UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended: December 31, 2022
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _____to _____
Commission File Number: 001-37606
ANAVEX LIFE SCIENCES CORP.
(Exact name of registrant as specified in its charter)
| Nevada | 98-0608404 |
| (State or other jurisdiction of | (IRS Employer |
| incorporation or organization) | Identification No.) |
630 5th Avenue, 20th Floor, New York, NY USA 10111
(Address of principal executive offices) (Zip Code)
1-844-689-3939
(Registrant’s telephone number, including area code)
Securities Registered Pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol | Name of Each Exchange on Which Registered | ||
| Common Stock Par Value $0.001 | AVXL | NASDAQ Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
☒ Yes ☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☒ | Accelerated filer | ☐ | |
| Non-accelerated filer | ☐ | Smaller reporting company | ☐ | |
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act
☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
☐ Yes ☒ No
Indicate the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date: 78,032,135 shares of common stock outstanding as of February 7, 2023.
TABLE OF CONTENTS
PART I – FINANCIAL INFORMATION F-1PART II – OTHER INFORMATION 18SIGNATURES 21
PART I – FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS
Anavex Life Sciences Corp.
Interim Condensed Consolidated Financial Statements Interim Condensed Consolidated Balance Sheets
December 31, 2022
(Unaudited)
F-
Anavex Life Sciences Corp.
As at December 31, 2022 and September 30, 2022
(Unaudited)
| December 31, | September 30, | |||||||
| 2022 | 2022 | |||||||
| (Unaudited) | ||||||||
| Assets | ||||||||
| Current | ||||||||
| Cash and cash equivalents | $ | 143,621,848 | $ | 149,157,861 | ||||
| Incentive and tax receivables | 4,094,431 | 3,192,580 | ||||||
| Prepaid expenses and other current assets | 655,643 | 354,162 | ||||||
| Total Assets | $ | 148,371,922 | $ | 152,704,603 | ||||
| Liabilities and Stockholders' Equity | ||||||||
| Current Liabilities | ||||||||
| Accounts payable | $ | 5,288,261 | $ | 3,824,777 | ||||
| Accrued liabilities - Note 4 | 7,042,822 | 5,944,953 | ||||||
| Deferred grant income - Note 3 | 916,763 | 443,831 | ||||||
| Total Liabilities | 13,247,846 | 10,213,561 | ||||||
| Commitments and Contingencies - Note 6 | ||||||||
| Capital stock | ||||||||
| Authorized: | ||||||||
| 10,000,000 preferred stock, par value $0.001 per share | — | — | ||||||
| 200,000,000 common stock, par value $0.001 per share | ||||||||
| Issued and outstanding: | ||||||||
| 78,032,135 common shares (September 30, 2022 - 77,942,815) | 78,033 | 77,944 | ||||||
| Additional paid-in capital | 393,581,544 | 387,976,881 | ||||||
| Accumulated deficit | (258,535,501 | ) | (245,563,783 | ) | ||||
| Total Stockholders' Equity | 135,124,076 | 142,491,042 | ||||||
| Total Liabilities and Stockholders' Equity | $ | 148,371,922 | $ | 152,704,603 | ||||
See Accompanying Notes to Condensed Consolidated Interim Financial Statements
F-
Anavex Life Sciences Corp.
Interim Condensed Consolidated Statement of Operations
For the three months ended December 31, 2022 and 2021
(Unaudited)
| 2022 | 2021 | |||||||
| Operating expenses | ||||||||
| General and administrative | $ | 3,317,036 | $ | 3,066,951 | ||||
| Research and development | 12,066,919 | 8,656,439 | ||||||
| Total operating expenses | 15,383,955 | 11,723,390 | ||||||
| Operating loss | (15,383,955 | ) | (11,723,390 | ) | ||||
| Other income | ||||||||
| Grant income | 25,000 | — | ||||||
| Research and development incentive income | 733,590 | 810,730 | ||||||
| Interest income, net | 1,267,618 | 4,910 | ||||||
| Foreign exchange gain, net | 365,983 | 55,363 | ||||||
| Total other income, net | 2,392,191 | 871,003 | ||||||
| Net loss before provision for income taxes | (12,991,764 | ) | (10,852,387 | ) | ||||
| Income tax recovery (expense), current | 20,046 | (29,980 | ) | |||||
| Net loss and comprehensive loss | $ | (12,971,718 | ) | $ | (10,882,367 | ) | ||
| Net Loss per share | ||||||||
| Basic and diluted | $ | (0.17 | ) | $ | (0.14 | ) | ||
| Weighted average number of shares outstanding | ||||||||
| Basic and diluted | 77,977,112 | 75,997,738 | ||||||
See Accompanying Notes to Condensed Consolidated Interim Financial Statements
F-
Anavex Life Sciences Corp.
Interim Condensed Consolidated Statement of Changes in Stockholders' Equity
For the three months ended December 31, 2022 and 2021
| Common Stock | ||||||||||||||||||||
| Additional | ||||||||||||||||||||
| Paid-in | Accumulated | |||||||||||||||||||
| Shares | Par Value | Capital | Deficit | Total | ||||||||||||||||
| Balance, October 1, 2022 | 77,942,815 | $ | 77,944 | $ | 387,976,881 | $ | (245,563,783 | ) | $ | 142,491,042 | ||||||||||
| Shares issued pursuant to exercise of stock options | 89,320 | 89 | 258,155 | — | 258,244 | |||||||||||||||
| Share based compensation | — | — | 5,346,508 | — | 5,346,508 | |||||||||||||||
| Net loss | — | — | — | (12,971,718 | ) | (12,971,718 | ) | |||||||||||||
| Balance, December 31, 2022 | 78,032,135 | $ | 78,033 | $ | 393,581,544 | $ | (258,535,501 | ) | $ | 135,124,076 | ||||||||||
| Balance, October 1, 2021 | 75,918,465 | $ | 75,920 | $ | 348,328,048 | $ | (197,585,864 | ) | $ | 150,818,104 | ||||||||||
| Shares issued pursuant to exercise of stock options | 137,134 | 137 | 373,360 | — | 373,497 | |||||||||||||||
| Shares issued under Sales Agreement, net of share issuance costs | 99,588 | 99 | 2,149,602 | — | 2,149,701 | |||||||||||||||
| Share based compensation | — | — | 3,908,771 | — | 3,908,771 | |||||||||||||||
| Net loss | — | — | — | (10,882,367 | ) | (10,882,367 | ) | |||||||||||||
| Balance, December 31, 2021 | 76,155,187 | $ | 76,156 | $ | 354,759,781 | $ | (208,468,231 | ) | $ | 146,367,706 | ||||||||||
See Accompanying Notes to Condensed Consolidated Interim Financial Statements
F-
Anavex Life Sciences Corp.
Interim Condensed Consolidated Statement of Cash Flows
For the three months ended December 31, 2022 and 2021
(Unaudited)
| 2022 | 2021 | |||||||
| Cash Flows used in Operating Activities | ||||||||
| Net loss | $ | (12,971,718 | ) | $ | (10,882,367 | ) | ||
| Adjustments to reconcile net loss to net cash used in operations: | ||||||||
| Stock-based compensation | 5,346,508 | 3,908,771 | ||||||
| Changes in working capital balances related to operations: | ||||||||
| Incentive and tax receivables | (901,851 | ) | 3,764,678 | |||||
| Prepaid expenses and deposits | (301,481 | ) | (22,311 | ) | ||||
| Accounts payable | 1,463,484 | (104,992 | ) | |||||
| Accrued liabilities | 1,097,869 | (164,734 | ) | |||||
| Deferred grant income | 472,932 | — | ||||||
| Net cash used in operating activities | (5,794,257 | ) | (3,500,955 | ) | ||||
| Cash Flows provided by Financing Activities | ||||||||
| Issuance of common shares | — | 2,312,785 | ||||||
| Share issue costs | — | (146,717 | ) | |||||
| Proceeds from exercise of stock options | 258,244 | 373,497 | ||||||
| Net cash provided by financing activities | 258,244 | 2,539,565 | ||||||
| Decrease in cash and cash equivalents during the period | (5,536,013 | ) | (961,390 | ) | ||||
| Cash and cash equivalents, beginning of period | 149,157,861 | 152,107,745 | ||||||
| Cash and cash equivalents, end of period | $ | 143,621,848 | $ | 151,146,355 | ||||
| Supplemental Cash Flow Information | ||||||||
| Cash paid for state and local minimum income taxes | $ | 50,077 | $ | — | ||||
See Accompanying Notes to Condensed Consolidated Interim Financial Statements
F-
Anavex Life Sciences Corp.
Notes to the Condensed Consolidated Interim Financial Statements
December 31, 2022
(Unaudited)
Note 1 Business Description
Business
Anavex Life Sciences Corp. (“Anavex” or the “Company”) is a clinical stage biopharmaceutical company engaged in the development of differentiated therapeutics by applying precision medicine to central nervous system (“CNS”) diseases with high unmet need. Anavex analyzes genomic data from clinical trials to identify biomarkers, which are used in the analysis of its clinical trials for the treatment of neurodegenerative and neurodevelopmental diseases.
The Company’s lead compound ANAVEX®2-73 is being developed to treat Alzheimer’s disease, Parkinson’s disease and potentially other central nervous system diseases, including rare diseases, such as Rett syndrome, a rare severe neurological monogenic disorder caused by mutations in the X-linked gene, methyl-CpG-binding protein 2 (“MECP2”).
Note 2 Basis of Presentation
Basis of Presentation
These accompanying unaudited interim condensed consolidated financial statements have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) and accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim reporting. Accordingly, certain information and note disclosures normally included in the annual financial statements in accordance with U.S. GAAP have been condensed or omitted pursuant to such rules and regulations. In the opinion of management, the disclosures are adequate to make the information presented not misleading.
These accompanying unaudited interim condensed consolidated financial statements reflect all adjustments, consisting of normal recurring adjustments, which in the opinion of management are necessary for fair presentation of the information contained herein. The consolidated balance sheet as of September 30, 2022 was derived from the audited annual financial statements but does not include all disclosures required by U.S. GAAP. The accompanying unaudited interim condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto included in the Company’s annual report on Form 10-K for the year ended September 30, 2022 filed with the SEC on November 28, 2022. The Company follows the same accounting policies in the preparation of interim reports.
Operating results for the three months ended December 31, 2022 are not necessarily indicative of the results that may be expected for the year ending September 30, 2023.
Liquidity
All of the Company’s potential drug compounds are in the clinical development stage and the Company cannot be certain that its research and development efforts will be successful or, if successful, that its potential drug compounds will ever be approved for sales to pharmaceutical companies or generate commercial revenues. To date, we have not generated any revenues from our operations. The Company expects the business to continue to experience negative cash flows from operations for the foreseeable future and cannot predict when, if ever, our business might become profitable.
F-
Anavex Life Sciences Corp.
Notes to the Interim Condensed Consolidated Financial Statements
December 31, 2022
(Unaudited)
Management believes that the current working capital position will be sufficient to meet the Company’s working capital requirements beyond the next 12 months after the date that these interim condensed consolidated financial statements are issued. The process of drug development can be costly, and the timing and outcomes of clinical trials are uncertain. The assumptions upon which the Company has based its estimates are routinely evaluated and may be subject to change. The actual amount of the Company’s expenditures will vary depending upon a number of factors including but not limited to the design, timing and duration of future clinical trials, the progress of the Company’s research and development programs and the level of financial resources available. The Company has the ability to adjust its operating plan spending levels based on the timing of future clinical trials.
Other than our rights related to the Sales Agreement (as defined below in Note 5), there can be no assurance that additional financing will be available to us when needed or, if available, that it can be obtained on commercially reasonable terms. If the Company is not able to obtain the additional financing on a timely basis, if and when it is needed, it will be forced to delay or scale down some or all of its research and development activities.
Use of Estimates
The preparation of financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses in the reporting period. The Company regularly evaluates estimates and assumptions related to accounting for research and development costs, incentive income receivable, valuation and recoverability of deferred tax assets, stock-based compensation, and loss contingencies. The Company bases its estimates and assumptions on current facts, historical experience, and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future results of operations will be affected.
The global pandemic resulting from the disease known as COVID-19, caused by a novel strain of coronavirus, did not have a material impact on the Company’s result of operations or financial condition for the quarter ended December 31, 2022. However, the future course of the pandemic could have adverse effects in the U.S and global economies and thus negatively impact our business and financial results.
Principles of Consolidation
These consolidated financial statements include the accounts of Anavex Life Sciences Corp. and its wholly-owned subsidiaries, Anavex Australia Pty Limited (“Anavex Australia”), a company incorporated under the laws of Australia, Anavex Germany GmbH, a company incorporated under the laws of Germany, and Anavex Canada Ltd., a company incorporated under the laws of the Province of Ontario, Canada. All inter-company transactions and balances have been eliminated.
F-
Anavex Life Sciences Corp.
Notes to the Interim Condensed Consolidated Financial Statements
December 31, 2022
(Unaudited)
Fair Value Measurements
The fair value hierarchy under GAAP is based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which are the following:
Level 1 - quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 - observable inputs other than Level 1, quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, and model-derived prices whose inputs are observable or whose significant value drivers are observable; and
Level 3 - assets and liabilities whose significant value drivers are unobservable by little or no market activity and that are significant to the fair value of the assets or liabilities.
At December 31, 2022 and September 30, 2022, the Company did not have any Level 3 assets or liabilities.
Basic income/(loss) per common share is computed by dividing net income/(loss) available to common stockholders by the weighted average number of common shares outstanding during the period. Diluted income/(loss) per common share is computed by dividing net income/(loss) available to common stockholders by the sum of (1) the weighted-average number of common shares outstanding during the period, (2) the dilutive effect of the assumed exercise of options and warrants using the treasury stock method and (3) the dilutive effect of other potentially dilutive securities. For purposes of the diluted net loss per share calculation, options and warrants are potentially dilutive securities and are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive.
As of December 31, 2022 loss per share excludes 13,525,296 (December 31, 2021: 11,403,769) potentially dilutive common shares related to outstanding options and warrants, as their effect was anti-dilutive.
Recently Adopted Accounting Pronouncements
In November 2021, the FASB issued ASU 2021-10, “Government Assistance (Topic 832): Disclosures by Business Entities about Government Assistance” (“ASU 2021-10”). ASU 2021-10 increases the disclosure requirements for annual reporting periods relating to material government assistance transactions on the entity’s financial statements and any significant terms and conditions of the agreements including commitments and contingencies. The new standard was effective for the Company on October 1, 2022 but only impacts annual financial statement footnote disclosures. The adoption of ASU 2021-10 is expected to impact the disclosures related to the research and development incentive income that the Company receives from the Australian Tax Office (“ATO”) for its clinical trials in Australia.
Note 3 Other Income
Grant Income
As of December 31, 2022, the Company had received $995,862 in a research grant awarded by the Michael J. Fox Foundation for Parkinson’s Research. The grant will be used to fund a clinical trial of the Company’s lead compound, ANAVEX®2-73 related to Parkinson’s disease. Of the total, $497,931 was received during the three months ended December 31, 2022 and $497,931 was received during the year ended September 30, 2021.
F-
Anavex Life Sciences Corp.
Notes to the Interim Condensed Consolidated Financial Statements
December 31, 2022
(Unaudited)
The grant income has been deferred when received and is being amortized to other income as the related research and development expenditures are incurred. During the three months ended December 31, 2022, the Company recognized $25,000 (2021: $0) of this grant on its statements of operations as grant income. At December 31, 2022, an amount of $916,763 (September 30, 2022: $443,831) of this grant is recorded as deferred grant income, representing the amount of this grant which has not yet been amortized to other income. The Company will recognize this income on its statement of operations as the relating expenditures are incurred to offset the income.
Research and development incentive income
Research and development incentive income represents the income earned by Anavex Australia, of the Australian research and development tax incentive credit (the “Tax Incentive Credit”).
During the three months ended December 31, 2022 the Company recorded research and development incentive income of $733,590 (AUD 1,116,618) (2021: $810,730 (AUD 1,116,362)) in respect of the Tax Incentive Credit for eligible research and development expenses incurred during the period.
The Company evaluates its eligibility under the tax incentive program as of each balance sheet date based on the most current and relevant data available. Although the Company believes that it complies with all the relevant conditions of the program, the Company may be subject to pre-issue review or audit by the ATO and, the ATO may have different interpretations of certain eligibility requirements. Currently, the Company’s tax incentive claims from 2018 to 2022 are open to potential review or audit by the ATO.
Note 4 Accrued Liabilities
The principal components of accrued liabilities consists of:
| Schedule of Accrued Liabilities | ||||||||
| December 31, | September 31, | |||||||
| 2022 | 2022 | |||||||
| Accrued clinical site and patient visits costs | $ | 2,212,464 | $ | 2,031,105 | ||||
| Accrued compensation and benefits | 1,249,205 | 1,297,337 | ||||||
| Fixed contract accruals | 1,076,630 | 417,414 | ||||||
| All other accrued liabilities | 2,504,523 | 2,199,097 | ||||||
| Total accrued liabilities | $ | 7,042,822 | $ | 5,944,953 | ||||
Note 5 Equity Offerings
Common Stock
Common shares are voting and are entitled to dividends as declared at the discretion of the Board of Directors (the “Board”).
Preferred Stock
The Company’s Board has the authority to issue preferred stock in one or more series and to fix the rights, preferences, privileges, restrictions and the number of shares constituting any series of the designation of the series.
F-
Anavex Life Sciences Corp.
Notes to the Interim Condensed Consolidated Financial Statements
December 31, 2022
(Unaudited)
Sales Agreement
The Company entered into a Controlled Equity Offering Sales Agreement on July 6, 2018, which was amended and restated on May 1, 2020 (the “Sales Agreement”) with Cantor Fitzgerald & Co. and SVB Leerink LLC (together the “Sales Agents”), pursuant to which the Company may offer and sell shares of common stock registered under an effective registration statement from time to time through the Sales Agents (the “Offering”).
Upon delivery of a placement notice based on the Company’s instructions and subject to the terms and conditions of the Sales Agreement, the Sales Agents may sell the Shares by methods deemed to be an “at the market offering” offering, in negotiated transactions at market prices prevailing at the time of sale or at prices related to such prevailing market prices, or by any other method permitted by law, including negotiated transactions, subject to the prior written consent of the Company. The Company is not obligated to make any sales of Shares under the Sales Agreement. The Company or Sales Agents may suspend or terminate the offering of Shares upon notice to the other party, subject to certain conditions. The Sales Agents will act as agent on a commercially reasonable efforts basis consistent with their normal trading and sales practices and applicable state and federal law, rules and regulations and the rules of Nasdaq.
The Company has agreed to pay the Sales Agents commissions for their services of up to 3.0% of the gross proceeds from the sale of the Shares pursuant to the Sales Agreement. The Company also agreed to provide the Sales Agents with customary indemnification and contribution rights. During the three months ended December 31, 2022, no shares were sold pursuant to the Offering. During the three months ended December 31, 2021, 99,588 shares were sold for gross proceeds of $2,312,785 (net proceeds of $2,149,701). At December 31, 2022, an amount of $142,407,882 (September 30, 2022: $142,407,882) was registered pursuant to an effective registration statement and remained available to be sold under the Sales Agreement.
Note 6 Commitments and Contingencies
Leases
During the three months ended December 31, 2022, the Company incurred office lease expense of $30,070 (2021: $4,845).
Employee 401(k) Benefit Plan
The Company has a defined-contribution savings plan under Section 401(k) of the Internal Revenue Code. The plan covers all United States based employees. United States based employees eligible to participate in the plan may contribute up to the current statutory limits under the Internal Revenue Service regulations. The 401(k) plan permits the Company to make additional matching contributions on behalf of contributing employees. During the three months ended December 31, 2022, the Company made $43,646 (2021: $22,682) in matching contributions under the 401(k) plan.
Litigation
The Company is subject to claims and legal proceedings that arise in the ordinary course of business. Such matters are inherently uncertain, and there can be no guarantee that the outcome of any such matter will be decided favorably to the Company or that the resolution of any such matter will not have a material adverse effect upon the Company’s consolidated financial statements. The Company does not believe that any of such pending claims and legal proceedings will have a material adverse effect on its consolidated financial statements.
F-
Anavex Life Sciences Corp.
Notes to the Interim Condensed Consolidated Financial Statements
December 31, 2022
(Unaudited)
Share Purchase Warrants
At December 31, 2022 and September 30, 2022, the Company had 160,000 warrants outstanding at a weighted average exercise price of $3.72 as follows:
| Schedule of share purchase warrants outstanding | |||||||
| Number | Exercise Price | Expiry Date | |||||
| 150,000 | $ | 3.17 | May 6, 2024 | ||||
| 10,000 | $ | 12.00 | April 21, 2026 | ||||
| 160,000 | |||||||
Stock–based Compensation Plan
2015 Stock Option Plan
On September 18, 2015, the Company’s Board approved a 2015 Omnibus Incentive Plan (the “2015 Plan”), which provided for the grant of stock options and restricted stock awards to directors, officers, employees and consultants of the Company.
The maximum number of our common shares reserved for issue under the plan was 6,050,553 shares, subject to adjustment in the event of a change of the Company’s capitalization.
2019 Stock Option Plan
On January 15, 2019, the Board approved the 2019 Omnibus Incentive Plan (the “2019 Plan”), which provides for the grant of stock options and restricted stock awards to directors, officers, employees, consultants and advisors of the Company.
The maximum number of our common shares reserved for issue under the plan was 6,000,000 shares, subject to adjustment in the event of a change of the Company’s capitalization.
During the year ended September 30, 2022, 406,453 options previously available under the 2019 Plan and the 2015 Plan became available under the 2022 Plan (as defined below).
2022 Stock Option Plan
On March 25, 2022, the Board approved the 2022 Omnibus Incentive Plan (the “2022 Plan”). The 2022 Plan was approved by stockholders on May 24, 2022. Under the terms of the 2022 Plan, 10,000,000 additional shares of Common Stock will be available for issuance under the plan. Any awards outstanding under a previous stock option plan will remain subject to and be paid under such plan, and any shares subject to outstanding awards under a previous plan that subsequently cease to be subject to such awards (other than by reason of settlement of the awards in shares) will automatically become available for issuance under the 2022 Plan.
The 2022 Plan provides that it may be administered by the Board, or the Board may delegate such responsibility to a committee. The exercise price will be determined by the Board at the time of grant and shall be at least equal to the fair market value on such date. If the grantee is a 10% stockholder on the grant date, then the exercise price shall not be less than 110% of fair market value of the Company’s shares of common stock on the grant date. Stock options may be granted under the 2022 Plan for an exercise period of up to ten years from the date of grant of the option or such lesser periods as may be determined by the Board, subject to earlier termination in accordance with the terms of the 2022 Plan. At December 31, 2022 2,378,000 options had been issued under the 2022 Plan and 8,078,453 options were available for issue under the 2022 Plan.
F-
Anavex Life Sciences Corp.
Notes to the Interim Condensed Consolidated Financial Statements
December 31, 2022
(Unaudited)
A summary of the status of Company’s outstanding stock options is presented below:
| Schedule of outstanding stock purchase options | |||||||||||||||||
| Weighted | Weighted | ||||||||||||||||
| Average | Average | Aggregate | |||||||||||||||
| Number of | Exercise | Grant Date | intrinsic value | ||||||||||||||
| Shares | Price ($) | Fair Value ($) | ($) | ||||||||||||||
| Outstanding, September 30, 2021 | 11,330,903 | 5.74 | 140,132,451 | ||||||||||||||
| Granted | 2,358,000 | 10.13 | 7.07 | ||||||||||||||
| Forfeited | (118,750 | ) | 6.86 | 5.23 | |||||||||||||
| Exercised | (400,537 | ) | 2.52 | 1.88 | 4,201,015 | ||||||||||||
| Outstanding, September 30, 2022 | 13,169,616 | 6.61 | 62,267,309 | ||||||||||||||
| Granted | 325,000 | 12.43 | 8.17 | ||||||||||||||
| Forfeited | (40,000 | ) | 7.51 | 2.52 | |||||||||||||
| Exercised | (89,320 | ) | 2.89 | 2.44 | 591,993 | ||||||||||||
| Outstanding, December 31, 2022 | 13,365,296 | 6.78 | 50,676,617 | ||||||||||||||
| Exercisable, December 31, 2022 | 8,795,293 | 4.51 | 45,930,627 | ||||||||||||||
The following summarizes information about stock options at December 31, 2022 by a range of exercise prices:
| Schedule Of Share-based Payment Arrangement, Option, Activity | Weighted | |||||||||||||||||||||||||
| Weighted | average | |||||||||||||||||||||||||
| average | exercise | |||||||||||||||||||||||||
| Number of | remaining | Weighted | price | |||||||||||||||||||||||
| Range of exercise prices | outstanding | contractual | average | Number of | options | |||||||||||||||||||||
| From | To | options | life (in years) | exercise price | vested options | vested | ||||||||||||||||||||
| $ | 0.92 | 2.96 | 3,850,742 | 4.86 | 2.29 | 3,844,908 | 2.29 | |||||||||||||||||||
| $ | 3.15 | 4.80 | 2,042,500 | 5.09 | 3.30 | 2,032,500 | 3.29 | |||||||||||||||||||
| $ | 5.04 | 8.98 | 3,927,054 | 5.80 | 6.29 | 2,374,554 | 6.31 | |||||||||||||||||||
| $ | 9.20 | 13.01 | 1,968,000 | 9.04 | 10.73 | 154,581 | 12.27 | |||||||||||||||||||
| $ | 13.22 | 24.58 | 1,577,000 | 8.24 | 18.51 | 388,750 | 18.80 | |||||||||||||||||||
| 13,365,296 | 8,795,293 | |||||||||||||||||||||||||
The weighted average grant date fair value of options vested during the three months ended December 31, 2022 was $4.40 (2021: $2.85). At December 31, 2022, the weighted average contractual life of options outstanding was 6.18 years (September 30, 2022: 6.40 years) and for options exercisable was 4.83 years (September 30, 2022: 5.1 years).
The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the quoted market price of the Company’s stock for the options that were in-the-money at December 31, 2022.
During the three months ended December 31, 2022, the Company recognized stock-based compensation expense of $5,346,508 (2021: $3,908,771) in connection with the issuance and vesting of stock options and warrants in exchange for services. These amounts have been included in general and administrative expenses and research and development expenses on the Company’s statement of operations as follows:
| Schedule of general and administrative expenses and research and development expenses | ||||||||
| 2022 | 2021 | |||||||
| General and administrative | $ | 1,742,524 | $ | 1,671,725 | ||||
| Research and development | 3,603,984 | 2,237,046 | ||||||
| Total stock-based compensation | $ | 5,346,508 | $ | 3,908,771 | ||||
F-
Anavex Life Sciences Corp.
Notes to the Interim Condensed Consolidated Financial Statements
December 31, 2022
(Unaudited)
An amount of approximately $16,340,941 in stock-based compensation is expected to be recorded over the remaining term of such options through fiscal 2025.
The fair value of each option award granted during the three months ended December 31, 2022 and 2021 is estimated on the date of grant using the Black Scholes option pricing model based on the following weighted average assumptions:
| Schedule of weighted average assumptions for fair value of each option award | ||||||||
| 2022 | 2021 | |||||||
| Risk-free interest rate | 4.07 | % | 1.37 | % | ||||
| Expected life of options (years) | 4.98 | 6.50 | ||||||
| Annualized volatility | 84.01 | % | 96.79 | % | ||||
| Dividend rate | 0.00 | % | 0.00 | % | ||||
The fair value of stock compensation charges recognized during the three months ended December 31, 2022 and 2021 was determined with reference to the quoted market price of the Company’s shares on the grant date.
Note 7 Subsequent Events
Subsequent to December 31, 2022, the Company entered into a Purchase Agreement (the “Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“LPC”), whereby the Company has the right, in its sole discretion, to sell to LPC up to $150,000,000 in value of shares of the Company’s common stock from time to time over a 36-month period pursuant to the terms of the Purchase Agreement.
F-
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
Forward-Looking Statements
This Quarterly Report on Form 10-Q includes forward-looking statements. All statements other than statements of historical facts contained in this Quarterly Report on Form 10-Q, including statements regarding our anticipated future clinical and regulatory milestone events, future financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. The words “believe,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “expect” “should,” “forecast,” “potential,” “predict”, “could,” “would,” “will,” “suggest,” “plan” and similar expressions, as they relate to us, are intended to identify forward-looking statements. Such forward-looking statements include, without limitation, statements regarding:
| ● | volatility in our stock price and in the markets in general; | |
| ● | our ability to successfully conduct preclinical studies and clinical trials for our product candidates; | |
| ● | our ability to raise additional capital on favorable terms and the impact of such activities on our stockholders and stock price; | |
| ● | the impact of the COVID-19 outbreak and its effect on us; | |
| ● | our ability to generate any revenue or to continue as a going concern; | |
| ● | our ability to execute our research and development plan on time and on budget; | |
| ● | our products candidates’ ability to demonstrate efficacy or an acceptable safety profile; | |
| ● | our ability to obtain the support of qualified scientific collaborators; | |
| ● | our ability, whether alone or with commercial partners, to successfully commercialize any of our product candidates that may be approved for sale; | |
| ● | our ability to identify and obtain additional product candidates; | |
| ● | our reliance on third parties in non-clinical studies and clinical trials; | |
| ● | our ability to defend against product liability claims; | |
| ● | our ability to safeguard against security breaches; | |
| ● | our ability to obtain and maintain sufficient intellectual property protection for our product candidates; | |
| ● | our ability to comply with our intellectual property licensing agreements; | |
| ● | our ability to defend against claims of intellectual property infringement; | |
| ● | our ability to comply with the maintenance requirements of the government patent agencies; | |
| ● | our ability to protect our intellectual property rights throughout the world; | |
| ● | competition; | |
| ● | the anticipated start dates, durations and completion dates of our ongoing and future clinical trials; | |
| ● | the anticipated designs of our future clinical trials; | |
| ● | our ability to attract and retain qualified employees; | |
| ● | the impact of Fast Track designation on receipt of actual FDA approval; | |
| ● | our anticipated future regulatory submissions and our ability to receive regulatory approvals to develop and market our product candidates, including any orphan drug or Fast Track designations; and | |
| ● | our anticipated future cash position and ability to obtain funding for our operations. |
We have based these forward-looking statements largely on our current expectations and projections about future events, including the responses we expect from the U.S. Food and Drug Administration, (“FDA”), and other regulatory authorities and financial trends that we believe may affect our financial condition, results of operations, business strategy, preclinical studies and clinical trials, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions including without limitation the risks described in “Risk Factors” in Part I, Item 1A of our Annual Report on Form 10-K filed with the Securities and Exchange Commission on November 28, 2022. These risks are not exhaustive. Other sections of this Quarterly Report on Form 10-Q include additional factors which could adversely impact our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. We cannot assure you that the events and circumstances reflected in the forward-looking statements will be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable laws including the securities laws of the United States, we assume no obligation to update or supplement forward-looking statements.
