000181838212-312025Q3FALSE16401640xbrli:sharesiso4217:USDiso4217:USDxbrli:shareshuma:purchaserhuma:employeehuma:segmenthuma:yearxbrli:purehuma:termhuma:votehuma:tranchehuma:planhuma:installmenthuma:action00018183822025-01-012025-09-300001818382us-gaap:CommonStockMember2025-01-012025-09-300001818382huma:RedeemableWarrantsEachWholeWarrantExercisableForOneShareOfCommonStockAtAnExercisePriceOf1150Member2025-01-012025-09-3000018183822025-11-0400018183822025-09-3000018183822024-12-310001818382us-gaap:ProductMember2025-07-012025-09-300001818382us-gaap:ProductMember2024-07-012024-09-300001818382us-gaap:ProductMember2025-01-012025-09-300001818382us-gaap:ProductMember2024-01-012024-09-300001818382us-gaap:ProductAndServiceOtherMember2025-07-012025-09-300001818382us-gaap:ProductAndServiceOtherMember2024-07-012024-09-300001818382us-gaap:ProductAndServiceOtherMember2025-01-012025-09-300001818382us-gaap:ProductAndServiceOtherMember2024-01-012024-09-3000018183822025-07-012025-09-3000018183822024-07-012024-09-3000018183822024-01-012024-09-300001818382us-gaap:CommonStockMember2024-12-310001818382us-gaap:AdditionalPaidInCapitalMember2024-12-310001818382us-gaap:RetainedEarningsMember2024-12-310001818382huma:PublicStockOfferingMemberus-gaap:CommonStockMember2025-01-012025-03-310001818382huma:PublicStockOfferingMemberus-gaap:AdditionalPaidInCapitalMember2025-01-012025-03-310001818382huma:PublicStockOfferingMember2025-01-012025-03-310001818382huma:ATMFacilityMemberus-gaap:CommonStockMember2025-01-012025-03-310001818382huma:ATMFacilityMemberus-gaap:AdditionalPaidInCapitalMember2025-01-012025-03-310001818382huma:ATMFacilityMember2025-01-012025-03-310001818382us-gaap:CommonStockMember2025-01-012025-03-310001818382us-gaap:AdditionalPaidInCapitalMember2025-01-012025-03-3100018183822025-01-012025-03-310001818382us-gaap:RetainedEarningsMember2025-01-012025-03-310001818382us-gaap:CommonStockMember2025-03-310001818382us-gaap:AdditionalPaidInCapitalMember2025-03-310001818382us-gaap:RetainedEarningsMember2025-03-3100018183822025-03-310001818382huma:ATMFacilityMemberus-gaap:CommonStockMember2025-04-012025-06-300001818382huma:ATMFacilityMemberus-gaap:AdditionalPaidInCapitalMember2025-04-012025-06-300001818382huma:ATMFacilityMember2025-04-012025-06-300001818382us-gaap:AdditionalPaidInCapitalMember2025-04-012025-06-3000018183822025-04-012025-06-300001818382us-gaap:RetainedEarningsMember2025-04-012025-06-300001818382us-gaap:CommonStockMember2025-06-300001818382us-gaap:AdditionalPaidInCapitalMember2025-06-300001818382us-gaap:RetainedEarningsMember2025-06-3000018183822025-06-300001818382huma:ATMFacilityMemberus-gaap:CommonStockMember2025-07-012025-09-300001818382huma:ATMFacilityMemberus-gaap:AdditionalPaidInCapitalMember2025-07-012025-09-300001818382huma:ATMFacilityMember2025-07-012025-09-300001818382us-gaap:AdditionalPaidInCapitalMember2025-07-012025-09-300001818382us-gaap:RetainedEarningsMember2025-07-012025-09-300001818382us-gaap:CommonStockMember2025-09-300001818382us-gaap:AdditionalPaidInCapitalMember2025-09-300001818382us-gaap:RetainedEarningsMember2025-09-300001818382us-gaap:CommonStockMember2023-12-310001818382us-gaap:AdditionalPaidInCapitalMember2023-12-310001818382us-gaap:RetainedEarningsMember2023-12-3100018183822023-12-310001818382huma:PublicStockOfferingMemberus-gaap:CommonStockMember2024-01-012024-03-310001818382huma:PublicStockOfferingMemberus-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-310001818382huma:PublicStockOfferingMember2024-01-012024-03-310001818382us-gaap:CommonStockMember2024-01-012024-03-310001818382us-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-3100018183822024-01-012024-03-310001818382us-gaap:RetainedEarningsMember2024-01-012024-03-310001818382us-gaap:CommonStockMember2024-03-310001818382us-gaap:AdditionalPaidInCapitalMember2024-03-310001818382us-gaap:RetainedEarningsMember2024-03-3100018183822024-03-310001818382us-gaap:CommonStockMember2024-04-012024-06-300001818382us-gaap:AdditionalPaidInCapitalMember2024-04-012024-06-3000018183822024-04-012024-06-300001818382us-gaap:RetainedEarningsMember2024-04-012024-06-300001818382us-gaap:CommonStockMember2024-06-300001818382us-gaap:AdditionalPaidInCapitalMember2024-06-300001818382us-gaap:RetainedEarningsMember2024-06-3000018183822024-06-300001818382huma:CommitmentSharesMemberus-gaap:CommonStockMember2024-07-012024-09-300001818382huma:CommitmentSharesMemberus-gaap:AdditionalPaidInCapitalMember2024-07-012024-09-300001818382huma:CommitmentSharesMember2024-07-012024-09-300001818382huma:EquityLineFinancingMemberus-gaap:CommonStockMember2024-07-012024-09-300001818382huma:EquityLineFinancingMemberus-gaap:AdditionalPaidInCapitalMember2024-07-012024-09-300001818382huma:EquityLineFinancingMember2024-07-012024-09-300001818382us-gaap:CommonStockMember2024-07-012024-09-300001818382us-gaap:AdditionalPaidInCapitalMember2024-07-012024-09-300001818382us-gaap:RetainedEarningsMember2024-07-012024-09-300001818382us-gaap:CommonStockMember2024-09-300001818382us-gaap:AdditionalPaidInCapitalMember2024-09-300001818382us-gaap:RetainedEarningsMember2024-09-3000018183822024-09-300001818382huma:PublicStockOfferingMember2025-01-012025-09-300001818382huma:PublicStockOfferingMember2024-01-012024-09-300001818382huma:ATMFacilityMember2025-01-012025-09-300001818382huma:ATMFacilityMember2024-01-012024-09-300001818382huma:EquityLineFinancingMember2025-01-012025-09-300001818382huma:EquityLineFinancingMember2024-01-012024-09-300001818382huma:RevenueInterestPurchaseAgreementMember2023-05-120001818382huma:RevenueInterestPurchaseAgreementMemberus-gaap:UnsecuredDebtMember2025-09-300001818382huma:RevenueInterestPurchaseAgreementMember2024-05-080001818382huma:RevenueInterestPurchaseAgreementMember2024-05-082024-05-080001818382huma:RevenueInterestPurchaseAgreementMember2024-08-140001818382huma:RevenueInterestPurchaseAgreementMember2025-09-172025-09-170001818382huma:RevenueInterestPurchaseAgreementMember2025-09-3000018183822024-09-240001818382huma:EquityLineFinancingMember2024-09-240001818382huma:EquityLineFinancingMember2024-09-242024-09-240001818382huma:EquityLineFinancingMember2025-09-300001818382huma:ATMFacilityMember2022-09-010001818382huma:ATMFacilityMember2025-09-3000018183822025-04-282025-04-280001818382huma:VascularTraumaMemberhuma:ReportableSegmentMember2025-07-012025-09-300001818382huma:VascularTraumaMemberhuma:ReportableSegmentMember2024-07-012024-09-300001818382huma:VascularTraumaMemberhuma:ReportableSegmentMember2025-01-012025-09-300001818382huma:VascularTraumaMemberhuma:ReportableSegmentMember2024-01-012024-09-300001818382huma:AVAccessMemberhuma:ReportableSegmentMember2025-07-012025-09-300001818382huma:AVAccessMemberhuma:ReportableSegmentMember2024-07-012024-09-300001818382huma:AVAccessMemberhuma:ReportableSegmentMember2025-01-012025-09-300001818382huma:AVAccessMemberhuma:ReportableSegmentMember2024-01-012024-09-300001818382huma:PADMemberhuma:ReportableSegmentMember2025-07-012025-09-300001818382huma:PADMemberhuma:ReportableSegmentMember2024-07-012024-09-300001818382huma:PADMemberhuma:ReportableSegmentMember2025-01-012025-09-300001818382huma:PADMemberhuma:ReportableSegmentMember2024-01-012024-09-300001818382huma:DirectExpensesMemberhuma:ReportableSegmentMember2025-07-012025-09-300001818382huma:DirectExpensesMemberhuma:ReportableSegmentMember2024-07-012024-09-300001818382huma:DirectExpensesMemberhuma:ReportableSegmentMember2025-01-012025-09-300001818382huma:DirectExpensesMemberhuma:ReportableSegmentMember2024-01-012024-09-300001818382huma:ExternalServicesMemberhuma:ReportableSegmentMember2025-07-012025-09-300001818382huma:ExternalServicesMemberhuma:ReportableSegmentMember2024-07-012024-09-300001818382huma:ExternalServicesMemberhuma:ReportableSegmentMember2025-01-012025-09-300001818382huma:ExternalServicesMemberhuma:ReportableSegmentMember2024-01-012024-09-300001818382huma:MaterialsAndSuppliesMemberhuma:ReportableSegmentMember2025-07-012025-09-300001818382huma:MaterialsAndSuppliesMemberhuma:ReportableSegmentMember2024-07-012024-09-300001818382huma:MaterialsAndSuppliesMemberhuma:ReportableSegmentMember2025-01-012025-09-300001818382huma:MaterialsAndSuppliesMemberhuma:ReportableSegmentMember2024-01-012024-09-300001818382huma:PayrollAndPersonnelExpensesMemberhuma:ReportableSegmentMember2025-07-012025-09-300001818382huma:PayrollAndPersonnelExpensesMemberhuma:ReportableSegmentMember2024-07-012024-09-300001818382huma:PayrollAndPersonnelExpensesMemberhuma:ReportableSegmentMember2025-01-012025-09-300001818382huma:PayrollAndPersonnelExpensesMemberhuma:ReportableSegmentMember2024-01-012024-09-300001818382huma:OtherResearchAndDevelopmentMemberhuma:ReportableSegmentMember2025-07-012025-09-300001818382huma:OtherResearchAndDevelopmentMemberhuma:ReportableSegmentMember2024-07-012024-09-300001818382huma:OtherResearchAndDevelopmentMemberhuma:ReportableSegmentMember2025-01-012025-09-300001818382huma:OtherResearchAndDevelopmentMemberhuma:ReportableSegmentMember2024-01-012024-09-300001818382huma:UnallocatedExpensesMemberhuma:ReportableSegmentMember2025-07-012025-09-300001818382huma:UnallocatedExpensesMemberhuma:ReportableSegmentMember2024-07-012024-09-300001818382huma:UnallocatedExpensesMemberhuma:ReportableSegmentMember2025-01-012025-09-300001818382huma:UnallocatedExpensesMemberhuma:ReportableSegmentMember2024-01-012024-09-300001818382huma:ReportableSegmentMember2025-07-012025-09-300001818382huma:ReportableSegmentMember2024-07-012024-09-300001818382huma:ReportableSegmentMember2025-01-012025-09-300001818382huma:ReportableSegmentMember2024-01-012024-09-300001818382us-gaap:ProductMember2025-09-300001818382us-gaap:ProductAndServiceOtherMember2025-09-300001818382us-gaap:ProductAndServiceOtherMember2024-12-310001818382us-gaap:DepositAccountMember2025-09-300001818382us-gaap:DepositAccountMember2024-12-310001818382huma:RevenueInterestPurchaseAgreementMember2024-12-310001818382us-gaap:EmployeeStockOptionMember2025-07-012025-09-300001818382us-gaap:EmployeeStockOptionMember2024-07-012024-09-300001818382us-gaap:EmployeeStockOptionMember2025-01-012025-09-300001818382us-gaap:EmployeeStockOptionMember2024-01-012024-09-300001818382us-gaap:WarrantMember2025-07-012025-09-300001818382us-gaap:WarrantMember2024-07-012024-09-300001818382us-gaap:WarrantMember2025-01-012025-09-300001818382us-gaap:WarrantMember2024-01-012024-09-300001818382huma:SaleOfStockOptionMember2025-07-012025-09-300001818382huma:SaleOfStockOptionMember2024-07-012024-09-300001818382huma:SaleOfStockOptionMember2025-01-012025-09-300001818382huma:SaleOfStockOptionMember2024-01-012024-09-300001818382huma:TPCInvestmentsIIILPAndTPCInvestmentSolutionsLPMemberhuma:OptionPurchaseAgreementMember2023-05-120001818382us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2025-09-300001818382us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2025-09-300001818382us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2025-09-300001818382us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2025-09-300001818382us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2025-09-300001818382us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2025-09-300001818382us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2025-09-300001818382us-gaap:FairValueMeasurementsRecurringMember2025-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2025-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2025-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2025-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberus-gaap:FairValueMeasurementsRecurringMember2025-09-300001818382huma:PrivatePlacementWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2025-09-300001818382huma:PrivatePlacementWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2025-09-300001818382huma:PrivatePlacementWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2025-09-300001818382huma:PrivatePlacementWarrantsMemberus-gaap:FairValueMeasurementsRecurringMember2025-09-300001818382huma:October2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2025-09-300001818382huma:October2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2025-09-300001818382huma:October2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2025-09-300001818382huma:October2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMember2025-09-300001818382huma:November2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2025-09-300001818382huma:November2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2025-09-300001818382huma:November2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2025-09-300001818382huma:November2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMember2025-09-300001818382us-gaap:StockOptionMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2025-09-300001818382us-gaap:StockOptionMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2025-09-300001818382us-gaap:StockOptionMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2025-09-300001818382us-gaap:StockOptionMemberus-gaap:FairValueMeasurementsRecurringMember2025-09-300001818382huma:JDRFAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2025-09-300001818382huma:JDRFAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2025-09-300001818382huma:JDRFAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2025-09-300001818382huma:JDRFAgreementMemberus-gaap:FairValueMeasurementsRecurringMember2025-09-300001818382us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-12-310001818382us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-12-310001818382us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-12-310001818382us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001818382us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-12-310001818382us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-12-310001818382us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-12-310001818382us-gaap:FairValueMeasurementsRecurringMember2024-12-310001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-12-310001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-12-310001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-12-310001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001818382huma:PrivatePlacementWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-12-310001818382huma:PrivatePlacementWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-12-310001818382huma:PrivatePlacementWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-12-310001818382huma:PrivatePlacementWarrantsMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001818382huma:October2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-12-310001818382huma:October2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-12-310001818382huma:October2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-12-310001818382huma:October2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001818382huma:November2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-12-310001818382huma:November2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-12-310001818382huma:November2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-12-310001818382huma:November2024RegisteredDirectOfferingWarrantsMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001818382us-gaap:StockOptionMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-12-310001818382us-gaap:StockOptionMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-12-310001818382us-gaap:StockOptionMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-12-310001818382us-gaap:StockOptionMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001818382huma:JDRFAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-12-310001818382huma:JDRFAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-12-310001818382huma:JDRFAgreementMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-12-310001818382huma:JDRFAgreementMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001818382huma:CommitmentSharesMemberhuma:EquityLineFinancingMember2024-09-242024-09-240001818382us-gaap:FairValueMeasurementsRecurringMemberhuma:EquityLineFinancingMember2024-09-240001818382huma:EquityLineFinancingMember2024-12-310001818382huma:ContingentEarnoutLiabilityMember2025-06-300001818382huma:ContingentEarnoutLiabilityMember2024-06-300001818382huma:ContingentEarnoutLiabilityMember2024-12-310001818382huma:ContingentEarnoutLiabilityMember2023-12-310001818382huma:ContingentEarnoutLiabilityMember2025-07-012025-09-300001818382huma:ContingentEarnoutLiabilityMember2024-07-012024-09-300001818382huma:ContingentEarnoutLiabilityMember2025-01-012025-09-300001818382huma:ContingentEarnoutLiabilityMember2024-01-012024-09-300001818382huma:ContingentEarnoutLiabilityMember2025-09-300001818382huma:ContingentEarnoutLiabilityMember2024-09-300001818382huma:ContingentEarnoutLiabilityMemberus-gaap:MeasurementInputExpectedTermMember2025-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberus-gaap:MeasurementInputExpectedTermMember2025-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberhuma:MeasurementInputRevenueForecastDiscountRateMember2025-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberhuma:MeasurementInputPayoffOfInstrumentDiscountRateMemberMember2025-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberhuma:MeasurementInputRevenueForecastDiscountRateMember2024-12-310001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberhuma:MeasurementInputPayoffOfInstrumentDiscountRateMemberMember2024-12-310001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2025-06-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2024-06-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2024-12-310001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2023-12-310001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2025-07-012025-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2024-07-012024-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2025-01-012025-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2024-01-012024-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2025-09-300001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2024-09-300001818382huma:October2024RegisteredDirectOfferingWarrantsMember2025-06-300001818382huma:October2024RegisteredDirectOfferingWarrantsMember2024-12-310001818382huma:November2024RegisteredDirectOfferingWarrantsMember2025-06-300001818382huma:November2024RegisteredDirectOfferingWarrantsMember2024-12-310001818382huma:October2024RegisteredDirectOfferingWarrantsMember2025-07-012025-09-300001818382huma:October2024RegisteredDirectOfferingWarrantsMember2025-01-012025-09-300001818382huma:November2024RegisteredDirectOfferingWarrantsMember2025-07-012025-09-300001818382huma:November2024RegisteredDirectOfferingWarrantsMember2025-01-012025-09-300001818382huma:October2024RegisteredDirectOfferingWarrantsMember2025-09-300001818382huma:November2024RegisteredDirectOfferingWarrantsMember2025-09-300001818382huma:PrivatePlacementWarrantsMember2025-06-300001818382huma:PrivatePlacementWarrantsMember2024-06-300001818382huma:PrivatePlacementWarrantsMember2024-12-310001818382huma:PrivatePlacementWarrantsMember2023-12-310001818382huma:PrivatePlacementWarrantsMember2025-07-012025-09-300001818382huma:PrivatePlacementWarrantsMember2024-07-012024-09-300001818382huma:PrivatePlacementWarrantsMember2025-01-012025-09-300001818382huma:PrivatePlacementWarrantsMember2024-01-012024-09-300001818382huma:PrivatePlacementWarrantsMember2025-09-300001818382huma:PrivatePlacementWarrantsMember2024-09-300001818382huma:ScientificAndManufacturingEquipmentMember2025-09-300001818382huma:ScientificAndManufacturingEquipmentMember2024-12-310001818382us-gaap:ComputerEquipmentMember2025-09-300001818382us-gaap:ComputerEquipmentMember2024-12-310001818382us-gaap:SoftwareDevelopmentMember2025-09-300001818382us-gaap:SoftwareDevelopmentMember2024-12-310001818382us-gaap:FurnitureAndFixturesMember2025-09-300001818382us-gaap:FurnitureAndFixturesMember2024-12-310001818382us-gaap:LeaseholdImprovementsMember2025-09-300001818382us-gaap:LeaseholdImprovementsMember2024-12-310001818382huma:RevenueInterestPurchaseAgreementMemberhuma:SubsequentInstallmentOneMember2024-03-112024-03-110001818382huma:RevenueInterestPurchaseAgreementMember2024-12-190001818382huma:RevenueInterestPurchaseAgreementMemberus-gaap:UnsecuredDebtMember2025-07-012025-09-300001818382huma:RevenueInterestPurchaseAgreementMemberhuma:PeriodTwoMember2023-05-120001818382huma:RevenueInterestPurchaseAgreementMemberhuma:PeriodThreeMember2023-05-120001818382huma:RevenueInterestPurchaseAgreementMember2024-08-142024-08-140001818382huma:RevenueInterestPurchaseAgreementMember2023-05-122023-05-120001818382us-gaap:StockOptionMemberus-gaap:FairValueMeasurementsRecurringMember2023-05-120001818382huma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMemberus-gaap:FairValueMeasurementsRecurringMember2023-05-120001818382huma:RevenueInterestPurchaseAgreementMemberhuma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2023-05-122023-05-120001818382huma:RevenueInterestPurchaseAgreementMember2025-07-012025-09-300001818382huma:RevenueInterestPurchaseAgreementMember2025-01-012025-09-300001818382huma:RevenueInterestPurchaseAgreementMember2024-07-012024-09-300001818382huma:RevenueInterestPurchaseAgreementMember2024-01-012024-09-300001818382huma:RevenueInterestPurchaseAgreementMemberhuma:EmbeddedDerivativeFinancialInstrumentsRevenueInterestPurchaseAgreementMember2024-03-112024-03-110001818382huma:RevenueInterestPurchaseAgreementMemberus-gaap:UnsecuredDebtMember2024-12-310001818382huma:RevenueInterestPurchaseAgreementMemberus-gaap:UnsecuredDebtMember2025-01-012025-03-310001818382huma:RevenueInterestPurchaseAgreementMemberus-gaap:UnsecuredDebtMember2025-03-310001818382huma:RevenueInterestPurchaseAgreementMemberus-gaap:UnsecuredDebtMember2025-04-012025-06-300001818382huma:RevenueInterestPurchaseAgreementMemberus-gaap:UnsecuredDebtMember2025-06-300001818382huma:TPCInvestmentsIIILPAndTPCInvestmentSolutionsLPMemberhuma:OptionPurchaseAgreementMember2024-02-292024-02-290001818382huma:TPCInvestmentsIIILPAndTPCInvestmentSolutionsLPMemberhuma:OptionPurchaseAgreementMember2024-12-310001818382huma:TPCInvestmentsIIILPAndTPCInvestmentSolutionsLPMemberhuma:OptionPurchaseAgreementMember2025-09-3000018183822025-09-2900018183822025-09-292025-09-290001818382huma:PublicStockOfferingMember2024-03-052024-03-0500018183822024-03-050001818382huma:PublicStockOfferingMember2025-03-272025-03-2700018183822025-03-270001818382us-gaap:OverAllotmentOptionMember2025-03-250001818382us-gaap:OverAllotmentOptionMember2025-03-252025-03-250001818382huma:EquityLineFinancingMember2024-09-242025-09-300001818382huma:EquityLineFinancingMember2025-07-012025-09-300001818382huma:October2024RegisteredDirectOfferingMember2024-10-042024-10-040001818382huma:October2024RegisteredDirectOfferingWarrantsMemberhuma:October2024RegisteredDirectOfferingMember2024-10-040001818382huma:October2024RegisteredDirectOfferingMember2024-10-040001818382huma:October2024RegisteredDirectOfferingMember2024-10-072024-10-070001818382huma:November2024RegisteredDirectOfferingMember2024-11-132024-11-130001818382huma:November2024RegisteredDirectOfferingWarrantsMemberhuma:November2024RegisteredDirectOfferingMember2024-11-130001818382huma:November2024RegisteredDirectOfferingMember2024-11-130001818382huma:November2024RegisteredDirectOfferingMember2024-11-152024-11-150001818382huma:October2025RegisteredDirectOfferingMemberus-gaap:SubsequentEventMember2025-10-062025-10-060001818382huma:October2025RegisteredDirectOfferingWarrantsMemberhuma:October2025RegisteredDirectOfferingMemberus-gaap:SubsequentEventMember2025-10-0600018183822025-06-290001818382huma:CommonStockReservedForContingentConsiderationSharesMember2025-09-300001818382huma:EquityLineFinancingMember2025-09-300001818382huma:CommonStockReservedForATMFacilityMember2025-09-300001818382huma:CommonStockReservedForOptionAgreementMember2025-09-300001818382us-gaap:EmployeeStockOptionMember2025-09-300001818382huma:OptionsAvailableForIssuanceUnderStockPlansMember2025-09-300001818382us-gaap:WarrantMember2025-09-300001818382huma:HumacyteIncMember2025-09-300001818382huma:HumacyteIncMember2024-12-310001818382huma:PrivatePlacementWarrantsMember2025-09-300001818382huma:PrivatePlacementWarrantsMember2024-12-310001818382huma:PublicWarrantsMember2025-09-300001818382huma:PublicWarrantsMember2024-12-310001818382huma:October2024RegisteredDirectOfferingWarrantsMember2025-09-300001818382huma:October2024RegisteredDirectOfferingWarrantsMember2024-12-310001818382huma:November2024RegisteredDirectOfferingWarrantsMember2025-09-300001818382huma:November2024RegisteredDirectOfferingWarrantsMember2024-12-310001818382huma:October2024RegisteredDirectOfferingWarrantsMember2025-04-050001818382huma:November2024RegisteredDirectOfferingWarrantsMember2025-05-140001818382us-gaap:WarrantMemberhuma:TermLoanAgreementMember2021-12-310001818382huma:PublicWarrantsMemberhuma:MergerAgreementMemberus-gaap:WarrantMemberhuma:AssumptionOfPubliclyTradedSecuritiesMember2021-08-262021-08-260001818382huma:PrivatePlacementWarrantsMemberhuma:MergerAgreementMemberus-gaap:WarrantMemberus-gaap:PrivatePlacementMember2021-08-262021-08-2600018183822021-08-260001818382huma:PublicWarrantsMember2021-08-260001818382huma:PublicWarrantsMemberus-gaap:MeasurementInputSharePriceMember2021-08-260001818382huma:PrivatePlacementWarrantsMember2021-08-260001818382huma:PrivatePlacementWarrantsMember2025-01-012025-09-300001818382huma:PrivatePlacementWarrantsMember2024-07-012024-09-300001818382huma:PrivatePlacementWarrantsMember2024-01-012024-09-300001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputSharePriceMember2025-09-300001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputSharePriceMember2024-12-310001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputExercisePriceMember2025-09-300001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputExercisePriceMember2024-12-310001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputExpectedTermMember2025-09-300001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputExpectedTermMember2024-12-310001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputPriceVolatilityMember2025-09-300001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputPriceVolatilityMember2024-12-310001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputRiskFreeInterestRateMember2025-09-300001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputRiskFreeInterestRateMember2024-12-310001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputExpectedDividendRateMember2025-09-300001818382huma:PrivatePlacementWarrantsMemberus-gaap:MeasurementInputExpectedDividendRateMember2024-12-310001818382huma:RegisteredDirectOfferingsMember2024-10-040001818382huma:RegisteredDirectWarrants180DayOctober2024Memberhuma:October2024RegisteredDirectOfferingMember2024-10-040001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberhuma:October2024RegisteredDirectOfferingMember2024-10-040001818382huma:October2024RegisteredDirectOfferingWarrantsMemberhuma:October2024RegisteredDirectOfferingMember2024-10-070001818382huma:October2024RegisteredDirectOfferingWarrantsMemberhuma:October2024RegisteredDirectOfferingMember2024-12-310001818382huma:October2024RegisteredDirectOfferingWarrantsMemberhuma:October2024RegisteredDirectOfferingMember2025-09-300001818382huma:October2024RegisteredDirectOfferingWarrantsMemberhuma:October2024RegisteredDirectOfferingMember2025-07-012025-09-300001818382huma:October2024RegisteredDirectOfferingWarrantsMemberhuma:October2024RegisteredDirectOfferingMember2025-01-012025-09-300001818382huma:RegisteredDirectWarrants180DayOctober2024Memberus-gaap:MeasurementInputSharePriceMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputSharePriceMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputSharePriceMember2024-12-310001818382huma:RegisteredDirectWarrants180DayOctober2024Memberus-gaap:MeasurementInputExercisePriceMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputExercisePriceMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputExercisePriceMember2024-12-310001818382huma:RegisteredDirectWarrants180DayOctober2024Memberus-gaap:MeasurementInputExpectedTermMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputExpectedTermMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputExpectedTermMember2024-12-310001818382huma:RegisteredDirectWarrants180DayOctober2024Memberus-gaap:MeasurementInputPriceVolatilityMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputPriceVolatilityMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputPriceVolatilityMember2024-12-310001818382huma:RegisteredDirectWarrants180DayOctober2024Memberus-gaap:MeasurementInputRiskFreeInterestRateMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputRiskFreeInterestRateMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputRiskFreeInterestRateMember2024-12-310001818382huma:RegisteredDirectWarrants180DayOctober2024Memberus-gaap:MeasurementInputExpectedDividendRateMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputExpectedDividendRateMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayOctober2024Memberus-gaap:MeasurementInputExpectedDividendRateMember2024-12-310001818382huma:RegisteredDirectWarrants180DayNovember2024Memberhuma:November2024RegisteredDirectOfferingMember2024-11-130001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberhuma:November2024RegisteredDirectOfferingMember2024-11-130001818382huma:November2024RegisteredDirectOfferingWarrantsMemberhuma:November2024RegisteredDirectOfferingMember2024-11-150001818382huma:November2024RegisteredDirectOfferingWarrantsMemberhuma:November2024RegisteredDirectOfferingMember2024-12-310001818382huma:November2024RegisteredDirectOfferingWarrantsMemberhuma:November2024RegisteredDirectOfferingMember2025-09-300001818382huma:November2024RegisteredDirectOfferingWarrantsMemberhuma:November2024RegisteredDirectOfferingMember2025-07-012025-09-300001818382huma:November2024RegisteredDirectOfferingWarrantsMemberhuma:November2024RegisteredDirectOfferingMember2025-01-012025-09-300001818382huma:RegisteredDirectWarrants180DayNovember2024Memberus-gaap:MeasurementInputSharePriceMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputSharePriceMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputSharePriceMember2024-12-310001818382huma:RegisteredDirectWarrants180DayNovember2024Memberus-gaap:MeasurementInputExercisePriceMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputExercisePriceMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputExercisePriceMember2024-12-310001818382huma:RegisteredDirectWarrants180DayNovember2024Memberus-gaap:MeasurementInputExpectedTermMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputExpectedTermMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputExpectedTermMember2024-12-310001818382huma:RegisteredDirectWarrants180DayNovember2024Memberus-gaap:MeasurementInputPriceVolatilityMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputPriceVolatilityMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputPriceVolatilityMember2024-12-310001818382huma:RegisteredDirectWarrants180DayNovember2024Memberus-gaap:MeasurementInputRiskFreeInterestRateMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputRiskFreeInterestRateMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputRiskFreeInterestRateMember2024-12-310001818382huma:RegisteredDirectWarrants180DayNovember2024Memberus-gaap:MeasurementInputExpectedDividendRateMember2024-12-310001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputExpectedDividendRateMember2025-09-300001818382huma:RegisteredDirectWarrants1640DayNovember2024Memberus-gaap:MeasurementInputExpectedDividendRateMember2024-12-310001818382huma:EarnoutSharesMember2021-08-260001818382huma:EarnoutSharesMemberhuma:ContingentConsiderationTrancheOneMember2021-08-260001818382huma:EarnoutSharesMemberhuma:ContingentConsiderationTrancheTwoMember2021-08-260001818382huma:EarnoutSharesMember2021-08-262021-08-260001818382huma:EarnoutSharesMemberus-gaap:MeasurementInputExpectedTermMember2021-08-260001818382huma:EarnoutSharesMember2025-09-300001818382huma:EarnoutSharesMember2024-12-310001818382huma:EarnoutSharesMember2025-07-012025-09-300001818382huma:EarnoutSharesMember2025-01-012025-09-300001818382huma:EarnoutSharesMember2024-07-012024-09-300001818382huma:EarnoutSharesMember2024-01-012024-09-300001818382us-gaap:MeasurementInputSharePriceMember2025-09-300001818382us-gaap:MeasurementInputSharePriceMember2024-12-310001818382us-gaap:MeasurementInputPriceVolatilityMember2025-09-300001818382us-gaap:MeasurementInputPriceVolatilityMember2024-12-310001818382us-gaap:MeasurementInputRiskFreeInterestRateMember2025-09-300001818382us-gaap:MeasurementInputRiskFreeInterestRateMember2024-12-310001818382us-gaap:MeasurementInputExpectedDividendRateMember2025-09-300001818382us-gaap:MeasurementInputExpectedDividendRateMember2024-12-310001818382us-gaap:MeasurementInputExpectedTermMember2025-09-300001818382us-gaap:MeasurementInputExpectedTermMember2024-12-310001818382us-gaap:EmployeeStockMember2025-01-012025-09-300001818382huma:LongTermIncentivePlan2021Member2025-01-012025-09-300001818382huma:LongTermIncentivePlan2021Member2024-01-012024-01-010001818382huma:LongTermIncentivePlan2021Member2025-01-012025-01-010001818382us-gaap:EmployeeStockMember2025-04-170001818382huma:LongTermIncentivePlan2021Member2025-09-3000018183822021-08-252021-08-250001818382huma:OmnibusIncentivePlan2015Member2025-09-300001818382huma:StockOptionPlan2005Member2025-09-300001818382us-gaap:EmployeeStockOptionMember2025-01-012025-09-300001818382srt:MinimumMemberus-gaap:EmployeeStockOptionMember2025-01-012025-09-300001818382srt:MaximumMemberus-gaap:EmployeeStockOptionMember2025-01-012025-09-300001818382us-gaap:EmployeeStockOptionMember2025-07-012025-09-300001818382us-gaap:EmployeeStockOptionMember2024-07-012024-09-300001818382us-gaap:EmployeeStockOptionMember2024-01-012024-09-300001818382us-gaap:ResearchAndDevelopmentExpenseMember2025-07-012025-09-300001818382us-gaap:ResearchAndDevelopmentExpenseMember2024-07-012024-09-300001818382us-gaap:ResearchAndDevelopmentExpenseMember2025-01-012025-09-300001818382us-gaap:ResearchAndDevelopmentExpenseMember2024-01-012024-09-300001818382us-gaap:SellingGeneralAndAdministrativeExpensesMember2025-07-012025-09-300001818382us-gaap:SellingGeneralAndAdministrativeExpensesMember2024-07-012024-09-300001818382us-gaap:SellingGeneralAndAdministrativeExpensesMember2025-01-012025-09-300001818382us-gaap:SellingGeneralAndAdministrativeExpensesMember2024-01-012024-09-3000018183822024-01-012024-12-310001818382huma:DecellularizedTissueEngineeringPatentRightsMemberhuma:LicenseAgreementMemberhuma:DukeUniversityMember2025-01-012025-09-300001818382huma:DecellularizedTissueEngineeringPatentRightsMemberhuma:LicenseAgreementMemberhuma:DukeUniversityMember2025-09-300001818382huma:DecellularizedTissueEngineeringPatentRightsMemberhuma:LicenseAgreementMemberhuma:DukeUniversityMember2024-01-012024-03-310001818382huma:DecellularizedTissueEngineeringPatentRightsMemberhuma:LicenseAgreementMemberhuma:DukeUniversityMember2024-12-310001818382huma:BVPPatentRightsMemberhuma:LicenseAgreementMemberhuma:YaleUniversityMember2019-08-012019-08-310001818382huma:TubularProsthesesPatentRightsMemberhuma:LicenseAgreementMemberhuma:YaleUniversityMember2019-08-012019-08-310001818382huma:YaleUniversityMemberhuma:LicenseAgreementMember2025-01-012025-09-300001818382huma:LicenseAgreementMemberhuma:YaleUniversityMemberhuma:RegulatoryMilestoneMember2025-01-012025-09-300001818382huma:LicenseAgreementMemberhuma:YaleUniversityMemberhuma:CommercialMilestoneMember2025-01-012025-09-300001818382huma:YaleUniversityMemberhuma:LicenseAgreementMember2025-09-300001818382huma:JDRFAgreementMember2023-04-010001818382huma:JDRFAgreementMemberhuma:FirstMilestonePaymentMember2023-04-012023-04-300001818382huma:JDRFAgreementMemberhuma:SecondMilestonePaymentMember2024-05-012024-05-300001818382huma:JDRFAgreementMemberhuma:ThirdMilestonePaymentMember2024-05-012024-05-300001818382huma:JDRFAgreementMember2025-09-302025-09-300001818382huma:JDRFAgreementMember2025-09-300001818382huma:JDRFAgreementMember2024-12-310001818382us-gaap:ResearchAndDevelopmentExpenseMember2025-04-012025-06-300001818382us-gaap:SellingGeneralAndAdministrativeExpensesMember2025-04-012025-06-300001818382us-gaap:PendingLitigationMemberhuma:ConsolidatedDerivativeActionMember2025-01-100001818382huma:FreseniusMedicalCareMemberus-gaap:RelatedPartyMember2018-06-012018-06-300001818382huma:FreseniusMedicalCareMemberus-gaap:RelatedPartyMember2021-08-262021-08-260001818382huma:FreseniusMedicalCareMemberus-gaap:RelatedPartyMember2021-08-012021-08-310001818382huma:FreseniusMedicalCareMemberus-gaap:RelatedPartyMember2021-08-310001818382huma:FreseniusMedicalCareMemberus-gaap:RelatedPartyMember2021-02-162021-02-160001818382huma:FreseniusMedicalCareMemberus-gaap:RelatedPartyMember2025-09-300001818382huma:FrenovaRenalResearchMemberus-gaap:RelatedPartyMember2025-01-012025-09-300001818382huma:FrenovaRenalResearchMemberus-gaap:RelatedPartyMember2025-07-012025-09-300001818382huma:FrenovaRenalResearchMemberus-gaap:RelatedPartyMember2025-09-300001818382huma:FreseniusMedicalCareDeutschlandGmbHMemberus-gaap:RelatedPartyMember2025-07-012025-09-300001818382huma:FreseniusMedicalCareDeutschlandGmbHMemberus-gaap:RelatedPartyMember2025-01-012025-09-300001818382huma:FreseniusMedicalCareDeutschlandGmbHMemberus-gaap:RelatedPartyMember2025-09-300001818382huma:FreseniusMedicalCareDeutschlandGmbHMemberus-gaap:RelatedPartyMember2024-12-310001818382huma:RegisteredDirectWarrants180DayOctober2025Memberhuma:October2025RegisteredDirectOfferingMemberus-gaap:SubsequentEventMember2025-10-060001818382huma:October2025RegisteredDirectOfferingMemberus-gaap:SubsequentEventMember2025-10-06
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
___________________________________________________________________________________________________________________________
FORM 10-Q
(Mark One)
x QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended September 30, 2025
OR
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ______ to ______.
Commission File Number: 001-39532
___________________________________________________________________________________________________
Humacyte, Inc.
(Exact name of registrant as specified in its charter)
___________________________________________________________________________________________________
|
|
|
|
|
|
|
|
|
| Delaware |
85-1763759 |
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer Identification No.) |
|
|
|
2525 East North Carolina Highway 54 |
|
| Durham, |
NC |
27713 |
| (Address of principal executive offices) |
(Zip code) |
(919) 313-9633
(Registrant’s telephone number, including area code)
Not Applicable
(Former name, former address and former fiscal year, if changed since last report)
___________________________________________________________________________________________________
Securities registered pursuant to 12(b) of the Act:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share |
|
HUMA |
|
The Nasdaq Stock Market LLC |
| Redeemable Warrants, each whole warrant exercisable for one share of Common Stock at an exercise price of $11.50 |
|
HUMAW |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Large accelerated filer |
o |
|
Accelerated filer |
o |
| Non-accelerated filer |
x |
|
Smaller reporting company |
x |
|
|
|
Emerging growth company |
x |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
As of November 4, 2025, 187,271,321 shares of common stock, par value $0.0001, were issued and outstanding.
Humacyte, Inc.
Quarterly Report on Form 10-Q
Table of Contents
FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q (“Quarterly Report”) contains forward-looking statements that involve substantial risks and uncertainties. “Forward-looking statements,” as that term is defined in the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”) are statements that are not historical facts and involve a number of risks and uncertainties. These statements include, without limitation, statements regarding the financial position, business strategy and the plans and objectives of management for future operations. These statements constitute projections, forecasts and forward-looking statements, and are not guarantees of performance. Such statements can be identified by the fact that they do not relate strictly to historical or current facts. When used therein, words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “strive,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. Such statements are based on the beliefs of, as well as assumptions made by and information currently available to, our management.
Forward-looking statements may include, for example, statements about:
•our plans and ability to commercialize SymvessTM (acellular tissue engineered vessel-tyod or “ATEVTM”) and, if approved by regulatory authorities, our product candidates, successfully and on our anticipated timelines;
•the degree of market acceptance of and the availability of third-party coverage and reimbursement for Symvess and, if approved by regulatory authorities, our product candidates;
•our ability to manufacture Symvess and, if approved by regulatory authorities, our product candidates, in sufficient quantities to satisfy our clinical trial and commercial needs;
•the expected size of the target populations for Symvess and, if approved by regulatory authorities, our product candidates;
•the anticipated benefits of our ATEVs relative to existing alternatives;
•our assessment of the competitive landscape;
•our plans and ability to execute product development, process development and preclinical development efforts successfully and on our anticipated timelines;
•our plans, anticipated timeline and ability to file applications for, and obtain marketing approvals from, the United States (“U.S.”) Food and Drug Administration (“FDA”) and other regulatory authorities, including the European Medicines Agency (“EMA”), for our ATEVs and product candidates;
•our ability to design, initiate and successfully complete clinical trials and other studies for our product candidates and our plans and expectations regarding our ongoing or planned clinical trials, including for our V007 and V012 Phase 3 clinical trials;
•our ability to execute and achieve the expected benefits of our cost-saving measures and whether our efforts will result in further actions or additional asset impairment charges that adversely affect our business;
•the outcome of our ongoing discussions with the FDA concerning the design of our clinical trials;
•our anticipated growth rate and market opportunities;
•our ability to use our proprietary scientific technology platform to build a pipeline of additional product candidates;
•the characteristics and performance of our ATEVs and the public perception thereof;
•our expectations regarding our strategic partnership with Fresenius Medical Care Holdings, Inc. (“Fresenius Medical Care”) to sell, market and distribute our 6 millimeter ATEV for certain specified indications and in specified markets, if approved by regulatory authorities;
•the performance of other third parties on which we rely, including our third-party manufacturers, our licensors, our suppliers and the organizations conducting our clinical trials;
•our ability to obtain and maintain intellectual property protection for our product candidates as well as our ability to operate our business without infringing, misappropriating or otherwise violating the intellectual property rights of others;
•our ability to maintain the confidentiality of our trade secrets, particularly with respect to our manufacturing process;
•our compliance with applicable laws and regulatory requirements, including FDA regulations, healthcare laws and regulations, and anti-corruption laws;
•our involvement in existing or potential claims and legal and administrative proceedings, and the merits, potential outcomes and effects of both existing and potential claims and legal and administrative proceedings, as well as regulatory determinations, on our business, prospects, financial condition and results of operations;
•our ability to attract, retain and motivate qualified personnel and to manage our growth effectively;
•our estimates regarding how long our existing cash and cash equivalents will be sufficient to fund our anticipated operating expenses, capital expenditures and debt service obligations;
•our future financial performance and capital requirements, including our ability to raise additional capital in the future;
•our ability to implement and maintain effective internal controls;
•the potential liquidity and trading of our securities; and
•the impact of the overall global economy and increasing interest rates and inflation on our business.
We caution readers not to place undue reliance on any such forward-looking statements, which speak only as of the date they are made. Any forward-looking statements are based on information current as of the date of this Quarterly Report and speak only as of the date on which such statements are made. Actual events or results may differ materially from the results, plans, intentions or expectations anticipated by these forward-looking statements as a result of a variety of factors, many of which are beyond our control. More information on factors that could cause actual results to differ materially from those anticipated is included from time to time in our reports filed with the Securities and Exchange Commission (the “SEC”), including, but not limited to, those described in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in this Quarterly Report and our Annual Report on Form 10-K for the year ended December 31, 2024, which we filed with the SEC on March 31, 2025. We disclaim any obligation, except as specifically required by law, to publicly update or revise any such statements to reflect any change in our expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.
PART I – FINANCIAL INFORMATION
Item 1. Financial Statements
Humacyte, Inc.
Condensed Consolidated Balance Sheets
(unaudited)
(in thousands except for share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30,
2025 |
|
December 31,
2024 |
| ASSETS |
|
|
|
| Current assets |
|
|
|
| Cash and cash equivalents |
$ |
19,488 |
|
|
$ |
44,937 |
|
| Inventory |
18,418 |
|
|
— |
|
| Accounts receivable |
1,078 |
|
|
180 |
|
| Prepaid expenses and other current assets |
2,367 |
|
|
2,742 |
|
|
|
|
|
|
|
|
|
| Total current assets |
41,351 |
|
|
47,859 |
|
|
|
|
|
| Restricted cash |
209 |
|
|
50,209 |
|
| Property and equipment, net |
19,857 |
|
|
23,063 |
|
| Finance lease right-of-use assets, net |
29,420 |
|
|
15,490 |
|
| Other long-term assets |
672 |
|
|
1,251 |
|
| Total assets |
$ |
91,509 |
|
|
$ |
137,872 |
|
|
|
|
|
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) |
|
|
|
| Current liabilities |
|
|
|
| Accounts payable |
$ |
9,575 |
|
|
$ |
4,490 |
|
| Accrued expenses |
10,264 |
|
|
11,424 |
|
| Finance lease obligation, current portion |
2,670 |
|
|
2,917 |
|
|
|
|
|
| Revenue interest liability, current portion |
3,072 |
|
|
885 |
|
| Other current liabilities |
— |
|
|
238 |
|
|
|
|
|
| Total current liabilities |
25,581 |
|
|
19,954 |
|
|
|
|
|
| Revenue interest liability, net of current portion |
17,674 |
|
|
63,354 |
|
| Contingent Earnout Liability |
21,807 |
|
|
70,961 |
|
| Finance lease obligation, net of current portion |
27,155 |
|
|
13,620 |
|
| Common stock warrant liabilities |
3,234 |
|
|
19,254 |
|
| Contingent derivative liability |
289 |
|
|
2,415 |
|
| Other long-term liabilities |
520 |
|
|
983 |
|
|
|
|
|
|
|
|
|
| Total liabilities |
96,260 |
|
|
190,541 |
|
|
|
|
|
| Commitments and contingencies (Note 12) |
|
|
|
|
|
|
|
| Stockholders’ equity (deficit) |
|
|
|
Preferred stock, $0.0001 par value; 20,000,000 shares designated as of September 30, 2025 and December 31, 2024; 0 shares issued and outstanding as of September 30, 2025 and December 31, 2024 |
— |
|
|
— |
|
Common stock, $0.0001 par value; 350,000,000 and 250,000,000 shares authorized as of September 30, 2025 and December 31, 2024, respectively; 158,835,303 and 130,027,509 shares issued and outstanding as of September 30, 2025 and December 31, 2024, respectively |
16 |
|
|
13 |
|
| Additional paid-in capital |
697,277 |
|
|
633,333 |
|
| Accumulated deficit |
(702,044) |
|
|
(686,015) |
|
| Total stockholders’ equity (deficit) |
(4,751) |
|
|
(52,669) |
|
| Total liabilities and stockholders’ equity (deficit) |
$ |
91,509 |
|
|
$ |
137,872 |
|
The accompanying notes are an integral part of these financial statements.
Humacyte, Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(unaudited)
(in thousands except for share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the
Three Months Ended September 30,
|
|
For the
Nine Months Ended September 30,
|
|
|
|
|
|
2025 |
|
2024 |
|
2025 |
|
2024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Revenue: |
|
|
|
|
|
|
|
|
|
|
|
| Product revenue, net |
$ |
703 |
|
|
$ |
— |
|
|
$ |
950 |
|
|
$ |
— |
|
|
|
|
|
Contract revenue |
50 |
|
|
— |
|
|
621 |
|
|
— |
|
|
|
|
|
| Total revenue |
753 |
|
|
— |
|
|
1,571 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
| Cost of goods sold |
260 |
|
|
— |
|
|
620 |
|
|
— |
|
|
|
|
|
| Research and development |
17,273 |
|
|
22,926 |
|
|
54,697 |
|
|
67,943 |
|
|
|
|
|
| Selling, general and administrative |
7,610 |
|
|
7,307 |
|
|
23,555 |
|
|
18,367 |
|
|
|
|
|
| Total operating expenses |
25,143 |
|
|
30,233 |
|
|
78,872 |
|
|
86,310 |
|
|
|
|
|
| Loss from operations |
(24,390) |
|
|
(30,233) |
|
|
(77,301) |
|
|
(86,310) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other income (expense), net: |
|
|
|
|
|
|
|
|
|
|
|
| Interest income |
590 |
|
|
911 |
|
|
2,084 |
|
|
3,252 |
|
|
|
|
|
| Change in fair value of Contingent Earnout Liability |
4,893 |
|
|
(8,489) |
|
|
49,154 |
|
|
(38,653) |
|
|
|
|
|
| Change in fair value of derivatives |
4,009 |
|
|
1,047 |
|
|
18,191 |
|
|
719 |
|
|
|
|
|
| Interest expense |
(2,612) |
|
|
(2,438) |
|
|
(8,157) |
|
|
(6,769) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total other income (expense), net |
6,880 |
|
|
(8,969) |
|
|
61,272 |
|
|
(41,451) |
|
|
|
|
|
| Net loss and comprehensive loss |
$ |
(17,510) |
|
|
$ |
(39,202) |
|
|
$ |
(16,029) |
|
|
$ |
(127,761) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss per share attributable to common stockholders, basic and diluted |
$ |
(0.11) |
|
|
$ |
(0.33) |
|
|
$ |
(0.11) |
|
|
$ |
(1.10) |
|
|
|
|
|
| Weighted-average shares outstanding used in computing net loss per share attributable to common stockholders, basic and diluted |
158,313,290 |
|
|
119,408,565 |
|
|
148,514,044 |
|
|
115,623,616 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these financial statements.
Humacyte, Inc.
Condensed Consolidated Statements of Changes in Stockholders’ Equity (Deficit)
(unaudited)
(in thousands except for share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
Additional
Paid-in Capital |
|
Accumulated
Deficit |
|
Total Stockholders’
Equity (Deficit)
|
|
Shares |
|
Amount |
|
|
|
Balance as of December 31, 2024 |
130,027,509 |
|
|
$ |
13 |
|
|
$ |
633,333 |
|
|
$ |
(686,015) |
|
|
$ |
(52,669) |
|
| Issuance of stock in public offering, net of issuance costs |
25,000,000 |
|
|
3 |
|
|
46,657 |
|
|
— |
|
|
46,660 |
|
| Issuance of stock under ATM Facility, net of issuance costs |
75,793 |
|
|
— |
|
|
370 |
|
|
— |
|
|
370 |
|
| Proceeds from the exercise of stock options |
15,514 |
|
|
— |
|
|
56 |
|
|
— |
|
|
56 |
|
| Stock-based compensation |
— |
|
|
— |
|
|
2,487 |
|
|
— |
|
|
2,487 |
|
Net income |
— |
|
|
— |
|
|
— |
|
|
39,139 |
|
|
39,139 |
|
| Balance as of March 31, 2025 |
155,118,816 |
|
|
$ |
16 |
|
|
$ |
682,903 |
|
|
$ |
(646,876) |
|
|
$ |
36,043 |
|
| Issuance of stock under ATM Facility, net of issuance costs |
1,224,077 |
|
|
— |
|
|
3,233 |
|
|
— |
|
|
3,233 |
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation |
— |
|
|
— |
|
|
2,434 |
|
|
|
|
2,434 |
|
Net loss |
— |
|
|
— |
|
|
— |
|
|
(37,658) |
|
|
(37,658) |
|
| Balance as of June 30, 2025 |
156,342,893 |
|
|
$ |
16 |
|
|
$ |
688,570 |
|
|
$ |
(684,534) |
|
|
$ |
4,052 |
|
| Issuance of stock under ATM Facility, net of issuance costs |
2,492,410 |
|
|
|
|
6,197 |
|
|
— |
|
|
6,197 |
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation |
— |
|
|
— |
|
|
2,510 |
|
|
— |
|
|
2,510 |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
(17,510) |
|
|
(17,510) |
|
Balance as of September 30, 2025 |
158,835,303 |
|
|
$ |
16 |
|
|
$ |
697,277 |
|
|
$ |
(702,044) |
|
|
$ |
(4,751) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
Additional
Paid-in Capital |
|
Accumulated
Deficit |
|
Total Stockholders’
Equity |
|
Shares |
|
Amount |
|
|
|
Balance as of December 31, 2023 |
103,673,728 |
|
|
$ |
10 |
|
|
$ |
550,850 |
|
|
$ |
(537,314) |
|
|
$ |
13,546 |
|
| Issuance of stock in public offering, net of issuance costs |
15,410,000 |
|
|
2 |
|
|
43,044 |
|
|
— |
|
|
43,046 |
|
| Proceeds from the exercise of stock options |
625 |
|
|
— |
|
|
2 |
|
|
— |
|
|
2 |
|
| Stock-based compensation |
— |
|
|
— |
|
|
1,454 |
|
|
— |
|
|
1,454 |
|
Net loss |
|
|
|
|
|
|
(31,896) |
|
|
(31,896) |
|
| Balance as of March 31, 2024 |
119,084,353 |
|
|
$ |
12 |
|
|
$ |
595,350 |
|
|
$ |
(569,210) |
|
|
$ |
26,152 |
|
| Proceeds from the exercise of stock options |
263,335 |
|
|
— |
|
|
787 |
|
|
— |
|
|
787 |
|
| Stock-based compensation |
— |
|
|
— |
|
|
1,437 |
|
|
— |
|
|
1,437 |
|
Net loss |
— |
|
|
— |
|
|
— |
|
|
(56,663) |
|
|
(56,663) |
|
| Balance as of June 30, 2024 |
119,347,688 |
|
|
$ |
12 |
|
|
$ |
597,574 |
|
|
$ |
(625,873) |
|
|
$ |
(28,287) |
|
| Issuance of commitment shares pursuant to Common Stock Purchase Agreement |
115,705 |
|
|
— |
|
|
708 |
|
|
— |
|
|
708 |
|
| Proceeds from sale of stock under Common Stock Purchase Agreement |
200,000 |
|
|
— |
|
|
1,013 |
|
|
— |
|
|
1,013 |
|
| Proceeds from the exercise of stock options |
179,547 |
|
|
|
|
495 |
|
|
— |
|
|
495 |
|
| Stock-based compensation |
|
|
|
|
1,552 |
|
|
— |
|
|
1,552 |
|
Net loss |
|
|
|
|
|
|
(39,202) |
|
|
(39,202) |
|
| Balance as of September 30, 2024 |
119,842,940 |
|
|
$ |
12 |
|
|
$ |
601,342 |
|
|
$ |
(665,075) |
|
|
$ |
(63,721) |
|
The accompanying notes are an integral part of these financial statements.
Humacyte, Inc.
Condensed Consolidated Statements of Cash Flows
(unaudited)
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Nine Months Ended September 30, |
|
2025 |
|
2024 |
| Cash flows from operating activities |
|
|
|
| Net loss |
$ |
(16,029) |
|
|
$ |
(127,761) |
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
| Depreciation expense |
3,987 |
|
|
3,819 |
|
| Stock-based compensation expense |
7,283 |
|
|
4,443 |
|
| Change in fair value of Contingent Earnout Liability |
(49,154) |
|
|
38,653 |
|
| Non-cash interest expense |
6,655 |
|
|
5,606 |
|
| Change in fair value of derivatives |
(18,191) |
|
|
(719) |
|
|
|
|
|
|
|
|
|
| Amortization expense |
1,570 |
|
|
1,563 |
|
| Non-cash operating lease costs |
44 |
|
|
39 |
|
|
|
|
|
| Changes in operating assets and liabilities: |
|
|
|
| Accounts receivable |
(898) |
|
|
— |
|
| Inventory |
(18,270) |
|
|
— |
|
| Prepaid expenses and other current assets |
441 |
|
|
396 |
|
| Accounts payable |
5,134 |
|
|
475 |
|
| Accrued expenses |
(1,446) |
|
|
1,980 |
|
| Operating lease obligation |
(44) |
|
|
(39) |
|
|
|
|
|
| Net cash used in operating activities |
(78,918) |
|
|
(71,545) |
|
|
|
|
|
| Cash flows from investing activities |
|
|
|
| Purchase of property and equipment |
(845) |
|
|
(1,509) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash used in investing activities |
(845) |
|
|
(1,509) |
|
|
|
|
|
| Cash flows from financing activities |
|
|
|
| Proceeds from issuance of stock in public offering, net of underwriting fees |
47,000 |
|
|
43,396 |
|
| Payments of costs related to public offering |
(340) |
|
|
(350) |
|
| Proceeds from issuance of stock under ATM Facility, net of issuance costs |
9,800 |
|
|
— |
|
| Proceeds from sale of stock under Common Stock Purchase Agreement |
— |
|
|
1,013 |
|
| Proceeds from the exercise of stock options |
56 |
|
|
1,284 |
|
| Proceeds from JDRF Agreement |
— |
|
|
240 |
|
| Payments of finance lease principal |
(2,157) |
|
|
(1,906) |
|
|
|
|
|
| Payments on revenue interest liability |
(45) |
|
|
— |
|
| Partial call payment under amended Revenue Interest Purchase Agreement |
(50,000) |
|
|
— |
|
| Proceeds from Revenue Interest Purchase Agreement |
— |
|
|
20,000 |
|
| Payments of transaction costs related to Revenue Interest Purchase Agreement |
— |
|
|
(500) |
|
| Net cash provided by financing activities |
4,314 |
|
|
63,177 |
|
|
|
|
|
| Net decrease in cash, cash equivalents and restricted cash |
(75,449) |
|
|
(9,877) |
|
| Cash, cash equivalents and restricted cash at the beginning of the period |
95,290 |
|
|
80,801 |
|
| Cash, cash equivalents and restricted cash at the end of the period |
$ |
19,841 |
|
|
$ |
70,924 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Supplemental disclosure of noncash activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Increase in right-of-use assets and lease liabilities |
$ |
14,965 |
|
|
$ |
— |
|
| Debt discount from embedded contingent derivative liability |
$ |
— |
|
|
$ |
1,552 |
|
| Issuance of commitment shares pursuant to Common Stock Purchase Agreement |
$ |
— |
|
|
$ |
708 |
|
|
|
|
|
The accompanying notes are an integral part of these financial statements.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
1. Organization and Description of Business
Organization
Humacyte, Inc. and subsidiaries (unless the context indicates otherwise, collectively, the “Company”) is pioneering the development and manufacture of off-the-shelf, universally implantable, bioengineered human tissues, advanced tissue constructs and organ systems with the goal of improving the lives of patients and transforming the practice of medicine. The Company is leveraging its regenerative medicine technology platform to develop proprietary product candidates for use in the treatment of diseases and conditions across a range of anatomic locations in multiple therapeutic areas.
Liquidity
Since its inception in 2004, the Company has incurred operating losses and negative cash flows from operations in each year. To date, the Company has financed its operations primarily through the sale of equity securities and convertible debt, proceeds from the reverse recapitalization described below, borrowings under loan facilities, proceeds from a revenue interest purchase agreement and, to a lesser extent, through governmental and other grants. At September 30, 2025 and December 31, 2024, the Company had an accumulated deficit of $702.0 million and $686.0 million, respectively. The Company’s operating losses were $77.3 million and $86.3 million for the nine months ended September 30, 2025 and 2024, respectively. Net cash flows used in operating activities were $78.9 million and $71.5 million during the nine months ended September 30, 2025 and 2024, respectively. Substantially all of the Company’s operating losses resulted from costs incurred in connection with the Company’s research and development programs and from general and administrative costs associated with the Company’s commercial launch and other operations. The Company expects to incur substantial operating losses and negative cash flows from operations for the foreseeable future as the Company advances its product candidates and commercial operations.
As further disclosed in Note 7, on May 12, 2023, Humacyte, Inc. and Global (defined in Note 2 — Summary of Significant Accounting Policies) entered into a Revenue Interest Purchase Agreement (the “Purchase Agreement”) with two purchasers, both affiliates of Oberland Capital Management LLC (the “Purchasers”), and another affiliate of Oberland Capital Management LLC (“Oberland”), as agent for the Purchasers (the “Agent”), to obtain financing with respect to the further development and commercialization of the Company’s ATEV, to repay the Company’s then-existing credit facility with Silicon Valley Bank (“SVB”), and for other general corporate purposes. As of September 30, 2025, $20.7 million was recorded as a revenue interest liability on the condensed consolidated balance sheets.
The Purchase Agreement contains customary representations and warranties and affirmative covenants for transactions of this type, including, among others, the provision of financial and other information to the Purchaser, notice to the Purchaser upon the occurrence of certain material events, and compliance with applicable laws. The Purchase Agreement also contains customary negative covenants, including certain restrictions on the ability to incur indebtedness and grant liens or security interests on assets. On February 18, 2024, the Company reached an agreement with the Purchasers and the Agent to waive certain breaches related to, and extend the deadline for, certain post-closing obligations under the Purchase Agreement, including the requirement for the Company to deliver a leasehold mortgage in favor of the Agent over the Company’s headquarters. On May 8, 2024, the Company agreed with the Purchasers to amend the Purchase Agreement to remove requirements related to the leasehold mortgage. In exchange for removing this requirement, the Company agreed to fund an account in the amount of $54.0 million, over which the Agent has certain consent and other rights to $50.0 million of the funds. The Company funded an account with the required $54.0 million on August 14, 2024.
On September 17, 2025, Humacyte, Inc. and Global entered into a second amendment to the Purchase Agreement (the “Purchase Agreement Amendment”) with the Purchasers and the Agent to amend the Purchase Agreement. In connection with the Purchase Agreement Amendment, the Company made a $50.0 million repayment under the Purchase Agreement (the “Purchase Agreement Amendment Payment”), funded from the restricted cash previously maintained for the benefit of the Agent. As a result of the Purchase Agreement Amendment Payment, the Company is no longer obligated to maintain $50.0 million of restricted cash in an account for the benefit of the Agent unless the Purchase Agreement has not been repaid in full by December 31, 2025, at which point the Company will be required to maintain $12.5 million in such account. As of September 30, 2025, no restricted cash related to the Purchase Agreement remains on the accompanying condensed consolidated balance sheets.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
As further disclosed in Note 9, on September 24, 2024, the Company entered into a common stock purchase agreement with Lincoln Park Capital Fund, LLC (“Lincoln Park”) for an equity line financing (the “Common Stock Purchase Agreement”). The Common Stock Purchase Agreement provides that, subject to the terms and conditions set forth therein, the Company has the sole right, but not the obligation, to sell to Lincoln Park shares of the Company’s common stock, par value $0.0001 per share (“Common Stock”), having an aggregate value of up to $50.0 million (the “Purchase Shares”) over a 24-month period. The Company controls the timing and amount of any sales of Purchase Shares to Lincoln Park pursuant to the Common Stock Purchase Agreement in its sole discretion. As of September 30, 2025, the Company had $47.5 million in remaining availability for sales of Common Stock under the Common Stock Purchase Agreement.
As further disclosed in Note 9, on September 1, 2022, the Company entered into an agreement with Jefferies LLC for the sale from time to time of up to $80.0 million of shares of Common Stock pursuant to a sales agreement (the “ATM Facility”). As of September 30, 2025, $62.7 million remained available under the ATM Facility.
As of September 30, 2025, the Company had available cash and cash equivalents of $19.5 million. As further disclosed in Note 12, on April 28, 2025, the Company implemented a cost reduction action to reduce its workforce by 30 employees, cease recruitment of additional planned new hires, and reduce other operating expenses. The Company undertook these cost reductions to improve its cash runway and to better align the Company’s organizational structure with its top business objectives. The Company believes its cash and cash equivalents on hand and existing capacity under its Common Stock Purchase Agreement will be sufficient to fund operations for at least twelve months from the issuance date of these interim financial statements. The future viability of the Company beyond that point is dependent on its ability to generate cash flows from the sale of Symvess and raise additional capital to finance its operations. The Company plans to seek additional funding through private or public equity financings, debt financings, debt refinancings or restructurings, collaborations, strategic alliances, and marketing, distribution or licensing arrangements. Adequate capital may not be available to the Company when needed or on acceptable terms. If the Company is unable to raise capital, it could be forced to delay, reduce, suspend or cease certain of its planned research and development programs or any future commercialization efforts, which would have a negative impact on its business, prospects, operating results and financial condition.
2. Summary of Significant Accounting Policies
Basis of Presentation
The Company has prepared the accompanying financial statements in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The Company’s condensed consolidated financial statements reflect the operations of the Company and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
Reverse Recapitalization
On August 26, 2021 (the “Closing Date”), Hunter Merger Sub, Inc. (“Merger Sub”), a wholly owned subsidiary of Alpha Healthcare Acquisition Corp. (“AHAC”) merged with Humacyte, Inc. (“Legacy Humacyte”), with Legacy Humacyte continuing as the surviving corporation and as a wholly-owned subsidiary of AHAC (the “Merger”). The Merger was accounted for as a reverse recapitalization in accordance with U.S. GAAP. As a result of the Merger, AHAC changed its name to Humacyte, Inc. and Legacy Humacyte changed its name to Humacyte Global, Inc. (“Global”).
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates in the financial statements include stock-based compensation costs, right-of-use assets, accruals for research and development activities, contingent earnout liability, revenue interest liability, derivatives, fair value of common stock warrants and income taxes. The Company evaluates its estimates and assumptions on an ongoing basis using historical experience and other factors and adjusts those estimates and assumptions when facts and circumstances dictate. Actual results could differ from those estimates.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Unaudited Interim Condensed Consolidated Financial Statements
The accompanying interim condensed consolidated financial statements and the related footnote disclosures are unaudited. These unaudited interim financial statements have been prepared on the same basis as the audited financial statements and, in management’s opinion, include all adjustments, consisting of only normal recurring adjustments, necessary for the fair statement of the Company’s financial position as of September 30, 2025 and its results of operations for the three and nine months ended September 30, 2025 and 2024, and cash flows for the nine months ended September 30, 2025 and 2024. The results of operations for the three and nine months ended September 30, 2025 are not necessarily indicative of the results to be expected for the year ending December 31, 2025 or any other period. The December 31, 2024 year-end condensed consolidated balance sheet was derived from audited annual financial statements but does not include all disclosures from the annual financial statements.
Certain information and footnote disclosures normally included in consolidated financial statements prepared in accordance with U.S. GAAP have been condensed or omitted pursuant to such rules and regulations. Accordingly, these condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements for the year ended December 31, 2024 and the related notes included in the Company’s Annual Report on Form 10-K, filed with the SEC on March 31, 2025 (the “Annual Report”), which provides a more complete discussion of the Company’s accounting policies and certain other information. There have been no significant changes to the significant accounting policies disclosed in Note 2 of the audited consolidated financial statements as of and for the years ended December 31, 2024 and 2023 included in the Company’s Annual Report.
Reclassifications
Certain amounts from prior periods have been reclassified to conform to the current period’s presentation. None of these reclassifications had a material impact on the Company’s condensed consolidated financial statements.
Segments
The Company is developing proprietary, bioengineered, acellular human tissues, advanced tissue constructs and organ systems that are designed to be used in the treatment of diseases and conditions across a range of anatomic locations in multiple therapeutic areas. The Company’s operations are managed and reported to its Chief Executive Officer, the Company’s chief operating decision maker (“CODM”), on a consolidated basis. The CODM evaluates financial performance, allocates resources and monitors budget versus actual results based on the Company’s condensed consolidated statements of operations. The measure of segment assets provided to and reviewed by the CODM is reported on the condensed consolidated balance sheets as total assets. Segment asset information is not used by the CODM to evaluate performance, allocate resources or make strategic decisions. Under the current organizational and reporting structure, the Company operates and manages its business on a consolidated basis as one reportable and operating segment.
As a single reportable segment entity, the Company’s segment performance measure is consolidated net loss. Consolidated net loss is used to monitor the budget versus actual results and to help make key operating decisions such as the allocation of budget between research and development and selling, general and administrative expenses. Significant segment expenses within net income (loss) include cost of goods sold, research and development and selling, general and administrative expenses, which are each separately presented on the Company’s condensed consolidated statements of operations. Other segment items within net income (loss) include interest income, interest expense, the change in fair value of the Company’s Contingent Earnout Liability and the change in fair value of derivatives.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Additional disaggregated significant segment expenses that are not separately presented on the Company’s condensed consolidated statements of operations are presented below.
Research and Development Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| ($ in thousands) |
2025 |
|
2024 |
|
2025 |
|
2024 |
| Direct Expenses |
|
|
|
|
|
|
|
| Vascular Trauma |
$ |
278 |
|
|
$ |
439 |
|
|
$ |
690 |
|
|
$ |
1,904 |
|
| AV Access |
1,612 |
|
|
1,927 |
|
|
4,356 |
|
|
4,952 |
|
| PAD |
— |
|
|
22 |
|
|
— |
|
|
161 |
|
| Total |
1,890 |
|
|
2,388 |
|
|
5,046 |
|
|
7,017 |
|
| Unallocated Expenses |
|
|
|
|
|
|
|
| External services |
1,569 |
|
|
1,915 |
|
|
4,466 |
|
|
5,340 |
|
| Materials and supplies |
4,903 |
|
|
5,451 |
|
|
13,289 |
|
|
17,470 |
|
| Payroll and personnel expenses |
8,316 |
|
|
9,621 |
|
|
26,948 |
|
|
27,763 |
|
| Other research and development expenses |
595 |
|
|
3,551 |
|
|
4,948 |
|
|
10,353 |
|
| Total |
15,383 |
|
|
20,538 |
|
|
49,651 |
|
|
60,926 |
|
| Total research and development expenses |
$ |
17,273 |
|
|
$ |
22,926 |
|
|
$ |
54,697 |
|
|
$ |
67,943 |
|
Direct expenses for the Company’s vascular trauma, arteriovenous (“AV”) access for hemodialysis and peripheral artery disease (“PAD”) indications include costs related to the Company’s clinical trials, including fees paid to clinical research organizations (“CROs”), consultants, clinical sites and investigators. Costs related to development activities which broadly support multiple programs using the Company’s technology platform, including personnel, materials and supplies cost prior to inventory capitalization, external services costs, and other internal expenses, such as facilities and overhead costs, are not allocated to individual research and development programs. Other research and development expenses reported in the table above include direct costs not identifiable with a specific product candidate, including costs associated with the Company’s research and development platform used across programs, process development, manufacturing analytics and preclinical research and development for prospective product candidates and new technologies.
Non-cash Operating Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| ($ in thousands) |
2025 |
|
2024 |
|
2025 |
|
2024 |
| Depreciation expense |
$ |
1,342 |
|
|
$ |
1,289 |
|
|
$ |
3,987 |
|
|
$ |
3,819 |
|
| Stock-based compensation expense |
2,510 |
|
|
1,552 |
|
|
7,431 |
|
|
4,443 |
|
Inventory
The Company capitalizes inventory when it concludes that commercialization and future economic benefit from the sale of products is probable. Prior to this conclusion, the Company expenses inventory as research and development expense in the condensed consolidated statements of operations and comprehensive income (loss) in the period incurred. The determination to capitalize inventory costs is based on various factors, including the product’s historical shelf life, the product’s current status in the development and regulatory approval process, results from related clinical trials, results from meetings with relevant regulatory agencies, potential obstacles to the approval process and viability of commercialization and market trends.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
In early 2025, based on the Company’s assessment of the legal and regulatory process related to Symvess, the Company concluded that it met the criteria to capitalize expenditures as inventory. The Company capitalized $18.4 million of inventory as of September 30, 2025 and none as of December 31, 2024. Inventory is stated at the lower of cost or net realizable value. The Company’s inventory is valued under the first in, first out method. The Company does not have an allowance for inventory obsolescence as of September 30, 2025. Cost of goods sold was $0.3 million and $0.6 million for the three and nine months ended September 30, 2025, respectively, and includes overhead related to unused production capacity, which was recorded as an expense during the period, as well as royalty expense related to the Company’s product sales. There was no cost of goods sold recognized during the three and nine months ended September 30, 2024.
Revenue Recognition
Revenue from Customers
Under Accounting Standards Codification 606, “Revenue from Contracts with Customers” (“ASC 606”), revenue is recognized when a customer obtains control of promised goods or services and is recognized in an amount that reflects the consideration that an entity expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that an entity determines are within the scope of ASC 606, the entity performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. ASC 606 also impacts certain other areas, such as the accounting for costs to obtain or fulfill a contract.
In addition, ASC 606 requires disclosure of the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers.
For contracts where the period between when the Company transfers a promised good or service to the customer and when the customer pays is one year or less, the Company has elected the practical expedient to not adjust the promised amount of consideration for the effects of a significant financing component.
Product Revenue
The Company’s current source of product revenue has been from U.S. sales of Symvess. The Company recognizes product revenue upon delivery of Symvess to the customer. Revenue is recognized based on the price stated in the approved contract or purchase order. There are no contractual rights of returns and replacements for damaged products are provided free of charge. Accounts receivable related to product sales was approximately $0.4 million as of September 30, 2025.
Contract Revenue
Contract revenue consists of revenue related to a single contract with a customer to recover contract expenses. The expenses incurred related to the contract are primarily classified as research and development expenses on the Company’s condensed consolidated statements of operations and comprehensive income (loss). The Company recognizes revenue associated with each performance obligation as the research and development services are provided using an input method, according to the actual costs incurred compared to the total costs expected to be incurred to satisfy the performance obligation. The transfer of control occurs as the program expenses are incurred and, in management’s judgment, is the best measure of progress towards satisfying each performance obligation. The transaction price is determined based on the milestones within the contract and there is no variable consideration. Accounts receivable related to the Company’s contract revenue was approximately $0.1 million and $0.2 million as of September 30, 2025 and December 31, 2024, respectively.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Concentration of Credit Risk
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents, including amounts classified as restricted cash. Total cash balances exceeded insured balances by the Federal Deposit Insurance Corporation as of September 30, 2025 and December 31, 2024. The Company believes it mitigates this risk by monitoring the financial stability of the institutions holding material cash and cash equivalents balances. The Company maintains the majority of these balances at a Global Systemically Important Bank, as designated by the Financial Stability Board. The Company has cash equivalents that are invested in highly rated money market funds that are invested only in obligations of the U.S. government and its agencies. The Company has not experienced any credit loss relating to its cash and cash equivalents.
The Company believes that credit risks associated with its customers and contractual partners are not significant and has not recorded an allowance for credit loss as of September 30, 2025.
Restricted Cash
The Company classifies as restricted cash all cash pledged as collateral to secure long-term obligations and all cash whose use is otherwise limited by contractual provisions. As of September 30, 2025 and December 31, 2024, $0.2 million in funds maintained in a separate deposit account to secure a letter of credit for the benefit of the lessor of the Company’s headquarters lease, and $0.1 million in cash balances held as collateral for the Company’s employee credit card program.
As of December 31, 2024, restricted cash consisted of $50.0 million maintained in an account that was not subject to the Company’s unilateral control, in accordance with the amended Purchase Agreement. On September 17, 2025, Humacyte, Inc. and Global entered into the Purchase Agreement Amendment with the Purchasers and the Agent to further amend the Purchase Agreement. In connection with the Purchase Agreement Amendment, the Company made the Purchase Agreement Amendment Payment of $50.0 million, funded from the restricted cash previously maintained for the benefit of the Agent. As a result of the Purchase Agreement Amendment Payment, the Company is no longer obligated to maintain $50.0 million of restricted cash in an account for the benefit of the Agent unless the Purchase Agreement has not been repaid in full by December 31, 2025, at which point the Company will be required to maintain $12.5 million in such account. As of September 30, 2025, no restricted cash related to the Purchase Agreement remains on the accompanying condensed consolidated balance sheets.
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported within the condensed consolidated balance sheets to the total of the amounts shown in the condensed consolidated statements of cash flows as of September 30, 2025 and December 31, 2024.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in thousands) |
|
September 30,
2025 |
|
December 31,
2024 |
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
19,488 |
|
|
$ |
44,937 |
|
| Restricted cash included in prepaid expenses and other current assets |
|
144 |
|
|
144 |
|
| Restricted cash included in long-term assets |
|
209 |
|
|
50,209 |
|
| Total cash, cash equivalents and restricted cash |
|
$ |
19,841 |
|
|
$ |
95,290 |
|
Leases
During the third quarter of 2025, the Company executed an amendment to its lease agreements that extended the lease term and modified key financial terms, including rent and related provisions. The amendment also resulted in a remeasurement of the related lease liability and right‑of‑use asset as of September 30, 2025, using the Company’s incremental borrowing rate at that date. See Note 8 — Leases for additional details regarding the amendment and its impact to the condensed consolidated financial statements.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Net Loss per Share Attributable to Common Stockholders
Basic net loss per share attributable to common stockholders is computed by dividing net loss attributable to common stockholders by the weighted-average number of shares of Common Stock outstanding during the period without consideration of potentially dilutive shares of Common Stock. Diluted net loss per share attributable to common stockholders reflects the potential dilution that could occur if securities or other contracts to issue Common Stock were exercised or converted into Common Stock or resulted in the issuance of Common Stock that then shared in the earnings of the Company unless inclusion of such shares would be anti-dilutive. The calculation of net loss per share also considers the effect of participating securities. The Common Stock warrants issued in the Company’s October 2024 and November 2024 registered direct offerings are considered participating securities and are included in the computation of net income per share pursuant to the two-class method. In applying the two-class method, during periods of net income, earnings are allocated to both Common Stock and participating securities based on their respective weighted-average shares outstanding for the period. During periods of net loss, no effect is given to participating securities since they do not share in the losses of the Company. As the Company has incurred losses for the three and nine months ended September 30, 2025 and 2024, basic and diluted net loss per share is the same for each period.
The following potential shares of Common Stock were excluded from the computation of diluted net loss per share for each period because including them would have had an antidilutive effect.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
|
2025 |
|
2024 |
|
2025 |
|
2024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Exercise of options under stock plan |
16,537,950 |
|
|
12,287,369 |
|
|
16,537,950 |
|
|
12,287,369 |
|
| Warrants to purchase Common Stock |
9,833,910 |
|
|
5,588,506 |
|
|
11,527,441 |
|
|
5,588,506 |
|
| Exercise of option by underwriters |
— |
|
|
— |
|
|
412,088 |
|
|
— |
|
The 15,000,000 Contingent Earnout Shares, as defined in Note 9, are excluded from the anti-dilutive table for all periods presented, as such shares are contingently issuable until the share price of the Company exceeds specified thresholds that have not yet been achieved, or upon the occurrence of a change in control. The shares subject to the Option Agreement, as defined in Note 7 — Revenue Interest Purchase Agreement, are excluded from the anti-dilutive table for all periods presented based on the Company’s assumption that the Option Agreement will not be exercised unless the Company’s stock price exceeds $7.50 per share, the minimum purchase price under the Option Agreement.
Other Risks and Uncertainties
The Company is subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, successful discovery and development of its product candidates, the success of clinical trials and other studies for its product candidates, including its ongoing V007 and V012 Phase 3 clinical trials, successful commercialization of Symvess and regulatory approval and commercialization of its product candidates, if approved, the expected size of the target populations for the Company’s product candidates, the degree of market acceptance of Symvess, and if approved by regulatory authorities, its product candidates, the availability of third-party coverage and reimbursement, development by competitors of new technological innovations, the ability to manufacture Symvess and its product candidates in sufficient quantities, expectations regarding the Company’s strategic partnerships, dependence on third parties, key personnel and the ability to attract and retain qualified employees, protection of proprietary technology and confidentiality of trade secrets, compliance with governmental regulations, the Company’s implementation and maintenance of effective internal controls, and the ability to secure additional capital to fund operations and the commercial success of its product candidates.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Product candidates currently under development will require extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel, and infrastructure and extensive compliance-reporting capabilities. Even if the Company’s commercialization efforts are successful, it is uncertain when, if ever, the Company will realize significant revenue from product sales, and the Company may depend on certain strategic relationships to distribute its products, including the Company’s strategic partnership with Fresenius Medical Care, to sell, market and distribute its 6 millimeter ATEV for certain specified indications outside the United States.
Recent Accounting Pronouncements
In December 2023, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2023-09, “Income Taxes (Topic 740), Improvements to Income Tax Disclosures” (“ASU 2023-09”). The FASB issued this update to improve the transparency and comparability of income tax disclosures, including requiring consistent categories and greater disaggregation of information in the rate reconciliation and further disaggregation of income taxes paid by jurisdiction. The enhanced disclosures required by ASU 2023-09 are effective for the Company beginning with its Annual Report on Form 10-K for the fiscal year ending December 31, 2025. The Company is currently evaluating the impact of adopting ASU 2023-09 on its consolidated financial statements and related disclosures.
In November 2024, the FASB issued ASU No. 2024-03, “Income Statement — Reporting Comprehensive Income — Expense Disaggregation Disclosures (Subtopic 220-40), Disaggregation of Income Statement Expenses” (“ASU 2024-03”). In January 2025, the FASB issued ASU No. 2025-01, “Income Statement — Reporting Comprehensive Income — Expense Disaggregation Disclosures (Subtopic 220-40), Clarifying the Effective Date” (“ASU 2025-01”). ASU 2024-03 requires additional disclosure about the nature and amounts of expenses included in certain expense captions presented on the income statement to enhance the transparency of the relevant expense captions. ASU 2024-03, as clarified by ASU 2025-01, is effective for annual reporting periods beginning after December 15, 2026 and interim reporting periods within annual reporting periods beginning after December 15, 2027, with early adoption permitted. Entities may elect to apply the amendments either prospectively or retrospectively. The Company is currently evaluating the impact of adopting ASU 2024-03 on its consolidated financial statements and related disclosures.
In September 2025, the FASB issued ASU No. 2025-07, “Derivatives and Hedging (Topic 815) and Revenue from Contracts with Customers (Topics 606): Derivatives Scope Refinements and Scope Clarification for Share-based Noncash Consideration from a Customer in a Revenue Contract” (“ASU 2025-07”). The amendments in this update are intended to reduce the cost and complexity of evaluating whether contracts with features based on the operations or activities of one of the parties to the contract are derivatives, improve the representation of the economics of those contracts in the financial statements, and reduce diversity in practice. This ASU also clarifies that share-based noncash consideration from a customer should initially be accounted for under Topic 606 until the right to receive or retain such consideration becomes unconditional, at which point financial instruments guidance may apply. This standard is effective for fiscal years beginning after December 15, 2026, including interim periods within those fiscal years. Early adoption is permitted. Entities may apply the amendments prospectively or on a modified retrospective basis. The Company is currently evaluating the impact of adopting ASU 2025-07 on its consolidated financial statements and related disclosures.
3. Fair Value Measurements
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in the principal or most advantageous market in an orderly transaction between market participants at the measurement date. ASC 820, Fair Value Measurement and Disclosures, establishes a hierarchy whereby inputs to valuation techniques used in measuring fair value are prioritized, or the fair value hierarchy. There are three levels to the fair value hierarchy based on reliability of inputs, as follows:
•Level 1 — Observable inputs that reflect unadjusted quoted prices for identical assets or liabilities in active markets.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
•Level 2 — Inputs other than quoted prices included in Level 1 that are observable for the asset or liability, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
•Level 3 — Unobservable inputs in which little or no market data exists, therefore requiring the Company to develop its own assumptions.
The Company’s money market funds are classified within Level 1 of the fair value hierarchy because they are valued using quoted market prices. The carrying values of cash, accounts receivable, prepaid expenses and other current assets, accounts payable, accrued expenses and other current liabilities as of September 30, 2025 and December 31, 2024 approximated their fair values due to the short-term nature of these items.
The Company evaluates assets and liabilities subject to fair value measurements on a recurring basis to determine the appropriate level at which to classify them for each reporting period, utilizing valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible. The determination requires significant judgments to be made by the Company.
The Company’s assets and liabilities that were measured at fair value on a recurring basis were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in thousands) |
|
Fair Value Measured as of September 30, 2025 |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
| Assets: |
|
|
|
|
|
|
|
|
| Cash equivalents (money market funds) |
|
$ |
12,166 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
12,166 |
|
| Common Stock Purchase Agreement derivative asset |
|
— |
|
|
672 |
|
|
— |
|
|
672 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total financial assets |
|
$ |
12,166 |
|
|
$ |
672 |
|
|
$ |
— |
|
|
$ |
12,838 |
|
| Liabilities: |
|
|
|
|
|
|
|
|
| Contingent Earnout Liability |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
21,807 |
|
|
$ |
21,807 |
|
| Contingent derivative liability |
|
— |
|
|
— |
|
|
289 |
|
|
289 |
|
| Private Placement Warrants liability |
|
— |
|
|
— |
|
|
101 |
|
|
101 |
|
| October 2024 RDO Warrants liability |
|
— |
|
|
— |
|
|
2,083 |
|
|
2,083 |
|
| November 2024 RDO Warrants liability |
|
— |
|
|
— |
|
|
1,050 |
|
|
1,050 |
|
| Option Agreement liability |
|
— |
|
|
— |
|
|
6 |
|
|
6 |
|
| JDRF Agreement derivative liability |
|
— |
|
|
— |
|
|
134 |
|
|
134 |
|
| Total financial liabilities |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
25,470 |
|
|
$ |
25,470 |
|
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in thousands) |
|
Fair Value Measured as of December 31, 2024 |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
| Assets: |
|
|
|
|
|
|
|
|
| Cash equivalents (money market funds) |
|
$ |
32,044 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
32,044 |
|
| Common Stock Purchase Agreement derivative asset |
|
$ |
— |
|
|
$ |
672 |
|
|
$ |
— |
|
|
$ |
672 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total financial assets |
|
$ |
32,044 |
|
|
$ |
672 |
|
|
$ |
— |
|
|
$ |
32,716 |
|
| Liabilities: |
|
|
|
|
|
|
|
|
| Contingent Earnout Liability |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
70,961 |
|
|
$ |
70,961 |
|
| Contingent derivative liability |
|
— |
|
|
— |
|
|
2,415 |
|
|
2,415 |
|
| Private Placement Warrants liability |
|
— |
|
|
— |
|
|
385 |
|
|
385 |
|
| October 2024 RDO Warrants liability |
|
— |
|
|
— |
|
|
12,437 |
|
|
12,437 |
|
| November 2024 RDO Warrants liability |
|
— |
|
|
— |
|
|
6,432 |
|
|
6,432 |
|
| Option Agreement liability |
|
— |
|
|
— |
|
|
64 |
|
|
64 |
|
| JDRF Agreement derivative liability |
|
— |
|
|
— |
|
|
121 |
|
|
121 |
|
| Total financial liabilities |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
92,815 |
|
|
$ |
92,815 |
|
The fair value of the Contingent Earnout Liability (defined in Note 9 — Stockholders’ Equity (Deficit) and Warrants), contingent derivative liability related to the Put Option (as defined in Note 7 — Revenue Interest Purchase Agreement and discussed below), Private Placement Warrants liability, liabilities associated with the Registered Direct Offering Warrants (each as defined in Note 9 — Stockholders’ Equity (Deficit) and Warrants), Option Agreement liability (as defined in Note 7 — Revenue Interest Purchase Agreement), and the derivative liability associated with the JDRF Agreement Disposition Payment (defined in Note 12 — Commitments and Contingencies) are based on significant unobservable inputs, which represent Level 3 measurements within the fair value hierarchy. The fair values of the Private Placement Warrants liability and the liabilities associated with the Registered Direct Offering Warrants are included in common stock warrant liabilities on the condensed consolidated balance sheets. The fair values of the Option Agreement liability and the derivative liability associated with the JDRF Agreement Disposition Payment are included in other long-term liabilities on the condensed consolidated balance sheets.
Common Stock Purchase Agreement
The Company evaluated the Common Stock Purchase Agreement and determined that the agreement should be accounted for in accordance with ASC 815-40, “Derivatives and Hedging — Contracts on an Entity’s Own Equity”. Accordingly, the Company recorded a derivative asset with an initial fair value based on the 115,705 shares of Common Stock issued to Lincoln Park as consideration for its irrevocable commitment to purchase up to $50.0 million in shares of Common Stock. The initial fair value of $0.7 million was based on the closing price of the Common Stock on September 24, 2024, which was $6.12 per share, and the derivative asset is reported as a component of long-term assets on the condensed consolidated balance sheets. Subsequent changes in the fair value of the derivative asset are dependent upon, among other things, changes in the closing share price of Common Stock, the quantity and purchase price of the shares purchased by Lincoln Park during the reporting period and the unused capacity under the Common Stock Purchase Agreement. The Common Stock Purchase Agreement is subsequently remeasured at each reporting date with changes in fair value recorded within Change in fair value of derivatives in the condensed consolidated statements of operations and comprehensive loss. There was no change in fair value of the derivative asset between December 31, 2024 and September 30, 2025 and the fair value of the Commitment Shares as of both September 30, 2025 and December 31, 2024 was $0.7 million.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Contingent Earnout Liability
The following table presents a summary of the changes in the fair value of the Contingent Earnout Liability:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in thousands) |
|
Contingent Earnout Liability |
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
|
2025 |
|
2024 |
|
2025 |
|
2024 |
|
|
|
|
|
|
|
|
|
| Fair value as of beginning of period |
|
$ |
(26,700) |
|
|
$ |
(68,080) |
|
|
$ |
(70,961) |
|
|
$ |
(37,916) |
|
| Change in fair value included in other income (expense), net |
|
4,893 |
|
|
(8,489) |
|
|
49,154 |
|
|
(38,653) |
|
| Fair value as of end of period |
|
$ |
(21,807) |
|
|
$ |
(76,569) |
|
|
$ |
(21,807) |
|
|
$ |
(76,569) |
|
In determining the fair value of the Contingent Earnout Liability, the Company used the Monte Carlo simulation value model using a distribution of potential outcomes on a monthly basis over a 10-year period prioritizing the most reliable information available. The assumptions utilized in the calculation were based on the achievement of certain stock price milestones, including the current Common Stock price, expected volatility, risk-free rate, expected term and expected dividend yield (see Note 9 — Stockholders’ Equity (Deficit) and Warrants). Contingent earnout payments involve certain assumptions requiring significant judgment and actual results can differ from assumed and estimated amounts.
Contingent Derivative Liability
The debt pursuant to the Purchase Agreement, as defined in Note 7, contains an embedded derivative related to the Put Option, as defined in Note 7, requiring bifurcation as a single compound derivative instrument. The Company estimated the fair value of the derivative liability using a “with-and-without” methodology. The “with-and-without” methodology involves valuing the whole instrument on an as-is basis and then valuing the instrument without the individual embedded derivative. The difference between the entire instrument with the embedded derivative compared to the instrument without the embedded derivative was the fair value of the derivative liability at issuance and each subsequent reporting period. In determining the fair value of the contingent derivative liability, the Company used the Monte Carlo simulation value model using a distribution of potential outcomes on a monthly basis over a 10-year period. The estimated probability and timing of underlying events triggering the exercisability of the Put Option contained within the Purchase Agreement, forecasted cash flows and the discount rates are significant unobservable inputs used to determine the estimated fair value of the entire instrument with the embedded derivative.
As of September 30, 2025, the discount rates used to calculate the value of the contingent derivative liability were 12.6% to calculate the present-value of the revenue forecast and 12.2% to calculate the present-value of the payoff of the Put Option. As of December 31, 2024, the discount rates used to calculate the value of the contingent derivative liability were 14.2% to calculate the present-value of the revenue forecast and 11.8% to calculate the present-value of the payoff of the Put Option. Changes in fair value of the contingent derivative liability are recognized as other income (expense) in the condensed consolidated statements of operations and comprehensive loss, classified in Change in fair value of derivatives.
The following table presents a summary of the changes in the fair value of the contingent derivative liability, which is classified as a Level 3 financial instrument:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent Derivative Liability |
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| ($ in thousands) |
|
2025 |
|
2024 |
|
2025 |
|
2024 |
|
|
|
|
|
|
|
|
|
| Fair value as of beginning of period |
|
$ |
(3,161) |
|
|
$ |
(4,266) |
|
|
$ |
(2,415) |
|
|
$ |
(2,636) |
|
| Fair value of embedded derivative upon issuance of debt |
|
— |
|
|
— |
|
|
— |
|
|
(1,552) |
|
| Change in fair value included in other income (expense), net |
|
2,872 |
|
|
1,161 |
|
|
2,126 |
|
|
1,083 |
|
| Fair value as of end of period |
|
$ |
(289) |
|
|
$ |
(3,105) |
|
|
$ |
(289) |
|
|
$ |
(3,105) |
|
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Registered Direct Offering Warrants Liabilities
The following table presents a summary of the changes in the fair value of the Registered Direct Offering Warrants liabilities, as defined in Note 9, during the three and nine months ended September 30, 2025:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
October 2024 RDO Warrants |
|
November 2024 RDO Warrants |
| ($ in thousands) |
|
Three Months Ended
September 30, 2025 |
|
Nine Months Ended September 30, 2025 |
|
Three Months Ended
September 30, 2025 |
|
Nine Months Ended September 30, 2025 |
| Fair value as of beginning of period |
|
$ |
(2,811) |
|
|
$ |
(12,437) |
|
|
$ |
(1,411) |
|
|
$ |
(6,432) |
|
|
|
|
|
|
|
|
|
|
| Change in fair value included in other income (expense), net |
|
728 |
|
|
10,354 |
|
|
361 |
|
|
5,382 |
|
| Fair value as of end of period |
|
$ |
(2,083) |
|
|
$ |
(2,083) |
|
|
$ |
(1,050) |
|
|
$ |
(1,050) |
|
In determining the fair value of the Registered Direct Offering Warrants liabilities, the Company used the Black-Scholes valuation model to estimate the fair value utilizing assumptions including the current Company stock price, expected volatility, risk-free rate, expected term and expected dividend yield (see Note 9 — Stockholders’ Equity (Deficit) and Warrants).
Private Placement Warrants Liability
The following table presents a summary of the changes in the fair value of the Private Placement Warrants liability:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Private Placement Warrants |
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| ($ in thousands) |
|
2025 |
|
2024 |
|
2025 |
|
2024 |
|
|
|
|
|
|
|
|
|
| Fair value as of beginning of period |
|
$ |
(136) |
|
|
$ |
(285) |
|
|
$ |
(385) |
|
|
$ |
(78) |
|
| Change in fair value included in other income (expense), net |
|
35 |
|
|
(89) |
|
|
284 |
|
|
(296) |
|
| Fair value as of end of period |
|
$ |
(101) |
|
|
$ |
(374) |
|
|
$ |
(101) |
|
|
$ |
(374) |
|
In determining the fair value of the Private Placement Warrants liability, the Company used the Monte Carlo simulation valuation model to estimate the fair value utilizing assumptions including the current Company stock price, expected volatility, risk-free rate, expected term and expected dividend yield (see Note 9 — Stockholders’ Equity (Deficit) and Warrants).
4. Inventory
Inventory is stated at the lower of cost or net realizable value and consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in thousands) |
|
September 30,
2025 |
|
December 31,
2024 |
|
|
|
|
|
| Raw materials |
|
$ |
5,679 |
|
|
$ |
— |
|
| Work in process |
|
12,098 |
|
|
— |
|
| Finished goods |
|
641 |
|
|
— |
|
| Inventory |
|
$ |
18,418 |
|
|
$ |
— |
|
As of September 30, 2025, the Company capitalized costs of $18.4 million associated with the manufacturing of Symvess as a result of regulatory approval and the Company’s determination that subsequent commercialization and future economic benefit from the sales of Symvess was probable.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
5. Property and Equipment, Net
Property and equipment, net consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in thousands) |
|
September 30,
2025 |
|
December 31,
2024 |
|
|
|
|
|
| Scientific and manufacturing equipment |
|
$ |
29,758 |
|
|
$ |
29,059 |
|
| Computer equipment |
|
105 |
|
|
100 |
|
| Software |
|
1,025 |
|
|
1,024 |
|
| Furniture and fixtures |
|
1,066 |
|
|
1,066 |
|
| Leasehold improvements |
|
27,967 |
|
|
27,901 |
|
|
|
|
|
|
|
|
59,921 |
|
|
59,150 |
|
| Accumulated depreciation |
|
(40,064) |
|
|
(36,087) |
|
| Property and equipment, net |
|
$ |
19,857 |
|
|
$ |
23,063 |
|
Depreciation expense totaled $1.3 million and $4.0 million for the three and nine months ended September 30, 2025, respectively, and $1.3 million and $3.8 million for the three and nine months ended September 30, 2024, respectively. All long-lived assets are maintained in the United States.
6. Accrued Expenses
Accrued expenses consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in thousands) |
|
September 30,
2025 |
|
December 31,
2024 |
|
|
|
|
|
| Accrued external research, development and manufacturing costs |
|
$ |
3,515 |
|
|
$ |
4,889 |
|
| Accrued employee compensation and benefits |
|
6,193 |
|
|
6,242 |
|
| Accrued professional fees |
|
430 |
|
|
293 |
|
| Accrued royalties |
|
126 |
|
|
— |
|
| Total |
|
$ |
10,264 |
|
|
$ |
11,424 |
|
7. Revenue Interest Purchase Agreement
Revenue Interest Purchase Agreement
On May 12, 2023, the Company and Global entered into the Purchase Agreement with the Purchasers, and another affiliate of Oberland, as agent for the Purchasers, to obtain financing with respect to the further development and commercialization of the Company’s ATEV, to repay the Company’s then-existing credit facility with SVB, and for other general corporate purposes. Pursuant to the Purchase Agreement, on May 12, 2023, the Purchasers purchased certain revenue interests (the “Revenue Interests”) from Global in exchange for an aggregate investment amount of up to $150.0 million (the “Investment Amount”) to be paid in multiple tranches. On May 12, 2023, the Company received an initial payment of $40.0 million, less certain transaction expenses, which was used to repay in full the Company’s then-existing obligations under the former loan agreement with SVB. In February 2024, the FDA accepted the Company’s Biologics License Application (“BLA”) for an indication in vascular trauma, and in accordance with the Purchase Agreement, on March 11, 2024, the Company drew a subsequent installment of $20.0 million.
The FDA granted full approval for the Company’s BLA on December 19, 2024, and the Company elected not to draw the additional $40.0 million that became available under the Purchase Agreement. As of December 31, 2024, the Company was not entitled to draw on any further installments under the Purchase Agreement. On September 17, 2025, the Company entered into the Purchase Agreement Amendment, which modified certain terms of the agreement and the Company made a partial call payment of $50.0 million to the Purchasers. The Company analyzed the changes to the agreement and concluded the amendment resulted in a modification for accounting purposes.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Pursuant to the Purchase Agreement, the Revenue Interests entitle the Purchasers to receive a royalty initially equal to 7.5% (the “Rate”) of global net sales of the Company’s products (subject to a lower rate for net sales by specified licensees outside the United States), to be paid on a calendar quarterly basis (the “Revenue Interest Payments”).
If the Purchasers do not receive cumulative Revenue Interest Payments equal to 100% of the amount funded to date (the “Cumulative Purchaser Payments”) by the last business day of 2028 (the “Test Date”), the Rate will increase to a rate that, had such increased rate applied during the period from May 12, 2023 through the Test Date, would have provided the Purchasers with cumulative Revenue Interest Payments equal to the Cumulative Purchaser Payments as of the Test Date. Additionally, Global will be required to pay the Purchasers an amount equal to 100% of the Cumulative Purchaser Payments as of the Test Date less the total Revenue Interest Payments made by Global to the Purchasers under the Purchase Agreement as of the Test Date. Global’s obligation to make Revenue Interest Payments terminates on the date on which the Purchasers have received Revenue Interest Payments of 150% of the Cumulative Purchaser Payments unless the Purchase Agreement is terminated earlier due to the Purchaser’s exercise of a Put Option, the Company’s exercise of a Call Option, or by mutual consent. However, if the Purchasers have not received such Revenue Interest Payments as of the Test Date, the Purchase Agreement will instead terminate on the date on which the Purchasers receive Revenue Interest Payments of 195% of the Cumulative Purchaser Payments.
Under the Purchase Agreement, Global has an option (the “Call Option”) to repurchase the Revenue Interests and terminate the Purchase Agreement at any time upon advance written notice. Additionally, the Purchasers have an option (the “Put Option”) to terminate the Purchase Agreement and to require Global to repurchase the Revenue Interests upon enumerated events such as a bankruptcy event, an uncured material breach, a material adverse effect or a change of control. Pursuant to the Purchase Agreement Amendment, if (i) the Put Option is exercised by May 12, 2026, or (ii) the Call Option is exercised on or prior to May 12, 2026, then in each case, the required repurchase price will be 175% of the Cumulative Purchaser Payments, minus the aggregate Revenue Interest Payments Global has made to the Purchasers as of such date and the amount of the Purchase Agreement Amendment Payment. If a Put Option or Call Option is exercised after May 12, 2026, the required repurchase price will be 195% of the Cumulative Purchaser Payments, subject to the same deductions and conditions described above. Additionally, the Company is permitted, at its option, to exercise its Call Option at a discounted repurchase price of $95.5 million, subject to the same deductions and conditions described above, so long as it is exercised on or prior to December 31, 2025, up to $7.5 million of which may be satisfied through the issuance of shares of the Company’s common stock, subject to the satisfaction of certain conditions.
The Purchase Agreement contains customary representations and warranties and affirmative covenants for transactions of this type, including, among others, the provision of financial and other information to the Purchaser, notice to the Purchaser upon the occurrence of certain material events, and compliance with applicable laws. The Purchase Agreement also contains customary negative covenants, including certain restrictions on the ability to incur indebtedness and grant liens or security interests on assets. On February 18, 2024, the Company reached an agreement with the Purchasers and the Agent to waive certain breaches related to, and extend the deadline for certain post-closing obligations under, the Purchase Agreement, including the requirement for the Company to deliver a leasehold mortgage in favor of the Agent over the Company’s headquarters. On May 8, 2024, the Company agreed with the Purchasers to amend the Purchase Agreement, the effect of which was to remove requirements related to the leasehold mortgage. In exchange for the removal of these requirements, the Company funded an account in an amount of $54.0 million on August 14, 2024, over which the Agent has certain consent and other rights to $50.0 million of the funds. In connection with the Purchase Agreement Amendment, the Company made the Purchase Agreement Amendment Payment of $50.0 million, funded from the restricted cash previously maintained for the benefit of the Agent. As a result of the Purchase Agreement Amendment Payment, the Company is no longer obligated to maintain $50.0 million of restricted cash in an account for the benefit of the Agent unless the Purchase Agreement has not been repaid in full by December 31, 2025, at which point the Company will be required to maintain $12.5 million in such account. As of September 30, 2025 and December 31, 2024, $0 and $50.0 million, respectively, were classified as restricted cash on the accompanying condensed consolidated balance sheets.
The Company has agreed to guarantee the payment in full of the obligations under the Purchase Agreement. The Company’s obligations under the parent company guaranty and Global’s obligations under the Purchase Agreement and the Revenue Interests are secured by a perfected security interest on substantially all of the Company’s and its subsidiaries’ assets.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
The Purchase Agreement is considered a sale of future revenues and accounted for as long-term debt recorded at amortized cost using the interest method.
The Company recorded a revenue interest liability related to the Purchase Agreement on the accompanying condensed consolidated balance sheet on the date the Company entered into the Purchase Agreement, net of a debt discount comprised of $2.1 million issuance costs and transaction costs, $0.1 million fair value allocated to the Option Agreement, defined below, and $2.4 million initial fair value of the bifurcated contingent derivative liability related to the Put Option. The revenue interest liability is based on the Company’s contractual repayment obligation to the Purchasers, based on the current estimates of future revenues, over the life of the Purchase Agreement. The Company imputes interest expense associated with this liability using the interest method. The effective interest rate is calculated based on the rate that would enable the debt to be repaid in full over the anticipated life of the arrangement. The interest rate on this liability may vary during the term of the agreement depending on a number of factors, including the level and expected timing of forecasted net sales. The Company evaluates the interest rate quarterly based on its current net sales forecasts. If the level and timing of any forecasted net sales and related payments change, the Company prospectively adjusts the effective interest and the related amortization of the liability and related issuance costs on a quarterly basis.
As of September 30, 2025 and December 31, 2024, $20.7 million and $64.2 million, respectively, was recorded as a revenue interest liability. As of September 30, 2025 and December 31, 2024, the current portion of the revenue interest liability was $3.1 million and $0.9 million, respectively. The estimated effective annual interest rate as of September 30, 2025 and December 31, 2024 was 52.1% and 13.7%, respectively. The Company recorded $2.3 million and $6.6 million in interest expense related to the Purchase Agreement for the three and nine months ended September 30, 2025, respectively, and recorded $2.0 million and $5.6 million in interest expense related to the Purchase Agreement for the three and nine months ended September 30, 2024, respectively. The Company incurred and paid $0.5 million of transaction costs during the nine months ended September 30, 2024 in connection with the Purchase Agreement. The transaction costs were capitalized to debt discount and are being amortized to interest expense over the estimated term of the debt, consistent with the issuance and transaction costs incurred in 2023 discussed above.
The Put Option under the Purchase Agreement that is exercisable by the Purchasers upon certain contingent events was determined to be an embedded derivative requiring bifurcation and separately accounted for as a single compound derivative instrument. At May 12, 2023, the Company recorded the initial fair value of the derivative liability of $2.4 million as a debt discount. On March 11, 2024, upon the issuance of the second installment of the Purchase Agreement of $20.0 million, the Company estimated the fair value of the embedded derivative and recorded a $1.6 million increase in fair value as a debt discount. The debt discount is being amortized to interest expense over the expected term of the debt using the interest method. See Note 3 — Fair Value Measurements for a further discussion of the fair value of the contingent derivative liability associated with the Put Option.
Revenue Interest Payments made as a result of the Company’s net product sales will reduce the revenue interest liability. During the three and nine months ended September 30, 2025, the Company recorded $0.7 million and $0.9 million of product sales revenue, respectively, and made revenue interest payments totaling $45 thousand across both periods. During the three and nine months ended September 30, 2024, the Company did not record any product sales revenue.
In addition, during the three and nine months ended September 30, 2025, the Company made a $50.0 million partial call payment on revenue interest liability in connection with the Purchase Agreement Amendment, reducing the revenue interest liability to $20.7 million as of September 30, 2025.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
The following table summarizes the revenue interest liability activity during the three and nine months ended September 30, 2025:
|
|
|
|
|
|
|
|
|
|
| ($ in thousands) |
|
|
|
Revenue interest liability at December 31, 2024 |
|
$ |
64,239 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest expense recognized |
|
2,123 |
|
|
|
|
|
|
| Revenue interest liability at March 31, 2025 |
|
66,362 |
|
|
| Interest expense recognized |
|
2,179 |
|
|
| Revenue interest liability at June 30, 2025 |
|
68,541 |
|
|
| Interest expense recognized |
|
2,250 |
|
|
| Revenue interest payments |
|
(45) |
|
|
| Payment under amended Purchase Agreement |
|
(50,000) |
|
|
Revenue interest liability at September 30, 2025 |
|
20,746 |
|
|
| Less current portion of revenue interest liability |
|
(3,072) |
|
|
Revenue interest liability, net of current portion at September 30, 2025 |
|
$ |
17,674 |
|
|
Option Agreement
In connection with the Purchase Agreement, the Company also entered into an option agreement with TPC Investments III LP and TPC Investment Solutions LP (the “Option Agreement”), which gave TPC Investments III LP and TPC Investment Solutions LP (the “Holders”) the right to purchase, in the aggregate, up to $10.0 million worth of shares of Common Stock (the “Option”) at a purchase price per share equal to the greater of $7.50, or the 15 day volume-weighted average price as of the exercise date, exercisable in cash only at any time prior to the earlier of (i) December 31, 2026 and (ii) the closing date of a corporate reorganization. The Holders also received certain registration rights relating to the shares underlying the Option pursuant to the Option Agreement. The Holders purchased $1,950,000 of shares of Common Stock in the 2024 Public Offering, as defined in Note 9, and as of September 30, 2025 and December 31, 2024, the Holders have the right to purchase up to $8,050,000 of shares of Common Stock under the Option Agreement.
The Option granted to the Holders represents a freestanding instrument separate from the purchaser commitments outlined in the Purchase Agreement. The Option Agreement does not qualify for the equity contract scope exception under ASC 815-40 and the Company recorded the Option as a liability (“Option Agreement liability”) on the condensed consolidated balance sheet at an initial fair value of $55 thousand, and subsequent changes in the fair value are recognized in the condensed consolidated statements of operations and comprehensive loss at each reporting date. The fair value of the Option Agreement liability as of September 30, 2025 and December 31, 2024 was $6 thousand and $64 thousand, respectively.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
8. Leases
On September 29, 2025, we executed a Sixth Amendment to our lease agreements. The amendment extends the non‑cancelable term to March 31, 2037, resets base rent effective January 1, 2026 with 3% annual escalations thereafter, and provides two rent abatement periods (January 1, 2026, through March 31, 2026, and January 1, 2027, through March 31, 2027). Accounting for the amendment resulted in remeasurement of the lease liability and right‑of‑use asset as of September 30, 2025 using our incremental borrowing rate at that date.
As a result, during the three and nine months ended September 30, 2025, the Company recorded a net non‑cash increase of $15.0 million to right‑of‑use assets and lease liabilities resulting from lease remeasurements associated with the lease modification.
As of September 30, 2025 and December 31, 2024, the Company had finance lease liabilities of $29.8 million and $16.5 million, respectively, and right-of-use assets of $29.4 million and $15.5 million, respectively. As of December 31, 2024, the Company had operating lease liabilities of $0.6 million, primarily included in other long-term liabilities with the remaining balance classified in other current liabilities, and related right-of-use assets of $0.6 million included in other long-term assets on the consolidated balance sheets. As of September 30, 2025, the Company had no operating lease liabilities or right-of-use assets on the condensed consolidated balance sheets.
The Company’s lease does not require any contingent rental payments, impose any financial restrictions, or contain any residual value guarantees. The Company’s lease includes renewal options and escalation clauses; renewal options have been included in the calculation of the lease liabilities and right-of-use assets as the Company is reasonably certain to exercise the options due to the specialized nature of the leased building. Variable expenses generally represent the Company’s share of the landlord’s operating expenses. The Company does not act as a lessor in any lease arrangements.
The following summarizes quantitative information about the Company’s leases:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| ($ in thousands) |
|
2025 |
|
2024 |
|
2025 |
|
2024 |
Finance lease cost |
|
|
|
|
|
|
|
|
| Amortization of right-of-use assets |
|
524 |
|
|
522 |
|
|
1,570 |
|
|
1,563 |
|
Interest on lease liabilities |
|
316 |
|
|
375 |
|
|
994 |
|
|
1,162 |
|
Total finance lease cost |
|
840 |
|
|
897 |
|
|
2,564 |
|
|
2,725 |
|
Operating lease cost |
|
$ |
26 |
|
|
$ |
26 |
|
|
$ |
79 |
|
|
$ |
79 |
|
Total lease cost |
|
$ |
866 |
|
|
$ |
923 |
|
|
$ |
2,643 |
|
|
$ |
2,804 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, 2025 |
|
Nine Months Ended September 30, 2024 |
| ($ in thousands) |
|
Finance
Leases |
|
Operating
Leases |
|
Finance
Leases |
|
Operating
Leases |
|
| Operating cash flows from leases |
|
$ |
994 |
|
|
$ |
79 |
|
|
$ |
1,162 |
|
|
$ |
79 |
|
| Financing cash flows from leases |
|
$ |
(2,157) |
|
|
$ |
— |
|
|
$ |
(1,906) |
|
|
$ |
— |
|
| Weighted-average remaining lease term |
|
9.98 |
|
0 |
|
3.82 |
|
4.36 |
| Weighted-average discount rate |
|
8.50 |
% |
|
— |
% |
|
8.50 |
% |
|
8.50 |
% |
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
As of September 30, 2025, the maturities of the Company’s finance lease liabilities were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
($ in thousands) |
|
Finance Leases |
|
|
| 2025 |
|
$ |
1,052 |
|
|
|
| 2026 |
|
2,428 |
|
|
|
| 2027 |
|
(301) |
|
(1) |
|
| 2028 |
|
2,500 |
|
|
|
2029 |
|
3,537 |
|
|
|
Thereafter |
|
53,001 |
|
|
|
Total |
|
62,217 |
|
|
|
Less: present value discount |
|
(32,392) |
|
|
|
Lease liabilities |
|
$ |
29,825 |
|
|
|
___________________________
(1)For the year ending 2027, the negative amount reflects a reimbursement from the lessor for tenant improvement costs. The reimbursement exceeds the scheduled lease payments for that period.
9. Stockholders’ Equity (Deficit) and Warrants
Public Offerings
On February 29, 2024, the Company entered into an underwriting agreement with Cowen and Company, LLC and Cantor Fitzgerald & Co., as representatives of the several underwriters named therein, relating to the issuance and sale in an underwritten offering (the “2024 Public Offering”) of 15,410,000 shares of Common Stock, which included a full exercise of the Underwriters’ option to purchase additional shares, at a price to the public of $3.00 per share. The net proceeds to the Company from the 2024 Public Offering were approximately $43.0 million after deducting underwriting discounts and commissions and offering expenses. The 2024 Public Offering closed on March 5, 2024.
On March 25, 2025, the Company entered into an underwriting agreement with TD Securities (USA) LLC, Barclays Capital Inc. and BTIG, LLC, as representatives of the several underwriters named therein, relating to the issuance and sale in an underwritten offering (the “2025 Public Offering”) of 25,000,000 shares of Common Stock, at a price to the public of $2.00 per share (the “Firm Shares”). The Company also granted the underwriters a 30-day option to purchase up to an additional 3,750,000 shares of Common Stock at the same price as the Firm Shares, which the underwriters did not exercise. The net proceeds to the Company from the 2025 Public Offering were approximately $46.7 million after deducting underwriting discounts and commissions and offering expenses. The 2025 Public Offering closed on March 27, 2025.
Equity Line Financing
On September 24, 2024, the Company entered into the Common Stock Purchase Agreement with Lincoln Park for an equity line financing, which provides that, subject to the terms and conditions set forth therein, the Company has the sole right, but not the obligation, to sell to Lincoln Park shares of Common Stock having an aggregate value of up to $50.0 million over a 24-month period. The Company controls the timing and amount of any sales of Purchase Shares to Lincoln Park pursuant to the Common Stock Purchase Agreement in its sole discretion. In consideration for entering into the Common Stock Purchase Agreement, the Company issued 115,705 shares of Common Stock (the “Commitment Shares”) to Lincoln Park. The Company did not receive any cash proceeds from the issuance of the Commitment Shares. The fair value of the Common Stock Purchase Agreement was measured on the issuance date based on the fair value of the Commitment Shares, which was the consideration given to Lincoln Park in exchange for entering into the agreement. The fair value of the Commitment Shares on the issuance date was determined to be $0.7 million based on the closing price of the Common Stock on September 24, 2024, which was $6.12 per share. The Company recognized the fair value of the Commitment Shares as a non-current asset as a component of other long-term assets on the condensed consolidated balance sheets. The Common Stock Purchase Agreement is subsequently remeasured at each reporting date with changes in fair value recorded within Change in fair value of derivatives in the condensed consolidated statements of operations and comprehensive loss. Through September 30, 2025, the Company has sold 500,000 shares to Lincoln Park for aggregate gross proceeds of $2.5 million and as of September 30, 2025, the Company had $47.5 million in remaining availability for sales of Common Stock under the Common Stock Purchase Agreement.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
There were no purchases under the Common Stock Purchase Agreement during the three and nine months ended September 30, 2025.
Registered Direct Offerings
On October 4, 2024, the Company entered into a securities purchase agreement with an institutional investor pursuant to which the investor purchased 5,681,820 shares of Common Stock and warrants to purchase up to 5,681,820 shares of Common Stock (the “October 2024 RDO Warrants”) in a registered direct offering (the “October 2024 Registered Direct Offering”). See below for additional information regarding the October 2024 RDO Warrants. The purchase price for one share of Common Stock and one warrant issued in the October 2024 Registered Direct Offering was $5.28. The net proceeds to the Company from the October 2024 Registered Direct Offering were approximately $28.0 million after deducting placement agent’s fees and offering expenses of approximately $2.0 million. The October 2024 Registered Direct Offering closed on October 7, 2024.
On November 13, 2024, the Company entered into a securities purchase agreement with an institutional investor pursuant to which the investor purchased 2,808,988 shares of Common Stock and warrants to purchase up to 2,808,988 shares of Common Stock (the “November 2024 RDO Warrants”) in a registered direct offering (the “November 2024 Registered Direct Offering”). See below for additional information regarding the November 2024 RDO Warrants. The purchase price for one share of Common Stock and one warrant issued in the November 2024 Registered Direct Offering was $5.34. The net proceeds to the Company from the November 2024 Registered Direct Offering were approximately $14.9 million after deducting offering expenses of approximately $0.1 million. The November 2024 Registered Direct Offering closed on November 15, 2024.
On October 6, 2025, the Company entered into a securities purchase agreement with an institutional investor pursuant to which the investor purchased 28,436,018 shares of Common Stock and warrants to purchase up to 28,436,018 shares of Common Stock (the “October 2025 RDO Warrants”) in a registered direct offering (the “October 2025 Registered Direct Offering”). See Note 14 — Subsequent Events for additional information.
ATM Facility
On September 1, 2022, the Company entered into the ATM Facility for the sale from time to time of up to $80.0 million of shares of Common Stock. During the three months ended September 30, 2025, the Company sold an aggregate of 2,492,410 shares of Common Stock under the ATM Facility at an average price of $2.64 per share for net proceeds of approximately $6.2 million. During the nine months ended September 30, 2025, the Company sold an aggregate of 3,792,280 shares of Common Stock under the ATM Facility at an average price of $2.72 per share for net proceeds of approximately $9.8 million. As of September 30, 2025, the Company had $62.7 million in remaining availability for sales of Common Stock under the ATM Facility.
Common Stock
In June 2025, the Company amended its Second Amended and Restated Certificate of Incorporation to increase the authorized number of shares of Common Stock from 250,000,000 to 350,000,000.
The holders of Common Stock are entitled to receive dividends from time to time as may be declared by the Company’s board of directors. Through September 30, 2025, no dividends have been declared. The Purchase Agreement limits the Company’s ability to pay cash dividends to the holders of Common Stock.
The holders of Common Stock are entitled to one vote for each share held with respect to all matters voted on by the common stockholders of the Company.
In the event of a reorganization of the Company, after payment to any preferred stockholders of their liquidation preferences, holders of Common Stock are entitled to share ratably in all remaining assets of the Company.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
As of September 30, 2025, the Company had reserved Common Stock for future issuances as follows:
|
|
|
|
|
|
|
|
|
|
September 30,
2025 |
|
| Common Stock reserved for Contingent Earnout Shares |
15,000,000 |
|
|
| Common Stock reserved for Common Stock Purchase Agreement |
12,000,000 |
|
|
| Common Stock reserved for ATM Facility |
14,874,124 |
|
|
| Common Stock reserved for Option Agreement |
1,073,333 |
|
(2) |
| Exercise of options outstanding under stock plans |
16,537,950 |
|
|
| Options available for issuance under stock plans |
8,037,751 |
|
|
|
|
|
| Warrants to purchase Common Stock |
9,833,910 |
|
|
|
77,357,068 |
|
|
___________________________
(2)Assumes the exercise of the $8,050,000 of shares of Common Stock remaining under the Option as provided for in the Option Agreement at the minimum purchase price of $7.50 per share.
Preferred Stock
The Company’s Second Amended and Restated Certificate of Incorporation provides the Company’s board of directors with the authority to issue preferred stock, par value $0.0001 per share, in one more series and to establish from time to time the number of shares to be included in each such series, by adopting a resolution and filing a certificate of designations. Voting powers, designations, preferences and relative, participating, optional, special and other rights shall be stated and expressed in such resolutions and the certificate of designations. There were 20,000,000 shares designated as preferred stock and none were outstanding as of September 30, 2025 and December 31, 2024.
