Document
Keros Therapeutics Presents Clinical Data from its KER-050 Program at the 65th American Society of Hematology Annual Meeting and Exposition
•KER-050 (elritercept) achieved durable transfusion independence in lower-risk MDS, including in patients with high transfusion burden
•Durable clinical responses were associated with improvements in patient-reported measures of fatigue
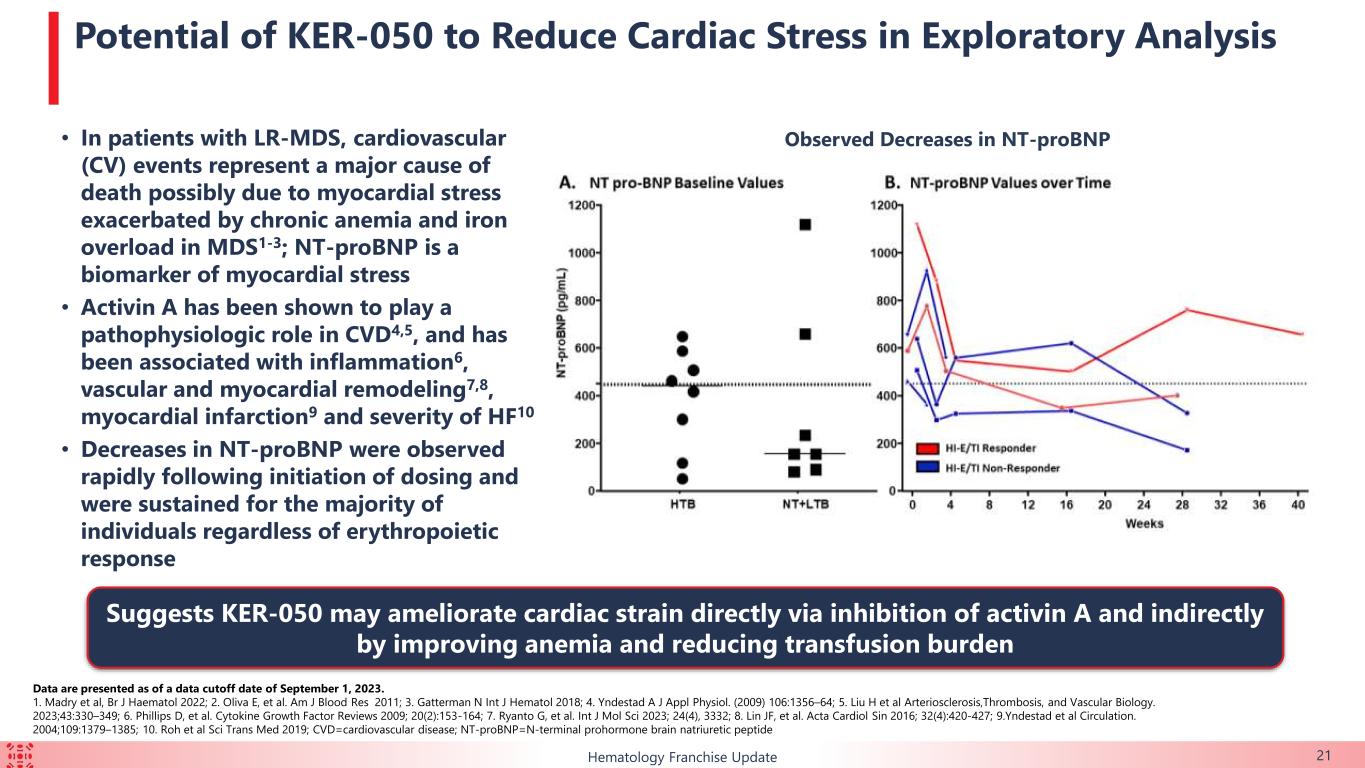
•Biomarker data demonstrate that KER-050 treatment has potential to reduce NT-proBNP a measure of cardiac stress
•Preliminary findings from ongoing Phase 2 clinical trial in myelofibrosis demonstrate that KER-050 can not only ameliorate ineffective hematopoiesis and address cytopenias, but also provide broader clinical benefit seen through reduction of spleen size and improved symptoms
•Keros will be hosting a conference call and webcast today, December 11, 2023, at 8:00 a.m. ET
LEXINGTON, Mass., Dec. 11, 2023 (GLOBE NEWSWIRE) -- Keros Therapeutics, Inc. (“Keros”) (Nasdaq: KROS), a clinical-stage biopharmaceutical company focused on developing and commercializing novel therapeutics to treat a wide range of patients with disorders that are linked to dysfunctional signaling of the transforming growth factor-beta (“TGF-ß”) family of proteins, today announced that it presented additional data from its two ongoing Phase 2 clinical trials of KER-050, one in patients with very low-, low-, or intermediate-risk myelodysplastic syndromes (“MDS”) and one in patients with myelofibrosis (“MF”), at the 65th American Society of Hematology (“ASH”) Annual Meeting and Exposition, held in person in San Diego and virtually from December 9 through 12, 2023. In addition, Keros presented preclinical data showing that a research form of KER-050 promoted erythropoiesis in an animal model of MF, as well as preclinical data evaluating the treatment effect of activin receptor-like kinase 2 inhibition in a mouse model of iron-refractory iron deficiency anemia.
“We believe the data presented at ASH from our ongoing KER-050 Phase 2 clinical trial in MDS supports the potential of KER-050 to ameliorate ineffective hematopoiesis and treat anemia and thrombocytopenia in difficult to treat patient populations, including those with greater transfusion burden and bone marrow dysfunction,” said Simon Cooper, MBBS, Chief Medical Officer of Keros. “Additionally, we are encouraged by the preliminary data from the lowest three dose cohorts from our ongoing Phase 2 clinical trial in MF. KER-050 treatment in combination with ruxolitinib and as KER-050 monotherapy led to improvements as assessed by changes in markers of hematopoiesis, reductions in spleen size and improvement in Total Symptom Score. We look forward to confirming this profile of benefit in the now enrolling dose confirmation part of our trial.”
