
November 2023 Corporate Presentation

Corporate Presentation 2 Statements contained in this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as "anticipates," "believes," "expects," "intends," “plans,” “potential,” "projects,” “would” and "future" or similar expressions are intended to identify forward-looking statements. Examples of these forward-looking statements include statements concerning: Keros’ expectations regarding its growth, strategy, progress and the design, objectives, expected results and timing of its preclinical studies and clinical trials for KER-050, KER-012 and KER-065; and the potential of Keros’ proprietary discovery approach. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties include, among others: Keros’ limited operating history and historical losses; Keros’ ability to raise additional funding to complete the development and any commercialization of its product candidates; Keros’ dependence on the success of its product candidates, KER-050, KER-012 and KER-065; that Keros may be delayed in initiating, enrolling or completing any clinical trials; competition from third parties that are developing products for similar uses; Keros’ ability to obtain, maintain and protect its intellectual property; and Keros’ dependence on third parties in connection with manufacturing, clinical trials and preclinical studies. These and other risks are described more fully in Keros’ filings with the Securities and Exchange Commission (“SEC”), including the “Risk Factors” section of the Company’s Quarterly Report on Form 10-Q, filed with the SEC on November 6, 2023, and its other documents subsequently filed with or furnished to the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Except to the extent required by law, Keros undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. Disclaimer
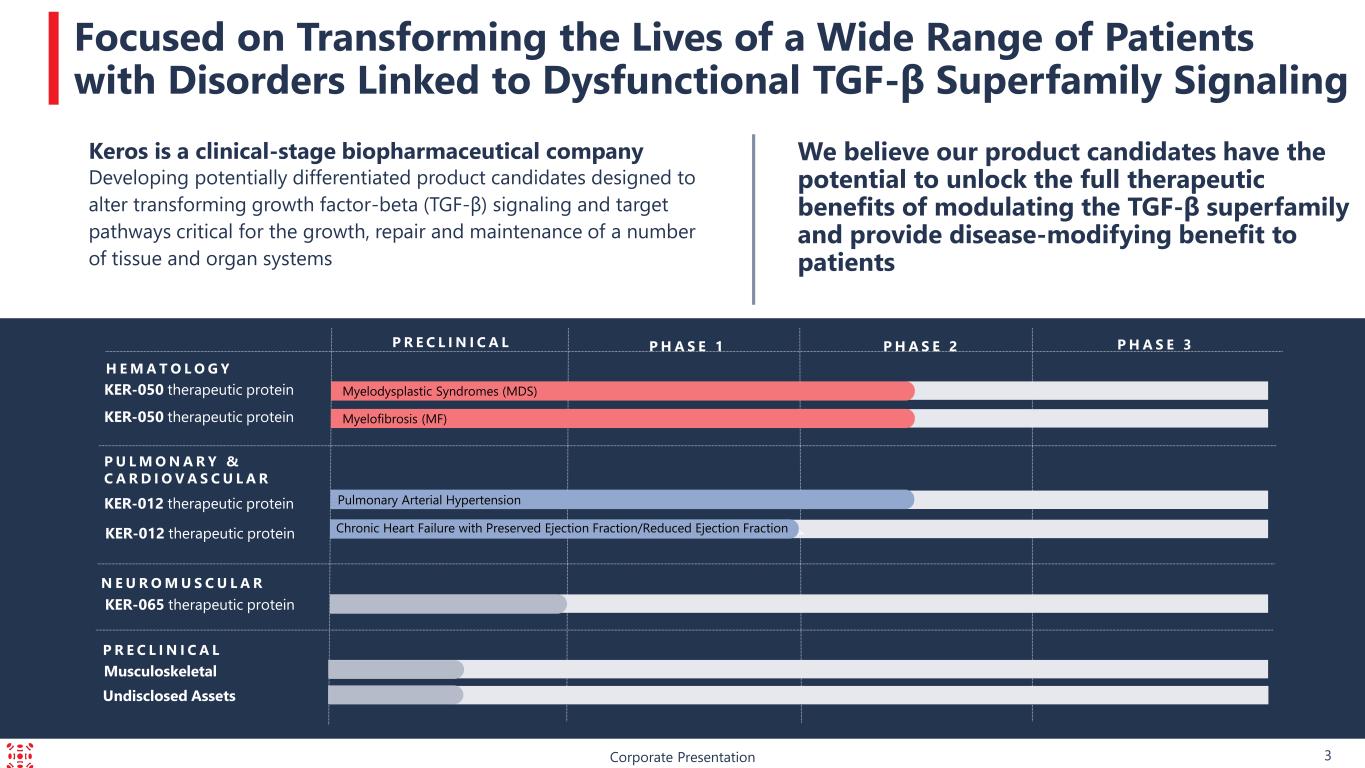
KER-065 therapeutic protein P R E C L I N I C A L Corporate Presentation 3 Focused on Transforming the Lives of a Wide Range of Patients with Disorders Linked to Dysfunctional TGF-β Superfamily Signaling We believe our product candidates have the potential to unlock the full therapeutic benefits of modulating the TGF-β superfamily and provide disease-modifying benefit to patients P H A S E 3P R E C L I N I C A L P H A S E 1 P H A S E 2 KER-050 therapeutic protein KER-050 therapeutic protein H E M A T O L O G Y KER-012 therapeutic protein P U L M O N A R Y & C A R D I O V A S C U L A R Myelodysplastic Syndromes (MDS) Myelofibrosis (MF) Pulmonary Arterial Hypertension Musculoskeletal 4 KER-012 therapeutic protein N E U R O M U S C U L A R Chronic Heart Failure with Preserved Ejection Fraction/Reduced Ejection Fraction Keros is a clinical-stage biopharmaceutical company Developing potentially differentiated product candidates designed to alter transforming growth factor-beta (TGF-β) signaling and target pathways critical for the growth, repair and maintenance of a number of tissue and organ systems Undisclosed Assets

B Hematology Franchise Corporate Presentation 4

B KER-050 5 Investigational Treatment for Anemia and Thrombocytopenia in Patients with Myelodysplastic Syndromes Ongoing Phase 2 Clinical Trial of KER-050 for the Treatment of Anemia in Patients with Very Low-, Low- or Intermediate-Risk Myelodysplastic Syndromes Corporate Presentation
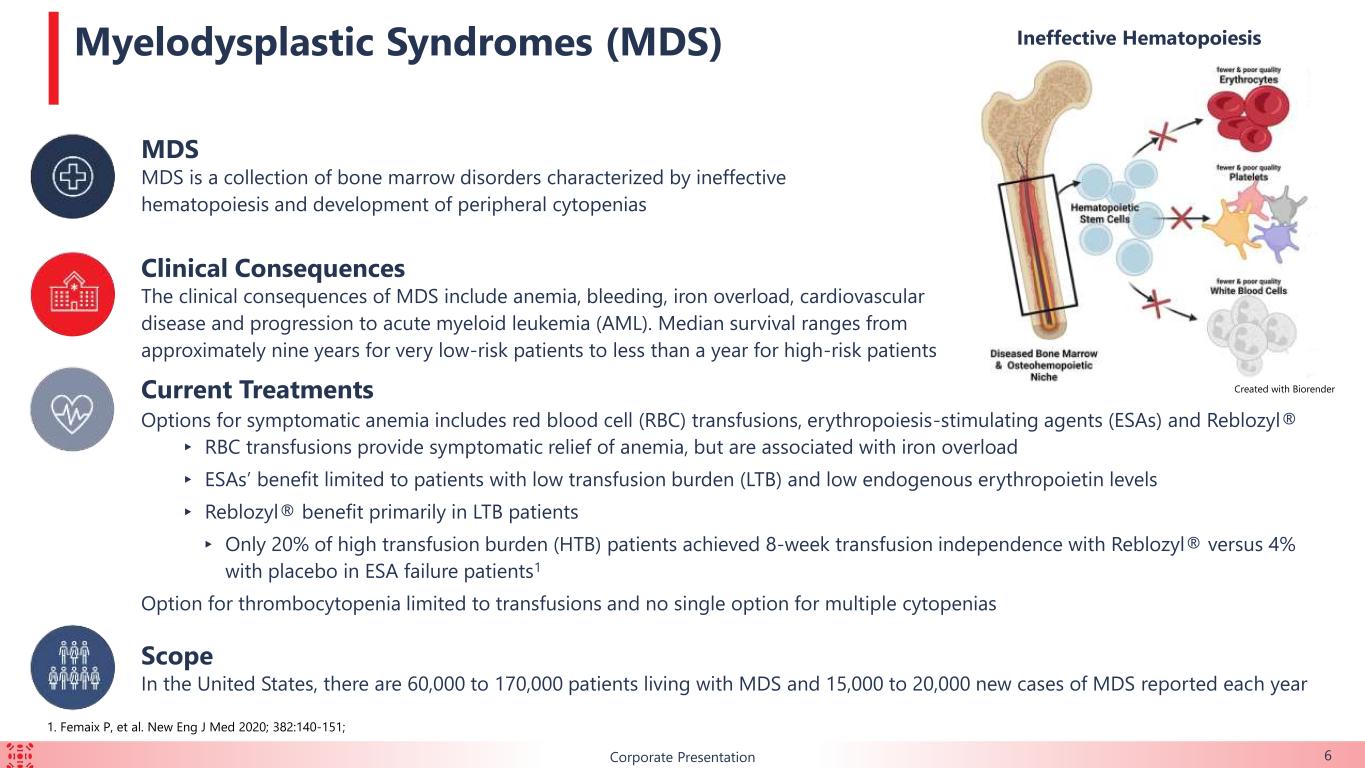
