UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2024
or
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___________ to __________
Commission file number: 001-15543

PALATIN TECHNOLOGIES, INC. |
(Exact name of registrant as specified in its charter) |
Delaware |
|
95-4078884 |
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
|
|
|
|
4B Cedar Brook Drive Cranbury, New Jersey |
|
08512 |
(Address of principal executive offices) |
|
(Zip Code) |
(609) 495‑2200
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class |
|
Trading Symbol |
|
Name of Each Exchange on Which Registered |
Common Stock, par value $.01 per share |
|
PTN |
|
NYSE American |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter (December 29, 2023): $55,737,667
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date (September 27, 2024): 19,548,167
PALATIN TECHNOLOGIES, INC.
Table of Contents
| 2 |
| Table of Contents |
Special Note Regarding Forward-Looking Statements
In this Annual Report on Form 10-K (this “Annual Report”) references to “we,” “our,” “us,” the “Company” or “Palatin” means Palatin Technologies, Inc. and its subsidiary.
Statements in this Annual Report, as well as oral statements that may be made by us or by our officers, directors, or employees acting on our behalf, that are not historical facts constitute “forward-looking statements,” which are made pursuant to the safe harbor provisions of Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”). The forward-looking statements in this Annual Report do not constitute guarantees of future performance. Investors are cautioned that statements that are not strictly historical facts contained in this Annual Report, including, without limitation, the following are forward looking statements:
|
· |
our significant operating losses since our inception and our need to obtain additional financing has caused management to determine there is substantial doubt regarding our ability to continue as a going concern; |
|
|
|
|
· |
our ability to obtain additional financing on terms acceptable to us, or at all, including unavailability of funds or delays in receiving funds as a result of economic disruptions; |
|
|
|
|
· |
our expectation that we will incur losses for the foreseeable future and may never achieve or maintain profitability; |
|
|
|
|
· |
our business, financial condition, and results of operations may be adversely affected by increases in costs of and delays in conducting human clinical trials and the performance of our contractors and suppliers, reduction in our productivity or the productivity of our contractors and suppliers, supply chain constraints, and labor shortages; |
|
|
|
|
· |
whether Cosette Pharmaceuticals, Inc. (“Cosette”), which acquired our Vyleesi® (the trade name for bremelanotide for treatment of hyperactive sexual desire disorder in premenopausal women) product in December 2023, will have sufficient sales to generate significant milestone payments under our purchase agreement with Cosette; |
|
|
|
|
· |
the results of clinical trials with our late-stage products, including co-administration of bremelanotide with tirzepatide, a GLP-1 agonist for treatment of obesity, which entered Phase 2 in the second quarter of calendar year 2024; a co-formulation of bremelanotide with a phosphodiesterase type 5 inhibitor (PDE5i) for treatment of erectile dysfunction in patients that do not respond to PDE5i monotherapy, scheduled to start a pharmacokinetic study in the first quarter of calendar year 2025; PL9643, an ophthalmic peptide solution for dry eye disease (“DED”), which completed Phase 3 clinical trials and announced top line results from the first Phase 3 clinical trial in the first quarter of calendar year 2024; PL8177, an oral peptide formulation for treatment of ulcerative colitis, which entered Phase 2 clinical trials in the third quarter of calendar year 2022; and a proof-of-concept melanocortin agonist clinical trial for diabetic nephropathy, which entered a Phase 2 clinical in the fourth quarter of calendar year 2022; |
|
|
|
|
· |
estimates of our expenses, future revenue and capital requirements; |
|
|
|
|
· |
our ability to achieve profitability; |
|
|
|
|
· |
our ability to advance product candidates into, and successfully complete, clinical trials; |
|
|
|
|
· |
the initiation, timing, progress and results of future preclinical studies and clinical trials, and our research and development programs; |
|
|
|
|
· |
the timing or likelihood of regulatory filings and approvals; |
|
|
|
|
· |
our expectations regarding the clinical efficacy and utility of our melanocortin agonist product candidates for treatment of inflammatory and autoimmune related diseases and disorders, including ocular indications; |
|
|
|
|
· |
our ability to compete with other products and technologies treating the same or similar indications as our product candidates; |
| 3 |
| Table of Contents |
|
· |
the ability of our contract manufacturers to perform their manufacturing activities for us in compliance with applicable regulations; |
|
|
|
|
· |
our ability to recognize the potential value of our licensing arrangements with third parties; |
|
|
|
|
· |
the potential to achieve revenues from the sale of our product candidates; |
|
|
|
|
· |
our ability to obtain adequate reimbursement from private insurers and other healthcare payers; |
|
|
|
|
· |
our ability to maintain product liability insurance at a reasonable cost or in sufficient amounts, if at all; |
|
|
|
|
· |
the performance and retention of our management team, senior staff professionals, other employees, and third-party contractors and consultants; |
|
|
|
|
· |
the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and technology in the United States and throughout the world; |
|
|
|
|
· |
our compliance with federal and state laws and regulations; |
|
|
|
|
· |
the timing and costs associated with obtaining regulatory approval for our product candidates; |
|
|
|
|
· |
the impact of fluctuations in foreign exchange rates; |
|
|
|
|
· |
the impact of any geopolitical instability, economic uncertainty, financial markets volatility, or capital markets disruption resulting from the ongoing military conflict between Russia and Ukraine, and any resulting effects on our revenue, financial condition, or results of operations; |
|
|
|
|
· |
the impact of legislative or regulatory healthcare reforms in the United States; |
|
|
|
|
· |
our ability to adapt to changes in global economic conditions as well as competing products and technologies; and |
|
|
|
|
· |
our ability to remain listed on the NYSE American stock exchange. |
Such forward-looking statements involve risks, uncertainties and other factors that could cause our actual results to be materially different from historical results or from any results expressed or implied by such forward-looking statements. Our future operating results are subject to risks and uncertainties and are dependent upon many factors, including, without limitation, the risks identified under the caption “Risk Factors” and elsewhere in this Annual Report, and any of those made in our other reports filed with the U.S. Securities and Exchange Commission (the “SEC”). Except as required by law, we do not intend, and undertake no obligation, to publicly update forward-looking statements to reflect events or circumstances after the date of this document or to reflect the occurrence of unanticipated events.
Trademarks and Trade Names
Palatin Technologies® is a registered trademarks of Palatin Technologies, Inc., and Palatin™ and the Palatin logo are trademarks of Palatin Technologies, Inc. Other trademarks referred to in this report are the property of their respective owners.
Risk Factors Summary
The following is a summary of the principal risks that could adversely affect our business, financial condition, operating results, cash flows or stock price. Discussion of the risks listed below, and other risks that we face, are discussed in the section titled “Risk Factors” in Part I, Item 1A of this Annual Report.
| 4 |
| Table of Contents |
Risks Related to Our Financial Results and Need for Financing
|
· |
Our management has determined that there is substantial doubt about our ability to continue as a going concern, which may hinder our ability to obtain future financing. |
|
|
|
|
· |
We have a history of substantial net losses, including a net loss of $29.7 million for the year ended June 30, 2024, and expect to incur substantial net losses over the next few years, and we may never achieve or maintain profitability. |
|
|
|
|
· |
We will need additional funding, including funding to complete clinical trials for our product candidates, which additional funding may not be available on acceptable terms, if at all. |
|
|
|
|
· |
We have a limited operating history upon which to base an investment decision. |
|
|
|
|
· |
Raising additional capital may cause dilution to existing stockholders, restrict our operations or require us to relinquish rights. |
Risks Related to Our Business, Strategy, and Industry
|
· |
Our product candidates, including PL9643 for dry eye disease and PL8177 for the treatment of ulcerative colitis, are still in the early stages of development and remain subject to clinical testing and regulatory approval. If we are unable to successfully develop and test our product candidates, we will not be successful. |
|
|
|
|
· |
If clinical trials for our product candidates are prolonged or delayed, we may be unable to commercialize our product candidates on a timely basis, which would require us to incur additional costs and delay our receipt of any revenue from potential product sales. |
|
|
|
|
· |
Even if our product candidates receive regulatory approval in the United States, they may never achieve market acceptance in the United States or approval outside the United States, in which case our business, financial condition and results of operations will be materially adversely affected. |
|
|
|
|
· |
If side effects emerge that can be linked to any of our product candidates (either while they are in development or after they are approved and on the market), we may be required to perform lengthy additional clinical trials, change the labeling of any such products, or withdraw such products from the market, any of which would hinder or preclude our ability to generate revenues. |
Risks Related to Government Regulation
|
· |
Both before and after marketing approval, our product candidates are subject to ongoing regulatory requirements and, if we fail to comply with these continuing requirements, we could be subject to a variety of sanctions and the sale of any approved commercial products could be suspended. |
|
|
|
Risks Related to the Ownership of Our Common Stock | ||
|
|
|
|
· |
Our stock price is volatile and may fluctuate in a way that is disproportionate to our operating performance and we expect it to remain volatile, which could limit investors’ ability to sell stock at a profit. |
|
|
|
|
· |
Because we do not anticipate paying any cash dividends on our common stock in the foreseeable future, capital appreciation, if any, will be our stockholders’ sole source of gains. |
|
|
|
|
· |
As of September 27, 2024, there were 11,385,637 shares of common stock underlying outstanding convertible preferred stock, options, restricted stock units and warrants. Stockholders may experience dilution from the conversion of preferred stock, exercise of outstanding options and warrants and vesting and delivery of restricted stock units. |
| 5 |
| Table of Contents |
PART I
Item 1. Business.
Our Business Overview
Palatin™ is a biopharmaceutical company developing first-in-class medicines based on molecules that modulate the activity of the melanocortin receptor (“MCr”) system. Our product candidates are targeted, receptor-specific therapeutics for the treatment of diseases with significant unmet medical need and commercial potential. Palatin’s strategy is to develop products and then form marketing collaborations with industry leaders to maximize product commercial potential.
The MCr system has effects on inflammation and immune system response, food intake, metabolism, and sexual function. There are five melanocortin receptors, MC1r through MC5r. Modulation of these receptors, through use of receptor-specific agonists, which activate receptor function, or receptor-specific antagonists, which block receptor function, can have significant pharmacological effects. We believe that our MC1r agonist peptides have broad anti-inflammatory effects and utilize mechanisms engaged by the endogenous melanocortin system in regulation of the immune system and resolution of inflammatory responses. We are also developing peptides that are active at more than one melanocortin receptor and small molecule MCr agonists.
Our primary focus is on the development of melanocortin receptor system treatments for obesity and for male sexual dysfunction. In the second quarter of calendar year 2024 we initiated a Phase 2 clinical study for the treatment of obesity with co-administration of the melanocortin agonist bremelanotide with tirzepatide, a GLP-1 (glucagon-like peptide-1) agonist, and plan to enroll up to 60 patients who are actively on tirzepatide with the primary endpoint of the trial to demonstrate safety and increased efficacy of co-administration of bremelanotide with tirzepatide in reducing body weight. We have initiated a clinical program for the evaluation of bremelanotide co-formulated with a phosphodiesterase type 5 inhibitor (PDE5i) for the treatment of erectile dysfunction (ED) in patients that do not respond to PDE5i monotherapy. A pharmacokinetics study is targeted for the first half of calendar year 2025, with a Phase 3 clinical trial in PDE5i non-responder ED patients expected to commence in the second half of calendar year 2025.
Our new product development activities in inflammation disease indications focus primarily on development of MCr peptides for ocular conditions, but also include conditions in the gut and kidney. Utilizing peptides which are agonists at MC1r, and in some instances agonists at additional melanocortin receptors, we are developing products to treat inflammatory and autoimmune diseases such as dry eye disease (also known as keratoconjunctivitis sicca), uveitis, diabetic retinopathy, and inflammatory bowel disease. We are actively engaged in discussions with potential partners and licensees that have the financial and operational resources to progress our products for ocular conditions through development, approval and commercialization.
Our U.S. Food and Drug Administration (“FDA”) approved melanocortin receptor agonist, Vyleesi, an “as needed” therapy used in anticipation of sexual activity and self-administered in the thigh or abdomen via a single-use subcutaneous auto-injector by premenopausal women with hypoactive sexual desire disorder (“HSDD”), was acquired by Cosette in December 2023. Vyleesi is the first FDA-approved melanocortin agent and the first and only FDA-approved as-needed treatment for premenopausal women with HSDD.
Our Business Strategy. Key elements of our business strategy include:
|
· |
Maintaining a team to create, develop and commercialize MCr products addressing unmet medical needs; |
|
|
|
|
· |
Entering into strategic alliances and partnerships with pharmaceutical companies to facilitate the development, manufacture, marketing, sale, and distribution of product candidates that we are developing; |
|
|
|
|
· |
Partially funding our product development programs with the cash flow generated from the sale of the Vyleesi product, including future milestone payments, as well as any future research, collaboration, or license agreements; and |
|
|
|
|
· |
Completing development and seeking regulatory approval of certain of our other product candidates. |
| 6 |
| Table of Contents |
Pipeline Overview
The following chart illustrates the status of our drug development programs and Vyleesi, an FDA approved product for the treatment of premenopausal women with acquired, generalized HSDD acquired by Cosette in December, 2023.
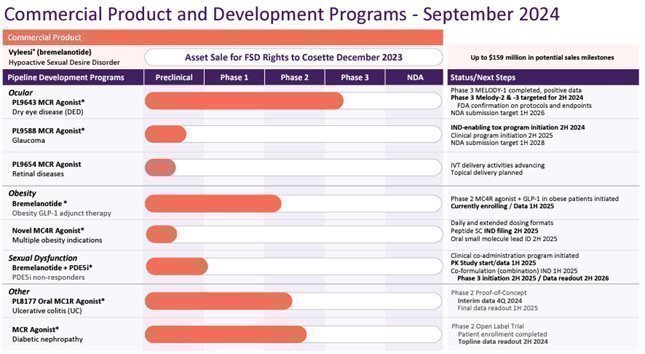
* These programs are planned but dependent on funding.
Melanocortin Receptor Programs
Our Current Product Development Strategy. We are developing products which incorporate a melanocortin agonist with another pharmaceutical agent, with an initial focus on an MC4r agonist plus a GLP-1 drug for use in obese patients, and on an MC4r agonist plus a PDE5i for use in treatment of erectile dysfunction in patients who do not respond to PDE5i monotherapy. We believe that use of two active agents may result in increased efficacy and decreased side effects.
We are designing and developing potent and highly selective MC1r agonist peptides and agonist peptides specific for more than one melanocortin receptor for treatment of a variety of inflammatory and autoimmune indications. We believe that our agonist peptides regulate certain inflammatory cytokines, and modulate the activities of immune cells, such as monocytes and T cells, to reduce immune response, and may utilize mechanisms engaged by the endogenous melanocortin system in regulation of the immune system and resolution of inflammatory responses.
Bremelanotide Co-administration with Tirzepatide (a GLP-1 agonist) to Treat Obesity. In August 2024, we initiated patient dosing for a clinical trial to investigate the safety, tolerability and effectiveness of co-administration of bremelanotide with tirzepatide in treatment of obesity. Prior clinical studies have demonstrated the role of bremelanotide in the regulation of energy storage and food intake, resulting in weight loss. Full patient enrollment in this Phase 2 trial is excepted later in calendar year 2024, with topline date readout expected in the first half of calendar year 2025. We are initiating Investigational New Drug (“IND”) enabling activities for a novel MC4r selective long-acting agonist later this year, and plan to file an IND in the second half of calendar year 2025.
Bremelanotide Co-formulated with a PDE5i for the Treatment of ED in Patients Not Adequately Responsive to PDE5i Monotherapy. We have co-formulated bremelanotide with a PDE5i drug and anticipate commencing a Phase 3 clinical study in PDE5i monotherapy non-responder ED patients in the second half of calendar year 2025. Prior clinical studies by Palatin have demonstrated the synergistic effects of combining bremelanotide with a PDE5i drug as a treatment for ED.
| 7 |
| Table of Contents |
PL9643 for Dry Eye Disease and Anti-Inflammatory Ocular Indications. PL9643, a peptide melanocortin agonist active at multiple MCrs, including MC1r and MC5r, is our lead clinical development candidate for anti-inflammatory ocular indications, including dry eye disease, which is also known as keratoconjunctivitis sicca. Dry eye disease is a syndrome with symptoms including irritation, redness, discharge and blurred vision. It may result from an autoimmune disease such as Sjögren’s syndrome, an ocular lipid or mucin deficiency, blink disorders, abnormal corneal sensitivity, or environmental factors. It is estimated to affect over 30 million people in the United States.
We have developed a PL9643 ophthalmic solution (topical eye drops) in a single use delivery device, and a Phase 3 pivotal clinical trial (“MELODY-1”) designed to support a New Drug Application (“NDA”) has been completed with positive top line results. PL9643 treatment demonstrated clinically meaningful (visual analog score reduction of >10 points from baseline) and statistically significant results for the co-primary symptom endpoint of pain (p<0.025) and multiple other symptom endpoints. PL9643 treatment for the co-primary sign endpoint and secondary sign endpoints demonstrated positive treatment effects over vehicle in the ITT population but did not achieve statistical significance. We concluded a positive Type C meeting with the FDA, confirming the acceptability of protocols and endpoints for signs and symptoms of PL9643 Phase 3 pivotal clinical trials for DED. The remaining Phase 3 clinical trial program consists of two Phase 3 studies, MELODY-2 and MELODY-3, and include sign and symptom endpoints in each study.
Oral PL8177 for Inflammatory Bowel Diseases. PL8177, a selective MC1r agonist peptide, is our lead clinical development candidate for inflammatory bowel diseases, including ulcerative colitis. We have completed subcutaneous dosing of human subjects in a Phase 1 single and multiple ascending dose clinical safety study, and a human microdose pharmacokinetic study to evaluate a polymer-encapsulated, delayed-release, oral formulation of PL8177.
For ulcerative colitis and other inflammatory bowel diseases, we administer PL8177 in our oral formulation to deliver PL8177 to the interior wall of the diseased bowel. PL8177 activates MC1r present on the interior wall of the bowel in ulcerative colitis and other inflammatory bowel diseases. We believe that PL8177 at MC1r in the bowel wall will maximize treatment effect while minimizing any systemic or off-target effects.
A Phase 2 study in ulcerative colitis using our polymer-encapsulated, delayed-release, oral formulation of PL8177 initiated patent enrollment in September 2022, and topline date is expected in the first half of calendar year 2025. The Phase 2 study is a multi-center, randomized, double-blind, placebo-controlled, adaptive design, parallel group of PL8177 study, with once daily oral dosing in adult ulcerative colitis subjects. The study uses an adaptive design with an interim assessment by an independent drug monitoring committee after the initial 16 subjects have completed the 8-week evaluation visit.
