UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): December 4, 2025
2025-11-25
Picard Medical, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-42801 | 86-3212894 | ||
|
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 1992 E Silverlake Tucson AZ, 85713 |
| (Address of principal executive offices, including zip code) |
Registrant’s telephone number, including area code: (520) 545-1234
Not
Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, par value $0.0001 per share | PMI | The NYSE American, LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. | Regulation FD Disclosure. |
On December 4, 2025, Duffy Elmer, Engineering Project Manager of Picard Medical, Inc. (the “Company”) presented in vitro data on the Emperor Total Artificial Heart at the 31st Annual Meeting of the International Society for Mechanical Circulatory Support in Vienna, Austria. A copy of the abstract that Mr. Elmer presented on is furnished as Exhibit 99.1 and the slide deck utilized during his presentation is furnished as Exhibit 99.2, each of which is incorporated by reference herein.
The information in this report and the exhibits attached hereto shall not be deemed to be “filed” for purposes of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, regardless of any general incorporation language in such filing.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
The following exhibits are being filed herewith:
| Exhibit No. | Description | |
| 99.1 | Abstract of The Emperor Total Artificial Heart, dated December 4, 2025. | |
| 99.2 | Presentation Slide Deck, dated December 4, 2025. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Picard Medical, Inc. | |||
| By: | /s/ Patrick NJ Schnegelsberg | ||
| Name: | Patrick NJ Schnegelsberg | ||
| Title: | Chief Executive Officer | ||
Dated: December 4, 2025
Exhibit 99.1
Dcvicc »csign Figure 1. Emperor Leverages STAH Materials and Construction, Eliminates External Drivers A 1A. The SynCardia Total Artificial Heart B 1B. The Emperor Total Artificial Heart The Emperor TAH maintains the use of the clinically - proven materials, components, and geometry of the STAH but eliminates the need for external pneumatic drivers . Design properties held in common are : Atrial Inflow Grafts Arterial Outflow Grafts Inflow/Outflow Connectors Mechanical Valves Blood Chamber Geometry Diaphragm Assemblies Ventricle Housings Mctko»s To simulate in vivo implant conditions, Emperor TAH prototypes were connected to a Donovan Mock Circulation Tank (MCT) . 2 Performance and function were evaluated and compared to the 50 cc STAH . Both systems were powered at 125 beats per minute (BPM), and cardiac output was assessed across a range of right atrial pressures to model preload response . Both systems underwent additional investigation of afterload sensitivity on the MCT by assessing cardiac output while increasing mean aortic pressure at 125 BPM while maintaining right atrial pressure at 10 mmHg . Further evaluation of the Emperor TAH was conducted in three acute porcine models . Following median sternotomy, cardiectomy, and device implantation, pressure lines were introduced into the atria and the aorta . A Swan - Ganz catheter was placed in the pulmonary artery to monitor flow rates . The device output was assessed for preload sensitivity and afterload independence in all three models by adjusting blood volume via the bypass circuit and vascular resistance via vasopressor administration . (Phenylephrine, 1 - 3 ug/kg/min IV) Figure 4. Emperor Exhibits Afterload Independence In Vitro In viẼío »citoimancc The Emperor TAH demonstrated increased cardiac output at a constant beat rate in response to increased right atrial pressure, indicating effective autoregulation in response to changing hemodynamic conditions . Overall autoregulation was comparable to that of the 50 cc STAH [see Figure 3 ] . The Emperor TAH reproduces the preload sensitivity of the pneumatic SynCardia Total Artificial Heart by replicating its fundamental principle of variable fill volume and full ejection . 6 1 5 4 Cardiac Output (L/min) 3 2 0 125 BPM 4 6 Emperor TAH STAH 8 10 12 Right Atrial Pressure (mmHg) 14 Figure 3. Emperor Exhibits Frank - Starling Autoregulation In Vitro 7 The Emperor TAH demonstrated sustained cardiac output at a constant beat rate in response to increased aortic pressure, demonstrating insensitivity to afterload . This performance reproduces the afterload independence of the pneumatic SynCardia Total Artificial Heart, as shown in Figure 4 . As a volume displacement pump, the STAH provides consistent flow against variable outflow pressures . The Emperor TAH, by replicating its mechanism of action, exhibits the same degreeof afterload independence . In vivo »citoimancc We report the first in vivo implantation of the Emperor TAH in a porcine model, conducted to assess surgical feasibility, hemodynamic performance, and anatomical compatibility during acute support . The evaluation aimed to confirm that the proven SynCardia blood - contacting system maintains physiologic performance when driven by the new electromechanical actuation platform .. Discussion In vitro testing showed that the Emperor TAH maintained constant cardiac output across increasing aortic pressures, demonstrating afterload independence equivalent to the pneumatic SynCardia Total Artificial Heart (Figure 4 ) . As a volume - displacement pump with identical chamber geometry, diaphragm, and valve architecture, the Emperor TAH replicates the STAH ’ s characteristic insensitivity to outflow pressure . Acute in vivo implantation further confirmed stable support and hemodynamic equivalence to the pneumatic platform, validating physiologic performance under electromechanical drive . These results reinforce the reliability of the shared blood - contacting architecture and establish a solid foundation for chronic large - animal studies and future clinical translation . Pcitoimancc Acute in vivo testing demonstrated stable support and hemodynamic equivalence to the pneumatic STAH, confirming that the Emperor TAH maintains the physiologic performance of the shared chamber, diaphragm, and valve system . 2025 Intio»uction The SynCardia Total Artificial Heart (STAH) is a clinically proven and highly effective treatment for end - stage biventricular heart failure . 1 With over 2 , 100 implants, it remains the only commercially available and FDA - approved total artificial heart on the market . The STAH provides robust and stable circulatory support, enabled by its pneumatic actuation system, which offers a broad range of autoregulation and afterload independence . 2 SynCardia is developing the Emperor Total Artificial Heart (Emperor TAH), a motor - driven evolution of the SynCardia STAH that preserves the proven blood - contacting architecture while replacing the external pneumatic driver with a compact electromechanical system . The device consists of two 50 cc STAH ventricles integrated with a novel mechanical actuation system engineered to replicate STAH physiology, enable full mobility, and serve as the foundation for a fully implantable platform . To validate functional continuity, we evaluated autoregulation equivalence between the pneumatic STAH and the mechanically driven Emperor TAH . All blood - contacting surfaces the pumping chamber geometry, polyurethane diaphragm, Syn - Hall inflow and outflow valves are identical to the FDA - approved SynCardia Total Artificial Heart . Only the actuation mechanism has been modernized from pneumatic to motor - driven pusher plate motion . This design preserves established biocompatibility, hemocompatibility, and flow performance while enabling next - generation implantability . Clinical durability of the legacy platform is well demonstrated, including a patient exceeding eight years of continuous TAH support, underscoring the robustness of the shared chamber, diaphragm, and valve system . 2 1 0 5 4 Cardiac Output (L/min) 3 80 125 BPM 85 Emperor TAH STAH RAP: 10 mmHg 90 95 100 Mean Aortic Pressure (mmHg) 105 110 A B STAH Emperor TAH C D 2A. STAH Diastole. Air is evacuated from behind the diaphragm, allowing blood to refill through the inflow valve 2B. STAH Systole. Air pressure is applied to the diaphragm, pushing blood through the outflow valve 2C. Emperor TAH Diastole. Pusher is evacuated from behind the diaphragm, allowing blood to refill through the inflow valve 2D. Emperor TAH Systole. Pusher is applied to the diaphragm, pushing blood through the outflow valve Mcckanism Figure 2. Mechanical Actuation Preserves “Partial Fill – Full Eject” Autoregulation ľkc Em»cioi ľotal 6ititicial Hcait 6 ne … t - geneí»tion, tull; - im ʷ l»nt»kle S;nC»ídi» Tot»l 6 ítitici»l He»ít D»fifiQ «lmgí* ( 1 ), An»íg M Simon ( 1 ), "oz ” ino»» a€»i ( 2 )( 3 ), «»waí» W BgЧЧgíЧon ( 2 ), SafiiQa ” A»»gzzalam ( 1 )( 4 ), MaЧЧ ” gw S Sc ” »zЧgí ( 1 ) 1 . SQnCaí»ia SQzЧgmz, LLC, "»czon, USA; 2 . AíЧifiicial HgaíЧ Pío«íam, Banngí UnivgíziЧQ Mg»ical CgnЧgí, "»czon, USA; 3 . Dg»aíЧmgnЧ ofi S»í«gíQ, UnivgíziЧQ ofi Aíi€ona Collg«g ofi Mg»icing, "»czon, USA; 4 . Dg»aíЧmgnЧ ofi Biomg»ical «n«inggíin«, UnivgíziЧQ ofi Aíi€ona, "»czon, USA 1. Copeland et al., N Engl J Med 2004;351:859 - 867 2. Crosby et al., ASAIO J. 2015 May - Jun; 61(3):274 – 281 Stabilization: Baseline MAP, RAP, LAP, PAP, device pressures, and outflow waveforms were recorded under steady - state conditions. Preload Manipulation : Venous return was reduced and restored via CPB reservoir adjustments to evaluate preload responsiveness through changes in pump output and atrial pressures . Afterload Challenge : Systemic vascular resistance was increased with a norepinephrine bolus to assess afterload tolerance and maintenance of balanced left – right pump output . Rate Challenge: Pump rate was increased stepwise to characterize performance across varying beat rates. Stu»Q »csign Four standardized test maneuvers were performed: 1 2 3 4
Exhibit 99.2
SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.

