FALSE000168954800016895482025-10-162025-10-16
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 16, 2025
PRAXIS PRECISION MEDICINES, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
| Delaware |
001-39620 |
47-5195942 |
|
(State or other jurisdiction
of incorporation)
|
(Commission
File Number)
|
(I.R.S. Employer
Identification No.)
|
Praxis Precision Medicines, Inc.
99 High Street, 30th Floor
Boston, Massachusetts 02110
(Address of principal executive offices, including zip code)
(617) 300-8460
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
|
|
|
|
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
|
|
|
|
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
|
|
|
|
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
|
|
|
|
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Title of each class |
|
Trade
Symbol(s)
|
|
Name of each exchange
on which registered
|
| Common Stock, $0.0001 par value per share |
|
PRAX |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item. 7.01. Regulation FD Disclosure.
On October 16, 2025, Praxis Precision Medicines, Inc. (the “Company”) published a corporate presentation announcing topline results from its Essential3 program of ulixacaltamide. The presentation is available in the “Investors + Media” portion of the Company’s website at investors.praxismedicines.com and a copy is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in Item 7.01 of this Form 8-K and Exhibit 99.1 attached hereto shall not be deemed filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall any of it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 8.01. Other Events.
On October 16, 2025, the Company announced positive topline results for the Phase 3 Essential3 program of ulixacaltamide in essential tremor (“ET”).
About the Essential3 Program Trial Design
The Essential3 Phase 3 program (NCT06087276) included two simultaneously enrolled studies utilizing a decentralized design conducted within the United States, where participants were allocated to the studies in a 2:1 blinded randomization (Study 1:Study 2).
Study 1 was a double-blind, parallel design, placebo-controlled study that enrolled 473 patients randomized 1:1 to receive either ulixacaltamide or placebo for 12 weeks. The primary endpoint was the change from baseline in mADL11 at Week 8.
Study 2 was a stable-responder randomized withdrawal study that enrolled 238 patients to receive ulixacaltamide for 8 weeks. Patients who improved by 3 points in the mADL11 from baseline were then randomized to receive either placebo or to continue receiving ulixacaltamide for an additional 4 weeks. The primary endpoint evaluated the proportion of patients who maintained response receiving ulixacaltamide versus placebo.
There were two additional pre-specified hypotheses evaluating combinations of arms in Study 1 and Study 2 using the change in mADL11 at Week 8. Hypothesis 3 compared the ulixacaltamide arms of Study 1 and Study 2 with the placebo arm of Study 1, and Hypothesis 4 compared the ulixacaltamide arm of Study 2 with the placebo arm of Study 1.
Key secondary endpoints in Studies 1 and 2 assessed the rate of disease improvement (slope of mADL11 change), the Patient Global Impression of change (“PGI-C”) and Clinical Global Impression of severity (“CGI-S”).
Summary of Essential3 Program Results
Study 1: Placebo-controlled Parallel Group Study Topline Efficacy Results
In Study 1, there was a statistically significant and clinically meaningful 4.3 point mean improvement in the mADL11 score at Week 8 (p<0.0001). The effect was sustained from Week 2 throughout the 12-week dosing period. All key secondary endpoints achieved statistical significance.
|
|
|
|
|
|
|
|
|
|
|
|
| Results Summary |
mITT population |
Ulixacaltamide
(n=199)
|
Placebo
(n=233)
|
p-value |
Primary Endpoint |
Day 56 CFB mADL11 |
-4.3 |
-1.7 |
<0.0001 |
Key Secondary Endpoints |
|
Rate of Disease Improvement, Baseline to Day 56 mADL11 |
-4.0 |
-1.7 |
<0.0001 |
PGI-C Day 56 |
3.3 |
3.9 |
<0.0001 |
CGI-S CFB to Day 56 |
-0.41 |
-0.12 |
0.0007 |
Select Sensitivity Analyses |
Imputation of missing data for primary analysis* |
-3.3 |
-1.6 |
0.0026 |
Day 84 CFB mADL11** |
-3.4 |
-1.9 |
0.0049 |
* Results from a pre-specified delta-adjusted tipping-point analysis remained statistically significant at the maximum tested shift (Δ = 2.5; p = 0.0026), exceeding the ~½ SD robustness criterion of Ratitch et al. (2013) and confirming strong resilience of the primary endpoint to non-MAR assumptions.
**Primary endpoint at the time of interim analysis (assessed as the average of Day 77 and Day 84)
Study 2: Randomized Withdrawal Study Topline Efficacy Results
In Study 2, after blinded exposure for 8 weeks with ulixacaltamide, patients meeting the responder criteria (n=80) were then randomized to continue receiving ulixacaltamide or switch to placebo for an additional 4 weeks. 55% of patients in the ulixacaltamide arm maintained response vs 33% in the placebo group (p=0.0369, OR=2.7 CI (1.06-6.92)). The first key secondary endpoint – rate of disease improvement – achieved statistical significance, and other secondary endpoints (PGI-C, CGI-S) were numerically in favor of ulixacaltamide, but not statistically significant.
|
|
|
|
|
|
|
|
|
|
|
|
| Results Summary |
| mITT population |
Ulixacaltamide
(n=40) |
Placebo
(n=40) |
p-value |
| Primary Endpoint |
| Maintenance of Response |
55% |
33% |
0.037 |
| Key Secondary Endpoints |
|
| Rate of Disease Improvement, RW Baseline to Day 84 |
2.8 |
5.2 |
0.004 |
| PGI-C Day 84 |
3.24 |
3.67 |
0.087 |
| CGI-S Day 56 to Day 84 |
0.39 |
0.73 |
0.055 |
| Select Exploratory Endpoint |
| PGI-S Day 56 to Day 84 |
0.24 |
0.59 |
0.027 |
Combined Study 1 and Study 2 Hypotheses
Hypothesis 3 and 4 further supported the precision of the effect of ulixacaltamide versus placebo.
•For Hypothesis 3, there was a 4.3 point improvement in mADL11 at Week 8 for the combined Studies 1 and 2 ulixacaltamide groups vs Study 1 placebo (p<0.0001).
•For Hypothesis 4, there was a 4.2 point improvement in mADL11 at Week 8 for the Study 2 ulixacaltamide group vs Study 1 placebo (p<0.0001), respectively.
Safety
Ulixacaltamide was generally well tolerated over 12 weeks of treatment. The most common (≥10% patients) treatment emergent adverse events (“TEAs”) were constipation, dizziness, euphoric mood, brain fog, headache, paraesthesia and insomnia. There were no deaths and no drug-related serious adverse events. Discontinuations were primarily due to TEAEs, with the most common being dizziness and brain fog.
|
|
|
|
|
|
|
|
|
|
|
|
Overview of Adverse Events |
|
Study 1 |
Study 2 |
Category |
Ulixacaltamide (n = 233) |
Placebo
(n = 234)
|
Ulixacaltamide
(n = 231)
|
Participants with any TEAE |
221 (94.9%) |
177 (75.6%) |
209 (90.5%) |
|
Participants with:
Mild TEAEs
|
98 (42.0%) |
89 (38.0%) |
87 (37.7%) |
Moderate TEAEs |
109 (46.8%) |
78 (33.3%) |
105 (45.5%) |
Severe TEAEs |
14 (6.0%) |
10 (4.3%) |
17 (7.4%) |
Participants with any SAE* |
2 (0.86%) |
8 (3.4%) |
4 (1.73%) |
Participants with drug-related TEAEs leading to discontinuation |
63 (27.0%) |
4 (1.7%) |
65 (28.1%) |
Discontinued from the study |
83 (35.6%) |
13 (5.6%) |
88 (38.1%) |
*not related to study drug The Company has submitted a pre-NDA meeting request to the FDA with plans to submit the NDA by early 2026, upon agreement with the agency.
Corporate Updates
The Company intends to share additional data from these studies at upcoming medical conferences and peer reviewed publications.
Forward-Looking Statements
This Current Report contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws, including statements regarding the clinical development of ulixacaltamide and the anticipated timing of regulatory submissions and interactions. The forward-looking statements included in this Current Report are subject to a number of risks, uncertainties and assumptions, including, without limitation, uncertainties inherent in clinical trials, the expected timing of submission for regulatory approval or review by governmental authorities and other risks as described in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024 and its other filings with the Securities and Exchange Commission. These statements are based only on facts currently known by the Company and speak only as of the date of this Current Report. As a result, you are cautioned not to rely on these forward-looking statements and the Company undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
|
|
|
|
|
|
|
|
|
|
Exhibit
No.
