May 25, 2023 Annual General Meeting English Convenience Translation: German language is the official version. Exhibit 99.2

Financial Development 2022 / Q1 2023 and Financial Outlook 2023 Jens Holstein, Chief Financial Officer2 1 Operations Development 2022 / Q1 2023 and Operations Outlook 2023 Prof. Dr. Ugur Sahin, Chief Executive Officer & Founder MANAGEMENT REPORT AGENDA NO. 1

1 Operations Development 2022 & Q1 2023 and Operations Outlook 2023 Prof. Dr. Ugur Sahin, CEO & Founder

This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: BioNTech's expected revenues and net profit related to sales of BioNTech's COVID-19 vaccine, referred to as COMIRNATY® where approved for use under full or conditional marketing authorization, in territories controlled by BioNTech's collaboration partners, particularly for those figures that are derived from preliminary estimates provided by BioNTech's partners; the rate and degree of market acceptance of BioNTech's COVID-19 vaccine and, if approved, BioNTech's investigational medicines; the initiation, timing, progress, results, and cost of BioNTech's research and development programs, including those relating to additional formulations of BioNTech's COVID-19 vaccine, and BioNTech's current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work and the availability of results; and BioNTech's estimates of commercial and other revenues, cost of sales, research and development expenses, sales and marketing expenses, general and administrative expenses, capital expenditures, income taxes, and shares outstanding. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this presentation are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond BioNTech’s control, and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to: BioNTech's pricing and coverage negotiations with governmental authorities, private health insurers and other third-party payors after BioNTech's initial sales to national governments; the future commercial demand and medical need for initial or booster doses of a COVID-19 vaccine; competition from other COVID-19 vaccines or related to BioNTech's other product candidates, including those with different mechanisms of action and different manufacturing and distribution constraints, on the basis of, among other things, efficacy, cost, convenience of storage and distribution, breadth of approved use, side-effect profile and durability of immune response; the timing of and BioNTech's ability to obtain and maintain regulatory approval for BioNTech's product candidates; the ability of BioNTech’s COVID-19 vaccine to prevent COVID-19 caused by emerging virus variants; BioNTech's and its counterparties’ ability to manage and source necessary energy resources; BioNTech's ability to identify research opportunities and discover and develop investigational medicines; the ability and willingness of BioNTech's third-party collaborators to continue research and development activities relating to BioNTech's development candidates and investigational medicines; the impact of the COVID-19 pandemic on BioNTech's development programs, supply chain, collaborators and financial performance; unforeseen safety issues and claims for potential personal injury or death arising from the use of BioNTech's COVID-19 vaccine and other products and product candidates developed or manufactured by BioNTech; BioNTech's and its collaborators’ ability to commercialize and market BioNTech's COVID-19 vaccine and, if approved, its product candidates; BioNTech's ability to manage its development and expansion; regulatory developments in the United States and foreign countries; BioNTech's ability to effectively scale BioNTech's production capabilities and manufacture BioNTech's products, including BioNTech's target COVID-19 vaccine production levels, and BioNTech's product candidates, risks relating to the global financial systems and markets; and other factors not known to BioNTech at this time. You should review the risks and uncertainties described under the heading “Risk Factors” in BioNTech’s report on Form 6-K for the period ended March 31, 2023 and in subsequent filings made by BioNTech with the SEC, which are available on the SEC’s website at https://www.sec.gov/. Except as required by law, BioNTech disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation in the event of new information, future developments or otherwise. These forward-looking statements are based on BioNTech’s current expectations and speak only as of the date hereof. This Slide Presentation Includes Forward-Looking Statements 4

COMIRNATY® ▼(the Pfizer-BioNTech COVID-19 vaccine) has been granted standard marketing authorization (MA) by the European Commission to prevent coronavirus disease 2019 (COVID-19) in the population aged 6 months and older. In people from 5 years of age and older the vaccine is administered as a 2-dose series, 3 weeks apart. Adults and adolescents from the age of 12 are given 30 micrograms per dose; children aged 5 to 11 years are given 10 micrograms per dose. There is a pediatric formulation containing 3 micrograms per dose available for infants and children 6 months to 4 years of age. In this age group, COMIRNATY can be given as primary vaccination consisting of three doses (of 3 micrograms each); the first two doses are given 3 weeks apart, followed by a third dose given at least 8 weeks after the second dose. In addition, the MA has been expanded to include a booster dose (third dose) of 30 micrograms at least 3 months after the second dose in individuals 12 years of age and older. A booster dose of COMIRNATY 10 micrograms may be given to children from 5 to 11 years of age at least 6 months after the primary vaccination course. A third primary course dose may be administered at least 28 days after the second dose to people aged 5 years and older with a severely weakened immune system. The European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use (CHMP) has completed its rigorous evaluation of COMIRNATY, concluding by consensus that sufficiently robust data on the quality, safety and efficacy of the vaccine are now available. COMIRNATY® ▼(the Pfizer-BioNTech COVID-19 vaccine), Bivalent: COMIRNATY Original/Omicron BA.1, COMIRNATY Original/Omicron BA.4-5 In addition, COMIRNATY has also been granted standard MA for two Omicron subvariant adapted vaccines: COMIRNATY Original/Omicron BA.1, which contains mRNA encoding for the spike protein of the wild-type and of the Omicron BA.1 subvariant of SARS-CoV-2; and COMIRNATY Original/Omicron BA.4-5, which contains mRNA encoding for the spike protein of the wild-type and of the Omicron BA.4/BA.5 subvariant of SARS-CoV-2. COMIRNATY Original/Omicron BA.1 or COMIRNATY Original/Omicron BA.4-5 (30 micrograms per dose) may be administered as a booster in people aged 12 years and older who have received at least a primary vaccination course against COVID-19. A booster dose of COMIRNATY Original/Omicron BA.4-5 (10 micrograms per dose) may be given to people aged from 5 years to 11 years after primary vaccination or a booster dose with a COVID-19 vaccine. There should be an interval of at least 3 months between administration of COMIRNATY Original/Omicron BA.1 or COMIRNATY Original/Omicron BA.4-5 and the last prior dose of a COVID-19 vaccine. IMPORTANT SAFETY INFORMATION: • Events of anaphylaxis have been reported. Appropriate medical treatment and supervision should always be readily available in case of an anaphylactic reaction following the administration of the vaccine. • There is an increased, but very rare risk (<1/10,000 cases) of myocarditis and pericarditis following vaccination with COMIRNATY. These conditions can develop within just a few days after vaccination and have primarily occurred within 14 days. They have been observed more often after the second vaccination, and more often in younger males. Available data suggest that the course of myocarditis and pericarditis following vaccination is not different from myocarditis or pericarditis in general. From post-marketing experience very rare adverse reactions of myocarditis and pericarditis, Rarecases of acute peripheral facial paralysis; uncommon incidence of insomnia, hyperhidrosis and night sweats, dizziness common incidence of vomiting, very common diarrhoea and unknown incidence (wcan not be estimated from available data) anaphylaxis, of paraesthesia, hypoaesthesia and erythema multiforme, extensive swelling of vaccinated limb, facial swelling (in vaccine recipients with a history of injection of dermatological fillers) and heavy menstrual bleeding(most case appeared to be non-serious and temporary in nature) have been identified after post-marketing experience.Anxiety-related reactions, including vasovagal reactions (syncope), hyperventilation or stress‐related reactions (e. g. dizziness, palpitations, increases in heart rate, alterations in blood pressure,paresthesia, hypoesthesia and sweating) may occur in association with the vaccination process itself. Stress-related reactions are temporary and resolve on their own. Individuals should be advised to bring symptoms to the attention of the vaccination provider for evaluation. It is important that precautions are in place to avoid injury from fainting. • Vaccination should be postponed in individuals suffering from acute severe febrile illness or acute infection. The presence of a minor infection and/or low-grade fever should not delay vaccination. • As with other intramuscular injections, the vaccine should be given with caution in individuals receiving anticoagulant therapy or those with thrombocytopenia or any coagulation disorder (such as haemophilia) because bleeding or bruising may occur following an intramuscular administration in these individuals. • The efficacy, safety and immunogenicity of the vaccine has not been assessed in immunocompromised individuals, including those receiving immunosuppressant therapy. The efficacy of COMIRNATY, COMIRNATY Original/Omicron BA.1 or COMIRNATY Original/Omicron BA.4-5 may be lower in immunosuppressed individuals. • As with any vaccine, vaccination with COMIRNATY, COMIRNATY Original/Omicron BA.1 or COMIRNATY Original/Omicron BA.4-5 may not protect all vaccine recipients. Individuals may not be fully protected until 7 days after their second dose of the vaccine. • Adverse reactions observed during clinical studies and identified after post authorization experience are listed below according to the following frequency categories: Very common (≥ 1/10), Common (≥ 1/100 to < 1/10), Uncommon (≥ 1/1,000 to < 1/100), Rare (≥ 1/10,000 to < 1/1,000), Very rare (< 1/10,000), • Very common side effects: injection site pain, injection site swelling, , headache, muscle pain, chills, joint pain, diarrhea, fever, chills, fatigue • Common side effects: injection site redness, nausea, vomiting • Uncommon side effects: enlarged lymph nodes (more frequently observed after the booster dose), feeling unwell, arm pain, insomnia, dizziness, injection site itching, allergic reactions such as rash,itching, urticaria or angioedema, feeling weak or lack of energy/sleepy, decreased appetite, excessive sweating, night sweats • Rare side effects: temporary one-sided facial drooping • Very rare side effects: inflammation of the heart muscle (myocarditis) or inflammation of the lining outside the heart (pericarditis), which can result in breathlessness, palpitations or chest pain. • Not known indicence (cannot be estimated from the available data): anaphylaxis, extensive swelling of vaccinated limbs; facial swelling, pins and needles/tingling, reduced sense of touch or sensation, a skin reaction that causes red spots or patches on the skin, heavy menstrual bleeding • A large amount of observational data from pregnant women vaccinated with the initially approved COMIRNATY vaccine during the second and third trimester have not shown an increase in adverse pregnancy outcomes. While data on pregnancy outcomes following vaccination during the first trimester are presently limited, no increased risk for miscarriage has been seen. COMIRNATY can be used during pregnancy. No effects on the breast-fed newborn/infant are anticipated since the systemic exposure of breast-feeding woman to the initially approved COMIRNATY vaccine is negligible. Observational data from women who were breast- feeding after vaccination have not shown a risk for adverse effects in breast-fed newborns/infants. COMIRNATY can be used during breast-feeding. • No data are available yet regarding the use of COMIRNATY Original/Omicron BA.1 or COMIRNATY Original/Omicron BA.4-5 during pregnancy. Since differences between products are confined to the spike protein sequence, and there are no clinically meaningful differences in reactogenicity between those COMIRNATY variant adapted vaccines that have been clinically evaluated, COMIRNATY Original/Omicron BA.1 or COMIRNATY Original/Omicron BA.4-5 can be used during pregnancy. • No data are available yet regarding the use of COMIRNATY Original/Omicron BA.1 or COMIRNATY Original/Omicron BA.4-5 during breast-feeding. Observational data from women who were breast-feeding after vaccination with the initially approved COMIRNATY vaccine have not shown a risk for adverse effects in breast-fed newborns/infants. COMIRNATY Original/Omicron BA.1 or COMIRNATY Original/Omicron BA.4-5 can be used during breast-feeding • Interactions with other medicinal products or concomitant administration of COMIRNATY, COMIRNATY Original/Omicron BA.1 or COMIRNATY Original/Omicron BA.4-5 with other vaccines has not been studied. • Animal studies with COMIRNATY Original do not indicate direct or indirect harmful effects with respect to reproductive toxicity. • In an analysis of Study 3 (Phase 2/3), 1,776 infants (1,178 Comirnaty 3 mcg and 598 placebo) were 6 to 23 months of age. The most frequent adverse reactions in infants 6 to 23 months of age that received any primary course dose included irritability (> 60%), drowsiness (> 40%), decreased appetite (> 30%), tenderness at the injection site (> 20%), injection site redness and fever (> 10%). • The most frequent adverse reactions in children 2 to 4 years of age that received any primary course dose included pain at injection site and fatigue (> 40%), injection site redness and fever (> 10%). • The overall safety profile of Comirnaty in participants 5 to 11 years of age was similar to that seen in participants 16 years of age and older. The most frequent adverse reactions in children 5 to 11 years of age that received 2 doses were injection site pain (> 80%), fatigue (> 50%), headache (> 30%), injection site redness and swelling (≥ 20%), myalgia, chills, and diarrhoea (> 10%). • The overall safety profile for the booster dose was similar to that seen after the primary course. The most frequent adverse reactions in children 5 to 11 years of age were injection site pain (> 70%), fatigue (> 40%), headache (> 30%), myalgia, chills, injection site redness and swelling (> 10%) • The overall safety profile of Comirnaty in adolescents 12 to 15 years of age was similar to that seen in participants 16 years of age and older. The most frequent adverse reactions in adolescents 12 to 15 years of age that received 2 doses were injection site pain (> 90%), fatigue and headache (> 70%), myalgia and chills (> 40%), arthralgia and pyrexia (> 20%) • The most frequent adverse reactions in participants 16 years of age and older that received 2 doses were injection site pain (> 80%), fatigue (> 60%), headache (> 50%), myalgia (> 40%), chills (> 30%), arthralgia (> 20%), pyrexia and injection site swelling (> 10%) and were usually mild or moderate in intensity and resolved within a few days after vaccination. A slightly lower frequency of reactogenicity events was associated with greater age. • The safety of a COMIRNATY Original/Omicron BA.1 booster dose in individuals from 18 to ≤ 55 years of age is extrapolated from safety data from a subset of 315 adults 18 to ≤ 55 years of age who received a booster (fourth dose) of Omicron BA.1 30 µg (monovalent) after completing 3 doses of COMIRNATY. The most frequent adverse reactions in these participants 18 to ≤ 55 years of age were injection site pain (> 70%), fatigue (> 60%), headache (> 40%), myalgia (> 30%), chills (> 30%) and arthralgia (> 20%). • In a subset from Study 4 ( Phase 3) , 305 adults > 55 years of age who had completed 3 doses of COMIRNATY, received a booster of COMIRNATY Original/Omicron BA.1 after receiving Dose 3. The overall safety profile for the COMIRNATY Original/Omicron BA.1 booster (fourth dose) was similar to that seen after the COMIRNATY booster (third dose). The most frequent adverse reactions in participants greater than 55 years of age were injection site pain (> 50%), fatigue (> 40%), headache (> 30%), myalgia (> 20%), chills and arthralgia (> 10%). No new adverse reactions were identified for COMIRNATY Original/Omicron BA.1. • The safety of a booster dose of COMIRNATY Original/Omicron BA.4-5 is inferred from safety data for a booster dose of COMIRNATY Original/Omicron BA.1 in individuals 18 years of age and older, as well as for a booster dose of COMIRNATY Original in individuals 5 years of age and older. • The duration of protection afforded by the vaccine is unknown as it is still being determined by ongoing clinical trials. As with any vaccine, vaccination with Comirnaty Original/Omicron BA.1 or COMIRNATY Original/Omicron BA.4-5 may not protect all vaccine recipients • For complete information on the safety of COMIRNATY, COMIRNATY Original/Omicron BA.1 and COMIRNATY Original/Omicron BA.4-5, always make reference to the approved Summary of Product Characteristics and Package Leaflet available in all the languages of the European Union on the EMA website. The black equilateral triangle ▼ denotes that additional monitoring is required to capture any adverse reactions. This will allow quick identification of new safety information. Individuals can help by reporting any side effects they may get. Side effects can be reported to EudraVigilance or directly to BioNTech using email medinfo@biontech.de, telephone +49 6131 9084 0, or via the website www.biontech.de 5 Safety Information