The Emperor Total Artificial Heart: A Next Generation, Fully Implantable Total Artificial Heart Duffy Elmer 1 , Andre R Simon 1 , Toshinobu Kazui 2,3 , Edward W Betterton 2 , Safiyah Abdessalam 1,4 , Matthew S Schuster 1 (1) SynCardia Systems, LLC, Tucson, USA; (2) Artificial Heart Program, Banner University Medical Center, Tucson, USA; (3) Department of Surgery, University of Arizona College of Medicine, Tucson, USA; and (4) Department of Biomedical Engineering, University of Arizona, Tucson, USA ISMCS December 4 th , 2025 Disclaimers SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved. General Picard Medical, Inc. (“PMI”) is making this presentation available in connection with an update on its business operations an d s trategies. The information contained in this presentation does not purport to be complete or to be all - inclusive or to contain all of the information that prospective investors may require. Prospective investors are encouraged to conduct the ir own analysis and review of information contained in this presentation as well as important additional information through the Securities and Exchange Commission’s (“SEC”) EDGAR system at www.sec.gov and on our website at www.picardmedical.com . Forward - Looking Statements This presentation contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other applicable securities laws. All statements other than statements of historical fact are forward - looking statements, including, but not limited to, statements regarding the Company’s future financial position, business str ate gy, budgets, projected costs, and plans and objectives of management for future operations. Words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “project,” “will,” “would,” “could,” “should,” and si mil ar expressions are intended to identify forward - looking statements. In addition, any statements that refer to projections, forecasts, or other characterizations of future events or circumstances, including any underlying assumptions, a re forward - looking statements. Forward - looking statements in this presentation may include risks and uncertainties which are provided to you and indicated from time to time as described in PMI’s most recent Forms 10 - Q and 8 - K fil ed or furnished with the SEC. You should also carefully consider the risks and uncertainties described and the information presented in or incorporated by reference into PMI’s registration statement and subsequent current, quarterly, an d a nnual reports filed with or furnished to the SEC. These filings or potential filings may identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward - looking statements referred to herein. These statements are based on current expectations, estimates and projections about the industry and markets in which the Company operates, as well as management’s beliefs and a ssu mptions. These statements are not guarantees of future performance and involve certain risks, uncertainties and assumptions that are difficult to predict. Actual results may differ materially from those expressed or imp lie d in the forward - looking statements. The Company undertakes no obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise, except as required by law. No Offer or Solicitation This presentation does not constitute an offer to sell or a solicitation of an offer to buy any securities of the Company, no r s hall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. Third - Party Information Certain information contained in this presentation has been obtained from published and non - published sources prepared by third parties, which in certain cases have not been independently verified. While such information is believed to be reliable, the Company makes no representation or warranty, express or implied, as to the accuracy or completeness of such inf ormation. Trademarks and Copyrights All trademarks, service marks and trade names appearing in this presentation are the property of their respective owners. The co ntents of this presentation are protected by copyright and may not be reproduced, distributed or used without the prior written consent of the Company. SynCardia Systems, LLC, together with its respective subsidiaries, directors, officers, employees, advisers, and agents, has not prepar ed , verified or approved the contents of this presentation and expressly disclaims any responsibility or liability for its accuracy, completeness, or adequacy. No representation, warranty, or understanding, express or implied, is mad e by any of the above parties regarding this presentation and no reliance should be placed on it in connection with any investment decision. All communications outside of this transaction - focused document should not be relied on and partic ipation in the transaction disclaims previous communications.
The SynCardia Total Artificial Heart (STAH) Over 2,100 Implants and Only FDA Approved Total Artificial Heart SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.
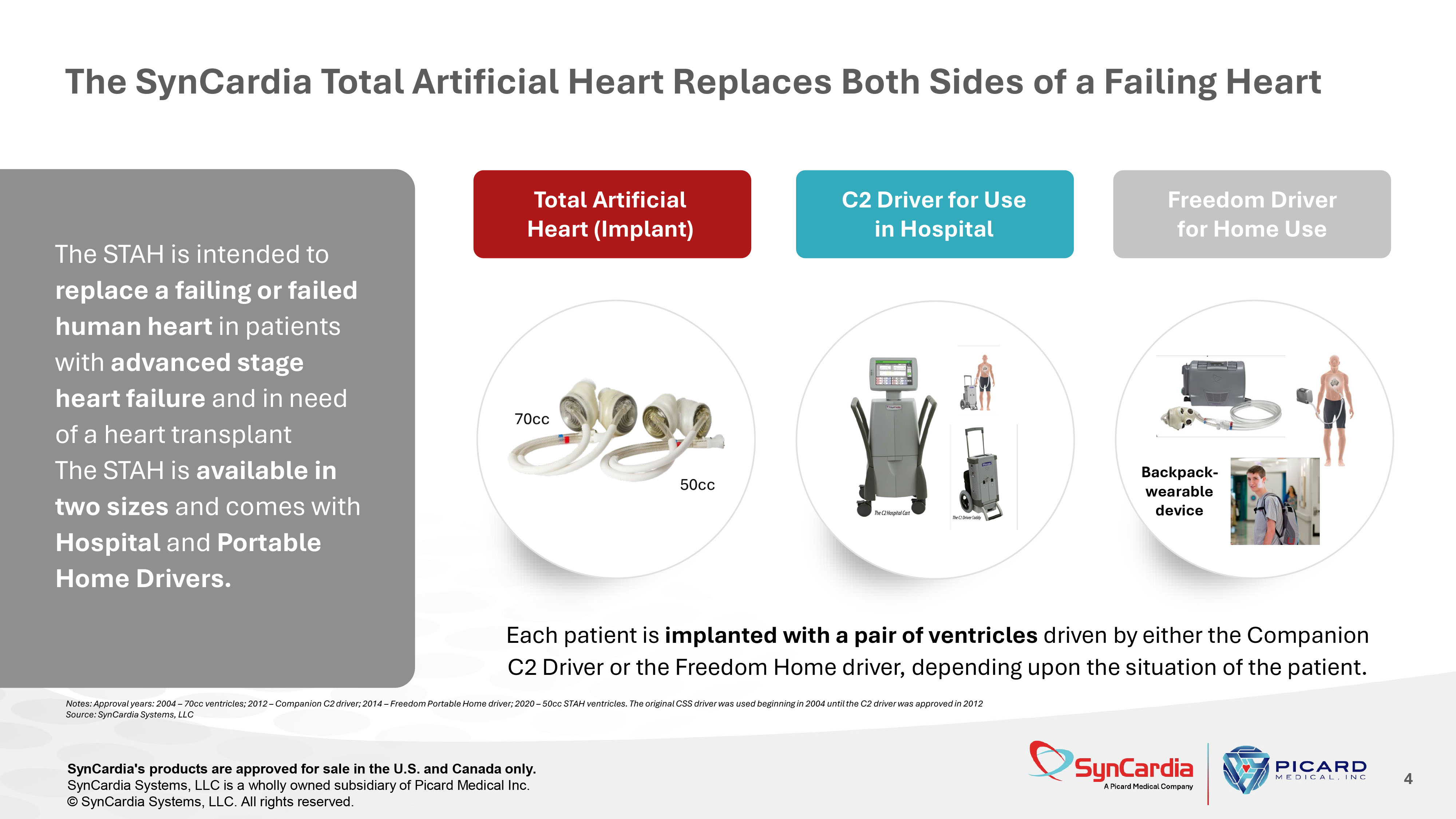
The SynCardia Total Artificial Heart Replaces Both Sides of a Failing Heart 4 Each patient is implanted with a pair of ventricles driven by either the Companion C2 Driver or the Freedom Home driver, depending upon the situation of the patient. The STAH is intended to replace a failing or failed human heart in patients with advanced stage heart failure and in need of a heart transplant The STAH is available in two sizes and comes with Hospital and Portable Home Drivers. Backpack - wearable device C2 Driver for Use in Hospital Freedom Driver for Home Use Total Artificial Heart (Implant) Notes: Approval years: 2004 – 70cc ventricles; 2012 – Companion C2 driver; 2014 – Freedom Portable Home driver; 2020 – 50cc STAH ventricles. The original CSS driver was used beginning in 2004 until the C2 driver was approved in 2012 Source: SynCardia Systems, LLC SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.
