Document
Keros Therapeutics Presents Clinical Trial and Preclinical Study Results from its KER-050 Program and Preclinical Data from its ALK2 Inhibitor Program at the 28th Annual Congress of the European Hematology Association
•Keros Therapeutics will be hosting a conference call and webcast today, June 9, 2023, at 8:00 a.m. Eastern time, to provide an update on its hematology franchise.
Lexington, Mass. – June 9, 2023 – Keros Therapeutics, Inc. (“Keros” or the “Company”) (Nasdaq: KROS), a clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of novel treatments for patients suffering from hematological, pulmonary and cardiovascular disorders with high unmet medical need, today announced that it presented additional data from its ongoing Phase 2 clinical trial of KER-050 in patients with very low-, low-, or intermediate-risk myelodysplastic syndromes (“MDS”), as well as preclinical data showing the potential of a research form of KER-050 (“RKER-050”) to restore erythropoiesis in an animal model of myelofibrosis (“MF”), at the 28th Annual Congress of the European Hematology Association (“EHA”), held in person and virtually June 8 through 15, 2023. In addition, Keros announced preclinical data evaluating activin receptor-like kinase-2 (“ALK2”) inhibition, as well as its combination with RKER-050, as potential treatment options for anemia of inflammation.
“We are pleased to present additional data from our ongoing Phase 2 clinical trial of KER-050 in MDS patients at EHA this year, which demonstrated durable hematological responses with longer-term treatment in a broad, lower-risk MDS patient population, including those with high transfusion burden,” said Jasbir S. Seehra, Ph.D., President and Chief Executive Officer of Keros. “Additionally, we are excited to announce that we have recently expanded this trial to include two cohorts of MDS patients with iron overload, which will enable us to further explore the potential of KER-050 to reduce iron overload and improve iron utilization in MDS patients. Separately, we believe that we will have sufficient data from this trial at the end of this year that will allow us to begin the process of engaging with regulators on the design of a Phase 3 clinical trial.”
Clinical Presentation
