Document
LENZ Therapeutics Announces Positive Topline Data from Phase 3 CLARITY Presbyopia Trials
–LNZ100 selected as lead candidate
–Primary endpoint was met with 71% of participants dosed with LNZ100 achieving three-lines or greater improvement at 3 hours
–Rapid onset and long duration shown with 71% of participants achieving three-lines or greater improvement at 30 minutes and 40% at 10 hours
–New Drug Application submission anticipated in mid-2024
–Company to host a conference call and webcast today at 8:00 a.m. ET
SAN DIEGO, CA – April 3, 2024 – LENZ Therapeutics, Inc. (Nasdaq: LENZ or “LENZ” or the “Company”), a late clinical-stage biopharmaceutical company focused on developing the first aceclidine-based eye drop to improve near vision in people with presbyopia, today announced positive topline results from its Phase 3 CLARITY study of two investigational formulations of aceclidine, LNZ100 and LNZ101, for the treatment of presbyopia, the inevitable loss of near vision that impacts the daily lives of nearly all people over 45.
In Phase 3 safety and efficacy trials (CLARITY 1 and 2), our lead product candidate LNZ100 (1.75% aceclidine) achieved the primary endpoints and key secondary endpoints, with statistically significant three-lines or greater improvement in Best Corrected Distance Visual Acuity (BCDVA) at near, without losing one-line or more in distance visual acuity. In the vehicle-controlled CLARITY 2 trial, the Day 1 results showed (all p<0.0001):
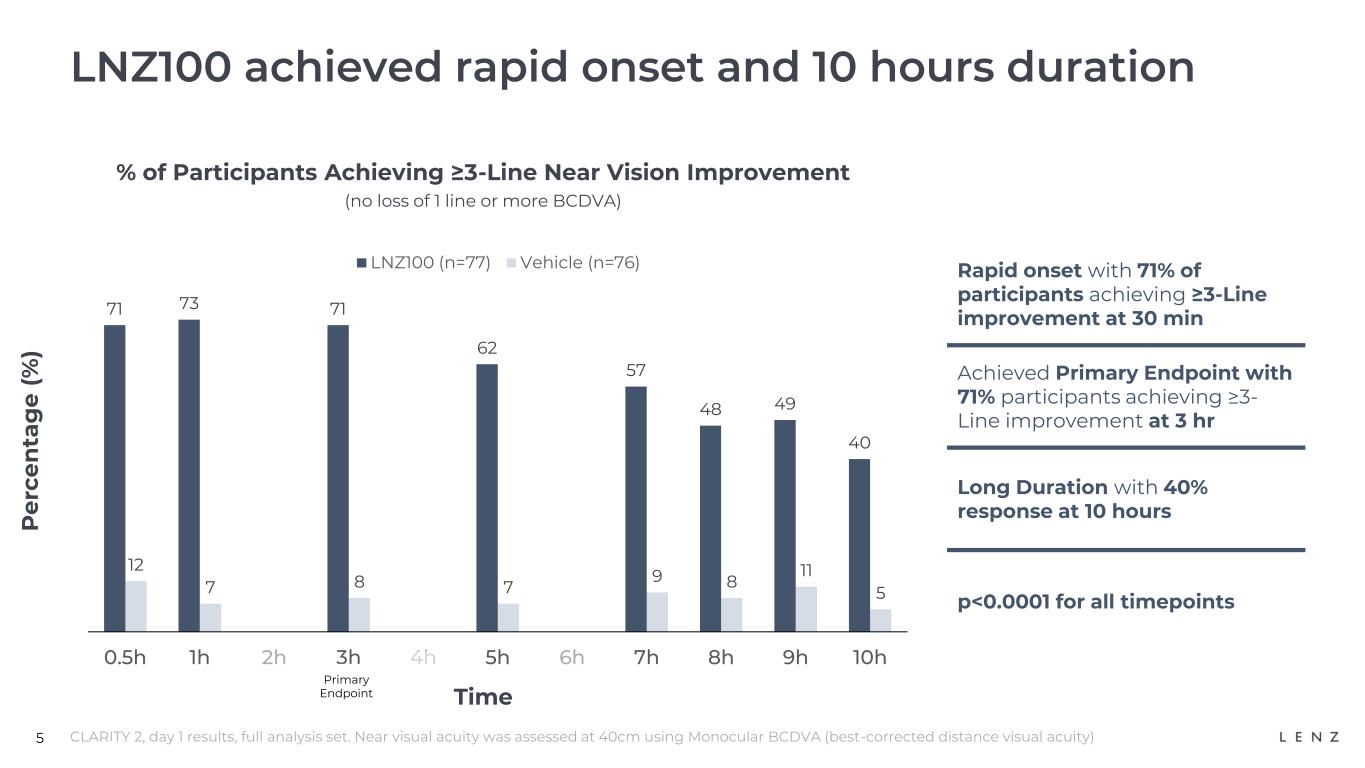
•Rapid onset: 71% achieved three-lines or greater improvement at 30 minutes.