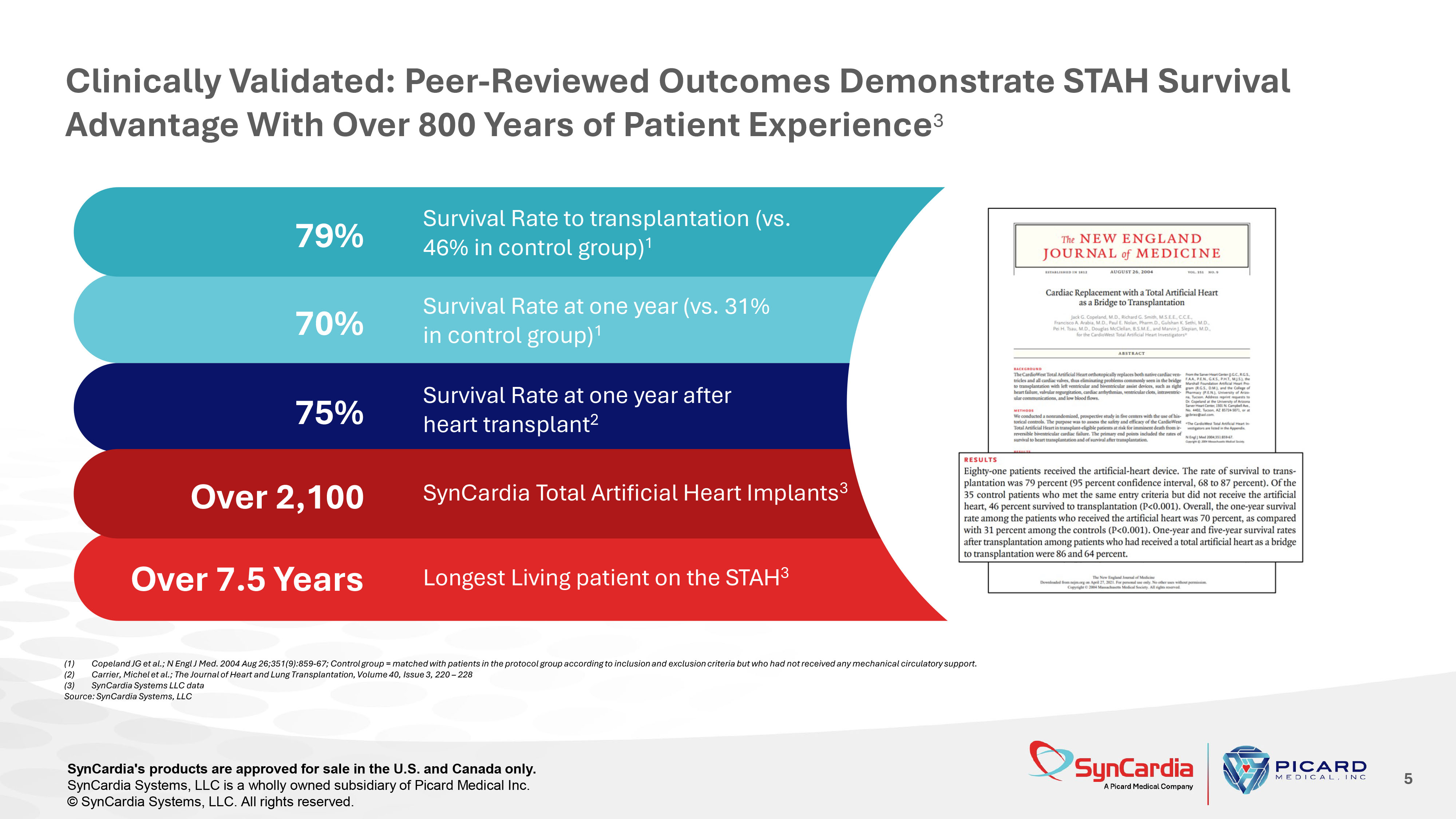
Survival Rate to transplantation (vs. 46% in control group) 1 79% Survival Rate at one year (vs. 31% in control group) 1 70% 75% Survival Rate at one year after heart transplant 2 Over 2,100 SynCardia Total Artificial Heart Implants 3 Over 7.5 Years Longest Living patient on the STAH 3 Clinically Validated: Peer - Reviewed Outcomes Demonstrate STAH Survival Advantage With Over 800 Years of Patient Experience 3 5 (1) Copeland JG et al.; N Engl J Med. 2004 Aug 26;351(9):859 - 67; Control group = matched with patients in the protocol group accordi ng to inclusion and exclusion criteria but who had not received any mechanical circulatory support. (2) Carrier, Michel et al.; The Journal of Heart and Lung Transplantation, Volume 40, Issue 3, 220 – 228 (3) SynCardia Systems LLC data Source: SynCardia Systems, LLC SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.