•KER-050 treatment improved markers of erythropoietic activity and hematopoiesis over six months which resulted in hematological responses across a broad, lower-risk MDS population
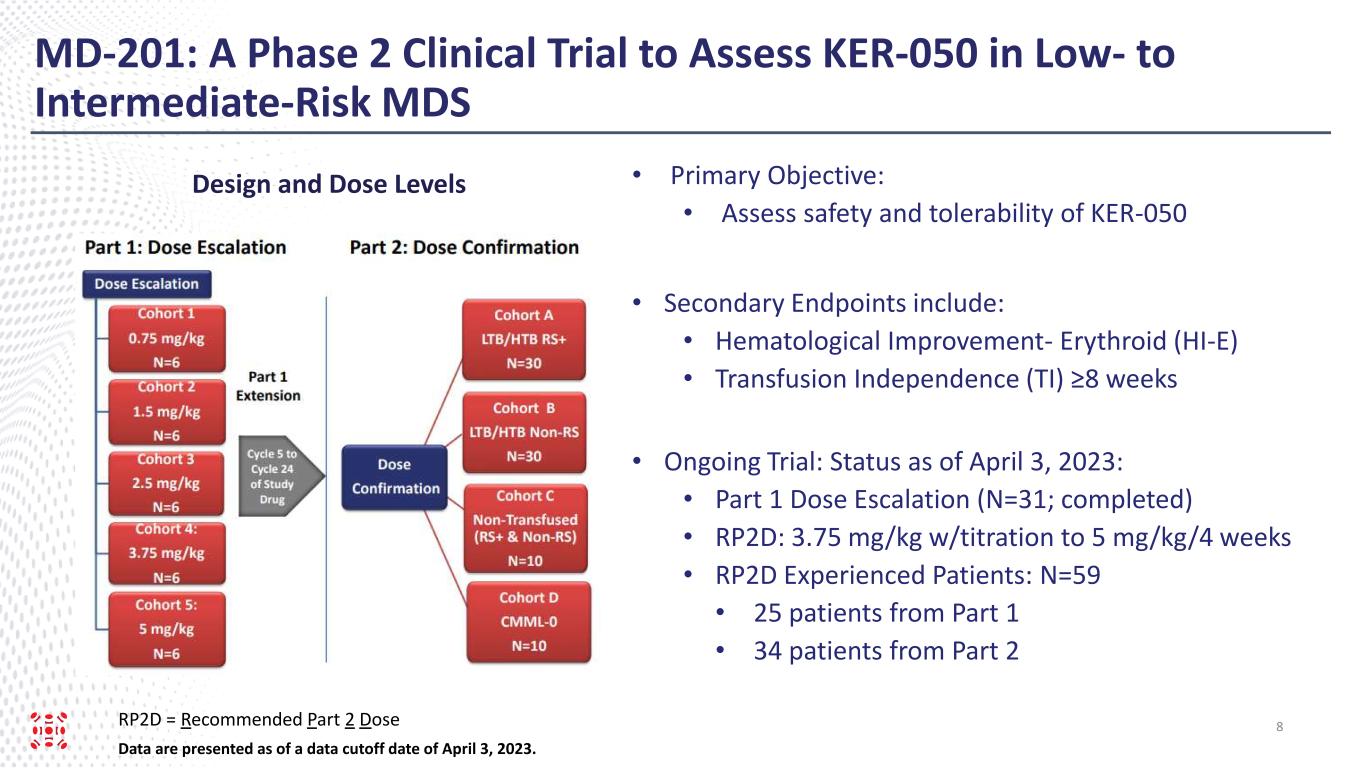
This ongoing, open-label, two-part, Phase 2 clinical trial is evaluating KER-050 in participants with very low-, low-, or intermediate-risk MDS. In Part 1, the dose escalation portion of the trial, enrollment was balanced approximately one-to-one between patients that did not have ring sideroblasts (“non-RS”) and patients that have ring sideroblasts (“RS positive”). Patients in Part 1 received KER-050 subcutaneously every 28 days for up to four cycles at the following dose levels: Cohort 1, 0.75 mg/kg; Cohort 2, 1.5 mg/kg; Cohort 3, 2.5 mg/kg; Cohort 4, 3.75 mg/kg; and Cohort 5, 5.0 mg/kg. In Part 2, the dose confirmation portion of the trial, an identical dosing schedule was followed, and patients initiated treatment at a starting dose of 3.75 mg/kg, the recommended Part 2 dose (“RP2D”), with the opportunity to dose escalate to 5.0 mg/kg or to down-titrate based on individual titration rules. Following completion of Part 1, eligible patients were given the opportunity to escalate up to the RP2D and receive long-term treatment with KER-050 for up to an additional 20 cycles (“Part 1 Extension”).
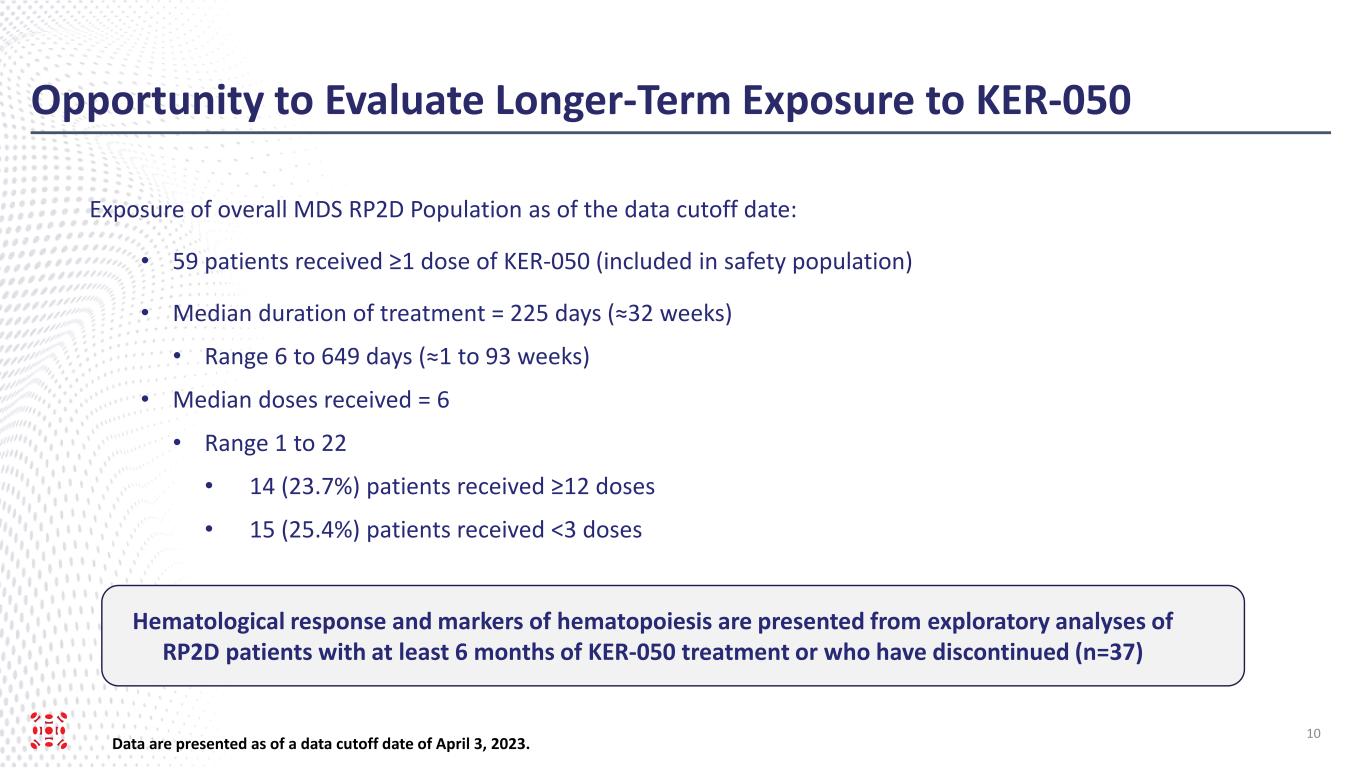
As of April 3, 2023 (the “data cut-off date”), 25 patients from Part 1, including the Part 1 Extension, and 34 patients from Part 2, had received at least one dose of KER-050 at RP2D (collectively, the “safety population”). Of these patients, 37 had completed at least 24 weeks of treatment or discontinued as of the data cut-off date (the “evaluated RP2D patients”). Data for hematological response and markers of hematopoiesis were presented from exploratory analyses of these evaluated RP2D patients.
Of the 59 patients in the safety population, 71.2% (n=42/59) were RS positive while 28.8% (n=17/59) were non-RS. The safety population included 12 non-transfused (“NT”), 16 low transfusion burden (“LTB”) and 31 high transfusion burden (“HTB”) patients.
