SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_____________________
FORM 8-K
_____________________
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of Earliest Event Reported): December 5, 2023
_____________________
COEPTIS THERAPEUTICS HOLDINGS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-39669 | 98-1465952 |
|
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
|
105 Bradford Rd, Suite 420 Wexford, Pennsylvania |
15090 | |
| (Address of principal executive offices) | (Zip Code) |
724-934-6467
(Registrant’s telephone number, including area code)
____________________________________________________________
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
|
Common Stock, par value $0.0001 per share |
COEP |
Nasdaq Capital Market |
||
| Warrants, each whole warrant exercisable for one-half of one share of Common Stock for $11.50 per whole share |
COEPW |
Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
|
|
| Item 7.01 | Regulation FD Disclosure |
On December 5, 2023, Coeptis Therapeutics Holdings, Inc. (the “Company” or “Coeptis”) posted a corporate presentation (the “Presentation”) to its website and it is available in the Presentations section of the Company’s website at https://coeptistx.com/presentation. A copy of the Presentation is included as Exhibit 99.1 to this Current Report on Form 8-K.
The Company intends to use the Presentation in presentations to investors and analysts from time to time in the future. The furnishing of the information in this Current Report on Form 8-K is not intended to, and does not, constitute a determination by the Company that the information in this Current Report on Form 8-K is material or complete, or that investors should consider this information before making an investment decision with respect to any security of the Company. The information in the materials is presented as of December 5, 2023, and the Company does not assume any obligation to update such information in the future.
The information in Item 7.01 of this Current Report on Form 8-K shall not be deemed to be “filed” for the purposes of Section 18 of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of such section, nor shall such information be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. | Description |
| 99.1 | Coeptis Therapeutics Holdings, Inc. Presentation |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
|
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Coeptis Therapeutics Holdings, Inc. | ||
| Date: December 5, 2023 | By: | /s/ David Mehalick |
|
David Mehalick Chief Executive Officer |
||
|
|
Exhibit 99.1

1 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Coeptis Therapeutics Holdings, Inc.

Corporate Overview NASDAQ: COEP C10050 V5.0 December - 2023 2 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Cautionary Note Regarding Forward - Looking Statements Certain statements in this Presentation, and statements by management or other persons acting by or on behalf of Coeptis made in connection with this Presentation, constitute “forward - looking statements” within the meaning of the safe harbor provisions of t he United States Private Securities Litigation Reform Act of 1995. Forward - looking statements are neither historical facts nor assurances of future performance. Because forward - looking statements relate to the future, they are inherently subject to significant known and unkno wn risks, uncertainties and other factors that are difficult to predict and are beyond the control of Coeptis. The actual results, lev el of activity, performance or achievements of Coeptis may be materially different from any future results, levels of activity, performance o r a chievements expressed or implied by these forwards - looking statements. Forward - looking statements generally are accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticip ate,” “intend,” “expect,” “should,” “would,” “plan,” “future,” “outlook,” and similar expressions that predict or indicate future events or t ren ds. All statements that are not statements of historical matters are forward - looking statements. The forward - looking statements made in this Presentation are based on Coeptis’ current assumptions and judgments regarding futur e events and results. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. Ma ny actual events and circumstances are beyond the control of Coeptis. Some important factors that could cause actual results to differ ma terially from those in any forward - looking statements could include changes in domestic and foreign business, market, financial, political and legal conditions. These forward - looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied upon as, a guarantee, an assurance, a prediction or a definitive statement of fact, probability or outcome.

3 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Important Legal Disclaimers No Offer or Solicitation: This Presentation is for informational purposes and does not constitute an offer to sell, a solicitation of an offer to buy, or a recommendation to purchase any security of Coeptis, or any of its affiliates nor shall there be any sale of securities, inv estment or other specific product in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qua lif ication under the securities laws of any such jurisdiction. No Representation: Neither Coeptis, nor any of its subsidiaries, shareholders, affiliates, representatives, control persons, partners, members, managers, directors, officers, employees, advisers or agents make any representation or warranty, express or implied, as to t he accuracy, completeness or reliability of the information contained in this Presentation. To the fullest extent permitted by law, in no ci rcumstances will Coeptis or any of its subsidiaries, shareholders, affiliates, representatives, control persons, partners, members, managers, dir ectors, officers, employees, advisers or agents be responsible or liable for any direct, indirect or consequential loss or loss of profit arisi ng from the use of this Presentation, its contents, its omissions, reliance on the information contained within it, or on opinions communicated in re lat ion thereto or otherwise arising in connection therewith. Date of Information: This Presentation speaks only as of the date hereof. Coeptis does not intend to update or otherwise revise this Presentation following its use, except the extent required by law. Coeptis makes no representation or warranty, express or i mpl ied, as to the accuracy or completeness of any of the information contained in this Presentation.