BCorporate Presentation 6 MDS MDS is a collection of bone marrow disorders characterized by ineffective hematopoiesis and development of peripheral cytopenias Clinical Consequences The clinical consequences of MDS include anemia, bleeding, iron overload, cardiovascular disease and progression to acute myeloid leukemia (AML). Median survival ranges from approximately nine years for very low-risk patients to less than a year for high-risk patients Scope In the United States, there are 60,000 to 170,000 patients living with MDS and 15,000 to 20,000 new cases of MDS reported each year Ineffective HematopoiesisMyelodysplastic Syndromes (MDS) Current Treatments Options for symptomatic anemia includes red blood cell (RBC) transfusions, erythropoiesis-stimulating agents (ESAs) and Reblozyl® ▸ RBC transfusions provide symptomatic relief of anemia, but are associated with iron overload ▸ ESAs’ benefit limited to patients with low transfusion burden (LTB) and low endogenous erythropoietin levels ▸ Reblozyl® benefit primarily in LTB patients ▸ Only 20% of high transfusion burden (HTB) patients achieved 8-week transfusion independence with Reblozyl® versus 4% with placebo in ESA failure patients1 Option for thrombocytopenia limited to transfusions and no single option for multiple cytopenias Created with Biorender 1. Femaix P, et al. New Eng J Med 2020; 382:140-151;
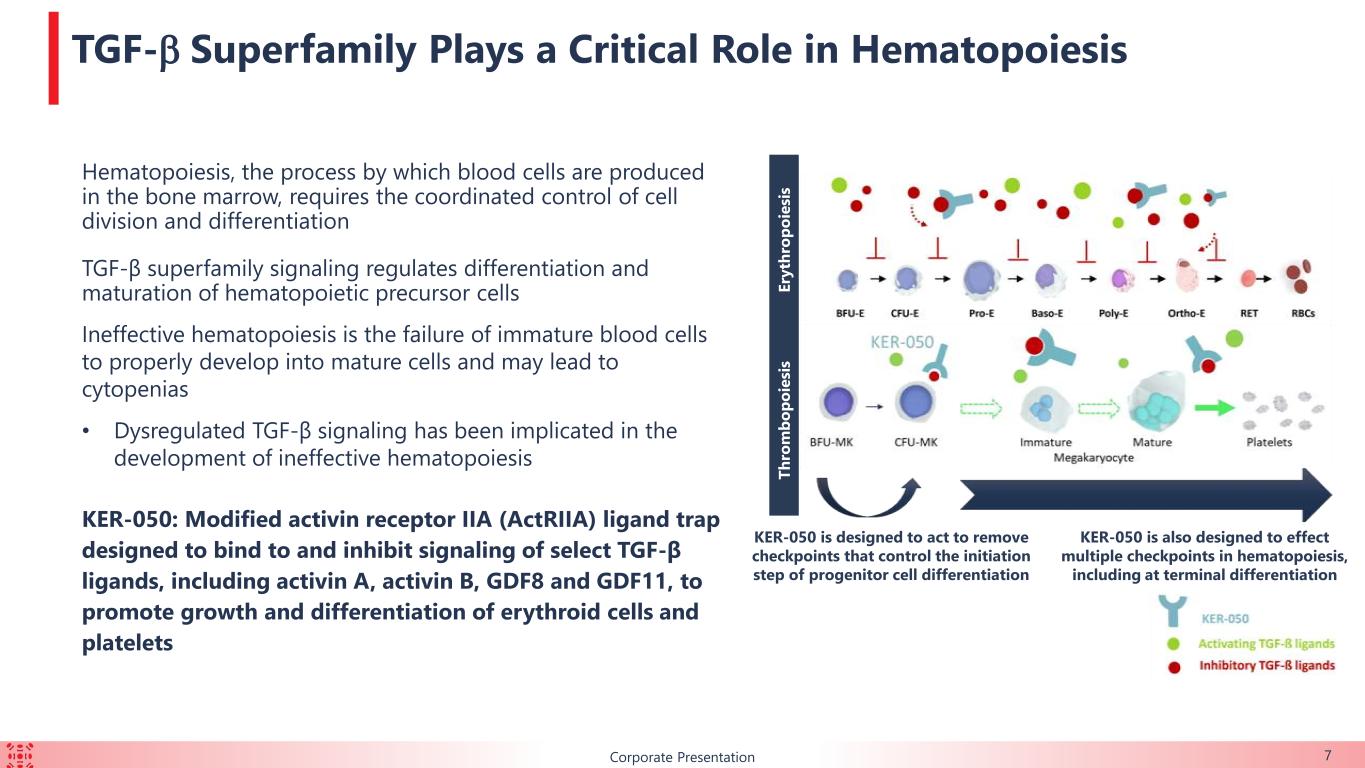
7 TGF-b Superfamily Plays a Critical Role in Hematopoiesis Hematopoiesis, the process by which blood cells are produced in the bone marrow, requires the coordinated control of cell division and differentiation TGF-β superfamily signaling regulates differentiation and maturation of hematopoietic precursor cells Ineffective hematopoiesis is the failure of immature blood cells to properly develop into mature cells and may lead to cytopenias • Dysregulated TGF-β signaling has been implicated in the development of ineffective hematopoiesis KER-050: Modified activin receptor IIA (ActRIIA) ligand trap designed to bind to and inhibit signaling of select TGF-β ligands, including activin A, activin B, GDF8 and GDF11, to promote growth and differentiation of erythroid cells and platelets Corporate Presentation E ry th ro p o ie si s T h ro m b o p o ie si s KER-050 is designed to act to remove checkpoints that control the initiation step of progenitor cell differentiation KER-050 is also designed to effect multiple checkpoints in hematopoiesis, including at terminal differentiation
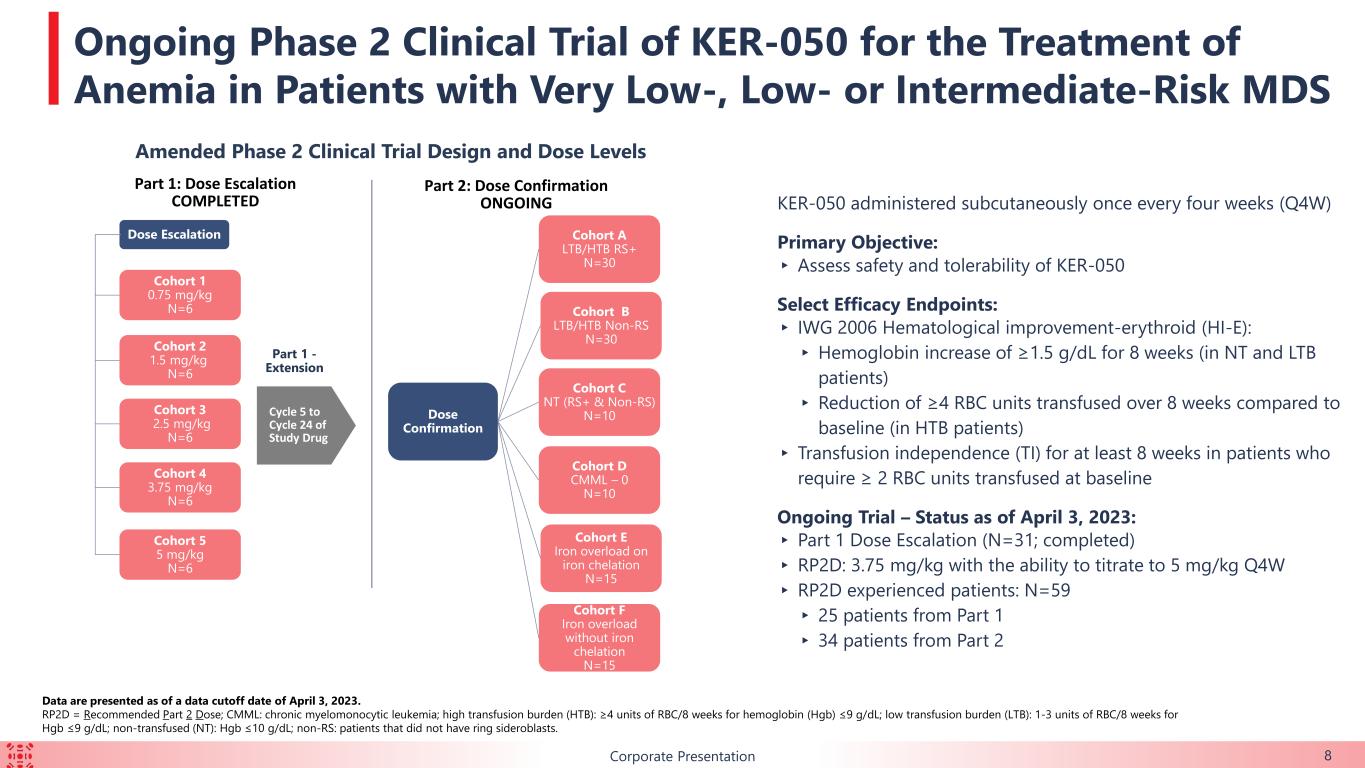
B KER-050 administered subcutaneously once every four weeks (Q4W) Primary Objective: ▸ Assess safety and tolerability of KER-050 Select Efficacy Endpoints: ▸ IWG 2006 Hematological improvement-erythroid (HI-E): ▸ Hemoglobin increase of ≥1.5 g/dL for 8 weeks (in NT and LTB patients) ▸ Reduction of ≥4 RBC units transfused over 8 weeks compared to baseline (in HTB patients) ▸ Transfusion independence (TI) for at least 8 weeks in patients who require ≥ 2 RBC units transfused at baseline Ongoing Trial – Status as of April 3, 2023: ▸ Part 1 Dose Escalation (N=31; completed) ▸ RP2D: 3.75 mg/kg with the ability to titrate to 5 mg/kg Q4W ▸ RP2D experienced patients: N=59 ▸ 25 patients from Part 1 ▸ 34 patients from Part 2 Corporate Presentation 8 Data are presented as of a data cutoff date of April 3, 2023. RP2D = Recommended Part 2 Dose; CMML: chronic myelomonocytic leukemia; high transfusion burden (HTB): ≥4 units of RBC/8 weeks for hemoglobin (Hgb) ≤9 g/dL; low transfusion burden (LTB): 1-3 units of RBC/8 weeks for Hgb ≤9 g/dL; non-transfused (NT): Hgb ≤10 g/dL; non-RS: patients that did