As of the data cut-off date, KER-050 was generally well tolerated by the 59 patients in the safety population. No patients had progressed to acute myeloid leukemia. There were two cases of fatal treatment-emergent adverse events (“TEAEs”) in the trial (cardiac failure and myocardial infarction), each of which were determined to be unrelated to treatment. Four additional patients experienced TEAEs that led to discontinuation of treatment. One case was deemed treatment related (injection site reaction), and in three patients, the events were determined to be unrelated to treatment (dyspnea, chronic obstructive pulmonary disease and cardiac failure congestive (in one patient), and nodular melanoma). The most commonly reported TEAEs (in ≥15% of patients) were COVID-19, diarrhea, dyspnea, fatigue, nausea and nosebleeds (epistaxis).
As of the data cut-off date, 51.4% (n=19/37) of the evaluated RP2D patients achieved an overall erythroid response over the first 24 weeks of treatment, which is defined as meeting one of the following two endpoints:
•Modified IWG 2006 Hematological improvement-erythroid (“HI-E”), which is defined as either:
◦a ≥ 1.5 g/dL mean increase in hemoglobin over any eight-week period on treatment compared with the eight-week period prior to Cycle 1, Day 1 in LTB and NT patients; or
◦a reduction by ≥ 4 RBC units transfused during any eight-week period on treatment, compared with the eight-week period prior to Cycle 1, Day 1 in HTB patients.
•Transfusion independence (“TI”) for at least eight weeks in transfusion-dependent patients who required ≥ 2 RBC units transfused at baseline.
Additional data from the evaluated RP2D patients, as of the data cut-off date, include:
•51.4% (n=19/37) of the evaluated RP2D patients achieved HI-E over an eight-week period during the first 24 weeks of treatment.
•42.3% (n=11/26) of the transfused RP2D patients receiving ≥ 2 RBC units at baseline achieved TI for at least eight weeks over the first 24 weeks of treatment. Of these 26 patients, 19 were RS positive and seven were non-RS.
◦42.1% (n=8/19) of these RS positive patients achieved TI for at least eight weeks over the first 24 weeks of treatment.
◦42.9% (n=3/7) of these non-RS patients achieved TI for at least eight weeks over the first 24 weeks of treatment.
•Of the transfused RP2D patients, 40.9% (n=9/22) of those who are HTB achieved TI for at least eight weeks during the first 24 weeks of treatment. Of these 22 patients, 17 were RS positive and five were non-RS.