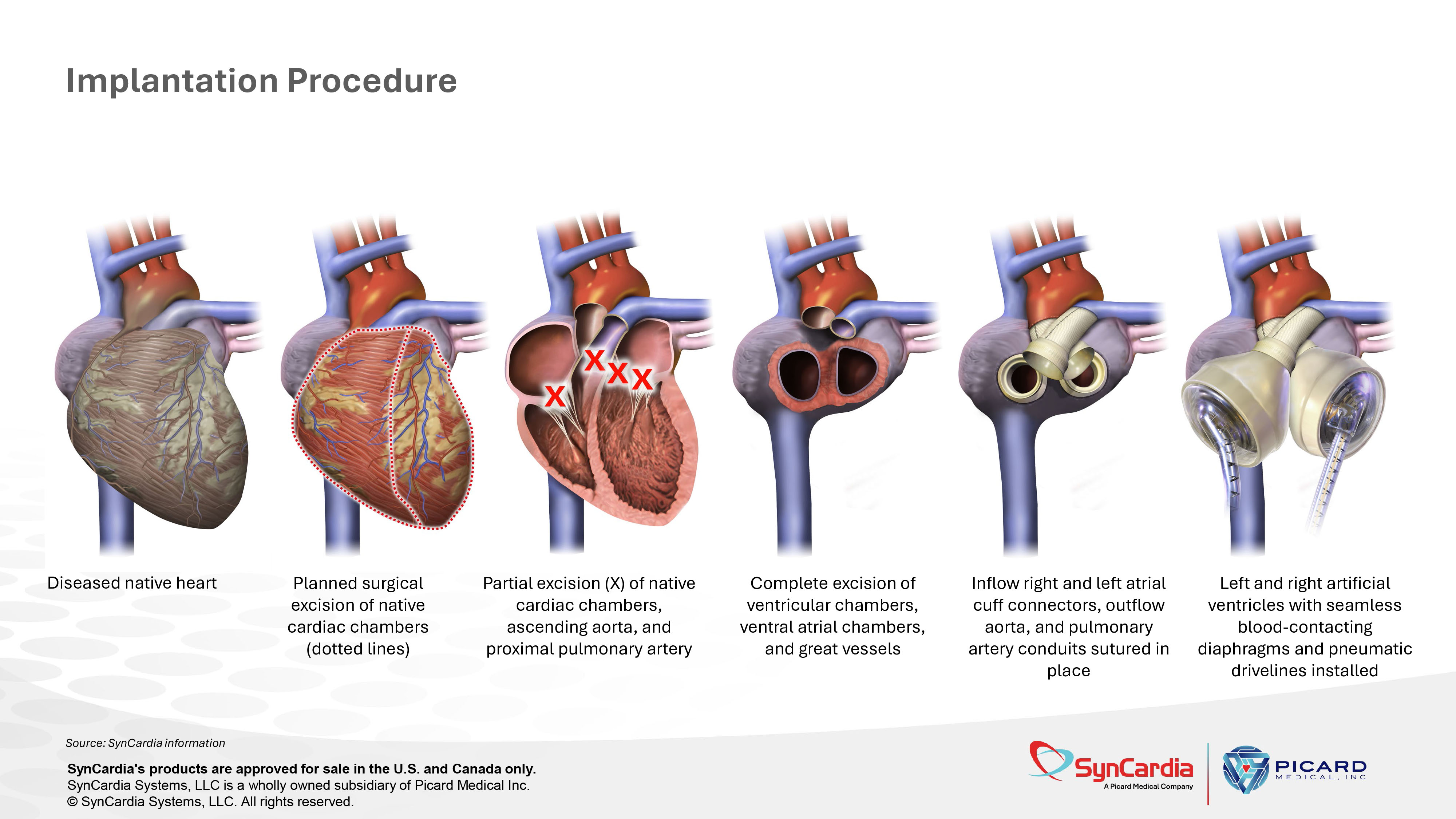
Diseased native heart Planned surgical excision of native cardiac chambers (dotted lines) Partial excision (X) of native cardiac chambers, ascending aorta, and proximal pulmonary artery Complete excision of ventricular chambers, ventral atrial chambers, and great vessels Inflow right and left atrial cuff connectors, outflow aorta, and pulmonary artery conduits sutured in place Left and right artificial ventricles with seamless blood - contacting diaphragms and pneumatic drivelines installed Implantation Procedure Source: SynCardia information SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.
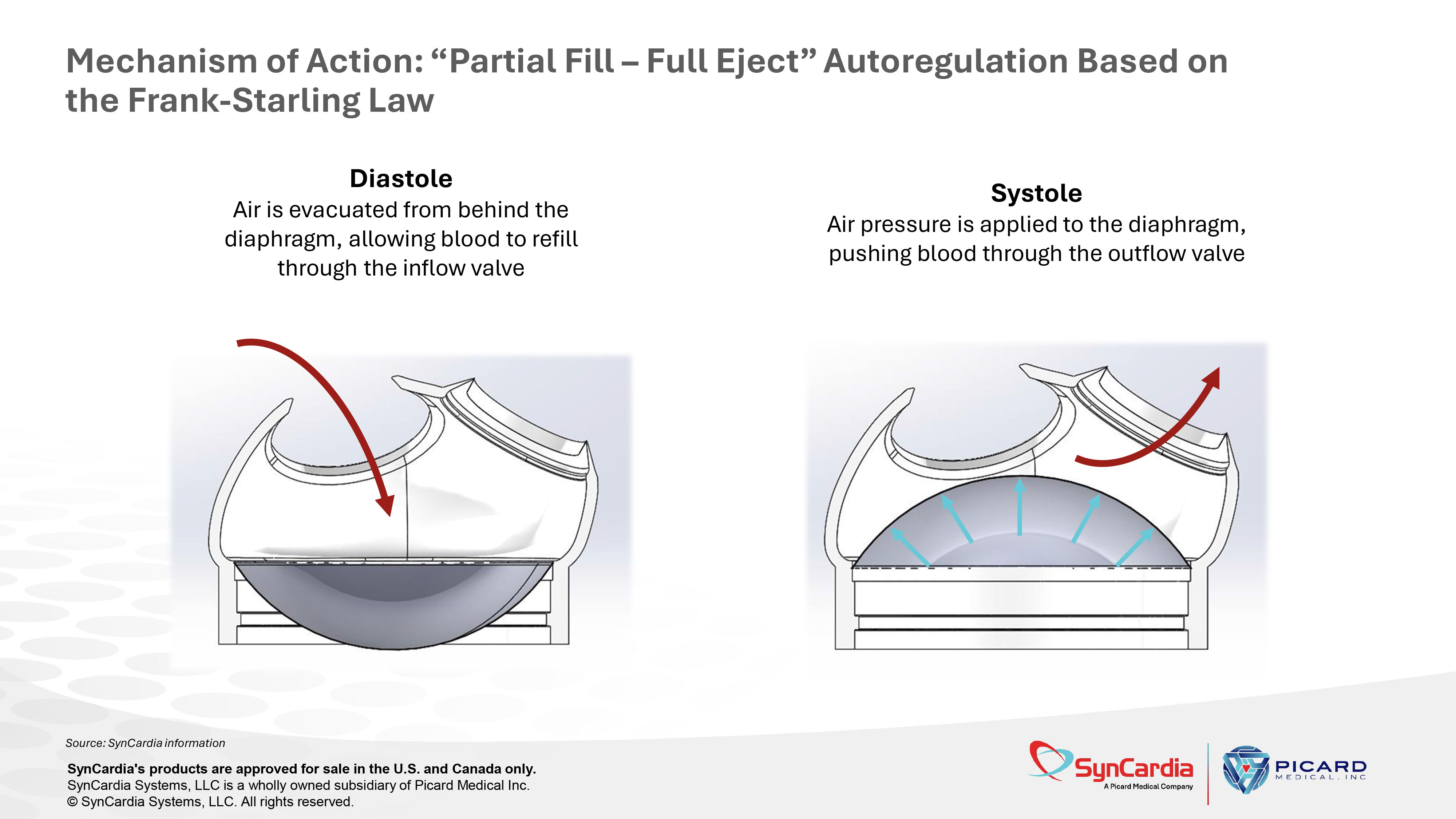
Diastole Air is evacuated from behind the diaphragm, allowing blood to refill through the inflow valve Systole Air pressure is applied to the diaphragm, pushing blood through the outflow valve Mechanism of Action: “Partial Fill – Full Eject” Autoregulation Based on the Frank - Starling Law Source: SynCardia information SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.
The Emperor Total Artificial Heart (ETAH) Fully Implantable TAH in Development as Alternative to Heart Transplantation SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.