•Primary endpoint: 71% achieved three-lines or greater improvement at 3 hours.
•Long duration: 40% achieved three-lines or greater improvement at 10 hours.
Near vision improvement was reproducible and consistent across both CLARITY 1 and 2 throughout the four-week study periods.
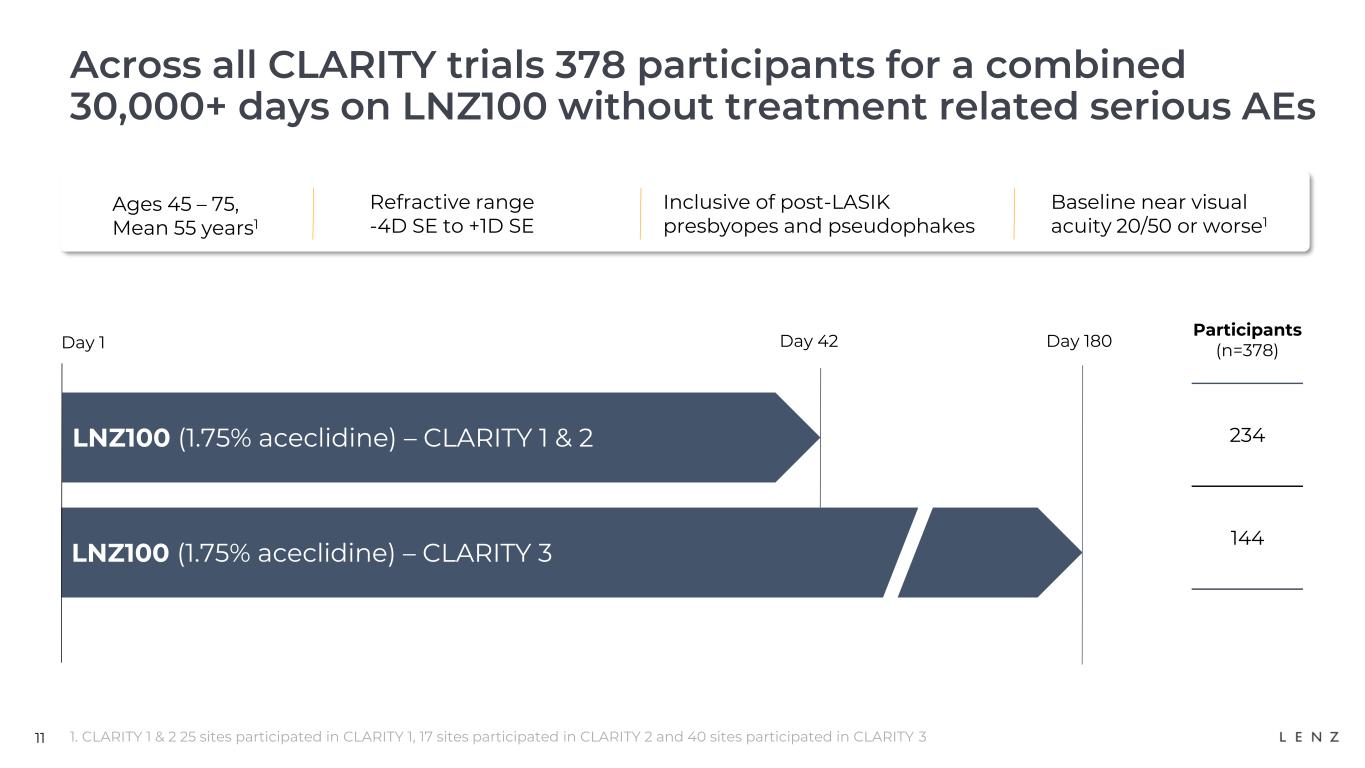
LNZ100 was well-tolerated with no serious treatment-related adverse events observed in the over 30,000 treatment days across all three CLARITY trials.