“The encouraging broad profile of KER-050 that we have observed supports its potential to treat not just the disease-associated cytopenias, but also impact the pathogenesis of the respective diseases, as supported by the observed improvements in bone health and reduction of cardiac stress,” said Jasbir S. Seehra, Ph.D., President and Chief Executive Officer. “We are excited by the results we presented including the durability of transfusion independence observed with KER-050 and to engage with regulators in the first half of next year and look forward to sharing the design of a Phase 3 clinical trial evaluating KER-050 in lower-risk MDS following that feedback.”
Clinical Presentations
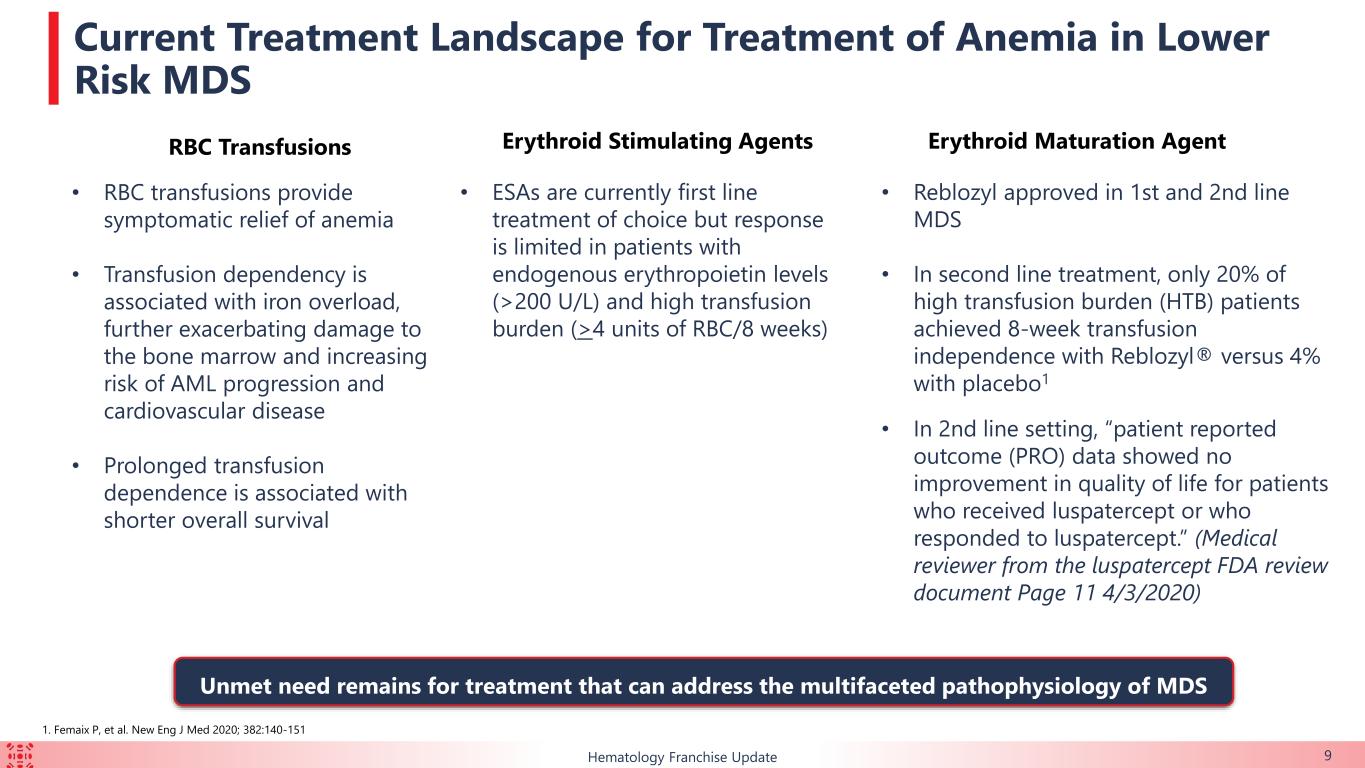
•Durable Clinical Benefit with KER-050 treatment: Findings From an Ongoing Phase 2 Study in Participants with Lower-Risk MDS
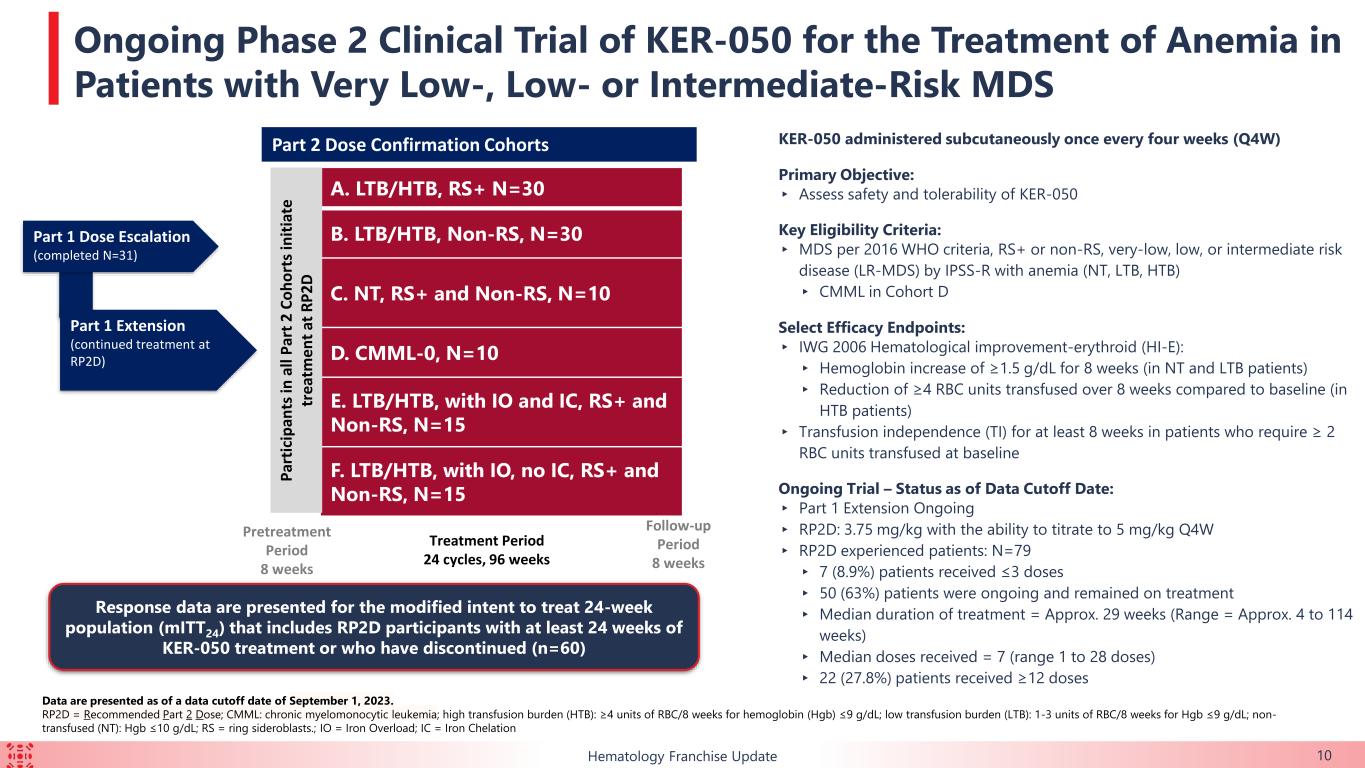
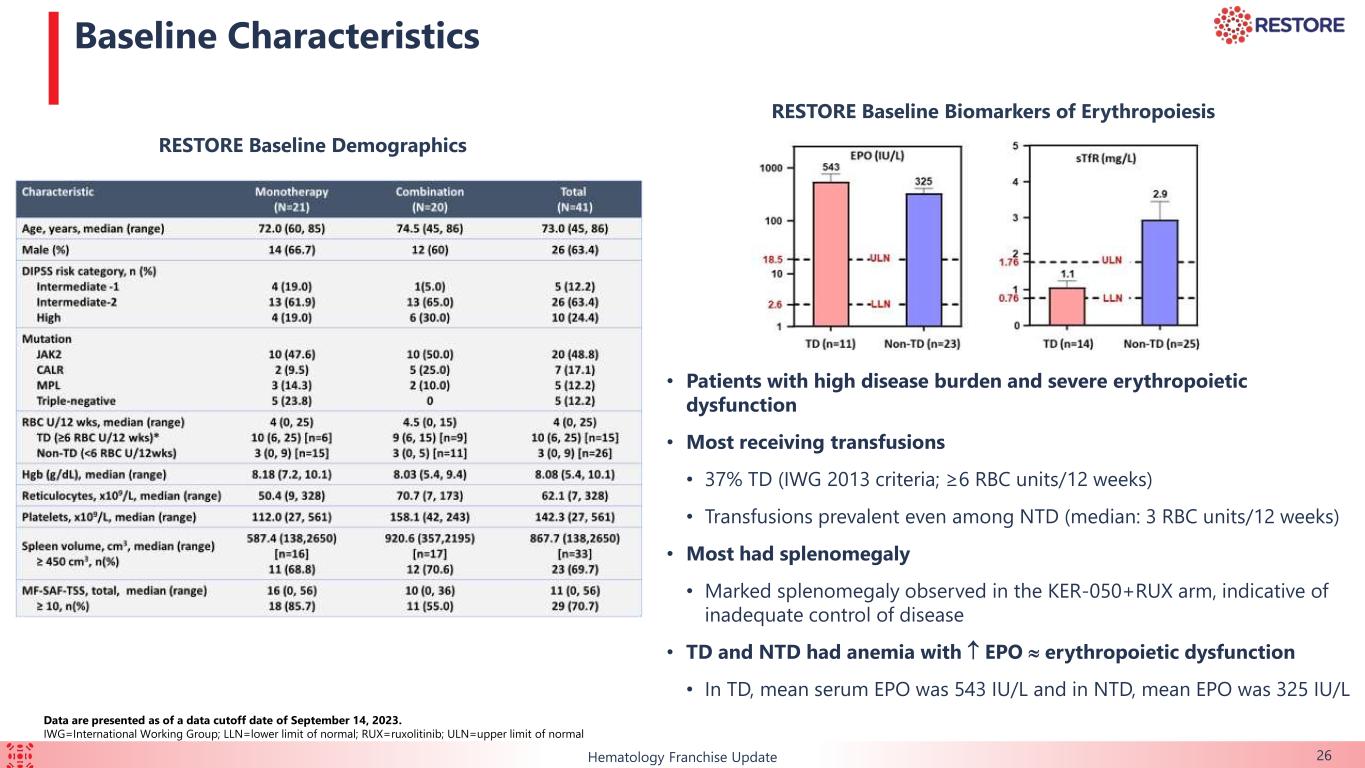
This ongoing, open-label, two-part, Phase 2 clinical trial is evaluating KER-050 in patients with very low-, low-, or intermediate-risk MDS. As of September 1, 2023 (the “data cut-off date”), 79 patients had received at least one dose of KER-050 at the recommended Part 2 dose (“RP2D”) (collectively, the “safety population”). Of these patients, 60 had completed at least 24 weeks of treatment or discontinued as of the data cut-off date (collectively, the modified intent to treat 24-week population, or the “mITT24 patients”). Data for hematological response and markers of hematopoiesis were presented from exploratory analyses of these mITT24 patients. All data presented from this trial is as of the data cut-off date.
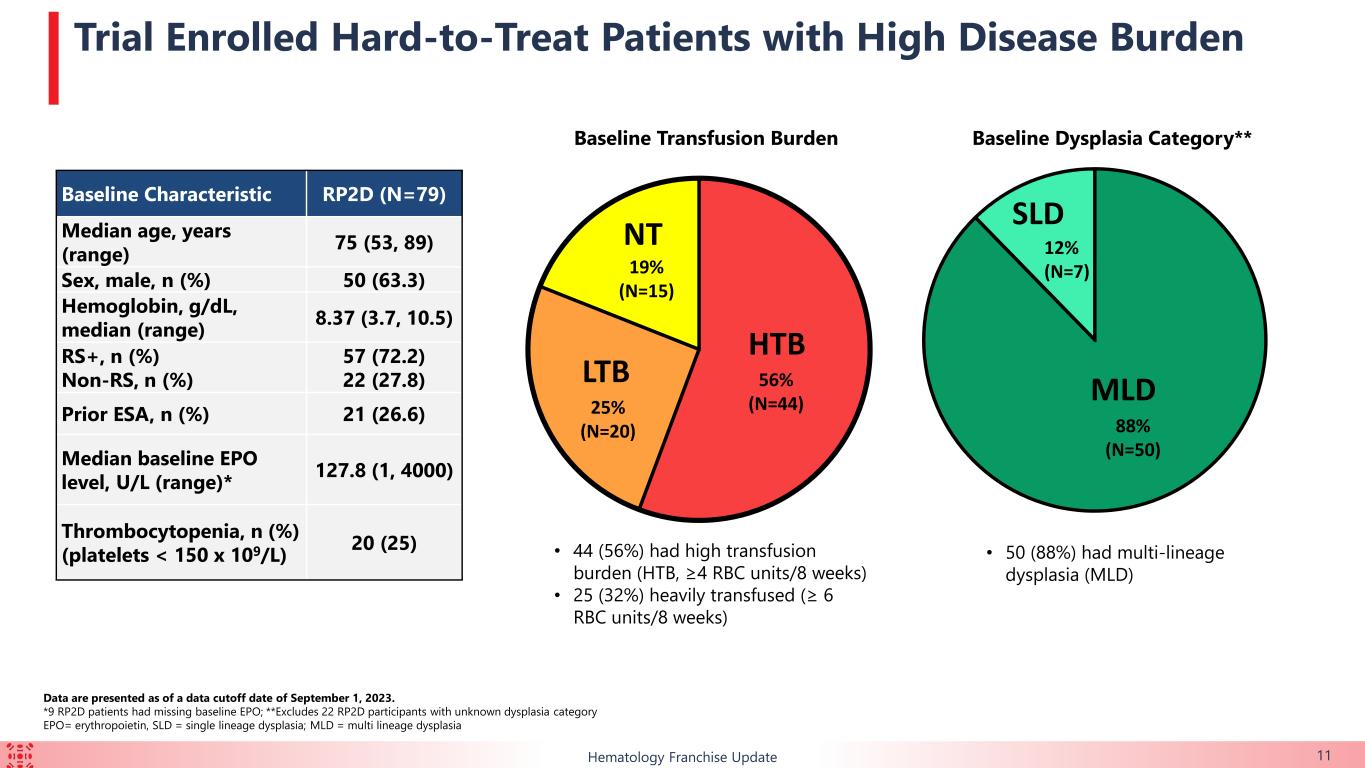
Of the 79 patients in the safety population, 55.7% (n=44) were high transfusion burden (“HTB”) while 25.3% (n=20) were low transfusion burden and 19.0% (n=15) were non-transfused (“NT”).
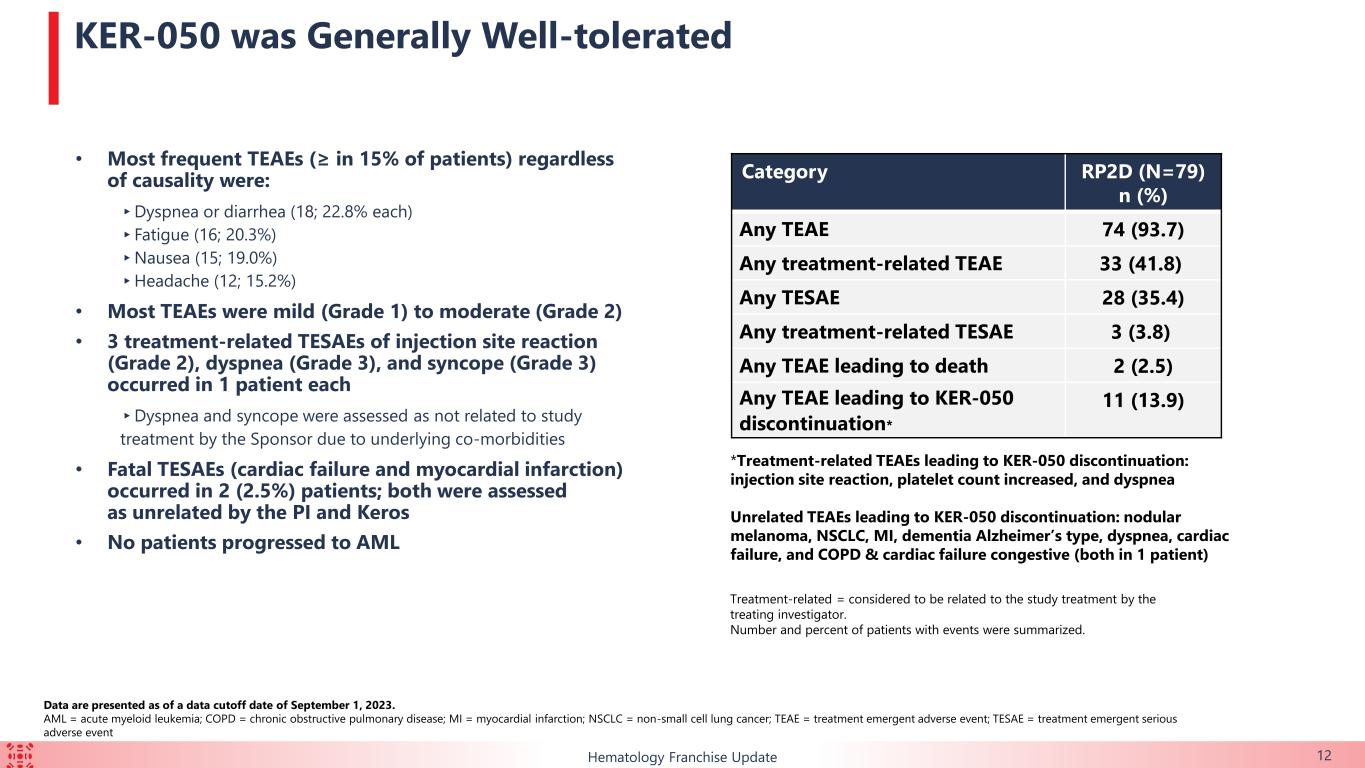
KER-050 was generally well tolerated by the 79 patients in the safety population. The most commonly reported treatment-emergent adverse events (“TEAEs”) (in ≥15% of patients) were diarrhea, dyspnea, fatigue, nausea and headache. No patients had progressed to acute myeloid leukemia.
