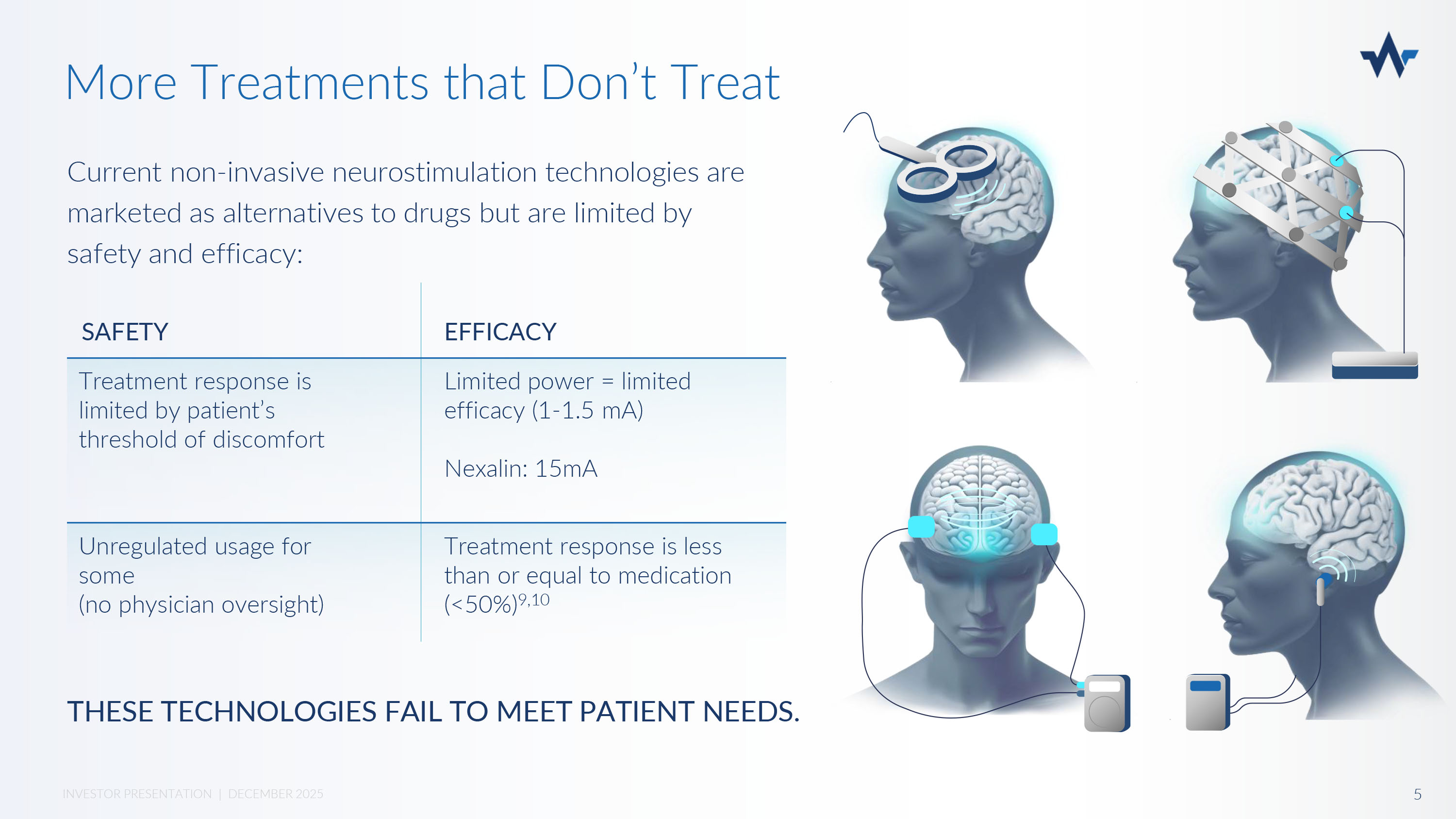
Drugs & Therapy Are Big Business Market Opportunity: $60 Billion and Growing TBI/PTSD (Military) 7 Depression 6 Alzheimer’s and Dementia 6 Anxiety 5 Insomnia 4 350M 330M 60M 360M 850M PATIENT POPULATION $ 16 B $ 22 B $ 5 B $ 12 B $ 5 B MARKET SIZE ($) • Low Efficacy • Dangerous Side Effects • Addiction • Lifetime of medication • Stigma DRUG TREATMENTS THAT DON’T TREAT: Prefer drug - free alternative 8 70% 4 INVESTOR PRESENTATION | DECEMBER 2025 Current non - invasive neurostimulation technologies are marketed as alternatives to drugs but are limited by safety and efficacy: More Treatments that Don’t Treat EFFICACY SAFETY Limited power = limited efficacy (1 - 1.5 mA) Nexalin: 15mA Treatment response is limited by patient’s threshold of discomfort Treatment response is less than or equal to medication (<50%) 9,10 Unregulated usage for some (no physician oversight) THESE TECHNOLOGIES FAIL TO MEET PATIENT NEEDS.
5 INVESTOR PRESENTATION | DECEMBER 2025

