
Annual Report 2024

2024 Annual Report including the Annual Financial Statements for the year ended December 31, 2024 This Annual Report is filed with the Dutch Authority for the Financial Markets (Stichting Autoriteit Financiële Markten, AFM). The following main items included in our annual report on Form 20-F for the year ended December 31, 2024 (2024 20-F) filed with the United States Securities and Exchange Commission (SEC) on or about the date of this Annual Report have not been included in this Annual Report: • Form 20-F cover page; • Item 7 – Major Shareholders and Related Party Transactions; • Item 10E – Taxation; • Item 16E – Purchases of Equity Securities by the Issuer and Affiliated Purchasers; • Item 16G – Corporate Governance; • Report of Independent Registered Public Accounting Firm in respect of Internal Control over Financial Reporting for the SEC filing; • Report of Independent Registered Public Accounting Firm in respect of the PCAOB audits of the 2024 financial statements for the SEC filing; • Exhibits; and • Signatures. The following main sections of our Annual Report have not been included in our 2024 20-F: • Shareholder Letter; • Outlook 2025; • Statement of the Board of Directors; • Risk Appetite and Control; • Share Classes and Principal Shareholders; • Non-Financial Information (including Sustainability Statement); • The Company Financial Statements under Section Financial Statements (prepared pursuant to Dutch law); • Independent auditor’s report - Report on the audit of the financial statements 2024 included in the Annual Report with respect to the AFM Filing; • Limited Assurance Report of the Independent Auditor on the Sustainability Statement; and • Glossary. Certain defined terms Unless otherwise indicated, “argenx,” “argenx SE,” “the Company,” “our company,” “we,” “us”, “our” our “Group” refer to argenx SE and its consolidated subsidiaries. argenx SE is a European public company (Societas Europaea) incorporated under the laws of the Netherlands with its statutory seat in Amsterdam, the Netherlands. It is publicly listed in Belgium and the United States of America (the U.S.) The applicable regulations with respect to public information and protection of investors, as well as the commitments we make to securities and market authorities, are described in this Annual Report. We own various trademark registrations and applications, and unregistered trademarks, including but not limited to VYVGART®, VYVGART HYTRULO™, VYVDURA®, ARGENX™, ABDEG™, NHANCE™, SIMPLE argenx Annual Report 2024 2

ANTIBODY™, ARGENXMEDHUB™, MG UNITED™, SHINING THROUGH CIDP™ and our corporate logo. Trade names, trademarks and service marks of other companies appearing in this Annual Report are the property of their respective holders. Solely for convenience, the trademarks and trade names in this Annual Report may be referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend to use or display other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship, any other companies. VYVGART® (efgartigimod alfa) (VYVGART) has been approved in the U.S., Japan, the European Union (the EU), the United Kingdom (UK), Switzerland, Israel, mainland China (Mainland China), Canada, South Korea and United Arab Emirates for the intravenous treatment of generalized myasthenia gravis (gMG). We have now commercialized VYVGART in the U.S., several countries in the EU, Japan, Mainland China (through our partner Zai Lab Ltd (Zai Lab)), Israel (through our Medison Pharma Ltd. (Medison)) and Canada. VYVGART is now also approved and launched in Japan for the treatment of ITP. VYVGART subcutaneous (SC) (efgartigimod alfa + hyaluronidase qvfc) (VYVGART SC) has been approved in the U.S. and China as VYVGART HYTRULO™ (VYVGART HYTRULO), in Japan as VYVDURA® (VYVDURA) and in the EU and the UK as VYVGART for the treatment of gMG. VYVGART SC has also been approved in Israel for the treatment of gMG. We have now commercialized VYVGART SC for gMG in the U.S. and China (as VYVGART HYTRULO), in Japan (as VYVDURA) and in several countries in the EU (as VYVGART). Pricing and reimbursement discussions for VYVGART SC remain ongoing in multiple other countries, including more countries in the EU. VYVGART SC has now also been approved in the U.S., China and Japan for the treatment of chronic inflammatory demyelinating polyneuropathy (CIDP). We have now commercialized VYVGART SC for CIDP in the U.S. and China (as VYVGART HYTRULO) and in Japan (as VYVDURA). For both VYVGART and VYVGART SC, we are aiming for further approvals and we are working to expand commercialization in other jurisdictions. Unless otherwise specified, references in this Annual Report to VYVGART should be read as references to VYVGART and/or VYVGART SC, including VYVGART HYTRULO in relation to the U.S. and China, VYVGART in relation to the EU and the UK and VYVDURA in relation to Japan, depending on the context. Basis of preparation of our audited consolidated financial statements Our consolidated financial statements are prepared in accordance with the IFRS® Accounting Standards (IFRS) as issued by the International Accounting Standards Board (IASB) as adopted by the EU (EU-IFRS) and in accordance with the legal requirements of Part 9 of Book 2 of the Dutch Civil Code. Our consolidated financial statements are presented in this Annual Report in U.S. dollars. All references in this Annual Report to “$,” “US$,” “U.S.$,” “U.S. dollars,” “dollars” and “USD” mean U.S. dollars and all references to “€,” “EUR,” and “euros” mean euros, unless otherwise noted. Throughout this Annual Report, references to ADSs mean American depositary shares (ADSs) or ordinary shares represented by ADSs, as the case may be. Forward-looking Statements This Annual Report contains certain forward-looking statements. A forward-looking statement is any statement that does not relate to historical facts or events or to facts or events as of the date of this Annual Report or that are derived from our management’s beliefs and assumptions based on information currently available to our management. Forward-looking statements are generally identified by the use of forward- looking words, such as “anticipate”, “aspire”, “believe”, “can”, “continue”, “could”, “estimate”, “expect”, “entail”, “hope”, “intend”, “is designed to”, “look forward to”, “may”, “might”, “objective”, “plan”, “potential”, “pursue”, “project”, “predict”, “seek”, “should”, “target”, “will” or other or comparable variations or the negative of such argenx Annual Report 2024 3

terms, or by discussion of strategy, plans, objectives, goals, future events or intentions, although not all forward-looking statements contain these identifying words. These statements relate to our future results of operations and financial positions, prospects, developments, growth, business strategies, plans and our objectives for future operations, results of clinical trials and regulatory approvals, and are based on analyses or forecasts of future developments and estimates of amounts not yet determinable. These forward-looking statements represent the view of management only as of the date of this Annual Report, and we expressly disclaim any obligation or undertaking to update, review or revise forward-looking statements (whether as a result of new information, future developments or otherwise), except as may be otherwise required by applicable law. The forward-looking statements in this Annual Report involve known and unknown risks, future events, assumptions, uncertainties and other factors that could cause our actual future results of operations and financial positions, prospects, developments, growth, business strategies, plans and our objectives for future operations, results of clinical trials and regulatory approvals to differ materially from those forecasted or suggested herein. Forward-looking statements include, but are not limited to, statements about: • the initiation, timing, progress, development and results of clinical trials of our product candidates, including new indications, alternative dosing regimens, treatment modalities, and methods of administration, including statements regarding when results or interim analysis of the clinical trials will be available or made public; • the expansion of our business, including the further development of our sales and marketing abilities and our IIP, and the value of our pipeline; • the potential attributes, benefits, and side effects of our products and product candidates, including new indications, alternative dosing regimens and treatment modalities, and their competitive position with respect to other alternative treatments; • our ability to advance product candidates into, and successfully complete, clinical trials; • our estimates of the number of patients who suffer from the diseases we are targeting and the number of patients that will enroll in our clinical trials; • the demand and commercialization of our products and product candidates, including new indications, alternative dosing regimens, treatment modalities, and methods of administration, if approved; • the anticipated timing or likelihood of market or regulatory decisions relating to or of our products, including new indications, alternative dosing regimens, treatment modalities, and methods of administration; • the anticipated pricing and reimbursement of our products and product candidates, if approved; • our plans to have various programs to help patients afford our products, including patient assistance and co-pay coupon programs for eligible patients; • our ability to establish sales, marketing and distribution capabilities for any of our products and product candidates that achieve regulatory approval; • our regulatory strategy and our ability to establish and maintain manufacturing arrangements for our products and product candidates; • the scope and duration of protection, including any exclusivity period, we are able to establish and maintain for intellectual property rights covering our products and product candidates, platform and technology, including our intention to seek patent term extensions where available; • our estimates regarding expenses, future revenues, cash flow, capital requirements and our needs for additional financing; • our expectation that we will benefit from the Belgian innovation income deduction; • our financial performance, including potential volatility in the price of our ordinary shares and ADSs; • the competition we face in our drug discovery, development, and commercialization efforts; • the rate and degree of market acceptance of our products and product candidates, if approved; • the potential benefits of our current collaborations, including the possibility to access partner technology platforms or capabilities; argenx Annual Report 2024 4

• our plans and ability to enter into or maintain current collaborations for additional programs or product candidates; • our plans and ability to enter into or maintain current new distribution partnerships; • our long-term growth strategy to develop and market additional products and product candidates, including efgartigimod for new indications, empasibrubart and ARGX-119; • the impact of government laws and regulations on our business; • our expectations with respect to the timing and amount of any dividends (if any); • our plans regarding our supply chain, including our reliance on third parties, including contract manufacturing organizations (CMOs); and • our business strategies, plans, projects, goals and targets and the timing, outcomes and benefits thereof. These include changes in general economic and business conditions. You should refer to Section 2 ”Risk Factors” of this Annual Report for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. You should read this Annual Report and the documents that we reference in this Annual Report and have filed as exhibits to the Annual Report completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements. Information regarding market and industry statistics contained in this Annual Report is included based on information available to us that we believe is accurate. Forecasts and other forward-looking information obtained from this available information is subject to the same qualifications and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services. In addition, statements that include “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and you are cautioned not to unduly rely upon these statements. argenx Annual Report 2024 5

Table of Contents To our Shareholders Shareholder Letter 10 2024 In Brief 11 2025 Outlook 23 1 Presentation of the Group 1.1 Company Profile 25 1.2 Strategy and Objectives 29 1.3 Our Products and Products Candidates 31 1.4 Collaborations and licenses 49 1.5 Manufacturing and Supply 56 1.6 Intellectual Property 56 1.7 Regulation 59 1.8 Documents on display 80 2 Risk Factors 2.1 Summary Risk Factors 82 2.2 Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications 84 2.3 Risk Factors Related to the Development and Clinical Testing of argenx’s Products and Product Candidates 91 2.4 Risk Factors Related to argenx’s Dependence on Third Parties 94 2.5 Risk Factors Related to Other Government Regulations 98 2.6 Risk Factors Related to argenx’s Financial Position 103 2.7 Risk Factors Related to argenx’s Business and Industry 104 2.8 Risk Factors Related to argenx’s Intellectual Property 107 2.9 Risk Factors Related to argenx’s Organization and Operations 111 2.10 Risk Factors Related to the ADSs 114 2.11 Risk Factors Related to being a Foreign Private Issuer or a Dutch Company 116 argenx Annual Report 2024 6

3 Corporate Governance 3.1 Dutch Corporate Governance Code 120 3.2 Management Structure 121 3.3 Report of the Non-Executive Directors 140 3.4 Remuneration Report and Compensation Statement 143 3.5 Corporate Governance – Nasdaq Listing Rules 183 3.6 Share Ownership 183 3.7 Insider Trading 183 3.8 Cybersecurity 184 3.9 Risk Appetite & Control 185 4 General Description of the Company and its Share Capital 4.1 Legal Information on the Company 190 4.2 Share Capital 190 4.3 Share Classes and Principal Shareholders 194 4.4 Limitations on the right to hold securities 196 4.5 General Meeting, Voting Rights and Admission 197 4.6 Anti-Takeover Provisions 199 4.7 Change of Control 199 4.8 Exchange Controls 199 4.9 Amendments of Articles of Association 199 4.10 Transparency Directive 199 4.11 Dutch Financial Reporting Supervision Act 200 4.12 Dividends and Other Distributions 200 4.13 Right to a surplus in the event of a liquidation 201 4.14 Material Modifications to the Rights of Security Holders and Use of Proceeds 201 4.15 Enforcement of civil liabilities 202 4.16 Controls and Procedures 203 4.17 Financial Calendar 2025 204 argenx Annual Report 2024 7

5 Operating and Financial Review and Prospects 5.1 Overview 206 5.2 Basis of presentation 208 5.3 Critical Accounting Judgements and Major Sources of Estimation Uncertainty 210 5.4 Results of Operation 212 5.5 Liquidity and Capital Resources 215 5.6 Research and development, patents and licenses 218 5.7 Trend information 218 5.8 Off-Balance Sheet Arrangements 219 5.9 Contractual Obligations 219 5.10 Information Regarding the Independent Auditor 219 5.11 Material Contracts and Related Party Transactions 219 5.12 Employees 222 5.13 Insurance 222 5.14 Legal and Arbitration Proceedings 222 5.15 Taxation 223 6 Financial Statements 6.1 Consolidated Financial Statements 242 6.2 Notes to the Consolidated Financial Statements 249 6.3 Company Financial Statements of argenx SE for the Year ended December 31, 2024 285 6.4 Other information 291 7 Non-Financial Information 7.1 Sustainability Statement 313 7.2 Sustainability Strategy 320 7.3 Environment 335 7.4 Social 352 7.5 Patients 357 7.6 Industry Specific Disclosures 364 7.7 Governance 369 7.8 Other Considerations 373 7.9 Appendix 375 8 Glossary 8.1 Cross Reference table for annual reporting requirements 383 8.2 Management Confirmations 384 8.3 Definitions 385 argenx Annual Report 2024 8

To our Shareholders Shareholder Letter 10 2024 In Brief 11 2025 Outlook 23 argenx Annual Report 2024 9

Shareholder Letter Dear Shareholder, As we reflect on 2024, we are filled with pride and gratitude for the remarkable progress and achievements that have defined this year for argenx. We are more committed than ever to transforming the treatment of severe autoimmune diseases and the milestones we have reached are a testament to the dedication and resilience of our entire team. This year, we made significant steps in expanding the reach and impact of VYVGART® (efgartigimod alfa- fcab), our first-in-class antibody fragment targeting FcRn. With approvals for both intravenous and subcutaneous formulations in multiple indications, including gMG, primary immune thrombocytopenia (ITP), and CIDP, we are now able to offer life-changing treatments to more than 10,000 patients globally. Our financial performance has been robust, with global product net sales reaching $2.2bn in 2024. With our continued commercial execution, we expect to reach sustainable profitability during 2025, giving us the financial flexibility to fuel the next generation of groundbreaking therapies. This growth reflects the strong demand for our innovative therapies and the successful execution of our strategic initiatives. We are particularly proud of the initial success of our CIDP launch, with approximately 1,000 patients on therapy in first two quarters of launch. Looking ahead, we remain focused on our Vision 2030, which aims to transform the treatment landscape for autoimmune diseases. Our goals include reaching at least 50,000 patients globally, advancing our pipeline to achieve 10 labelled indications, and bringing five new molecules into Phase 3 by 2030. This vision has already started to take shape as we continue to innovate on the patient experience with our pre- filled syringe (VYVGART SC) (with an expected Prescription Drug User Fee Act target action date (PDUFA Date) of April 10, 2025) and the auto-injector approval expected in 2027. We are also advancing our clinical programs bringing us to 10 Phase 3 clinical trials and 10 Phase 2 clinical trials across our 3 clinical assets (efgartigimod, empasiprubart, ARGX-119). We continue investing in our growing pipeline by progressing 4 INDs into Phase 1 in 2025. We are confident that our continued investment in innovation leading to differentiated antibody candidates will drive transformative outcomes for patients. None of this would be possible without the relentless commitment of all argonauts i.e., all employees, management and our board of directors (Board of Directors) to our mission and in particular the unwavering support of our shareholders. Your belief in our mission and your trust in our vision have been instrumental in our success. As we move forward, we remain committed to delivering value to our shareholders while making a meaningful difference in the lives of patients. Thank you for your continued support. Sincerely, Tim Van Hauwermeiren & Peter Verhaeghe argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Shareholder Letter 10 Peter Verhaeghe Tim van Hauwermeiren

2024 In Brief In 2024, we established our ‘Vision 2030’ outlining our long-term commitment to transform the treatment of severe autoimmune disease with VYVGART, empasiprubart and our expanding pipeline of antibody- based therapeutics. With our eyes set on 2030, we are targeting the treatment of 50,000 patients globally, securing 10 labeled indications across all approved medicines, and advancing five pipeline candidates into Phase 3 development. In 2024, we made important progress to reach this goal. We grew our global commercial footprint in gMG to reach more than 10,000 patients and we remain on track to expand into additional regions throughout 2025. We received FDA approval for VYVGART HYTRULO for the treatment of CIDP and have been working hard to bring VYVGART HYTRULO to CIDP patients, reaching approximately 1,000 patients by the end of 2024. This, together with our continued growth in MG, translated in $2.2 billion in product net sales in 2024. We look forward to continued commercial execution as we expand our patient reach through label enabling studies in seronegative gMG and ocular MG patient populations and we continue to innovate on the patient experience with our pre-filled syringe (PFS) with VYVGART SC, with an PDUFA Date of April 10, 2025. We made significant progress in evaluating efgartigimod across additional autoimmune diseases. We announced the GO-decisions for primary Sjögren’s disease (SjD) and three subsets of myositis (immune- mediated necrotizing myopathy (IMNM), anti-synthetase syndrome (ASyS), dermatomyositis (DM)), for which we are currently running Phase 3 clinical trials. We continue to evaluate efgartigimod in more than 10 additional indications, and this year, we added autoimmune encephalitis to the line-up. We are excited to add another indication in 2025. In 2024 we made significant progress with our second asset, empasiprubart (ARGX-117) targeting complement component 2 (C2). empasiprubart has now shown proof-of-concept in multifocal motor neuropathy (MMN) and has started its first Phase 3 in this indication. We additionally announced CIDP as the 4th indication for which we go straight to a Phase 3 clinical trial, expected to start in 2025. Beyond our first two assets, efgartigimod and empasiprubart, we worked to further advance our third clinical pipeline asset, ARGX-119, targeting muscle-specific kinase (MuSK). ARGX-119 has started its first proof-of- concept clinical trial in congenital myasthenic syndrome (CMS) and amyotrophic lateral sclerosis (ALS) this year and we have announced a 3rd indication, spinal muscular atrophy (SMA). We believe ARGX-119 has potential as a novel treatment modality in multiple serious indications. Our immunology innovation program (IIP) is a key driver for our future sustainable growth. This is reflected in 4 new investigational new drugs (INDs) that will start Phase 1 clinical trials in 2025 to continue to deliver immunology innovations to the patients who need them. argenx Annual Report 2024 2024 In Brief 11 Operational Highlights

Reach More Patients Globally with VYVGART • VYVGART is now approved in the U.S., Japan, the EU, the UK, Switzerland, Israel, Mainland China, Canada, South Korea, United Arab Emirates, Australia and Kuwait (through Genpharm Services FZ-LLC (Genpharm)) for the treatment of gMG. VYVGART is now also approved and launched in Japan for the treatment of ITP. VYVGART SC is now approved in the U.S., the EU, the UK, Japan, China (through Zai Lab), Australia and Kuwait (through Genpharm) for the treatment of gMG and in the U.S., Japan, and China for the treatment of CIDP. VYVGART is the only gMG treatment available as both an intravenous (IV) and a simple SC injection, providing choice to patients in how and where they are treated. • In 2024, we generated product net sales of $2.2 billion. • Pricing and reimbursement discussions for VYVGART and VYVGART SC remain ongoing in multiple jurisdictions, including in several countries in the EU, with new agreements in place in France, Luxembourg, Belgium, the Netherlands, Poland, Slovakia and Austria. • We filed for approval of VYVGART for gMG in Saudi Arabia and are expecting a decision on approval in 2025. • We filed for approval of VYVGART SC for CIDP in the EU and are expecting a decision on approval in 2025. • We received approval of the PFS for gMG in Europe on February 13, 2025. • We filed for approval of the PFS for gMG and CIDP in the U.S. with a PDUFA Date of April 10, 2025. We also filed for approval in Canada and Japan with expected decisions on approval in 2025. argenx Annual Report 2024 2024 In Brief 12 The science of co-creation drives our quest to engineer life-changing immunology solutions, the resilient spirit of patients fuels our urgency to deliver them. The infinity sign symbolizes our commitment to science and patients; it has no bounds. Our potential is infinite. Our purpose is immunology innovation.

Advance Extensive Pipeline We continue to demonstrate breadth and depth within our immunology pipeline and have advanced multiple pipeline-in-a-product candidates. With efgartigimod, we are furthering our leadership in neonatal Fc receptor (FcRn) and we are continuing its development in more than 10 indications today. Beyond efgartigimod, we are advancing our other clinical pipeline programs, including empasiprubart (C2 inhibitor) which has now shown proof-of-concept in MMN and initiated its first Phase 3 clinical trial, and is in Phase 2 POC clinical trials in delayed graft function (DGF) and DM. We also announced CIDP as the 4th indication for empasiprubart during our R&D Day on July 16, 2024 and plan to start a registrational study in CIDP evaluating empasiprubart head-to-head versus intravenous IgG (IVIg) in first half of 2025. In addition, we have initiated Phase 1b/2a clinical trials of ARGX-119, a MuSK agonist, in CMS congenital myasthenic syndromes and ALS. Four new pipeline candidates were nominated in 2024 from our immunology innovation program (IIP), including: ARGX-213, ARGX-121 and ARGX-220 and ARGX-109. Phase 1 results expected for ARGX-109 in second half of 2025 and for ARGX-213 and ARGX-121 in first half of 2026. argenx Annual Report 2024 2024 In Brief 13

Pioneer the FcRn Pathway with efgartigimod Neurology indications: • CIDP (ADHERE): following the positive topline results from the ADHERE clinical trial in CIDP, a supplemental biologics license application (sBLA) for efgartigimod SC was approved for the treatment of CIDP and launched in July 2024 in the U.S. We also received approval in Japan and China for the treatment of CIDP. • Myositis (ALKIVIA): In November 2024, we announced the GO decision to continue Phase 3 of the ALKIVIA clinical trial. ◦ The decision to continue clinical development of efgartigimod SC in each of the three myositis subtypes, including IMNM, ASyS and DM, is supported by the efficacy and safety results from the Phase 2 portion of the seamless Phase 2/3 ALKIVIA clinical trial. Overall, the clinical trial met its primary endpoint, demonstrating a statistically significant treatment effect in mean total improvement score (TIS) at week 24, and showed improvement across all six core set measures of the TIS in favor of efgartigimod SC compared to placebo. The observed safety and tolerability profile was consistent to that demonstrated with other clinical trials. ◦ Topline results expected 2H 2026. • TED (UplighTED): registrational clinical trials in thyroid eye disease (TED) ongoing with efgartigimod PFS. Topline results expected 2H 2026. • Seronegative gMG (ADAPT-SERON): registrational clinical trial in seronegative gMG patients ongoing with efgartigimod IV. Topline results expected 2H 2025. • Ocular MG (ADAPT-OCULUS): registrational clinical trial in ocular MG patients ongoing with efgartigimod PFS. Topline results expected 1H 2026. argenx Annual Report 2024 2024 In Brief 14 efgartigimod

Hematology/rheumatology indications: • ITP (ADVANCE-IV): positive clinical trial results formed the basis of approval in Japan for ITP, received on March 26, 2024. • ITP (ADVANCE-NXT): confirmatory clinical trial in ITP ongoing with efgartigimod IV in the U.S. Topline results expected in 2H 2026. • Primary SjD (RHO): following the analysis of topline data from the Phase 2 POC clinical trial through our partnership with IQVIA Ltd (IQVIA) in SjD we decided to continue the development of efgartigimod PFS to Phase 3 (UNITY), which was initiated at the end of 2024. Topline results expected in 2027. • Systemic Sclerosis (SSc): Phase 2 POC clinical trial ongoing. Topline results expected in 2H 2026. Nephrology indications: • Lupus Nephritis: Phase 2 POC clinical trial ongoing through our partnership with Zai Lab. Topline results expected in 2H 2025. • Antibody-mediated rejection: shAMRock Phase 2 POC clinical trial in antibody- mediated rejection (AMR) has been initiated. In 2024 we made the decision to stop development in PC-POTS (ALPHA), Bullous Pemphigoid (BALLAD) and membranous nephrology based on review of the Phase 2 data. argenx Annual Report 2024 2024 In Brief 15

Broaden Immunology Pipeline with empasiprubart and ARGX-119 empasiprubart (C2 inhibitor): • MMN (ARDA): based on the positive Phase 2 POC data of empasiprubart in MMN we have advanced empasiprubart into Phase 3 (EMPASSION). Topline results expected in 2H 2026. ◦ In January 2024, we reported positive clinical data from the first cohort of the Phase 2 POC ARDA clinical trial establishing POC in MMN. empasiprubart demonstrated a 91% reduction in the need for IVIg rescue compared to placebo [HR (95% CI) = 0.09 (0.2 : 0.44)]. ◦ In July 2024, we reported positive clinical data from the second cohort of the Phase 2 POC ARDA clinical trial confirming POC in MMN. empasiprubart demonstrated a 84% reduction in the need for IVIg rescue compared to placebo [HR: (95% CI) = 0.16 (0.02 : 1.54)]. ◦ Safety profile was consistent with Phase 1 data in both cohorts. ◦ We are also conducting a natural history study (IMMERSION) in MMN. • DGF (VARVARA) and DM (EMPACIFIC) Phase 2 POC clinical trials ongoing in DGF and DM. Topline results expected 2H 2025 and 1H 2026, respectively. • CIDP (EMVIGORATE): Phase 3 clinical trial in CIDP expected to start in 1H 2025. ARGX-119 (MuSK agonist): • Phase 1 dose-escalation clinical trial in healthy volunteers completed; data supports advancement in POC studies. • CMS: Phase 1b clinical trial started to assess early signal detection in patients with CMS. Topline results expected in 2H 2025. • ALS (reALiSe): Phase2a clinical trial started to assess early signal detection in patients with ALS. Topline results expected 1H 2026. argenx Annual Report 2024 2024 In Brief 16 empasiprubart ARGX-119

Build out the Innovation Ecosystem In January 2024, we announced the nomination of four new pipeline candidates, including: ARGX-213 targeting FcRn, furthering argenx’s leadership in this new class of medicine; ARGX-121 targeting Immunoglobulin A (IgA) and ARGX-220, which are first-in-class targets broadening argenx’s focus across the immune system; and ARGX-109, targeting IL-6, which plays an important role in inflammation. Preclinical work is ongoing in each candidate and the first healthy volunteer studies are expected to start in 2025. argenx Annual Report 2024 2024 In Brief 17

Corporate Achievements Dr. Brian Kotzin Dr. Brian Kotzin joined the Board of Directors in May 2024 as a non-executive director and chairperson of the research and development committee Mr. Peter Verhaeghe Mr. Peter Verhaeghe, who has served as a non- executive director since July 2014, was reappointed as a non-executive director and chairperson of the Board of Directors for a term of 2 years Dr. Pamela Klein Dr. Pamela Klein, who has served as a non- executive director since April 2016, was reappointed as a non-executive director for a term of 2 years 1,599 Expansion to 1,599 full-time employees (as of December 31, 2024) to support further growth of our business, including fully staffed commercial teams in the U.S., Europe, Japan and CanadaEmployees argenx Annual Report 2024 2024 In Brief 18

Financial Highlights $2.2 $1.0 billion billion Product net sales Research & development Transition to sustainable operating profitability in 2025 enables continued investment in innovation. argenx Annual Report 2024 2024 In Brief 19

argenx Annual Report 2024 This is the story of Nicola 20 The future belongs to those who dare to do more. Nicola “I think it's really important that people with MG are able to feel like they're still in control of their health.”

Living with an autoimmune disease can come with many unknowns. And trying to navigate the ups and downs may make you feel like you’re not in control of your own life. Nicola, who is living with myasthenia gravis (MG), says, “MG, as a disease, can take so much away from you. In terms of the symptoms themselves, it can feel like a loss of power in your own self in some ways.” But this didn’t sit well with Nicola. “Myasthenia gravis is such an unpredictable disease because everyone's symptoms are so different. On top of that, our symptoms will fluctuate day to day, week to week and sometimes even hour to hour.” She goes on to say, “Because of this, I think being able to have a sense of agency is so important, and you can have a say to help direct your treatment path.” Meet Nicola as she shares her journey with self-advocacy, why she uses the Myasthenia Gravis Activities of Daily Living (MG-ADL) scale and more. Being a self-advocate was something that Nicola herself had to practice. One of Nicola’s favorite instances of self-advocacy was when she was told that despite her diagnosis, she wasn’t the typical age to have MG. But because of the thorough research she did on her own, she knew that while MG can happen at any time, it commonly impacts young adult women under the age of 40. Looking back on that moment, Nicola thinks it may seem small, but it really wasn’t. Because she had been so hesitant to stand up for herself in the past and was frustrated by feeling that she lacked power in managing her MG, not only was it a win, but it was also a stepping stone for other opportunities in the future. She says, “I really like having agency over my healthcare and feeling like I'm in control.” What fuels her confidence with self-advocacy is her drive to keep learning. When she was first experiencing MG symptoms, she took it upon herself to read medical textbooks and join multiple MG support groups online. Not only were the groups good for emotional support, but she says the group members would also compare notes and share tips. She also has a “Myasthenia Gravis” alert set up on her internet browser so she can get notified whenever there’s new information and then talk about it with her healthcare provider. “I think it is so important to take a proactive approach to your health,” she says. Nicola’s advice on how to be your own best advocate? “Know the facts and the most current research about MG so you can properly articulate any questions or concerns.” She goes on to say, “It's important you have the confidence to stand firm when it comes to your experiences. And it’s especially helpful when you know that information to be true, whether that's through your own medical research or shared anecdotal evidence from other people living with MG.” But she acknowledges it’s not always easy and it’s perfectly OK to lean on your support system, “I always bring my boyfriend with me to appointments. It's so helpful to have someone else there to take notes and advocate for me, especially if I'm too short of breath to get out everything I need to say.” “I think it's really important that people with MG are able to feel like they're still in control of their health.” Nicola In step with her knowledge-is-power approach, Nicola embraces tools like the Myasthenia Gravis Activities of Daily Living scale (or MG-ADL). “It's so important for us to find resources that can help us be equipped with the best knowledge,” says Nicola. The MG-ADL scale is a tool that helps identify the impact MG has on a person’s daily life by providing an assessment of the severity of some common symptoms associated with MG. It’s made up of eight questions, six of which are about daily activities like breathing, brushing teeth and getting up out of a chair. argenx Annual Report 2024 This is the story of Nicola 21

The last two questions are eye-related (eyelid droop and double vision), because, as you may know, ocular symptoms are common in MG. To use the scale, you simply review each activity or item and give it a score from zero to three. A zero means you experienced normal function and a three means you experienced the greatest severity of symptoms. “I think the MG-ADL scale makes you stop and think about what’s really happening,” says Nicola. Sometimes there are symptoms Nicola has grown accustomed to, and she doesn’t recognize their impact on her day- to-day life until she sees the number written out on the scale. “Sitting and looking at the MG-ADL makes you realize either, ‘OK, I did have a few episodes this week and maybe I didn't notice it,’ or, ‘Hey, I haven't actually had a choking episode in a while!’ It’s really good to be able to see it in front of you.” Staying consistent with the scale may also be helpful when noticing changes. “I think the MG-ADL scale is really good for measuring your symptoms, so you can reflect back on where you were before, and compare it to where you are now,” reflects Nicola. I think all people living with MG could benefit from using the MG-ADL scale. Nicola’s self-advocacy and her MG-ADL score come together at her healthcare appointments. “I think the MG-ADL scale is a really good tool to start a conversation with your provider. You can write down the list of symptoms and discuss how they might be affecting you.” She adds, “I think it's a good starting point to be able to bring up certain issues with your physician and start that dialogue.” In fact, regularly tracking your symptoms may lead to more productive conversations with your healthcare team. It can also help you aim for improved daily abilities and, if possible, minimal or no symptoms (MSE). MSE means a person is experiencing minimal or almost no symptoms. “Minimal symptoms would be amazing,” Nicola says. “I couldn't think of anything better. I light up just thinking about it.” As a professional actor, dancer and singer, Nicola shares another aspiration: “I would also like to write, direct and act in a film about MG based on my experiences.” Her love of advocacy for herself and others comes with its own set of goals, too. “Some other goals of mine would be to improve MG awareness and ensure healthcare providers have access to critical information about MG patients in crisis.” “When living with an unpredictable disease,” she says, “Things are going to be…unpredictable!” Goal setting is important but so is giving yourself grace. “I think it's important to be patient with yourself when setting and achieving goals,” she advises. “Don't be discouraged when things don't happen the way you’d like or in the time that you planned, some things take longer than expected and that's OK!” argenx Annual Report 2024 This is the story of Nicola 22

2025 Outlook argenx Annual Report 2024 2025 Outlook 23

Presentation of the Group 1.1 Company Profile 25 1.2 Strategy and Objectives 29 1.3 Our Products and Product Candidates 31 1.4 Collaborations and licenses 49 1.5 Manufacturing and Supply 56 1.6 Intellectual Property 56 1.7 Regulation 59 1.8 Documents on display 80 argenx Annual Report 2024 24 1

1 Presentation of the Group 1.1 Company Profile 1.1.1 General We are a commercial-stage, global, fully-integrated biopharma company developing a deep pipeline of differentiated therapies for the treatment of severe autoimmune diseases. By combining our suite of antibody engineering technologies with the disease biology expertise of our research collaborators, we aim to translate immunology breakthroughs into a pipeline of novel antibody-based medicines through our discovery engine, the IIP. We developed and are commercializing the first approved FcRn blocker in more than 30 countries and we are evaluating efgartigimod in multiple serious autoimmune diseases. We are also advancing our second asset, empasiprubart, a C2 inhibitor, now in Phase 3. Several earlier stage experimental medicines, including ARGX-119, a MuSK agonist, are now in its first patient proof-of-concept studies. Our legal and commercial name is argenx SE. We were incorporated under the laws of the Netherlands on April 25, 2008, as a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid). From incorporation until August 28, 2009, our research and development activities were initially performed in the Netherlands, then Belgium, by argenx N.V. and its legal predecessors. Since August 28, 2009, all our research and development activities have been performed by our wholly-owned subsidiary, argenx BV, under a license provided by argenx N.V. Throughout this time, argenx BV assigned all resulting intellectual property to argenx N.V. On May 28, 2014, we converted to a Dutch public company with limited liability (naamloze vennootschap). On April 26, 2017, we converted to a Dutch European public company with limited liability (Societas Europaea or SE). On May 5, 2017, we transferred the legal ownership of all intellectual property rights of argenx SE to argenx BV, effective retroactively as of January 1, 2017. As a result, since January 1, 2017, (i) argenx BV holds all legal and economic ownership of our intellectual property rights, and (ii) the research and development agreement between argenx SE and argenx BV has been terminated. Our official seat is in Amsterdam, the Netherlands, and our registered office is at Laarderhoogtweg 25, 1101 EB Amsterdam, the Netherlands. We are registered with the trade register of the Dutch Chamber of Commerce under number 24435214. Our European legal entity identifier number (LEI) is 7245009C5FZE6G9ODQ71. Our telephone number is +31 (0) 10 70 38 441. Our website address is www.argenx.com. This website is not incorporated by reference in this Annual Report. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC at www.sec.gov. The registered agent for service of process in the U.S. is CT Corporation System, with an address at 111 8th Avenue, New York, NY 10011. Our ordinary shares are listed on the regulated market of Euronext Brussels in Belgium under ISIN NL0010832176 under the symbol “ARGX” since 2014 and ADSs, each representing one ordinary share in argenx (or a right to receive such share), are listed on the Nasdaq Global Select Market (Nasdaq) under the symbol “ARGX” since 2017. argenx SE is the parent entity of the Group and the sole shareholder of: • argenx B.V., a private company with limited liability (besloten vennootschap/société à responsabilité limitée) incorporated under the laws of Belgium, having its registered seat in Zwijnaarde, Belgium and its address at Industriepark-Zwijnaarde 7, 9052 Zwijnaarde, Belgium. argenx B.V. is the sole shareholder of: ◦ argenx US, Inc., incorporated under the laws of the state of Delaware, U.S., having its registered office in Wilmington, Delaware and its address at 33 Arch Street, Boston, Massachusetts 02110; ◦ argenx Japan KK., incorporated under the laws of Japan, having its registered office in Tokyo, Japan and its address at HULIC JP Akasaka Building 2-5-8, Akasaka, Minato-ku, Tokyo, 107-0052, Japan; argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Company Profile 25

◦ argenx Benelux B.V. (prior to October 31, 2022 known as argenx IIP BV), incorporated under the laws of Belgium, having its registered seat in Zwijnaarde, Belgium and its address at Industriepark- Zwijnaarde 7, 9052 Zwijnaarde, Belgium; ◦ argenx Switzerland, S.A., incorporated under the laws of Switzerland, having its registered office in Geneva, Switzerland, and its address at Rue du Pré-de-la-Bichette 1, 1202 Geneva, Switzerland; ◦ argenx France SAS, incorporated under the laws of France, having its registered office in Paris, France, and its address at Rue Camille Desmoulins 13, 92130 Issy-Les-Moulineaux, France; ◦ argenx UK Ltd., incorporated under the laws of the UK, having its registered office in Gerrards Cross, UK, and its address at Spaces Gerrards Cross Chalfont Park, Building 1 Gerrards Cross, SL9 0BG, UK; ◦ argenx Netherlands Services B.V., incorporated under the laws of the Netherlands, having its registered office in Laarderhoogteweg 25, 1101 EB Amsterdam, the Netherlands; ◦ argenx Germany GmbH, incorporated under the laws of Germany, having its registered office in Munich, Germany, and its address at Konrad-Zuse-Platz 8, 81829 Munich, Germany; ◦ argenx Canada Inc., incorporated under the laws of Ontario, having its registered office in Ontario, Canada and its address at 9131 Keele Street Suite A4, Vaughan, Ontario, Canada, L4K 0G7; ◦ argenx Italy S.r.l., incorporated under the laws of Italy, having its registered office in Milan, Italy and its address at Largo Francesco Richini 6 CAP, 20122 Milan, Italy; ◦ argenx Spain S.L., incorporated under the laws of Spain, having its registered office in Madrid, Spain and its address at Paseo dela Castellana 200, Planta 8a, Oficina 819, 28046 Madrid, Spain, with the branch office: argenx Spain S.L. - Sucursal em Portugal, organized under the laws of Portugal, having its registered office and address at Palácio Sottomayor, Rua Sousa Martins, nº1, 1º esquerdo 1050 217, Lisboa Portugal; ◦ argenx Australia Pty. Ltd., incorporated under the laws of Australia, having its registered office and address at Level 14, 2 Riverside Quay, Melbourne VIC 3006, Australia (since January 12, 2024); and, ◦ argenx Austria Services GmbH, incorporated under the laws of Austria, having its registered office and address at Graben 19, 4th & 5th floor Vienna A-1010 Austria. The following chart provides an overview of the Group as of the date of this Annual Report. Percentages refer to both the share of capital and voting rights. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Company Profile 26

argenx Corporate Legal Structure 1.1.2 Overview Our Medicines VYVGART is a first-in-class antibody fragment targeting FcRn. VYVGART is the only gMG treatment available as both an IV and a simple SC injection (VYVGART SC). VYVGART is approved in more than 30 countries and VYVGART SC is now approved in the U.S., the EU, the UK, Japan, Israel, China and in Australia for the treatment of gMG. VYVGART SC is now also approved in the U.S., China and Japan (as VYVDURA) for the treatment of CIDP. VYVDURA is also approved for the treatment of ITP in Japan. Our Pipeline • efgartigimod is an IgG1 antibody Fc fragment that has been engineered for increased affinity to FcRn compared to endogenous IgG. efgartigimod selectively reduces IgG by blocking FcRn-mediated IgG recycling without impacting antibody production, or affecting other parts of the immune system. It is approved in three indications, including gMG, CIDP and ITP, and is being evaluated in more than 10 additional serious autoimmune indications. • empasiprubart (C2 inhibitor): empasiprubart is a novel complement inhibitor targeting C2, blocking the function of both the classical and lectin pathways while leaving the alternative pathway intact. We believe empasiprubart has the potential to be a pipeline-in-a-product candidate and is being evaluated in 4 serious autoimmune diseases, of which 2 indications are in Phase 3. • ARGX-119 (MusK agonist): ARGX-119 is an agonist SIMPLE ANTIBODY™ to the MuSK receptor with potential in multiple neuromuscular indications. It is currently in proof-of-concept studies for CMS (Phase 1b clinical trial), ALS (Phase 2a) and will start in SMA in 2025. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Company Profile 27

• Preclinical Candidates: Four INDs to start Phase 1 studies in 2025: ◦ ARGX-213, targets FcRn, furthering argenx’s leadership in this new class of medicine ◦ ARGX-121 targeting IgA and ARGX-220 both broadening argenx’s focus across the immune system ◦ ARGX-109, targets IL-6, which plays an important role in inflammation • In addition to our wholly-owned pipeline, we have candidates that emerged from our IIP that we out- licensed to a partner for further development and for which we have milestone, royalty or profit-share agreements. These candidates include, amongst others: cusatuzumab (anti-CD70 antibody – OncoVerity), ARGX-112 (LP-0145 – anti-IL-22R antibody – LEO Pharma), ARGX-114 (AGMB-101 – agonistic anti-MET antibody – Agomab) and ARGX-115 (ABBV-151 – anti-GARP antibody – AbbVie). Immunology Innovation Program (IIP) Our IIP is central to our core business strategy of co-creation and innovation. The IIP also serves as our discovery engine to identify novel targets and together, in collaboration with our scientific and academic partners, to build potential new pipeline candidates. Every current pipeline candidate from both our wholly- owned and partnered pipeline emerged from an IIP collaboration. The IIP enables us to build our broad pipeline of products and product candidates and advance our long-term strategy to be a sustainable, integrated immunology company. Examples of our IIP programs include: • efgartigimod emerged from a collaboration with Professor Sally Ward at the University of Texas Southwestern Medical Center and later became one of the blueprints for our IIP collaborations. Professor Ward’s research identified the crucial role that FcRn plays in maintaining and distributing IgGs throughout the body. efgartigimod is a human IgG1 Fc fragment that is equipped with ABDEG™ mutations, which we in-licensed from the University of Texas Southwestern Medical Center. These proprietary mutations modified efgartigimod to increase its affinity for FcRn while retaining the pH- dependent binding that is characteristic of FcRn interactions with its natural ligand, endogenous IgG. • empasiprubart was built in collaboration with Broteio Pharma B.V. (Broteio). Broteio was launched in 2017 with support from Professor Erik Hack and the University of Utrecht, to conduct research demonstrating preclinical POC of the mechanism of action of empasiprubart. Professor Hack is a renowned researcher in the role of inflammation in disease, specifically in the complement system, and has contributed research and expertise to the approval of two complement inhibitors. His understanding of the mild phenotype associated with a natural C2 deficiency and C2’s unique positioning at the junction of the classical and lectin pathways led to our interest in engineering empasiprubart with our proprietary NHANCE™ mutations and LALA mutations. • ARGX-119 was built in collaboration with the Leiden University Medical Center (LUMC) and New York University (NYU) with support from teams led by Professor Verschuuren and Professor Steve Burden, respectively. Both groups have world-class expertise in unraveling the biological mechanism of neuromuscular disease and translating these insights from the lab to the patient. We bring to the collaboration our unique suite of antibody discovery and antibody engineering technologies and experience in clinical development to complement our partners’ expertise in disease and target biology. Our suite of technologies include amongst others our SIMPLE ANTIBODY™ platform technology and NHANCE™, ABDEG™, POTELLIGENT®, and DHS mutations that focus on engineering the Fc region of antibodies in order to augment their intrinsic therapeutic properties. For more information, please see Section “1.3.7 IIP”. Our Suite of Technologies • Through our IIP, we collaborate with scientific and academic partners to identify immunology breakthroughs and build potential pipeline candidates. This is done through co-creation. We bring to the collaboration our unique suite of antibody engineering technologies and experience in clinical development to complement our partners’ expertise in disease and target biology. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Company Profile 28

• SIMPLE ANTIBODY™ platform technology: Our proprietary SIMPLE ANTIBODY™ platform technology, based on the powerful llama immune system, allows us to exploit novel and complex disease biology targets. The platform sources antibody variable regions (V-regions) from the immune system of outbred llamas, each of which has a different genetic background. The llama produces highly diverse panels of antibodies with a high human homology, or similarity, in their V-regions when immunized with targets of human disease. Our SIMPLE ANTIBODY™ platform technology allows us to access and explore a broad target universe while potentially minimizing the long timelines associated with generating antibody candidates using traditional methods. • NHANCE™, ABDEG™, POTELLIGENT®, and DHS mutations focus on engineering the Fc region of antibodies in order to augment their intrinsic therapeutic properties. In addition, we obtained a non- exclusive research license and option from Chugai Pharmaceutical Co., Ltd. (Chugai) for the SMART-Ig® (‘Recycling Antibody’ and part of ‘Sweeping Antibody’) and ACT-Ig® (Antibody half-life extending) technologies. These technologies are designed to enable us to expand the therapeutic index of our product candidates, which is the ratio between toxic and therapeutic dose, by potentially modifying their half-life, tissue penetration, rate of disease target clearance and potency. In 2020, we also entered into a non-exclusive research agreement with the Clayton Foundation under which we may access the Clayton Foundation’s proprietary DHS mutations to extend the serum half-life of therapeutic antibodies. 1.2 Strategy and Objectives 1.2.1 Company’s Strategies Our goal is to transform the lives of at least 50,000 patients and their communities before 2030 by providing them with life-changing medicines built on scientific breakthroughs in immunology. To reach this goal, we plan to deliver a set of different strategies: • Maximize the VYVGART® opportunity: redefine treatment expectations for MG and CIDP, and deliver 6 additional labelled indications We plan to do this through our differentiated scientific and clinical activities and our commercial execution to drive VYVGART preference. Our PFS with PDUFA Date in April 2025 as a perfect example of our continued innovation for the patient experience. Beyond neurology, we plan to establish argenx and VYVGART in rheumatology as we prepare for data in Myositis and SjD, while also maximizing the therapeutic potential of VYVGART in other indications through the execution of multiple Phase 2 and Phase 3 studies. • Maximize the empasiprubart opportunity: establish its potential as a pipeline-in-a-product We plan to develop argenx as scientific leader in complement inhibitions and elevate the differentiation story. In particular, we have advanced the clinical development of empasiprubart in MMN, currently in Phase 3, DGF in the context of kidney transplants and DM, currently in Phase 2, and expect to start a registrational clinical trial for our fourth selected indication, CIDP in 2025. For both MMN and CIDP we will prepare for launches, building on key elements of the VYVGART playbook. • Build a sustainable, diversified portfolio of breakthrough and differentiated antibody-based products We plan to further advance ARGX-119 to a differentiated first-in-class MuSK agonist in multiple indications (CMS, ALS, SMA, 1 new indication), maximize our leadership position in the FcRn space through multiple generations of projects (e.g. ARGX-213), substantially grow our clinical portfolio of differentiated pipeline-in-a-product opportunities (ARGX-109, ARGX-121, ARGX-220, ARGX-213, other), create an exciting portfolio of promising new assets (through our IIP) and advance our clinical trial designs and speed. • Grow a unique, global biotech company by scaling the argenx Way: one company, one plan, on a mission to achieve the unthinkable We plan to embed the argenx Way throughout the organization, who we are through our cultural pillars and how we work through our operating principles. We want to demystify innovation and make it everyone’s business, strengthen the ‘winning’ competencies to share the future of argenx and advance our partnership approach to access. To be able to continue in this way, we plan to remain a magnet for talent and create unlimited opportunities for growth and development of our people, an important driver of developing the business. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Company Profile 29

• Ensure long-term sustainability We plan to continue to seek out, listen to and prioritize on behalf of the patient in all what we do, accelerate the science of immunology by being an active and trusted partner in the global immunology ecosystem through high-quality publications and patent applications, elevate and expand our relationships with regulatory, payors and policy stakeholders and create long- term shareholder value. • In our 2030 vision, we aim to build on our strong strategic pillars to have a continuous pipeline of innovation, strengthen our FcRn leadership and scale in a disciplined way. Our goal is to have 5 new molecules in Phase 3 development, 10 labelled indications and reaching 50,000 patients who are on treatment by 2030. 1.2.2 Competitive position We participate in a highly innovative industry characterized by a rapidly growing understanding of disease biology, quickly changing technologies, strong intellectual property barriers to entry, and a multitude of companies involved in the creation, development and commercialization of novel therapeutics. Many of these companies are highly sophisticated and often strategically collaborate with each other. Competition in the autoimmune field is intense and involves multiple monoclonal antibodies (mAbs), other biologics and small molecules either already marketed or in development by many different companies, including large pharmaceutical companies. We compete with a wide range of biopharmaceutical companies, who are developing products for the treatment of gMG, CIDP, ITP and other autoimmune diseases, including products that are in the same class as VYVGART, as well as products that are similar to some of our product candidates. We are aware of several FcRn inhibitors that are in clinical development or marketed. Competitive product launches may erode future sales of our products, including our existing products and those currently under development, or result in unanticipated product obsolescence. Such launches continue to occur, and potentially competitive products are in various stages of development. We could also face competition for use of limited international infusion sites, particularly in new markets as competitors launch new products. We cannot predict with accuracy the timing or impact of the introduction of competitive products that treat diseases and conditions like those treated by our products or product candidates. In addition, our competitors compete with us to recruit and retain qualified scientific and management personnel, establish clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, the development of our products. Please see the risk factor titled ”We face significant competition for our drug discovery and development efforts. for further details on the competition we face. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Strategy and Objectives 30
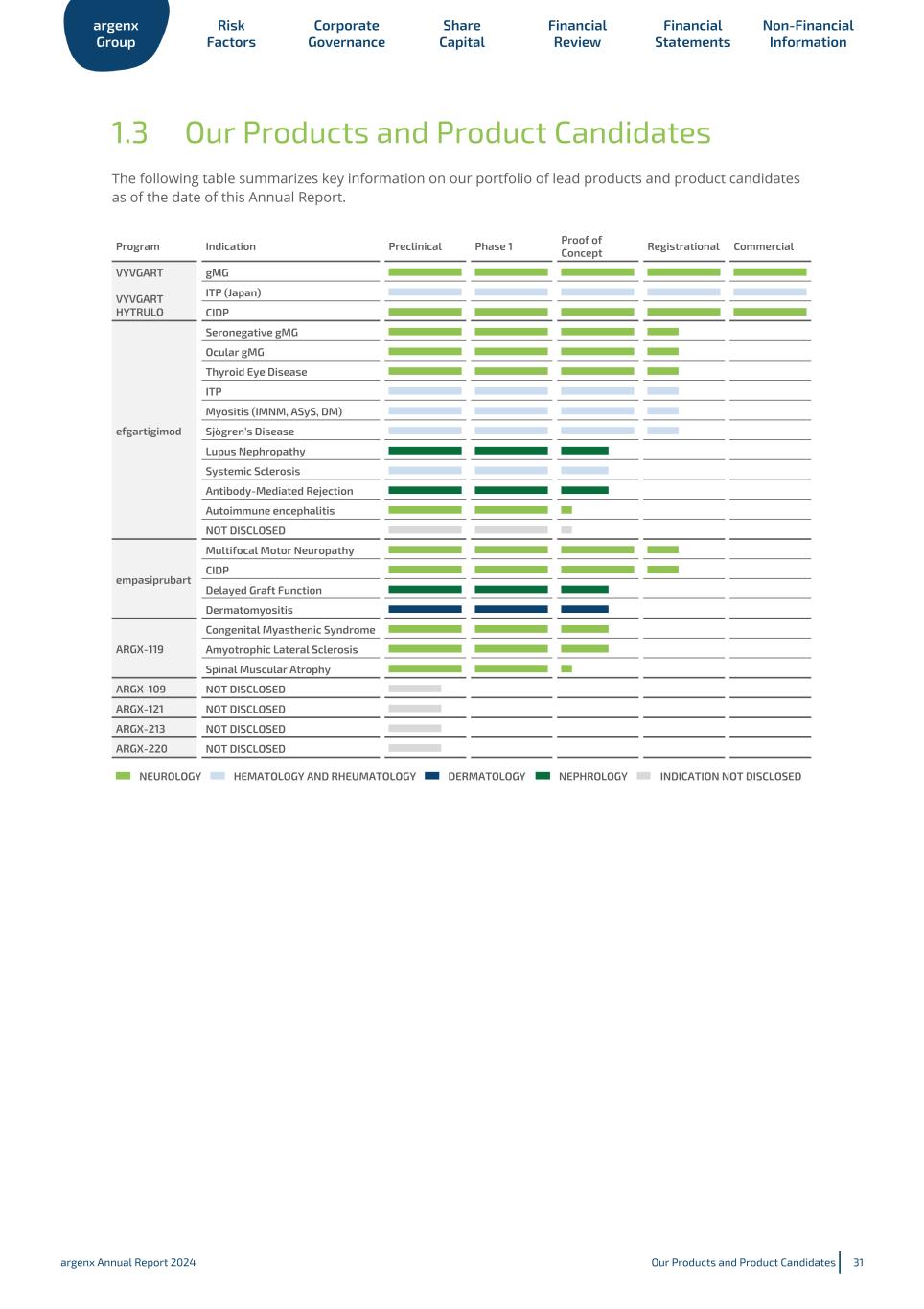
1.3 Our Products and Product Candidates The following table summarizes key information on our portfolio of lead products and product candidates as of the date of this Annual Report. Program Indication Preclinical Phase 1 Proof of Concept Registrational Commercial VYVGART VYVGART HYTRULO gMG ITP (Japan) CIDP efgartigimod Seronegative gMG Ocular gMG Thyroid Eye Disease ITP Myositis (IMNM, ASyS, DM) Sjögren’s Disease Lupus Nephropathy Systemic Sclerosis Antibody-Mediated Rejection Autoimmune encephalitis NOT DISCLOSED empasiprubart Multifocal Motor Neuropathy CIDP Delayed Graft Function Dermatomyositis ARGX-119 Congenital Myasthenic Syndrome Amyotrophic Lateral Sclerosis Spinal Muscular Atrophy ARGX-109 NOT DISCLOSED ARGX-121 NOT DISCLOSED ARGX-213 NOT DISCLOSED ARGX-220 NOT DISCLOSED NEUROLOGY HEMATOLOGY AND RHEUMATOLOGY DERMATOLOGY NEPHROLOGY INDICATION NOT DISCLOSED argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 31

1.3.1 VYVGART Approvals and Regulatory Plan Our two approved medicines, VYVGART and VYVGART SC, are FcRn blockers. VYVGART is approved in more than 30 countries for the treatment of adults with gMG who are anti-acetylcholine receptor (AChR) antibody positive (AChR-AB+) and for the treatment of adults with gMG who do not have sufficient response to steroids or non-steroidal immunosuppressive therapies (ISTs), including seronegative patients, in Japan. VYVGART is now also approved for the treatment of adult patients with ITP in Japan. Our second product, VYVGART SC, is a subcutaneous combination of efgartigimod alfa and recombinant human hyaluronidase PH20 (rHuPH20), Halozyme Therapeutics, Inc.’s (Halozyme) ENHANZE® SC drug delivery technology. It has been approved for the treatment of adults with gMG who are AChR-AB+ as VYVGART HYTRULO in the U.S. and China, as VYVGART in the EU, and as VYVGART SC in the UK, Israel. It has also been approved as VYVDURA in Japan for the treatment of adults with gMG who do not have sufficient response to steroids or non-steroidal ISTs, including seronegative patients. VYVGART SC has now also been approved for the treatment of adults with CIDP in the U.S. and China as VYVGART HYTRULO and in Japan as VYVDURA. More approvals and launches of both VYVGART and VYVGART SC in multiple jurisdictions and countries are planned following pricing and reimbursement negotiations. The following table summarizes the status of regulatory approvals and commercialization efforts for VYVGART IV, VYVGART SC and PFS as March 17, 2025: Product Product name Indication Geography Submission Approval Launched VYVGART IV VYVGART gMG US December 17, 2021 December 17, 2021 VYVGART gMG Europe August 10, 2022 Germany was the first European country to launch on September 1, 2022 VYVGART gMG Canada September 19, 2023 November 6, 2023 VYVGART gMG Israel April 24, 2023 Not marketed VYVGART gMG Japan January 20, 2022 May 9, 2022 VYVGART gMG The UK March 14, 2023 Not marketed VYVGART gMG China June 30, 2023 September 5, 2023 VYVGART gMG Australia February 24, 2025 Not available VYVGART gMG Kuwait February 19, 2025 Not available VYVGART gMG Saudi Arabia Submitted Not available Not available VYVGART gMG Korea (the Republic of) January 20, 2025 Not available VYVGART gMG United Arab Emirates October 30, 2024 Not marketed VYVGART gMG Switzerland October 3, 2024 Not marketed VYVGART ITP Japan March 26, 2024 On the market since launch of IV product Not available gMG Brazil Planned submission 2H 2025 Not available Not available Not available gMG Singapore Not available Not available Not available argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 32

VYVGART SC VYVGART HYTRULO gMG US June 20, 2023 June 20, 2023 VYVGART HYTRULO CIDP US June 21, 2024 On the market since launch of SC product VYVGART gMG Australia February 24, 2025 Not available VYVGART gMG Europe November 15, 2023 Germany was the first European country to launch on December 15, 2023 VYVGART CIDP Europe Submitted Expected in 2H Not available Not available gMG Switzerland February 10, 2025 Not available VYVGART gMG The UK February 6, 2024 Not marketed VYVGART SC gMG Israel September 23, 2024 Not marketed VYVGART HYTRULO gMG China July 9, 2024 December 3, 2024 VYVGART HYTRULO CIDP China November 5, 2024 On the market since launch of SC product VYVDURA gMG Japan January 18, 2024 April 17, 2024 VYVDURA CIDP Japan December 27, 2024 On the market since launch of SC product PFS VYVDURA gMG Japan Submitted Expected in 2H 2025 Not available VYVDURA CIDP Japan Submitted Expected in 2H 2025 Not available Not available gMG U.S. Submitted Expected in 1H 2025 Not available Not available CIDP U.S. Submitted Expected in 1H 2025 Not available VYVGART gMG Europe February 13, 2025 February 13, 2025 VYVGART CIDP Europe Submitted Expected in 1H 2025 Not available Not available gMG Canada Submitted Expected in 2H 2025 Not available Not available CIDP Canada Submitted Expected in 2H 2025 Not available Not available gMG The UK Submitted Not available Not available Commercialization We have established our own sales force in the U.S., Japan, Europe and Canada for VYVGART for the treatment of gMG and CIDP (where approved). We plan to expand our own sales and marketing capabilities and promote our products and product candidates in other regions if we decide there is a business case to do so after regulatory approval has been obtained. Development and commercialization may also be done through collaborations with third parties. In January 2021, we entered into an exclusive out-license agreement with Zai Lab (Zai Lab Agreement), a commercial- stage biopharmaceutical company, for the development and commercialization of efgartigimod in Greater China, (which includes Mainland China, Hong Kong, Taiwan and Macau, Greater China). Zai Lab announced approval of VYVGART in Mainland China in June 2023 for the treatment of adult gMG patients and in 2024 Zai Lab also announced the approval of VYVGART SC for gMG and CIDP. Under the Zai Lab Agreement, we received and continue to be eligible for certain sales-based milestone payments and royalties based on annual product net sales of efgartigimod in Greater China. In October 2021, we announced an exclusive distribution agreement with Medison to commercialize efgartigimod for gMG in Israel (Medison Agreement). Medison filed for and obtained approval for VYVGART in April 2023 and for VYVGART SC in September 2024. On June 6, 2022 we announced an exclusive multi- regional agreement with Medison to commercialize efgartigimod in 14 countries, including Poland, Hungary, Slovenia, Czech Republic, Romania, Bulgaria, Lithuania, Croatia, Slovakia, Estonia, Latvia, Greece, and Cyprus, for the treatment of adult patients with gMG (Medison Multi-Regional Agreement). argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 33

In January 2022, we entered into a partnership agreement with Genpharm, under which Genpharm shall purchase VYVGART from us for the resale in the Gulf Cooperation Council (comprising Saudi Arabia, Kuwait, the United Arab Emirates, Qatar, Bahrain and Oman) on an exclusive basis for Genpharm’s own account and own name (Genpharm Agreement). In 2023, we entered into the Handok Agreement for the distribution of VYVGART in South Korea and in 2024 we received approval for VYVGART in South Korea. We intend to sign additional distribution partnerships for other territories. In the U.S., argenx advertises certain products via digital and traditional media channels, including the internet and television. For a discussion of total revenues by geographic market, please see “Note 17 Segment Reporting” in our consolidated financial statements. Pre-Approval Access Program We are committed to improving the lives of people suffering from rare diseases. We are driven to discover new treatment approaches fueled by the resilience of patients to urgently deliver them. We aim to do this in partnership; we listen to patients, supporters and advocacy communities, and we hear their stories. Their insights guide us as we develop our investigational therapies and motivate us to advance the understanding of rare diseases. We have a Pre-Approval Access program (PAA) for patients with gMG which opened on February 21, 2021 for patients who are unable to participate in an ongoing clinical trial. In 2024, we approved access to this PAA for over 403 gMG patients in 14 countries. The PAA program remains open in countries where VYVGART is not yet launched or reimbursed. 1.3.2 efgartigimod (formerly ARGX-113) Development Mechanism of Action As shown in Figure 1, efgartigimod is a human IgG1 Fc fragment equipped with our ABDEG™ mutations that is designed to target the FcRn and reduce IgG. FcRn is foundational to the immune system and functions to recycle IgG, extending its serum half-life over other IgGs that are not recycled by FcRn. IgGs that bind to FcRn are rescued from lysosomal degradation. By binding to FcRn, efgartigimod can reduce IgG recycling and increase IgG degradation. Compared to alternative immunosuppressive approaches, such as B-lymphocyte (B-cell) depleting agents, efgartigimod acts in a highly selective manner. For efgartigimod, we now have an estimated 8,000 patients years of safety follow-up between clinical trials and real world experience. efgartigimod has been observed to significantly reduce concentrations of all IgG subtypes without decreasing levels of other IgGs or human serum albumin, which is also recycled by FcRn, discussed in more detail in the paragraph of this section on formulations below. Based on its mechanism of action in targeting FcRn to selectively reducing IgGs, efgartigimod has the potential to address a multitude of severe autoimmune diseases where pathogenic IgGs are believed to be mediators of disease. As of the end of 2024, we are evaluating efgartigimod in more than 10 serious autoimmune indications and plan to continue to expand into new indications. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 34

Figure 1: efgartigimod’s mechanism of action blocks the recycling of IgG antibodies and removes them from circulation Indication Selection Strategy We utilize the following strategy to select indications for efgartigimod: • We first start with a strong, unifying biological rationale. The indications in our pipeline are unified in that there exists a wide range of supportive evidence that demonstrates that each is IgG-mediated. This ranges from published literature, clinical trials with currently used therapies such as IVIg, PLEX, or rituximab, and other experiments, such as passive transfer models. • We also look at indications where a significant clinical or commercial opportunity exists. These are disease areas where there is a significant unmet need for innovation as patients are often not well- managed by current therapies and their respective side effects. • Furthermore, for each indication, there is a defined path forward with established precedent for how to run POC and registrational clinical trials with generally accepted clinical and regulatory endpoints. Formulations Overview We are developing two formulations of efgartigimod to address the needs of patients, physicians, and payers across indications and geographies, including efgartigimod IV (VYVGART) and efgartigimod SC (VYVGART SC). Scientific Publications We refer to our key scientific publications from our Phase 3 studies with either the IV or SC formulation in gMG, ITP and CIDP. • Publication in The Lancet Neurology of Phase 3 ADAPT study data in generalized myasthenia gravis: thelancet.com/journals/laneur/article/PIIS1474-4422(21)00159-9/abstract argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 35

• Publication in The Lancet of Phase 3 ADVANCE-IV study data in primary immune thrombocytopenia: thelancet.com/journals/lancet/article/PIIS0140-6736(23)01460-5/abstract • Publication in The Lancet Neurology of Phase 3 ADHERE study data in chronic inflammatory demyelinating polyneuropathy: thelancet.com/journals/laneur/article/PIIS1474-4422(24)00309-0/ abstract 1.3.3 efgartigimod Indications Clinical trial overview Clinical Trial Stage Indica tion Patients Primary Endpoint Status ADAPT Registrational gMG The proportion of responders based on the Myasthenia Gravis Activities of Daily Living (MG-ADL) score Marketed ADAPT-SC Registrational gMG The proportion of responders based on the Myasthenia Gravis Activities of Daily Living (MG-ADL) score Marketed ADAPT- SERON Registrational seronega tive gMG 110 MG-ADL total score change from baseline to day 29 (w4) Ongoing clinical trial results expected 2H 2025 ADAPT- OCULUS Registrational ocular MG 92-124 Change in MGII PRO ocular score from baseline to day 29 (w4) Ongoing clinical trial results expected 1H 2026 ADHERE Registrational CIDP 322 The hazard ratio for the time to first adjusted INCAT deterioration Marketed ADVANCE-IV Registrational ITP The proportion of patients that achieved sustained platelet response Marketed ADVANCE- NXT Registrational ITP 63 Extent of disease control (cumulative number of weeks over the planned 24- week treatment period with platelet counts of ≥ 50×109/L Ongoing clinical trial results expected in 2H 2026 BALLAD Registrational BP 98 The proportion of participants in complete remission while off oral corticosteroids for at least eight weeks at week 36 Clinical trial discontinued in 2024 ALKIVIA Registrational Myositis Target 240 The total improvement score (TIS) at the end of treatment period Ongoing clinical trial results expected in 2H 2026 RHO PoC Primary SjD Target 30 The proportion of responders to the Composite of Relevant endpoints for SjD (CRESS; response on ≥ three out of five items) at week 24 GO decision made in 2024 advanced in Phase 3 UNITY Registrational Primary SjD Target 580 The change from baseline on the ClinESSDAI score (w48) Ongoing clinical trial results expected in 2027 ALPHA PoC POTS post- COVID19 53 The co-primary endpoints are 1) COMPASS-31 and 2) the Malmö POTS Symptom score at the end of the 24- week treatment period Clinical trial discontinued in 2024 In partnership with Zai Lab PoC LN Target 60 The change in urine protein creatinine ratio from baseline to end of the treatment period Ongoing clinical trial results expected in 2H 2025 In partnership with Zai Lab PoC MN Target 70 The change in urine protein creatinine ratio from baseline to end of the treatment period in the anti-PLA2R Ab seropositive population Clinical trial discontinued in 2024 argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 36

uplighTED Registrational TED Target 108/trial Percentage of participants who were proptosis responders at week 24 Ongoing clinical trial results expected in 2H 2026 shAMRock PoC AMR Target 30 Safety and tolerability. Efficacy measures such as estimated glomerular filtration rate, histology and urine protein creatinine ratio are captured in the secondary endpoints Clinical trial has been initiated in 2024 ADAPT- JUNIOR IV Phase 2/3 gMG Target over 12 To confirm an age-adjusted optimum dose of efgartigimod IV and provide (model-predicted) evidence for a treatment response Ongoing clinical trial ADAPT- JUNIOR SC Phase 2/3 gMG Target over 12 To confirm an appropriate dose of efgartigimod PH20 SC in pediatric participants with gMG Ongoing clinical trial Other clinical trials PoC AAV Clinical trial discontinued in 2024 PoC SSc To be con firmed To be confirmed Ongoing clinical trial results expected in 2H 2026 gMG Overview gMG is a rare and chronic autoimmune disease where IgG autoantibodies disrupt communication between nerves and muscles, causing debilitating and potentially life-threatening muscle weakness. In myasthenia gravis (MG), IgG autoantibodies either bind and occupy or cross-link and internalize the receptor on the muscle cells, thereby preventing the binding of acetylcholine, the signal sent by the nerve cell. In addition, these autoantibodies can cause destruction of the neuromuscular junction by recruiting complement, a potent cell-destroying mechanism of the human immune system. The muscle weakness associated with MG usually presents initially in ocular muscles and can then spread into a generalized form affecting multiple muscles, known as gMG. Approximately 85% of people with MG progress to gMG within 24 months (source: Behin et al. New Pathways and Therapeutics Targets in Autoimmune Myasthenia Gravis. J Neuromusc Dis 5. 2018. 265-277). MG in the ocular form initially causes droopy eyelids and blurred or double vision due to partial paralysis of eye movements. As MG becomes generalized it affects muscles in the neck and jaw, causing problems in speaking, chewing and swallowing. MG can also cause weakness in skeletal muscles leading to problems in limb function. In the most severe cases, respiratory function can be weakened to the point where it becomes life-threatening. These respiratory crises occur at least once in the lives of approximately 15% to 20% of MG patients. The U.S. prevalence of MG is estimated at approximately 20 cases per 100,000 (source: Philips et al, Ann NY Acad Sci. 2003). Patients with confirmed AChR antibodies account for approximately 85% of the total gMG population (Behin et al. New Pathways and Therapeutics Targets in Autoimmune Myasthenia Gravis. J Neuromusc Dis 5. 2018. 265-277). In May 2020, we announced positive topline results from the pivotal ADAPT clinical trial of efgartigimod for the treatment of gMG. The topline results from the ADAPT clinical trial showed that efgartigimod was well- tolerated, demonstrated clinically meaningful improvements in strength and quality of life measures, and provided the option of an individualized dosing schedule for gMG patients. The full Phase 3 ADAPT results were published in The Lancet Neurology in July 2021. The data from the ADAPT clinical trial and the subsequent OLE (ADAPT+) formed the basis for the regulatory approvals of VYVGART in the U.S., Japan, the EU, Mainland China, Israel, the UK and Canada. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 37

On March 22, 2022, we announced positive topline results from the Phase 3 ADAPT-SC clinical trial, a registrational non-inferiority bridging clinical trial of efgartigimod SC for the treatment of gMG. efgartigimod SC achieved the primary endpoint of total IgG reduction from baseline at day 29, demonstrating statistical noninferiority to VYVGART IV formulation in gMG patients. Based on these results, we received regulatory approval in the U.S., the EU, China, Japan, Switzerland, the UK, Israel and Australia. Other clinical trials In 2024, we initiated registrational clinical trials to expand the VYVGART label into broader MG populations, including in seronegative gMG patients (ADAPT-SERON) and ocular MG patients (ADAPT-OCULUS). We also have clinical trials ongoing in pediatric gMG patients (ADAPT-JUNIOR) with efgartigimod IV and efgartigimod SC. CIDP Overview CIDP is a chronic autoimmune disorder of peripheral nerves and nerve roots caused by an autoimmune- mediated destruction of the myelin sheath, or myelin producing cells, insulating the axon of the nerves and enabling speed of signal transduction. The cause of CIDP is unknown, but abnormalities in both cellular and humoral immunity have been shown. CIDP is a chronic and progressive disease: onset and progression occur over at least eight weeks in contrast with the more acute Guillain-Barré-syndrome. Demyelination and axonal damage in CIDP lead to loss of sensory and/or motor neuron function, which can lead to weakness, sensory loss, imbalance and/or pain. U.S. prevalence for CIDP patients is 42,000 of whom 24,000 are treated patients. Most CIDP patients require treatment, the majority currently with IVIg. Glucocorticoids and plasma exchange are used to a lesser extent as they are either limited by side effects upon chronic use, in the case of glucocorticoids, or invasiveness of the procedure and access, which is restricted to specialized centers in case of plasma exchange. Alternative immunosuppressant agents are typically reserved for patients ineligible for or refractory to IVIg, glucocorticoids or plasma exchange. In July 2023, we announced positive topline results from the ADHERE clinical trial evaluating VYVGART SC (efgartigimod alfa and hyaluronidase-qvfc) in adults with CIDP. The clinical trial met its primary endpoint (p=0.000039), demonstrating a significantly lower risk of relapse with VYVGART SC compared to placebo (HR: 0.39 95% CI: 0.25; 0.61). 67% of patients in open-label Stage A demonstrated evidence of clinical improvement, indicating that IgG autoantibodies play a significant role in the underlying biology of CIDP. VYVGART SC was well-tolerated with a safety profile that is consistent with prior clinical trials and the known profile of VYVGART. The most frequent treatment-related adverse event was ISRs, which occurred in a lower percentage of patients than previous VYVGART SC clinical trials (20% in Stage A; 10% in Stage B). All ISRs were mild to moderate and resolved over time. 99% (226/249) of eligible patients continued to the ADHERE- Plus OLE clinical trial. Based on these results, we received regulatory approval in the U.S. in June 2024, in China in November 2024 and in Japan in December 2024. Regulatory review is currently ongoing in other jurisdictions including in the EU. Primary ITP Overview Primary ITP is an acquired autoimmune bleeding disorder, characterized by a low platelet count (<100×109/ L) in the absence of other causes associated with thrombocytopenia. In most patients, IgG autoantibodies directed against platelet receptors can be detected. They accelerate platelet clearance and destruction, inhibit platelet production, and impair platelet function, resulting in increased risk of bleeding and impaired quality of life. Primary ITP is differentiated from secondary ITP, which is associated with other illnesses, such as infections or autoimmune diseases, or which occurs after transfusion or taking other drugs, such as cancer drugs. Platelet deficiency, or thrombocytopenia, can cause bleeding in tissues, bruising and slow blood clotting after injury. Patients may suffer from depression and fatigue as well as side effects of existing therapies, impairing their quality of life. Current therapeutic approaches include non-specific immunosuppression (e.g., steroids and rituximab), inhibition of platelet clearance (e.g., splenectomy, IVIg, argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 38

anti-D globulin, and spleen tyrosine kinase inhibitor fostamatinib13) or stimulation of platelet production (e.g., thrombopoietin receptor agonist TPO-RA). Splenectomy remains the only treatment that provides sustained remission off therapy for one year or longer for a high proportion of patients. ITP affects approximately 72,000 patients in the U.S. (sources: Current Medical Research and Opinion, 25:12, 2961-2969; Am J Hematol. 2012 Sep; 87(9): 848–852; Pediatr Blood Cancer. 2012 Feb; 58(2): 216–220). Phase 3 ADVANCE Clinical Trials In 2019, the first of two registrational clinical trials, the ADVANCE clinical trial, was initiated to evaluate efgartigimod IV (VYVGART) for the treatment of primary ITP. The second registrational ADVANCE-SC clinical trial of efgartigimod SC for the treatment of primary ITP was initiated in 2020. In May 2022, we announced positive Phase 3 data from the ADVANCE clinical trial. Primary endpoint was met, demonstrating that a significantly higher proportion of patients with chronic ITP receiving VYVGART (17/78; 21.8%) compared to placebo (2/40; 5%) achieved a sustained platelet response (p=0.0316), defined as having platelet counts greater than or equal to 50x109/L on at least four of the last six scheduled visits between weeks 19 and 24 of treatment. There was also a statistically significant separation from placebo in key platelet-derived secondary endpoints. Additional secondary endpoint data from the ADVANCE clinical trial are consistent with primary and secondary platelet-derived endpoints and provide additional context on metrics that often drive treatment decisions, including on International Working Group responder status. VYVGART was well-tolerated in this 24-week clinical trial and the observed safety and tolerability profile was consistent with previous clinical trials. Results from ADVANCE-IV clinical trial were published in The Lancet in September 2023. In November 2023, results of the second registrational clinical trial as part of the ongoing ITP development program for VYVGART in adult patients with chronic and persistent ITP were announced. Patients were heavily pre-treated and 75% of patients had received three or more prior ITP therapies. The clinical trial did not meet the primary endpoint of a sustained platelet count response in chronic ITP patients. Secondary endpoints were also not met, including additional endpoints on International Working Group responder status and mean platelet count change from baseline. VYVGART SC was well-tolerated in ADVANCE-SC; the observed safety and tolerability profile was consistent with ADVANCE-IV and the confirmed safety profile of VYVGART and VYVGART SC. Based on the results of the ADVANCE-IV clinical trial we received regulatory approval for VYVGART for the treatment of adults with ITP in Japan in March 2024. We have initiated ADVANCE-NEXT in the U.S. with efgartigimod IV in ITP in 2024. Myositis Overview Myositis are a rare group of autoimmune diseases that can be muscle specific or affect multiple organs including the skin, joints, lung, gastrointestinal tract and heart. Myositis can be very severe and disabling and have a material impact on quality of life. Initially these Myositis were classified as either DM or polymyositis, but as the underlying pathophysiology of Myositis has become better understood, including through the identification of characteristic autoantibodies, new polymyositis subgroups have emerged. Two of these subtypes are IMNM and ASyS. Proximal muscle weakness is a unifying feature of each Myositis subset. IMNM is characterized by skeletal muscle weakness due to muscle cell necrosis. The muscle weakness is typically symmetrical – on both sides of the body – and affects proximal muscles including hips, thighs, upper arms, shoulder and neck. The muscle weakness can be severe and lead to difficulty in completing daily tasks. Characteristic autoantibodies of IMNM, include anti-signal recognition particle and anti-3- hydroxy-3-methylglutaryl-coenzyme A reductase autoantibodies. ASyS is characterized by muscle inflammation, inflammatory arthritis, interstitial lung disease, thickening and cracking of the hands (mechanic’s hands) and Raynaud phenomenon. Autoantibodies associated with ASyS attack tRNA synthetase enzymes and include anti-Jo-1 and anti-PL1 and PL-12 most commonly. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 39

DM is characterized by muscle inflammation and degeneration and skin abnormalities, including heliotrope rash, Gottron papules, erythematous, calcinosis and edema. DM is associated with Myositis-specific autoantibodies, including anti-Mi-2, anti-MDA-5, anti-TIF-1γ and others. There are no current FDA-approved therapies for IMNM or ASyS. IVIg (Octagam 10%) was approved by the FDA for the treatment of DM in July 2021. Myositis patients are most often treated with high-dose steroids. ALKIVIA Clinical Trial We initiated the registrational ALKIVIA clinical trial of efgartigimod SC for the treatment of Myositis in 2022. The clinical trial will enroll approximately 240 patients in three Myositis subtypes, IMNM, ASyS and DM. The clinical trial is being conducted in two Phases, with an analysis of the Phase 2 portion of the clinical trial, including 30 patients of each subtype, followed by conduct of the Phase 3 portion of the clinical trial only if a signal is observed in the Phase 2 portion of the clinical trial. The primary endpoint is the total improvement score (TIS) at the end of the treatment period. Key secondary endpoints include response rates at the end of treatment, time to response, and duration of response in TIS, as well as change from baseline in individual TIS components. Other secondary endpoints include quality of life and other functional scores. In November 2024, argenx announced our 'GO' decision to continue clinical development of efgartigimod SC in the seamless phase2/3 ALKIVIA clinical trial (ongoing) in all three Myositis subtypes following analysis of topline data from the Phase 2 portion of the clinical trial. The decision is supported by the efficacy and safety results from the Phase 2 portion of the seamless Phase 2/3 ALKIVIA clinical trial. Overall, the clinical trial met its primary endpoint, demonstrating a statistically significant treatment effect in mean TIS at Week 24, and showed improvement across all six core set measures of the TIS in favor of efgartigimod SC compared to placebo. The observed safety and tolerability profile was consistent to that demonstrated with other clinical trials. TED Overview TED is an autoimmune orbital disease associated with Graves’ disease and other autoimmune thyroid pathologies such as Hashimoto’s thyroiditis. TED is characterized by extraocular muscle enlargement, orbital adipose tissue expansion, and orbital inflammation, which can lead to proptosis, diplopia, or vision loss in severe cases. Persistent orbital symptoms often impair patient QoL long-term. Substantial nonclinical and clinical evidence supports thyrotropin receptor autoantibodies as causative in the pathology of TED. Clinical evidence supports the removal of autoantibodies as a mechanism for the treatment of TED. By reducing IgGs, including TED-associated pathogenic IgG autoantibodies, efgartigimod is expected to ease disease manifestations. Additionally, IgG reduction could address the underlying hyperthyroidism. Side effects and tolerability issues with current therapies, including steroids and teprotumumab (only FDA-approved biologic), are treatment limiting for many patients based on comorbidities and a significant unmet need remains for safe and convenient therapies. UplighTED Clinical Trials The UplighTED program aims to evaluate the efficacy and safety of weekly efgartigimod for SC administration in pre-filled syringe, coformulated with rHuPH20 (efgartigimod PH20 SC) in two randomized, placebo-controlled, double-blinded studies. Adult participants with moderate-to-severe active TED, with controlled baseline autoimmune thyroid pathology are dosed with 1000mg efgartigimod PH20 SC or placebo PH20 SC for 24 weeks and evaluated for proptosis response. At the end of the treatment period, participants will enter a follow-up observational period to assess safety, tolerability, and durability of efgartigimod PH20 SC treatment while off therapy or an open-label treatment period depending on their response to study treatment. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 40

SjD Overview SjD is a chronic, progressive autoimmune disease, characterized by lymphocytic infiltration and progressive destruction of exocrine glands. B-cells play a pivotal role in the development of the disease and this results amongst others in production of IgG autoantibodies, especially those which target SSA/Ro, SSB/La ribonuclear complexes. In addition to symptoms of dry eyes, dry mouth, chronic pain and fatigue, a substantial subset of patients suffer from extraglandular systemic disease. There are no FDA-approved treatments currently registered for the treatment of SjD. Phase 2 RHO Clinical Trial (in partnership with IQVIA) In March 2024, argenx announced its plan to continue the development of efgartigimod to Phase 3 in adults with primary SjD, following the analysis of topline data from the Phase 2 RHO clinical trial. • The decision to advance the clinical development of efgartigimod in SjD was supported by the safety, efficacy and biomarker results from the clinical trial. The observed safety and tolerability profile was consistent with other clinical trials. Efficacy assessments showed a treatment effect across multiple clinical endpoints, which were also consistent with biomarker data. ◦ In the RHO clinical trial, efgartigimod demonstrated increased response on composite endpoints (CRESS, STAR, ESSDAI (22-34%)). A response was observed in four out of five items of CRESS. ◦ The IgG reduction and biomarker data correlated to clinical benefit and efgartigimod was well tolerated and safe similar to other clinical trials. Phase 3 UNITY Clinical Trial (in partnership with IQVIA) In 2024, we initiated a Phase 3 clinical trial evaluating efgartigimod PH20 SC for the treatment of SjD. The Unity clinical trial is a randomized, placebo-controlled, double-blind clinical trial evaluating safety and efficacy of efgartigimod PH20 SC. The clinical trial plans to enroll 480 patients with at least moderate systemic disease (ClinESSDAI ≥6). Patients have to be on stable background treatment and positive for anti- SSA/Ro. At the end of the 48-week treatment period, participants who complete the clinical trial may roll over into an OLE. The primary endpoint is change from baseline in the clinESSDAI (Clinical ESSDAI). Key secondary endpoints will focus on patient-reported outcomes, ESSDAI (EULAR Sjögrens Syndrome Disease Activity Index), and STAR (Sjögren's Tool for Assessing Response, composite endpoint). LN Overview LN is an inflammatory autoimmune disease of the kidney and one of the most severe and common organ manifestations of the autoimmune disease systemic lupus erythematosus (SLE). In patients with SLE, approximately 25% to 50% have signs or symptoms of kidney disease at SLE onset. Approximately 40% to 60% of patients with SLE will develop renal involvement during the course of disease, with substantial morbidity or mortality. Pathogenic autoantibodies and complement deposits are critically involved in SLE pathogenesis and particularly LN, where renal deposition of immune complexes is a hallmark of the disease. Autoantibodies associated with LN include anti-dsDNA, anti-C1q, anti-cardiolipin, anti-Smith and anti-nuclear antibodies. 10-30% of LN patients progress to end-stage renal disease. Oral corticosteroids and broad immunosuppressants are current standards of care but are not uniformly effective. Belimumab (Benlysta) and voclosporin (Lupkynis) are approved by the FDA for the treatment of LN. Phase 2 POC Clinical Trial (in partnership with Zai Lab) In 2023, we initiated a POC clinical trial to evaluate the efficacy and safety of efgartigimod IV in Chinese patients with active LN. The clinical trial plans to enroll approximately 60 patients with LN class III or IV (with or without class V). The primary endpoint is the change in urine protein creatinine ratio from baseline to end of the treatment period. Key secondary endpoints include proportion of patients achieving complete and partial renal response at the end of treatment period and time to complete renal response and partial renal response. Other secondary endpoints include additional efficacy measurements, PK, PD, immunogenicity, biomarkers, safety, and quality of life assessments. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 41

Other efgartigimod Indications AMR AMR is an autoimmune disease that affects transplanted organs and can contribute to allograft loss. AMR in kidney allografts is driven by donor specific antibodies, which often target HLA antigens expressed by endothelial allograft cells. Through different mechanism, donor specific antibodies can induce microvascular inflammation, a histopathological hallmark of AMR. Microvascular inflammation leads to loss in organ function which, if continued, can result in allograft loss. The unmet need for an efficacious treatment is very high, as evidenced by AMR being the leading cause of kidney transplant failure. There are currently no approved therapies for treating AMR. Phase 2 shAMRock Clinical Trial Design In the end of 2024, we initiated a Phase 2 POC study to evaluate efgartigimod PFS in kidney transplant recipients with AMR. The clinical trial will enroll ~30 participants, randomized 1x1x1 across 2 treatment arms and placebo. Primary endpoint is safety and tolerability and secondary endpoints include efficacy endpoints around estimated glomerular filtration rate, kidney biopsy, urine protein creatinine ratio and survival. Partnerships for efgartigimod indications Zai Lab Limited Pursuant to the Zai Lab Agreement, Zai Lab obtained the exclusive right to develop and commercialize efgartigimod in Greater China. Zai Lab will also contribute patients to our global Phase 3 clinical trials of efgartigimod. Our Zai Lab strategic collaboration allows us to accelerate development of efgartigimod into new autoimmune indications with Zai Lab taking operational leadership of selected Phase 2 POC clinical trials. In 2022 Zai Lab initiated the Phase 2 POC clinical trials in MN and LN, which both fall within the emerging nephrology indications. Zai Lab also completed a Phase 1 PK/PD clinical trial to support the approval of efgartigimod for gMG in Mainland China and to obtain regulatory approvals to enroll Chinese patient into our global Phase 3 clinical trials. IQVIA On December 2, 2021 we entered into a strategic asset development agreement (Asset Development Agreement) with IQVIA. Pursuant to the Asset Development Agreement, IQVIA shall perform asset and indication development services for efgartigimod through an advanced outsourcing model. Such services include, but are not limited to, overall product indication development strategy, design of clinical trial protocol, set-up, execution and management of clinical development plans for an indication for efgartigimod selected by us. To enable and encourage fast and innovative delivery of the services by IQVIA, the Asset Development Agreement contains an innovative earn-back and bonus plan based upon the performance of IQVIA. Clinical trials that have been discontinued: • In May 2024, the decision was made to discontinue planned development of efgartigimod in AAV following the risk assessment of all ongoing clinical trials based on learnings from ADDRESS (PV) and ADVANCE SC (ITP) clinical trials. We determined the risk did not outweigh the benefit in AAV given the potentially unmanageable interference of background medications. • In June 2024, we announced results from the Phase 2 ALPHA clinical trial of efgartigimod in PC-POTS. Based on the results, we decided not to move forward development of efgartigimod in PC-POTS. • In October 2024, we announced the discontinuation of development of efgartigimod in MN. This decision was based on the observation that no clear signal was seen in the blinded data, which was part of an interim review by the executive data review team and the data and safety monitoring board. • Early January 2025, we announced our decision to discontinue development in BP based on results from 98 patients in the Phase 2 BALLAD clinical trial. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 42

1.3.4 empasiprubart (ARGX-117) Development Mechanism of Action empasiprubart is a differentiated therapeutic mAb targeting C2 equipped with our proprietary NHANCE™ mutations. By addressing a novel target at the intersection of the complement and lectin pathways of the complement cascade, we believe empasiprubart represents a broad pipeline opportunity across several severe autoimmune indications. Activation of the classical and lectin pathway of complement may contribute to tissue damage and organ dysfunction in a number of autoimmune inflammatory diseases and ischemia-reperfusion conditions. Targeting C2 also leaves the alternative pathway of the complement system intact, which is an important component of the innate defense system. empasiprubart exhibits both pH- and calcium dependent binding. These unique characteristics enable empasiprubart to capture free C2 in circulation and release it in the endosome to be sorted for degradation in the lysosome. empasiprubart is equipped with NHANCE™ mutations increasing its affinity for FcRn and allowing it to recycle back into circulation to capture more C2. In addition to an IV formulation, we have exclusive access to Halozyme’s ENHANZE® SC drug delivery technology for the C2 target. Figure 3: LEFT: empasiprubart exhibits both pH- and calcium dependent target binding. RIGHT: empasiprubart is equipped with NHANCE™ mutations increasing its affinity for FcRn at acidic pH and allowing it to recycle back into circulation. empasiprubart Indications MMN Overview MMN is a debilitating neuromuscular autoimmune disorder that is characterized by slowly progressive muscle weakness due to motor neuron degeneration. It mainly affects hands and forearms, mainly in males, and the median age of diagnosis is around 40 years. Diagnosis takes about a year and a half and is often misdiagnosed as ALS. There are estimated to be around 13,000 patients with MMN in the U.S. and this number is increasing. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 43

Specific pathophysiologic characteristics of MMN include the presence of IgM autoantibodies against the ganglioside GM1 and conduction block, i.e., impaired propagation of action potentials along the axon. GM1 is widely expressed in the nervous system by neurons, particularly around the nodes of Ranvier, and Schwann cells. IVIg is the only approved treatment for MMN and needs to be dosed frequently to address the disease’s progressive nature. Phase 2 POC ARDA Clinical Trial The Phase 2 POC ARDA clinical trial was a randomized, double-blinded, placebo-controlled multicenter clinical trial to evaluate the safety and tolerability, efficacy, PK, PD, and immunogenicity of two dose regimens of empasiprubart in adults with MMN. The primary endpoint was safety and tolerability. Additional endpoints included time to IVIg retreatment, biomarker analyses of C2 levels, and changes in measurements on key functional scores (modified medical research council -10 sum score, grip strength, MMN-RODS as well as several patient-reported quality of life outcome measures (fatigue severity score (FSS), chronic acquired polyneuropathy patient-reported index (CAP-PRI), and values of the patient global impression change scale). At the start of the year argenx announced positive data from the first cohort (n=16) of the Phase 2 POC ARDA clinical trial which was confirmed with the results of the second cohort (n=16) in July 2024, establishing POC in MMN. empasiprubart demonstrated a 91% reduction in the need for IVIg rescue compared to placebo [HR (95% CI) = 0.09 (0.02; 0.44)] in cohort 1 and a 84% reduction in the need for IVIg rescue compared to placebo [HR (95% CI) = 0.16 (0.02; 1.54)] in cohort 2. Based on these results argenx initiated the EMPASSION Phase 3 clinical trial evaluating empasiprubart in MMN at the end of 2024. Phase 3 EMPASSION Clinical Trial Design A Phase 3, randomized, double-blinded, double-dummy clinical trial evaluating the efficacy and safety of empasiprubart versus intravenous immunoglobulin in adults with multifocal motor neuropathy. The clinical trial comprises a screening period of up to 15 weeks, including a minimum of 2 IVIg cycles; a 24- week (6-month), randomized, double-blinded, double-dummy treatment period (part A) evaluating the efficacy and safety of empasiprubart vs IVIg continuation; a 24-month OLE period (part B); and a 15-month safety follow-up period starting after the last dose of IMP. The primary objective is to demonstrate the efficacy of empasiprubart compared to IVIg in improving functional ability. This will be measured by change from baseline in the 25-item MMN-RODS centile score at week 24. Additional key secondary endpoints include changes in measurements on key functional scores (modified medical research council -14 sum score, grip strength) as well as patient-reported quality of life outcome measures (polyneuropathy patient- reported index, and values of the patient global impression change scale and evaluation of manual dexterity using 9HPT. DGF Overview DGF, a complication after kidney transplantation, is defined as the need for dialysis in the first week after transplant. DGF occurs in up to 40% of patients receiving a deceased donor graft, and is associated with worse long-term transplant outcomes. DGF is often the clinical representation of ischemia reperfusion injury, in which the classical and lectin complement pathways play an important role, as shown by compelling evidence from both (in-house) in vitro and in vivo preclinical, and clinical trials. There are currently no approved therapies to reduce DGF risk. Furthermore, there is a well-established process to measure kidney function and DGF, and to establish POC and achieve registration. On this basis, combined with the significant unmet medical need, we have chosen DGF after kidney transplantation as second indication for empasiprubart. Phase 2 POC VARVARA Clinical Trial The Phase 2 POC VARVARA clinical trial was initiated in 2023 and is a randomized, placebo-controlled, double-blinded clinical trial to evaluate the efficacy, safety and tolerability of empasiprubart in improving allograft function in recipients at risk for DGF. The clinical trial will include approximately 102 recipients of argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 44

an at-risk deceased donor kidney. After a short screening period of < 24 hours, patients are randomly assigned in a 1:1 ratio to receive two doses of empasiprubart IV or placebo, of which one dose is administered during transplantation and one a week later. Participants receive standardized background induction and maintenance immunosuppression. They are evaluated for 52 weeks, with one additional safety follow-up visit in week 64. The primary endpoint is the estimated glomerular filtration rate at six months. Key secondary endpoints include DGF risk, safety, and PK, PD and immunogenicity. DM Overview Please refer to Section “1.3.3 efgartigimod Indications” (Myositis) for more information on DM. Phase 2 POC EMPACIFIC Clinical Trial The EMPACIFIC clinical trial is a Phase 2 POC, randomized, double-blinded, placebo-controlled, multicenter clinical trial to evaluate the safety, tolerability, and efficacy of multiple dose regimens of IV empasiprubart in adults with DM. A total of 56 adult participants with a clinical diagnosis of DM and active muscle disease will be randomized (1:1:1:1) to one of four treatment arms (three empasiprubart dose regimens and one placebo arm). Participants will receive loading doses on days 1 and 8, followed by maintenance doses every four weeks until the end of the 52-week treatment phase. The primary objective is to evaluate safety and tolerability. The secondary objective is to evaluate clinical efficacy, using the mean TIS at weeks 13, 25, and 52 as endpoint. CIDP Overview Please refer to Section “1.3.3 efgartigimod Indications ” (CIDP) for more information on CIDP. Phase 3 EMVIGORATE Clinical Trial In July 2024, we announced our plan to start a head-to-head Phase 3 development of empasiprubart versus IVIg in CIDP in 1H 2025. 1.3.5 ARGX-119 Development ARGX-119 is a humanized agonist mAb that specifically targets and activates MuSK to promote maturation and stabilization of the neuromuscular junction (NMJ). We plan to develop ARGX-119 in a range of neuromuscular diseases including CMS, a rare hereditary subtype of MG, ALS, and SMA, all severe neuromuscular indications. NMJs are specialized synapses formed between motor neurons and muscle cells, which are essential for the ability to move and breathe. At the NMJ, motor neurons release acetylcholine, which binds to AChRs on the muscle to initiate muscle contraction. Deficits in the NMJ can cause neuromuscular disorders, which can range in severity from mild to life-threatening skeletal muscle weakness. MuSK is an essential component for the formation and function of NMJs. ARGX-119 is the first and highly specific agonist mAb targeting human MuSK being developed for patients with neuromuscular disease, such as CMS and ALS. This mAb is derived from llamas and discovered using the argenx SIMPLE ANTIBODY™ platform technology. We developed ARGX-119 through our IIP program in collaboration with the world leading key opinion leaders on MuSK and the neuromuscular junction, including Professor Steve Burden from NYU and Professor Verschuuren from LUMC. In collaboration with Professor Burden, it was shown that ARGX-119 holds promising preclinical POC data in Dok7 congenital myasthenic syndrome, observed in a mouse model bearing the most common patient mutation and in ALS using ALS patient derived NMJ on-a-chip models. Based on these data, clinical development for ARGX-119 was initiated as activation of MuSK by ARGX-119 may stabilize, mature, and improve the function of the NMJ in patients with CMS or ALS, significantly reducing weakness and fatigability and improving quality of life. A Phase 1 dose-escalation clinical trial in healthy volunteers was completed in 2024; data support advancement in POC studies. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 45

A Phase 1b and 2a clinical trial in CMS and ALS respectively initiated in 2024 to assess early signal detection in patients. Early January 2025, we announced SMA as the third indication for ARGX-119 for which we expect to initiate a POC clinical trial in 2025. Phase 1b Clinical Trial Design The Phase 1b, multicenter, randomized, double-blinded, placebo-controlled clinical trial is designed to assess the safety, tolerability, PK, immunogenicity, and preliminary efficacy of ARGX-119 for the first time in participants with DOK7-CMS. The clinical trial is designed to demonstrate proof of biology for ARGX-119 through a preliminary evaluation of its efficacy with measures of muscle weakness and fatigability, activities of daily living, and patient-reported outcomes of global health in participants with DOK7-CMS. The clinical trial will be up to approximately 11 months long, comprising the following periods, a screening period: up to 28 days, a treatment period of 12 weeks and a follow-up period of approximately seven months. At baseline, eligible participants will be randomized in a 4:1 ratio to receive IV infusions of ARGX-119 or placebo. The primary objective is to evaluate the safety and tolerability of ARGX-119 in participants with DOK7 CMS. The secondary objective is to assess the PK, immunogenicity of ARGX-and efficacy of ARGX-119 in participants with DOK7 CMS. Phase 2a reALiSe Clinical Trial Design The ReALiSe clinical trial is a Phase 2a, double-blinded, randomized, placebo-controlled, and active- treatment extension clinical trial to assess the safety, tolerability, efficacy, pharmacokinetics, and immunogenicity of ARGX-119 in participants with amyotrophic lateral sclerosis. The clinical trial is designed to demonstrate proof of ARGX-119 activity through an evaluation of efficacy by assessing the impact of ARGX-119 on ALS disease outcomes, including muscle function in participants with ALS. Approximately 60 participants are planned to be enrolled, screened, and randomized 1:1:1:1 to one of three ARGX-119 intravenous dose arms or placebo IV for the double-blinded treatment period. This clinical trial will last up to 100 weeks. The clinical trial will contain the following periods: a screening period (up to four weeks), a double-blinded treatment period (24 weeks), an active-treatment extension period (48 weeks), and a safety- follow-up period (24 weeks). The primary objective is evaluate the safety and tolerability of ARGX-119 in participants with ALS. The secondary objective is to assess the efficacy of ARGX-119 on electrophysiological measures of disease progression in participants with ALS using MScan to measure motor unit number and other MScan-derived neurophysiological markers. MScan provides parameters that have been associated with ALS disease progression (i.e., motor unit loss and/or enlarged motor units due to reinnervation). Additional exploratory outcome measures, including SVC and ALSFRS-R, will be used to explore ARGX-119 impact on ALS-relevant outcome measures. 1.3.6 ARGX-213, ARGX-121, ARGX-220 and ARGX-109 Development We continue to invest in our discovery engine, the IIP, to drive long-term sustainable pipeline growth. Through the IIP, four new pipeline candidates were nominated in 2023, including: ARGX-213 targeting FcRn and furthering argenx’s leadership in this new class of medicine; ARGX-121 targeting IgA and ARGX-220, which broaden argenx’s focus across the immune system; and ARGX-109, targeting IL-6, which plays an important role in inflammation. Preclinical work is ongoing for each candidate and we expect Phase 1 results in 2025 and 2026. 1.3.7 Immunology Innovation Program (IIP) Co-creation Through our IIP, we collaborate with scientific and academic partners to identify immunology breakthroughs and build potential pipeline candidates. For more information, please refer to Section 1.1.2 ‘‘IIP”. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 46

Antibody Engineering and Other Technology Capabilities Our Proprietary SIMPLE ANTIBODY™ Platform Our proprietary SIMPLE ANTIBODY™ platform technology sources V-regions from conventional antibodies existing in the immune system of outbred llamas. Outbred llamas are those that have been bred from genetically diverse parents, and each has a different genetic background. The llama produces highly diverse panels of antibodies with a high human homology in their V-regions when immunized with human disease targets. We then combine these llama V-regions with Fc regions of fully human antibodies, resulting in antibodies that we then produce in industry-validated production cell lines. The resulting antibodies are diverse and, due to their similarity to human antibodies, we believe they are well suited to human therapeutic use. With this breadth of antibodies, we are able to test many different epitopes. Being able to test many different epitopes with our antibodies enables us to search for an optimized combination of safety, potency and species cross-reactivity with the potential for maximum therapeutic effect on disease. These antibodies are often cross-reactive with the rodent version of chosen disease targets. This rodent cross-reactivity enables more efficient preclinical development of our product candidates because most animal efficacy models are rodent-based. By contrast, most other antibody discovery platforms start with antibodies generated in inbred mice or synthetic antibody libraries, approaches that we believe are limited by insufficient antibody repertoires and limited diversity, respectively. Our SIMPLE ANTIBODY™ platform technology allows us to access and explore a broad target universe, including novel and complex targets, while minimizing the long timelines associated with generating antibody candidates using traditional methods. Our Antibody Engineering Technologies Through licensing we have obtained access to a broad range of antibody engineering technologies. NHANCE™, ABDEG™, POTELLIGENT® and the DHS mutations focus on engineering the Fc region of antibodies, while SMART-Ig® and ACT-Ig® technologies allow to make sweeping antibodies. Fc engineering can augment antibodies interactions with components of the immune system, thereby potentially expanding the therapeutic index of our product candidates by modifying their half-life, tissue penetration, rate of disease target clearance and potency. For example, our NHANCE™ and ABDEG™ engineering technologies enable us to modulate the interaction of the Fc region with FcRn, which is responsible for regulating half-life, tissue distribution and PD properties of IgG antibodies. Similarly, the POTELLIGENT® engineering technology modulates the interaction of the antibody Fc region with receptors located on specialized immune cells known as natural killer (NK) cells. These NK cells can destroy the target cell, resulting in enhanced antibody-dependent cell-mediated cytotoxicity (ADCC). NHANCE™ and ABDEG™: Modulation of Fc Interaction with FcRn. An illustration of the FcRn-mediated antibody recycling mechanism is shown in Figure 4. [1] Serum proteins, including IgG antibodies, are routinely removed from the circulation by cell uptake. [2] Antibodies can bind to FcRn, which serves as a dedicated recycling receptor in the endosomes, which have an acidic environment, and then [3A] return to the circulation by binding with their Fc region to FcRn. [3B] Unbound antibodies end up in the lysosomes and are degraded by enzymes. Because this Fc/ FcRn interaction is highly pH-dependent, antibodies tightly bind to FcRn at acidic pH (pH 6.0) in the endosomes but release again at neutral pH (pH 7. 4) in the circulation. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 47 Figure 4: The FcRn-mediated recycling mechanism

NHANCE™ NHANCE™ refers to two mutations that we introduce into the Fc region of an IgG antibody. NHANCE™ is designed to extend antibody serum half-life and increase tissue penetration. In certain cases, it is advantageous to engineer antibodies that remain in the circulation longer, allowing them to potentially exert a greater therapeutic effect or be dosed less frequently. As shown in Figure 5, [1] NHANCE™ antibodies bind to FcRn with higher affinity, specifically under acidic pH conditions. [2] Due to these tighter bonds, NHANCE™ FcRn-mediated antibody recycling is strongly favored over lysosomal degradation, although some degradation does occur. [3] NHANCE™ allows a greater proportion of antibodies to return to the circulation potentially resulting in increased bioavailability and reduced dosing frequency. ARGX-109, empasiprubart and a number of our discovery-stage programs utilize NHANCE™. Figure 5: NHANCE™ mutations favor the FcRn-mediated recycling of IgG antibodies. Figure 6: SIMPLE ANTIBODY™ and ABDEG™ platform technologies work in concert to sweep diseases targets. ABDEG™ ABDEG™ refers to five mutations that we introduce in the Fc region that increase its affinity for FcRn at both neutral and acidic pH. In contrast to NHANCE™, ABDEG™-modified Fc regions remain bound to FcRn if the pH changes, occupying FcRn with such high affinity that they deprive endogenous IgG antibodies of their recycling mechanism, leading to enhanced clearance of such antibodies by the lysosomes. Some diseases mediated by IgG antibodies are directed against self-antigens. These self-directed antibodies are referred to as autoantibodies. We use our ABDEG™ technology to reduce the level of these pathogenic autoantibodies in the circulation by increasing the rate at which they are cleared by the lysosomes. ABDEG™ is a component in a number of our products and product candidates, including efgartigimod. As shown in Figure 6, our ABDEG™ technology can also be used with our pH-dependent SIMPLE ANTIBODY™ generated antibodies in a mechanism referred to as sweeping. Certain antibodies generated through the SIMPLE ANTIBODY™ platform bind to their target in a pH-dependent manner. These antibodies [1] bind tightly to a target at neutral pH while in circulation, and [2] release the target at acidic pH in the endosome. [3] The unbound target is degraded in the lysosome. [4] However, when equipped with our ABDEG™ technology, the therapeutic antibodies remain tightly bound to FcRn at all pH levels and are not degraded themselves. Instead, they are returned to the circulation where they can bind new targets. We believe this is especially useful in situations where high levels of the target are circulating or where the target needs to be cleared very quickly from the system. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 48

POTELLIGENT® POTELLIGENT® modulates the interaction of the Fc region with the Fc gamma receptor IIIa located on specialized immune cells, known as NK cells. These NK cells can destroy the target cell, resulting in enhanced ADCC. POTELLIGENT® changes the Fc structure by excluding a particular sugar unit such that it enables a tighter fit with the Fc gamma receptor IIIa. The strength of this interaction is a key factor in determining the killing potential of NK cells. An independent publication reported that the exclusion of this sugar unit of the Fc region increases the ADCC-mediated cell-killing potential of antibodies by 10- to 1000- fold. cusatuzumab and ARGX-111 utilize POTELLIGENT® (source: Expert Opin Biol Ther 2006; 6:1161-1173; http://www.tandfonline.com/doi/full/10.1517/14712598.6.11.1161%20). SMART-Ig®, ACT-Ig® and DHS In 2020, we entered into a research license and option agreement with Chugai under which we may access Chugai’s SMART-Ig® and ACT-Ig®. In 2020, we also entered into a non-exclusive research agreement with the Clayton Foundation under which we may access the Clayton Foundation’s proprietary DHS mutations to extend the serum half-life of therapeutic antibodies. SC drug delivery technologies We have exclusive access to Halozyme’s ENHANZE® SC drug delivery technology for the FcRn and C2 targets and four additional targets. ENHANZE® has the potential to shorten drug administration time, reduce healthcare practitioner time, and offer additional flexibility and convenience for patients. In addition, in April 2021, we entered into the Elektrofi Agreement with Elektrofi to explore new SC formulations utilizing Elektrofi’s high concentration technology for efgartigimod, and up to one additional target. For more information on our collaborations, please refer to Section 1.4 “Collaborations and licenses”. Partnered Programs See Section 1.4 “Collaborations and licenses” for a description of collaboration and license agreements that we have entered into to further leverage our IIP. 1.4 Collaborations and licenses We follow a disciplined strategy to maximize the value of our pipeline. We plan to retain all development and commercialization rights to those products and product candidates that we believe we can commercialize successfully, if approved. We have partnered, and plan to continue to partner, to develop products and product candidates that we believe have promising utility in disease areas or have patient populations that may benefit from resources of other biopharmaceutical companies. We expect to continue to collaborate selectively with pharmaceutical and biotechnology companies to leverage our platform technology and accelerate product candidate development. We are also party to several license agreements under which we license patents, patent applications and other intellectual property to third parties. We have also entered into several license agreements under which we license patents, patent applications and other intellectual property from third parties. License agreements can relate to research and development and/or commercialization of the relevant product candidates (and technologies) or products. The licensed intellectual property covers some of our product candidates and some of the antibody engineering technologies that we use. Some of these licenses impose various diligence and financial payment obligations on us. We expect to continue to enter into these types of license agreements in the future. We have entered into multiple collaboration agreements with pharmaceutical partners and license agreements, as described below. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Our Products and Product Candidates 49

1.4.1 OncoVerity for cusatuzumab In 2022, we, the University of Colorado Anschutz Medical Campus and the University of Colorado Health (UCHealth) created an asset-centric spin-off, OncoVerity, Inc (OncoVerity), focused on optimizing and advancing the development of cusatuzumab, a novel anti-CD70 antibody, in acute myeloid leukemia (AML). OncoVerity is an entity of co-creation, combining the extensive translational biology insights from Dr. Clayton Smith, M.D. from the University of Colorado with our experience on the CD70/CD27 pathway. In 2023, we granted an exclusive license for cusatuzumab to OncoVerity and provided, together with a joint venture of University of Colorado Health and University License Equity Holdings, Inc. on the University of Colorado Anschutz Medical Campus, and funding for ongoing clinical development of cusatuzumab. In 2024, we participated in a further funding round to support the continued, ongoing, clinical development of cusatuzumab by OncoVerity. 1.4.2 Our Strategic Partnership with LEO Pharma for ARGX-112 (LP0145) In May 2015, we entered into a collaboration agreement with LEO Pharma A/S (LEO Pharma) to develop and commercialize ARGX-112 (LP0145) for the treatment of dermatologic indications involving inflammation (LEO Pharma Collaboration Agreement). ARGX-112 (LP0145) employs our SIMPLE ANTIBODY™ technology and blocks the IL-22R in order to neutralize the signaling of cytokines implicated in autoimmune diseases of the skin. LEO Pharma funded more than half of all product development costs up to clinical trial application (CTA) approval of a first product in a Phase 1 clinical trial, with our share of such costs capped, which was achieved in April 2018. Since then, LEO Pharma has been solely responsible for funding the clinical development of the program. In May 2021, CTA approval of a Phase 2a clinical trial for LP0145 was received. In September 2022, LEO Pharma, exercised its option to obtain, and was granted an exclusive, worldwide license to further develop and commercialize ARGX-112 against payment of a €5.0 million option fee to us. LEO Pharma assumed full responsibility for the continued development, manufacture and commercialization of such product and is subject to diligence obligations in respect of continuation of development and commercialization of such product. We are eligible to receive additional development, regulatory and commercial milestone payments in aggregate amount of up to €120.0 million, as well as tiered royalties on product sales at percentages ranging from the low single digits to the low teens, subject to customary reductions. Unless earlier terminated, the term of the LEO Pharma Collaboration Agreement ends upon the later of (i) the expiration of the last license granted under the agreement, and (ii) the fulfilment of all payment obligations under the agreement. LEO Pharma may terminate the LEO Pharma Collaboration Agreement for any reason upon prior written notice to us. LEO Pharma’s royalty payment obligations expire, on a product-by-product and country-by-country basis, upon the later of (i) a time when no valid claims covering such product, and (ii) (a) in major market countries with no composition of matter patent covering such product, the expiration of the data exclusivity period or (b) in countries that are not major market countries, a double-digit number of years after the first commercial sale of such product sold in that country. 1.4.3 Our Strategic Partnership with Zai Lab for efgartigimod Pursuant to the Zai Lab Agreement, Zai Lab obtained the exclusive right to develop and commercialize efgartigimod in Greater China. Zai Lab will also contribute patients to our global Phase 3 clinical trials of efgartigimod. Our Zai Lab strategic collaboration allows us to accelerate development of efgartigimod into new autoimmune indications with Zai Lab taking operational leadership of selected Phase 2 POC Clinical trials. We are eligible to receive a one-time sales based milestone and tiered royalties (mid-teen to low-twenties on a percentage basis) based on annual net sales of efgartigimod in Greater China thereafter. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Collaborations and licenses 50

1.4.4 Our Strategic Partnership with AbbVie for ARGX-115 (ABBV-151) In April 2016, we entered into a collaboration agreement with AbbVie to develop and commercialize ARGX-115 (ABBV-151) as a cancer immunotherapy against the novel target glycoprotein A repetitions predominant (GARP) (the AbbVie Collaboration Agreement). ARGX-115 (ABBV-151) employs our SIMPLE ANTIBODY™ platform technology and works by stimulating a patient’s immune system after a tumor has suppressed the immune system by co-opting immunosuppressive cells such as regulatory T cells. Under the terms of the AbbVie Collaboration Agreement, we were responsible for conducting and funding all ARGX-115 (ABBV-151) research and development activities up to completion of investigational new drug (IND) enabling studies. AbbVie has exercised its option and obtained a worldwide, exclusive license to the ARGX-115 (ABBV-151) program to develop and commercialize products and has assumed development obligations, including the sole responsibility for all research, development and regulatory costs relating to ARGX-115 (ABBV-151)- based products. Subject to the continuing progress of ARGX-115 (ABBV-151) by AbbVie, we are eligible to receive development, regulatory and commercial milestone payments in aggregate amounts of up to $110 million, $190 million and $325 million, respectively, as well as tiered royalties on product sales at percentages ranging from the mid-single digits to the lower teens, subject to customary reductions. Pursuant to the AbbVie Collaboration Agreement, we have the right, on a product-by-product basis, to co- promote ARGX-115 (ABBV-151) based products in the European Economic Area (EEA) and Switzerland and to combine the product with our own future oncology programs (if any). The co-promotion effort would be governed by a co-promotion agreement negotiated in good faith by the parties. Unless earlier terminated upon mutual agreement, for material breach or as otherwise specified in the AbbVie Collaboration Agreement, the term of the license agreement ends, with respect to the ARGX-115 (ABBV-151) program, upon fulfilment of all payment obligations under the agreement. AbbVie may terminate the AbbVie Collaboration Agreement for any reason upon prior written notice to us. AbbVie’s royalty payment obligations expire, on a product-by-product and country-by-country basis, on the date that is the later of (i) such time as there are no valid claims covering such product, (ii) expiration of regulatory or market exclusivity in respect of such product or (iii) 10 years after the first commercial sale of such product sold in that country under the AbbVie Collaboration Agreement. 1.4.5 Our Exclusive License with Elektrofi for efgartigimod In April 2021, we entered into the Elektrofi Agreement with Elektrofi to explore new SC formulations utilizing Elektrofi’s high concentration technology for efgartigimod, and up to one additional target. The Elektrofi-enabled formulations are aimed to promote additional optionality for patients through at-home and self-administration capabilities. Under the terms of the Elektrofi Agreement, we made an upfront payment and committed to future milestones payments across both targets pending achievement of pre-defined development, regulatory, and commercial milestones. Elektrofi is also eligible to receive a mid-single digit royalty on sales of commercialized products. 1.4.6 Our collaboration with Genmab In 2023, we entered into a collaboration with Genmab to jointly discover, develop and commercialize novel therapeutic antibodies with applications in immunology, as well as in oncology therapeutic areas. The multiyear collaboration is expected to leverage the antibody engineering expertise and knowledge of disease biology of both companies to accelerate the identification and development of novel antibody therapeutic candidates with a goal to address unmet patient needs in immunology and cancer. Under the terms of the collaboration, we and Genmab each have access to the suites of proprietary antibody technologies of both companies to advance the identification of lead antibody candidates against differentiated disease targets. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Collaborations and licenses 51

1.4.7 Our Non-Exclusive Research License and Option Agreement with Chugai for SMART-Ig® and ACT-Ig® In September 2020, we entered into a non-exclusive research license and option agreement with Chugai, allowing us to access Chugai’s SMART-Ig® and ACT-Ig® engineering technologies for conducting feasibility clinical trials. These technologies are designed to enable us to make sweeping antibodies and expand the therapeutic index of our product candidates, which is the ratio between toxic and therapeutic dose, by potentially modifying their half-life, tissue penetration, rate of disease target clearance and potency. 1.4.8 Our Non-exclusive License with the Clayton Foundation for DHS mutations In October 2020, we entered into a non-exclusive research agreement with the Clayton Foundation relating to the non-exclusive in-license for Clayton Foundation’s proprietary DHS mutations to extend the serum half-life of therapeutic candidates. 1.4.9 Our Exclusive License with Halozyme for ENHANZE® In February 2019, we entered into an in-license agreement with Halozyme for the use of certain patents, materials and know-how owned by Halozyme and relating to its ENHANZE®, for application in the field of prevention and treatment of human diseases (the ENHANZE® License Agreement). Pursuant to the ENHANZE® License Agreement, we were granted exclusive rights to apply ENHANZE® to biologic products against pre-specified targets, in order to research, develop and commercialize SC formulations of our therapeutic antibody-based product candidates. Our first therapeutic target for which we received an exclusive license from Halozyme was FcRn, which allows us to apply ENHANZE® to efgartigimod and any other product candidates selective and specific for FcRn. Moreover, the breadth of our exclusive license to FcRn precludes either Halozyme itself or any of its current or future partners from utilizing ENHANZE® in the context of an FcRn-targeted product. Our second therapeutic target for which we received an exclusive license from Halozyme was human C2 associated with the product candidate empasiprubart, which is being developed to treat severe autoimmune diseases. Pursuant to the ENHANZE® License Agreement, we also have the right to nominate future targets for an exclusive ENHANZE® license if the target in question has not already been licensed by Halozyme or is not already being pursued by Halozyme. In October 2020, we expanded our collaboration with Halozyme for ENHANZE® drug delivery technology to include three additional exclusive targets upon nomination bringing the total to six potential targets. From the effective date of the ENHANZE® License Agreement, we have a seven-year period in which to conduct research and preclinical trials on other target-specific molecules in combination with ENHANZE®. In September 2024, we expanded the existing global collaboration and license agreement with Halozyme by nominating four additional targets for a total of six, including FcRn and C2. The royalty rate for all products under the agreement is a tiered low-to-mid-single digit rate based on target and annual net sales until expiration of Halozyme's ENHANZE® related patents, when the rate will be reduced in one or more steps. Royalties will be paid for the longer of 10 years from the first commercial sale or until the expiration of the last valid claim of a co-formulation patent. Pursuant to the ENHANZE® License Agreement, we have the right to grant sublicenses to our subsidiaries and to third parties both for research/preclinical work (for example, to subcontractors) and for development and commercialization. Halozyme provides dedicated specialist support to us which it has accrued over 10 years of licensing ENHANZE® to its collaborators. We have diligence obligations with respect to the continuation of development and commercialization of product candidates, but we are not obligated to utilize ENHANZE® for every product candidate directed to a given exclusive target(s). argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Collaborations and licenses 52