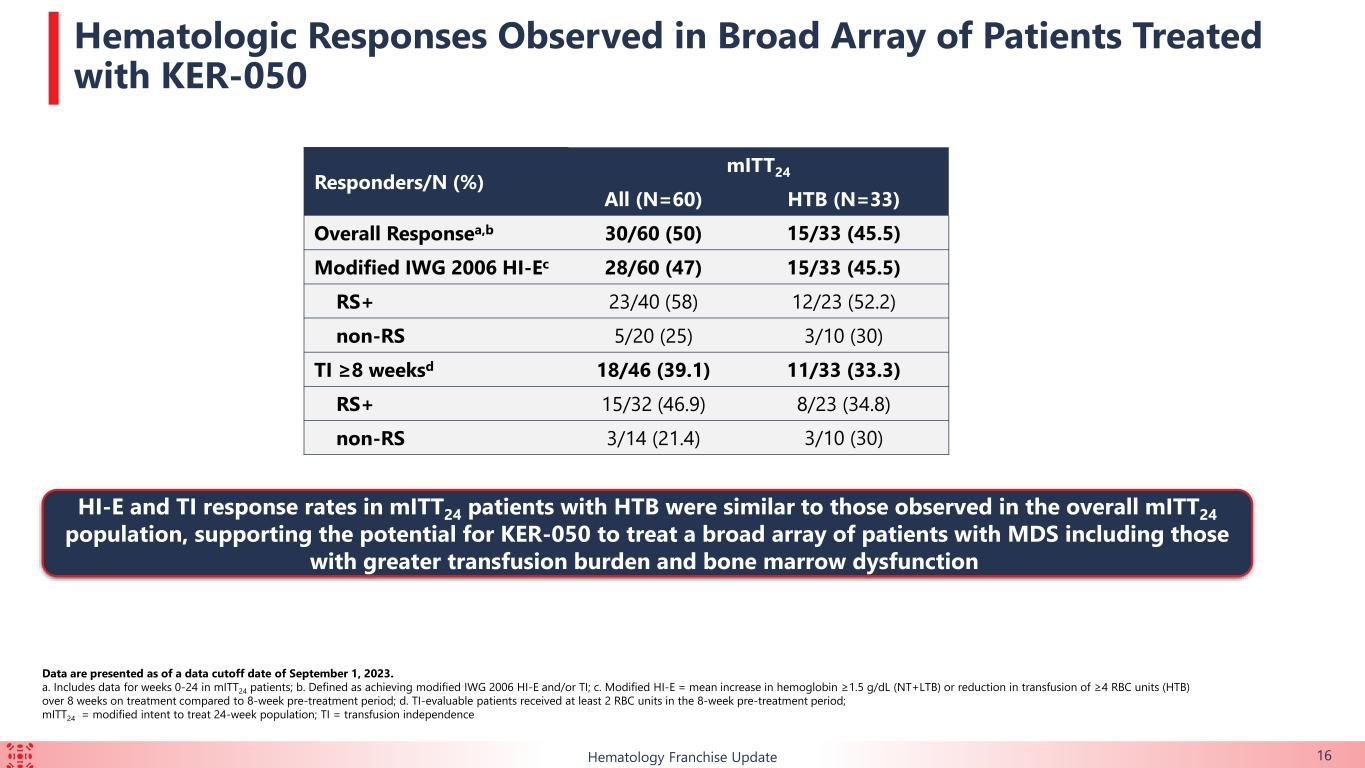
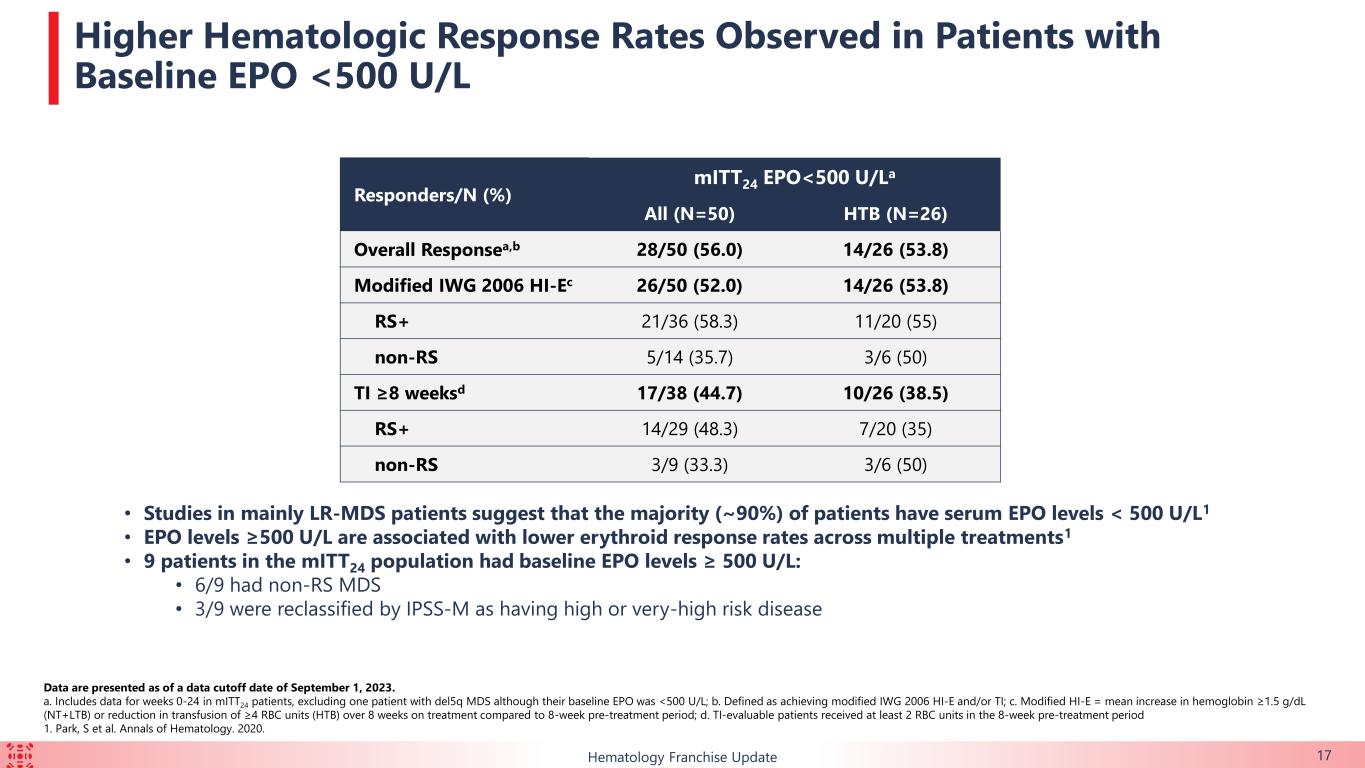
50% (n=30/60) of the mITT24 patients achieved an overall erythroid response over the first 24 weeks of treatment, which is defined as meeting either modified IWG 2006 Hematological improvement-erythroid (“HI-E”) or transfusion independence (“TI”) for at least eight weeks in transfusion-dependent patients who required ≥ 2 red blood cell (“RBC”) units transfused at baseline.
Additional data from the mITT24 patients include:
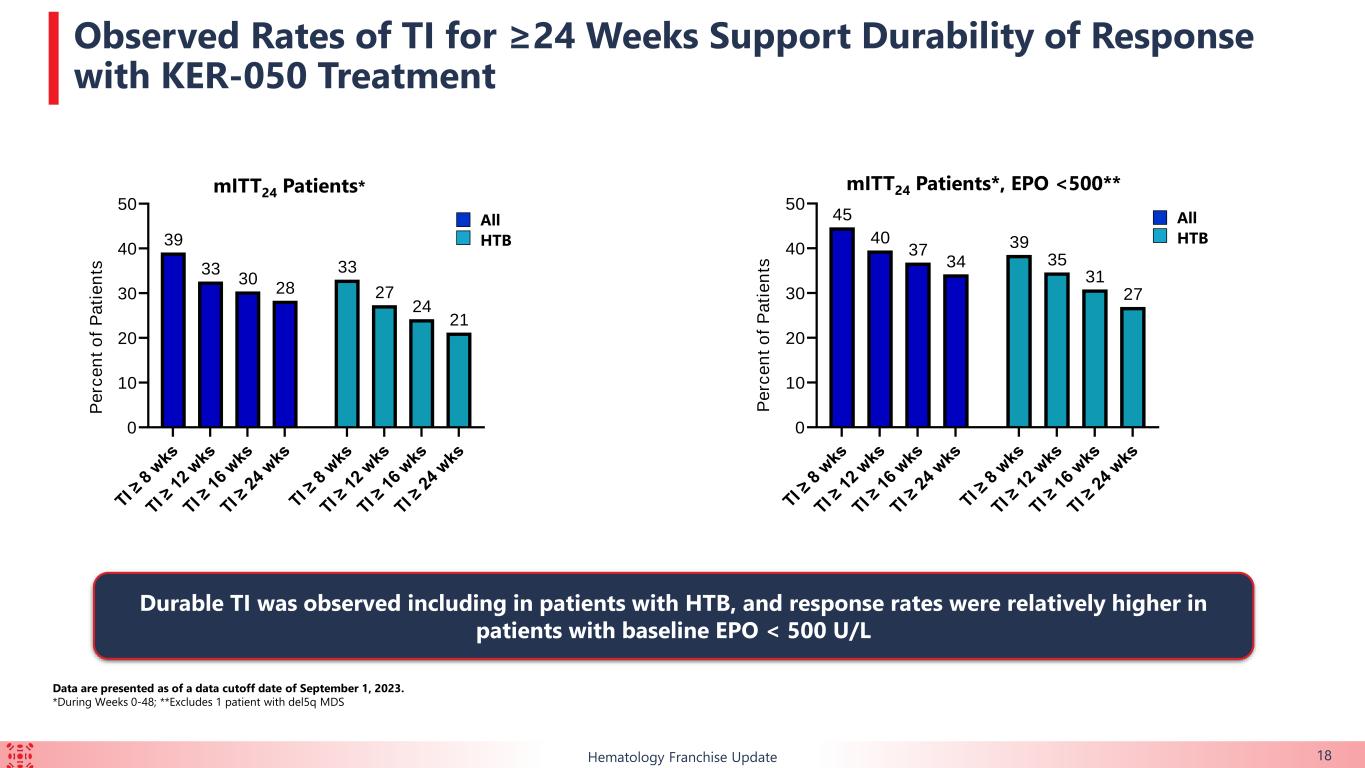
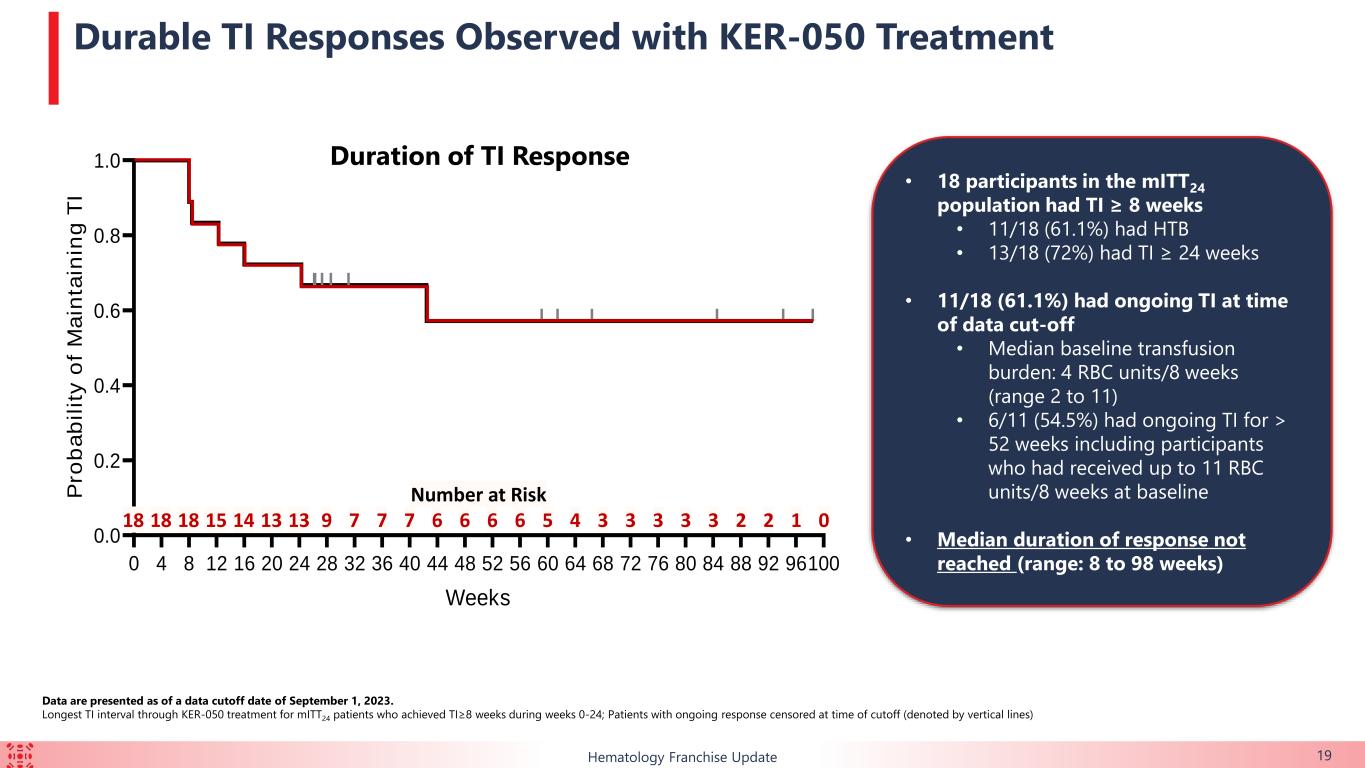
•39.1% (n=18/46) of the TI-evaluable patients achieved TI for at least eight weeks over the first 24 weeks of treatment. 13 of those 18 patients (72.2%) achieved TI for at least 24 weeks over the first 48 weeks of treatment.