Diabetic Nephropathy Proof-of-Concept Study. A Phase 2 proof-of-concept study is ongoing for diabetic nephropathy, with topline results expected in the fourth quarter of calendar year 2024. Diabetic nephropathy, also called diabetic kidney disease, is the most common cause of end-stage renal disease in the United States and other developed countries. A melanocortin pan agonist is administered by subcutaneous injection to patients taking conventional renin-angiotensin-aldosterone system (“RAAS”) inhibitors.
Melanocortin Peptides for Diabetic Retinopathy. We conducted preclinical studies with melanocortin peptides in diabetic retinopathy models and have selected a peptide candidate for further development work. We are working on a formulation for intravitreal and subcutaneous administration. If results support advancing the program, we will conduct required safety studies and manufacture drug product under Good Manufacturing Practices (“GMP”) regulations needed to file an IND and initiating clinical studies.
Ocular Research Programs. We are conducting research in several additional ocular areas, including both front of the eye and back of the eye indications, exploring use of our compounds to treat additional indications.
Technologies We Use
We used a rational drug design approach to discover and develop proprietary peptide, peptide mimetic and small molecule agonist compounds, focusing on the melanocortin receptor system. Computer-aided drug design models of receptors are optimized based on experimental results obtained with peptides and small molecules that we develop. With our approach, we believe we are developing an advanced understanding of the factors which drive agonism.
Competition
General. Our products under development will compete on the basis of quality, performance, cost effectiveness and application suitability with numerous established products and technologies. We have many competitors, including pharmaceutical, biopharmaceutical and biotechnology companies. Furthermore, there are several well-established products in our target markets that we will have to compete against. Other companies may also introduce products using new technologies that may be competitive with our proposed products. Most of the companies selling or developing competitive products have financial, technological, manufacturing and distribution resources significantly greater than ours and may represent significant competition for us. In addition, approved products may eventually face competition from generic versions that will sell at significantly reduced prices, be preferred by managed care and health insurance payers, and be eligible for automatic pharmacy substitution even when a prescriber writes a prescription for our product. The timing and extent of future generic competition is dependent upon both our intellectual property rights and the FDA regulatory process but cannot be accurately predicted.
| 8 |
| Table of Contents |
The pharmaceutical and biotechnology industries are characterized by extensive research efforts and rapid technological change. Many biopharmaceutical companies have developed or are working to develop products similar to ours or that address the same markets. Such companies may succeed in developing technologies and products that are more effective or less costly than any of those that we may develop. Such companies may be more successful than us in developing, manufacturing, and marketing products.
We cannot guarantee that we will be able to compete successfully in the future or that developments by others will not render our proposed products under development or any future product candidates obsolete or noncompetitive or that our collaborators or customers will not choose to use competing technologies or products.
Bremelanotide Co-administration with Tirzepatide (a GLP-1 agonist) to Treat Obesity. A large number of companies are developing products and therapies to combat obesity and diabetes, including Novo Nordisk, Sanofi, Merck, Eli Lilly, Roche, Pfizer, Regeneron and Altimmune. The recent extensive use of both Food and Drug Administration-approved and compounded versions of GLP-1 receptor agonist drug products, such as Wegovy and Ozempic (semaglutide) for the treatment of obesity, has significantly increased the competition in the obesity market. There are other melanocortin agonists marketed in the United States, including Imcivree®, the melanocortin agonist setmelanotide, marketed by Rhythm Pharmaceuticals and which is indicated for chronic weight management in adult and pediatric patients 6 years of age and older with monogenic or syndromic obesity. Many of our competitors and potential competitors have substantially greater financial, technological, research and development, marketing and personnel resources than we do. We cannot, with any accuracy, forecast when or if these companies are likely to bring their products and therapies to market in competition with those that we are pursuing.
Bremelanotide Co-formulated with a PDE5i for the Treatment of Erectile Dysfunction (“ED”) in Patients Not Adequately Responsive to PDE5i Monotherapy. PDE5i drugs, including sildenafil (Viagra®), tadalafil (Cialis®), vardenafil (Levitra®), and avanafil (Stendra®), are clinically indicated for the treatment of erectile dysfunction and are widely used throughout the world. Other therapies, including various devices and drugs, are in development. There are other melanocortin agonists marketed in the United States, including Imcivree®, the melanocortin agonist setmelanotide, marketed by Rhythm Pharmaceutical for chronic weight management. While competitors and potential competitors in treatment of refractory erectile dysfunction have substantially greater financial, technological, research and development, marketing and personnel resources than we do, there are no disclosed co-formulations of a melanocortin receptor 4 agonist and a PDE5i drug.
PL9643 for Anti-Inflammatory Ocular Indications. PL9643 is under development for dry eye diseases and may also have utility for other inflammatory ocular indications. Currently mild to moderate dry eye disease and other ocular inflammatory diseases may be treated with artificial tear eye drops, lubricating tear ointments, hot compresses or punctual plugs, and more severe disease may be treated with topical immunosuppressants such as cyclosporine ophthalmic emulsions, including Restasis® marketed in the United States by Allergan, Inc., or with drugs inhibiting inflammatory cell binding, such as lifitegrast, including Xiidra® marketed in the United States by Novartis. In addition, there are a number of drugs in clinical development for treatment of dry eye disease, with several agents reported to be in or have completed Phase 2 development. Products under development include perfluorohexyloctane, cyclosporine, TRPM8 selective agonist, aldehyde derivative, partial TrkA receptor agonist, cardiolipin peroxidation inhibitor, tumor necrosis factor antagonists, alpha-2 adrenergic receptor agonist, calcineurin inhibitors, and nicotinic receptor agonists, among others. There are no reported MC1r agonist drugs in clinical trials by third parties for dry eye disease. If one or more of these competing product candidates is approved and either treats the signs and symptoms of dry eye disease or reduces the frequency of flares of dry eye in patients, it could reduce the market for PL9643 for dry eye disease.
Oral PL8177 for Inflammatory Bowel Diseases/Ulcerative Colitis. FDA-approved drugs used in treatment of ulcerative colitis include aminosalicylates such as mesalazine and related drugs, immunosuppressive drugs such as cyclosporine and azathioprine, corticosteroids such as prednisone and other steroids, and various biologic drugs, including tumor necrosis factor inhibitors such as infliximab and adalimumab. There are a number of drugs in development for ulcerative colitis, including Janus kinase inhibitors, monoclonal antibodies specific for one or more immune system cytokine signaling molecules, FXR inhibitor, integrin inhibitors, S1P1 receptor modulators, anti-TL1A monoclonal antibodies, DHODH inhibitor, HIF-1a stabilizer, LANCL2 receptor modulator, and additional classes of immunomodulatory drugs. There are no reported MC1r agonist drugs in clinical trials for inflammatory bowel diseases, including ulcerative colitis. If one or more of the competing products under development are approved and can effectively treat ulcerative colitis with an acceptable side effect profile, such products could reduce the market for oral PL8177 for inflammatory bowel diseases, including ulcerative colitis.
| 9 |
| Table of Contents |
Diabetic Retinopathy. FDA-approved drugs used in treatment of diabetic retinopathy include steroids and anti-vascular endothelial growth factor compounds. At least two different antibody fragment products are marketed in the United States in which either aflibercept or ranibizumab is the active pharmaceutical ingredient. Additional vascular endothelial growth factor inhibitors are in clinical trials or in preclinical development. There are no reported MC1r agonist drugs in clinical trials for diabetic retinopathy. If one or more of the competing product candidates under development is approved and can treat diabetic retinopathy with an acceptable side effect profile, it could reduce the market for MC1r peptide products for this indication.
Melanocortin Receptor 1 Agonist Drug Products for Inflammatory and Autoimmune Diseases. Many inflammatory disease-related indications are treated using systemic steroids or immunosuppressant drugs, all of which have side effects that can be dose limiting. There are a number of approved biological drugs and other biological drugs under development for treatment of inflammatory disease-related indications, which typically affect only one pathway in the inflammatory response. Many of these drugs address symptoms, but do not resolve the underlying inflammatory or autoimmune disease process.
Patents and Proprietary Information
Patent Protection. Our success will depend in substantial part on our ability to obtain, defend and enforce patents, maintain trade secrets and operate without infringing upon the proprietary rights of others, both in the United States and abroad. We own a number of issued United States patents and have pending United States patent applications, many with issued or pending counterpart patents in selected foreign countries. We seek patent protection for our technologies and products in the United States and those foreign countries where we believe patent protection is commercially important.
We have filed patent applications under the Patent Cooperation Treaty claiming PL9643 and other peptides in development for ocular and inflammatory disease indications and have entered national stage prosecution in the United States, European Patent Office, Eurasian Patent Office, and broadly throughout the world. If one or more patents are granted, the patents will have a presumptive term until 2041. Until one or more product candidates covered by a claim of one of these patent applications are developed for commercialization, which may never occur, we cannot evaluate the duration of any potential patent term extension under the Hatch-Waxman Amendments.
We own five issued patents in the United States, and issued patents in Australia, Belgium, Brazil, Canada, China, France, Germany, Ireland, Israel, Japan, Korea, Mexico, New Zealand, Russia, South Africa, Sweden, Switzerland and the United Kingdom claiming highly selective MC1r agonist peptides, including for treatment of inflammation-related diseases and disorders and related indications. The presumptive term of the issued patents and pending patent applications is until 2030. Until one or more product candidates covered by a claim of one of these patent applications are developed for commercialization, which may never occur, we cannot evaluate the duration of any potential patent term extension under the Hatch-Waxman Amendments.
We have additional issued United States patents on melanocortin receptor specific peptides and small molecules, including patents on an alternative class of melanocortin receptor-specific peptides for treatment of sexual dysfunction and other indications, and on natriuretic peptide receptor agonist compounds, but we are not actively developing any product candidate covered by a claim of any of these patents.
In the event that a third party has also filed a patent application relating to an invention we claimed in a patent application, we may be required to participate in an interference proceeding adjudicated by the United States Patent and Trademark Office (“USPTO”) to determine priority of invention. The possibility of an interference proceeding could result in substantial uncertainties and cost, even if the eventual outcome is favorable to us. An adverse outcome could result in the loss of patent protection for the subject of the interference, subjecting us to significant liabilities to third parties, the need to obtain licenses from third parties at undetermined cost, or requiring us to cease using the technology. Additionally, the claims of our issued patents may be narrowed or invalidated by administrative proceedings, such as interference or derivation, inter partes review, post grant review or reexamination proceedings before the USPTO.
Future Patent Infringement. We do not know for certain that our commercial activities will not infringe upon patents or patent applications of third parties, some of which may not even have been issued. Although we are not aware of any valid United States patents which are infringed by our product candidates, we cannot exclude the possibility that such patents might exist or arise in the future. We may be unable to avoid infringement of any such patents and may have to seek a license, defend an infringement action, or challenge the validity of such patents in court. Patent litigation is costly and time consuming. If such patents are valid and we do not obtain a license under any such patents, or we are found liable for infringement, we may be liable for significant monetary damages, may encounter significant delays in bringing products to market, or may be precluded from participating in the manufacture, use or sale of products or methods of treatment covered by such patents.
| 10 |
| Table of Contents |
Proprietary Information. We rely on proprietary information, such as trade secrets and know-how, which is not patented. We have taken steps to protect our unpatented trade secrets and know-how, in part with confidentiality and intellectual property agreements with our employees, consultants and certain contractors. If our employees, scientific consultants, collaborators or licensees develop inventions or processes independently that may be applicable to our product candidates, disputes may arise about the ownership of proprietary rights to those inventions and processes. Such inventions and processes will not necessarily become our property but may remain the property of those persons or their employers. Protracted and costly litigation could be necessary to enforce and determine the scope of our proprietary rights.
If trade secrets are breached, our recourse will be solely against the person who caused the secrecy breach. This might not be an adequate remedy to us because third parties other than the person who causes the breach will be free to use the information without accountability to us. This is an inherent limitation of the law of trade secret protection.
U.S. Governmental Regulation of Pharmaceutical Products
General.
Regulation by governmental authorities in the United States and other countries will continue to significantly impact our research, product development, manufacturing and marketing of any pharmaceutical products. The nature and the extent to which regulations apply to us will vary depending on the nature of any such products. Our potential pharmaceutical products will require regulatory approval by governmental agencies prior to commercialization. The products we are developing are subject to federal regulation in the United States, principally by the FDA under the Federal Food, Drug, and Cosmetic Act (“FFDCA”), and by state and local governments, as well as ministries of health and other authorities in foreign governments. Such regulations govern or influence, among other things, the research, development, testing, manufacture, safety and efficacy requirements, labeling, storage, recordkeeping, licensing, advertising, promotion, distribution and export of products, manufacturing, and the manufacturing process. In many foreign countries, such regulations also govern the prices charged for products under their respective national social security systems and availability to consumers.
All drugs intended for human use are subject to regulation by the FDA in the United States and similar regulatory bodies in other countries. The steps ordinarily required by the FDA before an innovative new drug product may be marketed in the United States are similar to steps required in most other countries and include, but are not limited to:
|
· |
completion of preclinical laboratory tests, preclinical animal testing and formulation studies; |
|
|
|
|
· |
submission to the FDA of an IND, which must be in effect before clinical trials may commence; |
|
|
|
|
· |
clinical studies to evaluate safety and efficacy; |
|
|
|
|
· |
submission to the FDA of an NDA that includes preclinical data, clinical trial data and manufacturing information; |
|
|
|
|
· |
payment of substantial user fees for filing the NDA and other recurring user fees; |
|
|
|
|
· |
FDA review of the NDA; |
|
|
|
|
· |
satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities; and |
|
|
|
|
· |
FDA approval of the NDA, including approval of all product labeling. |
For new drug products or for combination products deemed to have a “drug” primary mode of action, primary review of the product will be conducted by the appropriate division within the FDA’s Center for Drug Evaluation and Research (“CDER”). For combination products, CDER will consult with the Center for Devices and Radiological Health to ensure that the device components of the product meet all applicable device requirements.
The research, development and approval process requires substantial time, effort and financial resources, and approvals may not be granted on a timely or commercially viable basis, if at all.
| 11 |
| Table of Contents |
Preclinical testing includes laboratory evaluations to characterize the product’s composition, impurities, stability, and mechanism of its pharmacologic effect, as well as animal studies to assess the potential safety and efficacy of each product. Preclinical safety tests must be conducted by laboratories that comply with FDA regulations regarding Good Laboratory Practices and the U.S. Department of Agriculture’s Animal Welfare Act. Violations of these laws and regulations can, in some cases, lead to invalidation of the tests, requiring such tests to be repeated and delaying approval of the NDA. The results of the preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND and are reviewed by the FDA before the commencement of human clinical trials. Unless the FDA objects to an IND by placing the study on clinical hold, the IND will go into effect 30 days following its receipt by the FDA. The FDA may authorize trials only on specified terms and may suspend ongoing clinical trials at any time on various grounds, including a finding that patients are being exposed to unacceptable health risks. If the FDA places a study on clinical hold, the sponsor must resolve all of the FDA’s concerns before the study may begin or continue. The IND application process may become extremely costly and substantially delay development of products. Similar restrictive requirements also apply in other countries. Additionally, positive results of preclinical tests will not necessarily indicate positive results in clinical trials.
Clinical trials involve the administration of the investigational product to humans under the supervision of qualified principal investigators. Our clinical trials must be conducted in accordance with Good Clinical Practice regulations under protocols submitted to the FDA as part of an IND. In addition, each clinical trial is approved and conducted under the auspices of an institutional review board (“IRB”) and requires the patients’ informed consent. An IRB considers, among other things, ethical factors, the safety of human subjects, and the possibility of liability of the institutions conducting the trial. The IRB at each institution at which a clinical trial is being performed may suspend a clinical trial at any time for a variety of reasons, including a belief that the test subjects are being exposed to an unacceptable health risk. As the sponsor, we can also suspend or terminate a clinical trial at any time.
Clinical development is typically conducted in three sequential phases, Phases 1, 2, and 3, involving clinical trials with increasing numbers of human subjects. These phases may sometimes overlap or be combined. Phase 1 trials are performed in a small number of healthy human subjects or subjects with the targeted condition, and involve testing for safety, dosage tolerance, absorption, distribution, metabolism and excretion. Phase 2 studies, which may involve up to hundreds of subjects, seek to identify possible adverse effects and safety risks, preliminary information related to the efficacy of the product for specific targeted diseases, dosage tolerance, and optimal dosage. Finally, Phase 3 trials may involve up to thousands of individuals, often at geographically dispersed clinical trial sites, and are intended to provide the data demonstrating the effectiveness and safety required for approval. Prior to commencing Phase 3 clinical trials many sponsors elect to meet with FDA officials to discuss the conduct and design of the proposed trial or trials.
In addition, federal law requires the listing, on a publicly available website, of detailed information on clinical trials for investigational drugs. Some states have similar or supplemental clinical trial reporting laws.
Success in early-stage animal studies and clinical trials does not necessarily assure success in later-stage clinical trials. Data obtained from animal studies and clinical activities are not always conclusive and may be subject to alternative interpretations that could delay, limit or even prevent regulatory approval.
All data obtained from the preclinical studies and clinical trials, in addition to detailed information on the manufacture and composition of the product, would be submitted in an NDA to the FDA for review and approval for the manufacture, marketing and commercial shipments of any of our products. FDA approval of the NDA is required before commercial marketing or non-investigational interstate shipment may begin in the United States. The FDA may also conduct an audit of the clinical trial data used to support the NDA.
The FDA may deny or delay approval of an NDA that does not meet applicable regulatory criteria. For example, the FDA may determine that the preclinical or clinical data or the manufacturing information does not adequately establish the safety and efficacy of the drug. The FDA has substantial discretion in the approval process and may disagree with an applicant’s interpretation of the data submitted in its NDA. The FDA can request additional information, seek clarification regarding information already provided in the submission or ask that new additional clinical trials be conducted, all of which can delay approval. Similar types of regulatory processes will be encountered as efforts are made to market any drug internationally. We will be required to assure product performance and manufacturing processes from one country to another.