not have ring sideroblasts. Ongoing Phase 2 Clinical Trial of KER-050 for the Treatment of Anemia in Patients with Very Low-, Low- or Intermediate-Risk MDS Amended Phase 2 Clinical Trial Design and Dose Levels Part 1: Dose Escalation COMPLETED Cycle 5 to Cycle 24 of Study Drug Part 1 - Extension Dose Escalation Cohort 1 0.75 mg/kg N=6 Cohort 2 1.5 mg/kg N=6 Cohort 3 2.5 mg/kg N=6 Cohort 4 3.75 mg/kg N=6 Cohort 5 5 mg/kg N=6 Part 2: Dose Confirmation ONGOING Dose Confirmation Cohort C NT (RS+ & Non-RS) N=10 Cohort B LTB/HTB Non-RS N=30 Cohort A LTB/HTB RS+ N=30 Cohort D CMML – 0 N=10 Cohort E Iron overload on iron chelation N=15 Cohort F Iron overload without iron chelation N=15
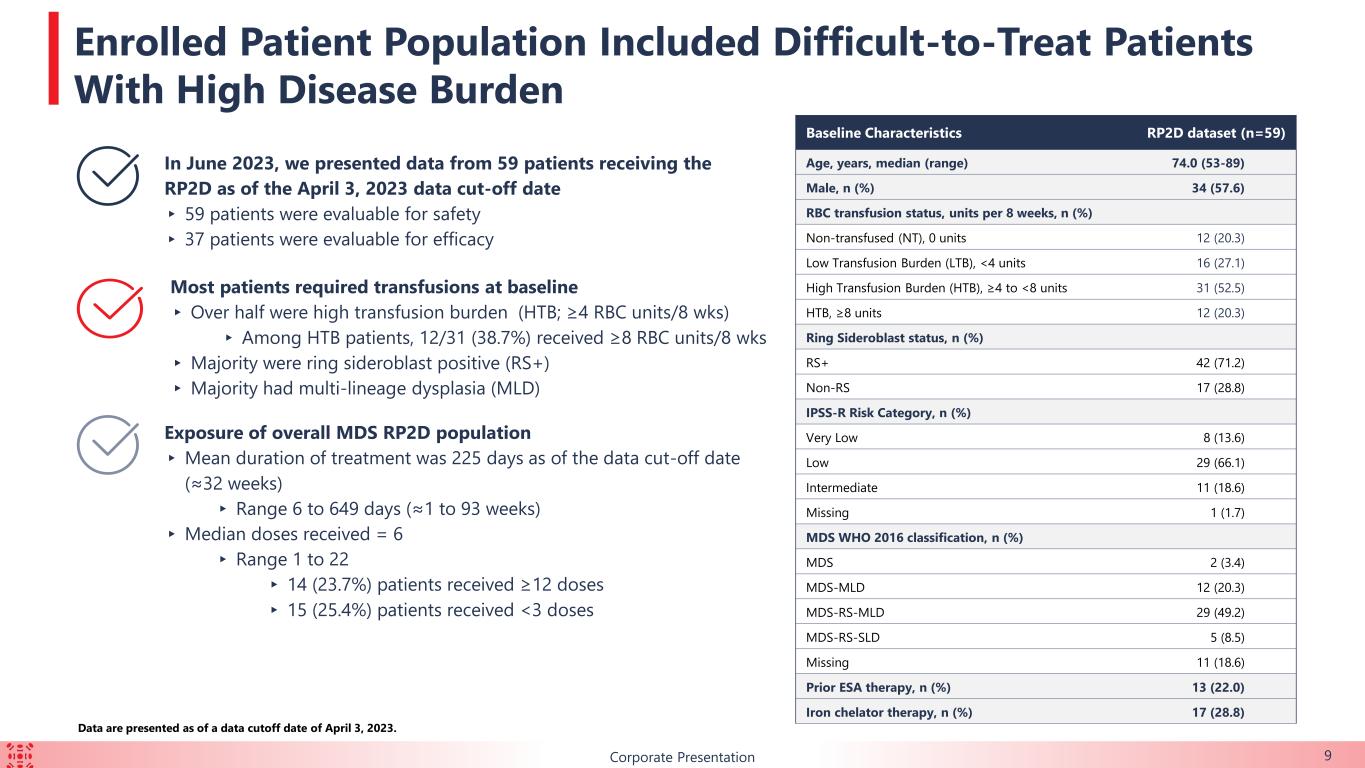
9Corporate Presentation Baseline Characteristics RP2D dataset (n=59) Age, years, median (range) 74.0 (53-89) Male, n (%) 34 (57.6) RBC transfusion status, units per 8 weeks, n (%) Non-transfused (NT), 0 units 12 (20.3) Low Transfusion Burden (LTB), <4 units 16 (27.1) High Transfusion Burden (HTB), ≥4 to <8 units 31 (52.5) HTB, ≥8 units 12 (20.3) Ring Sideroblast status, n (%) RS+ 42 (71.2) Non-RS 17 (28.8) IPSS-R Risk Category, n (%) Very Low 8 (13.6) Low 29 (66.1) Intermediate 11 (18.6) Missing 1 (1.7) MDS WHO 2016 classification, n (%) MDS 2 (3.4) MDS-MLD 12 (20.3) MDS-RS-MLD 29 (49.2) MDS-RS-SLD 5 (8.5) Missing 11 (18.6) Prior ESA therapy, n (%) 13 (22.0) Iron chelator therapy, n (%) 17 (28.8) In June 2023, we presented data from 59 patients receiving the RP2D as of the April 3, 2023 data cut-off date ▸ 59 patients were evaluable for safety ▸ 37 patients were evaluable for efficacy Most patients required transfusions at baseline ▸ Over half were high transfusion burden (HTB; ≥4 RBC units/8 wks) ▸ Among HTB patients, 12/31 (38.7%) received ≥8 RBC units/8 wks ▸ Majority were ring sideroblast positive (RS+) ▸ Majority had multi-lineage dysplasia (MLD) Exposure of overall MDS RP2D population ▸ Mean duration of treatment was 225 days as of the data cut-off date (≈32 weeks) ▸ Range 6 to 649 days (≈1 to 93 weeks) ▸ Median doses received = 6 ▸ Range 1 to 22 ▸ 14 (23.7%) patients received ≥12 doses ▸ 15 (25.4%) patients received <3 doses Data are presented as of a data cutoff date of April 3, 2023. Enrolled Patient Population Included Difficult-to-Treat Patients With High Disease Burden
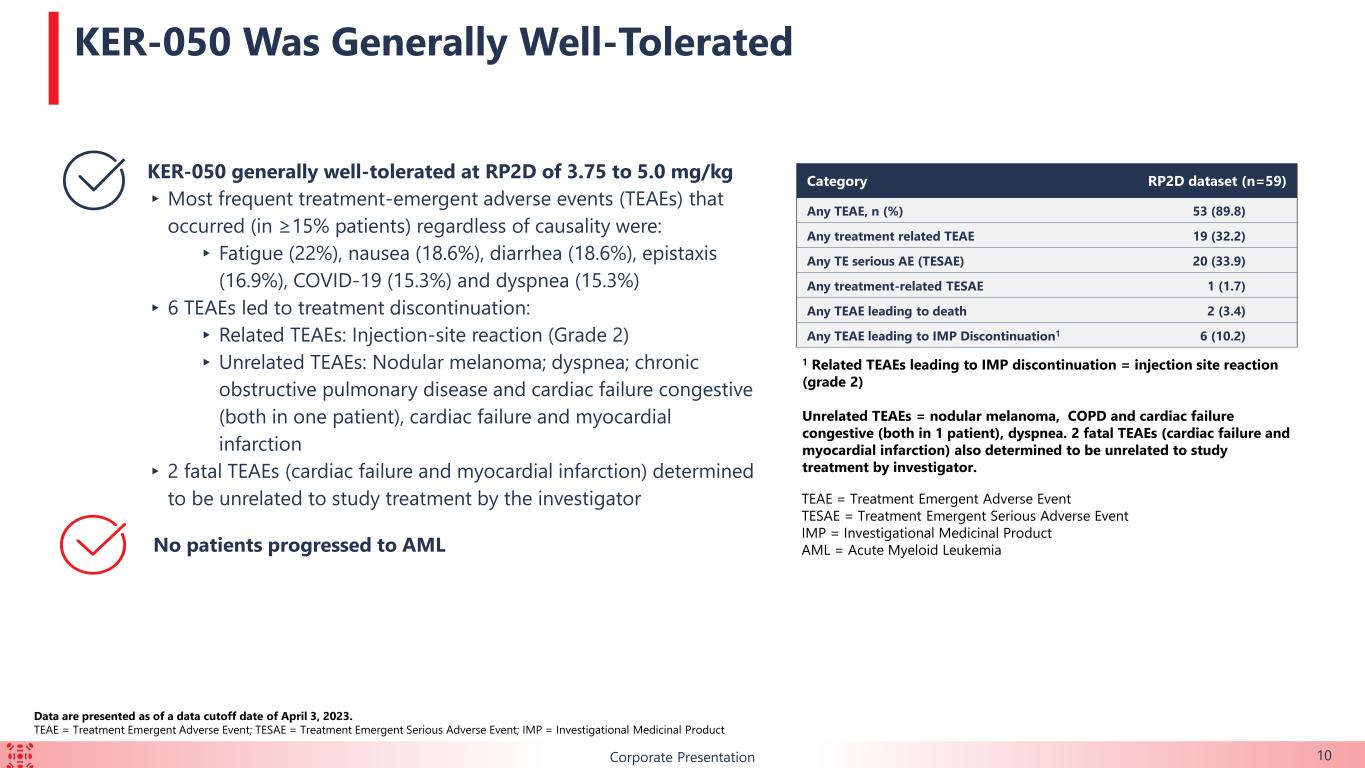
10Corporate Presentation Category RP2D dataset (n=59) Any TEAE, n (%) 53 (89.8) Any treatment related TEAE 19 (32.2) Any TE serious AE (TESAE) 20 (33.9) Any treatment-related TESAE 1 (1.7) Any TEAE leading to death 2 (3.4) Any TEAE leading to IMP Discontinuation1 6 (10.2) 1 Related TEAEs leading to IMP discontinuation = injection site reaction (grade 2) Unrelated TEAEs = nodular melanoma, COPD and cardiac failure congestive (both in 1 patient), dyspnea. 2 fatal TEAEs (cardiac failure and myocardial infarction) also determined to be unrelated to study treatment by investigator. KER-050 generally well-tolerated at RP2D of 3.75 to 5.0 mg/kg ▸Most frequent treatment-emergent adverse events (TEAEs) that occurred (in ≥15% patients) regardless of causality were: ▸ Fatigue (22%), nausea (18.6%), diarrhea (18.6%), epistaxis (16.9%), COVID-19 (15.3%) and dyspnea (15.3%) ▸ 6 TEAEs led to treatment discontinuation: ▸ Related TEAEs: Injection-site reaction (Grade 2) ▸Unrelated TEAEs: Nodular melanoma; dyspnea; chronic obstructive pulmonary disease and cardiac failure congestive (both in one patient), cardiac failure and myocardial