LNZ101 showed similar results, including achieving primary and secondary endpoints in both CLARITY 1 and 2, but did not show superiority to LNZ100. Based on these results, LENZ selected LNZ100 as its lead product candidate, for which it plans to submit a New Drug Application (NDA) in mid-2024.
“We are very pleased with the outcome of the CLARITY trials, and most importantly the strong efficacy and safety profile of LNZ100 observed in patients with presbyopia. We would like to thank our investigators, clinical sites, and all participants in our study”, said Eef Schimmelpennink, President and Chief Executive Officer of LENZ Therapeutics. “We believe these data support LNZ100 as a potential best-in-class therapy for the treatment of presbyopia. The high responder rate, rapid onset and long duration across a broad range of presbyopes ranging from 45 to 75 years of age and having a refractive range from -4.0 to +1.0D SE are consistent with features that patients are expecting from an effective treatment option. Based on these highly encouraging data, we will direct our focus towards our NDA submission in mid-2024 for LNZ100, and preparations for commercialization in second half of 2025 upon FDA approval, with the goal of moving closer to helping many of the 128 million people experiencing symptoms of presbyopia in the United States.”
“These positive CLARITY study data build on the compelling results from our INSIGHT trials, demonstrating a robust safety and efficacy profile for the use of aceclidine to treat presbyopia,” said Marc Odrich, Chief Medical Officer of LENZ Therapeutics. “Presbyopes often experience an abrupt change in their daily life as the symptoms become progressively pronounced starting in their mid-40s, when reading glasses or other corrective aids often become necessary to read, text or conduct close-up work. The statistically significant data and clinically meaningful outcomes observed in the CLARITY trials support the potential paradigm-shifting impact LNZ100 can have as an alternative and convenient therapeutic option to reading glasses.”
CLARITY Phase 3 Study Topline Data Highlights:
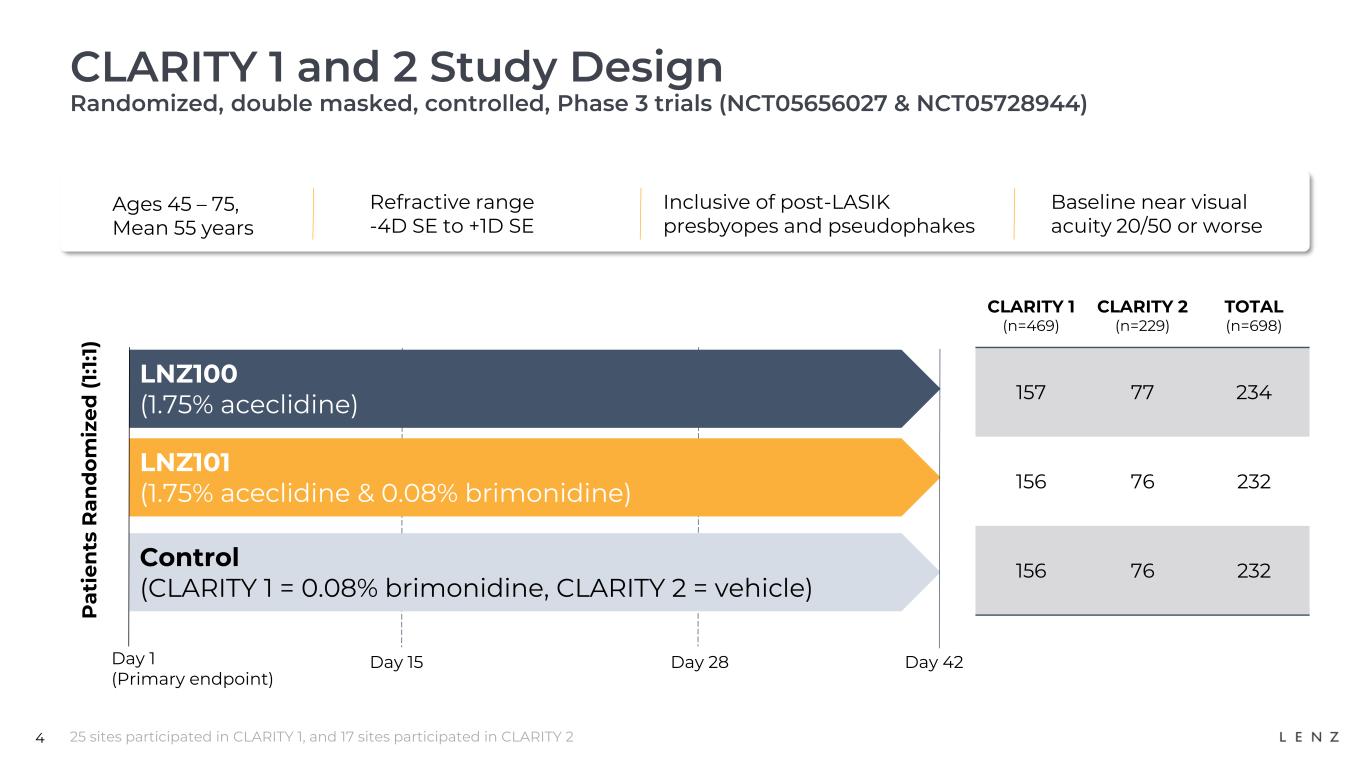