•Of the patients with HTB, 33.3% (n=11/33) achieved TI for at least eight weeks during the first 24 weeks of treatment. 7 of those 11 patients (63.6%) achieved TI for at least 24 weeks over the first 48 weeks of treatment.
•Of the patients with baseline erythropoietin level less than 500 U/L, 44.7% (n=17/38) achieved TI for at least eight weeks over the first 24 weeks of treatment. Of the patients with baseline erythropoietin level less than 500 U/L and HTB, 38.5% (n=10/26) achieved TI for at least eight weeks over the first 24 weeks of treatment.
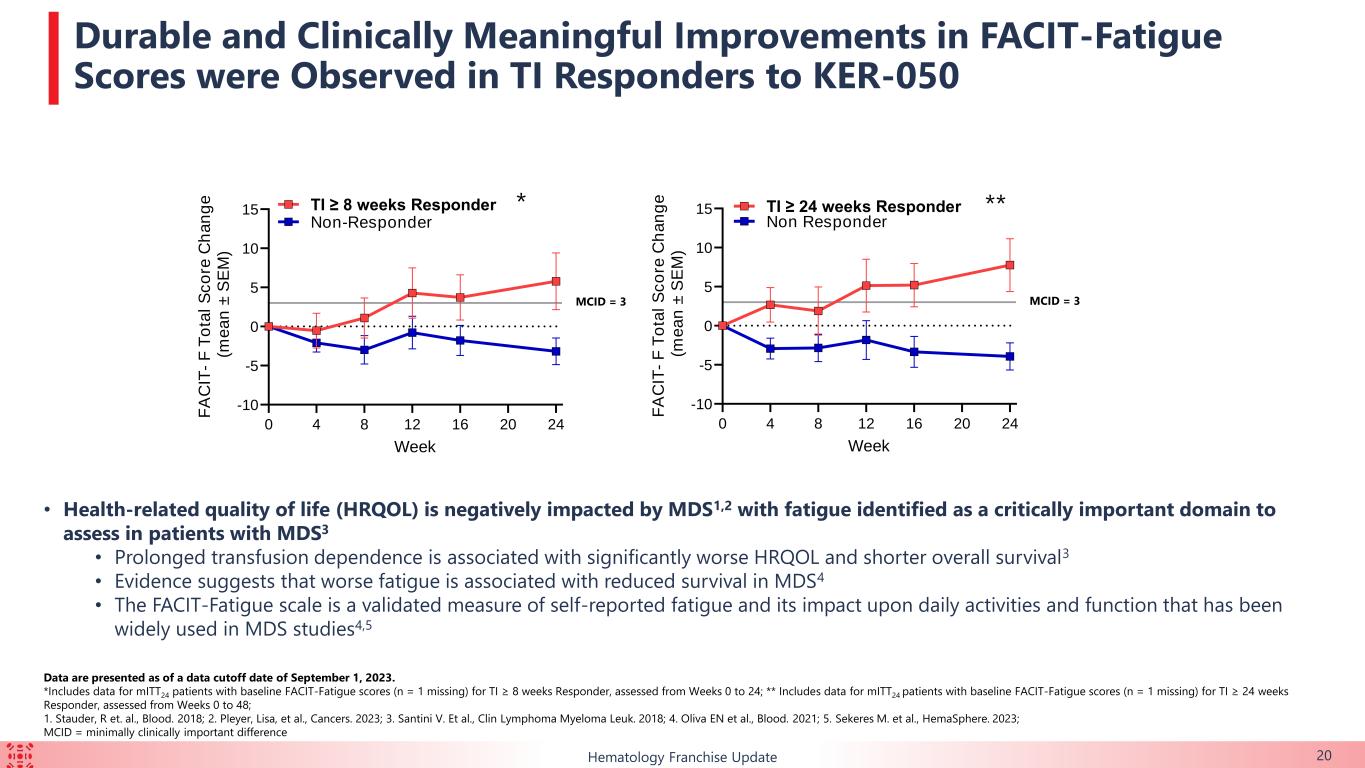
The FACIT-Fatigue scale, a measure of self-reported fatigue and its impact upon daily activities and function, was utilized to assess health-related quality of life in 45 of the mITT24 patients who were TI-evaluable and with baseline FACIT-F assessment. A difference of three in the FACIT-Fatigue scale is considered a minimally clinically important difference. In this group, patients who achieved TI had durable and clinically meaningful improvements in self-reported fatigue. At Week 24, patients achieving TI of eight weeks or longer within first 24 weeks had a mean score of 5.8 (n=10) versus patients who did not achieve TI who reported a mean score of -3.2 (n=11), for a mean difference of 9.0. At Week 24, patients achieving TI of 24 weeks or longer with first 48 weeks had a mean score of 7.8 (n=9) versus patients who did not achieve TI who reported a mean score of -3.9 (n=12), for a mean difference of 11.7.
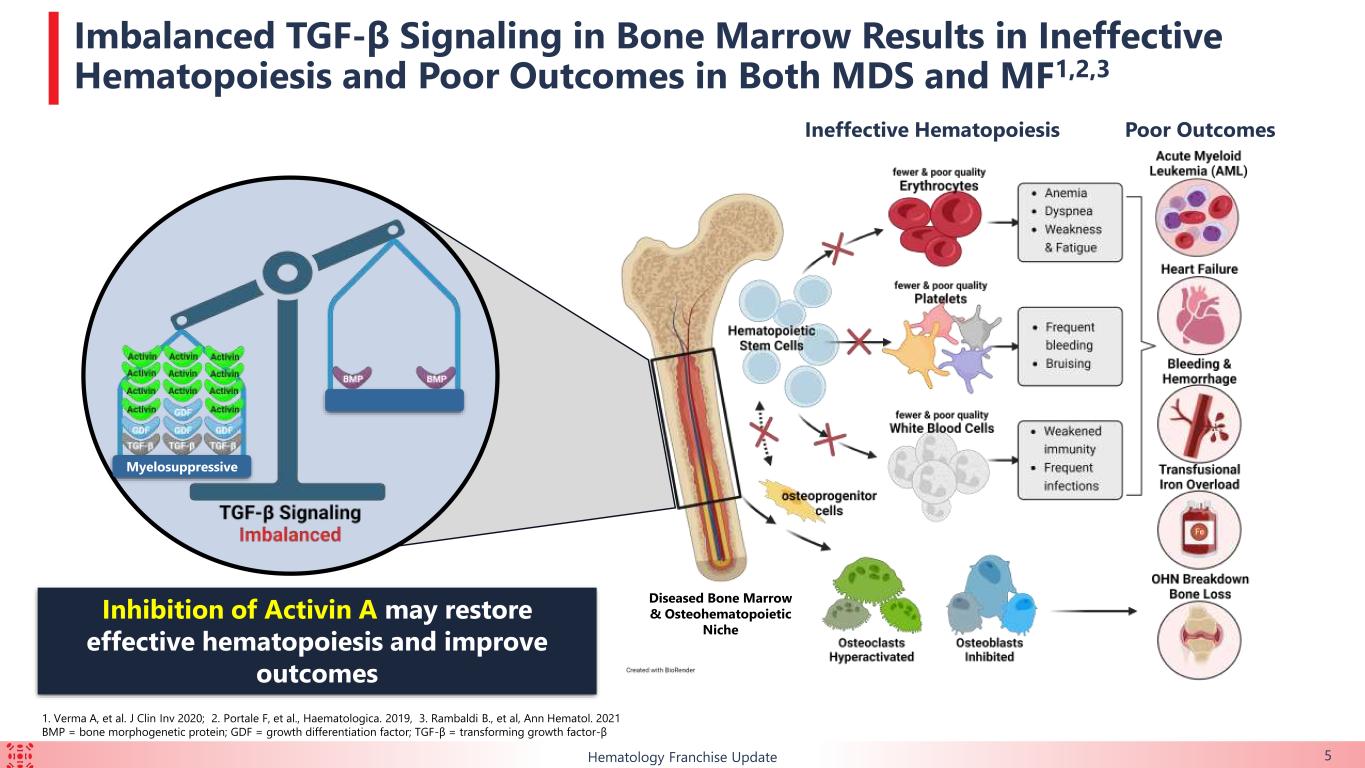
The majority of patients enrolled in this ongoing trial had HTB or multi-lineage dysplasia, indicating a difficult-to-treat trial population. Durable TI responses were observed in a broad range of patients with lower-risk MDS, including in those with HTB, which support the potential for KER-050 to ameliorate ineffective hematopoiesis across multiple lineages in patients with MDS. Patients who achieved TI showed clinically meaningful improvements in FACIT-Fatigue scores, indicating that KER-050 may improve quality of life in patients with lower-risk MDS.