infarction ▸ 2 fatal TEAEs (cardiac failure and myocardial infarction) determined to be unrelated to study treatment by the investigator No patients progressed to AML KER-050 Was Generally Well-Tolerated Data are presented as of a data cutoff date of April 3, 2023. TEAE = Treatment Emergent Adverse Event; TESAE = Treatment Emergent Serious Adverse Event; IMP = Investigational Medicinal Product TEAE = Treatment Emergent Adverse Event TESAE = Treatment Emergent Serious Adverse Event IMP = Investigational Medicinal Product AML = Acute Myeloid Leukemia
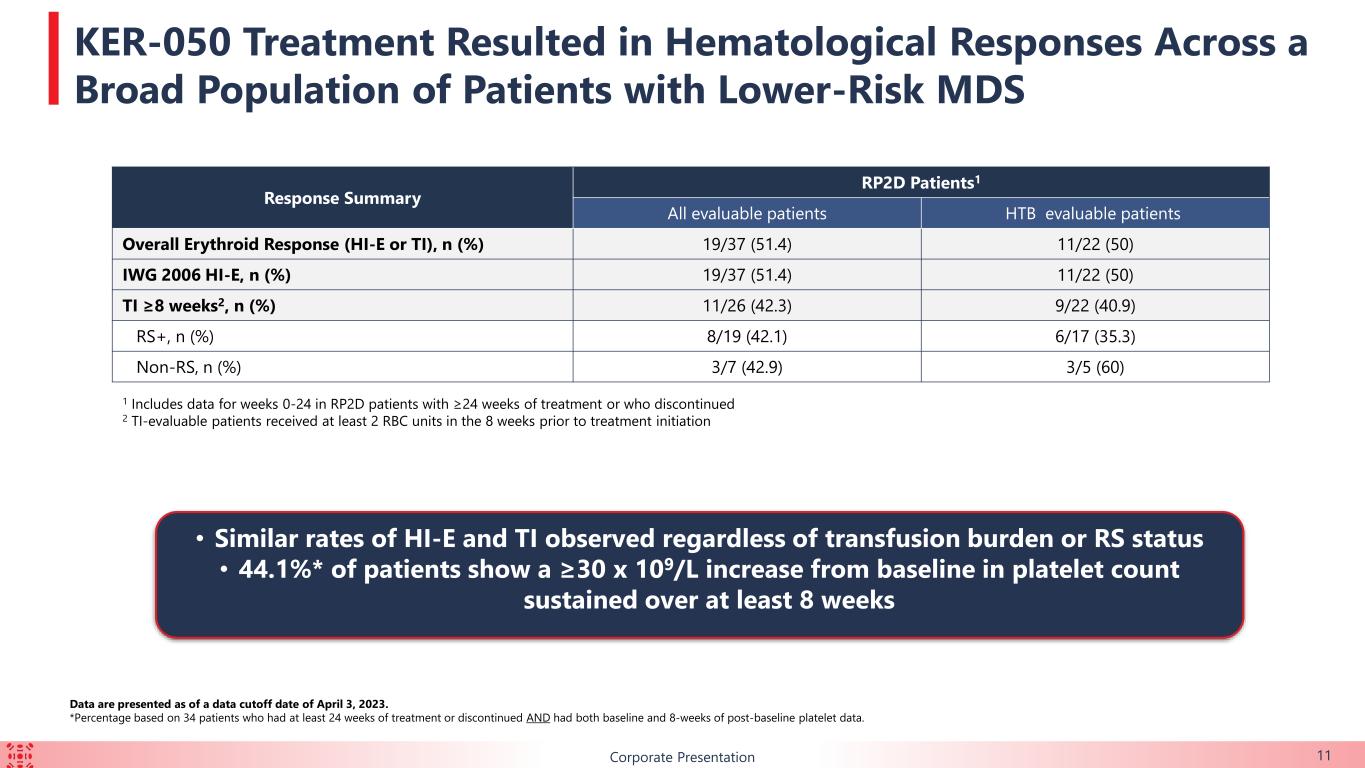
11Corporate Presentation Data are presented as of a data cutoff date of April 3, 2023. *Percentage based on 34 patients who had at least 24 weeks of treatment or discontinued AND had both baseline and 8-weeks of post-baseline platelet data. Response Summary RP2D Patients1 All evaluable patients HTB evaluable patients Overall Erythroid Response (HI-E or TI), n (%) 19/37 (51.4) 11/22 (50) IWG 2006 HI-E, n (%) 19/37 (51.4) 11/22 (50) TI ≥8 weeks2, n (%) 11/26 (42.3) 9/22 (40.9) RS+, n (%) 8/19 (42.1) 6/17 (35.3) Non-RS, n (%) 3/7 (42.9) 3/5 (60) 1 Includes data for weeks 0-24 in RP2D patients with ≥24 weeks of treatment or who discontinued 2 TI-evaluable patients received at least 2 RBC units in the 8 weeks prior to treatment initiation • Similar rates of HI-E and TI observed regardless of transfusion burden or RS status • 44.1%* of patients show a ≥30 x 109/L increase from baseline in platelet count sustained over at least 8 weeks KER-050 Treatment Resulted in Hematological Responses Across a Broad Population of Patients with Lower-Risk MDS
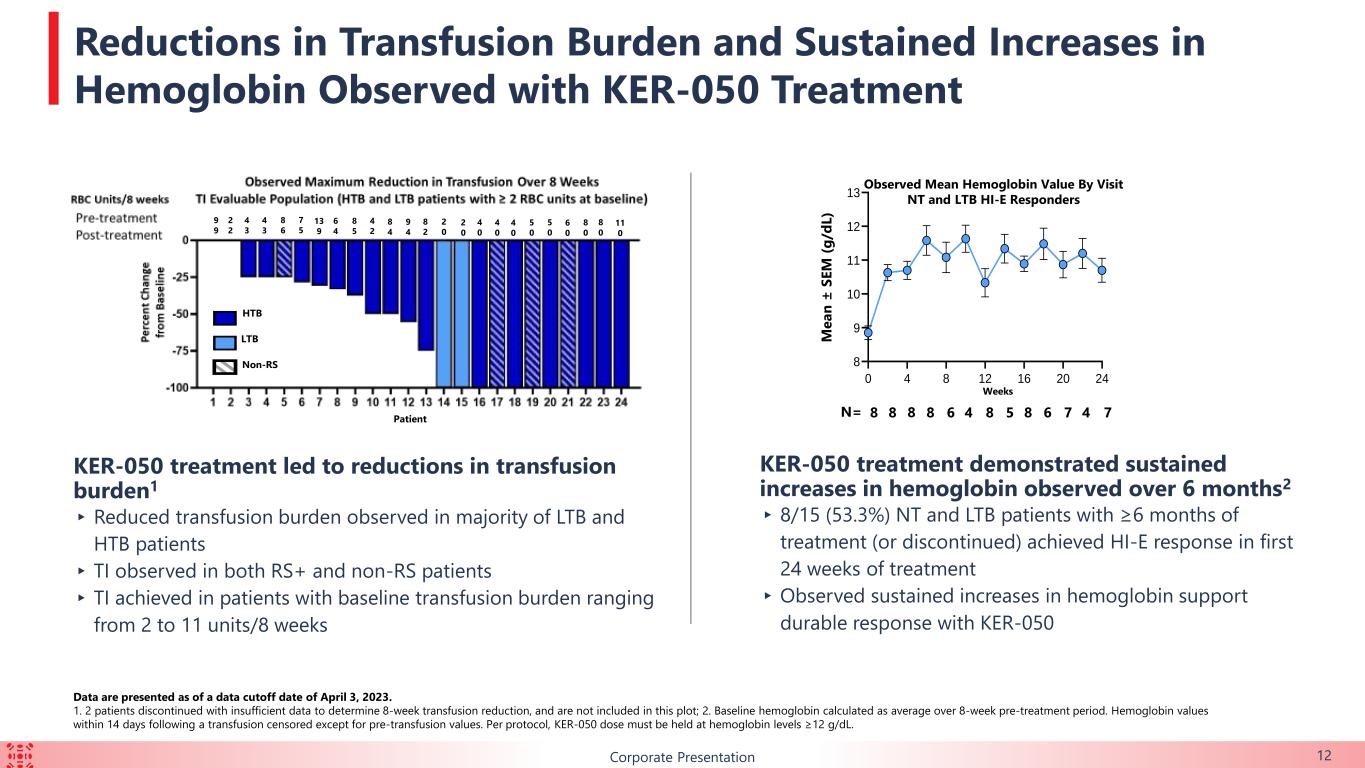
12Corporate Presentation Data are presented as of a data cutoff date of April 3, 2023. 1. 2 patients discontinued with insufficient data to determine 8-week transfusion reduction, and are not included in this plot; 2. Baseline hemoglobin calculated as average over 8-week pre-treatment period. Hemoglobin values within 14 days following a transfusion censored except for pre-transfusion values. Per protocol, KER-050 dose must be held at hemoglobin levels ≥12 g/dL. KER-050 treatment led to reductions in transfusion burden1 ▸ Reduced transfusion burden observed in majority of LTB and HTB patients ▸ TI observed in both RS+ and non-RS patients ▸ TI achieved in patients with baseline transfusion burden ranging from 2 to 11 units/8 weeks Patient Weeks KER-050 treatment demonstrated sustained increases in hemoglobin observed over 6 months2 ▸ 8/15 (53.3%) NT and LTB patients with ≥6 months of treatment (or discontinued) achieved HI-E response in first 24 weeks of treatment ▸Observed sustained increases in hemoglobin support durable response with KER-050 N= Observed Mean Hemoglobin Value By Visit NT and LTB HI-E Responders M e a n ± S E M ( g /d L ) 8 8 8 6 4 8 5 8 6 7 4 78 0 4 8 12 16 20 24 8 9 10 11 12 13 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 -100 -75 -50 -25 0 HTB LTB Non-RS Reductions in Transfusion Burden and Sustained Increases in Hemoglobin Observed with KER-050 Treatment 2 2 9 9 4 3 4 3 8 6 7 5 13 9 6 4 8 5 4 2 8 4 9 4 8 2 2 0 2 0 4 0 4 0 4 0 5 0 5 0 8 0 8 0 11 0 6 0
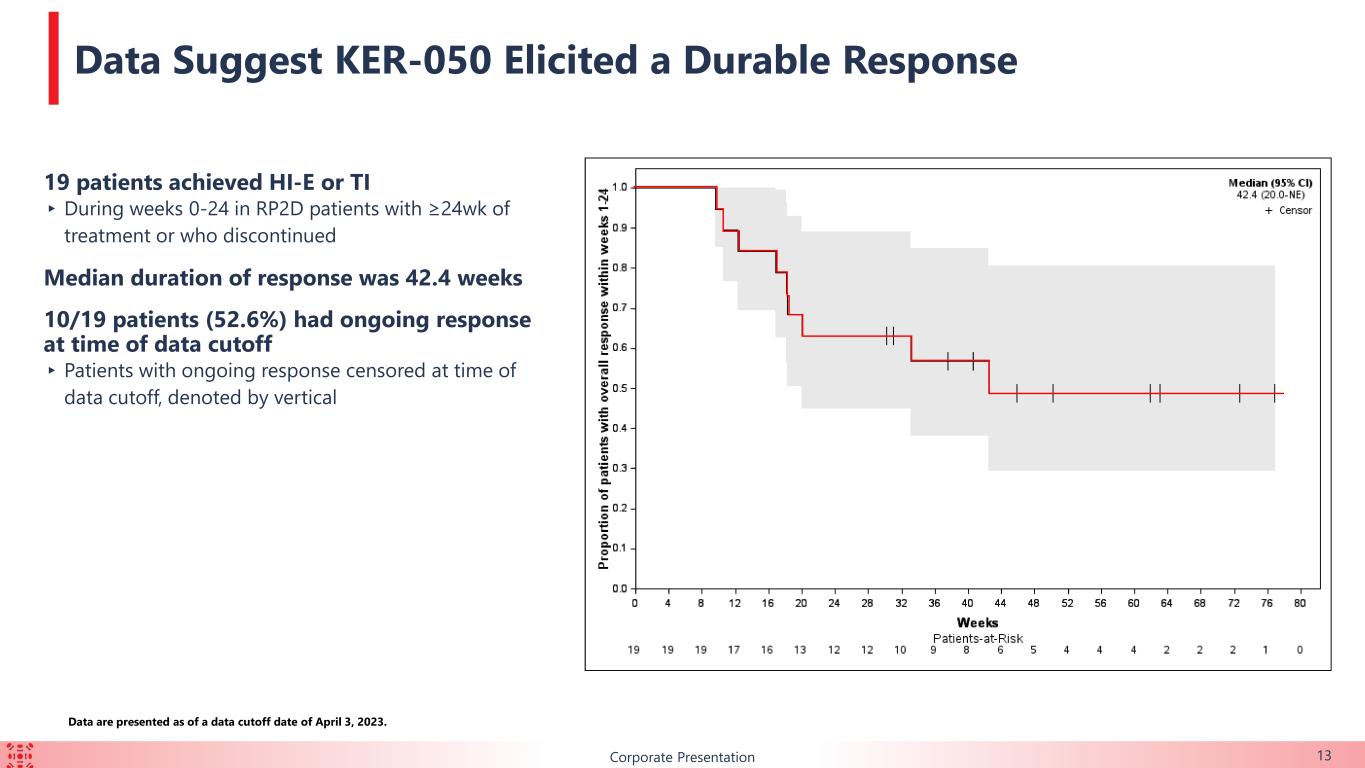
13Corporate Presentation Data are presented as of a data cutoff date of April 3, 2023. 19 patients achieved HI-E or TI ▸During weeks 0-24 in RP2D patients with ≥24wk of treatment or who discontinued Median duration of response was 42.4 weeks 10/19 patients (52.6%) had ongoing response at time of data cutoff ▸ Patients with ongoing response censored at time of data cutoff, denoted by vertical Data Suggest KER-050 Elicited a Durable Response

