
Innovation Series: R&D Day 2025 November 11th, 2025 Exhibit 99.1

Introductory Remarks Douglas Maffei, PhD, Vice President, Strategy and Investor Relations 1

3 This Slide Presentation Includes Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: BioNTech’s expected revenues and net profit/(loss) related to sales of BioNTech’s COVID-19 vaccine, referred to as COMIRNATY where approved for use under full or conditional marketing authorization, in territories controlled by BioNTech’s collaboration partners, particularly for those figures that are derived from preliminary estimates provided by BioNTech’s partners; the rate and degree of market acceptance of BioNTech’s COVID-19 vaccine and, if approved, BioNTech’s investigational medicines; expectations regarding anticipated changes in COVID-19 vaccine demand, including changes to the ordering environment and expected regulatory recommendations to adapt vaccines to address new variants or sublineages; the initiation, timing, progress, results, and cost of BioNTech’s research and development programs, including BioNTech’s current and future preclinical studies and clinical trials, including statements regarding the expected timing of initiation, enrollment, and completion of studies or trials and related preparatory work and the availability of results, and the timing and outcome of applications for regulatory approvals and marketing authorizations; BioNTech’s expectations regarding potential future commercialization in oncology, including goals regarding timing and indications; the targeted timing and number of additional potentially registrational trials, and the registrational potential of any trial BioNTech may initiate; discussions with regulatory agencies; BioNTech’s expectations with respect to intellectual property; the impact of BioNTech’s collaboration and licensing agreements, including BioNTech’s partnership with BMS; BioNTech’s planned acquisition of CureVac; the development, nature and feasibility of sustainable vaccine production and supply solutions; the deployment of AI across BioNTech’s preclinical and clinical operations; BioNTech's expectations with respect to tariff policy; BioNTech’s estimates of revenues, research and development expenses, selling, general and administrative expenses, and capital expenditures for operating activities; BioNTech's expectations regarding upcoming payments relating to litigation settlements; BioNTech's expectations for upcoming scientific and investor presentations; and BioNTech’s expectations of net profit / (loss). In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this presentation are based on BioNTech’s current expectations and beliefs of future events and are neither promises nor guarantees. You should not place undue reliance on these forward- looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond BioNTech’s control, and which could cause actual results to differ materially and adversely from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to: the uncertainties inherent in research and development, including the ability to meet anticipated clinical endpoints, commencement and/or completion dates for clinical trials, projected data release timelines, regulatory submission dates, regulatory approval dates and/or launch dates, as well as risks associated with preclinical and clinical data, including the data discussed in this release, and including the possibility of unfavorable new preclinical, clinical or safety data and further analyses of existing preclinical, clinical or safety data; the nature of the clinical data, which is subject to ongoing peer review, regulatory review and market interpretation; BioNTech’s pricing and coverage negotiations regarding its COVID-19 vaccine with governmental authorities, private health insurers and other third-party payors; the future commercial demand and medical need for initial or booster doses of a COVID-19 vaccine; the impact of tariffs and escalations in trade policy; competition from other COVID-19 vaccines or related to BioNTech’s other product candidates, including those with different mechanisms of action and different manufacturing and distribution constraints, on the basis of, among other things, efficacy, cost, convenience of storage and distribution, breadth of approved use, side-effect profile and durability of immune response; the timing of and BioNTech’s ability to obtain and maintain regulatory approval for its product candidates; the ability of BioNTech’s COVID- 19 vaccines to prevent COVID-19 caused by emerging virus variants; BioNTech’s and its counterparties’ ability to manage and source necessary energy resources; BioNTech’s ability to identify research opportunities and discover and develop investigational medicines; the ability and willingness of BioNTech’s third-party collaborators to continue research and development activities relating to BioNTech's development candidates and investigational medicines; the impact of COVID-19 on BioNTech’s development programs, supply chain, collaborators and financial performance; unforeseen safety issues and potential claims that are alleged to arise from the use of products and product candidates developed or manufactured by BioNTech; BioNTech’s and its collaborators’ ability to commercialize and market BioNTech’s COVID-19 vaccine and, if approved, its product candidates; BioNTech’s ability to manage its development and related expenses; regulatory and political developments in the United States and other countries; BioNTech’s ability to effectively scale its production capabilities and manufacture its products and product candidates; risks relating to the global financial system and markets; and other factors not known to BioNTech at this time. You should review the risks and uncertainties described under the heading “Risk Factors” in BioNTech’s Report on Form 6-K for the period ended September 30, 2025, and in subsequent filings made by BioNTech with the SEC, which are available on the SEC’s website at www.sec.gov. These forward-looking statements speak only as of the date hereof. Except as required by law, BioNTech disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation in the event of new information, future developments or otherwise. Furthermore, certain statements contained in this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and BioNTech’s own internal estimates and research. While BioNTech believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, any market data included in this presentation involves assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. While BioNTech believes its own internal research is reliable, such research has not been verified by any independent source. In addition, BioNTech is the owner of various trademarks, trade names and service marks that may appear in this presentation. Certain other trademarks, trade names and service marks appearing in this presentation are the property of third parties. Solely for convenience, the trademarks and trade names in this presentation may be referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. An abbreviation directory of defined terms can be found at the end of the presentation.

Innovation Series R&D Day 2025 Agenda 1 Introductory Remarks Douglas Maffei, PhD, Vice President, Strategy and Investor Relations 2 BioNTech’s Unique Approach to Innovation Prof. Uğur Şahin Co-founder and Chief Executive Officer 3 BioNTech’s Differentiated Clinical Strategy to Advance the Treatment of Solid Tumors Prof. Özlem Türeci, M.D. Co-founder and Chief Medical Officer 4 Establishing Pumitamig1 in Foundational Tumor Types Prof. Ilhan Celik, M.D. Vice President, Clinical Development Michael Wenger, M.D. Vice President, Clinical Development 5 Innovating Early-Stage Cancer Treatment with mRNA Cancer Immunotherapies Prof. Özlem Türeci, M.D. Co-founder and Chief Medical Officer 6 BioNTech’s Path to Value Creation Ramón Zapata Chief Financial Officer 7 Q&A Panel Discussion All Speakers Annemarie Hanekamp Chief Commercial Officer 1. Partnered with Bristol Myers Squibb.

