UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 13, 2024
Chromocell
Therapeutics Corporation
(Exact name of registrant as specified in its charter)
| Delaware | 001-41964 | 86-3335449 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
| 4400
Route 9 South, Suite
1000 Freehold, NJ |
07728 | |
| (Address of registrant’s principal executive office) | (Zip code) |
Registrant’s telephone number, including area code: 732-514-2636
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name
of each exchange on which registered |
||
| Common Stock, par value $0.0001 per share | CHRO | The NYSE American LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
|
|
| Item 7.01 | Regulation FD Disclosure. |
On March 13, 2023, Chromocell Therapeutics Corporation (the “Company”) will present at the virtual Sidoti Small Cap Conference held by Sidoti Events, LLC to prospective investors, analysts, industry participants and other attendees, which can be accessed live at Sidoti Events, LLC’s website. At the conference, the Company will provide an update on its product pipeline and discuss recent business developments, including its intended dose titration and Phase 2a clinical trials for systemic chronic pain and its pre-clinical trials for acute and chronic eye pain, both based on its proprietary compound, CC8464, and sublingual and intranasal programs under the exclusive licensing agreement with Benuvia Operations, LLC. The presentation materials furnished herewith as Exhibit 99.1 (the “Presentation Materials”) were used in connection with such presentation, are incorporated into this Item 7.01 by reference and will be posted on the Company’s website at https://www.chromocell.com. Information contained on the Company’s website is not incorporated by reference into and should not be considered to be part of this Current Report on Form 8-K (this “Form 8-K”).
The information contained in this Form 8-K under Item 7.01, including Exhibit 99.1 attached hereto, is deemed to be ”furnished” and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and shall not be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, whether made before or after the date hereof, except as shall be expressly set forth by specific reference to this Form 8-K in such filing. The information set forth in this Item 7.01 of this Form 8-K and Exhibit 99.1 attached hereto shall not be deemed an admission as to the materiality of any information in this Form 8-K that is required to be disclosed solely to satisfy the requirements of Regulation FD.
Forward-Looking Statements
Exhibit 99.1 attached hereto contains, and may indicate, forward-looking statements within the meaning of Section 27A of the Securities Act, Section 21E of the Exchange Act and as defined in the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements include, but are not limited to, statements that express the Company’s intentions, beliefs, expectations, strategies, predictions or any other statements related to the Company’s future activities, or future events or conditions, including without limitation, those statements relating to the timing, progress and results of preclinical and clinical trials for CC8464, its estimates regarding the potential market opportunity for CC8464, its ability to develop CC8464 and future compounds, its ability to protect its intellectual property and enforce its intellectual property rights, and its ability to execute its development strategy and sustain its competitive position in the Presentation Materials, and the Company’s clinical trials and/or trial results for its other products now or in the future, which can be identified by terminology such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict, “project,” “seek,” “should,” “target,” “will,” “would” and other similar expressions intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These statements are not historical facts and are based on current expectations, estimates and projections about the Company’s business based, in part, on assumptions made by its management. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict, many of which are beyond the Company’s control. Any forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date of this Form 8-K, except as required by applicable law.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Chromocell Therapeutics Corporation March 2024 Presentation | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
|
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: March 13, 2024 | CHROMOCELL THERAPEUTICS CORPORATION | |
| By: | /s/ Francis Knuettel II | |
| Name: Francis Knuettel II Title: Chief Executive Officer and Chief Financial Officer |
||
|
|
Exhibit 99.1