Warrants
The Company had the following Common Stock warrants outstanding:
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30,
2025 |
|
December 31,
2024 |
|
|
|
|
| Legacy Humacyte Common Stock Warrants |
411,006 |
|
|
411,006 |
|
| Private Placement Warrants |
177,500 |
|
|
177,500 |
|
| Public Warrants |
5,000,000 |
|
|
5,000,000 |
|
| October 2024 RDO Warrants |
2,840,910 |
|
|
5,681,820 |
|
| November 2024 RDO Warrants |
1,404,494 |
|
|
2,808,988 |
|
| Total Common Stock Warrants |
9,833,910 |
|
|
14,079,314 |
|
On April 5, 2025, October 2024 RDO Warrants to purchase 2,840,910 shares of Common Stock expired. On May 14, 2025, November 2024 RDO Warrants to purchase 1,404,494 shares of Common Stock expired. Other than as disclosed above, there were no issuances, exercises or expirations of warrants during the nine months ended September 30, 2025 or September 30, 2024. On October 6, 2025, in connection with the October 2025 Registered Direct Offering, the Company issued the October 2025 RDO Warrants to purchase 28,436,018 shares of Common Stock. See Note 14 — Subsequent Events for additional information.
Legacy Humacyte Common Stock Warrants
In connection with the Company’s former loan agreement with SVB, in 2021 the Company granted warrants to the lenders to purchase up to 411,006 shares of Common Stock at an exercise price of $10.28 per share (such warrants, “Legacy Humacyte Common Stock Warrants”). The Company recognized the fair value of the warrants within stockholders’ equity using a Black-Scholes valuation model, as the settlement of the warrants is indexed to the Common Stock.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Public and Private Placement Warrants
In connection with the Merger, the Company assumed 5,000,000 publicly-traded warrants (“Public Warrants”) and 177,500 private placement warrants issued to AHAC Sponsor LLC (the “Sponsor”), Oppenheimer & Co. Inc. and Northland Securities, Inc., in connection with AHAC’s initial public offering (“Private Placement Warrants” and, together with the Public Warrants, the “Common Stock Warrants”). The Common Stock Warrants entitle the holder to purchase one share of Common Stock at an exercise price of $11.50 per share. The Company evaluated the Common Stock Warrants to determine the appropriate financial statement classification upon the consummation of the Merger. The Common Stock Warrants are not mandatorily redeemable and are considered to be freestanding instruments as they are separately exercisable into Common Stock. As such, the Common Stock Warrants were not classified as liabilities under FASB ASC Topic 480, Distinguishing Liabilities from Equity. The Company then evaluated the Common Stock Warrants under FASB ASC Topic 815, Derivatives and Hedging.
Public Warrants
The Public Warrants are publicly traded and are exercisable for cash unless certain conditions occur, such as the failure to have an effective registration statement related to the shares issuable upon exercise or redemption by the Company under certain conditions, at which time the Public Warrants may be eligible for a cashless exercise. The Public Warrants may only be exercised for a whole number of shares and will expire five years after the completion of the Merger.
The Public Warrants are considered to be “indexed to the Company’s own stock.” The agreement provides that in the event of a tender or exchange offer made to and accepted by holders of more than 50% of the outstanding shares of Common Stock, all holders of the Common Stock Warrants (both the Public Warrants and the Private Placement Warrants) would be entitled to receive cash for all of their Common Stock Warrants. As the Company has a single class of Common Stock, a qualifying cash tender offer of more than 50% of the shares of Common Stock will always result in a change in control and would not preclude permanent equity classification of the Public Warrants. Based on this evaluation, the Company concluded that the Public Warrants met the criteria to be classified within stockholders’ equity. The Public Warrants were initially recognized as equity on the Closing Date at a fair value of $2.80 per share.
Private Placement Warrants
The Private Placement Warrants are non-redeemable for cash so long as they are held by the initial purchasers or their permitted transferees. If the Private Placement Warrants are held by someone other than the initial purchasers or their permitted transferees, the Private Placement Warrants are redeemable by the Company and exercisable by such holders on the same basis as the Public Warrants.
The agreement governing the Common Stock Warrants includes a provision, the application of which could result in a different settlement value for the Private Placement Warrants depending on their holder. Because the holder of an instrument is not an input into the pricing of a fixed-for-fixed option on the Common Stock, the Private Placement Warrants are not considered to be “indexed to the Company’s own stock” and therefore are not classified in stockholders’ equity. As the Private Placement Warrants met the definition of a derivative, the Company recorded these warrants as liabilities on the condensed consolidated balance sheet at fair value, with subsequent changes in their respective fair values recognized in the condensed consolidated statements of operations and comprehensive loss at each reporting date.
The Private Placement Warrants were initially recognized as a liability on the Closing Date, at a fair value of $0.6 million. See Note 3 — Fair Value Measurements for a summary of the change in the fair value of the Private Placement Warrants during the three and nine months ended September 30, 2025 and 2024. The remeasurement of the Private Placement Warrant liability to a fair value of $0.1 million as of September 30, 2025 from $0.4 million as of December 31, 2024 resulted in an insignificant non-cash gain for the three months ended September 30, 2025, and a gain of $0.3 million for the nine months ended September 30, 2025, compared to non-cash losses of $0.1 million and $0.3 million for the three and nine months ended September 30, 2024, respectively. The remeasurement of the Private Placement Warrant liability is classified within Change in fair value of derivatives in the condensed consolidated statements of operations and comprehensive loss.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
The Private Placement Warrants were valued using the following assumptions under the Monte Carlo simulation value model:
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30,
2025 |
|
December 31,
2024 |
| Market price of public stock |
$ |
1.74 |
|
$ |
5.05 |
| Exercise price |
$ |
11.50 |
|
$ |
11.50 |
| Expected term (years) |
0.90 |
|
1.65 |
| Expected share price volatility |
188.6 |
% |
|
128.0 |
% |
| Risk-free interest rate |
3.68 |
% |
|
4.22 |
% |
| Estimated dividend yield |
0 |
% |
|
0 |
% |
Registered Direct Offering Warrants
Collectively, the October 2024 RDO Warrants and the November 2024 RDO Warrants are referred to as the “Registered Direct Offering Warrants”. The Company evaluated the Registered Direct Offering Warrants to determine the appropriate financial statement classification upon issuance. The Registered Direct Offering Warrants are not mandatorily redeemable and are considered to be freestanding instruments as they are separately exercisable into shares of Common Stock. The Company is not required to transfer assets to settle the warrants, except potentially as a result of a fundamental transaction (defined in the Registered Direct Offering Warrants to include various merger and change in control transactions). As such, the Registered Direct Offering Warrants were not classified as liabilities under ASC 480. The Company then evaluated the Registered Direct Offering Warrants under ASC 815.
The agreements governing the Registered Direct Offering Warrants include a provision, which, if applied, could result in a different settlement value for the Registered Direct Offering Warrants. The Registered Direct Offering Warrants cannot be exercised if after the exercise the warrant holder would own more than 4.99% of the outstanding Common Stock (the “Beneficial Ownership Limitation”). The holder may elect to increase the Beneficial Ownership Limitation to 9.99%. The Beneficial Ownership Limitation constitutes an exercise contingency in that it limits or defers the exercise of some of the Registered Direct Offering Warrants if the limitation would otherwise be reached, depending on the number of shares of Common Stock that are outstanding. The exercise contingency is not based on either an observable market or an observable index, so it does not preclude the Registered Direct Offering Warrants from being considered indexed to the Company’s own stock.
In the event of a fundamental transaction, if the Registered Direct Offering Warrant holder elects to have the Company repurchase the applicable Registered Direct Offering Warrant(s), the Black-Scholes value of the Registered Direct Offering Warrant is calculated with adjustments to the stock price and volatility of the shares of Common Stock on the market. These are not standard adjustments in determining the fair value of an option. As the volatility adjustment provision violates the fixed-for-fixed rule, the Registered Direct Offering Warrants are not considered to be “indexed to the Company’s own stock” and therefore are not classified in stockholders’ equity. As the Registered Direct Offering Warrants meet the definition of a derivative, the Company recorded these warrants as liabilities on the consolidated balance sheet at fair value, with subsequent changes in their respective fair values recognized in the consolidated statements of operations and comprehensive loss at each reporting date.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
The Registered Direct Offering Warrants holders are entitled to participate in dividends and other distributions of assets to the same extent as if the holders held the number of shares of Common Stock issuable upon exercising the Registered Direct Offering Warrants. Therefore, the Registered Direct Offering Warrants are considered participating securities and are included in the computation of net income per share pursuant to the two-class method. In applying the two-class method, during periods of net income, earnings are allocated to both Common Stock and participating securities based on their respective weighted-average shares outstanding for the period. During periods of net loss, no effect is given to participating securities since they do not share in the losses of the Company.
October 2024 RDO Warrants
The October 2024 RDO Warrants were immediately exercisable. October 2024 RDO Warrants to purchase 2,840,910 shares of Common Stock had an exercise price of $5.28 per share, and expired 180 days from the date of issuance unexercised. The remaining October 2024 RDO Warrants to purchase 2,840,910 shares of Common Stock have an exercise price of $5.28 per share, and will expire 1,640 days from the date of issuance.
The October 2024 RDO Warrants were initially recognized as a liability on the issuance date at a fair value of $15.2 million and were subsequently remeasured to a fair value of $12.4 million as of December 31, 2024. The remeasurement of the October RDO Warrants liability to a fair value of $2.1 million as of September 30, 2025 resulted in a non-cash gain of $0.7 million and a non-cash gain of $10.4 million for the three and nine months ended September 30, 2025, respectively, classified within Change in fair value of derivatives in the condensed consolidated statements of operations and comprehensive loss. See Note 3 — Fair Value Measurements for a summary of the change in the fair value of the October 2024 RDO Warrants during the three and nine months ended September 30, 2025.
The October 2024 RDO Warrants were valued using the following assumptions under the Black-Scholes valuation model:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
180 Day Warrants |
|
1,640 Day Warrants |
|
|
|
|
|
|
|
December 31,
2024 |
|
September 30,
2025 |
|
December 31,
2024 |
|
|
|
|
Market price of public stock |
|
|
$ |
5.05 |
|
|
$ |
1.74 |
|
|
$ |
5.05 |
|
|
|
|
|
Exercise price |
|
|
$ |
5.28 |
|
|
$ |
5.28 |
|
|
$ |
5.28 |
|
|
|
|
|
Expected term (years) |
|
|
0.27 |
|
3.52 |
|
4.27 |
|
|
|
|
Expected share price volatility |
|
|
106.7 |
% |
|
93.3 |
% |
|
88.4 |
% |
|
|
|
|
Risk-free interest rate |
|
|
4.27 |
% |
|
3.58 |
% |
|
4.25 |
% |
|
|
|
|
Estimated dividend yield |
|
|
0 |
% |
|
0 |
% |
|
0 |
% |
|
|
|
|
November 2024 RDO Warrants
The November 2024 RDO Warrants were immediately exercisable. November 2024 RDO Warrants to purchase 1,404,494 shares of Common Stock had an exercise price of $5.34 per share, and expired 180 days from the date of issuance unexercised. The remaining November 2024 RDO Warrants to purchase 1,404,494 shares of Common Stock have an exercise price of $5.34 per share, and will expire 1,640 days from the date of issuance.
The November 2024 RDO Warrants were initially recognized as a liability on the issuance date at a fair value of $6.1 million and were subsequently remeasured to a fair value of $6.4 million as of December 31, 2024. The remeasurement of the November 2024 RDO Warrants liability to a fair value of $1.1 million as of September 30, 2025 resulted in a non-cash gain of $0.4 million and a non-cash gain of $5.4 million for the three and nine months ended September 30, 2025, respectively, classified within Change in fair value of derivatives in the condensed consolidated statements of operations and comprehensive loss. See Note 3 — Fair Value Measurements for a summary of the change in the fair value of the November 2024 RDO Warrants during the three and nine months ended September 30, 2025.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
The November 2024 RDO Warrants were valued using the following assumptions under the Black-Scholes valuation model:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
180 Day Warrants |
|
1,640 Day Warrants |
|
|
|
|
|
|
|
December 31,
2024 |
|
September 30,
2025 |
|
December 31,
2024 |
|
|
|
|
Market price of public stock |
|
|
$ |
5.05 |
|
|
$ |
1.74 |
|
|
$ |
5.05 |
|
|
|
|
|
Exercise price |
|
|
$ |
5.34 |
|
|
$ |
5.34 |
|
|
$ |
5.34 |
|
|
|
|
|
Expected term (years) |
|
|
0.37 |
|
3.62 |
|
4.37 |
|
|
|
|
Expected share price volatility |
|
|
106.7 |
% |
|
93.3 |
% |
|
88.4 |
% |
|
|
|
|
Risk-free interest rate |
|
|
4.22 |
% |
|
3.59 |
% |
|
4.25 |
% |
|
|
|
|
Estimated dividend yield |
|
|
0 |
% |
|
0 |
% |
|
0 |
% |
|
|
|
|
On October 6, 2025, in connection with the October 2025 Registered Direct Offering, the Company issued the October 2025 RDO Warrants to purchase 28,436,018 shares of Common Stock. See Note 14 — Subsequent Events for additional information.
Contingent Earnout Liability
Following the closing of the Merger (the “Closing”), former holders of Legacy Humacyte common and preferred shares are eligible to receive up to 15,000,000 additional shares of Common Stock (the “Contingent Earnout Shares”) in the aggregate, in two equal tranches of 7,500,000 shares of Common Stock per tranche. The first and second tranches are issuable if the closing volume weighted average price (“VWAP”) per share of Common Stock quoted on The Nasdaq Stock Market LLC (“Nasdaq”) (or the exchange on which the shares of Common Stock are then listed), is greater or equal to $15.00 and $20.00, respectively, over any 20 trading days within any 30 consecutive trading day period.
Upon the Closing, the contingent obligation to issue Contingent Earnout Shares was accounted for as a liability (“Contingent Earnout Liability”) because the triggering events that determine the number of Contingent Earnout Shares required to be issued include events that are not solely indexed to the Common Stock. The Contingent Earnout Shares are subsequently remeasured at each reporting date with changes in fair value recorded as a component of other income (expense), net in the condensed consolidated statements of operations and comprehensive loss. The estimated fair value of the total Contingent Earnout Shares at the Closing on August 26, 2021 was $159.4 million based on a Monte Carlo simulation valuation model using a distribution of potential outcomes on a monthly basis over a 10-year period using the most reliable information available.
See Note 3 — Fair Value Measurements for a summary of the change in the fair value of the Contingent Earnout Liability during the three and nine months ended September 30, 2025 and 2024. The remeasurement of the Contingent Earnout Liability to a fair value of $21.8 million as of September 30, 2025 from a fair value of $71.0 million as of December 31, 2024, resulted in a non-cash gain of $4.9 million and a non-cash gain of $49.2 million for the three and nine months ended September 30, 2025, respectively, compared to non-cash losses of $8.5 million and $38.7 million for the three and nine months ended September 30, 2024, respectively. The remeasurement of the Contingent Earnout Liability is classified within Change in fair value of Contingent Earnout Liability in the condensed consolidated statements of operations and comprehensive loss. The assumptions utilized in the calculations of fair value were based on the achievement of certain stock price milestones, including the current Common Stock price, expected volatility, risk-free rate, expected term and expected dividend yield.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Assumptions used in the valuations are described below:
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30,
2025 |
|
December 31,
2024 |
| Current stock price |
$ |
1.74 |
|
$ |
5.05 |
| Expected share price volatility |
85.8 |
% |
|
84.8 |
% |
| Risk-free interest rate |
4.16 |
% |
|
4.58 |
% |
| Estimated dividend yield |
0 |
% |
|
0 |
% |
| Expected term (years) |
10.00 |
|
10.00 |
10. Stock-based Compensation
At Closing, the 2021 Long-Term Incentive Plan, (the “2021 Plan”), and the 2021 Employee Stock Purchase Plan (the “ESPP”) became effective. Under the 2021 Plan, the Company can grant non-statutory stock options, incentive stock options, stock appreciation rights, restricted stock, restricted stock units, unrestricted stock, performance awards and other forms of awards. Under the ESPP, when and if implemented, eligible employees will be permitted to purchase shares of Common Stock at the lower of 85% of the closing trading price per share of Common Stock on the first day of the offering or 85% of the closing trading price per share on the exercise date, which will occur on the last day of each offering.
The 2021 Plan and ESPP provide that on January 1 of each year, the 2021 Plan and the ESPP reserve will automatically increase in an amount equal to the lesser of (a) 5% and 1%, respectively, of the number of shares of Common Stock outstanding on December 31 of the preceding year and (b) a number of shares of Common Stock determined by the Company’s board of directors. The 2021 Plan share reserve automatically increased on January 1, 2024 by 5,183,686 shares, which was equivalent to 5% of the number of shares of Common Stock outstanding on December 31, 2023. The 2021 Plan share reserve automatically increased on January 1, 2025 by 6,501,375 shares, which was equivalent to 5% of the number of shares of Common Stock outstanding on December 31, 2024. Since the inception of the ESPP, the Company’s board of directors determined that there would be no automatic increase in the number of shares reserved under the ESPP. Effective April 17, 2025, the Company’s board of directors reduced the number of shares reserved under the ESPP to zero shares of Common Stock. As of September 30, 2025, 8,037,751 shares of Common Stock were available under the 2021 Plan.
Prior to the Closing, Legacy Humacyte had two equity incentive plans, the 2015 Omnibus Incentive Plan, as amended (the “2015 Plan”), and the 2005 Stock Option Plan (the “2005 Plan”). As a result of the Merger, following the Closing, no further awards were granted under either the 2015 Plan or the 2005 Plan. All awards previously granted and outstanding as of the effective date of the Merger were adjusted to reflect the impact of the Merger, but otherwise retained their original terms. The shares underlying any award granted under the 2021 Plan or the 2015 Plan that are forfeited, cancelled or reacquired by the Company prior to vesting, that expire or that are paid out in cash rather than shares will become available for grant and issuance under the 2021 Plan. As of September 30, 2025, 13,200,091 and 3,337,859 shares of Common Stock remain reserved for outstanding options issued under the 2021 Plan and the 2015 Plan, respectively, and there were no shares of Common Stock outstanding under the 2005 Plan. The Company has sufficient authorized and unissued shares to issue Common Stock in satisfaction of any outstanding awards and any awards available for grant under the 2021 Plan.
The Company’s stock option plans allow for the grant of awards that the Company believes aid in aligning the interests of award recipients with those of its stockholders. The Company’s board of directors or compensation committee determines the specific terms of equity incentive grants, including the exercise price per share and vesting period for option awards. Option awards are granted with an exercise price equal to the fair market value of the Common Stock at the date of grant.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
The Company has granted options that include either a service-based or performance-based vesting condition, or both, and a 10-year contractual term. The service-based vesting condition for the plans is generally satisfied over 36 to 48 months from the date of grant. The performance-based vesting conditions are satisfied upon the attainment of certain product development milestones. The Company recognizes stock-based compensation expense based on the grant date fair value of the awards measured using the Black-Scholes option pricing model. Compensation expense related to awards with service-based vesting conditions is recognized on a straight-line basis over the requisite service period. Option valuation models, including the Black-Scholes option-pricing model, require the input of highly subjective assumptions, and changes in the assumptions used can materially affect the grant-date fair value of an award. These assumptions include the risk-free rate of interest, expected dividend yield, expected volatility, the expected term of the award, and the fair value of the underlying Common Stock on the date of grant. Forfeitures are accounted for as they occur.
Compensation expense related to awards with performance-based vesting conditions is recognized over the requisite service period using the accelerated attribution method to the extent achievement of the performance-based condition is probable. The Company does not recognize compensation expense related to awards with performance-based vesting conditions until it is probable that the performance-based vesting condition will be achieved. Forfeitures are accounted for as they occur.
Option awards under the Company’s option plans generally provide for accelerated vesting of the unvested portions of any option award in the event of an involuntary termination, as such term is defined in the relevant stock option agreement, of a grantee’s employment during the period that commences 30 days prior to the effective date of a corporate transaction and that ends 12 months following the effective date of such transaction. Additionally, the Company’s board of directors may, in its sole discretion, accelerate the vesting of any unvested stock options in the event of a corporate transaction.
The Company estimated the fair value of the stock options on the date of grant using the following assumptions in the Black-Scholes option-pricing model:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
|
2025 |
|
2024 |
|
2025 |
|
2024 |
|
|
|
|
|
|
|
|
| Estimated dividend yield |
0 |
% |
|
0 |
% |
|
0 |
% |
|
0 |
% |
| Expected share price volatility (weighted average and range, if applicable) |
95.7%
95.7% to 95.7%
|
|
92.8% |
|
92.8%
92.2% to 95.7%
|
|
91.8%
90.8% to 92.8%
|
| Risk-free interest rate (weighted average and range, if applicable) |
3.76%
3.76% to 3.76%
|
|
3.54% |
|
4.38%
3.76% to 4.45%
|
|
4.12%
3.54% to 4.41%
|
| Expected term of options (in years) |
6.25 |
|
6.25 |
|
6.25 |
|
6.25 |
•Fair Value of Common Stock. The fair value of the Common Stock has been determined based on the closing price of the shares on Nasdaq.
•Expected Term. The expected term represents the period that stock options are expected to be outstanding. The Company calculated the expected term using the simplified method for options, which is available where there is insufficient historical data about exercise patterns and post-vesting employment termination behavior. The simplified method is based on the vesting period and the contractual term for each grant, or for each vesting-tranche for awards with graded vesting. The mid-point between the vesting date and the maximum contractual expiration date is used as the expected term under this method. For awards with multiple vesting-tranches, the times from grant until the mid-points for each of the tranches may be averaged to provide an overall expected term.
•Expected Volatility. The expected volatility was determined based on a blended approach using the historical share volatility of the Common Stock and that of several publicly traded peer companies over a period of time equal to the expected term of the options, as the Company has a limited trading history. For purposes of identifying these peer companies, the Company considered the industry, stage of development, size and financial leverage of potential comparable companies.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
•Risk-Free Interest Rate. The risk-free interest rate was based on the yields of U.S. Treasury zero-coupon securities with maturities similar in duration to the expected term of the options.
•Expected Dividend Yield. The Company has not paid dividends on its Common Stock nor does it expect to pay dividends in the foreseeable future. Accordingly, the Company has estimated the dividend yield to be zero.
The following table shows a summary of stock-based compensation expense included in the condensed consolidated statements of operations and comprehensive loss for the three and nine months ended September 30, 2025 and 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| ($ in thousands) |
|
2025 |
|
2024 |
|
2025 |
|
2024 |
| Research and development |
|
$ |
989 |
|
|
$ |
635 |
|
|
$ |
3,038 |
|
|
$ |
2,110 |
|
| Selling, general and administrative |
|
1,521 |
|
|
917 |
|
|
4,393 |
|
|
2,333 |
|
| Total |
|
$ |
2,510 |
|
|
$ |
1,552 |
|
|
$ |
7,431 |
|
|
$ |
4,443 |
|
Approximately $0.1 million of stock-based compensation was capitalized to inventory as of September 30, 2025. As of September 30, 2025, unrecognized stock-based compensation cost for options was $26.8 million and is expected to be recognized over a weighted-average period of 2.7 years.
A summary of option activity under the Company’s stock option plans during the nine months ended September 30, 2025 is presented below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Shares |
|
Weighted
Average Exercise
Price Per Share |
|
Weighted
Average
Remaining
Contractual Term
(years) |
|
Aggregate
Intrinsic Value
(in thousands) |
Options outstanding at December 31, 2024 |
12,274,139 |
|
|
$ |
4.96 |
|
|
7.7 |
|
$ |
16,202 |
|
| Granted |
7,137,287 |
|
|
$ |
4.07 |
|
|
|
|
|
| Exercised |
(15,514) |
|
|
$ |
3.63 |
|
|
|
|
|
| Forfeited |
(2,857,962) |
|
|
$ |
4.20 |
|
|
|
|
|
Options outstanding at September 30, 2025 |
16,537,950 |
|
|
$ |
4.71 |
|
|
7.8 |
|
$ |
5 |
|
Vested and exercisable, September 30, 2025 |
6,677,814 |
|
|
$ |
5.89 |
|
|
6.1 |
|
$ |
— |
|
Vested and expected to vest, September 30, 2025 |
16,537,950 |
|
|
$ |
4.71 |
|
|
7.8 |
|
$ |
5 |
|
11. Income Taxes
The Company’s tax provision for interim periods is determined using an estimate of its annual effective tax rate, adjusted for discrete items, if any, that arise during the period. Each quarter, the Company updates its estimate of the annual effective tax rate and, if the estimated annual effective tax rate changes, the Company makes a cumulative adjustment in such period. No such adjustment was made as of September 30, 2025. The Company’s effective federal and state tax rate for the three and nine months ended September 30, 2025 and 2024 was 0%, primarily as a result of accumulating net operating losses for the fiscal year to date offset by the increase in the valuation allowance against the related deferred tax asset.
The Company did not record any income tax expense or benefit during the three and nine months ended September 30, 2025 and 2024. The Company has a net operating loss and has provided a valuation allowance against net deferred tax assets due to uncertainties regarding the Company’s ability to realize these assets. All losses before income taxes arose in the United States.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
On July 4, 2025, the One Big Beautiful Bill Act (“OBBBA”) was enacted. OBBBA amends the U.S. tax law including provisions related to bonus depreciation, domestic research and development expenses, and limitations on interest deductions. The OBBBA did not have a material impact to the Company's condensed consolidated financial statements and is not expected to have a material impact on the Company's year-end consolidated financial statements.
12. Commitments and Contingencies
Patent License Agreements
Duke University
In March 2006, the Company entered into a license agreement with Duke University (“Duke”), which was subsequently amended in 2011, 2014, 2015, 2018, 2019 and 2022 (as amended, the “Duke License Agreement”). Under the Duke License Agreement, Duke granted the Company a worldwide, exclusive, sublicensable license to certain patents related to decellularized tissue engineering, referred to as the patent rights, as well as a non-exclusive license to use and practice certain know-how related to the patent rights. The relevant licensed patent on decellularization of tissue expired in 2021. The Company has agreed to use commercially reasonable efforts to develop, register, market and sell products utilizing the patent rights, referred to as the licensed products. Any services provided to a third party utilizing licensed products are referred to as licensed services. The Company has also agreed to meet certain benchmarks in its development efforts, including as to development events, clinical trials, regulatory submissions and marketing approval, within specified timeframes. Under the Duke License Agreement, Duke retains the right to use the patent rights for its own educational and research purposes, and to provide the patent rights to other non-profit, governmental or higher-learning institutions for non-commercial purposes without paying royalties or other fees.
In connection with the Company’s entry into the Duke License Agreement, the Company granted equity consideration to Duke in the form of 52,693 shares of Common Stock. Under the Duke License Agreement, the Company also agreed to pay Duke:
•a low single-digit percentage royalty on eligible sales of licensed products and licensed services, plus a low double-digit percentage of any sublicensing revenue;
•an annual minimum royalty beginning in 2012, which increases in the calendar year immediately following the first commercial sale of licensed products or licensed services (whichever occurs first); and
•an additional amount in license fees, as certain milestones are met.
The Duke License Agreement remains effective until the later of (i) the last of the patent rights expires or (ii) four years after the Company’s first commercial sale, unless terminated earlier. Either party may terminate the agreement for fraud, willful misconduct or illegal conduct, or uncured material breach. Duke may terminate the agreement if the Company becomes insolvent. Duke may also terminate the license, convert the license into a non-exclusive license or seek assignment of any sublicense if the Company fails to reach diligence milestones within the applicable time period. If the Company abandons any claim, patent or patent application, its rights under the license with respect to such patent rights will be terminated in the territory in which the Company abandons such rights. The Company may terminate the license agreement unilaterally upon three months’ prior notice to Duke. The Company agrees to indemnify Duke against certain third-party claims.
In December 2023, the Company filed a BLA with the FDA for urgent arterial repair following extremity vascular trauma when synthetic graft is not indicated, and autologous vein use is not feasible. Based on the achievement of this milestone under the Duke License Agreement, the Company paid a $0.5 million license fee to Duke during the first quarter of 2024.
In December 2024, the FDA approved the Company’s BLA for urgent arterial repair following extremity vascular trauma when autologous vein use is not feasible. Based on the achievement of this milestone under the Duke License Agreement, the Company recorded $0.5 million of license expense payable in accrued expenses in the Company’s condensed consolidated balance sheets as of both September 30, 2025 and December 31, 2024. Other payments to Duke under the Duke License Agreement were immaterial during the periods presented.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Yale University
In August 2019, the Company entered into a license agreement with Yale University (“Yale”) that granted the Company a worldwide license to the patents related to the biovascular pancreas (“BVP”) product candidate (the “BVP License Agreement”). The license granted under the BVP License Agreement is exclusive in the field of engineered vascular tissues that deliver pancreatic islet cells to patients, except that it is subject to Yale’s non-exclusive right, on behalf of itself and all other non-profit academic institutions, to use the licensed products for research, teaching, and other non-commercial purposes. The Company has agreed to pay to Yale an annual maintenance fee, increasing between the first and fourth anniversaries of the BVP License Agreement up to a maximum of less than $0.1 million per year for this license.
In August 2019, the Company entered into a license agreement with Yale that granted the Company a worldwide license to the patents related to tubular prostheses (the “Tubular Prosthesis License Agreement”). The license granted under the Tubular Prosthesis License Agreement is exclusive in the field of engineered urinary conduits, engineered tracheas/airways, and engineered esophagi, except that it is subject to Yale’s non-exclusive right, on behalf of itself and all other non-profit academic institutions, to use the licensed products for research, teaching, and other non-commercial purposes. The Company has agreed to pay to Yale an annual maintenance fee, increasing between the first and fourth anniversaries of the Tubular Prosthesis License Agreement up to a maximum of less than $0.1 million per year for this license.
The Company has agreed to use reasonable commercial efforts to develop and commercialize the licensed patents and any licensed products and methods, and to use reasonable efforts to make the licensed products available to patients in low and low-middle income countries. The Company is also obligated to provide Yale periodically an updated and revised copy of its plan for each license, which must indicate progress of its development and commercialization. The Company may also sublicense the Company’s rights without Yale’s prior written consent, but such sublicense is subject to certain conditions.
In connection with its entry into the Tubular Prosthesis License Agreement, the Company paid Yale upfront cash fees. The Company has also agreed to pay Yale:
•annual maintenance fees, increasing annually until the fifth anniversary for the BVP License Agreement and until the fourth anniversary for the Tubular Prostheses License Agreement up to a maximum of less than $0.1 million per year;
•milestone payments upon achievement of certain regulatory and commercial milestones of $0.2 million and $0.6 million, respectively;
•a low single-digit percentage royalty on worldwide net sales, subject to reductions for third-party license fees; and
•a low double-digit percentage of sublicensing income.
If the Company or any of its future sublicensees bring a patent challenge against Yale or assists another party in bringing a patent challenge against Yale, the license fees described above will be subject to certain increases and penalties.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
The BVP License Agreement and Tubular Prosthesis License Agreement expire on a country-by-country basis on the date on which the last of the patents in such country expires, lapses or is declared invalid. Yale may terminate the BVP License Agreement and Tubular Prosthesis License Agreement if the Company fails to (i) provide written diligence reports, (ii) provide commercially reasonable diligence plans, (iii) implement the plans in accordance with the obligations under the agreements, or (iv) reach certain research and development milestones within the scheduled timeframe set forth in the agreements; however, any such termination right would be limited in scope to the country to which such failure relates. Yale may also terminate for the Company’s non-payment, uncured material breach, failure to obtain adequate insurance, bringing or assisting in bringing of a patent challenge against Yale, abandonment of the research and development of the Company’s products or insolvency. The Company may terminate the BVP License Agreement and Tubular Prosthesis License Agreement (i) on 90 days’ prior written notice to Yale, provided the Company is not in breach of the license agreements and has made all required payments to Yale thereunder and (ii) on written notice to Yale following an uncured material breach. With respect to the BVP License Agreement, the Company’s rights under the agreement will also terminate automatically with respect to a patent application or patent within the licensed patents in a specified country if, upon receipt of written notice from Yale, the Company does not agree to pay the patent filing, prosecution and maintenance fees incurred by Yale for such patent applications or patents in the specified country. Under certain circumstances, Yale may, at its option, convert the exclusive licenses to non-exclusive licenses if the Company declines to initiate certain infringement or interference proceedings with respect to the licensed patents. The Company has agreed to indemnify Yale against certain third-party claims. Payments to Yale under the BVP License Agreement and Tubular Prosthesis License Agreement were immaterial during the periods presented.
JDRF Agreement
On April 1, 2023, the Company entered into an Industry Discovery and Development Partnership Agreement with Breakthrough T1D (f/k/a JDRF International) (“JDRF,” and such agreement, the “JDRF Agreement”) to further develop and perform preclinical testing of the BVP, a product candidate designed to deliver insulin-producing islets using the ATEV as a means of treating patients with type 1 diabetes. According to the terms of the JDRF Agreement, JDRF will provide funding up to $0.8 million (“JDRF Award”) based on the achievement of certain research and development milestones related to the Company’s BVP. The JDRF Agreement refers to the total cumulative payments the Company has received from JDRF as of any point in time as the “Actual Award.”
The Company received the first milestone payment of $80 thousand in April 2023 upon execution of the JDRF Agreement. In May 2024, the Company received the second milestone payment of $90 thousand and the third milestone payment of $150 thousand, based upon the achievement of certain research and development milestones specified in the JDRF Agreement. As of September 30, 2025, the Actual Award totaled $320 thousand.
In accordance with the JDRF Agreement, the Company has agreed to pay JDRF:
•a one-time royalty in an amount equal to four times the Actual Award, to be paid in three equal installments following the first commercial sale of any product containing the Company’s technology identified in the JDRF Agreement;
•an additional royalty equal to the Actual Award at a specified payment date after net sales exceed $250 million; and
•in the event of a license, sale or transfer of the Company’s rights to the product’s technology identified in the JDRF Agreement or a change of control transaction, a payment equal to 10% of any license or purchase price payments received by the Company up to an amount equal to four times the Actual Award (the “Royalty Cap”), less any previous royalty payments paid towards the Royalty Cap (the “Disposition Payment”). The Disposition Payment was determined to meet the definition of an embedded derivative requiring bifurcation and is measured at fair value each reporting period with changes in fair value recognized as other income (expense) in the condensed consolidated statements of operations and comprehensive loss, classified in Change in fair value of derivatives.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
The JDRF Agreement expires on the date on which the Company has paid all of the royalty payments described above. Either party may terminate the JDRF Agreement for cause by providing the other party with written notice and allowing the other party 30 days to cure such breach. JDRF may terminate the JDRF Agreement without cause by providing 90 days’ notice to the Company at any time after April 1, 2024. Royalties based on previously received milestone payments would remain due after a termination by JDRF without cause. As the royalties are contractually required to be paid upon achieving these milestones even after the termination of the JDRF Agreement, the Company determined that the JDRF Actual Award payments are to be classified as a liability in the condensed consolidated balance sheets. The JDRF liability related to the Actual Award payments is reported at amortized cost and is included in other long-term liabilities in the condensed consolidated balance sheets. As of September 30, 2025 and December 31, 2024, the carrying value of the JDRF liability was $0.4 million and $0.3 million, respectively. There was an insignificant amount of interest expense related to the JDRF liability recorded during the three and nine months ended September 30, 2025 and September 30, 2024.
Workforce Reduction
On April 28, 2025, the Company implemented a cost reduction action to reduce its workforce by 30 employees, cease recruitment of additional planned new hires, and reduce other operating expenses. The Company undertook these cost reductions to improve cash runway and to better align the Company’s organizational structure with its top business objectives. Employee severance costs associated with this action were $0.7 million, which were expensed during the second quarter of 2025. Employee severance costs included $0.6 million recognized in research and development expenses and $0.1 million recognized in selling, general and administrative expenses on the Company’s condensed consolidated statements of operations and comprehensive income (loss). Of the total severance costs, $0.6 million were paid during the second quarter of 2025. The remaining accrued severance obligation was less than $0.1 million as of September 30, 2025 and is expected to be paid during the fourth quarter of 2025. There are no further costs associated with this cost reduction action expected to be incurred in the future.
Legal Matters
From time to time, the Company may be involved in various lawsuits, claims, assessments and proceedings, including securities, commercial, intellectual property, product liability, contractual, governmental, employment or other matters that arise in the normal course of business. The Company accrues a liability for a contingency when management believes information available prior to the issuance of the consolidated financial statements indicates it is probable a loss has been incurred as of the date of the consolidated financial statements and the amount of loss can be reasonably estimated. The Company adjusts its accruals to reflect the impact of negotiations, settlements, rulings, advice of legal counsel and other information and events pertaining to a particular case. Legal costs are expensed as incurred.
On November 18, 2024, James A. Cutshall filed a putative class action lawsuit, captioned Cutshall v. Humacyte, Inc., et al., No. 1:24-cv-00954 (the “Securities Litigation”), against the Company and certain of the Company’s officers in the United States District Court for the Middle District of North Carolina. The complaint in the Securities Litigation (the “Initial Complaint”) asserts claims under Sections 10(b) and 20(a) of the Exchange Act on behalf of a putative class of persons and entities that purchased or otherwise acquired securities of the Company between May 10, 2024 and October 17, 2024, based on allegations that the defendants made or were responsible for false or misleading statements and omissions related to the BLA for the vascular trauma indication and to alleged deficiencies at the Company’s Durham, North Carolina manufacturing facility. The Initial Complaint seeks a variety of relief, including unspecified compensatory damages, attorneys fees and costs. On January 31, 2025, the court appointed co-lead plaintiffs. On May 22, 2025, the co-lead plaintiffs filed the amended complaint in the Securities Litigation. The amended complaint expands the putative class to include persons and entities that purchased or otherwise acquired securities of the Company between August 14, 2023 and March 25, 2025. It alleges that the defendants made or were responsible for false or misleading statements and omissions related to the safety of Symvess, alleged deficiencies at the Company’s Durham, North Carolina manufacturing facility, and the Company’s financial condition and liquidity. On July 25, 2025, defendants moved to dismiss the amended complaint in its entirety and with prejudice. The court has yet to rule on the motion, which remains pending.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
On January 7 and 10, 2025, putative stockholders of the Company filed two verified stockholder derivative actions in the United States District Court for the Middle District of North Carolina, captioned Silva v. Sebelius, et al., No. 1:25-cv-00005 (the “Silva Action”) and Misko v. Niklason, et al., No. 1:25-cv-00028 (the “Misko Action”). Each of these derivative actions was brought on behalf of the Company against certain of its current or former directors and officers, as well as Ayabudge LLC. The complaints in each action assert claims for violations of Section 14(a) of the Exchange Act, breach of fiduciary duty, unjust enrichment, abuse of control, gross mismanagement, and waste of corporate assets, based on a variety of allegations including claims that the defendants are responsible for any damages sustained by the Company as a result of the Securities Litigation. The Misko Action also includes a claim for contribution against certain defendants under Sections 10(b) and 21(d) of the Exchange Act for any liability the Company may sustain as a result of the Securities Litigation. On February 18, 2025, the court issued an order consolidating the Silva Action and the Misko Action (collectively, the “Consolidated Derivative Action”) and staying the defendants’ obligation to respond to any complaint in the Consolidated Derivative Action pending the submission of a proposed scheduling order. On March 11, 2025, the parties entered a joint motion to stay the Consolidated Derivative Action pending final resolution of the Securities Litigation, which the court granted.
On December 19, 2024, the Company received a demand letter (the “2024 Demand Letter”) from a purported stockholder of the Company, demanding that the Board assert claims against certain of the Company’s current or former officers and directors for breach of fiduciary duty, gross mismanagement, corporate waste, unjust enrichment, aiding and abetting, violations of Section 14(a) of the Exchange Act, and insider trading, based on a variety of allegations including claims that the Company’s current and former officers and directors are responsible for any damages sustained by the Company as a result of the Securities Litigation. On January 24, 2025, the Board appointed a demand evaluation committee to evaluate the claims made in the 2024 Demand Letter and report back to the full Board. On February 19, 2025, the purported stockholder who sent the 2024 Demand Letter filed a stockholder derivative action in the United States District Court for the Middle District of North Carolina, captioned Olson v. Niklason, et al., No. 1:25-cv-00123 (the “Olson Action”), alleging that the Company had refused his demand. The complaint in the Olson Action asserts substantive claims and allegations that are substantially similar to those asserted in the Consolidated Derivative Action. On April 22, 2025, the parties filed a joint motion to stay the Olson Action pending final resolution of the Securities Litigation, which the court granted.
On May 19, 2025, the Company received a demand letter (the “2025 Demand Letter”) from a purported stockholder of the Company, making demands and stating allegations substantially similar to those in the 2024 Demand Letter. The letter was referred to the demand evaluation committee for evaluation, and the demand evaluation committee recommended that the board of directors of the Company defer action on the demand until after the resolution of the pending motion to dismiss in the securities class action. The board of directors accepted the demand evaluation committee’s recommendation, and counsel for the demand evaluation committee informed the shareholder of the board of director’s determination to defer action on the demand by letter dated September 24, 2025.
On June 9, 2025, a putative stockholder of the Company filed a verified stockholder derivative action in the United States District Court for the District of Delaware, captioned Dusci v. Bamforth, et al., No. 1:25-cv-00722 (the “Dusci Action”). The complaint in the Dusci Action asserts substantive claims and allegations that are substantively similar to those asserted in the Consolidated Derivative Action and Olson Action. On September 8, 2025, the parties field a joint stipulation to stay the Dusci Action pending final resolution of the Securities Litigation, which the court granted.
The Company disputes all claims asserted against it in the Securities Litigation and disputes that the plaintiffs in the Consolidated Derivative Action, the Dusci Action and Olson Action have standing to assert claims derivatively on its behalf. The Company is currently unable to estimate the potential loss or range of loss, if any, associated with these lawsuits, which could be material. Although there can be no assurance of the outcome of these lawsuits, based on information known by management, the Company has not accrued any material liabilities related to these lawsuits in the consolidated financial statements, as a negative outcome is deemed not probable, nor is any range of loss estimable as of September 30, 2025. Since the outcome of these matters cannot be predicted with certainty, any associated costs could have a material adverse effect on the Company’s consolidated results of operations, financial position or cash flows.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Indemnification
To the extent permitted under Delaware law, the Company has agreed to indemnify its directors and officers for certain events or occurrences while the director or officer is, or was serving, at the Company’s request in such capacity. The indemnification period covers all pertinent events and occurrences during the director’s or officer’s service. The maximum potential amount of future payments the Company could be required to make under these indemnification arrangements is not specified in such arrangements; however, the Company has director and officer insurance coverage that is intended to reduce its exposure and enable the Company to recover a portion of any potential future amounts the Company could be required to make. To date, the Company has not incurred any costs as a result of such obligations and has not accrued any liabilities related to such obligations in the condensed consolidated financial statements.
13. Related Party Transactions
Fresenius Medical Care investments and distribution agreement
In June 2018, the Company completed a $150 million financing transaction pursuant to which Fresenius Medical Care purchased shares of series D redeemable convertible preferred stock that at the Closing Date converted into 15,812,735 shares of Common Stock. In August 2021, Fresenius Medical Care invested $25 million as part of a private placement offering related to the Merger (the “PIPE Financing”) and received an additional 2.5 million shares of Common Stock.
In addition, the Company entered into a distribution agreement with Fresenius Medical Care in June 2018 which, as amended as of February 16, 2021, granted Fresenius Medical Care and its affiliates exclusive rights to develop outside the United States and European Union (the “EU”) and commercialize outside of the United States the Company’s 6 millimeter x 42 centimeter ATEV and all improvements thereto, and modifications and derivatives thereof (including any changes to the length, diameter or configuration of the foregoing), for use in vascular creation, repair, replacement or construction, including renal replacement therapy for dialysis access, the treatment of peripheral artery disease, and the treatment of vascular trauma, but excluding coronary artery bypass graft (“CABG”), pediatric heart surgery, or adhering pancreatic islet cells onto the outer surface of the distribution product for use in diabetic patients. Within the United States, Fresenius Medical Care will collaborate with the Company in its commercialization of the product in the field, including adoption of the distribution product as a standard of care in patients for which such use is supported by clinical results and health economic analyses.
The Company is responsible for developing and seeking regulatory approval for the distribution product in the field in the United States. For countries outside the United States, the parties agreed to use commercially reasonable efforts to satisfy certain agreed minimum market entry criteria for the distribution product in the field in such country. For the EU, once such criteria have been satisfied for the applicable country, or if the parties otherwise mutually agree to obtain regulatory approval for the distribution product in the field in the applicable country, the Company agreed to use commercially reasonable efforts to obtain such regulatory approval (other than pricing approval), and Fresenius Medical Care agreed to use commercially reasonable efforts to obtain the corresponding pricing approval. For the rest of the world (i.e., outside the United States and the EU), once such criteria have been satisfied for the applicable country, or if the parties otherwise mutually agree to obtain regulatory and pricing approval for the distribution product in the field in the applicable country, Fresenius Medical Care agreed to use commercially reasonable efforts to obtain such approvals, and the Company agreed to use commercially reasonable efforts to support Fresenius Medical Care in its efforts.
Under the distribution agreement, the Company grants an exclusive, sublicensable license to Fresenius Medical Care under the patents, know-how and regulatory materials controlled by the Company during the term to commercialize the distribution product in the field outside the United States, subject to the Company’s retained rights to carry out its obligations under the distribution agreement. The Company also grants a non-exclusive, sublicensable license to Fresenius Medical Care under the patents, know-how and regulatory materials controlled by the Company during the term to develop the distribution product in accordance with the terms of the distribution agreement. In addition, the Company grants to Fresenius Medical Care, among other things, a perpetual, irrevocable, non-exclusive sublicensable license under the patents and know-how that primarily relate to the distribution product or its manufacture and that were created, conceived or developed solely or jointly by or on behalf of Fresenius Medical Care in the performance of its activities under the distribution agreement.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
The distribution agreement provides that the Company will own all know-how and patents that primarily relate to the distribution product or its manufacture that are created, conceived or developed by or on behalf of either party in the performance of activities under the distribution agreement. Ownership of all other know-how, patents, materials and other intellectual property created, conceived or developed during the performance of activities under the distribution agreement will be determined in accordance with U.S. patent laws for determining inventorship.
The Company is obligated to make payments to Fresenius Medical Care based on a share of aggregate net sales by or on behalf of the Company of the distribution product in the United States in the field. Such revenue-share payments will be a percentage of net sales in the low double digits, without regard to the calendar year in which such net sales are attributable, until such time that the Company has paid to Fresenius Medical Care a certain total amount, at which time the revenue-share will decrease to a percentage of net sales in the mid-single digits. The amounts that Fresenius Medical Care will be obligated to pay the Company under the distribution agreement for sales of the distribution product in the field outside of the United States will vary. Fresenius Medical Care agreed to pay the Company initially, on a country-by-country basis for sales outside of the United States, the amount equal to the average cost of manufacturing the Company’s distribution product plus a fixed dollar amount per unit. Following a specified period, on a country-by-country basis outside of the United States, Fresenius Medical Care will pay the Company a fixed percentage of net sales for each unit sold in such country, such that the Company will receive more than half of such net sales.
The distribution agreement will generally continue on a country-by-country basis until the later of (a) the tenth anniversary of the launch date of the distribution product in the relevant country or (b) the expiration of the last-to-expire valid claim of specified patents in such country. Each party is permitted to terminate the distribution agreement for insolvency of, or, under certain circumstances, including various cure periods, material breach by the other party. Subject to a cure period, Fresenius Medical Care may also terminate the distribution agreement in its entirety or on a country-by-country basis (i) for certain withdrawals of regulatory approval or (ii) for termination or expiration of any of our in-licenses that is necessary for the exercise of Fresenius Medical Care’s rights, or the satisfaction of its obligations, under the distribution agreement. In addition, Fresenius Medical Care may terminate the distribution agreement for convenience on a country-by-country basis upon not less than 12 months’ written notice to the Company, although Fresenius Medical Care is not permitted to give such notice prior to the end of the second year following launch of the distribution product in such country. Each party is required to indemnify one another for certain third-party claims.
As of September 30, 2025, there were $0.1 million of royalties payable to Fresenius Medical Care based on the Company’s product revenue recognized during the three and nine months ended September 30, 2025.
Agreements with Frenova Renal Research
In June 2024, the Company entered into a master services agreement with Frenova Renal Research (“Frenova”), a subsidiary of Fresenius Medical Care, that sets forth the terms by which the Company may engage Frenova to provide certain services for projects, with the services for each project being described in a separate statement of work. As of September 30, 2025, Frenova was engaged to perform clinical research services related to the Company’s V012 Phase 3 clinical trial. During each of the three and nine months ended September 30, 2025, amounts expensed in relation to this agreement with Frenova were approximately $0.2 million. There was $0.1 million and an insignificant amount payable to Frenova as of September 30, 2025 and December 31, 2024, respectively, included in accrued expenses on the Company’s condensed consolidated balance sheets.
In July 2024, the Company entered into a service agreement with Fresenius Medical Care Deutschland GmbH (“Fresenius GmbH”), which provides medical scientific research services through Frenova. Frenova agreed to conduct a study to review patient data of adult hemodialysis patients who received treatment in certain European countries at dialysis centers that are part of Fresenius Medical Care AG. Fresenius Medical Care AG is the German parent company of Fresenius GmbH and ultimately of Fresenius Medical Care. During the three and nine months ended September 30, 2025, there was no expense recognized in relation to this agreement with Fresenius GmbH. As of September 30, 2025, no amount payable to Fresenius GmbH was included in accounts payable on the Company’s condensed consolidated balance sheets. As of December 31, 2024, there was less than $0.1 million payable to Fresenius GmbH included in accounts payable and less than $0.1 million payable to Fresenius GmbH included in accrued expenses on the Company’s condensed consolidated balance sheets.
Humacyte, Inc.
Notes to Condensed Consolidated Financial Statements
(unaudited)
Arrangements with Yale University
The Company’s President and Chief Executive Officer, Laura Niklason M.D., PhD., serves as an Adjunct Professor in Anesthesia at Yale University. As of September 30, 2025 and December 31, 2024, the Company was a party to license agreements with Yale University as described in Note 12 — Commitments and Contingencies above. Amounts expensed in relation to the license agreements with Yale University were insignificant during the three and nine months ended September 30, 2025 and 2024. There was an insignificant amount payable to Yale as of both September 30, 2025 and December 31, 2024 included in accounts payable on the Company’s condensed consolidated balance sheets.
14. Subsequent Events
Proceeds from Sales of Shares
On October 6, 2025, the Company entered into a securities purchase agreement with an institutional investor pursuant to which the investor purchased 28,436,018 shares of Common Stock and the October 2025 RDO Warrants to purchase up to 28,436,018 shares of Common Stock in the October 2025 Registered Direct Offering. The October 2025 RDO Warrants will become exercisable 180 days following the date of issuance, and will expire on April 7, 2031. The October 2025 RDO Warrants have an exercise price of $2.11 per share. The purchase price for one share of Common Stock and one October 2025 RDO Warrant was $2.11. The net proceeds to the Company from the October 2025 RDO Warrants were approximately $56.5 million after deducting placement agent’s fees and offering expenses of approximately $3.5 million. The October 2025 Registered Direct Offering closed on October 8, 2025.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our unaudited condensed consolidated financial statements and related notes appearing elsewhere in this Quarterly Report on Form 10-Q (“Quarterly Report”) and with our audited financial statements and the notes thereto included in our Annual Report. In addition, you should read the “Risk Factors” and “Information Regarding Forward-Looking Statements” sections of this Quarterly Report and our Annual Report for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Unless the context indicates otherwise, references in this Quarterly Report to the “Company,” “Humacyte,” “we,” “us,” “our” and similar terms refer to Humacyte, Inc. and its consolidated subsidiaries (Humacyte Global, Inc. and Humacyte Europe Limited).
Overview
We are a commercial-stage biotechnology platform company developing universally implantable, bioengineered human tissues at commercial scale, and in the first quarter of 2025 commenced the United States commercial launch of our first FDA-approved product. We believe our regenerative medicine technology has the potential to overcome limitations in existing standards of care and address the lack of significant innovation in products that support tissue repair, reconstruction and replacement. We are leveraging our novel, scalable technology platform to develop proprietary bioengineered, acellular human tissues for use in the treatment of diseases and conditions across a range of anatomic locations in multiple therapeutic areas.
We are initially using our proprietary, scientific technology platform to engineer and manufacture ATEVs. On December 19, 2024, the FDA granted full approval for the ATEV under the brand name SymvessTM for use in adults as a vascular conduit for extremity arterial injury when urgent revascularization is needed to avoid imminent limb loss, and autologous vein graft is not feasible. Our ATEVs are designed to be easily implanted into any patient without inducing a foreign body response or leading to immune rejection. We are developing a portfolio, or “cabinet”, of ATEVs with varying diameters and lengths. The ATEV cabinet would initially target the vascular repair, reconstruction and replacement market, including use in vascular trauma, AV access for hemodialysis and PAD. We are also developing the ATEV for CABG and pediatric heart surgery. Over the longer term, we are developing our ATEV for the delivery of cellular therapies, including pancreatic islet cell transplantation to treat Type 1 diabetes (our BioVascular Pancreas or BVP). We will continue to explore the application of our technology across a broad range of markets and indications, including the development of urinary conduit, trachea, esophagus and other novel cell delivery systems.
For the ATEV, we believe there is substantial clinical demand for safe and effective vascular conduits to replace and repair blood vessels throughout the body. Vascular injuries resulting from trauma are common in civilian and military populations, frequently resulting in the loss of either life or limb. Existing treatment options in the vascular repair, reconstruction and replacement market include the use of autologous vessels and synthetic grafts, which we believe suffer from significant limitations. For example, the use of autologous veins to repair traumatic vascular injuries can lead to significant morbidity associated with the surgical wounds created for vein harvest and prolonged times to restore blood flow to injured limbs, leading to an increased risk of complications such as amputation and reperfusion injury. In addition, in many instances of vascular trauma the patient may not have adequate vein available, or the time between injury and treatment is too long to make autologous graft repair feasible. Synthetic grafts are often contraindicated in the setting of vascular trauma due to wound contamination that contributes to higher infection risk and can lead to prolonged hospitalization and limb loss. Given the competitive advantages our ATEVs are designed to have over existing vascular substitutes, we believe that ATEVs have the potential to become the standard of care and lead to improved patient outcomes and lower healthcare costs.
In addition to extremity vascular trauma, we and our collaborators are currently conducting Phase 3 and Phase 2 trials of our 6 millimeter ATEV in AV access for hemodialysis and PAD. We were granted Fast Track designation by the FDA for our 6 millimeter ATEV for use in AV access for hemodialysis in 2014. We also received the first Regenerative Medicine Advanced Therapy (“RMAT”) designation from the FDA, for the creation of vascular access for performing hemodialysis, in March 2017. In May 2023, we were granted the RMAT designation for the ATEV for urgent arterial repair following extremity vascular trauma, and in June 2024, we were granted the RMAT designation for the ATEV for patients with advanced PAD. In addition, in 2018 our ATEV product candidate was assigned a priority designation by the Secretary of Defense under Public Law 115-92, enacted to expedite the FDA’s review of products that are intended to diagnose, treat or prevent serious or life-threatening conditions facing American military personnel.
In September 2023, we announced positive topline results from our V005 Phase 2/3 trial in vascular trauma, and in December 2023, we filed a BLA for urgent arterial repair following extremity vascular trauma when synthetic graft is not indicated, and autologous vein use is not feasible. In February 2024, the FDA accepted the BLA filing, granted priority review and set a Prescription Drug User Fee Act date of August 10, 2024. On August 9, 2024, the FDA informed us that it required additional time to complete its review of the BLA for the vascular trauma indication. On December 19, 2024, the FDA granted full approval for Symvess (acellular tissue engineered vessel-tyod) for use in adults as a vascular conduit for extremity arterial injury when urgent revascularization is needed to avoid imminent limb loss, and autologous vein graft is not feasible. In February 2025, the FDA completed its required review of commercial batch information for Symvess and authorized us to commence commercial shipments and we shipped our first commercial products in March 2025.
In April 2023, we announced completion of enrollment of our V007 Phase 3 trial of the ATEV for use in AV access for hemodialysis. In July 2024, we announced positive topline results from our V007 Phase 3 trial, where the ATEV met the primary endpoints in the study. If the interim results from our ongoing V012 Phase 3 trial in women are positive, we plan to submit a supplemental BLA for the ATEV to the FDA for an indication in AV access for hemodialysis in the second half of 2026.
We have incurred operating losses and negative cash flows from operations in each year since our inception in 2004. As of September 30, 2025 and December 31, 2024, we had an accumulated deficit of $702.0 million and $686.0 million, respectively, and working capital of $15.8 million and $27.9 million, respectively. Our operating losses were approximately $24.4 million and $77.3 million for the three and nine months ended September 30, 2025, respectively, and $30.2 million and $86.3 million for the three and nine months ended September 30, 2024, respectively.
Net cash flows used in operating activities were $78.9 million and $71.5 million during the nine months ended September 30, 2025 and 2024, respectively. Substantially all of our operating losses resulted from costs incurred in connection with our research and development programs and from selling, general and administrative costs associated with our operations. We expect to incur substantial operating losses and negative cash flows from operations for the foreseeable future as we begin to commercialize Symvess and advance our product candidates.
As of September 30, 2025, we had cash and cash equivalents of $19.5 million and restricted cash of $0.4 million. We believe our cash and cash equivalents on hand and existing capacity under our Common Stock Purchase Agreement will be sufficient to fund operations for at least 12 months from the date of this Quarterly Report. See Note 1 — Organization and Description of Business in the notes to our unaudited condensed consolidated financial statements included elsewhere in this Quarterly Report for additional information regarding this assessment.
Our need for additional capital will depend in part on the scope and costs of our development and commercial manufacturing activities, and the results of our commercial sales efforts. We recently received FDA approval to commercialize Symvess and have only recently generated revenue from the sale of commercialized products. Our ability to generate product revenue will depend on the successful development and commercialization of Symvess and our product candidates. Until such time, if ever, we expect to finance our operations through the use of existing cash and cash equivalents, the sale of equity, debt, financings, debt refinancings or restructurings or through potential collaborations with other companies, other strategic transactions or government and other grants. Adequate capital may not be available to us when needed or on acceptable terms. If we are unable to raise capital, we could be forced to delay, reduce, suspend or cease our research and development programs or any future commercialization efforts, which would have a negative impact on our business, prospects, operating results and financial condition. See “Risk Factors” for additional information.
We expect to continue to incur significant expenses and to increase operating losses for at least the next several years. We anticipate that our expenses will increase substantially as we seek to:
•commercialize Symvess via U.S. market launch for indications in vascular trauma and, if approved, in AV access for hemodialysis;
•obtain marketing approval for our 6 millimeter ATEV in additional indications involving vascular repair, reconstruction and replacement, including in AV access for hemodialysis;
•scale out our manufacturing facility to the extent required to satisfy potential market demand for Symvess in the U.S. and our product candidates, following receipt of any regulatory approval;
•continue our preclinical and clinical development efforts;
•maintain, expand and protect our intellectual property portfolio;
•add operational, financial and management information systems and personnel to support, among other things, our product development and commercialization efforts and operations; and
•continue operating as a public company, which includes higher costs associated with hiring additional personnel, director and officer insurance premiums, audit and legal fees and expenses for compliance with public company reporting requirements under the Exchange Act and rules implemented by the SEC and Nasdaq.
Recent Developments
On April 28, 2025, we implemented a cost reduction action to reduce our workforce by 30 employees, cease recruitment of additional planned new hires, and reduce other operating expenses. Substantially all of the employees impacted by this plan were notified beginning May 7, 2025. We undertook these cost reductions to improve cash runway and to better align the Company’s organizational structure with its top business objectives. We incurred aggregate charges representing one-time cash expenditure for severance and other employee termination benefits of approximately $0.7 million during the second quarter of 2025, of which $0.6 million was recognized in research and development expenses and $0.1 million was recognized in selling, general and administrative expenses on our condensed consolidated statements of operations and comprehensive income (loss). Humacyte estimates that it will incur savings due to the workforce and other operating cost reductions, and reduced capital expenditures, net of termination severance and benefits, totaling approximately $13.8 million in 2025 and up to approximately $38.0 million in 2026.
In October 2024, we submitted a New Technology Add-On Payment (“NTAP”) application for Symvess to the Centers for Medicare and Medicaid Services (“CMS”). In July 2025, CMS declined to approve our NTAP application. We may engage in discussions with CMS regarding the application, but there is no guarantee that CMS will reverse its decision within a given timeframe, or at all. We believe that the potential impact of the NTAP on commercial success is limited as only approximately 4.3% of vascular trauma patients are covered under Medicare reimbursement.
Components of Results of Operations
Revenue
We generate product revenue from commercial sales of Symvess in the United States. Contract revenue consists of revenue related to a single contract with a customer to recover contract expenses. Contract revenue associated with each performance obligation in the contract is recognized as the research and development services are provided according to the actual costs incurred compared to the total costs expected to be incurred to satisfy the performance obligation.
Prior to the recent commercialization of the ATEVs in the vascular trauma indication, all of our revenue was derived from government and other grants. From inception through September 30, 2025, we have been awarded grants, including grants from the California Institute of Regenerative Medicine (“CIRM”), the National Institutes of Health (“NIH”), and the Department of Defense, to support our development, production scaling and clinical trials of our product candidates.
We may generate revenue in the future from government and other grants, payments from future license or collaboration agreements and from product sales of our ATEVs in the vascular trauma indication and any of our product candidates that receive marketing approval. We expect that any revenue we generate will fluctuate from quarter to quarter. If we fail to complete the development of, or obtain marketing approval for, our product candidates in a timely manner, our ability to generate future revenue, and our results of operations and financial position, would be materially adversely affected.
Cost of goods sold
We have inventory for which the production costs were recorded as an expense in 2024 prior to FDA approval. We expect cost of goods sold to normalize after utilization of this inventory. If market acceptance of Symvess does not occur at all or on a timely basis prior to the inventory shelf-life expiration, we may be required to write-off some or all inventory, which could affect our financial requirements and financial results. Cost of goods sold includes overhead related to unused production capacity, which was recorded as an expense during the three and nine months ended September 30, 2025, as well as royalties expense based on our product revenue.
Research and Development Expenses
Since our inception, we have focused our resources on our research and development activities, including conducting preclinical studies and clinical trials, developing and refining our manufacturing process and activities related to regulatory filings for our product candidates. We recognize research and development expenses as they are incurred. Our research and development expenses consist primarily of:
•salaries and related overhead expenses for personnel in research and development functions, including stock-based compensation and benefits;
•fees paid to CROs and consultants, including in connection with our clinical trials, and other related clinical trial fees, such as for clinical site fees and investigator grants related to patient screening and treatment, conduct of clinical trials, laboratory work and statistical compilation and analysis;
•allocation of facility lease and maintenance costs;
•depreciation of leasehold improvements, laboratory equipment and computers;
•costs related to purchasing raw materials and producing our product candidates for clinical trials;
•costs related to compliance with regulatory requirements;
•costs related to our manufacturing development and expanded-capabilities initiatives; and
•license fees related to in-licensed technologies.
The majority of our research and development resources are currently focused on our Phase 2 and 3 clinical trials for our 6 millimeter ATEV and other work needed to obtain marketing approval for our 6 millimeter ATEV for use in AV access in hemodialysis and PAD in the United States. We have incurred and expect to continue to incur significant expenses in connection with these and our other clinical development efforts, including expenses related to regulatory filings, trial enrollment and conduct, data analysis, patient follow up and study report generation for our Phase 2 and Phase 3 clinical trials.
Direct expenses for our vascular trauma, AV access for hemodialysis and PAD indications include costs related to our clinical trials, including fees paid to CROs, consultants, clinical sites and investigators. Costs related to development activities which broadly support multiple programs using our technology platform, including personnel, materials and supplies, external services costs, and other internal expenses, such as facilities and overhead costs, are not allocated to individual research and development programs. Other research and development expenses include direct costs not identifiable with a specific product candidate, including costs associated with our research and development platform used across programs, process development, manufacturing analytics and preclinical research and development for prospective product candidates and new technologies.
The successful development of our preclinical and clinical product candidates is highly uncertain. At this time, we cannot estimate with any reasonable certainty the nature, timing or costs of the efforts that will be necessary to complete the remainder of the development of any of our preclinical or clinical product candidates or the period, if any, in which material net cash inflows from these product candidates may commence. This is due to the numerous risks and uncertainties associated with the development of our product candidates, including:
•the scope, rate of progress, expense and results of our preclinical development activities, our ongoing clinical trials and any additional clinical trials that we may conduct, and other research and development activities;
•successful patient enrollment in and the initiation and completion of clinical trials;
•the timing, receipt and terms of any marketing approvals from applicable regulatory authorities including the FDA and non-U.S. regulators;
•the extent of any required post-marketing approval commitments to applicable regulatory authorities;
•development and refinement of clinical and commercial manufacturing capabilities or making arrangements with third-party manufacturers in order to ensure that it or its third-party manufacturers are able to successfully manufacture our product;
•obtaining, maintaining, defending and enforcing patent claims and other intellectual property rights;
•significant and changing government regulations;
•launching commercial sales of Symvess and our product candidates, if approved, whether alone or in collaboration with others;
•the degree of market acceptance of Symvess and any product candidates that obtain marketing approval; and
•maintaining a continued acceptable safety profile following approval of Symvess in the vascular trauma indication and in any other indications for which approval may be granted, or for any of our product candidates, if approved.
A change in the outcome of any of these variables could lead to significant changes in the costs and timing associated with the development of our product candidates. For example, if the FDA or another regulatory authority were to require us to conduct clinical trials beyond those that we currently anticipate being required to conduct in order to complete the clinical development of any of our product candidates, or if we experience significant delays in the enrollment or the conduct of any of our clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development.
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist primarily of salaries and related costs for employees in executive, finance, human resources, commercialization, and administrative support functions, which also include stock-based compensation expenses and benefits for such employees. Other significant general and administrative expenses include facilities costs, professional fees for accounting and legal services and expenses associated with obtaining and maintaining patents.
We expect our general and administrative expenses will continue to increase for the foreseeable future to support our expanded infrastructure and increased costs of operating as a public company and as we commercialize Symvess in the United States and seek marketing approval for Symvess outside of the United States. These increases are expected to include increased employee-related expenses, increased sales and marketing expenses, and increased director and officer insurance premiums, audit and legal fees, and expenses for compliance with public company reporting requirements under the Exchange Act and rules implemented by the SEC, as well as Nasdaq rules.
Other Income (Expense), Net
Total other income (expense), net consists of (i) the change in fair value of the Contingent Earnout Liability that was accounted for as a liability as of the date of the Merger, and is remeasured to fair value at each reporting period, resulting in a non-cash gain or loss, (ii) interest income earned on our cash and cash equivalents, (iii) interest expense incurred on the Purchase Agreement (defined above) and finance leases, and (iv) the change in fair value of our derivative liabilities and asset including the private placement Common Stock warrant liabilities related to the Private Placement Warrants, which we assumed in connection with the Merger; Common Stock warrant liabilities related to our Registered Direct Offerings; the contingent derivative liability related to the Purchase Agreement; a liability related to a freestanding option agreement related to the Purchase Agreement; a derivative liability related to our agreement with JDRF; and a derivative asset related to our Common Stock Purchase Agreement, all of which are subject to remeasurement to fair value at each balance sheet date resulting in a non-cash gain or loss.
Results of Operations
Comparison of the Three Months Ended September 30, 2025 and 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Change |
| ($ in thousands) |
2025 |
|
2024 |
|
$ |
|
% |
| Revenue: |
|
|
|
|
|
|
|
| Product revenue, net |
$ |
703 |
|
|
$ |
— |
|
|
$ |
703 |
|
|
100 |
% |
Contract revenue |
50 |
|
|
— |
|
|
50 |
|
|
100 |
% |
| Total revenue |
753 |
|
|
— |
|
|
753 |
|
|
100 |
% |
|
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
| Cost of goods sold |
260 |
|
|
— |
|
|
260 |
|
|
100 |
% |
| Research and development |
17,273 |
|
|
22,926 |
|
|
(5,653) |
|
|
(25) |
% |
| Selling, general and administrative |
7,610 |
|
|
7,307 |
|
|
303 |
|
|
4 |
% |
| Total operating expenses |
25,143 |
|
|
30,233 |
|
|
(5,090) |
|
|
(17) |
% |
| Loss from operations |
(24,390) |
|
|
(30,233) |
|
|
5,843 |
|
|
(19) |
% |
|
|
|
|
|
|
|
|
| Other income (expense), net |
|
|
|
|
|
|
|
| Interest income |
590 |
|
|
911 |
|
|
(321) |
|
|
(35) |
% |
| Change in fair value of Contingent Earnout Liability |
4,893 |
|
|
(8,489) |
|
|
13,382 |
|
|
(158) |
% |
| Change in fair value of derivatives |
4,009 |
|
|
1,047 |
|
|
2,962 |
|
|
283 |
% |
| Interest (expense) income |
(2,612) |
|
|
(2,438) |
|
|
(174) |
|
|
7 |
% |
|
|
|
|
|
|
|
|
| Total other income (expense), net |
6,880 |
|
|
(8,969) |
|
|
15,849 |
|
|
(177) |
% |
| Net loss |
$ |
(17,510) |
|
|
$ |
(39,202) |
|
|
$ |
21,692 |
|
|
(55) |
% |
Revenue
Total revenue was $0.8 million for the three months ended September 30, 2025, compared to no revenue for the three months ended September 30, 2024. Revenue for the three months ended September 30, 2025 consisted of $0.7 million of product revenue from sales of Symvess in the United States and $0.1 million of revenue earned related to research and development services pursuant to a research contract with a large medical technology company.
Cost of goods sold
Cost of goods sold was $0.3 million for the three months ended September 30, 2025 and includes overhead related to unused production capacity which was recorded as an expense during the period. There was no cost of goods sold for the three months ended September 30, 2024.
Research and Development Expenses
The following table discloses the breakdown of research and development expenses for the periods indicated:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Change |
| ($ in thousands) |
2025 |
|
2024 |
|
$ |
|
% |
| Direct Expenses |
|
|
|
|
|
|
|
| Vascular Trauma |
$ |
278 |
|
|
$ |
439 |
|
|
$ |
(161) |
|
|
(37) |
% |
| AV Access |
1,612 |
|
|
1,927 |
|
|
(315) |
|
|
(16) |
% |
| PAD |
— |
|
|
22 |
|
|
(22) |
|
|
(100) |
% |
| Total |
1,890 |
|
|
2,388 |
|
|
(498) |
|
|
(21) |
% |
| Unallocated Expenses |
|
|
|
|
|
|
|
| External services |
1,569 |
|
|
1,915 |
|
|
(346) |
|
|
(18) |
% |
| Materials and supplies |
4,903 |
|
|
5,451 |
|
|
(548) |
|
|
(10) |
% |
| Payroll and personnel expenses |
8,316 |
|
|
9,621 |
|
|
(1,305) |
|
|
(14) |
% |
| Other research and development expenses |
595 |
|
|
3,551 |
|
|
(2,956) |
|
|
(83) |
% |
| Total |
15,383 |
|
|
20,538 |
|
|
(5,155) |
|
|
(25) |
% |
| Total research and development expenses |
$ |
17,273 |
|
|
$ |
22,926 |
|
|
$ |
(5,653) |
|
|
(25) |
% |
Research and development expenses were $17.3 million for the three months ended September 30, 2025, representing a decrease of $5.7 million, or 25%, from $22.9 million for the three months ended September 30, 2024. The decrease was driven by a $3.0 million decrease in other research and development expense and $1.0 million decrease in payroll and personnel expenses related to the capitalization of overhead costs associated with the commercial manufacturing of Symvess, a $0.3 million decrease in external services, a $0.5 million decrease in clinical trials expense, and a $0.5 million decrease in materials and supplies expense.
Selling, General and Administrative Expenses
Selling, general and administrative expenses were $7.6 million and $7.3 million for the three months ended September 30, 2025 and 2024, respectively. The increase in selling, general and administrative expenses compared to the prior period of $0.3 million, or 4% , was primarily driven by the commercial launch of Symvess, and included a $0.8 million increase in payroll and personnel expenses and $0.3 million increase in professional fees, partially offset by a $1.0 million decrease in external services and consulting expenses.
Total Other Income (Expense), net
Total other income, net was $6.9 million for the three months ended September 30, 2025 compared to total other expense, net of $9.0 million for the three months ended September 30, 2024. The increase in net income of $15.8 million was primarily attributable to a $13.4 million non-cash gain from the fair value remeasurement of the Contingent Earnout Liability and a $3.0 million non-cash gain from the fair value remeasurement of the derivative liabilities.
Comparison of the Nine Months Ended September 30, 2025 and 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, |
|
Change |
| ($ in thousands) |
2025 |
|
2024 |
|
$ |
|
% |
| Revenue: |
|
|
|
|
|
|
|
| Product revenue, net |
$ |
950 |
|
|
$ |
— |
|
|
950 |
|
|
100 |
% |
Contract revenue |
621 |
|
|
— |
|
|
621 |
|
|
100 |
% |
| Total revenue |
1,571 |
|
|
— |
|
|
1,571 |
|
|
100 |
% |
|
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
| Cost of goods sold |
620 |
|
|
— |
|
|
620 |
|
|
100 |
% |
| Research and development |
54,697 |
|
|
67,943 |
|
|
(13,246) |
|
|
(19) |
% |
| Selling, general and administrative |
23,555 |
|
|
18,367 |
|
|
5,188 |
|
|
28 |
% |
| Total operating expenses |
78,872 |
|
|
86,310 |
|
|
(7,438) |
|
|
(9) |
% |
| Loss from operations |
(77,301) |
|
|
(86,310) |
|
|
9,009 |
|
|
(10) |
% |
| Other income (expense), net: |
|
|
|
|
|
|
|
| Interest income |
2,084 |
|
|
3,252 |
|
|
(1,168) |
|
|
(36) |
% |
| Change in fair value of Contingent Earnout Liability |
49,154 |
|
|
(38,653) |
|
|
87,807 |
|
|
(227) |
% |
| Change in fair value of derivatives |
18,191 |
|
|
719 |
|
|
17,472 |
|
|
2430 |
% |
| Interest expense |
(8,157) |
|
|
(6,769) |
|
|
(1,388) |
|
|
21 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total other income (expense), net |
61,272 |
|
|
(41,451) |
|
|
102,723 |
|
|
(248) |
% |
| Net income (loss) |
$ |
(16,029) |
|
|
$ |
(127,761) |
|
|
$ |
111,732 |
|
|
(87) |
% |
Revenue
Total revenue was $1.6 million for the nine months ended September 30, 2025, compared to no revenue for the nine months ended September 30, 2024. Revenue for the nine months ended September 30, 2025 consisted of $0.9 million of product revenue from sales of Symvess in the United States and $0.6 million of revenue earned related to research and development services pursuant to a research contract with a large medical technology company.
Cost of goods sold
Cost of goods sold was $0.6 million for the nine months ended September 30, 2025 and includes overhead related to unused production capacity which was recorded as an expense during the period. There was no cost of goods sold for the nine months ended September 30, 2024.
Research and Development Expenses
The following table discloses the breakdown of research and development expenses for the periods indicated:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, |
|
Change |
| ($ in thousands) |
2025 |
|
2024 |
|
$ |
|
% |
| Direct Expenses |
|
|
|
|
|
|
|
| Vascular Trauma |
$ |
690 |
|
|
$ |
1,904 |
|
|
$ |
(1,214) |
|
|
(64) |
% |
| AV Access |
4,356 |
|
|
4,952 |
|
|
(596) |
|
|
(12) |
% |
| PAD |
— |
|
|
161 |
|
|
(161) |
|
|
(100) |
% |
| Total |
5,046 |
|
|
7,017 |
|
|
(1,971) |
|
|
(28) |
% |
| Unallocated Expenses |
|
|
|
|
|
|
|
| External services |
4,466 |
|
|
5,340 |
|
|
(874) |
|
|
(16) |
% |
| Materials and supplies |
13,289 |
|
|
17,470 |
|
|
(4,181) |
|
|
(24) |
% |
| Payroll and personnel expenses |
26,948 |
|
|
27,763 |
|
|
(815) |
|
|
(3) |
% |
| Other research and development expenses |
4,948 |
|
|
10,353 |
|
|
(5,405) |
|
|
(52) |
% |
| Total |
$ |
49,651 |
|
|
$ |
60,926 |
|
|
$ |
(11,275) |
|
|
(19) |
% |
| Total research and development expenses |
$ |
54,697 |
|
|
$ |
67,943 |
|
|
$ |
(13,246) |
|
|
(19) |
% |
Research and development expenses were $54.7 million for the nine months ended September 30, 2025, representing a decrease of $13.2 million, or 19%, from $67.9 million for the nine months ended September 30, 2024. The decrease was primarily driven by a $4.2 million decrease in materials and supplies expense, related to the capitalization of expenditures for inventory during the nine months ended September 30, 2025 following the commencement of commercial manufacturing to support the market launch of Symvess, a $2.0 million decrease in clinical trials expense, and a $5.4 million decrease in other research and development expenses related primarily to the capitalization of overhead costs associated with the commercial manufacturing of Symvess.
Selling, General and Administrative Expenses
Selling, general and administrative expenses were $23.6 million and $18.4 million for the nine months ended September 30, 2025 and 2024, respectively. The increase in general and administrative expenses compared to the prior period of $5.2 million, or 28%, was primarily driven by the commercial launch of Symvess. Significant increases in expenses included a $4.9 million increase in payroll and personnel expenses.
Total Other Income (Expense), net
Total other income, net was $61.3 million for the nine months ended September 30, 2025, compared to total other expense, net of $41.5 million for the nine months ended September 30, 2024. The $102.7 million change was primarily attributable to an $87.8 million non-cash gain from the fair value remeasurement of the Contingent Earnout Liability and a $17.5 million non-cash gain from the fair value remeasurement of derivative liabilities.
Liquidity and Capital Resources
Sources of Liquidity
We have historically financed our operations primarily through the sale of equity securities and convertible debt, borrowings under loan facilities, the Purchase Agreement, and, to a lesser extent, through grants from governmental and other agencies. Since our inception, we have incurred significant operating losses and negative cash flows. As of September 30, 2025 and December 31, 2024, we had an accumulated deficit of $702.0 million and $686.0 million, respectively.
As of September 30, 2025 and December 31, 2024, we had working capital of $15.8 million and $27.9 million, respectively. As of September 30, 2025 and December 31, 2024, we had cash and cash equivalents of $19.5 million and $44.9 million, respectively, and restricted cash of $0.4 million and $50.4 million, respectively. We funded the restricted cash account on August 14, 2024, in accordance with our amended Purchase Agreement, of which $50.0 million was not subject to our unilateral control. In connection with the Purchase Agreement Amendment, the Company made the Purchase Agreement Amendment Payment of $50.0 million, funded from the restricted cash previously maintained for the benefit of the Agent. As a result of the Purchase Agreement Amendment Payment, the Company is no longer obligated to maintain $50.0 million of restricted cash in an account for the benefit of the Agent unless the Purchase Agreement has not been repaid in full by December 31, 2025, at which point the Company will be required to maintain $12.5 million in such account.
As of September 30, 2025, we had $47.5 million in remaining availability for sales of Common Stock under our Common Stock Purchase Agreement with Lincoln Park and $62.7 million in remaining availability for sales of Common Stock under our ATM Facility.
As discussed above, on April 28, 2025, we implemented a cost reduction action to reduce our workforce by 30 employees, cease recruitment of additional planned new hires, and reduce other operating expenses. We undertook these cost reductions to improve cash runway and to better align the Company’s organizational structure with its top business objectives. We believe our cash and cash equivalents on hand and existing capacity under our Common Stock Purchase Agreement will be sufficient to fund operations for at least twelve months from the issuance date of these interim financial statements. The future viability of the Company beyond that point is dependent on our ability to generate cash flows from the sale of Symvess and raise additional capital to finance our operations. See Note 1 — Organization and Description of Business to our accompanying unaudited condensed consolidated financial statements included elsewhere in this Quarterly Report for additional information regarding our assessment. We believe that our longer-term working capital, planned research and development, capital expenditures and other general corporate funding requirements may be satisfied through the sale of equity, debt, financings, debt refinancings or restructurings or through potential collaborations with other companies, other strategic transactions or government or other grants. Our liquidity plans are subject to a number of risks and uncertainties, including those described in the sections entitled “Forward-Looking Statements” and “Risk Factors” in this Quarterly Report and our Annual Report. If we are unable to raise sufficient capital, we may be forced to delay, reduce, suspend or cease certain of our planned research and development programs or any future commercialization efforts, which would have a negative impact on our business, prospects, operating results and financial condition.
On February 29, 2024, we entered into an underwriting agreement in connection with the 2024 Public Offering. The net proceeds to us from the 2024 Public Offering were approximately $43.0 million, after deducting underwriting discounts and commissions and offering expenses. The 2024 Public Offering closed on March 5, 2024.
On September 24, 2024, we entered into the Common Stock Purchase Agreement with Lincoln Park for an equity line financing, which provides that, subject to the terms and conditions set forth in the Common Stock Purchase Agreement, we have the sole right, but not the obligation, to sell to Lincoln Park shares of Common Stock having an aggregate value of up to $50.0 million over a 24-month period. We control the timing and amount of any sales to Lincoln Park. As of September 30, 2025, we had completed sales of shares under the Common Stock Purchase Agreement that provided $2.5 million in gross proceeds, and as of September 30, 2025 we had $47.5 million in remaining availability for sales of our Common Stock under our Common Stock Purchase Agreement with Lincoln Park.
On October 4, 2024, we entered into a securities purchase agreement with an institutional investor pursuant to which the investor purchased approximately $30.0 million of Common Stock and warrants in the October 2024 Registered Direct Offering. The net proceeds to us from the October 2024 Registered Direct Offering were approximately $28.0 million, after deducting placement agent’s fees and offering expenses of approximately $2.0 million. The October 2024 Registered Direct Offering closed on October 7, 2024.
On November 13, 2024, we entered into a securities purchase agreement with an institutional investor pursuant to which the investor purchased approximately $15.0 million of Common Stock and warrants in the November 2024 Registered Direct Offering. The net proceeds to us from the November 2024 Registered Direct Offering were approximately $14.9 million after deducting offering expenses of approximately $0.1 million. The November 2024 Registered Direct Offering closed on November 15, 2024.
On March 25, 2025, we entered into an underwriting agreement in connection with the 2025 Public Offering. The net proceeds to us from the 2025 Public Offering were approximately $46.7 million, after deducting underwriting discounts and commissions and offering expenses. The 2025 Public Offering closed on March 27, 2025.
On October 6, 2025, we entered into a securities purchase agreement with institutional investors pursuant to which the investors purchased approximately $60.0 million worth of Common Stock and October 2025 RDO Warrants in the October 2025 Registered Direct Offering. The net proceeds to us from the October 2025 Registered Direct Offering were approximately $56.5 million, after deducting placement agent’s fees and offering expenses of approximately $3.5 million. The October 2025 Registered Direct Offering closed on October 8, 2025.
ATM Facility
On September 1, 2022, we entered into the ATM Facility for the sale from time to time of up to $80.0 million of shares of Common Stock. As of September 30, 2025, we have sold an aggregate of 5,125,876 shares of Common Stock under the ATM Facility at an average price of $3.38 per share for net proceeds of approximately $16.8 million after deducting sales commissions of approximately $0.5 million. As of September 30, 2025, we had $62.7 million in remaining availability for sales of Common Stock under the ATM Facility.
Material Cash Requirements
Our known material cash requirements include: (1) the purchase of supplies and services that are primarily for research and development; (2) repayments pursuant to the Purchase Agreement; (3) employee wages, benefits, and incentives; (4) financing and operating lease payments (for additional information see below), and (5) payments under our JDRF Agreement (see Note 12 — Commitments and Contingencies to our unaudited condensed consolidated financial statements contained elsewhere in this Quarterly Report). We have also entered into contracts with CROs primarily for clinical trials. These contracts generally provide for termination upon limited notice, and therefore we believe that our non-cancellable obligations under these agreements are not material. Moreover, we may be subject to additional material cash requirements that are contingent upon the occurrence of certain events, for example, legal contingencies, uncertain tax positions, and other matters.
As of September 30, 2025, we had non-cancellable purchase commitments of $24.2 million for supplies and services that are primarily for research and development. We have existing license agreements with Duke University and Yale University, a distribution agreement with Fresenius Medical Care and our JDRF Agreement. The amount and timing of any potential milestone payments, license fee payments, royalties and other payments that we may be required to make under these agreements are unknown or uncertain at September 30, 2025. For additional information regarding our agreement with Fresenius Medical Care, see Note 13 — Related Party Transactions to our unaudited condensed consolidated financial statements contained elsewhere in this Quarterly Report. For additional information regarding our agreements with Duke University, Yale University and JDRF, see Note 12 — Commitments and Contingencies to our unaudited condensed consolidated financial statements contained elsewhere in this Quarterly Report.
Revenue Interest Purchase Agreement
On May 12, 2023, we entered into the Purchase Agreement with the Purchasers and another affiliate of Oberland Capital Management LLC, as agent for the Purchasers, to obtain financing in respect to the further development and commercialization of our ATEV, to repay our then outstanding credit facility with SVB, and for other general corporate purposes. Pursuant to the Purchase Agreement and subject to customary closing conditions, the Purchasers have purchased certain revenue interests from us in exchange for an aggregate investment amount of up to $150.0 million. Under the terms of the Purchase Agreement, $40.0 million of the Investment Amount, less certain transaction expenses, was funded on May 12, 2023, which was used to repay in full and retire our indebtedness under our loan agreement with SVB, with the remaining proceeds funded to the Company. On March 11, 2024, $20.0 million of the Investment Amount was funded to the Company. On December 19, 2024, the FDA granted full approval for our BLA for the vascular trauma indication, and we elected not to draw the additional $40.0 million that became available under the Purchase Agreement. As of and subsequent to December 31, 2024, we are not entitled to draw on any further installments under the Purchase Agreement. See Note 7 — Revenue Interest Purchase Agreement to the condensed consolidated financial statements for additional details about this financing transaction.
On February 18, 2024, we agreed with the Purchasers and the Agent, to waive certain breaches related to, and extend the deadline for certain post-closing obligations under, the Purchase Agreement, including the requirement for us to deliver a leasehold mortgage in favor of the Agent over our headquarters. On May 8, 2024, we reached an agreement with the Purchasers to amend the Purchase Agreement to remove requirements related to the leasehold mortgage. In exchange for the removal of this requirement, on August 14, 2024 we funded an account in the amount of $54.0 million, over which the Agent has certain consent and other rights to $50.0 million of the funds. See Note 7 for further information.
On September 17, 2025, we entered into the Purchase Agreement Amendment with the Purchasers and the Agent to amend the Purchase Agreement. In connection with the Purchase Agreement Amendment, we made a $50 million repayment under the Purchase Agreement, funded from the restricted cash maintained for the benefit of the Agent. Additionally, pursuant to the Purchase Agreement Amendment, we are no longer obligated to maintain $50.0 million of restricted cash in an account for the benefit of the Agent unless the Purchase Agreement has not been repaid in full by December 31, 2025, at which point we will be required to maintain $12.5 million in such account. Under the Purchase Agreement, as of September 30, 2025, we had $20.7 million recorded as a revenue interest liability on our condensed consolidated financial statements. For additional information regarding the Purchase Agreement, see Note 7 — Revenue Interest Purchase Agreement to our unaudited condensed consolidated financial statements contained elsewhere in this Quarterly Report.
Leases
On September 29, 2025, we executed a Sixth Amendment to our lease agreements that extended the lease term and modified key financial terms. Additional details regarding the amendment and its impact are provided in Note 8 — Leases to the condensed consolidated financial statements. Our future contractual obligations under our lease agreement as of September 30, 2025 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ($ in thousands) |
Total |
|
Less than
1 year |
|
1 – 3 years |
|
3 – 5 years |
|
More than
5 years |
Finance leases |
$ |
62,217 |
|
|
$ |
2,670 |
|
|
$ |
2,152 |
|
|
$ |
7,128 |
|
|
$ |
50,267 |
|
|
|
|
|
|
|
|
|
|
|
Future Funding Requirements
We expect to incur significant expenses in connection with our ongoing activities as we seek to (i) commercialize Symvess and seek marketing approval for Symvess in additional indications and for our product candidates in the United States and to obtain marketing approval for our 6 millimeter ATEV outside of the United States, (ii) continue clinical development of our 6 millimeter ATEV for use in hemodialysis AV access and submit a BLA for FDA approval of an indication in AV access for hemodialysis, (iii) advance our pipeline in major markets, including PAD Phase 3 trials and continue preclinical development and advance to planned clinical studies in CABG and BVP for diabetes, and (iv) expand our manufacturing facility as required to satisfy market demand. We will need additional funding in connection with these activities.
Our future funding requirements, both short-term and long-term, will depend on many factors, including:
•the cost and timing of our future commercialization activities, including product manufacturing, marketing and distribution for Symvess in the United States, and any other product candidate for which we receive marketing approval in the future;
•the amount and timing of revenues that we receive from commercial sales of Symvess and any product candidates for which we receive marketing approval;
•the progress and results of our clinical trials and interpretation of those results by the FDA and other regulatory authorities;
•the cost, timing and outcome of regulatory review of our product candidates, particularly for marketing approval of Symvess outside of the United States and of our product candidates in the United States;
•the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for our additional product candidates;
•the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims; and
•the costs of operating as a public company, including hiring additional personnel as well as increased director and officer insurance premiums, audit and legal fees, and expenses for compliance with public company reporting requirements under the Exchange Act and rules implemented by the SEC and Nasdaq.
Until such time, if ever, as we are able to successfully commercialize Symvess and to develop and commercialize our product candidates, we expect to continue financing our operations through the sale of equity, debt, financings, debt refinancings or restructurings or through potential collaborations with other companies, other strategic transactions or government or other grants. Adequate capital may not be available to us when needed or on acceptable terms. We do not currently have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making acquisitions or capital expenditures. Debt financing would also result in fixed payment obligations. If we are unable to raise capital, we plan to implement a program that delays, reduces, suspends or ceases our planned capital expenditures, research and development programs or any future commercialization efforts, which would have a negative impact on our business, prospects, operating results and financial condition.
Our principal use of cash in recent periods has been primarily to fund our operations, including the clinical and preclinical development of our product candidates. Our future capital requirements, both short-term and long-term, will depend on many factors, including the progress and results of our clinical trials and preclinical development, timing and extent of spending to support development efforts, cost and timing of future commercialization activities, and the amount and timing of revenues that we receive from commercial sales.
See the section of our Annual Report entitled “Risk Factors” for additional risks associated with our substantial capital requirements.
Cash Flows
The following table shows a summary of our cash flows for each of the periods shown below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, |
|
|
| ($ in thousands) |
2025 |
|
2024 |
|
|
| Net loss |
$ |
(16,029) |
|
|
$ |
(127,761) |
|
|
|
Non-cash adjustments to reconcile net loss to net cash used in operating activities(1): |
(47,806) |
|
|
53,404 |
|
|
|
| Changes in operating assets and liabilities: |
(15,083) |
|
|
2,812 |
|
|
|
Net cash used in operating activities |
(78,918) |
|
|
(71,545) |
|
|
|
Net cash used in investing activities |
(845) |
|
|
(1,509) |
|
|
|
Net cash provided by financing activities |
4,314 |
|
|
63,177 |
|
|
|
| Net decrease in cash, cash equivalents and restricted cash |
$ |
(75,449) |
|
|
$ |
(9,877) |
|
|
|
| Cash, cash equivalents and restricted cash at the beginning of the period |
$ |
95,290 |
|
|
$ |
80,801 |
|
|
|
| Cash, cash equivalents and restricted cash at the end of the period |
$ |
19,841 |
|
|
$ |
70,924 |
|
|
|
___________________________
(1) Primarily includes depreciation, amortization related to our leases, stock-based compensation expense, non-cash interest expense related to our revenue interest liability and our JDRF Award liability, and the changes in fair value of our Contingent Earnout Liability and our derivative liabilities and asset.
Cash Flow from Operating Activities
The increase in net cash used in operating activities from the nine months ended September 30, 2024 to the nine months ended September 30, 2025 was primarily due to increased spending on preclinical, clinical and commercial activities as well as payroll and personnel expenses, expansion of clinical development of the ATEV for use in AV access, and our commercial launch of Symvess.
Cash Flow from Investing Activities
Net cash used in investing activities for the nine months ended September 30, 2025 and September 30, 2024 consisted of purchases of property and equipment.
Cash Flow from Financing Activities
Net cash provided by financing activities for the nine months ended September 30, 2025 consisted primarily of $46.7 million of net proceeds from our 2025 Public Offering and $9.8 million of net proceeds from the issuance of stock under our ATM Facility, partially offset by a $50.0 million payment on the revenue interest liability in connection with the Purchase Agreement Amendment. Net cash provided by financing activities for the nine months ended September 30, 2024 consisted primarily of $43.0 million of net proceeds from our 2024 Public Offering and $19.5 million of proceeds from the Purchase Agreement.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in SEC rules and regulations.
Critical Accounting Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our unaudited condensed consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of our unaudited condensed consolidated financial statements requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, revenues, and expenses, and disclosure of contingent liabilities. We base our estimates and assumptions on historical experience and other factors that we believe to be reasonable under the circumstances. We evaluate our estimates and assumptions on an ongoing basis. Although we believe that our estimates, assumptions, and judgments are reasonable, they are based upon information presently available. Actual results may differ significantly from these estimates based on different assumptions, judgments, or conditions.
An accounting estimate or assumption is considered critical if both (a) the nature of the estimate or assumption involves a significant level of estimation uncertainty, and (b) the impact within a reasonable range of outcomes of the estimate and assumption is material to our financial condition. There have been no material changes to our critical accounting policies and estimates as compared to those disclosed in our audited consolidated financial statements as of and for the years ended December 31, 2024 and 2023, included in our Annual Report.
Emerging Growth Company and Smaller Reporting Company Status
We are an “emerging growth company” as defined in the Jumpstart our Business Startups Act of 2012 (the “JOBS Act”), and may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies until it is no longer an emerging growth company. Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period afforded by the JOBS Act for the implementation of new or revised accounting standards. We expect to use the extended transition period and, therefore, while we are an emerging growth company we will not be subject to new or revised accounting standards at the same time that they become applicable to other public companies that are not emerging growth companies, unless we choose to early adopt a new or revised accounting standard. This may make it difficult or impossible to compare our financial results with the financial results of another public company because of the potential differences in accounting standards used.
Additionally, we are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K under the Exchange Act (“Regulation S-K”), and may continue to qualify as such even after we no longer qualify as an emerging growth company. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company if (1) the market value of Common Stock held by non-affiliates is less than $250 million as of the last business day of the second fiscal quarter, or (2) our annual revenues in our most recent fiscal year completed before the last business day of its second fiscal quarter are less than $100 million and the market value of Common Stock held by non-affiliates is less than $700 million as of the last business day of the second fiscal quarter.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
We qualify as a smaller reporting company, as defined by Item 10 of Regulation S-K and, thus, are not required to provide the information required by this Item.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is (i) recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding required disclosure.
As of September 30, 2025, our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of September 30, 2025.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act) during the three months ended September 30, 2025 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our management, including our Chief Executive Officer and Chief Financial Officer, believes that our disclosure controls and procedures and internal control over financial reporting are designed to provide reasonable assurance of achieving their objectives and are effective at the reasonable assurance level. However, our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all error and fraud. Any control system, no matter how well designed and operated, is based upon certain assumptions and can provide only reasonable, not absolute, assurance that its objectives will be met. Further, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within our company have been detected.
PART II – OTHER INFORMATION
Item 1. Legal Proceedings
On November 18, 2024, James A. Cutshall filed a putative class action lawsuit, captioned Cutshall v. Humacyte, Inc., et al., No. 1:24-cv-00954 (the “Securities Litigation”), against the Company and certain of the Company’s officers in the United States District Court for the Middle District of North Carolina. The complaint in the Securities Litigation (the “Initial Complaint”) asserts claims under Sections 10(b) and 20(a) of the Exchange Act on behalf of a putative class of persons and entities that purchased or otherwise acquired securities of the Company between May 10, 2024 and October 17, 2024, based on allegations that the defendants made or were responsible for false or misleading statements and omissions related to the BLA for the vascular trauma indication and to alleged deficiencies at the Company’s Durham, North Carolina manufacturing facility. The Initial Complaint seeks a variety of relief, including unspecified compensatory damages, attorneys fees and costs. On January 31, 2025, the court appointed co-lead plaintiffs. On May 22, 2025, the co-lead plaintiffs filed the amended complaint in the Securities Litigation. The amended complaint expands the putative class to include persons and entities that purchased or otherwise acquired securities of the Company between August 14, 2023 and March 25, 2025. It alleges that the defendants made or were responsible for false or misleading statements and omissions related to the safety of Symvess, alleged deficiencies at the Company’s Durham, North Carolina manufacturing facility, and the Company’s financial condition and liquidity. On July 25, 2025, defendants moved to dismiss the amended complaint in its entirety and with prejudice. The court has yet to rule on the motion, which remains pending.
On January 7 and 10, 2025, putative stockholders of the Company filed two verified stockholder derivative actions in the United States District Court for the Middle District of North Carolina, captioned Silva v. Sebelius, et al., No. 1:25-cv-00005 (the “Silva Action”) and Misko v. Niklason, et al., No. 1:25-cv-00028 (the “Misko Action”). Each of these derivative actions was brought on behalf of the Company against certain of its current or former directors and officers, as well as Ayabudge LLC. The complaints in each action assert claims for violations of Section 14(a) of the Exchange Act, breach of fiduciary duty, unjust enrichment, abuse of control, gross mismanagement, and waste of corporate assets, based on a variety of allegations including claims that the defendants are responsible for any damages sustained by the Company as a result of the Securities Litigation. The Misko Action also includes a claim for contribution against certain defendants under Sections 10(b) and 21(d) of the Exchange Act for any liability the Company may sustain as a result of the Securities Litigation. On February 18, 2025, the court issued an order consolidating the Silva Action and the Misko Action (collectively, the “Consolidated Derivative Action”) and staying the defendants’ obligation to respond to any complaint in the Consolidated Derivative Action pending the submission of a proposed scheduling order. On March 11, 2025, the parties entered a joint motion to stay the Consolidated Derivative Action pending final resolution of the Securities Litigation, which the court granted.
On December 19, 2024, the Company received a demand letter (the “2024 Demand Letter”) from a purported stockholder of the Company, demanding that the Board assert claims against certain of the Company’s current or former officers and directors for breach of fiduciary duty, gross mismanagement, corporate waste, unjust enrichment, aiding and abetting, violations of Section 14(a) of the Exchange Act, and insider trading, based on a variety of allegations including claims that the Company’s current and former officers and directors are responsible for any damages sustained by the Company as a result of the Securities Litigation. On January 24, 2025, the Board appointed a demand evaluation committee to evaluate the claims made in the 2024 Demand Letter and report back to the full Board. On February 19, 2025, the purported stockholder who sent the 2024 Demand Letter filed a stockholder derivative action in the United States District Court for the Middle District of North Carolina, captioned Olson v. Niklason, et al., No. 1:25-cv-00123 (the “Olson Action”), alleging that the Company had refused his demand. The complaint in the Olson Action asserts substantive claims and allegations that are substantially similar to those asserted in the Consolidated Derivative Action. On April 22, 2025, the parties filed a joint motion to stay the Olson Action pending final resolution of the Securities Litigation, which the court granted.
On May 19, 2025, the Company received a demand letter (the “2025 Demand Letter”) from a purported stockholder of the Company, making demands and stating allegations substantially similar to those in the 2024 Demand Letter. The letter was referred to the demand evaluation committee for evaluation, and the demand evaluation committee recommended that the board of directors of the Company defer action on the demand until after the resolution of the pending motion to dismiss in the securities class action. The board of directors accepted the demand evaluation committee’s recommendation, and counsel for the demand evaluation committee informed the shareholder of the board of director’s determination to defer action on the demand by letter dated September 24, 2025.
On June 9, 2025, a putative stockholder of the Company filed a verified stockholder derivative action in the United States District Court for the District of Delaware, captioned Dusci v. Bamforth, et al., No. 1:25-cv-00722 (the “Dusci Action”). The complaint in the Dusci Action asserts substantive claims and allegations that are substantively similar to those asserted in the Consolidated Derivative Action and Olson Action. On September 8, 2025, the parties field a joint stipulation to stay the Dusci Action, pending final resolution of the Securities Litigation, which the court granted.
The Company disputes all claims asserted against it in the Securities Litigation and disputes that the plaintiffs in the Consolidated Derivative Action, the Dusci Action and Olson Action have standing to assert claims derivatively on its behalf. The Company is currently unable to estimate the potential loss or range of loss, if any, associated with these lawsuits, which could be material.
See the section “Legal Matters” contained in Note 12 — Commitments and Contingencies in the notes to our accompanying condensed consolidated financial statements for additional information.
Item 1A. Risk Factors
Our risk factors are disclosed in Part I, Item 1A of our Annual Report. There have been no material changes during the nine months ended September 30, 2025 from, or updates to, the risk factors discussed in Part I, Item 1A,
Risk Factors of our Annual Report, except as follows:
We may not successfully execute or achieve the expected benefits of cost-saving measures that we have taken or may take in the future, and our efforts may result in further actions or additional asset impairment charges and adversely affect our business.
On April 28, 2025, we implemented a cost reduction action to reduce our workforce by 30 employees, cease recruitment of additional planned new hires, and reduce other operating expenses. We undertook these cost reductions to improve our cash runway and to better align our organizational structure with our top business objectives. From time to time, we also take actions intended to address the short-term health of our business as well as our long-term objectives based on our current estimates, assumptions and forecasts. These measures are subject to known and unknown risks and uncertainties, including whether we have targeted the appropriate areas for our cost-saving efforts and at the appropriate scale, and whether, if required in the future, we will be able to appropriately target any additional areas for our cost-saving efforts. As such, the actions we are taking in connection with our current cost saving measures and that we may decide to take in the future may not be successful in yielding our intended results and may not appropriately address either or both of the short-term and long-term strategy for our business. Implementation of our current and any other cost-saving initiatives may be costly and disruptive to our business, the expected costs and charges may be greater than we have forecasted, and the estimated cost savings may be lower than we have forecasted. Certain aspects of the cost saving measures, such as severance costs in connection with reducing our headcount, could negatively impact our cash flows. In addition, our initiatives could result in personnel attrition beyond our planned reduction in headcount or reduced employee morale, which could in turn adversely impact productivity, including through a loss of continuity, loss of accumulated knowledge or inefficiency during transitional periods, or our ability to attract highly skilled employees. Unfavorable publicity about us or any of our strategic initiatives could result in reputation harm and could diminish confidence in, and the use of, our ATEVs.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosures
None.
Item 5. Other Information
Director and Officer Trading Arrangements
During the three months ended September 30, 2025, no director or officer (as defined in Rule 16a-1(f) under the Exchange Act) of the Company adopted or terminated any “Rule 10b5-1 trading arrangement” or any “non Rule 10b5-1 trading arrangement,” as each term is defined in Item 408(a) of Regulation S-K.
Item 6. Exhibits
The following exhibits are filed as part of, or incorporated by reference into, this Quarterly Report on Form 10-Q.
|
|
|
|
|
|
|
|
|
|
Exhibit
Number
|
|
Description |
| 4.1 |
|
|
|
|
|
| 10.1 |
|
|
|
|
|
| 10.2* |
|
|
|
|
|
| 10.3* |
|
|
|
|
|
| 31.1* |
|
|
|
|
|
| 31.2* |
|
|
|
|
|
| 32.1** |
|
|
|
|
|
| 32.2** |
|
|
|
|
|
| 101* |
|
The following materials from Humacyte, Inc.’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2025, formatted in Inline XBRL (Inline eXtensible Business Reporting Language): (i) Condensed Consolidated Balance Sheets (unaudited), (ii) Condensed Consolidated Statements of Operations and Comprehensive Loss (unaudited), (iii) Condensed Consolidated Statements of Changes in Stockholders’ Equity (Deficit) (unaudited), (iv) Condensed Consolidated Statements of Cash Flows (unaudited), (v) Notes to Condensed Consolidated Financial Statements (unaudited), and (vi) Cover Page. |
|
|
|
| 104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
* Filed herewith.
** This exhibit is being furnished rather than filed, and shall not be deemed incorporated by reference into any filing, in accordance with Item 601 of Regulation S-K.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized on this 12th day of November, 2025.
|
|
|
|
|
|
|
|
|
|
|
|
|
HUMACYTE, INC. |
Date: November 12, 2025 |
By: |
/s/ Laura E. Niklason, M.D., Ph.D. |
|
|
Name: |
Laura E. Niklason, M.D., Ph.D. |
|
|
Title: |
President and Chief Executive Officer |
|
|
|
|
|
By: |
/s/ Dale A. Sander |
|
|
Name: |
Dale A. Sander |
|
|
Title: |
Chief Financial Officer, Chief Corporate Development Officer and Treasurer |
EX-10.2
2
huma-20250930x10qxex102.htm
EX-10.2
huma-20250930x10qxex102

