Document
BioNTech Announces First Quarter 2025 Financial Results
and Corporate Update
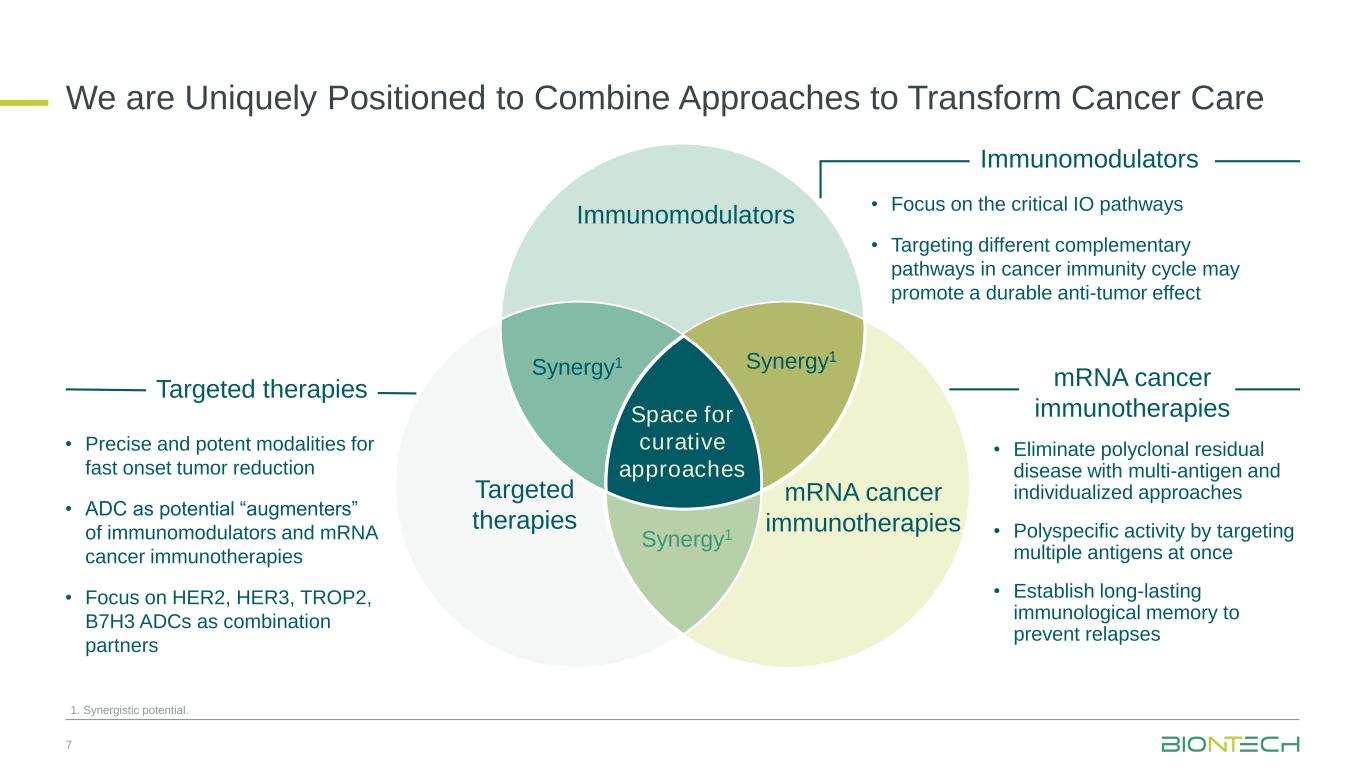
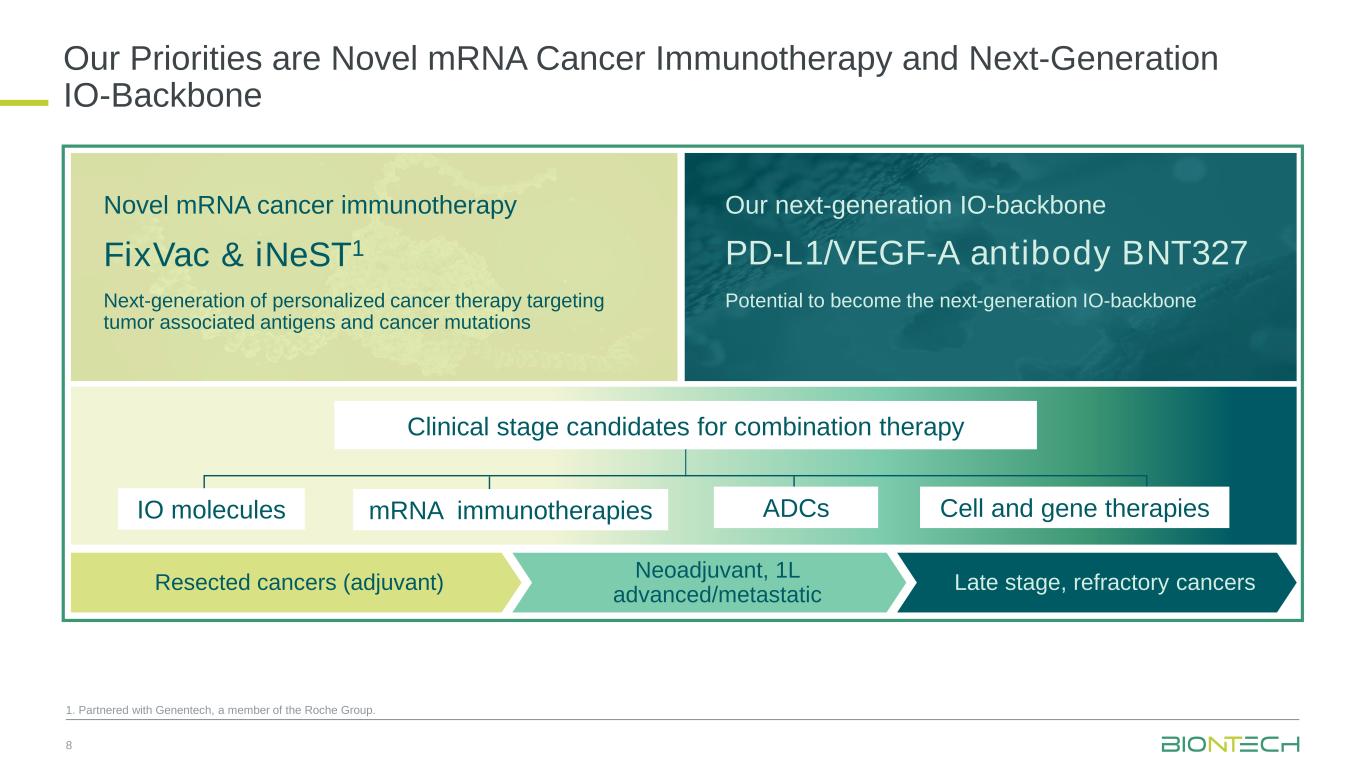
Continued oncology pipeline advancement with a strategic focus on two priority pan-tumor programs: next-generation immunomodulator BNT327, a bispecific antibody targeting PD-L1 and VEGF-A1, and mRNA cancer immunotherapies
•Presented multiple clinical updates across oncology pipeline underlining BioNTech’s combination strategy in oncology with first data presented for the novel combination of BNT327 plus antibody-drug conjugates (“ADCs”)
•Development and commercial preparation for a 2025/2026 season variant-adapted COVID-19 vaccine
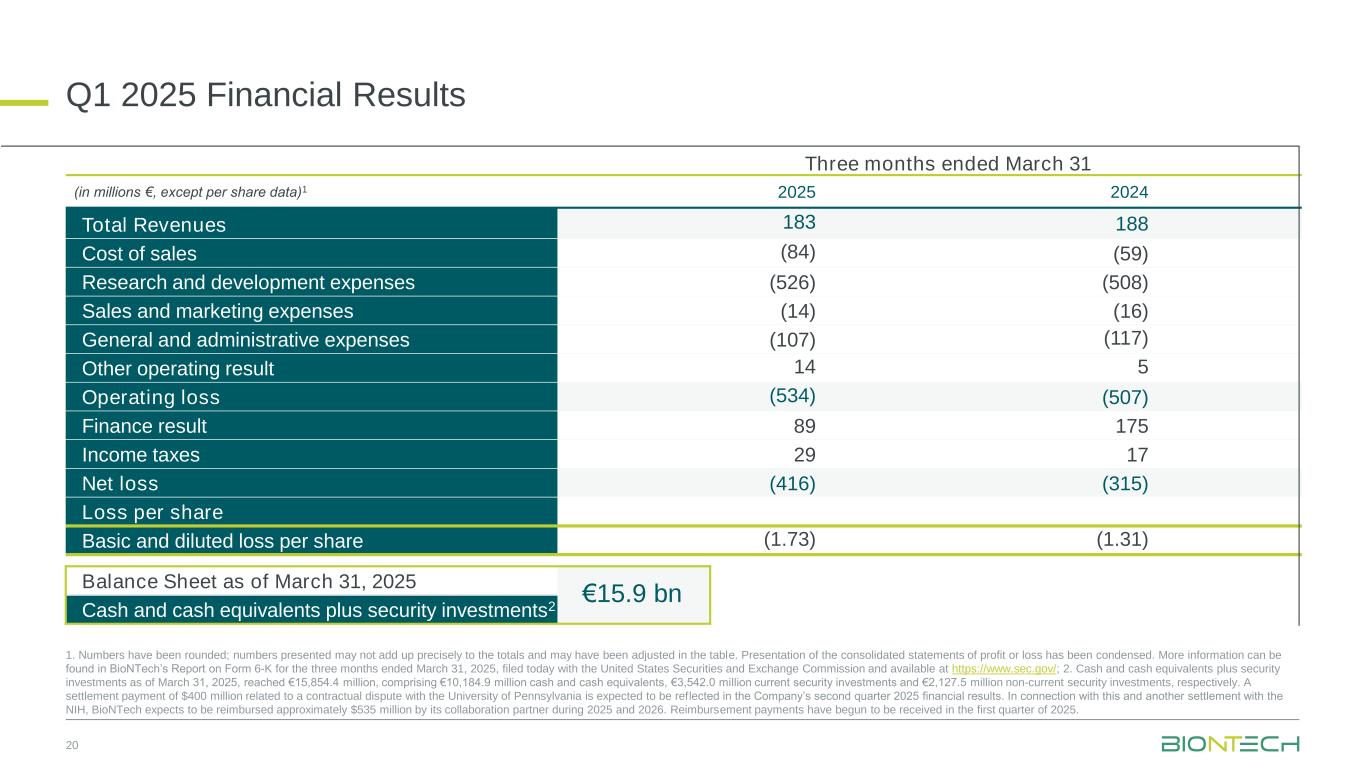
•First quarter 2025 revenues of €0.2 billion2, net loss of €0.4 billion and basic and diluted loss per share of €1.73 ($1.823)
•Maintained strong financial position with €15.9 billion in cash, cash equivalents and security investments as of March 31, 20254
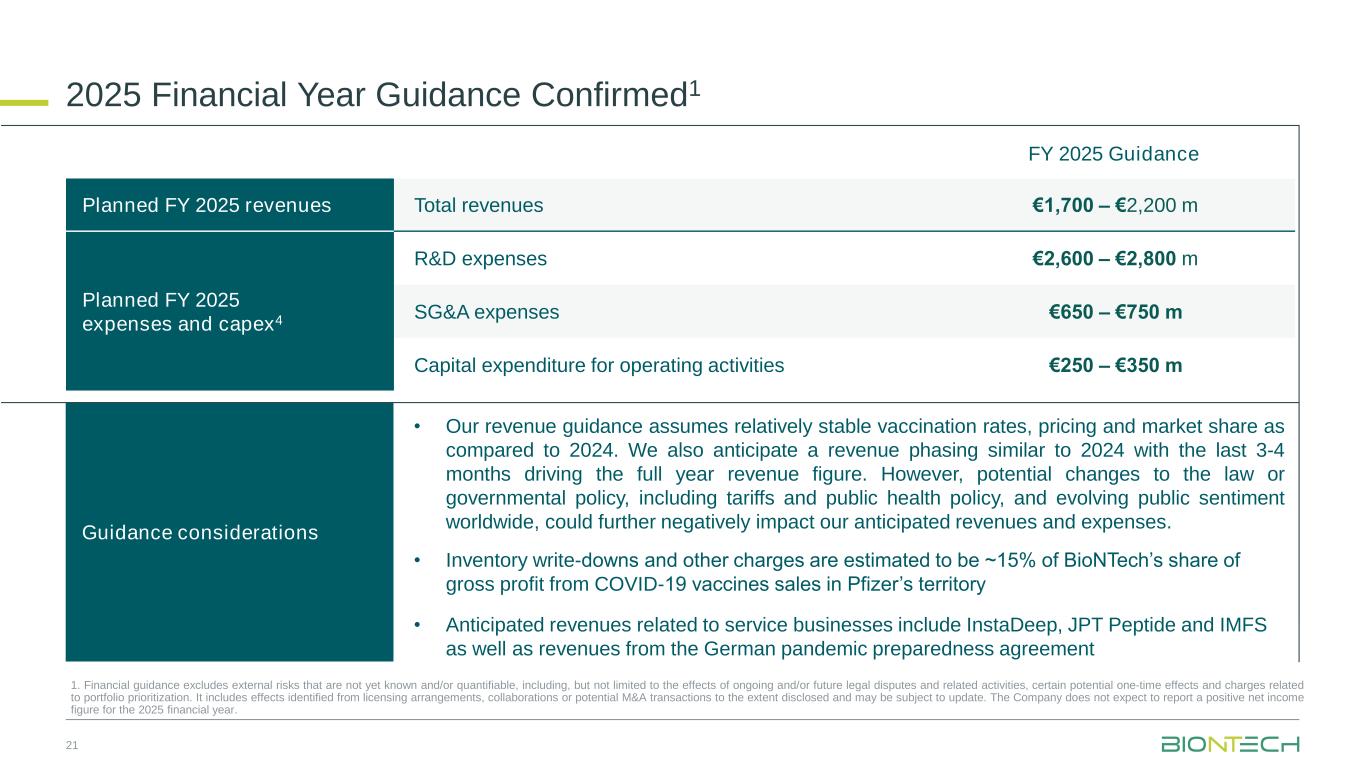
•Full year 2025 financial guidance confirmed
Conference call and webcast scheduled for May 5, 2025, at 8:00 a.m. EDT (2:00 p.m. CEST)
MAINZ, Germany, May 5, 2025 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, “BioNTech” or “the Company”) today reported financial results for the three months ended March 31, 2025 and provided an update on its corporate progress.
“In the first quarter of 2025, we demonstrated continued execution against our strategic focus areas, highlighted by data updates for our PD-L1xVEGF-A bispecific antibody candidate BNT327 and the progress in clinical evaluation of our focus programs and combination treatment approaches,” said Prof. Ugur Sahin, M.D., CEO and Co-Founder of BioNTech. “We will continue to focus on our key strategic programs as we remain steadfast in our vision to translate our science into survival for patients in need.”