Even if the FDA approves a product, it may limit the approved uses for the product as described in the product labeling, require that contraindications, warning statements or precautions be included in the product labeling, require that additional studies be conducted following approval as a condition of the approval, impose restrictions and conditions on product distribution, prescribing or dispensing in the form of a REMS, or otherwise limit the scope of any approval or limit labeling. Once it approves an NDA, the FDA may revoke or suspend the product approval if compliance with postmarketing regulatory commitments is not maintained or if problems occur after the product reaches the marketplace. In addition, the FDA may require postmarketing studies to monitor the effect of approved products and may limit further marketing of the product based on the results of these postmarketing studies. The FDA and other government agencies have broad postmarket regulatory and enforcement powers, including the ability to levy civil and criminal penalties, suspend or delay issuance of approvals, seize or recall products and revoke approvals.
| 12 |
| Table of Contents |
Pharmaceutical manufacturers, distributors and their subcontractors are required to register their facilities with the FDA and state agencies. Manufacturers are required to list their marketed drugs with the FDA, are subject to periodic inspection by the FDA’s current GMP regulations, and the product specifications set forth in the approved NDA. The GMP requirements for pharmaceutical products are extensive and compliance with them requires considerable time, resources and ongoing investment. The regulations require manufacturers and suppliers of raw materials and components to establish validated systems and to employ and train qualified employees to ensure that products meet high standards of safety, efficacy, stability, sterility (where applicable), purity, and potency. The requirements apply to all stages of the manufacturing process, including the synthesis, processing, sterilization, packaging, labeling, storage and shipment of the drug product. For all drug products, the regulations require investigation and correction of any deviations from GMP requirements and impose documentation requirements upon us and any third-party manufacturers that we may decide to use. Manufacturing establishments are subject to mandatory user fees, and to periodic unannounced inspections by the FDA and state agencies for compliance with all GMP requirements. The FDA is authorized to inspect manufacturing facilities without a warrant at reasonable times and in a reasonable manner.
We or our present or future suppliers may not be able to comply with GMP and other FDA regulatory requirements. Failure to comply with the statutory and regulatory requirements subjects the manufacturer and/or the NDA sponsor or distributor to possible legal or regulatory action, such as a delay or refusal to approve an NDA, suspension of manufacturing, seizure or recall of a product, or civil or criminal prosecution of the company or individual officers or employees.
Postmarketing Regulation.
Any drug products manufactured or distributed by us pursuant to FDA approvals, as well as the materials and components used in our products, are subject to pervasive and continuing regulation by the FDA, including:
|
· |
recordkeeping requirements; |
|
|
|
|
· |
periodic reporting requirements; |
|
|
|
|
· |
GMP requirements related to all stages of manufacturing, testing, storage, packaging, labeling and distribution of finished dosage forms of the product; |
|
|
|
|
· |
monitoring and reporting of adverse experiences with the product; and |
|
|
|
|
· |
advertising and promotional reporting requirements and restrictions. |
Adverse experiences with the product must be reported to the FDA and could result in the imposition of market restriction through labeling changes or product removal. Product approvals may be revoked if compliance with regulatory requirements is not maintained or if problems concerning safety or effectiveness of the product occur following approval. The FDA is developing a national electronic drug safety tracking system known as SENTINEL that may impose additional safety monitoring burdens, and enhanced FDA enforcement authority, beyond the extensive requirements already in effect. As a condition of NDA approval, the FDA may require post-approval testing and surveillance to monitor a product’s safety or efficacy. The FDA also may impose other conditions, including labeling restrictions which can materially impact the potential market and profitability of a product.
With respect to post-market product advertising and promotion, the FDA and other government agencies including the Department of Health and Human Services and the Department of Justice, and individual States, impose a number of complex regulations on entities that advertise and promote pharmaceuticals, including, among others, standards and restrictions on direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the Internet. The FDA has very broad enforcement authority under the FFDCA, and failure to abide by these regulations can result in administrative and judicial enforcement actions, including the issuance of a Warning Letter directing correction of deviations from FDA standards, a requirement that future advertising and promotional materials be pre-cleared by the FDA, False Claims Act prosecution based on alleged off-label marketing seeking monetary and other penalties, including potential exclusion of the drug and/or the company from participation in government health care programs, and state and federal civil and criminal investigations and prosecutions. Foreign regulatory bodies also strictly enforce these and other regulatory requirements and drug marketing may be prohibited in whole or in part in other countries.
| 13 |
| Table of Contents |
We, our collaborators, licensees or third-party contract manufacturers may not be able to comply with the applicable regulations. After regulatory approvals are obtained, the subsequent discovery of previously unknown problems, or the failure to maintain compliance with existing or new regulatory requirements, may result in:
|
· |
restrictions on the marketing or manufacturing of a product; |
|
|
|
|
· |
Warning Letters or Untitled Letters from the FDA asking us, our collaborators or third-party contractors to take or refrain from taking certain actions; |
|
|
|
|
· |
withdrawal of the product from the market; |
|
|
|
|
· |
the FDA’s refusal to approve pending applications or supplements to approved applications; |
|
|
|
|
· |
voluntary or mandatory product recall; |
|
|
|
|
· |
fines or disgorgement of profits or revenue; |
|
|
|
|
· |
suspension or withdrawal of regulatory approvals; |
|
|
|
|
· |
refusals to permit the import or export of products; |
|
|
|
|
· |
product seizure; and |
|
|
|
|
· |
injunctions or the imposition of civil or criminal penalties. |
We may also be subject to healthcare laws, regulations and enforcement and our failure to comply with any such laws, regulations or enforcement could adversely affect our business, operations and financial condition. Certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. We are subject to regulation by both the federal government and the states in which we or our partners conduct our business. The laws and regulations that may affect our ability to operate include:
|
· |
the federal Anti-Kickback Statute, which prohibits, among other things, any person or entity from knowingly and willfully offering, soliciting, receiving or providing any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce either the referral of an individual or in return for the purchase, lease, or order of any good, facility item or service, for which payment may be made, in whole or in part, under federal healthcare programs such as the Medicare and Medicaid programs; |
|
|
|
|
· |
federal civil and criminal false claims laws and civil monetary penalty laws, including, for example, the federal civil False Claims Act, which impose criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
|
|
|
|
· |
the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payer (e.g., public or private), knowingly and willfully embezzling or stealing from a health care benefit program, willfully obstructing a criminal investigation of a health care offense and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters; |
|
|
|
|
· |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and their implementing regulations, which impose obligations on covered entities, including healthcare providers, health plans, and healthcare clearinghouses, as well as their respective business associates that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| 14 |
| Table of Contents |
|
· |
the federal physician sunshine requirements under the Patient Protection and Affordable Care Act (“Affordable Care Act”), which require manufacturers of drugs, devices, biologics and medical supplies to report annually to the Centers for Medicare & Medicaid Services information related to payments and other transfers of value provided to physicians and teaching hospitals, and ownership and investment interests held by physicians and their immediate family members; and |
|
|
|
|
· |
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payer, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government, or otherwise restrict payments that may be provided to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to healthcare providers or marketing expenditures; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent health care reform legislation has strengthened these laws. For example, the Affordable Care Act, among other things, amended the intent requirement of the federal Anti-Kickback Statute and certain criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it. In addition, the Affordable Care Act provided that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
Achieving and sustaining compliance with these laws may prove costly. In addition, any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of the laws described above or any other governmental laws or regulations that apply to us, we may be subject to penalties, including administrative, civil and criminal penalties, damages, fines, disgorgement, the exclusion from participation in federal and state healthcare programs, individual imprisonment or the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our financial results.
Generic Competition.
Orange Book Listing. In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent whose claims cover the applicant’s product. Upon approval of a drug, the applicant identifies all patents that claim the approved product’s active ingredient(s), the drug product’s approved formulation, or an approved method of use of the drug. Each of the identified patents are then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an abbreviated new drug application (“ANDA”). An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown through bioequivalence testing, unless such testing is waived by the FDA, as is the case with some injectable drug products, to be therapeutically equivalent to the listed drug. Other than bioequivalence testing, ANDA applicants are not required to conduct, or submit results of, preclinical or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way are commonly referred to as “generic equivalents” to the listed drug and can usually be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book. Specifically, the applicant must certify either that: (1) the required patent information has not been filed (a Paragraph I Certification); (2) the listed patent has expired (a Paragraph II Certification); (3) the listed patent has not expired, but will expire on a particular date and the generic approval is being sought only after patent expiration (a Paragraph III Certification); or (4) the listed patent is invalid, unenforceable, or will not be infringed by the proposed generic product (a Paragraph IV Certification). In certain circumstances, the ANDA applicant may also elect to submit a “section (viii)” statement instead of a Paragraph IV Certification, certifying that its proposed ANDA label does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent. If the application contains only Paragraph I or Paragraph II Certifications, the ANDA may be approved as soon as FDA completes its review and concludes that all approval requirements have been met. If the ANDA contains one or more Paragraph III Certifications, the ANDA cannot not be approved until each listed patent for which a Paragraph III Certification was filed have expired.
| 15 |
| Table of Contents |
If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA holder and patent owner once the ANDA has been accepted for filing by the FDA. The patent owner or NDA holder may then commence a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months (the “30-month stay”), expiration of the patent, settlement of the lawsuit in which the patent owner admits that the patent is invalid or not infringed by the ANDA product, or a decision in the infringement case that holds the patent to be invalid or not infringed, or an order by the court shortening the 30-month stay due to actions by the patent holder to delay the litigation. In most circumstances, the NDA holder is only eligible for one 30-month stay against an ANDA.
If a patent infringement action is filed against an ANDA applicant, any settlement of the litigation must be submitted to the Federal Trade Commission (“FTC”). If the FTC believes the terms or effects of the settlement are anticompetitive, the FTC may bring an antitrust enforcement action against the parties. Private parties may also bring antitrust lawsuits against drug companies based on such patent litigation settlements.
The ANDA also will not be approved until any applicable non-patent regulatory exclusivity listed in the Orange Book for the referenced product has expired.
Regulatory Exclusivity. Upon NDA approval of a new chemical entity (“NCE”), which is a drug that contains no active moiety that has been approved by the FDA in any other NDA, that drug receives five years of marketing exclusivity during which the FDA cannot receive for review any ANDA seeking approval of a generic version of that drug. An ANDA containing a Paragraph IV Certification may be received by the FDA 4 years after the NCE drug’s approval, but any 30-month stay that ensues would be extended so that it expires seven and one half years after the NCE approval date, subject to early termination by reason of a court decision or settlement as described above.
Certain changes to an NDA drug, such as the addition of a new indication to the package insert, for which new clinical trials, conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the change, can be eligible for a three-year period of exclusivity during which the FDA cannot approve an ANDA for a generic drug that includes the change. An ANDA that contains a section (viii) statement to a method of use patent may be approved with labeling that omits the patented use before the use patent expires. Generic drugs approved with such a labeling carve out may be substituted by pharmacists for the original branded drug before the method of use patent expires.
Section 505(b)(2) NDAs. Most drug products obtain FDA marketing approval pursuant to an NDA or an ANDA. A third alternative is a special type of NDA, commonly referred to as a 505(b)(2) NDA, which enables the applicant to rely, in part, on the FDA’s previous approval of a similar product, or published literature, in support of its application.
505(b)(2) NDAs often provide an alternate path to FDA approval for new or improved formulations or new uses of previously approved products. A 505(b)(2) NDA may be used where at least some of the information required for approval comes from studies not conducted by, or for, the applicant and for which the applicant has not obtained a right of reference. If the 505(b)(2) applicant can establish that reliance on the FDA’s previous approval is scientifically appropriate, it may eliminate the need to conduct certain preclinical or clinical studies of the new product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved product. The FDA may then approve the new product candidate for all, or some, of the label indications for which the referenced product has been approved, as well as for any new indication or conditions of use sought by the Section 505(b)(2) applicant.
To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would. As a result, approval of a 505(b)(2) NDA can be stalled until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the expiration of any 30-month stay, subject to early termination of the stay as described above.
Changing Legal and Regulatory Landscape.
Periodically, legislation is introduced in the U.S. Congress that could change the statutory and regulatory provisions governing the approval, manufacturing and marketing of our drugs. In addition, the FFDCA, FDA regulations and guidance are often revised or reinterpreted by the FDA or the courts in ways that may significantly affect our business and products. We cannot predict whether or when legislation or court decisions impacting our business will be enacted or issued, what FDA regulations, guidance or interpretations may change, or what the impact of such changes, if any, may be in the future.
| 16 |
| Table of Contents |
Third-Party Reimbursements
Successful sales of our proposed products in the United States and other countries depend, in large part, on the availability of adequate reimbursement from third-party payers such as governmental entities, managed care organizations, health maintenance organizations (“HMOs”), and private insurance plans. Reimbursement by a third-party payer depends on a number of factors, including the payer’s determination that the product has been approved by the FDA for the indication for which the claim is being made, that it is neither experimental nor investigational, and that the use of the product is safe and efficacious, medically necessary, appropriate for the specific patient and cost effective.
Since reimbursement by one payer does not guarantee reimbursement by another, we or our licensees may be required to seek approval from each payer individually. Seeking such approvals is a time-consuming and costly process. Third-party payers routinely limit the products that they will cover and the amount of money that they will pay and, in many instances, are exerting significant pressure on medical suppliers to lower their prices.
Payers frequently employ a tiered system in reimbursing end users for pharmaceutical products, with tier designation affecting copay or deductible amounts. Vyleesi is classified as a Tier 3 drug by insurers covering Vyleesi. Thus, reimbursement is limited for Vyleesi for treatment of premenopausal women with HSDD. Flibanserin, sold under the trade name Addyi, is similarly classified as a Tier 3 drug. Less than full reimbursement by third-party payers may adversely affect the market acceptance of Vyleesi. Further, healthcare reimbursement systems vary from country to country, and third-party reimbursement might not be made available for Vyleesi for HSDD under other reimbursement systems.
Manufacturing and Marketing
To be successful, our proposed products will need to be manufactured in commercial quantities under GMP prescribed by the FDA and at acceptable costs. We do not have the facilities to manufacture any of our proposed products under GMP. We intend to rely on collaborators, licensees, or contract manufacturers for the commercial manufacture of our proposed products.
Our MC1r and MCr agonist product candidates are synthetic peptides. We have had a contract manufacturer make both the PL8177 and PL9643 peptides in suitable scale for toxicity studies and under GMP for clinical trial use. The PL8177 drug product oral formulation for ulcerative colitis has been manufactured for clinical trial use. While the production process for making peptide active pharmaceutical ingredient involves well-established technology, there are a limited number of manufacturers capable of scaling up to commercial quantities under GMP at acceptable costs. Additionally, scaling up to commercial quantities may involve production, purification, formulation and other problems not present in the scale of manufacturing done to date. Manufacturing drug product, such as the oral formulation of PL8177, similarly may involve production, formulation and other problems not present in manufacturing at clinical trial or laboratory scale.
The failure of any manufacturer or supplier to comply with FDA regulations, including GMP or medical device quality systems regulations (“QSR”), or to supply the device component or drug substance and services as agreed, would force us or our licensees to seek alternative sources of supply and could interfere with our and our licensees’ ability to deliver product on a timely and cost-effective basis or at all. Establishing relationships with new manufacturers or suppliers, any of whom must be FDA-approved, is a time-consuming and costly process.
Product Liability and Insurance
Our business may be affected by potential product liability risks that are inherent in the testing, manufacturing, marketing and use of our proposed products. We have liability insurance providing $10 million coverage in the aggregate as to certain product liability and commercialization risks and certain clinical trial risks.
Climate Change Related Regulation
Our operations are focused on research and development of pharmaceutical products, and a significant portion of this research and development is conducted outside our facilities, including by outsourced contract research organizations or universities conduct research and studies at multiple sites. We do not anticipate any regulation regarding climate change to impact our operations.
| 17 |
| Table of Contents |
There is the potential for more frequent and severe weather events that may impact the facilities of our contractors and suppliers. We cannot provide assurance that physical risks to the facilities of our contractors, suppliers and supply chain due to climate change will not occur in the future, but do not believe these potential risks are material to our operations at this time.
Employees
As of September 27, 2024, we employed 30 people full time, of whom 21 are engaged in research and development activities and 9 are engaged in administration and management, and did not have any part-time employees. While we have been successful in attracting skilled and experienced scientific personnel, competition for personnel in our industry is intense. None of our employees are covered by a collective bargaining agreement. All of our employees have executed confidentiality and intellectual property agreements. We consider relations with our employees to be good.
We rely on contractors and scientific consultants to work on specific research and development programs. We rely on independent organizations, advisors, and consultants to provide services, including aspects of manufacturing, testing, preclinical evaluation, clinical management, regulatory strategy, and market research. Our independent advisors, contractors and consultants sign agreements that provide for confidentiality of our proprietary information and that we have the rights to any intellectual property developed while working for us.
Corporate Information
We were incorporated under the laws of the State of Delaware on November 21, 1986 and commenced operations in the biopharmaceutical area in 1996. Our corporate offices are located at 4B Cedar Brook Drive, Cranbury, New Jersey 08512 and our telephone number is (609) 495-2200. We maintain an Internet site at www.palatin.com, where among other things, we make available free of charge on and through this website our Forms 3, 4 and 5, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) and Section 16 of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Our website and the information contained in it or connected to it are not incorporated into this Annual Report. The reference to our website is an inactive textual reference only.
The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC (www.sec.gov).
Item 1A. Risk Factors.
Risks Related to Our Financial Results and Need for Financing
Our management has determined that there is substantial doubt about our ability to continue as a going concern, which may hinder our ability to obtain future financing.
Our management has determined that there is substantial doubt about our ability to continue as a going concern because of our need to raise significant additional financing to complete clinical trials and development of our product candidates. Because we have not yet generated sufficient revenues from our operations, our ability to continue as a going concern is currently heavily dependent upon our ability to obtain additional financing to sustain our operations. Such financing may take the form of the issuance of common or preferred stock or debt securities or may involve bank financing. Our independent registered public accounting firm has issued their report, which includes an explanatory paragraph for going concern uncertainty on our consolidated financial statements as of and for the year ended June 30, 2024. The existence of a “going concern” conclusion may hinder our ability to obtain additional financing in the future. Currently, we have no commitments to obtain any additional financing, and there can be no assurance that financing will be available in amounts or on terms acceptable to us, if at all.
We have a history of substantial net losses, including a net loss of $29.7 million for the year ended June 30, 2024. We expect to incur substantial net losses over the next few years, and we may never achieve or maintain profitability.
As of June 30, 2024, we had an accumulated deficit of $441.8 million. We had $29.7 million in net loss for the year ended June 30, 2024, compared to $24.0 million in net loss for the year ended June 30, 2023. We may not achieve or sustain profitability in future years, depending on numerous factors, including whether and when development and product commercialization milestones are met, whether and when we enter into license agreements for any of our products under development, regulatory actions by the FDA and other regulatory bodies, the performance of our licensees, and market acceptance of our products.
| 18 |
| Table of Contents |
We expect to incur significant expenses as we continue our development of MC1r and MCr products. These expenses, among other things, have had and will continue to have an adverse effect on our stockholders’ equity, total assets and working capital.
