UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
________________________
FORM 8-K
________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 6, 2023
________________________
Xenetic Biosciences, Inc.
(Exact name of registrant as specified in charter)
| Nevada | 001-37937 | 45-2952962 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 945 Concord Street | |
| Framingham, Massachusetts | 01701 |
| (Address of principal executive offices) | (Zip Code) |
(781) 778-7720
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock, $0.001 par value per share | XBIO | The Nasdaq Stock Market | ||
| Purchase Warrants | XBIOW | The Nasdaq Stock Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
|
|
| Item 7.01. | Regulation FD Disclosure. |
Attached to this report as Exhibit 99.1 is the current corporate presentation of Xenetic Biosciences, Inc. (the “Company”), which the Company has prepared in anticipation of potential upcoming investor meetings. The presentation is furnished pursuant to this Item 7.01 and shall not be deemed filed in this or any other filing of the Company with the Securities and Exchange Commission, unless expressly incorporated by specific reference in any such filing.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Updated 2023 Corporate Presentation | |
| 104 | Cover Page Interactive Data File (formatted as inline XBRL). |
|
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| XENETIC BIOSCIENCES, INC. | |
| By: /s/ James Parslow | |
| Date: June 6, 2023 | Name: James Parslow |
| Title: Chief Financial Officer |
|
|
Exhibit 99.1

nasdaq : XBIO xeneticbio.com Corporate Presentation June 2023

This presentation contains forward - looking statements that we intend to be subject to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 . All statements contained in this presentation other than statements of historical facts may constitute forward - looking statements within the meaning of the federal securities laws . These statements can be identified by words such as "expects," "plans," "projects," "will," "may," "anticipates," "believes," "should," "intends," "estimates," and other words of similar meaning, including, but not limited to : all statements set forth under the “Investment Highlights” section of this presentation, including those relating to the DNase I technology platform ; our statements regarding DNase I providing opportunity to address multiple oncology indication ; our belief that DNase I has the potential to improve current cancer therapies ; our currently planned Phase 1 study ; our belief that we will be successful with respect to pancreatic cancer ; our belief that targeting solid tumors provides opportunities for significant upside ; our expectation of advancing with our collaboration with VolitionRX ; and all statements under the “Investment Summary” section, including statements relating to advancing the technology platform . Any forward - looking statements contained herein are based on current expectations and are subject to a number of risks and uncertainties . Many factors could cause our actual activities or results to differ materially from the activities and results anticipated in forward - looking statements . Important factors that could cause actual results to differ materially from such plans, estimates or expectations include, among others, ( 1 ) uncertainty of the expected financial performance of the Company ; ( 2 ) failure to realize the anticipated potential of the DNase I platform or XCART or PolyXen technologies ; ( 3 ) the ability of the Company to implement its business strategy ; ; and ( 4 ) other risk factors as detailed from time to time in the Company’s reports filed with the SEC, including its annual report on Form 10 - K, periodic quarterly reports on Form 10 - Q, periodic current reports on Form 8 - K and other documents filed with the SEC . The foregoing list of important factors is not exclusive . In addition, forward - looking statements may also be adversely affected by general market factors, general business and economic conditions, including potential adverse effects of public health issues such as the COVID - 19 pandemic, competitive product development, product availability, federal and state regulations and legislation, the regulatory process for new product candidates and indications, manufacturing issues that may arise, patent positions and litigation, among other factors . The forward - looking statements contained in this presentation speak only as of the date the statements were made, and the Company does not undertake any obligation to update forward - looking statements, except as required by law . Disclaimer The information contained in this presentation is provided for informational and discussion purposes only and is not, and may not be relied on in any manner as legal, business, financial, tax or investment advice or as an offer to sell or a solicitation of an offer to buy an interest in Xenetic Biosciences, Inc . or to participate in any trading strategy .