Financial Review for First Quarter 2025
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
in millions €, except per share data |
|
|
First Quarter 2025 |
|
|
First Quarter 2024 |
Revenues |
|
|
182.8 |
|
|
187.6 |
Net loss |
|
|
(415.8) |
|
|
(315.1) |
Basic and diluted loss per share |
|
|
(1.73) |
|
|
(1.31) |
Revenues reported were €182.8 million for the three months ended March 31, 2025, compared to €187.6 million for the comparative prior year period. Revenues during the first quarter of 2025 were mainly driven by revenues derived from BioNTech’s COVID-19 vaccine collaboration.
Cost of sales were €83.8 million for the three months ended March 31, 2025, compared to €59.1 million for the comparative prior year period. The change was mainly due to a positive impact of an inventory revaluation in the first quarter of 2024.
Research and development (“R&D”) expenses were €525.6 million for the three months ended March 31, 2025, compared to €507.5 million for the comparative prior year period. The increase was mainly driven by progressing late-stage clinical studies for candidates in BioNTech’s ADC and antibody portfolio.
Sales, general and administrative (“SG&A”) expenses5, in total, amounted to €120.6 million for the three months ended March 31, 2025, compared to €132.6 million for the comparative prior year period. The decrease was primarily driven by a reduction in external services.
Income taxes were realized with an amount of €29.6 million in tax income for the three months ended March 31, 2025, compared to €16.7 million in realized tax income for the comparative prior year period.