|
|
Description |
|
|
|
|
|
|
|
|
|
| 104 |
|
Cover Page Interactive Data File (embedded within the inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
PRAXIS PRECISION MEDICINES, INC. |
|
|
|
|
| Date: October 16, 2025 |
By: |
|
/s/ Marcio Souza |
|
|
|
Marcio Souza |
|
|
|
Chief Executive Officer |
EX-99.1
2
e3_toplinexfinaloctober2.htm
EX-99.1
e3_toplinexfinaloctober2

Essential3 Program Topline Results October 16, 2025

2 This presentation may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, including but not limited to express or implied statements regarding the current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, , including statements regarding the estimated market for our product candidates, if approved, our development plans, our preclinical and clinical results and other future conditions, including our cash runway, and the safety, efficacy, and regulatory and clinical design or progress, potential regulatory submissions, approvals and timing thereof of any of our product candidates. Any forward-looking statements in this presentation are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this presentation, including, without limitation, risks relating to: (i) the success and timing of our ongoing clinical trials, (ii) the success and timing of our product development activities and initiating clinical trials, (iii) the success and timing of our collaboration partners’ product development activities, (iv) the timing of and our ability to obtain and maintain regulatory approval of any of our product candidates, (v) our plans to research, discover and develop additional product candidates, (vi) our ability to enter into collaborations for the development of new product candidates, (vii) our ability to establish manufacturing capabilities, and our collaboration partners’ abilities to manufacture our product candidates and scale production, (viii) our ability to meet any specific milestones set forth herein, and (ix) the potential addressable market sizes for product candidates. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. For further information regarding the risks, uncertainties and other factors that may cause differences between our expectations and actual results, you should review the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2024 filed with the Securities and Exchange Commission (“SEC”) and our other filings with the SEC. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. Forward Looking Statements

3 First positive Phase 3 program for a drug in Essential Tremor Essential3 Program – Ulixacaltamide HCl Both studies in the Essential3 Program met their primary endpoints Generally well tolerated, with no drug-related SAEs Praxis has submitted a pre-NDA meeting request to the FDA

4 No specific drugs developed for ET currently approved An estimated 7 million people in the U.S. live with ET

5 With deep gratitude to the people living with ET who participated in our clinical studies, and to all the ET families and advocates who guide our work
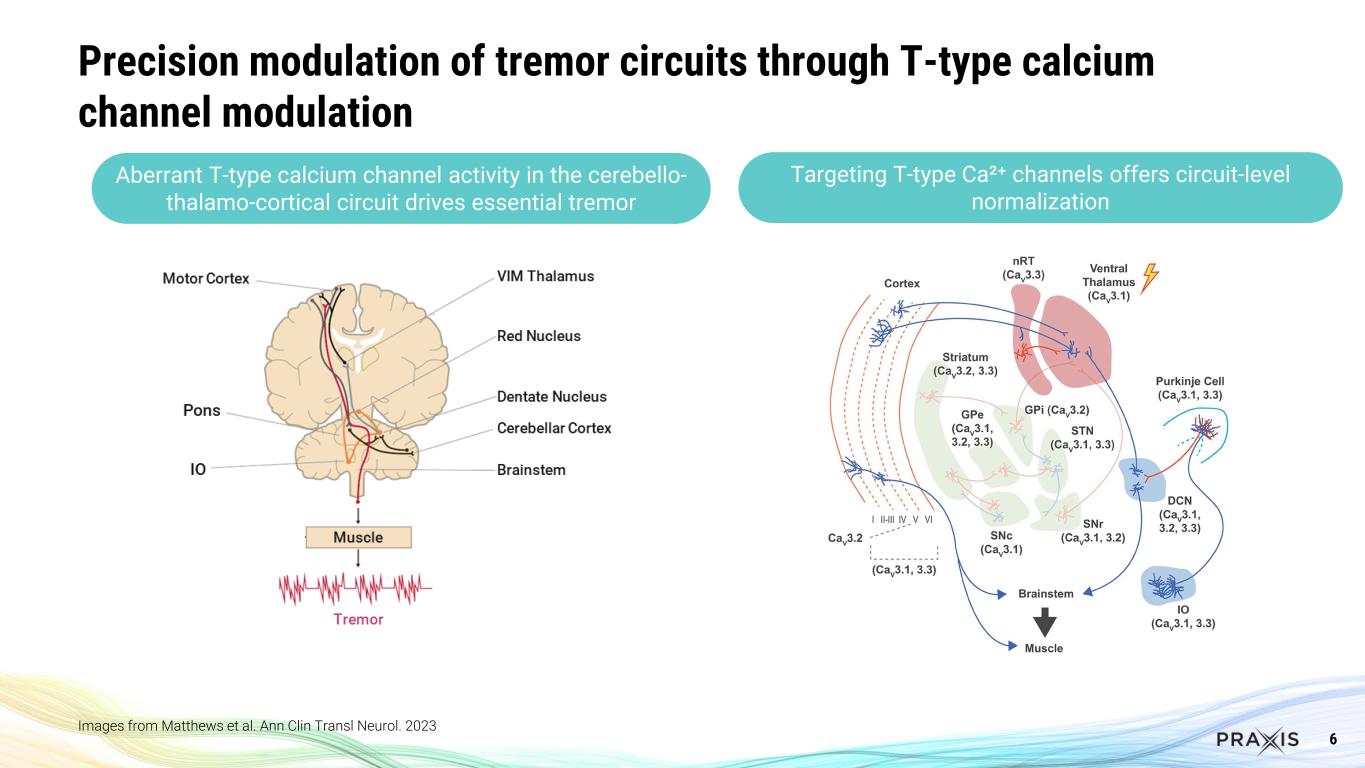
6 Precision modulation of tremor circuits through T-type calcium channel modulation Targeting T-type Ca²⁺ channels offers circuit-level normalization Aberrant T-type calcium channel activity in the cerebello- thalamo-cortical circuit drives essential tremor Images from Matthews et al. Ann Clin Transl Neurol. 2023

7 Essential3 Program Topline Results
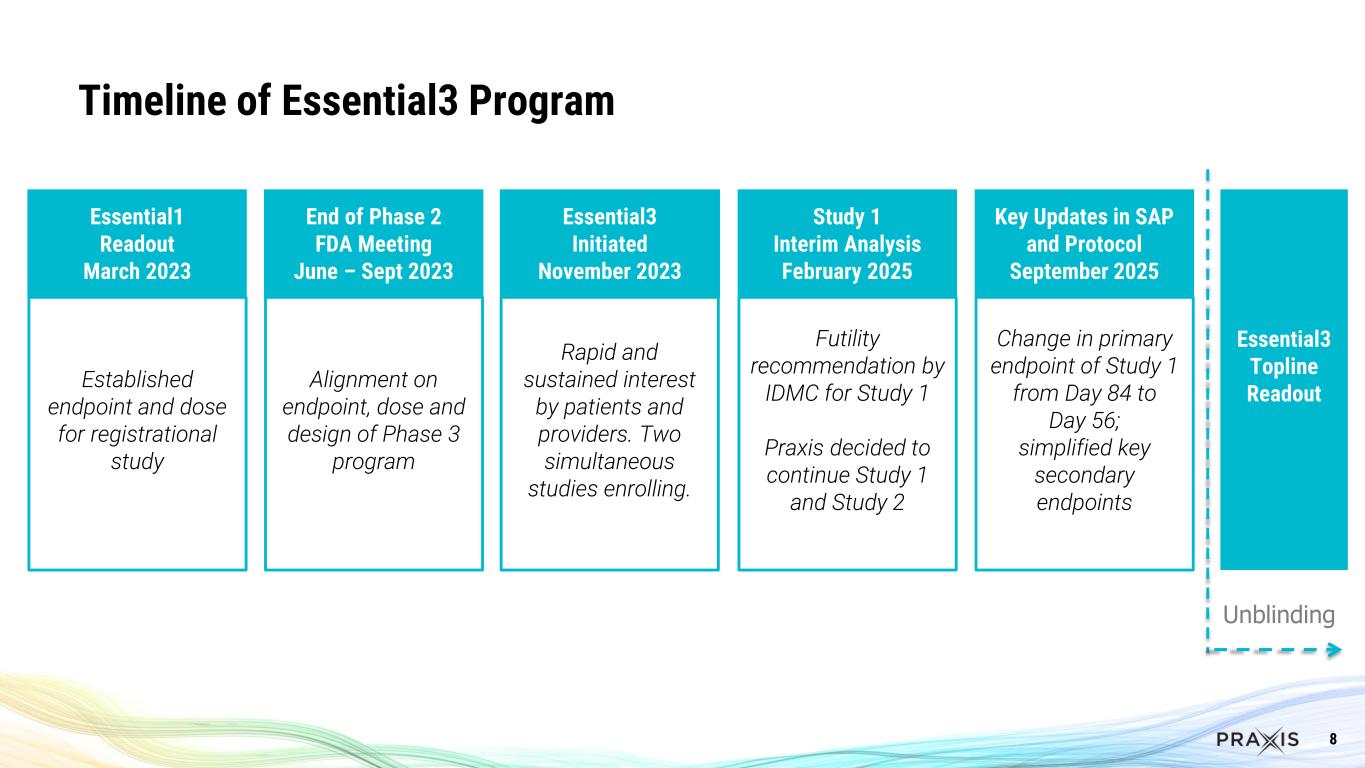
8 Timeline of Essential3 Program Essential1 Readout March 2023 Established endpoint and dose for registrational study End of Phase 2 FDA Meeting June – Sept 2023 Alignment on endpoint, dose and design of Phase 3 program Essential3 Initiated November 2023 Rapid and sustained interest by patients and providers. Two simultaneous studies enrolling. Study 1 Interim Analysis February 2025 Futility recommendation by IDMC for Study 1 Praxis decided to continue Study 1 and Study 2 Essential3 Topline Readout Key Updates in SAP and Protocol September 2025 Change in primary endpoint of Study 1 from Day 84 to Day 56; simplified key secondary endpoints Unblinding
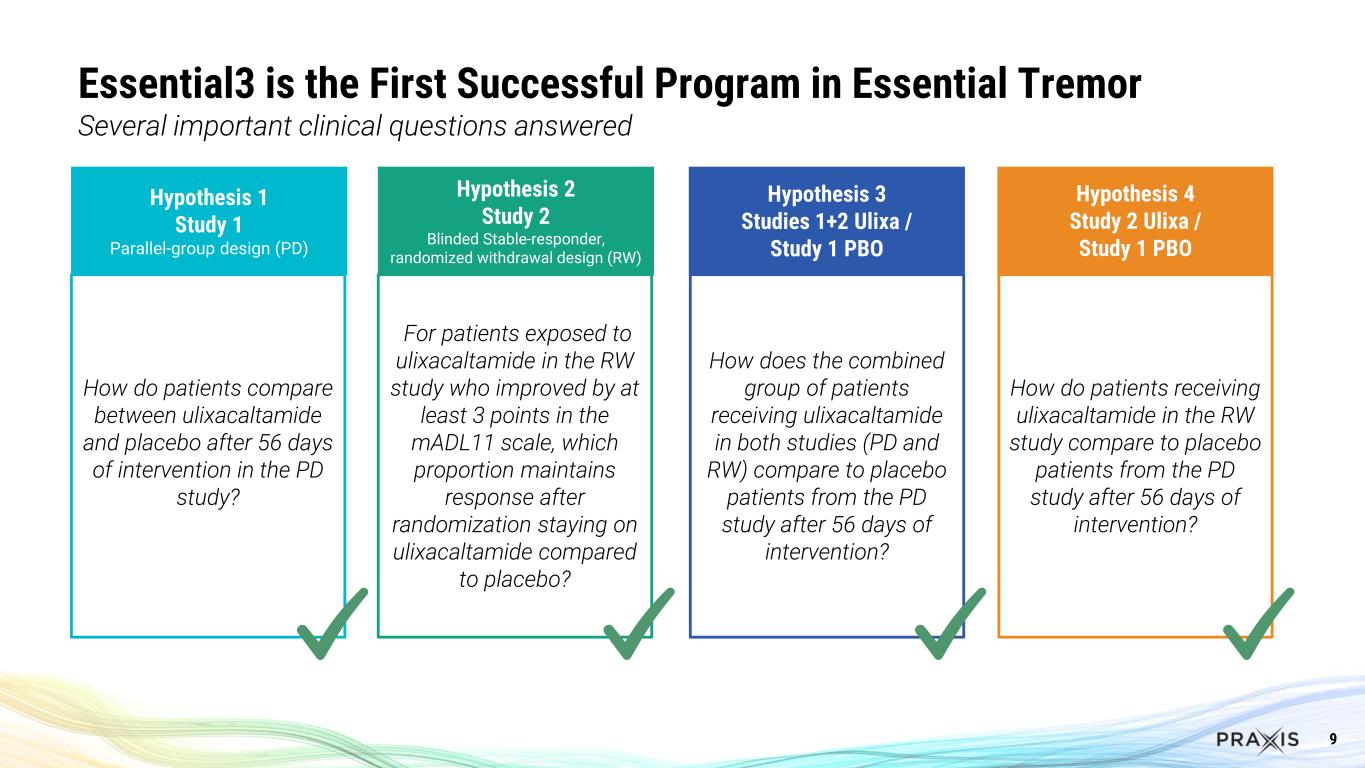
9 Essential3 is the First Successful Program in Essential Tremor Several important clinical questions answered Hypothesis 1 Study 1 Parallel-group design (PD) Hypothesis 2 Study 2 Blinded Stable-responder, randomized withdrawal design (RW) Hypothesis 3 Studies 1+2 Ulixa / Study 1 PBO Hypothesis 4 Study 2 Ulixa / Study 1 PBO How do patients compare between ulixacaltamide and placebo after 56 days of intervention in the PD study? For patients exposed to ulixacaltamide in the RW study who improved by at least 3 points in the mADL11 scale, which proportion maintains response after randomization staying on ulixacaltamide compared to placebo? How does the combined group of patients receiving ulixacaltamide in both studies (PD and RW) compare to placebo patients from the PD study after 56 days of intervention? How do patients receiving ulixacaltamide in the RW study compare to placebo patients from the PD study after 56 days of intervention?