BioNTech’s Unique Approach to Innovation Prof. Uğur Şahin CEO and Co-founder 2

Translating Science into Survival Building a Global Immunotherapy Powerhouse

7 BioNTech – Disruptive Tech-Bio Company with Pioneering Technologies Developed Through Full AI Integration Multiplatform oncology company Infectious diseases pipeline COVID-19 vaccine global impact Leader in integrated AI capabilities In-house manufacturing 16 >20Clinical programs Ongoing Phase 2 or 3 trials 7 Clinical programs in high unmet need indications 5 Billion doses distributed 4 Platforms including individualized mRNA and bispecific antibodies
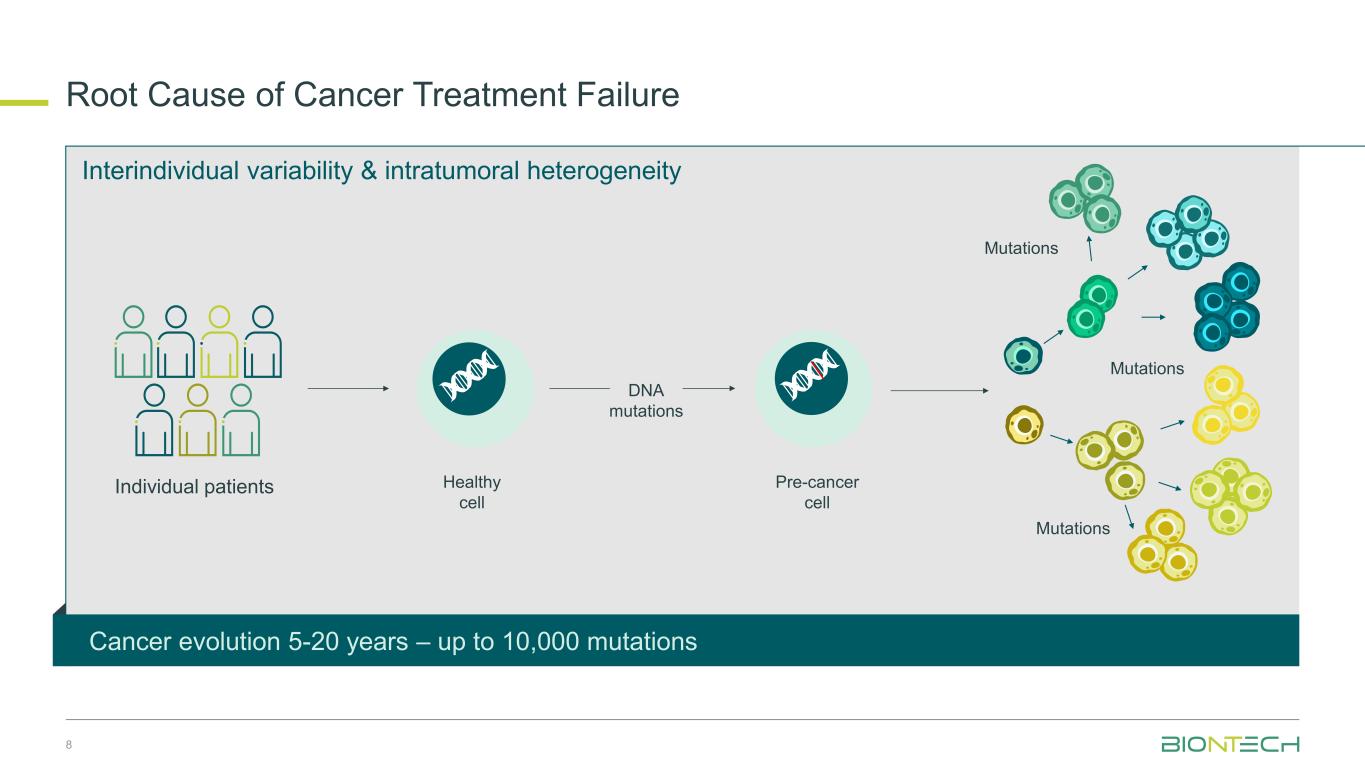
Interindividual variability & intratumoral heterogeneity Root cause of cancer treatment failureR ot Cause of Cancer Tre tm nt Failure Mutations Mutations Mutations Healthy cell Pre-cancer cell Individual patients DNA mutations Cancer evolution 5-20 years – up to 10,000 mutations 8
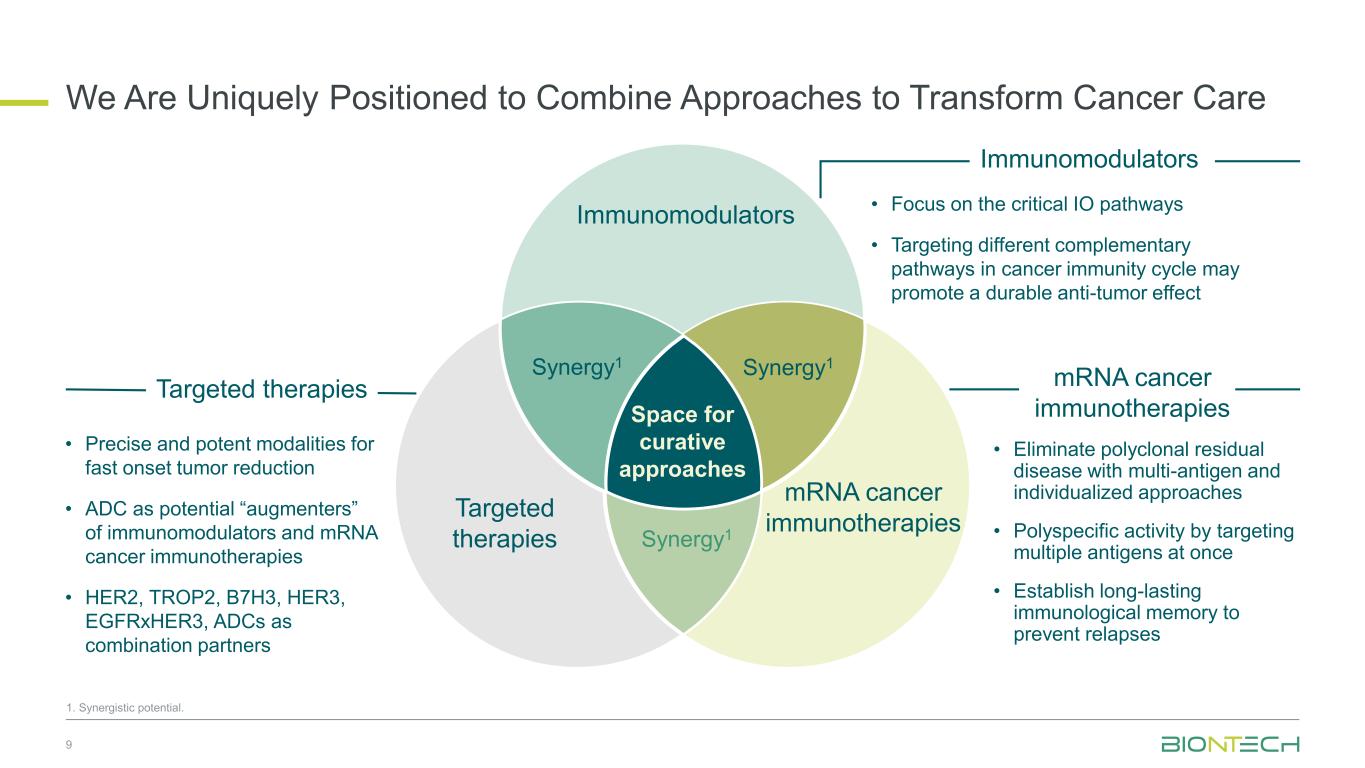
Space for curative approaches Immunomodulators mRNA cancer immunotherapies Targeted therapies Synergy1Synergy1 Synergy1 • Focus on the critical IO pathways • Targeting different complementary pathways in cancer immunity cycle may promote a durable anti-tumor effect • Eliminate polyclonal residual disease with multi-antigen and individualized approaches • Polyspecific activity by targeting multiple antigens at once • Establish long-lasting immunological memory to prevent relapses • Precise and potent modalities for fast onset tumor reduction • ADC as potential “augmenters” of immunomodulators and mRNA cancer immunotherapies • HER2, TROP2, B7H3, HER3, EGFRxHER3, ADCs as combination partners 9 Immunomodulators Targeted therapies mRNA cancer immunotherapies We Are Uniquely Positioned to Combine Approaches to Transform Cancer Care 1. Synergistic potential.
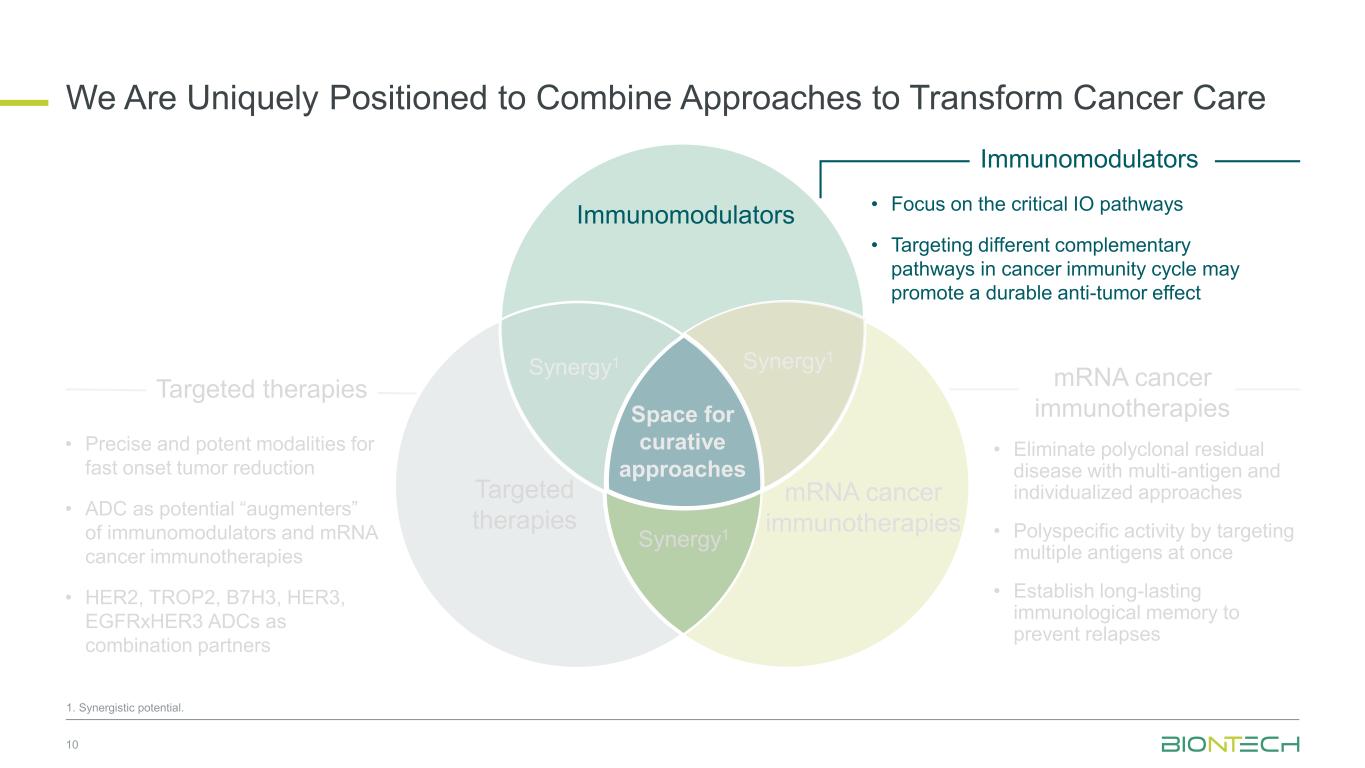
Space for curative approaches Immunomodulators mRNA cancer immunotherapies Targeted therapies Synergy1 Synergy1 Synergy1 • Focus on the critical IO pathways • Targeting different complementary pathways in cancer immunity cycle may promote a durable anti-tumor effect • Eliminate polyclonal residual disease with multi-antigen and individualized approaches • Polyspecific activity by targeting multiple antigens at once • Establish long-lasting immunological memory to prevent relapses • Precise and potent modalities for fast onset tumor reduction • ADC as potential “augmenters” of immunomodulators and mRNA cancer immunotherapies • HER2, TROP2, B7H3, HER3, EGFRxHER3 ADCs as combination partners 10 Immunomodulators Targeted therapies mRNA cancer immunotherapies We Are Uniquely Positioned to Combine Approaches to Transform Cancer Care 1. Synergistic potential.
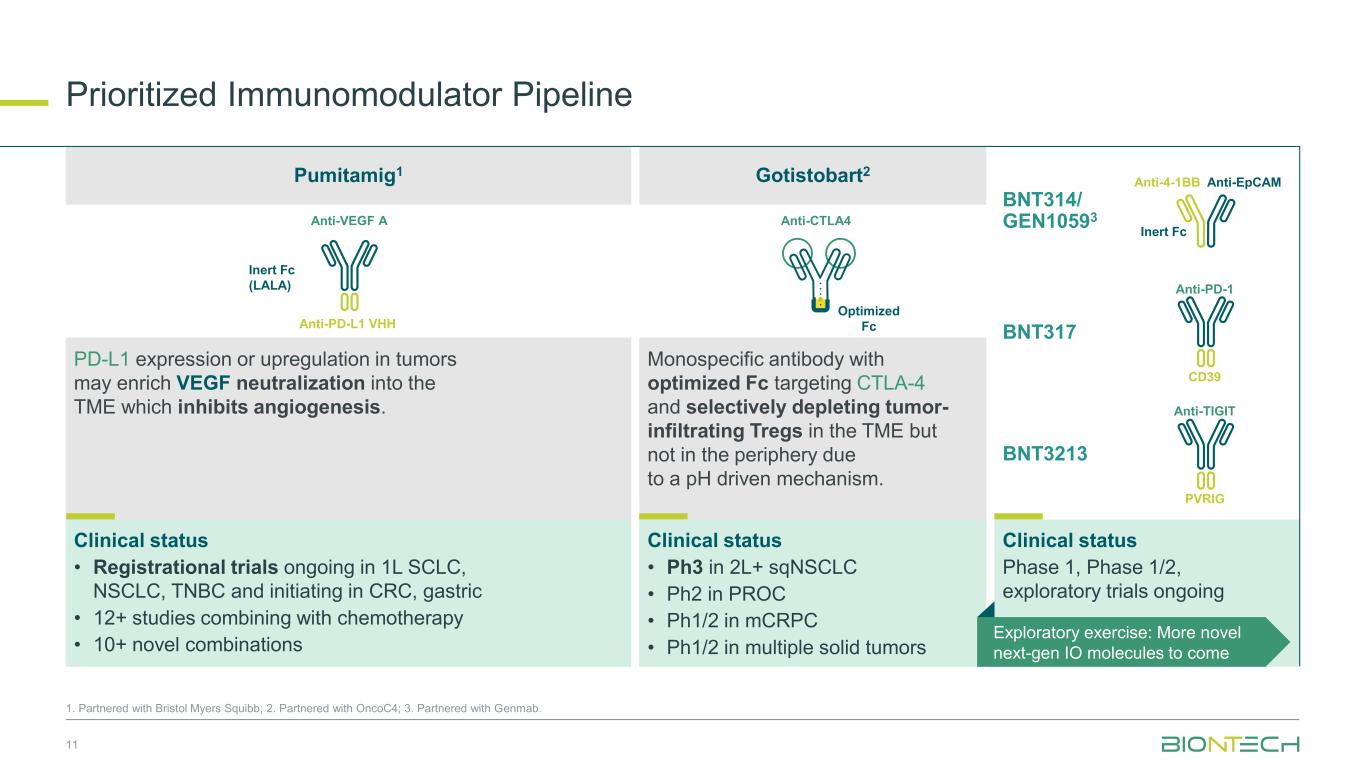
Prioritized Immunomodulator Pipeline 11 1. Partnered with Bristol Myers Squibb; 2. Partnered with OncoC4; 3. Partnered with Genmab. Anti-EpCAMAnti-4-1BB Inert Fc Anti-PD-1 CD39 Anti-TIGIT PVRIG Clinical status Phase 1, Phase 1/2, exploratory trials ongoing BNT314/ GEN10593 BNT3213 BNT317 Anti-VEGF A Anti-PD-L1 VHH Inert Fc (LALA) Anti-CTLA4 Optimized Fc PD-L1 expression or upregulation in tumors may enrich VEGF neutralization into the TME which inhibits angiogenesis. Monospecific antibody with optimized Fc targeting CTLA-4 and selectively depleting tumor- infiltrating Tregs in the TME but not in the periphery due to a pH driven mechanism. Clinical status • Ph3 in 2L+ sqNSCLC • Ph2 in PROC • Ph1/2 in mCRPC • Ph1/2 in multiple solid tumors Clinical status • Registrational trials ongoing in 1L SCLC, NSCLC, TNBC and initiating in CRC, gastric • 12+ studies combining with chemotherapy • 10+ novel combinations Exploratory exercise: More novel next-gen IO molecules to come Pumitamig1 Gotistobart2
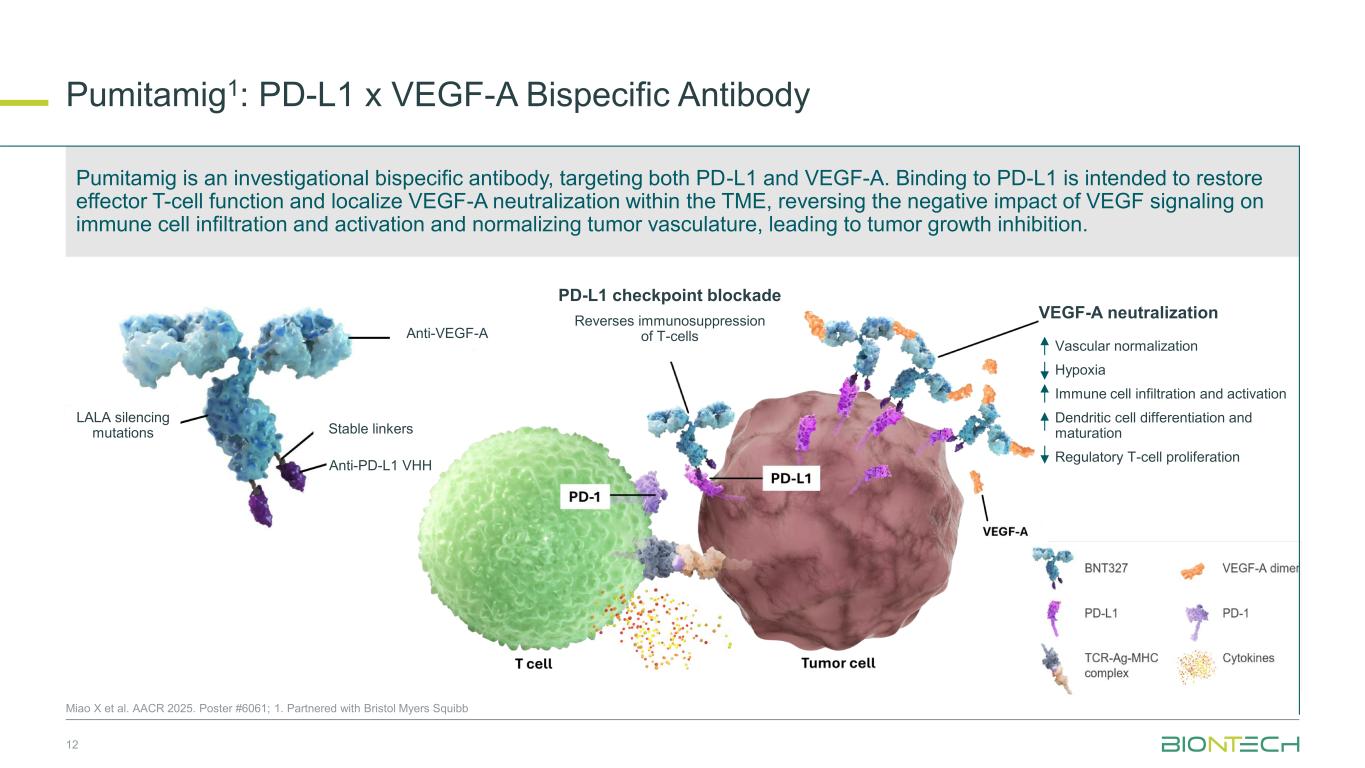
Pumitamig1: PD-L1 x VEGF-A Bispecific Antibody 12 Miao X et al. AACR 2025. Poster #6061; 1. Partnered with Bristol Myers Squibb Anti-PD-L1 VHH LALA silencing mutations Stable linkers Anti-VEGF-A PD-L1 checkpoint blockade Reverses immunosuppression of T-cells VEGF-A neutralization Vascular normalization Hypoxia Immune cell infiltration and activation Dendritic cell differentiation and maturation Regulatory T-cell proliferation Pumitamig is an investigational bispecific antibody, targeting both PD-L1 and VEGF-A. Binding to PD-L1 is intended to restore effector T-cell function and localize VEGF-A neutralization within the TME, reversing the negative impact of VEGF signaling on immune cell infiltration and activation and normalizing tumor vasculature, leading to tumor growth inhibition.
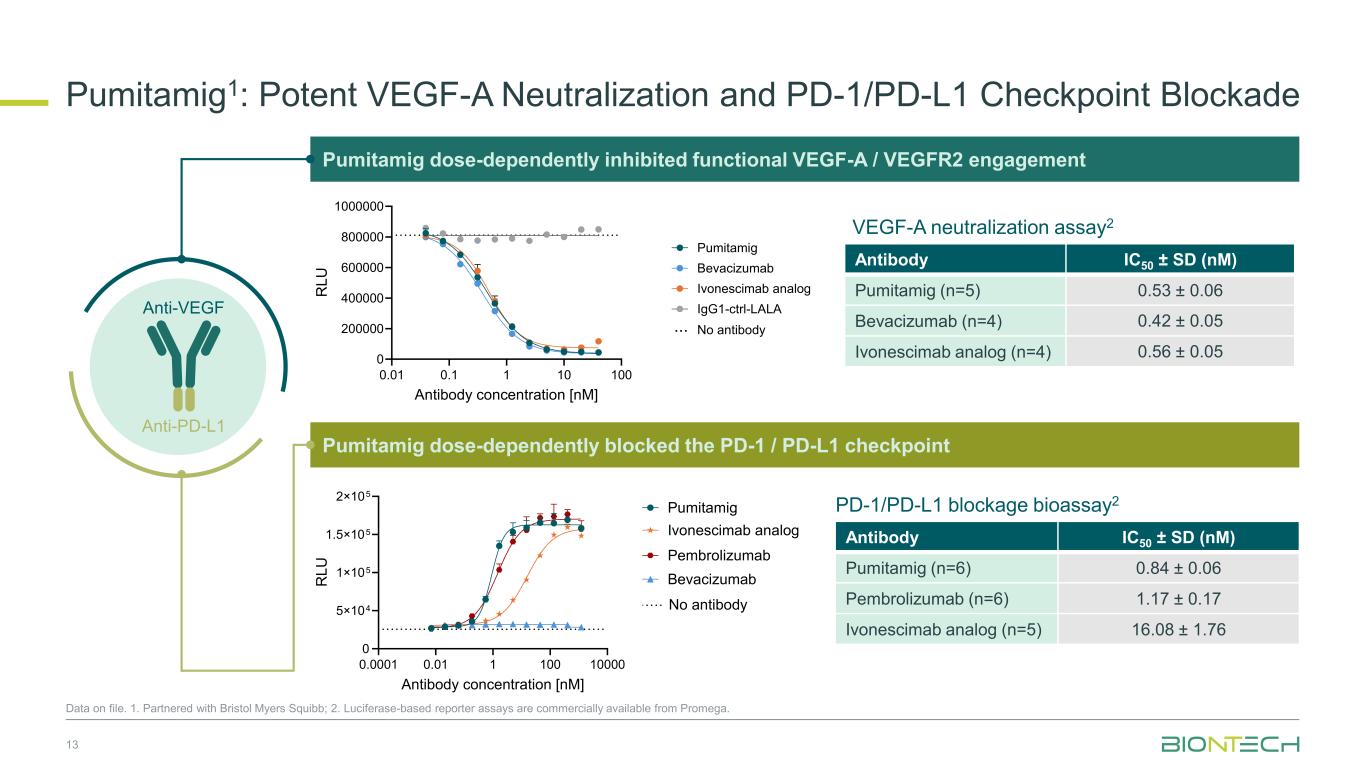
13 Anti-PD-L1 Anti-VEGFnti- F Pumitamig dose-dependently inhibited functional VEGF-A / VEGFR2 engagement 0.01 0.1 1 10 100 0 200000 400000 600000 800000 1000000 Antibody concentration [nM] R L U Pumitamig Bevacizumab Ivonescimab analog IgG1-ctrl-LALA No antibody... Antibody IC50 ± SD (nM) Pumitamig (n=5) 0.53 ± 0.06 Bevacizumab (n=4) 0.42 ± 0.05 Ivonescimab analog (n=4) 0.56 ± 0.05 Pumitamig dose-dependently blocked the PD-1 / PD-L1 checkpoint 0.0001 0.01 1 100 10000 0 5×104 1×105 1.5×105 2×105 Antibody concentration [nM] R L U Pumitamig Ivonescimab analog Bevacizumab Pembrolizumab No antibody Antibody IC50 ± SD (nM) Pumitamig (n=6) 0.84 ± 0.06 Pembrolizumab (n=6) 1.17 ± 0.17 Ivonescimab analog (n=5) 16.08 ± 1.76 VEGF-A neutralization assay2 PD-1/PD-L1 blockage bioassay2 Data on file. 1. Partnered with Bristol Myers Squibb; 2. Luciferase-based reporter assays are commercially available from Promega. Pumitamig1: Potent VEGF-A Neutralization and PD-1/PD-L1 Checkpoint Blockade
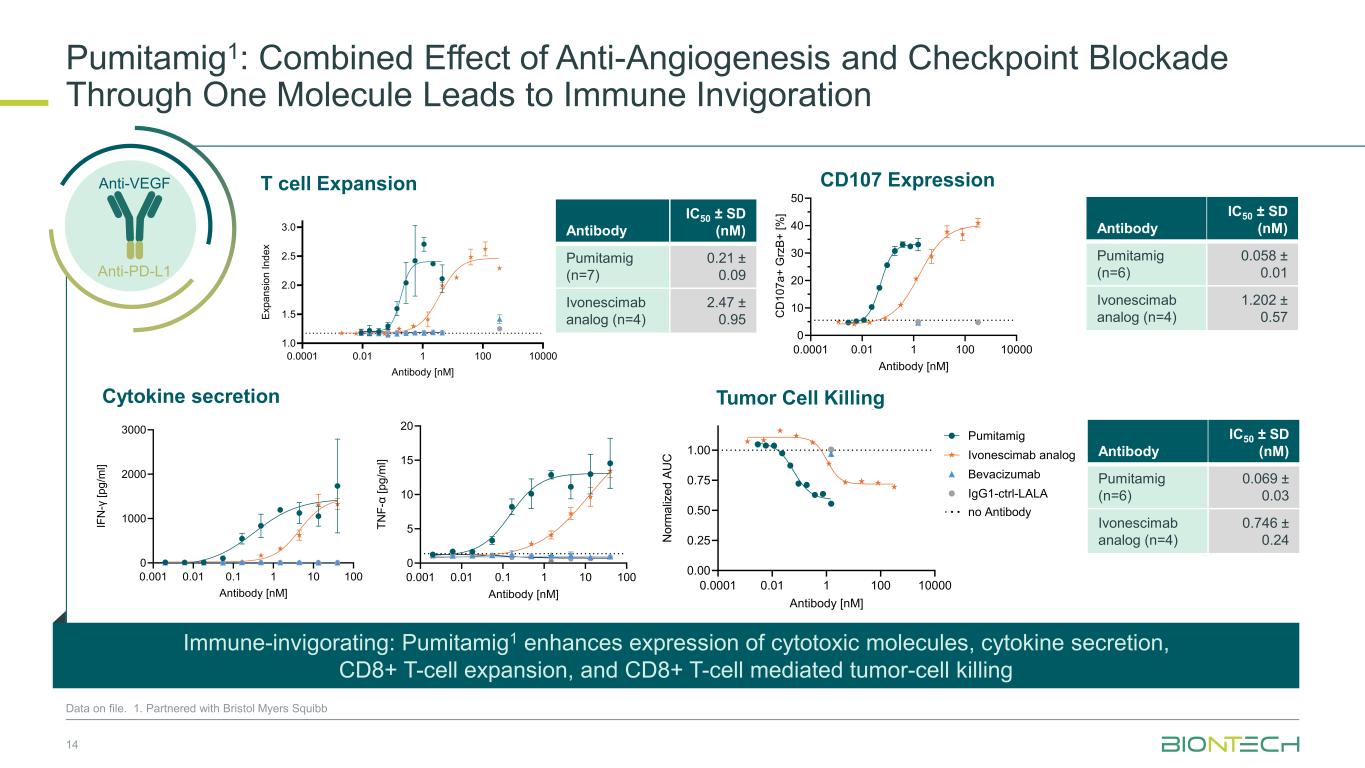
0.0001 0.01 1 100 10000 0 10 20 30 40 50 CD107a/GrzmB expression Antibody [nM] C D 1 0 7 a + G rz B + [ % ] 0.001 0.01 0.1 1 10 100 0 1000 2000 3000 Antibody [nM] IF N -γ [ p g /m l] 0.001 0.01 0.1 1 10 100 0 5 10 15 20 Antibody [nM] T N F -α [ p g /m l] 0.001 0.01 0.1 1 10 100 0 10 20 30 40 Antibody [nM] IL -2 [ p g /m l] T cell Expansion Cytokine secretion 0.0001 0.01 1 100 10000 1.0 1.5 2.0 2.5 3.0 Antibody [nM] E x p a n s io n I n d e x Pumitamig Ivonescimab analog Bevacizumab IgG1-ctrl-LALA no Antibody Data on file. 1. Partnered with Bristol Myers Squibb 14 Pumitamig1: Combined Effect of Anti-Angiogenesis and Checkpoint Blockade Through One Molecule Leads to Immune Invigoration Immune-invigorating: Pumitamig1 enhances expression of cytotoxic molecules, cytokine secretion, CD8+ T-cell expansion, and CD8+ T-cell mediated tumor-cell killing Anti-PD-L1 Anti-VEGFti- Antibody IC50 ± SD (nM) Pumitamig (n=7) 0.21 ± 0.09 Ivonescimab analog (n=4) 2.47 ± 0.95 Antibody IC50 ± SD (nM) Pumitamig (n=6) 0.069 ± 0.03 Ivonescimab analog (n=4) 0.746 ± 0.24 Antibody IC50 ± SD (nM) Pumitamig (n=6) 0.058 ± 0.01 Ivonescimab analog (n=4) 1.202 ± 0.57 0.0001 0.01 1 100 10000 0.00 0.25 0.50 0.75 1.00 Tumor-cell killing Antibody [nM] N o rm a liz e d A U C Pumitamig Ivonescimab analog Bevacizumab IgG1-ctrl-LALA no Antibody Tumor Cell Killi CD107 Expression
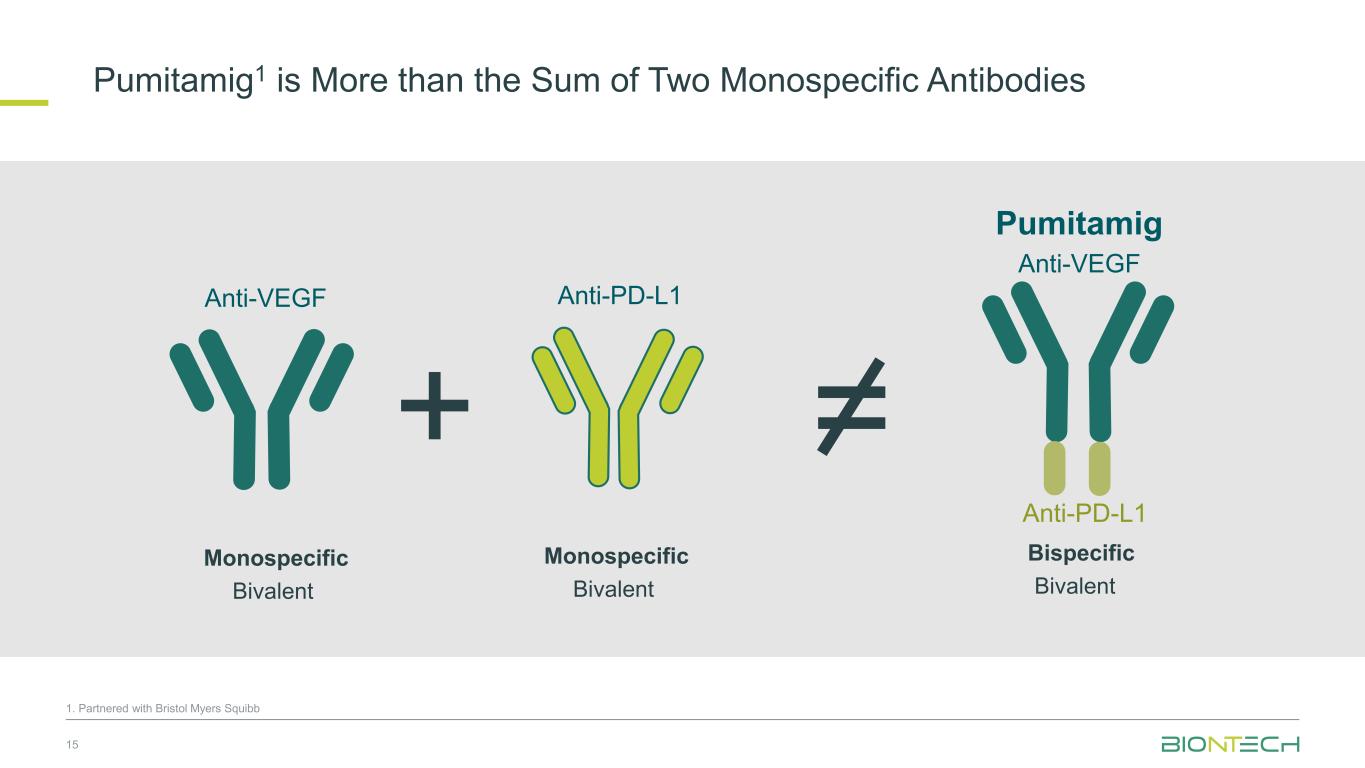
Pumitamig1 is More than the Sum of Two Monospecific Antibodies 15 Anti-VEGF Anti-PD-L1 Anti-VEGF Pumitamig Anti-PD-L1 + = Monospecific Bivalent Monospecific Bivalent Bispecific Bivalent 1. Partnered with Bristol Myers Squibb
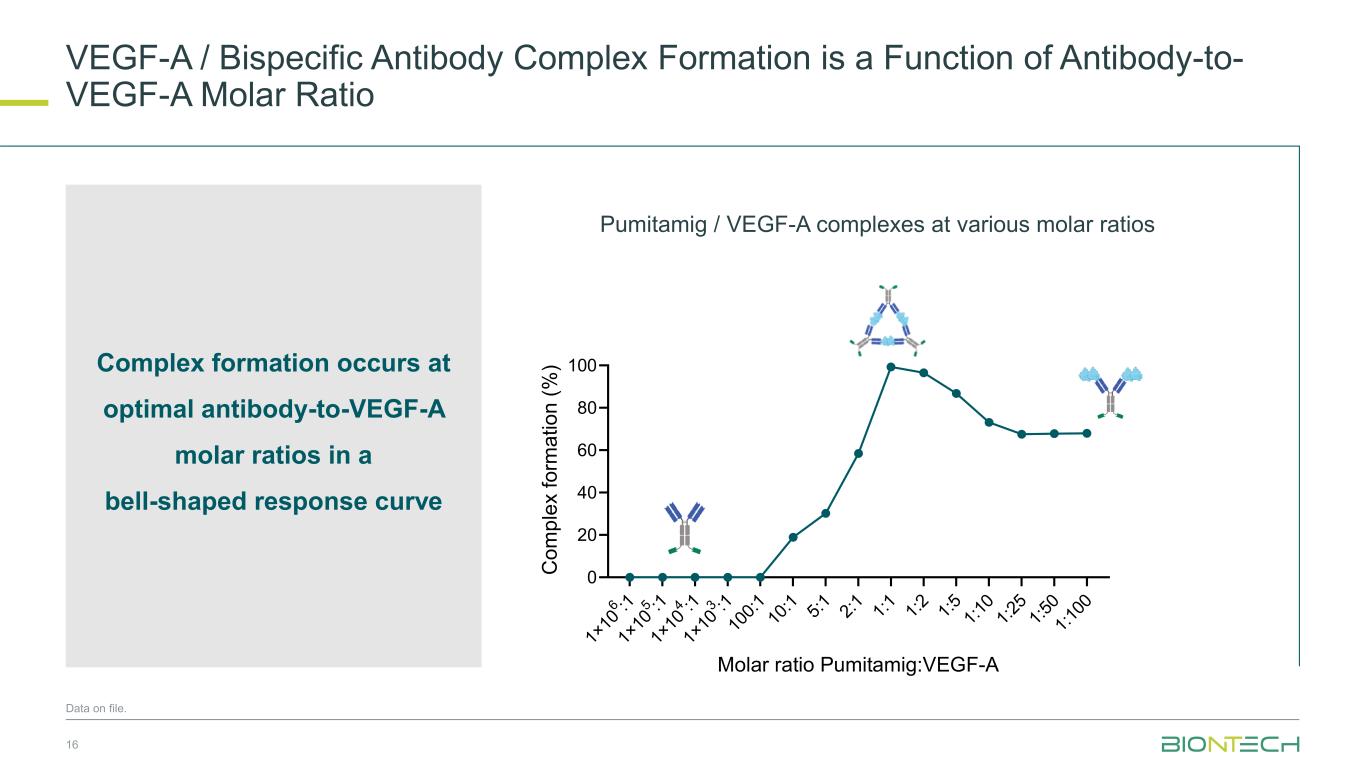
VEGF-A / Bispecific Antibody Complex Formation is a Function of Antibody-to- VEGF-A Molar Ratio 16 Data on file. Complex formation occurs at optimal antibody-to-VEGF-A molar ratios in a bell-shaped response curve 1× 10 6 :1 1× 10 5 :1 1× 10 4 :1 1× 10 3 :1 10 0: 1 10 :1 5: 1 2: 1 1: 1 1: 2 1: 5 1: 10 1: 25 1: 50 1: 10 0 0 20 40 60 80 100 Molar ratio Pumitamig:VEGF-A C o m p le x f o rm a ti o n ( % ) D. % Pumitamig/VEGF-A complexes at various molar ratios Pumitamig / VEGF-A complexes at various molar ratios
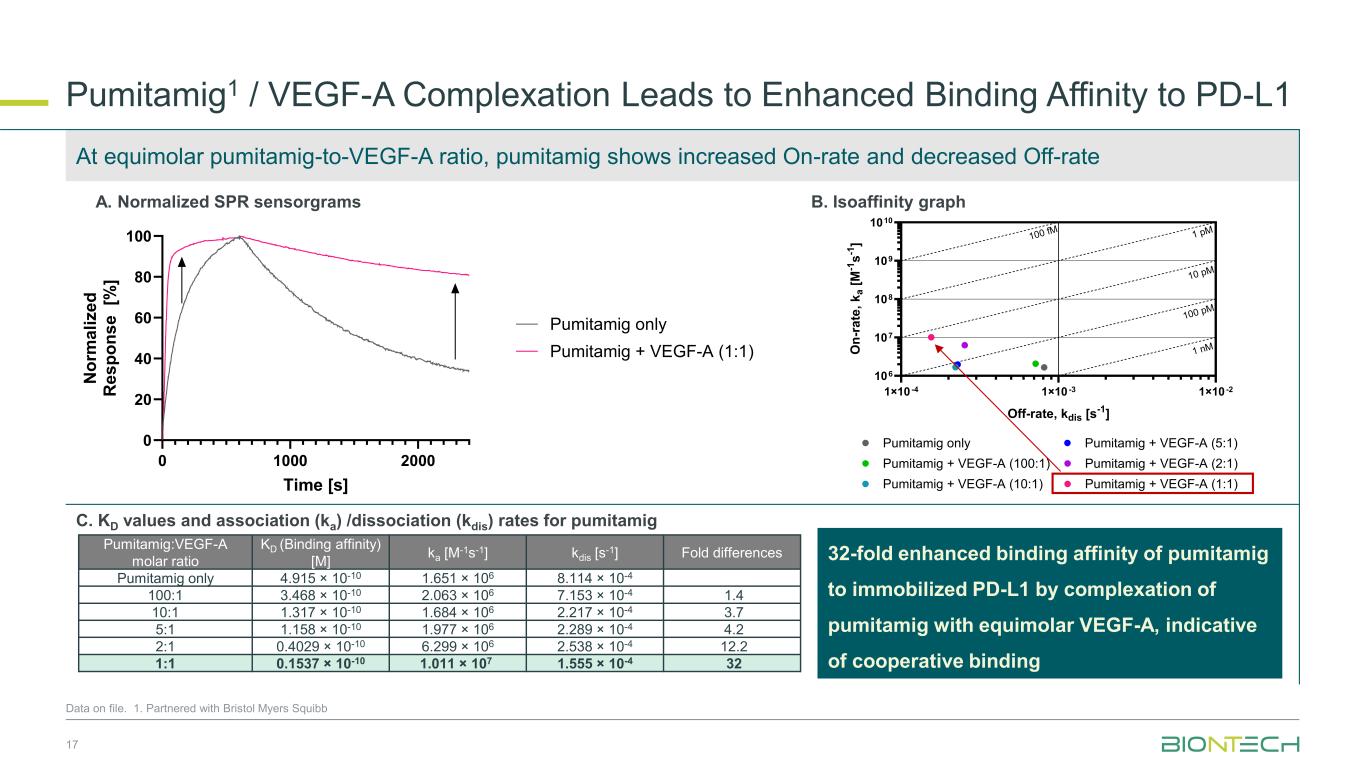
1×10 -4 1×10 -3 1×10 -2 106 107 108 109 1010 Off-rate, kdis [s-1] O n -r a te , k a [ M -1 s -1 ] Pumitamig only Pumitamig + VEGF-A (100:1) Pumitamig + VEGF-A (10:1) Pumitamig + VEGF-A (5:1) Pumitamig + VEGF-A (2:1) Pumitamig + VEGF-A (1:1) 100 fM 1 pM 10 pM 100 pM 1 nM Pumitamig1 / VEGF-A Complexation Leads to Enhanced Binding Affinity to PD-L1 17 Data on file. 1. Partnered with Bristol Myers Squibb 0 1000 2000 0 20 40 60 80 100 Time [s] N o rm a li z e d R e s p o n s e [% ] Pumitamig only Pumitamig + VEGF-A (1:1) Pumitamig:VEGF-A molar ratio KD (Binding affinity) [M] ka [M-1s-1] kdis [s-1] Fold differences Pumitamig only 4.915 × 10-10 1.651 × 106 8.114 × 10-4 100:1 3.468 × 10-10 2.063 × 106 7.153 × 10-4 1.4 10:1 1.317 × 10-10 1.684 × 106 2.217 × 10-4 3.7 5:1 1.158 × 10-10 1.977 × 106 2.289 × 10-4 4.2 2:1 0.4029 × 10-10 6.299 × 106 2.538 × 10-4 12.2 1:1 0.1537 × 10-10 1.011 × 107 1.555 × 10-4 32 A. Normalized SPR sensorgrams B. Isoaffinity graph C. KD values and association (ka) /dissociation (kdis) rates for pumitamig At equimolar pumitamig-to-VEGF-A ratio, pumitamig shows increased On-rate and decreased Off-rate 32-fold enhanced binding affinity of pumitamig to immobilized PD-L1 by complexation of pumitamig with equimolar VEGF-A, indicative of cooperative binding
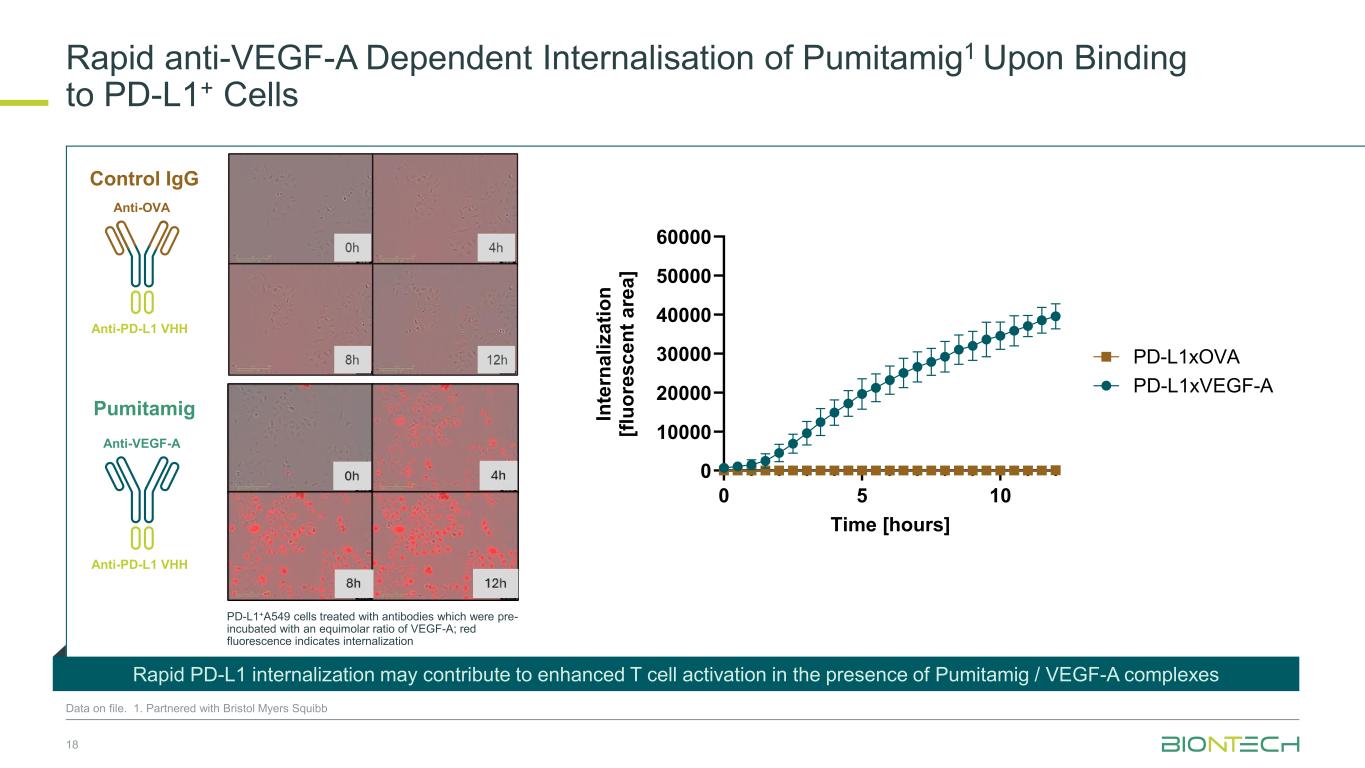
18 0 5 10 0 10000 20000 30000 40000 50000 60000 Time [hours] In te rn a li z a ti o n [f lu o re s c e n t a re a ] PD-L1xVEGF-A PD-L1xOVA Anti-VEGF-A Anti-PD-L1 VHH Anti-OVA Anti-PD-L1 VHH Rapid anti-VEGF-A Dependent Internalisation of Pumitamig1 Upon Binding to PD-L1+ Cells Data on file. 1. Partnered with Bristol Myers Squibb PD-L1+A549 cells treated with antibodies which were pre- incubated with an equimolar ratio of VEGF-A; red fluorescence indicates internalization Rapid PD-L1 internalization may contribute to enhanced T cell activation in the presence of Pumitamig / VEGF-A complexes Control IgG Pumitamig
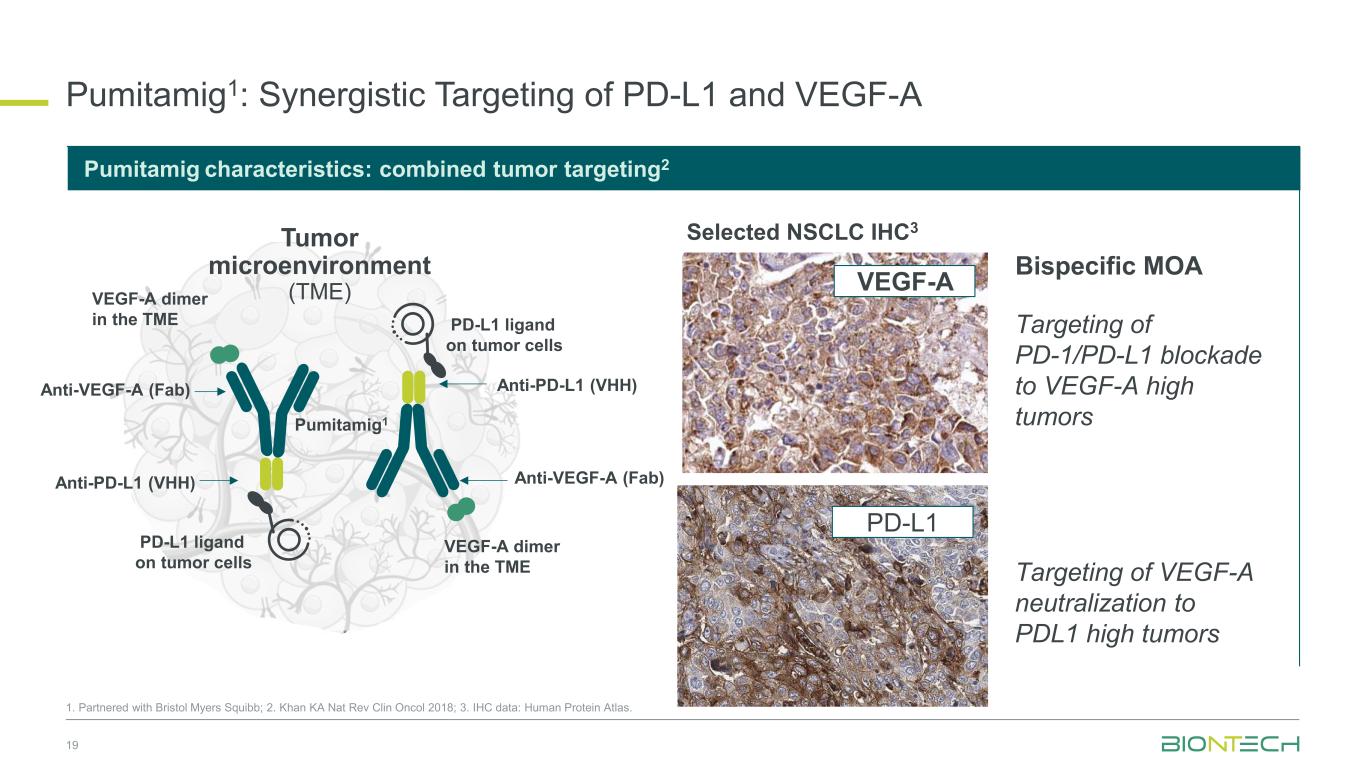
Pumitamig1: Synergistic Targeting of PD-L1 and VEGF-A 19 Targeting of PD-1/PD-L1 blockade to VEGF-A high tumors PD-L1 ligand binding on tumor cells Selected NSCLC IHC3 Bispecific MOA Anti-VEGF-A (Fab) Anti-PD-L1 (VHH) VEGF-A dimer in the TME PD-L1 ligand on tumor cells Tumor microenvironment (TME) Pumitamig1 VEGF-A dimer in the TME Anti-VEGF-A (Fab) Anti-PD-L1 (VHH) Pumitamig characteristics: combined tumor targeting2 PD-L1 ligand on tumor cells 1. Partnered with Bristol Myers Squibb; 2. Khan KA Nat Rev Clin Oncol 2018; 3. IHC data: Human Protein Atlas. VEGF-A PD-L1 Targeting of VEGF-A neutralization to PDL1 high tumors
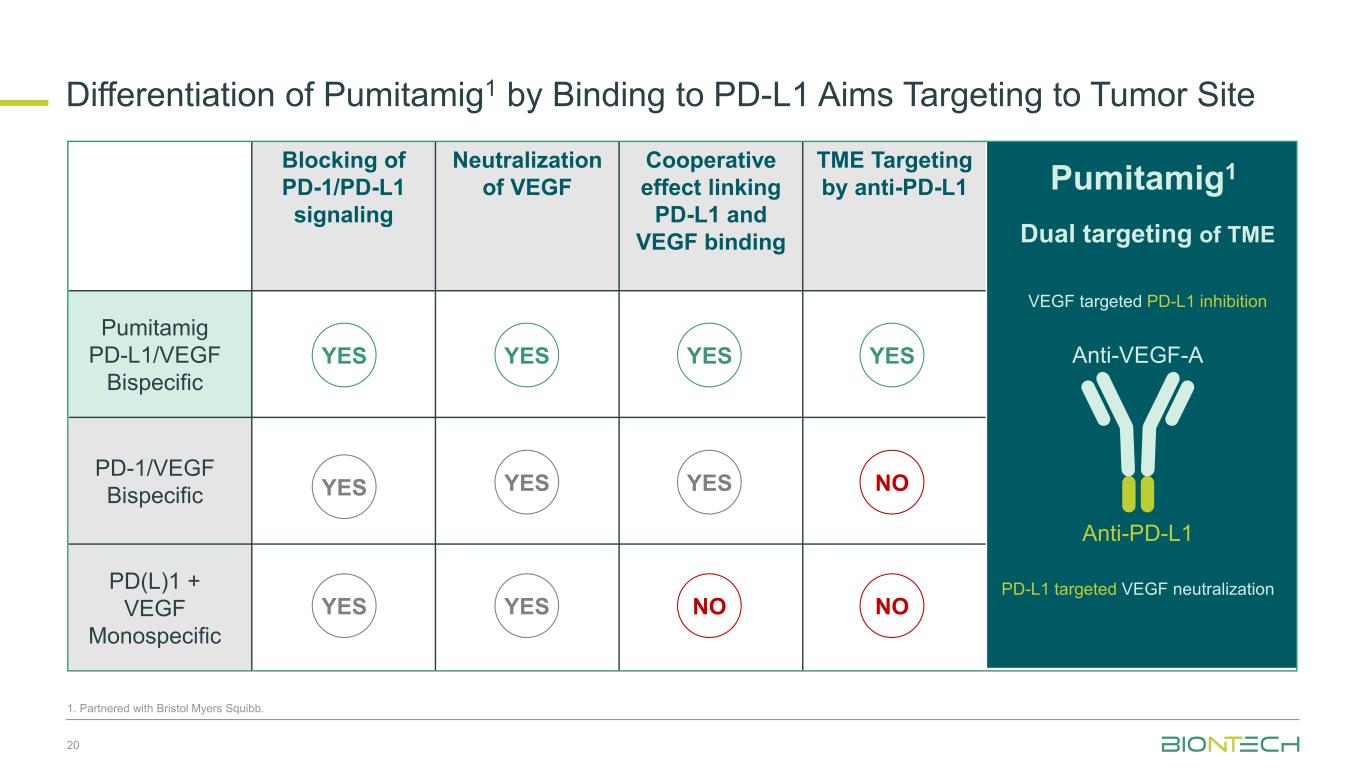
Blocking of PD-1/PD-L1 signaling Neutralization of VEGF Cooperative effect linking PD-L1 and VEGF binding TME Targeting by anti-PD-L1 Pumitamig PD-L1/VEGF Bispecific PD-1/VEGF Bispecific PD(L)1 + VEGF Monospecific Dual targeting of TME Anti-PD-L1 Anti-VEGF-AYES YES YES NO YESYES YES 1. Partnered with Bristol Myers Squibb. Differentiation of Pumitamig1 by Binding to PD-L1 Aims Targeting to Tumor Site 20 Pumitamig1 VEGF targeted PD-L1 inhibition PD-L1 targeted VEGF neutralization YES YES NOYES NO
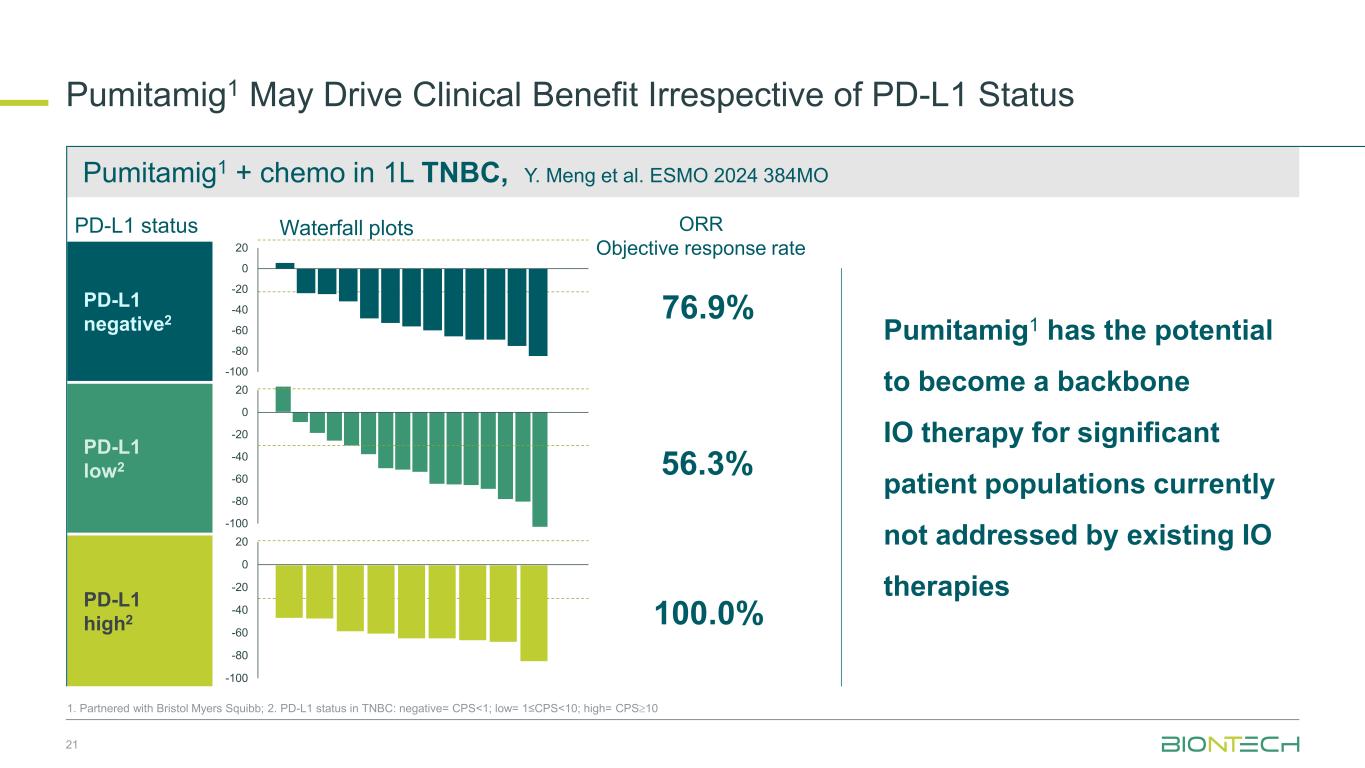
Pumitamig1 + chemo in 1L TNBC, Y. Meng et al. ESMO 2024 384MO Pumitamig1 May Drive Clinical Benefit Irrespective of PD-L1 Status 21 76.9% 56.3% 100.0% ORR Objective response rate Pumitamig1 has the potential to become a backbone IO therapy for significant patient populations currently not addressed by existing IO therapies PD-L1 negative2 PD-L1 low2 PD-L1 high2 -100 -80 -60 -40 -20 0 20 -100 -80 -60 -40 -20 0 20 -100 -80 -60 -40 -20 0 20 1. Partnered with Bristol Myers Squibb; 2. PD-L1 status in TNBC: negative= CPS<1; low= 1≤CPS<10; high= CPS10 Waterfall plotsPD-L1 status
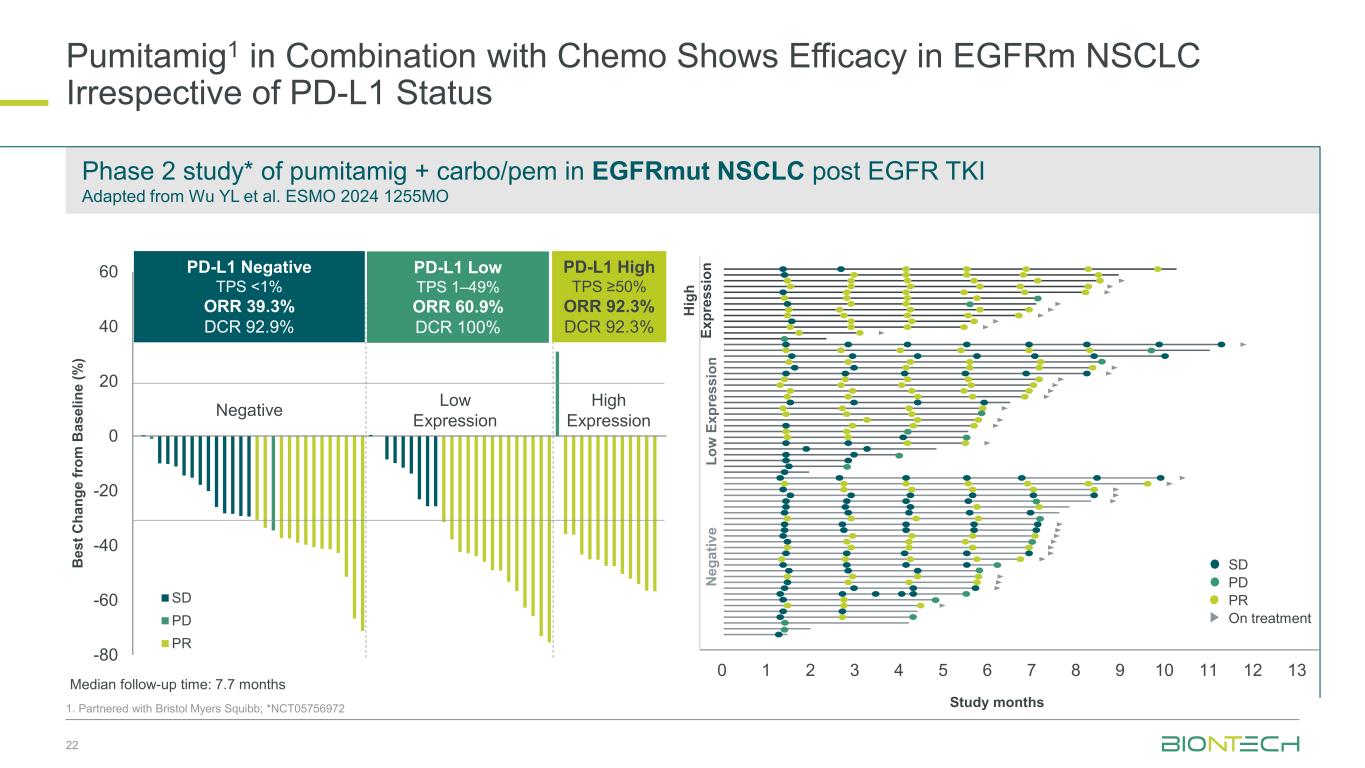
-80 -60 -40 -20 0 20 40 60 SD PD PR 22 1. Partnered with Bristol Myers Squibb; *NCT05756972 B e s t C h a n g e f ro m B a s e li n e ( % ) Negative Low Expression High Expression Study months 0 1 2 3 4 5 6 7 8 9 10 11 12 13 PD SD PR On treatment N e g a ti v e L o w E x p re s s io n H ig h E x p re s s io n Median follow-up time: 7.7 months PD-L1 Negative TPS <1% ORR 39.3% DCR 92.9% PD-L1 High TPS ≥50% ORR 92.3% DCR 92.3% PD-L1 Low TPS 1–49% ORR 60.9% DCR 100% Phase 2 study* of pumitamig + carbo/pem in EGFRmut NSCLC post EGFR TKI Adapted from Wu YL et al. ESMO 2024 1255MO Pumitamig1 in Combination with Chemo Shows Efficacy in EGFRm NSCLC Irrespective of PD-L1 Status
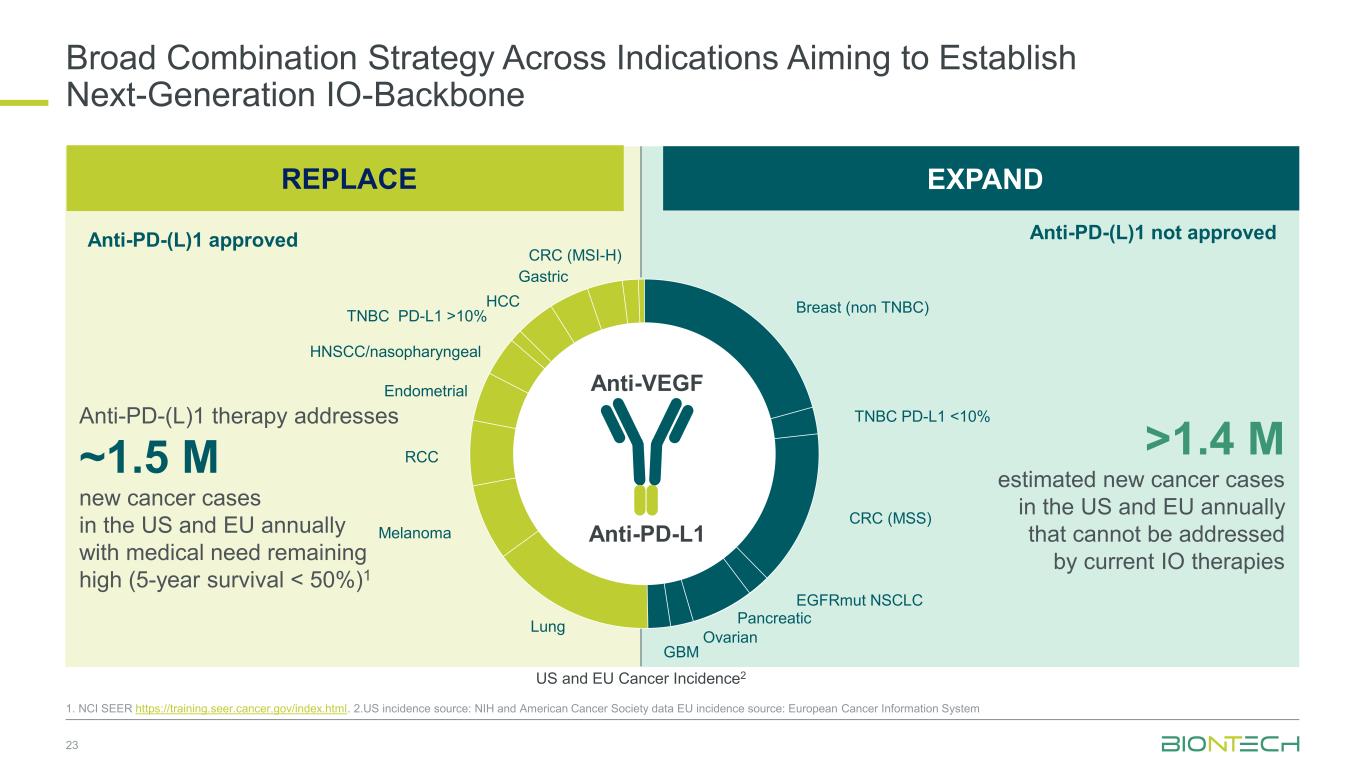
Broad Combination Strategy Across Indications Aiming to Establish Next-Generation IO-Backbone 23 1. NCI SEER https://training.seer.cancer.gov/index.html. 2.US incidence source: NIH and American Cancer Society data EU incidence source: European Cancer Information System Anti-PD-L1 Anti-VEGF Anti-PD-(L)1 therapy addresses ~1.5 M new cancer cases in the US and EU annually with medical need remaining high (5-year survival < 50%)1 >1.4 M estimated new cancer cases in the US and EU annually that cannot be addressed by current IO therapies EXPAND US and EU Cancer Incidence2 Breast (non TNBC) TNBC PD-L1 <10% CRC (MSS) EGFRmut NSCLC Pancreatic Ovarian GBM Lung Melanoma RCC Endometrial HNSCC/nasopharyngeal TNBC PD-L1 >10% HCC Gastric CRC (MSI-H) REPLACE Anti-PD-(L)1 approved Anti-PD-(L)1 not approved
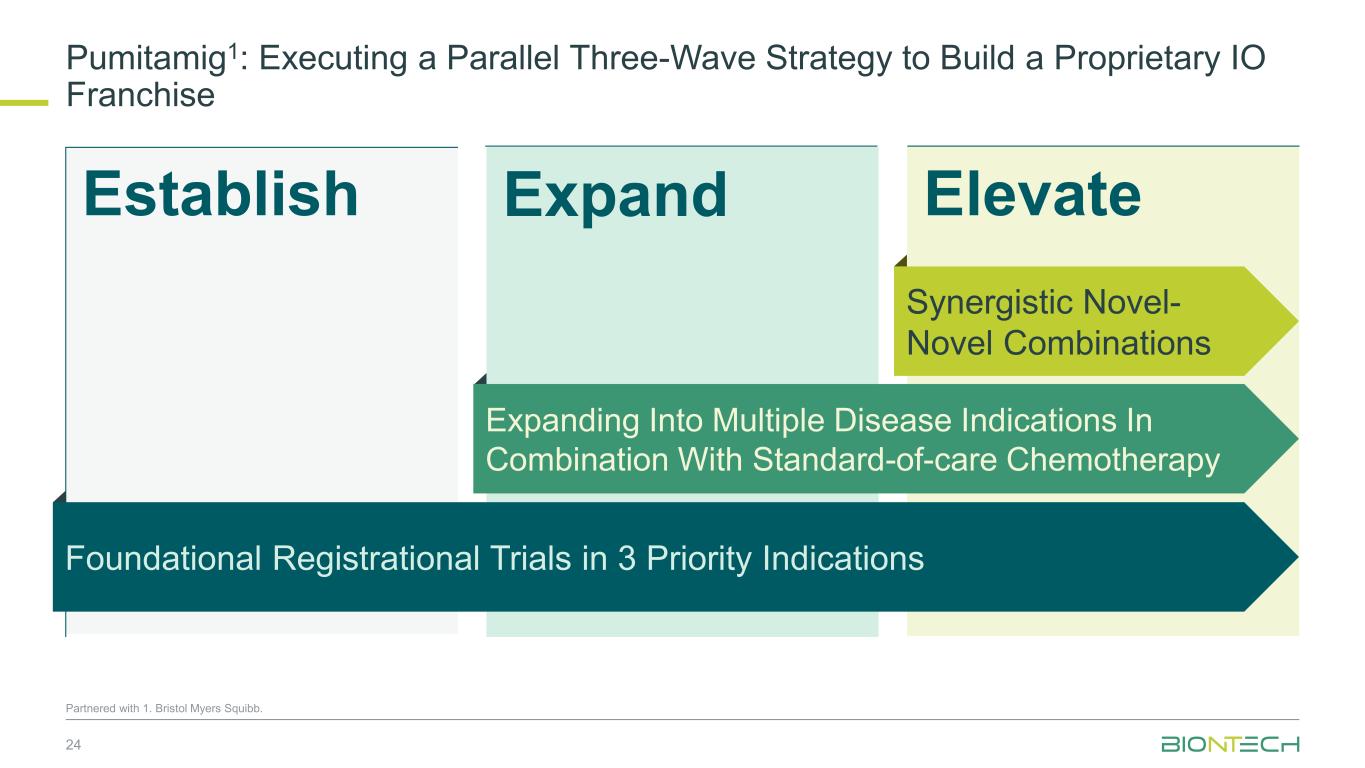
ElevateEstablish Pumitamig1: Executing a Parallel Three-Wave Strategy to Build a Proprietary IO Franchise 24 Partnered with 1. Bristol Myers Squibb. Expand Expanding Into Multiple Disease Indications In Combination With Standard-of-care Chemotherapy Synergistic Novel- Novel Combinations Foundational Registrational Trials in 3 Priority Indications
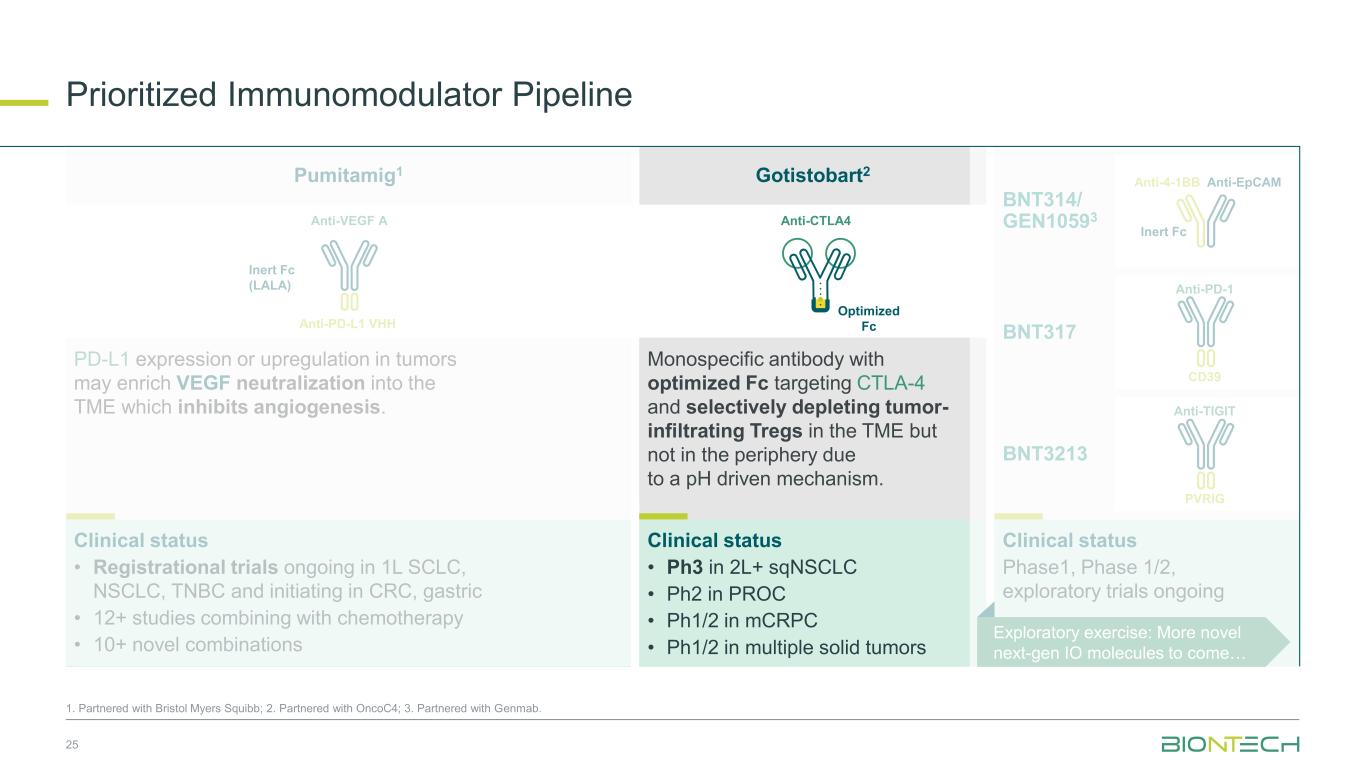
Prioritized Immunomodulator Pipeline 25 1. Partnered with Bristol Myers Squibb; 2. Partnered with OncoC4; 3. Partnered with Genmab. Anti-EpCAMAnti-4-1BB Inert Fc Anti-PD-1 CD39 Anti-TIGIT PVRIG Clinical status Phase1, Phase 1/2, exploratory trials ongoing BNT314/ GEN10593 BNT3213 BNT317 Anti-VEGF A Anti-PD-L1 VHH Inert Fc (LALA) Anti-CTLA4 Optimized Fc PD-L1 expression or upregulation in tumors may enrich VEGF neutralization into the TME which inhibits angiogenesis. Monospecific antibody with optimized Fc targeting CTLA-4 and selectively depleting tumor- infiltrating Tregs in the TME but not in the periphery due to a pH driven mechanism. Clinical status • Ph3 in 2L+ sqNSCLC • Ph2 in PROC • Ph1/2 in mCRPC • Ph1/2 in multiple solid tumors Clinical status • Registrational trials ongoing in 1L SCLC, NSCLC, TNBC and initiating in CRC, gastric • 12+ studies combining with chemotherapy • 10+ novel combinations Exploratory exercise: More novel next-gen IO molecules to come… Pumitamig1 Gotistobart2
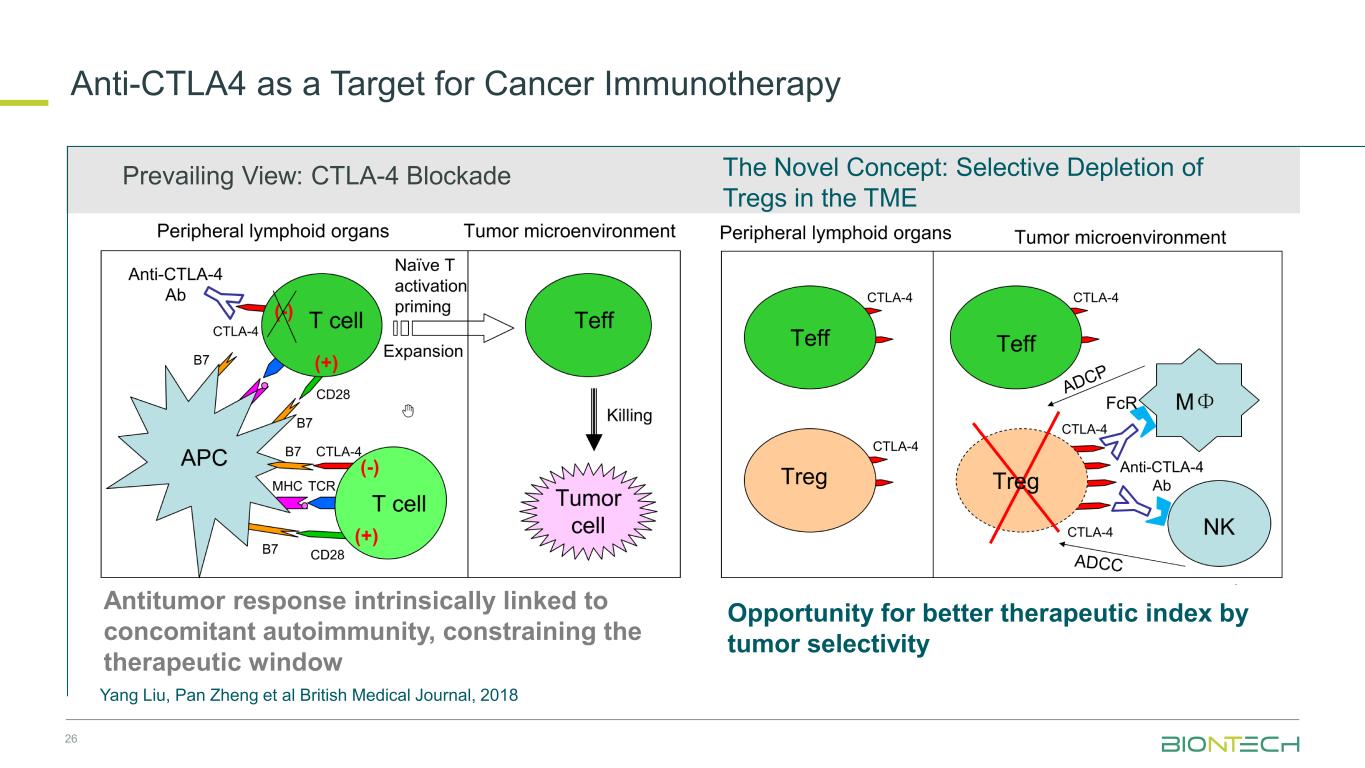
26 The Novel Concept: Selective Depletion of Tregs in the TME Prevailing View: CTLA-4 Blockade Antitumor response intrinsically linked to concomitant autoimmunity, constraining the therapeutic window Opportunity for better therapeutic index by tumor selectivity Yang Liu, Pan Zheng et al British Medical Journal, 2018 Anti-CTLA4 as a Target for Cancer Immunotherapy
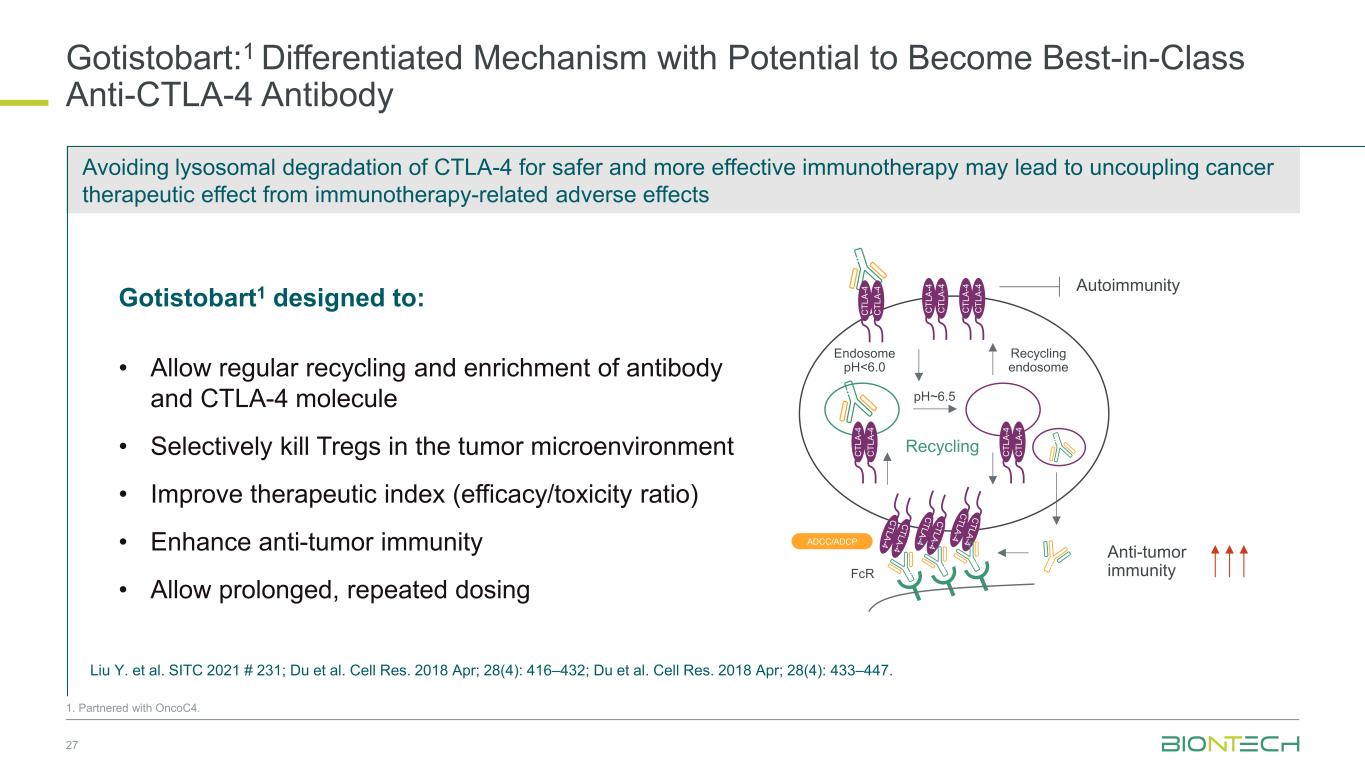
Avoiding lysosomal degradation of CTLA-4 for safer and more effective immunotherapy may lead to uncoupling cancer therapeutic effect from immunotherapy-related adverse effects Gotistobart:1 Differentiated Mechanism with Potential to Become Best-in-Class Anti-CTLA-4 Antibody 27 1. Partnered with OncoC4. Gotistobart1 designed to: • Allow regular recycling and enrichment of antibody and CTLA-4 molecule • Selectively kill Tregs in the tumor microenvironment • Improve therapeutic index (efficacy/toxicity ratio) • Enhance anti-tumor immunity • Allow prolonged, repeated dosing Endosome pH<6.0 Recycling endosome Recycling pH~6.5 FcR ADCC/ADCP Anti-tumor immunity Autoimmunity C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 C T L A -4 Liu Y. et al. SITC 2021 # 231; Du et al. Cell Res. 2018 Apr; 28(4): 416–432; Du et al. Cell Res. 2018 Apr; 28(4): 433–447.
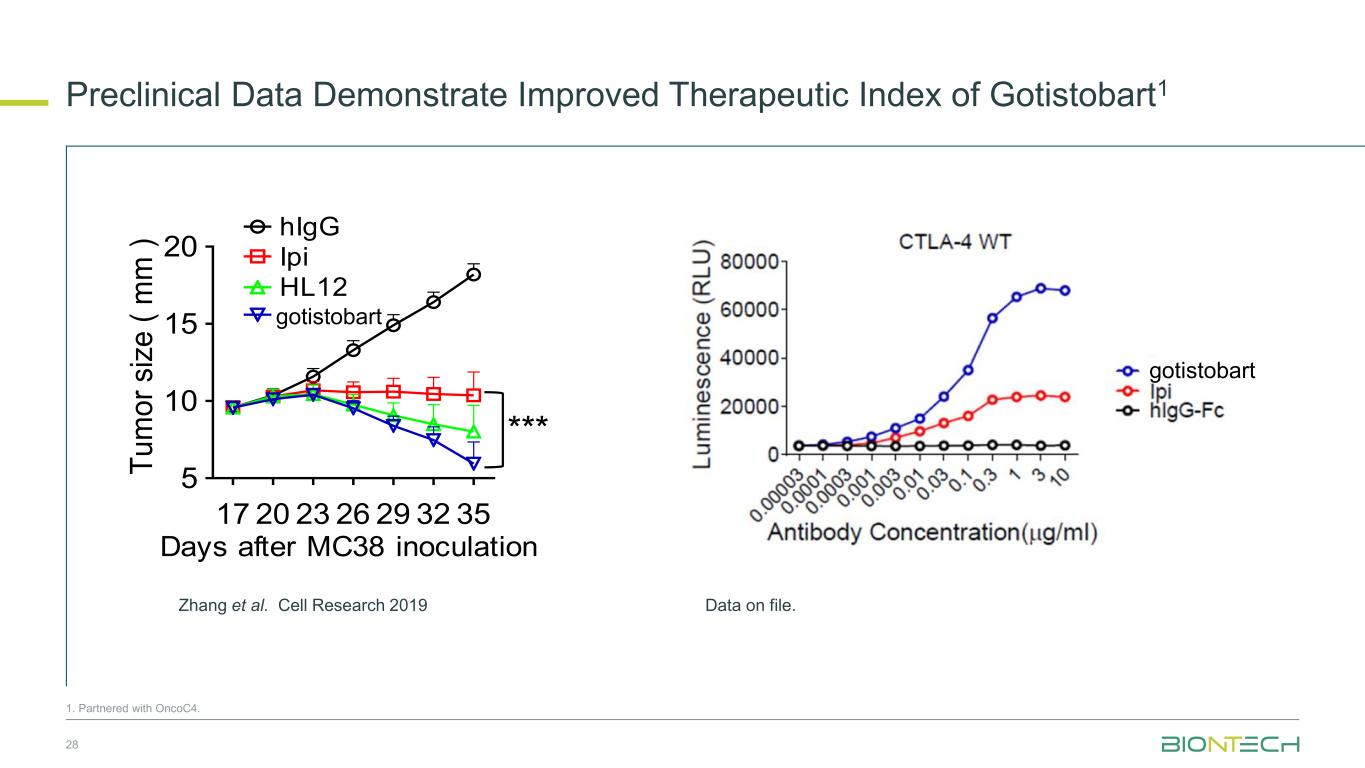
Preclinical Data Demonstrate Improved Therapeutic Index of Gotistobart1 28 Days after MC38 inoculation 17 20 23 26 29 32 35 5 10 15 20 hIgG Ipi HL12 HL32 Days after MC38 inoculation T u m o r si ze ( m m ) * hIg Ipi HL12 HL32 *** -100 0 500 1000 T u m o r v o lu m e c h a n g e f ro m b a s e lin e (% ) hIgG Ipi HL12 HL32 17 20 23 26 29 32 35 5 10 15 20 hIg Ipi. HL12 HL32 Days after MC38 inoculation T u m o r si ze ( m m ) Zhang et al. Cell Research 2019 gotistobart gotistobart 1. Partnered with OncoC4. Data on file.
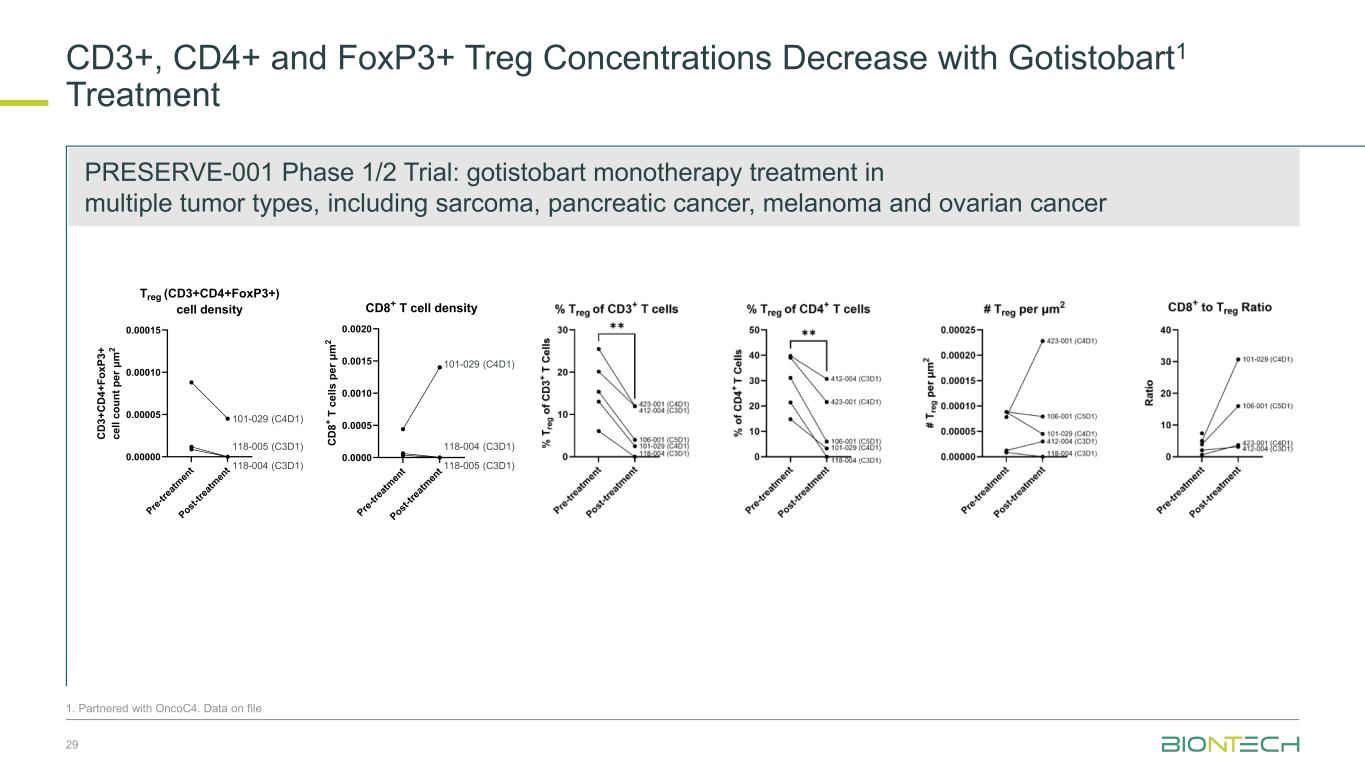
CD3+, CD4+ and FoxP3+ Treg Concentrations Decrease with Gotistobart1 Treatment 29 P re -t re at m en t P ost -t re at m en t 0.00000 0.00005 0.00010 0.00015 Treg (CD3+CD4+FoxP3+) cell density C D 3 + C D 4 + F o x P 3 + c e ll c o u n t p e r μ m 2 101-029 (C4D1) 118-004 (C3D1) 118-005 (C3D1) P re -t re at m en t P ost -t re at m en t 0.0000 0.0005 0.0010 0.0015 0.0020 CD8+ T cell density C D 8 + T c e ll s p e r μ m 2 101-029 (C4D1) 118-004 (C3D1) 118-005 (C3D1) 1. Partnered with OncoC4. Data on file PRESERVE-001 Phase 1/2 Trial: gotistobart monotherapy treatment in multiple tumor types, including sarcoma, pancreatic cancer, melanoma and ovarian cancer
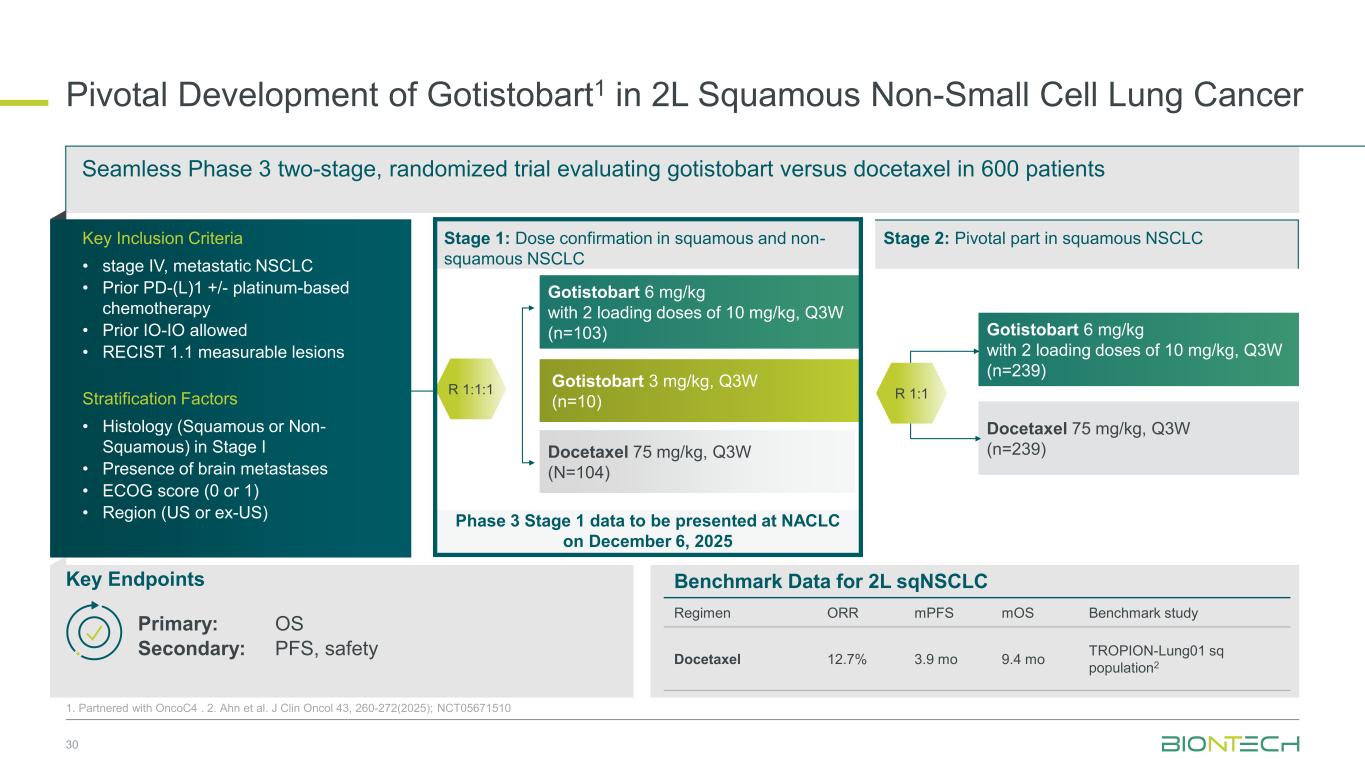
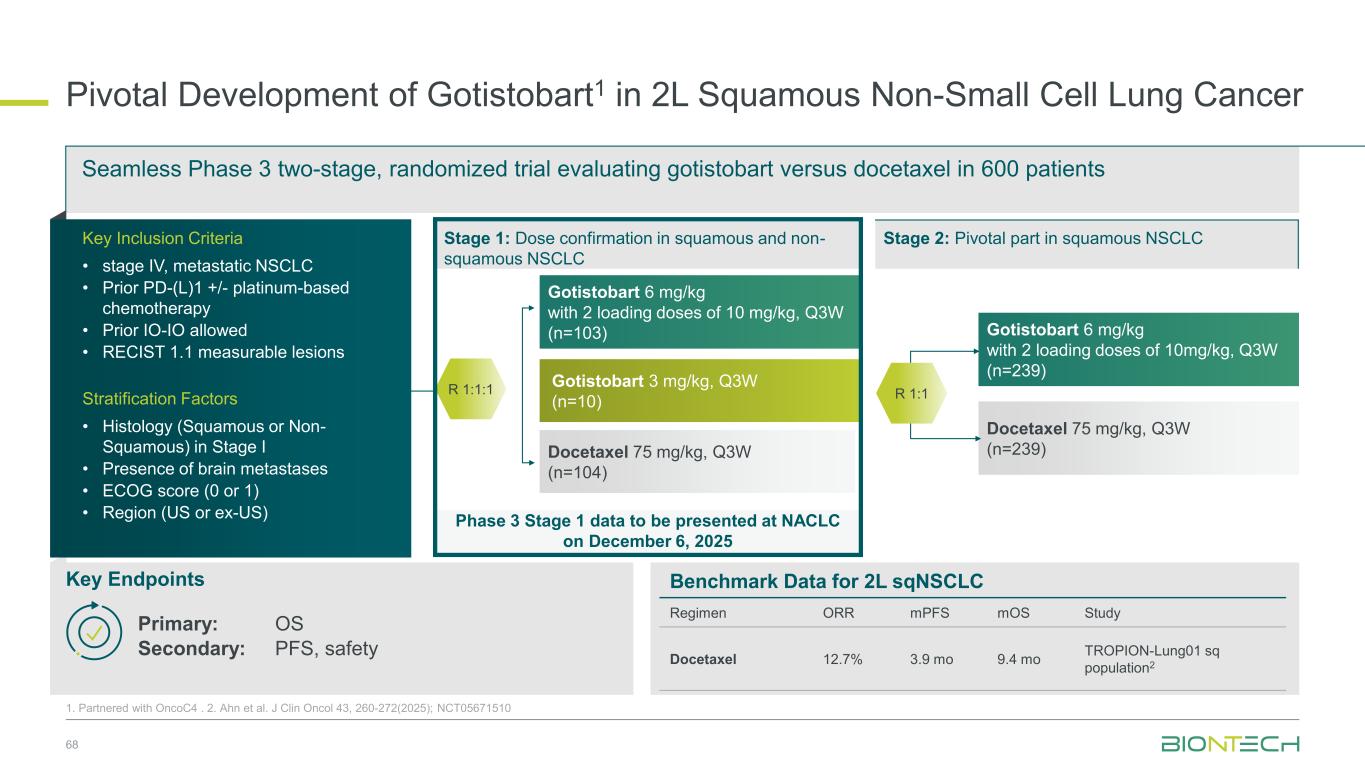
Phase 3 Stage 1 data to be presented at NACLC on December 6, 2025 Pivotal Development of Gotistobart1 in 2L Squamous Non-Small Cell Lung Cancer 30 1. Partnered with OncoC4 . 2. Ahn et al. J Clin Oncol 43, 260-272(2025); NCT05671510 Gotistobart 6 mg/kg with 2 loading doses of 10 mg/kg, Q3W (n=239) Docetaxel 75 mg/kg, Q3W (n=239) Gotistobart 6 mg/kg with 2 loading doses of 10 mg/kg, Q3W (n=103) Gotistobart 3 mg/kg, Q3W (n=10) Docetaxel 75 mg/kg, Q3W (N=104) Seamless Phase 3 two-stage, randomized trial evaluating gotistobart versus docetaxel in 600 patients Key Endpoints Primary: OS Secondary: PFS, safety Stage 1: Dose confirmation in squamous and non- squamous NSCLC R 1:1 Stage 2: Pivotal part in squamous NSCLCKey Inclusion Criteria • stage IV, metastatic NSCLC • Prior PD-(L)1 +/- platinum-based chemotherapy • Prior IO-IO allowed • RECIST 1.1 measurable lesions Stratification Factors • Histology (Squamous or Non- Squamous) in Stage I • Presence of brain metastases • ECOG score (0 or 1) • Region (US or ex-US) R 1:1:1 Benchmark Data for 2L sqNSCLC Regimen ORR mPFS mOS Benchmark study Docetaxel 12.7% 3.9 mo 9.4 mo TROPION-Lung01 sq population2
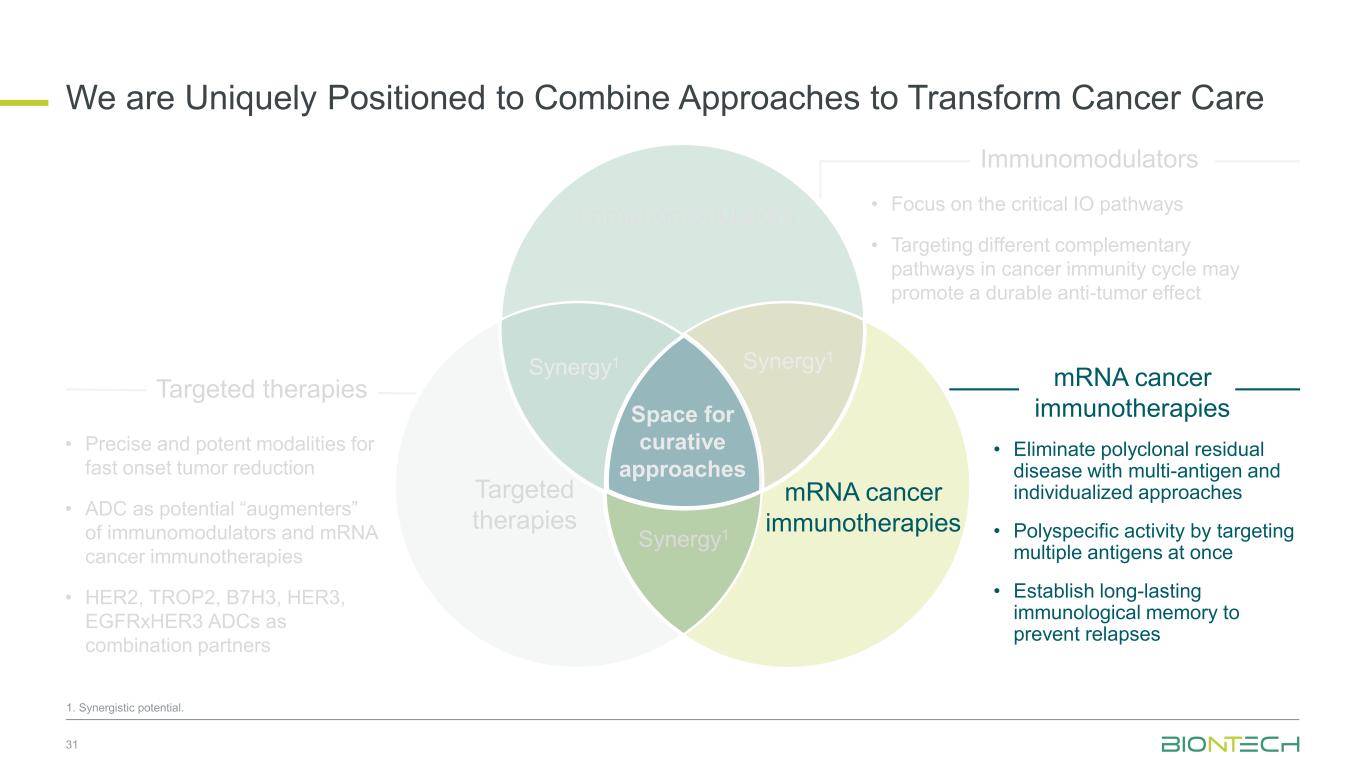
Space for curative approaches Immunomodulators mRNA cancer immunotherapies Targeted therapies Synergy1 Synergy1 Synergy1 • Focus on the critical IO pathways • Targeting different complementary pathways in cancer immunity cycle may promote a durable anti-tumor effect • Eliminate polyclonal residual disease with multi-antigen and individualized approaches • Polyspecific activity by targeting multiple antigens at once • Establish long-lasting immunological memory to prevent relapses • Precise and potent modalities for fast onset tumor reduction • ADC as potential “augmenters” of immunomodulators and mRNA cancer immunotherapies • HER2, TROP2, B7H3, HER3, EGFRxHER3 ADCs as combination partners 31 Immunomodulators Targeted therapies mRNA cancer immunotherapies We are Uniquely Positioned to Combine Approaches to Transform Cancer Care 1. Synergistic potential.
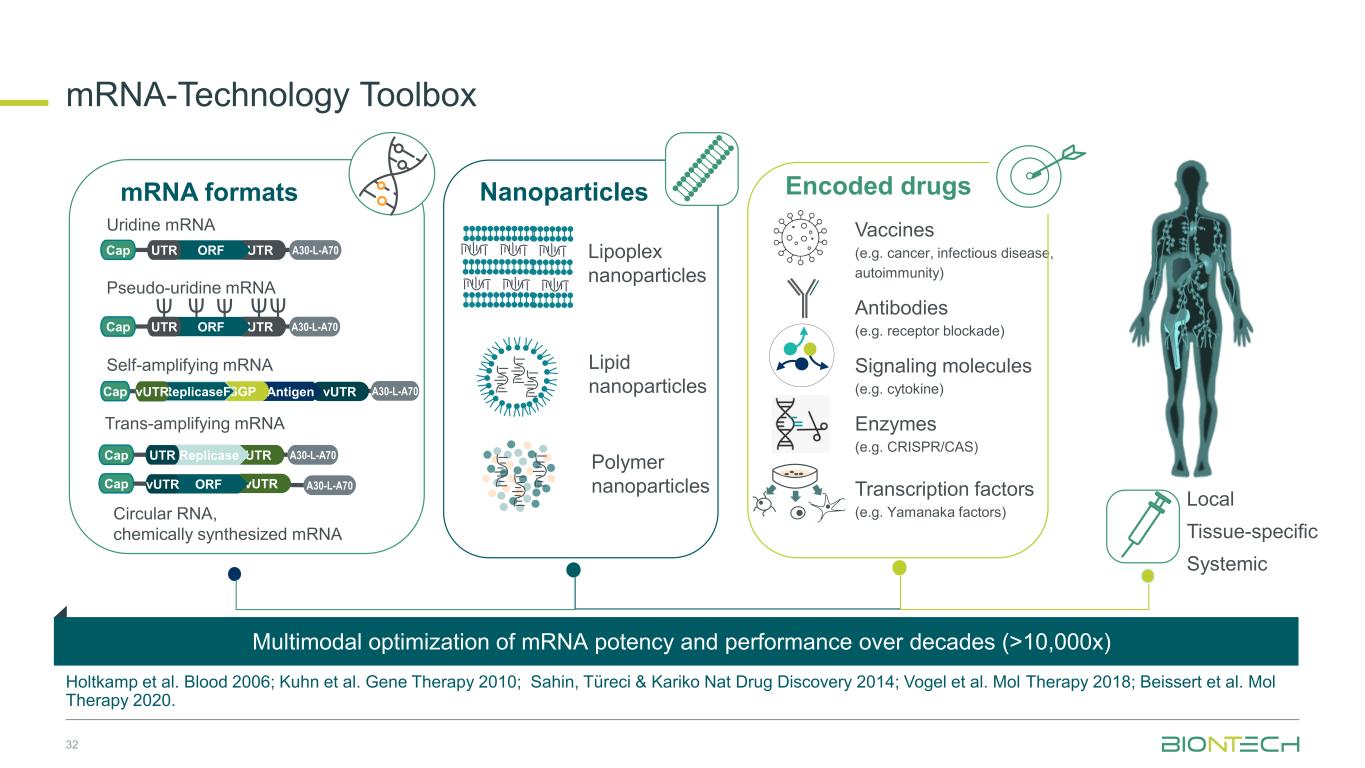
mRNA-Technology Toolbox Holtkamp et al. Blood 2006; Kuhn et al. Gene Therapy 2010; Sahin, Türeci & Kariko Nat Drug Discovery 2014; Vogel et al. Mol Therapy 2018; Beissert et al. Mol Therapy 2020. mRNA formats Local Tissue-specific Systemic Lipoplex nanoparticles Lipid nanoparticles Polymer nanoparticles Uridine mRNA Pseudo-uridine mRNA Self-amplifying mRNA Trans-amplifying mRNA Nanoparticles Vaccines (e.g. cancer, infectious disease, autoimmunity) Antibodies (e.g. receptor blockade) Signaling molecules (e.g. cytokine) Enzymes (e.g. CRISPR/CAS) Transcription factors (e.g. Yamanaka factors) Encoded drugs A30-L-A70Cap UTRORFUTR A30-L-A70Cap UTRORFUTR vUTR A30-L-A70Cap AntigenSGPReplicaseFvUTR A30-L-A70Cap UTRReplicaseUTR vUTR A30-L-A70Cap ORFvUTR Circular RNA, chemically synthesized mRNA Multimodal optimization of mRNA potency and performance over decades (>10,000x) 32
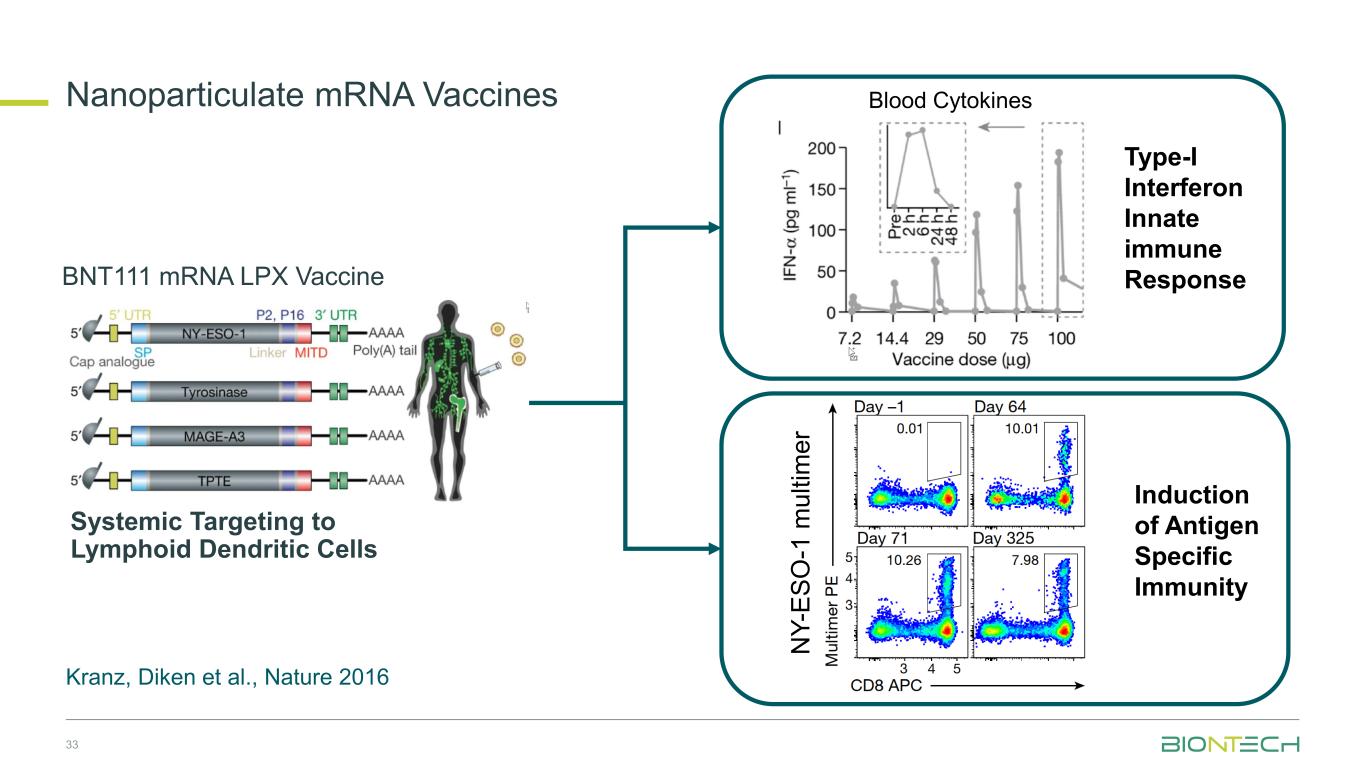
Type-I Interferon Innate immune Response N Y -E S O -1 m u lt im e r Induction of Antigen Specific Immunity Blood Cytokines BNT111 mRNA LPX Vaccine Systemic Targeting to Lymphoid Dendritic Cells Kranz, Diken et al., Nature 2016 33 Nanoparticulate mRNA Vaccines
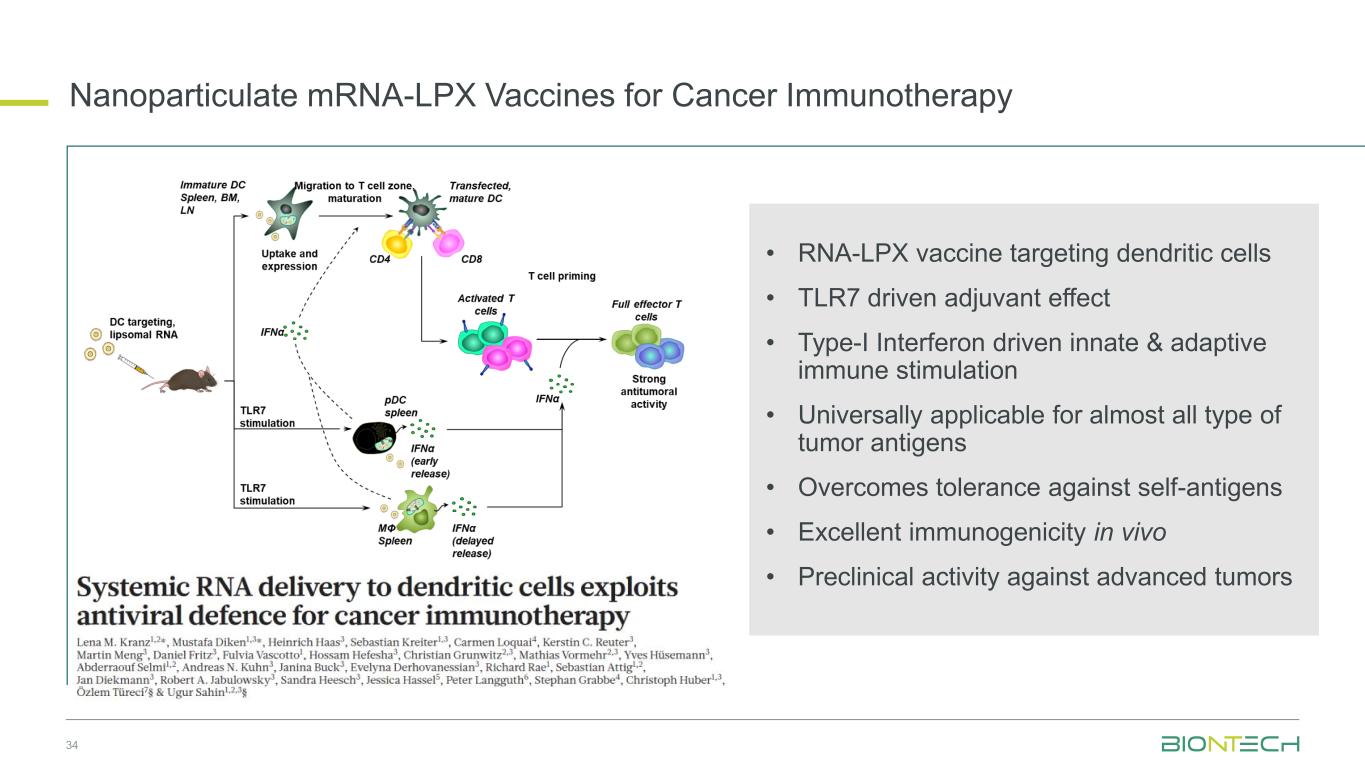
34 Nanoparticulate mRNA-LPX Vaccines for Cancer Immunotherapy • RNA-LPX vaccine targeting dendritic cells • TLR7 driven adjuvant effect • Type-I Interferon driven innate & adaptive immune stimulation • Universally applicable for almost all type of tumor antigens • Overcomes tolerance against self-antigens • Excellent immunogenicity in vivo • Preclinical activity against advanced tumors
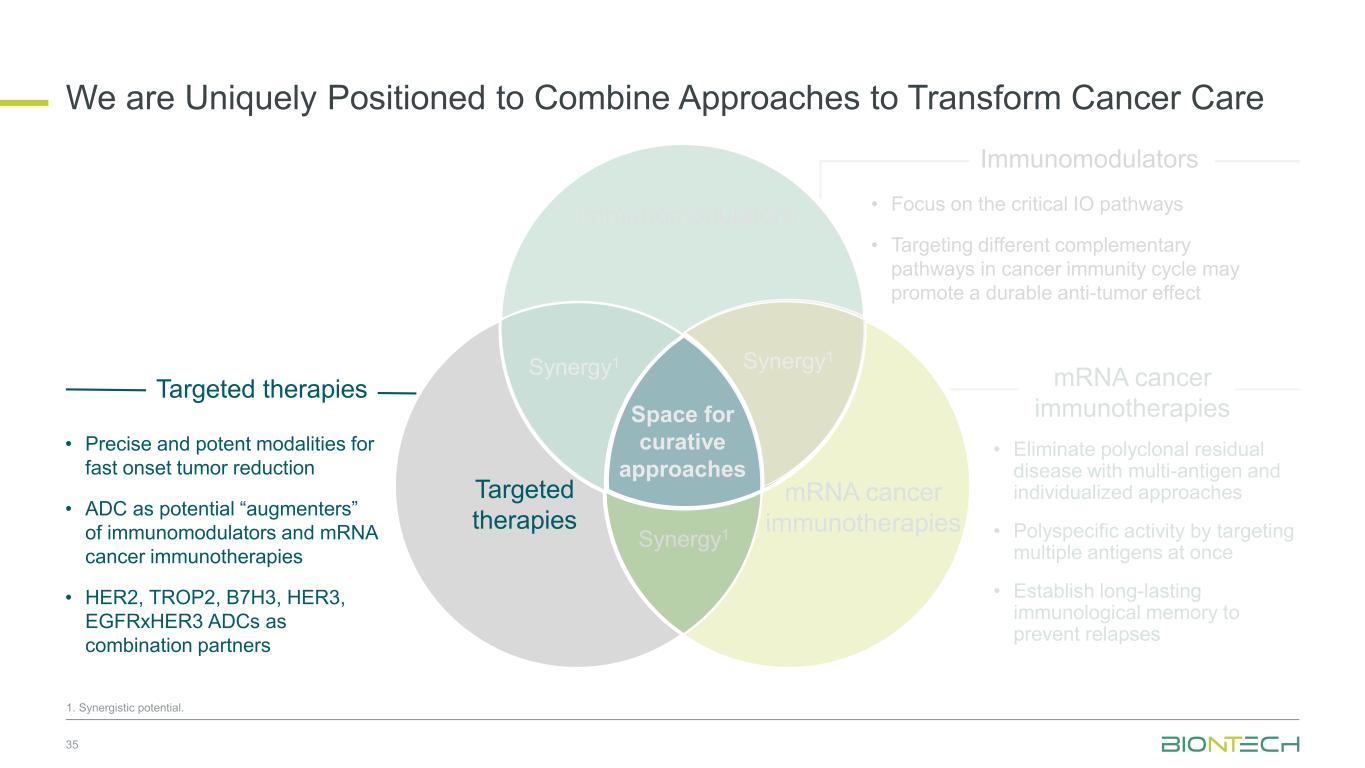
Space for curative approaches Immunomodulators mRNA cancer immunotherapies Targeted therapies Synergy1 Synergy1 Synergy1 • Focus on the critical IO pathways • Targeting different complementary pathways in cancer immunity cycle may promote a durable anti-tumor effect • Eliminate polyclonal residual disease with multi-antigen and individualized approaches • Polyspecific activity by targeting multiple antigens at once • Establish long-lasting immunological memory to prevent relapses • Precise and potent modalities for fast onset tumor reduction • ADC as potential “augmenters” of immunomodulators and mRNA cancer immunotherapies • HER2, TROP2, B7H3, HER3, EGFRxHER3 ADCs as combination partners 35 Immunomodulators Targeted therapies mRNA cancer immunotherapies We are Uniquely Positioned to Combine Approaches to Transform Cancer Care 1. Synergistic potential.
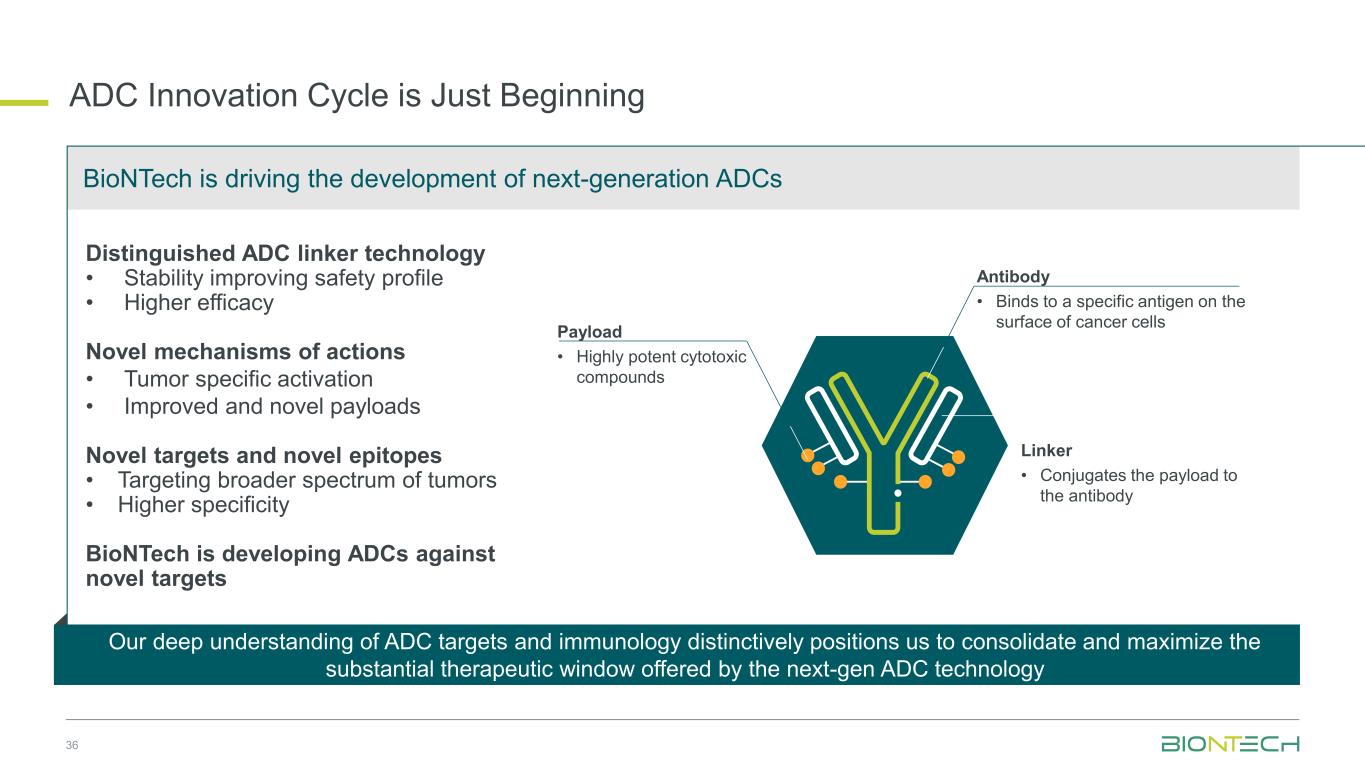
36 ADC Innovation Cycle is Just Beginning Distinguished ADC linker technology • Stability improving safety profile • Higher efficacy Novel mechanisms of actions • Tumor specific activation • Improved and novel payloads Novel targets and novel epitopes • Targeting broader spectrum of tumors • Higher specificity BioNTech is developing ADCs against novel targets Linker • Conjugates the payload to the antibody Antibody • Binds to a specific antigen on the surface of cancer cells Payload • Highly potent cytotoxic compounds BioNTech is driving the development of next-generation ADCs Our deep understanding of ADC targets and immunology distinctively positions us to consolidate and maximize the substantial therapeutic window offered by the next-gen ADC technology
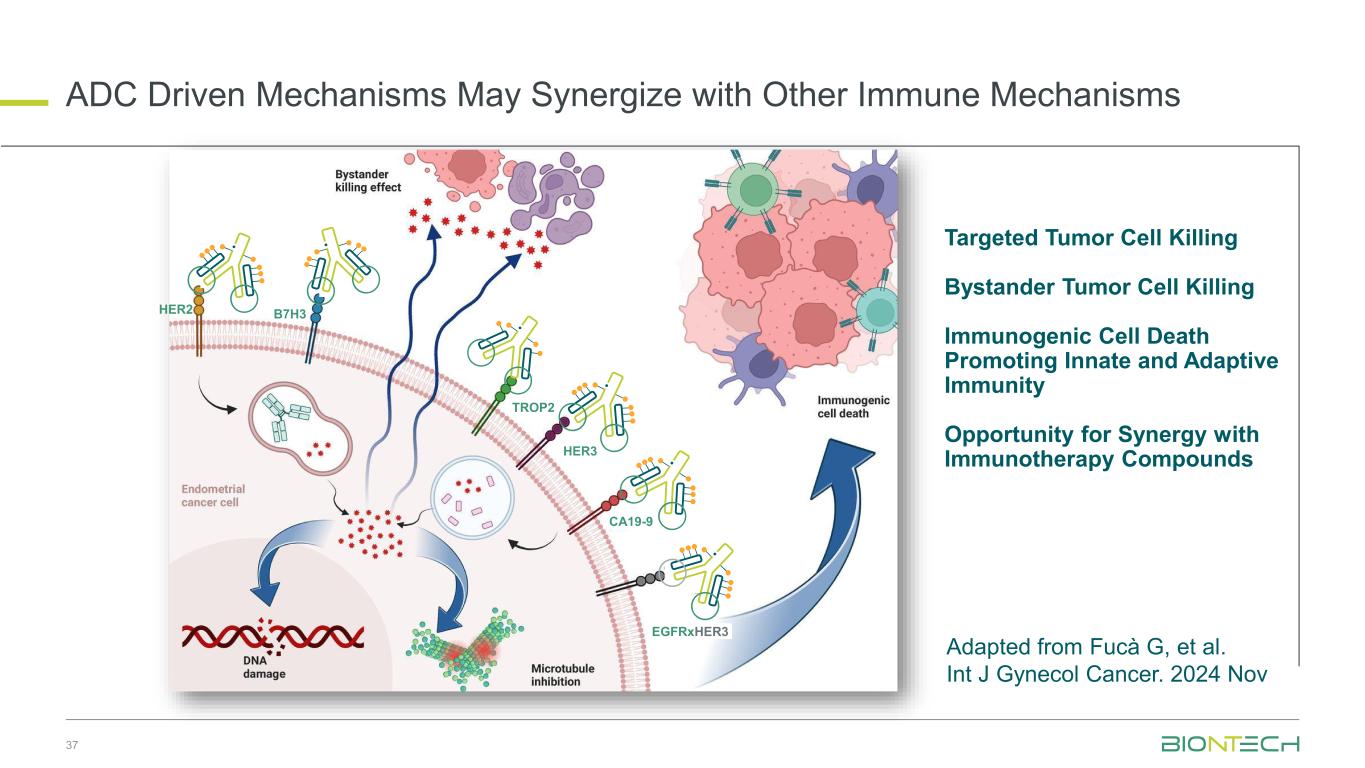
ADC Driven Mechanisms May Synergize with Other Immune Mechanisms 37 Adapted from Fucà G, et al. Int J Gynecol Cancer. 2024 Nov TROP2 HER3 CA19-9 EGFRxHER3 HER2 B7H3 Targeted Tumor Cell Killing Bystander Tumor Cell Killing Immunogenic Cell Death Promoting Innate and Adaptive Immunity Opportunity for Synergy with Immunotherapy Compounds
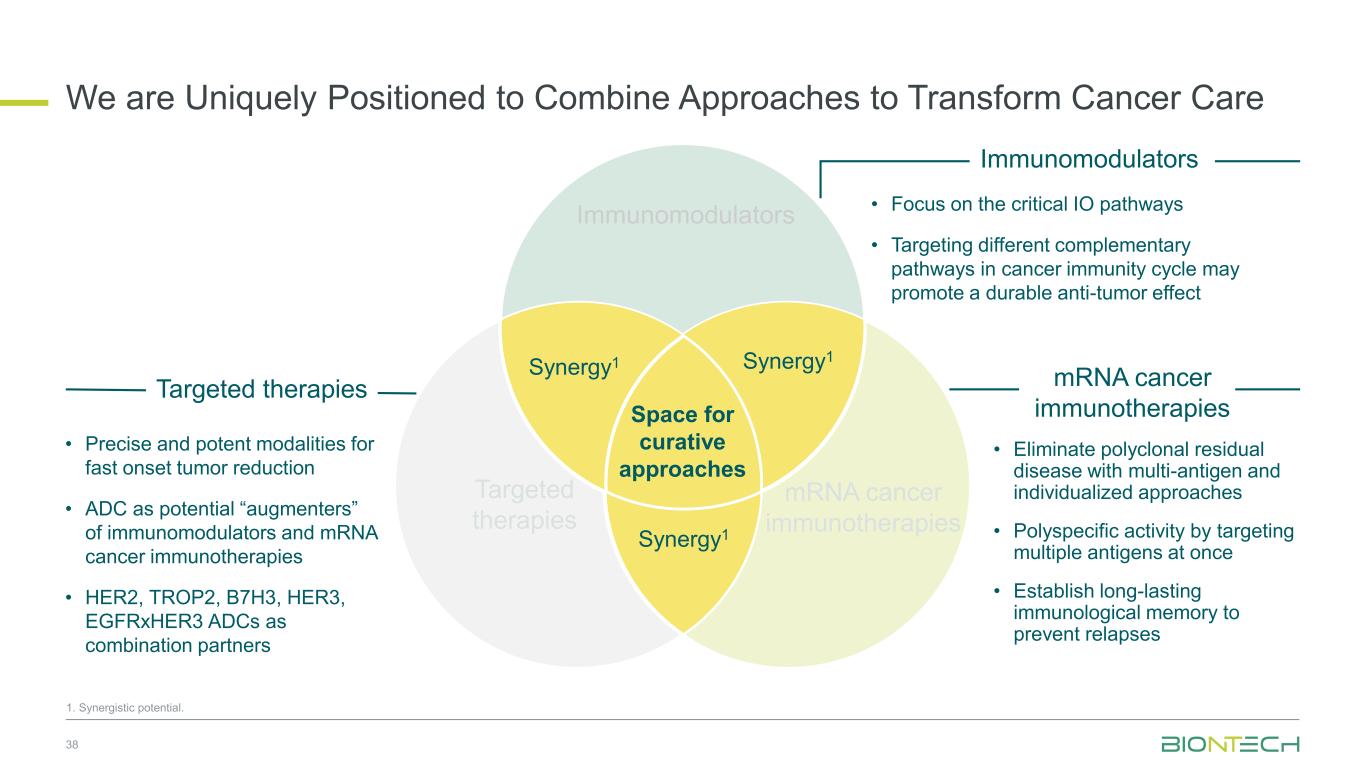
Space for curative approaches Immunomodulators mRNA cancer immunotherapies Targeted therapies Synergy1 Synergy1 Synergy1 • Focus on the critical IO pathways • Targeting different complementary pathways in cancer immunity cycle may promote a durable anti-tumor effect • Eliminate polyclonal residual disease with multi-antigen and individualized approaches • Polyspecific activity by targeting multiple antigens at once • Establish long-lasting immunological memory to prevent relapses • Precise and potent modalities for fast onset tumor reduction • ADC as potential “augmenters” of immunomodulators and mRNA cancer immunotherapies • HER2, TROP2, B7H3, HER3, EGFRxHER3 ADCs as combination partners 38 Immunomodulators Targeted therapies mRNA cancer immunotherapies We are Uniquely Positioned to Combine Approaches to Transform Cancer Care 1. Synergistic potential.
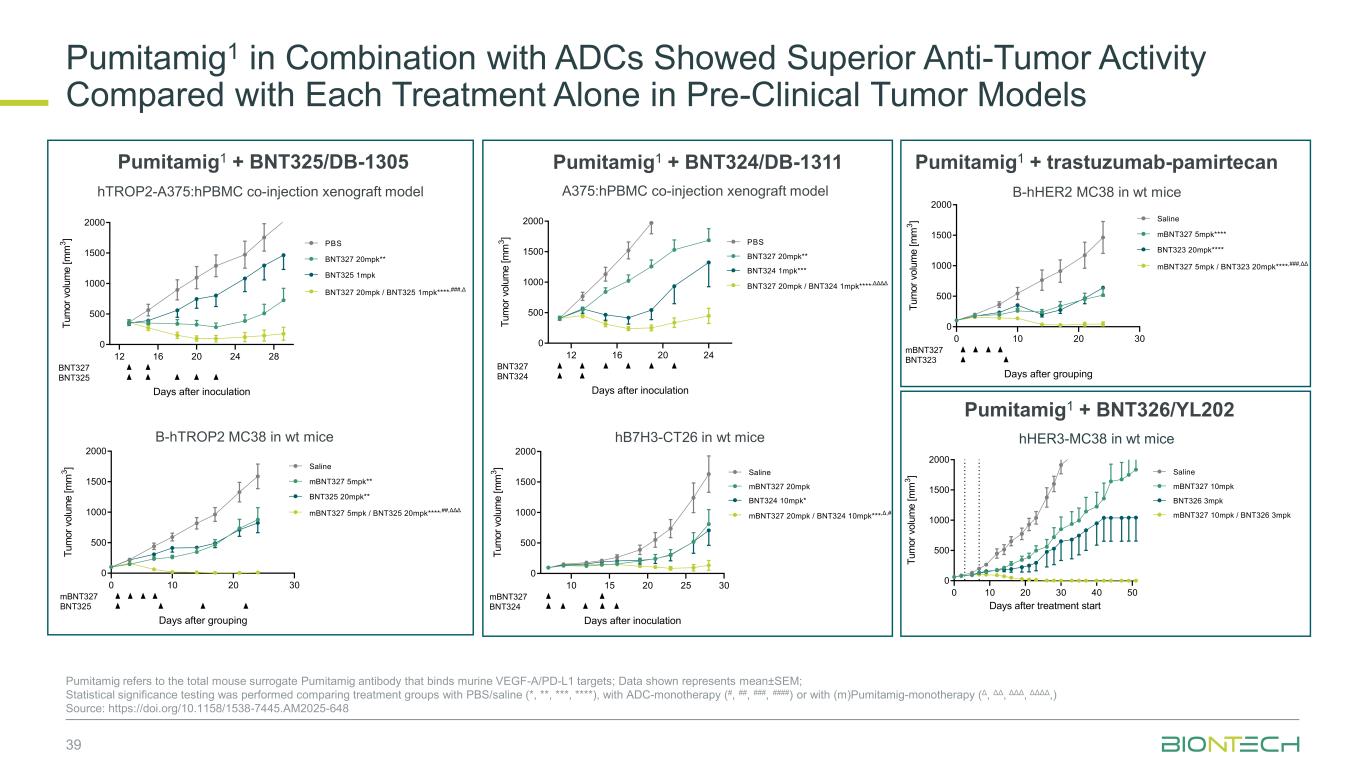
Pumitamig1 in Combination with ADCs Showed Superior Anti-Tumor Activity Compared with Each Treatment Alone in Pre-Clinical Tumor Models 39 Pumitamig refers to the total mouse surrogate Pumitamig antibody that binds murine VEGF-A/PD-L1 targets; Data shown represents mean±SEM; Statistical significance testing was performed comparing treatment groups with PBS/saline (*, **, ***, ****), with ADC-monotherapy (#, ##, ###, ####) or with (m)Pumitamig-monotherapy (Δ, ΔΔ, ΔΔΔ, ΔΔΔΔ,) Source: https://doi.org/10.1158/1538-7445.AM2025-648 hTROP2-A375:hPBMC co-injection xenograft model B-hTROP2 MC38 in wt mice A375:hPBMC co-injection xenograft model hHER3-MC38 in wt mice B-hHER2 MC38 in wt mice hB7H3-CT26 in wt mice Pumitamig1 + BNT325/DB-1305 Pumitamig1 + BNT324/DB-1311 Pumitamig1 + trastuzumab-pamirtecan Pumitamig1 + BNT326/YL202 0 10 20 30 40 50 0 500 1000 1500 2000 Days after treatment start T u m o r v o lu m e [ m m 3 ] mBNT327 10mpk Saline BNT326 3mpk mBNT327 10mpk / BNT326 3mpk 12 16 20 24 28 0 500 1000 1500 2000 Days after inoculation T u m o r vo lu m e [ m m 3 ] BNT327 20mpk** PBS BNT325 1mpk BNT327 20mpk / BNT325 1mpk**** ,###,Δ BNT327 BNT325 12 16 20 24 0 500 1000 1500 2000 Days after inoculation T u m o r v o lu m e [ m m 3 ] BNT327 20mpk** PBS BNT324 1mpk*** BNT327 20mpk / BNT324 1mpk**** ,ΔΔΔΔ BNT327 BNT324 0 10 20 30 0 500 1000 1500 2000 Days after grouping T u m o r v o lu m e [ m m 3 ] mBNT327 5mpk**** Saline BNT323 20mpk**** mBNT327 5mpk / BNT323 20mpk**** ,###,ΔΔ mBNT327 BNT323 0 10 20 30 0 500 1000 1500 2000 Days after grouping T u m o r v o lu m e [ m m 3 ] mBNT327 5mpk** Saline BNT325 20mpk** mBNT327 5mpk / BNT325 20mpk**** ,##,ΔΔΔ mBNT327 BNT325 10 15 20 25 30 0 500 1000 1500 2000 Days after inoculation T u m o r vo lu m e [ m m 3 ] mBNT327 20mpk Saline BNT324 10mpk* mBNT327 20mpk / BNT324 10mpk*** ,Δ,# mBNT327 BNT324
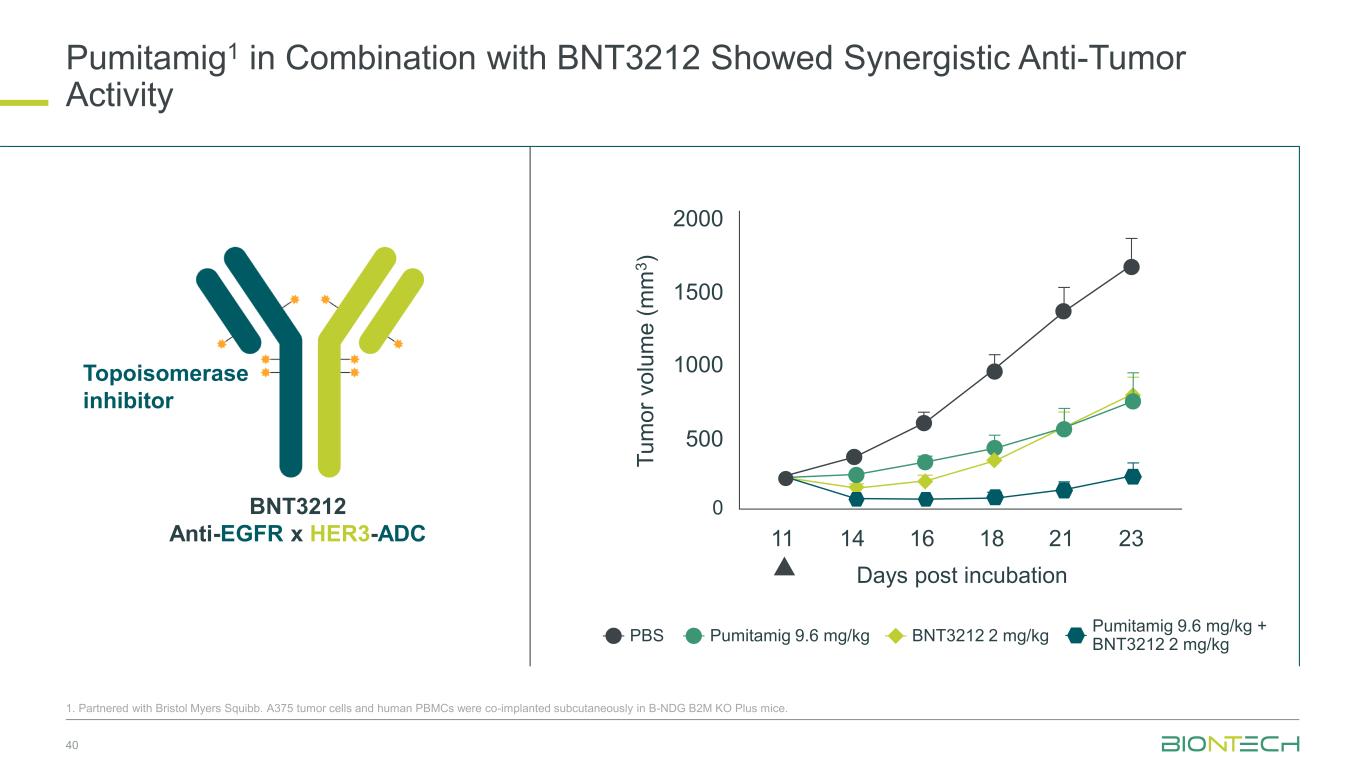
Pumitamig1 in Combination with BNT3212 Showed Synergistic Anti-Tumor Activity 40 1. Partnered with Bristol Myers Squibb. A375 tumor cells and human PBMCs were co-implanted subcutaneously in B-NDG B2M KO Plus mice. BNT3212 Anti-EGFR x HER3-ADC Topoisomerase inhibitor PBS Pumitamig 9.6 mg/kg BNT3212 2 mg/kg Pumitamig 9.6 mg/kg + BNT3212 2 mg/kg T u m o r v o lu m e ( m m 3 ) 2000 1500 1000 500 0 11 14 16 18 21 23 Days post incubation
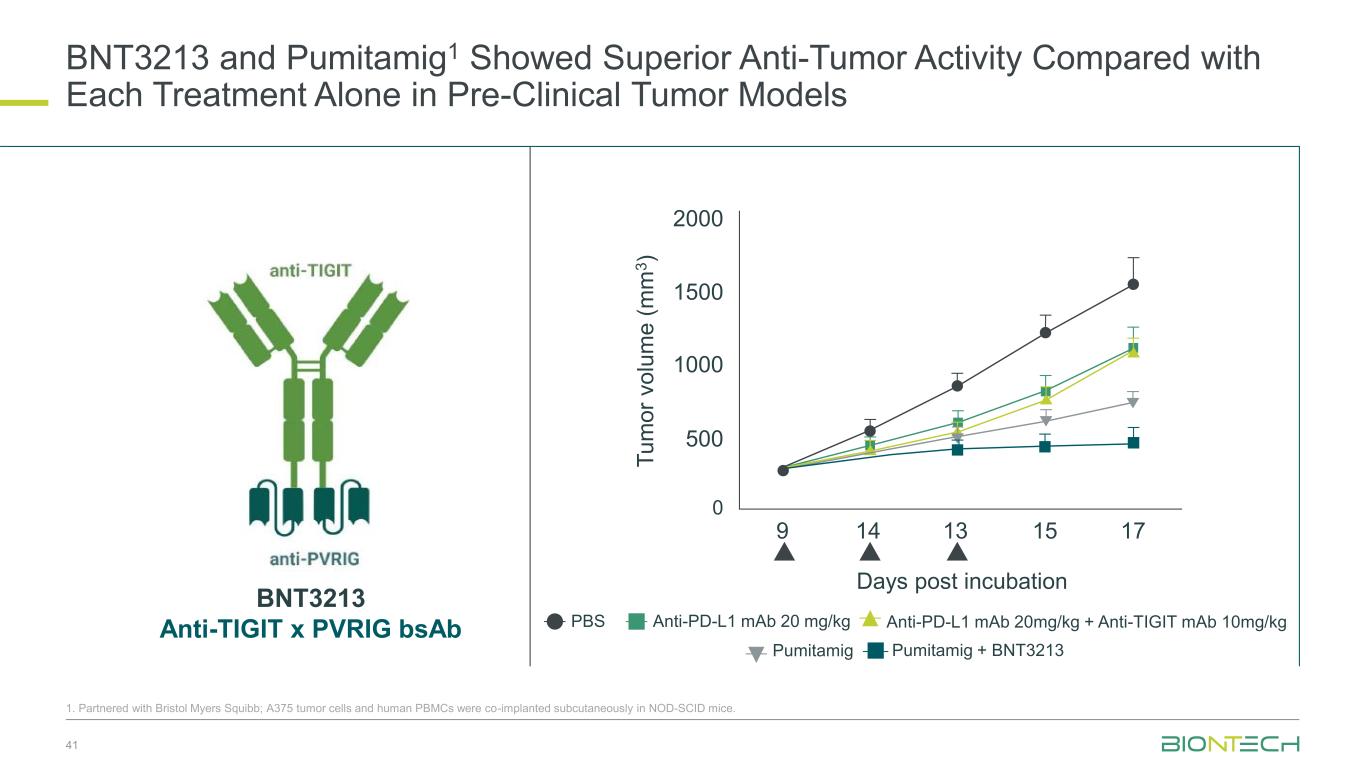
BNT3213 and Pumitamig1 Showed Superior Anti-Tumor Activity Compared with Each Treatment Alone in Pre-Clinical Tumor Models 41 1. Partnered with Bristol Myers Squibb; A375 tumor cells and human PBMCs were co-implanted subcutaneously in NOD-SCID mice. PBS Anti-PD-L1 mAb 20 mg/kg Anti-PD-L1 mAb 20mg/kg + Anti-TIGIT mAb 10mg/kg Pumitamig Pumitamig + BNT3213 2000 1500 1000 500 0 9 14 13 15 17 Days post incubation T u m o r v o lu m e ( m m 3 ) BNT3213 Anti-TIGIT x PVRIG bsAb
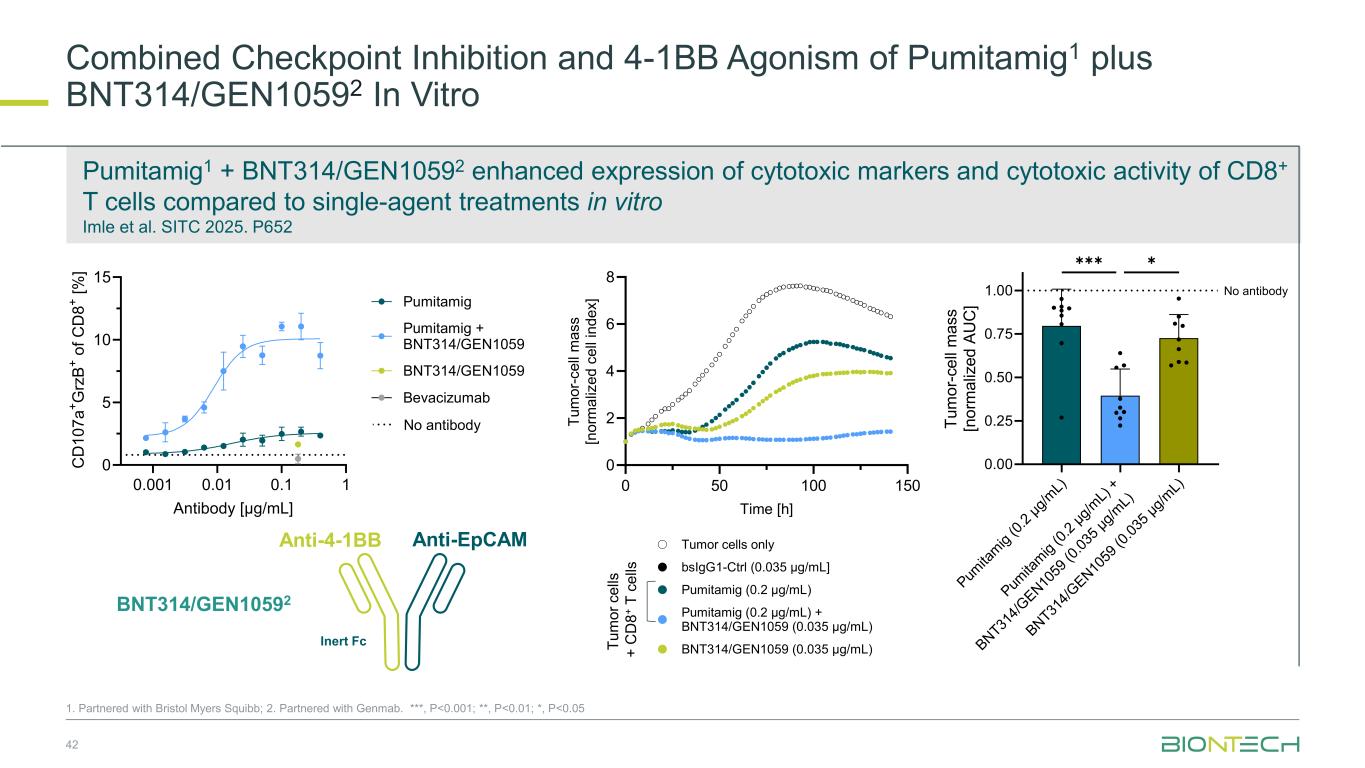
Combined Checkpoint Inhibition and 4-1BB Agonism of Pumitamig1 plus BNT314/GEN10592 In Vitro 42 1. Partnered with Bristol Myers Squibb; 2. Partnered with Genmab. ***, P<0.001; **, P<0.01; *, P<0.05 Pumitamig1 + BNT314/GEN10592 enhanced expression of cytotoxic markers and cytotoxic activity of CD8+ T cells compared to single-agent treatments in vitro Imle et al. SITC 2025. P652 T u m o r c e lls + C D 8 + T c e lls 0 50 100 150 0 2 4 6 8 Time [h] T u m o r- c e ll m a s s [n o rm a liz e d c e ll in d e x ] Atezolizumab (0.2 µg/mL) BNT314/GEN1059 (0.035 µg/mL) Pumitamig (0.2 µg/mL) + BNT314/GEN1059 (0.035 µg/mL) Pumitamig (0.2 µg/mL) bsIgG1-Ctrl (0.035 µg/mL] Tumor cells only 0.001 0.01 0.1 1 0 5 10 15 Antibody [µg/mL] C D 1 0 7 a + G rz B + o f C D 8 + [ % ] Bevacizumab BNT314/GEN1059 Pumitamig Pumitamig + BNT314/GEN1059 No antibody 0 50 0 150 0 2 4 6 8 Time [h] T u m o r- c e ll m a s s [n o rm a liz e d c e ll in d e x ] BNT314/GEN1059 (0.035 µg/mL) Pumitamig (0.2 µg/mL) + BNT314/GEN1059 (0.035 µg/mL) Pumitamig (0.2 µg/mL) bsIgG1-Ctrl (0.035 µg/mL] Tumor cells only Pum ita m ig (0 .2 µ g/ m L) Pum ita m ig (0 .2 µ g/ m L) + BN T31 4/ G E N 10 59 (0 .0 35 µ g/ m L) BN T31 4/ G E N 10 59 (0 .0 35 µ g/ m L) 0.00 0.25 0.50 0.75 1.00 T u m o r- c e ll m a s s [n o rm a liz e d A U C ] ✱✱✱✱ No antibody Anti-EpCAMAnti-4-1BB Inert Fc BNT314/GEN10592
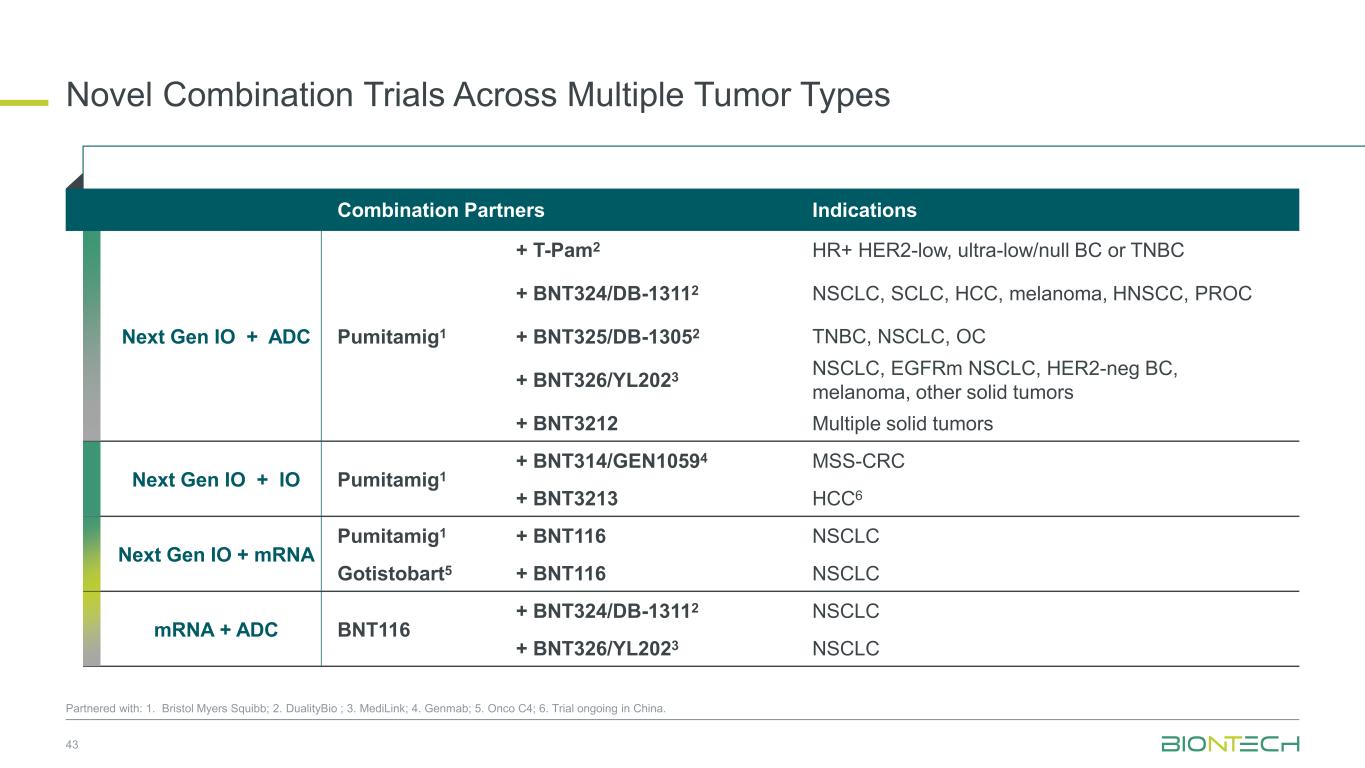
Novel Combination Trials Across Multiple Tumor Types 43 Partnered with: 1. Bristol Myers Squibb; 2. DualityBio ; 3. MediLink; 4. Genmab; 5. Onco C4; 6. Trial ongoing in China. Combination Partners Indications Next Gen IO + ADC Pumitamig1 + T-Pam2 HR+ HER2-low, ultra-low/null BC or TNBC + BNT324/DB-13112 NSCLC, SCLC, HCC, melanoma, HNSCC, PROC + BNT325/DB-13052 TNBC, NSCLC, OC + BNT326/YL2023 NSCLC, EGFRm NSCLC, HER2-neg BC, melanoma, other solid tumors + BNT3212 Multiple solid tumors Next Gen IO + IO Pumitamig1 + BNT314/GEN10594 MSS-CRC + BNT3213 HCC6 Next Gen IO + mRNA Pumitamig1 + BNT116 NSCLC Gotistobart5 + BNT116 NSCLC mRNA + ADC BNT116 + BNT324/DB-13112 NSCLC + BNT326/YL2023 NSCLC
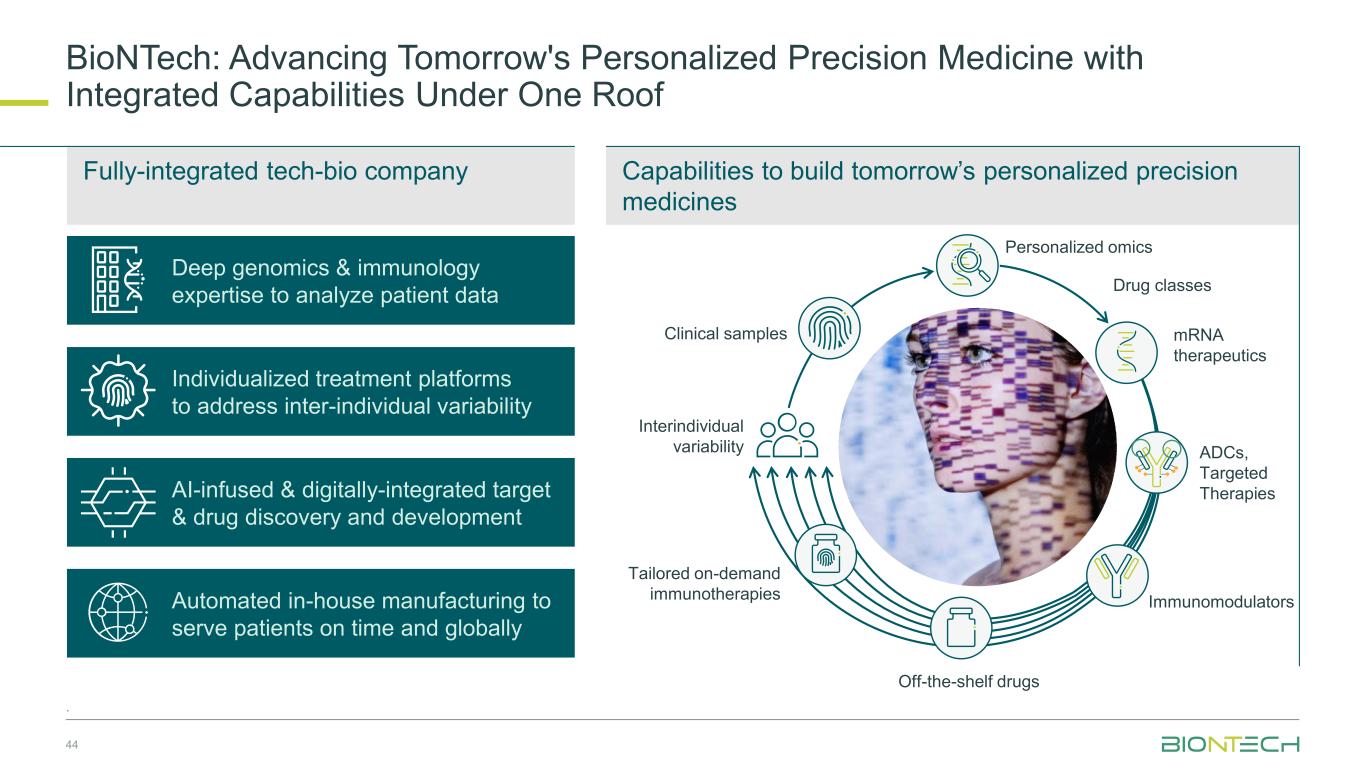
Capabilities to build tomorrow’s personalized precision medicines BioNTech: Advancing Tomorrow's Personalized Precision Medicine with Integrated Capabilities Under One Roof 44 . Automated in-house manufacturing to serve patients on time and globally Drug classes Interindividual variability Off-the-shelf drugs Tailored on-demand immunotherapies Clinical samples mRNA therapeutics Immunomodulators Personalized omics AI-infused & digitally-integrated target & drug discovery and development Individualized treatment platforms to address inter-individual variability Deep genomics & immunology expertise to analyze patient data Fully-integrated tech-bio company ADCs, Targeted Therapies f-the-shelf drugs
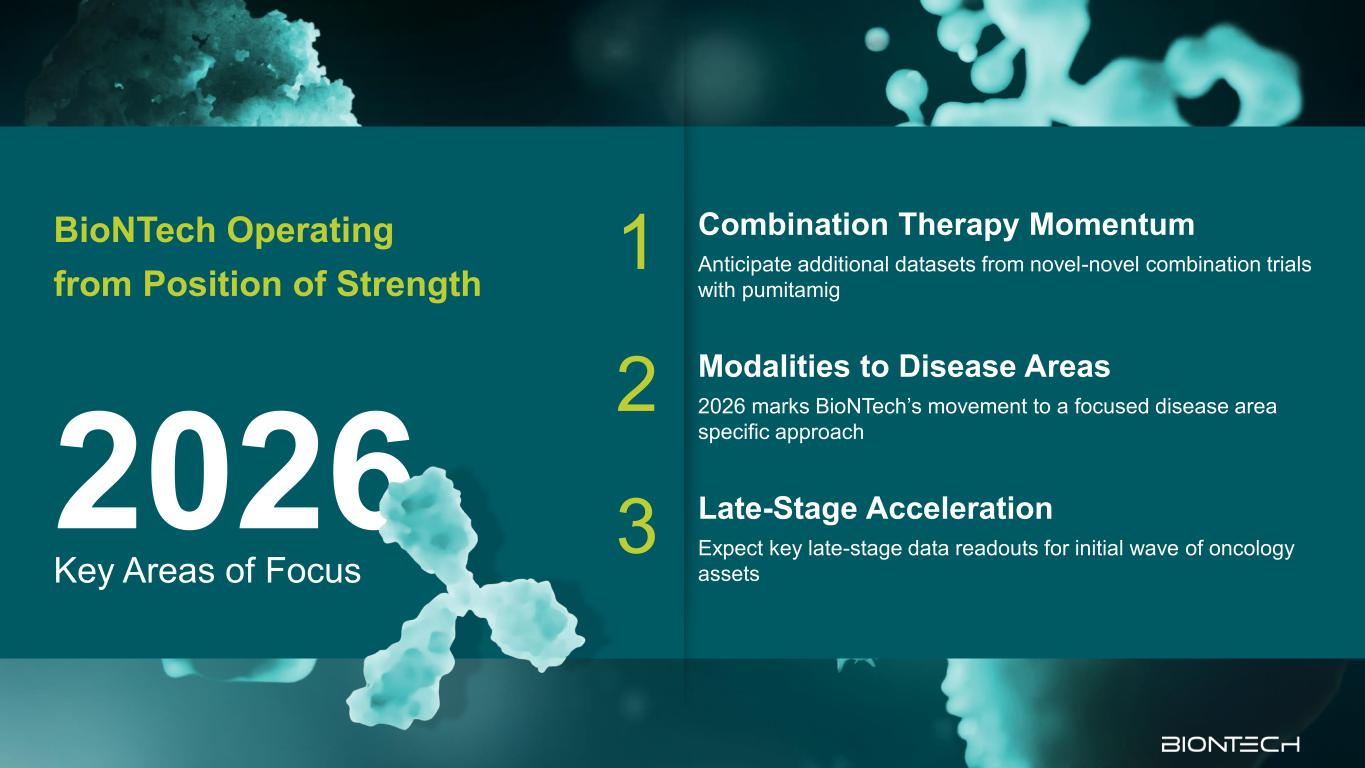
Key Areas of Focus 2026 BioNTech Operating from Position of Strength 1 Combination Therapy Momentum Anticipate additional datasets from novel-novel combination trials with pumitamig 2 Modalities to Disease Areas 2026 marks BioNTech’s movement to a focused disease area specific approach 3 Late-Stage Acceleration Expect key late-stage data readouts for initial wave of oncology assets