Forward Looking Statements 2 Investment Highlights 3 Focused on advancing proprietary technology platform to address multiple high - value cancer indications DNase I Oncology Platform Aimed at improving immunotherapies by targeting Neutrophil Extracellular Traps (NETs) DNase I – Our Innovative Solution DNase I is an enzyme that digests DNA and can eliminate NETs Exposes cancer cells to the immune system, chemotherapy and other targeted cancer treatments The Power of Leveraging DNase I The Problem NETs promote tumorigenesis and metastasis by shielding tumor cells from the immune system NETs can also contribute to resistance to chemotherapy, checkpoint inhibitors and radiotherapy 4 Innovative Oncology Pipeline Opportunity to Address Multiple Oncology Indications DNase I HIGHLIGHTS PHASE 2 PHASE 1 IND ENABLING PRECLINICAL INDICATIONS TECHNOLOGY PROGRAM Upcoming study to evaluate combination with chemo Pancreatic Carcinoma Systemic DNase I (+Chemo) XBIO - 015 Upcoming study to evaluate combination with ICIs Solid Tumors Systemic DNase I (+ICIs) Potential to enhance CAR T cell function in the tumor microenvironment Solid Tumors Systemic DNase I (+CAR T) Potential to enhance CAR T cell function in the tumor microenvironment Solid Tumors DNase I - Armored CAR T XBIO - 020


Leveraging DNase I to Target Neutrophil Extracellular Traps (NETs) 5

The Role of Neutrophil Extracellular Traps (NETs) 6 NETs are an Innate Immune Response to Kill Invading Pathogens NETs are composed of cell - free DNA, histones, neutrophil elastase, MMP - 9 and other proteins Neutrophil Cancer Cells Cancer Cells Caught in NET Elevated levels of NETs lead to inflammation and a pro - tumorigenic environment that potentiates coagulopathies and cancer progression NET Role of NETs in Cancer Progression 7 Primary Tumor Microenvironment NETs Neutrophil Secondary Metastatic Tumor Epithelial - Mesenchymal Transition (EMT) of primary tumor cells Circulating tumor cells are shielded by NETs, preventing attack by the immune system NETs can potentiate the establishment of metastatic niches



NETs Can Limit the Effectiveness of Current Cancer Therapies 8 Shaping of the Tumor Microenvironment (TME) Engaging in Pro - tumorigenic and Immunosuppressive Signaling, thereby Promoting Cancer Cell Proliferation, Invasion and Metastasis Promoting Hypercoagulability and Treatment - Associated Thrombosis Exacerbated by Chemotherapy Neutrophils Extracellular Traps Inhibition Improves PD - 1 Blockade Immunotherapy in Colorectal Cancer Hongji Zhang, Yu Wang, Amblessed Onuma , Jiayi He, Han Wang, Yujia Xia, Rhea Lal 5, Xiang Cheng, Gyulnara Kasumova , Zhiwei Hu, Meihong Deng, Joal D. Beane, Alex C. Kim, Hai Huang, and Allan Tsung Neutrophils Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress Samer Tohme , Hamza O. Yazdani , Ahmed B. Al - Khafaji , Alexis P. Chidi , Patricia Loughran , Kerri Mowen , Yanming Wang , Richard L. Simmons , Hai Huang , Allan Tsung Interleukin - 17 - Induced Neutrophil Extracellular Traps Mediate Resistance to Checkpoint Blockade in Pancreatic Cancer Yu Zhang , Vidhi Chandra , Erick Riquelme Sanchez , Prasanta Dutta , Pompeyo R Quesada , Amanda Rakoski , Michelle Zoltan , Nivedita Arora , Seyda Baydogan , William Horne , Jared Burks , Hanwen Xu , Perwez Hussain , Huamin Wang , Sonal Gupta , Anirban Maitra , Jennifer M Bailey , Seyed J Moghaddam , Sulagna Banerjee , Ismet Sahin , Pratip Bhattacharya , Florencia McAllister Neutrophil Extracellular Traps Promote Inflammation and Development of Hepatocellular Carcinoma in Nonalcoholic Steatohepatitis Dirk J van der Windt , Vikas Sud , Hongji Zhang , Patrick R Varley , Julie Goswami , Hamza O Yazdani , Samer Tohme , Patricia Loughran , Robert M O'Doherty , Marta I Minervini , Hai Huang , Richard L Simmons , Allan Tsung Citrullinated Histone H3, a Biomarker for Neutrophil Extracellular Trap Formation, Predicts the Risk of Mortality in Patients with Cancer Ella Grilz , Lisa - Marie Mauracher , Florian Posch , Oliver Königsbrügge , Sabine Zöchbauer - Müller , Christine Marosi , Irene Lang , Ingrid Pabinger , Cihan Ay The Literature Confirms the Presence of NETs is Associated with a Poor Prognosis 9 10 Systemic DNase I Mode of Action Primary Tumor Microenvironment NETs Neutrophil DNase I DNase I is an enzyme that digests DNA and can eliminate NETs thereby exposing cancer cells to the immune system, chemotherapy and other targeted cancer treatments Co - administered with Immune Checkpoint Inhibitors or Chemotherapy