•KER-050 Treatment Reduced Iron Overload and Increased Bone Specific Alkaline Phosphatase in Participants with Lower-risk MDS Supporting Potential to Restore Balance to the Osteohematopoietic Niche
Exploratory analysis of biomarkers that may indicate MDS disease modification were evaluated as of the data cut-off date in the ongoing Phase 2 clinical trial of KER-050 in patients with MDS. Observations from these biomarkers included improvements in:
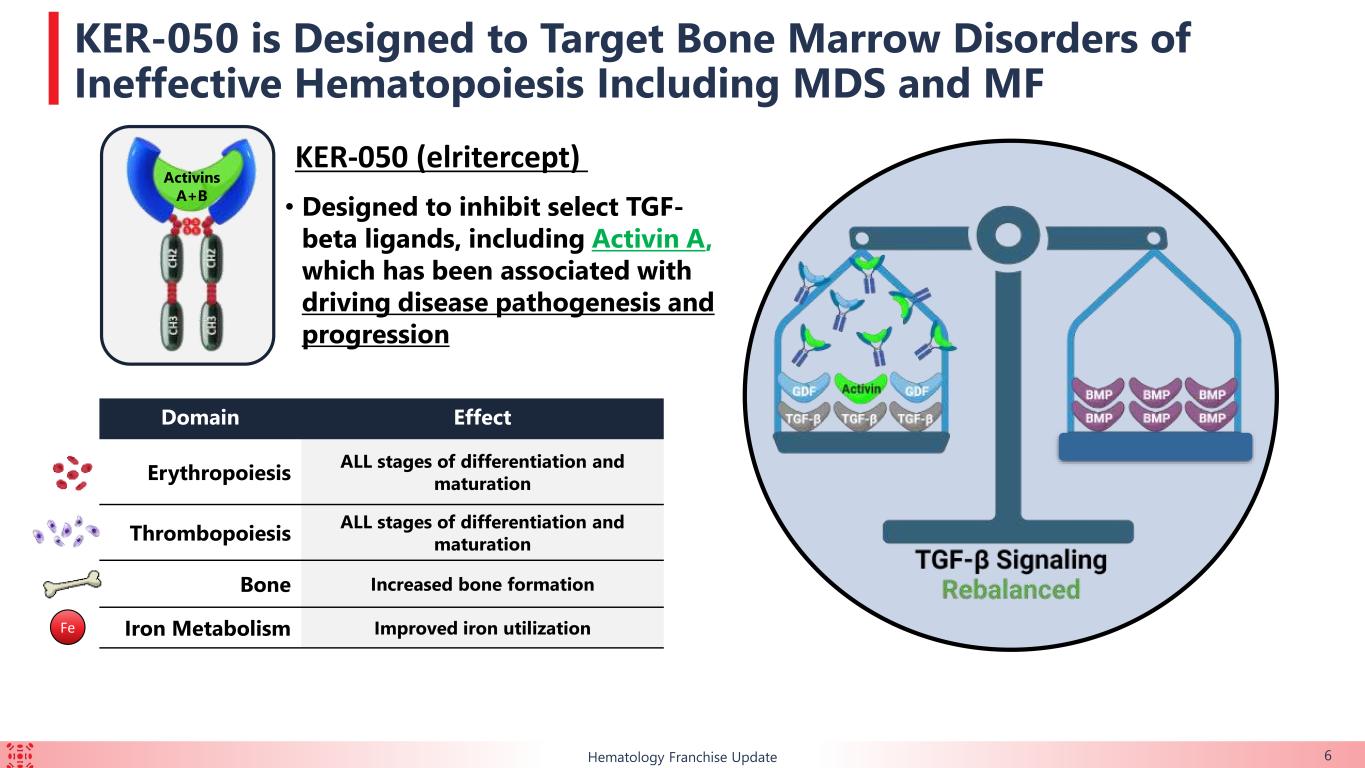
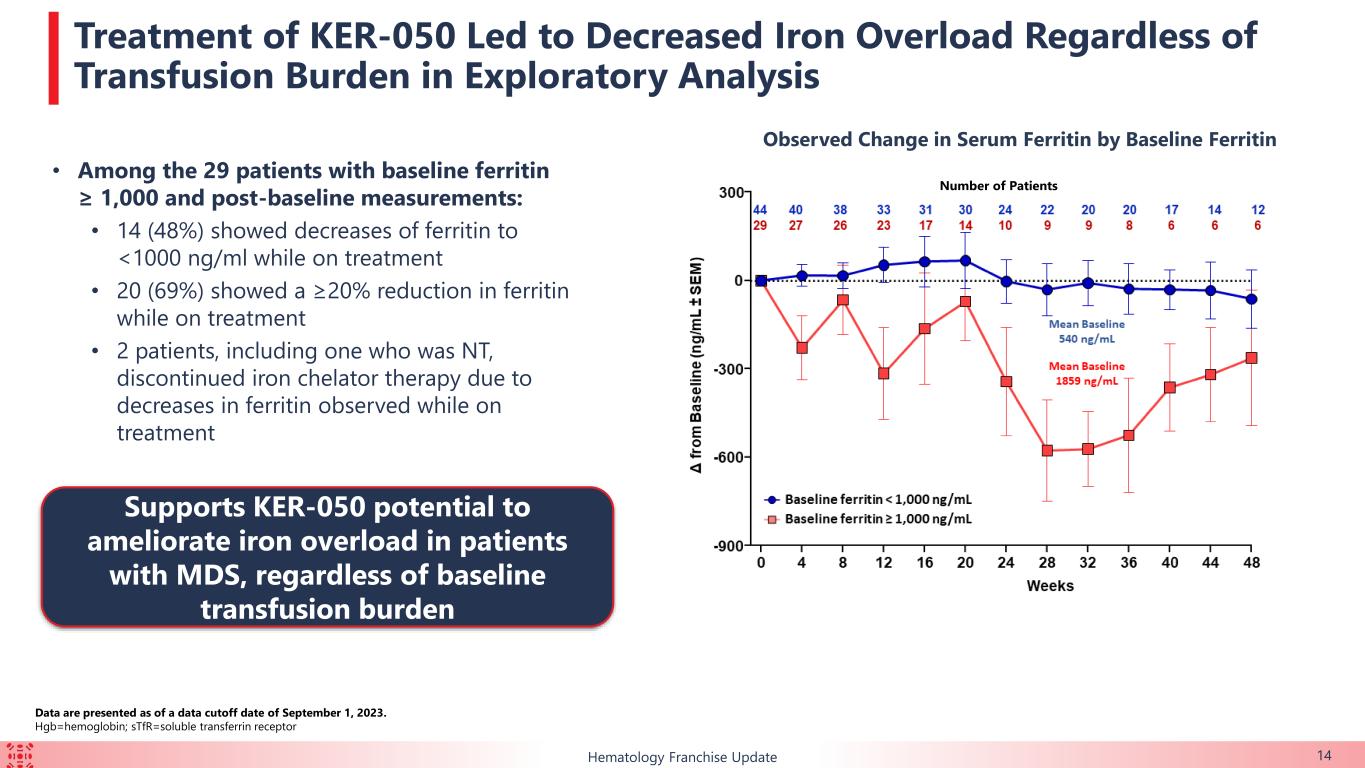
•Iron metabolism: 48.3% (n=14/29) of patients with baseline ferritin ≥ 1,000 ng/ml had a decreased ferritin to < 1000 ng/ml and 69.0% (n=20/29) of patients decreased ferritin by ≥20%. Two patients, including one who was NT, discontinued iron chelator therapy due to observed decreases in ferritin. These data support potential of KER-050 to ameliorate iron overload.
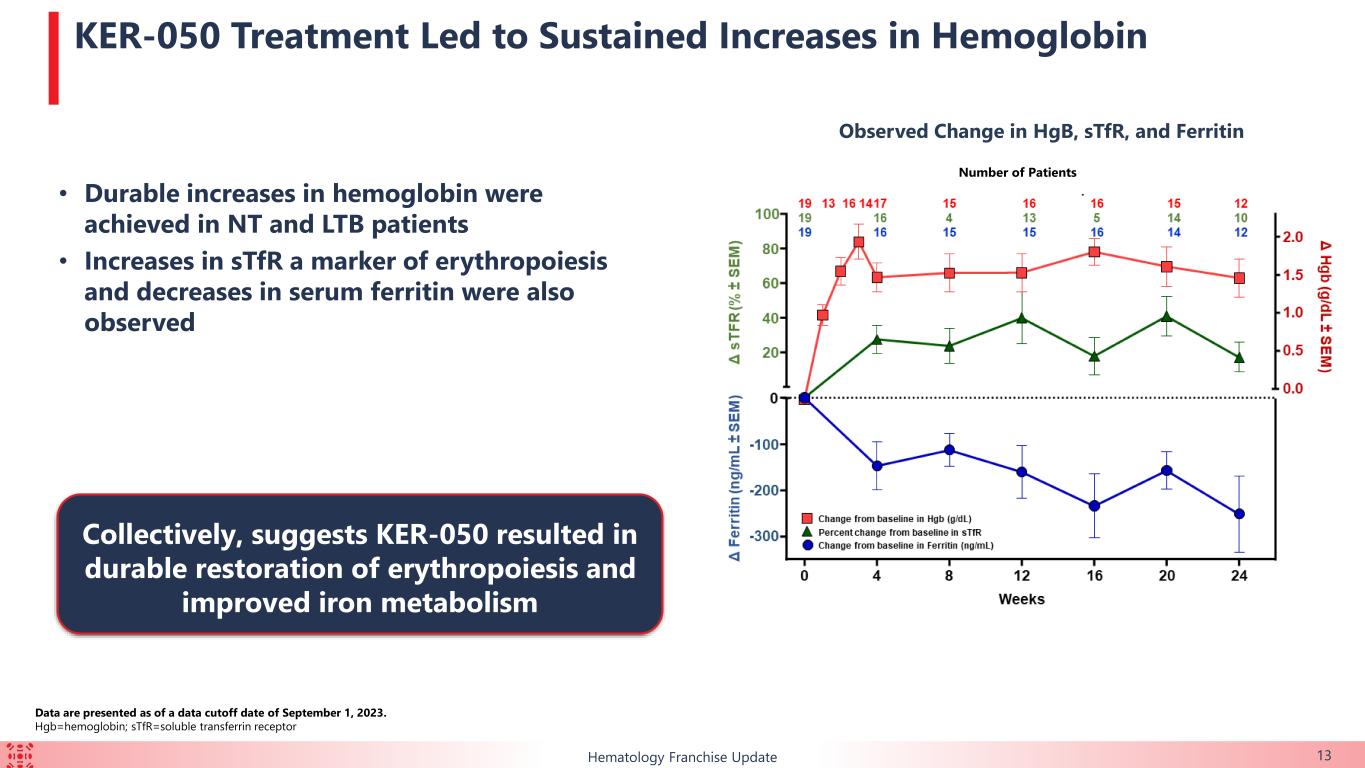
•Hematopoiesis: Sustained increases in hemoglobin for 24 weeks coincided with observed increases in soluble transferrin receptor and concomitant decreases in serum ferritin, suggesting KER-050 resulted in durable restoration of erythropoiesis and improved iron metabolism.