4 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Important Legal Disclaimers cont’d. This Presentation is not a substitute for any other document that Coeptis may file with the SEC, and this Presentation is qua lif ied in its entirety by such SEC filings. Readers may obtain free copies of all documents filed with the SEC by Coeptis and previously b y i ts wholly - owned subsidiary Coeptis Therapeutics, Inc. through the website maintained by the SEC at www.sec.gov. Risk Awareness. Any reader of this Presentation should be aware of the numerous risks facing Coeptis in the operation of its business and pursuit of its growth strategy. Those risks include those that are detailed in sections entitled “Risk Factors” in documents pr eviously or hereafter filed or furnished by Coeptis with the SEC, and you should carefully consider those risks and uncertainties, togeth er with the financial statements and related notes filed with the SEC. Note that there may be also additional risks that are currently n ot known about or that are currently believed to be immaterial may also impair its business, financial condition or results of operations. You sho uld review this Presentation and perform your own due diligence and consult with your own financial and legal advisors, with such risks in co nsi deration.

5 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Risk Factors There are a number of risks related to us and our operations. You should carefully review the risks described in our filings th at we make with the SEC from time to time. If any of these risks actually occurs, our business, financial condition, results of operations and pr osp ects would likely be materially, adversely affected. In that event, the trading price of our common stock could be adversely impacted, and you co uld lose part or all of your investment. Below is a summary of some of the principal risks we face: • We may not be able to successfully implement our growth strategy on a timely basis or at all; • We may have difficulties managing our anticipated growth, or we may not grow at all; • We have a history of losses, we expect to incur losses in the future and we may not be able to achieve or maintain profitabil ity ; • We may not be able to initiate and complete preclinical studies and clinical trials for our product candidates which could ad ver sely affect our business; • We may not be able to obtain and maintain the third - party relationships that are necessary to develop, commercialize and manufac ture some or all of our product candidates; • We may encounter difficulties in managing our growth, which could adversely affect our operations; • We need to obtain financing in order to continue our operations; • The drug development and approval process is uncertain, time - consuming and expensive; • Competition in the biotechnology and pharmaceutical industries may result in competing products, superior marketing of other pro ducts and lower revenues or profits for us; • Federal laws or regulations on drug importation could make lower cost versions of our future products available, which could adv ersely affect our revenues, if any; • The regulatory approval process is costly and lengthy, and we may not be able to successfully obtain all required regulatory app rovals; • Healthcare reform measures could adversely affect our business; • Protecting and defending against intellectual property claims may have a material adverse effect on our business; • If we are not able to retain our current senior management team and our scientific advisors or continue to attract and retain qu alified scientific, technical and business personnel, our business will suffer; and • We may not be able to maintain our listing on the Nasdaq Capital Market; and • There is a substantial doubt about our ability to continue as a going concern.