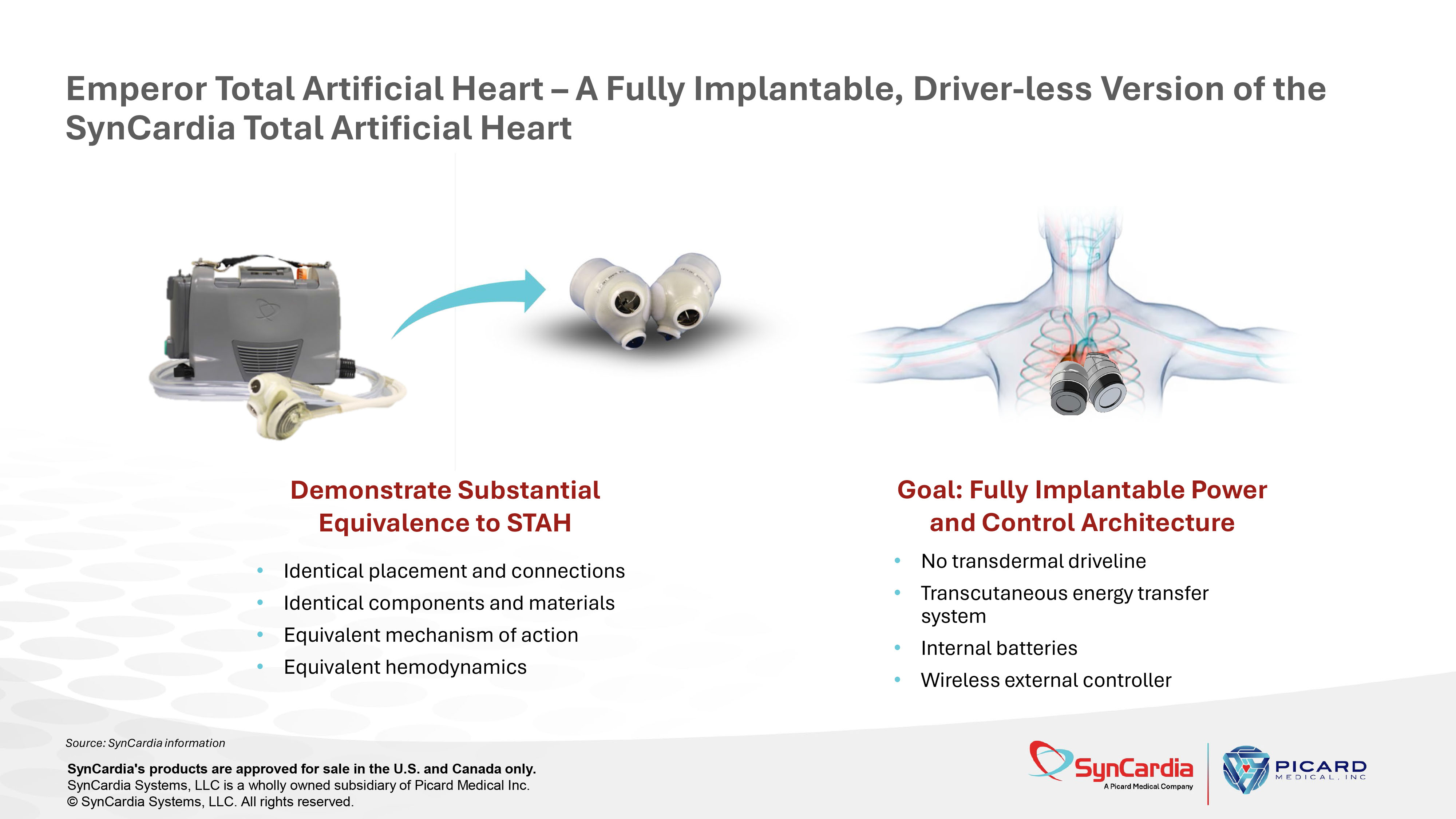
Source: SynCardia information Emperor Total Artificial Heart – A Fully Implantable, Driver - less Version of the SynCardia Total Artificial Heart • Identical placement and connections • Identical components and materials • Equivalent mechanism of action • Equivalent hemodynamics • No transdermal driveline • Transcutaneous energy transfer system • Internal batteries • Wireless external controller Demonstrate Substantial Equivalence to STAH Goal: Fully Implantable Power and Control Architecture SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.
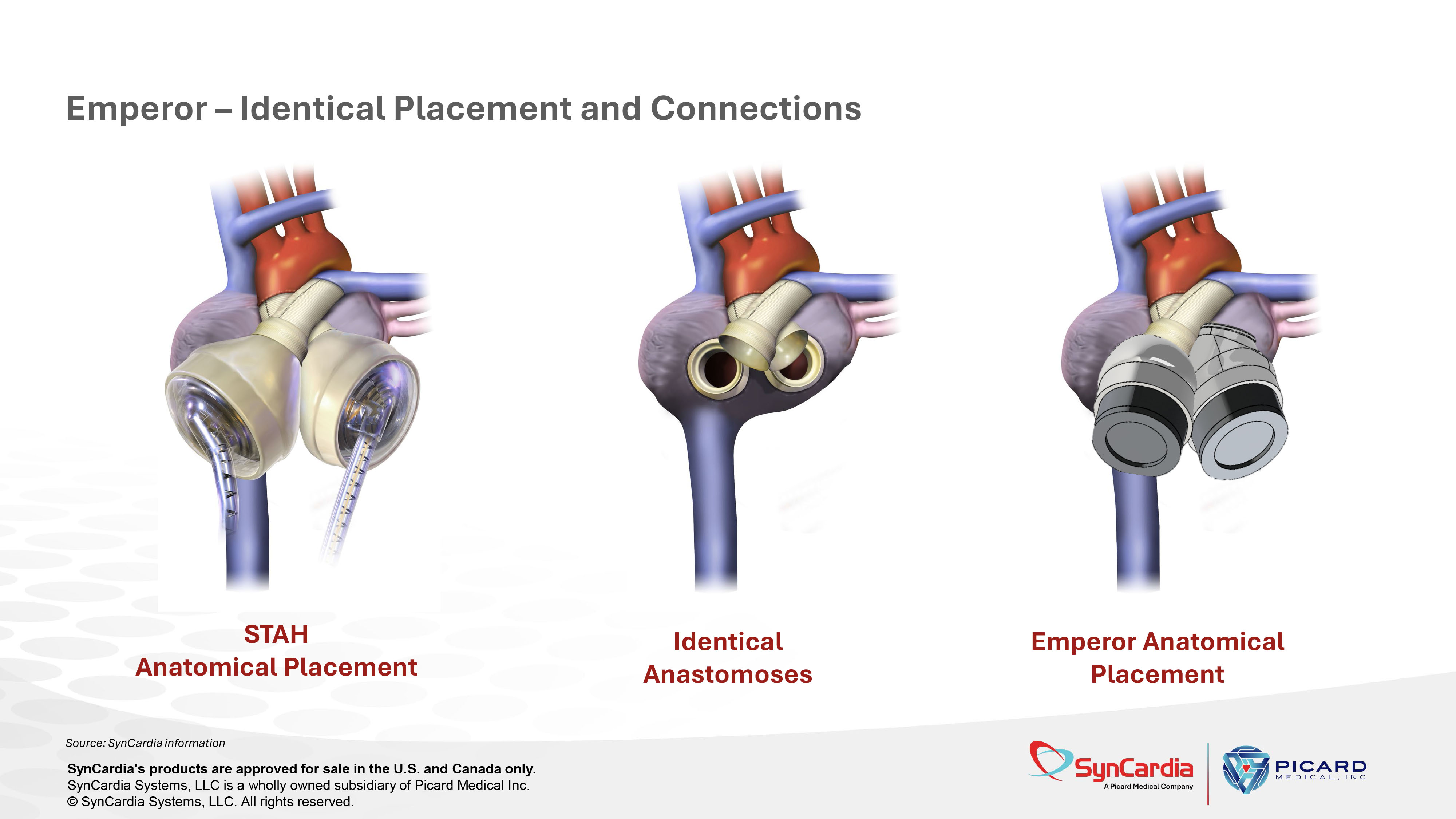
Source: SynCardia information Emperor – Identical Placement and Connections STAH Anatomical Placement Identical Anastomoses Emperor Anatomical Placement SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.