Nexalin is replacing all current neurostimulator technologies with its new Deep Intracranial Frequency Stimulation (DIFS ® ). The Future of Mental Health Treatment is Here x Undetectable to the human body x No patient discomfort or side effects x 15x more powerful than current neurostimulators* x Increased power allows for deeper penetration x Deeper penetration, better response x Treatment response based on clinical evidence *Compared to 1mA 6 INVESTOR PRESENTATION | DECEMBER 2025 Our DIFS device mirrors the brain’s natural energy rhythms to effectively reset and retrain networks associated with trauma.
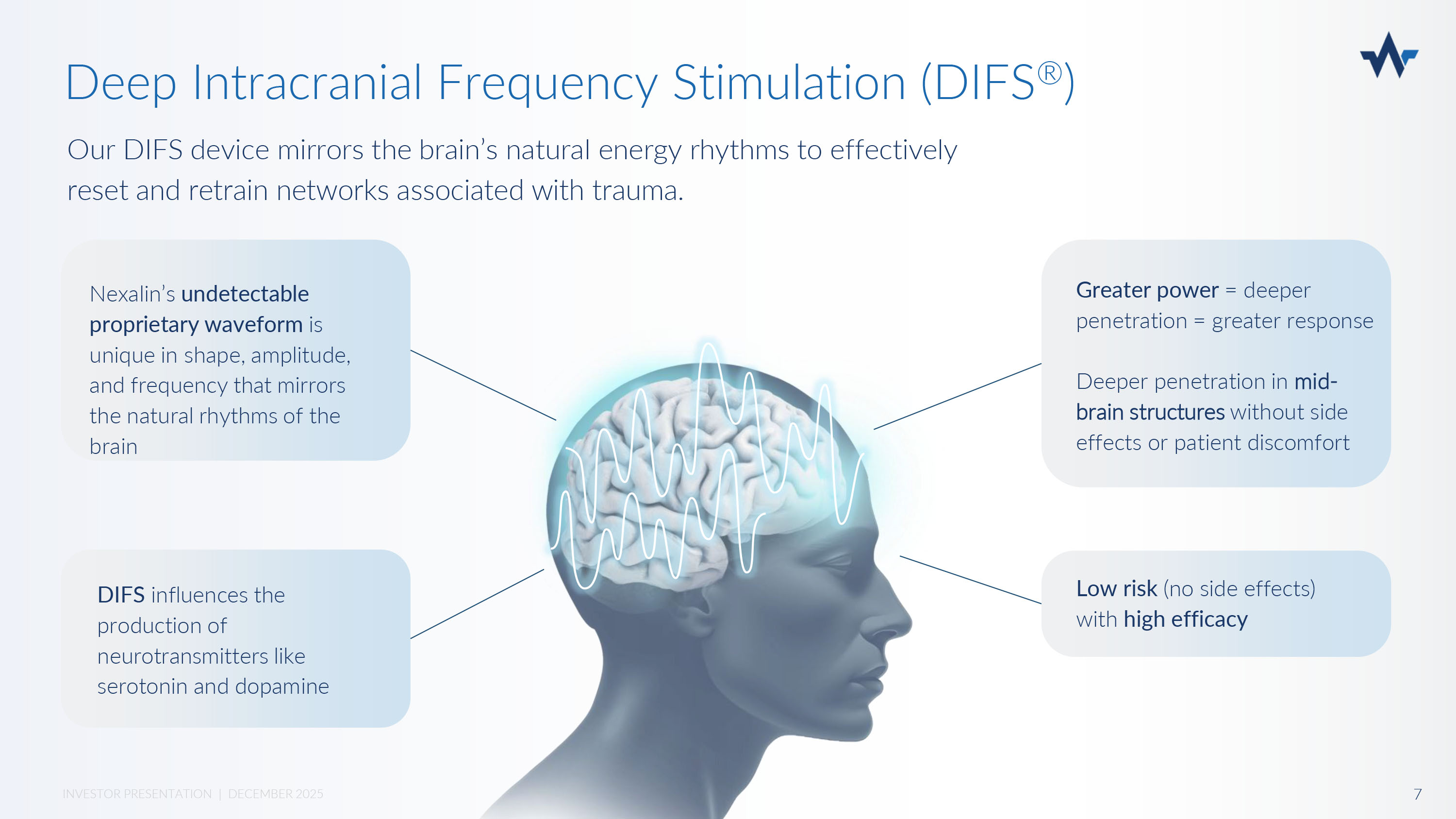
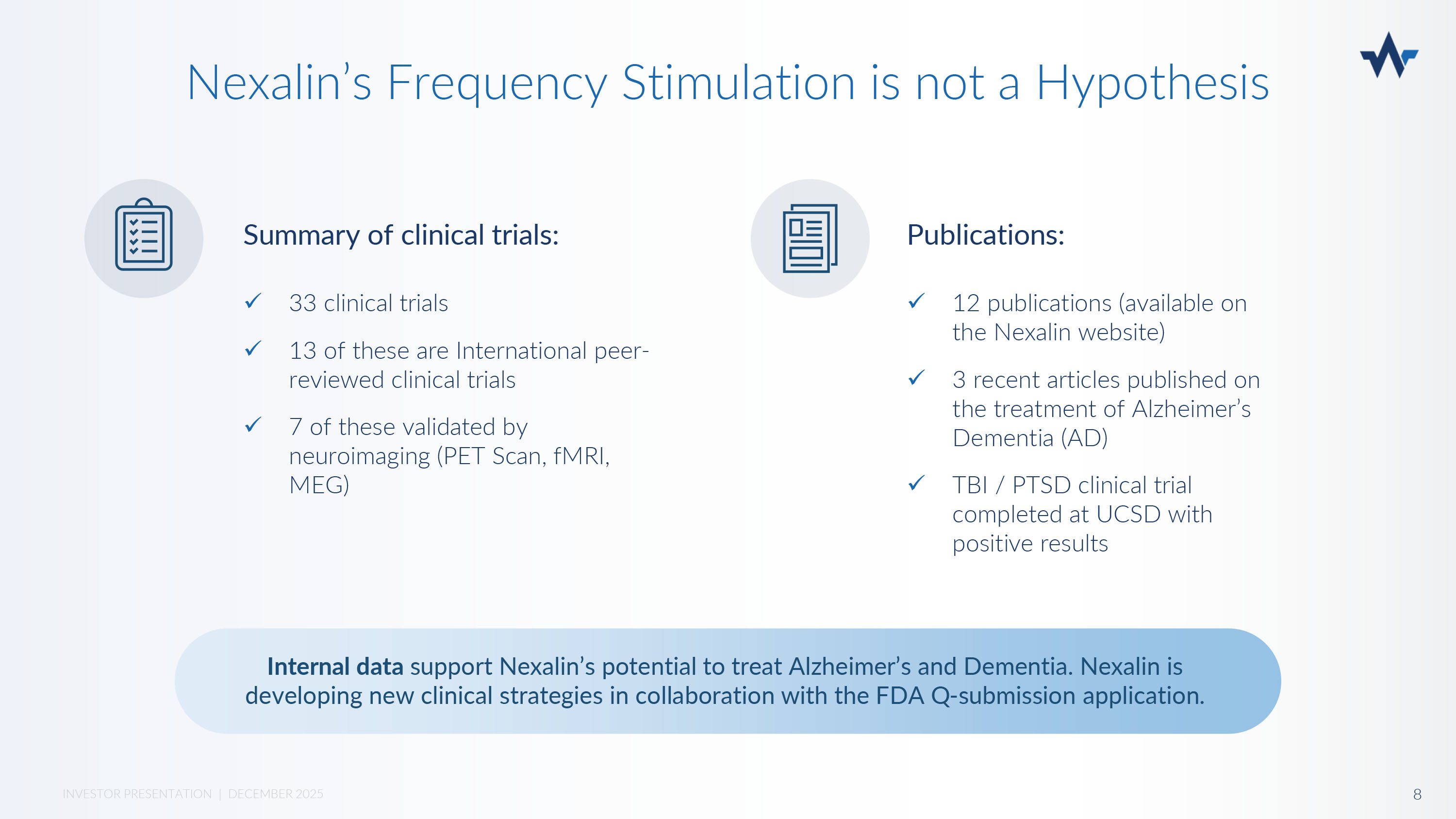
Deep Intracranial Frequency Stimulation (DIFS ® ) DIFS influences the production of neurotransmitters like serotonin and dopamine Nexalin’s undetectable proprietary waveform is unique in shape, amplitude, and frequency that mirrors the natural rhythms of the brain Greater power = deeper penetration = greater response 7 INVESTOR PRESENTATION | DECEMBER 2025 Deeper penetration in mid - brain structures without side effects or patient discomfort Low risk (no side effects) with high efficacy Summary of clinical trials: Nexalin’s Frequency Stimulation is not a Hypothesis x 33 clinical trials x 13 of these are International peer - reviewed clinical trials x 7 of these validated by neuroimaging (PET Scan, fMRI, MEG) Publications: x 12 publications (available on the Nexalin website) x 3 recent articles published on the treatment of Alzheimer’s Dementia (AD) x TBI / PTSD clinical trial completed at UCSD with positive results Internal data support Nexalin’s potential to treat Alzheimer’s and Dementia.
Nexalin is developing new clinical strategies in collaboration with the FDA Q - submission application. 8 INVESTOR PRESENTATION | DECEMBER 2025 PRODUCT AND STRATEGY 9 INVESTOR PRESENTATION | DECEMBER 2025