Net loss was €415.8 million for the three months ended March 31, 2025, compared to a net loss of €315.1 million for the comparative prior year period.
Cash and cash equivalents plus security investments as of March 31, 2025, reached €15,854.4 million, comprising €10,184.9 million in cash and cash equivalents, €3,542.0 million in current security investments and €2,127.5 million in non-current security investments.
Basic and diluted loss per share was €1.73 for the three months ended March 31, 2025, compared to a basic and diluted loss per share of €1.31 for the comparative prior year period.
Shares outstanding as of March 31, 2025, were 240,392,622, excluding 8,159,578 shares held in treasury.
“Our revenues for the first quarter reflect the seasonal demand for COVID-19 vaccines and are in line with our expectations,” said Jens Holstein, CFO of BioNTech. “BioNTech’s robust financial position empowers us to pursue our strategic goal of evolving into a leading biotech company with multiple oncology products by 2030.”
2025 Financial Year Guidance Confirmed6
|
|
|
|
|
|
|
|
|
Total revenues for the 2025 financial year |
|
€1,700 million - €2,200 million |
BioNTech expects its revenues for the full 2025 financial year to be in the range of €1,700 - €2,200 million and revenue phasing similar to 2024, primarily concentrated in the last three to four months, driving the full year revenue figure. The revenue guidance assumes: relatively stable vaccination rates, pricing levels and market share compared to 2024; estimated inventory write-downs and other charges by BioNTech’s collaboration partner Pfizer that negatively influence BioNTech’s revenues; anticipated revenues from a pandemic preparedness contract with the German government; and anticipated revenues from the BioNTech Group service businesses. Potential changes to the law or governmental policy, including tariffs and public health policy, and evolving public sentiment worldwide, could further negatively impact our anticipated revenues and expenses.
Planned 2025 Financial Year Expenses and Capex
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R&D expenses |
|
|
€2,600 million - €2,800 million |
|
SG&A expenses |
|
|
€650 million - €750 million |
|
Capital expenditures for operating activities |
|
|
€250 million - €350 million |
|
BioNTech expects to continuously focus investments on R&D and scaling the business for late-stage development and commercial readiness in oncology, while remaining cost-disciplined. Strategic capital allocation will continue to be a key driver of the Company’s trajectory. As part of BioNTech’s strategy, the Company may continue to evaluate appropriate corporate development opportunities with the aim of driving sustainable long-term growth and create future value.
The full interim unaudited condensed consolidated financial statements can be found in BioNTech’s Report on Form 6-K for the period ended March 31, 2025, filed today with the United States Securities and Exchange Commission (“SEC”) and available at www.sec.gov.
Endnotes
1 All abbreviations are compiled in an abbreviation directory at the end of this press release.
2 All numbers in this press release have been rounded.