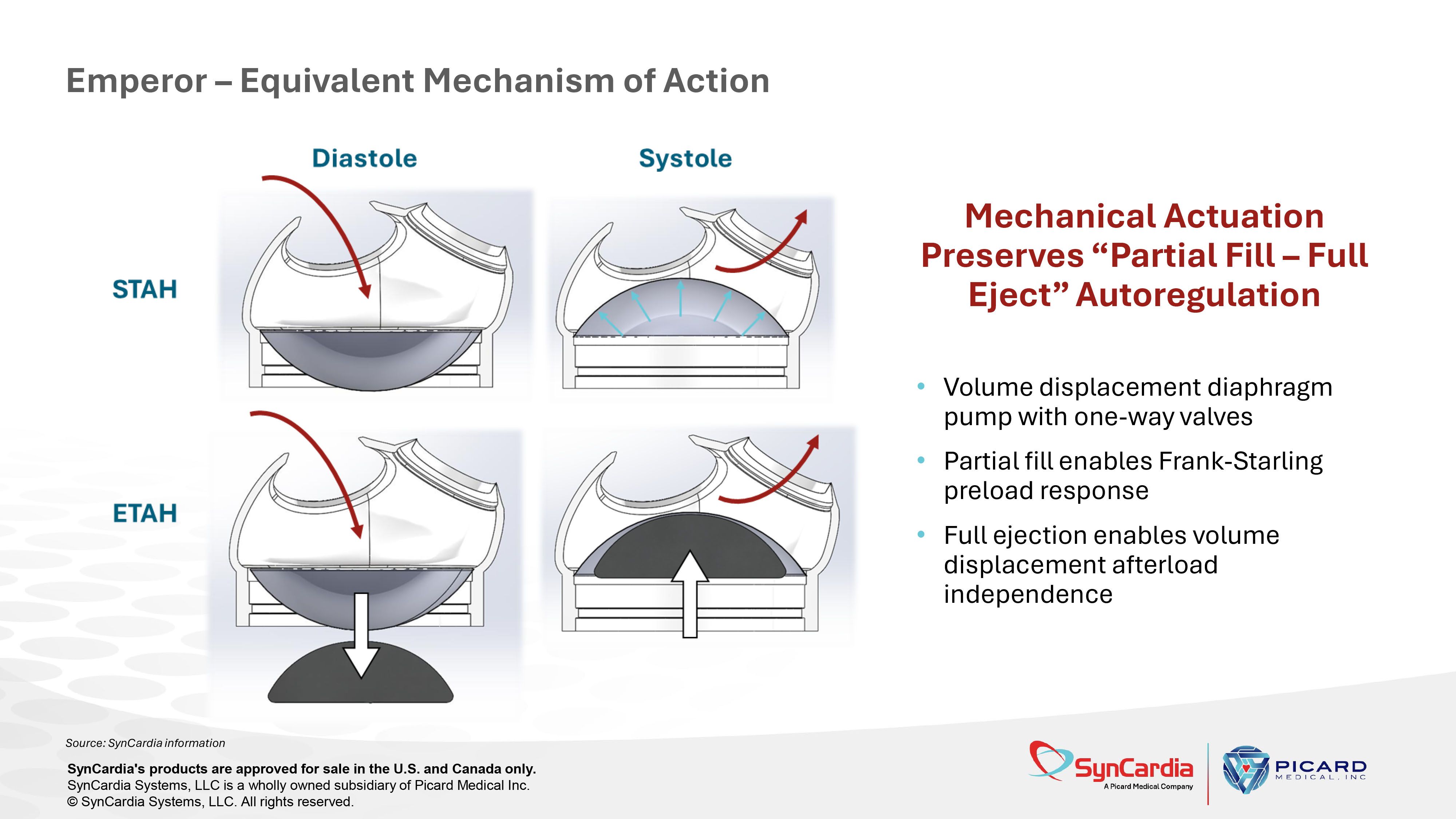
Mechanical Actuation Preserves “Partial Fill – Full Eject” Autoregulation • Volume displacement diaphragm pump with one - way valves • Partial fill enables Frank - Starling preload response • Full ejection enables volume displacement afterload independence Emperor – Equivalent Mechanism of Action SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.
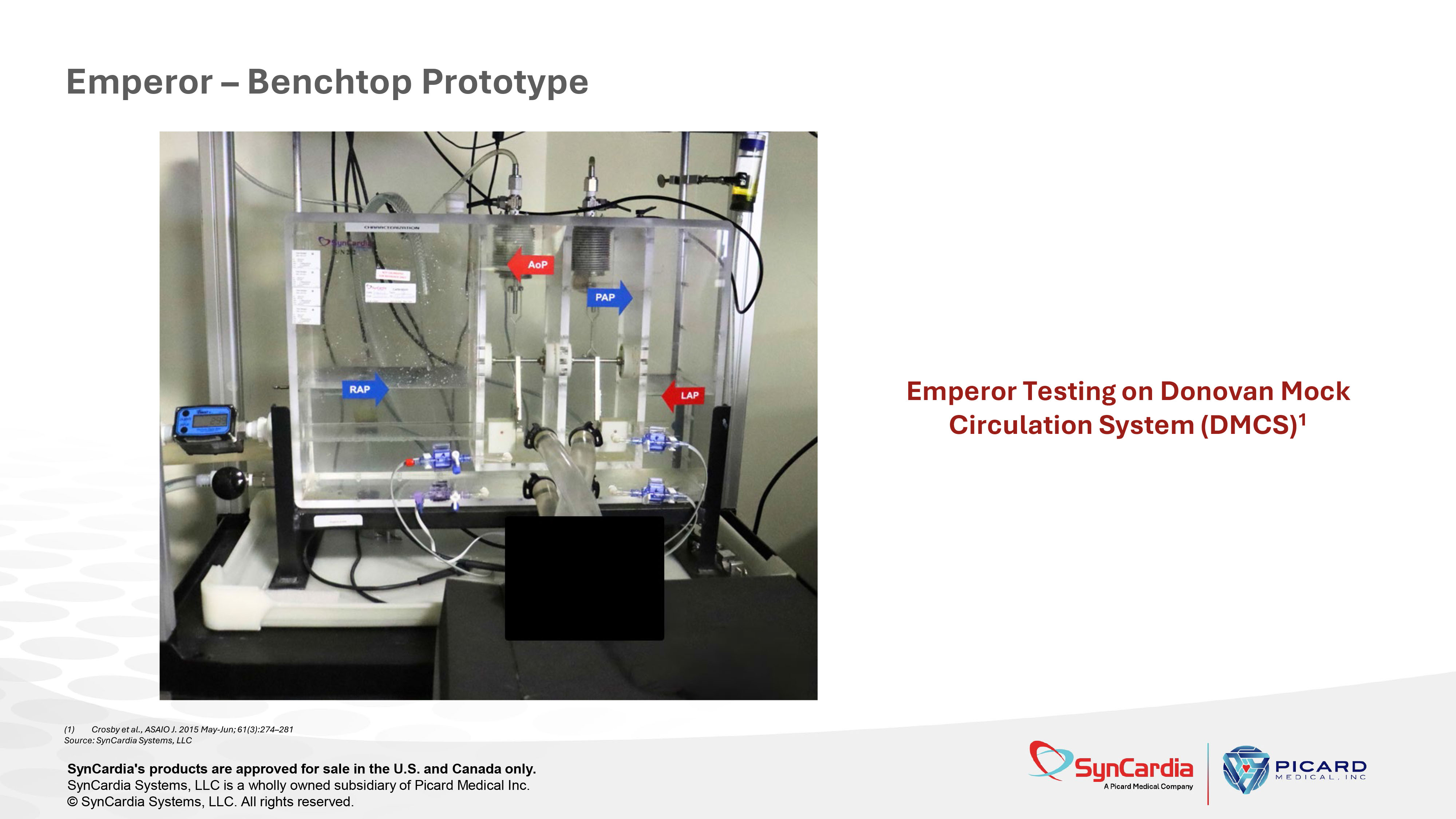
Source: SynCardia information Emperor – Benchtop Prototype (1) Crosby et al., ASAIO J. 2015 May - Jun; 61(3):274 – 281 Source: SynCardia Systems, LLC SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved. Emperor Testing on Donovan Mock Circulation System (DMCS) 1 Emperor TAH shows increased cardiac output at a constant beat rate in response to increased inflow right atrial pressure, demonstrating autoregulation in response to changing hemodynamics.