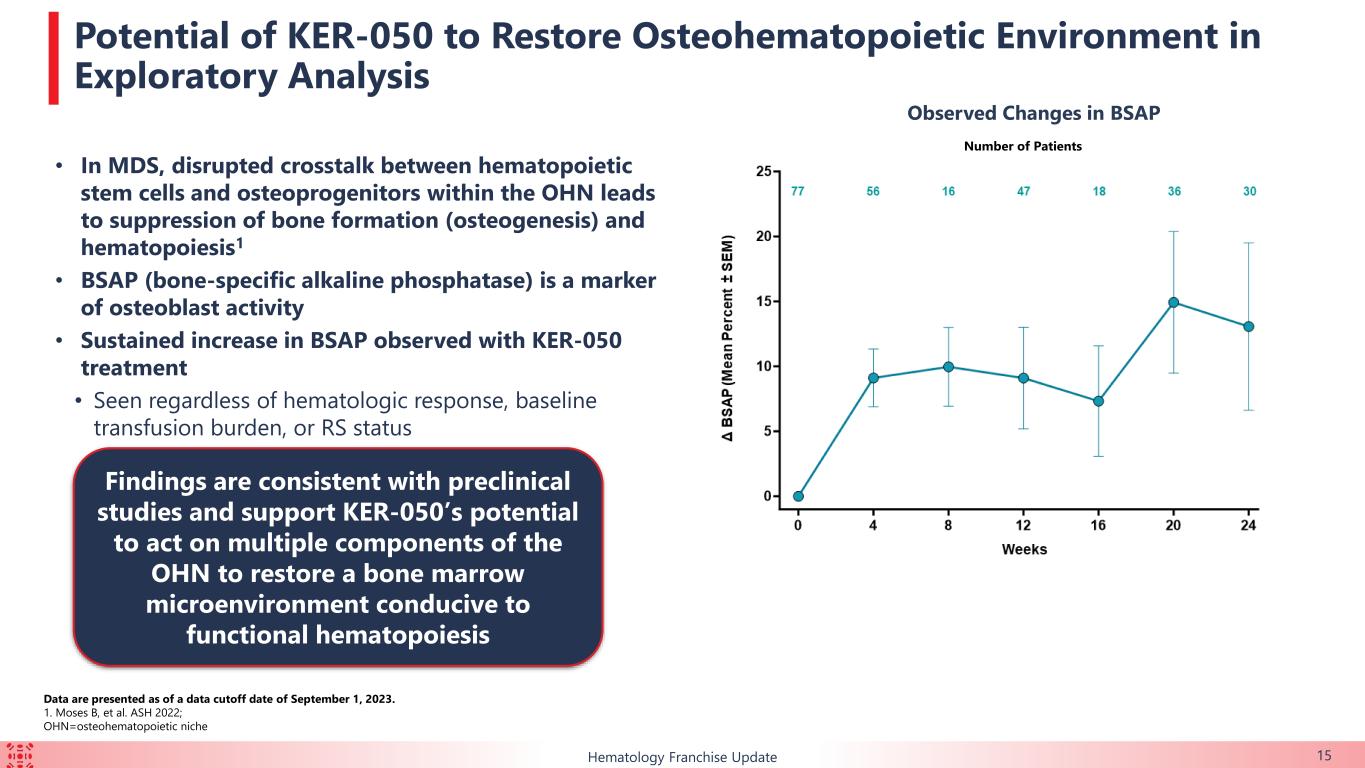
•Bone turnover: Increases in bone-specific alkaline phosphatase, a marker of osteoblast activity, were observed with KER-050 treatment regardless of hematological response, baseline transfusion burden or RS status, suggesting KER-050 can potentially restore a bone marrow microenvironment conducive to functional hematopoiesis.
•Cardiac stress: Levels of N-terminal prohormone of brain natriuretic protein, a biomarker of myocardial stress, decreased in both HI-E and/or TI responders and non-responders, suggesting that KER-050 may ameliorate cardiac strain directly via inhibition of activin A and indirectly by improving anemia and reducing transfusion burden.
Collectively, these exploratory data suggest that KER-050 has the potential to provide benefit to patients with MDS beyond treatment of anemia, such as reestablishing hematopoiesis across multiple cell lineages, restoring homeostasis within the osteohematopoietic niche and ameliorating myocardial strain.
•Modulation of TGF-β Superfamily Signaling by KER-050 Demonstrated Potential to Treat Myelofibrosis and Mitigate Ruxolitinib-Associated Cytopenias
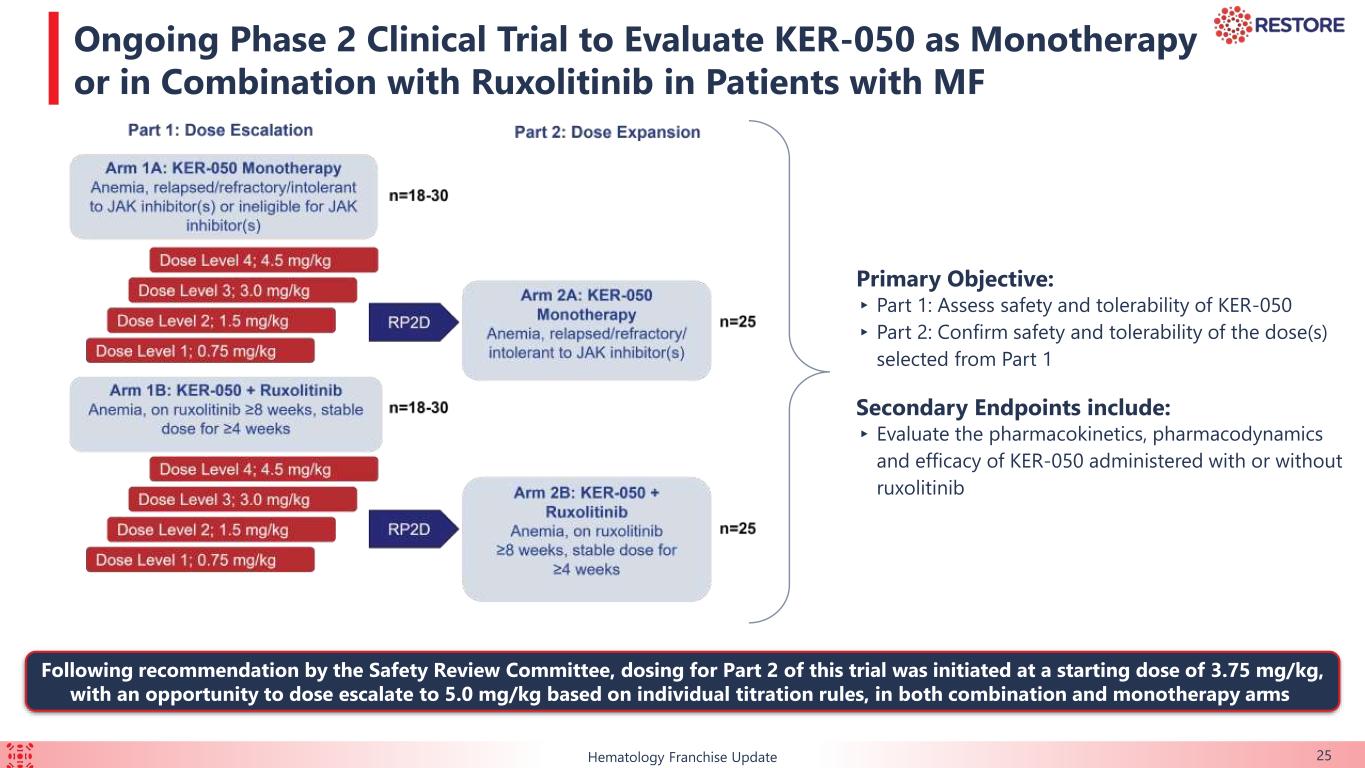
This ongoing, open-label, two-part Phase 2 clinical trial is evaluating KER-050 administered with or without ruxolitinib in patients with MF who have anemia and were either currently on, failed, or ineligible for ruxolitinib at baseline. Safety data are presented for all patients that received at least one dose of KER-050 in Part 1 (n=41) as of September 14, 2023. Evaluations of markers of hematopoiesis and anemia over 12 weeks, along with measurements of spleen volume and symptom scores (by the MF-symptom assessment form-Total Symptom Score, or “MF-SAF-TSS”) over 24 weeks, were presented for dose levels 1 through 3, ranging from 0.75 mg/kg to 3.0 mg/kg (collectively, the “efficacy evaluable patients”). Data for dose level 4 (4.5 mg/kg), the highest dose level being evaluated in Part 1, are not included due to limited exposure as of the data cutoff date. All data presented from this trial is as of the September 14, 2023 data cut-off date.
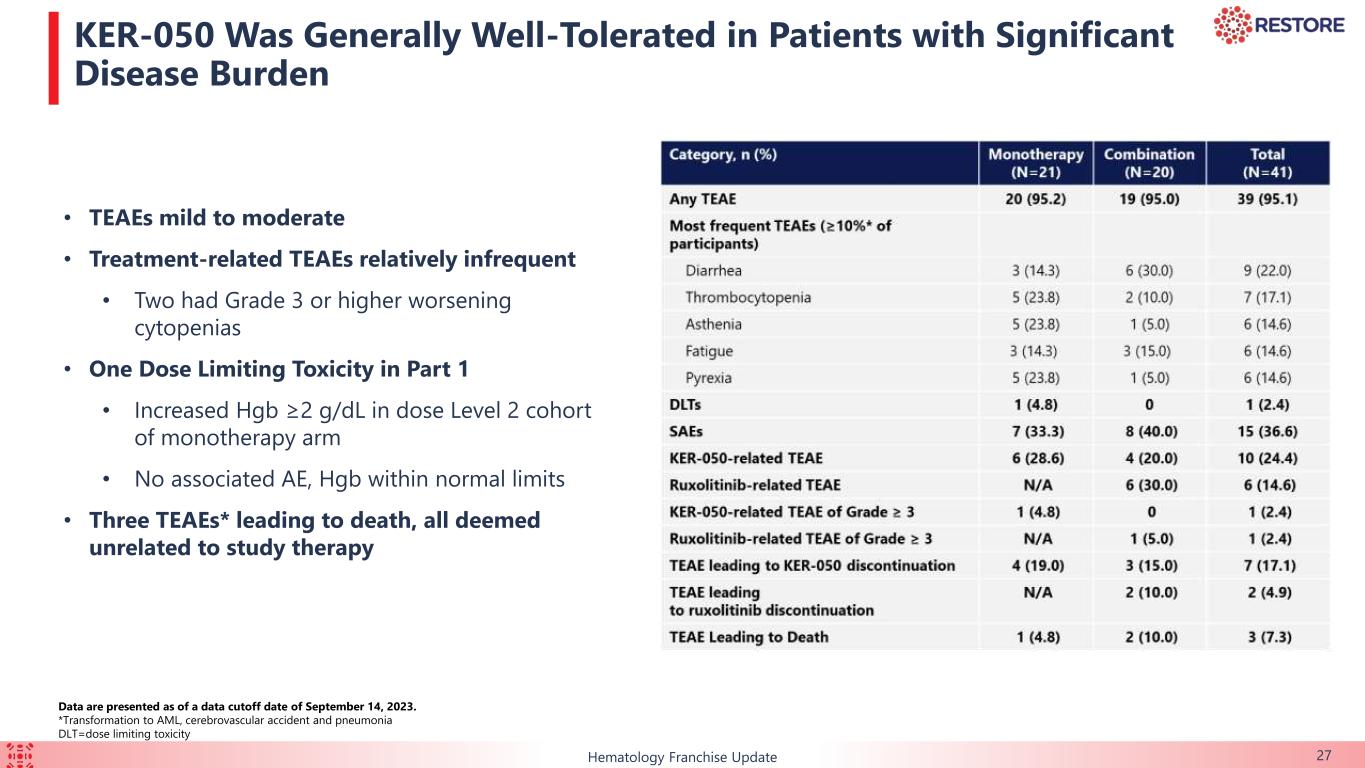
KER-050 was generally well tolerated by the safety population. There was one dose-limiting toxicity reported from a patient in the 1.5mg/kg dose level of the monotherapy arm. The patient had an increase in hemoglobin of at least 2 g/dL, which met protocol criteria for dose reduction at the end of cycle 1. There were no adverse events associated with this event, and the maximum observed hemoglobin remained within normal limits. There were three cases of fatal TEAEs in the trial that were each deemed unrelated to treatment. The most commonly reported TEAEs (in ≥10% of patients) were diarrhea, thrombocytopenia, asthenia (weakness), fatigue and pyrexia (fever). Treatment-related TEAEs were relatively infrequent, most of which were mild to moderate, with two patients experiencing Grade 3 or higher worsening cytopenias.