We sold our Vyleesi product to Cosette in December 2023 and have the potential to receive milestone payments based on sales of Vyleesi by Cosette. However, we do not anticipate receiving significant milestone payments for at least the next year from the issuance of this Annual Report and may never receive significant milestone payments. For the foreseeable future, we will have to fund our operations and capital expenditures from license, royalty and contract revenue under license agreements, existing cash balances and outside sources of financing, which may not be available on acceptable terms, if at all. We will not have product revenue from our products in development unless and until we receive approval from the FDA or other equivalent regulatory authorities outside the United States. We have devoted substantially all of our efforts to research and development, including preclinical and clinical trials. Because of the numerous risks associated with developing drugs, we are unable to predict the extent of future losses, whether or when any of our product candidates will become commercially available, or when we will become profitable, if at all.
We will need additional funding, including funding to complete clinical trials for our product candidates other than Vyleesi, which may not be available on acceptable terms, if at all.
We intend to focus future efforts on our bremelanotide combination products MC1r product candidates, primarily for ocular indications. As of June 30, 2024, we had cash, cash equivalents and marketable securities of $9.5 million, with current liabilities of $9.7 million. Based on our available cash, cash equivalents and marketable securities, we have concluded that substantial doubt exists about our ability to continue as a going concern for one year from the date our consolidated financial statements are issued and we are seeking additional funding to complete development activities and required clinical trials for our MC1r product candidates and, if those clinical trials are successful (which we cannot predict), to complete submission of required regulatory applications to the FDA.
We may raise additional funds through public or private equity or debt financings, collaborative arrangements on our product candidates, or other sources. However, such financing arrangements may not be available on acceptable terms, or at all. To obtain additional funding, we may need to enter into arrangements that require us to develop only certain of our product candidates or relinquish rights to certain technologies, product candidates and/or potential markets.
If we are unable to raise sufficient additional funds when needed, we may be required to curtail operations significantly, cease clinical trials and decrease staffing levels. We may seek to license, sell or otherwise dispose of our product candidates, technologies and contractual rights on the best possible terms available. Even if we are able to license, sell or otherwise dispose of our product candidates, technologies and contractual rights, it is likely to be on unfavorable terms and for less value than if we had the financial resources to develop or otherwise advance our product candidates, technologies and contractual rights ourselves.
Our future capital requirements depend on many factors, including:
|
· |
the expense and timing of obtaining regulatory approvals for our other product candidates; |
|
|
|
|
· |
the number and characteristics of any additional product candidates we develop or acquire; |
|
|
|
|
· |
the scope, progress, results and costs of researching and developing our future product candidates, and conducting preclinical and clinical trials; |
|
|
|
|
· |
the cost of commercialization activities if any future product candidates are approved for sale, including marketing, sales and distribution costs; |
|
|
|
|
· |
the cost of manufacturing any future product candidates and any products we successfully commercialize; |
|
|
|
|
· |
our ability to establish and maintain strategic collaborations, licensing or other arrangements and the terms and timing of such arrangements; |
|
|
|
|
· |
the degree and rate of market acceptance of any future approved products; |
|
|
|
|
· |
the emergence, approval, availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing products or treatments; |
|
|
|
|
· |
any product liability or other lawsuits related to our products; |
|
|
|
|
· |
the expenses needed to attract and retain skilled personnel; |
|
|
|
|
· |
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; and |
|
|
|
|
· |
the timing, receipt and amount of sales of, or royalties on, future approved products, if any. |
| 19 |
| Table of Contents |
We have a limited operating history upon which to base an investment decision.
Our operations are primarily focused on acquiring, developing and securing our proprietary technology, conducting preclinical and clinical studies and formulating and manufacturing, through contract manufacturers, our principal product candidates on a small-scale basis. These operations provide a limited basis for stockholders to assess our ability to commercialize our product candidates.
While we completed Phase 3 clinical trials on Vyleesi for HSDD in premenopausal women, together with AMAG filed an NDA on Vyleesi for HSDD with the FDA, and received approval on Vyleesi from the FDA, we have not yet demonstrated our ability to perform the functions necessary for the successful commercialization of any of our current product candidates. The successful commercialization of our product candidates will require us to perform a variety of functions, including:
|
· |
continuing to conduct preclinical development and clinical trials; |
|
|
|
|
· |
participating in regulatory approval processes; |
|
|
|
|
· |
formulating and manufacturing products, or having third parties formulate and manufacture products; |
|
|
|
|
· |
post-approval monitoring and surveillance of our products; |
|
|
|
|
· |
conducting sales and marketing activities, either alone or with a partner; and |
|
|
|
|
· |
obtaining additional capital. |
If we are unable to obtain regulatory approval of any of our product candidates, to successfully commercialize any products for which we receive regulatory approval or to obtain additional capital, we may not be able to recover our investment in our development efforts.
The clinical and commercial success of our product candidates will depend on a number of factors, including the following:
|
· |
the ability to raise additional capital on acceptable terms, or at all; |
|
|
|
|
· |
timely completion of our clinical trials, which may be significantly slower or cost more than we currently anticipate and will depend substantially upon the performance of third-party contractors; |
|
|
|
|
· |
whether we are required by the FDA or similar foreign regulatory agencies to conduct additional clinical trials beyond those planned to support the approval and commercialization of our product candidates or any future product candidates; |
|
|
|
|
· |
acceptance of our proposed indications and primary endpoint assessments relating to the proposed indications of our product candidates by the FDA and similar foreign regulatory authorities; |
|
|
|
|
· |
our ability to demonstrate to the satisfaction of the FDA and similar foreign regulatory authorities, the safety and efficacy of our product candidates or any future product candidates; |
|
|
|
|
· |
the prevalence, duration and severity of potential side effects experienced with our product candidates or future approved products, if any; |
|
|
|
|
· |
the timely receipt of necessary marketing approvals from the FDA and similar foreign regulatory authorities; |
|
|
|
|
· |
achieving and maintaining, and, where applicable, ensuring that our third-party contractors achieve and maintain, compliance with our contractual obligations and with all regulatory requirements applicable to our product candidates or any future product candidates or approved products, if any; |
|
|
|
|
· |
the ability of third parties with whom we contract to manufacture clinical trial and commercial supplies of our product candidates or any future product candidates, remain in good standing with regulatory agencies and develop, validate and maintain commercially viable manufacturing processes that are compliant with the FDA’s current GMP regulations; |
| 20 |
| Table of Contents |
|
· |
a continued acceptable safety profile and efficacy during clinical development and following approval of our product candidates or any future product candidates; |
|
|
|
|
· |
our ability to successfully commercialize our product candidates or any future product candidates in the United States and internationally, if approved for marketing, sale and distribution in such countries and territories, whether alone or in collaboration with others; |
|
|
|
|
· |
acceptance by physicians and patients of the benefits, safety and efficacy of our product candidates or any future product candidates, if approved, including relative to alternative and competing treatments; |
|
|
|
|
· |
our and our partners’ ability to establish and enforce intellectual property rights in and to our product candidates or any future product candidates; |
|
|
|
|
· |
our and our partners’ ability to avoid third-party patent interference or intellectual property infringement claims; and |
|
|
|
|
· |
our ability to develop, in-license or acquire additional product candidates or commercial-stage products that we believe can be successfully developed and commercialized. |
If we do not achieve one or more of these factors, many of which are beyond our control, in a timely manner or at all, we could experience significant delays or an inability to obtain regulatory approvals or commercialize our product candidates. Even if regulatory approvals are obtained, we may never be able to successfully commercialize any of our product candidates. Accordingly, we cannot assure our investors that we will be able to generate sufficient revenue through the sale of our product candidates or any future product candidates to continue our business.
Raising additional capital may cause dilution to existing stockholders, restrict our operations, or require us to relinquish rights.
We will seek the additional capital necessary to fund our operations through public or private equity offerings, collaboration agreements, debt financings, licensing arrangements or combinations of the foregoing. To the extent that we raise additional capital through the sale of equity or convertible debt securities, existing stockholders’ ownership interests will be diluted, and the terms may include liquidation or other preferences that adversely affect their rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures, or declaring dividends. If we raise additional funds through collaborations and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates or grant licenses on terms that are not favorable to us.
Risks Related to Our Business, Strategy, and Industry
The commercial success of Vyleesi for HSDD is a component of our corporate strategy, but we may not receive significant milestone payments under our purchase agreement with Cosette.
In December 2023 we sold Vyleesi to Cosette under a purchase agreement providing for contingent, sales-base milestone payments of up to $159 million. We do not know whether or to what extent Cosette will meet milestone payment benchmarks. Our near-term prospects, including our ability to finance our company and generate revenue, will be impacted by the successful commercialization of Vyleesi for HSDD by Cosette, as well as preclinical and clinical results with our future product candidates. The clinical and commercial success of Vyleesi by Cosette and our product candidates will depend on a number of factors, including the following:
|
· |
timely completion of, or need to conduct additional clinical trials and studies, for our product candidates, which may be significantly slower or cost more than we currently anticipate and will depend substantially upon the accurate and satisfactory performance of third-party contractors; |
|
|
|
|
· |
the ability to demonstrate to the satisfaction of the FDA the safety and efficacy of future product candidates through clinical trials; |
|
|
|
|
· |
whether we or our licensees are required by the FDA or other similar foreign regulatory agencies to conduct additional clinical trials to support the approval of Vyleesi and future product candidates; |
|
|
|
|
· |
Cosette’s ability to successfully manufacture Vyleesi; |
|
|
|
|
· |
Cosette’s success in educating physicians and patients about the benefits, administration and use of Vyleesi for HSDD; |
| 21 |
| Table of Contents |
|
· |
the prevalence and severity of adverse events experienced with Vyleesi for HSDD or any future product candidates or approved products; |
|
|
|
|
· |
the adequacy and regulatory compliance of the autoinjector device, supplied by an unaffiliated third party, used as part of the Vyleesi combination product; |
|
|
|
|
· |
the timely receipt of necessary marketing approvals from the FDA and similar foreign regulatory authorities; |
|
|
|
|
· |
our ability to raise additional capital on acceptable terms to achieve our goals; |
|
|
|
|
· |
achieving and maintaining compliance with all regulatory requirements applicable to Vyleesi for HSDD or any future product candidates or approved products; |
|
|
|
|
· |
the availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing treatments; |
|
|
|
|
· |
the effectiveness of our own or our future potential strategic collaborators’ marketing, sales and distribution strategy and operations; |
|
|
|
|
· |
the ability to manufacture clinical trial supplies of any future product candidates and to develop, validate and maintain a commercially viable manufacturing process that is compliant with current GMP; |
|
|
|
|
· |
Cosette’s ability to successfully commercialize Vyleesi for HSDD in the United States; |
|
|
|
|
· |
our ability to successfully commercialize any future product candidates, if approved for marketing and sale, whether alone or in collaboration with others; |
|
|
|
|
· |
our ability to enforce our intellectual property rights for any future product candidates; |
|
|
|
|
· |
our ability to avoid third-party patent interference or intellectual property infringement claims; |
|
|
|
|
· |
acceptance of Vyleesi for HSDD or any future product candidates, if approved, as safe and effective by patients and the medical community; and |
|
|
|
|
· |
a continued acceptable safety profile and efficacy of Vyleesi for HSDD or any future product candidates following approval. |
If we fail to satisfy any one of these prerequisites to our commercial success, many of which are beyond our control, in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our product candidates. Accordingly, we cannot assure investors that we will be able to generate sufficient revenue through the sale of any future product candidate, to continue our business. In addition to preventing us from executing our current business plan, any delays in our clinical trials, or inability to successfully commercialize our products could impair our reputation in the industry and the investment community and could hinder our ability to fulfill our existing contractual commitments. As a result, our share price would likely decline significantly, and we would have difficulty raising necessary capital for future projects.
Ongoing military conflict could cause geopolitical instability, economic uncertainty, financial markets volatility and capital markets disruption, which may adversely affect our revenue, financial condition, or results of operations.
Military conflict, such as the ongoing conflicts between Russia and Ukraine and between Israel and Hamas, may disrupt or otherwise adversely impact our operations and those of third parties upon which we rely. Related sanctions, export controls or other actions that have already been initiated or may in the future be initiated by nations including the U.S., the European Union or Russia (e.g., potential cyberattacks, disruption of energy flows, etc.) can adversely affect our business, our contract research organizations, and other third parties with which we conduct business. Resulting volatility, disruption, or deterioration in the credit and financial markets may further make any necessary debt or equity financing more difficult and more costly. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our business strategy, financial performance, and stock price and could require us to delay or abandon clinical development plans. In addition, there is a risk that one or more of our current service providers, manufacturers, or other partners may be adversely impacted by deteriorating economic conditions, which could directly affect our ability to attain our operating goals and to accurately forecast and plan our future business activities.
| 22 |
| Table of Contents |
Our product candidates including our combination products for treatment of obesity and ED, as well as PL9643 for dry eye disease and PL8177 for the treatment of ulcerative colitis, are still in the early stages of development and remain subject to clinical testing and regulatory approval. If we are unable to successfully develop and test our product candidates, we will not be successful.
Our product candidates, including the combination products bremelanotide and a PDE5i agent and bremelanotide and tirzepatide or another GLP-1 agonist, PL9643 for dry eye disease, and PL8177 for the treatment of ulcerative colitis, are at various stages of research and development, will require regulatory approval, and may never be successfully developed or commercialized. Our product candidates will require significant further research, development and testing before we can seek regulatory approval to market and sell them. We must demonstrate that our product candidates are safe and effective for use in patients in order to receive regulatory approval for commercial sale. Preclinical studies in animals, using various doses and formulations, must be performed before we can begin human clinical trials. Even if we obtain favorable results in the preclinical studies, the results in humans may be different. Numerous small-scale human clinical trials may be necessary to obtain initial data on a product candidate’s safety and efficacy in humans before advancing to large scale human clinical trials. We face the risk that the results of our trials in later phases of clinical trials may be inconsistent with those obtained in earlier phases. Adverse or inconclusive results could delay the progress of our development programs and may prevent us from filing for regulatory approval of our product candidates. Additional factors that could inhibit the successful development of our product candidates include:
|
· |
lack of effectiveness of any product candidate during clinical trials or the failure of our product candidates to meet specified endpoints; |
|
· |
failure to design appropriate clinical trial protocols; |
|
· |
uncertainty regarding proper dosing; |
|
· |
for injectable products, inability to develop or obtain a supplier for a suitable autoinjector device that meets the FDA’s medical device requirements; |
|
· |
insufficient data to support regulatory approval; |
|
· |
inability or unwillingness of medical investigators to follow our clinical protocols; |
|
· |
inability to add a sufficient number of clinical trial sites; or |
|
· |
the availability of sufficient capital to sustain operations and clinical trials. |
You should evaluate us in light of these uncertainties, difficulties and expenses commonly experienced by early stage biopharmaceutical companies, as well as unanticipated problems and additional costs relating to:
|
· |
product approval or clearance; |
|
· |
regulatory compliance; |
|
· |
good manufacturing practices; |
|
· |
intellectual property rights; |
|
· |
product introduction; and |
|
· |
marketing and competition. |
If clinical trials for our product candidates are prolonged or delayed, we may be unable to commercialize our product candidates on a timely basis, which would require us to incur additional costs and delay our receipt of any revenue from potential product sales.
We may be unable to commercialize our product candidates on a timely basis due to unexpected delays in our human clinical trials. Potential delaying events include:
|
· |
discovery of serious or unexpected toxicities or side effects experienced by study participants or other safety issues; |
|
|
|
|
· |
slower than expected rates of subject recruitment and enrollment rates in clinical trials resulting from numerous factors, including the prevalence of other companies’ clinical trials for their product candidates for the same indication, or clinical trials for indications for which patients do not as commonly seek treatment; |
|
|
|
|
· |
difficulty in retaining subjects who have initiated a clinical trial but may withdraw at any time due to adverse side effects from the therapy, insufficient efficacy, fatigue with the clinical trial process or for any other reason; |
|
|
|
|
· |
difficulty in obtaining IRB approval for studies to be conducted at each site; |
|
|
|
|
· |
delays in manufacturing or obtaining, or inability to manufacture or obtain, sufficient quantities of materials for use in clinical trials; |
| 23 |
| Table of Contents |
|
· |
inadequacy of or changes in our manufacturing process or the product formulation or method of delivery; |
|
|
|
|
· |
changes in applicable laws, regulations and regulatory policies; |
|
|
|
|
· |
delays or failure in reaching agreement on acceptable terms in clinical trial contracts or protocols with prospective contract research organizations (“CROs”), clinical trial sites and other third-party contractors; |
|
|
|
|
· |
failure of our CROs or other third-party contractors to comply with contractual and regulatory requirements or to perform their services in a timely or acceptable manner; |
|
|
|
|
· |
failure by us, our employees, our CROs or their employees or any partner with which we may collaborate or their employees to comply with applicable FDA or other regulatory requirements relating to the conduct of clinical trials or the handling, storage, security and recordkeeping for drug, medical device and biologic products; |
|
|
|
|
· |
delays in the scheduling and performance by the FDA of required inspections of us, our CROs, our suppliers, or our clinical trial sites, and violations of law or regulations discovered in the course of FDA inspections; |
|
|
|
|
· |
scheduling conflicts with participating clinicians and clinical institutions; or |
|
|
|
|
· |
difficulty in maintaining contact with subjects during or after treatment, which may result in incomplete data. |
Any of these events or other delaying events, individually or in the aggregate, could delay the commercialization of our product candidates and have a material adverse effect on our business, results of operations and financial condition.
We may not be able to secure and maintain relationships with research institutions and other organizations to conduct our clinical trials.
We rely on research institutions and other organizations to conduct our clinical trials, and we therefore have limited control over the timing and cost of clinical trials and our ability to recruit subjects. If we are unable to reach agreements with suitable research institutions or organizations on acceptable terms, or if any such agreement is terminated, we may be unable to quickly replace the research institution or organization with another qualified institution or organization on acceptable terms. We may not be able to secure and maintain suitable research institutions or organizations to conduct our clinical trials.
Even if our product candidates receive regulatory approval, they may never achieve market acceptance, in which case our business, financial condition and results of operation will be materially adversely affected.