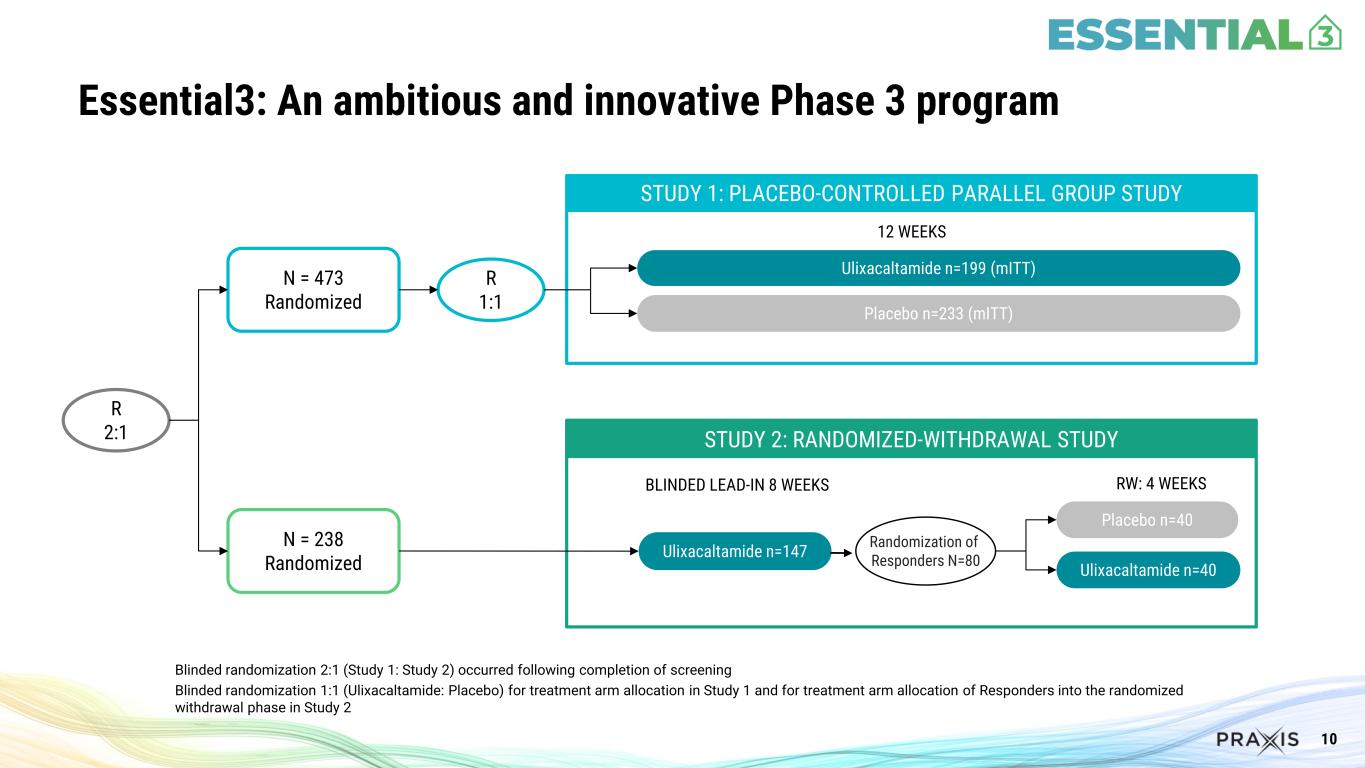
10 Essential3: An ambitious and innovative Phase 3 program Blinded randomization 2:1 (Study 1: Study 2) occurred following completion of screening Blinded randomization 1:1 (Ulixacaltamide: Placebo) for treatment arm allocation in Study 1 and for treatment arm allocation of Responders into the randomized withdrawal phase in Study 2 STUDY 1: PLACEBO-CONTROLLED PARALLEL GROUP STUDY Ulixacaltamide n=199 (mITT) Placebo n=233 (mITT) STUDY 2: RANDOMIZED-WITHDRAWAL STUDY Ulixacaltamide n=147 Randomization of Responders N=80 Ulixacaltamide n=40 Placebo n=40 BLINDED LEAD-IN 8 WEEKS RW: 4 WEEKS 12 WEEKS R 2:1 N = 473 Randomized N = 238 Randomized R 1:1
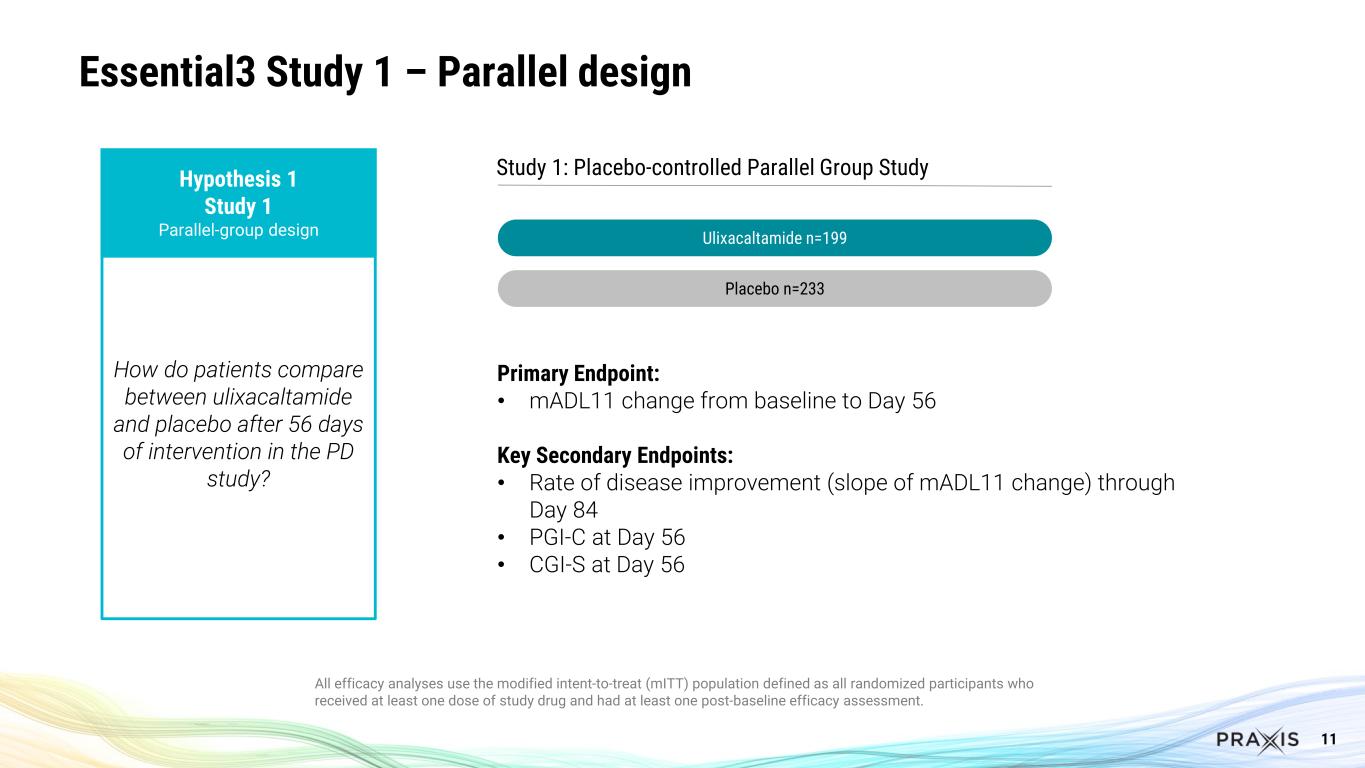
11 Essential3 Study 1 – Parallel design All efficacy analyses use the modified intent-to-treat (mITT) population defined as all randomized participants who received at least one dose of study drug and had at least one post-baseline efficacy assessment. Hypothesis 1 Study 1 Parallel-group design How do patients compare between ulixacaltamide and placebo after 56 days of intervention in the PD study? Study 1: Placebo-controlled Parallel Group Study Ulixacaltamide n=199 Placebo n=233 Primary Endpoint: • mADL11 change from baseline to Day 56 Key Secondary Endpoints: • Rate of disease improvement (slope of mADL11 change) through Day 84 • PGI-C at Day 56 • CGI-S at Day 56
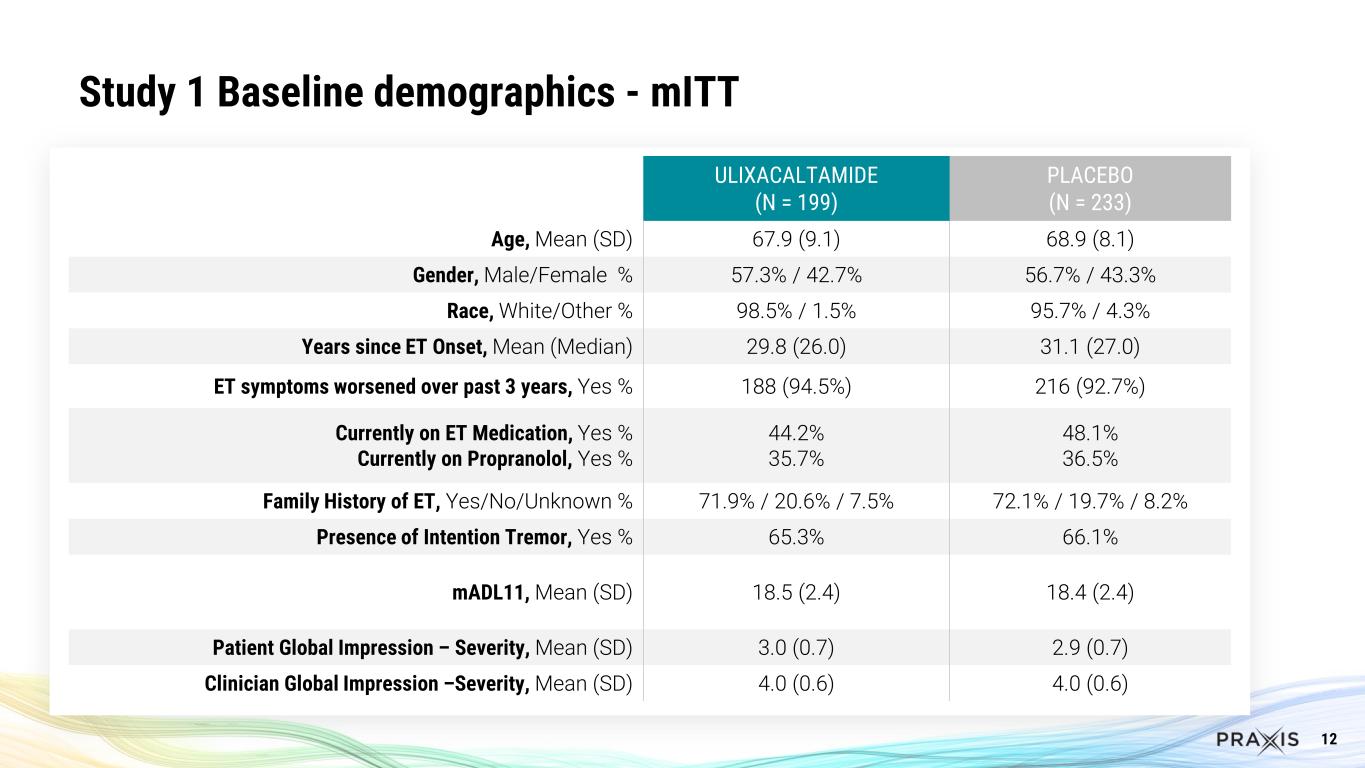
