UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): May 31, 2025
Actuate Therapeutics, Inc.
(Exact Name of Registrant as Specified in Charter)
| Delaware | 001-42139 | 47-3044785 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 1751 River Run, Suite 400 Fort Worth, Texas |
76107 |
|
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s Telephone Number, Including Area Code: (817) 887-8455
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
| Common Stock, par value $0.000001 per share | ACTU | The Nasdaq Stock Market LLC | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
|
|
Item 7.01 Regulation FD Disclosure.
On May 31, 2025, Actuate Therapeutics, Inc. (the “Company”) highlighted its topline Phase 2 (Actuate-1801 Part 3B) data of elraglusib in combination with gemcitabine/nab-paclitaxel (“GnP”) in first-line treatment of metastatic pancreatic cancer (“mPDAC”) in a presentation at the American Society of Clinical Oncology 2025 Annual Meeting, or the ASCO Presentation, and issued a press release concurrent with the ASCO Presentation. As previously announced on May 6, 2025, the Company’s topline Phase 2 data of elraglusib in combination with GnP met the primary endpoints of the trial and achieved statistical significance in topline results from its ongoing Phase 2 (Actuate-1801 Part 3B) trial in first-line treatment of mPDAC. A copy of the Company’s revised corporate presentation and copy of the press release are attached as Exhibits 99.1 and 99.2, respectively, to this Current Report.
In addition, the Company hosted a Key Opinion Leader (“KOL”) event for the investment community to review the data. The KOL event featured a fireside discussion moderated by Daniel Schmitt, President & Chief Executive Officer of Actuate, and included four distinguished KOLs: Tanios Bekaii-Saab, MD, FACP, Mayo Clinic College of Medicine and Science, Devalingam Mahalingam, MD, Northwestern University Feinberg School of Medicine, Rachna Shroff, MD, MS, FASCO, University of Arizona Cancer Center, and Colin Weekes, MD, PhD, Massachusetts General Hospital. A replay of the event is available on the Investor Relations section of the Actuate website at www.actuatetherapeutics.com.
The information furnished in this Item 7.01, including Exhibits 99.1 and 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Exchange Act or the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such a filing.
Item 8.01 Other Events.
On May 31, 2025, the Company announced details of its topline results from the Phase 2 (Actuate-1801 Part 3B) trial of elraglusib in combination with GnP versus GnP alone in first-line mPDAC.
The Actuate-1801 Part 3B study (NCT03678883) is a randomized, controlled Phase 2 trial of elraglusib in combination with GnP (“elraglusib/GnP”) versus GnP alone in first-line mPDAC. The trial enrolled 286 mPDAC patients with no prior systemic treatment for metastatic disease, who were randomized 2:1 to the elraglusib/GnP combination arm or the GnP arm. Elraglusib is administered at a dose of 9.3 mg/kg by IV infusion on day 1 of each week of a 28-day cycle. The primary endpoint for this study is median overall survival (“mOS”), with overall survival (“OS”) summarized throughout the study by estimates of 1-year survival. Secondary endpoints are DCR, ORR, PFS (each defined below) and adverse events.
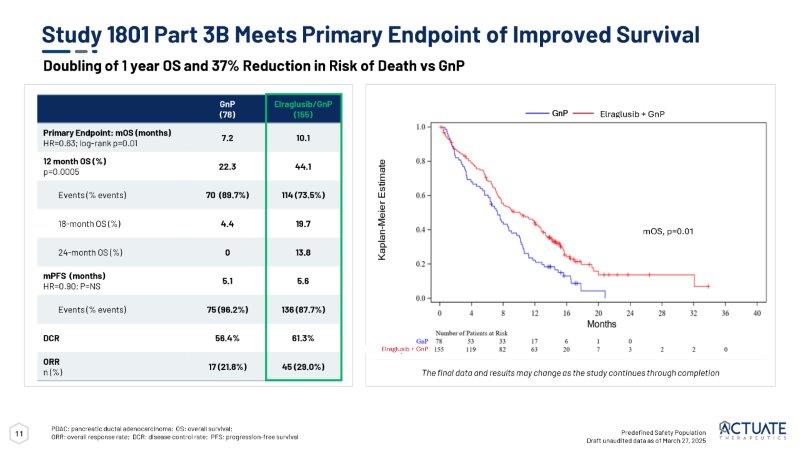
Topline data in the pre-specified safety population as of March 27, 2025 showed that the trial met its primary endpoint of improved mOS in patients in the elraglusib/GnP combination arm versus the GnP control arm. The analysis of topline data demonstrated treatment with elraglusib/GnP resulted in statistically significant increases in 1-year survival rate (p-value of 0.0005) and mOS (10.1 months vs 7.2 months, HR=0.63, log-rank p=0.01) with a 37% reduction in the risk of death versus treatment with GnP alone. The following table provides additional topline results from the study as of March 27, 2025:
|
Safety Population |
Elraglusib/GnP (n=155) |
GnP (n=78) |
|
Primary Endpoint: mOS (months) HR=0.63; log-rank p=0.01* |
10.1 | 7.2 |
| 12-month OS (%) p=0.0005* | 44.1 | 22.3 |
| Number (%) of death events | 114 (73.5) | 70 (89.7) |
| 18-month OS (%) | 19.7 | 4.4 |
| 24-month OS (%) | 13.8 | 0 |
_____________
*statistically significant
|
|
Moreover, Figure 1 below depicts the Kaplan-Meier estimate for mOS (10.1 months vs 7.2 months, HR=0.63, log-rank p=0.01), displaying a clear clinical survival benefit for patients treated in the elraglusib/GnP combination arm vs GnP alone arm.