We may terminate the ENHANZE® License Agreement at any time, either in its entirety or on a target-by- target basis, by sending Halozyme prior written notice. Absent early termination, the ENHANZE® License Agreement will automatically expire upon the expiry of our royalty payment obligations under the agreement. In the event the ENHANZE® License Agreement is terminated for any reason, the license granted to us would terminate but Halozyme would grant our sublicensees a direct license following such termination. In the event the ENHANZE® License Agreement is terminated other than for our breach, we would retain the right to sell licensed products then on hand for a certain period of time post-termination. As also set out in Section 3 “Corporate Governance” below, our non-executive director in the Board of Directors (Non-Executive Director) James Daly previously served as a non-executive member of the board of directors of Halozyme. Mr. Daly did not participate in any discussions and decision making relating to the ENHANZE® License Agreement. The ENHANZE® License Agreement with Halozyme was not a related party transaction in accordance with IAS 24 - Related Party Disclosures, since Mr. Daly, in his role as Non- Executive Director, did not control or have significant influence over argenx or Halozyme. 1.4.10 Our Exclusive License with Agomab for ARGX-114 (AGMB-101) In March 2019, we entered into an exclusive out-license with Agomab for the use of certain patent rights relating to our proprietary suite of technologies for the development and commercialization of a series of agonistic anti-MET SIMPLE ANTIBODY™ generated antibodies, including ARGX-114 (AGMB-101), a halofuginone-mimetic antibody directed against the MET receptor. Agomab is required to use commercially reasonable efforts to develop and commercialize at least one licensed product. In connection with our entry into this agreement, we received a profit-sharing certificate which entitles us to 20% of all distributions to Agomab’s shareholders (which shall be reduced to 10% following the filing of an IND and is subject to further adjustment upon the occurrence of certain financings). Upon the occurrence of a qualified initial public offering of Agomab, the profit-sharing certificate will automatically be converted into the equivalent number of ordinary shares in Agomab. This agreement is subject to mutual termination for material breach or insolvency and automatically expires upon the expiration of the last to expire of our licensed patent rights. 1.4.11 Our Exclusive License with Broteio for empasiprubart In March 2017, we entered into a collaboration with Broteio in connection with our IIP, to develop an antibody against a novel target in the complement cascade, empasiprubart (Broteio Agreement). Under the Broteio Agreement, we have jointly developed the complement-targeted antibody and established preclinical POC using our proprietary suite of technologies. Upon successful completion of these preclinical studies, we exercised an exclusive option to in-license the program in March 2018 and assumed responsibility for further development and commercialization. Pursuant to the Broteio Agreement, we are obligated to make milestone payments upon the occurrence of certain development milestones (up to an aggregate of €4.0 million), commercialization milestones (up to an aggregate of €10.0 million) and pay tiered royalties on net sales in the low single digits. We may terminate the Broteio Agreement for convenience upon 90 days prior written notice. The Broteio Agreement is also subject to mutual termination for material breach or insolvency and automatically expires upon the expiration of our financial obligations thereunder. 1.4.12 Our Exclusive License with VIB for ARGX-118 In November 2016, we entered into a collaboration under our IIP with VIB vzw (VIB) to develop antibodies against Galectin-10, the protein of Charcot-Ley-den Crystals, which play a major role in severe asthma and the persistence of mucus plugs, including ARGX-118 (VIB Agreement). Pursuant to the VIB Agreement, we are jointly developing antibodies against Galectin-10 using our proprietary suite of technologies. Upon successful completion of this initial research, we exercised an exclusive option to in-license the program and assumed responsibility for further development and commercialization. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Collaborations and licenses 53

Under the VIB Agreement, including as amended in November 2018, we are obligated to make milestone payments upon the occurrence of certain development milestones (up to an aggregate of €4.0 million), commercialization milestones (up to an aggregate of €11.0 million) and pay tiered royalties on net sales in the low single digits. We may terminate the VIB Agreement for convenience upon 90 days prior written notice. The VIB Agreement is also subject to mutual termination for material breach, insolvency or certain patent challenges and automatically expires upon the expiration of VIB’s licensed patent rights. 1.4.13 Our Exclusive License with the University of Texas for NHANCE™ and ABDEG™ In February 2012, we entered into an exclusive in-license with the Board of Regents of the University of Texas System (UT BoR) for the use of certain patent rights relating to the NHANCE™ platform for any use worldwide (the UT Agreement). The UT Agreement was amended on December 23, 2014 to also include certain additional patent rights relating to the ABDEG™ platform. Upon commercialization of any of our products that use the in-licensed patent rights, we will be obligated to pay UT BoR a percentage of net sales as a royalty until the expiration of any patents covering the product. This royalty varies with net sales volume and is subject to an adjustment for royalties we receive from a sublicensee of our rights under the UT Agreement, but in any event does not exceed 1%. In addition, we must make annual license maintenance payments to UT BoR until termination of the UT Agreement and we have assumed certain development and commercial milestone payment and reimbursement obligations. We also have diligence requirements with respect to development and commercialization of products which use the in-licensed patent rights. Pursuant to the UT Agreement, we may grant sublicenses to third parties. If we receive any non-royalty income in connection with such sublicenses, we must pay UT BoR a percentage of such income varying from low-middle single digits to middle-upper single digits depending on the nature of the sublicense. Such fees are waived if a sublicensee agrees to pay the milestone payments as set forth in the UT Agreement. We may unilaterally terminate the UT Agreement for convenience upon prior written notice. Absent early termination, the UT Agreement will automatically expire upon the expiration of all issued patents and filed patent applications within the patent rights covered by the UT Agreement. Our royalty payment obligations expire, on a product-by-product and country-by-country basis, at such time as there are no valid claims covering such product. 1.4.14 Our Non-Exclusive License with BioWa and Non-Exclusive Commercial Licenses with BioWa and Lonza for POTELLIGENT® In October 2010, we entered into a non-exclusive license agreement with BioWa, Inc. (BioWa) for the use of certain patents and know-how owned by BioWa and relating to its POTELLIGENT® platform technology, for use in the field of prevention and treatment of human diseases. Pursuant to this agreement, we are granted a non-exclusive right to use POTELLIGENT® to research and develop antibodies and products containing such antibodies using POTELLIGENT®. In 2013 and 2014, we entered into non-exclusive license agreements for POTELLIGENT® CHOK1SV with BioWa and Lonza Sales AG (Lonza) for the further development, manufacturing and commercialization of ARGX-110 and ARGX-111, respectively (the POTELLIGENT® License Agreements). Upon commercialization of our products developed using POTELLIGENT®, we will be obligated to pay BioWa and Lonza a percentage of net sales of a licensed product as a royalty. This royalty varies with net sales volume, ranging in the low single digits, and it is reduced by half if during the following 10 years from the first commercial sale of the product in a country the last valid claim within the licensed patent(s) that covers the product expires or ends. In addition, we must make annual research license maintenance payments which cease with commencement of our royalty payments to BioWa. We have diligence requirements with respect to the continuation of development and commercialization of products. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Collaborations and licenses 54

We have also assumed certain development, regulatory and commercial milestone payment obligations and must report on our progress toward achieving these milestones on an annual basis. Milestones to BioWa are to be paid on a commercial target-by-commercial target basis, and we are obligated to make milestone payments in aggregate amounts of up to $36 million per commercial target should we achieve annual global sales of over $1 billion. Pursuant to the POTELLIGENT® License Agreements, we have the right to grant sublicenses to third parties. BioWa retains a right of first negotiation for the exclusive right to develop and commercialize, in certain countries only, any product we develop using POTELLIGENT®. We may terminate the POTELLIGENT® License Agreements at any time by sending BioWa and Lonza prior written notice. Absent early termination, the POTELLIGENT® License Agreements will automatically expire upon the expiry of our royalty obligations under the POTELLIGENT® License Agreements. In the event a POTELLIGENT® License Agreement is terminated for any reason, the license granted to us would terminate but BioWa would grant our sublicensees a direct license following such termination. In the event the POTELLIGENT® License Agreement is terminated other than for our breach or insolvency, we would retain the right to sell licensed products then on hand for a certain period of time post-termination. 1.4.15 Our non-exclusive license with Lonza for Multi-product GS Xceed®-License On February 4, 2015, we entered into a non-exclusive multi-product in-license agreement with Lonza (the Multi-Product License) for use of Lonza’s proprietary glutamine synthetase gene expression system known as GS Xceed® consisting of Chinese hamster ovary cell line and the vectors for the manufacturing of drug substance. This system is used for the manufacturing of, amongst others, efgartigimod, empasiprubart and ARGX-119. Pursuant to the Multi-Product License, we have the right to grant sublicenses to certain pre-approved third parties without prior written consent of Lonza, but otherwise we must obtain Lonza’s prior written consent. We have assumed certain development, regulatory and commercial milestone payment obligations to Lonza. We are required to pay such milestones using this system. We may terminate the Multi-Product License on a product-by-product basis by giving Lonza prior written notice. Lonza may terminate the Multi-Product License solely in case of breach or insolvency events. Absent early termination, the Multi-Product License will automatically expire upon the expiry of the last valid claim for such product. We or our strategic partners would retain the right to sell the respective products then on hand post-termination. 1.4.16 Our Collaboration with Université Catholique de Louvain (UCL) and Sopartec S.A. (Sopartec) for GARP In January 2013, we entered into a collaboration and exclusive product license agreement with UCL and its technology transfer company Sopartec to discover and develop novel human therapeutic antibodies against GARP (GARP Agreement). Pursuant to the GARP Agreement, each party is responsible for all of its own costs in connection with the activities assigned to it under a mutually agreed research plan. In January 2015, we exercised the option we were granted under the GARP Agreement to enter into an exclusive, worldwide commercial in-license for use of certain GARP-related intellectual property rights owned by UCL and the Ludwig Institute for Cancer Research to further develop and commercialize licensed products, including the GARP-neutralizing antibody ARGX-115 which was discovered under the original collaboration (GARP License). Upon the expiration of the GARP Agreement, the GARP License will become a fully paid-up, perpetual worldwide exclusive license under the GARP intellectual property for any purpose, subject to UCL’s retention of non-commercial research rights. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Collaborations and licenses 55

Pursuant to the GARP License, we may grant sublicenses to third parties and affiliates of such third parties. In 2016, we entered into an exclusive collaboration and license agreement with AbbVie regarding ARGX-115. From any income we receive in connection with these sublicenses, such as in connection with AbbVie Collaboration Agreement, we must pay Sopartec a percentage of that income in the lower teen digit range. Royalty payment obligations expire on a product-by-product and country-by-country basis when there are no valid claims covering the ARGX-115 product. We also have diligence obligations with respect to the continued development and commercialization of ARGX-115 products. 1.4.17 Our Exclusive Licenses with NYU Langone Health and LUMC for ARGX-119 In 2019 and 2020, we entered into collaboration and exclusive license agreements with NYU Langone Health and LUMC, respectively, under our IIP to develop antibodies targeting the MuSK, for the treatment neuromuscular diseases, which play a major role at the neuromuscular junction (NYU and LUMC Agreements). Pursuant to the NYU and LUMC Agreements, we, NYU and LUMC jointly developed antibodies against MuSK using our proprietary suite of technologies. Under the NYU and LUMC Agreements, as amended, we are obligated to make milestone payments upon the occurrence of certain development milestones, commercialization milestones and pay tiered royalties on net sales in the low single digits. 1.5 Manufacturing and Supply We utilize third-party contract manufacturers who act in accordance with the FDA’s current good manufacturing practices (cGMPs) for the manufacture of drug substance and drug product. We continue to build our global network of contract manufacturers to support the development and commercialization of our products. We work with Lonza teams based in Slough, UK, Portsmouth, U.S., Singapore and Visp, Switzerland for activities relating to the development of cell banks, development of our manufacturing processes and the manufacturing of drug substance, thereby using validated and scalable systems broadly accepted in our industry. In 2022, we started our collaboration with FUJIFILM Diosynth Biotechnologies Denmark ApS (Fujifilm) based in Hillerød, Denmark, for activities relating to the large-scale manufacturing of efgartigimod drug substance. We use additional contract manufacturers to fill, label, package, store and distribute (investigational) drug products. 1.6 Intellectual Property 1.6.1 Introduction We strive to protect and maintain exclusivity for the proprietary technologies that we believe are important to our business, patients, and shareholders. We continue to pursue and maintain patent protection intended to cover core platform technologies incorporated into, or used to produce, our product candidates and commercial products. We will seek protection for our innovations in key global jurisdictions. We continue to focus our exclusivity strategies on all aspects of our assets, including our compositions of matter, methods of use for our approved products, and other inventions that are important to our business (e.g., the patient innovations described in our product labels/product inserts and our core manufacturing technologies). Our intellectual property portfolio continues to grow and keep pace with the innovations arising from our discovery, development, and commercial efforts. We expect the total volume of patent positions under our management to increase each year as our pipeline evolves. We currently oversee more than 500 pending applications and granted patents. More importantly, as we continue to innovate for patients, we will work to protect our patient innovations with new intellectual property filings to enable future reinvestment for patients. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Collaborations and licenses 56

In addition to patent protection, we rely on trademarks and trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection, including certain aspects of our llama immunization and antibody affinity maturation approaches. Our commercial success depends in part upon our ability to obtain and maintain exclusivity, including regulatory exclusivities, patent, and other proprietary protection for commercially important technologies, inventions and know-how related to our business. We will defend and enforce our intellectual property rights, particularly our patent rights, and preserve the confidentiality of our trade secrets while operating without infringing valid and enforceable intellectual property rights of others. Specifically, we are materially dependent on regulatory, patent and other proprietary protection related to our core platform technologies, described in Section 1.6.2 “Platform Technologies” below and our product candidates, as described in Section 1.6.3 “Our Internal Programs” below and Section 1.6.4 “Our Partnered Programs” below. The patent positions for biotechnology companies like us are generally uncertain and can involve complex legal, scientific, and factual issues. In addition, the coverage recited in the claims in a patent application can be significantly reduced before a patent is issued, and claim scope can be reinterpreted and even challenged after issuance. As a result, we cannot guarantee that any of our platform technologies and product candidates, or products will be protectable or remain protected by enforceable patents. We cannot predict whether pending patent applications will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient protection from competitors. Any patents we hold may be challenged, circumvented, or invalidated by third parties. The term of individual patents depends on the patent laws in the countries in which they are obtained. In most countries, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the U.S., the term of a patent covering an FDA-approved drug may be eligible for a limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984 (Hatch-Waxman Act) as compensation for the loss of patent term during the FDA regulatory review process as described in Section 1.7.1 “Licensure and Regulation of Biologics in the U.S” below. Similar provisions are available in the EU and in other jurisdictions to extend the term of a patent that covers an approved drug or its use. It is possible that issued U.S. patents covering each of our products/product candidates may be entitled to patent term extensions. If our product candidates receive FDA approval, we intend to apply for patent term extensions, if available, to extend the term of patents that cover the approved product candidates and/or their uses. We also intend to seek patent term extensions in any jurisdictions where available. There is no guarantee that the applicable authorities, including the FDA, will agree with our assessment of whether such extensions should be granted, and if granted, the length of such extensions. 1.6.2 Platform Technologies With regard to our platform technologies, we own or control intellectual property rights directed to our SIMPLE ANTIBODY™ discovery platform, the ABDEG™ and NHANCE™ technologies. With regard to our SIMPLE ANTIBODY™ discovery platform, we have a broad patent portfolio providing exclusivity on the SIMPLE ANTIBODY™ platform. We expect to enjoy exclusivity under this patent portfolio until between 2029 and 2033. With regard to the ABDEG™ platform, we co-own the technology with the University of Texas Southwestern Medical Center and enjoy certain exclusive license rights. We have a broad patent portfolio covering the composition of matter and uses of certain FcRn antagonists to achieve certain biological effects. The composition of matter and other relevant patents in the U.S. expire in 2036 whereas in many other countries the base expiry date is 2034. With regard to the NHANCE™ platform, we exclusively licensed two U.S. patents from the University of Texas Southwestern Medical Center with composition of matter claims directed to an IgG molecule comprising a variant human Fc domain, and method of use claims directed to a method of blocking FcRn function in a subject by providing to the subject such an IgG molecule. The U.S. patents are expected to expire earliest in 2027 to 2028. The patent family also includes a granted European patent. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Intellectual Property 57

1.6.3 Our Internal Programs efgartigimod efgartigimod incorporates the ABDEG™ platform technology. We anticipate several more patient innovations to evolve during development for which we will seek additional patent protection. Our ARGX-109 Product Candidate With regard to our wholly-owned ARGX-109 product candidate, we have one patent family with composition of matter claims directed to ARGX-109. The patent family has a base expiry date in 2033. We anticipate several more patient innovations to evolve during development for which we will seek additional patent protection. Furthermore, ARGX-109 incorporates or employs the SIMPLE ANTIBODY™ platform technology and the NHANCE™ platform technology. empasiprubart Product Candidate With regard to the empasiprubart product candidate, we own or have rights to multiple patent families (including one in-licensed patent family from Broteio) with several granted patents and pending patent applications in multiple jurisdictions in North America, South America, the EU and Asia, directed to composition of matter claims and method of treatment claims. The in-licensed patent family from Broteio has granted patents in several countries/regions and has a base expiry date in 2034. Additional patent families include granted patents with base expiry dates in 2039 and 2040. We anticipate several more patient innovations to evolve during development for which we will seek additional patent protection. empasiprubart product candidate incorporates or employs the NHANCE™ platform technology. Our ARGX-119 Product Candidate With regard to the ARGX-119 product candidate, we in-licensed patent families from/with NYU Langone Health, a U.S. medical center based in New York, and additional patent families from/with the LUMC, with a U.S. granted patent and several pending applications in multiple jurisdictions. We anticipate several more patient innovations to evolve during development for which we will seek additional patent protection. Our ARGX-118 Product Candidate With regard to the ARGX-118 product candidate, we co-own a patent portfolio with VIB, an inflammation research center in Ghent, Brussels, and Ghent University, with one U.S. granted patent and pending patent applications in multiple jurisdictions in North America, South America, the EU and Asia. The patent family has a base expiry date in 2039. 1.6.4 Our Partnered Programs Our cusatuzumab (ARGX-110) Product Candidate With regard to the cusatuzumab product candidate, we have a broad patent portfolio that include claims to the composition of matter, uses of the molecule, and other important inventions. The issued U.S. patents expire in 2032 and 2033, without taking a potential patent term extension into account. cusatuzumab incorporates or employs the SIMPLE ANTIBODY™ and POTELLIGENT® platform technologies. Our ARGX-115 (ABBV-151) Product Candidate With regard to the ARGX-115 (ABBV-151) product candidate that we co-own with, and exclusively license from, the Ludwig Institute for Cancer Research and UCL, we have a patent portfolio that includes a U.S. patent with a base expiry date in 2034, without taking a potential patent term extension into account. There is a second family with meaningful patent coverage to the composition of matter and epitope claims that are expected to expire in 2036 and 2038. Furthermore, ARGX-115 (ABBV-151) incorporates or employs the SIMPLE ANTIBODY™ platform technology. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Intellectual Property 58

Our ARGX-112 (LP-0145) Product Candidate With regard to the ARGX-112 (LP-0145) product candidate, we have one patent family with composition of matter claims directed to an antibody that binds human IL-22R. The patent family has a base expiry date in 2037. Furthermore, ARGX-112 (LP-0145) incorporates the SIMPLE ANTIBODY™ platform technology. 1.6.5 Trade Secret Protection In addition to patent protection, we rely on trade secret protection to ensure exclusivity for our proprietary information that is not amenable to, or that we do not consider appropriate for, patent protection, including, for example, certain aspects of our llama immunization and antibody affinity maturation approaches. However, trade secrets can be difficult to protect. Although we take steps to protect our proprietary information, including restricting access to our premises and our confidential information, as well as entering into agreements with our employees, consultants, advisors and potential collaborators, third parties may independently develop the same or similar proprietary information or may otherwise gain access to our proprietary information. As a result, we may be unable to meaningfully protect our trade secrets and proprietary information. 1.7 Regulation Government authorities in the U.S., at the federal, state and local level, and in the EU and its Member States, and other countries and jurisdictions, extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of pharmaceutical products, including biological products. In addition, many countries and jurisdictions regulate the pricing of pharmaceutical products. The processes for obtaining marketing approvals in the U.S. and in other countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial personnel and financial resources, and breach of which can result in enforcement activity under civil, administrative and / or criminal law. 1.7.1 Licensure and Regulation of Biologics in the U.S. In the U.S., biological products used for the prevention, treatment, or cure of a disease or condition in a human being are subject to regulation under the U.S. Federal Food, Drug, and Cosmetic Act (FDCA) and its implementing regulations. Biologics are approved for marketing under provisions of the Public Health Service Act (PHSA) via biologics license applications (BLAs). An applicant seeking approval to market and distribute a new biologic in the U.S. generally must satisfactorily complete each of the following steps: • preclinical laboratory tests, animal studies and formulation studies all performed in accordance with applicable requirements, including the GLPs; • submission to the FDA of an IND application for human clinical testing, which contains results of the preclinical tests, together with manufacturing information and analytical data and must become effective before human clinical trials may begin; • approval by an institutional review board (IRB) representing each clinical site before each clinical trial may be initiated; • performance of adequate and well-controlled human clinical trials to establish the safety, potency and purity of the product candidate for each proposed indication, in accordance with good clinical practices (GCPs); • preparation and submission to the FDA of a BLA for a biological product requesting marketing for one or more proposed indications, including submission of detailed information on the manufacture and composition of the product in clinical development and proposed labeling; argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Intellectual Property 59

• one or more FDA inspections of the manufacturing facility or facilities, including those of third parties, at which the product, or components thereof, are produced to assess compliance with cGMP requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, potency, quality and purity; • FDA inspections of the clinical trial sites and/or sponsor to assure compliance with GCPs, and the integrity of clinical data in support of the BLA; • payment of user fees and securing FDA approval of the BLA and licensure of the new biological product; and • compliance with any post-approval requirements, including the potential requirement to implement a risk evaluation and mitigation strategy (REMS) and any post-approval studies required by the FDA. Human Clinical Trials in Support of a BLA Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. Additional clinical trials may be required after approval. • Phase 1 clinical trials are initially conducted in a limited population to test the product candidate for safety, including adverse effects, dose tolerance, absorption, metabolism, distribution, excretion and PD in healthy humans or, in patients. • Phase 2 clinical trials are generally conducted in a limited patient population to identify possible adverse effects and safety risks, evaluate the efficacy of the product candidate for specific targeted indications and determine dose tolerance and optimal dosage. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger Phase 3 clinical trials. • Phase 3 clinical trials are undertaken within an expanded patient population to gather additional information about safety and effectiveness necessary to evaluate the overall benefit-risk relationship of the drug and to provide an adequate basis for physician labeling. A sponsor who wishes to conduct a clinical trial outside the U.S. may, but is not required to, obtain FDA clearance to conduct the clinical trial under an effective IND. If a foreign clinical trial is not conducted under an IND, the sponsor may submit data from the clinical trial to the FDA in support of the BLA so long as the clinical trial is well-designed and well-conducted in accordance with GCPs, including review and approval by an independent ethics committee, and the FDA is able to validate the clinical trial data through an onsite inspection, if necessary. In some cases, the FDA may approve a BLA for a product candidate but require the sponsor, or the sponsor may otherwise choose, to conduct additional clinical trials to further assess, amongst other things, the product candidate’s safety and effectiveness after approval. Such post-approval clinical trials are typically referred to as Phase 4 clinical trials. Failure to exhibit due diligence with regard to conducting required Phase 4 clinical trials could result in FDA enforcement, including withdrawal of approval for products. Review and Approval of a BLA The results of product candidate development, preclinical testing and clinical trials, including negative or ambiguous results as well as positive findings, are submitted to the FDA as part of a BLA requesting a license to market the product. The BLA also must contain extensive manufacturing information and detailed information on the composition of the product and proposed labeling as well as payment of a user fee, unless exempt. The FDA has 60 days after submission of the application to conduct an initial review to determine whether the BLA is sufficient to file based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. If the FDA determines the BLA is not sufficiently complete, it will refuse to file the BLA. Once the submission has been filed, the FDA begins an in-depth review of the application. Under the goals agreed to by the FDA under the PDUFA, the FDA has 10 months from the filing date in which to complete its initial review of a standard application and respond to the applicant, and six months from the filing date for an application granted priority review. The FDA does not always meet its PDUFA goal dates and they may be extended in certain circumstances. After the FDA’s evaluation of the application and accompanying information, including the results of any necessary inspections, the FDA will issue an approval letter, or a complete response letter. An approval argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 60

letter authorizes commercial marketing of the product with specific prescribing information for specific indications. Under the PHSA, the FDA may approve a BLA if it determines that the product is safe, pure and potent and the facility where the product will be manufactured meets standards designed to ensure that it continues to be safe, pure and potent. If the application is not approved, the FDA will issue a complete response letter, which will identify the deficiencies in the application. Sponsors that receive a complete response letter may resubmit to the FDA information addressing the issues identified by the FDA, withdraw the application, or request a hearing. Even if a BLA is resubmitted with data and information addressing the deficiencies, the FDA may decide that the BLA does not satisfy the criteria for approval. The FDA may also refer the application to an advisory committee, consisting of independent experts, for review, evaluation and recommendation as to whether the application should be approved, particularly when applications present difficult or novel questions of safety or efficacy. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. If the FDA approves a new product, it may require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, including REMS, to help ensure that the benefits of the product outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and/or elements to assure safe use. This can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patent registries. The FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs. After approval, many types of changes to the approved product, such as adding new indications, certain manufacturing changes, and additional labeling claims, are subject to further testing requirements and FDA review and approval. Expedited Development and Review Programs The FDA is authorized to designate products meeting certain criteria for expedited development and review programs. Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification, or the time period for FDA review or approval may not be shortened. The FDA may designate a product for fast track review if it is intended, whether alone or in combination with one or more other products, for the treatment of a serious or life-threatening disease or condition, and demonstrates the potential to address unmet medical needs for such a disease or condition. For fast track products, sponsors may have more frequent interactions with the FDA and the FDA may initiate review of sections of a fast track product’s application before the application is complete (rolling review). The sponsor must also provide, and the FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees. However, the FDA’s PDUFA clock for a rolling review application does not begin until the last section of the application is submitted. A product may be designated as a breakthrough therapy if it is intended, either alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to breakthrough therapies, including holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross-disciplinary project lead for the review team; and taking other steps to design the clinical trials in an efficient manner. Breakthrough therapy designation also comes with all of the benefits of fast-track designation. The FDA may designate a product for priority review if it is a product that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines, on a case-by-case basis, whether the proposed product represents a significant improvement when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 61

product reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes, or evidence of safety and effectiveness in a new subpopulation. A priority designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s goal for taking action on a marketing application from 10 months to six months after accepting the application for filing. The FDA may grant accelerated approval to a product for a serious or life-threatening condition that provides meaningful therapeutic advantage to patients over existing treatments based upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or on a clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality (IMM) and that is reasonably likely to predict an effect on IMM or other clinical benefit (intermediate clinical endpoint), taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. Products granted accelerated approval must meet the same statutory standards for safety and effectiveness as those granted traditional approval. For the purposes of accelerated approval, a surrogate endpoint is a marker, such as a laboratory measurement, radio-graphic image, physical sign, or other measure that is thought to predict clinical benefit but is not itself a measure of clinical benefit. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. An intermediate clinical endpoint is a measurement of a therapeutic effect that is considered reasonably likely to predict the clinical benefit of a product, such as an effect on IMM. The accelerated approval pathway is most often used in settings in which the course of a disease is long and an extended period of time is required to measure the intended clinical benefit of a product, even if the effect on the surrogate or intermediate clinical endpoint occurs rapidly. Thus, accelerated approval has been used extensively in the development and approval of products for treatment of a variety of cancers in which the goal of therapy is generally to improve survival or decrease morbidity and the duration of the typical disease course requires lengthy and sometimes large clinical trials to demonstrate a clinical or survival benefit. The accelerated approval pathway is usually contingent on a sponsor’s agreement to conduct, in a diligent manner, a post-approval confirmatory clinical trial or studies to verify and describe the product’s clinical benefit. These confirmatory clinical trials must be completed with due diligence, and the FDA may require that the confirmatory clinical trial be designed, initiated, and/or fully enrolled prior to, or within a certain period following, approval. The FDA must also specify the conditions of any required post-approval clinical trial. Sponsors are required to submit progress reports for required post-approval studies, and the failure to conduct with due diligence a required post-approval clinical trial, including a failure to meet any required conditions specified by the FDA, or to submit timely reports, are prohibited acts under the FDCA. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, would allow the FDA to withdraw the product from the market on an expedited basis. Unless otherwise informed by the FDA, all promotional materials for product candidates approved under accelerated approval are subject to prior review by the agency. Orphan Drug Designation and Exclusivity Orphan drug designation in the U.S. is designed to encourage sponsors to develop products intended for rare diseases or conditions. In the U.S., a rare disease or condition is statutorily defined as a condition that affects fewer than 200,000 individuals in the U.S. or that affects more than 200,000 individuals in the U.S. and for which there is no reasonable expectation that the cost of developing and making available the product for the disease or condition will be recovered from sales of the product in the U.S. An application for designation as an orphan product can be made any time prior to the filing of an application for approval to market the product. If the FDA grants orphan drug designation, the generic identity of the product and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation qualifies a company for tax credits. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process. If a product that has orphan drug designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other application to market the same drug for the same indication for seven years from the date of such approval, except in limited circumstances, such as a argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 62

showing of clinical superiority to the product with orphan exclusivity by means of greater effectiveness, greater safety or providing a major contribution to patient care, or if the holder of the orphan exclusivity is unable to supply the market. Competitors, however, may receive approval of either a different product for the same indication or the same product for a different indication, which could be used off-label in the orphan indication. Orphan drug exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval before we do for the same product, as defined by the FDA, for the same indication we are seeking approval, or if our product is determined to be contained within the scope of the approval of the competitor’s product for the same indication or disease. Post-Approval Regulation If regulatory approval for marketing of a product or new indication for an existing product is obtained, the sponsor will be required to comply with all post-approval regulatory requirements, including those that the FDA has imposed as part of the approval process. The sponsor will be required to report certain adverse reactions and production problems to the FDA, provide updated safety and efficacy information and comply with requirements concerning advertising and promotional labeling. Manufacturers and other parties involved in the drug supply chain for prescription drug and biological products must also comply with product tracking and tracing requirements and must notify the FDA of counterfeit, diverted, stolen and intentionally adulterated products or products that are otherwise unfit for distribution in the U.S. Manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMPs. Accordingly, the sponsor and its third-party manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs and other regulatory requirements. A biological product may also be subject to official lot release, meaning that the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official lot release, the manufacturer must submit samples of each lot, together with a release protocol showing a summary of the history of manufacture of the lot and the results of all of the manufacturer’s tests performed on the lot, to the FDA. The FDA may in addition perform certain confirmatory tests on lots of some products before releasing the lots for distribution. Finally, the FDA will conduct laboratory research related to the safety, purity, potency and effectiveness of biological products. Any distribution of biological products and samples must comply with the U.S. Prescription Drug Marketing Act and the PHSA. Once approval of a BLA is granted, the FDA may revoke or suspend the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. FDA also has authority to require post-market studies, in certain circumstances, on reduced effectiveness of a product and may require labeling changes related to new reduced effectiveness information. Other potential consequences for a failure to maintain regulatory compliance include, among other things: • restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market, or product recalls; • fines, untitled letters, or warning letters; • refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals; • product seizure or detention, or refusal to permit the import or export of products; or • injunctions or the imposition of civil or criminal penalties. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 63

Pediatric Studies and Exclusivity Under the Pediatric Research Equity Act of 2003, as amended (PREA), certain BLAs or supplements thereto must contain data that are adequate to assess the safety and effectiveness of the product for the claimed indications in all relevant pediatric sub-populations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. Sponsors must also submit an initial Pediatric Study Plan (PSP), within 60 days of an end-of-Phase 2 meeting or, if there is no such meeting, as early as practicable before the initiation of the Phase 3 or Phase 2/3 clinical trial. The initial PSP plans must contain an outline of the proposed pediatric clinical trial or studies the applicant plans to conduct, including clinical trial objectives and design, any deferral or waiver requests and other information required by regulation. The applicant and the FDA must agree upon a final plan. The FDA or the applicant may request an amendment to the plan at any time. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Unless otherwise required by regulation, PREA does not apply to a biologic for an indication for which orphan designation has been granted, except that PREA will apply to an original BLA for a new active ingredient that is orphan-designated if the biologic is a molecularly targeted cancer product intended for the treatment of an adult cancer and is directed at a molecular target that FDA determines to be substantially relevant to the growth or progression of a pediatric cancer. Pediatric exclusivity is another type of non-patent regulatory exclusivity in the U.S. and, if granted for a biologic, provides for the attachment of an additional six months of protection to the term of any existing regulatory exclusivity (i.e., reference product exclusivity and orphan drug exclusivity) that has at least 9 months left to expiration. This six-month exclusivity may be granted if a BLA sponsor submits reports of pediatric studies that fairly respond to a written request from the FDA for such studies, were conducted in accordance with commonly accepted scientific principles and protocols, and have been reported in accordance with filing requirements. Biosimilars and Exclusivity The Biologics Price Competition and Innovation Act (BPCIA) established a regulatory scheme authorizing the FDA to approve biosimilars and interchangeable biosimilars. Under the BPCIA, an applicant may submit an application for licensure of a biologic product that is “biosimilar to” or “interchangeable with” a previously approved biological product or “reference product.” For the FDA to approve a biosimilar product, it must find that the proposed biosimilar is highly similar to the reference product notwithstanding minor differences in clinically inactive components and that there are no clinically meaningful differences between the product and the reference product in terms of safety, purity, or potency. For the FDA to approve a biosimilar product as interchangeable with a reference product, the agency must find that the biosimilar product is biosimilar to the reference product and that it can be expected to produce the same clinical results as the reference product in any given patient, and (for products administered multiple times) that the biologic and the reference biologic may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic without such alternation or switch. Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date of approval of the reference product. The FDA may not approve a biosimilar product until 12 years from the date on which the reference product was approved. Even if a product is considered to be a reference product eligible for exclusivity, another company could market a competing version of that product if the FDA approves a full BLA for such product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. We note that patent positions may be available to preclude the introduction into commerce of such competing product independent of any FDA exclusivities. The BPCIA also created certain exclusivity periods for biosimilars approved as interchangeable products. Products deemed interchangeable by the FDA may be substituted by pharmacies as dictated by individual state law. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 64

U.S. Patent Term Restoration Depending upon the timing, duration, and specifics of FDA review and approval of our product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Act that permits restoration of the patent term of up to five years as compensation for patent term lost during the FDA regulatory review process. Patent-term restoration, however, cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date, and only those claims covering such approved product, a method for using it or a method for manufacturing it may be extended. The patent- term restoration period is generally one-half the time between the effective date of an IND and the submission date of a BLA plus the time between the submission date of a BLA and the approval of that application, except that the review period is reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved biologic is eligible for the extension and the application for the extension must be submitted within 60 days of approval from FDA and prior to the expiration of the patent. The U.S. Patent and Trademark Office (USPTO), in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we may apply for restoration of patent term for our currently owned or licensed patents to add patent life beyond the current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant BLA. 1.7.2 Regulation and Procedures Governing Approval of Medicinal Products in the European Union and the UK Similar to the U.S., the EU, and the UK comprehensively regulate, among other things, the development, manufacturing, placing on the market, advertising, distribution, import and export of medicinal products. Particularly, the placing on the market of a medicinal product for human use in the EU requires a marketing authorization (MA). Main Provisions governing medicinal products in the EU are Directive 2001/83/EC and Regulation (EC) No 726/2004 (each as amended). Regulation (EC) No 141/2000 and Regulation (EC) No. 847/2000 (each as amended) are also of particular relevance for orphan medicinal products. While directives need to be transposed into national law by member states of the EU (EU Member States) before they are applicable, regulations directly apply in the EU Member States once these have been enacted. The process governing approval of MA applications (MAA) for the placing on the market of medicinal products in the EU and the UK generally follows the same lines as in the U.S. It entails satisfactory completion of pharmaceutical development, pre-clinical trials and adequate and well-controlled clinical trials to establish the safety and efficacy of the medicinal product for each proposed indication. The EU also requires the submission to relevant competent authorities for clinical trials authorization and to the European Medicines Agency (EMA) or to competent authorities in EU member states and granting of such MA by the EU Commission or relevant national authorities before the medicinal product can be marketed and sold in the EU or the relevant EU Member States. The below mentioned principles and rules generally apply within the EEA, i.e., the EU including Iceland, Liechtenstein and Norway. Following the UK’s departure from the EU, a separate MA is required from the Medicines and Healthcare Products Regulatory Agency (the MHRA), the UK medicines regulator, in order to place medicinal products on the market in the Great Britain (England, Wales and Scotland), which has been extended to Northern Ireland following the Windsor Framework having taken effect (see below)). Under the recently introduced International Recognition Procedure (IRP), the MHRA may take into account decisions from the EMA (and certain other international regulators) when considering an application for an MA. In respect of Northern Ireland, the UK government and the EU have agreed to replace the Northern Ireland Protocol (pursuant to which the EU regulatory framework continued to apply to Northern Ireland) with the ‘Windsor Framework’. Under the Windsor Framework, the MHRA is responsible for approving all medicinal products destined for the entire UK market (including Northern Ireland), and the EMA no longer has any role in approving medicinal products destined for Northern Ireland. The medicines aspects of the Windsor Framework came into force on January 1, 2025. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 65

Clinical Trial Approval Both non-clinical and clinical data are generally required to support an MA for a medicinal product in the EU. Non-clinical investigations are performed to demonstrate the health or environmental safety of new biological substances. Non-clinical (pharmaco-toxicological) investigations must generally be conducted in compliance with the principles of good laboratory practice (GLP) as set forth in EU Directive 2004/10/EC (as amended). Clinical trials are comprehensively regulated under the Clinical Trials Regulation (EU) No 536/2014 (CTR), which entered into application on January 31, 2022, and (gradually) replaces the Clinical Trials Directive 2001/20/EC (CTD). By January 30, 2025, all still ongoing clinical trials under the CTD must be transitioned to the CTR. The CTR, aims to simplify and streamline the approval of clinical trials in the EU. As before, many of the legal obligations are on the so-called sponsor, which is defined as the individual, company, institution, or organization that takes responsibility for the initiation, for the management and for setting up the financing of a clinical trial. The sponsor must obtain an authorization from the competent authority in the EU Member State(s) in which the clinical trial will be conducted as well as an approval from the competent national ethics committee in accordance with relevant national legislation in each of the relevant member states, before the commencement of such clinical trial. The CTR also imposes requirements, among others, regarding the conduct of a clinical trial (which must be conducted in accordance with the protocol and good clinical practice to generate acceptable data for MA submission), safety reporting of adverse events and reactions, changes to clinical trials, protection and informed consent of clinical trial subjects. Clinical trials conducted outside the EEA must follow the principles set forth in EU legislation if their results are to be submitted in an application for an MA in the EU. Before its exit from the EU, the UK implemented the CTD into national law through the Medicines for Human Use (Clinical Trials) Regulations 2004 (as amended). The entry into application of the CTR took place after the UK’s departure from the EU, so it does not apply to Great Britain. The MHRA ran a consultation on reforms to the UK clinical trials legislation, the outcome of which was published in March 2023. New draft legislation was laid for consideration before the UK Parliament in mid-December 2024. The draft regulations include a 12-month implementation period. The UK’s new clinical trials regime is therefore expected to come into force in early 2026 or thereafter. Orphan Designation and Exclusivity Regulation (EC) No. 141/2000 and Regulation (EC) No. 847/2000 (each as amended) provide that a product can be designated as an orphan medicinal product by the EU Commission if its sponsor can establish: (i) that the product is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition, (ii) either (a) the prevalence of the condition is not more than five in ten thousand persons in the EU when the application is made, or (b) without incentives it is unlikely that the marketing of the product in the EU would generate sufficient return to justify the necessary investment in its development and (iii) there exists no satisfactory method of diagnosis, prevention, or treatment of the condition in question that has been authorized in the EU or, if such method exists, the product has to be of a significant benefit compared to products available for the condition. An orphan designation provides a number of benefits, including fee reductions and, regulatory assistance. If an MA is granted for an orphan medicinal product, this generally results in a ten-year period of market exclusivity for the approved orphan indication. It is, however, not possible to combine non-orphan and orphan indications within the same MA. Thus, for non-orphan indications treated with the same active pharmaceutical ingredient, a separate MA has to be sought. Alternatively, the orphan designation may be waived to allow for the addition of non-orphan indications to an existing MA. As a result, the approved medicinal product would no longer profit from the orphan designation’s benefits. During an orphan medicinal product’s market exclusivity period, neither the EMA, the EU Commission nor the EU Member States can accept an application or grant an MA for a “similar medicinal product.” A “similar medicinal product”, i.e., a medicinal product containing a similar active substance or substances as contained in an authorized orphan medicinal product, and which is intended for the same therapeutic indication. The market exclusivity period for the authorized therapeutic indication may, however, be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 66

criteria for orphan designation. For orphan medicinal products intended for pediatric use, the market exclusivity period may be prolonged by additional two years if they are authorized with a pediatric indication based on the results from studies conducted under an EMA-approved pediatric investigation plan or if they are authorized without a pediatric indication but the results of the studies conducted under the EMA-approved pediatric investigation plan are reflected in the summary of product characteristic and, if appropriate, in the package leaflet. Market exclusivity may also be revoked in very select cases, such as if (i) it is established that a similar medicinal product is safer, more effective or otherwise clinically superior; (ii) the MA holder (MAH) for the authorized orphan medicinal product consents to the second orphan application; or (iii) the MA holder for the authorized orphan medicinal product cannot supply sufficient quantities. Orphan designation must be requested before submitting an MAA and is reconfirmed during the MAA process. Orphan designation does not convey any advantage in, or shorten the duration of, the regulatory review and MA approval process. Since January 1, 2021, a separate process for orphan designation has applied in Great Britain. There is no pre-marketing authorization orphan designation (as there is in the EU) and the application for orphan designation is reviewed by the MHRA, at the time of an MAA for a UK or Great Britain marketing authorization. Until January 1, 2025, a UK-wide orphan MAA could only be considered in the absence of an active EU orphan designation. From January 1, 2025, MAs granted for products that fulfil UK orphan criteria are valid UK-wide (including in Northern Ireland), regardless of whether there is an EU orphan designation or EU authorization as an orphan medicinal product. The criteria are the same as in the EU and, following implementation of the Windsor Framework from January 1, 2025, apply to the whole of the UK. The criteria are that: the medicine must be intended for the treatment, prevention or diagnosis of life-threatening or chronically debilitating diseases; the prevalence of the condition must be no more than five in 10,000 persons in the UK or it must be unlikely that the medicine’s marketing would generate sufficient returns to justify the investment needed for its development; and there must be no satisfactory method of diagnosis, prevention or treatment of the condition concerned in the UK, or if such method exists the medicine must be of significant benefit to those affected by the condition. Marketing Authorization To obtain an MA for a medicinal product under the EU regulatory framework, an applicant must submit an MAA, either to the EMA using the centralized procedure or to competent authorities in the EU Member States using the other procedures (decentralized procedure, national procedure, or mutual recognition procedure). An MA may be granted only to an applicant established in the EU. Regulation (EC) No. 1901/2006 provides that prior to obtaining an MA in the EU, an applicant must demonstrate compliance with all measures included in an EMA-approved pediatric investigation plan, covering all subsets of the pediatric population, unless the EMA has granted a product-specific waiver, class waiver, or a deferral for one or more of the measures included in the pediatric investigation plan. The centralized procedure provides for the grant of a single MA by the EU Commission that is valid for all EEA Member States. Pursuant to Regulation (EC) No. 726/2004 (as amended), the centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy medicinal products (gene therapy, somatic cell therapy or tissue engineered products) and products with a new active substance indicated for the treatment of certain diseases, including products for the treatment of cancer and auto-immune diseases and other immune dysfunctions and neurodegenerative disorders. The centralized procedure is optional for certain other medicinal products. Under the centralized procedure, the EMA’s Committee for Medicinal Products for Human Use (CHMP) is responsible for conducting the assessment of a product to define its risk/benefit profile. The CHMP recommendation is then sent to the EU Commission, which adopts a decision binding in all EEA Member States. Under the centralized procedure, the maximum timeframe for the evaluation of an MA application is 210 days, excluding clock stops when additional information or written or oral explanation is to be provided by the applicant in response to questions asked by the CHMP, which can considerably extend the 210 days. Accelerated evaluation (150 days excluding clock stops) may be granted by the CHMP in exceptional cases, when a medicinal product is of major interest from the point of view of public health and, in particular, from the viewpoint of therapeutic innovation. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 67

MAs have an initial validity for five years, in principle, and they may be renewed after five years on the basis of a reevaluation of the risk benefit balance by the EMA, or by the competent authority of the EU Member State. Once renewed, the MA is valid for an unlimited period, unless the EU Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five- year renewal period. Any MA that is not followed by the placement of the medicinal product on the EU market or on the market of the authorizing EU Member State(s) within three years after authorization, or if the drug is removed from the market for three consecutive years, ceases to be valid. In Great Britain, centrally authorized products converted from EU to UK marketing authorizations will have the same renewal date. Following the departure of the UK from the EU, the UK is no longer covered by European centralized marketing authorizations issued by the EMA. As of January 1, 2025, the MHRA regulates medicines through UK-wide MAs and EU centralized MAs are not valid anywhere in the UK. Instead, medicines that were previously within scope of the EU centralized procedure are authorized by the MHRA under UK-wide MAs. European Data and Market Exclusivity In the EU, innovative medicinal products, approved on the basis of a complete independent data package, qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity (for the more comprehensive protections applying to orphan medicinal products, please refer to Section 1.8.2 “Orphan Designation and Exclusivity” above). The data exclusivity, if granted, prevents generic or biosimilar applicants from referencing the innovator’s preclinical and clinical trial data contained in the dossier of the reference product when applying for a generic or biosimilar MA in the EU, for a period of eight years from the date on which the reference product was first authorized in the EU. During the additional two-year period of market exclusivity, a generic or biosimilar MAA can be submitted, and the innovator’s data may be referenced, but no generic or biosimilar product can be marketed in the EU until the expiration of the market exclusivity period. The overall ten-year period will be extended to a maximum of 11 years if, during the first eight years of those 10 years, the MA holder obtains an MA for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are determined to bring a significant clinical benefit in comparison with currently approved therapies. There is no guarantee that a product will be considered by the EMA to be an innovative medicinal product, and products may not qualify for data exclusivity. Even if a product is considered to be an innovative medicinal product so that the innovator gains the prescribed period of data exclusivity, another company nevertheless could also market another version of the product if such company obtained an MA based on an MAA with a complete independent data package of pharmaceutical tests, preclinical tests and clinical trials. Similar arrangements apply in the UK. Regulatory Requirements after Marketing Authorization Following MA approval, the MA holder is required to comply with a range of requirements applicable to the manufacturing, marketing, promotion and sale of the medicinal product. These include compliance with the EU’s stringent pharmacovigilance or safety reporting rules under Directive 2001/83/EC and Regulation (EU) 726/2004 (each as amended) and the associated guideline on good pharmacovigilance practices (as amended), pursuant to which post-authorization studies and additional monitoring obligations can be imposed. In addition, the manufacturing of authorized medicinal products, for which a separate manufacturer’s license is mandatory, must also be conducted in strict compliance with the principles of good manufacturing practice (GMP) set forth in Commission Directive 2017/1572 GMP and comparable requirements of other regulatory bodies in the EU, which mandate the methods, facilities and controls used in manufacturing, processing and packing of products to assure their safety and identity. Further, the wholesale distribution of authorized medicinal products requires a separate distribution license and must be conducted in strict compliance with good distribution practice standards. Finally, the marketing and promotion of authorized medicinal products is strictly regulated under Directive 2001/83/EC, (as amended) and as transposed into national laws. Potential consequences for a failure to maintain regulatory compliance mainly depend on the relevant regulations in the EU Member States, but are, for example, in Germany, similar to those in the U.S. Please refer to Section 1.7.1. “Post-Approval Regulations” above. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 68

Proposal for new EU Pharmaceutical Legislation On April 26, 2023, the EU Commission has published a proposal for a new directive (COM/2023/192 final) and a new regulation (COM/2023/193 final), which would revise and replace the existing general pharmaceutical legislation, including e.g., Directive 2001/83/EC, as well as Regulations (EC) No. 726/2004, No. 141/2000, or No. 1901/2006 (EU Pharmaceutical Legislation). Proposed amendments include, among others, modifications to the orphan designation criteria as well as the introduction of a modulated framework for orphan market exclusivity. Regarding the latter, the regulation proposal envisages a shift to a staggered approach. Those orphan medicinal products that address a high unmet medical need shall still benefit from a market exclusivity period of ten years. Well-established use orphan medicinal products will have a five-year market exclusivity period. Nine years of market exclusivity shall apply for all other orphan medicinal products. In certain cases, exclusivity periods may be prolonged (e.g., obtaining of an MA for one or more new therapeutic indications). Other key points of the proposed new EU Pharmaceutical Legislation include new measures to prevent and mitigate medicine shortages, to simplify the market entry of generics and biosimilars and the introduction of a new data protection regime for medicinal products. The proposal remains to be agreed and adopted by the European Parliament and European Council and may therefore be substantially revised before adoption, which is not anticipated before early 2026. Brexit and the Regulatory Framework in the UK On January 31, 2020, the UK officially ceased being a Member State of the EU (Brexit). For a period thereafter, immediate arrangements applied governing pharmaceutical legislation in the UK. However, as from January 1, 2025, following the implementation of the Windsor Framework, the MHRA is now the only authority approving medicines for the UK market. The Windsor Framework replaced the Northern Ireland Protocol, under which the EU regulatory framework continued to apply in Northern Ireland, and made the following key regulatory changes for medicines: (i) removed EU licensing processes in relation to Northern Ireland for novel medicines; (ii) removed any requirement for EU Falsified Medicines Directive packaging, labelling and serialization barcode for medicines in Northern Ireland; and (iii) required all medicines placed on the UK market to be labelled ‘UK Only’, indicating they are not for sale in the Republic of Ireland or other EU countries. More broadly, with the exception of the CTR, the UK and the EU’s regimes for the marketing, promotion and sale of medicinal products remain aligned, as the UK’s Human Medicines Regulations 2012 (as amended) implemented prior EU legislation on these topics before Brexit, and remain in force post- Brexit. However, these regulatory regimes may diverge increasingly in future, now that the UK’s regulatory system is formally independent from the EU. 1.7.3 Regulation and Procedures Governing Approval of Medicinal Products in Japan In order to market any medical products in Japan, a company must comply with numerous and varying regulatory requirements regarding quality, safety and efficacy in the context, among other things, of clinical trials, marketing approval, commercial sales and distribution of products. A person who manufactures or markets medical products in Japan is subject to the supervision of the Ministry of Health, Labour and Welfare (MHLW), primarily under the Act on Securing Quality, Efficacy and Safety of Pharmaceuticals and Medical Devices (Pharmaceutical and Medical Devices Act). This entails the satisfactory completion of pharmaceutical development, preclinical studies and adequate and well-controlled clinical trials to establish the safety and efficacy of the medical product for each proposed indication. It also requires the filing of a notification of clinical trials with the Pharmaceuticals and Medical Devices Agency (Japan) (PMDA) and the obtaining of marketing approval from the relevant authorities before the product can be marketed and sold in the Japanese market. Business License Under the Pharmaceutical and Medical Devices Act, a company or individual must obtain a Marketing Authorization Holder (MAH) license from the MHLW to engage in the marketing or provision of medical products. This requirement applies to medical products that are either manufactured by the company itself outsourced to a third party for manufacturing or imported. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 69

To manufacture medical products for the Japanese market, a company must obtain a manufacturing license from the MHLW for each production facility. This license is separate from the marketing authorization and is required for both domestic and foreign manufacturing sites. Marketing Approval Under the Pharmaceutical and Medical Devices Act, it is generally required to obtain marketing approval from the MHLW for the marketing of each medical product. An application for marketing approval must be made through the PMDA, which implements a marketing approval review. Clinical Trial Under the Pharmaceutical and Medical Devices Act, it is required to file notification of clinical trials with the PMDA. The data of clinical trials and other pertinent data, which must be attached to an application for marketing approval, must be obtained in compliance with the standards established by the MHLW, such as GLPs and GCPs stipulated by the ministerial ordinances of the MHLW. Regulatory Requirements after Marketing Approval A MAH that has obtained marketing approval for a new pharmaceutical is subject to re-examination by the PMDA for a specified period after receiving marketing approval. Such re-examination period for VYVGART is stated to be 10 years after the marketing approval in January 2022. The purpose of this re-examination process is to ensure the safety and efficacy of a newly approved pharmaceutical by imposing on the MAH the obligation to gather clinical data for a certain period after the marketing approval was granted to enable the PMDA to re-examine the product. Results of use and other pertinent data must be attached to an application for a re-examination. An MAH that has obtained a marketing approval is also required to investigate, among other things, the results of use and to periodically report to the PMDA pursuant to the Pharmaceutical and Medical Devices Act. Price Regulation Japan's public medical insurance systems cover virtually the entire Japanese population. The public medical insurance system, however, does not cover any medical product which is not listed on the National Health Insurance (NHI) price list published by the Minister of the MHLW. Accordingly, an MAH of medical products must first have a new medical product listed on the NHI price list to obtain coverage under the public medical insurance system. VYVGART was listed on the NHI price list in April 2022 and the price was adjusted in February 2024. VYVDURA was listed in April 2024. The NHI price of a medical product is determined either by price comparison of comparable medical products with necessary adjustments for innovation, usefulness or size of the market; or, in the absence of comparable medical products, by the cost calculation method, determined after considering of the opinion of the manufacturer. Prices on the NHI price list are subject to revision, generally once every year, based on the actual prices at which the medical products are purchased by medical institutions. 1.7.4 Coverage, Pricing and Reimbursement Significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we may obtain regulatory approval. Even if our product candidates are approved for marketing, sales of such product candidates will depend, in part, on the extent to which third-party payors, including government health programs in the U.S. (such as Medicare and Medicaid), commercial health insurers, and managed care organizations, provide coverage and establish adequate reimbursement levels for such product candidates. Moreover, increasing efforts by governmental and third-party payors in the EU, the U.S. and other markets to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates. We expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 70

The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In the U.S. and markets in other countries, patients generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Adequate coverage and reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors is critical to new product acceptance. Patients are unlikely to use any product candidates we may develop unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of such product candidates. Factors payors consider in determining reimbursement are based on whether the product is (i) a covered benefit under its health plan; (ii) safe, effective and medically necessary; (iii) appropriate for the specific patient; (iv) cost-effective; and (v) neither experimental nor investigational. The Medicare and Medicaid programs increasingly are used as models for how private payors and other governmental payors develop their coverage and reimbursement policies for drugs and biologics. Some third-party payors may require pre-approval of coverage for new or innovative devices or drug therapies before they will reimburse healthcare providers who use such therapies. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our product candidates. No uniform policy for coverage and reimbursement for drug products exists among third-party payors in the U.S. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor including formulary tier placement and utilization management requirements (if any). As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. The position of a product on a formulary generally determines the co-payment that a patient will need to make to obtain the product and can strongly influence the adoption of a product by patients and physicians. Third-party payors may limit coverage to specific products on a formulary, which might not include all of the approved products for a particular indication. Additionally, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved or that cost- sharing will be acceptable for patients. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more of our products for which we or our collaborators receive marketing approval, less favorable coverage policies and reimbursement rates may be implemented in the future. Third-party payors are increasingly challenging the price and examining the medical necessity and cost- effectiveness of medical products and services and imposing controls to manage costs, especially drugs when an equivalent generic drug or a less expensive therapy is available. It is possible that a third-party payor may consider our product candidate and other therapies (in some cases even off-label treatments) as substitutable and only offer to reimburse patients for the less expensive product. Even if we show improved efficacy or improved convenience of administration with our product candidate, pricing of existing drugs may limit the amount we will be able to charge for our product candidate. These payors may deny or revoke the reimbursement status of a given drug product or establish prices for new or existing marketed products at levels that are too low to enable us to realize an appropriate return on our investment in product development. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our product candidates and may not be able to obtain a satisfactory financial return on products that we may develop. In Mainland China, VYVGART has been included in the NRDL for the treatment of adults with gMG who are AChR-AB+ after going through price negotiations with the National Healthcare Security Administration (NHSA) since January 2024, which means that the price of this drug can be (partly) reimbursed by the social security program of Mainland China for the treatment of this indication in accordance with relevant rules within certain period. According to the current regulations of Mainland China, if we want our products in addition to VYVGART to be included in the NRDL or want VYVGART to be included in the NRDL for the treatment of other indications, we will need to go through price negotiations with the NHSA, for which purpose we will likely need to significantly reduce their prices. Although the inclusion of our products in the NRDL may increase the demand for the relevant products, our potential revenue from the sales of these products may still decrease as a result of lower prices. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 71

Furthermore, rules and regulations regarding reimbursement change frequently, in some cases at short notice, and we believe that changes in these rules and regulations are likely. Outside the U.S., we will face challenges in ensuring obtaining adequate coverage and payment for any product candidates we may develop. Pricing of prescription pharmaceuticals is subject to governmental control in many countries. In order to secure coverage and reimbursement for any product that might be approved for sale, we have needed and may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, and the cost of these studies would be in addition to the costs required to obtain FDA or other comparable marketing approvals. Conducting such studies could be expensive, involve additional risk and result in delays in our commercialization efforts. Even after pharmacogenomic studies are conducted, product candidates may not be considered medically necessary or cost-effective. A decision by a third-party payor not to cover any product candidates we may develop could reduce physician utilization of such product candidates once approved and have a material adverse effect on our sales, results of operations and financial condition. Third-party reimbursement and coverage may not be adequate to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. The insurance coverage and reimbursement status of newly approved products for orphan diseases is particularly uncertain, and failure to obtain or maintain adequate coverage and reimbursement for any such product candidates could limit our ability to generate revenue. As noted above, in the U.S., we plan to have various programs to help patients afford our products, including patient assistance programs and co-pay coupon programs for eligible patients. More specifically, patients can enroll into MY VYVGART PATH™, a patient support program that provides personalized support from a nurse case manager and committed support team. In addition to providing support on questions on the treatment and on navigating the insurance process, the program provides a VYVGART Co-pay Program to eligible patients, aids in referring patients to charitable foundations that may be able to help with out-of- pocket costs and informs patients of financial assistance programs that may be available. The containment of healthcare costs also has become a priority of U.S. federal, state and international governments and the prices of pharmaceuticals have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot be sure that reimbursement will be available for any future product candidate that we commercialize and, if reimbursement is available, the level of reimbursement. In addition, many pharmaceutical manufacturers must calculate and report certain price reporting metrics to the government, such as average sales price and best price. Penalties may apply in some cases when such metrics are not submitted accurately and timely. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our potential revenue from the sale of any products for which we may obtain approval. The delivery of healthcare in the EU, including the establishment and operation of health services and the pricing and reimbursement of medicinal products, is almost exclusively a matter for national, rather than EU, provisions and regulations. National governments and health service providers have different priorities and approaches to the delivery of healthcare and the pricing and reimbursement of products in that context. Therefore, in the EU, pricing and reimbursement schemes vary widely from EU Member State to another. Some EU Member States provide that products may be marketed only after a reimbursement price has been agreed. Some EU Member States may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies (so called health technology assessments) in order to obtain reimbursement or pricing approval. EU Member States may approve a specific price for a product or may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. Other Member States allow companies to fix their own prices for products but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Recently, many EU Member States have increased the amount of discounts required on medicinal products and these efforts could continue as Member States attempt to further manage healthcare expenditures. For example, Germany recently introduced a specific discount on certain combination products with new active ingredients. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 72

The downward pressure on healthcare costs in general, particularly medicinal prescription products, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various EU Member States and parallel trade (arbitrage between low-priced and high-priced Member States) can further reduce prices. Special pricing and reimbursement rules may apply to orphan medicinal products. Inclusion of orphan drugs in reimbursement systems tend to focus on the medical usefulness, need, quality and economic benefits to patients and the healthcare system as for any drug. Acceptance of any medicinal product for reimbursement may come with cost, use and often volume restrictions, which again can vary by country. In addition, results-based rules of reimbursement may apply. There can be no assurance that any EU Member State that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products, if approved in those countries. Historically, products launched in the EU do not follow price structures of the U.S. and generally prices tend to be significantly lower. Outside the U.S., international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada and other countries has and will continue to put pressure on the pricing and usage of our product candidates. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medical products but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the U.S., the reimbursement for our products may be reduced compared with the U.S. and may be insufficient to generate commercially reasonable revenue and profits. 1.7.5 Government Pricing and Reimbursement Programs for Marketed Drugs in the U.S. Medicaid, the 340B Drug Pricing Program, and Medicare Federal law requires that a pharmaceutical manufacturer, as a condition of having its drug and biological products receive federal reimbursement under Medicaid and Medicare Part B, must pay rebates to state Medicaid programs for all units of its covered outpatient drugs dispensed to Medicaid beneficiaries and paid for by a state Medicaid program under either a fee-for-service arrangement or through a managed care organization. This federal requirement is effectuated through a Medicaid drug rebate agreement between the manufacturer and the Secretary of U.S. Department of Health and Human Services (HHS). The Centers for Medicare & Medicaid Services (CMS) administers the Medicaid drug rebate agreements, which provide, among other things, that the drug manufacturer will pay rebates to each state Medicaid agency on a quarterly basis and report certain price information on a monthly and quarterly basis. The rebates are based on prices reported to CMS by manufacturers for their covered outpatient drugs, including average manufacturer price (AMP) and best price. Effective January 1, 2024, the Medicaid total rebate amount is no longer capped at 100% of a covered outpatient drug’s AMP, which means that a manufacturer could pay a total rebate amount on a unit of the drug that is greater than the average price the manufacturer receives for the drug. The terms of participation in the Medicaid drug rebate program impose an obligation to correct the prices reported in previous quarters, as may be necessary. Any such corrections could result in additional or lesser rebate liability, depending on the direction of the correction. In addition to retroactive rebates, if a manufacturer were found to have knowingly submitted false information to the government, federal law provides for civil monetary penalties for failing to provide required information, late submission of required information, and false information. A manufacturer must also participate in a federal program known as the 340B drug pricing program in order for federal funds to be available to pay for the manufacturer’s drug and biological products under Medicaid and Medicare Part B. Under this program, the participating manufacturer agrees to charge certain safety net healthcare providers no more than an established discounted price for its covered outpatient drugs. The formula for determining the discounted price is defined by statute and is based on the AMP and the unit rebate amount as calculated under the Medicaid drug rebate program, discussed above. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 73

Manufacturers are required to report pricing information to the Health Resources and Services Administration on a quarterly basis. The Health Resources and Services Administration has also issued regulations relating to the calculation of the ceiling price as well as imposition of civil monetary penalties for each instance of knowingly and intentionally overcharging a 340B covered entity. Federal law also requires that manufacturers report data on a quarterly basis to CMS regarding the pricing of drugs that are separately reimbursable under Medicare Part B. These are generally drugs and biologics, such as injectable products, that are administered incident to a physician service and are not generally self- administered. The pricing information submitted by manufacturers is the basis for reimbursement to physicians and suppliers for drugs covered under Medicare Part B. Under the Inflation Reduction Act (IRA), manufacturers are also required to provide quarterly rebates for certain single-source drugs and biologics (including biosimilars) covered under Medicare Part B with prices that increase faster than the rate of inflation. This requirement started on January 1, 2023 for drugs approved on or before December 1, 2020 and begins six quarters after a drug is first marketed for all other drugs. As with the Medicaid drug rebate program, federal law provides for civil monetary penalties for failing to provide required information, late submission of required information, and false information. Additionally, the Infrastructure Investment and Jobs Act added a requirement, effective January 1, 2023, for manufacturers of certain single-source drugs (including biologics and biosimilars) separately paid for under Medicare Part B for at least 18 months and marketed in single-dose containers or packages (known as refundable single-dose containers or single-use package drugs) to provide annual refunds for any portions of the dispensed drug that are unused and discarded if those unused or discarded portions exceed an applicable percentage defined by statute or regulation. Manufacturers will be subject to periodic audits and those that fail to pay refunds for their refundable single-dose containers or single-use package drugs shall be subject to civil monetary penalties. Medicare Part D provides prescription drug benefits for seniors and people with disabilities. Beginning in 2025, the IRA eliminates the coverage gap phase and associated manufacturer discounts under Medicare Part D, significantly lowers the enrollee maximum out-of-pocket cost and establishes a new manufacturer discount program, which requires 10% discounts in the initial phase, and 20% discounts in the catastrophic phase. Although these discounts represent a lower percentage of enrollees’ costs than coverage gap discounts, the new manufacturer contribution during the catastrophic phase could be considerable for certain high-cost drugs and the total contributions by manufacturers to a Part D enrollee’s drug expenses may exceed those currently provided. The IRA also requires manufacturers to provide annual Medicare Part D rebates for single-source drugs and biological products with prices that increase faster than the rate of inflation. The IRA also allows HHS to directly negotiate the selling price of a statutorily specified number of drugs and biologics each year that CMS reimburses under Medicare Part B and Part D. Only high-expenditure single- source biologics that have been approved for at least 11 years (7 years for single-source drugs) can qualify for negotiation, with the negotiated price taking effect two years after the selection year. Negotiations for Medicare Part D products began in 2023 with the negotiated price taking effect in 2026, and negotiations for Medicare Part B products will begin in 2026 with the negotiated price taking effect in 2028. U.S. Federal Contracting and Pricing Requirements Manufacturers are also required to make their covered drugs, which are generally drugs approved under NDAs or BLAs, available to authorized users of the Federal Supply Schedule (FSS) of the General Services Administration. The law also requires manufacturers to offer deeply discounted FSS contract pricing for purchases of their covered drugs by the Department of Veterans Affairs, the Department of Defense, the Coast Guard, and the Public Health Service (including the Indian Health Service) in order for federal funding to be available for reimbursement or purchase of the manufacturer’s drugs under certain federal programs. FSS pricing to those four federal agencies for covered drugs must be no more than the Federal Ceiling Price (FCP), which is at least 24% below the Non-Federal Average Manufacturer Price (Non-FAMP) for the prior year. The Non-FAMP is the average price for covered drugs sold to wholesalers or other middlemen, net of any price reductions. The accuracy of a manufacturer’s reported Non-FAMPs, FCPs, or FSS contract prices may be audited by the government. Among the remedies available to the government for inaccuracies is recoupment of any overcharges to the four specified federal agencies based on those inaccuracies. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 74

If a manufacturer were found to have knowingly reported false prices, in addition to other penalties available to the government, the law provides for significant civil monetary penalties per incorrect item. Finally, manufacturers are required to disclose in FSS contract proposals all commercial pricing that is equal to or less than the proposed FSS pricing, and subsequent to award of an FSS contract, manufacturers are required to monitor certain commercial price reductions and extend commensurate price reductions to the government, under the terms of the FSS contract Price Reductions Clause. Among the remedies available to the government for any failure to properly disclose commercial pricing and/or to extend FSS contract price reductions is recoupment of any FSS overcharges that may result from such omissions. 1.7.6 Healthcare Law and Regulation Healthcare providers and third-party payors play a primary role in the recommendation and prescription of pharmaceutical products that are granted marketing approval. Our current and future arrangements with providers, researchers, consultants, third-party payors and customers are subject to broadly applicable federal and state fraud and abuse, anti-kickback, false claims, transparency and patient privacy laws and regulations and other healthcare laws and regulations that may constrain our business and/or financial arrangements. Restrictions under applicable federal and state healthcare laws and regulations include, without limitation, the following: • the U.S. federal Anti-Kickback Statute (AKS) prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering, or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, lease, order or recommendation of, any good facility, item, or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers formulary managers and other persons and entities on the other. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain activities from prosecution, the exceptions and safe harbors are drawn narrowly, and arrangements may be subject to scrutiny or penalty if they do not fully satisfy all elements of an available exception or safe harbor. A person or entity can be found guilty of violating the AKS without actual knowledge of the statute or specific intent to violate it. In addition, the government may assert that a claim including items or services resulting from a violation of the AKS constitutes a false or fraudulent claim for purposes of the federal False Claims Act or federal civil money penalties statute. Violations of the AKS carry potentially significant civil and criminal penalties, including imprisonment, fines, administrative civil monetary penalties, and exclusion from participation in federal healthcare programs. • the U.S. federal false claims and civil monetary penalties laws, including the civil False Claims Act and federal civil monetary penalty laws, which, among other things, impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the U.S. federal government, claims for payment or approval that are false or fraudulent, knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim or obligation to pay or transmit money to the federal government, or from knowingly making a false statement to avoid, decrease or conceal an obligation to pay money to the U.S. federal government. In addition, the government may assert that a claim including items and services resulting from a violation of the AKS constitutes a false or fraudulent claim for purposes of the False Claims Act. Manufacturers can be held liable under the False Claims Act even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. The False Claims Act also permits a private individual acting as a “whistleblower” to bring qui tam actions on behalf of the federal government alleging violations of the False Claims Act and to share in any monetary recovery. When an entity is determined to have violated the federal civil False Claims Act, the government may impose civil fines and penalties for each false claim, plus treble damages, and exclude the entity from participation in Medicare, Medicaid and other federal healthcare programs; argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 75

• the U.S. federal Health Insurance Portability and Accountability Act of 1996 (HIPAA) which imposes criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or obtaining by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the pay (e.g., public or private) or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters; similar to the AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; • HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (HITECH) and its implementing regulations, and as amended again by the Omnibus Rule in 2013, which imposes certain obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information without appropriate authorization by covered entities subject to the Final HIPAA Omnibus Rule, i.e., certain covered health plans, healthcare clearinghouses and healthcare providers, as well as their business associates, those independent contractors or agents of covered entities that perform certain services for or on their behalf involving the use or disclosure of individually identifiable health information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions; • the federal transparency requirements known as the federal Physician Payments Sunshine Act, under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the ACA), which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to CMS information related to payments and other transfers of value made by that entity to physicians (currently defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain non-physician providers such as physician assistants and nurse practitioners and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit required information may result in civil monetary penalties for all payments, transfers of value or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission; • federal government price reporting laws, which require us to calculate and report complex pricing metrics in an accurate and timely manner to government programs; • federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; • analogous state and local laws and regulations, including: state anti-kickback and false claims laws; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the U.S. federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state and local laws that require the licensure of sales representatives; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and pricing information; state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect; and state laws related to insurance fraud in the case of claims involving private insurers; and • EU, UK and other foreign law equivalents, including reporting requirements detailing interactions with and payments to healthcare providers and data privacy and security laws and regulations that may be more stringent than those in the U.S. State and foreign laws, including for example the EU General Data Protection Regulation (GDPR), also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. There are ambiguities as to what is required to comply with these state requirements and if we fail to comply with an applicable state law requirement, we could be subject to penalties. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 76

We have and will continue to spend substantial time and money to ensure that our business arrangements with third parties comply with applicable healthcare laws and regulations. Recent healthcare reform legislation has strengthened these federal and state healthcare laws. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Other laws that may affect our ability to operate include: • the anti-inducement law prohibits, among other things, the offering or giving of remuneration, which includes, without limitation, any transfer of items or services for free or for less than fair market value (with limited exceptions), to a Medicare or Medicaid beneficiary that the person know or should know is likely to influence the beneficiary’s selection of a particular supplier of items or services reimbursable by a federal or state governmental program; and • European and other foreign law equivalents of each of the laws, including reporting requirements detailing interactions with and payments to healthcare providers. In the U.S., to help patients afford our approved product, we may utilize programs to assist them, including patient assistance programs and co-pay coupon programs for eligible patients. Government enforcement agencies have shown increased interest in pharmaceutical companies’ product and patient assistance programs, including reimbursement support services, and a number of investigations into these programs have resulted in significant civil and criminal settlements. In addition, at least one insurer has directed its network pharmacies to no longer accept co-pay coupons for certain specialty drugs the insurer identified. Our co-pay coupon programs could become the target of similar insurer actions. In addition, in November 2013, the CMS issued guidance to the issuers of qualified health plans sold through the ACA’s marketplaces encouraging such plans to reject patient cost-sharing support from third parties and indicating that the CMS intends to monitor the provision of such support and may take regulatory action to limit it in the future. The CMS subsequently issued a rule requiring individual market qualified health plans to accept third-party premium and cost-sharing payments from certain government-related entities. In September 2014, the Office of the Inspector General of the HHS issued a Special Advisory Bulletin warning manufacturers that they may be subject to sanctions under the AKS and/or civil monetary penalty laws if they do not take appropriate steps to exclude Part D beneficiaries from using co-pay coupons. Accordingly, companies exclude these Part D beneficiaries from using co-pay coupons. Additionally, certain third-party payors are modifying benefit designs based on the availability of manufacturer cost-sharing assistance (e.g., copay accumulator or maximizer programs). Following a federal district court decision vacating the provisions of the 2021 Notice of Benefit and Payment Parameter final rule that provided health plans with discretion whether to include manufacturer assistance toward the cost-sharing limit, CMS stated its intent to address this issue in future rulemaking. It is possible that changes in insurer policies regarding co-pay coupons and/or the introduction and enactment of new legislation or regulatory action could restrict or otherwise negatively affect these patient support programs, which could result in fewer patients using affected products, and therefore could have a material adverse effect on our sales, business, and financial condition. Third-party patient assistance programs that receive financial support from companies have become the subject of enhanced government and regulatory scrutiny. The Office of the Inspector General of the HHS has established guidelines that suggest that it is lawful for pharmaceutical manufacturers to make donations to charitable organizations who provide co-pay assistance to Medicare patients, provided that such organizations, among other things, are bona fide charities, are entirely independent of and not controlled by the manufacturer, provide aid to applicants on a first-come basis according to consistent financial criteria and do not link aid to use of a donor’s product. However, donations to patient assistance programs have received some negative publicity and have been the subject of multiple government enforcement actions, related to allegations regarding their use to promote branded pharmaceutical products over other less costly alternatives. Specifically, in recent years, there have been multiple settlements resulting out of government claims challenging the legality of their patient assistance programs under a variety of federal and state laws. It is possible that we may make grants to independent charitable foundations that help financially needy patients with their premium, co-pay, and co-insurance obligations. If we choose to do so, and if we or our vendors or donation recipients are deemed to fail to comply with relevant laws, regulations or evolving government guidance in the operation of these programs, we could be subject to damages, fines, penalties, or other criminal, civil, or administrative sanctions or enforcement actions. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 77

We cannot ensure that our compliance controls, policies, and procedures will be sufficient to protect against acts of our employees, business partners, or vendors that may violate the laws or regulations of the jurisdictions in which we operate. Regardless of whether we have complied with the law, a government investigation could impact our business practices, harm our reputation, divert the attention of management, increase our expenses, and reduce the availability of foundation support for our patients who need assistance. Violations of these laws or any future enacted laws can subject us to criminal, civil and administrative sanctions including monetary penalties, damages, fines, disgorgement, individual imprisonment and exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, reputational harm, and we may be required to curtail or restructure our operations. Moreover, we expect that there will continue to be federal and state laws and regulations, proposed and implemented, that could impact our future operations and business. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Ensuring that our internal operations and future business arrangements with third parties comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations, agency guidance or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of the laws described above or any other governmental laws and regulations that may apply to us, we may be subject to significant penalties, including administrative, civil and criminal penalties, damages, fines, disgorgement, the exclusion from participation in federal and state healthcare programs, individual imprisonment, reputational harm, and the curtailment or restructuring of our operations, as well as additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws. Further, defending against any such actions can be costly and time-consuming, and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired. If any of the physicians or other providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs and imprisonment. If any of the above occur, our ability to operate our business and our results of operations could be adversely affected. 1.7.7 Healthcare Reform In the U.S., the EU and other foreign jurisdictions, there have been a number of legislative and regulatory changes to the healthcare systems that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs and improve the quality of healthcare. For example, the ACA, effective since March 2010, is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Healthcare reforms that have been adopted, and that may be adopted in the future, could result in further reductions in coverage and levels of reimbursement for pharmaceutical products, increases in rebates payable under U.S. government rebate programs and additional downward pressure on pharmaceutical product prices. As discussed above, in August 2022, the IRA was enacted codifying, among other things: a Medicare drug price negotiation program, under which HHS directly negotiates the selling price of statutorily specified number of Part B and Part D drugs and biologics each year; inflation rebates which penalizes drug manufacturers that increase prices of Medicare Part B and Part D drugs at a rate greater than the rate of inflation; and a redesign of the Part D benefit. The IRA permits the Secretary of HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. Manufacturers that fail to comply with the IRA may be subject to various penalties, including civil monetary penalties. The IRA also extends enhanced subsidies for individuals purchasing health insurance coverage in argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 78

ACA marketplaces through plan year 2025. These provisions will take began taking progressively starting in 2023, although certain policies have been subject to legal challenges. For example, the provisions related to the negotiation of selling prices of high-expenditure single-source drugs and biologics have been challenged in multiple lawsuits. Additionally, we cannot predict whether the U.S. Congress will amend the IRA or if the government will adopt new or different interpretations of the law in future guidance or rulemaking. However, at this time, the Trump administration is continuing to implement the IRA and to defend the law in litigation. While it is unclear how the IRA will be implemented in the future and the outcome of the litigation, it will likely have a significant impact on the pharmaceutical industry. We expect that additional U.S. federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures. Further, legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals, if any, of our product candidates, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post- marketing conditions and other requirements. Individual states in the U.S. have also become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, affordability review boards, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce the ultimate demand for our products or put pressure on our product pricing, which could negatively affect our business, results of operations, financial condition and prospects. In the EU, similar political, economic and regulatory developments may affect our ability to profitably commercialize our current or any future products. In addition to continuing pressure on prices and cost containment measures, legislative developments at the EU (such as the above-mentioned EU Pharmaceutical Legislation) or EU Member State level may result in significant additional requirements or obstacles that may increase our operating costs. In general the healthcare budgetary constraints in most EU Member States have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to commercialize any products for which we obtain marketing approval. In international markets, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the U.S. or abroad. If we or our collaborators are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we or our collaborators are not able to maintain regulatory compliance, our product candidates may lose any regulatory approval that may have been obtained and we may not sustain or achieve profitability in the future, which would adversely affect our business. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 79

1.7.8 Environmental Issues which may Influence the Use of our Material Fixed Assets Our primary research and development activities take place in our facilities in Zwijnaarde, Belgium. For these activities we require, and have obtained, the necessary environmental and biohazard permits from the responsible governments, required by us for the manner in which we use said facilities. 1.8 Documents on display We are subject to the information reporting requirements of the U.S. Securities Exchange Act of 1934, as amended (Exchange Act) applicable to foreign private issuers. Accordingly, we are required to file reports and other information with the SEC. Those reports may be inspected without charge at the locations described below. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. Nevertheless, we will file with the SEC an Annual Report containing financial statements that have been examined and reported on, with an opinion expressed by an independent registered public accounting firm. We maintain a corporate website at www.argenx.com. We make available on our website, free of charge, our Annual Report and the text of our reports on Form 6-K, including any amendments to these reports, as well as certain other SEC filings, as soon as reasonably practicable after they are electronically filed with or furnished to the SEC. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report. We have included our website address in this Annual Report solely as an inactive textual reference. The SEC maintains a website (www.sec.gov) that contains reports and other information regarding registrants, such as argenx SE, that file electronically with the SEC. With respect to references made in this Annual Report to any contract or other document of argenx SE, such references are not necessarily complete and you should refer to the exhibits attached or included elsewhere to this Annual Report for copies of the actual contract or document. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Regulation 80

Risk Factors 2.1 Summary Risk Factors 82 2.2 Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications 84 2.3 Risk Factors Related to the Development and Clinical Testing of argenx’s Products and Product Candidates 91 2.4 Risk Factors Related to argenx’s Dependence on Third Parties 94 2.5 Risk Factors Related to Other Government Regulations 98 2.6 Risk Factors Related to argenx’s Financial Position 103 2.7 Risk Factors Related to argenx’s Business and Industry 104 2.8 Risk Factors Related to argenx’s Intellectual Property 107 2.9 Risk Factors Related to argenx’s Organization and Operations 111 2.10 Risk Factors Related to the ADSs 114 2.11 Risk Factors Related to being a Foreign Private Issuer or a Dutch Company 116 argenx Annual Report 2024 81 2

2 Risk Factors Our business faces significant risks, including those described below. You should carefully consider all of the information set forth in this Annual Report and in our other filings with the SEC, including the following risk factors which we face and are faced by our industry. Our business, financial condition or results of operations could be materially and adversely affected if any of these risks occurs. These are not the only risks argenx faces. Additional risks and uncertainties not presently known to argenx or that it currently considers immaterial or not specific may also impair its business, results of operation and financial condition. This report also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially and adversely from those anticipated in these forward-looking statements as a result of certain factors including the risks described below and elsewhere in this Annual Report and our other SEC filings. See “Forward-Looking Statements”. 2.1 Summary Risk Factors Our ability to implement our business strategy is subject to numerous risks that you should be aware of before making an investment decision. These risks are described more fully below. These risks include, among others: • The commercial success of our products and product candidates, including in new indications or methods of administration, will depend on the degree of market acceptance. • We face significant competition for our drug discovery and development efforts. • We will face significant challenges in successfully commercializing our products and additional product candidates after they are launched. • Our products and product candidates for which we have obtained or intend to seek approval as biological products, including for new indications, may face biosimilar competition. • Enacted and future legislation could impact demand for our products which could impact our business and future results of operations. • We are subject to government pricing laws, regulation and enforcement. These laws affect the prices we may charge the government for our products and the reimbursement our customers may obtain from the government. Our failure to comply with these laws could harm our results, operations and/or financial conditions. • We may not obtain or maintain adequate pricing and coverage or reimbursement status for our products and product candidates. • If we fail to obtain orphan drug designation or we do not have valid and enforceable patents covering our products and their uses and product candidates and fail to obtain and/or maintain orphan drug exclusivity for our products or product candidates, our competitors may be able to sell products to treat the same conditions and our revenue may be reduced. • Failure to successfully identify, select and develop our products in other indications, or additional products or product candidates could impair our ability to grow. • Failure to successfully develop or obtain marketing approval for our products and product candidates could negatively impact our business. • Certain of our clinical trials have not succeeded, and may in the future also not succeed, and even if they succeed, we may not obtain regulatory approval for our products or product candidates or regulatory approval may be delayed. • If we decide to pursue accelerated approval for any of our product candidates, it may not lead to faster development or regulatory review or approval and we may still need to conduct additional clinical trials, which could increase the expense of obtaining, if at all, necessary marketing approvals. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Summary Risk Factors 82

• Our products and product candidates may have serious adverse, undesirable or unacceptable side effects, and we or others may identify undesirable or unacceptable side effects caused by any of our products or product candidates before and after they have received marketing approval. • If our target patient population is smaller than expected, we are unable to successfully enroll and retain patients in our clinical trials, or experience significant delays in doing so, we may not realize the full commercial potential of any products or product candidates. • We rely, and expect to continue to rely, on third parties to conduct some of our research activities and clinical trials and for parts of the development and commercialization of our existing and future research programs, products and product candidates. If our relationships with such third parties are not successful, our business may be adversely affected. • Disruptions caused by our reliance on third parties for our manufacturing process may delay or disrupt our business, product development and commercialization efforts. • Accuracy and timing of our financial reporting is partially dependent on information received from third- party partners, which we do not control. • We and our third-party manufacturers and suppliers may become exposed to liability, fines, penalties or other sanctions and substantial expenses in connection with environmental compliance or remediation activities. • We are subject to healthcare laws, regulation and potential enforcement. The failure to comply with these laws could harm our results, operations and/or financial conditions. • Our performance tracked by our Environmental, Social and Governance metrics is subject to risks and the outcomes may not achieve the anticipated benefits or align with new regulations and stakeholders’ expectations. • We expect to increase our expenses for the foreseeable future, and we may not be able to raise additional capital, be profitable or sustain net profitability in the future in order to fund our operations. • We may become exposed to costly and damaging liability claims and other litigation. • Our business and operations could suffer in the event of system failures or unauthorized or inappropriate use of or access to our systems. • We are highly dependent on public perception of our products. • We may be unable to adequately maintain, enforce or protect our intellectual property rights in products, product candidates and platform technologies which could adversely affect our ability to maximize the value for patients in our marketed products and product candidates. • Intellectual property litigation could lead to substantial resource diversion or issued patents could be found invalid, not infringed, or unenforceable if challenged in the applicable patent office or court. • Our future growth and ability to compete depends on maintaining our culture, retaining our key personnel and recruiting additional qualified personnel. • Global geo- and socio-political threats and macro-economic uncertainty and other unforeseen political crises could materially and adversely affect our business and financial performance. • Holders of our ADSs have fewer rights than our ordinary shareholders. • The price of our ADSs may be volatile and may fluctuate due to factors beyond our control. An active public trading market may not be sustained. • Claims of U.S. civil liabilities may not be enforceable against us or the members of our Senior Management Team and our Board of Directors. • As a foreign private issuer, we are exempt from various rules and regulations that a U.S. domestic public company would be required to follow, including those requirements under U.S. securities laws and Nasdaq listing standards. • We may lose our foreign private issuer status which would then require us to comply with the Exchange Act’s domestic reporting regime and cause us to incur significant legal, accounting and other expenses. • If we were to be classified as a passive foreign investment company for U.S. federal income tax purposes, this could result in adverse U.S. tax consequences to certain U.S. holders. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Summary Risk Factors 83

2.2 Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications The commercial success of our products and product candidates, including in new indications or methods of administration, will depend on the degree of market acceptance. Our products and product candidates, including for new indications or methods of administration, if and when approved and available on the market, may never achieve an adequate level of acceptance by physicians, patients, the medical community, or healthcare payors for us to be profitable or sustain net profitability in the future. This will depend on a number of factors, many of which are beyond our control, including, but not limited to: • consumer perceptions or publicity regarding our business or the efficacy, safety and quality of the products and product candidates in our profile, our clinical trials for new indications, or any similar products distributed by other companies, and the prevalence and severity of any adverse effects discovered before or after marketing approval has been received; • approval may be for indications, dosage and methods of administration or patient populations that are not as broad as intended or desired; • changes in the standard of care for the targeted indications for any product and product candidate; • relative availability, cost, and convenience of alternative approved therapies; • labeling may require significant use or distribution restrictions or safety warnings; • acceptance by physicians, public health bodies, patients and healthcare payors of each product as safe, effective and cost-effective; and • patients continued commitment required to receive periodic in-center infusions. In addition, because we are developing our products and product candidates for the treatment of different indications, negative results in a clinical trial evaluating the efficacy and safety of a product or product candidate for one indication, including by one of our competitors, could negatively impact the perception of the efficacy and safety of such product or product candidate in a different indication, which could have an adverse effect on our reputation, commercialization efforts and financial condition. Moreover, efforts to educate the medical community and third-party payors on the benefits of our products and product candidates may require significant resources and may never be successful. If our product candidates or methods of use of existing products or new indications fail to gain market acceptance, this will have a material adverse impact on our ability to generate revenues. Even if some products achieve market acceptance, they may not be able to retain market acceptance and/or the market may prove not to be large enough to allow us to generate significant revenues. We face significant competition for our drug discovery and development efforts. The market for pharmaceutical products is highly competitive and characterized by rapidly growing understanding of disease biology, quickly changing technologies, strong intellectual property barriers to entry, and a multitude of companies involved in the creation, development, and commercialization of novel therapeutics. Many of these companies are highly sophisticated and often strategically collaborate with each other. Competition in the autoimmune field is intense and involves multiple mAbs, other biologics and small molecules either already marketed or in development by many different companies including, but not limited to, large pharmaceutical companies such as AbbVie, Inc. (AbbVie), Amgen, Inc. , Biogen Inc. , GlaxoSmithKline plc , F. Hoffman-La Roche AG (Roche) and Janssen Pharmaceuticals, Inc. now part of Johnson & Johnson Innovation, Inc. (Johnson & Johnson). argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications 84

In addition, these and other pharmaceutical companies have mAbs or other biologics in clinical development for the treatment of autoimmune diseases. Currently, our commercial revenue is generated by VYVGART and VYVGART SC in gMG, CIDP and ITP (Japan only). We face and expect to continue to face intense competition from other biopharmaceutical companies, who have launched or are developing products for the treatment of gMG and/or CIDP and other autoimmune diseases, including products that are in the same class as VYVGART, as well as products that are similar to some of our product candidates. Competition for other (potential) future indications is also fierce, with significant development in almost all of the indications we are currently developing or planning to develop for our product or product candidates. For example, we are aware of several neonatal Fc receptor (FcRn) inhibitors that are in clinical development and one FcRn inhibitor, Rystiggo (rozanolixizumab-noli), which was approved in June 2023. We are also aware that AstraZeneca plc is selling Soliris and Ultomiris for the treatment of adult patients with gMG who are AChR-AB+ and that UCB is selling Rystiggo for the treatment of adult patients with gMG who are AchR-AB+ or MuSK-AB+ and Zilbrysq for the treatment of adult patients with gMG who are AchR-AB+. Roche, Novartis AG, CSL Behring, Grifols, S.A., Curavac, Inc., Takeda Pharmaceutical Co Ltd, RemeGen Co, Immunovant, Inc., Cartesian Therapeutics, Inc., Horizon Therapeutics plc, Regeneron Pharmaceuticals, Inc., Alnylam Pharmaceuticals, Inc., Sanofi S.A. and Johnson & Johnson, among others, are developing drugs that may have utility for the treatment of myasthenia gravis (MG) and/or CIDP. Any negative side effects or safety concerns from one of our competitors’ products may adversely affect our business. Competitive product launches may erode future sales of our products, including our existing products and those currently under development, or result in unanticipated product obsolescence. Such launches continue to occur, and potentially competitive products are in various stages of development. We could also face competition for use of limited international infusion sites, particularly in new markets as competitors launch new products. We cannot predict with accuracy the timing or impact of the introduction of competitive products that treat diseases and conditions like those treated by our products or product candidates. In addition, our competitors and potential competitors compete with us in recruiting and retaining qualified scientific, clinical research and development and management personnel, establishing clinical trial sites, registering patients for clinical trials, as well as in acquiring technologies complementary to, or necessary for, the development of our products. There can be no assurance that our competitors are not currently developing, or will not in the future develop, technologies and products that are equally or more effective, are more economically attractive, and can be administered more easily than any of our current or future technologies or products. Competing products or technology platforms may gain faster or greater market acceptance than our products or technology platforms and medical advances or rapid technological development by competitors may result in our products and product candidates or technology platforms becoming non- competitive or obsolete before we are able to recover our research and development and commercialization expenses. If we, our products and product candidates or our technology platforms do not compete effectively, it is likely to have a material adverse effect on our business, financial condition and results of operation. We will face significant challenges in successfully commercializing our products and additional product candidates after they are launched. The commercialization of VYVGART in new indications or other product candidates once approved, or entrance of any of our products or product candidates into new markets will require us to further expand our sales and marketing organization, enter into collaboration arrangements with third parties, outsource certain functions to third parties, or use some combination of each. We have built, and continue to expand, our sales forces in certain of the countries where VYVGART is approved and plan to further develop our sales and marketing capabilities to promote our products, and product candidates, including new indications, if and when marketing approval has been obtained in other relevant jurisdictions. Even if we successfully expand our sales and marketing capabilities, either on our own or in collaboration with third parties, we may fail to launch or market our products effectively. Recruiting and training a specialized sales force is expensive and the costs of expanding an independent sales, marketing and/or promotion organization could be greater than we anticipate. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications 85

We could further encounter difficulties in our sales or marketing, due to regulatory actions, shut-downs, work stoppages or strikes, approval delays, withdrawals, recalls, penalties, supply disruptions, shortages or stock-outs at our facilities or third-party facilities that we rely on, reputational harm, the impact to our facilities due to pandemics or natural or man-made disasters, including as a result of climate change, product liability, and/or unanticipated costs. In addition, recruiting and training a sales force is time- consuming and could delay any product launch. In the event that any such launch is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel. We have entered into distribution agreements with Medison, Zai Lab, Genpharm and Handok to perform sales and marketing services in Israel, Central and Eastern Europe, Mainland China, the Gulf Cooperation Council and South Korea, respectively. Under these agreements, our product revenues or the profitability of these product revenues could be lower than if we were to market and sell the products that we develop ourselves. Such distribution agreements may place the commercialization of our products outside of our control, including over the amount or timing of resources that our distribution partners devote to our products. Furthermore, our distributors’ willingness or ability to comply with and complete their obligations under our arrangements may be adversely affected by business combinations or significant changes in our distributors’ business strategies. In addition, we may not succeed in entering into arrangements with third parties to sell and market our products or may be unable to do so on terms that are favorable to us. Our products and product candidates for which we have obtained or intend to seek approval as biological products, including for new indications, may face biosimilar competition. In the U.S., the Biologics Price Competition and Innovation Act (BPCIA) created an abbreviated approval pathway for biological products that are demonstrated to be “biosimilar” to or interchangeable with a U.S. FDA-licensed reference biological product. However, during the 12-year regulatory exclusivity period applicable to reference biological products, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials of their product. We believe that any of our product candidates approved as a biological product under a BLA in the U.S. should qualify for the Biologics Price Competition and Innovation Act 12-year period of exclusivity, as is the case with VYVGART and VYVGART HYTRULO. The base regulatory exclusivity period for VYVGART and VYVGART HYTRULO is expected to extend until December 2033 in the U.S. whereas regulatory protection in the EU is expected to expire in August 2032 in the EEA and March 2033 in the UK. However, in the U.S., there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for competition by biosimilar products sooner than anticipated. The same applies to the EU, as there is also a risk that this exclusivity could be shortened due to legislative actions. We are aware that some of our competitors may be actively developing competing or biosimilar products for VYVGART and VYVGART HYTRULO, including for CIDP, for which VYVGART HYTRULO received FDA approval in 2024. It is possible our competitors will be successful in developing biosimilar or interchangeable products for our products and product candidates, and the approval of such competing products may lead to substantial competition in the market, a decrease in sales, or force us to make VYVGART or VYVGART HYTRULO available at lower prices due to competitive pressures. Moreover, an interchangeable biosimilar product, once approved, may be substituted under existing state laws for any one of our reference products. In addition, the Further Consolidated Appropriations Act, 2020, which incorporated the framework from the Creating and Restoring Equal Access To Equivalent Samples (CREATES) legislation, allows biosimilar developers to obtain access to reference biological products, which may facilitate the development of biosimilars to our products. If competing or biosimilar products are approved, the market position of our products for existing and recently approved indications may be adversely affected. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications 86

In the EU, biosimilars are evaluated for marketing authorization pursuant to a set of general and product class-specific guidelines and in coming years, the European Commission may further revise relevant legislation and lessen the amount of data and market exclusivity available for medicinal products. In addition, some EU Member States have adopted, or are considering the adoption of, biosimilar uptake measures or may impose automatic price reductions upon market entry of one or more biosimilar competitors. While the degree of competitive effects of biosimilar competition among EU Member States may vary, continuation of policies promoting biosimilar products in the EU and in EU Member States could erode market share or introduce competitive pricing pressures for our products and product candidates. Enacted and future legislation could impact demand for our products which could impact our business and future results of operations. In the U.S., the UK, the EU and other jurisdictions, there have been a number of legislative and regulatory changes to the healthcare systems that could affect our future results of operations. Governmental regulations that mandate price controls or limitations on patient access to our products or establish prices paid by government entities or programs for our products could impact our business, and our future results of operations could be adversely affected by changes in such regulations or policies. For example, if the European Commission’s recent proposal to revise the EU’s pharmaceutical legislation is adopted in the form proposed, we may be affected by a decrease in data and market exclusivity for our products and product candidates in the EEA. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs in general and the cost of pharmaceuticals in particular. The IRA, enacted in August 2022, allows, among other things, the HHS to directly negotiate the price of a statutorily specified number of high-expenditure drugs and biologics each year that the CMS reimburses under Medicare Part B and Part D. In August 2023, CMS announced the first 10 Part D selected drugs for negotiation, with maximum fair prices taking effect in 2026. In January 2025, CMS announced an additional 15 Part D drugs selected for negotiation, with maximum fair prices taking effect in 2027. Negotiations for Medicare Part B products will begin in 2026 with the negotiated price taking effect in 2028. The Medicare drug price negotiation program is currently subject to legal challenges and we cannot predict the outcome of those cases. At this time, the Trump administration is continuing to implement the IRA and to defend the law in litigation. The IRA also penalizes drug manufacturers that increase prices of Medicare Part D and Part B drugs at a rate greater than the rate of inflation relative to a benchmark period. The IRA also capped out-of-pocket spending for Medicare Part D enrollees and made other Part D benefit design changes beginning in 2024. Beginning in 2025, the IRA eliminated the coverage gap (and the Coverage Gap Discount Program), lowered the enrollee maximum out-of-pocket cost to $2,000, and established a new manufacturer discount program, which requires manufacturers to provide discounts on their applicable drugs equal to 10% in the initial phase, and 20% in the catastrophic phase of the Part D benefit. Although these discount percentages are lower than coverage gap discounts, the new catastrophic phase discounts could be considerable for certain high-cost drugs and may exceed those coverage gap discounts previously provided. These Part D design changes also increase costs to Part D plans and may incentivize Part D plans to exclude certain drugs from their formularies, which could affect the supply, demand, and pricing of our product and product candidates. The HHS has and will continue to issue and update guidance and rulemaking as these IRA programs are implemented. We cannot predict how the HHS will interpret the IRA in the future, or whether the U.S. Congress will enact legislation that amends the law. However, at this time, the Trump administration is continuing to implement the IRA. Manufacturers that fail to comply with the IRA may be subject to significant penalties, including civil monetary penalties and excise taxes. The IRA also extends enhanced subsidies for individuals purchasing health insurance coverage in ACA (as defined below) marketplaces through plan year 2025. Thus, while the full economic impact of IRA is unknown at this time, the law’s passage is likely to affect the pricing of our products and product candidates. The adoption of restrictive price controls in new jurisdictions, more restrictive controls in existing jurisdictions, the adoption of these lower prices by commercial payors, or the failure to obtain or maintain timely or adequate pricing could also adversely impact revenue. We expect pricing pressures will continue globally. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications 87

Further, at the U.S. state level, legislatures are increasingly enacting laws and implementing regulations designed to control pharmaceutical and biological product pricing, including price or reimbursement constraints, discount requirements, price transparency reporting, and programs designed to encourage importation from other countries and bulk purchasing. States are also enacting laws modeled on federal policies, such as the IRA and the 340B drug discount program. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, including pharmaceuticals, which could result in reduced demand for our products and product candidates or additional pricing pressures. It is too early to predict whether and how the policies and priorities of the new U.S. presidential administration could materially impact the regulation governing our products and product candidates. The EU, on the other hand, has reopened the entire legislative framework for medicinal products. On April 26, 2023, the European Commission has published its proposal for a new directive (COM/2023/192 final) and a new regulation (COM/2023/193 final), which would revise and replace the existing general pharmaceutical legislation, including e.g., Directive 2001/83/EC, as well as Regulations (EC) No. 726/2004, No. 141/2000, or No. 1901/2006 (EU Pharmaceutical Legislation). This proposal is currently undergoing the ordinary legislative procedure in the European Parliament and Council of the European Union and is therefore still subject to changes. If at all, the EU Pharmaceutical Legislation is expected to be implemented at the earliest in the next few years. Prevention and mitigation of medicine shortages, simplification of the market entry of generics and biosimilars, the reduction of the regulatory burden (e.g., by increased digitalization) and the implementation of a new regime for data and / or market exclusivity (e.g., by reducing the minimum period while introducing factors that, if met, prolong protections for MA holders) are among the major objectives pursued by the European Commission. Pending the outcome of the legislative procedure, the impact could be positive with respect to certain regulatory processes. There could, however, also be a negative impact on innovative pharma and biotech companies such as argenx due to shorter baseline regulatory and orphan exclusivities if the proposal is not amended. Following its exit from the EU, the UK is not required to reflect future changes to EU Pharmaceutical Legislation in its own domestic regulatory regime (subject to ongoing alignment in respect of pharmaceuticals marketed in Northern Ireland). However, other legislative and regulatory changes to the healthcare systems that could affect our future results of operations are possible. We are subject to government pricing laws, regulation and enforcement. These laws affect the prices we may charge the government for our products and the reimbursement our customers may obtain from the government. Our failure to comply with these laws could harm our results, operations and/or financial conditions. In the U.S., we are required to participate in various government programs for our products to be reimbursed or purchased by the federal government. We participate in programs such as the Medicaid Drug Rebate Program, the 340B drug discount program, Medicare Part B, Medicare Part D and the U.S. Department of Veterans Affairs Federal Supply Schedule pricing program. The requirements vary by program, but we are, among other things, required to enter into agreements with and calculate and report prices and other information to certain government agencies, charge no more than statutorily mandated ceiling prices and calculate and pay rebates and refunds for certain products. The calculations are complex and are often subject to interpretation by us, governmental agencies and the courts. If we determine that the prices we reported were in error, we may be required to restate those prices and pay additional rebates or refunds to the extent we understated the rebate or overcharged the government due to the error. Additionally, there are penalties associated with submission of incorrect pricing or other data by the specified deadline, as well as potential allegations under the False Claims Act and other laws and regulations. Recently enacted legislation in the U.S. has imposed additional rebates under government programs. For example, effective January 1, 2024, under the American Rescue Plan of 2021, the cap on Medicaid drug rebates at 100 percent of the average manufacturer price was eliminated, which may require pharmaceutical manufacturers to pay more in Medicaid rebates than they receive on the sale of products. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications 88

In addition, the Infrastructure Investment and Jobs Act, effective January 1, 2023, requires manufacturers of certain single-source drugs (including biologics and biosimilars) separately paid for under Medicare Part B for at least 18 months and marketed in single-dose containers or packages (known as refundable single- dose containers or single-use package drugs) to provide refunds for discarded units that exceed a defined applicable percentage. Manufacturers that fail to pay such refunds shall be subject to civil monetary penalties. This requirement applies to VYVGART, and potentially other of our products in the future. As a result, we owe refunds to CMS starting this year. Although we will evaluate options to reduce the amount of refunds owed, pursuing any such actions will be time-consuming and costly. Even if we invest resources to reduce the amount of refunds owed to CMS, it is possible that we will be delayed or unsuccessful in achieving a reduction worthy of our investment. Maintaining compliance with these government price reporting and discounting obligations is time- consuming and costly, and a failure to comply can result in substantial fines, penalties, all of which could adversely impact our financial results. We may not obtain or maintain adequate pricing and coverage or reimbursement status for our products and product candidates. Sales of VYVGART and VYVGART SC and our product candidates, if approved, will depend, in part, on the extent to which third-party payors, including government health programs in the U.S. (such as Medicare Parts B and D and Medicaid) and other countries, commercial health insurers, and managed care organizations, provide coverage and establish adequate reimbursement levels for such products and product candidates. Patients generally rely on third-party payors to reimburse all or part of the associated healthcare costs, and are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. In the U.S., no uniform policy of coverage and reimbursement for products exists among commercial third- party payors. Commercial third-party payors decide which products they will pay for and establish reimbursement levels, often relying upon Medicare coverage policy and payment limitations. However, decisions regarding the extent of coverage, formulary tier placement, utilization management requirements (including step therapy), and the amount of reimbursement to be provided for any product candidate that we develop through approval will be made on a plan-by-plan basis. Even under U.S. government healthcare programs such as Medicare and Medicaid, coverage and reimbursement policies can vary significantly. Medicare Part D is administered by commercial insurance companies under contract with the CMS, and their coverage and reimbursement policies may vary, subject to certain statutory and regulatory requirements. Additionally, Medicaid programs vary from state to state in their coverage policies and reimbursement rates, subject to certain federal requirements. Further, from time to time, typically on an annual basis, payment rates are updated and revised by third-party payors. Such updates could impact the demand for our products, to the extent that patients who are prescribed our products, if approved, are not separately reimbursed for the cost of the product. The process for determining whether a third-party payor will provide coverage for a product may be separate from the process for setting the price of a product or for establishing the reimbursement rate that such a payor will pay for the product. Even if we do obtain adequate levels of reimbursement, third-party payors, such as government or private healthcare insurers, carefully review and increasingly question the coverage of, and challenge the prices charged for, products. Increasingly, third-party payors are requiring that biopharmaceutical companies provide them with predetermined discounts from list prices and are challenging the prices charged for products. We may also be required to conduct expensive pharmacoeconomic studies to justify the coverage and the amount of reimbursement for particular medications. We cannot be sure that coverage and reimbursement will be available for any product that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Moreover, coverage policies and third-party payor reimbursement rates may change at any time. Therefore, even if favorable coverage and reimbursement status is attained for one or more products for which we receive marketing approval in one or more indications, less favorable coverage policies and reimbursement rates may be implemented in the future. For instance, even though favorable coverage and reimbursement status has been attained for VYVGART for the treatment of gMG in the U.S., access to VYVGART for any other indication may be reduced or restricted by limited payor coverage due to treatment criteria, which may prevent us from realizing its full commercial potential. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications 89

In addition, the coverage and reimbursement levels for our products for the treatment in one indication may have an adverse impact on the coverage and reimbursement levels of such products or product candidates in other indications for which marketing approval has previously been or may subsequently be obtained. Inadequate coverage or reimbursement may diminish or prevent altogether any significant demand for our products and/or may prevent us entirely from entering certain markets or indications, which would prevent us from generating significant revenues or sustaining net profitability in the future, which would adversely affect our business, financials and results of operations. In many foreign countries, pricing, coverage, and level of reimbursement of prescription drugs are subject to governmental control, and we and our collaborators may be unable to obtain coverage, pricing, and/or reimbursement on terms that are favorable to us or necessary for us or our collaborators to successfully commercialize our marketed products in those countries. In some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing and reimbursement vary widely from country to country, and may take into account the clinical effectiveness, cost, and service impact of existing, new, and emerging drugs and treatments. For example, the EU provides options for EU Member States to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. An EU Member State may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. Our results of operations may suffer if we or our collaborators are unable to market our products in foreign countries or if coverage and reimbursement for our marketed products in foreign countries is limited or delayed. If we fail to obtain orphan drug designation or we do not have valid and enforceable patents covering our products and their uses and product candidates and fail to obtain and/or maintain orphan drug exclusivity for our products or product candidates, our competitors may be able to sell products to treat the same conditions and our revenue may be reduced. We have and may from time to time seek orphan drug designation in the U.S., Japan, or the EU for certain indications addressed by our products and product candidates. With regard to these designations or future designations we may obtain, we may not be the first to obtain marketing approval of these drugs for such indication due to the uncertainties associated with developing therapeutic products, and we may not obtain orphan exclusivity upon approval. In addition, exclusive marketing rights in the U.S. may be limited if we seek approval for an indication broader than the orphan-designated indication, or may be lost if the FDA later determines that the request for designation was materially defective or if we are unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition. Further, even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs with different active moieties or different principal molecular structural features can be approved for the same condition. Even after an orphan drug is approved, the MHRA, the EMA, the FDA, the MHLW (collectively, the Relevant Regulatory Authorities) or other comparable regulatory authorities can subsequently approve the same drug with the same principal molecular structural features for the same condition if the regulator concludes that the later drug is safer, more effective, or makes a major contribution to patient care. Further, in the U.S., a September 2021 Eleventh Circuit decision in Catalyst Pharmaceuticals, Inc. vs. Becerra regarding interpretation of the Orphan Drug Act exclusivity provisions as applied to drugs approved for orphan indications narrower than the drug’s orphan designation could significantly broaden the scope of orphan drug exclusivity for such products. In January 2023, the FDA, however, issued a Federal Register notice clarifying its approach to orphan drug exclusivity following the Catalyst decision. Consistent with the court’s decision, the FDA set aside its approval of the drug at issue in the case, but announced that, while complying with the court’s order in Catalyst, the FDA intended to continue to apply its regulations tying the scope of orphan-drug exclusivity to the uses or indications for which a drug is approved to matters beyond the scope of that order. Legislation has also been introduced that may reverse the Catalyst decision but its passage is uncertain at this time. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications 90

2.3 Risk Factors Related to the Development and Clinical Testing of argenx’s Products and Product Candidates Failure to successfully identify, select and develop our products in other indications, or additional products or product candidates could impair our ability to grow. Our long-term growth strategy entails developing and marketing additional products and product candidates, including efgartigimod for new indications, empasibrubart and ARGX-119. This requires substantial resources, whether or not any product candidates or new indications are ultimately identified. The success of this strategy depends partly upon our ability to identify, select, develop, and ultimately, commercialize promising product candidates. We are heavily dependent on precise, accurate and reliable scientific data to identify, select and develop promising product candidates and products. Our business decisions may therefore be adversely influenced by inaccurate, improper or fraudulent scientific data, including data sourced from third parties. Even with accurate scientific data, our technology platforms may fail to discover and to generate additional products and products candidates, that are suitable for further development. Even if we identify additional product candidates, they may not be suitable for clinical development as a result of harmful side effects, limited efficacy or other characteristics that indicate that it is unlikely to be a product that will receive approval by the Relevant Regulatory Authorities, and other comparable regulatory authorities or achieve market acceptance. For example, we have previously announced that certain clinical trials did not meet their primary endpoints. We consequently decided not to pursue additional development in pemphigus and plan to prioritize clinical development of efgartigimod in its ongoing severe autoimmune indications. If we do not successfully identify, develop and commercialize product candidates and VYVGART in new indications based upon our technological approach, we may not be able to obtain product or collaboration revenues in future periods. Obtaining regulatory approval for our products and product candidates is inherently uncertain. To obtain the requisite regulatory approvals to market and sell any of our products and product candidates, we or our collaborators for such candidates must successfully demonstrate that our products are safe and effective in humans. Clinical trials are expensive and can take many years to complete, and their outcome is inherently uncertain. Further, success in early clinical trials or in one indication does not guarantee success in later clinical trials or in other indications. Failure to successfully develop or obtain marketing approval for our products and product candidates could negatively impact our business. The time required to obtain approval by the Relevant Regulatory Authorities and other comparable regulatory authorities is unpredictable but typically takes many years, if obtained at all, following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion or interpretation of the regulatory authorities. This lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval to market any of our product candidates, including for new indications. We may experience delays in our ongoing or planned clinical trials, for a large variety of reasons outside our control in complying with regulatory approvals which can adversely affect the timing of clinical trials, including as described in Section 2.5 “Risk Factors Related to Other Government Regulations — All aspects of our business ranging from preclinical, clinical trials, marketing and commercialization are highly regulated and any delay by relevant regulatory authorities could jeopardize our development and approval process or result in other suspensions, refusals or withdrawal of approvals.” In addition, ongoing efforts by the Trump administration to limit the size of the FDA and other agencies of HHS, including through reductions in staff, may further increase the unpredictability in approval timelines for our products and product candidates. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to the Development and Clinical Testing of argenx’s Products and Product Candidates 91

For example, on February 11, 2025, President Trump issued an executive order on workforce optimization, seeking to reduce the size of the federal workforce through large-scale reductions in force and by placing limitations on the number of new employee hires. Whether this executive order and other similar Trump administration efforts to reduce the federal work force will have an adverse effect on FDA’s ability to timely review drug and biologic product applications remains uncertain. If we are unable to obtain regulatory approval of our products and product candidates on a timely basis or at all, our business, financial operations and/or financial condition may be impacted. Certain of our clinical trials have not succeeded, and may in the future also not succeed, and even if they succeed, we may not obtain regulatory approval for our products or product candidates or regulatory approval may be delayed. Certain of our clinical trials have not succeeded, and may in the future also not succeed. We could experience operational challenges as we undertake an increasing number of clinical trials, including those conducted in countries outside the EU, UK and the U.S. that may subject us to further delays and expenses as a result of increased shipment costs, additional regulatory requirements and the engagement of non-EU, non-UK and non-U.S. contract research organizations (CROs), as well as expose us to risks associated with clinical investigators and institutions who apply different standards of diagnosis, screening and medical care or are otherwise unfamiliar with standards and requirements imposed by the Relevant Regulatory Authorities. If we experience delays in the completion of, or termination of, any clinical trial of our products or product candidates, our commercial prospects may be harmed. Any delays in completing our clinical trials may increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates or result in the development of our product candidates being stopped early. Significant clinical trial delays could also allow our competitors to bring products to market before we do or shorten any periods during which we have the exclusive right to commercialize our products and product candidates. Even if clinical trials are initiated, our development efforts may not be successful. Even if we obtain positive results from preclinical trials or initial clinical trials, we may not achieve the same success in future clinical trials, which may negatively impact the price of our ordinary shares or ADSs. Regulatory approval of our products or product candidates may be delayed or refused for many reasons, including for reasons outside our control such as: • the Relevant Regulatory Authorities or other comparable regulatory authorities may disagree with the design or implementation of our clinical trials; • we may be unable to demonstrate, to the satisfaction of the Relevant Regulatory Authorities or other comparable regulatory authorities, that our product candidates are safe, pure, potent and effective for any of their proposed indications; • the results of clinical trials may not meet the level of statistical significance required by the Relevant Regulatory Authorities or other comparable regulatory authorities for approval; • the chemistry, manufacturing and controls information submitted in an application is insufficient; and • the facilities of third-party manufacturers with which we contract for the manufacture of our product candidates are not adequate to support approval of our product candidates. Any of these occurrences may harm our business, results of operations and financial condition significantly. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to the Development and Clinical Testing of argenx’s Products and Product Candidates 92

If we decide to pursue accelerated approval for any of our product candidates, it may not lead to faster development or regulatory review or approval and we may still need to conduct additional clinical trials, which could increase the expense of obtaining, if at all, necessary marketing approvals. Recently, the accelerated approval pathway has come under scrutiny by various stakeholders, and the FDORA revised the requirements for this pathway. Although this legislation did not change the standard for accelerated approval, the FDA is now authorized to require a post-approval clinical trial to be underway prior to approval or within a specified time period following approval, and must specify conditions of any required post-approval clinical trial. FDORA also requires sponsors to submit progress reports for required post-approval studies. Failure to conduct due diligence for required post-approval studies is deemed a prohibited act under the FDCA. FDORA also details procedures the FDA must follow to withdraw an accelerated approval on an expedited basis, including where the required post-approval studies are not conducted with due diligence or fail to verify clinical benefit, other evidence demonstrates that the product is not shown to be safe or effective under the conditions of use, or the sponsor disseminates false or misleading promotional materials with respect to the product. If we decide to pursue accelerated approval for any of our product candidates, the failure to obtain accelerated approval (or the withdrawal of any accelerated approval) could result in a longer time period to commercialization of such product candidate, if any, and could increase the cost of development of such product candidate and harm our competitive position in the marketplace. For example, if standard of care were to evolve or if any of our competitors were to receive approval for a drug or biological product for a disease or condition for which we are seeking accelerated approval before we receive accelerated approval, we may not be able to demonstrate that our product candidate provides a meaningful advantage over other available therapies and accelerated approval may not occur. Our products and product candidates may have serious adverse, undesirable or unacceptable side effects, and we or others may identify undesirable or unacceptable side effects caused by any of our products or product candidates before and after they have received marketing approval. Undesirable side effects that may be caused by our product candidates, or by the combination of our product candidates with other medical products, could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in more restrictive labeling or the delay or denial of regulatory approval by the Relevant Regulatory Authorities or other comparable regulatory authorities. We have observed adverse events and treatment emergent adverse events in our clinical trials, and we may see additional adverse events and treatment emergent adverse events in our ongoing and future clinical trials. Such side effects may be more serious than those observed to date, and as a result, our ongoing and future clinical trials may be negatively impacted. Moreover, as we seek to develop product candidates, including products in new indications, patients may experience new or more serious effects. Drug-related side effects caused by any of our products or product candidates that we or others identify could, among other things, affect patient recruitment, the ability of enrolled patients to complete the clinical trial, result in potential product liability claims, damage sales of our existing products, result in significant reputational damage for us and our product development, and other issues including the delay of other programs. They can also cause the Relevant Regulatory Authorities or other comparable regulatory authorities to withdraw approvals or revoke licenses of such products and require us to take such products off the market, require the addition of labeling statements, specific warnings, or a contraindication or other modification of the product labeling, request the issuance of safety alerts, require a REMS to ensure that the benefits of the product outweigh its risks, and/or require us to change the way the product is administered, conduct additional clinical trials or change the labeling of the product. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to the Development and Clinical Testing of argenx’s Products and Product Candidates 93

If our target patient population is smaller than expected, we are unable to successfully enroll and retain patients in our clinical trials, or experience significant delays in doing so, we may not realize the full commercial potential of any products or product candidates. Currently, we mainly develop products or product candidates for the treatment of rare diseases for which the target patient population can be small. If the actual number of patients with these disorders is smaller than we expected, we may encounter difficulties in enrolling sufficient patients in our clinical trials, thereby delaying or preventing development and approval of our products or product candidates. Physicians, who are an important source of patients for clinical trials, may also be less familiar with these rare diseases and may therefore fail to identify these conditions in their patients and therefore may not refer them to our clinical trials. Patient enrollment, a significant factor in the timing of clinical trials, depends on many factors, including the size and nature of the patient population, eligibility criteria for the clinical trial, the proximity of patients to clinical sites, competition for patient recruitment from competing clinical trials, the design of the clinical trial, the availability of alternate approved therapies for the indication the clinical trial is investigating, and clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies. We compete with other companies to enroll target patient populations, as set forth in Section 2.1 “Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications—We face significant competition for our drug discovery and development efforts.” Even if product candidates obtain significant market share for their approved indications, because certain potential target populations are small, we may never recoup our investment in such product candidate without obtaining regulatory approval for additional indications for such product candidates. Even once enrolled, we may be unable to retain a sufficient number of patients to complete any of our clinical trials. In addition, any negative results we may report in clinical trials of our drug candidates may make it difficult or impossible to recruit and retain patients in other clinical trials of that same drug candidate. Delays in the completion of any clinical trial of our product candidates will increase our costs, slow down our product candidate development and approval process and delay or potentially jeopardize our ability to commence product sales and generate revenue. In addition, some of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates. 2.4 Risk Factors Related to argenx’s Dependence on Third Parties We rely, and expect to continue to rely, on third parties to conduct some of our research activities and clinical trials and for parts of the development and commercialization of our existing and future research programs, products and product candidates. If our relationships with such third parties are not successful, our business may be adversely affected. We have relied upon and plan to continue to rely upon third parties, including independent clinical investigators, CROs, CMOs and other third-party service providers with the applicable protocol, legal and regulatory requirements and scientific standards, and our reliance on these third parties does not relieve us of our regulatory responsibilities. To the extent our collaborators or the CROs or investigators fail to enroll participants for our clinical trials, fail to conduct the clinical trial to GCP standards or in full compliance with legal and regulatory requirements or are delayed for a significant time in the execution of clinical trials, including achieving full enrollment, we may be affected by increased costs, program delays or both, which may harm our business. In addition, we are, and expect to continue to be, dependent on partnerships with partners and licensees relating to the development and commercialization of our existing and future research programs, products and product candidates. We currently have collaborative research relationships with various argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to the Development and Clinical Testing of argenx’s Products and Product Candidates 94

pharmaceutical companies such as AbbVie, Zai Lab, Genmab and with various academic and research institutions worldwide for the development of product candidates resulting from such collaborations. We also have distribution agreements in place with Medison, Genpharm and Handok for the distribution of VYVGART. We had, have and will continue to have discussions on potential partnering opportunities with various pharmaceutical companies. If we fail to enter into or maintain collaborations on reasonable terms or at all, our ability to develop our existing or future research programs and product candidates and to commercialize our existing or future products could be delayed, the commercial potential of our products could change and our costs of development and commercialization could increase. While we have agreements governing our relationships with these third parties, we have limited influence over their actual performance and control only certain aspects of their activities. If independent investigators, third-party service providers or CROs fail to devote sufficient resources to the development of our product candidates, or if their performance is substandard, it may delay or compromise the prospects for approval and commercialization of any product candidates that we develop. In addition, regulatory authorities enforce GCP requirements through periodic inspections of clinical trial sponsors, principal investigators and clinical trial sites. If we, our investigators or any of our CROs fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the Relevant Regulatory Authorities or comparable regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. Upon inspection by a given regulatory authority, such regulatory authority may determine that our clinical trials do not fully comply with GCP regulations, which may require us to repeat clinical trials and delay the regulatory approval process. Our collaborative partners may not adhere to or may terminate collaboration agreements with all associated consequences or disagree on the interpretation of contractual terms. We may not be able to control our collaborative partners’ compliance with all applicable requirements for the commercialization of our products, which could adversely affect such commercialization and the profitability of such products. Failures by our collaborative partners to meet their contractual, regulatory, or other obligations to us, or any disruption in the relationships between us and our collaborative partners, could have a material adverse effect on our product pipeline and business. We face significant competition in establishing successful relationships with third-party service providers and appropriate collaborative partners. These third-party service providers may have contractual relationships with other entities, some of which may be our competitors, which may draw their time and resources away from our programs. In addition, some of our third-party service providers or CROs have the ability to terminate their respective agreements with us, and if such agreements terminate, we may not be able to enter into arrangements with alternative CROs or investigators or to do so on commercially reasonable terms. In addition, we may not be able to find appropriate collaboration partners. Our ability to reach a definitive agreement for a partnership will depend, among other things, upon an assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed partnership and the proposed collaborator’s evaluation of a number of factors. These factors may include the design or results of clinical trials, the likelihood of regulatory approval, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership regardless of the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a partnership could be more attractive than the one with us. In addition, in the U.S., legislative, executive and regulatory proposals were recently enacted or are pending to, among other things, prevent drug shortages, improve pandemic preparedness and reduce the dependency of the United States on foreign supply chains and manufacturing. While we are still assessing these developments, they could impact our selection and utilization of CMOs, vendors and other suppliers and could have a material adverse impact on our business, financial condition and results of operations. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Dependence on Third Parties 95