CLARITY is a Phase 3 multi-center, double-masked, randomized, controlled, efficacy and safety study for LNZ100 and LNZ101 for the treatment of presbyopia. It is comprised of two six-week efficacy trials, CLARITY 1 and 2, and a six-month safety trial, CLARITY 3. The trials enrolled a total of 1,059 participants ranging from ages 45 to 75, and a refractive range of -4.0D SE to +1.0D SE, and included users who previously had LASIK surgery or are pseudophakes. The primary efficacy endpoint in both CLARITY 1 and 2 is the percentage of participants who achieve three-lines or greater improvement in BCDVA at near without losing one-line (5 letters or more of distance vision) at 3 hours post-treatment.
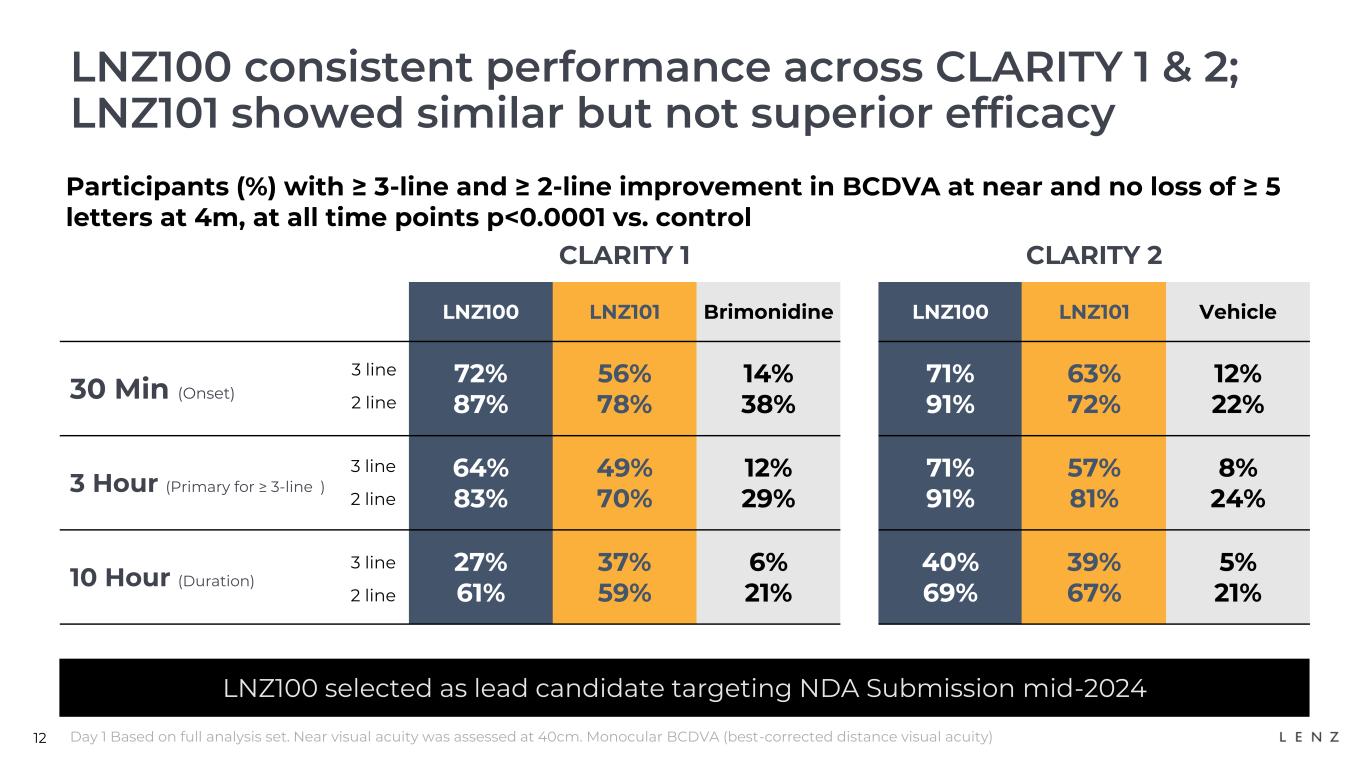
In both the vehicle-controlled CLARITY 2 trial and the brimonidine-controlled CLARITY 1 trial, our lead product candidate LNZ100 (1.75% aceclidine) achieved all primary and secondary near vision improvement endpoints, including demonstrating (in all cases, p<0.0001):
•Rapid onset: at 30 minutes, for CLARITY 2, 71% and 91% of participants achieved three- and two-lines or greater improvement, respectively, and for CLARITY 1, 72% and 87% of participants achieved three- and two-lines or greater improvement, respectively;