3 Calculated applying the average foreign exchange rate for the three months ended March 31, 2025, as published by the German Central Bank (Deutsche Bundesbank).
4 A settlement payment of $400 million related to a contractual dispute with the University of Pennsylvania is expected to be reflected in the Company’s second quarter 2025 financial results. In connection with this and another settlement with the National Institutes of Health (“NIH”), BioNTech expects to be reimbursed approximately $535 million by its collaboration partner during 2025 and 2026. Reimbursement payments have begun with the first payment received by BioNTech in the first quarter of 2025.
5 Sales, general and administrative expenses (“SG&A”) include sales and marketing expenses as well as general and administrative expenses.
6 Financial guidance excludes external risks that are not yet known and/or quantifiable, including, but not limited to the effects of ongoing and/or future legal disputes and related activities, certain potential one-time effects and charges related to portfolio prioritization. It includes effects identified from licensing arrangements, collaborations or potential M&A transactions to the extent disclosed and may be subject to update. The Company does not expect to report a positive net income figure for the 2025 financial year.
Operational Review for the First Quarter 2025, Key Post Period-End Events and 2025 Outlook
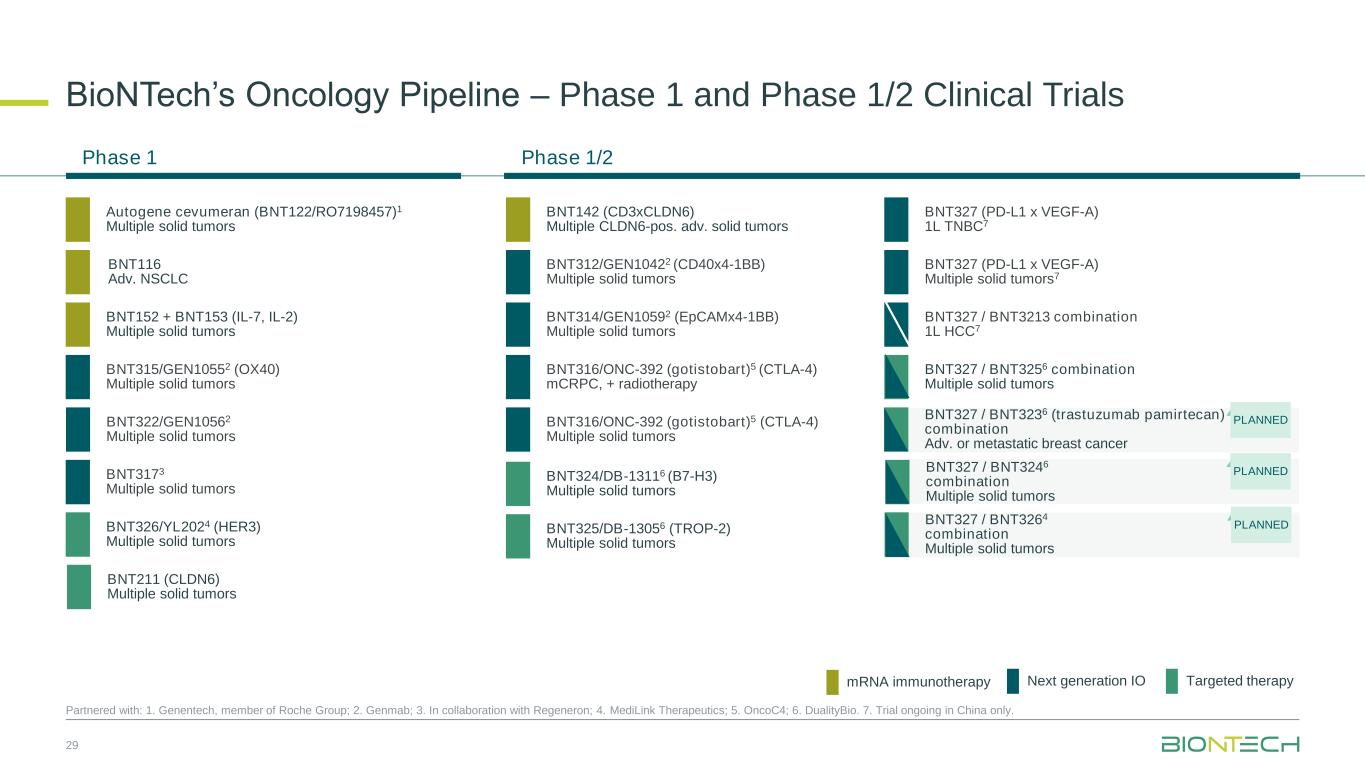
Selected Oncology Pipeline Updates
Next-Generation Immunomodulators
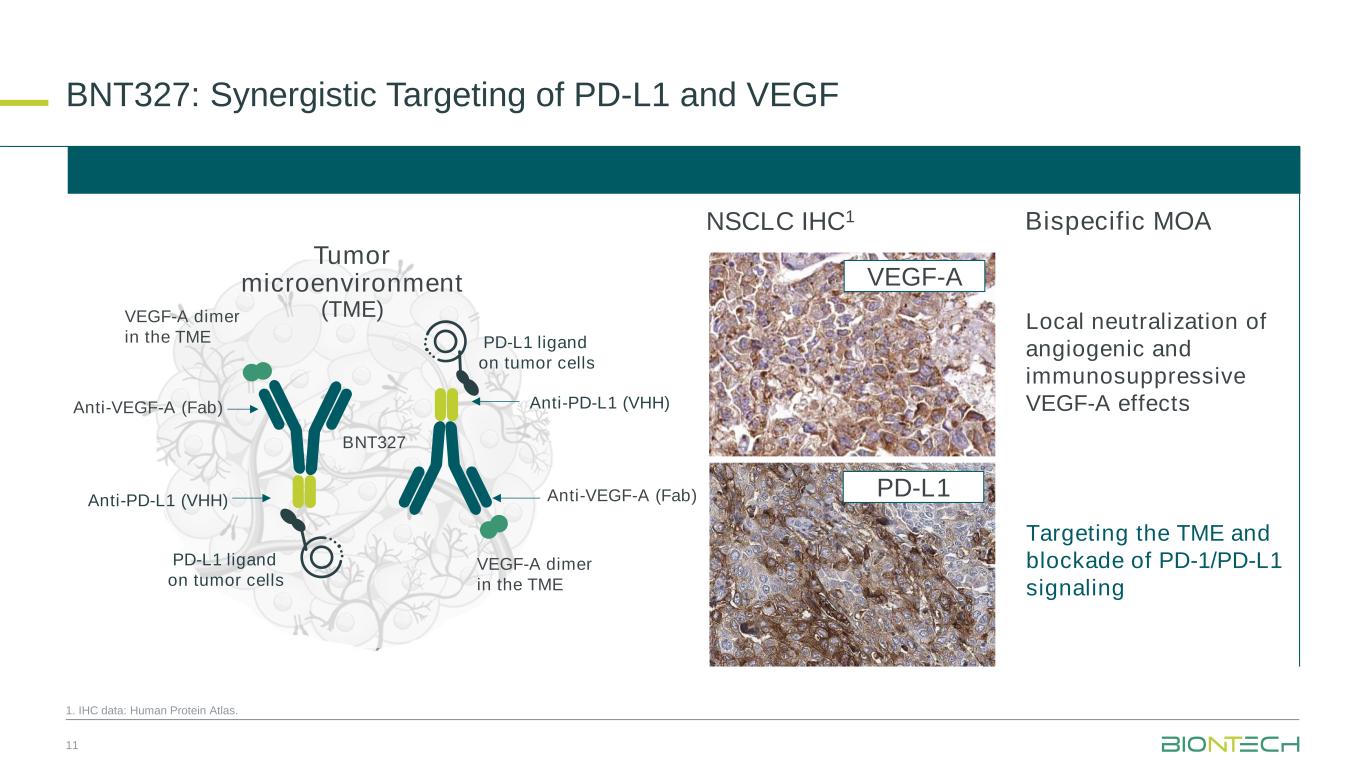
BNT327, formerly also known as PM8002, is a bispecific antibody candidate combining PD-L1 checkpoint inhibition with VEGF-A neutralization.
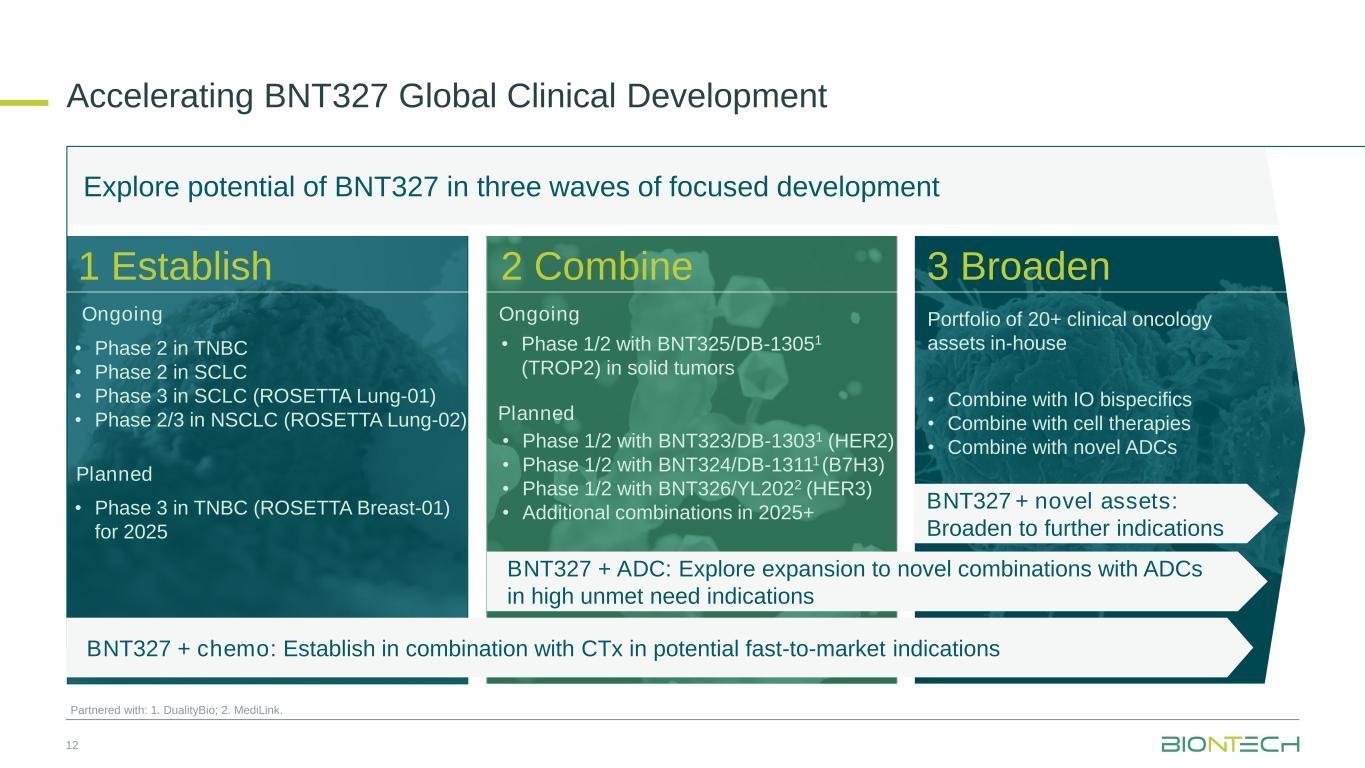
•In March 2025, at the European Lung Cancer Congress (“ELCC”), preliminary data from two Phase 2 clinical trials conducted in China in first-line extensive-stage small cell lung cancer (“ES-SCLC”) and second-line small cell lung cancer (“SCLC”) were presented.
◦Preliminary data from the ongoing single-arm, open-label Phase 2 clinical trial (NCT05844150) evaluating BNT327 in combination with chemotherapy as a first-line treatment in patients with ES-SCLC showed anti-tumor activity and an acceptable safety profile with no new safety signals beyond those typically described for chemotherapy agents and anti-PD-(L)1 and anti-VEGF monotherapies. These data were the first presented for BNT327 as a potential first-line treatment in ES-SCLC supporting the ongoing global randomized Phase 3 clinical trial ROSETTA Lung-01 (NCT06712355).
◦Preliminary data from the ongoing Phase 2 clinical trial (NCT05879068) evaluating BNT327 in combination with chemotherapy as a second-line treatment in patients with SCLC showed anti-tumor activity, which was observed regardless of prior immuno-oncology treatment, and an acceptable safety profile.
•In April 2025, at the American Association for Cancer Research (“AACR”) Annual Meeting 2025, first data were presented for the novel combination of the PD-L1xVEGF-A bispecific antibody candidate BNT327 with various ADC candidates.
◦Interim data from the ongoing Phase 1/2 clinical trial (NCT05438329) evaluating BNT327 with BNT325/DB-1305, a TROP2-targeting ADC candidate being developed in collaboration with Duality Biologics (Suzhou) Co.