Figure 1: Actuate-1801 Part 3B: Kaplan-Meier Estimate for mOS as of March 27, 2025 (Topline data cut-off).
In addition, topline data showed there were numerically improved overall response rates (“ORR”), median progression-free survival (“PFS”) and disease control rate (“DCR”) in the elraglusib/GnP combination arm versus the GnP arm as noted in the below table.
|
Safety Population |
Elraglusib/GnP (n=155) |
GnP (n=78) |
| ORR (%) | 29.0 | 21.8 |
| PFS (months) | 5.6 | 5.1 |
| DCR (%) | 61.3 | 56.4 |
Moreover, the trial also met its primary safety endpoint. Treatment-emergent adverse events (“TEAEs”) and Serious Adverse Events (“SAEs”) in the elraglusib/GnP combination arm were similar to those observed in the GnP arm, indicating a favorable risk-benefit profile for the elraglusib/GnP combination. Treatment-related adverse events (“TRAEs”) were mostly Grade 1-2, with the most frequent TRAEs observed (in about two-thirds of patients) being transient visual impairments that were reversible and non-progressive. Also, while Grade 3 or higher neutropenia was observed, similar rates of febrile neutropenia and sepsis were observed in both treatment arms.
The topline data noted herein should not be relied upon as a final analysis and is subject to change once full data analysis is complete.
|
|
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Corporate Presentation dated May 31, 2025 | |
| 99.2 | Press Release dated May 31, 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
Forward-Looking Statements
This Current Report contains forward-looking statements about us, including our and other parties’ clinical trials and development plans, and our industry. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” or the negative of these terms or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. All statements, other than statements related to present facts or current conditions or of historical facts, contained in this Current Report are forward-looking statements. Accordingly, these statements involve estimates, assumptions, substantial risks and uncertainties which could cause actual results to differ materially from those expressed in them, including but not limited to that preliminary and unpublished data may be subject to change and further interpretation following the availability of more data or following a more comprehensive review of the data and should not be relied upon as a final analysis; the risk that clinical trial data are subject to differing interpretations and assessments by regulatory authorities and within the medical community; clinical and preclinical drug development involves a lengthy and expensive process with uncertain timelines and outcomes, results of prior preclinical studies and early clinical trials are not necessarily predictive of future results, and elraglusib may not achieve positive clinical results or favorable preclinical results, and we may not be able to make regulatory submissions or receive regulatory approval on a timely basis, if at all; that we may not successfully enroll additional patients or establish or advance plans for further development, including through conversations with the FDA or EMA and the standards such bodies may impose for such development; that elraglusib could be associated with side effects, adverse events or other properties or safety risks, which could delay or preclude regulatory approval, cause us to suspend or discontinue clinical trials or result in other negative consequences; our reliance on third parties to conduct our non-clinical studies and our clinical trials; our reliance on third-party licensors and ability to preserve and protect our intellectual property rights; that we face significant competition from other biotechnology and pharmaceutical companies; our ability to fund development activities, including because our financial condition raises substantial doubt as to our ability to continue as a going concern and we require additional capital to finance our operations beyond the second quarter of fiscal year 2025, and a failure to obtain this necessary capital in the near term on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our development programs, commercialization efforts or other operations. In addition, any forward-looking statements are qualified in their entirety by reference to the factors discussed under the heading “Item 1A. Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on March 13, 2025 and other filings with the SEC. Because the risk factors referred to above could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us or on our behalf, you should not place undue reliance on any forward-looking statements. Further, any forward-looking statement speaks only as of the date on which it is made. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Unless legally required, we do not undertake any obligation to release publicly any revisions to such forward-looking statements to reflect events or circumstances after the date of this Current Report or to reflect the occurrence of unanticipated events.
|
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Actuate Therapeutics, Inc. | ||
| Date: June 2, 2025 | By: | /s/ Daniel M. Schmitt |
| Name: Daniel M. Schmitt | ||
| Title: President and Chief Executive Officer | ||
|
|
Exhibit 99.1

Corporate Overview May 31, 2025

This presentation contains forward - looking statements about us, including our clinical trials and development plans, and our industry, that are based on management’s beliefs and assumptions and on information currently available to our management . The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” o r the negative of these terms o r other comparable terminology are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words . All statements, other than statements related to present facts o r current conditions o r of historical facts, contained in this presentation are forward - looking statements . Accordingly, these statements involve estimates, assumptions, substantial risks and uncertainties which could cause actual results to differ materially from those expressed in them, including bu t not limited to that we have incurred significant operating losses, and we expect that we will incur significant operating losses for the foreseeable future ; that our financial condition raises substantial doubt as to our ability to continue as a going concern and we require additional capital to finance our operations beyond the second quarter of fiscal year 2025 , and a failure to obtain this necessary capital in the near term on acceptable terms, o r at all, could force us to delay, limit, reduce o r terminate our development programs, commercialization efforts o r other operations ; that we have a high risk of never generating revenue o r becoming profitable or, if we achieve profitability, we may not be able to sustain it ; that clinical and preclinical drug development involves a lengthy and expensive process with uncertain timelines and outcomes, and results of prior preclinical studies and early clinical trials are not necessarily predictive of future results, and elraglusib may not achieve favorable results in clinical trials o r preclinical studies, and we may not be able to make regulatory submissions o r receive regulatory approval on a timely basis, if at all ; that we may not successfully enroll additional patients o r establish o r advance plans for phase 2 o r other development, including through conversations with the FDA o r EMA and the standards such bodies may impose for such development ; that regulatory approval processes may involve delays, unfavorable determinations o r other challenges due to various factors, including government funding, staffing and political uncertainties ; the risk that clinical trial data are subject to differing interpretations and assessments by regulatory authorities and within the medical community ; that elraglusib could be associated with side effects, adverse events o r other properties o r safety risks, which could delay o r preclude regulatory approval, cause us to suspend o r discontinue clinical trials o r result in other negative consequences ; that this presentation includes preliminary and unpublished data which may be subject to change following the availability of more data o r following a more comprehensive review of the data and should not be relied upon as a final analysis ; that we do not have, and may never have, any approved products on the market and our business is highly dependent upon receiving approvals from various U . S . and international governmental agencies and will be severely harmed if we are not granted approval to manufacture and sell our product candidates ; our reliance on third parties to conduct our non - clinical studies and our clinical trials ; our reliance on third - party licensors and ability to preserve and protect our intellectual property rights ; that we currently depend entirely on the success of elraglusib, which is our only product candidate, and if we are unable to advance elraglusib in clinical development, obtain regulatory approval and ultimately commercialize elraglusib, o r experience significant delays in doing so, our business will be materially harmed ; that we face significant competition from other biotechnology and pharmaceutical companies ; that we may not be successful in our efforts to investigate elraglusib in additional indications and we may expend our limited resources to pursue a new product candidate o r a particular indication for elraglusib and fail to capitalize on product candidates o r indications that may be more profitable o r for which there is a greater likelihood of success ; that the termination of third - party licenses could adversely affect our rights to important compounds o r technologies ; and our ability to fund development activities , including because our financial condition raises substantial doubt as to our ability to continue as a going concern and we require additional capital to finance our operations beyond the second quarter of fiscal year 2025 , and a failure to obtain this necessary capital in the near term on acceptable terms, o r at all, could force us to delay, limit, reduce o r terminate our development programs, commercialization efforts o r other operations . You are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date hereof, and we undertake no obligation to update such statements to reflect events that occur o r circumstances that exist after the date hereof . In addition, any forward - looking statements are qualified in their entirety by reference to the factors discussed under the heading “Risk Factors” in our Annual Report on Form 10 - K filed with the SEC on March 13 , 2025 , our Quarterly Report on Form 10 - Q for the quarter ended March 31 , 2025 , filed with the SEC on May 15 , 2025 , and other filings with the SEC . This presentation also contain estimates and other statistical data that we obtained from industry publications and research and studies conducted by third parties relating to market size and growth and other data about our industry . This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates .

1 Forward - Looking Statements 2 IPO: August 2024 HEADQUARTERS: Fort Worth, TX Advancing a potentially class - leading GSK - 3 β inhibitor, elraglusib with a novel, multimodal MOA, in multiple advanced cancer Phase 2 trials Clinical responses (CRs/PRs) and Disease Control observed across cancer histologies Extended survival and increased responses are observed in mPDAC and relapsed/refractory Ewing sarcoma.

Preliminary evidence of clinical benefit has also been observed in patients with metastatic melanoma and relapsed/refractory colorectal and lung cancer Oral version of elraglusib successfully evaluated in Healthy Volunteer Phase 1 • Phase 1 dose escalation study planned in advanced cancer patients Broad composition of matter IP protection and development incentives • Orphan Drug Designations for pancreatic and other cancer types; Fast Track Designation for pancreatic cancer Company Highlights NASDAQ: ACTU 3 Decreased immune evasion T Cells and NK Cells Activation Reduction of fibrosis Accumulation of activated T cells in and through str o m A a Inhibition of epithelial - mesenchymal transition (EMT) Inhibition of cell proliferation and survival Apoptosis GSK - 3 β Elraglusib: Multimodal MOA Supported by Clinical Data • Elraglusib is an ATP - competitive inhibitor of GSK - 3 β • GSK - 3 β has been shown to potentially contribute to tumor progression in many treatment naive and refractory/resistant tumors • Pleiotropic effects as signaling adaptor • Elraglusib downregulates well - credentialed molecular pathways that can lead to chemotherapy and drug resistance • NF - kB pathway - anti - apoptotic protein expression • Alterations in TGF - b and pro - inflammatory cytokines suggest role in fibrosis in addition to immunomodulation • DDR pathways (ATR/ATM) including mismatch repair (PMS2) • Increase responsiveness of resistant/refractory tumors to chemo and immune therapy - ”cold” tumors turned to “hot” • Inhibition of oncogenic epithelial - mesenchymal transitions DDR: DNA Damage Response Source, Walz et al., Clinical Cancer Research 2017; DOI: 10.1158/1078 - 0432.

elraglusib 4 Milestones Phase 3 Phase 2 Phase 1 Preclinical Program Elraglusib Injection (IV) - ASCO presentation of topline data: Q2 2025 - FDA Type B pre - NDA meeting request: 2H 2025* Adult Actuate - 1801 Part 3B Pancreatic Cancer (with GnP) 1st line metastatic In Planning TBD** Adult – Phase 3 or Confirmatory Trial (if required)* Pancreatic Cancer (with GnP) 1st line metastatic Topline Data: 2H 2025 Pediatric Actuate - 1902 Advanced Refractory Cancers - Ewing Sarcoma Cohort Only Elraglusib (oral tablet) TBD** TBD** In Planning In Planning Adult Actuate - 2401 Adult /Pediatric Phase 1 in Advanced, refractory solid cancers Advanced, refractory cancers (solid and hematological) Strategic Pipeline Growth for GSK - 3 β Associated Diseases * The Company plans to request a pre - NDA meeting with the FDA in the second half of 2025 to align on a path towards product registration **Contingent upon future funding GnP: gemcitabine/nab - paclitaxel Ongoing Trial Fast track designation 5 Phase 1/2 Study Design for Elraglusib Injection (IV) Establishes process for transition from elraglusib (9 - ING - 41) Monotherapy (Part 1) to evaluation of multiple chemotherapy combinations (Part 2) to Phase 2 efficacy studies (Part 3) under one protocol 1 Clinical Study Actuate - 1801 1 Database for 1801 Parts 1 - 3A locked; final CSR in development GnP: gemcitabine/nab - paclitaxel Avg 3+ lines prior therapy (n=67) BOR: CR, PR, SDs DCR (16 wks): 42% 48% of patients went on to subsequent therapies Simon Two - Stage Trial (International) Randomized Controlled Trial (International) 1801 - Part 1 Elraglusib FIH Monotherapy Dose Escalation 1801 - Part 2 Elraglusib Dose Escalation in Combination with Standard Dosing Chemotherapy All patients required to have previously failed the combination chemo prior to enrollment Elraglusib + Gemcitabine Elraglusib + GnP Elraglusib + Carboplatin Elraglusib + Lomustine Elraglusib + Irinotecan Elraglusib + Paclitaxel Carboplatin Elraglusib + Doxorubicin Elraglusib + Pemetrexed Carboplatin 1801 - Part 3 Company Sponsored Phase 2 Studies 1801 - Part 3A First Line mPDAC GnP + elraglusib 1801 - Part 3B First Line mPDAC GnP + elraglusib