FIFTH AMENDMENT TO LEASE THIS FIFTH AMENDMENT TO LEASE (this "Fifth Amendment") is made as of September '/~ 2019, by and between ARE-NC REGION NO. 5, LLC, a Delaware limited liability company ("Landlord"), and HUMACYTE, INC., a Delaware corporation ("Tenant"). RECITALS A. Landlord and Tenant are parties to that certain Lease Agreement dated as of December 31, 2015, as amended by that certain letter agreement dated January 29, 2016, and as further amended by that certain First Amendment to Lease dated as of September 30, 2016, as further amended by that certain Second Amendment to Lease dated as of February 8, 2017 (the "Second Amendment"), as further amended by that certain Third Amendment to Lease dated as of April 21, 2017, and as further amended by that certain Fourth Amendment to Lease dated as of October 31, 2017 (as amended, the "Lease"). Pursuant to the Lease, Tenant leases that certain building located at 2525 E. NC Highway 54, Durham, North Carolina premises containing approximately 82,996 rentable square feet (the "Premises"). The Premises are more particularly described in the Lease. Capitalized terms used herein without definition shall have the meanings defined for such terms in the Lease. B. Landlord and Tenant desire, subject to the terms and conditions set forth below, to amend the Lease as provided for in this Fifth Amendment. NOW, THEREFORE, in consideration of the foregoing Recitals, which are incorporated herein by this reference, the mutual promises and conditions contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, Landlord and Tenant hereby agree as follows: 1. Base Term. Commencing on the date of this Fifth Amendment, the defined term "Base Term" on page 1 of the Lease shall be·'deleted in its entirety and replaced with the following: "A term (i) beginning, with respect to the Original Premises and the Expansion Premises, on the Commencement Date and ending on March 31, 2027, and (ii) beginning, with respect to the Second Expansion Premises, on the Second Expansion Premises Commencement Date and ending on May 31, 2028." For the avoidance of doubt, the terms of Section 6 of the Second Amendment with respect to Tenant's election to exercise its Extension Right under Section 40 of the Lease shall continue to apply. , 2. Base Rent. Attached to this Fifth Amendment as Exhibit A is a schedule of Base Rent payable under the Lease. 3. Tl Allowances. Attached to this Fifth Amendment as Exhibit B is a schedule of the Additional Rent payable in connection with the Tl Allowance, the Additional Tl Allowance, the Second Expansion Premises Tl Allowance and the Additional Tl Fund. 4. OFAC. Tenant is and, to Tenant's knowledge, all beneficial owners of Tenant are currently (a) in compliance with and shall at all times during the Term of the Lease remain in compliance with the regulations of the Office of Foreign Assets Control ("OFAC") of the U.S. Department of Treasury and any statute, executive order, or regulation relating thereto (collectively, the 11 OFAC Rules"), (b) not listed on, and shall not during the term of the Lease be listed on, the Specially Designated Nationals and Blocked Persons List, Foreign Sanctions Evaders List, or the Sectoral Sanctions Identification List, which are all maintained by OFAC and/or on any other similar list maintained by OFAC or other governmental authority pursuant to any authorizing statute, executive order, or 732914860.2 (ffi:\ Copyright© 2005, Alexandria Real fatale Equities, Inc. ALL ~ RIGHTS RESERVED. Confidential und Propriet:iry - Do Nol A L E x A N D R I A. Copy Dt' Distribute. Ale:<:ru1driu and lhe Ale,andriu L(lgo :ire registered trodemnrks of Alru:nn<lrin Real Estate Equities, Inc.