Disruptions caused by our reliance on third parties for our manufacturing process may delay or disrupt our business, product development and commercialization efforts. We do not have the ability to internally source the raw materials necessary to produce our products or product candidates, and do not currently have, nor do we plan to acquire, the infrastructure or capability internally to manufacture our products or product candidates and depend on a worldwide supply chain and third parties for both. Disruptions caused by our reliance on such third-party suppliers, service providers and manufacturers may delay or disrupt our business, product development and commercialization efforts. Reliance on Third-Party Suppliers and Service Providers For some of our raw materials, we rely on a single source of supply and there are limited supplies of the raw materials. If prices increased, or we were to experience an unexpected loss of supply of or if any supplier was unable to meet our demand for any of our products and product candidates, including for example if VYVGART is approved for additional indications, we could experience delays in our research or planned clinical trials or risk shortages in commercial supply which could materially impact our revenue potential. These issues could be exacerbated by pressure on the supply chain, for example due to power shortages, telecommunications failures, natural disasters such as floods, hurricanes and wildfires, extreme weather conditions, public health crises, changed laws or regulations or geopolitical events, including trade disputes or economic sanctions enacted as a result of international conflict. The cost of our raw materials may also increase based on increased tariffs on foreign exports. As we continue to grow our business we may need to establish additional sources of supply for our products. The lead time needed to establish a relationship with a new supplier can be lengthy and require us to devote substantial time and resources. The time and effort to qualify a new supplier could result in additional costs, or delays, which could adversely affect our business. Additionally, certain of the raw materials required in the manufacture and the formulation of our products and product candidates may be derived from biological sources, including mammalian tissues, bovine serum and human serum albumin. There are certain European regulatory restrictions on using these biological source materials including rigorous testing requirements, which could limit or delay production. Regulatory authorities may require additional studies if we adopt a new supplier. If there are changes in the regulation requirements that our suppliers are unable to meet, our clinical development or commercial activities may be delayed or interrupted. We may not be able to engage a back-up or alternative supplier or service provider in a timely manner or at all if any of these third parties were to cease or interrupt production or otherwise fail to supply these materials, products, or services to us for any reasons, including due to regulatory requirements or actions (including recalls), adverse financial developments at or affecting the supplier, failure by the supplier to comply with cGMPs, contamination, business interruptions, or labor shortages or disputes. Interruptions in the supply of these materials, products or services may also result from international conflict, trade disputes or economic sanctions enacted by, or imposed on, the U.S., the UK, the EU or any other country or region. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Dependence on Third Parties 96

Reliance on Third-Party Manufacturing We rely on and expect to continue to rely on CMOs. We also rely on certain third parties to perform filling, finishing, distribution, laboratory testing and other services related to the manufacture and supply of our products and product candidates. We do not control the manufacturing process at our CMOs and are completely dependent on them for the production of our products and product candidates in accordance with relevant regulations (such as cGMPs), we are responsible for ensuring that our products comply with regulatory requirements. If our CMOs cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the Relevant Regulatory Authorities or other comparable regulatory authorities, our business could be adversely affected, including an inability to initiate or continue clinical trials of product candidates under development, delay in submitting regulatory applications, or receiving regulatory approvals for product candidates, including new indications, subjecting third-party manufacturing facilities to additional inspections by regulatory authorities, requirements to cease distribution or to recall batches of our products or product candidates and an inability to meet commercial demands for our marketed products. Most notably, we contract with Lonza for their manufacturing sites in Slough, UK, Portsmouth, U.S, Singapore and Visp, Switzerland as well as with Fujifilm, based in Denmark for activities relating to the development of cell banks, development of our manufacturing processes and the manufacturing of drug substance. We use additional contract manufacturers to fill, test, label, package, store and distribute our (investigational) drug products. Our products and product candidates are biologics and require multiple processing steps that are more difficult than those required for most small molecule chemical pharmaceuticals. While we work with our CMOs and partners on optimization, strengthening and upscaling our manufacturing, problems with these manufacturing processes, such as capacity issues, or even minor deviations from the normal process or from the materials used in the manufacturing process, which may not be detectable by us in a timely manner, could lead to manufacturing failures or product defects, resulting in lot failures, product recalls, product liability claims and insufficient inventory. We face risks inherent in relying on limited CMOs, as any failure in their ability to successfully manufacture our products or product candidates as described above or any disruption, such as supply shortages or disruptions of raw materials, fires, pandemics, natural hazards or acts of vandalism at the CMO could significantly interrupt our manufacturing capability. Alternative production plans in place or disaster- recovery facilities available to us may not be sufficient. In case of a disruption, we may have to establish additional alternative manufacturing sources. This would require substantial investment on our part, which we may not be able to obtain on commercially acceptable terms or at all. Additionally, we may experience significant manufacturing delays as we build or locate replacement facilities and seek and obtain necessary regulatory approvals. If this occurs, we will be unable to satisfy manufacturing needs on a timely basis, if at all. Also, operating any new facilities may be more expensive than operating at our current facilities. Further, business interruption insurance may not adequately compensate us for any losses that may occur, and we would have to bear the additional cost of any disruption. For these reasons, a significant disruptive event of the manufacturing facility could have drastic consequences, including placing our financial stability at risk. Accuracy and timing of our financial reporting is partially dependent on information received from third-party partners, which we do not control. We have collaborated, and plan to continue to collaborate, with third parties, including distributor and licensing partners, on certain product candidates. As part of some of these collaborations, our collaboration partners are responsible for providing us with financial information regarding specific projects, including funds spent, liabilities incurred and expected future costs, on which we rely for our own financial reporting. If our collaboration partners fail to provide us with the necessary financial information within the agreed upon timeframes, or if such financial information proves inaccurate, it would adversely impact the timing and accuracy of our own financial reporting. Any inaccuracy in our financial reporting could cause investors to lose confidence in our financial reporting. This in turn may lead to reputational damage or affect our ability to obtain, and the terms of, any future financing, which may harm our business. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Dependence on Third Parties 97

We and our third-party manufacturers and suppliers may become exposed to liability, fines, penalties or other sanctions and substantial expenses in connection with environmental compliance or remediation activities. Our third-party manufacturers’ and suppliers’ operations, including research, development, testing and manufacturing activities, are subject to numerous environmental, health and safety laws and regulations. These laws and regulations govern, among other things, the controlled use, handling, release and disposal of and the maintenance of a registry for, hazardous materials and biological materials, laboratory procedures and exposure to pathogens. We do not have control over our manufacturers’ or suppliers’ compliance with environmental, health and safety laws and regulations. If we, or they fail to comply with such laws and regulations, we could be subject to liability, fines, penalties or other sanctions and incur substantial expenses to comply or remediate the activities. We face a risk of environmental liability inherent in our current and historical activities, including liability relating to releases of or exposure to hazardous or biological materials. Environmental, health and safety laws and regulations are becoming more stringent. We may be required to incur substantial expenses in connection with future environmental compliance or remediation activities, in which case, our production and development efforts may be interrupted or delayed, and our financial condition and results of operations may be materially adversely affected. 2.5 Risk Factors Related to Other Government Regulations We are subject to healthcare laws, regulation and enforcement. The failure to comply with these laws could harm our results, operations and/or financial condition. Our current and future operations are and may become directly, or indirectly through our customers and third-party payors, subject to various U.S. federal and state, EU, Japanese, Chinese, UK, Canadian and Israeli healthcare laws, and healthcare laws of other jurisdictions in which we conduct our business. This includes, but is not limited to, the U.S. FDCA, the U.S. False Claims Act and EU Directive 2001/83/EC. EU Directive 2001/83/EC provides that where medicinal products are being promoted to persons qualified to prescribe or supply them, no gifts, pecuniary advantages or benefits of any kind may be supplied, offered or promised to such persons, except under certain circumstances. This provision was also transposed into the Human Medicines Regulations 2012 in the UK. Healthcare providers, physicians and others play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Healthcare laws also impact our arrangements with healthcare professionals who participate in our clinical research programs, healthcare professionals and others who recommend, purchase, or provide our approved products, and other parties through which we market, sell and distribute our products for which we obtain marketing approval. Therefore, the healthcare laws we are subject to may impact, among other things, our proposed sales, marketing and education programs and constrain our business and financial arrangements with third-party payors. For example, the provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order or use of medical products is generally not permitted in countries that form part of the EU, or the UK. Some EU Member States have enacted laws explicitly prohibiting the provision of these types of benefits and advantages to induce or reward improper performance generally, and the UK has enacted similar restrictions. Infringement of these laws can result in substantial fines and imprisonment, as well as associated reputational harm. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. The shifting compliance environment and the need to maintain robust and expandable systems to comply with multiple jurisdictions with different compliance or reporting requirements increases the possibility that we or our collaborative partners may run afoul of one or more of these requirements. We continue to argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Dependence on Third Parties 98

expand, enhance and refine our internal ethics and compliance function and program to ensure compliance with the different healthcare laws and regulations. As we continue to grow our headcount to support our business, we face increased compliance risk as we need to train and supervise additional personnel to comply with relevant healthcare laws and regulations. This involves substantial costs and, notwithstanding our investment, there can be no assurance that our policies and procedures will be followed at all times or will effectively detect and/or prevent all compliance violations by our employees, consultants, subcontractors, agents and partners. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations applicable to us, we may be subject to significant civil, criminal and administrative investigations, penalties, damages, fines, disgorgement, imprisonment, exclusion of drugs from government funded healthcare programs, such as Medicare and Medicaid in the U.S., additional reporting requirements and oversight, reputational harm and the curtailment or restructuring of our operations. Managing such investigations and defending against or appealing any such actions or penalties can be costly and time-consuming and may require significant financial and personnel resources. Therefore, even if we are successful in managing any such governmental investigations and/or defending against or appealing any such actions or penalties that may be brought against or imposed upon us, our business may be impaired. Efforts to ensure that our business arrangements with third parties comply with applicable healthcare laws and regulations also involve substantial costs. The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform. In the U.S., federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Ensuring business arrangements comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time and resource consuming and can divert a company’s attention from the business. All aspects of our business, including preclinical research, clinical trials, marketing and commercialization, are highly regulated, and any delay by relevant regulatory authorities could jeopardize our development and approval process and/or result in suspensions of marketing authorizations, refusals to approve our products, or withdrawal of existing approvals. Before we can commence clinical trials for a product candidate, we must complete extensive preclinical testing to support our IND or planned IND applications in the U.S. or Japan, or our clinical trial applications (CTAs) in the UK or in the EU, or comparable applications in other jurisdictions. We cannot be sure that we will be able to submit INDs or CTAs or comparable applications for our development programs on the timelines we expect, if at all. We also cannot guarantee that submission of INDs or CTAs or comparable applications will result in the Relevant Regulatory Authorities or other regulatory authorities allowing clinical trials to begin. Clinical trials must be conducted in accordance with Relevant Regulatory Authorities' and other comparable regulatory authorities’ legal requirements and regulations and are subject to oversight by these governmental agencies as well as IRBs and ethics committees. In addition, clinical trials must be conducted in compliance with GCPs and clinical supplies of our products and product candidates must be produced under cGMPs and other regulations. We could encounter delays if a clinical trial is suspended or terminated, by us, by the IRB or ethics committee, by the data review committee or data safety monitoring board for such clinical trial, or by the Relevant Regulatory Authorities or other comparable regulatory authorities. Such authorities may impose a suspension or termination due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or clinical trial site by the Relevant Regulatory Authorities or other comparable regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, including those relating to the class to which our products and product candidates belong, failure to demonstrate a benefit from using the product or product candidate, changes in governmental regulations or administrative actions, or lack of adequate funding to continue the clinical trial. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Other Government Regulations 99

If we experience delays in the completion of, or termination of, any clinical trial of our products or product candidates, the costs of our clinical programs may increase, the commercial prospects of our products and product candidates may be harmed, and our ability to generate product revenues from any of these products and product candidates may be delayed. Significant clinical trial delays could also allow our competitors to bring products to market before we do, or shorten any periods during which we have the exclusive right to commercialize our products and product candidates. Moreover, we must obtain separate regulatory approvals in each jurisdiction where we want to market, and approval by one regulatory authority does not ensure approval by any other regulatory authority. As approval procedures can vary among countries and may change over time, this can require additional clinical testing, and the time required to obtain approval may differ. We can provide no assurances that such approval will be obtained on the timeline that we expect or at all. In addition, we anticipate submitting applications for approval of VYVGART in new indications, but can provide no assurances that such applications will be accepted or that we will receive approval on our anticipated timeline, or at all. If VYVGART or any new formulations of VYVGART are not approved in one or more jurisdictions including beyond the countries where VYVGART is approved, or if such approvals are significantly delayed, it could have a material adverse effect on our business. It is possible that none of our other existing product candidates or any product candidates we may seek to develop in the future will ever obtain regulatory approval in any other jurisdiction for any indication. Even if approval is obtained, the Relevant Regulatory Authorities or other comparable regulatory authorities may approve the product for fewer or more limited indications or patient sub-segments than requested and/or with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product. Further, the Relevant Regulatory Authorities or other comparable regulatory authorities may impose extensive and ongoing unique regulatory requirements, such as granting approval contingent on the performance of costly post-marketing clinical trials, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product. The costs of compliance with all Relevant Regulatory Authorities' and other applicable authorities' regulations, requirements or guidelines could be substantial, and failure to comply could result in sanctions, including fines, injunctions, civil penalties, denial of applications for marketing authorization of our products, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products, operating restrictions and criminal prosecutions, any of which could significantly increase our and/or our collaborative partners’ costs or delay or prevent the development and commercialization of our product candidates. At this time, we cannot guarantee or know the exact nature, precise timing and detailed costs of the efforts that will be necessary to complete the remainder of the development of our research programs and product candidates. We are subject to privacy laws, regulation and potential enforcement. The failure to comply with these laws could harm our results, operations and/or financial conditions. Privacy laws, regulation and potential enforcement are particularly relevant to our business as we collect, store and process patient data, including sensitive health data as well as human biological samples such as blood or tissue, in the context of our clinical development activities, post-marketing approval monitoring obligations, and associated activities. We also collaborate on a regular basis with third parties where we may seek to use data collected by third parties on our or their behalf, or we may seek to share data collected by us with such third parties to further our research or commercial initiatives. The GDPR imposes a broad range of strict requirements on companies, including with respect to cross- border transfers of personal data and imposes substantial penalties in the event of non-compliance. We face uncertainty as to the exact interpretation of the requirements under the GDPR, and we may be unsuccessful in implementing all measures required by data protection authorities or courts in interpretation of the GDPR. In addition, national laws of EU Member States may partially deviate from the GDPR and impose different obligations from country to country. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Other Government Regulations 100

Also, in the field of handling genetic data, the GDPR specifically allows EU Member States’ laws to impose additional and more specific requirements or restrictions, and European national laws have historically differed quite substantially in this field, leading to additional uncertainty. Apart from the GDPR, privacy laws continue to evolve and expand in Europe. For example, violations of the Directive 2002/58/EC of the European Parliament and of the Council of July 12, 2002 (as amended, the e-Privacy Directive) can result in administrative measures, including fines, or criminal sanctions. The EU is in the process of developing a new e-Privacy Regulation to replace the e-Privacy Directive, and the new e-Privacy Regulation may impose additional obligations and risk for our business. Following its departure from the EU, the UK has maintained in force substantially equivalent provisions to the GDPR (UK GDPR). Similar concerns as those described above with respect to GDPR apply to our compliance with the UK GDPR and other UK data protection rules as well. Beyond the EU and UK, privacy and data protection laws and regulations continue to develop and expand around the world, including in other jurisdictions in which we operate, such as the U.S., Japan, and Canada. Such laws and regulations impose increasing restrictions and obligations on the processing of personal data, including sensitive personal data such as genetic data. For example, in the U.S., the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, and state privacy laws, such as the California Consumer Privacy Act of 2018, as amended, and the Washington My Health My Data Act of 2023, impose obligations on covered businesses, including, but not limited to, providing specific disclosures in privacy notices and affording residents certain rights related to their personal data. Privacy laws continue expanding globally and may require us to modify our data collection or processing practices and to incur significant expenses associated with our compliance efforts. We continue to evaluate and consider whether and how to incorporate artificial intelligence solutions into certain aspects of our business, which may pose significant risks, including to data privacy. The legal regulatory regime relating to artificial intelligence is uncertain and evolving, and compliance with existing and new laws and regulations governing artificial intelligence may give rise to significant costs, which could increase our operating expenses. Further, these compliance obligations may make it harder for us to conduct our business using artificial intelligence, require us to change our business practices, or prevent or limit our use of artificial intelligence, which could make our business less efficient or put us at a competitive disadvantage. Implementation of artificial intelligence into our business could pose certain risks to our patients or partners. For example, if we incorporate artificial intelligence into our business, our use of artificial intelligence may result in cybersecurity incidents that implicate the personal data of our patients or partners. In addition, if we are investigated by a data protection authority, we may face fines and other penalties. Any such investigation or charges by data protection authorities could have a negative effect on our existing business and on our ability to attract and retain new clients or pharmaceutical partners. We may also experience hesitancy, reluctance, or refusal by clients or pharmaceutical partners to continue to use our products and solutions due to the potential risk exposure as a result of the current and future data protection obligations. Such clients or pharmaceutical partners may also view any alternative approaches to compliance as being too costly, too burdensome, too legally uncertain, or otherwise objectionable and therefore decide not to do business with us. Any of the foregoing could harm our business, prospects, financial condition and results of operations. Failure to comply with anti-corruption laws and regulations, anti-money laundering laws and regulations, economic sanctions and/or export control regulations and other laws governing our operations could have an adverse impact on our business. We are or may become subject to various laws and regulations regarding anti-corruption, anti-money laundering, economic sanctions, investment restrictions, anti-fraud and export control regulations issued by multiple jurisdictions. These include the UK Bribery Act 2010 and the U.S. Foreign Corrupt Practices Act of 1977, as amended, which prohibits, among other things, payments, offers, or promises made for the purpose of improperly influencing any act or decision of a foreign official. The nature of our business means that we engage in significant interactions with foreign officials. In the UK, from September 1, 2025, it will also be an offense under the Economic Crime and Corporate Transparency Act 2023 for a large argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Other Government Regulations 101

organization to fail to prevent certain fraudulent activities by an associated person (such as an employee, agent, or subsidiary), unless it can demonstrate that it had reasonable prevention procedures in place to prevent the fraudulent activity. We are also subject to economic sanctions and export control rules and regulations imposed by multiple jurisdictions, including the U.S., UK, and EU. Any change in export or import regulations, economic sanctions regulations or related legislation, shift in the enforcement or scope of existing regulations, or change in the countries, governments, persons or technologies targeted by such regulations, could decrease our ability to manufacture, import, export or sell our products internationally, which could adversely affect our business. We have mechanisms in place to promote compliance with such rules and regulations. However, there can be no assurance that our policies and procedures will be followed at all times or will effectively detect and/ or prevent violations of applicable compliance regimes by our employees, consultants, sub-contractors, agents and partners. In the event of non-compliance, we could be subject to substantial civil or criminal penalties, including economic sanctions against us, incarceration for responsible employees and managers, the possible loss of export or import privileges, reputational harm, and resulting loss of revenue and profits, which could have a material adverse impact on our business, financial conditions and operations. Our performance tracked by our Environmental, Social and Governance metrics is subject to risks and the outcomes may not achieve the anticipated benefits or align with new regulations and stakeholders’ expectations. There has been an increasing focus from stakeholders and regulators relating to environmental, social and governance (ESG) matters across all industries in recent years. The standards and stakeholder expectations continue to evolve and criteria to evaluate ESG practices may change rapidly. We are subject to evolving rules, including the European Union’s Corporate Sustainability Reporting Directive (CSRD). We may also be subject to other U.S. state specific legislation, such as California’s recently enacted climate disclosure laws, which will require in-scope companies to report on greenhouse gas emissions, climate-related financial risks, and the use of carbon offsets and emissions reduction claims relating to their cooperate operations or products. The future of the California climate disclosure law is uncertain, and two of three are subject to ongoing litigation. The CSRD increases the depth of required disclosures. The specific information required to be reported on is set out in the European Sustainability Reporting Standards (ESRS). The Dutch government is in the process of implementing the CSRD into Dutch legislation. In 2025, we published our first CSRD report in alignment with the CSRD and the ESRS for the financial year 2024 and this year’s Annual Report is the first time we have reported a more extensive set of prescribed ESG data points. Performance tracked by our ESG metrics is also highly dependent on third-parties, such as our suppliers and CROs, that we do not control, which may adversely affect our reputation. In response to new ESG initiatives and regulations we may voluntarily elect, or be required, to adopt strategies, policies, or procedures related to ESG matters. Such efforts could divert management’s attention from central operational matters and cause us to expend significant capital and human resources. Moreover, increasingly, different stakeholder groups have divergent views on ESG matters, which increases the risk that any action or lack thereof with respect to sustainability or ESG matters will be perceived negatively by at least some stakeholders and adversely impact our business and reputation. The current sociopolitical landscape has led to rapid and unpredictable shifts in public sentiment, which has resulted in dynamics that increase the risk of reputational damage, boycotts and shifts in consumer behavior that could adversely affect our business and reputation. Reports could also lead to the disclosure of information that may have a negative impact on our operations and reputation which may lead to additional exposure. Failure to accurately comply with any sustainability reporting obligations may result in enforcement actions, sanctions, fines and penalties, reputational harm or private litigation. . We may become exposed to liability and substantial expenses in connection with environmental compliance or remediation activities. Our operations, including our research, development, testing and third-party manufacturing activities, are subject to numerous environmental, health and safety laws and regulations and for which we may become liable. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Other Government Regulations 102

If we or one of our CMOs or third-party distributors, manufacturers, licensees or co-marketers fail to comply with such laws and regulations, such failure could result in substantial fines, penalties or other sanctions which could also bring significant reputational loss to our business. We face a risk of environmental liability inherent in our current and historical activities, including liability relating to releases of our exposure to hazardous or biological materials. Furthermore, environmental, health and safety laws and regulations are becoming more stringent. Both us and our CMOs may be required to incur substantial expenses in connection with future environmental compliance or remediation activities, in which case, our production and development efforts may be interrupted or delayed, and our financial condition and results of operations may be materially adversely affected. 2.6 Risk Factors Related to argenx’s Financial Position We expect to increase our expenses for the foreseeable future, and we may not be able to raise additional capital, be profitable or sustain net profitability in the future in order to fund our operations. We intend to continue to conduct research and development, preclinical testing, clinical trials and regulatory compliance activities as well as the continued commercialization of VYVGART and other products candidates, for current and future indications, and we intend to continue our efforts to expand our sales, marketing and distribution infrastructure. We anticipate that our operating expenses will increase as we execute our strategic objectives and as we experience delays or encounter issues relating thereto, including failed clinical trials, ambiguous clinical trial results, safety issues or other regulatory challenges. To be profitable or sustain net profitability in the future, we must succeed in commercializing products that generate significant product net sales. Our future results of operations and profitability may fluctuate from period to period, and we will need to generate significant revenues to be profitable or sustain net profitability in the future. We may not be able to generate these revenues, and we may never achieve profitability on a sustained basis in the future. If we do not succeed in sustaining profitability, we would not be able to use deferred tax assets against taxable profits which would result in a de-recognition of our deferred tax asset balance. To finance our operations, we may need to raise additional capital through a combination of public or private equity or debt financings or other sources, which may include collaborations with third parties. Our ability to raise additional funds on acceptable terms or at all will depend on financial, economic and market conditions and other factors, over which we may have no or limited control. If we are unable to raise additional capital if and when needed, or if the terms are not acceptable, our business strategy could be impacted, and we may be forced to delay, reduce or terminate one or more of our research or development programs or the commercialization of any of our products or product candidates, including new indications, or be unable to expand our operations or otherwise capitalize on our business opportunities, all of which may have a material adverse impact on our business, financial condition and results of operations. Our assets, earnings and cash flows and the investment of our cash and cash equivalents may be subject to risks which may cause losses and affect the liquidity of these investments. We invest our cash in accordance with an established internal investment policy. Currently, substantially all of our available cash and cash equivalents and current financial assets are invested in either current accounts, savings accounts, term accounts or highly liquid money market funds. Any future investments may include term deposits, corporate bonds, commercial paper, certificates of deposit, government securities and money market funds in accordance with our cash investment policy. These investments may be subject to general credit, liquidity, market, inflation, foreign currency and interest rate risks and we may realize losses in the fair value of these investments or a complete loss of these investments. The argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to Other Government Regulations 103

aforementioned risks associated with our cash flows and investment portfolio may adversely affect our results of operations, liquidity and financial condition. Due to the international scope of our operations, our assets, earnings and cash flows are influenced by movements in exchange rates of several currencies, particularly the euro and Japanese Yen. Our revenue from outside of the U.S. will increase as our products, whether commercialized by us or our business partners or our collaborators gain marketing approval in such jurisdictions. If the U.S. dollar weakens against a specific foreign currency, our revenues will increase, having a positive impact on net income, but our overall expenses will increase, having a negative impact. Conversely, if the U.S. dollar strengthens against a specific foreign currency, our revenues will decrease, having a negative impact on net income, but our overall expenses will decrease, having a positive impact. Continued volatility in foreign exchange rates is likely to impact our operating results and financial condition. 2.7 Risk Factors Related to argenx’s Business and Industry Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, or insider trading violations, which could significantly harm our business. We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with governmental regulations, comply with healthcare fraud and abuse and anti-kickback laws and regulations in the U.S. and other markets, failure to report financial information or data accurately or disclose unauthorized activities to us, among others. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of material information, including improper trading based upon, information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We maintain a global compliance program and remain focused on its evolution and enhancement. Our program includes efforts such as risk assessment and monitoring, fostering a culture encouraging employees and third parties to raise good faith questions or concerns, and defined processes and systems for reviewing and remediating allegations and identified potential concerns. It is not always possible, however, to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions. We may become exposed to costly and damaging liability claims. We are exposed to potential product liability and professional indemnity risks that are inherent in the research, development, manufacturing, marketing and use of pharmaceutical products and marketing of human therapeutic products. The current and future use of products and product candidates by us and our collaborators in clinical trials and the sale of any approved products may further expose us to liability claims. If any of our products or product candidates were to cause adverse side effects during clinical trials or after approval of the product candidate, we may be exposed to substantial liabilities. These claims might be made by patients who use the product, healthcare providers, pharmaceutical companies, physicians, payors, caregivers, investors, employees, government agencies, or our collaborators or others selling such products. Physicians and patients may not comply with any warnings that identify known potential adverse effects and patients who should not use our product candidates. Any claims against us, regardless of their merit, could be difficult and costly to defend and could materially adversely affect the market for our products and product candidates or any prospects for commercialization of our products and product candidates. Any such claims, regardless of their merit, could also adversely affect our reputation and the trust that physician and patients place in our products. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Financial Position 104

Product liability risk in the EU will increase in the future once plaintiff-friendly reforms, such as Directive (EU) 2024/2853 (Product Liability Directive), take effect. The Product Liability Directive introduces claimant- friendly changes. This includes, for instance, the expansion of the definition of “damage” (e.g. by including medically recognized psychological harm), creating rebuttable presumptions as to defect and causation to help claimants prove their case (e.g. if the claimant faces excessive difficulties to prove this due to scientific complexity) and abolishing minimum or maximum financial thresholds for claims. The Product Liability Directive, like its predecessor, provides that claims shall expire if the injured person does not initiate proceedings within ten years after the defective product was placed on the market. However, it extends this longstop period to 25 years if this is due to the latency of the underlying personal injury. Member States must transpose the Product Liability Directive into national law by December 2026. Regardless of the merits or eventual outcome, litigation or liability claims may result in: • decreased demand for our products due to negative public perception; • damage to our reputation; • withdrawal of clinical trial participants or difficulties in recruiting new clinical trial participants; • initiation of investigations by regulators; • costs to defend or settle the related litigation; • a diversion of management’s time and our resources; • substantial monetary awards to clinical trial participants or patients; • product recalls, withdrawals or labeling, marketing or promotional restrictions; • loss of revenues from product sales; and • the inability to successfully commercialize our product candidates, if approved. Although we maintain product liability insurance, we may not be able to maintain insurance coverage at a reasonable cost or to obtain adequate insurance coverage to satisfy any liability that may arise. Product liability claims could delay or prevent completion of our clinical development programs. In addition, claims made by patients, healthcare professionals or others might not be fully covered by product liability insurance and could result in investigations of the safety of our products or product candidates or may result in recalls. If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, our assets may not be sufficient to cover such claims and our business, financial condition and results of operations would be adversely affected. In the ordinary course of business we may also face substantial, complex or extended litigation that could cause us to incur significant costs and distract our management. This is especially relevant for biopharmaceutical companies. Such litigation or proceedings could substantially increase our operating expenses and could adversely affect our business. We may engage in strategic transactions, including acquisitions, collaborations, licenses or investments in other companies or technologies, and we may not realize the benefits of such transactions. We may enter into strategic transactions, including acquisitions, collaborations, licenses or investments for or in other companies or technologies that complement or augment our existing business and facilitate our access to new products, research projects or geographical areas. However, we may not be able to identify appropriate targets or enter into such transactions under satisfactory conditions. In addition, we may need additional funding to finance these transactions including through issuances of public or private equity or convertible debt securities, which could be dilutive to our shareholders and ADS holders. Integrating any newly acquired companies, business, technologies or products could be expensive, time- consuming, and may never be successful. Integration efforts often take a significant amount of time, place a significant strain on managerial, operational and financial resources, result in loss of key personnel and could prove to be more difficult or expensive than we predict. The diversion of our management’s attention and any delay or difficulties encountered in connection with any future transactions we may consummate could result in the disruption of our ongoing business or inconsistencies in standards and controls that argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Business and Industry 105

could negatively affect our ability to maintain third-party relationships. We cannot assure that we will achieve the expected synergies to justify any such transaction, which could have a material adverse effect on our business, financial condition, results of operations and future growth prospects and our investors’ ability to realize on their investment. Our business and operations could suffer in the event of system failures or unauthorized or inappropriate use of or access to our systems. We are increasingly dependent on our information technology systems and infrastructure for our business. We collect, store and transmit sensitive information including intellectual property, proprietary business information, including highly sensitive clinical trial data, and personal data in connection with business operations. The secure maintenance of this information is critical to our operations and business strategy. Some of this information could be an attractive target of criminal attack or unauthorized access and use by third parties with a wide range of motives and expertise, including organized criminal groups, “hacktivists”, patient groups, disgruntled current or former employees and others. Cyber-attacks are of ever-increasing levels of sophistication, and despite our security measures, our information technology and infrastructure may be vulnerable to such attacks or may be breached, including due to employee error or malfeasance. Although we are making significant efforts to maintain the security and integrity of our information systems and are exploring various measures to manage the risk of a security breach or disruption, there can be no assurance that our security efforts and measures will be effective or that attempted security breaches or disruptions would not be successful or damaging. Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage or interruption from computer viruses, unauthorized or inappropriate access or use, natural disasters, pandemics, terrorism, war (including the ongoing conflict in Ukraine and the ongoing conflict in Israel and the Gaza Strip), and telecommunication and electrical failures. For example, the loss of pre-clinical trial data or data from completed or ongoing clinical trials for our product candidates could result in delays in our regulatory filings and development efforts, as well as delays in the commercialization of our products, and significantly increase our costs. To the extent that any disruption, security breach or unauthorized or inappropriate use or access to our systems were to result in a loss of or damage to our data, or inappropriate disclosure of confidential, personal or proprietary information, we could incur notification obligations to affected individuals and government agencies, liability, including potential lawsuits from patients, collaborators, employees, stockholders or other third parties and liability under foreign, federal and state laws that protect the privacy and security of personal data, and the development and potential commercialization of our product candidates could be delayed. Not all of our contracts contain limitations of liability, and even where they do, there can be no assurance that limitations of liability in our contracts are sufficient to protect us from liabilities, damages or claims related to our data privacy and security obligations. We cannot be sure that our insurance coverage will be adequate or sufficient to protect us from or to mitigate liabilities arising out of our privacy and security practices. We are highly dependent on public perception of our products. We are highly dependent upon consumer perceptions of the safety and quality of our products. We could be adversely affected if we, or any of our collaborators, are subject to negative publicity or if any of our products or any similar products distributed by other companies prove to be, or are asserted to be, harmful to patients, or for example, be deemed cruel to animals. In addition, if patients have negative perceptions of our products and our competitors are successful in developing biosimilar or interchangeable products for our products and product candidates, patients may choose the other biosimilar or interchangeable products sold by our competitors. Because of our dependence upon consumer perception, any adverse publicity associated with illness or other adverse effects resulting from patients’ use or misuse of our products or any similar products distributed by other companies, or adverse results reported by us, our collaborators or other companies relating to clinical studies may subject our products to class warnings or negatively impact our public perception of our products and product candidates, which could have a material adverse impact on our business, prospects, financial condition and results of operations. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Business and Industry 106

2.8 Risk Factors Related to argenx’s Intellectual Property We may be unable to adequately maintain, enforce or protect our intellectual property rights in products, product candidates and platform technologies which could adversely affect our ability to maximize the value for patients in our marketed products and product candidates. Our commercial success depends in part on obtaining and maintaining patents and other forms of intellectual property rights for our products, product candidates and platform technologies. Failure to obtain, maintain, enforce, protect, or extend adequate patent and other intellectual property rights, which can be challenging and costly, could adversely affect our ability to develop and market our products and product candidates and reduce any competitive advantage we may have. We cannot be certain that patents will be issued or granted with respect to applications that are currently pending and we may not be the first to file patent applications related to our product candidates and products. The scope of patent protection that the European Patent Office and the USPTO will grant with respect to products in our product pipeline is uncertain and may vary. It is possible that the European Patent Office and USPTO will not allow broad claims that cover molecules closely related to our products and product candidates as well as the specific molecule and competitors may be free to market substantially similar molecules if granted approval, thereby reducing our market potential. We and our current or future licensors, licensees or collaboration partners may not be able to prepare, file, prosecute and maintain all necessary or desirable patent applications at a reasonable cost or in a timely manner. Our current and future licensors’, licensees’ or collaboration partners’ ability to ensure the issuance, scope, validity, enforceability and commercial value of licensed technology is uncertain and we may need to rely on them to obtain costly additional IP licenses. Additionally, such parties may not fully comply with applicable patent rules or laws, which could result in loss of patent rights, or such parties may disagree with us as to the strategy for prosecution, maintenance or enforcement of any such patent rights. Filing, prosecuting, and defending patents on product candidates in all jurisdictions throughout the world would be prohibitively expensive and the laws of certain jurisdictions may not protect our rights to the same extent as the laws of the U.S., UK or EU. We may face difficulties in enforcing patent rights in the future, including in certain jurisdictions where we have not yet filed patent applications. Competitors may use our and our licensors’ or collaboration partners’ technologies in jurisdictions where we have not obtained patent protection, or where broad research exemptions are available, to develop their own products and may export otherwise infringing products to territories where we, our licensors or collaboration partners have patent protection, but where enforcement is not as strong as that in the U.S., UK and the EU. In such cases, we would have little effective recourse to prevent such products from competing with ours. In addition, some countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties, and other countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Intellectual Property 107

Intellectual property litigation could lead to substantial resource diversion or issued patents could be found invalid, not infringed, or unenforceable if challenged in the applicable patent office or court. Our patents may remain open to invalidity challenges after allowance or grant, whereby third parties can challenge the scope or validity of such granted patent. In the course of such proceedings, we may be compelled to limit the scope of patent claims thus challenged or may lose the claims altogether. We may elect to initiate adversarial proceedings in order to enforce or defend any intellectual property rights owned by or licensed to us, or to determine or challenge the scope or validity of intellectual property rights of third parties to protect our competitive position. We may need to divert substantial time and resources to the enforcement and protection of our or our collaboration partners’ intellectual property rights. In addition, the outcomes are uncertain and any remedies or damages awarded may not be meaningful. An adverse ruling of non-infringement, limiting claim scope, or invalidating one or more of our issued patents could allow third parties to commercialize our products after the expiration of our market exclusivity or use our platform technologies to compete directly with us, without payment to us. We may be subject to claims challenging the inventorship or ownership of our intellectual property or be required to make additional payments to secure intellectual property from collaborators. Many of our consultants and employees, including in the senior management team (consisting of our CEO and senior personnel reporting directly to the CEO) (Senior Management Team), were previously employed at other competing or potentially competing biotechnology or pharmaceutical companies and some have executed proprietary rights, non-disclosure and non-competition agreements in connection with such previous employment. Although we take measures to ensure third parties, consultants and employees do not use such proprietary information in their work for us, we may be subject to claims that we or these consultants and employees have improperly used or disclosed confidential information or intellectual property of their former employer. Additionally, many of our collaborators do not commit to assigning all intellectual property arising out of our collaborations to us and, instead, grant us options to acquire intellectual property or commit to making such intellectual property available to us at a fair price. As such, we may be required to make additional payments to secure valuable intellectual property rights under our existing collaborations or become subject to inventorship disputes. In addition, although we take steps to ensure that our collaborators do not use our intellectual property rights other than for the purposes of our collaboration, there may be instances where former or current collaborators or other third parties nevertheless apply for or obtain patent protection for inventions to which we believe we have rights, in whole or in part. In such cases, we may elect to assert our ownership of such intellectual property. There is no guarantee that we will be successful in asserting such claims. In addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in ensuring effective assignment of intellectual property under such agreements. Our assignment agreements may not be self-executing, or may be breached, and we may be forced to bring or defend against claims to assert ownership of such intellectual property. There is no guarantee we will be successful in pursuing such claims, which could result in us paying monetary damages or losing valuable personnel or intellectual property rights. Third-party intellectual property rights could adversely affect our ability to commercialize our products and product candidates. Our competitive position may suffer if valid and enforceable third-party intellectual property rights cover our products, product candidates, manufacturing processes, or those of our partners. In such cases, our freedom to develop or commercialize products or product candidates may require obtaining a license, designing around third party intellectual property rights with significant time and materials costs, or invalidating the third party rights. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Intellectual Property 108

If our products are found to infringe a valid and enforceable patent claim, we and our partners could be prevented from continuing to develop or commercialize the affected product without an appropriate license, which may be costly or unavailable on commercially reasonable terms, if at all. Similarly, other companies may have filed patent applications or have patents directed toward similar targets for certain of our products and we may not be aware of unpublished pending patent applications or patent applications that are amended to cover our products or platform technologies. Even if we or our partners can obtain the appropriate license, it may be non-exclusive, thereby providing our competitors with opportunity to access the same licensed technology. If the breadth and scope of protection provided by our or our partners’ patents, licensed patents or patent applications is threatened or limited, it could dissuade companies from future collaborations with us to license, develop or commercialize products and product candidates which would have an adverse effect on our competitive business position. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, some of our confidential information could be disclosed in any such proceedings. We may not be successful in obtaining or maintaining necessary rights to our products and product candidates through acquisitions and in-licenses. We may be unable to acquire or in-license third-party intellectual property rights necessary or useful for development or commercialization of our product, product candidates or technology. We sometimes collaborate with U.S. and non-U.S. academic institutions and typically receive an option to negotiate a license to the institution’s proprietary interest in any collaboration technology. However, we may be unable to successfully negotiate such license and the institution may offer such intellectual property rights to third parties thereby blocking our ability to pursue the applicable program. In addition, our competitor companies may be unwilling to license desirable or necessary intellectual property rights to us, or we may be otherwise unable to license or acquire other third party intellectual property rights on commercially reasonable terms which could negatively impact current development or hinder our ability to pursue development of new programs. Under our existing licenses, failure to comply with our obligations thereunder could result in termination of such licenses, thereby limiting our ability to develop and commercialize products covered by such licensed technology. Moreover, despite our efforts to comply with our contractual obligations, our licensors could conclude we have materially breached any such agreement and we could incur significant costs and disruption to our business defending against any breach alleged by the licensor. Moreover, several of our existing license agreements are sub-licenses from third parties. We have little control if our licensors fail to comply with their obligations under their upstream license agreements, whereby the original third-party licensor may have the right to terminate the original license and possibly our sub-license. In such cases, we may not be able to procure a direct license covering such intellectual property possibly materially affecting our ability to develop and commercialize certain products and product candidates. If our brand protection strategies, including the filing, prosecution and enforcement of trademarks and trade names, are not adequately executed, we may not be able to build name recognition for approved products in our markets of interest in line with our strategic priorities. Third parties may seek to oppose, attempt to cancel our trademark applications, or challenge, infringe or circumvent our registered and unregistered trademarks, including through counterfeiting of our products. In the event that our trademarks are successfully challenged, we may not be able to use these trademarks to continue to effectively market our branded products and could be forced to rebrand them, which could result in loss of their brand recognition or require us to devote resources to develop new brand profiles. Such efforts could also hinder our efforts to commit to and deliver on strategic internal initiatives. If we attempt to enforce our trademarks or assert trademark infringement claims, a court may determine that our trademarks are invalid or unenforceable or that the party against whom we have asserted argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Intellectual Property 109

trademark infringement has superior rights with respect to such marks. If we are unable to establish name recognition and adequately protect and enforce our trademark portfolio, we may not be able to compete effectively in the market or build brand recognition for new products globally. We may not be able to obtain protection under the U.S. Drug Price Competition and Patent Term Restoration Act of 1984 (Hatch-Waxman Act) and similar non- U.S. legislation for extending the term of patents covering each of our products and product candidates. Depending upon the timing, duration and conditions of FDA marketing approval of our product candidates, one or more of our U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Act and similar legislation in the EU and the Asia Pacific region. However, the patent term extension under the Hatch Waxman Act cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, and only one patent applicable to an approved drug may be extended. In the EU, the term of a patent for a medicinal product can be extended by a supplementary protection certificate (SPC), but not beyond a maximum of five years (except for patents for products for pediatric use, for which the term can be extended by a further six months). However, products containing a compound for which an SPC has already been granted are not eligible for SPC protection. Therefore, we will not be able to extend the term of a patent relating to a medical compound for which an SPC has already been granted, even if the patented product relates to a different medical use of that compound. If we are unable to obtain patent term extension, the term of any such extension is less than we request, or the statutes governing patent term extension are amended to reduce the term of such extensions, our patent exclusivity for that product will be shortened and our competitors may obtain approval to market competing products sooner than we expect. Changes in patent laws or patent jurisprudence could diminish the value of patents in general thereby impairing our ability to protect our products. Changes in patent law across jurisdictions, or changes in any relevant government’s enforcement procedure may weaken our ability to obtain new patents or to enforce rights in our owned and licensed patents. For example, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. Relatedly, the U.S. Congress is considering multiple draft bills that, if passed, may have a significant impact on U.S. patent laws. Any such changes by the U.S. Congress or U.S. courts and the relevant law-making bodies in other countries may materially affect our patents or patent applications and we cannot predict the effects of future changes in patent law. We may be unable to protect the confidentiality of our trade secrets and their disclosure to competitors, harming our market position. In addition to patent protection, we rely on trade secret protection for our proprietary information, including, for example, certain aspects of our llama immunization and antibody affinity maturation approaches. However, while we take appropriate measures to prevent misappropriation of our trade secrets and restrict access to them internally, trade secrets are difficult to protect. Despite requiring our licensors, collaborators, suppliers, consultants and advisors to execute confidentiality agreements, we cannot fully protect against willful or inadvertent unauthorized disclosure of our trade secrets by such counterparties to competitors, and we may not be able to secure adequate legal or equitable remedies to prevent our competitors from using these trade secrets. Any such disclosure, whether willful or inadvertent, could enable our competitors to duplicate or build upon our technology. In addition, enforcing a claim that a third party unlawfully misappropriated trade secrets is expensive, time- consuming and the outcome is unpredictable, and the enforceability of the underlying confidentiality agreements protecting these trade secrets may vary across jurisdictions. Further, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, they could use such technology to compete with us. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Intellectual Property 110

2.9 Risk Factors Related to argenx’s Organization and Operations Our future growth and ability to compete depends on maintaining our culture, retaining our key personnel and recruiting additional qualified personnel. We believe that our corporate culture has been, and will continue to be a key contributor to our success. However, as we continue to grow and evolve, our ability to foster our key values - innovation, co-creation, empowerment, excellence and humility that we believe are important to support our growth - may be impacted. For example, investors, regulators, customers, employees and other stakeholders continue to focus on ESG matters, including in workforce policies and initiatives and we may not be able to meet the different expectations and demands of all our stakeholders in that respect, which could result in adverse publicity, harm our reputation and negatively impact our ability to attract, retain and motivate qualified employees and our future success. As we implement more complex organizational structures, and increase our headcount to support the growth in our business, we may find it increasingly difficult to maintain the beneficial aspects of our corporate culture, which could similarly negatively impact our ability to attract, retain and motivate qualified employees and our future success. As a global organization in a highly competitive and specialized industry, our success also depends upon the continued contributions of our key management, scientific, medical and technical personnel, many of whom have been instrumental for us and have substantial experience with our product and related technologies. These key management individuals include the members of the Board of Directors and Senior Management Team. Difficulties in recruiting or the loss of key managers, scientific, medical or technical personnel could delay our research and development activities. In addition, it may be difficult to attract and retain highly qualified management, scientific and medical personnel, particularly if we expand into fields that will require additional skills. As a Dutch company listed on Euronext Brussels in addition to Nasdaq, our remuneration practices and policies may be limited by local governance rules or shareholder guidance for EU companies. Such limitations may make it more difficult to successfully compete for key talent in a number of markets with differing remuneration practices and policies compared to our competitors. For example, the Dutch Corporate Governance Code 2022 (DCGC) places certain limitations on the ability to grant equity incentives to Non-Executive Directors, while Belgian law requires Non-Executive Directors to receive part of their remuneration in the forms of shares, but not stock options. The DCGC also places limitations on amount of severance payment permitted in the event of dismissal. Many other biotechnology and pharmaceutical companies and academic institutions that we compete against for qualified personnel have greater financial and other resources, different risk profiles and a longer history in the industry than we do. Additionally, an inflationary environment, combined with the tight labor market for the recruitment and retention of skilled workers, could make it more costly for us to attract or retain employees. In order to meet the compensation expectations of our prospective and current employees due to inflationary factors, we may be required to increase our operating costs. Therefore, we might not be able to attract or retain these key persons on conditions that are economically acceptable. Global geo- and socio-political threats and macro-economic uncertainty and other unforeseen political crises could materially and adversely affect our business and financial performance. Many geo- and socio-political threats and macro-economic uncertainties are outside of our control and could adversely affect consumer confidence and disposable income levels, increase difficulty in forecasting our financial results and have other impacts on our business and financial performance. Such geo- and socio-political threats could also result in volatility in stock markets in general, causing our stock to have extreme price and volume fluctuations unrelated to our business and financial performance. Such geo- and socio-political threats and uncertainties include: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Organization and Operations 111

• general economic and market conditions, including instability resulting from inflationary pressures, increasing interest rates and the ongoing Russia-Ukraine and Middle East conflicts; • geopolitical events, including natural disasters, public health issues (including pandemics), acts of war (such as the Russia-Ukraine and Middle East conflicts), and terrorism; • economic and trade sanctions, import and export regulations, customs, outbound investment • restrictions, changes in trade agreements, trade barriers or other restrictions on foreign trade, and changes in trade regulations and restrictions, including between the U.S. and other countries; • global or regional economic conditions that impact companies and customers with which we do business; • political or social unrest, economic instability, repression, or human rights issues; • disruptions in supply chains; • risks related to other government regulation or required compliance with local laws; and • consumer and commercial credit availability, unemployment, and consumer debt levels; • local licensing and reporting obligations. Due to our international operations and the fact that we run clinical trials in a large number of jurisdictions, the eruption of global conflicts may negatively impact our ability to conduct or complete clinical trials in the affected regions, which could adversely affect our business and financial performance. For example, on June 12, 2024, the U.S. Department of the Treasury’s Office of Foreign Assets Control issued General License 6D to replace General License 6C. General License 6D authorizes “clinical trials and other medical research activities” that would otherwise be prohibited by U.S. sanctions targeting Russia, and General License 6D does not have an expiration date. Additionally, the conflict between Russia and Ukraine and the sanctions imposed upon Russia by the U.S., the UK, and the EU, among others could have a material adverse impact on our business, financial conditions and operations. The sanctions and export controls landscape is evolving and may change unexpectedly at any time. We also perform development activities in a number of countries exposed to geopolitical risk and if conflicts in those countries were to escalate further and impact neighboring countries, it could impact our development activities in those countries. Changes in U.S.-Mainland China relations, including tariffs, export controls, sanctions, and other regulations may adversely impact our collaboration with Zai Lab in Mainland China, Hong Kong, Taiwan and Macau (together, Greater China). The U.S. government has taken steps and continues to take steps with regard to U.S.-Mainland China relations that will impact companies with connections to the U.S. or Mainland China, including by imposing tariffs affecting certain products manufactured in Mainland China, imposing certain sanctions on individuals and entities in the Mainland China, and issuing statements indicating enhanced review of companies with significant Mainland China-based operations. The U.S. government may pass laws that could potentially severely restrict our ability to contract with certain Chinese biotechnology companies without losing our ability to contract with or receive funding from the U.S. government. Such restrictions could have an adverse impact on our operations. Several countries are considering or have implemented tariffs, trade barriers or restrictions, as well as other measures impacting cross-order commerce, which could negatively affect our business, financial conditions and results of operations, including by negatively impacting our revenues from product sales or our cost of goods sold. The U.S. federal government has implemented tariffs on certain foreign goods and may implement additional or revised tariffs in the future. Such actions could give rise to an escalation of trade measures by the U.S. and impacted countries. Developments with regard to the timing and manner in which tariffs will be implemented; the amount, scope, and nature of tariffs; the countries subject to new or additional tariffs imposed by the U.S.; tariffs imposed by other countries on goods imported from the U.S.; and other wide-ranging retaliatory measures are rapidly evolving and may change unexpectedly at any time. Any new legislation, executive orders, tariffs, export controls, sanctions and/or other regulations that may be implemented, any unfavorable government policies on international trade, including tariffs and export controls, the renegotiation of existing trade agreements, any increased scrutiny on companies with significant Mainland China-based operations, and any retaliatory actions taken by the U.S., EU, Chinese or argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Organization and Operations 112

other governments due to trade tensions could have an adverse effect on our business, including the development and commercialization of products containing argenx-licensed material. Further, general political uncertainty may have an adverse impact on our business, financial condition and results of operations. For example, significant political events in the U.S. may cast uncertainty on global financial and economic markets, especially following the recent U.S. presidential election. We face risks related to natural disasters and public health issues, that could negatively affect our business and financial condition. Our business could be adversely impacted by the effects of catastrophic global events including natural disasters such as earthquakes, fires, hurricanes, tornados, floods or significant power outages and public health crises, such as the COVID-19 pandemic. For example, the manufacturing of all of our products and product candidates requires using cells which are stored in a cell bank. We have one master cell bank for each product manufactured in accordance with cGMPs. However, it is possible that we could lose multiple cell banks and have our manufacturing significantly impacted by the need to replace these cell banks, which could materially adversely affect our business, prospects, financial condition and results of operations. Public health issues could also negatively affect our business and financial condition. We operate and conduct our clinical trials globally. We cannot presently predict the scope and severity of any potential future business shutdowns or disruptions as a result of public health issues. If we or any of the third parties with whom we engage, including the suppliers, contract manufacturers, clinical trial sites, regulators and other third parties, were to experience shutdowns, quarantines, or other business disruptions due to natural disasters or global public health issues, it may impair our or our third-party partners’ ability to initiate clinical trials and recruit and retain patients, particularly if quarantine or travel restrictions impede healthcare provider or patient movement, impact the usability of the data due to treatment interruptions and require protocol amendments. In addition, regulatory authorities may restrict their operations or be delayed in their operations during a pandemic, the outbreak of new variants or other public health issues, including further to travel restrictions which could adversely affect our ability to obtain regulatory approval for and to commercialize our products and product candidates and have a material adverse effect on our business and financial results. We may encounter difficulties efficiently managing our growth and our increasing development, regulatory, and sales and marketing capabilities, which could disrupt our operations. We have grown, and expect to continue to grow, significantly in the number of employees and scope of operations over recent years, particularly in the areas of drug research, drug development, regulatory affairs, and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. In particular, we must efficiently leverage our own sales and marketing capabilities in order to launch or market our products candidates effectively. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. Our limited financial, manufacturing and management resources, could cause us to forgo or delay the pursuit of opportunities with potential product candidates that later prove to have greater market potential, fail to capitalize on viable commercial products or profitable market opportunities or relinquish rights to such product candidates through collaborations, licensing or royalty arrangements in circumstances where it would have been more advantageous for us to retain sole development and commercialization rights. Any inability to manage growth could delay the execution of our strategic objectives or disrupt our operations, which in turn could materially harm our business and prospects. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Organization and Operations 113

We are exposed globally to unanticipated changes in tax laws and regulations, adjustments to our tax provisions, exposure to additional tax liabilities, or adjustments of our tax assets. As a company active in research and development, we have benefited from certain research and development tax incentives including tax credits and a payroll withholding tax exemption. We also expect to benefit from the Belgian innovation income deduction. The determination of our provision for income taxes and other tax liabilities requires judgment, including the adoption of certain accounting policies and our determination of whether our deferred tax assets are, and will remain, fully available in future periods. We cannot guarantee that our interpretation of applicable tax laws (including with respect to our eligibility for, or our calculation of, tax incentives such as the Belgian R&D tax credit, the Belgian payroll withholding tax exemption for R&D personnel, the Belgian innovation income deduction and similar tax incentives in other jurisdictions in which we have material operations or sales), our transfer pricing policies or our organizational and operational structure will not be questioned by the relevant tax authorities, or that the relevant tax laws and regulations, or the interpretation thereof, including through tax rulings, will not be subject to change. Our effective tax rates could be adversely affected, now or in the future, by changes in tax laws, treaties and regulations or the interpretation thereof by the relevant tax authorities in countries where we have material operations. A successful challenge to tax positions in Belgium or other country where we have material operations may lead to adjustments in the amounts recorded in our financial statements and could have a significant impact on our effective tax rate and on our deferred tax assets. An increase of the effective tax rates could have an adverse effect on our business, financial position, results of operations and cash flows. In 2021, the Organisation of Economic Co-operation and Development (OECD) published a proposal that included a global minimum tax (Pillar Two). To date, many jurisdictions are in various stages of implementation of Pillar Two rules. Based on current information, management expects that the Company will be subject to the Pillar Two Directive and domestic laws in 2025, as it is the year the Company has met all requirements under the Pillar Two legislation. The Company does not expect the Pillar Two rule to have a material impact on the effective tax rate of the Group. In case of a change of control, we could be exposed to the risk of losing any unused tax credit and innovation income deduction. Furthermore, if any legislator decides to eliminate, or change the conditions for claiming such tax incentives, or reduce the scope or the rate of such incentives, any of which it could decide to do at any time, our results of operations could be adversely affected including through the de- recognition of deferred tax assets. 2.10 Risk Factors Related to the ADSs Holders of our ADSs have fewer rights than our ordinary shareholders. Except as described in this Annual Report or any deposit agreements, holders of ADSs are not treated as our shareholders unless they withdraw the ordinary shares underlying their ADSs in accordance with the deposit agreement and applicable laws and regulations. The depositary, or its nominee, is the holder of the ordinary shares underlying the ADSs. ADSs are transferable on the books of the depositary. The depositary may refuse to deliver, transfer or register transfers of ADSs. Temporary delays in the cancellation of ADSs and withdrawal of the underlying ordinary shares may arise. In addition, ADS holders may not be able to cancel their ADSs and withdraw the underlying ordinary shares when they owe money for fees, taxes and similar charges and when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited securities. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to argenx’s Organization and Operations 114

Holders of ADSs may vote them in person or by proxy in accordance with applicable laws and regulations and our articles of association (Articles of Association). We cannot guarantee that holders of ADSs will receive the voting materials in time to ensure that they can instruct the depositary to vote the ordinary shares underlying their ADSs. Holders of our ADSs may not be able to exercise their right to give voting instructions or to vote in person or by proxy and they may not have any recourse against the depositary or us if their ordinary shares are not voted as they have requested or if their shares cannot be voted. The price of our ADSs may be volatile and may fluctuate due to factors beyond our control. An active public trading market may not be sustained. The stock markets in general, and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. The trading price of our ADSs depends on a number of factors, including those described elsewhere in this “Risk Factors” section, many of which are beyond our control and may not be related to our operating performance, which may limit or prevent investors from readily selling their ADSs or ordinary shares and may otherwise negatively affect the liquidity of our ADSs and ordinary shares. We provide guidance regarding our cash and expenses, which are inherently uncertain. Any guidance that we provide may not always be accurate or may vary. If we fail to meet our guidance, or if we have to revise such guidance, the price of our ADSs or ordinary shares could decline. Sales of a substantial number of ADSs or our ordinary shares in the public market, or the perception that these sales might occur, could depress the market price of ADSs or our ordinary shares and could impair the market price of our securities or our ability to raise capital through the sale of additional equity securities. In addition, an active public trading market for our ADSs may not be sustained. Further, fluctuations in exchange rates may also impact the price of our ADSs and ordinary shares which may result in heavy trading by investors seeking to exploit such differences, or impact the proceeds holders receive. If securities or industry analysts cease coverage of us, or publish inaccurate or unfavorable research about our business, the price of our ADSs or ordinary shares and our trading volume could decline. The trading market for the ADSs and ordinary shares depends in part on the research and reports that securities or industry analysts publish about us or our business. We do not have control over these analysts. If no or too few securities or industry analysts cover us, the trading price of our ADSs and ordinary shares would likely be negatively affected. If one or more of the analysts who cover us downgrade our ADSs or ordinary shares or publish inaccurate or unfavorable research about our business, the price of our ADSs or ordinary shares would likely decline. If we fail to maintain an effective system of disclosure controls and internal control over financial reporting, our ability to comply with applicable regulations could be impaired, and the trading price of our ADSs may be negatively impacted. We are required to comply with various corporate governance and financial requirements under the Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing rules of the Nasdaq Global Market (the Nasdaq listing Rules) and requirements, and other applicable securities rules and regulations. We are required to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting and an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur the expense of remediation. Moreover, any failure to maintain internal control over financial reporting or any material weaknesses or significant deficiency thereover, could result in a loss of investors’ in the accuracy, completeness and reliability of our financial statements, subject us to sanctions or investigations, or negatively impact the trading price of our ADSs or ordinary shares. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to the ADSs 115

2.11 Risk Factors Related to being a Foreign Private Issuer or a Dutch Company The risks in this subsection that relate to our status as a foreign private issuer will change if we lose our status as a foreign private issuer under U.S. law. We are a Dutch European public company with limited liability. The rights of our shareholders may be different from the rights of shareholders in companies governed by the laws of U.S. jurisdictions. We are a Dutch European public company with limited liability (Societas Europeae ). The rights of shareholders and the responsibilities of members of our Board of Directors may be different from the rights and obligations of shareholders in companies governed by the laws of U.S. jurisdictions. As a result of these differences between Dutch corporate law and our Articles of Association, on the one hand, and the U.S. federal and state laws, on the other hand, in certain instances, our shareholders and holders of our ADSs could receive less protection than they would as shareholders or ADS holders of a listed U.S. company. For example, provisions of our Articles of Association may make it more difficult for a third party to acquire control of us or effect a change in our Board of Directors. We have adopted several provisions that may have the effect of making a takeover of our Company more difficult or less attractive. These provisions could discourage potential takeover attempts that other shareholders may consider to be in their best interest and could adversely affect the market price of our securities. Holders of our ordinary shares outside the Netherlands, and holders of ADSs may not be able to exercise pre-emptive rights or preferential subscription rights, respectively. In the event of an increase in our share capital, holders of our ordinary shares are generally entitled under Dutch law to full pre-emptive rights, unless these rights are excluded either by a resolution of the shareholders at a General Meeting, or by a resolution of the Board of Directors (if the Board of Directors has been designated by the shareholders at a General Meeting for this purpose). However, making pre-emptive rights available to holders of ordinary shares or ADSs representing ordinary shares also requires compliance with applicable securities laws in the jurisdictions where holders of those securities are located, which we may be unable or unwilling to do. In particular, holders of ordinary shares or ADSs located in the U.S. would not be able to participate in a pre-emptive rights offering unless we registered the securities to which the rights relate under the U.S. Securities Act of 1933, as amended (Securities Act) or an exemption from the registration requirements. In addition, ADS holders would not be able to participate in a pre-emptive rights offering unless we made arrangements with the depositary to extend that offering to holders of ADSs, which we are not required to do. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to being a Foreign Private Issuer or a Dutch Company 116

Claims of U.S. civil liabilities may not be enforceable against us or the members of our Senior Management Team and our Board of Directors. A significant amount of our assets are located outside the U.S. The majority of the members of our Senior Management Team and our directors are not U.S. residents and we do not have significant assets in the U.S. As a result, it may not be possible, or more difficult, for investors to enforce against them or us in U.S. courts, including judgments predicated upon the civil liability provisions of the U.S. federal securities laws. There are no treaties between the U.S. with either the Netherlands or Belgium providing for the reciprocal recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. Consequently, a final judgment for payment given by a court in the U.S. based on civil liability, whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in the Netherlands or in Belgium unless the underlying claim was re-litigated before a Dutch or Belgian court of competent jurisdiction. This will depend on the applicable Dutch or Belgian national rules. In addition, there is doubt as to whether a Dutch or Belgian court would impose civil liability on us or the members of our management or of our Board of Directors in an original action predicated solely upon the U.S. federal securities laws brought in a court of competent jurisdiction against us, our management or directors. As a foreign private issuer, we are exempt from various rules and regulations that a U.S. domestic public company would be required to follow, including those requirements under U.S. securities laws and Nasdaq listing standards. As a “foreign private issuer” defined in the SEC’s rules and regulations, we are not subject to all of the disclosure and corporate governance requirements applicable to companies organized within the United States. For example, are exempt from certain provisions of the U.S. Securities Exchange Act of 1934, as amended (Exchange Act), that are applicable to U.S. domestic public companies. We are subject to Dutch laws and regulations with regard to such matters. While we furnish quarterly unaudited financial information to the SEC on Form 6-K, the information we furnish to the SEC is less extensive and less timely compared to that required to be filed with the SEC by U.S. domestic issuers. As a foreign private issuer listed on Nasdaq, we are subject to corporate governance listing standards. However, we are permitted to rely on home country governance requirements and certain exemptions thereunder. Certain of our corporate governance practices may differ significantly from the Nasdaq corporate governance listing standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards. As a Dutch public company with limited liability, we are not obligated to, and do not comply with, all the best practice provisions of the DCGC, which may affect shareholders’ rights. We are required to disclose in our annual report, filed in the Netherlands, whether we comply with the provisions of the DCGC. If we do not comply with the provisions of the DCGC (for example, because of a conflicting Nasdaq Listing Rule or otherwise), we must list the reasons for any deviation from the DCGC in our annual report filed in the Netherlands. We may lose our foreign private issuer status which would then require us to comply with the Exchange Act’s domestic reporting regime and cause us to incur significant legal, accounting and other expenses. In order to maintain our current status as a foreign private issuer, either (a) a majority of our ordinary shares must be either directly or indirectly owned of record by non-residents of the U.S. or (b)(i) a majority of our executive officers or directors may not be U.S. citizens or residents, (ii) more than 50% of our assets cannot be located in the U.S. and (iii) our business must be administered principally outside the U.S. As of February 19, 2025, we believe at least 50% of our outstanding ordinary shares were held by U.S. residents (assuming that all our ordinary shares represented by ADSs were held by residents of the U.S.). The regulatory and compliance costs to us as a U.S. domestic issuer may be significantly higher than those we incur as a foreign private issuer. We also expect that if we were required to comply with the rules and regulations applicable to U.S. domestic issuers, it would make it more difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified members of our Board of Directors. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to being a Foreign Private Issuer or a Dutch Company 117

If we were to be classified as a passive foreign investment company for U.S. federal income tax purposes, this could result in adverse U.S. tax consequences to certain U.S. holders. If the Company is classified as a passive foreign investment company (PFIC) for any taxable year, U.S. investors may be subject to adverse U.S. federal income tax consequences described below under Section 5.15.1 “Taxation – U.S. Federal Income Tax Considerations – Passive Foreign Investment Company Considerations” The Company will be classified as a PFIC for U.S. federal income tax purposes for any taxable year in which, taking into account a pro rata portion of the income and assets of 25% or more owned subsidiaries, either (i) at least 75% of its gross income consists of “passive income” or (ii) at least 50% of the average quarterly value of its assets is attributable to assets that produce, or are held for the production of, passive income. Based on our historic and anticipated operations, the composition of our income and the projected composition and estimated fair market values of our assets, we do not believe that we were a PFIC for our most recent taxable year and do not expect to be classified as a PFIC for the current taxable year or for the foreseeable future. However, our status as a PFIC is a factual determination made on an annual basis, and we cannot provide any assurances regarding our PFIC status for the current or future taxable years. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Factors Related to being a Foreign Private Issuer or a Dutch Company 118

Corporate Governance 3.1 Dutch Corporate Governance Code 120 3.2 Management Structure 121 3.3 Report of the Non-Executive Directors 140 3.4 Remuneration Report and Compensation Statement 144 3.5 Corporate Governance – Nasdaq Listing Rules 183 3.6 Share Ownership 183 3.7 Insider Trading 183 3.8 Cybersecurity 184 3.9 Risk Appetite & Control 185 argenx Annual Report 2024 119 3

3 Corporate Governance 3.1 Dutch Corporate Governance Code As a European public company (Societas Europaea) incorporated under the laws of the Netherlands, we are subject to the DCGC. A copy of the DCGC can be found on www.mccg.nl. The DCGC is based on the notion that a company is a long-term alliance between the various stakeholders of the company. Stakeholders are groups and individuals who, directly or indirectly, influence – or are influenced by – the attainment of our objectives: employees, shareholders and other lenders, suppliers, customers and other stakeholders. The DCGC is based on a “comply or explain” principle. Accordingly, companies are required to state the extent to which they comply with the principles and best practice provisions of the DCGC in their annual report and, where they do not comply with them, why and to what extent they deviate from them. We acknowledge the importance of good corporate governance and we fully endorse the underlying principles of the DCGC, which is reflected in a policy that complies with the best practice provisions as stated in the DCGC (the Board By-Laws). However, we deviate from the best practice provisions in the areas set out below, for the reasons explained in this section. These deviations all relate to our remuneration practices, which are in line with our remuneration policy as approved by our annual General Meeting held in 2021. • Pursuant to best practice provisions 3.1.2 under vi of the DCGC, shares should be held for at least five years after they are awarded. Whereas we do have minimum holding requirements requiring our directors and executive management to hold minimum levels of ownership in the company during their time in function and for a period thereafter, we do not have a generic restriction on selling shares within the five years after they are granted. We regularly benchmark our equity incentive practices, and note that an all out selling restriction of five years post grant is significantly more strict than the selling restrictions applied by a large majority of our global competitors for talent. We believe our overall cliff vesting periods of four years for RSUs and three years for stock options, combined with minimum holding requirements after the vesting period, effectively ensure long term alignment of interest and we do not expect to implement a general five year holding requirement for all equity in the foreseeable future. • Pursuant to best practice provision 3.2.3. of the DCGC, the severance payment in the event of dismissal should not exceed one year’s base compensation. Our remuneration policy provides that a severance payment equal to 18 months base compensation to our chief executive officer and statutory executive director (CEO or Executive Director). The severance component of the remuneration package is, like all other components, benchmarked against and aligned with the severance components as identified within our global reference group. On this topic, considering the importance of competitive remuneration for our ability to attract and retain highly qualified persons, alignment with our global reference group is prioritized over compliance with this best practice provision 3.2.3. Whereas we do not envision adapting the existing contractual arrangements with our current CEO on this point, the 2025 Draft Remuneration Policy (as defined below) proposes a severance arrangement not exceeding 12 months for new Executive Directors. • Pursuant to best practice provision 3.3.2. of the DCGC, Non-Executive Directors should not be granted any shares or rights to shares as remuneration. We note that the ‘best practices’ and usages regarding granting equity incentives to Non-Executive Directors vary significantly between the key jurisdictions in which we operate. For example, we have our primary listing in, and conduct a significant part of our operations in, Belgium and the Belgian Corporate Governance Code requires that Non-Executive Directors receive part of their remuneration in the form of shares. When recruiting qualified Non- Executive Directors, we are competing against other companies who like us, have a major U.S. stock exchange listing, and face the corresponding stringent regulatory and legal environments. We, like our peers, need Non-Executive Directors who can navigate these complex requirements along with the personal liability and responsibility that comes with it. We benchmark our remuneration for Non- Executive Directors against our global reference group, selected on the basis of objective criteria that we disclose. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Dutch Corporate Governance Code 120

We realize that granting equity to Non-Executive Directors is viewed differently in the Dutch context and is a deviation from the (comply-or-explain) best practice provisions in the DCGC. However, considering our international peer group and considering that the corporate governance code principles in our country of primary listing (Belgium) actually require paying part of the non-executive fees in the form of equity, as a well-considered deviation from the DCGC, we pay part of our remuneration for Non- Executive Directors in the form of equity. This also ensures alignment of interest between our Non- Executive Directors and our shareholders. We do not expect to change this practice in the foreseeable future. 3.2 Management Structure 3.2.1 General As at December 31, 2024, we have a one-tier board structure consisting of 1 Executive Director and 9 Non- Executive Directors, and a Senior Management Team responsible for the day-to-day operations. Set out below is a summary of certain provisions of Dutch corporate law as of the date of this Annual Report, as well as a summary of relevant information concerning our Board of Directors and certain provisions of our Articles of Association and the Board By-Laws. This summary does not purport to give a complete overview and should be read in conjunction with and is qualified in its entirety by reference to the relevant provisions of Dutch law as in force on the date of this Annual Report, the Articles of Association and Board By-Laws. The Articles of Association are available in the governing Dutch language and an unofficial English translation thereof, and the Board By-Laws are available in English, on our website. 3.2.2 Statement of the Board of Directors Responsibilities for the Financial Statements and Management Report In accordance with Article 5:25c(2)(c) of the Dutch Financial Supervision Act (Wet toezicht financiële verslaggeving) (DFSA), the Board of Directors hereby certifies that, to the best of our knowledge, our consolidated financial statements as of December 31, 2024, prepared in accordance with IFRS as adopted by the EU, and with the legal requirements applicable in the Netherlands, give a true and fair view of the assets, liabilities, financial position and profit or loss of the company and the undertakings included in the consolidation taken as a whole, and that the management report includes a fair review of the development and performance of the business and the position of argenx and the undertakings included in the consolidation taken as a whole, together with a description of the principal risks and uncertainties that they face. Responsibility for this Annual Report The Board of Directors declares that the information contained in this Annual Report, including our consolidated financial statements as of December 31, 2024 and the management report, is, to the best of its knowledge, in accordance with the facts and contains no omission likely to affect its import. The Board of Directors is responsible for the information given in this Annual Report. In Control Statement Our Board of Directors is responsible for the oversight of our risk management activities and has specifically designated the audit and compliance committee (the Audit and Compliance Committee) to assist our Board of Directors in this task and prepare recommendations in this respect to the Board of Directors. While our Board of Directors oversees our risk management, our Senior Management Team is responsible for day-to-day risk management processes. Our Board of Directors expects our Senior Management Team to consider risk and risk management in each business decision, to proactively develop and monitor risk management strategies and processes for day-to-day activities and to effectively argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Dutch Corporate Governance Code 121

implement risk management strategies adopted by the Board of Directors. We believe this division of responsibilities is the most effective approach for addressing the risks we face. 3.2.3 Board of Directors Responsibilities Pursuant to the Dutch Civil Code (DCC), our Board of Directors is collectively responsible for our general affairs. Our Board of Directors, our Executive Director as well as our Non-Executive Directors, define our strategy (as further set out in Section 1.2 “Strategy and objectives”). Our strategy is regularly discussed and monitored at our Board of Directors meetings. Please refer to Section 7.2.5 “ESG Governance and Oversight” for more details regarding our governance and oversight with regard to sustainability matters. Pursuant to our Articles of Association, our Board of Directors will divide its duties among its members, with our day-to-day management entrusted to the Executive Director(s). The Non-Executive Directors are tasked with supervising our management and advising the Executive Director(s). In addition, both the Executive Director(s) and the Non-Executive Directors must perform the duties assigned to them pursuant to the Articles of Association. The division of tasks within our Board of Directors is determined (and amended, if necessary) by our Board of Directors. Each director has a duty to properly perform the duties assigned to him or her and to act in our corporate interest. Under Dutch law, the corporate interest extends to the interests of all corporate stakeholders, such as shareholders, creditors, employees and other stakeholders. Composition, Appointment and Dismissal The Articles of Association provide that our Board of Directors will consist of our Executive Director(s) and Non-Executive Directors. The number of Executive Directors must at all times be less than the number of Non-Executive Directors. The number of directors, as well as the number of Executive Directors and Non- Executive Directors, is determined by our Board of Directors, provided that the Board of Directors must consist of at least three members. Our directors are appointed by the General Meeting for a period of four years as either Executive Directors or as Non-Executive Directors. This four-year term aligns with best practice 2.2.1 of the DCGC, which stipulates that executive and Non-Executive Directors may be appointed for a maximum period of four years. We believe that appointing directors for a four-year term, rather than for example annual (re-)appointments, promotes stability and continuity within the Board of Directors. It also allows deserving candidates to be appointed for more than one year, enhancing our position in recruitment processes, as longer appointment periods are generally more attractive to candidates. Additionally, it contributes to the Board of Directors' and, by extension, the Company's ability to focus on long-term goals, in line with the DCGC's principle that a company's strategy should aim for sustainable long-term value creation. In accordance with best practice provision 2.2.1 of the DCGC, Executive Directors may be reappointed for periods not more than four years at a time. In accordance with best practice provision 2.2.2 of the DCGC, Non-Executive Directors may be reappointed once for a period of four years, after which the Non-Executive Director may be reappointed again for a period of two years, which reappointment may be extended by at most two years. In the event of a reappointment after an eight-year period, reasons will be given in the report of the Board of Directors. The Board of Directors is required to make one or more proposals for each seat on our Board of Directors to be filled. A resolution to nominate a director by our Board of Directors (with support from the remuneration and nomination committee (the Remuneration and Nomination Committee) may be adopted by a simple majority of the votes cast. Our Board of Directors conducts evaluations of all its directors and director candidates to create a well- rounded board, designed to promote long-term shareholder value creation through strong leadership and oversight. The Board of Directors recognizes that directors who serve on the board for longer terms can be valuable sources of continuity, understanding of the business and historical insight. Our Board of Directors designates one Executive Director as CEO and may grant other titles to Executive Directors (if appointed). Our Board of Directors also designates a Non-Executive Director as chairperson of argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 122

the Board of Directors and a Non-Executive Director as vice chairperson of the Board of Directors. The legal relationship between an executive member of the Board of Directors and argenx SE will not be considered as an employment agreement. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 123

Employment agreements between an Executive Director and a Group company (other than argenx SE) are permitted. In the absence of an employment agreement, members of a board of directors generally do not enjoy the same protection as employees under Dutch labor law. For a discussion of date of expiration of the current term of office and the period during which the person has served in that office, see Section 3.2.4 “Non-Executive Directors” and Section 3.2.5 “Senior Management Team”. Except for the arrangements described in Section 5.11.2 “Related Party Transactions”, subsection “Agreements with Our Senior Management Team”, there are no arrangements or understanding between us and any of the Executive Directors providing for benefits upon termination of their employment, other than as required by applicable law. In addition, the contracts between us and our Non-Executive Directors do not provide for any benefits upon termination. In addition, the Company is not party to any agreement with a director or employee providing for compensation if his or her employment is terminated because of a public takeover offer in respect of the Company. As a foreign private issuer, under the Nasdaq Listing Rules, we are not required to have a majority independent directors on our Board of Directors, except that Audit and Compliance Committee is required to consist fully of independent directors. However, our Board of Directors has determined that, taking into account any applicable committee independence standards, all of our Non-Executive Directors, including the members of Audit and Compliance Committee, are “independent directors” under Rule 10A-3 of the Exchange Act and the applicable rules of Nasdaq and of the DCGC. In making such determination, our Board of Directors considered the relationships that each Non-Executive Director has with us and all other facts and circumstances our Board of Directors deemed relevant in determining the director’s independence, including the number of ordinary shares beneficially owned by the director and his or her affiliated entities (if any). The DCGC requires that the composition of Non-Executive Directors is such that the members are able to operate independently and critically vis-à-vis one another, the Executive Directors, and any particular interests involved. As of the date of this Annual Report, all Non-Executive Directors meet the independence criteria contained in the DCGC. Therefore, in the opinion of the Non-Executive Directors, the composition of our Non-Executive Directors complies with the independence requirements of best practice provisions 2.1.7 to 2.1.9 of the DCGC. Our Board of Directors has consequently also determined that all members of our committees are independent under the applicable rules of the DCGC. As of the date of this Annual Report (or in any period before), none of the members of our Board of Directors and Senior Management Team has or has had a family relationship with any other member of our Board of Directors or Senior Management Team. Directors may be suspended or removed by the General Meeting at any time, with or without cause, by means of a resolution passed by a simple majority of the votes cast. Pursuant to the Dutch Civil Code, Executive Directors may also be suspended by the board of directors. A suspension of an Executive Director by the board of directors may be discontinued by the shareholders at any time at a General Meeting. Diversity In accordance with applicable Dutch legislation, we are required to report annually to the Social Economic Council (Sociaal-Economische Raad) on (i) the gender ratio, i.e., the male and female Executive Directors and Non-Executive Directors, as well as employees in managerial positions at the end of the financial year, (ii) the Company’s self-imposed appropriate and ambitious targets in the form of a target figure to make the ratio between the number of male and female Executive Directors and Non-Executive Directors, as well as in categories of employees in managerial positions to be determined by the Company, more balanced, and (iii) the plan of action to achieve these targets. If we have not complied with one or more of the foregoing, we are required to report on the reasons for this non-compliance. As of December 31, 2024, our Board of Directors consisted of 10 directors, including 1 Executive Director and 9 Non-Executive Directors. Of the directors who chose to disclose their gender, the Board of Directors contained 6 male directors and 3 female directors (Non-Executive Directors), translating into a 60.00% male / 30.00% female balance for our full Board of Directors (compared to 5 males and 3 females (Non- argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 124

Executive Directors) (55.56%/33.33%) as of December 31, 2023) and a 66.67% male / 33.33% female balance for our Non-Executive Directors (compared to 62.50% male/37.50% female as of December 31, 2023). As of December 31, 2024, , our Company leadership team consisted of 57 persons, comprised of a mix of 24 males and 28 females, (42% / 49% respectively) while 5 positions remained vacant. Compared to 31 persons as of December 31, 2023, comprised of a mix of 19 males and 12 females, (61% / 39% respectively). Our leadership consists of all full time employees reporting directly to our CEO, as well as all (other) leaders of our largest functions and projects. Each of these positions is characterized by a high impact across the organization, leading a global and cross functional team and having a global reach. We estimate that as of December 31, 2024, 58% of our workforce were female and 42% were male (compared to 58% female and 42% male as of December 31, 2023). Committees In accordance with the DCGC, our Non-Executive Directors can set up specialized committees to analyze specific issues and advise the Non-Executive Directors on those issues and prepare resolutions with respect thereto. The committees are advisory bodies only, and the decision-making remains within the collegial responsibility of the Board of Directors. The Non-Executive Directors determine the terms of reference of each committee with respect to the organization, procedures, policies and activities of the committee. Our Non-Executive Directors have established and appointed (i) an Audit and Compliance Committee; and (ii) the Remuneration and Nomination Committee. The composition and function of these committees complies with all applicable requirements of Euronext Brussels, the DCGC, the Exchange Act, the exchange on which the ordinary shares and the ADSs are listed and U.S. SEC rules and regulations. Only Non-Executive Directors qualify for membership of these committees. The audit and compliance committee and the Remuneration and Nomination Committee may not be chaired by the chairperson of the Board of Directors or by a former Executive Director of the Company. In addition to the aforementioned legally required subcommittees, our Board of Directors may also opt to incorporate informal committees consisting of Non-Executive Directors and other internal and external persons in argenx, in order to facilitate discussions and act as a sounding board on specific projects, as well as on a more permanent basis. Our Board of Directors has incorporated a research and development committee and a commercialization committee. Meetings and decision-making Our Board By-Laws describe, inter alia, the procedure for holding meetings of the Board of Directors, for the decision-making by the Board of Directors and the Board of Directors’ operating procedures. In accordance with our Articles of Association, our Board of Directors meets at least once every three months to discuss the state of affairs within the Company and the expected developments. Under our Board By-Laws, the members of our Board of Directors must endeavor, insofar as is possible, to ensure that resolutions are adopted unanimously. Where unanimity cannot be achieved and Dutch law, the Articles of Association or the Board By-Laws do not prescribe a larger majority, all resolutions of our Board of Directors must be adopted by a simple majority of the votes cast in a meeting at which at least a majority of the members of our Board of Directors then in office are present or represented. The Articles of Association provide that in case of a tie of votes, the chairperson does not have a casting vote and as such the proposal will be rejected in case of a tie. Under the Board By-Laws, some specific matters require approval of the majority of the Non-Executive Directors. These matters are set out in Schedule 1 of our Board By-Laws. Our Board By-Laws are available on our website. The Non-Executive Directors may also determine that certain other matters shall require approval of a certain majority of the Non-Executive Directors. Such matters shall be clearly specified and notified to the Executive Director(s) in writing. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 125

Resolutions of the Board of Directors may also be adopted outside of a meeting in writing, provided that all directors in office (in respect of whom no conflict of interest exists as referred to in the Articles of Association) have consented in writing to this manner of decision-making. A director may issue a proxy for a specific Board of Directors meeting to another director in writing. A director having a direct or indirect personal interest that conflicts with the interest of the Company and its affiliated enterprise has a conflict of interest. Each director shall inform all other directors of a conflict of interest without delay. A director shall not participate in the deliberations and decision-making process in relation to an item if he has a conflict of interest with respect thereto. In such case, the other directors shall resolve the item. In case because of this no resolution can be adopted by the Executive Directors, the Non- Executive Directors will resolve on the matter. In case because of this no resolution can be adopted by the Non-Executive Directors, the Board of Directors will resolve on the matter as if there were no conflict of interest. The Executive Director(s) are required to be asked their vision on their own remuneration in accordance with best practice provision 3.2.2 of the DCGC but may not participate in the adoption of resolutions (including any deliberations in respect of such resolutions) relating to their remuneration. Audit and Compliance Committee Our Audit and Compliance Committee consists of four members: Mr. Steve Krognes (chairperson), Mr. Peter Verhaeghe, Anthony Rosenberg and James Daly. Our Board of Directors previously established that Mr. Peter Verhaeghe, Anthony Rosenberg, James Daly and Mr. Steve Krognes satisfy the independence requirements set forth in Rule 10A-3 of the Exchange Act and that Mr. Steve Krognes qualifies as “audit committee financial experts” as defined by SEC rules and Article 39 paragraph 1 of Directive 2014/56/EU of the European Parliament and of the Council of April 16, 2014 amending Directive 2006/43/EC on statutory audits of annual accounts and consolidated accounts and has the requisite financial sophistication under the applicable Nasdaq rules and regulations. Further, our Board of Directors established that the composition of the Audit and Compliance Committee meets the requirements under the Dutch Decree on Establishing Audit Committees. Our Audit and Compliance Committee assists our Board of Directors in overseeing the accuracy and integrity of our accounting, financial and non-financial (including sustainability) reporting processes and audits and reviews of our consolidated financial statements as well as non-financial statements, the implementation and effectiveness of an internal control system and our compliance with legal and regulatory requirements, the independent auditors’ qualifications and independence and the performance of the independent auditors. Our Audit and Compliance Committee is also responsible for monitoring the status of, and compliance with, our global ethics and compliance program and meets with the head of our ethics and compliance function at least quarterly to discuss the status and overall effectiveness of the program as well as any issues or incidents that occurred and remedial actions needed (if applicable). The committee furthermore oversees climate-related risks and supervises the status of the Company’s cybersecurity program and regularly (at least quarterly) discusses the status thereof with our Senior Management Team. Our Audit and Compliance Committee is governed by a charter that complies the Nasdaq Listing Rules and the DCGC and is publicly available on our website. It is responsible for, among other things, establishing methods and procedures for supervising, and where necessary requiring improvements of, our financial reporting, risk management, ethics and compliance and organization for the purpose of making appropriate recommendations to our Board of Directors in that regard. Our Audit and Compliance Committee meets as often as is required for its proper functioning, but at least four times a year and at least once a year meets separately with our independent auditor. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 126

Our Audit and Compliance Committee reports regularly to our Board of Directors on the exercise of its functions. It informs our Board of Directors about all areas in which action or improvement is necessary in its opinion and produces recommendations concerning the necessary steps or resolutions that need to be taken. The audit review and the reporting on that review cover us and our subsidiaries as a whole. The members of the Audit and Compliance Committee are entitled to receive all information which they need for the performance of their function, from our Board of Directors and employees. Every member of the Audit and Compliance Committee shall exercise this right in consultation with the chairperson of the Audit and Compliance Committee. Please refer to Section 3.3.5 “Report Audit and Compliance Committee” for an overview of the number of meetings and attendance rates. Remuneration and Nomination Committee We have established a Remuneration and Nomination Committee, which serves as both the remuneration committee and selection and appointment committee as prescribed by the DCGC. Our Remuneration and Nomination Committee currently consists of three members: Dr. Donald deBethizy (chairperson), Peter Verhaeghe and Dr. Ana Cespedes. As announced on February 27, 2025, Donald deBethizy will retire from the Board of Directors after the annual General Meeting to be held on May 27, 2025 (the 2025 General Meeting). Consequently, Dr. Ana Cespedes will succeed Dr. Donald deBethizy as the chairperson of the Remuneration and Nomination Committee and Mr. Steve Krognes will become a member of the Remuneration and Nomination Committee. Our Remuneration and Nomination Committee is responsible for, among other things: • regularly reviewing the remuneration policy and practices in light of all relevant circumstances and benchmarks, and recommending to the Non-Executive Directors the remuneration of the individual Executive Directors; • advising our Board of Directors in respect of the remuneration for the Non-Executive Directors; • preparing the remuneration report to be included in our annual report; and • drawing up selection criteria and appointment procedures for directors and making proposals for appointment and re-appointment of the directors. The Remuneration and Nomination Committee consists of at least three members. The Remuneration and Nomination Committee meets as often as is required for its proper functioning, but at least once per year to evaluate its functioning. Please refer to Section 3.3.6 “Report Remuneration and Nomination Committee” for an overview of the number of meetings and attendance rates. Informal subcommittees Research and Development Committee The research and development committee consists of members of our Board of Directors and other persons, which composition may vary from time to time. Currently, the research and development committee consists of three members who are also members of our Board of Directors: Dr. Brian Kotzin (chairperson), Dr. Donald deBethizy and Dr. Pamela Klein. Non-board member advisors of the research and development committee include David Lacey, Prof. Hans de Haard and Wim Parys. Ad-hoc participants to the committee meetings include a variety of employees and/or external advisors, depending on the needs of the committee and the topics under discussion. As announced on February 27, 2025, Dr. Donald deBethizy will retire from the Board of Directors after the 2025 General Meeting. The Board of Directors will examine the options for a replacement. The research and development committee is responsible for, among other things: • monitoring and overseeing our research and development goals, strategies and measures; • serving as a sounding board to our research and development management, general management and Board of Directors; and argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 127

• performing strategic reviews of our key research and development programs. The research and development committee also promotes transparency in R&D practices, ensuring that findings, both positive and negative, are reported accurately and openly, and reviews, comments on and makes recommendations in respect of our non-financial reporting on R&D related topics to the Audit and Compliance Committee and/or the Board of Directors. All members of the research and development committee shall have adequate industrial, academic and/or practical experience with the research and development of biopharmaceuticals. Our research and development committee meets as often as is required for its proper functioning, but typically meets at least once prior to each meeting of our Board of Directors and reports regularly to our Board of Directors on the outcome of its deliberations, including any recommendations to the Board of Directors or the Senior Management Team. The chairperson of our research and development committee reports to our Board of Directors on the research and development committee’s discussions and strategic advice after each meeting on all matters within its duties and responsibilities. Please refer to Section 3.3.7 “Report Research and Development Committee” for an overview of the number of meetings and attendance rates. Commercialization Committee Our commercialization committee consists of members of our Board of Directors and other persons, which composition may vary from time to time. As of the date of this Annual Report, the commercialization committee consists of three permanent members: James Daly (chairperson), Anthony Rosenberg and Camilla Sylvest. Keith Woods serves as a non-board member advisor of the committee. The commercialization committee is responsible for, among other things: • reviewing and guiding the global sales and marketing strategy to ensure optimal product uptake and sustained growth and promoting innovation within commercialization efforts; • overseeing the global product launch strategy and supervising all stages of product lifecycle; and • reviewing our partnerships and collaborations. Our commercialization committee meets as often as is required for its proper functioning and in practice meets at least once per quarter. The commercialization committee reports regularly to our Board of Directors on the outcome of its strategic reviews and any recommendations to the Board of Directors or Senior Management Team. Please refer to Section 3.3.8 “Report Commercialization Committee” for an overview of the number of meetings and attendance rates. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 128

3.2.4 Non-Executive Directors Our Board of Directors as at December 31, 2024 comprised the following 9 Non-Executive Directors: Mr. Peter Verhaeghe Peter Verhaeghe has served as a member and chairperson of the board of arGEN-X B.V. since October 2008 and as Non- Executive Director on our Board of Directors since July 2014. Mr. Verhaeghe is the managing partner of VVGB Advocaten- Avocats, a corporate finance law and tax law firm, a position he has held since July 1999. He is currently lead counsel to a number of Belgian, Dutch, French, U.S. and Swiss life sciences companies. Mr. Verhaeghe has served on the boards of directors of Participatiemaatschappij Vlaanderen NV since May 2018 and miDiagnostics NV since April 2020. He has also served as chairman of the board of Haretis SA (Luxembourg) since March 2011 and as chairman of the LP & advisory committee of Bioqube Factory Fund I NV since September 2020. Mr. Verhaeghe previously served as a member of the board of directors of CzechPak Manufacturing s.r.o., Innogenetics NV (now Fujirebio Europe N.V.), Tibotec-Virco NV, and Biocartis SA. He was also the president of the board of directors of Merisant France SAS, a member of the management board of Merisant Company 2 S.à. rl., and chairman of the board of directors of PharmaNeuroBoost NV. Mr. Steve Krognes Steve Krognes has served as a member of our Board of Directors and as a chairperson of our Audit and Compliance Committee since February 2023. Mr. Krognes also serves on the boards of directors of Guardant Health, Inc., Denali Therapeutics, Inc., and Pliant Therapeutics, Inc. In September 2023, he also was appointed to the board of directors of ClayvstBio. He previously served on the boards of directors of RLS Global AB and Corvus Pharmaceuticals, Inc. and Gritstone Bio, Inc. Mr. Krognes was the chief financial officer of Denali Therapeutics, Inc. from 2015 until retiring from that position in April 2022. Mr. Krognes led successful financings for Denali Therapeutics, Inc., including its initial public offering in 2017, and contributed significantly to the company’s strategy, growth and strong financial position. His extensive leadership experience in the biotech and pharmaceutical industries includes 12 years in total at Roche and Genentech, Inc., during which Mr. Krognes served as chief financial officer of Genentech, Inc. for six years and global head of Roche’s mergers & acquisition team for six years. He also chaired the Genentech Access to Care Foundation and represented Genentech on the board and executive committee of the California Life Science Association. Before that, Mr. Krognes worked as an investment banker at Goldman Sachs, as a management consultant at McKinsey & Company, and as a venture capitalist in Scandinavia. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 129

Dr. Donald deBethizy Donald deBethizy has served as a member of our Board of Directors since May 2015. Dr. deBethizy has 30 years of experience in research and development, as well as financial, business and operating management, and board work in the biotechnology and consumer products industries. He is the president of White City Consulting ApS, also known as Custom Coaching) a consulting company that specializes in advising technology-focused companies. Dr. deBethizy currently serves on the boards of directors of Lophora ApS and Proterris, Inc. and as a board advisor for Cereno Scientific AB. Previously, Dr. deBethizy served as president and CEO of Santaris Pharma A/S until October 2014, when the company was sold to Roche. From March 1997 to June 2012, Dr. deBethizy was co-founder and CEO of Targacept, Inc., a U.S. biotechnology company listed on Nasdaq. From June 2012 to May 2013, he was special advisor to the chairman of Targacept, Inc.’s board of directors. From May 2013 to November 2014, Dr. deBethizy served as executive chairman of Contera Pharma ApS until it was sold to Bukwang Pharma, and from July 2015 to November 2017, he served as chairman of Rigontec GmbH until it was sold to Merck Inc. He previously served as chairman of the boards of directors of Albumedix Ltd (sold to Sartorius AG in September 2022), Saniona AB, and TME Pharma NV and AG. Dr. deBethizy was also a member of the boards of directors of Asceneuron SA, Serendex Pharmaceuticals A/S, Enbiotix Inc., Ligocyte Pharmaceuticals until it was sold to Takeda Pharmaceutical Co Ltd, Biosource Inc., and NOXXON Pharma N.V. Dr. deBethizy has held adjunct appointments at Wake Forest University Babcock School of Management, Wake Forest University School of Medicine, and Duke University. Dr. Pamela Klein Pamela Klein has served as a member of our Board of Directors since April 2016. Since 2008, Dr. Klein has been a principal and founder of PMK BioResearch, a company offering strategic consulting in oncology drug development to corporate boards, management teams and the investment community. She has also been a venture partner in Ysios Capital Partners, SGIEC, S.A.U. since 2023. She currently serves as a member of the board of directors of several companies including Shasqi and Patrys Ltd; as well as various scientific advisor boards. In 2023, Dr. Klein also joined the boards of directors of Frontier Medicines Corp, Ona Therapeutics SL, and Sardona Therapeutics, Inc. Previously, Dr. Klein served on the board of directors of Sardona Therapeutics, Inc., F-Star Therapeutics, Inc. until March 2023, Jiya Acquisition Corp, and Spring Bank Pharmaceuticals, Inc. until its merger with F-Star Therapeutics in July 2020. Dr. Klein previously spent seven years at the National Cancer Institute as research director of the NCI-Navy Breast Center, after which she joined Genentech as vice president of development until 2001. She also served as chief medical officer for Intellikine, Inc., which was acquired by Takeda American Holdings. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 130

Anthony Rosenberg Anthony Rosenberg has served as a member of our Board of Directors since April 2017. He currently serves as chief executive officer of TR Advisory Services GmbH, his own consultancy firm advising on business development, licensing, and mergers and acquisitions. Mr. Rosenberg also currently serves as chairman of the boards of directors of NUCLIDIUM AG, Oculis SA and Cullinan Therapeutics Inc. Previously Mr. Rosenberg held the positions of Managing Director at MPM Capital, a venture capital firm (2015 until 2020); head of M&A and Licensing of Novartis International (2013 to 2015); and head of business development and licensing at Novartis Pharma (2005 to 2012). Mr. Rosenberg also previously served on the boards of directors of SiO2 Material Science (until March 2023), Radius Health Inc., TriNetX, Inc., iOmx Therapeutics AG, and Clinical Ink, Inc. James Daly James Daly has served as a member of our Board of Directors since May 2018. Mr. Daly currently also serves as a director of Acadia Pharmaceuticals, Inc. and Madrigal Pharmaceuticals, Inc. He was formerly a member of the board of Halozyme, Bellicum Pharmaceuticals, Inc. and Chimerix, Inc. In 1985, he joined GlaxoSmithKline where he held various positions, including senior vice president of the respiratory division with full responsibility for sales, marketing and medical affairs. Mr. Daly moved to Amgen Inc. in 2001 where he was senior vice president for the North America commercial operations until 2011. In 2012, he joined Incyte Corp, a publicly-traded company focused on oncology and inflammation, where he was chief commercial officer until June 2015. Camilla Sylvest Camilla Sylvest has served as a member of our Board of Directors since September 2022. Ms. Sylvest currently serves as the executive vice president of commercial strategy and corporate affairs of Novo Nordisk A/S. Ms. Sylvest has more than 28 years of working experience within Novo Nordisk A/S and was based in Switzerland, Denmark, Germany, Malaysia, and Mainland China. Over the years, Ms. Sylvest has headed up Novo Nordisk A/S affiliates of growing size and complexity in Europe. She was also corporate vice president of the business area Oceania and Southeast Asia and senior vice president and general manager of the Novo Nordisk A/S region of Mainland China. Ms. Sylvest also serves as a member of the board of Danish Crown A/S. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 131

Dr. Ana Cespedes Ana Cespedes has served as a member of our Board of Directors since December 2022. Dr. Cespedes is currently the chief operating officer of the International AIDS Vaccine Initiative, a global organization dedicated to developing accessible vaccines and antibodies for infectious diseases. She will resign from the International AIDS Vaccine Initiative as of the end of March 2025 and she will become the chief executive officer of Vitamin Angels at the end of March 2025. Prior to joining the International AIDS Vaccine Initiative, Dr. Cespedes held several roles at Merck KGaA, most recently serving as global head of strategy and engagement, government, and public affairs. She founded and led the global market access and pricing function for the company and worked with stakeholders to communicate the clinical, economic, and societal value of innovative medicines. Prior to that, Dr. Cespedes led the first integrated corporate affairs group at Serono Iberia and Merck Spain, was managing director of the Spanish branch of the company’s nonprofit organization, and worked as a senior consultant at Arthur Andersen. Dr. Cespedes is a founding member of the National Congress of Corporate Affairs in Spain, the London School of Economics Market Access Academy, and the Cooperation for Oncology Data. She is also the founder of Living Mindfulness S.L. Dr. Cespedes is also a member of the steering committee of ProPatiens Institute. Dr. Brian Kotzin Brian Kotzin has served as a member of our Board of Directors and as a chairperson of our research and development committee since May 7, 2024. He is a former member of the board of directors at Vera Therapeutics, Inc., Rigel Pharmaceuticals, Inc. and Kyverna Therapeutics, Inc. He served as Senior Vice President for Nektar Therapeutics, Inc. from April 2017 to June 2023, and has held various leadership positions at Nektar Therapeutics, Inc., including serving as Chief Medical Officer and Head of Clinical Development from January 2021 to September 2021 and again from May 2022 to June 2023. He currently is the interim chief medical officer at Nektar Therapeutics, Inc. From 2004 to 2015, Dr. Kotzin was Vice President, Global and Clinical Development and Head, Inflammation Therapeutic Area at Amgen Inc., directing the global development efforts for product candidates in the inflammation area. During his employment at Amgen Inc, he also served as Vice President of Translational Sciences and Head of Medical Sciences from 2006 to 2011. Prior to entering the life sciences industry, Dr. Kotzin held several positions as a professor at the University of Colorado Health Sciences Center, where his research focused on immunopathogenesis of inflammatory diseases. He has also held leadership roles at several national organizations, including as a member of the American College of Rheumatology (ACR) Board of Directors, Member and Chairperson of the NIH Immunological Sciences Study Section, Chairperson of the NIH Autoimmunity Centers of Excellence, and Member of the Board of Directors for the Federation of Clinical Immunology Societies. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 132

The following table sets forth certain information with respect to the current non-executive members of our Board of Directors, including their ages, as at December 31, 2024: Name Age Gender Position Nationality Date of Initial Appointment Date of last (re-) Appointment Term expiration Mr. Peter Verhaeghe 66 M Non-Executive Director (chairperson) Belgium October 15, 2008 May 7, 2024 2026 Mr. Steve Krognes 56 M Non-Executive Director U.S. and Norway February 27, 2023 February 27, 2023 2027 Dr. Donald deBethizy 74 M Non-Executive Director (vice- chairperson) U.S. May 13, 2015 May 2, 2023 2025 1) Dr. Pamela Klein 63 F Non-Executive Director U.S. April 28, 2016 May 7, 2024 2026 Anthony Rosenberg 71 M Non-Executive Director UK April 26, 2017 May 11, 2021 2025 James Daly 63 M Non-Executive Director U.S. May 8, 2018 May 10, 2022 2026 Camilla Sylvest 52 F Non-Executive director Denmark September 8, 2022 September 8, 2022 2026 Dr. Ana Cespedes 51 F Non-Executive director Spain December 12, 2022 December 12, 2022 2026 Dr. Brian Kotzin 76 M Non-Executive Director U.S. May 7, 2024 May 7, 2024 2028 1) On February 27, 2025, it was announced that Dr. Donald deBethizy will retire from the Board of Directors after the 2025 General Meeting. Anthony Rosenberg will succeed him as the vice-chairperson of the Board of Directors. The address for our Non-Executive Directors is our registered office, Laarderhoogtweg 25, 1101 EB Amsterdam, the Netherlands. The following table sets forth the companies and partnerships of which the current non-executive members of our Board of Directors have been a member of the administrative, management or supervisory bodies or partner at any time in the previous five years, indicating whether or not the individual is still a member of the administrative, management or supervisory bodies or partner, as of the date of this Annual Report, other than argenx or our subsidiaries: Mr. Peter Verhaeghe • VVGB Advocaten – Avocats • Participatiemaatschappij Vlaanderen NV • miDiagnostics NV • Tibotec-Virco NV • Merisant France SAS • Merisant Company 2 sàrl • CzechPak Manufacturing s. r. o. Mr. Steve Krognes • Denali Therapeutics Inc. • Guardant Health, Inc. • Pliant Therapeutics, Inc. • ClavystBio • RLS Global AB • Corvus Pharmaceuticals Inc. • Gritstone Bio, Inc. Dr. Donald deBethizy • White City Consulting ApS • Cereno Scientific AB • Protteris, Inc. • Lophora ApS • Rigotec GmbH • TME Pharma NV and AG • Saniona AB • Albumedix Ltd. • Asceneuron SA • Albumin Holdings ApS • Innovent LLC Dr. Pamela Klein • Ysios Capital Partners, SGIEC, S.A.U. • Shasqi • Patrys Ltd. • Frontier Medicines • Ona Therapeutics • F-Star Therapeutics, Inc. • Jiya Acquisition Corp. • Sardona Therapeutics, Inc. • I-MAB Name Current Past argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 133

Anthony Rosenberg • TR Advisory Services GmbH • Cullinan Therapeutics Inc • Oculis SA • NUCLIDIUM AG • Radius Health, Inc. • Clinical Ink, Inc. • MPM Capital • SiO2 Material Science James Daly • Acadia Pharmaceuticals Inc. • Madrigal Pharmaceuticals, Inc. • Chimerix, Inc. • Coherus Biosciences • Halozyme Therapeutics, Inc. • Bellicum Pharmaceuticals, Inc. Camilla Sylvest • Novo Nordisk A/S • Danish Crown A/S • World Diabetes Foundation Dr. Ana Cespedes • International AIDS Vaccine Initiative (IAVI) • ProPatiens Institute • Living Mindfulness S.L. Dr. Brian Kotzin • Biora Therapeutics, Inc. • Genascence Corporation • Nektar Therapeutics, Inc. • Vera Therapeutics, Inc. • Kyverna Therapeutics, Inc. • Rigel Pharmaceuticals, Inc. Name Current Past argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 134

3.2.5 Senior Management Team Our Senior Management Team acts as our executive management. Of these people, only our CEO, Mr. Tim Van Hauwermeiren, is part of our Board of Directors as Executive Director. Our Senior Management Team comprised of the following persons in 2024 and on the date of this Annual Report (appointment and retirement dates noted as applicable). Tim Van Hauwermeiren Tim Van Hauwermeiren co-founded our Company in 2008 and has served as our CEO since July 2008. He has served as a member of our Board of Directors since July 2014. Mr. Van Hauwermeiren has almost 30 years of general management and business development experience across the life sciences and consumer goods sectors. He also serves on the boards of directors of iTeos Therapeutics, Inc. and Lexeo Therapeutics, Inc. Karen Massey Karen Massey has served as our COO since March 2023. Ms. Massey has over 20 years of experience in the pharmaceutical and biotechnology industry, including in commercial, product development, corporate strategy, and innovation roles. Prior to joining argenx, Ms. Massey was with Genentech (Roche Group) for over nine years, where she most recently served as senior vice president of product development and global clinical operations and previously held various commercial leadership roles across marketing and business operations, including as the vice president of the multiple sclerosis and neuromyelitis optica business. Ms. Massey started her biopharmaceutical career in marketing at Pfizer Inc., and returned there, after two years as a management consultant at Bain & Company, to take on leadership positions in corporate strategy and sales and as a commercial lead in Latin America. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 135

Karl Gubitz Karl Gubitz has served as our CFO since June 2021. Mr. Gubitz previously worked at Pfizer Inc. for nearly 20 years, most recently as vice president of finance within the global oncology business. Within Pfizer Inc., Mr. Gubitz held country, regional, and global positions, and consistently delivered top- line growth. He managed teams of over 250 colleagues in financial leadership roles within the global internal medicine and global innovative products businesses. Prior to joining Pfizer Inc. in 2003, Mr. Gubitz held various management roles at PricewaterhouseCoopers LLP. Dr. Peter Ulrichts Peter Ulrichts has served as our chief scientific officer since January 2023. In this role, he oversees the development of all clinical and pre-clinical compounds within our pipeline. Dr. Ulrichts previously served in various roles at the Company since he joined us in 2010, including, most recently, as our head of clinical science. As a research scientist, Dr. Ulrichts was involved in the development of various therapeutic antibodies for the treatment of cancer and autoimmune diseases. In 2013, he headed the development of our FcRn antagonist efgartigimod until the first-in-human clinical trial. He subsequently transitioned to become the lead scientist of our efgartigimod program. Malini Moorthy Malini Moorthy has served as our general counsel since February 2022. She has over 25 years of extensive global legal and compliance experience in the biopharmaceutical and medical device industries. She was most recently senior vice president and chief deputy general counsel of legal, compliance, and government affairs at Medtronic plc, where she played a pivotal role in shaping and driving enterprise and functional strategies. Before joining Medtronic plc, Ms. Moorthy spent four years at Bayer Corporation as the head of global litigation and investigations and 10 years at Pfizer Inc., where she progressed to lead civil litigation globally. Ms. Moorthy began her career as a law firm associate, first with McCarthy Tétrault LLP and Genest Murray Desbrisay Lamek LLP in Toronto, Canada and then Salans LLP (now Dentons US LLP) in New York City. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 136

Dr. Luc Truyen Luc Truyen has served as our chief medical officer since April 2022 and previously served as our head of research and development operations management from September 2021 to April 2022. Prior to this, Dr. Truyen was with Johnson & Johnson (and its subsidiary companies) for over 20 years holding various leadership positions, primarily within neuroscience. In his most recent position prior to joining argenx, Dr. Truyen was global head of development and external affairs for neuroscience, managing the strategy and delivery of the early and late portfolio of assets for mood disorders, schizophrenia, and neurodegenerative and neuroinflammatory disorders. Besides Dr. Truyen’s strong track record in clinical development resulting in several globally innovative drug approvals, his broad-based experience also includes leading global clinical development operations for the whole Johnson & Johnson pharmaceutical group as well as serving as the head of research and development and chief medical officer of Janssen Alzheimer Immunotherapy Research & Development LLC, an internal spin- out from Johnson & Johnson. Arjen Lemmen Arjen Lemmen joined argenx in 2016 and has served as our vice president of corporate development & strategy since 2019. He has successfully executed several transactions including a number of programs within the IIP. Prior to joining the Company, Mr. Lemmen served as a corporate finance specialist at Kempen & Co NV focusing on mergers and acquisitions, equity capital markets and strategic advisory transactions in the European life sciences industry. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 137

Andria Wilk Andria Wilk joined argenx as global head of quality in January 2020. Ms. Wilk has more than 25 years of experience in quality assurance within the pharmaceutical industry. Most recently, Ms. Wilk served as senior director, head of medical, regulatory & clinical quality assurance at H Lundbeck A/S, where she managed the global medical, regulatory & clinical quality assurance group based in the EU, U.S., and Asia. In this role, she was responsible for the global audit programs and quality assurance support for all clinical trial and post-marketing activities and related computerized systems. Prior to H Lundbeck A/S, she held various quality assurance positions of increasing responsibility within AstraZeneca plc, Takeda Global Research, Development Centre Europe, and Astellas Pharma Inc. The following table sets forth certain information with respect to the members of our Senior Management Team, including their ages, as of December 31, 2024: Name Age Position Nationality Date of Initial Appointment Tim Van Hauwermeiren 52 CEO and Executive Director Belgium July 15, 2008 Karen Massey 46 COO Australia March 13, 2023 Karl Gubitz 55 CFO South Africa June 1, 2021 Dr. Peter Ulrichts 45 Chief Scientific Officer Belgium January 1, 2023 Malini Moorthy 55 General Counsel Canada February 14, 2022 Dr. Luc Truyen 60 Chief Medical Officer Belgium April 1, 2022 Arjen Lemmen 40 Vice-President Corporate Development & Strategy The Netherlands May 1, 2016 Andria Wilk 52 Global Head of Quality UK January 13, 2020 The address for our Senior Management Team is Industriepark-Zwijnaarde 7, 9052 Zwijnaarde (Ghent), Belgium. The following table sets forth the companies and partnerships of which the members of our Senior Management Team (or persons who have been members of our Senior Management Team in 2024) have been a member of the administrative, management or supervisory bodies or partner at any time in the previous five years, indicating whether or not the individual is still a member of the administrative, management or supervisory bodies or partner, as of the date of this Annual Report, other than argenx or our subsidiaries: Tim Van Hauwermeiren Iteos Therapeutics, Inc. Aelin Therapeutics NV Lexeo Therapeutics, Inc. RayzeBio, Inc. Karen Massey – Genentech, Inc. Karl Gubitz – Pfizer Inc. Dr. Peter Ulrichts – – Malini Moorthy – Medtronic plc Dr. Luc Truyen – Johnson & Johnson Arjen Lemmen OncoVerity Inc. – Andria Wilk European Forum for Good Clinical Practice (EFGCP) – Name Current Past argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 138

3.2.6 Conflict-of-Interest and Related Party Transactions Directors must immediately report any (potential) direct or indirect personal interest in a matter that conflicts with the interests of the Company and the business connected with it to the chairperson of our Board of Directors and to the other directors. The Non-Executive Directors will decide, without the director concerned being present, whether there is a conflict of interest. A director will not participate in any discussions and decision making if he or she has a conflict of interest in the matter being discussed. In case because of this no resolution can be adopted by the Executive Directors, the Non-Executive Directors will resolve on the matter. Decisions to enter into transactions in which there are conflicts of interest with directors that are of material significance to us or to the relevant director require the approval of the Non-Executive Directors. Dutch law provides that transactions with related parties are material if they are (a) not entered into in the ordinary course of our business or (b) not concluded on normal market terms. The Board of Directors has established an internal procedure to periodically assess whether transactions are concluded in the ordinary course of business and on normal market terms. Material transactions must be made public by the Company at the time the transaction is entered into. Transactions with related parties are considered material if (i) information on the transaction qualifies as inside information under the MAR and (ii) such transaction is entered into with one or more holders of shares in the Company representing at least 10% of issued share capital, or a member of our Board of Directors. Transactions that are individually non- material, but which are entered into with the same related party during the same fiscal year, must be evaluated in the aggregate to determine if they are material. There are no arrangements or understandings in place with major shareholders, customers, suppliers or others pursuant to which any member of our Board of Directors or Senior Management Team has been appointed. There are no conflicts of interests between the Company and any administrative, management and supervisory bodies and Senior Management Team, nor are there any potential conflicts of interests of the members of our Board of Directors and Senior Management Team between any duties to the Company and their private interests and or other duties. In connection with our initial U.S. public offering, we entered into a related party transaction policy. 3.2.7 Code of Business Conduct and Ethics We adopted a Code of Business Conduct and Ethics (Code of Conduct), that is applicable to all of our employees and directors. The Code of Conduct is available on our website at www.argenx.com/investors/ governance/rules-codes-compliance. The Audit and Compliance Committee of our Board of Directors is responsible for overseeing the Code of Conduct and is required to approve any waivers of the Code of Conduct for employees and directors. We expect that any amendments to the Code of Conduct, and any waivers of its requirements, will be disclosed on our website. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Structure 139

3.3 Report of the Non-Executive Directors 3.3.1 Meetings Our Board of Directors had 5 formal meetings in the course of 2024. The meetings were held in the months February, May, July, October and December. The committees of the Board of Directors also convened regularly and at least once per quarter.. Please refer to Sections ”Report Audit and Compliance Committee” to “Report Commercialization Committee” below for the separate reports of the committees. All Board of Director meetings and 16 out of 19 formal committee meetings were also attended by Mr. Van Hauwermeiren, as executive director. In addition, several members of the Senior Management Team were invited to discuss specific items included on the Board of Director and committee meetings’ agendas. 3.3.2 Attendance Record Board of Director Meetings In 2024, 5 Board of Directors meetings were held. The meeting attendance rate for our directors is set out in the table below. Name Number of meetings attended in 2024 since appointment (and up to resignation, as applicable) Attendance % Mr. Peter Verhaeghe (chairperson) 5 100% Tim Van Hauwermeiren 5 100% Mr. Steve Krognes 5 100% Dr. Donald deBethizy (vice-chairperson) 5 100% Dr. Pamela Klein 5 100% Anthony Rosenberg 5 100% James Daly 5 100% Camilla Sylvest 5 100% Dr. Ana Cespedes 5 100% Dr. Brian Kotzin 1) 4 100% 1) Dr. Brian Kotzin was appointed to the Board of Directors as of May 7, 2024. In 2024, all of the 5 Board of Directors meetings with solely the Non-Executive Directors being present were held as closed sessions at the beginning or the end of other meetings. These meetings were attended by all Non-Executive Directors appointed at such time. Name Number of meetings attended in 2024 since appointment Attendance % Mr. Peter Verhaeghe (chairperson) 5 100% Dr. Donald deBethizy (vice-chairperson) 5 100% Dr. Pamela Klein 5 100% Anthony Rosenberg 5 100% James Daly 5 100% Camilla Sylvest 5 100% Dr. Ana Cespedes 5 100% Dr. Brian Kotzin 1) 4 100% 1) Dr. Brian Kotzin was appointed to the Board of Directors as of May 7, 2024. 3.3.3 Activities The agenda for the Board of Directors centers around the key business objectives for long-term value creation and the key risks involved, as well as the manner in which the Senior Management Team implements our strategy including our research and development pipeline and the commercialization of our products, our culture to ensure proper monitoring by the Non-Executive Directors, our financial position as well as the results of our subsidiaries, significant investment proposals, yearly budgets, the argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Report of the Non-Executive Directors 140

internal risk management and control system, talent development, succession planning and remuneration and appointment matters. In 2024, the Board of Directors primarily discussed the Company's innovation mission and objectives and reviewed the scientific pipeline and regulatory developments for all product candidates, ensuring the required progression thereof. The Board of Directors furthermore reviewed and discussed the commercialization strategies and opportunities, contributing to our successful product launches and sales ramp-up. The Board of Directors also spent a significant amount of time on talent development and succession planning, both for the senior leaders within the Company (within and beyond the Senior Management Team) and the Board of Directors. This lead to the appointment of Dr. Brian Kotzin as a Non- Executive Director and chairperson of the research and development committee and the renewal of the appointment of Dr. Pamela Klein and Mr. Peter Verhaeghe as Non-Executive Directors and Mr. Peter Verhaeghe's reappointment as chairperson of the Board of Directors. A lot of time was also spent discussing and evaluating the Company's rapid growth and how to maintain our unique company culture through these periods of growth. Finally, the Board of Directors spent time discussing our ESG journey and shareholder feedback on say-on-pay and how to address that. 3.3.4 Board Evaluation The Board of Directors evaluates its functioning and the functioning of its committees and of each individual director annually. The evaluation process is performed with the help of an external professional board evaluation consultant. In 2024, the evaluation was performed by Nasdaq Governance Solutions. The evaluation includes preparing specific questionnaires focusing on the skills and competences most relevant to us, and the most material board topics and challenges we face. The written questionnaire is then followed up by one-to-one interviews with the representative of Nasdaq Governance Solutions with each member of the Board of Directors, followed by a debrief and discussion held with the external evaluator and the entire Board of Directors both in writing (in form of a report) and in the form of a live discussion of the evaluation report aimed at distilling specific learnings and conclusions. Based on the self-evaluation performed, the Non-Executive Directors concluded that the Board of Directors and its committees had properly discharged their responsibilities during 2024. The Board of Directors identified certain strengths and weaknesses and adopted a plan for further board development and succession in 2025. All directors consider the Board of Directors to be a high performing, engaged, open and transparent board. The importance to preserve this was highlighted by Nasdaq Governance Solutions. All Non-Executive Directors consider fostering further development and education of great importance, which can be furthered in 2025 through advisory board sessions, deep-dives and other educational courses. 3.3.5 Report Audit and Compliance Committee The Audit and Compliance Committee reports regularly to our Board of Directors on the exercise of its functions. It informs our Board of Directors about all areas in which action or improvement is necessary in its opinion and produces recommendations concerning the necessary steps that need to be taken. The audit review and the reporting on that review cover the Company and its subsidiaries as a whole. In 2024, the main points of discussion at the meetings were the 2023 consolidated financial statements and press release as well as interim consolidated financial statements and press releases, internal audit and external auditors’ reports, the review of quarterly forecasts, updates on tax priorities, cash management, CSRD implementation (including finalization of the double materiality assessment), the Company’s ethics and compliance program, the Company’s cyber security program and the Company’s privacy program. In 2024, 6 formal Audit and Compliance Committee meetings were held. The meeting attendance rate for our Non-Executive Directors is set out in the table below. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Report of the Non-Executive Directors 141

Name Number of meetings attended in 2024 since appointment Attendance % Mr. Steve Krognes (chairperson) 6 100% Mr. Peter Verhaeghe 6 100% Anthony Rosenberg 6 100% James Daly 6 100% 3.3.6 Report Remuneration and Nomination Committee The Remuneration and Nomination Committee assists the Board of Directors by, amongst other matters, regularly reviewing our remuneration policy, preparing remuneration proposals and periodically assessing the size and composition of the Board of Directors, as well as preparing the policy of the Senior Management Team on the selection criteria and appointment procedures for the Senior Management Team. During their deliberations in 2024, the main topics of discussion were long-term succession and development planning for key company leadership. The key themes in 2024 were around our evolving remuneration practices and needs in light of stakeholder feedback and engagement, leading up to a proposed revised remuneration policy in 2024 and preparations for a further revised policy to be proposed in 2025. In 2024, 6 formal Remuneration and Nomination Committee meetings were held. The meeting attendance rate for our Directors is set out in the table below. Name Number of meetings attended in 2024 since appointment Attendance % Dr. Donald deBethizy (chairperson) 6 100% Mr. Peter Verhaeghe 5 83.33% Dr. Ana Cespedes 6 100% 3.3.7 Report Research and Development Committee The research and development committee functions as a sounding board to our research and development management, general management and the Board of Directors, and monitors our research and development goals, strategies and measures. In 2024, the committee held 4 formal meetings, in which it focused mainly on the vision and strategy on science, the Company’s research and development pipeline including its preclinical and clinical stage product-candidates, potential future indications for its commercial stage products and developments in relation to our IIP. The meeting attendance rate for our directors is set out in the table below. Name Number of meetings attended in 2024 since appointment Attendance % Dr. Brian Kotzin (chairperson) 1) 4 100% Dr. Donald deBethizy 4 100% Dr. Pamela Klein 4 100% David Lacey (chairperson) 2) 4 100% 1) Dr. Brian Kotzin was appointed chairperson of the research and development committee as of May 7, 2024. 2) David Lacey resigned as chairperson effective May 7, 2024 and was replaced by Dr. Brian Kotzin effective May 7, 2024. He attended the other meetings in 2024 as an advisor to the Board of Directors. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Report of the Non-Executive Directors 142