BioNTech’s Differentiated Clinical Strategy to Advance the Treatment of Solid Tumors Prof. Özlem Türeci, M.D. Chief Medical Officer and Co-founder 3
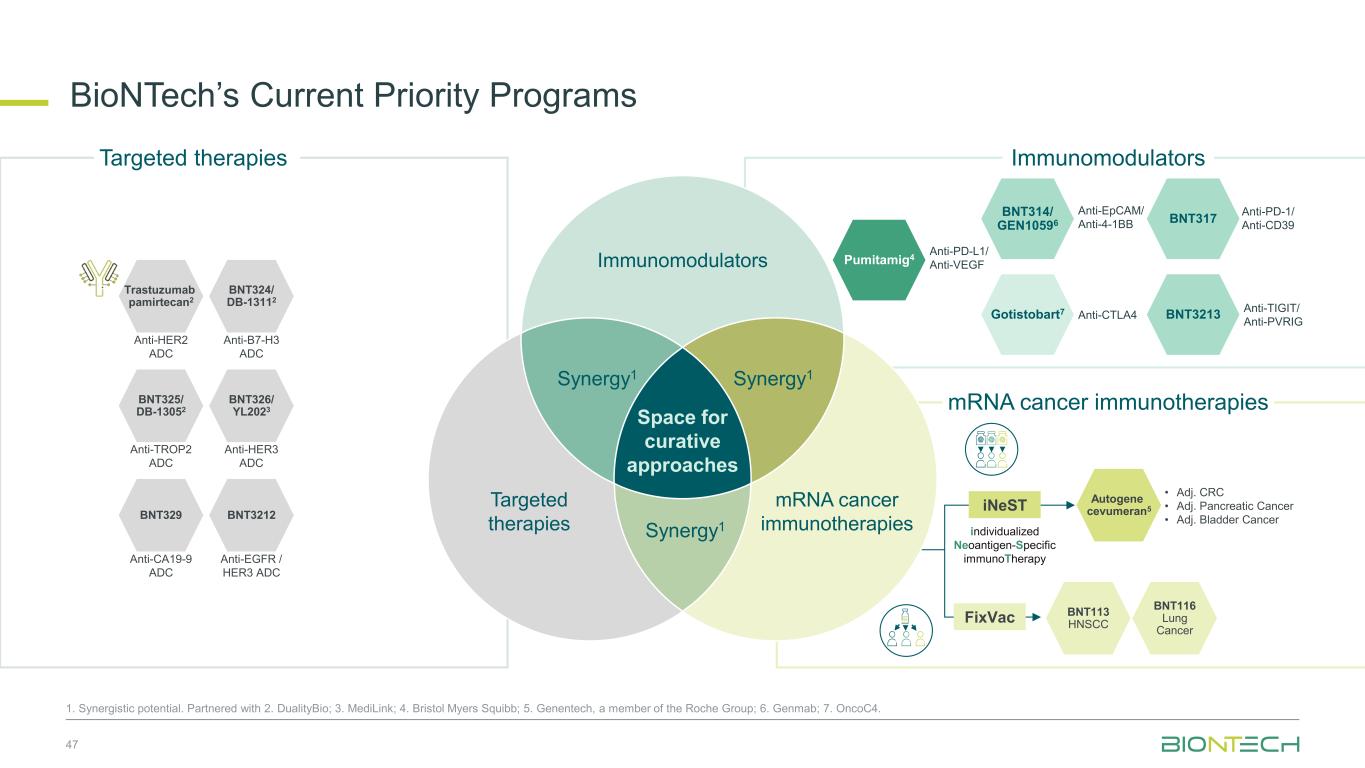
47 1. Synergistic potential. Partnered with 2. DualityBio; 3. MediLink; 4. Bristol Myers Squibb; 5. Genentech, a member of the Roche Group; 6. Genmab; 7. OncoC4. Immunomodulators mRNA cancer immunotherapies Gotistobart7 BNT314/ GEN10596 Pumitamig4 Anti-CTLA4 Anti-EpCAM/ Anti-4-1BB Anti-PD-L1/ Anti-VEGF BNT324/ DB-13112 Trastuzumab pamirtecan2 BNT325/ DB-13052 BNT326/ YL2023 Anti-HER2 ADC Anti-B7-H3 ADC Anti-TROP2 ADC Anti-HER3 ADC BNT317 BNT3213 Anti-TIGIT/ Anti-PVRIG BNT329 BNT3212 Anti-CA19-9 ADC Anti-EGFR / HER3 ADC Targeted therapies Immunomodulators Space for curative approaches Synergy1 Targeted therapies mRNA cancer immunotherapies Synergy1Synergy1 iNeST FixVac individualized Neoantigen-Specific immunoTherapy BNT113 HNSCC BNT116 Lung Cancer Autogene cevumeran5 • Adj. CRC • Adj. Pancreatic Cancer • Adj. Bladder Cancer BioNTech’s Current Priority Programs Anti-PD-1/ Anti-CD39
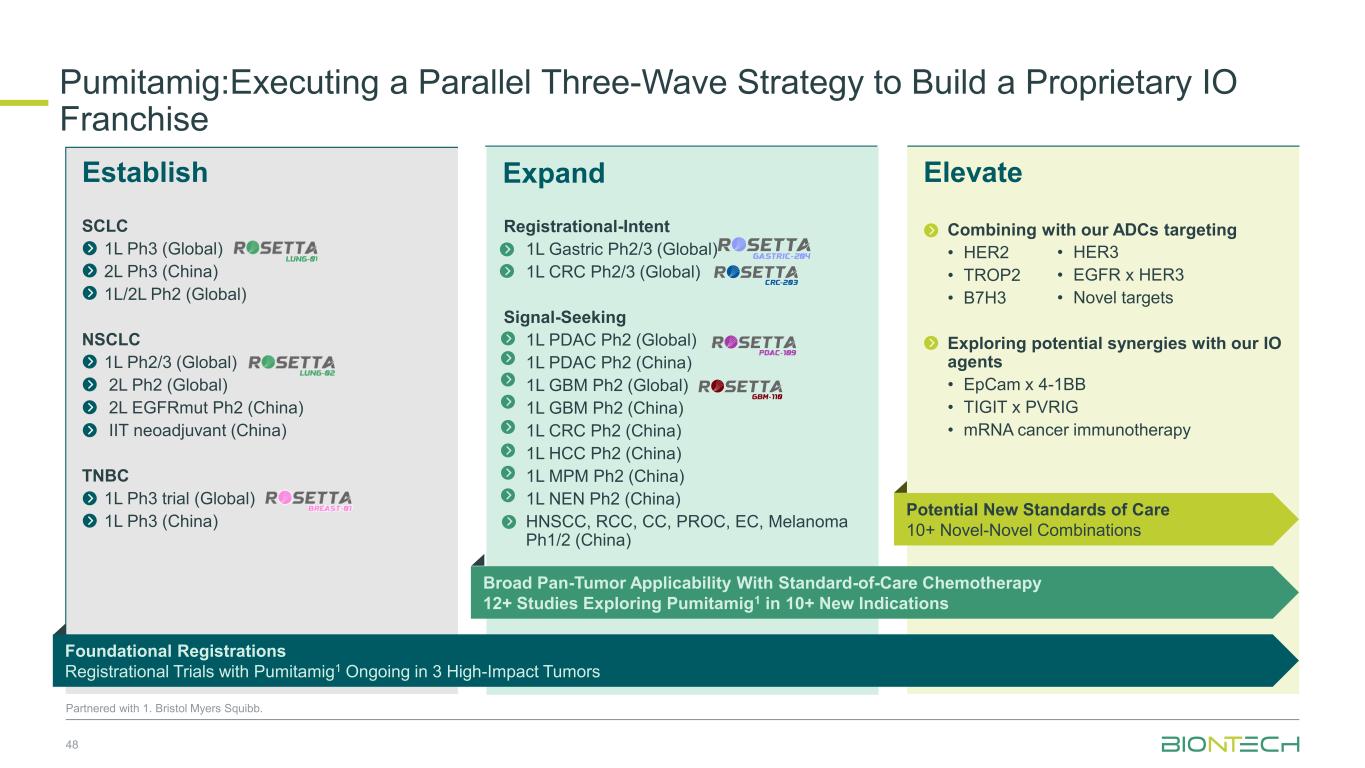
ElevateEstablish SCLC 1L Ph3 (Global) 2L Ph3 (China) 1L/2L Ph2 (Global) NSCLC 1L Ph2/3 (Global) 2L Ph2 (Global) 2L EGFRmut Ph2 (China) IIT neoadjuvant (China) TNBC 1L Ph3 trial (Global) 1L Ph3 (China) Pumitamig:Executing a Parallel Three-Wave Strategy to Build a Proprietary IO Franchise 48 Partnered with 1. Bristol Myers Squibb. Expand Combining with our ADCs targeting • HER2 • TROP2 • B7H3 Exploring potential synergies with our IO agents • EpCam x 4-1BB • TIGIT x PVRIG • mRNA cancer immunotherapy Registrational-Intent 1L Gastric Ph2/3 (Global) 1L CRC Ph2/3 (Global) Signal-Seeking 1L PDAC Ph2 (Global) 1L PDAC Ph2 (China) 1L GBM Ph2 (Global) 1L GBM Ph2 (China) 1L CRC Ph2 (China) 1L HCC Ph2 (China) 1L MPM Ph2 (China) 1L NEN Ph2 (China) HNSCC, RCC, CC, PROC, EC, Melanoma Ph1/2 (China) • HER3 • EGFR x HER3 • Novel targets Foundational Registrations Registrational Trials with Pumitamig1 Ongoing in 3 High-Impact Tumors Broad Pan-Tumor Applicability With Standard-of-Care Chemotherapy 12+ Studies Exploring Pumitamig1 in 10+ New Indications Potential New Standards of Care 10+ Novel-Novel Combinations
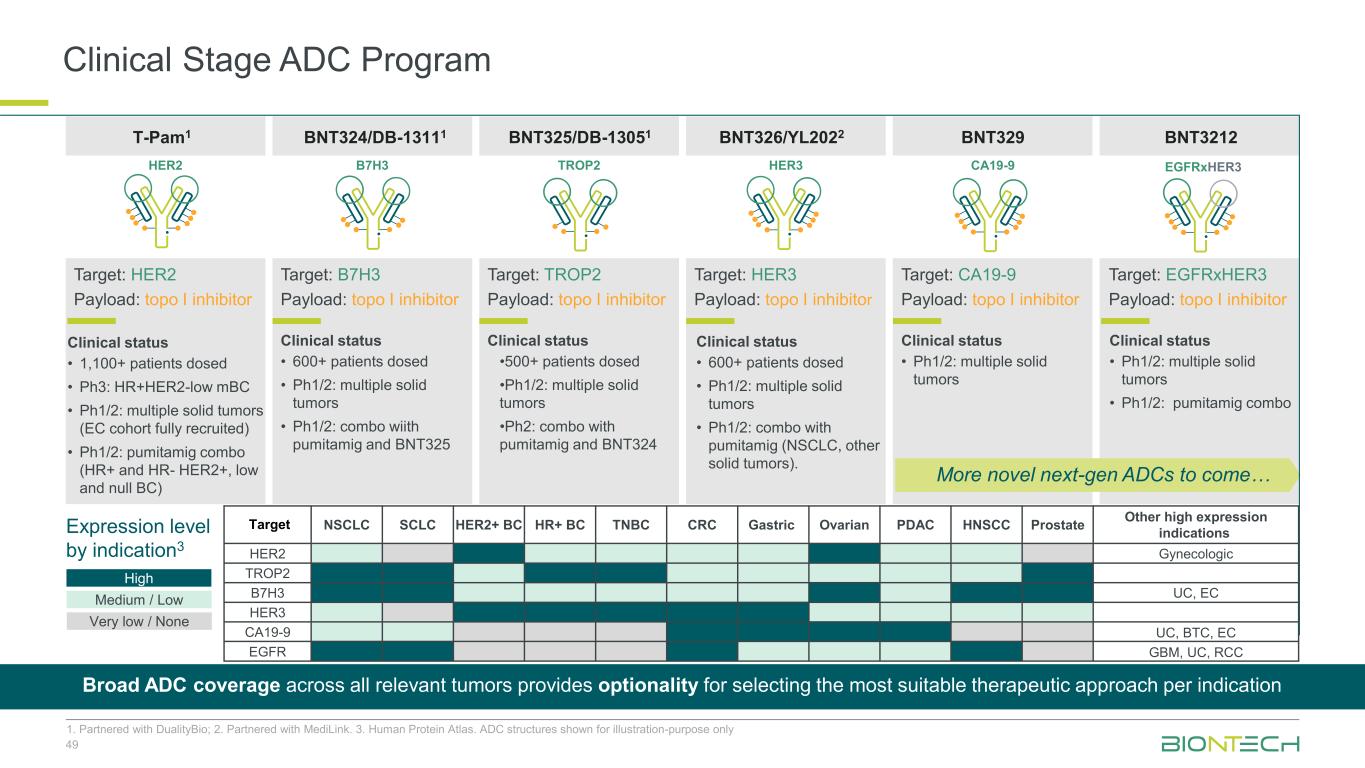
Clinical Stage ADC Program 49 1. Partnered with DualityBio; 2. Partnered with MediLink. 3. Human Protein Atlas. ADC structures shown for illustration-purpose only BNT324/DB-13111T-Pam1 BNT325/DB-13051 BNT326/YL2022 Clinical status • 1,100+ patients dosed • Ph3: HR+HER2-low mBC • Ph1/2: multiple solid tumors (EC cohort fully recruited) • Ph1/2: pumitamig combo (HR+ and HR- HER2+, low and null BC) Clinical status • 600+ patients dosed • Ph1/2: multiple solid tumors • Ph1/2: combo wiith pumitamig and BNT325 Clinical status •500+ patients dosed •Ph1/2: multiple solid tumors •Ph2: combo with pumitamig and BNT324 Clinical status • 600+ patients dosed • Ph1/2: multiple solid tumors • Ph1/2: combo with pumitamig (NSCLC, other solid tumors). HER2 B7H3 TROP2 HER3 Target: TROP2 Payload: topo I inhibitor Target: B7H3 Payload: topo I inhibitor Target: HER3 Payload: topo I inhibitor Target: HER2 Payload: topo I inhibitor BNT329 Clinical status • Ph1/2: multiple solid tumors CA19-9 Target: CA19-9 Payload: topo I inhibitor BNT3212 Clinical status • Ph1/2: multiple solid tumors • Ph1/2: pumitamig combo Target: EGFRxHER3 Payload: topo I inhibitor EGFRxHER3 More novel next-gen ADCs to come… Target NSCLC SCLC HER2+ BC HR+ BC TNBC CRC Gastric Ovarian PDAC HNSCC Prostate Other high expression indications HER2 Gynecologic TROP2 B7H3 UC, EC HER3 CA19-9 UC, BTC, EC EGFR GBM, UC, RCC Expression level by indication3 High Medium / Low Very low / None Broad ADC coverage across all relevant tumors provides optionality for selecting the most suitable therapeutic approach per indication
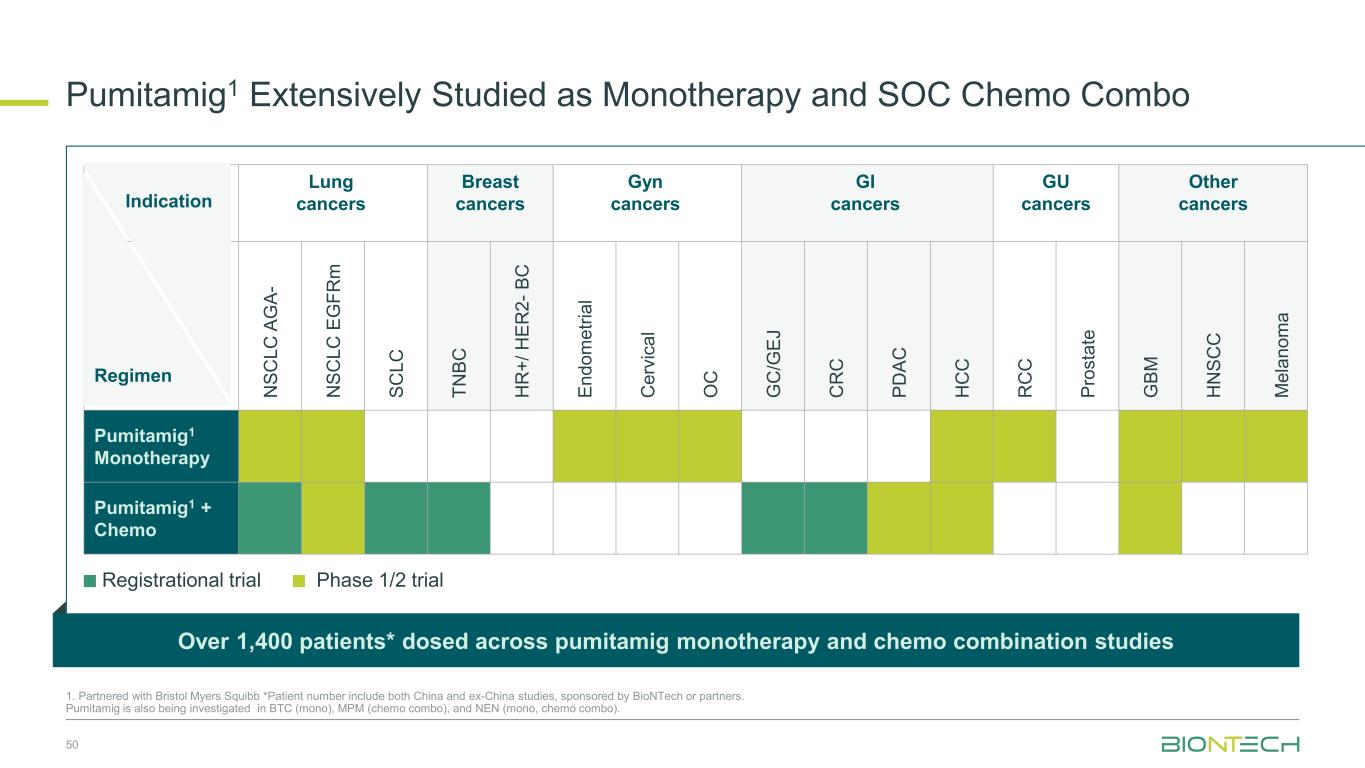
Lung cancers Breast cancers Gyn cancers GI cancers GU cancers Other cancers N S C L C A G A - N S C L C E G F R m S C L C T N B C H R + / H E R 2 - B C E n d o m e tr ia l C e rv ic a l O C G C /G E J C R C P D A C H C C R C C P ro s ta te G B M H N S C C M e la n o m a Pumitamig1 Monotherapy Pumitamig1 + Chemo Regimen Pumitamig1 Extensively Studied as Monotherapy and SOC Chemo Combo 50 1. Partnered with Bristol Myers Squibb *Patient number include both China and ex-China studies, sponsored by BioNTech or partners. Pumitamig is also being investigated in BTC (mono), MPM (chemo combo), and NEN (mono, chemo combo). Over 1,400 patients* dosed across pumitamig monotherapy and chemo combination studies Indication Registrational trial Phase 1/2 trial
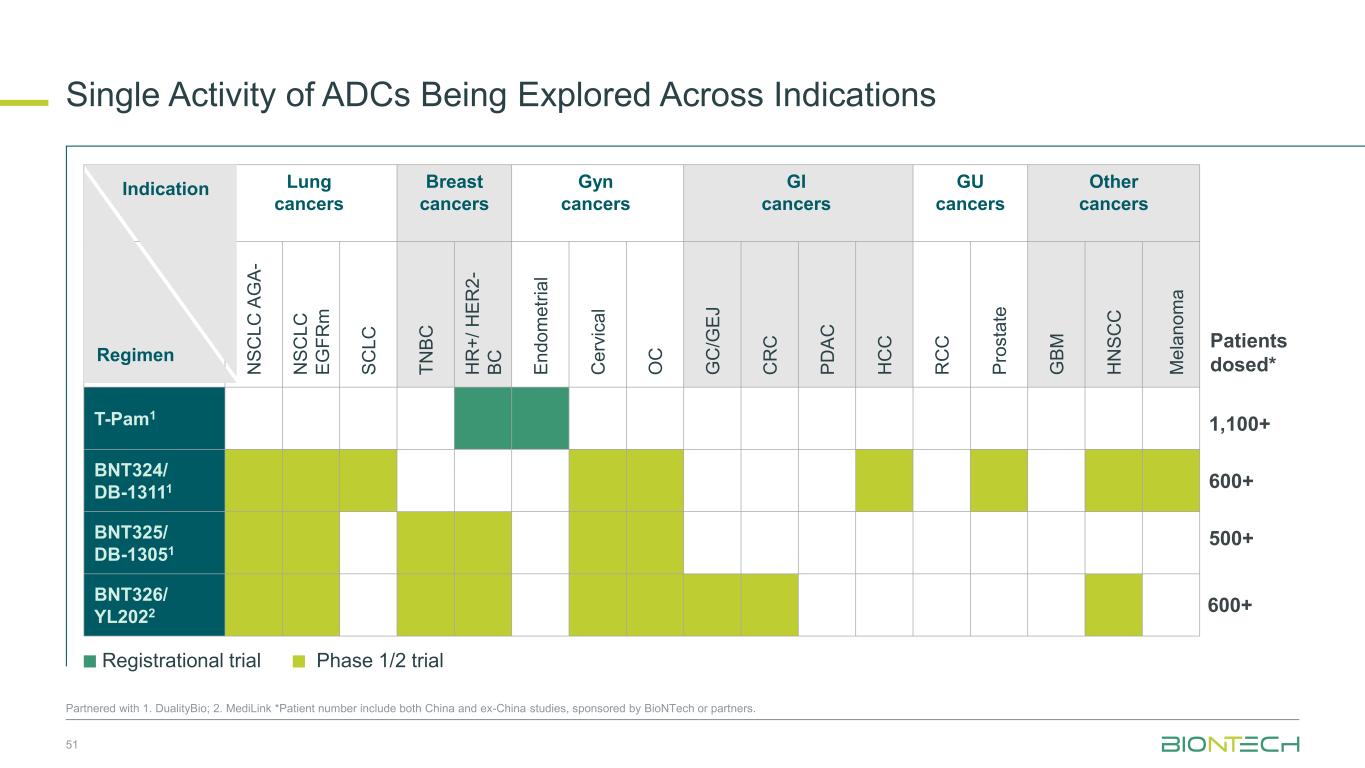
Lung cancers Breast cancers Gyn cancers GI cancers GU cancers Other cancers N S C L C A G A - N S C L C E G F R m S C L C T N B C H R + / H E R 2 - B C E n d o m e tr ia l C e rv ic a l O C G C /G E J C R C P D A C H C C R C C P ro s ta te G B M H N S C C M e la n o m a T-Pam1 BNT324/ DB-13111 BNT325/ DB-13051 BNT326/ YL2022 Regimen Single Activity of ADCs Being Explored Across Indications 51 Partnered with 1. DualityBio; 2. MediLink *Patient number include both China and ex-China studies, sponsored by BioNTech or partners. Indication Registrational trial Phase 1/2 trial 1,100+ Patients dosed* 600+ 500+ 600+
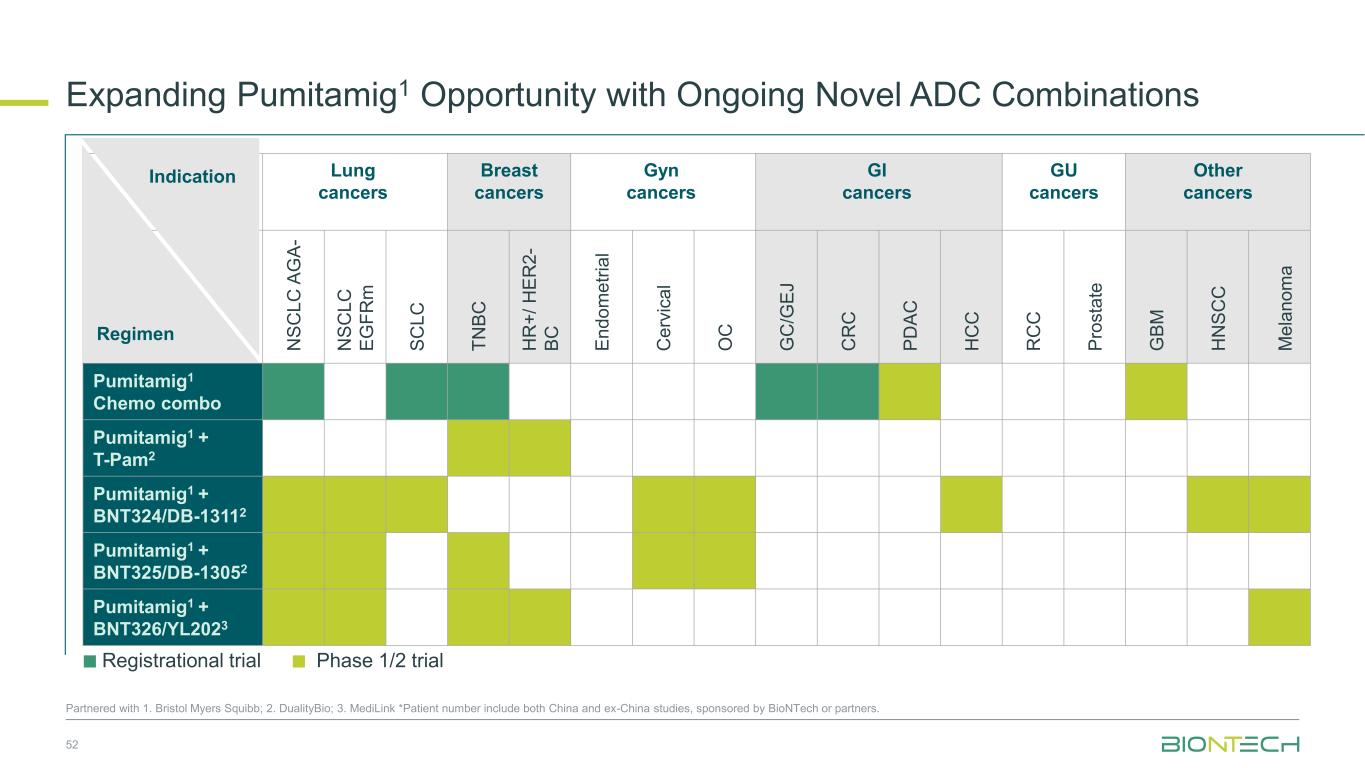
Lung cancers Breast cancers Gyn cancers GI cancers GU cancers Other cancers N S C L C A G A - N S C L C E G F R m S C L C T N B C H R + / H E R 2 - B C E n d o m e tr ia l C e rv ic a l O C G C /G E J C R C P D A C H C C R C C P ro s ta te G B M H N S C C M e la n o m a Pumitamig1 Chemo combo Pumitamig1 + T-Pam2 Pumitamig1 + BNT324/DB-13112 Pumitamig1 + BNT325/DB-13052 Pumitamig1 + BNT326/YL2023 Expanding Pumitamig1 Opportunity with Ongoing Novel ADC Combinations 52 Partnered with 1. Bristol Myers Squibb; 2. DualityBio; 3. MediLink *Patient number include both China and ex-China studies, sponsored by BioNTech or partners. Regimen Indication Registrational trial Phase 1/2 trial
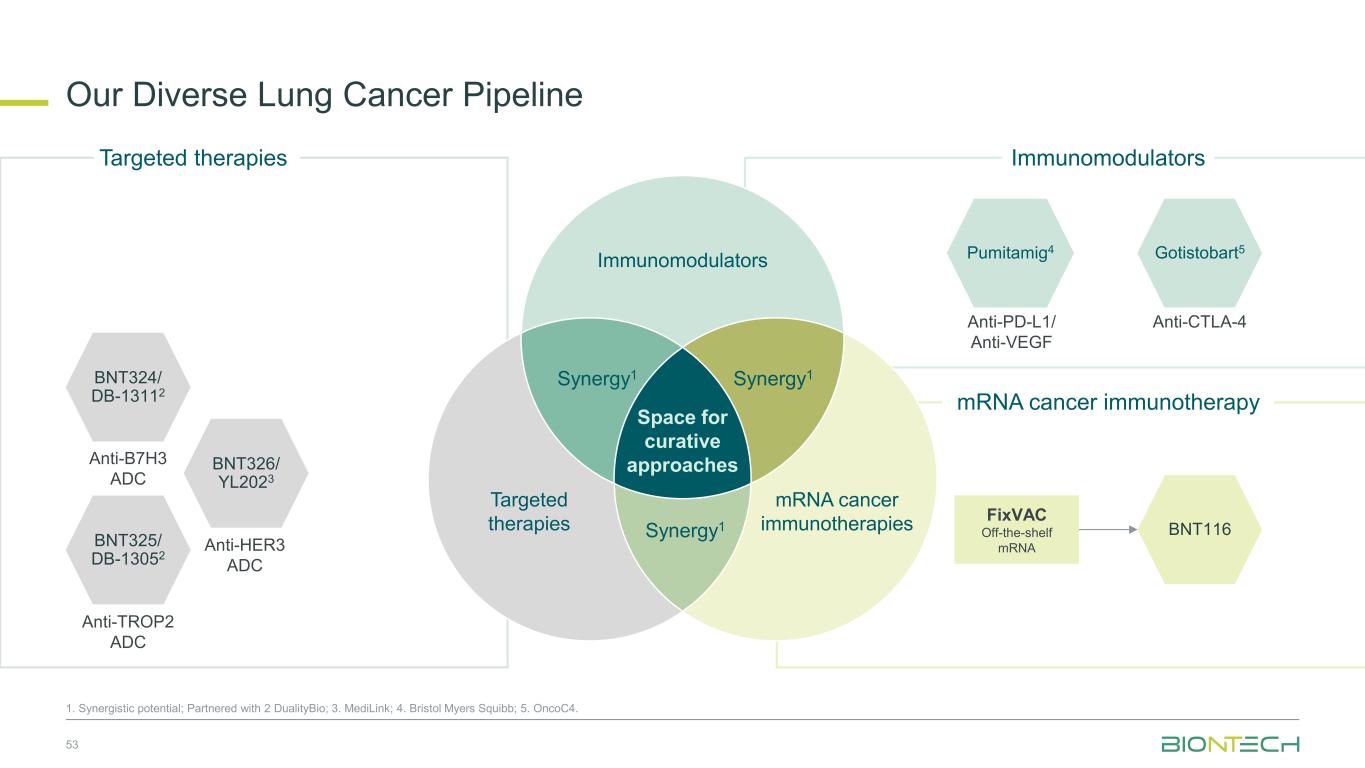
Our Diverse Lung Cancer Pipeline 53 1. Synergistic potential; Partnered with 2 DualityBio; 3. MediLink; 4. Bristol Myers Squibb; 5. OncoC4. BNT324/ DB-13112 Anti-B7H3 ADC BNT325/ DB-13052 Anti-TROP2 ADC BNT326/ YL2023 Anti-HER3 ADC Targeted therapies Immunomodulators Space for curative approaches Synergy1 Targeted therapies mRNA cancer immunotherapies Synergy1Synergy1 Anti-PD-L1/ Anti-VEGF Pumitamig4 Gotistobart5 Anti-CTLA-4 BNT116 FixVAC Off-the-shelf mRNA Immunomodulators mRNA cancer immunotherapy
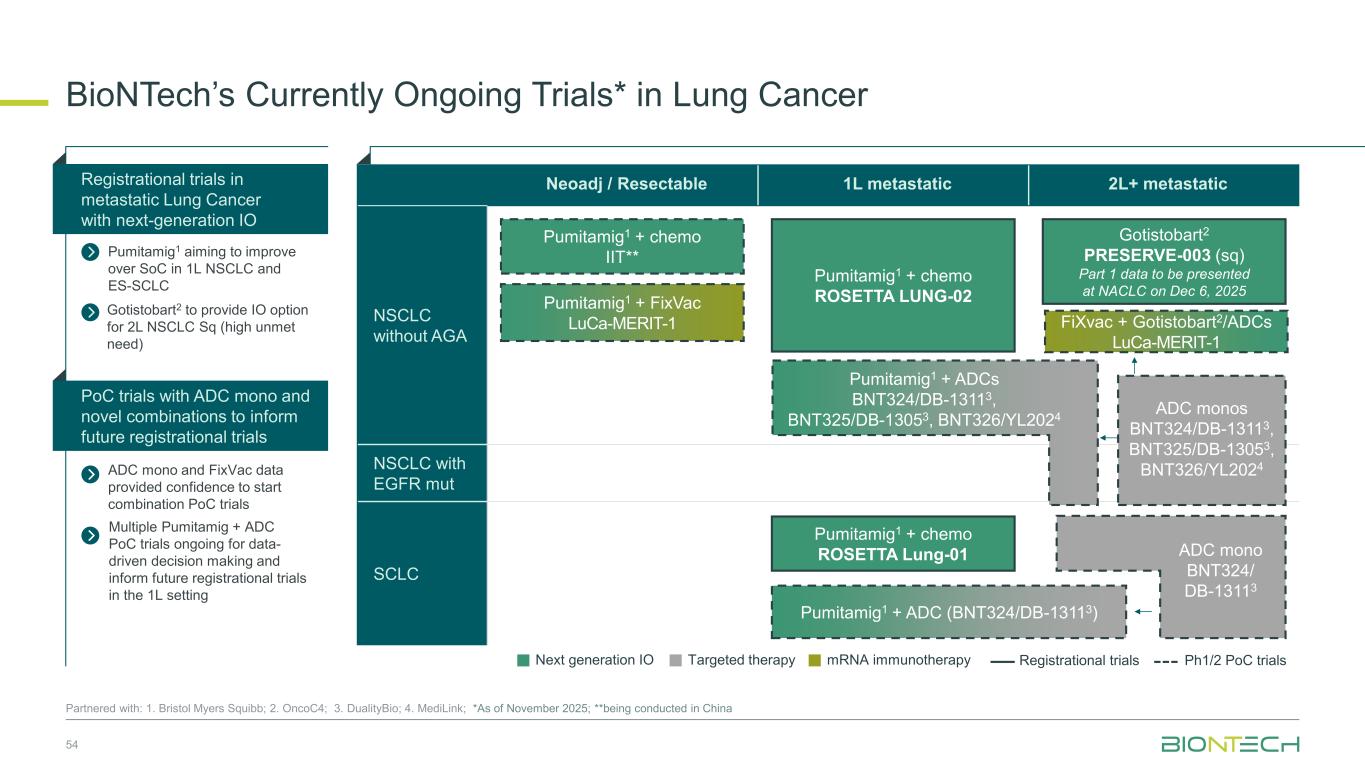
Neoadj / Resectable 1L metastatic 2L+ metastatic NSCLC without AGA NSCLC with EGFR mut SCLC BioNTech’s Currently Ongoing Trials* in Lung Cancer 54 Partnered with: 1. Bristol Myers Squibb; 2. OncoC4; 3. DualityBio; 4. MediLink; *As of November 2025; **being conducted in China ADC mono and FixVac data provided confidence to start combination PoC trials Registrational trials in metastatic Lung Cancer with next-generation IO Pumitamig1 aiming to improve over SoC in 1L NSCLC and ES-SCLC PoC trials with ADC mono and novel combinations to inform future registrational trials Next generation IO Targeted therapy mRNA immunotherapy Registrational trials Ph1/2 PoC trials Gotistobart2 PRESERVE-003 (sq) Part 1 data to be presented at NACLC on Dec 6, 2025 FiXvac + Gotistobart2/ADCs LuCa-MERIT-1 Pumitamig1 + chemo ROSETTA Lung-01 Pumitamig1 + chemo IIT** Pumitamig1 + chemo ROSETTA LUNG-02 ADC monos BNT324/DB-13113, BNT325/DB-13053, BNT326/YL2024 Pumitamig1 + FixVac LuCa-MERIT-1 Pumitamig1 + ADCs BNT324/DB-13113, BNT325/DB-13053, BNT326/YL2024 ADC mono BNT324/ DB-13113 Pumitamig1 + ADC (BNT324/DB-13113) Gotistobart2 to provide IO option for 2L NSCLC Sq (high unmet need) Multiple Pumitamig + ADC PoC trials ongoing for data- driven decision making and inform future registrational trials in the 1L setting
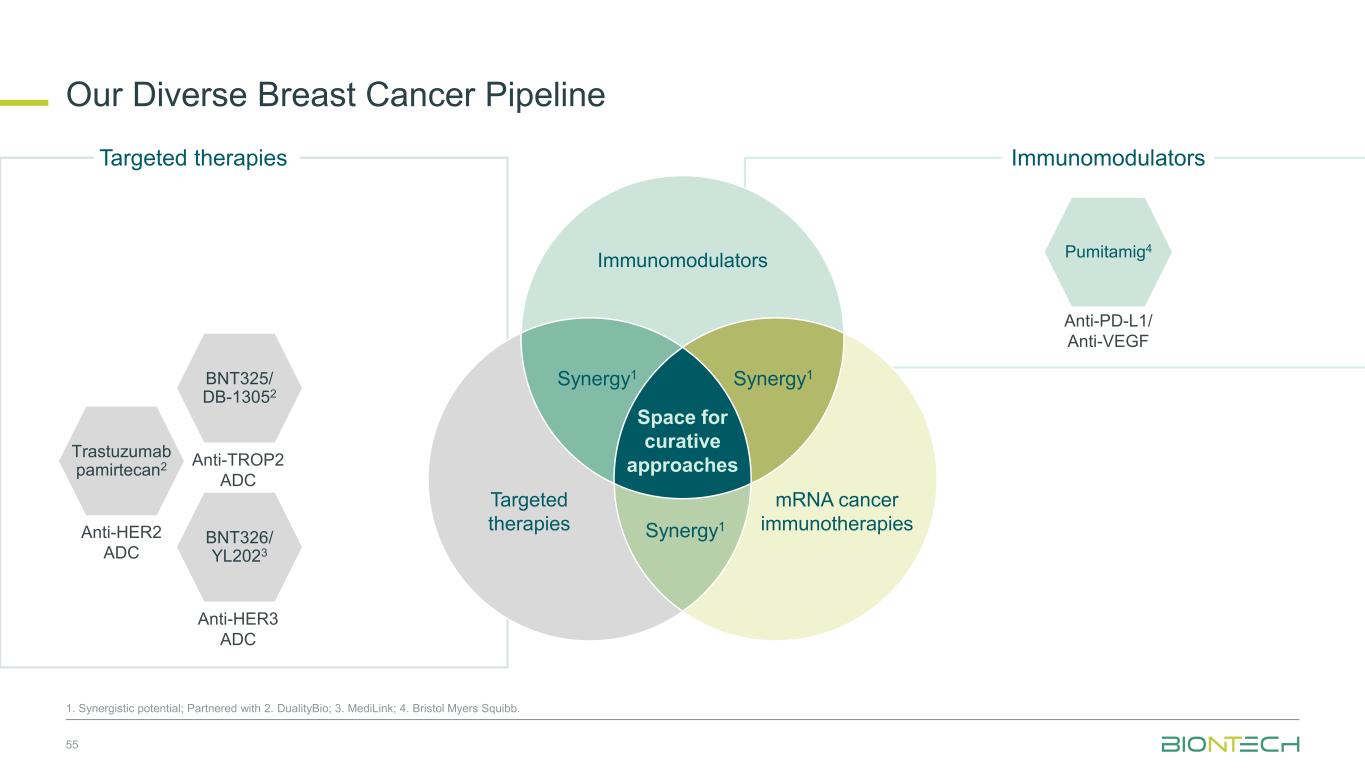
Immunomodulators Our Diverse Breast Cancer Pipeline 55 1. Synergistic potential; Partnered with 2. DualityBio; 3. MediLink; 4. Bristol Myers Squibb. Trastuzumab pamirtecan2 Anti-HER2 ADC BNT326/ YL2023 Anti-HER3 ADC Targeted therapies BNT325/ DB-13052 Anti-TROP2 ADC Anti-PD-L1/ Anti-VEGF Pumitamig4 Immunomodulators Space for curative approaches Synergy1 Targeted therapies mRNA cancer immunotherapies Synergy1Synergy1
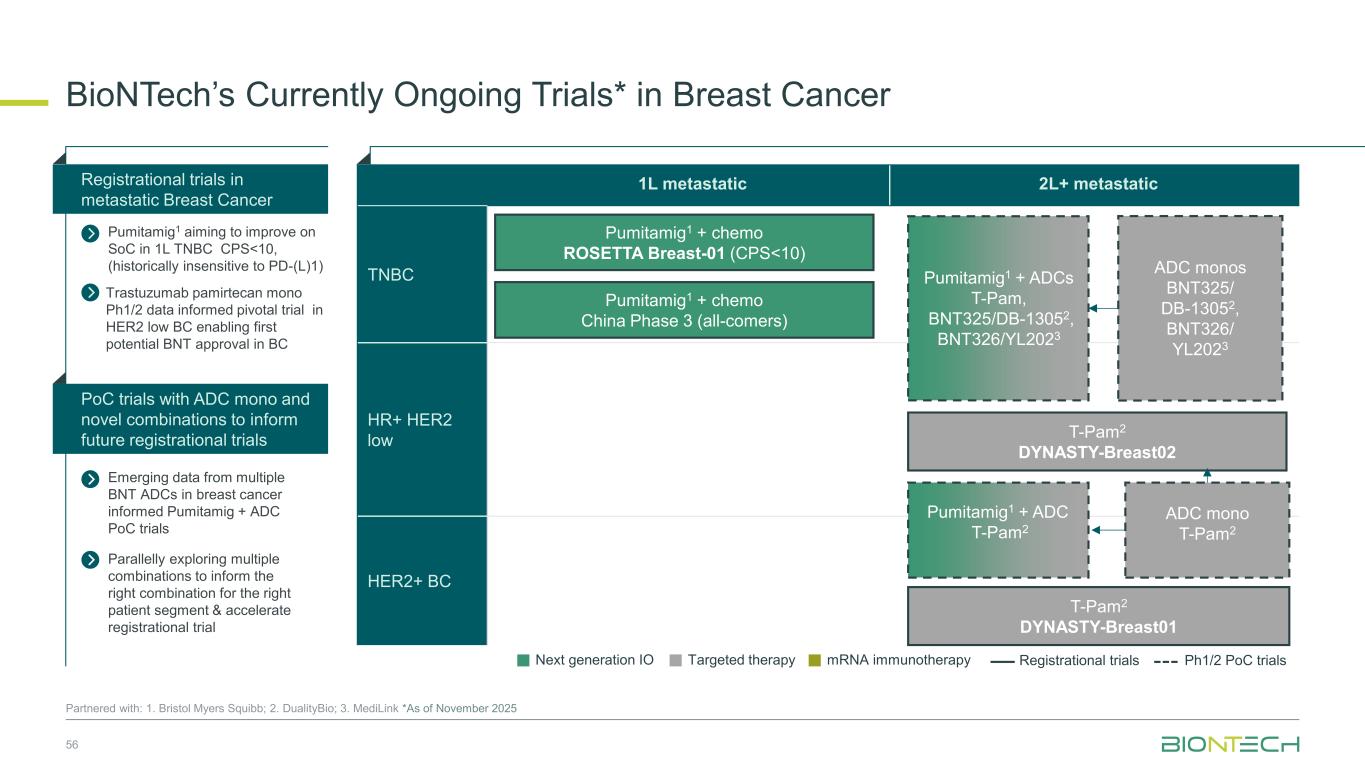
1L metastatic 2L+ metastatic TNBC HR+ HER2 low HER2+ BC BioNTech’s Currently Ongoing Trials* in Breast Cancer 56 Partnered with: 1. Bristol Myers Squibb; 2. DualityBio; 3. MediLink *As of November 2025 Pumitamig1 + chemo ROSETTA Breast-01 (CPS<10) Pumitamig1 + chemo China Phase 3 (all-comers) Emerging data from multiple BNT ADCs in breast cancer informed Pumitamig + ADC PoC trials Registrational trials in metastatic Breast Cancer Pumitamig1 aiming to improve on SoC in 1L TNBC CPS<10, (historically insensitive to PD-(L)1) PoC trials with ADC mono and novel combinations to inform future registrational trials Next generation IO Targeted therapy mRNA immunotherapy Registrational trials Ph1/2 PoC trials T-Pam2 DYNASTY-Breast02 ADC monos BNT325/ DB-13052, BNT326/ YL2023 ADC mono T-Pam2 Pumitamig1 + ADCs T-Pam, BNT325/DB-13052, BNT326/YL2023 Pumitamig1 + ADC T-Pam2 Trastuzumab pamirtecan mono Ph1/2 data informed pivotal trial in HER2 low BC enabling first potential BNT approval in BC Parallelly exploring multiple combinations to inform the right combination for the right patient segment & accelerate registrational trial T-Pam2 DYNASTY-Breast01
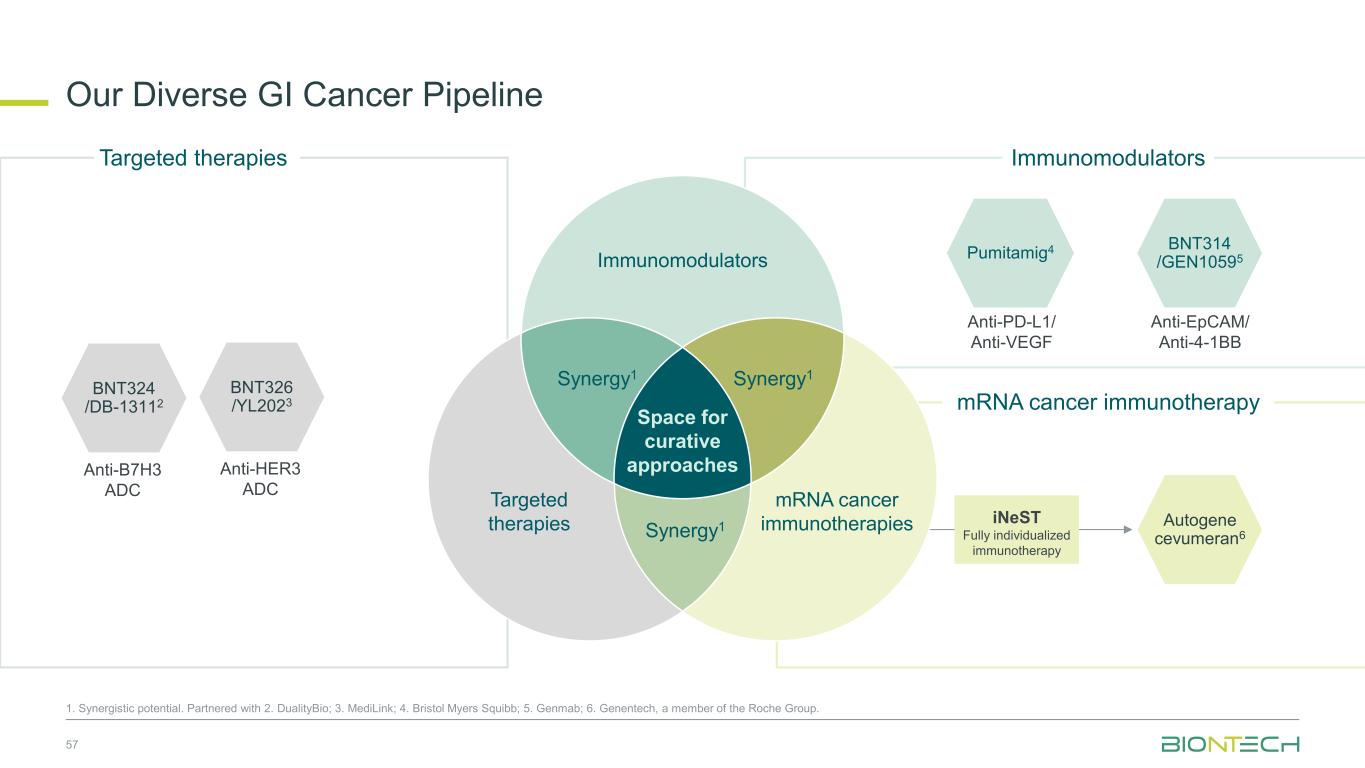
Anti-PD-L1/ Anti-VEGF Pumitamig4 Autogene cevumeran6 Immunomodulators mRNA cancer immunotherapy Our Diverse GI Cancer Pipeline 57 1. Synergistic potential. Partnered with 2. DualityBio; 3. MediLink; 4. Bristol Myers Squibb; 5. Genmab; 6. Genentech, a member of the Roche Group. Targeted therapies iNeST Fully individualized immunotherapy BNT314 /GEN10595 Anti-EpCAM/ Anti-4-1BB BNT326 /YL2023 Anti-HER3 ADC BNT324 /DB-13112 Anti-B7H3 ADC Immunomodulators Space for curative approaches Synergy1 Targeted therapies mRNA cancer immunotherapies Synergy1Synergy1
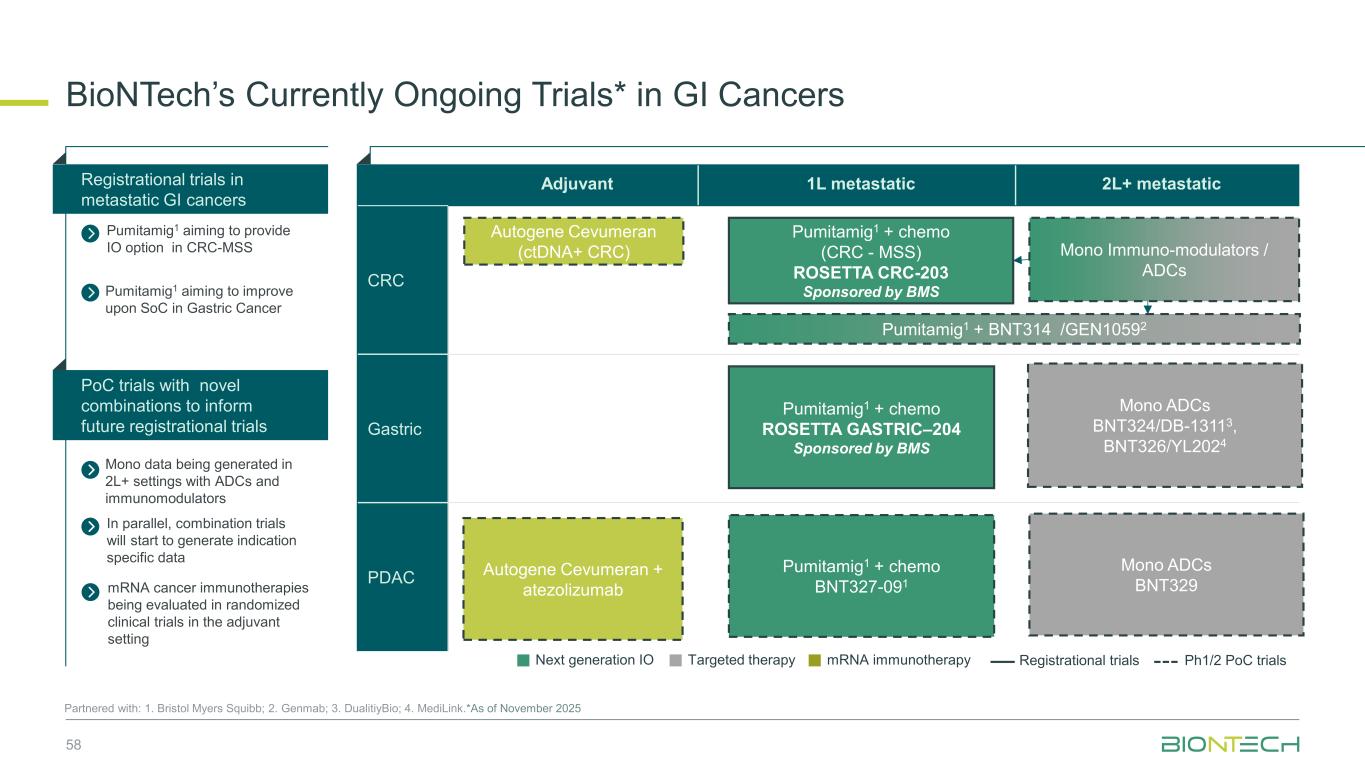
Adjuvant 1L metastatic 2L+ metastatic CRC Gastric PDAC BioNTech’s Currently Ongoing Trials* in GI Cancers 58 Partnered with: 1. Bristol Myers Squibb; 2. Genmab; 3. DualitiyBio; 4. MediLink.*As of November 2025 Pumitamig1 + chemo (CRC - MSS) ROSETTA CRC-203 Sponsored by BMS Pumitamig1 + chemo BNT327-091 Pumitamig1 + chemo ROSETTA GASTRIC–204 Sponsored by BMS Mono Immuno-modulators / ADCs Pumitamig1 + BNT314 /GEN10592 Mono ADCs BNT329 Mono data being generated in 2L+ settings with ADCs and immunomodulators Pumitamig1 aiming to provide IO option in CRC-MSS PoC trials with novel combinations to inform future registrational trials Registrational trials in metastatic GI cancers Mono ADCs BNT324/DB-13113, BNT326/YL2024 Pumitamig1 aiming to improve upon SoC in Gastric Cancer In parallel, combination trials will start to generate indication specific data Next generation IO Targeted therapy mRNA immunotherapy Registrational trials Ph1/2 PoC trials Autogene Cevumeran + atezolizumab Autogene Cevumeran (ctDNA+ CRC) mRNA cancer immunotherapies being evaluated in randomized clinical trials in the adjuvant setting

Establishing Pumitamig in Foundational Tumor Types Prof. Ilhan Celik, M.D. Vice President, Clinical Development Michael Wenger, M.D. Vice President, Clinical Development 4

Thoracic Cancer
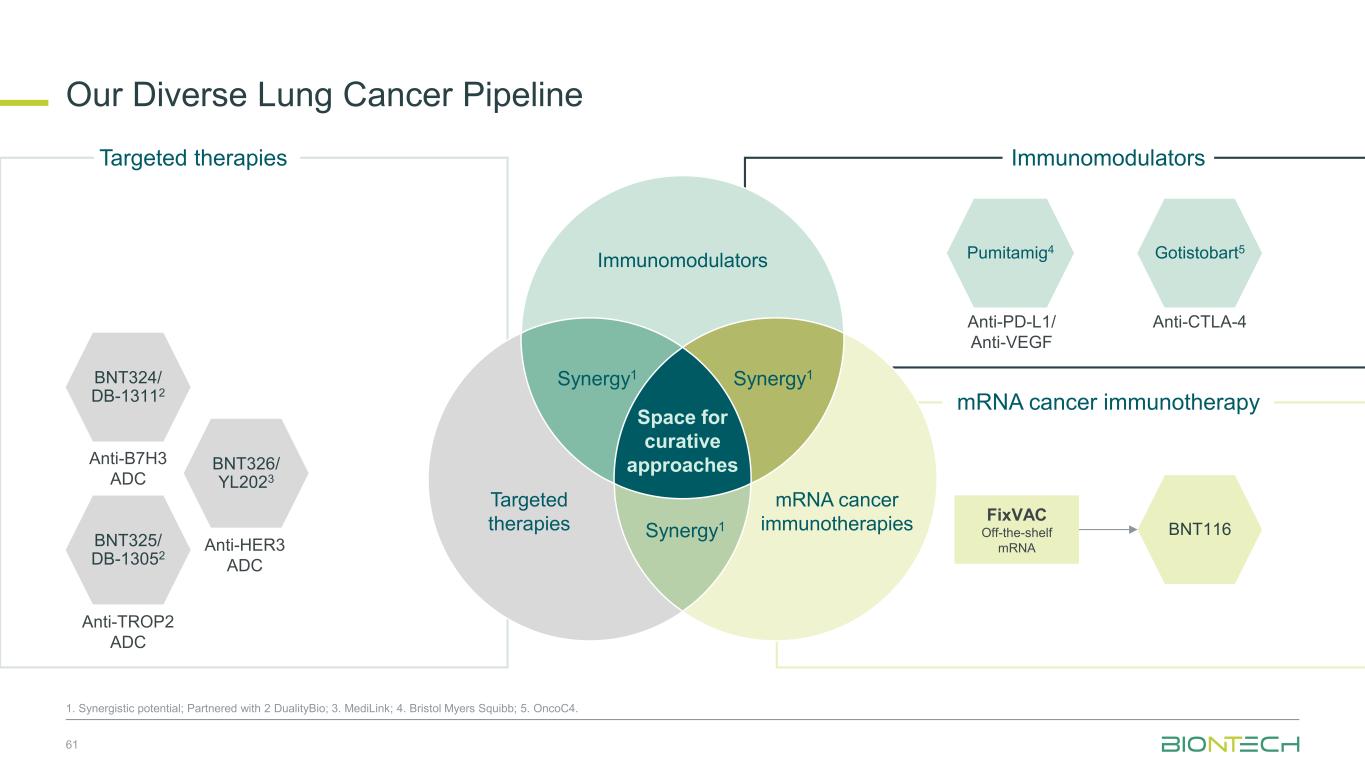
Our Diverse Lung Cancer Pipeline 61 1. Synergistic potential; Partnered with 2 DualityBio; 3. MediLink; 4. Bristol Myers Squibb; 5. OncoC4. BNT324/ DB-13112 Anti-B7H3 ADC BNT325/ DB-13052 Anti-TROP2 ADC BNT326/ YL2023 Anti-HER3 ADC Targeted therapies Immunomodulators Space for curative approaches Synergy1 Targeted therapies mRNA cancer immunotherapies Synergy1Synergy1 Anti-PD-L1/ Anti-VEGF Pumitamig4 Gotistobart5 Anti-CTLA-4 BNT116 FixVAC Off-the-shelf mRNA Immunomodulators mRNA cancer immunotherapy
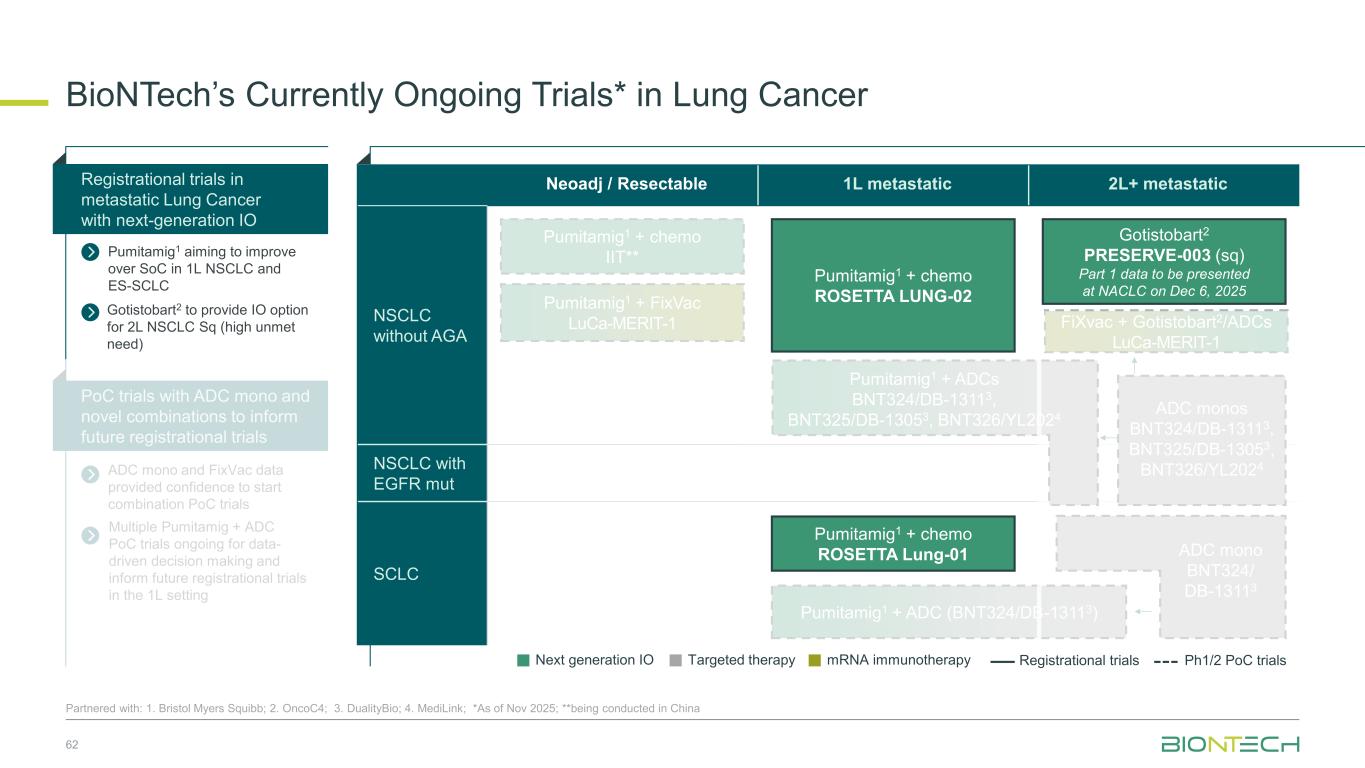
Neoadj / Resectable 1L metastatic 2L+ metastatic NSCLC without AGA NSCLC with EGFR mut SCLC BioNTech’s Currently Ongoing Trials* in Lung Cancer 62 Partnered with: 1. Bristol Myers Squibb; 2. OncoC4; 3. DualityBio; 4. MediLink; *As of Nov 2025; **being conducted in China ADC mono and FixVac data provided confidence to start combination PoC trials Registrational trials in metastatic Lung Cancer with next-generation IO Pumitamig1 aiming to improve over SoC in 1L NSCLC and ES-SCLC PoC trials with ADC mono and novel combinations to inform future registrational trials Next generation IO Targeted therapy mRNA immunotherapy Registrational trials Ph1/2 PoC trials Gotistobart2 PRESERVE-003 (sq) Part 1 data to be presented at NACLC on Dec 6, 2025 FiXvac + Gotistobart2/ADCs LuCa-MERIT-1 Pumitamig1 + chemo ROSETTA Lung-01 Pumitamig1 + chemo IIT** Pumitamig1 + chemo ROSETTA LUNG-02 ADC monos BNT324/DB-13113, BNT325/DB-13053, BNT326/YL2024 Pumitamig1 + FixVac LuCa-MERIT-1 Pumitamig1 + ADCs BNT324/DB-13113, BNT325/DB-13053, BNT326/YL2024 ADC mono BNT324/ DB-13113 Pumitamig1 + ADC (BNT324/DB-13113) Gotistobart2 to provide IO option for 2L NSCLC Sq (high unmet need) Multiple Pumitamig + ADC PoC trials ongoing for data- driven decision making and inform future registrational trials in the 1L setting
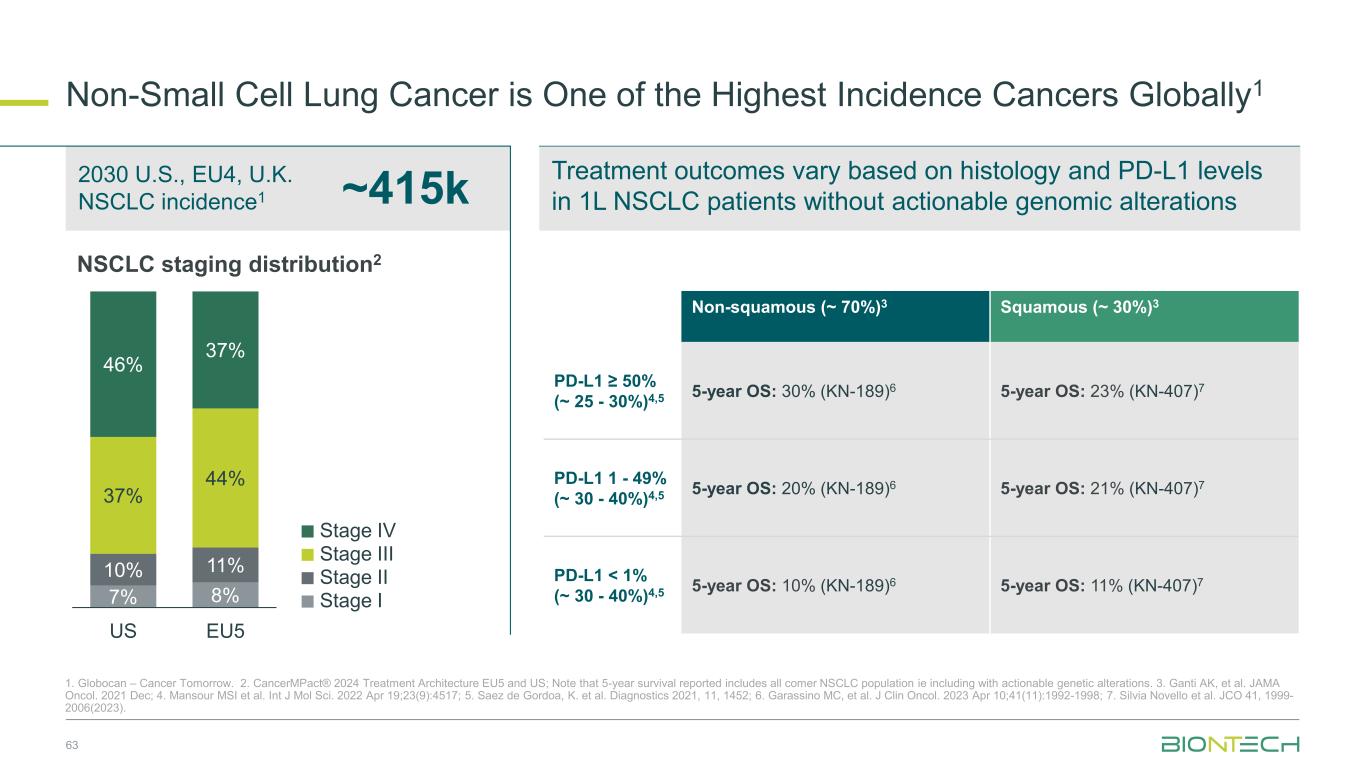
Treatment outcomes vary based on histology and PD-L1 levels in 1L NSCLC patients without actionable genomic alterations Non-Small Cell Lung Cancer is One of the Highest Incidence Cancers Globally1 63 1. Globocan – Cancer Tomorrow. 2. CancerMPact® 2024 Treatment Architecture EU5 and US; Note that 5-year survival reported includes all comer NSCLC population ie including with actionable genetic alterations. 3. Ganti AK, et al. JAMA Oncol. 2021 Dec; 4. Mansour MSI et al. Int J Mol Sci. 2022 Apr 19;23(9):4517; 5. Saez de Gordoa, K. et al. Diagnostics 2021, 11, 1452; 6. Garassino MC, et al. J Clin Oncol. 2023 Apr 10;41(11):1992-1998; 7. Silvia Novello et al. JCO 41, 1999- 2006(2023). ~415k 2030 U.S., EU4, U.K. NSCLC incidence1 Non-squamous (~ 70%)3 Squamous (~ 30%)3 PD-L1 ≥ 50% (~ 25 - 30%)4,5 5-year OS: 30% (KN-189)6 5-year OS: 23% (KN-407)7 PD-L1 1 - 49% (~ 30 - 40%)4,5 5-year OS: 20% (KN-189)6 5-year OS: 21% (KN-407)7 PD-L1 < 1% (~ 30 - 40%)4,5 5-year OS: 10% (KN-189)6 5-year OS: 11% (KN-407)7Stage II Stage III Stage IV Stage I NSCLC staging distribution2 7% 8% 10% 11% 37% 44% 46% 37% US EU5
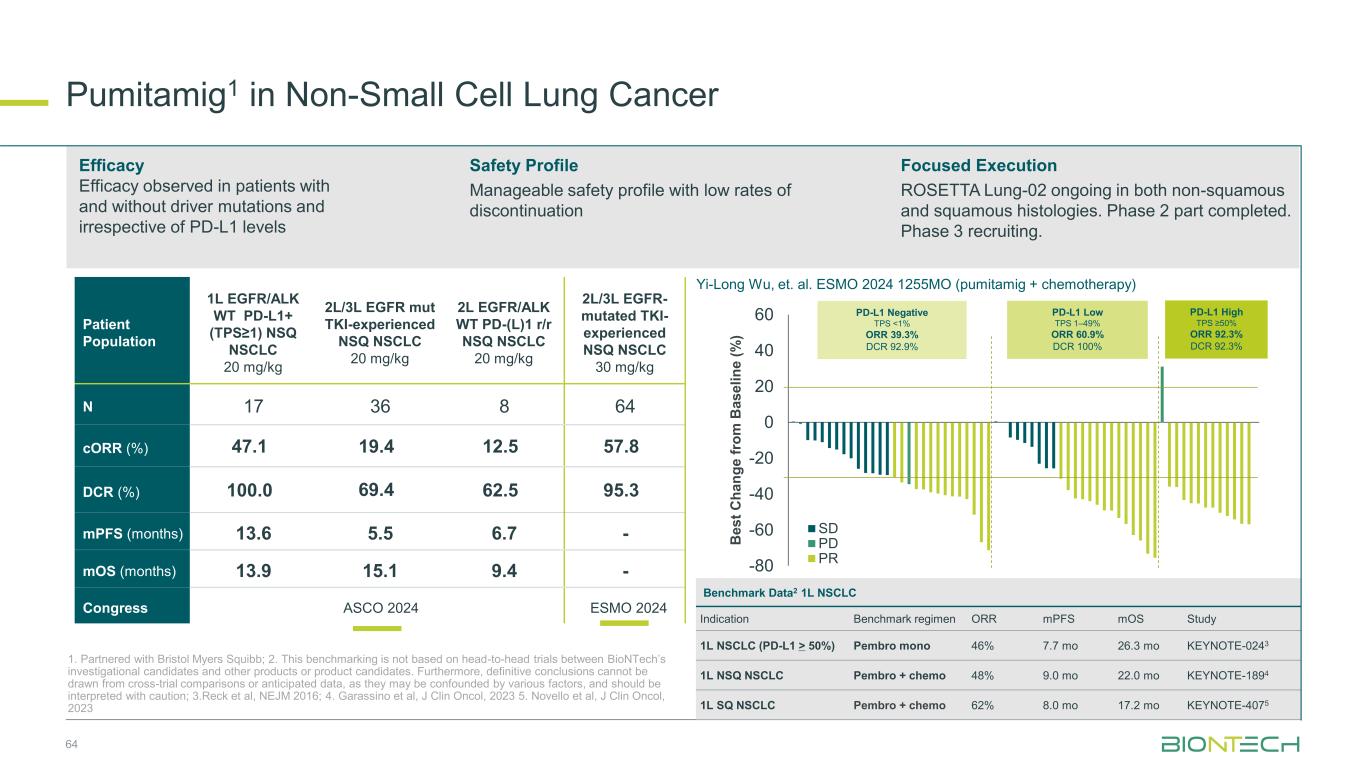
64 Patient Population 1L EGFR/ALK WT PD-L1+ (TPS≥1) NSQ NSCLC 20 mg/kg 2L/3L EGFR mut TKI-experienced NSQ NSCLC 20 mg/kg 2L EGFR/ALK WT PD-(L)1 r/r NSQ NSCLC 20 mg/kg 2L/3L EGFR- mutated TKI- experienced NSQ NSCLC 30 mg/kg N 17 36 8 64 cORR (%) 47.1 19.4 12.5 57.8 DCR (%) 100.0 69.4 62.5 95.3 mPFS (months) 13.6 5.5 6.7 - mOS (months) 13.9 15.1 9.4 - Congress ASCO 2024 ESMO 2024 Efficacy Efficacy observed in patients with and without driver mutations and irrespective of PD-L1 levels Safety Profile Manageable safety profile with low rates of discontinuation Focused Execution ROSETTA Lung-02 ongoing in both non-squamous and squamous histologies. Phase 2 part completed. Phase 3 recruiting. Pumitamig1 in Non-Small Cell Lung Cancer 1. Partnered with Bristol Myers Squibb; 2. This benchmarking is not based on head-to-head trials between BioNTech’s investigational candidates and other products or product candidates. Furthermore, definitive conclusions cannot be drawn from cross-trial comparisons or anticipated data, as they may be confounded by various factors, and should be interpreted with caution; 3.Reck et al, NEJM 2016; 4. Garassino et al, J Clin Oncol, 2023 5. Novello et al, J Clin Oncol, 2023 -80 -60 -40 -20 0 20 40 60 SD PD PR B e s t C h a n g e f ro m B a s e li n e ( % ) PD-L1 Negative TPS <1% ORR 39.3% DCR 92.9% PD-L1 High TPS ≥50% ORR 92.3% DCR 92.3% PD-L1 Low TPS 1–49% ORR 60.9% DCR 100% Yi-Long Wu, et. al. ESMO 2024 1255MO (pumitamig + chemotherapy) Benchmark Data2 1L NSCLC Indication Benchmark regimen ORR mPFS mOS Study 1L NSCLC (PD-L1 > 50%) Pembro mono 46% 7.7 mo 26.3 mo KEYNOTE-0243 1L NSQ NSCLC Pembro + chemo 48% 9.0 mo 22.0 mo KEYNOTE-1894 1L SQ NSCLC Pembro + chemo 62% 8.0 mo 17.2 mo KEYNOTE-4075
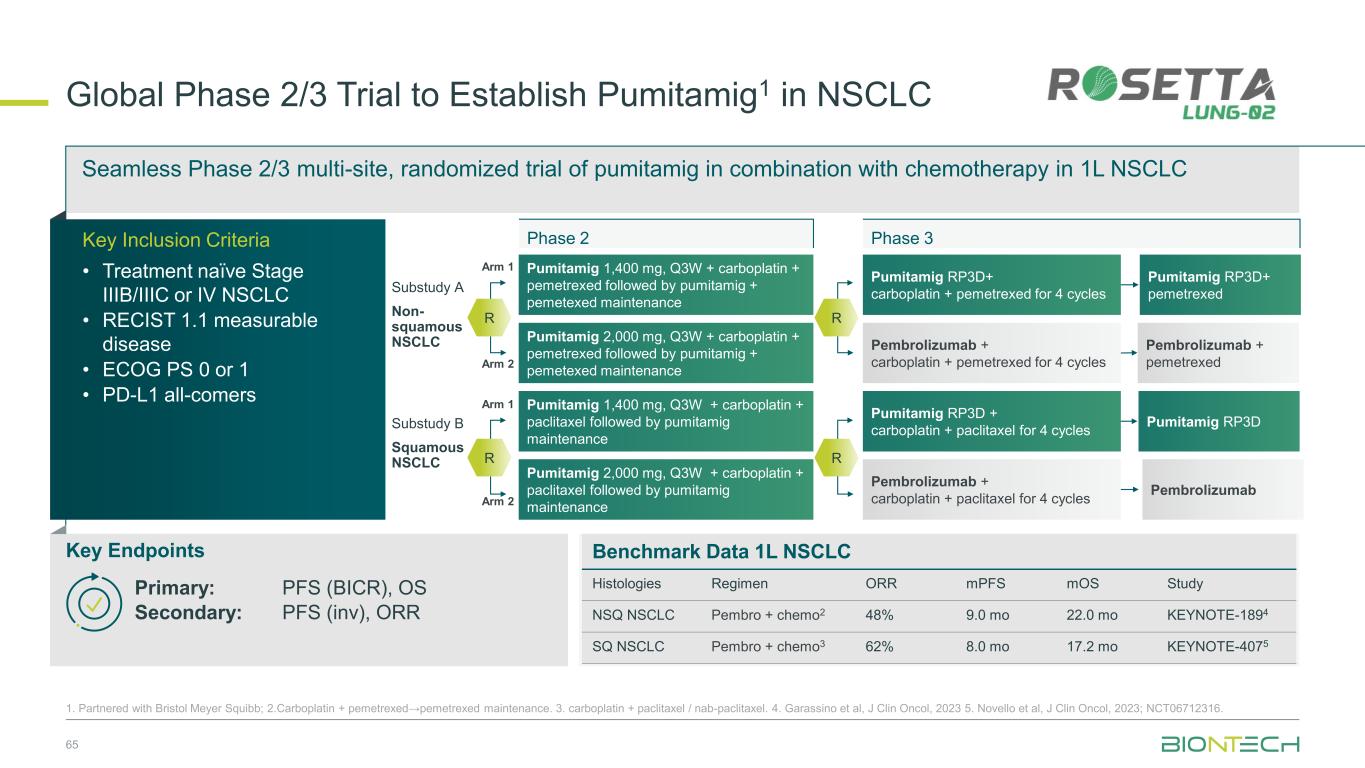
Seamless Phase 2/3 multi-site, randomized trial of pumitamig in combination with chemotherapy in 1L NSCLC Benchmark Data 1L NSCLC Histologies Regimen ORR mPFS mOS Study NSQ NSCLC Pembro + chemo2 48% 9.0 mo 22.0 mo KEYNOTE-1894 SQ NSCLC Pembro + chemo3 62% 8.0 mo 17.2 mo KEYNOTE-4075 Global Phase 2/3 Trial to Establish Pumitamig1 in NSCLC 65 1. Partnered with Bristol Meyer Squibb; 2.Carboplatin + pemetrexed→pemetrexed maintenance. 3. carboplatin + paclitaxel / nab-paclitaxel. 4. Garassino et al, J Clin Oncol, 2023 5. Novello et al, J Clin Oncol, 2023; NCT06712316. Key Endpoints Key Inclusion Criteria • Treatment naïve Stage IIIB/IIIC or IV NSCLC • RECIST 1.1 measurable disease • ECOG PS 0 or 1 • PD-L1 all-comers Primary: PFS (BICR), OS Secondary: PFS (inv), ORR Phase 2 Phase 3 Pumitamig 2,000 mg, Q3W + carboplatin + pemetrexed followed by pumitamig + pemetexed maintenance Arm 1 Arm 2 Pumitamig RP3D+ pemetrexed Pembrolizumab + pemetrexed Pumitamig RP3D Pembrolizumab Pumitamig RP3D+ carboplatin + pemetrexed for 4 cycles Pembrolizumab + carboplatin + pemetrexed for 4 cycles Pumitamig RP3D + carboplatin + paclitaxel for 4 cycles Pembrolizumab + carboplatin + paclitaxel for 4 cycles Pumitamig 1,400 mg, Q3W + carboplatin + pemetrexed followed by pumitamig + pemetexed maintenance Pumitamig 1,400 mg, Q3W + carboplatin + paclitaxel followed by pumitamig maintenance Pumitamig 2,000 mg, Q3W + carboplatin + paclitaxel followed by pumitamig maintenance Substudy A Non- squamous NSCLC Substudy B Squamous NSCLC R R R Arm 1 Arm 2 R