•At 3 hours (primary endpoint for three-lines): for CLARITY 2, 71% and 91% of participants achieved three- and two-lines or greater improvement, respectively, and for CLARITY 1, 64% and 83% of participants achieved three- and two-lines or greater improvement, respectively; and
•Long duration: at 10 hours, for CLARITY 2, 40% and 69% of participants achieved three- and two-lines or greater improvement, respectively, and for CLARITY 1, 27% and 61% of participants achieved three- and two-lines or greater improvement, respectively.
Near vision improvement was reproducible and consistent across both CLARITY 1 and 2 throughout the four-week study periods.
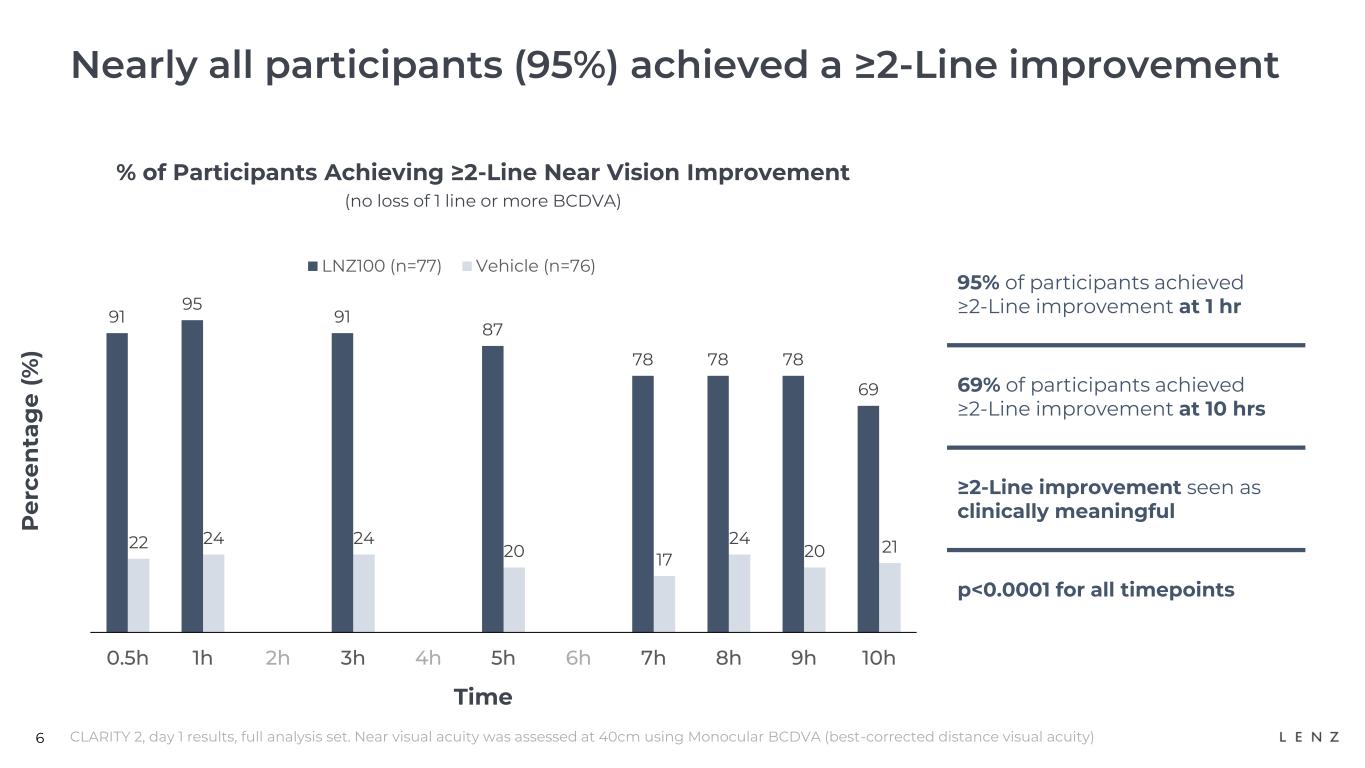
Additionally, in CLARITY 2, nearly all (95%) participants who received LNZ100 achieved the clinically meaningful two-lines or greater improvement (p<0.0001) at 1 hour post-treatment.
LNZ100 also demonstrated statistically significant (p<0.0001) improvement of 2-4 letters on distance vision in normal light and no negative impact to distance vision in low light at all time points.
