
Corporate Overview January 2024

The material in this presentation regarding Tourmaline Bio, Inc. (“we,” “us” or the “Company”) is for informational purposes only. This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding the timing of initiation, progress and results of the Company’s current and future preclinical and clinical trials for its product candidates, including TOUR006; the therapeutic potential of TOUR006; the timing and likelihood of seeking regulatory approval for the Company’s product candidates, including TOUR006; the timing of submitting investigational new drug applications and other regulatory documents; the Company’s ability to achieve planned milestones; the competitive landscape for the Company’s product candidates; and the Company’s estimates regarding expenses, future revenue, capital requirements, cash runway and needs for additional financing. The words “may,” “will,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Many factors may cause differences between current expectations and actual results, including, but not limited to, unexpected safety or efficacy data observed during preclinical or clinical studies, clinical site activation rates or clinical trial enrollment rates that are lower than expected, changes in the regulatory environment, changes in expected or existing competition, unexpected litigation or other disputes, and other risks and uncertainties, including those described in the section titled “Risk Factors” in the Company’s most recent filings with the Securities and Exchange Commission. The forward-looking statements included in this presentation represent the Company’s views as of the date of this presentation. The Company undertakes no obligation to update the information contained in this presentation to reflect new events or circumstances, except as required by law. In addition, certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. This presentation contains trademarks, services marks, trade names and copyrights of the Company and other companies, which are the property of their respective owners. The use or display of third parties’ trademarks, service marks, trade name or products in this presentation is not intended to, and does not imply, a relationship with the Company, or an endorsement of sponsorship by the Company. Solely for convenience, the trademarks, service marks and trade names referred to in this presentation may appear with the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that the Company will not assert, to the fullest extent under applicable law, their rights or the right of the applicable licensor to these trademarks, service marks and trade name. Disclaimer 2

Our mission 3 We are driven by our mission to develop transformative medicines that dramatically improve the lives of patients with life-altering immune and inflammatory diseases

Experienced leadership team 4 Ryan Robinson, CPA Interim Chief Financial Officer Sandeep Kulkarni, MD Co-founder and Chief Executive Officer Gerhard Hagn Senior Vice President, Head of Commercial & BD Brad Middlekauff, JD Chief Business Officer and General Counsel Ryan Iarrobino Senior Vice President, Product Development Yung Chyung, MD Chief Medical Officer Emil deGoma, MD Senior Vice President, Medical Research Susan Dana Jones, PhD Chief Technology Officer Dora Rau Senior Vice President, Head of Quality Kevin Johnson, PhD Chief Regulatory Officer Management Team Board of Directors Clay Siegall, PhD Chairman Caley Castelein, MD Aaron Kantoff Mark McDade Sapna Srivastava, PhD Parvinder Thiara Sandeep Kulkarni, MD
TOUR006 has demonstrated best-in-class potential: long-acting, low immunogenicity, and low- volume subcutaneous administration Two strategic paths to significant value creation: FcRn+ and cardiovascular inflammation Key highlights 5 An IL-6 renaissance is underway: new insights emerging about a broad range of indications where IL-6 may be clinically validated A late-stage clinical company: topline data from pivotal Phase 2b spiriTED trial and Phase 2 CV trial both expected in H1 2025, pivotal Phase 3 TED trial also expected to commence in H2 2024 Accomplished leadership team: extensive experience developing and commercializing antibodies for immune and inflammatory diseases Well-financed: cash expected to fund operations through 2026, enabling the delivery of key milestones for both strategic paths
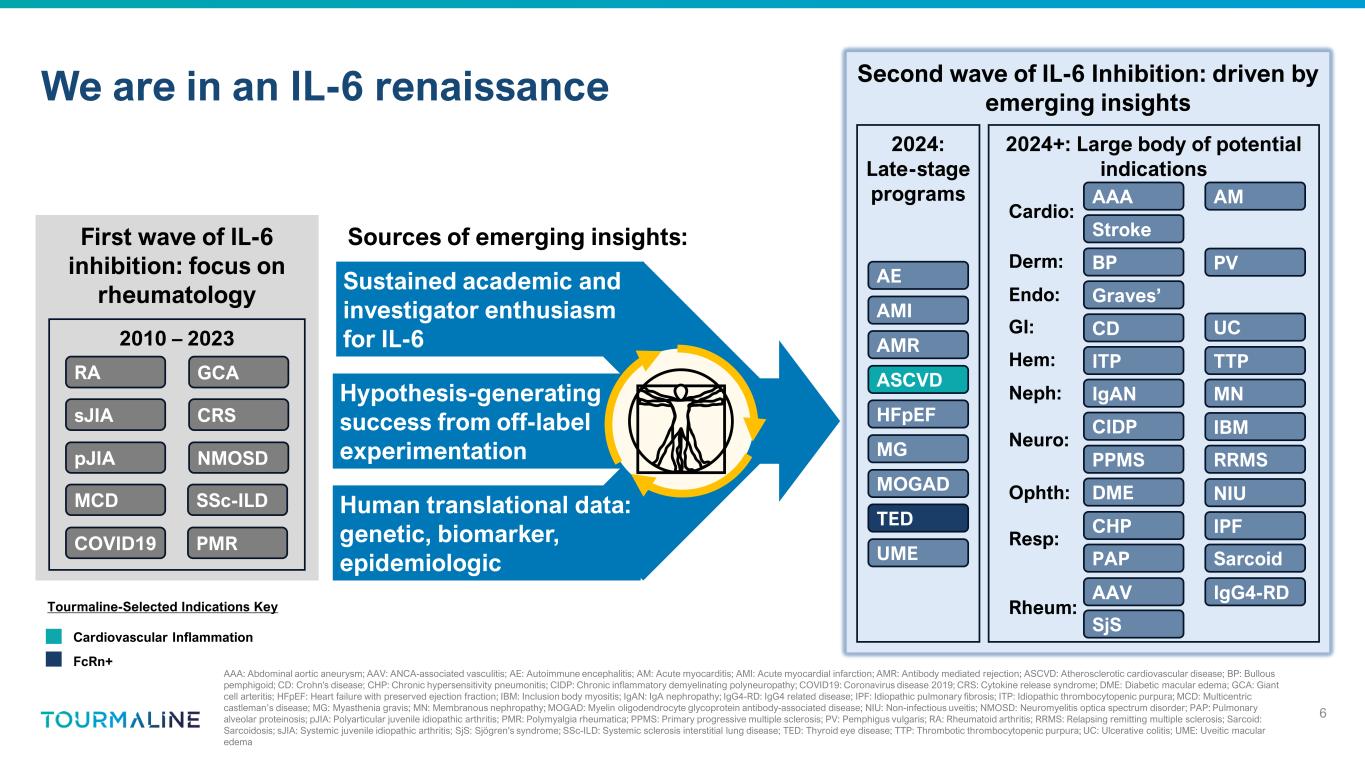
We are in an IL-6 renaissance 6 AAA: Abdominal aortic aneurysm; AAV: ANCA-associated vasculitis; AE: Autoimmune encephalitis; AM: Acute myocarditis; AMI: Acute myocardial infarction; AMR: Antibody mediated rejection; ASCVD: Atherosclerotic cardiovascular disease; BP: Bullous pemphigoid; CD: Crohn's disease; CHP: Chronic hypersensitivity pneumonitis; CIDP: Chronic inflammatory demyelinating polyneuropathy; COVID19: Coronavirus disease 2019; CRS: Cytokine release syndrome; DME: Diabetic macular edema; GCA: Giant cell arteritis; HFpEF: Heart failure with preserved ejection fraction; IBM: Inclusion body myositis; IgAN: IgA nephropathy; IgG4-RD: IgG4 related disease; IPF: Idiopathic pulmonary fibrosis; ITP: Idiopathic thrombocytopenic purpura; MCD: Multicentric castleman’s disease; MG: Myasthenia gravis; MN: Membranous nephropathy; MOGAD: Myelin oligodendrocyte glycoprotein antibody-associated disease; NIU: Non-infectious uveitis; NMOSD: Neuromyelitis optica spectrum disorder; PAP: Pulmonary alveolar