regulation, and (c) not a person or entity with whom a U.S. person is prohibited from conducting business under the OFAC Rules. 5. Brokers. Landlord and Tenant each represents and warrants that it has not dealt with any broker, agent or other person (collectively, "Broker") in connection with the transaction reflected in this Fifth Amendment and that no Broker brought about this transaction. Landlord and Tenant each hereby agrees to indemnify and hold the other harmless from and against any claims by any Broker claiming a commission or other form of compensation by virtue of having dealt with Tenant or Landlord, as applicable, with regard to this Fifth Amendment. 6. Miscellaneous. a. This Fifth Amendment is the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior and contemporaneous oral and written agreements and discussions. This Fifth Amendment may be amended only by an agreement in writing, signed by the parties hereto. b. This Fifth Amendment is binding upon and shall inure to the benefit of the parties hereto, and their respective successors and assigns. c. This Fifth Amendment may be executed in 2 or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts may be delivered via facsimile, electronic mail (including pdf or any electronic signature process complying with the U.S. federal ESIGN Act of 2000) or other transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes. Electronic signatures shall be deemed original signatures for purposes of this Fifth Amendment and all matters related thereto, with such electronic signatures having the same legal effect as original signatures. d. Except as amended by this Fifth Amendment, the Lease is hereby ratified and confirmed and all other terms of the Lease shall remain in full force and effect, unaltered and unchanged by this Fifth Amendment. In the event of any conflict between the provisions of this Fifth Amendment and the provisions of the Lease, the provisions of this Fifth Amendment shall prevail. Whether or not specifically amended by this Fifth Amendment, all of the terms and provisions of the Lease are hereby amended to the extent necessary to give effect to the purpose and intent of this Fifth Amendment. 732914860.2 [Signatures are on the next page] 2 Pl"\ Copyright@ 2005, Ale~andria Real Estaic Equities, Inc. ALL ~ RIGHTS RESERVED. Conficlcntiill and Proprietary-Do Nol AL t x AN D RI A, Cofy or DisLribule. AleKwidria ~cl the Ale~andria ~go are regtslcrccl trudcmnrks of Alcxnndna Reul Esmte Eqmtics, Inc.