Squamous NSCLC Remains an Area of High Unmet Need 66 1. CancerMPact; 2. Clarivate / Clarivate Survey| By 2030 55k squamous patients start in 1L (non-AGA population) 1 ~30% continue into 2L treatment and are IO addressable Limited treatment options for patients without actionable genetic alterations in squamous NSCLC Amongst NSCLC, metastatic squamous NSCLC is seen as #1 area of unmet need for improving treatment amongst NSCLC2 In 2L, current chemo- based SOC shows 10 months median OS in clinical trials <25% respond to 2L chemo-based SOC (docetaxel ± ramucirumab) Multiple efforts failed to improve therapeutic outcome in 2L squamous NSCLC in recent years
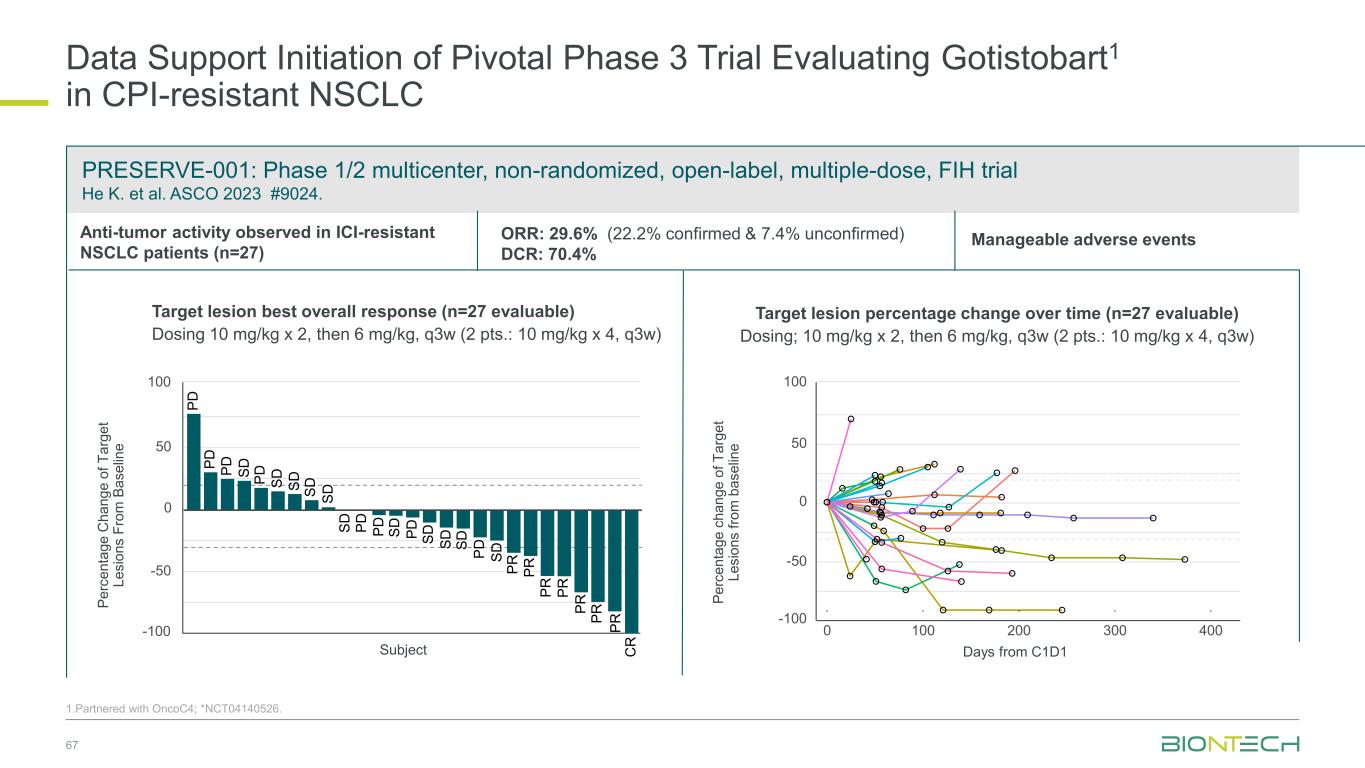
PRESERVE-001: Phase 1/2 multicenter, non-randomized, open-label, multiple-dose, FIH trial He K. et al. ASCO 2023 #9024. Data Support Initiation of Pivotal Phase 3 Trial Evaluating Gotistobart1 in CPI-resistant NSCLC 67 Target lesion best overall response (n=27 evaluable) Dosing 10 mg/kg x 2, then 6 mg/kg, q3w (2 pts.: 10 mg/kg x 4, q3w) Target lesion percentage change over time (n=27 evaluable) Dosing; 10 mg/kg x 2, then 6 mg/kg, q3w (2 pts.: 10 mg/kg x 4, q3w) Days from C1D1 -100 -50 0 50 100 0 100 200 300 400 P e rc e n ta g e c h a n g e o f T a rg e t L e s io n s fr o m b a s e lin e Anti-tumor activity observed in ICI-resistant NSCLC patients (n=27) -100 -50 0 50 100 S D S D S D S D C R P R P R P D P D S D P R P D P D S D P D S DP D P R S D S D S D S D P R P D P RP R P D Subject P e rc e n ta g e C h a n g e o f T a rg e t L e s io n s F ro m B a s e lin e Manageable adverse eventsORR: 29.6% (22.2% confirmed & 7.4% unconfirmed) DCR: 70.4% Phase 3 trial in NSCLC ongoing 1.Partnered with OncoC4; *NCT04140526.
Phase 3 Stage 1 data to be presented at NACLC on December 6, 2025 Pivotal Development of Gotistobart1 in 2L Squamous Non-Small Cell Lung Cancer 68 1. Partnered with OncoC4 . 2. Ahn et al. J Clin Oncol 43, 260-272(2025); NCT05671510 Gotistobart 6 mg/kg with 2 loading doses of 10mg/kg, Q3W (n=239) Docetaxel 75 mg/kg, Q3W (n=239) Gotistobart 6 mg/kg with 2 loading doses of 10 mg/kg, Q3W (n=103) Gotistobart 3 mg/kg, Q3W (n=10) Docetaxel 75 mg/kg, Q3W (n=104) Seamless Phase 3 two-stage, randomized trial evaluating gotistobart versus docetaxel in 600 patients Key Endpoints Primary: OS Secondary: PFS, safety Stage 1: Dose confirmation in squamous and non- squamous NSCLC R 1:1 Stage 2: Pivotal part in squamous NSCLCKey Inclusion Criteria • stage IV, metastatic NSCLC • Prior PD-(L)1 +/- platinum-based chemotherapy • Prior IO-IO allowed • RECIST 1.1 measurable lesions Stratification Factors • Histology (Squamous or Non- Squamous) in Stage I • Presence of brain metastases • ECOG score (0 or 1) • Region (US or ex-US) R 1:1:1 Benchmark Data for 2L sqNSCLC Regimen ORR mPFS mOS Study Docetaxel 12.7% 3.9 mo 9.4 mo TROPION-Lung01 sq population2
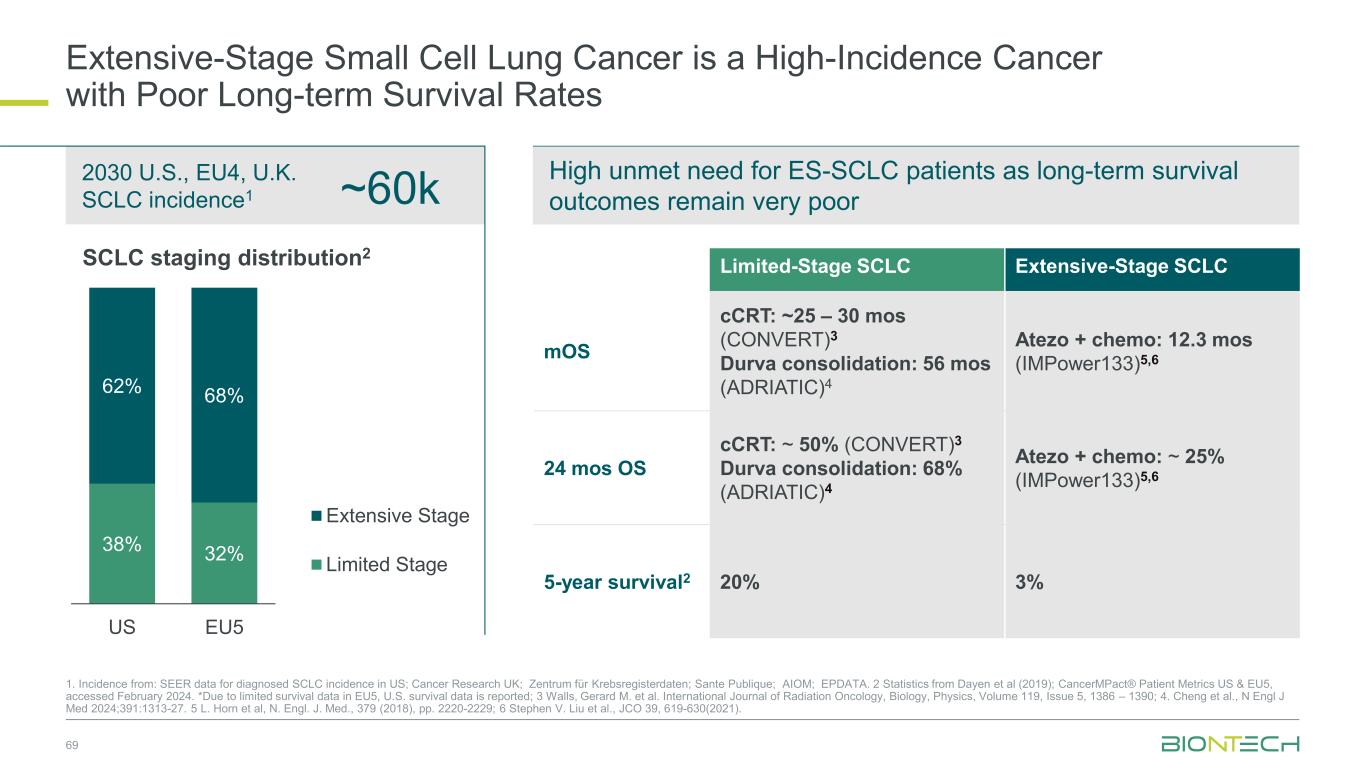
69 ~60k 2030 U.S., EU4, U.K. SCLC incidence1 1. Incidence from: SEER data for diagnosed SCLC incidence in US; Cancer Research UK; Zentrum für Krebsregisterdaten; Sante Publique; AIOM; EPDATA. 2 Statistics from Dayen et al (2019); CancerMPact® Patient Metrics US & EU5, accessed February 2024. *Due to limited survival data in EU5, U.S. survival data is reported; 3 Walls, Gerard M. et al. International Journal of Radiation Oncology, Biology, Physics, Volume 119, Issue 5, 1386 – 1390; 4. Cheng et al., N Engl J Med 2024;391:1313-27. 5 L. Horn et al, N. Engl. J. Med., 379 (2018), pp. 2220-2229; 6 Stephen V. Liu et al., JCO 39, 619-630(2021). Extensive-Stage Small Cell Lung Cancer is a High-Incidence Cancer with Poor Long-term Survival Rates 38% 32% 62% 68% US EU5 Extensive Stage Limited Stage SCLC staging distribution2 Limited-Stage SCLC Extensive-Stage SCLC mOS cCRT: ~25 – 30 mos (CONVERT)3 Durva consolidation: 56 mos (ADRIATIC)4 Atezo + chemo: 12.3 mos (IMPower133)5,6 24 mos OS cCRT: ~ 50% (CONVERT)3 Durva consolidation: 68% (ADRIATIC)4 Atezo + chemo: ~ 25% (IMPower133)5,6 5-year survival2 20% 3% High unmet need for ES-SCLC patients as long-term survival outcomes remain very poor
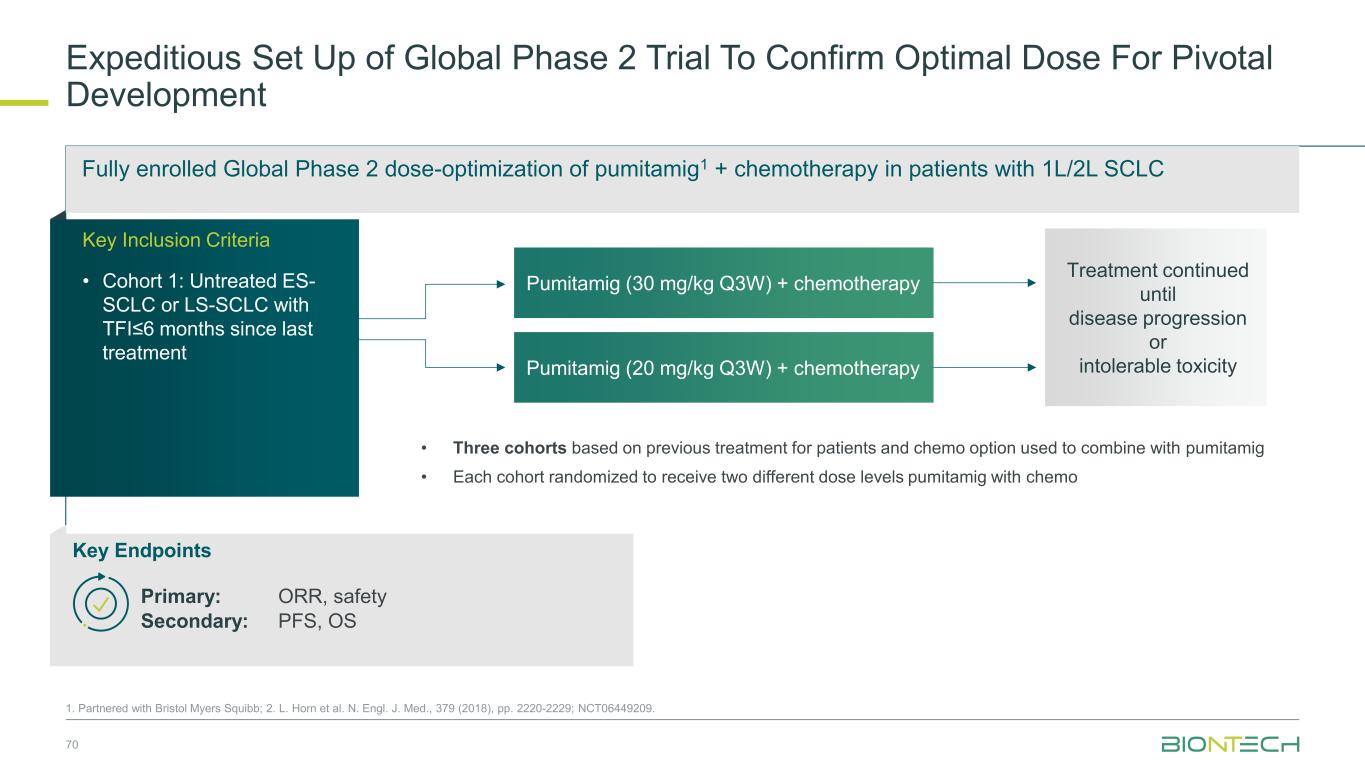
Fully enrolled Global Phase 2 dose-optimization of pumitamig1 + chemotherapy in patients with 1L/2L SCLC Expeditious Set Up of Global Phase 2 Trial To Confirm Optimal Dose For Pivotal Development 70 1. Partnered with Bristol Myers Squibb; 2. L. Horn et al. N. Engl. J. Med., 379 (2018), pp. 2220-2229; NCT06449209. Pumitamig (30 mg/kg Q3W) + chemotherapy Pumitamig (20 mg/kg Q3W) + chemotherapy • Three cohorts based on previous treatment for patients and chemo option used to combine with pumitamig • Each cohort randomized to receive two different dose levels pumitamig with chemo Treatment continued until disease progression or intolerable toxicity Key Endpoints Primary: ORR, safety Secondary: PFS, OS Key Inclusion Criteria • Cohort 1: Untreated ES- SCLC or LS-SCLC with TFI≤6 months since last treatment
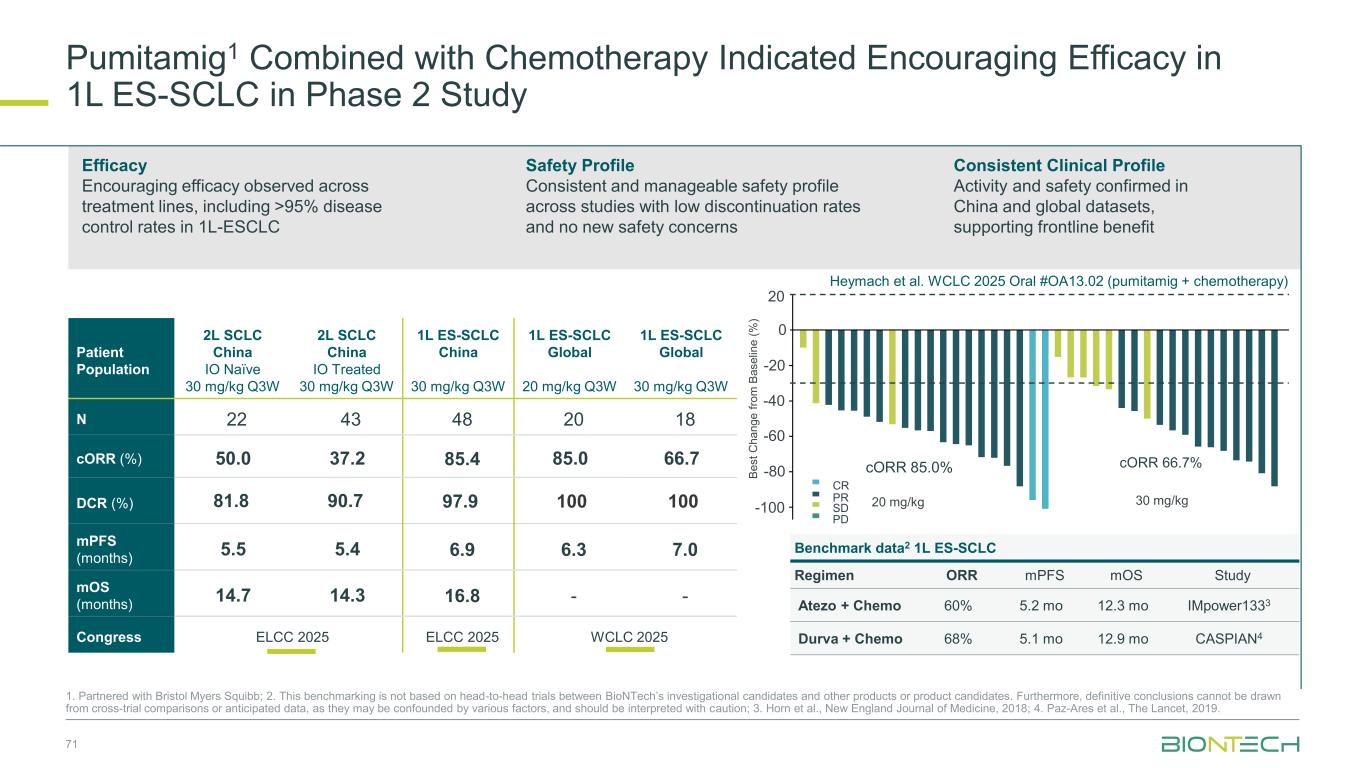
71 cORR 66.7%cORR 85.0% 20 0 -20 -40 -60 -80 -100 30 mg/kg20 mg/kg B e s t C h a n g e f ro m B a s e lin e ( % ) PD SD PR CR Patient Population 2L SCLC China IO Naïve 30 mg/kg Q3W 2L SCLC China IO Treated 30 mg/kg Q3W 1L ES-SCLC China 30 mg/kg Q3W 1L ES-SCLC Global 20 mg/kg Q3W 1L ES-SCLC Global 30 mg/kg Q3W N 22 43 48 20 18 cORR (%) 50.0 37.2 85.4 85.0 66.7 DCR (%) 81.8 90.7 97.9 100 100 mPFS (months) 5.5 5.4 6.9 6.3 7.0 mOS (months) 14.7 14.3 16.8 - - Congress ELCC 2025 ELCC 2025 WCLC 2025 Efficacy Encouraging efficacy observed across treatment lines, including >95% disease control rates in 1L-ESCLC Safety Profile Consistent and manageable safety profile across studies with low discontinuation rates and no new safety concerns Consistent Clinical Profile Activity and safety confirmed in China and global datasets, supporting frontline benefit Heymach et al. WCLC 2025 Oral #OA13.02 (pumitamig + chemotherapy) Pumitamig1 Combined with Chemotherapy Indicated Encouraging Efficacy in 1L ES-SCLC in Phase 2 Study 1. Partnered with Bristol Myers Squibb; 2. This benchmarking is not based on head-to-head trials between BioNTech’s investigational candidates and other products or product candidates. Furthermore, definitive conclusions cannot be drawn from cross-trial comparisons or anticipated data, as they may be confounded by various factors, and should be interpreted with caution; 3. Horn et al., New England Journal of Medicine, 2018; 4. Paz-Ares et al., The Lancet, 2019. Benchmark data2 1L ES-SCLC Regimen ORR mPFS mOS Study Atezo + Chemo 60% 5.2 mo 12.3 mo IMpower1333 Durva + Chemo 68% 5.1 mo 12.9 mo CASPIAN4
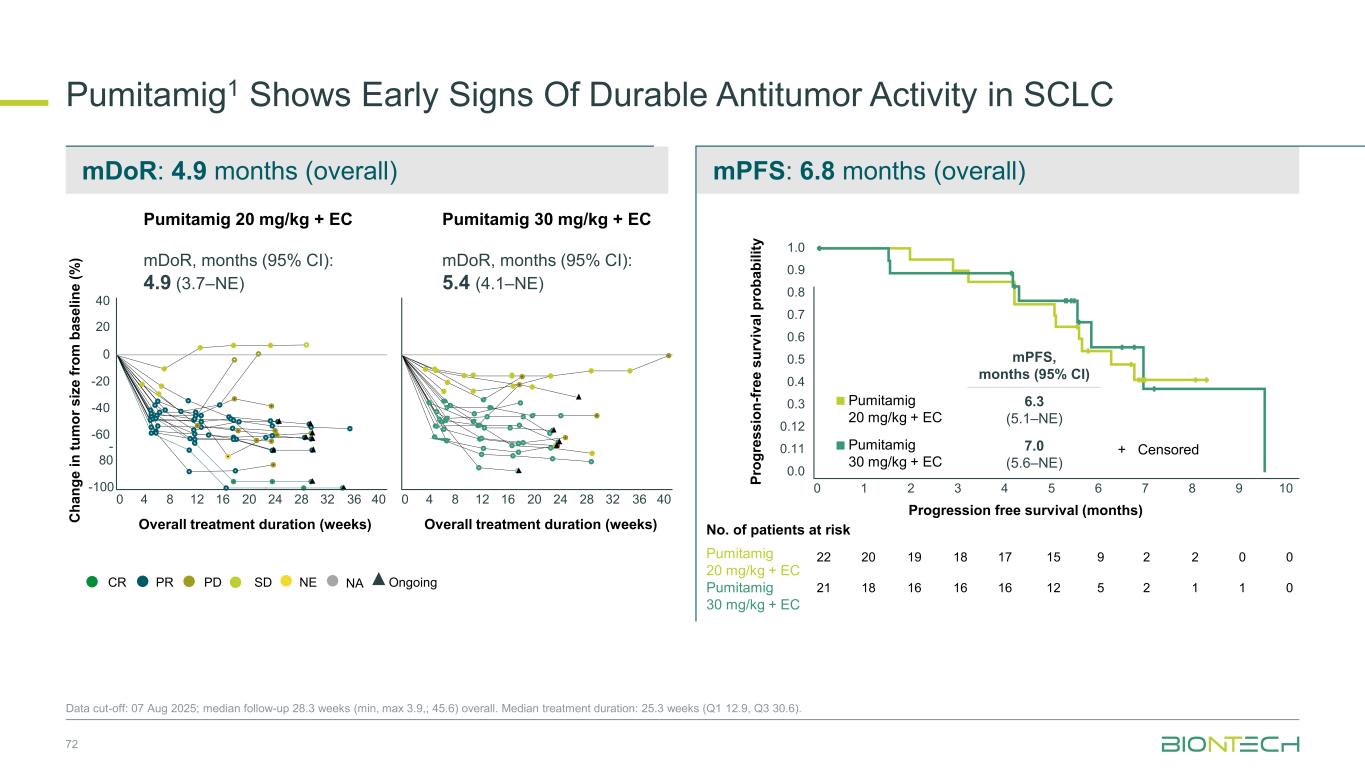
mDoR: 4.9 months (overall) mPFS: 6.8 months (overall) Pumitamig1 Shows Early Signs Of Durable Antitumor Activity in SCLC Data cut-off: 07 Aug 2025; median follow-up 28.3 weeks (min, max 3.9,; 45.6) overall. Median treatment duration: 25.3 weeks (Q1 12.9, Q3 30.6). Overall treatment duration (weeks) Overall treatment duration (weeks)C h a n g e i n t u m o r s iz e f ro m b a s e li n e ( % ) PDPRCR SD NE OngoingNA Pumitamig 20 mg/kg + EC mDoR, months (95% CI): 4.9 (3.7–NE) Pumitamig 30 mg/kg + EC mDoR, months (95% CI): 5.4 (4.1–NE) mPFS, months (95% CI) Pumitamig 20 mg/kg + EC 6.3 (5.1–NE) Pumitamig 30 mg/kg + EC 7.0 (5.6–NE) P ro g re s s io n -f re e s u rv iv a l p ro b a b il it y + Censored Progression free survival (months) Pumitamig 20 mg/kg + EC Pumitamig 30 mg/kg + EC No. of patients at risk 22 21 20 18 19 16 18 16 17 16 15 12 9 5 2 2 2 1 0 1 0 0 40 20 0 -20 -40 -60 - 80 -100 0 4 8 12 16 20 24 28 32 36 40 0 4 8 12 16 20 24 28 32 36 40 0 1 2 3 4 0.0 0.11 0.12 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 5 6 7 8 9 10 72
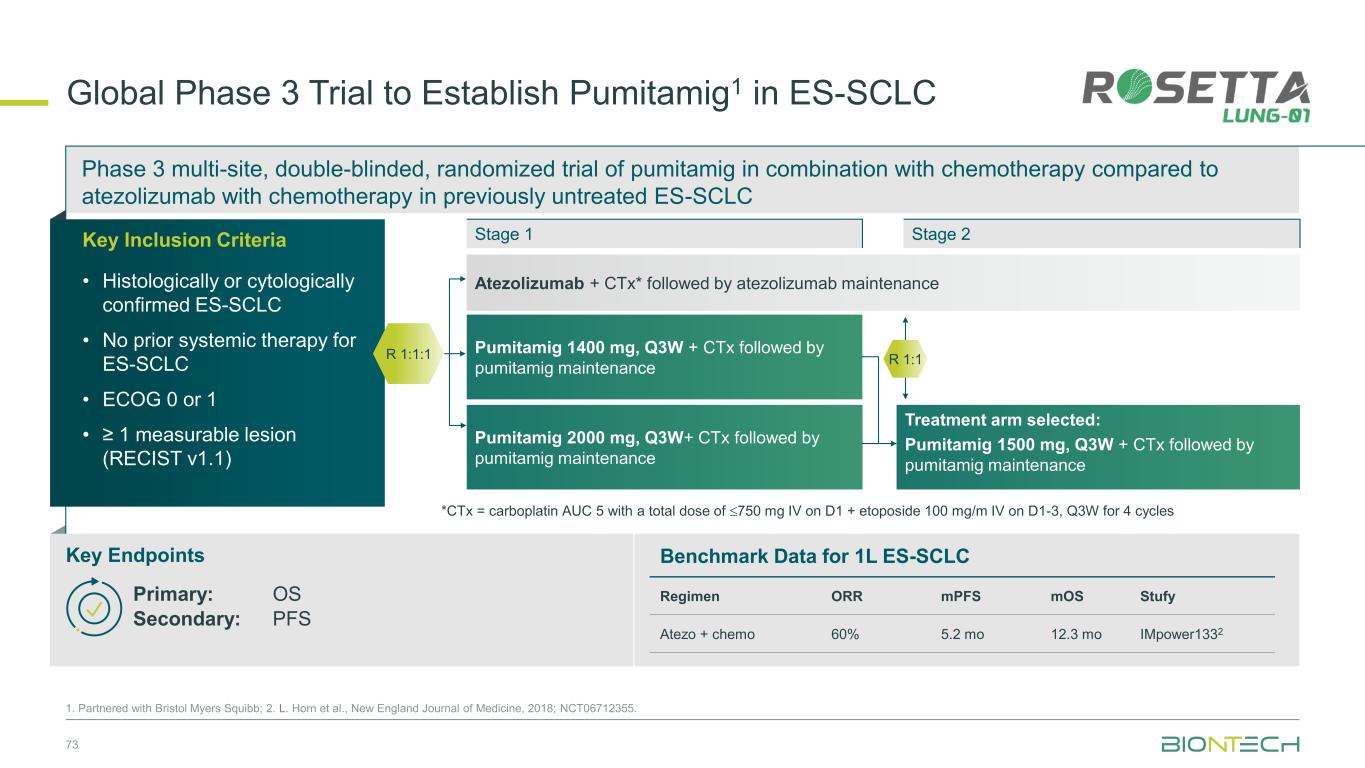
Phase 3 multi-site, double-blinded, randomized trial of pumitamig in combination with chemotherapy compared to atezolizumab with chemotherapy in previously untreated ES-SCLC 73 1. Partnered with Bristol Myers Squibb; 2. L. Horn et al., New England Journal of Medicine, 2018; NCT06712355. Global Phase 3 Trial to Establish Pumitamig1 in ES-SCLC Key Endpoints Key Inclusion Criteria • Histologically or cytologically confirmed ES-SCLC • No prior systemic therapy for ES-SCLC • ECOG 0 or 1 • ≥ 1 measurable lesion (RECIST v1.1) Stage 1 R 1:1:1 Benchmark Data for 1L ES-SCLC Regimen ORR mPFS mOS Stufy Atezo + chemo 60% 5.2 mo 12.3 mo IMpower1332 Stage 2 Primary: OS Secondary: PFS Atezolizumab + CTx* followed by atezolizumab maintenance Pumitamig 1400 mg, Q3W + CTx followed by pumitamig maintenance Treatment arm selected: Pumitamig 1500 mg, Q3W + CTx followed by pumitamig maintenance Pumitamig 2000 mg, Q3W+ CTx followed by pumitamig maintenance *CTx = carboplatin AUC 5 with a total dose of 750 mg IV on D1 + etoposide 100 mg/m IV on D1-3, Q3W for 4 cycles R 1:1
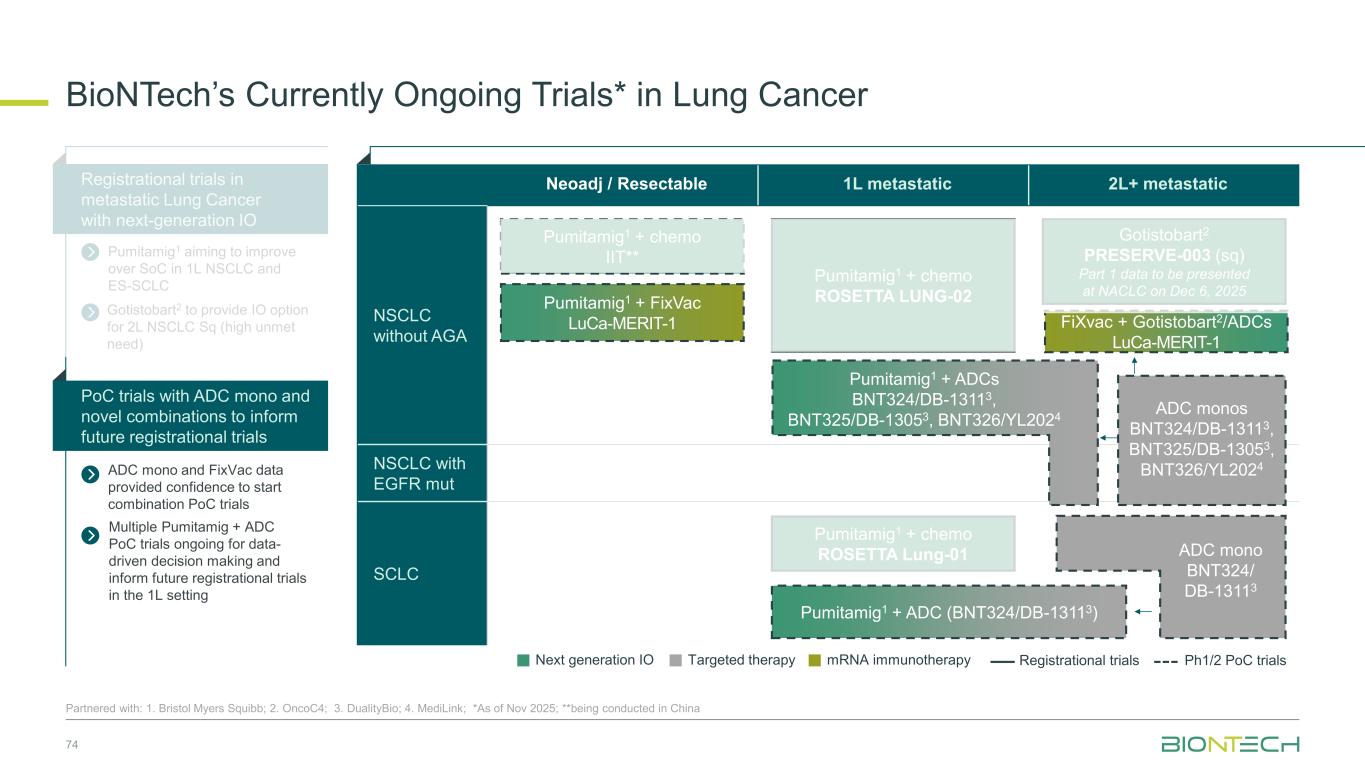
Neoadj / Resectable 1L metastatic 2L+ metastatic NSCLC without AGA NSCLC with EGFR mut SCLC BioNTech’s Currently Ongoing Trials* in Lung Cancer 74 Partnered with: 1. Bristol Myers Squibb; 2. OncoC4; 3. DualityBio; 4. MediLink; *As of Nov 2025; **being conducted in China ADC mono and FixVac data provided confidence to start combination PoC trials Registrational trials in metastatic Lung Cancer with next-generation IO Pumitamig1 aiming to improve over SoC in 1L NSCLC and ES-SCLC PoC trials with ADC mono and novel combinations to inform future registrational trials Next generation IO Targeted therapy mRNA immunotherapy Registrational trials Ph1/2 PoC trials Gotistobart2 PRESERVE-003 (sq) Part 1 data to be presented at NACLC on Dec 6, 2025 FiXvac + Gotistobart2/ADCs LuCa-MERIT-1 Pumitamig1 + chemo ROSETTA Lung-01 Pumitamig1 + chemo IIT** Pumitamig1 + chemo ROSETTA LUNG-02 ADC monos BNT324/DB-13113, BNT325/DB-13053, BNT326/YL2024 Pumitamig1 + FixVac LuCa-MERIT-1 Pumitamig1 + ADCs BNT324/DB-13113, BNT325/DB-13053, BNT326/YL2024 ADC mono BNT324/ DB-13113 Pumitamig1 + ADC (BNT324/DB-13113) Gotistobart2 to provide IO option for 2L NSCLC Sq (high unmet need) Multiple Pumitamig + ADC PoC trials ongoing for data- driven decision making and inform future registrational trials in the 1L setting
Anti-PD-L1/ Anti-VEGF Pumitamig4 Gotistobart5 Anti-CTLA-4 Immunomodulators Our Diverse Lung Cancer Pipeline 75 1. Synergistic potential; Partnered with 2 DualityBio; 3. MediLink; 4. Bristol Myers Squibb; 5. OncoC4. BNT116 FixVAC Off-the-shelf mRNA mRNA cancer immunotherapy BNT324/ DB-13112 Anti-B7H3 ADC BNT325/ DB-13052 Anti-TROP2 ADC BNT326/ YL2023 Anti-HER3 ADC Targeted therapies Space for curative approaches Synergy1 Synergy1 Targeted therapies Immunomodulators mRNA cancer immunotherapies Synergy1
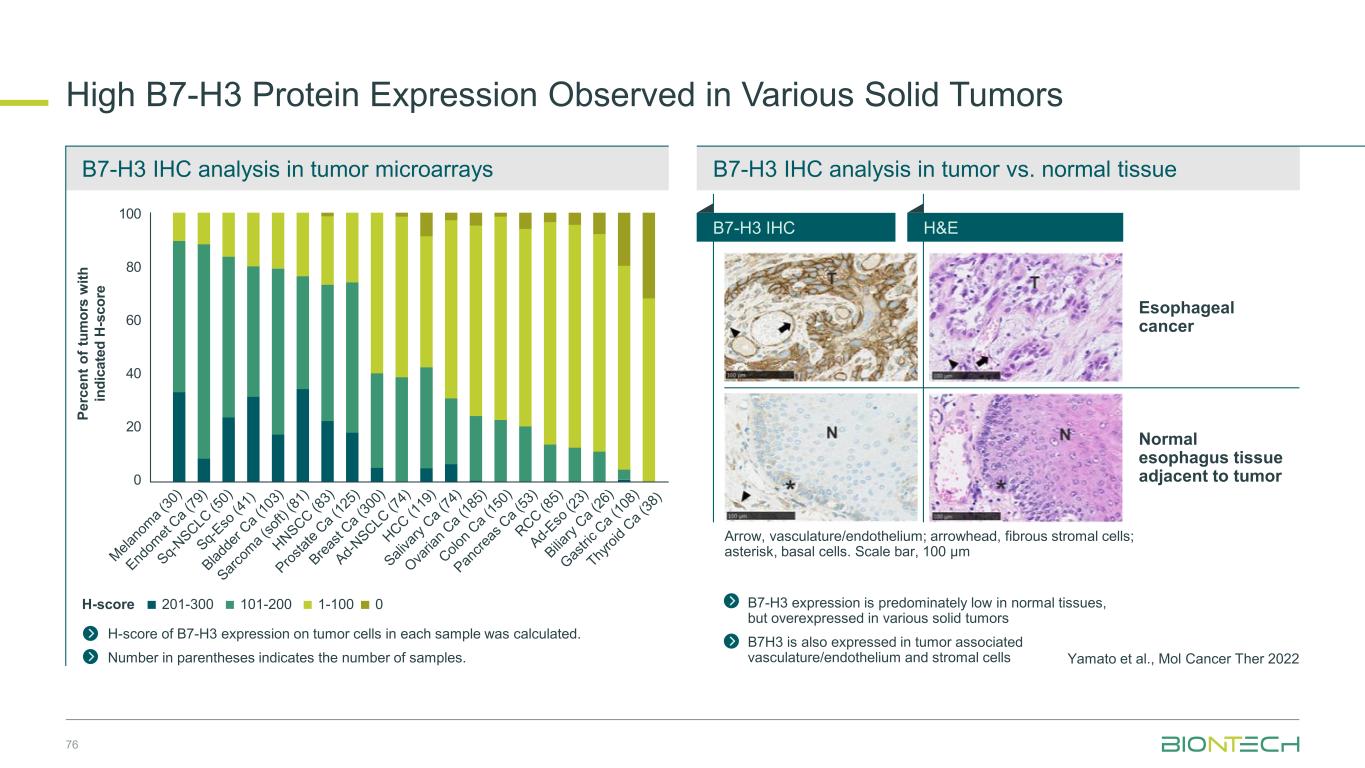
B7-H3 IHC analysis in tumor vs. normal tissueB7-H3 IHC analysis in tumor microarrays High B7-H3 Protein Expression Observed in Various Solid Tumors 76 Yamato et al., Mol Cancer Ther 2022 H-score of B7-H3 expression on tumor cells in each sample was calculated. Number in parentheses indicates the number of samples. Esophageal cancer Normal esophagus tissue adjacent to tumor B7-H3 expression is predominately low in normal tissues, but overexpressed in various solid tumors B7H3 is also expressed in tumor associated vasculature/endothelium and stromal cells Arrow, vasculature/endothelium; arrowhead, fibrous stromal cells; asterisk, basal cells. Scale bar, 100 μm P e rc e n t o f tu m o rs w it h in d ic a te d H -s c o re 1-100101-200201-300 0H-score 100 80 60 40 20 0 B7-H3 IHC H&E
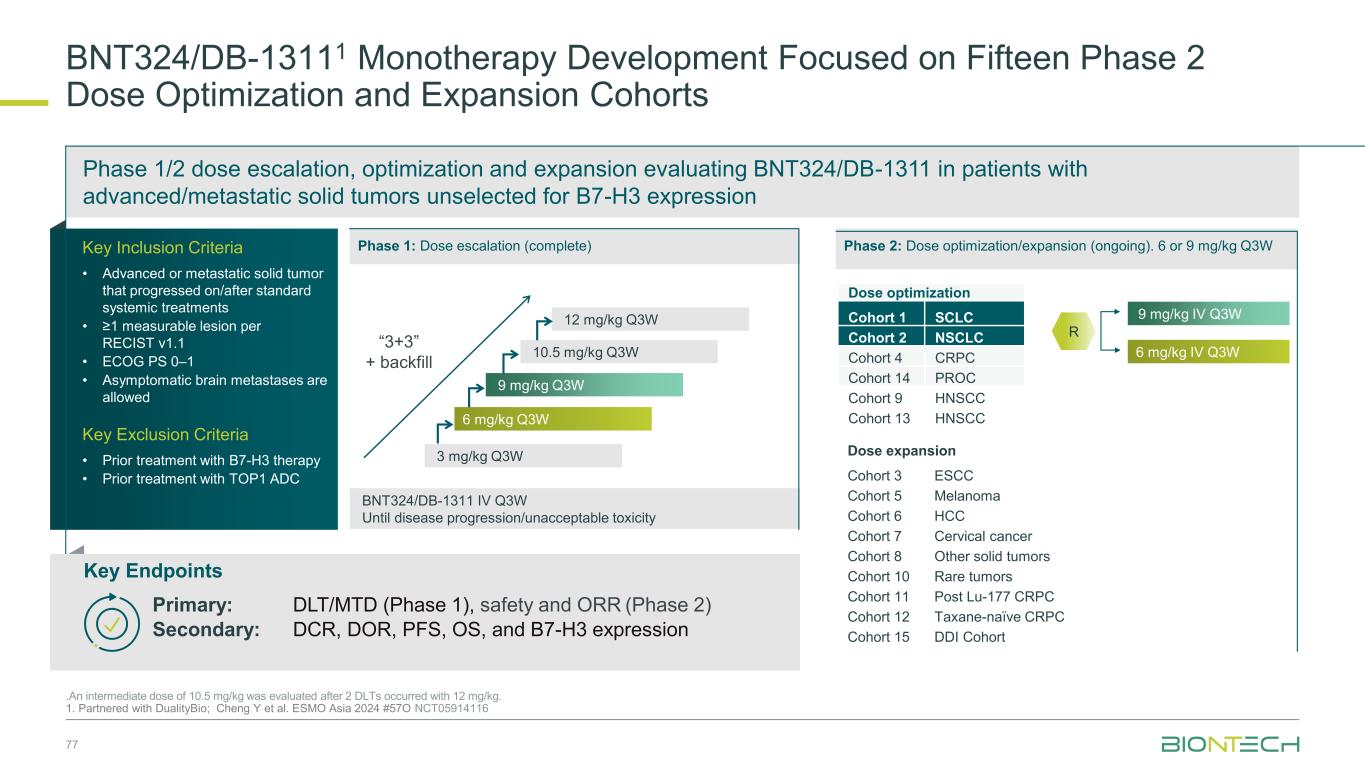
BNT324/DB-13111 Monotherapy Development Focused on Fifteen Phase 2 Dose Optimization and Expansion Cohorts .An intermediate dose of 10.5 mg/kg was evaluated after 2 DLTs occurred with 12 mg/kg. 1. Partnered with DualityBio; Cheng Y et al. ESMO Asia 2024 #57O NCT05914116 BNT324/DB-1311 IV Q3W Until disease progression/unacceptable toxicity 12 mg/kg Q3W 9 mg/kg Q3W 6 mg/kg Q3W 10.5 mg/kg Q3W 3 mg/kg Q3W “3+3” + backfill 6 mg/kg IV Q3W 9 mg/kg IV Q3W Key Inclusion Criteria • Advanced or metastatic solid tumor that progressed on/after standard systemic treatments • ≥1 measurable lesion per RECIST v1.1 • ECOG PS 0–1 • Asymptomatic brain metastases are allowed Key Exclusion Criteria • Prior treatment with B7-H3 therapy • Prior treatment with TOP1 ADC Phase 1/2 dose escalation, optimization and expansion evaluating BNT324/DB-1311 in patients with advanced/metastatic solid tumors unselected for B7-H3 expression R Dose optimization Cohort 1 SCLC Cohort 2 NSCLC Cohort 4 CRPC Cohort 14 PROC Cohort 9 HNSCC Cohort 13 HNSCC Phase 1: Dose escalation (complete) Phase 2: Dose optimization/expansion (ongoing). 6 or 9 mg/kg Q3W Dose expansion Cohort 3 ESCC Cohort 5 Melanoma Cohort 6 HCC Cohort 7 Cervical cancer Cohort 8 Other solid tumors Cohort 10 Rare tumors Cohort 11 Post Lu-177 CRPC Cohort 12 Taxane-naïve CRPC Cohort 15 DDI Cohort Key Endpoints Primary: DLT/MTD (Phase 1), safety and ORR (Phase 2) Secondary: DCR, DOR, PFS, OS, and B7-H3 expression 77
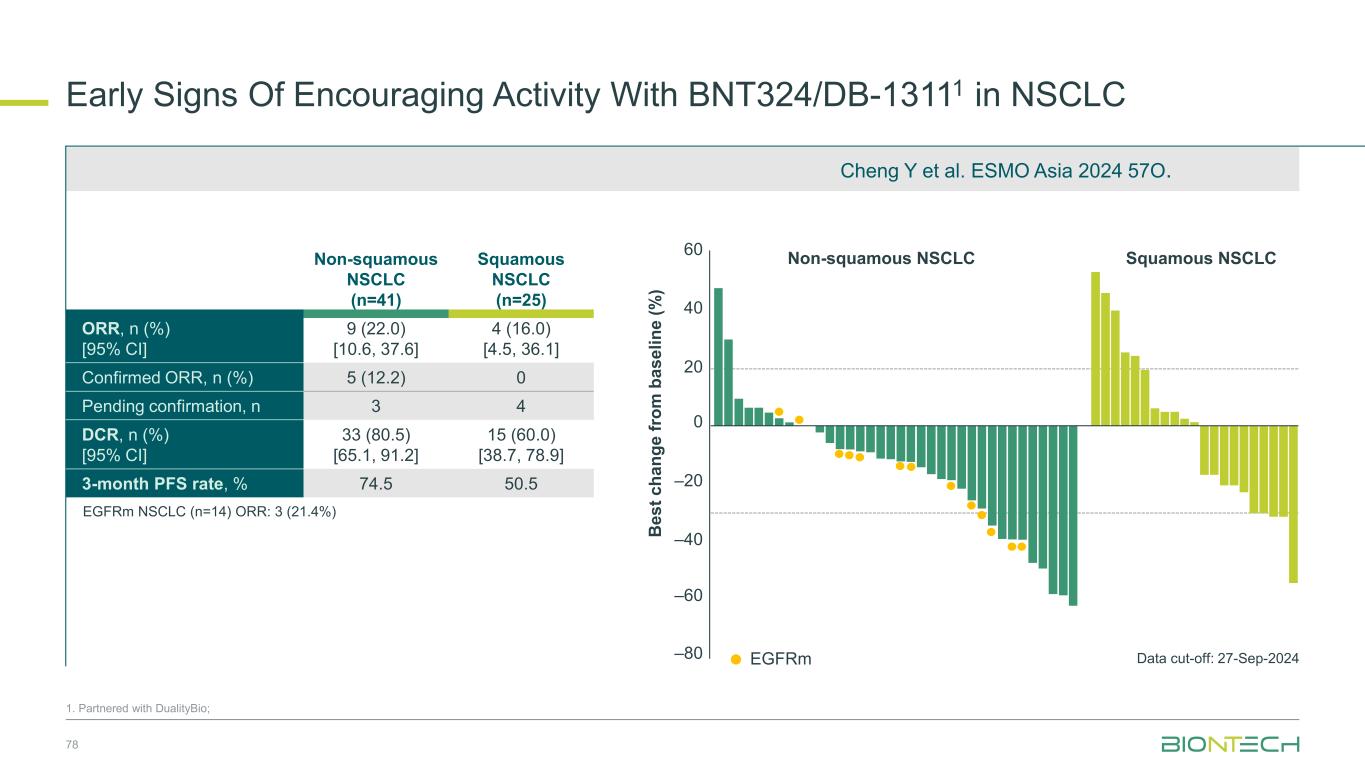
Early Signs Of Encouraging Activity With BNT324/DB-13111 in NSCLC 1. Partnered with DualityBio; EGFRm EGFRm NSCLC (n=14) ORR: 3 (21.4%) 60 40 –20 0 –40 –60 –80 20 Data cut-off: 27-Sep-2024 Non-squamous NSCLC Squamous NSCLC 78 Non-squamous NSCLC (n=41) Squamous NSCLC (n=25) ORR, n (%) [95% CI] 9 (22.0) [10.6, 37.6] 4 (16.0) [4.5, 36.1] Confirmed ORR, n (%) 5 (12.2) 0 Pending confirmation, n 3 4 DCR, n (%) [95% CI] 33 (80.5) [65.1, 91.2] 15 (60.0) [38.7, 78.9] 3-month PFS rate, % 74.5 50.5 B e s t c h a n g e f ro m b a s e li n e ( % ) Cheng Y et al. ESMO Asia 2024 57O.
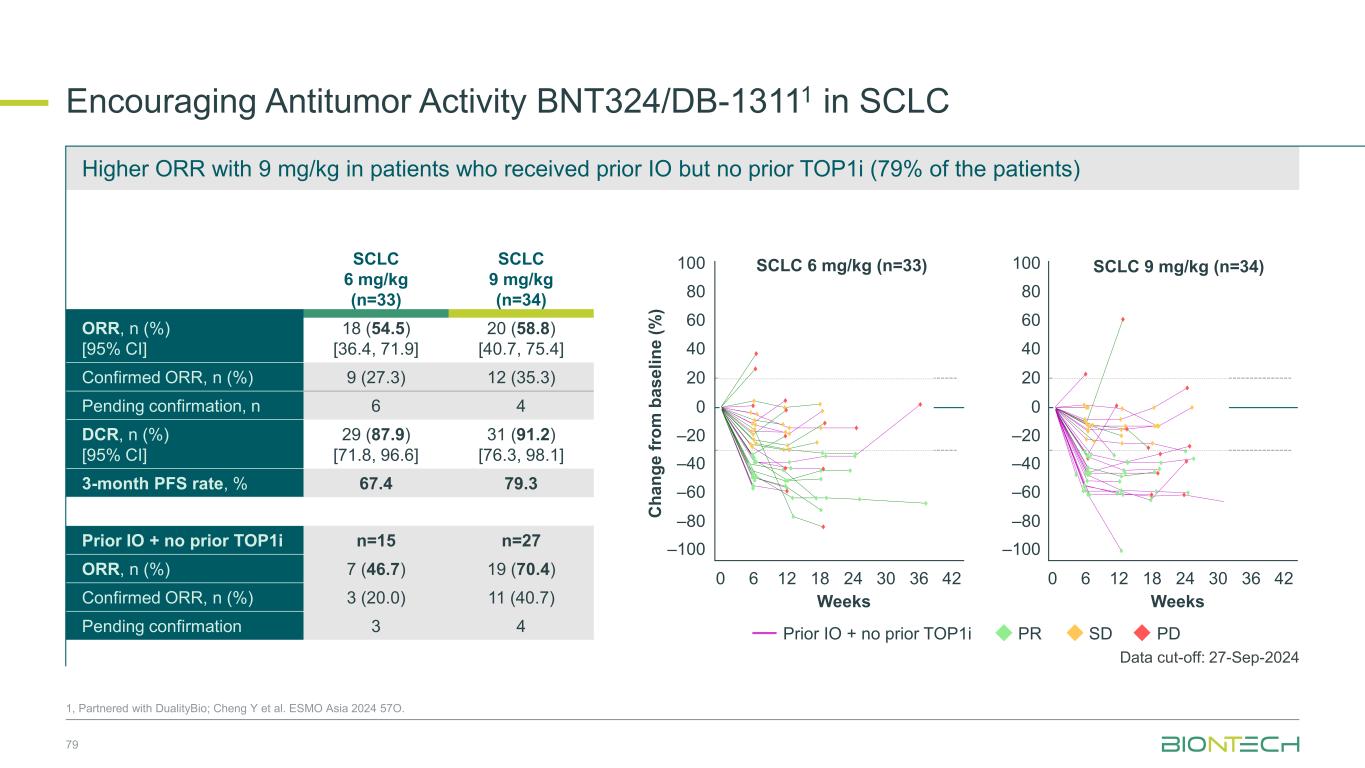
SCLC 6 mg/kg (n=33) SCLC 9 mg/kg (n=34) ORR, n (%) [95% CI] 18 (54.5) [36.4, 71.9] 20 (58.8) [40.7, 75.4] Confirmed ORR, n (%) 9 (27.3) 12 (35.3) Pending confirmation, n 6 4 DCR, n (%) [95% CI] 29 (87.9) [71.8, 96.6] 31 (91.2) [76.3, 98.1] 3-month PFS rate, % 67.4 79.3 Prior IO + no prior TOP1i n=15 n=27 ORR, n (%) 7 (46.7) 19 (70.4) Confirmed ORR, n (%) 3 (20.0) 11 (40.7) Pending confirmation 3 4 Encouraging Antitumor Activity BNT324/DB-13111 in SCLC 1, Partnered with DualityBio; Cheng Y et al. ESMO Asia 2024 57O. SCLC 6 mg/kg (n=33) SCLC 9 mg/kg (n=34) C h a n g e f ro m b a s e li n e ( % ) 100 80 60 40 20 0 –20 –40 –60 –80 –100 Weeks Weeks 0 6 12 18 24 30 36 420 6 12 18 24 30 36 42 100 80 60 40 20 0 –20 –40 –60 –80 –100 PR SD PDPrior IO + no prior TOP1i Data cut-off: 27-Sep-2024 79 Higher ORR with 9 mg/kg in patients who received prior IO but no prior TOP1i (79% of the patients) Program Name: P:\01_OngoingProjects\C_244_ONC_DB_1311_O_1001_SA\24EOP2OCT\Programs\TFL\Original\f_14_2_2_1.sas, Creation Date and Time: 19NOV2024 16:26 SCLC = small cell lung cancer. Weeks = (Date of tumor scan - first dose date + 1)/7. Footnote: Efficacy Analysis Set Figure 14.2.2.1.p12.eas.s1 Spider Plot for Percentage Change from Baseline on Sum of Target Lesion Diameter for SCLC (Dose Escalation + Expansion Part) 9 mg/kg Q3W, N=34 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 Weeks -100 -80 -60 -40 -20 0 20 40 60 80 100 P e r c e n t a g e C h a n g e f r o m B a s e l i n e ( % ) PDSDPRResponse: prior IO and no prior TOP1i 6 mg/kg Q3W, N=33 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 Weeks -100 -80 -60 -40 -20 0 20 40 60 80 100 P e r c e n t a g e C h a n g e f r o m B a s e l i n e ( % ) prior IO and no prior TOP1i Program Name: P:\01_OngoingProjects\C_244_ON DB_1311 O_100 _SA\24EOP2OCT\Programs\TFL\Original\f_14_2_2_1.sas, Creation Date and Tim : 19NOV2024 16: 6 SCLC = small cell ung cancer. Weeks = (Date of tumor scan - first dose date + 1)/7. Footnote: Efficacy Analysis Set Figure 14.2.2.1.p1 eas s Spider Plot for Percentag Change from Baseline on Sum of Target Lesion Diameter for SCLC (Dose Escalation + Expansion Part) 9 mg/kg Q3W, N=34 0 3 6 9 12 15 18 2 24 27 30 33 36 39 42 45 Weeks -100 -80 -60 -40 -20 0 20 40 60 80 100 P e r c e n t a g e C h a n g e f r o m B a s e l i n e ( % ) PDSDPRResponse: prior IO and no p ior TOP1i 6 mg/kg Q3W, N=33 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 Weeks -100 -80 -60 -40 -20 0 20 40 60 80 100 P e r c e n t a g e C h a n g e f r o m B a s e l i n e ( % ) prior IO and no prior TOP1i
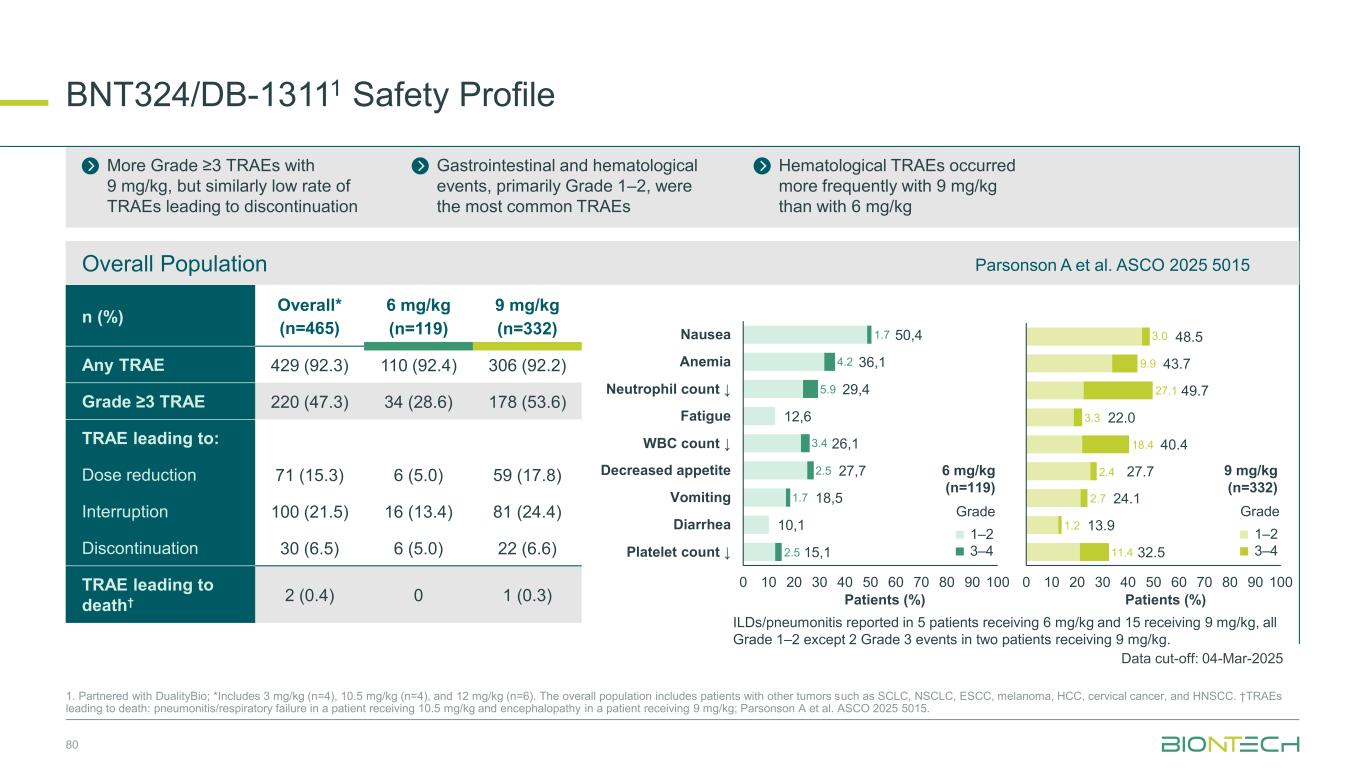
BNT324/DB-13111 Safety Profile 80 1. Partnered with DualityBio; *Includes 3 mg/kg (n=4), 10.5 mg/kg (n=4), and 12 mg/kg (n=6). The overall population includes patients with other tumors such as SCLC, NSCLC, ESCC, melanoma, HCC, cervical cancer, and HNSCC. †TRAEs leading to death: pneumonitis/respiratory failure in a patient receiving 10.5 mg/kg and encephalopathy in a patient receiving 9 mg/kg; Parsonson A et al. ASCO 2025 5015. n (%) Overall* (n=465) 6 mg/kg (n=119) 9 mg/kg (n=332) Any TRAE 429 (92.3) 110 (92.4) 306 (92.2) Grade ≥3 TRAE 220 (47.3) 34 (28.6) 178 (53.6) TRAE leading to: Dose reduction 71 (15.3) 6 (5.0) 59 (17.8) Interruption 100 (21.5) 16 (13.4) 81 (24.4) Discontinuation 30 (6.5) 6 (5.0) 22 (6.6) TRAE leading to death† 2 (0.4) 0 1 (0.3) More Grade ≥3 TRAEs with 9 mg/kg, but similarly low rate of TRAEs leading to discontinuation Gastrointestinal and hematological events, primarily Grade 1–2, were the most common TRAEs Hematological TRAEs occurred more frequently with 9 mg/kg than with 6 mg/kg 48.5 43.7 49.7 22.0 40.4 27.7 24.1 13.9 32.5 0 10 20 30 40 50 60 70 80 90 100 50,4 36,1 29,4 12,6 26,1 27,7 18,5 10,1 15,1 0 10 20 30 40 50 60 70 80 90 100 Nausea Anemia Neutrophil count ↓ Fatigue WBC count ↓ Decreased appetite Vomiting Diarrhea Platelet count ↓ 1.7 4.2 5.9 3.4 2.5 1.7 2.5 11.4 1.2 2.7 18.4 3.3 2.4 27.1 9.9 3.0 1–2 3–4 Grade 1–2 3–4 Grade ILDs/pneumonitis reported in 5 patients receiving 6 mg/kg and 15 receiving 9 mg/kg, all Grade 1–2 except 2 Grade 3 events in two patients receiving 9 mg/kg. Overall Population Patients (%) Patients (%) 6 mg/kg (n=119) 9 mg/kg (n=332) Data cut-off: 04-Mar-2025 Parsonson A et al. ASCO 2025 5015
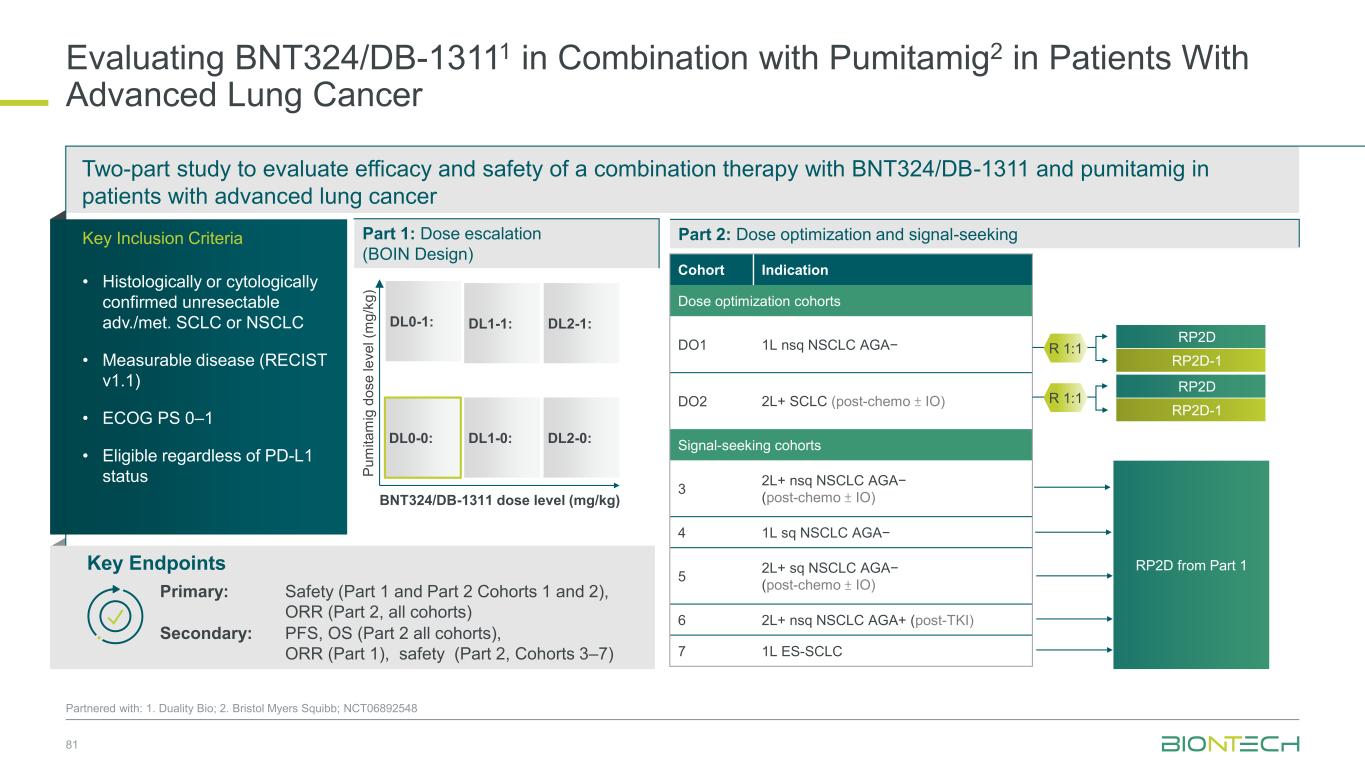
Two-part study to evaluate efficacy and safety of a combination therapy with BNT324/DB-1311 and pumitamig in patients with advanced lung cancer 81 Partnered with: 1. Duality Bio; 2. Bristol Myers Squibb; NCT06892548 Evaluating BNT324/DB-13111 in Combination with Pumitamig2 in Patients With Advanced Lung Cancer Key Endpoints Key Inclusion Criteria • Histologically or cytologically confirmed unresectable adv./met. SCLC or NSCLC • Measurable disease (RECIST v1.1) • ECOG PS 0–1 • Eligible regardless of PD-L1 status Part 2: Dose optimization and signal-seeking Primary: Safety (Part 1 and Part 2 Cohorts 1 and 2), ORR (Part 2, all cohorts) Secondary: PFS, OS (Part 2 all cohorts), ORR (Part 1), safety (Part 2, Cohorts 3–7) RP2D from Part 1 Part 1: Dose escalation (BOIN Design) DL0-0: DL1-0: DL1-1: DL2-0: DL0-1: DL2-1: BNT324/DB-1311 dose level (mg/kg) P u m it a m ig d o s e l e v e l (m g /k g ) Cohort Indication Dose optimization cohorts DO1 1L nsq NSCLC AGA− DO2 2L+ SCLC (post-chemo ± IO) Signal-seeking cohorts 3 2L+ nsq NSCLC AGA− (post-chemo ± IO) 4 1L sq NSCLC AGA− 5 2L+ sq NSCLC AGA− (post-chemo ± IO) 6 2L+ nsq NSCLC AGA+ (post-TKI) 7 1L ES-SCLC RP2D-1 RP2D R 1:1 RP2D-1 RP2D R 1:1
Anti-PD-L1/ Anti-VEGF Pumitamig4 Gotistobart5 Anti-CTLA-4 Immunomodulators Our Diverse Lung Cancer Pipeline 82 1. Synergistic potential; Partnered with 2 DualityBio; 3. MediLink; 4. Bristol Myers Squibb; 5. OncoC4. BNT116 FixVAC Off-the-shelf mRNA mRNA cancer immunotherapy BNT324/ DB-13112 Anti-B7H3 ADC BNT325/ DB-13052 Anti-TROP2 ADC BNT326/ YL2023 Anti-HER3 ADC Targeted therapies Space for curative approaches Synergy1 Synergy1 Targeted therapies Immunomodulators mRNA cancer immunotherapies Synergy1
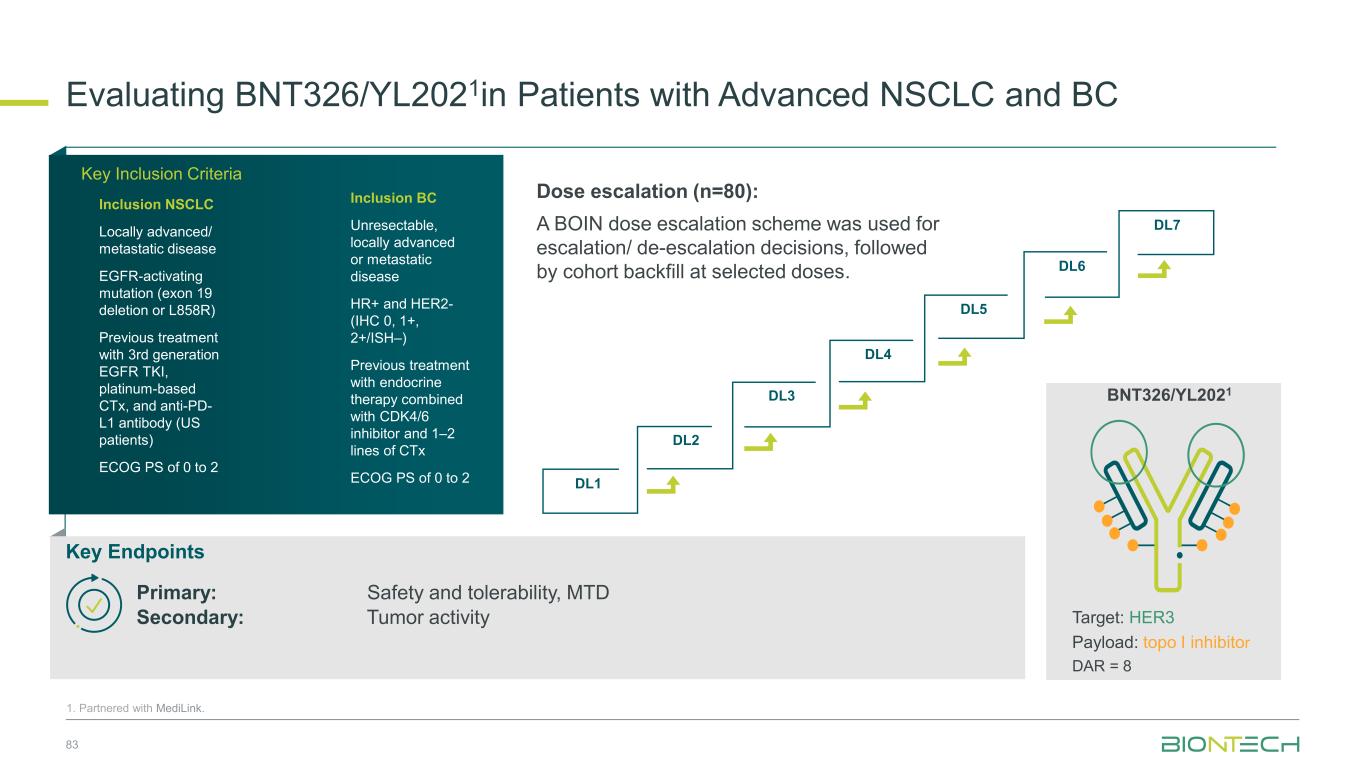
DL1 DL2 DL3 DL4 DL5 DL6 DL7 Dose escalation (n=80): A BOIN dose escalation scheme was used for escalation/ de-escalation decisions, followed by cohort backfill at selected doses. Evaluating BNT326/YL2021in Patients with Advanced NSCLC and BC 83 1. Partnered with MediLink. Key Inclusion Criteria Inclusion NSCLC Locally advanced/ metastatic disease EGFR-activating mutation (exon 19 deletion or L858R) Previous treatment with 3rd generation EGFR TKI, platinum-based CTx, and anti-PD- L1 antibody (US patients) ECOG PS of 0 to 2 Inclusion BC Unresectable, locally advanced or metastatic disease HR+ and HER2- (IHC 0, 1+, 2+/ISH–) Previous treatment with endocrine therapy combined with CDK4/6 inhibitor and 1–2 lines of CTx ECOG PS of 0 to 2 Key Endpoints Primary: Safety and tolerability, MTD Secondary: Tumor activity Target: HER3 Payload: topo I inhibitor DAR = 8 BNT326/YL2021
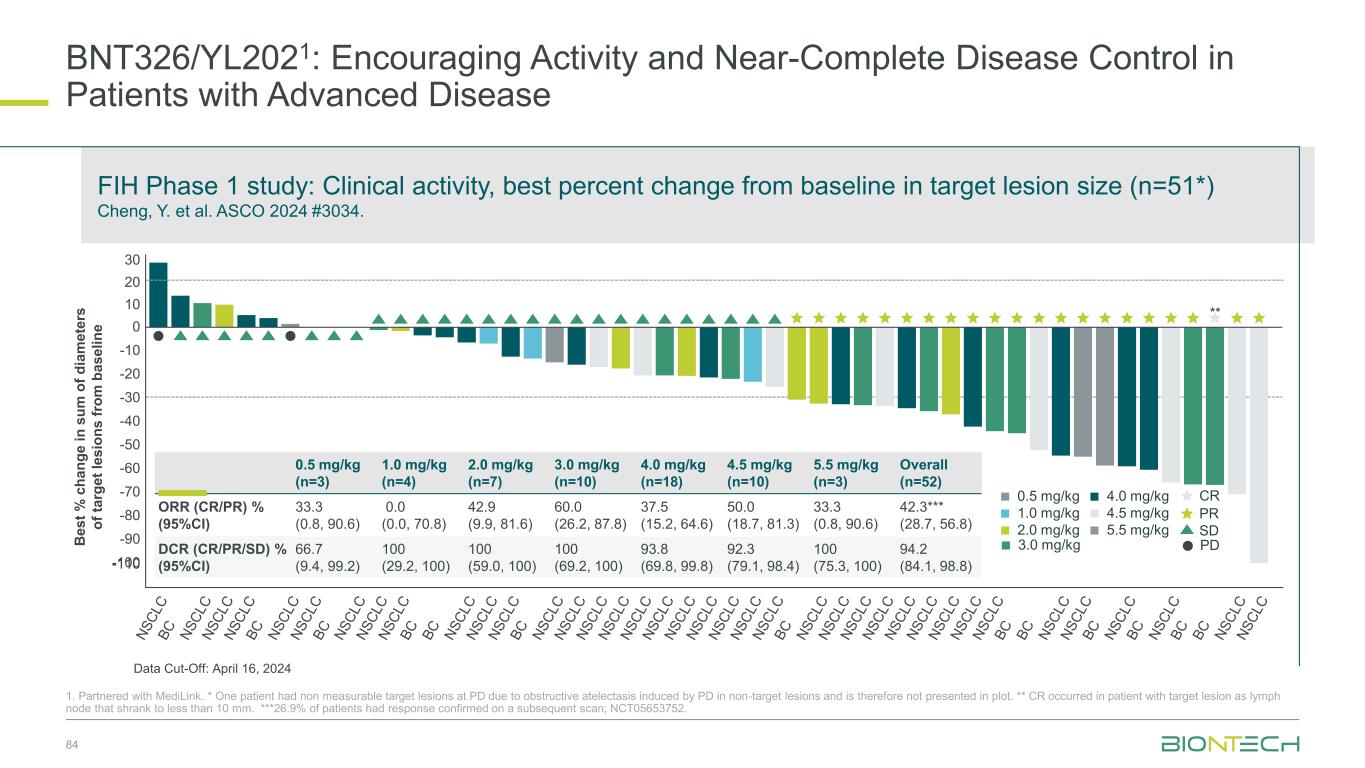
BNT326/YL2021: Encouraging Activity and Near-Complete Disease Control in Patients with Advanced Disease 84 1. Partnered with MediLink. * One patient had non measurable target lesions at PD due to obstructive atelectasis induced by PD in non-target lesions and is therefore not presented in plot. ** CR occurred in patient with target lesion as lymph node that shrank to less than 10 mm. ***26.9% of patients had response confirmed on a subsequent scan; NCT05653752. FIH Phase 1 study: Clinical activity, best percent change from baseline in target lesion size (n=51*) Cheng, Y. et al. ASCO 2024 #3034. B e s t % c h a n g e i n s u m o f d ia m e te rs o f ta rg e t le s io n s f ro m b a s e li n e 30 20 10 0 -20 -30 -40 -50 -60 -70 -90 -80 -100 0.5 mg/kg (n=3) 1.0 mg/kg (n=4) 2.0 mg/kg (n=7) 3.0 mg/kg (n=10) 4.0 mg/kg (n=18) 4.5 mg/kg (n=10) 5.5 mg/kg (n=3) Overall (n=52) ORR (CR/PR) % (95%CI) 33.3 (0.8, 90.6) 0.0 (0.0, 70.8) 42.9 (9.9, 81.6) 60.0 (26.2, 87.8) 37.5 (15.2, 64.6) 50.0 (18.7, 81.3) 33.3 (0.8, 90.6) 42.3*** (28.7, 56.8) DCR (CR/PR/SD) % (95%CI) 66.7 (9.4, 99.2) 100 (29.2, 100) 100 (59.0, 100) 100 (69.2, 100) 93.8 (69.8, 99.8) 92.3 (79.1, 98.4) 100 (75.3, 100) 94.2 (84.1, 98.8) -10 4.0 mg/kg 2.0 mg/kg 1.0 mg/kg 0.5 mg/kg 4.5 mg/kg 5.5 mg/kg SD PR CR ** 1 PD3.0 mg/kg Data Cut-Off: April 16, 2024
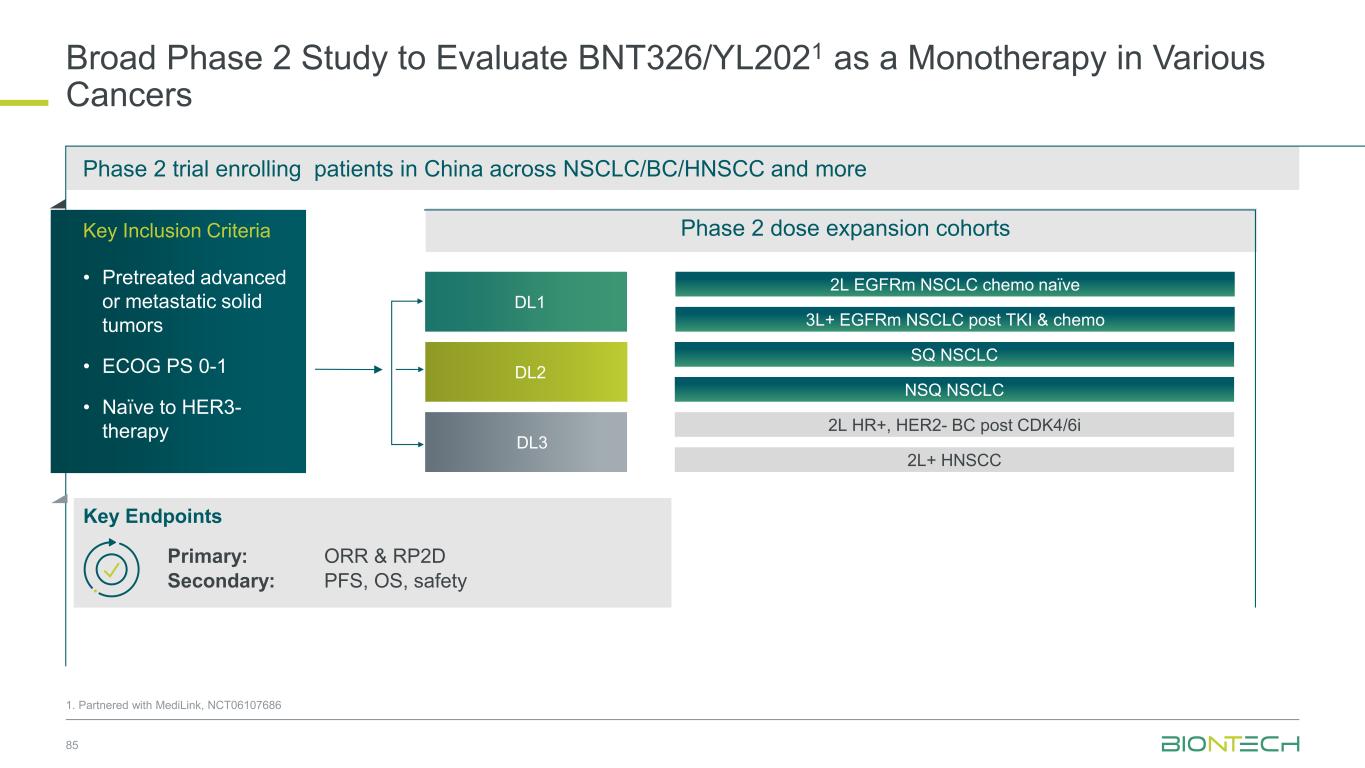
Broad Phase 2 Study to Evaluate BNT326/YL2021 as a Monotherapy in Various Cancers 85 Phase 2 trial enrolling patients in China across NSCLC/BC/HNSCC and more 3L+ EGFRm NSCLC post TKI & chemo 2L EGFRm NSCLC chemo naïve 2L HR+, HER2- BC post CDK4/6i Key Inclusion Criteria • Pretreated advanced or metastatic solid tumors • ECOG PS 0-1 • Naïve to HER3- therapy Key Endpoints 2L+ HNSCC SQ NSCLC NSQ NSCLC DL2 DL3 DL1 Phase 2 dose expansion cohorts 1. Partnered with MediLink, NCT06107686 Primary: ORR & RP2D Secondary: PFS, OS, safety
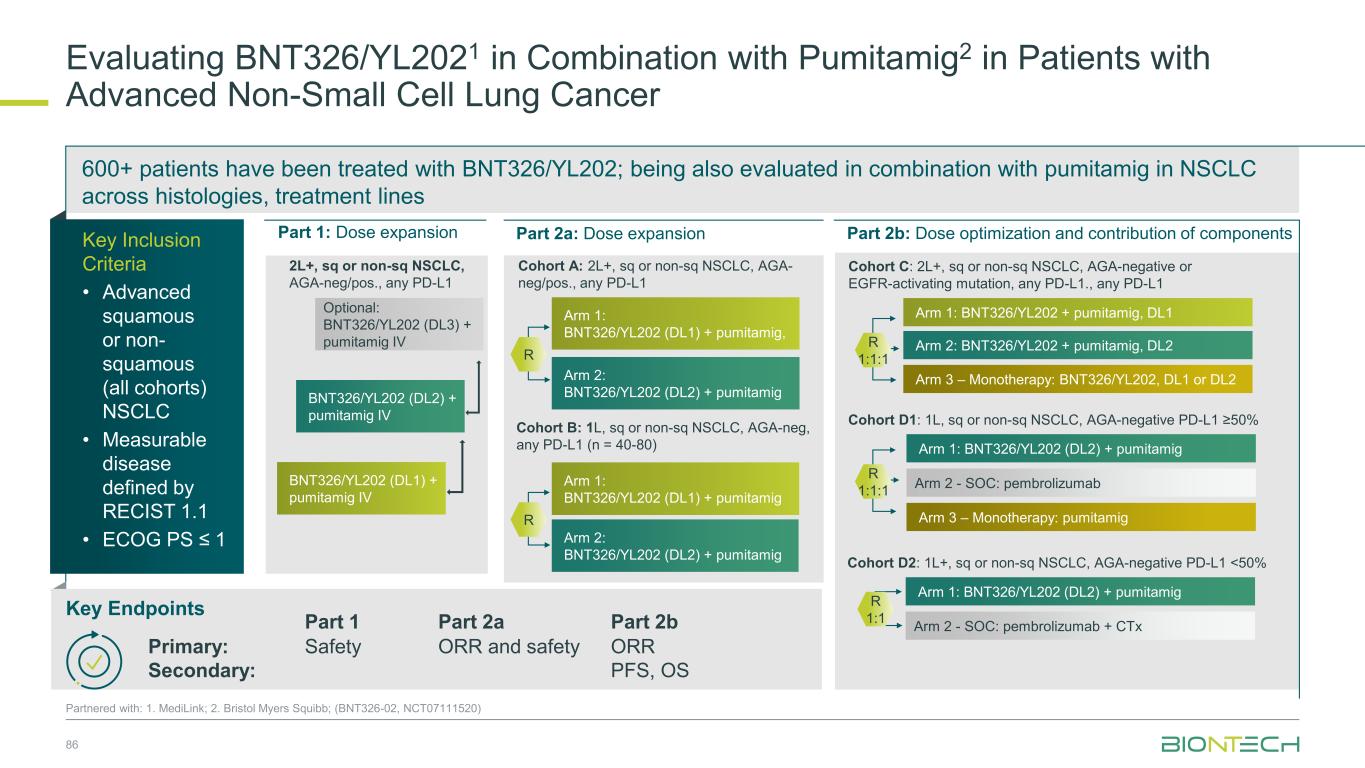
600+ patients have been treated with BNT326/YL202; being also evaluated in combination with pumitamig in NSCLC across histologies, treatment lines 86 Partnered with: 1. MediLink; 2. Bristol Myers Squibb; (BNT326-02, NCT07111520) Evaluating BNT326/YL2021 in Combination with Pumitamig2 in Patients with Advanced Non-Small Cell Lung Cancer Part 1: Dose expansion Part 2a: Dose expansion Part 2b: Dose optimization and contribution of components Optional: BNT326/YL202 (DL3) + pumitamig IV BNT326/YL202 (DL2) + pumitamig IV BNT326/YL202 (DL1) + pumitamig IV 2L+, sq or non-sq NSCLC, AGA-neg/pos., any PD-L1 Cohort B: 1L, sq or non-sq NSCLC, AGA-neg, any PD-L1 (n = 40-80) Cohort C: 2L+, sq or non-sq NSCLC, AGA-negative or EGFR-activating mutation, any PD-L1., any PD-L1 Cohort A: 2L+, sq or non-sq NSCLC, AGA- neg/pos., any PD-L1 Arm 1: BNT326/YL202 (DL1) + pumitamig, R Arm 2: BNT326/YL202 (DL2) + pumitamig Arm 2: BNT326/YL202 + pumitamig, DL2 Arm 1: BNT326/YL202 + pumitamig, DL1 Arm 3 – Monotherapy: BNT326/YL202, DL1 or DL2 R 1:1:1 Arm 2 - SOC: pembrolizumab Arm 1: BNT326/YL202 (DL2) + pumitamig Arm 3 – Monotherapy: pumitamig Cohort D1: 1L, sq or non-sq NSCLC, AGA-negative PD-L1 ≥50% Arm 2 - SOC: pembrolizumab + CTx Arm 1: BNT326/YL202 (DL2) + pumitamig R 1:1 Cohort D2: 1L+, sq or non-sq NSCLC, AGA-negative PD-L1 <50% R Arm 1: BNT326/YL202 (DL1) + pumitamig Arm 2: BNT326/YL202 (DL2) + pumitamig Key Inclusion Criteria • Advanced squamous or non- squamous (all cohorts) NSCLC • Measurable disease defined by RECIST 1.1 • ECOG PS ≤ 1 Key Endpoints Part 1 Part 2a Part 2b Primary: Secondary: Safety ORR and safety ORR PFS, OS R 1:1:1
BNT324/ DB-13112 Anti-B7H3 ADC BNT325/ DB-13052 Anti-TROP2 ADC BNT326/ YL2023 Anti-HER3 ADC Anti-PD-L1/ Anti-VEGF Pumitamig4 Gotistobart5 Anti-CTLA-4 Targeted therapies Our Diverse Lung Cancer Pipeline 87 1. Synergistic potential; Partnered with 2 DualityBio; 3. MediLink; 4. Bristol Myers Squibb; 5. OncoC4. Immunomodulators Targeted therapies Synergy1 BNT116 FixVAC Off-the-shelf mRNA mRNA cancer immunotherapy Immunomodulators Space for curative approaches Synergy1 mRNA cancer immunotherapies Synergy1
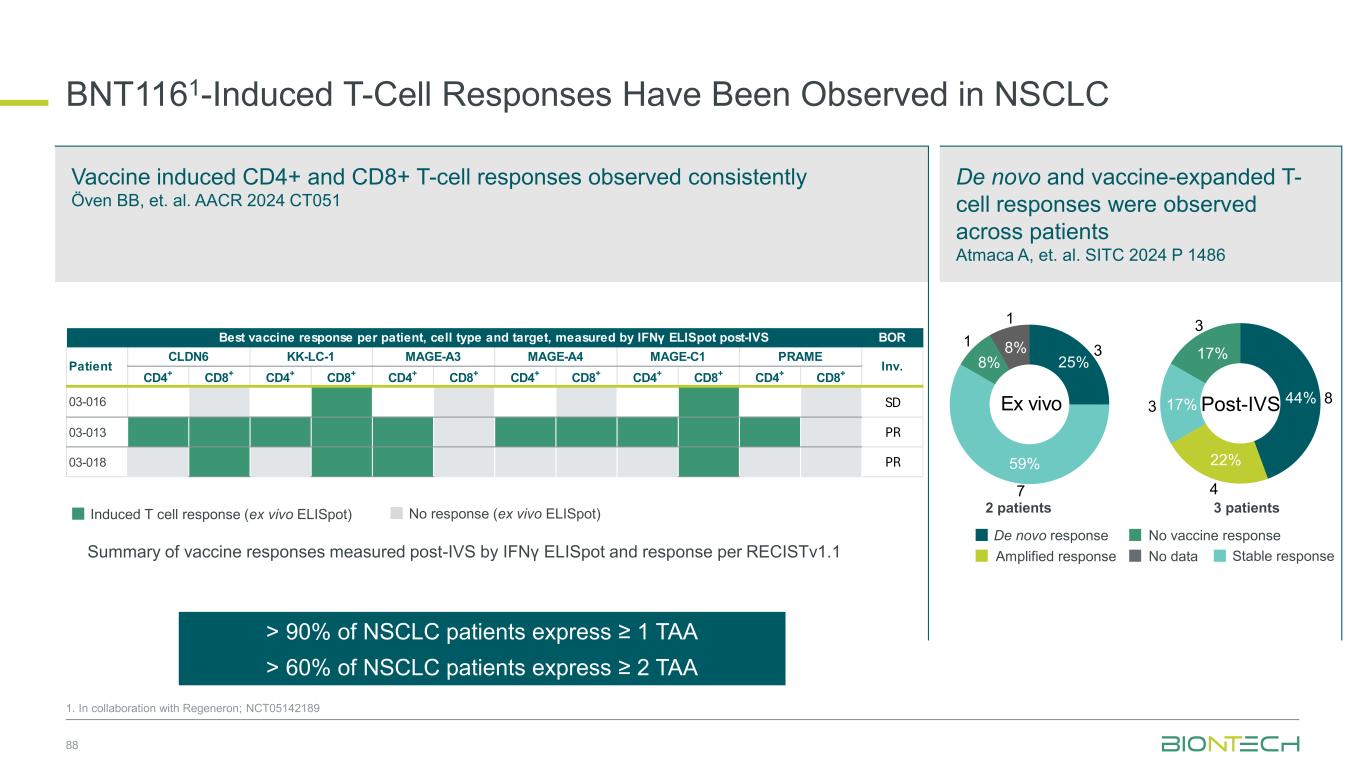
Induced T cell response (ex vivo ELISpot) De novo and vaccine-expanded T- cell responses were observed across patients Atmaca A, et. al. SITC 2024 P 1486 BNT1161-Induced T-Cell Responses Have Been Observed in NSCLC 88 Vaccine induced CD4+ and CD8+ T-cell responses observed consistently Öven BB, et. al. AACR 2024 CT051 No response (ex vivo ELISpot) BOR CD4+ CD8+ CD4+ CD8+ CD4+ CD8+ CD4+ CD8+ CD4+ CD8+ CD4+ CD8+ 03-016 SD 03-013 PR 03-018 PR Inv. Best vaccine response per patient, cell type and target, measured by IFNγ ELISpot post-IVS Patient CLDN6 KK-LC-1 MAGE-A3 MAGE-A4 MAGE-C1 PRAME De novo response Amplified response No vaccine response No data 2 patients 3 patients De novo response Stable response No vaccine response No data Ex vivo 8% 8% 25% 59% 1 1 3 7 De novo response Stable response No vaccine response Post-IVS17% 17% 44% 22% 3 3 8 4 Amplified response Stable responseSummary of vaccine responses measured post-IVS by IFNγ ELISpot and response per RECISTv1.1 1. In collaboration with Regeneron; NCT05142189 > 90% of NSCLC patients express ≥ 1 TAA > 60% of NSCLC patients express ≥ 2 TAA
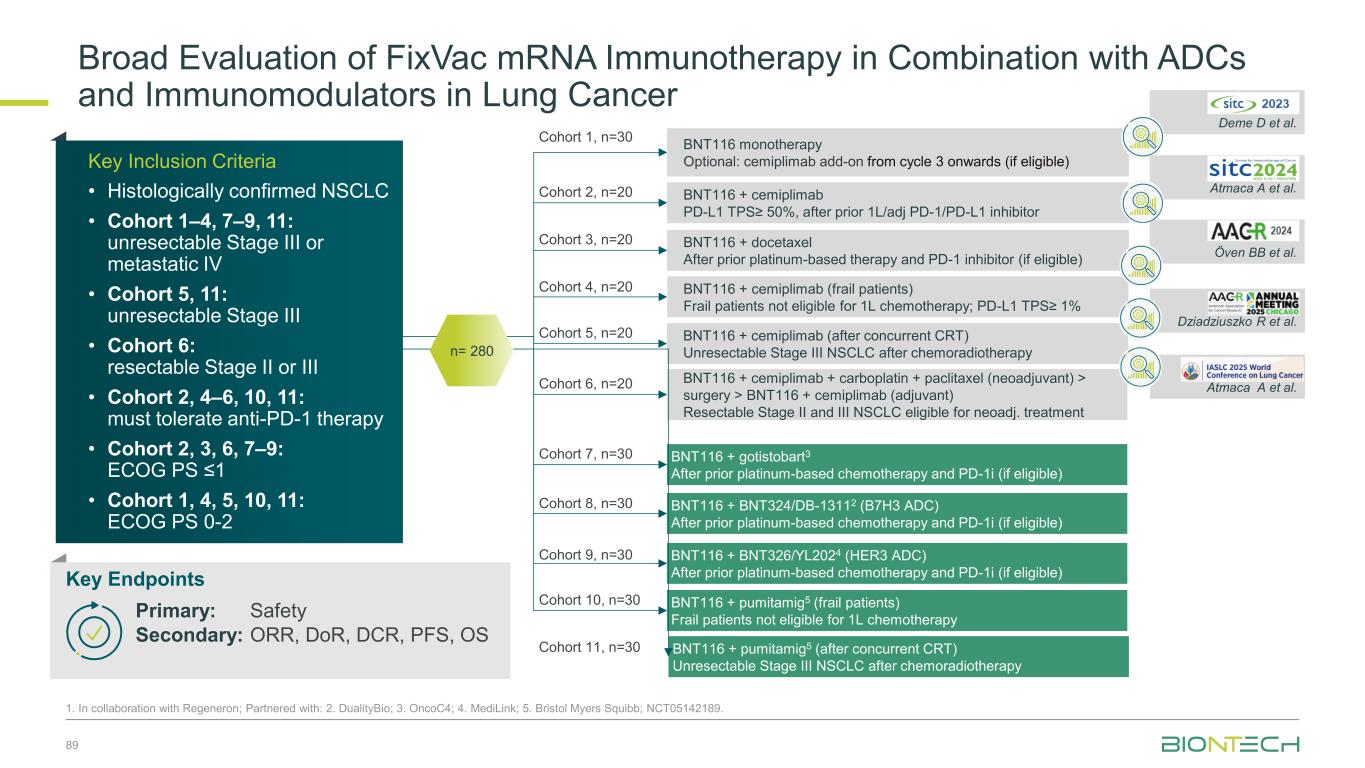
Broad Evaluation of FixVac mRNA Immunotherapy in Combination with ADCs and Immunomodulators in Lung Cancer 1. In collaboration with Regeneron; Partnered with: 2. DualityBio; 3. OncoC4; 4. MediLink; 5. Bristol Myers Squibb; NCT05142189. Cohort 1, n=30 BNT116 + cemiplimab (frail patients) Frail patients not eligible for 1L chemotherapy; PD-L1 TPS≥ 1% BNT116 monotherapy Optional: cemiplimab add-on from cycle 3 onwards (if eligible) BNT116 + cemiplimab PD-L1 TPS≥ 50%, after prior 1L/adj PD-1/PD-L1 inhibitor BNT116 + docetaxel After prior platinum-based therapy and PD-1 inhibitor (if eligible) BNT116 + cemiplimab (after concurrent CRT) Unresectable Stage III NSCLC after chemoradiotherapy BNT116 + cemiplimab + carboplatin + paclitaxel (neoadjuvant) > surgery > BNT116 + cemiplimab (adjuvant) Resectable Stage II and III NSCLC eligible for neoadj. treatment BNT116 + gotistobart3 After prior platinum-based chemotherapy and PD-1i (if eligible) BNT116 + BNT324/DB-13112 (B7H3 ADC) After prior platinum-based chemotherapy and PD-1i (if eligible) BNT116 + BNT326/YL2024 (HER3 ADC) After prior platinum-based chemotherapy and PD-1i (if eligible) Deme D et al. Atmaca A et al. Öven BB et al. Dziadziuszko R et al. Atmaca A et al. BNT116 + pumitamig5 (frail patients) Frail patients not eligible for 1L chemotherapy BNT116 + pumitamig5 (after concurrent CRT) Unresectable Stage III NSCLC after chemoradiotherapy n= 280 Cohort 2, n=20 Cohort 3, n=20 Cohort 4, n=20 Cohort 5, n=20 Cohort 6, n=20 Cohort 7, n=30 Cohort 8, n=30 Cohort 9, n=30 Cohort 10, n=30 Cohort 11, n=30 • Patients with resected PDAC • No prior systemic anti- cancer treatment for PDAC • No evidence of disease after surgery Key Inclusion Criteria • Histologically confirmed NSCLC • Cohort 1–4, 7–9, 11: unresectable Stage III or meta tatic IV • Cohort 5, 11: unresectable Stage III • Cohort 6: resectable Stage II or III • Cohort 2, 4–6, 10, 11: must tolerate anti-PD-1 therapy • Cohort 2, 3, 6, 7–9: ECOG PS ≤1 • Cohort 1, 4, 5, 10, 11: ECOG PS 0-2 Key Endpoints Primary: Safety Secondary: ORR, DoR, DCR, PFS, OS 89
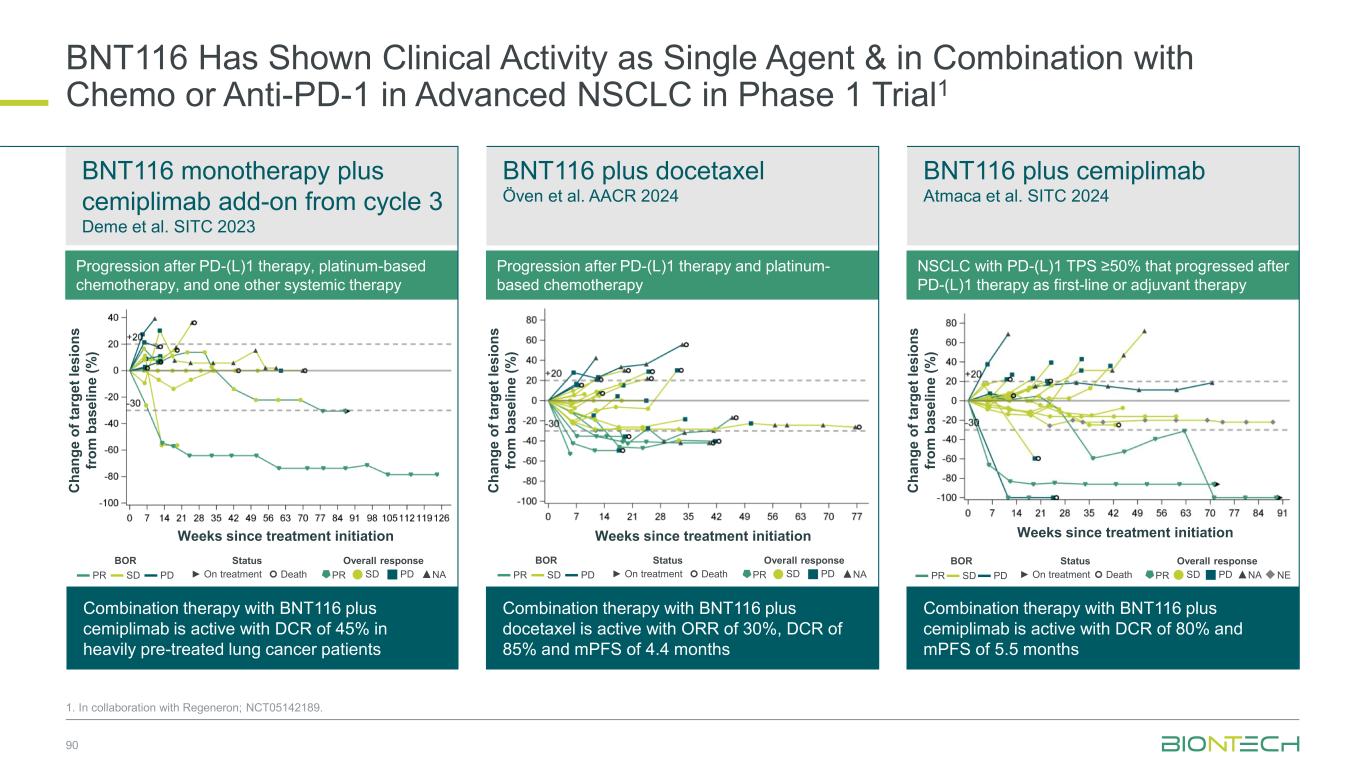
BNT116 Has Shown Clinical Activity as Single Agent & in Combination with Chemo or Anti-PD-1 in Advanced NSCLC in Phase 1 Trial1 90 1. In collaboration with Regeneron; NCT05142189. Progression after PD-(L)1 therapy, platinum-based chemotherapy, and one other systemic therapy Progression after PD-(L)1 therapy and platinum- based chemotherapy BNT116 monotherapy plus cemiplimab add-on from cycle 3 Deme et al. SITC 2023 BNT116 plus docetaxel Öven et al. AACR 2024 BNT116 plus cemiplimab Atmaca et al. SITC 2024 Combination therapy with BNT116 plus cemiplimab is active with DCR of 45% in heavily pre-treated lung cancer patients Combination therapy with BNT116 plus docetaxel is active with ORR of 30%, DCR of 85% and mPFS of 4.4 months Combination therapy with BNT116 plus cemiplimab is active with DCR of 80% and mPFS of 5.5 months NSCLC with PD-(L)1 TPS ≥50% that progressed after PD-(L)1 therapy as first-line or adjuvant therapy Weeks since treatment initiation Weeks since treatment initiation Weeks since treatment initiation C h a n g e o f ta rg e t le s io n s fr o m b a s e li n e ( % ) C h a n g e o f ta rg e t le s io n s fr o m b a s e li n e ( % ) C h a n g e o f ta rg e t le s io n s fr o m b a s e li n e ( % ) BOR Status Overall response ▬ PR ▬ SD ▬ PD ► On treatment Death PR SD PD ▲NA BOR Status Overall response ▬ PR ▬ SD ▬ PD ► On treatment Death PR SD PD ▲NA BOR Status Overall response ▬ PR▬ SD▬ PD ► On treatment Death PR SD PD ▲NA◆NE
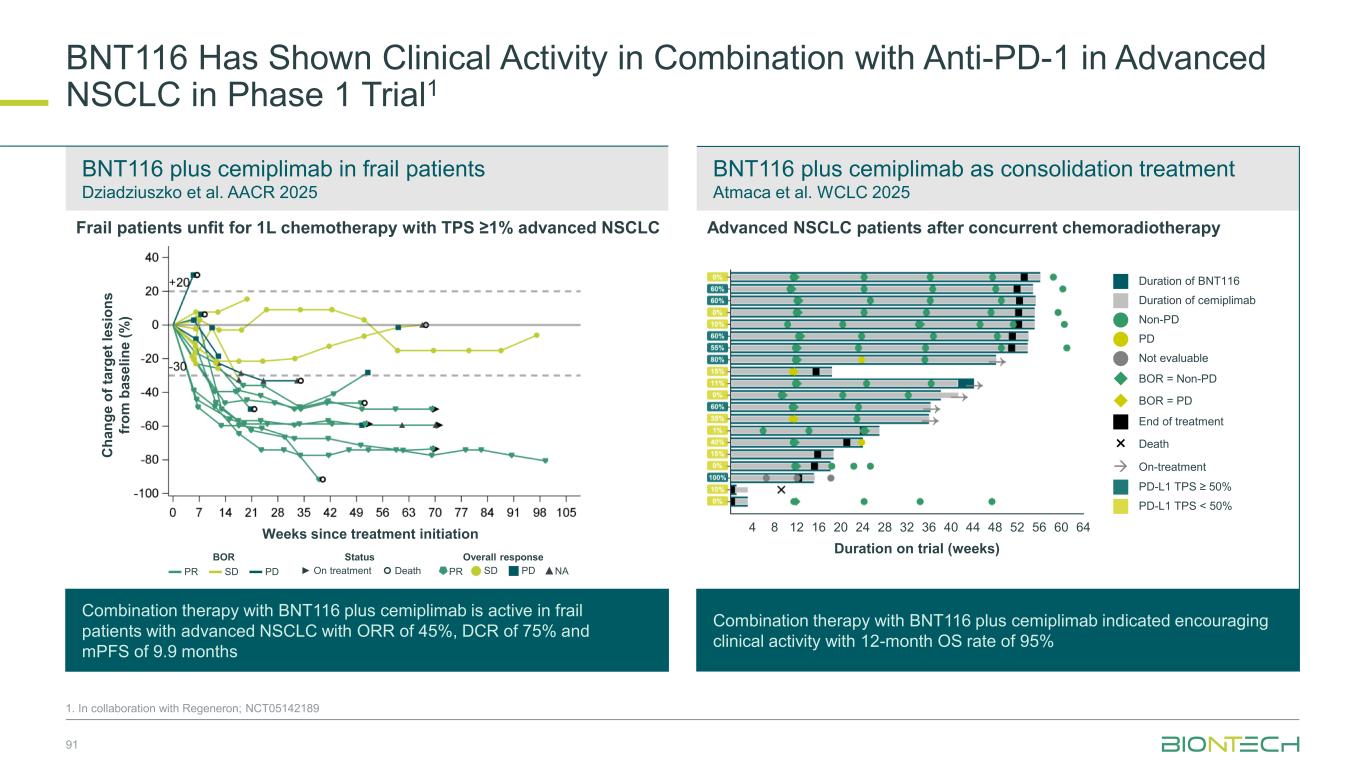
BNT116 plus cemiplimab in frail patients Dziadziuszko et al. AACR 2025 BNT116 plus cemiplimab as consolidation treatment Atmaca et al. WCLC 2025 BNT116 Has Shown Clinical Activity in Combination with Anti-PD-1 in Advanced NSCLC in Phase 1 Trial1 91 1. In collaboration with Regeneron; NCT05142189 Frail patients unfit for 1L chemotherapy with TPS ≥1% advanced NSCLC Combination therapy with BNT116 plus cemiplimab is active in frail patients with advanced NSCLC with ORR of 45%, DCR of 75% and mPFS of 9.9 months Combination therapy with BNT116 plus cemiplimab indicated encouraging clinical activity with 12-month OS rate of 95% Weeks since treatment initiation C h a n g e o f ta rg e t le s io n s fr o m b a s e li n e ( % ) Duration on trial (weeks) 4 12 20 24 28 32 36 44 48 60 648 5216 40 56 Duration of BNT116 Duration of cemiplimab Non-PD PD Not evaluable ◆ BOR = Non-PD ◆ BOR = PD End of treatment × Death → On-treatment PD-L1 TPS ≥ 50% PD-L1 TPS < 50% BOR Status Overall response ▬ PR ▬ SD ▬ PD ► On treatment Death PR SD PD ▲NA Advanced NSCLC patients after concurrent chemoradiotherapy