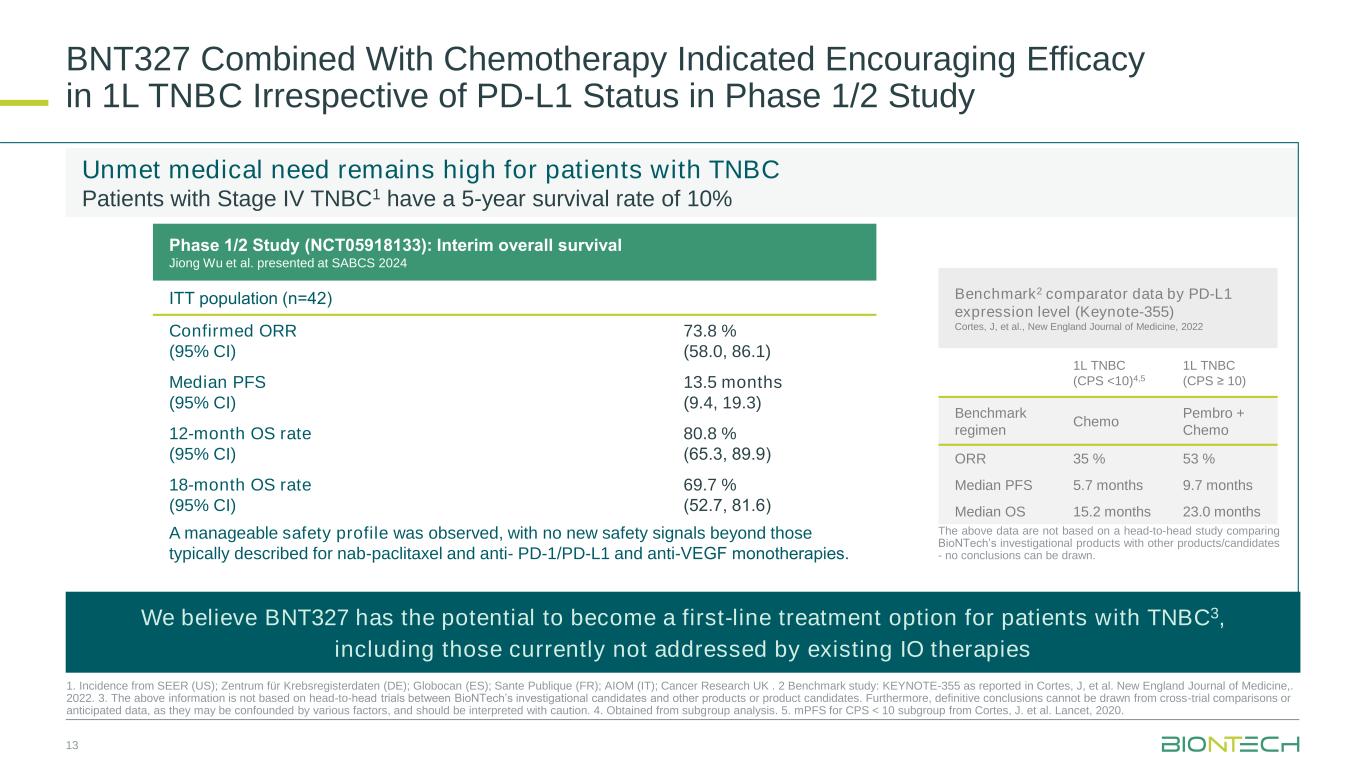
Ltd. (“DualityBio”), in patients with advanced/metastatic solid tumors showed a manageable safety profile and early signs of anti-tumor activity in a cohort with patients with platinum-resistant ovarian cancer (“PROC”). Across the 13 efficacy evaluable patients with PROC, seven patients achieved partial response and three stable disease. Responses were also observed in patients with non-small cell lung cancer (“NSCLC”) or triple-negative breast cancer (“TNBC”).
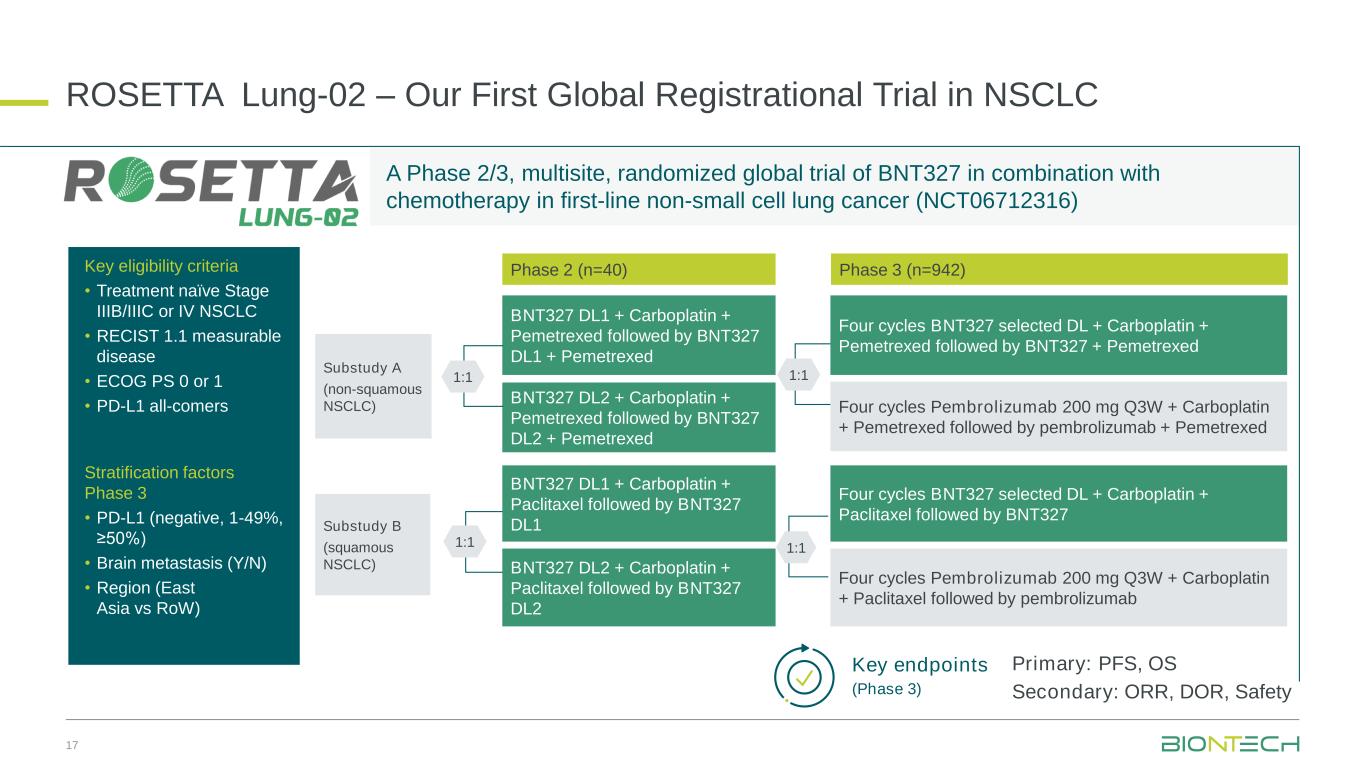
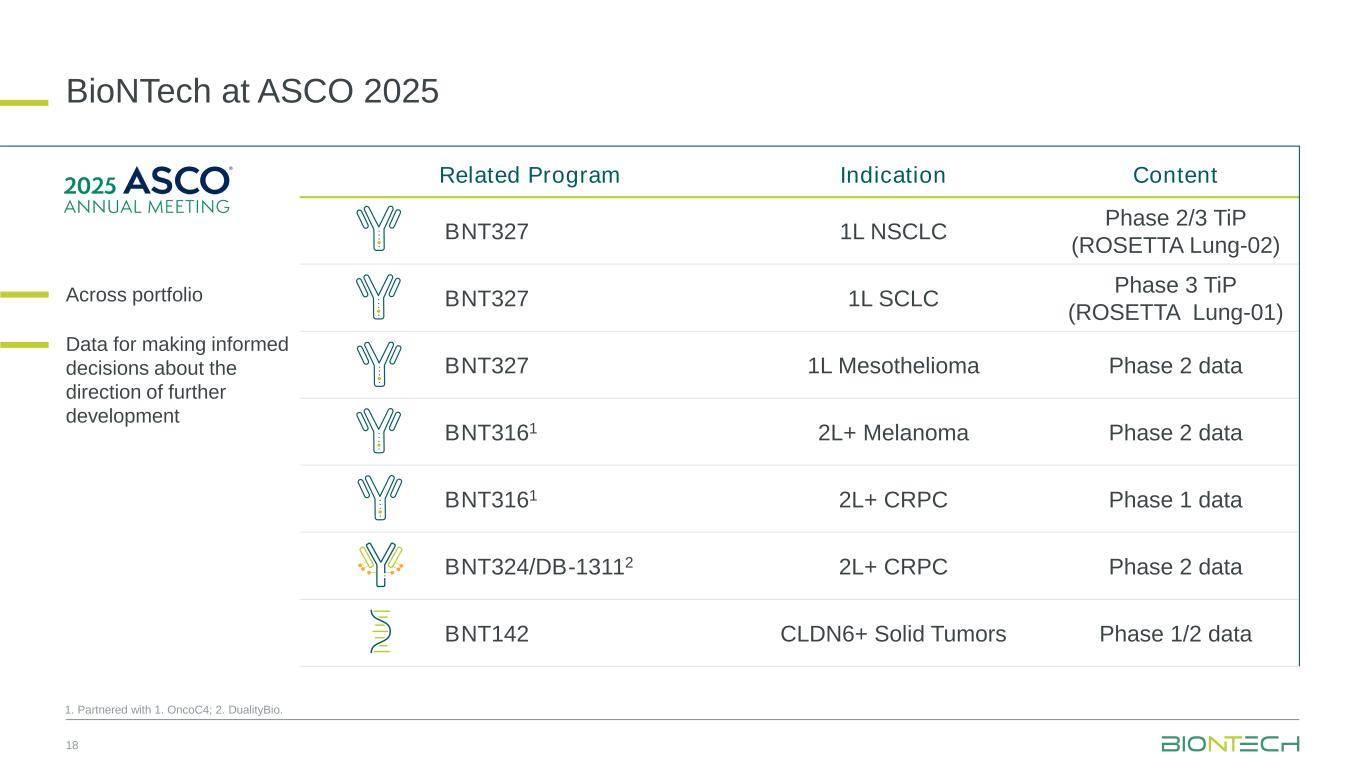
•First data from an ongoing Phase 2 clinical trial (NCT05918107) in first-line mesothelioma and two trial-in-progress posters for the ongoing global Phase 3 and Phase 2/3 clinical trials, ROSETTA Lung-01 (NCT06712355) and ROSETTA Lung-02 (NCT06712316) respectively, will be presented at the American Society for Clinical Oncology (“ASCO”) Annual Meeting taking place from May 30 to June 3, 2025 in Chicago, Illinois.