12 Study 1 Baseline demographics - mITT ULIXACALTAMIDE (N = 199) PLACEBO (N = 233) Age, Mean (SD) 67.9 (9.1) 68.9 (8.1) Gender, Male/Female % 57.3% / 42.7% 56.7% / 43.3% Race, White/Other % 98.5% / 1.5% 95.7% / 4.3% Years since ET Onset, Mean (Median) 29.8 (26.0) 31.1 (27.0) ET symptoms worsened over past 3 years, Yes % 188 (94.5%) 216 (92.7%) Currently on ET Medication, Yes % Currently on Propranolol, Yes % 44.2% 35.7% 48.1% 36.5% Family History of ET, Yes/No/Unknown % 71.9% / 20.6% / 7.5% 72.1% / 19.7% / 8.2% Presence of Intention Tremor, Yes % 65.3% 66.1% mADL11, Mean (SD) 18.5 (2.4) 18.4 (2.4) Patient Global Impression – Severity, Mean (SD) 3.0 (0.7) 2.9 (0.7) Clinician Global Impression –Severity, Mean (SD) 4.0 (0.6) 4.0 (0.6)
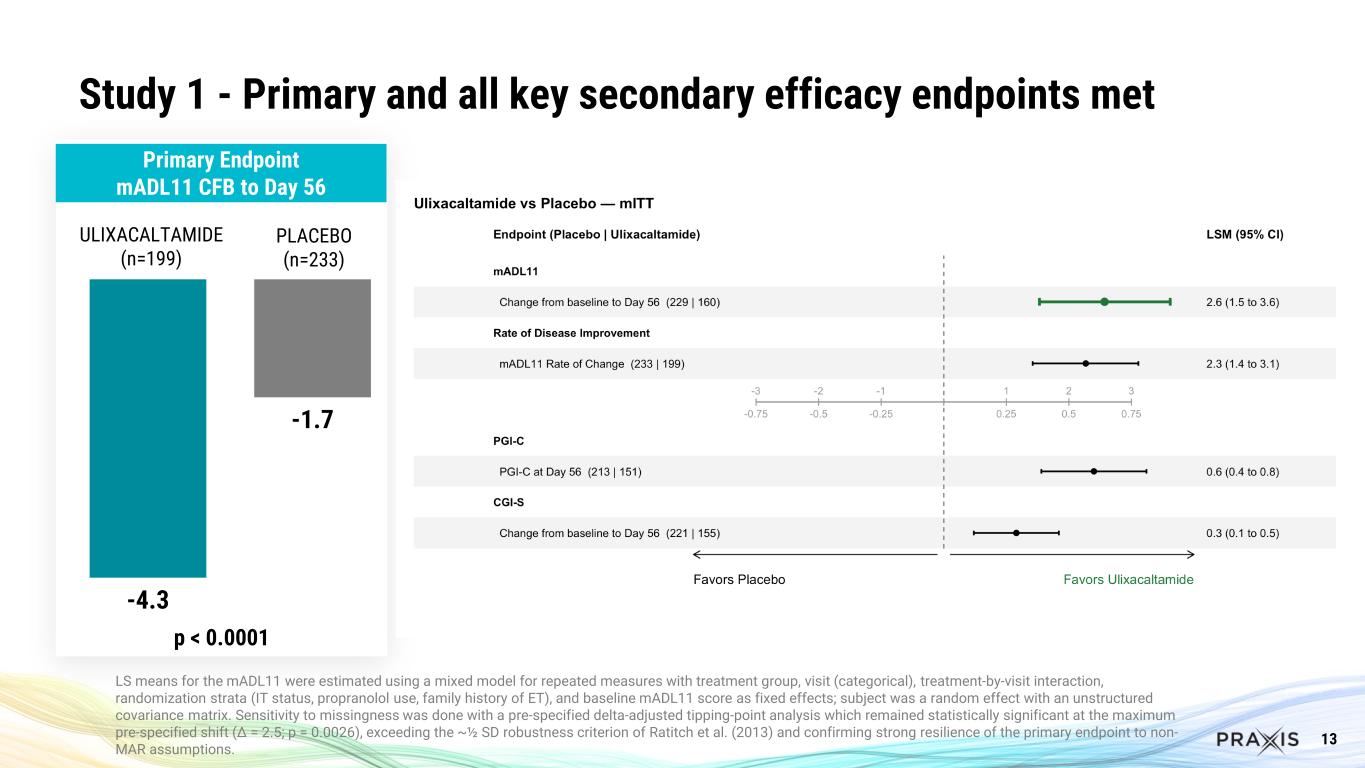
13 Study 1 - Primary and all key secondary efficacy endpoints met -4.3 -1.7 p < 0.0001 ULIXACALTAMIDE (n=199) PLACEBO (n=233) LS means for the mADL11 were estimated using a mixed model for repeated measures with treatment group, visit (categorical), treatment-by-visit interaction, randomization strata (IT status, propranolol use, family history of ET), and baseline mADL11 score as fixed effects; subject was a random effect with an unstructured covariance matrix. Sensitivity to missingness was done with a pre-specified delta-adjusted tipping-point analysis which remained statistically significant at the maximum pre-specified shift (Δ = 2.5; p = 0.0026), exceeding the ~½ SD robustness criterion of Ratitch et al. (2013) and confirming strong resilience of the primary endpoint to non- MAR assumptions. Primary Endpoint mADL11 CFB to Day 56
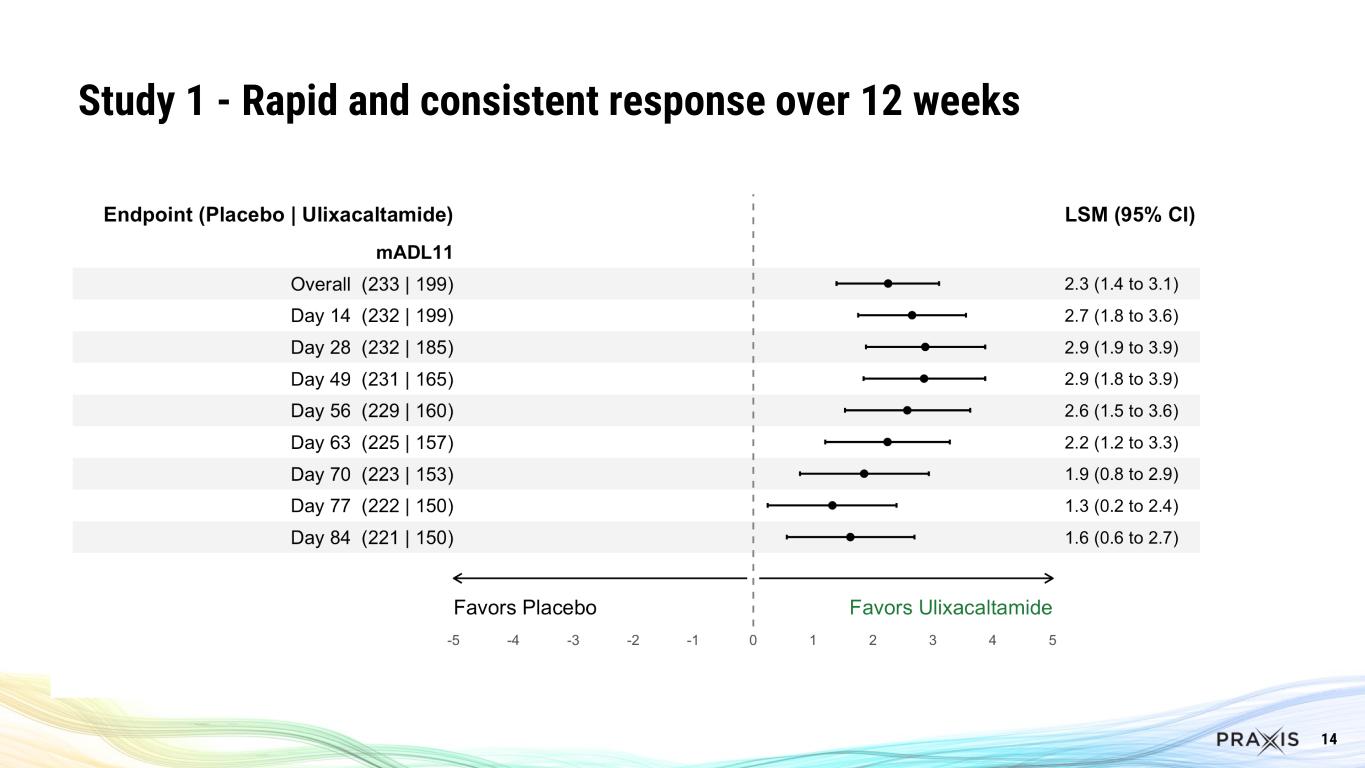
14 Study 1 - Rapid and consistent response over 12 weeks
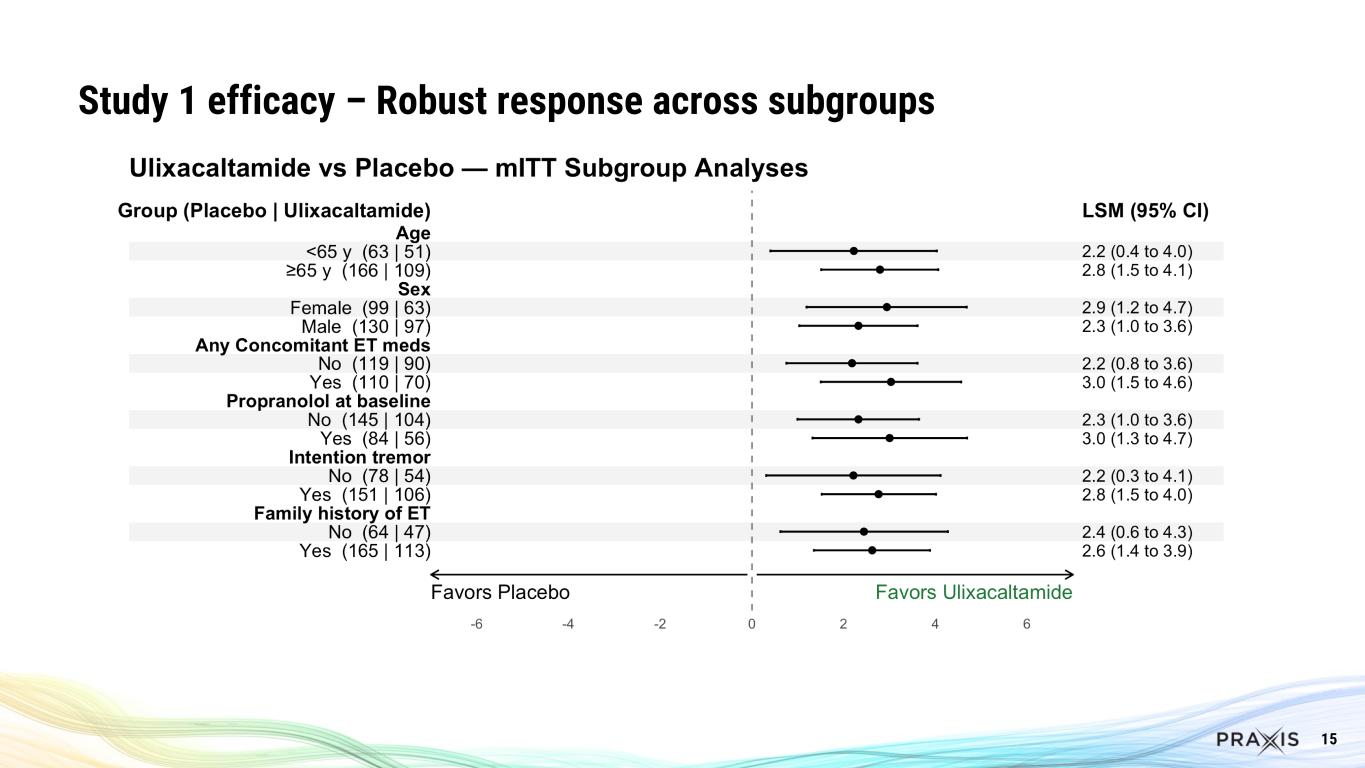
15 Study 1 efficacy – Robust response across subgroups
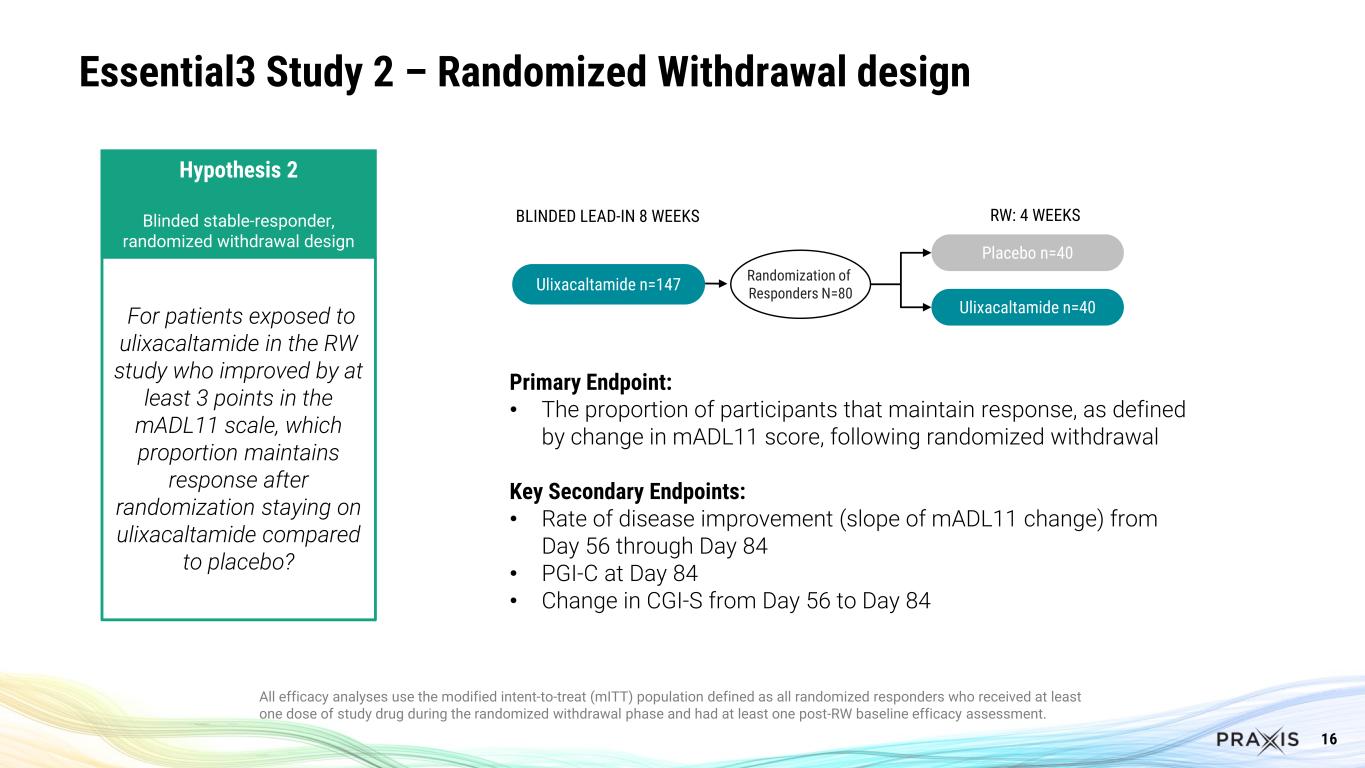