As used in this Quarterly Report on Form 10-Q, the terms “we,” “us,” “our,” “Company”, and “Anavex” mean Anavex Life Sciences Corp., unless the context clearly indicates otherwise.
Our Current Business
Anavex Life Sciences Corp. is a clinical stage biopharmaceutical company engaged in the development of differentiated therapeutics by applying precision medicine to central nervous system (“CNS”) diseases with high unmet need. We analyze genomic data from clinical trials to identify biomarkers, which we use in the analysis of our clinical trials.
Our lead product candidate, ANAVEX®2-73, is being developed to treat Alzheimer’s disease, Parkinson’s disease and potentially other central nervous system diseases, including rare diseases, such as Rett syndrome, a rare severe neurological monogenic disorder caused by mutations in the X-linked gene, methyl-CpG-binding protein 2 (“MECP2”).
We currently have two core programs and two seed programs. Our core programs are at various stages of clinical and preclinical development, in neurodegenerative and neurodevelopmental diseases.
The following table summarizes key information about our programs:
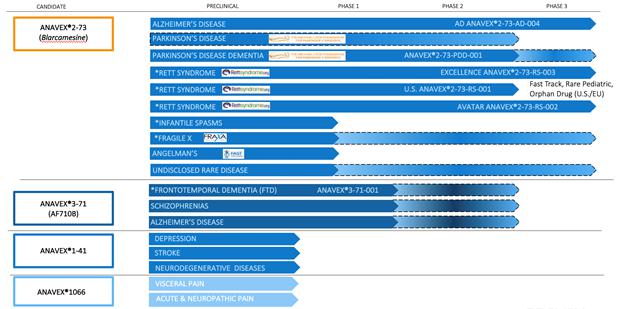
* = Orphan Drug Designation by the FDA; Dashed lines indicate planned clinical trials to-date
Anavex has a portfolio of compounds varying in sigma-1 receptor (SIGMAR1) binding activities. The SIGMAR1 gene encodes the SIGMAR1 protein, which is an intracellular chaperone protein with important roles in cellular communication. SIGMAR1 is also involved in transcriptional regulation at the nuclear envelope and restores homeostasis and stimulates recovery of cell function when activated. In order to validate the ability of our compounds to activate quantitatively the SIGMAR1, we performed, in collaboration with Stanford University, a quantitative Positron Emission Tomography (PET) imaging scan in mice, which demonstrated a dose-dependent ANAVEX®2-73 target engagement or receptor occupancy with SIGMAR1 in the brain.
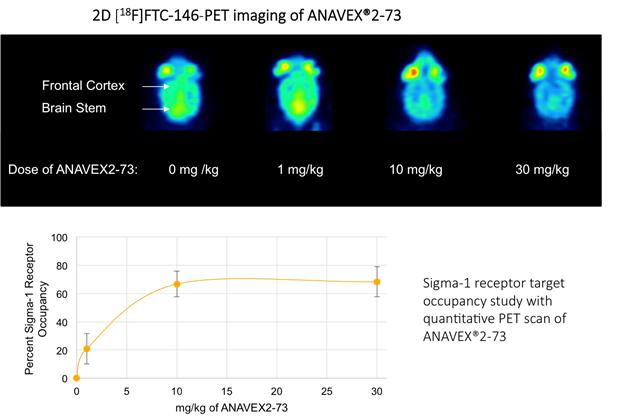
Source: Reyes S et al., Sci Rep. 2021 Aug 25; 11(1):17150
Cellular Homeostasis
Many diseases are possibly directly caused by chronic homeostatic imbalances or cellular stress of brain cells. In pediatric diseases, such as Rett syndrome or infantile spasms, the chronic cellular stress is possibly caused by the presence of a constant genetic mutation. In neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, chronic cellular stress is possibly caused by age-correlated buildup of cellular insult and hence chronic cellular stress. Specifically, defects in homeostasis of protein or ribonucleic acid (“RNA”) lead to the death of neurons and dysfunction of the nervous system. The spreading of protein aggregates resulting in a proteinopathy, a characteristic found in Alzheimer’s and Parkinson’s diseases that results from disorders of protein synthesis, trafficking, folding, processing or degradation in cells. The clearance of macromolecules in the brain is particularly susceptible to imbalances that result in aggregation and degeneration in nerve cells. For example, Alzheimer’s disease pathology is characterized by the presence of amyloid plaques, and neurofibrillary tangles, which are aggregates of hyperphosphorylated Tau protein that are a marker of other diseases known as tauopathies as well as inflammation of microglia. With the SIGMAR1 activation through SIGMAR1 agonists like ANAVEX®2-73, our approach is to restore cellular balance (i.e. homeostasis). Therapies that correct defects in cellular homeostasis might have the potential to halt or delay neurodevelopmental and neurodegenerative disease progression.
ANAVEX®2-73-specific Biomarkers
As part of some of our clinical trials, we have incorporated a genomic analysis to better understand potential populations for whom our clinical programs might benefit. In our clinical trials, a full genomic analysis of Alzheimer’s disease patients treated with ANAVEX®2-73 has helped us identify actionable genetic variants. A significant impact of the genomic biomarkers SIGMAR1, the direct target of ANAVEX®2-73 and COMT, a gene involved in memory function, on the drug response level was identified, leading to an early ANAVEX®2-73-specific biomarker hypothesis. We believe that excluding patients with SIGMAR1 identified biomarker variant (approximately 10%-20% of the population) in prospective studies would identify approximately 80%-90% patients that would display clinically significant improved functional and cognitive scores. The consistency between the identified DNA and RNA data related to ANAVEX®2-73, which are considered independent of Alzheimer’s disease pathology, as well as multiple endpoints and time-points, provides support for the potential precision medicine clinical development of ANAVEX®2-73 by using genetic biomarkers identified within the trial population itself to target patients who are most likely to respond to ANAVEX®2-73 treatment. We may in the future utilize such an approach in Alzheimer’s disease as well as indications like Parkinson’s disease dementia or Rett syndrome in which ANAVEX®2-73 is currently being studied.
Clinical Trials Overview
Alzheimer’s Disease
In November 2016, we completed a Phase 2a clinical trial, consisting of Part A and Part B, which lasted a total of 57 weeks, for ANAVEX®2-73 in mild-to-moderate Alzheimer’s patients. This open-label randomized trial in Australia met both primary and secondary endpoints and was designed to assess the safety and exploratory efficacy of ANAVEX®2-73 in 32 patients. ANAVEX®2-73 targets sigma-1 and muscarinic receptors, which have been shown in preclinical studies to reduce stress levels in the brain believed to restore cellular homeostasis and to reverse the pathological hallmarks observed in Alzheimer’s disease. In October 2017, we presented positive pharmacokinetic (“PK”) and pharmacodynamic (“PD”) data from the Phase 2a clinical trial, which established a concentration-effect relationship between ANAVEX®2-73 and trial measurements. These measures obtained from all patients who participated in the entire 57 weeks include exploratory cognitive and functional scores as well as biomarker signals of brain activity. Additionally, the clinical trial appeared to show that ANAVEX®2-73 activity was enhanced by its active metabolite (ANAVEX19-144), which also targets the SIGMAR1 receptor and has a half-life approximately twice as long as the parent molecule.
Two consecutive trial extensions for the Phase 2a trial have allowed participants who completed the 52-week Part B of the trial to continue taking ANAVEX®2-73, providing an opportunity to gather extended safety data for a cumulative time period of five years. In August 2020, patients completing these Phase 2a trial extensions were granted continued access to treatment with ANAVEX®2-73 through the Australian Government Department of Health – Therapeutic Goods Administration’s compassionate use Special Access Scheme.
A larger Phase 2b/3 double-blind, placebo-controlled trial of ANAVEX®2-73 in Alzheimer’s disease commenced in August 2018. The Phase 2b/3 trial enrolled 509 patients, which were treated with a convenient once-daily oral formulation of ANAVEX®2-73 for 48 weeks, randomized 1:1:1 to two different ANAVEX®2-73 doses or placebo. The trial took place at 52 sites across North America, Europe and Australia. Primary and secondary endpoints to assess safety and both cognitive and functional efficacy, were measured through the Alzheimer’s Disease Assessment Scale – Cognitive Subscale test (“ADAS-Cog”), Alzheimer’s Disease Cooperative Study – Activities of Daily Living (“ADCS-ADL”) and Clinical Dementia Rating – Sum of Boxes for cognition and function (“CDR-SB”). In addition to the primary endpoints, the ANAVEX®2-73 Phase 2b/3 trial design incorporated pre-specified statistical analyses related to potential genomic precision medicine biomarkers previously identified in the ANAVEX®2-73 Phase 2a clinical trial. The trial completed in mid-2022 and, in December 2022, the Company presented positive topline results from the Phase 2b/3 clinical trial.
ANAVEX®2-73 met the co-primary endpoints ADAS-Cog and ADCS-ADL and key secondary endpoint CDR-SB. ANAVEX®2-73 treatment slowed decline of cognition and function in patients with early Alzheimer’s disease over 48 weeks. Patients treated with ANAVEX®2-73 had 1.84 times higher odds, or likelihood, to improve cognitively compared to placebo, with a ADAS-Cog score threshold change of -0.5 points or better [Odds Ratio = 1.84 (p = 0.015)]. At clinically significant levels of improvement in function (ADCS-ADL score threshold change of +3.5 points or better), patients treated with ANAVEX®2-73 had 2.67 times higher odds, or likelihood, to improve function compared to placebo [Odds Ratio = 2.67 (p = 0.0255)]. Additionally, treatment with ANAVEX®2-73 reduced cognitive decline at end of treatment, measured with the ADAS-Cog, as compared to placebo, by 45%, representing a treatment difference in mean score change of -1.85 points (p=0.033). Compared to placebo, ANAVEX®2-73 reduced clinical decline of cognition and function by 27% with mean score difference of -0.42 points (p=0.040) as measured by the CDR-SB. ANAVEX®2-73 was generally safe and well tolerated. All statistical analyses were performed by outside consultancy companies.
In October 2019, we initiated a long-term open label extension study of ANAVEX®2-73, entitled the ATTENTION-AD trial, for patients who have completed the 48-week Phase 2b/3 placebo-controlled trial referenced above. This trial extension for an additional two years gives patients the opportunity to continue their treatment.
Rett Syndrome
In February 2016, we presented positive preclinical data for ANAVEX®2-73 in Rett syndrome, a rare neurodevelopmental disease. The data demonstrated dose related and significant improvements in an array of behavioral and gait paradigms in a mouse model with an MECP2-null mutation that causes neurological symptoms that mimic Rett syndrome. The study was funded by the International Rett Syndrome Foundation (“Rettsyndrome.org”). In January 2017, we were awarded a financial grant from Rettsyndrome.org of a minimum of $0.6 million to cover some of the costs of a multicenter Phase 2 clinical trial of ANAVEX®2-73 for the treatment of Rett syndrome. This award was received in quarterly instalments which commenced during fiscal 2018.
In March 2019, we commenced the first Phase 2 clinical trial in a planned Rett syndrome program of ANAVEX®2-73 for the treatment of Rett syndrome. The clinical trials are being conducted in a range of patient age demographics and geographic regions, utilizing an oral liquid once-daily formulation of ANAVEX®2-73.
The first Phase 2 trial, (ANAVEX®2-73-RS-001), which took place in the United States, was completed in December 2020. This trial was a randomized double-blind, placebo-controlled safety, tolerability, PK and efficacy trial of oral liquid ANAVEX®2-73 formulation in 25 adult female patients with Rett syndrome over a 7-week treatment period including ANAVEX®2-73-specific genomic precision medicine biomarkers. The primary endpoint of the trial was safety. The dosing of 5 mg ANAVEX®2-73 was well-tolerated and demonstrated dose-proportional PK. All secondary efficacy endpoints of the trial showed statistically significant and clinically meaningful response in the Rett Syndrome Behaviour Questionnaire (“RSBQ”) response, when compared to placebo, in the intent to treat (“ITT”) cohort (all participants, p = 0.011). 66.7% of ANAVEX®2-73 treated subjects showed a statistically significant improvement in RSBQ response as compared to 10% of the subjects on placebo in the ITT cohort (all participants, p = 0.011). ANAVEX®2-73 treatment resulted in a sustained improvement in Clinical Global Impression Improvement (CGI-I) response throughout the 7-week clinical trial, when compared to placebo in the ITT cohort (all participants, p = 0.014). Consistent with previous ANAVEX®2-73 clinical trials, patients carrying the common form of the SIGMAR1 gene treated with ANAVEX®2-73 experienced stronger improvements in the prespecified efficacy endpoints.
The second, international trial of ANAVEX®2-73 for the treatment of Rett syndrome, called the AVATAR trial, commenced in June 2019. This trial took place in Australia and the United Kingdom using a higher dose than the U.S. based Phase 2 trial for Rett syndrome. The trial was a Phase 3 randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of ANAVEX®2-73 in 33 adult patients over a 7-week treatment period including ANAVEX®2-73 specific precision medicine biomarkers. Based upon the input from the successful U.S. Phase 2 Rett syndrome trial (ANAVEX®2-73-RS-001), we updated the endpoints for the AVATAR trial (ANAVEX®2-73-RS-002) to appropriately assess the clinically meaningful outcome following International Conference on Harmonization (ICH) guidelines. These updates were approved by the respective regulatory authorities in the U.K. and in Australia, respectively, where the AVATAR trial was conducted.
The data from the AVATAR trial was released in February 2022. The clinical trial met all primary and secondary efficacy and safety endpoints, with consistent improvements in primary efficacy endpoint, RSBQ response (p = 0.037), and secondary efficacy endpoints, ADAMS (p = 0.010) and CGI-I (p = 0.037) response. Efficacy endpoints demonstrated statistically significant and clinically meaningful reductions in Rett syndrome symptoms. Convenient once daily oral liquid doses of up to 30 mg of ANAVEX®2-73 were also well tolerated with good medication compliance. All patients who participated in the trial were eligible to receive ANAVEX®2-73 under a voluntary open label extension protocol.
In July 2020, we commenced the third trial of ANAVEX®2-73 for the treatment of Rett syndrome, called the EXCELLENCE trial. This Phase 2/3 trial in pediatric patients with Rett syndrome includes trial sites in Australia, the United Kingdom and Canada, and will evaluate the safety and efficacy of ANAVEX®2-73 in approximately 84 pediatric patients, aged 5 to 18, over a 12-week treatment period incorporating ANAVEX®2-73 specific precision medicine biomarkers. This trial completed enrollment in February 2023, exceeding the original enrollment target and topline results are expected in the second half of 2023. All patients who participate in the trial will be eligible to receive ANAVEX®2-73 under a voluntary open label extension protocol, which is currently ongoing.
Parkinson’s Disease
In September 2016, we presented positive preclinical data for ANAVEX®2-73 in an animal model of Parkinson’s disease, which demonstrated significant improvements on behavioral, histopathological, and neuroinflammatory endpoints. The study was funded by the Michael J. Fox Foundation. Additional data announced in October 2017 indicated that ANAVEX®2-73 induced robust neurorestoration in experimental Parkinsonism. We believe the encouraging results we have gathered in this preclinical model, coupled with the favorable profile of this product candidate in the Alzheimer’s disease trial, support the notion that ANAVEX®2-73 has the potential to treat Parkinson’s disease dementia.
In October 2020, we completed a double-blind, randomized, placebo-controlled proof-of-concept Phase 2 trial with ANAVEX®2-73 in Parkinson’s disease dementia in Spain and Australia, to study the effect of the compound on both the cognitive and motor impairment of Parkinson’s disease. The Phase 2 trial enrolled approximately 132 patients for 14 weeks, randomized 1:1:1 to two different ANAVEX®2-73 doses, 30 mg and 50 mg, or placebo. The ANAVEX®2-73 Phase 2 Parkinson’s disease dementia trial design incorporated genomic precision medicine biomarkers identified in the ANAVEX®2-73 Phase 2a Alzheimer’s disease trial.
The trial demonstrated that ANAVEX®2-73 was safe and well tolerated in oral doses up to 50 mg once daily. The results showed clinically meaningful, dose-dependent, and statistically significant improvements in the Cognitive Drug Research (“CDR”) computerized assessment system analysis. Treatment with ANAVEX®2-73 also resulted in clinically meaningful improvements as measured by the global composite score of Parkinson’s disease symptom severity, MDS-Unified Parkinson’s Disease Rating Scale total score on top of standard of care including dopaminergic therapy, levodopa and other anti-PD medications after 14 weeks of treatment, suggesting ANAVEX®2-73’s potential capability of slowing and reversing symptoms that progress in Parkinson’s disease. In addition, the trial confirmed the precision medicine approach of targeting SIGMAR1 as a genetic biomarker in response to ANAVEX®2-73 may result in improved clinical outcomes.
In January 2021, we were awarded a research grant of $1.0 million from The Michael J. Fox Foundation for Parkinson’s Research to develop ANAVEX®2-73 for the treatment of Parkinson’s disease. The award will explore utilization of PET imaging biomarkers to enable measurement of target engagement and pathway activation of the SIGMAR1 with clinically relevant doses including in people with Parkinson’s disease.
Frontotemporal Dementia
In July 2020, we commenced the First-in-Human Phase 1 clinical trial of ANAVEX®3-71. ANAVEX®3-71 was previously granted orphan drug designation for the treatment of Frontotemporal Dementia (“FTD”) by the FDA. ANAVEX®3-71 is an orally administered small molecule targeting sigma-1 and M1 muscarinic receptors that is designed to be beneficial for neurodegenerative diseases. In preclinical studies, ANAVEX®3-71 demonstrated disease-modifying activity against the major hallmarks of Alzheimer’s disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid and tau pathologies, as well as beneficial effects on mitochondrial dysfunction and neuroinflammation.
The Phase 1 clinical trial was a prospective double-blind, randomized, placebo-controlled trial in Australia. A total of 36 healthy male and female subjects were included. Single escalating doses of ANAVEX®3-71 were administered in order to evaluate the safety, tolerability, and PK of ANAVEX®3-71 and the effects of food and gender on its PK in healthy volunteers.
The trial met its primary and secondary endpoints of safety, with no serious adverse events (“SAEs”) or dose-limiting toxicities observed. ANAVEX®3-71 was well tolerated in all cohorts receiving ANAVEX®3-71 in single doses ranging from 5 mg to 200 mg daily with no SAEs and no significant lab abnormalities in any subject. In the trial, ANAVEX®3-71 exhibited linear PK. Its pharmacokinetics was also dose proportional for doses up to 160 mg. Gender had no effect on the PK of the drug and food had no effect on the bioavailability of ANAVEX®3-71. The trial also met the secondary objective of characterizing the effect of ANAVEX®3-71 on electrocardiogram (“ECG”) parameters. There were no clinically significant ECG parameters throughout the trial. Participant QTcF measures were normal across all dose groups with no difference between ANAVEX®3-71 and placebo.
Based on these results, and ANAVEX®3-71’s pre-clinical profile, we intend to advance ANAVEX®3-71 into a biomarker-driven clinical development dementia program for the treatment of schizophrenia, FTD and Alzheimer’s disease, evaluating longitudinal effect of treatment with ANAVEX®3-71. We believe the results of these clinical trials and preclinical study could serve as a basis for advancing into respective registration trials in the U.S.
Our Pipeline
Our research and development pipeline includes ANAVEX®2-73 currently in three different clinical trial indications, and several other compounds in different stages of clinical and pre-clinical development.
Our proprietary SIGMACEPTOR™ Discovery Platform produced small molecule drug candidates with unique modes of action, based on our understanding of sigma receptors. Sigma receptors may be targets for therapeutics to combat many human diseases, both of neurodegenerative nature, including Alzheimer’s disease, as well as of neurodevelopmental nature, like Rett syndrome. When bound by the appropriate ligands, sigma receptors influence the functioning of multiple biochemical signals that are involved in the pathogenesis (origin or development) of disease. Multiple viruses including SARS-CoV-2 (COVID-19) induce cellular stress by intrinsic mitochondrial apoptosis and other related cellular processes, in order to ensure survival and replication. Hence, it is possible that SIGMAR1 could play a role in modulating the cellular response to viral infection and ameliorate pathogenesis.
Compounds that have been subjects of our research include the following:
ANAVEX®2-73 (blarcamesine)
We believe ANAVEX®2-73 may offer a disease-modifying approach in neurodegenerative and neurodevelopmental diseases by activation of SIGMAR1. ANAVEX®2-73 is being developed in an oral liquid once-daily formulation for rare diseases such as Rett syndrome as well as an oral once-daily capsule formulation for diseases such as Alzheimer’s disease.
In Rett syndrome, administration of ANAVEX®2-73 in liquid form resulted in both significant and dose related improvements in an array of behavioral paradigms in the MECP2 HET Rett syndrome disease model. In addition, in a further experiment sponsored by Rettsyndrome.org, ANAVEX®2-73 was evaluated in automatic visual response and respiration tests in 7-month old mice, an age at which advanced pathology is evident. Vehicle-treated MECP2 mice demonstrated fewer automatic visual responses than wild-type mice. Treatment with ANAVEX®2-73 for four weeks significantly increased the automatic visual response in the MECP2 Rett syndrome disease mice. Additionally, chronic oral dosing daily for 6.5 weeks of ANAVEX®2-73 starting at ~5.5 weeks of age was conducted in the MECP2 HET Rett syndrome disease mouse model assessed the different aspects of muscular coordination, balance, motor learning and muscular strengths, some of the core deficits observed in Rett syndrome. Administration of ANAVEX®2-73 resulted in both significant and dose related improvements in an array of these behavioral paradigms in the MECP2 HET Rett syndrome disease model.
In May 2016 and June 2016, the FDA granted Orphan Drug Designation to ANAVEX®2-73 for the treatment of Rett syndrome and infantile spasms, respectively. In November 2019, the FDA granted to ANAVEX®2-73 the Rare Pediatric Disease (RPD) designation for the treatment of Rett syndrome. The RPD designation is intended to encourage the development of treatments for rare pediatric diseases.
Further, in February 2020, the FDA granted Fast Track designation for the ANAVEX®2-73 clinical development program for the treatment of Rett syndrome. The FDA Fast Track program is designed to facilitate and expedite the development and review of new drugs to address unmet medical needs in the treatment of serious and life-threatening conditions.
For Parkinson’s disease, data demonstrates significant improvements and restoration of function in a disease modifying animal model of Parkinson’s disease. Significant improvements were seen on all measures tested: behavioral, histopathological, and neuroinflammatory endpoints. In October 2020, we completed a double-blind, randomized, placebo-controlled proof-of-concept Phase 2 trial with ANAVEX®2-73 in Parkinson’s disease dementia, to study the effect of the compound on both the cognitive and motor impairment of Parkinson’s disease. The Phase 2 trial enrolled approximately 132 patients for 14 weeks, randomized 1:1:1 to two different ANAVEX®2-73 doses, 30mg and 50mg, or placebo. The ANAVEX®2-73 Phase 2 Parkinson’s disease dementia trial design incorporated genomic precision medicine biomarkers identified in the ANAVEX®2-73 Phase 2a Alzheimer’s disease trial.
The trial demonstrated that ANAVEX®2-73 was safe and well tolerated in oral doses up to 50mg once daily. The results showed clinically meaningful, dose-dependent, and statistically significant improvements in the CDR computerized assessment system analysis. We anticipate conducting further clinical trials of ANAVEX®2-73 in Parkinson’s disease dementia after submitting the results of the trial to the FDA to obtain regulatory guidance.
In Alzheimer’s disease animal models, ANAVEX®2-73 has shown pharmacological, histological and behavioral evidence as a potential neuroprotective, anti-amnesic, anti-convulsive and anti-depressive therapeutic agent, due to its potent affinity to SIGMAR1 and moderate affinities to M1-4 type muscarinic receptors. In addition, ANAVEX®2-73 has shown a potential dual mechanism which may impact amyloid, tau pathology and inflammation. In a transgenic Alzheimer’s disease animal model Tg2576, ANAVEX®2-73 induced a statistically significant neuroprotective effect against the development of oxidative stress in the mouse brain, as well as significantly increased the expression of functional and synaptic plasticity markers that is apparently amyloid-beta independent. It also statistically alleviated the learning and memory deficits developed over time in the animals, regardless of sex, both in terms of spatial working memory and long-term spatial reference memory.
Based on the results of pre-clinical testing, we initiated and completed a Phase 1 single ascending dose (SAD) clinical trial of ANAVEX®2-73. In this Phase 1 SAD trial, the maximum tolerated single dose was defined per protocol as 55-60 mg. This dose is above the equivalent dose shown to have positive effects in mouse models of Alzheimer’s disease. There were no significant changes in laboratory or ECG parameters. ANAVEX®2-73 was well tolerated below the 55-60 mg dose with only mild adverse events in some subjects. Observed adverse events at doses above the maximum tolerated single dose included headache and dizziness, which were moderate in severity and reversible. These side effects are often seen with drugs that target CNS conditions, including Alzheimer’s disease.
In November 2016, we completed a Phase 2a clinical trial for ANAVEX®2-73, for the treatment of Alzheimer’s disease. The open-label randomized trial was designed to assess the safety and exploratory efficacy of ANAVEX®2-73 in 32 patients with mild-to-moderate Alzheimer’s disease. The Phase 2a trial met both primary and secondary objectives of the trial.
In July 2018, we presented the results of a genomic DNA and RNA evaluation of the participants in the Phase 2a clinical trial. More than 33,000 genes were analyzed using unbiased, data driven, machine learning, artificial intelligence (AI) system for analyzing DNA and RNA data in patients treated with ANAVEX®2-73. The analysis identified genetic variants that impacted response to ANAVEX®2-73, among them variants related to the SIGMAR1, the target for ANAVEX®2-73. Results showed that trial participants with the common SIGMAR1 wild type gene variant, which is estimated to be about 80% of the population worldwide, demonstrated improved cognitive (MMSE) and the functional (ADCS-ADL) scores. The results from this evaluation supported the continued evaluation of genomic information in subsequent clinical trials, since these signatures can now be applied to neurological indications tested in future clinical trials with ANAVEX®2-73 including Alzheimer’s disease, Parkinson’s disease dementia and Rett syndrome.
ANAVEX®2-73 data met prerequisite information in order to progress into a Phase 2b/3 placebo-controlled trial. On July 2, 2018, the Human Research Ethics Committee in Australia approved the initiation of our Phase 2b/3, double-blind, randomized, placebo-controlled 48-week safety and efficacy trial of ANAVEX®2-73 for the treatment of early Alzheimer’s disease. Clinical trial sites in Canada, the United Kingdom, the Netherlands and Germany were also added. This Phase 2b/3 trial design incorporates inclusion of genomic precision medicine biomarkers identified in the ANAVEX®2-73 Phase 2a trial.
The trial completed and, in December 2022, the Company presented positive topline results from the Phase 2b/3 clinical trial. ANAVEX®2-73 met the co-primary endpoints ADAS-Cog and ADCS-ADL and key secondary endpoint CDR-SB as further described above under Clinical Trials Overview – Alzheimer’s Disease.
We believe preclinical data from our studies also supports further research into the use of ANAVEX®2-73 as a potential platform drug for other neurodegenerative diseases beyond Alzheimer’s disease, Parkinson’s disease or Rett syndrome, more specifically, epilepsy, infantile spasms, Fragile X syndrome, Angelman syndrome, multiple sclerosis, and, more recently, tuberous sclerosis complex (TSC). ANAVEX®2-73 demonstrated significant improvements in all of these indications in the respective preclinical animal models.
In a preclinical study sponsored by the Foundation for Angelman Syndrome, ANAVEX®2-73 was assessed in a mouse model for the development of audiogenic seizures. The results indicated that ANAVEX®2-73 administration significantly reduced audiogenic-induced seizures in mice. In a study sponsored by FRAXA Research Foundation regarding Fragile X syndrome, data demonstrated that ANAVEX®2-73 restored hippocampal brain-derived neurotrophic factor (BDNF) expression to normal levels. BDNF under-expression has been observed in many neurodevelopmental and neurodegenerative pathologies. BDNF signaling promotes maturation of both excitatory and inhibitory synapses. ANAVEX®2-73 normalization of BDNF expression could be a contributing factor for the positive preclinical data observed in both neurodevelopmental and neurodegenerative disorders like Angelman and Fragile X syndromes.
In addition, preclinical data to-date also indicates that ANAVEX®2-73 has the potential to demonstrate protective effects of mitochondrial enzyme complexes during pathological conditions, which, if impaired, may play a role in the pathogenesis of neurodegenerative and neurodevelopmental diseases.