proteinosis; pJIA: Polyarticular juvenile idiopathic arthritis; PMR: Polymyalgia rheumatica; PPMS: Primary progressive multiple sclerosis; PV: Pemphigus vulgaris; RA: Rheumatoid arthritis; RRMS: Relapsing remitting multiple sclerosis; Sarcoid: Sarcoidosis; sJIA: Systemic juvenile idiopathic arthritis; SjS: Sjögren's syndrome; SSc-ILD: Systemic sclerosis interstitial lung disease; TED: Thyroid eye disease; TTP: Thrombotic thrombocytopenic purpura; UC: Ulcerative colitis; UME: Uveitic macular edema First wave of IL-6 inhibition: focus on rheumatology 2010 – 2023 RA sJIA pJIA PMR SSc-ILD COVID19 MCD GCA NMOSD CRS Second wave of IL-6 Inhibition: driven by emerging insights 2024: Late-stage programs 2024+: Large body of potential indications NIU Hem: GI: Neph: Resp: Neuro: Rheum: Ophth: Endo: Derm: AAV IgG4-RD SjS CHP IPF PAP Sarcoid DME CIDP IBM PPMS RRMS IgAN MN ITP TTP CD UC Graves’ BP PV Cardio: AAA AM AMI Stroke AMR AE ASCVD MG MOGAD HFpEF TED UME Sustained academic and investigator enthusiasm for IL-6 Hypothesis-generating success from off-label experimentation Human translational data: genetic, biomarker, epidemiologic Sources of emerging insights: Tourmaline-Selected Indications Key Cardiovascular Inflammation FcRn+
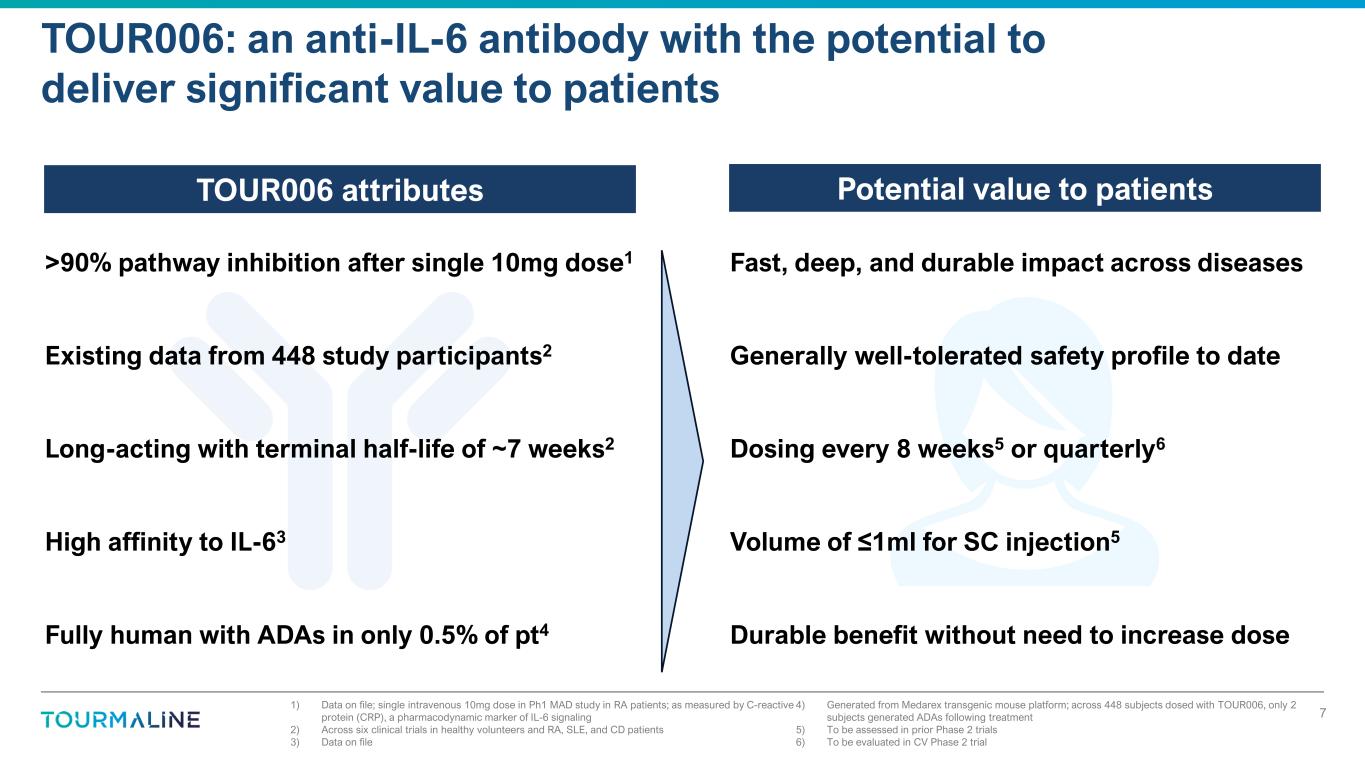
TOUR006: an anti-IL-6 antibody with the potential to deliver significant value to patients TOUR006 attributes Potential value to patients 1) Data on file; single intravenous 10mg dose in Ph1 MAD study in RA patients; as measured by C-reactive protein (CRP), a pharmacodynamic marker of IL-6 signaling 2) Across six clinical trials in healthy volunteers and RA, SLE, and CD patients 3) Data on file 4) Generated from Medarex transgenic mouse platform; across 448 subjects dosed with TOUR006, only 2 subjects generated ADAs following treatment 5) To be assessed in prior Phase 2 trials 6) To be evaluated in CV Phase 2 trial >90% pathway inhibition after single 10mg dose1 Existing data from 448 study participants2 Long-acting with terminal half-life of ~7 weeks2 High affinity to IL-63 Fully human with ADAs in only 0.5% of pt4 Fast, deep, and durable impact across diseases Generally well-tolerated safety profile to date Dosing every 8 weeks5 or quarterly6 Volume of ≤1ml for SC injection5 Durable benefit without need to increase dose 7

AE: autoimmune encephalitis; AMI: acute myocardial infarction; ASCVD: atherosclerotic cardiovascular disease; CKD: chronic kidney disease; MG: myasthenia gravis; Satra: satralizumab; Zilti: ziltivekimab; Two strategic paths to unlock major value creation 8 FcRn+ Cardiovascular Inflammation IL-6 Renaissance Emerging insights implicating IL-6 in a wide range of rare & large diseases TOUR006 Long-acting Low immunogenicity Low volume, SC TOUR006 has the potential to be a superior therapy for a wide range of autoantibody- driven diseases vs. FcRn inhibitors TOUR006 has the potential to transform the care of high-risk patients by targeting key inflammatory pathways driving cardiovascular disease + H1 2025: CV Phase 2 topline data 2025: Zilti ASCVD in CKD Phase 3 (ZEUS) topline data H1 2025: spiriTED topline data 2025: Satra AE Phase 3 (CIELO) topline data H1 2024: Satra MG Phase 3 (LUMINESCE) topline data 2026: Zilti AMI Phase 3 (ARTEMIS) topline data Key expected readouts Key expected readouts Milestones key: Internal External
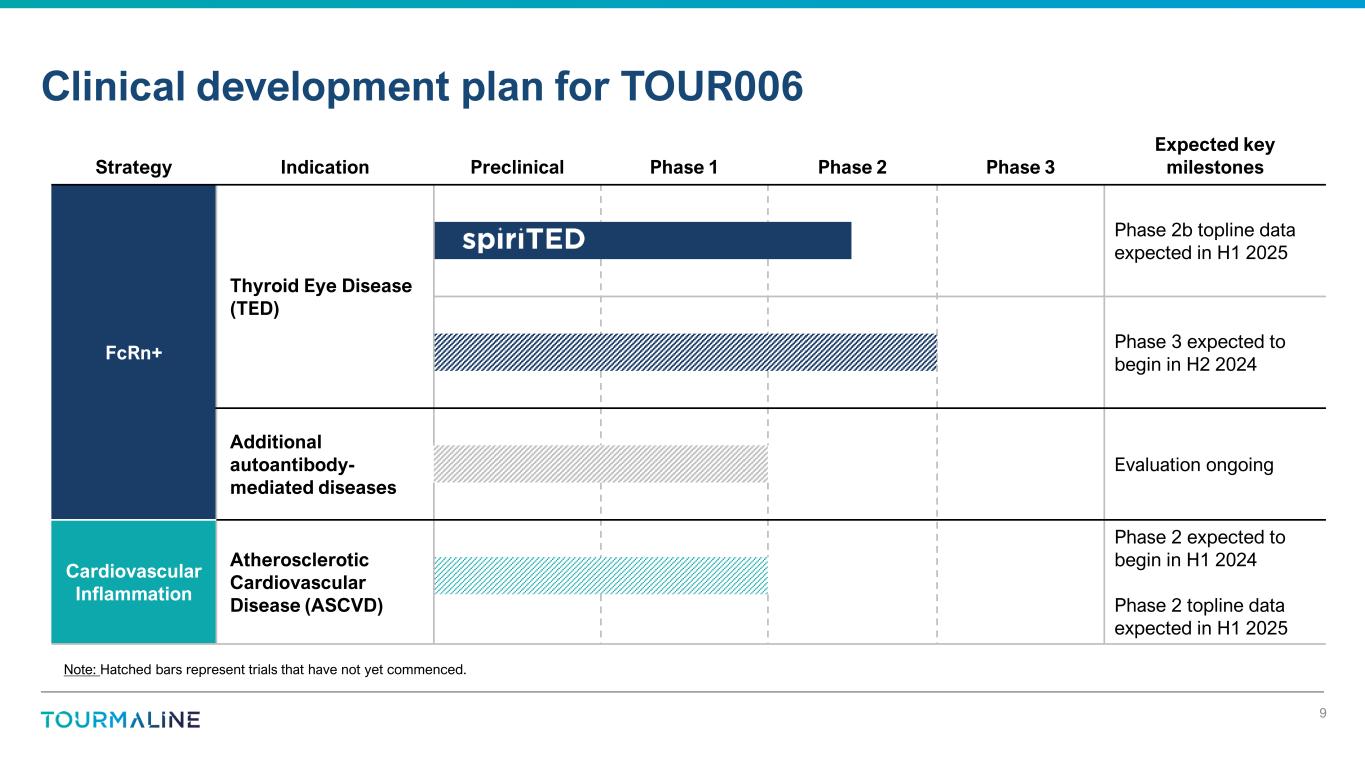
Strategy Indication Preclinical Phase 1 Phase 2 Phase 3 Expected key milestones FcRn+ Thyroid Eye Disease (TED) Phase 2b topline data expected in H1 2025 Phase 3 expected to begin in H2 2024 Additional autoantibody- mediated diseases Evaluation ongoing Cardiovascular Inflammation Atherosclerotic Cardiovascular Disease (ASCVD) Phase 2 expected to begin in H1 2024 Phase 2 topline data expected in H1 2025 Clinical development plan for TOUR006 9 Note: Hatched bars represent trials that have not yet commenced.
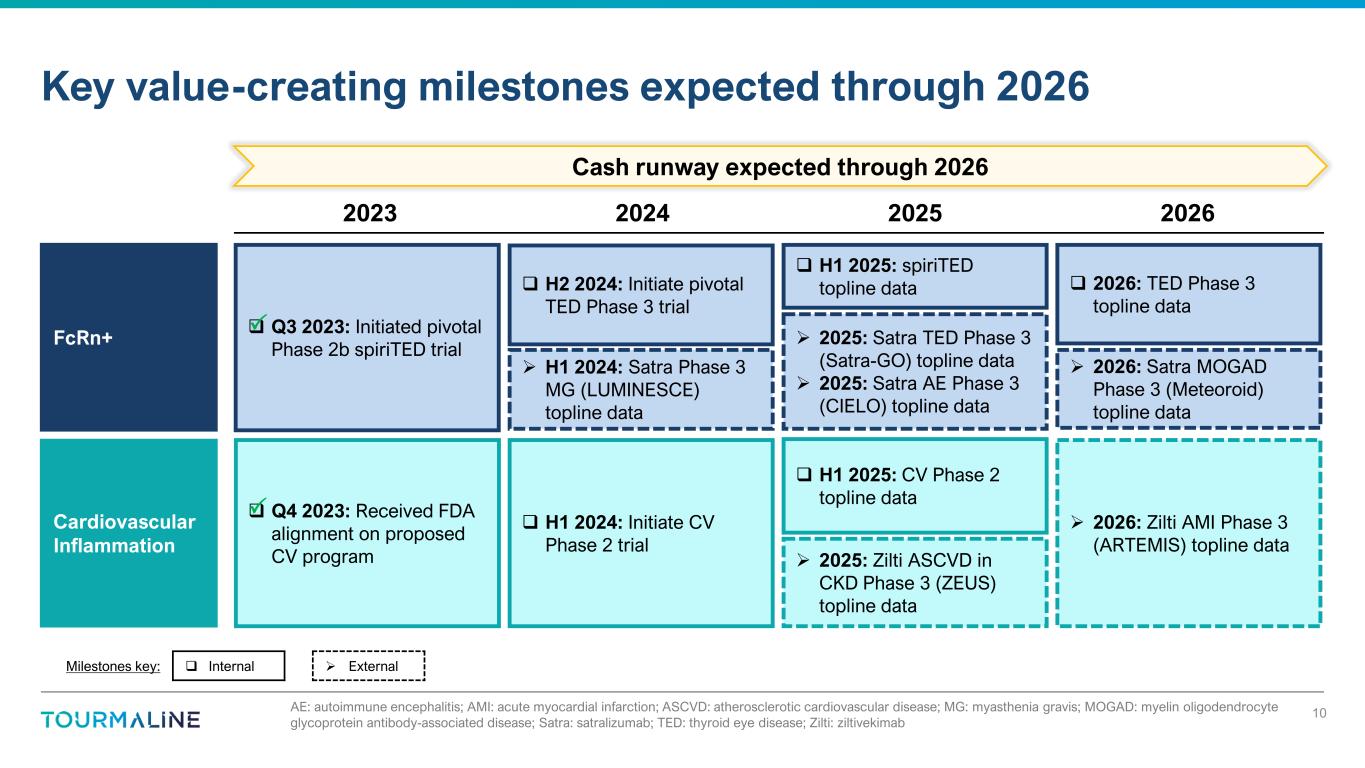
Milestones key: Internal External AE: autoimmune encephalitis; AMI: acute myocardial infarction; ASCVD: atherosclerotic cardiovascular disease; MG: myasthenia gravis; MOGAD: myelin oligodendrocyte glycoprotein antibody-associated disease; Satra: satralizumab; TED: thyroid eye disease; Zilti: ziltivekimab Key value-creating milestones expected through 2026 10 Q3 2023: Initiated pivotal Phase 2b spiriTED trial Q4 2023: Received FDA alignment on proposed CV program H1 2024: Initiate CV Phase 2 trial H1 2025: CV Phase 2 topline data Cash runway expected through 2026 FcRn+ Cardiovascular Inflammation H2 2024: Initiate pivotal TED Phase 3 trial H1 2025: spiriTED topline data 2026: TED Phase 3 topline data 2026: Zilti AMI Phase 3 (ARTEMIS) topline data H1 2024: Satra Phase 3 MG (LUMINESCE) topline data 2025: Satra TED Phase 3 (Satra-GO) topline data 2025: Satra AE Phase 3 (CIELO) topline data 2026: Satra MOGAD Phase 3 (Meteoroid) topline data 2025: Zilti ASCVD in CKD Phase 3 (ZEUS) topline data 2023 2024 2025 2026

FcRn+
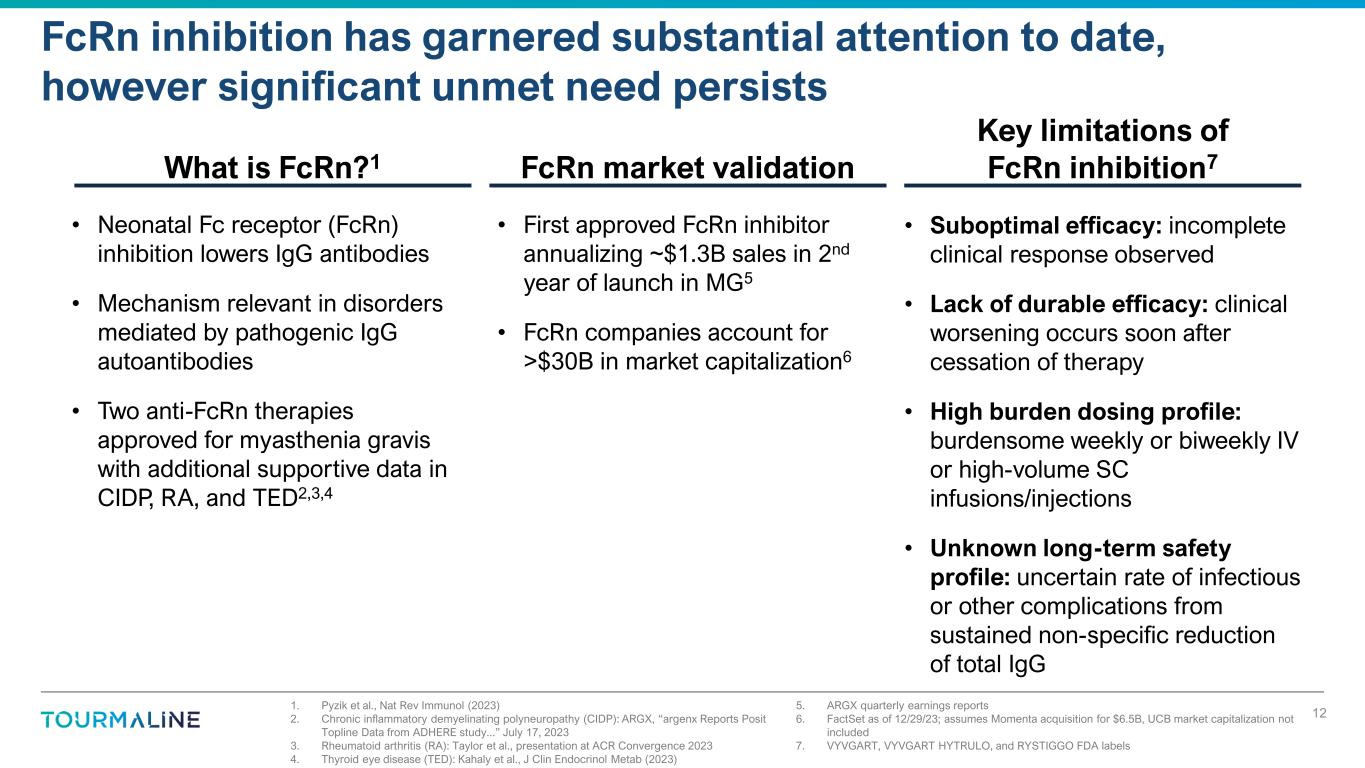