6 Elraglusib Opportunity Novel GSK - 3 β inhibitor that targets multiple molecular pathways in cancer cells but also impacts the TME and immune response Pancreatic Cancer Metastatic pancreatic cancer is highly aggressive and accounts for approximately 80 - 85% of all pancreatic cancer diagnoses. Projected market growth to >$5 billion by 2030 Survival Rate and Economic Burden The prognosis remains poor with a 5 - year survival rate of less than 10% and a high economic burden with annual treatment costs exceeding $100,000 per patient Current Treatment FOLFIRINOX and gemcitabine with nab - paclitaxel (GnP) are standard but offer limited survival benefits. Elraglusib is currently in clinical trials with FOLFIRINOX and GnP Elraglusib Potential in Metastatic Pancreatic Cancer (mPDAC) Pancreatic Cancer Treatment Market Size Report, 2030, https://www.grandviewresearch.com/industry - analysis/pancreatic - cancer - treatment - market ; Hu JX, Zhao CF, Chen WB, Liu QC, Li QW, Lin YY, Gao. World J Gastroenterol. 2021 Jul 21;27(27):4298 - 4321. doi: 10.3748/wjg.v27.i27.4298. PMID: 34366606; PMCID: PMC8316912. Soefje SA. Managing the economic impact of advanced pancreatic cancer. Am J Manag Care. 2019 Jan;25(1 Suppl):S11 - S16. PMID: 30681820.
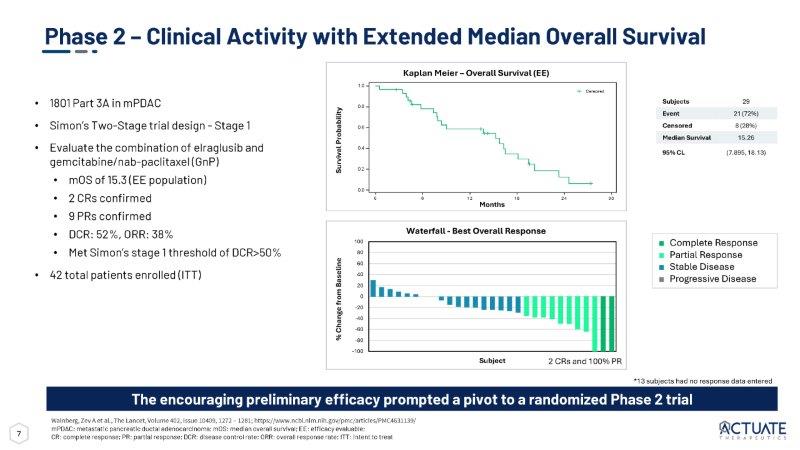
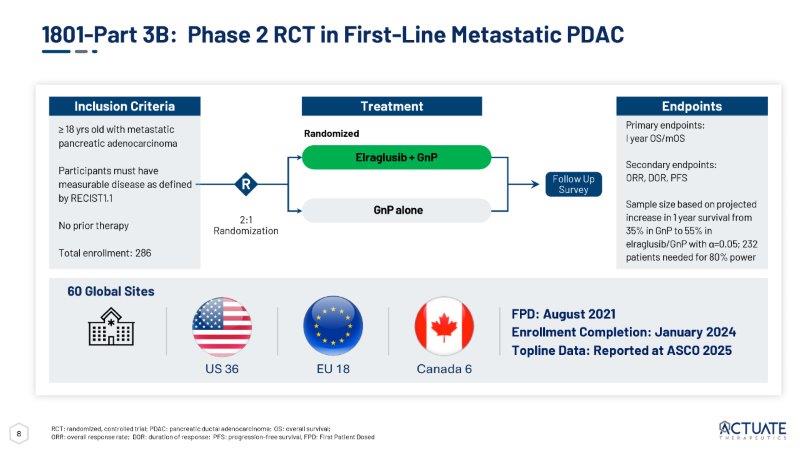
7 Phase 2 – Clinical Activity with Extended Median Overall Survival • 1801 Part 3A in mPDAC • Simon’s Two - Stage trial design - Stage 1 • Evaluate the combination of elraglusib and gemcitabine/nab - paclitaxel (GnP) • mOS of 15.3 (EE population) • 2 CRs confirmed • 9 PRs confirmed • DCR: 52%, ORR: 38% • Met Simon’s stage 1 threshold of DCR>50% • 42 total patients enrolled (ITT) Wainberg, Zev A et al., The Lancet, Volume 402, Issue 10409, 1272 – 1281; https:// www.ncbi.nlm.nih.gov/pmc/articles/PMC4631139/ mPDAC: metastatic pancreatic ductal adenocarcinoma; mOS: median overall survival; EE: efficacy evaluable; CR: complete response; PR: partial response; DCR: disease control rate; ORR: overall response rate; ITT: intent to treat The encouraging preliminary efficacy prompted a pivot to a randomized Phase 2 trial 100 80 60 40 20 0 - 20 - 40 - 60 - 80 - 100 Subject *13 subjects had no response data entered ■ Complete Response ■ Partial Response ■ Stable Disease ■ Progressive Disease Waterfall - Best Overall Response Kaplan Meier – Overall Survival (EE) 29 Subjects 21 (72%) Event 8 (28%) Censored 15.26 Median Survival (7.895, 18.13) 95% CL Survival Probability % Change from Baseline 0.8 0.6 0.4 0.2 0.0 1.0 0 6 1 2 1 8 2 4 Censored 3 0 Months 2 CRs and 100% PR 8 2:1 Randomization R ≥ 18 yrs old with metastatic pancreatic adenocarcinoma Participants must have measurable disease as defined by RECIST1.1 No prior therapy Total enrollment: 286 Inclusion Criteria Endpoints Primary endpoints: I year OS/mOS Secondary endpoints: ORR, DOR, PFS Sample size based on projected increase in 1 year survival from 35% in GnP to 55% in elraglusib/GnP with α =0.05; 232 patients needed for 80% power Randomized Treatment Elraglusib + GnP GnP alone Follow Up Survey 1801 - Part 3B: Phase 2 RCT in First - Line Metastatic PDAC RCT: randomized, controlled trial; PDAC: pancreatic ductal adenocarcinoma; OS: overall survival; ORR: overall response rate; DOR: duration of response; PFS: progression - free survival, FPD: First Patient Dosed 60 Global Sites FPD: August 2021 Enrollment Completion: January 2024 Topline Data: Reported at ASCO 2025 EU 18 US 36 Canada 6

9 PDAC: A Disease with Urgent Unmet Needs • Most patients with PDAC present with advanced or metastatic disease with poor survival • Median survival remains < 1 year, with real world data for mOS ranging from 6 - 9 months • Apart from PARP inhibitors as maintenance therapy in a subset of patients with gBRAC1/2 metastatic PDAC patients, there is an urgent need for more novel therapeutic targets for PDAC patients 1 year OS (%) mOS (months) Comparison Study 35.0 vs 22.0 8.5 vs 6.7 HR 0.72 ; p< 0.001 GnP vs Gem MPACT (Von Hoff et al. 2013) 39.5 vs 45.6 9.2 vs 11.1 HR 0.83 ; p< 0.036 GnP vs NALIRIFOX NAPOLI - 3 (Wainberg et al., 2024) N/A 6.87 vs 9.27; p< 0.001 GnP vs FFX retrospective, nonrandomized Klein - Brill et al. (2022) N/A GnP 6.9 (3.6 - 9.8) FFX 9.2 (4.7 - 11.4) GnP and FFX Real - world review Cockrum et al. (2025)

10 Predefined Safety Population Draft unaudited data as of March 27, 2025 The final data and results may change as the study continues through completion Phase 2 RCT Patient Demographics Elraglusib + GnP (n=155) GnP (n=78) Demographics Sex 75 (48.4%) 35 (44.9%) Female 80 (51.6%) 43 (55.1%) Male Age (years) 155 (100%) 78 (100%) n (%) 65.1 (9.1) 66.2 (9.9) Mean (S.D.) 65.0 68.0 Median 42.0, 86.0 42.0, 85.0 Min, Max Race 5 (3.2%) 2 (2.6%) Asian 7 (4.5%) 6 (7.7%) Black or African American 128 (82.6%) 65 (83.3%) White 1 (0.6%) 0 Multiracial 14 (9.0%) 5 (6.4%) Unknown/Not Reported Ethnicity 8 (5.2%) 0 Hispanic or Latino 141 (91.0%) 77 (98.7%) Not Hispanic or Latino 6 (3.9%) 1 (1.3%) Unknown/Not Reported Body Surface Area (BSA) (m2) 154 (99.4%) 78 (100%) n (%) 1.82 (0.22) 1.83 (2.23) Mean (S.D.) 1.81 1.82 Median 1.30, 2.41 1.31, 2.77 Min, Max Elraglusib + GnP (n=155) GnP (n=78) Demographics Eastern Cooperative Oncology Group Performance Status 64 (41.3%) 31 (39.7%) 0 89 (57.4%) 45 (57.7%) 1 2 (1.3%) 2 (2.6%) 2 Disease Status 109 (70.3%) 59 (75.6%) Metastatic at Initial Diagnosis 154 (99.4%) 77 (98.7%) Metastatic at Study Entry Site of Metastases 123 (79.4%) 68 (87.2%) Pancreas 112 (72.3%) 61 (78.2%) Liver 69 (44.5%) 27 (34.6%) Lymph Node 58 (37.4%) 26 (33.3%) Lung RCT: randomized, controlled trial 11 Study 1801 Part 3B Meets Primary Endpoint of Improved Survival PDAC: pancreatic ductal adenocarcinoma; OS: overall survival; ORR: overall response rate; DCR: disease control rate; PFS: progression - free survival Elraglusib/GnP (155) GnP (78) 10.1 7.2 Primary Endpoint: mOS (months) HR=0.63; log - rank p=0.01 44.1 22.3 12 month OS (%) p=0.0005 114 (73.5%) 70 (89.7%) Events (% events) 19.7 4.4 18 - month OS (%) 13.8 0 24 - month OS (%) 5.6 5.1 mPFS (months) HR=0.90; P=NS 136 (87.7%) 75 (96.2%) Events (% events) 61.3% 56.4% DCR 45 (29.0%) 17 (21.8%) ORR n (%) Predefined Safety Population Draft unaudited data as of March 27, 2025 mOS, p=0.01 Doubling of 1 year OS and 37% Reduction in Risk of Death vs GnP Elraglusib + GnP mOS, p=0.01 Elraglusib + GnP The final data and results may change as the study continues through completion