In addition, preclinical data on ANAVEX®2-73 related to multiple sclerosis indicates that ANAVEX®2-73 may promote remyelination in multiple sclerosis disease. Further, our data also demonstrates that ANAVEX®2-73 has the potential to provide protection for oligodendrocytes (“OL’s”) and oligodendrocyte precursor cells (“OPC’s”), as well as central nervous system neurons in addition to helping repair by increasing OPC proliferation and maturation in tissue culture.
In March 2018, we presented preclinical data of ANAVEX®2-73 in a genetic mouse model of tuberous sclerosis complex (“TSC”). TSC is a rare genetic disorder characterized by the growth of numerous benign tumors in many parts of the body with a high incidence of seizures. The preclinical data demonstrated that treatment with ANAVEX®2-73 significantly increased survival and reduced seizures in those mice.
ANAVEX®3-71
ANAVEX®3-71 is a clinical drug candidate with a novel mechanism of action via SIGMAR1 activation and M1 muscarinic allosteric modulation, which has been shown to enhance neuroprotection and cognition in Alzheimer’s disease models. ANAVEX®3-71 is a CNS-penetrable potential disease modifying treatment for cognitive impairments. We believe it is effective in very small doses against the major Alzheimer’s hallmarks in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid and tau pathologies, and also has beneficial effects on inflammation and mitochondrial dysfunctions. ANAVEX®3-71 indicates extensive therapeutic advantages in Alzheimer’s and other protein-aggregation-related diseases given its ability to enhance neuroprotection and cognition via SIGMAR1 activation and M1 muscarinic allosteric modulation.
A preclinical study examined the response of ANAVEX®3-71 in aged transgenic animal models and showed a significant reduction in the rate of cognitive deficit, amyloid beta pathology and inflammation with the administration of ANAVEX®3-71. In April 2016, the FDA granted Orphan Drug Designation to ANAVEX®3-71 for the treatment of FTD.
During pathological conditions ANAVEX®3-71 demonstrated the formation of new synapses between neurons (synaptogenesis) without causing an abnormal increase in the number of astrocytes. In neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease, synaptogenesis is believed to be impaired. Additional preclinical data presented also indicates that in addition to reducing oxidative stress, ANAVEX®3-71 has the potential to demonstrate protective effects of mitochondrial enzyme complexes during pathological conditions, which, if impaired, are believed to play a role in the pathogenesis of neurodegenerative and neurodevelopmental diseases.
In July 2020, we commenced the first Phase 1 clinical trial of ANAVEX®3-71. The trial took place in Australia and was a double-blind, randomized, placebo-controlled, Phase 1 trial to evaluate safety and tolerability, and PK of oral escalating doses of ANAVEX®3-71 including effects of food and gender in healthy volunteers. The trial met its primary and secondary endpoints of safety, respectively, with no serious adverse events (SAEs) or dose-limiting toxicities observed, as more fully described above under Clinical Trials Overview – Frontotemporal Dementia.
Based on these results, and ANAVEX®3-71 pre-clinical profile, the Company intends to advance ANAVEX®3-71 into a biomarker-driven clinical development dementia program for the treatment of schizophrenia, FTD and Alzheimer’s disease, evaluating longitudinal effect of treatment with ANAVEX®3-71. We believe the results of this clinical trial and preclinical study could serve as a basis for advancing into respective registration trials in the U.S.
ANAVEX®1-41
ANAVEX®1-41 is a sigma-1 agonist. Pre-clinical tests revealed significant neuroprotective benefits (i.e., protects nerve cells from degeneration or death) through the modulation of endoplasmic reticulum, mitochondrial and oxidative stress, which damages and impairs cell viability. In addition, in animal models, ANAVEX®1-41 prevented the expression of caspase-3, an enzyme that plays a key role in apoptosis (programmed cell death) and loss of cells in the hippocampus, the part of the brain that regulates learning, emotion and memory. These activities involve both muscarinic and SIGMAR1 systems through a novel mechanism of action.
Preclinical data presented also indicates that ANAVEX®1-41 has the potential to demonstrate protective effects of mitochondrial enzyme complexes during pathological conditions, which, if impaired, are believed to play a role in the pathogenesis of neurodegenerative and neurodevelopmental diseases.
ANAVEX®1066
ANAVEX®1066, a mixed sigma-1/sigma-2 ligand, is designed for the potential treatment of neuropathic and visceral pain. ANAVEX®1066 was tested in two preclinical models of neuropathic and visceral pain that have been extensively validated in rats. In the chronic constriction injury model of neuropathic pain, a single oral administration of ANAVEX®1066 dose-dependently restored the nociceptive threshold in the affected paw to normal levels while leaving the contralateral healthy paw unchanged. Efficacy was rapid and remained significant for two hours. In a model of visceral pain, chronic colonic hypersensitivity was induced by injection of an inflammatory agent directly into the colon and a single oral administration of ANAVEX®1066 returned the nociceptive threshold to control levels in a dose-dependent manner. Companion studies in rats demonstrated the lack of any effects on normal gastrointestinal transit with ANAVEX®1066 and a favorable safety profile in a battery of behavioral measures.
ANAVEX®1037
ANAVEX®1037 is designed for the treatment of prostate and pancreatic cancer. It is a low molecular weight, synthetic compound exhibiting high affinity for SIGMAR1 at nanomolar levels and moderate affinity for sigma-2 receptors and sodium channels at micromolar levels. In advanced pre-clinical studies, this compound revealed antitumor potential. It has also been shown to selectively kill human cancer cells without affecting normal/healthy cells and also to significantly suppress tumor growth in immune-deficient mice models. Scientific publications highlight the possibility that these ligands may stop tumor growth and induce selective cell death in various tumor cell lines. Sigma receptors are highly expressed in different tumor cell types. Binding by appropriate sigma-1 and/or sigma-2 ligands can induce selective apoptosis. In addition, through tumor cell membrane reorganization and interactions with ion channels, we believe our drug candidates may play an important role in inhibiting the processes of metastasis (spreading of cancer cells from the original site to other parts of the body), angiogenesis (the formation of new blood vessels) and tumor cell proliferation.
ANAVEX®1037 is currently in the pre-clinical and clinical testing stages of development, and there is no guarantee that the activity demonstrated in pre-clinical models will be shown in human testing.
We continue to identify and initiate discussions with potential strategic and commercial partners to most effectively advance our programs and increase stockholder value. Further, we may acquire or develop new intellectual property and assign, license, or otherwise transfer our intellectual property to further our goals.
Our Target Indications
We are developing compounds with potential application to two broad categories and several specific indications, including:
Central Nervous System Diseases
| ● | Alzheimer’s disease – In 2022, an estimated 6.5 million Americans were suffering from Alzheimer’s disease. The Alzheimer’s Association® estimates that by 2050, this number is expected to rise to 12.7 million Americans. Medications on the market today treat only the symptoms of Alzheimer’s disease and do not have the ability to stop its onset or its progression. We believe that there is an urgent and unmet need for both a disease modifying cure for Alzheimer’s disease as well as for better symptomatic treatments. |
| ● | Parkinson’s disease – Parkinson’s disease is a progressive disease of the nervous system marked by tremors, muscular rigidity, and slow, imprecise movement. It is associated with degeneration of the basal ganglia of the brain and a deficiency of the neurotransmitter dopamine. Parkinson’s disease currently is estimated to afflict more than 10 million people worldwide, typically middle-aged and elderly people. The Parkinson’s disease market is expected to reach $11.5 billion by 2029, according to GlobalData. |
| ● | Rett syndrome – Rett syndrome is a rare X-linked genetic neurological and developmental disorder that affects the way the brain develops, including protein transcription, which is altered and as a result leads to severe disruptions in neuronal homeostasis. It is considered a rare, progressive neurodevelopmental disorder and is caused by a single mutation in the MECP2 gene. Because males have a different chromosome combination from females, boys who have the genetic MECP2 mutation are affected in devastating ways. Most of them die before birth or in early infancy. For females who survive infancy, Rett syndrome leads to severe impairments, affecting nearly every aspect of the child’s life; severe mental retardation, their ability to speak, walk and eat, sleeping problems, seizures and even the ability to breathe easily. Rett syndrome affects approximately 1 in every 10,000-15,000 females. |
| ● | Depression – Depression is a major cause of morbidity worldwide according to the World Health Organization. The global antidepressant drug market is projected to reach $21 Billion by 2030 according to Allied Market Research. Pharmaceutical treatment for depression has been historically dominated by blockbuster brands. However, the dominance of the leading brands is waning, largely due to an increase in the number of approvals for antidepressant drugs. |
| ● | Epilepsy – Epilepsy is a common chronic neurological disorder characterized by recurrent unprovoked seizures. These seizures are transient signs and/or symptoms of abnormal, excessive or synchronous neuronal activity in the brain. According to the Centers for Disease Control and Prevention, in 2015 epilepsy affected 3.4 million Americans. Today, epilepsy is often controlled, but not cured, with medication that is categorized as older traditional anti-epileptic drugs and second generation anti-epileptic drugs. Because epilepsy afflicts sufferers in different ways, there is a need for drugs used in combination with both traditional anti-epileptic drugs and second generation anti-epileptic drugs. |
| ● | Neuropathic Pain – We define neuralgia, or neuropathic pain, as pain that is not related to activation of pain receptor cells in any part of the body. Neuralgia is more difficult to treat than some other types of pain because it does not respond well to normal pain medications. Special medications have become more specific to neuralgia and typically fall under the category of membrane stabilizing drugs or antidepressants. |
Cancer
| ● | Malignant Melanoma – Predominantly a skin cancer, malignant melanoma can also occur in melanocytes found in the bowel and the eye. Malignant melanoma accounts for a large majority of skin cancer deaths. The treatment includes surgical removal of the tumor, adjuvant treatment, chemo and immunotherapy, or radiation therapy. According to iHealthcareAnalyst, Inc. the worldwide malignant melanoma market is expected to grow to $7.5 billion by 2029. |
| ● | Prostate Cancer – Specific to men, prostate cancer is a form of cancer that develops in the prostate, a gland in the male reproductive system. Cancer cells may metastasize from the prostate to other parts of the body, particularly the bones and lymph nodes. Drug therapeutics for prostate cancer are expected to increase to nearly $10.1 billion by the end of 2030 according to Market Research Future. |
| ● | Pancreatic Cancer – Pancreatic cancer is a malignant neoplasm of the pancreas. In the United States, approximately 62,000 new cases of pancreatic cancer will be diagnosed this year and approximately 50,000 patients will die as a result of their cancer, according to the American Cancer Society. Sales predictions by Market Data Forecast predict that the market for the global pharmaceutical treatment of pancreatic cancer will increase to $3.73 billion by 2027. |
Patents, Trademarks and Intellectual Property
We hold ownership or exclusive rights to seventeen U.S. patents, nineteen U.S. patent applications, and various PCT or ex-U.S. patent applications relating to our drug candidates, methods associated therewith, and to our research programs.
We own one issued U.S. patent entitled “ANAVEX®2-73 and certain anticholinesterase inhibitors composition and method for neuroprotection” claims a composition of matter of ANAVEX®2-73 directed to a novel and synergistic neuroprotective compound combined with donepezil and other cholinesterase inhibitors. This patent is expected to expire in June 2034, absent any patent term extension for regulatory delays. We own three issued U.S. patents each with claims directed to crystalline forms of ANAVEX®2-73. The first of these three patents claims crystalline forms of ANAVEX®2-73, dosage forms and compositions containing crystalline ANAVEX®2-73, and methods of treatment for Alzheimer’s disease using them. This patent is expected to expire in July 2036, absent any patent term extension for regulatory delays. The second of these three patents claims pharmaceutical compositions containing a crystalline form of ANAVEX®2-73, and methods of treatment for Alzheimer’s disease using the compositions. This patent is expected to expire in June 2037, absent any patent term extension for regulatory delays. The third of these three patents claims pharmaceutical compositions containing a crystalline form of ANAVEX®2-73, and methods of treatment for Alzheimer’s disease using the compositions. This patent is expected to expire in June 2037, absent any patent term extension for regulatory delays. We also own two issued U.S. patents for seizure treatment. The first of these two patents claims methods and dosage forms for treating seizures, the dosage forms containing a low-dose anti-epilepsy drug combined with either: (i) ANAVEX®2-73 and its active metabolite ANAVEX®19-144; or (ii) ANAVEX®19-144. The second of these two patents further claims a combination seizure treatment involving administration of an anti-epilepsy drug combined with (i) ANAVEX®19-144, or (ii) ANAVEX 19-144® and ANAVEX 2-73®. Both patents are expected to expire in October 2035, absent any patent term extension for regulatory delays. We also own three issued U.S. patents with claims directed to treating neurodevelopmental disorders. These patents claim methods for treating a neurodevelopmental disorder, multiple sclerosis, or their related biochemical and functional abnormalities by administering ANAVEX®2-73, ANAVEX®19-144, and/or ANAVEX®1-41 (another sigma receptor ligand similar to ANAVEX®2-73), or compositions thereof. All three patents are expected to expire in January 2037, absent any patent term extension for regulatory delays. In addition, we own one issued U.S. Patent with claims directed to methods of treating melanoma with a compound related to ANAVEX®2-73. This patent is expected to expire in February 2030, absent any patent term extension for regulatory delays. We also own an issued U.S. patent that claims crystalline forms of ANAVEX®19-144, dosage forms and compositions containing the crystalline forms of ANAVEX®19-144, and methods of treatment for Alzheimer’s disease. This patent is expected to expire in July 2036, absent any patent term extension for regulatory delays. Further, we own one issued U.S. Patent with claims directed to methods of treating cardiac dysfunction with ANAVEX®2-73. This patent is expected to expire in July 2038, absent any patent term extension for regulatory delays. Additionally, we own one issued U.S. Patent with claims directed to methods of treating insomnia or anxiety with ANAVEX®2-73, ANAVEX®19-144, and/or ANAVEX®1-41. This patent is expected to expire in September 2038, absent any patent term extension for regulatory delays.
We also own two issued U.S. patents related to ANAVEX®1066. The first of these two patents claims methods for treating or preventing pain using (+) ANAVEX®1066 isomer. The second patent claims methods for treating or preventing pain using (-) ANAVEX®1066 isomer. Both patents are expected to expire in November 2036, absent any patent term extension for regulatory delays.
For ANAVEX®2-73, ANAVEX®19-144, ANAVEX®1-41, and ANAVEX®1066, we also have granted or pending applications in Australia, Canada, China, Europe, Japan, and Hong Kong, which are expected to expire after 2035.
With regard to ANAVEX®3-71, we own exclusive rights to two issued U.S. patents with claims respectively directed to the ANAVEX®3-71 compound and methods of treating various diseases including Alzheimer’s with the same. These patents are expected to expire in April 2030, and January 2030, respectively, absent any patent term extension for regulatory delays. We also own exclusive rights to related patents or applications that are granted or pending in Australia, Canada, China, Europe, Japan, Korea, New Zealand, Russia, and South Africa, which are expected to expire in January 2030.
We also own other patent applications directed to enantiomers, crystals, formulations, uses, and patient selection methods that may provide additional protection for one or more of our product candidates.
We regard patents and other intellectual property rights as corporate assets. Accordingly, we attempt to optimize the value of intellectual property in developing our business strategy including the selective development, protection, and exploitation of our intellectual property rights. In addition to filings made with intellectual property authorities, we protect our intellectual property and confidential information by means of carefully considered processes of communication and the sharing of information, and by the use of confidentiality and non-disclosure agreements and provisions for the same in contractor’s agreements. While no agreement offers absolute protection, such agreements provide some form of recourse in the event of disclosure, or anticipated disclosure.
Our intellectual property position, like that of many biomedical companies, is uncertain and involves complex legal and technical questions for which important legal principles are unresolved. For more information regarding challenges to our existing or future patents, see “Risk Factors” in Part I, Item 1A of our Annual Report on Form 10-K filed with the Securities and Exchange Commission on November 28, 2022.
Financial Overview
The following discussion should be read in conjunction with our interim condensed consolidated financial statements and related notes thereto contained elsewhere in this report. Past operating results are not necessarily indicative of results that may occur in future periods. The discussion contains forward-looking statements, which involve a number of risks and uncertainties. See “Forward Looking Statements” included elsewhere in this report.
We are in the development stage and have not earned any revenues since our inception in 2004. We do not anticipate earning any revenues until we can establish an alliance with other companies to develop, co-develop, license, acquire or market our products.
Our operating costs consist primarily of research and development activities including the cost of clinical trials and clinical supplies as well as clinical drug manufacturing and formulation. Research and development expenses also include personnel related costs such as salaries and wages, and third-party contract research organization (CRO) expenses in support of these clinical trials. Personnel costs include salaries and wages, benefits, and non-cash stock-based compensation charges associated with options and other equity awards granted to employees and consultants who are directly engaged in support of our research and development activities.
General and administrative expenses consist of personnel costs, expenses for outside professional services and expenses associated with operating as a public company. Personnel costs consist of salaries and wages, benefits and stock-based compensation for general and administrative personnel. Outside professional services and public company expenses include expenses related to compliance and reporting, additional insurance expenses, audit and SOX compliance, expenses associated with patent research, applications and filings, investor and stockholder relations activities and other administrative expenses and professional services.
Comparison of the three months ended December 31, 2022 and 2021
Operating Expenses
Total operating expenses for the quarter ended December 31, 2022 were $15.4 million, compared to $11.7 million for the comparable quarter ended December 31, 2021.
Our research and development expenses for the three months ended December 31, 2022 were $12.1 million, as compared to $8.7 million for the three months ended December 31, 2021. The increase in research and development expenses is primarily related to (i) an increase in personnel costs of $0.7 million and an increase in stock based compensation expense of $1.4 million as a result of the expansion of our team engaged in research and development activities, (ii) an increase of approximately $0.7 million over the comparable period, relating to the manufacture of additional clinical trial supplies for both our ANAVEX®2-73 and ANAVEX®3-71 programs and (iii) an increase in costs associated with expanded enrollment in our EXCELLENCE trial.
The following table summarizes our research and development expenses for the quarter ended December 31, 2022 and 2021 (in thousands):
| 2022 | 2021 | |||||||
| Cost of external service providers | $ | 6,054 | $ | 4,258 | ||||
| Personnel costs | 2,381 | 1,649 | ||||||
| Stock-based compensation | 3,604 | 2,237 | ||||||
| License fees | — | 500 | ||||||
| Other common costs | 28 | 12 | ||||||
| Total research and development costs | $ | 12,067 | $ | 8,656 | ||||
During the quarter ended December 31, 2022 and 2021, external service providers cost by product candidate was as follows (in thousands):
| 2022 | 2021 | |||||||
| ANAVEX®2-73 | $ | 5,240 | $ | 3,908 | ||||
| ANAVEX®3-71 | 712 | 324 | ||||||
| All other product candidates | — | 2 | ||||||
| Other external service provider costs | 102 | 24 | ||||||
| Total external service provider costs | $ | 6,054 | $ | 4,258 | ||||
General and administrative expenses were $3.3 million for the three months ended December 31, 2022, as compared to $3.1 million for the same quarter of fiscal 2022, an increase of $0.2 million mostly relating to an increase in professional and IP related costs.
During the quarter, we utilized cash and cash equivalents of $5.8 million to fund our operations, compared to $3.5 million during comparable quarter in fiscal 2022. Our cash position was $143.6 million at December 31, 2022 compared to $149.2 million at our fiscal year ended September 30, 2022.
We expect to continue an increase in our research and development expenditures as we advance our ANAVEX®2-73 clinical trials, including planned advancement of ANAVEX®2-73 for Parkinson’s disease program, planned initiation of a Fragile X clinical program, ongoing extension trials of our current clinical programs, continued advancement of our other pipeline compounds such as ANAVEX®3-71, and as we continue to add additional staffing to manage and support these clinical initiatives.
Other income (net)
The net amount of other income for the three months ended December 31, 2022 was $2.4 million as compared to $0.9 million for the comparable three months ended December 31, 2021. The increase in other income for the quarter is primarily related to an increase in return on excess cash invested in cash equivalents as well as a foreign exchange gain relating to the recovery of the Australian dollar relative to the US dollar during the period.
Net loss
Net loss for the current quarter was $13.0 million, or $0.17 per share, as compared to $10.9 million, or $0.14 per share in the comparative quarter of fiscal 2022. The increase in net loss for the quarter is primarily related to increased research and development fees as discussed above.
Liquidity and Capital Resources
Working Capital
| December 31, 2022 | September 30, 2022 | |||||||
| Current Assets | $ | 148,371,922 | $ | 152,704,603 | ||||
| Current Liabilities | 13,247,846 | 10,213,561 | ||||||
| Working Capital | $ | 135,124,076 | $ | 142,491,042 | ||||
At December 31, 2022, we had current assets of $148.4 million, a decrease of $4.3 million from September 30, 2022. The decrease in current assets relates mostly to cash used in operations of $5.8 million for the quarter ended December 31, 2022.
At December 31, 2022, we had current liabilities of $13.2 million, an increase of $3.0 million from September 30, 2022. A portion of this increase relates to an increase in deferred grant income of $0.5 million relating to amounts received during the quarter for a research grant awarded by the Michael J. Fox Foundation for Parkinson’s Research. Other increases in current liabilities relate to amounts owed at period end for manufacturing of additional clinical trial supplies for use in future studies.
We intend to continue to use our capital resources to advance our clinical trials for ANAVEX®2-73 and ANAVEX®3-71, and to perform work necessary to prepare for future development of our pipeline compounds.
Cash Flows
The following table summarizes cash flows during the three months ended December 31, 2022 and 2021:
| 2022 | 2021 | |||||||
| Net cash flows used in operating activities | $ | (5,794,257 | ) | $ | (3,500,955 | ) | ||
| Net cash flows from financing activities | 258,244 | 2,539,565 | ||||||
| Decrease in cash and cash equivalents | $ | (5,536,013 | ) | $ | (961,390 | ) | ||
Cash flow used in operating activities
Net cash used in operating activities for the three months ended December 31, 2022 was $5.8 million, compared to $3.5 million during the comparable quarter ended December 31, 2021. The principal reason for this increase in net cash used in operating activities in the current period is due to the receipt of the Company’s annual research and development incentive income refund in the comparable quarter.
Cash flow provided by financing activities
Cash provided by financing activities for the quarter ended December 31, 2022 was $0.3 million, attributable to the exercise of employee stock options, while cash provided by financing activities for the quarter ended December 31, 2021 was $2.5 million, primarily attributable to the sale of common shares at various market prices under the Sales Agreement, described below.
Other Financings
Controlled Equity Offering Sales Agreement
On May 1, 2020, we entered into an Amended and Restated Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. and SVB Leerink LLC (the “Sales Agents”), pursuant to which we may offer and sell shares of common stock registered under an effective registration statement from time to time through the Sales Agents (the “At-the-Market Offering”).
Upon delivery of a placement notice based on our instructions and subject to the terms and conditions of the Sales Agreement, the Sales Agents may sell shares of common stock by methods deemed to be an “at the market offering”, in negotiated transactions at market prices prevailing at the time of sale or at prices related to such prevailing market prices, or by any other method permitted by law, including negotiated transactions, subject to our prior written consent. We are not obligated to make any sales of shares under the Sales Agreement. We or the Sales Agents may suspend or terminate the At-the-Market Offering upon notice to the other party, subject to certain conditions. The Sales Agents will act as agents on a commercially reasonable efforts basis consistent with their normal trading and sales practices, applicable state and federal law, and rules and regulations and the rules of Nasdaq.
We have agreed to pay the Sales Agents’ commissions for their services of 3.0% of the gross proceeds from the sale of shares of common stock pursuant to the Sales Agreement. We have also agreed to provide the Sales Agents with customary indemnification and contribution rights.
No shares were sold during the quarter ended December 31, 2022 under the Sales Agreement.
During the quarter ended December 31 2021, 99,588 shares were sold under the Sales Agreement for gross proceeds of $2.3 million (net proceeds of $2.1 million after deducting commissions and offering expenses).
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to our stockholders.
CRITICAL ACCOUNTING POLICIES
We prepare our interim condensed consolidated financial statements in accordance with accounting principles generally accepted in the United States of America and make estimates and assumptions that affect our reported amounts of assets, liabilities, revenue and expenses, and the related disclosures of contingent liabilities. We base our estimates on historical experience and other assumptions that we believe are reasonable in the circumstances. Actual results may differ from these estimates.
There have been no significant changes in the critical accounting policies and estimates described in our Annual Report on Form 10-K for the year ended September 30, 2022, as filed with the SEC on November 28, 2022.
RECENT ACCOUNTING PRONOUNCEMENTS
Please refer to Note 2 “Recent Accounting Pronouncements” in notes to our Interim Condensed Consolidated Financial Statements included in this Form 10-Q.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISKS.
Not applicable
ITEM 4. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to provide reasonable assurance that material information required to be disclosed in our periodic reports filed under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and to provide reasonable assurance that such information is accumulated and communicated to our management, our chief executive officer and our principal financial officer, to allow timely decisions regarding required disclosure.
We carried out an evaluation, under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rule 13a 15(e) under the Exchange Act, as of the end of the period covered by this Quarterly Report on Form 10-Q. Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective as of December 31, 2022.
Changes in Internal Control over Financial Reporting
During the quarter ended December 31, 2022, there were no changes to our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a 15(d) or 15d 15 (d) of the Exchange Act that materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
PART II – OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
We know of no material pending legal proceedings, other than ordinary routine litigation incidental to our business, to which our Company or our subsidiaries are a party or of which any of their property is subject. There are no proceedings in which any of our directors, officers or affiliates, or any registered or beneficial stockholder holding more than 5% of our shares, is an adverse party or has a material interest adverse to our or our subsidiaries’ interest.
ITEM 1A. RISK FACTORS
There have been no material changes to the risk factors discussed in “Risk Factors” in Part I, Item 1A of our Annual Report on Form 10-K for the fiscal year ended September 30, 2022, filed with the SEC on November 28, 2022.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
During the period covered by this Quarterly Report on Form 10-Q, we have not sold any equity securities that were not registered under the Securities Act of 1933 that were not previously reported in a Current Report on Form 8-K.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None
ITEM 4.
Not applicable.
ITEM 5. OTHER INFORMATION
MINE SAFETY DISCLOSURES On February 3, 2023 (the “Execution Date”), the Company entered into a purchase agreement (the “Purchase Agreement”) and a registration rights agreement (“RRA”) with Lincoln Park Capital Fund, LLC (“LPC”), an Illinois limited liability company, whereby the Company has the right, in its sole discretion, to sell to LPC up to $150,000,000 in shares of the Company’s common stock, par value $0.001 per share (“Common Stock”) from time to time over a 36 month period pursuant to the terms of the Purchase Agreement.
On any business day and subject to certain customary conditions, the Company may direct LPC to purchase up to 200,000 shares of Common Stock (such purchases, “Regular Purchases”). The amount of a Regular Purchase may increase under certain circumstances based on the market price of the Common Stock; provided, however, that LPC’s committed obligation under any Regular Purchase shall not exceed $4,000,000. The purchase price of shares of Common Stock will be based on the then prevailing market prices of such shares at the time of sales as described in the Purchase Agreement. There are no limits on the price per share that LPC may pay to purchase Common Stock under the Purchase Agreement. In addition, if the Company has directed LPC to purchase the full amount of Common Stock available as a Regular Purchase on a given day, it may direct LPC to purchase additional amounts as “accelerated purchases” and “additional accelerated purchases” as set forth in the Purchase Agreement.
The Purchase Agreement limits the Company’s sale shares of Common Stock to LPC to 15,606,426 shares of Common Stock, representing 19.99% of the shares of the Common Stock outstanding on the date of the Purchase Agreement unless (i) shareholder approval is obtained to issue more than such amount or (ii) the average price of all applicable sales of Common Stock to LPC under the Purchase Agreement equals or exceeds the lower of (A) the closing price of the Common Stock on the Nasdaq Capital Market immediately preceding the Execution Date or (B) the average of the closing price of the Common Stock on the Nasdaq Capital Market for the five Business Days immediately preceding the Execution Date.
The Purchase Agreement also prohibits the Company from directing LPC to purchase any shares of Common Stock if those shares, when aggregated with all other shares of Common Stock then beneficially owned by Lincoln Park and its affiliates, would result in LPC and its affiliates having beneficial ownership, at any single point in time, of more than 4.99% of the then total outstanding shares of Common Stock, as calculated pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended, and Rule 13d-3 thereunder.
The Purchase Agreement contains customary representations, warranties, covenants, closing conditions and indemnification and termination provisions by, among and for the benefit of the parties. LPC has covenanted not to cause or engage in any manner whatsoever, any direct or indirect short selling or hedging of the Company’s Common Stock. The Purchase Agreement does not limit the Company’s ability to raise capital from other sources at its sole discretion; provided, however, that the Company shall not enter into any “Variable Rate Transaction” as defined in the Purchase Agreement, including the issuance of any floating conversion rate or variable priced equity-like securities, but excluding any “At-the-Market” offering with a registered broker-dealer, during the 36 month term of the Purchase Agreement.
In consideration for entering into the Purchase Agreement, the Company will issue to LPC 75,000 shares of Common Stock as a commitment fee and shall issue up to 75,000 shares pro rata as up to $150,000,000 of shares of our common stock are purchase by LPC at our discretion from time to time, in accordance with the Purchase Agreement. The Purchase Agreement may be terminated by the Company at any time at its discretion without any cost to the Company.
Actual sales of shares of Common Stock to LPC under the Purchase Agreement will depend on a variety of factors to be determined by the Company from time to time, including, among others, market conditions, the trading price of the Common Stock and determinations by the Company as to the appropriate sources of funding for the Company and its operations.
The net proceeds under the Purchase Agreement to the Company will depend on the frequency and prices at which the Company sells shares of its stock to LPC. The Company expects that any proceeds received by the Company from such sales to LPC will be used for working capital and general corporate purposes.
ITEM 6. EXHIBIT
* Filed herewith.