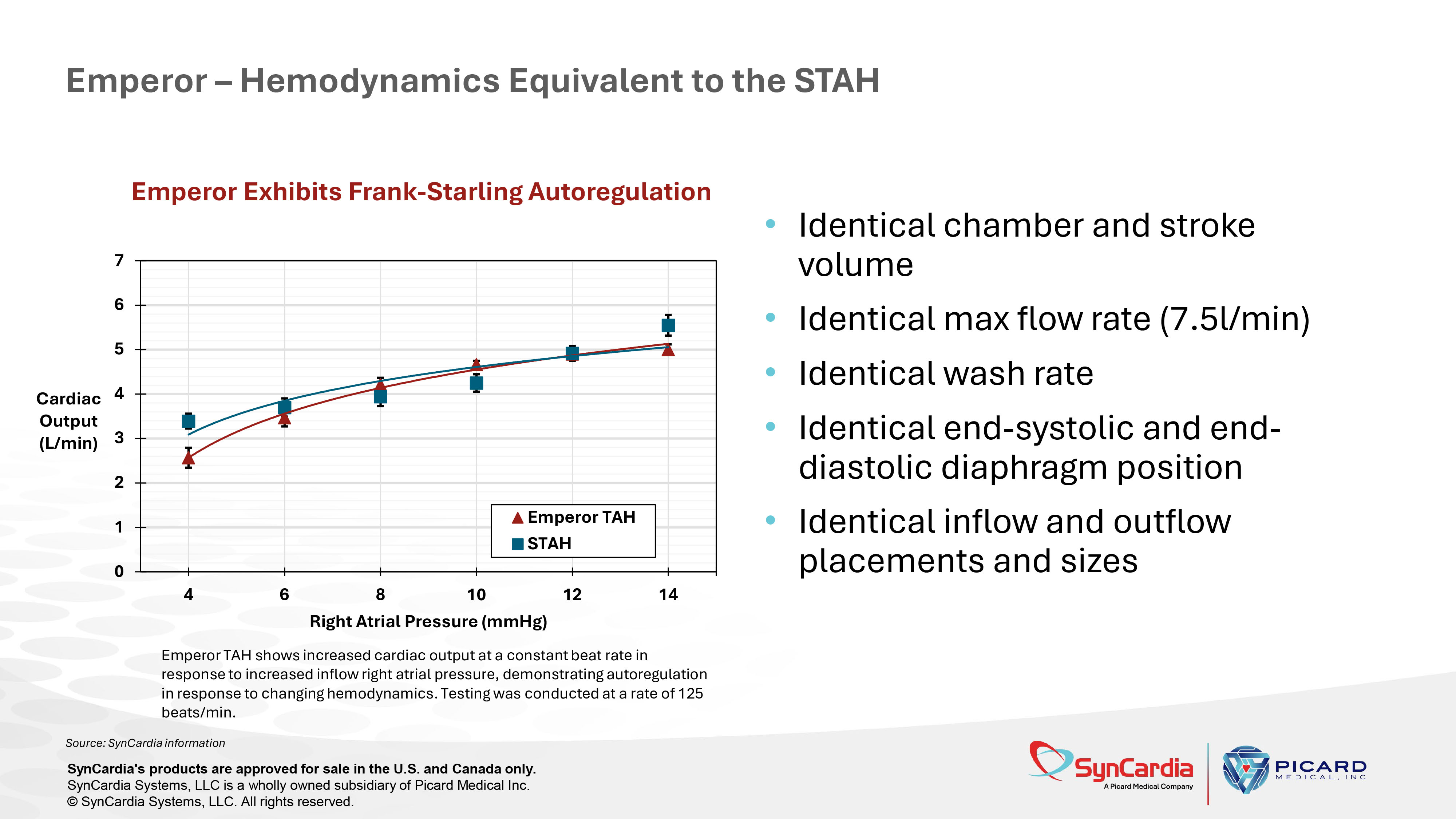
Testing was conducted at a rate of 125 beats/min. • Identical chamber and stroke volume • Identical max flow rate (7.5l/min) • Identical wash rate • Identical end - systolic and end - diastolic diaphragm position • Identical inflow and outflow placements and sizes Emperor – Hemodynamics Equivalent to the STAH Source: SynCardia information SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.
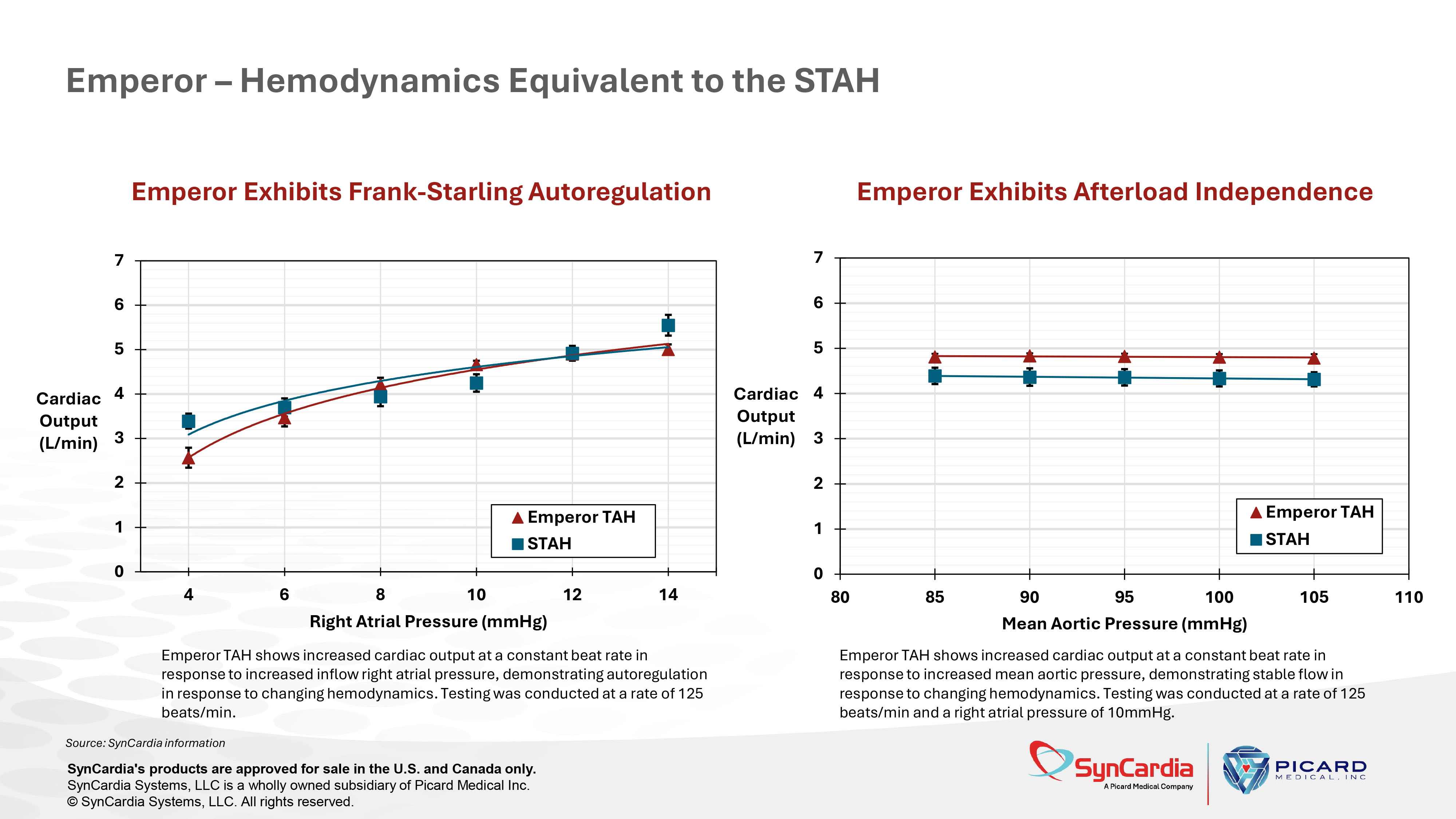
Emperor Exhibits Frank - Starling Autoregulation 0 1 2 3 4 5 6 7 4 6 8 10 12 14 Cardiac Output (L/min) Right Atrial Pressure (mmHg) Emperor TAH STAH Emperor – Hemodynamics Equivalent to the STAH Emperor Exhibits Frank - Starling Autoregulation Source: SynCardia information SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved. Emperor TAH shows increased cardiac output at a constant beat rate in response to increased inflow right atrial pressure, demonstrating autoregulation in response to changing hemodynamics. Testing was conducted at a rate of 125 beats/min. 0 1 2 3 4 5 6 7 80 85 90 95 100 105 110 Cardiac Output (L/min) Mean Aortic Pressure (mmHg) Emperor TAH STAH 0 1 2 3 4 5 6 7 4 6 8 10 12 14 Cardiac Output (L/min) Right Atrial Pressure (mmHg) Emperor TAH STAH Emperor Exhibits Afterload Independence Emperor TAH shows increased cardiac output at a constant beat rate in response to increased mean aortic pressure, demonstrating stable flow in response to changing hemodynamics. Testing was conducted at a rate of 125 beats/min and a right atrial pressure of 10mmHg.