DNase I Has the Potential to Improve Current Cancer Therapies 11 Overcome T cell exclusion and immunosuppressive signals by the tumor microenvironment (TME) Improve side effect profiles of current ChemoRx DNase I Improves Efficacy of PD - 1 Blockade 12 Combination of DNase I and anti - PD - 1 mAb resulted in the lowest tumor volume growth, superior to either DNase I or anti - PD - 1 alone Systemic administration of DNase I improves the efficacy of PD - 1 blockade to reduce the growth of cancer in MC38 colorectal cancer cell model Control Anti - PD - 1 DNase I DNase I+ Anti - PD - 1 10 Days after tumor injection 21 Days after tumor injection 10 Days after tumor injection 400 Bioluminescence Radiance (photos*10 7 /sec) 300 200 100 0 4000 3000 2000 1000 0 Bioluminescence Radiance (photos*10 7 /sec) 21 Days after tumor injection * * * * * * * * * * * ns * * * * * * * * * * * ns Zhang, H.; Wang, Y.; Onuma , A.; He, J.; Wang, H.; Xia, Y.; Lal, R.; Cheng, X.; Kasumova , G.; Hu, Z.; Deng, M.; Beane, J.D.; Kim, A.C.; Huang, H.; Tsung, A. Neutrophils Extracellular Traps Inhibition Improves PD - 1 Blockade Immunotherapy in Colorectal Cancer. Cancers 2021, 13, 5333.
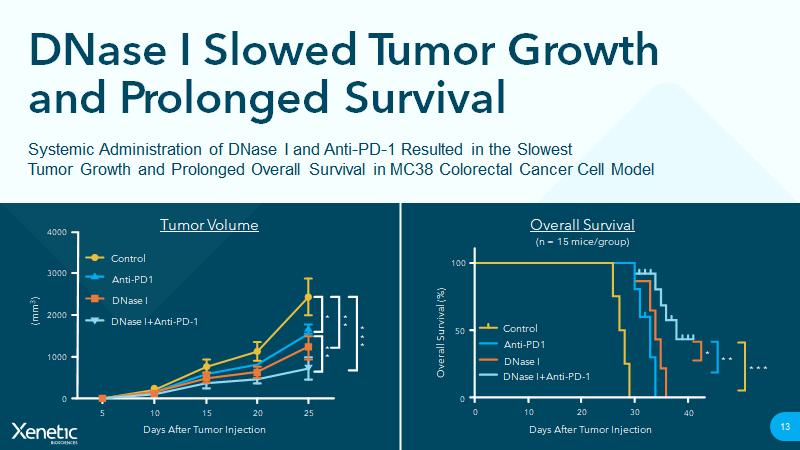
https://doi.org/10.3390/cancers13215333 DNase I Slowed Tumor Growth and Prolonged Survival 13 Systemic Administration of DNase I and Anti - PD - 1 Resulted in the Slowest Tumor Growth and Prolonged Overall Survival in MC38 Colorectal Cancer Cell Model Overall Survival Control Anti - PD1 DNase I DNase I+Anti - PD - 1 100 Overall Survival (%) Days After Tumor Injection 10 * 50 0 (n = 15 mice/group) 0 20 30 40 * * * * * Tumor Volume Control Anti - PD1 DNase I DNase I+Anti - PD - 1 4000 3000 2000 1000 (mm 3 ) Days After Tumor Injection 5 10 15 20 25 0 * * * * * * * * DNase I for the Treatment of Pancreatic Carcinoma 14 Advancing Toward First - In - Human Study


Initially Targeting Pancreatic Carcinoma 15 Multi - Billion - Dollar Indication with Significant Unmet Need 5 - year survival for advanced stage patients: ~3% 1 Early detection is currently not feasible – most patients are diagnosed at advanced stages Deadliest Cancer in the United States 1 3 rd Diagnosed Annually 2 ~62,000 Deaths Annually 2 ~50,000 Projected Market by 2025 3 $4.8B 1. U.S. Department of Health and Human Services. (n.d.). Common cancer sites - Cancer stat facts. SEER. Retrieved March 17, 2023, f rom https://seer.cancer.gov/statfacts/html/common.html 2. NIH National Cancer Institute, Surveillance, Epidemiology and End Results Program,Cancer Stat Facts: Pancreatic Cancer, https://seer.cancer.gov/statfacts/html/pancreas.html 3. Grand View Research, Inc. (n.d.). Global pancreatic cancer treatment market size report, 2025.