** Furnished herewith.
| SIGNATURES |
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
ANAVEX LIFE SCIENCES CORP.
| /s/Christopher Missling, PhD | |
| Christopher Missling, PhD | |
| Chief Executive Officer | |
| (Principal Executive Officer) | |
| Date: February 7, 2023 |
| /s/Sandra Boenisch | |
| Sandra Boenisch, CPA, CGA | |
| Principal Financial Officer | |
| (Principal Financial and Accounting Officer) | |
| Date: February 7, 2023 |
21
EXHIBIT 4.1
REGISTRATION RIGHTS AGREEMENT
REGISTRATION RIGHTS AGREEMENT (this “Agreement”), dated as of February 3, 2023 by and between ANAVEX LIFE SCIENCES CORP., a Nevada corporation (the “Company”), and LINCOLN PARK CAPITAL FUND, LLC, an Illinois limited liability company (together with its permitted assigns, the “Buyer”). Capitalized terms used herein and not otherwise defined herein shall have the respective meanings set forth in the Purchase Agreement by and between the parties hereto, dated as of the date hereof (as amended, restated, supplemented or otherwise modified from time to time, the “Purchase Agreement”).
WHEREAS:
The Company has agreed, upon the terms and subject to the conditions of the Purchase Agreement, to sell to the Buyer up to One Hundred Fifty Million Dollars ($150,000,000) of Purchase Shares and to induce the Buyer to enter into the Purchase Agreement, the Company has agreed to provide certain registration rights under the Securities Act of 1933, as amended, and the rules and regulations thereunder, or any similar successor statute (collectively, the “Securities Act”), and applicable state securities laws.
NOW, THEREFORE, in consideration of the promises and the mutual covenants contained herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Company and the Buyer hereby agree as follows:
1. DEFINITIONS.
As used in this Agreement, the following terms shall have the following meanings:
a. “Investor” means the Buyer, any transferee or assignee thereof to whom a Buyer assigns its rights under this Agreement in accordance with Section 8 and who agrees to become bound by the provisions of this Agreement, and any transferee or assignee thereof to whom a transferee or assignee assigns its rights under this Agreement in accordance with Section 8 and who agrees to become bound by the provisions of this Agreement.
b. “Person” means any individual or entity including but not limited to any corporation, a limited liability company, an association, a partnership, an organization, a business, an individual, a governmental or political subdivision thereof or a governmental agency.
c. “Register,” “Registered,” and “Registration” refer to a registration effected by preparing and filing one or more registration statements of the Company in compliance with the Securities Act and pursuant to Rule 415 under the Securities Act or any successor rule providing for offering securities on a continuous basis (“Rule 415”), and the declaration or ordering of effectiveness of such registration statement(s) by the United States Securities and Exchange Commission (the “SEC”).
d. “Registrable Securities” means all of the Purchase Shares which have been, or which may, from time to time be issued, including without limitation all of the Commitment Shares which have been or which may, from time to time, be issued or become issuable to the Investor under the Purchase Agreement (without regard to any limitation or restriction on purchases), and any and all shares of capital stock issued or issuable with respect to the Purchase Shares or the Commitment Shares or the Purchase Agreement as a result of any stock split, stock dividend, recapitalization, exchange or similar event or otherwise, without regard to any limitation on purchases under the Purchase Agreement.
e. “Registration Statement” means the Shelf Registration Statement and any other registration statement of the Company that Registers Registrable Securities, including a New Registration Statement, as amended when each became effective, including all documents filed as part thereof or incorporated by reference therein, and including any information contained in a Prospectus subsequently filed with the SEC.
f. “Shelf Registration Statement” means the Company’s existing registration statement on Form S-3 ASR (File No. 333-259788) and including any registration statement filed in connection therewith under Rule 462(b) pursuant to the Securities Act (a “462(b) Registration Statement”).
2. REGISTRATION.
a. Mandatory Registration. The Company agrees that it shall, within the time required under Rule 424(b) under the Securities Act, file with the SEC the Initial Prospectus Supplement pursuant to Rule 424(b) under the Securities Act specifically relating to the transactions contemplated by, and describing the material terms and conditions of, the Transaction Documents, containing information previously omitted at the time of effectiveness of the Registration Statement in reliance on Rule 430B under the Securities Act, and disclosing all information relating to the transactions contemplated hereby required to be disclosed in the Registration Statement and the Prospectus as of the date of the Initial Prospectus Supplement, including, without limitation, information required to be disclosed in the section captioned “Plan of Distribution” in the Prospectus. The Investor acknowledges that it will be identified in the Initial Prospectus Supplement as an underwriter within the meaning of Section 2(a)(11) of the Securities Act. The Company shall permit the Investor to review and comment upon the Initial Prospectus Supplement at least two (2) Business Days prior to their filing with the SEC, the Company shall give due consideration to all such comments, and the Company shall not file the Initial Prospectus Supplement with the SEC in a form to which the Investor reasonably objects. The Investor shall use its reasonable best efforts to comment upon the Initial Prospectus Supplement within one (1) Business Day from the date the Investor receives the final pre-filing draft version thereof from the Company. The Investor shall furnish to the Company such information regarding itself, the Securities held by it and the intended method of distribution thereof, including any arrangement between the Investor and any other Person relating to the sale or distribution of the Securities, as shall be reasonably requested by the Company in connection with the preparation and filing of the Initial Prospectus Supplement, and shall otherwise cooperate with the Company as reasonably requested by the Company in connection with the preparation and filing of the Initial Prospectus Supplement with the SEC.
b. Effectiveness. The Company shall use its reasonable best efforts to keep the Registration Statement effective under the Securities Act (including through any necessary renewals), and to keep the Registration Statement and the Prospectus current and available (including through any necessary renewals) for issuances and sales of all possible Registrable Securities by the Company to the Investor, and for the sale of all of the Registrable Securities by the Investor, at all times until the earlier of (i) the date on which the Investor shall have sold all the Registrable Securities and no Available Amount remains and (ii) the earlier of (A) 180 days following the Maturity Date and (B) the nine months following the termination of the Purchase Agreement (the “Registration Period”). Without limiting the generality of the foregoing, during the Registration Period, the Company shall (a) take all action necessary to cause the Common Stock to continue to be Registered as a class of securities under Section 12(b) of the Exchange Act and shall not take any action or file any document (whether or not permitted by the Exchange Act) to terminate or suspend such registration and (b) file or furnish on or before their respective due dates all reports and other documents required to be filed or furnished by the Company pursuant to Sections 13(a), 13(c), 14, 15(d) or any other provision of or under the Exchange Act, and shall not take any action or file any document (whether or not permitted by the Exchange Act) to terminate or suspend its reporting and filing obligations under the Exchange Act. The Registration Statement (including any amendments or supplements thereto and prospectuses contained therein) shall not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements therein, in the light of the circumstances then existing, not misleading. In the event that the Company files a Rule 462(b) Registration Statement, such Rule 462(b) Registration Statement shall have been filed and automatically effective pursuant to Rule 462 under the Securities Act prior to the filing of the Initial Prospectus Supplement under Section 2(a) hereto.
c. Sufficient Number of Shares Registered. In the event the number of shares available under the Shelf Registration Statement, including any 462(b) Registration Statement, is insufficient to cover all of the Registrable Securities, the Company shall, to the extent necessary and permissible, file a new Registration Statement (a “New Registration Statement”), so as to cover all of such Registrable Securities as soon as practicable, but in any event not later than ten (10) Business Days after the necessity therefor arises, subject to any limits that may be imposed by the SEC pursuant to Rule 415 under the Securities Act. The Company shall use its reasonable best efforts to cause such New Registration Statement to become effective as soon as practicable following the filing thereof.
d. Offering. If the staff of the SEC (the “Staff”) or the SEC seeks to characterize any offering pursuant to a Registration Statement as constituting an offering of securities that does not permit such Registration Statement be used by the Investor under Rule 415 at then-prevailing market prices (and not fixed prices), or if after the filing of the Initial Prospectus Supplement with the SEC pursuant to Section 2(a), the Company is otherwise required by the Staff or the SEC to reduce the number of Registrable Securities registered under the Shelf Registration Statement, then the Company shall reduce the number of Registrable Securities to be registered under the Shelf Registration Statement (with the prior consent, which shall not be unreasonably withheld, of the Investor and its legal counsel as to the specific Registrable Securities to be removed therefrom) until such time as the Staff and the SEC shall so permit such Registration Statement to be used as aforesaid. In the event of any reduction in Registrable Securities pursuant to this paragraph, the Company shall file one or more New Registration Statements in accordance with Section 2(c) until such time as all Registrable Securities have been included in Registration Statements that have been declared effective and the prospectuses contained therein is available for use by the Investor. Notwithstanding any provision herein or in the Purchase Agreement to the contrary, the Company’s obligations to register Registrable Securities (and any related conditions to the Investor’s obligations) shall be qualified as necessary to comport with any requirement of the SEC or the Staff as addressed in this Section 2(d).
3. RELATED OBLIGATIONS.
With respect to the Registration Statement and whenever any Registrable Securities are to be Registered pursuant to Section 2 including on the Shelf Registration Statement or any New Registration Statement, the Company shall use its reasonable best efforts to effect the registration of the Registrable Securities in accordance with the intended method of disposition thereof and, pursuant thereto, the Company shall have the following obligations:
a. Notifications. The Company will notify the Investor promptly of the time when any subsequent amendment to the Shelf Registration Statement or any New Registration Statement, other than documents incorporated by reference, has been filed with the SEC and/or has become effective or where a receipt has been issued therefor or any subsequent supplement to a Prospectus has been filed and of any request by the SEC for any amendment or supplement to the Registration Statement, any New Registration Statement or any Prospectus or for additional information.
b. Amendments. The Company will prepare and file with the SEC, promptly upon the Investor’s request, any amendments or supplements to the Shelf Registration Statement, any New Registration Statement or any Prospectus, as applicable, that, in the reasonable opinion of the Investor and the Company, may be necessary or advisable in connection with any acquisition or sale of Registrable Securities by the Investor (provided, however, that the failure of the Investor to make such request shall not relieve the Company of any obligation or liability hereunder).
c. Investor Review. The Company will not file any amendment or supplement to the Registration Statement, any New Registration Statement or any Prospectus, other than documents incorporated by reference, relating to the Investor, the Registrable Securities or the transactions contemplated hereby unless (A) the Investor shall have been advised and afforded the opportunity to review and comment thereon at least two (2) Business Days prior to filing with the SEC, (B) the Company shall have given due consideration to any comments thereon received from the Investor or its counsel, and (C) the Investor has not reasonably objected thereto (provided, however, that the failure of the Investor to make such objection shall not relieve the Company of any obligation or liability hereunder), and the Company will furnish to the Investor at the time of filing thereof a copy of any document that upon filing is deemed to be incorporated by reference into the Registration Statement or any Prospectus, except for those documents available via EDGAR.
d. Form S-3. The Company will cause each amendment or supplement to the Prospectus, other than documents incorporated by reference, to be filed with the SEC as required pursuant to the rules of Form S-3.
e. Copies Available. The Company will furnish to the Investor and its counsel (at the expense of the Company) copies of the Registration Statement, the Prospectus (including all documents incorporated by reference therein), any Prospectus Supplement, any New Registration Statement and all amendments and supplements to the Registration Statement, the Prospectus or any New Registration Statement that are filed with the SEC during the Registration Period (including all documents filed with or furnished to the SEC during such period that are deemed to be incorporated by reference therein), in each case as soon as reasonably practicable upon the Investor’s request and in such quantities as the Investor may from time to time reasonably request and, at the Investor’s request, will also furnish copies of the Prospectus to each exchange or market on which sales of the Registrable Securities may be made; provided, however, that the Company shall not be required to furnish any document (other than the Prospectus) to the Investor to the extent such document is available on EDGAR.
f. Qualification. The Company shall take all such action, if any, as is reasonably necessary in order to obtain an exemption for or to qualify (i) the issuance of the Commitment Shares and the sale of the Purchase Shares to the Investor under the Purchase Agreement and (ii) any subsequent resale of all Commitment Shares and all Purchase Shares by the Investor, in each case, under applicable securities or “Blue Sky” laws of the states of the United States in such states as is reasonably requested by the Investor during the Registration Period, and shall provide evidence of any such action so taken to the Investor. During the Registration Period, the Company shall promptly notify the Investor of the receipt by the Company of any notification with respect to the suspension of the registration or qualification of any of the Registrable Securities for sale under the securities or “blue sky” laws of any jurisdiction in the United States or its receipt of actual notice of the initiation or threat of any proceeding for such purpose.
g. Notification of Stop Orders; Material Changes. The Company shall advise the Investor promptly (but in no event later than 24 hours) and shall confirm such advice in writing, in each case: (i) of the Company’s receipt of notice of any request by the SEC or any other federal or state governmental authority for amendment of or a supplement to the Registration Statement or any Prospectus or for any additional information; (ii) of the Company’s receipt of notice of the issuance by the SEC or any other federal or state governmental authority of any stop order suspending the effectiveness of the Registration Statement or prohibiting or suspending the use of the Prospectus or Prospectus Supplement, or any New Registration Statement, or of the Company’s receipt of any notification of the suspension of qualification of the Registrable Securities for offering or sale in any jurisdiction or the initiation or contemplated initiation of any proceeding for such purpose; and (iii) of the Company becoming aware of the happening of any event, which makes any statement of a material fact made in the Registration Statement or any Prospectus untrue or which requires the making of any additions to or changes to the statements then made in the Registration Statement or any Prospectus in order to state a material fact required by the Securities Act to be stated therein or necessary in order to make the statements then made therein (in the case of any Prospectus, in light of the circumstances under which they were made) not misleading, or of the necessity to amend the Registration Statement or any Prospectus to comply with the Securities Act or any other law. The Company shall not be required to disclose to the Investor the substance or specific reasons of any of the events set forth in clauses (i) through (iii) of the immediately preceding sentence, but rather, shall only be required to disclose that the event has occurred. If at any time the SEC, or any other federal or state governmental authority shall issue any stop order suspending the effectiveness of the Registration Statement or prohibiting or suspending the use of the Prospectus or Prospectus Supplement, the Company shall use its reasonable best efforts to obtain the withdrawal of such order at the earliest possible time. The Company shall furnish to the Investor, without charge, a copy of any correspondence from the SEC or the staff of the SEC, or any other federal or state governmental authority to the Company or its representatives relating to the Shelf Registration Statement, any New Registration Statement or any Prospectus, or Prospectus Supplement as the case may be. The Company shall not deliver to the Investor any Regular Purchase Notice, Accelerated Purchase Notice or Additional Accelerated Purchase Notice, and the Investor shall not be obligated to purchase any shares of Common Stock under this Agreement, during the continuation or pendency of any of the foregoing events. If at any time the SEC shall issue any stop order suspending the effectiveness of the Registration Statement or prohibiting or suspending the use of the Prospectus or any Prospectus Supplement, the Company shall use its reasonable best efforts to obtain the withdrawal of such order at the earliest possible time. The Company shall furnish to the Investor, without charge, a copy of any correspondence from the SEC or the staff of the SEC to the Company or its representatives relating to the Registration Statement or the Prospectus, as the case may be.
h. Listing on the Principal Market. The Company shall promptly secure the listing, or conditional listing as applicable, of all of the Purchase Shares and Commitment Shares to be issued to the Investor hereunder on the Principal Market (subject to standard listing conditions, if any, for transactions of this nature, official notice of issuance and the Exchange Cap) and upon each other national securities exchange or automated quotation system, if any, upon which the Common Stock are then listed, and shall maintain, so long as any Common Stock shall be so listed, such listing of all such Registrable Securities from time to time issuable hereunder. The Company shall use commercially reasonable efforts to maintain the listing of the Common Stock on the Principal Market and shall comply in all respects with the Company’s reporting, filing and other obligations under the bylaws or rules and regulations of the Principal Market. The Company shall not take any action that would reasonably be expected to result in the delisting or suspension of the Common Stock on the Principal Market. The Company shall promptly, and in no event later than the following Business Day, provide to the Investor copies of any notices it receives from any Person regarding the continued eligibility of the Common Stock for listing on the Principal Market; provided, however, that the Company shall not provide the Investor copies of any such notice that the Company reasonably believes constitutes material non-public information and that the Company would not be required to publicly disclose such notice in any report or statement filed with the SEC under the Exchange Act (including on Form 8-K) or the Securities Act. The Company shall pay all fees and expenses in connection with satisfying its obligations under this Section 3(h).
i. Delivery of Shares. The Company shall cooperate with the Investor to facilitate the timely preparation and delivery of DWAC Shares (not bearing any restrictive legend) representing the Registrable Securities to be offered pursuant to the Shelf Registration Statement or any New Registration Statement and enable such DWAC Shares to be in such denominations or amounts as the Investor may reasonably request and registered in such names as the Investor may request.
j. Transfer Agent. The Company shall at all times maintain the services of the Transfer Agent with respect to its Common Stock.
k. Approvals. The Company shall use its reasonable best efforts to cause the Registrable Securities covered by any Registration Statement to be Registered with or approved by such other governmental agencies or authorities in the United States as may be necessary to consummate the disposition of such Registrable Securities.
l. Confirmation of Effectiveness. If reasonably requested by the Investor at any time, the Company shall deliver to the Investor a written confirmation from Company’s counsel of whether or not the effectiveness of such Registration Statement has lapsed at any time for any reason (including, without limitation, the issuance of a stop order) and whether or not the Registration Statement is currently effective and available to the Company for sale of all of the Registrable Securities.
m. Further Assurances. The Company agrees to take all other reasonable actions as necessary and reasonably requested by the Investor to expedite and facilitate disposition by the Investor of Registrable Securities pursuant to any Registration Statement.
n. Suspension of Sales. The Investor agrees that, upon receipt of any notice from the Company of the existence of any suspension or stop order as set forth in Section 3(f) or 3(g), the Investor will immediately discontinue disposition of Registrable Securities pursuant to any Registration Statement covering such Registrable Securities until the Investor’s receipt of the copies of a notice regarding the resolution or withdrawal of the suspension or stop order as contemplated by Section 3(f) or 3(g). Notwithstanding anything to the contrary, the Company shall cause its transfer agent to promptly deliver to the Investor DWAC Shares without any restrictive legend in accordance with the terms of the Purchase Agreement in connection with any sale of Registrable Securities with respect to which the Investor has entered into a contract for sale prior to the Investor’s receipt of a notice from the Company of the happening of any event of the kind described in Section 3(f) or 3(g) and for which the Investor has not yet settled.
4. OBLIGATIONS OF THE INVESTOR.
a. Investor Information. The Company shall notify the Investor in writing of the information the Company reasonably requires from the Investor in connection with any registration statement hereunder. The Investor shall furnish to the Company such information regarding itself, the Registrable Securities held by it and the intended method of disposition of the Registrable Securities held by it as shall be reasonably required to effect the registration of such Registrable Securities and shall execute such documents in connection with such registration as the Company may reasonably request.
b. Investor Cooperation. The Investor agrees to cooperate with the Company as reasonably requested by the Company in connection with the preparation and filing of any amendments and supplements to any Registration Statement or New Registration Statement hereunder.
5. EXPENSES OF REGISTRATION.
All reasonable expenses of the Company, other than sales or brokerage commissions, and fees and disbursements of counsel for, and other expenses of, the Investor, incurred in connection with registrations, filings or qualifications pursuant to Sections 2 and 3, including, without limitation, all registration, listing and qualifications fees, printers and accounting fees, and fees and disbursements of counsel for the Company, shall be paid by the Company.
6. INDEMNIFICATION.
a. To the fullest extent permitted by law, the Company will, and hereby does, indemnify, hold harmless and defend the Investor, each Person, if any, who controls the Investor, the members, the directors, officers, partners, employees, members, managers, agents, representatives of the Investor and each Person, if any, who controls the Investor within the meaning of the Securities Act or the Securities Exchange Act of 1934, as amended (the “Exchange Act”) (each, an “Indemnified Person”), against any losses, claims, damages, liabilities, judgments, fines, penalties, charges, costs, reasonable attorneys’ fees, amounts paid in settlement (with the consent of the Company, such consent not to be unreasonably withheld) or reasonable expenses, joint or several, (collectively, “Claims”) reasonably incurred in investigating, preparing or defending any action, claim, suit, inquiry, proceeding, investigation or appeal taken from the foregoing by or before any court or governmental, administrative or other regulatory agency, body or the SEC, whether pending or threatened, whether or not an indemnified party is or may be a party thereto (“Indemnified Damages”), to which any of them may become subject insofar as such Claims (or actions or proceedings, whether commenced or threatened, in respect thereof) arise out of or are based upon: (i) any untrue statement or alleged untrue statement of a material fact in the Shelf Registration Statement, any New Registration Statement or any post-effective amendment thereto or in any filing made in connection with the qualification of the offering under the securities or other “blue sky” laws of any jurisdiction in which Registrable Securities are offered (“Blue Sky Filing”), or the omission or alleged omission to state a material fact required to be stated therein or necessary to make the statements therein not misleading, (ii) any untrue statement or alleged untrue statement of a material fact contained in the final Prospectus the omission or alleged omission to state therein any material fact necessary to make the statements made therein, in light of the circumstances under which the statements therein were made, not misleading, (iii) any violation or alleged violation by the Company of the Securities Act, the Exchange Act, any other law, including, without limitation, any state securities law, or any rule or regulation thereunder relating to the offer or sale of the Registrable Securities pursuant to the Shelf Registration Statement or any New Registration Statement or (iv) any material violation by the Company of this Agreement (the matters in the foregoing clauses (i) through (iv) being, collectively, “Violations”). The Company shall reimburse each Indemnified Person promptly as such expenses are incurred and are due and payable, for any reasonable out-of-pocket legal fees or other reasonable expenses incurred by them in connection with investigating or defending any such Claim. Notwithstanding anything to the contrary contained herein, the indemnification agreement contained in this Section 6(a): (i) shall not apply to a Claim by an Indemnified Person arising out of or based upon a Violation which occurs in reliance upon and in conformity with information about the Investor furnished in writing to the Company by such Indemnified Person expressly for use in connection with the preparation of the Registration Statement, any New Registration Statement, the Prospectus or any such amendment thereof or supplement thereto, in each case if the foregoing was timely made available by the Company pursuant to Section 3(c) or Section 3(e); (ii) with respect to any superseded prospectus, shall not inure to the benefit of any such Person from whom the Person asserting any such Claim purchased the Registrable Securities that are the subject thereof (or to the benefit of any other Indemnified Person) if the untrue statement or omission of material fact contained in the superseded prospectus was corrected in the revised prospectus, as then amended or supplemented, if such revised prospectus was timely made available by the Company pursuant to Section 3(c) or Section 3(e), and the Indemnified Person was promptly advised in writing not to use the incorrect prospectus prior to the use giving rise to a violation and such Indemnified Person, notwithstanding such advice, used it; (iii) shall not be available to the extent such Claim is based on a failure of the Investor to deliver or to cause to be delivered the prospectus made available by the Company, if such prospectus was timely made available by the Company pursuant to Section 3(c) or Section 3(e); and (iv) shall not apply to amounts paid in settlement of any Claim if such settlement is effected without the prior written consent of the Company, which consent shall not be unreasonably withheld. Such indemnity shall remain in full force and effect regardless of any investigation made by or on behalf of the Indemnified Person and shall survive the transfer of the Registrable Securities by the Investor pursuant to Section 8.
b. In connection with the Shelf Registration Statement, any New Registration Statement or Prospectus, the Investor agrees to indemnify, hold harmless and defend, to the same extent and in the same manner as is set forth in Section 6(a), the Company, each of its directors, each of its officers who signed the Shelf Registration Statement or signs any New Registration Statement, each Person, if any, who controls the Company within the meaning of the Securities Act or the Exchange Act (collectively and together with an Indemnified Person, an “Indemnified Party”), against any Claim or Indemnified Damages to which any of them may become subject, under the Securities Act, the Exchange Act or otherwise, insofar as such Claim or Indemnified Damages arise out of or are based upon any Violation, in each case to the extent, and only to the extent, that such Violation occurs in reliance upon and in conformity with written information about the Investor and furnished to the Company by the Investor expressly for use in connection with such Registration Statement; and, subject to Section 6(d), the Investor will reimburse any legal or other expenses reasonably incurred by them in connection with investigating or defending any such Claim; provided, however, that the indemnity agreement contained in this Section 6(b) and the agreement with respect to contribution contained in Section 7 shall not apply to amounts paid in settlement of any Claim if such settlement is effected without the prior written consent of the Investor, which consent shall not be unreasonably withheld; provided, further, however, that the Investor shall be liable under this Section 6(b) for only that amount of a Claim or Indemnified Damages as does not exceed the net proceeds to the Investor as a result of the sale of Registrable Securities pursuant to such Registration Statement. Such indemnity shall remain in full force and effect regardless of any investigation made by or on behalf of such Indemnified Party and shall survive the transfer of the Registrable Securities by the Investor pursuant to Section 8.
c. Promptly after receipt by an Indemnified Person or Indemnified Party under this Section 6 of notice of the commencement of any action or proceeding (including any governmental action or proceeding) involving a Claim, such Indemnified Person or Indemnified Party shall, if a Claim in respect thereof is to be made against any indemnifying party under this Section 6, deliver to the indemnifying party a written notice of the commencement thereof, and the indemnifying party shall have the right to participate in, and, to the extent the indemnifying party so desires, jointly with any other indemnifying party similarly notified, to assume control of the defense thereof with counsel mutually satisfactory to the indemnifying party and the Indemnified Person or the Indemnified Party, as the case may be; provided, however, that an Indemnified Person or Indemnified Party shall have the right to retain its own counsel with the fees and expenses to be paid by the indemnifying party, if, in the reasonable opinion of counsel retained by the indemnifying party, the representation by such counsel of the Indemnified Person or Indemnified Party and the indemnifying party would be inappropriate due to actual or potential differing interests between such Indemnified Person or Indemnified Party and any other party represented by such counsel in such proceeding. The Indemnified Party or Indemnified Person shall cooperate fully with the indemnifying party in connection with any negotiation or defense of any such action or claim by the indemnifying party and shall furnish to the indemnifying party all information reasonably available to the Indemnified Party or Indemnified Person which relates to such action or claim. The indemnifying party shall keep the Indemnified Party or Indemnified Person fully apprised at all times as to the status of the defense or any settlement negotiations with respect thereto. No indemnifying party shall be liable for any settlement of any action, claim or proceeding effected without its written consent, provided, however, that the indemnifying party shall not unreasonably withhold, delay or condition its consent. No indemnifying party shall, without the consent of the Indemnified Party or Indemnified Person, consent to entry of any judgment or enter into any settlement or other compromise which does not include as an unconditional term thereof the giving by the claimant or plaintiff to such Indemnified Party or Indemnified Person of a release from all liability in respect to such claim or litigation. Following indemnification as provided for hereunder, the indemnifying party shall be subrogated to all rights of the Indemnified Party or Indemnified Person with respect to all third parties, firms or corporations relating to the matter for which indemnification has been made. The failure to deliver written notice to the indemnifying party within a reasonable time of the commencement of any such action shall not relieve such indemnifying party of any liability to the Indemnified Person or Indemnified Party under this Section 6, except to the extent that the indemnifying party is prejudiced in its ability to defend such action.
d. The indemnification required by this Section 6 shall be made by periodic payments of the amount thereof during the course of the investigation or defense, as and when bills are received or Indemnified Damages are incurred. Any Person receiving a payment pursuant to this Section 6 which person is later determined to not be entitled to such payment shall return such payment to the person making it.
e. The indemnity agreements contained herein shall be in addition to (i) any cause of action or similar right of the Indemnified Party or Indemnified Person against the indemnifying party or others, and (ii) any liabilities the indemnifying party may be subject to pursuant to the law.
7. CONTRIBUTION.
To the extent any indemnification by an indemnifying party is prohibited or limited by law, the indemnifying party agrees to make the maximum contribution with respect to any amounts for which it would otherwise be liable under Section 6 to the fullest extent permitted by law; provided, however, that: (i) no seller of Registrable Securities guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the Securities Act) shall be entitled to contribution from any seller of Registrable Securities who was not guilty of fraudulent misrepresentation; and (ii) contribution by any seller of Registrable Securities shall be limited in amount to the net amount of proceeds received by such seller from the sale of such Registrable Securities.
8. ASSIGNMENT OF REGISTRATION RIGHTS.
The Company shall not assign this Agreement or any rights or obligations hereunder without the prior written consent of the Investor; provided, however, that any transaction, whether by merger, reorganization, restructuring, consolidation, financing or otherwise, whereby the Company remains the surviving entity immediately after such transaction shall not be deemed an assignment. The Investor may not assign its rights under this Agreement without the prior written consent of the Company, other than to an affiliate of the Investor controlled by Jonathan Cope or Josh Scheinfeld, in which case the assignee must agree in writing to be bound by the terms and conditions of this Agreement.
9. AMENDMENT OF REGISTRATION RIGHTS.
No provision of this Agreement may be amended or waived by the parties from and after the date that is one Business Day immediately preceding the initial filing of the Initial Prospectus Supplement with the SEC. Subject to the immediately preceding sentence, no provision of this Agreement may be (i) amended other than by a written instrument signed by both parties hereto or (ii) waived other than in a written instrument signed by the party against whom enforcement of such waiver is sought. Failure of any party to exercise any right or remedy under this Agreement or otherwise, or delay by a party in exercising such right or remedy, shall not operate as a waiver thereof.
10. MISCELLANEOUS.
a. Notices. Any notices, consents, waivers or other communications required or permitted to be given under the terms of this Agreement must be in writing and will be deemed to have been delivered: (i) upon receipt, when delivered personally; (ii) upon receipt, when sent by facsimile or email (provided confirmation of transmission is mechanically or electronically generated and kept on file by the sending party); or (iii) one (1) Business Day after deposit with a nationally recognized overnight delivery service, in each case properly addressed to the party to receive the same. The addresses for such communications shall be:
If to the Company:
Anavex Life Sciences Corp.