•Abstract title: First report of efficacy and safety results from a Phase 2 trial evaluating BNT327/PM8002 plus chemotherapy (chemo) as first-line treatment (1L) in unresectable malignant mesothelioma
•Abstract title: A global Phase III, double-blind, randomized trial of BNT327/PM8002 plus chemotherapy (chemo) compared to atezolizumab plus chemo in patients (pts) with first-line (1L) extensive-stage small cell lung cancer (ES-SCLC)
•Abstract title: A global Phase 2/3, randomized, open-label trial of BNT327/PM8002 in combination with chemotherapy (chemo) in first-line (1L) non-small cell lung cancer (NSCLC)
mRNA Cancer Immunotherapies
BNT116 is based on BioNTech’s fully owned, off-the-shelf FixVac platform, and is designed to elicit an immune response to six tumor-associated antigens that were identified to be frequently expressed in NSCLC.
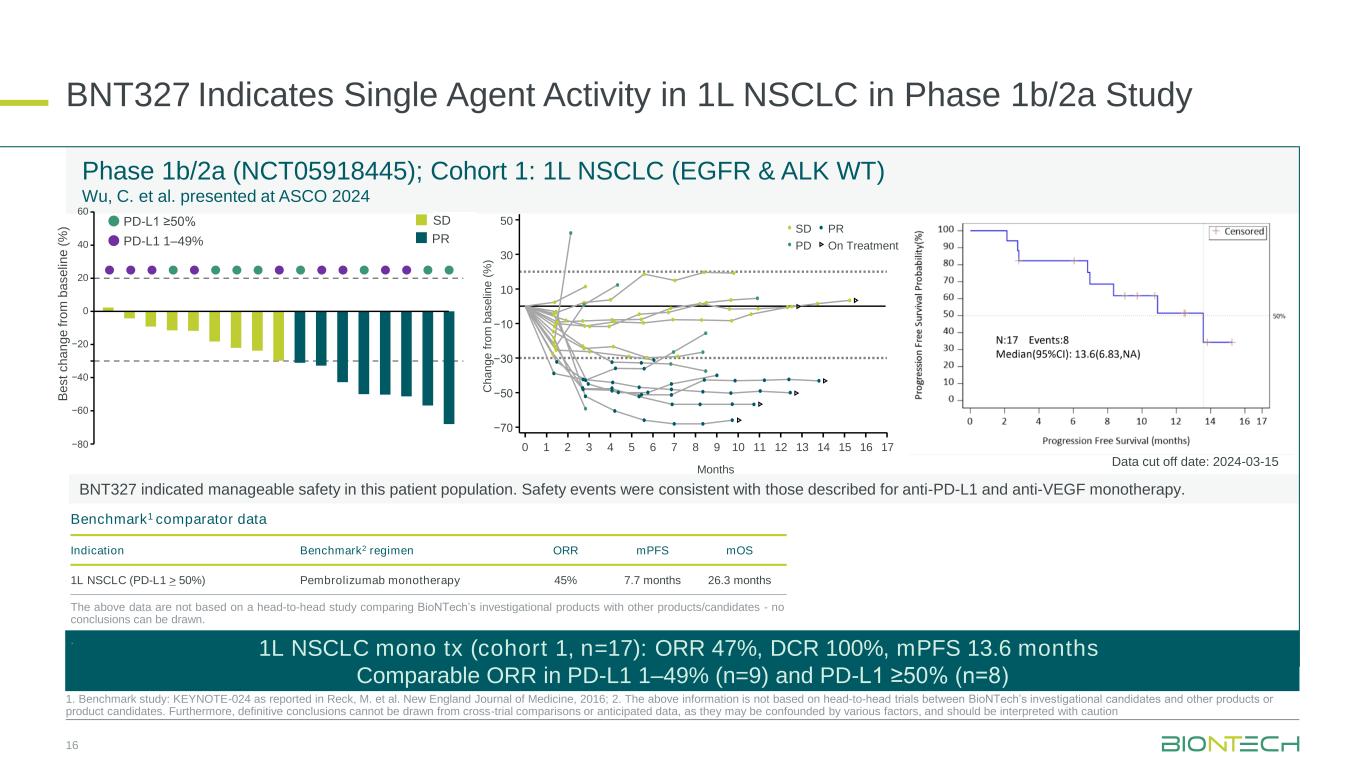
•In April 2025, at the AACR Annual Meeting 2025, preliminary data from a cohort with frail patients were presented from the ongoing Phase 1 clinical trial LuCa-MERIT-1 (NCT05142189) evaluating BNT116 in combination with cemiplimab in patients with PD-L1 positive (TPS≥1%) unresectable Stage III or metastatic Stage IV NSCLC who are not eligible for chemotherapy as first-line treatment. The preliminary data showed anti-tumor activity, consistent immune response induction and a manageable safety profile.
Antibody-Drug Conjugates
BNT324/DB-1311 is an B7H3-targeted ADC candidate that is being developed in collaboration with DualityBio. The program has received Fast Track designation from the U.S. Food and Drug Administration (“FDA”) for the treatment of patients with advanced castration-resistant prostate cancer (“CRPC”) whose disease has progressed while undergoing or after undergoing standard systemic regimens. In addition, the program has received Orphan Drug designation from the FDA for the treatment of patients with advanced esophageal squamous cell carcinoma.
•Preliminary data from the ongoing Phase 1/2 clinical trial (NCT05914116) evaluating BNT324/DB-1311 in patients with advanced solid tumors, including patients with previously treated castration-resistant prostate cancer (“CRPC”), will be presented at the 2025 ASCO Annual Meeting.
•Abstract title: DB‑1311/BNT324 (a novel B7H3 ADC) in patients with heavily pretreated castrate-resistant prostate cancer (CRPC)
BNT325/DB-1305 is a TROP2-targeted ADC candidate that is being developed in collaboration with DualityBio. BNT325/DB-1305 received Fast Track designation from the FDA for the treatment of patients with platinum-resistant ovarian epithelial cancer, fallopian tube cancer, or primary peritoneal cancer who have received one to three prior systemic treatment regimens.
•In March 2025, at the Society of Gynecologic Oncology (“SGO”) Annual Meeting, preliminary data were presented from an ongoing Phase 1/2 clinical trial (NCT05438329) evaluating BNT325/DB-1305 in signal-seeking cohorts across various cancer indications, including PROC. The data from a cohort of patients with PROC showed a manageable safety profile and early signs of anti-tumor activity.