1. Pyzik et al., Nat Rev Immunol (2023) 2. Chronic inflammatory demyelinating polyneuropathy (CIDP): ARGX, “argenx Reports Posit Topline Data from ADHERE study...” July 17, 2023 3. Rheumatoid arthritis (RA): Taylor et al., presentation at ACR Convergence 2023 4. Thyroid eye disease (TED): Kahaly et al., J Clin Endocrinol Metab (2023) 5. ARGX quarterly earnings reports 6. FactSet as of 12/29/23; assumes Momenta acquisition for $6.5B, UCB market capitalization not included 7. VYVGART, VYVGART HYTRULO, and RYSTIGGO FDA labels FcRn inhibition has garnered substantial attention to date, however significant unmet need persists 12 • Neonatal Fc receptor (FcRn) inhibition lowers IgG antibodies • Mechanism relevant in disorders mediated by pathogenic IgG autoantibodies • Two anti-FcRn therapies approved for myasthenia gravis with additional supportive data in CIDP, RA, and TED2,3,4 What is FcRn?1 • Suboptimal efficacy: incomplete clinical response observed • Lack of durable efficacy: clinical worsening occurs soon after cessation of therapy • High burden dosing profile: burdensome weekly or biweekly IV or high-volume SC infusions/injections • Unknown long-term safety profile: uncertain rate of infectious or other complications from sustained non-specific reduction of total IgG Key limitations of FcRn inhibition7FcRn market validation • First approved FcRn inhibitor annualizing ~$1.3B sales in 2nd year of launch in MG5 • FcRn companies account for >$30B in market capitalization6
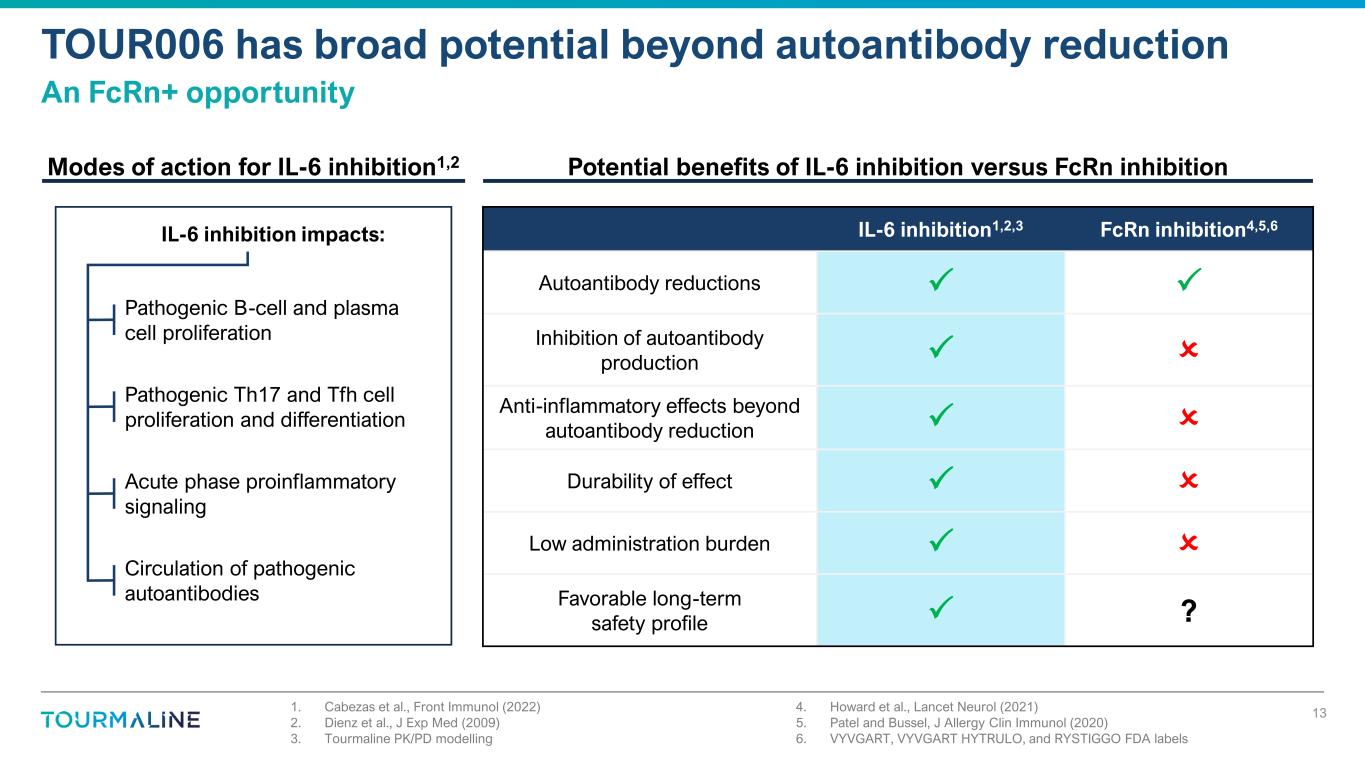
1. Cabezas et al., Front Immunol (2022) 2. Dienz et al., J Exp Med (2009) 3. Tourmaline PK/PD modelling 4. Howard et al., Lancet Neurol (2021) 5. Patel and Bussel, J Allergy Clin Immunol (2020) 6. VYVGART, VYVGART HYTRULO, and RYSTIGGO FDA labels TOUR006 has broad potential beyond autoantibody reduction An FcRn+ opportunity 13 Potential benefits of IL-6 inhibition versus FcRn inhibitionModes of action for IL-6 inhibition1,2 IL-6 inhibition1,2,3 FcRn inhibition4,5,6 Autoantibody reductions Inhibition of autoantibody production Anti-inflammatory effects beyond autoantibody reduction Durability of effect Low administration burden Favorable long-term safety profile ? Pathogenic B-cell and plasma cell proliferation Pathogenic Th17 and Tfh cell proliferation and differentiation Acute phase proinflammatory signaling Circulation of pathogenic autoantibodies IL-6 inhibition impacts:
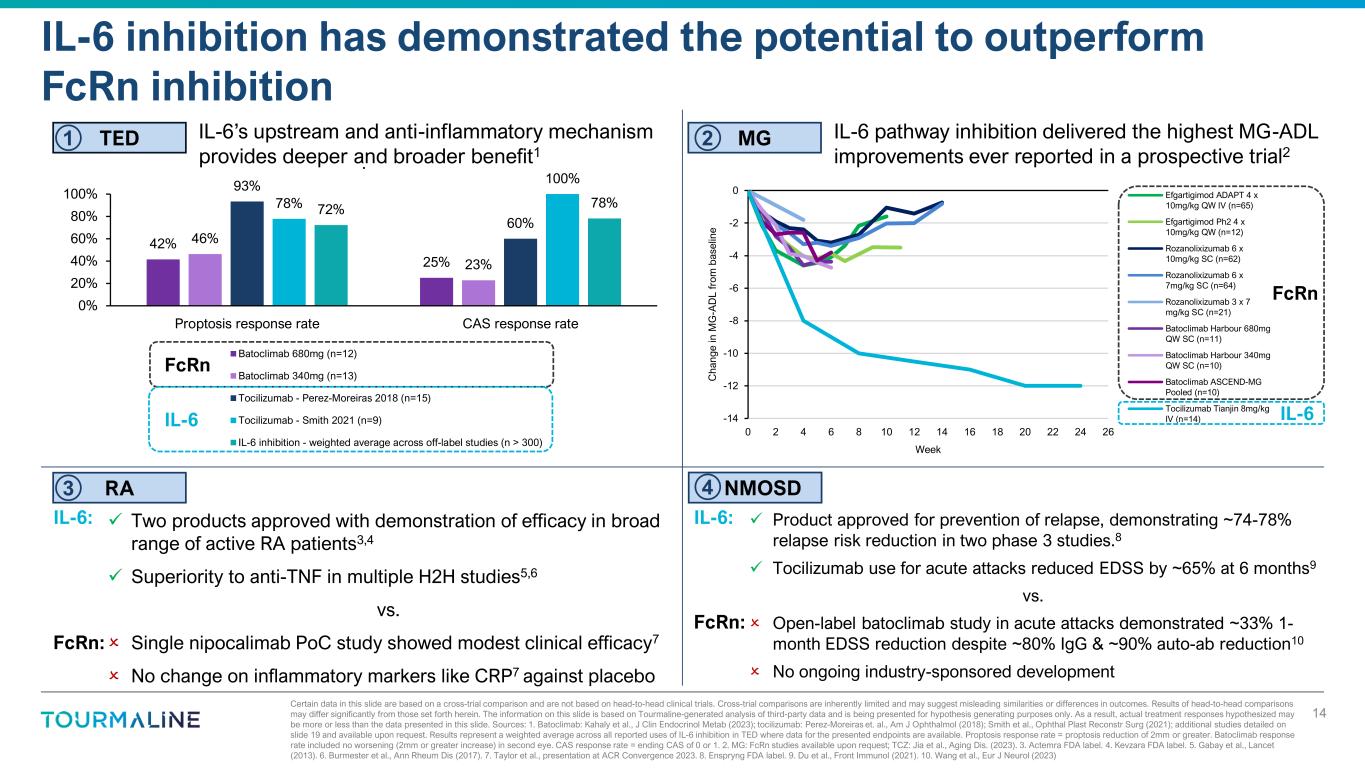
42% 25% 46% 23% 93% 60% 78% 100% 72% 78% 0% 20% 40% 60% 80% 100% Proptosis response rate CAS response rate . Batoclimab 680mg (n=12) Batoclimab 340mg (n=13) Tocilizumab - Perez-Moreiras 2018 (n=15) Tocilizumab - Smith 2021 (n=9) IL-6 inhibition - weighted average across off-label studies (n > 300) -14 -12 -10 -8 -6 -4 -2 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 C ha ng e in M G -A D L fr om b as el in e Week Efgartigimod ADAPT 4 x 10mg/kg QW IV (n=65) Efgartigimod Ph2 4 x 10mg/kg QW (n=12) Rozanolixizumab 6 x 10mg/kg SC (n=62) Rozanolixizumab 6 x 7mg/kg SC (n=64) Rozanolixizumab 3 x 7 mg/kg SC (n=21) Batoclimab Harbour 680mg QW SC (n=11) Batoclimab Harbour 340mg QW SC (n=10) Batoclimab ASCEND-MG Pooled (n=10) Tocilizumab Tianjin 8mg/kg IV (n=14) Certain data in this slide are based on a cross-trial comparison and are not based on head-to-head clinical trials. Cross-trial comparisons are inherently limited and may suggest misleading similarities or differences in outcomes. Results of head-to-head comparisons may differ significantly from those set forth herein. The information on this slide is based on Tourmaline-generated analysis of third-party data and is being presented for hypothesis generating purposes only. As a result, actual treatment responses hypothesized may be more or less than the data presented in this slide. Sources: 1. Batoclimab: Kahaly et al., J Clin Endocrinol Metab (2023); tocilizumab: Perez-Moreiras et. al., Am J Ophthalmol (2018); Smith et al., Ophthal Plast Reconstr Surg (2021); additional studies detailed on slide 19 and available upon request. Results represent a weighted average across all reported uses of IL-6 inhibition in TED where data for the presented endpoints are available. Proptosis response rate = proptosis reduction of 2mm or greater. Batoclimab response rate included no worsening (2mm or greater increase) in second eye. CAS response rate = ending CAS of 0 or 1. 2. MG: FcRn studies available upon request; TCZ: Jia et al., Aging Dis. (2023). 3. Actemra FDA label. 4. Kevzara FDA label. 5. Gabay et al., Lancet (2013). 6. Burmester et al., Ann Rheum Dis (2017). 7. Taylor et al., presentation at ACR Convergence 2023. 8. Enspryng FDA label. 9. Du et al., Front Immunol (2021). 10. Wang et al., Eur J Neurol (2023) IL-6 inhibition has demonstrated the potential to outperform FcRn inhibition 14 NMOSD MGTED RA IL-6 pathway inhibition delivered the highest MG-ADL improvements ever reported in a prospective trial2 IL-6’s upstream and anti-inflammatory mechanism provides deeper and broader benefit1 Product approved for prevention of relapse, demonstrating ~74-78% relapse risk reduction in two phase 3 studies.8 Tocilizumab use for acute attacks reduced EDSS by ~65% at 6 months9 vs. Open-label batoclimab study in acute attacks demonstrated ~33% 1- month EDSS reduction despite ~80% IgG & ~90% auto-ab reduction10 No ongoing industry-sponsored development Two products approved with demonstration of efficacy in broad range of active RA patients3,4 Superiority to anti-TNF in multiple H2H studies5,6 vs. Single nipocalimab PoC study showed modest clinical efficacy7 No change on inflammatory markers like CRP7 against placebo FcRn IL-6 FcRn IL-6 IL-6: FcRn: IL-6: FcRn: 1 2 3 4