630 5th Avenue, 20th Floor
New York, New York 10111
Telephone: (844) 689-3939
E-mail: cmissling@anavexcorp.com
Attention: Christopher Missling, PhD., CEO
With a copy to (which shall not constitute notice or service of process):
K&L Gates LLP
200 S. Biscayne Blvd., Ste. 3900
Miami, Florida 33131
Telephone: (305) 539-3306
Facsimile: (305) 358-7095
E-mail: clayton.parker@klgates.com
Attention: Clayton E. Parker, Esq.
If to the Investor:
Lincoln Park Capital Fund, LLC
440 North Wells, Suite 410
Chicago, Illinois 60654
Telephone: (312) 822-9300
Facsimile: (312) 822-9301
E-mail: jscheinfeld@lpcfunds.com/jcope@lpcfunds.com
Attention: Josh Scheinfeld/Jonathan Cope
If to the Transfer Agent:
Nevada Agency and Transfer Company
50 West Liberty Street, Suite 880
Reno, NV 89501
Tel: (775) 322-0626
Fax: (775) 322-5623
E-mail: tiffany@natco.org
Attention: Tiffany Baxter
or at such other address and/or facsimile number and/or to the attention of such other person as the recipient party has specified by written notice given to each other party three (3) Business Days prior to the effectiveness of such change. Written confirmation of receipt (A) given by the recipient of such notice, consent, waiver or other communication, (B) mechanically or electronically generated by the sender’s facsimile machine or email account containing the time, date, recipient facsimile number or email address, as applicable, and an image of the first page of such transmission or (C) provided by a nationally recognized overnight delivery service, shall be rebuttable evidence of personal service, receipt by facsimile or receipt from a nationally recognized overnight delivery service in accordance with clause (i), (ii) or (iii) above, respectively.
b. Governing Law. The corporate laws of the State of Nevada shall govern all issues concerning the relative rights of the Company and its stockholders. All other questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be governed by the internal laws of the State of Illinois, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of Illinois or any other jurisdictions) that would cause the application of the laws of any jurisdictions other than the State of Illinois. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting the State of Illinois, County of Cook, for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding is improper. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof to such party at the address for such notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. If any provision of this Agreement shall be invalid or unenforceable in any jurisdiction, such invalidity or unenforceability shall not affect the validity or enforceability of the remainder of this Agreement in that jurisdiction or the validity or enforceability of any provision of this Agreement in any other jurisdiction. EACH PARTY HEREBY IRREVOCABLY WAIVES ANY RIGHT IT MAY HAVE, AND AGREES NOT TO REQUEST, A JURY TRIAL FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR IN CONNECTION HEREWITH OR ARISING OUT OF THIS AGREEMENT OR ANY TRANSACTION CONTEMPLATED HEREBY.
c. Integration. This Agreement and the Purchase Agreement and the exhibits and schedules hereto and thereto constitute the entire agreement among the parties hereto with respect to the subject matter hereof and thereof. There are no restrictions, promises, warranties or undertakings, other than those set forth or referred to herein and therein. This Agreement and the Purchase Agreement supersede all prior agreements and understandings among the parties hereto with respect to the subject matter hereof and thereof.
d. Survival. Subject to the requirements of Section 8, this Agreement shall inure to the benefit of and be binding upon the successors and permitted assigns of each of the parties hereto.
e. Headings. The headings in this Agreement are for convenience of reference only and shall not limit or otherwise affect the meaning hereof.
f. Counterparts. This Agreement may be executed in identical counterparts, each of which shall be deemed an original but all of which shall constitute one and the same agreement. This Agreement, once executed by a party, may be delivered to the other party hereto by facsimile transmission or by e-mail in a “.pdf” format data file of a copy of this Agreement bearing the signature of the party so delivering this Agreement.
g. Further Assurances. Each party shall do and perform, or cause to be done and performed, all such further acts and things, and shall execute and deliver all such other agreements, certificates, instruments and documents, as the other party may reasonably request in order to carry out the intent and accomplish the purposes of this Agreement and the consummation of the transactions contemplated hereby.
h. No Strict Construction. The language used in this Agreement will be deemed to be the language chosen by the parties to express their mutual intent and no rules of strict construction will be applied against any party.
i. No Third Party Benefits. This Agreement is intended for the benefit of the parties hereto and their respective successors and permitted assigns, and is not for the benefit of, nor may any provision hereof be enforced by, any other Person, except with respect to Section 6 hereto.
* * * * * *
IN WITNESS WHEREOF, the parties have caused this Registration Rights Agreement to be duly executed as of day and year first above written.
THE COMPANY:
ANAVEX LIFE SCIENCES CORP.
| By: | /s/ Christopher Missling | |
| Name: | Christopher Missling, PhD. | |
| Title: | Chief Executive Officer |
BUYER:
LINCOLN PARK CAPITAL FUND, LLC
BY: LINCOLN PARK CAPITAL, LLC
BY: ROCKLEDGE CAPITAL CORPORATION
| By: | /s/ Josh Scheinfeld | |
| Name: | Josh Scheinfeld | |
| Title: | President |
11
EXHIBIT 10.1
PURCHASE AGREEMENT
THIS PURCHASE AGREEMENT (the “Agreement”), dated as of February 3, 2023 by and between ANAVEX LIFE SCIENCES CORP., a Nevada corporation (the “Company”), and LINCOLN PARK CAPITAL FUND, LLC, an Illinois limited liability company (the “Investor”). Capitalized terms used herein and not otherwise defined herein are defined in Section 1 hereof.
WHEREAS:
Subject to the terms and conditions set forth in this Agreement, the Company wishes to sell to the Investor, and the Investor wishes to buy from the Company, up to One Hundred Fifty Million Dollars ($150,000,000) of the Company’s common stock, $0.001 par value per share (the “Common Stock”). The shares of Common Stock to be purchased hereunder are referred to herein as the “Purchase Shares.”
NOW THEREFORE, in consideration of the mutual covenants contained in this Agreement, and for other good and valuable consideration, the receipt and adequacy of which are hereby acknowledged, the Company and the Investor hereby agree as follows:
1. CERTAIN DEFINITIONS.
For purposes of this Agreement, the following terms shall have the following meanings:
(a) “Accelerated Purchase Date” means, with respect to any Accelerated Purchase made pursuant to Section 2(b) hereof, the Business Day immediately following the applicable Purchase Date with respect to the corresponding Regular Purchase referred to in Section 2(b) hereof.
(b) “Accelerated Purchase Minimum Price Threshold” means, with respect to any Accelerated Purchase made pursuant to Section 2(b) hereof, any minimum per share price threshold set forth in the applicable Accelerated Purchase Notice.
(c) “Accelerated Purchase Notice” means, with respect to any Accelerated Purchase made pursuant to Section 2(b) hereof, an irrevocable written notice from the Company to the Investor directing the Investor to buy a specified Accelerated Purchase Share Amount on the applicable Accelerated Purchase Date pursuant to Section 2(b) hereof at the applicable Accelerated Purchase Price on the Accelerated Purchase Date for such Accelerated Purchase in accordance with this Agreement, and specifying any Accelerated Purchase Minimum Price Threshold determined by the Company and including the Accelerated Purchase Share Estimate.
(d) “Accelerated Purchase Price” means, with respect to an Accelerated Purchase made pursuant to Section 2(b) hereof, the lower of ninety-six percent (96%) of (i) the VWAP for the period beginning at 9:30:01 a.m., Eastern time, on the applicable Accelerated Purchase Date, or such other time publicly announced by the Principal Market as the official open (or commencement) of trading on the Principal Market on such applicable Accelerated Purchase Date (the “Accelerated Purchase Commencement Time”), and ending at the earliest of (A) 4:00:00 p.m., Eastern time, on such applicable Accelerated Purchase Date, or such other time publicly announced by Principal Market as the official close of trading on the Principal Market on such applicable Accelerated Purchase Date, (B) such time, from and after the Accelerated Purchase Commencement Time for such Accelerated Purchase, that the total number (or volume) of shares of Common Stock traded on the Principal Market has exceeded the applicable Accelerated Purchase Share Volume Maximum, and (C) such time, from and after the Accelerated Purchase Commencement Time for such Accelerated Purchase, that the Sale Price has fallen below the applicable Accelerated Purchase Minimum Price Threshold (such earliest of (i)(A), (i)(B) and (i)(C) above, the “Accelerated Purchase Termination Time”), and (ii) the Closing Sale Price of the Common Stock on such applicable Accelerated Purchase Date (each to be appropriately adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction).
(e) “Accelerated Purchase Share Amount” means, with respect to an Accelerated Purchase made pursuant to Section 2(b) hereof, the number of Purchase Shares directed by the Company to be purchased by the Investor in an Accelerated Purchase Notice, which number of Purchase Shares shall not exceed the lesser of (i) 1,000% of the number of Purchase Shares directed by the Company to be purchased by the Investor pursuant to the corresponding Regular Purchase Notice for the corresponding Regular Purchase referred to in clause (i) of the second sentence of Section 2(b) hereof (subject to the Purchase Share limitations contained in Section 2(b) hereof) and (ii) an amount equal to (A) the Accelerated Purchase Share Percentage multiplied by (B) the total number (or volume) of shares of Common Stock traded on the Principal Market during the period on the applicable Accelerated Purchase Date beginning at the Accelerated Purchase Commencement Time for such Accelerated Purchase and ending at the Accelerated Purchase Termination Time for such Accelerated Purchase.
(f) “Accelerated Purchase Share Estimate” means, with respect to any Accelerated Purchase made pursuant to Section 2(b) hereof, a good faith estimate by the Company of the number of Purchase Shares that the Investor shall have the obligation to buy pursuant to the Accelerated Purchase Notice.
(g) “Accelerated Purchase Share Percentage” means, with respect to any Accelerated Purchase made pursuant to Section 2(b) hereof, thirty percent (30%).
(h) “Accelerated Purchase Share Volume Maximum” means, with respect to an Accelerated Purchase made pursuant to Section 2(b) hereof, a number of shares of Common Stock equal to (i) the applicable Accelerated Purchase Share Amount to be purchased by the Investor pursuant to the applicable Accelerated Purchase Notice for such Accelerated Purchase, divided by (ii) the Accelerated Purchase Share Percentage (to be appropriately adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction).
(i) “Additional Accelerated Purchase Date” means, with respect to an Additional Accelerated Purchase made pursuant to Section 2(c) hereof, the Business Day (i) that is the Accelerated Purchase Date with respect to the corresponding Accelerated Purchase referred to in Section 2(b) hereof and (ii) on which the Investor receives, prior to 1:00 p.m., Eastern time, on such Business Day, a valid Additional Accelerated Purchase Notice for such Additional Accelerated Purchase in accordance with this Agreement.
(j) “Additional Accelerated Purchase Minimum Price Threshold” means, with respect to an Additional Accelerated Purchase made pursuant to Section 2(c) hereof, any minimum per share price threshold set forth in the applicable Additional Accelerated Purchase Notice.
(k) “Additional Accelerated Purchase Notice” means, with respect to an Additional Accelerated Purchase made pursuant to Section 2(c) hereof, an irrevocable written notice from the Company to the Investor directing the Investor to purchase the applicable Additional Accelerated Purchase Share Amount at the Additional Accelerated Purchase Price for such Additional Accelerated Purchase in accordance with this Agreement, and specifying any Additional Accelerated Purchase Minimum Price Threshold determined by the Company and including the Accelerated Purchase Share Estimate.
(l) “Additional Accelerated Purchase Price” means, with respect to an Additional Accelerated Purchase made pursuant to Section 2(c) hereof, the lower of ninety-six percent (96%) of (i) the VWAP for the period on the applicable Additional Accelerated Purchase Date, beginning at the latest of (A) the applicable Accelerated Purchase Termination Time with respect to the corresponding Accelerated Purchase referred to in Section 2(c) hereof on such Additional Accelerated Purchase Date, (B) the applicable Additional Accelerated Purchase Termination Time with respect to the most recently completed prior Additional Accelerated Purchase on such Additional Accelerated Purchase Date, as applicable, and (C) the time at which all Purchase Shares subject to all prior Accelerated Purchases and Additional Accelerated Purchases (as applicable), including, without limitation, those that have been effected on the same Business Day as the applicable Additional Accelerated Purchase Date with respect to which the applicable Additional Accelerated Purchase relates, have theretofore been received by the Investor as DWAC Shares in accordance with this Agreement (such latest of (i)(A), (i)(B) and (i)(C) above, the “Additional Accelerated Purchase Commencement Time”), and ending at the earliest of (X) 4:00 p.m., Eastern time, on such Additional Accelerated Purchase Date, or such other time publicly announced by the Principal Market as the official close of trading on the Principal Market on such Additional Accelerated Purchase Date, (Y) such time, from and after the Additional Accelerated Purchase Commencement Time for such Additional Accelerated Purchase, that the total number (or volume) of shares of Common Stock traded on the Principal Market has exceeded the applicable Additional Accelerated Purchase Share Volume Maximum, and (Z) such time, from and after the Additional Accelerated Purchase Commencement Time for such Additional Accelerated Purchase, that the Sale Price has fallen below the applicable Additional Accelerated Purchase Minimum Price Threshold (if any) (such earliest of (i)(X), (i)(Y) and (i)(Z) above, the “Additional Accelerated Purchase Termination Time”), and (ii) the Closing Sale Price of the Common Stock on such Additional Accelerated Purchase Date (each to be appropriately adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction).
(m) “Additional Accelerated Purchase Share Amount” means, with respect to an Additional Accelerated Purchase made pursuant to Section 2(c) hereof, the number of Purchase Shares directed by the Company to be purchased by the Investor on an Additional Accelerated Purchase Notice, which number of Purchase Shares shall not exceed the lesser of (i) 1,000% of the number of Purchase Shares directed by the Company to be purchased by the Investor pursuant to the corresponding Regular Purchase Notice for the corresponding Regular Purchase referred to in Section 2(c) hereof (subject to the Purchase Share limitations contained in Section 2(a) hereof) and (ii) an amount equal to (A) the Additional Accelerated Purchase Share Percentage multiplied by (B) the total number (or volume) of shares of Common Stock traded on the Principal Market during the period on the applicable Additional Accelerated Purchase Date beginning at the Additional Accelerated Purchase Commencement Time for such Additional Accelerated Purchase and ending at the Additional Accelerated Purchase Termination Time for such Additional Accelerated Purchase.
(n) “Additional Accelerated Purchase Share Estimate” means, with respect to any Additional Accelerated Purchase made pursuant to Section 2(c) hereof, a good faith estimate by the Company of the number of Purchase Shares that the Investor shall have the obligation to buy pursuant to the Additional Accelerated Purchase Notice.
(o) “Additional Accelerated Purchase Share Percentage” means, with respect to an Additional Accelerated Purchase made pursuant to Section 2(c) hereof, thirty percent (30%).
(p) “Additional Accelerated Purchase Share Volume Maximum” means, with respect to an Additional Accelerated Purchase made pursuant to Section 2(c) hereof, a number of shares of Common Stock equal to (i) the applicable Additional Accelerated Purchase Share Amount properly directed by the Company to be purchased by the Investor in the applicable Additional Accelerated Purchase Notice for such Additional Accelerated Purchase, divided by (ii) the Additional Accelerated Purchase Share Percentage (to be appropriately adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction).
(q) “Alternate Adjusted Regular Purchase Share Limit” means, with respect to a Regular Purchase made pursuant to Section 2(a) hereof, the maximum number of Purchase Shares which, taking into account the applicable per share Purchase Price therefor calculated in accordance with this Agreement, would enable the Company to deliver to the Investor, on the applicable Purchase Date for such Regular Purchase, a Regular Purchase Notice for a Purchase Amount equal to, or as closely approximating without exceeding, Four Hundred Thousand Dollars ($400,000).
(r) “Available Amount” means, initially, One Hundred Fifty Million Dollars ($150,000,000) in the aggregate, which amount shall be reduced by the Purchase Amount each time the Investor purchases shares of Common Stock pursuant to Section 2 hereof.
(s) “Average Price” means a price per Purchase Share (rounded to the nearest tenth of a cent) equal to the quotient obtained by dividing (i) the aggregate gross purchase price paid by the Investor for all Purchase Shares purchased pursuant to this Agreement, by (ii) the aggregate number of Purchase Shares issued pursuant to this Agreement.
(t) “Bankruptcy Law” means Title 11, U.S. Code, or any similar federal or state law for the relief of debtors.
(u) “Base Price” means a price per Purchase Share equal to the sum of (i) the Signing Market Price and (ii) $ $0.106 (subject to adjustment for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction that occurs on or after the date of this Agreement).
(v) “Base Prospectus” means the Company’s final base prospectus, dated September 24, 2021, a preliminary form of which is included in the Registration Statement, including the documents incorporated by reference therein.
(w) “Business Day” means any day on which the Principal Market is open for trading, including any day on which the Principal Market is open for trading for a period of time less than the customary time.
(x) “Closing Sale Price” means, for any security as of any date, the last closing sale price for such security on the Principal Market as reported by the Principal Market.
(y) “Confidential Information” means any information disclosed by either party to the other party, either directly or indirectly, in writing, orally or by inspection of tangible objects (including, without limitation, documents, prototypes, samples, plant and equipment), which is designated as “Confidential,” “Proprietary” or some similar designation. Information communicated orally shall be considered Confidential Information if such information is confirmed in writing as being Confidential Information within ten (10) Business Days after the initial disclosure. Confidential Information may also include information disclosed to a disclosing party by third parties. Confidential Information shall not, however, include any information which (i) was publicly known and made generally available in the public domain prior to the time of disclosure by the disclosing party; (ii) becomes publicly known and made generally available after disclosure by the disclosing party to the receiving party through no action or inaction of the receiving party; (iii) is already in the possession of the receiving party without confidentiality restriction at the time of disclosure by the disclosing party as shown by the receiving party’s files and records immediately prior to the time of disclosure; (iv) is obtained by the receiving party from a third party without a breach of such third party’s obligations of confidentiality; (v) is independently developed by the receiving party without use of or reference to the disclosing party’s Confidential Information, as shown by documents and other competent evidence in the receiving party’s possession; or (vi) is required by law to be disclosed by the receiving party, provided that the receiving party gives the disclosing party prompt written notice of such requirement prior to such disclosure and assistance in obtaining an order protecting the information from public disclosure.
(z) “Custodian” means any receiver, trustee, assignee, liquidator or similar official under any Bankruptcy Law.
(aa) “DTC” means The Depository Trust Company, or any successor performing substantially the same function for the Company.
(bb) “DWAC Shares” means shares of Common Stock that are (i) issued in electronic form, (ii) freely tradable and transferable and without restriction on sale and (iii) timely credited by the Company to the Investor’s or its designee’s specified Deposit/Withdrawal at Custodian (DWAC) account with DTC under its Fast Automated Securities Transfer (FAST) Program, or any similar program hereafter adopted by DTC performing substantially the same function.
(cc) “Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
(dd) “Fully Adjusted Regular Purchase Share Limit” means, with respect to any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction from and after the date of this Agreement, the Regular Purchase Share Limit (as defined in Section 2(a) hereof) in effect on the applicable date of determination, after giving effect to the full proportionate adjustment thereto made pursuant to Section 2(a) hereof for or in respect of such reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction.
(ee) “Initial Prospectus Supplement” means the prospectus supplement of the Company relating to the Securities, including the accompanying Base Prospectus, to be prepared and filed by the Company with the SEC pursuant to Rule 424(b)(5) under the Securities Act and in accordance with Section 5(a) hereof, together with all documents and information incorporated therein by reference.
(ff) “Material Adverse Effect” means any material adverse effect on (i) the enforceability of any Transaction Document, (ii) the results of operations, assets, business or financial condition of the Company and its Subsidiaries, taken as a whole, other than any material adverse effect that resulted exclusively from (A) any change in the United States or foreign economies or securities or financial markets in general that does not have a disproportionate effect on the Company and its Subsidiaries, taken as a whole, (B) any change that generally affects the industry in which the Company and its Subsidiaries operate that does not have a disproportionate effect on the Company and its Subsidiaries, taken as a whole, (C) any change arising in connection with earthquakes, hostilities, acts of war, sabotage or terrorism or military actions or any escalation or material worsening of any such hostilities, acts of war, sabotage or terrorism or military actions existing as of the date hereof, (D) any action taken by the Investor, its affiliates or its or their successors and assigns with respect to the transactions contemplated by this Agreement, (E) the effect of any change in applicable laws or accounting rules that does not have a disproportionate effect on the Company and its Subsidiaries, taken as a whole, or (F) any change resulting from compliance with terms of this Agreement or the consummation of the transactions contemplated by this Agreement, or (iii) the Company’s ability to perform in any material respect on a timely basis its obligations under any Transaction Document to be performed as of the date of determination.
(gg) “Maturity Date” means the first day of the month immediately following the thirty-six (36) month anniversary of the Commencement Date.
(hh) “PEA Period” means the period commencing at 9:30 a.m., Eastern time, on the fifth (5th) Business Day immediately prior to the filing of any post-effective amendment to the Registration Statement (as defined herein) or New Registration Statement (as such term is defined in the Registration Rights Agreement), and ending at 9:30 a.m., Eastern time, on the Business Day immediately following, the effective date of any post-effective amendment to the Registration Statement (as defined herein) or New Registration Statement (as such term is defined in the Registration Rights Agreement).
(ii) “Person” means an individual or entity including but not limited to any limited liability company, a partnership, a joint venture, a corporation, a trust, an unincorporated organization and a government or any department or agency thereof.
(jj) “Principal Market” means the NASDAQ Capital Market (or any nationally recognized successor thereto); provided, however, that in the event the Company’s Common Stock is ever listed or traded on, the NASDAQ Global Market, the NASDAQ Global Select Market, the New York Stock Exchange, the NYSE American, the NYSE Arca, or the OTC Bulletin Board the OTCQX or OTCQB operated by the OTC Markets Group, Inc. (or any nationally recognized successor to any of the foregoing), then the “Principal Market” shall mean such other market or exchange on which the Company’s Common Stock is then listed or traded.
(kk) “Prospectus” means the Base Prospectus, as supplemented by any Prospectus Supplement (including the Initial Prospectus Supplement), including the documents and information incorporated by reference therein.
(ll) “Prospectus Supplement” means any prospectus supplement to the Base Prospectus (including the Initial Prospectus Supplement) filed with the SEC pursuant to Rule 424(b) under the Securities Act in connection with the transactions contemplated by this Agreement, including the documents and information incorporated by reference therein.
(mm) “Purchase Amount” means, with respect to any Regular Purchase, any Accelerated Purchase or any Additional Accelerated Purchase made hereunder, the portion of the Available Amount to be purchased by the Investor pursuant to Section 2 hereof.
(nn) “Purchase Date” means, with respect to any Regular Purchase made pursuant to Section 2(a) hereof, the Business Day on which the Investor receives a valid Regular Purchase Notice for such Regular Purchase in accordance with this Agreement; provided that if a Regular Purchase Notice is delivered by 8:30 a.m. Eastern time, the Purchase Date for such Regular Purchase shall be the prior Business Day.
(oo) “Purchase Price” means, with respect to any Regular Purchase made pursuant to Section 2(a) hereof, the lower of: (i) the lowest Sale Price on the applicable Purchase Date and (ii) the arithmetic average of the three (3) lowest Closing Sale Prices for the Common Stock during the ten (10) consecutive Business Days ending on the Business Day immediately preceding such Purchase Date (in each case, to be appropriately adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction that occurs on or after the date of this Agreement).
(pp) “Registration Rights Agreement” means that certain Registration Rights Agreement, of even date herewith between the Company and the Investor.
(qq) “Registration Statement” has the meaning set forth in the Registration Rights Agreement.
(rr) “Regular Purchase Notice” means, with respect to any Regular Purchase pursuant to Section 2(a) hereof, an irrevocable written notice from the Company to the Investor directing the Investor to buy such applicable amount of Purchase Shares at the applicable Purchase Price as specified by the Company therein on the applicable Purchase Date for such Regular Purchase.
(ss) “Sale Price” means any trade price for the shares of Common Stock on the Principal Market as reported by the Principal Market.
(tt) “SEC” means the U.S. Securities and Exchange Commission.
(uu) “Securities” means, collectively, the Purchase Shares and the Commitment Shares.
(vv) “Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
(ww) “Signing Market Price” means $10.908, representing the consolidated closing bid price of the Common Stock on The NASDAQ Capital Market on the date of this Agreement, representing the lower of (i) the closing price of the Common Stock on the Nasdaq Capital Market immediately preceding the date of this Agreement or (ii) the average of the closing price of the Common Stock on the Nasdaq Capital Market for the five Business Days immediately preceding the signing of this Agreement.
(xx) “Subsidiary” means any Person the Company wholly-owns or controls, or in which the Company, directly or indirectly, owns a majority of the voting stock or similar voting interest, in each case that would be disclosable pursuant to Item 601(b)(21) of Regulation S-K promulgated under the Securities Act.
(yy) “Transaction Documents” means, collectively, this Agreement and the schedules and exhibits hereto, the Registration Rights Agreement and the schedules and exhibits thereto, and each of the other agreements, documents, certificates and instruments entered into or furnished by the parties hereto in connection with the transactions contemplated hereby and thereby.
(zz) “Transfer Agent” means Nevada Agency and Transfer Company, or such other Person who is then serving as the transfer agent for the Company in respect of the Common Stock.
(aaa) “VWAP” means in respect of an applicable Accelerated Purchase Date and an Additional Accelerated Purchase Date, as applicable, the volume weighted average price of the Common Stock on the Principal Market, as reported on the Principal Market or by another reputable source such as Bloomberg, L.P.
2. PURCHASE OF COMMON STOCK.
Subject to the terms and conditions set forth in this Agreement, the Company has the right, but not the obligation, to sell to the Investor, and the Investor, in the Company’s sole and absolute discretion, has the obligation to purchase from the Company, Purchase Shares as follows:
(a) Commencement of Regular Sales of Common Stock. Upon the satisfaction of the conditions set forth in Sections 7 and 8 hereof (the “Commencement” and the date of satisfaction of such conditions the “Commencement Date”) and thereafter, the Company shall have the right, but not the obligation, to direct the Investor, by its delivery to the Investor of a Regular Purchase Notice from time to time, to purchase up to Two Hundred Thousand (200,000) Purchase Shares, subject to adjustment as set forth below in this Section 2(a) (such maximum number of Purchase Shares, as may be adjusted from time to time, the “Regular Purchase Share Limit”), at the Purchase Price on the Purchase Date (each such purchase a “Regular Purchase”); provided, however, that if, after giving effect to the full proportionate adjustment to the Regular Purchase Share Limit therefor, the Fully Adjusted Regular Purchase Share Limit then in effect would preclude the Company from delivering to the Investor a Regular Purchase Notice hereunder for a Purchase Amount (calculated by multiplying (X) the number of Purchase Shares equal to the Fully Adjusted Regular Purchase Share Limit, by (Y) the Purchase Price per Purchase Share covered by such Regular Purchase Notice on the applicable Purchase Date therefor) equal to or greater than Two Hundred Thousand Dollars ($200,000), the Regular Purchase Share Limit for such Regular Purchase Notice shall not be fully adjusted to equal the applicable Fully Adjusted Regular Purchase Share Limit, but rather the Regular Purchase Share Limit for such Regular Purchase Notice shall be adjusted to equal the applicable Alternate Adjusted Regular Purchase Share Limit as of the applicable Purchase Date for such Regular Purchase Notice; and provided, further, however, that the Investor’s committed obligation under any single Regular Purchase, other than any Regular Purchase with respect to which an Alternate Adjusted Regular Purchase Share Limit shall apply, shall not exceed Four Million Dollars ($4,000,000), unless the parties mutually agree to increase the Regular Purchase Share Limit on any Purchase Date up to Two Million (2,000,000) Purchase Shares. If the Company delivers any Regular Purchase Notice for a Purchase Amount in excess of the limitations contained in the immediately preceding sentence, such Regular Purchase Notice shall be void ab initio to the extent of the amount by which the amount of Purchase Shares set forth in such Regular Purchase Notice exceeds the amount of Purchase Shares which the Company is permitted to include in such Regular Purchase Notice in accordance herewith, and the Investor shall have no obligation to purchase such excess Purchase Shares in respect of such Regular Purchase Notice; provided that the Investor shall remain obligated to purchase the amount of Purchase Shares which the Company is permitted to include in such Regular Purchase Notice. The Company may deliver Regular Purchase Notices to the Investor as often as every Business Day, and upon mutual agreement of the parties may deliver multiple Purchase Notices in a Business Day, in each case as long as the Company has not failed to deliver Purchase Shares subject to all prior Regular Purchases, Accelerated Purchases and Additional Accelerated Purchases, including, without limitation, those that have been effected on the same Business Day as the applicable Purchase Date, have theretofore been received by the Investor as DWAC Shares in accordance with this Agreement. Notwithstanding the foregoing, the Company shall not deliver any Regular Purchase Notices during the PEA Period.