12 Administrative Analysis of 1801 Part 3B OS Swim Plot The final data and results may change as the study continues through completion GnP + weekly elraglusib: n=155 (8 on treatment, 114 events) GnP : n= 78 (0 on treatment, 70 events) Predefined Safety Population Draft unaudited data as of March 27, 2025 13 Predefined Safety Population Data as of March 27, 2025 Phase 2 RCT in First - Line Metastatic PDAC - Best Overall Response The final data and results may change as the study continues through completion.
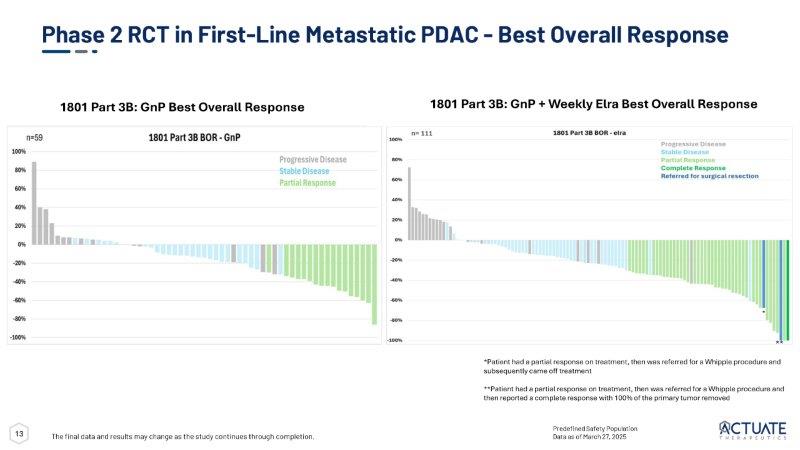
1801 Part 3B: GnP + Weekly Elra Best Overall Response 1801 Part 3B: GnP Best Overall Response * ** *Patient had a partial response on treatment, then was referred for a Whipple procedure and subsequently came off treatment **Patient had a partial response on treatment, then was referred for a Whipple procedure and then reported a complete response with 100% of the primary tumor removed 14 Elraglusib + GnP Data Show OS Benefit Across Key Subgroups The final data and results may change as the study continues through completion Predefined Safety Population Data as of April 27, 2025 Draft unaudited data Elraglusib + GnP Better Elraglusib + GnP,


15 The final data and results may change as the study continues through completion Subgroup of Patients Treated for One Cycle (4 weeks) - Significant Benefit in OS PDAC: pancreatic ductal adenocarcinoma; OS: overall survival; ORR: overall response rate; DCR: disease control rate; PFS: progression - free survival Elraglusib/GnP (116) GnP (58) 12.5 8.5 Primary Endpoint: mOS (months) HR=0.57; log - rank p=0.018 52.5 28.3 12 - month OS (%) 85 (73.3%) 50 (86.2%) Events (% events) 21.5 0 18 - month OS (%) 12.1 0 24 - month OS (%) 6.9 5.6 mPFS (months) HR=0.78; P=NS 105 (90.5%) 55 (94.8%) Events (% events) 53.4% 44.8% DCR 44 (37.9%) 17 (29.3%) ORR n (%) Predefined Safety Population Draft unaudited data as of March 27, 2025 Near Doubling of 1 year OS and 43% Reduction in Risk of Death vs GnP Elraglusib + GnP mOS, p=0.018 16 Patients with Liver Metastases - Significant Benefit in mOS and mPFS 2.5X Increase in 1 Year Survival and a 38% Reduction in Risk of Death Elraglusib/GnP (114) GnP (61) 8.3 6.6 Primary Endpoint: mOS (months) HR=0.62; log - rank p=0.008 39.2 15.2 12 month OS (%) p=0.0003 92 (80.7%) 56 (91.8%) Events (% events) 13.6 0 18 - month OS (%) 0 0 24 - month OS (%) 4.9 3.9 mPFS (months) HR=0.72; P=0.039 104 (91.2%) 59 (96.7%) Events (% events) 36.8% 27.9% DCR 34 (29.8%) 12 (19.7%) ORR n (%) Predefined Safety Population Draft unaudited data as of March 27, 2025 * z - test PDAC: pancreatic ductal adenocarcinoma; OS: overall survival; ORR: overall response rate; DCR: disease control rate; PFS: progression - free survival mOS, p=0.008 Elraglusib + GnP The final data and results may change as the study continues through completion