AUTHORIZED USE IN THE U.S. COMIRNATY® (COVID-19 Vaccine, mRNA) • COMIRNATY® (COVID-19 Vaccine, mRNA) is an FDA-approved COVID-19 vaccine for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 12 years of age and older. It is also authorized as a third primary series dose to individuals 12 years of age and older who have certain kinds of immunocompromise • The COVID-19 vaccine is FDA authorized under Emergency Use Authorization (EUA) for use in individuals 6 months and older to provide: • the first 2 doses of the 3-dose primary series for children 6 months through 4 years of age. • a 2-dose primary series to individuals 5 years through 11 years of age • a third primary series dose to individuals 5 years and older with certain kinds of immunocompromise Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) • Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) is FDA-authorized under Emergency Use Authorization (EUA) to prevent COVID-19 as: • the third dose of the 3-dose primary series following 2 doses of the monovalent* Pfizer-BioNTech COVID-19 Vaccine in children 6 months through 4 years of age; or • a single booster dose in children 6 months through 4 years of age at least 2 months after completion of primary vaccination with 3 doses of the monovalent Pfizer-BioNTech COVID-19 Vaccine; or • a single booster dose at least 2 months after completion of either primary vaccination with any authorized or approved COVID-19 vaccine or receipt of the most recent booster dose with any authorized or approved monovalent COVID-19 vaccine in individuals 5 years of age and older. EMERGENCY USE AUTHORIZATION Emergency uses of the vaccines have not been approved or licensed by FDA but have been authorized by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) in individuals aged 6 months and older for the Pfizer-BioNTech COVID-19 Vaccine and 5 years and older for the Pfizer-BioNTech COVID-19 Vaccine, Bivalent. The emergency uses are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of the medical product under Section 564(b)(1) of the FD&C Act unless the declaration is terminated or authorization revoked sooner. IMPORTANT SAFETY INFORMATION Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), COMIRNATY® (COVID-19 Vaccine, mRNA) and Pfizer-BioNTech COVID-19 Vaccine • Do not administer Pfizer-BioNTech COVID-19 Vaccine to individuals with known history of a severe allergic reaction (e.g., anaphylaxis) to any component of the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent. • Warnings: • Management of Acute Allergic Reactions: Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent. • Monitor Pfizer-BioNTech COVID-19 Vaccine recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention (CDC) guidelines (https://www.cdc.gov/vaccines/covid-19/clinicalconsiderations/managing-anaphylaxis.html) • Myocarditis and Pericarditis: Postmarketing safety data with Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent are relevant because these vaccines are manufactured using the same process. • Postmarketing data with authorized or approved Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent, demonstrate increased risks of myocarditis and pericarditis, particularly within the first week following receipt of the second primary series dose or first booster dose, with most booster doses likely administered at least 5 months after completing primary vaccination. For the Pfizer-BioNTech COVID-19 Vaccine, the observed risk is higher among adolescent males and adult males under 40 years of age than among females and older males, and the observed risk is highest in males 12 through 17 years of age. Although some cases required intensive care support, available data from short-term follow-up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae. The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html). • Syncope • Syncope (fainting) may occur in association with administration of injectable vaccines, in particular in adolescents. Procedures should be in place to avoid injury from fainting. • Altered Immunocompetence • Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent. • Limitation of Effectiveness • Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent may not protect all vaccine recipients. • Adverse reactions reported with the vaccine include: • Adverse Reactions in Clinical Trials • Adverse reactions following administration of a booster dose of the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent that have been reported in clinical trials include injection site pain, fatigue, headache, muscle pain, chills, joint pain, injection site swelling, fever, injection site redness, lymphadenopathy, nausea, malaise, pain in extremity, rash, decreased appetite, vomiting, diarrhea (see Full EUA Prescribing Information). • Adverse Reactions Identified in Post Authorization Experience • Severe allergic reactions, including anaphylaxis, and other hypersensitivity reactions (e.g., rash, pruritus, urticaria, angioedema), diarrhea, vomiting, pain in extremity (arm), syncope, and dizziness have been reported following administration of the Pfizer-BioNTech COVID-19 Vaccine. • Myocarditis and pericarditis have been reported following administration of the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent. • Additional adverse reactions, some of which may be serious, may become apparent with post-authorization use of the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent. • Use with Other Vaccines • There is no information on the co-administration of the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent, with other vaccines. 6 Safety Information