16 Essential3 Study 2 – Randomized Withdrawal design All efficacy analyses use the modified intent-to-treat (mITT) population defined as all randomized responders who received at least one dose of study drug during the randomized withdrawal phase and had at least one post-RW baseline efficacy assessment. Primary Endpoint: • The proportion of participants that maintain response, as defined by change in mADL11 score, following randomized withdrawal Key Secondary Endpoints: • Rate of disease improvement (slope of mADL11 change) from Day 56 through Day 84 • PGI-C at Day 84 • Change in CGI-S from Day 56 to Day 84 Ulixacaltamide n=147 Randomization of Responders N=80 Ulixacaltamide n=40 Placebo n=40 BLINDED LEAD-IN 8 WEEKS RW: 4 WEEKS Hypothesis 2 Blinded stable-responder, randomized withdrawal design For patients exposed to ulixacaltamide in the RW study who improved by at least 3 points in the mADL11 scale, which proportion maintains response after randomization staying on ulixacaltamide compared to placebo?
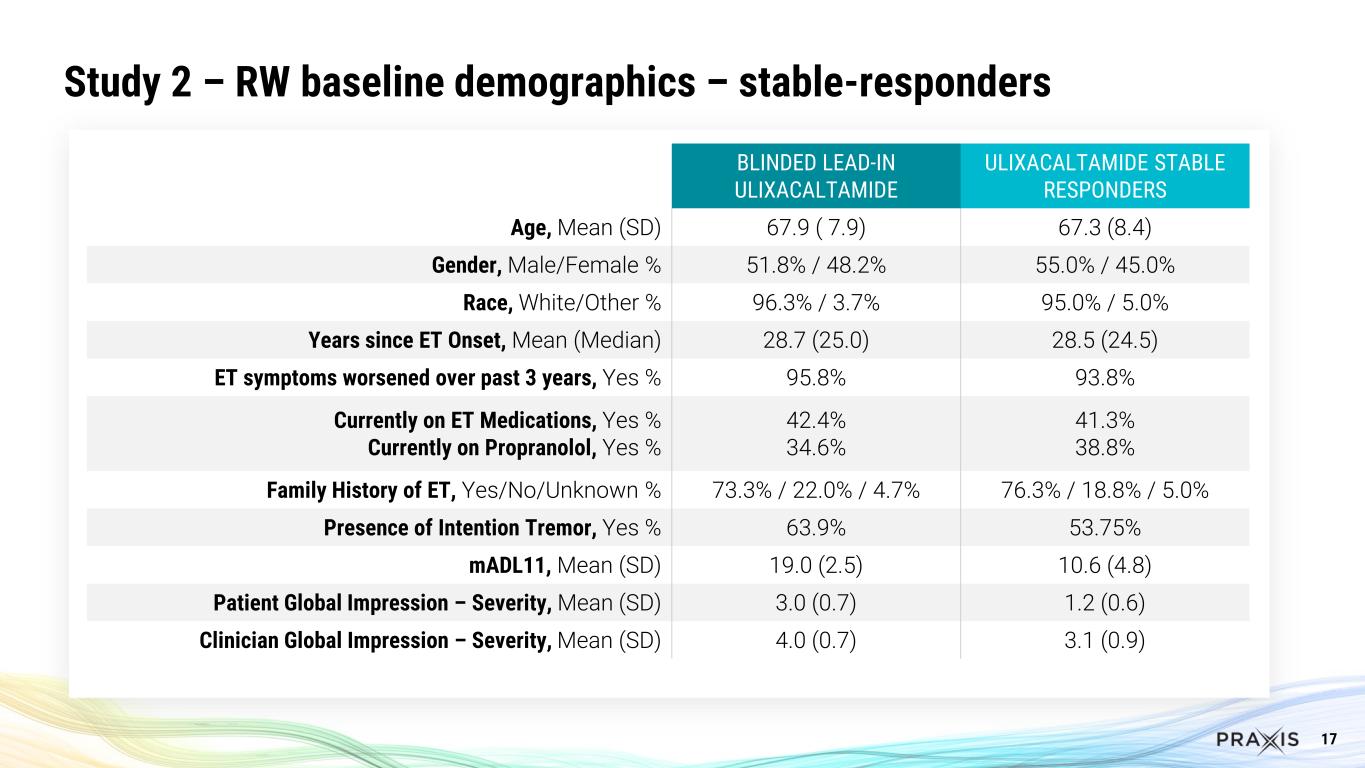
17 Study 2 – RW baseline demographics – stable-responders BLINDED LEAD-IN ULIXACALTAMIDE ULIXACALTAMIDE STABLE RESPONDERS Age, Mean (SD) 67.9 ( 7.9) 67.3 (8.4) Gender, Male/Female % 51.8% / 48.2% 55.0% / 45.0% Race, White/Other % 96.3% / 3.7% 95.0% / 5.0% Years since ET Onset, Mean (Median) 28.7 (25.0) 28.5 (24.5) ET symptoms worsened over past 3 years, Yes % 95.8% 93.8% Currently on ET Medications, Yes % Currently on Propranolol, Yes % 42.4% 34.6% 41.3% 38.8% Family History of ET, Yes/No/Unknown % 73.3% / 22.0% / 4.7% 76.3% / 18.8% / 5.0% Presence of Intention Tremor, Yes % 63.9% 53.75% mADL11, Mean (SD) 19.0 (2.5) 10.6 (4.8) Patient Global Impression – Severity, Mean (SD) 3.0 (0.7) 1.2 (0.6) Clinician Global Impression – Severity, Mean (SD) 4.0 (0.7) 3.1 (0.9)