3.3.8 Report Commercialization Committee The commercialization committee functions as a sounding board on branded and unbranded strategic marketing plans for the Board of Directors. In 2024, the committee held 3 formal meetings, in which it focused mainly on the execution of our launch of VYVGART in CIDP, the execution of our launch of VYVGART in ITP in Japan and gMG in several other jurisdictions as well as the preparation for potential future launches, subject to obtaining further approvals. The meeting attendance rate for our directors is set out in the table below. Name Number of meetings attended in 2024 since appointment Attendance % James Daly (chairperson) 3 100% Anthony Rosenberg 3 100% Camilla Sylvest 3 100% argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Report of the Non-Executive Directors 143

3.4 Remuneration Report and Compensation Statement 3.4.1 Letter of the Chairperson of the Remuneration and Nomination Committee Dear Stakeholders, The Remuneration and Nomination Committee is pleased to present the 2024 remuneration report and compensation statement (the 2024 Remuneration Report). This report outlines the Remuneration and Nomination Committee’s role and activities over the past financial year and provides an outlook for 2025. It also explains the efforts made to continuously align our remuneration framework with the interests of the Board of Directors and those of our stakeholders, ensuring sustainable value creation as argenx evolves. In line with our current remuneration policy, approved in 2021 (the 2021 Remuneration Policy), and in anticipation of the revised policy we are submitting for approval at the 2025 General Meeting (the Proposed 2025 Remuneration Policy), this report highlights our commitment to a remuneration structure that fosters performance-based remuneration and transparency on targets and long-term alignment. Both policies are designed to ensure that the compensation of the Board of Directors remains closely tied to the Company’s strategic goals and stakeholder interests. The CEO’s remuneration, in particular, is structured to include a well-balanced mix of short-term and long- term incentives. This approach rewards not only immediate achievements, but also sustained progress in our business strategies, individual objectives, and key strategic non-financial metrics that we believe underpin our long-term mission and Vision 2030. On behalf of the Board of Directors, I am pleased to provide insight into how the Company’s achievements and continued progress in 2024 have shaped the remuneration of our CEO, COO, and CFO (the Named Executive Officers or NEOs), as well as our Non-Executive Directors. This report details the implementation of the 2021 Remuneration Policy, prepared in accordance with the DCGC and the draft, non-binding disclosure guidelines of the European Commission. It reflects our commitment to transparency and alignment with best governance practices while ensuring that our remuneration framework supports the Company’s strategic objectives and long-term value creation. Looking Back on 2024 In 2024, we conducted our annual comprehensive base pay review for our Named Executive Officers, to ensure our compensation framework remains competitive and aligned with industry benchmarks. Based on the recommendation of the Remuneration and Nomination Committee, the Board of Directors approved the following compensation changes: • The CEO’s base pay increased by 15% to EUR 700,000 ($757,680). While the broader workforce has received progressive increases in line with Company guidelines over the past two years, the CEO’s base pay remained unchanged between 2022 and 2023, at his personal request. We believe that the approved base pay increase was necessary to maintain fairness, ensure market competitiveness, and recognize our CEO’s exceptional leadership in delivering sustained value to our shareholders. Even after this increase, the CEO’s base pay remains at the 27th percentile of our newly defined global peer group. • The COO and CFO each received a 6% base pay increase, reflecting their critical roles and contributions to the Company’s continued growth and success. These adjustments reinforce our commitment to a balanced, performance-driven remuneration structure that supports long-term value creation while maintaining fairness and transparency. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 144

Company Performance As detailed in our Shareholder Letter, 2024 was a year marked by remarkable progress and significant achievements such as receiving regulatory approval in Japan for VYVGART for the treatment of adults with ITP and receiving FDA approval for VYVGART HYTRULO for the treatment of CIDP patients. Our Senior Management Team, including the Named Executive Officers, navigated multiple hurdles while capitalizing on strategic opportunities. We established our ‘Vision 2030’, a long-term commitment to transforming the treatment of severe autoimmune diseases through innovative therapies such as VYVGART and VYVGART HYTRULO, empasiprubart, and our expanding pipeline of antibody-based therapeutics. Over the past year, we have made substantial progress in our ambitious target to treat 50,000 patients globally, secure 10 labelled indications, and advance five pipeline candidates into Phase 3 development by 2030. Notably, we reached over 10,000 gMG patients, expanded our global footprint with multiple approvals for gMG and CIDP (approximately touching approximately1,000 patients), and initiated label-enabling studies that further our reach in the market. We have also advanced several key assets: • efgartigimod has progressed, with GO-decisions announced for SjD to enter into a Phase 3 clinical trial and for Myositis to continue the Phase 3 clinical trial based on the Phase 2 data. • empasiprubart has advanced into a Phase 3 clinical trial for MMN and a Phase 3 clinical trial for CIDP will commence in 1H 2025. • ARGX-119 has entered proof-of-concept clinical trials in CMS and ALS. • Four new INDs advancing into Phase 1 clinical trials, further underpinning our commitment to delivering immunology innovations. Financially, our strong performance is reflected in reaching $2.2 billion in product net sales, an impressive increase from $1.2 billion in 2023, along with significant advancements in our clinical pipeline, including 10 Phase 2 clinical trials and 10 Phase 3 clinical trials. These achievements have enabled us to meet or exceed all quantitative short-term incentive targets, while qualitative metrics relating to building a robust organization were also achieved. Moreover, the Remuneration and Nomination Committee appreciates the strategic shift driven by our management’s focus on operational and commercial excellence. This focus has well positioned argenx among its peers to capitalize on emerging opportunities, particularly in light of the positive Phase 2 proof- of-concept data for empasiprubart, which has paved the way for its advancement into Phase 3 clinical trial for MMN (EMPASSION). 2021-2024 Performance and Long-Term Incentive Plan Outcome As a shareholder, you will be pleased that in 2024, the Euronext Brussels share price rose by 74.7% from €343.50 per share on the last trading day of 2023 to €600.00 per share on December 31, 2024. In a three- year long-term incentive (LTI) period between December 31, 2021 and December 31, 2024, the share price rose by approximately 90.3%, from €315.30 to €600.00 per share. Stakeholder Engagement and Looking Forward to 2025 Shareholders play a crucial role in our success by providing invaluable support and fostering strong partnerships that are essential to our growth. We deeply appreciate their continued commitment and strive to keep them well-informed, ensuring a lasting and productive relationship. In 2024 and 2025 to date, we have actively engaged with our investor community on several topics, including on the 2023 remuneration report (which led to a positive voting outcome of 58.6%), the proposed 2024 remuneration policy (which led to a voting outcome of 68.9% where a 75% majority was required) and the Proposed 2025 Remuneration Policy. In 2024, we held over 70 dedicated meetings with shareholders. As at the date of this Annual Report, we have conducted more than 20 meetings in 2025. These discussions focused on key remuneration events and have been instrumental in driving continuous improvements in our remuneration practices. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 145

Key points raised during these interactions include: • We received concerns from stakeholders they were not able to determine if and how pay-for- performance was embedded in our remuneration. To address this feedback, this 2024 Remuneration Report includes enhanced disclosure on the 2024 performance targets and corresponding pay-out for the NEOs. We have also introduced prospective disclosure on the short-term incentive (STI) metrics set for 2025 and for the newly introduced performance share units (PSUs) against a threshold-target- maximum framework. In the Proposed 2025 Remuneration Policy, we will commit to this enhanced prospective disclosure against a threshold-target-maximum framework going forward. • Feedback indicated that at this stage in the evolution of the Company stock options could be perceived as performance-based incentives, potentially compromising the objectivity of our Non-Executive Directors. To respond to feedback from a number of stakeholders and upholding the highest standards of governance and independence, we decided to no longer grant stock options to Non-Executive Directors as from 2024. Instead, we transitioned to a remuneration structure based on fixed fees and non-performance-based equity compensation, namely RSUs. This new structure is aligned with best practices while maintaining fairness and transparency. • Historically, stock price appreciation was considered an inherent performance target in stock option grants. However, in response to shareholder feedback, the Proposed 2025 Remuneration Policy introduces a more structured, performance-driven approach to LTI. Under the revised framework, stock options will be limited to a maximum of 50% of the Executive Director’s total LTI grant, while at minimum 50% will be allocated as PSUs, tied to predefined performance criteria beyond share price appreciation. To further reinforce this performance-based approach, RSUs have been eliminated, ensuring that the entire LTI is 100% ‘at-risk’. This fully aligns Executive Director rewards with long-term value creation and strategic objectives. This refined approach strengthens the link between Executive Director compensation and sustained company performance while addressing shareholder concerns regarding performance measurement. • Some stakeholders expressed concerns regarding the perceived lack of a cap on the LTI awards. In response, the Proposed 2025 Remuneration Policy introduces a clearly defined cap on total awards as a multiple of base pay. Notably, the Founder CEO has requested that his target and maximum LTI opportunities be set at 7x and 10x base pay, respectively. Future incoming Executive Directors will also be subject to capped LTI awards, ensuring consistency, transparency, and alignment with stakeholder expectations while maintaining a competitive and performance-driven compensation structure. • Investor feedback prompted a revision of the vesting profile for stock option grants to enhance alignment with market best practices. In line with our peers, the Proposed 2025 Remuneration Policy introduces a three-year cliff vest for the CEO’s stock option grants, replacing the previous monthly vesting schedule that began one year after the grant date. Additionally, the newly introduced PSUs will also be subject to a three-year cliff vesting schedule, reinforcing a long-term commitment to performance and shareholder value creation. • Concerns were raised regarding the Board of Directors’ discretion in granting an additional $98,368 (25% of the target incentive) in 2023 to the CEO in recognition of the successful execution of the Company’s business plan. While this discretionary adjustment was deemed appropriate in this instance due to the significant over achievement of business results, we acknowledge the importance of transparency and a more detailed disclosure of pay-for-performance approach. In the event of any future discretionary adjustments will be accompanied by detailed disclosure of the performance targets and corresponding payouts to ensure clear alignment between compensation and measurable achievements. More information on how we further specifically addressed stakeholder concerns, including from our latest engagement in 2025, will be included in the explanatory notes of the Proposed 2025 Remuneration Policy. We will continue to engage actively with our key stakeholders and proxy advisors throughout 2025 and onwards, remaining available to address any questions or concerns regarding corporate governance and executive compensation. Moreover, we foster an open dialogue within our organization, guided by our unified culture and core values of co-creation, humility, excellence, empowerment and innovation. This underpins our vision for long-term value creation while balancing the interests of all stakeholders. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 146

Based on ongoing conversations with our shareholders and the positive feedback received regarding the performance of our Named Executive Officers and the Company’s overall results, I am confident that our current and proposed remuneration policies effectively support argenx’s strategic and operational objectives. On behalf of the Remuneration and Nomination Committee, Dr. Donald deBethizy Chairperson, Remuneration and Nomination Committee 3.4.2 Introduction In compliance with article 2:135b of the Dutch Civil Code, the European Shareholder Rights Directive and the DCGC, this 2024 Remuneration Report contains information on how we implemented our 2021 Remuneration Policy for the Board of Directors in financial year 2024. Remuneration in 2025 This 2024 Remuneration Report also provides early insight on remuneration that will be set in financial year 2025, for example with respect to benchmarking and peer group selection and prospectively regarding STI and LTI targets. This 2024 Remuneration Report also includes information on the Proposed 2025 Remuneration Policy, which will be submitted to the 2025 General Meeting for a binding vote and which will be available shortly after publication of this Annual Report. Please refer to Section 3.4.10 “Looking Forward” for more information. Statement of voting at general meetings The table below sets out the votes on the remuneration reports and compensation statement of the past years as well as the votes on the 2021 Remuneration Policy during the annual General Meeting held in 2021. This 2024 Remuneration Report as well as the Proposed 2025 Remuneration Policy, which will be available shortly after this Annual Report, will be put to an advisory and binding vote, respectively, at the 2025 General Meeting to be held on May 27, 2025. Resolution Percentage of votes cast for the resolution Resolution to approve the remuneration report (2024 AGM) 58.6% Resolution to approve the remuneration report (2023 AGM) 44.1% Resolution to approve the remuneration report (2022 AGM) 51.9% Resolution to approve the remuneration report (2021 AGM) 76.6% Resolution to amend the remuneration policy (2021 AGM) 76.6% 3.4.3 2024 Remuneration 2021 Remuneration Policy The 2021 Remuneration Policy is designed to reward contributions toward achieving Company objectives and generating long-term stakeholder value. Its primary goal is to offer competitive remuneration packages that align with market practices in the key regions where the Company competes for talent, ensuring strong support for the Company’s long-term business strategy. To maintain market competitiveness, the Company conducts regular reviews, typically annually but at least once every three years, of the total remuneration of members of the Board of Directors and members of the Senior Management Team. These reviews assess both compensation levels and program design, benchmarking against a carefully selected group of reference companies. Under the 2021 Remuneration Policy, total compensation is structured to align with or slightly exceed the market median for fixed compensation, benefits, and short-term variable incentives. The LTI component argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 147

consists of equity grants, with award sizes positioned between the 50th and 75th percentile of the global reference group. The 2021 Remuneration Policy was approved at the 2021 General Meeting with a 76.6% majority vote and is available on the Company’s website at https://www.argenx.com/investors/governance/remuneration- policy. Benchmarking and peer group selection in 2024 Under the Proposed 2025 Remuneration Policy, the methodology of benchmarking and peer group selection is different than described below for determination of 2024 remuneration. Please refer to Section 3.4.9 “Peer Group Selection” where we describe the updated benchmarking process, the objective peer group selection criteria and outcome for remuneration in 2025 in connection with the Proposed 2025 Remuneration Policy. The 2024 remuneration was determined following a comprehensive benchmarking exercise conducted in the third quarter of 2023 in collaboration with AON Radford, an independent third-party compensation advisor. To ensure a globally competitive compensation structure, the Company benchmarked against both U.S. and European peer groups, reflecting its position as a global company competing for top talent in both regions. This approach supports the execution of the Company’s business strategy while aligning executive pay with long-term sustainable value creation for stakeholders. The following criteria were used to select the peer group for the 2024 remuneration as part of the Company’s benchmark performed in the third quarter of 2023, ahead of setting the long-term incentive schemes of 2024 in December 2023 and the annual cash compensation for 2024 in the first quarter of 2024: • Sector: Biotechnology and Pharmaceutical industries • Stage of development: market companies • Market Capitalization: 1/4x – 3x argenx’s 30-day average market value as of November 2023, corresponding with a market capitalization between $5 - 60 billion • Revenue: 1/4x – 3x argenx’s trailing 12 months revenue, corresponding with an annual revenue between $160 million -$2 billion • Headcount (secondary criteria): 1/3x – 3x argenx’s projected financial year ended December 31, 2023 corresponding with a headcount between 400-4,000 employees With the goal of arriving at a sufficiently sized U.S. and EU peer group of at least 15 peer companies disclosing detailed compensation information, a number of companies were added to the European peer group following a qualitative review by AON Radford to identify companies with relevant similarities in business model and therapeutic focus. This led to the following selection of peer companies used by us in the 2023 benchmark for the 2024 remuneration: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 148

Company name Country of Headquarters ACADIA Pharmaceuticals Inc. USA Alnylam Pharmaceuticals, Inc. USA Amicus Therapeutics, Inc. USA BeiGene, Ltd. Cayman Islands BioMarin Pharmaceutical Inc. USA Blueprint Medicines Corporation USA CRISPR Therapeutics AG Switzerland Exelixis, Inc. USA Incyte Corporation USA Intra-Cellular Therapies, Inc. USA Ionis Pharmaceuticals, Inc. USA Neurocrine Biosciences, Inc. USA Sarepta Therapeutics, Inc. USA Seagen Inc. (formerly Seattle Genetics, Inc.) USA United Therapeutics Corporation USA Award levels For 2024, the Board of Directors sets award levels based on the outcome of the benchmarking exercise, in accordance with the 2021 Remuneration Policy, which contains the following framework: Non-Executive Directors Senior Management Team (including the CEO) Cash-based compensation (base pay + STI) 50th percentile of the companies in Reference Peer Group 50th percentile of the companies in Reference Peer Group Equity-based compensation (LTI) 50th percentile of the companies in Reference Peer Group Between the 50th to 75th percentile of the companies in Reference Peer Group 3.4.4 Application of the 2021 Remuneration Policy in 2024 Named Executive Officer Remuneration during 2024 This chapter contains a detailed overview of the remuneration paid for the year ended December 31, 2024 to the NEOs. Of the NEOs, only the CEO is a statutory director in the Board of Directors. The remuneration of the NEOs in 2024 consisted of base pay and benefits, STI pay in the form of variable cash remuneration, and LTI pay in the form of Company equity, consisting of stock options and restricted stock units (RSUs). Total Named Executive Officer Remuneration The majority of NEO compensation is provided in the form of variable remuneration, which is a combination of performance-dependent (short-term cash incentives) and stock options and service- dependent (RSUs) compensation. Variable (short-term) compensation allows the Board of Directors to set challenging annual objectives aligning the priorities of the NEOs with the short-term strategic objectives of the Company. Company equity in the form of stock options provides an incentive to the NEOs to contribute to Company (stock price) value increase over the long-term (3 years) vesting period of the stock options. Company equity in the form of RSUs also provides an incentive for value creation over the long-term (4 years) vesting period of the RSUs. The combination of variable pay in the form of cash-award STI pay, and stock option based and RSU based LTI pay, ensures a balanced incentive for short-term focus on and performance of near-term strategic targets, while contributing to sustainable long-term value creation and ensuring long-term commitment (retention) of the executive. In addition, the Company provides severance arrangements as applicable for the industry and pension and fringe benefits, including a performance based corporate bonus for all employees in the Company of up to €3,948 ($4,266) in accordance with Belgian practice. Moreover, in accordance with the DCGC, when determining the remuneration package of argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 149

the executives, scenario analyses are performed annually and taken into account in setting the total remuneration levels and target and maximum awards under the STI and LTI plans. Base pay In 2024, compared to 2023, the base pay of the NEOs was increased in line with the total argenx employee population annual base pay increase guidelines (CEO +15%, COO +6%, CFO +6%). The increases for the NEOs followed a review of the individual’s performance over the preceding year(s), in light of comprehensive analysis of benchmark data showing the relative positioning of base salaries compared to the relevant external and internal peers. This process ensures that the Company’s compensation packages are a fair reflection of individual performance while also remaining competitive and aligned with the market. The merit principles and base pay increase framework applied are identical to those applicable to all employees in the organization and are based on the individuals’ performance and contributions over the preceding period. With respect to the CEO, at his request his base pay remained unchanged between 2022 and 2023; accordingly, the increase in 2024 was the first increase since then. This increase was deemed necessary to ensure fairness in light of industry benchmarks and to recognize the continued outstanding commitment and performance of the CEO in delivering exceptional value to our shareholders. Even with this adjustment, the CEO places only at the 27th percentile of our new global peer group for base pay. With respect to the COO, the Board of Directors recognized the outstanding performance and the achievement and over achievement of short-term targets. Consequently, and in line with pay practice applied consistently across the wider workforce, the COO’s base pay was increased by 6.1% in 2024 versus 2023. The increase consisted of a merit increase and an additional base pay increase to recognize her exceptional performance mentioned above, her competitive placement versus the benchmark data and her critical role in our ongoing success. With respect to the CFO, the Board of Directors recognized outstanding performance for the second year in a row in 2024, including achievement and over achievement of short-term targets, and established that the CFO’s base pay was still below the midpoint for base pay for CFOs in the reference peer group. Consequently, and in line with pay practice applied consistently across the wider workforce, the CFO’s base pay was increased by 6% in 2024 versus 2023, consisting of a merit increase and an additional base pay increase to move the CFO closer to the benchmarked midpoint. The base pay increase was consistent with the base pay increase of 6% between 2023 and 2022. Please refer to Section 3.4.11 “Total Named Executive Officer Remuneration” for an overview of the total value of the remuneration paid to the NEOs for the last 3 years. Variable Cash The NEOs were eligible for a variable cash payment for the performance of pre-defined short-term performance targets in 2024, with the target variable cash compensation set as a percentage of their base pay (60% for CEO, 50% for COO and 40% for CFO). The Board of Directors has set a cap of 200% pay-out per target, and a 200% overall pay-out cap. The Board of Directors evaluated the pay-out of each target, with ‘at target’, ‘maximum per target’ and ‘actual pay-out’ explained in detail in the table below. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 150

CEO When considering the variable pay pay-out of the CEO, the Board of Directors primarily reviewed whether the key objectives of the Company’s business plan for 2024 were achieved. Personal targets set for the CEO, in addition to his overall responsibility for delivering the business plan, were the following: Performance Metric and Weighting Measurement (how the Board of Directors evaluated the target) Threshold Target Max Achievement Vesting Actual pay-out (USD) 1) Deliver continued VYVGART growth (25%) • Global annual operating budget revenue targets ($ targets) (75%) • New launches (patient on drug target (25%) 80% of annual operating budget target 100% annual operating budget target 120% annual operating budget target > 120% annual operating budget target 50% 227,304 Advance the Pipeline (25%) 10 high quality IIP programs per OGSM definition • 4 INDs on track for 2025 • MMN on accelerated path • 8 IIP Programs and • 3 INDs on track and • IND accepted • 10 IIP programs and • 4 INDs on track and • First site activated Minimum 2 out of 3: • 12 IIP programs and/or • 5 INDs on track and/or • First patient randomized • 11 IIP programs delivered • 5 INDs on track • First patient randomized 50% 113,652 Embed our culture and innovation mission (25%) • Integrating our newly hired people through dedicated culture/ways of working sessions organized with our global managers and culture champions • Champion four innovation initiatives N/A • Key hires successfully onboarded and • 4 innovation initiatives championed N/A • Key hires successfully onboarded and • 5 innovation initiatives championed 25% 227,304 Talent development (25%) Internal leadership talent pool assessed, increased and enhanced through direct personal involvement in the Personal Development Plan of undisclosed number of key high potentials and future Company leaders 25 35 50 > 50 50% 227,304 1) Amounts paid out in Euro have been converted to USD using the average rate for the period of 1.0824 EUR/USD. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 151

COO When considering the variable pay pay-out of the COO, the Board of Directors primarily reviewed whether the following key objectives of the Company’s business plan for which the COO had key responsibilities for 2024 were achieved. Personal targets set for the COO, in addition to her overall responsibility for delivering commercial performance, were the following: Performance Metric and Weighting Measurement (how the Board of Directors evaluated the target) Threshold Target Max Achievement Vesting Actual pay-out (USD) 1) Deliver continued VYVGART growth (25%) • Global annual operating budget revenue targets ($ targets) (75%) • New launches (patient on drug target (25%) 80% of annual operating budget target 100% annual operating budget target 120% annual operating budget target > 120% annual operating budget target 50% 168,741 PFS with self-administration delivered according to plan, maintaining subcutaneous Gen-1 option (25%) FDA acceptance FDA acceptance FDA acceptance with no concerns and review on track FDA acceptance with PDUFA date < 6 months FDA acceptance with no concerns and review on track 25% 84,371 Scale commercial engine by leveraging the new operating model (25%) Successful onboarding of key hires and cross-functional indication teams delivering their OGSMs Key hires successfully onboarded with max 2 attritions Key hires successfully onboarded with no attrition AND 80% of OGSM targets delivered Key hires successfully onboarded with no attrition AND 90% of OGSM targets delivered • Key hires successfully onboarded with no attrition AND • 90% of OGSM targets delivered 50% 168,741 Integrate our newly hired colleagues to the argenx culture and operating principles, leveraging the operating excellence model to create a global network of leaders (25%) Measured by operating excellence model self-assessment At least 3 significant, global operational wins by applying operating principles At least 5 significant, global operational wins by applying operating principles At least 7 significant, global operational wins by applying operating principles At least 7 significant, global operational wins by applying operating principles 50% 168,741 1) Amounts paid out in Swiss Franc have been converted to USD using the average rate for the period of 1.1363 CHF/USD. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 152

CFO When considering the variable pay pay-out of the CFO, the Board of Directors primarily reviewed whether the key commercial and operational objectives of the Company’s business plan for 2024 were achieved. The personal targets set for the CFO, in addition to his overall responsibility for delivering commercial performance, were the following: Deliver continued VYVGART growth (25%) • Global annual operating budget revenue targets ($ targets) (75%) • New launches (patient on drug target) (25%) 80% of annual operating budget target 100% annual operating budget target 120% annual operating budget target > 120% annual operating budget target 50% 110,600 Ensure internal and external alignment of expectations and grow investor base (25%) • Analyst expectations vs actual revenue disclosed per quarter, measured by average % change in Nasdaq stock price on the trading day of earnings communications (50%) • Add generalist shareholders to the top 35 list (50%) On average no more than 20% change and 1 generalist shareholder added On average no more than 10% change and 2 generalist shareholder added On average no more than 5% change and 4 generalist shareholders added Average change of 4% and 4 generalist shareholders added 50% 110,600 ERP process simplification & time reduction (25%): • Simplify project management module; embed forecasting capabilities and insight generation • Global process owner automation & centralization Measured by successful completion of projects and internal customer feedback 75% of projects successfully delivered 85% of projects successfully delivered 100% of projects successfully delivered 85% of projects delivered 25% 55,300 Protect and preserve company and critical assets (25%): • Strong audit ratings from internal & external audits • Partnership with audit and compliance committee • Sustainable future tax rate • Working capital • Audit and compliance committee partnership: measured by feedback • Sustainable future tax rate: measured by filings of key rulings (US Bilateral Advance Pricing Arrangements, expanded Belgian Innovation Income Deduction ruling for ARGX-113 IV & SC for CIDP and ITP, Switzerland, and Japan) • Working capital: measured against agreed terms • No major findings external • Audit and Compliance Committee rates partnership with CFO as "strong" (7+) • US ruling filed • Annual operating budget + 10% • No major findings internal & external audit • Audit and Compliance Committee rates partnership as very strong (8+) • US + Japan rulings filed • Annual operating budget • No major findings internal & external audit and no minor findings external • Audit and Compliance Committee rates partnership as excellent (9+) • US / Japan / Switzerland rulings filed • Annual operating budget -10% • No major internal & external audit findings • Audit and Compliance Committee rates partnership as 9+ • US and Japan rulings filed • Annual operating budget -10% 25% 55,300 Performance Metric and Weighting Measurement (how the Board of Directors evaluated the target) Threshold Target Max Achievement Vesting Actual pay-out (USD) argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 153

Corporate Bonus All employees are eligible to annually earn a performance based corporate bonus with a maximum value of €3,948 ($4,266) per year, based on three equally weighted Company-wide goals. In 2024, the targets focused on (i) continued increased cybersecurity awareness, (ii) building argenx together by bringing the cultural pillars and operating principles to life through participation in Culture Labs, and (iii) supporting development and growth of all employees through personal development plans. A pay-out of €3,350 ($3,636) was made to all employees. Equity In 2024, the Company granted a mix of stock options and RSUs to the NEOs. The number of instruments to be granted in the course of 2024 was determined pursuant to the annual benchmark exercise performed with the help of AON Radford. This benchmark exercise takes place in the third quarter of each calendar year. Determination of target value of CEO equity grant For the 2023 CEO equity granted on the first business day in July 2023, being July 3, 2023, the determination of the target value for the CEO followed the below steps: • The total target value of $6,986,986 was established in the third quarter of 2022. • Immediately thereafter, the target value was subsequently converted into a fixed number of stock options and RSUs to be granted on the grant date of July 3, 2023. • For the conversion into a fixed number of stock options; a Black-Scholes value of $151.03 per stock option was used based on the 30-day average stock price of $366.58 before July 22, 2022. Based on this valuation, the number of stock options to be granted on July 3, 2023 was fixed at 30,000 stock options. • For the conversion into a fixed number of RSUs; the value of $366.58 per RSU was based on the 30-day average stock price of $366.58 before July 22, 2022. Based on this valuation, the number of RSUs to be granted on July 3, 2023 was fixed at 6,700 RSUs. • Consequently, the fixed number of stock options and RSUs, 30,000 and 6,700 respectively, were embedded in the 2023 equity allocation scheme. • On the grant date of July 3, 2023, the stock price was $389.73 compared to $366.58 on the date on which the number of stock options and RSUs were fixed following conversion of the target value. • This resulted in a value at grant on July 3, 2023 of $8,084,605 compared to the target value of $6,986,986 in the third quarter of 2022. This difference is explained by the stock price increase in the intervening period.1) • Consequently, by fixing the number of equity instruments in the third quarter of 2022 while the grant takes place in the second quarter of the next year, any positive or negative fluctuations in the stock price between the third quarter of 2022 and July 3, 2023 were not taken into account. The same determination methodology was followed between the approval of the 2021 Remuneration Policy and 2024. Updated determination of target value of CEO equity grant as of 2024 As referenced in the 2023 remuneration report, the Company took concrete steps in 2024 to close the time gap between the benchmarking exercise when determining the target value. The below steps have been followed in 2024 for the determination of the target value for the CEO: • The total target value of $7,080,000 was established in the third quarter of 2023. • In contrast to 2023, the target value was not immediately converted into a fixed number of stock options and RSUs. 1) These amounts do not reflect the actual economic value realized by the beneficiary. Amounts included represent the expenses with respect to the assumptions used in the Black-Scholes model differ between Belgian beneficiaries versus non-Belgian beneficiaries, resulting in the CEO’s stock based compensation expenses to be higher than other beneficiaries. For a description of the assumptions used, see ”Note 13 Share-Based Payments“ in the 2023 consolidated financial statements. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 154

• Instead, the number of stock options was calculated by dividing the target value through the then applicable Black-Scholes value based on 30 calendar days preceding the 15th day of the month in which the grant occurs (the Reference Date), rounded up to the nearest whole number granted as stock options. • The numbers of RSUs was calculated by dividing the target value through the average closing price 30 calendar days preceding the Reference Date, rounded up to the nearest whole number granted as RSUs. • The 30-calendar day average closing price on June 15, 2024 was $375.68. • Consequently, 18,279 stock options and 6,672 RSUs were granted on the grant date of June 28, 2024. • The stock price on the day preceding the grant of June 28, 2024 was $445.76 compared to the 30- calendar day average of $375.68 on the Reference Date of June 15, 2024. • This resulted in a total value at grant on June 28, 2024 of $6,209,313 compared to the target value of $5,080,000 on the Reference Date of June 15, 2024. This difference is explained by the stock price increase in the intervening period.2) • Even though the time period between the valuation date and grant date has been drastically reduced from 8 months to 2 weeks, stock fluctuations whether positive or negative, will still influence the grant value compared to the target value. For instance, in 2024, we announced the FDA approval of VYVGART HYTRULO for CIDP on June 21, 2024, which positively influenced the stock price between June 15, 2024 and July 1, 2024. The above determination methodology will be applied going forward, irrespective of whether the Proposed 2025 Remuneration Policy will be approved. It will for the first time be used for the determination of the 2025 target value for stock options and PSUs. 2) These amounts do not reflect the actual economic value realized by the beneficiary. Amounts included represent the expenses with respect to the assumptions used in the Black-Scholes model differ between Belgian beneficiaries versus non-Belgian beneficiaries, resulting in the CEO’s stock based compensation expenses to be higher than other beneficiaries. For a description of the assumptions used, see “Note 13 Share-Based Payments“ in the consolidated financial statements. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 155

The following table sets out the number, value and key terms of equity instruments granted to the NEOs in 2024: RSUs granted in 2024 Stock options granted in 2024 Name # RSUs Key terms Value at grant in $ Benchmark value in $ # Stock options Exercise price in € Exercise price in $ Key terms Value at grant in $ 1) Benchmark value in $ 1) Total Tim Van Hauwermeiren, CEO 6,762 RSUs vest and are settled in 4 equal installments of 25% over a 4 year period 3,014,500 2,540,348 18,279 416.40 445.76 1/3 vests after year 1 2/3 vest in monthly installments in year 2 and 3 Options not exercisable until the 4th calendar year after the grant year 3,194,813 2,540,050 6,209,313 Karen Massey, COO 4,712 2,100,610 1,770,204 12,738 416.40 445.76 1/3 vests after year 1 2/3 vest in monthly installments in year 2 and 3 2,018,973 1,770,072 4,119,583 Karl Gubitz, CFO 4,712 2,100,610 1,770,204 12,738 416.40 445.76 2,018,973 1,770,072 4,119,583 1) Amounts shown represent the expenses with respect to stock options measured using the Black-Scholes model. For a description of the assumptions used in valuing these awards, see “Note 13 Share-Based Payments” to our Consolidated Financial Statements. Based on the approved 2024 equity allocation scheme, the total equity target value for Tim Van Hauwermeiren is equal to $5,080,000 and the total equity target value for Karen Massey and Karl Gubitz is equal to $3,040,000 for each. The CEO, COO and CFO received their respective equity grants at target value converted into a number of stock options and RSUs on the Reference Date of the 30-days average share price of $375.68 per share preceding the Reference Date and the Black-Scholes model fair market value of $138.96 per stock option. This results in the number of stock options and RSUs shown above. The amounts shown above represent the actual value received at the grant date of June 28, 2024 at which date the Company’s share price was equal to $445.76. The difference of $70.08 per share is explained by the stock price increase in the intervening period primarily due to approval of VYVGART HYTRULO for CIDP in the U.S. by the FDA. For more information on the CEO equity grant, please refer to “Determination of target value of CEO equity grant” included in “Equity“ above. The fair market value based on the Black-Scholes model for Tim Van Hauwermeiren is $174.78 and the fair market value for the COO and CFO is $158.50. These amounts do not reflect the actual economic value realized by the beneficiary. Amounts shown represent the expenses with respect to the stock options awards granted in 2024 measured using the Black-Scholes model with unobservable assumptions. The assumptions used in the fair valuation differ between Belgian beneficiary versus non-Belgian beneficiary. For a description of the assumptions used in valuing these awards, see Note 13 Share-Based Payments to our Consolidated Financial Statements. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 156

Pension and fringe benefits The benefits paid to the NEOs are jurisdiction dependent. For the CEO, these included benefits customary in the Belgian market, and which are standard components of Belgian-based employee packages: pension contributions, a hospitalization insurance, a representation allowance and a company car. The Company pension contribution percentage of base pay for the CEO is equal to the Company pension contribution percentage for all employees in Belgium. For the COO, these included benefits customary in the Swiss market, and which are standard components of Switzerland-based employee packages: car allowance, lunch allowance, health insurance allowance, representation allowance and pension contributions For the CFO, these included benefits customary in the U.S. market, and which are standard components of our U.S.- based employee packages: a company-administered health and 401k plan, with a 4% company match. Equity holding requirements for Named Executive Officers In 2023, the Company implemented the following holding requirements for the Named Executive Officers : • CEO: 3x base pay • Other NEOs: 1x base pay The minimum equity stake has to be built up over a maximum of five years and continues to apply for the duration of employment and for two years thereafter. Severance arrangements In accordance with our 2021 Remuneration Policy, the CEO has an 18-month notice period for termination (or alternatively, 12 months severance in lieu of notice). For the other NEOs, no contractual arrangements have been made for severance. In the year ended December 31, 2024, no severance payments were granted to the NEOs. Clawback policy In the event that any variable remuneration (cash or equity) is paid to members of the Senior Management Team, including the NEOs, based on financial information which later proves to be incorrect and leads to an accounting restatement (i) due to the material noncompliance of the Company with any financial reporting requirement under applicable securities laws, including any required accounting restatement to correct an error in previously issued financial statements of the Company that is material to the previously issued financial statements of the Company, or (ii) that corrects an error that is not material to previously issued financial statements of the Company, but would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period, then the difference between the paid compensation and the compensation which would have been payable without such accounting restatement, shall be claimed back from the executive, all as further set out in the Executive Compensation Clawback Policy, as adopted by the Board of Directors on July 25, 2023. In the year ended December 31, 2024, no variable remuneration was clawed back and no variable remuneration was adjusted (retroactively). If the Proposed 2025 Remuneration Policy is approved at the 2025 General Meeting, new management agreements that are entered into with the Company will not have notice periods exceeding 12 months unless otherwise required by local law. No severance arrangements will be paid in the event of serious culpable or negligent behavior on the part of an Executive Director being dismissed. We also will not pay severance if the agreement is terminated at the initiative of an Executive Director, other than due to serious culpable conduct or neglect on the part of the Company. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 157

3.4.5 Remuneration of Other Members of the Senior Management Team For the purposes of U.S. governance reporting requirements, all senior level employees reporting directly to the CEO qualify as the Company’s ‘executives’. The remuneration disclosures in relation to this more extensive group of senior personnel (excluding the NEOs) in this 2024 Remuneration Report is presented on an aggregated basis, with the exception of equity remuneration, which is presented on an individual basis. Aggregate compensation for other members of the Senior Management Team The following table sets forth information regarding aggregate compensation paid to members of the Senior Management Team (other than the NEOs) during the year ended December 31, 2024. (in $) Compensation Base pay 2,563,047 Variable STI 1) 1,376,604 Compensation in the form of stock options 10,525,234 Compensation in the form of RSUs 8,994,991 Other benefits 2) 4,095,467 Total 27,555,343 1) Variable STI includes a performance based Company wide corporate bonus of $3,636 per member of the Senior Management Team. 2) Other benefits consists of the lease of a company car, employer-paid medical insurance premiums, pension contributions, social security costs and allowance. In 2024, employer social security costs were impacted by the increase of share-price at year end against the share-price as of December 31, 2023. For more information on equity granted to members of the Senior Management Team (other than the NEOs), during 2024, please refer to Section 3.4.12 Summary of Other members of the Senior Management Team” below. 3.4.6 Non-Executive Director Remuneration Pursuant to the 2021 Remuneration Policy, the remuneration of the Non-Executive Directors consists of (i) a cash retainer fee calculated on the basis of their membership or chairpersonship of the Board of Directors and/or its committees, and (ii) a long-term equity incentive in the form of stock options and RSUs. One of the key points raised by stakeholders in respect of the 2021Remuneration Policy was that granting stock options to Non-Executive Directors may be perceived as performance-based remuneration, potentially affecting the objectivity of our Non-Executive Directors. To address this concern, the Company decided to no longer grant stock options to Non-Executive Directors as of 2024. Consequently, in 2024, the remuneration of the Non-Executive Directors consisted of cash retainer fees and RSUs. Our Proposed 2025 Remuneration Policy will formally reflect this change. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 158
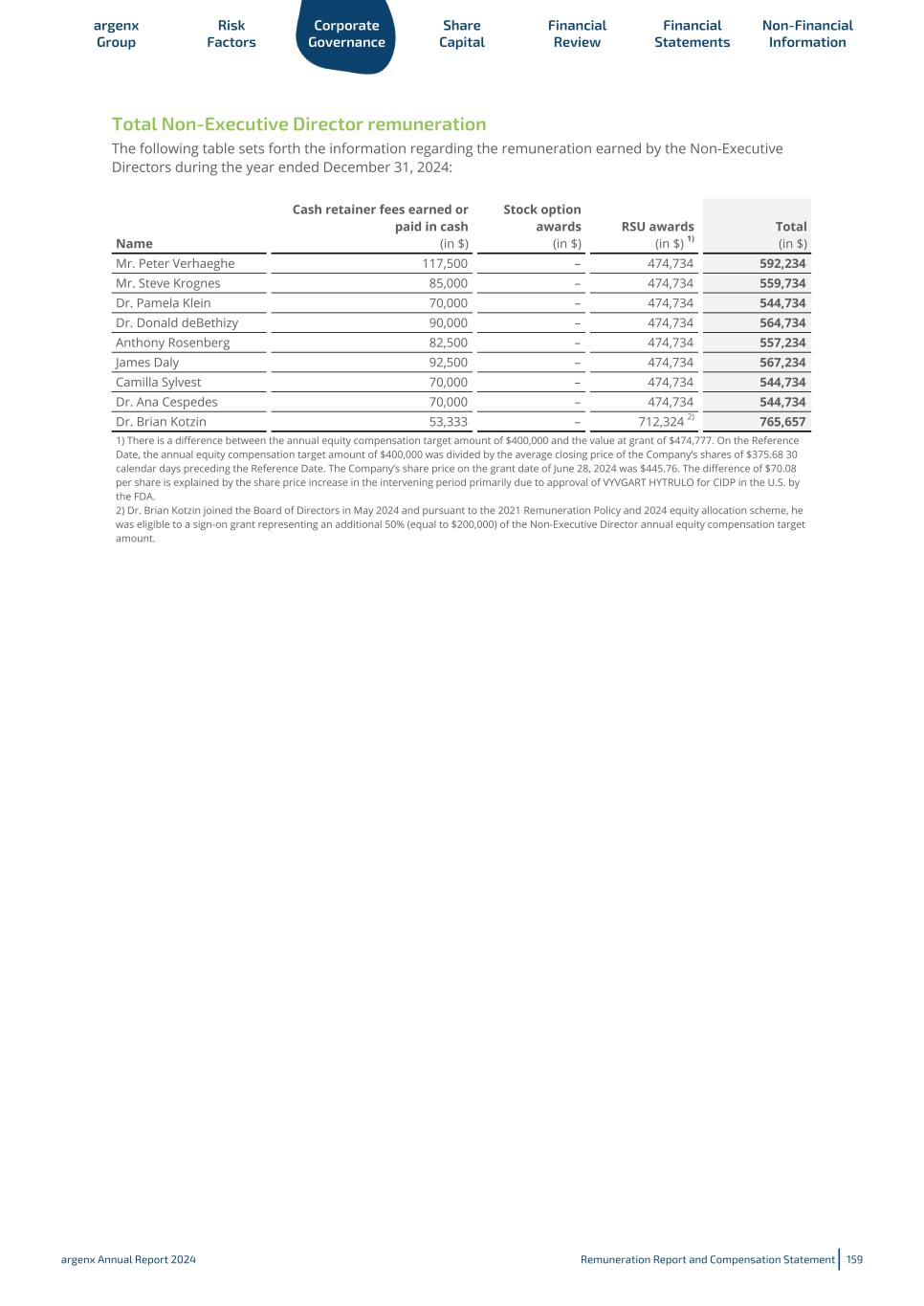
Total Non-Executive Director remuneration The following table sets forth the information regarding the remuneration earned by the Non-Executive Directors during the year ended December 31, 2024: Name Cash retainer fees earned or paid in cash (in $) Stock option awards (in $) RSU awards (in $) 1) Total (in $) Mr. Peter Verhaeghe 117,500 – 474,734 592,234 Mr. Steve Krognes 85,000 – 474,734 559,734 Dr. Pamela Klein 70,000 – 474,734 544,734 Dr. Donald deBethizy 90,000 – 474,734 564,734 Anthony Rosenberg 82,500 – 474,734 557,234 James Daly 92,500 – 474,734 567,234 Camilla Sylvest 70,000 – 474,734 544,734 Dr. Ana Cespedes 70,000 – 474,734 544,734 Dr. Brian Kotzin 53,333 – 712,324 2) 765,657 1) There is a difference between the annual equity compensation target amount of $400,000 and the value at grant of $474,777. On the Reference Date, the annual equity compensation target amount of $400,000 was divided by the average closing price of the Company’s shares of $375.68 30 calendar days preceding the Reference Date. The Company’s share price on the grant date of June 28, 2024 was $445.76. The difference of $70.08 per share is explained by the share price increase in the intervening period primarily due to approval of VYVGART HYTRULO for CIDP in the U.S. by the FDA. 2) Dr. Brian Kotzin joined the Board of Directors in May 2024 and pursuant to the 2021 Remuneration Policy and 2024 equity allocation scheme, he was eligible to a sign-on grant representing an additional 50% (equal to $200,000) of the Non-Executive Director annual equity compensation target amount. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 159

Annual cash The Board of Directors has set the annual cash retainer fees, including for members of the Audit and Compliance Committee, the research and development committee, the Remuneration and Nomination Committee and the commercial committee and, in each case, the additional remuneration for the respective chairperson as follows. In 2024, the annual cash retainer fees were at the 50th percentile of cash remuneration in the peer group for 2024 remuneration. In $ Relevant body Position Fees in $ Mr. Peter Verhaeghe Mr. Steve Krognes Dr. Pamela Klein Dr. Donald deBethizy Anthony Rosenberg James Daly Camilla Sylvest Dr. Ana Cespedes Dr. Brian Kotzin Board of Directors Chairperson 95,000 95,000 – – – – – – – Member 60,000 – 60,000 60,000 60,000 60,000 60,000 60,000 60,000 40,000 Audit & Compliance Committee Chairperson 25,000 – 25,000 – – – – – – – Member 12,500 12,500 – – – 12,500 12,500 – – – Remuneration & Nomination Committee Chairperson 20,000 – – – 20,000 – – – – – Member 10,000 10,000 – – – – – – 10,000 – Commercial Committee Chairperson 20,000 – – – – – 20,000 – – – Member 10,000 – – – – 10,000 – 10,000 – – Research & Development Committee Chairperson 20,000 – – – – – – – – 13,333 Member 10,000 – – 10,000 10,000 – – – – – Total 117,500 85,000 70,000 90,000 82,500 92,500 70,000 70,000 53,333 argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 160
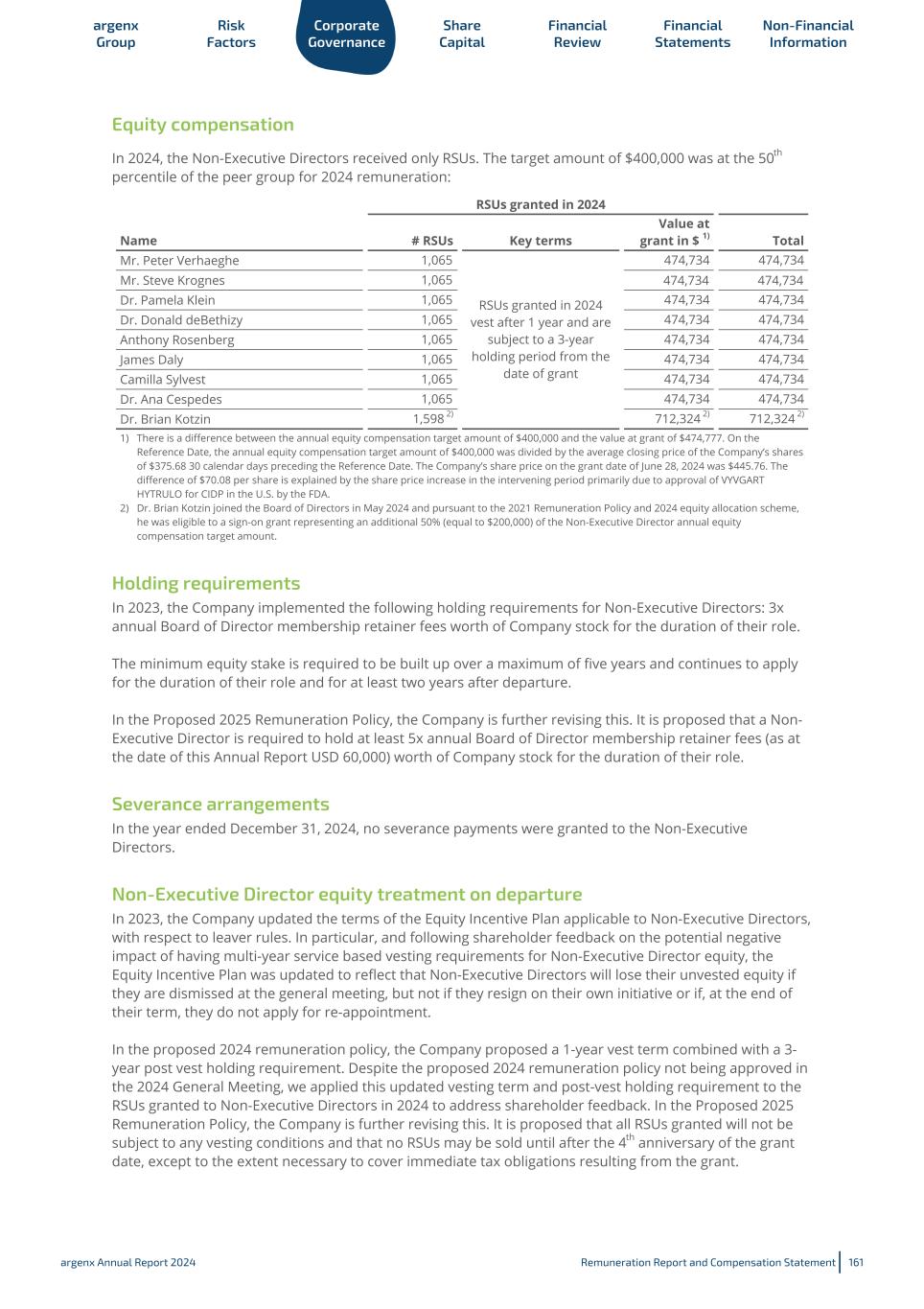
Equity compensation In 2024, the Non-Executive Directors received only RSUs. The target amount of $400,000 was at the 50th percentile of the peer group for 2024 remuneration: RSUs granted in 2024 Name # RSUs Key terms Value at grant in $ 1) Total Mr. Peter Verhaeghe 1,065 RSUs granted in 2024 vest after 1 year and are subject to a 3-year holding period from the date of grant 474,734 474,734 Mr. Steve Krognes 1,065 474,734 474,734 Dr. Pamela Klein 1,065 474,734 474,734 Dr. Donald deBethizy 1,065 474,734 474,734 Anthony Rosenberg 1,065 474,734 474,734 James Daly 1,065 474,734 474,734 Camilla Sylvest 1,065 474,734 474,734 Dr. Ana Cespedes 1,065 474,734 474,734 Dr. Brian Kotzin 1,598 2) 712,324 2) 712,324 2) 1) There is a difference between the annual equity compensation target amount of $400,000 and the value at grant of $474,777. On the Reference Date, the annual equity compensation target amount of $400,000 was divided by the average closing price of the Company’s shares of $375.68 30 calendar days preceding the Reference Date. The Company’s share price on the grant date of June 28, 2024 was $445.76. The difference of $70.08 per share is explained by the share price increase in the intervening period primarily due to approval of VYVGART HYTRULO for CIDP in the U.S. by the FDA. 2) Dr. Brian Kotzin joined the Board of Directors in May 2024 and pursuant to the 2021 Remuneration Policy and 2024 equity allocation scheme, he was eligible to a sign-on grant representing an additional 50% (equal to $200,000) of the Non-Executive Director annual equity compensation target amount. Holding requirements In 2023, the Company implemented the following holding requirements for Non-Executive Directors: 3x annual Board of Director membership retainer fees worth of Company stock for the duration of their role. The minimum equity stake is required to be built up over a maximum of five years and continues to apply for the duration of their role and for at least two years after departure. In the Proposed 2025 Remuneration Policy, the Company is further revising this. It is proposed that a Non- Executive Director is required to hold at least 5x annual Board of Director membership retainer fees (as at the date of this Annual Report USD 60,000) worth of Company stock for the duration of their role. Severance arrangements In the year ended December 31, 2024, no severance payments were granted to the Non-Executive Directors. Non-Executive Director equity treatment on departure In 2023, the Company updated the terms of the Equity Incentive Plan applicable to Non-Executive Directors, with respect to leaver rules. In particular, and following shareholder feedback on the potential negative impact of having multi-year service based vesting requirements for Non-Executive Director equity, the Equity Incentive Plan was updated to reflect that Non-Executive Directors will lose their unvested equity if they are dismissed at the general meeting, but not if they resign on their own initiative or if, at the end of their term, they do not apply for re-appointment. In the proposed 2024 remuneration policy, the Company proposed a 1-year vest term combined with a 3- year post vest holding requirement. Despite the proposed 2024 remuneration policy not being approved in the 2024 General Meeting, we applied this updated vesting term and post-vest holding requirement to the RSUs granted to Non-Executive Directors in 2024 to address shareholder feedback. In the Proposed 2025 Remuneration Policy, the Company is further revising this. It is proposed that all RSUs granted will not be subject to any vesting conditions and that no RSUs may be sold until after the 4th anniversary of the grant date, except to the extent necessary to cover immediate tax obligations resulting from the grant. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 161

3.4.7 Pay Ratios Overall pay ratios The total expense for the non-equity remuneration paid to the CEO (being the only statutory Executive Director on the Board of Directors) for the year ended December 31, 2024, totalled $1,598,471. The table below shows the evolution over the past five years of CEO compensation, the performance of the Company’s stock price and the median remuneration on a full-time equivalent basis (annualized for the employees who joined or left us during the year) of employees, other than the CEO: 2020 2021 2022 2023 2024 Base pay of the CEO (EUR) € 525,000 551,250 606,368 606,368 700,000 Base pay of the CEO (USD) $ 553,167 580,825 638,901 655,787 757,680 Non-equity remuneration of the CEO (USD) (base pay, short-term cash incentive, pension contributions and other compensation elements) $ 1,144,301 1,285,136 1,443,925 1,285,056 1,598,471 Total remuneration of the CEO (USD) (non-equity remuneration, STI and LTI) $ 8,160,745 7,263,828 7,778,298 11,944,835 1) 7,807,786 Non-equity median salary paid to employees (USD) $ 163,062 157,349 153,193 159,500 180,543 Non-equity remuneration ratio employee/CEO 14% 12 % 11 % 12% 2) 11 % Average remuneration paid to Non- Executive Director (USD) $ 57,925 54,484 48,587 59,230 81,204 Number of employees on December 31 336 650 843 1,148 1,599 Share price at end of year Euronext (EUR) on December 31 € 242.00 315.30 348.30 343.50 600.00 Share price at end of year Euronext (USD) on December 31 $ 296.96 357.11 371.50 379.57 623.34 1) Based on the approved 2023 equity allocation scheme, the total equity target value for Tim Van Hauwermeiren is equal to $6,986,986. Please refer to Section “Determination of target value of CEO equity grant included in “Equity ” above for more information on the variation in granted equity value between 2023 and 2024. 2) The increase in the remuneration ratio between the CEO and other employees between 2022 and 2023 is caused by the increase in salary of employees when base salary of the CEO remained unchanged. The comparison of non-equity compensation above is made between the compensation paid to the CEO, the Company’s sole statutory Executive Director on the Board of Directors, and the median compensation paid to employees. The Company has opted to compare non-equity salaries, because whereas the number of stock options granted is linked to the overall size of remuneration packages granted, the value of equity components depends on the evolution of the Company’s share price, volatility and the risk-free rate, which is unknown at the time of grant and as such the forward-looking valuation methods for stock options normally do not provide an accurate representation of actual economic value granted. In the assumptions used, the fair valuation differs between a Belgian beneficiary versus a non-Belgian beneficiary. For a description of the assumptions used in valuing these awards, please refer to “Note 13 Share-Based Payments“ in Section 6 “Consolidated Financial Statements”. Regional pay ratios Due to the global spread of employees over multiple continents, it is deemed relevant to also include the above comparison separately to U.S. employees, EU employees and Japanese employees. Due to the overall higher compensation level in the business segment in the U.S. compared to the EU, there is a significant difference in the pay ratio when the CEO’s compensation is compared to the median compensation of all employees, compared to employees in the U.S. The following information is provided for reference purposes: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 162

Ratio of non-equity compensation of the median employee compared to the CEO for the year ended December 31, 2024 All employees 11 % European employees 7 % North-America Employees 16 % Japan employees 5 % Total employment costs (excluding any costs related stock options and RSUs) paid in the year ended December 31, 2024 was split between regions as follows: Total employment costs in the year ended December 31, 2024 (in millions of $) Europe 186.7 North-America 204.9 Japan 13.9 Share-based payment ratios 2020 2021 2022 2023 2024 Stock options granted to the CEO 50,000 25,000 25,000 30,000 18,279 Median stock options granted to employees 2,900 981 900 600 306 Ratio employee/CEO for stock options 6 % 4 % 4 % 2 % 2 % RSUs granted to the CEO N/A 5,700 5,700 6,700 6,762 Median RSUs granted to the employees N/A 200 200 94 148 Ratio employee/CEO for RSUs N/A 4 % 4 % 1 % 2 % Median number of stock options granted to Non-Executive Directors 10,000 2,700 2,700 1,600 N/A Median stock options granted to employees 2,900 981 900 600 306 Ratio Non-Executive Directors/employee stock options 29 % 36 % 33 % 38 % N/A Median number of RSUs granted to Non- Executive Directors N/A 600 600 350 1,124 Ratio Non-Executive Directors/employee RSUs N/A 33 % 33 % 27 % 13 % 3.4.8 Other Disclosures Remuneration by subsidiaries In the year ended December 31, 2024, no remuneration was granted and allocated by subsidiaries or other companies whose financials are consolidated, other than the regular remuneration payments made by the entities with whom members of Senior Management Team have their employment contracts. No loans or guarantees In the year ended December 31, 2024, no loans were granted to members of our Senior Management Team and Non-Executive Directors and no guarantees or the like have been granted in favor of any member of Senior Management Team or the Board of Directors. Deviations In the year ended December 31, 2024, the Company did not deviate from the decision-making process for the implementation of the 2021 Remuneration Policy for the NEOs and Non-Executive Directors and no deviations took place from the 2021 Remuneration Policy. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 163

Key terms of equity plan applicable to grants in 2024 Stock options granted pursuant to the Equity Incentive Plan shall vest with respect to one third of the shares upon the first anniversary of the date of grant, with the remaining two thirds vesting in 24 equal monthly installments with the stock options fully vesting upon the third anniversary of the date of grant, subject, in each case, to the optionee’s continued status as a service provider. Stock options are exercisable when vested, and in any case not after the stock option expiration date included in each individual stock option grant, which is 10 years, or in the case of Belgian tax resident employees, at their election either five years or 10 years from the date of grant. Each stock option shall be granted with an exercise price equal to the fair market value upon the date of grant and shall have a term equal to five or 10 years from the date of grant. Optionees may prefer to elect the five-year period as this may limit their personal tax obligations in respect of the stock option in respect to the jurisdiction where stock options are taxed at grant, compared to a ten-year stock option. Stock options granted to Belgian tax resident beneficiaries (including the CEO) are not exercisable prior to the fourth year following the year of the grant. Stock options granted to Non-Executive Directors vest at once on the third anniversary of the date of grant. RSUs granted under the Equity Incentive Plan shall vest over a period of four years with respect to one fourth of the shares upon each anniversary of the date of grant. At the time of vesting, the holder of such RSUs receives shares in the share capital of the Company for free equal to the number of RSUs vested minus a certain number of shares required to cover employee taxes payable by us on behalf of the holder of RSUs, if applicable. In 2024, the Equity Incentive Plan was updated to reflect stakeholder feedback in relation to RSUs granted to Non-Executive Directors. RSUs granted in 2024 to Non-Executive Directors vest after one year instead of four years and are subject to a three-year holding period. Unvested equity incentives shall vest in the event of a (i) sale, merger, consolidation, tender offer or similar acquisition of shares or other transaction or series of related transactions as a result of which a change in control occurs, (ii) sale or other disposition of all or substantially all of the Company’s assets or (iii) the Company’s dissolution and/or liquidation. The Board of Directors, upon approval of a majority of the Non-Executive Directors, may amend or terminate the Equity Incentive Plan or may amend the terms of the Equity Incentive Plan, or any outstanding stock options or RSUs, provided that the Company will compensate any affected individual for any direct negative impact of such amendment. We plan to amend the Equity Incentive Plan to reflect the Proposed 2025 Remuneration Policy if it is approved at the 2025 General Meeting. 3.4.9 Peer group selection We have rapidly evolved and will continue to evolve into a fully integrated immunology company with a strong presence globally. To thrive and continue building the organization, we need executive and non- executive talent with a deep understanding of the global market in which we operate. We therefore compete for global talent. This is why we have established a global peer group focused on six key criteria that reflect the companies we benchmark against in attracting and retaining top talent. In connection with the benchmark exercise for 2025 remuneration ahead of setting fixed and variable pay levels, the following criteria were used for the first time in the third quarter of 2024 to select the new global peer group (the Peer Group). Compared to the European and Global peer groups used for the determine of the remuneration until 2024, the 2025 Peer Group consists of 15 companies. We deem a minimum of 15 companies appropriate, because (i) our industry tends to evolve quickly, with companies emerging and disappearing (due to mergers or otherwise) relatively often, and (ii) we deem it relevant to have a certain consistency in the companies comprising our peer group over the longer term. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 164

If there are not 15 companies meeting each of the criteria, we will include in our reference group all companies that meet the criteria, and supplement with companies that meet all but one criterion. The least relevant criterion will be dropped first, in the order as displayed below (from most to least relevant). 1. Sector Biopharmaceutical companies, excluding diagnostics and animal health companies Biopharmaceutical companies have characteristic pay and incentive structures compared to other industries. Within the biopharmaceutical industry, excluding diagnostics and animal health companies is appropriate because the talent focus of such companies is different and therefore they are not typically our competitors for talent. In addition, their pay structures tend to differ from those in our industry making these less relevant comparators. 2. Listing location Listed on a major US Stock Exchange Being listed on a major US stock exchange brings additional complexity, expertise requirements and potential liabilities to company officers and directors, which is typically reflected in a different pay structure of executives and board members serving on US listed companies, versus companies without a US listing. Our benchmark exercise shows that having a listing on a major US stock exchange tends to have a more significant relevance for pay structure applied by companies than does location of headquarters, which is why we do not apply a ‘location of headquarters’ filter. 3. Innovation focus At least 25% of revenue is spent on R&D Innovation focused, R&D driven companies tend to have a typical remuneration structure which differs from companies who focus on commercializing external innovations. To ensure we continue to be able to compete with other innovators, we limit our peer group selection to other companies who continue to significantly invest in their R&D activities. 4. Global reach Generates product revenues both within and outside the US Leading commercial operations both inside and outside the United States puts unique demands on the skills and expertise of key individuals, in addition to the strain of splitting their time and efforts across continents. For this reason, we compare pay practices to other global companies instead of companies with mostly local activities. 5. Revenue 1/4 – 4x of our annual revenue We compare ourselves to organizations that also have significant product revenues, as a reflection of overall size and complexity of the organization. Using a relatively wide range for this metric is appropriate to ensure we include relevant peers while ensuring a level of stability in the peer group over time. In setting the range, we also considered the rapid development in our own revenues since our first year of product commercialization (2023) and our internal revenue projections for the immediate future. 6. Market Cap 1/4 – 4x our market cap (based on 30 day average closing price) Whereas market cap can give some indication of overall size and complexity of comparator organizations, we also recognize that companies in our sector tend to have volatile stock prices and market cap can vary significantly even throughout a given calendar year.1) Using a relatively wide range for this metric is appropriate to ensure we include relevant peers while ensuring a level of stability in the peer group year-over- year. By going as low as 1/4x our value and as high as 4x our value, we aim to ensure that we are not positioning ourselves on either end of the peer group for this metric, to avoid establishing a peer group that is considered aspirational. Finally, given that market cap is influenced by a range of factors that do not necessarily correlate to the organization’s size or complexity or talent needs, we deem this the least relevant filter. If we are unable to reach our minimum of 15 peer companies meeting all selection criteria, we will include companies who meet all other criteria and are closest to our market cap criterion (but no more than 2x the high end of our range) in order to make up the 15. If we cannot make up to 15 applying this modified filter, we will include companies who are outside of but close to our revenue filter limits. 1) As at March 17, 2025, the delta between our 52-week high and low was approximately 104%, https://live.euronext.com/ nl/product/equities/NL0010832176-XBRU. Selection Criterion in order of relevance Range of Peers based on Criterion Relevance of criterion argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 165

Company Name Country of Headquarters Alnylam Pharmaceuticals, Inc. USA Amicus Therapeutics, Inc. USA Ascendis Pharma A/S Denmark BeiGene, Ltd. Cayman Islands Biogen Inc. USA BioMarin Pharmaceutical Inc. USA BioNTech SE Germany Blueprint Medicines Corporation USA Genmab SE Denmark Incyte Corporation USA Insmed Inc. USA Jazz Pharmaceuticals plc Ireland Moderna, Inc. USA Sarepta Therapeutics, Inc. USA Ultragenyx Pharmaceutical Inc. USA 3.4.10 Looking Forward Increased disclosure During our stakeholder outreach in relation to our proposed 2024 remuneration policy as well as the Proposed 2025 Remuneration Policy, we received feedback that the disclosure on STI and LTI in previous remuneration reports was not consistent with best practices. Consequently, stakeholders were not able to determine if and how pay-for-performance was embedded in our remuneration. To address this feedback, in the Proposed 2025 Remuneration Policy we therefore commit to a more detailed prospective disclosure for both the STI and LTI and retrospective disclosure against a threshold-target-maximum framework, including actual achievement and corresponding payout. To showcase our commitment to address stakeholder feedback, this 2024 Remuneration Report contains the prospective disclosure on the STI and LTI for the Named Executive Officers despite the Proposed 2025 Remuneration Policy not having been approved yet. STI CEO Distinction Under the current 2021 Remuneration Policy, the annual STI opportunity for the CEO consists of an at- target opportunity of 60% of base pay, and a maximum opportunity of 120% of base pay. The Proposed 2025 Remuneration Policy does not include any change to the current founder CEO’s 2025 STI opportunity. Therefore, for 2025 the STI opportunity remains equal to 60% of base pay at target and a maximum payout of 120% of base pay. In order to remain competitive in attracting, motivating and retaining any future Executive Director (including a future CEO), we target competitive remuneration levels in the Proposed 2025 Remuneration Policy. Therefore, and based on the Peer Group benchmark data, the annual STI opportunity for a future Executive Director will be up to 100% of base pay at target and a maximum payout of up to 200% of base pay. The majority of the targets under the Proposed 2025 Remuneration Policy will be quantitative in nature and at least 50% of the total STI opportunity for an Executive Director will be linked to financial performance metrics. Qualitative targets will be milestone-based to the extent possible. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 166

STI The top priorities identified in 2025 for the CEO include delivering continued VYVGART growth, advancing the pipeline, further embedding our culture and innovation mission by making it everyone’s business and ensuring business continuity by having a succession plan in place for senior key leader. The following metrics apply for 2025, which will be reported in more detail along with their final assessment and payout as part of the remuneration report on financial year ended December 31, 2025, to be published in 2026. Performance metric Target Measurement (how the Board of Directors will evaluate the metric and why it has been chosen) Threshold Target Max Revenue (50%) Deliver continued VYVGART growth Annual operating budget revenue target delivered and successful PFS self-administration approval and launch in US Targets and Executive Director achievement will be disclosed retroactively in the 2025 remuneration report Pipeline (20%) Advance the pipeline MG combo clinical trial launched Q3 Nominate 2 new ARGX-xxx candidates and graduate 3 discovery projects to lead identification (PPD) Innovation (20%) Embed our culture and innovation mission Champion key innovation projects All variable pay eligible employees have 1x performance goal linked to innovation Key innovations recognized, celebrated and cascaded throughout the Company Scaling the argenx way (10%) Talent development Succession plan in place for key senior leaders COO The top priorities identified in 2025 for the COO include delivering continued VYVGART growth growth and leading digital transformation and scaling the argenx way. The following metrics apply for 2025, which will be reported in more detail along with their final assessment and payout as part of the remuneration report on financial year ended December 31, 2025, to be published in 2026. Performance metric Target Measurement (how the Board of Directors will evaluate the metric and why it has been chosen) Threshold Target Max Revenue (40%) Deliver continued VYVGART growth Annual operating budget revenue target delivered and successful PFS self-administration approval and launch in US Targets and COO achievement will be disclosed retroactively in the 2025 remuneration report Pipeline acceleration (20%) Not disclosed Not disclosed Digital transformation (20%) Embed our culture and innovation mission Successful onboarding of Business Information Systems (BIS) leader and deliver on the BIS OGSM Scaling the argenx way 20%) Talent development Successful onboarding of key hires and leadership teams' their OGSM Elevate the operational excellence community to a leadership community and their OGSM delivered argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 167

CFO The top priorities identified in 2025 for the CFO include delivering continued VYVGART growth, delivering profit and loss leadership and to further drive productivity. The following metrics apply for 2025, which will be reported in more detail along with their final assessment and payout as part of the remuneration report on financial year ended December 31, 2025, to be published in 2026. Performance metric Target Measurement (how the Board of Directors will evaluate the metric and why it has been chosen) Threshold Target Max Revenue (30%) Deliver continued VYVGART growth Annual operating budget revenue target delivered and successful PFS self-administration approval and launch in US Targets and CFO achievement will be disclosed retroactively in the 2025 remuneration report P&L (25%) Financial performance Target effective tax rate in 2025 in line with annual operating budget Digital transformation (25%) Internal financial systems Time required to close the quarter reduced by 50% Annual operating budget process transformation Financial accounts automation Scaling the argenx way (20%) Strategic organizational growth Management headcount growth LTI PSU During our stakeholder outreach in relation to our proposed 2024 remuneration policy, the vast majority of the feedback we received on the introduction of PSUs was positive. This was confirmed in the outreach relating to the Proposed 2025 Remuneration Policy. Irrespective of the Proposed 2025 Remuneration Policy being approved at 2025 General Meeting, we will introduce PSUs. The LTI grant will therefore consist of 50% stock options and 50% PSUs. PSUs are granted on the last business day of June, i.e., on June 30 in 2025. PSUs have a 3-year performance period and in 2025 will therefore cover the period between January 1, 2025 and December 31, 2027. PSUs will have a 3-year cliff vest. The performance metrics will be challenging long-term goals essential for the Company’s success and will be set within the following framework: • at least 50% of the pay opportunity will be linked to financial performance metrics such as revenue growth; • at least 40% of the pay opportunity will be linked to innovation and pipeline development metrics, such as delivering clinical and regulatory milestones; and • up to 10% of the pay opportunity will be linked to people and culture metrics essential for sustainable, long-term value creation. The grant value of the PSUs will be determined after publication of this Annual Report on June 30, 2025, in accordance with the determination methodology described under Section 3.4.4 “Total Named Executive Officer Remuneration — Equity” above. Further details on the PSU grant will be included in the 2025 remuneration report, to be published in 2026. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 168

2025 PSU grant performance metrics The below performance metrics will apply to the 2025 PSU grant for all Named Executive Officers and all others members of Senior Management Team. Performance Metric Target Measurement (how the Board of Directors will evaluate the metric and why it has been chosen) Threshold Target Max Maximize the VYVGART opportunity (65%) 2027 annual revenue (50%) Minimum product net sales of undisclosed amount Targets and Executive Director achievement will be disclosed retroactively in the 2027 remuneration report, published in 2028 gMG Label Expansion (15%) Seronegative gMG and ocular gMG approved by the FDA Build a portfolio of breakthrough antibody-based products (15%) FDA submissions (15%) Undisclosed number of indications approved or submitted to the FDA Ensure long-term sustainability as an independent company (10%) Pipeline progression (10%) Undisclosed number of pipeline assets into phase 2 and/or undisclosed number of additional pipeline assets IND / clinical trial application submitted Scaling the argenx way (10%) Talent retention (10%) Three-year average voluntary employee turnover equal to or below 8% argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 169

3.4.11 Summary of Named Executive Officer Remuneration Total remuneration Named Executive Officers The following table sets forth the total value of the remuneration paid to the NEOs for the last three years: (in $) Base pay 1) Base pay in % change vs the prior year 1) Sign on bonus Corporate bonus Variable short-term incentive Variable cash as % of maximum opportunity Compensation in the form of stock options 2) Compensation in the form of RSUs Other benefits 3) % fixed (of total) 4) Total CEO - Tim Van Hauwermeiren 2024 757,680 15 % – – 795,563 60 % 3,194,813 3,014,500 45,230 10 % 7,807,786 2023 655,787 – % – – 590,215 60 % 8,084,605 5) 2,575,174 39,054 6 % 11,944,835 2022 638,901 10 % – – 766,682 60 % 4,174,684 2,159,689 38,342 9 % 7,778,298 COO - Karen Massey 6) 2024 655,657 37 % – 3,636 573,593 50 % 2,018,973 2,100,610 842,014 24 % 6,194,483 2023 481,471 N/A 338,000 7) 2,921 467,662 50 % 3,939,093 2,296,517 127,393 8 % 7,653,057 CFO - Karl Gubitz 2024 553,000 7 % – 3,636 331,800 40 % 2,018,973 2,100,610 260,571 15 % 5,268,590 2023 516,043 6 % – 3,556 260,866 40 % 2,626,062 1,287,587 62,798 12 % 4,756,913 2022 487,600 79 % – 3,745 243,800 40 % 2,623,633 1,356,048 91,203 12 % 4,806,030 COO - Keith Woods 8) 2023 305,022 (48) % – – – – – – 46,034 100 % 351,056 2022 583,774 5 % – 3,745 583,774 50 % 2,601,982 1,364,014 205,032 15 % 5,342,321 1) The base pay of the CEO is paid in EUR (for 2024 the base pay exchange rate used in this table is 1.0824 EUR/USD ), the base pay of the COO is paid in CHF (for 2024 the base pay exchange rate used in this table is 1.1363 CHF/USD). The base pay of the CFO is paid in USD. The percentage presenting the change in base pay is calculated using the currency of payment. 2) Amounts shown represent the expenses with respect to stock options measured using the Black-Scholes model. For a description of the assumptions used in valuing these awards, see “Note 13 Share-Based Payments” to our Consolidated Financial Statements. 3) Other benefits consists of the lease of a company car, employer-paid medical insurance premiums, pension contributions, social security costs and other allowances. In 2024, employer social security costs were impacted by the increase of share-price at year end against the share-price as of December 31, 2023. 4) Fixed compensation is considered as base pay and other benefits. 5) Based on the approved 2024 equity allocation scheme, the total equity target value for Tim Van Hauwermeiren is equal to $5,080,000 and the total equity target value for Karen Massey and Karl Gubitz is equal to $3,040,000 for each. The CEO, COO and CFO received their respective equity grants at target value converted into a number of stock options and RSUs on the Reference Date of the 30-days average share price of $375.68 per share preceding the Reference Date and the Black-Scholes model fair market value of $138.96 per stock option. This results in the number of stock options and RSUs shown above. The amounts shown above represent the actual value received at the grant date of June 28, 2024 at which date the Company’s share price was equal to $445.76. The difference of $70.08 per share is explained by the stock price increase in the intervening period primarily due to approval of VYVGART HYTRULO for CIDP in the U.S. by the FDA. For more information on the CEO equity grant, please refer to “Determination of target value of CEO equity grant” included in “Equity“ above. The fair market value based on the Black-Scholes model for Tim Van Hauwermeiren is $174.78 and the fair market value for the COO and CFO is $158.50. These amounts do not reflect the actual economic value realized by the beneficiary. Amounts shown represent the expenses with respect to the stock options awards granted in 2024 measured using the Black-Scholes model with unobservable assumptions. The assumptions used in the fair valuation differ between Belgian beneficiary versus non-Belgian beneficiary. For a description of the assumptions used in valuing these awards, see Note 13 Share-Based Payments to our Consolidated Financial Statements. 6) Karen Massey joined as COO in March 2023, and consequently no comparison to 2022 is available. Ms. Massey’s remuneration shows the remuneration paid for the period March 13, 2023 through December 31, 2023. Her 2023 variable pay pay-out has been pro-rated to reflect this as well. The increase year over year for 2024 is not representative as it is comparing to a partial work year. 7) In 2023, the Company paid a sign-on bonus to Karen Massey to allow the Company to make an overall competitive offer of employment and in recognition of lost corporate benefits as a result of early departure at Ms. Massey’s previous employer. Ensuring a competitive offer in this way and securing Ms. Massey as the Company’s new COO was deemed by the Board of Directors to be in the best interest of the Company and its stakeholders. 8) Keith Woods resigned as COO March 2023 and his employment relationship ended on June 30, 2023 and consequently the remuneration numbers show his remuneration for the period January 1, 2023 through June 30, 2023. No equity award or variable pay was paid to Mr. Woods in the year ended December 31, 2023. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 170

Stock option overview Named Executive Officers The table below shows (i) the stock options held as of January 1, 2024, (ii) the stock options granted to the NEOs which vested during the year ended December 31, 2024, (iii) the number of stock options scheduled to vest in the years ending December 31, 2025, December 31, 2026 and December 31, 2027 and (iv) the respective exercise price of such stock options. Each stock option was granted pursuant to the Equity Incentive Plan: Information regarding the reported financial year Opening Balance During the Year Closing balance Name of Directors, Position Specification plan Performance Period Award Date Vesting date End of retention period Exercise Period Exercise price of stock option (€) Stock options held at the beginning of the period Stock options awarded Stock options exercised Stock options forfeited Stock options vested Stock options subjected to a service period Stock options awarded and unvested Stock options held at the end of the year Stock options subjected to a retention period Tim Van Hauwermeiren, CEO Equity incentive plan 21/12/2018 - 01/12/2021 21/12/2018 (1) 31/12/2021 01/01/2022 - 21/12/2028 86.32 80,000 – – – – – – 80,000 – 20/12/2019 - 01/12/2022 20/12/2019 (1) 31/12/2022 01/01/2023 - 20/12/2029 135.75 80,000 – – – – – – 80,000 – 21/12/2020 - 01/12/2023 21/12/2020 (1) 31/12/2023 01/01/2024 - 21/12/2030 247.60 50,000 – – – – – – 50,000 – 24/12/2021 - 01/12/2024 24/12/2021 (1) 31/12/2024 01/01/2025 - 24/12/2031 309.20 25,000 – – – 8,333 – – 25,000 – 23/12/2022 - 01/12/2025 23/12/2022 (1) 31/12/2025 01/01/2026 - 23/12/2032 359.60 25,000 – – – 8,334 8,333 8,333 25,000 25,000 03/07/2023 - 01/07/2026 03/07/2023 (1) 31/12/2026 01/01/2027 - 03/07/2033 355.40 30,000 – – – 14,167 15,833 15,833 30,000 30,000 28/06/2024 - 01/06/2027 28/06/2024 (1) 31/12/2027 01/01/2028 - 28/06/2034 416.40 – 18,279 – – – 18,279 18,279 18,279 18,279 Total 290,000 18,279 – – 30,834 42,445 42,445 308,279 73,279 Karen Massey, COO Equity incentive plan 03/07/2023 - 01/07/2026 03/07/2023 (1) N/A 03/07/2024 - 03/07/2033 355.40 22,500 – – – 10,625 11,875 11,875 22,500 – 28/06/2024 - 01/06/2027 28/06/2024 (1) N/A 28/06/2025 - 28/06/2034 416.40 – 12,738 – – – 12,738 12,738 12,738 – Total 22,500 12,738 – – 10,625 24,613 24,613 35,238 – Karl Gubitz, CFO Equity incentive plan 01/07/2021 - 01/07/2024 01/07/2021 (1) N/A 01/07/2022 - 01/07/2031 255.10 24,000 – – – 4,667 – – 24,000 – 01/07/2022 - 01/07/2025 01/07/2022 (1) N/A 01/07/2023 - 01/07/2032 357.50 16,000 – – – 5,333 3,111 3,111 16,000 – 03/07/2023 - 01/07/2026 07/03/2023 (1) N/A 03/07/2024 - 03/07/2033 355.40 15,000 – – – 7,083 7,917 7,917 15,000 – 28/06/2024 - 01/06/2027 28/06/2024 (1) N/A 28/06/2025 - 28/06/2034 416.40 – 12,738 – – 12,738 12,738 12,738 – Total 55,000 12,738 – – 17,083 23,766 23,766 67,738 – 1) 1/3rd of the stock options vests on the first anniversary of the date of grant and the remaining 2/3rd vest in equal installments (24 in total) over the next two years, each time upon the 1st day of each next month. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 171

RSU overview Named Executive Officers The table below shows (i) the RSUs held as of January 1, 2024, (ii) the RSUs granted to the NEOs which vested during the year ended December 31, 2024 and (iii) the number of RSUs scheduled to vest in the years ending December 31, 2025, December 31, 2026, December 31, 2027 and December 31, 2028. Each RSU was granted pursuant to the Equity Incentive Plan: Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Vesting period Award Date Vesting date End of retention period RSUs held at the beginning of the year RSUs awarded RSU Forfeited RSUs vested RSUs subject to a service condition RSUs awarded and unvested RSUs held at the closing of the year RSUs subject to a retention period Tim van Hauwermeiren, CEO 24/12/2021 - 24/12/2025 24/12/2021 (1) N/A 2,850 – – 1,425 – 1,425 1,425 – 23/12/2022 - 23/12/2026 23/12/2022 (1) N/A 4,275 – – 1,425 – 2,850 2,850 – 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 6,700 – – 1,675 – 5,025 5,025 – 28/06/2024 - 28/06/2028 28/06/2024 (1) N/A – 6,762 – – 6,762 6,762 – Total 13,825 6,762 – 4,525 – 16,062 16,062 – Karen Massey, COO 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 5,025 – – 1,256 – 3,769 3,769 – 28/06/2024 - 28/06/2028 28/06/2024 (1) N/A – 4,712 – – – 4,712 4,712 – Total 5,025 4,712 – 1,256 – 8,481 8,481 – Karl Gubitz, CFO 01/07/2021 - 01/07/2025 01/07/2021 (1) N/A 2,700 – – 1,350 – 1,350 1,350 – 01/07/2022 - 01/07/2026 01/07/2022 (1) N/A 2,700 – – 900 – 1,800 1,800 – 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 3,350 – – 837 – 2,513 2,513 – 28/06/2024 - 28/06/2028 28/06/2024 (1) N/A – 4,712 – – – 4,712 4,712 – Total 8,750 4,712 – 3,087 – 10,375 10,375 – 1) RSUs vest over a period of four years with 1/4th of the total grant vesting at each anniversary of the date of grant. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 172

3.4.12 Summary of other members of the Senior Management Team Stock options overview other members of the Senior Management Team The following table sets forth information regarding stock option and RSU awards granted to members of the Senior Management Team during the year ended December 31, 2024: RSUs granted in 2024 Stock options granted in 2024 Name # RSUs Key terms Value at grant in $ # Stock options Exercise price in € Exercise price in $ Key terms Value at grant in $ 1) Total Peter Ulrichts 4,712 RSUs vest and are settled in 4 equal installments of 25% over a 4 year period. 2,100,421 12,738 416.40 445.76 1/3 vests after year 1 2/3 vest in monthly installments in year 2 and 3. 2,740,705 4,841,126 Malini Moorthy 4,712 2,100,421 12,738 416.40 445.76 2,018,973 4,119,394 Luc Truyen 4,712 2,100,421 12,738 416.40 445.76 2,250,490 4,350,911 Arjen Lemmen 4,712 2,100,421 12,738 416.40 445.76 2,740,705 4,841,126 Andria Wilk 1,331 593,307 3,599 416.40 445.76 774,360 1,367,667 1) These amounts do not reflect the actual economic value realized by the beneficiary. Amounts shown represent the expenses with respect to the Stock options awards granted in 2024 measured using the Black-Scholes model with unobservable assumptions. The assumptions used in the fair valuation differ between Belgian beneficiary versus non-Belgian beneficiary. The fair value of Belgian beneficiary was higher than non-Belgian beneficiary resulting in stock based compensation expense to be higher for Belgian beneficiaries than other beneficiaries. For a description of the assumptions used in valuing these awards, see “Note 13 Share-Based Payments“ to our consolidated financial statements in section “Consolidated Financial Statements”. The table below shows (i) the stock options held as of January 1, 2024, (ii) the stock options granted to members of Senior Management Team (other than the NEOs) which vested during the year ended December 31, 2024, (iii) the number of stock options scheduled to vest in the years ending December 31, 2025, December 31, 2026 and December 31, 2027 and (iv) the respective exercise price of such stock options. Each stock option was granted pursuant to the Equity Incentive Plan: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 173

Peter Ulrichts, CSO Equity incentive plan 20/12/2019 - 01/12/2022 20/12/2019 (1) 31/12/2022 01/01/2023 - 20/12/2029 135.75 5,000 – 1,000 – – – – 4,000 – 21/12/2020 - 01/12/2023 21/12/2020 (1) 31/12/2023 01/01/2024 - 21/12/2030 247.60 9,900 – 2,249 – – – – 7,651 – 24/12/2021 - 01/12/2024 24/12/2021 (1) 31/12/2024 01/01/2025 - 24/12/2026 309.20 3,420 – – – 1,140 – – 3,420 – 23/12/2022 - 01/12/2025 23/12/2022 (1) 31/12/2025 01/01/2026 - 23/12/2027 359.60 16,000 – – – 3,812 3,811 3,811 16,000 16,000 03/07/2023 - 01/07/2026 03/07/2023 (1) 31/12/2026 01/01/2027 - 03/07/2028 355.40 15,000 – – – 7,083 7,917 7,917 15,000 15,000 28/06/2024 - 01/06/2027 28/06/2024 (1) 31/12/2027 01/01/2028 - 28/06/2034 416.40 – 12,738 – – 2,782 9,956 9,956 12,738 12,738 Total 49,320 12,738 3,249 – 14,817 21,684 21,684 58,809 43,738 Malini Moorthy, Legal Counsel Equity incentive plan 01/04/2022 - 01/04/2025 01/04/2022 (1) N/A 01/04/2023 - 01/04/2032 282.50 16,500 – 10,000 – 8,000 2,667 2,667 6,500 – 03/07/2023 - 01/07/2026 03/07/2023 (1) N/A 03/07/2024 - 03/07/2033 355.40 15,000 – – – 7,083 7,917 7,917 15,000 – 28/06/2024 - 01/06/2027 28/06/2024 (1) N/A 01/01/2028 - 28/06/2034 416.40 – 12,738 – – – 12,738 12,738 12,738 – Total 31,500 12,738 10,000 – 15,083 23,322 23,322 34,238 – Luc Truyen, CMO Equity incentive plan 01/10/2021 - 01/10/2024 01/10/2021 (1) 31/12/2024 01/01/2025 - 01/10/2026 259.5 24,000 – – – 6,667 – – 24,000 – 23/12/2022 - 01/12/2025 23/12/2022 (1) 31/12/2025 01/01/2026 - 23/12/2027 359.6 16,000 – – – 5,334 5,333 5,333 16,000 16,000 03/07/2023 - 01/07/2026 03/07/2023 (1) 31/12/2026 01/01/2027 - 03/07/2028 355.4 15,000 – – – 7,083 7,917 7,917 15,000 15,000 28/06/2024 - 01/06/2027 28/06/2024 (1) 31/12/2027 01/01/2028 - 28/06/2034 416.4 – 12,738 – – – 12,738 12,738 12,738 12,738 Total 55,000 12,738 – – 19,084 25,988 25,988 67,738 43,738 Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Specificat ion plan Performance period Award date Vesting date End of retention period Exercise period Exercise price of stock option in € Stock options held at the begin ning of the year Stock options awarded Stock options exercised Stock options forfeited Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 174

Arjen Lemmen, Vice President of Corporate Development & Strategy Equity incentive plan 28/06/2018 - 01/06/2021 28/06/2018 (1) 31/12/2021 01/01/2022 - 28/06/2028 80.82 695 – 695 – – – – – – 21/12/2018 - 01/12/2021 21/12/2018 (1) 31/12/2021 01/01/2022 - 21/12/2028 86.32 15,952 – 15,952 – – – – – – 20/12/2019 - 01/12/2022 20/12/2019 (1) 31/12/2022 01/01/2023 - 20/12/2029 135.75 37,555 – 37,555 – – – – – – 21/12/2020 - 01/12/2023 21/12/2020 (1) 31/12/2023 01/01/2024 - 21/12/2030 247.60 50,000 – 2,326 – – – – 47,674 – 24/12/2021 - 01/12/2024 24/12/2021 (1) 31/12/2024 01/01/2025 - 24/12/2031 309.20 16,000 – – – 5,333 – – 16,000 – 23/12/2022 - 01/12/2025 23/12/2022 (1) N/A 23/12/2023 - 23/12/2032 359.60 16,000 – – – 5,333 5,333 5,333 16,000 – 03/07/2023 - 01/07/2026 03/07/2023 (1) N/A 03/07/2024 - 03/07/2033 355.40 15,000 – – – 7,084 7,917 7,917 15,000 – 28/06/2024 - 01/06/2027 28/06/2024 (1) 31/12/2027 01/01/2028 - 28/06/2034 416.40 – 12,738 – – – 12,738 12,738 12,738 12,738 Total 151,202 12,738 56,528 – 17,750 25,988 25,988 107,412 12,738 Andria Wilk, Global Head of Quality Equity incentive plan 21/12/2020 - 01/12/2023 21/12/2020 (1) 31/12/2023 01/01/2024 - 21/12/2025 247.60 9,900 – 9,813 – – – – 87 – 24/12/2021 - 01/12/2024 24/12/2021 (1) 31/12/2024 01/01/2025 - 24/12/2031 309.20 4,446 – – – 756 – – 4,446 – 23/12/2022 - 01/12/2025 23/12/2022 (1) 31/12/2025 01/01/2026 - 23/12/2027 359.60 4,600 – – – 1,127 1,032 1,032 4,600 4,600 03/07/2023 - 01/07/2026 03/07/2023 (1) 31/12/2026 01/01/2027 - 03/07/2033 355.40 4,600 – – – 1,809 1,915 1,915 4,600 3,830 28/06/2024 - 01/06/2027 28/06/2024 (1) 31/12/2027 01/01/2028 - 28/06/2034 416.40 – 3,599 – – 786 2,813 2,813 3,599 2,813 Total 23,546 3,599 9,813 – 4,478 5,760 5,760 17,332 11,243 1) 1/3rd of the stock options vests on the first anniversary of the date of grant and the remaining 2/3rd vest in equal installments (24 in total) over the next two years, each time upon the 1st day of each next month. Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Specificat ion plan Performance period Award date Vesting date End of retention period Exercise period Exercise price of stock option in € Stock options held at the begin ning of the year Stock options awarded Stock options exercised Stock options forfeited Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 175

RSU overview other members of the Senior Management Team The table below shows (i) the RSUs held as of January 1, 2024, (ii) the RSUs granted to members of Senior Management Team (other than the NEOs) which vested during the year ended December 31, 2024 and (iii) the number of RSUs scheduled to vest in the years ending December 31, 2025, December 31, 2026, December 31, 2027 and December 31, 2028. Each RSU was granted pursuant to the Equity Incentive Plan: Peter Ulrichts, CSO 24/12/2021 - 24/12/2025 24/12/2021 (1) N/A 380 – – 190 – 190 190 23/12/2022 - 23/12/2026 23/12/2022 (1) N/A 2,700 – – 900 – 1,800 1,800 – 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 3,350 – – 837 – 2,513 2,513 – 28/06/2024 - 27/06/2028 28/06/2024 (1) N/A – 4,712 – – – 4,712 4,712 – Total 6,430 4,712 – 1,927 – 9,215 9,215 – Malini Moorthy, General Counsel 01/04/2022 - 01/04/2026 01/04/2022 (1) N/A 4,050 – – 1,350 – 2,700 2,700 – 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 3,350 – – 837 – 2,513 2,513 – 28/06/2024 - 27/06/2028 28/06/2024 (1) N/A – 4,712 – – – 4,712 4,712 – Total 7,400 4,712 – 2,187 – 9,925 9,925 – Luc Truyen, CMO 01/10/2021 - 01/10/2025 01/10/2021 (1) N/A 2,700 – – 1,350 – 1,350 1,350 – 23/12/2022 - 23/12/2026 23/12/2022 (1) N/A 2,700 – – 900 – 1,800 1,800 – 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 3,350 – – 837 – 2,513 2,513 – 28/06/2024 - 27/06/2028 28/06/2024 (1) N/A – 4,712 – – – 4,712 4,712 – Total 8,750 4,712 – 3,087 – 10,375 10,375 – Arjen Lemmen, Vice President of Corporate Development & Strategy 24/12/2021 - 24/12/2025 24/12/2021 (1) N/A 1,800 – – 900 – 900 900 – 23/12/2022 - 23/12/2026 23/12/2022 (1) N/A 2,700 – – 900 – 1,800 1,800 – 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 3,350 – – 837 – 2,513 2,513 – 28/06/2024 - 27/06/2028 28/06/2024 (1) N/A – 4,712 – – – 4,712 4,712 – Total 7,850 4,712 – 2,637 – 9,925 9,925 – Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Vesting period Award date Vesting date End of retention period RSU’s held at the beginning of the year RSUs awarded RSUs forfeited RSUs vested RSUs subject to a service condition RSUs awarded and unvested RSUs held at the closing of the year RSUs subject to a retention period argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 176

Andria Wilk, Global Head of Quality 24/12/2021 - 24/12/2025 24/12/2021 (1) N/A 494 – – 247 – 247 247 – 23/12/2022 - 23/12/2026 23/12/2022 (1) N/A 750 – – 250 – 500 500 – 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 1,000 – – 250 – 750 750 – 28/06/2024 - 27/06/2028 28/06/2024 (1) N/A – 1,331 – – – 1,331 1,331 – Total 2,244 1,331 – 747 – 2,828 2,828 – 1) RSUs vest over a period of four years with 1/4th of the total grant vesting at each anniversary of the date of grant. Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Vesting period Award date Vesting date End of retention period RSU’s held at the beginning of the year RSUs awarded RSUs forfeited RSUs vested RSUs subject to a service condition RSUs awarded and unvested RSUs held at the closing of the year RSUs subject to a retention period argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 177

3.4.13 Summary of Non-Executive Director Equity compensation Stock Option overview Non-Executive Directors The table below shows (i) the stock options held at January 1, 2024, (ii) the stock options granted to the Non- Executive Directors which have vested during the year ended December 31, 2024, (iii) the number of stock options scheduled to vest in the years ending December 31, 2025 December 31, 2026 December 31, 2027and (iv) the respective exercise price of such stock options. No stock options were granted in 2024 to Non- Executive Directors and consequently Dr. Brian Kotzin does not hold any stock options and is as such not included in the below table. Mr. Peter Verhaeghe 18/12/2014 - 18/12/2017 18/12/2014 (1) 31/12/2017 01/01/2018 - 18/12/2024 7.17 2,000 – 2,000 – – – – – 18/06/2016 - 18/06/2019 18/06/2016 (1) 31/12/2019 01/01/2020 - 18/06/2026 11.38 10,000 – 6,000 – – – 4,000 – 21/12/2018 - 21/12/2021 21/12/2018 (1) 31/12/2021 01/01/2022 - 21/12/2028 86.32 10,000 – – – – – 10,000 – 20/12/2019 - 20/12/2022 20/12/2019 (1) 31/12/2022 01/01/2023 - 20/12/2029 135.75 10,000 – – – – – 10,000 – 21/12/2020 - 21/12/2023 21/12/2020 (1) 31/12/2023 01/01/2024 - 21/12/2030 247.60 10,000 – – – – – 10,000 – 24/12/2021 - 24/12/2024 24/12/2021 (2) 31/12/2024 01/01/2025 - 24/12/2031 309.20 2,700 – – 2,700 – – 2,700 – 23/12/2022 - 23/12/2025 23/12/2022 (2) 31/12/2025 01/01/2026 - 23/12/2032 359.60 2,700 – – – – 2,700 2,700 2,700 03/07/2023 - 03/07/2026 03/07/2023 (2) 31/12/2026 01/01/2027 - 03/07/2033 355.40 1,600 – – – – 1,600 1,600 1,600 Total 49,000 – 8,000 2,700 – 4,300 41,000 4,300 Mr. Steve Krognes 03/04/2023 - 03/04/2026 03/04/2023 (2) 31/12/2026 03/04/2024 - 03/04/2033 340.70 2,400 – 2,400 2,400 2,400 Total 2,400 – – – – 2,400 2,400 2,400 Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors Performance period Award date Vesting date End of retention period Exercise period Grant price in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 178

Dr. Pamela Klein 21/12/2018 - 21/12/2021 21/12/2018 (1) N/A 21/12/2019 - 21/12/2028 86.32 1,500 – 1,500 – – – – – 20/12/2019 - 20/12/2022 20/12/2019 (1) N/A 20/12/2020 - 20/12/2029 135.75 10,000 – 2,500 – – – 7,500 – 21/12/2020 - 21/12/2023 21/12/2020 (1) N/A 21/12/2021 - 21/12/2030 247.60 10,000 – – – – – 10,000 – 24/12/2021 - 24/12/2024 24/12/2021 (2) 31/12/2024 24/12/2022 - 24/12/2031 309.20 2,700 – – 2,700 – – 2,700 – 23/12/2022 - 23/12/2025 23/12/2022 (2) 31/12/2025 23/12/2023 - 23/12/2032 359.60 2,700 – – – – 2,700 2,700 2,700 03/07/2023 - 03/07/2026 03/07/2023 (2) 31/12/2026 03/07/2024 - 03/07/2033 355.40 1,600 – – – – 1,600 1,600 1,600 Total 28,500 – 4,000 2,700 – 4,300 24,500 4,300 Dr. Donald deBethizy 18/06/2016 - 18/06/2019 18/06/2016 (1) N/A 18/06/2017 - 18/06/2026 11.38 10,000 – – – – – 10,000 – 21/12/2018 - 21/12/2021 21/12/2018 (1) N/A 21/12/2019 - 21/12/2028 86.32 10,000 – – – – – 10,000 – 20/12/2019 - 20/12/2022 20/12/2019 (1) N/A 20/12/2020 - 20/12/2029 135.75 10,000 – – – – – 10,000 – 21/12/2020 - 21/12/2023 21/12/2020 (1) N/A 21/12/2021 - 21/12/2030 247.60 10,000 – – – – – 10,000 – 24/12/2021 - 24/12/2024 24/12/2021 (2) 31/12/2024 24/12/2022 - 24/12/2031 309.20 2,700 – – 2,700 – – 2,700 – 23/12/2022 - 23/12/2025 23/12/2022 (2) 31/12/2025 23/12/2023 - 23/12/2032 359.60 2,700 – – – – 2,700 2,700 2,700 03/07/2023 - 03/07/2026 03/07/2023 (2) 31/12/2026 03/07/2024 - 03/07/2033 355.40 1,600 – – – – 1,600 1,600 1,600 Total 47,000 – – 2,700 – 4,300 47,000 4,300 Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors Performance period Award date Vesting date End of retention period Exercise period Grant price in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 179

Anthony Rosenberg 13/12/2016 - 13/12/2019 13/12/2016 (1) N/A 13/12/2017 - 13/12/2026 14.13 15,000 – 7,200 – – – 7,800 – 21/12/2018 - 21/12/2021 21/12/2018 (1) N/A 21/12/2019 - 21/12/2028 86.32 10,000 – – – – – 10,000 – 20/12/2019 - 20/12/2022 20/12/2019 (1) N/A 20/12/2020 - 20/12/2029 135.75 8,840 – – – – – 8,840 – 21/12/2020 - 21/12/2023 21/12/2020 (1) N/A 21/12/2021 - 21/12/2030 247.60 3,640 – – – – – 3,640 – 24/12/2021 - 24/12/2024 24/12/2021 (2) 12/31/2024 24/12/2022 - 24/12/2031 309.20 2,700 – – 2,700 – – 2,700 – 23/12/2022 - 23/12/2025 23/12/2022 (2) 12/31/2025 23/12/2023 - 23/12/2032 359.60 2,700 – – – – 2,700 2,700 2,700 03/07/2023 - 03/07/2026 03/07/2023 (2) 12/31/2026 03/07/2024 - 03/07/2033 355.40 1,600 – – – – 1,600 1,600 1,600 Total 44,480 – 7,200 2,700 – 4,300 37,280 4,300 James Daly 21/12/2020 - 21/12/2023 21/12/2020 (1) N/A 21/12/2021 - 21/12/2030 247.60 10,000 – – – – – 10,000 – 24/12/2021 - 24/12/2024 24/12/2021 (2) 12/31/2024 24/12/2022 - 24/12/2031 309.20 2,700 – – 2,700 – – 2,700 – 23/12/2022 - 23/12/2025 23/12/2022 (2) 12/31/2025 23/12/2023 - 23/12/2032 359.60 2,700 – – – – 2,700 2,700 2,700 03/07/2023 - 03/07/2026 03/07/2023 (2) 12/31/2026 03/07/2024 - 03/07/2033 355.40 1,600 – – – – 1,600 1,600 1,600 Total 17,000 – – 2,700 – 4,300 17,000 4,300 Camilla Sylvest 03/10/2022 - 03/10/2025 03/10/2022 (2) 12/31/2025 03/10/2023 - 03/10/2032 368.50 4,050 – – – – 4,050 4,050 4,050 03/07/2023 - 03/07/2026 03/07/2023 (2) 12/31/2026 03/07/2024 - 03/07/2033 355.40 1,200 – – – – 1,200 1,200 1,200 Total 5,250 – – – – 5,250 5,250 5,250 Dr. Ana Cespedes 23/12/2022 - 23/12/2025 23/12/2022 (2) 12/31/2025 23/12/2023 - 23/12/2032 359.60 4,050 – – – – 4,050 4,050 4,050 03/07/2023 - 03/07/2026 03/07/2023 (2) 12/31/2026 03/07/2024 - 03/07/2033 355.40 800 – – – – 800 800 800 Total 4,850 – – – – 4,850 4,850 4,850 1) 1/3rd of the stock options vests on the first anniversary of the date of grant and the remaining 2/3rd vest in equal monthly installments (24 in total) over the next two years, each time upon the 1st day of each next month. 2) Stock options vest upon third anniversary of the grant. Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors Performance period Award date Vesting date End of retention period Exercise period Grant price in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 180

RSU overview Non-Executive Directors The table below shows (i) the RSUs held at January 1, 2024, (ii) the RSUs granted to the Non-Executive Directors which have vested during the year ended December 31, 2024 and (iii) RSUs scheduled to vest in the years ending December 31, 2025, December 31, 2026 December 31, 2027 December 31, 2028(in number of RSUs). RSUs granted to Non-Executive Directors before 2024 vest over a period of four years with 1/4th of the total grant vesting at each anniversary of the grant date. RSUs granted to Non-Executive Directors in 2024 will all vest on the 1st anniversary of the grant date in 2025 and are subject to a holding period of 3 years. Mr. Peter Verhaeghe 24/12/2021 - 24/12/2025 24/12/2021 (1) N/A 300 – 150 – 150 150 23/12/2022 - 23/12/2026 23/12/2022 (1) N/A 450 – 150 – 300 300 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 350 – 87 – 263 263 28/06/2024 - 28/06/2025 28/06/2024 (1) 28/06/2028 – 1065 – – 1,065 1,065 Total 1,100 1,065 387 – 1,778 1,778 Mr. Steve Krognes 03/04/2023 - 03/04/2027 03/04/2023 (1) N/A 525 – – – 394 394 28/06/2024 - 28/06/2025 28/06/2024 (1) 28/06/2028 – 1065 – – 1,065 1,065 Total 525 1,065 131 – 1,459 1,459 Dr. Pamela Klein 24/12/2021 - 24/12/2025 24/12/2021 (1) N/A 300 – 150 – 150 150 23/12/2022 - 23/12/2026 23/12/2022 (1) N/A 450 – 150 – 300 300 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 350 – 87 – 263 263 28/06/2024 - 28/06/2025 28/06/2024 (1) 28/06/2028 – 1065 – – 1,065 1,065 Total 1,100 1,065 387 – 1,778 1,778 Dr. Donald deBethizy 24/12/2021 - 24/12/2025 24/12/2021 (1) N/A 300 – 150 – 150 150 23/12/2022 - 23/12/2026 23/12/2022 (1) N/A 450 – 150 – 300 300 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 350 – 87 – 263 263 28/06/2024 - 28/06/2025 28/06/2024 (1) 28/06/2028 – 1065 – – 1,065 1,065 Total 1,100 1,065 387 – 1,778 1,778 Anthony Rosenberg 24/12/2021 - 24/12/2025 24/12/2021 (1) N/A 300 – 150 – 150 150 23/12/2022 - 23/12/2026 23/12/2022 (1) N/A 450 – 150 – 300 300 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 350 – 87 – 263 263 28/06/2024 - 28/06/2025 28/06/2024 (1) 28/06/2028 – 1065 – – 1,065 1,065 Total 1,100 1,065 387 – 1,778 1,778 James Daly 24/12/2021 - 24/12/2025 24/12/2021 (1) N/A 300 – 150 – 150 150 23/12/2022 - 23/12/2026 23/12/2022 (1) N/A 450 – 150 – 300 300 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 350 – 87 – 263 263 28/06/2024 - 28/06/2025 28/06/2024 (1) 28/06/2028 – 1065 – – 1,065 1,065 Total 1,100 1,065 387 – 1,778 1,778 Information regarding the reported financial year Opening balance During the Year Closing balance Name of member of Board of Directors Vesting period Award date Vesting date End of holding period RSUs held at the beginning of the year RSUs awarded RSUs vested RSUs subject to a service condition RSUs awarded and unvested RSUs held at the closing of the year argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 181

Camilla Sylvest 03/10/2022 - 03/10/2026 03/10/2022 (1) N/A 675 – 225 – 450 450 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 263 – 66 – 197 197 28/06/2024 - 28/06/2025 28/06/2024 (1) 28/06/2028 – 1065 – – 1,065 1,065 Total 938 1,065 291 – 1,712 1,712 Dr. Ana Cespedes 23/12/2022 - 23/12/2026 23/12/2022 (1) N/A 675 – 225 – 450 450 03/07/2023 - 03/07/2027 03/07/2023 (1) N/A 175 – 44 – 131 131 28/06/2024 - 28/06/2025 28/06/2024 (1) 28/06/2028 – 1065 – – 1,065 1,065 Total 850 1,065 269 – 1,646 1,646 Dr. Brian Kotzin 28/06/2024 - 28/06/2028 28/06/2024 (1) 28/06/2028 – 1,598 – – 1,598 1,598 Total – 1,598 – – 1,598 1,598 1) RSUs granted before 2024 vest over a period of four years with 1/4th of the total grant vesting at each anniversary of the date of grant. RSUs granted to Non-Executive Directors in 2024 will all vest on the 1st anniversary of the grant date in 2025 and are subject to a holding period of 3 years. Information regarding the reported financial year Opening balance During the Year Closing balance Name of member of Board of Directors Vesting period Award date Vesting date End of holding period RSUs held at the beginning of the year RSUs awarded RSUs vested RSUs subject to a service condition RSUs awarded and unvested RSUs held at the closing of the year argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Remuneration Report and Compensation Statement 182

3.5 Corporate Governance – Nasdaq Listing Rules As a foreign private issuer, the Nasdaq Listing Rules include certain accommodations in the corporate governance requirements that allow foreign private issuers to follow “home country” corporate governance practices in lieu of the otherwise applicable Nasdaq corporate governance standards. We intend to rely on certain exemptions for foreign private issuers and to follow Dutch corporate governance practices in lieu of the Nasdaq corporate governance rules. The following is a summary of the significant ways in which our corporate governance practices differ from those required by the Nasdaq Listing Rules with which we are not required to comply: • Quorum at Shareholder Meetings. In accordance with Dutch law and generally accepted business practices in the Netherlands, our Articles of Association do not provide quorum requirements generally applicable to general meetings of shareholders. To that extent, our practice varies from the requirement of Nasdaq Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock. • Solicitation of Proxies. Although we must provide shareholders with an agenda and other relevant documents ahead of any General Meeting, Dutch law does not have a regulatory regime for the solicitation of proxies, and the solicitation of proxies is not a generally accepted business practice in the Netherlands. Thus, our practice varies from the requirement of Nasdaq Listing Rule 5620(b). • Shareholder Approval. We follow certain Dutch shareholder approval requirements for the issuance of securities in connection with certain events such as the acquisition of stock or assets of another company, the establishment of or amendments to equity-based compensation plans for employees, a change of control of us and certain private placements. To this extent, our practice varies from the requirements of Nasdaq Rule 5635, which generally requires an issuer to obtain shareholder approval for the issuance of securities in connection with such events. • Distribution of Annual Reports. We do not follow Nasdaq Listing Rule 5250(d), which requires companies to make available copies of their annual reports containing audited financial statements to their shareholders. The distribution of our annual reports to shareholders is not required under Dutch corporate law or Dutch securities laws. Furthermore, it is generally accepted business practice for Dutch companies not to distribute annual reports. In part, this is because the Dutch system of bearer shares has made it impractical to keep a current list of holders of the bearer shares in order to distribute the annual reports. Instead, we make our Annual Report available at our corporate head office in the Netherlands (and at the offices of our Dutch listing agent as stated in the convening notice for the meeting) no later than 42 days prior to convocation of any annual General Meeting. In addition, we post a copy of our annual reports on our website prior to our annual General Meeting. 3.6 Share Ownership For information regarding the share ownership of our directors and members of our executive committee, please refer to Section “Remuneration Report and Compensation Statement” and Section “Share Classes and Principal Shareholders”, Subsection “Major Shareholders”. 3.7 Insider Trading We have an insider trading policy in place that complies with MAR. The insider trading policy is intended to maintain confidentiality of inside information (as defined under MAR), refrain from market manipulation and comply with the obligations of argenx under MAR, the Exchange Act and other applicable securities laws. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Corporate Governance – Nasdaq Listing Rules 183

3.8 Cybersecurity 3.8.1 Information Security Risk Management and Strategy Our approach to risk management is designed to identify, assess, prioritize and manage major risk exposures that could affect our ability to execute our corporate strategy and fulfill our business objectives. As part of our information security and privacy program, the Information Security and Management System (the ISMS), we perform risk assessments in which we map and prioritize information security risks identified through the processes described below, including risks associated with our use of third-party service providers. These assessments inform our ISMS strategies and oversight processes and are included with other enterprise risks as part of our broader enterprise risk management. We view information security risks as one of the key risks categories we face. IT system vendors are subject to security review and audits. For more information regarding the cybersecurity-related risks we face, please refer to Section 2.7.4 “Our business and operations could suffer in the event of system failures or unauthorized or inappropriate use of or access to our systems”. Our processes for assessing, identifying and managing information security risks and vulnerabilities are embedded across our business as part of our ISMS. Among other things, we conduct audits and tests of our information systems (including review and assessment by independent third-party advisors, who assess and report on the maturity of our security measures and help identify areas for continued focus and improvement) and review information security threat information published by government entities and other organizations in which we participate. We conduct training on data security matters for our employees to be aware and vigilant against potential data security risks and data privacy is incorporated into our overall compliance training, such as through privacy-specific training for employees and contractors. Phishing training is also implemented regularly, which includes mock phishing emails to test employee vigilance. In addition, employees are required to read and acknowledge information security policies that are relevant to their specific role. We also have implemented and maintain information security incident response plans, which include processes to triage, assess severity for, escalate, contain, investigate and remediate information security incidents, as well as to comply with potentially applicable legal obligations and mitigate brand and reputational damage. 3.8.2 Information Security Governance and Oversight Our ISMS enables our Board of Directors to establish a mutual understanding with our Senior Management Team of the effectiveness of our information security risk management practices and capabilities, including the division of responsibilities for reviewing our information security risk exposure and risk tolerance, tracking emerging information risks and ensuring proper escalation of certain key risks for periodic review by the Board of Directors and its committees. As part of its broader risk oversight activities, the Board of Directors oversees risks from information security threats, both directly and through the Audit and Compliance Committee . The Audit and Compliance Committee also oversees our internal control over financial reporting. As an element of its cybersecurity oversight activities, the Audit and Compliance Committee regularly reviews the results of our enterprise risk assessments, including information security risk assessments, as well as management's strategies to detect, monitor and manage such risks and related risk assessment and risk management policies. Our ISMS contains provisions regarding reporting to the Global Risk Management Committee. Additionally, the data protection officer provides regular updates to the Senior Management Team, and the Audit and Compliance Committee as a component of the Audit and Compliance Committee’s compliance updates. The data protection officer also regularly reports to the Global Corporate Compliance Committee, the Global Risk Management Committee and the General Counsel on matters such as the status of the organizational privacy plan, data breaches and routine programs. In addition to these regularly scheduled updates from the data protection officer, the Global Head of Business Information Systems reports to the Audit and Compliance Committee or the full Board of Directors, as appropriate, on how certain information security risks are being managed and progress towards agreed mitigation goals, as well as any potential material risks from cybersecurity threats that have been detected by the information security team. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Cybersecurity 184

Our information security team is responsible for day-to-day identification, assessment and management of the information security risks we face. Our Global Head of Business Information Systems has 33 years of experience in information management systems and the managers reporting to the Global Head of Business Information Systems have over 40 cumulative years of experience in information security. Our incident response and data breach procedures are designed for the timely detection, reporting, and investigation of all security incidents, as well as the timely notification of any reportable breaches (including any material cybersecurity incidents and personal data breaches) to the competent authorities and the timely communication to the affected individuals, where relevant. We maintain records of breaches on our quarterly corporate risk dashboard and our personal data breach register, and we monitor and regularly report our security and data breach metrics to the Senior Management Team, including the Audit and Compliance Committee , the global corporate compliance committee, and the global risk management committee. In addition to the ordinary-course Board of Directors and Audit and Compliance Committee reporting and oversight described above, we also maintain disclosure controls and procedures designed for prompt reporting to the Board of Directors and timely public disclosure, as appropriate, of material events covered by our risk management framework, including information security risks. 3.9 Risk Appetite & Control Before reading this section, please carefully review the following cautionary statement: In this section we will make the required disclosures regarding our risk appetite and mitigating actions. We fully take the risk mitigation actions and risk management described in this section into account while preparing the description of the main risks and uncertainties we face, as set out in Section “Risk Factors”. Any mitigating language used in this section does not have any impact on the risks and uncertainties we face or their potential adverse effects as they are described in Section “Risk Factors”. Section “Risk Factors” describes the main risks and uncertainties we face already fully having taken into account our risk management and the risk mitigating actions described herein. 3.9.1 Introduction This Section 3 provides a general description of our willingness to mitigate the risks and uncertainties we face (also called our ‘risk appetite’), and to give a description of the mitigating actions we have taken with regard to our most relevant risks. 3.9.2 General Description of Our Risk Appetite Our risk appetite serves as a guideline to determine the measures we may take in mitigating some of the risks and uncertainties we face. Our risk appetite is aligned with our strategy and priorities. The business we operate in is inherently high-risk. In general, we are willing, and in our view required, to take significant risks to be able to operate successfully in our line of business. Some of the risks and uncertainties we face are entirely outside of our control whereas others may be influenced or mitigated. The process of developing, implementing and improving risk management procedures remains an ongoing effort. In accordance with guideline 400.110c of the Dutch Counsel for Annual Reporting (Raad voor de Jaarverslaggeving), this risk management section provides an overview of the risk mitigating actions taken or planned to be taken by us. The mentioning of these mitigating actions may not in any way be viewed as an implied or express guarantee that such mitigation will in practice be effective in limiting the risk exposure and/or the potential damage to us from any such risk materializing. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Cybersecurity 185

3.9.3 Controlling Actions We Take with Regard to Our Most Relevant Risks and Uncertainties The following is a description of the main risks and uncertainties we face (being the first risk of each category of risk factors set out in Section “Risk Factors”) and a description of the measures we took to control them. A description of the expected impact upon materialization of these risks is included for each risk in Section “Risk Factors”. RISK FACTOR MEASURES TAKEN TO CONTROL THESE RISKS The commercial success of our products and product candidates, including in new indications or methods of administration, will depend on the degree of market acceptance. We plan to focus on the successful commercialization of the products and product candidates after they are launched. We aim to expand our sales and marketing organization, enter into collaboration arrangements with third parties, outsource certain functions to third parties, or use some combination of each to promote market acceptance of our products and product candidates. We have already built, and continue to expand, our sales forces in certain of the countries where VYVGART is approved and plan to further develop our sales and marketing capabilities to promote our products, and product candidates, including new indications or methods of administration. Failure to successfully identify, select and develop our products in other indications, or additional products or product candidates could impair our ability to grow. We remain committed to using technology and contracting with parties that are able to achieve the level of sophistication we need to accurately and reliably identify, select and develop efgartigimod in other indications, additional products or product candidates. We expect our spending to continue to increase as we continue to expand drug inventory for our products and the progress of our clinical-stage pipeline, including ongoing clinical trials for several indications with efgartigimod, empasiprubart and ARGX-119 and expand our global commercial infrastructure. We rely, and expect to continue to rely, on third parties to conduct some of our research activities and clinical trials and for parts of the development and commercialization of our existing and future research programs, products and product candidates. If our relationships with such third parties are not successful, our business may be adversely affected. We endeavor to meet our contractual obligations and any relevant milestone achievements under our collaboration contracts, maintain a rich pipeline of possible collaboration partners as well as foster good relationships with existing and potential future collaboration partners in order to limit reliance on a limited number of collaboration partners. Furthermore, third-party contractor selection and management is subject to our quality management system. Customary contractual agreements are put in place in an effort to protect us from under-performance. We are typically spreading operational risks over various service providers. Project management belongs to our core internal competences. Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, or insider trading violations, which could significantly harm our business. We have adopted a Code of Conduct, that is applicable to all of our employees and directors, which addresses the key risks related to potential breaches of ethical standards. All employees have accepted and are trained (and retrained annually) on our Code of Conduct. We expect all newcomers to accept, and commit to, the contents of the Code of Conduct. To increase compliance and ensure our colleagues know where to go with questions on the Code of Conduct and its application, we have established the argenx COMPASS Helpline, where our employees can raise any concerns they may have regarding potential violations of our policy confidentially or anonymously (to the extent allowed by law). argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Appetite & Control 186

We may be unable to adequately maintain, enforce or protect our intellectual property rights in products, product candidates and platform technologies which could adversely affect our ability to maximize the value for patients in our marketed products and product candidates. We strive to protect the proprietary technologies that we believe are important to our business, including pursuing and maintaining patent protection intended to cover the platform technologies incorporated into, or used to produce, our product candidates, the compositions of matter of our product candidates and their methods of use, as well as other inventions that are important to our business. In addition to patent protection, we also rely on trademarks and trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection, including certain aspects of our llama immunization and antibody affinity maturation approaches. Our future growth and ability to compete depends on maintaining our culture, retaining our key personnel and recruiting additional qualified personnel. We offer competitive remuneration packages and share- based incentives in the form of the Equity Incentive Plan. We perform periodic benchmark analyses with an external service provider to ensure the competitiveness of the compensation offered to our key personnel in comparison to other (reference group) companies. We pay close attention to creating an environment that supports the further development of the talents of our key people. Our corporate culture, including fostering our key values, has been, and will be, a key contributor to our success. We therefore continue to build and promote internal programs focusing on our corporate culture and our five key values. 3.9.4 Material Impact of Risk Materialization in 2024 During the period between January 1, 2024 and December 31, 2024, we did not identify any material impact as a result of materialization of previously identified risks and uncertainties. 3.9.5 Financial Risks and Controls In running our business, we seek to implement a sustainable policy regarding internal control and risk management. Our Board of Directors has delegated an active role to our Audit and Compliance Committee in the design, implementation and monitoring of an internal risk management and control system to manage the significant risks to which we are exposed. Our financial reporting is structured within a tight framework of budgeting, reporting and forecasting. A distinction is made between reports for internal and external use. External reporting at group level consists of an annual report (in the form of this Annual Report), including financial statements audited by the independent auditor, as well semi-annual reporting and quarterly updates, containing summarized financial information. The external reports are based on the internal financial reporting. Internal financial reporting consists of extensive consolidated monthly reports in which current developments are compared to the monthly (cumulative) budgets and previous forecasts. In addition, each quarter we reiterate or update our forecast for the annual results, including the cash flow position at the end of the year. The quarterly budgets are part of the annual group budget, which is prepared every year by our Senior Management Team and approved by our Board of Directors. Our specialized finance and administration department are primarily responsible for evaluating the draft internal and external reporting, before these are finally approved by our Board of Directors. Our Board of Directors discusses the financial results of the group at all formal board meetings, which meetings are minuted. Our internal controls over financial reporting are a subset of internal controls and include policies and procedures that: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Appetite & Control 187

• pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; • provide reasonable assurance that transactions are recorded as necessary to permit preparation of our financial statements in accordance with IFRS as issued by the International Accounting Standards Board and as adopted by the EU, and that receipts and expenditures are being made only by authorized persons; and • provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. Since we have securities registered with the SEC and are a large accelerated filer within the meaning of Rule 12b-2 of the Exchange Act, we need to assess the effectiveness of our internal controls over financial reporting and provide a report on the results of our assessment. Our Board of Directors reviewed its internal controls over financial reporting based on criteria established in the Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and engaged an external advisor to help assess the effectiveness of its controls. 3.9.6 Recent or Current Developments in our System of Risk Management We pay attention to pro-active risk management by continuing to have the evaluation of our core risks and uncertainties as a standing discussion topic for our Board of Directors. In addition, in 2024, we have added quarterly updates for specific risks to our Board of Directors agendas, including cyber security, privacy and healthcare compliance risks. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Risk Appetite & Control 188

General Description of the Company and its Share Capital 4.1 Legal Information on the Company 190 4.2 Share Capital 190 4.3 Share Classes and Principal Shareholders 194 4.4 Limitations on the right to hold securities 196 4.5 General Meeting, Voting Rights and Admission 197 4.6 Anti-Takeover Provisions 199 4.7 Change of Control 199 4.8 Exchange Controls 199 4.9 Amendments of Articles of Association 199 4.10 Transparency Directive 199 4.11 Dutch Financial Reporting Supervision Act 200 4.12 Dividends and Other Distributions 200 4.13 Right to a surplus in the event of a liquidation 201 4.14 Material Modifications to the Rights of Security Holders and Use of Proceeds 201 4.15 Enforcement of civil liabilities 202 4.16 Controls and Procedures 203 4.17 Financial Calendar 2025 204 argenx Annual Report 2024 189 4

4 General Description of the Company and its Share Capital 4.1 Legal Information on the Company 4.1.1 General We were incorporated on April 25, 2008 in the Netherlands and under Dutch law. Our commercial name is ‘argenx’ and since April 26, 2017, our corporate name is ‘argenx SE’. We are a European public company (Societas Europaea or SE), with our corporate seat in Amsterdam, the Netherlands, are registered with the trade register of the Dutch Chamber of Commerce under number 24435214. Our registered office is at Laarderhoogtweg 25, 1101 EB Amsterdam, the Netherlands and our telephone number is +31 (0) 10 70 38 441. Our website address is http://www.argenx.com. Our LEI is 7245009C5FZE6G9ODQ71. Our ordinary shares are listed on Euronext Brussels under ISIN NL0010832176 under the symbol “ARGX” since July 10, 2014. The ADSs are listed on Nasdaq, under the symbol “ARGX” since May 18, 2017. 4.1.2 Statutory/Corporate Objectives Pursuant to Article 3 of our Articles of Association, our corporate objectives are, amongst others: (a) to exploit, including all activities relating to research, development, production, marketing and commercial exploitation; biological, chemical or other products, processes and technologies in the life sciences sector in general, and more specifically in the diagnostic, pharmaceutical, medical, cosmetic, chemical and agricultural sector; (b) to design and develop instruments which may be used in medical diagnosis and affiliated areas; (c) the worldwide distribution of, sale of and rendering services relating to our products and subsidiaries directly to customers as well as through third parties; and (d) to act as the holding company of the Group. 4.2 Share Capital 4.2.1 Authorized and Issued Share Capital Under Dutch Law, a company’s authorized share capital sets out the maximum amount and number of shares that it may issue without amending its articles of association. Our Articles of Association provide for an authorized share capital in the amount of €9.0 million divided into 90 million shares, each with a nominal value of €0.10. All issued and outstanding shares have been fully paid up and the shares are held in dematerialized form. As of December 31, 2024 our issued and paid up share capital amounted to €6,076,096 ($7,226,856), represented by 60,760,957 ordinary shares with a nominal value of €0.10, each representing an identical fraction of our share capital. As of December 31, 2024, neither we nor any of our subsidiaries held any of our own shares. During the year ended December 31, 2024 and as of the date of this Annual Report, we did not purchase any shares in the Company. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Legal Information on the Company 190

4.2.2 Stock Options and Restricted Stock Units In addition to the shares already outstanding, we have granted stock options which upon exercise will lead to an increase in the number of our outstanding shares. 42,243 stock options were granted on April 1, 2024, 660,166 on June 28, 2024, 33,529 on September 30, 2024 and 20,296 on December 31, 2024. A total of 4,300,760 stock options (where each stock option entitles the holder to subscribe for one new ordinary share) were outstanding and granted as of December 31, 2024. Upon exercise of these 4,300,760 stock options, we will receive a total amount of €1.2 billion ($1.2 billion) in stock option exercise price, thereby increasing our share capital and share premium by the same amount. Further, we have granted RSUs which upon vesting will lead to an increase in the number of our outstanding shares. 29,747 RSUs were granted on April 1, 2024, 282,253 on June 28, 2024, 21,726 on September 30, 2024 and 14,960 on December 31, 2024. A total of 615,360 RSUs (where the holder receives an equal number of new ordinary shares, minus a certain number of shares required to cover certain costs, if applicable) were outstanding and granted as of December 31, 2024. Apart from the stock options and RSUs granted under our Equity Incentive Plan, we do not currently have other stock options, RSUs, options to purchase securities, convertible securities or other rights to subscribe for or purchase securities outstanding. For stock option information through December 31, 2024, see ”Note 13 Share-Based Payments” in our consolidated financial statements which are appended to our Annual Report for the year ended December 31, 2024”. 4.2.3 American Depositary Shares In connection with our initial public offering on Nasdaq, the Bank of New York Mellon, as depositary, registered and delivered ADSs. Each ADS represents one share (or a right to receive one share) deposited with ING Bank N.V., as custodian for the depositary in the Netherlands. Each ADS also represents any other securities, cash or other property which may be held by the depositary. The deposited shares together with our other securities, cash and other property held by the depositary, are referred to as the deposited securities. The depositary’s office at which the ADSs are administered is located at 101 Barclay Street, New York, New York 10286. The Bank of New York Mellon’s principal executive office is located at 225 Liberty Street, New York, New York 10286. A deposit agreement among us, the depositary, ADS holders and all other persons indirectly or beneficially holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Share Capital 191

4.2.4 Fees and Charges Persons depositing or withdrawing shares or ADS holders must pay: For: $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates $.05 (or less) per ADS Any cash distribution to ADS holders A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs Distribution of securities distributed to holders of deposited securities (including rights) that are distributed by the depositary to ADS holders $.05 (or less) per ADS per calendar year Depositary services Registration or transfer fees Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares Expenses of the depositary Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement) Converting foreign currency to USDs Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes As necessary Any charges incurred by the depositary or its agents for servicing the deposited securities As necessary The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may collect any of its fees by deduction from any cash distribution payable (or by selling a portion of securities or other property distributable) to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid. From time to time, the depositary may make payments to us to reimburse us for costs and expenses generally arising out of establishment and maintenance of the ADS program, waive fees and expenses for services provided to us by the depositary or share revenue from the fees collected from ADS holders. In performing its duties under the deposit agreement, the depositary may use brokers, dealers, foreign currency dealers or other service providers that are owned by or affiliated with the depositary and that may earn or share fees, spreads or commissions. The depositary may convert currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and not as agent, advisor, broker or fiduciary on behalf of any other person and earns revenue, including, without limitation, transaction spreads, that it will retain for its own account. The revenue is based on, among other things, the difference between the exchange rate assigned to the currency conversion made under the deposit agreement and the rate that the depositary or its affiliate receives when buying or selling foreign currency for its own account. The depositary makes no representation that the exchange rate used or obtained in any currency conversion under the deposit agreement will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable to ADS holders, subject to the depositary’s obligations under the deposit agreement. The methodology used to determine exchange rates used in currency conversions is available upon request. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Share Capital 192

New shares created during 2024 As a result of the exercise of stock options and vesting of RSUs under our Equity Incentive Plan, 1,566,469 new shares were created in 2024. The following table shows the developments in our share capital for the year ended December 31, 2024 and on February 19, 2025: Number of shares outstanding on December 31, 2022 55,395,856 Number of shares outstanding on December 31, 2023 59,194,488 Exercise of stock options 1,478,225 Vesting of RSUs 88,244 Number of shares outstanding on December 31, 2024 60,760,957 Exercise of stock options in January 2025 223,971 Exercise of stock options in February 2025 5,929 Number of shares outstanding on February 19, 2025 60,990,857 4.2.5 Issue of Shares The Articles of Association provide that shares may be issued or rights to subscribe for our shares may be granted pursuant to a resolution of the General Meeting, or alternatively, by our Board of Directors if so designated by the General Meeting. If the Board of Directors is designated by the General Meeting to issue shares or grant rights to subscribe for shares, the shareholders are not permitted to also do so as long as the designation of the Board of Directors is in effect. A resolution of the General Meeting to issue shares, to grant rights to subscribe for shares or to designate our Board of Directors as the corporate body authorized to do so can only take place at the proposal of our Board of Directors. Designation by resolution of the General Meeting cannot be withdrawn unless determined otherwise at the time of designation. The scope and duration of our Board of Directors’ authority to issue shares or grant rights to subscribe for shares (such as granting stock options or issuing convertible bonds) is determined by a resolution of the General Meeting and relates, at the most, to all unissued shares in our authorized capital at the relevant time. The duration of this authority may not exceed a period of five years. A resolution of our Board of Directors to issue shares and to grant rights to subscribe for shares can only be taken with the consent of the majority of the Non-Executive Directors. The 2024 General Meeting designated our Board of Directors as the corporate body competent to issue additional shares and grant rights to subscribe for shares up to a maximum of 10% of the outstanding capital at the date of the 2024 General Meeting, and to limit or exclude pre-emptive rights of shareholders for such shares with the prior consent of the majority of the Non-Executive Directors for a period of 18 months. 4.2.6 Pre-Emption Rights Dutch law and the Articles of Association give shareholders pre-emptive rights to subscribe on a pro rata basis for any issue of new shares or, upon a grant of rights, to subscribe for shares. Holders of shares have no pre-emptive rights upon (i) the issue of shares against a payment in kind (being a contribution other than in cash); (ii) the issue of shares to our employees or the employees of a member of our group; and (iii) the issue of shares to persons exercising a previously granted right to subscribe for shares. Pursuant to the Articles of Association, the General Meeting may restrict or exclude the pre-emptive rights of shareholders. A resolution of the General Meeting to restrict or exclude the pre-emptive rights or to designate our Board of Directors as our corporate body authorized to do so, may only be adopted on the proposal of our Board of Directors with the consent of the majority of the Non-Executive Directors, and requires a majority of at least two-thirds of the votes cast, if less than 50% of our issued and outstanding share capital is present or represented at the General Meeting. A resolution of our Board of Directors to restrict or exclude pre-emptive rights can only be taken with the consent of the majority of the Non-Executive Directors. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Share Capital 193

The designation of our Board of Directors as the body competent to restrict or exclude the pre-emptive rights may not exceed a period of five years. Designation by resolution of the shareholders at a General Meeting cannot be withdrawn unless determined otherwise at the time of designation. Please refer to section “Issue of Shares” with respect to the current right of the Board of Directors to limit or exclude pre-emptive rights. 4.2.7 Acquisition of Shares in our Capital We may not subscribe for our own shares on issue. We may acquire fully paid-up shares at any time for no consideration or, if: • our shareholders’ equity less the payment required to make the acquisition, does not fall below the sum of called-up and paid-in share capital and any statutory reserves; • we and our subsidiaries would thereafter not hold shares or hold a pledge over shares with an aggregate nominal value exceeding 50% of our issued share capital; and • our Board of Directors has been authorized thereto by the General Meeting. As part of the authorization, the General Meeting must specify the number of shares that may be repurchased, the manner in which the shares may be acquired and the price range within which the shares may be acquired. An authorization by the General Meeting to our Board of Directors for the repurchase of shares can be granted for a maximum period of 18 months. No authorization of the General Meeting is required if ordinary shares are acquired by us with the intention of transferring such ordinary shares to our employees under the Equity Incentive Plan. A resolution of our Board of Directors to repurchase shares can only be taken with the consent of the majority of the Non-Executive Directors. Shares held by us in our own share capital do not carry a right to any distribution. Please refer to Section 4.5 “General Meeting, Voting Rights and Admission" with respect to the exercising voting rights for the shares held by us. 4.2.8 Reduction of Share Capital The shareholders at a General Meeting may, upon a proposal by our Board of Directors, resolve to reduce the issued share capital by cancelling shares or by amending the Articles of Association to reduce the nominal value of the shares. 4.3 Share Classes and Principal Shareholders As at February 19, 2025 our issued share capital amounted to €6,099,085.70 and was represented by 60,990,857 ordinary shares. There is only one class of shares (ordinary shares, including ordinary shares represented by ADSs), and there are no special rights attached to any of the ordinary shares, nor special shareholder rights, including voting rights, for any of our shareholders. Each shareholder has one vote. 4.3.1 Disclosure of holdings Pursuant to the DFSA, any person who, directly or indirectly, acquires or disposes of an (actual or deemed) interest in the capital, voting rights or gross short position of the Company must immediately give written notice to the AFM by means of a standard form, if, as a result of such acquisition or disposal, the percentage of capital interest or voting rights held by such person meets, exceeds or falls below the following thresholds: 3%, 5%, 10%, 15%, 20%, 25%, 30%, 40%, 50%, 60%, 75% and 95%. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Share Capital 194

Any person whose interest in the capital, voting rights or gross short position in the Company meets, exceeds or falls below one or several of the above-mentioned thresholds due to a change in the Company’s outstanding capital, or in voting rights attached to the shares as notified to the AFM by the Company, should notify the AFM no later than the fourth trading day after the AFM has published the notification by the Company. Furthermore, each director must notify the AFM of each change in the number of shares he or she holds and of each change in the number of votes he or she is entitled to cast in respect of our issued and outstanding share capital, immediately after the relevant change. The AFM does not issue separate public announcements of the notifications. It does, however, keep a public register of and publishes all notifications made pursuant to the DFSA at its website (www.afm.nl). Third parties can request to be notified automatically by email of changes to the public register in relation to a particular company’s shares or a particular notifying party. Non-compliance with these notification obligations is an economic offence and may lead to criminal prosecution. The AFM may impose administrative penalties for non-compliance, and the publication thereof. In addition, a civil court can impose measures against any person who fails to notify or incorrectly notifies the AFM of matters required to be notified. A claim requiring that such measures be imposed may be instituted by us, or by one or more of our shareholders who alone or together with others represent at least 3% of our issued and outstanding share capital of or voting rights. Shareholders are advised to consult with their own legal advisors to determine whether the notification obligations apply to them. 4.3.2 Short positions Pursuant to EU Regulation No. 236/2012, each person (legal entities as well as natural persons) holding a net short position attaining 0.2% of our issued share capital must report it to the AFM. Each subsequent increase of this position by 0.1% above 0.2% will also have to be reported. Each net short position equal to 0.5% of our issued share capital and any subsequent increase of that position by 0.1% will be made public via the AFM short selling register. To calculate whether a natural person or legal person has a net short position, their short positions and long positions must be set off. A short transaction in a share can only be contracted if a reasonable case can be made that the shares sold can actually be delivered, which requires confirmation of a third party that the shares have been located. The notification shall be made no later than 15:30 central European time on the following trading day. Furthermore, each person holding a gross short position in relation to our issued share capital that reaches, exceeds or falls below one of the following thresholds: 3%, 5%, 10%, 15%, 20%, 25%, 30%, 40%, 50%, 60%, 75% and 95%, must immediately give written notice to the AFM. If a person’s gross short position reaches, exceeds or falls below one of the above mentioned thresholds as a result of a change in our issued share capital, such person is required to make a notification not later than on the fourth trading day after the AFM has published our notification in the public register of the AFM. The AFM keeps a public register of the short selling notifications. Shareholders are advised to consult with their own legal advisors to determine whether any of the above short selling notification obligations apply to them. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Share Classes and Principal Shareholders 195

4.3.3 Major Shareholders The following table sets forth information with respect to the beneficial ownership of our ordinary shares for persons and entities that have notified the AFM of their substantial interest in the Company of 3% or more of our total outstanding ordinary shares at February 19, 2025. Name of beneficial owner Shares beneficially owned 3% or greater shareholders* Number of shares Capital interest Number of voting rights Voting interest Artisan Investments GP LLC 3,015,2431) 5.00% 3,015,243 1) 5.00% BlackRock, Inc. 3,685,398 2) 6.11% 4,200,942 2) 6.96% Capital Research and Management Company – –% 1,837,683 3) 3.07% FMR LLC 6,076,044.40 4) 10.00% 6,054,847.40 4) 9.97% Janus Henderson Group plc 1,784,723 5) 3.02% 1,784,723 5) 3.02% T. Rowe Price Group, Inc. 6,022,043 6) 9.98% 5,895,601 6) 9.77% Wellington Management Group LLP – –% 2,150,704 7) 3.62% 1) Consisting of 215,293 ordinary shares and 2,799,950, according to the AFM filing, depositary receipts (on which, according to the AFM filing, an equal number of voting rights can be exercised by this entity). 2) Consisting of 2,674,291 ordinary shares (on which, according to the AFM filing, 3,084,810 voting rights can be exercised by this entity), 1,010,752, according to the AFM filing, depositary receipts (on which, according to the AFM filing, 1,115,620 voting rights can be exercised by this entity) and 355 contracts for difference (on which, according to the AFM filing, 512 voting rights can be exercised by this entity). 3) Consisting of voting rights on 206,694 ordinary shares and 1,630,989 ADSs. 4) Consisting of 6,076,044 ordinary shares (on which, according to the AFM filing, 6,054,847 voting rights can be exercised by this entity). 5) Consisting of 10,882 ordinary shares and 1,773,841 ADSs. 6) Consisting of 19,386 ordinary shares (on which, according to the AFM filing, 19,156 voting rights can be exercised by this entity) and 6,002,657 ADSs (on which, according to the AFM filing, 5,876,445 voting rights can be exercised by this entity). 7) Consisting of voting rights on 1,819,494 ordinary shares, 330,691 ADSs and 519 total equity return swap. * Based on the number of securities reported in, and at the time of, the most recent transparency notification filed with the AFM. Actual interests may differ as the holder of a substantial interest is only obliged to notify the AFM of any change in the percentage of share capital and/or voting rights if such holder, directly or indirectly, reaches, exceeds or falls below any of the above mentioned thresholds. The total number of stock options and RSUs outstanding at February 19, 2025 amounts to 4,999,378 stock options and 439,161 RSUs. As of the date of this Annual Report, we are not directly or indirectly owned or controlled by any shareholder, whether individually or acting in concert. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company. Other than as publicly disclosed through AFM filings or Schedule 13D or 13G filings filed with the SEC and any amendments thereof, and other than changes in percentage ownership as a result of the shares issued in connection with our initial and follow-on U.S. public offerings, we are not aware of any significant change in the percentage ownership held by the major shareholders listed above. The number of record holders in the U.S. is not representative of the number of beneficial holders nor is it representative of where such beneficial holders are resident since many of these ordinary shares were held by brokers or other nominees. At February 19, 2025, assuming that all of our ordinary shares represented by ADSs are held by residents of the U.S., we estimate that approximately 52.18% of our outstanding ordinary shares were held in the U.S. by approximately one institutional holder of record, which is the Bank of New York Mellon as depositary of the ADSs. As of the date of this Annual Report, as far as we are aware, there are no direct or indirect relationships between us and any of our significant shareholders. 4.4 Limitations on the right to hold securities Neither Dutch law nor our Articles of Association impose any general limitation on the right of non- residents or foreign persons to hold our securities or exercise voting rights on our securities other than those limitations that would generally apply to all shareholders. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Share Classes and Principal Shareholders 196

4.5 General Meeting, Voting Rights and Admission General Meetings are held at the place where the Company has its official seat (being Amsterdam) or at Schiphol Airport (municipality of Haarlemmermeer), the Netherlands. The Articles of Association provide that at least one annual General Meeting shall be held within six months after the close of each fiscal year. Additional extraordinary General Meetings may be held whenever our Board of Directors deems such to be necessary. Shareholders representing alone or in aggregate at least one-tenth of our issued and outstanding share capital may, pursuant to the Dutch Civil Code, request that a General Meeting be convened. If our Board of Directors has not taken the steps necessary to ensure that a General Meeting will be held within the relevant statutory period after the request, the requesting persons may, at his/her/their request, be authorized by a court in preliminary relief proceedings to convene a General Meeting. We will give notice of any General Meeting by publication on our website and furthermore, to the extent required, in another manner in accordance with the applicable stock exchange regulations. The notice convening any General Meeting must include, among other items, an agenda indicating the place and date of the meeting, the items for discussion and voting, the proceedings for registration including the registration date, as well as any proposals for the agenda made by the Board of Directors or shareholders holding at least 3% of the issued share capital. For an annual General Meeting, the agenda shall include, among other things, the adoption of the annual accounts, appropriation of our profits and proposals relating to the composition of our Board of Directors. Pursuant to Dutch law, shareholders holding at least 3% of our issued and outstanding share capital have a right to request our Board of Directors to include items on the agenda of any General Meeting. Our Board of Directors must agree to these requests, provided that (i) the request was made in writing and motivated, and (ii) the request was received by the chair person of our Board of Directors at least 60 days prior to the date of a General Meeting. No resolutions shall be adopted on items other than those which have been included in the agenda. In accordance with the DCGC, a shareholder may include an item on the agenda only after consulting our Board of Directors in that respect. If one or more shareholders intends to request that an item be put on the agenda that may result in a change in the Company’s strategy, our Board of Directors may invoke a response time of a maximum of 180 days until the day of a General Meeting. In addition, pursuant to the Dutch Civil Code, our Board of Directors may invoke a statutory cooling-off period up to a maximum of 250 days (wettelijke bedenktijd). For the Company, this will apply in case: • shareholders request our Board of Directors to have a General Meeting consider a proposal for the appointment, suspension or dismissal of one or more directors, or a proposal for the amendment of one or more provisions in the Articles of Association relating thereto; or • a public offering of shares in the capital of the Company is announced or made without the bidder and the Company having been reached agreement about the offering; and • only if our Board of Directors also considers the relevant situation to be substantially contrary to the interests of the Company and its affiliated enterprises. If our Board of Directors invokes such a cooling-off period, this causes the powers of the General Meeting to appoint, suspend or dismiss directors (and to amend the Articles of Association in this respect) to be suspended. General Meetings are presided over by the chairperson of the Board of Directors or, if he/she is absent, by the vice chairperson of the Board of Directors. If both the chairperson and the vice chairperson are absent, the Non-Executive Directors present at the General Meeting shall appoint one of them to be chairperson. In General Meetings, members of the Board of Directors have an advisory vote. The chairperson of the General Meeting may decide at his/her discretion to admit other persons to the General Meeting. The external auditor of the Company shall attend a General Meeting in which the annual accounts are discussed. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 General Meeting, Voting Rights and Admission 197

Our Board of Directors must give notice of a General Meeting, by at least such number of days prior to the day of the meeting as required by Dutch law, which is currently 42 days. Shareholders (as well as other persons with voting rights or meeting rights) may attend a General Meeting, to address the General Meeting and, in so far as they have such right, to exercise voting rights pro rata to its shareholding, either in person or by proxy. Shareholders may exercise these rights, if they are the holders of shares on the registration date which is currently the 28th day before the day of a General Meeting, and they or their proxy have notified our Board of Directors of their intention to attend a General Meeting in writing at the address and by the date specified in the notice of said meeting. All shareholders, and each usufructuary and pledgee to whom the right to vote on our shares accrues, are entitled, in person or represented by a proxy authorized in writing, to attend and address a General Meeting and exercise voting rights pro rata to their shareholding. Shareholders may exercise their rights if they are the holders of our shares on the record date as required by Dutch law, which is currently the 28th day before the day of a General Meeting, and they or their proxy have notified us of their intention to attend such General Meeting in writing or by any other electronic means that can be reproduced on paper ultimately at a date set for that purpose by our Board of Directors which date may not be earlier than the seventh day prior to such General Meeting, specifying such person’s name and the number of shares for which such person may exercise the voting rights and/or meeting rights at such General Meeting. The convocation notice shall state the record date and the manner in which the persons entitled to attend a General Meeting may register and exercise their rights. Each ordinary share confers the right on the holder to cast one vote at the General Meeting. Shareholders may vote by proxy. The voting rights attached to any shares held by us are suspended as long as they are held in treasury. Nonetheless, the holders of a right of usufruct (vruchtgebruik) in shares belonging to another and the holders of a right of pledge in respect of ordinary shares held by us are not excluded from any right they may have to vote on such ordinary shares, if the right of usufruct (vruchtgebruik) or the right of pledge was granted prior to the time such ordinary share was acquired by us. We may not cast votes in respect of a share in respect of which there is a right of usufruct (vruchtgebruik) or a right of pledge. Shares which are not entitled to voting rights pursuant to the preceding sentences will not be taken into account for the purpose of determining the number of shareholders that vote and that are present or represented, or the amount of the share capital that is provided or that is represented at a General Meeting. Decisions of the General Meeting are taken by an absolute majority of votes cast, except where Dutch law or the Articles of Association provide for a qualified majority or unanimity. In accordance with Dutch law and generally accepted business practices, our Articles of Association do not provide quorum requirements generally applicable to a General Meeting. To this extent, our practice varies from the requirement of Nasdaq Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock. One General Meeting was held in 2024At the 2024 General Meeting, our annual report and annual accounts for the year ended December 31, 2023 were approved, the allocation of losses of the year ended December 31, 2023 to the retained earnings of the Company was approved, Dr. Brian Kotzin was appointed as a Non- Executive Director to the Board of Directors for a term of four years, Mr. Peter Verhaeghe was reappointed as a Non-Executive Director to the Board of Directors for a term of two years, Dr. Pamela Klein was reappointed as a Non-Executive Director to the Board of Directors for a term of two years, the amendment of the Articles of Association was approved, the Board of Directors was authorized to issue shares and grant rights to subscribe for shares in our share capital for up to 10% of the outstanding share capital at the date of the meeting and for a period of 18 months from the meeting and to limit or exclude statutory pre- emptive rights with regard to such (rights to subscribe for) shares, the appointment of Deloitte Accountants B.V. as the Company’s auditor for the year ended December 31, 2024 was approved, and the appointment of EY Accountants B.V. as the Company’s auditor for the year ended December 31, 2025 was approved. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 General Meeting, Voting Rights and Admission 198

4.6 Anti-Takeover Provisions Various protective measures are possible and permissible within the boundaries set by Dutch law and Dutch case law. We have not implemented specific measures with the aim of deterring takeover attempts. However, we have adopted several provisions that may have the effect of making a takeover of argenx more difficult or less attractive, including requirements that certain matters, including an amendment of our Articles of Association, may only be brought to our shareholders for a vote upon a proposal by our Board of Directors. No takeover bid has been instigated by third parties in respect of our equity during the current or previous fiscal years. 4.7 Change of Control The Company is not a party to any significant agreements which will take effect, be altered or terminated upon a change of control of the Company as a result of a public offer. 4.8 Exchange Controls Under Dutch law, subject to the 1977 Sanction Act (Sanctiewet 1977) or otherwise by international sanctions, there are no exchange control restrictions on investments in, or payments on, shares (except as to cash amounts). There are no special restrictions in our Articles of Association or Dutch law that limit the right of shareholders who are not citizens or residents of the Netherlands to hold or vote shares. 4.9 Amendments of Articles of Association The shareholders at a General Meeting may amend the Articles of Association, at the proposal of our Board of Directors, with the consent of the majority of the Non-Executive Directors. A resolution by the shareholders at a General Meeting to amend the Articles of Association requires a simple majority of the votes cast in a meeting in which at least half of our issued and outstanding capital is present or represented, or at least two-thirds of the votes cast, if less than half of our issued and outstanding capital is present or represented at that meeting. The 2024 General Meeting approved the amendment of our current Articles of Association to align with current Dutch law and practice. The Articles of Association were amended pursuant to the notarial deed of partial amendment of the Articles of Association, executed on May 7, 2024. The full text of the Articles of Association and an unofficial English translation thereof are available on our website (www.argenx.com/ investors). 4.10 Transparency Directive We are a European public company with limited liability (Societas Europaea or SE) incorporated and existing under the laws of the Netherlands. The Netherlands is our EU home member state (lidstaat van herkomst) for the purposes of Directive 2004/109/EC (as amended by Directive 2013/50/EU), or the Transparency Directive, as a consequence of which we are subject to the DFSA in respect to certain ongoing transparency and disclosure obligations. In addition, as long as our shares are listed on Euronext Brussels and the ADSs on Nasdaq, we are required to disclose any regulated information which has been disclosed pursuant to the DFSA as well as in accordance with the Belgian Law of May 2, 2007, the Belgian Royal Decree of November 14, 2007 as well as Nasdaq Listing Rules. We must publish our annual accounts within four months after the end of each financial year and our half-yearly figures within two months after the end of the first six months of each financial year. Within five calendar days after adoption of our annual accounts, we must file our adopted annual accounts with the AFM. Pursuant to the DFSA, we will be required, among other things, to make public without delay any change in the rights attaching to our shares or any rights to subscribe our shares. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Anti-Takeover Provisions 199

4.11 Dutch Financial Reporting Supervision Act Pursuant to the Dutch Financial Reporting Supervision Act (Wet toezicht financiële verslaggeving), the AFM supervises the application of financial reporting standards and has an independent right to (i) request an explanation from the Company regarding its application of the applicable financial reporting standards if, based on publicly known facts or circumstances, it has reason to doubt that the issuer’s financial reporting meets such standards and (ii) make a notification to the Company that its financial reports do not meet the applicable financial reporting standards, which notification may be accompanied by a recommendation to the Company to issue a press release on the subject matter. If the Company does not comply with such a request or recommendation, the AFM may request the Enterprise Chamber of the Court of Appeal in Amsterdam (Ondernemingskamer van het Gerechtshof te Amsterdam) (the Enterprise Chamber) to order the Company to (a) provide an explanation regarding its application of the applicable financial reporting standards to its financial reports or (b) prepare its financial reports in accordance with the Enterprise Chamber’s instructions. This Annual Report also concerns the annual financial reporting within the meaning of 5:25c(2) DFSA. 4.12 Dividends and Other Distributions Pursuant to Dutch law and the Articles of Association, the distribution of profits will take place following the adoption of our annual accounts, from which we will determine whether such distribution is permitted. We may only make distributions to the shareholders, whether from profits or from its freely distributable reserves, only insofar as its shareholders’ equity exceeds the sum of the paid-up and called-up share capital plus the reserves required to be maintained by Dutch law. The General Meeting may determine which part of our profits will be added to the reserves in consideration of our reserves and dividends policy. The remaining part of the profits after the addition to the reserves will be at the disposal of the General Meeting. Distributions of dividends will be made pro rata to the nominal value of each share. Subject to Dutch law and the Articles of Association, our Board of Directors, with the consent of the majority of the Non-Executive Directors, may resolve to distribute an interim dividend if it determines such interim dividend to be justified by our profits. For this purpose, our Board of Directors must prepare an interim statement of assets and liabilities. Such interim statement shall show our financial position not earlier than on the first day of the third month before the month in which the resolution to make the interim distribution is announced. An interim dividend can only be paid if (a) an interim statement of assets and liabilities is drawn up showing that the funds available for distribution are sufficient, and (b) our shareholders’ equity exceeds the sum of the paid-up and called-up share capital plus the reserves required to be maintained by Dutch law. Our Board of Directors, with the consent of the majority of the Non-Executive Directors, may resolve that we make distributions to shareholders from one or more of our freely distributable reserves, other than by way of profit distribution, subject to the due observance of our policy on reserves and dividends. Any such distributions will be made pro rata to the nominal value of each share. Dividends and other distributions shall be made payable not later than the date determined by our Board of Directors. Claims to dividends and other distributions not made within five years from the date that such dividends or distributions became payable, will lapse and any such amounts will be considered to have been forfeited to us (verjaring). argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Dutch Financial Reporting Supervision Act 200

Our Board of Directors has declared a series of interim distributions on account of the Company’s freely distributable reserves for such amounts as was required to pay up the aggregate nominal value of all such shares that were issued to holders of vested RSUs, all in accordance with our Equity Incentive Plan. In accordance with Dutch law, our Board of Directors prepared and filed an interim simplified balance sheet demonstrating that there were sufficient freely distributable reserves for such interim distributions. Such interim simplified balance sheet was filed with the Dutch trade register. The aggregate amount of these interim distributions amounted to approximately €8,825 ($9,170) in 2024. Other than these interim distributions, we have not paid or declared any cash dividends on our ordinary shares, and we do not anticipate paying any cash dividends in the foreseeable future. All of our outstanding shares have the same dividend rights. We intend to retain all available funds and any future earnings to fund the development and expansion of our business. Even if future operations lead to significant levels of distributable profits, we currently intend that any earnings will be reinvested in our business and that cash dividends will not be paid until we have an established revenue stream to support continuing cash dividends. In addition, payment of any future dividends to shareholders would be subject to shareholder approval at a General Meeting, upon proposal of our Board of Directors, which proposal would be subject to the approval of the majority of the Non- Executive Directors after taking into account various factors including our business prospects, cash requirements, financial performance and new product development. Our Articles of Association, as available on our website, contain the provision on the distribution of profits in article 20 (profits, distributions and losses). 4.13 Right to a surplus in the event of a liquidation Any surplus remaining after settlement of all debts and liquidation costs will be distributed to the shareholders in proportion to the nominal value of their shareholdings. 4.14 Material Modifications to the Rights of Security Holders and Use of Proceeds On July 18, 2023, we entered into an Underwriting Agreement with J.P. Morgan Securities LLC, Morgan Stanley & Co. LLC, Goldman Sachs & Co. LLC, BofA Securities, Inc., Cowen and Company, LLC, as representatives of the several underwriters named therein, relating to a global offering of an aggregate of 2,244,899 ordinary shares of the Company, nominal value €0.10 per share, including ordinary shares represented by ADSs, comprised of (i) 1,580,981 ADSs at a public offering price of $490.00 per ADS in the U.S. and countries outside the EEA and (ii) 663,918 ordinary shares at an offering price of €436.37 per ordinary share in a concurrent private placement in the EEA to certain legal entities all of which are qualified investors within the meaning of Regulation 2017/1129 of the European Parliament and of the Council of June 14, 2017, as amended. The offering was made pursuant to our effective shelf registration statement on Form F-3ASR (File No. 333-258251) filed on July 29, 2021, as supplemented by a preliminary prospectus supplement dated July 17, 2023, filed with the SEC on July 17, 2023, and a final prospectus supplement dated July 18, 2023, filed with the SEC on July 20, 2023. In connection with this offering, we granted the underwriters a 30-day option to purchase up to 336,734 additional ordinary shares (which may be represented by ADSs), which was exercised in full. The net proceeds to us from the sale of the ADSs and ordinary shares in this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by the Company, was $1.2 billion (€1.1 billion). The offering closed on July 24, 2023. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Dividends and Other Distributions 201

We have not used any of the net proceeds from the offering to make payments, directly or indirectly, to any director, officer or general partner of ours or to their associates, persons owning 10% or more of any class of our equity securities, or to any of our affiliates. We have invested the net proceeds from the offering in cash and cash equivalents and current financial assets. There has been no material change in our planned use of the net proceeds from the offering as described in our final prospectus supplement filed pursuant to Rule 424(b)(5) under the Securities Act with the SEC on July 20, 2023 (File No.333-258251). The registration statement was effective on July 29, 2021. 4.15 Enforcement of civil liabilities We are a European public company with limited liability (Societas Europaea or SE) incorporated under the laws of the Netherlands. Substantially all of our assets are located outside the U.S. The majority of our directors reside outside the U.S. As a result, it may not be possible for investors to effect service of process within the U.S. upon such persons or to enforce against them or us in U.S. courts, including judgments predicated upon the civil liability provisions of the federal securities laws of the U.S. The U.S. and the Netherlands currently do not have a treaty providing for the reciprocal recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. Consequently, a final judgment for payment given by a court in the U.S., whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in the Netherlands. In order to obtain a judgment which is enforceable in the Netherlands, the party in whose favor a final and conclusive judgment of the U.S. court has been rendered will be required to file its claim with a court of competent jurisdiction in the Netherlands. Such party may submit to the Dutch court the final judgment rendered by the U.S. court. This court will have a level of discretion in its assessment of the judgment rendered by the relevant U.S. court. On the basis of case law by the Dutch Supreme Court, Dutch courts will in principle have to give conclusive effect to a final and enforceable judgment of such court in respect of the contractual obligations thereunder without re-examination or re-litigation of the substantive matters adjudicated upon, provided that: (i) the U.S. court involved accepted jurisdiction on the basis of internationally recognized grounds to accept jurisdiction, (ii) the proceedings before such court being in compliance with principles of proper procedure (behoorlijke rechtspleging), (iii) such judgment not being contrary to the public policy of the Netherlands and (iv) such judgment not being incompatible with a judgment given between the same parties by a Netherlands court or with a prior judgment given between the same parties by a foreign court in a dispute concerning the same subject matter and based on the same cause of action, provided such prior judgment fulfills the conditions necessary for it to be given binding effect in the Netherlands. Dutch courts may deny the recognition and enforcement of punitive damages or other awards that do not fit to the Dutch legal order. Moreover, a Dutch court may reduce the amount of damages granted by a U.S. court and recognize damages only to the extent that they are necessary to compensate actual losses or damages. Enforcement and recognition of judgments of U.S. courts in the Netherlands are solely governed by the provisions of the Dutch Civil Procedure Code. Original actions or actions for the enforcement of judgments of U.S. courts relating to the civil liability provisions of the federal or state securities laws of the U.S. are not directly enforceable in Belgium. The U.S. and Belgium currently do not have a treaty providing for reciprocal recognition and enforcement of judgments, other than arbitral awards, in civil and commercial matters. Consequently, a final judgment for payment given by a court in the U.S., whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in Belgium. In order for a final judgment for the payment of money rendered by U.S. courts based on civil liability to produce any effect on Belgian soil, it is accordingly required that this judgment be recognized and be declared enforceable by a Belgian court pursuant to the relevant provisions of the PIL Code. Recognition or enforcement does not imply a review of the merits of the case and is irrespective of any reciprocity requirement. A U.S. judgment will, however, not be recognized or declared enforceable in Belgium if it infringes upon one or more of the grounds for refusal which are exhaustively listed in article 25 of the PIL Code. In addition to recognition or enforcement, a judgment by a federal or state court in the U.S. against us may also serve as evidence in a similar action in a Belgian court if it meets the conditions required for the authenticity of judgments according to the law of the state where it was rendered. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Material Modifications to the Rights of Security Holders and Use of Proceeds 202

In addition, with regard to enforcements by legal proceedings in Belgium (including the recognition of foreign court decisions in Belgium), a registration tax at the rate of 3% of the amount of the judgment is payable by the debtor, if the sum of money which the debtor is ordered to pay by a Belgian court, or by a foreign court judgment that is either (i) automatically enforceable and registered in Belgium, or (ii) rendered enforceable by a Belgian court, exceeds €12,500. The registration tax is payable by the debtor. The debtor is liable for the payment of the registration tax, in the proportion determined by the decision ordering payment or liquidation or determining priority for creditors made or established against it. The debtor(s) are jointly and severally liable in the event that they are ordered to pay jointly and severally. A stamp duty is payable as of the second certified copy of an enforcement judgment rendered by a Belgian court, with a maximum of €1,450. Dutch and Belgian civil procedure differ substantially from U.S. civil procedure in a number of respects. Insofar as the production of evidence is concerned, U.S. law and the laws of several other jurisdictions based on common law provide for pre-trial discovery, a process by which parties to the proceedings may prior to trial compel the production of documents by adverse or third parties and the deposition of witnesses. Evidence obtained in this manner may be decisive in the outcome of any proceeding. No such pre-trial discovery process exists under Dutch or Belgian law. Subject to the foregoing and service of process in accordance with applicable treaties, investors may be able to enforce in the Netherlands or Belgium judgments in civil and commercial matters obtained from U.S. federal or state courts. However, no assurance can be given that those judgments will be enforceable. In addition, it is doubtful whether a Dutch or Belgian court would accept jurisdiction and impose civil liability in an original action commenced in the Netherlands or Belgium and predicated solely upon U.S. federal securities laws. 4.16 Controls and Procedures 4.16.1 Disclosure Controls and Procedures Our management, with the participation of our CEO and CFO, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Exchange Act Rule 13a-15(b) as of December 31, 2024. While there are inherent limitations to the effectiveness of any system of disclosure controls and procedures, including the possibility of human error and the circumvention or overriding of the controls and procedures, our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives. Based upon our evaluation, as of December 31, 2024, our CEO and CFO have concluded that the disclosure controls and procedures, in accordance with Exchange Act Rule 13a-15(e), are (i) effective at the level of reasonable assurance in ensuring that information required to be disclosed in the reports that are filed or submitted under the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in the Commission’s rules and forms, and (ii) are effective at the level of reasonable assurance in ensuring that information to be disclosed in the reports that are filed or submitted under the Exchange Act is accumulated and communicated to the management of our company, including our CEO and CFO, to allow timely decisions regarding required disclosure. 4.16.2 Management’s Annual Report on Internal Control Over Financial Reporting Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as such term is defined in Rule 13a-15(f) and Rule 15d-15(f) under the Exchange Act). Our internal control over financial reporting is a process designed, under the supervision of our CEO and CFO, to provide reasonable assurance regarding the reliability of financial reporting and preparation of financial statements for external reporting purposes in accordance with IFRS, as issued by the IASB. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Enforcement of civil liabilities 203

Our internal control over financial reporting includes policies and procedures that pertain to the maintenance of records that, in reasonable detail, accurately and fairly, reflect transactions and dispositions of assets, provide reasonable assurance that transactions are recorded in the manner necessary to permit the preparation of financial statements in accordance with IFRS, as issued by the IASB, and that receipts and expenditures are only carried out in accordance with the authorization of our management and directors, and provide reasonable assurance regarding the prevention or timely detection of any unauthorized acquisition, use or disposition of our assets that could have a material effect on our consolidated financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect all misstatements. Moreover, projections of any evaluation of the effectiveness of internal control to future periods are subject to a risk that controls may become inadequate because of changes in conditions and that the degree of compliance with the policies or procedures may deteriorate. Our management has assessed the effectiveness of internal control over financial reporting based on the Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in 2013. Based on this assessment, our management has concluded that our internal control over financial reporting as of December 31, 2024 was effective. 4.16.3 Changes in Internal Control Over Financial Reporting During the period covered by this Annual Report, we have not made any change to our internal control over financial reporting that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. 4.17 Financial Calendar 2025 May 8, 2025 First Quarter 2025 Financial Results and Business Update May 27, 2025 Annual General Meeting of Shareholders in Amsterdam, the Netherlands July 31, 2025 Half Year and Second Quarter 2025 Financial Results and Business Update October 30, 2025 Third Quarter 2025 Financial Results and Business Update argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Controls and Procedures 204

Operating and Financial Review and Prospects "Operating and Financial Review and Prospects” should be read together with the information in our financial statements and related notes included elsewhere in this Annual Report. The following discussion is based on our financial information prepared in accordance with the International Financial Reporting Standards as issued by the IASB’s and adopted by the European Union (EU-IFRS) and in accordance with the legal requirements of Part 9 of Book 2 of the Dutch Civil Code. The following discussion includes forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those described in section “Risk Factors” and elsewhere in this Annual Report. See “Forward-Looking Statements” in this Annual Report. 5.1 Overview 206 5.2 Basis of presentation 208 5.3 Critical Accounting Judgments and Major Sources of Estimation Uncertainty 210 5.4 Results of Operations 212 5.5 Liquidity and Capital Resources 215 5.6 Research and development, patents and licenses 218 5.7 Trend information 218 5.8 Off-Balance Sheet Arrangements 219 5.9 Contractual Obligations 219 5.10 Information Regarding the Independent Auditor 219 5.11 Material Contracts and Related Party Transactions 219 5.12 Employees 222 5.13 Insurance 222 5.14 Legal and Arbitration Proceedings 222 5.15 Taxation 223 argenx Annual Report 2024 205 5

5 Operating and Financial Review and Prospects 5.1 Overview Since our inception in 2008, we have focused most of our financial resources and efforts towards developing our SIMPLE ANTIBODY™ Platform and antibody engineering technologies, identifying potential product candidates, establishing process, development and manufacturing capabilities for our product candidates and advancing multiple discovery programs into the clinic. In 2022, we executed on our global launch of VYVGART our first-in-class neonatal FcRn blocker for intravenous use, which is now approved in the U.S., Japan, the EU, Canada, China and other EMEA jurisdictions for gMG. In 2023, we launched VYVGART SC, the first-and-only neonatal FcRn blocker administered by subcutaneous injection. As of the year ended December 31, 2024, it is now approved in the U.S., Japan, the EU, Canada, China and other EMEA jurisdictions for gMG. In 2024, we successfully started the sale of VYVGART SC for the treatment of CIDP in the U.S. and obtained approval in China and Japan. The commercialization of VYVGART IV and VYVGART SC generated global product net sales of $2.2 billion in 2024 as compared to $1.2 billion in 2023. On our research and development, we continue towards advancing a deep pipeline of both clinical and preclinical-stage product candidates for the treatment of severe autoimmune diseases. Leveraging our technology suite and clinical expertise, we have advanced several candidates into late-stage clinical development and we currently have multiple programs in the discovery stage. As of December 31, 2024 and December 31, 2023, we had cash and cash equivalents amounting to $1.5 billion and $2.0 billion, respectively; in addition to current financial assets of $1.9 billion and $1.1 billion, respectively. Our Statement of Financial Position shows total assets of $6.2 billion for the year ended December 31, 2024, compared to $4.5 billion for the year ended December 31, 2023. The main reason for the material change in balance sheet total is the operational growth of the Company in the period. Since our inception, we have incurred significant operating losses. For the year ended December 31, 2024 the Company recorded its first annual profit for the year of $833 million. In 2023, the Company recorded a loss for the year of $295 million. As of December 31, 2024, we had accumulated losses of $1.6 billion. VYVGART and VYVGART SC are the only approved products we currently have. We expect our expenses to continue to increase as we continue to execute registrational and proof-of- concept studies across efgartigimod, empasiprubart and ARGX-119, as well as the continued investment in our IIP. We anticipate that our expenses will increase if and as we execute on the following elements. Research and development activities: • Execute the registrational study of efgartigimod in three myositis subsets (IMNM, ASyS, and DM); • Execute the Phase 3 clinical studies of efgartigimod in pediatric, seronegative and ocular MG; • Execute the Phase 4 study switching CIDP patients from IVIg to VYVGART SC; • Execute the confirmatory study of efgartigimod in primary ITP; • Execute the registrational studies of efgartigimod in TED; • Execute the registrational study of efgartigimod in SjD; • Execute the Phase 2 study of efgartigimod in LN with our partner Zai Lab; argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Overview 206

• Execute the Phase 2 studies of efgartigimod in AMR, SSc and AIE; • Execute the Phase 3 studies of empasiprubart in MMN and CIDP; • Execute the Phase 2 studies of empasiprubart in DGF and DM; • Execute the Phase 1b/Phase 2a studies of ARGX-119 in CMS and ALS, respectively; • Execute on the launch of Phase 2 study of ARGX-119 in SMA; • Continue the research and development of our other clinical and preclinical-stage product candidates and discovery stage programs; and • Seek regulatory approvals for any product candidates, including new indications, that successfully complete clinical trials. Pre-commercial and commercial activities: • Continue the build-out of our sales, marketing and distribution infrastructure and scale-up of manufacturing capabilities for the commercial expansion of VYVGART and VYVGART SC and any other product candidate, including new indications, for which we may obtain approval; and • Expand our global reach enabling us to commercialize any product candidates, including new indications, for which we may obtain regulatory approval. Other activities: • Seek to enhance our technology platform and discover and develop additional product candidates; • Maintain, expand and protect our intellectual property portfolio, including litigation costs associated with defending against alleged patent infringement claims; • Add clinical, scientific, operational, financial and management information systems and personnel, including personnel to support our product development and potential future commercialization efforts; and, • Experience any delays or encounter any issues, including failed studies, ambiguous clinical trial results, safety issues or other regulatory challenges. We expect that the costs of development and commercialization might also increase due to current and future collaborations with research and development partners as well as commercial partners. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Overview 207

5.2 Basis of presentation 5.2.1 Foreign Currency Transactions Functional and presentation currency Items included in the consolidated financial statements of each of the entities are valued using the currency of their economic environment in which the entity operates. The consolidated financial statements are presented in USD ($), which is the Company’s functional and presentation currency. 5.2.2 Revenue from sale of product Revenue from the sale of products is recognized at an amount that reflects the consideration that the Company expects to be entitled to receive in exchange for transferring goods to a customer, at the time when the customer obtains control of the goods rendered, this means when the customer has the ability to direct the use of the asset. The consideration that is committed in a contract with a customer can include fixed amounts, variable amounts, or both. The amount of the consideration may vary due to discounts, rebates, returns, chargebacks or other similar items. Contingent consideration is included in the transaction price when it is highly probable that the amount of revenue recognized is not subject to future significant reversals. Our product net sales mainly consist of sales of VYVGART and VYVGART SC in the U.S., Japan, EMEA and China. Product net sales are recognized once we satisfy the performance obligation at a point in time under the revenue recognition criteria in accordance with IFRS 15 Revenue from contracts with customers. Revenue arising from the commercial sale of VYVGART and VYVGART SC is presented under ‘‘Note 17 Segment Reporting’’ in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. In accordance with IFRS 15, such revenue is recognized when the product is physically transferred, in accordance with the delivery and acceptance terms agreed with the customer. Payment of the transaction price is payable at the point the customer obtains the legal title to the goods. 5.2.3 Revenue from Collaboration and License Agreements Revenues to date have consisted principally of milestones, license fees, non-refundable upfront fees and research and development service fees in connection with collaboration and license agreements. We recognize revenue when the customer obtains control of promised goods or services, in an amount that reflects the consideration that we expect to receive in exchange for those goods and services. In order to determine revenue recognition for agreements that we determine to be in the scope of IFRS 15, we followed the IFRS 15 5-step model. The Company has only recognized revenue from its collaboration with Zai Lab in the current year. Under the collaboration agreement, the Company provides clinical and commercial supply to Zai Lab. The Company concludes to recognize such sales as revenue given that the Company acts as principal in the transaction as the risk related to inventory is borne by the Company until the inventory is transferred to Zai Lab. The revenue related to clinical supply is recorded under line item “Collaboration revenue”. The revenue related to commercial supply is recorded under line item “Product net sales” in the Consolidated Statements of Profit or Loss. The income related to royalties or sales-based milestones on sales made in China is recorded under line item “Collaboration revenue”. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Basis of presentation 208

5.2.4 Research and Development Expenses Research and development expenses consist principally of: • external research and development expenses related to (i) chemistry, manufacturing and control costs for our product candidates, both for preclinical and clinical testing, all of which is conducted by specialized contract manufacturers, (ii) fees and other costs paid to CROs in connection with preclinical testing and the performance of clinical trials for our product candidates, (iii) costs associated with regulatory submissions and approvals, QA and pharmacovigilance and (iv) costs associated with post- approval clinical trials; • personnel expenses related to compensation of research and development staff and related expenses, including salaries, benefits and share-based payment expenses; • Business Information Systems (BIS) related expenses; and • other expenses. Our research and development expenses may vary substantially from period to period based on the timing of our research and development activities, including the timing of the initiation of clinical trials, material used in R&D phase and enrollment of patients in clinical trials. Research and development expenses are expected to increase as we advance the clinical development of efgartigimod, empasiprubart ARGX-119 and further advance the research and development of our other early-stage pipeline candidates. The successful development of our product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing and estimated costs of the efforts that will be necessary to complete the development of, or the period, if any, in which material net cash inflows may commence from any of our product candidates. This is due to numerous risks and uncertainties associated with developing drugs, as further described in “Section 2 Risk Factors”. 5.2.5 Selling, General and Administrative Expenses Selling, general and administrative expenses consist primarily of: • personnel expenses related to compensation of commercial and enabling staff and related expenses, including salaries, benefits and share-based payment expenses; • professional fees related to commercial and enabling functions; • Board of Directors expenses consisting of directors’ fees, travel expenses and share-based compensation for non-executive board members; • marketing and promotional activities related to the global commercialization of VYVGART and VYVGART SC for the treatment of gMG, CIDP and ITP (in Japan); and • other Selling, general and administrative expenses, including leasing costs, office expenses and travel costs. We expect our general and administrative expenses to increase as we continue to support our growth. Such costs include increases in our personnel, additional BIS-related expenses, and expenses and costs associated with compliance with the regulations governing public companies. We expect our selling and marketing expenses to increase due to marketing and promotional activities with respect to the ongoing commercial launch of VYVGART, VYVGART SC and preparation of commercial launch of our other product candidates. 5.2.6 Financial Income (Expense) Financial income mainly reflects interest earned on our cash and cash equivalents and current financial assets and net gains on our cash and cash equivalents and current financial assets held at fair value through profit or loss. Financial expense corresponds mainly to interest expenses arising from lease liabilities. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Basis of presentation 209

5.2.7 Exchange Gains (Losses) Our exchange gains (losses) relate to (i) our transactions denominated in foreign currencies, mainly in euro, and which generate exchange gains or losses and (ii) the translation at the reporting date of assets and liabilities denominated in foreign currencies into USD, which is our functional and presentation currency. For more information on currency exchange fluctuations on our business, please see “Note 25 Financial Risk Management’’ in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. We have no derivative financial instruments to hedge interest rate and foreign currency risk. 5.2.8 Income Tax Benefit (Expense) For the year ended December 31, 2024 the Company recognized its Belgian based deferred tax assets. For more information on income taxes and deferred taxes, please see “Note 23 Income taxes” in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. 5.3 Critical Accounting Judgments and Major Sources of Estimation Uncertainty In the application of the Company’s accounting policies, which are described above, the Company is required to make judgments, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the revision affects both current and future periods. 5.3.1 Critical accounting judgment Deferred Tax Assets The Company recognizes deferred tax assets if management assesses that these tax assets are recoverable in the future. This judgment is made on an ongoing basis, considering actual results, forecasts, and business plans for the look-forward period. The Company has exercised a Critical Accounting Judgement with respect to defining the number of years of forecasted future taxable profits to be considered as reliable as positive evidence towards its estimate on recognition of deferred tax assets. The Company has aligned the duration of its deferred tax assessment with the time horizon of its annual operating plan and long-range predictive estimates. In the fourth quarter of the year ended December 31, 2024, the Company reassessed the body of evidence as part of its 2025 budgeting and forecasting cycle noting the shift of positive evidence outweighing negative evidence. Such positive evidence, includes significant revenue growth in the U.S. based Product Net Sales, as well as, expectations regarding future operating and taxable profits. The Company expects its future revenues to sustain its investments in its clinical and pre-clinical pipeline. This evaluation was done alongside the evolution of the external competitive landscape of our commercialized products, and the positive evidence following our execution in the second half of 2024 on the approvals of VYVGART HYTRULO for CIDP. Our evaluation of the evidence for the current reporting period is further detailed in “Note 23 Income taxes” which presents the balance of $708 million in net deferred tax assets for argenx BV as of December 31, 2024. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Basis of presentation 210

The Company considers the evaluation of all available positive and negative evidence used in its overall recognition conclusion to be a Critical Accounting Judgment. The Company has determined that a key estimate in exercising this judgement is related to the growth of U.S. based Product Net Sales. The forecasts for future growth in this market are important for the utilization of deferred tax assets against taxable profits by argenx BV. Based on sensitivities and analysis over assumptions in the model, the Company has determined that other inputs are less sensitive or significant to the recognition of our deferred tax assets. The Company’s current sensitivities do not exceed the look- forward period. The Critical Accounting Judgement and the key estimate relating to deferred tax assets are limited to the Company’s subsidiary argenx BV which holds these assets. 5.3.2 Major sources of estimation uncertainty Sales rebates and reserves Product Sales are recognized when the Company has transferred control of the goods to the customer. Product Sales are subject to various deductions, which are primarily composed of rebates to government agencies, distributors, health insurance companies and managed healthcare organizations to arrive to ‘‘Product net sales’’. Certain deductions from Product Sales are subject to payment based on claims after the initial recognition of the sale due to the time lag between the point of sale and receipt of a claim. Upon initial recognition of the Product Sales, the Company recognizes a liability for the variable consideration based on the Company’s best estimate of expected claims. This estimate is a source of complexity and uncertainty as the Company estimates the transaction price based upon contracts with customers, healthcare providers, payors and government agencies, regulated discounts applicable to government-funded programs, historical experience of claims received, estimated payer mix, and other relevant factors. These open claims are recorded as liabilities under “Sales rebates and reserves’’ in the ‘‘Consolidated Statements of Financial Position’’. The Company reviews these liabilities at each reporting period to take into account potential changes in the programs, the volume of claims and/or the most probable final outcome associated to each sale. In line with IFRS 15, the Company applies constraint in recognition of variable compensation on Product Net Sales. Due to the nature of these liabilities it is not practicable to give meaningful sensitivity estimates due to the large volume of variables that contribute to the overall rebates, chargebacks, and other incentives as outlined in “Note 2.17 Product net sales”. Future events could cause the assumptions within our valuation models to change and materially affect the future results of the Company. Please refer to “Note 14 Trade and Other Payables” for the movement over the period and the ending balance of the sales rebates and reserves. No other Critical accounting judgments and major sources of estimation uncertainty have been made in the current period by the Company. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Critical Accounting Judgments and Major Sources of Estimation Uncertainty 211

5.4 Results of Operations 5.4.1 Comparison of Years Ended December 31, 2024 and 2023 Year Ended December 31, (in thousands of $ except for shares and EPS) 2024 2023 % Change Product net sales 2,185,883 1,190,783 84% Collaboration revenue 4,348 35,533 (88%) Other operating income 61,808 42,278 46% Total operating income 2,252,039 1,268,594 78% Cost of sales (227,289) (117,835) 93% Research and development expenses (983,423) (859,492) 14% Selling, general and administrative expenses (1,055,337) (711,905) 48% Loss from investment in a joint venture (7,644) (4,411) 73% Total operating expenses (2,273,693) (1,693,643) 34% Operating loss (21,654) (425,049) (95%) Financial income 157,509 107,386 47% Financial expense (2,464) (906) 172% Exchange (losses)/gains (48,211) 14,073 (443%) Profit/(Loss) for the year before taxes 85,180 (304,496) 128% Income tax benefit 747,860 9,443 7819% Profit/(Loss) for the year 833,040 (295,053) 382% Weighted average number of shares outstanding 59,855,585 57,169,253 Basic profit/(loss) per share (in $) 13.92 (5.16) Weighted average number of shares for purpose of diluted profit/(loss) per share 65,177,815 57,169,253 Diluted profit/(loss) per share (in $) 12.78 (5.16) Product net sales Year Ended December 31, (in thousands of $) 2024 2023 United States 1,895,919 1,046,592 Japan 89,389 56,432 China 39,177 14,907 Rest of the World 161,398 72,852 Total product net sales 2,185,883 1,190,783 Our product net sales have increased in the U.S. and other countries as the Company continues to execute on the global commercialization of VYVGART and VYVGART SC and obtain further approvals worldwide. Collaboration Revenue Year Ended December 31, (in thousands of $) 2024 2023 % Change AbbVie – 30,000 (100%) Zai Lab 4,348 5,533 (21%) Total collaboration revenue 4,348 35,533 (88%) Our collaboration revenue decreased by $31 million to $4 million for the year ended December 31, 2024, compared to $36 million for the year ended December 31, 2023. The collaboration revenue recognized in the year ended December 31, 2024 was mainly the result of the clinical supply of product and royalties on product net sales of VYVGART in Greater China through Zai Lab. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Results of Operations 212

Other Operating Income Year Ended December 31, (in thousands of $) 2024 2023 % Change Research and development incentives 46,106 27,815 66% Payroll tax rebates 11,855 11,925 (1%) Grants 13 2,538 (99%) Change in fair value on non-current financial assets 3,834 – 100% Total other operating income 61,808 42,278 46% Other operating income increased by $20 million to $62 million for the year ended December 31, 2024, compared to $42 million for the year ended December 31, 2023. The $20 million increase was primarily driven by: • the increase in research and development incentives due to a Belgian research and development tax incentive scheme, as a result of the overall increased research and development costs incurred; • the increase in payroll tax rebates for the year ended December 31, 2024, as a result of higher research and development personnel expenses eligible for rebates for the year ended December 31, 2024; and • an increase of $4 million due to the change in fair value on our profit share in AgomAb for the year ended December 31, 2024. For more information regarding governmental policies that could affect our operations, see “Business Overview” and “Healthcare Law and Regulation”. Research and Development Expenses Year Ended December 31, (in thousands of $) 2024 2023 % Change External research and development expenses 605,082 483,192 25% Personnel expenses 310,992 226,344 37% BIS expenses 34,012 19,935 71% Materials and consumables 5,863 4,057 45% Depreciation and amortization 6,204 105,546 (94%) Other expenses 21,270 20,418 4% Total Research and development expenses 983,423 859,492 14% Our research and development expenses totaled $983 million and $859 million for the years ended December 31, 2024 and 2023, respectively. The increase of$124 million in 2024 as compared to 2023 is primarily driven by Personnel expenses and External research and development expenses. This is offset by the decrease in Depreciation and amortization resulting from the use of a Priority Review Voucher (PRV) for priority review by the FDA of its VYVGART HYTRULO for use in CIDP in 2023. Personnel expenses relate to internal and external R&D personnel. The expenses also include share-based compensation expenses related to the grant of stock options and RSUs to our research and development employees. We employed on average 805 full-time equivalents in our research and development functions in the year ended December 31, 2024, compared to 607 in the year ended December 31, 2023. Our external research and development expenses for the year ended December 31, 2024 totaled to $605 million, compared to $483 million for the year ended December 31, 2023. The expenses reflect clinical trial costs and manufacturing expenses related to the development of our product candidate portfolio. The table below provides additional detail on our external research and development expenses by program: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Results of Operations 213

Year Ended December 31, (in thousands of $) 2024 2023 % Change efgartigimod 405,347 361,676 12% empasiprubart 86,254 47,636 81% ARGX-119 26,098 13,731 90% cusatuzumab 10,856 14,298 (24%) Other programs 76,527 45,851 67% Total 605,082 483,192 25% External research and development expenses for our lead product efgartigimod totaled $405 million for the year ended December 31, 2024, compared to $362 million for the year ended December 31, 2023 relating to the efforts in evaluating in more than 15 severe autoimmune diseases including MG, CIDP and ITP. External research and development expenses for empasiprubart totaled $86 million for the year ended December 31, 2024 compared to $48 million for the year ended December 31, 2023. This increase of $39 million was due to the ramp-up of Phase 2 clinical trials in MMN, DGF, DM and CIDP. External research and development expenses for ARGX-119 increased by $12 million to $26 million for the year ended December 31, 2024 as we continue to invest in proof-of-concept studies ongoing in ALS and CMS. Our investments in other programs have increased by $31 million with four new pipeline candidate nominations to IIP alongside our continued Discovery programs. Selling, general and administrative Expenses Year Ended December 31, (in thousands of $) 2024 2023 % Change Personnel expenses 424,916 303,033 40% Marketing services 306,987 202,146 52% Professional fees 170,215 108,820 56% BIS expenses 27,295 20,408 34% Supervisory board 9,724 8,362 16% Depreciation and amortization 3,149 2,366 33% Other expenses 92,163 55,506 66% Total Selling, general and administrative expenses 1,055,337 711,905 48% The increase in our Selling, general and administrative expenses for the year ended December 31, 2024 was principally resulting from: • increased professional and marketing fees, including promotional and marketing costs primarily due to the scaling of our commercial operations relating to VYVGART and VYVGART SC; • increased costs of personnel expenses is related to planned increase in the headcount of our Selling, general and administrative employees recruited to strengthen our enabling functions and the scaling of our commercial operations relating to VYVGART and VYVGART SC; and • continued investment in our IT infrastructure. We employed on average 835 full-time equivalents in our selling, general and administrative functions in the year ended December 31, 2024, compared to 681 in the year ended December 31, 2023. Financial Income and (Expense) For the year ended December 31, 2024, financial income amounted to $158 million compared to $107 million for the year ended December 31, 2023. The increase of $50 million in 2024 related primarily to the capital increase of our financial assets. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Results of Operations 214

Exchange Gains (Losses) Exchange losses totaled $48 million for the year ended December 31, 2024, compared to exchange gains of $14 million for the year ended December 31, 2023. The losses were mainly attributable to unrealized exchange rate losses on the cash and cash equivalents, in addition to current financial assets position in euro during the year ended December 31, 2024. 5.5 Liquidity and Capital Resources 5.5.1 Sources of Funds The table below sets forth our capitalization as of December 31, 2024 on an actual basis based on the information available in our Consolidated Financial Statements: (in thousands of $) As of December 31, 2024 Shareholder equity 5,498,283 Share capital 7,227 Share premium 5,948,916 Legal reserve(s) 1) 126,832 Retained earnings (1,571,804) Other reserves 987,112 Total 5,498,283 1) Legal reserves are the amount of translation differences. Since our inception in 2008, we have invested most of our resources in developing our product candidates, building our intellectual property portfolio, developing our supply chain, conducting business planning, raising capital and providing general and administrative support for these operations. To date, we have funded our operations through public and private placements of equity securities, upfront, milestone and expense reimbursement payments received from our collaborators, funding from governmental bodies, proceeds from exercise of employee stock options and interest income from the investment of our cash and cash equivalents, in addition to current financial assets. Through December 31, 2024, we have raised gross proceeds of $5.9 billion from private and public offerings of equity securities. We currently have two products approved by the FDA, VYVGART and VYVGART SC; therefore, our commercial operations have also started to contribute to the funding of our operations. We have made product net sales of $2.2 billion during the twelve months ended December 31, 2024. As we continue to invest in innovation and the our cash flows may fluctuate, are difficult to forecast and will depend on many factors. We have no ongoing material financing commitments, such as lines of credit or guarantees, that are expected to affect our liquidity over the next five years, other than leases and commitments as part of our operations, which are detailed in “Note 28 Commitments” in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. For more information as to the risks associated with our future funding needs, see “Risk Factors — Risk Factors Related to argenx’s Financial Position and Need for Additional Capital”. For more information as to our financial instruments, please see “Note 25 Financial Risk Management” in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Results of Operations 215

5.5.2 Cash Flows Comparison for the Years Ended December 31, 2024 and 2023 The table below summarizes our cash flows for the years ended December 31, 2024 and 2023. Year Ended December 31, (in thousands of $) 2024 2023 Variance Cash and cash equivalents at the beginning of the year 2,048,844 800,740 1,248,104 Net cash flows used in operating activities (82,747) (420,327) 337,580 Net cash flows from/(used in) investing activities (717,594) 308,210 (1,025,804) Net cash flows from financing activities 279,759 1,336,727 (1,056,968) Exchange gains/(losses) on cash and cash equivalents (28,326) 23,494 (51,820) Cash and cash equivalents at the end of the year 1,499,936 2,048,844 (548,908) As of December 31, 2024, the Company had $1.5 billion of cash and cash equivalents compared to $2.0 billion as of December 31, 2023. For more information, please see “Note 11 Cash and Cash Equivalents” in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. Net Cash Used in Operating Activities Net cash outflow used in our operating activities decreased by $338 million to a net outflow of $83 million for the year ended December 31, 2024, compared to a net outflow of $420 million for the year ended December 31, 2023. The decrease in net cash used in operating activities results primarily from an increase in product net sales of VYVGART and VYVGART SC, partly offset by: i. the increase in research and development expenses incurred in relation to the manufacturing and clinical development activities of efgartigimod and the advancement of other clinical, preclinical and discovery-stage product candidates; ii. the increase in personnel expenses, marketing expenses and consulting expenses incurred for the commercial growth of VYVGART and VYVGART SC; and iii. the further increase in working capital as a result of our inventory levels, including prepaid inventory Net Cash Used in/from Investing Activities Investing activities for the year ended December 31, 2024, consist primarily of the net movements in current financial assets of $754 million, and interests received, partly offset by payments related to regulatory and sales based milestones to Halozyme and investment in OncoVerity, resulting in a cash outflow of $718 million. Investing activities for the year ended December 31, 2023, consist primarily of net movements in current financial assets of $272 million, and purchase of a priority review voucher for $102 million, partly offset by payments related to regulatory and sales based milestones to Halozyme and investment in OncoVerity, resulting in a cash inflow of $308 million. Net Cash Provided by Financing Activities The net cash inflow from financing activities was $280 million for the year ended December 31, 2024, compared to a net cash inflow of $1.3 billion for the year ended December 31, 2023. The net cash inflows were mainly attributed to proceeds received from the exercise of stock options in 2024 as compared to inflows from issuance of new shares in 2023. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Liquidity and Capital Resources 216

5.5.3 Operating and Capital Expenditure Requirements We have achieved after-tax profitability as of December 31, 2024, mainly resulting from the recognition of deferred tax assets held by argenx BV, a subsidiary of the Company. As of the year ended December 31, 2024, we had accumulated losses of $1.6 billion. Our losses resulted principally from costs incurred in research and development, preclinical testing and clinical development of our research programs, and from selling, general and administrative costs associated with commercial expansion. We anticipate that our operating expenses will increase as we intend to continue to conduct research and development and continue our efforts to expand our sales, marketing and distribution infrastructure. Although we have generated product net sales of $2.2 billion from global product net sales of VYVGART and VYVGART SC for the treatment of gMG and CIDP for the year ended December 31, 2024, which supports our current profitability, we can provide no assurances that we will be able to be profitable or sustain net profitability in the future based on these indications alone or that we will be able to receive regulatory approval of and commercialize VYVGART and VYVGART SC in other indications or in other countries. On the basis of current assumptions, we expect that our existing cash and cash equivalents and current financial assets will enable us to fund our operating expenses and capital expenditure requirements through at least the next twelve months. Due to the numerous risks and uncertainties associated with the development and commercialization of efgartigimod and our other product candidates and discovery stage programs and because the extent to which we may enter into collaborations with third parties for the development of these product candidates is unknown, we are unable to estimate the amounts of increased capital outlays and operating expenses associated with completing the research and development of our product candidates. Our future capital requirements for efgartigimod and our other product candidates and discovery stage programs will depend on many factors, including: • the progress, timing and completion of preclinical testing and clinical trials for our current or any future product candidates; • the number of potential new product candidates we identify and decide to develop; • the time and costs involved in obtaining regulatory approval for our product candidates and any delays we may encounter as a result of evolving regulatory requirements or adverse results with respect to any of our product candidates; • selling and marketing activities undertaken in connection with the commercialization of VYVGART, VYVGART SC or potential commercialization of any of our current or any future product candidates, if approved, and costs involved in the creation of an effective sales and marketing organization; • manufacturing activities undertaken for VYVGART, VYVGART SC and potential commercialization of any of our current or any future product candidates, if approved, and costs involved in the creation of an effective supply chain; • the costs involved in growing our organization to the size needed to allow for the research, development and potential commercialization of our current or any future product candidates; • the costs involved in filing patent applications and maintaining and enforcing patents or defending against claims or infringements raised by third parties; • the maintenance of our existing collaboration agreements and entry into new collaboration agreements; and • developments related to the global economic uncertainties and political instability. For more information as to the risks associated with our future funding needs, see “Risk Factors — Risk Factors Related to argenx’s Financial Position and Need for Additional Capital". argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Liquidity and Capital Resources 217

5.5.4 Cash Investment Policy The Company has adopted a policy whereby cash and cash equivalents and current financial assets are invested with several highly reputable banks and financial institutions. The main purpose of the Cash Investment Policy is to preserve the available cash and to ensure sufficient short-term liquidity at all times. Therefore, the Company holds its cash, cash equivalents and current financial assets mainly with banks which are independently rated A- or higher. Amounts of cash held with banks rated lower than A- are limited to insignificant balances. The maximum amount and tenor of time deposits depends on the rating of the counterparty bank. The Company also holds cash equivalents in the form of money market funds with a low historical volatility. These money market funds are highly liquid investments and can be readily convertible into a known amount of cash. The Company has adopted a policy whereby money market funds must have a minimum rating of A of which 95% should have a AAA-rating. For more information as to our treasury policy and liquidity, please see “Note 25 Financial Risk Management” in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. 5.5.5 Working capital statement As of December 31, 2024, the Company had $1.5 billion of cash and cash equivalents and $1.9 billion of current financial assets, while the shareholder equity amounted to $5.5 billion and the non-current and current Lease liabilities to $33 million and $7 million, respectively. In accordance with item 3.1 of Annex 11 of the Commission Delegated Regulation (EU) 2019/980 we make the following statement: In our opinion, the working capital of the Company is sufficient for the Company’s present requirements, at least for a period of 12 months from the date of this Annual Report. 5.6 Research and development, patents and licenses For a discussion of our research and development policies, see the “Overview” and “Results of Operations” within Section 5. 5.7 Trend information Other than as disclosed elsewhere in this Annual Report, we are not aware of any trends, uncertainties, demands, commitments or events for the current financial period that are reasonably likely to have a material effect on our net revenues, income, profitability, liquidity, capital resources or prospects, or that caused the disclosed financial information to be not necessarily indicative of future operating results or financial conditions. There has been no significant change in the financial performance or the financial position of the Group since the balance sheet date of December 31, 2024. For more information, please refer to “Overview”, “Operating Results”,“Liquidity and Capital Resources” within Section 5 and to ‘‘Note 28 Commitments’’ in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Liquidity and Capital Resources 218

5.8 Off-Balance Sheet Arrangements We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the applicable rules and regulations, such as relationships with unconsolidated entities or financial partnerships, which are often referred to as structured finance or special purpose entities, established for the purpose of facilitating financing transactions that are not required to be reflected on our balance sheets. 5.9 Contractual Obligations For a discussion of contractual obligations, please see ‘‘Note 28 Commitments” in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. 5.10 Information Regarding the Independent Auditor The audited consolidated financial statements as of and for the year ended December 31, 2024 and 2023 and 2022 have been audited by our independent auditor, Deloitte Accountants B.V. (Deloitte), who rendered an unqualified audit report on these financial statements. The partner of Deloitte who signed the auditors’ reports is a member of the Netherlands Institute of Chartered Accountants (Koninklijke Nederlandse Beroepsorganisatie van Accountants). The office of Deloitte is located at Wilhelminakade 1, 3072 AP Rotterdam, the Netherlands. 5.11 Material Contracts and Related Party Transactions 5.11.1 Material Contracts Our material contracts are described in sections “Collaborations and Licenses”. 5.11.2 Related Party Transactions Since January 1, 2024, we have not entered into any transactions with any related parties which are, as a single transaction or in their entirety, material to us other than that which is described in ‘‘Note 26 Related Party Transactions“ in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. In addition, since January 1, 2024, there has not been, nor is there currently proposed, any material transaction or series of similar material transactions to which we were or are a party in which any of the members of our Board of Directors or senior management, holders of more than 10% of any class of our voting securities, or any member of the immediate family of any of the foregoing persons, had or will have a direct or indirect material interest, other than the compensation and shareholding arrangements we describe in section “Share Classes and Principal Shareholders”, and the transactions we describe below. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Off-Balance Sheet Arrangements 219

Transactions with Related Companies From time to time, in the ordinary course of our business, we may contract for services from companies in which certain of the members of our senior management or directors may serve as director or advisor. The costs of these services are negotiated on an at arm’s length basis and none of these arrangements are material to us. Agreements with Our Senior Management Team Other than as set forth in this Annual Report, there are no arrangements or understandings in place with major shareholders, customers, suppliers or others pursuant to which any member of our Board of Directors or Senior Management Team has been appointed. We have entered into a management agreement with Tim Van Hauwermeiren as our CEO, our sole executive director. The key terms of his agreement are as follows: Tim Van Hauwermeiren Fixed-base compensation $757,680 Short-term variable compensation A target of 60% of the fixed-base compensation based on previously determined bonus targets established by the non-executive directors Pension contributions 1) $29,118 Duration Indefinite 1) Amounts shown represent pension contributions paid during the year ended December 31, 2024. We may terminate Mr. Van Hauwermeiren’s services upon 18 months’ notice, or payment of 18 months’ pro-rated base compensation in lieu of notice. Mr. Van Hauwermeiren would be entitled to the same payment in lieu of notice in the event he terminates his services with us in circumstances in which it cannot reasonably be expected for him to continue providing services to us (and after our failure to remedy such conditions after being provided at least 14 days’ notice). Mr. Van Hauwermeiren would also be entitled to payment in lieu of notice in the event he terminated his services with us in certain cases of our failure to comply with obligations under applicable law or his agreement (and after our failure to remedy such non- compliance, if non-deliberate, after being provided at least 14 days’ notice). In these cases, there will be a full acceleration of the vesting of any outstanding stock options held by Mr. Van Hauwermeiren. There will be no notice period or payment in lieu of notice in certain cases of Mr. Van Hauwermeiren’s failure to comply with obligations under applicable law or his agreement. Mr. Van Hauwermeiren may be dismissed immediately as an executive director. Karl Gubitz, our chief financial officer, has an employment contract with our subsidiary, argenx US Inc., for an indefinite term. Karen Massey, our chief operating officer has an employment contract with our subsidiary, argenx Switzerland SA, for an indefinite term. Peter Ulrichts, our chief scientific officer has an employment contract with our subsidiary, argenx BV, for an indefinite term. Arjen Lemmen, our vice president corporate development and strategy, has an employment contract with our subsidiary, argenx BV, for an indefinite term. We may terminate his employment contract at any time, subject to a notice period and a severance payment of at least 12 months. Mr. Lemmen entered into a secondment agreement with argenx BV, under which Mr. Lemmen was seconded from argenx BV to argenx US Inc. in the U.S. from August 2022 until July 2024. In connection with his secondment, Mr. Lemmen received a housing, a schooling and a cost of living allowance. Andria Wilk, our global head of quality, has an employment contract with our subsidiary, argenx BV, for an indefinite term. Malini Moorthy, our general counsel has an employment contract with our subsidiary, argenx US, for an indefinite term. Ms. Moorthy has also entered into a secondment agreement with argenx US, under which argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Material Contracts and Related Party Transactions 220

Ms. Moorthy was seconded from argenx US to argenx BV and was based in Belgium for the period of April 1, 2023 through December 31, 2024. This secondment was extended through December 31, 2026. Luc Truyen, our head of research and development management operations and our chief medical officer, has an employment contract with our subsidiary, argenx US Inc. for an indefinite term. Mr. Truyen entered into a secondment agreement with argenx US Inc., under which Mr. Truyen has been seconded from argenx US Inc. to argenx BV and is based in Belgium for the period of April 1, 2022 through November 30, 2026 (unless otherwise extended by the parties). Indemnification Agreements In connection with our initial U.S. public offering, we entered into indemnification agreements with each of our non-executive directors and each member of our Senior Management Team. We have entered into such agreements with each new non-executive director or member of our senior management when they have joined us since our initial U.S. public offering. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to non-executive directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. Related Party Transactions Policy In connection with our initial U.S. public offering, we entered into a related party transaction policy. Our Code of Conduct and our Board Rules also include specific rules of transactions with related parties. Property, plants and equipment Our principal executive, operational offices and laboratory space located in Zwijnaarde, Belgium. In 2024, we added new office space in Zwijnaarde. The total future cash flows related to these leases are represented below in Note 21 Leases in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. We also lease office space in Amsterdam (the Netherlands), Boston (U.S.), Tokyo (Japan), Geneva (Switzerland), Munich (Germany), Issy-Les-Moulineaux (France), Vaughan, Ontario (Canada), Gerrards Cross (UK) and Milan (Italy). In addition, our lease liabilities include a lease plan for company cars with maturity dates up to four years. For a discussion of contractual obligations, please see Note 28 Commitments in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2024. Below are the key facilities worldwide leased as of December 31, 2024: Facility location Use Approx. size (m2) Lease expiry Zwijnaarde, Belgium (leased) Operations and Laboratory Space 4,951 September 30, 2031 Zwijnaarde, Belgium (leased) Office Space 3,765 March 31, 2037 Boston, Massachusetts (leased) Office Space 2,379 August 31, 2030 Tokyo, Japan (leased) Office Space 546 January 17, 2027 Environment, Health and Safety Our primary research and development activities take place in our facilities in Zwijnaarde, Belgium. For these activities we require, and have obtained, the necessary environmental and biohazard permits from the responsible governments, required by us for the manner in which we use said facilities. See section “Risk Factors”. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Material Contracts and Related Party Transactions 221

5.12 Employees As of December 31, 2024, we had 1,599 employees and 774 consultants, which we refer to as “contingent workers”. At each date shown below, we had the following number of employees, broken out by department and geography. As of December 31, 2024 2023 2022 Function: Selling, general and administrative 955 495 476 Research and development 644 653 367 Total 1,599 1,148 843 Geography: U.S. 694 454 340 Belgium 466 355 363 Japan 139 116 75 Switzerland 49 28 15 UK 44 37 – Germany 41 25 11 France 38 40 11 The Netherlands 34 22 – Italy 33 27 – Spain 32 20 – Canada 19 16 5 Rest of the World/Remote 10 8 23 Total 1,599 1,148 843 Collective bargaining agreements (CBAs) can be entered into in Belgium at the national, industry, or company levels. These CBAs are binding on both employers and employees. We have no trade union representation or CBAs at the company level, but we are subject to the national and chemical industry CBAs. The CBAs currently applicable to us relate to employment conditions such as wages, working time, job security, innovation and supplementary pensions. We have not had, and do not anticipate having, disputes on any of these subjects. CBAs may, however, change the employment conditions of our employees in the future and hence adversely affect our employment relationships. 5.13 Insurance We maintain an insurance portfolio that is common and appropriate for our business. Our main insurances are commercial general liability insurances, including products liability insurance, director and officer liability insurance and our maritime insurance covering the risk of loss of product during transit and storage. 5.14 Legal and Arbitration Proceedings From time to time we may become involved in legal, governmental or arbitration proceedings or be subject to claims arising in the ordinary course of our business. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors. During the previous 12 months, there have not been any legal, governmental or arbitration proceedings (including any such proceedings which are pending or threatened of which we are aware) which may have, or have had in the recent past, significant effects on argenx and/or the Group’s financial position or profitability. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Employees 222

5.15 Taxation This summary does not consider your particular circumstances. We urge you to consult your own independent tax advisors about the income, capital gains and/or transfer tax consequences to you in light of your particular circumstances of purchasing, holding and disposing of ordinary shares or ADSs. 5.15.1 U.S. Federal Income Tax Considerations The following discussion is a summary under present law of certain material U.S. federal income tax considerations relating to the ownership and disposition of ADSs by a U.S. holder (as defined below). This summary addresses only the U.S. federal income tax considerations for U.S. holders that hold ADSs as capital assets (generally, property held for investment) and use the U.S. dollar as their functional currency. This summary does not address all U.S. federal income tax matters that may be relevant to a particular U.S. holder and is not a substitute for tax advice. This summary does not address tax considerations applicable to a holder of ADSs that may be subject to special tax rules including, without limitation, banks, financial institutions or insurance companies, brokers, dealers or traders in securities, currencies, commodities, or notional principal contracts, traders in securities that elect to mark-to-market, tax-exempt entities or organizations, including “individual retirement accounts” or “Roth IRAs”, real estate investment trusts, regulated investment companies, persons that hold the ADSs as part of a “hedging,” “integrated” or “conversion” transaction or as a position in a “straddle”, partnerships (including entities or arrangements classified as partnerships for U.S. federal income tax purposes) or other pass-through entities (including S- corporations), or persons that will hold the ADSs through such an entity, certain former citizens or long- term residents of the United States, persons that received the ADSs as compensation for the performance of services, persons subject to special tax accounting rules as a result of any item of gross income with respect to the shares being taken into account in an applicable financial statement, and holders that own directly, indirectly, or through attribution 10% or more of the voting power or value of our ordinary shares and ADSs. This summary does not address U.S. federal taxes other than the income tax (such as the Medicare surtax on net investment income, the estate, gift, or alternative minimum tax), any election to apply Section 1400Z-2 of the U.S. Internal Revenue Code of 1986, as amended (the Code) to gains recognized with respect to ADSs, or any U.S. state, local, or non-U.S. tax considerations of the ownership and disposition of ADSs. For the purposes of this summary, a “U.S. holder” is a beneficial owner of ADSs that is (or is treated as), for U.S. federal income tax purposes, (i) an individual who is a citizen or resident of the United States, (ii) a corporation, or any other entity treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof, or the District of Columbia, (iii) an estate, the income of which is subject to U.S. federal income taxation regardless of its source, or a trust, if a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons have the authority to control all of the substantial decisions of such trust. If a partnership (or other entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds ADSs, the U.S. federal income tax consequences relating to an investment in those ADSs will depend in part upon the status of the partner and the activities of the partnership. A partnership that holds ADSs should consult its tax advisor regarding the U.S. federal income tax considerations for it and for its partners of owning and disposing of ADSs in its and their particular circumstances. In general, a U.S. holder that owns ADSs will be treated as the beneficial owner of the underlying shares represented by those ADSs for U.S. federal income tax purposes. Accordingly, no gain or loss will generally be recognized if a U.S. holder exchanges ADSs for the underlying shares represented by those ADSs. Persons considering an investment in the ADSs should consult their own tax advisors as to the particular tax consequences applicable to them relating to the ownership and disposition of ADSs, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 223

Distributions Although we do not currently plan to pay dividends, and subject to the discussion under “Passive Foreign Investment Company Considerations” below, the gross amount of distributions paid with respect to our ordinary shares including Dutch or Belgian tax withheld therefrom, if any (other than pro rata distribution), generally will be included in a U.S. holder’s gross income as foreign source ordinary dividend income when actually or constructively received to the extent such distribution is paid out of our current and accumulated earnings and profits as determined under U.S. federal income tax principles. Distributions in excess of our current and accumulated earnings and profits will be treated as a non-taxable return of capital and will be applied against and reduce, the U.S. holder’s adjusted tax basis in ADSs (but not below zero) and distributions in excess of earnings and profits and a U.S. holder’s adjusted tax basis will generally be taxable to the U.S. holder as either long-term or short-term capital gain depending upon whether the U.S. holder has held the ADSs for more than one year as of the time such distribution is received. However, since we do not calculate our earnings and profits under U.S. federal income tax principles, it is expected that any distribution will be reported as a dividend, even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain. Our dividends will not be eligible for the dividends-received deduction generally allowed to U.S. corporations. Dividends paid to non-corporate U.S. holders that satisfy a minimum holding period (during which they are not protected from the risk of loss) and certain other requirements may qualify for the preferential favorable tax rates applicable to qualified dividend income, provided that we are a “qualified foreign corporation” and we are not a PFIC as to the non-corporate U.S. holder in the taxable year of the dividend or the preceding taxable year. A qualified foreign corporation includes a non-U.S. corporation that is eligible for the benefits of a comprehensive income tax treaties with the United States. A non-U.S. corporation also will be considered to be a qualified foreign corporation with respect to any dividend it pays on shares which are readily tradable on an established securities market in the United States. Our ADSs are listed on Nasdaq, which is an established securities market in the United States, and we expect our ADSs to be readily tradable on Nasdaq. However, there can be no assurance that the ADSs will be considered readily tradable on an established securities market in the United States in any taxable year. U.S. holders should consult their own tax advisors regarding the application of these rules given their particular circumstances. If dividends are subject to Dutch or Belgian withholding tax, a U.S. holder may be entitled, subject to generally applicable limitations, to claim a U.S. foreign tax credit for Dutch or Belgian withholding tax imposed at the appropriate rate. U.S. holders who do not elect to claim a credit for any foreign income taxes paid or accrued during the taxable year may instead claim a deduction of such taxes. The rules relating to the foreign tax credit are complex and recent changes to the foreign tax credit rules that apply to foreign taxes paid or accrued in taxable years beginning after December 27, 2021 introduced additional requirements and limitations. Each U.S. holder should consult its own tax advisors regarding the foreign tax credit rules. In general, the amount of a distribution paid to a U.S. holder in a foreign currency will be the dollar value of the foreign currency calculated by reference to the applicable exchange rate on the day the U.S. holder receives the distribution, regardless of whether the foreign currency is converted into USDs at that time. Any foreign currency gain or loss a U.S. holder realizes on a subsequent conversion of foreign currency into USDs will be U.S. source ordinary income or loss. If dividends received in a foreign currency are converted into USDs on the day they are received, a U.S. holder should not be required to recognize foreign currency gain or loss in respect of the dividend. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 224

Sale, Exchange or Other Taxable Disposition of ADSs Subject to the discussion under “Passive Foreign Investment Company Considerations” below, a U.S. holder will generally recognize capital gain or loss on the sale, exchange or other taxable disposition of ADSs in an amount equal to the difference between the amount realized from such sale or exchange and the U.S. holder’s adjusted basis in the ADSs, each amount determined in USD. The adjusted tax basis in ADSs generally will be equal to the USD cost of such ADSs. Any such capital gain or loss generally will be long- term capital gain or loss if the U.S. holder’s holding period for such ADSs exceeds one year as of the date of sale or other disposition. Long-term capital realized by a non-corporate U.S. holder is generally eligible for a preferential reduced rates. The deductibility of capital losses for U.S. federal income tax purposes is subject to certain limitations. Any such gain or loss that a U.S. holder recognizes generally will be treated as U.S. source income or loss for foreign tax credit limitation purposes. Passive Foreign Investment Company Considerations In general, a non-U.S. corporation will be classified as a passive foreign investment company, or PFIC, for any taxable year in which, after applying certain look-through rules with respect to certain dividends, rents, interest or royalties received from its affiliates and taking into account its proportionate share of the income and assets of its 25% or more owned subsidiaries, either: (i) at least 75% of its gross income is “passive income” or (ii) at least 50% of the average quarterly value of its total gross assets is attributable to cash in excess of working capital requirements or assets that produce “passive income” or are held for the production of “passive income”. Passive income for this purpose generally includes dividends, interest, royalties, rents, gains from commodities and securities transactions, the excess of gains over losses from the disposition of assets which produce passive income. While we are treated as a publicly traded company for these purposes, the value of our assets, including goodwill and other intangibles, will be based on their fair market value, which will depend on the market value of our ordinary shares and ADSs, which are subject to change. Based on our historic and anticipated operations, the composition of our income and the projected composition and estimated fair market values of our assets, we do not believe that we were a PFIC for our most recent taxable year and do not expect to be classified as a PFIC for the current taxable year or for the foreseeable future. However, our possible status as a PFIC is a factual determination made annually after the close of each taxable year and, therefore, may be subject to change. Accordingly, there can be no assurance that we will not be a PFIC for any year in which a U.S. holder holds ADSs. The Company does not intend to provide any annual assessments of its PFIC status. If we were to be classified as a PFIC for any taxable year during which a U.S. holder owns ADSs, gain recognized on a sale or other disposition (including certain pledges) of such U.S. holder’s ADSs would be allocated ratably over such U.S. holder’s holding period. Amounts allocated to the taxable year of the sale or disposition and to any year before we became a PFIC would be taxed as ordinary income and the amount allocated to each other taxable year would be subject to tax at the highest rate in effect for individuals or corporations, as appropriate, for that taxable year, and an interest charge will be imposed on the resulting tax liability for each such year. In addition, to the extent that distributions received by a U.S. holder on its ADSs in any taxable year exceed 125% of the average of the annual distributions on such holder’s ADSs received during the preceding three taxable years (or, if shorter, the U.S. holder’s holding period), such excess distributions will be subject to taxation in the same manner. Furthermore, dividends that are not excess distributions would not be eligible for the preferential tax rate applicable to qualified dividend income received by individuals and certain other non-corporate persons. If the Company is a PFIC for any taxable year during which you own ADSs, the Company will generally continue to be treated as a PFIC with respect to you for all succeeding years during which you own the ADSs, even if the Company ceases to meet the threshold requirements for PFIC status. Certain elections may be available that will result in alternative treatments (such as mark-to-market treatment) of the Shares. U.S. holders should consult their own tax advisors concerning the Company’s possible PFIC status and the consequences to them if the Company were a PFIC for any taxable year, including whether any of these elections will be available, and, if so, what the consequences of the alternative treatments will be in your particular circumstances. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 225

Backup Withholding and Information Reporting U.S. holders generally will be subject to information reporting requirements with respect to dividends on ADSs and on the proceeds from the sale, exchange or disposition of the ADSs that are paid within the United States or through U.S.- related financial intermediaries, unless the U.S. holder is a corporation or other “exempt recipient.” In addition, U.S. holders may be subject to backup withholding on such payments, unless the U.S. holder provides a correct taxpayer identification number and a duly executed IRS Form W-9 or otherwise establishes an exemption. Backup withholding is not an additional tax, and the amount of any backup withholding will be allowed as a credit against a U.S. holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS. Foreign Asset Reporting Certain U.S. holders who are individuals and certain entities controlled by individuals may be required to report information relating to an interest in ADSs, subject to certain exceptions (including an exception for shares held in accounts maintained by U.S. financial institutions) by filing IRS Form 8938 (Statement of Specified Foreign Financial Assets) with their federal income tax return. Investors who fail to report required information could become subject to substantial penalties. U.S. holders are urged to consult their tax advisors regarding their information reporting obligations, if any, with respect to their ownership and disposition of the ADSs. 5.15.2 Material Dutch Tax Consequences The following summary outlines certain material Dutch tax consequences in connection with the acquisition, ownership and disposal of the ADSs. All references in this summary to the Netherlands and Dutch law are to the European part of the Kingdom of the Netherlands and its law, respectively, only. The summary does not purport to present any comprehensive or complete picture of all Dutch tax aspects that could be of relevance to the acquisition, ownership and disposal of the ADSs by a (prospective) holder of the ADSs. Depending on the particular situation of a holder of ADSs, this summary may not describe all potentially relevant Dutch tax consequences in light of such a holder of ADSs’ (specific) circumstances. The summary is based on the tax laws and practice of the Netherlands as in effect on the date of this Annual Report, which are subject to changes that could prospectively or retrospectively affect the Dutch tax consequences. This summary does not address the Dutch tax consequences for a holder of ADSs that is considered to be affiliated (gelieerd) to the Company within the meaning of the Dutch Withholding Tax Act 2021 (Wet bronbelasting 2021). Generally, a holder of ADSs is considered to be affiliated to the Company for these purposes if (i) it has a qualifying interest in the Company, (ii) the Company has a qualifying interest in such party, or (iii) a third party has a qualifying interest in both the Company and such party. A party is equated with any qualifying unity (kwalificerende eenheid) of parties of which it forms part. A qualifying unity is defined as entities that have been established and/or are acting jointly with the primary purpose, or one of the primary purposes, to avoid the imposition of tax on one or more of such entities, for example where the controlling interest (to be) held is divided into various non-controlling interests with the primary purpose, or one of the primary purposes, to avoid the aforementioned tax. A qualifying interest is an interest that allows the holder to have a decisive influence over the other party’s decisions, in such a way that it is able to determine the activities of the other party. A party is in any case considered to have a qualifying interest in another party if it (directly or indirectly) owns more than 50 per cent. of the voting rights in such other party. For purposes of Dutch income and corporate income tax, shares, or certain other assets, which may include depositary receipts in respect of shares, legally owned by a third party such as a trustee, foundation or similar entity or arrangement, a “Third Party”, may under certain circumstances have to be allocated to the (deemed) settlor, grantor or similar originator, the “Settlor”, or, upon the death of the Settlor, such Settlor’s beneficiaries, the “Beneficiaries”, in proportion to their entitlement to the estate of the Settlor of such trust or similar arrangement, the “Separated Private Assets”. The summary does not address the Dutch tax consequences of a holder of the ADSs who is an individual and who has a substantial interest (aanmerkelijk belang) in the Company. Generally, a holder of the ADSs will have a substantial interest in the Company if such holder of the ADSs, whether alone or together with argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 226

such holder’s spouse or partner and/or certain other close relatives, holds directly or indirectly, or as Settlor or Beneficiary of Separated Private Assets (i) (x) the ownership of, (y) certain other rights, such as usufruct, over, or (z) rights to acquire (whether or not already issued), shares (including the ADSs) representing 5% or more of the total issued and outstanding capital (or the issued and outstanding capital of any class of shares) of the Company or (ii) (x) the ownership of, or (y) certain other rights, such as usufruct over, profit participating certificates (winstbewijzen) that relate to 5% or more of the annual profit of the Company or to 5% or more of the liquidation proceeds of the Company. In addition, a holder of the ADSs has a substantial interest in the Company if such holder, whether alone or together with such holder’s spouse or partner and/or certain other close relatives, has the ownership of, or other rights over, shares, or depositary receipts in respect of shares, in, or profit certificates issued by, the Company that represent less than 5% of the relevant aggregate that either (a) qualified as part of a substantial interest as set forth above and where shares, or depositary receipts in respect of shares, profit certificates and/or rights there over have been, or are deemed to have been, partially disposed of, or (b) have been acquired as part of a transaction that qualified for non-recognition of gain treatment. Furthermore, this summary does not address the Dutch tax consequences of a holder of the ADSs who: • is an individual and receives income or realizes capital gains in respect of the ADSs in connection with such holder’s employment activities or in such holder’s capacity as (former) board member or (former) supervisory board member; • is a resident of any non-European part of the Kingdom of the Netherlands; or • falls within the scope of the Dutch Minimum Taxation Act 2024 (Wet minimumbelasting 2024). Dividend Withholding Tax General The Company is generally required to withhold dividend withholding tax imposed by the Netherlands at a rate of 15% on dividends distributed by the Company in respect of our ordinary shares underlying the ADSs. The expression “dividends distributed by the Company” as used herein includes, but is not limited to: (a) distributions in cash or in kind, deemed and constructive distributions and repayments of paid-in capital (gestort kapitaal) not recognized for Dutch dividend withholding tax purposes; (b) liquidation proceeds, proceeds of redemption of our ordinary shares or, as a rule, consideration for the repurchase of our ordinary shares by the Company in excess of the average paid-in capital recognized for Dutch dividend withholding tax purposes; (c) the par value of our ordinary shares issued to a holder of our ordinary shares or an increase of the par value of our ordinary shares, to the extent that it does not appear that a contribution, recognized for Dutch dividend withholding tax purposes, has been made or will be made; and (d) partial repayment of paid-in capital, recognized for Dutch dividend withholding tax purposes, if and to the extent that there are net profits (zuivere winst), unless (i) the shareholders at a General Meeting have resolved in advance to make such repayment and (ii) the par value of our ordinary shares concerned has been reduced by an equal amount by way of an amendment of our articles of association. Holders of the ADSs Resident in the Netherlands A holder of the ADSs that is an individual that is resident or deemed to be resident in the Netherlands for Dutch tax purposes is generally entitled, subject to the anti-dividend stripping rules described below, to a full credit against its income tax liability, or a full refund, of the Dutch dividend withholding tax. A holder of the ADSs that is a legal entity that is resident or deemed to be resident in the Netherlands for Dutch tax purposes is generally entitled, subject to the anti-dividend stripping rules described below, to a full credit against its corporate income tax liability of the Dutch dividend withholding tax. If and to the extent such legal entity cannot credit the full amount of Dutch dividend withholding tax in a given year, the Dutch dividend withholding tax may be carried forward and credited against its corporate income tax liability in subsequent years (without time limitation). argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 227

A holder of the ADSs that is a legal entity that is resident or deemed to be resident in the Netherlands for Dutch tax purposes that is exempt from Dutch corporate income tax but that is not qualifying exempt investment institution (vrijgestelde beleggingsinstelling), is generally entitled, subject to the anti-dividend stripping rules described below, to an exemption at source (subject to the completion of necessary procedural formalities) or a full refund of Dutch dividend withholding tax on dividends received. The same generally applies to holders of the ADSs that are neither resident nor deemed to be resident in the Netherlands for Dutch tax purposes if the ADSs are attributable to a permanent establishment in the Netherlands of such non-resident holder. Holders of the ADSs Resident Outside the Netherlands A holder of the ADSs that is resident in a country for tax purposes with which the Netherlands has a tax treaty in effect, may, depending on the terms of such tax treaty and subject to the anti-dividend stripping rules described below, be eligible for a full or partial exemption from, or full or partial refund of, Dutch dividend withholding tax on dividends received. A holder of the ADSs, that is a legal entity (a) tax resident in (i) an EU Member State, (ii) Iceland, Norway or Liechtenstein, or (iii) a country with which the Netherlands has concluded a tax treaty that includes an article on dividends and (b) that is in its state of residence under the terms of a tax treaty concluded with a third state, not considered to be resident for tax purposes in a country with which the Netherlands has not concluded a tax treaty that includes an article on dividends (i.e., not an EU Member State, Iceland, Norway or Liechtenstein), is generally entitled, subject to the anti-abuse rules and the anti-dividend stripping rules described below, to a full exemption from Dutch dividend withholding tax on dividends received if it holds an interest of at least 5% (in shares or, in certain cases, in voting rights) in the Company or if it holds an interest of less than 5%, in either case where, had the holder of the ADSs been a Dutch resident, it would have had the benefit of the participation exemption (this may include a situation where another related party holds an interest of 5% or more in the Company). The full exemption from Dutch dividend withholding tax on dividends received by a holder of the ADSs, that is a legal entity (a) tax resident in (i) an EU Member State, (ii) Iceland, Norway or Liechtenstein, or (iii) a country with which the Netherlands has concluded a tax treaty that includes an article on dividends is not granted if (x) the interest held by such holder (i) is held with the avoidance of Dutch dividend withholding tax of another person as (one of) the main purpose(s) and (ii) forms part of an artificial structure or series of structures (such as structures which are not put into place for valid business reasons reflecting economic reality), or (y) the holder of ADSs has a similar function to a qualifying investment institution (fiscale beleggingsinstelling) or a qualifying exempt investment institution (vrijgestelde beleggingsinstelling). A holder of the ADSs, that is an entity tax resident in (i) an EU Member State or (ii) Iceland, Norway or Liechtenstein, or (iii) in a jurisdiction which has an arrangement for the exchange of tax information with the Netherlands (and such holder as described under (iii) holds the ADSs as a portfolio investment (i.e., such holding is not acquired with a view to the establishment or maintenance of lasting and direct economic links between the holder of the ADSs and the Company and does not allow the holder of the ADSs to participate effectively in the management or control of the Company)), which is exempt from tax in its country of residence and does not have a similar function to a qualifying investment institution (fiscale beleggingsinstelling) or a qualifying exempt investment institution (vrijgestelde beleggingsinstelling), and that would have been exempt from Dutch corporate income tax if it had been a resident of the Netherlands, is generally entitled, subject to the anti-dividend stripping rules described below, to an exemption at source (subject to the completion of necessary procedural formalities) or a full refund of Dutch dividend withholding tax on dividends received. This exemption of full refund will in general benefit certain foreign pension funds, government agencies and certain government controlled commercial entities. No exemption, reduction, credit or refund of Dutch dividend withholding tax will be granted if the recipient of the dividend paid by the Company is not considered the beneficial owner (uiteindelijk gerechtigde) of the dividend. A recipient of a dividend is in any case not considered the beneficial owner of the dividend pursuant to the anti-dividend stripping rules if, as a consequence of a combination of transactions and tested at group level, (i) a person (other than the holder of the dividend coupon), directly or indirectly, partly or wholly benefits from the dividend, (ii) such person directly or indirectly retains or acquires a comparable interest in the ADSs, and (iii) such person is entitled to a less favorable exemption, refund or credit of dividend withholding tax than the recipient of the dividend distribution. The term “combination of argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 228

transactions” includes transactions that have been entered into by parties related to the recipient of the dividend, that have been entered into in the anonymity of a regulated stock market, the sole acquisition of one or more dividend coupons and the establishment of short-term rights or enjoyment on the ADSs (e.g., usufruct). The burden of proof to demonstrate that the recipient of a dividend qualifies as the beneficial owner of such dividend lies with the recipient, unless the amount of the withheld dividend withholding tax in respect of such recipient in the relevant calendar is €1,000 or less. Holders of the ADSs Resident in the U.S. Dividends distributed by the Company to U.S. resident holders of the ADSs that are eligible for benefits under the Convention between the Netherlands and the U.S. for the avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to Taxes and Income, dated December 18, 1992 as amended by the protocol of March 8, 2004 (U.S. Tax Treaty), generally will be entitled to a reduced dividend withholding tax rate of 5% in case of certain U.S. corporate shareholders owning at least 10% of the Company’s total voting power. Certain U.S. pension funds and tax-exempt organizations may qualify for a complete exemption from Dutch dividend withholding tax. Under the U.S. Tax Treaty such benefits are generally available to U.S. residents if such resident is the beneficial owner of the dividends, provided that such shareholder does not have an enterprise or an interest in an enterprise that is, in whole or in part, carried on through a permanent establishment or permanent representative in the Netherlands and to which enterprise or part of an enterprise the ADSs are attributable. A person may, however, not claim the benefits of the U.S. Tax Treaty if such person’s entitlement to such benefits is limited by the provisions of Article 26 (the limitation on benefits provision) of the U.S. Tax Treaty. The reduced dividend withholding tax rate can generally be applied at source upon the distribution of the dividends, provided that the proper forms have been filed in advance of the distribution. In the case of certain tax-exempt organizations, as a general rule, the so-called refund method applies; only when certain administrative conditions have been fulfilled may such tax-exempt organization use the exemption method. Irrespective of meeting the conditions of the relevant provisions of the U.S. Tax Treaty, dividends distributed by the Company to a U.S. resident holder (i) who is a legal entity resident in the U.S. and (ii) that is in the U.S. under the terms of a tax treaty with a third state not considered to be resident for tax purposes in a country with which the Netherlands has not concluded a tax treaty that includes an article on dividends (not being an EU Member State, Iceland, Norway or Liechtenstein), are generally, subject to the anti-dividend stripping rules described above, fully exempt from Dutch dividend withholding tax if the U.S. resident holder of ADSs holds an interest of at least 5% in the Company or if it holds an interest of less than 5%, in either case where, had the holder of ADSs been a Dutch resident, it would have had the benefit of the participation exemption (this may include a situation where another related party holds an interest of 5% or more in the Company). The full exemption from Dutch dividend withholding tax on dividends received by a U.S. holder of ADSs that is a legal entity is however not granted if (x) the interest held by such U.S. holder (i) is held with the avoidance of Dutch dividend withholding tax of another person as (one of) the main purpose(s) and (ii) forms part of an artificial structure or series of structures (such as structures which are not put into place for valid business reasons reflecting economic reality) or (y) the U.S. holder of ADSs has a similar function to a qualifying investment institution (fiscale beleggingsinstelling) or a qualifying exempt investment institution (vrijgestelde beleggingsinstelling). Taxes on Income and Capital Gains Holders of the ADSs Resident in the Netherlands: Individuals A holder of the ADSs, who is an individual resident or deemed to be resident in the Netherlands for Dutch tax purposes will be subject to regular Dutch income tax on the income derived from the ADSs and the gains realized upon the acquisition, redemption and/or disposal of the ADSs by the holder thereof, if: (a) such holder of the ADSs has an enterprise or an interest in an enterprise, to which enterprise the ADSs are attributable; and/or (b) such income or capital gain forms “a benefit from miscellaneous activities” (resultaat uit overige werkzaamheden) which, for instance, would be the case if the activities with respect to the ADSs exceed “normal active asset management” (normaal, actief vermogensbeheer) or if income and gains are derived from the holding, whether directly or indirectly, of (a combination of) shares, debt claims or other rights argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 229

(together, a “lucrative interest” (lucratief belang)) that the holder thereof has acquired under such circumstances that such income and gains are intended to be remuneration for work or services performed by such holder (or a related person), whether within or outside an employment relation, where such lucrative interest provides the holder thereof, economically speaking, with certain benefits that have a relation to the relevant work or services. If either of the abovementioned conditions (a) or (b) applies, income derived from the ADSs and the gains realized upon the acquisition, redemption and/or disposal of the ADSs will in general be subject to Dutch income tax at the progressive rates up to 49.5%. If the abovementioned conditions (a) and (b) do not apply, a holder of the ADSs who is an individual, resident or deemed to be resident in the Netherlands for Dutch tax purposes will not be subject to taxes on income and capital gains in the Netherlands. Instead, such individual is generally taxed at a flat rate of 36% on deemed income from “savings and investments” (sparen en beleggen), which deemed income is determined on the basis of the amount included in the individual’s “yield basis” (rendementsgrondslag) at the beginning of the calendar year (minus a tax-free threshold; the yield basis minus such threshold being the tax basis). For 2025, the deemed income derived from savings and investments will be a percentage of the tax basis up to 5.88% that is determined based on the actual allocation of (i) savings, (ii) other investments, and (iii) debts/liabilities within the individual’s yield basis. The tax-free threshold for 2025 is €57,684. The percentages to determine the deemed income will be reassessed every year. A holder of the ADSs that is able to demonstrate that its tax liability is determined on the basis of the deemed income derived from savings and investments that exceeds the “actual returns” (werkelijk rendement) of such individual may under certain circumstances elect to be taxed on the basis of such “actual returns” (werkelijk rendement) iinstead. These rules are subject to ongoing litigation and may therefore change. A holder of ADSs may need to file (protective) appeals to any assessments based on these rules to benefit from any beneficial case law. Holders of the ADSs Resident in the Netherlands: Corporate Entities The income derived from the ADSs and the gains realized upon the acquisition, redemption and/or disposal of the ADSs by any holder of the ADSs that is an entity subject to corporate income tax in the Netherlands is generally subject to Dutch corporate income tax levied at a rate of 25.8% (19% over profits up to and including €200,000), unless, and to the extent that, the participation exemption (deelnemingsvrijstelling) applies. Holders of the ADSs Resident Outside the Netherlands: Individuals A holder of the ADSs who is an individual, not resident or deemed to be resident in the Netherlands will not be subject to any Dutch taxes on income derived from the ADSs and the gains realized upon the acquisition, redemption and/or disposal of the ADSs, unless: (a) such holder has an enterprise or an interest in an enterprise that is, in whole or in part, carried on through a permanent establishment (vaste inrichting) or a permanent representative (vaste vertegenwoordiger) in the Netherlands and to which enterprise or part of an enterprise, as the case may be, the ADSs are attributable; or (b) such income or capital gain forms a “benefit from miscellaneous activities in the Netherlands” (resultaat uit overige werkzaamheden in Nederland) which would for instance be the case if the activities in the Netherlands with respect to the ADSs exceed “normal active asset management” (normaal, actief ver mogensbeheer) or if such income and gains are derived from the holding, whether directly or indirectly, of (a combination of) shares, debt claims or other rights (together, a “lucrative interest” (lucratief belang)) that the holder thereof has acquired under such circumstances that such income and gains are intended to be remuneration for work or services performed by such holder (or a related person), in whole or in part, in the Netherlands, whether within or outside an employment relation, where such lucrative interest provides the holder thereof, economically speaking, with certain benefits that have a relation to the relevant work or services. If either of the abovementioned conditions (a) or (b) applies, income derived from the ADSs and the gains realized upon the acquisition, redemption and/or disposal of the ADSs will in general be subject to Dutch income tax at the progressive rates up to 49.5%. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 230

Holders of the ADSs Resident Outside the Netherlands: Legal and Other Entities A holder of the ADSs, that is not an individuals and that is not resident or deemed to be resident in the Netherlands for corporate income tax purposes, will not be subject to any Dutch taxes on income derived from the ADSs and the gains realized upon the acquisition, redemption and/or disposal of the ADSs, unless: • such holder has an enterprise or an interest in an enterprise that is, in whole or in part, carried on through a permanent establishment (vaste inrichting) or a permanent representative (vaste vertegenwoordiger) in the Netherlands and to which enterprise or part of an enterprise, as the case may be, the ADSs are attributable; or • such holder has a substantial interest (aanmerkelijk belang) in the Company, that (i) is held with the avoidance of Dutch income tax of another person as (one of) the main purpose(s) and (ii) forms part of an artificial structure or series of structures (such as structures which are not put into place for valid business reasons reflecting economic reality). If one of the abovementioned conditions applies, income derived from the ADSs and the gains realized upon the acquisition, redemption and/or disposal of the ADSs will, in general, be subject to Dutch regular corporate income tax, levied at a rate of 25.8% (19% over profits up to and including €200,000), unless, and to the extent that, with respect to a holder as described under (a), the participation exemption (deelnemingsvrijstelling) applies. Gift, Estate and Inheritance Taxes Holders of the ADSs Resident in the Netherlands Gift tax may be due in the Netherlands with respect to an acquisition of the ADSs by way of a gift by a holder of the ADSs who is resident or deemed to be resident of the Netherlands at the time of the gift. Inheritance tax may be due in the Netherlands with respect to an acquisition or deemed acquisition of the ADSs by way of an inheritance or bequest on the death of a holder of the ADSs who is resident or deemed to be resident of the Netherlands, or in case of a gift by an individual who at the date of the gift was neither resident nor deemed to be resident in the Netherlands, such individual dies within 180 days after the date of the gift, while that individual, at the time of the individual’s death, is resident or deemed to be resident in the Netherlands. For purposes of Dutch gift and inheritance tax, an individual with the Dutch nationality will be deemed to be resident in the Netherlands if such individual has been resident in the Netherlands at any time during the 10 years preceding the date of the gift or such individual’s death. For purposes of Dutch gift tax, an individual not holding the Dutch nationality will be deemed to be resident of the Netherlands if such individual has been resident in the Netherlands at any time during the 12 months preceding the date of the gift. Holders of the ADSs Resident Outside the Netherlands No gift, estate or inheritance taxes will arise in the Netherlands with respect to an acquisition of the ADSs by way of a gift by, or on the death of, a holder of the ADSs who is neither resident nor deemed to be resident of the Netherlands, unless, in the case of a gift of the ADSs by an individual who at the date of the gift was neither resident nor deemed to be resident in the Netherlands, such individual dies within 180 days after the date of the gift, while being resident or deemed to be resident in the Netherlands. Certain Special Situations For purposes of Dutch gift, estate and inheritance tax, (i) a gift by a third party will be construed as a gift by the settlor, and (ii) upon the death of the settlor, as a rule such settlor’s beneficiaries will be deemed to have inherited directly from the settlor. Subsequently, such beneficiaries will be deemed the settlor, grantor or similar originator of the separated private assets for purposes of the Dutch gift, estate and inheritance tax in case of subsequent gifts or inheritances. For the purposes of the Dutch gift and inheritance tax, a gift that is made under a condition precedent is deemed to have been made at the moment such condition precedent is satisfied. If the condition precedent is fulfilled after the death of the donor, the gift is deemed to be made upon the death of the donor. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 231

Value Added Tax No Dutch value added tax will arise in respect of or in connection with the subscription, issue, placement, allotment or delivery of the ADSs. Other Taxes and Duties No Dutch registration tax, capital tax, custom duty, transfer tax, stamp duty or any other similar documentary tax or duty, other than court fees, will be payable in the Netherlands in respect of or in connection with the subscription, issue, placement, allotment or delivery of the ADSs. Residency A holder of the ADSs will not be treated as a resident, or a deemed resident, of the Netherlands for tax purposes by reason only of the acquisition, or the holding, of the ADSs or the performance by the Company under the ADSs. 5.15.3 Material Belgian Tax Consequences The paragraphs below present a summary of certain Belgian federal income tax consequences of the ownership and disposal of ADSs by an investor. This summary does not describe the tax treatment of investors that are subject to special rules, such as banks, insurance companies, collective investment undertakings, dealers in securities or currencies, persons that hold, or will hold, ADSs as a position in a straddle, share-repurchase transaction, conversion transactions, synthetic security or other integrated financial transactions. The summary is based on laws, treaties and regulatory interpretations in effect in Belgium on the date of this Annual Report, all of which are subject to change, including changes that could have retroactive effect. Investors should appreciate that, as a result of evolutions in law or practice, the eventual tax consequences may be different from what is stated below. For the purposes of this summary, a resident investor is: • an individual subject to Belgian personal income tax (personenbelasting/impôt des personnes physiques), i.e., (i) an individual having its domicile in Belgium, (ii) when not having its domicile in Belgium, an individual having its seat of wealth in Belgium, or (iii) an individual assimilated to a resident for purposes of Belgian tax law; • a company subject to Belgian corporate income tax (vennootschapsbelasting/impôt des sociétés), i.e., a corporate entity having its principal establishment, administrative seat or effective place of management in Belgium (and that is not excluded from the scope of the Belgian corporate income tax); or • a legal entity subject to the Belgian tax on legal entities (rechtspersonenbelasting/impôt des personnes morales), i.e., a legal entity other than a company subject to Belgian corporate income tax having its principal establishment, administrative seat or effective place of management in Belgium. A non-resident investor is any person that is not a Belgian resident investor. Investors should consult their own advisors regarding the tax consequences of an investment in the ADSs in light of their particular situation, including the effect of any state, local or other national laws, treaties and regulatory interpretations thereof. Dividends For Belgian income tax purposes, the gross amount of all benefits paid on or attributed to the ADSs is generally treated as a dividend distribution. By way of exception, the repayment of capital carried out in accordance with applicable Dutch company law provisions is not treated as a dividend distribution to the extent that such repayment is imputed on fiscal capital. This fiscal capital includes, in principle, the actual paid-up statutory share capital and, subject to certain conditions, the paid-up share premiums and the cash amounts subscribed to at the time of the issue of profit-sharing certificates. However, a repayment of capital is not fully imputed on fiscal capital if the company also has certain reserves. Indeed, in such case, a reimbursement of capital is proratedly imputed on, on the one hand, fiscal capital and, on the other hand, taxed reserves (whether or not incorporated in capital) and tax-exempt reserves incorporated in capital argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 232

(according to a specific priority rule). The part imputed on the reserves is treated as a dividend distribution subject to applicable tax rules. In general, a Belgian withholding tax of (currently) 30% is normally levied on dividends by any intermediary established in Belgium that is in any way involved in the processing of the payment of non-Belgian sourced dividends (e.g., a Belgian financial institution). For this purpose, “dividends” also include the price paid in case of a redemption of ADSs (after deduction of the part of the fiscal capital represented by the redeemed ADSs) and, in the event of our liquidation, any amounts distributed in excess of the fiscal capital. However, no withholding tax will be triggered in case of a redemption which is carried out on a stock exchange and meets certain conditions. Further, the withholding tax rate is subject to such relief as may be available under applicable domestic or tax treaty provisions. Under Belgian law, non-Belgian dividend withholding tax is not creditable against Belgian income tax and is not reimbursable to the extent that it exceeds Belgian income tax. Please refer to “Taxation—Material Dutch Tax Consequences—Dividend Withholding Tax” for a description of withholding tax that may be imposed on dividends by the Netherlands. Belgian Resident Individuals For Belgian resident individuals who acquire and hold ADSs as a private investment, the Belgian dividend withholding tax fully discharges their personal income tax liability. If (and only if) the dividend income would be declared in the personal income tax return, it will be taxed at the lower of the generally applicable 30% Belgian withholding tax rate on dividends or, in case globalization is more advantageous, at the progressive personal income tax rates applicable to the taxpayer’s overall declared income. The first €859 (for income year 2025) (amount applicable per year and per taxpayer) of the reported ordinary dividend income will be exempt from tax, subject to certain conditions. For the avoidance of doubt, all reported dividends (not only dividends distributed on our ADSs) are taken into account to assess whether the said maximum amount is reached. If the dividends are reported, the Belgian dividend withholding tax levied at source may be credited against the personal income tax due and is reimbursable to the extent that it exceeds the personal income tax due, provided that the dividend distribution does not result in a reduction in value of or a capital loss on our ADSs. The latter condition is not applicable if the individual can demonstrate that it has held ADSs in full legal ownership for an uninterrupted period of 12 months prior to the payment or attribution of the dividends. Belgian resident individual investors who acquire and hold the ADSs for professional purposes must always declare the dividend income in their personal income tax return and will be taxable at the investor’s personal income tax rate increased with local surcharges. Belgian withholding tax levied may be credited against the personal income tax due and is reimbursable to the extent that it exceeds the income tax due, subject to two conditions: (i) the taxpayer must own the ADSs in full legal ownership on the dividend record date and (ii) the dividend distribution may not result in a reduction in value of or a capital loss on the ADSs. The latter condition is not applicable if the investor can demonstrate that it has held the full legal ownership of the ADSs for an uninterrupted period of 12 months prior to the payment or attribution of the dividends. Belgian Resident Companies Dividends received by Belgian resident companies are exempt from Belgian withholding tax provided that the investor satisfies the identification requirements in Article 117, §11 of the Royal Decree implementing the ITC. For Belgian resident companies, the gross dividend income (after deduction of any non-Belgian withholding tax but including any Belgian withholding tax) must be declared in the corporate income tax return and will be subject to a corporate income tax rate of 25%, except that a reduced corporate income tax rate of 20% applies to small companies and medium sized enterprises (as defined by Article 2, §1, 5°, c) bis ITC) on the first €100,000 of taxable profits (subject to certain conditions). argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 233