OUR VISION USING THE FULL POTENTIAL OF THE IMMUNE SYSTEM TO DEVELOP NEW IMMUNOTHERAPIES AND VACCINES
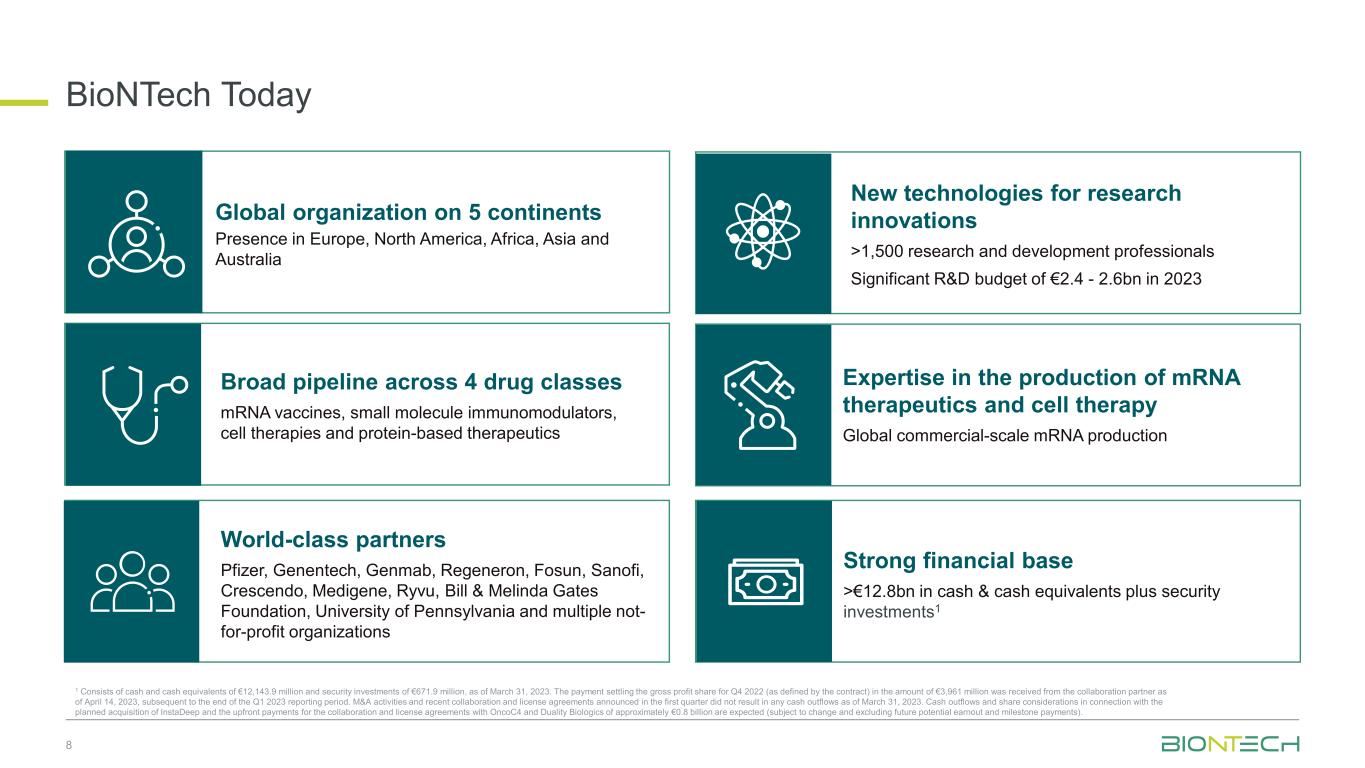
BioNTech Today 8 Global organization on 5 continents Presence in Europe, North America, Africa, Asia and Australia Broad pipeline across 4 drug classes mRNA vaccines, small molecule immunomodulators, cell therapies and protein-based therapeutics Expertise in the production of mRNA therapeutics and cell therapy Global commercial-scale mRNA production World-class partners Pfizer, Genentech, Genmab, Regeneron, Fosun, Sanofi, Crescendo, Medigene, Ryvu, Bill & Melinda Gates Foundation, University of Pennsylvania and multiple not- for-profit organizations Strong financial base >€12.8bn in cash & cash equivalents plus security investments1 New technologies for research innovations >1,500 research and development professionals Significant R&D budget of €2.4 - 2.6bn in 2023 1 Consists of cash and cash equivalents of €12,143.9 million and security investments of €671.9 million, as of March 31, 2023. The payment settling the gross profit share for Q4 2022 (as defined by the contract) in the amount of €3,961 million was received from the collaboration partner as of April 14, 2023, subsequent to the end of the Q1 2023 reporting period. M&A activities and recent collaboration and license agreements announced in the first quarter did not result in any cash outflows as of March 31, 2023. Cash outflows and share considerations in connection with the planned acquisition of InstaDeep and the upfront payments for the collaboration and license agreements with OncoC4 and Duality Biologics of approximately €0.8 billion are expected (subject to change and excluding future potential earnout and milestone payments).
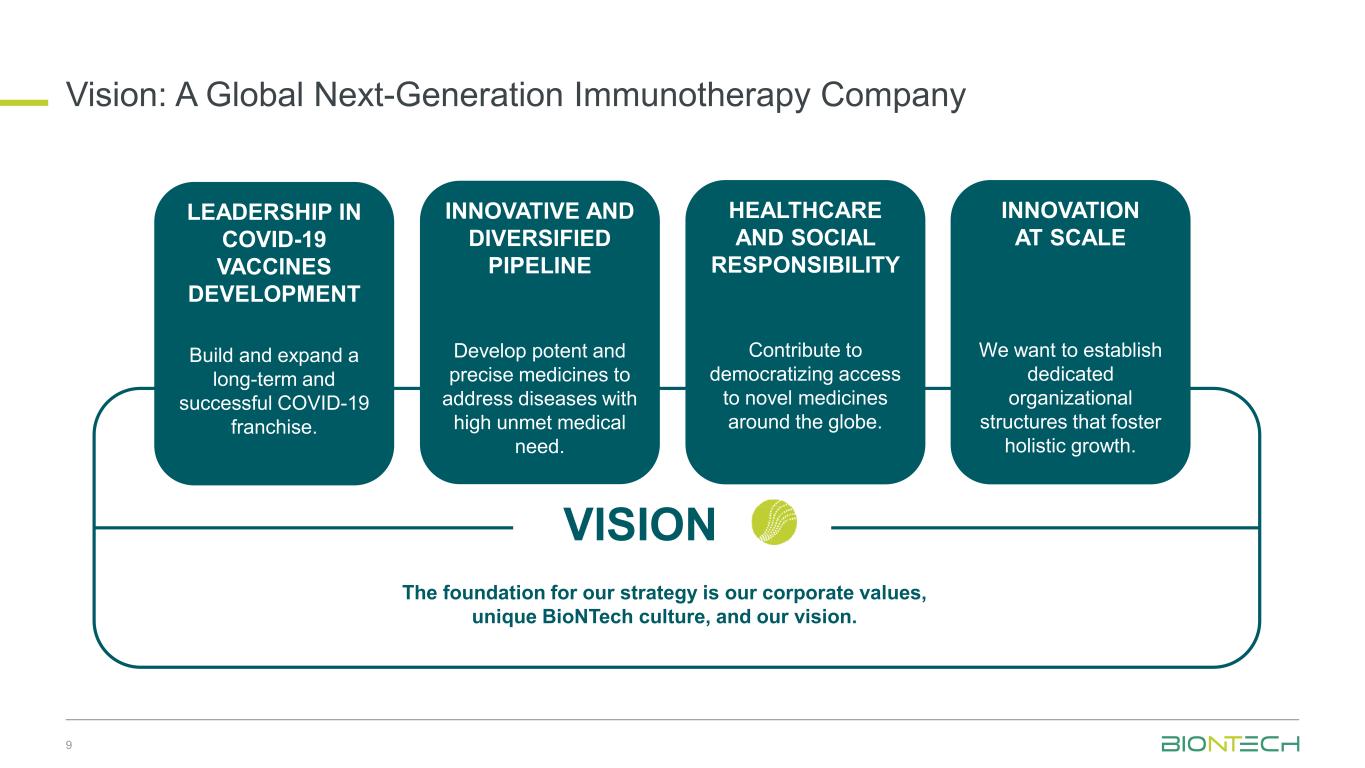
Vision: A Global Next-Generation Immunotherapy Company 9 INNOVATIVE AND DIVERSIFIED PIPELINE Develop potent and precise medicines to address diseases with high unmet medical need. INNOVATION AT SCALE We want to establish dedicated organizational structures that foster holistic growth. HEALTHCARE AND SOCIAL RESPONSIBILITY Contribute to democratizing access to novel medicines around the globe. VISION The foundation for our strategy is our corporate values, unique BioNTech culture, and our vision. LEADERSHIP IN COVID-19 VACCINES DEVELOPMENT Build and expand a long-term and successful COVID-19 franchise.

10 *Excluding studies with Comirnaty. IND = Investigational new drug; FIH = First-in-human. Advancing Towards Our Vision Infectious diseases 6 Phase 2 trials Globally first-to-market BA.4-5-adapted COVID-19 vaccine 7 programs in 8 clinical trials* 20 programs in 25 clinical trials Deepen COVID-19 vaccine leadership Multiple oncology and infectious disease product launches in next 3-5 years Mid-term goalsDriving transformation today Long-term vision 5-10 IND submissions per year Next-generation and combination COVID-19 vaccines Approved products across various disease areas Cardiovascular diseases Neurodegenerative diseases Autoimmune diseases Oncology We aim to be a multi-product global biotechnology leader with multiple approved products to help make individual cancer therapies available and address health challenges worldwide 1 Phase 2 trial 1 Phase 3 trial 4 new FIH programs 5 new FIH programs

BioNTech Achievements in 2022 & 2023 11 Goal: To deliver long-term value to patients, shareholders, and society Launch of variant- adapted vaccine and further label expansion Development of advanced oncology programs and expansion of early-stage pipeline Ramped up R&D investment and made strategic investments in AI technologies and capabilities Acquired complementary, synergistic technologies, infrastructure, and product candidates Expanded global organization in Europe, the U.S., Asia, and Africa

2022 & 2023: Global Growth 12 Memorandum of Understanding for new collaboration Local cooperation BioNTech site2 Manufacturing site2 United Kingdom Collaboration with UK government with the target to deliver personalized therapies to up to 10,000 patients by end of 2030 Israel Pandemic preparedness and development of innovative medicines Singapore Commercial-scale mRNA manufacturing Australia mRNA research center and clinical manufacturing facility Taiwan Clinical trial hub for mRNA-based cancer immunotherapies >4,500 professionals globally1 >1,900 new hires in 2022 >80 nationalities 36 average age 50 % of total workforce are female Rwanda, Senegal Establishment of mRNA manufacturing facilities1 Employment data as of April 2023 2 Sites may be existing or planned