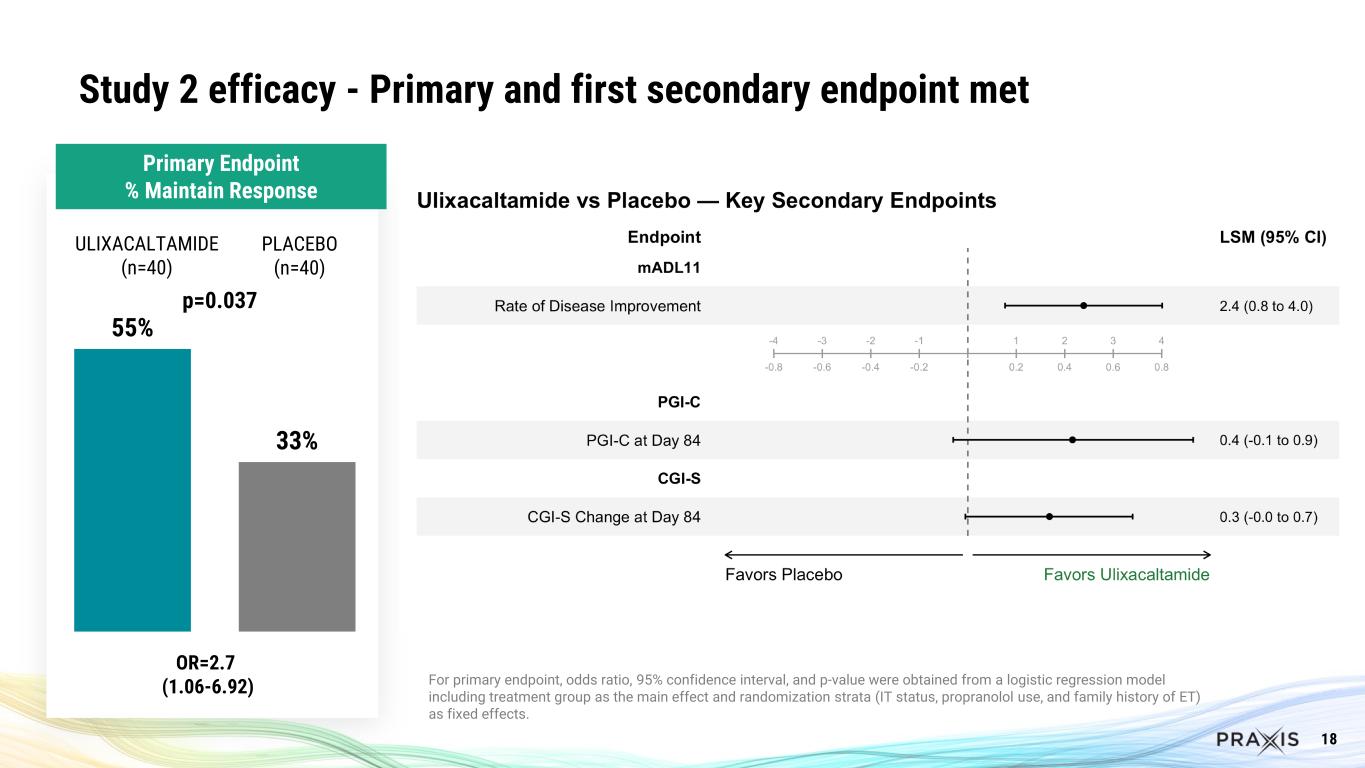
18 Study 2 efficacy - Primary and first secondary endpoint met 55% 33% p=0.037 ULIXACALTAMIDE (n=40) PLACEBO (n=40) OR=2.7 (1.06-6.92) For primary endpoint, odds ratio, 95% confidence interval, and p-value were obtained from a logistic regression model including treatment group as the main effect and randomization strata (IT status, propranolol use, and family history of ET) as fixed effects. Primary Endpoint % Maintain Response
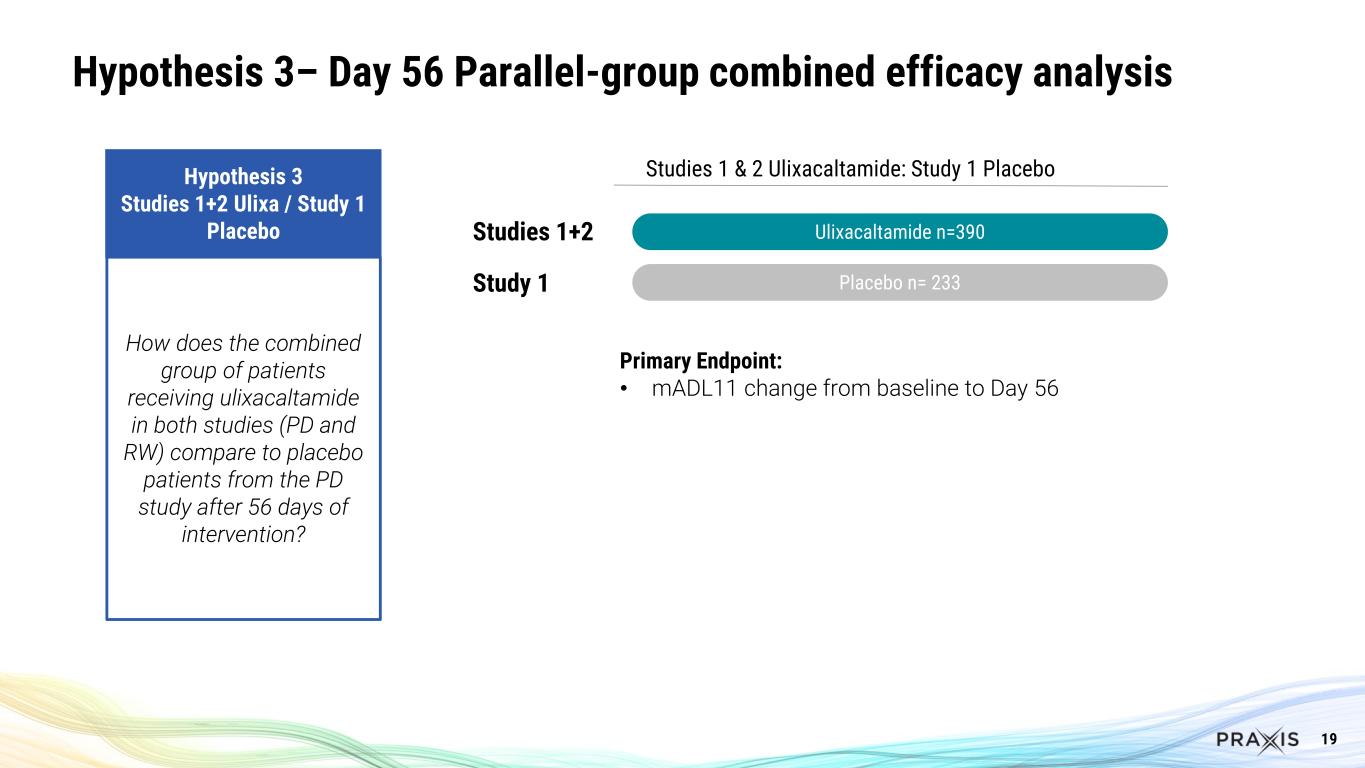
19 Hypothesis 3– Day 56 Parallel-group combined efficacy analysis Studies 1 & 2 Ulixacaltamide: Study 1 Placebo Ulixacaltamide n=390 Placebo n= 233 Studies 1+2 Study 1 Hypothesis 3 Studies 1+2 Ulixa / Study 1 Placebo How does the combined group of patients receiving ulixacaltamide in both studies (PD and RW) compare to placebo patients from the PD study after 56 days of intervention? Primary Endpoint: • mADL11 change from baseline to Day 56
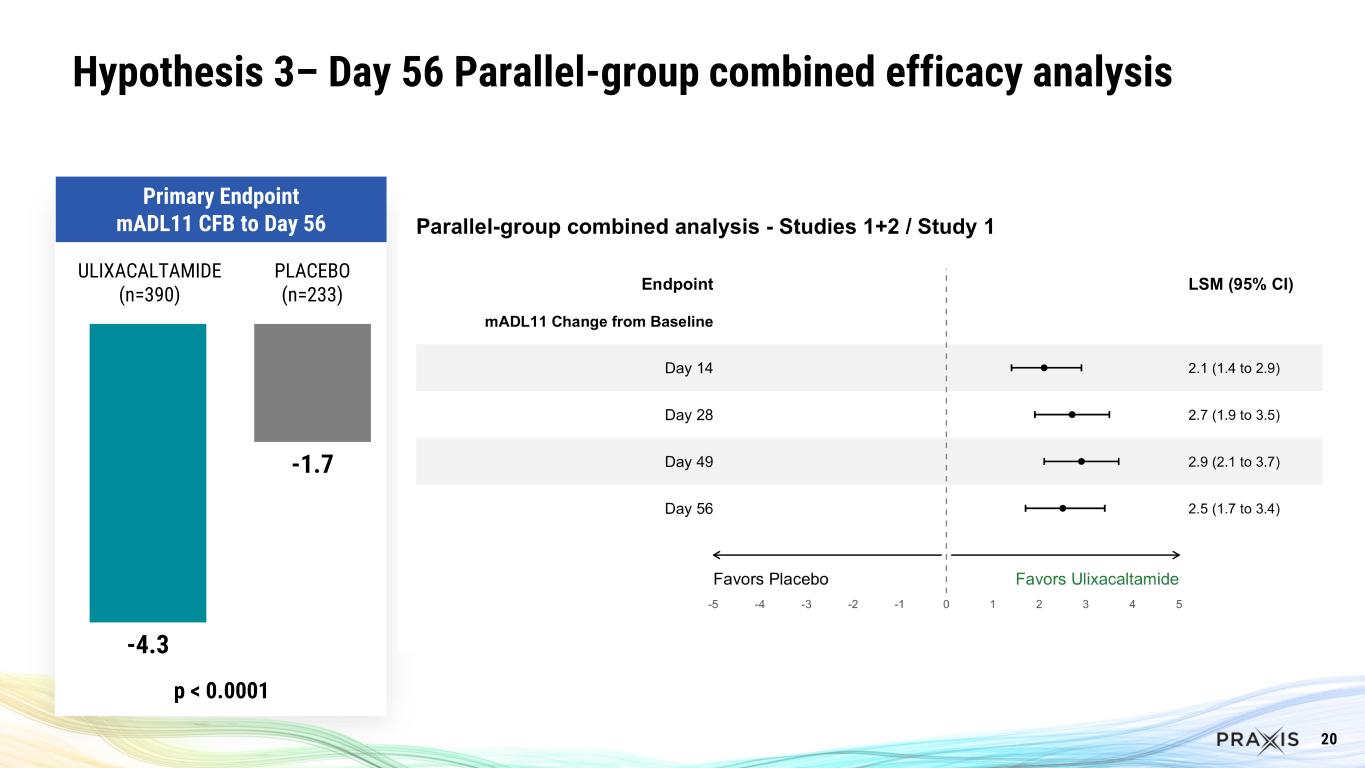
20 -4.3 -1.7 p < 0.0001 ULIXACALTAMIDE (n=390) PLACEBO (n=233) Primary Endpoint mADL11 CFB to Day 56 Hypothesis 3– Day 56 Parallel-group combined efficacy analysis
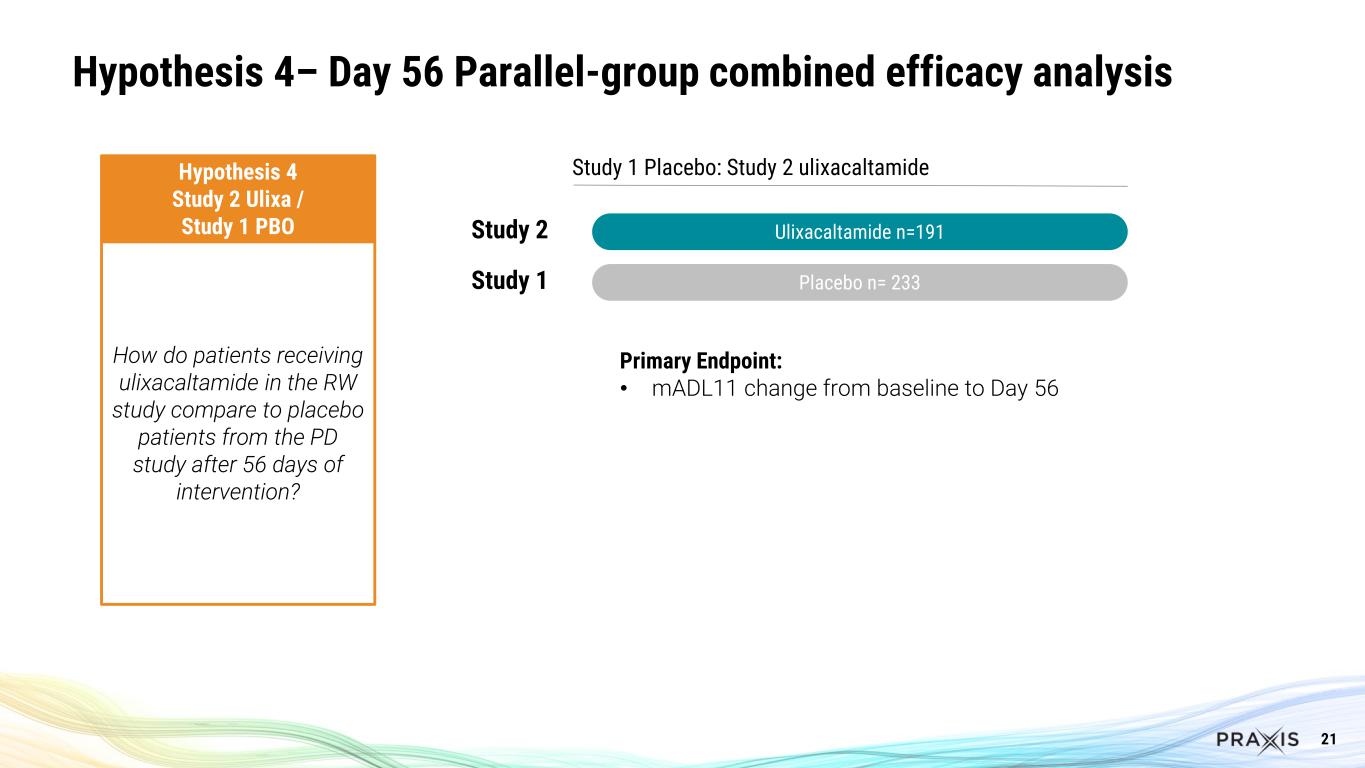
21 Study 1 Placebo: Study 2 ulixacaltamide Ulixacaltamide n=191 Placebo n= 233 Hypothesis 4 Study 2 Ulixa / Study 1 PBO How do patients receiving ulixacaltamide in the RW study compare to placebo patients from the PD study after 56 days of intervention? Study 2 Study 1 Hypothesis 4– Day 56 Parallel-group combined efficacy analysis Primary Endpoint: • mADL11 change from baseline to Day 56