(b) Accelerated Purchases. Subject to the terms and conditions of this Agreement, beginning on the Commencement Date, in addition to purchases of Purchase Shares as described in Section 2(a) above, the Company shall also have the right, but not the obligation, to direct the Investor, by its delivery to the Investor of an Accelerated Purchase Notice from time to time in accordance with this Agreement, to purchase the applicable Accelerated Purchase Share Amount at the Accelerated Purchase Price on the Accelerated Purchase Date therefor in accordance with this Agreement (each such purchase, an “Accelerated Purchase”); provided, however, that the Investor shall not be required to buy a number of Purchase Shares in an Accelerated Purchase that exceeds the Accelerated Purchase Share Estimate. The Company may deliver an Accelerated Purchase Notice to the Investor only on a Purchase Date on which (i) the Company also properly submitted a Regular Purchase Notice providing for a Regular Purchase of not less than 50,000 shares and (ii) if all Purchase Shares subject to all prior Regular Purchases, Accelerated Purchases and Additional Accelerated Purchases, including, without limitation, those that have been effected on the same Business Day as the applicable Accelerated Purchase Date with respect to which the applicable Accelerated Purchase relates, have theretofore been received by the Investor as DWAC Shares in accordance with this Agreement; provided, further, however, that the parties may mutually agree to increase the Accelerated Purchase Share Amount on any Accelerated Purchase Date at the applicable Accelerated Purchase Price. Each Accelerated Purchase Notice must be accompanied by the Accelerated Purchase Share Estimate for such Accelerated Purchase, and such Accelerated Purchase Notice shall be void ab initio to the extent of the amount by which the number of Purchase Shares set forth in such Accelerated Purchase Notice exceeds the number of Purchase Shares set forth in the Accelerated Purchase Share Estimate. The Investor shall immediately return to the Company such number of Purchase Shares issued pursuant to the Accelerated Purchase Share Estimate that exceeds the Accelerated Purchase Share Amount. Within one (1) Business Day after each Accelerated Purchase Date, the Accelerated Purchase Share Amount and the applicable Accelerated Purchase Price shall be set forth on a confirmation of the Accelerated Purchase to be provided to the Company by the Investor (an “Accelerated Purchase Confirmation”). Notwithstanding the foregoing, the Company shall not deliver any Accelerated Purchase Notices during the PEA Period.
(c) Additional Accelerated Purchases. Subject to the terms and conditions of this Agreement, beginning one (1) Business Day following the Commencement Date and thereafter, in addition to purchases of Purchase Shares as described in Section 2(a) and Section 2(b) above, the Company shall also have the right, but not the obligation, to direct the Investor, by its timely delivery to the Investor of an Additional Accelerated Purchase Notice on an Additional Accelerated Purchase Date in accordance with this Agreement, to purchase the applicable Additional Accelerated Purchase Share Amount at the applicable Additional Accelerated Purchase Price therefor in accordance with this Agreement (each such purchase, an “Additional Accelerated Purchase”); provided, however, that the Investor shall not be required to buy a number of Purchase Shares in an Additional Accelerated Purchase that exceeds the Additional Accelerated Purchase Share Estimate. The Company may deliver multiple Additional Accelerated Purchase Notices to the Investor on an Additional Accelerated Purchase Date; provided, however, that the Company may deliver an Additional Accelerated Purchase Notice to the Investor only (i) on a Business Day that is also the Accelerated Purchase Date for an Accelerated Purchase with respect to which the Company properly submitted to the Investor an Accelerated Purchase Notice in accordance with this Agreement on the applicable Purchase Date for a Regular Purchase of a number of Purchase Shares not less than the Regular Purchase Share Limit then in effect in accordance with this Agreement and (ii) if all Purchase Shares subject to all prior Regular Purchases, Accelerated Purchases and Additional Accelerated Purchases, including, without limitation, those that have been effected on the same Business Day as the applicable Additional Accelerated Purchase Date with respect to which the applicable Additional Accelerated Purchase relates, have theretofore been received by the Investor as DWAC Shares in accordance with this Agreement and provided, further, however, that the parties may mutually agree to increase the Additional Accelerated Purchase Share Amount applicable to any Accelerated Purchase, and all of the Purchase Shares subject to such increased Accelerated Purchase shall be purchased by the Investor at the Accelerated Purchase Price for such increased Accelerated Purchase in accordance with this Agreement. Each Additional Accelerated Purchase Notice must be accompanied by the Additional Accelerated Purchase Share Estimate for such Additional Accelerated Purchase, and such Additional Accelerated Purchase Notice shall be void ab initio to the extent of the amount by which the number of Purchase Shares set forth in such Additional Accelerated Purchase Notice exceeds the number of Purchase Shares set forth in the Additional Accelerated Purchase Share Estimate. The Investor shall immediately return to the Company such number of Purchase Shares issued pursuant to the Additional Accelerated Purchase Share Estimate that exceeds the Accelerated Purchase Share Amount. Within one (1) Business Day after each Additional Accelerated Purchase Date, the Additional Accelerated Purchase Share Amount and the applicable Additional Accelerated Purchase Price shall be set forth on a confirmation of the Additional Accelerated Purchase to be provided to the Company by the Investor (an “Additional Accelerated Purchase Confirmation”).
(d) Payment for Purchase Shares. For each Regular Purchase, the Investor shall pay to the Company an amount equal to the Purchase Amount with respect to such Regular Purchase as full payment for such Purchase Shares via wire transfer of immediately available funds on the same Business Day that the Investor receives such Purchase Shares, if such Purchase Shares are received by the Investor before 1:00 p.m., Eastern time, or, if such Purchase Shares are received by the Investor after 1:00 p.m., Eastern time, the next Business Day. For each Accelerated Purchase and each Additional Accelerated Purchase, the Investor shall pay to the Company an amount equal to the Purchase Amount with respect to such Accelerated Purchase and Additional Accelerated Purchase, respectively, as full payment for such Purchase Shares via wire transfer of immediately available funds on the third Business Day following the date that the Investor receives such Purchase Shares. The Company shall not issue any fraction of a share of Common Stock upon any Regular Purchase, Accelerated Purchase or Additional Accelerated Purchase. If the issuance would result in the issuance of a fraction of a share of Common Stock, the Company shall round such fraction of a share of Common Stock up or down to the nearest whole share. All payments made under this Agreement shall be made in lawful money of the United States of America or wire transfer of immediately available funds to such account as the Company may from time to time designate by written notice in accordance with the provisions of this Agreement. Whenever any amount expressed to be due by the terms of this Agreement is due on any day that is not a Business Day, the same shall instead be due on the next succeeding day that is a Business Day.
(e) Beneficial Ownership Limitation. Notwithstanding anything to the contrary contained in this Agreement, the Company shall not issue or sell, and the Investor shall not purchase or acquire, any shares of Common Stock under this Agreement which, when aggregated with all other shares of Common Stock then beneficially owned by the Investor and its affiliates (as calculated pursuant to Section 13(d) of the Exchange Act and Rule 13d-3 promulgated thereunder), would result in the beneficial ownership by the Investor and its affiliates of more than 4.99% of the then issued and outstanding shares of Common Stock (the “Beneficial Ownership Limitation”). Upon the written or oral request of the Investor, the Company shall promptly (but not later than 24 hours) confirm orally or in writing to the Investor the number of shares of Common Stock then outstanding. The Investor and the Company shall each cooperate in good faith in the determinations required hereby and the application hereof. The Investor’s written certification to the Company of the applicability of the Beneficial Ownership Limitation, and the resulting effect thereof hereunder at any time, shall be conclusive with respect to the applicability thereof and such result absent manifest error.
(f) Compliance with Principal Market Rules.
(i) Exchange Cap. Subject to Section 2(f)(ii) below, the Company shall not issue or sell any shares of Common Stock pursuant to this Agreement, and the Investor shall not purchase or acquire any shares of Common Stock pursuant to this Agreement, to the extent that after giving effect thereto, the aggregate number of shares of Common Stock that would be issued pursuant to this Agreement and the transactions contemplated hereby would be equal or greater to 15,606,426 shares of Common Stock, representing 19.99% of the shares of Common Stock outstanding on the date of this Agreement (which number of shares shall be reduced, on a share-for-share basis, by the number of shares of Common Stock issued or issuable pursuant to any transaction or series of transactions that may be aggregated with the transactions contemplated by this Agreement under applicable rules of the Nasdaq Capital Market or any other Principal Market on which the Common Stock may be listed or quoted) (the “Exchange Cap”), unless and until the Company elects to solicit stockholder approval of the issuance of Common Stock as contemplated by this Agreement and the stockholders of the Company have in fact approved such issuance in accordance with the applicable rules and regulations of the Nasdaq Capital Market, any other Principal Market on which the Common Stock may be listed or quoted, and the Company’s Articles of Incorporation, as amended and as in effect on the date hereof (the “Articles of Incorporation”), and the Company’s Bylaws, as amended and as in effect on the date hereof (the “Bylaws”). For the avoidance of doubt, the Company may, but shall be under no obligation to, request its stockholders to approve the issuance of Common Stock as contemplated by this Agreement; provided, that if stockholder approval is not obtained in accordance with this Section 2(f)(i), the Exchange Cap shall be applicable for all purposes of this Agreement and the transactions contemplated hereby at all times during the term of this Agreement (except as set forth in Section 2(f)(ii) below).
(ii) At-Market Transaction. Notwithstanding Section 2(f)(i) above and subject to the prior approval of the Nasdaq Capital Market or any other Principal Market on which the Common Stock may be listed or quoted (to the extent required), the Exchange Cap shall not be applicable for any purposes of this Agreement and the transactions contemplated hereby, solely to the extent that (and only for so long as) the Average Price shall equal or exceed the Base Price and in accordance with any other applicable rules of the Nasdaq Capital Market or any other Principal Market on which the Common Stock may be listed or quoted (it being hereby acknowledged and agreed that the Exchange Cap shall be applicable for all purposes of this Agreement and the transactions contemplated hereby at all other times during the term of this Agreement, unless the stockholder approval referred to in Section 2(f)(i) is obtained).
(iii) General. The Company shall not issue any Securities pursuant to this Agreement if such issuance would reasonably be expected to result in (A) a violation of the Securities Act or (B) a breach of the rules and regulations of the Principal Market. Furthermore, the Company agrees that it shall not issue any Securities pursuant to this Agreement if, at the time of such issuance (Y) the effectiveness of the Registration Statement registering the Securities has lapsed for any reason (including, without limitation, the issuance of a stop order or similar order) or (Z) the Registration Statement is unavailable for the sale by the Company to the Investor (or the resale by the Investor, as the case may be) of any or all of the Securities to be issued to the Investor under the Transaction Documents. The provisions of this Section 2(f) shall be implemented in a manner otherwise than in strict conformity with the terms hereof only if necessary to ensure compliance with the Securities Act and the rules and regulations of the Principal Market.
3. INVESTOR’S REPRESENTATIONS AND WARRANTIES.
The Investor represents and warrants to the Company that as of the date hereof and as of the Commencement Date:
(a) Organization, Authority. The Investor is an entity duly organized, validly existing and in good standing under the laws of the jurisdiction of its organization, with the requisite power and authority to enter into and to consummate the transactions contemplated by this Agreement and otherwise to carry out its obligations hereunder and thereunder.
(b) Accredited Investor Status. The Investor is an “accredited investor” as that term is defined in Rule 501(a)(3) of Regulation D promulgated under the Securities Act.
(c) Information. The Investor understands that its investment in the Securities involves a high degree of risk. The Investor (i) is able to bear the economic risk of an investment in the Securities including a total loss thereof, (ii) has such knowledge and experience in financial and business matters that it is capable of evaluating the merits and risks of the proposed investment in the Securities and (iii) has had an opportunity to ask questions of and receive answers from the officers of the Company concerning the financial condition and business of the Company and others matters related to an investment in the Securities. Neither such inquiries nor any other due diligence investigations conducted by the Investor or its representatives shall modify, amend or affect the Investor’s right to rely on the Company’s representations and warranties contained in Section 4 below. The Investor has sought such accounting, legal and tax advice as it has considered necessary to make an informed investment decision with respect to its acquisition of the Securities. The Investor acknowledges and agrees that the Company neither makes nor has made any representations or warranties with respect to the transactions contemplated hereby other than those specifically set forth in Section 4 hereof.
(d) No Governmental Review. The Investor understands that no U.S. federal or state agency or any other government or governmental agency has passed on or made any recommendation or endorsement of the Securities or the fairness or suitability of an investment in the Securities nor have such authorities passed upon or endorsed the merits of the offering of the Securities.
(e) Validity; Enforcement. This Agreement has been duly and validly authorized, executed and delivered on behalf of the Investor and is a valid and binding agreement of the Investor enforceable against the Investor in accordance with its terms, subject as to enforceability to general principles of equity and to applicable bankruptcy, insolvency, reorganization, moratorium, liquidation and other similar laws relating to, or affecting generally, the enforcement of applicable creditors’ rights and remedies.
(f) Residency. The Investor is a resident of the State of Illinois.
(g) Certain Transactions and Confidentiality. Other than consummating the transactions contemplated hereunder, Investor has not, nor has any Person acting on behalf of or pursuant to any understanding with Investor, directly or indirectly executed any purchases or sales, including short sales, of the securities of the Company during the period commencing as of the time that Investor first received a term sheet (written or oral) as of the Company or any other Person representing the Company setting forth the material terms of the transactions contemplated hereunder and ending immediately prior to the execution hereof. Other than to other Persons party to this Agreement, Investor has maintained the confidentiality of all disclosures made to it in connection with this transaction (including the existence and terms of this transaction).
(h) No Short Selling. The Investor represents and warrants to the Company that at no time prior to the date of this Agreement has any of the Investor, its agents, representatives or affiliates engaged in or effected, in any manner whatsoever, directly or indirectly, any (i) “short sale” (as such term is defined in Rule 200 of Regulation SHO of the Exchange Act) of the Common Stock or (ii) hedging transaction, which establishes a net short position with respect to the Common Stock.
4. REPRESENTATIONS AND WARRANTIES OF THE COMPANY.
The Company represents and warrants to the Investor that as of the date hereof and as of the Commencement Date:
(a) Organization and Qualification. The Company and each of its Subsidiaries is an entity duly incorporated or otherwise organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation or organization, with the requisite corporate power and authority to own and use its properties and assets and to carry on its business as currently conducted. Neither the Company, nor any of its Subsidiaries is in violation or default of any of the provisions of its respective certificate or Articles of Incorporation, bylaws or other organizational or charter documents, except as would not reasonably be expected to have a Material Adverse Effect. Each of the Company and its Subsidiaries is duly qualified to conduct business and is in good standing as a foreign corporation or other entity in each jurisdiction in which the nature of the business conducted or property owned by it makes such qualification necessary, except where the failure to be so qualified or in good standing, as the case may be, could not have or reasonably be expected to result in a Material Adverse Effect and no proceeding has been instituted in any such jurisdiction revoking, limiting or curtailing or seeking to revoke, limit or curtail such power and authority or qualification. The Company has no Subsidiaries except as set forth in Schedule 4(a) hereof.
(b) Authorization; Enforcement; Validity. (i) The Company has the requisite corporate power and authority to enter into and perform its obligations under this Agreement and each of the other Transaction Documents, and to issue the Securities in accordance with the terms hereof and thereof, (ii) the execution and delivery of the Transaction Documents by the Company and the consummation by it of the transactions contemplated hereby and thereby, including without limitation, the issuance of the Commitment Shares (as defined below in Section 5(e)) and the reservation for issuance and the issuance of the Purchase Shares issuable under this Agreement, have been duly authorized by the Company’s Board of Directors and no further consent or authorization is required by the Company, its Board of Directors or its stockholders, (iii) this Agreement has been, and each other Transaction Document shall be on the Commencement Date, duly executed and delivered by the Company and (iv) this Agreement constitutes, and each other Transaction Document upon its execution on behalf of the Company, shall constitute, the valid and binding obligations of the Company enforceable against the Company in accordance with their terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency, reorganization, moratorium, liquidation or similar laws relating to, or affecting generally, the enforcement of creditors’ rights and remedies. The Board of Directors of the Company has approved resolutions (the “Signing Resolutions”) to authorize this Agreement and the transactions contemplated hereby. The Signing Resolutions are valid, in full force and effect and have not been modified or supplemented in any respect. Except as set forth in this Agreement, no other approvals or consents of the Company’s Board of Directors, any authorized committee thereof, and/or stockholders is necessary under applicable laws and the Company’s Articles of Incorporation and/or Bylaws to authorize the execution and delivery of this Agreement or any of the transactions contemplated hereby, including, but not limited to, the issuance of the Commitment Shares and the issuance of the Purchase Shares.
(c) Capitalization. As of the date hereof, the authorized capital stock of the Company is set forth in Schedule 4(c) hereof. Except as disclosed in the SEC Documents (as defined below), (i) no shares of the Company’s capital stock are subject to preemptive rights or any other similar rights or any liens or encumbrances suffered or permitted by the Company, (ii) there are no outstanding debt securities, (iii) there are no outstanding options, warrants, scrip, rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities or rights convertible into, any shares of capital stock of the Company or any of its Subsidiaries, or contracts, commitments, understandings or arrangements by which the Company or any of its Subsidiaries is or may become bound to issue additional shares of capital stock of the Company or any of its Subsidiaries or options, warrants, scrip, rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities or rights convertible into, any shares of capital stock of the Company or any of its Subsidiaries, (iv) there are no agreements or arrangements under which the Company or any of its Subsidiaries is obligated to register the sale of any of their securities under the Securities Act (except the Registration Rights Agreement), (v) there are no outstanding securities or instruments of the Company or any of its Subsidiaries which contain any redemption or similar provisions, and there are no contracts, commitments, understandings or arrangements by which the Company or any of its Subsidiaries is or may become bound to redeem a security of the Company or any of its Subsidiaries, (vi) there are no securities or instruments containing anti-dilution or similar provisions that will be triggered by the issuance of the Securities as described in this Agreement and (vii) the Company does not have any stock appreciation rights or “phantom stock” plans or agreements or any similar plan or agreement. The Company has furnished to the Investor true and correct copies of the Articles of Incorporation, , and the Bylaws, , and summaries of the terms of all securities convertible into or exercisable for Common Stock, if any, and copies of any documents containing the material rights of the holders thereof in respect thereto.
(d) Issuance of Securities. Upon issuance and payment therefor in accordance with the terms and conditions of this Agreement, the Purchase Shares shall be validly issued, fully paid and nonassessable and free from all taxes, liens, charges, restrictions, rights of first refusal and preemptive rights with respect to the issue thereof, with the holders being entitled to all rights accorded to a holder of Common Stock. 10,000,000 shares of Common Stock have been duly authorized and reserved for issuance upon purchase under this Agreement as Purchase Shares. 75,000 shares of Common Stock (subject to equitable adjustment for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction) have been duly authorized and reserved for issuance as Initial Commitment Shares (as defined below in Section 5(e)) in accordance with this Agreement. The Initial Commitment Shares shall be validly issued, fully paid and nonassessable and free from all taxes, liens, charges, restrictions, rights of first refusal and preemptive rights with respect to the issue thereof, with the holders being entitled to all rights accorded to a holder of Common Stock. 75,000 shares of Common Stock (subject to equitable adjustment for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction) have been duly authorized and reserved for issuance as Additional Commitment Shares (as defined below in Section 5(e)) in accordance with this Agreement. When issued in accordance with this Agreement, the Additional Commitment Shares shall be validly issued, fully paid and nonassessable and free from all taxes, liens, charges, restrictions, rights of first refusal and preemptive rights with respect to the issue thereof, with the holders being entitled to all rights accorded to a holder of Common Stock. The Securities are being issued pursuant to the Registration Statement, and the issuance of the Securities has been registered by the Company pursuant to the Securities Act. Upon receipt of the Purchase Shares and the Commitment Shares, the Investor will have good and marketable title to such Securities and such Securities will be immediately freely tradable on the Principal Market by any holder who is not an “affiliate” under the Securities Act.
(e) No Conflicts. The execution, delivery and performance of the Transaction Documents by the Company and the consummation by the Company of the transactions contemplated hereby and thereby (including, without limitation, the reservation for issuance and issuance of the Purchase Shares and the Commitment Shares) will not (i) result in a violation of the Articles of Incorporation, any Certificate of Designations, Preferences and Rights of any outstanding series of preferred stock of the Company or the Bylaws or (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which the Company or any of its Subsidiaries is a party, or result in a violation of any law, rule, regulation, order, judgment or decree (including federal and state securities laws and regulations and the rules and regulations of the Principal Market applicable to the Company or any of its Subsidiaries) or by which any property or asset of the Company or any of its Subsidiaries is bound or affected, except in the case of conflicts, defaults, terminations, amendments, accelerations, cancellations and violations under clause (ii), which could not reasonably be expected to result in a Material Adverse Effect. Neither the Company nor its Subsidiaries is in violation of any term of or in default under its Articles of Incorporation, any Certificate of Designation, preferences and rights of any outstanding series of preferred stock of the Company or Bylaws or their organizational charter or bylaws, respectively. Neither the Company, nor any of its Subsidiaries is in violation of any term of or is in default under any material contract, agreement, mortgage, indebtedness, indenture, instrument, judgment, decree or order or any statute, rule or regulation applicable to the Company or its Subsidiaries, except for possible conflicts, defaults, terminations or amendments that could not reasonably be expected to have a Material Adverse Effect. The business of the Company and its Subsidiaries is not being conducted, and shall not be conducted, in violation of any law, ordinance, regulation of any governmental entity, except for possible violations, the sanctions for which either individually or in the aggregate could not reasonably be expected to have a Material Adverse Effect. Except as specifically contemplated by this Agreement and as required under the Securities Act or applicable state securities laws and the rules and regulations of the Principal Market and under the Corporate Financing Rule 5110 of the Financial Industry Regulatory Authority (FINRA), the Company is not required to obtain any consent, authorization or order of, or make any filing or registration with, any court or governmental agency or any regulatory or self-regulatory agency in order for it to execute, deliver or perform any of its obligations under or contemplated by the Transaction Documents in accordance with the terms hereof or thereof. Except as set forth elsewhere in this Agreement, all consents, authorizations, orders, filings and registrations which the Company is required to obtain pursuant to the preceding sentence shall be obtained or effected on or prior to the Commencement Date. Since one year prior to the date hereof, the Company has not received nor delivered any notices or correspondence from or to the Principal Market. The Principal Market has not commenced any delisting proceedings against the Company.
(f) SEC Documents; Financial Statements. The Company has filed all reports, schedules, forms, statements and other documents required to be filed by the Company under the Securities Act and the Exchange Act, including pursuant to Section 13(a) or 15(d) thereof, for the 24 months preceding the date hereof (or such shorter period as the Company was required by law or regulation to file such material) (the foregoing materials, including the exhibits thereto and documents incorporated by reference therein, being collectively referred to herein as the “SEC Documents”) on a timely basis or has received a valid extension of such time of filing and has filed any such SEC Documents prior to the expiration of any such extension. As of their respective dates and to the Company’s knowledge, the SEC Documents complied in all material respects with the requirements of the Securities Act and the Exchange Act, as applicable. None of the SEC Documents, when filed, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. The financial statements of the Company included in the SEC Documents comply in all material respects with applicable accounting requirements and the rules and regulations of the SEC with respect thereto as in effect at the time of filing. Such financial statements have been prepared in accordance with United States generally accepted accounting principles applied on a consistent basis during the periods involved (“GAAP”), except as may be otherwise specified in such financial statements or the notes thereto and except that unaudited financial statements may not contain all footnotes required by GAAP, and fairly present in all material respects the financial position of the Company and its consolidated Subsidiaries as of and for the dates thereof and the results of operations and cash flows for the periods then ended, subject, in the case of unaudited statements, to normal, immaterial, year-end audit adjustments. Except as set forth in the SEC Documents, the Company has received no notices or correspondence from the SEC for the one year preceding the date hereof. The SEC has not commenced any enforcement proceedings against the Company or any of its Subsidiaries.
(g) Absence of Certain Changes. Except as disclosed in the SEC Documents, since September 30, 2022, there has been no material adverse change in the business, properties, operations, financial condition or results of operations of the Company or its Subsidiaries. The Company has not taken any steps, and does not currently expect to take any steps, to seek protection pursuant to any Bankruptcy Law nor does the Company or any of its Subsidiaries have any knowledge or reason to believe that its creditors intend to initiate involuntary bankruptcy or insolvency proceedings.
(h) Absence of Litigation. There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of the Company or any of its Subsidiaries, threatened against or affecting the Company, the Common Stock or any of the Company’s or its Subsidiaries’ officers or directors in their capacities as such, which could reasonably be expected to have a Material Adverse Effect.
(i) Acknowledgment Regarding Investor’s Status. The Company acknowledges and agrees that the Investor is acting solely in the capacity of arm’s length purchaser with respect to the Transaction Documents and the transactions contemplated hereby and thereby. The Company further acknowledges that the Investor is not acting as a financial advisor or fiduciary of the Company (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated hereby and thereby and any advice given by the Investor or any of its representatives or agents in connection with the Transaction Documents and the transactions contemplated hereby and thereby is merely incidental to the Investor’s purchase of the Securities. The Company further represents to the Investor that the Company’s decision to enter into the Transaction Documents has been based solely on the independent evaluation by the Company and its representatives and advisors.
(j) No Aggregated Offering. Neither the Company, nor any of its affiliates, nor any Person acting on its or their behalf has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would cause this offering of the Securities to be aggregated with prior offerings by the Company in a manner that would require stockholder approval pursuant to the rules of the Principal Market on which any of the securities of the Company are listed or designated. The issuance and sale of the Securities hereunder does not contravene the rules and regulations of the Principal Market.
(k) Intellectual Property Rights. Except as disclosed in the Registration Statement or the SEC Documents, the Company and its Subsidiaries own or possess adequate rights or licenses to use all material trademarks, trade names, service marks, service mark registrations, service names, patents, patent rights, copyrights, inventions, licenses, approvals, governmental authorizations, trade secrets and rights necessary to conduct their respective businesses as now conducted. None of the Company’s material trademarks, trade names, service marks, service mark registrations, service names, patents, patent rights, copyrights, inventions, licenses, approvals, government authorizations, trade secrets or other intellectual property rights have expired or terminated, or, by the terms and conditions thereof, could expire or terminate within two years from the date of this Agreement, except as would not reasonably be expected to have a Material Adverse Effect. The Company and its Subsidiaries do not have any knowledge of any infringement by the Company or its Subsidiaries of any material trademark, trade name rights, patents, patent rights, copyrights, inventions, licenses, service names, service marks, service mark registrations, trade secret or other similar rights of others, or of any such development of similar or identical trade secrets or technical information by others, and there is no claim, action or proceeding being made or brought against, or to the Company’s knowledge, being threatened against, the Company or its Subsidiaries regarding trademark, trade name, patents, patent rights, invention, copyright, license, service names, service marks, service mark registrations, trade secret or other infringement, which could reasonably be expected to have a Material Adverse Effect.
(l) Environmental Laws. To the Company’s knowledge, the Company and its Subsidiaries (i) are in compliance with any and all applicable foreign, federal, state and local laws and regulations relating to the protection of human health and safety, the environment or hazardous or toxic substances or wastes, pollutants or contaminants (“Environmental Laws”), (ii) have received all permits, licenses or other approvals required of them under applicable Environmental Laws to conduct their respective businesses and (iii) are in compliance with all terms and conditions of any such permit, license or approval, except where, in each of the three foregoing clauses, the failure to so comply could not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect.
(m) Title. Except as disclosed in the Registration Statement or SEC Documents, the Company and its Subsidiaries have good and marketable title in fee simple to all real property owned by them and good and marketable title in all personal property owned by them that is material to the business of the Company and its Subsidiaries, in each case free and clear of all liens, encumbrances and defects (“Liens”) and, except for Liens as do not materially affect the value of such property and do not materially interfere with the use made and proposed to be made of such property by the Company and its Subsidiaries and Liens for the payment of federal, state or other taxes, the payment of which is neither delinquent nor subject to penalties. Any real property and facilities held under lease by the Company and its Subsidiaries are held by them under valid, subsisting and enforceable leases with which the Company and its Subsidiaries are in compliance with such exceptions as are not material and do not interfere with the use made and proposed to be made of such property and buildings by the Company and its Subsidiaries.
(n) Insurance. The Company and each of its Subsidiaries are insured by insurers of recognized financial responsibility against such losses and risks and in such amounts as management of the Company believes to be prudent and customary in the businesses in which the Company and its Subsidiaries are engaged. Neither the Company nor any such Subsidiary has been refused any insurance coverage sought or applied for and neither the Company nor any such Subsidiary has any reason to believe that it will not be able to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may be necessary to continue its business at a cost that would not materially and adversely affect the condition, financial or otherwise, or the earnings, business or operations of the Company and its Subsidiaries, taken as a whole.
(o) Regulatory Permits. Except as disclosed in the Registration Statement or SEC Documents, the Company and its Subsidiaries possess all material certificates, authorizations and permits issued by the appropriate federal, state or foreign regulatory authorities necessary to conduct their respective businesses, and neither the Company nor any such Subsidiary has received any notice of proceedings relating to the revocation or modification of any such certificate, authorization or permit, except, in the case of each of the two foregoing clauses, as would not reasonably be expected to have a Material Adverse Effect.
(p) Tax Status. The Company and each of its Subsidiaries has made or filed all federal and state income and all other material tax returns, reports and declarations required by any jurisdiction to which it is subject (unless and only to the extent that the Company and each of its Subsidiaries has set aside on its books provisions reasonably adequate for the payment of all unpaid and unreported taxes) and has paid all taxes and other governmental assessments and charges that are material in amount, shown or determined to be due on such returns, reports and declarations, except those being contested in good faith and has set aside on its books provision reasonably adequate for the payment of all taxes for periods subsequent to the periods to which such returns, reports or declarations apply, and except as would not reasonably be expected to have a Material Adverse Effect. There are no unpaid taxes in any material amount claimed to be due by the taxing authority of any jurisdiction, and the officers of the Company know of no basis for any such claim.
(q) Transactions With Affiliates. Except as set forth in the SEC Documents, none of the officers or directors of the Company and, to the knowledge of the Company, none of the employees of the Company is presently a party to any transaction with the Company or any Subsidiary (other than for services as employees, officers and directors), including any contract, agreement or other arrangement providing for the furnishing of services to or by, providing for rental of real or personal property to or from, or otherwise requiring payments to or from any officer, director or such employee or, to the knowledge of the Company, any entity in which any officer, director, or any such employee has a substantial interest or is an officer, director, trustee or partner, in each case in excess of $120,000 other than for (i) payment of salary or consulting fees for services rendered, (ii) reimbursement for expenses incurred on behalf of the Company and (iii) other employee benefits, including stock option agreements under any stock option plan of the Company.