Corporate and Commercial Update for the First Quarter 2025
•Earlier today, BioNTech announced that the Supervisory Board has appointed Ramón Zapata-Gomez to the Management Board as Chief Financial Officer (“CFO”) effective July 1, 2025. He will join BioNTech from Novartis AG’s global biomedical research organization where he has been serving as CFO since 2022. Ramón Zapata will succeed Jens Holstein, who, as previously planned and announced, will retire at the end of his term on June 30, 2025.
•In February 2025, BioNTech completed the acquisition of Biotheus, obtaining full global rights to BNT327 and to all other candidates from Biotheus’ pipeline, as well as to its in-house antibody generation platform and bispecific ADC capability. The transaction amounted to an upfront consideration of $800 million, plus additional performance-based payments of up to $150 million.
•BioNTech and Pfizer developed, manufactured and delivered their JN.1 and KP.2-adapted COVID-19 vaccines, which have received multiple regulatory approvals, including full approvals, authorizations for emergency or temporary use, or marketing authorizations, in more than 40 countries and regions. BioNTech is now focused on preparing for variant strain vaccine adaptation to be ready for commercial launch ahead of the upcoming 2025/2026 vaccination season, pending approvals.
Upcoming Investor and Analyst Events
•Annual General Meeting: May 16, 2025
•Innovation Series R&D Day: November 11, 2025
Conference Call and Webcast Information
BioNTech invites investors and the general public to join a conference call and webcast with investment analysts today, May 5, 2025, at 8:00 a.m. EDT (2:00 p.m. CEST) to report its financial results and provide a corporate update for the first quarter of 2025.
To access the live conference call via telephone, please register via this link. Once registered, dial-in numbers and a PIN number will be provided.
The slide presentation and audio of the webcast will be available via this link.
Participants may also access the slides and the webcast of the conference call via the “Events & Presentations” page of the Investor section of the Company’s website at www.BioNTech.com. A replay of the webcast will be available shortly after the conclusion of the call and archived on the Company’s website for 30 days following the call.
About BioNTech
Biopharmaceutical New Technologies (BioNTech) is a global next generation immunotherapy company pioneering novel investigative therapies for cancer and other serious diseases. BioNTech exploits a wide array of computational discovery and therapeutic modalities with the intent of rapid development of novel biopharmaceuticals. Its diversified portfolio of oncology product candidates aiming to address the full continuum of cancer includes mRNA cancer immunotherapies, next-generation immunomodulators and targeted therapies such as antibody-drug conjugates (ADCs) and innovative chimeric antigen receptor (CAR) T cell therapies. Based on its deep expertise in mRNA development and in-house manufacturing capabilities, BioNTech and its collaborators are researching and developing multiple mRNA vaccine candidates for a range of infectious diseases alongside its diverse oncology pipeline. BioNTech has established a broad set of relationships with multiple global and specialized pharmaceutical collaborators, including Duality Biologics, Fosun Pharma, Genentech, a member of the Roche Group, Genevant, Genmab, MediLink, OncoC4, Pfizer and Regeneron.
For more information, please visit www.BioNTech.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: BioNTech’s expected revenues and net profit/(loss) related to sales of BioNTech’s COVID-19 vaccine in territories controlled by BioNTech’s collaboration partners, particularly for those figures that are derived from preliminary estimates provided by BioNTech’s partners; the rate and degree of market acceptance of BioNTech’s COVID-19 vaccine and, if approved, BioNTech’s investigational medicines; expectations regarding anticipated changes in COVID-19 vaccine demand, including changes to the ordering environment and expected regulatory recommendations to adapt vaccines to address new variants or sublineages; the initiation, timing, progress, results, and cost of BioNTech’s research and development programs, including BioNTech’s current and future preclinical studies and clinical trials, including statements regarding the expected timing of initiation, enrollment, and completion of studies or clinical trials and related preparatory work and the availability of results, and the timing and outcome of applications for regulatory approvals and marketing authorizations; BioNTech’s expectations regarding potential future commercialization in oncology, including goals regarding timing and indications; the targeted timing and number of additional potentially registrational clinical trials, and the registrational potential of any clinical trial BioNTech may initiate; discussions with regulatory agencies; BioNTech’s expectations with respect to intellectual property; the impact of BioNTech’s collaboration and licensing agreements; the development, nature and feasibility of sustainable vaccine production and supply solutions; the deployment of AI across BioNTech’s preclinical and clinical operations; BioNTech’s expectations with respect to tariff policy; BioNTech’s estimates of revenues, research and development expenses, selling, general and administrative expenses and capital expenditures for operating activities; BioNTech’s expectations regarding upcoming payments relating to litigation settlements; BioNTech’s expectations for upcoming scientific and investor presentations; and BioNTech’s expectations of net profit / (loss). In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words.
The forward-looking statements in this press release are based on BioNTech’s current expectations and beliefs of future events, and are neither promises nor guarantees. You should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond BioNTech’s control and which could cause actual results to differ materially and adversely from those expressed or implied by these forward-looking statements.
These risks and uncertainties include, but are not limited to: the uncertainties inherent in research and development, including the ability to meet anticipated clinical endpoints, commencement and/or completion dates for clinical trials, projected data release timelines, regulatory submission dates, regulatory approval dates and/or launch dates, as well as risks associated with preclinical and clinical data, including the data discussed in this release, and including the possibility of unfavorable new preclinical, clinical or safety data and further analyses of existing preclinical, clinical or safety data; the nature of the clinical data, which is subject to ongoing peer review, regulatory review and market interpretation; BioNTech’s pricing and coverage negotiations regarding its COVID-19 vaccine with governmental authorities, private health insurers and other third-party payors; the future commercial demand and medical need for initial or booster doses of a COVID-19 vaccine; the impact of tariffs and escalations in trade policy; competition from other COVID-19 vaccines or related to BioNTech’s other product candidates, including those with different mechanisms of action and different manufacturing and distribution constraints, on the basis of, among other things, efficacy, cost, convenience of storage and distribution, breadth of approved use, side-effect profile and durability of immune response; the timing of and BioNTech’s ability to obtain and maintain regulatory approval for its product candidates; the ability of BioNTech’s COVID-19 vaccines to prevent COVID-19 caused by emerging virus variants; BioNTech’s and its counterparties’ ability to manage and source necessary energy resources; BioNTech’s ability to identify research opportunities and discover and develop investigational medicines; the ability and willingness of BioNTech’s third-party collaborators to continue research and development activities relating to BioNTech's development candidates and investigational medicines; the impact of COVID-19 on BioNTech’s development programs, supply chain, collaborators and financial performance; unforeseen safety issues and potential claims that are alleged to arise from the use of products and product candidates developed or manufactured by BioNTech; BioNTech’s and its collaborators’ ability to commercialize and market BioNTech’s COVID-19 vaccine and, if approved, its product candidates; BioNTech’s ability to manage its development and related expenses; regulatory and political developments in the United States and other countries; BioNTech’s ability to effectively scale its production capabilities and manufacture its products and product candidates; risks relating to the global financial system and markets; and other factors not known to BioNTech at this time. You should review the risks and uncertainties described under the heading “Risk Factors” in BioNTech’s Report on Form 6-K for the period ended March 31, 2025 and in subsequent filings made by BioNTech with the SEC, which are available on the SEC’s website at www.sec.gov. These forward-looking statements speak only as of the date hereof. Except as required by law, BioNTech disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise.