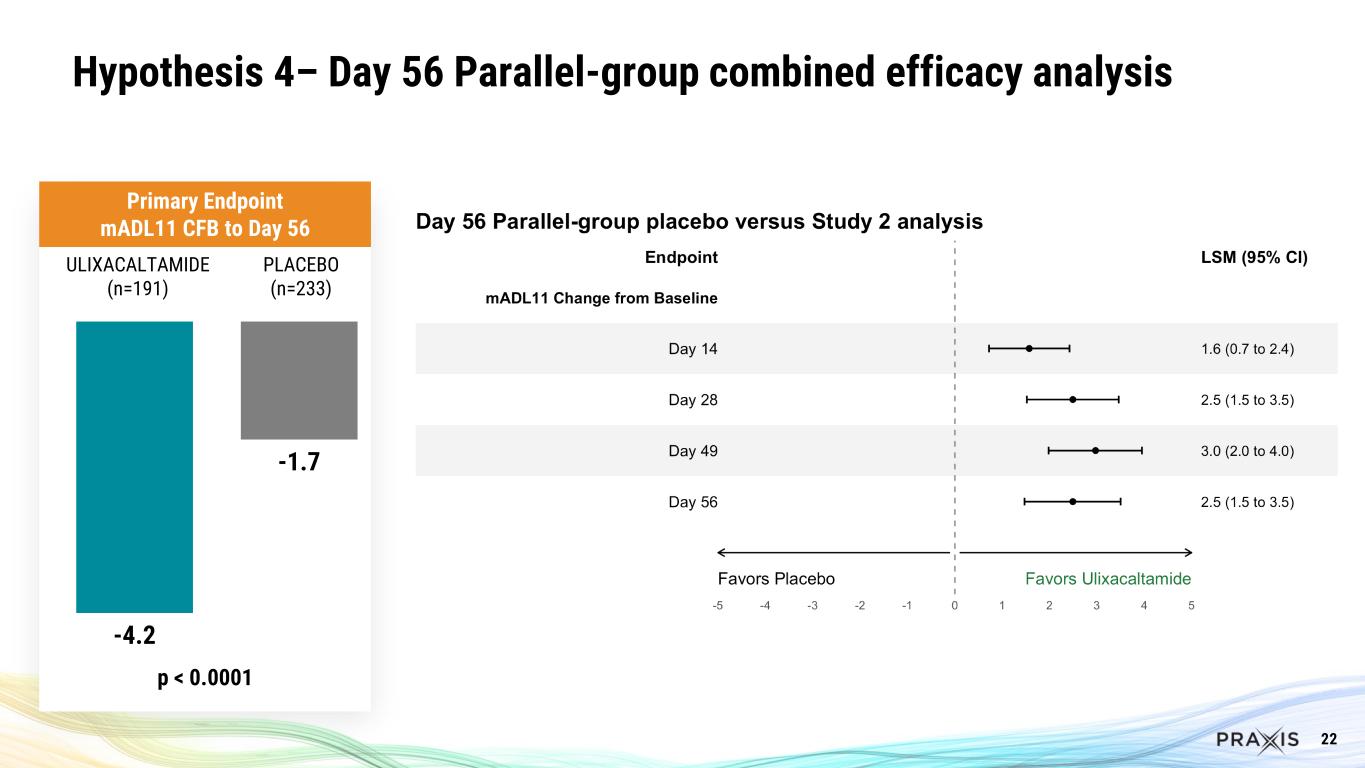
22 -4.2 -1.7 p < 0.0001 ULIXACALTAMIDE (n=191) PLACEBO (n=233) Primary Endpoint mADL11 CFB to Day 56 Hypothesis 4– Day 56 Parallel-group combined efficacy analysis
23 • No change in overall safety profile and no new signals identified • Most common TEAEs (≥10%) in participants treated with ulixacaltamide were constipation, dizziness, euphoric mood, brain fog, headache, paraesthesia and insomnia. • Discontinuations were primarily due to AEs, with most common due to dizziness and brain fog • Majority of TEAEs were mild to moderate in severity • No SAEs related to ulixacaltamide Safety across studies remains consistent
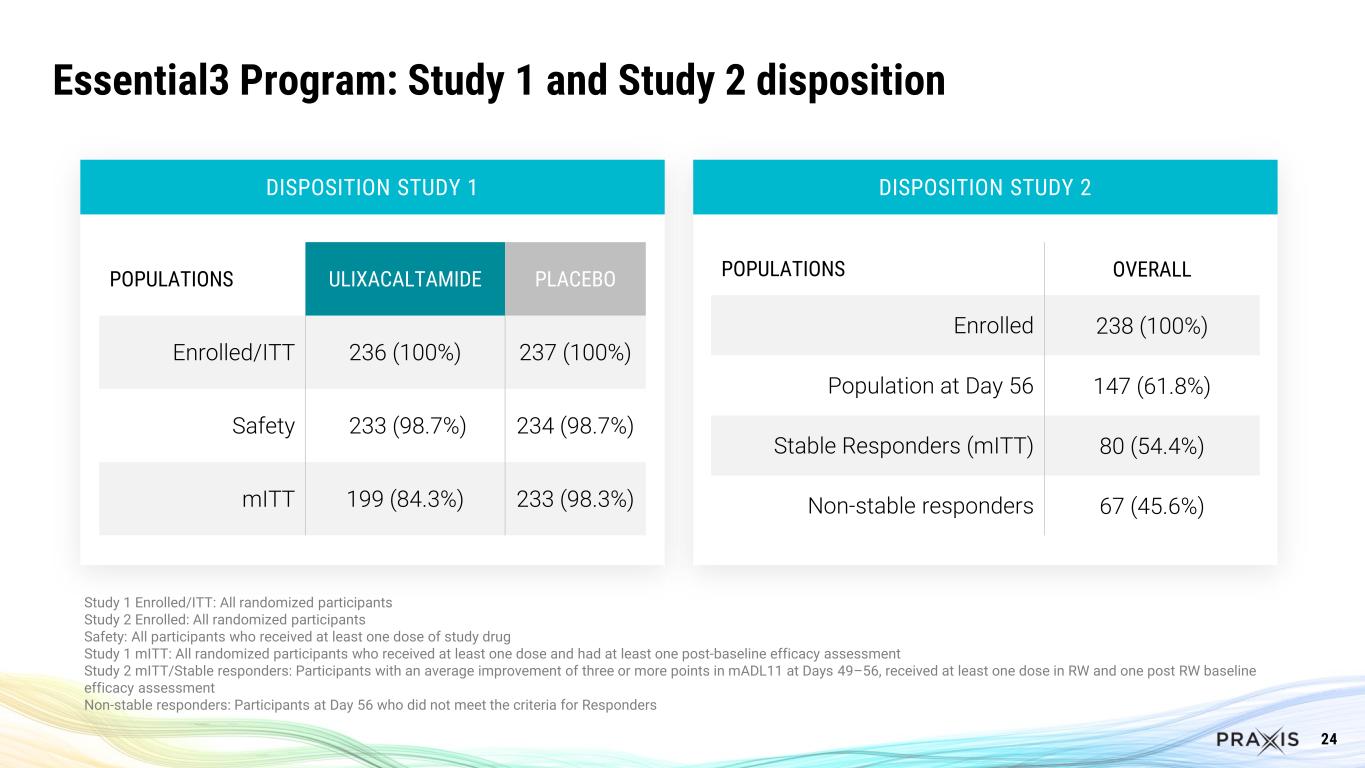
24 Essential3 Program: Study 1 and Study 2 disposition Study 1 Enrolled/ITT: All randomized participants Study 2 Enrolled: All randomized participants Safety: All participants who received at least one dose of study drug Study 1 mITT: All randomized participants who received at least one dose and had at least one post-baseline efficacy assessment Study 2 mITT/Stable responders: Participants with an average improvement of three or more points in mADL11 at Days 49–56, received at least one dose in RW and one post RW baseline efficacy assessment Non-stable responders: Participants at Day 56 who did not meet the criteria for Responders DISPOSITION STUDY 2DISPOSITION STUDY 1 POPULATIONS OVERALL Enrolled 238 (100%) Population at Day 56 147 (61.8%) Stable Responders (mITT) 80 (54.4%) Non-stable responders 67 (45.6%) POPULATIONS ULIXACALTAMIDE PLACEBO Enrolled/ITT 236 (100%) 237 (100%) Safety 233 (98.7%) 234 (98.7%) mITT 199 (84.3%) 233 (98.3%)