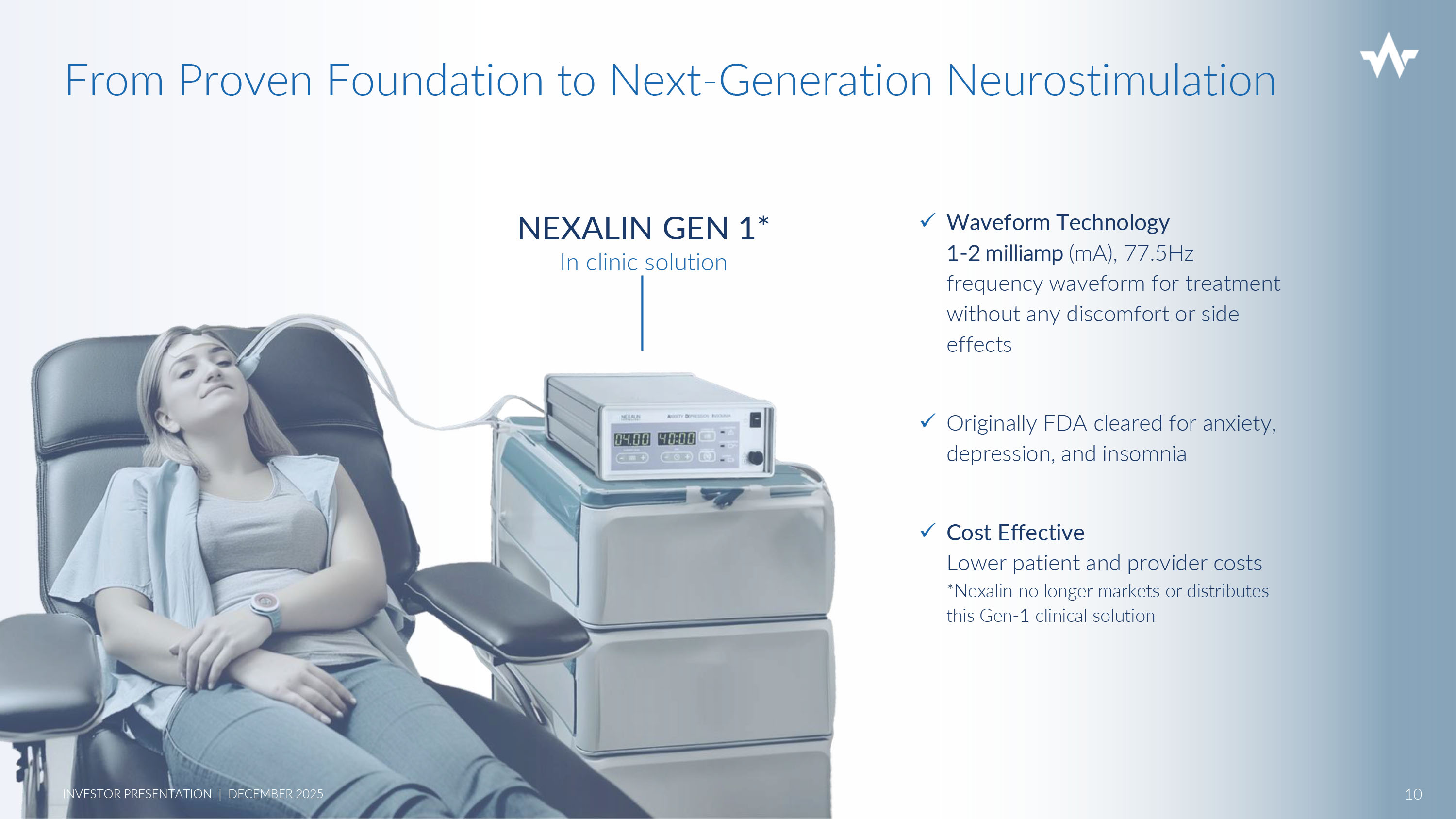
x Waveform Technology 1 - 2 milliamp (mA), 77.5Hz frequency waveform for treatment without any discomfort or side effects x Originally FDA cleared for anxiety, depression, and insomnia x Cost Effective Lower patient and provider costs *Nexalin no longer markets or distributes this Gen - 1 clinical solution NEXALIN GEN 1* In clinic solution From Proven Foundation to Next - Generation Neurostimulation 10 INVESTOR PRESENTATION | DECEMBER 2025 More advanced, powerful technology Enhanced access through virtual clinic GEN 1 - FDA Anxiety, Depression, Insomnia Three regulatory pathways leading to commercialization x Mood disorders – Anxiety, Depression, Insomnia x Alzheimer’s and Dementia x TBI/PTSD (military) OVERALL GROWTH STRATEGY GEN 2 - SYNC Clinical Device GEN 3 - HALO Remote Device 11 INVESTOR PRESENTATION | DECEMBER 2025
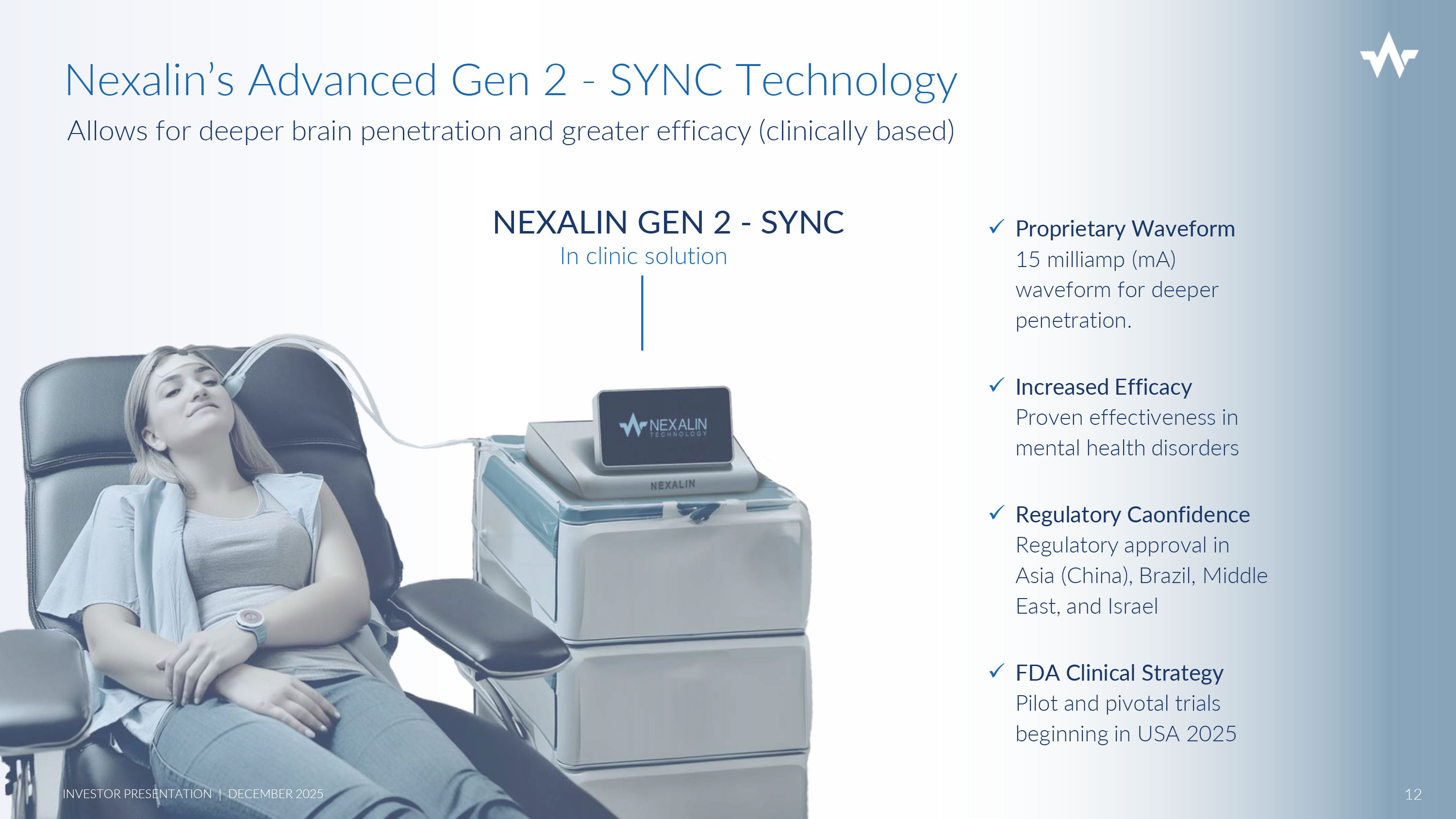
x Proprietary Waveform 15 milliamp (mA) waveform for deeper penetration. x Increased Efficacy Proven effectiveness in mental health disorders x Regulatory Caonfidence Regulatory approval in Asia (China), Brazil, Middle East, and Israel x FDA Clinical Strategy Pilot and pivotal trials beginning in USA 2025 NEXALIN GEN 2 - SYNC In clinic solution Nexalin’s Advanced Gen 2 - SYNC Technology Allows for deeper brain penetration and greater efficacy (clinically based) 12 INVESTOR PRESENTATION | DECEMBER 2025 Nexalin’s GEN 3 - HALO headset is coming… BRINGING ADVANCED, NON - INVASIVE TREATMENT INTO THE HOME 13