6 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved COEPTIS THERAPEUTICS We are a Pittsburgh, PA based clinical stage biotechnology company founded by an experienced team developing innovative cell therapy platforms in oncology and other diseases .
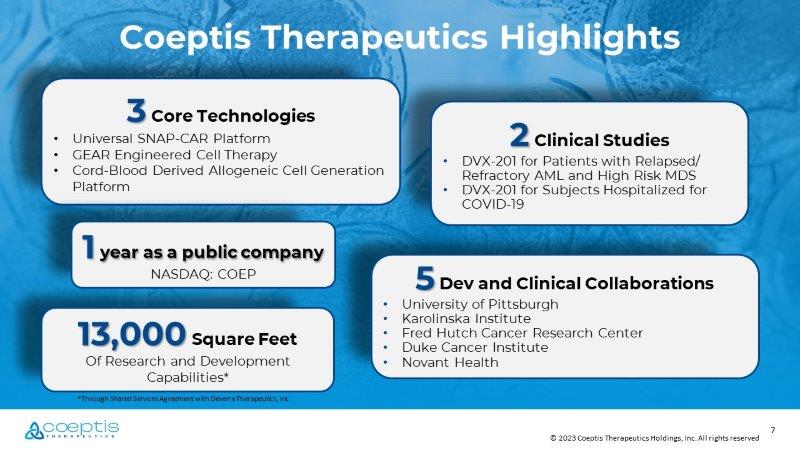
7 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved 5 Dev and Clinical Collaborations • University of Pittsburgh • Karolinska Institute • Fred Hutch Cancer Research Center • Duke Cancer Institute • Novant Health 2 Clinical Studies • DVX - 201 for Patients with Relapsed/ Refractory AML and High Risk MDS • DVX - 201 for Subjects Hospitalized for COVID - 19 13,000 Square Feet Of Research and Development Capabilities* 3 Core Technologies • Universal SNAP - CAR Platform • GEAR Engineered Cell Therapy • Cord - Blood Derived Allogeneic Cell Generation Platform Coeptis Therapeutics Highlights *Through Shared Services Agreement with Deverra Therapeutics, Inc 1 year as a public company NASDAQ: COEP 8 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Corporate Milestones 2021 Secured ownership rights to GEAR from VyGen - Bio 2022 IPO listed on NASDAQ (COEP) 2023 Expanded SNAP - CAR License 2022 License SNAP - CAR from University of Pittsburgh 2023 Licensed DVX - 201 clinical programs and immune cell generation platform from Deverra Therapeutics 2023 Onboard Deverra R&D site 2024 Anticipated close - out of DVX - 201 clinical trials


9 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Diverse Pipeline PHASE 2 PHASE 1 PRE - CLINICAL TARGET INDICATION PROGRAM Relapsed/Refractory Acute Myeloid Leukemia & High Risk MDS DVX201 - AML - 01 ( Allo ) Acute Viral Respiratory Diseases DVX201 - COV - 01 ( Allo ) Multiple Myeloma CD38 - GEAR - NK (TBD) Solid Tumors SNAP - CAR - T (Auto) Heme Malignancies Solid Tumors SNAP - CAR - NK ( Allo ) Oncology Engineered - MAC ( Allo ) We are focused on creating best - in - class modified and un - modified cell therapies, with a focus on leveraging: • Allogeneic model when possible • Clinically - tested, cord - blood derived starting material 10 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Proprietary Allogeneic Immune Effector Cell Generation Platform • FDA - regulated cord blood as source material • Established pooled donor manufacturing and cryopreservation (2 FDA approved INDs fo r NK cell product, DVX201 ) • Reproducible, scalable, very low COGs and simple clinical delivery • Clinical safety data in two indications using unmodified NK cells * SNAP - CAR, GEAR engineering
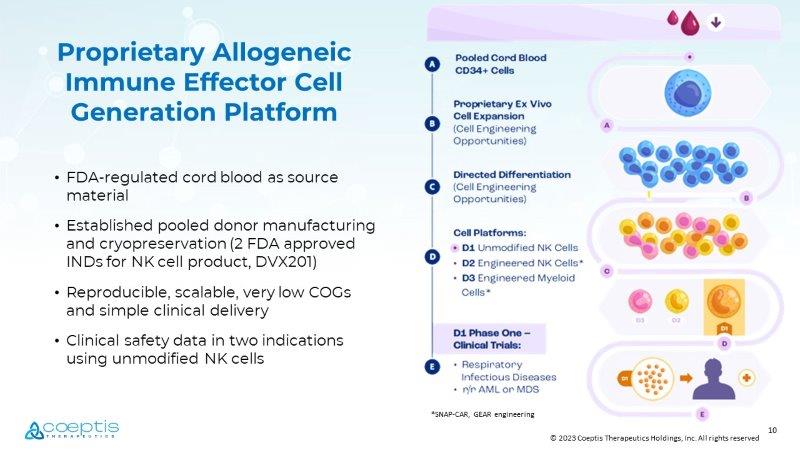

11 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved DVX - 201: Phase 1 Clinical Trials of a First - in - Human Pooled Donor Unmodified NK Cell Product • Indication 1 : COVID Pneumonia (NCT04900454), Cell Therapy in Subjects Hospitalized for COVID - 19 • Dosing : Single dose, MTD study; 100, 300, 900M CD56+ cells/dose • PI : Dr. Josh Hill, Fred Hutchinson Cancer Research Center/University of WA Preliminary Clinical Results to Date: • Enrollment complete, 3+3 MTD design • N= 9 patients enrolled • All dose levels tolerated • No DLTs, no CRS, no infusion toxicities • Clinical correlatives include: Persistence, immune activation studies, cytokines • Indication 2 : Relapsed/Refractory AML & High Risk MDS/MPN (NCT04901416), Cell Infusions in Patients with Relapsed/ Refractory AML and High Risk MDS • Dosing : Multiple dose, MTD study (2 doses/patient); 100, 300, 900M CD56+ cells/dose • PI : Dr. Tom LeBlanc, Duke University • Study Chair : Dr.