Emperor – First - in - Animal Feasibility Testing SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved. • Successfully implanted in three porcine models, each separated from bypass without complications • Delivered full and stable circulatory support with efficient energy use and no technical issues during observation • Demonstrated physiologic performance aligned with human cardiac function • Produced stable hemodynamics with reliable preload sensitivity and afterload independence • Replicated the Frank Starling response through natural adjustment of pump output • Met all laboratory performance goals established for the Emperor platform Source: SynCardia information
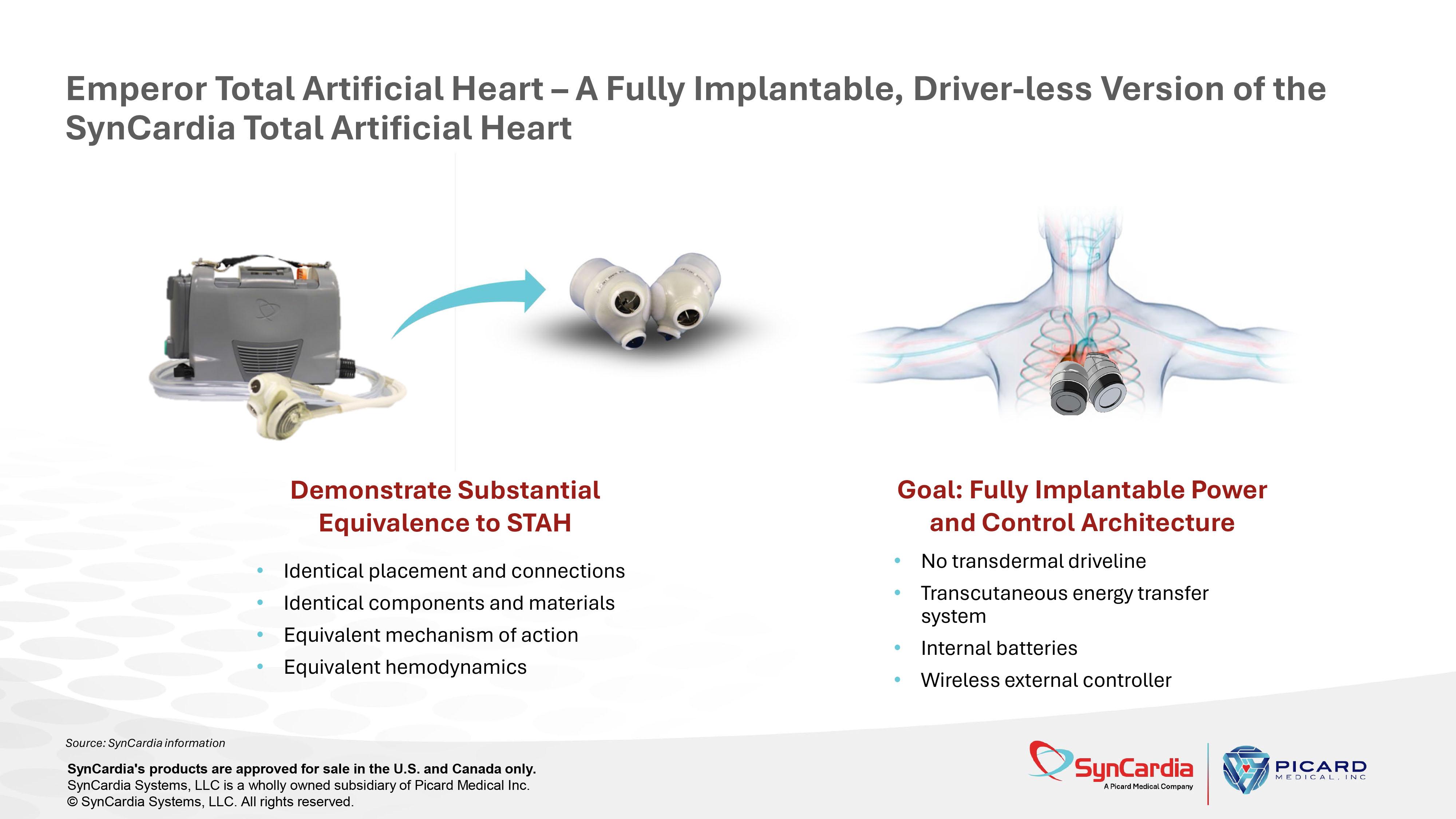
Source: SynCardia information Emperor Total Artificial Heart – A Fully Implantable, Driver - less Version of the SynCardia Total Artificial Heart • Identical placement and connections • Identical components and materials • Equivalent mechanism of action • Equivalent hemodynamics • No transdermal driveline • Transcutaneous energy transfer system • Internal batteries • Wireless external controller Demonstrate Substantial Equivalence to STAH Goal: Fully Implantable Power and Control Architecture SynCardia's products are approved for sale in the U.S. and Canada only. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved.

About SynCardia Systems, LLC SynCardia Systems, LLC, a Picard Medical Company, is headquartered in Tucson, AZ. We manufacture and distribute the world’s f irs t and only US Food and Drug Administration (FDA) and Health Canada approved total artificial heart. The SynCardia Total Artificial Heart is the most widely used and extensively studied artific ial heart in the world. The SynCardia Total Artificial Heart (STAH) The STAH has been successfully implanted in over 2,000 patients suffering from advanced stage heart failure. The implant is a vai lable in two sizes to accommodate patients’ diverse physiologies. The STAH is approved for sale in the US and in Canada where it is indicated for use in cardiac transplant - eligible patients at risk of imminent death from biventricular failure. EU and international approvals are pending. For additional information and label information, visit us at www.syncardia.com. About Heart Failure* Heart failure (HF) is a growing burden for the United States and other developed countries due to the aging of their populati ons . Approximately 6.7 million individuals had HF in 2023 and the prevalence of advanced stage HF has been estimated to be as high as 25% of the total HF populations. Approximately 300,000 (5%) of advanced st age HF are refractory to guideline directed therapy. Medical Advice Disclaimer The information, including but not limited to, text, graphics, images, and other components contained in this material are fo r i nformational purposes only. No information in this material is intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or a qualified health care provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of information you have read in this material. * Bozkurt B, et al., J Card Fail. 2023 Oct;29(10):1412 - 1451; (2) Abouezzeddine et al., Congest Heart Fail. 2011 Jul - Aug;17(4):160 - 8. SynCardia Systems, LLC is a wholly owned subsidiary of Picard Medical Inc. © SynCardia Systems, LLC. All rights reserved. SynCardia's products are approved for sale in the U.S. and Canada only. “Freedom” is a registered trademark of SynCardia Systems, LLC. “SynCardia Systems”, the SynCardia logo, “Giving the Gift of Time”, “Saving Lives, one Heart at a Time”, “Companion 2 Driver ” are trademarks of SynCardia Systems, LLC. NYSE: PMI