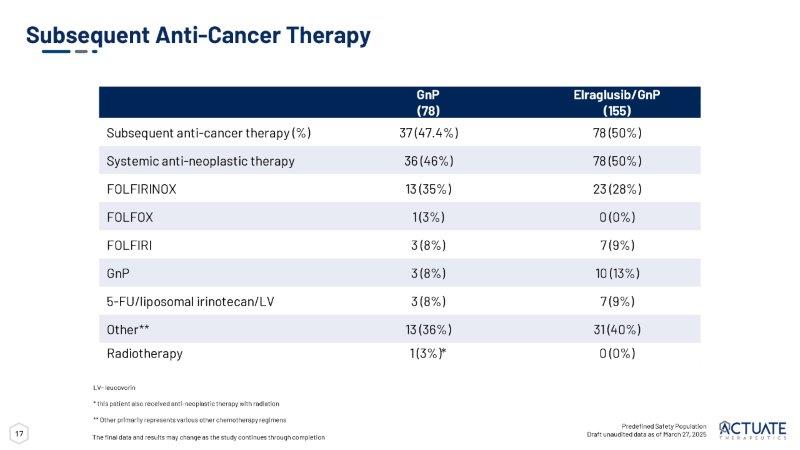
17 Subsequent Anti - Cancer Therapy Predefined Safety Population Draft unaudited data as of March 27, 2025 LV - leucovorin * this patient also received anti - neoplastic therapy with radiation ** Other primarily represents various other chemotherapy regimens The final data and results may change as the study continues through completion Elraglusib/GnP GnP (155) (78) 78 (50%) 37 (47.4%) Subsequent anti - cancer therapy (%) 78 (50%) 36 (46%) Systemic anti - neoplastic therapy 23 (28%) 13 (35%) FOLFIRINOX 0 (0%) 1 (3%) FOLFOX 7 (9%) 3 (8%) FOLFIRI 10 (13%) 3 (8%) GnP 7 (9%) 3 (8%) 5 - FU/liposomal irinotecan/LV 31 (40%) 13 (36%) Other ** 0 (0%) 1 (3%)* Radiotherapy 18 Actuate 1801 Part 3B (ongoing) TEAEs of Any Grade Reported in 20% of Patients Treated with elraglusib Safety Profile of Elraglusib in Combination with GnP • Overall rate of a TEAE and/or an SAE observed were similar in the elraglusib + GnP - treated patients as compared to GnP - treated patients • Treatment discontinuation due to TEAEs were similar across the treatment groups • Visual impairment and fatigue were major TEAEs attributed to elraglusib as a single agent in 1801 Part 1 and were mild to moderate in the 1801 3B 1 • Transient visual impairment described as transient alterations in color and skin tones under fluorescent light • No permanent changes to eye structure or vision Key Takeaways 1 Carneiro et al.

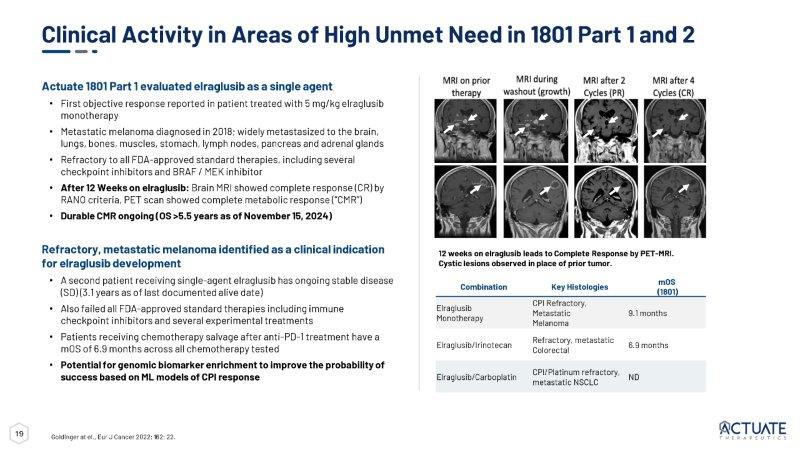
Clin Cancer Res 2024 Feb 1;30(3):522 - 531 *Includes Preferred Terms (PT) neutropenia and neutrophil count decreased ** Includes PT anemia and hemoglobin decreased ***Includes PT thrombocytopenia and platelet count decreased Predefined Safety Population Draft unaudited data as of March 27, 2025 TEAE: Treatment - Emergent Adverse Event The final data and results may change as the study continues through completion 19 mOS (1801) Key Histologies Combination 9.1 months CPI Refractory, Metastatic Melanoma Elraglusib Monotherapy 6.9 months Refractory, metastatic Colorectal Elraglusib/Irinotecan ND CPI/Platinum refractory, metastatic NSCLC Elraglusib/Carboplatin 12 weeks on elraglusib leads to Complete Response by PET - MRI. Cystic lesions observed in place of prior tumor. Clinical Activity in Areas of High Unmet Need in 1801 Part 1 and 2 Actuate 1801 Part 1 evaluated elraglusib as a single agent • First objective response reported in patient treated with 5 mg/kg elraglusib monotherapy • Metastatic melanoma diagnosed in 2018; widely metastasized to the brain, lungs, bones, muscles, stomach, lymph nodes, pancreas and adrenal glands • Refractory to all FDA - approved standard therapies, including several checkpoint inhibitors and BRAF / MEK inhibitor • After 12 Weeks on elraglusib: Brain MRI showed complete response (CR) by RANO criteria, PET scan showed complete metabolic response (“CMR”) • Durable CMR ongoing (OS >5.5 years as of November 15, 2024) Refractory, metastatic melanoma identified as a clinical indication for elraglusib development • A second patient receiving single - agent elraglusib has ongoing stable disease (SD) (3.1 years as of last documented alive date) • Also failed all FDA - approved standard therapies including immune checkpoint inhibitors and several experimental treatments • Patients receiving chemotherapy salvage after anti - PD - 1 treatment have a mOS of 6.9 months across all chemotherapy tested • Potential for genomic biomarker enrichment to improve the probability of success based on ML models of CPI response Goldinger at el., Eur J Cancer 2022; 162: 22.

20 Elraglusib Oral Formulations Provide Similar Drug Exposures to IV • Decreased cost of manufacturing at commercial scale compared to IV formulations • Phase 1 Study of Oral Solution in Normal Healthy Volunteers (NHV) showed > 50 % bioavailability vs IV after a single dose • Exposure and pharmacodynamic effects exhibited in fed/fasted patients • Oral Solid > 95 % bioavailability vs IV when dosed with food • Phase 1 dose escalation study using Elraglusib Oral Tablet in advanced cancer patients (not healthy volunteers) in planning stage Elraglusib Arithmetic Mean Concentration - Time Profiles 21 Key Near Term Anticipated Development Plans and Milestones For Accelerating Registration in mPDAC 2026 2025 Jan Mar Jun mPDAC: Initiate planning of Phase 3 or Confirmatory Trial mPDAC: Phase 2 Topline data mPDAC: Part 3A publication mPDAC: ASCO Presentation Sep Dec mPDAC: FDA Type B pre - NDA Meeting to discuss P2 data supportive NDA 2 1: Referencing IND previously filed with DO2 2: Contingent on future discussions with the FDA and future funding mPDAC: Initiate Phase 3 or Confirmatory Trial (if required) 2 Jan Jun mPDAC: Start Rolling / RTOR NDA (P2 Data) 2 mPDAC: DO3 IND Submission 1 mPDAC: FDA Type B pre - NDA Meeting Request