IN WITNESS W HEREOF, the parties hereto have executed this Fifth Amendment as of the day and year first above written. 732914860.2 TENANT: HUMACYTE, INC., a Delaware corporation By:_·b~r"--rl..~-----·l"--_ 1.,, ,6_· u_· _j;.,J._-~--,--· ~_"'_· · 'P"r'"";.::_:J _ _ _ lts: __ ~_,,t+.y"-1"c._ ..c__t:_ t:.__--_ ...h._~_"-_·_e.....:,.(_4A _ c_ W__,__,··c_·;::.._. _ _ __ _ LANDLORD: ARE-NC REGION NO. 5, LLC, a Delaware limited liability company By: ALEXANDRIA REAL ESTATE EQUITIES, L.P., a Delaware limited partnership, 3 managing member By: ARE-QRS CORP., a Maryland corporation, general partner \ Senior Vice President RE Legal Affairs nf:\ Copyright @2005, Alexandria Real Estate Equilic:.s, [nc. ALL ~ RIGHTS RESERVED. Confidential und Proprietnry - Do Not A L r: X" N D It 1 "· Copy or Distribute. Ale~nm!ria and \he Alernndriu Logo ure registered trncfomnrks of Alexandria Reul Estate Equities, lnc ,

Base Rent suite 100 04/01 /17 - 03/31 /18 $74,507.25 04/01/18 - 03/31/19 $76,369.93 04/01/19 - 03/31/20 $78,279.18 04/01/20 - 03/31/21 $80,236.16 04/01/21 - 03/31/22 $82,242.06 04/01/22 - 03/31/23 $84,298.11 04/01/23 - 03/31/24 $86,405.57 04/01/24 - 03/31/25 $88,565.71 04/01/25 - 03/31/26 $90,779.85 04/01/26 - 03/31/27 $93,049.35 Base Rent suite 200A 05/01/17 - 05/31/17 $22,442.00 06/01/17 - 05/31/18 $- 06/01/18 - 05/31/19 $22,863.50 06/01/19 - 05/31/20 $23,435.09 06/01/20 - 05/31/21 $24,020.96 06/01 /21 - 05/31 /22 $24,621.49 06/01 /22 - 05/31 /23 $25,237.03 06/01 /23 - 05/31 /24 $25,867.95 06/01/24 - 05/31/25 $26,514.65 06/01 /25 - 05/31 /26 $27,177.52 06/01 /26 - 05/31 /27 $27,856.95 06/01 /27 - 05/31 /28 $28,553.38 732914860.2 Exhibit A Base Rent suite 225 $20,580.29 $21,094.80 $21,622.17 $22,162.72 $22,716.79 $23,284.71 $23,866.83 $24,463.50 $25,075.09 $25,701.96 A-1 suite 250 Total $25,392.67 $120,480.21 $26,027.48 $ 123,492.21 $26,678.17 $ 126,579.52 $27,345.12 $129,744.00 $28,028.75 $132,987.60 $28,729.47 $136,312.29 $29,447.71 $139,720.11 $30,183.90 $143,213.10 $30,938.50 $ 146,793.44 $31,711.96 $ 150,463.27 ~ Copyright© 2005, Alexandria Real Esta lo Equities, Inc. ALL ~ RIGHTS RESERVED. Confidential "nd Proprietary- Do Nol A I Ex AN D R I A, Copy or Dislrioule. Ale,undrla and U1e Alexundria Luso are registered tmdcmarks uf Alexandria Real E.~tate Equities, Jnc.