1. Lazarus, Best Pract. Res. Clin. Endocrinol. Metab. (2012) 2. Bartalena et al., Front. Endocrinol. (2020) 4. Tourmaline market research 3. Horizon Q3 2022 earnings call TED: our beachhead indication to validate TOUR006’s FcRn+ potential in autoantibody-driven diseases 15 High unmet medical need with significant market opportunity • Potentially sight-threatening disease characterized by proptosis, double-vision, and pain • ~30k new patients each year in the U.S. (average age at diagnosis is ~45)1,2 • ~80%3 of eligible TED patients not receiving an FDA-approved treatment due to significant limitations: risk of permanent hearing impairment / loss, limited durability, and inconvenience / complexity4 Extensive clinical validation that IL-6 inhibition may address key unmet needs • 40+ publications with 300+ patients demonstrate the therapeutic potential of IL-6 pathway inhibition in TED • IL-6 may play a more central and upstream role in TED pathogenesis than IGF-1R or FcRn TOUR006 has best-in-disease potential in TED • Deep inhibition of IL-6 pathway offers potential for durable efficacy across many endpoints • Existing clinical database supports the potential for a well-tolerated profile at selected doses • Q8W dosing allows for a patient-friendly, low burden treatment course 1 2 3

1. Safety issues: Risk of potentially permanent hearing loss2 2. Limited durability: Growing real-world effectiveness data reveals larger than anticipated number of non-responders / high relapse rate3,4 3. High level of inconvenience & complexity: – IV Q3W (n=8)2 but limited access to infusion centers5 – Numerous visits and high time commitment (HCPs and patients)5 – Need for serial audiograms, as per label2,6 – Burdensome reimbursement approval process7 1. Horizon company reports and filings 2. TEPEZZA FDA label 3. Kahaly et al., Thyroid (2021) (ATA 2021 presentation) 4. Rosenblatt et al., Ophthalmic Plast Reconstr Surg (2023) 5. Tourmaline market research 6. Chow and Silkiss, BMJ Case Rep (2022) 7. Horizon Therapeutics Public Ltd. Co. Q2 2023 Form 10-Q IGF-1R class limitations present a significant opportunity for novel therapeutic approaches in TED 16 Sales ($M)1 TEPEZZA U.S. revenues have been stagnating since 2021… $24 $166 $287 $344 $2 $453 $616 $590 $502 $480 $491 $494 $405 $446 $453 -- $100 $200 $300 $400 $500 $600 $700 Q1 2020 Q2 2020 Q3 2020 Q4 2020 Q1 2021 Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 Q3 2022 Q4 2022 Q1 2023 Q2 2023 Q3 2023 …due to real-world experience

1. Horizon Q3 2022 earnings call; LTM = last twelve months 2. Tourmaline market research; endo = endocrinologist; ophth = ophthalmologist 3. AAMC 2022 Physician Specialty Data Report 4. Hussey and Tao. Orbit (2022) Despite an FDA-approved medicine, vast majority of moderate-to- severe TED patients remain untreated Most TED patients are not receiving TEPEZZA… …or only get it relatively late in the treatment journey2 Endo (~8.2k3) monitors thyroid levels, treats mainly mild TED Ophth (~18.9k3) manages moderate-severe ocular symptoms Oculoplastic Surgeons (~1.2k4) manages severe disease and/or inadequate response to steroids Mild Moderate Severe / Sight-Threatening Watch & wait supplemented with artificial tears Steroids short-term (IV, oral, or periocular) supplemented with prism lenses and artificial tears TEPEZZA 17 Surgery 0% 20% 40% 60% 80% 100% High Proptosis, High CAS TED Patients % o f P at ie nt s Untreated 16,000 80% Treated with Tepezza 4,000 20% Simplified Treatment Journey2TEPEZZA US LTM penetration1
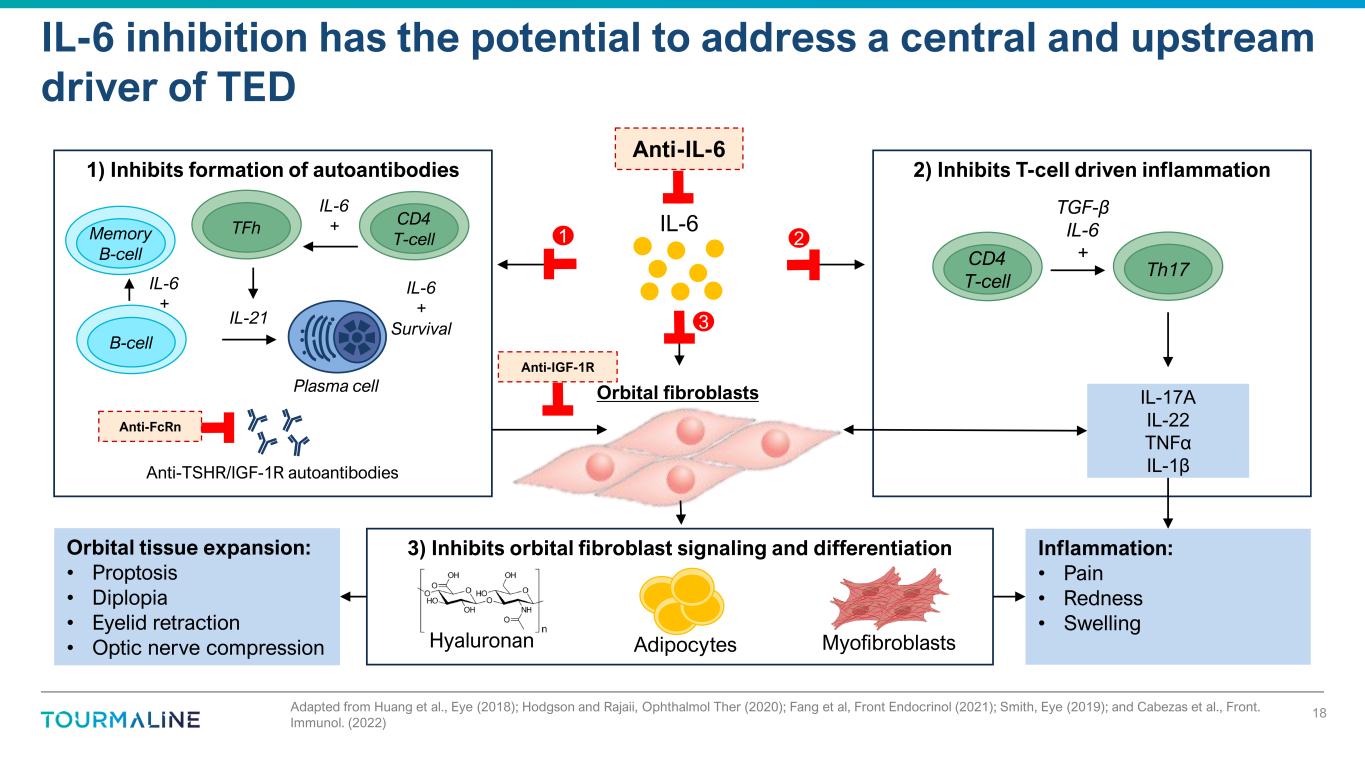
Adapted from Huang et al., Eye (2018); Hodgson and Rajaii, Ophthalmol Ther (2020); Fang et al, Front Endocrinol (2021); Smith, Eye (2019); and Cabezas et al., Front. Immunol. (2022) IL-6 inhibition has the potential to address a central and upstream driver of TED Adipocytes MyofibroblastsHyaluronan 3) Inhibits orbital fibroblast signaling and differentiation Inflammation: • Pain • Redness • Swelling Orbital tissue expansion: • Proptosis • Diplopia • Eyelid retraction • Optic nerve compression Anti-TSHR/IGF-1R autoantibodies IL-6 Orbital fibroblasts 2) Inhibits T-cell driven inflammation1) Inhibits formation of autoantibodies CD4 T-cell TFhMemory B-cell B-cell IL-6 + IL-6 + IL-6 + Survival Plasma cell IL-21 Th17 CD4 T-cell TGF-β IL-6 + IL-17A IL-22 TNFα IL-1β Anti-FcRn Anti-IGF-1R Anti-IL-6 1 3 2 18

Over 40 publications demonstrate the therapeutic potential of IL-6 pathway inhibition in TED • 300+ mostly steroid- refractory patients • Late IL-6 inhibition (>9 months post symptom onset) when disease may have exited active phase • Exposure to IL-6 inhibition may have been suboptimal (<6 months) We believe many of these reports may underestimate the true efficacy of IL-6 blockade Proptosis response rate is generally defined in the data outlined here as a ≥2 mm proptosis improvement in the worse eye at baseline without any worsening in the other eye. CAS response rate is generally defined in the data outlined here as a CAS of 0 or 1. Studies referenced in this table represent investigator-led studies and were not designed with the intent of generating evidence for an approval of tocilizumab or sarilumab in TED. The majority of these studies were not designed with power to detect statistical significance. Retro: retrospective. Obs: observational. Prosp: prospective. RCR: randomized controlled trial. CS: case series. CR: case report. NR: not reported. NR: not reported. NS: not significant. CI: clear improvement. Tepro: teprotumumab. Publications available upon request 19 Study Details Key Endpoints First author