B KER-050 14 Investigational Treatment for Anemia and Thrombocytopenia in Patients with Myelofibrosis Ongoing Phase 2 Open-Label Clinical Trial to Evaluate the Safety and Efficacy of KER-050 as Monotherapy or in Combination with Ruxolitinib in Participants with Myelofibrosis Corporate Presentation
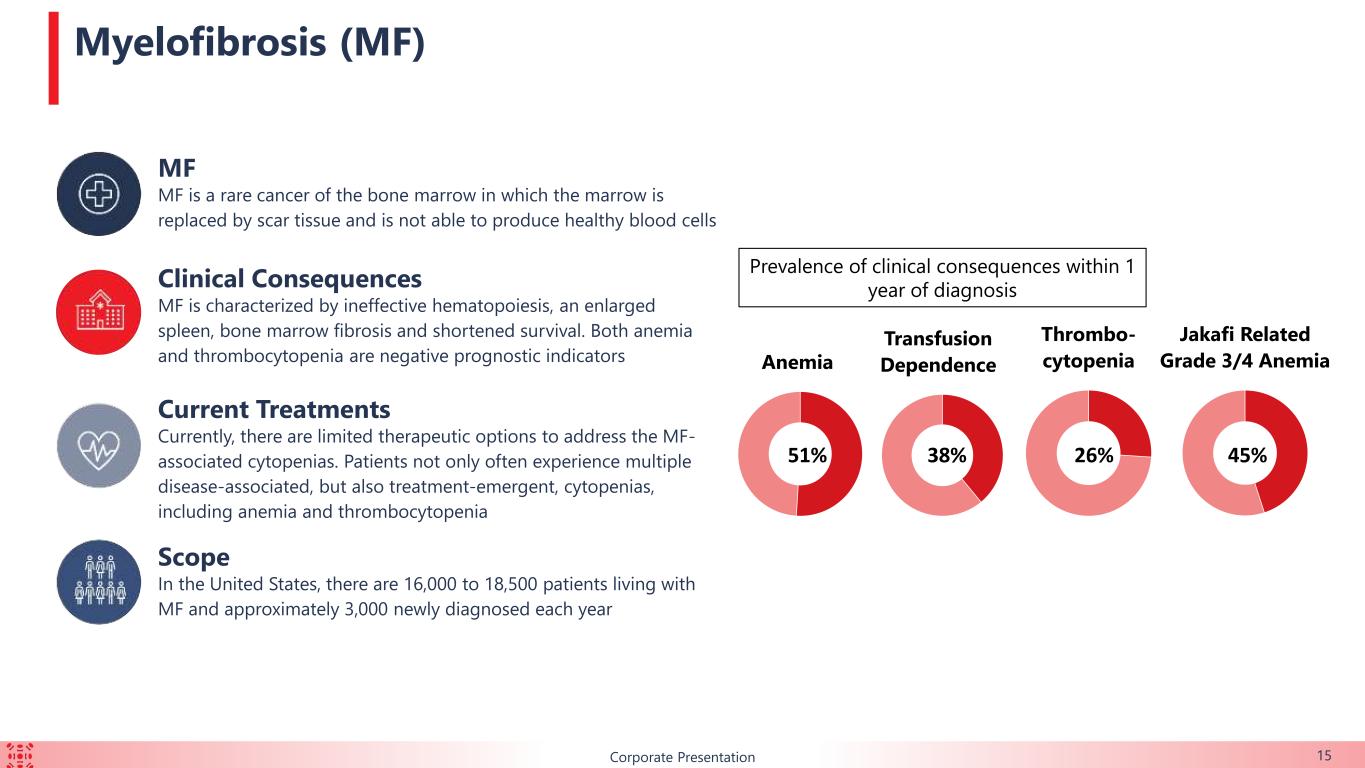
BCorporate Presentation 15 MF MF is a rare cancer of the bone marrow in which the marrow is replaced by scar tissue and is not able to produce healthy blood cells Clinical Consequences MF is characterized by ineffective hematopoiesis, an enlarged spleen, bone marrow fibrosis and shortened survival. Both anemia and thrombocytopenia are negative prognostic indicators Current Treatments Currently, there are limited therapeutic options to address the MF- associated cytopenias. Patients not only often experience multiple disease-associated, but also treatment-emergent, cytopenias, including anemia and thrombocytopenia Scope In the United States, there are 16,000 to 18,500 patients living with MF and approximately 3,000 newly diagnosed each year Myelofibrosis (MF) Prevalence of clinical consequences within 1 year of diagnosis 51% Anemia 38% Transfusion Dependence 26% Thrombo- cytopenia Jakafi Related Grade 3/4 Anemia 45%
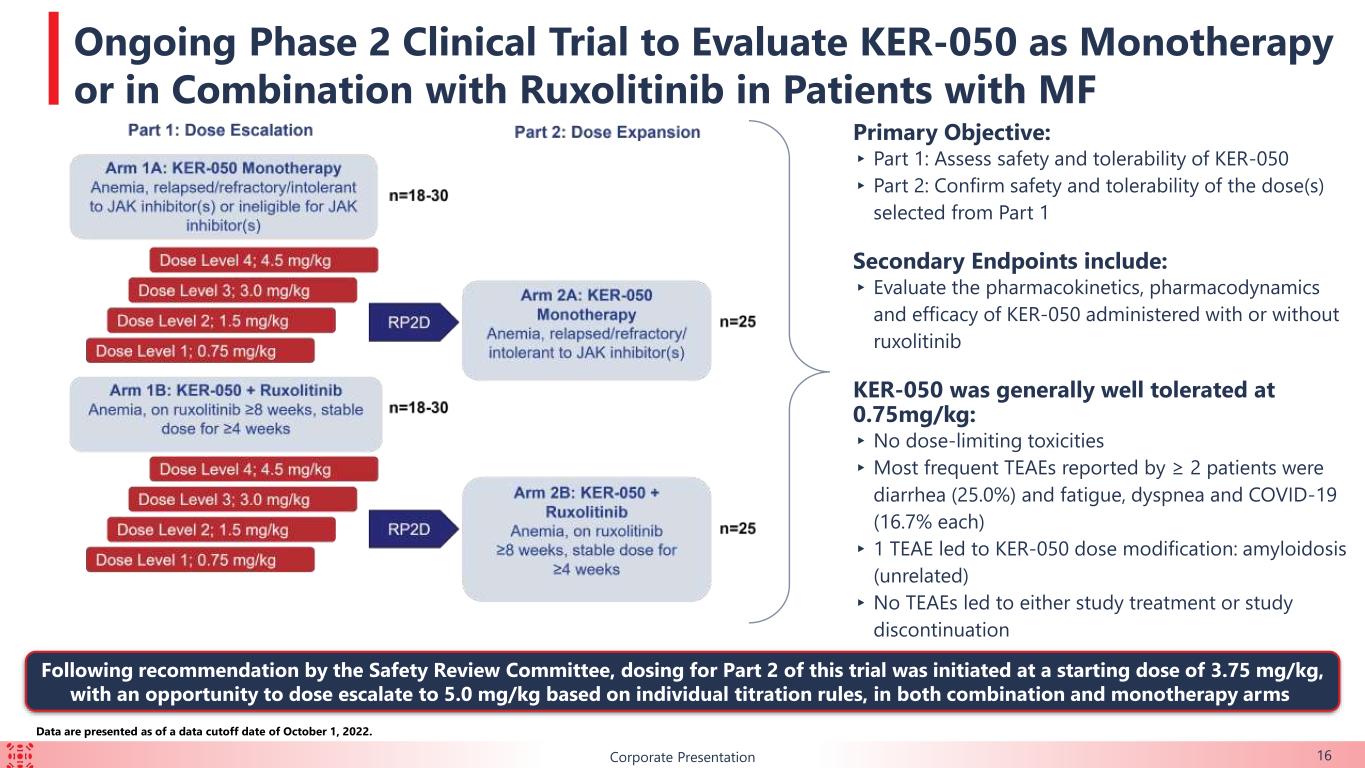
BCorporate Presentation 16 Data are presented as of a data cutoff date of October 1, 2022. Primary Objective: ▸ Part 1: Assess safety and tolerability of KER-050 ▸ Part 2: Confirm safety and tolerability of the dose(s) selected from Part 1 Secondary Endpoints include: ▸ Evaluate the pharmacokinetics, pharmacodynamics and efficacy of KER-050 administered with or without ruxolitinib KER-050 was generally well tolerated at 0.75mg/kg: ▸No dose-limiting toxicities ▸Most frequent TEAEs reported by ≥ 2 patients were diarrhea (25.0%) and fatigue, dyspnea and COVID-19 (16.7% each) ▸ 1 TEAE led to KER-050 dose modification: amyloidosis (unrelated) ▸No TEAEs led to either study treatment or study discontinuation Ongoing Phase 2 Clinical Trial to Evaluate KER-050 as Monotherapy or in Combination with Ruxolitinib in Patients with MF Following recommendation by the Safety Review Committee, dosing for Part 2 of this trial was initiated at a starting dose of 3.75 mg/kg, with an opportunity to dose escalate to 5.0 mg/kg based on individual titration rules, in both combination and monotherapy arms
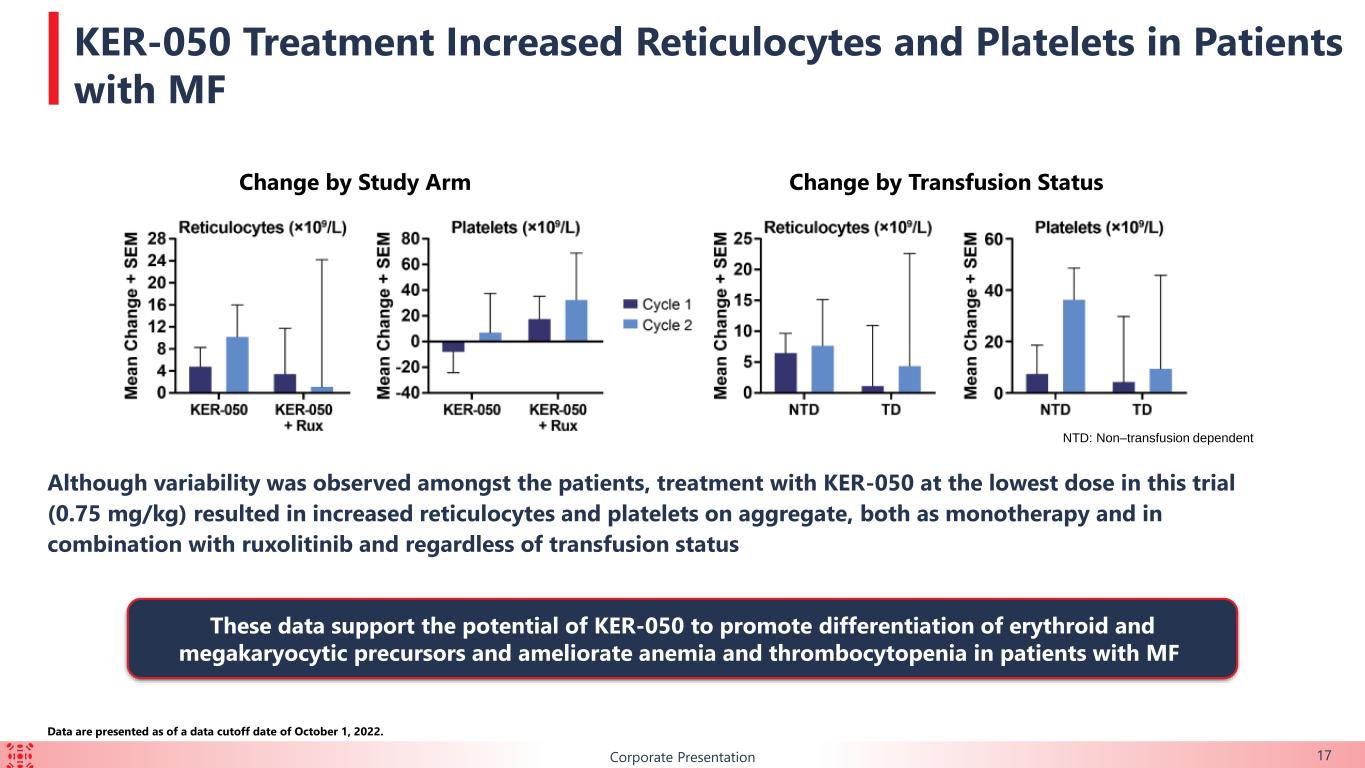
BCorporate Presentation 17 Change by Study Arm Change by Transfusion Status NTD: Non–transfusion dependent Although variability was observed amongst the patients, treatment with KER-050 at the lowest dose in this trial (0.75 mg/kg) resulted in increased reticulocytes and platelets on aggregate, both as monotherapy and in combination with ruxolitinib and regardless of transfusion status KER-050 Treatment Increased Reticulocytes and Platelets in Patients with MF Data are presented as of a data cutoff date of October 1, 2022. These data support the potential of KER-050 to promote differentiation of erythroid and megakaryocytic precursors and ameliorate anemia and thrombocytopenia in patients with MF
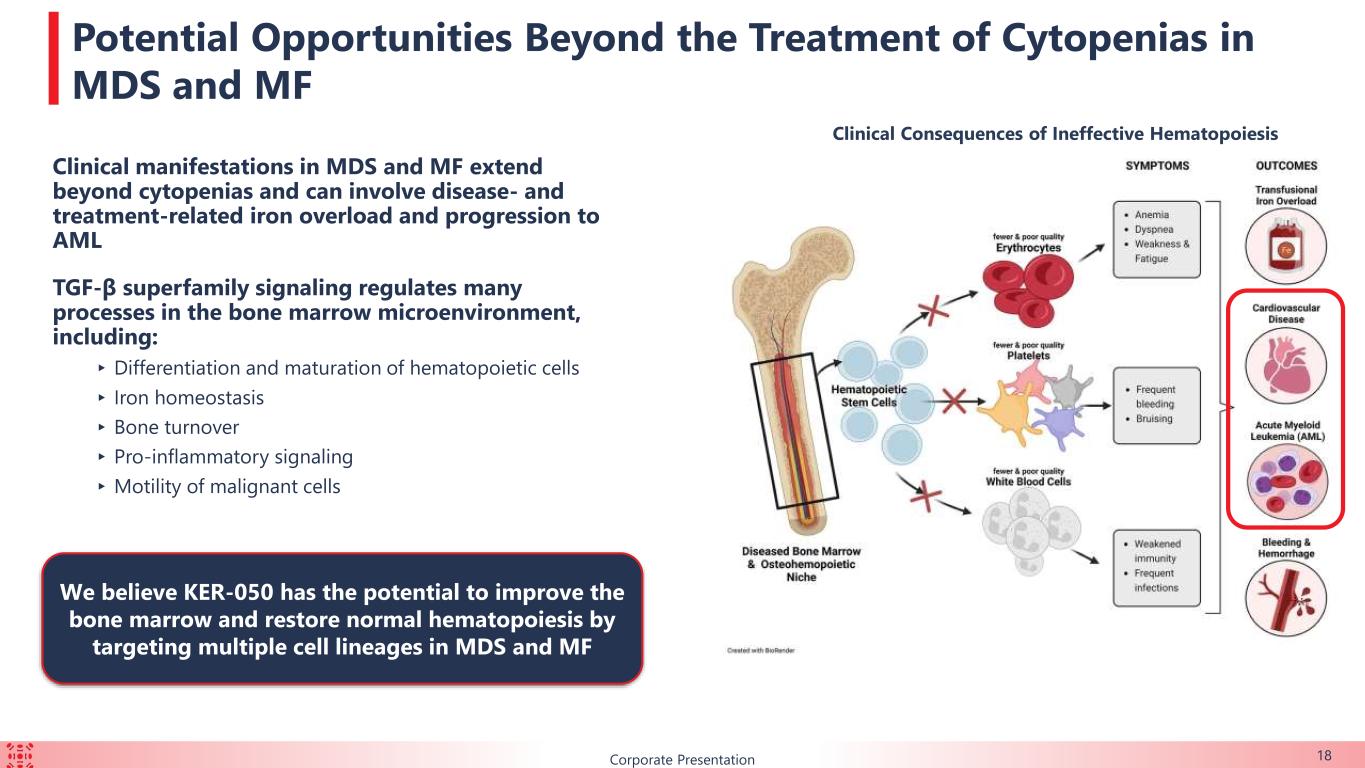