-l u.> Iv 'Cl .i:,. 00 °' 0 N co I -l,. X >ill ~IQ ?' ;;in~Q ~-~ ~~ !sq;i~ "-oc!~ ~ ~-~~ g- 5' f¾l 8 ~*at Cf;;. o., fa.ti'• X ~§~l ~~~;; Re ~ o ~-o..[E.. :,;, s:-'" Ul O fl ;:I 6'" a>o..o Uln_gi * s ]. ~: ..81 et;;; l), §. ~9 ;' ii'"a or> ;;.~;~ !' o gr A-Tl Allowance Original Premises Annual Escalation Rate A - Tl Allowance $ Additional Rent Rate $ Annual Additional Rent $ Monthly Additional Rent $ 2.50% annual escalation rate 1,408,840 billing for Tl spend above $35/rsf ($20 x 74,442 rsf (with ste 100 not remeasured)) 0.1585 per rsf of the Original Premises per year 223,301 18,608.43 Building BillingGrou12 Unit From To Gross Amount 16013 16013 100/250/225 05/01/17 03/31/18 $ 18,608.43 16013 16013 100/250/225 04/01/18 03/31/19 $ 19,073.64 16013 16013 100/250/225 04/01/19 03/31/20 $ 19,550.48 16013 16013 100/250/225 04/01/20 03/31/21 $ 20,039.24 16013 16013 100/250/225 04/01/21 03/31/22 $ 2.0,540.22 16013 16013 100/250/225 04/01/22 03/31/23 $ 21,053.73 16013 16013 100/250/225 04/01/23 03/31/24 $ 21,580.07 16013 16013 100/250/225 04/01/24 03/31/25 $ 22,119.57 16013 16013 100/250/225 04/01/25 03/31/26 $ 22,672.56 16013 16013 100/250/225 04/01/26 03/31/27 $ 23,239.38 suite ill allocated 100 43,531 $ 11,499.44 250 14,864 $ 3,926.57 225 12,047 $ 3,182.42 70.442 $ 18.608.43 fil. Pay Term M 01 )> M D1 C. M Dl C. m ;:; M D1 s· >< ::r M D1 :::, a: M Dl !. ;:; M Dl ::0 OJ M D1 (D M D1 :::, -M D1 esqbru 3.31.19 illlluici~~~mark $ 11,786.93 A $ 4,024.74 A s 3,261.98 A $ ... ~073.64