Belgian resident companies can generally (although subject to certain limitations) deduct 100% of the gross dividend received from their taxable income (Dividend Received Deduction) provided that at the time of a dividend payment or attribution: (i) the Belgian resident company holds ADSs representing at least 10% of our share capital or a participation with an acquisition value of at least €2,500,000 (it being understood that only one out of the two tests must be satisfied); (ii) the shares representing our share capital have been or will be held in full ownership for an uninterrupted period of at least one year; and (iii) the conditions described in Article 203 of the ITC (relating to the taxation of the underlying distributed income and the absence of abuse), or the Article 203 of the ITC Taxation Condition, are met (Conditions for Dividend Received Deduction). Conditions (i) and (ii) above are, in principle, not applicable for dividends received by an investment company within the meaning of Article 2, §1, 5°, f) ITC. The Conditions for the application of the Dividend Received Deduction Regime depend on a factual analysis and for this reason the availability of this regime should be verified upon each dividend distribution. On January 31, 2025, as part of the “Federal Government Agreement 2025-2029”, the new Belgian federal government announced that the threshold of €2,500,000 in condition (i) above will be raised to €4,000,000. In addition, for “large companies” to meet the condition (i) based on the acquisition value of their participation, such participation would also need to have the nature of a “fixed financial asset” (the possibility to meet condition (i) via a 10% participation remains applicable). Large companies are companies that, on a consolidated basis and for at least two of the last three closed accounting periods, employed an average of more than 250 full-time equivalents and exceeded one of the following threshold: (i) a turnover (excluding VAT) of €50,000,000 or (ii) a balance sheet total of €43,000,000. This change might therefore have an impact on the tax treatment of dividends received from the ADSs below the said thresholds. This change must first be adopted by the Belgian parliament before it can become law, the timing of which is uncertain but may occur before the end of 2025. Any Belgian dividend withholding tax levied at source can be credited against the ordinary Belgian corporate income tax and is reimbursable to the extent it exceeds such corporate income tax, subject to two conditions: (i) the taxpayer must own the ADSs in full legal ownership on the dividend record date and (ii) the dividend distribution does not result in a reduction in value of or a capital loss on the ADSs. The latter condition is not applicable: (i) if the taxpayer can demonstrate that it has held the ADSs in full legal ownership for an uninterrupted period of 12 months immediately prior to the payment or attribution of the dividends or (ii) if, during that period, the ADSs never belonged to a taxpayer other than a Belgian resident company or a non-resident company that has, in an uninterrupted manner, invested the ADSs in a PE in Belgium. Belgian resident Organizations for Financing Pensions For organizations for financing pensions (OFPs) i.e., Belgian pension funds incorporated under the form of an OFP (organisme voor de financiering van pensioenen/organisme de financement de pensions) within the meaning of Article 8 of the Belgian Law of October 27, 2006, dividend income is generally tax exempt. Subject to certain limitations, any Belgian dividend withholding tax levied at source may be credited against the corporate income tax due and is reimbursable to the extent that it exceeds the corporate income tax due. Belgian (or foreign) OFPs not holding the ADSs for an uninterrupted period of 60 days in full ownership results in a rebuttable presumption that the arrangement (or a series of arrangements) is not genuine (kunstmatig/pas authentique) and has been put in place for the main purpose or one of the main purposes of obtaining this withholding tax credit. The withholding tax exemption will in such case not apply and/or any Belgian dividend withholding tax levied at source on the dividends will in such case not be credited against the corporate income tax, unless counterproof is provided that the arrangement or series of arrangements are genuine. Other Belgian resident Taxable Legal Entities For taxpayers subject to the Belgian income tax on legal entities, the Belgian dividend withholding tax in principle fully discharges their income tax liability. If the dividend is paid outside Belgium without the intervention of a Belgian paying agent and without the deduction of Belgian withholding tax, the legal entity is in principle required to declare and pay the 30% withholding tax to the Belgian tax authorities. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 234

Belgian Non-Resident Individuals and Companies Dividend payments on the ADSs through a professional intermediary in Belgium will, in principle, be subject to the 30% withholding tax, unless the shareholder is resident in a country with which Belgium has concluded a double taxation agreement and delivers the requested affidavit. Non-resident investors can also obtain an exemption of Belgian dividend withholding tax if they are the owners or usufructors of the ADSs and they deliver an affidavit confirming that they have not allocated the ADSs to business activities in Belgium and that they are non-residents, provided that the dividend is paid through a Belgian credit institution, stock market company or recognized clearing or settlement institution. If the ADSs are acquired by a non-resident investor in connection with a business in Belgium, the investor must report any dividends received, which are taxable at the applicable non-resident individual or corporate income tax rate, as appropriate. Any Belgian withholding tax levied at source can be credited against the non-resident individual or corporate income tax and is reimbursable to the extent it exceeds the income tax due, subject to two conditions: (i) the taxpayer must own the ADSs in full legal ownership on the dividend record date and (ii) the dividend distribution does not result in a reduction in value of or a capital loss on the ADSs. The latter condition is not applicable if (i) the non-resident individual or the non-resident company can demonstrate that the ADSs were held in full legal ownership for an uninterrupted period of 12 months immediately prior to the payment or attribution of the dividends or (ii) with regard to non- resident companies only, if, during the said period, the ADSs have not belonged to a taxpayer other than a resident company or a non-resident company which has, in an uninterrupted manner, invested the ADSs in a Belgian PE. Non-resident companies that have invested the ADSs in a Belgian establishment can deduct up to 100% of the gross dividends included in their taxable profits if, at the date dividends are paid or attributed, the Conditions for Dividend Received Deduction are satisfied. Application of the Dividend Received Deduction depends, however, on a factual analysis to be made upon each distribution and its availability should be verified upon each distribution. Capital Gains and Losses on ADSs Belgian Resident Individuals In principle, Belgian resident individuals acquiring the ADSs as a private investment should not be subject to Belgian capital gains tax on the disposal of the ADSs; capital losses are not tax deductible. On January 31, 2025, as part of the “Federal Government Agreement 2025-2029”, the new Belgian federal government announced its intention to introduce a “general solidarity contribution” on capital gains on financial assets, including shares and ADSs. The contribution’s rate would be 10% and would apply on capital gains realized after its entry into force and only on capital gains accrued as of this date (historical capital gains remain exempt). Capital losses on financial assets would be deductible from capital gains realized in the same taxable year (without possibility of loss carry forward). The regime would include an exemption of the first €10,000 (indexed) on an annual basis. A special regime (a higher exemption and lower rates) would apply to capital gains on substantial holdings of at least 20%. The introduction of this 10% contribution may therefore materially affect the taxation of capital gains on ADSs realized by Belgian Resident Individuals. However, this change must first be adopted by the Belgian parliament before it can become law. Although the timing is unclear, the regime is currently expected to enter into force on January 1, 2026. Under the current legislation, capital gains realized in a private (i.e., non-professional) context on the transfer for consideration of shares by a private individual, are taxable at 33% (plus local surcharges) if the capital gain is deemed to be realized outside the scope of the normal management of the individual’s private estate. Capital losses are, however, not tax deductible in such event. Although we can infer from the “Federal Government Agreement 2025-2029” that this capital gains tax regime will, in principle, remain unaffected, it remains unclear how it would interact with the announced general solidarity contribution referred to above. Gains realized by Belgian resident individuals upon the redemption of the ADSs or upon our liquidation are generally taxable as a dividend. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 235

Under the current legislation, Belgian resident individuals who hold the ADSs for professional purposes are taxable at the ordinary progressive personal income tax rates (plus local surcharges) on any capital gains realized upon the disposal of the ADSs, except for shares held for more than five years, which are taxable at a separate rate of 16.5% or (if the capital gain is realized in the framework of the cessation of activities and under certain circumstances) 10% (in each case plus local surcharges). Capital losses on the ADSs incurred by Belgian resident individuals who hold the ADSs for professional purposes are in principle tax deductible. Although we can infer from the “Federal Government Agreement 2025-2029” that this capital gains tax regime will, in principle, remain unaffected, it remains unclear how it would interact with the announced general solidarity contribution referred to above. Belgian Resident Companies Belgian resident companies are normally not subject to Belgian capital gains taxation on gains realized upon the disposal of our ADSs provided that the Conditions for Dividend Received Deduction (see above under “Dividends—Belgian Resident Companies”) are met. In this respect, the announced changes to the Conditions for Dividend Received Deduction (see above under “Dividend—Belgian Resident Companies”) may affect the tax treatment of capital gains on shares for Belgian Resident Companies. If one of the Conditions for Dividend Received Deduction is not met, the capital gains realized upon the disposal of our ADSs by a Belgian resident company are taxable at the ordinary corporate income tax rate of, currently, 25%, unless the reduced corporate income tax rate of 20% on the first €100,000 of taxable profits applies (see above). Capital losses on our ADSs incurred by resident companies are as a general rule not tax deductible. Our ADSs held in the trading portfolios (handelsportefeuille/portefeuille commercial) of qualifying credit institutions, investment enterprises and management companies of collective investment undertakings which are subject to the Royal Decree of 23 September 1992 on the annual accounts of credit institutions, investment firms and management companies of collective investment undertakings (Koninklijk besluit van 23 september 1992 op de jaarrekening van de kredietinstellingen, de beleggingsondernemingen en de beheervennootschappen van instellingen voor collectieve belegging/ arrêté royal du 23 septembre 1992 relatif aux comptes annuels des établissements de crédit, des entreprises d’investissement et des sociétés de gestion d’organismes de placement collectif) are subject to a different regime. The capital gains on such shares are taxable at the ordinary corporate income tax rate of 25%. Capital losses on such shares are tax deductible. Internal transfers to and from the trading portfolio are assimilated to a realization. Capital gains realized by Belgian resident companies (both ordinary Belgian resident companies and qualifying credit institutions, investment enterprises and management companies of collective investment undertakings) upon the redemption of our ADSs or upon our liquidation are, in principle, subject to the same taxation regime as dividends. Refer to section “Taxation — Dividends”. Belgian resident OFPs OFPs are, in principle, not subject to Belgian capital gains taxation realized upon the disposal of the ADSs, and capital losses are not tax deductible. Capital gains realized by Belgian OFPs upon the redemption of ADSs or upon our liquidation will in principle be taxed as dividends. Other Belgian Taxable Legal Entities Belgian resident legal entities subject to the legal entities income tax are, in principle, not subject to Belgian capital gains taxation on the disposal of ADSs. Capital gains realized by Belgian resident legal entities upon the redemption of ADSs or upon our liquidation will in principle be taxed as dividends. Capital losses on ADSs incurred by Belgian resident legal entities are not tax deductible. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 236

Belgian Non-Resident Individuals and Companies Non-resident individuals or companies are, in principle, not subject to Belgian income tax on capital gains realized upon disposal of the ADSs, unless such ADSs are held as part of a business conducted in Belgium through a Belgian establishment. In such a case, the same principles apply as described with regard to Belgian individuals (holding the shares for professional purposes) or Belgian companies. Non-resident individuals who do not use the shares for professional purposes and who have their fiscal residence in a country with which Belgium has not concluded a tax treaty or with which Belgium has concluded a tax treaty that confers the authority to tax capital gains on the ADSs to Belgium, might be subject to tax in Belgium if the capital gains are obtained or received in Belgium and arise from transactions which are to be considered speculative or beyond the normal management of one’s private estate. Refer to Section “Taxation— Capital Gains and Losses on ADSs - Belgian Resident Individuals". Such non-resident individuals might therefore be obliged to file a tax return and should consult their own tax advisor. However, Belgium has concluded tax treaties with more than 95 countries which generally provide for a full exemption from Belgian capital gains taxation on such gains realized by residents of those countries. Capital gains realized by non-resident individuals or non-resident companies upon the redemption of ADSs or upon our liquidation will, in principle, be subject to the same taxation regime as dividends. Tax on Stock Exchange Transactions Upon the issue of the ADSs (primary market), no Tax on Stock Exchange Transactions (taks op beursverrichtingen/taxe sur opérations de bourse) is due. The purchase and the sale and any other acquisition or transfer for consideration of ADSs (secondary market transactions) is subject to the Tax on Stock Exchange Transactions if (i) it is executed in Belgium through a professional intermediary, or (ii) deemed to be executed in Belgium, which is the case if the order is directly or indirectly made to a professional intermediary established outside of Belgium, either by private individuals with habitual residence in Belgium, or legal entities for the account of their seat or establishment in Belgium (both, a Belgian Investor). The Tax on Stock Exchange Transactions is levied at a rate of 0.35% of the purchase price, capped at €1,600 per transaction and per party. A separate tax is due by each party to the transaction, and both taxes are collected by the professional intermediary. However, if the intermediary is established outside of Belgium, the tax will in principle be due by the Belgian Investor, unless that Belgian Investor can demonstrate that the tax has already been paid. Professional intermediaries established outside of Belgium can, subject to certain conditions and formalities, appoint a Belgian Stock Exchange Tax Representative, which will be liable for the Tax on Stock Exchange Transactions in respect of the transactions executed through the professional intermediary. If the Stock Exchange Tax Representative would have paid the Tax on Stock Exchange Transactions due, the Belgian Investor will, as per the above, no longer be the debtor of the Tax on Stock Exchange Transactions. No Tax on Stock Exchange Transactions is due on transactions entered into by the following parties, provided they are acting for their own account: (i) professional intermediaries described in Article 2, 9° and 10° of the Belgian Law of August 2, 2002; (ii) insurance companies described in Article 2, §1 of the Belgian Law of July 9, 1975; (iii) professional retirement institutions referred to in Article 2, 1° of the Belgian Law of October 27, 2006 concerning the supervision on institutions for occupational pension; (iv) collective investment institutions; (v) regulated real estate companies; and (vi) Belgian non-residents provided they deliver a certificate to their financial intermediary in Belgium confirming their non-resident status. The EU Commission adopted on February 14, 2013 the Draft Directive on a Financial Transaction Tax (FTT). The Draft Directive currently stipulates that once the FTT enters into force, the Participating Member States shall not maintain or introduce taxes on financial transactions other than the FTT (or VAT as provided in the Council Directive 2006/112/EC of November 28, 2006 on the common system of value added tax). For Belgium, the Tax on Stock Exchange Transactions should thus be abolished once the FTT enters into force. Due to the lack of progress in the negotiations on the Draft Directive, the European Commission announced that it would endeavor to present a proposal for a new own resource based on the FTT by June 2024 (with a view to its introduction by 1 January 2026). The European Commission has, however, not published any proposals so far. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 237

Annual Tax on Securities Accounts The Belgian Annual Tax on Securities Accounts is a subscription tax, levied on securities accounts and not on the holders thereof. A securities account is defined as an account on which financial instruments can be credited and debited. The tax applies to securities accounts held both in Belgium and abroad when the account holder is a Belgian resident or when the account forms part of the assets of a Belgian establishment of a non-Belgian resident. The tax applies to natural persons residing in Belgium, as well as to companies and legal entities (subject to the tax for legal entities) that are established in Belgium. The tax is also applicable to securities accounts held by non-Belgian residents (both natural persons and legal persons) if the securities account is held in Belgium. If the applicable double tax treaty however allocates the right to tax capital to the jurisdiction of residence, Belgium would be prevented from applying the Annual Tax on Securities Accounts to the Belgian securities accounts held by non-Belgian residents. As described above, the tax applies whether or not the account is held in Belgium if the account forms part of the assets of a Belgian establishment of a non-Belgian resident. The Annual Tax on Securities Accounts is applicable to securities accounts of which the average value of the assets amounts to more than €1,000,000 during the reference period. In principle, this reference period starts on 1 October and ends on 30 September of the following year. The aforementioned threshold is assessed on the average value of the assets in the securities account at reference points within the reference period (in principle December 31st, March 31st, June 30th and September 30th). The threshold is assessed per securities account and not per account holder. The applicable tax rate is 0.15%, which is levied on the average value of the assets held in the securities account that exceeds the €1,000,000 threshold. It is however limited to 10% of the difference between the average value and the threshold of €1,000,000, in order to avoid that the Annual Tax on Securities Accounts would result in reducing the value of the securities account below the €1,000,000 threshold. The Annual Tax is in principle withheld, reported and paid by the Belgian intermediary. If the intermediary is established outside of Belgium, the tax must in principle be reported and paid by the account holder, unless the account holder can demonstrate that the tax has already been reported and paid by an intermediary. Intermediaries established outside of Belgium can, subject to certain conditions and formalities, appoint a Belgian Annual Tax on Securities Accounts Representative, which will be liable for reporting and paying the Annual Tax on Securities Accounts in respect of securities accounts in scope of the Annual Tax that are held through such intermediaries. If the Annual Tax on Securities Accounts Representative would have paid the Annual Tax on Securities Accounts due, the account holder will, as per the above, no longer be the debtor of the Annual Tax on Securities Accounts. The Annual Tax on Securities Accounts is however not applicable to securities accounts held by certain categories of account holders active in the financial or fund sector, as listed in the relevant legislation (e.g. credit institutions, insurance companies, investment companies, and certain collective investment undertakings). These exemptions do however not apply if a non-qualifying third party has a direct or indirect claim on the value of the securities account. Prospective investors are strongly advised to seek their own professional advice in relation to the possible impact of the Annual Tax on Securities Accounts on their own personal tax position. 5.15.4 Enforcement of civil liabilities We are a European public company with limited liability (Societas Europaea or SE) incorporated under the laws of the Netherlands. A majority of our assets are located outside the U.S. As a result, it may not be possible for investors to effect service of process within the U.S. upon such persons or to enforce against them or us in U.S. courts, including judgments predicated upon the civil liability provisions of the federal securities laws of the U.S. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 238

The U.S. and the Netherlands currently do not have a treaty providing for the reciprocal recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. Consequently, a final judgment for payment given by a court in the U.S., whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in the Netherlands. In order to obtain a judgment which is enforceable in the Netherlands, the party in whose favor a final and conclusive judgment of the U.S. court has been rendered will be required to file its claim with a court of competent jurisdiction in the Netherlands. Such party may submit to the Dutch court the final judgment rendered by the U.S. court. This court will have a level of discretion in its assessment of the judgment rendered by the relevant U.S. court. On the basis of case law by the Dutch Supreme Court, Dutch courts will in principle have to give conclusive effect to a final and enforceable judgment of such court in respect of the contractual obligations thereunder without re-examination or re-litigation of the substantive matters adjudicated upon, provided that: (i) the U.S. court involved accepted jurisdiction on the basis of internationally recognized grounds to accept jurisdiction, (ii) the proceedings before such court being in compliance with principles of proper procedure (behoorlijke rechtspleging), (iii) such judgment not being contrary to the public policy of the Netherlands and (iv) such judgment not being incompatible with a judgment given between the same parties by a Netherlands court or with a prior judgment given between the same parties by a foreign court in a dispute concerning the same subject matter and based on the same cause of action, provided such prior judgment fulfills the conditions necessary for it to be given binding effect in the Netherlands. Dutch courts may deny the recognition and enforcement of punitive damages or other awards that do not fit to the Dutch legal order. Moreover, a Dutch court may reduce the amount of damages granted by a U.S. court and recognize damages only to the extent that they are necessary to compensate actual losses or damages. Enforcement and recognition of judgments of U.S. courts in the Netherlands are solely governed by the provisions of the Dutch Civil Procedure Code. Original actions or actions for the enforcement of judgments of U.S. courts relating to the civil liability provisions of the federal or state securities laws of the U.S. are not directly enforceable in Belgium. The U.S. and Belgium currently do not have a treaty providing for reciprocal recognition and enforcement of judgments, other than arbitral awards, in civil and commercial matters. Consequently, a final judgment for payment given by a court in the U.S., whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in Belgium. In order for a final judgment for the payment of money rendered by U.S. courts based on civil liability to produce any effect on Belgian soil, it is accordingly required that this judgment be recognized and be declared enforceable by a Belgian court pursuant to the relevant provisions of the PIL Code. Recognition or enforcement does not imply a review of the merits of the case and is irrespective of any reciprocity requirement. A U.S. judgment will, however, not be recognized or declared enforceable in Belgium if it infringes upon one or more of the grounds for refusal which are exhaustively listed in article 25 of the PIL Code. In addition to recognition or enforcement, a judgment by a federal or state court in the U.S. against us may also serve as evidence in a similar action in a Belgian court if it meets the conditions required for the authenticity of judgments according to the law of the state where it was rendered. In addition, with regard to enforcements by legal proceedings in Belgium (including the recognition of foreign court decisions in Belgium), a registration tax at the rate of 3% of the amount of the judgment is payable by the debtor, if the sum of money which the debtor is ordered to pay by a Belgian court, or by a foreign court judgment that is either (i) automatically enforceable and registered in Belgium, or (ii) rendered enforceable by a Belgian court, exceeds €12,500. The registration tax is payable by the debtor. The debtor is liable for the payment of the registration tax, in the proportion determined by the decision ordering payment or liquidation or determining priority for creditors made or established against it. The debtor(s) are jointly and severally liable in the event that they are ordered to pay jointly and severally. A stamp duty is payable as of the second certified copy of an enforcement judgment rendered by a Belgian court, with a maximum of €1,450. Dutch and Belgian civil procedure differ substantially from U.S. civil procedure in a number of respects. Insofar as the production of evidence is concerned, U.S. law and the laws of several other jurisdictions based on common law provide for pre-trial discovery, a process by which parties to the proceedings may prior to trial compel the production of documents by adverse or third parties and the deposition of witnesses. Evidence obtained in this manner may be decisive in the outcome of any proceeding. No such pre-trial discovery process exists under Dutch or Belgian law. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 239

Subject to the foregoing and service of process in accordance with applicable treaties, investors may be able to enforce in the Netherlands or Belgium judgments in civil and commercial matters obtained from U.S. federal or state courts. However, no assurance can be given that those judgments will be enforceable. In addition, it is doubtful whether a Dutch or Belgian court would accept jurisdiction and impose civil liability in an original action commenced in the Netherlands or Belgium and predicated solely upon U.S. federal securities laws. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Taxation 240

Financial Statements 6.1 Consolidated Financial Statements 242 6.2 Notes to the Consolidated Financial Statements 249 6.3 Company Financial Statements of argenx SE for the Year ended December 31, 2024 285 6.4 Other information 291 argenx Annual Report 2024 241 6

6 Financial Statements 6.1 Consolidated Financial Statements 6.1.1 Consolidated Statements of Financial Position As of December 31, (in thousands of $) Note 2024 2023 2022 Assets Non-current assets Property, plant and equipment 4 43,517 22,675 16,234 Intangible assets 5 181,445 125,228 174,901 Deferred tax assets 23 924,299 97,211 79,222 Research and development incentive receivables 94,854 76,706 47,488 Investment in a joint venture 26 9,268 9,912 1,323 Prepaid expenses 23,643 47,327 – Other non-current assets 6 42,393 39,662 40,894 Total non-current assets 1,319,419 418,721 360,064 Current assets Inventories 7 407,233 310,550 228,353 Prepaid expenses 8 187,948 134,072 76,022 Trade and other receivables 9 904,471 496,687 275,697 Research and development incentive receivables 4,625 2,584 1,578 Financial assets 10 1,878,890 1,131,000 1,391,808 Cash and cash equivalents 11 1,499,936 2,048,844 800,740 Total current assets 4,883,103 4,123,737 2,774,197 Total assets 6,202,522 4,542,458 3,134,261 The accompanying notes form an integral part of these consolidated financial statements. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Consolidated Financial Statements 242

As of December 31, (in thousands of $) Note 2024 2023 2022 Equity and liabilities Equity 12 Equity attributable to owners of the parent Share capital 7,227 7,058 6,640 Share premium 5,948,916 5,651,497 4,309,880 Translation differences 126,832 131,543 129,280 Accumulated losses (1,571,804) (2,404,844) (2,109,791) Other reserves 987,112 712,253 477,691 Total equity 5,498,283 4,097,507 2,813,699 Non-current liabilities Provisions for employee benefits 1,803 1,449 870 Lease liabilities 21 32,520 15,354 9,009 Deferred tax liabilities 23 – 5,155 8,406 Total non-current liabilities 34,323 21,958 18,285 Current liabilities Lease liabilities 21 6,533 4,646 3,417 Trade and other payables 14 649,993 414,013 295,679 Tax liabilities 23 13,390 4,334 3,181 Total current liabilities 669,916 422,993 302,277 Total liabilities 704,239 444,951 320,562 Total equity and liabilities 6,202,522 4,542,458 3,134,261 The accompanying notes form an integral part of these consolidated financial statements. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Consolidated Financial Statements 243

6.1.2 Consolidated Statements of Profit or Loss Year Ended December 31, (in thousands of $ except for shares and EPS) Note 2024 2023 2022 Product net sales 17 2,185,883 1,190,783 400,720 Collaboration revenue 15 4,348 35,533 10,026 Other operating income 16 61,808 42,278 34,520 Total operating income 2,252,039 1,268,594 445,267 Cost of sales 7 (227,289) (117,835) (29,431) Research and development expenses 18 (983,423) (859,492) (663,366) Selling, general and administrative expenses 19 (1,055,337) (711,905) (472,132) Loss from investment in a joint venture 26 (7,644) (4,411) (677) Total operating expenses (2,273,693) (1,693,643) (1,165,607) Operating loss (21,654) (425,049) (720,341) Financial income 22 157,509 107,386 27,665 Financial expense 22 (2,464) (906) (3,906) Exchange (losses)/gains 22 (48,211) 14,073 (32,732) Profit/(Loss) for the year before taxes 85,180 (304,496) (729,314) Income tax benefit 23 747,860 9,443 19,720 Profit/(Loss) for the year 833,040 (295,053) (709,594) Profit/(Loss) for the year attributable to: Owners of the parent 833,040 (295,053) (709,594) Weighted average number of shares outstanding 24 59,855,585 57,169,253 54,381,371 Weighted average number of shares for purpose of diluted profit/ (loss) per share 24 65,177,815 57,169,253 54,381,371 Basic profit/(loss) per share (in $) 24 13.92 (5.16) (13.05) Diluted profit/(loss) per share (in $) 24 12.78 (5.16) (13.05) The accompanying notes form an integral part of these consolidated financial statements. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Consolidated Financial Statements 244

6.1.3 Consolidated Statements of Comprehensive Income or Loss Year Ended December 31, (in thousands of $) Note 2024 2023 2022 Profit/(Loss) for the year 833,040 (295,053) (709,594) Items that may be reclassified subsequently to profit or loss, net of tax Currency translation differences, arisen from translating foreign activities (4,711) 2,263 (2,404) Items that will not be reclassified subsequently to profit or loss, net of tax Fair value gain/(loss) on investments in equity instruments designated as FVTOCI 6 (648) (1,915) (18,267) Other comprehensive profit/(loss), net of income tax (5,359) 348 (20,671) Total comprehensive profit/(loss) attributable to: Owners of the parent 827,681 (294,705) (730,266) The accompanying notes form an integral part of these consolidated financial statements. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Consolidated Financial Statements 245

6.1.4 Consolidated Statements of Cash Flows Operating profit/(loss) (21,654) (425,049) (720,341) Adjustments for non-cash items Amortization of intangible assets 5 10,282 105,674 99,766 Depreciation of property, plant and equipment 4 7,245 5,633 4,576 Provisions for employee benefits 432 573 459 Expense recognized in respect of share-based payments 13 235,179 232,974 157,026 Fair value gains on financial assets at fair value through profit or loss 6 (3,834) – (4,256) Loss from investment in a joint venture 26 7,644 4,411 677 Other non-cash (benefit)/expenses (277) 2,074 – 235,017 (73,710) (462,093) Movements in current assets/liabilities (Increase)/decrease in trade and other receivables 9 (423,112) (185,694) (222,260) (Increase)/decrease in inventories 7 (95,996) (83,030) (119,277) (Increase)/decrease in other current assets (56,154) (59,024) (18,294) Increase/(decrease) in trade and other payables 14 246,336 95,600 329 Movements in non-current assets/liabilities (Increase)/decrease in other non-current assets 6 (19,930) (29,416) (16,220) (Increase)/decrease in non-current prepaid expense 23,683 (47,327) – Net cash flows used in operating activities, before interest and taxes (90,156) (382,601) (837,815) Interest paid (392) (211) (851) Income taxes received/(paid) 7,801 (37,515) (24,141) Net cash flows used in operating activities (82,747) (420,327) (862,807) Purchase of intangible assets 5 (66,500) (43,000) (102,986) Purchase of property, plant and equipment 4 (1,801) (812) (837) Purchase of current financial assets 10 (2,183,542) (1,271,730) (1,694,046) Sale of current financial assets 10 1,429,600 1,543,999 1,325,540 Interest received 111,649 92,753 13,146 Investment in a joint venture 26 (7,000) (13,000) (2,000) Net cash flows from/(used in) investing activities (717,594) 308,210 (461,184) Principal elements of lease payments 21 (7,638) (3,801) (4,165) Proceeds from issue of new shares, gross amount 12 – 1,196,731 760,953 Issue costs paid 12 – (821) (781) Exchange (losses)/gains from currency conversion on proceeds from issue of new shares – (1,507) 410 Payment of employee withholding taxes relating to restricted stock unit awards (21,868) (12,138) (5,855) Proceeds from exercise of stock options 12 309,265 158,263 93,195 Net cash flows from financing activities 279,759 1,336,727 843,757 Increase/(decrease) in cash and cash equivalents (520,582) 1,224,610 (480,234) Cash and cash equivalents at the beginning of the year 2,048,844 800,740 1,334,676 Exchange gains/(losses) on cash and cash equivalents (28,326) 23,494 (53,702) Cash and cash equivalents at the end of the year 1,499,936 2,048,844 800,740 Year Ended December 31, (in thousands of $) Note 2024 2023 2022 The accompanying notes form an integral part of these consolidated financial statements. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Consolidated Financial Statements 246

6.1.5 Consolidated Statements of Changes in Equity Balance on January 1, 2022 6,233 3,462,775 (1,400,197) 131,684 373,019 (39,290) 2,534,224 2,534,224 Loss for the year (709,594) (709,594) (709,594) Other comprehensive income/(loss) (2,404) (18,267) (20,671) (20,671) Total comprehensive income/(loss) for the year – – (709,594) (2,404) – (18,267) (730,266) (730,266) Income tax benefit from excess tax deductions related to share-based payments 3,946 3,946 3,946 Share-based payment 158,282 158,282 158,282 Issue of share capital 294 760,659 760,953 760,953 Transaction costs for equity issue (781) (781) (781) Exercise of stock options 113 93,082 93,195 93,195 Ordinary shares withheld for payment of employees’ withholding tax liability (5,855) (5,855) (5,855) Balance on December 31, 2022 6,640 4,309,880 (2,109,791) 129,280 535,247 (57,557) 2,813,699 2,813,699 Loss for the year (295,053) (295,053) (295,053) Other comprehensive income/(loss) 2,263 (1,915) 348 348 Total comprehensive income/(loss) for the year – – (295,053) 2,263 – (1,915) (294,705) (294,705) Income tax benefit from excess tax deductions related to share-based payments 2,310 2,310 2,310 Share-based payment 234,168 234,168 234,168 Issue of share capital 288 1,196,444 1,196,732 1,196,732 Transaction costs for equity issue (821) (821) (821) Exercise of stock options 130 158,133 158,263 158,263 Ordinary shares withheld for payment of employees’ withholding tax liability (12,139) (12,139) (12,139) Balance on December 31, 2023 7,058 5,651,497 (2,404,844) 131,543 771,725 (59,472) 4,097,507 4,097,507 Profit for the year 833,040 833,040 833,040 Other comprehensive income/(loss) (4,711) (648) (5,359) (5,359) Total comprehensive income/(loss) for the year – – 833,040 (4,711) – (648) 827,681 827,681 Income tax benefit from excess tax deductions related to share-based payments 39,650 39,650 39,650 Share-based payment 235,856 235,856 235,856 (in thousands of $) Attributable to owners of the parent Share capital Share premium Accumulated losses Translation differences Share-based payment and income tax deduction on share- based payments Fair value movement on investment in equity instruments designated as at FVTOCI Total equity attributable to owners of the parent Total equity argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Consolidated Financial Statements 247

Exercise of stock options 169 319,288 319,457 319,457 Ordinary shares withheld for payment of employees’ withholding tax liability (21,869) (21,869) (21,869) Balance on December 31, 2024 7,227 5,948,916 (1,571,804) 126,832 1,047,231 (60,119) 5,498,283 5,498,283 Please refer to ‘‘Note 12 Share Capital and Share Premium’’ for more information on the share capital and movement in number of shares. See also ‘‘Note 13 Share-Based Payments’’ for more information on the share-based payments. The accompanying notes form an integral part of these consolidated financial statements. (in thousands of $) Attributable to owners of the parent Share capital Share premium Accumulated losses Translation differences Share-based payment and income tax deduction on share- based payments Fair value movement on investment in equity instruments designated as at FVTOCI Total equity attributable to owners of the parent Total equity argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Consolidated Financial Statements 248

6.2 Notes to the Consolidated Financial Statements 1. General Information about the Company argenx SE is a Dutch European public company with limited liability incorporated under the laws of the Netherlands. The company (COC 24435214) has its official seat in Amsterdam, The Netherlands, and its registered office is at Laarderhoogtweg 25, 1101 EB Amsterdam, the Netherlands. An overview of the company and its subsidiaries (the Company) are described in ‘‘Note 30 Overview of Consolidation Scope’’. argenx SE is a publicly traded company with ordinary shares listed on Euronext Brussels under the symbol “ARGX” since July 2014 and with American Depositary Shares listed on Nasdaq under the symbol “ARGX” since May 2017. 2. Material Accounting Policy Information The Company’s material accounting policies are summarized below. 2.1 Statement of compliance and basis of preparation The consolidated financial statements are prepared in accordance with the IFRS® Accounting Standards (IFRS) as adopted by the European Union (EU-IFRS) and in accordance with the legal requirements of Part 9 of Book 2 of the Dutch Civil Code. The consolidated financial statements provide a general overview of the Company’s activities and the results achieved. They present fairly the entity’s financial position, its financial performance and cash flows, on a going concern basis. The material accounting policy information applied in the preparation of the above consolidated financial statements are set out below. All amounts are presented in thousands of US dollar, unless otherwise indicated, rounded to the nearest $ ‘000. The consolidated financial statements have been approved for issue by the Company’s Board of Directors (the “Board”) on March 19, 2025. 2.2 Adoption of new and revised standards New standards and interpretations applicable for the annual period beginning on January 1, 2024 In the current year, the Group has assessed and adopted amendments to IFRS that are mandatorily effective for accounting periods that begin on or after January 1, 2024. Their adoption has not had any material impact on the disclosures or on the amounts reported in these consolidated financial statements. New standards and interpretations issued, but not yet applicable for the annual period beginning on January 1, 2024 • IFRS 18 Presentation and Disclosures in Financial Statements IFRS 18 replaces IAS 1, carrying forward many of the requirements in IAS 1 unchanged and complementing them with new requirements. In addition, some IAS 1 paragraphs have been moved to IAS 8 Accounting Policies, Changes in Accounting Estimates and Errors and IFRS 7 Financial Instruments: Disclosures. Furthermore, the IASB has made minor amendments to IAS 7 Statement of Cash Flows and IAS 33 Earnings per Share. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 249

IFRS 18 introduces new requirements to: • present specified categories and defined subtotals in the statement of profit or loss • provide disclosures on management-defined performance measures (MPMs) in the notes to the financial statements • improve aggregation and disaggregation. An entity is required to apply IFRS 18 for annual reporting periods beginning on or after January 1, 2027, with earlier application permitted. The amendments to IAS 7 and IAS 33, as well as the revised IAS 8 and IFRS 7, become effective when an entity applies IFRS 18. IFRS 18 requires retrospective application with specific transition provisions. • Amendments to the Classification and Measurement of Financial Instruments (Amendments to IFRS 9 and IFRS 7) The amendments address matters identified during the post-implementation review of the classification and measurement requirements of IFRS 9 Financial Instruments. The amendments are effective for reporting periods beginning on or after January 1, 2026. The Group continues to evaluate the impacts of the application of these amendments on the consolidated financial statements in future periods. We have not early adopted any standard, interpretation, or amendment that has been issued but is not yet effective. 2.3 Basis of consolidation The consolidated financial statements include the financial statements of the Company and entities controlled by the Company (its subsidiaries). Control is achieved when the Company: • has power over the investee; • is exposed, or has rights, to variable returns from its involvement with the investee; and • has the ability to use its power to affect its returns. The Company reassesses whether or not it controls an investee if facts and circumstances indicate that there are changes to one or more of the three elements of control listed above. The results of the subsidiaries are included in the consolidated statements of profit or loss and consolidated statements of other comprehensive income or loss from the effective date of acquisition up to the date when control ceases to exist. When necessary, adjustments are made to the financial statements of subsidiaries to bring their accounting policies into line with those used by other members of the Group. All intercompany transactions and unrealized gains on transactions between group companies are eliminated. Unrealized losses are also eliminated unless the transaction provides evidence of an impairment of the transferred asset. 2.4 Foreign currency transactions 2.4.1 Functional and presentation currency Items included in the consolidated financial statements of each of the entities are valued using the currency of their economic environment in which the entity operates. The consolidated financial statements are presented in USD ($), which is the Company’s functional and presentation currency. 2.4.2 Transactions and balances Transactions in foreign currencies are translated at the exchange rate ruling at the date of the transaction. Monetary assets and liabilities denominated in foreign currencies are translated at the exchange rate ruling at the reporting date. Foreign exchange differences arising on translation are recognized in the argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 250

consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss as “Exchange (losses)/gains”. Non-monetary assets and liabilities denominated in foreign currencies are translated at the foreign exchange rate applicable at the date of the transaction. 2.4.3 Financial statements of foreign entities For foreign entities using a different functional currency than USD: • assets and liabilities for each balance sheet presented are translated at the closing rate at the date of the balance sheet; • income and expenses for each statement presenting profit or loss and statements of other comprehensive income or loss are translated at average exchange rates. 2.5 Intangible assets 2.5.1 Internally generated intangible assets Expenditure on research activities is recognized as an expense in the period in which it is incurred. 2.5.2 Acquired In-Process R&D and Acquired R&D available for use Upfront payments and development milestone payments for “Acquired In-Process R&D” obtained through in-licensing arrangements are capitalized as intangible assets under “Acquired In-Process R&D” upon meeting the IAS 38 capitalization criteria. These intangibles are considered as intangible assets with definite useful lives and are carried at cost less amortization and accumulated impairment losses. The Company has not started to amortize “Acquired In-Process R&D” as they are not available for use until regulatory approval has been obtained, but they are evaluated for potential impairment on an annual basis or when facts and circumstances may indicate a risk of impairment. Any impairment charge is recorded in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss under “Research and development expense”. Once an asset included in “Acquired In-Process R&D” has received marketing approval from a regulatory authority, it is recorded under “Acquired R&D available for use” category. Regulatory milestone payments and sales-based milestone payments for R&D obtained through in-licensing arrangements acquired are capitalized intangible assets under “Acquired R&D available for use” upon meeting the IAS 38 capitalization criteria. All intangibles classified under “Acquired R&D available for use” are considered as intangible assets with finite useful lives and are carried at cost less accumulated amortization and accumulated impairment losses. “Acquired R&D available for use” is evaluated for potential impairment when the Company identifies indications based on facts and circumstances of the asset. Any impairment charge is recorded in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss under “Cost of sales”. “Acquired R&D available for use” is amortized under “Cost of sales” on a straight-line basis over the estimated useful life, being the longer of the current patent protection life of the acquired R&D and patent protection life of the combined product. 2.5.3 Other intangible assets Other intangible assets could include a Priority Review Voucher (PRV) which the Company can use to obtain the priority review by the FDA for one of its future regulatory submissions or may sell or transfer to a third party. The PRV is initially measured at cost and annually reviewed for impairment when events or circumstances indicate that the carrying value may not be recoverable. Any impairment charge is recorded in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss under “Research and development expenses.” Using the PRV results in amortization recorded in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss under “Research and development expenses” and subsequent derecognition of the intangible asset. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 251

2.6 Research and development incentives receivables The current and non-current research and development incentive receivables relate to refunds resulting from research and development incentives on Research and development expenses in Belgium and are credited to the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss under the line “Other operating income” when the relevant expenditure has been incurred and there is a reasonable assurance that the research and development incentives will be received. 2.7 Inventories Inventories are carried at cost or net realizable value, whichever is lowest. Cost comprises of costs of purchase, costs of conversion and other costs incurred in bringing the inventories to their present location and condition. If the expected sales price less completion costs to execute sales (net realizable value) is lower than the carrying amount, a write-down is recognized for the amount by which the carrying amount exceeds its net realizable value. Included in inventory are products which could, besides commercial activities, be used in preclinical and clinical programs, and free-of-charge, compassionate use and pre-approval access program. These products are expensed either through “Research & development expenses” or “Selling, general and administrative expenses”. We capitalize inventory costs associated with products prior to the regulatory approval of these products, or for inventory produced in production facilities not yet approved, when it is highly probable that the pre- approval inventories will be sellable. The determination to capitalize is based on the particular facts and circumstances relating to the expected regulatory approval of the product or production facility being considered. The assessment of whether or not the product is considered highly probable to be sellable is made and includes, but is not limited to, how far a particular product or facility has progressed along the approval process, any known safety or efficacy concern and other impediments. Previously capitalized costs related to pre-launch inventories could be required to be written down upon a change in such judgement or due to a denial or delay of approval by regulatory bodies, a delay in commercialization or other potential factors, which will be recorded under “Research and development expenses” in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss. 2.8 Trade and other receivables Trade and other receivables are designated as financial assets measured at amortized cost. They are initially measured either at their invoiced amounts or at transaction price, in the absence of a significant financing component less adjustments for estimated revenue deductions such as rebates, chargebacks and returns. All receivables are subsequently measured at amortized cost, which generally corresponds to nominal value less expected credit loss provision. Loss allowance for expected credit losses are established using a simplified approach of forward-looking expected credit loss model (ECL), which includes possible default events on the trade receivables over the entire holding period of the trade receivable. These provisions represent the difference between the trade receivable’s carrying amount in the consolidated statements of financial position and the estimated collectible amount. Charges for loss allowance for expected credit losses are recorded under “Selling, general and administrative expenses” in the consolidated statements of profit or loss and consolidated statements of other comprehensive income or loss. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 252

2.9 Current financial assets Current financial assets measured at amortized costs comprise of term accounts that have an initial maturity equal or less than twelve months, but exceeding three months. Current financial assets measured at fair value through profit or loss comprise of money market funds. Interests on Current financial assets are reported under Cash Flow from investment activities under “Interest received” 2.10 Cash and cash equivalents Cash are financial assets measured at amortized cost and comprise of cash at bank. Cash equivalents measured at amortized cost comprise of term accounts that have an initial maturity of less than three months that are subject to an insignificant risk of changes in values. Cash equivalents measured at fair value through profit or loss comprise of money market funds that are readily convertible to cash and are subject to insignificant risk of changes in value and which are used by the Company in the management of its short-term commitments. The Company applies judgement at each reporting period on the classification of its money market funds. Cash and cash equivalents exclude restricted cash, which is presented in the consolidated statements of financial position under the line “Other non-current assets”. Interests on Cash and cash equivalents is reported under Cash Flow from investment activities under “Interest received”. 2.11 Trade and other payables Trade and other payables are comprised of liabilities that are due less than one year from the balance sheet date and are in general not interest bearing and settled on an ongoing basis during the financial year. They also include accrued expenses related to the Company’s research and development activities, sales rebates and reserves and short-term employee benefits. Trade and other payables are initially measured at their transaction price, which are subsequent to initial recognition measured at amortized cost. Short-term employee benefits include payables and accruals for salaries and bonuses to be paid to the employees of the Company. They are recognized as expenses for the period in which employees perform the corresponding services. 2.12 Leases The Company assesses whether a contract is or contains a lease, at inception of the contract. The Company recognizes a right-of-use asset and a corresponding lease liability with respect to all lease arrangements in which it is the lessee, except for short-term leases (defined as leases with a lease term of 12 months or less) and leases of low value assets. For short-term leases, the Company recognizes the lease payments as an operating expense on a straight-line basis over the term of the lease unless another systematic basis is more representative of the time pattern in which economic benefits from the leased assets are consumed. The lease liability is initially measured at the present value of the lease payments that are not paid at the commencement date, discounted by using the rate implicit in the lease. If this rate cannot be readily determined, the lessee uses its incremental borrowing rate. The lease liability is subsequently measured by increasing the carrying amount to reflect interest on the lease liability (using the effective interest method) and by reducing the carrying amount to reflect the lease payments made. The lease liability is presented as a separate line in the consolidated statements of financial position. The right-of-use assets comprise the initial measurement of the corresponding lease liability, lease payments made at or before the commencement day, less any lease incentives received and any initial direct costs. They are subsequently measured at cost less accumulated depreciation and impairment losses. Right-of-use assets are depreciated over the shorter period of lease term and useful life of the argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 253

underlying asset. If a lease transfers ownership of the underlying asset or the cost of the right-of-use asset reflects that the Company expects to exercise a purchase option, the related right-of-use asset is depreciated over the useful life of the underlying asset. The right-of-use assets are presented in the consolidated statements of financial position under the caption “Property, plant and equipment”. 2.13 Financial instruments Financial instruments are initially recognized either at fair value or at transaction price and subsequently measured at either amortized cost or fair value under IFRS 9 on the basis of both the Company’s model for managing the financial assets and the contractual cash flow characteristics of the financial asset. A financial asset is classified as current when the cash flows expected to flow from the instrument mature within one year. Profit share in AgomAb Therapeutics NV: The Company holds investments in non-current financial assets, which based on IFRS 9, are designated as financial assets at fair value through profit or loss. The fair value of listed investments is based upon the closing price of such securities at each reporting date. As there is no active market for this equity instrument, the Company establishes the fair value by using valuation techniques. The changes to the fair valuation is recorded under “Other operating income” in the consolidated statements of profit or loss. Shares of Zai Lab Ltd: Based on IFRS 9, the Company irrevocably elected to designate this specific investment as a financial asset at fair value through OCI as the participation is not held for trading purposes nor contingent consideration recognized by an acquirer in a business combination. The investment is recorded under “Other non-current assets” in consolidated statements of financial position and changes to the fair valuation is recorded under “Fair value gain/(loss) on investments in equity instruments designated as at FVTOCI” in the consolidated statements of other comprehensive income or loss. 2.14 Shareholder’s equity An equity instrument is any contract that evidences a residual interest in the assets of an entity after deducting all of its liabilities. Equity instruments issued by the Company are recognized at the proceeds received, net of direct issue costs. The Company has never distributed any dividends to its shareholders. As of December 31, 2024, no profits were available for distribution. 2.15 Share-based payments Equity-settled share-based payments to employees and others providing similar services are measured at the fair value of the equity instruments at the acceptance date. Equity settled share based payments includes expenses related to stock options and restricted stock units granted by the Company. The fair value determined at the acceptance date of the equity-settled share-based payments is expensed on a straight-line basis over the vesting period, based on the Company’s estimate of equity instruments that will eventually vest, with a corresponding increase in equity. At the end of each reporting period, the Company revises its estimate of the number of equity instruments expected to vest. The impact of the revision of the original estimates, if any, is recognized in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss such that the cumulative expense reflects the revised estimate, with a corresponding adjustment to the equity-settled share-based payment reserve. The share-based payment expense is recorded in the “Consolidated Statements of Profit or Loss” depending on the nature of the services provided by each beneficiary. 2.16 Income taxes Income tax in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss represents the total of the current tax and deferred tax. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 254

The current tax is based on taxable profit for the year. Taxable profit differs from profit as reported in the consolidated statements of profit or loss and consolidated statements of other comprehensive income or loss as it excludes items of income or expense that are taxable or deductible in other years and items that are never taxable or deductible. The Company’s liability for current tax is calculated using tax rates that have been enacted or substantively enacted by the end of the reporting period. Deferred tax is recognized on temporary differences between the carrying amounts of assets and liabilities in the consolidated financial statements and the corresponding tax basis used in the computation of taxable profit. The Company recognizes deferred tax assets, including the tax base of tax loss carryforwards, if management assesses that these tax assets can be offset against positive taxable profits in the future. This judgment is made on an ongoing basis, considering actual results, budgets, and business plans for the coming years. The realization of deferred tax assets depends on all available factors as of reporting date. The carrying amount of deferred tax assets is reviewed at the end of each reporting period and reduced to the extent that it is not probable that sufficient taxable profits will be available to allow all or part of the asset to be recovered. Deferred tax assets and liabilities are offset if there is a legally enforceable right to offset. Deferred tax assets and liabilities are measured at the tax rates that are expected to apply in the period in which the liability is settled or the asset realized, based on tax rates (and tax laws) that have been enacted or substantially enacted by the end of the reporting period. The Company records uncertain tax positions in accordance with IAS 12 Income Taxes using the 2-step test whereby (1) the Company determines whether it is probable that the tax positions will be accepted by relevant taxing authorities, and (2) for those tax positions that are not probable that a tax authority will accept in full the position, the Company recognizes uncertain tax positions using either the most likely amount or the expected value, depending on specific facts and circumstances. 2.17 Product net sales Revenue from the sale of products is recognized at an amount that reflects the consideration that the Company expects to be entitled to receive in exchange for transferring goods to a customer, at the time when the customer obtains control of the goods rendered, this means when the customer has the ability to direct the use of the asset. The consideration that is committed in a contract with a customer can include fixed amounts, variable amounts, or both. The amount of the consideration may vary due to discounts, rebates, returns, chargebacks or other similar items. Contingent consideration is included in the transaction price when it is highly probable that the amount of revenue recognized is not subject to future significant reversals. Product net sales are recognized once we satisfy the performance obligation at a point in time under the revenue recognition criteria in accordance with IFRS 15 Revenue from contracts with customers. Revenue arising from the commercial sale of commercial product is presented in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss under “Product net sales”. In accordance with IFRS 15, such revenue is recognized when the product is physically transferred, in accordance with the delivery and acceptance terms agreed with the customer. Payment of the transaction price is payable at the point the customer obtains the legal title to the goods. The amount of revenue recognized reflects the various types of price reductions or rights of return offered by the Company to its customers. Such price reductions and rights of return qualify as variable consideration under IFRS 15. Products sold are covered by various Government and State programs (such as Medicare and Medicaid) under which products are sold at a discount. Rebates are granted to healthcare authorities, and under contractual arrangements with certain customers. Some wholesalers are entitled to chargeback incentives based on the selling price to the end customer, under specific contractual arrangements. Rebates, chargebacks and other incentives are recognized in the period in which the underlying sales are recognized as a reduction of product sales. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 255

The significant components of variable consideration are as follows: Co-payment assistance: We provide co-payment assistance to patients who have commercial insurance and meet certain eligibility requirements. We use the expected-value method for estimating co-payment assistance based on estimates of program redemption using data provided by third-party administrators. Estimates for the co-payment assistance are adjusted quarterly to reflect actual experience. We record an accrued liability for unredeemed co-payment assistance related to products for which control has been transferred to customers. Chargebacks: Chargebacks are discounts that occur when contracted parties purchase directly from a specialty distributor. Contracted parties, which currently consist primarily of Public Health Service Institutions and federal government entities purchasing via the Federal Supply Schedule, generally purchase the product at a discounted price. The specialty distributor, in turn, charges back the difference between the price initially paid by the specialty distributor and the discounted price paid to the specialty distributor by the contracted parties to the Company. The reserves for chargeback are based on known sales to contracted parties. We establish the reserves for chargebacks in the same period that the related revenue is recognized, resulting in an accrued liability and reduction of product gross sales. Rebates: We are subject to government mandated rebates for Medicaid Drug Rebate Program, Medicare Part D Prescription Drug Benefit Program, and other government health care programs in the U.S. Rebate amounts are based upon contractual agreements or legal requirements with public sector benefit providers. We use the expected-value method for estimating these rebates. The expected utilization of rebates is estimated based on third-party data from the specialty pharmacies and specialty distributor. Estimates for these rebates are adjusted quarterly to reflect the most recent information. We record an accrued liability and reduction of product sales for unpaid rebates related to products for which control has been transferred to customers. Medicare Part D Coverage Gap: The Medicare Part D coverage gap is a federal program to subsidize the costs of prescription drugs for Medicare beneficiaries in the U.S., which mandates manufacturers to fund a portion of the Medicare Part D insurance coverage gap for prescription drugs sold to eligible patients. Funding of the coverage gap is generally invoiced and paid in arrears. We estimate the impact of the Medicare Part D coverage gap using the expected-value method based on an amount expected to be incurred for the current quarter’s activity, plus an accrual balance for known prior quarters. Estimates for the impact of the Medicare Part D coverage gap are adjusted quarterly to reflect actual experience. We record an accrued liability for unpaid reserves related to the Medicare Part D coverage gap. Distributor fees: The specialty distributor provides distribution services to the Company for a fee, based on a contractually determined fixed percentage of sales. As the services being provided by the specialty distributor are not distinct, the recurring service fees paid to specialty distributors are treated as variable consideration and a reduction to the transaction price. We estimate these distributor fees and record such estimates in the same period the related revenue is recognized, resulting in a reduction of product gross sales. We record an accrued liability for unpaid distributor fees. Value-based arrangements (VBAs): VBAs are arrangements with third party payers where the Company will pay the third-party payers rebates and other fees on eligible purchases of the Company’s product. In consideration for the rebates and fees paid, the third-party Payers will cover its’ patient purchases made of the Company’s products. The structure of the rebates and fees are largely structured based on volume of product purchased. The rebates and fees paid will be treated as variable consideration and a reduction to the transaction price. We use the expected-value method for estimating the ultimate rebate and fee paid, which are based on the volume of product sold. We apply the applicable rebate rate against a payer mix factor for the relevant patient populations and to the vials sold in the effective plan year of the rebate to derive a liability recorded. Estimates for these agreements are adjusted quarterly to reflect the most recent information. We record an accrued liability for unpaid value-based agreements. The estimated amounts described above are recognized in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss within “Product net sales” as a reduction of gross sales, and within “Trade and other payables” in the consolidated statements of financial position. They are subject to regular review and adjustment as appropriate based on the most recent data available to management. Each of the above items require significant estimates, judgement and argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 256

information obtained from external sources. If management’s estimates differ from actual results, we will record adjustments that would affect product sales in the period of adjustment. 2.18 Collaboration and license agreements The Company has currently two active collaboration and license agreements in scope of IFRS 15: Zai Lab Under the collaboration agreement, the Company provides clinical and commercial supply to Zai Lab. The Company concludes to recognize such sales as revenue given that the Company acts as principal in the transaction as the risk related to inventory is borne by the Company until the inventory is transferred to Zai Lab. The revenue related to clinical supply is recorded under line item “Collaboration revenue”. The revenue related to commercial supply is recorded under line item “Product net sales” in the Consolidated Statements of Profit or Loss. The income related to royalties on sales made in China is recorded under line item “Collaboration revenue”. AbbVie For the collaboration agreement with AbbVie the Company has determined that the transfer of license combined with the performance of research and development activities represent one single performance obligation. The Company concluded that the license is not distinct in the context of the contract. The transaction price is composed of a fixed part, being an upfront license fee, and a variable part, being milestone payments and cost reimbursements for research and development activities delivered. Milestone payments are only included in the transaction price to the extent it is highly probable that a significant reversal in the amount of cumulative revenue recognition will not occur when the uncertainty associated with the variable consideration is subsequently resolved. Management estimates the amount to be included in the transaction price upon achievement of the milestone event. Sales-based milestones and sales-based royalties are a part of the Company’s arrangements but are not yet included in its revenues. The transaction price has been allocated to the single performance obligation and revenues have been recognized over the estimated service period based on an input model, being the percentage of completion method. The upfront license fee has been fully recognized since 2021 as the performance obligation has been fulfilled at that time. Milestone payments that become highly probable after the performance obligation has been fulfilled are therefore recognized at that point in time. 2.19 Cost of Sales Cost of sales are recognized when the associated revenue from product net sales is recognized. Cost of sales include material, manufacturing costs and other costs attributable to production, including shipping costs relevant amortizations, as well as royalties payable on sold products. 3. Critical accounting judgments and major sources of estimation uncertainty In the application of the Company’s accounting policies, which are described above, the Company is required to make judgments, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the revision affects both current and future periods. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 257

Major sources of estimation uncertainty Sales rebates and reserves Product Sales are recognized when the Company has transferred control of the goods to the customer. Product Sales are subject to various deductions, which are primarily composed of rebates to government agencies, distributors, health insurance companies and managed healthcare organizations to arrive to ‘‘Product net sales’’. Certain deductions from Product Sales are subject to payment based on claims after the initial recognition of the sale due to the time lag between the point of sale and receipt of a claim. Upon initial recognition of the Product Sales, the Company recognizes a liability for the variable consideration based on the Company’s best estimate of expected claims. This estimate is a source of complexity and uncertainty as the Company estimates the transaction price based upon contracts with customers, healthcare providers, payors and government agencies, regulated discounts applicable to government-funded programs, historical experience of claims received, estimated payer mix, and other relevant factors. These open claims are recorded as liabilities under “Sales rebates and reserves’’ in the ‘‘Consolidated Statements of Financial Position’’. The Company reviews these liabilities at each reporting period to take into account potential changes in the programs, the volume of claims and/or the most probable final outcome associated to each sale. In line with IFRS 15, the Company applies constraint in recognition of variable compensation on Product Net Sales. Due to the nature of these liabilities it is not practicable to give meaningful sensitivity estimates due to the large volume of variables that contribute to the overall rebates, chargebacks, and other incentives as outlined in “Note 2.17 Product net sales”. Future events could cause the assumptions within our valuation models to change and materially affect the future results of the Company. Please refer to “Note 14 Trade and Other Payables” for the movement over the period and the ending balance of the sales rebates and reserves. Critical accounting judgment Deferred Tax Assets The Company recognizes deferred tax assets if management assesses that these tax assets are recoverable in the future. This judgment is made on an ongoing basis, considering actual results, forecasts, and business plans for the look-forward period. The Company has exercised a Critical Accounting Judgement with respect to defining the number of years of forecasted future taxable profits to be considered as reliable as positive evidence towards its estimate on recognition of deferred tax assets. The Company has aligned the duration of its deferred tax assessment with the time horizon of its annual operating plan and long-range predictive estimates. In the fourth quarter of the year ended December 31, 2024, the Company reassessed the body of evidence as part of its 2025 budgeting and forecasting cycle noting the shift of positive evidence outweighing negative evidence. Such positive evidence, includes significant revenue growth in the U.S. based Product Net Sales, as well as, expectations regarding future operating and taxable profits. The Company expects its future revenues to sustain its investments in its clinical and pre-clinical pipeline. This evaluation was done alongside the evolution of the external competitive landscape of our commercialized products, and the positive evidence following our execution in the second half of 2024 on the approvals of VYVGART HYTRULO for CIDP. Our evaluation of the evidence for the current reporting period is further detailed in “Note 23 Income taxes” which presents the balance of $708 million in net deferred tax assets for argenx BV as of December 31, 2024. The Company considers the evaluation of all available positive and negative evidence used in its overall recognition conclusion to be a Critical Accounting Judgment. The Company has determined that a key estimate in exercising this judgement is related to the growth of U.S. based Product Net Sales. The forecasts for future growth in this market are important for the utilization of deferred tax assets against taxable profits by argenx BV. Based on sensitivities and analysis over assumptions in the model, the Company has determined that other inputs are less sensitive or significant argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 258

to the recognition of our deferred tax assets. The Company’s current sensitivities do not exceed the look- forward period. The Critical Accounting Judgement and the key estimate relating to deferred tax assets are limited to the Company’s subsidiary argenx BV which holds these assets. No other Critical accounting judgments and major sources of estimation uncertainty have been made in the current period by the Company. 4. Property, Plant and Equipment (in thousands of $) IT, office and lab equipment Right-of- use assets Buildings Right-of- use assets Vehicles Leasehold improve ments Leased equipment Total Cost On January 1, 2022 7,938 16,462 3,075 1,981 346 29,802 Additions 962 3,353 905 – – 5,219 Disposals (105) – – – – (105) Currency translation adjustment (635) – – – – (635) On December 31, 2022 8,160 19,815 3,980 1,981 346 34,282 Additions 937 8,770 2,327 48 – 12,082 Disposals (202) – (757) (54) – (1,013) On December 31, 2023 8,895 28,585 5,550 1,975 346 45,350 Additions 1,039 20,639 5,492 982 – 28,152 Disposals (220) (234) (333) – – (787) On December 31, 2024 9,714 48,990 10,709 2,957 346 72,715 Depreciation and impairment On January 1, 2022 (4,565) (6,774) (1,411) (1,093) (116) (13,958) Depreciation (1,388) (2,179) (735) (257) (35) (4,593) Disposals 90 – – – – 90 Currency translation adjustment 408 5 1 1 – 414 On December 31, 2022 (5,454) (8,948) (2,145) (1,350) (150) (18,047) Depreciation (1,539) (2,839) (971) (189) (36) (5,574) Disposals 189 – 757 – – 946 On December 31, 2023 (6,804) (11,787) (2,359) (1,539) (186) (22,675) Depreciation (1,252) (3,657) (2,067) (234) (35) (7,245) Disposals 155 234 333 – – 722 On December 31, 2024 (7,901) (15,210) (4,093) (1,773) (221) (29,198) Carrying Amount On December 31, 2022 2,706 10,867 1,835 631 196 16,234 On December 31, 2023 2,091 16,798 3,191 436 160 22,675 On December 31, 2024 1,813 33,780 6,615 1,184 125 43,517 Depreciation is recognized as from the moment when the asset is ready for its intended use so as to depreciate the cost of the assets less their residual values over their useful lives, using the straight-line method. Unless revised due to specific changes in the estimated useful life, annual depreciation rates are as follows: • Office and lab equipment: three to five years • IT equipment: three years argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 259

Depreciation of right-of-use assets is done over the expected duration of the lease including lease extensions where applicable. As of December 31, 2024, there are no material commitments to acquire property, plant and equipment. Furthermore, no items of property, plant and equipment are pledged. See “Note 21 Leases” for information for leases where the Company is a lessee. 5. Intangible Assets (in thousands of $) Acquired R&D available for use Acquired In-Process R&D Software & databases Other Intangibles Total Cost On January 1, 2022 – 70,180 3,353 99,058 172,591 Additions – – 992 102,000 102,992 Disposals – – (5) – (5) Derecognition – – – (99,058) (99,058) On December 31, 2022 – 70,180 4,340 102,000 176,519 Additions 56,000 – – – 56,000 Derecognition – – – (102,000) (102,000) Reclassification 52,931 (52,931) – – – On December 31, 2023 108,931 17,249 4,340 – 130,520 Additions 36,500 30,000 – – 66,500 Derecognition – – – – – Reclassification – – – – – On December 31, 2024 145,431 47,249 4,340 – 197,020 Amortization and impairment On January 1, 2022 – – (907) – (907) Amortization – – (711) (99,058) (99,768) Derecognition – – – 99,058 99,058 On December 31, 2022 – – (1,618) – (1,618) Amortization (3,392) – (282) (102,000) (105,674) Derecognition – – – 102,000 102,000 On December 31, 2023 (3,392) – (1,900) – (5,292) Amortization (10,069) – (213) (10,282) Derecognition – – – – – On December 31, 2024 (13,461) – (2,113) – (15,575) Carrying Amount On December 31, 2022 – 70,180 2,722 102,000 174,901 On December 31, 2023 105,539 17,249 2,440 – 125,228 On December 31, 2024 131,970 47,249 2,226 – 181,445 Acquired In-Process R&D is mainly related to the in-licensing of the ENHANZE® drug delivery technology from Halozyme. In line with its accounting policies, the Company has capitalized the upfront payment upon commencement of the in-license agreement. In June 2023, the Company obtained the FDA approval for VYVGART HYTRULO. During the year ended December 31, 2023, upon obtaining regulatory approval, $53 million has been moved from “Acquired In-Process R&D” to “Acquired R&D available for use”. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 260

In 2024, the Company extended its collaboration with Halozyme and nominated four new targets to be in- licensed to the ENHANZE® drug delivery technology. The cost of the license was capitalized as “Acquired In- Process R&D”. Further, the additions to “Acquired R&D available for use” are related to sales-based milestones triggered during 2024. The “Acquired R&D available for use” are amortized under “Cost of sales” on a straight-line basis over their useful life. The Company performs an annual impairment review on the intangible assets. This review did not result in the recognition of an impairment charge for the years ended December 31, 2024, 2023 and 2022. In the fourth quarter of 2023, the Company utilized the PRV submitted with the sBLA filing for VYVGART HYTRULO for the treatment of CIDP, which resulted in the amortization of $102 million of intangible assets which is recognized under “Research and development expenses” within the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss and subsequent derecognition of $102 million of intangibles included under “Other intangibles” on the consolidated statements of financial position. As of December 31, 2024, there are no material commitments to acquire intangible assets, except as set forth in “Note 28 Commitments”. No intangible assets are pledged as security for liabilities nor are there any intangible assets whose title is restricted. 6. Other Non-Current Assets Other non-current assets consisted of non-current restricted cash and financial assets held at fair value through profit or loss or through OCI. As of December 31, (in thousands of $) 2024 2023 2022 Non-current restricted cash 1,964 2,419 1,736 Non-current financial assets held at fair value through profit or loss 25,549 21,715 21,715 Non-current financial assets held at fair value through OCI 14,880 15,528 17,443 Total other non-current assets 42,393 39,662 40,894 Non-current restricted cash on December 31, 2024 was mainly composed of deposit guarantees paid under the lease agreements for the laboratory and offices of the Company. Non-current financial assets held at fair value through profit or loss is comprised of the profit share in AgomAb Therapeutics NV. In March 2019, the Company entered into a license agreement with AgomAb Therapeutics NV for the use of HGF-mimetic SIMPLE Antibodies™, developed under the Company’s Immunology Innovative Program. In exchange for granting this license, the Company received a profit share in AgomAb Therapeutics NV. Since AgomAb Therapeutics NV is a private company, the valuation of the profit share is based on the post-money valuation coming from its most recent financing round. In June 2022, AgomAb Therapeutics NV secured €38 million as a result of the extension of Series B. The Company used the post-money valuation of this Series B financing round and the number of outstanding shares in determining the fair value of the profit-sharing instrument, which resulted in a change in fair value of non-current financial assets of €4 million recorded through profit or loss in 2022. In October 2023, AgomAb Therapeutics NV secured €100 million as a result of a Series C financing round. The Company’s profit share was diluted, but resulting in no change of the fair value. In October 2024, AgomAb Therapeutics NV secured $89 million as a result of a Series D financing round. The Company’s profit share was diluted and resulted in an increase of the fair value. Fair value changes on non-current financial assets with fair value through profit or loss are recognized in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss under “Other operating income”. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 261

As part of the license agreement for the development and commercialization for efgartigimod in Greater China, in 2021 the Company obtained, amongst others, 568,182 newly issued Zai Lab shares calculated at a price of €132 per share. The fair value of the equity instrument at reporting date is determined by reference to the closing price of such securities at each reporting date (classified as level 1 in the fair value hierarchy). The Company made the irrevocable election to recognize subsequent changes in fair value through OCI under “Fair value gain/(loss) on investments in equity instruments designated as at FVTOCI”. The table below illustrates these non-current financials assets at fair value through profit or loss or OCI as of December 31, 2024, 2023 and 2022. As of December 31, (in thousands of $) 2024 2023 2022 Cost on January 1 76,659 76,659 76,659 Additions of the year – – – Cost on December 31 76,659 76,659 76,659 Fair value adjustments on January 1 (39,416) (37,501) (23,490) Fair value adjustment of the year through profit or loss 3,834 – 4,256 Fair value adjustment of the year through OCI (648) (1,915) (18,267) Fair value adjustment on December 31 (36,230) (39,416) (37,501) Net book value on December 31 40,429 37,243 39,158 7. Inventories As of December 31, (in thousands of $) 2024 2023 2022 Raw materials and consumables 337,832 240,836 126,046 Inventories in process 26,357 47,074 65,016 Finished goods 43,044 22,640 37,291 Total inventories 407,233 310,550 228,353 The cost of inventories, which is recognized under “Cost of sales” in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss, amounted to $168 million for the year ended December 31, 2024 (compared to $101 million for the year ended December 31, 2023 and $29 million for the year ended December 31, 2022). The Company has pre-launch inventory awaiting regulatory approval amounting to $4.5 million as of the current year end. 8. Prepaid Expenses (Current) The current prepaid expenses are composed of prepayments which are detailed below: As of December 31, (in thousands of $) 2024 2023 2022 Prepaid research and development expenses 110,249 71,201 44,905 Prepaid inventory 34,753 22,460 11,667 Prepaid software 18,564 6,240 4,309 Prepaid advertising expenses 9,463 19,933 13,479 Other prepaid expenses 14,919 14,238 1,662 Total prepaid expenses 187,948 134,072 76,022 argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 262

9. Trade and Other Receivables The trade and other receivables are composed of receivables which are detailed below: As of December 31, (in thousands of $) 2024 2023 2022 Trade receivables 817,707 417,994 241,228 Tax receivables 40,886 63,605 20,526 Interest receivables 40,214 13,126 12,918 Other receivables 5,664 1,962 1,025 Total trade and other receivables 904,471 496,687 275,697 The carrying amounts of trade and other receivables approximate their respective fair values. On December 31, 2024, 2023 and 2022, we did not have a material provision for expected credit losses. Please also refer to “Note 25 Financial Risk Management” for more information on the financial risk management. 10. Financial Assets — Current These current financial assets relate to term accounts with an initial maturity longer than three months and less than 12 months and money market funds that do not qualify as cash equivalents as they are not expected to be used to meet short-term commitments. As of December 31, (in thousands of $) 2024 2023 2022 Money market funds – – 46,162 Term accounts 1,878,890 1,131,000 1,345,646 Total current financial assets 1,878,890 1,131,000 1,391,808 On December 31, 2024, the current financial assets included $104 million (€100 million) held in EUR which could generate a foreign currency exchange gain or loss in the financial results in accordance with the fluctuations of the USD/EUR exchange rate as the Company’s functional currency is USD. Please also refer to “Note 25 Financial Risk Management” for more information on the financial risk management. 11. Cash and Cash Equivalents As of December 31, (in thousands of $) 2024 2023 2022 Money market funds 1,394,409 1,678,100 669,147 Term accounts 100,000 350,000 54,116 Cash and bank balances 5,527 20,744 77,477 Total cash and cash equivalents 1,499,936 2,048,844 800,740 Cash and cash equivalents comprise of cash and bank balances, term accounts with an original maturity not exceeding three months and money market funds that are readily convertible to cash and are subject to an insignificant risk of changes in value. Cash positions are invested with preferred financial partners, which are considered to be high quality financial institutions with sound credit ratings to reduce credit risk. On December 31, 2024, the cash and cash equivalents included $653 million (€628 million) held in EUR, and $2 million (¥257 million) held in JPY which could generate a foreign currency exchange gain or loss in the argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 263

financial results in accordance with the fluctuations of the USD/EUR and USD/JPY exchange rates as the Company’s functional currency is USD. Please also refer to “Note 25 Financial Risk Management” for more information on the financial risk management. 12. Share Capital and Share Premium As of December 31, 2024, the Company’s share capital was represented by 60,760,957 shares. All shares were issued, fully paid up and of the same class. The table below summarizes the share issuances as a result of offerings, exercise of stock options and the vesting of restricted stock units under the Company’s Employee Stock Option Plan. Roll forward of number of shares outstanding: Number of shares outstanding on January 1, 2022 51,668,315 Exercise of stock options 1,024,626 Vesting of RSUs 19,581 Global public offering on Euronext and Nasdaq on March 23, 2022 2,333,334 Overallotment option exercised by underwriters on March 29, 2022 350,000 Number of shares outstanding on December 31, 2022 55,395,856 Exercise of stock options 1,137,439 Vesting of RSUs 79,560 Global public offering on Nasdaq on July 18, 2023 2,244,899 Over-allotment option exercised by underwriters on July 19, 2023 336,734 Number of shares outstanding on December 31, 2023 59,194,488 Exercise of stock options 1,478,225 Vesting of RSUs 88,244 Number of shares outstanding on December 31, 2024 60,760,957 On July 18, 2023, argenx SE offered 2,244,899 of its ordinary shares through a global offering which consisted of 1,580,981 ADSs in the U.S. at a price of $490.00 per ADS, before underwriting discounts and commissions and offering expenses; and 663,918 ordinary shares in the European Economic Area at a price of €436.37 per share, before underwriting discounts and commissions and offering expenses. On July 19, 2023, the underwriters of the offering exercised their overallotment option to purchase 336,734 additional ADSs in full. As a result, argenx SE received $1.3 billion in gross proceeds from this offering, decreased by $66 million of underwriter discounts and commissions, and offering expenses, of which $1 million has been deducted from equity. The total net cash proceeds from the offering amounted to $1.2 billion. On May 7, 2024, at the annual general meeting, the shareholders of the Company approved the authorization to the Board to issue up to a maximum of 10% of the then-outstanding share capital, for a period of 18 months. On December 31, 2024, an amount of €461,348, represented by 4,613,483 shares, still remained available under the authorization to issue shares as granted to the Board by the shareholders of the Company. 13. Share-Based Payments The Company has an equity incentive plan for the employees, key consultants, board members, senior management and key outside advisors (“key persons”) of the Company and its subsidiaries. In accordance with the terms of the plan, as approved by shareholders, employees may be granted stock options and/or restricted stock units. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 264

13.1 Stock Options The stock options are granted to key persons of the Company and its subsidiaries. The stock options may be granted to purchase ordinary shares at an exercise price. The stock options have been granted free of charge. Each employee’s stock option converts into one ordinary share of the Company upon exercise. The stock options carry neither rights to dividends nor voting rights. Stock options may be exercised at any time from the date of vesting to the date of their expiry. The stock options granted vest, in principle, as follows: • 1/3rd of the total stock options granted will vest on the first anniversary of the granting of the stock options, and • 1/36th of the total stock options granted will vest on the first day of each month following the first anniversary of the granting of the stock options. Stock options granted to non-executive directors vest on the third anniversary of the date of grant. Upon leave of the key persons stock options must be exercised before the later of (i) 90 days after the last working day at argenx, or (ii) March 31 of the fourth year following the date of grant of those stock options, and in any case no later than the expiration date of the option. In order to pre-finance the taxes that are paid upon the grant of stock options, Belgian employees have the ability, in exchange for the taxes due upon the grant of the stock options, to transfer the economic benefits related to part of those stock options to a third party. In the year ending December 31, 2024, the economic benefits of 20,823 stock options, for which accelerated vesting applies, were transferred to a third party. No other conditions are attached to stock options. The following stock option arrangements were in existence during the current and prior years and which are exercisable at the end of each period presented: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 265

2024 2.53 – 3,308 19,743 2024 4.10 – 532 5,127 2024 7.45 – 81,500 214,800 2025 11.89 400 1,600 2,000 2025 9.84 78,690 99,326 101,861 2026 11.82 14,000 24,400 30,000 2026 11.92 93,378 97,972 99,772 2026 14.68 103,859 111,811 115,211 2027 19.13 35,046 38,434 42,509 2027 21.99 152,085 225,852 303,867 2023 83.96 – – 12,111 2028 83.96 7,370 13,890 19,490 2023 89.68 – – 124,338 2028 89.68 190,011 225,457 264,392 2024 117.90 – 26,171 110,774 2029 117.90 44,158 71,573 110,756 2024 141.03 – 104,176 202,852 2029 141.03 275,154 370,566 537,110 2025 124.18 3,758 16,712 16,712 2030 124.18 30,675 50,801 71,486 2025 203.78 7,926 126,331 127,731 2030 203.78 79,691 160,677 223,812 2025 208.01 5,629 31,424 32,100 2030 208.01 47,908 78,534 117,790 2025 257.23 90,425 202,205 202,475 2030 257.23 351,911 559,173 620,014 2026 243.52 23,491 23,491 23,491 2031 243.52 19,486 27,201 35,214 2026 265.02 59,527 59,626 60,890 2031 265.02 96,888 128,600 167,406 2026 269.59 45,044 45,228 45,862 2031 269.59 39,359 62,138 81,311 2027 293.49 13,876 13,957 14,976 2032 293.49 34,773 58,255 79,155 2028 310.32 6,043 – – 2033 310.32 61,806 79,305 – 2025 321.23 – 16,000 – 2026 321.23 80,179 80,425 80,833 2031 321.23 169,196 226,520 286,353 2028 353.95 15,014 15,014 – 2033 353.95 36,065 43,856 – 2028 369.23 121,071 127,490 – 2033 369.23 415,859 495,821 – 2027 371.41 57,118 58,091 61,816 2032 371.41 144,505 192,291 238,532 2027 373.59 134,748 136,459 137,778 2032 373.59 249,755 347,765 370,354 2029 380.34 3,291 – – 2034 380.34 37,642 – – 2029-2034 ²⁾ 618.56 20,296 – – 4,300,760 5,118,949 5,511,767 Outstanding stock options on December 31, Expiry date Exercise price per stock options (in $) ¹⁾ 2024 2023 2022 1) Amounts have been converted to USD at the closing rate as of December 31, 2024. 2) In December 2024, the Company granted stock options for which the Belgian taxed beneficiaries had a 60-day period to choose between a contractual term of five or ten years. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 266

2024 2023 2022 Number of stock options Weighted average exercise price ¹⁾ (in $) Number of stock options Weighted average exercise price ¹⁾ (in $) Number of stock options Weighted average exercise price ¹⁾ (in $) Outstanding as of January 1 5,118,949 255.41 5,511,767 205.02 5,619,113 164.33 Granted 756,234 451.63 844,011 395.92 1,021,642 375.58 Exercised (1,478,225) 206.43 (1,137,439) 142.31 (1,025,780) 92.62 Forfeited (96,198) 367.18 (99,390) 356.57 (103,208) 273.93 Outstanding as of December 31 4,300,760 283.29 5,118,949 255.41 5,511,767 205.02 Exercisable as of December 31 2,492,709 203.36 3,030,486 179.22 3,983,960 148.11 1) Amounts have been converted to USD at the closing rate of the respective period. The weighted average share price at the date of exercise of options exercised during the year ended December 31, 2024 was $498.6, compared to $456.8 during the year ended December 31, 2023 and $336.5 during the year ended December 31, 2022. The weighted average remaining contractual life of the stock options outstanding amounted to 5.89 years on December 31, 2024 compared to 5.90 years on December 31, 2023 and 6.20 years on December 31, 2022. The table below shows the weighted average remaining contractual life for each range of exercise price: Exercise price (in $) Outstanding on December 31, 2024 Weighted average remaining contractual life (in years) 9.84 - 11.89 79,090 0.95 11.82 - 14.68 211,237 1.67 19.13 - 21.99 187,131 2.87 83.96 - 89.68 197,381 3.96 117.90 - 141.03 319,312 4.91 124.18 - 257.23 617,923 5.16 243.52 - 321.23 533,170 4.79 293.49 - 382.83 705,359 6.22 310.32 - 478.52 714,875 7.55 380.34 - 618.56 735,282 8.84 The fair market value of the stock options has been determined based on the Black-Scholes model using the following unobservable assumptions: • The expected volatility, determined on the basis of the implied volatility of the share price over the expected life of the option. • The expected option life, calculated as the estimated duration until exercise, taking into account the specific features of the plans. Below is an overview of the parameters used in relation to the determination of the fair value of the grants during 2024: Stock options granted in April 2024 June 2024 September 2024 December 2024 ¹⁾ Number of options granted 42,243 660,166 33,529 20,296 Average Fair value of options (in $) ²⁾ 112.14 - 156.49 158.50 - 215.16 188.85 - 298.99 187.13 - 236.00 Share price (in $) ²⁾ 365.56 - 396.30 437.41 - 492.86 543.68 - 656.53 623.34 Exercise price (in $) ²⁾ 396.30 445.76 535.95 618.56 Expected volatility 35.53 - 39.04% 35.17 - 36.16% 33.33 - 35.61% 34.37 - 34.47% Average Expected option life (in years) 4.30 - 6.49 4.16 - 6.35 4.05 - 6.24 3.88 - 6.07 Risk-free interest rate 2.66 - 3.02% 2.48 - 2.87% 2.06 - 2.24% 2.22 - 2.27% Expected dividends –% –% –% –% argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 267

1) In December 2024, the Company granted a total of 20,296 stock options of which 3,158 stock options to Belgian taxed beneficiaries. Belgian taxed beneficiaries can choose between a contractual term of five or ten years. The expected option life ranges between 3.88 and 6.07 years. This estimate will be reassessed once the acceptance period of 60 days has passed and the beneficiaries will have made a choice between a contractual term of five or ten years. The total fair value of the grant to Belgian taxed beneficiaries would be below $1 million irrespective of 100% of the stock options of Belgian taxed beneficiaries with a contractual term of five years or 100% of the stock options of Belgian beneficiaries with a contractual term of ten years. 2) Amounts have been converted to USD at the applicable rate prevailing at the grant date. Below is an overview of the parameters used in relation to the determination of the fair value of grants during 2023: Stock options granted in April 2023 July 2023 October 2023 December 2023 ¹⁾ Number of options granted 61,056 629,121 74,529 79,305 Average Fair value of options (in $) ²⁾ 158.21 - 196.18 176.44 - 271.59 123.94 - 209.04 161.88 - 200.55 Share price (in $) ²⁾ 361.64 - 401.21 380.81 - 521.19 439.42 - 491.75 371.36 - 403.77 Exercise price (in $) ²⁾ 370.34 387.35 485.01 329.26 Expected volatility 41.00 - 42.18% 36.22 - 43.99% 35.35 - 36.67% 36.21 - 38.64% Average Expected option life (in years) 4.00 - 6.50 4.00 - 6.50 4.00 - 6.50 4.00 - 6.50 Risk-free interest rate 2.96 - 3.14% 2.90 - 3.03% 2.80 - 3.44% 2.40 - 2.81% Expected dividends –% –% –% –% 1) In December 2023, the Company granted a total of 79,305 stock options. Belgian beneficiaries could choose between a contractual term of five or ten years impacting the parameters used in determination of the fair value of the grant. Once the acceptance period of 60 days has passed in which the beneficiaries made a choice between a contractual term of five or ten years years, the parameters and fair value used in the financial year ending December 31, 2023 has been reassessed. 2) Amounts have been converted to USD at the applicable rate prevailing at the grant date. Below is an overview of the parameter used in relation to the determination of the fair value of grants during 2022: Stock options granted in April 2022 July 2022 October 2022 December 2022 Number of options granted 102,081 311,311 100,118 508,132 Average Fair value of options (in $) ¹⁾ 111.27 - 140.23 153.45 - 190.53 136.66 - 169.96 127.68 - 163.94 Share price (in $) ¹⁾ 320.84 - 321.06 378.11 - 397.92 352.97 - 376.01 368.69 - 377.61 Exercise price (in $) ¹⁾ 312.22 372.69 359.80 381.97 Expected volatility 39.18 - 40.87% 41.30 - 43.10% 39.64 - 45.97% 39.74 - 40.26% Average Expected option life (in years) 4.00 - 6.50 4.00 - 6.50 4.00 - 6.50 4.00 - 6.50 Risk-free interest rate 1.05 - 1.62% 1.77 - 2.28% 2.57 - 2.80% 3.09 - 3.29% Expected dividends –% –% –% –% 1) Amounts have been converted to USD at the applicable rate prevailing at the grant date. The total share-based payment expense related to stock options recognized in the consolidated statements of profit or loss totaled $147 million for the year ended December 31, 2024, compared to $164 million for the year ended December 31, 2023 and $120 million for the year ended December 31, 2022. 13.2 Restricted Stock Units (RSUs) The RSUs are granted to key persons of the Company and its subsidiaries. The RSUs have been granted free of charge. Each employee’s RSUs converts into one ordinary share of the Company upon vesting. The RSUs carry neither rights to dividends nor voting rights. RSUs once converted into ordinary shares, may be sold at any time from the date of vesting, have no expiry date and may be held by the participant without limitation. The fair value of RSUs is based on the closing sale price of the Company’s common stock on the day prior to the date of issuance. RSUs vest over a period of four years with 1/4th of the total grant vesting at each anniversary of the date of grant. The following restricted stock units arrangements were in existence during the current and prior years: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 268
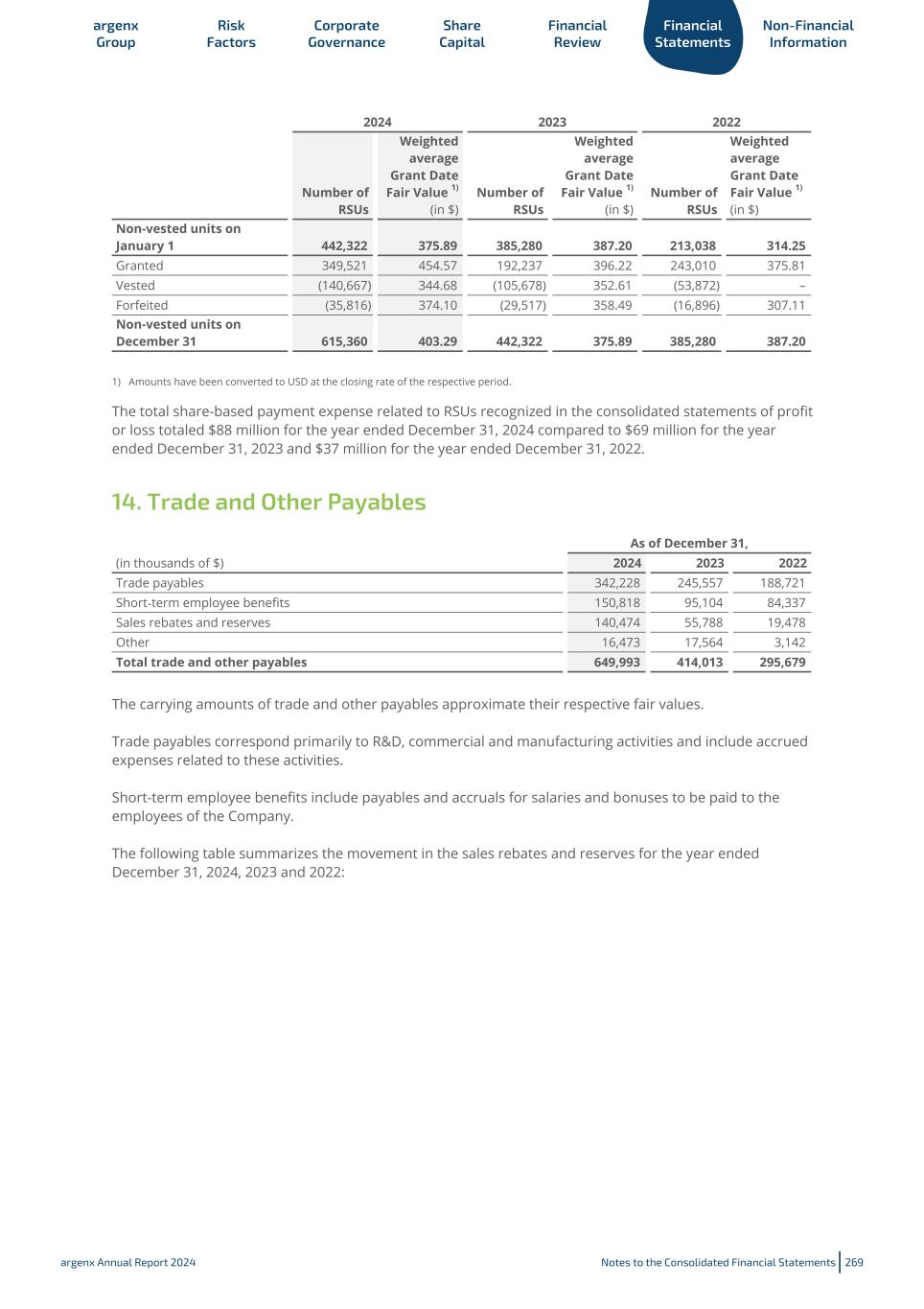
2024 2023 2022 Number of RSUs Weighted average Grant Date Fair Value 1) (in $) Number of RSUs Weighted average Grant Date Fair Value 1) (in $) Number of RSUs Weighted average Grant Date Fair Value 1) (in $) Non-vested units on January 1 442,322 375.89 385,280 387.20 213,038 314.25 Granted 349,521 454.57 192,237 396.22 243,010 375.81 Vested (140,667) 344.68 (105,678) 352.61 (53,872) – Forfeited (35,816) 374.10 (29,517) 358.49 (16,896) 307.11 Non-vested units on December 31 615,360 403.29 442,322 375.89 385,280 387.20 1) Amounts have been converted to USD at the closing rate of the respective period. The total share-based payment expense related to RSUs recognized in the consolidated statements of profit or loss totaled $88 million for the year ended December 31, 2024 compared to $69 million for the year ended December 31, 2023 and $37 million for the year ended December 31, 2022. 14. Trade and Other Payables As of December 31, (in thousands of $) 2024 2023 2022 Trade payables 342,228 245,557 188,721 Short-term employee benefits 150,818 95,104 84,337 Sales rebates and reserves 140,474 55,788 19,478 Other 16,473 17,564 3,142 Total trade and other payables 649,993 414,013 295,679 The carrying amounts of trade and other payables approximate their respective fair values. Trade payables correspond primarily to R&D, commercial and manufacturing activities and include accrued expenses related to these activities. Short-term employee benefits include payables and accruals for salaries and bonuses to be paid to the employees of the Company. The following table summarizes the movement in the sales rebates and reserves for the year ended December 31, 2024, 2023 and 2022: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 269

(in thousands of $) Rebates and chargebacks Distribution fees, product returns Total sales rebates and reserves Balance on January 1, 2022 – – – Current estimate related to the sales made in the current year 35,426 10,740 46,166 (Credits or payments related to sales made during the year) (20,028) (6,661) (26,689) Balance on December 31, 2022 15,398 4,079 19,478 Current estimate related to the sales made in the current year 123,542 26,427 149,969 Adjustment for prior year sales (4,041) (883) (4,924) (Credits or payments related to sales made during the year) (78,327) (20,722) (99,049) (Credits or payments related to sales made during prior year) (6,910) (2,775) (9,685) Balance on December 31, 2023 49,662 6,126 55,788 Current estimate related to the sales made in the current period 285,863 50,239 336,102 Adjustment for prior year sales (10,912) (162) (11,074) (Credits or payments related to sales made during the year) (170,391) (39,104) (209,495) (Credits or payments related to sales made during prior year) (26,811) (4,036) (30,847) Balance on December 31, 2024 127,411 13,063 140,474 15. Collaboration Revenue Year Ended December 31, (in thousands of $) 2024 2023 2022 Zai Lab 4,348 5,533 4,238 AbbVie – 30,000 – Other – – 5,788 Total collaboration revenue 4,348 35,533 10,026 For the years ended December 31, 2024, the collaboration revenue was generated under the agreement with Zai Lab. This note should be read alongside ‘‘Note 2.18 Collaboration and license agreements’’. AbbVie In April 2016, the Company entered into a collaboration agreement with AbbVie to develop and commercialize ARGX-115 (ABBV-151). In October 2023, the Company achieved the second development milestone upon initiation of a non-pivotal clinical trial, triggering a $30 million payment. Subject to the continuing progress of ARGX-115 (ABBV-151) by AbbVie, the Company is eligible to receive future development, regulatory and commercial milestone payments in aggregate amounts of up to $50 million, $190 million and $325 million, respectively, as well as tiered royalties on sales at percentages ranging from the mid-single digits to the lower teens, subject to customary reductions. 16. Other Operating Income Year Ended December 31, (in thousands of $) 2024 2023 2022 Research and development incentives 46,106 27,815 19,502 Payroll tax rebates 11,855 11,925 8,576 Grants 13 2,538 2,186 Change in fair value on non-current financial assets 3,834 – 4,256 Total other operating income 61,808 42,278 34,520 16.1 Research and development incentives The Company has accounted for tax incentives following a research and development tax incentive scheme in Belgium according to which the incentive will be refunded after a 5 years period, if not offset against the current tax payable over the period. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 270

16.2 Payroll tax rebates The Company accounted for payroll tax rebates as a reduction in withholding income taxes for its highly qualified personnel employed in its research and development department. 17. Segment Reporting The Company manages its activities and operates as one business unit which is reflected in its organizational structure and internal reporting. The Company does not distinguish in its internal reporting different segments, neither business nor geographical segments. The chief operating decision-maker is the Board of Directors. Following table summarizes the product net sales by country of sales based on the country of the entity that recognizes product net sales: Year Ended December 31, (in thousands of $) 2024 2023 2022 United States 1,895,919 1,046,592 377,659 Japan 89,389 56,432 15,764 China 39,177 14,907 – Netherlands 153 – – Rest of the World 161,245 72,852 7,297 Total product net sales 2,185,883 1,190,783 400,720 The Company sells its products through a limited number of distributors and wholesalers. Five U.S. customers represent approximately 87% of the product net sales during the twelve months ended December 31, 2024 (compared to 86% and 91% for four customers the same period in 2023 and 2022 respectively). Collaboration revenue is generated by external customers with their main registered office geographically located as shown in the table below: Year Ended December 31, (in thousands of $) 2024 2023 2022 China 4,348 5,533 4,238 United States – 30,000 – Denmark – – 5,365 Other – – 424 Total collaboration revenue 4,348 35,533 10,026 The non-current assets including property, plant and equipment and intangible assets are presented geographically as shown in the table below: Year Ended December 31, (in thousands of $) 2024 2023 2022 Netherlands – – – Belgium 209,758 138,252 186,923 United States 11,557 6,219 2,275 Japan 2,242 2,971 1,938 Rest of the World 1,405 461 130 Total non-current assets 224,962 147,903 191,136 argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 271

18. Research and Development Expenses Year Ended December 31, (in thousands of $) 2024 2023 2022 External research and development expenses 605,082 483,192 366,955 Personnel expenses 310,992 226,344 162,010 BIS expenses 34,012 19,935 12,678 Materials and consumables 5,863 4,057 2,396 Depreciation and amortization 6,204 105,546 102,132 Other expenses 21,270 20,418 17,194 Total Research and development expenses 983,423 859,492 663,366 19. Selling, General and Administrative Expenses Year Ended December 31, (in thousands of $) 2024 2023 1) 2022 1) Personnel expenses 424,916 303,033 234,740 Marketing services 306,987 202,146 115,950 Professional fees 170,215 108,820 62,620 BIS expenses 27,295 20,408 17,431 Facilities and occupancy expenses 20,888 11,264 9,627 Supervisory board 9,724 8,362 6,912 Depreciation and amortization 3,149 2,366 2,211 Other expenses 92,163 55,506 22,641 Total Selling, general and administrative expenses 1,055,337 711,905 472,132 1) Comparative figures have been presented to be consistent with the one adopted in the current year. 20. Personnel Expenses The personnel expenses mentioned in ‘‘Note 18 Research and Development Expenses” and ‘‘Note 19 Selling, General and Administrative Expenses’’ above are as follows: Year Ended December 31, (in thousands of $) 2024 2023 2022 Short-term employee benefits - Salaries 410,184 266,482 216,847 Short-term employee benefits - Social Security 30,856 19,231 16,274 Post-employment benefits 12,330 7,758 5,406 Termination benefits 2,498 1,089 401 Share-based payment 228,142 226,830 151,912 Employer social security contributions share-based payments 51,898 7,987 5,910 Total personnel expenses 735,908 529,377 396,750 The post-employment benefits relate to the pension plans the Company has in place for its employees. The average number of full-time equivalents (FTE) by function is presented below: Year Ended December 31, Average Number of FTEs 2024 2023 2022 Research and development 805 607 475 Selling, general and administrative 835 681 442 Total number of FTEs 1,639 1,289 917 argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 272

21. Leases The statements of financial position shows the following amounts relating to leases: Year Ended December 31, (in thousands of $) 2024 2023 2022 Right-of-use assets Buildings 33,780 16,798 10,867 Vehicles 6,615 3,191 1,835 Equipment 125 160 196 40,520 20,149 12,897 Lease liabilities Current 6,533 4,646 3,417 Non-current 32,520 15,354 9,009 39,053 20,000 12,426 Additions to the right-of-use assets amounted to $26 million for the year ended December 31, 2024, compared to $11 million and $4 million for the years ended December 31, 2023 and 2022 respectively. The table below shows a maturity analysis of the lease liabilities as on December 31, 2024: (in thousands of $) Less than 1 year 1-3 years 3-5 years More than 5 years Total contrac tual cash flows Carrying value Lease liabilities 8,047 14,499 11,171 11,829 45,547 39,053 The consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss shows the following amounts relating to leases: Year Ended December 31, (in thousands of $) 2024 2023 2022 Depreciation charges Buildings 3,657 2,839 2,179 Vehicles 2,067 971 735 Equipment 35 36 35 5,759 3,846 2,949 Interest expense (included in finance cost) 2,072 693 1,343 Expense relating to short-term leases 1,517 1,517 732 Expense relating to leases of low-value assets that are not shown above as short-term leases 40 40 21 The total cash outflow for leases in 2024, 2023 and 2022 was $8 million, $4 million and $4 million respectively. The Company did not enter into any lease agreement with variable lease payments or residual value guarantees. The Company has leases that include extension options. These options provide flexibility in managing the leased assets and align with the Company’s business needs. The Company exercises judgement in deciding whether it is reasonably certain that the extension options will be exercised. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 273

22. Financial Result and Exchange Gains/(Losses) Year Ended December 31, (in thousands of $) 2024 2023 2022 Interest income 138,740 92,962 24,741 Net gain on cash equivalents & current financial assets held at fair value through profit or loss and cash equivalents 18,769 14,424 2,924 Financial income 157,509 107,386 27,665 Net loss on cash equivalents & current financial assets held at fair value through profit or loss and cash equivalents – (2) (1,713) Other financial expense (2,464) (904) (2,193) Financial expense (2,464) (906) (3,906) Realized exchange (losses)/gains (5,444) 29 (3,743) Unrealized exchange (losses)/gains (42,767) 14,044 (28,989) Exchange (losses)/gains (48,211) 14,073 (32,732) The exchange losses of $48 million for the year ended December 31, 2024 were primarily attributable to unrealized exchange rate gains on the cash and cash equivalents and current financial assets position in EUR due to the fluctuation of the EUR/USD exchange rate over the period. 23. Income taxes Income taxes recognized in the income statements can be detailed as follows: Year Ended December 31, (in thousands of $) 2024 2023 2022 Current year (53,462) (9,592) (27,162) Income tax prior years (383) (2,080) (12) Current tax (expense)/benefit (53,845) (11,672) (27,174) Recognition of deferred tax assets 724,700 – – Originating and reversal of temporary differences 77,005 21,115 46,894 Deferred tax benefit 801,705 21,115 46,894 Total tax benefit 747,860 9,443 19,720 The difference between the provision for income taxes and the amount that would result from applying the Dutch statutory tax rate to income before provision for income taxes is as follows: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 274

Year Ended December 31, (in thousands of $) 2024 2023 2022 (Profit)/Loss before taxes (85,180) 304,496 729,314 Income tax (expense)/benefit calculated at the Dutch statutory federal income tax rates (21,977) 78,560 188,163 Effect of intercompany asset deal/transaction – 396 (112,200) Effect of expenses not deductible in determining taxable results (5,383) (2,674) (1,570) Effect of share based payment expenses that are not deductible in determining taxable results (13,151) (43,040) (27,043) Effect of stock issue expenses that are not taxable in determining taxable results – 18,620 11,412 Effect of concessions 102,823 87,123 18,263 Effect of change of (de)recognition of deferred tax assets on tax losses 187,361 (2,282) (194) Effect of different tax rates in jurisdictions in which the company operates 4,169 (3,509) (5,566) Effect of change of (de)recognition of deferred tax assets 535,598 (124,457) (51,320) Effect of foreign exchange translation (38,307) – – Other 1) (3,273) 706 (225) Income tax (expense)/benefit recognized in the consolidated statements of profit or loss 747,860 9,443 19,720 1) Comparative figures have been presented to be consistent with the one adopted in the current year. Deferred tax assets are recognized to the extent that it is probable that sufficient taxable profits will be available in the look-forward period. The Company believes that it is probable that sufficient future taxable profits will be generated to support the recognized deferred tax asset for tax losses carried forward in Belgium. As part of its assessment, the Company has taken into account recent taxable profits or losses, forecasted operating profits and taxable earnings, U.S. based Product Net Sales from the commercialization of the VYVGART franchise, evolution of the external competitive landscape, and likelihood that factors contributing to past losses will not recur, while considering the risks and uncertainties associated with those forecasts. We consider that the management forecasts used specifically in this assessment to be reasonable, based on historical accuracy and alignment with external market data. The Company considered ordering rules established by tax legislation Belgium noting that under Belgian tax legislation, tax losses and Innovation Income Deduction can be carried forward indefinitely. Based on the weight of available evidence, in the fourth quarter of 2024, the Company recognized a consolidated tax benefit for previously unrecognized net deferred tax assets existing as of December 31, 2023 amounting to $725 million. As of December 31, 2024, the Company’s balance of net deferred tax assets for argenx BV totaled $708 million. During 2022, argenx Benelux BV transferred certain pipeline activities to argenx BV through a transfer of assets, (hereafter referred to as “asset deal”), for a total amount of $449 million. As a result of the asset deal, argenx Benelux BV realized a capital gain on this intellectual property, which results in the rate reconciling item categorized as “effect of intercompany asset deal/transaction”. The amount of deferred tax assets and liability by type of temporary difference can be detailed as follows: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 275

As of December 31, 2024 (in thousands of $) Assets Liabilities Net Deferred tax assets/(liabilities) Innovation income deduction 122,306 – 122,306 Net operating loss carryforwards 177,599 – 177,599 Capitalized R&D expenses 312,420 – 312,420 Intangible assets 100,321 – 100,321 Accruals and allowances 25,037 – 25,037 Share-based payments 71,481 – 71,481 Profit in inventory 110,474 – 110,474 Other tax carryforwards 8,874 8,874 Property, plant and equipment 3,392 (3,012) 380 Non-current fixed assets – (6,289) (6,289) Other 2,265 (569) 1,696 Netting by taxable entity (9,870) 9,870 – Net deferred tax assets 924,299 – 924,299 As of December 31, 2023 (in thousands of $) Assets Liabilities Net Deferred tax assets/(liabilities) Accruals and allowances 13,189 – 13,189 Share-based payments 23,310 – 23,310 Profit in inventory 52,026 – 52,026 Other tax carryforwards 6,339 – 6,339 Property, plant and equipment 2,136 (1,550) 586 Non-current fixed assets – (5,155) (5,155) Other 1,760 – 1,760 Netting by taxable entity (1,549) 1,550 1 Net deferred tax assets/(liabilities) 97,211 (5,155) 92,056 As of December 31, 2022 (in thousands of $) Assets Liabilities Net Deferred tax assets/(liabilities) Accruals and allowances 8,884 – 8,884 Share-based payments 26,887 – 26,887 Profit in inventory 29,711 – 29,711 R&D capitalized expense 11,316 – 11,316 Property, plant and equipment 856 (549) 307 Intangible assets – (3,430) (3,430) Non-current fixed assets – (4,975) (4,975) Other 2,117 – 2,117 Netting by taxable entity (549) 549 – Net deferred tax assets/(liabilities) 79,222 (8,406) 70,817 argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 276

The change in net deferred taxes recorded in the consolidated statements of financial position can be detailed as follows: (in thousands of $) Deferred tax assets Deferred tax liabilities Balance on January 1, 2024 97,211 (5,155) Recognized in profit or loss 758,264 5,155 Recognized in equity 30,846 – Effects of change in foreign exchange rate 37,978 – Balance on December 31, 2024 924,299 – (in thousands of $) Deferred tax assets Deferred tax liabilities Balance on January 1, 2023 79,222 (8,406) Recognized in profit or loss 17,685 3,430 Recognized in equity 381 – Effects of change in foreign exchange rate (77) (179) Balance on December 31, 2023 97,211 (5,155) (in thousands of $) Deferred tax assets Deferred tax liabilities Balance on January 1, 2022 32,191 (6,438) Recognized in profit or loss 49,075 (2,180) Recognized in equity (1,960) – Effects of change in foreign exchange rate (84) 212 Balance on December 31, 2022 79,222 (8,406) The Company also has unrecognized tax losses carried forward in the Netherlands in the amount of $46 million as of December 31, 2024, compared to $33 million on December 31, 2023 and $35 million on December 31, 2022. These losses carried forward do not have an expiration date based upon the applicable enacted tax legislation in the Netherlands. As of December 31, 2024, the Company has $125 million of undistributed earnings attributable to foreign subsidiaries for which no provision for deferred tax liabilities have been recognized because the Company has control over the timing of the reversal of the temporary differences and there are no plans of distributions in the foreseeable future. On 23 May 2023, the International Accounting Standards Board (the IASB or Board) issued International Tax Reform – Pillar Two Model Rules – Amendments to IAS 12 which clarified the application of IAS 12 Income Taxes arising from tax law enacted or substantively enacted to implement the OECD/G20 Inclusive Framework on Base Erosion and Profit Shifting Pillar Two model rules. Based on current information, management expects that the Company will be subject to the Pillar Two Directive and implementing domestic laws in 2025, as it is the year the Company has met all requirements under the Pillar Two legislation. The company is currently in the process of determining the impact, if any, for 2025. Based on the preliminary analysis, we do not expect the Pillar Two Rules to have a material impact on our effective tax rate. It is unclear if the Pillar Two model rules create additional temporary differences, whether to remeasure deferred taxes for the Pillar Two model rules, and which tax rate to use to measure deferred taxes. In response to this unclarity, the amendments mentioned above introduced a mandatory temporary exception to the requirements of IAS 12 under which a company does not recognize or disclose information about deferred tax assets and liabilities related to the Pillar Two model rules. We continue to apply the temporary exception for the year ended December 31, 2024. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 277

24. Earnings per Share Year Ended December 31 (in thousands of $ except for shares and EPS) 2024 2023 2022 Profit/(Loss) for the period 833,040 (295,053) (709,594) Weighted average number of shares outstanding 59,855,585 57,169,253 54,381,371 Basic profit/(loss) per share (in $) 13.92 (5.16) (13.05) Weighted average number of shares for purpose of diluted profit/(loss) per share 65,177,815 57,169,253 54,381,371 Diluted profit/(loss) per share (in $) 12.78 (5.16) (13.05) Profit/(loss) per ordinary share is calculated by dividing the profit/(loss) for the period by the weighted average number of ordinary shares during the year. Diluted profit/(loss) per share is calculated by adjusting the weighted average number of shares by in the money outstanding dilutive stock options and RSUs. As the Company reported a net loss in 2023 and 2022, stock options and RSUs have an anti-dilutive effect rather than a dilutive effect. As such, there is no difference between basic and diluted loss per ordinary share for those periods. 25. Financial Risk Management The financial risks are managed centrally. The Company coordinates the access to national and international financial markets and considers and manages continuously the financial risks concerning the Company’s activities. These relate to credit risk, liquidity risk, interest rate risk and currency risk. The Company does not buy or trade financial instruments for speculative purposes. Categories of financial assets and liabilities: Measurement category Carrying amount on December 31 (in thousands of $) 2024 2023 2022 Financial assets - non-current FVTPL 25,549 21,715 21,715 Financial assets - non-current FVTOCI 14,880 15,528 17,443 Research and development incentive receivables - non- current Amortized cost 94,854 76,706 47,488 Restricted cash - non-current Amortized cost 1,964 2,419 1,736 Trade and other receivables Amortized cost 904,471 496,687 275,697 Financial assets - current FVTPL – – 46,162 Financial assets - current Amortized cost 1,878,890 1,131,000 1,345,646 Research and development incentive receivables - current Amortized cost 4,625 2,584 1,578 Cash and bank balances Amortized cost 5,527 20,744 77,477 Cash equivalents FVTPL 1,394,409 1,678,100 669,147 Cash equivalents Amortized cost 100,000 350,000 54,116 Trade and other payables Amortized cost 649,993 414,013 295,679 The carrying amounts of trade and other payables and trade and other receivables are considered to be the same as their fair values, due to their short-term nature. Financial assets held at fair value through profit or loss or OCI Financial assets held at fair value through profit or loss or OCI consisted of equity instruments of listed and non-listed companies and money market funds. The Company has no restrictions on the sale of these equity instruments and the assets are not pledged under any of its liabilities. These instruments are classified as financial assets held at fair value through profit or loss or OCI which qualify for: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 278

• Level 1 fair value measurement with respect to current financial assets and cash equivalents based upon the closing price (net asset value) of such securities at each reporting date. • Level 3 fair value measurement with respect to non-current financial assets. The market price of these financial instruments might face fluctuations and might be affected by a variety of factors, such as the global economic situation. Current financial assets and cash equivalents include collective investment funds denominated in € and $ of which the underlying investments include bonds and other international debt securities. Based on the weighted average maturity of the underlying instruments, amongst others, these investments are either classified as current financial assets or cash equivalents. The maximum exposure to credit risk is the carrying amount at reporting date. The Company carried the following assets at fair value on December 31, 2024, 2023 and 2022 respectively: As of December 31, 2024 (in thousands of $) Level 1 Level 2 Level 3 Non-current financial assets 14,880 – 25,549 Cash and cash equivalents 1,394,409 – – Assets carried at fair value 1,409,289 – 25,549 As of December 31, 2023 (in thousands of $) Level 1 Level 2 Level 3 Non-current financial assets 15,528 – 21,715 Cash and cash equivalents 1,678,100 – – Assets carried at fair value 1,693,628 – 21,715 As of December 31, 2022 (in thousands of $) Level 1 Level 2 Level 3 Non-current financial assets 17,443 – 21,715 Current financial assets 46,162 – – Cash and cash equivalents 669,147 – – Assets carried at fair value 732,752 – 21,715 During the disclosed calendar year, no transfers occurred between the applicable categories. Non-current financial assets – Level 3 In March 2019, the Company entered into a license agreement with AgomAb Therapeutics NV for the use of HGF-mimetic SIMPLE Antibodies™, developed under the Company’s Immunology Innovative Program. In exchange for granting this license, the Company received a profit share in AgomAb Therapeutics NV. The changes in the value of this investment are detailed in ‘‘Note 6 Other Non-Current Assets’’. Non-current financial assets – Level 1 In January 2021, as part of the license agreement for the development and commercialization for efgartigimod in Greater China, the Company obtained, amongst others, 568,182 newly issued Zai Lab shares calculated at a price of $132 per share. The fair value of the equity instrument at period-end is determined by reference to the closing price of such securities at each reporting date (classified as level 1 in the fair value hierarchy), resulting in a change in fair value. The Company made the irrevocable election to recognize subsequent changes in fair value through OCI. Capital risk The Company manages its capital to ensure that it will be able to continue as a going concern. The capital structure of the Company consists of equity attributed to the holders of equity instruments of the Company, such as capital, reserves and accumulated losses as mentioned in the consolidated statements of changes in equity. The Company makes the necessary adjustments in light of changes in the economic circumstances, risks associated to the different assets and the projected cash needs of the current and projected research activities. On December 31, 2024, cash and cash equivalents amounted to $1.5 billion, current financial assets amounted to $1.9 billion and total capital amounted to $5.5 billion. The current argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 279

cash situation and the anticipated cash generation and usage are the most important parameters in assessing the capital structure. The Company’s objective is to maintain the capital structure at a level to be able to finance its activities for at least twelve months. Cash income from operations is taken into account and, if needed and possible, the Company can issue new shares or enter into financing agreements. Credit risk Credit risk refers to the risk that a counterparty will default on its contractual obligations resulting in financial loss to the Company. The Company has adopted a policy of only dealing with creditworthy counterparties and obtaining sufficient collateral, where appropriate, as a means of mitigating the risk of financial loss from defaults. Concentrations in credit risk are determined based on an analysis of counterparties and their importance on the overall outstanding contractual obligations at year-end. The Company's commercial revenue are concentrated as discussed in “Note 17 Segment Reporting”, on a limited number of U.S. customers with high quality creditworthiness. The Company sets customer specific credit limits in order to reduce credit risk from commercial payers. The Company applied the IFRS 9 simplified approach to measuring expected credit losses which uses a lifetime expected loss allowance for all receivables. To measure the expected credit losses, receivables have been grouped based on credit risk characteristics and the days past due. The provision for expected credit losses was not significant given that there have been no credit losses over the last three years and the high quality nature of the Company’s customers. Cash and cash equivalents and current financial assets are invested with several highly reputable banks and financial institutions. The main purpose of the Cash Investment Policy is to preserve the available cash and to ensure sufficient short-term liquidity at all times. Therefore, the Company holds its cash and cash equivalents, in addition to current financial assets mainly with banks which are independently rated A- or higher. Amounts of cash held with banks rated lower than A- are limited to insignificant balances. The maximum amount and tenor of time deposits depends on the rating of the counterparty bank. The Company also holds cash equivalents in the form of money market funds with a low historical volatility. These money market funds are highly liquid investments and can be readily convertible into a known amount of cash. The company has adopted a policy whereby money market funds must have a minimum rating of A, and whereby 95% of its money market funds should have a AAA-rating. Liquidity risk The Company manages liquidity risk by maintaining adequate reserves, by continuously monitoring forecast and actual cash flows, and by matching the maturity profile of financial assets and liabilities. The Company’s main sources of cash are the sale of commercial product and exercise of stock options. This cash is invested in savings accounts, term accounts and money market funds. These money market funds represent the majority of the Company’s available sources of liquidity. Since all of these are immediately tradable and convertible in cash they have an important mitigating effect on any short-term liquidity risk. As of December 31, 2024, the Company had lines of credit totaling $16 million with the banks which were not used as of year end. Interest rate risk The only variable interest-bearing financial instruments are cash and cash equivalents and current financial assets. Changes in interest rates may cause variations in interest income resulting from short-term interest- bearing assets. Lower short-term interests may have a negative impact on the interest income of the Company. For the year ended December 31, 2024, if applicable interest rates would increase/decrease by 25 basis points, this would have a positive/negative impact of $8 million (compared to $8 million for the year ended December 31, 2023 and $6 million for the year ended December 31, 2022). argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 280

Foreign exchange risk The Company undertakes transactions denominated in foreign currencies, causing exposures to exchange rate fluctuations. The Company is mainly exposed to the Euro, Japanese yen, British pound and Swiss franc. To limit this risk, the Company attempts to align incoming and outgoing cash flows in currencies other than USD. The net exposure to exchange differences of the monetary assets (being from cash and cash equivalents, in addition to current financial assets) of the Company at the end of the reporting period are as follows: As of December 31, (in thousands of $) 2024 2023 2022 EUR 756,676 923,773 613,866 JPY 1,640 8,232 5,613 GBP 11 7 59,026 CHF 18 193 3,832 CAD 3 266 657 Other currencies 7 10 13 On December 31, 2024, if the EUR would have strengthened/weakened versus the USD by 10%, this would have had a negative/positive impact of $76 million, compared to $92 million and $61 million on December 31, 2023 and December 31, 2022, respectively. On December 31, 2024, if other currencies would have strengthen/weakened against the USD by 10%, this would have had no significant impact. 26. Related Party Transactions 26.1 Relationship and transactions with joint venture entity In 2022, the University of Colorado Anschutz Medical Campus and the University of Colorado Health (UCHealth) created an asset-centric spin-off, OncoVerity, Inc (OncoVerity), focused on optimizing and advancing the development of cusatuzumab, a novel anti-CD70 antibody, in acute myeloid leukemia (AML). OncoVerity is an entity of co-creation, combining the extensive translational biology insights from Dr. Clayton Smith, M.D. from the University of Colorado with our experience on the CD70/CD27 pathway. argenx contributed $7 million in 2024 ($13 million and $2 million in 2023 and 2022 respectively). The investment has been accounted under IAS 28 Investment in associates and Joint Ventures using the equity method of accounting and has been designated as an “Investment in a joint venture” in the consolidated statements of financial position. The share of net loss resulting from investment in joint ventures is presented in consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss in line “Loss from investment in a joint venture”. The cash contributions made by the Company to the Joint Venture is reported under Cash flow from investing activities under “Investment in a joint venture”. 26.2 Relationship and transactions with subsidiaries See ‘‘Note 30 Overview of Consolidation Scope’’ for an overview of the consolidated companies of the group, which are all wholly-owned subsidiaries of argenx SE. Balances and transactions between the Company and its subsidiaries, which are related parties of the Company, have been eliminated on consolidation and are not disclosed in this note. 26.3 Relationship and transactions with key personnel The Company’s key management personnel consists of the members of the management team and the members of the board of directors. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 281

Remuneration of key management personnel On December 31, 2024, the Senior Management Team consisted of 8 members: Chief Executive Officer, Chief Operating Officer, Chief Financial Officer, Chief Scientific Officer, General Counsel, Chief Medical Officer, Vice President Corporate Development and Strategy and Global Head of Quality Assurance. They provide their services on a full-time basis. On December 31, 2024, the board of directors consisted of 10 members: Mr. Peter Verhaeghe, Dr. Donald deBethizy, Dr. Pamela Klein, Anthony Rosenberg, James Daly, Camilla Sylvest, Dr. Brian Kotzin, Dr. Ana Cespedes, Mr. Steve Krognes and Tim Van Hauwermeiren. Only the Chief Executive Officer is a member of both the Senior Management Team and the board of directors. The Chief Executive Officer does not receive any remuneration for his board membership, as this is part of his total remuneration package in his capacity as member of the Senior Management Team. The remuneration package of the members of key management personnel comprises: Year Ended December 31, (in thousands of $, except for the number of stock options & RSUs) 2024 2023 2022 Remuneration of key management personnel Short-term benefits for the Senior Management Team Gross salary 4,529 4,161 4,199 Variable pay 3,084 2,816 3,077 Employer social security 1,473 807 1,015 Other short term benefits 672 545 372 Termination Benefits – – – Post-employment benefits for the Senior Management Team 274 167 104 Cost of stock options granted in the year for the Senior Management Team 17,758 27,983 18,393 Cost of restricted stock units granted in the year for the Senior Management Team 16,211 11,694 9,594 Employer social security cost related to stock options 2,825 (494) 1,101 Total benefits for key management personnel 46,826 47,679 37,855 Numbers of stock options granted in the year Senior Management Team 98,306 132,100 117,600 Numbers of restricted stock units granted in the year Senior Management Team 36,365 30,425 26,500 Remuneration of Non-Executive Directors Board fees and other short-term benefits for Non-Executive Directors 731 533 437 Cost of stock options granted in the year for Non-Executive Directors – 2,280 3,643 Cost of restricted stock units granted in the year for Non-Executive Directors 4,511 1,034 1,850 Total benefits for Non-Executive Directors 5,242 3,847 5,929 Numbers of stock options granted in the year Non-Executive Directors – 12,400 21,600 Numbers of restricted stock units granted in the year Non-Executive Directors 10,118 2,713 4,800 Other No loans, quasi-loans or other guarantees were given by the Company or any of its subsidiaries to members of the board of directors or the Senior Management Team. We have not entered into transactions with the Company’s key management personnel, other than as described above with respect to remuneration arrangements relating to the exercise of their mandates as members of the Senior Management Team and the board of directors. 27. Contingencies The Company is currently not facing any outstanding claims or litigation that may have a significant adverse impact on the Company’s consolidated financial position. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 282