18 Potential Opportunities Beyond the Treatment of Cytopenias in MDS and MF Clinical manifestations in MDS and MF extend beyond cytopenias and can involve disease- and treatment-related iron overload and progression to AML TGF-β superfamily signaling regulates many processes in the bone marrow microenvironment, including: ▸Differentiation and maturation of hematopoietic cells ▸ Iron homeostasis ▸ Bone turnover ▸ Pro-inflammatory signaling ▸Motility of malignant cells Corporate Presentation We believe KER-050 has the potential to improve the bone marrow and restore normal hematopoiesis by targeting multiple cell lineages in MDS and MF Clinical Consequences of Ineffective Hematopoiesis

Pulmonary and Cardiovascular Franchise Corporate Presentation 19

KER-012 Corporate Presentation 20 Investigational Treatment for Pulmonary Arterial Hypertension (PAH) and for Cardiovascular Disorders Ongoing Randomized, Phase 2, Double-blind, Placebo-Controlled Clinical Trial to Evaluate the Safety and Efficacy of KER-012 in Combination with Background Therapy in Adult Participants with Pulmonary Hypertension
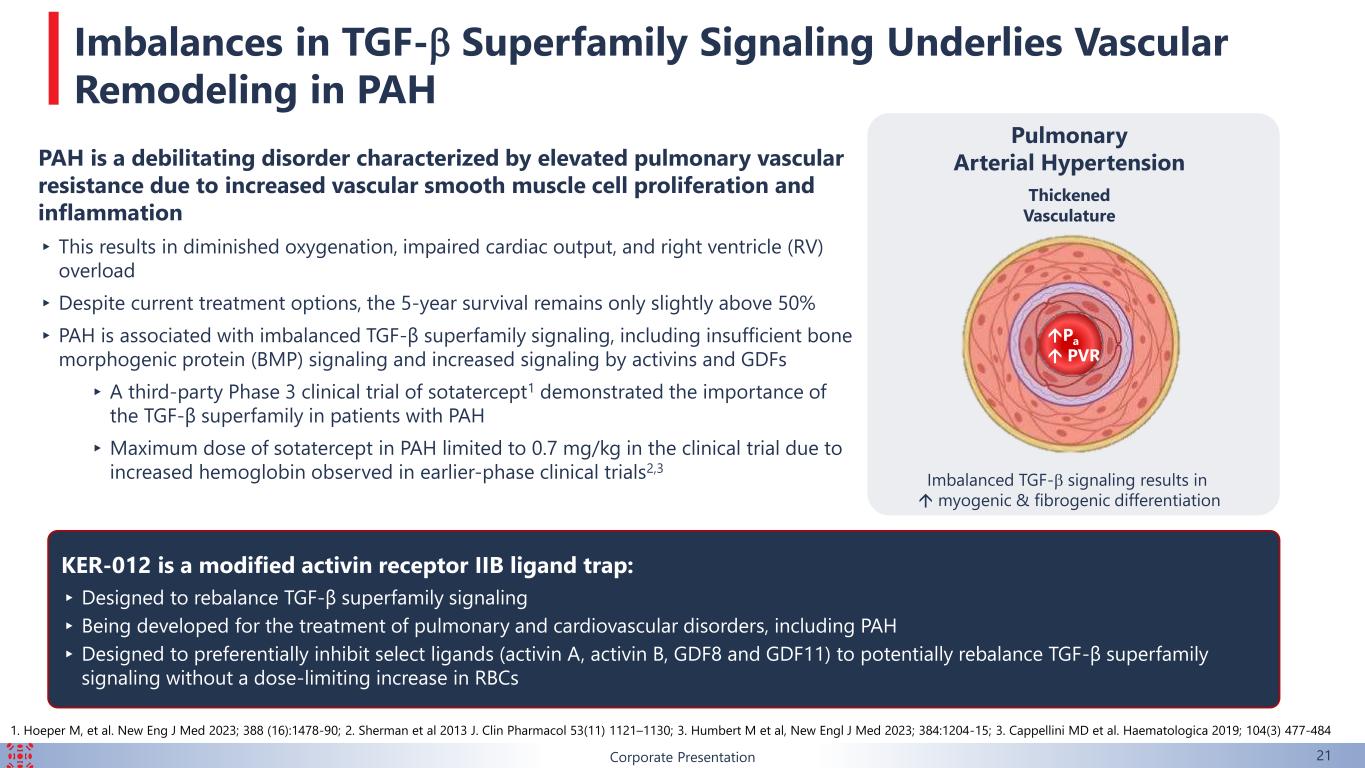
Corporate Presentation 21 KER-012 is a modified activin receptor IIB ligand trap: ▸Designed to rebalance TGF-β superfamily signaling ▸ Being developed for the treatment of pulmonary and cardiovascular disorders, including PAH ▸Designed to preferentially inhibit select ligands (activin A, activin B, GDF8 and GDF11) to potentially rebalance TGF-β superfamily signaling without a dose-limiting increase in RBCs Thickened Vasculature Pulmonary Arterial Hypertension Pa PVR Imbalanced TGF-b signaling results in myogenic & fibrogenic differentiation PAH is a debilitating disorder characterized by elevated pulmonary vascular resistance due to increased vascular smooth muscle cell proliferation and inflammation ▸ This results in diminished oxygenation, impaired cardiac output, and right ventricle (RV) overload ▸Despite current treatment options, the 5-year survival remains only slightly above 50% ▸ PAH is associated with imbalanced TGF-β superfamily signaling, including insufficient bone morphogenic protein (BMP) signaling and increased signaling by activins and GDFs ▸A third-party Phase 3 clinical trial of sotatercept1 demonstrated the importance of the TGF-β superfamily in patients with PAH ▸Maximum dose of sotatercept in PAH limited to 0.7 mg/kg in the clinical trial due to increased hemoglobin observed in earlier-phase clinical trials2,3 Imbalances in TGF-b Superfamily Signaling Underlies Vascular Remodeling in PAH 1. Hoeper M, et al. New Eng J Med 2023; 388 (16):1478-90; 2. Sherman et al 2013 J. Clin Pharmacol 53(11) 1121–1130; 3. Humbert M et al, New Engl J Med 2023; 384:1204-15; 3. Cappellini MD et al. Haematologica 2019; 104(3) 477-484
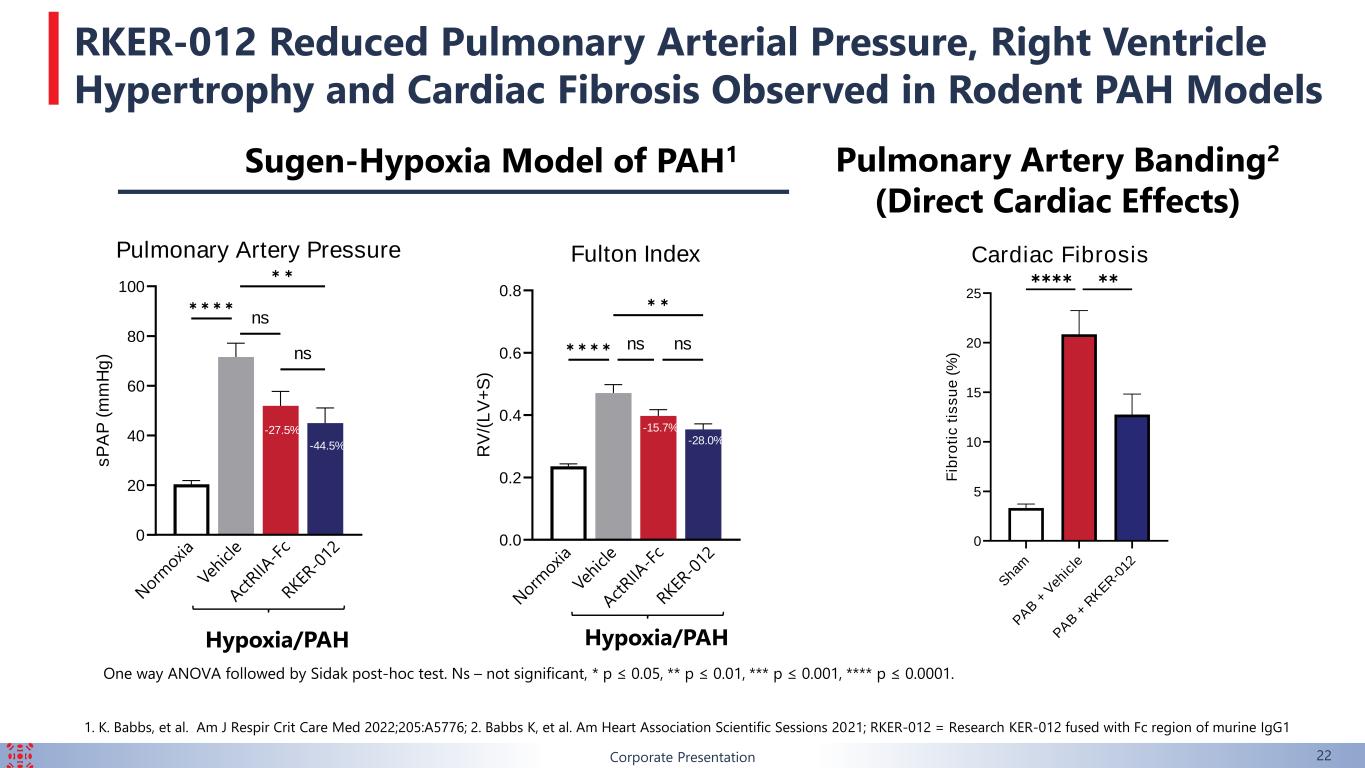
1. K. Babbs, et al. Am J Respir Crit Care Med 2022;205:A5776; 2. Babbs K, et al. Am Heart Association Scientific Sessions 2021; RKER-012 = Research KER-012 fused with Fc region of murine IgG1 0.0 0.2 0.4 0.6 0.8 Fulton Index R V /( L V + S ) -15.7% -28.0% ✱ ✱ ✱ ✱ ns ✱ ✱ ns 0 20 40 60 80 100 Pulmonary Artery Pressure s P A P ( m m H g ) -27.5% -44.5% ✱ ✱ ✱ ✱ ns ✱ ✱ ns One way ANOVA followed by Sidak post-hoc test. Ns – not significant, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. Hypoxia/PAH Hypoxia/PAH Sugen-Hypoxia Model of PAH1 Pulmonary Artery Banding2 (Direct Cardiac Effects) Cardiac Fibrosis S ham P A B + V eh ic le P A B + R K E R -0 12 0 5 10 15 20 25 F ib ro ti c t is s u e ( % ) -38.9% ✱✱✱✱ ✱✱ 22Corporate Presentation RKER-012 Reduced Pulmonary Arterial Pressure, Right Ventricle Hypertrophy and Cardiac Fibrosis Observed in Rodent PAH Models