Year Study type N treated Proptosis response rate CAS response rate* % autoantibody reduction Perez-Moreiras 2021 Retro 54 78 89 75 Sánchez-Bilbao 2020 Obs 48 NR NR NR Atienza-Mateo 2018 Retro 29 NR NR NR Pérez-Moreiras 2014 Prosp 18 72 100 76 Pérez-Moreiras 2018 RCT 15 93 60 NS de la Fuente Bursón 2020 Retro 15 NR NR NR Pereira 2023 Retro 14 NR NR NR Boutzios 2023 Obs 12 NR NR 84 Pampín-Sánchez 2022 Retro 11 75 73 NR Moi 2022 Retro 10 CI 80 75 Cortez 2022 Prosp 10 10 100 81 Silkiss 2020 CS 9 CI 56 74 Smith 2021 Retro 9 78 100 54 Bielefeld 2019 Obs 8 NR NR NR Ceballos-Marcias Jose 2020 CS 8 NR 75 41 Bennedjai 2020 Retro 7 NR NR 73 Moás 2022 Obs 7 NR NR 92 Toro-Tobon 2023 Retro 6 50 NR NR de Pablo Gomez 2018 CS 5 NR 60 NR Navarrete 2022 Retro 5 NR NR NR Ribi 2017 CS 3 33 67 NR Maldiney 2020 CS 3 67 NR NR Stevens 2022 Retro 3 100 67 NR Russell 2017 CS 2 NR 0 NR Study Details Key Endpoints First author Year Study type N treated Proptosis response rate CAS response rate* % autoantibody reduction Sy 2017 CS 2 CI 50 69 Copperman 2019 CS 2 100 0 NR Coy 2019 CS 2 NR 50 NR Park 2021 CS 2 100 100 NR Abeillon-du Payrat2022 CS 2 100 50 NR Butnaru 2013 CR 1 NR 100 NR Gómez Rodríguez 2014 CR 1 NR 100 NR Bielefeld 2017 CR 1 CI NR NR Canas 2018 CR 1 100 NR NR Pascual-Camps 2018 CR 1 NR NR NR Garreta Fontelles 2019 CR 1 NR NR 93 Mehmet 2020 CR 1 0 NR NR Kaplan 2020 CR 1 NR 0 85 Cayon-Blanco 2020 CR 1 NR 100 NR Tran 2020 CS 1 NR NR NR Ruiz 2021 CR 1 NR NR NR Albrashdi 2022 CR 1 100 NR NR Cezara 2022 CR 1 NR 0 NR Mohamed 2022 CS 1 0 0 NR Moleiro 2022 CR 1 100 NR 86 Almazrouei 2023 CR 1 NR NR NR Cuculescu 2023 CR 1 CI 0 NR Nirmalan 2023 CS 1 NR NR NR Weighted Mean 72% 78% 74% Smith 2017 (tepro Phase 2) 71% 69% N/A Douglas 2020 (tepro Phase 3) 83% 59% N/A
The characteristics presented reflect outcomes that may not be representative of TOUR006. The results of past clinical trials may not be indicative of future results, and the results of future or ongoing clinical trials may not demonstrate some or any of the characteristics presented. 20 Deep & broad efficacy • Meaningful reduction of proptosis • Important improvement of CAS and diplopia Durable • Inhibition of production of anti-TSHR auto-antibodies • Durable response, in part due to low immunogenicity Patient-friendly • SC, ≤1ml injections, every 8 weeks • Finite treatment for most of patients with flexibility where needed Well-tolerated • Well tolerated safety profile, manageable with routine monitoring • Lack of permanent or irreversible side effects Potential target profile of TOUR006 Market research indicates TOUR006’s potential to become the optimal first-line TED therapy
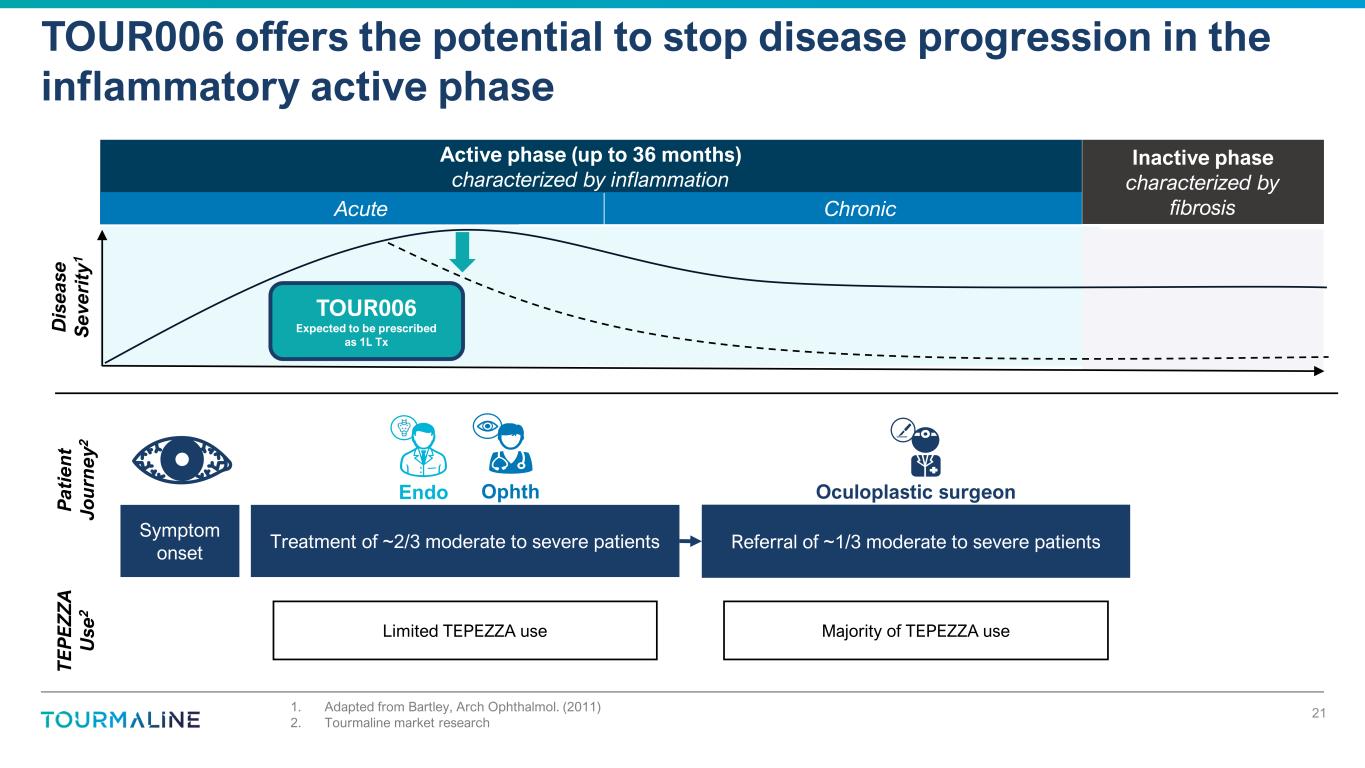
Inactive phase characterized by fibrosis Active phase (up to 36 months) characterized by inflammation 1. Adapted from Bartley, Arch Ophthalmol. (2011) 2. Tourmaline market research TOUR006 offers the potential to stop disease progression in the inflammatory active phase 21 D is ea se S ev er it y1 TOUR006 Expected to be prescribed as 1L Tx T E P E Z Z A U se 2 Limited TEPEZZA use Majority of TEPEZZA use Symptom onset Treatment of ~2/3 moderate to severe patients Referral of ~1/3 moderate to severe patients P at ie n t Jo u rn ey 2 OphthEndo Oculoplastic surgeon Acute Chronic
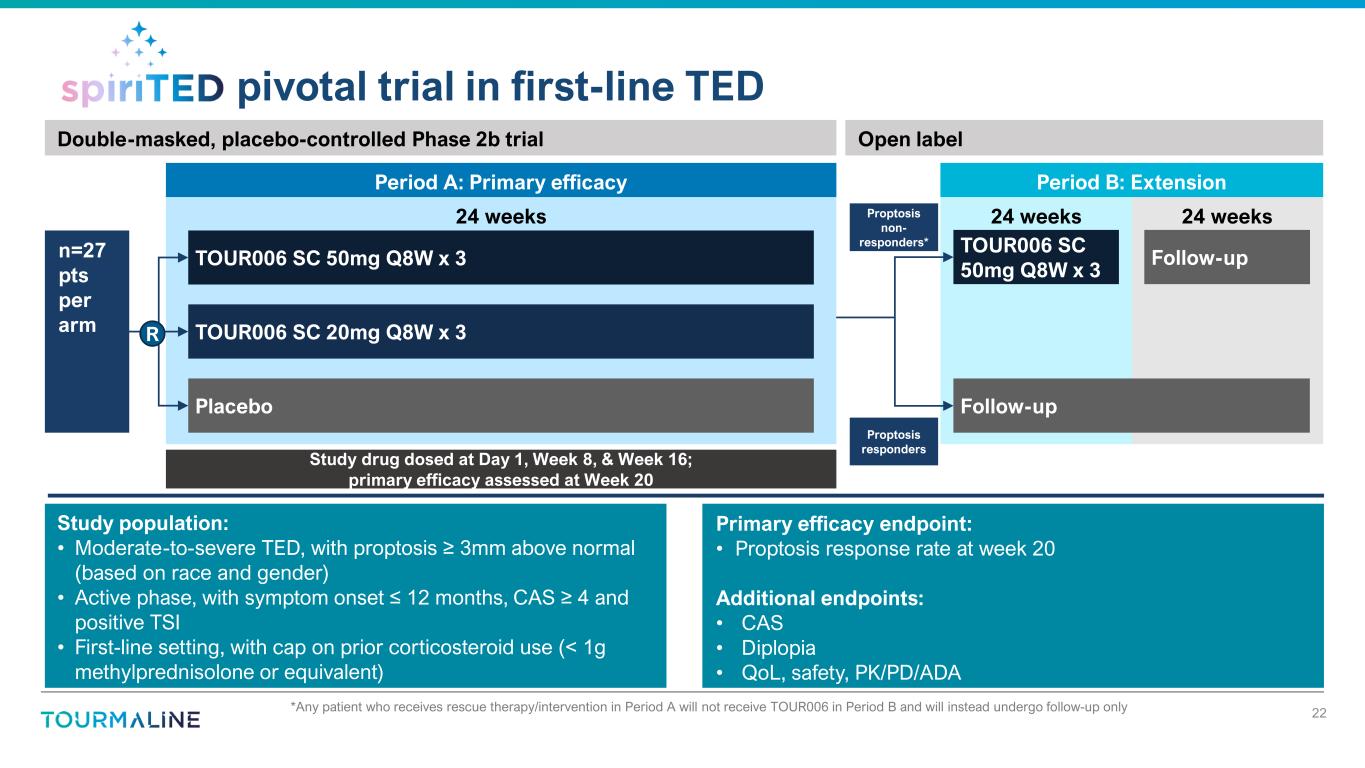
24 weeks24 weeks24 weeks pivotal trial in first-line TED n=27 pts per arm TOUR006 SC 50mg Q8W x 3 TOUR006 SC 20mg Q8W x 3 Period A: Primary efficacy Study population: • Moderate-to-severe TED, with proptosis ≥ 3mm above normal (based on race and gender) • Active phase, with symptom onset ≤ 12 months, CAS ≥ 4 and positive TSI • First-line setting, with cap on prior corticosteroid use (< 1g methylprednisolone or equivalent) Primary efficacy endpoint: • Proptosis response rate at week 20 Additional endpoints: • CAS • Diplopia • QoL, safety, PK/PD/ADA Double-masked, placebo-controlled Phase 2b trial Placebo R 22Any patient who receives rescue therapy/intervention in Period A will not receive TOUR006 in Period B and will instead undergo follow-up only* TOUR006 SC 50mg Q8W x 3 Follow-up Period B: Extension Proptosis non- responders* Proptosis responders Open label Study drug dosed at Day 1, Week 8, & Week 16; primary efficacy assessed at Week 20 Follow-up

Cardiovascular Inflammation

TOUR006 is poised to capitalize on emerging insights into targeting IL-6-driven inflammation in cardiovascular diseases 24 Emerging breakthrough insights support IL-6-driven inflammation as a key driver of CV diseases, particularly ASCVD, a leading cause of death worldwide TOUR006’s potentially best-in-class profile with quarterly subcutaneous administration will be evaluated in Phase 2 study TOUR006 is anticipated to be Phase 3-ready for CV disease in 2025 and well-positioned to capitalize on key external de-risking events
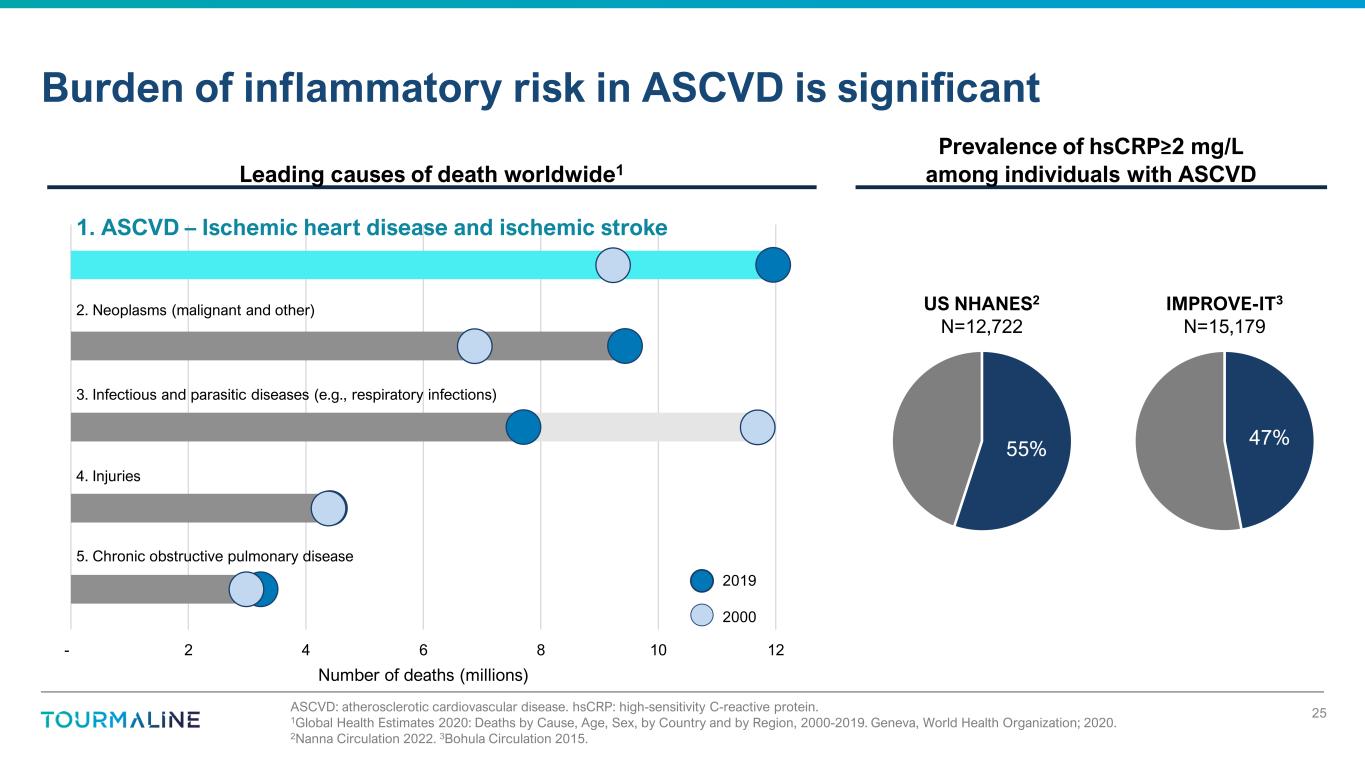
- 2 4 6 8 10 12 Number of deaths (millions) ASCVD: atherosclerotic cardiovascular disease. hsCRP: high-sensitivity C-reactive protein. 1Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2019. Geneva, World Health Organization; 2020. 2Nanna Circulation 2022. 3Bohula Circulation 2015. Burden of inflammatory risk in ASCVD is significant 25 1. ASCVD – Ischemic heart disease and ischemic stroke 2. Neoplasms (malignant and other) 3. Infectious and parasitic diseases (e.g., respiratory infections) 4. Injuries 5. Chronic obstructive pulmonary disease 2019 2000 55% US NHANES2 N=12,722 47% IMPROVE-IT3 N=15,179 Leading causes of death worldwide1 Prevalence of hsCRP≥2 mg/L among individuals with ASCVD