28. Commitments At balance sheet date, there were no commitments signed for the acquisition of property, plant and equipment. In February 2019, the Company entered into a global collaboration and license agreement with Halozyme Therapeutics., which was later amended in September 2020 and again in September 2024. Under the terms of the agreement, the Company will pay up to $95 million to achievement of specific regulatory and sales-based milestones related specifically to its FcRn target. This amount represents the maximum amount that would be paid if all milestones would be achieved but excludes variable royalty payments based on unit sales. Further, the Company will pay up to $78 million per other non-FcRn target subject to achievement of specified development, regulatory and sales-based milestones. This amount represents the maximum amount that would be paid per target if all milestones would be achieved but excludes variable royalty payments based on unit sales. The Company has a total of six nominated targets under this agreement including its FcRn target. The Company’s manufacturing commitments with Lonza, its drug substance manufacturing contractor, relate to the ongoing execution of the biologic license application (BLA) services for efgartigimod and its manufacturing activities related to the potential future commercialization. In December 2018, the Company signed its first commercial supply agreement with Lonza related to the reservation of commercial drug substance supply capacity for efgartigimod. In the aggregate, the Company has outstanding commitments for efgartigimod under the commercial supply agreements of $496 million. As of December 31, 2024, the Company had a line of credit totaling $16 million with the banks. 29. Audit Fees The following auditors’ fees were expensed in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income or loss: Year Ended December 31, (in thousands of $) 2024 2023 2022 Audit fees 1) 2,657 1,979 1,394 Audit-related fees 597 330 380 Total 3,254 2,309 1,774 1) Audit services performed by Deloitte Accountants B.V. as the external auditor referred to in Section 1 of the Dutch Accounting Firms Oversight Act (Wta) as well as by the Deloitte network. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 283

30. Overview of Consolidation Scope The parent company argenx SE is domiciled in The Netherlands. The Company, argenx SE, has one subsidiary, argenx BV, which is based in Belgium. argenx BV has fourteen subsidiaries. Details of the Company’s consolidated entities at the end of the reporting period are as follows: Name Country Participation argenx SE The Netherlands 100% argenx B.V. Belgium 100% argenx Benelux B.V. Belgium 100% argenx US, Inc. USA 100% argenx Switzerland, S.A. Switzerland 100% argenx Japan KK. Japan 100% argenx France SAS France 100% argenx Germany GmbH Germany 100% argenx Canada Inc. Canada 100% argenx UK Ltd. United Kingdom 100% argenx Netherlands Services B.V. The Netherlands 100% argenx Italy S.r.l. Italy 100% argenx Spain S.L. Spain 100% argenx Australia Pty. Ltd. Australia 100% argenx Spain S.L. - Sucursal em Portugal Portugal 100% argenx Austria Services GmbH Austria 100% 31. Events After the Balance Sheet Date No events have occurred after the balance sheet date that could have a material impact on the consolidated financial statements. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Notes to the Consolidated Financial Statements 284

6.3 Company Financial Statements of argenx SE for the Year ended December 31, 2024 6.3.1 Signatures of Executive and Non-Executive Directors In accordance with Article 2:101 of the Dutch Civil Code, the annual accounts were signed by all executive and Non-Executive Directors on March 19, 2025. 6.3.2 Company Financial Statements of argenx SE For argenx SE For the year ended December 31, 2024 Company Balance Sheet on December 31, 2024 of argenx SE As of December 31, (in thousands of $) Note 2024 2023 Assets Non-current assets Financial fixed assets 2 Investments in Group Companies 4,796,972 3,703,280 Other financial assets 1 Total financial fixed assets 4,796,972 3,703,281 Total non-current assets 4,796,972 3,703,281 Current assets Receivables 3 705,832 369,640 Cash and cash equivalents 4 6 28,744 Total current assets 705,838 398,384 Total assets 5,502,810 4,101,665 Equity and liabilities Equity 5 Share capital 7,227 7,058 Share premium 5,948,916 5,651,497 Accumulated losses (1,571,804) (2,404,845) Reserve for share-based payments 985,180 749,324 Translation reserves 126,832 131,543 Other reserves 1,932 (37,073) Total equity 5,498,283 4,097,505 Current liabilities 6 Accounts payable 388 266 Intercompany payables 2,426 2,127 Taxes payable 53 925 Accrued expenses 1,660 841 Other payables – – Total liabilities 4,527 4,159 Total equity and liabilities 5,502,810 4,101,665 argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Company Financial Statements of argenx SE for the Year ended December 31, 2024 285

Company Profit and Loss Account for the Year Ended December 31, 2024 of argenx SE Year Ended December 31, (in thousands of $) Note 2024 2023 Intercompany recharges – – Total operating income – – General & adminstrative expenses (21,594) (19,303) Total operating expenses (21,594) (19,303) Operating result (21,594) (19,303) Financial income and expense 7 2,161 19,378 Share in result of subsidiaries 8 852,450 (294,476) Result before taxation 833,017 (294,402) Taxation on result of ordinary activities 23 (652) Result after taxation 833,040 (295,053) 6.3.3 Notes to the Company Financial Statements of argenx SE 1. Acounting information and Policies 1.1 Basis of Preparation The company financial statements of argenx SE (hereafter: the Company) have been prepared in accordance with Part 9, Book 2 of the Dutch Civil Code. In accordance with article 362 sub8, Book 2 of the Dutch Civil Code, the company’s financial statements are prepared based on the accounting principles of recognition, measurement and determination of profit, as applied in the Consolidated IFRS financial statements. 1.2 Summary of Significant Accounting Policies In case no other policies are mentioned, refer to the accounting policies as described in the summary of significant accounting policies in the Consolidated IFRS financial statements. For an appropriate interpretation, the company financial statements of argenx SE should be read in conjunction with the Consolidated IFRS financial statements. Participating Interests in Group Companies Participating interests in group companies are valued using the equity method, applying the IFRS accounting policies endorsed by the European Union. Following the adoption of IFRS 9 by the group, and our interpretation of the Dutch Accounting Standard 100.108, the company shall, upon identification of a credit loss on an intercompany loan and/or receivable, eliminate the carrying amount of the intercompany loan and/or receivable for the value of the identified credit loss. Result of Participating Interests The share in the result of participating interests consists of the share of the Company in the result of these participating interests. In so far as gains or losses on transactions involving the transfer of assets and liabilities between the Company and its participating interests or between participating interests themselves can be considered unrealized, they have not been recognized. All amounts are presented in thousands of USD, unless stated otherwise. The balance sheet and income statement references have been included. These refer to the notes herein. 2. Financial Fixed Assets The Company has a Belgian subsidiary, argenx BV, which carries out the research and development activities of the Group and is the supplier of commercial product to entities within the Group. argenx B.V. has fourteen subsidiaries, argenx US, Inc., argenx Benelux B.V., argenx Japan KK., argenx Australia Pty. Ltd., argenx Switzerland, S.A., argenx Germany GmbH, argenx France SAS, argenx Canada Inc., argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Company Financial Statements of argenx SE for the Year ended December 31, 2024 286

argenx Netherlands Services B.V., argenx UK Ltd., argenx Italy S.r.l., argenx Spain S.L., argenx Spain S.L. - Sucursal em Portugal, argenx Austria Services GmbH. The financial fixed assets mainly consist of the 100% participation in argenx BV registered at Industriepark- Zwijnaarde 7, 9052 Zwijnaarde, Belgium. The movement in financial fixed assets is as follows: As of December 31, (in thousands of $) 2024 2023 Investments in Group Companies Opening balance 3,703,279 2,583,759 Share of result of investments 852,450 (294,476) Share-based payment expenses of investments 228,819 228,023 Fair value gain on financial assets held at FVTPL – (67,200) Capital increase in subsidiaries – 1,262,653 Changes booked directly in equity at the subsidiary level 12,425 (9,480) Ending balance 4,796,973 3,703,279 Receivable/(payable) on Group companies – – Investments in Group companies 4,796,973 3,703,279 Other financial assets Opening balance 1 1 Change in the period (1) – Ending balance – 1 Total financial fixed assets 4,796,973 3,703,280 3. Receivables As of December 31, (in thousands of $) 2024 2023 Other receivables 704,814 368,543 Prepaid expenses 1,018 964 Interest receivables – 133 Total receivables 705,832 369,640 Receivables fall due in less than one year. The fair value of the receivables approximates the nominal value, due to their short-term character. Other receivables are short-term receivables from argenx BV. 4. Cash and Cash equivalents As of December 31, (in thousands of $) 2024 2023 Money market funds – 28,736 Current bank accounts 6 8 Total cash in banks 6 28,744 argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Company Financial Statements of argenx SE for the Year ended December 31, 2024 287
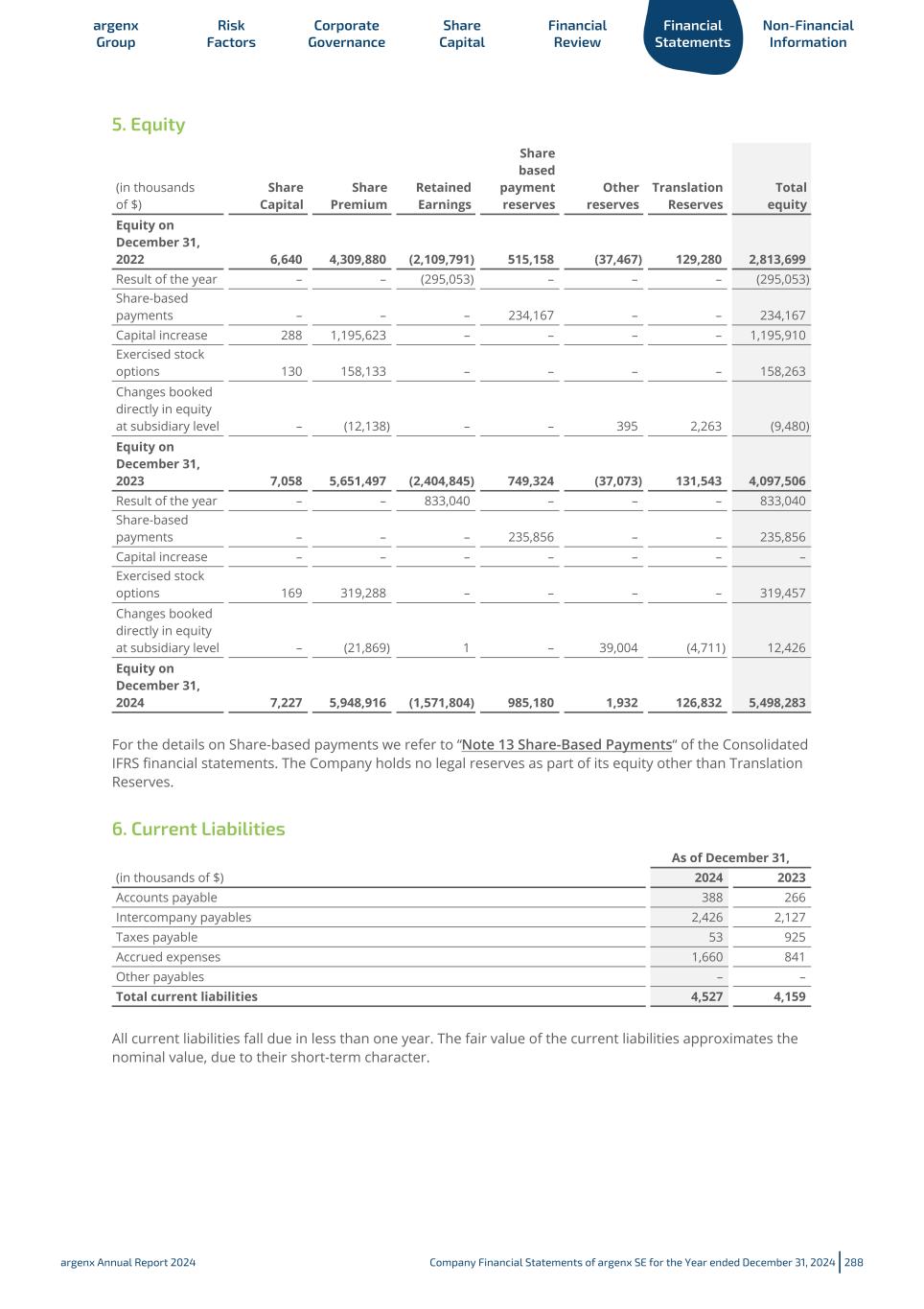
5. Equity (in thousands of $) Share Capital Share Premium Retained Earnings Share based payment reserves Other reserves Translation Reserves Total equity Equity on December 31, 2022 6,640 4,309,880 (2,109,791) 515,158 (37,467) 129,280 2,813,699 Result of the year – – (295,053) – – – (295,053) Share-based payments – – – 234,167 – – 234,167 Capital increase 288 1,195,623 – – – – 1,195,910 Exercised stock options 130 158,133 – – – – 158,263 Changes booked directly in equity at subsidiary level – (12,138) – – 395 2,263 (9,480) Equity on December 31, 2023 7,058 5,651,497 (2,404,845) 749,324 (37,073) 131,543 4,097,506 Result of the year – – 833,040 – – – 833,040 Share-based payments – – – 235,856 – – 235,856 Capital increase – – – – – – – Exercised stock options 169 319,288 – – – – 319,457 Changes booked directly in equity at subsidiary level – (21,869) 1 – 39,004 (4,711) 12,426 Equity on December 31, 2024 7,227 5,948,916 (1,571,804) 985,180 1,932 126,832 5,498,283 For the details on Share-based payments we refer to “Note 13 Share-Based Payments“ of the Consolidated IFRS financial statements. The Company holds no legal reserves as part of its equity other than Translation Reserves. 6. Current Liabilities As of December 31, (in thousands of $) 2024 2023 Accounts payable 388 266 Intercompany payables 2,426 2,127 Taxes payable 53 925 Accrued expenses 1,660 841 Other payables – – Total current liabilities 4,527 4,159 All current liabilities fall due in less than one year. The fair value of the current liabilities approximates the nominal value, due to their short-term character. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Company Financial Statements of argenx SE for the Year ended December 31, 2024 288

7. Financial Result and exchange Gains/(Losses) As of December 31, (in thousands of $) 2024 2023 Net gains on investments held at FVTPL 88 7,343 Fees collected from ADS holders 509 500 Interest on intercompany current account 6,236 3,893 Dividend income – – Financial income 6,833 11,736 Other financial expenses (8) (29) Financial expenses (8) (29) Exchange gains/(losses) (4,664) 7,671 Financial income and expense 2,161 19,378 8. Share in Result of Subsidiaries The Company has one Belgian subsidiary, argenx BV, which carries out the research and development activities of the Group and its commercial supply. Year ended December 31, (in thousands of $) 2024 2023 argenx BV 852,450 (307,191) argenx Benelux BV – 12,656 Total share in result of subsidiaries 852,450 (294,476) 9. Other Disclosures Contingent Liabilities The contingent liabilities of the Company consist of a rental agreement for office space in Amsterdam for an amount of $21,276 per annum until 2026. Related-Party Transactions All legal entities that can be controlled, jointly controlled or significantly influenced are considered as a related party. Also, entities which can control the company are considered a related party. In addition, directors, other key management of argenx SE and close relatives are regarded as related parties. Other than the intercompany cross-charges, there were no related party transactions. Remuneration Remuneration of the Executive Director for 2024 and 2023 is as follows: (in $) 2024 2023 Base pay 757,679 655,787 Variable short-term incentive 795,563 590,215 Stock options granted 3,194,813 8,084,605 Restricted stock units (RSUs) granted 3,014,500 2,575,174 Pension contributions 29,118 22,821 Other 16,112 16,232 Total remuneration of the executive director 7,807,785 11,944,835 Part of the remuneration of the Executive Director is being paid by subsidiaries of argenx SE. See “Note 26 Related Party Transactions“ of the notes to the Consolidated IFRS financial statements for the remuneration of non-executive Board of directors. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Company Financial Statements of argenx SE for the Year ended December 31, 2024 289

Information Relating to Employees During the year 2024, the Company had an average of 0.25 FTE (2023: 0.20 FTE). Auditor’s Fees See “Note 29 Audit Fees“ of the notes to the Consolidated IFRS financial statements. Proposal for Appropriation of the Result The Company reported a profit for the year of $833 million for the year ended on December 31, 2024. The Board of Directors proposes to carry forward the net profit of the year 2024 to the accumulated losses. Anticipating the approval of the financial statements by the shareholders at the annual general meeting of shareholders, this proposal has already been reflected in the 2024 financial statements. Events after the balance sheet date For the events after balance sheet date, we refer to “Note 31 Events After the Balance Sheet Date“ of the Consolidated IFRS financial statements. Amsterdam, March 20, 2025 The Director Tim Van Hauwermeiren, CEO argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Company Financial Statements of argenx SE for the Year ended December 31, 2024 290

6.4 Other information 6.4.1 Provision in the articles of association governing the appropriation of results 1. The company shall have a policy on reserves and dividends which shall be determined and may be amended by the board of directors. The adoption and thereafter each material change of the policy on reserves and dividends shall be discussed at the general meeting under a separate agenda item. 2. From the profits, shown in the annual accounts, as adopted, the board of directors shall determine which part shall be reserved. Any profits remaining thereafter shall be at the disposal of the general meeting. The board of directors shall make a proposal for that purpose. A proposal to pay a dividend shall be dealt with as a separate agenda item at the general meeting. 3. Distribution of dividends on the shares shall be made in proportion to the nominal value of each share. 4. Distributions may be made only insofar as the Company’s equity exceeds the amount of the paid in and called up part of the issued capital, increased by the reserves which must be kept by virtue of the law. 5. If a loss was suffered during any one year, the board of directors may resolve to offset such loss by writing it off against a reserve which the company is not required to keep by virtue of the law. 6. The distribution of profits shall be made after the adoption of the annual accounts, from which it appears that the same is permitted. 7. The board of directors may, subject to due observance of the policy of the Company on reserves and dividends, resolve to make an interim distribution, provided the requirement of paragraph 4 of this article has been complied with, as shown by interim accounts. Such interim accounts shall show the financial position of the Company not earlier than on the first day of the third month before the month in which the resolution to make the interim distribution is announced. Such interim accounts shall be signed by all members of the board of directors. If the signature of one or more of them is missing, this shall be stated and reasons for this omission shall be given. The interim accounts shall be deposited in the offices of the trade register within eight days after the day on which the resolution to make the interim distribution has been announced. 8. At the proposal of the board of directors, the general meeting may resolve to make a distribution on shares wholly or partly not in cash but in shares. 9. The board of directors may, subject to due observance of the policy of the Company on reserves and dividends, resolve that distributions to holders of shares shall be made out of one or more reserves. 10. A claim of a shareholder for payment of a distribution shall be barred after five years have elapsed. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 291

6.4.2 Independent Auditor's report To the shareholders and the Board of Directors of argenx SE Report on the audit of the financial statements for the year ended December 31, 2024 included in The Annual Report Our opinion We have audited the financial statements for the year ended December 31, 2024 of argenx SE, based in Amsterdam, the Netherlands. The financial statements comprise the consolidated financial statements and the company financial statements. In our opinion: • The accompanying consolidated financial statements give a true and fair view of the financial position of argenx SE as at December 31, 2024, and of its result and its cash flows for 2024 in accordance with the IFRS® Accounting Standards (IFRS) as adopted by the European Union (EU-IFRS) and in accordance with Part 9 of Book 2 of the Dutch Civil Code. • The accompanying company financial statements give a true and fair view of the financial position of argenx SE as at December 31, 2024, and of its result for 2024 in accordance with Part 9 of Book 2 of the Dutch Civil Code. The consolidated financial statements comprise: 1. The consolidated statements of financial position as at December 31, 2024. 2. The following statements for 2024: the consolidated statements of profit or loss, the consolidated statements of comprehensive income or loss, the consolidated statements of cash flows and the consolidated statements of changes in equity. 3. The notes comprising material accounting policy information and other explanatory information. The company financial statements comprise: 1. The company balance sheet as at December 31, 2024. 2. The company profit and loss account for 2024. 3. The notes comprising a summary of the accounting policies and other explanatory information. Basis for our opinion We conducted our audit in accordance with Dutch law, including the Dutch Standards on Auditing. Our responsibilities under those standards are further described in the 'Our responsibilities for the audit of the financial statements' section of our report. We are independent of argenx SE in accordance with the EU Regulation on specific requirements regarding statutory audit of public-interest entities, the Wet toezicht accountantsorganisaties (Wta, Audit firms supervision act), the Verordening inzake de onafhankelijkheid van accountants bij assurance-opdrachten (ViO, Code of Ethics for Professional Accountants, a regulation with respect to independence) and other relevant independence regulations in the Netherlands. Furthermore, we have complied with the Verordening gedrags- en beroepsregels accountants (VGBA, Dutch Code of Ethics for Professional Accountants). We believe the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion. Information in support of our opinion We designed our audit procedures in the context of our audit of the financial statements as a whole and in forming our opinion thereon. The following information in support of our opinion was addressed in this context, and we do not provide a separate opinion or conclusion on these matters. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 300

Materiality Based on our professional judgement we determined the materiality for the financial statements as a whole at USD 71,000,000 (USD 50,000,000 in prior year). The materiality is based on 3.5% of operating expenses excluding cost of sales and excluding the loss from investment in joint venture, consistent with prior year. We have also taken into account misstatements and/or possible misstatements that in our opinion are material for the users of the financial statements for qualitative reasons. We agreed with the Board of Directors that misstatements in excess of USD 3,550,000 (USD 2,500,000 in prior year), which are identified during the audit, would be reported to them, as well as smaller misstatements that in our view must be reported on qualitative grounds. Scope of the group audit argenx SE is at the head of a group of entities. The financial information of this group is included in the consolidated financial statements of argenx SE. Because we are ultimately responsible for the opinion, we are also responsible for directing, supervising and performing the group audit. In this respect we have determined the nature and extent of the audit procedures to be carried out for group entities. The audit procedures on all group entities have been performed by the group engagement team without using the work of other auditors. By performing the procedures mentioned above at group entities we have been able to obtain sufficient and appropriate audit evidence about the group's financial information to provide an opinion on the consolidated financial statements. Audit approach fraud risks We identified and assessed the risks of material misstatements of the financial statements due to fraud. During our audit we obtained an understanding of the entity and its environment and the components of the system of internal control, including the risk assessment process and management's process for responding to the risks of fraud and monitoring the system of internal control and how the Board of Directors exercises oversight, as well as the outcomes. We evaluated management’s fraud risk assessment. We evaluated the design and relevant aspects of the system of internal control and in particular the fraud risk assessment, as well as among others the code of conduct, whistle blower procedures and incident registration. We evaluated the design and the implementation and tested the operating effectiveness, of internal controls designed to mitigate fraud risks. As part of our process of identifying fraud risks, we evaluated fraud risk factors with respect to financial reporting fraud, misappropriation of assets and bribery and corruption in close co-operation with our forensic specialists. We evaluated whether these factors indicate that a risk of material misstatement due to fraud is present. We identified the following fraud risks and performed the following specific procedures: We identified a risk of material misstatement due to fraud related to revenue recognition. The risk exists that the Company did not accurately record the US Sales rebates and reserves because of a materially incorrect estimation of the rebate mix of Medicare, Medicaid, Discarded Drug Refund and Value Based Agreements (VBA). Reference is made to the section 'Our key audit matters' for our procedures performed. We furthermore identified a risk of material misstatement due to fraud related to management override of controls. Management is in a unique position to perpetrate fraud because of management’s ability to manipulate accounting records and prepare fraudulent financial statements by overriding controls that otherwise appear to be operating effectively. We tested the relevant controls related to US Sales rebates and reserves and related to management override of controls. We tested the appropriateness of journal entries recorded in the general ledger and other adjustments made in the preparation of the financial statements. In addition, we assessed whether there were any significant unusual transactions outside the normal course of business. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 301

We incorporated elements of unpredictability in our audit. We also considered the outcome of our other audit procedures and evaluated whether any findings were indicative of fraud or non-compliance. We considered available information and made enquiries of relevant management team members, (including the Chief Executive Officer, the Chief Operating Officer, the Chief Financial Officer, General Counsel, Global Head of Quality, Chief Scientific Officer, Chief Medical Officer, Vice President Corporate Development & Strategy, Global Head of Technical Operations, Vice President Corporate Communications & Investor Relations, Global Head of Human Resources) and the Board of Directors (including the Chair of the Audit and Compliance Committee). We evaluated whether the selection and application of accounting policies by the group, particularly those related to subjective measurements and complex transactions, may be indicative of fraudulent financial reporting. We evaluated whether the judgments and decisions made by management in making the accounting estimates included in the financial statements indicate a possible bias that may represent a risk of material misstatement due to fraud. Management insights, estimates and assumptions that might have a major impact on the financial statements are disclosed in Note 3 Critical accounting judgement and major sources of estimation uncertainty of the financial statements. We performed a retrospective review of management judgments and assumptions related to significant accounting estimates reflected in prior year financial statements. We evaluated the reasonableness of management’s estimates with respect to the US Sales rebates and reserves. Reference is made to the section 'Our key audit matters'. For transactions of interest, for instance in relation to donations made to patient charities, we evaluated whether the business rationale of the transactions suggests that they may have been entered into to engage in activities in relation to bribery and corruption. This did not lead to indications for fraud potentially resulting in material misstatements. Audit approach compliance with laws and regulations We assessed the laws and regulations relevant to the Company through discussion with the General Counsel and the Head of Global Quality, reading minutes and reports of internal audit. We involved our forensic specialists in this evaluation. As a result of our risk assessment procedures, and while realizing that the effects from non-compliance could considerably vary, we considered the following laws and regulations: (corporate) tax law, the requirements under IFRS® Accounting Standards (IFRS) as adopted by the European Union (EU-IFRS) and Part 9 of Book 2 of the Dutch Civil Code with a direct effect on the financial statements as an integrated part of our audit procedures, to the extent material for the financial statements. We obtained sufficient appropriate audit evidence regarding provisions of those laws and regulations generally recognized to have a direct effect on the financial statements. Apart from these, argenx SE is subject to other laws and regulations where the consequences of non- compliance could have a material effect on amounts and/or disclosures in the financial statements, for instance, through imposing fines or litigation. Given the nature of argenx SE's business and the complexity of healthcare regulations, there is a risk of non-compliance with the requirements of such laws and regulations. In addition, we considered major laws and regulations applicable to listed companies. Our procedures are more limited with respect to these laws and regulations that do not have a direct effect on the determination of the amounts and disclosures in the financial statements. Compliance with these laws and regulations may be fundamental to the operating aspects of the business, to argenx SE’s ability to continue its business, or to avoid material penalties (e.g., compliance with healthcare regulations) and therefore non-compliance with such laws and regulations may have a material effect on the financial statements. Our responsibility is limited to undertaking specified audit procedures to help identify non- compliance with those laws and regulations that may have a material effect on the financial statements. Our procedures are limited to (i) inquiry of management, the Board of Directors, and others within argenx SE as to whether argenx SE is in compliance with such laws and regulations and (ii) inspecting argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 302

correspondence, if any, with the relevant licensing or regulatory authorities to help identify non-compliance with those laws and regulations that may have a material effect on the financial statements. Naturally, we remained alert to indications of (suspected) non-compliance throughout the audit. Finally, we obtained written representations that all known instances of (suspected) fraud or non- compliance with laws and regulations have been disclosed to us. Audit approach going concern We are responsible for obtaining reasonable assurance that the Company is able to continue as a going concern. Management is responsible to assess the Company’s ability to continue as a going concern and disclosing in the financial statements any events or circumstances that may cast significant doubt on the Company’s ability to continue as a going concern. As explained in Note 2.1 Statement of compliance and basis of preparation’ and Note 25 Financial Risk Management of the financial statements, management has prepared the financial statements of argenx SE based on the going concern assumption. No events or circumstances have been identified which cause significant doubt about the Company’s ability to continue its operations (going concern). Our procedures to evaluate the going concern assessment of management include: • Consider whether management’s assessment of going concern contains all relevant information of which we are aware as a result of our audit and review of the other information. In addition, we inquired with management about the key assumptions underlying the going concern assessment. • Inquiry with management regarding their knowledge of events and/or circumstances beyond the period of management’s assessment. • We reconciled the cash and cash equivalents position as used in the going concern assessment to the audited position at December 31, 2024. • We evaluated management’s financial forecasts and analysis prepared for a period of at least 12 months from the date of preparation of the financial statements. This included consideration of the reasonableness of key underlying assumptions by evaluating historically realized and future expected operating and capital expenditure as well as evaluating mathematical accuracy of the assessment. • We evaluated the adequacy of disclosures made in the financial statements in respect of going concern. Our audit procedures did not produce results that were inconsistent with management’s assumptions and judgments in applying the going concern assumption. Our key audit matters Key audit matters are those matters that, in our professional judgement, were of most significance in our audit of the financial statements. We have communicated the key audit matters to the Board of Directors. The key audit matters are not a comprehensive reflection of all matters discussed. Trade and Other Payables | US Sales rebates and reserves — Refer to Notes 2.17, 3 and 14 to the financial statements Key Audit Matter Description The Company recognizes product net sales, relating to the sale of the products VYVGART and VYVGART SC. These product net sales are accounted for in accordance with IFRS 15 Revenue from Contracts with Customers (“IFRS 15”), whereby the sale of these products to customers is recognized for an amount that reflects the consideration to which the Company expects to be entitled in exchange for these goods. The majority of the product gross sales are in the United States of America, which are subject to reduction for significant components of variable consideration primarily composed mandatory rebates to government agencies, distributors, health insurance companies and managed healthcare organizations. Together, we refer to these deductions as US Sales rebates and reserves, as included in Notes 2.17, 3 and 14 to the financial statements. The US sales rebates and reserves recorded by the Company represent estimates of the related obligations that will be settled in a future period. The main sources of significant estimation uncertainty are the payer argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 303

mix, representing the portion of total sales that will be made into each payer channel, and the time lag between the point of sale and receipt of a claim. The significant assumptions and estimates used to determine the liability for the variable considerations are based upon contracts with customers, healthcare providers, payers and government agencies, regulated discounts applicable to government-funded programs, historical experience of claims received, estimated payer mix and other relevant factors. Given the complexity of this estimate, auditing this estimate required both extensive audit effort and a high degree of auditor judgment when performing auditing procedures and evaluating the results of those procedures, and therefore we identified the US Sales rebates and reserves as a key audit matter. How the Key Audit Matter Was Addressed in the Audit Our audit procedures related to the US Sales rebates and reserves included the following, among others: • We evaluated the key revenue contracts and key supply chain contracts, including evaluation of the accounting treatment of the US Sales rebates and reserves and the disclosures thereof in accordance with IFRS 15. • We evaluated the independent service auditor reports for the service providers used by the Company to process US Sales rebates and reserves on behalf of the Company. • We evaluated the appropriateness and consistency of the Company’s methodology and assumptions in developing the US Sales rebates and reserves, including testing the completeness and accuracy of the underlying data used by management in their estimates. • We tested significant assumptions and key inputs used to calculate the US Sales rebates and reserves, including the payer mix, by comparing to historical data, testing the historical accuracy of estimates made by management and evaluating the impact of changes in government legislation. • We tested the mathematical accuracy of the US Sales rebates and reserves calculation. • We tested the rebate claims received during the financial year against source documentation. Observations The scope and nature of the audit procedures we performed was sufficient and appropriate to address the risks of material misstatement related to the US Sales rebates and reserves. Deferred Tax Assets | Recognition of deferred tax assets in Belgium — Refer to Notes 2.16, 3 and 13 to the financial statements Key Audit Matter Description The Company recognizes deferred tax assets for the carryforward of unused tax losses, innovation income deductions, and other timing differences, to the extent that it is probable that future taxable profit will be available against which the unused tax losses and unused tax credits can be utilized. Under applicable tax law in Belgium, tax losses accumulated do not expire and are recoverable against future taxable income. In the fourth quarter of the year ended December 31, 2024, the Company concluded sufficient evidence was available to recognize the deferred tax assets held by argenx B.V., a subsidiary of the Company in Belgium. The balance of net deferred tax assets related to argenx B.V. is USD 708 million at December 31, 2024. The recognition of argenx B.V.’s deferred tax asset requires subjective and complex auditor judgment due to a history of recent losses. Significant judgment is applied by management to evaluate whether there is convincing evidence that future taxable profits will be available against which the deferred tax assets can be utilized. This includes the evaluation of all available evidence, including historical performance, the external competitive landscape and the forecasted taxable profits within the look-forward period. A key estimate used to determine whether sufficient future taxable profits will be available is the forecasted US product net sales in the look-forward period. This assessment, which includes the length of the look-forward period utilized, requires significant management judgment. Given the complexity of this judgment, auditing the recognition of the deferred tax assets required both extensive audit effort and a high degree of auditor judgment when performing auditing procedures and evaluating the results of those procedures. Therefore, we identified the recognition of the deferred tax assets in Belgium as a key audit matter. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 304

How the Key Audit Matter Was Addressed in the Audit Our audit procedures related to the recognition of the deferred tax assets in Belgium included the following, among others: • We tested the effectiveness of internal controls over management's valuation of deferred tax assets in Belgium, including the controls over the budget approval and assessment of the likelihood of future taxable profits being available during the look-forward period. • We evaluated the appropriateness of the Company’s methodology to determine whether sufficient future taxable profits will be available, including assessment of the number of years of forecasted future taxable profits used. • We involved tax specialists to assess whether the recognition of the deferred tax assets is in accordance with the applicable accounting standards, the methodology applied to determine future taxable profits is consistent with Belgium tax legislation and the deferred tax assets are calculated in accordance with Belgium tax legislation. • We tested key assumptions and inputs used to determine forecasted taxable profits, specifically the growth of US product net sales by comparing this against historical performance of the Company, the approved budget and external sources. • We assessed the Company’s ability to estimate taxable profits accurately by evaluating the historical accuracy of forecasted commercial results made in the prior year in relation to the actual results incurred in the current year. • We tested the mathematical accuracy of the model used to determine forecasted taxable profits. Observations The scope and nature of the audit procedures we performed was sufficient and appropriate to address the risks of material misstatement related to the recognition of the deferred tax assets in Belgium. Report on the other information included in The Annual Report The Annual Report contains other information, in addition to the financial statements and our auditor's report thereon. The other information consists of: • Management’s Report, including, among others, the Remuneration Report and Compensation Statement, and Non-Financial Information. • Other Information as required by Part 9 of Book 2 of the Dutch Civil Code. Based on the following procedures performed, we conclude that the other information: • Is consistent with the financial statements and does not contain material misstatements. • Contains all the information regarding the management report and the other information as required by Part 9 of Book 2 of the Dutch Civil Code. We have read the other information. Based on our knowledge and understanding obtained through our audit of the financial statements or otherwise, we have considered whether the other information contains material misstatements. By performing these procedures, we comply with the requirements of Part 9 of Book 2 of the Dutch Civil Code and the Dutch Standard 720. The scope of the procedures performed is substantially less than the scope of those performed in our audit of the financial statements. Management is responsible for the preparation of the other information, including Management’s Report in accordance with Part 9 of Book 2 of the Dutch Civil Code, and the other information as required by Part 9 of Book 2 of the Dutch Civil Code. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 305

Report on other legal and regulatory requirements and ESEF Engagement We were engaged by the Board of Directors as auditor of argenx SE on May 13, 2015, as of the audit for the year 2015 and have operated as statutory auditor ever since that financial year. No prohibited non-audit services We have not provided prohibited non-audit services as referred to in Article 5(1) of the EU Regulation on specific requirements regarding statutory audit of public-interest entities. European Single Electronic Format (ESEF) argenx SE has prepared its annual report in ESEF. The requirements for this are set out in the Delegated Regulation (EU) 2019/815 with regard to regulatory technical standards on the specification of a single electronic reporting format (hereinafter: the RTS on ESEF). In our opinion, the annual report, prepared in XHTML format, including the (partly) marked-up consolidated financial statements, as included in the reporting package by argenx SE complies in all material respects with the RTS on ESEF. Management is responsible for preparing the annual report including the financial statements in accordance with the RTS on ESEF, whereby management combines the various components into one single reporting package. Our responsibility is to obtain reasonable assurance for our opinion whether the annual report in this reporting package complies with the RTS on ESEF. We performed our examination in accordance with Dutch law, including Dutch Standard 3950N 'Assurance- opdrachten inzake het voldoen aan de criteria voor het opstellen van een digitaal verantwoordingsdocument' (assurance engagements relating to compliance with criteria for digital reporting). Our examination included amongst others: • Obtaining an understanding of the company's financial reporting process, including the preparation of the reporting package. • Identifying and assessing the risks that the annual report does not comply in all material respects with the RTS on ESEF and designing and performing further assurance procedures responsive to those risks to provide a basis for our opinion, including: ◦ obtaining the reporting package and performing validations to determine whether the reporting package containing the Inline XBRL instance and the XBRL extension taxonomy files has been prepared in accordance with the technical specifications as included in the RTS on ESEF; ◦ examining the information related to the consolidated financial statements in the reporting package to determine whether all required mark-ups have been applied and whether these are in accordance with the RTS on ESEF. Description of responsibilities regarding the financial statements Responsibilities of Management and the Board of Directors for the financial statements Management is responsible for the preparation and fair presentation of the financial statements in accordance with IFRS® Accounting Standards (IFRS) and the interpretations issued by the IASB’s International Financial Reporting Interpretation Committee as adopted by the European Union (EU-IFRS) and Part 9 of Book 2 of the Dutch Civil Code. Furthermore, management is responsible for such internal control as management determines is necessary to enable the preparation of the financial statements that are free from material misstatement, whether due to fraud or error. As part of the preparation of the financial statements, management is responsible for assessing the company's ability to continue as a going concern. Based on the financial reporting frameworks mentioned, management should prepare the financial statements using the going concern basis of accounting unless argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 306

management either intends to liquidate the company or to cease operations, or has no realistic alternative but to do so. Management should disclose events and circumstances that may cast significant doubt on the company's ability to continue as a going concern in the financial statements. The Board of Directors is responsible for overseeing the company's financial reporting process. Our responsibilities for the audit of the financial statements Our objective is to plan and perform the audit assignment in a manner that allows us to obtain sufficient and appropriate audit evidence for our opinion. Our audit has been performed with a high, but not absolute, level of assurance, which means we may not detect all material errors and fraud during our audit. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of these financial statements. The materiality affects the nature, timing and extent of our audit procedures and the evaluation of the effect of identified misstatements on our opinion. We have exercised professional judgement and have maintained professional scepticism throughout the audit, in accordance with Dutch Standards on Auditing, ethical requirements and independence requirements. Our audit included among others: • Identifying and assessing the risks of material misstatement of the financial statements, whether due to fraud or error, designing and performing audit procedures responsive to those risks, and obtaining audit evidence that is sufficient and appropriate to provide a basis for our opinion. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. • Obtaining an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company's internal control. • Evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates and related disclosures made by management. • Concluding on the appropriateness of management's use of the going concern basis of accounting, and based on the audit evidence obtained, whether a material uncertainty exists related to events or conditions that may cast significant doubt on the company's ability to continue as a going concern. If we conclude that a material uncertainty exists, we are required to draw attention in our auditor's report to the related disclosures in the financial statements or, if such disclosures are inadequate, to modify our opinion. Our conclusions are based on the audit evidence obtained up to the date of our auditor's report. However, future events or conditions may cause the company to cease to continue as a going concern. • Evaluating the overall presentation, structure and content of the financial statements, including the disclosures. • Evaluating whether the financial statements represent the underlying transactions and events in a manner that achieves fair presentation. Because we are ultimately responsible for the opinion, we are also responsible for directing, supervising and performing the group audit. In this respect we have determined the nature and extent of the audit procedures to be carried out for group entities. Decisive were the size and/or the risk profile of the group entities or operations. On this basis, we selected group entities for which an audit or review had to be carried out on the complete set of financial information or specific items. We communicate with the Board of Directors regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant findings in internal control that we identified during our audit. In this respect we also submit an additional report to the Board of Directors in accordance with Article 11 of the EU Regulation on specific requirements regarding statutory audit of argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 307

public-interest entities. The information included in this additional report is consistent with our audit opinion in this auditor's report. We provide the Board of Directors with a statement that we have complied with relevant ethical requirements regarding independence, and to communicate with them all relationships and other matters that may reasonably be thought to bear on our independence, and where applicable, related safeguards. From the matters communicated with the Board of Directors, we determine the key audit matters: those matters that were of most significance in the audit of the financial statements. We describe these matters in our auditor's report unless law or regulation precludes public disclosure about the matter or when, in extremely rare circumstances, not communicating the matter is in the public interest. Rotterdam, March 20, 2025 Deloitte Accountants B.V. V.A.J. Fruytier 6.4.3 Limited Assurance Report of the Independent Auditor on the Sustainability Statement To the shareholders and the Board of Directors of argenx SE Our conclusion We have performed a limited assurance engagement on the Sustainability Statement for the year ending December 31, 2024 of argenx SE based in Amsterdam (hereinafter: the company) included in section ‘Non- Financial Information’ of the accompanying management report including the information incorporated in the Sustainability Statement by reference (hereinafter: the Sustainability Statement). Based on our procedures performed and the assurance evidence obtained, nothing has come to our attention that causes us to believe that the Sustainability Statement is not, in all material respects: • Prepared in accordance with the European Sustainability Reporting Standards (ESRS) as adopted by the European Commission and in accordance with the double materiality assessment process carried out by the company to identify the information reported pursuant to the ESRS. • Compliant with the reporting requirements provided for in Article 8 of Regulation (EU) 2020/852 (Taxonomy Regulation). Basis for our conclusion We have performed our limited assurance engagement on the Sustainability Statement in accordance with Dutch law, including Dutch Standard 3810N ‘Assurance-opdrachten inzake duurzaamheidsverslaggeving’ (Assurance engagements relating to sustainability reports) which is a specified Dutch Standard that is based on the International Standard on Assurance Engagements (ISAE) 3000 ‘Assurance engagements other than audits or reviews of historical financial information’. Our responsibilities in this regard are further described in the section ‘Our responsibilities for the limited assurance engagement on the Sustainability Statement’ of our report. We are independent of argenx SE in accordance with the ‘Verordening inzake de onafhankelijkheid van accountants bij assurance-opdrachten’ (ViO, Code of Ethics for Professional Accountants, a regulation with respect to independence) and other relevant independence regulations in the Netherlands. Furthermore, we have complied with the ‘Verordening gedrags- en beroepsregels accountants’ (VGBA, Dutch Code of Ethics for Professional Accountants). The ViO and VGBA are at least as demanding as the International code of ethics for professional accountants (including International independence standards) of the International Ethics Standards Board for Accountants (the IESBA Code). We believe that the assurance evidence we have obtained is sufficient and appropriate to provide a basis for our conclusion. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 308

Emphasis of matters Emphasis on the most significant uncertainties affecting the quantitative metrics and monetary amounts We draw attention to the paragraphs ‘Estimation Techniques and Data Limitations’ and ‘Digitalization and Data Collection Enhancements’ in section 7.1.2 ‘General Basis for Preparation (BP-1)’, and to the paragraphs ‘Value Chain Estimations’ and ‘Sources of Estimation and Outcome Uncertainty’ in section 7.1.3 ‘Disclosures in relation to specific circumstances (BP-2)’ of the Sustainability Statement that identifies the quantitative metrics that are subject to a high level of measurement uncertainty and discloses information about the sources of measurement uncertainty and the assumptions, approximations and judgements the company has made in measuring these in compliance with the ESRS. The comparability of sustainability information between entities and over time may be affected by the lack of historical sustainability information in accordance with the ESRS and by the absence of a uniform practice on which to draw, to evaluate and measure this information. This allows for the application of different, but acceptable, measurement techniques, especially in the initial years. Emphasis on the double materiality assessment process We draw attention to the paragraph ‘Evolving Sustainability Reporting: Embracing CSRD and ESRS’ in section 7.1.1 ‘General Information’ of the Sustainability Statement. This disclosure explains that argenx SE considers due diligence and double materiality assessment as an ongoing process. Due diligence is an on-going practice that responds to and may trigger changes in the company’s strategy, business model, activities, business relationships, operating, sourcing and selling contexts. The double materiality assessment process may also be impacted in time by sector-specific standards to be adopted or developments in stakeholder expectations, regulatory developments, changes in risk management or new business developments. The Sustainability Statement may not include every impact, risk and opportunity or additional entity-specific disclosure that each individual stakeholder (group) may consider important in its own particular assessment. Our conclusion is not modified in respect of these matters. Comparative information not subject to assurance procedures The comparative information in the Sustainability Statement prepared in accordance with ESRS has not been subject to reasonable or limited assurance. Our conclusion is not modified in respect of this matter. Limitations to the scope of our assurance engagement In reporting forward-looking information in accordance with the ESRS, management of the company is required to prepare the forward-looking information on the basis of disclosed assumptions about events that may occur in the future and possible future actions by the company. The actual outcome is likely to be different since anticipated events frequently do not occur as expected. Forward-looking information relates to events and actions that have not yet occurred and may never occur. We do not provide assurance on the achievability of this forward-looking information. Our conclusion is not modified in respect of this matter. Responsibilities of management board and the board of directors for the Sustainability Statement Management is responsible for the preparation of the Sustainability Statement in accordance with the ESRS, including the double materiality assessment process carried out by the company as the basis for the Sustainability Statement and disclosure of material impacts, risks and opportunities in accordance with the ESRS. As part of the preparation of the Sustainability Statement, management is responsible for compliance with the reporting requirements provided for in Article 8 of Regulation (EU) 2020/852 (Taxonomy Regulation). Management is also responsible for selecting and applying additional entity-specific disclosures to enable users to understand the company’s sustainability-related impacts, risks or opportunities and for determining that these additional entity-specific disclosures are suitable in the circumstances and in accordance with the ESRS. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 309

Furthermore, management is responsible for such internal control as it determines necessary to enable the preparation of the Sustainability Statement that is free from material misstatement, whether due to fraud or error. The board of directors is responsible for overseeing the sustainability reporting process including the double materiality assessment process carried out by the company. Our responsibilities for the limited assurance engagement on the Sustainability Statement Our responsibility is to plan and perform the limited assurance engagement in a manner that allows us to obtain sufficient appropriate assurance evidence for our conclusion. Our assurance engagement is aimed to obtain a limited level of assurance that the Sustainability Statement is free from material misstatements. The procedures vary in nature and timing, and are less in extent than a reasonable assurance engagement. Consequently, the level of assurance obtained in a limited assurance engagement is substantially lower than the assurance that would have been obtained had a reasonable assurance engagement been performed. We apply the applicable quality management requirements pursuant to the ‘Nadere voorschriften kwaliteitsmanagement’ (NV KM, regulations for quality management) and the International Standard on Quality Management (ISQM) 1, and accordingly maintain a comprehensive system of quality management including documented policies and procedures regarding compliance with ethical requirements, professional standards and other relevant legal and regulatory requirements. Our limited assurance engagement included among others: • Performing inquiries and an analysis of the external environment and obtaining an understanding of relevant sustainability themes and issues, the characteristics of the company, its activities and the value chain and its key intangible resources in order to assess the double materiality assessment process carried out by the company as the basis for the Sustainability Statement and disclosure of all material sustainability-related impacts, risks and opportunities in accordance with the ESRS. • Obtaining through inquiries a general understanding of the internal control environment, the company’s processes for gathering and reporting entity-related and value chain information, the information and the company’s risk assessment process relevant to the preparation of the Sustainability Statement and for identifying the company’s activities, determining eligible and aligned economic activities and prepare the disclosures provided for in Article 8 of Regulation (EU) 2020/852 (Taxonomy Regulation), without obtaining assurance information about the implementation, or testing the operating effectiveness, of controls. • Assessing the double materiality assessment process carried out by the company and identifying and assessing areas of the Sustainability Statement, including the disclosures provided for in Article 8 of Regulation (EU) 2020/852 (Taxonomy Regulation) where misleading or unbalanced information or material misstatements, whether due to fraud or error, are likely to arise (‘selected disclosures’). We designed and performed further assurance procedures aimed at assessing that the Sustainability Statement is free from material misstatements responsive to this risk analysis. • Considering whether the description of the double materiality assessment process in the Sustainability Statement made by management appears consistent with the process carried out by the company. • Performing analytical review procedures on quantitative information in the Sustainability Statement, including consideration of data and trends. • Assessing whether the company’s methods for developing estimates are appropriate and have been consistently applied for selected disclosures. We considered data and trends; however, our procedures did not include testing the data on which the estimates are based or separately developing our own estimates against which to evaluate management’s estimates. • Analyzing, on a limited sample basis, relevant internal and external documentation available to the company (including publicly available information or information from actors throughout its value chain) for selected disclosures. • Reading the other information in the annual report to identify material inconsistencies, if any, with the Sustainability Statement. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 310

• Considering whether: ◦ the disclosures provided to address the reporting requirements provided for in Article 8 of Regulation (EU) 2020/852 (Taxonomy Regulation) for each of the environmental objectives, reconcile with the underlying records of the company and are consistent or coherent with the Sustainability Statement and appear reasonable, in particular whether the eligible economic activities meet the cumulative conditions to qualify as aligned and whether the technical screening criteria are met; and ◦ the key performance indicators disclosures have been defined and calculated in accordance with the Taxonomy reference framework as defined in Appendix 1 Glossary of Terms of the CEAOB Guidelines on limited assurance on sustainability reporting adopted on 30 September 2024 and in compliance with the reporting requirements provided for in Article 8 of Regulation (EU) 2020/852 (Taxonomy Regulation), including the format in which the activities are presented. • Considering the overall presentation, structure and the fundamental qualitative characteristics of information (relevance and faithful representation: complete, neutral and accurate) reported in the Sustainability Statement, including the reporting requirements provided for in Article 8 of Regulation (EU) 2020/852 (Taxonomy Regulation). • Considering, based on our limited assurance procedures and evaluation of the assurance evidence obtained, whether the Sustainability Statement as a whole is free from material misstatements and prepared in accordance with the ESRS. Rotterdam, March 20, 2025 Deloitte, Accountants B.V. V.A.J. Fruytier argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other information 311

Non-Financial Information 7.1 Sustainability Statement 313 7.2 Sustainability Strategy 320 7.3 Environment 335 7.4 Social 352 7.5 Industry Specific Disclosures 364 7.6 Governance 369 7.7 Other Considerations 373 7.8 Appendix 375 argenx Annual Report 2024 312 7

7 Non-Financial Information 7.1 Sustainability Statement 7.1.1 General Information Sustainability Statement Introduction At argenx, we are on a mission to transform the lives of patients by translating immunology breakthroughs into novel antibody-based medicines. Our mission aims to promote a responsible approach to bringing medicines to patients. In 2024, we upheld our commitment to transparency and accountability as required by the European Union’s Non-Financial Reporting Directive (the NFRD), as implemented into Dutch legislation, which requires us to publish a non-financial statement as part of our management report, which statement should include, to the extent necessary for a proper understanding of our development, results, position, and the impact of our activities, disclosures on (i) our business model; (ii) our policies (if applicable), including the due diligence procedures applied, as well as the results of this policy, with regard to: (a) environmental, social and employee matters; (b) respect for human rights; and (c) the fight against corruption and bribery. In addition, we are required to describe the main risks related to the matters referred to in (i) through (iii) above arising from our operations, including, where relevant and proportionate, our business relationships, products or services that are likely to have negative impacts on those matters and how we manage those risks. We are also required to disclose the non-financial performance indicators that are of importance to our specific business activities. In addition, the European Union’s Corporate Sustainability Reporting Directive (CSRD) entered into force in January 2023. Being a Directive, all EU Member States and EEA countries are required to implement the CSRD into domestic law, with the deadline for such implementation being 6 July 2024. Several EU member states, including Belgium, have transposed and implemented the CSRD into domestic law. The Netherlands has not done so yet. In 2024, draft legislation was proposed in the Netherlands and on 13 January 2025, the legislative proposal to implement the CSRD was submitted to the Second Chamber of Dutch Parliament for parliamentary proceedings. Being a company governed under Dutch law, the Dutch domestic law implementing the CSRD will apply to us. In anticipation of implementation into Dutch domestic law with expected applicability in respect of annual reports starting financial year 2024, companies in scope of CSRD have been urged by the financial markets supervisory authority in the Netherlands to prepare and be ready for CSRD compliant reporting for their annual report 2024. In expectation of the CSRD being applicable to our Annual Report, and, in line with market practice in relation to annual reports for 2024 in the Netherlands and Belgium, we have prepared our sustainability statement based on the general principles of the CSRD for the first time and integrated such sustainability statement into our Annual Report. This chapter of our annual report is our CSRD Sustainability Statement and is prepared in accordance with the European Sustainability Reporting Standards (ESRS), reflecting our dedication to comprehensive and accurate sustainability reporting. Additionally, we reference the Sustainability Accounting Standards Board (SASB) standards. This statement addresses the concerns and interests of various affected stakeholders including patients, healthcare communities, employees, investors, and business partners. This statement is guided by the material topics identified in our double materiality assessment. Going forward, we will enhance the measurement and reporting of these topics, further embedding them into our performance framework. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Statement 313

Evolving Sustainability Reporting: Embracing CSRD and ESRS This year marks the first time we are reporting to CSRD. Like many of our industry peers, we are navigating this substantial new regulatory landscape with the aim of delivering meaningful activities and comprehensive reporting on ESG topics. We anticipate that our reporting on these topics will evolve significantly in the coming years, as we consider our reporting due diligence and double materiality assessment (DMA) an ongoing process. As we build and refine our processes and policies, we will delve deeper into areas where we can make the most impact, and we expect industry best practices to emerge over time. The CSRD and the ESRS guide sustainability reporting practices at argenx and determine what we must disclose. The CSRD largely supersedes the details of previous years’ ESG reporting, if disclosures are related to ESG reporting topics, however, where appropriate, we have integrated industry-specific SASB metrics, as included in our previous ESG reporting, where relevant for material topics. We also want to provide transparency on how we allocate our ESG reporting resources. We are mindful of our limited resources and the need to prioritize where we focus our ESG efforts. We focus our efforts on areas that we believe have the greatest impact on our mission, including innovation, and improving patient outcomes. Our primary focus remains on ESG topics that are closely aligned with our mission to improve patient outcomes. We aspire that regulatory reporting requirements on ESG topics do not divert our attention or that of our stakeholders from our mission. Content ESRS 2 BP-1 General basis for preparation of sustainability statement General Basis for Preparation (BP-1) BP-2 Disclosures in relation to specific circumstances Disclosures in Relation to Specific Circumstances (BP-2) GOV-1 The role of administrative, management and supervisory bodies ESG Governance and Oversight (GOV-1) GOV-2 Information provided to and sustainability matters addressed by the undertaking’s administrative, management and supervisory bodies Management of Material Risks, Impacts and Opportunities by Administrative, Management and Supervisory Bodies (GOV-1, GOV-2) GOV-3 Integration of sustainability-related performance in incentive schemes Integration of Sustainability-Related Performance in Incentive Schemes (GOV-3) GOV-4 Statement on due diligence Due Diligence (GOV-4) GOV-5 Risk management and internal controls over sustainability reporting Risk Management and Internal Controls Over Sustainability Reporting (GOV-5) SBM-1 Strategy, business model and value chain Sustainability and Our Business Model (SBM-1) SBM-2 Interests and views of stakeholders Stakeholder Engagement (SBM-2) SBM-3 Material impacts, risks and opportunities and their interaction with strategy and business model Double Materiality Assessment Result (ESRS 2 SBM-3) IRO-1 Description of process to identify and assess material impacts, risks, and opportunities Our Double Materiality Assessment (IRO-1, IRO-2) IRO-2 Disclosure requirements in ESRS covered by the undertaking’s sustainability statement Our Double Materiality Assessment (IRO-1, IRO-2) Incorporation by reference SBM-1-40 a (i) Products and services 1.1.2 Our Medicines GOV-1-20 (c) Board of director’s skills and expertise 3.2.4 Non-Executive Directors EU Taxonomy Denominator for calculation of turnover Note 17 Product net sales and Note 15 Collaboration revenue EU Taxonomy Denominator for CapEx calculation Note 4 Property, plant and equipment and Note 5 Intangible Assets EU Taxonomy Denominator for OpEx calculation Note 18 Research and Development Expenses E1-6 53 GHG Intensity based on net revenue 6.1.2 Consolidated Statements of Profit or Loss S1 Employee Data Note 20 Personnel Expenses S1-16 97 (b) Remuneration Note 20 Personnel Expenses Disclosure Requirements Section argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Statement 314

S4-1 Coverage, pricing, and reimbursement 1.7.4 Coverage, pricing and reimbursement and 1.7.5 Government Pricing and Reimbursement Programs for Marketed Drugs in the U.S. ESRS E1 - Climate Change ESRS 2, GOV-3 Integration of sustainability-related performance in incentive schemes Sustainability-Related Performance in Incentive Schemes E1-1 Transition Plan for Climate Change Mitigation Transition Plan for Climate Change Mitigation (E1-1, E1-2, E1-3, E1-4) ESRS 2 SBM-3 Material Impacts, Risks and Opportunities and Their Interaction with Strategy and Business Model Environmental Topics (ESRS 2 SBM-3); Climate Risk Assessment (ESRS 2 IRO-1, ESRS 2 SBM-3) ESRS 2, IRO-1 Description of the processes to identify and assess material impacts, risks, and opportunities Climate Risk Assessment (ESRS 2 IRO-1, ESRS 2 SBM-3) E1-2 Policies related to climate change mitigation and adaptation Transition Plan for Climate Change Mitigation (E1-1, E1-2, E1-3, E1-4); Transition Risks Screening (E1-2) E1-3 Actions and resources in relation to climate change policies Transition Plan for Climate Change Mitigation (E1-1, E1-2, E1-3, E1-4) E1-4 Targets related to climate change mitigation and adaptation Transition Plan for Climate Change Mitigation (E1-1, E1-2, E1-3, E1-4) E1-5 Energy consumption and mix Energy Consumption and Mix (E1-5) E1-6 Gross Scopes 1, 2, 3 and Total GHG emissions Gross Scopes 1, 2, 3 and Total GHG Emissions (GHG Intensity Based on Net Revenue) (E1-6) E5 – Circular Economy ESRS 2 SBM-3 Material impacts, risks and opportunities and their interaction with strategy and business model Waste Impacts (ESRS 2 SBM-3) E5-1 Policies related to resource use and circular economy Waste Management (E5-1, E5-2, E5-3) E5-2 Actions and resources related to resource use and circular economy Waste Management (E5-1, E5-2, E5-3) E5-3 Targets related to resource use and circular economy Waste Management (E5-1, E5-2, E5-3) E5-5 Resource outflows Resource outflows (E5-5) S1 - Own Workforce ESRS 2 SBM-2 Interests and views of stakeholders Employee Engagement (ESRS 2 SBM-2, S1-2) ESRS 2 SBM-3 Material impacts, risk and opportunities and their interaction with strategy and business models (unless described in the cross-cutting section) Introduction to Workforce at argenx (ESRS 2 SBM-3) S1-1 Policies relating to own workforce Talent Strategy, Management, and Development (S1-1, S1-3, S1-4, S1-5, S1-13, MDR-P, MDR-A, MDR-T); Human Rights (S1-1); Anti-Discrimination (S1-1, S1-17); Inclusion (S1-1) S1-2 Processes for engaging with workers about impacts Employee Engagement (ESRS 2 SBM-2, S1-2) S1-3 Processes to remediate impacts & channels to raise concerns Talent Strategy, Management, and Development (S1-1, S1-3, S1-4, S1-5, S1-13, MDR-P, MDR-A, MDR-T) S1-4 Taking action on material negative impacts, advancing positive impacts, and approaches to mitigating material risks and pursuing material opportunities relating to own workforce Talent Strategy, Management, and Development (S1-1, S1-3, S1-4, S1-5, S1-13, MDR-P, MDR-A, MDR-T) S1-5 Targets Talent Strategy, Management, and Development (S1-1, S1-3, S1-4, S1-5, S1-13, MDR-P, MDR-A, MDR-T) S1-6 Characteristics of employees Characteristics of the Undertaking’s Employees (S1-6) S1-7 Characteristics of non-employees in the workforce Characteristics of non-employees in the workforce (S1-7) Disclosure Requirements Section argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Statement 315

S1-9 Diversity metrics Our Employee Data S1-13 Training and skills development metrics Training and Skills Development Metrics (S1-13); Talent Development (S1-13) S1-14 Health and safety metrics Other Considerations 2. Employee Health and Safety S1-17 Incidents, complaints and severe human rights impacts Anti-Discrimination (S1-1, S1-17) S4 - Consumers and End-Users ESRS 2 SBM-3 Materials, impacts, risks and opportunities and their interaction with strategy and business model Introduction to Patients at argenx (ESRS 2 SBM-3) S4-1 Policies Patient Health & Safety (S4-1, S4-2, S4-3, S4-4, S4-5, MDR-P, MDR-A, MDR-T); Human Rights (S4-1, MDR-P); Access to Quality Information & Responsible Marketing (S4-1, S4-2, S4-3, S4-4, S4-5, MDR-P, MDR-A, MDR-T); Access to Medicines (S4-1, S4-3, S4-5, MDR-P); Data Privacy (S4-1, S4-3, MDR-P, MDR-A, MDR-T) S4-2 Processes for engaging with consumers about impact Patient Health & Safety (S4-1, S4-2, S4-3, S4-4, S4-5, MDR-P, MDR-A, MDR-T);Patient Engagement; Remediation Processes and Consumer Feedback Channels (S4-2, S4-3, MDR-A); Access to Quality Information & Responsible Marketing (S4-1, S4-2, S4-3, S4-4, S4-5, MDR-P, MDR-A, MDR-T); Patient Advocacy (S4-2, MDR-A) S4-3 Processes to remediate negative impacts/ channels to raise concerns Patient Health & Safety (S4-1, S4-2, S4-3, S4-4, S4-5, MDR-P, MDR-A, MDR-T); Remediation Processes and Consumer Feedback Channels (S4-2, S4-3, MDR-A); Access to Medicines (S4-1, S4-3, S4-5, MDR-P); Data Privacy (S4-1, S4-3, MDR- P, MDR-A, MDR-T) S4-4 Taking action on material IROs Patient Health & Safety (S4-1, S4-2, S4-3, S4-4, S4-5, MDR-P, MDR-A, MDR-T); Access to Quality Information & Responsible Marketing (S4-1, S4-2, S4-3, S4-4, S4-5, MDR-P, MDR-A, MDR-T) S4-5 Targets Patient Health & Safety (S4-1, S4-2, S4-3, S4-4, S4-5, MDR-P, MDR-A, MDR-T); Access to Quality Information & Responsible Marketing (S4-1, S4-2, S4-3, S4-4, S4-5, MDR-P, MDR-A, MDR-T); Access to Medicines (S4-1, S4-3, S4-5, MDR-P) G1 - Business Conduct SBM-3 Materials, impacts, risks and opportunities and their interaction with strategy and business model Corporate Culture (ESRS 2 SBM-3, G1-1) G1-1 Business conduct policies and corporate culture Corporate Culture (ESRS 2 SBM-3, G1-1); Code of Conduct and Business Ethics (G1-1, G1-3, MDR-P); Global Anti-Bribery and Anti-Corruption Policy (G1-1, G1-3, MDR-P, MDR-A, MDR-T) G1-2 Management of relationships with suppliers Supply Chain Management (G1-2, MDR-A) G1-3 Prevention and detection of corruption and bribery Code of Conduct and Business Ethics (G1-1, G1-3, MDR-P); Global Anti-Bribery and Anti-Corruption Policy (G1-1, G1-3, MDR-P, MDR-A, MDR-T); Whistleblower Protections (G1-3, MDR-A) G1-4 Incidents of corruption of bribery Incidents of corruption and bribery (G1-4) G1-6 Payment practices Payment practices (G1-6, MDR-P, MDR-A) Disclosure Requirements Section argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Statement 316

7.1.2 General Basis for Preparation This statement outlines our ESG principles, and the methodologies employed to measure our progress, ensuring transparency and accountability in our operations. This report demonstrates our ongoing efforts to positively impact patients, our employees, and communities. Basis of Sustainability Statement In preparing our 2024 Sustainability Statement, we have ensured that the scope of consolidation aligns with that of our financial statements. Value Chain Coverage Our sustainability statement offers a deep dive into how we innovate and bring our medicines to patients. In 2024, we conducted a double materiality assessment, which is a cornerstone of CSRD-aligned reporting and required by the ESRS regulation. Our double materiality assessment encompassed a comprehensive review of our actual and potential impacts, risks, and opportunities across the entirety of our operations and value chain. This included both upstream activities (e.g., suppliers, raw material sourcing, and production) and downstream activities (e.g., distribution, use of products, and product end-of-life management). By incorporating these dimensions, we have a holistic understanding of our value chain's sustainability impacts, risks, and opportunities (as those terms are defined under the ESRS glossary). Where possible, we have provided data from both upstream and downstream activities in our sustainability disclosures. This includes environmental value chain data for the GHG emissions inventory and waste figures reported. We acknowledge that sustainability progress is dependent on various external factors, including supplier engagement, regulatory developments, and industry-wide collaboration. Achieving our sustainability goals requires continuous engagement with value chain partners, and their commitment to transparency and data sharing is critical to improving the quality of our disclosures. Omission of Information In the preparation of this statement, we have not exercised the option to omit specific pieces of information that pertain to intellectual property, knowledge, know-how, or the results of innovation. Scope and Boundaries The reporting period and entities included in this report are consistent with the scope of our financial statements. The 2024 Sustainability Statement for argenx has been prepared in alignment with the ESRS. The data published in this report covers the period from January 1, 2024, to December 31, 2024. Methodology The data collection process for our 2024 Sustainability Statement has been designed to ensure compliance with ESRS. The process involves several key steps to maintain the accuracy, reliability, and transparency of the reported data. Additionally, to comply with legal requirements, we engaged our statutory auditor, Deloitte Accountants B.V., to provide limited assurance as outlined in the independent assurance report. We also use the industry-specific frameworks where applicable, specifically SASB’s Biotechnology & Pharmaceuticals standard. This alignment enables us to provide stakeholders with comparable and meaningful insights into our sustainability performance, demonstrating our commitment to accountability. Future iterations of our Sustainability Statement may undergo methodological refinements as the applicable legal and regulatory framework, such as the CSRD omnibus package, as well as regulatory guidance, industry standards, and best practices evolve. Estimation Techniques and Data Limitations In preparing the 2024 Sustainability Statement, several assumptions and estimation techniques were employed to provide representative data. Given the complexity and scope of sustainability data, certain data points were based on estimates or assumptions due to the unavailability of precise information. This argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Statement 317 BP-1

includes instances where third-party data is not yet available. For example, Scope 3 GHG emissions calculations rely on supplier-provided data, industry averages, and extrapolated estimates where direct reporting is not possible. We recognize that methodologies for calculating sustainability data, including carbon accounting and climate risk scenario analysis, are continually evolving. As such, future reports may include refined methodologies or adjustments to previously reported figures. Where such updates occur, we will disclose changes in methodology and their impact on reported metrics. Digitalization and Data Collection Enhancements Additionally, our implementation of a digital data management tool in 2024 has significantly enhanced the data collection and validation process. This tool allows for the efficient aggregation and analysis of data, reducing the reliance on manual data entry and minimizing the potential for errors. However, despite these advancements, our ability to obtain high-quality data remains dependent on supplier transparency, industry data availability, and regulatory developments. Where data limitations exist, we apply reasonable estimations, and these are clearly documented within the report. 7.1.3 Disclosures in relation to specific circumstances Time Horizons The time horizons applied are the same as those defined by the ESRS for reporting purposes: • Short-term: The period covered by the undertaking's financial statements, typically one year. • Medium-term: From the end of the short-term period up to five years. • Long-term: More than five years. Value Chain Estimations We have instituted a system to estimate and monitor value chain data. Our identified metrics encompass critical areas such as energy usage, GHG emissions, and waste data. The preparation basis for these metrics involves utilizing industry average data and supplier surveys to estimate upstream and downstream impacts accurately. We have instituted a system to estimate and monitor value chain data. Our identified metrics encompass critical areas such as energy usage, GHG emissions, and waste data. The preparation basis for these metrics involves utilizing industry average data and supplier surveys to estimate upstream and downstream impacts accurately. Sources of Estimation and Outcome Uncertainty In our reporting, we have identified areas where there exists a degree of uncertainty, primarily attributed to external factors. These areas include greenhouse gas (GHG) and waste management data due to the availability of environmental data. Despite these challenges, we have made informed assumptions and judgments, assuming stable market conditions and consistent consumer demand, and utilizing the best-available environmental data to craft a report that reflects our standing and strategy. Where significant measurement uncertainties exist, these are disclosed alongside our metrics. We recognize that the forward-looking information presented in this report is subject to uncertainty. This is primarily due to the dynamic nature of market conditions and environmental factors that influence our business operations. Despite this, we remain committed to adapting and evolving to meet these challenges head-on. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Statement 318 BP-2

Policy Availability argenx policies referenced throughout this statement are available on our internal document-sharing platform. Policies that apply to external stakeholders are also available on the argenx website. Changes in Preparation or Presentation of Sustainability Information & Reporting Errors in Prior Periods Given this is our first year of preparing a Sustainability Statement under the CSRD framework, there are no changes in preparation or presentation of sustainability information from prior years. As our sustainability reporting matures, we cannot exclude that future refinements may lead to restatements of previously reported data. These restatements may occur due to improved methodologies, updated emissions factors, changes in organizational boundaries, or the correction of prior reporting errors. Any such restatements, if deemed material, may be disclosed in future reports, along with explanations of the reasons for the revisions as appropriate. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Statement 319

7.2 Sustainability Strategy 7.2.1 Sustainability and Our Business Model Our mission is to transform the lives of patients by translating immunology breakthroughs into novel antibody-based medicines. From the development of innovative therapies to our collaboration with stakeholders, we are committed to addressing the needs of diverse patient groups across geographical regions and prioritizing the development of treatments that make a meaningful difference in health outcomes. Additionally, we regularly engage with healthcare providers, regulators, and patient advocacy groups to ensure our efforts align with the needs of patients and contribute to global health improvements. Our sustainability strategy emphasizes non-financial performance data and discloses metrics that offer insight into our long-term sustainability commitment and global community role. We view sustainability as a strategic enabler, driving innovation, resilience, and long-term societal impact. We are refining our approach to sustainability, with the scope of our disclosures informed by the results of our DMA. Currently, our primary focus is on establishing baseline data, which aims to lay the foundation for a comprehensive and impactful sustainability strategy that integrates long-term objectives across all aspects of our operations. Our Business Model and Sustainability Our business model centers on the discovery, development, and commercialization of innovative immunology therapies designed to transform the lives of patients suffering from severe autoimmune diseases. Leveraging proprietary antibody engineering technology, we have developed a robust pipeline of therapeutic candidates to address unmet medical needs globally. As of December 31, 2024, we had 1,599 employees, reflecting the significant expanse of our operations and innovation pipeline, and have total operating income of $2.3 billion. Products and Services For a description of our products, please refer to Section 1.1.2 "Our Medicines" of this Annual Report. We serve a diverse range of significant markets and customer groups globally. We aim to comply with all regional regulations and market specific restrictions. Market access for our products is subject to market approval by the competent regulatory authorities of the relevant jurisdiction. Our Value Chain Our value chain is a structured network that encompasses our approach to 1) Research & Development (R&D) and 2) Commercial operations. Within our value chain, R&D encompasses upstream activities such as early-stage research, the sourcing of biotechnology and raw materials. Core operations within this part of the value chain include preclinical studies, clinical trials, collaboration with external partners, such as Contract Manufacturing Organizations (CMOs), focused on manufacturing investigational drug substances, and Contract Research Organizations (CROs), focused on drug discovery and development processes. This part of the value chain culminates in regulatory submissions that pave the way for eventual commercialization. Commercial operations include upstream activities such as manufacturing processes to produce finished products and collaboration with our external CMOs who support bulk production, packaging, and labeling of products within our value chain. Downstream activities within this part of the value chain include global distribution, patient support programs, and the responsible management of products at the end of their lifecycle. Core operations within our value chain include sales and marketing activities that engage downstream payers. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 320 SBM-1

Our Impact on Patients Our business model, founded on discovery, development, and commercialization, leverages proprietary antibody engineering technology to create a robust pipeline of therapeutic candidates. Medicines such as VYVGART and VYVGART SC significantly improve the quality of life and functionality for autoimmune patients, benefiting both patients and healthcare providers. By prioritizing patient health and treatment efficacy, we contribute to broader social well-being and alleviate pressures on healthcare systems worldwide, underlining our commitment to stakeholder welfare. 7.2.2 Our Double Materiality Assessment Under the European Sustainability Reporting Standards regulation, companies are required to undertake a double materiality assessment (considering both impact and financial sustainability matters). In 2024, we conducted an initial double materiality analysis aligned with the CSRD and ESRS standards. This assessment aimed to identify the most pertinent environmental, social, and governance topics that could present financial risks and opportunities for argenx (outside-in perspective), while evaluating the impact of our activities on people and the environment (inside-out perspective). Our 2024 Materiality Assessment Followed a Structured Approach: Defining Scope and Objective of the Materiality Assessment The initial phase involved defining our primary activities, mapping the value chain, and determining geographical scope. The following entities were subject to the materiality review: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 321 IRO-1 IRO-2

Name of the Company Registered Office Country % Ownership argenx Australia Pty Ltd Level 14, 2 Riverside Quay Melbourne VIC 3006 Australia 100% argenx Austria Services GmbH Graben 19, 4th & 5th floor, Vienna, A-1010, Austria Austria 100% argenx Benelux BV Industriepark-Zwijnaarde 7, 9052 Zwijnaarde, Belgium Belgium 100% argenx BV Industriepark-Zwijnaarde 7, 9052 Zwijnaarde, Belgium Belgium 100% argenx Canada Inc 9131 Keele Street Suite A4, Vaughan, Ontario, Canada, L4K 0G7 Canada 100% argenx France SAS Rue Camille Desmoulins 13, 92130 Issy-Les- Moulineaux, France France 100% argenx Germany GMBH Konrad-Zuse-Platz 8, 81829 Munich, Germany Germany 100% argenx Italy S.r.L. Largo Francesco Richini 6, 20122 Milan, Italy Italy 100% argenx Japan KK HULIC JP Akasaka Building 2-5-8, Akasaka, Minato-ku, Tokyo, 107-0052, Japan Japan 100% argenx Netherlands Services BV Laarderhoogtweg 25, 1101 EB Amsterdam, the Netherlands Netherlands 100% argenx SE Laarderhoogtweg 25, 1101 EB Amsterdam, the Netherlands Netherlands 100% argenx Spain S.L Sucursal em Portugal Palácio Sottomayor, Rua Sousa Martins, nº. 1, 1º esquerdo, 1050 217 Lisboa, Portugal Portugal 100% argenx Spain S.L. Paseo Dela Castellana 200, Planta 8a, Oficina 819 28046 Madrid Spain 100% argenx Switzerland SA Rue du Pré-de-la-Bichette 4, 1202 Geneva, Switzerland Switzerland 100% argenx UK Ltd. Spaces Gerrards Cross Chalfont Park, Building 1 Gerrargs Cross, SL9 0BG, UK UK 100% argenx US Inc. 33 Arch Street, Boston, Massachusetts 02110 US 100% OncoVerity, Inc Aurora, Colorado, United States United States 50% To learn more about our consolidation scope, refer to “Note 30 Overview of Consolidation Scope. The double materiality assessment also incorporated a value chain mapping exercise, which identified the most relevant upstream and downstream activities, relationships, and sectors affecting our operations. We evaluated our Tier One suppliers and main customer base, split by geographic locations, to ensure the assessment accurately captured impacts across the value chain. Using ESRS guidelines, topics were mapped and clustered according to their relevance across the value chain, resulting in a tailored list of ESG topics for assessment that ensures compliance with CSRD requirements. To ensure a focused assessment on high-risk activities and relationships, our value chain mapping included identifying areas where heightened risks could arise due to specific operational, business, or geographic factors. This targeted focus enables us to assess our operations and partnerships effectively, incorporating geographic and sector-specific insights to understand potential adverse impacts. When considering the CSRD perimeter, argenx concluded that all entities fall within their direct operations. However, this scope did not include OncoVerity, as it is considered part of the value chain. argenx holds a 50% non-controlling interest in OncoVerity, which remains an unconsolidated entity. Our new subsidiaries in Portugal and Austria were incorporated after the double materiality assessment process concluded therefore, these subsidiaries were not directly considered as part of our business context for the purpose of impact, risk, and opportunity identification. However, given these new subsidiaries do not differ in their purpose it was agreed that the final material impacts, risks, and opportunities were representative of the activities of these newer subsidiaries. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 322

Identifying Topics and Impacts, Risks, and Opportunities: Preliminary Identification Process We conducted an initial evaluation of our business context, encompassing operations, products, services, locations, customers, suppliers, and material inputs, to identify a preliminary list of potential sustainability matters. This evaluation was integrated with the extensive list of sustainability matters outlined in the ESRS sector-agnostic sub-topics, along with our previously established sustainability priorities. The outcome of this process is an initial compilation of potential material sustainability matters. Stakeholder Engagement To define material matters in the context of ESG double materiality, we employed a stakeholder engagement strategy focused on direct internal stakeholder engagement, supplemented by indirect external stakeholder engagement. Engagement methods included: • Direct methods: Semi-structured interviews and workshops. • Indirect methods: Desk research. Insights were gathered from stakeholders in our key operating regions and complemented by localized analyses in specific countries. We did not include direct consultation with external stakeholders. As a proxy, however, our staff represented external groups such as suppliers, patients, and sector associations. Through engagement and dialogue with these stakeholders, our team has a detailed understanding of their views. Internal stakeholders included employees, members of our Board of Directors, and the executive team. The stakeholder groups represented by argenx employees included Human Resources, Facilities and Environment, Health and Safety, Technical Operations, Global Patient Advocacy and Policy, Legal, Compliance and Intellectual Property, Investor Relations, Global Clinical Operations, Finance, Internal Controls, Commercial team, Research & Development, Internal Audit, and Global Sourcing & Alliance Management. Below is an overview of our stakeholder engagement process, outlining the various stakeholder groups and the representative groups that conveyed their input that informed our double materiality assessment. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 323

Stakeholder Category Stakeholder Group Stakeholder Group Representative Affected Stakeholder Employees Human Resources and Employee Safety Local Residents/Communities Not Applicable — Desktop Research Manufacturers/Suppliers (CMO, CRO, Partners) Clinical Procurement Healthcare Community (a.o Patients, Collaborators and Patient Organizations) Patient Advocacy Board of Directors, CSR Steering Committee (Environmental Expert) Corporate Governance Shareholders/Investors Investor Relations Landlords Facilities and Environment, Health and Safety Service Providers Pre-clinical, Clinical, and Commercial Transporters Operations, Procurement and Supply Chain Team User of Sustainability Report Shareholders/Investors Investor Relations Rating Agencies Finance and Controls Authorities Government Relations Financial Institutions/Banks Finance and Controls Patients and Patient Organizations Patient Advocacy Internal Experts Finance Team Finance and Controls Human Capital Team Human Resources Commercial Team Commercial Operations, Procurement and Supply Chain Team CMC-Supply Chain R&D Team 1. Science/research 2. Development Legal and Compliance Team Legal and Compliance Animal Welfare Animal Welfare Scientific and Clinical Development Teams Scientific and Clinical Development Teams Privacy IT, Business Information Systems and Cybersecurity, External Counsel External Experts Consultants (PwC) PwC Doctors, Researchers 1. Science/Research 2. Development NGOs/Federations/Authorities Not Applicable — Desktop Research and advising from PwC Assessment and Due Diligence argenx synthesized stakeholder insights and external sources–such as existing risk frameworks, corporate strategy documents, sector reports, and scientific studies–to validate and refine the list of potential impacts, risks, and opportunities. Analytical tools like a proprietary IRO tool that presents IROs derived from sector- specific SASB standards and ESRS Sustainability matters, supported the identification of sector-specific material topics. Use of this tool provided structure and orientation for the materiality analysis, guiding the assessment process. There are currently no other formalized policies that incorporate international guidelines, other than those mentioned in our Code of Conduct. Materiality Assessment Methodology Quantitative Scoring Sustainability matters were prioritized through the scoring criteria aligned with the ESRS guidance: • Impact materiality: Impacts were scored (one to five) on scale (how grave the impact is), scope (how widespread the impact is), and irremediable character (how difficult a negative impact is to remediate) with a severity score calculated as the mean of scale, scope, and irremediable character. • Financial materiality: Risks and opportunities were scored on likelihood and potential financial impact. Risks and opportunities considered financial, operational, reputational and/or legal factors. The mean score of financial impact and likelihood was used for prioritization. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 324

Example: The impact “inability to ensure the safety of clinical trial participants” scored five out of five for scale, three out of five for scope, and five out of five for irremediable character, yielding a severity score of 4.3. Threshold Review We evaluated three thresholds (three, three and a half, and four out of a possible five) to identify material IROs. The threshold of four out of five was chosen as we believe that this best represented the most material sustainability matters for argenx across environment, own workforce, consumers, and end-users and governance. Following this process, our team overseeing the materiality assessment reviewed IROs that both did and did not meet the threshold as part of the validation process. It was determined that five additional IROs that were initially below the threshold should be included as material, based on key stakeholder views on sustainability matters. Validation and Governance Findings were validated by argenx's Senior Management Team and the Board of Directors to ensure alignment with strategic objectives. Strategic Integration of Material IROs The relationship between material IROs and argenx’s strategy and business model is fundamental to its sustainability efforts. The material IROs were tested against our existing risk management criteria and the latest corporate strategy for alignment to help position argenx to address key ESG challenges and opportunities. For any risk factors that were relevant to ESG matters and aligned with an IRO, they have been included in our Sustainability Statement if they passed the scoring threshold. Ongoing Monitoring and Adaptation As part of ongoing enhancement of our materiality assessment, the material IROs were further reviewed by our ESG leads, with support from internal expertise on supply chain, operations, and compliance teams, to enhance the IROs relevance to argenx's business activities. The language was refined for conciseness and clarity. Stakeholders were re-engaged to provide feedback and validate previous scoring and justifications. This process led to modifications in the material IROs and subsequently, the ESRS topics that we are currently reporting on. 7.2.3 Double Materiality Assessment Result As part of our double materiality assessment (which requires consideration of both impact and financial sustainability matters) that is required by the European Sustainability Reporting Standards regulation, argenx has identified several related material topics across the ESRS topics. We have described these in the following tables in accordance with the requirements of ESRS 2 SBM-3. That being so, all of our initiatives are reviewed to ensure compliance with local laws, and individual decisions are always based on merit, consistent with applicable laws. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 325 ESRS 2 SBM-3

E1 Climate Change Sub-Topic & Sub-sub-topic Classification Value Chain; Time Horizon Material Impact, Risk, or Opportunity Description Climate Change Mitigation Energy Negative Impact (actual) Entire Value Chain Short Term Indirect emissions from upstream and downstream activities, including manufacturing, purchased goods and services, transportation and distribution of raw materials and products (to Europe, Japan, and the USA), waste generated in operations, and end-of-life treatment of products. These processes also require substantial energy, often sourced from fossil fuels, which amplifies the carbon footprint associated with the reporting company’s supply chain. We serve a diverse range of significant markets and customers globally, with key partners located in the USA, Japan, Germany, and China. Partnerships, CROs, as well as CMOs and other key suppliers are critical to advancing argenx's drug development pipeline. Energy used by these partners for refrigeration, heating, ventilation, air conditioning (HVAC) and lighting is dependent on fossil-based fuels leading to GHG emissions that have a negative effect on the environment. As such, in 2024, we actively engaged with 21 suppliers leading to 35% primary GHG data. Climate Change Mitigation Negative Impact (actual) Own Operations Short Term Direct operational emissions and energy usage from company owned sources (i.e., facilities and fleet) contribute to the negative effects of climate change. argenx’s fleet of transportation vehicles and operation of office buildings/facilities (i.e., R&D facilities) are a necessary part of our efforts in developing, commercializing, and distributing immunology therapies aimed at treating severe autoimmune diseases. However, these activities also produce emissions that are harmful for the environment. To address this, in 2024, we enhanced our GHG emissions inventory process. E5 Resource Use and Circular Economy Sub-Topic & Sub-sub-topic Classification Value Chain; Time Horizon Material Impact, Risk, or Opportunity Description Waste Negative Impact (actual) Upstream/ Downstream Short Term The disposal of single-use products, disposable medical devices, and hazardous waste (e.g., expired medications, chemical solvents, contaminated packaging, laboratory waste, and manufacturing byproducts) contributes to significant waste generation, resource depletion, and environmental and health risks when improperly managed. argenx’s work in the research and innovation phase of immunology therapies results in the disposal of single use products and disposable medical devices, contributing to waste generation. These materials are typically discarded after one use for health and safety purposes. As such, we are exploring to what extent disposable consumables (i.e., plastic) can be replaced by reusable consumables (i.e., glass). argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 326

S1 Own Workforce Sub-Topic & Sub-sub-topic Classification Value Chain; Time Horizon Material Impact, Risk, or Opportunity Description Equal Treatment and Opportunities for All Equal Treatment, Diversity Positive Impact (actual) Own Operations Short Term A positive, diverse, and inclusive work environment that ensures equal treatment of all employees–regardless of origin, gender, sexual orientation, or religion–promotes fairness, and strengthens teamwork and collaboration. argenx fosters an inclusive work environment where everyone feels safe and encouraged to contribute, regardless of employee origin, gender, sexual orientation, or religion. We strongly believe a diverse culture broadens the scope of ideas and creativity essential to developing and delivering innovative therapies to patients, while leading to better work outcomes for employees. Equal Treatment and Opportunities for All Training and Skills Development Positive Impact (actual) Own Operations Short Term Investments in employee learning and development through training programs fosters employee well-being and prepares employees to meet future challenges while making them feel a greater sense of purpose and belonging. argenx encourages all employees to participate in a Personal Development Program aimed at building employees’ individual strengths in support of long-term career aspirations and goals. The development program focuses on empowering employees in their roles, while promoting a greater sense of purpose, belonging, and worthiness across argenx. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 327

S4 Consumers and End-Users Personal Safety of Consumers and/or End- Users Health and Safety Negative Impact (potential) Upstream Downstream Short Term Inability to ensure the safety of clinical trial participants and patients can have severe impacts on users’ health. A key component of argenx’s business model is the discovery and development of medicinal treatments. To launch a drug successfully and safely in the market, we must test the drug by performing clinical trials. We manage our clinical trials through a combination of internal oversight and collaboration with external partners. We ensure the health and safety of clinical trial participants and patients through rigorous compliance with regulatory standards, including Good Clinical Practices (GCPs). Clinical trials undergo thorough review and approval by Institutional Review Boards (IRBs) or Ethics Committees, which assess trial design, patient safety measures, and ethical considerations. Additionally, independent Data Safety Monitoring Boards oversee trials, monitors safety data, and make recommendations to modify or halt trials if necessary. These measures help identify and address safety concerns proactively, minimizing risks to participants and maintaining the integrity of clinical trials. Personal Safety of Consumers and/or End- Users Health and Safety Risk (Reputational) Entire Value Chain Short Term Risk of reputational damage from clinical trials' or patients' claims as a result of adverse events observed during clinical trials, including unforeseen reactions, if not appropriately addressed. A critical component of argenx’s business model is the development of medicinal treatments, which involves conducting clinical trials to ensure safety and efficacy. Our trials are governed by stringent internal controls and partnerships with external stakeholders to ensure oversight and compliance. To mitigate reputational risks, we adhere to regulatory standards, including Good Clinical Practices (GCPs), to transparently report and manage adverse events. Independent monitoring bodies, such as Data Safety Monitoring Boards, assess safety data and recommend actions as needed. These measures minimize risks, uphold clinical integrity, and protect our reputation. Social Inclusion of Consumers and/or End- Users Access to Products and Services Positive Impact (actual) Downstream Medium Term Increased and better access to products and services (including through affordable pricing) can improve health and longevity for more patients. argenx’s business model prioritizes the development and delivery of innovative treatments that address unmet medical needs. The company ensures global access through contractual partnerships with healthcare systems, payors, and product distributors, supported by patient-affordability programs. These initiatives reduce financial barriers and seek to expand access to therapies, improving health outcomes and patient longevity. By reducing these barriers, we demonstrate adaptability and resilience in addressing healthcare challenges while fostering sustainable growth. Social Inclusion of Consumers and/or End- Users Access to Products and Services Opportunity (Financial/ Operation) Downstream Medium Term Increased and better access to medicines through improving commercial/ distribution channels and improving the affordability and pricing may lead to a growth in market capacity. argenx’s business model supports the development and distribution of innovative treatments with a focus on accessibility. The company collaborates with distribution partners, healthcare systems and payors to optimize supply chains and reduce barriers to access. Initiatives such as rebating and innovative value-based agreements coupled with market-specific patient-affordability programs enable us to reach more patients with our life changing products, in turn driving market expansion and improving global health outcomes in the rare disease space. These efforts enhance resilience by addressing access barriers while supporting sustainable growth and market capacity. Sub-Topic & Sub-sub-topic Classification Value Chain; Time Horizon Material Impact, Risk, or Opportunity Description argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 328

Social Inclusion of Consumers and/or End- Users Responsible Marketing Practices Risk (Reputational/ Financial) Entire Value Chain Medium Term Off-label promotion exposes pharmaceutical companies to legal, financial, and reputational risks, inviting regulatory scrutiny and liability. argenx ensures compliance with all regulatory requirements governing the promotion of its products. Training programs and internal policies are in place to mitigate risks associated with off-label promotion. The relevant materials are reviewed to ensure alignment with approved product indications and global standards, such as those set by the FDA and EMA. Through these measures, we protect our reputation, minimize legal liabilities, and maintain stakeholder trust while reinforcing resilience against regulatory scrutiny. Information- Related Impacts for Consumers and/or End- Users Access to Quality Information Negative Impact (potential) Downstream Short Term Misleading or inaccurate information relating to products can lead to improper use, including dangerous interactions with other medications or incorrect usage and dosage. Ensuring accurate and transparent communication of product information is integral to argenx’s business model. The company employs robust internal processes and collaborates with regulatory authorities to verify the accuracy and accessibility of all product-related materials. Compliance with global standards, such as those set by the FDA and EMA, is ensured through rigorous review processes. We also provide training for internal personnel who communicate with healthcare professionals, to ensure truthfulness and accuracy in the information they provide. Information- Related Impacts for Consumers and/or End- Users Access to Quality Information Risk (Reputational/ Financial) Own Operations Short Term Risk of product misinformation and false claims, which can result in a loss of support from stakeholders (i.e., patients, doctors, pharmacists) and non- compliance, significant fines, and settlements. argenx’s business model emphasizes transparency and accuracy in product communication. The company has established a framework to ensure that all claims are substantiated and align with global regulatory requirements. The relevant materials undergo rigorous internal review processes to prevent misinformation and ensure compliance with standards such as FDA and EMA guidelines. These efforts protect the company from reputational damage, regulatory penalties, and financial liabilities while maintaining stakeholder trust and ensuring appropriate access to medicines. Information- Related Impacts for Consumers and/or End- Users Privacy Risk Own Operations Short Term Exposing sensitive patient information risks fines and penalties, lawsuits, remediation costs and reputational damage Protecting sensitive patient information is integral to argenx’s operations and compliance framework. The company adheres to all applicable data privacy regulations, including the GDPR, and implements stringent internal controls, information security as well as cybersecurity measures to safeguard patient data. Regular risk assessments, incident response protocols, and employee training programs further mitigate the risk and potential impact of data breaches. These measures ensure compliance, protect stakeholder trust, and reinforce the company’s resilience against legal, financial, and reputational risks. Sub-Topic & Sub-sub-topic Classification Value Chain; Time Horizon Material Impact, Risk, or Opportunity Description argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 329
G1 Business Conduct Corporate Culture Negative Impact (potential) Entire Value Chain Short Term Unethical practices (e.g., harassment, discrimination, corruption, fraud, safety issues) as a result of poor corporate culture. Poor corporate culture can compromise patient care, erode trust in healthcare practitioners, and negatively impact employee livelihood. As such, we prioritize and incorporate our five cultural pillars throughout the business – innovation, co-creation, empowerment, excellence, and humility. These pillars provide the foundation for a high-integrity environment where employees can operate at their fullest potential. Protection of Whistle- Blowers Negative Impact (potential) Entire Value Chain Short Term An inability to protect whistleblowers against retaliation prevents the identification and remediation of incidents towards employees and patients, impacting engagement, safety, and trust. Failing to protect whistleblowers against retaliation undermines employee engagement, compromises patient trust, and increases the risk of incidents going unaddressed. Employees may feel discouraged from raising concerns about potential issues, dangers or wrongdoings relating to our business, eroding our ability to sustain growth and innovation in a highly regulated and trust-dependent industry. In 2024, we updated the Global Speak-Up Policy which complies with Directive (EU (2019/1937) since its effective date. Employees are regularly trained and reminded of the Speak-Up Policy, which is also part of the onboarding package for every new employee. Management of Relationships with Suppliers and Payment Practices Towards Suppliers Negative Impact (potential) Own Operations Short Term Poor relationships with suppliers and inconsistent payment practices (as a result of argenx failing to pay suppliers on time) may impact the reliability and consistency of suppliers activities, interfering with supply chains, affecting R&D and medical distribution to those in need of treatment. argenx utilizes third-party CMOs who act in accordance with the FDA’s current good manufacturing practices (cGMPs) for the manufacturing of drug substance and drug product. Poor relationship management with CMOs may lead to delays in delivering essential materials and medications, resulting in serious health consequences for patients. Recognizing the critical importance of supplier relationships, we take a meticulous approach when forming supplier arrangements, prioritizing direct contact over intermediaries to ensure reliability and transparency. Management of Relationships with Suppliers and Payment Practices Towards Suppliers Risk (Operational) Entire Value Chain Short Term Poor supplier relationship management can lead to risks such as low- quality products, supply chain disruptions, financial losses (e.g., increased costs and interest from late payments), non- compliance with supplier agreements and payment terms, and reputational damage, resulting in a loss of trust and credibility. Co-creation is at the core of argenx’s business model. As such, actively managing supplier relationships is critical to our success. Non-compliance and disruptions with suppliers can escalate costs, strain partnerships, and jeopardize our reputation and long-term strategic goals in a highly competitive market. This can pose significant challenges to our activities, potentially disrupting operational efficiency and causing reputational and financial repercussions. Recognizing the critical importance of supplier relationships, we take a meticulous approach when forming supplier arrangements, prioritizing direct contact over intermediaries to ensure reliability and transparency. Sub-Topic & Sub-sub-topic Classification Value Chain; Time Horizon Material Impact, Risk, or Opportunity Description argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 330

Entity Specific Sub-Topic & Sub-sub-topic Classification Value Chain; Time Horizon Material Impact, Risk, or Opportunity Description Product Quality and Safety Negative Impact (potential) Downstream Short Term Poor product quality can directly impact the effectiveness of treatments and overall health of patients. argenx is dedicated to building a foundation of loyalty and trust with its stakeholders on the safety and quality of our products. Compliance standards with various international regulatory bodies and intensive internal QA processes are key to assuring strong product quality. Product quality testing is performed on every batch of products to ensure sufficient quality control is integrated throughout the process. In addition, a full batch record review is performed to ensure the product is manufactured in line with the applicable regulatory requirements. Innovation and R&D Positive Impact (actual) Downstream Medium Term Successful innovation helps find new treatments for current diseases and address unmet needs, allowing more patients to be treated. argenx’s goal is to deliver immunology innovations to patients that are both first-in-class and best-in- class to transform the lives of people with serious autoimmune diseases. At every step of the drug development process, we combine our leading antibody engineering capabilities with disease biology insights from collaborators to identify and develop innovative new treatments. Product Traceability (Counterfeit Drugs) Negative Impact (potential) Entire Value Chain Short Term A lack of product traceability and transparency can lead to counterfeit drugs or materials, endangering patient health. A lack of product traceability in the pharmaceutical value chain poses serious risks to patient safety as counterfeit drugs or materials may infiltrate the supply chain, compromising patient health. In compliance with national legislation like the United States Drug Supply Chain Security Act and the European Union Falsified Medicines Directive, all commercial argenx products are coded with serial numbers at unit, case and pallet levels and transported in tamper-evident seals to effectively trace products and prevent physical product tampering. Product traceability initiatives are critical for argenx in ensuring the health and safety of all patients. 7.2.4 Stakeholder Engagement We engage with various stakeholders, including patients, healthcare providers, employees, suppliers, and investors, to integrate their needs and expectations into our strategy and business model. Currently, stakeholder engagement is managed through our business units, and through cross-functional teams and communities focused on our alliances, partnerships, healthcare professionals and patients, amongst other stakeholders. as we do not have a formal policy or dedicated function for this. While we did not directly consult external stakeholders for the double materiality assessment, their views were represented through our business units, which engage and dialogue with them. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 331 SBM-2

Stakeholder Engagement Purpose Outcomes Patients We host regular patient panels and listening sessions where patients share their experiences and challenges dealing with rare autoimmune conditions. Patient panels and listening sessions Strengthen patient communities as well as deepen our own ability to identify and address unmet clinical needs. Patients • Advance our understanding of rare disease via listening sessions • inform the development of treatments Healthcare Providers We engage with healthcare providers for clinical research, advisory services and speaking engagements. Engaging with healthcare providers helps us advance research, gain expert insights, and share medical knowledge. Healthcare Providers • Inform the development of treatments • Improve patient outcomes. Employees Our employee communications and engagement team connect with employees through engagement sessions such as Culture Lab sessions. Employee engagement sessions foster colleague unity, gather insights to enhance employee experience, and promote our Cultural Pillars Employees • Shape the agendas of company-wide meetings. • Bolster engagement with cultural pillars as culture champions • Inform the development of offerings via focus groups. • Engage through company- wide communications through various channels Suppliers Since 2024, our supply chain management team, in collaboration with an external vendor, has sent questionnaires to selected suppliers to gather emissions data. Supplier engagement Informs our GHG inventory via emissions data gathered Suppliers • Support our understanding of Scope 3 emissions. Investors Our investor relations team regularly engages with shareholders on ESG matters Our investor engagements provide us insights into key ESG topics and responsible business practices Investors • inform our sustainability strategy and communication 7.2.5 ESG Governance and Oversight ESG Governance Our governance structure is designed to provide robust oversight and strategic guidance. Our Board of Directors, our highest governance body, is a one-tier board under Dutch law. Our Board of Directors, with 90% independent members, is collectively responsible for our general affairs, including for governance and oversight with regard to sustainability matters. Given the breadth of topics covered under the umbrella of ESG, we have strategically allocated ESG oversight responsibilities across our Board of Directors' specialized committees. The Audit and Compliance Committee holds ultimate responsibility for ensuring the integrity and design of our external sustainability reporting. Specific ESG themes are managed by designated committees, as depicted in the chart below. The members of our Board of Directors bring extensive experience from the biotechnology and pharmaceutical industries, with significant expertise in developing and commercializing innovative therapies. Their strong track records in global markets, particularly in North America, Europe, and Asia, supports argenx’s strategic goals and international expansion. We prioritize a diverse and inclusive board of directors, encompassing a broad spectrum of perspectives, experiences, backgrounds, expertise, and gender. Please refer to "Section 3.2.4. Non-Executive Directors" for more information on the Board of Directors' skills and expertise. Pursuant to our Articles of Association, our Board of Directors has entrusted day-to-day management to the executive director, being our CEO. Our CEO leads a diverse and experienced senior management team, of which seven members report directly to our CEO, who is responsible for day-to-day management of the overall corporate strategy and the integration of sustainability matters therein. Within the senior management team, our general counsel has primary responsibility for management oversight regarding sustainability matters and helps to guide our sustainability strategy and disclosures, in addition to argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 332 GOV-1

coordinating with the Board of Directors on sustainability work streams. ESG considerations are also part of the agenda of our Global Risk Management and ESG Committee. In light of new ESG legislation, such as the EU CSRD, the Board of Directors, the Audit and Compliance Committee, and senior management have discussed how to best prepare argenx for these changes. Consistent with our outsourcing model to supplement our internal team with partners who bring unique expertise and/or fill resource capacity gaps, it was concluded that we would hire highly experienced external consultants and advisors to supplement capacity and fill any gaps in sustainability expertise. This collaboration with external sustainability consultants enhances our governance framework. As such, while leveraging internal knowledge of social responsibility and governance best practices, we engage with external advisors for environmental regulation expertise. By leveraging both internal and external expertise, we aim to stay abreast of the latest trends and best practices, effectively overseeing and advancing our sustainability initiatives. Employees are not formally represented within the administrative, management, and supervisory bodies. Nevertheless, regular meetings, feedback sessions, and representation in committees help to ensure that their voices are heard and considered in decision-making processes. This approach aims to foster an environment where all stakeholders can contribute to the company’s approach to sustainability. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 333

Management of Material Risks, Impacts and Opportunities by Administrative, Management and Supervisory Bodies In 2024, our senior management team provided quarterly updates and reports during scheduled Board of Directors meetings, ensuring that the Board of Directors and the Audit and Compliance Committee were informed of the progress on the journey towards CSRD compliance. Generally speaking, the Board of Directors and the Audit and Compliance Committee’s agenda contains topics that directly or indirectly relate to our material impacts, risks, opportunities. Key material impacts, risks, and opportunities addressed during 2024 included regulatory compliance, corporate culture, scientific innovation and affordability and pricing. Our Board of Directors has industry expertise and knowledge, enabling effective oversight and guidance of ethical business practices. The Board of Directors in general, and the Audit and Compliance Committee specifically, have significant experience in developing and implementing compliance frameworks, monitoring anti-corruption and bribery measures, and ensuring adherence to international regulatory standards. The Audit and Compliance Committee actively collaborates with the Ethics & Compliance function to support the organization’s integrity framework. As part of the company’s Code of Conduct and Business Ethics, the Board of Directors receives quarterly updates on anti-bribery and anti-corruption matters and other compliance-related topics. This close alignment with Compliance aims to ensure that emerging risks and opportunities in business conduct are effectively addressed. To learn more about our Board of Directors, see Section “3.2.4 Non-Executive Directors”. Integration of Sustainability-Related Performance in Incentive Schemes In 2024, the short-term and long-term incentive compensation for our Board of Directors and senior management did not include performance metrics specifically tied to sustainability performance. Due Diligence In 2024, we continued to build/enhance our due diligence process within our sustainability framework to manage ESG risks and opportunities, aligning with the ESRS requirements. The double materiality assessment has been documented and reviewed by the Risk Management Committee and validated by the Audit and Compliance Committee and the Board. Risk Management and Internal Controls Over Sustainability Reporting In 2024, we improved our risk management for sustainability reporting and have identified several main risks categories, including regulatory, and legal and compliance. To address this, we have enhanced our sustainability reporting framework by establishing an internal team and engaging external experts. In this first year of CSRD reporting, we have built systems and tools to ensure our reporting is thorough and accurate. We are committed to continuous improvement and will implement measures to address any identified inefficiencies in the future. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Sustainability Strategy 334 GOV-1 GOV-2 GOV-4 GOV-3 GOV-5

7.3 Environment 7.3.1 Introduction to Environmental Topics at argenx We recognize our responsibility to act as good stewards of the planet we all share. Our approach is centered on understanding and addressing our impact on the environment, from energy and greenhouse gas emissions to waste. 7.3.2 Climate Change Mitigation Climate Risk Assessment Physical Risks Overview In 2024, we conducted our first scenario-based climate assessment to evaluate exposure to climate-related physical risks. This assessment screened forty-three key locations across our value chain, including offices, contract manufacturers, and suppliers, selected based on their strategic importance. Out of 43 locations, 12 locations were office sites, 28 locations were supplier sites, and three locations were customer sites. These locations were globally distributed (across North America, Europe, UK, and Asia Pacific). Eight hazards—extreme heat, coastal flooding, pluvial flooding, riverine flooding, wildfires, water stress, drought, and cyclones—were analyzed across three-time horizons (historical, 2030s, and 2050s) and three climate scenarios (SSP1, SSP2, and SSP5) using IPCC AR6-aligned datasets. Final results were reported considering which hazards are the most impactful in the short, medium, and long term (i.e., baseline, 2030, and 2050-time horizons) for the SSP5 scenario. As a quantitative iteration, the assessment was conducted at the inherent level, without considering existing adaptations or mitigations. Future assessments will incorporate existing adaptations and mitigations to refine our understanding of climate risks. Sensitivity to hazards was evaluated based on past experiences and potential impacts on our assets. Scenario analysis results can be used to inform strategic and risk management decisions regarding resilience measures, such as enhancing contingency plans, diversifying supply sources, and relocating from high-risk areas to low-risk areas. For instance, our offices are less sensitive to climate hazards, as business interruption is less likely to occur if an office is impacted by a hazard, while a higher impact could be felt if our contract manufacturers were impacted by climate events. Transition Risks Overview In 2024, we conducted our first scenario-based climate transition risk assessment. The assessment took a qualitative approach to examining transition risk exposure levels regarding four transition risk categories: policy and legal, market, technology, and reputation. For each of these categories, several sub-categories of risk were assessed in order to gain further insight into specific risks that might impact us. In total, 13 subcategories of risk were assessed. Related to policy and legal risks, four were assessed: greenhouse gas emissions pricing, enhanced emissions reporting obligations, mandates on and regulation of existing products and services, and exposure to litigation. For the market risk category, three subcategories were assessed: changing customer behavior, increased cost of raw materials, and uncertainty in market signals. Under the technology risk category, three subcategories were assessed: substitution of existing products and services, unsuccessful investment in new technologies, and costs to transition to lower emissions technology. Finally, under the reputation risk category, three subcategories were assessed: shift in consumer preferences, stigmatization of sector, and increased shareholder concern. These risk categories and sub-categories were drawn from the Taskforce on Climate-Related Disclosures’ (TCFD) framework. The assessment used data and information regarding argenx emissions, revenue, market presence, and stakeholder priorities combined with scenarios and data from the International Energy Agency (IEA) to gauge inherent transition risk exposure levels across IEA scenarios and three-time horizons - 2030, 2040, and 2050. The three IEA scenarios used were the Stated Policies Scenario (STEPS), Announced Pledges Scenario (APS), and the Net Zero Emissions by 2050 Scenario (NZE). Of these scenarios, the Net Zero argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 335 ESRS 2 SBM-3 ESRS 2 IRO-1 ESRS 2 SBM-3

Scenario is recommended to inform company strategy and is often required to comply with climate disclosures, so particular attention was paid to the results under this scenario. This transition risk assessment provided high level climate risk exposure levels, which may inform future analyses and provide a foundation for argenx to build understanding of climate-related risks. This and future assessments may be able to inform our broader risk management strategy and enable argenx to put measures in place to monitor and manage these risks effectively. Time Horizons Scenario analysis was used in our climate risk assessments to inform how exposure could evolve over time. Both physical and transition risk screenings used three different warming scenarios to assess potential exposure across various futures. Time horizons have been defined as part of the analysis for both physical and transition risks. The short-term time horizon has been defined as up to five years in the future. The medium-term time horizon is between five to 15 years, while the long-term time horizon is more than 15 years. The time horizons identified for climate risk assessment differ from those defined for the rest of the IROs and are based on climate scenario data and timelines as recommended by TCFD. Physical Risks Screening The physical risk screening used three scenarios from the Intergovernmental Panel on Climate Change (IPCC) - SSP1 (below 2°C), SSP2 (2°C-4°C), and SSP5 (3.3°C-5.7°C), with the main focus on SSP5 as this is the scenario under which the world would be expected to see the highest impacts from climate change. Models from the Coupled Model Intercomparison Project (CMIP) were used to complete this analysis. The analysis looked at three-time horizons, assessing baseline exposure based on historical observations, and projecting possible exposure in the future by 2030 and 2050. Both the projected exposure to the risk in different time horizons and the change in exposure from baseline were considered when determining whether a climate hazard could have a substantive impact on argenx. Transition Risks Screening The transition risk screening used three scenarios from the IEA - Stated Policies scenario, Announced Pledges scenario, and Net Zero scenario, with the main focus on the Net Zero scenario because this is the scenario under which the highest transition risks are expected to be seen. These scenarios provide a range of assumptions that were used in the screening to inform the magnitude of potential impacts that we could see in the future. These scenarios provide medium to long-term energy trend projections, which allows argenx to explore the potential implications of various policy choices, investment trends, and technology dynamics. The assessment undertook a structured approach that included assigning a baseline score for each risk reflecting our current exposure based on our current climate actions, business model, and the industry we operate in. To project this exposure into the future, risks were assigned proxy indicators from the IEA to assess how exposure could evolve over time by 2030, 2040, and 2050 under different possible scenarios based on the change in these proxies. Results On the basis of the foregoing assessment and our understanding of our business, we identified the following climate-related physical and transition risks as the most relevant to our operations among the risks that were screened. Given their likelihood to affect our business, these risks were not identified as material according to our double materiality assessment. • Climate-Related Regulations: Emissions reporting standards are emerging globally. There is an increased risk of regulations that we will be exposed to, which requires additional resources and costs to address. In Europe, the CSRD regulation mandates that companies report on their social and environmental impacts from a double materiality perspective. Similar regulations are being considered and adopted in other regions. For instance, in the US, California’s SB 219 bill is asking companies to report their emissions and climate risks, and in Australia the Climate-related Reporting Bill is asking companies to report climate impacts. Non-compliance poses potential risks, including litigation and reputational damage. The risks associated with climate-related regulations could also increasingly impact argenx through increased costs for compliance. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 336

• Increased Costs of Raw Materials: The risk of increased cost and decreased availability of raw materials was identified as relevant and could potentially emerge in the short to medium term as fossil fuel-based materials could be taxed, which would raise the price of such materials that we use in operations and increase procurement costs. Moreover, supply chain disruptions due to climate events could result in decreased availability of raw materials, which could make it more difficult for argenx to procure these materials. For a limited number of our raw materials, we rely on a single source of supply. We try to mitigate the risk of scarcity by avoiding single-source suppliers to ensure we can source our materials from alternative suppliers if needed. This allows us to have access to different suppliers who are impacted by climate events to varying degrees and at different times based on their geographies. The following risks were identified as potentially relevant to argenx at an inherent level, as exposure to these risks was found to be higher. • Physical Risk (Acute): Certain sites are expected to face extreme heat under a high warming scenario starting in 2030 and increasing by 2050. However, the sensitivity of our operations to extreme heat is low due to existing measures, so the impact is not expected to be significant. For example, our offices have HVAC systems to address heat and ensure employee well-being, and our R&D facility has backup generators to prevent product spoilage during power outages that may occur due to grid overloads. • Physical Risk (Chronic): Water stress relating to water withdrawal, which includes domestic, industrial, irrigation and livestock uses, is expected to impact certain sites under a high warming scenario, but this exposure is not expected to change significantly over time. Our sites are already adapted to this level of exposure as they were designed to be suitable for existing water withdrawal conditions. Water stress relating to water withdrawal could increase water utility costs, but this impact would be minimal for our offices, where water is mainly used for sanitation. Our contract manufacturers who are directly exposed would bear most of the increased costs, with likely only part of the cost increase passed down to argenx due to indirect exposure. argenx is applying the phase-in relief outlined in ESRS 1 Appendix C which allows the omission of information relating to the anticipated financial effects associated with each identified material physical and transition risk. We will however, continue to refine the existing analysis to better understand these potential risks to argenx by addressing the requirements to disclose associated financial effect in future sustainability statements. Transition Plan for Climate Change Mitigation As we are required to expressly note under paragraph 17 of ESRS E1-1, we have not yet developed a climate transition plan for climate change mitigation, nor have we developed policies, actions and targets in this respect. 7.3.3 Emissions Material E1 impacts are described in this section include: • Indirect emissions from upstream and downstream activities, including manufacturing, purchased goods and services, transportation and distribution of raw materials and products (to Europe, Japan, and the USA), waste generated in operations, and end-of-life treatment of products. These processes also require substantial energy, often sourced from fossil fuels, which amplifies the carbon footprint associated with the reporting company’s supply chain (Impact). • Direct operational emissions and energy usage from company-owned sources (i.e., facilities and fleet) contribute to the negative effects of climate change (Impact). At argenx, we define our organizational boundaries using the Operational Control approach per the World Resource Institute (WRI)/World Business Council for Sustainable Development (WBCSD) GHG Protocol. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 337

Under this approach, we account for 100% of the GHG emissions from operations that we control. Emissions from our joint venture, OncoVerity, have been included in our Scope 3, Category 15 Investments emissions. Scope 1 Emissions Scope 1 emissions include all direct GHG emissions associated with sources owned or controlled by the company. argenx Scope 1 emissions are associated with leased employee vehicles. All our sites are leased, so purchased heating and cooling are captured under Scope 2 as defined within GHG Protocol Scope 2 Guidance, the Identifying Scope 2 Emissions and Setting the Scope 2 Boundary section. Scope 2 Emissions Scope 2 emissions include indirect GHG emissions that result from the consumption of purchased or acquired energy, such as electricity, heating, cooling, and electricity purchased to charge leased electric vehicles used by employees. These emissions are classified as indirect because the emissions do not occur in our assets, but rather at the location where the electricity is generated from input fuels. Although argenx does not own or control the sources, these emissions are a consequence of our activities. We gather actual invoiced utility data for its properties for which we have operational control. In instances where data is not available, consumption is estimated using floor area and energy intensities from the Better Building Partnership (BBP) and the World Bank. For facilities using actual data, consumption data is obtained by facility personnel from landlords. utility bills, or supplier invoices. Electricity emissions are calculated using the total energy consumption per site multiplied by the appropriate region- or country-specific emission factor. The location-based calculation uses factors from sources such as IEA, CO2emissiefactoren, EPA eGRID, and others. We do not currently procure renewable energy; therefore, the market-based electricity emissions reflect the residual mix where available and utilize the location-based factors where a residual factor is not available following the GHG Protocol market-based emission factor hierarchy. Emissions from heating and cooling such as those from HVAC equipment or a basement boiler are calculated using the annual facility consumption for these sources and appropriate emission factors from sources such as the UK Department for Energy Security and Net Zero (DESNZ) and global warming potentials from the IPCC Assessment Report 6. Scope 2 Accounting Principles Location-based emissions represent the average emissions from energy generated and consumed within the geographic regions where argenx operates, primarily using grid-average emission factors. Market- based emissions, on the other hand, reflect the emissions associated with argenx's electricity purchasing decisions. Emissions are calculated using actual data when available and estimates when data is not available. These estimates are sourced from the International Energy Agency (IEA) and the Department for Environment, Food & Rural Affairs (DEFRA). Residual factors are used for market-based calculations where available. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 338

Scope 3 Emissions Scope 3 emissions allow for the accounting of all other indirect emissions. In line with GHG best practices as defined by WBCSD and GHG Protocol, we engaged partners to produce an emissions inventory for Scope 3 data through primary data collection. This resulted in 35% of emissions across Scope 3 coming from supplier specific data. Our Scope 3 emissions include the following categories and methodologies: • Category 1 – Purchased Goods and Services: We utilize a hybrid approach pulling in supplier specific data where available, where a supplier specific spend-based emissions factor was used. 42% of emissions for PG&S came from supplier specific data. Where supplier data was not available cash-out categories were are matched to appropriate Environmentally Extended Input-Output (EEIO) emission factors. Emissions are calculated by multiplying the annual category spend data by the appropriate inflation adjusted Environmental Protection Agency (EPA) Supply Chain Emissions Factors. • Category 2 – Capital Goods: Capital Goods covers all upstream (i.e., cradle-to-gate) emissions from the production of capital goods purchased or acquired by argenx in the reporting year. We utilize a spend- based EEIO analysis to calculate emissions. • Category 3 – Fuel- and Energy-Related Activities Not Included in Scope 1 or Scope 2: Fuel- & Energy- Related Activities (FERA) emissions represent the upstream emissions of fuels and energy used in our Scope 1 and 2. Data collected for FERA includes all fuel and electricity data gathered for Scope 1 and 2 and utilizes an average data approach to calculate emissions. • Category 4 – Upstream Transportation and Distribution: As required by the Science-Based Targets Initiative (SBTi), these transportation emissions are calculated on a well-to-wheel (WTW) basis. This includes the extraction, refinement, and distribution of fuels as well as the combustion of that fuel. We utilize a hybrid methodology, relying on supplier provided emission data where available, distance-based approach when primary supplier data is not available, and spend data if neither of those are available. 14% of emissions for Category 4 came from supplier specific data. • Category 5 – Waste Generated in Operation: To calculate emissions from Waste Generated in Operations, we utilize a waste-type-specific approach. Where available, we provide total weight of waste broken down by material type and disposal method per facility. If the data is not available, facility waste is estimated by the number of employees tied to that location and regional waste intensities. If the disposal method is not known, EU average disposal patterns are used. • Category 6 – Business Travel: Our employee business travel data is provided for air, personal car mileage reimbursement, rail, and other modes such as ride-share/rental car/taxi. As required by the SBTi, these transportation emissions are calculated on a WTW basis. This includes the extraction, refinement, and distribution of fuels as well as the combustion of that fuel for travel. Where available, we utilize a distance-based approach and a spend-based approach if distance is not available. Emissions from hotel stays are an optional portion of this category and have been included using a spend-based approach. argenx exports air travel data from Business Travel Insights, detailing the distance per flight and cabin class. To calculate emissions, we categorize individual flights by haul length (short, medium, long) based on the total distance traveled. The mileage for each haul type and cabin class is then multiplied by the corresponding DEFRA emissions factors for well-to-tank (WTT) and tank-to-wheel (TTW) emissions. • Category 7 – Employee Commute and Work from Home (WFH): Employee Commute is calculated using a distance-based methodology based on location information and assumed modes of transport from regional transportation patterns. As required by the SBTi, these transportation emissions are calculated on a WTW basis. This includes the extraction, refinement, and distribution of fuels as well as the combustion of that fuel for travel. Emissions from teleworking (work from home) are an optional portion of this category and have been included by estimating the incremental increase in energy consumption associated with working from home and employee frequency of remote work. Emissions from employee use of leased vehicles for commuting is excluded as this is already captured in Scope 1. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 339