(r) Application of Takeover Protections. The Company and its Board of Directors have taken or will take prior to the Commencement Date all necessary action, if any, in order to render inapplicable any control share acquisition, business combination, poison pill (including any distribution under a rights agreement) or other similar anti-takeover provision under the Articles of Incorporation or the laws of the state of its incorporation which is or could become applicable to the Investor as a result of the transactions contemplated by this Agreement, including, without limitation, the Company’s issuance of the Securities and the Investor’s ownership of the Securities.
(s) Disclosure. Except with respect to the material terms and conditions of the transactions contemplated by the Transaction Documents that will be timely publicly disclosed by the Company, the Company confirms that neither it nor any other Person acting on its behalf has provided the Investor or its agents or counsel with any information that it believes constitutes or might constitute material, non-public information which is not otherwise disclosed in the Registration Statement or the SEC Documents. The Company understands and confirms that the Investor will rely on the foregoing representation in effecting purchases and sales of securities of the Company. All of the disclosure furnished by or on behalf of the Company to the Investor regarding the Company, its business and the transactions contemplated hereby, taken as a whole, including the disclosure schedules to this Agreement, is true and correct in all material respects and does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading. The press releases disseminated by the Company during the twelve (12) months preceding the date of this Agreement taken as a whole did not as of their issue date contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made and when made, not misleading. The Company acknowledges and agrees that the Investor neither makes nor has made any representations or warranties with respect to the transactions contemplated hereby other than those specifically set forth in Section 3 hereof.
(t) Foreign Corrupt Practices. Neither the Company, nor to the knowledge of the Company, any agent or other Person acting on behalf of the Company, has (i) directly or indirectly, used any funds for unlawful contributions, gifts, entertainment or other unlawful expenses related to foreign or domestic political activity, (ii) made any unlawful payment to foreign or domestic government officials or employees or to any foreign or domestic political parties or campaigns from corporate funds, (iii) failed to disclose fully any contribution made by the Company (or made by any Person acting on its behalf of which the Company is aware) which is in violation of law, or (iv) violated in any material respect any provision of the Foreign Corrupt Practices Act of 1977, as amended.
(u) Registration Statement. The Company has prepared and filed with the SEC in accordance with the provisions of the Securities Act the Registration Statement. The Registration Statement was declared effective by order of the SEC on September 24, 2021. The Registration Statement is effective pursuant to the Securities Act and available for the issuance of the Securities thereunder, and the Company has not received any written notice that the SEC has issued or intends to issue a stop order or other similar order with respect to the Registration Statement or the Prospectus or that the SEC otherwise has (i) suspended or withdrawn the effectiveness of the Registration Statement or (ii) issued any order preventing or suspending the use of the Prospectus or any Prospectus Supplement, in either case, either temporarily or permanently or intends or has threatened in writing to do so. The “Plan of Distribution” section of the Prospectus permits the issuance of the Securities hereunder. At the time the Registration Statement and any amendments thereto became effective, at the date of this Agreement and at each deemed effective date thereof pursuant to Rule 430B(f)(2) of the Securities Act, the Registration Statement and any amendments thereto complied and will comply in all material respects with the requirements of the Securities Act and did not and will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein not misleading; and the Base Prospectus and any Prospectus Supplement thereto, at the time such Base Prospectus or such Prospectus Supplement thereto was issued and on the Commencement Date, complied and will comply in all material respects with the requirements of the Securities Act and did not and will not contain an untrue statement of a material fact or omit to state a material fact necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading; provided that this representation and warranty does not apply to statements in or omissions from any Prospectus Supplement made in reliance upon and in conformity with information relating to the Investor furnished to the Company in writing by or on behalf of the Investor expressly for use therein. The Company meets all of the requirements for the use of a registration statement on Form S-3 pursuant to the Securities Act for the offering and sale of the Securities contemplated by this Agreement without reliance on General Instruction I.B.6. of Form S-3, and the SEC has not notified the Company of any objection to the use of the form of the Registration Statement pursuant to Rule 401(g)(1) of the Securities Act. The Registration Statement, as of its effective date, meets the requirements set forth in Rule 415(a)(1)(x) pursuant to the Securities Act. At the earliest time after the filing of the Registration Statement that the Company or another offering participant made a bona fide offer (within the meaning of Rule 164(h)(2) of the Securities Act) relating to any of the Securities, the Company was not and is not an Ineligible Issuer (as defined in Rule 405 of the Securities Act). The Company has not distributed any offering material in connection with the offering and sale of any of the Securities, and, until the Investor does not hold any of the Securities, shall not distribute any offering material in connection with the offering and sale of any of the Securities, to or by the Investor, in each case, other than the Registration Statement or any amendment thereto, the Prospectus or any Prospectus Supplement required pursuant to applicable law or the Transaction Documents. The Company has not made, and agrees that unless it obtains the prior written consent of the Investor it will not make, an offer relating to the Securities that would constitute a “free writing prospectus” as defined in Rule 405 under the Securities Act. The Company shall comply with the requirements of Rules 164 and 433 under the Securities Act applicable to any such free writing prospectus consented to by the Investor, including in respect of timely filing with the SEC, legending and record keeping.
(v) DTC Eligibility. The Company, through the Transfer Agent, currently participates in the DTC Fast Automated Securities Transfer (FAST) Program and the Common Stock can be transferred electronically to third parties via the DTC Fast Automated Securities Transfer (FAST) Program.
(w) Sarbanes-Oxley. The Company is in material compliance with all provisions of the Sarbanes-Oxley Act of 2002, as amended, which are applicable to it as of the date hereof.
(x) Certain Fees. No brokerage or finder’s fees or commissions are or will be payable by the Company to any broker, financial advisor or consultant, finder, placement agent, investment banker, bank or other Person with respect to the transactions contemplated by the Transaction Documents. The Investor shall have no obligation with respect to any fees or with respect to any claims made by or on behalf of other Persons for fees that may be due in connection with the transactions contemplated by the Transaction Documents.
(y) Investment Company. The Company is not, and immediately after receipt of payment for the Securities will not be, an “investment company” within the meaning of the Investment Company Act of 1940, as amended.
(z) Listing and Maintenance Requirements. The Common Stock is registered pursuant to Section 12(b) of the Exchange Act, and the Company has taken no action designed to, or which to its knowledge is likely to have the effect of, terminating the registration of the Common Stock pursuant to the Exchange Act nor has the Company received any notification that the SEC is currently contemplating terminating such registration. The Company has not, in the twelve (12) months preceding the date hereof, received any notice from the Principal Market to the effect that the Company is not in compliance with the listing or maintenance requirements of the Principal Market. The Company is, and has no reason to believe that it will not in the foreseeable future continue to be, in compliance with all such listing and maintenance requirements.
(aa) Accountants. The Company’s accountants are set forth in the SEC Documents and, to the knowledge of the Company, such accountants are an independent registered public accounting firm as required by the Securities Act.
(bb) No Market Manipulation. The Company has not, and to its knowledge no Person acting on its behalf has, (i) taken, directly or indirectly, any action designed to cause or to result in the stabilization or manipulation of the price of any security of the Company to facilitate the sale or resale of any of the Securities, (ii) sold, bid for, purchased, or, paid any compensation for soliciting purchases of, any of the Securities, or (iii) paid or agreed to pay to any Person any compensation for soliciting another to purchase any other securities of the Company.
(cc) Shell Company Status. The Company is not currently, and has never been, an issuer identified in Rule 144(i)(1) under the Securities Act.
5. COVENANTS.
(a) Filing of Current Report and Initial Prospectus Supplement. The Company agrees that it shall, within the time required under the Exchange Act, file with the SEC a report, which may be on Form 8-K or in a periodic report on Form 10-Q, relating to the transactions contemplated by, and describing the material terms and conditions of, the Transaction Documents (the “Report”). The Company further agrees that it shall, within the time required under Rule 424(b) under the Securities Act, file with the SEC the Initial Prospectus Supplement pursuant to Rule 424(b) under the Securities Act specifically relating to the transactions contemplated by, and describing the material terms and conditions of, the Transaction Documents, containing information previously omitted at the time of effectiveness of the Registration Statement in reliance on Rule 430B under the Securities Act, and disclosing all information relating to the transactions contemplated hereby required to be disclosed in the Registration Statement and the Prospectus as of the date of the Initial Prospectus Supplement, including, without limitation, information required to be disclosed in the section captioned “Plan of Distribution” in the Prospectus. The Investor acknowledges that it will be identified in the Initial Prospectus Supplement as an underwriter within the meaning of Section 2(a)(11) of the Securities Act. The Company shall permit the Investor to review and comment upon the Report, but only such sections of the Report that relate to the transactions contemplated herein, and the Initial Prospectus Supplement at least two (2) Business Days prior to their filing with the SEC, the Company shall give due consideration to all such comments, and the Company shall not file the Report or the Initial Prospectus Supplement with the SEC in a form to which the Investor reasonably objects. The Investor shall use its reasonable best efforts to comment upon the Report and the Initial Prospectus Supplement within one (1) Business Day from the date the Investor receives the final pre-filing draft version thereof from the Company. The Investor shall furnish to the Company such information regarding itself, the Securities held by it and the intended method of distribution thereof, including any arrangement between the Investor and any other Person relating to the sale or distribution of the Securities, as shall be reasonably requested by the Company in connection with the preparation and filing of the Current Report and the Initial Prospectus Supplement, and shall otherwise cooperate with the Company as reasonably requested by the Company in connection with the preparation and filing of the Current Report and the Initial Prospectus Supplement with the SEC.
(b) Blue Sky. The Company shall take all such action, if any, as is reasonably necessary in order to obtain an exemption for or to register or qualify (i) the issuance of the Commitment Shares and the sale of the Purchase Shares to the Investor under this Agreement and (ii) any subsequent resale of all Commitment Shares and all Purchase Shares by the Investor, in each case, under applicable securities or “Blue Sky” laws of the states of the United States in such states as is reasonably requested by the Investor from time to time, and shall provide evidence of any such action so taken to the Investor.
(c) Listing/DTC. The Company shall promptly secure the listing of all of the Purchase Shares and Commitment Shares to be issued to the Investor hereunder on the Principal Market (subject to official notice of issuance) and upon each other national securities exchange or automated quotation system, if any, upon which the Common Stock is then listed, and shall maintain, so long as any shares of Common Stock shall be so listed, such listing of all such Securities from time to time issuable hereunder. The Company shall maintain the listing of the Common Stock on the Principal Market and shall comply in all respects with the Company’s reporting, filing and other obligations under the bylaws or rules and regulations of the Principal Market. Neither the Company, nor any of its Subsidiaries shall take any action that would reasonably be expected to result in the delisting or suspension of the Common Stock on the Principal Market. The Company shall promptly, and in no event later than the following Business Day, provide to the Investor copies of any notices it receives from any Person regarding the continued eligibility of the Common Stock for listing on the Principal Market; provided, however, that the Company shall not provide the Investor copies of any such notice that the Company reasonably believes constitutes material non-public information, and the Company would not be required to publicly disclose such notice in any report or statement filed with the SEC under the Exchange Act (including on Form 8-K) or the Securities Act. The Company shall pay all fees and expenses in connection with satisfying its obligations under this Section 5(c). The Company shall take all action necessary to ensure that its Common Stock can be transferred electronically as DWAC Shares.
(d) Prohibition of Short Sales and Hedging Transactions. The Investor agrees that beginning on the date of this Agreement and ending on the date of termination of this Agreement as provided in Section 11, the Investor and its agents, representatives and affiliates shall not in any manner whatsoever enter into or effect, directly or indirectly, any (i) “short sale” (as such term is defined in Rule 200 of Regulation SHO of the Exchange Act) of the Common Stock or (ii) hedging transaction, which establishes a net short position with respect to the Common Stock.
(e) Issuance of Commitment Shares. In consideration for the Investor’s execution and delivery of this Agreement, the Company shall cause the Transfer Agent to issue no later than the date of the filing of the Initial Prospectus Supplement 75,000 shares of Common Stock (the “Initial Commitment Shares”) directly to the Investor and shall deliver to the Transfer Agent the Irrevocable Transfer Agent Instructions in the form as set forth in Section 6 hereto. For the avoidance of doubt, all of the Initial Commitment Shares shall be fully earned as of the date of this Agreement, whether or not the Commencement shall occur or any Purchase Shares are purchased by the Investor under this Agreement and irrespective of any termination of this Agreement. In connection with each Regular Purchase and each Accelerated Purchase of Purchase Shares hereunder, the Company shall issue to the Investor a number of shares of Common Stock (the “Additional Commitment Shares” and, together with the Initial Commitment Shares, the “Commitment Shares”) equal to the product of (x) 75,000 and (y) the Purchase Amount Fraction. The “Purchase Amount Fraction” shall mean a fraction, the numerator of which is the Purchase Amount purchased by the Investor with respect to such Regular Purchase and Accelerated Purchase (as applicable) of Purchase Shares and the denominator of which is One Hundred Fifty Million Dollars ($150,000,000). The Additional Commitment Shares shall be issued to the Investor on the same Business Day as Purchase Shares are issued to the Investor in connection with the applicable Regular Purchase, Accelerated Purchase and Additional Accelerated Purchase (as applicable) in accordance with Section 2. In no event shall the amount of the Additional Commitment Shares to be issued under this Agreement exceed 75,000 shares of Common Stock, provided that such Additional Commitment Shares shall be equitably adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction.
(f) Due Diligence; Non-Public Information. The Investor shall have the right, from time to time as the Investor may reasonably deem appropriate and upon reasonable notice to the Company, to perform reasonable due diligence on the Company during normal business hours. The Company and its officers and employees shall provide information and reasonably cooperate with the Investor in connection with any reasonable request by the Investor related to the Investor’s due diligence of the Company; provided however, that the Company will not disclose any material non-public information to the Investor. Each party hereto agrees not to disclose any Confidential Information of the other party to any third party and shall not use the Confidential Information for any purpose other than in connection with, or in furtherance of, the transactions contemplated hereby. Each party hereto acknowledges that the Confidential Information shall remain the property of the disclosing party and agrees that it shall take all reasonable measures to protect the secrecy of any Confidential Information disclosed by the other party. The receiving party may disclose Confidential Information to the extent such information is required to be disclosed by law, regulation or order of a court of competent jurisdiction or regulatory authority, provided that the receiving party shall promptly notify the disclosing party when such requirement to disclose arises, and shall cooperate with the disclosing party so as to enable the disclosing party to: (i) seek an appropriate protective order; and (ii) make any applicable claim of confidentiality in respect of such Confidential Information; and provided, further, that the receiving party shall disclose Confidential Information only to the extent required by the protective order or other similar order, if such an order is obtained, and, if no such order is obtained, the receiving party shall disclose only the minimum amount of such Confidential Information required to be disclosed in order to comply with the applicable law, regulation or order. In addition, any such Confidential Information disclosed pursuant to this Section 5(f) shall continue to be deemed Confidential Information. Notwithstanding anything in this Agreement to the contrary, the Company and the Investor agree that neither the Company, nor any other Person acting on its behalf shall provide the Investor or its agents or counsel with any information that constitutes or might constitute material, non-public information, unless a simultaneous public announcement thereof is made by the Company in the manner contemplated by Regulation FD. In the event of a breach of the foregoing covenant by the Company or any Person acting on its behalf (as determined in the reasonable good faith judgment of the Investor), in addition to any other remedy provided herein or in the other Transaction Documents, the Investor shall have the right to make a public disclosure, in the form of a press release, public advertisement or otherwise, of such material, non-public information without the prior approval by the Company; provided the Investor shall have first provided notice to the Company that it believes it has received information that constitutes material, non-public information, the Company shall have at least 24 hours to publicly disclose such material, non-public information prior to any such disclosure by the Investor, and the Company shall have failed to publicly disclose such material, non-public information within such time period. The Investor shall not have any liability to the Company, any of its Subsidiaries, or any of their respective directors, officers, employees, stockholders or agents, for any such disclosure. The Company understands and confirms that the Investor shall be relying on the foregoing covenants in effecting transactions in securities of the Company.
(g) Purchase Records. The Investor and the Company shall each maintain records showing the remaining Available Amount at any given time and the dates and Purchase Amounts for each Regular Purchase, Accelerated Purchase and Additional Accelerated Purchase or shall use such other method, reasonably satisfactory to the Investor and the Company.
(h) Taxes. The Company shall pay any and all transfer, stamp or similar taxes that may be payable with respect to the issuance and delivery of any shares of Common Stock to the Investor made under this Agreement.
(i) Effective Registration Statement; Current Prospectuses. The Company shall use its reasonable best efforts to keep the Registration Statement effective pursuant to Rule 415 promulgated under the Securities Act, and to keep the Registration Statement and the Prospectus current and available for issuances and sales of all of the Securities by the Company to the Investor, and for the resale by the Investor as prescribed by Registration Rights Agreement, the Company shall comply with all applicable federal, state and foreign securities laws in connection with the offer, issuance and sale of the Securities contemplated by the Transaction Documents. Without limiting the generality of the foregoing, neither the Company, nor any of its officers, directors or affiliates will take, directly or indirectly, any action designed or intended to stabilize or manipulate the price of any security of the Company, or which would reasonably be expected to cause or result in, stabilization or manipulation of the price of any security of the Company.
(j) Stop Orders. The Company shall advise the Investor promptly (but in no event later than 24 hours) and shall confirm such advice in writing: (i) of the Company’s receipt of notice of any request by the SEC for amendment of or a supplement to the Registration Statement, the Prospectus, any Prospectus Supplement or for any additional information with respect thereto; (ii) of the Company’s receipt of notice of the issuance by the SEC of any stop order suspending the effectiveness of the Registration Statement or prohibiting or suspending the use of the Prospectus or any Prospectus Supplement, or of the Company’s receipt of any notification of the suspension of qualification of the Securities for offering or sale in any jurisdiction or the initiation or contemplated initiation of any proceeding for such purpose; and (iii) of the Company becoming aware of the happening of any event, which makes any statement of a material fact made in the Registration Statement, the Prospectus or any Prospectus Supplement untrue or which requires the making of any additions to or changes to the statements then made in the Registration Statement, the Prospectus or any Prospectus Supplement in order to state a material fact required by the Securities Act to be stated therein or necessary in order to make the statements then made therein (in the case of the Prospectus or any Prospectus Supplement, in light of the circumstances under which they were made) not misleading, or of the necessity to amend the Registration Statement or supplement the Prospectus or any Prospectus Supplement to comply with the Securities Act or any other law. The Company shall not be required to disclose to the Investor the substance or specific reasons of any of the events set forth in clauses (i) through (iii) of the immediately preceding sentence, but rather, shall only be required to disclose that the event has occurred. The Company shall not deliver to the Investor any Regular Purchase Notice, Accelerated Purchase Notice or Additional Accelerated Purchase Notice, and the Investor shall not be obligated to purchase any shares of Common Stock under this Agreement, during the continuation or pendency of any of the foregoing events. If at any time the SEC shall issue any stop order suspending the effectiveness of the Registration Statement or prohibiting or suspending the use of the Prospectus or any Prospectus Supplement, the Company shall use its reasonable best efforts to obtain the withdrawal of such order at the earliest possible time. The Company shall furnish to the Investor, without charge, a copy of any correspondence from the SEC or the staff of the SEC to the Company or its representatives relating to the Registration Statement or the Prospectus, as the case may be.
(k) Amendments to Registration Statement; Prospectus Supplements. Except as provided in this Agreement and other than periodic and current reports required to be filed pursuant to the Exchange Act, the Company shall not file with the SEC any amendment to the Registration Statement or any supplement to the Base Prospectus that refers to the Investor, the Transaction Documents or the transactions contemplated thereby (including, without limitation, any Prospectus Supplement filed in connection with the transactions contemplated by the Transaction Documents), in each case with respect to which (a) the Investor shall not previously have been advised and afforded the opportunity to review and comment thereon at least two (2) Business Days prior to filing with the SEC, as the case may be, (b) the Company shall not have given due consideration to any comments thereon received from the Investor or its counsel, or (c) the Investor shall reasonably object, unless the Company reasonably has determined that it is necessary to amend the Registration Statement or make any supplement to the Prospectus to comply with the Securities Act or any other applicable law or regulation, in which case the Company shall promptly (but in no event later than 24 hours) so inform the Investor, the Investor shall be provided with a reasonable opportunity to review and comment upon any disclosure referring to the Investor, the Transaction Documents or the transactions contemplated thereby, as applicable, and the Company shall promptly furnish to the Investor a copy thereof. In addition, for so long as, in the reasonable opinion of counsel for the Investor, the Prospectus is required to be delivered in connection with any acquisition or sale of Securities by the Investor, the Company shall not file any Prospectus Supplement with respect to the Securities without furnishing to the Investor as many copies of such Prospectus Supplement, together with the Prospectus, as the Investor may reasonably request.
(l) Prospectus Delivery. The Company consents to the use of the Prospectus (and of each Prospectus Supplement thereto) in accordance with the provisions of the Securities Act and with the securities or “blue sky” laws of the jurisdictions in which the Securities may be sold by the Investor, in connection with the offering and sale of the Securities and for such period of time thereafter as the Prospectus is required by the Securities Act to be delivered in connection with sales of the Securities. The Company will make available to the Investor upon request, and thereafter from time to time will furnish to the Investor, as many copies of the Prospectus (and each Prospectus Supplement thereto) as the Investor may reasonably request for the purposes contemplated by the Securities Act within the time during which the Prospectus is required by the Securities Act to be delivered in connection with sales of the Securities. If during such period of time any event shall occur that in the reasonable judgment of the Company and its counsel, or in the reasonable judgment of the Investor and its counsel, is required to be set forth in the Registration Statement, the Prospectus or any Prospectus Supplement or should be set forth therein in order to make the statements made therein (in the case of the Prospectus or any Prospectus Supplement, in light of the circumstances under which they were made) not misleading, or if in the reasonable judgment of the Company and its counsel, or in the reasonable judgment of the Investor and its counsel, it is otherwise necessary to amend the Registration Statement or supplement the Prospectus or any Prospectus Supplement to comply with the Securities Act or any other applicable law or regulation, the Company shall forthwith prepare and, subject to Section 5(k) above, file with the SEC an appropriate amendment to the Registration Statement or an appropriate Prospectus Supplement and in each case shall expeditiously furnish to the Investor, at the Company’s expense, such amendment to the Registration Statement or such Prospectus Supplement, as applicable, as may be necessary to reflect any such change or to effect such compliance. The Company shall have no obligation to separately advise the Investor of, or deliver copies to the Investor of, the SEC Documents, all of which the Investor shall be deemed to have notice.
(m) Aggregation. From and after the date of this Agreement, neither the Company, nor or any of its affiliates will, and the Company shall use its reasonable best efforts to ensure that no Person acting on their behalf will, directly or indirectly, make any offers or sales of any security or solicit any offers to buy any security, under circumstances that would cause this offering of the Securities by the Company to the Investor to be aggregated with other offerings by the Company in a manner that would require stockholder approval pursuant to the rules of the Principal Market on which any of the securities of the Company are listed or designated, unless stockholder approval is obtained before the closing of such subsequent transaction in accordance with the rules of such Principal Market.
(n) Use of Proceeds. The Company will use the net proceeds from the offering as described in the Prospectus.
(o) Other Transactions. The Company shall not enter into, announce or recommend to its stockholders any agreement, plan, arrangement or transaction in or of which the terms thereof would restrict, materially delay, conflict with or impair the ability or right of the Company to perform its obligations under the Transaction Documents, including, without limitation, the obligation of the Company to deliver the Purchase Shares and the Commitment Shares to the Investor in accordance with the terms of the Transaction Documents.
(p) Required Filings Relating to Purchases. To the extent required under the Securities Act or under interpretations by the SEC thereof, as promptly as practicable after the close of each of the Company’s fiscal quarters (or on such other dates as required under the Securities Act or under interpretations by the SEC thereof), the Company shall prepare a Prospectus Supplement, which will set forth the number of Purchase Shares sold to the Investor during such quarterly period (or other relevant period), the purchase price for such Purchase Shares and the net proceeds received by the Company from such sales, and shall file such Prospectus Supplement with the SEC pursuant to Rule 424(b) under the Securities Act (and within the time periods required by Rule 424(b) and Rule 430B under the Securities Act). If any such quarterly Prospectus Supplement is not required to be filed under the Securities Act or under interpretations by the SEC thereof, the Company shall disclose the information referenced in the immediately preceding sentence in its annual report on Form 10-K or its quarterly report on Form 10-Q (as applicable) in respect of the quarterly period that ended immediately before the filing of such report in which sales of Purchase Shares were made to the Investor under this Agreement, and file such report with the SEC within the applicable time period required by the Exchange Act. The Company shall not file any Prospectus Supplement pursuant to this Section 5(p), and shall not file any report containing disclosure relating to such sales of Purchase Shares, unless a copy of such Prospectus Supplement or disclosure has been submitted to the Investor a reasonable period of time before the filing and the Investor has not reasonably objected thereto (it being acknowledged and agreed that the Company shall not submit any portion of any Form 10-K or Form 10-Q other than the specific disclosure relating to any sales of Purchase Shares). The Company shall also furnish copies of all such Prospectus Supplements to each exchange or market in the United States on which sales of the Purchase Shares may be made as may be required by the rules or regulations of such exchange or market, if applicable.
(q) Limitation on Variable Rate Transactions. From and after the date of this Agreement until the Maturity Date (irrespective of any earlier termination of this Agreement), the Company shall be prohibited from effecting or entering into an agreement to effect any issuance by the Company or any of its Subsidiaries of Common Stock or Common Stock Equivalents (or a combination of units thereof) involving a Variable Rate Transaction other than with the Investor. “Variable Rate Transaction” means an “equity line of credit” or substantially similar transaction whereby an investor is irrevocably bound to purchase securities over a period of time from the Company at a price based on the market price of the Company’s Common Stock at the time of each such purchase, provided, however, that this Section 5(q) shall not be deemed to prohibit the issuance and sale of Common Stock pursuant to an “at-the-market offering” by the Company exclusively through a registered broker-dealer acting as agent of the Company pursuant to a written agreement between the Company and such registered broker-dealer.
6. TRANSFER AGENT INSTRUCTIONS.
On the Commencement Date, the Company shall issue to the Transfer Agent (and any subsequent transfer agent) irrevocable instructions, in the form substantially similar to those used by the Investor in substantially similar transactions, to issue the Purchase Shares and the Commitment Shares in accordance with the terms of this Agreement (the “Irrevocable Transfer Agent Instructions”). All Securities to be issued to or for the benefit of the Investor pursuant to this Agreement shall be issued as DWAC Shares. The Company warrants to the Investor that no instruction other than the Irrevocable Transfer Agent Instructions referred to in this Section 6 will be given by the Company to the Transfer Agent with respect to the Securities, and the Securities shall otherwise be freely transferable on the books and records of the Company.
7. CONDITIONS TO THE COMPANY’S RIGHT TO COMMENCE SALES OF SHARES OF COMMON STOCK.
The right of the Company hereunder to commence sales of the Purchase Shares on the Commencement Date is subject to the satisfaction or waiver of each of the following conditions:
(a) The Investor shall have executed each of the Transaction Documents and delivered the same to the Company;
(b) No stop order with respect to the Registration Statement shall be pending or threatened by the SEC;
(c) All federal, state, local and foreign governmental laws, rules and regulations applicable to the transactions contemplated by the Transaction Documents and necessary for the execution, delivery and performance of the Transaction Documents and the consummation of the transactions contemplated thereby in accordance with the terms thereof shall have been complied with, and all consents, authorizations and orders of, and all filings and registrations with, all federal, state, local and foreign courts or governmental agencies and all federal, state, local and foreign regulatory or self-regulatory agencies necessary for the execution, delivery and performance of the Transaction Documents and the consummation of the transactions contemplated thereby in accordance with the terms thereof shall have been obtained or made, including, without limitation, in each case those required prior to the commencement of sales of Purchase Shares under the Securities Act, the Exchange Act, applicable state securities or “Blue Sky” laws or applicable rules and regulations of the Principal Market or otherwise required by the SEC, the Principal Market, or any state securities regulators;
(d) No statute, regulation, order, decree, writ, ruling or injunction shall have been enacted, entered, promulgated, threatened or endorsed by any federal, state or local court or governmental authority of competent jurisdiction which prohibits the consummation of or which would materially modify or delay any of the transactions contemplated by the Transaction Documents;
(e) All Securities to be issued by the Company to the Investor under the Transaction Documents shall have been approved for listing on the Principal Market in accordance with the applicable rules and regulations of the Principal Market, subject only to official notice of issuance; and
(f) The representations and warranties of the Investor shall be true and correct in all material respects as of the date hereof and as of the Commencement Date as though made at that time.