Regulatory approval for the marketing and sale of any of our product candidates does not assure the product’s commercial success. Any approved product will compete with other products manufactured and marketed by major pharmaceutical and other biotechnology companies. If any of our product candidates are approved by the FDA and do not achieve adequate market acceptance, our business, financial condition, and results of operations will be materially adversely affected. The degree of market acceptance of any such product will depend on a number of factors, including:
|
· |
perceptions by members of the healthcare community, including physicians, about the safety and effectiveness of any such product; |
|
|
|
|
· |
cost-effectiveness relative to competing products and technologies; |
|
|
|
|
· |
availability of reimbursement for our products from third-party payers such as health insurers, HMOs and government programs such as Medicare and Medicaid; and |
|
|
|
|
· |
advantages over alternative treatment methods. |
Even if our product candidates receive regulatory approval in the United States, we may never receive approval or commercialize our products outside of the United States.
In order to market any products outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks detailed above regarding FDA approval in the United States as well as other risks. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. Failure to obtain regulatory approval in other countries or any delay or setbacks in obtaining such approval would impair our ability to develop foreign markets for our product candidates and may have a material adverse effect on our results of operations and financial condition.
| 24 |
| Table of Contents |
If side effects emerge that can be linked to any of our product candidates (either while they are in development or after they are approved and on the market), we may be required to perform lengthy additional clinical trials, change the labeling of any such products, or withdraw such products from the market, any of which would hinder or preclude our ability to generate revenues.
If we identify side effects or other problems occur in future clinical trials, we may be required to terminate or delay clinical development of the product candidate. Furthermore, even if any of our product candidates receive marketing approval, as greater numbers of patients use a drug following its approval, if the incidence of side effects increases or if other problems are observed after approval that were not seen or anticipated during pre-approval clinical trials, or if the incidence of side effects increase or other problems are observed with Vyleesi, a number of potentially significant negative consequences could result, including:
|
· |
regulatory authorities may withdraw their approval of the product; |
|
· |
we may be required to reformulate such products or change the way the product is manufactured; |
|
· |
we may become the target of lawsuits, including class action suits; and |
|
· |
our reputation in the marketplace may suffer resulting in a significant drop in the sales of such products. |
Any of these events could substantially increase the costs and expenses of developing, commercializing, and marketing any such product candidates or could harm or prevent sales of any approved products.
We may not be able to keep up with the rapid technological change in the biotechnology and pharmaceutical industries, which could make any future approved products obsolete and reduce our revenue.
Biotechnology and related pharmaceutical technologies have undergone and continue to be subject to rapid and significant change. Our future will depend in large part on our ability to maintain a competitive position with respect to these technologies. Our competitors may render our technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages in our drug discovery process that we believe we derive from our research approach and proprietary technologies. In addition, any future products that we develop, including our clinical product candidates, may become obsolete before we recover expenses incurred in developing those products, which may require that we raise additional funds to continue our operations.
Competing products and technologies may make our proposed products noncompetitive.
There are a number of products approved for use in treating inflammatory diseases and indications, and other products are being developed, including products in clinical trials. The dry eye disease and ocular inflammatory disease markets are highly competitive, with a number of marketed products and products reported to be in late-stage clinical trials. Similarly, the inflammatory bowel disease and ulcerative colitis markets are highly competitive, with a number of marketed products and products reported to be in late-stage clinical trials.
In general, the biopharmaceutical industry is highly competitive. We are likely to encounter significant competition with respect to MC1r product candidates and MCr product candidates. Most of our competitors have substantially greater financial and technological resources than we do. Many of them also have significantly greater experience in research and development, marketing, distribution, and sales than we do. Accordingly, our competitors may succeed in developing, marketing, distributing, and selling products and underlying technologies more rapidly than we can. These competitive products or technologies may be more effective and useful or less costly than Vyleesi or our MC1r product candidates and MCr product candidates. In addition, academic institutions, hospitals, governmental agencies, and other public and private research organizations are also conducting research and may develop competing products or technologies on their own or through strategic alliances or collaborative arrangements.
| 25 |
| Table of Contents |
We rely on third parties over whom we have no control to conduct preclinical studies, clinical trials and other research for our product candidates and their failure to timely perform their obligations could significantly harm our product development.
We have limited research and development staff. We rely on third parties and independent contractors, such as researchers at CROs and universities, in certain areas that are particularly relevant to our research and product development plans. We engage such researchers to conduct our preclinical studies, clinical trials and associated tests. These outside contractors are not our employees and may terminate their engagements with us at any time. In addition, we have limited control over the resources that these contractors devote to our programs, and they may not assign as great a priority to our programs or pursue them as diligently as we would if we were undertaking such programs ourselves. There is also competition for these relationships, and we may not be able to maintain our relationships with our contractors on acceptable terms. If our third-party contractors do not carry out their duties under their agreements with us, fail to meet expected deadlines or fail to comply with appropriate standards for preclinical or clinical research, our ability to develop our product candidates and obtain regulatory approval on a timely basis, if at all, may be materially adversely affected.
Production and supply of our product candidates depend on contract manufacturers over whom we have no control, with the risk that we may not have adequate supplies of our product candidates or products.
We do not have the facilities to manufacture our early-stage potential products such as bremelanotide in combination with a PDE5i, bremelanotide in combination with tirzepatide, PL8177, PL9643, PL9654 and other melanocortin receptor agonist compounds for use in preclinical studies and clinical trials. Contract manufacturers must perform these manufacturing activities in a manner that complies with FDA regulations. Our ability to control third-party compliance with FDA requirements is limited to contractual remedies and rights of inspection. The manufacturers of our potential products and their manufacturing facilities will be subject to continual review and periodic inspections by the FDA and other authorities where applicable, and must comply with ongoing regulatory requirements, including FDA regulations concerning GMP. Failure of third-party manufacturers to comply with GMP, medical device QSR, or other FDA requirements may result in enforcement action by the FDA. Failure to conduct their activities in compliance with FDA regulations could delay our development programs or negatively impact our ability to receive FDA approval of our potential products. Establishing relationships with new suppliers, who must be FDA-approved, is a time-consuming and costly process.
If we are unable to establish sales and marketing capabilities within our organization or enter into and maintain agreements with third parties to market and sell our product candidates, we may be unable to generate product revenue.
If any of our products candidates are approved by the FDA or other regulatory authorities, we must enter into agreements with third parties to market these product candidates or develop marketing, distribution and selling capacity and expertise, which will be costly and time consuming, or enter into agreements with other companies to provide these capabilities. We may not be able to enter into suitable agreements on acceptable terms, if at all. Engaging a third party to perform these services could delay the commercialization of any of our product candidates, if approved for commercial sale. If we are unable to establish adequate sales, marketing, and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and our business would suffer. In addition, if we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenues are likely to be lower than if we could market and sell any products that we develop ourselves.
We may need to hire additional employees in order to commercialize our product candidates in the future. Any inability to manage future growth could harm our ability to commercialize our product candidates, increase our costs and adversely impact our ability to compete effectively.
To commercialize our product candidates, we will need to hire or contract with experienced sales and marketing personnel to sell and market those product candidates that we decide to commercialize, and we will need to expand the number of our managerial, operational, financial and other employees to support commercialization. Competition exists for qualified personnel in the biopharmaceutical field.
Future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively.
Our ability to achieve revenues from the sale of our products will depend, in part, on our ability to obtain adequate reimbursement from private insurers and other healthcare payers.
Our ability to successfully commercialize our products in development, will depend, in significant part, on the extent to which we or our marketing partners can obtain reimbursement for our products and also reimbursement at appropriate levels for the cost of our products. Obtaining reimbursement from governmental payers, insurance companies, HMOs and other third-party payers of healthcare costs is a time-consuming and expensive process.
| 26 |
| Table of Contents |
Even if we receive regulatory approval for our products in Europe, we may not be able to secure adequate pricing and reimbursement in Europe for us or any strategic partner to achieve profitability.
Even if one or more of our products are approved in Europe, we may be unable to obtain appropriate pricing and reimbursement for such products. In most European markets, demand levels for healthcare in general and for pharmaceuticals in particular are principally regulated by national governments. Therefore, pricing and reimbursement for our products will have to be negotiated on a “Member State by Member State” basis according to national rules, as there does not exist a centralized European process. As each Member State has its own national rules governing pricing control and reimbursement policy for pharmaceuticals, there are likely to be uncertainties attaching to the review process, and the level of reimbursement that national governments are prepared to accept. In the current economic environment, governments and private payers or insurers are increasingly looking to contain healthcare costs, including costs on drug therapies. If we are unable to obtain adequate pricing and reimbursement for our products in Europe, we or a potential strategic partner or collaborator may not be able to cover the costs necessary to manufacture, market and sell the product, limiting or preventing our ability to achieve profitability.
We may incur substantial liabilities and may be required to limit commercialization of our products in response to product liability lawsuits.
The testing and marketing of medical products entails an inherent risk of product liability. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our products or cease clinical trials. Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of pharmaceutical products we develop, alone or with corporate collaborators. We currently carry $10.0 million liability insurance in the aggregate as to certain product liability and commercialization risks and certain clinical trial risks. We, or any corporate collaborators, may not in the future be able to obtain insurance at a reasonable cost or in sufficient amounts, if at all. Even if our agreements with any future corporate collaborators entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
Our internal computer systems, or those of our third-party contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.
In the ordinary course of our business, we collect, store and transmit confidential information. Despite the implementation of security measures, our internal computer systems and those of our third-party contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. We rely on industry accepted measures and technology to secure confidential and proprietary information maintained on our computer systems. However, these measures and technology may not adequately prevent security breaches. While we do not believe that we have experienced any such system failure, accident, or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a loss of clinical trial data for our product candidates that could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Cyberattacks are increasing in their frequency, sophistication, and intensity. Cyberattacks could include the deployment of harmful malware, denial-of-service attacks, social engineering, and other means to affect service reliability and threaten the confidentiality, integrity and availability of information. Significant disruptions of our information technology systems or security breaches could adversely affect our business operations and/or result in the loss, misappropriation, and/or unauthorized access, use or disclosure of, or the prevention of access to, confidential information (including trade secrets or other intellectual property, proprietary business information and personal information), and could result in financial, legal, business, and reputational harm to us. To the extent that any disruption or security breach results in a loss of or damage to our data or applications or other data or applications relating to our technology, intellectual property, research and development or product candidates, or inappropriate disclosure of confidential or proprietary information, we could incur liabilities and the further development of our product candidates could be delayed.
| 27 |
| Table of Contents |
We may use artificial intelligence in our business, and challenges with properly managing its use, as well as uncertainty regarding the legal landscape surrounding the use of artificial intelligence ("AI”) could result in reputational harm, competitive harm, and legal liability, and adversely affect our results of operations.
We incorporate AI solutions into our platform, and these applications may become important in our operations over time. There are significant risks involved in utilizing AI and no assurance can be provided that the usage of such AI will enhance our business or assist our business in being more efficient or profitable. Known risks of AI currently include inaccuracy, bias, toxicity, intellectual property infringement or misappropriation, data privacy and cybersecurity and data provenance. In addition, AI may have errors or inadequacies that are not easily detectable. AI may also be subject to data herding and interconnectedness (i.e., multiple market participants utilizing the same data), which may adversely impact our business. If the data used to train AI or the content, analyses, or recommendations that AI applications assist in producing are or are alleged to be deficient, inaccurate, incomplete, overbroad or biased, our business, financial condition, and results of operations may be adversely affected. The legal landscape and subsequent legal protection for the use of AI remains uncertain, and development of the law in this area could impact our ability to enforce our proprietary rights or protect against infringing uses. If we do not have sufficient rights to use the data on which AI relies or to the outputs produced by AI applications, we may incur liability through the violation of certain laws, third-party privacy or other rights or contracts to which we are a party. Our use of AI applications may also, in the future, result in cybersecurity incidents that implicate the personal data of customers or patients. Any such cybersecurity incidents related to our use of AI applications could adversely affect our reputation and results of operations.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We may in the future employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants, and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee’s former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
As we begin commercializing any of our products in the United States, our operations may be directly, or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, and physician sunshine laws and regulations. These laws may impact, among other things, our proposed sales, marketing, and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
|
· |
the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from soliciting, receiving, offering or providing remuneration, directly or indirectly, in return for or to induce either the referral of an individual for, or the purchase order or recommendation of, any item or services for which payment may be made under a federal health care program such as the Medicare and Medicaid programs; |
|
|
|
|
· |
federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent; |
|
|
|
|
· |
HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
|
|
|
|
· |
HIPAA, as amended by the Health Information Technology and Clinical Health Act, and its implementing regulations, which imposes certain requirements relating to the privacy, security, and transmission of individually identifiable health information; |
|
|
|
|
· |
The federal physician sunshine requirements under the Affordable Care Act, which require manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; and |
|
|
|
|
· |
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers, state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
| 28 |
| Table of Contents |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent health care reform legislation has strengthened these laws. For example, the Affordable Care Act, among other things, amends the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. Moreover, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
We are highly dependent on our management team, senior staff professionals and third-party contractors and consultants, and the loss of their services could materially adversely affect our business.
We rely on our relatively small management team and staff as well as various contractors and consultants to provide critical services. Our ability to execute our clinical programs for PL8177, PL9643 and our other preclinical programs for MC1r and MC4r peptide or small molecule drug candidates depends on our continued retention and motivation of our management and senior staff professionals, including executive officers and senior members of product development and management, including commercialization, who possess significant technical expertise and experience and oversee our development and commercialization programs. If we lose the services of existing key personnel, our development programs could be adversely affected if suitable replacement personnel are not recruited quickly. Our success also depends on our ability to develop and maintain relationships with contractors, consultants, and scientific advisors.
There is competition for qualified personnel, contractors, and consultants in the pharmaceutical industry, which makes it difficult to attract and retain the qualified personnel, contractors and consultants necessary for the development and growth of our business. Our failure to attract and retain such personnel, contractors and consultants could have a material adverse effect on our business, results of operations and financial condition.
Risks Related to Government Regulation
Both before and after marketing approval, our product candidates are subject to ongoing regulatory requirements and, if we fail to comply with these continuing requirements, we could be subject to a variety of sanctions and the sale of any approved commercial products could be suspended.
Both before and after regulatory approval to market a particular product candidate, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising and promotion and record keeping related to the product candidates are subject to extensive regulatory requirements. If we fail to comply with the regulatory requirements of the FDA and other applicable U.S. and foreign regulatory authorities, we could be subject to administrative or judicially imposed sanctions, including:
|
· |
restrictions on the products or manufacturing process; |
|
· |
warning letters; |
|
· |
civil or criminal penalties; |
|
· |
fines; |
|
· |
injunctions; |
|
· |
imposition of a Corporate Integrity Agreement requiring heightened monitoring of our compliance functions, overseen by outside monitors, and enhanced reporting requirements to, and oversight by, the FDA and other government agencies; |
|
· |
product seizures or detentions and related publicity requirements; |
|
· |
suspension or withdrawal of regulatory approvals; |
|
· |
regulators or IRBs may not authorize us or any potential future collaborators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
|
· |
total or partial suspension of production; and |
|
· |
refusal to approve pending applications for marketing approval of new product candidates. |
| 29 |
| Table of Contents |
Changes in the regulatory approval policy during the development period, changes in or the enactment of additional regulations or statutes, or changes in the regulatory review for each submitted product application may cause delays in the approval or rejection of an application. Even if the FDA approves a product candidate, the approval may impose significant restrictions on the indicated uses, conditions for use, labeling, advertising, promotion, marketing and/or production of such product, and may impose ongoing requirements for post-approval studies, including additional research and development and clinical trials. The approval may also impose REMS on a product if the FDA believes there is a reason to monitor the safety of the drug in the marketplace. REMS may include requirements for additional training for health care professionals, safety communication efforts and limits on channels of distribution, among other things. The sponsor would be required to evaluate and monitor the various REMS activities and adjust them if need be. The FDA also may impose various civil or criminal sanctions for failure to comply with regulatory requirements, including withdrawal of product approval.
Furthermore, the approval procedure and the time required to obtain approval varies among countries and can involve additional testing beyond that required by the FDA. Approval by one regulatory authority does not ensure approval by regulatory authorities in other jurisdictions. The FDA has substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical, clinical or other studies.
In addition, varying interpretations of the data obtained for preclinical and clinical testing could delay, limit or prevent regulatory approval of a product candidate. Even if we submit an application to the FDA for marketing approval of a product candidate, it may not result in marketing approval from the FDA.
We do not expect to receive regulatory approval for the commercial sale of any of our product candidates that are in development in the near future, if at all. The inability to obtain FDA approval or approval from comparable authorities in other countries for our product candidates would prevent us or any potential future collaborators from commercializing these product candidates in the United States or other countries.
The regulatory approval process is lengthy, expensive and uncertain, and may prevent us from obtaining the approvals that we require.
Government authorities in the United States and other countries extensively regulate the advertising, labeling, storage, record-keeping, safety, efficacy, research, development, testing, manufacture, promotion, marketing, and distribution of drug products. Drugs are subject to rigorous regulation in the United States by the FDA and similar regulatory bodies in other countries. The steps ordinarily required by the FDA before a new drug may be marketed in the United States include:
|
· |
completion of non-clinical tests including preclinical laboratory and formulation studies and animal testing and toxicology; |
|
· |
submission to the FDA of an IND application, which must become effective before clinical trials may begin, and which may be placed on “clinical hold” by the FDA, meaning the trial may not commence, or must be suspended or terminated prior to completion; |
|
· |
performance of adequate and well-controlled Phase 1, 2 and 3 human clinical trials to establish the safety and efficacy of the drug for each proposed indication, and potentially post-approval or Phase 4 studies to further define the drug’s efficacy and safety, generally or in specific patient populations; |
|
· |
submission to the FDA of an NDA that must be accompanied by a substantial “user fee” payment; |
|
· |
FDA review and approval of the NDA before any commercial marketing or sale; and |
|
· |
compliance with post-approval commitments and requirements. |
| 30 |
| Table of Contents |
Satisfaction of FDA pre-market approval requirements for new drugs typically takes a number of years and the actual time required for approval may vary substantially based upon the type, complexity and novelty of the product or disease to be treated by the drug. The results of product development, preclinical studies and clinical trials are submitted to the FDA as part of an NDA. The NDA also must contain extensive manufacturing information, demonstrating compliance with applicable GMP requirements. Once the submission has been accepted for filing, the FDA generally has twelve months to review the application and respond to the applicant. Such response may be an approval or may be a “complete response letter” outlining additional data or steps that must be completed prior to further FDA review of the NDA. The review process is often significantly extended by FDA requests for additional information or clarification. Success in early-stage clinical trials does not assure success in later stage clinical trials. Data obtained from clinical trials is not always conclusive and may be susceptible to varying interpretations that could delay, limit or prevent regulatory approval. The FDA may refer the NDA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved, but the FDA is not bound by the recommendation of the advisory committee. The FDA may deny or delay approval of applications that do not meet applicable regulatory criteria or if the FDA determines that the clinical data do not adequately establish the safety and efficacy of the drug. Therefore, our proposed products could take a significantly longer time than we expect or may never gain approval. If regulatory approval is delayed or never obtained, our business, financial condition and results of operations would be materially adversely affected.