Partnered with: 1. Bristol Myers Squibb; 2. OncoC4. Ongoing and Next Steps | Thoracic Cancer Establishing pumitamig as a potential frontline treatment for lung cancer Exploring novel combinations to identify potential future standards-of-care Providing new options for IO experienced sqNSCLC ROSETTA Lung-011 Pumitamig + chemotherapy in 1L ES-SCLC ROSETTA Lung-021 Pumitamig + chemotherapy in 1L NSCLC PRESERVE-0032 Gotistobart2 in 2L+ sqNSCLC Part 1 non-pivotal data to be presented at IASLC-NACLC on December 6, 2025 Pumitamig1/ FixVac + ADCs in metastatic disease Pumitamig1 + FixVac in early disease Novel combination data to be presented in 2026 92

Coffee Break 15 minutes

Breast Cancer
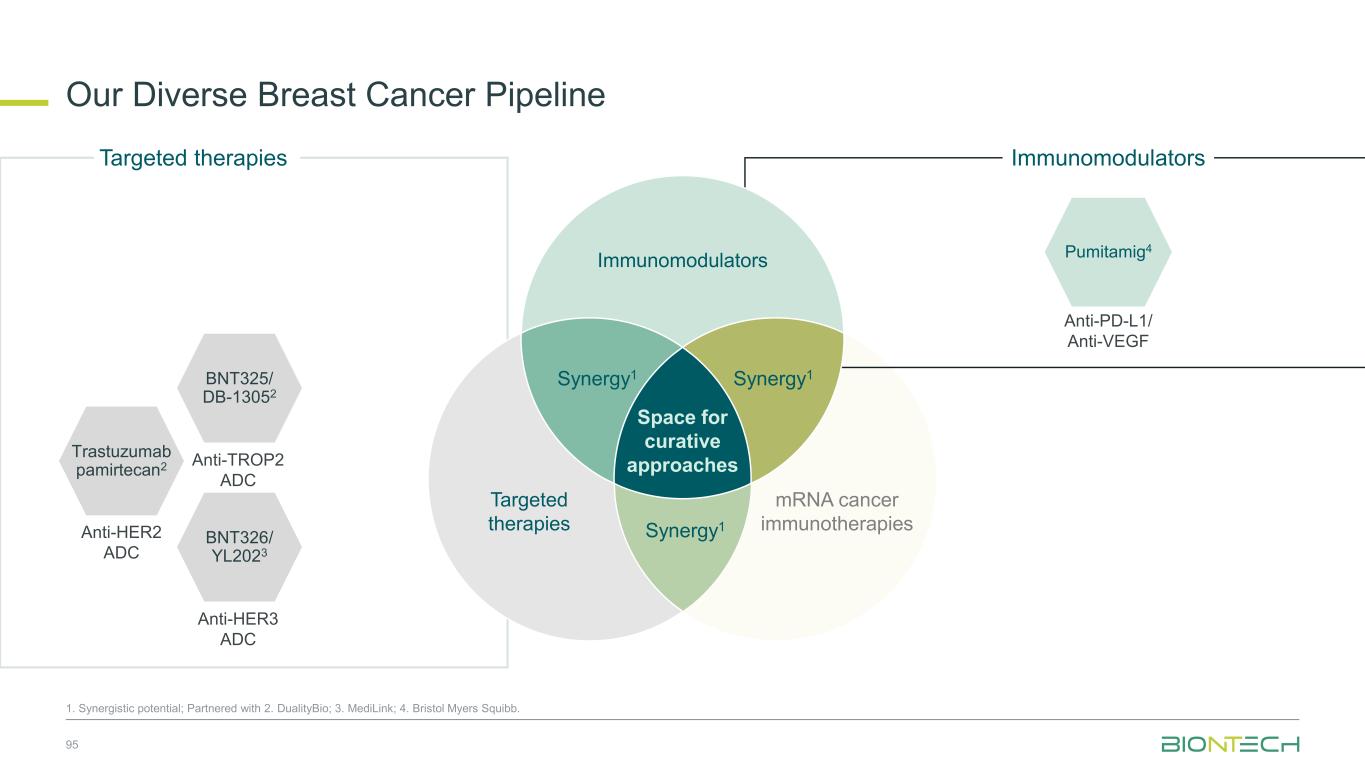
Our Diverse Breast Cancer Pipeline 95 1. Synergistic potential; Partnered with 2. DualityBio; 3. MediLink; 4. Bristol Myers Squibb. Trastuzumab pamirtecan2 Anti-HER2 ADC BNT326/ YL2023 Anti-HER3 ADC Targeted therapies BNT325/ DB-13052 Anti-TROP2 ADC mRNA cancer immunotherapies Immunomodulators Anti-PD-L1/ Anti-VEGF Pumitamig4 Immunomodulators Space for curative approaches Synergy1 Targeted therapies Synergy1Synergy1
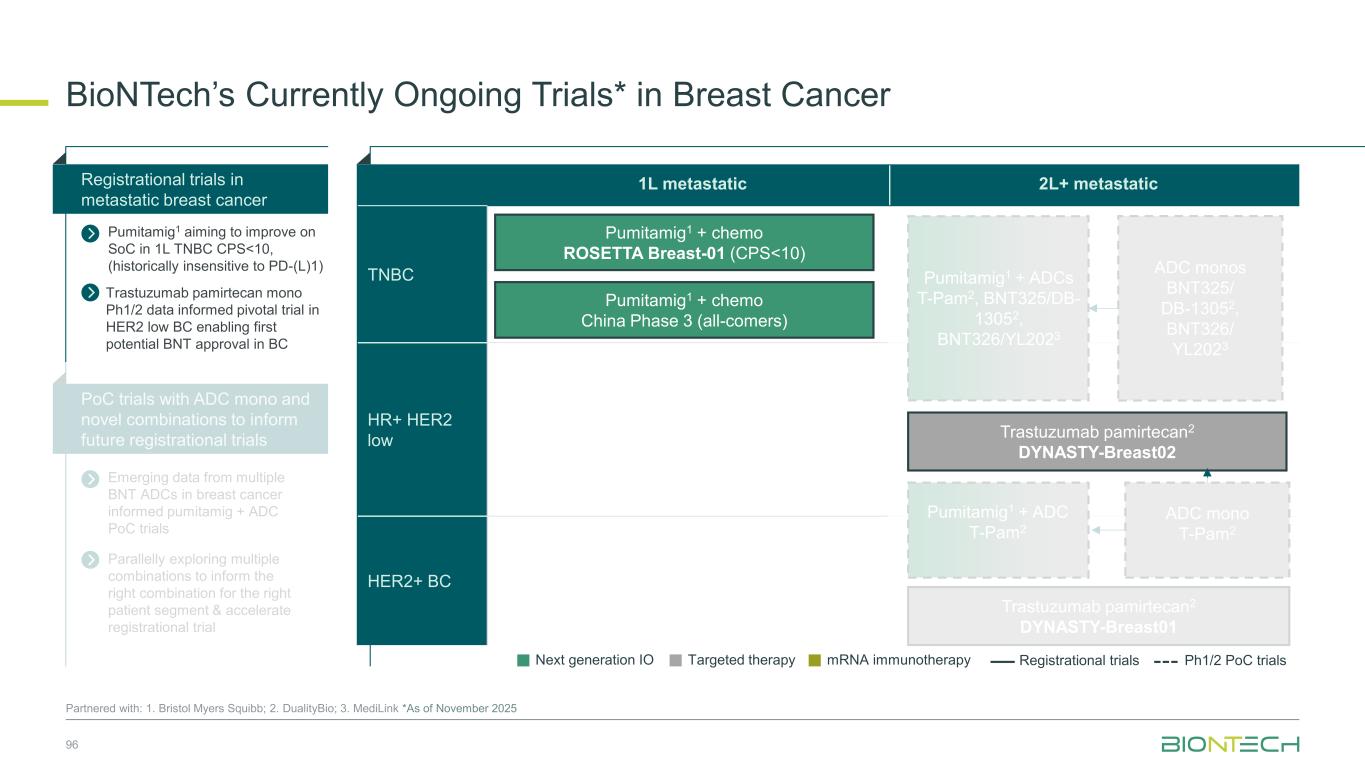
1L metastatic 2L+ metastatic TNBC HR+ HER2 low HER2+ BC BioNTech’s Currently Ongoing Trials* in Breast Cancer 96 Partnered with: 1. Bristol Myers Squibb; 2. DualityBio; 3. MediLink *As of November 2025 Pumitamig1 + chemo ROSETTA Breast-01 (CPS<10) Pumitamig1 + chemo China Phase 3 (all-comers) Emerging data from multiple BNT ADCs in breast cancer informed pumitamig + ADC PoC trials Registrational trials in metastatic breast cancer Pumitamig1 aiming to improve on SoC in 1L TNBC CPS<10, (historically insensitive to PD-(L)1) PoC trials with ADC mono and novel combinations to inform future registrational trials Next generation IO Targeted therapy mRNA immunotherapy Registrational trials Ph1/2 PoC trials Trastuzumab pamirtecan2 DYNASTY-Breast02 ADC monos BNT325/ DB-13052, BNT326/ YL2023 Trastuzumab pamirtecan mono Ph1/2 data informed pivotal trial in HER2 low BC enabling first potential BNT approval in BC Parallelly exploring multiple combinations to inform the right combination for the right patient segment & accelerate registrational trial Trastuzumab pamirtecan2 DYNASTY-Breast01 Pumitamig1 + ADC T-Pam2 Pumitamig1 + ADCs T-Pam2, BNT325/DB- 13052, BNT326/YL2023 ADC mono T-Pam2
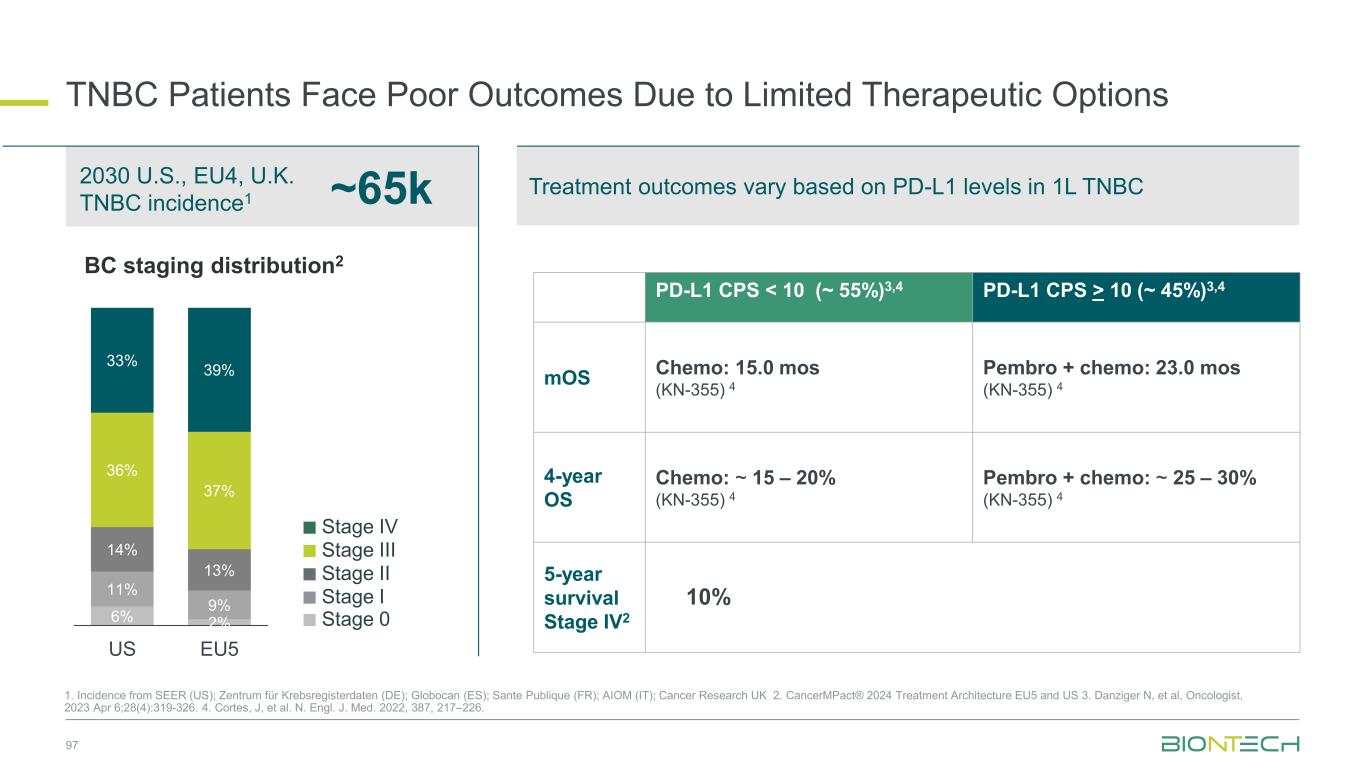
Treatment outcomes vary based on PD-L1 levels in 1L TNBC TNBC Patients Face Poor Outcomes Due to Limited Therapeutic Options 97 1. Incidence from SEER (US); Zentrum für Krebsregisterdaten (DE); Globocan (ES); Sante Publique (FR); AIOM (IT); Cancer Research UK 2. CancerMPact® 2024 Treatment Architecture EU5 and US 3. Danziger N, et al, Oncologist, 2023 Apr 6;28(4):319-326. 4. Cortes, J, et al. N. Engl. J. Med. 2022, 387, 217–226. 6% 2% 11% 9% 14% 13% 36% 37% 33% 39% US EU5 BC staging distribution2 PD-L1 CPS < 10 (~ 55%)3,4 PD-L1 CPS > 10 (~ 45%)3,4 mOS Chemo: 15.0 mos (KN-355) 4 Pembro + chemo: 23.0 mos (KN-355) 4 4-year OS Chemo: ~ 15 – 20% (KN-355) 4 Pembro + chemo: ~ 25 – 30% (KN-355) 4 5-year survival Stage IV2 ~65k2030 U.S., EU4, U.K. TNBC incidence1 10% Stage II Stage III Stage IV Stage I Stage 0
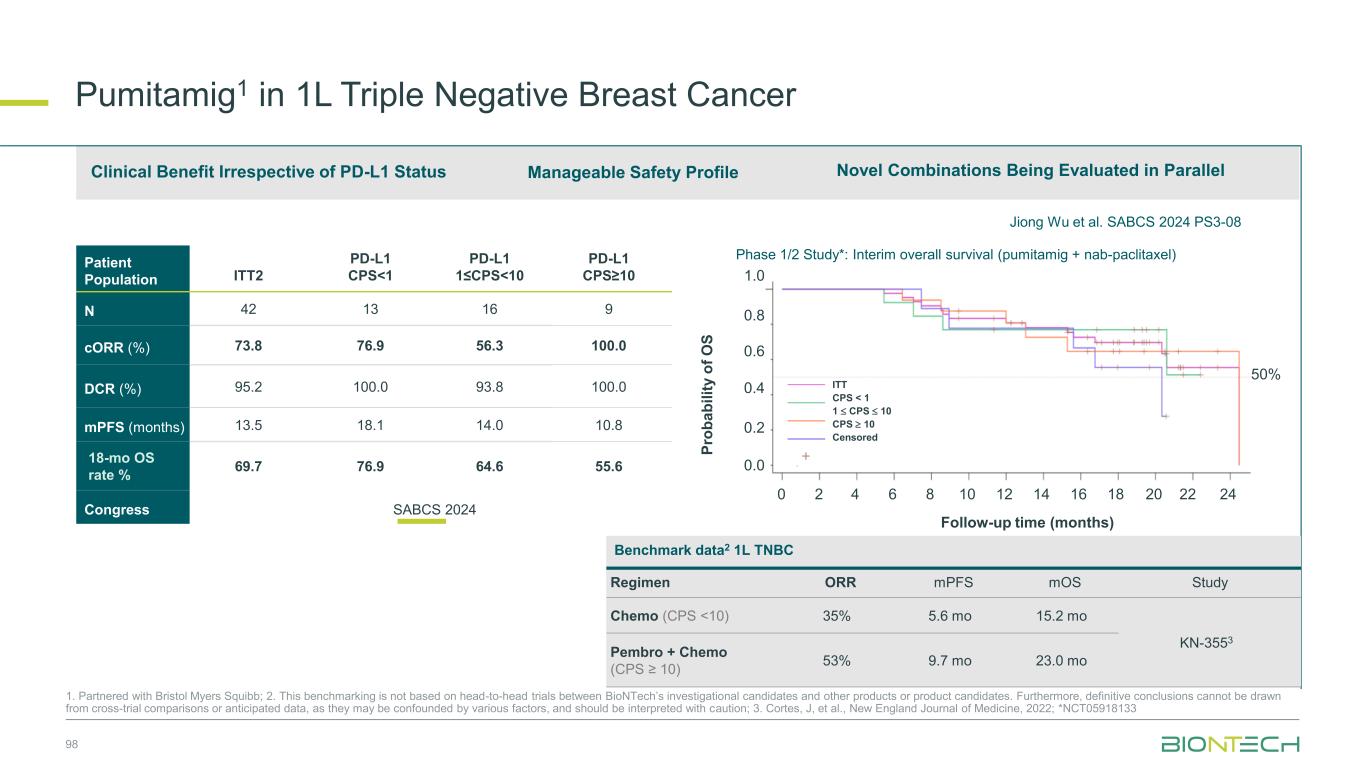
98 Patient Population ITT2 PD-L1 CPS<1 PD-L1 1≤CPS<10 PD-L1 CPS≥10 N 42 13 16 9 cORR (%) 73.8 76.9 56.3 100.0 DCR (%) 95.2 100.0 93.8 100.0 mPFS (months) 13.5 18.1 14.0 10.8 18-mo OS rate % 69.7 76.9 64.6 55.6 Congress SABCS 2024 Novel Combinations Being Evaluated in ParallelManageable Safety ProfileClinical Benefit Irrespective of PD-L1 Status Jiong Wu et al. SABCS 2024 PS3-08 Pumitamig1 in 1L Triple Negative Breast Cancer 1. Partnered with Bristol Myers Squibb; 2. This benchmarking is not based on head-to-head trials between BioNTech’s investigational candidates and other products or product candidates. Furthermore, definitive conclusions cannot be drawn from cross-trial comparisons or anticipated data, as they may be confounded by various factors, and should be interpreted with caution; 3. Cortes, J, et al., New England Journal of Medicine, 2022; *NCT05918133 0 2 4 6 8 10 12 14 16 18 20 22 24 Follow-up time (months) P ro b a b il it y o f O S 1.0 0.8 0.6 0.4 0.2 0.0 50% + ITT CPS < 1 1 CPS 10 CPS 10 Censored Phase 1/2 Study*: Interim overall survival (pumitamig + nab-paclitaxel) Benchmark data2 1L TNBC Regimen ORR mPFS mOS Study Chemo (CPS <10) 35% 5.6 mo 15.2 mo KN-3553 Pembro + Chemo (CPS ≥ 10) 53% 9.7 mo 23.0 mo
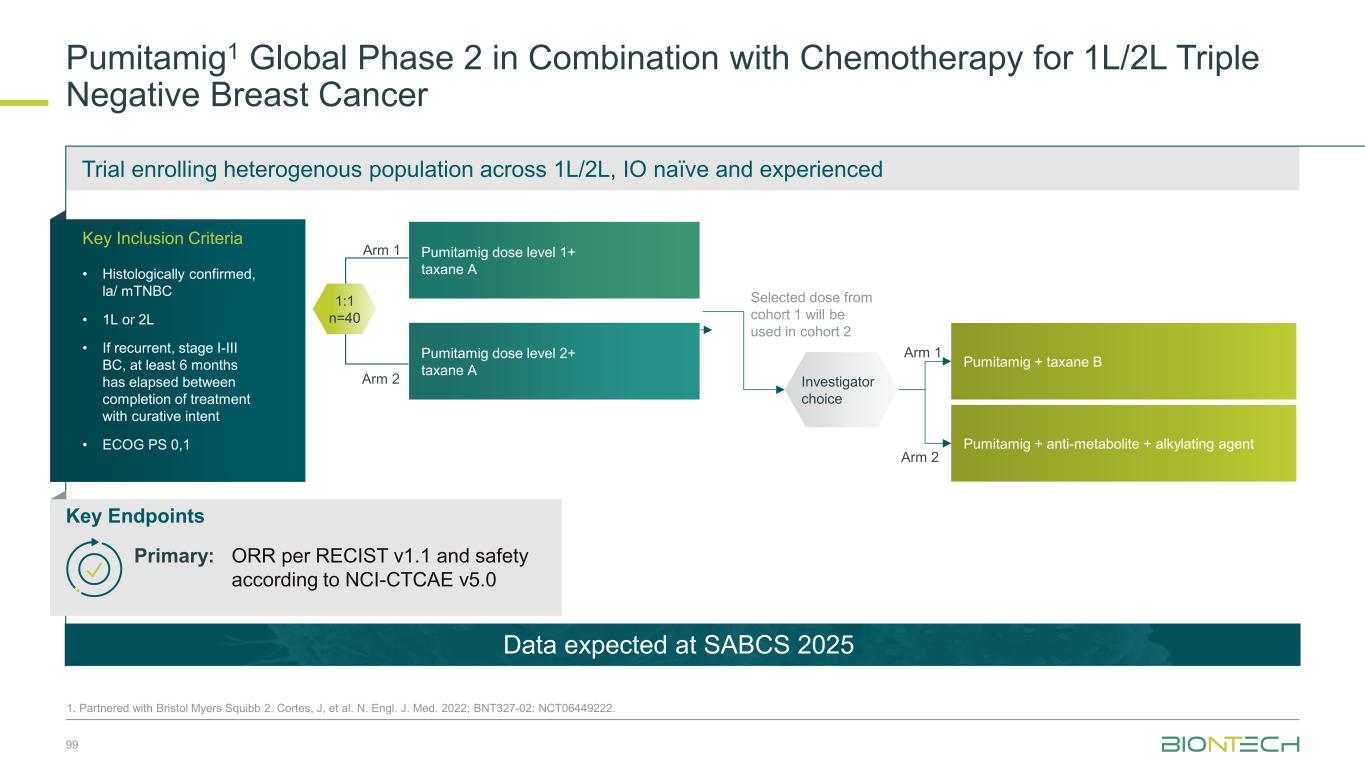
Trial enrolling heterogenous population across 1L/2L, IO naïve and experienced Arm 1 Arm 2 Pumitamig1 Global Phase 2 in Combination with Chemotherapy for 1L/2L Triple Negative Breast Cancer 99 1. Partnered with Bristol Myers Squibb 2. Cortes, J, et al. N. Engl. J. Med. 2022; BNT327-02: NCT06449222. Pumitamig dose level 1+ taxane A Pumitamig dose level 2+ taxane A 1:1 n=40 Selected dose from cohort 1 will be used in cohort 2 Arm 1 Arm 2 Investigator choice Pumitamig + taxane B Pumitamig + anti-metabolite + alkylating agent Data expected at SABCS 2025 Key Inclusion Criteria • Histologically confirmed, la/ mTNBC • 1L or 2L • If recurrent, stage I-III BC, at least 6 months has elapsed between completion of treatment with curative intent • ECOG PS 0,1 Key Endpoints Primary: ORR per RECIST v1.1 and safety according to NCI-CTCAE v5.0
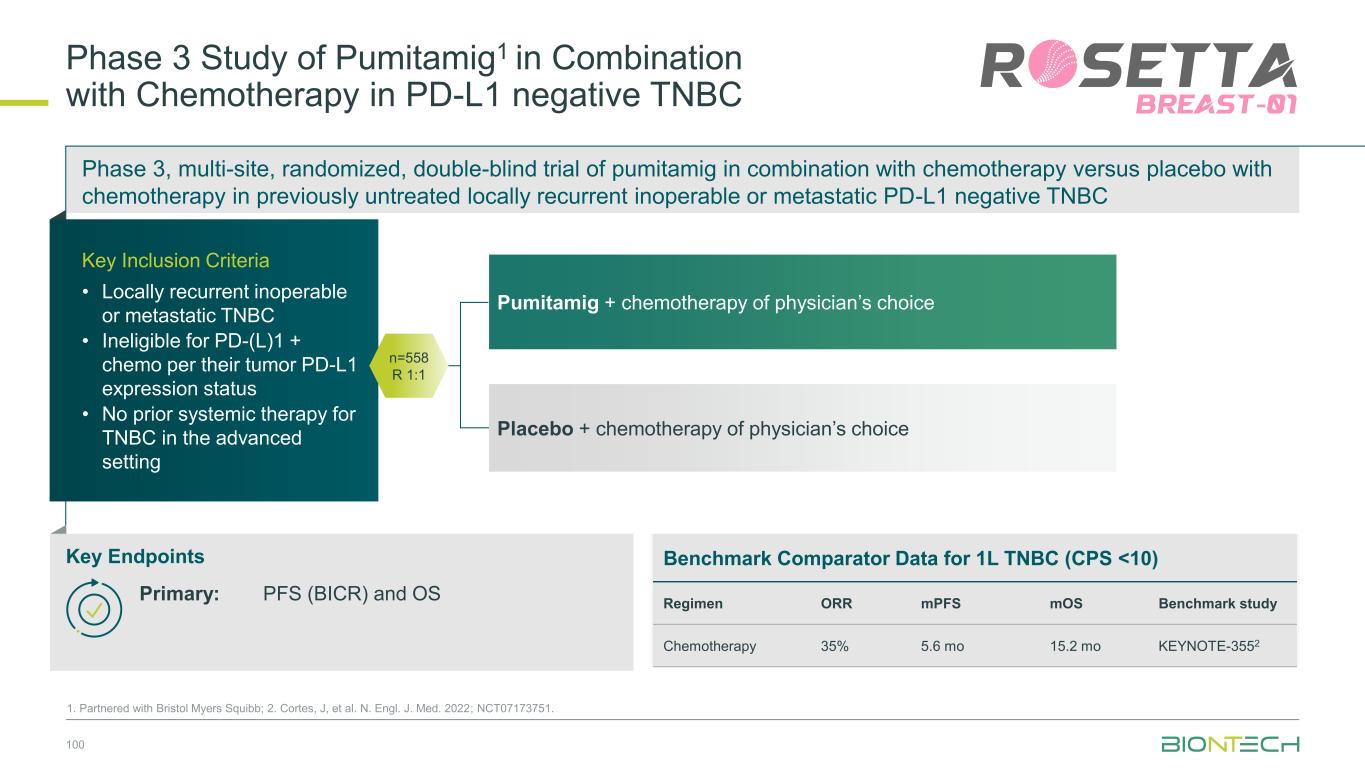
Phase 3, multi-site, randomized, double-blind trial of pumitamig in combination with chemotherapy versus placebo with chemotherapy in previously untreated locally recurrent inoperable or metastatic PD-L1 negative TNBC 100 1. Partnered with Bristol Myers Squibb; 2. Cortes, J, et al. N. Engl. J. Med. 2022; NCT07173751. Pumitamig + chemotherapy of physician’s choice Placebo + chemotherapy of physician’s choice Phase 3 Study of Pumitamig1 in Combination with Chemotherapy in PD-L1 negative TNBC Key Inclusion Criteria • Locally recurrent inoperable or metastatic TNBC • Ineligible for PD-(L)1 + chemo per their tumor PD-L1 expression status • No prior systemic therapy for TNBC in the advanced setting Key Endpoints n=558 R 1:1 Benchmark Comparator Data for 1L TNBC (CPS <10) Regimen ORR mPFS mOS Benchmark study Chemotherapy 35% 5.6 mo 15.2 mo KEYNOTE-3552 Primary: PFS (BICR) and OS
Our Diverse Breast Cancer Pipeline 101 1. Synergistic potential; Partnered with 2. DualityBio; 3. MediLink; 4. Bristol Myers Squibb. Trastuzumab pamirtecan2 Anti-HER2 ADC BNT326/ YL2023 Anti-HER3 ADC Targeted therapies BNT325/ DB-13052 Anti-TROP2 ADC mRNA cancer immunotherapies Immunomodulators Anti-PD-L1/ Anti-VEGF Pumitamig4 Immunomodulators Space for curative approaches Synergy1 Targeted therapies Synergy1Synergy1
Evaluating T-Pam1 in Patients With Advanced HER2-Expressing Solid Tumors 1. Partnered with DualityBio; NCT05150691.. 102 10.0 mg/kg 12.0 mg/kg 8.0 mg/kg 7.0 mg/kg 6.0 mg/kg 4.4 mg/kg 2.2 mg/kg Both HER2 overexpression and HER2 low (IHC3+,2+,1+ or ISH positive) endometrial carcinoma HR+/HER2 Low (IHC2+ /ISH negative, or IHC1+) breast cancer HER2+ (IHC3+, IHC2+/ISH positive) breast cancer NSCLC with activating HER2 mutation HER2+ or HR+/HER2-low breast cancer with treatment failure of trastuzumab deruxtecan (HER2+ BC; HR+/HER2-low BC) (RP2D=8 mg/kg) RP2D/ MTD Key Inclusion Criteria • Pretreated advanced or metastatic solid tumors • HER2-positive or HER2-expressing cancers • Previous systemic therapies • ECOG PS 0-1 Key Endpoints Safety, tolerability, pharmacokinetic, preliminary anti- tumor activity at the selected MTD/RP2D Part 1: Dose escalation (n=88 patients) HER2 IHC 3+, IHC 2+, IHC 1+ or ISH+, or HER2 amplification and mutation by NGS) Part 1: Dose escalation (n=88 patients) Trastuzumab-treated HER2+ (IHC3+, IHC2+/ISH positive) gastric or GEJ adenocarcinoma, esophageal carcinoma and CRC
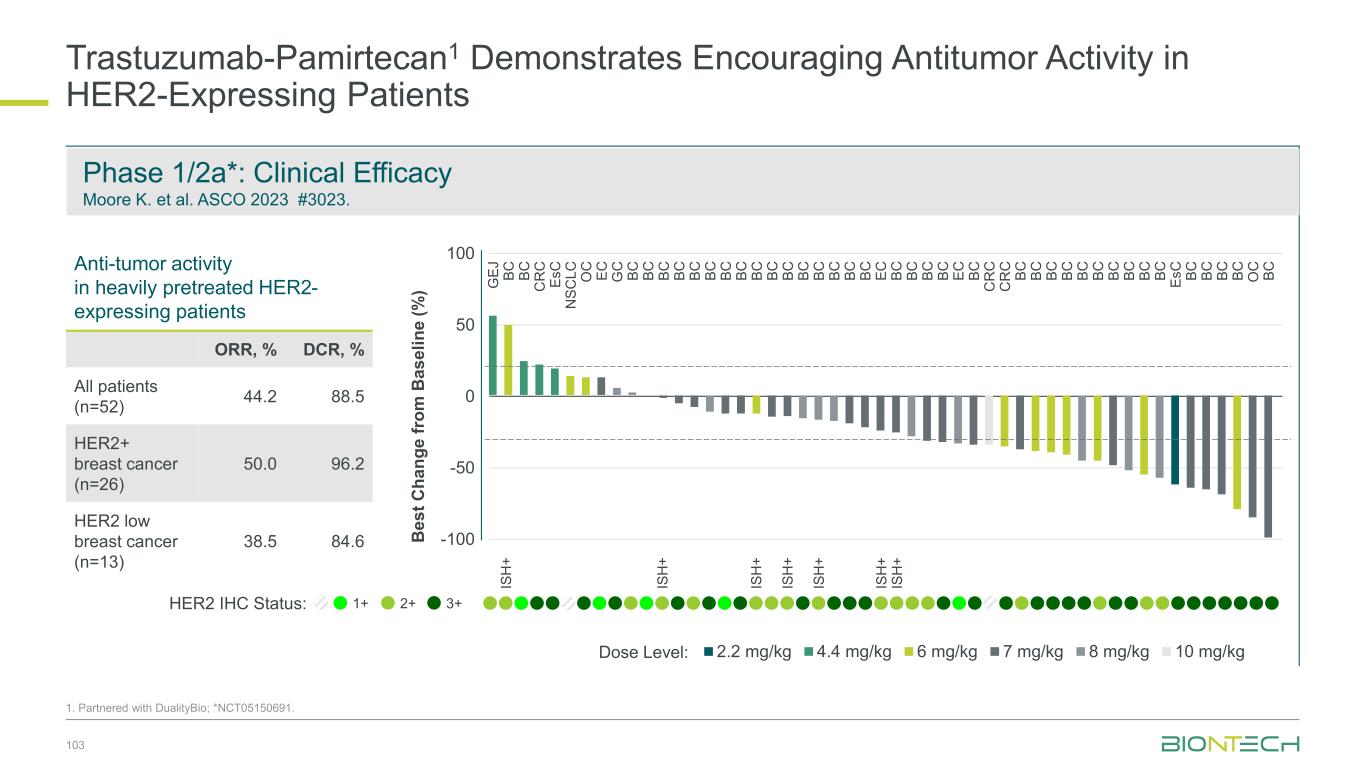
Trastuzumab-Pamirtecan1 Demonstrates Encouraging Antitumor Activity in HER2-Expressing Patients 103 1. Partnered with DualityBio; *NCT05150691. Anti-tumor activity in heavily pretreated HER2- expressing patients ORR, % DCR, % All patients (n=52) 44.2 88.5 HER2+ breast cancer (n=26) 50.0 96.2 HER2 low breast cancer (n=13) 38.5 84.6 Dose Level: 3+1+ 2+HER2 IHC Status: -100 -50 0 50 100 2.2 mg/kg 4.4 mg/kg 6 mg/kg 7 mg/kg 8 mg/kg 10 mg/kg B e s t C h a n g e f ro m B a s e li n e ( % ) IS H + IS H + IS H + IS H + IS H + IS H + IS H + G E J B C C R C E s C C R C G C B C B C B C B C B C B C E C B C B C B C E s C B C N S C L C O C B C C R C B C B C B C B C B C B C E C B C B C B C B C B C B C B C B C B C E C B C B C B C B C B C B C B C B C B C O C B C B C Phase 1/2a*: Clinical Efficacy Moore K. et al. ASCO 2023 #3023.
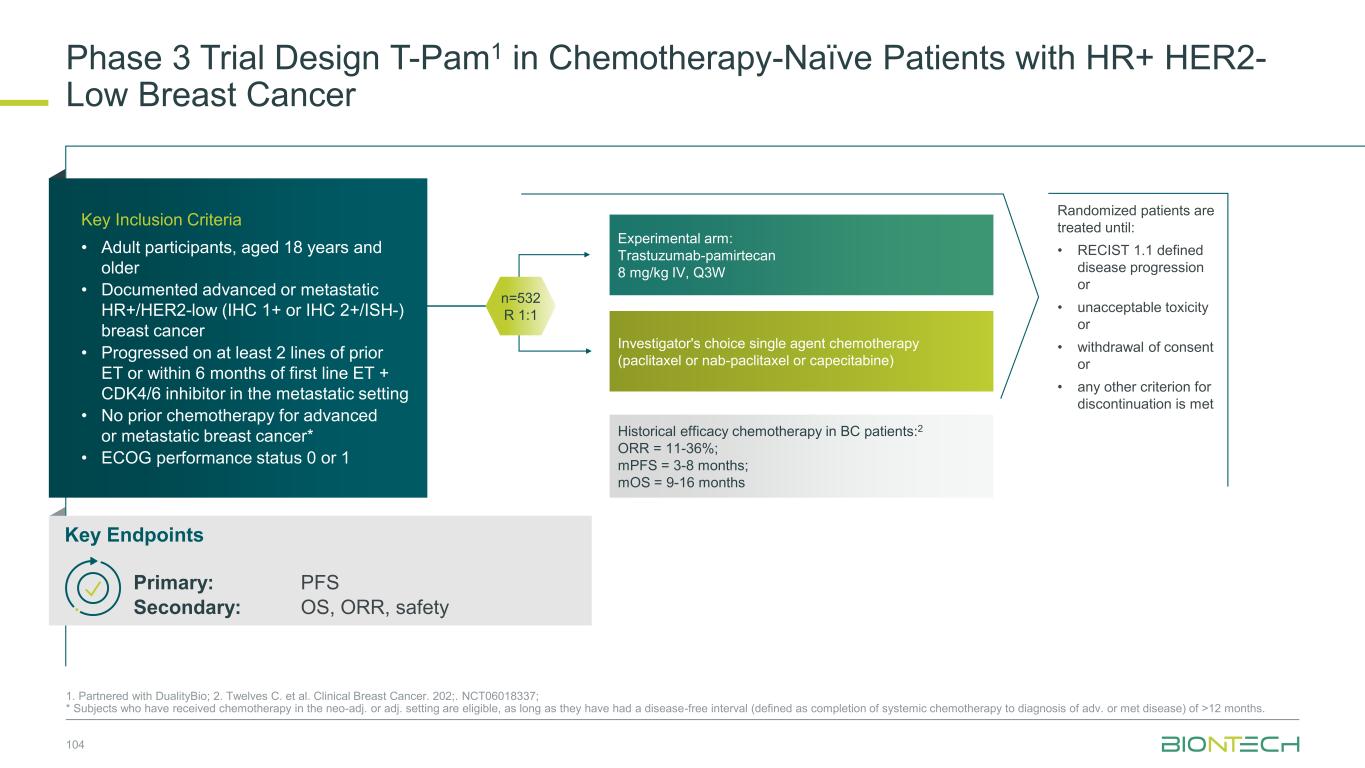
Investigator's choice single agent chemotherapy (paclitaxel or nab-paclitaxel or capecitabine) Phase 3 Trial Design T-Pam1 in Chemotherapy-Naïve Patients with HR+ HER2- Low Breast Cancer 104 1. Partnered with DualityBio; 2. Twelves C. et al. Clinical Breast Cancer. 202;. NCT06018337; * Subjects who have received chemotherapy in the neo-adj. or adj. setting are eligible, as long as they have had a disease-free interval (defined as completion of systemic chemotherapy to diagnosis of adv. or met disease) of >12 months. Randomized patients are treated until: • RECIST 1.1 defined disease progression or • unacceptable toxicity or • withdrawal of consent or • any other criterion for discontinuation is met n=532 R 1:1 Experimental arm: Trastuzumab-pamirtecan 8 mg/kg IV, Q3W Historical efficacy chemotherapy in BC patients:2 ORR = 11-36%; mPFS = 3-8 months; mOS = 9-16 months Key Inclusion Criteria • Adult participants, aged 18 years and older • Documented advanced or metastatic HR+/HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer • Progressed on at least 2 lines of prior ET or within 6 months of first line ET + CDK4/6 inhibitor in the metastatic setting • No prior chemotherapy for advanced or metastatic breast cancer* • ECOG performance status 0 or 1 Key Endpoints Primary: PFS Secondary: OS, ORR, safety
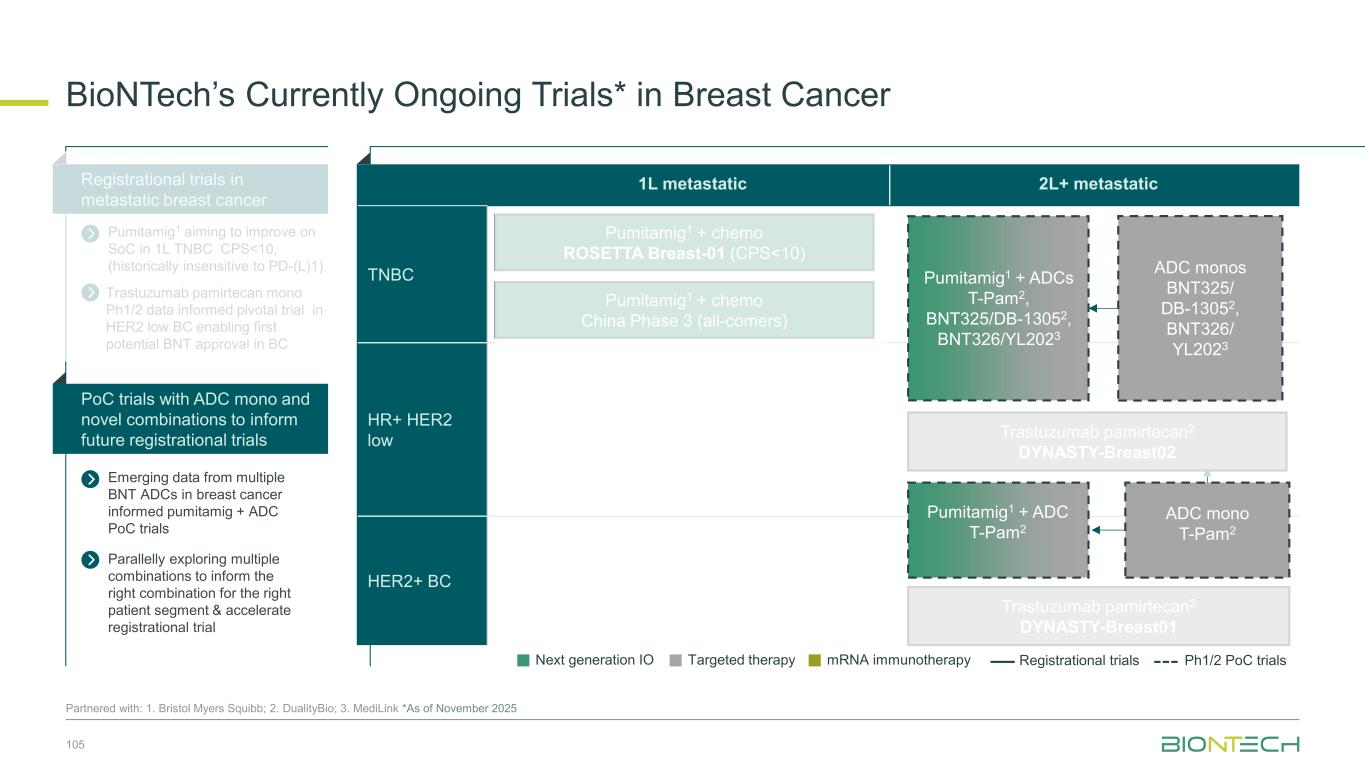
1L metastatic 2L+ metastatic TNBC HR+ HER2 low HER2+ BC BioNTech’s Currently Ongoing Trials* in Breast Cancer 105 Partnered with: 1. Bristol Myers Squibb; 2. DualityBio; 3. MediLink *As of November 2025 Pumitamig1 + chemo ROSETTA Breast-01 (CPS<10) Pumitamig1 + chemo China Phase 3 (all-comers) Emerging data from multiple BNT ADCs in breast cancer informed pumitamig + ADC PoC trials Registrational trials in metastatic breast cancer Pumitamig1 aiming to improve on SoC in 1L TNBC CPS<10, (historically insensitive to PD-(L)1) PoC trials with ADC mono and novel combinations to inform future registrational trials Next generation IO Targeted therapy mRNA immunotherapy Registrational trials Ph1/2 PoC trials Trastuzumab pamirtecan2 DYNASTY-Breast02 ADC monos BNT325/ DB-13052, BNT326/ YL2023 ADC mono T-Pam2 Pumitamig1 + ADCs T-Pam2, BNT325/DB-13052, BNT326/YL2023 Pumitamig1 + ADC T-Pam2 Trastuzumab pamirtecan mono Ph1/2 data informed pivotal trial in HER2 low BC enabling first potential BNT approval in BC Parallelly exploring multiple combinations to inform the right combination for the right patient segment & accelerate registrational trial Trastuzumab pamirtecan2 DYNASTY-Breast01
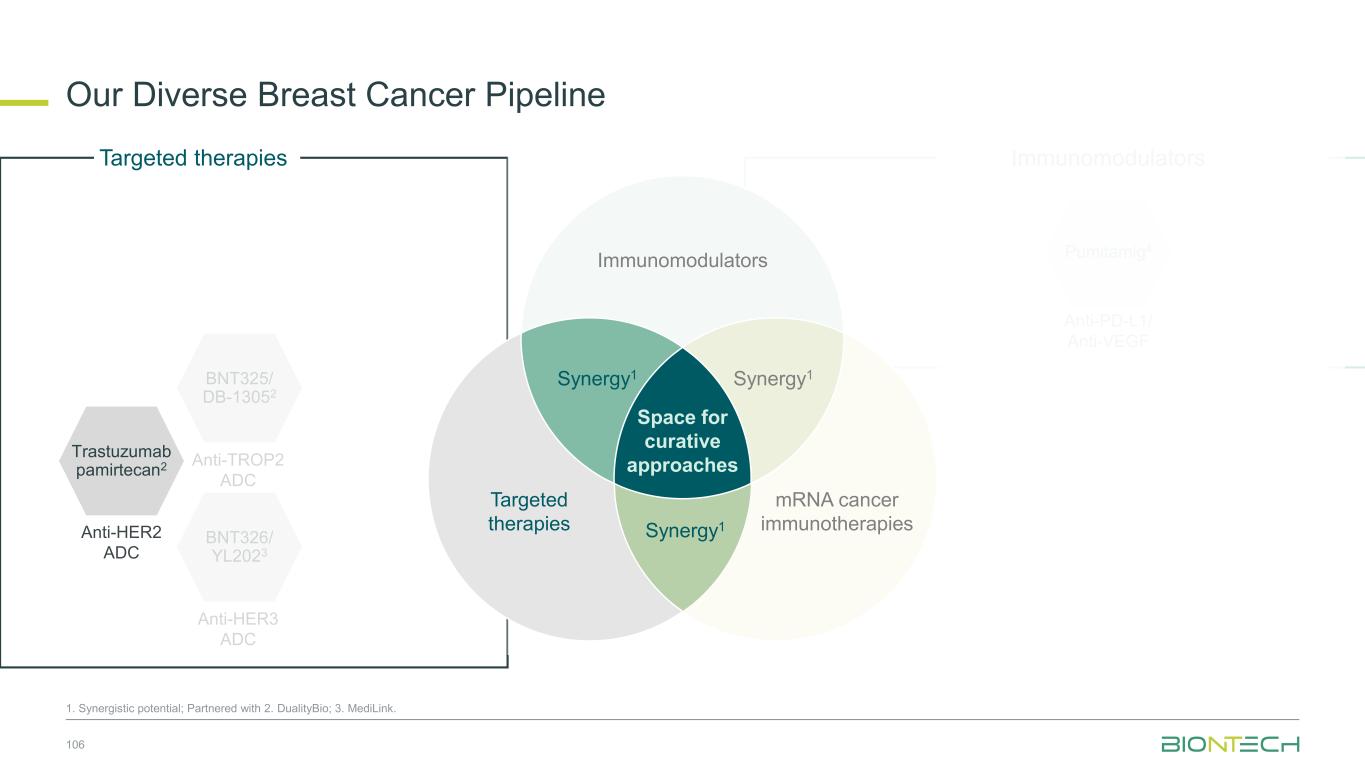
Immunomodulators Our Diverse Breast Cancer Pipeline 106 1. Synergistic potential; Partnered with 2. DualityBio; 3. MediLink. BNT326/ YL2023 Anti-HER3 ADC Targeted therapies BNT325/ DB-13052 Anti-TROP2 ADC Anti-PD-L1/ Anti-VEGF Pumitamig4 Space for curative approaches Synergy1 Targeted therapies Synergy1 Immunomodulators mRNA cancer immunotherapies Synergy1 Trastuzumab pamirtecan2 Anti-HER2 ADC
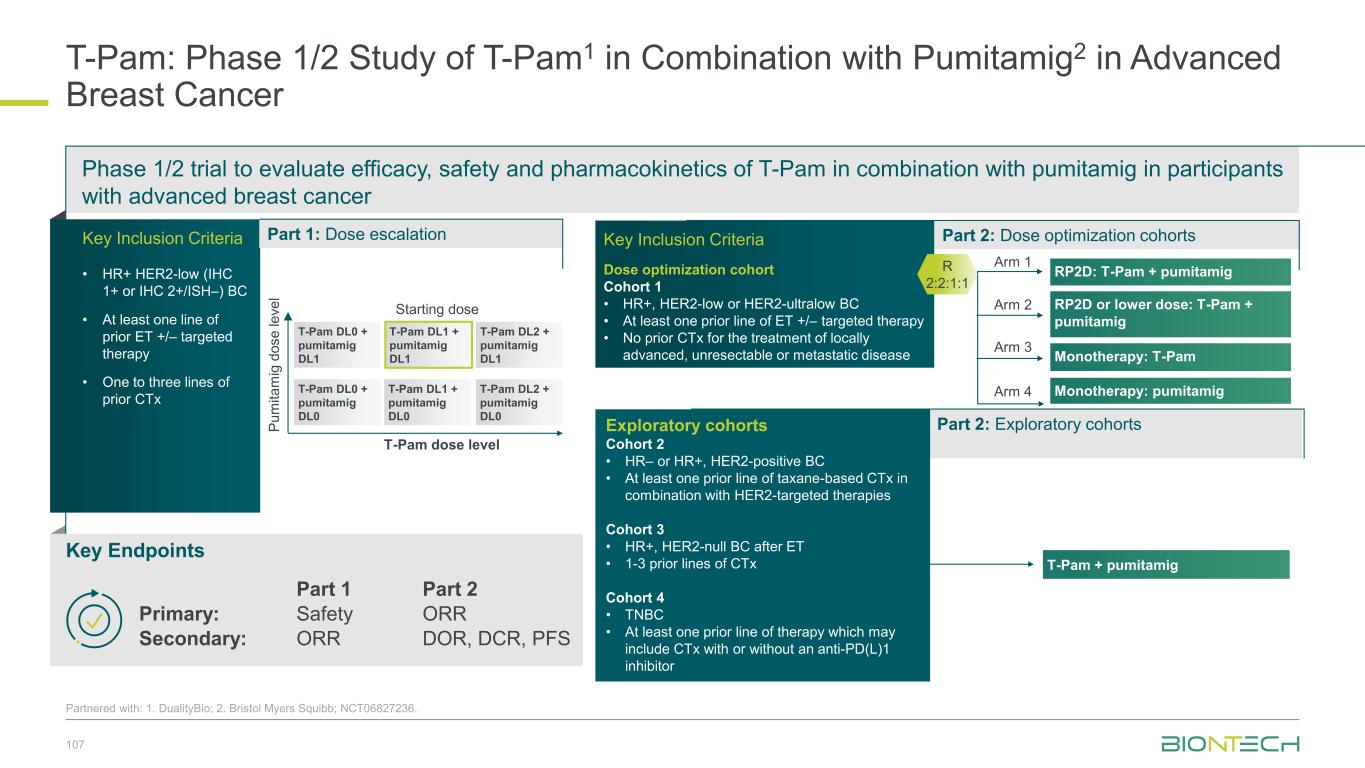
Phase 1/2 trial to evaluate efficacy, safety and pharmacokinetics of T-Pam in combination with pumitamig in participants with advanced breast cancer 107 Partnered with: 1. DualityBio; 2. Bristol Myers Squibb; NCT06827236. T-Pam: Phase 1/2 Study of T-Pam1 in Combination with Pumitamig2 in Advanced Breast Cancer Key Endpoints Key Inclusion Criteria • HR+ HER2-low (IHC 1+ or IHC 2+/ISH–) BC • At least one line of prior ET +/– targeted therapy • One to three lines of prior CTx Part 1: Dose escalation Part 2: Dose optimization cohorts Part 1 Part 2 Primary: Safety ORR Secondary: ORR DOR, DCR, PFS RP2D: T-Pam + pumitamig RP2D or lower dose: T-Pam + pumitamig Monotherapy: T-Pam Monotherapy: pumitamig Key Inclusion Criteria Dose optimization cohort Cohort 1 • HR+, HER2-low or HER2-ultralow BC • At least one prior line of ET +/– targeted therapy • No prior CTx for the treatment of locally advanced, unresectable or metastatic disease R 2:2:1:1 Arm 1 Arm 2 Arm 3 Arm 4T-Pam DL0 + pumitamig DL0 T-Pam DL1 + pumitamig DL0 T-Pam DL1 + pumitamig DL1 T-Pam DL2 + pumitamig DL0 T-Pam DL0 + pumitamig DL1 T-Pam DL2 + pumitamig DL1 T-Pam dose level P u m it a m ig d o s e l e v e l Starting dose Part 2: Exploratory cohortsExploratory cohorts Cohort 2 • HR– or HR+, HER2-positive BC • At least one prior line of taxane-based CTx in combination with HER2-targeted therapies Cohort 3 • HR+, HER2-null BC after ET • 1-3 prior lines of CTx Cohort 4 • TNBC • At least one prior line of therapy which may include CTx with or without an anti-PD(L)1 inhibitor T-Pam + pumitamig
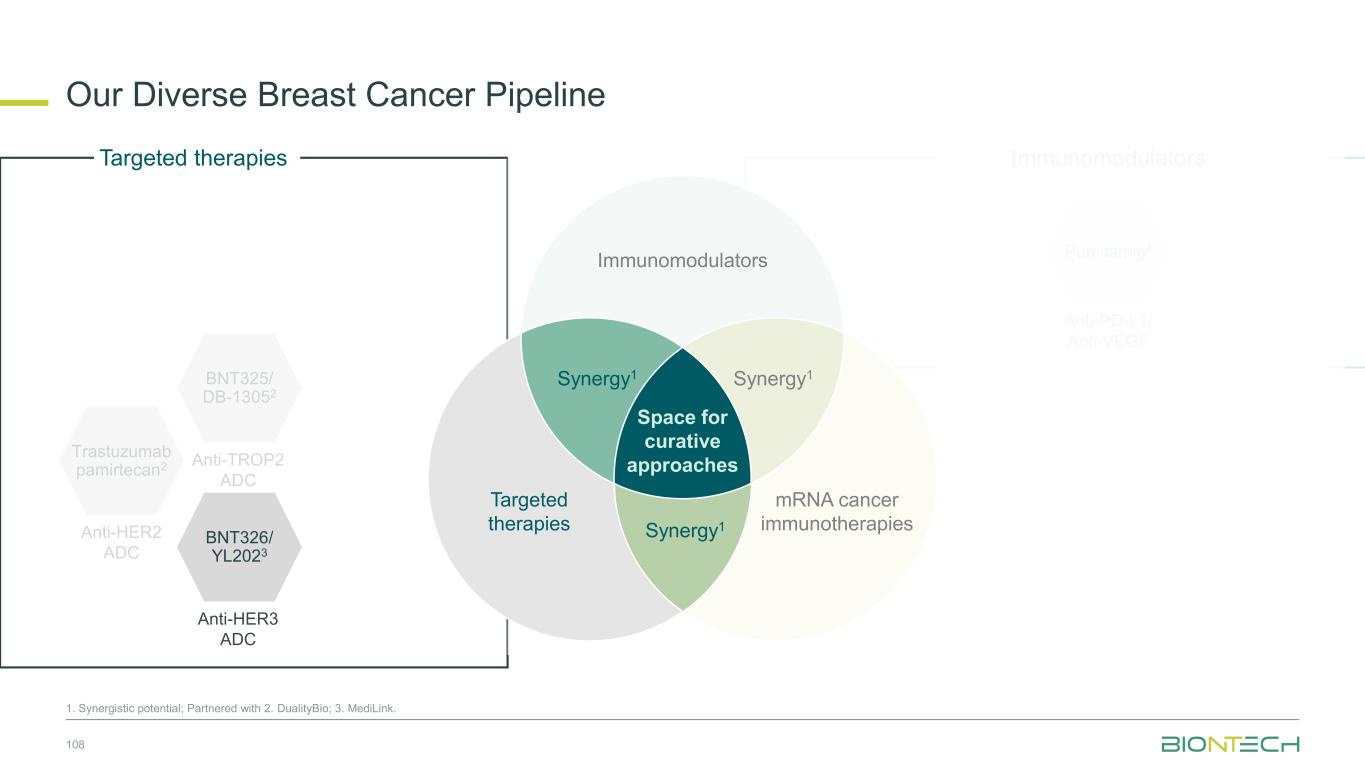
Immunomodulators Our Diverse Breast Cancer Pipeline 108 1. Synergistic potential; Partnered with 2. DualityBio; 3. MediLink. Trastuzumab pamirtecan2 Anti-HER2 ADC Targeted therapies BNT325/ DB-13052 Anti-TROP2 ADC Anti-PD-L1/ Anti-VEGF Pumitamig4 Space for curative approaches Synergy1 Targeted therapies Synergy1 Immunomodulators mRNA cancer immunotherapies Synergy1 BNT326/ YL2023 Anti-HER3 ADC
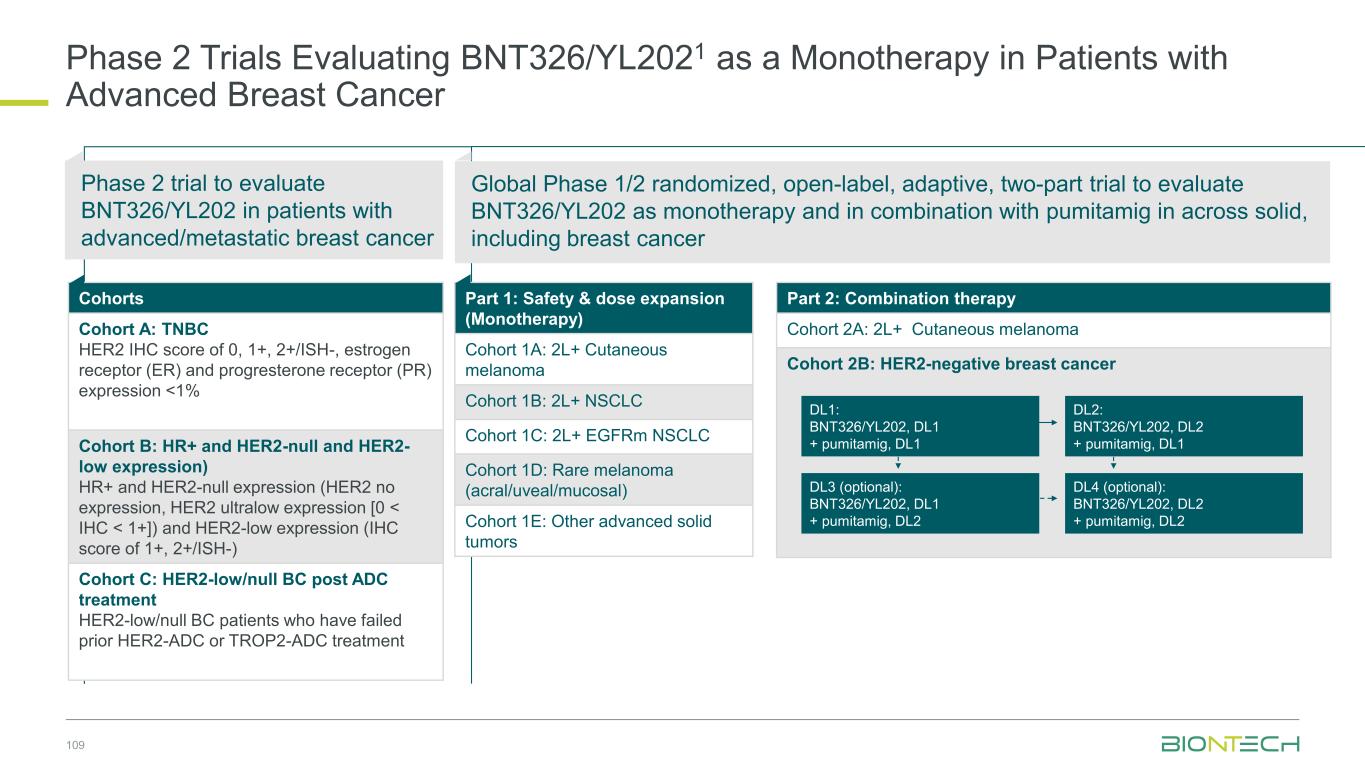
Phase 2 Trials Evaluating BNT326/YL2021 as a Monotherapy in Patients with Advanced Breast Cancer 109 Cohorts Cohort A: TNBC HER2 IHC score of 0, 1+, 2+/ISH-, estrogen receptor (ER) and progresterone receptor (PR) expression <1% Cohort B: HR+ and HER2-null and HER2- low expression) HR+ and HER2-null expression (HER2 no expression, HER2 ultralow expression [0 < IHC < 1+]) and HER2-low expression (IHC score of 1+, 2+/ISH-) Cohort C: HER2-low/null BC post ADC treatment HER2-low/null BC patients who have failed prior HER2-ADC or TROP2-ADC treatment Phase 2 trial to evaluate BNT326/YL202 in patients with advanced/metastatic breast cancer Global Phase 1/2 randomized, open-label, adaptive, two-part trial to evaluate BNT326/YL202 as monotherapy and in combination with pumitamig in across solid, including breast cancer Part 1: Safety & dose expansion (Monotherapy) Cohort 1A: 2L+ Cutaneous melanoma Cohort 1B: 2L+ NSCLC Cohort 1C: 2L+ EGFRm NSCLC Cohort 1D: Rare melanoma (acral/uveal/mucosal) Cohort 1E: Other advanced solid tumors Part 2: Combination therapy Cohort 2A: 2L+ Cutaneous melanoma Cohort 2B: HER2-negative breast cancer DL2: BNT326/YL202, DL2 + pumitamig, DL1 DL4 (optional): BNT326/YL202, DL2 + pumitamig, DL2 DL1: BNT326/YL202, DL1 + pumitamig, DL1 DL3 (optional): BNT326/YL202, DL1 + pumitamig, DL2