8. CONDITIONS TO THE INVESTOR’S OBLIGATION TO PURCHASE SHARES OF COMMON STOCK.
The obligation of the Investor to buy Purchase Shares under this Agreement is subject to the satisfaction or waiver of each of the following conditions on or prior to the Commencement Date and, once such conditions have been initially satisfied, there shall not be any ongoing obligation to satisfy such conditions after the Commencement has occurred:
(a) The Company shall have executed each of the Transaction Documents and delivered the same to the Investor;
(b) The Common Stock shall be listed or quoted on the Principal Market, trading in the Common Stock shall not have been within the last 365 days suspended by the SEC or the Principal Market for one or more Business Days, and all Securities to be issued by the Company to the Investor pursuant to this Agreement shall have been approved for listing or quotation on the Principal Market in accordance with the applicable rules and regulations of the Principal Market, subject only to official notice of issuance;
(c) The Investor shall have received the opinion letter of the Company’s corporate legal counsel dated as of the Commencement Date substantially in the form agreed to prior to the date of this Agreement by the Company’s legal counsel and the Investor’s legal counsel;
(d) The representations and warranties of the Company shall be true and correct in all material respects (except to the extent that any of such representations and warranties is already qualified as to materiality in Section 4 above, in which case, such representations and warranties shall be true and correct without further qualification) as of the date hereof and as of the Commencement Date as though made at that time (except for representations and warranties that speak as of a specific date, which shall be true and correct as of such date) and the Company shall have performed, satisfied and complied with the covenants, agreements and conditions required by the Transaction Documents to be performed, satisfied or complied with by the Company at or prior to the Commencement Date. The Investor shall have received a certificate, executed by the CEO, President or CFO of the Company, dated as of the Commencement Date, to the foregoing effect in the form attached hereto as Exhibit A;
(e) The Board of Directors of the Company shall have adopted the Signing Resolutions, which shall be in full force and effect without any amendment or supplement thereto as of the Commencement Date;
(f) As of the Commencement Date, the Company shall have reserved out of its authorized and unissued Common Stock, (i) solely for the purpose of effecting purchases of Purchase Shares hereunder, 10,000,000 shares of Common Stock; and (ii) solely for the purpose of effecting the issuance of Additional Commitment Shares hereunder 75,000 shares of Common Stock;
(g) The Irrevocable Transfer Agent Instructions shall have been delivered to and acknowledged in writing by the Company and the Company’s Transfer Agent (or any successor transfer agent), and the Commitment Shares required to be issued on the Commencement Date in accordance with Section 5(e) hereof shall have been issued directly to the Investor electronically as DWAC Shares;
(h) The Company shall have delivered to the Investor a certificate evidencing the incorporation and good standing of the Company in the State of Nevada issued by the Secretary of State of the State of Nevada as of a date within ten (10) Business Days of the Commencement Date;
(i) The Company shall have delivered to the Investor a certified copy of the Articles of Incorporation as certified by the Secretary of State of the State of Nevada within ten (10) Business Days of the Commencement Date;
(j) The Company shall have delivered to the Investor a secretary’s certificate executed by the Secretary of the Company, dated as of the Commencement Date, in the form attached hereto as Exhibit B;
(k) The Registration Statement shall continue to be effective and no stop order with respect to the Registration Statement shall be pending or threatened by the SEC. The Company shall have a maximum dollar amount of Common Stock registered under the Registration Statement which is sufficient to issue to the Investor not less than (i) the full Available Amount worth of Purchase Shares plus (ii) all of the Commitment Shares. The Current Report and the Initial Prospectus Supplement each shall have been filed with the SEC, as required pursuant to Section 5(a), and copies of the Prospectus shall have been delivered to the Investor in accordance with Section 5(l) hereof. The Prospectus shall be current and available for issuances and sales of all of the Securities by the Company to the Investor, and for the resale of all of the Securities by the Investor. Any other Prospectus Supplements required to have been filed by the Company with the SEC under the Securities Act at or prior to the Commencement Date shall have been filed with the SEC within the applicable time periods prescribed for such filings under the Securities Act. All reports, schedules, registrations, forms, statements, information and other documents required to have been filed by the Company with the SEC at or prior to the Commencement Date pursuant to the reporting requirements of the Exchange Act shall have been filed with the SEC within the applicable time periods prescribed for such filings under the Exchange Act;
(l) No Suspension Event has occurred, or any event which, after notice and/or lapse of time, would become a Suspension Event has occurred;
(m) All federal, state and local governmental laws, rules and regulations applicable to the transactions contemplated by the Transaction Documents and necessary for the execution, delivery and performance of the Transaction Documents and the consummation of the transactions contemplated thereby in accordance with the terms thereof shall have been complied with, and all consents, authorizations and orders of, and all filings and registrations with, all federal, state and local courts or governmental agencies and all federal, state and local regulatory or self-regulatory agencies necessary for the execution, delivery and performance of the Transaction Documents and the consummation of the transactions contemplated thereby in accordance with the terms thereof shall have been obtained or made, including, without limitation, in each case those required under the Securities Act, the Exchange Act, applicable state securities or “Blue Sky” laws or applicable rules and regulations of the Principal Market, or otherwise required by the SEC, the Principal Market or any state securities regulators;
(n) No statute, regulation, order, decree, writ, ruling or injunction shall have been enacted, entered, promulgated, threatened or endorsed by any federal, state, local or foreign court or governmental authority of competent jurisdiction which prohibits the consummation of or which would materially modify or delay any of the transactions contemplated by the Transaction Documents; and
(o) No action, suit or proceeding before any federal, state, local or foreign arbitrator or any court or governmental authority of competent jurisdiction shall have been commenced or threatened, and no inquiry or investigation by any federal, state, local or foreign governmental authority of competent jurisdiction shall have been commenced or threatened, against the Company, or any of the officers, directors or affiliates of the Company, seeking to restrain, prevent or change the transactions contemplated by the Transaction Documents, or seeking material damages in connection with such transactions.
9. INDEMNIFICATION.
In consideration of the Investor’s execution and delivery of the Transaction Documents and acquiring the Securities hereunder and in addition to all of the Company’s other obligations under the Transaction Documents, the Company shall defend, protect, indemnify and hold harmless the Investor and all of its affiliates, stockholders, officers, directors, members, managers, employees and direct or indirect investors and any of the foregoing Person’s agents or other representatives (including, without limitation, those retained in connection with the transactions contemplated by this Agreement) (collectively, the “Indemnitees”) from and against any and all actions, causes of action, suits, claims, losses, costs, penalties, fees, liabilities and damages, and expenses in connection therewith (irrespective of whether any such Indemnitee is a party to the action for which indemnification hereunder is sought), and including reasonable attorneys’ fees and disbursements (the “Indemnified Liabilities”), incurred by any Indemnitee as a result of, or arising out of, or relating to (a) any misrepresentation or breach of any representation or warranty made by the Company in the Transaction Documents or any other certificate, instrument or document contemplated hereby or thereby, (b) any breach of any covenant, agreement or obligation of the Company contained in the Transaction Documents or any other certificate, instrument or document contemplated hereby or thereby, or (c) any cause of action, suit or claim brought or made against such Indemnitee and arising out of or resulting from the execution, delivery, performance or enforcement of the Transaction Documents or any other certificate, instrument or document contemplated hereby or thereby, other than, in the case of clause (c), with respect to Indemnified Liabilities which directly and primarily result from the fraud, gross negligence or willful misconduct of an Indemnitee. The indemnity in this Section 9 shall not apply to amounts paid in settlement of any claim if such settlement is effected without the prior written consent of the Company, which consent shall not be unreasonably withheld, conditioned or delayed. To the extent that the foregoing undertaking by the Company may be unenforceable for any reason, the Company shall make the maximum contribution to the payment and satisfaction of each of the Indemnified Liabilities which is permissible under applicable law. Payment under this indemnification shall be made within thirty (30) days from the date the Investor makes written request for it. A certificate containing reasonable detail as to the amount of such indemnification submitted to the Company by the Investor shall be conclusive evidence, absent manifest error, of the amount due from the Company to the Investor. If any action shall be brought against any Indemnitee in respect of which indemnity may be sought pursuant to this Agreement, such Indemnitee shall promptly notify the Company in writing, and the Company shall have the right to assume the defense thereof with counsel of its own choosing reasonably acceptable to the Indemnitee. Any Indemnitee shall have the right to employ separate counsel in any such action and participate in the defense thereof, but the fees and expenses of such counsel shall be at the expense of such Indemnitee, except to the extent that (i) the employment thereof has been specifically authorized by the Company in writing, (ii) the Company has failed after a reasonable period of time to assume such defense and to employ counsel or (iii) in such action there is, in the reasonable opinion of such separate counsel, a material conflict on any material issue between the position of the Company and the position of such Indemnitee, in which case the Company shall be responsible for the reasonable fees and expenses of no more than one such separate counsel.
10. SUSPENSION EVENTS.
A “Suspension Event” shall be deemed to have occurred at any time as any of the following events occurs:
(a) the effectiveness of the Registration Statement registering the Securities lapses for any reason (including, without limitation, the issuance of a stop order or similar order) or the Registration Statement or the Prospectus is unavailable for the sale by the Company to the Investor (or the resale by the Investor) of any or all of the Securities to be issued to the Investor under the Transaction Documents (including, without limitation, as a result of any failure of the Company to satisfy all of the requirements for the use of a registration statement on Form S-3 pursuant to the Securities Act for the offering and sale of the Securities contemplated by this Agreement), and such lapse or unavailability continues for a period of ten (10) consecutive Business Days or for more than an aggregate of thirty (30) Business Days in any 365-day period;
(b) the suspension of the Common Stock from trading on the Principal Market for a period of three (3) consecutive Business Days, provided that the Company may not direct the Investor to purchase any shares of Common Stock during any such suspension;
(c) the delisting of the Common Stock from the NASDAQ Capital Market; provided, however, that the Common Stock is not immediately thereafter trading on the New York Stock Exchange, the NASDAQ Global Market, he NASDAQ Global Select Market, the NYSE American, the NYSE Arca or the OTC Bulletin Board, OTCQX or OTCQB operated by the OTC Markets Group, Inc. (or nationally recognized successor to any of the foregoing). If at any time after the Commencement Date, the Exchange Cap is reached unless and until stockholder approval is obtained pursuant to Section 2(f) hereof. The Exchange Cap shall be deemed to be reached at such time if, upon submission of a Regular Purchase Notice or Accelerated Purchase Notice under this Agreement, the issuance of such shares of Common Stock would exceed that number of shares of Common Stock which the Company may issue under this Agreement without breaching the Company’s obligations under the rules or regulations of the Principal Market;
(d) the failure for any reason by the Transfer Agent to issue (i) the Additional Commitment Shares to the Investor within three (3) Business Days after the date on which the Investor is entitled to receive such Additional Commitment Shares pursuant to Section 5(e) hereof and (ii) Purchase Shares to the Investor within three (3) Business Days after the applicable Purchase Date, Accelerated Purchase Date or Additional Accelerated Purchase Date (as applicable) on which the Investor is entitled to receive such Securities;
(e) the Company breaches any representation, warranty, covenant or other term or condition under any Transaction Document if such breach could have a Material Adverse Effect and except, in the case of a breach of a covenant which is reasonably curable, only if such breach continues for a period of at least five (5) Business Days;
(f) if any Person commences a proceeding against the Company pursuant to or within the meaning of any Bankruptcy Law;
(g) if the Company, pursuant to or within the meaning of any Bankruptcy Law, (i) commences a voluntary case, (ii) consents to the entry of an order for relief against it in an involuntary case, (iii) consents to the appointment of a Custodian of it or for all or substantially all of its property, or (iv) makes a general assignment for the benefit of its creditors or is generally unable to pay its debts as the same become due;
(h) a court of competent jurisdiction enters an order or decree under any Bankruptcy Law that (i) is for relief against the Company in an involuntary case, (ii) appoints a Custodian of the Company or for all or substantially all of its property, or (iii) orders the liquidation of the Company or any Subsidiary; or
(i) if at any time the Company is not eligible to transfer its Common Stock electronically as DWAC Shares.
In addition to any other rights and remedies under applicable law and this Agreement, so long as a Suspension Event has occurred and is continuing, or if any event which, after notice and/or lapse of time, would become a Suspension Event, has occurred and is continuing, the Company shall not deliver to the Investor any Regular Purchase Notice or Accelerated Purchase Notice.
11. TERMINATION
This Agreement may be terminated only as follows:
(a) If pursuant to or within the meaning of any Bankruptcy Law, the Company commences a voluntary case or any Person commences a proceeding against the Company, a Custodian is appointed for the Company or for all or substantially all of its property, or the Company makes a general assignment for the benefit of its creditors (any of which would be a Suspension Event as described in Sections 10(f), 10(g) and 10(h) hereof), this Agreement shall automatically terminate without any liability or payment to the Company (except as set forth below) without further action or notice by any Person.
(b) In the event that the Commencement shall not have occurred on or before March 20, 2023 due to the failure to satisfy the conditions set forth in Sections 7 and 8 above with respect to the Commencement, either the Company or the Investor shall have the option to terminate this Agreement at the close of business on such date or thereafter without liability of any party to any other party (except as set forth below); provided, however, that the right to terminate this Agreement under this Section 11(b) shall not be available to any party if such party is then in breach of any covenant or agreement contained in this Agreement or any representation or warranty of such party contained in this Agreement fails to be true and correct such that the conditions set forth in Section 7(c) or Section 8(e), as applicable, could not then be satisfied.
(c) At any time after the Commencement Date, the Company shall have the option to terminate this Agreement for any reason or for no reason by delivering notice (a “Company Termination Notice”) to the Investor electing to terminate this Agreement without any liability whatsoever of any party to any other party under this Agreement (except as set forth below). The Company Termination Notice shall not be effective until one (1) Business Day after it has been received by the Investor.
(d) This Agreement shall automatically terminate on the date that the Company sells and the Investor purchases the full Available Amount as provided herein, without any action or notice on the part of any party and without any liability whatsoever of any party to any other party under this Agreement (except as set forth below).
(e) If, for any reason or for no reason, the full Available Amount has not been purchased in accordance with Section 2 of this Agreement by the Maturity Date, this Agreement shall automatically terminate on the Maturity Date, without any action or notice on the part of any party and without any liability whatsoever of any party to any other party under this Agreement (except as set forth below).
Except as set forth in Sections 11(a) (in respect of a Suspension Event under Sections 10(f), 10(g) and 10(h)), 11(d) and 11(e), any termination of this Agreement pursuant to this Section 11 shall be effected by written notice from the Company to the Investor, or the Investor to the Company, as the case may be, setting forth the basis for the termination hereof. The representations and warranties and covenants of the Company and the Investor contained in Sections 3, 4, 5, and 6 hereof, the indemnification provisions set forth in Section 9 hereof and the agreements and covenants set forth in Sections 10, 11 and 12 shall survive the Commencement and any termination of this Agreement. No termination of this Agreement shall (i) affect the Company’s or the Investor’s rights or obligations under (A) this Agreement with respect to pending Regular Purchases, Accelerated Purchases and Additional Accelerated Purchases, and the Company and the Investor shall complete their respective obligations with respect to any pending Regular Purchases, Accelerated Purchases and Additional Accelerated Purchases under this Agreement and (B) the Registration Rights Agreement, which shall survive any such termination, or (ii) be deemed to release the Company or the Investor from any liability for intentional misrepresentation or willful breach of any of the Transaction Documents.
12. MISCELLANEOUS.
(a) Governing Law; Jurisdiction; Jury Trial. The corporate laws of the State of Nevada shall govern all issues concerning the relative rights of the Company and its stockholders. All other questions concerning the construction, validity, enforcement and interpretation of this Agreement and the other Transaction Documents shall be governed by the internal laws of the State of Illinois, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of Illinois or any other jurisdictions) that would cause the application of the laws of any jurisdictions other than the State of Illinois. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in the State of Illinois, County of Cook, for the adjudication of any dispute hereunder or under the other Transaction Documents or in connection herewith or therewith, or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding is improper. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof to such party at the address for such notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. EACH PARTY HEREBY IRREVOCABLY WAIVES ANY RIGHT IT MAY HAVE, AND AGREES NOT TO REQUEST, A JURY TRIAL FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR IN CONNECTION HEREWITH OR ARISING OUT OF THIS AGREEMENT OR ANY TRANSACTION CONTEMPLATED HEREBY.
(b) Counterparts. This Agreement may be executed in two or more identical counterparts, all of which shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to the other party; provided that a facsimile signature or signature delivered by e-mail in a “.pdf” format data file shall be considered due execution and shall be binding upon the signatory thereto with the same force and effect as if the signature were an original signature.
(c) Headings. The headings of this Agreement are for convenience of reference and shall not form part of, or affect the interpretation of, this Agreement.
(d) Severability. If any provision of this Agreement shall be invalid or unenforceable in any jurisdiction, such invalidity or unenforceability shall not affect the validity or enforceability of the remainder of this Agreement in that jurisdiction or the validity or enforceability of any provision of this Agreement in any other jurisdiction.
(e) Entire Agreement. The Transaction Documents supersede all other prior oral or written agreements between the Investor, the Company, their affiliates and Persons acting on their behalf with respect to the subject matter thereof, and this Agreement, the other Transaction Documents and the instruments referenced herein contain the entire understanding of the parties with respect to the matters covered herein and therein and, except as specifically set forth herein or therein, neither the Company nor the Investor makes any representation, warranty, covenant or undertaking with respect to such matters. The Company acknowledges and agrees that is has not relied on, in any manner whatsoever, any representations or statements, written or oral, other than as expressly set forth in the Transaction Documents.
(f) Notices. Any notices, consents or other communications required or permitted to be given under the terms of this Agreement must be in writing and will be deemed to have been delivered: (i) upon receipt when delivered personally; (ii) upon receipt when sent by facsimile or email (provided confirmation of transmission is mechanically or electronically generated and kept on file by the sending party); or (iii) one Business Day after deposit with a nationally recognized overnight delivery service, in each case properly addressed to the party to receive the same. The addresses for such communications shall be:
If to the Company:
Anavex Life Sciences Corp.
630 5th Avenue, 20th Floor
New York, New York 10111
Telephone: (844) 689-3939
E-mail: cmissling@anavexcorp.com
Attention: Christopher Missling, PhD., CEO
With a copy to (which shall not constitute notice or service of process):
K&L Gates, LLP
200 S. Biscayne Blvd., Ste. 3900
Miami, Florida 33131
Telephone: (305) 539-3306
Facsimile: (305) 358-7095
E-mail: clayton.parker@klgates.com
Attention: Clayton E. Parker, Esq.
If to the Investor:
Lincoln Park Capital Fund, LLC
440 North Wells, Suite 410
Chicago, IL 60654
Telephone: (312) 822-9300
Facsimile: (312) 822-9301
E-mail: jscheinfeld@lpcfunds.com/jcope@lpcfunds.com
Attention: Josh Scheinfeld/Jonathan Cope
If to the Transfer Agent:
Nevada Agency and Transfer Company
50 West Liberty Street, Suite 880
Reno, NV 89501
Telephone: (775) 322-0626
Fax: (775) 322-5623
E-mail: tiffany@natco.org
Attention: Tiffany Baxter
or at such other address and/or facsimile number and/or to the attention of such other Person as the recipient party has specified by written notice given to each other party three (3) Business Days prior to the effectiveness of such change. Written confirmation of receipt (A) given by the recipient of such notice, consent or other communication, (B) mechanically or electronically generated by the sender’s facsimile machine or email account containing the time, date, and recipient facsimile number or email address, as applicable, and an image of the first page of such transmission or (C) provided by a nationally recognized overnight delivery service, shall be rebuttable evidence of personal service, receipt by facsimile or receipt from a nationally recognized overnight delivery service in accordance with clause (i), (ii) or (iii) above, respectively.
(g) Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their respective successors and assigns. The Company shall not assign this Agreement or any rights or obligations hereunder without the prior written consent of the Investor, including by merger or consolidation. The Investor may not assign its rights or obligations under this Agreement.
(h) No Third Party Beneficiaries. This Agreement is intended for the benefit of the parties hereto and their respective permitted successors and assigns, and is not for the benefit of, nor may any provision hereof be enforced by, any other Person.
(i) Publicity. The Company shall afford the Investor and its counsel with the opportunity to review and comment upon, shall consult with the Investor and its counsel on the form and substance of, and shall give due consideration to all such comments from the Investor or its counsel on, any press release, SEC filing or any other public disclosure by or on behalf of the Company relating to the Investor, its purchases hereunder or any aspect of the Transaction Documents or the transactions contemplated thereby, not less than 24 hours prior to the issuance, filing or public disclosure thereof. The Investor must be provided with a final version of any such press release, SEC filing or other public disclosure at least 24 hours prior to any release, filing or use by the Company thereof; provided, however, that the Company’s obligations pursuant to this Section 12(i) shall not apply if the form and substance of such press release, SEC filing, or other public disclosure relating to the Investor, its purchases hereunder or any aspect of the Transaction Documents or the transactions contemplated thereby previously have been publicly disclosed by the Company in compliance with this Section 12(i). The Company agrees and acknowledges that its failure to fully comply with this provision constitutes a Material Adverse Effect.
(j) Further Assurances. Each party shall do and perform, or cause to be done and performed, all such further acts and things, and shall execute and deliver all such other agreements, certificates, instruments and documents, as the other party may reasonably request in order to consummate and make effective, as soon as reasonably possible, the Commencement, and to carry out the intent and accomplish the purposes of this Agreement and the consummation of the transactions contemplated hereby.
(k) No Financial Advisor, Placement Agent, Broker or Finder. The Company represents and warrants to the Investor that it has not engaged any financial advisor, placement agent, broker or finder in connection with the transactions contemplated hereby. The Investor represents and warrants to the Company that it has not engaged any financial advisor, placement agent, broker or finder in connection with the transactions contemplated hereby. The Company shall be responsible for the payment of any fees or commissions, if any, of any financial advisor, placement agent, broker or finder relating to or arising out of the transactions contemplated hereby. The Company shall pay, and hold the Investor harmless against, any liability, loss or expense (including, without limitation, attorneys’ fees and out of pocket expenses) arising in connection with any such claim.
(l) No Strict Construction. The language used in this Agreement will be deemed to be the language chosen by the parties to express their mutual intent, and no rules of strict construction will be applied against any party.
(m) Remedies, Other Obligations, Breaches and Injunctive Relief. The Investor’s remedies provided in this Agreement, including, without limitation, the Investor’s remedies provided in Section 9, shall be cumulative and in addition to all other remedies available to the Investor under this Agreement, at law or in equity (including a decree of specific performance and/or other injunctive relief), no remedy of the Investor contained herein shall be deemed a waiver of compliance with the provisions giving rise to such remedy and nothing herein shall limit the Investor’s right to pursue actual damages for any failure by the Company to comply with the terms of this Agreement. The Company acknowledges that a breach by it of its obligations hereunder will cause irreparable harm to the Investor and that the remedy at law for any such breach may be inadequate. The Company therefore agrees that, in the event of any such breach or threatened breach, the Investor shall be entitled, in addition to all other available remedies, to an injunction restraining any breach, without the necessity of showing economic loss and without any bond or other security being required.
(n) Enforcement Costs. In the event of a dispute arising out of or relating to this Agreement, if a court of competent jurisdiction determines in a final, non-appealable order that a party has breached this Agreement, then in addition to any other available remedies, the non-breaching party shall be entitled to (in addition to all other available remedies), and the breaching party shall be liable for, the reasonable legal fees and expenses incurred by the non-breaching party in connection with such dispute, including any appeals in connection therewith.
(o) Waivers. No provision of this Agreement may be waived other than in a written instrument signed by the party against whom enforcement of such waiver is sought. No failure or delay in the exercise of any power, right or privilege hereunder shall operate as a waiver thereof, nor shall any single or partial exercise of any such power, right or privilege preclude other or further exercise thereof or of any other right, power or privilege.
** Signature Page Follows **
IN WITNESS WHEREOF, the Investor and the Company have caused this Purchase Agreement to be duly executed as of the date first written above.
THE COMPANY:
ANAVEX LIFE SCIENCES CORP.
| By: | /s/ Christopher Missling | |
| Name: | Christopher Missling, PhD. | |
| Title: | Chief Executive Officer |
INVESTOR:
LINCOLN PARK CAPITAL FUND, LLC
BY: LINCOLN PARK CAPITAL, LLC
BY: ROCKLEDGE CAPITAL CORPORATION
| By: | /s/ Josh Scheinfeld | |
| Name: | Josh Scheinfeld | |
| Title: | President |
| SCHEDULES | |
| Schedule 4(a) | Subsidiaries |
| AnavexAustralia Pty Limited | |
| AnavexGermany GmbH | |
| AnavexCanada Ltd. | |
| Schedule 4(c) | Capitalization |
| See attached. | |
| EXHIBITS | |
| Exhibit A | Form of Officer’s Certificate |
| Exhibit B | Form of Secretary’s Certificate |
EXHIBIT A
FORM OF OFFICER’S CERTIFICATE
This Officer’s Certificate (“Certificate”) is being delivered pursuant to Section 8(e) of that certain Purchase Agreement dated as of February 3, 2023 (“Purchase Agreement”), by and between ANAVEX LIFE SCIENCES CORP. , a Nevada corporation (the “Company”), and LINCOLN PARK CAPITAL FUND, LLC (the “Investor”). Terms used herein and not otherwise defined shall have the meanings ascribed to them in the Purchase Agreement.
The undersigned, ___________, ______________ of the Company, hereby certifies as follows:
1. I am the _____________ of the Company and make the statements contained in this Certificate;
2. The representations and warranties of the Company are true and correct in all material respects (except to the extent that any of such representations and warranties is already qualified as to materiality in Section 4 of the Purchase Agreement, in which case, such representations and warranties are true and correct without further qualification) as of the date when made and as of the Commencement Date as though made at that time (except for representations and warranties that speak as of a specific date, in which case such representations and warranties are true and correct as of such date);
3. The Company has performed, satisfied and complied in all material respects with covenants, agreements and conditions required by the Transaction Documents to be performed, satisfied or complied with by the Company at or prior to the Commencement Date.
4. The Company has not taken any steps, and does not currently expect to take any steps, to seek protection pursuant to any Bankruptcy Law nor does the Company or any of its Subsidiaries have any knowledge or reason to believe that its creditors intend to initiate involuntary bankruptcy or insolvency proceedings.
IN WITNESS WHEREOF, I have hereunder signed my name on this ___ day of ___________.
| Name: | |
| Title: |
The undersigned as Secretary of ANAVEX LIFE SCIENCES CORP., a Nevada corporation, hereby certifies that ___________ is the duly elected, appointed, qualified and acting ________ of _________ and that the signature appearing above is his genuine signature.
| Secretary |
EXHIBIT B
FORM OF SECRETARY’S CERTIFICATE
This Secretary’s Certificate (“Certificate”) is being delivered pursuant to Section 8(k) of that certain Purchase Agreement dated as of February 3, 2023 (“Purchase Agreement”), by and between ANAVEX LIFE SCIENCES CORP. , a Nevada corporation (the “Company”) and LINCOLN PARK CAPITAL FUND, LLC (the “Investor”), pursuant to which the Company may sell to the Investor up to One Hundred Fifty Million Dollars ($150,000,000) of the Company’s Common Stock, $0.001 par value per share (the “Common Stock”). Terms used herein and not otherwise defined shall have the meanings ascribed to them in the Purchase Agreement.
The undersigned, ____________, Secretary of the Company, hereby certifies as follows:
1. I am the Secretary of the Company and make the statements contained in this Secretary’s Certificate.
2. Attached hereto as Exhibit A and Exhibit B are true, correct and complete copies of the Company’s Bylaws (“Bylaws”) and Articles of Incorporation (“Charter”), in each case, as amended through the date hereof, and no action has been taken by the Company, its directors, officers or stockholders, in contemplation of the filing of any further amendment relating to or affecting the Bylaws or Charter.
3. Attached hereto as Exhibit C are true, correct and complete copies of the resolutions duly adopted by the Board of Directors of the Company on _____________, at which a quorum was present and acting throughout. Such resolutions have not been amended, modified or rescinded and remain in full force and effect and such resolutions are the only resolutions adopted by the Company’s Board of Directors, or any committee thereof, or the stockholders of the Company relating to or affecting (i) the entering into and performance of the Purchase Agreement, or the issuance, offering and sale of the Purchase Shares and the Commitment Shares and (ii) and the performance of the Company of its obligation under the Transaction Documents as contemplated therein.
4. As of the date hereof, the authorized, issued and reserved capital stock of the Company is as set forth on Exhibit D hereto.
IN WITNESS WHEREOF, I have hereunder signed my name on this ___ day of ____________
| Secretary |
The undersigned as ___________ of ANAVEX LIFE SCIENCES CORP., a Nevada corporation, hereby certifies that ____________ is the duly elected, appointed, qualified and acting Secretary of _________, and that the signature appearing above is his genuine signature.
35
Exhibit 31.1
CERTIFICATION
I, Christopher Missling, certify that:
1. I have reviewed this Quarterly Report on Form 10-Q for the three months ended December 31, 2022 of Anavex Life Sciences Corp. (the “registrant”);
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a–15(e) and 15d–15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a–15(f) and 15d–15(f)) for the registrant and have:
(a) designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b) any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
| Date: February 7, 2023 | |
| /s/Christopher Missling, PhD | |
| Christopher Missling, PhD | |
| Chief Executive Officer, President and Secretary | |
| (Principal Executive Officer) |
Exhibit 31.2
CERTIFICATION
I, Sandra Boenisch, certify that:
1. I have reviewed this Quarterly Report on Form 10-Q for the three months ended December 31, 2022 of Anavex Life Sciences Corp. (the “registrant”);
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a–15(e) and 15d–15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a–15(f) and 15d–15(f)) for the registrant and have:
(a) designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b) any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
| Date: February 7, 2023 | |
| /s/Sandra Boenisch | |
| Sandra Boenisch, CPA, CGA | |
| Principal Financial Officer, Treasurer | |
| (Principal Financial and Accounting Officer) |
Exhibit 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of Anavex Life Sciences Corp. (the “Company”) on Form 10-Q for the three months ended December 31, 2022 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned, in the capacities and on the date indicated below, hereby certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to the best of our knowledge:
(1) the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
| Date: February 7, 2023 | /s/Christopher Missling, PhD |
| Christopher Missling, PhD | |
| Chief Executive Officer, President, Secretary | |
| (Principal Executive Officer) |
| /s/Sandra Boenisch | |
| Sandra Boenisch, CPA, CGA | |
| Principal Financial Officer, Treasurer | |
| (Principal Financial and Accounting Officer) |
The foregoing certification is being furnished solely to accompany the Report pursuant to 18 U.S.C. § 1350, and is not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is not to be incorporated by reference into any filing of the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing. A signed original of this written statement required by Section 906, or other document authenticating, acknowledging, or otherwise adopting the signature that appears in typed form within the electronic version of this written statement required by Section 906, has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.