◦35.3% (n=6/17) of these RS positive HTB patients achieved TI for at least eight weeks.
◦60.0% (n=3/5) of these non-RS HTB patients achieved TI for at least eight weeks.
•Of the 19 patients who achieved HI-E or TI during the first 24 weeks of treatment (the “HI-E or TI Responders”), 10 of them (52.6%) had ongoing response as of the data cutoff date.
◦The median duration of response for the HI-E or TI Responders was 42.4 weeks.
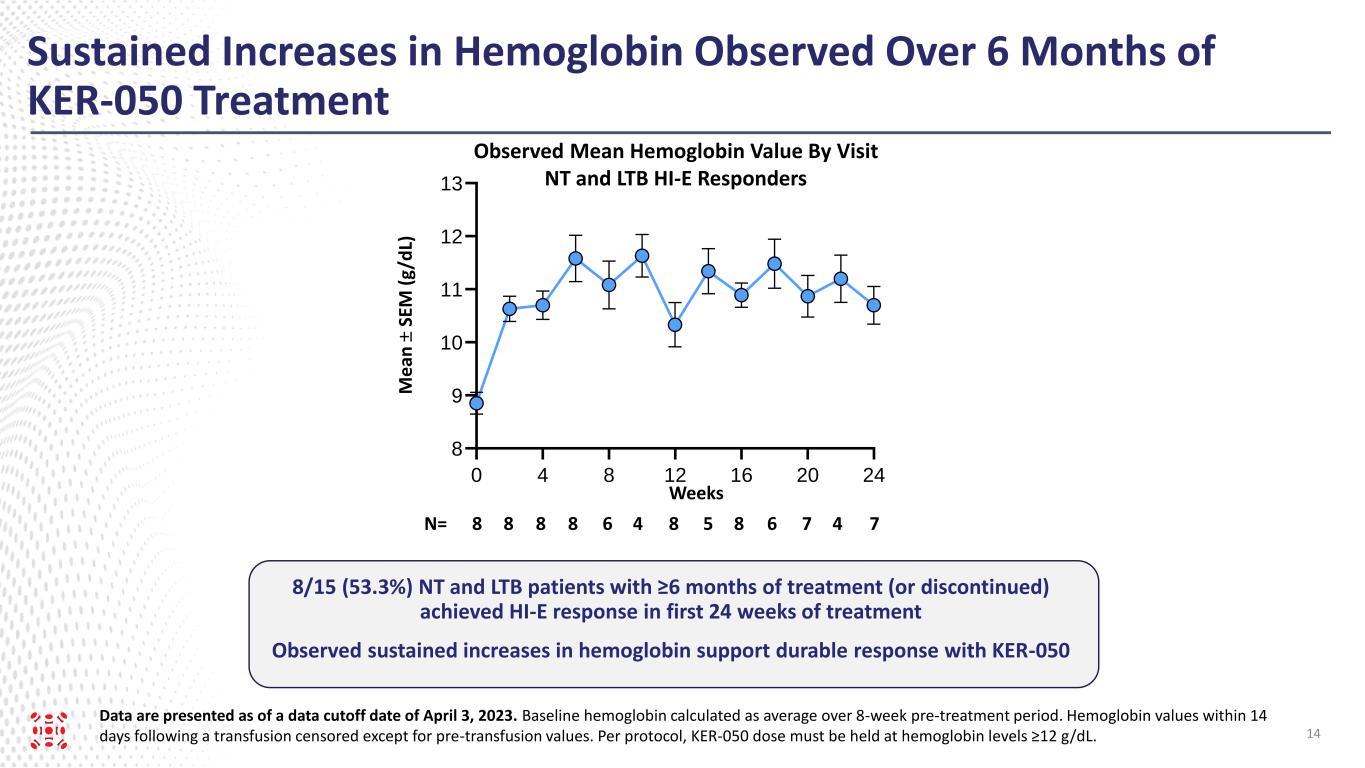
As of the data cut-off date, 44.1% (n=15/34) of the evaluated RP2D patients who had at least eight weeks of post-baseline platelet measurements exhibited sustained increases in platelet counts from baseline over at least eight weeks. Additionally, sustained increases in hemoglobin were observed over six months of treatment with KER-050 in the LTB and NT HI-E responders.