Additional data from the efficacy evaluable patients include:
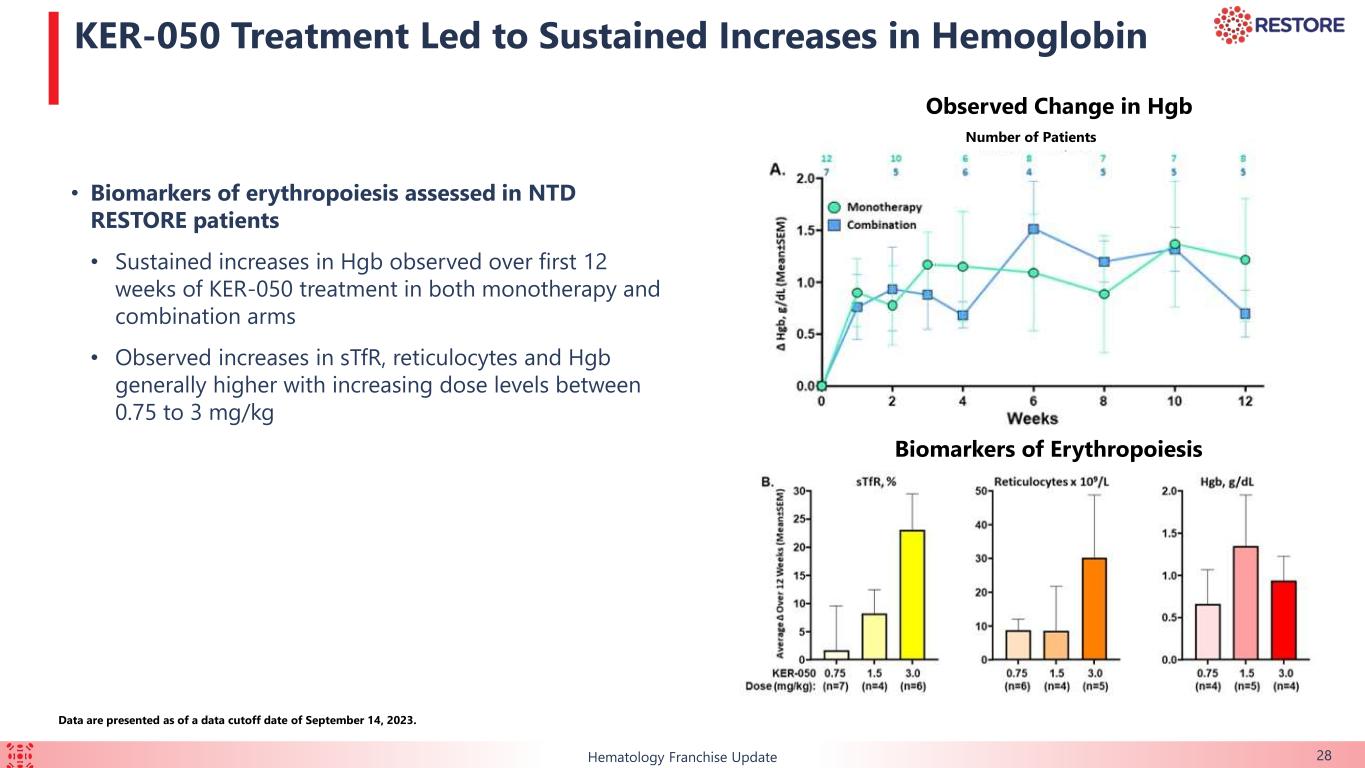
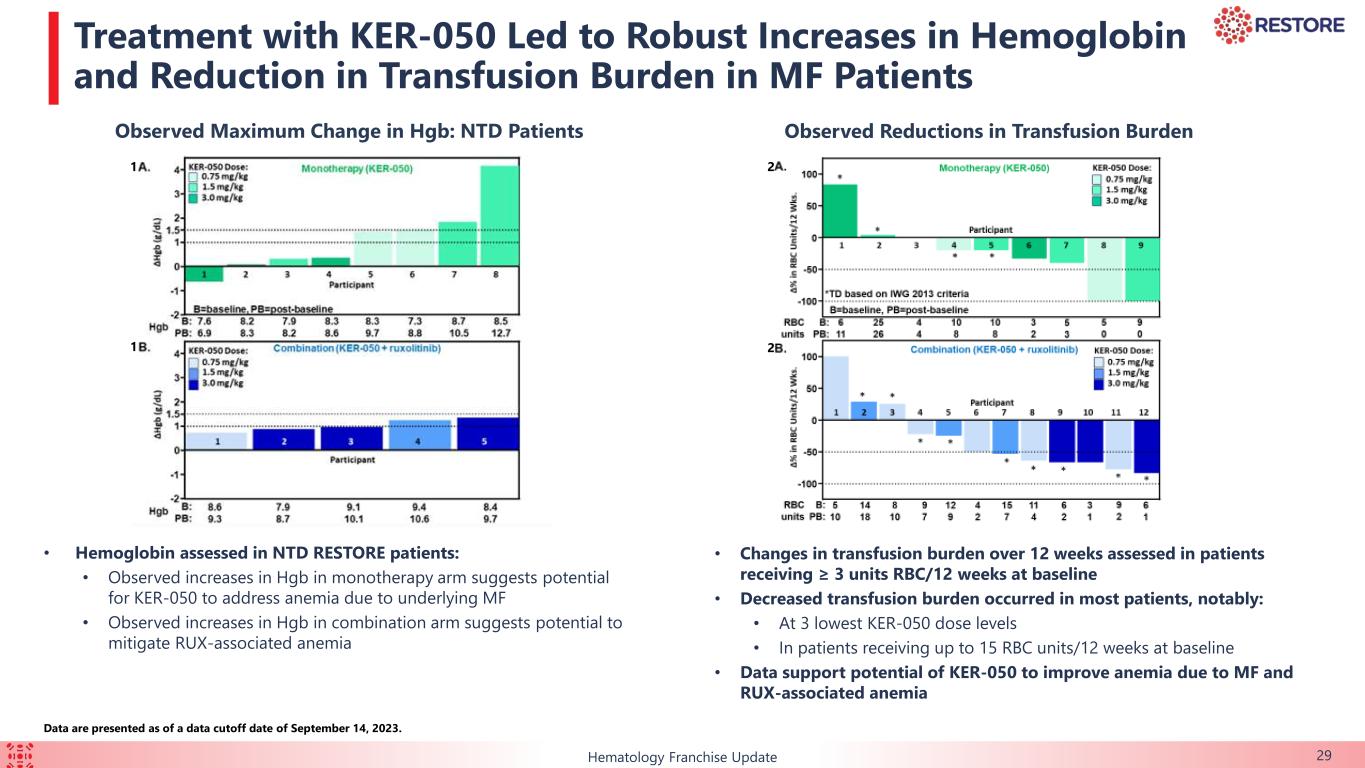
•Increases in hemoglobin were observed in non-transfusion dependent patients in both arms, suggesting that KER-050 has the potential to address anemia due to MF and ruxolitinib-associated anemia.
◦Additionally, most patients had reductions in transfusion burden, including patients receiving up to 15 RBC units per 12 weeks at baseline.
•Non-transfusion dependent patients, who received a median of three RBC units per 12 weeks at baseline, experienced sustained increases in hemoglobin within the first 12 weeks of treatment in both the monotherapy and combination arms (pooled across dose cohorts).
◦Additionally, observed increases in soluble transferrin receptor, reticulocytes and hemoglobin were generally higher with increasing dose levels between 0.75 mg/kg to 3.0 mg/kg (pooled across both monotherapy and combination arms at each dose level).
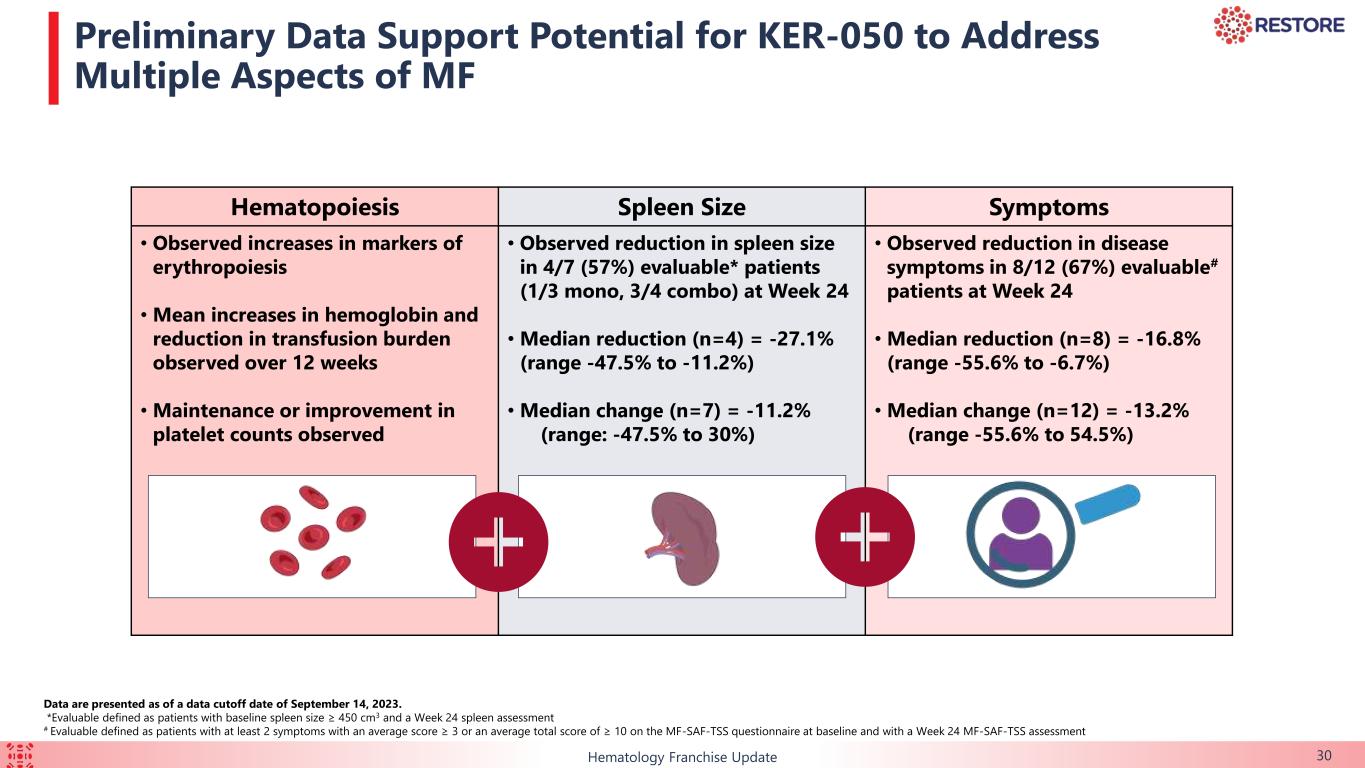
•At week 24, reduction in spleen size was observed in 57.1% (n=4/7) of patients with baseline spleen size ≥ 450 cm3 and a week 24 spleen assessment, including one of three patients in the monotherapy arm and three of four patients in the combination arm.
•At week 24, decrease in disease symptoms was observed in 66.7% (n=8/12) of patients with at least two symptoms with an average score ≥ 3 or an average total score of ≥ 10 on the MF-SAF-TSS questionnaire at baseline and a week 24 MF-SAF-TSS assessment.
The data support the potential of KER-050 to ameliorate ineffective hematopoiesis and address cytopenias due to MF and associated with ruxolitinib, and provide broader clinical benefit in patients as observed by the reduction in spleen size and improvement in symptoms.