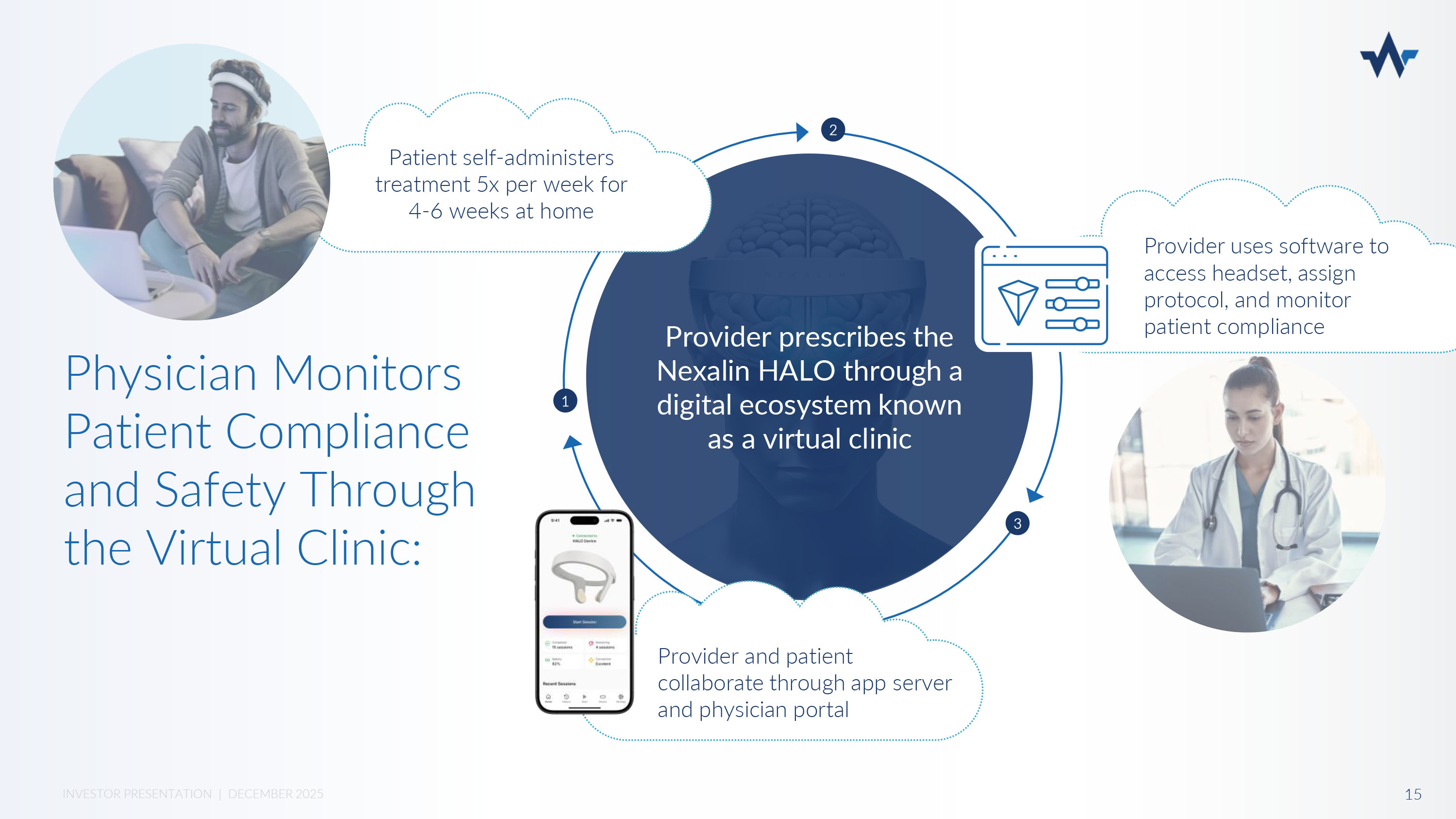
NEXALIN GEN 3 - HALO x Same Increased Efficacy as Gen 2 – SYNC x Convenient At Home Treatment through App and Virtual Clinic x FDA Q - submissions filed, collaborating with FDA on clinical design strategies x FDA application expected 2026 In home virtual clinic solution Allows patients to receive advanced neurostimulation treatment in their home with clinical supervision Nexalin’s Gen 3 - HALO Device 14 INVESTOR PRESENTATION | DECEMBER 2025 Provider prescribes the Nexalin HALO through a digital ecosystem known as a virtual clinic Physician Monitors Patient Compliance and Safety Through the Virtual Clinic : Provider uses software to access headset, assign protocol, and monitor patient compliance 3 1 2 Provider and patient collaborate through app server and physician portal Patient self - administers treatment 5x per week for 4 - 6 weeks at home 15 INVESTOR PRESENTATION | DECEMBER 2025

Key: – Commercial Clearance – Developing Business Strategy – Future Strategy Development IN SUMMARY: In Market (JV) China, Brazil, Oman Pilot and Pivotal FDA Clinical Trials in USA Pilot and Pivotal Clinical Trials Internationally FDA De Novo Clinical Trial Strategy USA Nexalin Portfolio Poised For Rapid Commercial Growth 2025 2026 2027 INSOMNIA 16 INVESTOR PRESENTATION | DECEMBER 2025 DEPRESSION ANXIETY Alzheimer’s and DEMENTIA TBI/PTSD (Military) GEN 2 SYNC GEN 3 HALO N/A I N T E R N A T I O N A L U S A I N T E R N A T I O N A L U S A MARKET AND BUSINESS MODEL 17 INVESTOR PRESENTATION | DECEMBER 2025