Ongoing and Next Steps | Breast Cancer Partnered with: 1. Bristol Myers Squibb; 2. DualityBio. Establishing pumitamig1 as a potential frontline treatment for TNBC Evaluating novel pumitamig1 + ADC combinations to bring checkpoint inhibition to additional breast cancer subtypes and treatment settings Establishing trastuzumab pamirtecan2 as a backbone for HER2-expressing breast cancers 110 ROSETTA Breast-011 Pumitamig + chemotherapy in 1L TNBC CPS < 10 China Phase 3 Pumitamig + chemotherapy in 1L TNBC Data expected in 2026 Pumitamig1 + ADCs Novel combination data expected in 2026 DYNASTY Breast-022 Trastuzumab pamirtecan in chemo naïve HR+, HER-2 low BC Data expected in 2026

Gynecologic Cancers
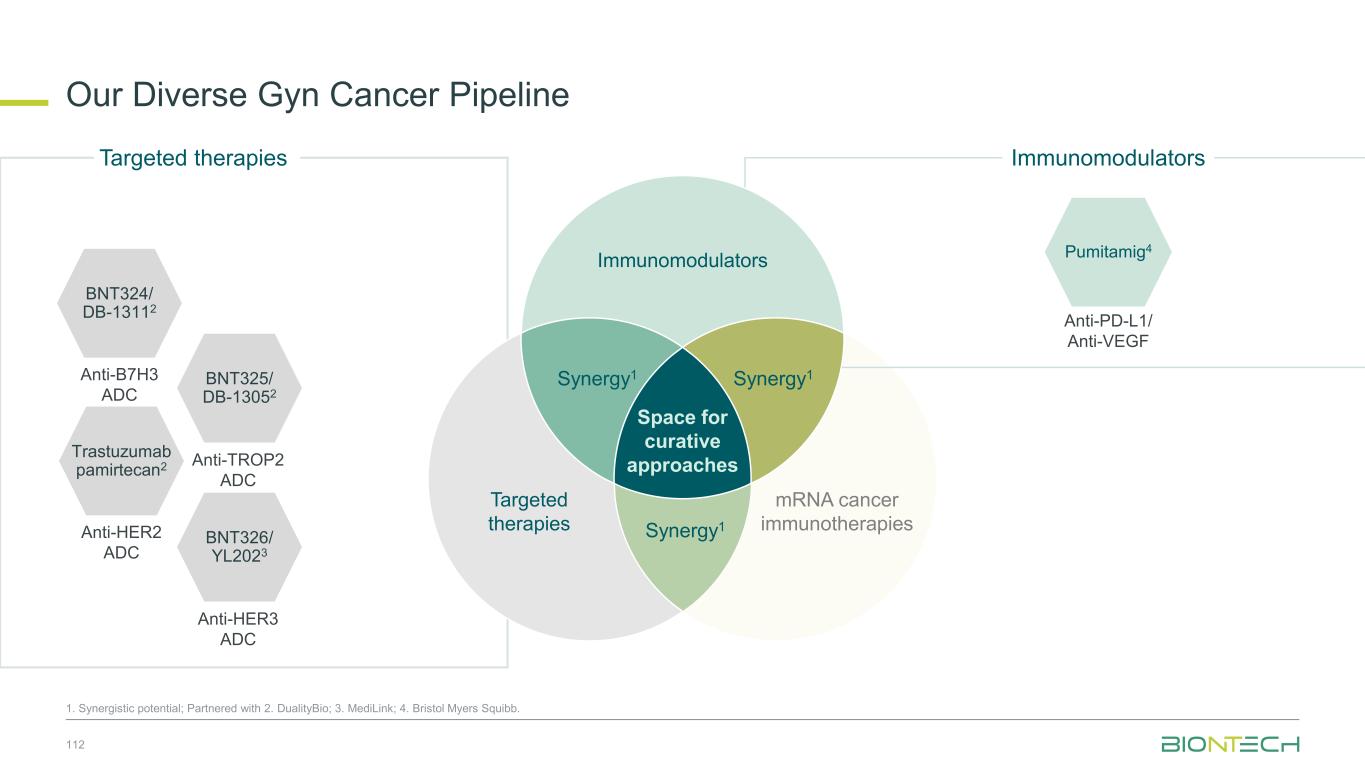
Our Diverse Gyn Cancer Pipeline 112 1. Synergistic potential; Partnered with 2. DualityBio; 3. MediLink; 4. Bristol Myers Squibb. Trastuzumab pamirtecan2 Anti-HER2 ADC BNT326/ YL2023 Anti-HER3 ADC Targeted therapies BNT325/ DB-13052 Anti-TROP2 ADC mRNA cancer immunotherapies Immunomodulators Anti-PD-L1/ Anti-VEGF Pumitamig4 Immunomodulators Space for curative approaches Synergy1 Targeted therapies Synergy1Synergy1 BNT324/ DB-13112 Anti-B7H3 ADC
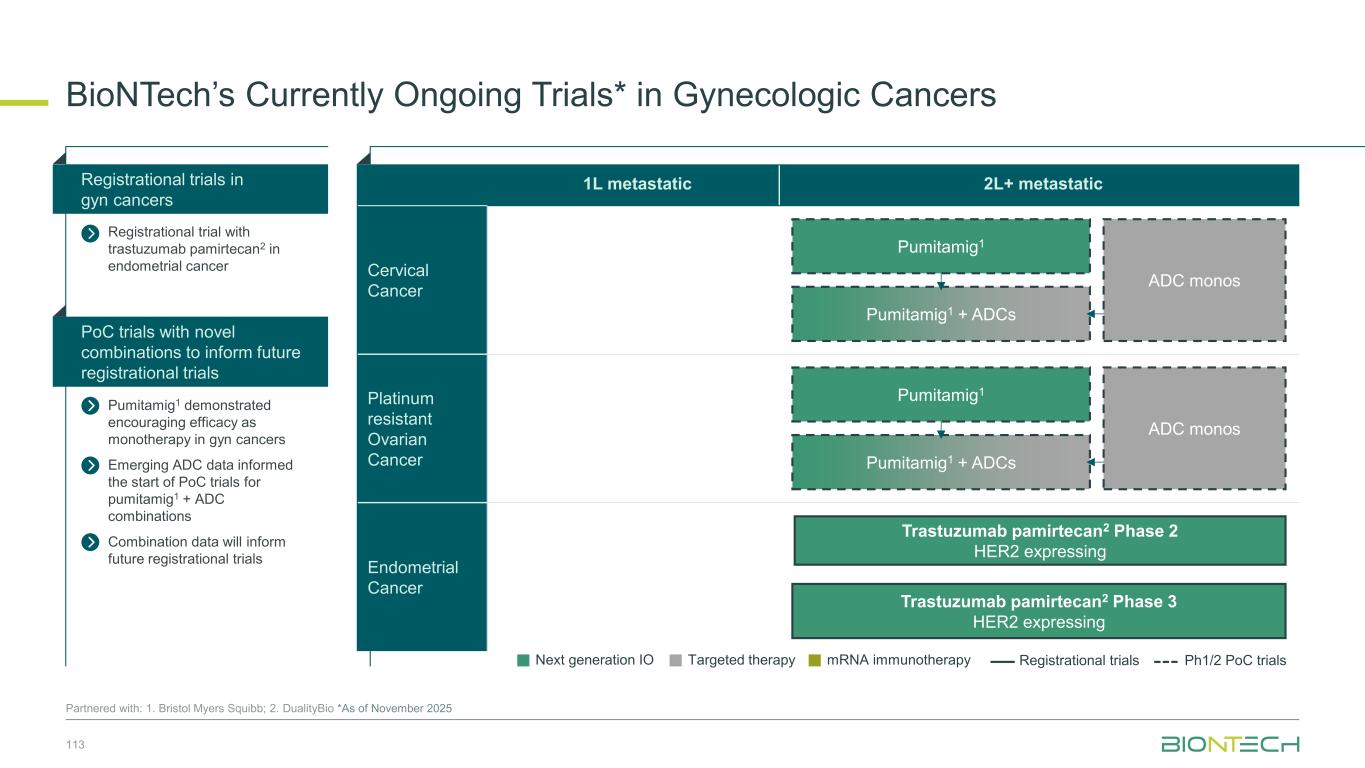
BioNTech’s Currently Ongoing Trials* in Gynecologic Cancers 113 Partnered with: 1. Bristol Myers Squibb; 2. DualityBio *As of November 2025 Pumitamig1 demonstrated encouraging efficacy as monotherapy in gyn cancers Emerging ADC data informed the start of PoC trials for pumitamig1 + ADC combinations Combination data will inform future registrational trials Registrational trial with trastuzumab pamirtecan2 in endometrial cancer PoC trials with novel combinations to inform future registrational trials Registrational trials in gyn cancers 1L metastatic 2L+ metastatic Cervical Cancer Platinum resistant Ovarian Cancer Endometrial Cancer Next generation IO Targeted therapy mRNA immunotherapy Registrational trials Ph1/2 PoC trials ADC monos Pumitamig1 Pumitamig1 + ADCs ADC monos Pumitamig1 Pumitamig1 + ADCs Trastuzumab pamirtecan2 Phase 2 HER2 expressing Trastuzumab pamirtecan2 Phase 3 HER2 expressing
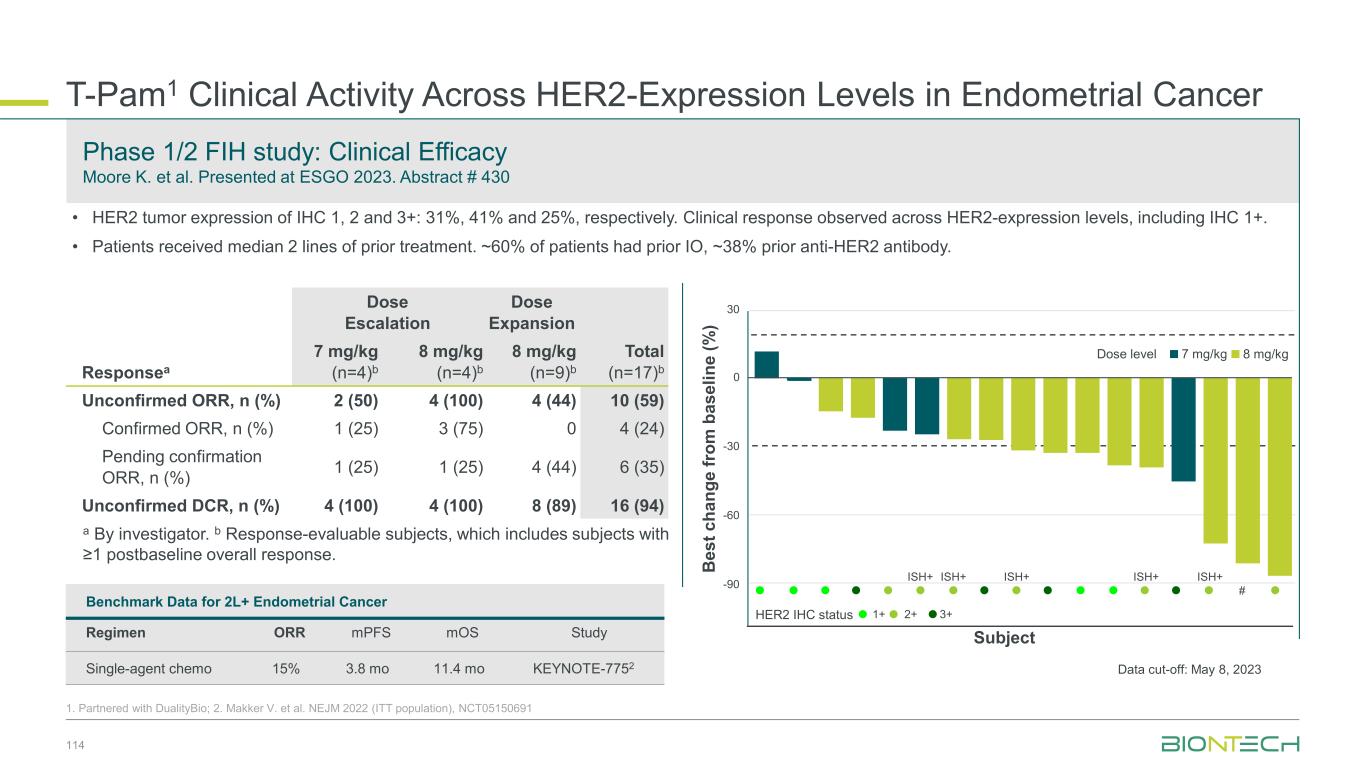
T-Pam1 Clinical Activity Across HER2-Expression Levels in Endometrial Cancer 114 1. Partnered with DualityBio; 2. Makker V. et al. NEJM 2022 (ITT population), NCT05150691 Phase 1/2 FIH study: Clinical Efficacy Moore K. et al. Presented at ESGO 2023. Abstract # 430 Responsea Dose Escalation Dose Expansion Total (n=17)b 7 mg/kg (n=4)b 8 mg/kg (n=4)b 8 mg/kg (n=9)b Unconfirmed ORR, n (%) 2 (50) 4 (100) 4 (44) 10 (59) Confirmed ORR, n (%) 1 (25) 3 (75) 0 4 (24) Pending confirmation ORR, n (%) 1 (25) 1 (25) 4 (44) 6 (35) Unconfirmed DCR, n (%) 4 (100) 4 (100) 8 (89) 16 (94) a By investigator. b Response-evaluable subjects, which includes subjects with ≥1 postbaseline overall response. B e s t c h a n g e f ro m b a s e li n e ( % ) ISH+ ISH+ ISH+ ISH+ ISH+ # Subject 30 0 -30 -60 -90 Dose level 7 mg/kg 8 mg/kg 1+ 2+ 3+HER2 IHC status • HER2 tumor expression of IHC 1, 2 and 3+: 31%, 41% and 25%, respectively. Clinical response observed across HER2-expression levels, including IHC 1+. • Patients received median 2 lines of prior treatment. ~60% of patients had prior IO, ~38% prior anti-HER2 antibody. Benchmark Data for 2L+ Endometrial Cancer Regimen ORR mPFS mOS Study Single-agent chemo 15% 3.8 mo 11.4 mo KEYNOTE-7752 Data cut-off: May 8, 2023
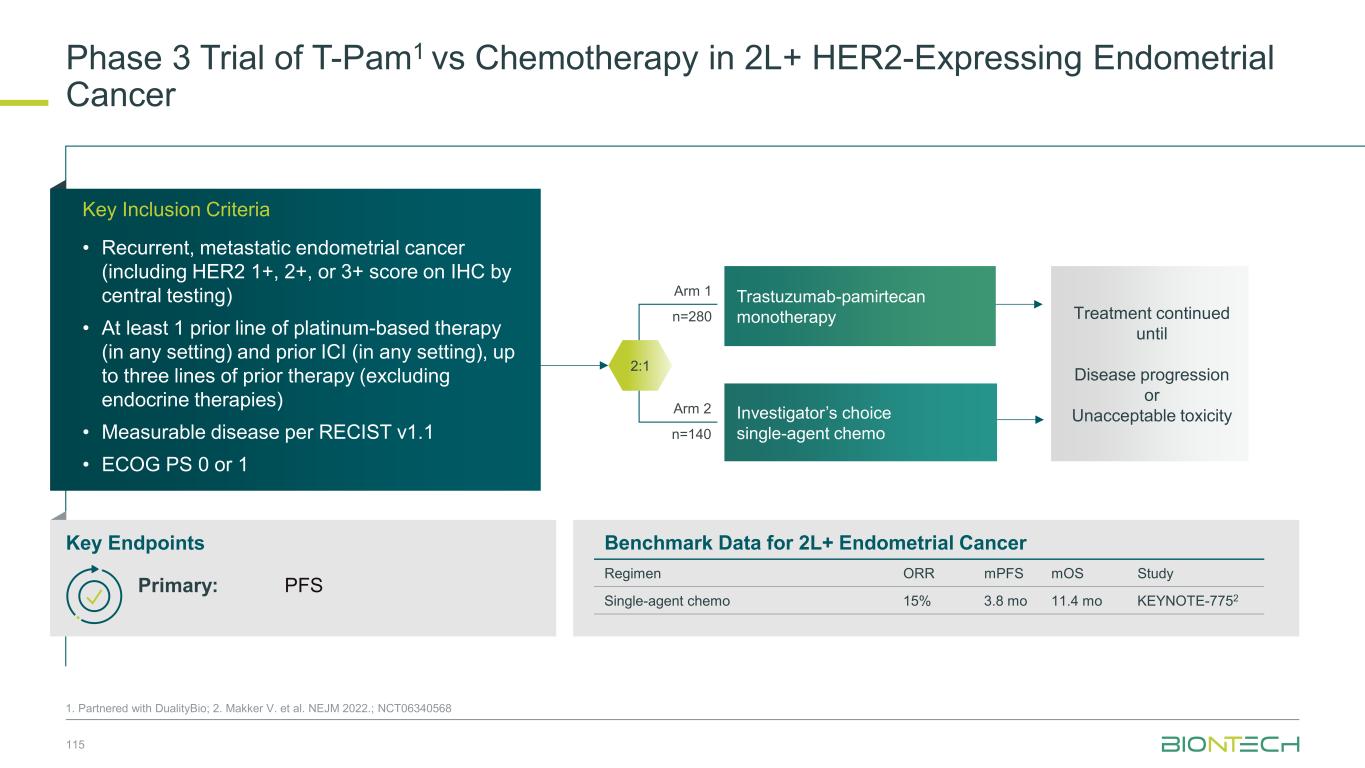
Phase 3 Trial of T-Pam1 vs Chemotherapy in 2L+ HER2-Expressing Endometrial Cancer 115 1. Partnered with DualityBio; 2. Makker V. et al. NEJM 2022.; NCT06340568 Arm 1 n=280 Arm 2 n=140 Trastuzumab-pamirtecan monotherapy Investigator’s choice single-agent chemo 2:1 Treatment continued until Disease progression or Unacceptable toxicity Key Inclusion Criteria • Recurrent, metastatic endometrial cancer (including HER2 1+, 2+, or 3+ score on IHC by central testing) • At least 1 prior line of platinum-based therapy (in any setting) and prior ICI (in any setting), up to three lines of prior therapy (excluding endocrine therapies) • Measurable disease per RECIST v1.1 • ECOG PS 0 or 1 Key Endpoints Benchmark Data for 2L+ Endometrial Cancer Regimen ORR mPFS mOS Study Single-agent chemo 15% 3.8 mo 11.4 mo KEYNOTE-7752 Primary: PFS
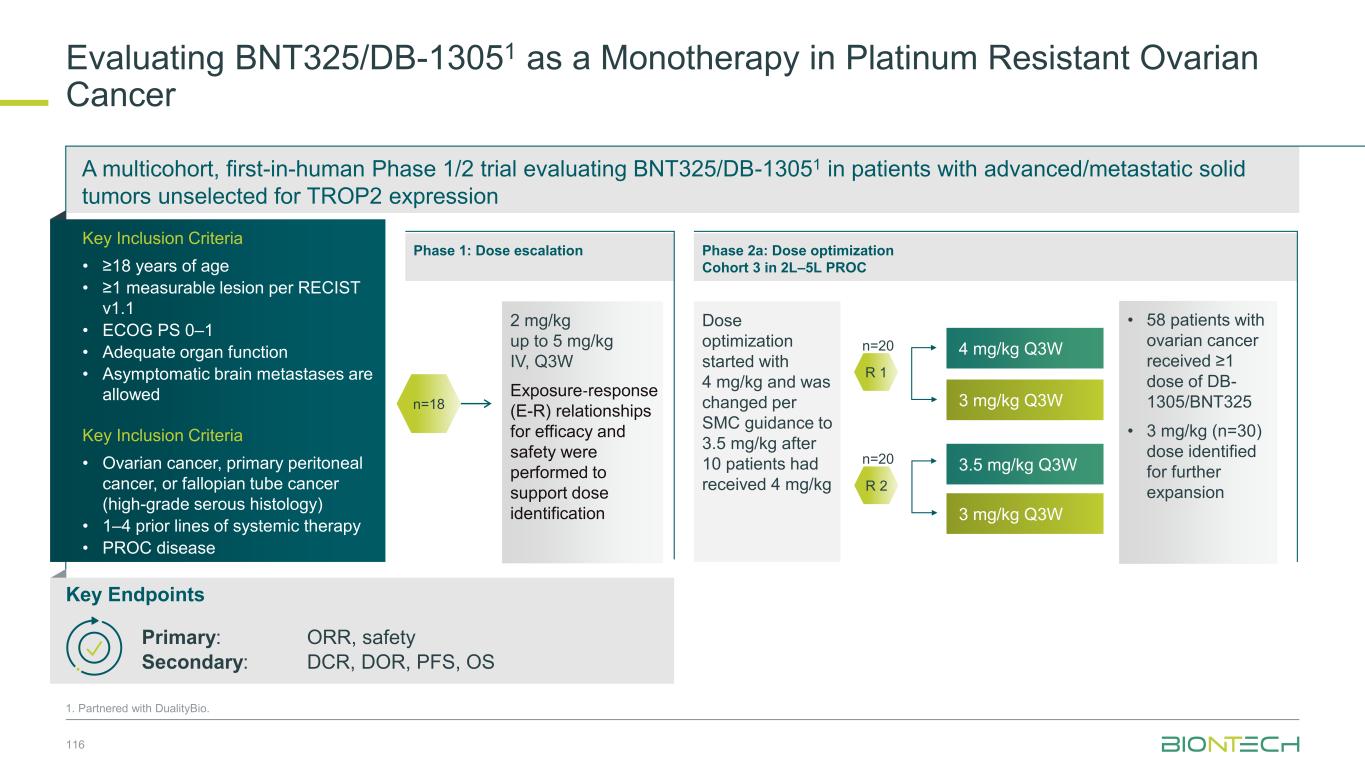
A multicohort, first-in-human Phase 1/2 trial evaluating BNT325/DB-13051 in patients with advanced/metastatic solid tumors unselected for TROP2 expression Evaluating BNT325/DB-13051 as a Monotherapy in Platinum Resistant Ovarian Cancer 116 1. Partnered with DualityBio. Key Endpoints Primary: ORR, safety Secondary: DCR, DOR, PFS, OS Key Inclusion Criteria • ≥18 years of age • ≥1 measurable lesion per RECIST v1.1 • ECOG PS 0–1 • Adequate organ function • Asymptomatic brain metastases are allowed Key Inclusion Criteria • Ovarian cancer, primary peritoneal cancer, or fallopian tube cancer (high-grade serous histology) • 1–4 prior lines of systemic therapy • PROC disease R 1 4 mg/kg Q3W 3 mg/kg Q3W n=20 R 2 n=20 3.5 mg/kg Q3W 3 mg/kg Q3W Dose optimization started with 4 mg/kg and was changed per SMC guidance to 3.5 mg/kg after 10 patients had received 4 mg/kg Phase 2a: Dose optimization Cohort 3 in 2L–5L PROC Phase 1: Dose escalation 2 mg/kg up to 5 mg/kg IV, Q3W Exposure‐response (E-R) relationships for efficacy and safety were performed to support dose identification n=18 • 58 patients with ovarian cancer received ≥1 dose of DB- 1305/BNT325 • 3 mg/kg (n=30) dose identified for further expansion
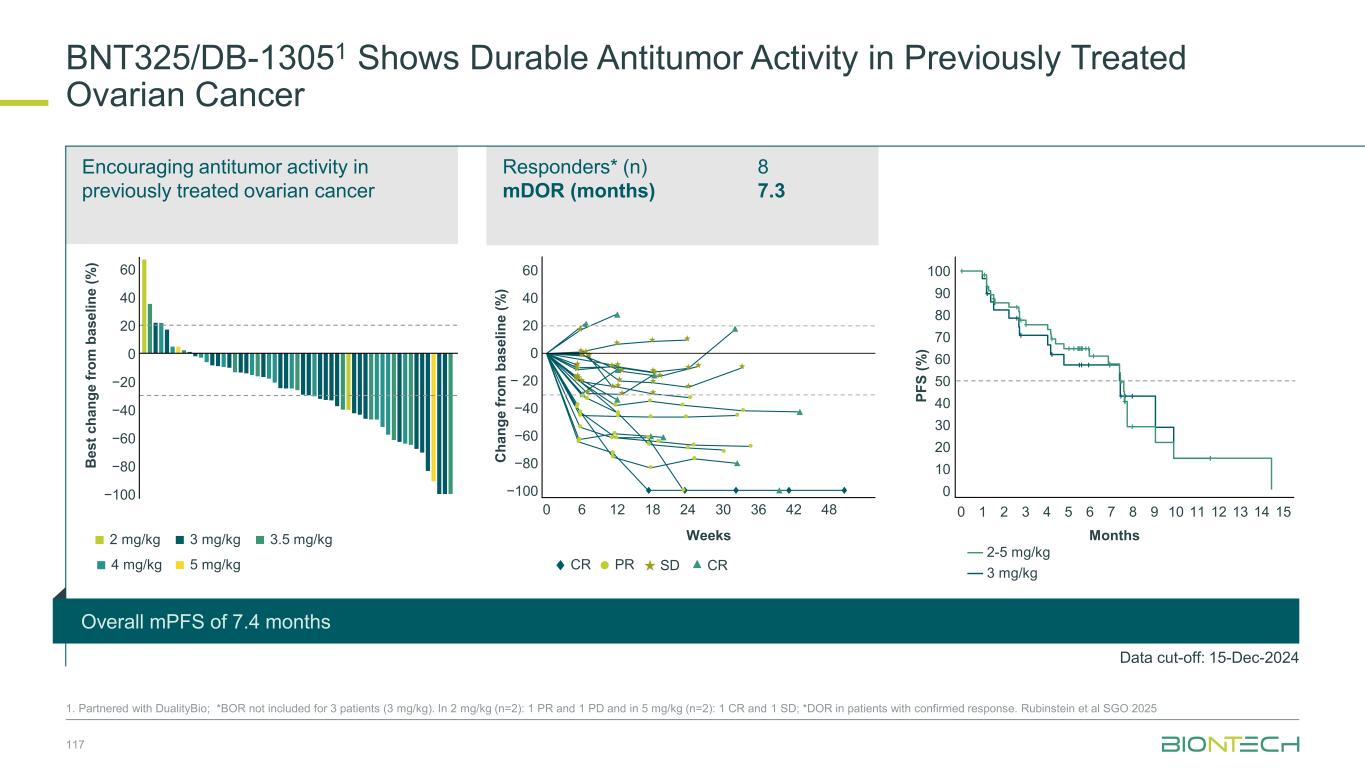
Overall mPFS of 7.4 months Responders* (n) mDOR (months) 8 7.3 Encouraging antitumor activity in previously treated ovarian cancer BNT325/DB-13051 Shows Durable Antitumor Activity in Previously Treated Ovarian Cancer 1. Partnered with DualityBio; *BOR not included for 3 patients (3 mg/kg). In 2 mg/kg (n=2): 1 PR and 1 PD and in 5 mg/kg (n=2): 1 CR and 1 SD; *DOR in patients with confirmed response. Rubinstein et al SGO 2025 117 117 −100 60 40 20 0 −20 −40 −60 −80B e s t c h a n g e f ro m b a s e li n e ( % ) 2 mg/kg 3 mg/kg 3.5 mg/kg 4 mg/kg 5 mg/kg C h a n g e f ro m b a s e li n e ( % ) −100 60 40 0 − 20 −40 −60 −80 20 0 6 12 18 24 30 36 42 48 CR PR Weeks SD CR 100 90 70 40 30 20 10 0 80 60 50 0 2 4 6 158 10 11 12 131 3 5 7 9 14 P F S ( % ) Months Data cut-off: 15-Dec-2024 3 mg/kg 2-5 mg/kg
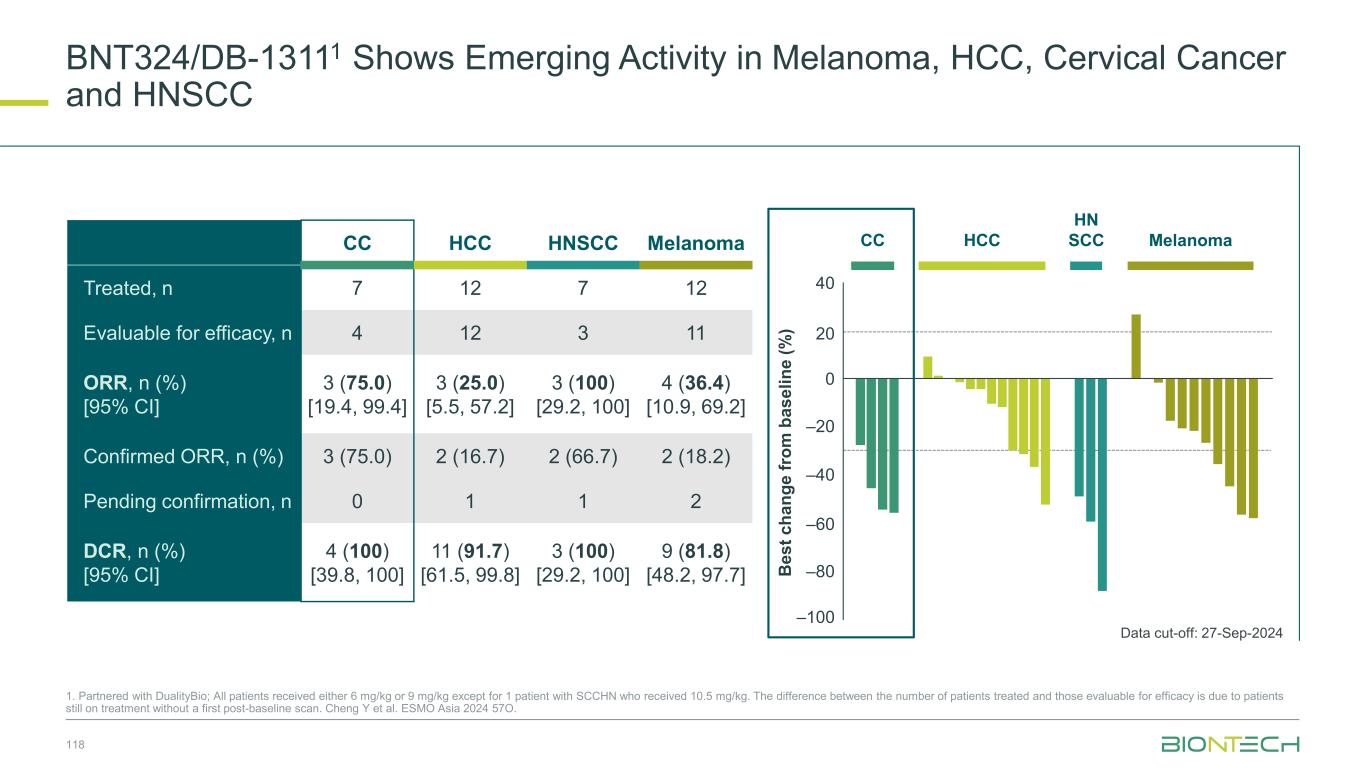
BNT324/DB-13111 Shows Emerging Activity in Melanoma, HCC, Cervical Cancer and HNSCC 118 1. Partnered with DualityBio; All patients received either 6 mg/kg or 9 mg/kg except for 1 patient with SCCHN who received 10.5 mg/kg. The difference between the number of patients treated and those evaluable for efficacy is due to patients still on treatment without a first post-baseline scan. Cheng Y et al. ESMO Asia 2024 57O. Data cut-off: 27-Sep-2024 CC HCC HNSCC Melanoma Treated, n 7 12 7 12 Evaluable for efficacy, n 4 12 3 11 ORR, n (%) [95% CI] 3 (75.0) [19.4, 99.4] 3 (25.0) [5.5, 57.2] 3 (100) [29.2, 100] 4 (36.4) [10.9, 69.2] Confirmed ORR, n (%) 3 (75.0) 2 (16.7) 2 (66.7) 2 (18.2) Pending confirmation, n 0 1 1 2 DCR, n (%) [95% CI] 4 (100) [39.8, 100] 11 (91.7) [61.5, 99.8] 3 (100) [29.2, 100] 9 (81.8) [48.2, 97.7] CC HCC HN SCC Melanoma B e s t c h a n g e f ro m b a s e li n e ( % ) 40 –20 0 –40 –60 –100 20 –80
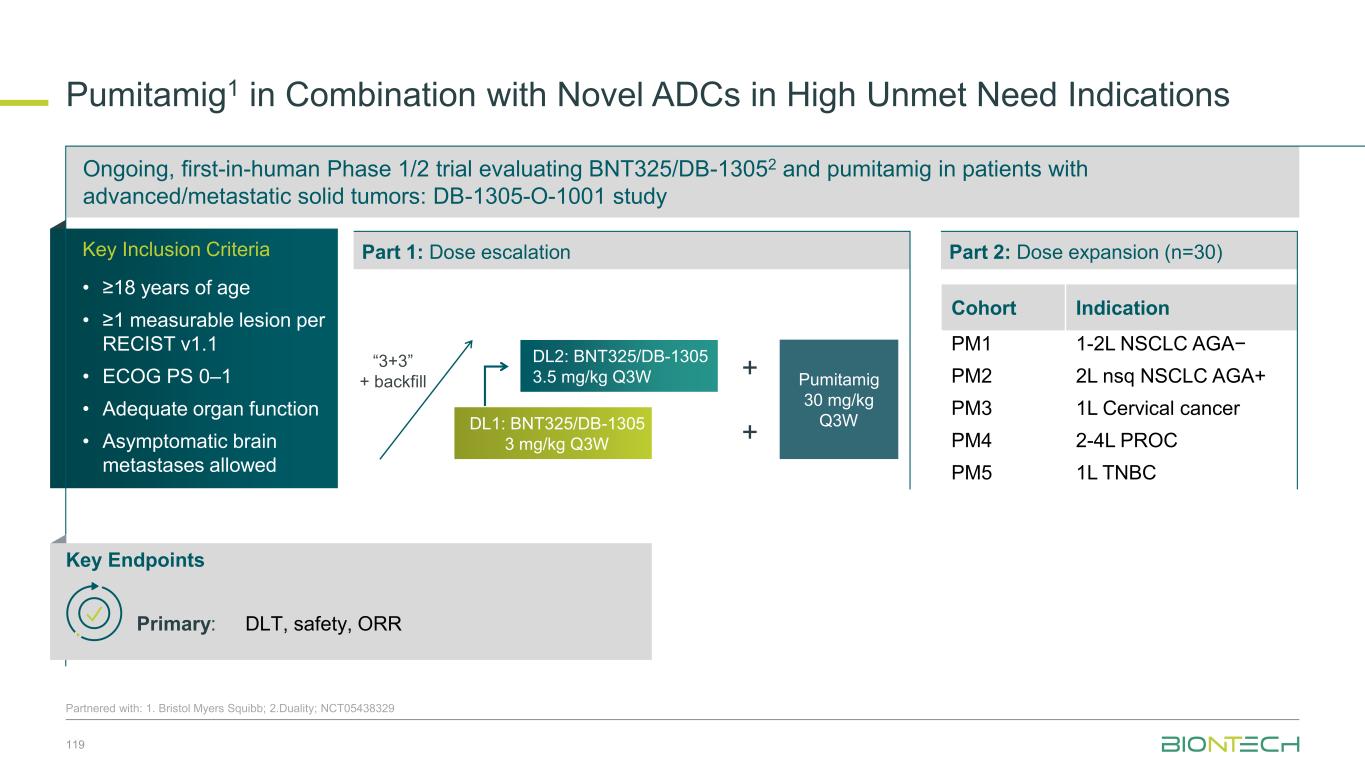
Pumitamig1 in Combination with Novel ADCs in High Unmet Need Indications Partnered with: 1. Bristol Myers Squibb; 2.Duality; NCT05438329 DL1: BNT325/DB-1305 3 mg/kg Q3W DL2: BNT325/DB-1305 3.5 mg/kg Q3W “3+3” + backfill Key Inclusion Criteria • ≥18 years of age • ≥1 measurable lesion per RECIST v1.1 • ECOG PS 0–1 • Adequate organ function • Asymptomatic brain metastases allowed Ongoing, first-in-human Phase 1/2 trial evaluating BNT325/DB-13052 and pumitamig in patients with advanced/metastatic solid tumors: DB-1305-O-1001 study Cohort Indication PM1 1-2L NSCLC AGA− PM2 2L nsq NSCLC AGA+ PM3 1L Cervical cancer PM4 2-4L PROC PM5 1L TNBC Part 1: Dose escalation Part 2: Dose expansion (n=30) Key Endpoints Primary: DLT, safety, ORR Pumitamig 30 mg/kg Q3W + + 119
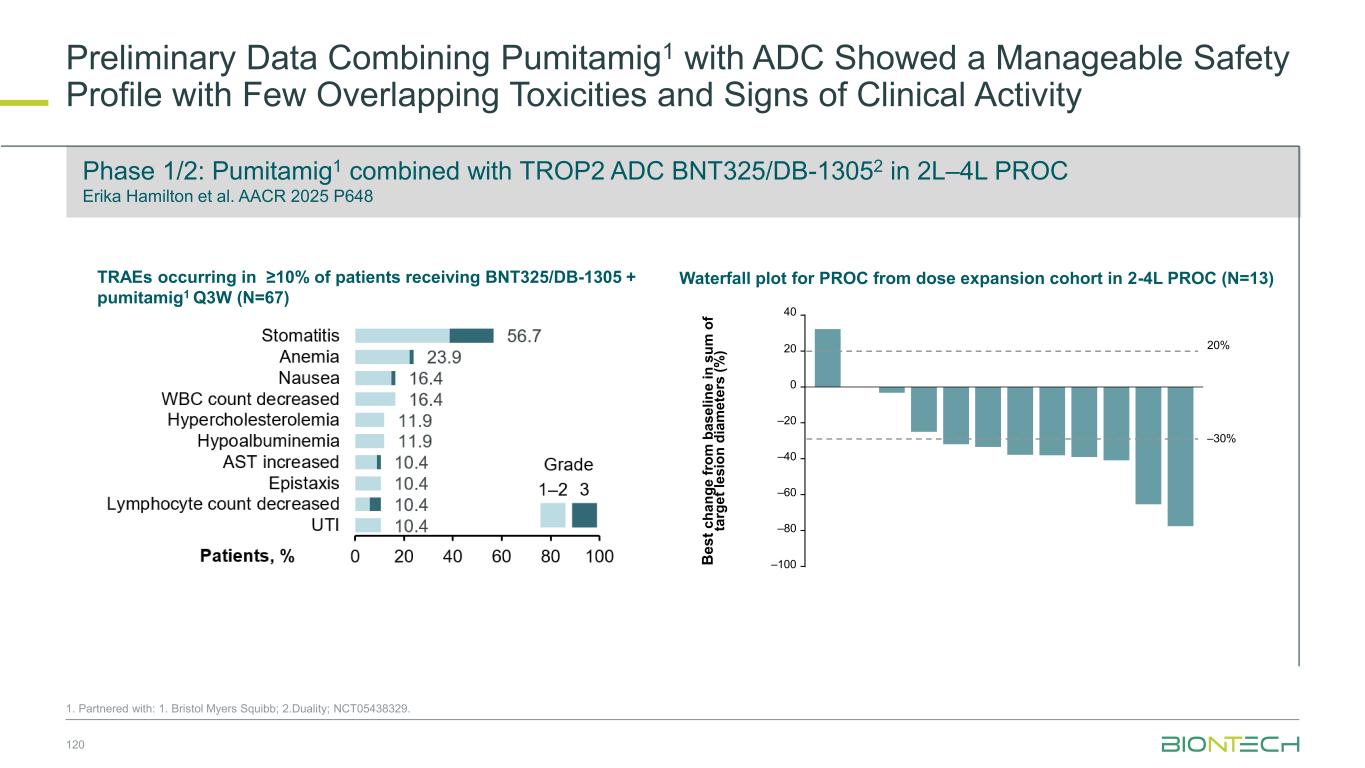
Preliminary Data Combining Pumitamig1 with ADC Showed a Manageable Safety Profile with Few Overlapping Toxicities and Signs of Clinical Activity 120 Phase 1/2: Pumitamig1 combined with TROP2 ADC BNT325/DB-13052 in 2L–4L PROC Erika Hamilton et al. AACR 2025 P648 1. Partnered with: 1. Bristol Myers Squibb; 2.Duality; NCT05438329. 20% 0 –20 –40 –60 –80 –100B e s t c h a n g e f ro m b a s e li n e i n s u m o f ta rg e t le s io n d ia m e te rs ( % ) –30% 20 40 Waterfall plot for PROC from dose expansion cohort in 2-4L PROC (N=13)TRAEs occurring in ≥10% of patients receiving BNT325/DB-1305 + pumitamig1 Q3W (N=67)
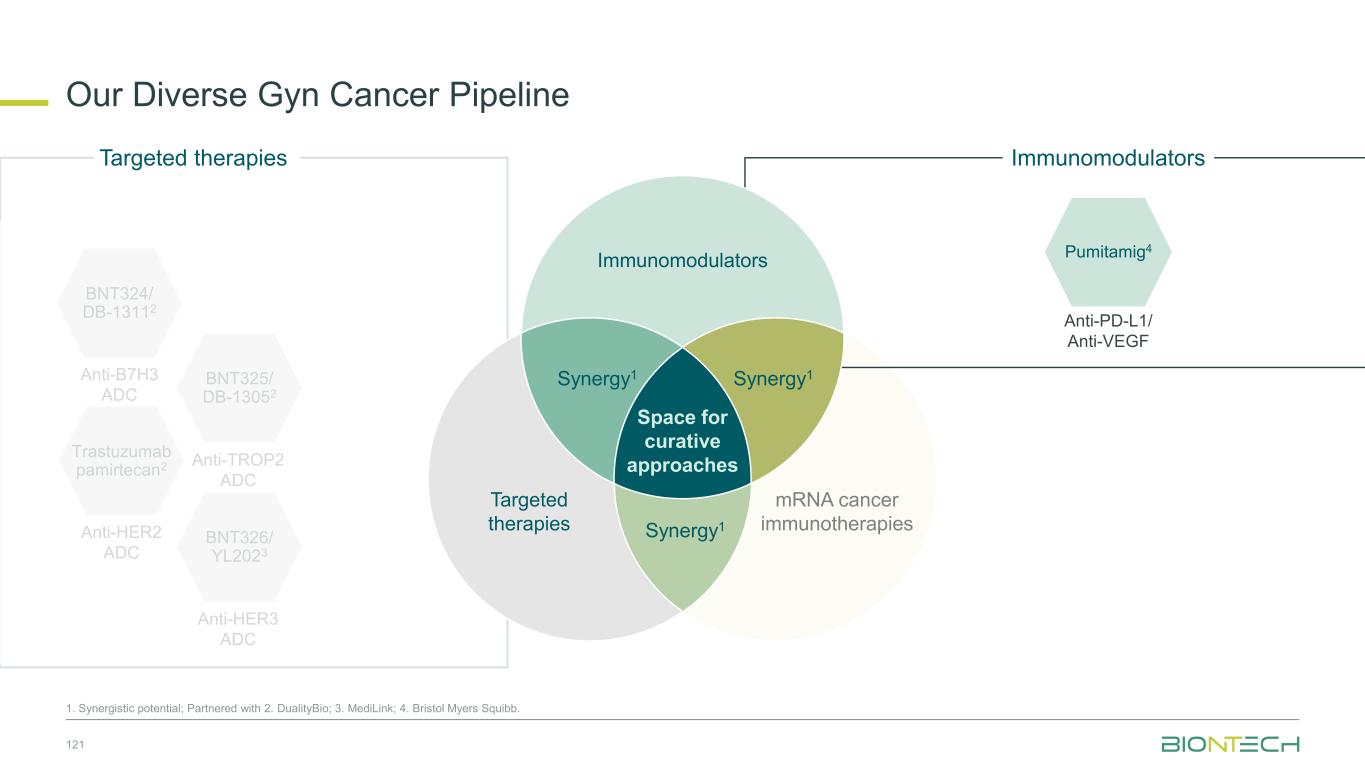
Our Diverse Gyn Cancer Pipeline 121 1. Synergistic potential; Partnered with 2. DualityBio; 3. MediLink; 4. Bristol Myers Squibb. Trastuzumab pamirtecan2 Anti-HER2 ADC BNT326/ YL2023 Anti-HER3 ADC Targeted therapies BNT325/ DB-13052 Anti-TROP2 ADC mRNA cancer immunotherapies Immunomodulators Anti-PD-L1/ Anti-VEGF Pumitamig4 Immunomodulators Space for curative approaches Synergy1 Targeted therapies Synergy1Synergy1 BNT324/ DB-13112 Anti-B7H3 ADC
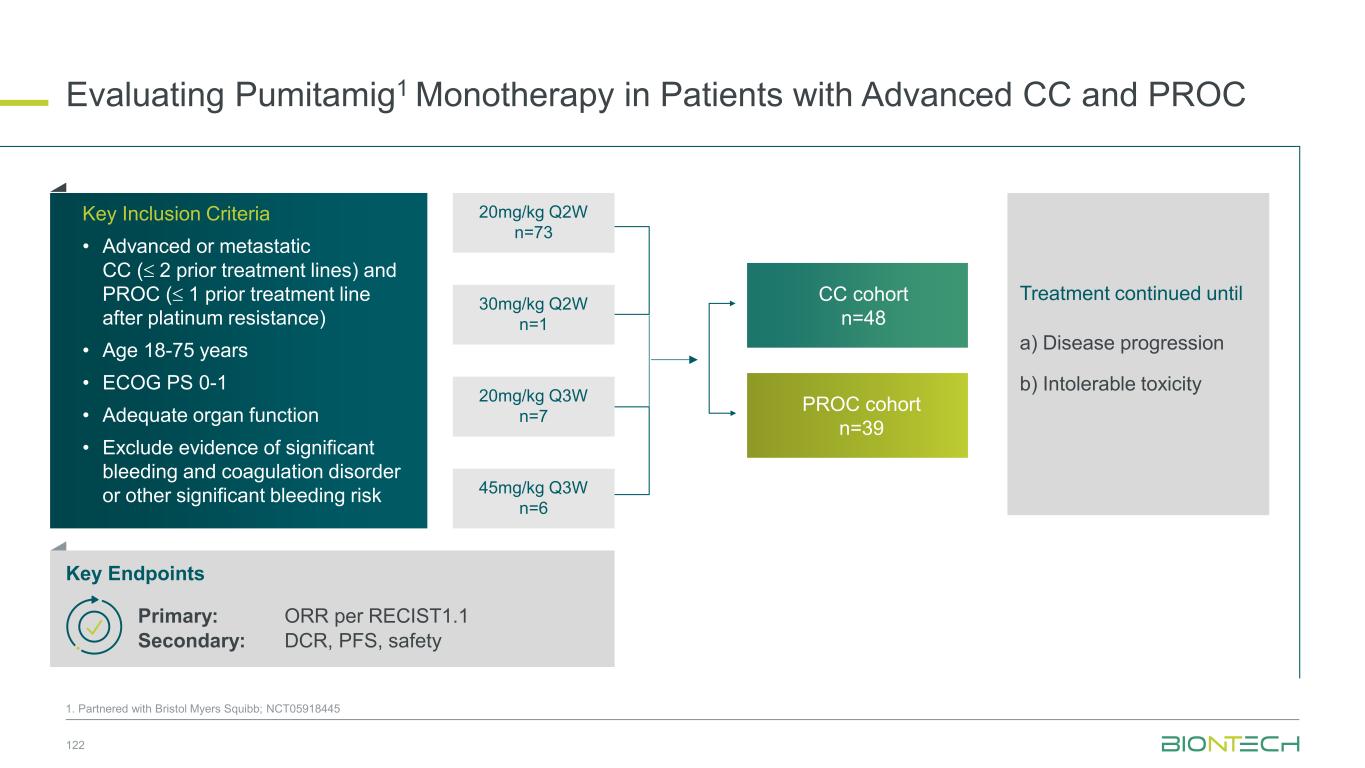
Evaluating Pumitamig1 Monotherapy in Patients with Advanced CC and PROC 1. Partnered with Bristol Myers Squibb; NCT05918445 122 Key Inclusion Criteria • Advanced or metastatic CC ( 2 prior treatment lines) and PROC ( 1 prior treatment line after platinum resistance) • Age 18-75 years • ECOG PS 0-1 • Adequate organ function • Exclude evidence of significant bleeding and coagulation disorder or other significant bleeding risk CC cohort n=48 PROC cohort n=39 Treatment continued until a) Disease progression b) Intolerable toxicity Key Endpoints Primary: ORR per RECIST1.1 Secondary: DCR, PFS, safety 20mg/kg Q2W n=73 30mg/kg Q2W n=1 20mg/kg Q3W n=7 45mg/kg Q3W n=6
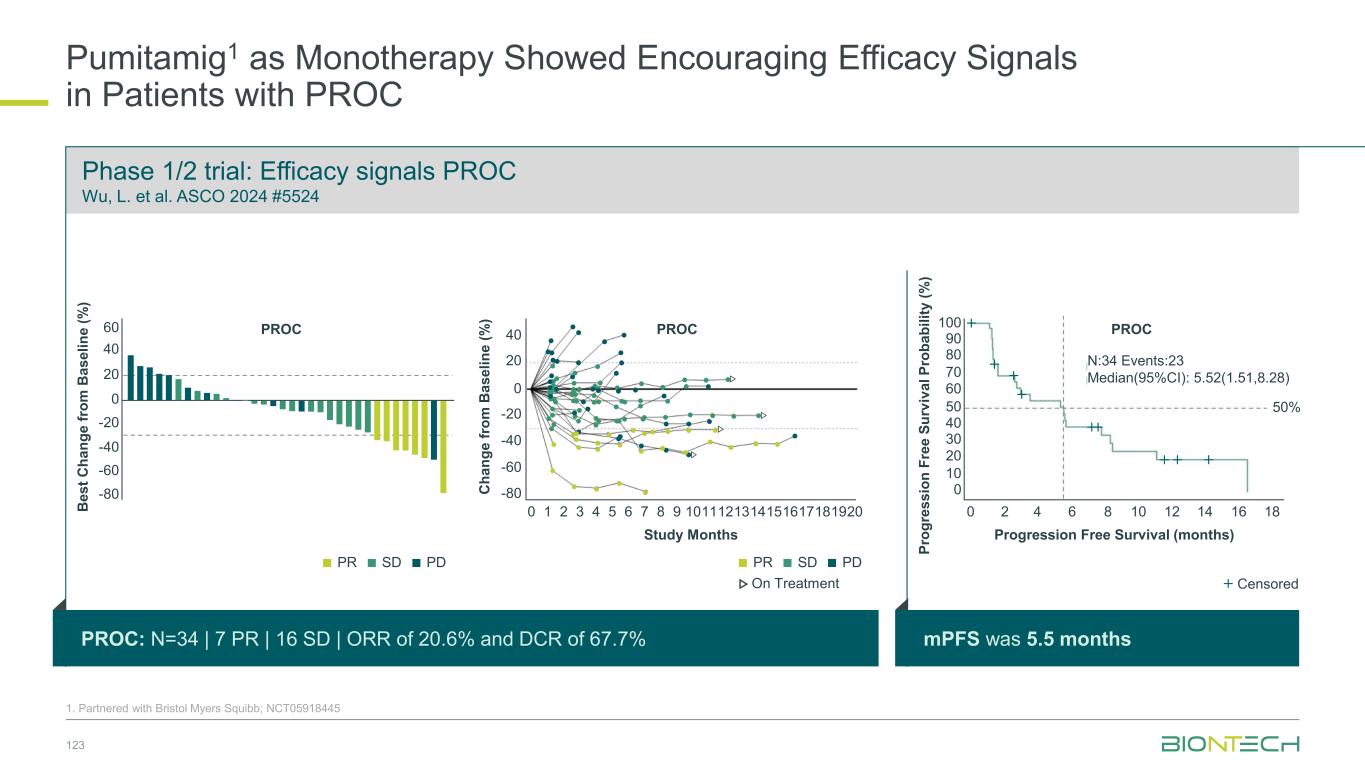
Pumitamig1 as Monotherapy Showed Encouraging Efficacy Signals in Patients with PROC 123 1. Partnered with Bristol Myers Squibb; NCT05918445 Phase 1/2 trial: Efficacy signals PROC Wu, L. et al. ASCO 2024 #5524 PROC: N=34 | 7 PR | 16 SD | ORR of 20.6% and DCR of 67.7% mPFS was 5.5 months B e s t C h a n g e f ro m B a s e li n e ( % ) PROC C h a n g e fr o m B a s e li n e ( % ) 40 20 0 -20 -40 -60 -80 0 1 2 3 4 5 6 7 8 9 1011121314151617181920 Study Months 40 20 0 -20 -40 -60 -80 60 PROC PDSDPR On Treatment PDSDPR 0 P ro g re s s io n F re e S u rv iv a l P ro b a b il it y ( % ) 90 70 60 40 30 20 10 100 80 50 0 2 4 6 8 10 12 14 16 18 Progression Free Survival (months) Censored PROC N:34 Events:23 Median(95%CI): 5.52(1.51,8.28) 50%
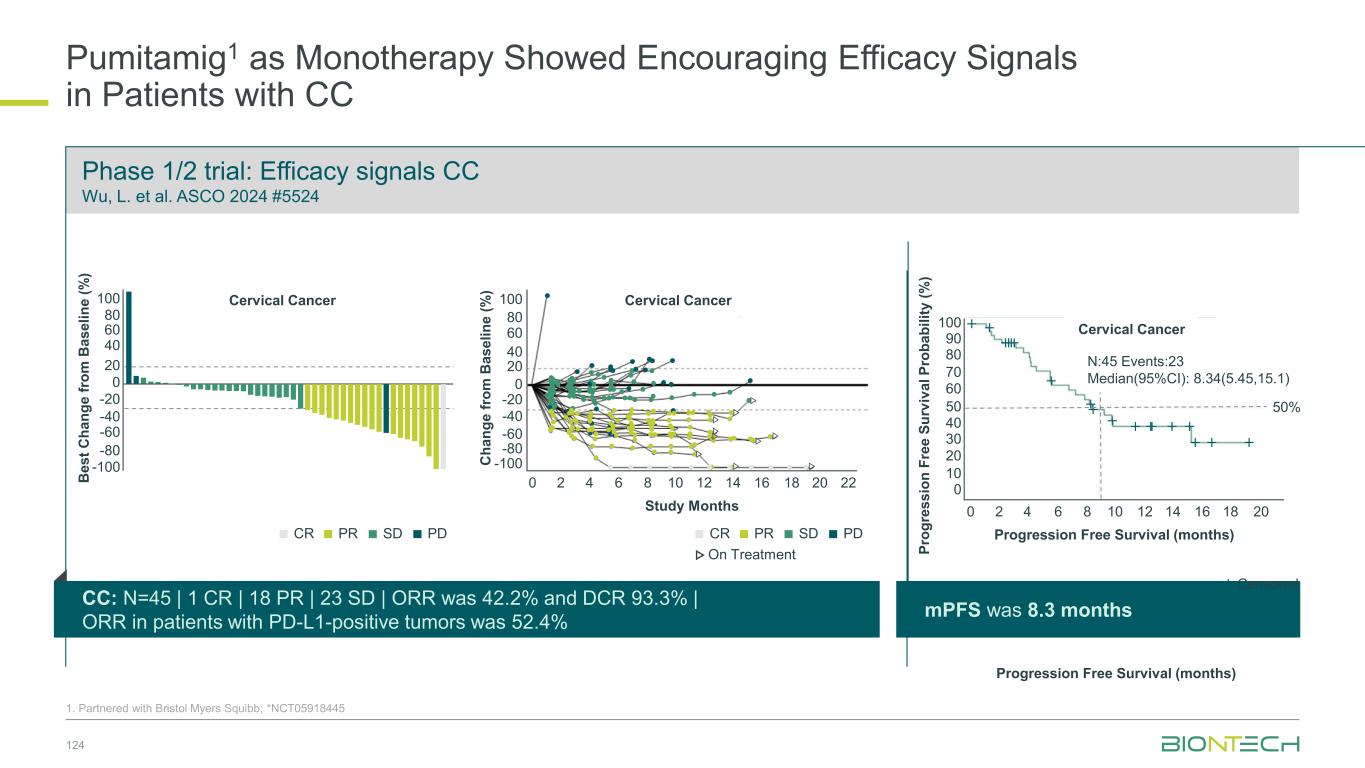
Pumitamig1 as Monotherapy Showed Encouraging Efficacy Signals in Patients with CC 124 1. Partnered with Bristol Myers Squibb; *NCT05918445 Phase 1/2 trial: Efficacy signals CC Wu, L. et al. ASCO 2024 #5524 CC: N=45 | 1 CR | 18 PR | 23 SD | ORR was 42.2% and DCR 93.3% | ORR in patients with PD-L1-positive tumors was 52.4% mPFS was 8.3 months B e s t C h a n g e fr o m B a s e li n e ( % ) 80 40 20 -20 -40 -60 -80 100 60 0 -100 Cervical Cancer C h a n g e fr o m B a s e li n e ( % ) 100 0 2 4 6 8 10 12 14 16 18 20 80 60 40 20 0 -20 -40 -60 -80 -100 22 Study Months Cervical Cancer PDSDPRCR Progression Free Survival (months) On Treatment PDSDPRCR 0 P ro g re s s io n F re e S u rv iv a l P ro b a b il it y ( % ) 90 70 60 40 30 20 10 100 80 50 0 2 4 6 8 10 12 14 16 18 20 Progression Free Survival (months) Censored 50% Cervical Cancer N:45 Events:23 Median(95%CI): 8.34(5.45,15.1)
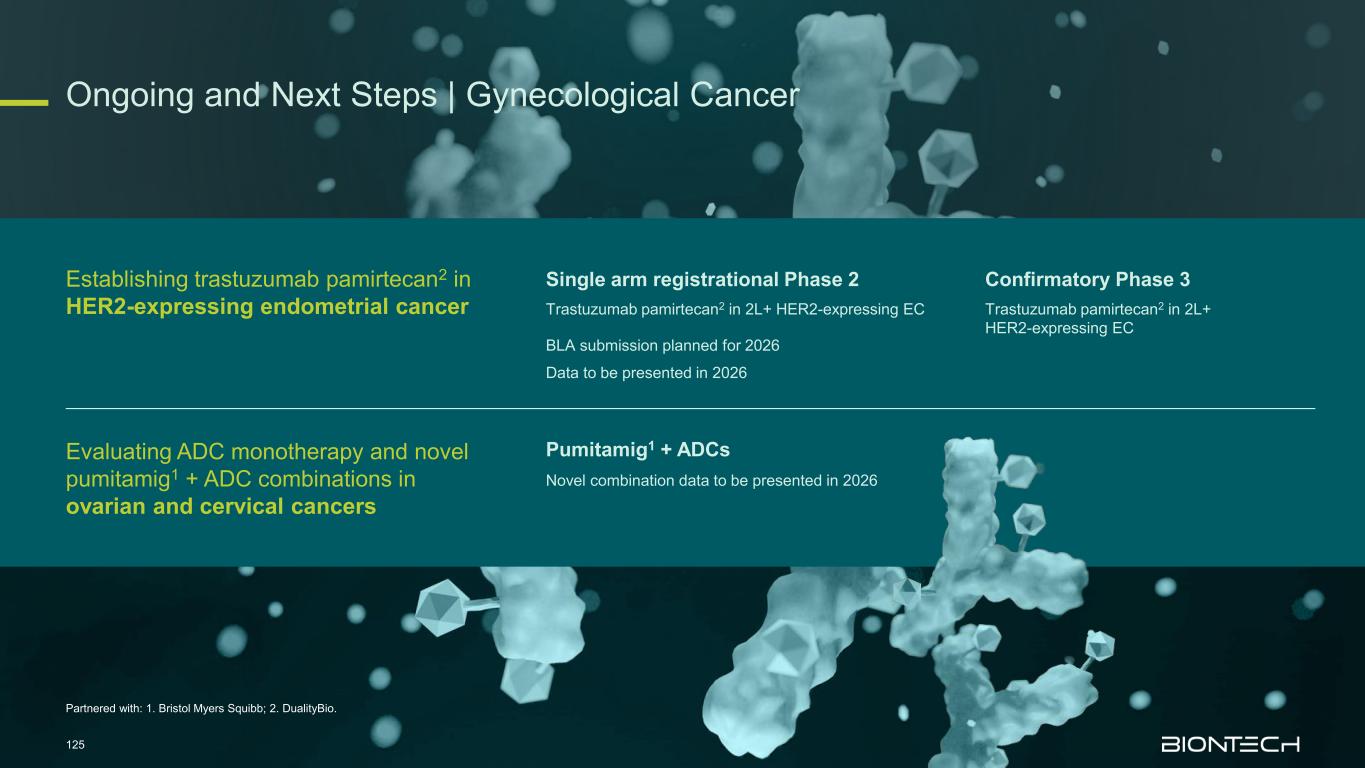
Partnered with: 1. Bristol Myers Squibb; 2. DualityBio. Ongoing and Next Steps | Gynecological Cancer Establishing trastuzumab pamirtecan2 in HER2-expressing endometrial cancer Evaluating ADC monotherapy and novel pumitamig1 + ADC combinations in ovarian and cervical cancers Pumitamig1 + ADCs Novel combination data to be presented in 2026 125 Single arm registrational Phase 2 Trastuzumab pamirtecan2 in 2L+ HER2-expressing EC Confirmatory Phase 3 Trastuzumab pamirtecan2 in 2L+ HER2-expressing EC BLA submission planned for 2026 Data to be presented in 2026