13 Global Social Responsibility at Our Core 13 Democratizing Access to Novel Medicines and Upholding Social Responsibility COVID-19 vaccine delivery to LMICs • 1.7 billion doses of COVID-19 vaccine in total to low- and middle-income countries (LMICs) delivered in line with demand2 For diseases with medical care gaps Development programs for infectious diseases in support of UN Sustainable Development Goal 3: HSV-2, tuberculosis, malaria and shingles Sustainable, scalable mRNA manufacturing • March 2023: first BioNTainer arrived in Kigali, Rwanda • Aim of building BioNTainers for other partner countries Australia, Senegal, and Israel 13 Environmental & climate protection • Currently under review by SBTi1: BioNTech's short- term climate targets by 2030 • Submitted targets: absolute emission reduction of 42% by 2030 for Scope 1/2 and supplier engagement target for Scope 3 • Analysis of qualitative/financial climate risks according to TCFD3 completed, measures being implemented CSR governance and regulation • CSR core strategy: integration of sustainability in all responsible business areas • Human rights due diligence: Implementation of all global regulatory requirements (esp. LkSG) • Projects launched to implement CSRD regulation § 1. LkSG: Gesetz über die unternehmerischen Sorgfaltspflichten zur Vermeidung von Menschenrechtsverletzungen in Lieferketten 2. SBTi: Science Based Targets initiative 3. TCFD: Task Force on Climate-related Financial Disclosures 4. Stand December 2022

COVID-19 VACCINE GLOBAL LEADERSHIP

2022: Continued Leadership against COVID-19 15 1 Partnered with Pfizer 2 As of Dec. 16, 2022 3 Pfizer/BioNTech cumulative global COVID-19 market share across reporting countries; CDC, ECDC OWID data as of Nov 2022 4 in the U.S., EU and United Kingdom Invoiced ~2 billion doses of vaccine Shipped ~550 million doses of variant-adapted vaccine2 ~2 months from recommendations of regulatory authorities to vaccine delivery COMIRNATY market share: ~64%3 First to market BA.4-5 variant-adapted vaccine1 Broadest label amongst COVID-19 vaccines4

COVID-19 Franchise: Building for Continued Success 16 Comprehensive COVID-19 research program Ability to rapidly roll out new vaccines at commercial scale within months Investments in combination vaccines and next- generation COVID-19 vaccines Leveraging AI and ML for pandemic preparedness Development of a variant-adapted vaccine for 2023 1 AI = Artificial Intelligence; ML = Machine Learning
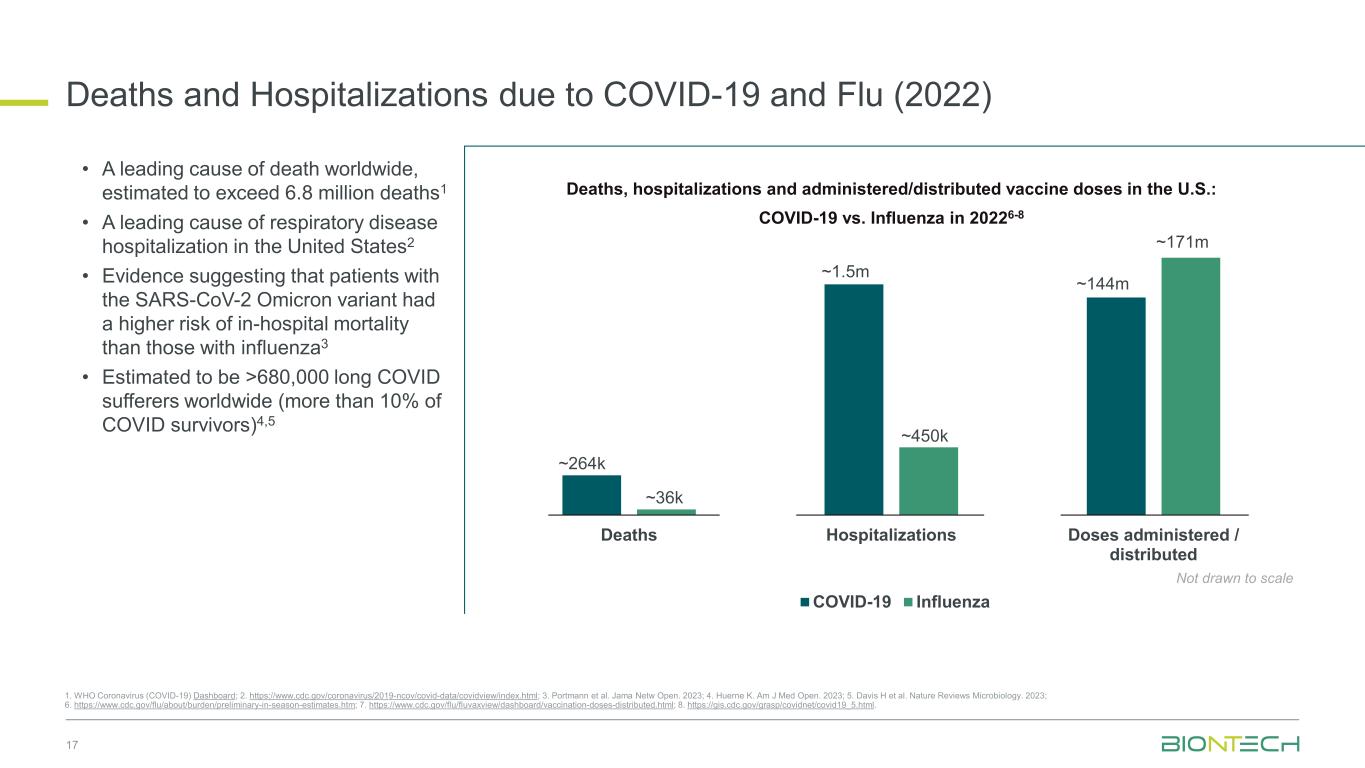
Deaths and Hospitalizations due to COVID-19 and Flu (2022) 17 1. WHO Coronavirus (COVID-19) Dashboard; 2. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html; 3. Portmann et al. Jama Netw Open. 2023; 4. Huerne K. Am J Med Open. 2023; 5. Davis H et al. Nature Reviews Microbiology. 2023; 6. https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm; 7. https://www.cdc.gov/flu/fluvaxview/dashboard/vaccination-doses-distributed.html; 8. https://gis.cdc.gov/grasp/covidnet/covid19_5.html. Deaths Hospitalizations Doses administered / distributed COVID-19 Influenza ~264k ~36k ~1.5m ~450k ~144m ~171m Not drawn to scale Deaths, hospitalizations and administered/distributed vaccine doses in the U.S.: COVID-19 vs. Influenza in 20226-8 • A leading cause of death worldwide, estimated to exceed 6.8 million deaths1 • A leading cause of respiratory disease hospitalization in the United States2 • Evidence suggesting that patients with the SARS-CoV-2 Omicron variant had a higher risk of in-hospital mortality than those with influenza3 • Estimated to be >680,000 long COVID sufferers worldwide (more than 10% of COVID survivors)4,5