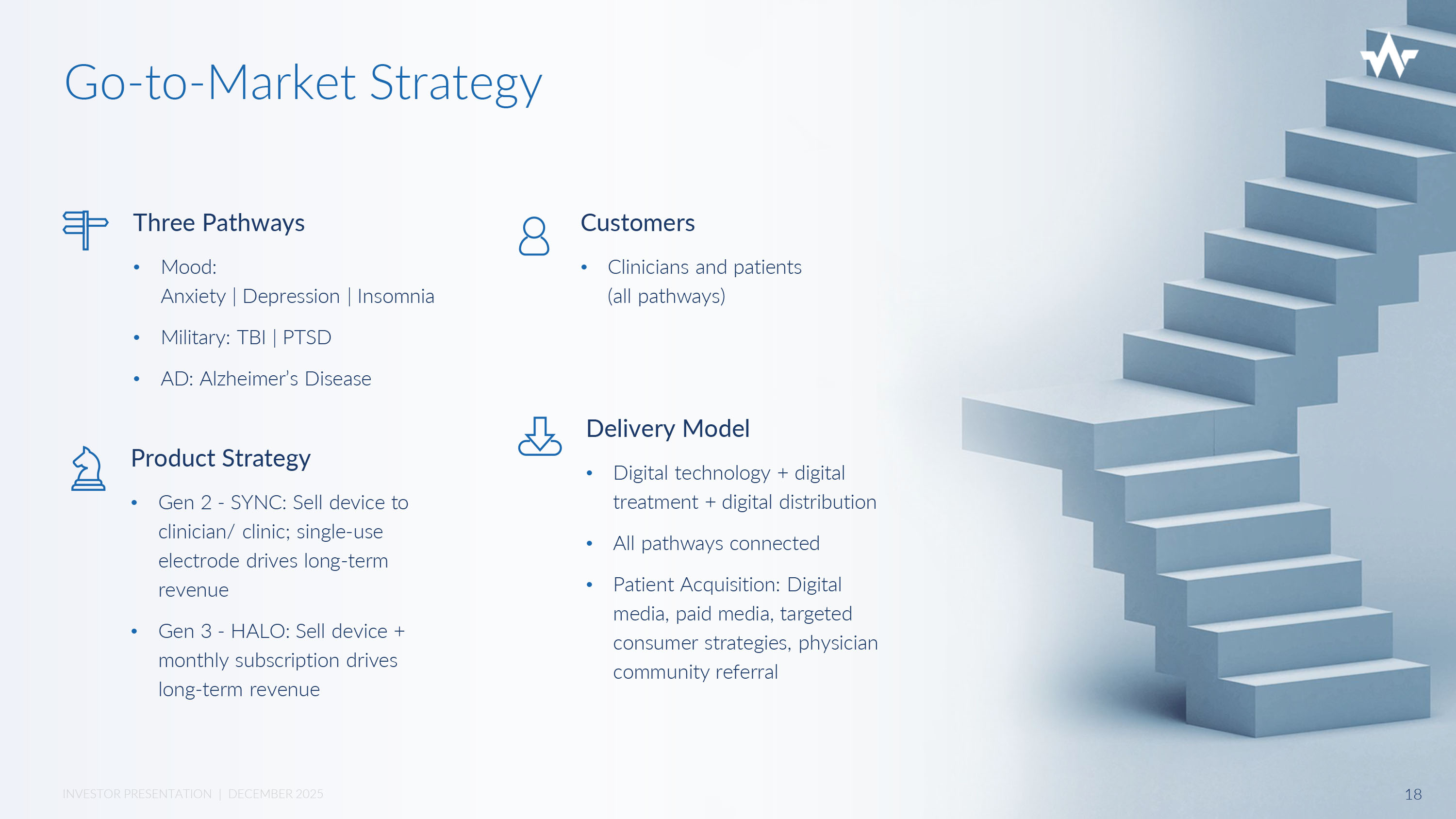
Go - to - Market Strategy Three Pathways • Mood: Anxiety | Depression | Insomnia • Military: TBI | PTSD • AD: Alzheimer’s Disease Customers • Clinicians and patients (all pathways) Delivery Model • Digital technology + digital treatment + digital distribution • All pathways connected • Patient Acquisition: Digital media, paid media, targeted consumer strategies, physician community referral Product Strategy • Gen 2 - SYNC: Sell device to clinician/ clinic; single - use electrode drives long - term revenue • Gen 3 - HALO: Sell device + monthly subscription drives long - term revenue 18 INVESTOR PRESENTATION | DECEMBER 2025 Nexalin is currently collaborating with FDA on clinical study design and strategy for De Novo clinical treatment application National and International Regulatory Strategy National: FDA applications (treatment, breakthrough) filing strategy: • First tranche - Insomnia, Alzheimer’s & Dementia • Second tranche - TBI/PTSD, Depression • Third tranche (in development) – Anxiety, Addiction, Chronic Pain International • Current clearance with NMPA in Asia (China): Depression, Insomnia • Current ANVISA approval in South America (Brazil): Anxiety, Depression, Insomnia • Current clearance with Ministry of Health in Middle East (Oman): Anxiety, Depression, Insomnia • Current clearance with Ministry of Health in Israel: Anxiety, Depression, Insomnia 19 INVESTOR PRESENTATION | DECEMBER 2025

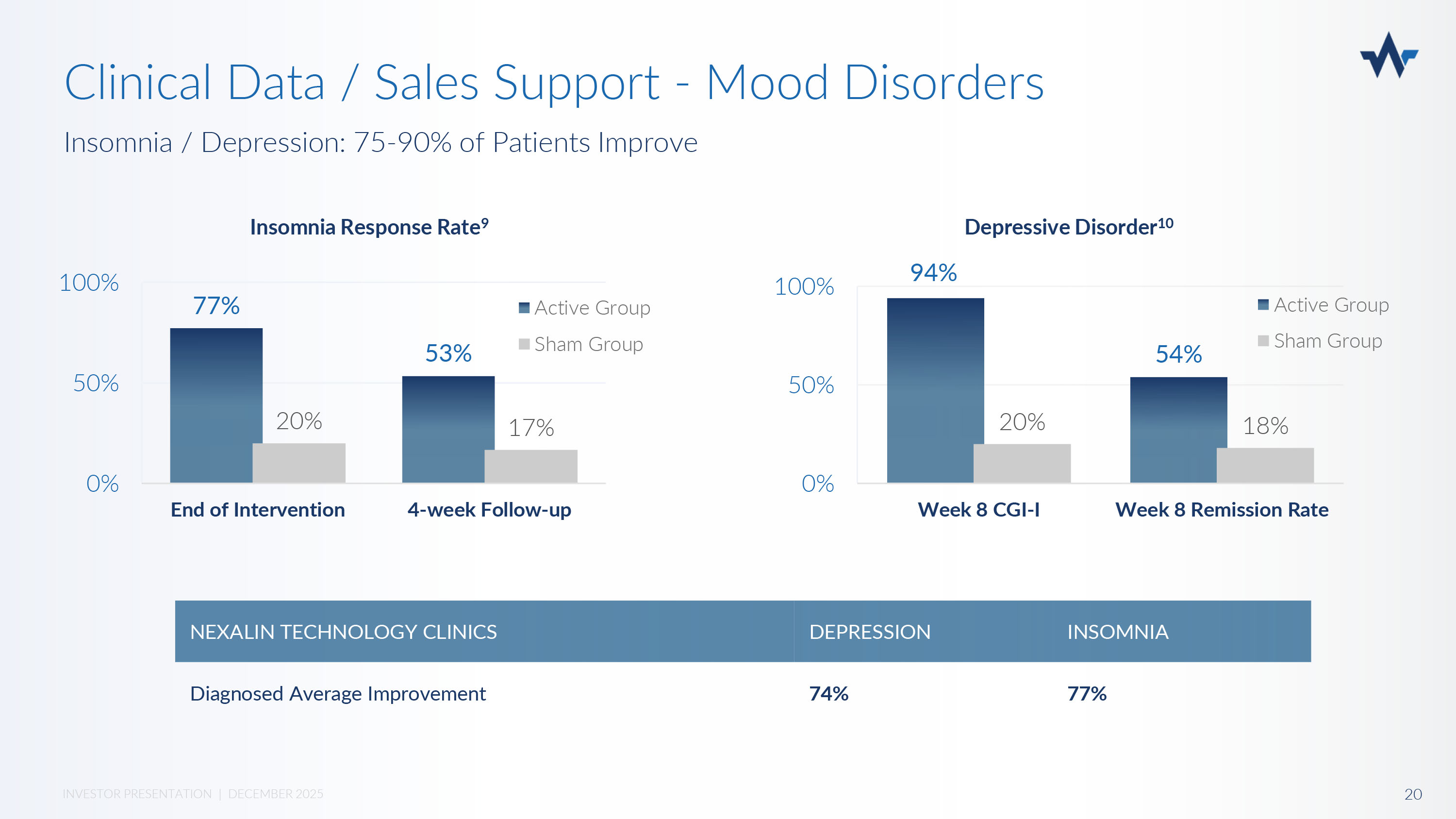
77% 53% 20% 17% 0% 50% 100% End of Intervention 4 - week Follow - up Active Group Sham Group 94% 54% 20% 18% 0% 50% 100% Week 8 CGI - I Week 8 Remission Rate Active Group Sham Group Insomnia Response Rate 9 Depressive Disorder 10 INSOMNIA DEPRESSION NEXALIN TECHNOLOGY CLINICS 77% 74% Diagnosed Average Improvement Insomnia / Depression: 75 - 90% of Patients Improve 20 INVESTOR PRESENTATION | DECEMBER 2025 Clinical Data / Sales Support - Mood Disorders David Lasseter Deputy Assistant Secretary of Defense for Policy.