Gastrointestinal Cancers
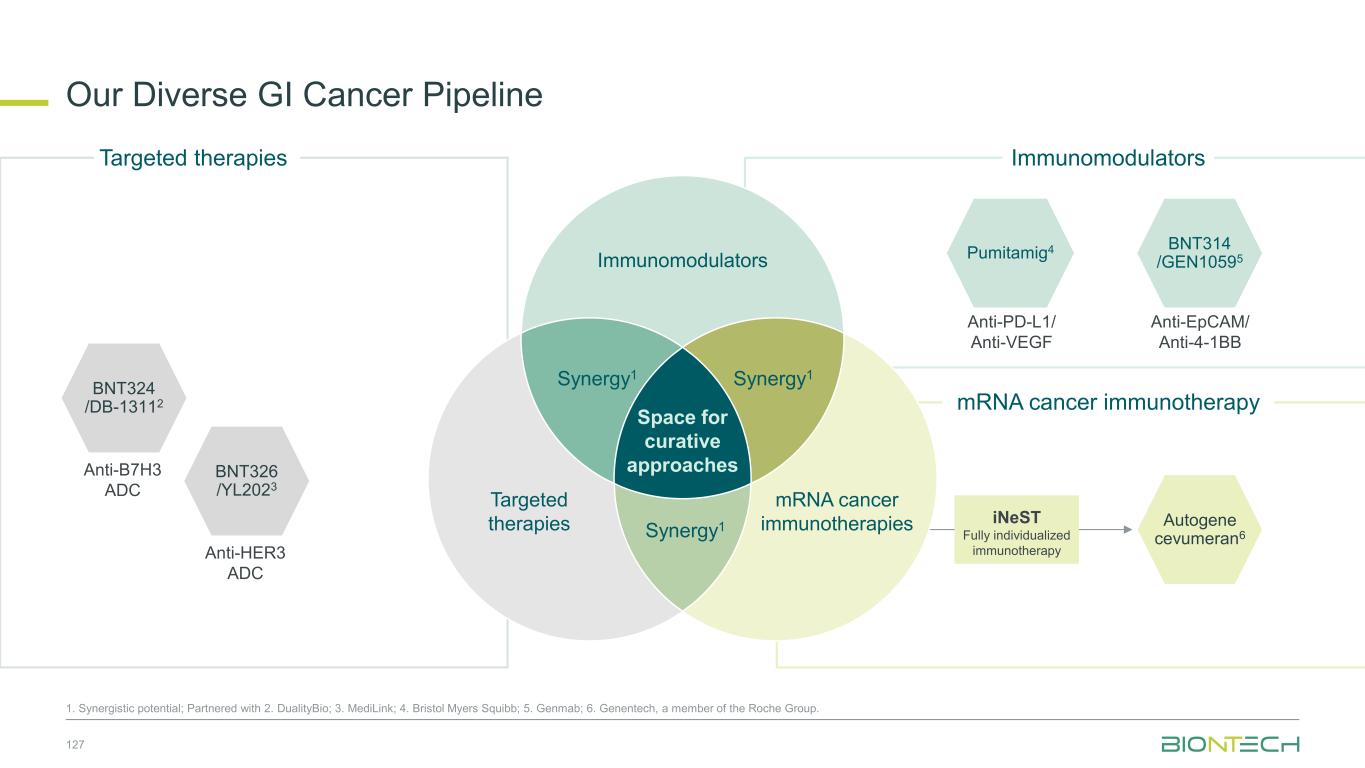
Anti-PD-L1/ Anti-VEGF Pumitamig4 Autogene cevumeran6 Immunomodulators mRNA cancer immunotherapy Our Diverse GI Cancer Pipeline 127 1. Synergistic potential; Partnered with 2. DualityBio; 3. MediLink; 4. Bristol Myers Squibb; 5. Genmab; 6. Genentech, a member of the Roche Group. Targeted therapies iNeST Fully individualized immunotherapy BNT314 /GEN10595 Anti-EpCAM/ Anti-4-1BB BNT326 /YL2023 Anti-HER3 ADC BNT324 /DB-13112 Anti-B7H3 ADC Immunomodulators Space for curative approaches Synergy1 Targeted therapies mRNA cancer immunotherapies Synergy1Synergy1
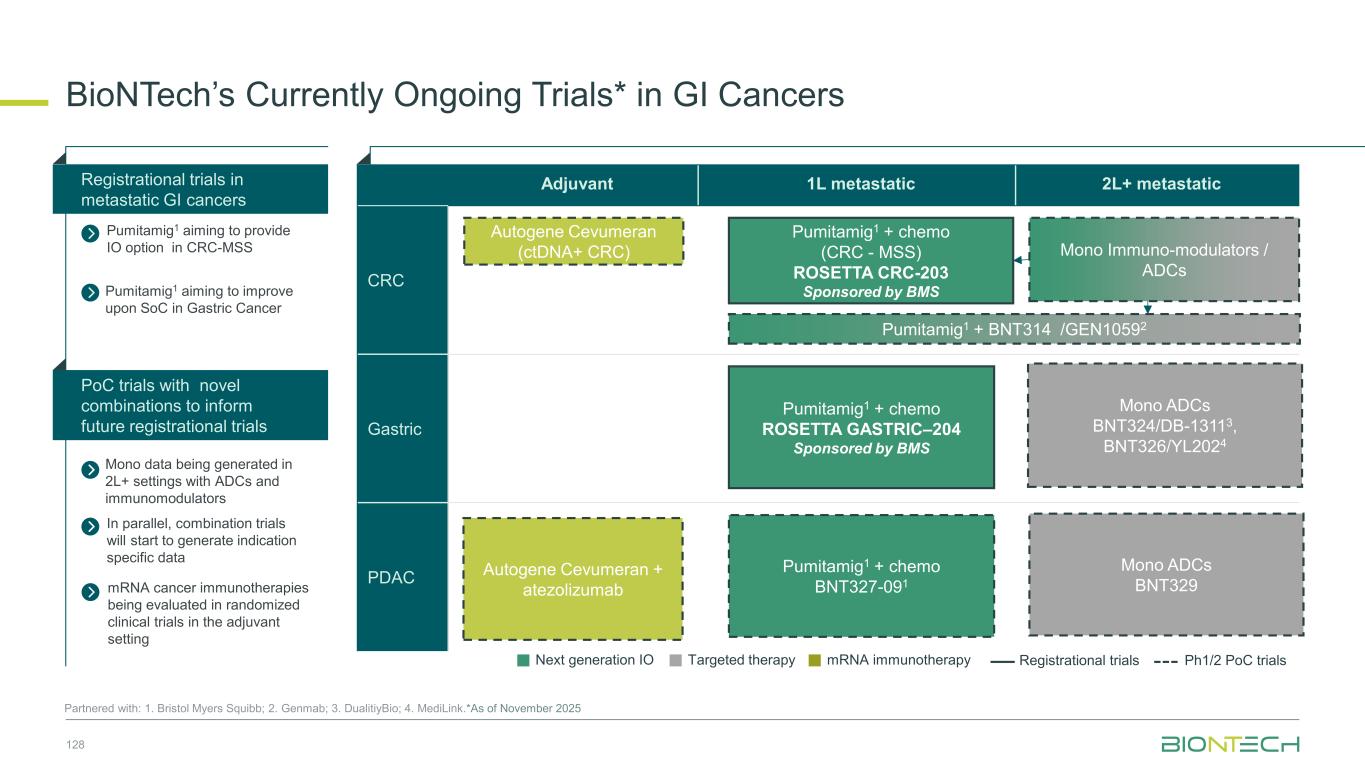
Adjuvant 1L metastatic 2L+ metastatic CRC Gastric PDAC BioNTech’s Currently Ongoing Trials* in GI Cancers 128 Partnered with: 1. Bristol Myers Squibb; 2. Genmab; 3. DualitiyBio; 4. MediLink.*As of November 2025 Pumitamig1 + chemo (CRC - MSS) ROSETTA CRC-203 Sponsored by BMS Pumitamig1 + chemo BNT327-091 Pumitamig1 + chemo ROSETTA GASTRIC–204 Sponsored by BMS Mono Immuno-modulators / ADCs Pumitamig1 + BNT314 /GEN10592 Mono ADCs BNT329 Mono data being generated in 2L+ settings with ADCs and immunomodulators Pumitamig1 aiming to provide IO option in CRC-MSS PoC trials with novel combinations to inform future registrational trials Registrational trials in metastatic GI cancers Mono ADCs BNT324/DB-13113, BNT326/YL2024 Pumitamig1 aiming to improve upon SoC in Gastric Cancer In parallel, combination trials will start to generate indication specific data Next generation IO Targeted therapy mRNA immunotherapy Registrational trials Ph1/2 PoC trials Autogene Cevumeran + atezolizumab Autogene Cevumeran (ctDNA+ CRC) mRNA cancer immunotherapies being evaluated in randomized clinical trials in the adjuvant setting
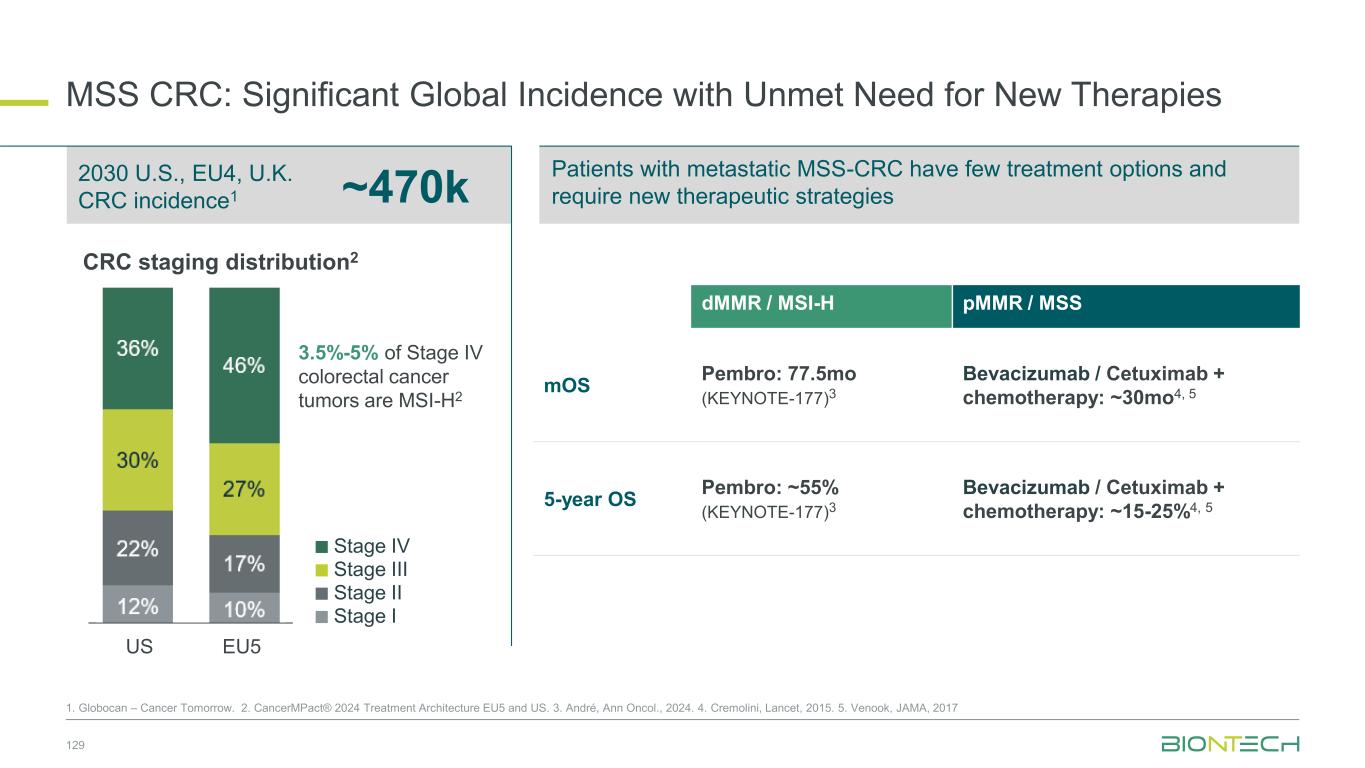
Patients with metastatic MSS-CRC have few treatment options and require new therapeutic strategies MSS CRC: Significant Global Incidence with Unmet Need for New Therapies 129 1. Globocan – Cancer Tomorrow. 2. CancerMPact® 2024 Treatment Architecture EU5 and US. 3. André, Ann Oncol., 2024. 4. Cremolini, Lancet, 2015. 5. Venook, JAMA, 2017 ~470k 2030 U.S., EU4, U.K. CRC incidence1 7% 8% 10% 11% 37% 44% 46% 37% US EU5 dMMR / MSI-H pMMR / MSS mOS Pembro: 77.5mo (KEYNOTE-177)3 Bevacizumab / Cetuximab + chemotherapy: ~30mo4, 5 5-year OS Pembro: ~55% (KEYNOTE-177)3 Bevacizumab / Cetuximab + chemotherapy: ~15-25%4, 5 3.5%-5% of Stage IV colorectal cancer tumors are MSI-H2 CRC staging distribution2 Stage II Stage III Stage IV Stage I
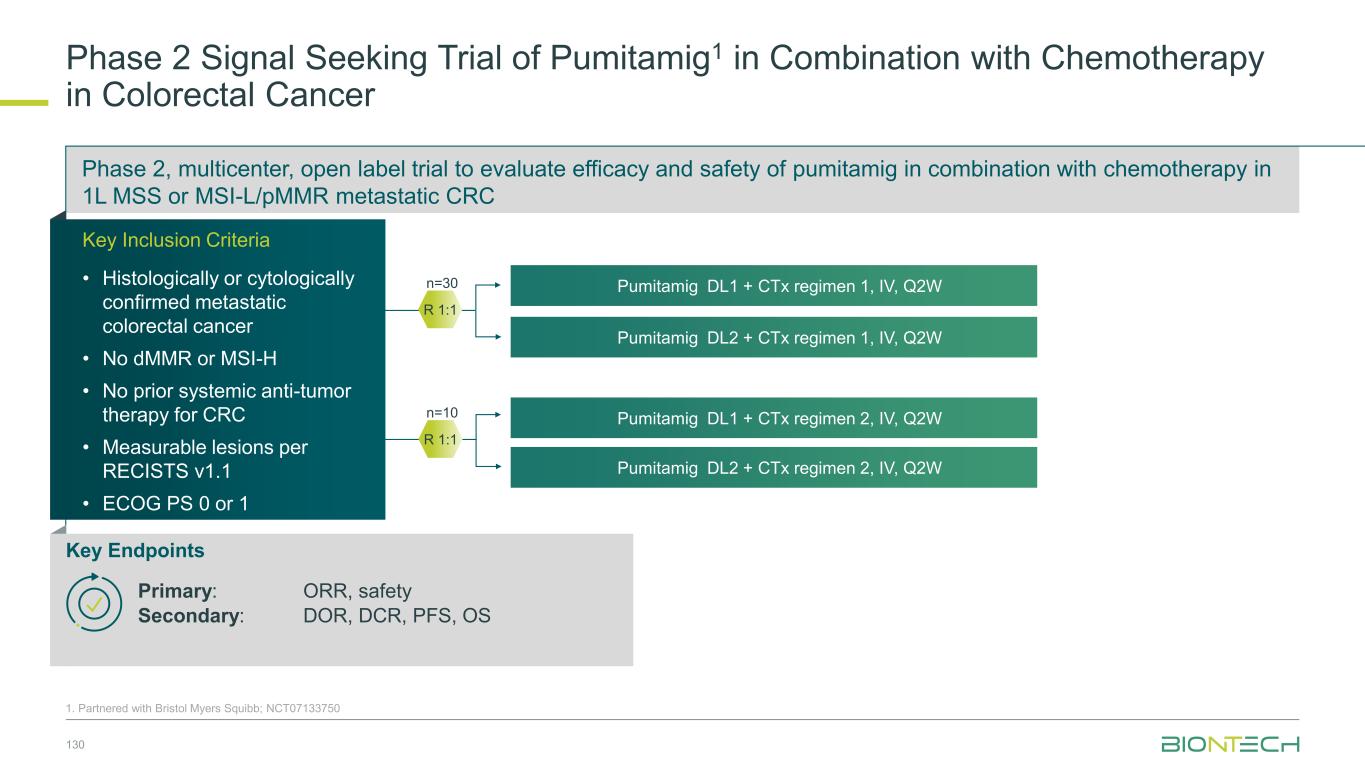
Phase 2, multicenter, open label trial to evaluate efficacy and safety of pumitamig in combination with chemotherapy in 1L MSS or MSI-L/pMMR metastatic CRC Phase 2 Signal Seeking Trial of Pumitamig1 in Combination with Chemotherapy in Colorectal Cancer 130 1. Partnered with Bristol Myers Squibb; NCT07133750 Key Endpoints Primary: ORR, safety Secondary: DOR, DCR, PFS, OS R 1:1 Pumitamig DL1 + CTx regimen 1, IV, Q2W Pumitamig DL2 + CTx regimen 1, IV, Q2W Key Inclusion Criteria • Histologically or cytologically confirmed metastatic colorectal cancer • No dMMR or MSI-H • No prior systemic anti-tumor therapy for CRC • Measurable lesions per RECISTS v1.1 • ECOG PS 0 or 1 R 1:1 n=30 n=10 Pumitamig DL1 + CTx regimen 2, IV, Q2W Pumitamig DL2 + CTx regimen 2, IV, Q2W
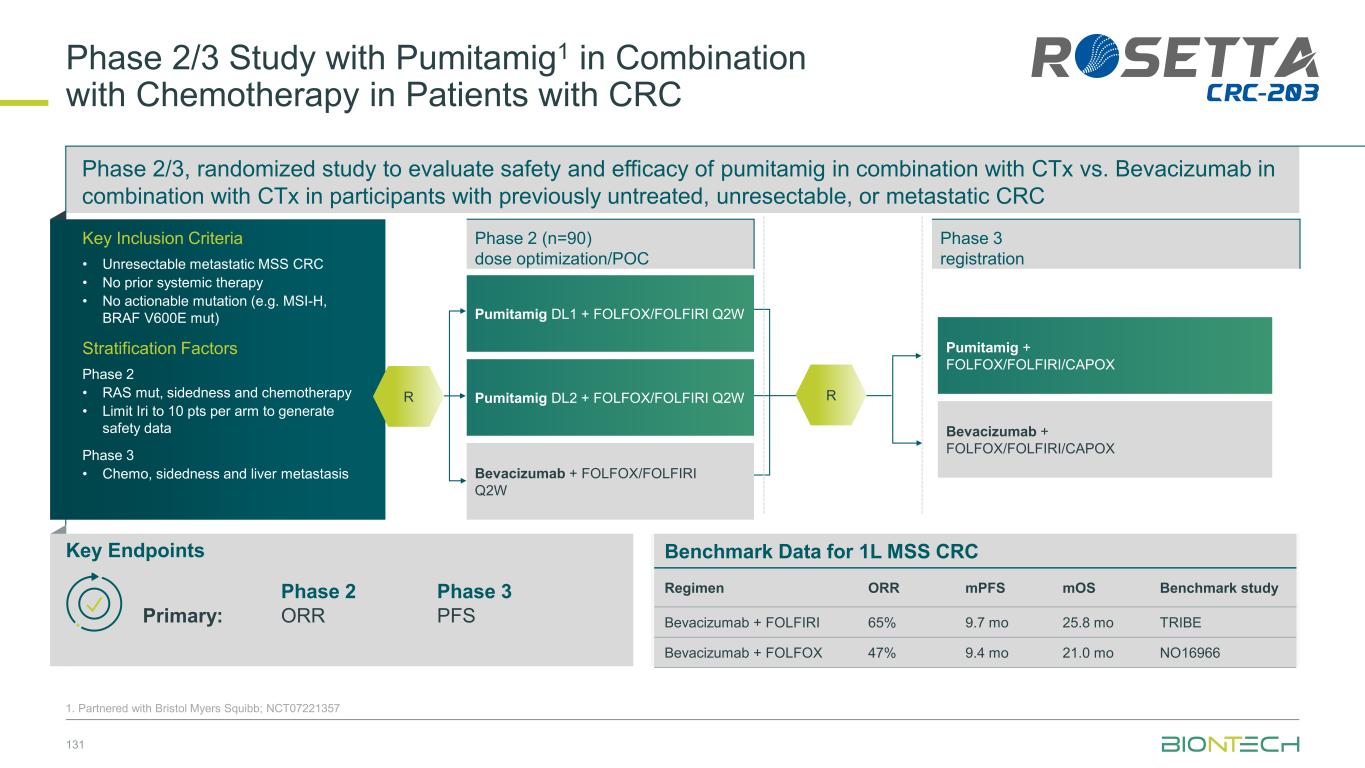
Phase 2/3, randomized study to evaluate safety and efficacy of pumitamig in combination with CTx vs. Bevacizumab in combination with CTx in participants with previously untreated, unresectable, or metastatic CRC Phase 2/3 Study with Pumitamig1 in Combination with Chemotherapy in Patients with CRC 131 1. Partnered with Bristol Myers Squibb; NCT07221357 Pumitamig + FOLFOX/FOLFIRI/CAPOX Bevacizumab + FOLFOX/FOLFIRI/CAPOX Pumitamig DL1 + FOLFOX/FOLFIRI Q2W Pumitamig DL2 + FOLFOX/FOLFIRI Q2W Bevacizumab + FOLFOX/FOLFIRI Q2W Phase 2 (n=90) dose optimization/POC Phase 3 registration R Benchmark Data for 1L MSS CRC Regimen ORR mPFS mOS Benchmark study Bevacizumab + FOLFIRI 65% 9.7 mo 25.8 mo TRIBE Bevacizumab + FOLFOX 47% 9.4 mo 21.0 mo NO16966 Key Endpoints Phase 2 Phase 3 Primary: ORR PFS Key Inclusion Criteria • Unresectable metastatic MSS CRC • No prior systemic therapy • No actionable mutation (e.g. MSI-H, BRAF V600E mut) Stratification Factors Phase 2 • RAS mut, sidedness and chemotherapy • Limit Iri to 10 pts per arm to generate safety data Phase 3 • Chemo, sidedness and liver metastasis R
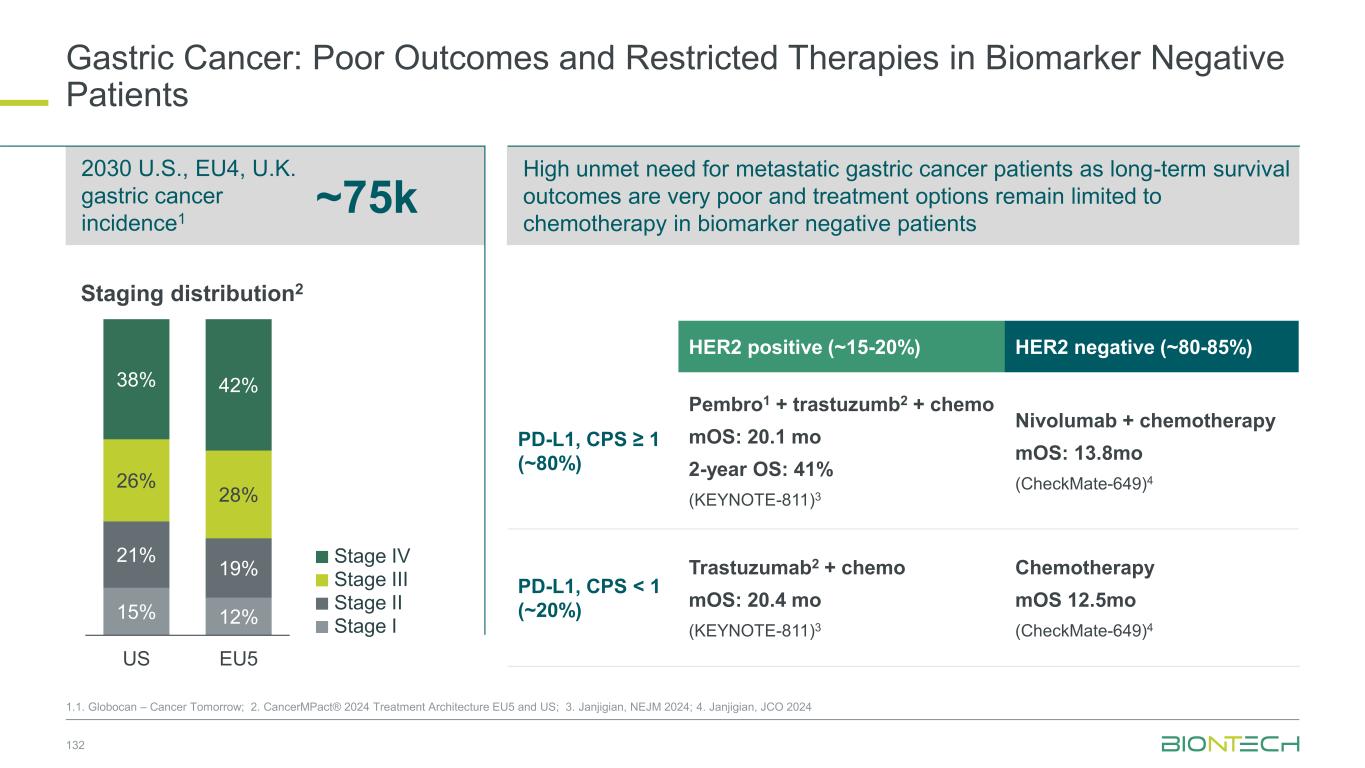
132 ~75k 2030 U.S., EU4, U.K. gastric cancer incidence1 1.1. Globocan – Cancer Tomorrow; 2. CancerMPact® 2024 Treatment Architecture EU5 and US; 3. Janjigian, NEJM 2024; 4. Janjigian, JCO 2024 Gastric Cancer: Poor Outcomes and Restricted Therapies in Biomarker Negative Patients 15% 12% 21% 19% 26% 28% 38% 42% US EU5 Staging distribution2 HER2 positive (~15-20%) HER2 negative (~80-85%) PD-L1, CPS ≥ 1 (~80%) Pembro1 + trastuzumb2 + chemo mOS: 20.1 mo 2-year OS: 41% (KEYNOTE-811)3 Nivolumab + chemotherapy mOS: 13.8mo (CheckMate-649)4 PD-L1, CPS < 1 (~20%) Trastuzumab2 + chemo mOS: 20.4 mo (KEYNOTE-811)3 Chemotherapy mOS 12.5mo (CheckMate-649)4 High unmet need for metastatic gastric cancer patients as long-term survival outcomes are very poor and treatment options remain limited to chemotherapy in biomarker negative patients Stage II Stage III Stage IV Stage I
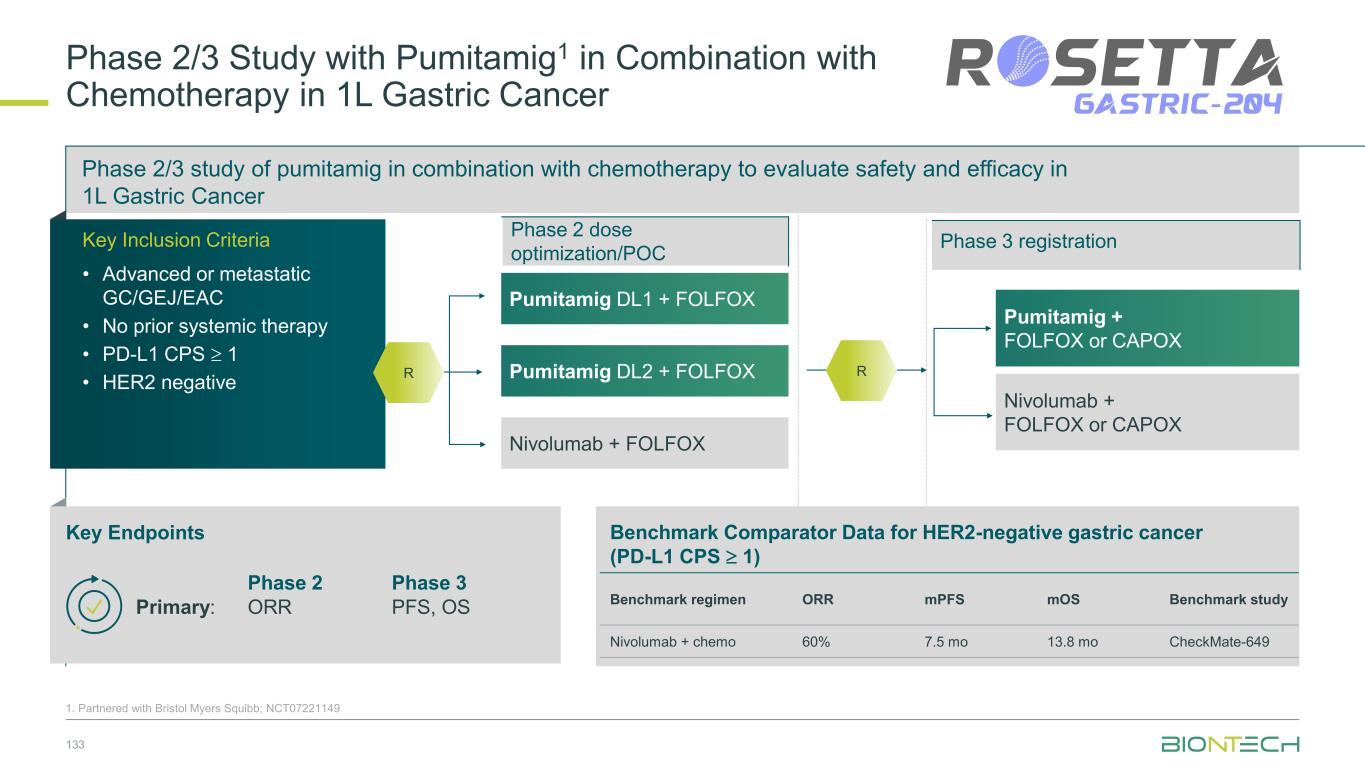
Phase 2/3 Study with Pumitamig1 in Combination with Chemotherapy in 1L Gastric Cancer 133 1. Partnered with Bristol Myers Squibb; NCT07221149 Pumitamig + FOLFOX or CAPOX Nivolumab + FOLFOX or CAPOX Pumitamig DL1 + FOLFOX Pumitamig DL2 + FOLFOX Nivolumab + FOLFOX Phase 2/3 study of pumitamig in combination with chemotherapy to evaluate safety and efficacy in 1L Gastric Cancer Key Inclusion Criteria • Advanced or metastatic GC/GEJ/EAC • No prior systemic therapy • PD-L1 CPS 1 • HER2 negative Key Endpoints Benchmark Comparator Data for HER2-negative gastric cancer (PD-L1 CPS 1) Benchmark regimen ORR mPFS mOS Benchmark study Nivolumab + chemo 60% 7.5 mo 13.8 mo CheckMate-649 Phase 2 Phase 3 Primary: ORR PFS, OS R Phase 2 dose optimization/POC Phase 3 registration R
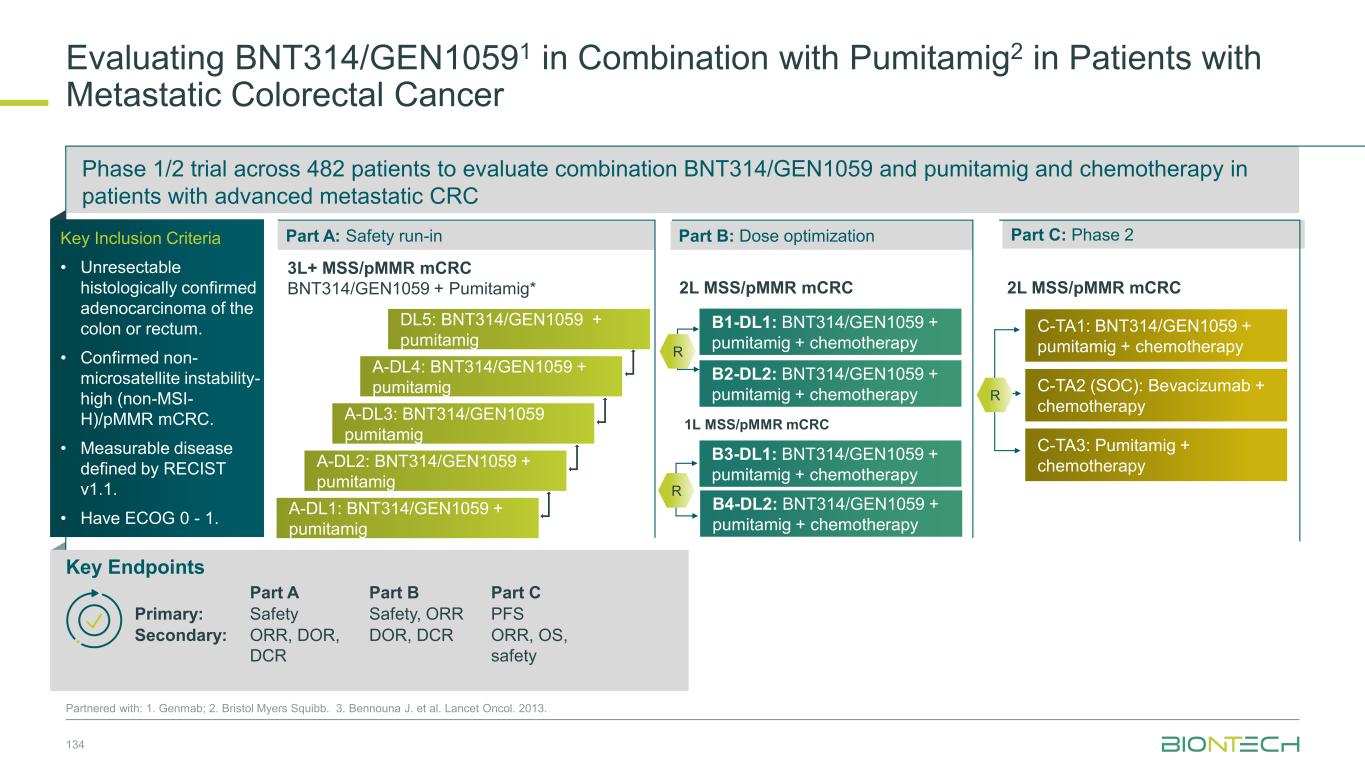
Phase 1/2 trial across 482 patients to evaluate combination BNT314/GEN1059 and pumitamig and chemotherapy in patients with advanced metastatic CRC 134 Partnered with: 1. Genmab; 2. Bristol Myers Squibb. 3. Bennouna J. et al. Lancet Oncol. 2013. Evaluating BNT314/GEN10591 in Combination with Pumitamig2 in Patients with Metastatic Colorectal Cancer Key Endpoints Key Inclusion Criteria • Unresectable histologically confirmed adenocarcinoma of the colon or rectum. • Confirmed non- microsatellite instability- high (non-MSI- H)/pMMR mCRC. • Measurable disease defined by RECIST v1.1. • Have ECOG 0 - 1. Part A Part B Part C Primary: Safety Safety, ORR PFS Secondary: ORR, DOR, DCR DOR, DCR ORR, OS, safety Part A: Safety run-in Part B: Dose optimization Part C: Phase 2 DL5: BNT314/GEN1059 + pumitamig A-DL4: BNT314/GEN1059 + pumitamig A-DL3: BNT314/GEN1059 pumitamig A-DL2: BNT314/GEN1059 + pumitamig A-DL1: BNT314/GEN1059 + pumitamig 3L+ MSS/pMMR mCRC BNT314/GEN1059 + Pumitamig* 2L MSS/pMMR mCRC B2-DL2: BNT314/GEN1059 + pumitamig + chemotherapy B1-DL1: BNT314/GEN1059 + pumitamig + chemotherapy R B3-DL1: BNT314/GEN1059 + pumitamig + chemotherapy 1L MSS/pMMR mCRC R B4-DL2: BNT314/GEN1059 + pumitamig + chemotherapy C-TA2 (SOC): Bevacizumab + chemotherapy C-TA1: BNT314/GEN1059 + pumitamig + chemotherapy C-TA3: Pumitamig + chemotherapy R 2L MSS/pMMR mCRC
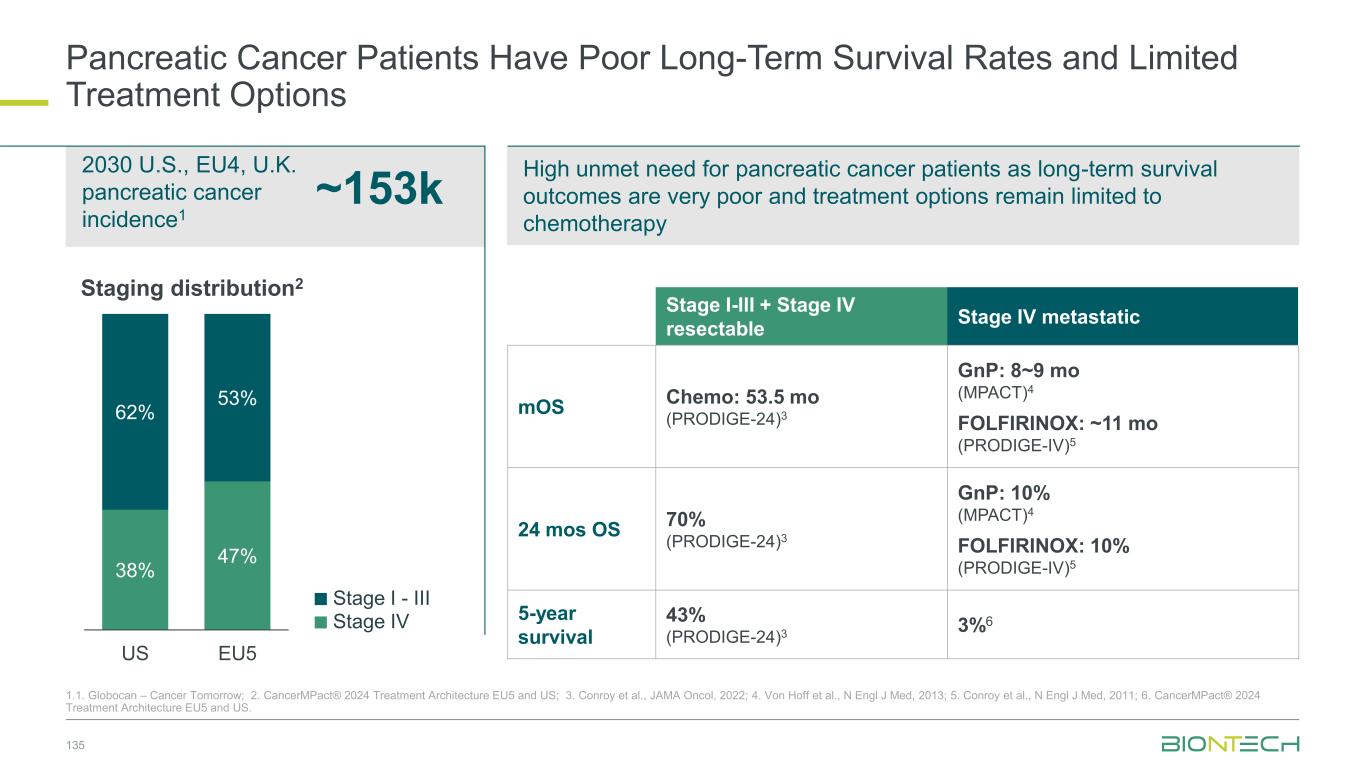
135 ~153k 2030 U.S., EU4, U.K. pancreatic cancer incidence1 1.1. Globocan – Cancer Tomorrow; 2. CancerMPact® 2024 Treatment Architecture EU5 and US; 3. Conroy et al., JAMA Oncol, 2022; 4. Von Hoff et al., N Engl J Med, 2013; 5. Conroy et al., N Engl J Med, 2011; 6. CancerMPact® 2024 Treatment Architecture EU5 and US. Pancreatic Cancer Patients Have Poor Long-Term Survival Rates and Limited Treatment Options 38% 47% 62% 53% US EU5 Staging distribution2 Stage I-III + Stage IV resectable Stage IV metastatic mOS Chemo: 53.5 mo (PRODIGE-24)3 GnP: 8~9 mo (MPACT)4 FOLFIRINOX: ~11 mo (PRODIGE-IV)5 24 mos OS 70% (PRODIGE-24)3 GnP: 10% (MPACT)4 FOLFIRINOX: 10% (PRODIGE-IV)5 5-year survival 43% (PRODIGE-24)3 3%6 High unmet need for pancreatic cancer patients as long-term survival outcomes are very poor and treatment options remain limited to chemotherapy Stage IV Stage I - III
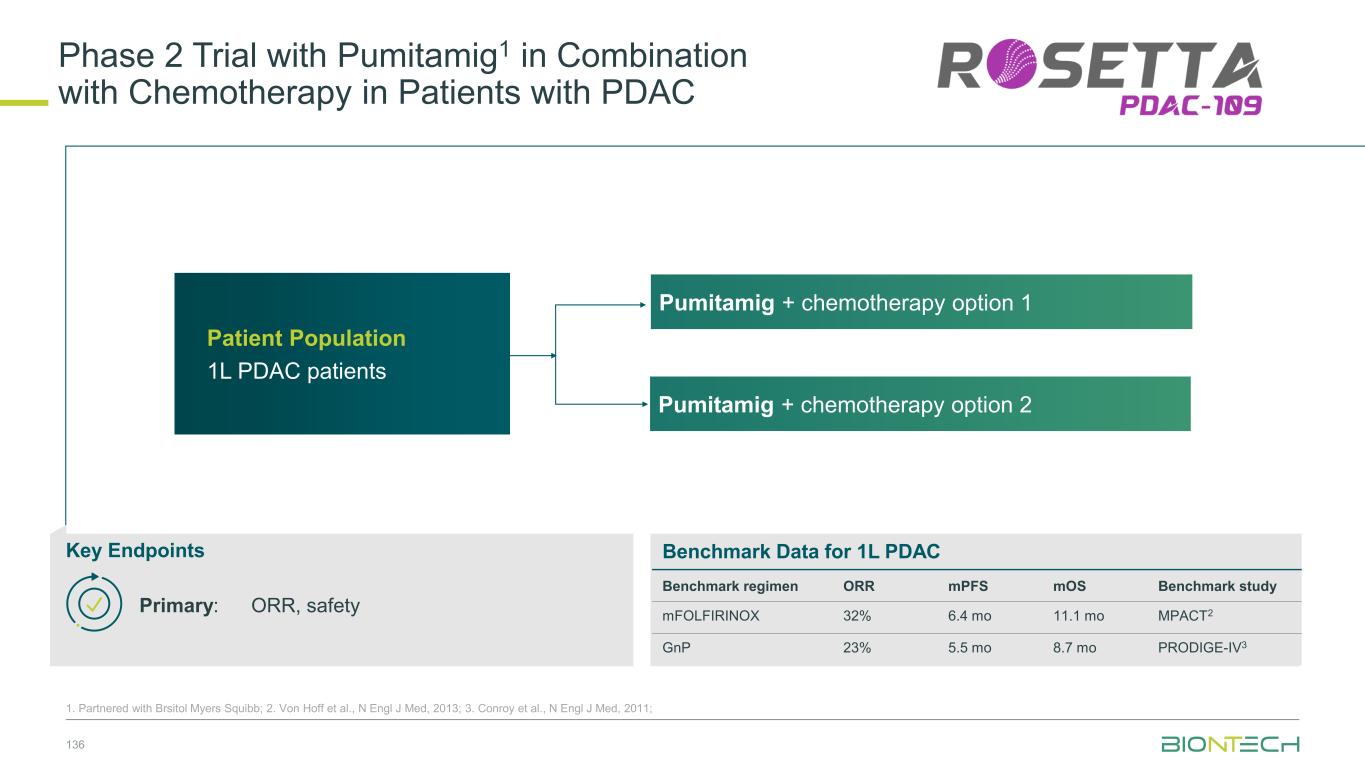
Phase 2 Trial with Pumitamig1 in Combination with Chemotherapy in Patients with PDAC 136 Patient Population 1L PDAC patients Key Endpoints Benchmark Data for 1L PDAC Benchmark regimen ORR mPFS mOS Benchmark study mFOLFIRINOX 32% 6.4 mo 11.1 mo MPACT2 GnP 23% 5.5 mo 8.7 mo PRODIGE-IV3 Primary: ORR, safety Pumitamig + chemotherapy option 1 Pumitamig + chemotherapy option 2 1. Partnered with Brsitol Myers Squibb; 2. Von Hoff et al., N Engl J Med, 2013; 3. Conroy et al., N Engl J Med, 2011;
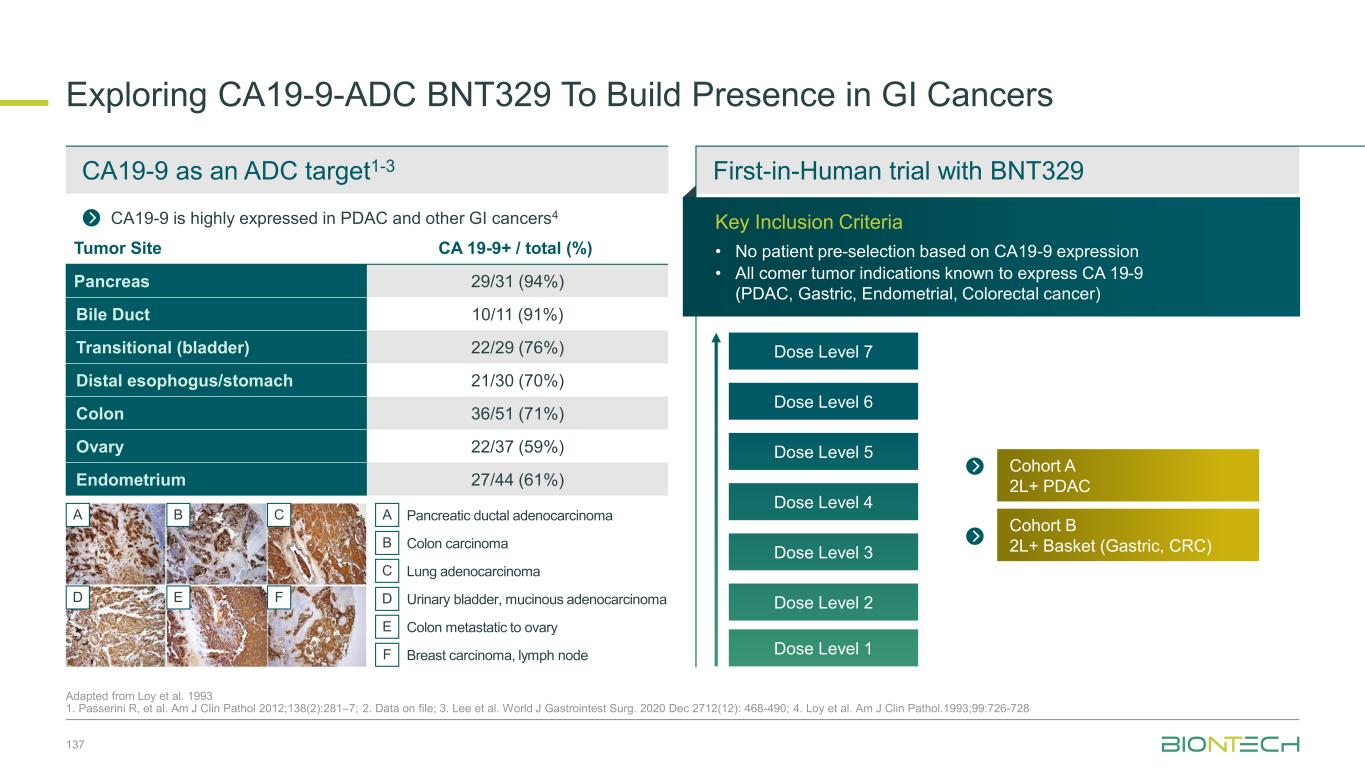
Exploring CA19-9-ADC BNT329 To Build Presence in GI Cancers 137 Adapted from Loy et al. 1993 1. Passerini R, et al. Am J Clin Pathol 2012;138(2):281–7; 2. Data on file; 3. Lee et al. World J Gastrointest Surg. 2020 Dec 2712(12): 468-490; 4. Loy et al. Am J Clin Pathol.1993;99:726-728 CA19-9 is highly expressed in PDAC and other GI cancers4 ExpansionDose escalation CA19-9 as an ADC target1-3 First-in-Human trial with BNT329 Key Inclusion Criteria • No patient pre-selection based on CA19-9 expression • All comer tumor indications known to express CA 19-9 (PDAC, Gastric, Endometrial, Colorectal cancer) Tumor Site CA 19-9+ / total (%) Pancreas 29/31 (94%) Bile Duct 10/11 (91%) Transitional (bladder) 22/29 (76%) Distal esophogus/stomach 21/30 (70%) Colon 36/51 (71%) Ovary 22/37 (59%) Endometrium 27/44 (61%) A B C D E F Pancreatic ductal adenocarcinomaA B C D E F Colon carcinoma Lung adenocarcinoma Urinary bladder, mucinous adenocarcinoma Colon metastatic to ovary Breast carcinoma, lymph node Dose Level 6 Dose Level 7 Dose Level 5 Dose Level 4 Dose Level 3 Dose Level 2 Dose Level 1 Cohort B 2L+ Basket (Gastric, CRC) Cohort A 2L+ PDAC

Partnered with: 1. Bristol Myers Squibb. Ongoing and Next Steps | Gastrointestinal Cancer 138 Establishing pumitamig1 in gastrointestinal cancers Evaluating novel pumitamig1 + immunomodulator and ADC monotherapy in late-stage disease ROSETTA CRC1 Pumitamig1 + chemotherapy in 1L MSS-CRC ROSETTA Gastric-2041 Pumitamig1 + chemotherapy in 1L HER2-, PD-L1+ gastric Signal-seeking Phase 2 Pumitamig1 + chemotherapy in 1L PDAC Pumitamig1 + immunomodulator ADCs

Genitourinary Cancers
Anti-PD-L1/ Anti-VEGF Pumitamig3 Gotistobart4 Anti-CTLA-4 Immunomodulators Our Diverse GU Cancer Pipeline 140 1. Synergistic potential; Partnered with: 2. DualityBio; 3. Bristol Myers Squibb; 4. OncoC4. Targeted therapies Immunomodulators Space for curative approaches Synergy1 Targeted therapies mRNA cancer immunotherapies Synergy1Synergy1 BNT324 /DB-13112 Anti-B7H3 ADC
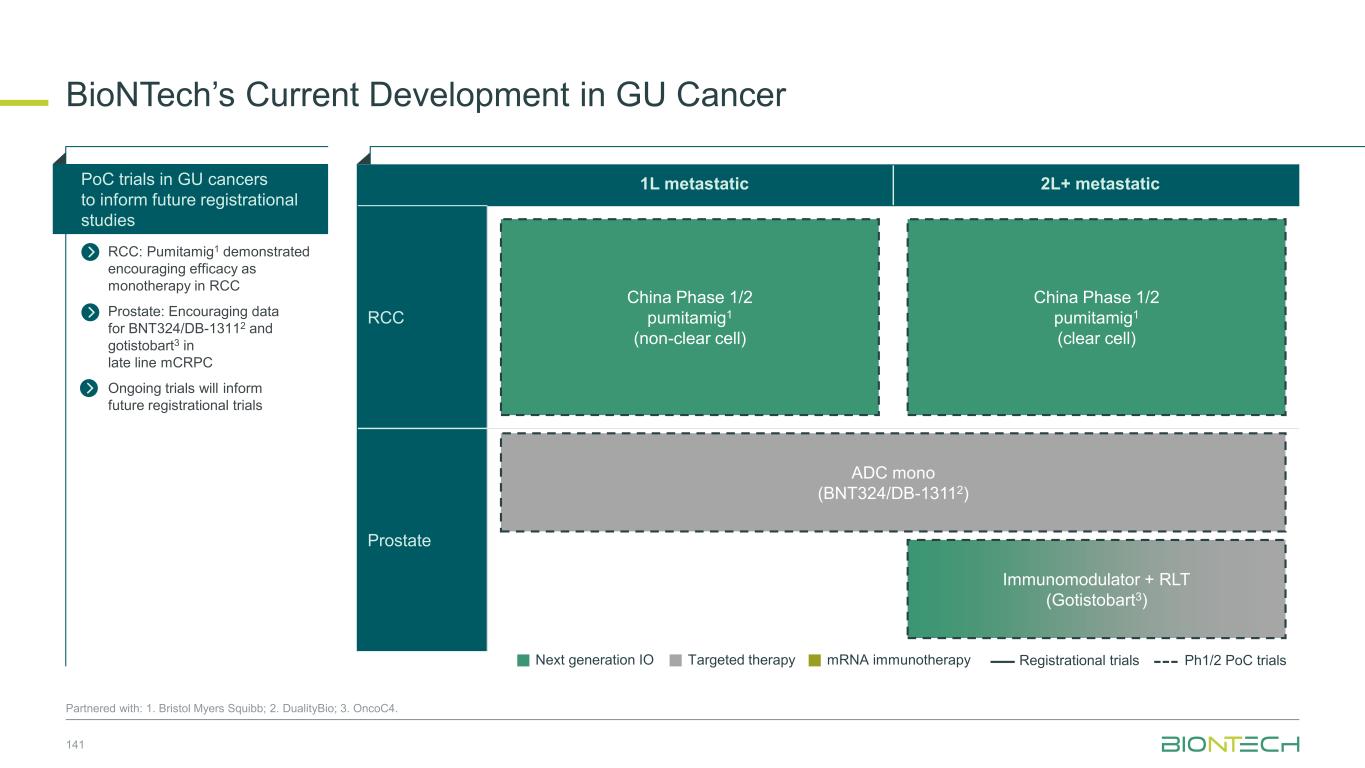
1L metastatic 2L+ metastatic RCC Prostate BioNTech’s Current Development in GU Cancer 141 Partnered with: 1. Bristol Myers Squibb; 2. DualityBio; 3. OncoC4. China Phase 1/2 pumitamig1 (non-clear cell) China Phase 1/2 pumitamig1 (clear cell) Immunomodulator + RLT (Gotistobart3) PoC trials in GU cancers to inform future registrational studies RCC: Pumitamig1 demonstrated encouraging efficacy as monotherapy in RCC Prostate: Encouraging data for BNT324/DB-13112 and gotistobart3 in late line mCRPC Ongoing trials will inform future registrational trials Next generation IO Targeted therapy mRNA immunotherapy Registrational trials Ph1/2 PoC trials ADC mono (BNT324/DB-13112)
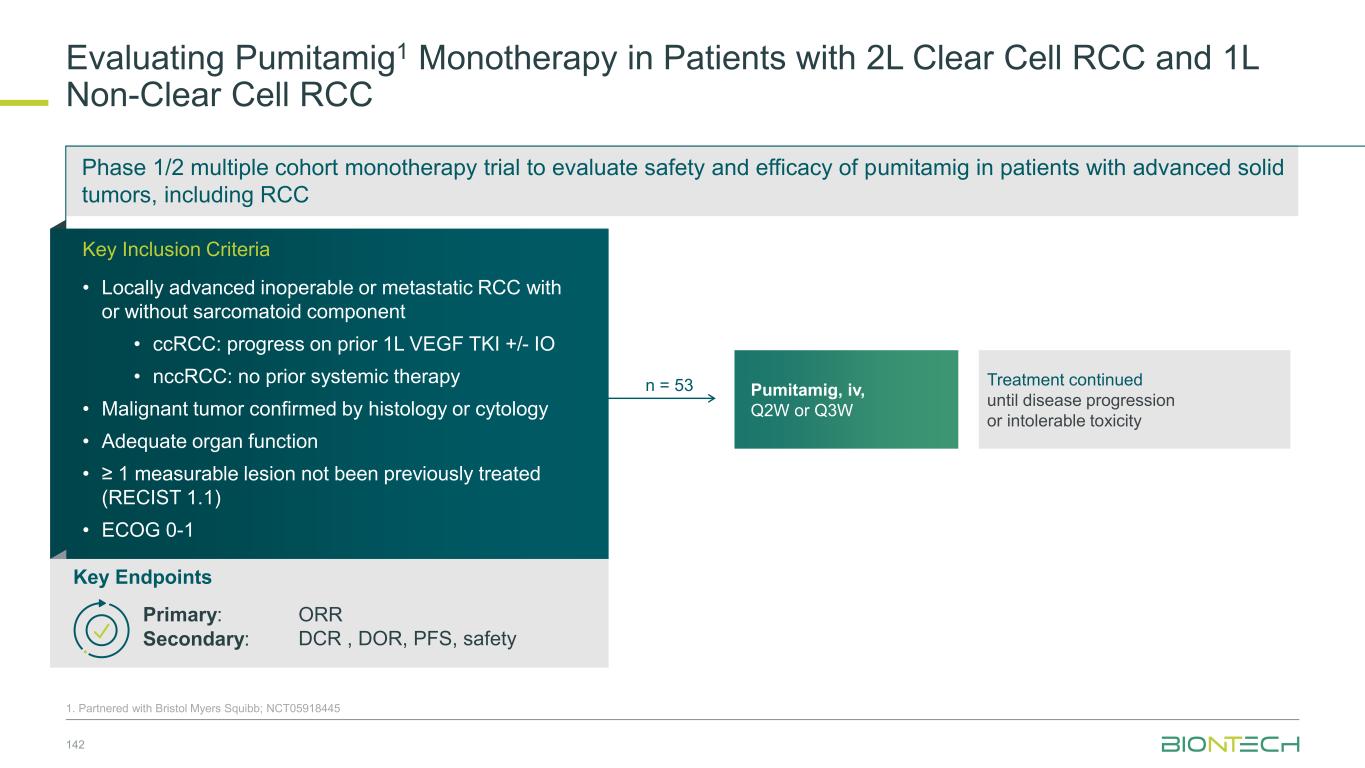
Phase 1/2 multiple cohort monotherapy trial to evaluate safety and efficacy of pumitamig in patients with advanced solid tumors, including RCC Evaluating Pumitamig1 Monotherapy in Patients with 2L Clear Cell RCC and 1L Non-Clear Cell RCC 1. Partnered with Bristol Myers Squibb; NCT05918445 Confidential Treatment continued until disease progression or intolerable toxicity Pumitamig, iv, Q2W or Q3W Key Endpoints Primary: ORR Secondary: DCR , DOR, PFS, safety Key Inclusion Criteria • Locally advanced inoperable or metastatic RCC with or without sarcomatoid component • ccRCC: progress on prior 1L VEGF TKI +/- IO • nccRCC: no prior systemic therapy • Malignant tumor confirmed by histology or cytology • Adequate organ function • ≥ 1 measurable lesion not been previously treated (RECIST 1.1) • ECOG 0-1 n = 53 142
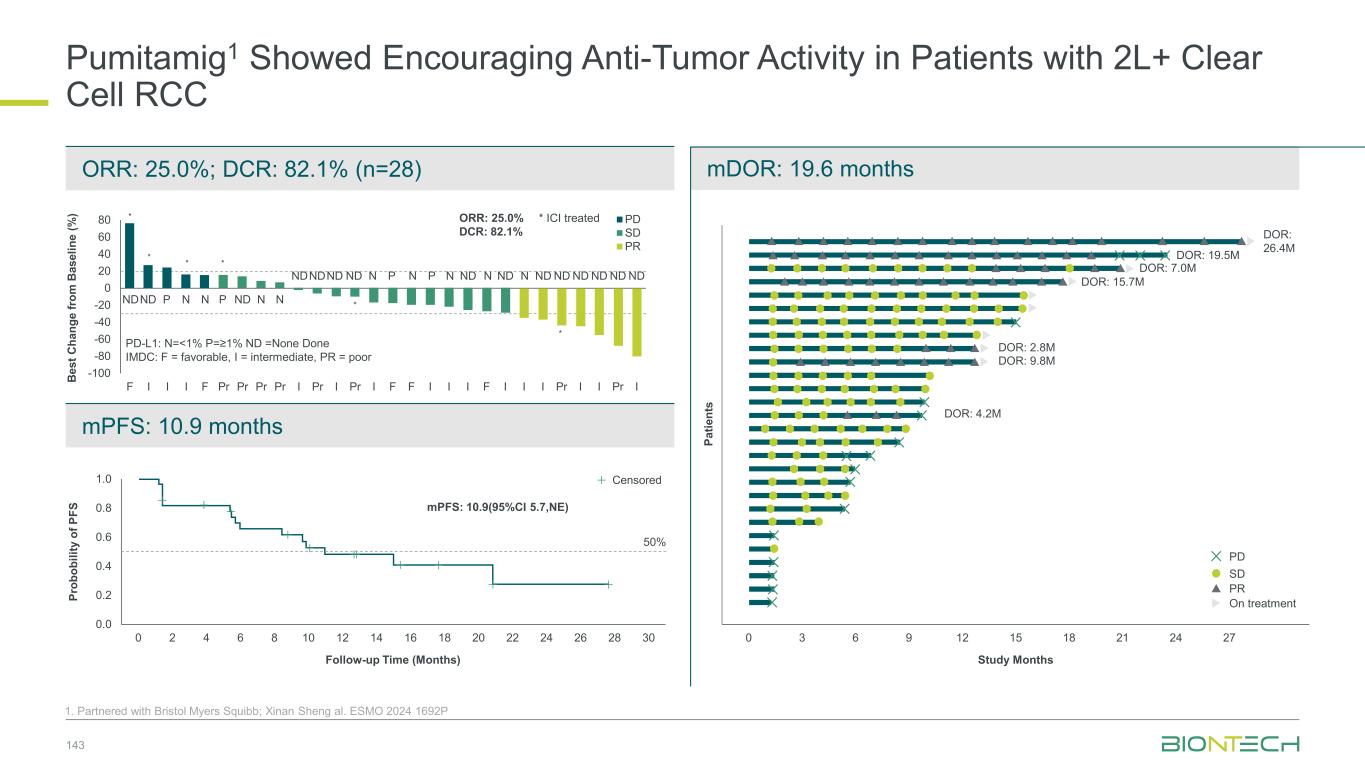
mPFS: 10.9 months Pumitamig1 Showed Encouraging Anti-Tumor Activity in Patients with 2L+ Clear Cell RCC 143 1. Partnered with Bristol Myers Squibb; Xinan Sheng al. ESMO 2024 1692P ORR: 25.0%; DCR: 82.1% (n=28) mDOR: 19.6 months P ro b o b il it y o f P F S 0.0 0.2 0.4 0.6 0.8 1.0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 Follow-up Time (Months) 50% Censored mPFS: 10.9(95%CI 5.7,NE) 0 3 6 9 12 15 18 21 24 27 Study Months DOR: 26.4M DOR: 19.5M DOR: 7.0M DOR: 15.7M DOR: 2.8M DOR: 9.8M DOR: 4.2M P a ti e n ts PD SD PR On treatment B e s t C h a n g e f ro m B a s e li n e ( % ) -100 -80 -60 -40 -20 0 20 40 60 80 F I I I F Pr Pr Pr Pr I Pr I Pr I F F I I I F I I I Pr I I Pr I PD SD PR * * * * * * NDND P N N P ND N N NDNDND ND N P N P N ND N ND N ND ND ND ND ND ND PD-L1: N=<1% P=≥1% ND =None Done IMDC: F = favorable, I = intermediate, PR = poor * ICI treatedORR: 25.0% DCR: 82.1%
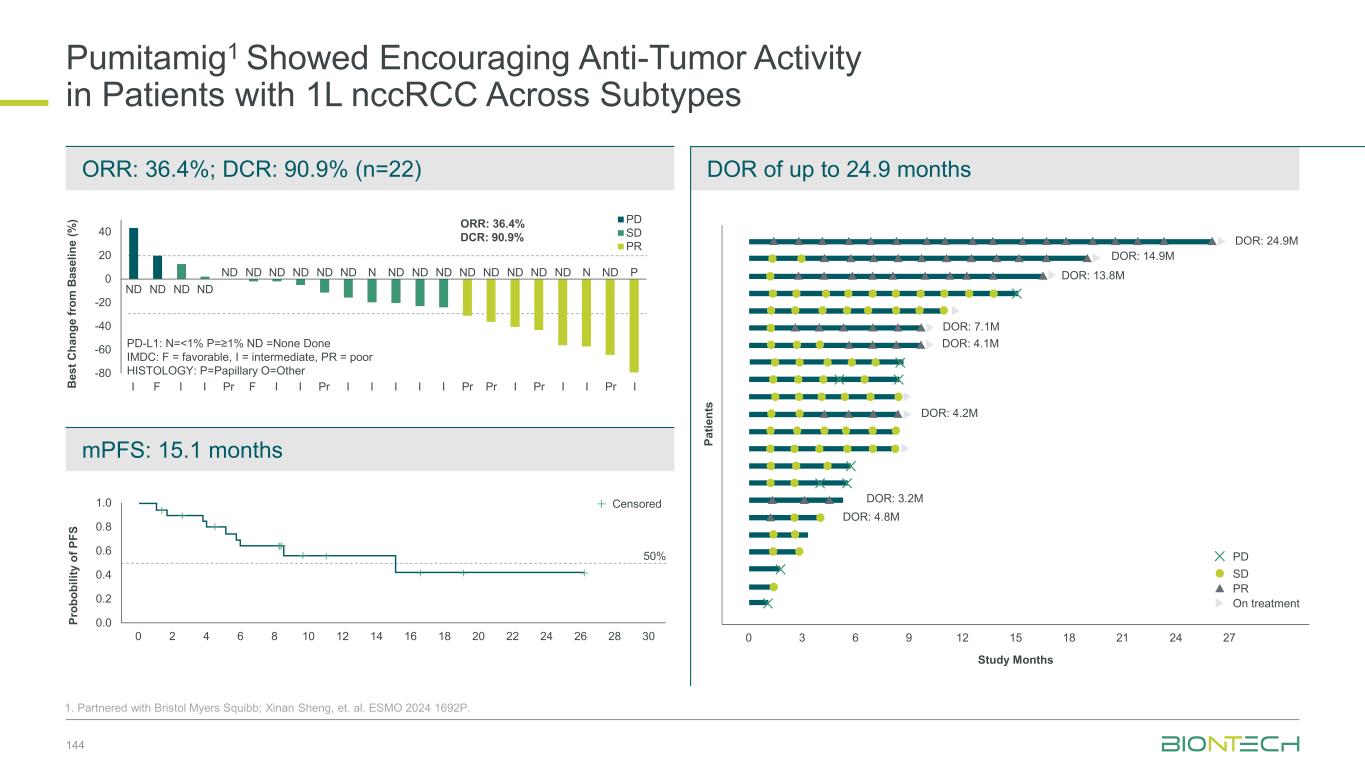
Pumitamig1 Showed Encouraging Anti-Tumor Activity in Patients with 1L nccRCC Across Subtypes 144 1. Partnered with Bristol Myers Squibb; Xinan Sheng, et. al. ESMO 2024 1692P. mPFS: 15.1 months ORR: 36.4%; DCR: 90.9% (n=22) DOR of up to 24.9 months 0 3 6 9 12 15 18 21 24 27 Study Months DOR: 24.9M DOR: 14.9M DOR: 13.8M DOR: 7.1M DOR: 4.1M DOR: 4.2M DOR: 3.2M DOR: 4.8M PD SD PR On treatment P a ti e n ts B e s t C h a n g e fr o m B a s e li n e ( % ) -80 -60 -40 -20 0 20 40 I F I I Pr F I I Pr I I I I I Pr Pr I Pr I I Pr I PD SD PR PD-L1: N=<1% P=≥1% ND =None Done IMDC: F = favorable, I = intermediate, PR = poor HISTOLOGY: P=Papillary O=Other ORR: 36.4% DCR: 90.9% ND ND ND ND ND ND ND ND ND ND N ND ND ND ND ND ND ND ND N ND P P ro b o b il it y o f P F S 0.0 0.2 0.4 0.6 0.8 1.0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 Follow-up Time (Months) 50% Censored 22 19 16 12 12 5 4 4 3 2 1 1 1 1 0No. at risk
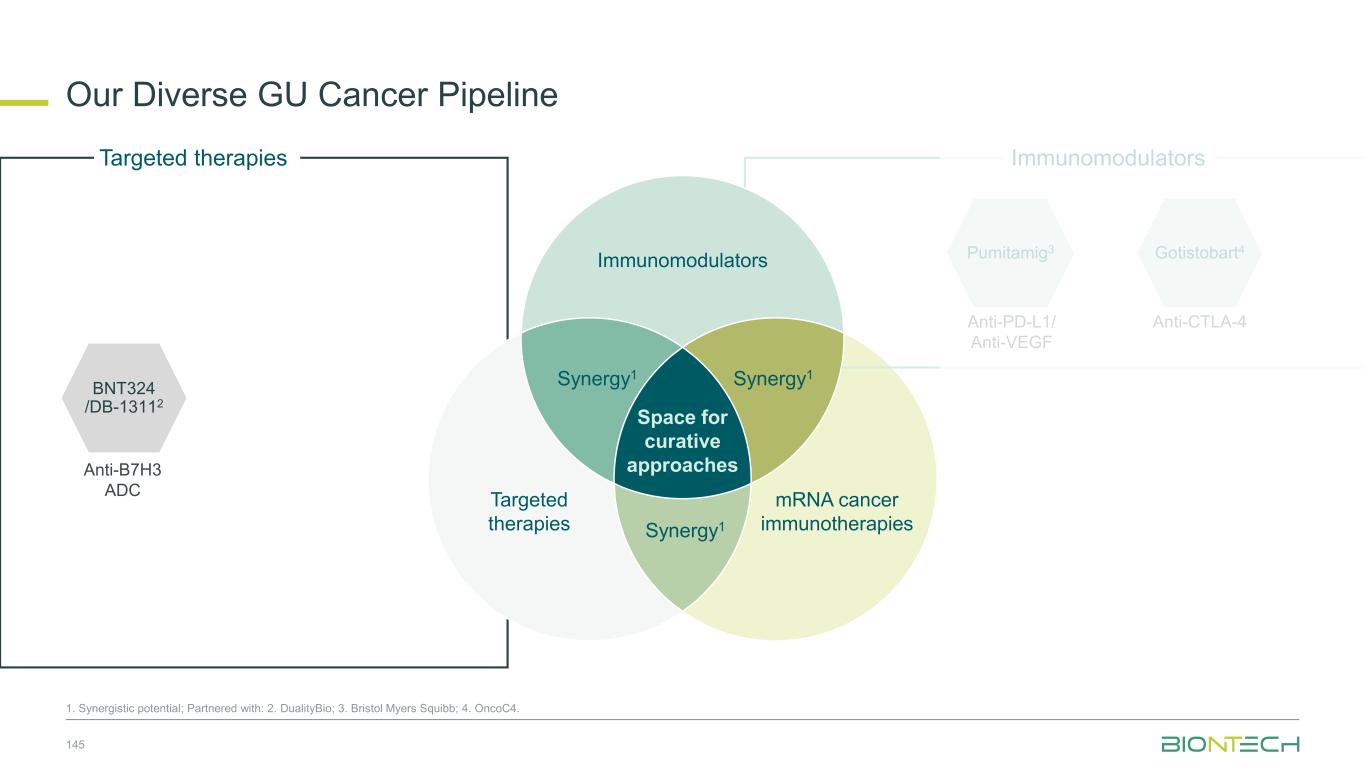
Anti-PD-L1/ Anti-VEGF Pumitamig3 Gotistobart4 Anti-CTLA-4 Immunomodulators Our Diverse GU Cancer Pipeline 145 1. Synergistic potential; Partnered with: 2. DualityBio; 3. Bristol Myers Squibb; 4. OncoC4. Targeted therapies Immunomodulators Space for curative approaches Synergy1 Targeted therapies mRNA cancer immunotherapies Synergy1Synergy1 BNT324 /DB-13112 Anti-B7H3 ADC
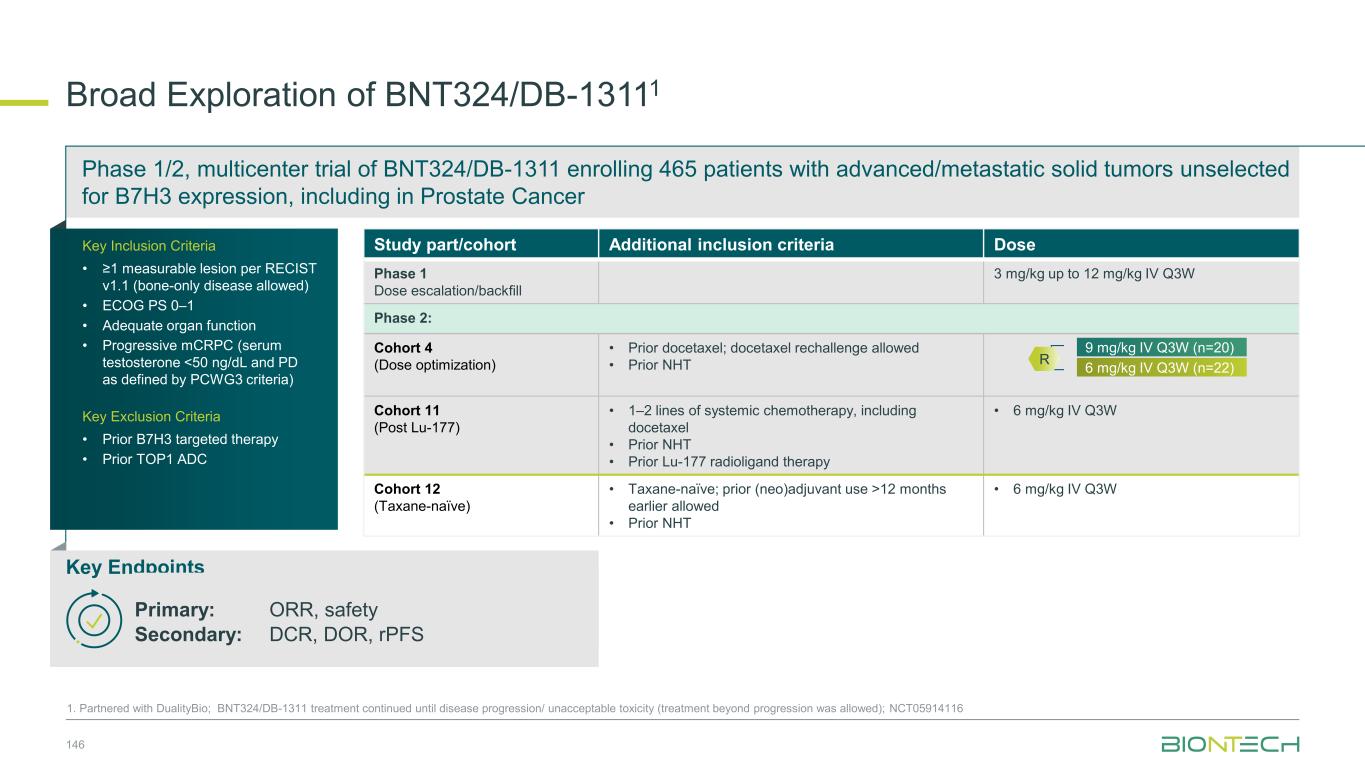
Phase 1/2, multicenter trial of BNT324/DB-1311 enrolling 465 patients with advanced/metastatic solid tumors unselected for B7H3 expression, including in Prostate Cancer Broad Exploration of BNT324/DB-13111 146 1. Partnered with DualityBio; BNT324/DB-1311 treatment continued until disease progression/ unacceptable toxicity (treatment beyond progression was allowed); NCT05914116 Key Endpoints Primary: ORR, safety Secondary: DCR, DOR, rPFS Key Inclusion Criteria • ≥1 measurable lesion per RECIST v1.1 (bone-only disease allowed) • ECOG PS 0–1 • Adequate organ function • Progressive mCRPC (serum testosterone <50 ng/dL and PD as defined by PCWG3 criteria) Key Exclusion Criteria • Prior B7H3 targeted therapy • Prior TOP1 ADC Study part/cohort Additional inclusion criteria Dose Phase 1 Dose escalation/backfill 3 mg/kg up to 12 mg/kg IV Q3W Phase 2: Cohort 4 (Dose optimization) • Prior docetaxel; docetaxel rechallenge allowed • Prior NHT Cohort 11 (Post Lu-177) • 1–2 lines of systemic chemotherapy, including docetaxel • Prior NHT • Prior Lu-177 radioligand therapy • 6 mg/kg IV Q3W Cohort 12 (Taxane-naïve) • Taxane-naïve; prior (neo)adjuvant use >12 months earlier allowed • Prior NHT • 6 mg/kg IV Q3W 6 mg/kg IV Q3W (n=22) 9 mg/kg IV Q3W (n=20) R
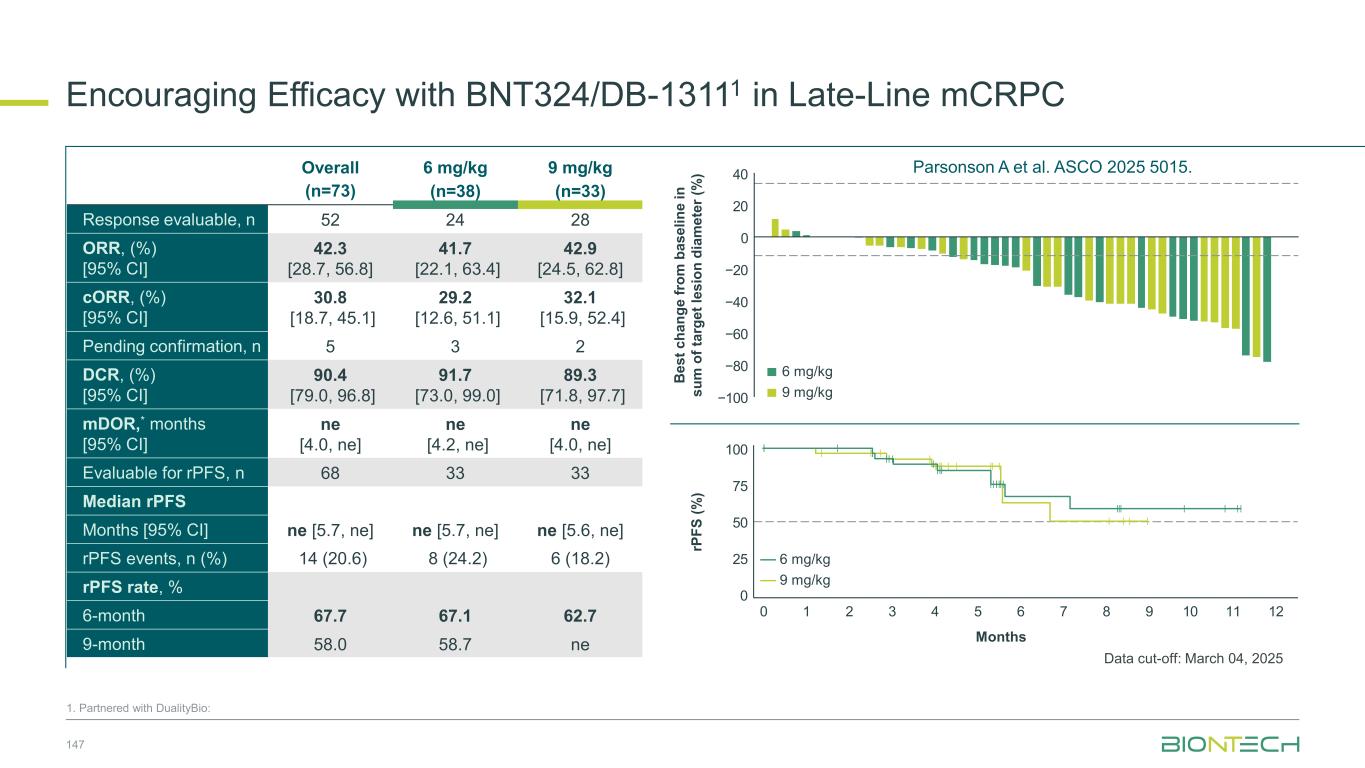
Encouraging Efficacy with BNT324/DB-13111 in Late-Line mCRPC 147 1. Partnered with DualityBio: Overall (n=73) 6 mg/kg (n=38) 9 mg/kg (n=33) Response evaluable, n 52 24 28 ORR, (%) [95% CI] 42.3 [28.7, 56.8] 41.7 [22.1, 63.4] 42.9 [24.5, 62.8] cORR, (%) [95% CI] 30.8 [18.7, 45.1] 29.2 [12.6, 51.1] 32.1 [15.9, 52.4] Pending confirmation, n 5 3 2 DCR, (%) [95% CI] 90.4 [79.0, 96.8] 91.7 [73.0, 99.0] 89.3 [71.8, 97.7] mDOR,* months [95% CI] ne [4.0, ne] ne [4.2, ne] ne [4.0, ne] Evaluable for rPFS, n 68 33 33 Median rPFS Months [95% CI] ne [5.7, ne] ne [5.7, ne] ne [5.6, ne] rPFS events, n (%) 14 (20.6) 8 (24.2) 6 (18.2) rPFS rate, % 6-month 67.7 67.1 62.7 9-month 58.0 58.7 ne B e s t c h a n g e f ro m b a s e li n e i n s u m o f ta rg e t le s io n d ia m e te r (% ) −80 −60 −40 −20 0 20 40 −100 9 mg/kg 6 mg/kg rP F S (% ) 25 50 75 100 0 1 2 3 4 5 6 7 8 9 10 11 12 0 9 mg/kg 6 mg/kg Months Data cut-off: March 04, 2025 Parsonson A et al. ASCO 2025 5015.
Partnered with: 1. Bristol Myers Squibb. Ongoing and Next Steps | Genitourinary Cancer Exploring pumitamig in GU cancers Evaluating ADCs and novel combinations in GU cancers ADCs China Phase 1/2 Pumitamig1 in 2L+ ccRCC and 1L nccRCC 148