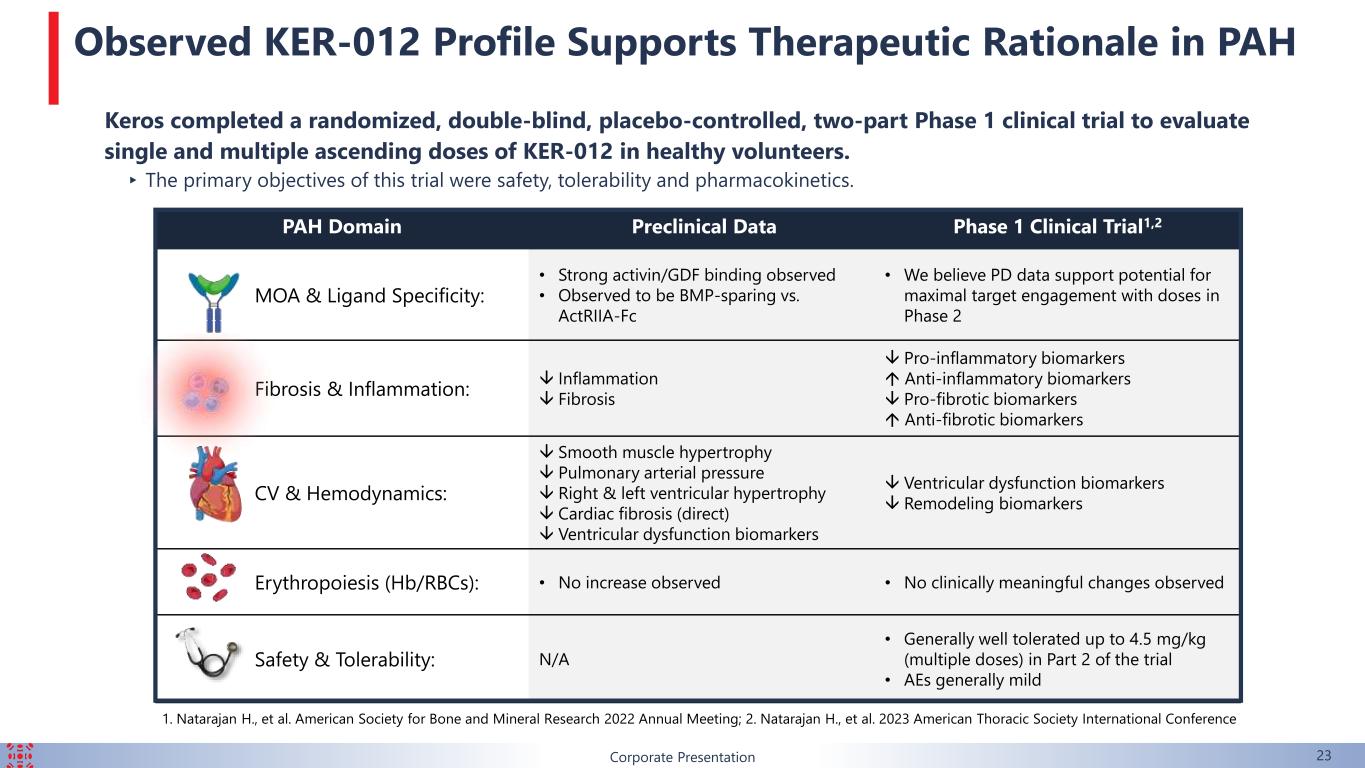
PAH Domain Preclinical Data Phase 1 Clinical Trial1,2 MOA & Ligand Specificity: • Strong activin/GDF binding observed • Observed to be BMP-sparing vs. ActRIIA-Fc • We believe PD data support potential for maximal target engagement with doses in Phase 2 Fibrosis & Inflammation: Inflammation Fibrosis Pro-inflammatory biomarkers Anti-inflammatory biomarkers Pro-fibrotic biomarkers Anti-fibrotic biomarkers CV & Hemodynamics: Smooth muscle hypertrophy Pulmonary arterial pressure Right & left ventricular hypertrophy Cardiac fibrosis (direct) Ventricular dysfunction biomarkers Ventricular dysfunction biomarkers Remodeling biomarkers Erythropoiesis (Hb/RBCs): • No increase observed • No clinically meaningful changes observed Safety & Tolerability: N/A • Generally well tolerated up to 4.5 mg/kg (multiple doses) in Part 2 of the trial • AEs generally mild Corporate Presentation 23 Keros completed a randomized, double-blind, placebo-controlled, two-part Phase 1 clinical trial to evaluate single and multiple ascending doses of KER-012 in healthy volunteers. ▸ The primary objectives of this trial were safety, tolerability and pharmacokinetics. 1. Natarajan H., et al. American Society for Bone and Mineral Research 2022 Annual Meeting; 2. Natarajan H., et al. 2023 American Thoracic Society International Conference Observed KER-012 Profile Supports Therapeutic Rationale in PAH
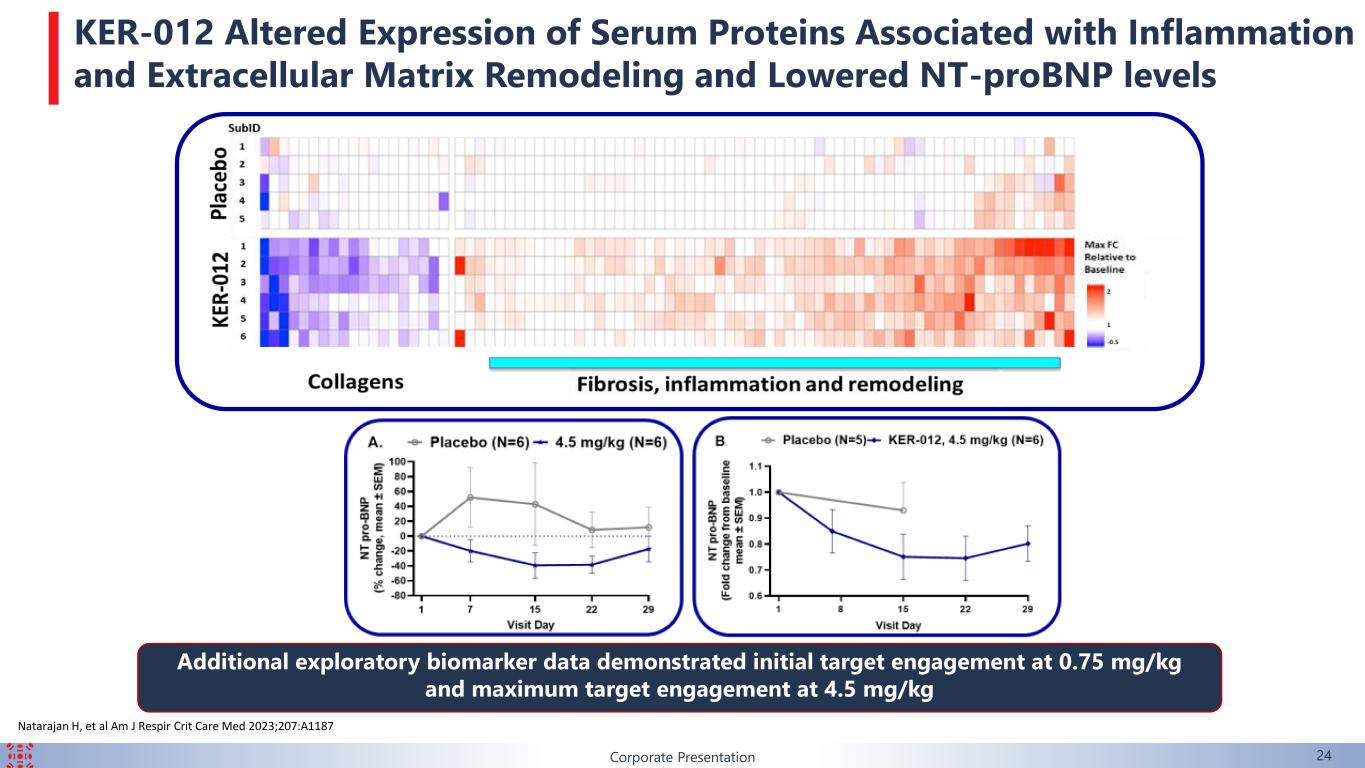
Natarajan H, et al Am J Respir Crit Care Med 2023;207:A1187 Additional exploratory biomarker data demonstrated initial target engagement at 0.75 mg/kg and maximum target engagement at 4.5 mg/kg Corporate Presentation 24 KER-012 Altered Expression of Serum Proteins Associated with Inflammation and Extracellular Matrix Remodeling and Lowered NT-proBNP levels
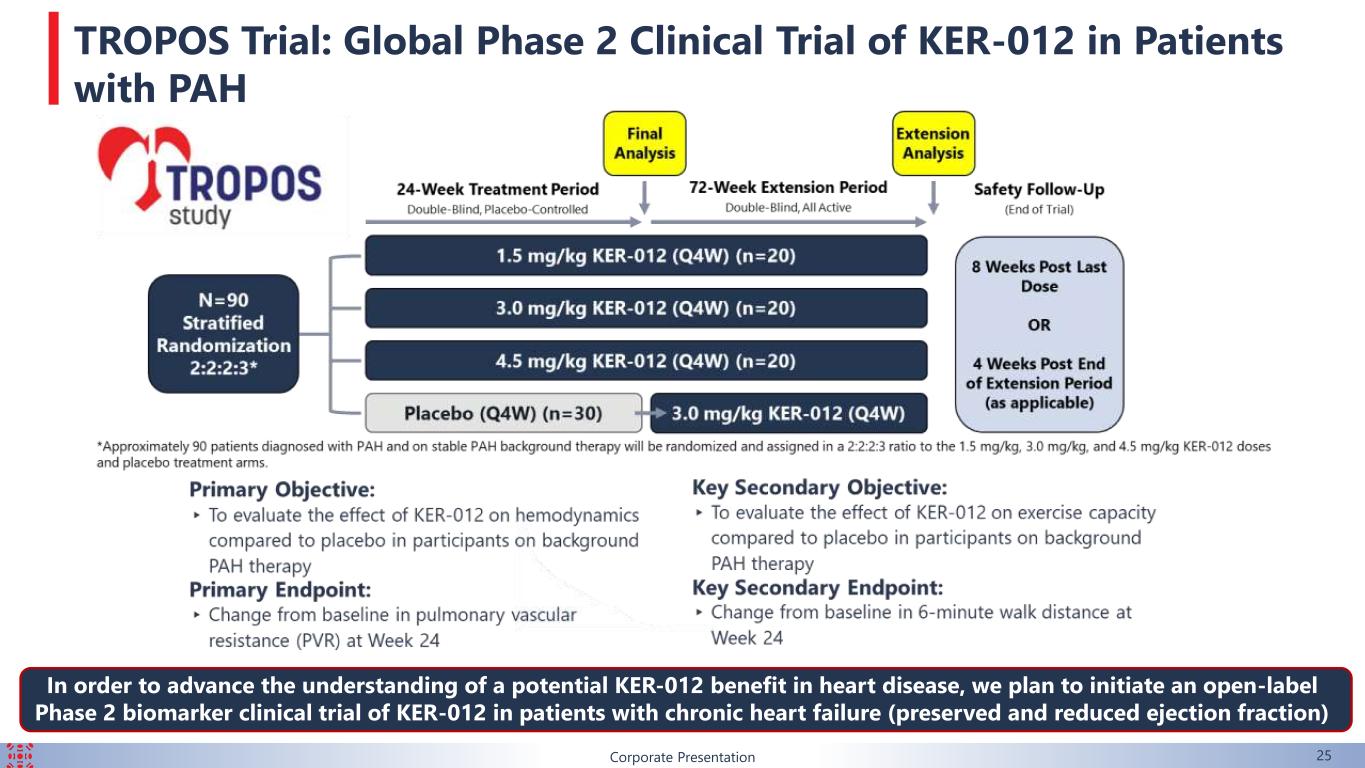
Corporate Presentation 25 TROPOS Trial: Global Phase 2 Clinical Trial of KER-012 in Patients with PAH In order to advance the understanding of a potential KER-012 benefit in heart disease, we plan to initiate an open-label Phase 2 biomarker clinical trial of KER-012 in patients with chronic heart failure (preserved and reduced ejection fraction)

Neuromuscular Franchise Corporate Presentation 26

Corporate Presentation 27 Muscle loss can occur as a consequence of many factors, including neuromuscular disease, disuse, aging and as a side effect of some therapies In neuromuscular diseases, muscle loss can result in muscle weakness and, with increased severity, can lead to loss of ambulation, reliance on a wheelchair, swallowing difficulties, respiratory muscle weakness and death ▸ Decline in muscle mass can also be associated with secondary osteoporosis and metabolic consequences, including obesity and insulin resistance TGF-b pathway signaling regulates skeletal muscle, fat and bone Activins and myostatin are powerful negative regulators of skeletal muscle Muscle Loss and the TGF-b Pathway

KER-065 Corporate Presentation 28 Designed to Address Neuromuscular Diseases, with an initial focus on Duchenne Muscular Dystrophy (DMD)
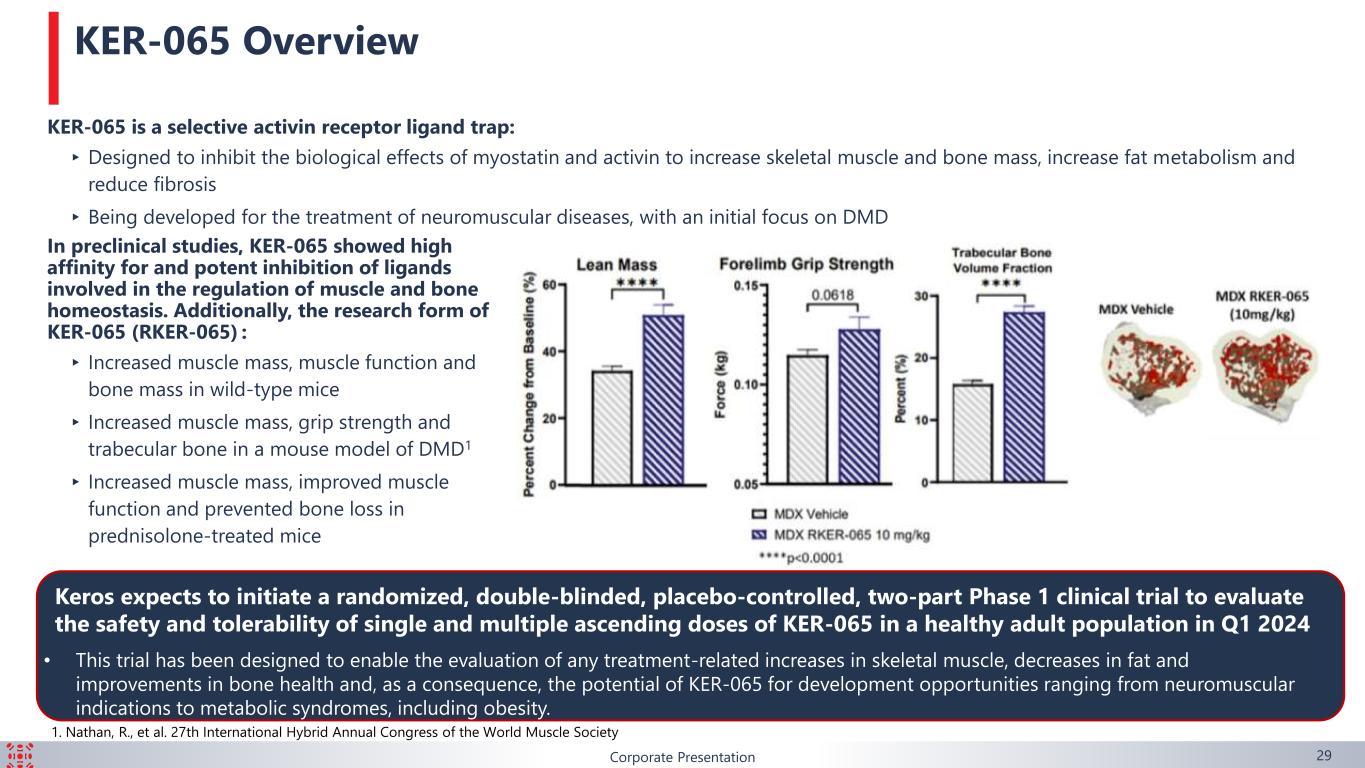