CHROMOCELL THERAPEUTICS CORPORATION 1 CHROMOCELL THERAPEUTICS CORPORATION Potential Breakthrough Drug for Non - Opioid Pain Treatment Therapies CHROMOCELL THERAPEUTICS CORPORATION 2 Legal Disclaimer This presentation of Chromocell Therapeutics Corporation (“we”, “us”, “our” or the “Company”) contains “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act and other securities laws . Words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict, “project,” “seek,” “should,” “target,” “will,” “would” or similar expressions and the negatives of those term are intended to identify forward - looking statements . Forward - looking statements reflect management’s current expectations, are based on judgments and assumptions, are inherently uncertain and are subject to risks, uncertainties and other factors, which could cause the Company’s actual results, performance or achievements to differ materially from expected future results, performance or achievements expressed or implied in those forward - looking statements . Examples of these forward - looking statements and the related risks, uncertainties and other factors include, but are not limited to, the following : the timing, progress and results of preclinical and clinical trials for CC 8464 , its estimates regarding the potential market opportunity for CC 8464 , its ability to develop CC 8464 and future compounds, its ability to protect its intellectual property and enforce its intellectual property rights, and its ability to execute its development strategy and sustain its competitive position . Actual future results and trends may differ materially depending on a variety of factors, including, but not limited to, the Company’s limited operating history, the Company’s ability to develop CC 8464 , the Company’s ability to establish its market development capabilities to commercialize its products and generate any revenue, and the Company’s ability to obtain regulatory approval of CC 8464 . Forward - looking statements are provided to allow potential investors the opportunity to understand management’s beliefs and opinions in respect of the future so that they may use such beliefs and opinions as one factor in evaluating an investment . These statements are not guarantees of future performance and undue reliance should not be placed on them . Any forward - looking statement in this presentation, in any related presentation supplement and in any related free writing presentation reflects our current view with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our business, results of operations, industry and future growth . You should read this presentation with the understanding that our actual future results may be materially different from any future results expressed or implied by these forward - looking statements . Except as required by law, we assume no obligation to update or revise these forward - looking statements for any reason, even if new information becomes available in the future .

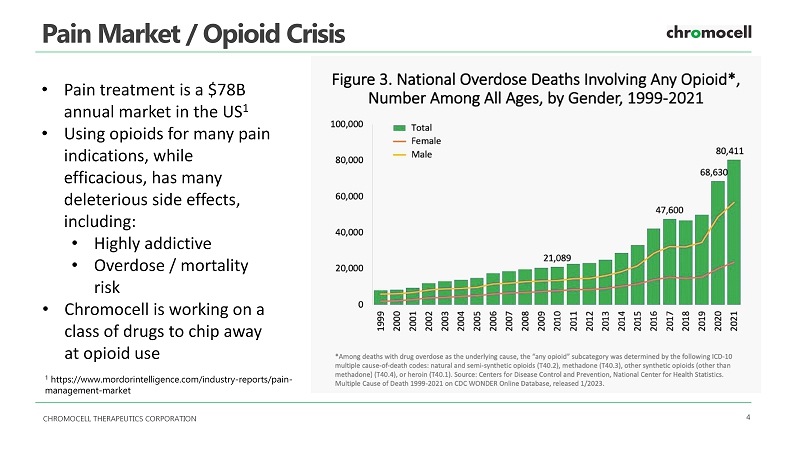
CHROMOCELL THERAPEUTICS CORPORATION 3 Investment Highlights Overview • Developing patented non - opioid pain treatment therapies • Proprietary compound with good patent protection Clinical / Pre - Clinical • Phase I and toxicology completed • Pre - clinical programs are topical, and expect swifter and cheaper development process Strategy • After achieving Phase II success, review out - licensing / sales / JV options • Exploring international joint ventures Platform • Build a pipeline of programs initially focused on non - opioid pain treatment therapies CHROMOCELL THERAPEUTICS CORPORATION 4 Pain Market / Opioid Crisis • Pain treatment is a $78B annual market in the US 1 • Using opioids for many pain indications, while efficacious, has many deleterious side effects, including: • Highly addictive • Overdose / mortality risk • Chromocell is working on a class of drugs to chip away at opioid use 1 https:// www.mordorintelligence.com /industry - reports/pain - management - market