Conference Call and Webcast Information
Keros will host a conference call and webcast today, December 11, 2023, at 8:00 a.m. Eastern time, to discuss the additional data from its two ongoing Phase 2 clinical trials of KER-050, one in patients with MDS and one in patients with MF, which was presented at the 65th ASH Annual Meeting and Exposition.
The conference call will be webcast live at: https://event.webcasts.com/starthere.jsp?ei=1645187&tp_key=cad9574144. The live teleconference may be accessed by dialing (877) 407-0309 (domestic) or (201) 389-0853 (international). An archived version of the call will be available in the Investors section of the Keros website at https://ir.kerostx.com/ for 90 days following the conclusion of the call.
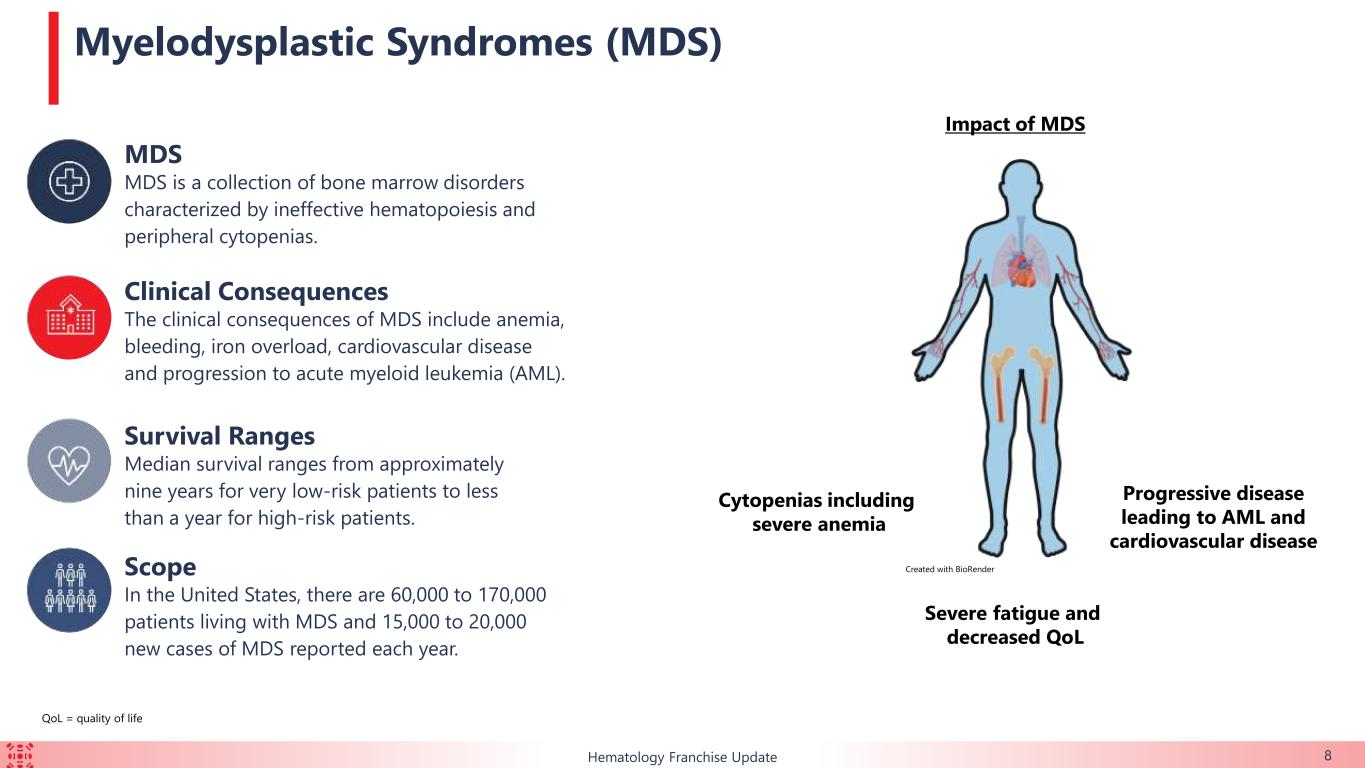
About the Ongoing Phase 2 Clinical Trial of KER-050 in Patients with MDS (NCT04419649)
Keros is conducting an open label, two-part, multiple ascending dose Phase 2 clinical trial to evaluate KER-050 in patients with very low-, low-, or intermediate-risk MDS who either have or have not previously received treatment with an erythroid stimulating agent.
The primary objective of this trial is to assess the safety and tolerability of KER-050 in patients with MDS that are RS positive or non-RS. The primary objective of Part 2 of this trial is confirmation of the safety and tolerability of the RP2D (3.75 mg/kg and 5.0 mg/kg). The secondary objectives of this trial are to evaluate the pharmacokinetics, pharmacodynamics and efficacy of KER-050.
About the Ongoing Phase 2 Clinical Trial of KER-050 in Patients with MF-Associated Cytopenias (RESTORE trial)
Keros is conducting an open label, two-part, multiple ascending dose Phase 2 clinical trial to evaluate KER-050 as a monotherapy and in combination with ruxolitinib in patients with MF-associated cytopenias.
The primary objective of this trial is to assess the safety and tolerability of KER-050 in patients with MF-associated cytopenias. The primary objective of Part 2 of this trial is confirmation of the safety and tolerability of the RP2D (3.75 mg/kg and 5.0 mg/kg). The secondary objectives of this trial are to evaluate the pharmacokinetics, pharmacodynamics and efficacy of KER-050 administered with or without ruxolitinib.
About KER-050
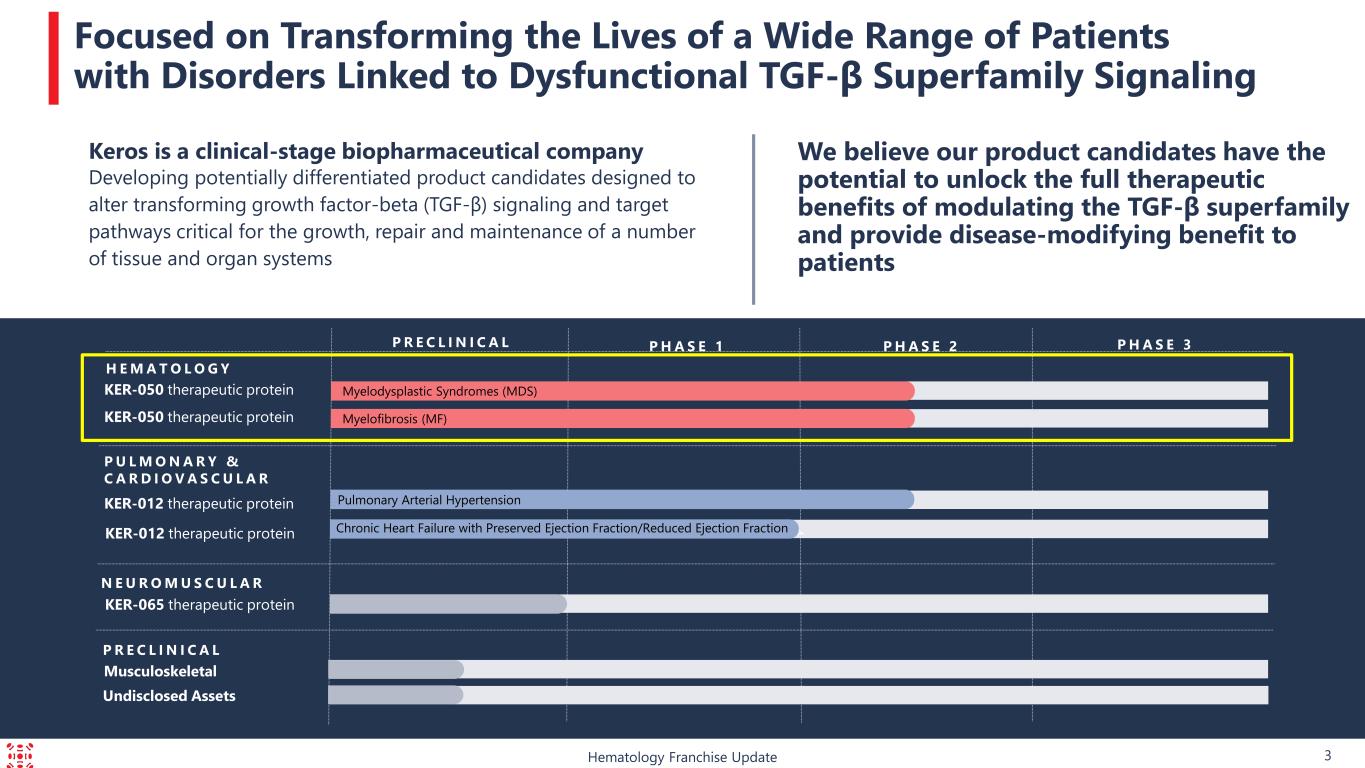
Keros’ lead protein therapeutic product candidate, KER-050, is an engineered ligand trap comprised of a modified ligand-binding domain of the TGF-ß receptor known as activin receptor type IIA that is fused to the portion of the human antibody known as the Fc domain. KER-050 is being developed for the treatment of low blood cell counts, or cytopenias, including anemia and thrombocytopenia, in patients with MDS and in patients with MF.
About Keros Therapeutics, Inc.
Keros is a clinical-stage biopharmaceutical company focused on developing and commercializing novel therapeutics to treat a wide range of patients with disorders that are linked to dysfunctional signaling of the TGF-ß family of proteins. We are a leader in understanding the role of the TGF-ß family of proteins, which are master regulators of the growth, repair and maintenance of blood cells and a number of tissues, including bone, skeletal muscle, adipose and heart tissue. By leveraging this understanding, we have discovered and are developing large and small molecules that have the potential to provide meaningful and potentially disease-modifying benefit to patients. Keros’ lead protein therapeutic product candidate, KER-050 (elritercept), is being developed for the treatment of low blood cell counts, or cytopenias, including anemia and thrombocytopenia, in patients with MDS and in patients with MF. Keros’ second product candidate, KER-012, is being developed for the treatment of pulmonary arterial hypertension and for the treatment of cardiovascular disorders. Keros’ third product candidate, KER-065, is being developed for the treatment of neuromuscular diseases, with an initial focus on Duchenne muscular dystrophy.
Cautionary Note Regarding Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as “believes,” “expects,” “intends,” “plans,” “potential,” “projects,” “would” and “future” or similar expressions such as “look forward” are intended to identify forward-looking statements. Examples of these forward-looking statements include statements concerning: Keros’ expectations regarding its growth, strategy, progress and the design, objectives and timing of its clinical trials for KER-050, including its regulatory plans; the potential of KER-050 to treat beyond MF- and MDS-associated cytopenias to have a direct effect on the pathogenesis of MF and MDS, respectively; the potential of KER-050 to ameliorate ineffective hematopoiesis across multiple lineages in patients with MDS and to improve quality of life in patients with lower-risk MDS; and the potential of KER-050 to address anemia due to MF and ruxolitinib-associated anemia. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties include, among others: Keros’ limited operating history and historical losses; Keros’ ability to raise additional funding to complete the development and any commercialization of its product candidates; Keros’ dependence on the success of its product candidates, KER-050, KER-012 and KER-065; that Keros may be delayed in initiating, enrolling or completing any clinical trials; competition from third parties that are developing products for similar uses; Keros’ ability to obtain, maintain and protect its intellectual property; and Keros’ dependence on third parties in connection with manufacturing, clinical trials and preclinical studies.
These and other risks are described more fully in Keros’ filings with the Securities and Exchange Commission (“SEC”), including the “Risk Factors” section of the Company’s Quarterly Report on Form 10-Q, filed with the SEC on November 6, 2023, and its other documents subsequently filed with or furnished to the SEC. All forward-looking statements contained in this press release speak only as of the date on which they were made. Except to the extent required by law, Keros undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Investor Contact:
Justin Frantz
jfrantz@kerostx.com
617-221-6042