Retrieved March 17, 2023, from https://www.grandviewresearch.com/industry - analysis/pancreatic - cancer - treatment - market Currently Planned Phase 1 Study 16 Multicenter, dose escalation and dose - expansion in subjects with locally advanced or metastatic solid tumors IV administration of rhDNase I Monotherapy dose escalation followed by expansion in two cohorts Combined with chemotherapy for pancreatic cancer patients Combined with immunotherapy for patients with other solid tumor indications Primary Endpoints: safety, tolerability, efficacy, MTD and recommended Phase 2 dose Secondary Endpoints: PK, Efficacy (ORR by RECIST)

Key Drivers for Success 17 Pancreatic Cancer is a Challenging Indication but We Believe We Will Be Successful Relatively Low Hurdle for Demonstrating Clinical Meaningfulness ORR > 50% or PFS > 9 Months Would be Meaningful Improvement to Current SOC Ipsen’s NAPOLI - 3 Study 1 NALIRIFOX demonstrated 42% ORR vs. 36% ORR for nab - paclitaxel and gemcitabine mPFS for NALIRIFOX was 7.4 months vs. 5.6 months for nab - paclitaxel and gemcitabine 1L PDAC has 40% ORR, 7.5 months PFS, 11.1 months OS 1. Ipsen presents phase III napoli 3 trial of Onivyde ® regimen demonstrating positive survival results in previously untreated metastatic pancreatic ductal adenocarcinoma at ASCO GI. Ipsen. (2023, May 26). https://www.ipsen.com/press - releases/ipsen - presents - phase - iii - napoli - 3 - trial - of - onivyde - regim en - demonstrating - positive - survival - results - in - previously - untreated - metastatic - pancreatic - ductal - adenocarcinoma - at - asco - gi/ Application Across a Number of Solid Tumors 18 ~1.9 million new solid tumor cases in the U.S. in 2022 1 ~.6 million solid tumor related deaths in the U.S. in 2022 1 New Cases Annually 1 New Cases Annually 1 New Cases Annually 1 ~290K ~343K ~254K Breast Gastrointestinal Lung 1. 2022, American Cancer Society, Inc. Surveillance and health Equity Science



DNase I Armored CAR T 19 Targeting Solid Tumors Provides Opportunities for Significant Upside 20 DNase I Armored CAR T for Solid Tumors Requirements for Successful T Cell Therapies in Solid Tumors Find the tumor Infiltrate and persist in tumor Maintain cytotoxic function Barriers to Success in the Tumor Microenvironment Physical barriers (e.g., extracellular matrix or NETs) impeding infiltration and occluding tumor cell contact Immunosuppressive signaling from bioactive elements within the TME 21 DNase I - Armored CAR T for Solid Tumors Primary Tumor Microenvironment NETs DNase I CAR T CAR T cells that deliver DNase I while maintaining CAR T tumor killing function DNase I digests DNA, clearing NETs and allowing tumor access to CAR T


DNase I Armored CAR T: Proof of Concept 22 Ability to Design CAR T Cells That Deliver DNase I While Maintaining CAR T Function 0.00 0.05 0.10 0.15 0.20 0.25 0.30 0 500000 1000000 1500000 2000000 [DNase I] (mg/L) # of CAR T cells DNase I levels in culture media anti - HER2 CAR T DNase I - armored anti - HER2 CAR T -20% -10% 0% 10% 20% 30% 40% 50% CTL assay (LDH), 27h E:T Ratio Cytotoxicity 2:1 5:1 Target Cell Line: BT - 474 (HER2 + ) anti - HER2 CAR T DNase I - armored anti - HER2 CAR T Control CAR T 10000:5000 25000:5000 Secrete DNase I Retain Cytotoxic Function HER2 - Targeting , DNase I - Armored CAR T Cells :