CHROMOCELL THERAPEUTICS CORPORATION 5 Pipeline Three active clinical / pre - clinical programs Asset Indication Preclinical Phase 1 Phase 2 Phase 3 Neuropathic Pain Erythromelalgia Eye Pain Acute Pain / Migraine CHROMOCELL THERAPEUTICS CORPORATION 6 Management Team Frank Knuettel | CEO & Chief Financial Officer M r . Knuettel has 30 years of management experience in growing early - stage companies . He has raised more than $ 300 million via venture, public equity and debt offerings and managed more than 15 mergers and acquisition transactions along with large - scale licensing transactions with fortune 50 companies . Mr . Knuettel holds numerous board positions, at both public and private companies, including ECOM Medical, Relativity Acquisition Corp . (RACY) and Capstone Technologies Group Inc (OTC : CATG) . He holds an MBA from The Wharton School and a BA from Tufts University . Dr. Eric Lang | Chief Medical Officer Dr . Lang is an Anesthesiologist and Pain Management Specialist with over 25 years of experience in the pharmaceutical industry . During his pharmaceutical career, he has had both broad - based drug and device development expertise in a variety of therapeutic areas . Dr . Lang has experience in designing development programs from early translational stages through phase III including the successful filing of several recent INDs and NDAs . Dr . Lang began his career with J&J and later worked for Novartis, Javelin Pharmaceuticals, Grunenthal USA, Covance, EnteraBio and Nevakar Inc . Dr . Lang received his MD from Ben Gurion University, Israel and completed post graduate training at Emory University in Atlanta .

CHROMOCELL THERAPEUTICS CORPORATION 7 CHROMOCELL THERAPEUTICS CORPORATION Clinical & Competitive Background CHROMOCELL THERAPEUTICS CORPORATION 8 Sodium channels are our bodies “electrical circuits” Non - selective (e.g.

carbamazepine) block all of the NaV channel subtypes CHROMOCELL THERAPEUTICS CORPORATION 9 Why is NaV1.7 a Good Target for Pain Treatment? Insensitivity to Pain Lack of NaV 1.7 (Rare condition initially described in family from Pakistan) Severe Pain Excessive NaV 1.7 activity (e.g.


Erythromelalgia) Genetic validation suggests that suppressing NaV 1.7 is an attractive pharmacological target for pain management Congenital Insensitivity to Pain Spectrum of NaV1.7 Activity Severe Pain CHROMOCELL THERAPEUTICS CORPORATION 10 CC8464 – Development Status Preclinical • Potent ( nM ) inhibitor of human NaV1.7; Subtype selective • Demonstrated in vivo efficacy in several rodent models of pain: Acute, chronic neuropathic, inflammatory, visceral and post - surg ical • No CNS and muscle/motor dysfunction effects CMC • Drug Substance: scaled up, cGMP API available • Drug Product: tablet (active, 3 strengths and placebo) available for Phase 2. Potency verification to be confirmed. Tox • Did not exhibit genotoxicity • Tox data supports up to 3 - month dosing in human clinical trials Clinical • Phase 1 completed • Occurrence of rashes may be addressed with gradual dose - escalation protocols • Clinical data supports a Proof of Concept (“POC”) EM study • Seek orphan drug designation and apply for breakthrough status CHROMOCELL THERAPEUTICS CORPORATION 11 CHROMOCELL THERAPEUTICS CORPORATION Development Plan – Systemic Chronic Pain


CHROMOCELL THERAPEUTICS CORPORATION 12 Phase 1 Results / Dose - Escalation Study Phase 1 Results • No significant dose related trends or apparent differences compared to placebo in laboratory assessments, vital signs or ECG and no dose escalation stopping criteria were met • A moderate drug - induced rash in 6 of 159 subjects (4%), was the only clinically significant dose limiting safety finding • Following detailed review and discussions with dermatology experts and the FDA, we proposed a gradual dose escalation regime and the FDA accepted - Marketed drugs with rash Adverse Event have reduced the incidence rate by up to 75% by administering with dose escalation Anticipated Dose Escalation Study Goal is to demonstrate that dose escalation regime decrease occurrence of rashes Screening - 42 to - 1 days 25 mg QD 50 mg QD 100mg (50 mg BID) 200 mg (100 mg BID) 400mg (200 mg BID) 800mg (400 mg BID) Week 1 Week 2 Week 3 Week 8 Week 7 Week 6 Week 4 Week 5 Expect the Occurrence of Rashes to be Addressed with a Dose Escalation Regime CHROMOCELL THERAPEUTICS CORPORATION 13 Idiopathic Small Fiber Neuropathy Overview Symptoms and Background Most such cases present in adults ≥50 years of age and progress slowly over months to years.