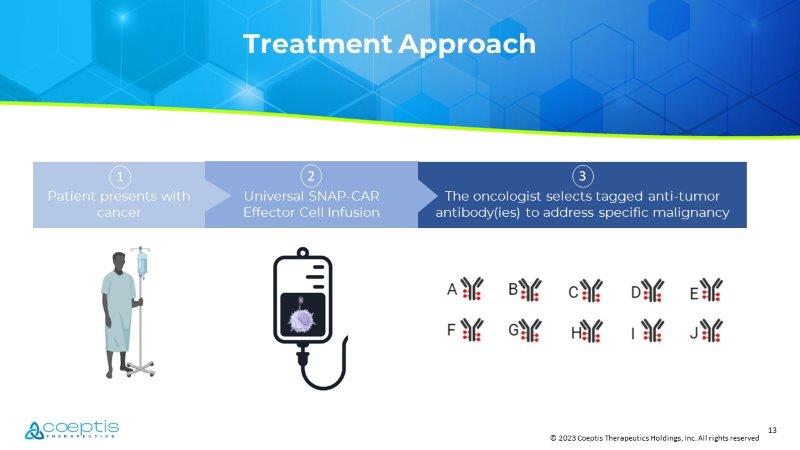
David Rizzieri, Novant Preliminary Clinical Results to Date: • Enrollment ongoing (Open to accrual at Duke and Novant) • 2 dose levels completed; currently at dose level 900M • No DLTs, LPLV expected 4Q23 12 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Proprietary Platform Technology: A monoclonal antibody conjugated (tagged) to a benzyl guanine linker which is specifically recognized by the custom SNAP - CAR effector cells Potential to generate a variety of SNAP - CAR effector cells (T cells and NK cells) in the autologous or allogeneic settings Advantages: Multiple targets and indications: By using universal SNAP - CAR cells and multiple anti - tumor antibodies, hematological malignancies and solid tumors can likely be targeted Tunable Activity: toxicity (ex: cytokine release syndrome) may be avoided by titrating the antibody dose SNAP - CAR: A Universal CAR Platform 13 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Patient presents with cancer Universal SNAP - CAR Effector Cell Infusion The oncologist selects tagged anti - tumor antibody( ies ) to address specific malignancy Treatment Approach 1 2 3

14 © 2023 Coeptis Therapeutics Holdings, Inc.
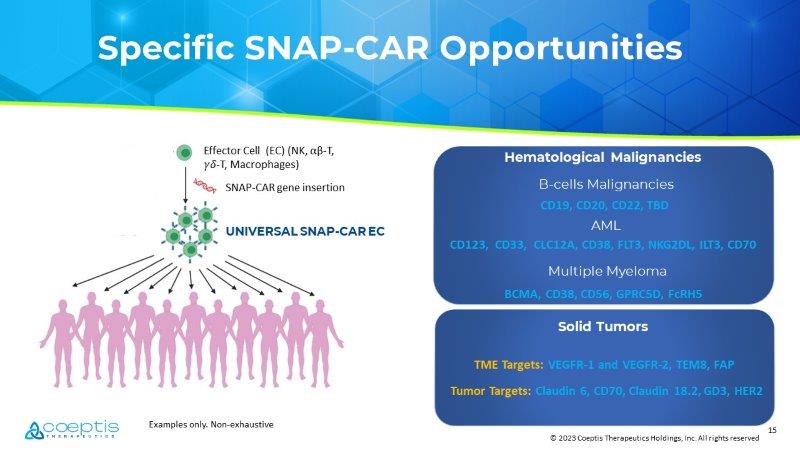
All rights reserved Ability to Target Both Heme Malignancies and Solid Tumors with a Single Bank of SNAP - CAR - ECs Hematological Malignancies Solid Tumors TBD TBD TUMOR MICROENVORNMENT CANCER CELL Examples Only 15 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Specific SNAP - CAR Opportunities Effector Cell (EC) (NK, ⍺ β - T, ߛ ߜ - T, Macrophages) SNAP - CAR gene insertion UNIVERSAL SNAP - CAR EC Multiple Myeloma B - cells Malignancies Solid Tumors CD19, CD20, CD22, TBD CD123, CD33, CLC12A, CD38, FLT3, NKG2DL, ILT3, CD70 TME Targets: VEGFR - 1 and VEGFR - 2, TEM8, FAP Tumor Targets: Claudin 6, CD70, Claudin 18.2, GD3, HER2 AML BCMA, CD38, CD56, GPRC5D, FcRH5 Hematological Malignancies Examples only.