ASCVD: atherosclerotic cardiovascular diseases. Figure adapted from Libby Cells 2021 and Libby Int J Cardiol 2018. Pro-inflammatory responses • Atherogenesis • Leukocyte recruitment • Activation of adaptive immunity Fibrinogen (promotes clotting) Plasminogen activator inhibitor-1 (inhibits fibrinolysis) C-reactive protein (marker of inflammation) Serum amyloid A (remodels HDL) Thrombus formation and stabilization 26 IL-6 is central to inflammation driving ASCVD
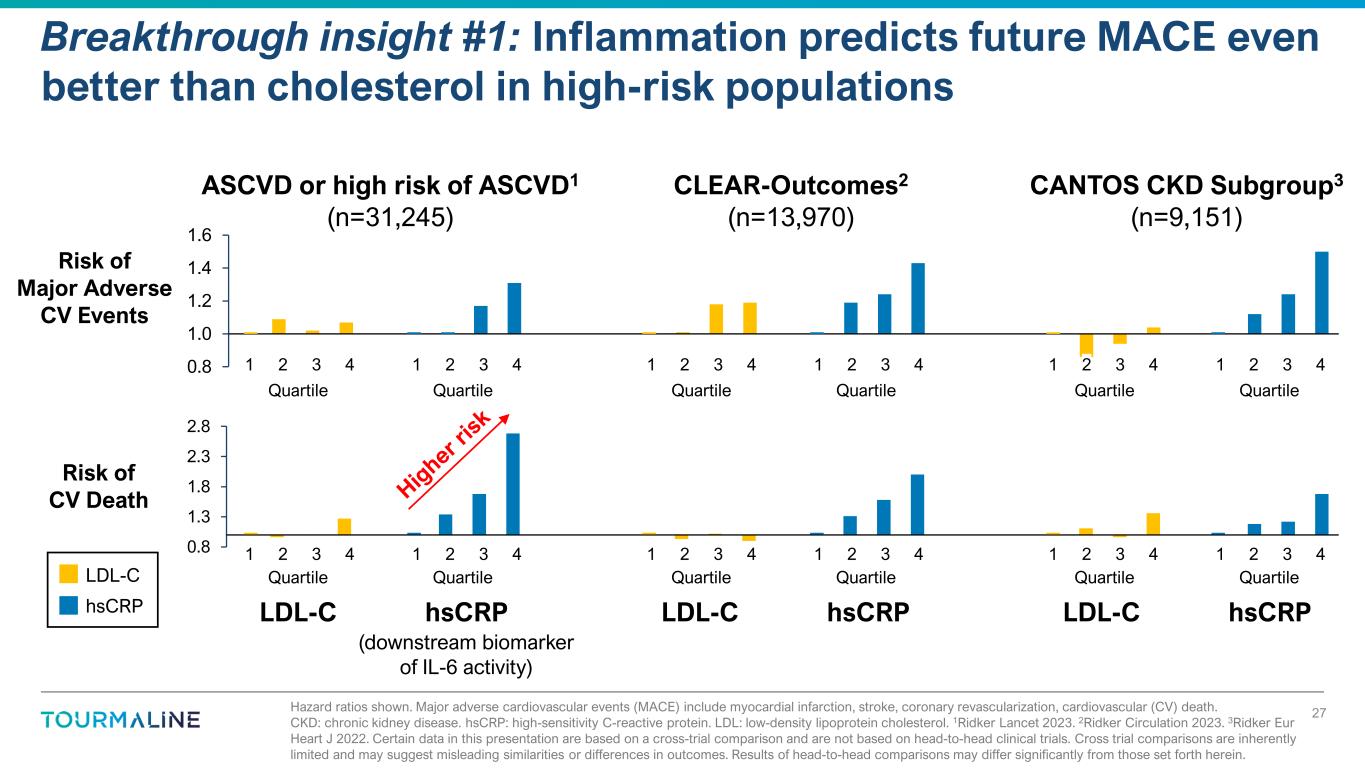
Hazard ratios shown. Major adverse cardiovascular events (MACE) include myocardial infarction, stroke, coronary revascularization, cardiovascular (CV) death. CKD: chronic kidney disease. hsCRP: high-sensitivity C-reactive protein. LDL: low-density lipoprotein cholesterol. 1Ridker Lancet 2023. 2Ridker Circulation 2023. 3Ridker Eur Heart J 2022. Certain data in this presentation are based on a cross-trial comparison and are not based on head-to-head clinical trials. Cross trial comparisons are inherently limited and may suggest misleading similarities or differences in outcomes. Results of head-to-head comparisons may differ significantly from those set forth herein. Breakthrough insight #1: Inflammation predicts future MACE even better than cholesterol in high-risk populations 27 0.8 1.0 1.2 1.4 1.6 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 LDL-C hsCRP Risk of Major Adverse CV Events ASCVD or high risk of ASCVD1 (n=31,245) LDL-C hsCRP LDL-C hsCRP 0.8 1.3 1.8 2.3 2.8 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Risk of CV Death (downstream biomarker of IL-6 activity) CANTOS CKD Subgroup3 (n=9,151) CLEAR-Outcomes2 (n=13,970) 1 2 3 41 2 3 4 1 2 3 41 2 3 4 1 2 3 41 2 3 4 1 2 3 41 2 3 4 1 2 3 41 2 3 4 1 2 3 41 2 3 4 uartile uartile uartile uartile uartile uartile Quartile Quartile Quartile Quartile Quartile Quartile hsCRP LDL-C
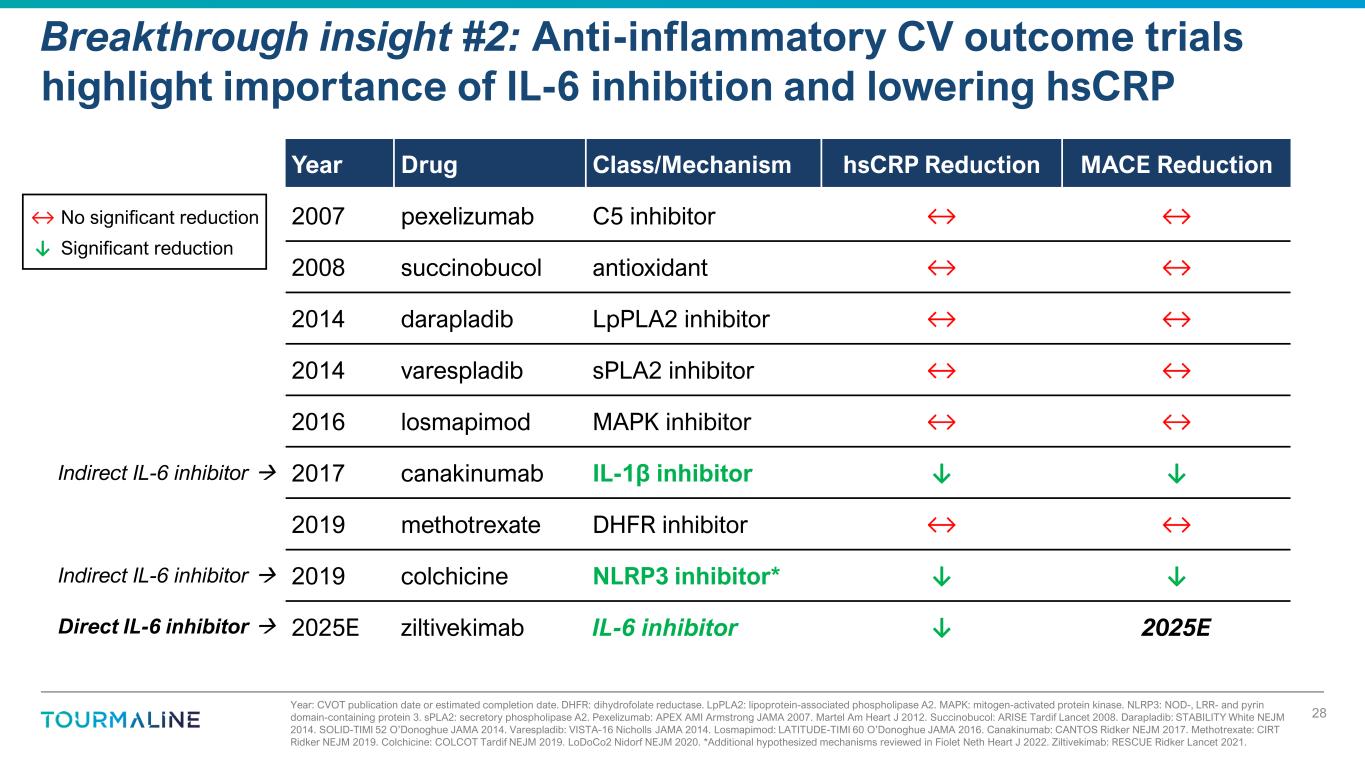
Year: CVOT publication date or estimated completion date. DHFR: dihydrofolate reductase. LpPLA2: lipoprotein-associated phospholipase A2. MAPK: mitogen-activated protein kinase. NLRP3: NOD-, LRR- and pyrin domain-containing protein 3. sPLA2: secretory phospholipase A2. Pexelizumab: APEX AMI Armstrong JAMA 2007. Martel Am Heart J 2012. Succinobucol: ARISE Tardif Lancet 2008. Darapladib: STABILITY White NEJM 2014. SOLID-TIMI 52 O’Donoghue JAMA 2014. Varespladib: VISTA-16 Nicholls JAMA 2014. Losmapimod: LATITUDE-TIMI 60 O’Donoghue JAMA 2016. Canakinumab: CANTOS Ridker NEJM 2017. Methotrexate: CIRT Ridker NEJM 2019. Colchicine: COLCOT Tardif NEJM 2019. LoDoCo2 Nidorf NEJM 2020. *Additional hypothesized mechanisms reviewed in Fiolet Neth Heart J 2022. Ziltivekimab: RESCUE Ridker Lancet 2021. Breakthrough insight #2: Anti-inflammatory CV outcome trials highlight importance of IL-6 inhibition and lowering hsCRP 28 Year Drug Class/Mechanism hsCRP Reduction MACE Reduction 2007 pexelizumab C5 inhibitor 2008 succinobucol antioxidant 2014 darapladib LpPLA2 inhibitor 2014 varespladib sPLA2 inhibitor 2016 losmapimod MAPK inhibitor 2017 canakinumab IL-1β inhibitor ↓ ↓ 2019 methotrexate DHFR inhibitor 2019 colchicine NLRP3 inhibitor* ↓ ↓ 2025E ziltivekimab IL-6 inhibitor ↓ 2025E Indirect IL-6 inhibitor Indirect IL-6 inhibitor Direct IL-6 inhibitor ↓ Significant reduction No significant reduction
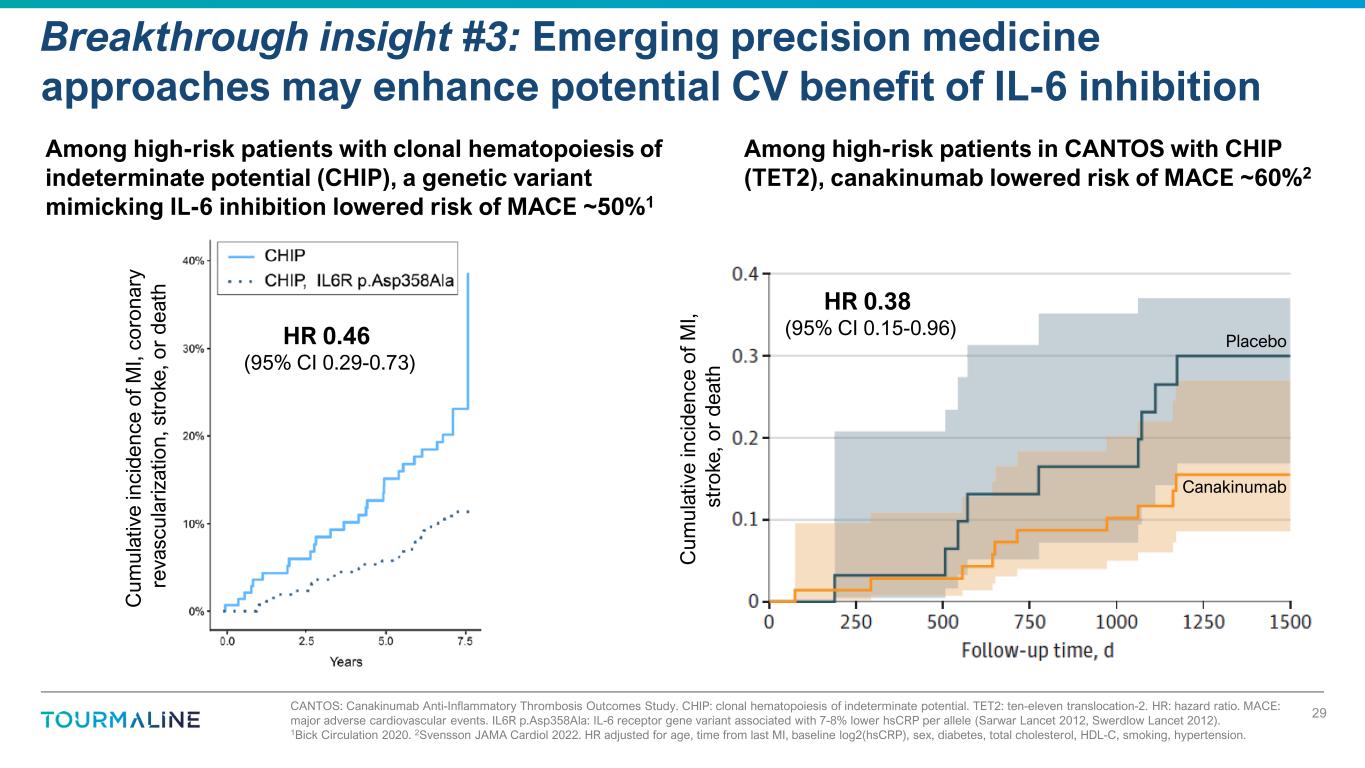
CANTOS: Canakinumab Anti-Inflammatory Thrombosis Outcomes Study. CHIP: clonal hematopoiesis of indeterminate potential. TET2: ten-eleven translocation-2. HR: hazard ratio. MACE: major adverse cardiovascular events. IL6R p.Asp358Ala: IL-6 receptor gene variant associated with 7-8% lower hsCRP per allele (Sarwar Lancet 2012, Swerdlow Lancet 2012). 1Bick Circulation 2020. 2Svensson JAMA Cardiol 2022. HR adjusted for age, time from last MI, baseline log2(hsCRP), sex, diabetes, total cholesterol, HDL-C, smoking, hypertension. Breakthrough insight #3: Emerging precision medicine approaches may enhance potential CV benefit of IL-6 inhibition 29 Among high-risk patients in CANTOS with CHIP (TET2), canakinumab lowered risk of MACE ~60%2 C um ul at iv e in ci de nc e of M I, st ro ke , o r d ea th HR 0.38 (95% CI 0.15-0.96) Placebo Canakinumab HR 0.46 (95% CI 0.29-0.73) C um ul at iv e in ci d en ce o f M I, co ro na ry re va sc ul ar iz at io n, s tr ok e, o r de at h Among high-risk patients with clonal hematopoiesis of indeterminate potential (CHIP), a genetic variant mimicking IL-6 inhibition lowered risk of MACE ~50%1
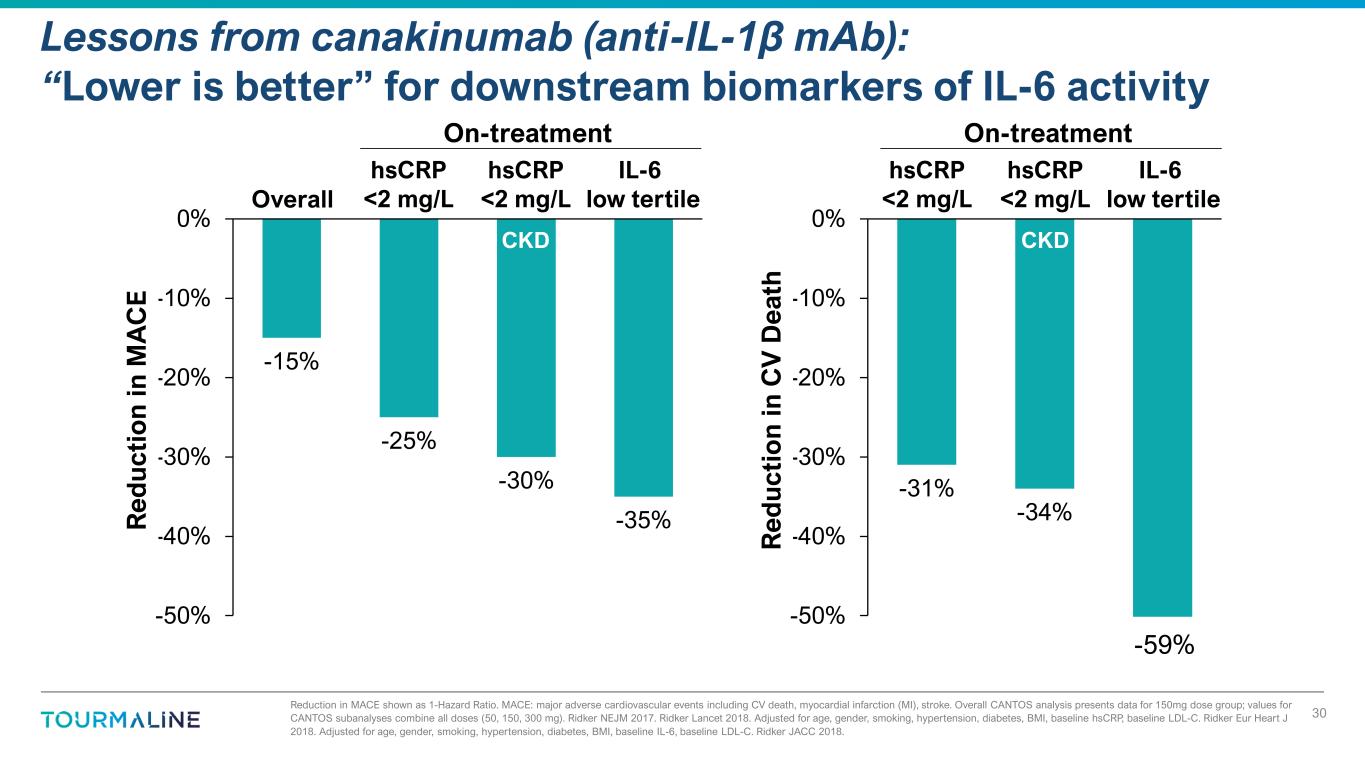
Reduction in MACE shown as 1-Hazard Ratio. MACE: major adverse cardiovascular events including CV death, myocardial infarction (MI), stroke. Overall CANTOS analysis presents data for 150mg dose group; values for CANTOS subanalyses combine all doses (50, 150, 300 mg). Ridker NEJM 2017. Ridker Lancet 2018. Adjusted for age, gender, smoking, hypertension, diabetes, BMI, baseline hsCRP, baseline LDL-C. Ridker Eur Heart J 2018. Adjusted for age, gender, smoking, hypertension, diabetes, BMI, baseline IL-6, baseline LDL-C. Ridker JACC 2018. Lessons from canakinumab (anti-IL-1β mAb): “Lower is better” for downstream biomarkers of IL-6 activity 30 -15% -25% -30% -35% -50% -40% -30% -20% -10% 0% R ed u ct io n in M A C E On-treatment IL-6 low tertile hsCRP <2 mg/L CKD Overall hsCRP <2 mg/L -31% -34% -50% -40% -30% -20% -10% 0% R ed u ct io n in C V D ea th On-treatment IL-6 low tertile hsCRP <2 mg/L CKD hsCRP <2 mg/L -59%