22 Steven D. Reich, MD – Sr VP, Clinical Development and Acting Chief Medical Officer • Oncology drug development executive leader for commercial clinical development and strategy • Directed multi - national medical research groups within pharmaceutical/biotechnology companies and CRO • Lead investigator for Phase I - III trials and designed and managed Phase I - IV trials for industrial sponsors • Headed the clinical research programs leading to multiple US, Canadian, and European drug approvals • Epogen, Targretin, Panretin, Fludara, Inlyta Seasoned and Successful Leadership Experienced leadership team with demonstrated ability to develop and commercialize cancer drugs Daniel M. Schmitt – Chief Executive Officer and Founder • 30+ years of biotechnology and pharmaceutical experience across senior executive roles • Led and contributed to the successful development and launch of multiple pharmaceutical products • Exosurf, Zovirax, Valtrex, Adenoscan, Ambisome, Duraclon, Campath, Abraxane, enTrust • Executed ~1B+ in milestone value through licensing, acquisition, and development deals Andrew Mazar, PhD – Chief Operating Officer and Scientific Co - Founder • Co - founder, Chief Scientific Officer and Director, Monopar Therapeutics, Inc. (Nasdaq: MNPR) • Entrepreneur - in - Residence; Professor of Pharmacology; Founding Director, Center for Developmental Therapeutics, Northwestern University • Chief Scientific Officer, Attenuon, LLC • Internationally recognized expert in cancer metastasis and translational oncology • Eleven drugs from discovery through Phase 2 • >250 peer - reviewed publications and book chapters and inventor on > 70 patents • Serial entrepreneur with seven start - ups founded Paul Lytle – Chief Financial Officer • 30+ years of finance and accounting experience • 25+ years of public company experience for Nasdaq listed companies • Served as co - founder, CFO, and director for multiple biotech companies • Raised in excess of $500 million in net proceeds from various equity and debt offerings 23 Extensive data on activity from leading research institutions and promising clinical data in multiple cancer histologies Developing elraglusib to address therapeutic shortcomings in key difficult - to - treat and refractory tumors Focus on mediating cancer cell survival and chemoresistance through regulation of NF - kB and regulating antitumor immune response Expansive, global patent portfolio with significant exclusivity runway Multiple key regulatory designations available (Fast Track, Orphan Drug) with registration path clinical trials underway and in development Distinguished leadership and recognized world leading scientific advisory team Leading Therapeutic Profile Significant Unmet Needs Complementary Mechanisms of Action Robust IP Portfolio Clearly Defined Regulatory Path Seasoned Leadership Team Investment Highlights