Innovating Early- Stage Cancer Treatment with mRNA Cancer Immunotherapies Prof. Özlem Türeci, M.D. Chief Medical Officer and Co-founder 5
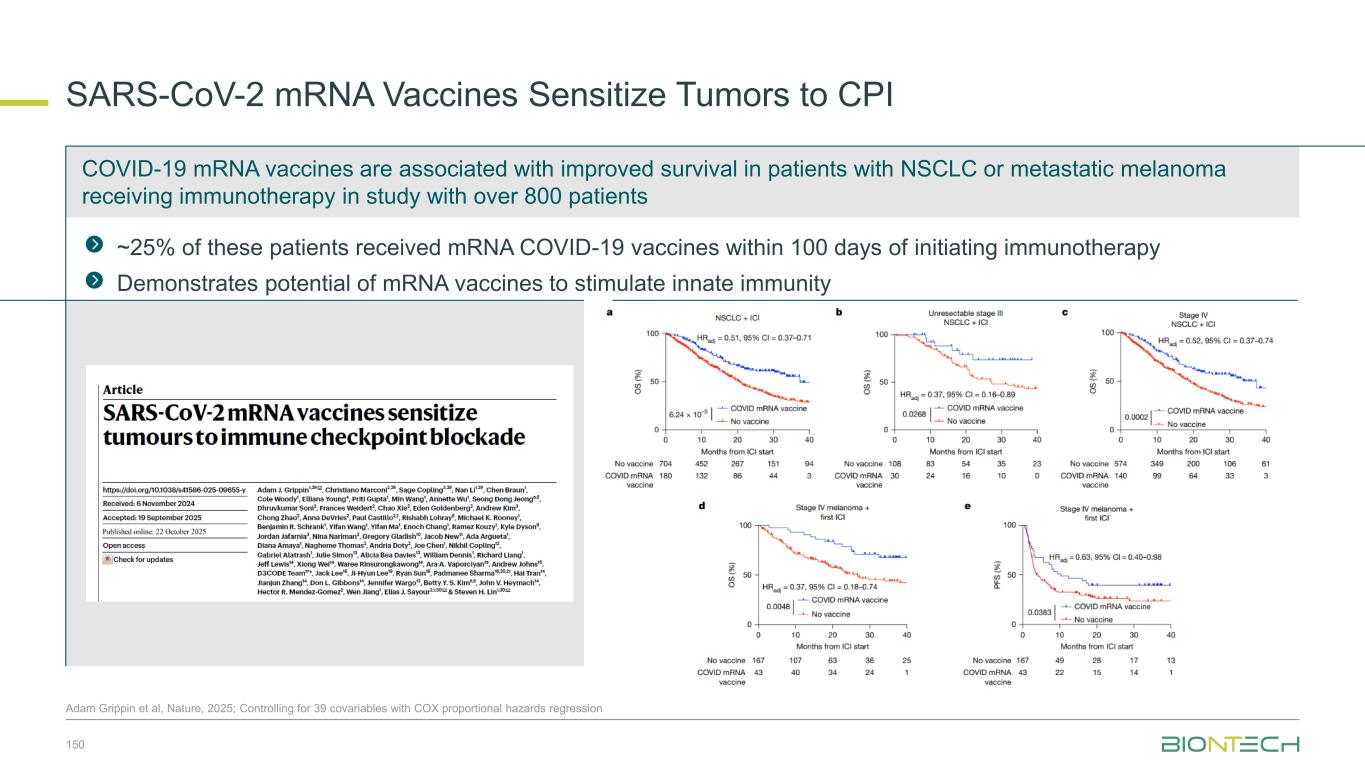
SARS-CoV-2 mRNA Vaccines Sensitize Tumors to CPI Adam Grippin et al, Nature, 2025; Controlling for 39 covariables with COX proportional hazards regression ~25% of these patients received mRNA COVID-19 vaccines within 100 days of initiating immunotherapy Demonstrates potential of mRNA vaccines to stimulate innate immunity COVID-19 mRNA vaccines are associated with improved survival in patients with NSCLC or metastatic melanoma receiving immunotherapy in study with over 800 patients 150
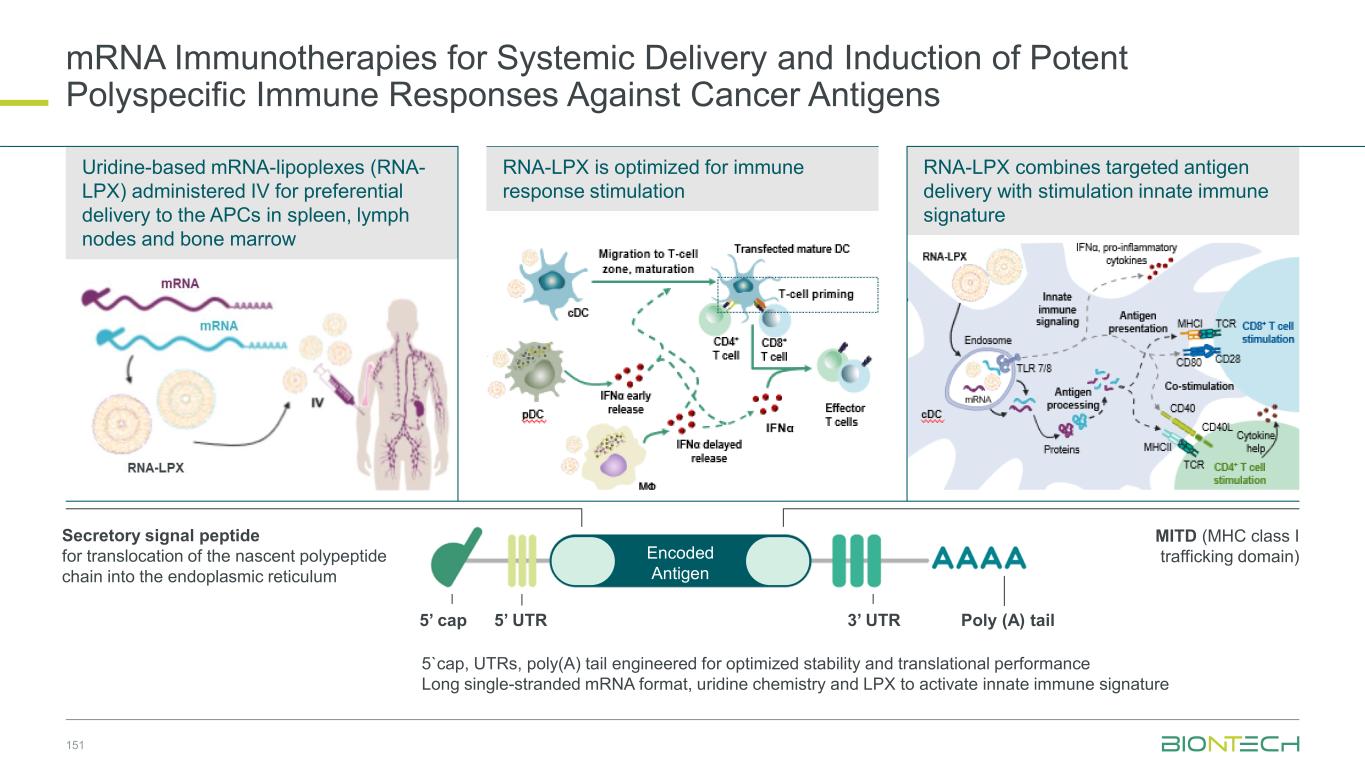
151 Uridine-based mRNA-lipoplexes (RNA- LPX) administered IV for preferential delivery to the APCs in spleen, lymph nodes and bone marrow RNA-LPX is optimized for immune response stimulation RNA-LPX combines targeted antigen delivery with stimulation innate immune signature Secretory signal peptide for translocation of the nascent polypeptide chain into the endoplasmic reticulum MITD (MHC class I trafficking domain)Encoded Antigen 5’ UTR5’ cap 3’ UTR Poly (A) tail 5`cap, UTRs, poly(A) tail engineered for optimized stability and translational performance Long single-stranded mRNA format, uridine chemistry and LPX to activate innate immune signature mRNA Immunotherapies for Systemic Delivery and Induction of Potent Polyspecific Immune Responses Against Cancer Antigens
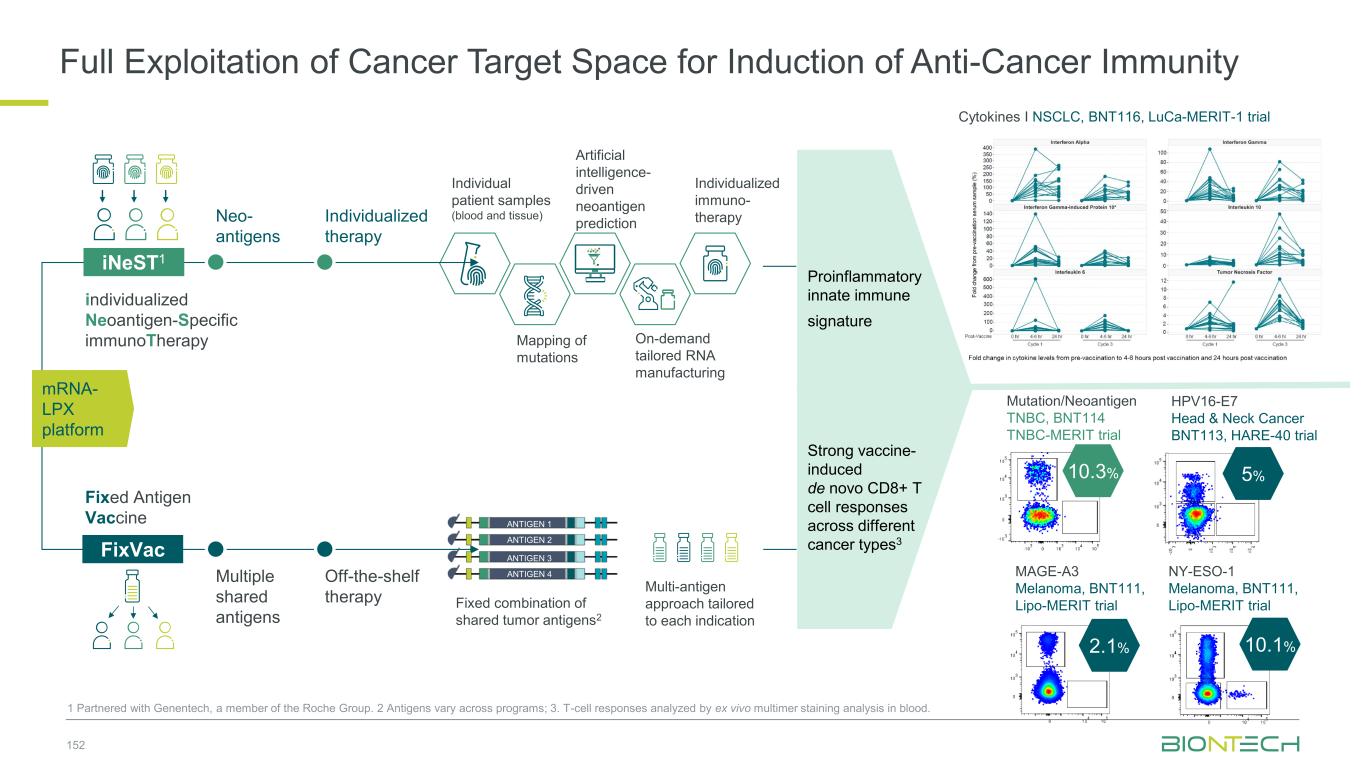
152 Individual patient samples (blood and tissue) Artificial intelligence- driven neoantigen prediction On-demand tailored RNA manufacturing Individualized immuno- therapy Mapping of mutations Fixed combination of shared tumor antigens2 Multi-antigen approach tailored to each indication Neo- antigens Individualized therapy Multiple shared antigens Off-the-shelf therapy iNeST1 FixVac individualized Neoantigen-Specific immunoTherapy Fixed Antigen Vaccine ANTIGEN 1 ANTIGEN 2 ANTIGEN 3 ANTIGEN 4 10.3% 10.1% HPV16-E7 Head & Neck Cancer BNT113, HARE-40 trial Mutation/Neoantigen TNBC, BNT114 TNBC-MERIT trial 5% MAGE-A3 Melanoma, BNT111, Lipo-MERIT trial 2.1% NY-ESO-1 Melanoma, BNT111, Lipo-MERIT trial mRNA- LPX platform Proinflammatory innate immune signature Strong vaccine- induced de novo CD8+ T cell responses across different cancer types3 1 Partnered with Genentech, a member of the Roche Group. 2 Antigens vary across programs; 3. T-cell responses analyzed by ex vivo multimer staining analysis in blood. Cytokines I NSCLC, BNT116, LuCa-MERIT-1 trial Full Exploitation of Cancer Target Space for Induction of Anti-Cancer Immunity
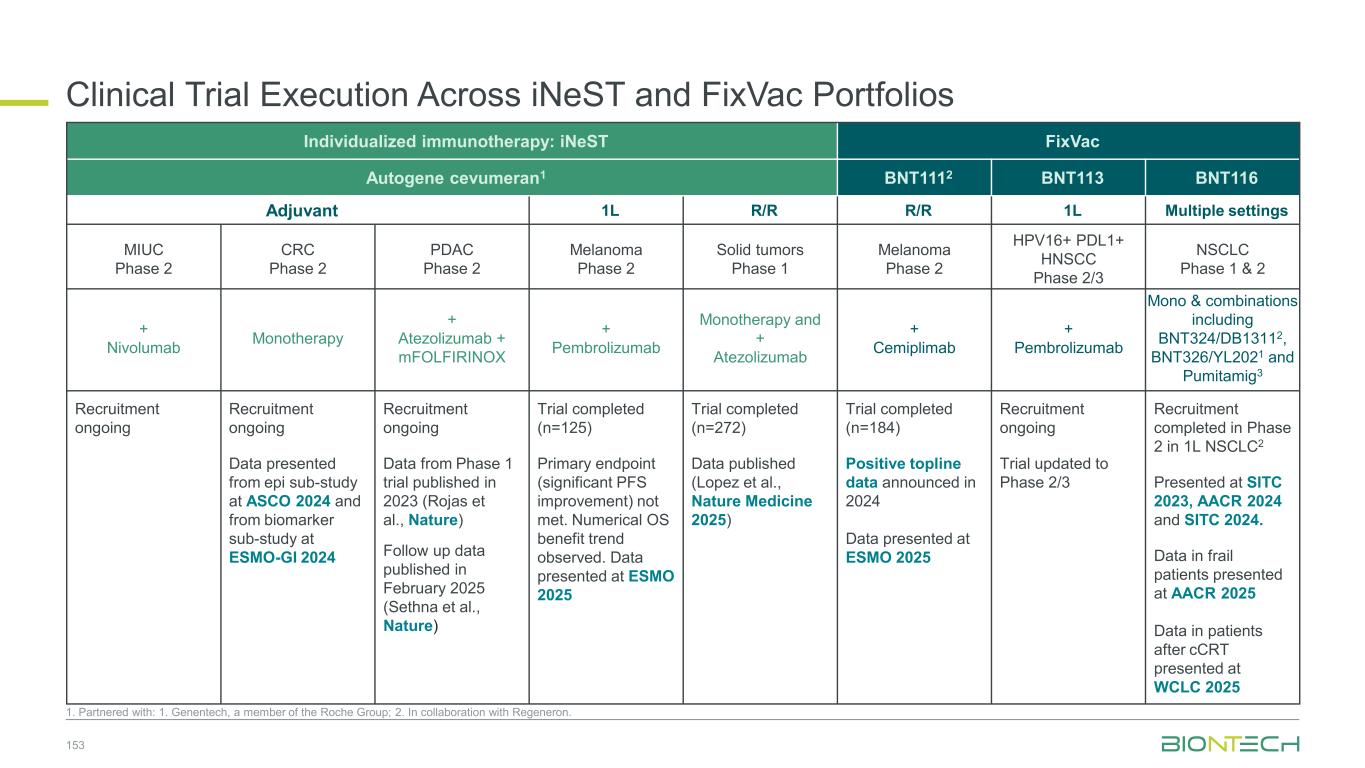
153 1. Partnered with: 1. Genentech, a member of the Roche Group; 2. In collaboration with Regeneron. Individualized immunotherapy: iNeST FixVac Autogene cevumeran1 BNT1112 BNT113 BNT116 Adjuvant 1L R/R R/R 1L Multiple settings MIUC Phase 2 CRC Phase 2 PDAC Phase 2 Melanoma Phase 2 Solid tumors Phase 1 Melanoma Phase 2 HPV16+ PDL1+ HNSCC Phase 2/3 NSCLC Phase 1 & 2 + Nivolumab Monotherapy + Atezolizumab + mFOLFIRINOX + Pembrolizumab Monotherapy and + Atezolizumab + Cemiplimab + Pembrolizumab Mono & combinations including BNT324/DB13112, BNT326/YL2021 and Pumitamig3 Recruitment ongoing Recruitment ongoing Data presented from epi sub-study at ASCO 2024 and from biomarker sub-study at ESMO-GI 2024 Recruitment ongoing Data from Phase 1 trial published in 2023 (Rojas et al., Nature) Follow up data published in February 2025 (Sethna et al., Nature) Trial completed (n=125) Primary endpoint (significant PFS improvement) not met. Numerical OS benefit trend observed. Data presented at ESMO 2025 Trial completed (n=272) Data published (Lopez et al., Nature Medicine 2025) Trial completed (n=184) Positive topline data announced in 2024 Data presented at ESMO 2025 Recruitment ongoing Trial updated to Phase 2/3 Recruitment completed in Phase 2 in 1L NSCLC2 Presented at SITC 2023, AACR 2024 and SITC 2024. Data in frail patients presented at AACR 2025 Data in patients after cCRT presented at WCLC 2025 Clinical Trial Execution Across iNeST and FixVac Portfolios
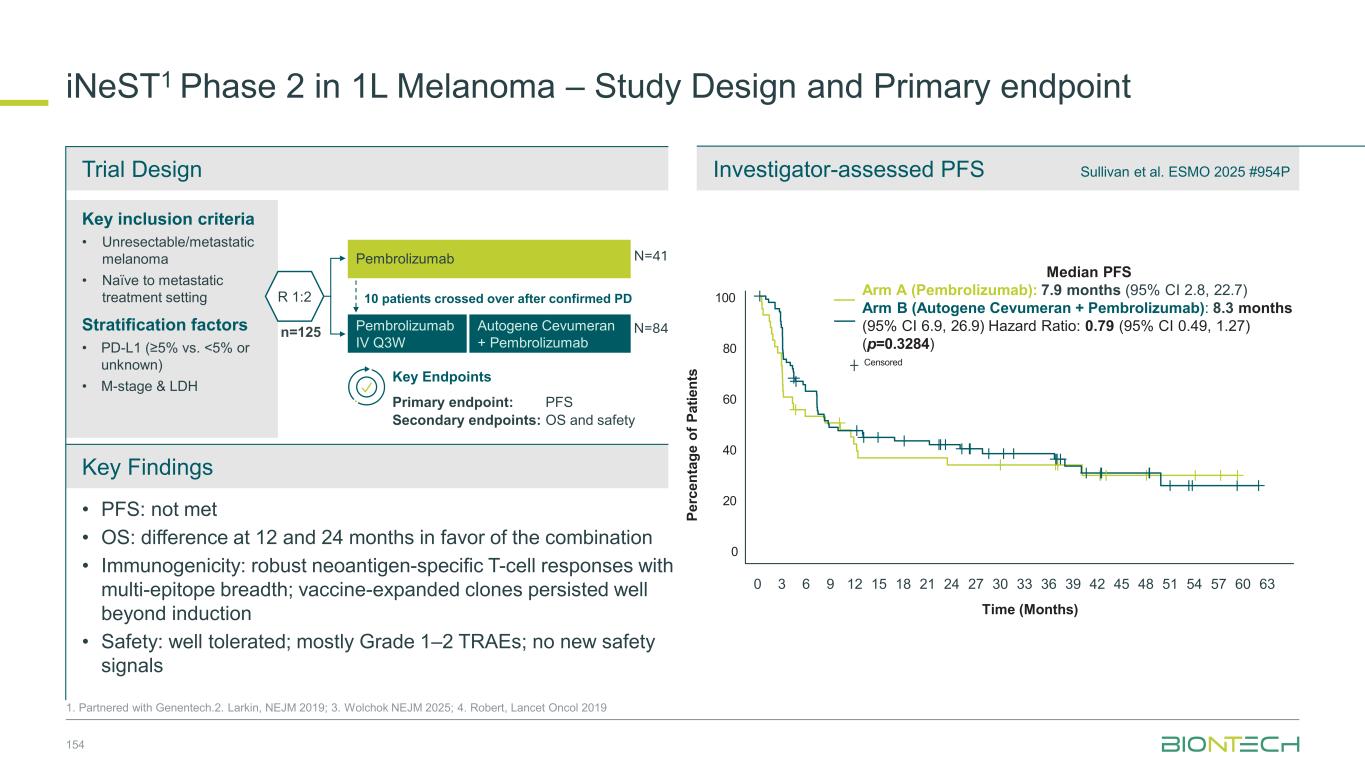
Key inclusion criteria • Unresectable/metastatic melanoma • Naïve to metastatic treatment setting Stratification factors • PD-L1 (≥5% vs. <5% or unknown) • M-stage & LDH Trial Design Investigator-assessed PFS Key Findings iNeST1 Phase 2 in 1L Melanoma – Study Design and Primary endpoint 154 1. Partnered with Genentech.2. Larkin, NEJM 2019; 3. Wolchok NEJM 2025; 4. Robert, Lancet Oncol 2019 • PFS: not met • OS: difference at 12 and 24 months in favor of the combination • Immunogenicity: robust neoantigen-specific T-cell responses with multi-epitope breadth; vaccine-expanded clones persisted well beyond induction • Safety: well tolerated; mostly Grade 1–2 TRAEs; no new safety signals N=41 Key Endpoints P e rc e n ta g e o f P a ti e n ts Time (Months) 100 80 0 20 40 60 Censored 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 63 Median PFS Arm A (Pembrolizumab): 7.9 months (95% CI 2.8, 22.7) Arm B (Autogene Cevumeran + Pembrolizumab): 8.3 months (95% CI 6.9, 26.9) Hazard Ratio: 0.79 (95% CI 0.49, 1.27) (p=0.3284) R 1:2 n=125 Pembrolizumab Autogene Cevumeran + Pembrolizumab Pembrolizumab IV Q3W 10 patients crossed over after confirmed PD N=84 Sullivan et al. ESMO 2025 #954P Primary endpoint: PFS Secondary endpoints: OS and safety
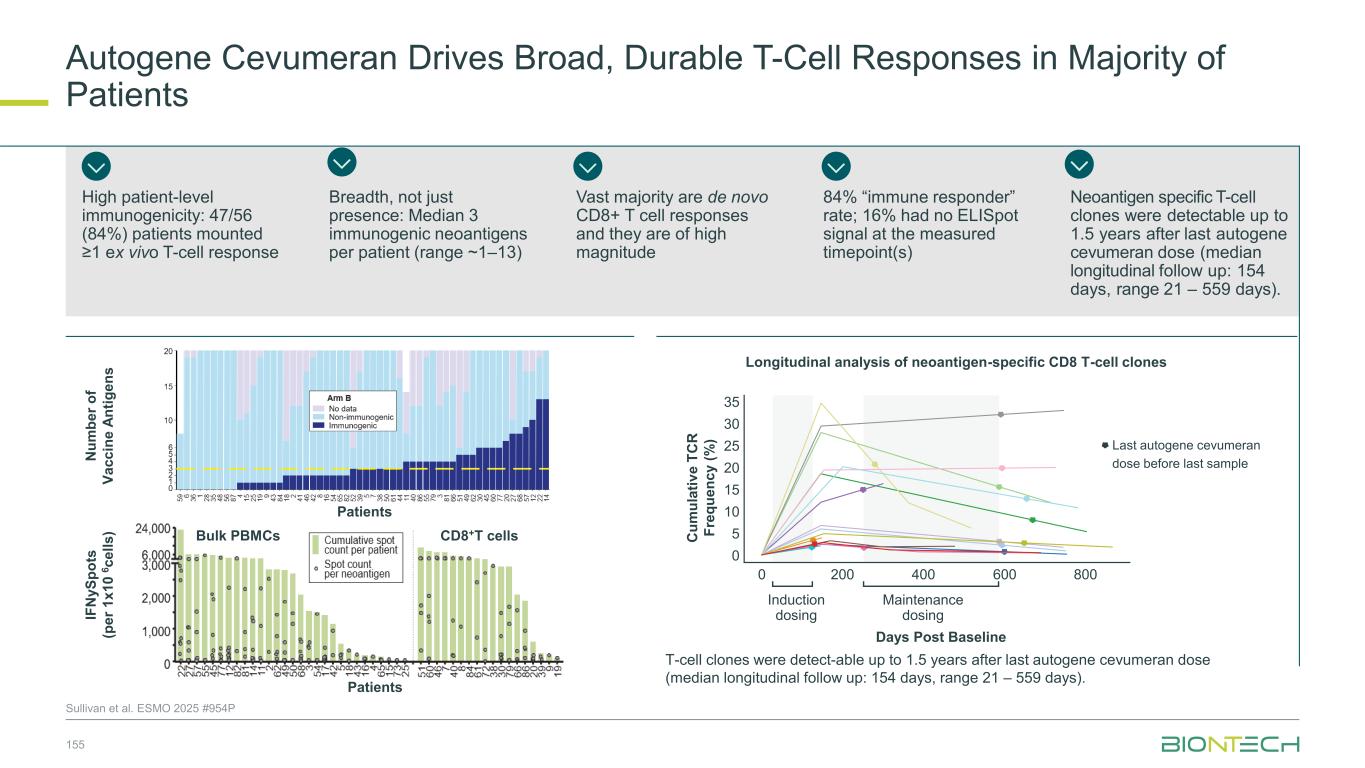
Bulk PBMCs CD8+T cells Patients Longitudinal analysis of neoantigen-specific CD8 T-cell clones Sullivan et al. ESMO 2025 #954P High patient-level immunogenicity: 47/56 (84%) patients mounted ≥1 ex vivo T-cell response N u m b e r o f V a c c in e A n ti g e n s IF N y S p o ts (p e r 1 x 1 0 6 c e ll s ) Patients Breadth, not just presence: Median 3 immunogenic neoantigens per patient (range ~1–13) Vast majority are de novo CD8+ T cell responses and they are of high magnitude 84% “immune responder” rate; 16% had no ELISpot signal at the measured timepoint(s) Neoantigen specific T-cell clones were detectable up to 1.5 years after last autogene cevumeran dose (median longitudinal follow up: 154 days, range 21 – 559 days). C u m u la ti v e T C R F re q u e n c y ( % ) Days Post Baseline 35 30 25 20 15 10 5 0 T-cell clones were detect-able up to 1.5 years after last autogene cevumeran dose (median longitudinal follow up: 154 days, range 21 – 559 days). 0 200 400 600 800 Induction dosing Maintenance dosing Last autogene cevumeran dose before last sample Autogene Cevumeran Drives Broad, Durable T-Cell Responses in Majority of Patients 155
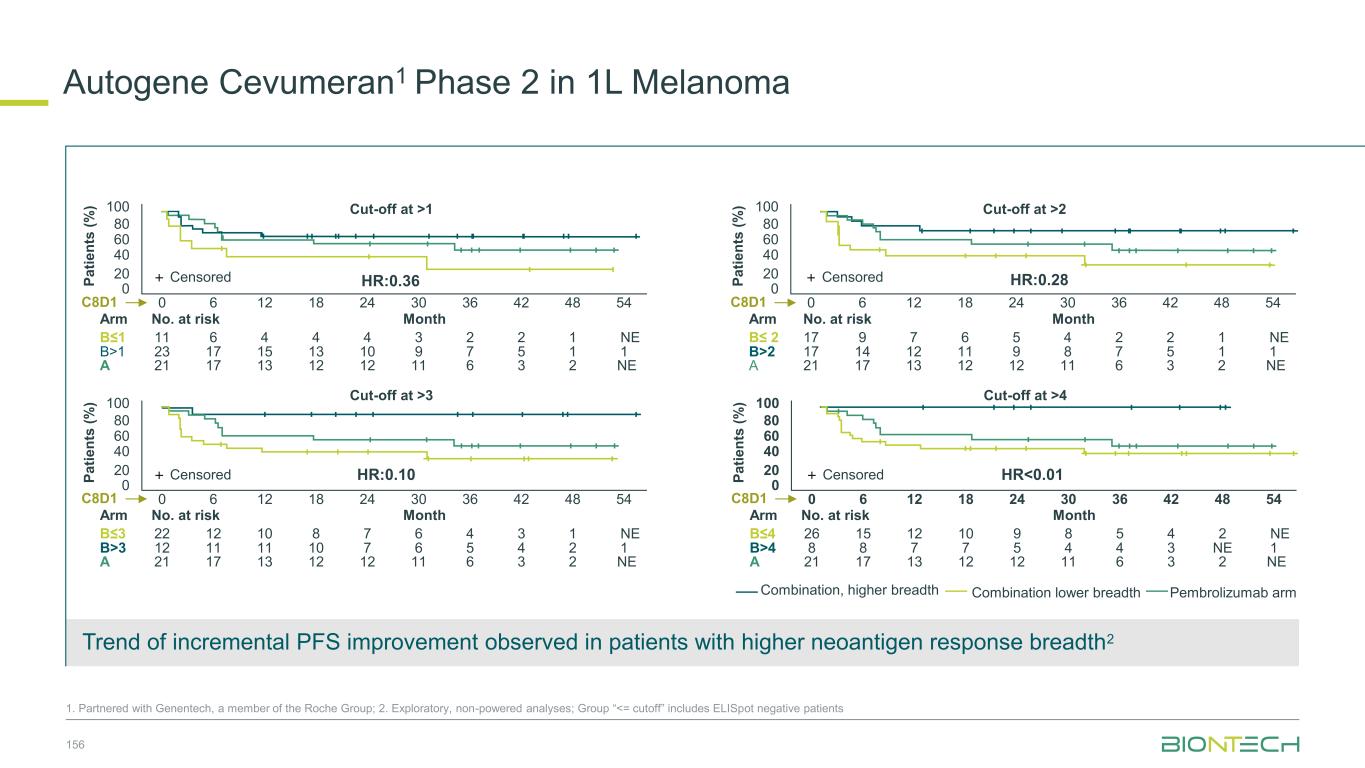
156 1. Partnered with Genentech, a member of the Roche Group; 2. Exploratory, non-powered analyses; Group “<= cutoff” includes ELISpot negative patients Trend of incremental PFS improvement observed in patients with higher neoantigen response breadth2 0 0 6 12 20 40 60 80 100 2418 30 36 42 48 54 P a ti e n ts ( % ) HR<0.01 Cut-off at >4 Month 26 15 12 910 8 5 4 2 NE 21 17 13 1212 11 6 3 2 NE 8 8 7 57 4 4 3 NE 1 B≤4 B>4 A No. at riskArm C8D1 Censored HR:0.28 Cut-off at >2 0 0 6 12 20 40 60 80 100 2418 30 36 42 48 54 Month 17 9 7 56 4 2 2 1 NE 21 17 13 1212 11 6 3 2 NE 17 14 12 911 8 7 5 1 1 B≤ 2 B>2 A No. at riskArm C8D1 CensoredP a ti e n ts ( % ) P a ti e n ts ( % ) Month 11 6 4 44 3 2 2 1 NE 21 17 13 1212 11 6 3 2 NE 23 17 15 1013 9 7 5 1 1 B≤1 B>1 A No. at riskArm C8D1 0 0 6 12 20 40 60 80 100 2418 30 36 42 48 54 HR:0.36 Cut-off at >1 Censored P a ti e n ts ( % ) HR:0.10 Cut-off at >3 Month 22 12 10 78 6 4 3 1 NE 21 17 13 1212 11 6 3 2 NE 12 11 11 710 6 5 4 2 1 B≤3 B>3 A No. at riskArm C8D1 0 0 6 12 20 40 60 80 100 2418 30 36 42 48 54 Censored Combination, higher breadth Combination lower breadth Pembrolizumab arm Autogene Cevumeran1 Phase 2 in 1L Melanoma
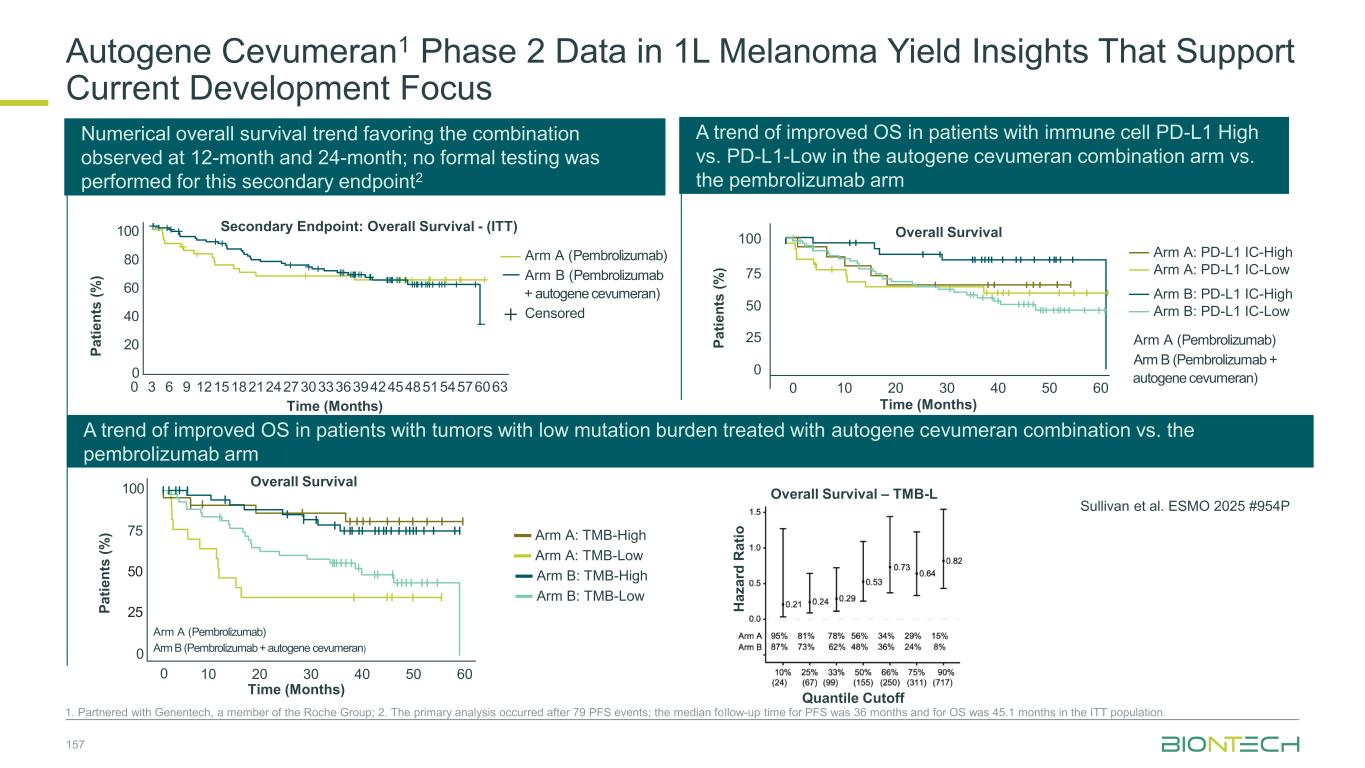
Autogene Cevumeran1 Phase 2 Data in 1L Melanoma Yield Insights That Support Current Development Focus 1. Partnered with Genentech, a member of the Roche Group; 2. The primary analysis occurred after 79 PFS events; the median follow-up time for PFS was 36 months and for OS was 45.1 months in the ITT population. A trend of improved OS in patients with immune cell PD-L1 High vs. PD-L1-Low in the autogene cevumeran combination arm vs. the pembrolizumab arm A trend of improved OS in patients with tumors with low mutation burden treated with autogene cevumeran combination vs. the pembrolizumab arm Time (Months) Overall Survival P a ti e n ts ( % ) Arm A (Pembrolizumab) Arm B (Pembrolizumab + autogene cevumeran) Arm A: PD-L1 IC-High Arm A: PD-L1 IC-Low Arm B: PD-L1 IC-High Arm B: PD-L1 IC-Low 0 10 20 30 40 50 60 0 25 50 75 100 Sullivan et al. ESMO 2025 #954P Numerical overall survival trend favoring the combination observed at 12-month and 24-month; no formal testing was performed for this secondary endpoint2 Time (Months) P a ti e n ts ( % ) 100 80 0 20 40 60 0 3 6 9 121518212427303336394245485154576063 Secondary Endpoint: Overall Survival - (ITT) Overall Survival – TMB-L Quantile Cutoff H a z a rd R a ti o Arm A (Pembrolizumab) Arm B (Pembrolizumab + autogene cevumeran) Censored 100 75 0 25 50 Time (Months) P a ti e n ts ( % ) Arm A (Pembrolizumab) Arm B (Pembrolizumab + autogene cevumeran) Arm A: TMB-High Arm A: TMB-Low Arm B: TMB-High Arm B: TMB-Low Overall Survival 0 10 20 30 40 50 60 157

Partnered with: 1. Bristol Myers Squibb; 2. Genentech, member of the Roche Group; 3. OncoC4. Ongoing and Next Steps | mRNA Cancer Immunotherapies 158 Evaluating autogene cevumeran2 in adjuvant-stage disease Evaluating novel combinations for FixVac Phase 2 Autogene cevumeran2 in adjuvant ctDNA+ stage II (high risk) / stage III MSS-CRC Phase 2 Autogene cevumeran2 + chemotherapy + atezolizumab in adjuvant PDAC Update planned for 2026 Phase 2 Autogene cevumeran2 + chemotherapy + nivolumab in adj. MIUC Phase 2/3 BNT113 + pembrolizumab in HPV16+, PD-L1+ 1L HNSCC BNT116 + pumitamig1 BNT116 + ADCs BNT116 + gotistobart3 Data expected in 2026 Novel combination in 2026

BioNTech’s Path to Value Creation Ramón Zapata Chief Financial Officer 6
✓ Launched variant-adapted COVID-19 vaccine ✓ Leading COVID-19 market share globally (>50%) ✓ >20 phase 2 and 3 oncology trials ongoing ✓ 30+ novel-novel combination cohorts ongoing across tumors ✓ Completed Biotheus acquisition ✓ Strategic BMS partnership to maximize pumitamig ✓ Increased 2025 revenue guidance to €2.6-2.8 billion1 ✓ €16.7 billion in cash, cash equivalents and securities2 Maintained Leadership in the COVID-19 Space Advanced Key Oncology Pan-Tumor Programs and Clinical Execution Executed Key Strategic Partnerships and Acquisitions Strengthened Financial Position to Drive Sustained Innovation 1 BioNTech increased revenue guidance and now expects its revenues for the full 2025 financial year to be in the range of €2,600 - €2,800 million, from previous range of €1,700 - €2,200 million; please refer to 3Q25 earnings press release and quarterly report on Form 6-K for risks and uncertainties. 2. As of September 30, 2025. Key 2025 Achievements Position BioNTech for Continued Oncology Innovation and Future Growth 160
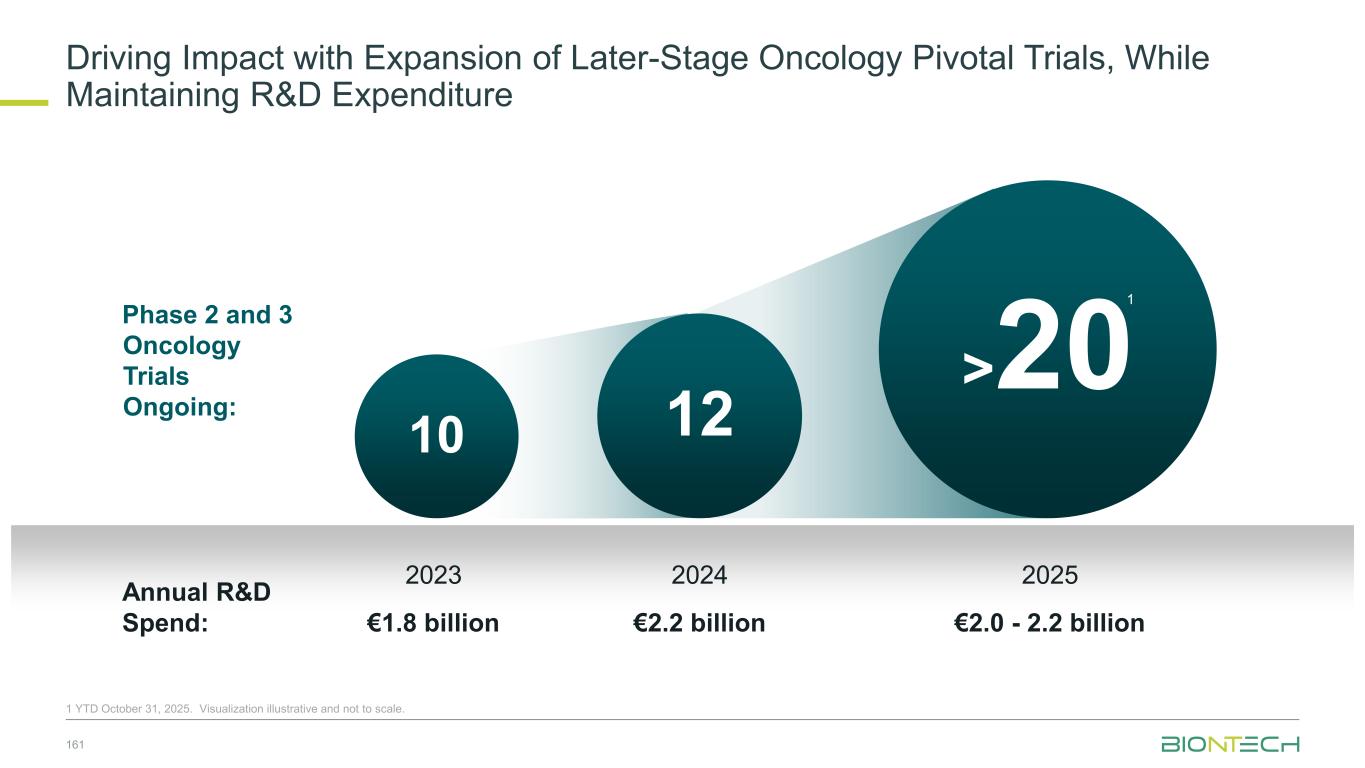
Driving Impact with Expansion of Later-Stage Oncology Pivotal Trials, While Maintaining R&D Expenditure 161 1 YTD October 31, 2025. Visualization illustrative and not to scale. 1210 Phase 2 and 3 Oncology Trials Ongoing: Annual R&D Spend: 2023 €1.8 billion 2024 €2.2 billion 2025 €2.0 - 2.2 billion >20 1
Financial Levers Enable BioNTech’s Dynamic R&D Investment 162 R&D Investment Control Levers Active portfolio management strategy sets high bar for late- stage investment and balance with high risk/reward programs Innovative, tailored partnerships to advance priority programs cost effectively, while strengthening P&L Early-stage programs empowered with dedicated budgets and opportunistic biotech in-licensing agreements
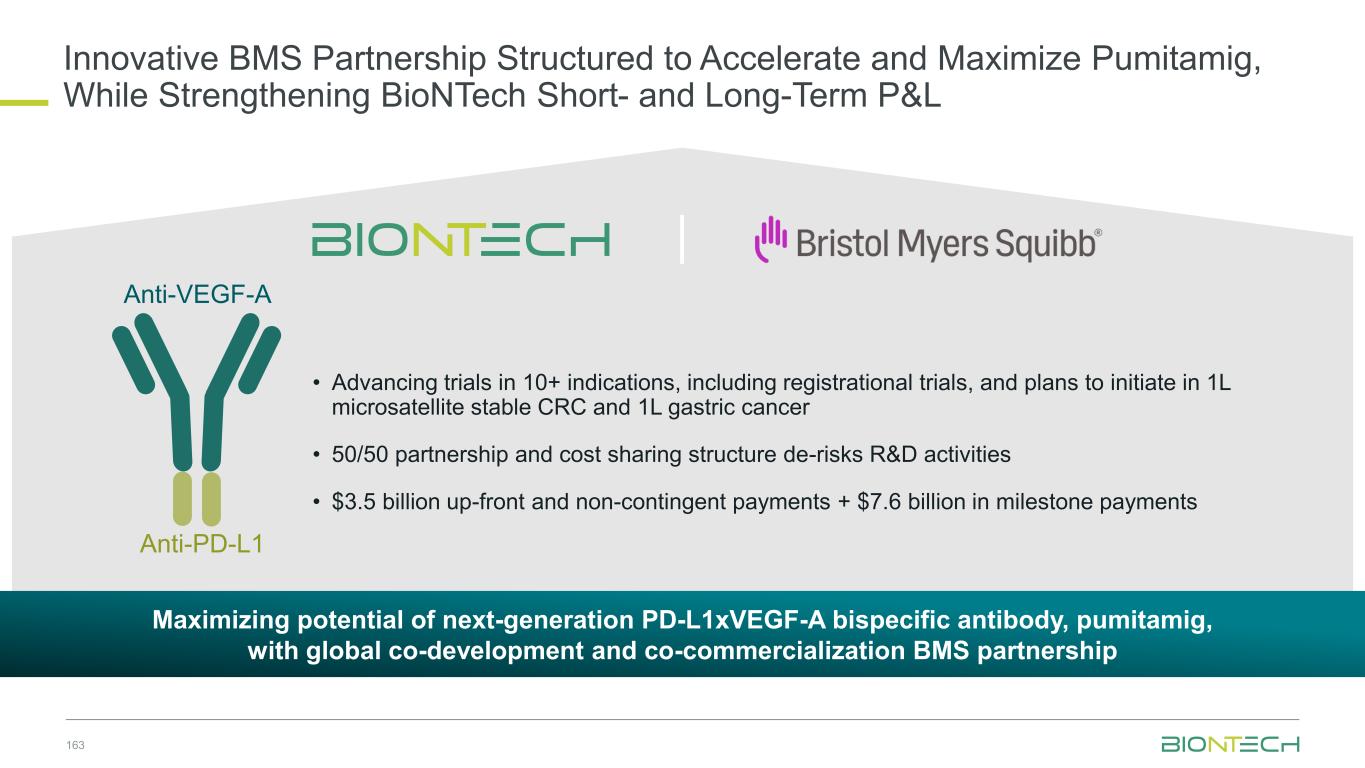
163 Innovative BMS Partnership Structured to Accelerate and Maximize Pumitamig, While Strengthening BioNTech Short- and Long-Term P&L Maximizing potential of next-generation PD-L1xVEGF-A bispecific antibody, pumitamig, with global co-development and co-commercialization BMS partnership • Advancing trials in 10+ indications, including registrational trials, and plans to initiate in 1L microsatellite stable CRC and 1L gastric cancer • 50/50 partnership and cost sharing structure de-risks R&D activities • $3.5 billion up-front and non-contingent payments + $7.6 billion in milestone payments Anti-PD-L1 Anti-VEGF-A

BioNTech Key Principles on the Path to Value Creation 164 Speed and Scalability: Commercial Readiness and First-to-Market Approach Ensure operational agility and organizational readiness to scale rapidly, build commercial infrastructure, prioritize speed to market Financial Efficiency: Ensuring Cash Runway and Capital Resilience Strong financial position provides strategic optionality Optimizing Productivity: Do More with Less Through Operational Excellence Focus resources on highest priority programs, optimize cost base to support sustainable development trajectory Strategic Portfolio Management: Shift to Later-Stage De-risked Programs Shifting pipeline focus to later-stage programs with higher POS and de-risk through partnerships 164
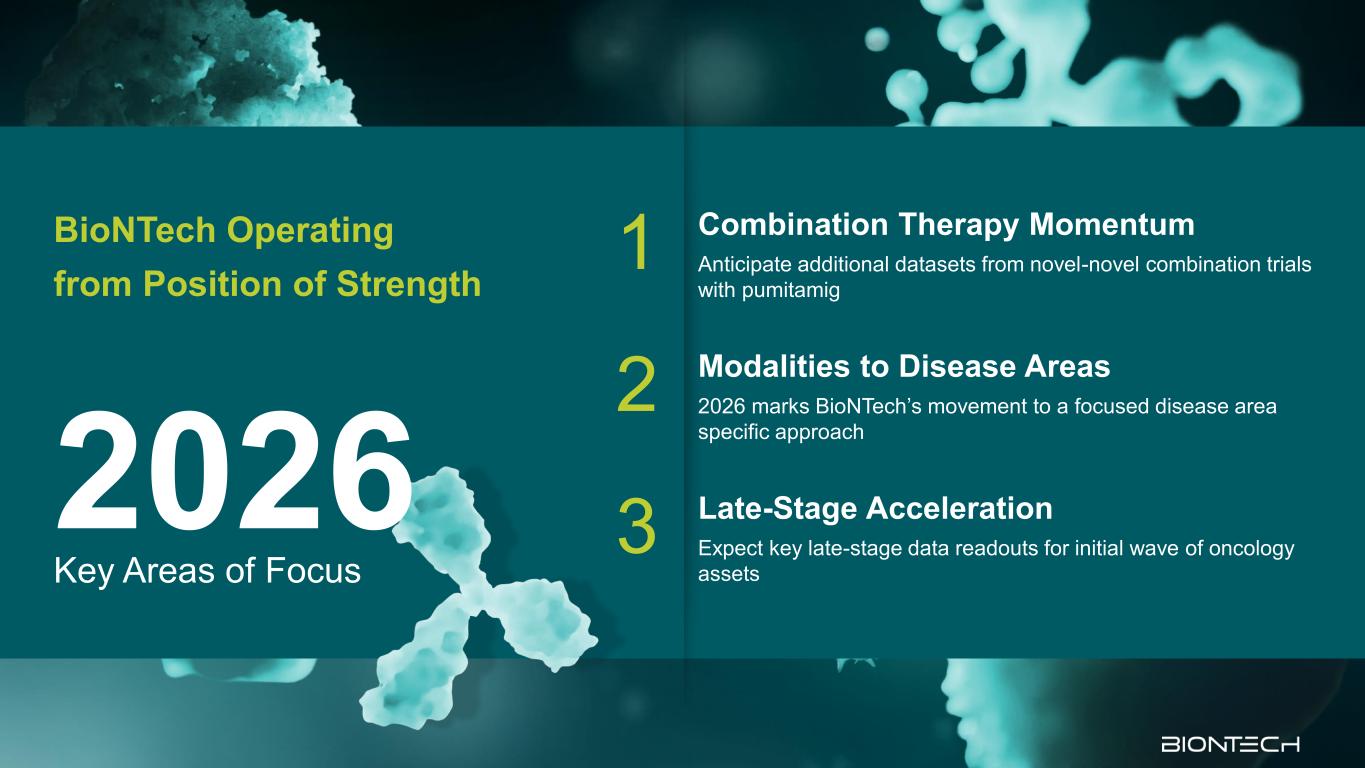
Key Areas of Focus BioNTech Operating from Position of Strength 1 Combination Therapy Momentum Anticipate additional datasets from novel-novel combination trials with pumitamig 2 Modalities to Disease Areas 2026 marks BioNTech’s movement to a focused disease area specific approach 3 Late-Stage Acceleration Expect key late-stage data readouts for initial wave of oncology assets 2026

Prof. Uğur Şahin, M.D. Chief Executive Officer Co-founder Annemarie Hanekamp Chief Commercial Officer Prof. Özlem Türeci, M.D. Chief Medical Officer Co-founder Ramón Zapata Chief Financial Officer Michael Wenger, M.D. Vice President, Clinical Development Prof. Ilhan Celik, M.D. Vice President, Clinical Development Q&A Panel Discussion 166

Thank you

Appendix
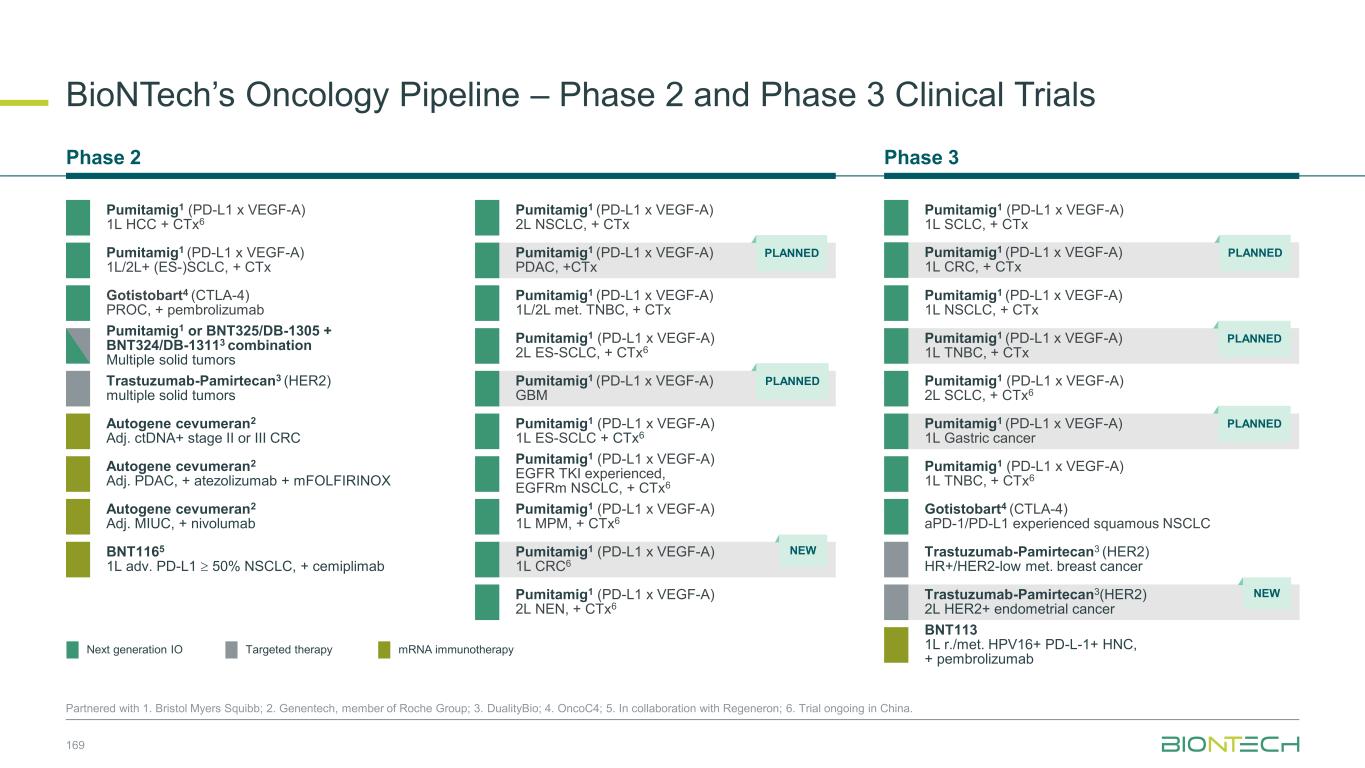
BioNTech’s Oncology Pipeline – Phase 2 and Phase 3 Clinical Trials Partnered with 1. Bristol Myers Squibb; 2. Genentech, member of Roche Group; 3. DualityBio; 4. OncoC4; 5. In collaboration with Regeneron; 6. Trial ongoing in China. Gotistobart4 (CTLA-4) PROC, + pembrolizumab Autogene cevumeran2 Adj. ctDNA+ stage II or III CRC BNT1165 1L adv. PD-L1 50% NSCLC, + cemiplimab Autogene cevumeran2 Adj. PDAC, + atezolizumab + mFOLFIRINOX Autogene cevumeran2 Adj. MIUC, + nivolumab Phase 3Phase 2 mRNA immunotherapyNext generation IO Targeted therapy Trastuzumab-Pamirtecan3 (HER2) multiple solid tumors 169 Pumitamig1 (PD-L1 x VEGF-A) 2L NSCLC, + CTx Pumitamig1 or BNT325/DB-1305 + BNT324/DB-13113 combination Multiple solid tumors Gotistobart4 (CTLA-4) aPD-1/PD-L1 experienced squamous NSCLC Trastuzumab-Pamirtecan3 (HER2) HR+/HER2-low met. breast cancer Pumitamig1 (PD-L1 x VEGF-A) 2L SCLC, + CTx6 Pumitamig1 (PD-L1 x VEGF-A) 1L TNBC, + CTx6 Pumitamig1 (PD-L1 x VEGF-A) 1L SCLC, + CTx Pumitamig1 (PD-L1 x VEGF-A) 1L NSCLC, + CTx Pumitamig1 (PD-L1 x VEGF-A) 1L TNBC, + CTx PLANNED Trastuzumab-Pamirtecan3(HER2) 2L HER2+ endometrial cancer NEW BNT113 1L r./met. HPV16+ PD-L-1+ HNC, + pembrolizumab Pumitamig1 (PD-L1 x VEGF-A) 1L/2L met. TNBC, + CTx Pumitamig1 (PD-L1 x VEGF-A) 1L/2L+ (ES-)SCLC, + CTx Pumitamig1 (PD-L1 x VEGF-A) 2L ES-SCLC, + CTx6 Pumitamig1 (PD-L1 x VEGF-A) 1L ES-SCLC + CTx6 Pumitamig1 (PD-L1 x VEGF-A) EGFR TKI experienced, EGFRm NSCLC, + CTx6 Pumitamig1 (PD-L1 x VEGF-A) 1L MPM, + CTx6 Pumitamig1 (PD-L1 x VEGF-A) 2L NEN, + CTx6 Pumitamig1 (PD-L1 x VEGF-A) 1L CRC6 NEW Pumitamig1 (PD-L1 x VEGF-A) 1L CRC, + CTx PLANNED Pumitamig1 (PD-L1 x VEGF-A) 1L Gastric cancer PLANNED Pumitamig1 (PD-L1 x VEGF-A) GBM PLANNED Pumitamig1 (PD-L1 x VEGF-A) PDAC, +CTx PLANNED Pumitamig1 (PD-L1 x VEGF-A) 1L HCC + CTx6
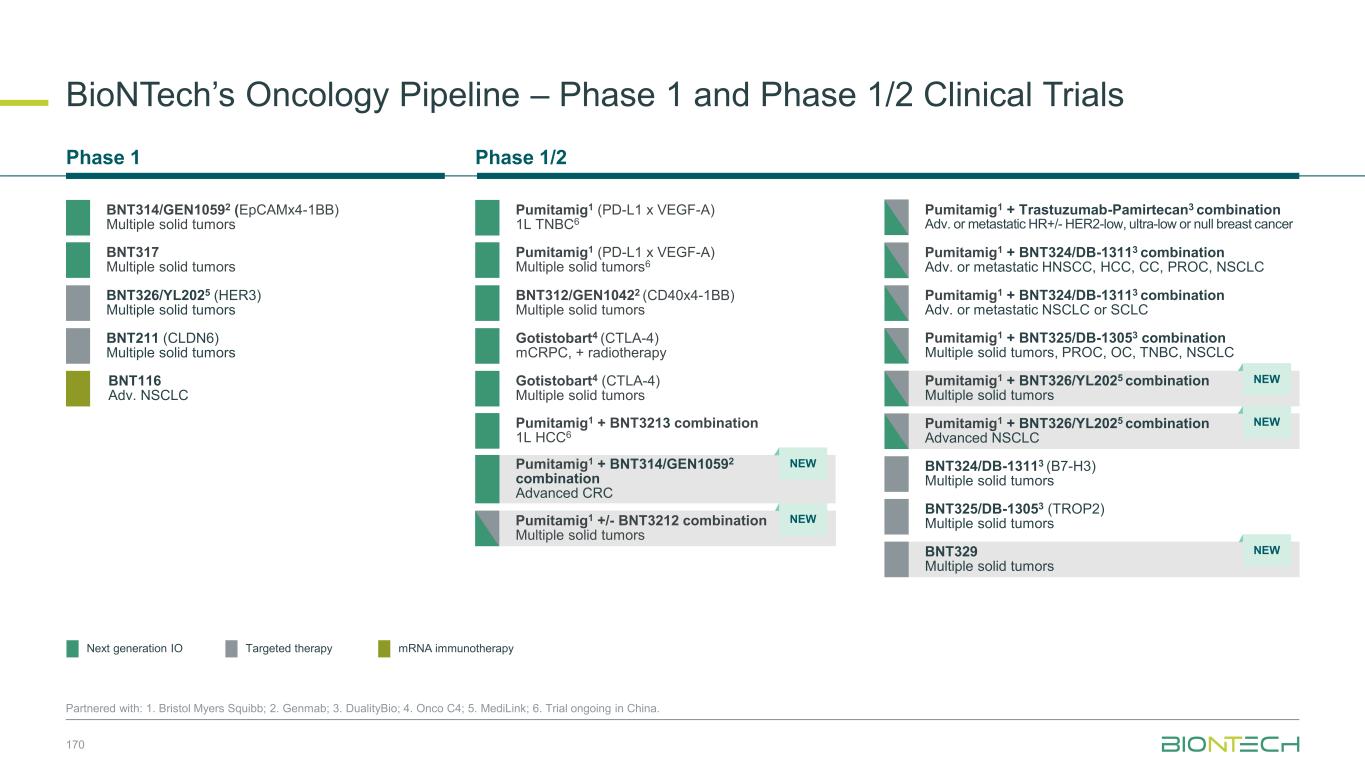
BioNTech’s Oncology Pipeline – Phase 1 and Phase 1/2 Clinical Trials 170 Partnered with: 1. Bristol Myers Squibb; 2. Genmab; 3. DualityBio; 4. Onco C4; 5. MediLink; 6. Trial ongoing in China. Phase 1/2Phase 1 BNT312/GEN10422 (CD40x4-1BB) Multiple solid tumors Gotistobart4 (CTLA-4) Multiple solid tumors BNT116 Adv. NSCLC BNT211 (CLDN6) Multiple solid tumors BNT314/GEN10592 (EpCAMx4-1BB) Multiple solid tumors BNT326/YL2025 (HER3) Multiple solid tumors BNT317 Multiple solid tumors Gotistobart4 (CTLA-4) mCRPC, + radiotherapy Pumitamig1 (PD-L1 x VEGF-A) 1L TNBC6 Pumitamig1 (PD-L1 x VEGF-A) Multiple solid tumors6 Pumitamig1 + BNT3213 combination 1L HCC6 BNT325/DB-13053 (TROP2) Multiple solid tumors BNT324/DB-13113 (B7-H3) Multiple solid tumors BNT329 Multiple solid tumors NEW Pumitamig1 + BNT325/DB-13053 combination Multiple solid tumors, PROC, OC, TNBC, NSCLC Pumitamig1 + BNT324/DB-13113 combination Adv. or metastatic HNSCC, HCC, CC, PROC, NSCLC Pumitamig1 + BNT324/DB-13113 combination Adv. or metastatic NSCLC or SCLC Pumitamig1 + BNT326/YL2025 combination Multiple solid tumors Pumitamig1 + Trastuzumab-Pamirtecan3 combination Adv. or metastatic HR+/- HER2-low, ultra-low or null breast cancer Pumitamig1 + BNT326/YL2025 combination Advanced NSCLC NEW NEW Pumitamig1 +/- BNT3212 combination Multiple solid tumors NEW mRNA immunotherapyNext generation IO Targeted therapy Pumitamig1 + BNT314/GEN10592 combination Advanced CRC NEW
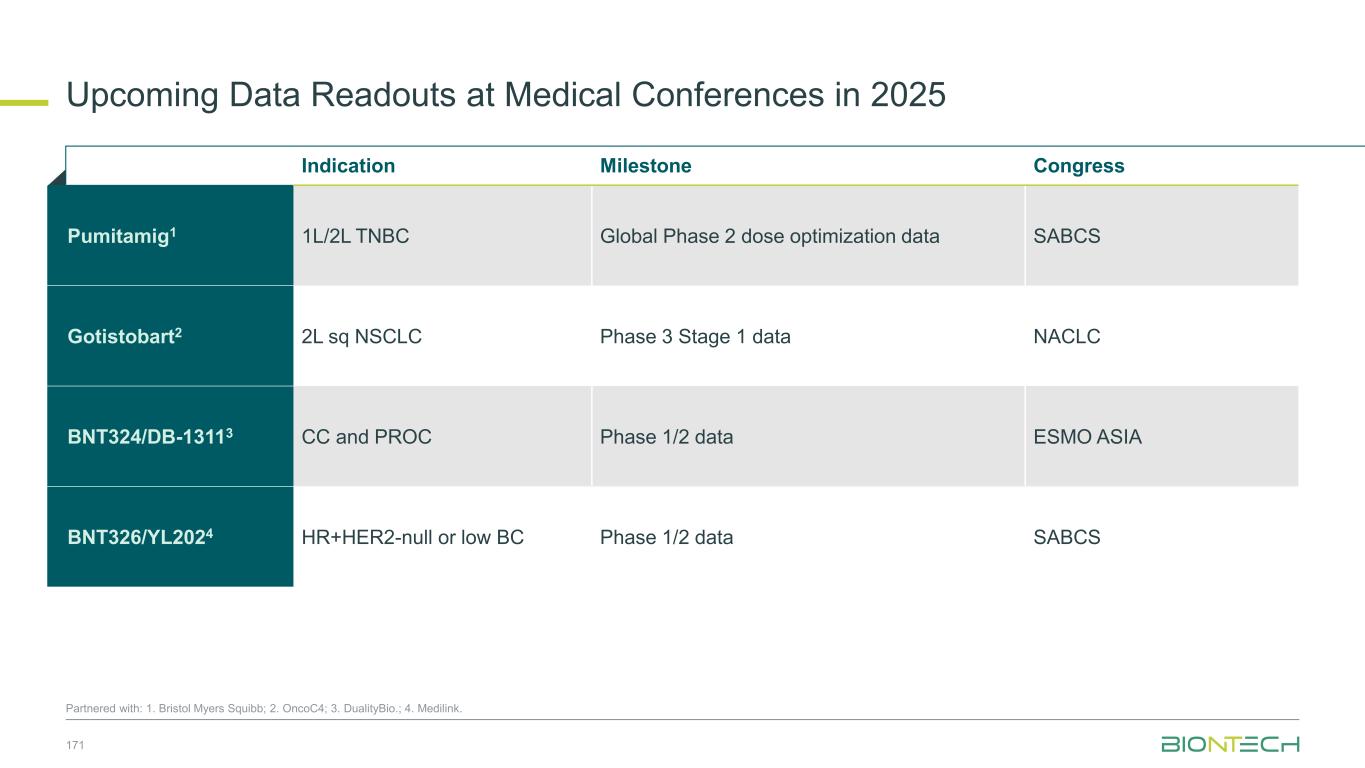
Upcoming Data Readouts at Medical Conferences in 2025 171 Indication Milestone Congress Pumitamig1 1L/2L TNBC Global Phase 2 dose optimization data SABCS Gotistobart2 2L sq NSCLC Phase 3 Stage 1 data NACLC BNT324/DB-13113 CC and PROC Phase 1/2 data ESMO ASIA BNT326/YL2024 HR+HER2-null or low BC Phase 1/2 data SABCS Partnered with: 1. Bristol Myers Squibb; 2. OncoC4; 3. DualityBio.; 4. Medilink.
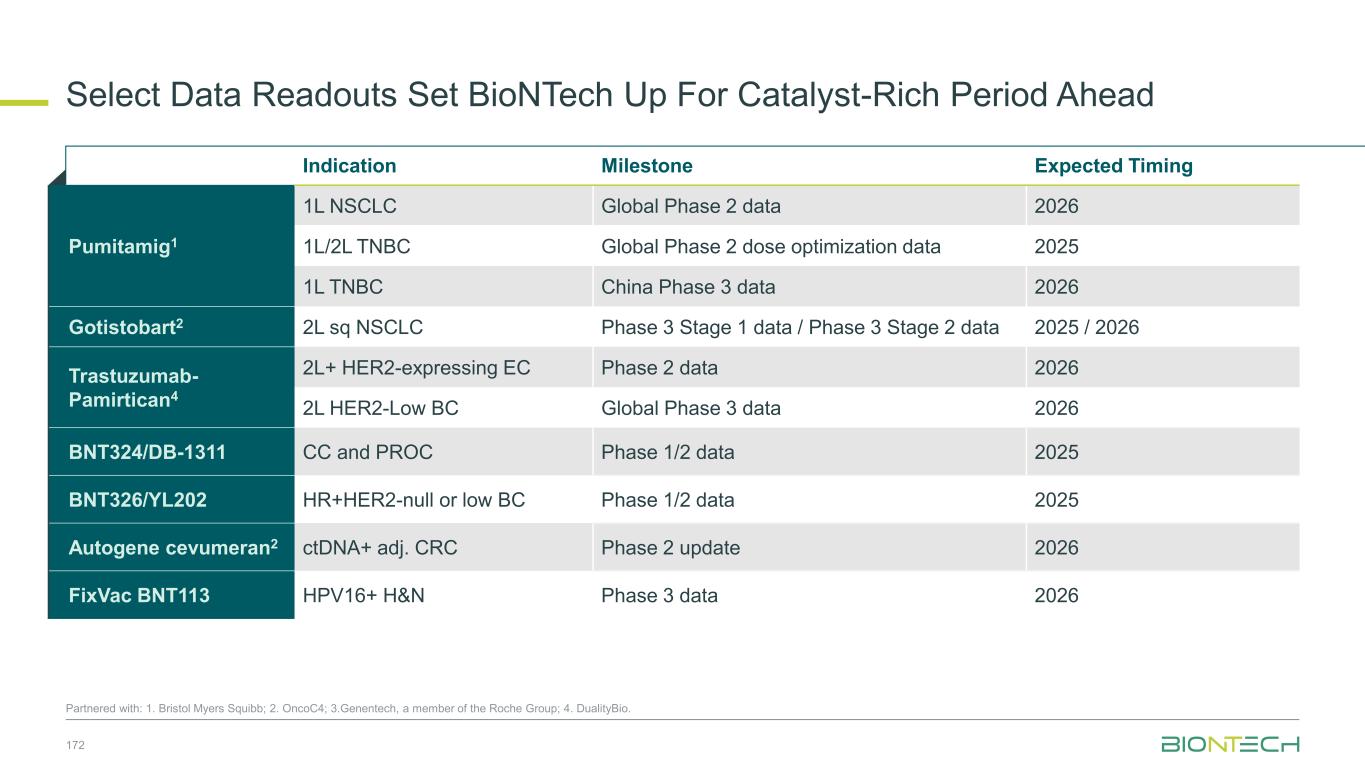
Select Data Readouts Set BioNTech Up For Catalyst-Rich Period Ahead 172 Partnered with: 1. Bristol Myers Squibb; 2. OncoC4; 3.Genentech, a member of the Roche Group; 4. DualityBio. Indication Milestone Expected Timing Pumitamig1 1L NSCLC Global Phase 2 data 2026 1L/2L TNBC Global Phase 2 dose optimization data 2025 1L TNBC China Phase 3 data 2026 Gotistobart2 2L sq NSCLC Phase 3 Stage 1 data / Phase 3 Stage 2 data 2025 / 2026 Trastuzumab- Pamirtican4 2L+ HER2-expressing EC Phase 2 data 2026 2L HER2-Low BC Global Phase 3 data 2026 BNT324/DB-1311 CC and PROC Phase 1/2 data 2025 BNT326/YL202 HR+HER2-null or low BC Phase 1/2 data 2025 Autogene cevumeran2 ctDNA+ adj. CRC Phase 2 update 2026 FixVac BNT113 HPV16+ H&N Phase 3 data 2026
Abbreviation Directory (1) 4-1BB CD137 CCA Cholangiocarcinoma EC Endometrial cancer n L nth line cCRT Concurrent chemoradiotherapy ECOG Eastern Cooperative Oncology Group AACR American Association for Cancer Research CDx Cluster of differentiation EGFR Epidermal growth factor receptor (bs)AB (bispecific) Antibody CDK4/6 Cyclin-dependent 4/6 ELCC European Lung Cancer Congress ADA Anti-drug antibody ChIP Chromatin Immunoprecipitation EORTC Europ. Organanisation. for Research and Treatment of Cancer (bs)ADC (bispecific) Antibody-drug conjugate CI Confidence interval EpCAM Epithelial cell adhesion molecule ADCC Antibody-dependent cell-mediated cytotoxicity CLDN6 Claudin 6 ESCC Esophageal squamous cell carcinoma ADCP Antibody-dependent cellular phagocytosis CPI Checkpoint inhibitor ESMO European Society for Medical Oncology adj. Adjuvant CPS Combined positive score ESMO GI European Society for Medical Oncology Gastrointestinal AE Adverse event CR Complete response ES-SCLC Extensive-stage small cell lung cancer AGA Actionable oncogenic alteration CRC Colorectal cancer ESO Esophageal AI Artificial intelligence CRPC Castration resistant prostate cancer ET Endocrine therapy ALK Anaplastic large-cell lymphoma kinase (c)CRT (Concurrent) Chemoradiation therapy EU European Union AST Aspartate aminotransferase ctDNA Circulating tumor DNA Fab Fragment antigen binding ASCO American Society of Clinical Oncology CTLA Cytotoxic T-lymphocyte-associated protein Fc(R) Fragment crystallizable region AU Absorvance unit ctrl Control FDA Food and Drug Administration AUC Area under curve CTx Chemotherapy FIH First in human B7-H3 B7 Homolog 3 DAR Drug-antibody ratio FixVac Fixed Antigen Vaccine BC Breast cancer DC Dendritic cell FoxP3 Forkhead-Box-Protein P3 BICR Blinded independent central review DCR Disease control rate GBM Glioblastoma multiforme BLA Biologics License Applications DDI Drug-drug interaction GC/GEJ Gastric/Gastro-esophageal junction cancer BMS Bristol Myers Squibb DFS Disease-free survival GI Gastrointestinal BOIN Bayesian optimal interval DL Dose level GnP Gemcitabine plus nab-paclitaxel BOR Best overall response DLT Dose limiting toxicity GrzmB Granzyme B B-RAF Serin/Threonin-Kinase dMMR Deficient mismatch repair GTEx Genotype-Tissue Expression BTC Biliary tract cancer DNA Desoxyribonucleic acid GU Genitourinary C1D1 Cycle 1 day 1 DO Dose optimization Gyn Gynecological CA 19-9 Carbohydrate antigen 19-9 DoR Duration of response h Human CC Cervical cancer EAC Esophageal adenocarcinoma H&E Hematoxylin and Eosin 173
Abbreviation Directory (2) H&N Head and neck JCO Journal of Clinical Oncology NCI PRO- National Cancer Institute Patient Reported Outcome Common HCC Hepatocellular carcinoma KD Dissociation constant CTCAE Terminology Criteria for Adverse Events HER2/3 Human epidermal growth factor receptor 2/3 KK-LC-1 Kita-Kyushu lung cancer antigen 1 NCI SEER National Cancer Institute Surveillance, Epidemiology, and End HLA Human leukocyte antigen LALA IgG1 variant L234A/L235A Results HNSCC Head and neck squamous cell carcinoma LDH Lactate dehydrogenase NCT National clinical trial HPV Human papilloma virus LOD Limit of detection NE Not evaluable for response HR Hormone receptor LPX Lipoplex NEJM The New England Journal of Medicine IASLC International Association for the Study of Lung LUAD Lung adenocarcinoma NEN Neuroendocrine neoplasm Cancer LUSC Lung squamous carcinoma NGS Next generation sequencing IC Immune checkpoint MAGE-A3 Melanoma antigen A3 NHT Novel hormonal therapy IC50 Half maximal inhibitory concentration MEKi Mitogen-activated protein kinase kinase NIH National Institutes of Health ICI Immune checkpoint inhibitor MHC Major histocompatibility complex NOD SCID Non-Obese Diabetic-Severe Combined Immunodeficiency IFN Interferon MITD Microtubule interacting and trafficking NCT National clinical trial IgG Immunoglobulin G domain NE Not evaluable for response IHC Immunohistochemistry MIUC Muscle-invasive urothelial carcinoma NEJM The New England Journal of Medicine IIT Investigator initiated trial mo Months NEN Neuroendocrine neoplasm ILD Interstitial lung disease MOA Mechanism of Action NGS Next generation sequencing IL-x Interleukin x MΦ Macrophage NHT Novel hormonal therapy IMDC International Metastatic Renal Cell Carcinoma mono Monotherapy NIH National Institutes of Health Database Consortium MPM Malignant pleural mesothelioma NOD SCID Non-Obese Diabetic-Severe Combined Immunodeficiency iNeST Individualized NeoAntigen-Specific Therapy MRD Minimal residual disease NR Not reached IO Immuno-oncology mRNA Messenger ribonucleic acid NSCLC Non-small cell lung cancer Ipi Ipilimumab MSI-H(L) High(low)-frequence microsatellite OC Ovarian cancer ISH In-situ hybridization Instability OP Operation ITT Intention to treat MSS Microsatellite stability (c)ORR (confirmed) Objective response rate iv Intravenously MTD Maximum tolerated dose OS Overall survival IvS in vitro stimulation NA Not applicable OVA Ovalbumin JAMA Journal of the American Medical NACLC Nort America conference on Lung Cancer P&L Profit and loss statement Association NCI National Cancer Institute PARP Poly (ADP-ribose) polymerase 174
Abbreviation Directory (3) PBMC Peripheral blood mononuclear cell RP2/3D Recommended phase 2/3 dose TLR Toll-like receptor PBS Phosphate buffered saline RPL18 Ribosomal Protein L18 TMB-H (or L) Tumor mutational burden-high or low PCWG3 Prostate Cancer Working Group 3 R/R Relapsed/refractory TME Tumor microenvironment PD Progressive disease RT-qPCR Real-time quantitative polymerase chain TNBC Triple-negative breast cancer PD Pharmacodynamics reaction TNF Tumor necrosis factor PDAC Pancreatic ductal adenocarcinoma SABCS San Antonio Breast Cancer Symposium TOP1 Topoisomerase I PD-(L)1 Programmed cell death protein (ligand) 1 SCCHN Squamous cell carcinoma of head and neck TPS Tumor proportion score pembro Pembrolizumab (ES)SCLC (Extensive/ stage) small cell lung cancer TPTE Transmembrane phosphatase with tensin homology PFS Progression-free survival SD Standard deviation TRAE Treatment-related adverse event pH Potentia hydrogenii SD Stable disease Treg Regulatory T cell Ph x (clinical) Phase x SEC Selenocysteinyl-tRNA TRON Helmholtz Institute for Translational Oncology PK Pharmacokinetics SEC United States Securities and Exchange TROP2 Trophoblast cell-surface antigen 2 pMMR Proficient mismatch repair Commission TYR Tyrosine PMX Pemetrexed SEER Surveillance, epidemiology, and end results UC Urothelial cancer PoC Proof of concept SEM Standard error of the mean UK United Kingdom POS Point of sale SITC Society of Immunotherapy of Cancer ULN Upper limit of normal PR Partial response SNP Single Nucleotide Polymorphism U.S. United States PR Progesterone receptor SoC Standard of care UTI Urinary tract infection PRAME Preferentially expressed antigen in melanoma SPR Surface Plasmon Resonance UTR Untranslated region PROC Platinum-resistant ovarian cancer (N)Sq (non-)squamous VEGF(R) - A Vascular endothelial growth factor (receptor) A PVRIG Poliovirus receptor-related immunoglobulin TAA Tumor-associated antigen VHH Heavy chain variable R Randomized TCGA The Cancer Genome Atlas WCLC World Conference of Lung Cancer RAS Rat sarcoma TCR T-cell receptor WHO World Health Organization RCC Renal cell carcinoma TEA Tissue engineering acoustophoretic WT Wild type R&D Research and development TFI Treatment-free interval YTD Year to date RECIST Response Evaluation Criteria in Solid TIGIT T cell immunoreceptor with Ig and ITIM Tumors domains RLT Radioligand therapy TIL Tumor-infiltrating lymphocytes RLU Relative light units TKI Tyrosine kinase inhibitor 175














































































































































