Additional data from the exploratory analysis of biomarkers of erythropoiesis and iron overload (“IO”) were also presented, with data from the evaluated RP2D patients. Key observations from this analysis, as of the data cut-off date, are as follows:
•Soluble transferrin receptor levels generally increased with KER-050 treatment in HI-E or TI Responders, while ferritin levels generally decreased.
•As of the data cut-off date, 18 patients had baseline ferritin of >500 ng/mL. Of those 18 patients, eight (44.4%) had a maximum mean decrease of ≥250 ng/mL over any 12-week period during the first six months of treatment with KER-050, suggesting potential for KER-050 to reduce IO in the most impacted patients.
Based on these additional data, this trial has been expanded to include two cohorts of MDS patients with iron overload either with or without iron chelation, which would allow Keros to further evaluate the potential of KER-050 to reduce serum ferritin, an indicator of iron overload, in that MDS patient population.
Preclinical Presentations
•A modified activin receptor type II ligand trap RKER-050 restored erythropoiesis in a mouse model of myelofibrosis
RKER-050 was tested in a mouse model of advanced MF. Male MF mice with established anemia were administered either vehicle or 10 mg/kg of RKER-050 twice weekly for 12 weeks. Healthy male mice received only vehicle.
The vehicle-treated MF mice continued to exhibit a significant or trending decrease in RBC parameters, including RBCs, hemoglobin and hematocrit, compared to healthy controls. Relative to vehicle-treated MF mice, RKER-050-treated MF mice had a significant recovery of those RBC parameters, demonstrating that RKER-050 fully reversed anemia in this MF mouse model.
In the bone marrow, vehicle-treated MF mice showed a significant reduction in erythroid progenitors compared to healthy controls. Treatment with RKER-050 significantly increased certain erythroid progenitors in MF mice compared to vehicle-treated MF mice, suggesting bone marrow erythropoiesis was increased with RKER-050 treatment. Additionally, RKER-050-treated MF mice also had increased erythroid progenitors in the spleen compared to vehicle-treated MF mice, which may be due to the severe state of the disease-impacted bone marrow microenvironment in this MF mouse model.
Additionally, RKER-050 significantly reduced megakaryocyte progenitors in the bone marrow compared to the elevated levels observed in vehicle-treated MF mice, suggesting RKER-050 may positively influence the megakaryocyte lineage. However, platelets in the RKER-050-treated MF mice were not significantly increased compared to vehicle-treated MF mice at this advanced stage of disease.
These results suggest that RKER-050 can promote erythropoiesis and reduce aberrant megakaryocyte progenitor proliferation in the bone marrow in this MF mouse model. Keros believes that KER-050 has the potential to treat patients with MF and other hematological diseases where ineffective hematopoiesis occurs.
•Combining ALK2 inhibition with a modified activin receptor IIA ligand trap provided additive benefits in resolving anemia in a mouse model of anemia of inflammation
This preclinical study evaluated whether RKER-050 combined with RKER-216, an investigational neutralizing antibody to ALK2, could ameliorate anemia in a mouse model of induced chronic kidney disease (“CKD”) representative of anemia of inflammation (“AI”).
To induce a model of CKD, mice were fed a diet containing 0.2% adenine and 40 ppm iron for five weeks. After AI was confirmed, CKD mice were treated with 3 mg/kg of RKER-216 or vehicle twice a week for four weeks. In a separate study, CKD mice received twice weekly treatment of 3 mg/kg of RKER-216 or vehicle in combination with once weekly treatment of 7.5 mg/kg of RKER-050 or vehicle for four weeks.
•RKER-216-treated CKD mice exhibited a >95% decrease in serum hepcidin, decreases in spleen iron retention and an increase in transferrin saturation compared to vehicle-treated CKD mice. RKER-216-treated CKD mice also showed improvements in hemoglobin and RBC production compared to vehicle-treated CKD mice. Taken together, these data suggest that the observed increase in iron availability resulting from the administration of RKER-216 may be sufficient for improving iron-restricted erythropoiesis in this AI model.
•While RKER-216 monotherapy improved hemoglobin and RBC levels in CKD mice relative to vehicle-treated CKD mice, combination treatment with RKER-050 resulted in a greater magnitude of increase in both hematological parameters. No significant differences in serum hepcidin, spleen iron, or transferrin saturation were observed between CKD mice receiving combination therapy or monotherapy.
By targeting ALK2 inhibition to suppress hepcidin, RKER-216 increased iron availability for erythropoiesis and partially rescued anemia in CKD mice. Separately, the combination of RKER-216 and RKER-050 maximized the hematologic recovery in this AI model, which supports the potential benefits of this combination therapy.