Reduction in MACE shown as 1-Hazard Ratio. MACE: major adverse cardiovascular events including CV death, myocardial infarction (MI), stroke except for: ODYSSEY OUTCOMES (all death, MI, ischemic stroke); COLCOT (CV death, MI, stroke, resuscitated cardiac arrest); LoDoCo2 (CV death, MI, ischemic stroke); main CANTOS analysis presents data for 150mg dose group; values for CANTOS subanalyses combine all doses (50, 150, 300mg); control arms were placebo + background SoC. Certain data in this slide are based on a cross-trial comparison and are not based on head-to-head clinical trials. Cross-trial comparisons are inherently limited and may suggest misleading similarities or differences in outcomes. Results of head-to-head comparisons may differ significantly from those set forth herein. NNT (number needed to treat) values obtained from absolute risk extracted from Kaplan-Meier figures via webplotdigitizer, unless directly reported. NNT for IL-6 < median shown; not reported for IL-6 low tertile. The information on this slide is based on Tourmaline-generated analysis of third-party data and is being presented for hypothesis generating purposes only. As a result, the actual MACE risk reduction hypothesized may be more or less than the data presented in this slide. Publications available upon request. Lessons from canakinumab (anti-IL-1β mAb): Robust inhibition of IL-6 pathway has transformative potential in ASCVD 31 -30% -35% -14% -15% -15% -20% -20% -26% -28% -50% -40% -30% -20% -10% 0% R ed u ct io n in M A C E IL-6 low tertile hsCRP <2 mg/L CKD Alirocumab ODYSSEY Bempedoic acid CLEAR Colchicine COLCOT Evolocumab FOURIER Semaglutide SELECT Icosapent ethyl REDUCE-IT Colchicine LoDoCo2 3-year estimates for NNT (number needed to treat) support transformative potential and efficient Phase 3 study designs 14 20 63 CANTOS: On-treatment 57368350nr85
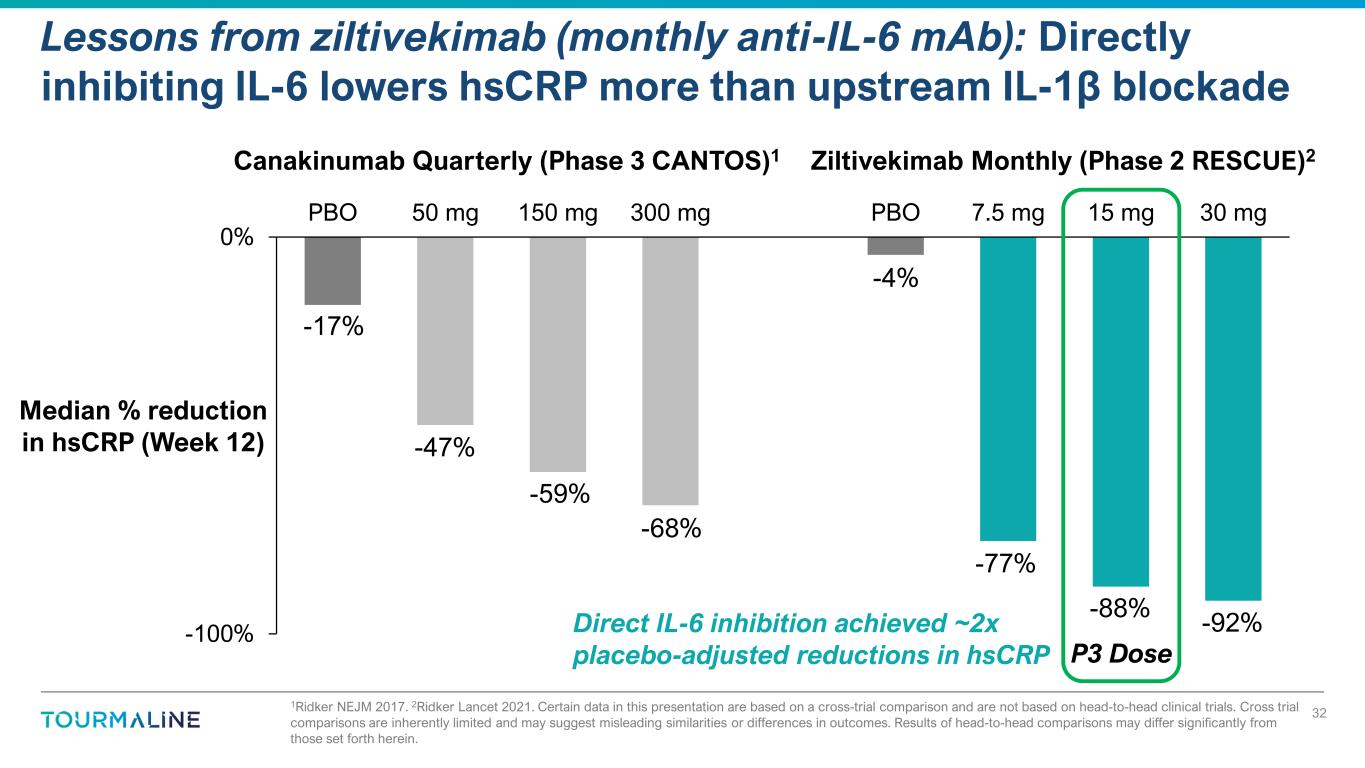
1Ridker NEJM 2017. 2Ridker Lancet 2021. Certain data in this presentation are based on a cross-trial comparison and are not based on head-to-head clinical trials. Cross trial comparisons are inherently limited and may suggest misleading similarities or differences in outcomes. Results of head-to-head comparisons may differ significantly from those set forth herein. Lessons from ziltivekimab (monthly anti-IL-6 mAb): Directly inhibiting IL-6 lowers hsCRP more than upstream IL-1β blockade 32 -100% 0% PBO 50 mg 150 mg 300 mg PBO 7.5 mg 15 mg 30 mg Median % reduction in hsCRP (Week 12) Ziltivekimab Monthly (Phase 2 RESCUE)2Canakinumab Quarterly (Phase 3 CANTOS)1 P3 Dose -47% -59% -77% -92%-88% -68% Direct IL-6 inhibition achieved ~2x placebo-adjusted reductions in hsCRP -4% -17%
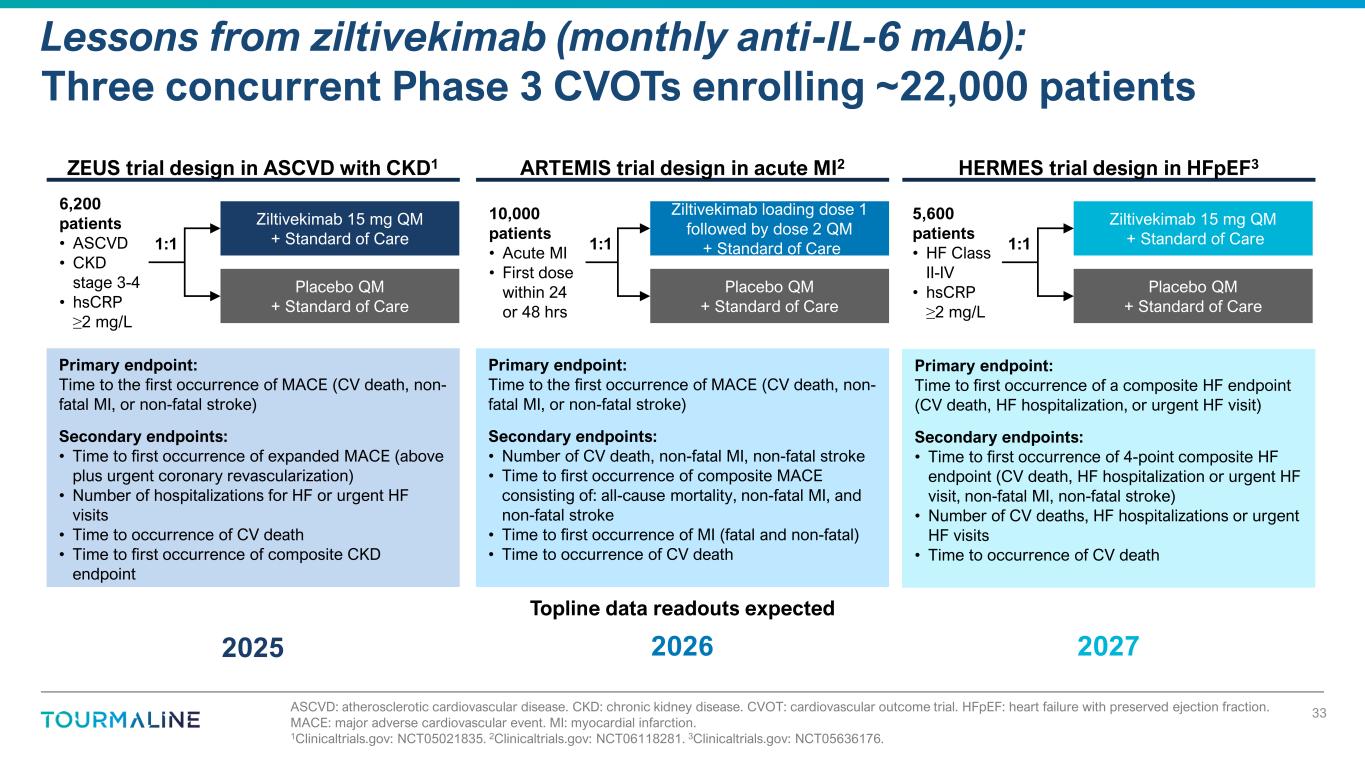
ASCVD: atherosclerotic cardiovascular disease. CKD: chronic kidney disease. CVOT: cardiovascular outcome trial. HFpEF: heart failure with preserved ejection fraction. MACE: major adverse cardiovascular event. MI: myocardial infarction. 1Clinicaltrials.gov: NCT05021835. 2Clinicaltrials.gov: NCT06118281. 3Clinicaltrials.gov: NCT05636176. Lessons from ziltivekimab (monthly anti-IL-6 mAb): Three concurrent Phase 3 CVOTs enrolling ~22,000 patients 33 Ziltivekimab 15 mg QM + Standard of Care Placebo QM + Standard of Care 6,200 patients • ASCVD • CKD stage 3-4 • hsCRP ≥2 mg/L 1:1 ZEUS trial design in ASCVD with CKD1 Primary endpoint: Time to the first occurrence of MACE (CV death, non- fatal MI, or non-fatal stroke) Secondary endpoints: • Time to first occurrence of expanded MACE (above plus urgent coronary revascularization) • Number of hospitalizations for HF or urgent HF visits • Time to occurrence of CV death • Time to first occurrence of composite CKD endpoint HERMES trial design in HFpEF3 Primary endpoint: Time to first occurrence of a composite HF endpoint (CV death, HF hospitalization, or urgent HF visit) Secondary endpoints: • Time to first occurrence of 4-point composite HF endpoint (CV death, HF hospitalization or urgent HF visit, non-fatal MI, non-fatal stroke) • Number of CV deaths, HF hospitalizations or urgent HF visits • Time to occurrence of CV death ARTEMIS trial design in acute MI2 Ziltivekimab loading dose 1 followed by dose 2 QM + Standard of Care Placebo QM + Standard of Care 10,000 patients • Acute MI • First dose within 24 or 48 hrs 1:1 Primary endpoint: Time to the first occurrence of MACE (CV death, non- fatal MI, or non-fatal stroke) Secondary endpoints: • Number of CV death, non-fatal MI, non-fatal stroke • Time to first occurrence of composite MACE consisting of: all-cause mortality, non-fatal MI, and non-fatal stroke • Time to first occurrence of MI (fatal and non-fatal) • Time to occurrence of CV death Ziltivekimab 15 mg QM + Standard of Care Placebo QM + Standard of Care 5,600 patients • HF Class II-IV • hsCRP ≥2 mg/L 1:1 Topline data readouts expected 2025 2026 2027