-...l w tv \0 ~ 00 °' 0 iv co I N ~~ 0~ ,. ;i n~Q ~-~ ~~ ! g v1~ i~~@ ~i~~ 3 "' :,it;; IP fi <• is. r:,:,,. B_~!='[ ► H>g_ [~ 51 ~- H 
-...J w ts ~ 00 °' 0 N OJ I <.,..) ;:~ ;0 C - Tl Allowance ( rsf adjustment} Annual Escalation Rate C- Tl Allowance {rsf adjustment} $ Original Premises Additional Rent Rate $ Annual Additional Rent $ Monthly Additional Rent $ 2.50% annual escalation rate 1,660 billing for Tl spend above $35/rsf on the incremetal rsf adjustment for ste 100 0.1585 perrsf of the Original Premises per year 263 21.93 Building Billing Graue Unit From To Gross Amount 16013 16013 100/2.50/225 05/01/17 03/31/18 $ 21.93 16013 16013 100/250/225 04/01/18 03/31/19 $ 22.47 16013 16013 100/250/225 04/01/19 03/31/20 $ 23.04 16013 16013 100/250/225 04/01/20 03/31/21 $ 23.61 16013 16013 100/250/225 04/01/21 03/31/22 $ 24.20 16013 16013 100/250/225 04/01/22 03/31/23 $ 24.81 16013 16013 100/250/2.25 04/01/23 03/31/24 $ 25.43 16013 16013 100/250/225 04/01/24 03/Sl/25 s 26.06 16013 16013 100/250/225 04/01/25 03/31/26 $ 26.71 16013 16013 100/250/225 04/01/26 03/31/27 $ 27.38 suite rsf allocated fil Pay Term M D1 M D1 M D1 M D1 M 01 M D1 M D1 M 01 M D1 M D1 esc thru 3.31.19 myuirx tick rnark 100 83 $ 21.93 $ 22.47 250 $ $ 225 $ s 83 ~ 21.93 $ 22.47 H~i--------------------------------------'-~====---------- ~ Et ~,g- c., 0 g- ;;i ~- (g@ g- 5' !ll 8 P;;i; C v.>c,- ~[~~ fiP1i a~·~; HF l:11:i-,, ~ a.;;.E.~ i[lf i.~l! g·i ~~ -;__t=' 0 > ~~~~
--..;J w N '-0 ~ 00 0\ 0 N OJ I .i::>-, ~E)--· ~ ,.1 0 "' > cl Qt:9 Q ,~ §l] a~ tiit ~~~~ H~g :l = ~ lJt ~~§~ ~n· ~ ~!lQ[ ~ §: ~ ~~ 5- t!' g ~ g-b·e. :,d 5- ;;- ~ [;a~ ni.w i; g_ :I.§, f.f!-~ :... ~ 0 ► P~r:: D -ATI Allowance 2 Annual Escalation Rate D - ATI Allow~nce $ Expansion Premises Additional Rent Rate $ Annual Additional Rent $ Monthly Additional Rent $ First Amendment Section 4 I 2.50% annual escalation rate 2,381,764 ATI AHowance for Original and 1st Expansion Premises (100,225,250} 0.1585 per rsf of the Original Premises per year 377,5,10 31,459_13 Building Billing Group Unit From IQ Gross Amount fil: Pay Term 16013 16013 100/250/225 08/01/17 03/31/18 $ 31,459.13 M D1 16013 16013 100/25p/225 04/01/18 03/31/19 $ 32,245.61 M D1 16013 16013 100/250/225 04/01/19 03/31/20 $ 33,051.75 M D1 16013 16013 100/25,0/225 04/01/20 03/31/21 $ 33,878.04 M Dl 16013 16013 100/250/225 04/01/21 03/31/22 $ 34,724.99 M D1 16013 16013 100/250/225 04/01/22 03/31/23 $ 35,593.12 M 01 16013 16013 100/250/225 04/01/23 03/31/24 $ 36,482.94 M D1 16013 16013 100/250/225 04/01/24 03/31/25 $ 37,395.02 M Dl 16013 16013 100/250/225 04/01/25 03/31/26 $ 38,329-89 M D1 16013 16013 100/250/225 04/01/26 03/31/27 s 39,288.14 M D1 suite rsf allocated esc thru 3 .31.19 inquiry tick m:irk 100 43,614 $ 19,454.92 $ 19,941.30 D 250 14,864 $ 6,630_39 $ 6,796.15 225 12,04.7 $ 5,373.81 s 5,508.16 70~ 31,459_13 $ 32,245.61
-.J w N '-0 :;;: 00 0\ 0 t,,J co I 01 ~~ 0 ,. E -ATI Allowance Expansion Premises Annual Escalation Rate E - ATI Allowance $ Additional Rent Rate $ Annual Additional Rent $ Monthly Additional Rent $ 2.50% annual escalation rate 408,449 ATI Allowance for Orginal and 1st Expansion Premises (100, 225, 250} 0.1585 per rsf of the Expansion Premises per year 64,739 5,395 Building BillingGroui;i Unit From To Gross Amouot 16013 16013 100/250/225 09/Ql/17 03/31/18 $ 5,394.93 16013 16013 100/250/225 04/01/18 03/31/19 $ 5,529.80 16013 16013 100 /250/22.5 04/01/19 03/31/20 $ 5,668.05 16013 16013 100/250/225 04/01/20 03/31/21 $ 5,809.75 16013 16013 100/250/225 04/01/21 03/31/22 $ 5,954.99 16013 16013 100/250/225 04/01/22 03/31/23 $ 6,103.87 16013 16013 100/250/225 04/01/23 03/31/24 $ 6,256.46 16013 16013 100/250/225 04/01/24 03/31/25 $ 6,412.88 16013 16013 100/250/225 04/01/25 03/31/26 $ 6,573.20 16013 16013 100/250/225 04/01/26 03/31/27 $ 6,737.53 suite rsf allocated fil: Pay Term M D1 M D1 M D1 M D1 M Dl M D1 M D1 M D1 M D1 M Dl esc thru 3.31.19 inquiJy1ick mark 100 43,614 $ 3,336.33 $ 3,419.74 E 250 14,864 $ 1,137.05 $ 1,165.47 225 12,047 $ 921.56 $ 944.59 70.525 $ 5,394.93 _S 5,529.80 ?" ~.~a.g i;"" :X:'-< i~~~ ;;iii.";@ n~8-----~:~; -------------------------------------------------~~===~~~i~~~~~~~=~~~~--~- ~GP[ HH r.: :?. ~ fil~ ~gp ::J a.;·~ Hif t11;;->os ~iH'. f~f~ )" '\3 0 f' ,;<i; 0 ► po~~

--..I w N '-0 :;;:: 00 O"I 0 tv a:, I CJ) ;:: ;~ ZI@ 0 ?- F -ATI Allowance 2.rid Expansion Premises Annual Escalation Rate F-ATI Allowance $ Additional Rent Rate $ Annual Additional Rent $ Monthly Additional Rent $ 2.50% annual escalation rate 384,595 Additional Tl Allowance 2nd Expansion Premises (200A) 0.1585 per rsf of the 2nd Expansion Premises per year 60,958 5,080 Building Billing Groui:; Unit From 16013 16013 200A 09/01/17 16013 16013 200A 06/01/18 160i3 16013 200A 06/01/19 16013 16013 200A 06/01/20 16013 16013 200A 06/01/?.1 16013 16013 200A 06/01/22 16013 16013 200A 06/01/23 16013 16013 200A 06/01/24 16013 16013 200A 06/01/25 16013 16013 200A 06/01/26 16013 16013 200A 06/01/27 IQ Gross Amount .fil: Pay Term 05/31/18 $ 5,079.86 M 01 05/31/19 $ 5,206.85 M D1 05/31/20 $ 5,337.02 M D1 05/31/21 $ 5,470.45 M D1 05/31/i2 $ 5,60,7.21 M D1 05/31/23 $ SJ47.39 M D1 05/31/24 $ 5,891.08 M D1 05/31/25 $ 6,038.35, M D1 05/31/26 $ 6,189.31 M DI 05/31/27 $ 6,344.0,4 M D1 08/31/27 $ 6,502.65 M DI ilJ!l.!!lrl.!i!:kmark F ~ti~~ l _____________________________________________________________________________ _ fg ~J c..o ~ [~·ilj@ G g' !2 8 !H~ "';,,gj::: ~f~§ :!.':§ g g. H ~ ~ g. [ i e. l<lS:-., U1 !~P i §1.f f~f~ ~°a o· -;.....e,:, a ► ~ 0 fF
-.J w N I.O :j;'. 00 0\ 0 iv OJ I ---1 ~ ;~.-: .. z~ Cl "' > ;; Q2:!Q ~-~ g~ a g ~~ ~ ¥- ~ e} g_ 5. !ii 8 H~Y' hi:ii ..., ~ (l ~ ~l~g: [ ~- g~ ~·8. [[ ?O 5-:, ~ e.;. ~ ~ ffl-g i;::, ::1. -· ll1~]J il bi ·s-a z f'.: p E. t""" G - Admin Rent Annual Escalation Rate G-Admln Rent $ 2nd Expansion Premises Additional Rent Rate $ Annual Additional Rent $ Monthly Additional Rent $ 2.50% annual escalation rate 188,860 Administrative Rent (1% of Hard Cost) 0.1585 per rsf of the 2nd Expansion Premises per year 29,934 2,494.53 Building Billing GrOU[! Unit From 16013 16013 200A 09/01/17 16013 16013 200A 06/01/18 16013 16013 200A 06/01/19 16013 16013 200A 06/0i/20 16013 16013 200A 06/01/21 16013 16013 200A 06/01/22 16013 16013 200A 06/01/23 16013 16013 200A 06/01/24 16013 16013 200A 06/01/25 16013 16013 200A 06/01/26 16013 16013 200A 06/01/27 IQ Gross Amount fil Pay Term 05/31/18 $ 2,494.53 M 01 05/31/19 $ 2,556.89 M D1 05/31/20 $ 2,620.81 M Dl 05/31/21 $ 2,685.33 M Dl 05/31/22 $ 2,753.49 M D1 05/31/23 $ 2,822.33 M 01 05/31/24 $ 2,892.89 M 01 05/31/25 $ 2,965.21 M 01 OS/31/26 $ 3,039.34 M 01 05/31/27 $ 3:,115.32 M 01 08/31/27 $ 3,193.21 M D1 ;.,~ G
-.l w C5 '.j;'. 00 CJ\ 0 l'-1 rn I 0) X >£8\ ~w "' > .g 1?c1Q ;;;·~ g~ "_ ., n. [~(/)~ H-$5M ATI 4th Amendment ii' a·;e1 g-g, !;] 8 ~ i <:1" hB;'-----·~~ ~g_ H~~ °'s g" ~·c. [~ :tlS-.,, l);l ~~~~ J~j.f f}!{ :..-i~c ► ~ ri ~ f= Annual Escalation Rate gross allowance by lessor calculation of maximum Tl ftlnd / square foot $ Incremental Increase in Base Rent per year $ Additional Rent with respect to Tl Allowance per month $ Suite 100 200A 225 250 2.50% annual es~alation rate 5,000,000 60.2439 9.8186 67,908.71 From RSF 43,614 12,471 12,047 14;864 82;996 %ofRSF 52.55% 15.03% 14.52% 17.91% 100.00% 12/01/17 04/01/18 04/01/19 04/01/20 04/01/21 04/01/22 04/01/23 Q4/0l/24 04/01/25 04/01/26 12/01/17 04/01/18 04/01/19 04/01/20 04/01/21 04/01/22 04/01/23 04/01/24 04/01/25 04/01/26 To 03/31/18 03/3'1/19 03/31/20 03/~1/21 03/31/22- 03/31/23 03/31/24 03/31/25 03/31/26 03/31/27 05/31/18 05/31/19 05/31/20 05/31/21 05/31/22 05/31/23 05/31/24 05/31/25 05/31/26 03/31/27 Suitel00 35;686 36,578 37,492 38,430 39,390 40,375 41,384 42,419 43,480 44,567 Suite 200A 10,204 10,45~ 10,72;1. 10,989 11,253 11,545 11,833 12,129 12,433 iZ,743 Suite 225 9;as1 10,103 i0,356 10;615 10;880 11,152 11,431 11,717 12,010 12,3-10 Suite 250 12,162 12,466 12,778 13,097 13,425· 13,760 14,104 14,457 14,818 15,189
EX-10.3
3
huma-20250930x10qxex103.htm
EX-10.3
huma-20250930x10qxex103

1 SIXTH AMENDMENT TO LEASE THIS SIXTH AMENDMENT TO LEASE (this “Sixth Amendment”) is made as of September ___, 2025 (the “Effective Date”), by and between ARE-NC REGION NO. 5, LLC, a Delaware limited liability company (“Landlord”), and HUMACYTE, INC., a Delaware corporation (“Tenant”). RECITALS A. Landlord and Tenant are parties to that certain Lease Agreement dated as of December 31, 2015, as amended by that certain letter agreement dated January 29, 2016, that certain First Amendment to Lease dated as of September 30, 2016, that certain Second Amendment to Lease dated as of February 8, 2017, that certain Third Amendment to Lease dated as of April 21, 2017, that certain Fourth Amendment to Lease dated as of October 31, 2017, that certain letter agreement dated as of January 14, 2019 (the “2019 Letter Agreement”), and that certain Fifth Amendment to Lease dated September 4, 2019 (“Fifth Amendment”) (as amended, the “Lease”). Pursuant to the Lease, Tenant leases that certain building located at 2525 E. NC Highway 54, Durham, North Carolina premises containing approximately 82,996 rentable square feet (the “Premises”). The Premises are more particularly described in the Lease. Capitalized terms used herein without definition shall have the meanings defined for such terms in the Lease. B. The Term of the Lease with respect to (i) the Original Premises and the Expansion Premises is scheduled to expire on March 31, 2027, and (ii) the Second Expansion Premises is scheduled to expire on March 31, 2028. C. Pursuant to Section 3 of the Fifth Amendment, Tenant is required to pay certain Additional Rent in connection with the TI Allowance, the Additional TI Allowance, the Second Expansion Premises TI Allowance and the Additional TI Fund (collectively, the “TI Rent”). D. Additionally, as of the Effective Date, (i) Landlord has performed certain capital improvements and repairs at the Project relating to (1) chiller replacement, (2) the Building’s main entry, (3) the parking lots, (4) paving work, and (5) roof replacement (collectively, the “CapEx Improvements”), and (ii) pursuant to Section 5 of the Lease, Tenant has been paying (and is required to continue paying) to Landlord the amortized costs of such CapEx Improvements as set forth in Section 5 of the Lease (such amortized costs, the “CapEx Costs”). E. Landlord and Tenant desire, subject to the terms and conditions set forth below, to amend the Lease to, among other things, (i) extend the Term of the Lease with respect to the entire Premises through March 31, 2037 (the “Sixth Amendment Expiration Date”), (ii) on January 1, 2026 (the “Sixth Amendment Adjustment Date”), modify certain rental amounts payable by Tenant under the Lease, including, without limitation, Base Rent and Operating Expenses, (iii) subject to further conditions set forth herein, on the Sixth Amendment Adjustment Date, forgive (a) the balance of the TI Rent remaining unpaid as of the Sixth Amendment Adjustment Date (such amount, the “Outstanding TI Rent”) and (b) the balance of the CapEx Costs remaining unpaid as of the Sixth Amendment Adjustment Date (such amount, the “Outstanding CapEx Costs”), and (iv) grant Tenant one right to extend the Term of the Lease for a period of 5 years. NOW, THEREFORE, in consideration of the foregoing Recitals, which are incorporated herein by this reference, the mutual promises and conditions contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, Landlord and Tenant hereby agree as follows: 1. Term. The Term of the Lease with respect to the entire Premises is hereby extended through the Sixth Amendment Expiration Date. Tenant’s occupancy of the Premises through the Sixth Amendment Expiration Date shall be on an “as-is” basis. Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E 29

2 2. Base Rent. Tenant shall continue paying Base Rent with respect to the Original Premises, Expansion Premises and Second Expansion Premises as set forth in Exhibit A to the Fifth Amendment through December 31, 2025. Notwithstanding anything to the contrary contained in the Lease, on the Sixth Amendment Adjustment Date, Tenant shall commence paying Base Rent with respect to the entire Premises in the amount of $39.00 per rentable square foot of the Premises per year. Base Rent with respect to the entire Premises shall thereafter be increased on each annual anniversary of the Sixth Amendment Adjustment Date (each, a “Sixth Amendment Rent Adjustment Date”) by multiplying the Base Rent payable immediately before such Sixth Amendment Rent Adjustment Date by 3% (the “Sixth Amendment Rent Adjustment Percentage”) and adding the resulting amount to the Base Rent payable immediately before such Sixth Amendment Rent Adjustment Date. Base Rent, as so adjusted, shall thereafter be due as provided herein. Notwithstanding anything to the contrary contained herein, so long as Tenant is not in Default under the Lease, Base Rent shall be abated during (i) the period commencing on January 1, 2026, through March 31, 2026 (the “Initial Base Rent Abatement Period”), and (ii) the period commencing on January 1, 2027, through March 31, 2027 (the “Subsequent Base Rent Abatement Period”; with the Initial Base Rent Abatement Period, collectively, the “Base Rent Abatement Periods”). Tenant shall commence paying full Base Rent on the day immediately following the expiration of the Initial Base Rent Abatement Period and the Subsequent Base Rent Abatement Period, as applicable. For the avoidance of doubt, during the Base Rent Abatement Periods, Tenant shall be required to pay administration rent each month equal to the amount of the administration rent that Tenant would have been required to pay in the absence of there being Base Rent Abatement Periods. 3. Forgiven Rent. a. Outstanding TI Rent. Tenant shall continue paying TI Rent as set forth in Exhibit B to the Fifth Amendment through December 31, 2025. Notwithstanding anything to the contrary contained in the Lease, so long as no Default occurs under the Lease during the period commencing on the Effective Date through December 31, 2025, then, on the Sixth Amendment Adjustment Date, Landlord shall forgive the Outstanding TI Rent, in the total amount of $2,757,038.77, and Tenant shall thereafter have no obligation to pay such amount to Landlord. For the avoidance of doubt, if any Default occurs under the Lease during the period commencing on the Effective Date through December 31, 2025, then Tenant shall be required to continue paying the Outstanding TI Rent as set forth in Exhibit B to the Fifth Amendment. b. Outstanding CapEx Costs. Tenant shall continue paying the CapEx Costs pursuant to the Lease through December 31, 2025. Notwithstanding anything to the contrary contained in the Lease, so long as no Default occurs under the Lease during the period commencing on the Effective Date through December 31, 2025, then, on the Sixth Amendment Adjustment Date, Landlord shall forgive the Outstanding CapEx Costs, in the total amount of $187,916.23, and Tenant shall thereafter have no obligation to pay such amount to Landlord. For the avoidance of doubt, if any Default occurs under the Lease during the period commencing on the Effective Date through December 31, 2025, then Tenant shall be required to continue paying the Outstanding CapEx Costs pursuant to Section 5 of the original Lease. 4. Tenant Improvements. Commencing on the Effective Date, Tenant shall have the right to construct the Tenant Improvements within the Premises pursuant to the terms and conditions of the work letter attached hereto as Exhibit A (the “Sixth Amendment Work Letter”). 5. Building System Improvements. Commencing on the Sixth Amendment Adjustment Date, Landlord shall make available to Tenant a tenant improvement allowance in the amount of $4,500,000.00 (the “Building System Improvement Allowance”), for the costs to repair and/or replace, as applicable, certain Building Systems to be mutually agreed upon by Landlord and Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

3 Tenant pursuant to the terms of this Section 5 (the “Building System Improvements”). The Building System Improvements shall be selected by Tenant, subject to Landlord’s reasonable approval, from the list of Building Systems described on Exhibit B-1 attached hereto, or such other Building Systems as may otherwise be mutually agreed to in writing by Landlord and Tenant in each party’s reasonable discretion (not to be unreasonably withheld, conditioned or delayed). Subject to the foregoing sentence, the Building System Improvements shall be performed by Tenant in phases pursuant to a schedule, plans and specifications, and a scope of work mutually acceptable to Landlord and Tenant. Tenant acknowledges and agrees that Landlord shall have reasonable approval (not to be unreasonably withheld, conditioned or delayed) over: (x) whether a Building System will be repaired or replaced as part of the Building System Improvements, and (y) the order in which Building Systems are repaired and/or replaced, as applicable, as part of each phase of the Building System Improvements; provided, however, Landlord shall in each case consider input from Tenant regarding Tenant’s operating needs at the Building. The Building System Improvements shall be performed in accordance with the specifications set forth on Exhibits B-1 and B-2; provided, however, that the parties acknowledge and agree that the specifications described on Exhibit B-2 are preliminary in nature only and subject to change as mutually agreed upon by Landlord and Tenant. The Building System Improvement Allowance shall be available only for the costs of the Building System Improvements. Tenant shall pay to Landlord administration rent in the amount of 1% of the total costs of the Building System Improvements, which shall be paid to Landlord out of the Building System Improvement Allowance in a proportional amount with each Tenant draw request for reimbursement of costs incurred by Tenant in performing the Building System Improvements. Except for Tenant’s right to reimbursement by Landlord of the Building Systems Improvement Allowance subject to and in accordance with the terms of this Section 5, Tenant shall be solely responsible for all of the costs of the Building System Improvements. Tenant acknowledges that the Building System Improvements may not be removed from the Premises by Tenant at any time during the Term or upon the expiration or earlier termination of the Term. The Building System Improvements shall be treated as Alterations and shall be undertaken pursuant to Section 12 of the original Lease and this Section 5. The contractors and engineers for the Building System Improvements shall be selected by Tenant, subject to Landlord’s approval, which approval shall not be unreasonably withheld, conditioned or delayed. Landlord hereby approves of the contractors, subcontractors and engineers identified on Exhibit B-3 attached hereto. Prior to the commencement of each phase of the Building System Improvements, Tenant shall deliver to Landlord (i) a copy of any contract with Tenant’s contractors, engineers and consultants performing any part of such phase of Building System Improvements for Landlord’s review and approval (which approval shall not be unreasonably withheld, conditioned or delayed), and (ii) certificates of insurance from any contractor performing any part of such phase of the Building System Improvements evidencing industry standard commercial general liability, automotive liability, “builder’s risk”, and workers’ compensation insurance. Landlord shall be named a third party beneficiary of any contract entered into by Tenant with any engineer, any consultant, any contractor or any subcontractor, and of any warranty made by any contractor or any subcontractor. Tenant shall cause the contractors for the Building System Improvements to provide a certificate of insurance naming Landlord and Alexandria Real Estate Equities, Inc. as additional insureds for the contractor’s liability coverages required above. Prior to the commencement of each phase of the Building System Improvements, Tenant shall also obtain a detailed breakdown of the costs incurred or that will be incurred, in connection with the performance of such phase of the Building System Improvements (each, a “Building Systems Budget”), and deliver a copy of such Building Systems Budget to Landlord for Landlord’s approval, which shall not be unreasonably withheld, conditioned or delayed. Each Building Systems Budget shall be based upon the finalized Building System Improvements approved by Landlord for such phase of the Building System Improvements work, including, without limitation, the finalized schedule, plans and specifications, and scope of work thereof. Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

4 During the course of design and construction of the Building System Improvements, Landlord shall reimburse Tenant for the cost of the Building System Improvements once a month against a draw request on Landlord’s standard form, containing evidence of payment of the applicable costs and such certifications, lien waivers (including a conditional lien release for each progress payment and unconditional lien releases for the prior month’s progress payments), inspection reports and other matters as Landlord customarily obtains, to the extent of Landlord’s approval thereof for payment, no later than 45 days following receipt and approval (not to be unreasonably withheld, conditioned or delayed) by Landlord of such draw request. Upon completion of the Building System Improvements (and prior to any final disbursement of the Building System Improvement Allowance), Tenant shall deliver to Landlord the following items: (i) sworn statements setting forth the names of all contractors and subcontractors who did work on the Building System Improvements and final unconditional lien waivers from all such contractors and subcontractors; (ii) if applicable, “as built” plans for the Building System Improvements; and (iii) reasonably acceptable invoices for the Building System Improvements showing the costs thereof and proof of payment. Landlord shall reimburse Tenant for the cost of the Building System Improvements up to the then-remaining balance of the Building System Improvement Allowance, within 45 days after receipt and approval (not to be unreasonably withheld, conditioned or delayed) by Landlord of all of the items referred to in the preceding sentence. Notwithstanding the foregoing, if at any time and from time-to-time the then current costs of the Building System Improvements exceed the remaining unexpended Building System Improvement Allowance, Tenant shall be required to pay 100% of all outstanding excess costs as a condition precedent to Landlord’s obligation to fund any remaining portion of the Building System Improvement Allowance. If Tenant fails to pay, or is late paying such excess costs, Landlord shall have all of the rights and remedies set forth in the Lease for nonpayment of Rent (including, but not limited to, the right to interest at the Default Rate and the right to assess a late charge). The Building System Improvement Allowance shall only be available for use by Tenant for the performance of the Building System Improvements beginning on the date this Sixth Amendment is mutually executed by the parties, through December 31, 2027 (the “Outside Allowance Date”). Any portion of the Building System Improvement Allowance which has not been disbursed on or before the Outside Allowance Date shall be forfeited and shall not be available for use by Tenant. In no event shall Landlord have any obligation to fund any portion of the Building System Improvement Allowance during any period that Tenant is in Default under the Lease. Notwithstanding anything to the contrary contained in this Sixth Amendment, subject to the terms of this paragraph, Tenant may elect to apply a portion of the Building System Improvement Allowance, not to exceed $360,000.00 in the aggregate, towards the costs incurred by Tenant with respect to the Prior Building System Improvements (as hereinafter defined). Tenant may elect to apply the Building System Improvement Allowance towards the costs of the Prior Building System Improvements, if at all, by delivering written notice thereof to Landlord no later than the Outside Allowance Date, which notice shall include (i) the total amount of the Building System Improvement Allowance that Tenant desires to apply towards the costs of Prior Building System Improvements, (ii) reasonably acceptable invoices for the applicable Prior Building System Improvements showing the costs thereof and proof of payment, (iii) to the extent not already delivered by Tenant to Landlord, copies of all documents required to be delivered to Landlord pursuant to Sections 12 and 13 of the Lease, and (iv) any other items reasonably requested by Landlord. No later than 45 days following Landlord’s receipt and approval (not to be unreasonably withheld, conditioned or delayed) of the foregoing documents, Landlord shall reimburse Tenant for such approved Prior Building System Improvements costs (not to exceed $360,000.00 in the aggregate). For the avoidance of doubt, the Building System Improvement Allowance shall be reduced by the amount reimbursed by Landlord to Tenant for the cost of Prior Building System Improvements, if any. As used herein, “Prior Building System Improvements” means the costs incurred by Tenant to replace and/or repair Building Systems, as reasonably approved by Landlord, during the period commencing on January 1, 2024, through the date immediately prior to the Effective Date. Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

5 Notwithstanding anything to the contrary contained in the Sixth Amendment Work Letter, if the cost of the Building System Improvements exceeds the Building System Improvement Allowance, then, subject to Landlord’s reasonable review and approval, which approval shall not be unreasonably withheld, conditioned or delayed, Tenant may elect to apply all or any portion of the remaining Total TI Allowance (as defined in the Sixth Amendment Work Letter) for which Tenant has not requested disbursement towards the cost of the Building System Improvements. Tenant may elect to apply the remaining Total TI Allowance for which Tenant has not requested disbursement towards the Building System Improvements, if at all, by delivering written notice thereof to Landlord no later than the Outside Allowance Date. Such notices shall include (i) the total amount of the Total TI Allowance that Tenant desires to apply towards such Building System Improvements costs, (ii) reasonably acceptable invoices for such applicable Building System Improvements showing the costs thereof and proof of payment, and (iii) any other items reasonably requested by Landlord. No later than 45 days following Landlord’s receipt and approval (not to be unreasonably withheld, conditioned or delayed) of all of the foregoing documents, Landlord shall apply such approved portion of the Total TI Allowance towards the costs of the Building System Improvements. For the avoidance of doubt, the Total TI Allowance shall be reduced by the amount applied towards the costs of the Building System Improvements, if any. 6. Operating Expenses. a. Operating Expenses Definition. Commencing on the Sixth Amendment Adjustment Date, the definition of the term “Operating Expenses” set forth in the second paragraph of Section 5 of the original Lease shall be deleted from the beginning of such paragraph through the words “excluding only,” and such language shall be replaced with the following: “The term “Operating Expenses” means all costs and expenses of any kind or description whatsoever incurred or accrued each calendar year by Landlord with respect to the Project including, without limitation, (1) Taxes (as defined in Section 9), (2) insurance, (3) the cost of any common amenities now or hereafter located in, on or otherwise serving the Project, if any, as may exist from time to time, as determined by Landlord in Landlord’s sole and absolute discretion (the “Project Amenities”), including, without limitation, commercially reasonable subsidies which Landlord may provide in connection with the Project Amenities, (4) the Project Amenities Share of the Submarket Amenities Operating Expenses (as defined in Section 7 of the Sixth Amendment), (5) the cost of repairs, improvements and replacements, provided that to the extent that such repairs, improvements and/or replacements are reasonably determined by Landlord in accordance with sound real estate accounting principles to be capital in nature (each, a “Capital Expenditure”), such costs shall be amortized, with reasonable interest thereon, over the lesser of 10 years and the useful life of such Capital Expenditure, as reasonably determined by Landlord taking into account all relevant factors including, without limitation, the 24/7 operation of the Building, and (6) the costs of Landlord’s third party property manager or, if there is no third party property manager, administration rent in the amount of 4% of Base Rent, excluding only:” b. [Intentionally Deleted]. c. Controllable Operating Expenses. Notwithstanding anything to the contrary contained in Section 5 of the Lease, commencing on January 1, 2027, the fourth paragraph of Section 5 of the original Lease shall be deleted in its entirety and shall be replaced with the following: “From and after the expiration of the Controllable Operating Expense Base Year (i.e., January 1, 2027), that part of Operating Expenses which is comprised of Controllable Operating Expenses (as defined below) shall be increased by no more than 5% per year. For the avoidance of doubt, the first annual 5% increase shall be calculated using the actual amount of Controllable Operating Expenses incurred during the Controllable Operating Expense Base Year. Such limitation of 5% per year on increases shall be cumulative year Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

6 to year, so that if in any year the increase in cumulative Operating Expenses is more or less than 5%, then the difference between 5% and the actual percentage increase in that year may be carried forward to any future year, and may be applied in such future year to increase the actual percentage increase (even if more than 5% for such year) subject to the limitation that Controllable Operating Expenses shall not have increased by more than 5% compounded annually since the expiration of the Controllable Operating Expense Base Year. As used herein, the “Controllable Operating Expense Base Year” shall mean the period commencing on January 1, 2026, through December 31, 2026. “Controllable Operating Expenses” shall mean those Project Operating Expenses for which increases are reasonably within the control of Landlord, and shall specifically not include, without limitation, Taxes, assessments, refuse and or trash removal, insurance, collectively bargained union wages, electricity and other utilities. There shall be no limitation on the amount of increase from year to year on Project Operating Expenses which are not Controllable Operating Expenses.” 7. Tenant’s Repair and Maintenance Obligations. The parties acknowledge and agree that, as of the Effective Date, (x) all Building Systems at the Project constitute Exclusive Building Systems (as defined in Section 13 of the original Lease), and (y) Tenant is responsible, at its sole cost and expense (excluding the Building System Improvement Allowance which is being provided pursuant to Section 5 above with respect to the Building System Improvements), for performing all of Landlord’s maintenance, repair and replacement obligations with respect to the Exclusive Building Systems pursuant to and in accordance with the requirements set forth in the third paragraph of Section 13 of the original Lease, including, without limitation, any capital repairs and replacements of the Exclusive Building Systems, subject to Landlord’s prior written consent. The parties further acknowledge and agree that the 2019 Letter Agreement is hereby terminated and is null and void and of no further force or effect. 8. Submarket Amenities. (a) Generally. During the Term, an affiliate of Landlord (“ARE Landlord Affiliate”) may construct certain common amenities at property owned by such ARE Landlord Affiliate and located at or within the vicinity of the project commonly known as “The Alexandria Center for Sustainable Technologies” (the “Affiliate Project”), which may include, without limitation, shared conferencing facilities (“Shared Conference Facilities”) and/or a fitness center (collectively, the “Submarket Amenities”) for non- exclusive use by (a) Tenant, (b) other tenants of the Project, (c) Landlord, (d) the tenants of ARE Landlord Affiliate, (e) ARE Landlord Affiliate, (f) other affiliates of Landlord and Alexandria Real Estate Equities, Inc. (“ARE”), (g) the tenants of such other affiliates of Landlord and ARE, and (h) any other parties permitted by ARE Landlord Affiliate (collectively, “Users”). Landlord, ARE Landlord Affiliate, ARE, and all affiliates of Landlord, ARE Landlord Affiliate and ARE may be referred to collectively herein as the “ARE Parties.” Notwithstanding anything to the contrary contained herein, Tenant acknowledges and agrees that ARE Landlord Affiliate shall have the right, in its sole discretion, to construct any Submarket Amenities desired by ARE Landlord Affiliate at the Affiliate Project but not make all or a portion of such Submarket Amenities available for use by some or all currently contemplated Users. ARE Landlord Affiliate shall have the sole right to determine all matters related to the Submarket Amenities including, without limitation, relating to the type, design and construction thereof. Tenant acknowledges and agrees that Landlord has not made any representations or warranties regarding the development of any of the Submarket Amenities and that Tenant is not entering into this Lease relying on the construction and completion of the Submarket Amenities or with an expectation that the Submarket Amenities will ever be constructed and/or made available to Tenant. (b) License. Commencing on the date that all or a portion of the Submarket Amenities are made available for use by Users (the “Submarket Amenities Commencement Date”), and so long as the Affiliate Project and the Project continue to be owned by affiliates of ARE, Tenant shall have the non- Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