Non - exhaustive 16 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved IMMUNOTHERAPY CHALLENGES Various immunotherapies for CD38+ tumors are designed to find and kill cells that express the CD38 antigen; therefore, CD38+ NK cells are likely to become collateral damage and with their eradication, the overall anti - tumor response is suboptimal. THE GOAL To protect CD38+ Natural Killer (NK) cells so that functional disease - targeting NK cells will not be eradicated, enabling their co - existence with CD38 targeting therapies, and thus allowing for complementary tumor killing and immune surveillance. A NOVEL COMBINATORIAL APPROACH Modified NK cells that are co - administered with select monoclonal antibodies and/or other CD38 targeting immunotherapies are in pre - clinical development to test ability to enhance and maximize tumor kill via combinatorial approaches otherwise not possible. CD38 - GEAR - NK Cells (Gene Edited Antibody Resistant) Animations herein are provided as visual aids to help articulate hypothesized proof - of - concept in a general manner and do not depict precise scientific mechanisms - of - action.

17 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Animations herein are provided as visual aids to help articulate hypothesized proof - of - concept in a general manner and do not de pict precise scientific mechanisms - of - action. Anti - CD38 mAbs (Infused antibodies from current cancer treatments) bind to CD38 proteins and kill both CD38+ Cancer cells and our body’s Natural Killer Cells. CD38 - GEAR - NK are modified, NK cells that we believe can avoid being ablated by therapies designed to target the CD38 antigen, potentially enabling the combination of passive immunity with innate active immunity to more efficiently target and eradicate CD38+ malignancies.

CURRENT ANTI - CD38 PATHWAY PATHWAY WITH GEAR - NK (CD38) CELL THERAPY 18 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved COEPTIS MANAGEMENT TEAM Dave Mehalick Co - Founder, President & CEO 30 years of diverse business experience in healthcare, information technology and finance including consulting, capital markets, private equity, and investments Christine Sheehy Vice President of Compliance and Corporate Secretary 30 years of finance and operational experience, mainly in pharmaceutical and life science startup companies leading design and development of global systems Lara Ionescu Silverman, PhD VP of CMC and Clinical Operations 10+ years of gene and cell therapy experience including roles in CMC, early - stage clinical testing, and technical product and process development. Dan Yerace Co - Founder & VP Operations 10+ years of pharmaceutical experience including roles in global supply chain, operations, business development, and procurement Colleen Delaney MD, MSc CSO & CMO World - renowned expert in cell and gene therapy research with 20+ years' experience in the translation of scientific discovery to clinical practice, including all aspects of cell therapy product development Brian Cogley Chief Financial Officer 15 + years of corporate financial experience in life sciences, pharmaceuticals, and financial services, expertise in asset management, and investments 19 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Coeptis Scientific Advisory Board Head of the Gene and Cell Therapy Group, Division of Hematology, Department of Medicine, Karolinska Institutet, Karolinska University Hospital, Stockholm Evren Alici M.D., Ph.D. Arnika K. Wagner, Ph.D. Assistant Professor, Department of Medicine, Karolinska Institutet, Karolinska University Hospital, Stockholm Former Dean of Research, Karolinska Institutet and founder of the Center for Infectious Medicine, Department of Medicine, Karolinska Institutet, Karolinska University Hospital, Stockholm Hans - Gustaf Ljunggren, M.D., Ph.D.


20 © 2023 Coeptis Therapeutics Holdings, Inc. All rights reserved Coeptis Public Relations Contact: Daniel Kontoh - Boateng Tiberend Strategic Advisors dboateng@tiberend.com coeptistx.com