Growing budgets and interest for military mental health services. Current emphasis is on treatment for traumatic brain injury and combat PTSD. William A. Hudson, Jr. General Counsel Department of Veterans Affairs 21 INVESTOR PRESENTATION | DECEMBER 2025 General Wesley Clark NATO Supreme Allied Commander Sales Channel Support - Military TBI and PTSD Congressman Don Davis House Armed Services Committee Nexalin Military & Government Advisory Board Clinical Data Summary Clinical Data / Sales Support - Alzheimer’s and Dementia Cognitive Improvement Treated patients showed: • Cognitive improvements (MMSE and MoCA) • Stronger hippocampal brain - network activity, Two key markers that decline early in Alzheimer’s disease.
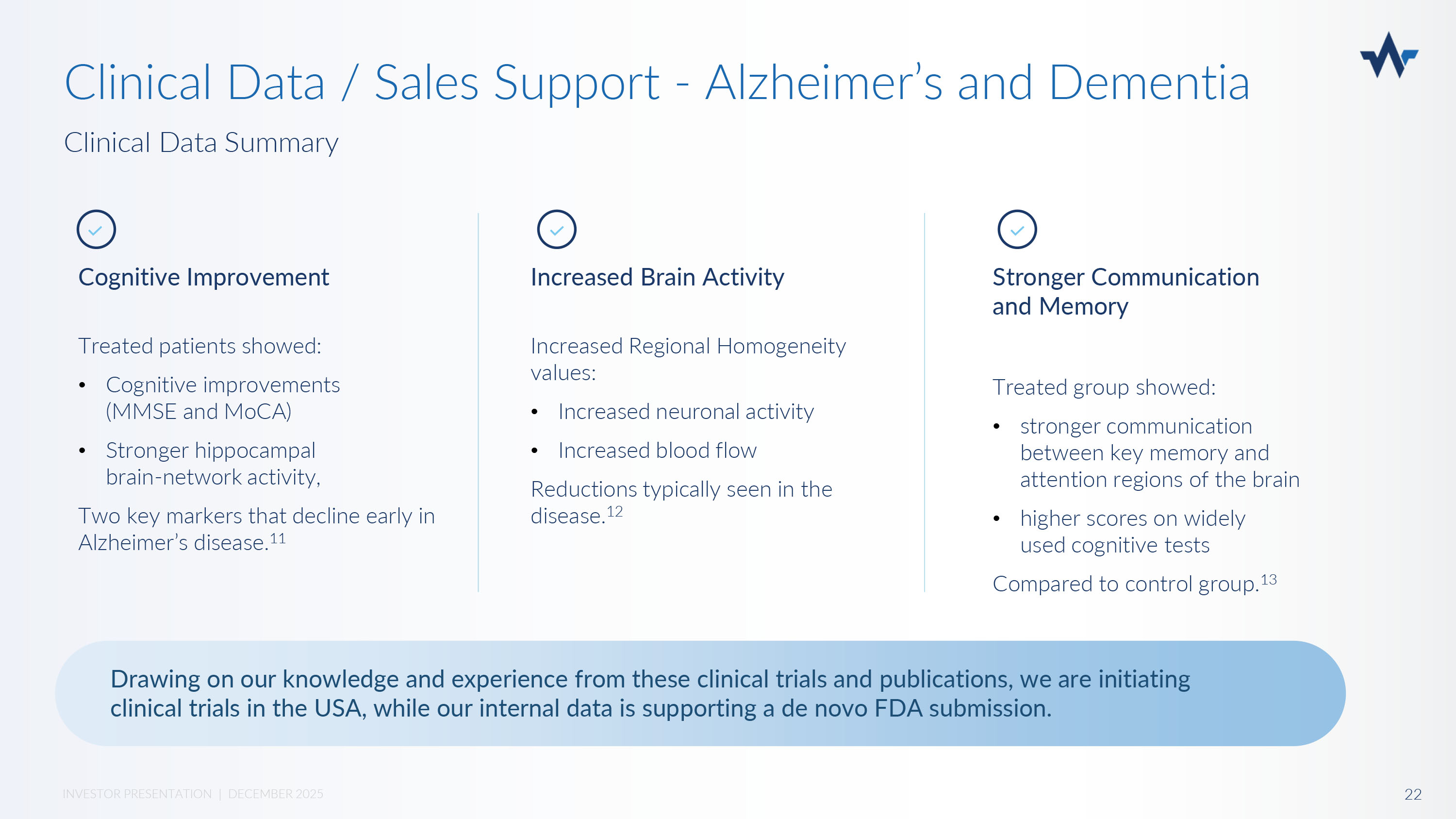
11 Increased Brain Activity Increased Regional Homogeneity values: • Increased neuronal activity • Increased blood flow Reductions typically seen in the disease. 12 Stronger Communication and Memory Treated group showed: • stronger communication between key memory and attention regions of the brain • higher scores on widely used cognitive tests Compared to control group. 13 Drawing on our knowledge and experience from these clinical trials and publications, we are initiating clinical trials in the USA, while our internal data is supporting a de novo FDA submission.

22 INVESTOR PRESENTATION | DECEMBER 2025 Validated by internal and peer - reviewed data in Alzheimer’s and dementia, Nexalin has convened a scientific advisory board of top specialists to guide development and accelerate market adoption: Alzheimer’s and Dementia Advisory Board Robert J. Rothstein, M.D. Abraham Scheer, M.D. Academic Affiliations: Brian Goldstein, M.D. 23 INVESTOR PRESENTATION | DECEMBER 2025 Global Market $ 1B Global Growth Strategy USA 24 INVESTOR PRESENTATION | DECEMBER 2025 South America Middle East China Israel

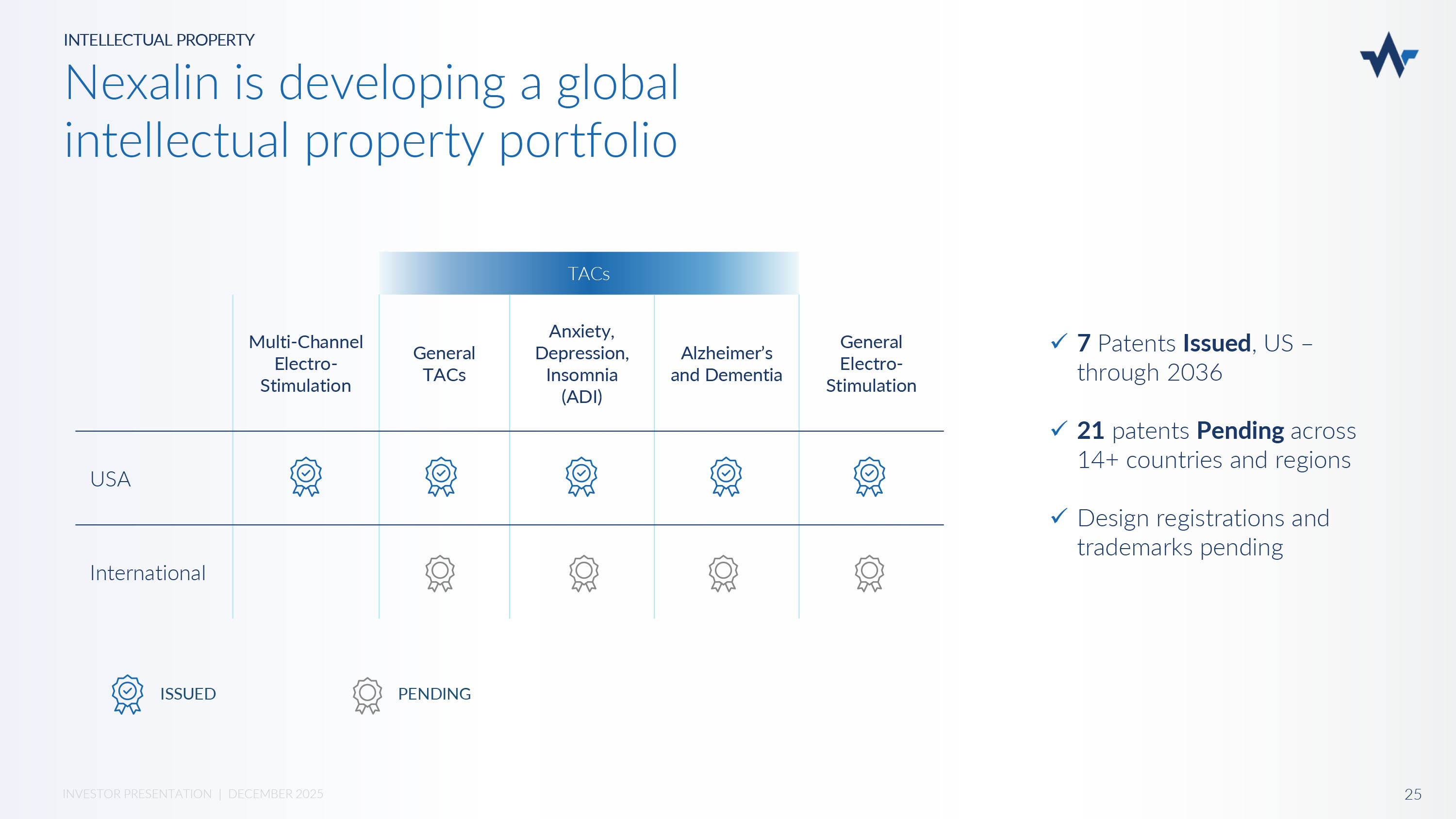
General Electro - Stimulation TACs Alzheimer’s and Dementia Anxiety, Depression, Insomnia (ADI) General TACs Multi - Channel Electro - Stimulation USA International PENDING ISSUED x 7 Patents Issued , US – through 2036 x 21 patents Pending across 14+ countries and regions x Design registrations and trademarks pending INTELLECTUAL PROPERTY Nexalin is developing a global intellectual property portfolio 25 INVESTOR PRESENTATION | DECEMBER 2025 NEXALIN FINANCIALS 26 INVESTOR PRESENTATION | DECEMBER 2025