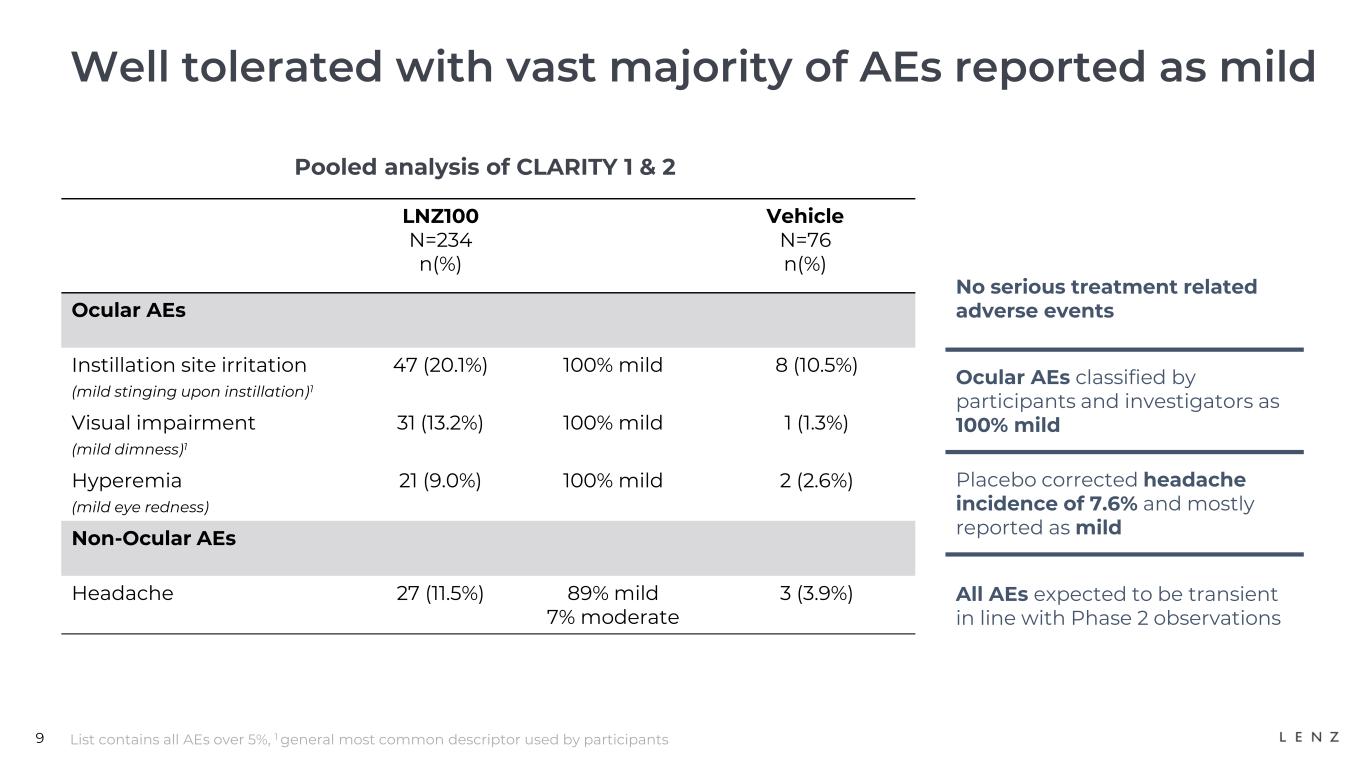
LNZ100 was well-tolerated with no treatment-related serious adverse events observed in the over 30,000 treatment days across all three CLARITY trials. The only reported adverse events with an incidence at 5% or more were installation site irritation, visual impairment and hyperemia which were 100% characterized by participants as mild, and headaches which were characterized as mild by 89% of participants.
We believe these adverse events were transient, consistent with those observed in previous trials.
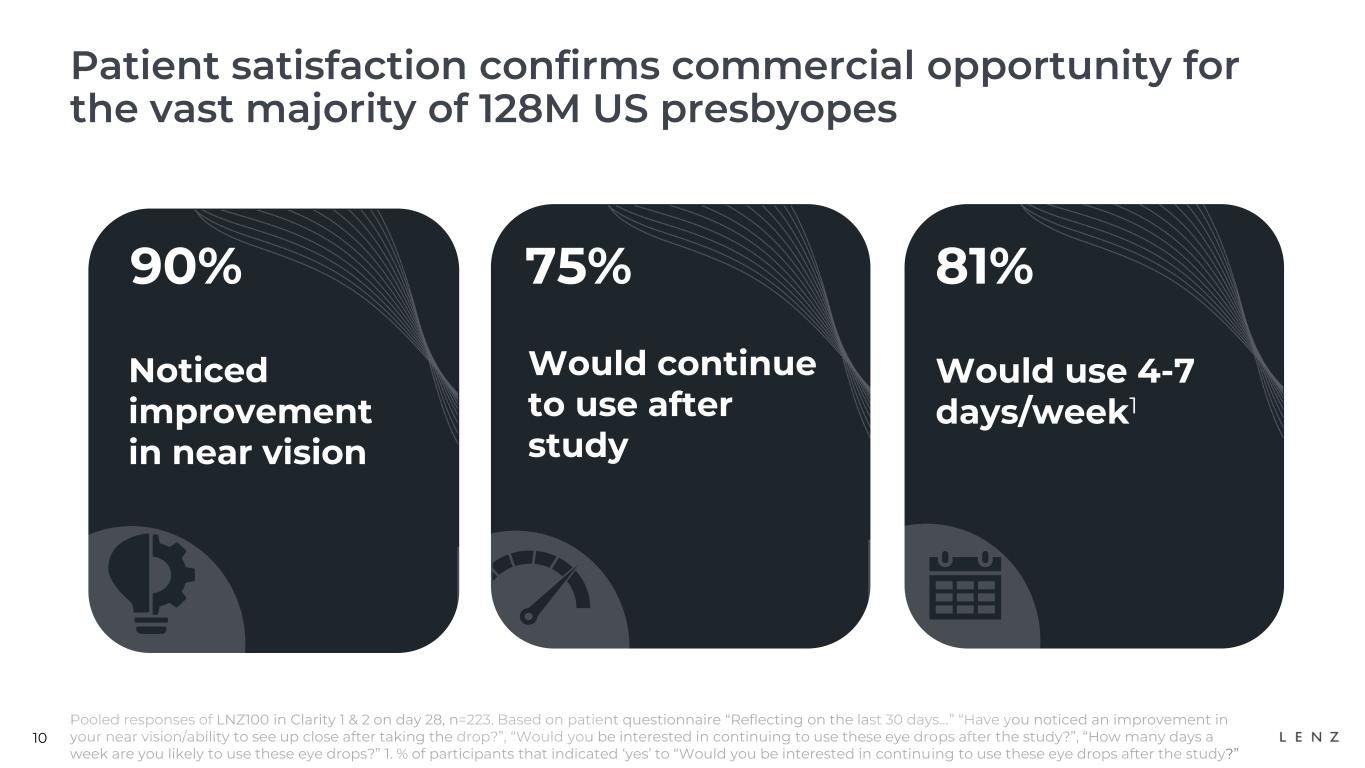
On day 28 of the CLARITY 1 and 2 trials, 223 participants who received LNZ100 were surveyed on their experience. 90% indicated they noticed an improvement in their near vision and 75% said they would want to continue to use these eye drops after the study, of which 81% noted they expected to use them 4-7 days a week. We believe these patient survey outcomes further confirm the potential commercial opportunity for LNZ100.