About the Ongoing Phase 2 Clinical Trial of KER-050 in Patients with MDS (NCT04419649)
Keros is conducting an open label, two-part, multiple ascending dose Phase 2 clinical trial to evaluate KER-050 in participants with very low-, low-, or intermediate-risk MDS who either have or have not previously received treatment with an erythroid stimulating agent.
The primary objective of this trial is to assess the safety and tolerability of KER-050 in participants with MDS that are RS positive or non-RS. The primary objective of Part 2 of this trial is confirmation of the safety and tolerability of the RP2D (3.75 mg/kg and 5.0 mg/kg). The secondary objectives of this trial are to evaluate the pharmacokinetics, pharmacodynamics and efficacy of KER-050.
Conference Call and Webcast Information
The Company will host a conference call and webcast today, June 9, 2023, at 8:00 a.m. Eastern time, to discuss updates to and additional data from its hematology franchise, including the additional results from the ongoing Phase 2 clinical trial of KER-050 presented at the 28th Annual Congress of EHA.
The conference call will be webcast live at https://event.webcasts.com/starthere.jsp?ei=1615749&tp_key=a5c7667d07. The live teleconference may be accessed by dialing (877) 405-1224 (domestic) or (201) 389-0848 (international). An archived version of the call will be available in the Investors section of the Keros website at https://ir.kerostx.com/ for 90 days following the conclusion of the call.
About KER-050
Keros’ lead protein therapeutic product candidate, KER-050, is an engineered ligand trap comprised of a modified ligand-binding domain of the transforming growth factor-beta receptor known as activin receptor type IIA that is fused to the portion of the human antibody known as the Fc domain. KER-050 is being developed for the treatment of low blood cell counts, or cytopenias, including anemia and thrombocytopenia, in patients with myelodysplastic syndromes, or MDS, and in patients with MF.
About Keros Therapeutics, Inc.
Keros is a clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of novel treatments for patients suffering from hematological, pulmonary and cardiovascular disorders with high unmet medical need. Keros is a leader in understanding the role of the transforming growth factor-beta family of proteins, which are master regulators of red blood cell and platelet production as well as of the growth, repair and maintenance of a number of tissues, including blood vessels and heart tissue. Keros’ lead protein therapeutic product candidate, KER-050, is being developed for the treatment of low blood cell counts, or cytopenias, including anemia and thrombocytopenia, in patients with MDS and in patients with MF. Keros’ lead small molecule product candidate, KER-047, is being developed for the treatment of functional iron deficiency. Keros’ third product candidate, KER-012, is being developed for the treatment of pulmonary arterial hypertension and for the treatment of cardiovascular disorders.
Cautionary Note Regarding Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as "anticipates," "believes," "expects," "intends," “plans,” “potential,” "projects,” “would” and "future" or similar expressions are intended to identify forward-looking statements. Examples of these forward-looking statements include statements concerning: Keros’ expectations regarding its growth, strategy, progress and the design, objectives and timing of its clinical trials for KER-050, including its regulatory plans; the potential of KER-050 to reduce serum ferritin in MDS patients with iron overload; the potential of KER-050 to treat patients with MF and other hematological diseases where ineffective hematopoiesis occurs; the potential of RKER-050 to promote erythropoiesis and reduce aberrant megakaryocyte proliferation in the bone marrow; and the potential benefits of combining RKER-050 with RKER-216 to treat anemia resulting from CKD. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties include, among others: Keros’ limited operating history and historical losses; Keros’ ability to raise additional funding to complete the development and any commercialization of its product candidates; Keros’ dependence on the success of its product candidates, KER-050, KER-047 and KER-012; that Keros may be delayed in initiating, enrolling or completing any clinical trials; competition from third parties that are developing products for similar uses; Keros’ ability to obtain, maintain and protect its intellectual property; and Keros’ dependence on third parties in connection with manufacturing, clinical trials and preclinical studies.
These and other risks are described more fully in Keros’ filings with the Securities and Exchange Commission (“SEC”), including the “Risk Factors” section of the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 4, 2023, and its other documents subsequently filed with or furnished to the SEC. All forward-looking statements contained in this press release speak only as of the date on which they were made. Except to the extent required by law, Keros undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Investor Contact:
Justin Frantz
jfrantz@kerostx.com
617-221-6042