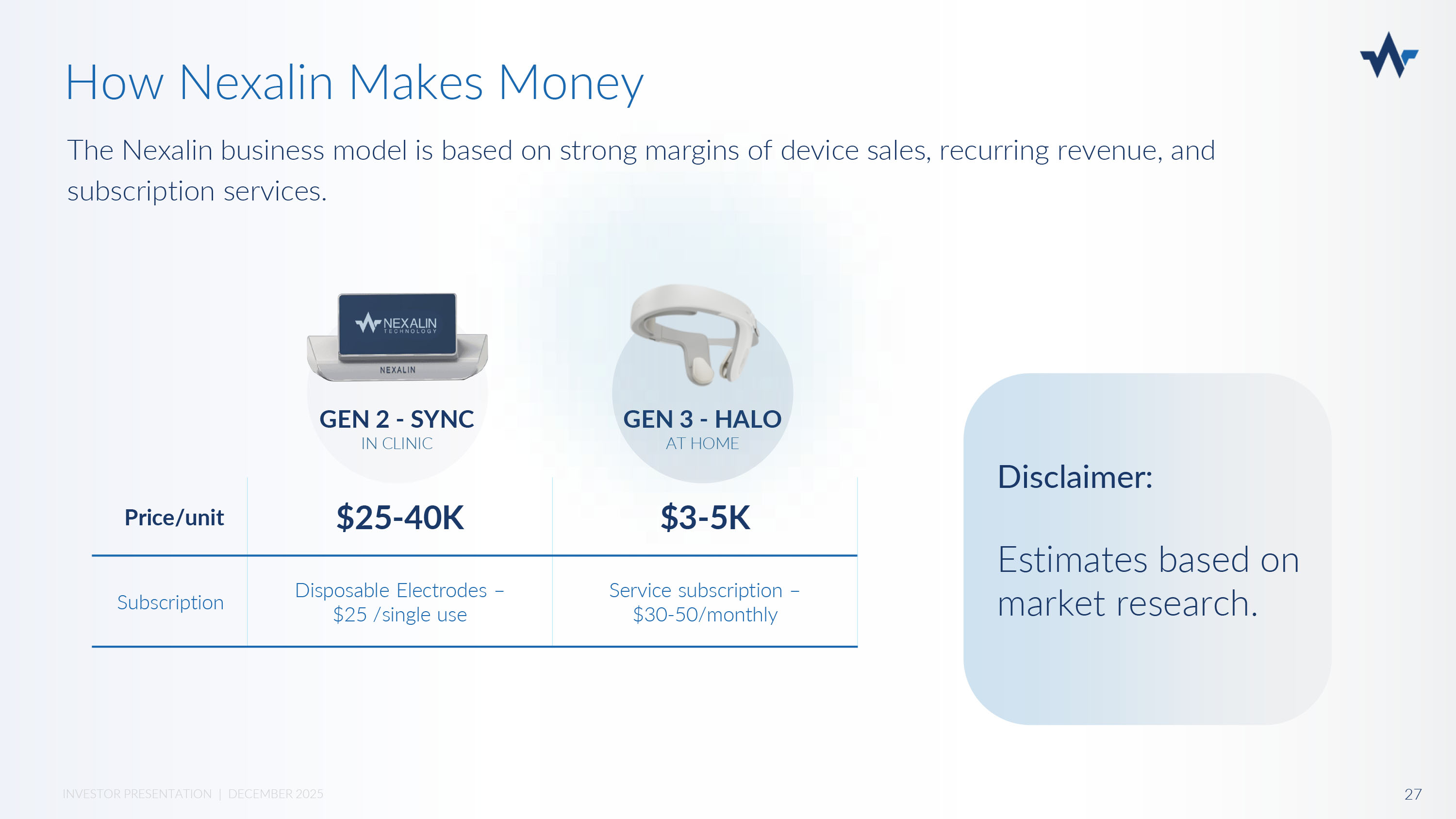
GEN 2 - SYNC IN CLINIC GEN 3 - HALO AT HOME $3 - 5K $25 - 40K Price/unit Service subscription – $30 - 50/monthly Disposable Electrodes – $25 /single use Subscription The Nexalin business model is based on strong margins of device sales, recurring revenue, and subscription services. How Nexalin Makes Money Disclaimer: Estimates based on market research.