Based on these results, LENZ Therapeutics anticipates submitting a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for LNZ100 as a treatment for presbyopia mid-2024.
Additional results from the CLARITY study will be presented at future medical meetings.
Conference Call Information
The Company will host a conference call and webcast today, Wednesday, April 3, 2024, at 8:00 a.m. ET to discuss the topline results. The live webcast and materials from today’s conference call can be accessed here and on the LENZ Therapeutics website at www.LENZ-tx.com in the Investors & Media section or by calling 877-315-3033 or 215-268-9883. A replay of the webcast will be archived and available for 30 days following the event.
About Presbyopia
Presbyopia is the inevitable loss of near vision associated with aging and impacts the daily lives of nearly all people over 45. In the United States, the estimated addressable population who suffer from this condition, known as presbyopes, is 128 million, almost four times the number of individuals suffering from dry eye disease and three times the number of individuals suffering from childhood myopia, macular degeneration, diabetic retinopathy, and glaucoma combined.
About LENZ Therapeutics
LENZ Therapeutics is a late clinical-stage biopharmaceutical company focused on developing the first aceclidine-based eye drop to improve vision in patients diagnosed with presbyopia. LENZ’s product candidate LNZ100 is a preservative-free, single-use, once-daily eye drop containing aceclidine. The Phase 3 CLARITY trials of LNZ100 were completed in March 2024 and NDA submission is planned in mid-2024. LENZ is committed to commercializing a potential best-in-class pharmaceutical presbyopia solution that enhances vision for “all eyes, all day.” LENZ is headquartered in San Diego, California. For more information, visit: LENZ-tx.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of federal securities laws. You can identify forward-looking statements by words such as “may,” “will,” “could,” “can,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “poised,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, but not all forward-looking statements will contain these words. Forward-looking statements in this press release include, but are not limited to, statements regarding the potential of LNZ100 to have best-in-class performance; our plans relating to the clinical development of our product candidates, including statements regarding the timing, presentation and reporting of data from our clinical trials and studies and the timing of a potential NDA submission for LNZ100; the size of the addressable population for our product candidates; our expectations regarding the commercial opportunity and beneficial characteristics of our product candidates; and our plans regarding commercialization of LNZ100, if approved.
These statements are based on numerous assumptions concerning the development of LENZ’s products and target markets and involve substantial risks, uncertainties and other factors that may cause actual results, levels of activity, performance or achievement to be materially different from the information expressed or implied by these forward-looking statements, including those risk factors described in the final 424B3 proxy statement/prospectus filed with the SEC on February 13, 2024. We cannot assure you that the forward-looking statements in this press release or the assumptions upon which they are based will prove to be accurate. The forward-looking statements in this press release are as of the date of this press release. Except as otherwise required by applicable law, LENZ disclaims any duty to update any forward-looking statements. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this press release.
Contacts:
Dan Chevallard
LENZ Therapeutics
IR@LENZ-Tx.com
Janhavi Mohite
Stern Investor Relations, Inc.
janhavi.mohite@sternir.com