Advancing with Collaboration Partner, VolitionRX 23 Developing Proprietary Adoptive Cell Therapies Potentially Targeting Multiple Solid Cancer Types DNase I - Armored CAR T Nu.Q ® Technology Expect Volition to fund research program and two parties to share proceeds from commercialization or licensing of any products arising from the collaboration Intellectual Property and Exclusivity 24 Systemic DNase I DNase I - Armored CAR T IP Portfolio Co - administration of Systemic DNase I with ICIs, Radiation, Chemo Orphan Designation DNase I for pancreatic cancer IP Portfolio Co - administration of Systemic DNase I with CAR T DNase I - secreting CAR T cells


Team with Proven Expertise 25 Jeffrey F. Eisenberg Chief Executive Officer & Director Life Sciences executive with over 25 years of successful track record in value creation in both private and public companies; fo rmer CEO of Noven Pharmaceuticals, responsible for leading 2 product launches and Noven’s Novogyne Women’s Health joint venture with Novartis Curtis Lockshin , Ph.D. Chief Scientific Officer 25 years Biotech/Pharma management experience, including discovery, preclinical and clinical development and commercial manuf act uring; former CEO of SciVac Therapeutics, CTO of VBI Vaccines and VP of Corporate R&D Initiatives for OPKO Health James F. Parslow , MBA, CPA Chief Financial Officer Over 30 years of experience providing financial and business leadership to biotech, manufacturing, technology, business - to - busin ess e - commerce and cleantech industries Scott N. Cullison Business Development Over 20 years of experience in the pharmaceutical industry with a broad range of expertise across business development, allia nce management, commercialization, product management, R&D program team leadership, and strategic planning. Reid P. Bissonnette, Ph.D.

Translational Research and Development Over 25 years of experience in small molecule drug discovery and development and biotherapeutics; well - established translational scientist, drug hunter and senior manager of Oncology and Inflammation drug R&D Dr. Allan Tsung Chair of the Department of Surgery at the University of Virginia School of Medicine and Director of the Cancer Therapeutics program at the University of Virginia Comprehensive Cancer Center; specializes in treating patients with liver, bile duct and pancreatic cancer Dr. Jonathan Spicer Associate Professor of Surgery at McGill University and Medical Director of the McGill University Health Center (MUHC) Thoracic Oncology Network; recognized as a leader in understanding how neutrophils impact cancer progression, in particular, the role of NETs in cancer biology Dr. Guenther Koehne Internationally recognized cancer specialist and current Chief of Blood & Marrow Transplant and Hematologic Oncology at the Miami Cancer Institute Dr. Matthew Frigault Medical Oncologist in the Hematologic Malignancy Program at the Massachusetts General Hospital Cancer Center, as well as Assistant Director of the Cellular Immunotherapy Program; serves as an Instructor at Harvard Medical School Dr. Maksim Mamonkin Assistant Professor, Pathology and Immunology and an independent faculty member at the Center for Cell and Gene Therapy at Baylor College of Medicine Scientific Advisory Board 26 Financial Snapshot NASDAQ: XBIO 27 Receiving royalties on net sales through licensing arrangement in the field of blood coagulation disorders from legacy assets Average Volume 2 Shares Outstanding 3 Market Cap 2 Cash Balance 1 ~23K ~1.5M ~$5M ~$12M 1.

As of March 31, 2023 2. As of May 30, 2023, with closing price of $3.15 3. As of May 12, 2023

28 Key Upcoming Milestones 2024 - 2025 Activities 2022 - 2023 Activities Assets • Phase 1 study start x Engaged Catalent, preeminent CDMO for clinical manufacturing x IP supporting the use of DNase I in cancer • Dose escalation and expansion data available • Enhance preclinical data set x IND - enabling GLP Tox studies in 2 species for systemic DNase I • Business Development x Cell line & established cGMP process and manufacturing • Academic Collaborations Investment Summary 29 Advancing Proprietary Technology Platform Aimed at Improving Immunotherapies by Targeting Neutrophil Extracellular Traps (NETs) DNase I oncology platform has the potential to improve the efficacy of current cancer therapies Initially targeting pancreatic carcinoma, a multi - billion - dollar indication with significant unmet need Multiple key value - driving milestones expected over the next 12 - 24 months

nasdaq : XBIO xeneticbio.com Investor Relations JTC Team 833 - 475 - 8247 xbio@jtcir.com