Corporate Presentation 29 KER-065 is a selective activin receptor ligand trap: ▸Designed to inhibit the biological effects of myostatin and activin to increase skeletal muscle and bone mass, increase fat metabolism and reduce fibrosis ▸ Being developed for the treatment of neuromuscular diseases, with an initial focus on DMD In preclinical studies, KER-065 showed high affinity for and potent inhibition of ligands involved in the regulation of muscle and bone homeostasis. Additionally, the research form of KER-065 (RKER-065) : ▸ Increased muscle mass, muscle function and bone mass in wild-type mice ▸ Increased muscle mass, grip strength and trabecular bone in a mouse model of DMD1 ▸ Increased muscle mass, improved muscle function and prevented bone loss in prednisolone-treated mice KER-065 Overview Keros expects to initiate a randomized, double-blinded, placebo-controlled, two-part Phase 1 clinical trial to evaluate the safety and tolerability of single and multiple ascending doses of KER-065 in a healthy adult population in Q1 2024 • This trial has been designed to enable the evaluation of any treatment-related increases in skeletal muscle, decreases in fat and improvements in bone health and, as a consequence, the potential of KER-065 for development opportunities ranging from neuromuscular indications to metabolic syndromes, including obesity. 1. Nathan, R., et al. 27th International Hybrid Annual Congress of the World Muscle Society
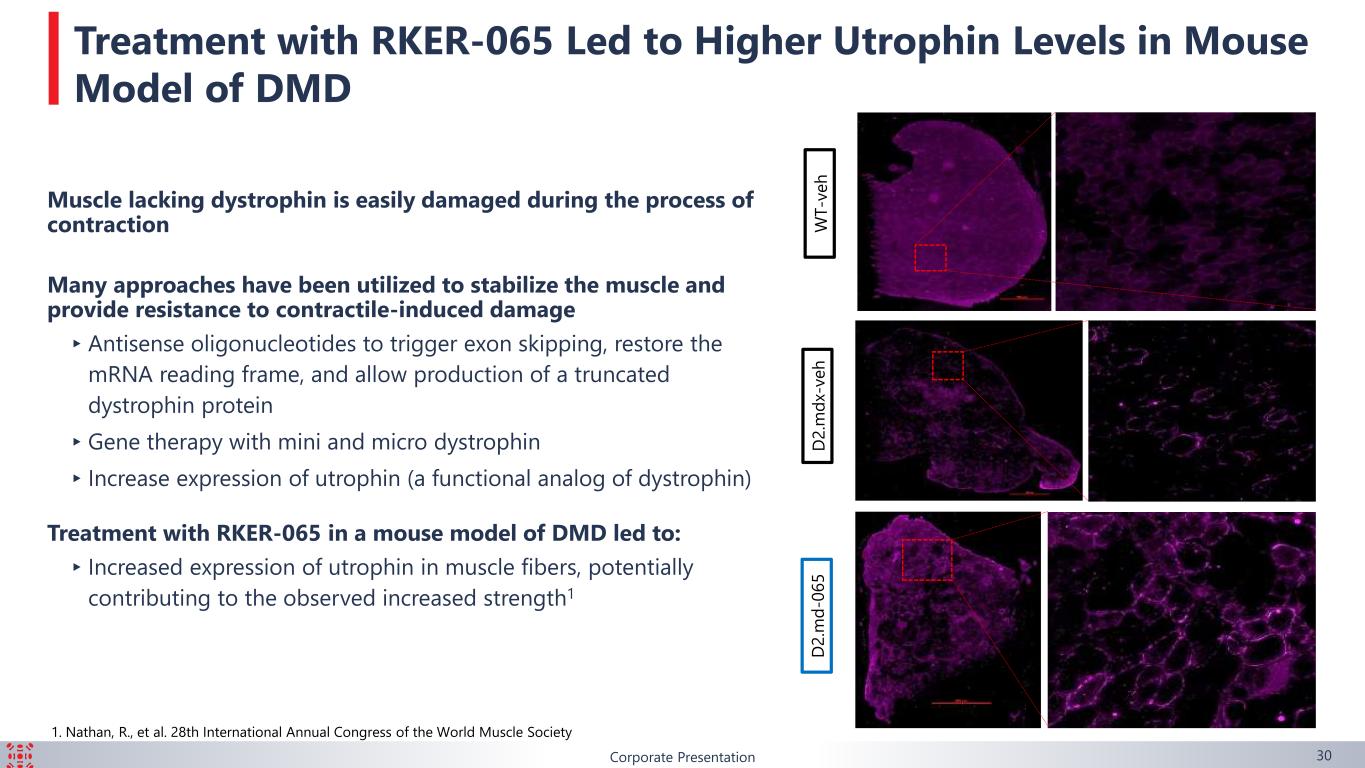
Corporate Presentation 30 W T -v e h D 2 .m d x- v e h D 2 .m d -0 6 5 Treatment with RKER-065 Led to Higher Utrophin Levels in Mouse Model of DMD Muscle lacking dystrophin is easily damaged during the process of contraction Many approaches have been utilized to stabilize the muscle and provide resistance to contractile-induced damage ▸Antisense oligonucleotides to trigger exon skipping, restore the mRNA reading frame, and allow production of a truncated dystrophin protein ▸Gene therapy with mini and micro dystrophin ▸Increase expression of utrophin (a functional analog of dystrophin) Treatment with RKER-065 in a mouse model of DMD led to: ▸Increased expression of utrophin in muscle fibers, potentially contributing to the observed increased strength1 1. Nathan, R., et al. 28th International Annual Congress of the World Muscle Society

Corporate Presentation 31 Proprietary Discovery Approach

Corporate Presentation 32 We have developed a proprietary library of ActRII ligand traps by combining sequences from ActRIIA and ActRIIB ▸ We have engineered molecules that are designed to have the therapeutic properties of either or both parent molecules ▸ Our ActRII program has produced a broader pipeline of engineered ligand traps and currently contains more than 20 unique variants in preclinical development ▸ KER-065 was nominated out of this proprietary library of ActRII ligand traps for clinical development This discovery approach has the potential to identify additional molecules with differentiated profiles from existing third-party products and product candidates ▸ Pipeline of preclinical assets: musculoskeletal; metabolic; other undisclosed indications Proprietary Discovery Approach

Corporate Presentation 33 ▸KER-050 ▸Complete enrollment in transfusion-dependent cohorts in Phase 2 MDS trial H1 2024 ▸Announce additional data from Part 2 of Phase 2 MDS trial ASH 2023 ▸Announce dose escalation data from Phase 2 MF trial ASH 2023 ▸KER-012 ▸Initiate Phase 2 open-label biomarker trial in patients with chronic heart failure H2 2023 with preserved ejection fraction and in such patients with reduced ejection fraction ▸KER-065 ▸Commence Phase 1 healthy volunteer trial Q1 2024 Anticipated Key Milestones ASH 2023 = 65th American Society of Hematology Annual Meeting and Exposition
