Some of our products or product candidates may be used in combination with a drug delivery device, such as an injector or other delivery system. Medical products containing a combination of new drugs, biological products or medical devices are regulated as “combination products” in the United States. A combination product generally is defined as a product comprised of components from two or more regulatory categories (e.g., drug/device, device/biologic, drug/biologic). Each component of a combination product is subject to the requirements established by the FDA for that type of component, whether a new drug, biologic or device. In order to facilitate pre-market review of combination products, the FDA designates one of its centers to have primary jurisdiction for the pre-market review and regulation of the overall product based upon a determination by the FDA of the primary mode of action of the combination product. The determination whether a product is a combination product or two separate products is made by the FDA on a case-by-case basis. Our product candidates intended for use with such devices, or expanded indications that we may seek for our products used with such devices, may not be approved or may be substantially delayed in receiving approval if the devices do not gain and/or maintain their own regulatory approvals or clearances. Where approval of the drug product and device is sought under a single application, the increased complexity of the review process may delay approval. In addition, because these drug delivery devices are provided by single source unaffiliated third-party companies, we are dependent on the sustained cooperation and effort of those third-party companies both to supply the devices, maintain their own regulatory compliance, and, in some cases, to conduct the studies required for approval or other regulatory clearance of the devices. We are also dependent on those third-party companies continuing to maintain such approvals or clearances once they have been received. Failure of third-party companies to supply the devices, to successfully complete studies on the devices in a timely manner, or to obtain or maintain required approvals or clearances of the devices, and maintain compliance with all regulatory requirements, could result in increased development costs, delays in or failure to obtain regulatory approval and delays in product candidates reaching the market or in gaining approval or clearance for expanded labels for new indications.
Upon approval, a product candidate may be marketed only in those dosage forms and for those indications approved by the FDA. Once approved, the FDA may withdraw the product approval if compliance with regulatory requirements is not maintained or if problems occur after the product reaches the marketplace. In addition, the FDA may require postmarketing studies, referred to as Phase 4 studies, to monitor the approved products in a specific subset of patients or a larger number of patients than were required for product approval and may limit further marketing of the product based on the results of these post-market studies. The FDA has broad post-market regulatory and enforcement powers, including the ability to seek injunctions, levy fines and civil penalties, criminal prosecution, withdraw approvals and seize products or request recalls.
If regulatory approval of any of our product candidates is granted, it will be limited to certain disease states or conditions, patient populations, duration, or frequency of use, and will be subject to other conditions as set forth in the FDA-approved labeling. Adverse experiences with the product must be reported to the FDA and could result in the imposition of market restriction through labeling changes or in product removal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following approval.
| 31 |
| Table of Contents |
Outside the United States, our ability to market our product candidates will also depend on receiving marketing authorizations from the appropriate regulatory authorities. The foreign regulatory approval process generally includes all of the risks associated with FDA approval described above. The requirements governing the conduct of clinical trials and marketing authorization vary widely from country to country. At present, foreign marketing authorizations are applied for at a national level, although within the European Community (“EC”), registration procedures are available to companies wishing to market a product to more than one EC member state. If the regulatory authority is satisfied that adequate evidence of safety, quality and efficiency has been presented, a marketing authorization will be granted. If we do not obtain, or experience difficulties in obtaining, such marketing authorizations, our business, financial condition and results of operations may be materially adversely affected.
Legislative or regulatory healthcare reforms in the United States may make it more difficult and costly for us to obtain regulatory clearance or approval of any future product candidates and to produce, market and distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in Congress, and court decisions are issued, that could significantly change the statutory provisions governing the regulatory clearance or approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of Vyleesi for HSDD or any future product candidates. We cannot determine what effect changes in regulations, statutes, court decisions, legal interpretation or policies, when and if promulgated, enacted, issued or adopted may have on our business in the future. Such changes could, among other things:
|
· |
require changes to manufacturing methods; |
|
· |
require recall, replacement or discontinuance of one or more of our products; |
|
· |
require additional recordkeeping; |
|
· |
limit or restrict our ability to engage in certain types of marketing or promotional activities; |
|
· |
alter or eliminate the scope or terms of any currently available regulatory exclusivities; and |
|
· |
restrict or eliminate our ability to settle any patent litigation we may bring against potential generic competitors. |
Each of these would likely entail substantial time and cost and could materially harm our business and our financial results. In addition, delays in receipt of or failure to receive regulatory clearances or approvals for any future products would harm our business, financial condition, and results of operations.
Changes in healthcare policy could adversely affect our business.
Our industry is highly regulated, and changes in law may adversely impact our business, operations, or financial results. In the U.S., there have been and continue to be a number of legislative initiatives to contain healthcare costs. For example, the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010 (the “PPACA”) is a sweeping measure intended to, among other things, expand healthcare coverage within the U.S., primarily through the imposition of health insurance mandates on employers and individuals and expansion of the Medicaid program. Several provisions of the law have affected us and increased certain of our costs. Since its enactment, there have been executive, judicial, and congressional challenges to certain aspects of the PPACA. In addition, other legislative changes have been adopted since the PPACA was enacted. Some of these changes have resulted in additional reductions in Medicare and other healthcare funding.
We anticipate that the PPACA, as well as other healthcare reform measures that may be adopted in the future in the U.S. or abroad, may result in more rigorous coverage criteria and an additional downward pressure on the reimbursement our customers may receive for our products. Recently there has been heightened governmental scrutiny in countries worldwide over the manner in which manufacturers set prices for their marketed products.
In the U.S., there have been several recent congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the cost of drugs under Medicare, and reform government program reimbursement methodologies for drug products. For example, at the federal level, during the former Trump administration there were multiple executive orders issued, initiatives implemented and calls for legislation from Congress to reduce drug prices, increase competition and reduce out of pocket costs of drugs for patients. The likelihood of implementation of any of the former Trump administration healthcare reform initiatives is uncertain, particularly in light of the Biden administration. Any reduction in reimbursement from Medicare and other government programs may result in a similar reduction in payments from private payers. In addition, individual states in the U.S. have also increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Moreover, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs.
| 32 |
| Table of Contents |
Legally mandated price controls on payment amounts by governmental and private third-party payers or other restrictions could harm our business, results of operations, financial condition, and prospects. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products.
For more information regarding government healthcare reform, see “U.S. Governmental Regulation of Pharmaceutical Products” in Part I, Item 1 of this Annual Report.
Risks Related to Our Intellectual Property
If we fail to adequately protect or enforce our intellectual property rights or secure rights to patents of others, the value of our intellectual property rights would diminish.
Our success, competitive position and future revenues will depend in part on our ability and the abilities of our licensors to obtain and maintain patent protection for our products, methods, processes, and other technologies, to preserve our trade secrets, to prevent third parties from infringing on our proprietary rights and to operate without infringing the proprietary rights of third parties. We cannot predict:
|
· |
the degree and range of protection any patents will afford us against competitors, including whether third parties will find ways to invalidate or otherwise circumvent our patents; |
|
· |
if and when patents will be issued; |
|
· |
whether or not others will obtain patents claiming aspects similar to those covered by our patents and patent applications; and |
|
· |
whether we will need to initiate litigation or administrative proceedings, which may be costly whether we win or lose. |
If our products, methods, processes, and other technologies infringe the proprietary rights of other parties we could incur substantial costs and we may have to:
|
· |
obtain licenses, which may not be available on commercially reasonable terms, if at all; |
|
· |
redesign our products or processes to avoid infringement; |
|
· |
stop using the subject matter claimed in the patents held by others; |
|
· |
pay damages; or |
|
· |
defend litigation or administrative proceedings, which may be costly whether we win or lose, and which could result in a substantial diversion of our management resources. |
We may become involved in lawsuits to protect or enforce our patents or other intellectual property or the patents of our licensors, which could be expensive and time consuming.
Competitors may infringe our intellectual property, including our patents or the patents of our licensors. As a result, we may be required to file infringement claims to stop third-party infringement or unauthorized use. This can be expensive, particularly for a company of our size, and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patent claims do not cover its technology or that the factors necessary to grant an injunction against an infringer are not satisfied.
An adverse determination of any litigation or other proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
| 33 |
| Table of Contents |
Interference, derivation, or other proceedings brought at the USPTO may be necessary to determine the priority or patentability of inventions with respect to our patent applications or those of our licensors or collaborators. Litigation or USPTO proceedings brought by us may fail or may be invoked against us by third parties. Even if we are successful, domestic, or foreign litigation or USPTO or foreign patent office proceedings may result in substantial costs and distraction to our management. We may not be able, alone or with our licensors or collaborators, to prevent misappropriation of our proprietary rights, particularly in countries where the laws may not protect such rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or other proceedings, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation or proceedings. In addition, during the course of this kind of litigation or proceedings, there could be public announcements of the results of hearings, motions or other interim proceedings or developments or public access to related documents. If investors perceive these results to be negative, the market price for our common stock could be significantly harmed.
If we infringe or are alleged to infringe intellectual property rights of third parties, our business could be harmed.
Our research, development and commercialization activities may infringe or otherwise violate or be claimed to infringe or otherwise violate patents owned or controlled by other parties. There may also be patent applications that have been filed but not published that, when issued as patents, could be asserted against us. These third parties could bring claims against us that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit.
As a result of patent infringement claims, or to avoid potential claims, we may choose or be required to seek licenses from third parties. These licenses may not be available on acceptable terms, or at all. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties or both, and the rights granted to us might be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms, if at all.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical industry. In addition to infringement claims against us, we may become a party to other patent litigation and other proceedings, including interference, derivation or post-grant proceedings declared or granted by the USPTO and similar proceedings in foreign countries, regarding intellectual property rights with respect to our current or future products. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Patent litigation and other proceedings may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could impair our ability to compete in the marketplace. The occurrence of any of the foregoing could have a material adverse effect on our business, financial condition, or results of operations.
Our patent applications and the enforcement or defense of our issued patents may be impacted by the application of or changes in U.S. and foreign standards.
The standards that the USPTO and foreign patent offices use to grant patents are not always applied predictably or uniformly and can change. Consequently, our pending patent applications may not be allowed and, if allowed, may not contain the type and extent of patent claims that will be adequate to conduct our business as planned. Additionally, any issued patents we currently own or obtain in the future may have a shorter patent term than expected or may not contain claims that will permit us to stop competitors from using our technology or similar technology or from copying our product candidates. Similarly, the standards that courts use to interpret patents are not always applied predictably or uniformly and may evolve, particularly as new technologies develop. In addition, changes to patent laws in the United States or other countries may be applied retroactively to affect the validation enforceability, or term of our patent. For example, the U.S. Supreme Court has recently modified some legal standards applied by the USPTO in examination of U.S. patent applications, which may decrease the likelihood that we will be able to obtain patents and may increase the likelihood of challenges to patents we obtain or license. In addition, changes to the U.S. patent system have come into force under the Leahy-Smith America Invents Act, or the Leahy-Smith Act, which was signed into law in September 2011. The Leahy-Smith Act included significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted and also affect patent litigation. Under the Leahy-Smith Act, the United States transitioned in March 2013 to a “first to file” system in which the first inventor to file a patent application will be entitled to the patent. Third parties are allowed to submit prior art before the issuance of a patent by the USPTO, and may become involved in opposition, derivation, reexamination, inter partes review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, which could adversely affect our competitive position.
| 34 |
| Table of Contents |
While we cannot predict with certainty the impact the Leahy-Smith Act or any potential future changes to the U.S. or foreign patent systems will have on the operation of our business, the Leahy-Smith Act and such future changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, results of operations, financial condition and cash flows and future prospects.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States and in some cases may even force us to grant a compulsory license to competitors or other third parties. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
In addition, our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in domestic and foreign intellectual property laws.
If we are unable to keep our trade secrets confidential, our technologies and other proprietary information may be used by others to compete against us.
In addition to our reliance on patents, we attempt to protect our proprietary technologies and processes by relying on trade secret laws and agreements with our employees and other persons who have access to our proprietary information. These agreements and arrangements may not provide meaningful protection for our proprietary technologies and processes in the event of unauthorized use or disclosure of such information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, our competitors may independently develop substantially equivalent technologies and processes or gain access to our trade secrets or technology, either of which could materially or adversely affect our competitive position.
Risks Related to the Ownership of Our Common Stock
Our stock price is volatile and may fluctuate in a way that is disproportionate to our operating performance and we expect it to remain volatile, which could limit investors’ ability to sell stock at a profit.
The volatile price of our stock makes it difficult for investors to predict the value of their investment, to sell shares at a profit at any given time or to plan purchases and sales in advance. A variety of factors may affect the market price of our common stock. These include, but are not limited to:
| 35 |
| Table of Contents |
|
· |
publicity regarding actual or potential clinical results relating to products under development by our competitors or us; |
|
· |
delay or failure in initiating, completing or analyzing preclinical or clinical trials or unsatisfactory designs or results of these trials; |
|
· |
interim decisions by regulatory agencies, including the FDA, as to clinical trial designs, acceptable safety profiles and the benefit/risk ratio of products under development; |
|
· |
achievement or rejection of regulatory approvals by our competitors or by us; |
|
· |
announcements of technological innovations or new commercial products by our competitors or by us; |
|
· |
developments concerning proprietary rights, including patents; |
|
· |
developments concerning our collaborations; |
|
· |
regulatory developments in the United States and foreign countries; |
|
· |
economic or other crises and other external factors; |
|
· |
period-to-period fluctuations in our revenue and other results of operations; |
|
· |
changes in the structure of healthcare payment systems or other actions that affect the effective reimbursement rates for treatment regimens containing our products; |
|
· |
changes in financial estimates and recommendations by securities analysts following our business or our industry; |
|
· |
sales of our common stock, or the perception that such sales could occur; and |
|
· |
the other factors described in this “Risk Factors” section. |
We will not be able to control many of these factors, and we believe that period-to-period comparisons of our financial results will not necessarily be indicative of our future performance. If our revenues, if any, in any particular period do not meet expectations, we may not be able to adjust our expenditures in that period, which could cause our operating results to suffer further. If our operating results in any future period fall below the expectations of securities analysts or investors, our stock price may fall by a significant amount.
For the 12-month period ended June 30, 2024, the price of our stock has been volatile, ranging from a high of $5.65 per share to a low of $1.43 per share. In addition, the stock market in general, and the market for biotechnology companies in particular, has experienced extreme price and volume fluctuations that may have been unrelated or disproportionate to the operating performance of individual companies. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance.
As a public company in the United States, we are subject to the Sarbanes-Oxley Act of 2002 (“Sarbanes-Oxley”). Our internal control over financial reporting was not effective as of June 30, 2024, and failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of Sarbanes-Oxley could have a material adverse effect on our business and share price.
Companies that file reports with the SEC, including us, are subject to the requirements of Section 404 of Sarbanes-Oxley. Section 404 requires management to establish and maintain a system of internal control over financial reporting. Ensuring that we have adequate internal financial and accounting controls and procedures in place to produce accurate financial statements on a timely basis is a costly and time-consuming effort that needs to be re-evaluated frequently.
Our management’s assessment of internal control over financial reporting and concluded that, as of June 30, 2024, our internal control over financial reporting was ineffective due to a material weakness in our controls over the accounting for complex financial instruments. While we have processes to identify and appropriately apply applicable accounting requirements, we plan to enhance these processes to better evaluate our research and understanding of the nuances of the complex accounting standards that apply to our financial statements.
Any failure to maintain such internal control could adversely impact our ability to report our financial position and results from operations on a timely and accurate basis. If our financial statements are not accurate, investors may not have a complete understanding of our operations. Likewise, if our financial statements are not filed on a timely basis, we could be subject to sanctions or investigations by the stock exchange on which our ordinary shares are listed, the SEC or other regulatory authorities. In either case, there could result a material adverse effect on our business. Failure to timely file will cause us to be ineligible to utilize short-form registration statements, which may impair our ability to obtain capital in a timely fashion to execute our business strategies or issue shares to effect an acquisition. Ineffective internal control could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our securities.
| 36 |
| Table of Contents |
We can give no assurance that the measures we have taken and plan to take in the future will remediate the material weaknesses identified or that any additional material weaknesses or restatements of financial results will not arise in the future due to a failure to implement and maintain adequate internal control over financial reporting or circumvention of these controls. In addition, even if we are successful in strengthening our controls and procedures, in the future those controls and procedures may not be adequate to prevent or identify irregularities or errors or to facilitate the fair presentation of our financial statements.
If securities or industry analysts do not publish research or publish unfavorable research about our business, our stock price and trading volume could decline.
As a smaller company, it may be difficult for us to attract or retain the interest of equity research analysts. A lack of research coverage may adversely affect the liquidity of and market price of our common stock. We do not have any control of the equity research analysts or the content and opinions included in their reports. The price of our stock could decline if one or more equity research analysts downgrade our stock or issue other unfavorable commentary or research. If one or more equity research analysts ceases coverage of us, or fails to publish reports on us regularly, demand for our stock could decrease, which in turn could cause our stock price or trading volume to decline.
Holders of our Series A Preferred Stock may have interests different from our common stockholders.
We are permitted under our certificate of incorporation to issue up to 10,000,000 shares of preferred stock. We can issue shares of our preferred stock in one or more series and can set the terms of the preferred stock without seeking any further approval from our common stockholders. As of September 27, 2024, there are 4,030 shares of Series A Preferred Stock outstanding. Each share of Series A Preferred Stock is convertible at any time, at the option of the holder, and such conversion could dilute the value of our common stock to current stockholders and could adversely affect the market price of our common stock. The conversion price decreases if we sell common stock (or equivalents) for a price per share less than the conversion price or less than the market price of the common stock and is also subject to adjustment upon the occurrence of a merger, reorganization, consolidation, reclassification, stock dividend or stock split which results in an increase or decrease in the number of shares of common stock outstanding. Upon (i) liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, (ii) sale or other disposition of all or substantially all of the assets of the Company, or (iii) any consolidation, merger, combination, reorganization or other transaction in which the Company is not the surviving entity or in which the shares of common stock constituting in excess of 50% of the voting power of the Company are exchanged for or changed into other stock or securities, cash and/or any other property, after payment or provision for payment of the debts and other liabilities of the Company, the holders of Series A Preferred Stock will be entitled to receive, pro rata and in preference to the holders of any other capital stock, an amount per share equal to $100 plus accrued but unpaid dividends, if any
Because we do not anticipate paying any cash dividends on our common stock in the foreseeable future, capital appreciation, if any, will be our stockholders’ sole source of gains.