7 exclusive right to the use of the available Submarket Amenities in common with other Users pursuant to the terms of this Section 7. To the extent that the Submarket Amenities include a fitness center, fitness center passes shall be issued to Tenant for all full time employees of Tenant employed at the Premises. (c) Operating Expenses. As used in the Lease, “Submarket Amenities Operating Expenses” shall mean all costs and expenses of any kind or description whatsoever incurred or accrued each calendar year with respect to the Submarket Amenities (including, without limitation, commercially reasonable subsidies which ARE Landlord Affiliate or its affiliates may provide in connection with the Submarket Amenities), not including costs or expenses in connection with the design or construction of the Submarket Amenities or the cost of correcting defects in the construction of the Submarket Amenities. The “Project Amenities Share” shall mean the Project’s share of the Submarket Amenities Operating Expenses, which shall be allocated as reasonably and equitably determined by the ARE Landlord Affiliate between and among the Affiliate Project, the Project and any other projects owned or operated by ARE Parties leased in whole or in part to Users granted the right under their respective leases (or other applicable occupancy agreement(s)) to all or portion of the Submarket Amenities. In no event shall the Project Amenities Share include any Submarket Amenities Operating Expenses for any Submarket Amenities that are not made available for use by Tenant. (d) Shared Conference Facilities. Use by Tenant of the Shared Conference Facilities shall be in common with other users with scheduling procedures reasonably determined by ARE Landlord Affiliate or ARE Landlord Affiliate’s then designated event operator (“Conferencing Operator”). Tenant’s use of the Shared Conference Facilities shall be subject to the payment by Tenant of a fee, which shall be payable as directed by ARE Landlord Affiliate to either ARE Landlord Affiliate or Conferencing Operator, equal to the quoted rates for the usage of the Shared Conference Facilities in effect at the time of Tenant’s scheduling. Tenant’s use of the conference rooms in the Shared Conference Facilities shall be subject to availability and ARE Landlord Affiliate (or, if applicable, Conferencing Operator) reserves the right to exercise its reasonable discretion in the event of conflicting scheduling requests among Users. (e) Rules and Regulations. Tenant shall be solely responsible for paying the cost of any and all ancillary services (e.g., audio visual equipment) provided to Tenant, at Tenant’s request, and the cost of any and all goods and services provided to Tenant, at Tenant’s request, by any food services operators and/or any third party vendors at the Affiliate Project. Tenant shall use the Submarket Amenities (including, without limitation, the Shared Conference Facilities) in compliance with all applicable Legal Requirements and any reasonable rules and regulations imposed by ARE Landlord Affiliate or Landlord from time to time (of which Tenant is provided advance written notice) and in a manner that will not interfere with the rights of other Users, which rules and regulations shall be enacted and enforced in a non-discriminatory manner and may include, (i) the required use by Users of one or more food and beverage operators designated by ARE Landlord Affiliate, (ii) usage of and compliance with reservations systems governing the use of Shared Conference Facilities and other facilities, (iii) the payment of additional costs in connection with the after- hours usage of shared conference rooms and other facilities, and (iv) access card entry requirements. The use of the Submarket Amenities other than the Shared Conference Facilities by employees of Tenant shall be in accordance with the terms and conditions of the standard licenses, indemnification and waiver agreements reasonably required by ARE Landlord Affiliate or the operator of the Submarket Amenities to be executed by all persons wishing to use such Submarket Amenities. Neither the ARE Landlord Affiliate nor Landlord (nor, if applicable, any other affiliate of Landlord) shall have any liability or obligation for the breach of any rules or regulations by other Users with respect to the Submarket Amenities. Tenant shall not make any alterations, additions, or improvements of any kind to the Shared Conference Facilities, the Submarket Amenities or the Affiliate Project. Tenant acknowledges and agrees that ARE Landlord Affiliate shall have the right at any time and from time to time to reconfigure, relocate, modify or remove any of the Submarket Amenities at the Affiliate Project and/or to revise, expand or discontinue any of the services (if any) provided in connection with the Submarket Amenities. Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

8 (f) Waiver of Liability and Indemnification. Tenant warrants that it will use reasonable care to prevent damage to property and injury to persons while at the Affiliate Project. Tenant acknowledges and agrees that the indemnity and waivers set forth in Section 16 of the Lease shall apply with respect Landlord, ARE and ARE Landlord Affiliate in connection with (i) the use of the Submarket Amenities by Tenant or any Tenant Parties, and (ii) any entry by Tenant and/or any Tenant Parties onto the Affiliate Project. The provisions of this Section 7(f) shall survive the expiration or earlier termination of the Lease. (g) Insurance. Tenant shall cause all insurance to be carried by Tenant pursuant to the terms of Section 17 of the Lease to apply with respect to Tenant’s use of the Submarket Amenities. 9. Extension Right. As of the Effective Date, Section 40(a) of the original Lease is hereby deleted in its entirety and replaced with the following: “(a) Extension Right. Tenant shall have one right (the “Extension Right”) to extend the term of the Lease for 5 years (the “Extension Term”) on the same terms and conditions as the Lease (other than with respect to Base Rent, the Work Letter and the Sixth Amendment Work Letter) by giving Landlord written notice of its election to exercise each Extension Right at least months 9 prior, and no earlier than 12 months prior, to the then- current expiration date of the Term of the Lease. Upon the commencement of the Extension Term, Base Rent shall be payable at the Market Rate (as defined below). Base Rent shall thereafter be adjusted on each annual anniversary of the commencement of the Extension Term by a percentage as determined by Landlord and agreed to by Tenant at the time the Market Rate is determined. As used herein, “Market Rate” shall mean the rate that comparable landlords of comparable buildings have accepted in current transactions from non-equity (i.e., not being offered equity in the buildings) and nonaffiliated tenants of similar financial strength for space of comparable size, quality (including all Tenant Improvements, Alterations and other improvements) and floor height in Class A laboratory/office buildings in the Raleigh- Durham area of North Carolina for a comparable term, with the determination of the Market Rate to take into account all relevant factors, including tenant inducements, views, available amenities (including, without limitation, the Project Amenities, the Submarket Amenities (if any), age of the Building, age of mechanical systems serving the Premises), parking costs, leasing commissions, allowances or concessions, if any. Notwithstanding the foregoing, the Market Rate shall in no event be less than the Base Rent payable as of the date immediately preceding the commencement of the Extension Term increased by 3%. In addition, Landlord may impose a market rent (as reasonably determined by Landlord) for the parking rights provided hereunder. If, on or before the date which is 240 days prior to the expiration of the Term of the Lease, Tenant has not agreed with Landlord’s determination of the Market Rate and the rent escalations during the Extension Term after the parties have negotiated in good faith, Tenant shall be deemed to have elected arbitration as described in Section 40(b). Tenant acknowledges and agrees that, if Tenant has elected to exercise the Extension Right by delivering notice to Landlord as required in this Section 40(a), Tenant shall have no right thereafter to rescind or elect not to extend the Term of the Lease for the Extension Term.” 10. OFAC. Tenant is and, to Tenant’s knowledge, all beneficial owners of Tenant are currently (a) in compliance with and shall at all times during the Term of the Lease remain in compliance with the regulations of the Office of Foreign Assets Control (“OFAC”) of the U.S. Department of Treasury and any statute, executive order, or regulation relating thereto (collectively, the “OFAC Rules”), (b) not listed on, and shall not during the term of the Lease be listed on, the Specially Designated Nationals and Blocked Persons List, Foreign Sanctions Evaders List, or the Sectoral Sanctions Identification List, which are all maintained by OFAC and/or on any other similar list maintained by OFAC or other governmental authority pursuant to any authorizing statute, executive order, or Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

9 regulation, and (c) not a person or entity with whom a U.S. person is prohibited from conducting business under the OFAC Rules. 11. Brokers. Landlord and Tenant each represents and warrants that it has not dealt with any broker, agent or other person (collectively, “Broker”) in connection with the transaction reflected in this Sixth Amendment and that no Broker brought about this transaction, other than Davis Moore Advisors and Innovation Commercial. Landlord and Tenant each hereby agrees to indemnify and hold the other harmless from and against any claims by any Broker, other than Davis Moore Advisors and Innovation Commercial, claiming a commission or other form of compensation by virtue of having dealt with Tenant or Landlord, as applicable, with regard to this Sixth Amendment. 12. Miscellaneous. a. This Sixth Amendment is the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior and contemporaneous oral and written agreements and discussions. This Sixth Amendment may be amended only by an agreement in writing, signed by the parties hereto. b. This Sixth Amendment is binding upon and shall inure to the benefit of the parties hereto, and their respective successors and assigns. c. This Sixth Amendment may be executed in 2 or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts may be delivered via electronic mail (including pdf or any electronic signature process complying with the U.S. federal ESIGN Act of 2000) or other transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes. Electronic signatures shall be deemed original signatures for purposes of this Sixth Amendment and all matters related thereto, with such electronic signatures having the same legal effect as original signatures. d. Except as amended by this Sixth Amendment, the Lease is hereby ratified and confirmed and all other terms of the Lease shall remain in full force and effect, unaltered and unchanged by this Sixth Amendment. In the event of any conflict between the provisions of this Sixth Amendment and the provisions of the Lease, the provisions of this Sixth Amendment shall prevail. Whether or not specifically amended by this Sixth Amendment, all of the terms and provisions of the Lease are hereby amended to the extent necessary to give effect to the purpose and intent of this Sixth Amendment. [Signatures are on the next page] Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

10 IN WITNESS WHEREOF, the parties hereto have executed this Sixth Amendment as of the day and year first above written. TENANT: HUMACYTE, INC., a Delaware corporation By: Its: □ I hereby certify that the signature, name, and title above are my signature, name and title. LANDLORD: ARE-NC REGION NO. 5, LLC, a Delaware limited liability company By: Alexandria Real Estate Equities, L.P., a Delaware limited partnership, managing member By: ARE-QRS Corp., a Maryland corporation, general partner By: Its: Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E X Dale Sander Chief Financial Officer Mark Hikin VP - Real Estate Legal Affairs

11 Exhibit A SIXTH AMENDMENT WORK LETTER THIS SIXTH AMENDMENT WORK LETTER dated September ______, 2025 (this “Work Letter”) is made and entered into by and between ARE-NC REGION NO. 5, LLC, a Delaware limited liability company (“Landlord”), and HUMACYTE, INC., a Delaware corporation (“Tenant”), and is attached to and made a part of the Lease Agreement dated as of December 31, 2015, as amended by that certain letter agreement dated January 29, 2016, and as further amended by that certain First Amendment to Lease dated as of September 30, 2016, that certain Second Amendment to Lease dated as of February 8, 2017, that certain Third Amendment to Lease dated as of April 21, 2017, that certain Fourth Amendment to Lease dated as of October 31, 2017, that certain Fifth Amendment to Lease dated September 4, 2019, and that certain Sixth Amendment to Lease dated of even date herewith (the “Sixth Amendment”) (as amended, the “Lease”). Any initially capitalized terms used but not defined herein shall have the meanings given them in the Lease. 1. General Requirements. (a) Tenant’s Authorized Representative. Tenant designates Greg Miller (such individual, “Tenant’s Representative”) as the only person authorized to act for Tenant pursuant to this Work Letter. Landlord shall not be obligated to respond to or act upon any request, approval, inquiry or other communication (“Communication”) from or on behalf of Tenant in connection with this Work Letter unless such Communication is in writing from Tenant’s Representative. Tenant may change Tenant’s Representative at any time upon not less than 5 business days advance written notice to Landlord. The email address for Tenant’s Representative named in this Section 1(a) is gmiller@humacyte.com. (b) Landlord’s Authorized Representative. Landlord designates Chuck Blatchley and Leon Kislowski (either such individual acting alone, “Landlord’s Representative”) as the only persons authorized to act for Landlord pursuant to this Work Letter. Tenant shall not be obligated to respond to or act upon any request, approval, inquiry or other Communication from or on behalf of Landlord in connection with this Work Letter unless such Communication is in writing from Landlord’s Representative. Landlord may change either Landlord’s Representative at any time upon not less than 5 business days advance written notice to Tenant. The email address for Landlord’s Representative named in this Section 1(b) are cblatchley@are.com and lkislowski@are.com. (c) Architects, Consultants and Contractors. Landlord and Tenant hereby acknowledge and agree that the architect (the “TI Architect”) for the Tenant Improvements (as defined in Section 2(a) below), the general contractor for the Tenant Improvements (the “General Contractor”), and any subcontractors for the Tenant Improvements shall be selected by Tenant, subject to Landlord’s approval, which approval shall not be unreasonably withheld, conditioned or delayed. Landlord shall be named a third party beneficiary of any contract entered into by Tenant with the TI Architect, any consultant, any contractor or any subcontractor, and of any warranty made by any contractor or any subcontractor. 2. Tenant Improvements. (a) Tenant Improvements Defined. As used herein, “Tenant Improvements” shall mean all improvements to the Premises of a fixed and permanent nature reflected in the TI Construction Drawings (as defined in Section 2(d) below) and any demolition of existing improvements necessary for the construction of the Tenant Improvements. Tenant shall be obligated to design and construct the Tenant Improvements with respect to the entire Premises in accordance with the terms of this Work Letter. Other than funding the Total TI Allowance (as defined below) as provided herein, Landlord shall not have any obligation whatsoever with respect to the finishing of the Premises for Tenant’s use and occupancy. (b) Tenant’s Space Plans. Following the date of this Work Letter, Tenant shall deliver to Landlord schematic drawings and outline specifications (the “Space Plans”) detailing Tenant’s Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E 29

12 requirements for the Tenant Improvements. Not more than 10 business days thereafter, Landlord shall deliver to Tenant the written objections, questions or comments of Landlord and the TI Architect with regard to the Space Plans. Tenant shall cause the Space Plans to be revised to address such written comments and shall resubmit said drawings to Landlord for approval within 10 business days thereafter. Such process shall continue until Landlord has approved the Space Plans. (c) Design Development Drawings. Following the approval of the Space Plans by Landlord, Tenant shall cause the TI Architect to prepare and deliver to Landlord preliminary permit set plans and specifications for development of the Tenant Improvements (the “Design Development Drawings”), which Design Development Drawings shall be prepared substantially in accordance with the approved Space Plans. Landlord shall deliver its written comments to the Design Development Drawings to Tenant not later than 10 business days after Landlord’s receipt of the same; provided, however, that Landlord may not disapprove any matter that is consistent with the Space Plans. Tenant and the TI Architect shall consider all such comments in good faith and shall, within 10 business days after receipt, notify Landlord how Tenant proposes to respond to such comments. Any disputes in connection with such comments shall be resolved in accordance with Section 2(e) hereof. Provided that the design reflected in the Design Development Drawings is consistent with the Space Plans, Landlord shall approve the Design Development Drawings submitted by Tenant. Landlord’s approval of the Design Development Drawings shall not be unreasonably withheld, conditioned or delayed. Once approved by Landlord, subject to the provisions of Section 4 below, Tenant shall not materially modify the Design Development Drawings. (d) Working Drawings. Following the approval of the Design Development Drawings by Landlord, Tenant shall cause the TI Architect to prepare and deliver to Landlord for review and comment construction plans, specifications and drawings for the Tenant Improvements (“TI Construction Drawings”), which TI Construction Drawings shall be prepared substantially in accordance with the Design Development Drawings. Tenant shall be solely responsible for ensuring that the TI Construction Drawings reflect Tenant’s requirements for the Tenant Improvements. Landlord shall deliver its written comments on the TI Construction Drawings to Tenant not later than 10 business days after Landlord’s receipt of the same; provided, however, that Landlord may not disapprove any matter that is consistent with the Design Development Drawings. Tenant and the TI Architect shall consider all such comments in good faith and shall, within 10 business days after receipt, notify Landlord how Tenant proposes to respond to such comments. Any disputes in connection with such comments shall be resolved in accordance with Section 2(e) hereof. Provided that the design reflected in the TI Construction Drawings is consistent with the Design Development Drawings, Landlord shall approve the TI Construction Drawings submitted by Tenant. Landlord’s approval of the TI Construction Drawings shall not be unreasonably withheld, conditioned or delayed. Once approved by Landlord, subject to the provisions of Section 4 below, Tenant shall not materially modify the TI Construction Drawings except as may be reasonably required in connection with the issuance of the TI Permit (as defined in Section 3(a) below). (e) Approval and Completion. If any dispute regarding the design of the Tenant Improvements is not settled within 10 business days after notice of such dispute is delivered by one party to the other, Tenant may make the final decision regarding the design of the Tenant Improvements, provided (i) Tenant acts reasonably and such final decision is either consistent with or a compromise between Landlord’s and Tenant’s positions with respect to such dispute, (ii) that all costs and expenses resulting from any such decision by Tenant shall be payable out of the TI Fund (as defined in Section 5(d) below), and (iii) Tenant’s decision will not affect the Building structure or any Building Systems (in which case Landlord shall make the final decision). 3. Performance of the Tenant Improvements. (a) Commencement and Permitting of the Tenant Improvements. Tenant shall commence construction of the Tenant Improvements upon obtaining and delivering to Landlord a building permit (the “TI Permit”) authorizing the construction of the Tenant Improvements consistent with the TI Construction Drawings approved by Landlord. The cost of obtaining the TI Permit shall be payable from the TI Fund. Landlord shall assist Tenant in obtaining the TI Permit. Prior to the commencement of the Tenant Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

13 Improvements, Tenant shall deliver to Landlord a copy of Tenant’s contract with the TI Architect and a copy of Tenant’s contract with the General Contractor and certificates of insurance from the TI Architect and General Contractor evidencing industry standard commercial general liability, automotive liability, “builder’s risk”, and workers’ compensation insurance. Tenant shall cause the General Contractor to provide a certificate of insurance naming Landlord, Alexandria Real Estate Equities, Inc., and Landlord’s lender (if any) as additional insureds for the General Contractor’s liability coverages required above. (b) Selection of Materials, Etc. Where more than one type of material or structure is indicated on the TI Construction Drawings approved by Tenant and Landlord, the option will be within Tenant’s reasonable discretion if the matter concerns the Tenant Improvements, and within Landlord’s reasonable discretion if the matter concerns the structural components of the Building or any Building Systems. (c) Tenant Liability. Tenant shall be responsible for correcting any deficiencies or defects in the Tenant Improvements. (d) Substantial Completion. Tenant shall substantially complete or cause to be substantially completed the Tenant Improvements in a good and workmanlike manner, in accordance with the TI Permit subject, in each case, to Minor Variations and normal “punch list” items of a non-material nature which do not interfere with the use of the Premises (“Substantial Completion” or “Substantially Complete”). Upon Substantial Completion of the Tenant Improvements, Tenant shall require the TI Architect and the General Contractor to execute and deliver, for the benefit of Tenant and Landlord, a Certificate of Substantial Completion in the form of the American Institute of Architects (“AIA”) document G704. For purposes of this Work Letter, “Minor Variations” shall mean any modifications reasonably required: (i) to comply with all applicable Legal Requirements and/or to obtain or to comply with any required permit (including the TI Permit); (ii) to comport with good design, engineering, and construction practices which are not material; or (iii) to make reasonable adjustments for field deviations or conditions encountered during the construction of the Tenant Improvements. 4. Changes. Any changes requested by Tenant to the Tenant Improvements which would affect the Building structure or Building Systems (or which would reasonably be expected to affect the Building structure or Building Systems) (“Changes”) after the delivery and approval by Landlord of the TI Construction Drawings, shall be requested and instituted in accordance with the provisions of this Section 4 and shall be subject to the written approval of Landlord, which approval shall not be unreasonably withheld, conditioned or delayed. (a) Tenant’s Right to Request Changes. If Tenant shall request Changes, Tenant shall request such Changes by notifying Landlord in writing in substantially the same form as the AIA standard change order form (a “Change Request”), which Change Request shall detail the nature and extent of any such Change. Such Change Request must be signed by Tenant’s Representative. Landlord shall review and approve or disapprove such Change Request within 10 business days thereafter, provided that Landlord’s approval shall not be unreasonably withheld, conditioned or delayed. (b) Implementation of Changes. If Landlord approves such Change, Tenant may cause the approved Change to be instituted. If any TI Permit modification or change is required as a result of such Change, Tenant shall promptly provide Landlord with a copy of such TI Permit modification or change. 5. Costs. (a) Budget For Tenant Improvements. Before the commencement of construction of the Tenant Improvements, Tenant shall obtain a detailed breakdown, by trade, of the costs incurred or that will be incurred, in connection with the design and construction of the Tenant Improvements (the “Budget”), and deliver a copy of the Budget to Landlord for Landlord’s approval, which shall not be unreasonably withheld or delayed. The Budget shall be based upon the TI Construction Drawings approved by Landlord. The Budget shall include a payment to Landlord of administrative rent (“Administrative Rent”) equal to 2% of the TI Costs (as hereinafter defined) for monitoring and inspecting the construction of the Tenant Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

14 Improvements, which sum shall be payable from the TI Fund. Such Administrative Rent shall include, without limitation, all out-of-pocket costs, expenses and fees incurred by or on behalf of Landlord arising from, out of, or in connection with, such monitoring of the construction of the Tenant Improvements, and shall be payable out of the TI Fund. (b) Total TI Allowance. Commencing on April 1, 2027, Landlord shall provide to Tenant a tenant improvement allowance (“Total TI Allowance”) of $45.00 per rentable square foot of the Premises. The Total TI Allowance shall be disbursed in accordance with this Work Letter. Except as expressly provided in Section 5 of the Sixth Amendment, Tenant shall have no right to the use or benefit (including any reduction to or payment of Base Rent) of any portion of the Total TI Allowance not required for the payment of TI Costs. Tenant shall have no right to any portion of the Total TI Allowance that is not disbursed before December 31, 2028. In addition to the TI Allowance, commencing on April 1, 2027, Landlord shall reimburse Tenant for the actual, out-of-pocket costs incurred by Tenant in connection with preparing an initial test fit for the Premises, but in no event shall Landlord be required to pay any costs in excess of $0.15 per rentable square foot of the Premises for such test fit (the “Test-Fit Allowance”). Landlord shall reimburse Tenant for such costs within 60 days after Landlord’s receipt of invoices reflecting the cost of such test-fit for the Premises and proof of payment thereof (which in no event shall occur prior to April 1, 2027). Tenant shall have no right to any portion of the Test-Fit Allowance that is not disbursed before December 31, 2028. (c) Costs Includable in TI Fund. The TI Fund shall be used solely for the payment of the cost of demolition of any existing improvements in the Premises and for the payment of design, permits and construction costs in connection with the construction of the Tenant Improvements, including, without limitation, the cost of electrical power and other utilities used in connection with the construction of the Tenant Improvements, the cost of preparing the Space Plans, the Design Development Drawings and the TI Construction Drawings, all costs set forth in the Budget, including Landlord’s Administrative Rent, and the cost of Changes (collectively, “TI Costs”). Notwithstanding anything to the contrary contained herein, the TI Fund shall not be used to purchase any furniture, personal property or other non-Building Systems materials or equipment, including, but not be limited to, Tenant’s voice or data cabling, non-ducted biological safety cabinets and other scientific equipment not incorporated into the Tenant Improvements. For the avoidance of doubt, the parties acknowledge and agree that although the Total TI Allowance will not be made available for Tenant’s use until April 1, 2027, Tenant shall have the right to apply the Total TI Allowance towards TI Costs incurred hereunder during the period commencing on the Effective Date of the Lease through March 31, 2027 (in addition to TI Costs incurred hereunder from and after April 1, 2027). (d) Excess TI Costs. Landlord shall have no obligation to bear any portion of the cost of any of the Tenant Improvements except to the extent of the Total TI Allowance. If at any time and from time- to-time, the remaining TI Costs under the Budget exceed the remaining unexpended Total TI Allowance (“Excess TI Costs”), monthly disbursements of the Total TI Allowance shall be made on a “pari passu” basis in the proportion that the remaining Total TI Allowance bear to the outstanding TI Costs under the Budget, and Tenant shall fund the balance of each such monthly draw. For purposes of any litigation instituted with regard to such amounts, those amounts required to be paid by Tenant will be deemed Rent under the Lease. The Total TI Allowance and Excess TI Costs are herein referred to as the “TI Fund.” Notwithstanding anything to the contrary set forth in this Section 5(d), Tenant shall be fully and solely liable for the TI Costs and the cost of Minor Variations in excess of the Total TI Allowance. (e) Payment for TI Costs. Commencing on April 1, 2027, subject to the terms of Section 5(e), Landlord shall reimburse Tenant for TI Costs once a month against a draw request in Landlord’s standard form, containing evidence of payment of such TI Costs by Tenant and such certifications, lien waivers (including a conditional lien release for each progress payment and unconditional lien releases for the prior month’s progress payments), inspection reports and other matters as Landlord customarily obtains, to the extent of Landlord’s approval thereof for payment, no later than 45 days following receipt of such draw request. Upon completion of the Tenant Improvements (and prior to any final disbursement of the TI Fund), Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

15 Tenant shall deliver to Landlord: (i) sworn statements setting forth the names of all contractors and first tier subcontractors who did the work and final, unconditional lien waivers from all such contractors and first tier subcontractors; (ii) as-built plans (one copy in print format and two copies in electronic CAD format) for such Tenant Improvements; (iii) a certification of substantial completion in Form AIA G704, (iv) a certificate of occupancy for the Premises; and (v) copies of all operation and maintenance manuals and warranties affecting the Premises. (f) Tenant Improvement Progress Reports. Commencing on the date that Tenant commences the design and construction of the Tenant Improvements, then on or before the 10th day of each calendar month thereafter during the course of design and construction of the Tenant Improvements, Tenant shall deliver to Landlord a Tenant Improvement progress report in the form of Schedule 1 completed to provide all of the most up-to-date information regarding Tenant’s progress with respect the design and construction of the Tenant Improvements in addition to the corresponding AIA forms G702 and G703 (or their reasonable equivalents), if applicable, for all contracted costs. Concurrently with each progress report, Tenant shall also deliver to Landlord a forecast in the form of Schedule 2 completed to provide the projected remaining TI Costs. 6. Miscellaneous. (a) Consents. Whenever consent or approval of either party is required under this Work Letter, that party shall not unreasonably withhold, condition or delay such consent or approval, except as may be expressly set forth herein to the contrary. (b) Modification. No modification, waiver or amendment of this Work Letter or of any of its conditions or provisions shall be binding upon Landlord or Tenant unless in writing signed by Landlord and Tenant. (c) No Default Funding. In no event shall Landlord have any obligation to fund any portion of the Total TI Allowance during any period that Tenant is in Default under the Lease. (d) Notices. Notwithstanding anything to the contrary contained in the Lease or in the Work Letter, notices between Tenant’s Representative and Landlord’s Representative may be delivered via email. Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

16 Schedule 1 Tenant Improvement Progress Report Project Address: _____________________________ Certification Period: ______________ 1. Original Project Budget $__________________ 2. Net change by Change Orders/Update to budget $__________________ 3. Current budget to date (Line 1 ± 2) $__________________ 4. Total costs incurred to date $__________________ 5. Remaining balance to budget (Line 3 less Line 4) $__________________ Certification signature: ______________________ Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

17 Schedule 2 TI Cost Forecast Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

1 Exhibit B See Attached Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E
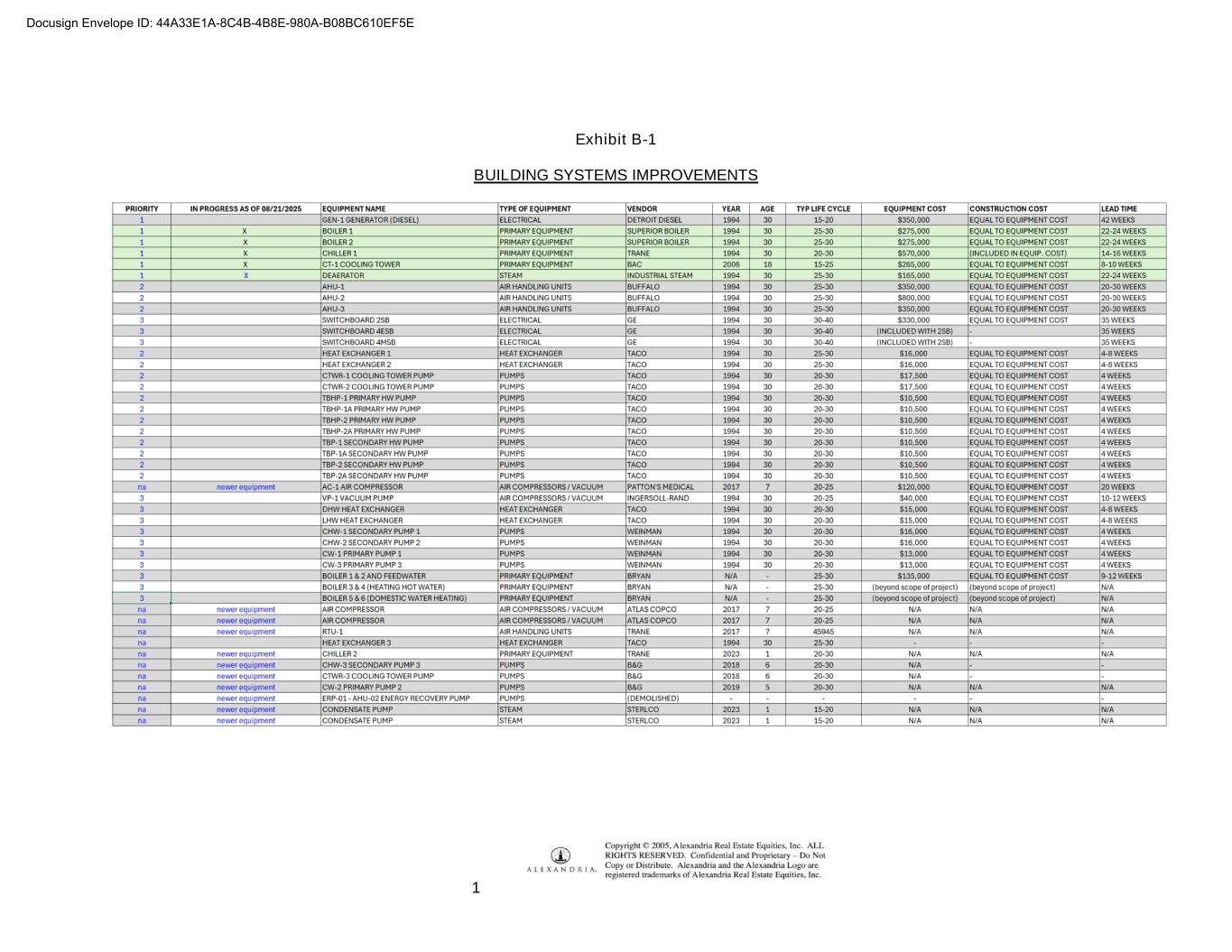
1 Exhibit B-1 BUILDING SYSTEMS IMPROVEMENTS Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

1 Exhibit B-2 Building Improvements Specifications Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E
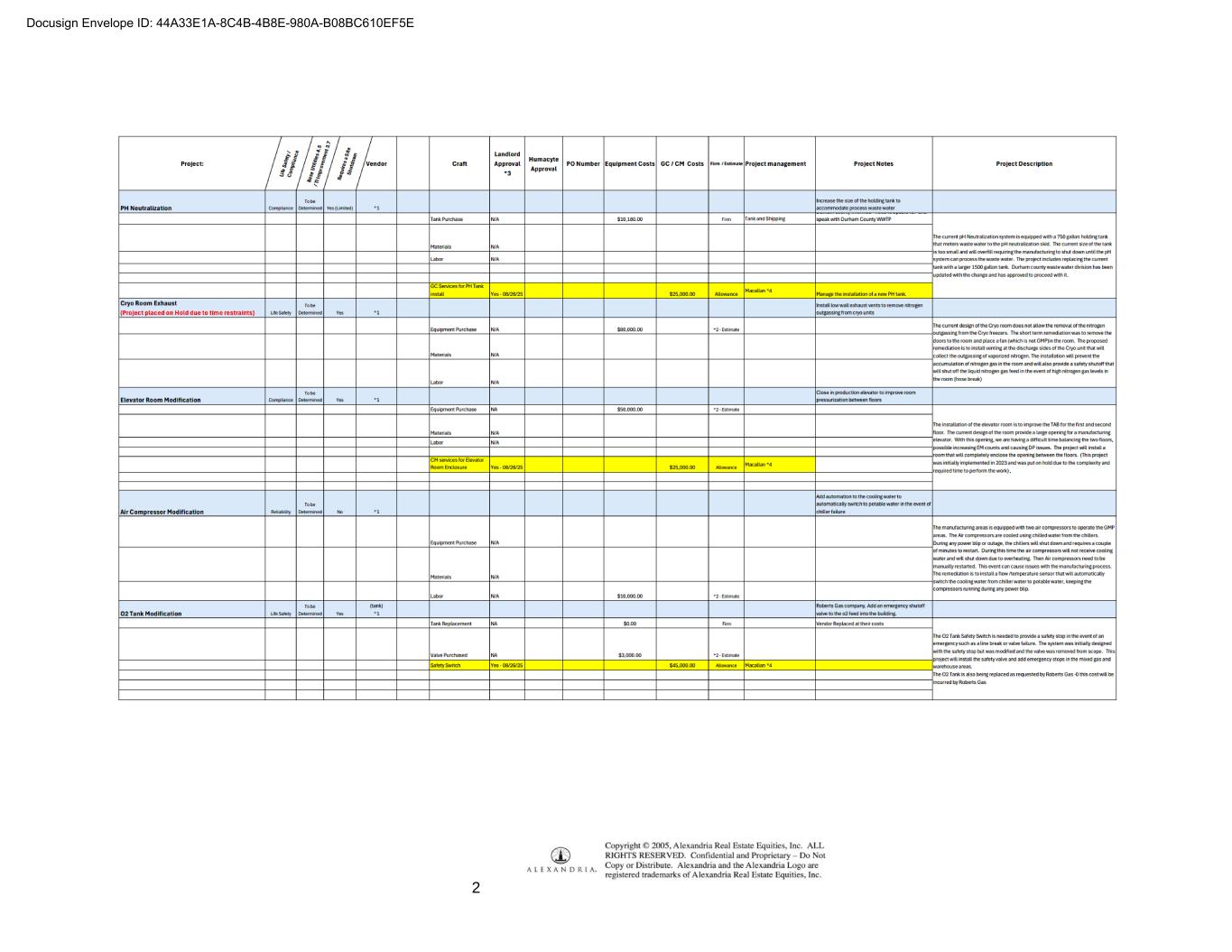
2 Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

3 Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E

1 Exhibit B-3 Contractor and Engineers for Building System Improvements Humacyte – Winter Shutdown projects: a) Construction Manager / Project Manager: Bryan Wall Macallan Construction 7711 Welborn Street – Suite 103 Raleigh NC 27615 919-420-7801 He will be bringing the following Subs under his supervision: b) Boiler · C&C Boilers · Carolina Electric · Daniels Concrete · Hard Rock Concrete Core Drilling c) O2 - Oxygen Tank Farm Repairs · Roberts Gas · Carolina Electric · CK Plumbing d) PH Neutralization System · Piedmont Welding e) HEPA Replacement · Full Spectrum HEPA Cert · Research Air TAB f) Chiller · Brady Services (Project is Turnkey) g) Cooling Tower · Brady Services (Project is Turnkey) h) Cryo Room Carolina Electronic Carolina Electric i) Elevator Room Cover Macallan Docusign Envelope ID: 44A33E1A-8C4B-4B8E-980A-B08BC610EF5E
EX-31.1
4
huma-20250930x10qxex311.htm
EX-31.1
Document
Exhibit 31.1
CERTIFICATION
I, Laura E. Niklason, certify that:
1.I have reviewed this Quarterly Report on Form 10-Q of Humacyte, Inc. for the quarter ended September 30, 2025;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
|
|
|
|
|
|
Date: November 12, 2025 |
By: |
/s/ Laura E. Niklason |
|
|
Name: |
Laura E. Niklason, M.D., Ph.D. |
|
|
Title: |
President and Chief Executive Officer |
EX-31.2
5
huma-20250930x10qxex312.htm
EX-31.2
Document
Exhibit 31.2
CERTIFICATION
I, Dale A. Sander, certify that:
1.I have reviewed this Quarterly Report on Form 10-Q of Humacyte, Inc. for the quarter ended September 30, 2025;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
|
|
|
|
|
|
Date: November 12, 2025 |
By: |
/s/ Dale A. Sander |
|
|
Name: |
Dale A. Sander |
|
|
Title: |
Chief Financial Officer, Chief Corporate Development Officer and Treasurer |
EX-32.1
6
huma-20250930x10qxex321.htm
EX-32.1
Document
Exhibit 32.1
CERTIFICATION
In connection with the Quarterly Report on Form 10-Q of Humacyte, Inc. (the “Company”) for the quarter ended September 30, 2025 (the “Report”), as filed with the Securities and Exchange Commission on the date hereof, I, Laura E. Niklason, President and Chief Executive Officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to the best of my knowledge:
1.The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
2.The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
|
|
|
|
|
|
Date: November 12, 2025 |
By: |
/s/ Laura E. Niklason |
|
|
Name: |
Laura E. Niklason, M.D., Ph.D. |
|
|
Title: |
President and Chief Executive Officer |
EX-32.2
7
huma-20250930x10qxex322.htm
EX-32.2
Document
Exhibit 32.2
CERTIFICATION
In connection with the Quarterly Report on Form 10-Q of Humacyte, Inc. (the “Company”) for the quarter ended September 30, 2025 (the “Report”), as filed with the Securities and Exchange Commission on the date hereof, I, Dale A. Sander, Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to the best of my knowledge:
1.The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
2.The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
|
|
|
|
|
|
Date: November 12, 2025 |
By: |
/s/ Dale A. Sander |
|
|
Name: |
Dale A. Sander |
|
|
Title: |
Chief Financial Officer, Chief Corporate Development Officer and Treasurer |


