The Passion to Pursue More
Exhibit 99.2

Actuate Therapeutics Presents Topline Elraglusib Phase 2 Data at ASCO 2025 Annual
Meeting: Trial Meets Primary Endpoint of Median Overall Survival and Doubles 1-Year
Survival in First-Line Treatment of Metastatic Pancreatic Cancer
| - | Phase 2 (Actuate-1801 Part 3B) trial meets primary endpoint and demonstrates a clinically meaningful increase in median overall survival (10.1 months vs 7.2 months; log-rank p=0.01) in previously untreated patients with metastatic pancreatic ductal adenocarcinoma (mPDAC) receiving elraglusib/GnP | |
| - | Risk of death was reduced by 37% (HR=0.63) in patients treated with elraglusib/GnP | |
| - | Data featured as an oral presentation at the ASCO Annual Meeting | |
| - | Company plans to engage with FDA in the second half of 2025 to align on a path towards product registration | |
| - | Company to host KOL event today at 6:30 pm CDT to discuss 1801 Part 3B results |
CHICAGO, IL and FORT WORTH, TX, May 31, 2025 – Actuate Therapeutics, Inc. (NASDAQ: ACTU) (“Actuate” or the “Company”), a clinical-stage biopharmaceutical company focused on developing therapies for the treatment of high-impact, difficult-to-treat cancers through the inhibition of glycogen synthase kinase-3 beta (GSK-3β), today presented topline results from the Phase 2 (Actuate-1801 Part 3B) trial of elraglusib in combination with gemcitabine/nab-paclitaxel (GnP) in previously untreated patients with metastatic pancreatic ductal adenocarcinoma (mPDAC) at the American Society of Clinical Oncology (ASCO) Annual Meeting.
Abstract Title: Preliminary results from the randomized phase 2 study (1801 Part 3B) of elraglusib in combination with gemcitabine/nab-paclitaxel (GnP) versus GnP
alone in patients with previously untreated metastatic pancreatic ductal
adenocarcinoma (mPDAC).
Abstract Number: 4006
Session Title: Gastrointestinal Cancer—Gastroesophageal,
Pancreatic, and Hepatobiliary
Presenter: Devalingam Mahalingam, MD, PhD
Oral Presentation Date and Time: Saturday, May 31, 2025, 4:48 PM
CDT
The trial met its primary endpoint of improved median overall survival. Median overall survival (mOS) increased by almost three months (10.1 months vs 7.2 months, HR=0.63, log-rank p=0.01) with a 37% reduction in the risk of death and strong statistical significance, representing a clinically meaningful advance in the potential treatment of mPDAC. Patients receiving elraglusib with GnP achieved a 12-month survival rate in 44.1% of patients treated—double that of GnP alone (22.3%).
Dr. Deva Mahalingam, MD, PhD, Northwestern University Feinberg School of Medicine, and lead principal investigator of the 1801 Part 3B trial commented, “Pancreatic cancer continues to represent one of the highest unmet medical needs with no major treatment advances in recent years, bar some molecular targets in small subsets of patients. New mechanisms of action are urgently needed. The addition of elraglusib to the existing chemotherapy backbone of gemcitabine and nab-paclitaxel is promising and may represent a meaningful therapeutic advance for patients with pancreatic cancer.”
Daniel Schmitt, President & Chief Executive Officer of Actuate added, “We significantly improved mOS, cut the risk of death by 37%, and doubled the 12-month survival rate. Combined with a manageable safety profile and strong emerging mechanistic insights, these results reinforce the transformative potential of our GSK-3β inhibitor program. Based on the clear clinical benefit and well-tolerated safety profile, we intend to engage with the FDA and EMA in the second half of this year to align on a path to registration. We believe this enables us to move rapidly towards commercialization and delivery of this first-in-class therapy to patients with an urgent unmet need.”
|
|
Efficacy
| · | Figure 1 below depicts the Kaplan-Meier estimate for mOS (10.1 months vs 7.2 months, HR=0.63, log-rank p=0.01) with a 37% reduction in the risk of death, displaying a clear clinical survival benefit for patients treated in the elraglusib/GnP combination arm vs GnP alone arm. |
Figure 1: Actuate-1801 Part 3B: Kaplan-Meier Estimate for mOS as of March 27, 2025 (Topline data cut-off).
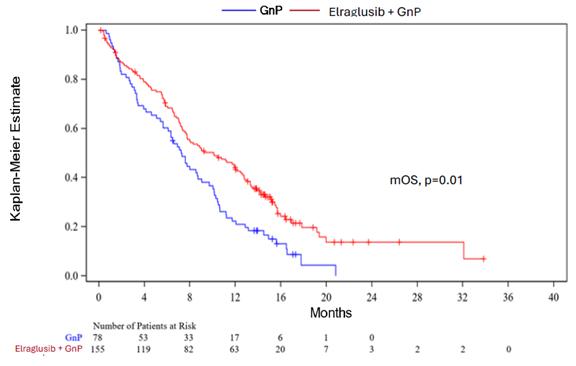
| · | In addition to the improved mOS and one-year survival rate, a continued survival benefit was also observed at eighteen and twenty-four months (survival rates of 19.7% vs 4.4% and 13.8% vs 0% in the elraglusib/GnP combination arm vs the GnP arm, respectively). | |
| · | The elraglusib/GnP combination treatment also resulted in numerically improved overall response rates (29.0% in the elraglusib/GnP combination arm vs 21.8% in the GnP arm) and improvements in median progression-free survival and median duration of response of 5.6 months vs 5.1 months, and 5.5 months vs 4.0 months in the elraglusib/GnP combination arms vs GnP arms, respectively. |
Safety and Biomarker Findings
| · | The trial also met its primary safety endpoint. Treatment-emergent adverse events (TEAEs) and Serious Adverse Events (SAEs) in the elraglusib/GnP combination arm were similar to those observed in the GnP arm, indicating a favorable risk-benefit profile for the elraglusib/GnP combination. |
| o | Treatment-related adverse events (TRAEs) were mostly Grade 1-2, with the most frequent TRAEs observed (in about two-thirds of patients) being transient visual impairments that were reversible and non-progressive | |
| o | While Grade 3 or higher neutropenia was observed, similar rates of febrile neutropenia and sepsis were observed in both treatment arms. |
| · | Pre-dose cytokine analysis that suggested lower baseline levels of key immune modulators, including CCL3, IL-1α, IL-18, TGF-β, and TRAIL R3, were correlated with improved 1-year survival. | |
| · | Increased CD8-positive and granzyme B-positive T cells, increased NK cells, and decreased myeloid-derived suppressor cells were observed in tumor biopsies only from elraglusib-treated patients. This exploratory result confirms elraglusib proposed immune modulating mechanism of action in patients with mPDAC. |
|
|
KOL Event
Actuate will host a KOL event for the investment community today, May 31, 2025, at 6:30 PM CDT to review the data. The webinar will feature a fireside discussion moderated by Daniel Schmitt, President & Chief Executive Officer of Actuate, and will include four distinguished KOLs: Tanios Bekaii-Saab, MD, FACP, Mayo Clinic College of Medicine and Science; Devalingam Mahalingam, MD, Northwestern University Feinberg School of Medicine; Rachna Shroff, MD, MS, FASCO, University of Arizona Cancer Center; and Colin Weekes, MD, PhD, Massachusetts General Hospital.
Event Details:
| Date and Time: | Saturday, May 31, 2025, at 6:30 pm CDT |
| Format: | In-person and via live webcast |
| Registration: | Click here |
A replay of the event will be available on the Investor Relations section of the Actuate website.
About Actuate-1801 Part 3B Study
The Actuate-1801 Part 3B study (NCT03678883) is a randomized, controlled Phase 2 trial of elraglusib with GnP versus GnP alone in first-line mPDAC. The trial enrolled 286 mPDAC patients with no prior systemic treatment for metastatic disease, who were randomized 2:1 to the elraglusib treatment arm (elraglusib + GnP) or the control arm (GnP alone). Elraglusib is administered at a dose of 9.3 mg/kg by IV infusion on Day 1 of each week of a 28-day cycle. The primary endpoint for this study is median overall survival, with OS summarized throughout the study by estimates of 1-year survival. Secondary endpoints are DCR, ORR, PFS, and AE.
Inhibition of GSK-3β may inhibit tumor growth and improve survival through several complimentary mechanisms that include enhancement
of chemotherapy activity, activation of innate anti-tumor immunity, and regulation of gene expression, leading to alterations in tumor
metabolism and Epithelial-to-Mesenchymal Transition (EMT).
About Actuate Therapeutics, Inc.
Actuate is a clinical-stage biopharmaceutical company focused on developing therapies for the treatment of high-impact, difficult-to-treat cancers. Actuate’s lead investigational drug, elraglusib (a novel GSK-3β inhibitor), targets molecular pathways in cancer that are involved in promoting tumor growth and resistance to conventional cancer drugs such as chemotherapy through the inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and DNA Damage Response (DDR). Elraglusib may also mediate anti-tumor immunity through the regulation of multiple immune checkpoints and immune cell function. For additional information, please visit the Company’s website at http://www.actuatetherapeutics.com.
|
|
Forward-Looking Statements
This press release contains forward-looking statements about us, including our and other parties’ clinical trials and development plans, and our industry. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” or the negative of these terms or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. All statements, other than statements related to present facts or current conditions or of historical facts, contained in this press release are forward-looking statements. Accordingly, these statements involve estimates, assumptions, substantial risks and uncertainties which could cause actual results to differ materially from those expressed in them, including but not limited to that preliminary and unpublished data may be subject to change and further interpretation following the availability of more data or following a more comprehensive review of the data and should not be relied upon as a final analysis; the risk that clinical trial data are subject to differing interpretations and assessments by regulatory authorities and within the medical community; clinical and preclinical drug development involves a lengthy and expensive process with uncertain timelines and outcomes, results of prior preclinical studies and early clinical trials are not necessarily predictive of future results, and elraglusib may not achieve positive clinical results or favorable preclinical results, and we may not be able to make regulatory submissions or receive regulatory approval on a timely basis, if at all; that we may not successfully enroll additional patients or establish or advance plans for further development, including through conversations with the FDA or EMA and the standards such bodies may impose for such development; that elraglusib could be associated with side effects, adverse events or other properties or safety risks, which could delay or preclude regulatory approval, cause us to suspend or discontinue clinical trials or result in other negative consequences; our reliance on third parties to conduct our non-clinical studies and our clinical trials; our reliance on third-party licensors and ability to preserve and protect our intellectual property rights; that we face significant competition from other biotechnology and pharmaceutical companies; our ability to fund development activities, including because our financial condition raises substantial doubt as to our ability to continue as a going concern and we require additional capital to finance our operations beyond the second quarter of fiscal year 2025, and a failure to obtain this necessary capital in the near term on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our development programs, commercialization efforts or other operations. In addition, any forward-looking statements are qualified in their entirety by reference to the factors discussed under the heading “Item 1A. Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on March 13, 2025, our Quarterly Report on Form 10-Q for the quarter ended March 31, 2025, filed with the SEC on May 15, 2025, and other filings with the SEC. Because the risk factors referred to above could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us or on our behalf, you should not place undue reliance on any forward-looking statements. Further, any forward-looking statement speaks only as of the date on which it is made. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Unless legally required, we do not undertake any obligation to release publicly any revisions to such forward-looking statements to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events.
Investor Contact
Mike Moyer
Managing Director
LifeSci Advisors, LLC
mmoyer@lifesciadvisors.com
|
|