DIVERSIFIED PRODUCT PIPELINE

Technology Agnostic Innovation Engine 19 1 mRNA encoded cancer-targeting antibodies and cytokines. AI = Artificial intelligence; CAR = chimeric antigen receptor; TLR = Toll-like receptor; TCR = T cell receptor; STING = stimulator of interferon genes. AI & Machine Learning – Internal capabilities & InstaDeepCore principles of our technology strategy Individualized therapiesEnabling technology STING AGONISTS Ryvu collaboration ANTIBODY- DRUG CONJUGATES Duality collaboration * SELECTIVE TLR-7 AGONISM TARGETED CANCER THERAPIES RIBOLYSIN Precision antibacterials Phagomed acquisition OFF-THE- SHELF mRNA CANCER VACCINES FixVac INFECTIOUS DISEASE VACCINES Prophylactic and therapeutic vaccines INDIVIDUA- LIZED mRNA CANCER VACCINES INDIVIDUALIZED TCR-THERAPY INDIVIDUALIZED EX VIVO T-CELL THERAPY SOLID TUMOR CAR-T Ideal CAR-T- cell targets MULTI- TARGET TCR Internal capabilities & Medigene collaborationCARVac mRNA vaccine boosted CAR-T-cells LIPID FORMULATIONS Internal capabilities IMMUNO- THERAPY TARGET DISCOVERY SMALL MOLECULES NEXT GEN IMMUNO- MODULATORS Mono and Bispecific Abs RIBO- LOGICALS1 RiboCytokines RiboMabs mRNA ENCODED HUMABODIES Crescendo collaboration ANTIBODIESCELL & GENE THERAPIES mRNA TECHNOLOGYTechnology agnostic approach rooted in deep fundamental understanding of biology Build novel platforms with the ability to produce multiple product candidates Open up new combination opportunities which leverage synergistic modes of action Enable individualized treatment
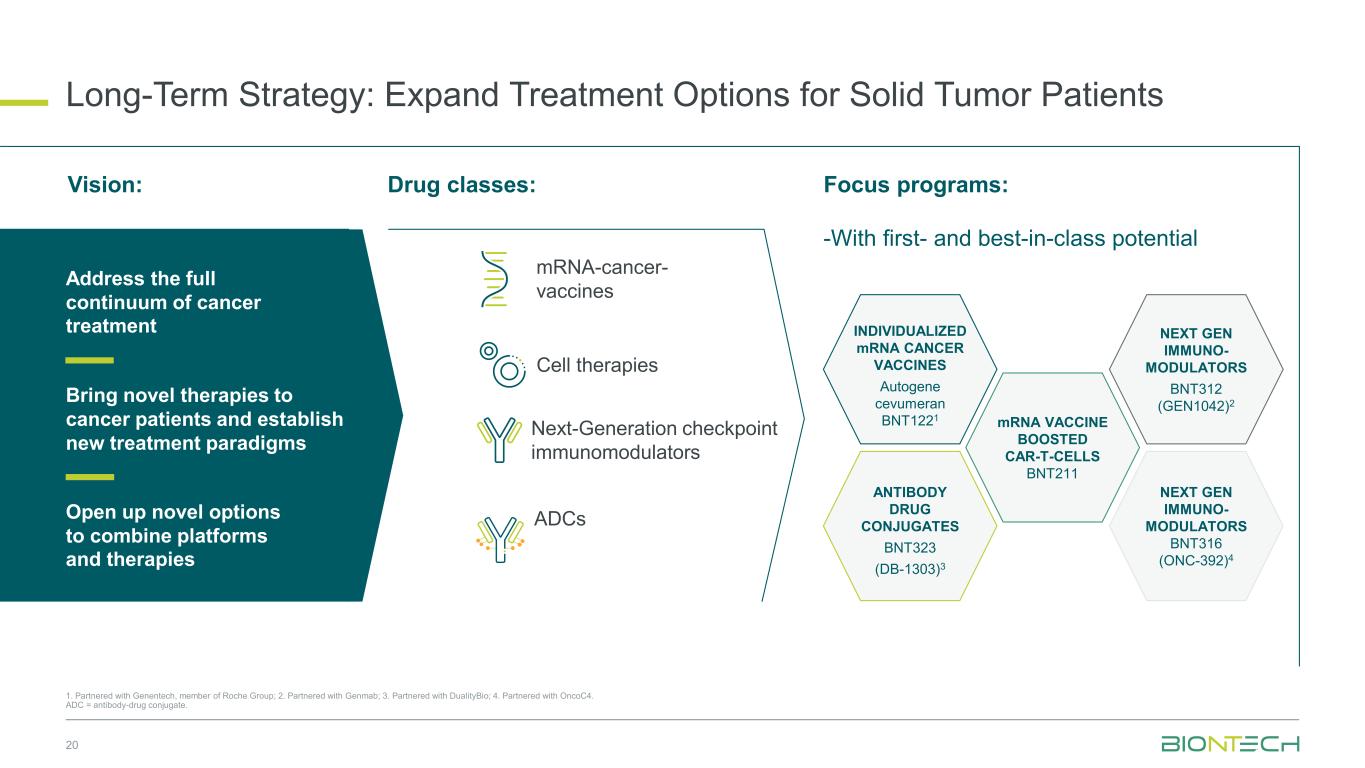
Long-Term Strategy: Expand Treatment Options for Solid Tumor Patients 20 1. Partnered with Genentech, member of Roche Group; 2. Partnered with Genmab; 3. Partnered with DualityBio; 4. Partnered with OncoC4. ADC = antibody-drug conjugate. mRNA-cancer- vaccines Cell therapies ADCs Next-Generation checkpoint immunomodulators Focus programs:Vision: Drug classes: -With first- and best-in-class potential Open up novel options to combine platforms and therapies Address the full continuum of cancer treatment Bring novel therapies to cancer patients and establish new treatment paradigms aPD1-R/R melanoma, + Pembro INDIVIDUALIZED mRNA CANCER VACCINES Autogene cevumeran BNT1221 mRNA VACCINE BOOSTED CAR-T-CELLS BNT211 NEXT GEN IMMUNO- MODULATORS BNT312 (GEN1042)2 ANTIBODY DRUG CONJUGATES BNT323 (DB-1303)3 NEXT GEN IMMUNO- MODULATORS BNT316 (ONC-392)4
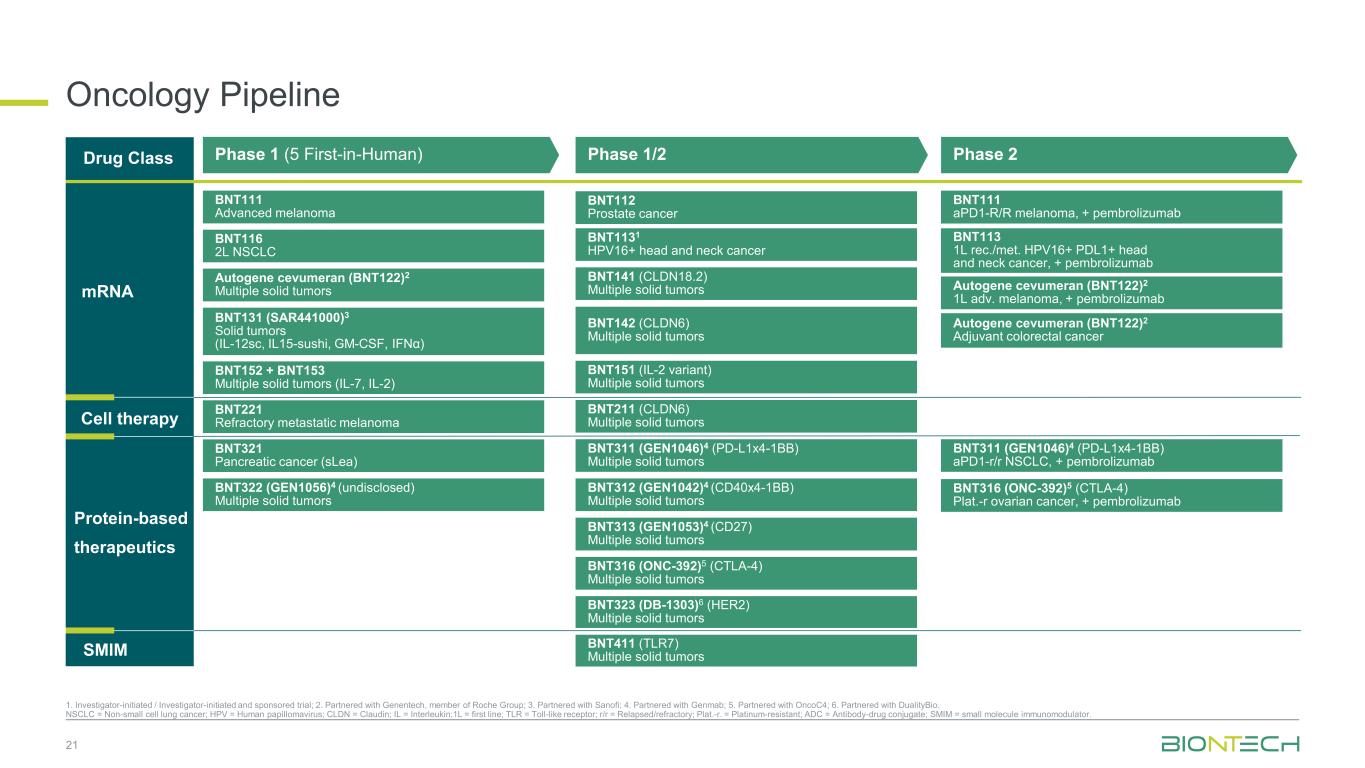
Oncology Pipeline 21 1. Investigator-initiated / Investigator-initiated and sponsored trial; 2. Partnered with Genentech, member of Roche Group; 3. Partnered with Sanofi; 4. Partnered with Genmab; 5. Partnered with OncoC4; 6. Partnered with DualityBio. NSCLC = Non-small cell lung cancer; HPV = Human papillomavirus; CLDN = Claudin; IL = Interleukin;1L = first line; TLR = Toll-like receptor; r/r = Relapsed/refractory; Plat.-r. = Platinum-resistant; ADC = Antibody-drug conjugate; SMIM = small molecule immunomodulator. Phase 1 (5 First-in-Human) BNT112 Prostate cancer BNT116 2L NSCLC BNT111 aPD1-R/R melanoma, + pembrolizumab BNT113 1L rec./met. HPV16+ PDL1+ head and neck cancer, + pembrolizumab Autogene cevumeran (BNT122)2 1L adv. melanoma, + pembrolizumab BNT131 (SAR441000)3 Solid tumors (IL-12sc, IL15-sushi, GM-CSF, IFNα) BNT141 (CLDN18.2) Multiple solid tumors BNT151 (IL-2 variant) Multiple solid tumors BNT152 + BNT153 Multiple solid tumors (IL-7, IL-2) BNT211 (CLDN6) Multiple solid tumors BNT221 Refractory metastatic melanoma BNT311 (GEN1046)4 (PD-L1x4-1BB) Multiple solid tumors BNT321 Pancreatic cancer (sLea) BNT411 (TLR7) Multiple solid tumors BNT311 (GEN1046)4 (PD-L1x4-1BB) aPD1-r/r NSCLC, + pembrolizumab BNT312 (GEN1042)4 (CD40x4-1BB) Multiple solid tumors BNT313 (GEN1053)4 (CD27) Multiple solid tumors BNT322 (GEN1056)4 (undisclosed) Multiple solid tumors BNT316 (ONC-392)5 (CTLA-4) Multiple solid tumors Autogene cevumeran (BNT122)2 Adjuvant colorectal cancer BNT142 (CLDN6) Multiple solid tumors Phase 1/2 BNT316 (ONC-392)5 (CTLA-4) Plat.-r ovarian cancer, + pembrolizumab BNT1131 HPV16+ head and neck cancer BNT111 Advanced melanoma Drug Class mRNA Cell therapy Protein-based therapeutics Autogene cevumeran (BNT122)2 Multiple solid tumors SMIM Phase 2 BNT323 (DB-1303)6 (HER2) Multiple solid tumors