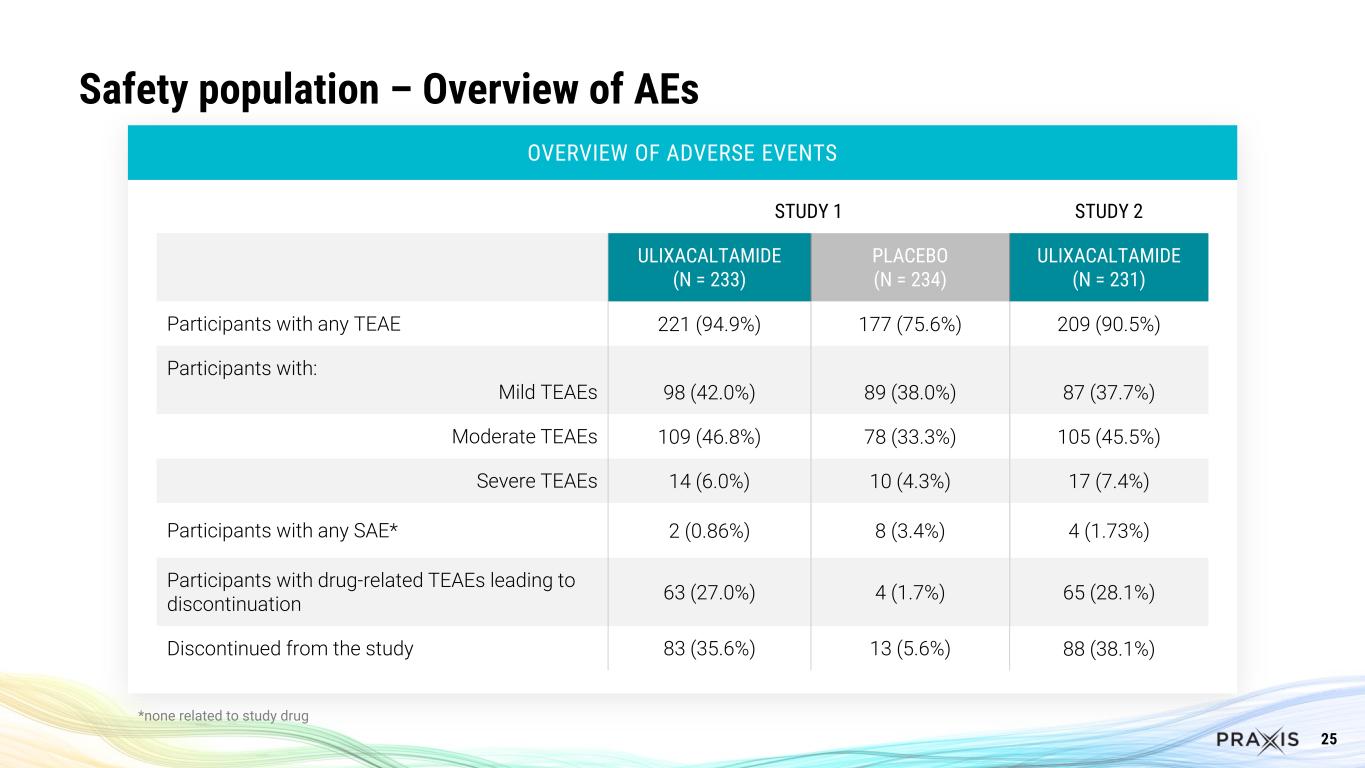
25 Safety population – Overview of AEs OVERVIEW OF ADVERSE EVENTS STUDY 1 STUDY 2 ULIXACALTAMIDE (N = 233) PLACEBO (N = 234) ULIXACALTAMIDE (N = 231) Participants with any TEAE 221 (94.9%) 177 (75.6%) 209 (90.5%) Participants with: Mild TEAEs 98 (42.0%) 89 (38.0%) 87 (37.7%) Moderate TEAEs 109 (46.8%) 78 (33.3%) 105 (45.5%) Severe TEAEs 14 (6.0%) 10 (4.3%) 17 (7.4%) Participants with any SAE* 2 (0.86%) 8 (3.4%) 4 (1.73%) Participants with drug-related TEAEs leading to discontinuation 63 (27.0%) 4 (1.7%) 65 (28.1%) Discontinued from the study 83 (35.6%) 13 (5.6%) 88 (38.1%) *none related to study drug
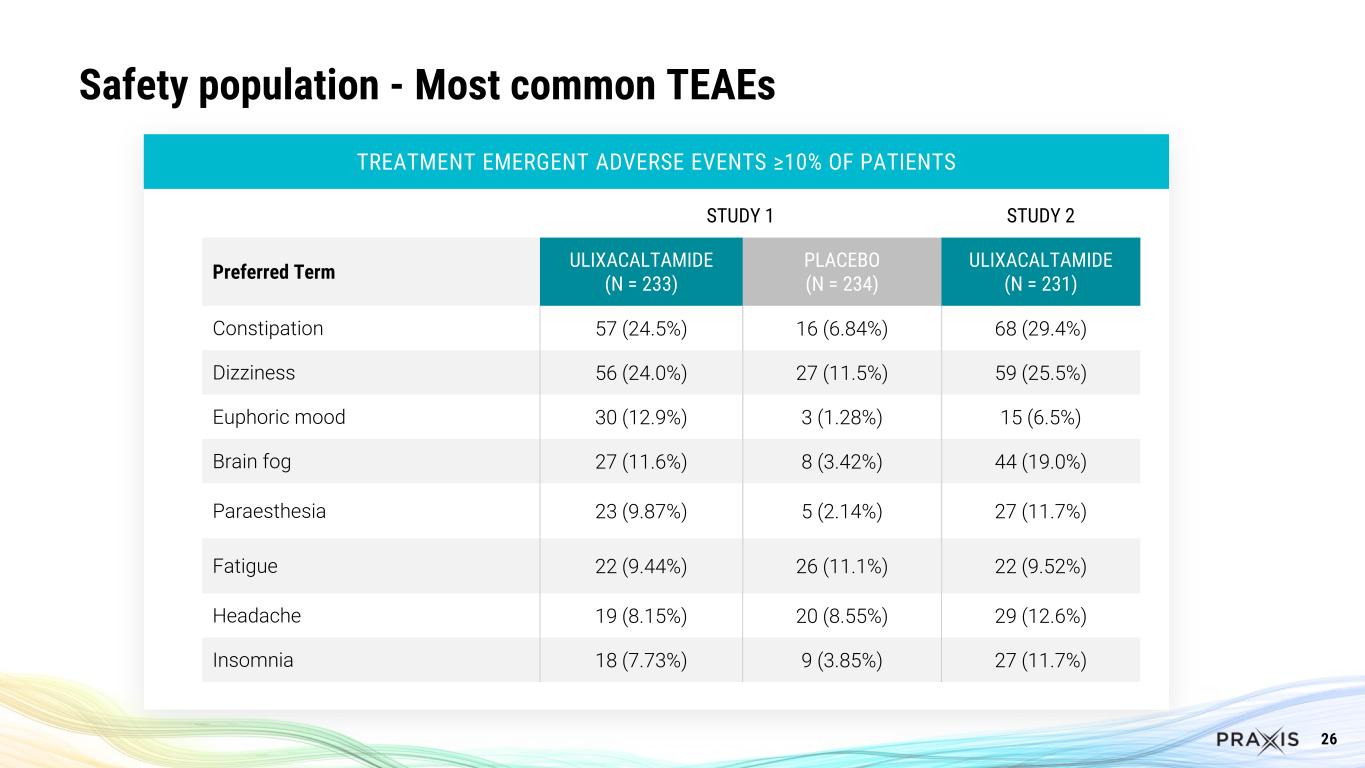
26 Safety population - Most common TEAEs TREATMENT EMERGENT ADVERSE EVENTS ≥10% OF PATIENTS STUDY 1 STUDY 2 Preferred Term ULIXACALTAMIDE (N = 233) PLACEBO (N = 234) ULIXACALTAMIDE (N = 231) Constipation 57 (24.5%) 16 (6.84%) 68 (29.4%) Dizziness 56 (24.0%) 27 (11.5%) 59 (25.5%) Euphoric mood 30 (12.9%) 3 (1.28%) 15 (6.5%) Brain fog 27 (11.6%) 8 (3.42%) 44 (19.0%) Paraesthesia 23 (9.87%) 5 (2.14%) 27 (11.7%) Fatigue 22 (9.44%) 26 (11.1%) 22 (9.52%) Headache 19 (8.15%) 20 (8.55%) 29 (12.6%) Insomnia 18 (7.73%) 9 (3.85%) 27 (11.7%)

27 One step closer to delivering life-altering treatments to ET patients First positive Phase 3 program for a drug in Essential Tremor Praxis has submitted a pre-NDA meeting request to the FDA and, upon agreement with the agency, expect to file an NDA in early 2026 Will share additional data in upcoming medical conferences and scientific journals


