We do not anticipate paying any cash dividends in the foreseeable future and intend to retain future earnings, if any, for the development and expansion of our business. Our outstanding Series A Preferred Stock, consisting of 4,030 shares on September 27, 2024, provides that we may not pay a dividend or make any distribution to holders of any class of stock unless we first pay a special dividend or distribution of $100 per share to the holders of the Series A Preferred Stock. In addition, the terms of existing or future agreements may limit our ability to pay dividends. As a result, capital appreciation, if any, of our common stock will be our stockholders’ sole source of gain for the foreseeable future.
Anti-takeover provisions of Delaware law and our charter documents may make potential acquisitions more difficult and could result in the entrenchment of management.
We are incorporated in Delaware. Anti-takeover provisions of Delaware law and our charter documents may make a change in control or efforts to remove management more difficult. Also, under Delaware law, our board of directors may adopt additional anti-takeover measures. Under Section 203 of the Delaware General Corporation Law, a corporation may not engage in a business combination with an “interested stockholder” for a period of three years after the date of the transaction in which the person first becomes an “interested stockholder,” unless the business combination is approved in a prescribed manner.
We are authorized to issue up to 300,000,000 shares of common stock. To the extent that we sell or otherwise issue authorized but currently unissued shares, this could have the effect of making it more difficult for a third party to acquire a majority of our outstanding voting stock.
| 37 |
| Table of Contents |
Our charter authorizes us to issue up to 10,000,000 shares of preferred stock and to determine the terms of those shares of stock without any further action by our stockholders. If we exercise this right, it could be more difficult for a third party to acquire a majority of our outstanding voting stock.
In addition, our equity incentive plans generally permit us to accelerate the vesting of options and other stock rights granted under these plans in the event of a change of control. If we accelerate the vesting of options or other stock rights, this action could make an acquisition more costly.
The application of these provisions could have the effect of delaying or preventing a change of control, which could adversely affect the market price of our common stock.
We are a smaller reporting company and the reduced disclosure requirements applicable to smaller reporting companies may make our common stock less attractive to investors.
We are currently a “smaller reporting company” as defined in the Exchange Act. Smaller reporting companies are able to provide simplified executive compensation disclosures in their filings and have certain other decreased disclosure obligations in their SEC filings. We cannot predict whether investors will find our common stock less attractive because of our reliance on the smaller reporting company exemption. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
As of September 27, 2024, there were 11,385,637 shares of common stock underlying outstanding convertible preferred stock, options, restricted stock units and warrants. Stockholders may experience dilution from the conversion of preferred stock, exercise of outstanding options and warrants and vesting and delivery of restricted stock units.
As of September 27, 2024, holders of our outstanding dilutive securities had the right to acquire the following amounts of underlying common stock:
|
· |
5,333 shares issuable on the conversion of our immediately convertible Series A Preferred Stock, subject to adjustment, for no further consideration; |
|
|
|
|
· |
2,249,102 shares issuable upon exercise of stock options with a weighted-average exercise price of $6.12 per share; |
|
|
|
|
· |
859,613 shares issuable under restricted stock units which will vest on dates between June 22, 2025 and June 4, 2028, subject to the fulfillment of service or performance conditions; |
|
|
|
|
· |
279,700 shares of common stock which have vested under restricted stock unit agreements, but are subject to provisions to delay delivery; |
|
|
|
|
· |
66,666 shares of common stock issuable upon exercise of warrants at an exercise price of $12.50 per share, and expire on May 11, 2026; |
|
|
|
|
· |
2,727,273 shares of common stock issuable upon exercise of Series A warrants at an exercise price of $1.88 per share, and expire on June 24, 2029; |
|
|
|
|
· |
2,122,642 shares of common stock issuable upon exercise of Series B warrants at an exercise price of $1.88 per share. 498,441 Series B warrants expire June 24, 2024 and 1,624,201 Series B warrants are subject to stockholder approval and will expire on the five-year anniversary from the date of stockholder approval; |
|
|
|
|
· |
1,831,503 shares of common stock issuable upon exercise of common warrants at an exercise price of $5.46 per share, and that expire on February 1, 2028; |
|
|
|
|
· |
91,575 shares of common stock issuable upon exercise of the placement agent warrants issued to the placement agent or its designees as compensation in connection with the Company’s February 1, 2024 offering, with an exercise price of $6.825 per share, and an expiration date of February 1, 2028; |
|
|
|
|
· |
90,909 shares of common stock issuable upon exercise of the placement agent warrants issued to the placement agent or its designees as compensation in connection with the Company’s October 2022 offering, with an exercise price of $6.875 per share, and an expiration date of October 31, 2027; |
|
|
|
|
· |
943,396 shares issuable upon exercise of warrants issued in the Company’s October 2023 Offering, with an exercise price of $2.12 per share, and that expire on April 24, 2029; |
|
|
|
|
· |
117,925 shares of common stock issuable upon exercise of the placement agent warrants issued to the placement agent or its designees as compensation in connection with the October 2023 Offering, with an exercise price of $2.65 per share, and an expiration date of October 20, 2028; and |
|
|
|
|
· |
223,538 shares of common stock available for future issuance under our 2011 Stock Incentive Plan. |
| 38 |
| Table of Contents |
If the holders convert, exercise, or receive these securities, or similar dilutive securities we may issue in the future, stockholders may experience dilution in the net book value of their common stock. In addition, the sale or availability for sale of the underlying shares in the marketplace could depress our stock price. We have registered or agreed to register for resale substantially all of the underlying shares listed above. Holders of registered underlying shares could resell the shares immediately upon issuance, which could result in significant downward pressure on our stock price.
We are currently not in compliance with the continued listing standards of the NYSE American. If we fail to regain compliance with the NYSE American listing standards, our common stock could be de-listed from the NYSE American.
Our common stock is listed on the NYSE American, a national securities exchange, under the symbol “PTN”. As a result, we are subject to NYSE American’s listing standards, which generally mandate that we meet certain requirements relating to stockholders’ equity, market capitalization, aggregate market value of publicly held shares and distribution requirements.
On October 10, 2023, Palatin received a notice from the staff of NYSE American LLC (the “NYSE American”) that Palatin was not in compliance with the Exchange’s continued listing standards under Section 1003(a)(i) and (ii) of the NYSE American Company Guide. Section 1003(a)(i) requires a listed company to have stockholders’ equity $2 million or more if the listed company has reported losses from continuing operations and/or net losses in two of its three most recent fiscal years, and Section 1003(a)(ii) requires a listed company to have stockholders’ equity of $4 million or more if the listed company has reported losses from continuing operations and/or net losses in three of its four most recent fiscal years. Palatin is now subject to the procedures and requirements of Section 1009 of the NYSE American Company Guide. Palatin had until November 9, 2023, to submit a plan (the “Plan”) of actions it has taken or will take to regain compliance with the continued listing standards by April 10, 2025.
Palatin has timely delivered a Plan to the Exchange. The Exchange has accepted the Plan, and Palatin will be able to continue its listing during the Plan period and will be subject to periodic reviews including quarterly monitoring for compliance with the Plan until it has regained compliance.
There can be no assurance that Palatin will be able to meet milestones set forth in the Plan between now and April 10, 2025.
If we fail to regain compliance with the continued listing requirements of the NYSE American, the NYSE American may take steps to de-list our common stock. If the NYSE American de-lists our securities for trading on its exchange, we could face significant material adverse consequences, including:
|
· |
a limited availability of market quotations for our securities; |
|
· |
reduced liquidity with respect to our securities; |
|
· |
a determination that our shares of common stock are “penny stock” which will require brokers trading in our shares of common stock to adhere to more stringent rules, possibly resulting in a reduced level of trading activity in the secondary trading market for our shares of common stock; |
|
· |
a limited amount of news and analyst coverage for our company; and |
|
· |
a decreased ability to issue additional securities or obtain additional financing in the future. |
Such a de-listing would likely have a negative effect on the price of our common stock and would impair our investors’ ability to sell or purchase our common stock when investors wish to do so.
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” Our common shares are considered to be covered securities because they are listed on the NYSE American. Although the states are preempted from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. Further, if we were no longer listed on the NYSE American, our common stock would not be deemed covered securities and we would be subject to regulation in each state in which we offer our securities.
| 39 |
| Table of Contents |
Item 1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
We have established certain processes for identifying, evaluating, and managing material risks from cybersecurity threats as a part of our overall technology management strategy. These processes are designed and reassessed on a periodic basis to help protect our technology assets and operations from internal and external security threats.
We engage third parties to assess the effectiveness of our cybersecurity and technology management strategy and continue to seek to implement new, and improve existing, processes regularly to adjust for changes in technology, internal or external threats, business strategy, and regulatory requirements. We, and our third parties, have deployed managed detection and response services to monitor our technology infrastructure and information systems for possible threats. Our technology management strategy also includes ongoing security training and education for employees regarding threats, including their role and responsibility in detecting and responding to such threats.
The Company’s employees are required to sign confidentiality agreements in an effort to, among other things, help to ensure cybersecurity. The Company has taken measures to better ensure that key employees are aware of data security threats (including cybersecurity threats), and Company security policies and procedures, as appropriate. Improper or illegitimate use of the Company’s information system resources or violation of the Company’s information security policies and procedures may result in disciplinary action.
In the last two fiscal years, we have not identified cybersecurity threats that have materially affected, or are reasonably likely to materially affect, our business, results of operations, or financial condition. Although we proactively attempt to prevent all threats, we are unable to eliminate all risk from cybersecurity threats or provide assurance that we have not experienced an undetected cybersecurity incident. For more information about these risks, please see Item 1A. Risk Factors “Our internal computer systems, or those of our third-party contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.”
While our board of directors is responsible for oversight and risk management in general, our audit committee provides oversight of our technology management strategy to ensure that cybersecurity threats and risks are identified, evaluated, and managed. The audit committee receives periodic updates from our management team regarding the overall state of our technology management strategy and any relevant risks from cybersecurity threats and cybersecurity incidents. Our management team is responsible for assessing and managing the material risks from cybersecurity threats.
Item 2. Properties
Our corporate offices are located at 4B Cedar Brook Drive, Cedar Brook Corporate Center, Cranbury, NJ 08512, where we lease approximately 10,000 square feet of office space under a lease that expires in June 2025. We also lease approximately 3,600 square feet of laboratory space in the Township of South Brunswick, NJ under a lease that expires in October 2026. We believe our present facilities are adequate for our current needs. The corporate offices include private offices, meeting rooms and workspaces for all administrative personnel and over half of the research and development personnel, with the remainder of research and development personnel stationed at the laboratory facility. We do not own any real property.
Item 3. Legal Proceedings
We are involved, from time to time, in various claims and legal proceedings arising in the ordinary course of our business. As of the date of this filing, we are not aware that we are a party to any pending or threatened legal proceeding or proceeding by a governmental authority.
Item 4. Mine Safety Disclosures
Not applicable.
| 40 |
| Table of Contents |
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Our common stock has been listed on NYSE American under the symbol “PTN” since December 21, 1999. It previously traded on The Nasdaq SmallCap Market under the symbol “PLTN.”
On September 27, 2024 we had approximately 90 record holders of common stock and the closing sales price of our common stock as reported on the NYSE American was $0.84 per share. The aggregate market value of the common and non-voting common equity held by non-affiliates on such date, computed by reference to the closing sales price of our common stock on that date, was $16,051,587.
Issuer purchases of equity securities. In certain instances we provide our employees with the option to withhold shares to satisfy tax withholding amounts due from the employees upon the vesting of restricted stock units and stock options in connection with our 2011 Stock Incentive Plan. There were no shares withheld during the quarter ended June 30, 2024, at the direction of the employees as permitted under the 2011 Stock Incentive Plan, in order to pay the minimum amount of tax liability owed by the employee from the vesting of those units and options.
Dividends and dividend policy. We have never declared or paid any dividends. We currently intend to retain earnings, if any, for use in our business. We do not anticipate paying dividends in the foreseeable future.
Dividend restrictions. Our outstanding Series A Preferred Stock, consisting of 4,030 shares on September 27, 2024, provides that we may not pay a dividend or make any distribution to holders of any class of stock unless we first pay a special dividend or distribution of $100 per share to the holders of the Series A Preferred Stock.
Equity compensation plan information. Reference is made to the information contained in the Equity Compensation Plan table contained in Item 11 of this Annual Report.
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis should be read in conjunction with the consolidated financial statements and notes to the consolidated financial statements filed as part of this Annual Report.
Forward-Looking Statements
The following discussion and analysis contains forward-looking statements within the meaning of the federal securities laws. You are urged to carefully review our description and examples of forward-looking statements included earlier in this Annual Report on Form 10-K (this “Annual Report”) immediately prior to Part I, under the heading “Special Note Regarding Forward-Looking Statements.” Forward-looking statements are subject to risk that could cause actual results to differ materially from those expressed in the forward-looking statements. You are urged to carefully review the disclosures we make concerning risks and other factors that may affect our business and operating results, including those made in Part I, Item 1A of this Annual Report, and any of those made in our other reports filed with the SEC. You are cautioned not to place undue reliance on the forward-looking statements included herein, which speak only as of the date of this document. We do not intend, and undertake no obligation, to publish revised forward-looking statements to reflect events or circumstances after the date of this document or to reflect the occurrence of unanticipated events.
Introduction
Palatin Technologies, Inc. is a biopharmaceutical company developing first-in-class medicines based on molecules that modulate the activity of the melanocortin receptor system. The Company’s product candidates are targeted, receptor-specific therapeutics for the treatment of diseases with significant unmet medical need and commercial potential.
Our prior commercial product, Vyleesi®, was approved by the U.S. Food and Drug Administration (“FDA”) in June 2019 for the treatment of hypoactive sexual desire disorder (“HSDD”) in premenopausal women. This product was acquired by Cosette Pharmaceuticals, Inc. (“Cosette”) on December 19, 2023.
Our new product development activities focus primarily on use of bremelanotide, or other MC4r agonists, with tirzepatide, a GLP-1 agonist for treatment of obesity, which entered Phase 2 in the second quarter of calendar year 2024, and a co-formulation of bremelanotide with a PDE5i for treatment of erectile dysfunction in patients that do not respond to PDE5i monotherapy. We are also developing MC1r agonists, with potential to treat inflammatory and autoimmune diseases, such as dry eye disease, uveitis, diabetic retinopathy, and inflammatory bowel disease.
| 41 |
| Table of Contents |
Critical Accounting Policies and Estimates
Our significant accounting policies are described in Note 2 to the consolidated financial statements included in this Annual Report. We believe that our accounting policies and estimates relating to revenue recognition, the carrying value of inventory, purchase commitment liabilities, accrued expenses, and stock-based compensation are the most critical.
Revenue Recognition (Prior to the sale of Vyleesi)
We recognize product revenues in accordance with Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers. The provisions of ASC Topic 606 require the following steps to determine revenue recognition: (1) Identify the contract(s) with a customer; (2) Identify the performance obligations in the contract; (3) Determine the transaction price; (4) Allocate the transaction price to the performance obligations in the contract; and (5) Recognize revenue when (or as) the entity satisfies a performance obligation.
In accordance with ASC Topic 606, we recognize product revenue when our performance obligation is satisfied by transferring control of the product to a customer. Per our contracts with customers, control of the product is transferred upon the conveyance of title, which occurs when the product is sold to and received by a customer. Trade accounts receivable due to us from contracts with our customers are stated separately in the consolidated balance sheet, net of various allowances as described in the Trade Accounts Receivable policy in Note 2- Summary of Significant Accounting Policies in the accompanying consolidated financial statements.
Product revenues consisted of sales of Vyleesi in the United States prior to the sale of the Vyleesi product line to Cosette Pharmaceuticals, Inc. (“Cosette”) in December 2023. Prior to the sale of Vyleesi product line, we sold Vyleesi to a specialty pharmacy at the wholesale acquisition cost with payment made within approximately 30 days. In addition to distribution agreements with customers, we had entered into arrangements with healthcare payers that provide for privately negotiated rebates, chargebacks, and discounts with respect to the purchase of our products.
We recorded product revenues net of allowances for direct and indirect fees, discounts, co-pay assistance programs, estimated chargebacks, and rebates. Certain of these allowances represent estimates of the related obligations and, as such, knowledge and judgement are required when estimating the impact of these allowances on gross product sales for a reporting period. If any of our judgments made during a reporting period are not indicative or accurate estimates of our future experience, our results could be materially affected. Product sales are also subject to return rights, which have not been significant to date.
Purchase Commitment Liabilities
Losses on firm commitment contractual obligations are recognized based upon the terms of the respective agreement and similar factors considered for the write-down of inventory, including expected sales requirements as determined by internal sales forecasts.
Accrued Expenses
Third parties perform a significant portion of our development activities. We review the activities performed under all contracts each quarter and accrue expenses and the amount of any reimbursement to be received from our collaborators based upon the estimated amount of work completed considering milestones achieved. Estimating the value or stage of completion of certain services requires judgment based on available information. If we do not identify services performed for us but not billed by the service-provider, or if we underestimate or overestimate the value of services performed as of a given date, reported expenses will be understated or overstated.
Stock-Based Compensation
We expense the fair value of stock options and other equity awards granted to employees and nonemployees for services. Compensation costs for stock-based awards with time-based vesting are determined using the quoted market price of our common stock on the grant date or for stock options, the value determined utilizing the Black-Scholes option pricing model, and are recognized on a straight-line basis, while awards containing a market condition are valued using multifactor Monte Carlo simulations and are recognized over the derived service period. Compensation costs for awards containing a performance condition are determined using the quoted price of our common stock on the grant date or for stock options, the value is determined utilizing the Black Scholes option pricing model and are recognized based on the probability of achievement of the performance condition over the service period. The Black-Scholes option pricing model requires us to make estimates of expected volatility and interest rates, which we estimate based on prior experience and public sources of information. The expected term of the option used is based upon the simplified method, which represents the average of the vesting and contractual term. Compensation expense is not adjusted for subsequent changes in the estimates used to calculate fair value or for actual experience. Forfeitures are recognized as they occur. As the amount and timing of compensation expense to be recorded in future periods may be affected by the achievement of performance conditions and employee terminations, stock-based compensation may vary significantly period to period.
| 42 |
| Table of Contents |
See Note 3 to the consolidated financial statements included in this Annual Report for a description of recent accounting pronouncements that affect us.