Infectious Diseases: Important Growth Area Addressing High Medical and Global Health Need 22 Ongoing clinical programs: All figures from World Health Organization (WHO) factsheets. https://www.who.int/news-room/fact-sheets (accessed April 14, 2023). 1.Partnership with Pfizer; 2. Cooperation with PFE and subject to agreement with our partners; 3. Exclusive license to Pfizer; 4. Cooperation with University of Pennsylvania; 5. Cooperation with Bill & Melinda Gates Foundation. HSV = Herpes simplex virus • COVID-191 • COVID-19+Influenza2 • Influenza3 • HSV-24 • Malaria • Tuberculosis5 • Shingles1 Influenza 290,000 – 650,000 deaths annually worldwide Shingles ~95% of the population >50 years is at risk of developing shingles HSV-2 ~491 million people aged 15 – 49 infected worldwide Once infected, HSV stays in the body for life with recurring symptomatic outbreaks Malaria ~247 million cases in 2021 worldwide ~691,000 deaths in 2021 (of which 82% in children <5 years from African regions) Tuberculosis ~10.6 million cases in 2021 worldwide ~1.6 million deaths in 2021 worldwide
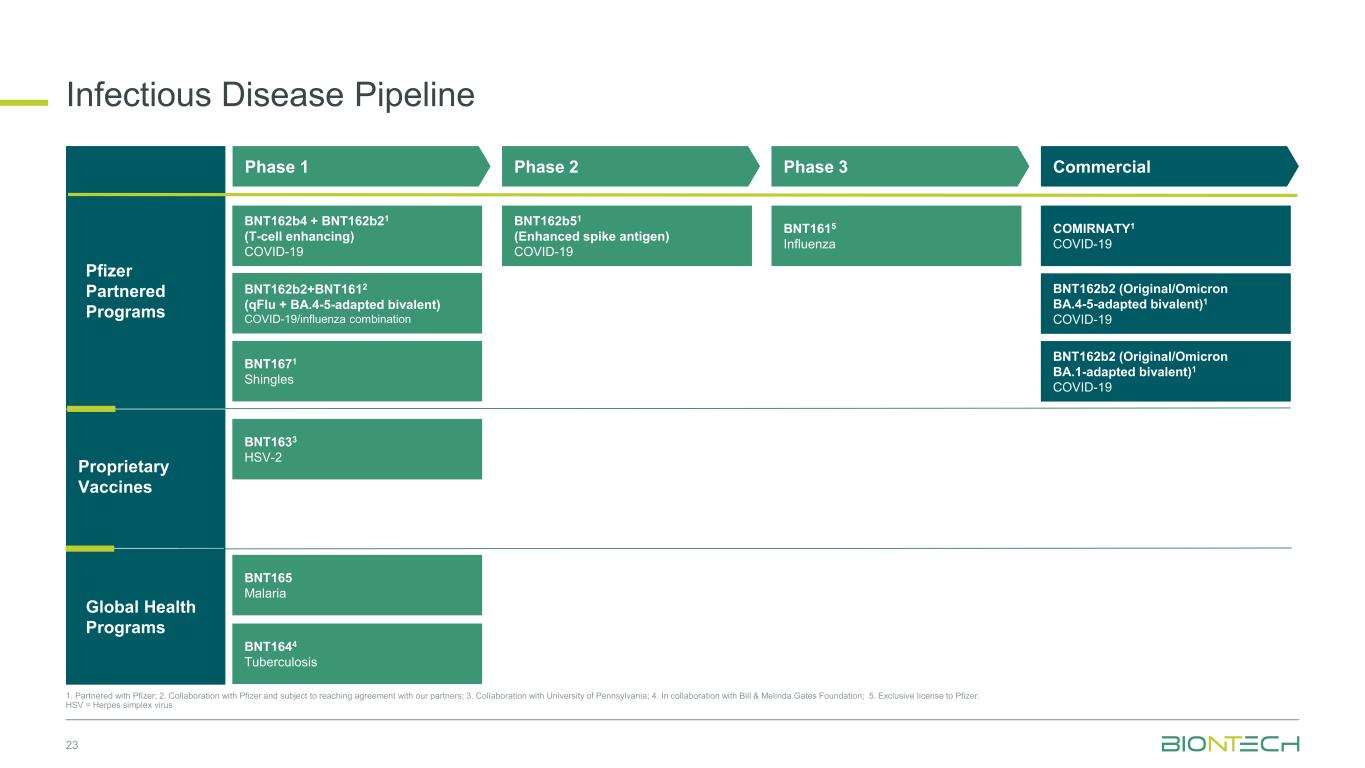
Phase 1 BNT162b2 (Original/Omicron BA.4-5-adapted bivalent)1 COVID-19 Pfizer Partnered Programs COMIRNATY1 COVID-19 BNT162b2 (Original/Omicron BA.1-adapted bivalent)1 COVID-19 BNT162b51 (Enhanced spike antigen) COVID-19 BNT162b4 + BNT162b21 (T-cell enhancing) COVID-19 BNT1633 HSV-2 Infectious Disease Pipeline 23 1. Partnered with Pfizer; 2. Collaboration with Pfizer and subject to reaching agreement with our partners; 3. Collaboration with University of Pennsylvania; 4. In collaboration with Bill & Melinda Gates Foundation; 5. Exclusive license to Pfizer. HSV = Herpes simplex virus BNT162b2+BNT1612 (qFlu + BA.4-5-adapted bivalent) COVID-19/influenza combination BNT1615 Influenza BNT1671 Shingles Phase 2 CommercialPhase 3 BNT165 Malaria Proprietary Vaccines BNT1644 Tuberculosis Global Health Programs

OUTLOOK 2023
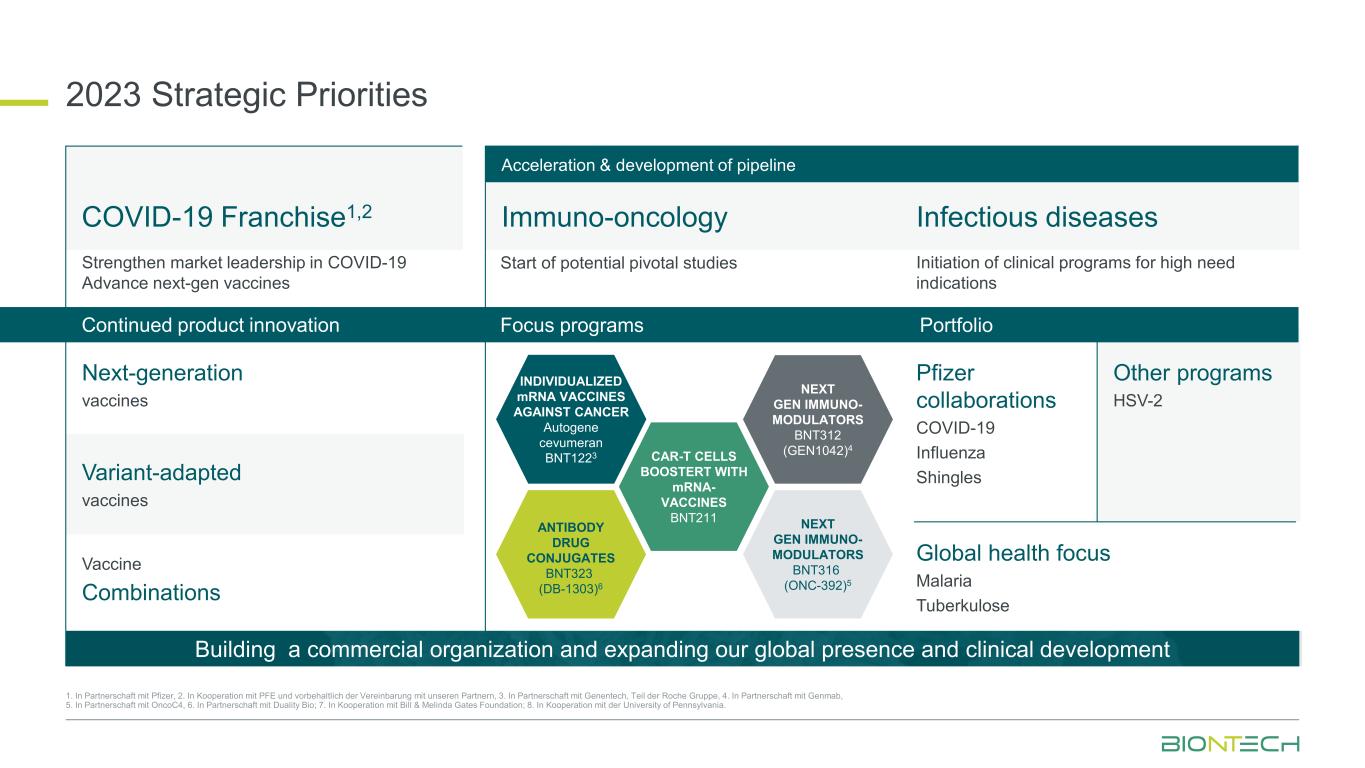
Immuno-oncology Infectious diseases 2023 Strategic Priorities 1. In Partnerschaft mit Pfizer, 2. In Kooperation mit PFE und vorbehaltlich der Vereinbarung mit unseren Partnern, 3. In Partnerschaft mit Genentech, Teil der Roche Gruppe, 4. In Partnerschaft mit Genmab, 5. In Partnerschaft mit OncoC4, 6. In Partnerschaft mit Duality Bio; 7. In Kooperation mit Bill & Melinda Gates Foundation; 8. In Kooperation mit der University of Pennsylvania. Strengthen market leadership in COVID-19 Advance next-gen vaccines Start of potential pivotal studies Initiation of clinical programs for high need indications Variant-adapted vaccines Next-generation vaccines Vaccine Combinations Pfizer collaborations COVID-19 Influenza Shingles Other programs HSV-2 Global health focus Malaria Tuberkulose Building a commercial organization and expanding our global presence and clinical development Acceleration & development of pipeline COVID-19 Franchise1,2 Focus programs PortfolioContinued product innovation INDIVIDUALIZED mRNA VACCINES AGAINST CANCER Autogene cevumeran BNT1223 CAR-T CELLS BOOSTERT WITH mRNA- VACCINES BNT211 NEXT GEN IMMUNO- MODULATORS BNT312 (GEN1042)4 ANTIBODY DRUG CONJUGATES BNT323 (DB-1303)6 NEXT GEN IMMUNO- MODULATORS BNT316 (ONC-392)5

2 Financial Development 2022 & Q1 2023 and Financial Outlook 2023 Jens Holstein, CFO
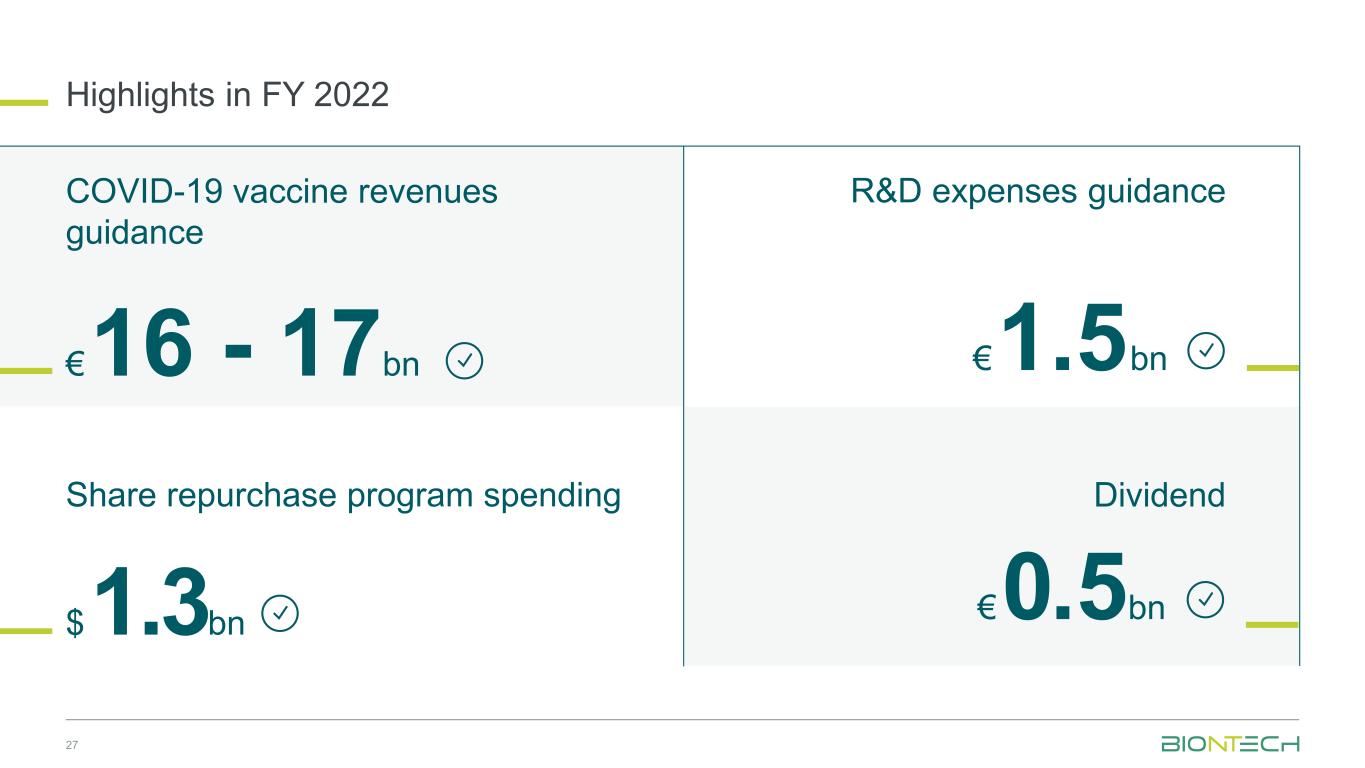
Highlights in FY 2022 27 R&D expenses guidance $1.3bn €1.5bn€16 - 17bn €0.5bn COVID-19 vaccine revenues guidance Share repurchase program spending Dividend