The symptoms are typically sensory, involving paresthesia, numbness, or pain. Electrodiagnostic studies show a primarily axonal polyneuropathy. Proposed but unproven causes include impaired glucose tolerance, hypertension, dyslipidemia, and increased oxidative stress Treatment Gabapentin and tricyclic antidepressants may help. Other medications have also been tried. Continued unmet medical need. Prevalence Estimated between 20,000 – 80,000 patients in the US 1 Polyneuropathy refers to a generalized, relatively homogeneous process affecting many peripheral nerves, with the distal nerves usually affected most prominently . Polyneuropathy is typically characterized by symmetric distal sensory loss, burning sensations, or weakness .

No specific cause is identified in up to 46 percent of patients with polyneuropathy 1 Source: https://www.uptodate.com/contents/overview - of - polyneuropathy/print CHROMOCELL THERAPEUTICS CORPORATION 14 Phase 2a POC Small Fiber Neuropathy* Strategy and Endpoints Our strategy sets two key goals for POC studies with CC8464: • Obtain results demonstrating that CC8464 is a viable therapeutic for EM patients • Demonstrate that CC8464 and its blockage of NaV1.7 is a viable therapeutic option in patients with idiopathic small fiber neuropathy. A randomized, double - blind trial in ~240 patients is anticipated. Primary Endpoint for POC Studies • Demonstrate pain reduction in this orphan populations Secondary Endpoints for POC Studies • Demonstration of efficacy in this orphan populations suggests that CC8464 may be developed for additional pain indications * The Company is planning to conduct a Phase 2 a study and the concept presented herein remains subject to change .

CHROMOCELL THERAPEUTICS CORPORATION 15 Erythromelalgia Overview Symptoms Erythema (heat), Pain (Usually severe burning pain but may include pins and needles or itching), Swelling, Change in perspira tio n and discoloration Types of EM Primary EM (Inherited or Sporadic SCN9A mutations + non genetic or uncharacterized) or Secondary EM (Related to an underlying disease, toxin or drug induced) Treatment No known currently approved treatments and off - label treatments often ineffective Prevalence Estimated between 4,000 – 50,000 EM patients in the US with patents covering approximately 2.2 billion people worldwide Neurovascular condition affecting the feet, hands, face, or other parts of the body triggered by warmth, physical activity or stress Intolerance to exercise, warm baths/showers and clothing In severe cases, the disease may lead to depression, anxiety and suicidal tendencies .

CHROMOCELL THERAPEUTICS CORPORATION 16 Phase 2a Pilot EM Proof of Concept Study (POC)* * The Company is planning to conduct a Phase 2 a study and the concept presented herein remains subject to change . An open label study of CC8464 in the treatment of Erythromelalgia Screening Randomization CC8464 Follow - Up • N = approximately 10 (current plan, may be adjusted) • Flare induction after each dose • Primary endpoint is reduction of pain during flare • Secondary endpoints include additional pain endpoints, neuropathy scores, and time to flare Induce Flare CHROMOCELL THERAPEUTICS CORPORATION 17 CHROMOCELL THERAPEUTICS CORPORATION Development Plan – Eye Pain


CHROMOCELL THERAPEUTICS CORPORATION 18 Eye Pain Eye pain is common with both acute and chronic etiologies that include : Corneal Induced Chronic Pain from Dry Eye/NOP, Post PRK surgery, second eye cataract surgery, acute corneal abrasion, Ectropion/Entropion, Acute closed angle glaucoma, Uveitis, Iritis/Scleritis Existing therapies include topical NSAIDS (e . g . Bromfenac ) and topical steroids . Chronic use of local anesthetic drops is contraindicated and dangerous .

Prevalence – Corneal Abrasion Example • Very frequent • There are over 5 million cases of corneal abrasion in the United States every year CHROMOCELL THERAPEUTICS CORPORATION 19 Eye Pain Treatment Program Plan Strategy • Develop an eye drop formula with CC - 8464 with initial 3 - month stability • Demonstrate ophthalmologic safety in rabbits • Consider animal efficacy model or progress immediately to a POC trial in humans Potential model for POC Study • Demonstrate pain reduction patients post PRK surgery • Alternative human pain models are still being considered CHROMOCELL THERAPEUTICS CORPORATION 20 CHROMOCELL THERAPEUTICS CORPORATION Sublingual Programs


CHROMOCELL THERAPEUTICS CORPORATION 21 Sublingual Diclofenac Spray Toxicology Diclofenac is a well characterized NSAID with well understood safety and efficacy Sublingually delivered NSAID for faster relief with no need for swallowing Exclusive Worldwide License Chromocell has an exclusive license from Benuvia Operations, LLC to a patented formulation of sublingual diclofenac Regulatory 505B2 development pathway Preliminary human PK data suggest that it may have a faster onset of action than oral diclofenac which will be important for the treatment of acute pain/migraine Indications Potential options for clinical use include: post - operative pain, severe migraine, pain associated with trauma, etc.