CONTACTS
Investor Relations
Michael Horowicz
Investors@biontech.de
Media Relations
Jasmina Alatovic
Media@biontech.de
Target Overview
|
|
|
|
|
|
Anti-PD-(L)1 |
Anti-programmed cell death protein (death-ligand) 1 |
PD-L1 |
Programmed death-ligand 1 |
TROP2 |
Trophoblast cell-surface antigen 2 |
VEGF-A |
Vascular endothelial growth factor A |
Interim Condensed Consolidated Statements of Profit or Loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months ended
March 31,
|
|
|
|
|
|
|
|
|
|
|
2025 |
|
|
2024 |
(in millions €, except per share data) |
|
|
(unaudited) |
|
|
(unaudited) |
Revenues |
|
|
182.8 |
|
|
187.6 |
Cost of sales |
|
|
(83.8) |
|
|
(59.1) |
Research and development expenses |
|
|
(525.6) |
|
|
(507.5) |
Sales and marketing expenses |
|
|
(13.7) |
|
|
(15.6) |
General and administrative expenses |
|
|
(106.9) |
|
|
(117.0) |
Other operating expenses |
|
|
(48.5) |
|
|
(23.9) |
Other operating income |
|
|
61.6 |
|
|
28.3 |
Operating loss |
|
|
(534.1) |
|
|
(507.2) |
Finance income |
|
|
122.6 |
|
|
180.1 |
Finance expenses |
|
|
(33.9) |
|
|
(4.7) |
Loss before tax |
|
|
(445.4) |
|
|
(331.8) |
Income taxes |
|
|
29.6 |
|
|
16.7 |
Net loss |
|
|
(415.8) |
|
|
(315.1) |
Loss per share |
|
|
|
|
|
|
Basic and diluted loss per share |
|
|
(1.73) |
|
|
(1.31) |
Interim Condensed Consolidated Statements of Financial Position
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
(in millions €) |
|
|
2025 |
|
|
2024 |
Assets |
|
|
(unaudited) |
|
|
|
Non-current assets |
|
|
|
|
|
|
Goodwill |
|
|
375.9 |
|
|
380.6 |
Other intangible assets |
|
|
1,511.2 |
|
|
790.4 |
Property, plant and equipment |
|
|
1,026.1 |
|
|
935.3 |
Right-of-use assets |
|
|
241.9 |
|
|
248.1 |
Contract assets |
|
|
7.9 |
|
|
9.8 |
Other financial assets |
|
|
2,285.8 |
|
|
1,254.0 |
Other non-financial assets |
|
|
22.6 |
|
|
26.3 |
Deferred tax assets |
|
|
85.5 |
|
|
81.7 |
Total non-current assets |
|
|
5,556.9 |
|
|
3,726.2 |
Current assets |
|
|
|
|
|
|
Inventories |
|
|
254.4 |
|
|
283.3 |
Trade and other receivables |
|
|
956.5 |
|
|
1,463.9 |
Contract assets |
|
|
10.0 |
|
|
10.0 |
Other financial assets |
|
|
3,924.9 |
|
|
7,021.7 |
Other non-financial assets |
|
|
234.3 |
|
|
212.7 |
Income tax assets |
|
|
60.5 |
|
|
50.0 |
Cash and cash equivalents |
|
|
10,184.9 |
|
|
9,761.9 |
Total current assets |
|
|
15,625.5 |
|
|
18,803.5 |
Total assets |
|
|
21,182.4 |
|
|
22,529.7 |
|
|
|
|
|
|
|
Equity and liabilities |
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
Share capital |
|
|
248.6 |
|
|
248.6 |
Capital reserve |
|
|
1,447.4 |
|
|
1,398.6 |
Treasury shares |
|
|
(8.2) |
|
|
(8.6) |
Retained earnings |
|
|
18,682.2 |
|
|
19,098.0 |
Other reserves |
|
|
(1,443.4) |
|
|
(1,325.5) |
Total equity |
|
|
18,926.6 |
|
|
19,411.1 |
Non-current liabilities |
|
|
|
|
|
|
Lease liabilities, loans and borrowings |
|
|
237.5 |
|
|
214.7 |
Other financial liabilities |
|
|
150.9 |
|
|
46.9 |
Provisions |
|
|
20.8 |
|
|
20.9 |
Contract liabilities |
|
|
182.9 |
|
|
183.0 |
Other non-financial liabilities |
|
|
85.1 |
|
|
87.5 |
Deferred tax liabilities |
|
|
44.1 |
|
|
42.4 |
Total non-current liabilities |
|
|
721.3 |
|
|
595.4 |
Current liabilities |
|
|
|
|
|
|
Lease liabilities, loans and borrowings |
|
|
55.5 |
|
|
39.5 |
Trade payables and other payables |
|
|
443.8 |
|
|
426.7 |
Other financial liabilities |
|
|
443.4 |
|
|
1,443.4 |
Income tax liabilities |
|
|
5.4 |
|
|
4.5 |
Provisions |
|
|
121.9 |
|
|
144.8 |
Contract liabilities |
|
|
294.5 |
|
|
294.9 |
Other non-financial liabilities |
|
|
170.0 |
|
|
169.4 |
Total current liabilities |
|
|
1,534.5 |
|
|
2,523.2 |
Total liabilities |
|
|
2,255.8 |
|
|
3,118.6 |
Total equity and liabilities |
|
|
21,182.4 |
|
|
22,529.7 |
Interim Condensed Consolidated Statements of Cash Flows
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months ended
March 31,
|
|
|
|
|
|
|
|
|
|
|
2025 |
|
|
2024 |
(in millions €) |
|
|
(unaudited) |
|
|
(unaudited) |
Operating activities |
|
|
|
|
|
|
Net loss |
|
|
(415.8) |
|
|
(315.1) |
Income taxes |
|
|
(29.6) |
|
|
(16.7) |
Loss before tax |
|
|
(445.4) |
|
|
(331.8) |
Adjustments to reconcile profit before tax to net cash flows: |
|
|
|
|
|
|
Depreciation and amortization of property, plant, equipment, intangible assets and right-of-use assets |
|
|
42.8 |
|
|
38.3 |
Share-based payment expenses |
|
|
22.1 |
|
|
16.3 |
Net foreign exchange differences |
|
|
48.3 |
|
|
(28.7) |
(Gain) / Loss on disposal of property, plant and equipment |
|
|
(0.1) |
|
|
— |
Finance income excluding foreign exchange differences |
|
|
(122.6) |
|
|
(174.9) |
Finance expense excluding foreign exchange differences |
|
|
7.9 |
|
|
4.7 |
Government grants |
|
|
(14.5) |
|
|
(9.1) |
Other non-cash (income) / loss |
|
|
(14.3) |
|
|
— |
Unrealized (gain) / loss on derivative instruments at fair value through profit or loss |
|
|
(11.3) |
|
|
1.7 |
Working capital adjustments: |
|
|
|
|
|
|
Decrease in trade and other receivables, contract assets and other assets |
|
|
520.7 |
|
|
498.2 |
Decrease in inventories |
|
|
33.8 |
|
|
12.3 |
Decrease in trade payables, other financial liabilities, other liabilities, contract liabilities, refund liabilities and provisions |
|
|
(971.0) |
|
|
(288.0) |
Interest received and realized gains from cash and cash equivalents |
|
|
118.6 |
|
|
199.4 |
Interest paid and realized losses from cash and cash equivalents |
|
|
(3.1) |
|
|
(3.7) |
Income tax paid |
|
|
(12.2) |
|
|
(258.8) |
Share-based payments |
|
|
(3.6) |
|
|
(2.4) |
Government grants received |
|
|
23.2 |
|
|
9.2 |
Net cash flows used in operating activities |
|
|
(780.7) |
|
|
(317.3) |
Investing activities |
|
|
|
|
|
|
Purchase of property, plant and equipment |
|
|
(48.9) |
|
|
(58.5) |
Proceeds from sale of property, plant and equipment |
|
|
0.5 |
|
|
— |
Purchase of intangible assets and right-of-use assets |
|
|
(569.2) |
|
|
(78.4) |
Acquisition of subsidiaries and businesses, net of cash acquired |
|
|
(78.5) |
|
|
— |
Investment in other financial assets |
|
|
(2,507.7) |
|
|
(4,895.1) |
Proceeds from maturity of other financial assets |
|
|
4,450.6 |
|
|
2,727.6 |
Net cash flows from / (used in) investing activities |
|
|
1,246.8 |
|
|
(2,304.4) |
Financing activities |
|
|
|
|
|
|
Repayment of loans and borrowings |
|
|
(4.5) |
|
|
— |
Payments related to lease liabilities |
|
|
(9.3) |
|
|
(7.8) |
Net cash flows used in financing activities |
|
|
(13.8) |
|
|
(7.8) |
Net increase / (decrease) in cash and cash equivalents |
|
|
452.3 |
|
|
(2,629.5) |
Change in cash and cash equivalents resulting from exchange rate differences |
|
|
(16.1) |
|
|
6.8 |
Change in cash and cash equivalents resulting from other valuation effects |
|
|
(13.2) |
|
|
(64.4) |
Cash and cash equivalents at the beginning of the period |
|
|
9,761.9 |
|
|
11,663.7 |
Cash and cash equivalents as of March 31 |
|
|
10,184.9 |
|
|
8,976.6 |