Key financial figures for FY 2022 28 1. BioNTech's share of profit is estimated as further described in the 2022 Annual Report based on preliminary data exchanged between Pfizer and BioNTech. Any changes in the estimated share of the collaborator's gross profit are recorded prospectively; 2. The payment settling the gross profit share for the third quarter of 2022 (as defined by the contract) in the amount of €1,816.5 million was received from the collaboration partner as of January 12, 2023, subsequent to the end of the reporting period. €13.9bn €17.3bn €13.6bn €37.77 Total revenues1 Operating cashflow Diluted EPS Cash and cash equivalents2

29 FY 2022 Guidance vs. Actuals 1. BioNTech's share of profit is estimated as further described in the 2022 Annual Report based on preliminary data exchanged between Pfizer and BioNTech. Any changes in the estimated share of the collaborator's gross profit are recorded prospectively; 2. Reduction in cash-effective tax rate due to IAS 12.68c as a result of tax deductibility of share-based payment settlement. Updated guidance (as published in Q3 2022 Financial Results and Corporate Update) Actual FY 2022 COVID-19 vaccine revenues Estimated BioNTech COVID-19 vaccine revenues1 €16 – 17 bn €17.1 bn Expenses and capex R&D expenses €1,400 – 1,500 m €1,537 m SG&A expenses €450 – 550 m €544 m Capital expenditure €450 – 550 m €363 m Tax assumptions BioNTech Group estimated annual effective income tax rate ~ 27% (IFRS) ~ 27% (cash-effective)2 ~ 24%

30 FY 2022 Financial Results – Profit and Loss 1. Numbers have been rounded, numbers presented may not add up precisely to the totals and may have been adjusted in the table context. Presentation of the consolidated statements of profit and loss has been condensed; 2. BioNTech's share of profit is estimated as further described in the 2022 Annual Report based on preliminary data exchanged between Pfizer and BioNTech. Any changes in the estimated share of the collaborator's gross profit are recorded prospectively. (in millions €, except per share data)1 Year ended December 31, 2022 2021 Commercial revenues2 17,194.6 18,874.0 Research & development revenues 116.0 102.7 Total revenues 17,310.6 18,976.7 Cost of sales (2,995.0) (2,911.5) Research and development expenses (1,537.0) (949.2) Sales and marketing expenses (59.5) (50.4) General and administrative expenses (484.7) (285.8) Other operating income less expenses 408.3 504.0 Operating income 12,642.7 15,283.8 Finance income less expenses 311.4 (237.4) Income taxes (3,519.7) (4,753.9) Profit for the period 9,434.4 10,292.5 Earnings per share Basic profit for the period per share 38.78 42.18 Diluted profit for the period per share 37.77 39.63
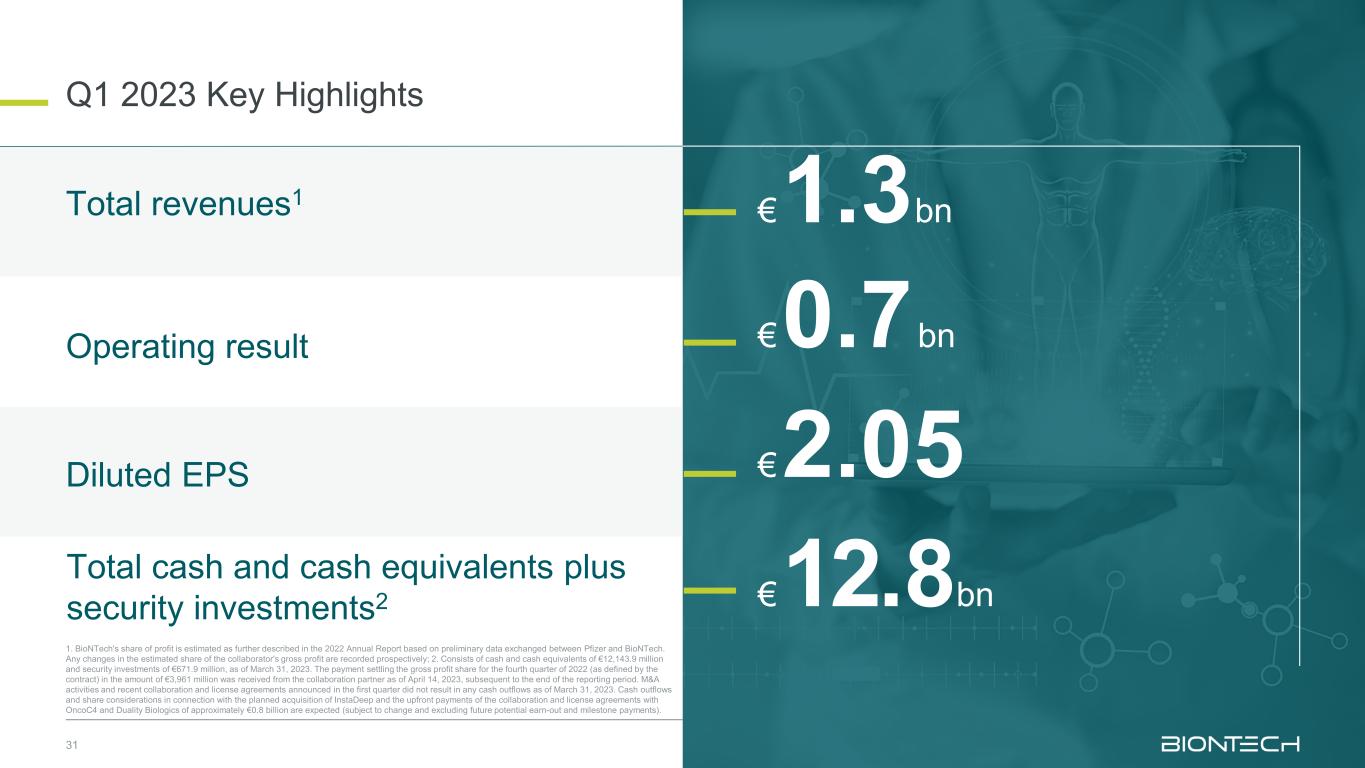
31 Q1 2023 Key Highlights 1. BioNTech's share of profit is estimated as further described in the 2022 Annual Report based on preliminary data exchanged between Pfizer and BioNTech. Any changes in the estimated share of the collaborator's gross profit are recorded prospectively; 2. Consists of cash and cash equivalents of €12,143.9 million and security investments of €671.9 million, as of March 31, 2023. The payment settling the gross profit share for the fourth quarter of 2022 (as defined by the contract) in the amount of €3,961 million was received from the collaboration partner as of April 14, 2023, subsequent to the end of the reporting period. M&A activities and recent collaboration and license agreements announced in the first quarter did not result in any cash outflows as of March 31, 2023. Cash outflows and share considerations in connection with the planned acquisition of InstaDeep and the upfront payments of the collaboration and license agreements with OncoC4 and Duality Biologics of approximately €0.8 billion are expected (subject to change and excluding future potential earn-out and milestone payments). €12.8bn €1.3bn €0.7bn €2.05 Total revenues1 Operating result Diluted EPS Total cash and cash equivalents plus security investments2