CHROMOCELL THERAPEUTICS CORPORATION 22 Sublingual Rizatriptan (Maxalt) and Ondansetron (Zofran) Rizatriptan (Maxalt) Treatment of acute migraine. Negates the need to swallow which is difficult during a migraine and may offer a more rapid onset. Sublingually delivered for potentially faster onset with no need for swallowing Exclusive Worldwide License Chromocell has an exclusive license from Benuvia Operations, LLC Regulatory 505B2 development pathway Ondansetron (Zofran) Treatment of nausea caused by many factors. Negates the need to swallow which is difficult while nauseated and may offer a more rapid onset.


CHROMOCELL THERAPEUTICS CORPORATION 23 CHROMOCELL THERAPEUTICS CORPORATION Board of Directors & Scientific Advisory Board CHROMOCELL THERAPEUTICS CORPORATION 24 Board of Directors Todd Davis | Chairman Mr . Davis is CEO and a member of the Board of Directors of Ligand Pharmaceuticals and has nearly 30 years of experience in biopharmaceutical and life sciences operations and investing . He has been involved in over $ 3 billion of healthcare financings including growth equity, public equity turnarounds, structured debt and royalty acquisitions . He has led, structured and closed more than 40 intellectual property licenses, as well as royalty and hybrid royalty - debt transactions . Mr . Davis is a navy veteran and holds a B . S . from the U . S . Naval Academy and an M . B . A . from Harvard University . Ezra Friedberg Mr . Friedberg has served as a member of our Board since May 2021 . Ezra is a seasoned investor with more than twenty years of investing experience in both public and private companies . He invests actively in the biotech space and has served on the board of directors of Humanigen (HGEN), a clinical - stage biopharmaceutical company which develops monoclonal antibodies . Mr . Friedberg is a graduate of Johns Hopkins University . Dr. Richard Malamut Dr . Malamut is currently CMO at MedinCell Inc . He has extensive experience focusing on early clinical development in Neurology, Psychiatry and Analgesia at Collegium Pharmaceuticals, Braeburn Pharmaceuticals, Teva, Bristol - Myers Squibb and AstraZeneca . Dr . Malamut earned his medical degree from Hahnemann University and completed both a residency in Neurology and a fellowship in Neuromuscular disease . He worked as a board - certified neurologist and has more than 50 publications in the fields of pain medicine, neuromuscular disease, autonomic disease, and neurodegenerative disease . Chia - Lin Simmons Ms . Simmons is the CEO of LogicMark , Inc . (Nasdaq : LGMK), the former CEO at LookyLoo and a former executive at Google, Harman International and Amazon . She is a current Board Member of New Energy Nexus, an international NGO that support clean energy entrepreneurs . Ms . Simmons graduated Magna cum Laude and Phi Beta Kappa from U . C . San Diego . She received her MBA from Cornell University, where she was a Park Leadership Fellow and her JD from George Mason University School of Law .

CHROMOCELL THERAPEUTICS CORPORATION 25 Scientific Advisory Board (“SAB”) Stephen G. Waxman | MD, PhD, Yale School of Medicine, Chairman • Bridget Marie Flaherty Professor of Neurology, Neuroscience, and Pharmacology • Chair, Department of Neurology ( 1986 - 2009 ), Yale University School of Medicine • Director, Center for Neuroscience & Regeneration Research, Yale Robert H. Dworkin | PhD, University of Rochester Medical Center • Adjunct Senior Scientist, Department of Anesthesiology, Critical Care & Pain Management, Hospital for Special Surgery Research Institute, New York, NY • Director, Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks, and Pediatric Anesthesia Safety Initiative public - private partnership with the FDA • Editorial Boards : Canadian Journal of Pain, Journal of Pain