• Category 8 – Upstream Leased Assets: Our use of shared workspaces falls within this category. Data is gathered and emissions are calculated following the approach outlined in the sections for Scopes 1 & 2. • Category 9 – Downstream Transportation and Distribution: This category represents emissions from outbound transportation that is not paid for by argenx. This is calculated using a distance-based approach. As required by the SBTi, these transportation emissions are calculated on a WTW basis. This includes the extraction, refinement, and distribution of fuels as well as the combustion of that fuel. • Category 12– End-of-Life Treatment of Sold Products: End-of-Life Treatment includes emissions from the disposal of sold products. Sales and product & packaging weight data is gathered. Since we sell a regulated medical product, it is assumed that all the drug is used, but the packaging data utilizes regional assumptions of disposal patterns. • Category 14 – Franchises: We granted a license to Zai Lab to sell and distribute VYVGART in the Chinese market in return for sales-based royalties and a one-time sales-based milestone. The franchise-specific method is utilized by gathering Zai Lab Scope 1 & 2 emissions allocated to argenx. • Category 15 – Investments: This includes a joint venture with OncoVerity calculated using the average data method. In addition, this also includes investment in Zai Lab calculated using the investment- specific method. In addition, this also includes investment in Zai Lab calculated using the investment- specific method. The following categories have been excluded: • Category 10 – Processing of Sold Products: We do not sell intermediate products. • Category 11 – Use of Sold Products: We do not sell products that consume energy. • Category 13 – Downstream Leased Assets: We do not have assets that have been leased to other entities. Scope 3 Accounting Principles The following accounting principles describe the methodology used to calculate scope 3 emissions by applicable category: • Category 1 — Purchased Goods and Services: We used a hybrid approach, combining supplier-specific and spend-based. Spend emissions were sourced from the EPA Supply Chain Emission Factors. Our spend-based methodology relies upon cash-based and cost-incurred financial data as the underlying source for these calculations. • Category 2 — Capital Goods: We used a spend based approach using factors from the EPA Supply Chain Emission Factors. • Category 3 — Fuel and Energy Related Activities: We used calculations based on our Scope 1 and 2 consumption, with fuel factors from DEFRA and electricity factors sourced from the IEA and country- specific sources where available. • Category 4— Upstream Transport: We used a hybrid approach that incorporated supplier data including distance data and transportation cost. Calculations are WTW, with distance emission factors from DEFRA and spend factors from the EPA Supply Chain Emission Factors. • Category 5 — Waste in Operations: We used actual waste data by stream where available, and estimated where data was not available, with emission factors from DEFRA. • Category 6 — Business Travel: We used a distance-based approach where data was available and a spend-based approach where data was not available. Calculations are WTW. Our spend-based methodology relies upon our cash-out financial data as the underlying source for these calculations. • Category 7 — Employee Commute: We used average calculated distance and regional commute patterns, as well as WTW calculations and emission factors from DEFRA. • Category 8 — Upstream Leased Assets: We used actual activity data where available, and used estimates where data was not available. We used emission factors primarily from the IEA and DEFRA. Residual factors were used for market-based calculations where available. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 340

• Category 9 — Downstream Transport: We used a distance-based method, with WTW calculations and emission factors from DEFRA. • Category 12 — End of Life Treatment of Sold Products: We used an average data approach with assumed disposal patterns and DEFRA emission factors. • Category 14 — Franchises: We used a franchise-specific method using primary allocated emission data. • Category 15 — Investments: We used an average data method with factors from the EPA Supply Chain Emission Factors. Gross Scopes 1, 2, 3 and Total GHG emissions (GHG Intensity Based on Net Revenue) Metric Name 2024 (tCO2e) Scope 1 GHG Emissions Total (gross) scope 1 GHG emissions 3,788 Percentage of Scope 1 GHG emissions from regulated emission trading schemes – Scope 2 GHG Emissions Gross location-based Scope 2 greenhouse gas emissions 494 Gross market-based Scope 2 greenhouse gas emissions 534 Significant Scope 3 GHG Emissions Total (gross) scope 3 GHG emissions 227,447 Purchased Goods and Services 183,781 Capital Goods 1,906 Fuel and energy-related activities 1,190 Upstream transportation and distribution 24,556 Waste generated in operations 2 Business travel 13,340 Employee commuting 1,370 Upstream leased assets 33 Downstream transportation 313 End of life treatment of sold products – Franchises 251 Investments 705 Total GHG Emissions Total GHG emissions (location-based) (tCO2eq) 231,700 Total GHG emissions (market-based) (tCO2eq) 231,769 GHG Intensity Total GHG emissions (location-based) per net revenue (tCO2eq/Monetary unit) 0.000103 Total GHG emissions (market-based) per net revenue (tCO2eq/Monetary unit) 0.000103 Note 1: The GHG intensity is calculated as the total GHG emissions divided by total operating income. The reported figure for total operating income can be found in the Section 6.1.2 “Consolidated Statements of Profit or Loss” within the financial statements. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 341 E1-6

7.3.4 Energy Energy Consumption and Mix Metric Name Unit 2024 Consumption of purchased or acquired electricity, heat, steam, or cooling from fossil sources Kilowatt hours 2,318,527 Total energy consumption from fossil sources Kilowatt hours 15,670,878 Share of fossil sources in total energy consumption % 100% Total energy consumption from nuclear sources MWh – Share of consumption from nuclear sources in total energy consumption % – Fuel consumption from renewable sources MWh – Consumption of purchased or acquired electricity, heat, steam, and cooling from renewable sources MWh – Consumption of self-generated non-fuel renewable energy MWh – Total energy consumption from renewable sources MWh – Share of renewable sources in total energy consumption % – Total Energy Consumption Kilowatt hours 17,989,405 7.3.5 Resource Use and Circular Economy Material E5 impacts are described in this section include: • The disposal of single-use products, disposable medical devices, and hazardous waste (e.g., expired medications, chemical solvents, contaminated packaging, laboratory waste, and manufacturing byproducts) contributes to significant waste generation, resource depletion, and environmental and health risks when improperly managed. (Impact) Waste Management At argenx, we recognize the importance of responsible waste management in our operations. The disposal of single-use products in research, development, and pharmaceutical administration, along with associated hazardous waste (e.g., expired medications, chemical solvents, contaminated packaging, laboratory waste, and manufacturing by-products), significantly contributes to waste generation, resource depletion, and environmental and health risks when improperly managed. While resource use and circular economy impacts emerged in our climate risk assessment of our facilities and supply chain partners, we will not disclose financial effects or data from our value chain in the first year, in accordance with CSRD transitional reliefs. Currently, we do not have a formal policy addressing material impacts, risks, and opportunities related to resource use and the circular economy. The actions described in E5-2-17 are not currently tracked. Recognizing the importance of addressing these issues, we are considering drafting and socializing a policy in 2025, to tackle the material impacts identified in our 2024 double materiality assessment and future IRO refinement. This policy would focus on reducing overall waste generation, improving waste diversion from landfills and incineration, and supporting suppliers in decreasing waste in their operations. Three key business units were identified as central to resource use and circular economy impacts, risks, and opportunities related to our products and services, and the waste they generate: • Facilities and EH&S: This includes argenx-owned and operated facilities such as offices and labs, as well as the procurement of new facility space and office and lab supplies. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 342 E5-3 E5-2 E1-5

• Supply Chain - Logistics and Transportation: Encompasses transportation, warehousing, and distribution activities contracted by argenx and executed by third-party suppliers. • Supply Chain - Products and Packaging: Involves the development and manufacturing of drug substances and products, also contracted by argenx and conducted by third-party suppliers. Additionally, we are evaluating business-critical activities, including material sourcing, through a Business Impact Assessment. This ongoing process aims to identify potential risks and ensure business continuity, guided by insights from internal stakeholder surveys. While material inflows were not calculated during this reporting cycle, we have data on packaging weights for products sold for 2024 as follows: • Packaging: 8,922 kilograms of fiber-based packaging and paper leaflets • Glass Vials: 8,723 kilograms, including labels, flip tops, and stoppers Waste Impacts In 2024, argenx identified a negative impact associated with resource use and circularity, including single use products (i.e., disposal of single-use products, disposable medical devices, and hazardous waste) contributes to waste generation, resource depletion and environmental and health risks when improperly managed. We are committed to supporting the efficient use of resources through various initiatives across our operations. The manufacturing, transportation, warehousing, and distribution of our products and packaging are managed by contracted suppliers, limiting our direct control over these processes. Our single lab in Ghent, Belgium, ensures waste is managed according to strict national laws, with all lab employees receiving waste segregation training. Most of our facilities are office spaces, many of which are leased coworking spaces where waste management is handled by the landlord, generating minimal non- hazardous office waste. Specific initiatives across our operations include: • Facilities and Ways of Working: At our Ghent lab, employees are trained in the proper segregation of bio-contaminated, hazardous, and non-hazardous waste, ensuring compliance with legal standards, and minimizing associated risks. Additionally, we recycle polystyrene for reuse in the building industry, improving downstream waste diversion. We offer refillable beverage stations to reduce single-use waste and work with a partner to recycle cups, improving diversion. Our employee-led Ghent Campus Team proposes and implements ESG initiatives across four key areas: innovation, facilities, people, and energy. • Supply Chain - Logistics and Transportation: All drug substances are shipped in TOPA boxes, which are reusable up to ten times and returned to vendors after use. Drug products are shipped in TOPA boxes (46% of total shipments), Va-Q-tainers (50% of total shipments) with an indefinite lifespan, or unpackaged in temperature-controlled vehicles (4% of total shipments). This practice supports waste reduction and efficient resource use in our upstream value chain for distribution and transportation. Reusable containers have always been used in our drug substance and drug product transportation. • Supply Chain - Products and Packaging: In Japan, we have replaced instructional paper leaflets with details online, reducing downstream waste from our products. Resource Outflows We produce two pharmaceutical products: Vyvgart (IV) and Vyvgart SC. Both products are packaged similarly, with the drug contained in a glass vial featuring a label, flip tops, and stopper. These vials are then placed in a paperboard box along with a paper leaflet that provides administration instructions. Due to the nature of these products, considerations such as durability, reusability, repairability, disassembly, and refurbishment are not applicable. While the paperboard box, paper leaflet, and glass vial are technically recyclable, they are often not recycled in clinical settings due to limited segregation and collection, particularly concerning bio-contaminated waste. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 343 ESRS 2 SBM-3 E5-5

(a) Relevant Waste Streams for argenx Outflows: (b) Materials in Each Stream Recycling streams Hard plastic Hard plastic Glass Glass bottles and jars Paper and cardboard Paper, cardboard, includes shredded paper PMD Plastic, metal, drink cartons Mixed recycling Hard plastic, metal, glass E-waste Electronic waste, lab devices (end of life, defective) Plastic Film Plastic films Organic waste Food waste (canteen waste) EfW (energy from waste) or incineration Residual non-hazardous waste Typical non-hazardous, non-recyclable material including packaging, blister packs, foils, composite packaging *Medical waste Biologically contaminated waste, plain medium, LB-agar, sharps, contaminated tissue, and wrappings *Non halogenated solvents Iso propanol, acetone, ethanol *Inorganic acids Hydrochloric acid, pH buffers, sulfuric acid *Lab waste (toxic) Contaminated plastics (empty recipients) *Lab waste (corrosive) Materials contaminated with corrosive chemicals, acids, bases (empty recipients) Landfill (only applicable in some regions) Residual non-hazardous waste Other Polystyrene Polystyrene *Hazardous waste streams The data gathered on our operational footprint was gathered from various sources: • Actual waste data for argenx labs and offices at the Ghent campus, provided by waste service providers, represents the largest waste footprint among all argenx facilities. • For the US and Tokyo offices, waste data was supplied by the building landlords, with the proportion of argenx waste calculated based on square footage. • For smaller offices, waste estimates were derived using existing waste data and calculations based on headcount or desk count, supplemented by publicly available figures on waste generation and composition. Metric Non- Hazardous Waste Hazardous Waste Diverted from Disposal Total tons prepared for reuse – – Total tons directed to recycling 14.78 – Total tons directed to other recovery operations 4.98 1.49 Disposal Total tons directed to incineration 9.98 6.38 Total tons directed to landfill 0.99 – Total tons directed to other disposal methods – – Total tons 30.73 7.87 Total waste (non-hazardous and hazardous) 38.60 argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 344

Metric Unit Hazardous Waste Total tons diverted from disposal 1.49 Total tons directed to disposal 6.38 Non-hazardous Waste Total tons diverted from disposal 19.76 Total tons directed to disposal 10.97 Radioactive Waste Total tons diverted from Disposal – Total tons directed to Disposal – Total Waste Diverted from disposal % 55% Directed to disposal % 45% 7.3.6 EU Taxonomy Introduction to the EU Taxonomy Regulation The EU Taxonomy is a classification system for environmentally sustainable economic activities. By setting out the overarching conditions and criteria for an activity to be considered sustainable, EU Taxonomy seeks to direct investments into sustainable activities, increase transparency and improve comparability. The EU Taxonomy Regulation identifies six environmental objectives: 1. Climate change mitigation 2. Climate change adaptation 3. Sustainable use and protection of water and marine resources 4. Transition to a circular economy 5. Pollution prevention and control 6. Protection and restoration of biodiversity and ecosystems As a non-financial undertaking, argenx is required to disclose the proportion of its turnover, capital expenditure and operational expenditure associated with Taxonomy-eligible or Taxonomy-aligned economic activities listed under these six environmental objectives. Compliance with the EU Taxonomy Regulation In 2024, argenx progressed its efforts towards developing an EU Taxonomy framework that aligned with regulatory disclosures and market best practices. We took a comprehensive approach to evaluating eligibility and alignment for the reported KPIs, allowing us to enhance our insights and drive greater clarity and precision in our outcomes. As a result, we adjusted the methodology applied in 2023 to align with the approach used in 2024, ensuring consistency and comparability across periods. Eligibility and Alignment Eligibility In 2024, argenx conducted the Taxonomy assessment by reviewing all activities listed under the six environmental objectives – covering the Climate, Environmental and Complementary Climate Delegated Acts. Potentially eligible activities were identified through an initial screening process of all activities and finalized based on the activity descriptions in the Delegated Acts. Two environmental objectives and two activities were identified as relevant to argenx, 1.2. Manufacture of medicinal products (Pollution prevention and control) and 6.5. Transport by motorbikes, passenger cars and light commercial vehicles (Climate Change Mitigation). These correspond to argenx’s turnover derived argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 345

from sales of medicinal products (associated with 1.2. Manufacture of medicinal products), R&D activities (associated with 1.2. Manufacture of medicinal products), and leases of vehicles (associated with activity 6.5. Transport by motorbikes, passenger cars and light commercial vehicles). argenx eligible activities Economic Activity Environmental objective Description of argenx’s economic activities KPI 1.2. Manufacture of medicinal products Pollution prevention and control Contract manufacturing of medicinal products Research and development activities related to medicinal products Turnover, OpEx 6.5. Transport by motorbikes, passenger cars and light commercial vehicles Climate change mitigation Leasing off vehicles CapEx Alignment The Taxonomy assessment was conducted in co-operation with legal, financial, and ESG experts at argenx, with additional support from external specialists. In 2024, argenx conducted a stringent assessment of whether it meets the Minimum Safeguards criteria as laid out in the Final Report on Minimum Safeguards published by the EU Platform on Sustainable Finance in October 2022. argenx is considered compliant with criteria related to corruption, taxation, and fair competition as per its Global Tax Policy and Code of Business Conduct and Ethics, which covers human rights, anti-corruption, and bribery as well as fair competition. argenx has not been found in breach of the Minimum Safeguards. Whilst argenx currently does not have a Human Rights Due Diligence Process that would be fully aligned with the six steps of UNGPs and OECD guidelines, as required by the Minimum Safeguards criteria, we remain fully committed to respecting human rights and to selecting partners who share the same vision. Based on this outcome, full alignment with Taxonomy requirements for turnover and OpEx associated with activity 1.2. Manufacture of medicinal products, or CapEx associated with activity 6.5. Transport by motorbikes, passenger cars and light commercial vehicles could not be proved at this time. Thus, argenx has reported 0% alignment for Turnover, CapEx and OpEx KPIs. KPI Eligible (USD million) Aligned (USD million) Non-eligible (USD million) 2023 2024 2023 2024 2023 2024 Turnover 1,190.8 (97.1%) 2,185.9 (99.8%) – (0%) – (0%) 35.5 (2.9%) 4.3 (0.2%) CapEx 2.3 (3.4%) 5.5 (5.8%) – (0%) – (0%) 65.8 (96.6%) 89.2 (94.2%) OpEx 483.2 (99.8%) 605.1 (99.9%) – (0%) – (0%) 0.9 (0.2%) 0.7 (0.1%) Accounting Policy Turnover Turnover consists of net turnover derived from products or services. In 2024, argenx revised its approach to assessing taxonomy eligibility for turnover based on additional guidance from the Commission regarding the consideration of subcontractor revenue. Based on the guidance, if the entity controls the economic activity performed by a subcontractor and recognizes the revenue as its own, it can be considered eligible. While third-party contract manufacturers (CMs) are engaged to produce the medicinal products, argenx controls the economic activity performed and therefore fully recognizes the revenue from sales of the manufactured products under the principles set out in IFRS 15. As such, contract manufacturing is included in the KPI calculation and all net product sales are considered eligible under activity 1.2. Manufacture of argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 346

medicinal products. Thus, the numerator consists of the external product net sales (associated with activity 1.2. Manufacture of medicinal products) and totals $2.2 billion. Our denominator for calculation of turnover KPI, covering product net sales and collaboration revenue (as listed in Annex I, point 1.1.1 of Disclosures Delegated Act), totals $2.2 billion. Refer to ”Note 17 Segment Reporting“ and ”Note 15 Collaboration Revenue” in the consolidated financial statements. CapEx CapEx covers additions to tangible and intangible assets including right-of-use assets during the fiscal year considered before depreciation, amortization, and any re-measurements. argenx has considered leased vehicles that result in the recognition of a right-of-use of asset and are recognized under IFRS 16 Leases as eligible CapEx per the definition in Taxonomy Disclosures Delegated Act. All leased vehicles are considered eligible under 6.5. Transport by motorbikes, passenger cars and light commercial vehicles. Thus, the numerator for the CapEx KPI consists of additions to leased vehicles (associated with activity 6.5. Transport by motorbikes, passenger cars and light commercial vehicles), and totals $5.5 million. The denominator for the CapEx KPI calculation covers additions to tangible and intangible assets during the fiscal year (as listed in Annex I, point 1.1.2.1 of Disclosures Delegated Act), totaling $94.7 million. Refer to “Note 4 Property, Plant and Equipment” and “Note 5 Intangible Assets” in the consolidated financial statements. OpEx OpEx covers direct non-capitalized costs related to research and development, building renovation measures, short-term lease, maintenance and repair, and any other direct expenditures relating to the day- to-day servicing of assets of property, plant, and equipment. argenx has considered its direct costs related to research and development associated with activity 1.2. Manufacture of medicinal products as eligible OpEx. Research and development are a key activity in argenx’s strategic business model and value chain. It consists of multi-phase clinical trials, regulatory approval processes, research of pre-clinical stage product candidates, and discovery stage programs, all with the eventual goal to manufacture medicinal products and treat patients globally. For 2024, specifically, R&D related to evaluating the use of efgartigimod in 15 severe autoimmune diseases (including MG, CIDP, and ITP), empasiprubart is currently being evaluated in four diseases, proof-of-concept studies in ARGX-119, and other pre-clinical research, were considered eligible OpEx. Thus, the numerator for OpEx consists of direct research and development expenses related to VYVGART, ARGX-117, ARGX-119 and other pre-clinical candidates (associated with activity 1.2. Manufacture of medicinal products) and totals $605.1 million Our denominator for calculation of OpEx KPI, covering research and development, maintenance, and repair (as listed in Annex I, point 1.1.3.1 of Disclosures Delegated Act), totals $605.8 million. Refer to “Note 18 Research and Development Expenses” in the consolidated financial statements. Maintenance and repair are included under “Note 18 Research and development expenses’’ and ‘‘Note 19 Selling, general, and administrative expenses” in the consolidated financial statements. Double counting is avoided as none of the eligible activities contribute to multiple environmental objectives and each KPI only includes one eligible activity. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 347

Nuclear and fossil gas related activities Row Nuclear energy related activities 1 The undertaking carries out, funds, or has exposures to research, development, demonstration and deployment of innovative electricity generation facilities that produce energy from nuclear processes with minimal waste from the fuel cycle. NO 2 The undertaking carries out, funds, or has exposures to construction and safe operation of new nuclear installations to produce electricity or process heat, including for the purposes of district heating or industrial processes such as hydrogen production, as well as their safety upgrades, using best available technologies. NO 3 The undertaking carries out, funds, or has exposures to safe operation of existing nuclear installations that produce electricity or process heat, including for the purposes of district heating or industrial processes such as hydrogen production from nuclear energy, as well as their safety upgrades. NO Fossil gas related activities 4 The undertaking carries out, funds, or has exposures to construction or operation of electricity generation facilities that produce electricity using fossil gaseous fuels. NO 5 The undertaking carries out, funds, or has exposures to construction, refurbishment, and operation of combined heat/cool and power generation facilities using fossil gaseous fuels. NO 6 The undertaking carries out, funds, or has exposures to construction, refurbishment and operation of heat generation facilities that produce heat/cool using fossil gaseous fuels. NO argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 348

Financial year 2024 2024 Substantial Contribution Criteria DNSH criteria ('Does No Significant Harm') (h) Economic Activities (1) Code (a) (2) Turnover (3) Proportion of Turnover, year 2024 (4) Clim ate change M itigation (5) Clim ate change A daptation (6) W ater (7) Pollution (8) Circular Econom y (9) Biodiversity (10) Clim ate change M itigation (11) Clim ate change A daptation (12) W ater (13) Pollution (14) Circular Econom y (15) Biodiversity (16) M inim um Safeguards (17) Proportion of Taxonomy aligned (a.1.) or eligible (A.2.) Turnover, year 2023 (18) Category enabling activity (19) Category transitional activity (20) USD (thousands) % Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y/N Y/N Y/N Y/N Y/N Y/N Y/N % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) Turnover of environmentally sustainable activities (Taxonomy- aligned) (A.1) – –% –% –% –% –% –% –% –% Of which Enabling – –% –% –% –% –% –% –% –% E Of which Transitional – –% –% –% T A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) EL; N/ EL (f) EL; N/ EL (f) EL; N/ EL (f) EL; N/ EL (f) EL; N/ EL (f) EL; N/ EL (f) Manufacture of medicinal products PPC 1.2. 2,185,883 99.8% N/EL N/EL N/EL EL N/EL N/EL 97% Turnover of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) 2,185,883 99.8% –% –% –% 99.8% –% –% 97.1% A. Turnover of Taxonomy eligible activities (A.1 + A.2) 2,185,883 99.8% –% –% –% 99.8% –% –% 97.1% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES Turnover of Taxonomy-non-eligible activities 4,348 0.2% Total 2,190,231 100% argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 349

Financial year 2024 2024 Substantial Contribution Criteria DNSH criteria ('Does No Significant Harm') (h) Economic Activities (1) Code (a) (2) CapEx (3) Proportion of CapEx, year 2024 (4) Clim ate change M itigation (5) Clim ate change A daptation (6) W ater (7) Pollution (8) Circular Econom y (9) Biodiversity (10) Clim ate change M itigation (11) Clim ate change A daptation (12) W ater (13) Pollution (14) Circular Econom y (15) Biodiversity (16) M inim um Safeguards (17) Proportion of Taxonomy aligned (a.1.) or eligible (A.2.) CapEx, year 2023 (18) Category enabling activity (19) Category transitional activity (20) USD (thousands) % Y; N; N/EL (b) (c) Y; N; N/EL (b) (c) Y; N; N/EL (b) (c) Y; N; N/EL (b) (c) Y; N; N/EL (b) (c) Y; N; N/EL (b) (c) Y/N Y/N Y/N Y/N Y/N Y/N Y/N % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) CapEx of environmentally sustainable activities (Taxonomy- aligned) (A.1) – –% –% –% –% –% –% –% –% Of which Enabling – –% –% –% –% –% –% –% –% E Of which Transitional – –% –% –% T A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) EL; N/ EL EL; N/ EL EL; N/ EL EL; N/ EL EL; N/ EL EL; N/ EL Transport by motorbikes, passenger cars and light commercial vehicles CCM 6.5 5,492 5.8% EL N/EL N/EL N/EL N/EL N/EL 3.4% CapEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) 5,492 5.8% 5.8% –% –% –% –% –% 3.4% A. CapEx of Taxonomy eligible activities (A.1 + A.2) 5,492 5.8% 5.8% –% –% –% –% –% 3.4% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES CapEx of Taxonomy-non-eligible activities 89,160 94.2% Total 94,652 100.0% argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 350
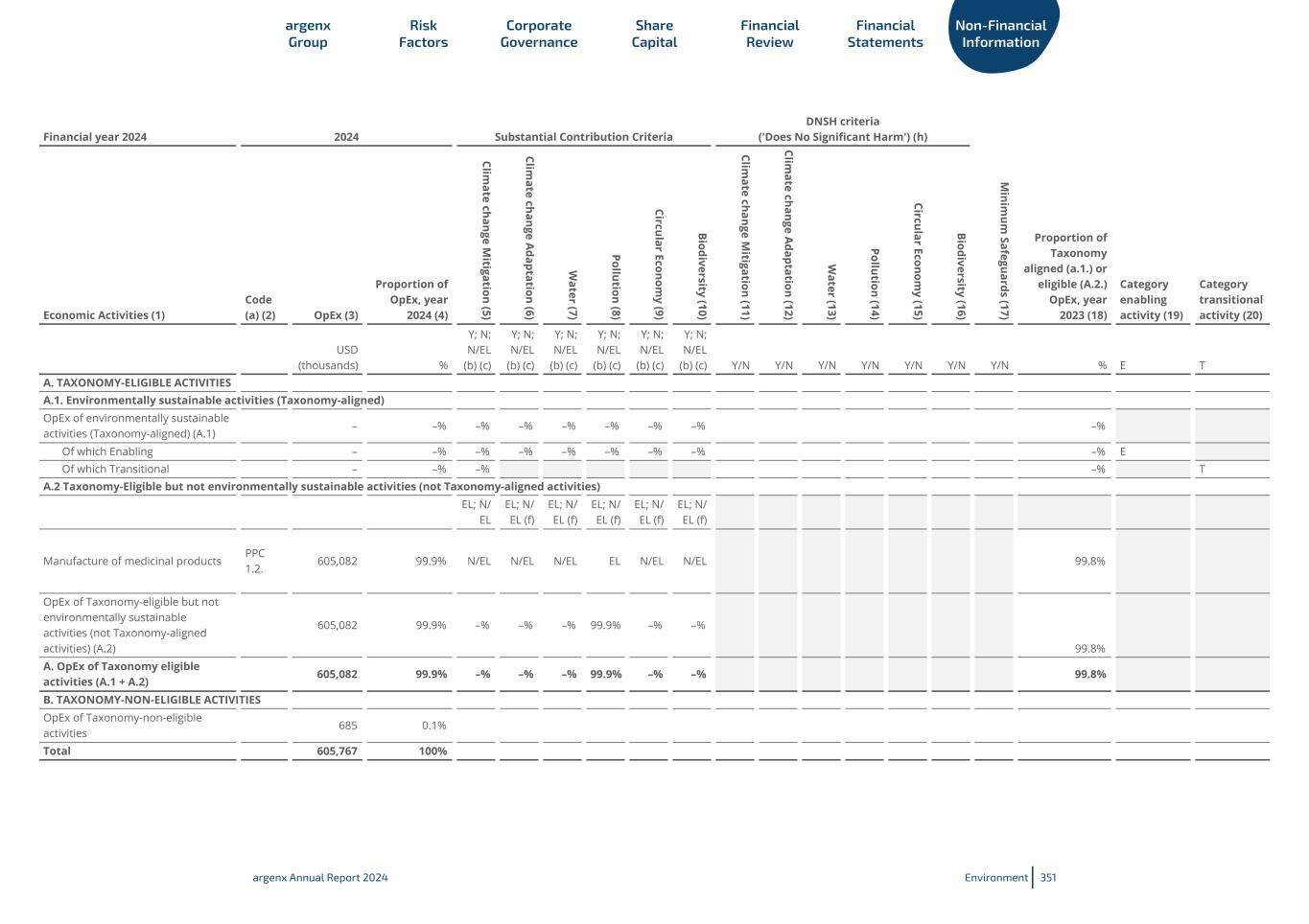
Financial year 2024 2024 Substantial Contribution Criteria DNSH criteria ('Does No Significant Harm') (h) Economic Activities (1) Code (a) (2) OpEx (3) Proportion of OpEx, year 2024 (4) Clim ate change M itigation (5) Clim ate change A daptation (6) W ater (7) Pollution (8) Circular Econom y (9) Biodiversity (10) Clim ate change M itigation (11) Clim ate change A daptation (12) W ater (13) Pollution (14) Circular Econom y (15) Biodiversity (16) M inim um Safeguards (17) Proportion of Taxonomy aligned (a.1.) or eligible (A.2.) OpEx, year 2023 (18) Category enabling activity (19) Category transitional activity (20) USD (thousands) % Y; N; N/EL (b) (c) Y; N; N/EL (b) (c) Y; N; N/EL (b) (c) Y; N; N/EL (b) (c) Y; N; N/EL (b) (c) Y; N; N/EL (b) (c) Y/N Y/N Y/N Y/N Y/N Y/N Y/N % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) OpEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) – –% –% –% –% –% –% –% –% Of which Enabling – –% –% –% –% –% –% –% –% E Of which Transitional – –% –% –% T A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) EL; N/ EL EL; N/ EL (f) EL; N/ EL (f) EL; N/ EL (f) EL; N/ EL (f) EL; N/ EL (f) Manufacture of medicinal products PPC 1.2. 605,082 99.9% N/EL N/EL N/EL EL N/EL N/EL 99.8% OpEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) 605,082 99.9% –% –% –% 99.9% –% –% 99.8% A. OpEx of Taxonomy eligible activities (A.1 + A.2) 605,082 99.9% –% –% –% 99.9% –% –% 99.8% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES OpEx of Taxonomy-non-eligible activities 685 0.1% Total 605,767 100% argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Environment 351

7.4 Social 7.4.1 Introduction to Workforce at argenx Innovation is important to our business, and we see investing in our team as a significant aspect of driving innovation. We regard all our collaborators — whether employees (including contingent workers), independent consultants, or partners — as valuable to our company. Talent Strategy, Management, and Development S1-1 S1-3 S1-4 S1-5 S1-13 MDR-P MDR-A MDR-T Material S1 IROs described in this section. • Investments in employee learning and development through training programs foster employee well-being and prepares employees to meet future challenges while making them feel a greater sense of purpose and belonging (Impact). • A positive, diverse, and inclusive work environment that ensures equal treatment of all employees–regardless of origin, gender, sexual orientation, or religion–promotes fairness, and strengthens teamwork and collaboration (Impact) argenx was founded on a model of co-creation and this core element of our entrepreneurial spirit remains widespread across the organization today, underpinning every step of our drug discovery, R&D, and commercialization processes. We aim to make everyone feel valued and empowered to reach our goals together with the objective to create an environment where our talent aligns with our mission, shares ownership of achievements, and contributes to our growth. Several functions, including human resources, legal & compliance, and our communications team, are dedicated to supporting our approach to talent at argenx. That being so, all of our initiatives are reviewed to ensure compliance with local laws, and individual decisions are always based on merit, consistent with applicable laws. Talent Development We work to create a workplace environment in which employees are equipped and empowered to do their best, while also having the support and resources to continue developing. To promote this continuous development, we offer formal leadership programs, development plans, and access to learning resources that help employees grow and advance their skills. Our Managing Training standard operating procedure outlines the training opportunities at argenx to guide how training is managed at argenx and provides a framework for how training is assigned. The policy refers to the roles of employees in ensuring trainings are distributed and conducted. This applies to all our employees, consultants, interns, and contractors. The Head of Quality is accountable, with support from the Quality team. We are committed to empowering our employees to reach their full potential through a personalized approach to development. Our employees are strongly encouraged to initiate a Personal Development Plan (PDP), which is designed to guide them on a strength-based development journey. This plan is paired with an e-learning program, offering educational materials tailored to their role and individual development objectives. In 2024, 99% of employees who opted into the program completed their e-learning courses. We focus on developing leaders that understand the complexities of our business and will lead our teams in line with our Cultural Pillars. To help our current and future leaders advance their skills, we have developed the following leadership-focused programs: argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 352 ESRS 2 SBM-3 S1-13

• Essentials Leadership Development Program: In 2024, 76 employees participated in the Essentials Leadership Development Program. • Emerging Leadership Development Program: In 2024, we launched three cohorts with a total of 40 participants. • LEAD: In 2024, 15 employees participated in the program, participating in summits and capstone projects. We monitor the effectiveness of these programs through participant surveys. We recognize that feedback is essential for employee development and continuous growth. Employees are encouraged to give and request feedback continuously throughout the year, rather than relying on an annual review process. We provide training to help teams understand how to give feedback following a Situation-Behavior-Impact (SBI) feedback model. For more information on our policies and procedures related to employee feedback channels, visit the Governance section of this statement. Employee Engagement Our engagement with employees occurs through direct and open forums, including dialogue sessions, focus groups, quarterly company-wide meetings, CEO introduction meetings and new hire check-ins, as detailed below. • Employees are invited to submit questions in advance to shape the agendas of our quarterly Company Wide Meeting “the Corporate Update” and invited to ask questions live during the Q&A portion of the meeting. • We have built a network of 44 employee ‘culture champions’ who host cultural dialogue sessions on an ongoing basis (employees can sign-up), this network includes both colleagues who have been nominated or have volunteered, the input from these sessions is used to shape employee programs and help guide leadership discussions around areas of opportunity to for employee engagement. • We have established office and campus site teams that host focus groups quarterly on various topics (wellbeing, development, and other offerings) to help shape offerings and discuss topics of interest. • New hire check-ins are hosted by members of the HR team following the first three months. We also engage employees through various ongoing communication channels, including our internal social networking site ‘Engage’ where all employees are invited to share thoughts, ideas and ask questions. Additionally, the Employee Communications team shares company-wide news weekly and shares a monthly ‘In Case You Missed It’ email covering key updates. To ensure employees are aware of key updates, employees receive a corresponding email every time we issue a press release. To assess the effectiveness of employee engagement, we use different forums to gather feedback, including through Focus group discussions and in the questions and comments submitted by employees ahead of our quarterly Corporate Update meetings. We also analyze how employees engage with content posted on our communication channels. argenx does not currently have a separate process to gain insight into the perspectives of particularly vulnerable groups or employees. Human Rights We endeavor to comply with international labor standards and applicable labor and employment laws in every region where we operate. This includes, but is not limited to, the prohibition of child exploitation and child labor, forced, bonded, or indentured labor and involuntary prison labor, harsh or inhumane treatment, the threat thereof, or any form of modern slavery or human trafficking. We do not have a policy or program that manages impacts related to human rights. We engage with third parties who align with our values and endeavour to not conduct business with any individual or organization that participates in activities we prohibit. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 353 S1-1 ESRS 2 SBM-2 S1-2

In 2024, we had no severe human rights issues and incidents. We also did not incur any material fines, penalties, and compensation for severe human rights issues and incidents connected to own workforce. Anti-Discrimination We uphold the right to freedom of association and maintain zero tolerance for workplace discrimination. Our Code of Conduct and Business Ethics outlines our policies for preventing discrimination, including but not limited to race, religion, color, political convictions, sex, language, pregnancy, ethnic or national origin, civil state, social status, sexual orientation, handicap, or age. Additionally, our workforce policies apply to all employees and non-employees. In 2024, we did not incur any fines, penalties, or compensation for damages as a result of the incidents and complaints related to discrimination. That being so, all of our initiatives are reviewed to ensure compliance with local laws, and individual decisions are always based on merit, consistent with applicable laws. Our anti-retaliation policy strictly prohibits any form of discrimination against employees who raise complaints. For more details on our anti-retaliation policies and procedures, as well as how we enable employees to speak up, visit the Governance section of this statement. In our ongoing commitment to maintaining a safe workplace, our organization experienced the reporting of eleven incidents alleging discrimination or harassment within our workforce in 2024. Each incident was reviewed and/or formally investigated and addressed. There have been no substantiated findings of unlawful discrimination or harassment as a result. Regardless, argenx will continue to foster a respectful environment for all employees. Inclusion Pursuant to the required disclosure under ESRS S1-1, para 17, we disclose that in 2022, we adopted our Diversity, Equity and Inclusion policy, which outlines our approach to our workforce, which supports our ability to achieve our strategic goals. With our policy, we aim to have a workforce that is composed of members that can provide broad and complementary perspectives of the various business goals and strategic objectives of our company while also complying with the requirement we are subject to pursuant to the Dutch Civil Code and the Dutch Decree on the Content of the Management Report to disclose our diversity policy with respect to our directors and employees in managerial positions to the Dutch Social and Economic Council (Sociaal Economische Raad). The policy is applicable to our global operations and is overseen by our Head of HR and General Counsel. To promote an inclusive recruitment process we follow a standardized process across the organization. Our personal development program is aimed at building on individuals’ strengths to benefit the broader team. We focus promotion, training, and career-development solely on job-related criteria such as skills. Our employee resource groups also support the objectives of our policy through programming that promotes dialogue around how to further advance inclusion. 7.4.2 Our Employee Data In 2024, the total number of employees by headcount was 1,599. During the reporting period, 96 employees left the company and we hired 524 new employees. Our turnover rate in 2024 was 6.7%, calculated using average headcount as the denominator. We have used our internal employee database, Workday, to compile the data. To learn more about our employee data, see "Note 20 Personnel Expenses" within this annual report. Training and Skills Development Metrics argenx has applied transitional relief in respect of ESRS S1-13 for the first year of preparation of its Sustainability Statement. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 354 S1-1 S1-13 S1-1 S1-17

Characteristics of employees in our workforce Employee headcount by gender Gender Number of employees (Head count) Percentage Female 957 59.8 Male 642 40.2 (Note: Not reported signifies when employees have not made a selection) Employee headcount in countries with at least fifty employees Country Number of Employees (Head Count) Belgium 466 Japan 139 United States 694 Other 300 Total 1599 (Note: Entities with fewer than 50 employees are consolidated and categorized as "others.") Information on employees by contract type, broken down by gender (head count) Metric Female Male Other Not reported Total Number of employees 957 642 – – 1599 Number of permanent employees 956 641 – – 1597 Number of temporary employees 1 1 – – 2 Number of non-guaranteed hours employees – – – – – Age Distribution Across Our Workforce Age Group Number of Employees (Head Count) Under 30 years old 95 30-50 years old 982 Over 50 years old 522 Total 1599 Characteristics of non-employees in the workforce argenx has applied transitional relief in respect of S1-7 for the first year of preparation of its sustainability statement. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 355 S1-6 S1-9 S1-7

Gender Distribution of Top Management Gender Number Percentage Female 28 49.1 Male 24 42.1 Not reported – – Other 5 8.8 Total 57 100.0 Note: Top management refers to the leadership team which is comprised of: the senior management team, major global and commercial leaders, major development project leaders, and key R&D leaders. ‘Other’ refers to vacant positions at top management level. Remuneration Metrics (Pay Gap and Total Remuneration) The remuneration ratio above is defined under ESRS and is presented differently under the Remuneration and Compensation report included in the Corporate Governance Chapter in Section 3 of this Annual Report. The figure for 2024 is 22.9. Our commitment to pay equity is deeply rooted in our core values and cultural foundation. We ensure that our remuneration practices are fair, reflecting team and individual impact. They are also based on skills and market competitiveness relevant to the responsibilities held. The gender pay gap as presented in the below table reflects the adjusted gender pay gap for comparable positions, responsibilities, skill sets and experiences following the ESRS methodology but clustered in the following five categories: The gender pay gap as presented in the below table reflects the adjusted gender pay gap for comparable positions, responsibilities, skill sets and experiences following the ESRS methodology but clustered in the following five categories: • Individual contributor; • Managers; • Directors; • Vice-Presidents; • Executives (excluding the CEO). The metric is calculated as the average male gross hourly pay level less the average female gross hourly pay level expressed as a percentage of the average male gross hourly pay level (Average gross hourly pay for male employees - Average gross hourly pay for female employees) / Average gross hourly pay for male employees) * 100. Using this methodology the gender pay gaps range from -6.0 % to +1 % with a weighted average of 5.4 % in favor of women, reflecting our commitment and continuous monitoring of the core principles as laid out above. Level Gender Pay Gap Individual Contributors (5.1%) Managers (6.0%) Directors (5.6%) Vice-Presidents 1.0% Executives excluding CEO (2.7%) Weighted average gender pay gap (5.4%) argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 356 S1-9 S1-16

We believe that examining gender pay gaps on a purely total population basis without adequate detail and precision, as required by ESRS, does not offer a meaningful metric or insight into the fairness of our employee compensation. It disregards experience, seniority, and cost-of-living differences by country. We believe that the unadjusted gender pay gap ratio provides an inaccurate and overly simplistic representation of a complex measure. If all relevant factors are disregarded, the value for 2024 would be 18%, as calculated under ESRS. 7.4.3 Introduction to Patients at argenx Driven by patient determination to overcome chronic disease challenges, we innovate for individuals with severe autoimmune diseases. We aim to advance autoimmune disease understanding and deliver transformative treatments globally by pioneering immunology innovations and understanding patient needs. 7.4.4 Patient Health & Safety S4-1 S4-2 S4-3 S4-4 S4-5 MDR-P MDR-A MDR-T Material S4 IROs described in this section include: • Inability to ensure the safety of clinical trial participants and patients can have severe impacts on users’ health condition (Impact). • Risk of reputational damage from clinical trials' or patients' claims because of adverse events observed during clinical trials, including unforeseen reactions, if not appropriately addressed (Risk). Patient health and safety are important to argenx at all stages of the product lifecycle. Due to the involvement in clinical trials and the marketing of medicinal products, we adhere to the worldwide pharmacovigilance regulations and guidelines to promote patient safety. Patient health and safety at argenx are managed through the Global Patient Safety Policy and a series of processes detailed in specific control documents, such as standard operating procedures and work instructions. The Chief Medical Officer is ultimately accountable for the implementation of this policy. The Global Patient Safety Policy includes the following commitments: • Prevent and mitigate any harm arising from the use of argenx products. • Protect individual and public health based on comprehensive medical and scientific analyses of available safety information. • Conduct patient safety activities and responsibilities in compliance with legal and regulatory requirements. • Transparently communicate significant safety findings that impact the benefit-risk balance of argenx products to all applicable parties (e.g., health authorities, patients, and healthcare providers). • Have an adequate number of dedicated resources, processes, equipment, and systems in place for the timely assessment and escalation of safety issues and submissions of safety reports and/or safety information to health authorities. • Ensure that applicable third parties are made aware of and are compliant with their adverse event reporting obligations. • Instruct all internal and external parties to report safety information (i.e., adverse events within 24 hours or no later than one business day). • Advance argenx’s pharmacovigilance capabilities using new or evolving technologies in data science and computing. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 357 ESRS 2 SBM-3

Our procedures to safeguard patient health and safety globally, in line with the worldwide pharmacovigilance regulations and standards, include: • Collecting and reporting patient adverse events. • Conducting signal detection, which is a set of activities that identifies safety signals and evaluates the potential associations between medicinal products and adverse events and making the appropriate recommendations. • Characterizing the risks based on accumulated safety data and making the update to the risk management plans when warranted. • Preparing aggregate safety analyses for submission to authorities. • Providing accurate product information to prescribers and patients, reflecting the safety profile, and including any necessary mitigation measures. • Maintaining transparent communication with prescribers and patients regarding safety issues. We do not have specific goals or targets related to patient health and safety. However, we monitor the safety of our products through metrics, including the metrics listed in the Drug Safety table below, which are reviewed regularly. There were no enforcement actions taken in response to violations of GMP or equivalent standards Preventive and corrective actions are implemented when defined thresholds are not met. Each instance is recorded in our internal system, and a CAPA (Corrective Action; Preventive Action) is created. This CAPA outlines immediate corrective measures, root cause investigation, and future preventive steps to avoid recurrence. Timelines for the CAPA are provided to support immediate correction of the issue and another timeline is provided for preventative activities. In 2024, we did not receive any FDA Safety Notices, had no units recalled, and issued no recalls. Metric description SASB Reference Value Products listed in public medical product safety or adverse event alert databases. HC-BP-250a.1 Vyvgart is listed in the European Medicines Agency’s list of medicinal products under additional monitoring Number of fatalities associated with products. HC-BP-250a.2 – Number of FDA safety notices N/A – Remediation Processes and Consumer Feedback Channels Patients can report adverse events or other concerns through various channels. These include informing their physician or nurse, contacting MyVyvgartPath Nurse Case Managers (for enrolled, post-prescription patients in the US only), or reaching out to patient advocacy representatives (argenx employees). Reports can also be made via our website, other monitored websites, email, phone, or social media. For information on our approach to anti-retaliation and whistleblower protections, see the Governance section of this Sustainability Statement. Information on adverse events or quality issues occurring at any stage of the product lifecycle (including clinical trials, Pre-Approval Access, and post-marketing) are reported through various communication channels to Global Patient Safety and Global Quality. These reports are managed in accordance with the worldwide pharmacovigilance regulations, guidelines, and quality procedures. For US patients enrolled in the MyVyvgartPath post-prescription patient support program, discussions about symptoms and patient experiences are conducted. Our nurse case managers are trained to identify and report adverse events and product quality issues on behalf of the patient. We aim to follow up on all reports of suspected adverse events in a timely and appropriate manner. A set of processes is in place to manage reported adverse events in compliance with pharmacovigilance regulations and standards. argenx regularly monitors received safety information and assesses whether the safety information and mitigation measures included in the product information are adequate or require updates. Additionally, further minimization measures beyond the product information are considered if necessary. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 358

We evaluate the performance and effectiveness of our pharmacovigilance systems through various activities, such as compliance monitoring and quality management system audits. We closely monitor the safety profile and risk-benefit balance of its products incorporating all available data. This includes reviews occurring approximately quarterly and as needed by the internal Benefit-Risk Committee for each approved and investigational product, as well as a multidisciplinary labeling working group and a Global Labeling Committee. All findings are documented, with progress reported on and monitored in subsequent meetings. The Benefit-Risk Committee and Global Labeling Committee are comprised of senior representatives from all relevant functions. 7.4.5 Access to Quality Information & Responsible Marketing S4-1 S4-2 S4-3 S4-4 S4-5 MDR-P MDR-A MDR-T Material S4 IROs described in this section include: • Misleading or inaccurate information relating to products can lead to improper use, including dangerous interactions with other medications or incorrect usage and dosage (Impact). • Off-label promotion exposes pharmaceutical companies to legal, financial, and reputational risks, inviting regulatory scrutiny and liability (Risk). • Risk of product misinformation and false claims can result in a loss of support from stakeholders (i.e., patients, doctors, pharmacists), non-compliance, significant fines, and settlements (Risk). Our Code of Conduct and Business Ethics governs the promotion of our products, complying with all applicable laws, regulations, and ethical standards. All marketing and promotional activities must align with approved product indications and regulatory guidelines. Our Global Commercial Materials and Medical Education Materials Review Committee policy addresses our responsible marketing practices and access to appropriate scientific information, including prohibiting off-label promotion of medicines. Through this policy, we require product promotional communications and medical educational materials to undergo an internal review process by the relevant Materials Review Committee (MRC) or Medical Materials Review Committee (MMRC) before they can be used. Each review committee consists of personnel from multiple functions, namely Medical, Legal, and Regulatory, and ensures our external communications about our products and therapeutic areas are accurate, truthful, non-misleading, consistent with product labeling where applicable, scientifically substantiated, and appropriately balanced between risks and benefits. While all our stakeholders are responsible for following the policy, the responsible heads of Regulatory, Legal, Marketing, and Medical Affairs per region oversee the implementation of the policy. In addition to the review process, Ethics and Compliance supports the implementation of the policy by providing training on how to use approved materials and messaging in a compliant way. The Global Commercial Materials and Medical Education Materials Review Committee policy is designed to maintain ongoing compliance with applicable legal and regulatory requirements governing advertising, promotion, and scientific communications, making it challenging to track effectiveness quantitatively. We ensure that the review committee is comprised of highly qualified professionals with expertise in the relevant Legal, Regulatory, and Medical disciplines. There are currently no targets related to responsible marketing. Patients may report concerns about marketing practices to state and federal regulatory authorities. Upon learning of such concerns, we would fully consider the allegations to determine whether any changes to the materials are warranted. The company can modify its communications on a voluntary basis at any time. Additionally, materials are typically approved for a limited time of use, after which they are re-reviewed to confirm that the information remains current, scientifically accurate, properly substantiated, and consistent argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 359

with regulatory-approved labeling where applicable. As previously described, compliance monitoring and internal audits are conducted to maintain strict adherence and to avoid potential deviations. In 2024, argenx did not incur any monetary losses as a result of legal proceedings related to ethical marketing. Patient Advocacy In 2024, the Patient Advocacy function, overseen by the Global Vice President of Patient Advocacy, was responsible for facilitating patient engagements. We collaborate with patient advocacy organizations worldwide that focus on rare diseases and marginalized communities. We have a global patient advocacy team for engaging with these communities. Feedback from patients has led to various argenx-led advocacy actions and initiatives aimed at improving patient outcomes, including the following: • Disease education engagement through platforms such as MG-United and Shining Through CIDP websites in the United States, sharing content on disease awareness, lifestyle information, patient mentors, training modules, resources, guidance for newly diagnosed patients, and holistic health tips like recipes, exercise, and emotional wellness. • All United for MG, founded in 2023, raises awareness about gMG in Europe and strengthens patients' and caregivers' rights for improved quality of life, better care, and access to resources. • Podcast series “Untold Stories: Life with a Severe Autoimmune Condition,” designed to tell the stories of patients from underserved or marginalized communities who are living with a severe autoimmune condition. • Educational materials on the disease and treatment produced by argenx, provided to patients through their physicians and published on platforms such as access-by-registration websites for each of the three indications available in Japan. Metric description Value Note Number of patient advocacy projects undertaken or organizations engaged with 1) 45 1 1) The number of patient advocacy projects includes patient panels as described in the “Patient Engagement” section below, and excludes broader patient advocacy projects. Patients and Human Rights We do not have specific policy related to patients beyond the general approach outlined in the Code of Conduct and Business Ethics. The argenx Code of Conduct and Business Ethics includes a provision on safeguarding the health and safety of study participants in clinical trials, including the protection of human rights. We are committed to the following: • Act ethically to protect the human rights, dignity, privacy, and personal information of individuals involved in research. • Obtain the appropriate informed consent from everyone taking part in an argenx-sponsored clinical trial. • Fully train clinical investigators and other site staff on relevant study protocols and other clinical trial requirements and routinely monitor clinical trial sites to ensure their compliance with these standards. We adhere to the European Federation of Pharmaceutical Industries and Associations (EFPIA) Code of Conduct. This code outlines how member companies should interact with healthcare professionals, healthcare organizations, and patient organizations, ensuring the responsible promotion of medicinal products while upholding high ethical standards in their interactions. We comply with all internationally accepted standards that apply to our clinical trials, including the ICH Guidelines for Good Clinical Practice and the ethical principles articulated in the Declaration of Helsinki, as well as applicable local laws and regulations. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 360 S4-2 MDR-A S4-1 MDR-P

Access to Medicines Material S4 IROs described in this section include: • Increased and better access to products and services (including through affordable pricing) can improve health and longevity for more patients (Impact). • Increased and better access to medicines through improving commercial/ distribution channels and improving the affordability and pricing may lead to a growth in market capacity (Opportunity). We have a Pre-Approval Access Global policy, overseen by our Chief Medical Officer, which outlines the principles for Pre-Approval Access (PAA) for an unapproved product. The policy's scope encompasses all global PPA requests and activities, including those conducted by partners contracted by argenx. Our Post-Trial Access Policy governs our Post-Trial Access program, detailing the evaluation and approval process for Post Trial Access (PTA) to an investigational product. The policy applies to all PTA requests for investigational products from patients previously enrolled in an argenx clinical trial. Our Global Pricing Committee Charter establishes the foundation for our pricing decisions and supports our commitment to serving patients while maintaining the ability to innovate. This includes bringing new products to market that meet medical and societal needs, while considering the impact of pricing decisions on external stakeholders, such as patients, providers, payers, and other healthcare system actors. The Global Pricing Committee is responsible for implementing the Global Pricing Committee charter. To increase access to medicines, we have established a patient support program called MyVyvgartPath which assists with education and navigation of the complex insurance process in the United States. This program provides patients with education about insurance coverage, potential financial assistance programs, commercial co-pay support, and possible out-of-pocket costs. We collaborate with distribution partners, healthcare systems and payers to optimize supply chains and reduce barriers to access (e.g., not having a product listed on formulary, unfavorable medical policies, new to market blocks, prior authorization requirements, and step edits). Initiatives, such as market-specific patient affordability programs, help argenx to reach more patients and improve global health outcomes in the rare disease space. In the United States, we participate in Medicare and Medicaid programs which contributes to affordability as it involves a series of out-of-pocket caps for the patient and manufacturer contributions in Part D as mandated by the United States Government. We also participate in the 340B program, which provides upfront product discounts to covered entity healthcare providers serving low-income communities. In cases where a patient has a life-threatening or serious condition for which a clinical trial is not an option, or the patient has exhausted all currently available treatment options, their licensed treating physician may request access to an investigational medicine under development at argenx. When reviewing an unsolicited request for treatment, we will consider providing access to investigational medicines through an argenx Pre-Approval Access program if the request meets all the requirements of the Pre-Approval Access Policy. Additionally, patients who are actively enrolled in an argenx clinical trial may qualify for Post-Trial Access if they will experience a temporary gap in treatment until the medication is approved and reimbursed in their country. The Post-Trial Access Program is managed by Medical Affairs and Evidence Generation, with the Chief Medical Officer overseeing the policy's implementation. Please refer to Section 1.7.4 "Coverage, Pricing and Reimbursement” for additional information regarding pricing and the uncertainty around the coverage and reimbursement status of or products and/or product candidates. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 361 S4-1 S4-3 S4-5 MDR-P

There are currently no targets in relation to access to medicines. At present, the effectiveness of these actions is not formally tracked. Metric description SASB Reference Value Note List of products on the WHO List of Prequalified Medicinal Products as part of its Prequalification of Medicines Programme (PQP). HC-BP-240a.2 – Number of patients approved for PAA for their gMG patients N/A 70 1 Percentage change in: (1) list price and (2) net price of product with largest increase compared to previous reporting period HC-BP-240b.3 (1) Percentage change in list price: 2% (2) Percentage change in net price: 1% 2 Percentage change in: (1) weighted average list price and (2) weighted average net price across product portfolio compared to previous reporting period HC-BP-240b.2 (1) Percentage change in weighted average list price: -1% (2) Percentage change in weighted average net price: -5% 3 1) The metric shows the number of new patients approved during 2024. 2) The metric shows the annualized percentage change in list price and the name of the product with the largest increase in list price compared to the previous reporting period. The calculation includes countries that contribute to aggregate 95% of product net sales. 3) The metric shows the annualized percentage change in the revenue-weighted average list price increase across all the entity’s products sold globally during the reporting period. The calculation includes countries that contribute to aggregate 95% of product net sales. The net price is based on all products in the Company’s global portfolio over the year, weighted based on sales made in the fiscal year. Data Privacy Material S4 IROs described in this section include: • Exposing sensitive patient information risks fines and penalties, lawsuits, remediation costs and reputational damage (Risk). Recognizing the importance of data privacy, we take steps to protect sensitive patient information and have implemented policies to enhance protection from both technical and organizational perspectives throughout the information lifecycle. Regarding patient data, the Global Data Privacy Policy outlines data privacy principles and safeguards, the role of the Data Protection Officer (DPO), data security considerations for data subjects (including patients), and the collection and use of sensitive personal data. The DPO reports to the General Counsel and is responsible for the implementation of the Global Data Privacy Policy. Committees such as the Global Compliance Committee (GCC) and the Audit and Compliance Committee oversee the privacy program. The Information Security Policy includes specific technical measures reviewed by the Information Security and Privacy team in accordance with applicable laws and regulations (e.g., access control, password protection, encryption). It also features increased strictness in cases where our personnel are expected to process sensitive patient information (e.g., limited access, pseudonymization, limited disclosure). From an organizational perspective, we have established internal teams, such as the Privacy Steering Committee, to keep the company's privacy practices accurate and up to date throughout the organization. Additionally, specific teams are formed for particular occurrences, such as the Incident Response team, which addresses security incidents, including data breaches, to report and mitigate them as much as possible. Controls are in place for any vendor-owned assets that process personal information and are used for the benefit of argenx. This is managed through a Computerized System Impact Assessment, which may lead to a Data Protection Impact Assessment if the privacy risk is considered higher. Additionally, we maintain a database of potential or actual data breaches where patient sensitive information might have been compromised. Over the past year, no data breaches were detected within argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 362 S4-1 S4-3 MDR-P MDR-A MDR-T

argenx that involved the exposure of sensitive patient information. Where data breaches occurred, they were external, involving our vendors, and had limited impact. Due to their limited impact from a privacy perspective, none of these data breaches were reportable to authorities or data subjects under applicable law. In 2025, we aim to further improve and optimize our data privacy and security readiness by minimizing data breaches through our internal organizational processes. To achieve this, we review, update, and enhance our current policies and procedures on a regular basis. The detection, notification, and overall management of security incidents and data breaches, as outlined in procedural documents such as the Security Incident Management and the Personal Data Breach Procedure, are kept up-to-date and aligned with current laws, regulations, and best practices. This includes meeting response and notification timeline requirements and implementing appropriate corrective and mitigation measures. Moreover, we aim to exercise further control over our vendors' technical and organizational measures to protect patient information. This includes updating contractual terms, such as Data Processing Agreements, and enhancing the overall management of third-party risk assessment. Vendor engagement questionnaires and validation processes are updated to include questions related to the vendors’ privacy and security practices. There are no measurable targets for patient data privacy. However, the Global Compliance Committee team monitors the overall progress of the privacy action plan, meeting quarterly each year. Patient Engagement We are dedicated to improving the lives of people with severe autoimmune diseases by listening to patients, supporters, and advocacy communities, with the gathering of perspectives and insights as a key part of our strategy. This approach helps advance the understanding of rare diseases and aims to deliver new treatments to as many patients as possible. Patient advocacy approaches vary across regions to comply with local regulations. In 2024, we engaged with patients through the following key channels, guiding argenx’s strategy and empowering patients: • Patient panels: Conducted 45 times in 2024, initiated by indication development teams to gather patient insights on clinical trial protocols, ICF reviews, patient journeys, and other clinical development needs • Multifocal Motor Neuropathy patient survey • Myasthenia Gravis Advocacy Leadership Council: Met once per quarter • Autoimmune round table: Developed in 2024 for non-MG groups, held three times in its inaugural year. • Annual Patient Summits: Bring patients together in person for focus groups, brainstorming, and keynote sessions. • Collaboration with national and regional patient advocacy groups: Share industry insights and best practices and align on priorities for the year ahead. • CIDP advisory board: Helps understand the burden of illness, real-world evidence from patients, and the societal impact of the disease across Europe. In the United States, argenx engages directly with patients and their representatives on key topics such as health, safety, and market access throughout the product lifecycle. In Europe and other regions, argenx collaborates with third-party service providers, such as nurses, who have direct access to patients. These nurses are trained by argenx on disease and treatment protocols to help patients through their treatment journey and are involved in pharmacovigilance data collection. Patient engagement occurs at all stages of the product lifecycle, including with both clinical trial patients during development and with commercial patients before and after prescription. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Social 363 S4-2

7.5 Industry Specific Disclosures 7.5.1 Introduction to Industry Specific Disclosures We are dedicated to upholding our standards of innovation, quality, and product safety in order to mitigate risks, accelerate scientific breakthroughs and provide high-quality, safe, and differentiated medicines. Innovation Material entity specific IROs described in this section include: • Successful innovation helps find new treatments for current diseases and address unmet needs, allowing more patients to be treated (Impact). Description of Innovation at argenx We strive to innovate on behalf of patients, and we do this through a model of co-creation, combining our antibody-engineering capabilities with the expertise of leading disease biologists to address novel targets and develop innovative, first-in-class therapies. While there are no formal policies or targets relating to innovation, our Vision 2030, which was announced at the R&D day in the summer of 2024, outlines our plans to transform the treatment of autoimmune diseases through a continuous pipeline of innovation. Through this vision, we aim to treat fifty thousand patients with our medicines, secure ten labeled indications across our approved medicines, and advance five pipeline candidates into Phase Three development by 2030. To foster a continuous pipeline of innovation and achieve the goals outlined in our ‘Vision 2030’, argenx has set the following priorities for 2025: • Expand the global VYVGART opportunity by reaching more patients broadly across MG, CIDP and ITP through additional regulatory approvals and continuous evidence generation. • Launch VYVGART SC as a pre-filled syringe to innovate on the patient experience and move earlier in the MG and CIDP treatment paradigms. • Execute ten registrational and ten proof-of-concept studies to fuel pipeline growth across efgartigimod, empasiprubart and ARGX-119. • Advance four new molecules into Phase One development, expanding the next wave of innovation. • Generate sustainable value through continued investment in the Immunology Innovation Program, focused on antibody-based medicines with pipeline-in-a-product potential. We measure progress through a set of strategic priorities aligned with our overarching vision. Our focus on innovation helps us to find new treatments for severe autoimmune diseases, as demonstrated through VYVGART, empasiprubart, and our expanding pipeline of antibody-based therapeutics. This includes plans to advance our offerings around myasthenia gravis (MG) and chronic inflammatory demyelinating polyneuropathy (CIDP), advance the next wave of indications through late-stage development to reach patients, and invest in bringing forward new pipeline candidates. Our innovative efforts have resulted in the continuous development of drug candidates and a cadence of wholly owned argenx-nominated programs into our portfolio. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Industry Specific Disclosures 364

Topic Accounting Metric SASB Code Value Note Number of Clinical Trial Patients Treated Clinical trial patients treated with our own pipeline candidates in 2024 HC-BP-000.A 1052 1 Number of Drugs Number of drugs in portfolio HC-BP-000.B 7 2 Number of drugs in research and development (Phases 1-3) HC-BP-000.B 3 3 Innovation and R&D Year-to-date R&D expense in line with our annual report (IFRS) N/A $983,423,000 Pipeline candidates currently out-licensed to our partners. N/A 4 Number of programs that have been tested in humans since inception N/A 9 4 Number of Research and development employees N/A 644 5 Active clinical trials N/A 33 6 1) Clinical trial patients include effectively treated subjects in our studies, any patient with at least one recorded IMP in the period under review is included. We count all participants, regardless of if they had placebo or active, or are still blinded. It also aggregates across different routes of administration. Healthy volunteers are excluded. 2) “Number of drugs” counted in the portfolio included argenx-nominated drug candidate programs in IND-enabling studies, clinical development or marketed as a commercial product that are not partnered. 3) “Number of drugs in research and development (Phases 1-3)” include argenx-nominated drug candidate programs that are in phase 1, 2 or 3 and are not partnered. 4) This includes all argenx-nominated programs that have been tested in humans since inception and includes partnered products. 5) Number of employees is disclosed in headcount, as of December 31, 2024. 6) Clinical trials include all clinical trials and observational studies that were active in 2024, including those starting and concluding during the year. Phase 1, 2 and 3 studies only run in the period under review. Based on gADAM database, which starts from the gSDTM raw clinical data that we have available and that is refreshed regularly. 7.5.2 Product Quality Material entity specific IROs described in this section include: • Poor product quality can directly impact the effectiveness of treatments and overall health of patients (Impact). Description of Quality at argenx Quality is a core value at argenx and drives our innovation by promoting consistency, reliability, and compliance throughout our processes. This commitment results in high standard products for patients. At argenx, quality is a shared responsibility, and we work together to make data-informed decisions for the benefit of patients worldwide. United in our commitment to improving the quality of life for patients, we deliver quality, safe, and life changing products. Our Quality policy is a formal statement outlining our commitment to quality. It communicates senior management’s commitment and strategic intent regarding quality standards, compliance, and customer satisfaction. The Quality policy acts as a guiding principle for the organization, influencing decisions and behavior at all levels. This includes putting all procedures and processes in place to prevent, mitigate, and remediate poor product quality, directly impacting the effectiveness of treatments and overall health of patients. The Quality policy sets out the following objectives: • Champion a strong quality culture across the organization. • Demonstrate strong and visible support for the quality management system and its implementation throughout the organization. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Industry Specific Disclosures 365

• Encourage participation in and promotion of quality responsibilities among all employees through consistent and effective communication. • Establish and maintain processes to promote traceable, transparent, and trustworthy data, enabling product integrity and patient safety. • Comply with applicable laws, quality regulations, standards, and internal requirements. • Create an effective and qualitative oversight of any third party acting on behalf of argenx. • Empower employees through continuous learning to achieve the required standards for excellent quality management and to elevate every employee to his/her highest potential. • Foster a culture of continuous improvement and innovation where we challenge ourselves to increase the effectiveness of our processes and quality performance. The Quality policy applies to all argenx internal and external activities related to research, development, preclinical development, clinical development, manufacturing, distribution, marketing, and commercialization of our products. It also covers oversight and appropriate monitoring controls of outsourced processes of third parties and collaborators such as manufacturing, distribution, preclinical trials, and collaborations. It is intended to ensure consistency, efficiency, reliability, compliance, data integrity, and ensures the health and safety of patients and the highest quality of products. The senior management team, including the Global Head of Quality, have overall accountability for the implementation of the policy. We maintain quality control and assurance measures throughout the product lifecycle, from research and development to manufacturing and distribution, to secure the safety and efficacy of our products. By adhering to stringent regulatory requirements and continuously monitoring the performance of our manufacturing processes, we aim to minimize risks and enhance patient outcomes. Actions on the respective policies are covered as part of the day-to-day operations in the Quality Management System. This is covered via the core quality processes, which cover aspects such as internal/ external deviations, complaints, and Corrective Action and Preventative Action (CAPA) procedures. Corrective actions are actions that are performed after an investigation to correct the root cause of the deviation and to prevent a recurrence of the deviation. Preventive actions are actions that are performed to prevent deviation from happening. A Product Quality Complaint (PQC) is any written, electronic, or oral communication that contains potential defects related to identity, quality, purity, strength, durability, reliability, safety, effectiveness, or performance of a Product released for distribution or used in a clinical trial. While no specific targets are currently in place for product quality, we track the effectiveness of our policies and procedures through the Quality Management Review (QMR) Process. The QMR is a key component of the quality management system(s) and provides a high level overview of the effectiveness and suitability of the quality management system within the organization that shows if expectations are being correctly anticipated, met and/or exceeded and processes are effective and in a state of control. It also plays a pivotal role in proactively identifying, scientifically evaluating, and controlling potential risk to quality. In 2024, we had zero commercial product and clinical batch recalls and zero commercial supply stock outs. 15/25 (60%) CAPAs from Continuous Improvement and Quality Risk Management were preventive. Our original Annual Audit Plan for 2024 included 31 external audits for GMP/GDP, which includes audits executed at an argenx third party, performed by either an external auditor or an argenx employee trained as an auditor. During the year, our audit schedule was adjusted quarterly based on evolving needs, for example, as new vendors were identified during the year. Through this process to adjust our audit schedule, 11 additional external audits were added during the year. Of the total 42 external audits, 33 (79%) were completed in 2024 and in all cases, vendors continued to be qualified. The remaining audits were either re-prioritized, based on risk, to be addressed in future years or deemed no longer relevant if the vendor no longer provided services to argenx. There were no inspections related to clinical trial management and pharmacovigilance that resulted in voluntary remediation or regulatory actions against the entity. All inspections passed without critical findings or FDA 483 OAIs. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Industry Specific Disclosures 366

We continuously monitor the safety profile of our investigational products and comply with adverse event reporting to health authorities worldwide. Additionally, we have maintained a consistent supply to patients in clinical trials, with no supply disruptions. Topic Accounting Metric SASB Code argenx disclosure Note (1) Number of recalls issued, (2) total units recalled. HC-BP-250a.3 1) 0 2) 0 Total amount of product accepted for take back, reuse, or disposal. HC-BP-250a.4 0.051 tons 1 Number of enforcement actions taken in response to violations of GMP or equivalent standards, by type 2. HC-BP-250a.5 – Percentage of CAPAs considered preventative N/A 60% 2 Percentage of audits completed on vendors involved in manufacturing, testing and distribution of argenx products and product candidates. N/A 79% 3 1) The weight of unused products is based on the number of VYVGART and VYVGART SC vials returned globally in the reporting year, converted into metric tons. VYVGART and VYVGART SC vials are assumed to weigh 59 and 49 grams, respectively. 2) 15 out of 25 (=60%) of the Corrective Action and Preventative Action procedures (CAPAs) are preventative. 3) This is based on 33 out of the planned 42 product quality audits being completed in 2024. 7.5.3 Product Traceability and Counterfeit Drugs Material entity specific IROs described in this section include: • A lack of product traceability and transparency can lead to counterfeit drugs or materials, endangering patient health (Impact). Description of Product Traceability and Counterfeit Drugs at argenx Product traceability is a crucial component of our mission and helps reinforce trust with stakeholders, particularly our patients. We are committed to maintaining the integrity and safety of our products through product traceability, anti-tampering, and counterfeit prevention mechanisms. In compliance with national legislation (e.g., DSCSA in the U.S. and Falsified Medicines Directive (FMD) in the EU), all commercial argenx products are serialized at the unit, case, and pallet levels. Each sellable unit features a unique random serial number, product code, lot number, and expiration date, both in human- readable format and encoded in a 2D data matrix barcode. All products are tamper-evident sealed, and shipping systems and trucks are sealed with numbered seals during transit to prevent tampering. Our EU Serialization Policy outlines the processes and procedures involved in the product lifecycle as it relates to our EU FMD serialization program. The policy applies to our Global Commercial Supply Chain, Commercial Product Quality, and EU Distribution and covers our pharmaceutical prescription product SKUs in the EU supply chain (EU-Falsified Medicines Directive participating markets, including EU 27, Northern Ireland, EEA, Switzerland). Our Head of Logistics Product Quality and EU RP are accountable for implementation of the policy. Our US Serialization Policy outlines the process and procedures involved in the product lifecycle as it relates to our DSCSA serialization program. The policy applies to our Global Commercial Supply Chain, Commercial Quality, US Distribution, Business Information Systems (BIS) and Chemistry, Manufacturing and Controls (CMC), and covers our pharmaceutical prescription product SKUs in the US supply chain. The policy is reflected in the supplier responsibilities outlined in quality agreements and contracts. The Global Commercial Supply Chain is responsible for DSCSA Serialization Program implementation. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Industry Specific Disclosures 367

In Japan, traceability is addressed through mandatory GS1 Standard Barcodes which includes a GS1 Databar Limited Composite Symbol on packaging. This symbol includes product code (GTIN), expiration date, and lot number (batch number). This allows pharmacies and hospitals to check the authenticity of the drug. During batch manufacturing (outsourced to CMOs), all processing steps are documented to have a full processing history and lot genealogy of consumed raw materials, which is also maintained in the CMO’s quality system. An ERP system is used to provide full lot genealogy and traceability of intermediates and finished products owned by argenx, located at its CMOs, third-party warehouses and third-party logistics providers (3PL). For downstream distribution, the entity can rely on the distributors and wholesalers’ systems to trace products to the end customer. We also have procedures in place to mitigate the risk of falsified or counterfeit medicine. This includes a consistent reporting process for any suspicions of falsified or counterfeit products. When a case is reported, the impacted batches are separated and quarantined, an investigation is conducted, and the relevant stakeholders in the supply chain, as well as competent authorities, are informed in case of confirmed counterfeit products. Market actions are taken in consultation with competent authorities. While no specific targets are currently in place, we track the effectiveness of our policies and procedures through an annual mock recall. This exercise tests the accuracy and efficiency of our recall process, including product traceability and responsiveness. In 2024, we had no incidents of falsified or counterfeit medicine reported and no actions that led to raids, seizures, arrests, or criminal charges related to counterfeit products. Product Traceability Metrics Topic Accounting Metric SASB Code argenx disclosure Counterfeit Drugs Description of methods and technologies used to maintain traceability of products throughout the supply chain and prevent counterfeiting. HC-BP-260a.1 See Product Traceability and Counterfeit Drugs Discussion of process for alerting customers and business partners to potential or known risks associated with counterfeit product HC-BP-260a.2 See Product Traceability and Counterfeit Drugs Number of actions that led to raids, seizure, arrests, or filing of criminal charges related to counterfeit products. HC-BP-260a.3 – Number of falsified or counterfeit medicine incidents reported N/A – argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Industry Specific Disclosures 368

7.6 Governance 7.6.1 Introduction to Corporate Culture at argenx We have put in place governance structures and mechanisms to promote accountability, adaptability, and alignment with our values. 7.6.2 Business Ethical Conduct Material G1 IROs described in this section include: • Unethical practices (e.g., harassment, discrimination, corruption, fraud, safety issues) as a result of poor corporate culture (Impact). • An inability to protect whistleblowers against retaliation prevents the identification and remediation of incidents towards employees and patients, impacting engagement, safety, and trust (Impact). Our culture, established by our company founders, is defined by five Cultural Pillars (who we are): innovation, co-creation, empowerment, excellence, and humility. These pillars are complemented by the expectations for employees to: • Follow the rules: Employees are expected to know and comply with the laws, regulations and Company policies that apply to their job role and the countries in which they operate. If local laws or policies are more restrictive than those outlined in the Code of Conduct and Business Ethics, employees are expected to follow the more restrictive local requirements. • Exercise good judgement: Employees are expected to conduct business with honesty and integrity and in a manner that protects argenx’s reputation. • Ask questions: If employees are uncertain about any of the laws, regulations or policies that apply to their role or the country in which they operate, employees should talk to their manager, Legal, Compliance, or HR business partner. These expectations are communicated to employees through the Code of Conduct and Business Ethics. We expect argenx colleagues to promptly raise any concerns regarding violations or potential violations of our policy by notifying their manager, Human Resources, Legal, Compliance, or the argenx COMPASS helpline. A key way in which we develop our culture, activating these pillars and operating principles across our teams is through Culture Lab sessions. These voluntary sessions are designed to unify and connect colleagues, gather insights to help improve employee experience, and activate all Argonauts to live our Cultural Pillars. Culture is also discussed and fostered through additional channels, including Corporate Updates, team meetings, and communications across various platforms. At argenx, we reinforce our corporate culture through training and awareness campaigns, and an Ethics & Compliance team that supports ethical behavior, accountability, and adherence to policies and procedures. There are currently no targets related to corporate culture and currently no further actions planned for the future, beyond actions already described in this section. Code of Conduct and Business Ethics Our commitment to ethical business practices ties to regulatory requirements and is also integral to maintaining stakeholder trust and fostering meaningful innovation. The Ethics & Compliance program aims to guide, promote, and embed a robust ethics and compliance culture. By systematizing our ethical argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Governance 369 ESRS 2 SBM-3 G1-1 G1-1 G1-3 MDR-P

business guidelines, we provide guidance for all employees and our network of partners, collaborators, and vendors. Our Code of Conduct and Business Ethics delineates our responsibilities to our employees and the diverse stakeholders we serve, including patients, healthcare professionals, customers, regulators, investors, and the communities where we live and work. The Code of Conduct and Business Ethics’ purpose is to summarize the standards we are all expected to meet and to provide guidance regarding compliance- related questions or situations encountered during the workday. The Code of Conduct and Business Ethics applies to all individuals conducting business on behalf of argenx worldwide. In 2024, 92% of employees completed the Code of Conduct and Business Ethics training. New members of the Board of Directors are walked through both the Code of Conduct and insider trading policy as part of their onboarding. Due to the nature of our business, our approach to ethics also includes specific policies related to interactions with healthcare professionals (HCPs). Interactions with HCPs are guided by the Interactions with Members of the Healthcare Community Policy, which includes provisions on fair market value compensation, appropriate documentation, and adherence to anti-bribery and anti-corruption laws. In addition to this HCP-specific policy, argenx’s Code of Conduct and Business Ethics governs interactions with HCPs by requiring compliance with all applicable laws, regulations, and ethical standards. The Code of Conduct and Business Ethics requires that all employee engagements with HCPs are conducted with transparency, integrity, and respect for their independence, prohibiting any improper influence or incentives. This aims to ensure unbiased decisions in patient care. Training programs and transactional monitoring and auditing by the Ethics and Compliance team reinforce these standards by building employee awareness of policies, demonstrating an expectation to adhere to policies, and verifying implementation of policies. Global Anti-Bribery and Anti-Corruption Policy We have implemented policies and procedures to prevent, detect, and address potential allegations of corruption or bribery. These, which either came into effect before or during 2024, include the argenx Code of Conduct and Business Ethics, Global Anti-Bribery and Corruption Policy, and Speak Up & Anti-Retaliation Policy. These are supported by regular training, monitoring, and reporting via our COMPASS Helpline for effective implementation and adherence. We have established a program for investigating allegations or incidents of corruption and bribery that is focused on thoroughness, fairness, and confidentiality. Investigations follow a standardized process guided by the Ethics & Compliance Global Investigations Procedure, Ethics and Compliance Investigations Summary, Speak Up & Anti-Retaliation Policy, and Code of Conduct and Business Ethics. All reports are reviewed and investigated in accordance with applicable policies and legal requirements. Employees are trained in reporting concerns and are assured of a non-retaliatory environment to encourage transparency and cooperation. Our Ethics & Compliance function oversees the COMPASS Helpline, which is our centralized reporting channel where reporters have the option to do so anonymously. argenx takes reports of violations of its Code of Conduct and Business Ethics, policies, and procedures very seriously and is committed to taking prompt action to review the facts and remediate issues when necessary. The E&C function is responsible for managing compliance investigations. Additional stakeholders, including Legal and Human Resources, are consulted for input and support where appropriate. Investigations are tracked from intake to remediation and documented in a Compliance Investigation Report. Allegations or concerns received through the various reporting channels shall be promptly communicated to E&C for triage. If an allegation or concern is cross-functional and involves issues relevant to more than one function (e.g., includes HR-related issues or potential law violations), E&C will confer with HR and/or Legal to determine which business function will lead the investigation. Upon intake, E&C will ensure that reporters receive confirmation that the report has been received and an acknowledgment that reports of misconduct are taken very seriously. If, based on the initial evaluation, it is determined that no further investigation is required, E&C will document that the matter will be closed. If, during the initial evaluation, it is determined that the report is one whose investigation should be directed and conducted by Legal, then Legal will lead the investigation. All reasonable steps are taken to maintain the confidentiality of the investigation, involving only those individuals with a need to know. If the Reporter wishes to remain anonymous, their anonymity will be protected to the fullest extent possible unless disclosure is required by law or necessary to conduct the investigation and any related proceedings. Reports are shared quarterly with the GCC and the Audit and Compliance Committee. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Governance 370 G1-1 G1-3 MDR-P MDR-A MDR-T

We provide anti-corruption and anti-bribery training programs to ensure all employees understand and adhere to ethical standards. These mandatory programs are tailored to the nature of each role and include policy reviews, approvals, and training. Training and monitoring mechanisms support preparedness and compliance across the organization. In 2024, 89.3% of employees completed the Global Anti-Bribery and Corruption Policy training. This training figure includes employees, contractors and consultants. Our Ethics and Compliance team also conducts periodic monitoring of activities, with the purpose of ensuring adherence to internal policies and procedures. This includes live monitoring, transaction monitoring, and auditing records. Live monitoring of speaker events is used to ensure promotional materials are consistent with our branding and regulatory guidelines. Live monitoring also includes field rides, advisory boards, congresses, and conferences. Transaction monitoring involves a retrospective review of records and documentation related to activities such as sponsorships, donations, grants, speaker programs, speaking engagements, and fee-for-service engagements to identify and correct any deviations. Additionally, our ACC oversees the Internal Audit function which conducts audits to prevent, identify and mitigate activities which might imply any bribery or corruption risks. In 2024, internal audits topics included external funding, speaker bureau programs, externally sponsored research, research services, and HCP expenses incurred through third parties. There are currently no further anti-bribery and anti-corruption actions planned for the future, beyond actions already described in this section. Percentage of functions-at-risk covered by prevention of bribery and corruption training programs Functions-at-risk % Covered by training programs Finance 100% of functions at risk are covered by our argenx training programs. Human Resources 100% of functions at risk are covered by our argenx training programs. Procurement 100% of functions at risk are covered by our argenx training programs. Sales and Marketing 100% of functions at risk are covered by our argenx training programs. Supply Chain Management 100% of functions at risk are covered by our argenx training programs. Incidents of Corruption and Bribery In 2024, we reported zero convictions for violations of anti-corruption and anti-bribery laws within our operations and argenx did not incur any monetary losses as a result of legal proceedings related to corruption and bribery. We track investigations in accordance with our Speak Up policy. There were no value chain violations within our hotline, and we did not incur any fines related to such violations. Whistleblower Protections We endeavor to comply with applicable global legal requirements to protect whistleblowers, ensuring individuals who report concerns in good faith are safeguarded from retaliation. Measures detailed in our Code of Conduct and Business Ethics and Speak Up & Anti-Retaliation Policy protect whistleblowers across all regions of operations. These policies outline that no argenx employee may retaliate against any Reporter or any person assisting a Reporter, cooperating with an investigation, responding to a request from regulators or government authorities, or exercising a legally protected right to report evidence of violations. Any person who retaliates will face disciplinary action, up to and including termination of employment, revocation of site access, or discontinuance of services. This is also documented in our Ethics & Compliance Investigations Procedure provided as supporting evidence. Whistleblowers can report incidents of corruption and bribery via our whistleblower hotline (insert link www.argenxhelpline.com) which is externally managed to ensure reporters stay anonymous. There are currently no targets related to protecting whistleblowers and currently no further actions planned for the future, beyond actions already described in this section. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Governance 371 G1-4 G1-3 MDR-A

7.6.3 Supply Chain Management Material G1 IROs described in this section include: • Poor relationships with suppliers and inconsistent payment practices (as a result of argenx failing to pay suppliers on time) may impact the reliability and consistency of suppliers’ activities, interfering with supply chains, affecting R&D and medical distribution to those in need of treatment (Impact). • Poor supplier relationship management can lead to risks such as low-quality products, supply chain disruptions, financial losses (e.g., increased costs and interest from late payments), non-compliance with supplier agreements and payment terms, and reputational damage, resulting in a loss of trust and credibility (Risk). We recognize the important role our supply chain plays and have developed a supply chain management approach to mitigate risks and maintain strong relationships with our suppliers. This approach focuses on defining expectations and implementing processes to monitor and manage supply chain performance. There is no formal target to measure the management of suppliers. Supplier management involves a vendor qualification process to evaluate supplier services and compliance with regulatory and argenx requirements. We carry out reference checks and screening of new vendors to ensure our suppliers meet the standard we expect. We also have the Global Sourcing and Vendor Alliance Management team which manages oversight of relationships with suppliers, along with each functional area who manages the relationships with third parties that they request. We engage suppliers directly through regular meetings with business owners who oversee the operational aspects of service performance and delivery. Outsourcing is a key business strategy for argenx, enabling us to leverage external expertise and resources. Partnership governance is set up with key suppliers and defines the collaboration among both partners from strategy and tactical through to operational, including governance structure, meeting structure, and responsible persons. This governance structure is continuously evaluated. Our collaboration with suppliers connects to our pillar of co-creation, which allows us to achieve more than we could individually. Co-creation enables us to bring additional perspectives to our work. We have established a third-party management working group to focus on early risk detection, compliance control, master data integration and process simplification. The goal is to build strong third-party risk management processes. We also incorporate risk management into the supply chain management approach through our Supply Chain Maps (SCM) process. SCM describes the supply chain for VYVGART and is used to visualize its logistics and QA requirements. Suppliers across our supply chain, from development vendors, manufacturing, and distribution to our commercial and tech vendors, undergo a qualification and monitoring process, including periodic audits, to help ensure the expected level of quality is maintained. Furthermore, business reviews are conducted periodically to evaluate and manage performance. Escalation and resolution of potential quality issues occurs through the governance channels. If issues such as low-quality products, supply chain disruptions and non-compliance with supplier agreements and payment terms are identified (e.g., through audits), corrective action plans will be implemented and followed up. In case of consistent unacceptable low-quality performance without any resolution and improvement, a vendor relationship may be terminated. Our Code of Conduct and Business Ethics states we will select all third parties on clear, objective criteria including, but not limited to, price and quality of goods or services, capability, reputation, and past performance. For relevant suppliers, we undertake applicable due diligence before entering business arrangements, including a review of the third party’s Code of Conduct and Business Ethics. With regards to social criteria, this due diligence process includes whether our suppliers are in adherence with mandatory argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Governance 372 G1-2 MDR-A

regulatory requirements including requirements for worker’s rights. There is currently no environmental criteria used for the selection of suppliers. There are currently no further supply chain management actions planned for the future, beyond actions already described in this section Payment Practices We view payment practices as fundamental part of managing vendor relationships although we currently do not have a formal payment practices policy. argenx's payment practices, including average payment time compared to standard payment terms, were calculated using data extracted from the AP application in Oracle and included all invoices paid to registered vendors, excluding employees and IC payments. The average payment time was calculated by dividing the total number of days taken to pay invoices by the total number of paid invoices, segmented by business unit, region, and the entire group. The standard payment terms are 30 days in instances where other terms have not been contractually agreed upon, and 76% of all invoices were paid within this timeframe. There are no outstanding legal proceedings for late payments. On average, argenx paid suppliers 30.2 days after the invoice date. There is no separate policy for preventing late payments to small or medium-sized enterprises (SMEs). To prevent late payments, argenx follows standardized processes for master vendor data, purchasing, invoicing, and payments. Weekly payment runs were conducted for all European and US entities, with Oracle-approved invoices included in the payment run, payment proposals reviewed and approved by the global Treasury manager and approved payment runs then executed in our treasury management software. In Japan, all duly approved invoices were paid once a month on a fixed date at the end of the month. 7.7 Other Considerations In addition to the disclosure of material topics in our Sustainability Statement, we have included the following select voluntary disclosures. These are other topics that argenx chooses to disclose and are topics that have not been deemed material as part of our ESRS materiality assessment process. 7.7.1 Animal Welfare Policy We have an Animal Welfare Policy that provides guidance and defines key principles (replace, reduce, and refine) aimed at promoting and safeguarding the welfare of animals used in research by or on behalf of argenx. The policy is assigned as a read and acknowledges requirements for all employees. 7.7.2 Employee Health and Safety Although we do not have a formal workplace accident prevention policy, we have implemented processes to prevent work-related accidents. In our labs, we train and educate employees on safe chemical handling, waste management, and biosafety practices. In 2024, we reported no fatalities due to work-related injuries and one recordable work-related accident. We do not track or record cases of work-related ill health among employees or non-employees. argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Governance 373 G1-6 MDR-P MDR-A

Metric description Value Number of cases of recordable work-related ill health of employees Not currently recorded nor recordable Number of cases of recordable work-related ill health of non-employees Not currently recorded nor recordable Number of days lost to work-related injuries and fatalities from work-related accidents related to employees 7 Number of fatalities in own workforce as result of work-related injuries 0 Number of recordable work-related accidents for own workforce 1 Percentage of own workforce who are covered by health and safety management system based on legal requirements and (or) recognized standards or guidelines and which have been internally audited and (or) audited or certified by external party 0 Percentage of people in its own workforce who are covered by health and safety management system based on legal requirements and (or) recognized standards or guidelines 0 Days Away, Restricted, or Transferred Rate (DART) 0 Number of days lost to incidents causing permanent injury 0 Number of days lost to incidents causing temporary injury 7 Number of days lost due to injury (LTIR) 0 Number of incidents causing permanent injury 0 Number of incidents causing temporary injury 1 Total Recordable Incident Rate (TRIR) 0.000018 argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Other Considerations 374

7.8 Appendix 7.8.1 EU Legislation Data Points EU List of datapoints in cross-cutting and topical standards that derive from other EU legislation ESRS 2 GOV-1 21 (d) Board's gender diversity SFDR, Benchmark regulation ESRS 2 GOV-1 21 (e) Percentage of board members who are independent Benchmark regulation ESRS 2 GOV-4 30 Statement on sustainability due diligence SFDR ESRS 2 SBM-1 40 (d) i Involvement in activities related to fossil fuel activities SFDR, Pillar 3, Benchmark regulation Not relevant ESRS 2 SBM-1 40 (d) ii Involvement in activities related to chemical production SFDR, Benchmark regulation Not relevant ESRS 2 SBM-1 40 (d) iii Involvement in activities related to controversial weapons SFDR, Benchmark regulation Not relevant ESRS 2 SBM-1 40 (d) iv Involvement in activities related to cultivation and production of tobacco Benchmark regulation Not relevant ESRS E1-1 14 Transition plan to reach climate neutrality by 2050 EU Climate Law ESRS E1-1 16 (g) Undertakings excluded from Paris-aligned Benchmarks Pillar 3, Benchmark regulation ESRS E1-4 34 GHG emission reduction targets SFDR, Pillar 3, Benchmark regulation ESRS E1-5 38 Energy consumption from fossil sources disaggregated by sources (only high climate impact sectors) SFDR ESRS E1-5 37 Energy consumption and mix SFDR ESRS E1-5 40-43 Energy intensity associated with activities in high climate impact sectors SFDR ESRS E1-6 44 Gross Scope 1, 2, 3 and Total GHG emissions SFDR, Pillar 3, Benchmark regulation ESRS E1-6 53-55 Gross GHG emissions intensity SFDR, Pillar 3, Benchmark regulation ESRS E1-7 56 GHG removals and carbon credits EU Climate Law Not stated (phase-in) ESRS E1-9 66 Exposure of the benchmark portfolio to climate- related physical risks Benchmark regulation ESRS E1-9 66 (a) Disaggregation of monetary amounts by acute and chronic physical risk Pillar 3 ESRS E1-9 66 (c) Location of significant assets at material physical risk Pillar 3 ESRS E1-9 67 (c) Breakdown of the carrying value of its real estate assets by energy-efficiency classes Pillar 3 ESRS E1-9 69 Degree of exposure of the portfolio to climate- related opportunities Benchmark regulation ESRS E2-4 28 Amount of each pollutant listed in Annex II of the E- PRTR Regulation emitted to air, water and soil SFDR ESRS E3-1 9 Water and marine resources SFDR Not material ESRS E3-1 13 Dedicated policy SFDR Not material ESRS E3-4 28 (c) Total water recycled and reused paragraph SFDR Not material Disclosure requirement Data point Description Regulation Section (state if not material) argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Appendix 375

ESRS E3-4 29 Total water consumption in m 3 per net revenue on own operations SFDR Not material ESRS 2 SBM-3 - E4 paragraph 16 (a) i 16 (a) i SFDR Not material ESRS 2 SBM-3 - E4 paragraph 16 (b) 16 (b) SFDR Not material ESRS 2 SBM-3 - E4 paragraph 16 (c) 16 (c) SFDR Not material ESRS E4-2 24 (b) Sustainable land / agriculture practices or policies SFDR Not material ESRS E4-2 24 (c) Sustainable oceans / seas practices or policies SFDR Not material ESRS E4-2 24 (d) Policies to address deforestation SFDR Not material ESRS 2 SBM3 14 (g) Risk of incidents of child labour SFDR ESRS S1-1 20 Human rights policy commitments SFDR ESRS S1-1 21 21 Sustainability due diligence policies on issues addressed by the fundamental International Labor Organisation Conventions 1 to 8 Pillar 3 ESRS S1-1 22 Processes and measures for preventing trafficking in human beings SFDR ESRS S1-1 23 Workplace accident prevention policy or management system SFDR ESRS S1-3 32 (c) Grievance/complaints handling mechanisms SFDR ESRS S1-14 88 (b), (c) Number of fatalities and number and rate of work- related accidents SFDR, Pillar 3 ESRS S1-14 88 (e) Number of days lost to injuries, accidents, fatalities or illness SFDR ESRS S1-16 97 (a) Unadjusted gender pay gap SFDR, Pillar 3 ESRS S1-16 97 (b) Excessive CEO pay ratio SFDR ESRS S1-17 103 (a) Incidents of discrimination SFDR ESRS S1-17 104 (a) Non-respect of UNGPs on Business and Human Rights and OECD Guidelines SFDR, Benchmark regulation ESRS 2 SBM-3 – S2 11 (b) Significant risk of child labour or forced labour in the value chain SFDR Not material ESRS S2-1 17 Human rights policy commitments SFDR Not material ESRS S2-1 18 Policies related to value chain workers SFDR Not material ESRS S2-1 Non-respect of UNGPs on Business and Human Rights principles and OECD guidelines paragraph 19 19 Non-respect of UNGPs on Business and Human Rights principles and OECD guidelines SFDR, Benchmark regulation Not material ESRS S2-1 19 Due diligence policies on issues addressed by the fundamental International Labor Organisation Conventions 1 to 8 Benchmark regulation Not material ESRS S2-4 36 Human rights issues and incidents connected to its upstream and downstream value chain SFDR Not material ESRS S3-1 16 Human rights policy commitments SFDR Not material ESRS S3-1 17 Non-respect of UNGPs on Business and Human Rights, ILO principles or OECD guidelines SFDR, Benchmark regulation Not material Disclosure requirement Data point Description Regulation Section (state if not material) argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Appendix 376

ESRS S3-4 36 Human rights issues and incidents SFDR Not material ESRS S4-1 16 Policies related to consumers and end-users SFDR ESRS S4-1 17 Non-respect of UNGPs on Business and Human Rights and OECD guidelines SFDR, Benchmark regulation ESRS S4-4 35 Human rights issues and incidents SFDR ESRS G1-1 10 (b) United Nations Convention against Corruption SFDR ESRS G1-1 10 (d) Protection of whistleblowers SFDR ESRS G1-4 24 (a) Fines for violation of anti-corruption and anti-bribery laws SFDR, Benchmark regulation ESRS G1-4 24 (b) Standards of anti-corruption and anti-bribery SFDR Disclosure requirement Data point Description Regulation Section (state if not material) 7.8.2 SASB SASB Table HC-BP-000.A Number of clinical trial patients treated. 1052 HC-BP-000.B Number of drugs in research and development (Phase 1-3). 3 (Phase 1: 0 Phase 2: 2 Phase 3: 1) HC-BP-000.B Number of drugs in portfolio. 7 HC-BP-210a.1 Discussion, by region, of management process for ensuring quality and patient safety during clinical trials. We ensured the continuous monitoring of the safety profile of our investigational products and ensured compliance with adverse event reporting to health authorities worldwide. We also ensured supply to patients on clinical trials and have had no supply disruption. HC-BP-210a.2 Number of inspections related to clinical trial management and pharmacovigilance that resulted in (2) regulatory or administrative actions taken against the entity. 0 HC-BP-210a.2 Number of inspections related to clinical trial management and pharmacovigilance that resulted in (1) entity voluntary remediation. 0 SASB Reference Metric description argenx response argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Appendix 377

HC-BP-240a.2 List of products on the WHO List of Prequalified Medicinal Products as part of its Prequalification of Medicines Programme (PQP). 0 HC-BP-250a.1 Products listed in public medical product safety or adverse event alert databases. Vyvgart is listed in the European Medicines Agency’s list of medicinal products under additional monitoring HC-BP-250a.2 Number of fatalities associated with products. 0 HC-BP-250a.3 (2) Total units recalled. 0 HC-BP-250a.3 (1) Number of recalls issued. 0 HC-BP-250a.4 Total amount of product accepted for takeback, reuse, or disposal. 0.051 HC-BP-250a.5 Number of enforcement actions taken in response to violations of good manufacturing practices (GMP) or equivalent standards, by type. 0 HC-BP-260a.1 Description of methods and technologies used to maintain traceability of products throughout the supply chain and prevent counterfeiting. To the extent this is required by national legislation (e.g. DSCSA in U.S. and FMD in EU), all commercial argenx products are serialized at unit, case and pallet (aggregated level). On each sellable unit a unique random serial number is printed as well as a product code, lot number and expiration date, both in human readable format and encoded in a 2D data matrix barcode. All products are tamper-evident sealed and shipping systems and trucks are sealed with numbered seals during transit to prevent product tampering. During batch manufacturing (outsourced to CMOs), all processing steps are documented to have a full processing history and lot genealogy of consumed raw materials, which is also maintained in the CMO’s quality system. An ERP system is used to provide full lot genealogy and traceability of intermediates and finished products owned by argenx, located at its CMOs, third party warehouses and 3PL. For downstream distribution, the entity can rely on the distributors and wholesaler’s systems to trace product to the end customer. A yearly mock up recall also takes place for which traceability is tested. argenx has a procedure in place to ensure that all suspicions of falsified or counterfeit medicine are reported in a consistent manner. When a case is reported, the impacted batches will be separated and quarantined, an investigation will be performed and the impacted stakeholders in the supply chain will be informed, as well as the relevant competent authorities in case of confirmed counterfeit product. Market actions will be taken in consultation with the competent authority. SASB Reference Metric description argenx response argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Appendix 378

HC-BP-260a.2 Discussion of process for alerting customers and business partners to potential or known risks associated with counterfeit products. argenx has a procedure in place to ensure that all suspicions of falsified or counterfeit medicine are reported in a consistent manner. When a case is reported, the impacted batches will be separated and quarantined, an investigation will be performed and the impacted stakeholders in the supply chain will be informed, as well as the relevant competent authorities in case of confirmed counterfeit product. Market actions will be taken in consultation with the competent authority. HC-BP-260a.3 Number of actions that led to raids, seizure, arrests, or filing of criminal charges related to counterfeit products. 0 HC-BP-270a.1 Total amount of monetary losses as a result of legal proceedings associated with false marketing claims. No monetary losses as a result of legal proceedings. No documentation to provide as there were no losses. HC-BP-270a.2 Description of code of ethics governing promotion of off- label use of products. argenx’s Code of Ethics and related policies strictly govern the promotion of its products, ensuring compliance with all applicable laws, regulations, and ethical standards. It explicitly prohibits the promotion of off-label use of products. All marketing and promotional activities must align with approved product indications and regulatory guidelines. SASB Reference Metric description argenx response argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Appendix 379

HC-BP-330a.1 Discussion of talent recruitment and retention efforts for scientists and research and development staff. Talent Attraction argenx takes a value- and team-based approach to talent acquisition. This means we not only prioritize the requisite knowledge and skills when hiring, but also seek alignment with our cultural pillars to strengthen the organization. In other words, we hire for attitude and train for skill. This commitment to building cohesive and value-driven teams is essential for fostering innovation and sustainability. Recognizing the importance of diversity in our global teams, we actively seek the greatest talent from around the world. Our leaders’ continuous commitment helps us to attract the brightest minds with equally collaborative spirits, in every function from R&D to legal. All argonauts serve as ambassadors of our culture, which is the best recruiting tool to rely on the broad network of our veteran teams. This approach helps to ensure that our workforce reflects the global nature of the biotech industry and brings diverse perspectives to drive our innovation forward. Employee Development & Retention We know our mission is a large part of why people choose to work at argenx; we also know that our mission alone will not keep top talent satisfied forever. We draw on our cultural pillars of innovation, co-creation, empowerment, excellence and humility to create an environment in which argonauts not only do their best — their best can become even better. The scientific breakthroughs we pursue on behalf of patients do not happen quickly — it can take years of research to bring an effective therapy to market. As a result, we need argenx to be a place where people want to work for the long-term, and where they can continue to grow their skills and impact over time. Throughout 2024, we deepened several initiatives to cultivate our team’s leadership skills while living up to our values. -Essentials Leadership Development Program: We aim to help all rising leaders appreciate the full complexities of the biotech business and how we can continue to improve decision-making across the company. - Personal Development Plans (PDP): One of our core operating principles is that we develop our business by developing our people. It’s critical that argonauts share a continuous growth mindset in pursuit of innovation, which means we must attract people who want to take on new challenges. We take a strengths-based approach to PDPs that allows argonauts to flex and grow within their current roles, while opening doors to new roles with new experiences on their argenx journey. 99% of our people completed the PDP e- learning. - Feedback training based on SBI (Situation Behaviour Impact) was launched in 2024. 76% of argonauts were trained. - Communications Council: To live up to our promise of “one company, one purpose,” we bring together argonauts spanning teams, management levels and geographies on our Communications Council. This group embodies our spirit of co-creation and collaboration by reviewing company-wide internal communication as well as social media programs and provides a channel for candid feedback and conversation. - Culture Labs: We also bring argonauts together for in-person and virtual Culture Lab sessions. In 2024, 1246 argonauts took part in at least one of these sessions to deepen our collective understanding of our cultural pillars. Our goal is to continue scaling and extending with a focus on our Operating Principles and on Innovation. HC-BP-510a.1 Total amount of monetary losses as a result of legal proceedings associated with corruption and bribery. 0 SASB Reference Metric description argenx response argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Appendix 380

HC-BP-510a.2 Description of code of ethics governing interactions with health care professionals. argenx’s Code of Ethics governs interactions with healthcare professionals (HCPs) to ensure compliance with all applicable laws, regulations, and ethical standards. The code requires that all engagements with HCPs are conducted with transparency, integrity, and respect for their independence. It prohibits any improper influence or incentives, ensuring that decisions regarding patient care remain unbiased and in the best interest of patients. Interactions are guided by policies such as the Interactions with HCC Policy, which includes provisions on fair market value compensation for services, appropriate documentation, and adherence to anti-bribery and anti-corruption laws. Training programs, regular monitoring, and internal audits reinforce these standards. HC-BP-240b.3 Percentage change in: (1) list price and (2) net price of product with largest increase compared to previous reporting period (1) Percentage change in list price: 2% (2) Percentage change in net price: 1% HC-BP-240b.2 Percentage change in: (1) weighted average list price and (2) weighted average net price across product portfolio compared to previous reporting period (1) Percentage change in weighted average list price: -1% (2) Percentage change in weighted average net price: -5% SASB Reference Metric description argenx response argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Appendix 381

Glossary 8.1 Cross Reference table for annual reporting requirements 383 8.2 Management Confirmations 384 8.3 Definitions 385 argenx Annual Report 2024 382 8

8 Glossary 8.1 Cross Reference table for annual reporting requirements The following list of cross references identifies where each item required for us to disclose in our yearly financial report can be found in this Annual Report. Source of Requirement Topic Location Article 2:391 DCC, RJ 400, RJ 405 Report on the Company’s activities Shareholder Letter Presentation of the Group Corporate structure General description of the Company and its Share Capital Board of Directors report Corporate Governance Primary risks and uncertainties Risk Factors Risk appetite & control Risk Appetite & Control Analysis of financial condition and results Operating and Financial Review Information on research and development activities Our Products and Product Candidates Collaborations and licenses Forward looking paragraph 2025 Outlook Compensation statements and remuneration report Remuneration Report and Compensation Statement RJ 430 Key figures, ratios etc. Operating and Financial Review Article 2:392 DCC/RJ 410 Auditor’s opinion Attached to the 2024 Annual Report included herein Articles of association on the distribution of profits Articles of Association on Profits, distributions and losses List of subsidiaries Company Profile – Group Structure Decree on contents of board report (Besluit inhoud bestuursverslag) Article 2:391 sub 5 DCC Corporate governance code comply-or-explain Dutch Corporate Governance Code, “Comply or Explain” Main elements of financial management & control systems in connection with the company’s financial reporting Financial Risks and Controls Functioning of the general meeting General Meeting and Voting Rights Composition and functioning of the board of directors and its committees Board of Directors Non-Executive Directors Article 10 Decree Takeover Directive (Besluit artikel 10 overnamerichtlijn), Article 2:391 sub 5 DCC Capital structure General description of the Company and its Share Capital Board of Directors Change of Control Article 2:391 sub 5 DCC Principal shareholders Share Classes and Principal Shareholders Particular shareholder rights and limitations thereof General Meetings and Voting Rights Procedure for appointment of board members Management Structure Procedure for amending the articles of association Amendment of Articles of Association Authority of the board of directors to issue or acquire shares Issue of Shares Acquisition of Shares in our Capital Material arrangements, to which the company is a party, in relation to a public offer Anti-Takeover Provisions RJ = Guidelines on Annual Reporting (Richtlijnen voor de Jaarverslaggeving) argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Cross Reference table for annual reporting requirements 383

8.2 Management Confirmations With due regard to best practice provision 1.4.3 of the DCGC, we confirm that: i. This Annual Report provides sufficient insights into any failings in the effectiveness of the internal risk management and control systems, with regard to the risks as referred to in best practice provision 1.2.1 of the DCGC, as is further substantiated in Section 2 “Risk Factors” and Section 3 “Corporate Governance”. ii. The risk- and control systems described herein, particularly in paragraph 3.9.5 “Financial Risks and Controls” provide reasonable assurance that the financial reporting does not contain any material inaccuracies; iii. We confirm that we expect that our existing cash and cash equivalents and current financial assets will enable us to fund our operating expenses and capital expenditure requirements through at least the next 12 months. On the basis of the current state of affairs, it is justified that the financial reporting is prepared on a going concern basis; and iv. This Annual Report, particularly Section 2 “Risk Factors” states the material risks, as referred to in best practice provision 1.2.1 and the uncertainties, to the extent that they are relevant to the expectation of our continuity for the period of 12 months after the preparation of this Annual Report. The aforementioned statement does not in any way limit the relevance or applicability of the Risk Factors set out in this Annual Report to the aforementioned period of 12 months. Signed on behalf of argenx SE argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Management Confirmations 384
8.3 Definitions The following explanations are intended to assist the general reader to understand certain terms used in this Annual Report. The definitions set out below apply throughout this Annual Report, unless the context requires otherwise. 2021 Remuneration Policy 2021 Remuneration policy 2024 20-F Form 20-F for the year ended December 31, 2024 2024 General Meeting annual General Meeting held on May 7, 2024 2024 Remuneration Report the 2024 remuneration report and compensation statement 2025 General Meeting the annual General Meeting that will take place on May 27, 2025 2025 Remuneration Policy the Company’s draft 2025 remuneration policy, which will be submitted for approval at the 2025 General Meeting AAV ANCA-associated vasculitis AbbVie AbbVie, Inc. AbbVie Collaboration Agreement the collaboration agreement with AbbVie, Inc. to develop and commercialize ARGX-115 (ABBV-151) as a cancer immunotherapy against the novel target GARP ACA the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 Accounting Directive Directive 2013/34/EU AChR anti-acetylcholine receptor AChR-AB+ AChR antibody positive ADCC antibody-dependent cell-mediated cytotoxicity ADSs American depositary shares AFM the Dutch Authority for the Financial Markets (Stichting Autoriteit Financiële Markten) AKS the U.S. federal Anti-Kickback Statute ALS amyotrophic lateral sclerosis AMP average manufacturer price AMR antibody-mediated rejection Annual Report this annual report argenx or the Company argenx SE argenx Activities the argenx activities identified as core activities for the purposes of the EU Taxonomy Legal Framework, such activities being research and development and marketing of pharmaceutical products and wholesale thereof Article 8 CDR Commission Delegated Regulation (EU) 2021/2178 of July 6, 2021 supplementing Regulation (EU) 2020/852 of the European Parliament and of the Council by specifying the content and presentation of information to be disclosed by undertakings subject to Articles 19a or 29a of Directive 2013/34/EU concerning environmentally sustainable economic activities, and specifying the methodology to comply with that disclosure obligation Articles of Association our current articles of association Asset Development Agreement the asset development agreement entered into with IQVIA ASyS anti-synthetase syndrome Audit and Compliance Committee the audit and compliance committee of the Board of Directors AV anti-neutrophil cytoplasmic antibody-associated Vasculitis B-cell B-lymphocyte BioWa BioWa, Inc BIS Business Information Systems BLA biologics license application Board By-Laws the rules adopted by our Board of Directors that describe the procedure for holding meetings of the Board of Directors, for the decision-making by the Board of Directors and the Board of Directors’ operating procedures Board of Directors consisting of our Executive Director(s) and our Non-Executive Directors. BPCIA the U.S. Biologics Price Competition and Innovation Act Term Definition argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Definitions 385

Broteio Broteio Pharma B.V. Broteio Agreement collaboration agreement entered into with Broteio C2 component 2 CapEx capital expenditure CBAs collective bargaining agreement CEO chief executive officer CFO chief financial officer cGMPs current good manufacturing practices CHMP Committee for Medicinal Products for Human Use Chugai Chugai Pharmaceutical Co., Ltd. CIDP chronic inflammatory demyelinating polyneuropathy Climate Delegated Act Commission Delegated Regulation (EU) 2021/2139 of June, 4 2021 supplementing Regulation (EU) 2020/852 of the European Parliament and of the Council by establishing the technical screening criteria for determining the conditions under which an economic activity qualifies as contributing substantially to climate change mitigation or climate change adaptation and for determining whether that economic activity causes no significant harm to any of the other environmental objectives CMOs contract manufacturing organizations CMS Congenital myasthenic syndrome or Centers for Medicare & Medicaid, as the context dictates Code of Conduct our Code of Business Conduct and Business Ethics COO chief operating officer COMP European Medicines Authority’s Committee for Orphan Medicinal Products CRmin minimal dose of steroids CRO contract research organization CSRD Directive (EU) 2022/2464 of the European Parliament and of the Council of December, 14 2022 amending Regulation (EU) No 537/2014, Directive 2004/109/EC, Directive 2006/43/ EC and Directive 2013/34/EU, as regards corporate sustainability reporting CTA clinical trial application CTD Clinical Trials Directive 2001/20/EC CTR EU Regulation No 536/2014 of the European Parliament and of the Council of April, 16 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC (clinical trials regulation) DCC Dutch Civil Code (Burgerlijk Wetboek) DCGC the Dutch Corporate Governance Code 2022 Deloitte Deloitte Accountants B.V. DFSA Dutch Financial Supervision Act (Wet op het financieel toezicht) DGF delayed graft function DHS dehydrated hereditary stomatocytosis Dividend Received Deduction deduction of 100% of the gross dividend received from taxable income DM dermatomyositis DSA donor specific antibodies e-Privacy Directive Directive 2002/58/EC of the European Parliament and of the Council of July 12, 2002 ECL expected credit loss EEA European Economic Area Elektrofi Elektrofi, Inc. Elektrofi Agreement collaboration and license agreement entered into with Elektrofi EMA European Medicines Authority ENHANZE® ENHANZE technology ENHANZE® License Agreement in-license agreement with Halozyme, Inc. Enterprise Chamber the Dutch Enterprise Chamber of the Amsterdam Court of Appeal (Ondernemingskamer van het Gerechtshof te Amsterdam) Environmental Delegated Act Delegated Regulation (EU) 2023/2486 of June 27, 2023 Term Definition argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Definitions 386

Equity Incentive Plan the equity incentive plan as adopted by our Board of Directors on December 18, 2014, which was approved by the General Meeting on May 13, 2015, and amended by the General Meeting on April 28, 2016, and November 25, 2019, and the Board of Directors on December 18, 2019, November 5, 2020, December 15, 2021 and on February 27, 2023 and on February 28, 2024. ESG environmental, social and corporate governance EU European Union EU-IFRS IFRS® Accounting Standards (IFRS) as issued by the IASB and adopted by the EU EU Taxonomy Legal Framework the EU Taxonomy Regulation, the Climate Delegated Act, the Environmental Delegated Act, the Article 8 CDR and ancillary legislation currently applicable to us EU Taxonomy Regulation Regulation (EU) 2020/852 of the European Parliament and of the Council of June 18, 2020 on the establishment of a framework to facilitate sustainable investment, and amending Regulation (EU) 2019/2088 Euronext Brussels the regulated market operated by Euronext Brussels SA/NV, a regulated market within the meaning of Directive 2014/65/EU of the European Parliament and of the Council of May 15, 2014, on markets in financial instruments amending Council Directives 2004/39/ EC, Directive 85/611/EEC, 93/6/EEC and Directive 2000/12/EC of the European Parliament and of the Council and repealing Council Directive 93/22/EEC (MiFID II) Exchange Act the U.S. Securities Exchange Act of 1934, as amended Executive Director an executive director in the Board of Directors Fc antibody region interacting with cell surface Fc receptors FCP Federal Ceiling Price FcRn neonatal Fc receptor FDA U.S. Food and Drug Administration FDCA the U.S. Federal Food, Drug, and Cosmetic Act FDORA Food and Drug Omnibus Reform Act FSS Federal Supply Schedule FTT Financial Transaction Tax Fujifilm FUJIFILM Diosynth Biotechnologies Denmark ApS GARP glycoprotein A repetitions predominant GARP Agreement a collaboration and exclusive product license agreement with UCL and its technology transfer company Sopartec GARP License exclusive, worldwide commercial in-license for use of certain GARP-related intellectual property rights owned by UCL and the Ludwig Institute for Cancer Research GCPs good clinical practices GDPR Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016, on the protection of natural persons with regard to the processing of personal data and on the free movement of such data General Meeting any general meeting of shareholders of argenx SE (i.e., any annual general meeting and any extraordinary general meeting) Genmab Genmab A/S Genpharm Genpharm Services FZ-LLC Genpharm Agreement partnership agreement entered into with Genpharm Services FZ-LLC GloBE Rules model rules in respect of Pillar Two GLPs good laboratory practices gMG generalized myasthenia gravis GMP good manufacturing practice Greater China Mainland China, Hong Kong, Taiwan and Macau Group argenx SE together with its subsidiaries Halozyme Halozyme Therapeutics, Inc. Handok Handok Inc. Handok Agreement an VGART commercial and distribution agreement entered into with Handok Hatch-Waxman Act the U.S. Drug Price Competition and Patent Term Restoration Act of 1984 HHS U.S. Department of Health and Human Services HIPAA the U.S. federal Health Insurance Portability and Accountability Act of 1996 IASB International Accounting Standards Board IFRS IFRS® Accounting Standards Term Definition argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Definitions 387

IgA Immunoglobulin A IgD Immunoglobulin D IgG Immunoglobulin G IgM Immunoglobulin M IIP immunology innovation program IMM irreversible morbidity or mortality IMNM immune-mediated necrotizing myopathy IND investigational new drug IQVIA IQVIA LTD IRA Inflation Reduction Act IRB institutional review board ISMS Information Security and Management System ISTs immunosuppressive therapies ITC Belgian Income Tax Code ITP immune thrombocytopenia IV intravenous IVIg intravenous IgG Johnson & Johnson Johnson & Johnson Innovation, Inc. KPI key performance indicator LEI European legal entity identifier number LEO Pharma Pharma LEO Pharma A/S LEO Pharma Collaboration Agreement collaboration agreement with LEO Pharma A/S LN lupus nephritis Lonza Lonza Sales AG LUMC Leiden University Medical Center MA marketing authorization MAA marketing authorization application mAb monoclonal antibody MAH marketing authorization holder Mainland China mainland China MADs multiple ascending doses MAR Regulation (EU) No 596/2014 of the European Parliament and of the Council of April 2014 on market abuse (market abuse regulation) and repealing Directive 2003/6/EC European Parliament and of the Council and Commission Directives 2003/124/EC, 2003/ EC and 2004/72/EC, and the rules and regulations promulgated pursuant thereto Medison Medison Pharma Ltd. Medison Agreement exclusive distribution agreement with Medison Pharma Ltd. to commercialize efgartigimod in Israel Medison Multi-Regional Agreement multi-regional agreement with Medison Pharma Ltd. to commercialize efgartigimod in 14 countries MG myasthenia gravis MG-ADL Myasthenia Gravis Activities of Daily Living MHLW Ministry of Health, Labour and Welfare MHRA Medicines and Healthcare products Regulatory Agency MMN multifocal motor neuropathy MN membranous nephropathy MSE minimal symptom expression Multi-Product License a non-exclusive multi-product in-license agreement with Lonza MuSK muscle-specific kinase Myositis idiopathic inflammatory myopathies Nasdaq the Nasdaq Global Select Market Nasdaq Listing Rules the listing rules of the Nasdaq Global Market NDA new drug application NEO named executive officer Term Definition argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Definitions 388

NFRD Directive 2014/95/EU of the European Parliament and the Council of 22 October 2014 amending Directive 2013/34/EU as regards disclosure of non-financial and diversity information by certain large undertakings and groups NHI National Health Insurance NK natural killer NMJ neuro muscular junction Non-Executive Director a non-executive director in the Board of Directors Non-FAMP Non-Federal Average Manufacturer Price NRDL National Reimbursement Drug List NYU New York University NYU and LUMC Agreement collaboration and exclusive license agreements with NYU Langone Health and LUMC OCI other comprehensive income OFPs organizations for financing pensions OLE open-label extension OpEx operating expenditure PAA pre-approval access program PD pharmacodynamic PDUFA Prescription Drug User Fee Act PFIC passive foreign investment company Pharmaceutical and Medical Devices Act the Act on Securing Quality, Efficacy and Safety of Pharmaceuticals and Medical Devices PHSA the U.S. Public Health Service Act PIL Code Belgian Code of private international law Pillar Two the project, worked on by the OECD in recent years, aimed at reforming the international tax system by, among other matters ensuring large multinational enterprises pay a minimum level of tax in each of the jurisdictions in which they operate Pillar Two Directive Directive (EU) 2022/2523 on ensuring a global minimum level of taxation for multinational enterprise groups and large-scale domestic groups in the Union PK pharmacokinetic PMDA Pharmaceuticals and Medical Devices Agency (Japan) POC proof-of-concept POTELLIGENT® License Agreements non-exclusive license agreements for POTELLIGENT® CHOK1SV with BioWa and Lonza POTS post-COVID-19 Postural Orthostatic Tachycardia Syndrome Post-COVID-19 PREA Pediatric Research Equity Act of 2003, as amended Product Liability Directive Directive (EU) 2024/2853 PSU performance share unit PV pemphigus vulgaris PVAS pemphigus vulgaris activity score Relevant Regulatory Authorities the MHRA, EMA, FDA, MHLW Reference Date 30 calendar days preceding the 15th day of the month in which the grant of stock option occurs Remuneration and Nomination Committee remuneration and nomination committee of the Board of Directors REMS risk evaluation and mitigation strategy rHuPH20 recombinant human hyaluronidase PH20 Roche F. Hoffman-La Roche AG RSUs restricted stock units sBLA supplemental Biologics License Application SC subcutaneous SEC the U.S. Securities and Exchange Commission Securities Act the U.S. Securities Act of 1933, as amended Senior Management Team the Company’s senior management team consisting of our CEO and senior personnel reporting directly to the CEO SjD sjögren’s disease Term Definition argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Definitions 389

SLE systemic lupus erythematosus SMA spinal muscular atrophy Sopartec Sopartec S.A. System Lonza Sales AG’s proprietary glutamine synthetase gene expression system known as GS Xceed® Taxonomy Environmental Objectives the six objectives included in the EU Taxonomy Regulation, being: (i) climate change mitigation, (ii) climate change adaption, (iii) sustainable use of protection of water and marine resources, (iv) transition to a circular economy, (v) pollution prevention, and (vi) protection and restoration of biodiversity and ecosystems TED thyroid eye disease TIS total improvement score Transparency Directive Directive 2004/109/EC of the European Parliament and of the Council of December 15, 2004, on the harmonization of transparency requirements in relation to information about issuers whose securities are admitted to trading on a regulated market and amending Directive 2001/34/EC and the rules and regulations promulgated pursuant thereto, as amended by various directives including 2013/50/EU UCL Université Catholique de Louvain UK the United Kingdom UK GDPR legal framework adopted by the United Kingdom substantially equivalent to the Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016, on the protection of natural persons with regard to the processing of personal data and on the free movement of such data U.S. the United States of America USPTO the United States Patent and Trademark Office U.S. Tax Treaty Convention between the Netherlands and the U.S. for the avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to Taxes and Income, dated December 18, 1992 as amended by the protocol of March 8, 2004 UT Agreement an exclusive in-license with the Board of Regents of the University of Texas System UT BoR Board of Regents of the University of Texas System VIB Agreement collaboration agreement entered into with VIB vzw V-regions antibody variable regions VYVDURA VYVDURA® VYVGART VYVGART® (efgartigimod alfa) VYVGART HYTRULO VYVGART HYTRULO™ VYVGART SC VYVGART subcutaneous (efgartigimod alfa + hyaluronidase qvfc) we, us or our argenx SE together with its wholly-owned subsidiaries and, as applicable, its former wholly-owned subsidiaries Zai Lab Zai Lab Ltd Zai Lab Agreement collaboration agreement with Zai Lab Ltd, relating to an exclusive out-license for the development and commercialization of efgartigimod in Greater China Zai Lab Payments $75.0 million upfront payment under the collaboration with Zai Lab Ltd in the form of 568,182 newly issued Zai Lab shares calculated at a price of $132.0 per share, a $75.0 million guaranteed non-creditable, non-refundable development cost-sharing payment and a $25.0 million milestone payment in connection with FDA approval of VYVGART Term Definition argenx Group Risk Factors Corporate Governance Share Capital Financial Review Financial Statements Non-Financial Information argenx Annual Report 2024 Definitions 390

Contact us via argenx.com/contact-us You can find the annual report 2024 online at reports.argenx.com/2024






























































































































































































































































































































































