32 Q1 2023 Financial Results – Profit and Loss 1. Numbers have been rounded, numbers presented may not add up precisely to the totals and may have been adjusted in the table context. Presentation of the unaudited interim consolidated statements of profit and loss has been condensed; 2. BioNTech's share of profit is estimated as further described in the 2022 Annual Report based on preliminary data exchanged between Pfizer and BioNTech. Any changes in the estimated share of the collaborator's gross profit are recorded prospectively. (in millions €, except per share data)1 Three months ended March 31, 2023 2022 Commercial revenues2 1,276.5 6,362.2 Research & development revenues 0.5 12.4 Total revenues 1,277.0 6,374.6 Cost of sales (96.0) (1,294.1) Research and development expenses (334.0) (285.8) Sales and marketing expenses (12.2) (14.3) General and administrative expenses (119.4) (90.8) Other operating income less expenses (61.0) 63.1 Operating income 654.4 4,752.7 Finance income less expenses 53.3 265.4 Income taxes (205.5) (1,319.3) Profit for the period 502.2 3,698.8 Earnings per share Basic profit for the period per share 2.07 15.13 Diluted profit for the period per share 2.05 14.24
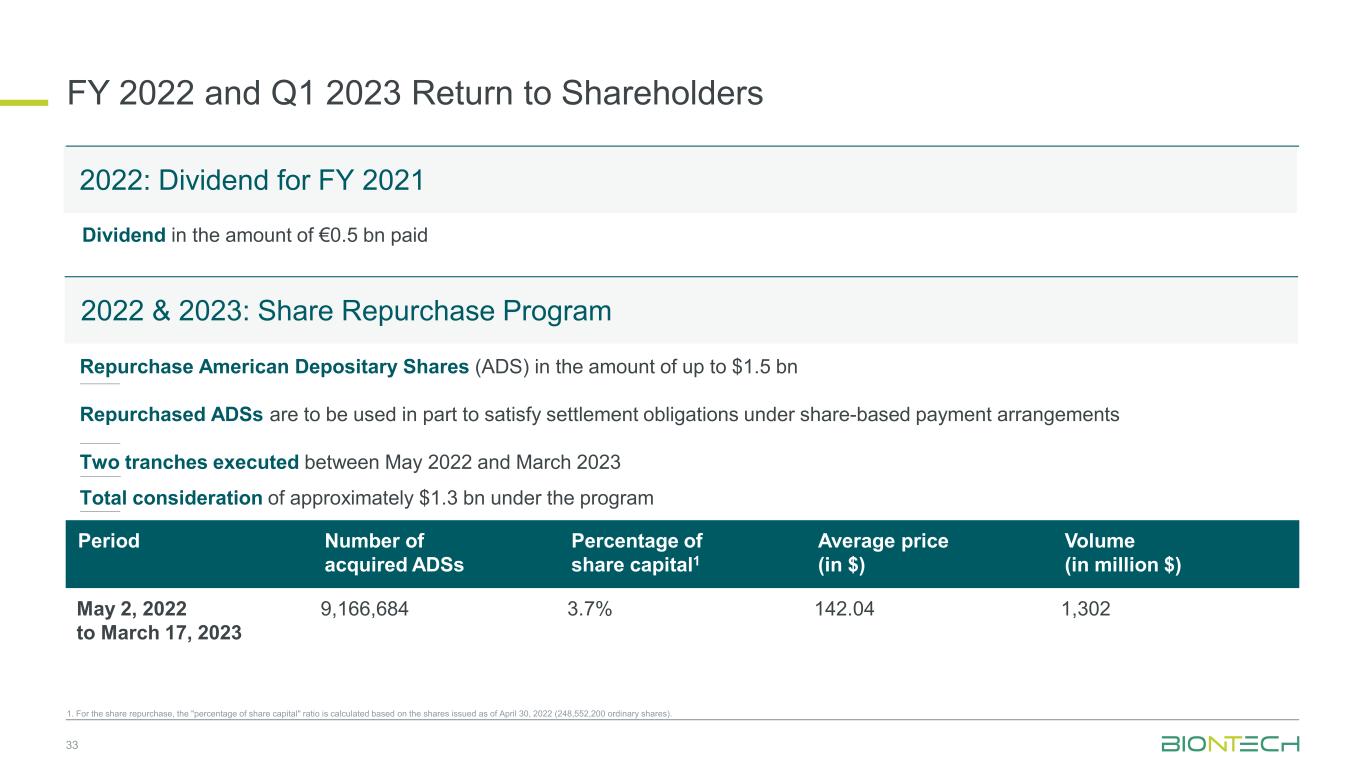
33 FY 2022 and Q1 2023 Return to Shareholders 1. For the share repurchase, the "percentage of share capital" ratio is calculated based on the shares issued as of April 30, 2022 (248,552,200 ordinary shares). 2022: Dividend for FY 2021 Period Number of acquired ADSs Percentage of share capital1 Average price (in $) Volume (in million $) May 2, 2022 to March 17, 2023 9,166,684 3.7% 142.04 1,302 2022 & 2023: Share Repurchase Program Dividend in the amount of €0.5 bn paid Repurchase American Depositary Shares (ADS) in the amount of up to $1.5 bn Repurchased ADSs are to be used in part to satisfy settlement obligations under share-based payment arrangements Two tranches executed between May 2022 and March 2023 Total consideration of approximately $1.3 bn under the program
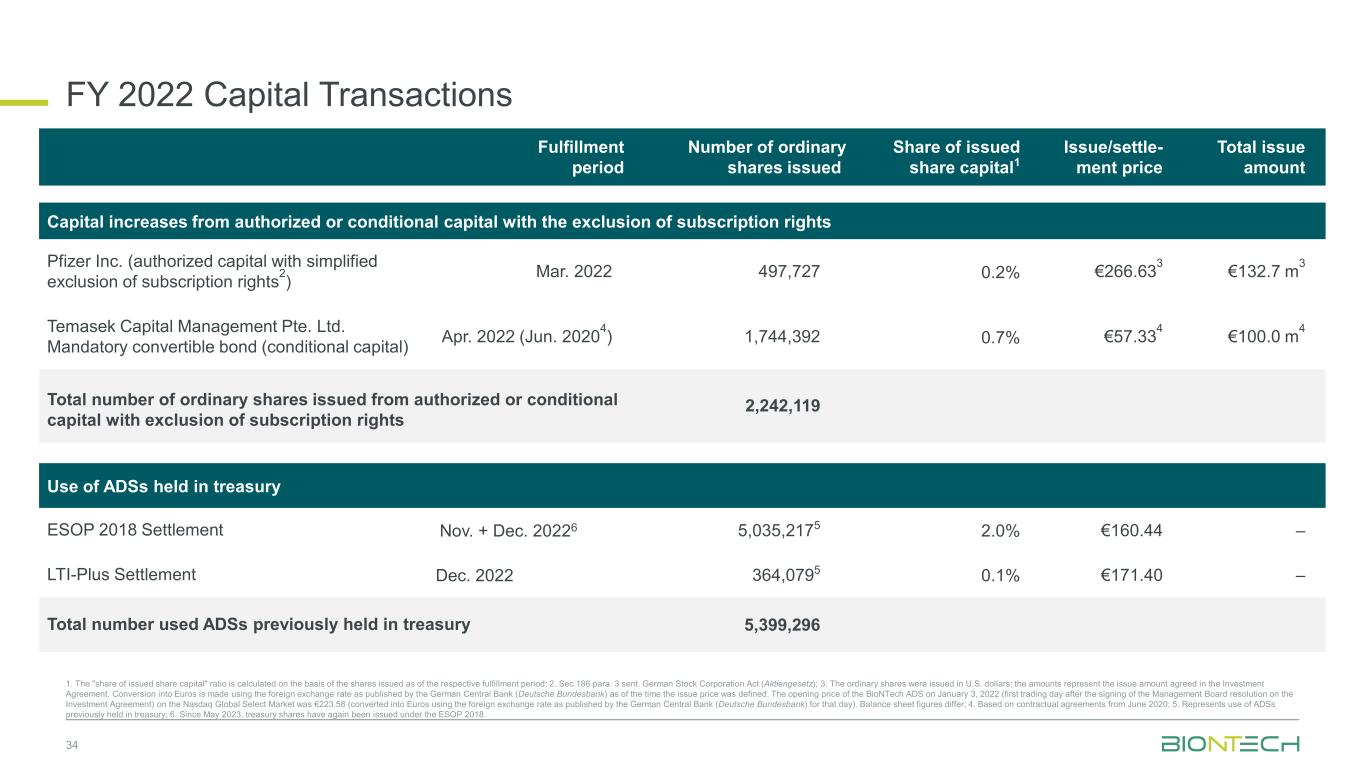
34 1. The "share of issued share capital" ratio is calculated on the basis of the shares issued as of the respective fulfillment period; 2. Sec.186 para. 3 sent. German Stock Corporation Act (Aktiengesetz); 3. The ordinary shares were issued in U.S. dollars; the amounts represent the issue amount agreed in the Investment Agreement. Conversion into Euros is made using the foreign exchange rate as published by the German Central Bank (Deutsche Bundesbank) as of the time the issue price was defined. The opening price of the BioNTech ADS on January 3, 2022 (first trading day after the signing of the Management Board resolution on the Investment Agreement) on the Nasdaq Global Select Market was €223.58 (converted into Euros using the foreign exchange rate as published by the German Central Bank (Deutsche Bundesbank) for that day). Balance sheet figures differ; 4. Based on contractual agreements from June 2020; 5. Represents use of ADSs previously held in treasury; 6. Since May 2023, treasury shares have again been issued under the ESOP 2018. Fulfillment period Number of ordinary shares issued Share of issued share capital1 Issue/settle- ment price Total issue amount Capital increases from authorized or conditional capital with the exclusion of subscription rights Pfizer Inc. (authorized capital with simplified exclusion of subscription rights2) Mar. 2022 497,727 0.2% €266.633 €132.7 m3 Temasek Capital Management Pte. Ltd. Mandatory convertible bond (conditional capital) Apr. 2022 (Jun. 20204) 1,744,392 0.7% €57.334 €100.0 m4 Total number of ordinary shares issued from authorized or conditional capital with exclusion of subscription rights 2,242,119 Use of ADSs held in treasury ESOP 2018 Settlement Nov. + Dec. 20226 5,035,2175 2.0% €160.44 – LTI-Plus Settlement Dec. 2022 364,0795 0.1% €171.40 – Total number used ADSs previously held in treasury 5,399,296 FY 2022 Capital Transactions

FY 2023 Financial Guidance 35 Key Assumptions and Considerations Expected increase in demand for a new, adapted vaccine with a simultaneous reduction in the number of primary and booster vaccinations. Assumption of seasonal demand, majority of revenues expected in second half of 2023. Expected transition from an advanced purchase agreement environment to commercial market ordering starting in 2023 and a regulatory recommendation to adapt the COVID-19 vaccines to newly circulating variants or sublineages of SARS-CoV-2. Revenue guidance reflects expected deliveries under existing or committed supply contracts and anticipated sales through traditional commercial orders. Renegotiation of the existing supply contract with the European Commission is ongoing, with the possibility of spreading dose supplies over several years and/or reducing volumes.

2023 FY Guidance Reiterated1 36 COVID-19 vaccine revenues for FY 2023 Estimated BioNTech COVID-19 vaccine revenues ~ €5 bn Planned FY 2023 expenses and capex R&D expenses2 €2,400 – 2,600 m SG&A expenses €650 – 750 m Capital expenditure for operating activities3 €500 – 600 m Estimated FY 2023 tax assumptions BioNTech Group estimated annual cash-effective income tax rate ~ 27% 2. Numbers include effects identified from additional collaborations or potential M&A transactions to the extent disclosed and identified and will be updated as needed; 3. Numbers exclude potential effects caused by or driven from collaborations or M&A transactions. 1.Numbers reflect current base case projections and are calculated based on constant currency rates;

37 FY 2023 Capital Allocation Framework R&D activities M&A and business development Main focus remains on the acceleration of our R&D activities in oncology and infectious diseases. Strengthen pipeline, technology platforms and digital capabilities by collaborations and potential complementary M&A. Return capital to shareholders New share repurchase program of up to $0.5bn during 2023.

Thank you