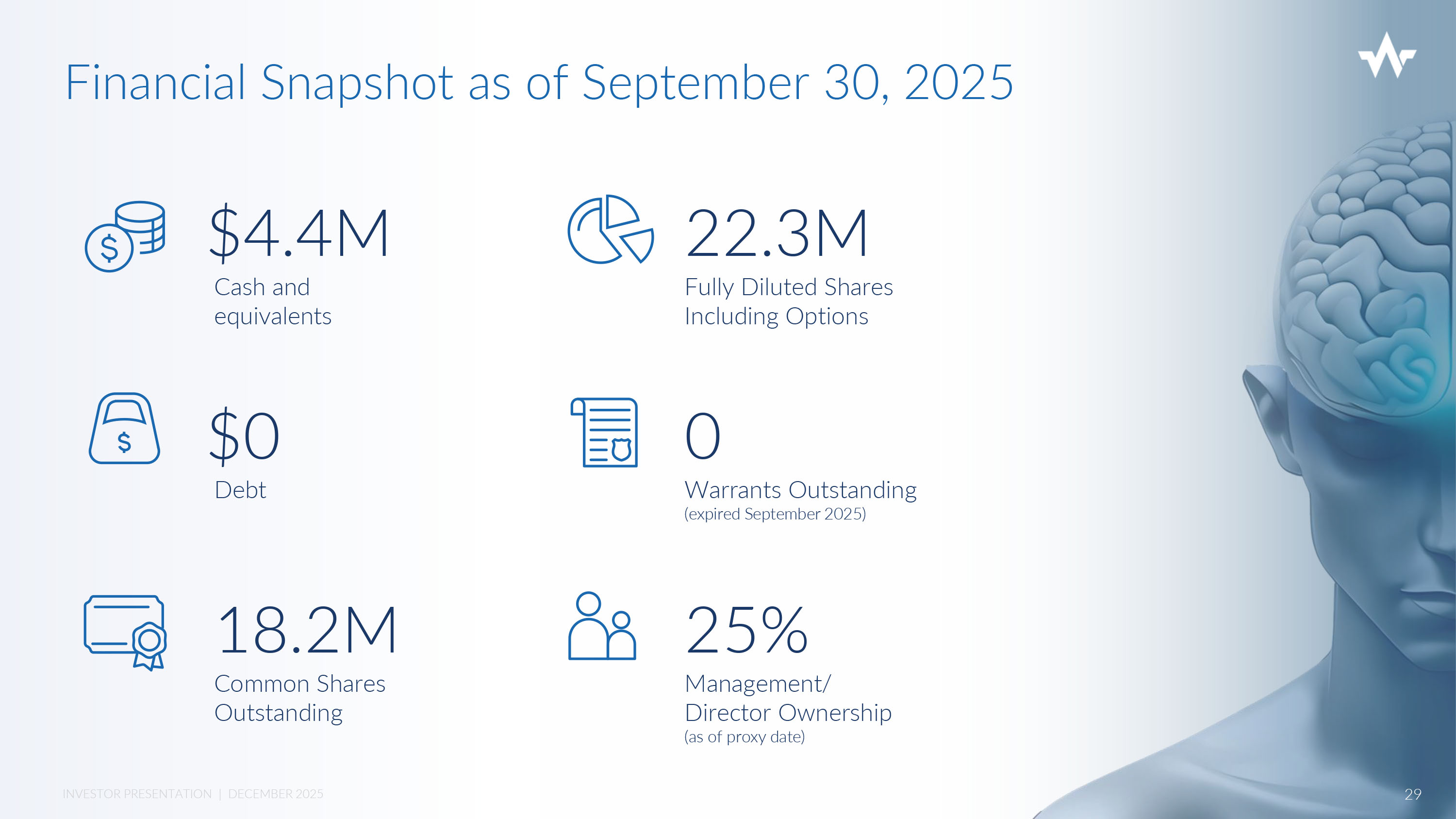
27 INVESTOR PRESENTATION | DECEMBER 2025 Nexalin Investment Summary Near - Term Gen 2 - SYNC revenue from initial Global and China JV Proprietary, effective & safe non - invasive treatment option in growing mental health market US Trials on Gen 2 – SYNC and Gen 3 – HALO expanding indications across mental health spectrum De Novo classification expected with FDA Military research and support for TBI and PTSD New Innovative Gen 3 - HALO In - Home Virtual Clinic: strongest in market 28 INVESTOR PRESENTATION | DECEMBER 2025 Financial Snapshot as of September 30, 2025 Cash and equivalents $4.4M $0 Debt 18.2M Common Shares Outstanding 22.3M Fully Diluted Shares Including Options 0 Warrants Outstanding (expired September 2025) 25% Management/ Director Ownership (as of proxy date) 29 INVESTOR PRESENTATION | DECEMBER 2025

HARNESSING THE POWER OF ADVANCED DIGITAL NEUROSTIMULATION TO IMPROVE MENTAL HEALTH NEXALIN NASDAQ: NXL Mark White President and Chief Executive Officer 30 INVESTOR PRESENTATION | DECEMBER 2025 Mark White President and Chief Executive Officer • Leads Nexalin’s executive team and provides leadership across global strategy, R&D, manufacturing, and treatment applications.

• Previously founded and operated clinics and addiction centres. • Pioneer in digital medical innovation, specializing in neuro - stimulation technologies. Justin Van Fleet Chief Financial Officer • 20+ years experience in public accounting with deep expertise in assurance services, IPOs, mergers, regulatory compliance, high - integrity reporting, and complex financial transactions. • Trusted advisor to boards and SEC - reporting clients. • Recognized for regulatory compliance and high - integrity reporting. • Licensed CPA (NY, NJ). Carolyn Shelton Chief Operations Officer and Executive VP of Regulatory, Quality and Clinical • 30+ years’ experience in global regulatory, quality, and clinical leadership. • Proven success in ensuring FDA, EU, and international compliance. • Driving Nexalin’s mission to advance neuro health through innovative solutions. Nexalin Leadership 31 INVESTOR PRESENTATION | DECEMBER 2025 Reynold Yordy Chief Technology Officer • 20+ years as a healthcare technology executive and entrepreneur.

• Early career at Eli Lilly supporting FDA - regulated clinical trials and analytics. • Senior roles in product, strategy, and data science at IBM Tivoli, Fujifilm Medical, and NetDirector (EHR/HL7 integrations). • Co - founder of Greenlight Ventures, launching platforms including The Recovery Platform, Health Logix, and OCHI. Zach Herrmann Chief Marketing Officer 32 INVESTOR PRESENTATION | DECEMBER 2025 • 15+ years in healthcare and technology marketing, brand development, and growth strategy. • Led campaigns for med - tech, telehealth, and behavioral health ventures, including The Recovery Platform and Health Logix. • Expertise in go - to - market strategy, investor communications, digital advertising, and partnerships. • Skilled at translating complex clinical technologies into clear, compelling value propositions. David Owens M.D. Chief Medical Officer • 30+ years experience in neuroradiology and neuroimaging with expertise in imaging for PTSD and TBI. • Medical Director of Radiology Consultation Services. • M.D. with degrees in Chemistry and Physics. • Senior member, American Society of Radiology.

Nexalin Leadership 1. Benjafield AV, Kuniyoshi FS, Malhotra A, et al. Estimation of the global prevalence and burden of insomnia: a systematic literature review - based analysis. Sleep Medicine Reviews. 2025;82:102121 - 102121. do i:https://doi.org/10.1016/j.smrv.2025.102121 2. World Health Organization 3. Yan J, Wang C, Sun B. Global, regional, and national burdens of traumatic brain injury from 1990 to 2021. Front Public Health. 2025;13:1556147. Published 2025 Apr 14. doi:10.3389/fpubh.2025.1556147 4. Market Research Future 5. Yahoo Finance 6. Grand View Research 7. S&S Insider 8. McHugh, R. K., Whitton, S. W., Peckham, A. D., Welge, J. A., & Otto, M. W. (2013). Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta - analytic review. The Journal of clinical psychiatry, 74(6) 9. Insomnia Clinical Trial - Effect of Transcranial Alternating Current Stimulation for the Treatment of Chronic Insomnia: A Randomized, Double - Blind, Parallel - Group, Placebo - Controlled Clinical Trial. https://pubmed.ncbi.nlm.nih.gov/31846980/ 10. Depression Clinical Trial - Transcranial alternating current stimulation for treating depression: a randomized controlled trial. https://pubmed.ncbi.nlm.nih.gov/35353887/ 11. Tang Y, Xing Y, Sun L, et al. TRanscranial AlterNating current stimulation FOR patients with mild Alzheimer's Disease (TRANSFORM - AD): a randomized controlled clinical trial. Alzheimers Res Ther. 2024;16(1):203. Published 2024 Sep 12. doi:10.1186/s13195 - 024 - 01570 - 0 12. Wang T, Yan S, Shan Y, et al. Altered Neuronal Activity Patterns of the Prefrontal Cortex in Alzheimer's Disease After Transcranial Alternating Current Stimulation: A Resting - State fMRI Study. J Alzheimers Dis. 2024;101(3):901 - 912. doi:10.3233/JAD - 240400 13. Wang T, Yan S, Shan Y, et al. Modulation of Cortical and Hippocampal Functional MRI Connectivity Following Transcranial Alternating Current Stimulation in Mild Alzheimer Disease. Radiology. 2025;315(3):e241463. doi:10.1148/radiol.241463 Sources/References 33 INVESTOR PRESENTATION | DECEMBER 2025