CD: Crohn’s disease, CKD: chronic kidney disease, HD: hemodialysis, NDD: non-dialysis dependent, SLE: systemic lupus erythematosus. 1. Incidence of ADAs in repeat-dose studies calculated as reported per dosing arm. 2. Route of administration in planned or ongoing studies in patients with or at high-risk of ASCVD. 3. Clinicaltrials.gov NCT03926117. 4. Pergola JASN 2021. 5. Ridker Lancet 2021. 6. Wada J Cardiol 2023. 7. Clinicaltrials.gov NCT01490450. 8. Clinicaltrials.gov NCT01545050. 9. Weinblatt Arthritis Rheum 2015. 10. Clinicaltrials.gov NCT05485961. Data reported in publications or on clinicaltrials.gov as detailed above. No head-to-head studies have been conducted between the mabs shown here, which have each been evaluated in different populations. TOUR006 offers best-in-class potential in ASCVD 34 TOUR006 Ziltivekimab Clazakizumab Company Monoclonal antibody fully human (IgG2) Medarex UltiMAb mouse fully human (IgG1k, YTE mutation) phage display humanized rabbit (IgG1k) Anti-drug antibodies1 0-1% 6-13%3,4 0-10%7-9 Route of administration2 SC 0.6 mL SC5,6 1.0 mL IV10 Longest dosing intervals in completed studies Q8W (SLE, CD) Q4W (NDD-CKD)5,6 Q4W10 (HD-CKD) Targeted dosing intervals Quarterly Monthly Monthly
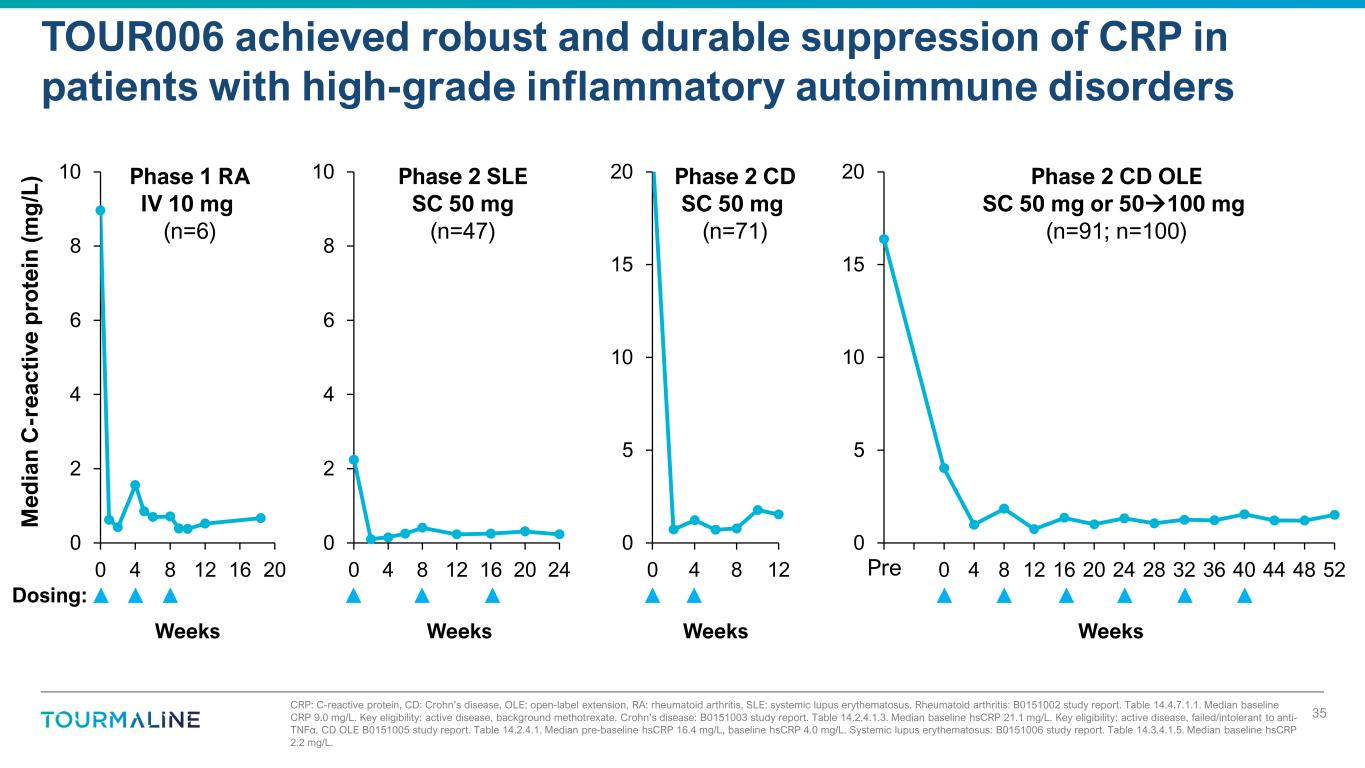
CRP: C-reactive protein, CD: Crohn’s disease, OLE: open-label extension, RA: rheumatoid arthritis, SLE: systemic lupus erythematosus. Rheumatoid arthritis: B0151002 study report. Table 14.4.7.1.1. Median baseline CRP 9.0 mg/L. Key eligibility: active disease, background methotrexate. Crohn’s disease: B0151003 study report. Table 14.2.4.1.3. Median baseline hsCRP 21.1 mg/L. Key eligibility: active disease, failed/intolerant to anti- TNF . CD OLE B0151005 study report. Table 14.2.4.1. Systemic lupus erythematosus: B0151006 study report. Table 14.3.4.1.5. Median baseline hsCRP 2.2 mg/L. α Median pre-baseline hsCRP 16.4 mg/L, baseline hsCRP 4.0 mg/L. TOUR006 achieved robust and durable suppression of CRP in patients with high-grade inflammatory autoimmune disorders 35 Dosing: 0 2 4 6 8 10 0 4 8 12 16 20 0 5 10 15 20 0 4 8 12 Phase 1 RA IV 10 mg (n=6) Phase 2 CD SC 50 mg (n=71) M ed ia n C -r ea ct iv e p ro te in (m g /L ) Weeks Weeks 0 5 10 15 20 -8 -4 0 4 8 12 16 20 24 28 32 36 40 44 48 52 Phase 2 CD OLE SC 50 mg or 50100 mg (n=91; n=100) Pre Weeks 0 2 4 6 8 10 0 4 8 12 16 20 24 Phase 2 SLE SC 50 mg (n=47) Weeks
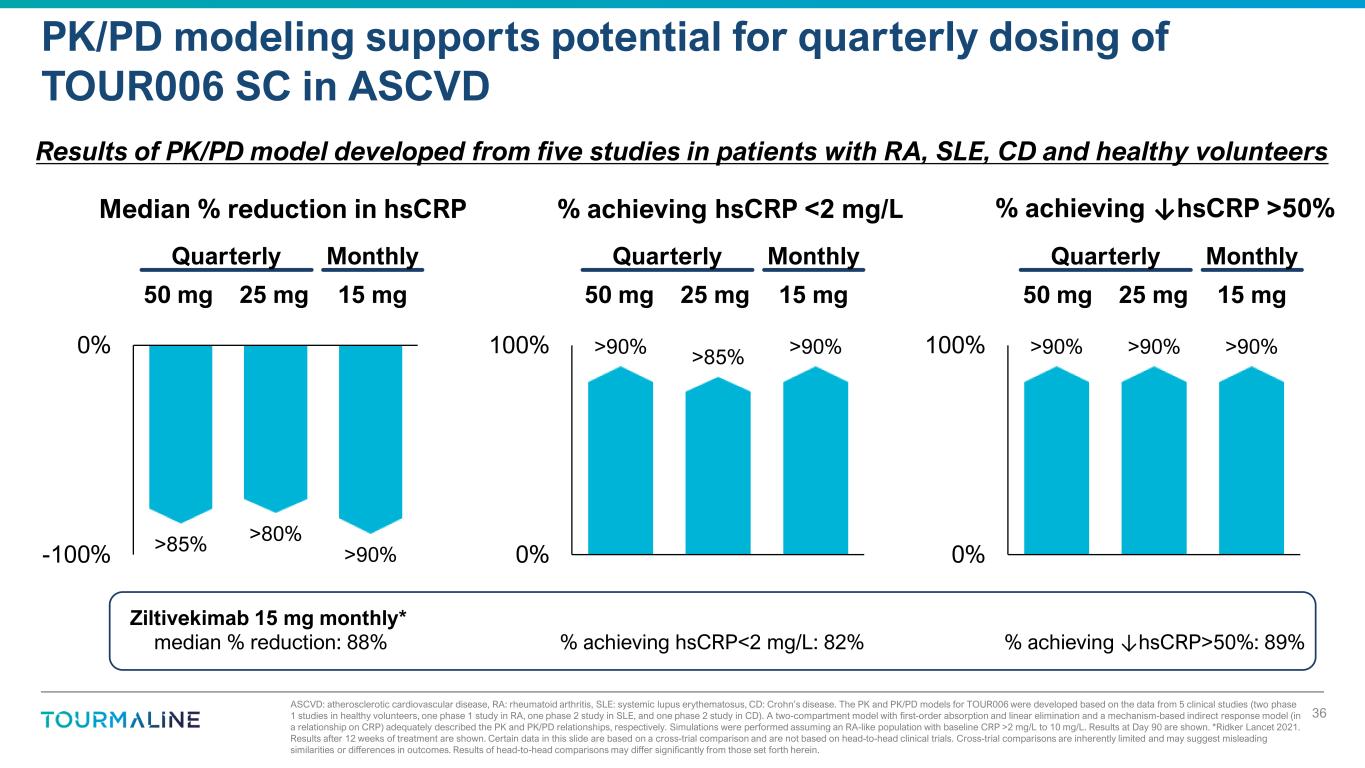
ASCVD: atherosclerotic cardiovascular disease, RA: rheumatoid arthritis, SLE: systemic lupus erythematosus, CD: Crohn’s disease. The PK and PK/PD models for TOUR006 were developed based on the data from 5 clinical studies (two phase 1 studies in healthy volunteers, one phase 1 study in RA, one phase 2 study in SLE, and one phase 2 study in CD). A two-compartment model with first-order absorption and linear elimination and a mechanism-based indirect response model (in a relationship on CRP) adequately described the PK and PK/PD relationships, respectively. Simulations were performed assuming an RA-like population with baseline CRP >2 mg/L to 10 mg/L. Results at Day 90 are shown. *Ridker Lancet 2021. Results after 12 weeks of treatment are shown. Certain data in this slide are based on a cross-trial comparison and are not based on head-to-head clinical trials. Cross-trial comparisons are inherently limited and may suggest misleading similarities or differences in outcomes. Results of head-to-head comparisons may differ significantly from those set forth herein. PK/PD modeling supports potential for quarterly dosing of TOUR006 SC in ASCVD 36 Median % reduction in hsCRP 15 mg50 mg 25 mg % achieving hsCRP <2 mg/L % achieving ↓hsCRP >50% Quarterly Monthly Results of PK/PD model developed from five studies in patients with RA, SLE, CD and healthy volunteers Ziltivekimab 15 mg monthly* median % reduction: 88% % achieving hsCRP<2 mg/L: 82% % achieving ↓hsCRP>50%: 89% 15 mg50 mg 25 mg Quarterly Monthly 15 mg50 mg 25 mg Quarterly Monthly >85% >80% >90%-100% 0% >90% >85% >90% 0% 100% >90% >90% >90% 0% 100%
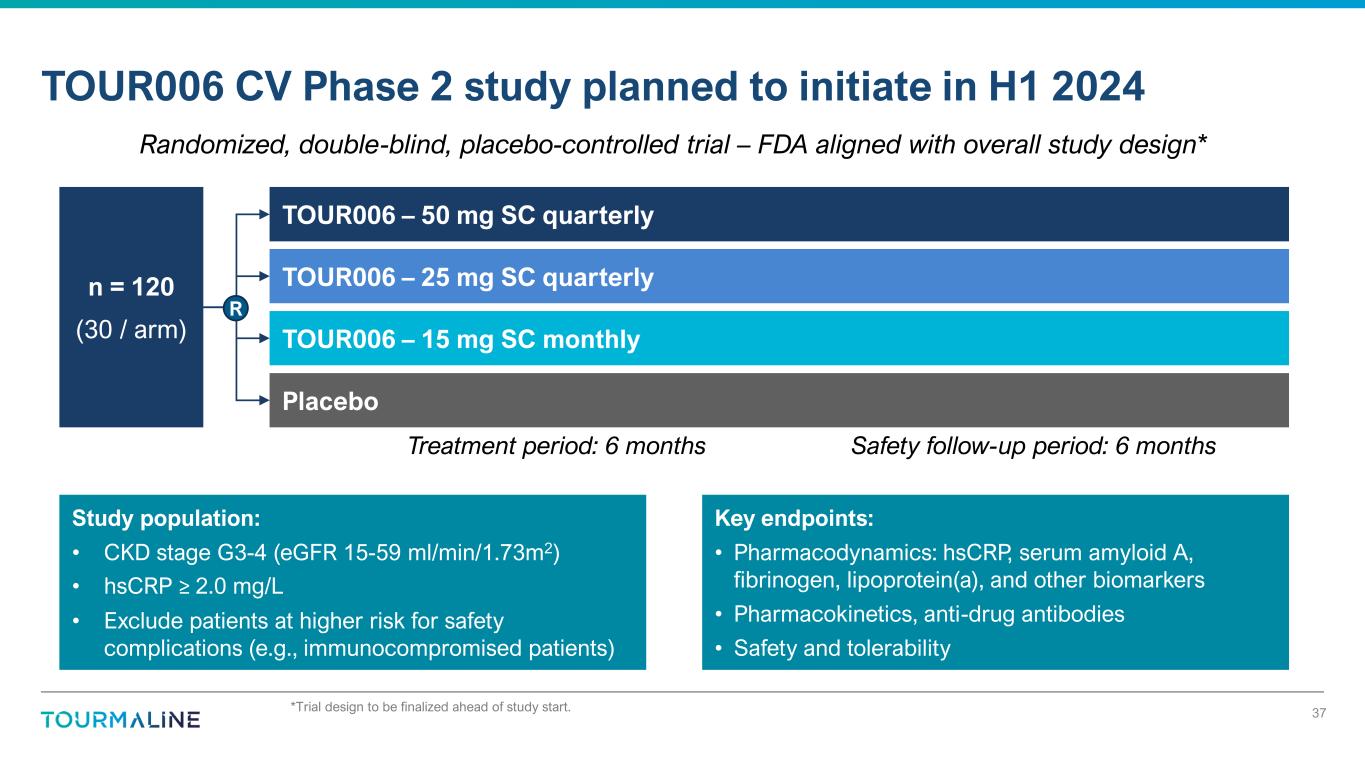
n = 120 (30 / arm) *Trial design to be finalized ahead of study start. TOUR006 CV Phase 2 study planned to initiate in H1 2024 37 TOUR006 – 50 mg SC quarterly TOUR006 – 25 mg SC quarterly TOUR006 – 15 mg SC monthly Study population: • CKD stage G3-4 (eGFR 15-59 ml/min/1.73m2) • hsCRP ≥ 2.0 mg/L • Exclude patients at higher risk for safety complications (e.g., immunocompromised patients) Key endpoints: • Pharmacodynamics: hsCRP, serum amyloid A, fibrinogen, lipoprotein(a), and other biomarkers • Pharmacokinetics, anti-drug antibodies • Safety and tolerability Randomized, double-blind, placebo-controlled trial – FDA aligned with overall study design* R Placebo Treatment period: 6 months Safety follow-up period: 6 months

AE: autoimmune encephalitis; AMI: acute myocardial infarction; ASCVD: atherosclerotic cardiovascular disease; CKD: chronic kidney disease; MG: myasthenia gravis; Satra: satralizumab; Zilti: ziltivekimab; Two strategic paths to unlock major value creation 38 FcRn+ Cardiovascular Inflammation IL-6 Renaissance Emerging insights implicating IL-6 in a wide range of rare & large diseases TOUR006 Long-acting Low immunogenicity Low volume, SC TOUR006 has the potential to be a superior therapy for a wide range of autoantibody- driven diseases vs. FcRn inhibitors TOUR006 has the potential to transform the care of high-risk patients by targeting key inflammatory pathways driving cardiovascular disease + H1 2025: CV Phase 2 topline data 2025: Zilti ASCVD in CKD Phase 3 (ZEUS) topline data H1 2025: spiriTED topline data 2025: Satra AE Phase 3 (CIELO) topline data H1 2024: Satra MG Phase 3 (LUMINESCE) topline data 2026: Zilti AMI Phase 3 (ARTEMIS) topline data Key expected readouts Key expected readouts Milestones key: Internal External








