Results of Operations
Year Ended June 30, 2024 Compared to the Year Ended June 30, 2023:
Revenue – For the fiscal year ended June 30, 2024 (“fiscal 2024”) we recognized $4,490,090 of product revenue, net of allowances. For the fiscal year ended June 30, 2023 (“fiscal 2023”) we recognized $4,850,678 of product revenue, net of allowances, and $3,000 in license and contract revenue pursuant to our license agreement with Fosun. The decrease in net revenue is a result of the sale of Vyleesi’s worldwide rights to Cosette during fiscal 2024.
Cost of Products Sold – Cost of products sold was $97,637 for fiscal 2024 compared to $418,470 for fiscal 2023. The decrease in cost of products sold is a result of the sale of Vyleesi’s worldwide rights to Cosette during fiscal 2024.
Research and Development – Total research and development expenses, including general research and development spending, were $22,400,372 for fiscal 2024 compared to $22,630,577 for fiscal 2023. The decrease is a result of lower spending on our MCr programs.
Research and development expenses related to our Vyleesi, MCr programs, and other preclinical programs were $15,512,149 for fiscal 2024 compared to $16,202,432 for fiscal 2023. The decrease is primarily related to a decrease in spending on our MCr programs.
The amounts of program spending above exclude general research and development spending, which were $6,888,233 for fiscal 2024 compared to $6,428,145 for fiscal 2023. The increase in general research and development spending is primarily attributable to increased compensation costs.
Cumulative spending from inception to June 30, 2024 was approximately $311,900,000 on our Vyleesi program and approximately $234,000,000 on all our other programs (which include PL8177, PL9643, other melanocortin receptor agonists and terminated programs). Due to various risk factors described herein under “Risk Factors,” including the difficulty in currently estimating the costs and timing of future Phase 1 clinical trials and larger-scale Phase 2 and Phase 3 clinical trials for any product under development, we cannot predict with reasonable certainty when, if ever, a program will advance to the next stage of development or be successfully completed, or when, if ever, related net cash inflows will be generated.
Selling, General and Administrative – Selling, general and administrative expenses, which consist of costs related to Vyleesi in addition to compensation and related costs, were $12,270,046 for fiscal 2024, compared to $15,290,836 for fiscal 2023. The decrease is primarily attributable to $1,912,243 of selling expenses related to Vyleesi in fiscal 2024 compared to $4,621,001 of selling expenses related to Vyleesi in fiscal 2023.
Gain on Purchase Commitment - Gain on purchase commitments was $1,027,322 for fiscal 2023 as a result of the Company amending the minimum purchase commitment that was previously reserved under the Lonza Agreement.
Gain on Sale of Vyleesi – On December 19, 2023, the Company entered into an asset purchase agreement (the “Cosette Purchase Agreement”) with Cosette pursuant to which Cosette acquired from the Company worldwide rights to Vyleesi. As a result of the transaction, the Company recorded a gain of $7,781,844 on the sale of Vyleesi for the year ended June 30, 2024. The gain represents the upfront purchase price of $9,500,000 less the cost of net assets transferred to the purchaser.
Other Income (Expense)– Total other income (expense), net was ($7,239,992) for fiscal 2024 compared to $3,747,143 for fiscal 2023. For fiscal 2024, we recognized an increase in the fair value of warrant liabilities of $6,962,562, offering expense of $696,912 and interest expense of $17,114 offset by investment income of $376,843 and unrealized foreign currency gain of $59,753. For fiscal 2023, we recognized a decrease in the fair value of warrant liabilities of $4,620,911 and investment income of $691,981 offset by $1,115,765 of offering expenses, $429,971 of unrealized foreign currency loss and $20,013 of interest expense.
Income Tax Benefit – Income tax benefit for fiscal 2023 was $4,674,999 as a result of the Company selling New Jersey state net operating losses (“NOLs”) and R&D credits.
| 43 |
| Table of Contents |
Effects of Inflation - We do not believe that inflation has had a material impact on our business, revenues or operating results during the periods presented.
Liquidity and Capital Resources
Since inception, we have generally incurred net operating losses, primarily related to spending on our research and development programs. We have financed our net operating losses primarily through debt and equity financings and amounts received under collaborative and license agreements.
Our product candidates are at various stages of development and will require significant further research, development, and testing and some may never be successfully developed or commercialized. We may experience uncertainties, delays, difficulties, and expenses commonly experienced by early-stage biopharmaceutical companies, which may include unanticipated problems and additional costs relating to:
|
· |
the development and testing of products in animals and humans; |
|
· |
dependance on third party contractors and collaborators for part of our research and development; |
|
· |
ability to attract and retain experienced personnel; |
|
· |
product approval or clearance; |
|
· |
regulatory compliance; |
|
· |
good manufacturing practices (“GMP”) compliance; |
|
· |
intellectual property rights; |
|
· |
product introduction; |
|
· |
marketing, sales, and competition; and |
|
· |
obtaining sufficient capital. |
Failure to enter into or successfully perform under collaboration agreements and obtain timely regulatory approval for our product candidates and indications would impact our ability to generate revenues and could make it more difficult to attract investment capital for funding our operations. Any of these possibilities could materially and adversely affect our operations and require us to curtail or cease certain programs.
During fiscal 2024, net cash used in operating activities was $31,461,441 compared to net cash used in operating activities of $29,271,346 in fiscal 2023. The increase in cash used in operations in fiscal 2024 compared with fiscal 2023 was a result of a higher net loss in fiscal 2023 due to a change in fair value off warrant liabilities, offset by the gain on the sale of Vyleesi and working capital changes.
During fiscal 2024, net cash provided by investing activities was $12,450,364 which consisted of $9,500,000 related to proceeds from the sale of Vyleesi and $2,992,890 for the maturity of marketable securities offset by $42,526 used for the purchases of property and equipment. During fiscal 2023, net cash used in investing activities was $3,426,757 which consisted of $2,992,830 used for the purchase of marketable securities and $433,927 of leasehold improvements.
During fiscal 2024, net cash provided by financing activities was $20,548,891 which consisted of proceeds from the sale of common stock and warrants, net of issuance costs of $14,693,779 and the exercise of outstanding warrants of $6,045,642 offset by payment of withholding taxes related to restricted stock units of $56,401, and payment of finance lease obligations of $106,392. During fiscal 2023, net cash provided by financing activities was $10,748,591 which consisted of proceeds from the sale of common stock and warrants, net of issuance costs of $10,995,497 and the exercise of outstanding warrants of $78 offset by payment of withholding taxes related to restricted stock units of $146,062, and payment of finance lease obligations of $100,922.
We had a net loss for fiscal 2024 of $29,736,113. We may not attain profitability in future years, which is dependent on numerous factors, including, but not limited to whether and when development and sales milestones are met, regulatory actions by the FDA and other regulatory bodies, the performance of our licensees, and market acceptance of our products.
We expect to incur significant expenses as we continue to develop our MCr product candidates. These expenses, among other things, have had and will continue to have an adverse effect on our stockholders’ equity, total assets, and working capital.
We have incurred cumulative negative cash flows from operations since our inception, and have expended, and expect to continue to expend in the future, substantial funds to complete our planned product development efforts. Continued operations are dependent upon existing licenses, including royalties and milestones, to complete equity or debt financing activities and enter into additional licensing or collaboration arrangements. As of June 30, 2024, our cash, cash equivalents and marketable securities were $9,527,396 with current liabilities of $9,657,681.
| 44 |
| Table of Contents |
Our obligations include aggregate lease obligations of $426,556 for the year ending June 30, 2025 and $163,782 for the years ending June 30, 2026 and 2027, and aggregate inventory purchase commitments of $1,976,450 which include $944,150 in current liabilities as of June 30, 2024 and $1,032,300 included in other long-term liabilities.
We intend to utilize existing capital resources for general corporate purposes and working capital, including preclinical and clinical development of our MC1r and MC4r programs, and development of other portfolio products.
Based on our June 30, 2024, cash and cash equivalents, we have concluded that substantial doubt exists about our ability to continue as a going concern for one year from the date our consolidated financial statements are issued. We are evaluating strategies to obtain additional funding for future operations which include but are not limited to obtaining equity financing, issuing debt, or reducing planned expenses. A failure to raise additional funding or to effectively implement cost reductions could harm our business, results of operations, and future prospects. If we are not able to secure adequate additional funding in future periods, we would be forced to make additional reductions in certain expenditures. This may include liquidating assets and suspending or curtailing planned programs. We may also have to delay, reduce the scope of, suspend, or eliminate one or more research and development programs or its commercialization efforts or pursue a strategic transaction. If we are unable to raise capital when needed or enter into a strategic transaction, then we may be required to cease operations, which could cause our stockholders to lose all or part of their investment. Based on our current existing cash and cash equivalents as of the date of this filing will be sufficient to fund currently anticipated operating expenses through the second half of calendar year 2024.
We will need additional funding to complete required clinical trials for our product candidates and development programs and, if those clinical trials are successful (which we cannot predict), to complete submission of required regulatory applications to the FDA. However, the COVID-19 pandemic and its resulting impact to economic conditions may negatively impact our operations, including possible effects on our financial condition, ability to access the capital markets on attractive terms or at all, liquidity, operations, suppliers, industry, and workforce. We will continue to evaluate the impact that these events could have on the operations, financial position, and the results of operations and cash flows during fiscal year 2025 and beyond.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Not applicable.
| 45 |
| Table of Contents |
Item 8. Financial Statements and Supplementary Data.
Table of Contents
Consolidated Financial Statements
The following consolidated financial statements are filed as part of this Annual Report:
|
|
Page |
|
|
|
|
|
|
47 |
|
|
|
|
|
|
|
48 |
|
|
|
|
|
|
|
49 |
|
|
|
|
|
|
|
50 |
|
|
|
|
|
|
|
51 |
|
|
|
|
|
|
|
52 |
|
| 46 |
| Table of Contents |
Report of Independent Registered Public Accounting Firm
To the To the Stockholders and Board of Directors
Palatin Technologies, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Palatin Technologies, Inc. and subsidiary (the Company) as of June 30, 2024 and 2023, the related consolidated statements of operations, changes in redeemable convertible preferred stock and stockholders’ (deficiency) equity, and cash flows forthe years then ended, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2024 and 2023, and the results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company hasincurred operating losses and negative cash flows from operations since inception and will need additional funding to complete planned product development efforts that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Evaluation of accrued external research and development expenses
As discussed in Notes 2 and 13 to the consolidated financial statements, the costs of research and development activities are charged to expense as incurred, which includes accrued external research and development expenses incurred under contracts with third parties. At the end of each quarter, the Company reviews the activities performed under all contracts and accrues expenses based upon the estimated amount of work completed considering milestones achieved. Accrued external research and development expenses were comprised of accrued clinical/regulatory costs and other research related expenses of $1,509,797 and $65,972, respectively as of June 30, 2024.
We identified the evaluation of the sufficiency of audit evidence over accrued external research and development expenses as a critical audit matter. Evaluating the sufficiency of audit evidence obtained over accrued external research and development expenses, including the estimated amount of work completed by third parties, required subjective auditor judgement due to the nature and extent of evidence available.
The following are the primary procedures we performed to address this critical audit matter. We applied auditor judgment to determine the nature and extent of procedures to be performed over accrued external research and development expenses. For a sample of accrued external research and development expenses, we evaluated management’s estimate of the amount of work remaining to be completed by comparing it to relevant third-party contracts, invoices, and communications. For a selection of third-party invoices and communications received after year-end, we compared the amounts to the relevant estimate of costs incurred or estimate of the amount of work completed by third parties as determined by management. We evaluated the sufficiency of audit evidence obtained by assessing the results of procedures performed, including the appropriateness of the nature and extent of such evidence.
/s/ KPMG LLP |
|
|
|
|
We have served as the Company’s auditor since 2002.
Philadelphia, Pennsylvania
September 30, 2024
| 47 |
| Table of Contents |
PALATIN TECHNOLOGIES, INC. | ||||||||
and Subsidiary | ||||||||
Consolidated Balance Sheets | ||||||||
|
|
|
|
|
|
|
||
|
|
June 30, 2024 |
|
|
June 30, 2023 |
|
||
ASSETS |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ | 9,527,396 |
|
|
$ | 7,989,582 |
|
Marketable securities |
|
|
- |
|
|
|
2,992,890 |
|
Accounts receivable |
|
|
- |
|
|
|
2,915,760 |
|
Inventories |
|
|
- |
|
|
|
526,000 |
|
Prepaid expenses and other current assets |
|
|
242,272 |
|
|
|
1,897,281 |
|
Total current assets |
|
|
9,769,668 |
|
|
|
16,321,513 |
|
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
|
388,361 |
|
|
|
684,910 |
|
Right-of-use assets - operating leases |
|
|
527,321 |
|
|
|
876,101 |
|
Other assets |
|
|
56,916 |
|
|
|
56,916 |
|
Total assets |
|
$ | 10,742,266 |
|
|
$ | 17,939,440 |
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ DEFICIENCY |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
$ | 4,101,929 |
|
|
$ | 4,303,527 |
|
Accrued expenses |
|
|
4,185,046 |
|
|
|
6,511,059 |
|
Short-term operating lease liabilities |
|
|
380,542 |
|
|
|
354,052 |
|
Short-term finance lease liabilities |
|
|
46,014 |
|
|
|
106,392 |
|
Other current liabilities |
|
|
944,150 |
|
|
|
3,856,800 |
|
Total current liabilities |
|
|
9,657,681 |
|
|
|
15,131,830 |
|
|
|
|
|
|
|
|
|
|
Long-term operating lease liabilities |
|
|
163,782 |
|
|
|
544,323 |
|
Long-term finance lease liabilities |
|
|
- |
|
|
|
46,014 |
|
Other long-term liabilities |
|
|
1,032,300 |
|
|
|
2,083,200 |
|
Warrant liabilities |
|
|
- |
|
|
|
1,850,544 |
|
Total liabilities |
|
|
10,853,763 |
|
|
|
19,655,911 |
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (Note 14) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingently redeemable warrants |
|
|
- |
|
|
|
263,400 |
|
|
|
|
|
|
|
|
|
|
Stockholders’ deficiency: |
|
|
|
|
|
|
|
|
Preferred stock of $0.01 par value – authorized 10,000,000 shares: shares issued and outstanding designated as follows: |
|
|
|
|
|
|
|
|
Series A Convertible: authorized 4,030 shares as of June 30, 2024: issued and outstanding 4,030 shares as of June 30, 2024 and June 30, 2023 |
|
|
40 |
|
|
|
40 |
|
Common stock of $0.01 par value – authorized 300,000,000 shares: |
|
|
|
|
|
|
|
|
issued and outstanding 17,926,640 shares as of June 30, 2024 and 11,656,714 shares as of June 30, 2023 |
|
|
179,266 |
|
|
|
116,567 |
|
Additional paid-in capital |
|
|
441,475,747 |
|
|
|
409,933,959 |
|
Accumulated deficit |
|
|
(441,766,550 | ) |
|
|
(412,030,437 | ) |
Total stockholders’ deficiency |
|
|
(111,497 | ) |
|
|
(1,979,871 | ) |
Total liabilities and stockholders’ deficiency |
|
$ | 10,742,266 |
|
|
$ | 17,939,440 |
|
The accompanying notes are an integral part of these consolidated financial statements
| 48 |
| Table of Contents |
PALATIN TECHNOLOGIES, INC. | ||||||||
and Subsidiary | ||||||||
Consolidated Statements of Operations | ||||||||
|
|
|
|
|
|
|
||
|
|
Year Ended June 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
|
|
|
|
|
|
|
||
REVENUES |
|
|
|
|
|
|
||
Product revenue, net |
|
$ | 4,490,090 |
|
|
$ | 4,850,678 |
|
License and contract |
|
|
- |
|
|
|
3,000 |
|
Total revenues |
|
|
4,490,090 |
|
|
|
4,853,678 |
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
Cost of products sold |
|
|
97,637 |
|
|
|
418,470 |
|
Research and development |
|
|
22,400,372 |
|
|
|
22,630,577 |
|
Selling, general and administrative |
|
|
12,270,046 |
|
|
|
15,290,836 |
|
Gain on sale of Vyleesi |
|
|
(7,781,844 | ) |
|
|
- |
|
Gain on purchase commitment |
|
|
- |
|
|
|
(1,027,322 | ) |
Total operating expenses |
|
|
26,986,211 |
|
|
|
37,312,561 |
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
|
(22,496,121 | ) |
|
|
(32,458,883 | ) |
|
|
|
|
|
|
|
|
|
OTHER INCOME (EXPENSE) |
|
|
|
|
|
|
|
|
Investment income |
|
|
376,843 |
|
|
|
691,981 |
|
Foreign currency gain (loss) |
|
|
59,753 |
|
|
|
(429,971 | ) |
Interest expense |
|
|
(17,114 | ) |
|
|
(20,013 | ) |
Offering expenses |
|
|
(696,912 | ) |
|
|
(1,115,765 | ) |
Change in fair value of warrant liabilities |
|
|
(6,962,562 | ) |
|
|
4,620,911 |
|
Total other income (expense), net |
|
|
(7,239,992 | ) |
|
|
3,747,143 |
|
|
|
|
|
|
|
|
|
|
Loss before income taxes |
|
|
(29,736,113 | ) |
|
|
(28,711,740 | ) |
Income tax benefit |
|
|
- |
|
|
|
4,674,999 |
|
NET LOSS |
|
$ | (29,736,113 | ) |
|
$ | (24,036,741 | ) |
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per common share |
|
$ | (2.02 | ) |
|
$ | (2.21 | ) |
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding used in computing basic and diluted net loss per common share |
|
|
14,697,096 |
|
|
|
10,890,159 |
|
The accompanying notes are an integral part of these consolidated financial statements
| 49 |
| Table of Contents |
PALATIN TECHNOLOGIES, INC. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
and Subsidiary | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Consolidated Statements of Changes in Redeemable Convertible Preferred Stock and Stockholders’ (Deficiency) Equity | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
|
|
|
|
Redeemable Convertible Preferred Stock |
|
|
Stockholders' Equity |
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
|
Contigently redeemable |
|
|
Series B |
|
|
Series C |
|
|
Escrowed |
|
|
Series A Convertible Preferred Stock |
|
|
Common Stock |
|
|
Additional paid-in |
|
|
Accumulated |
|
|
|
||||||||||||||||||||||||||||||
|
|
warrants |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
|
|
Amount |
|
|
Proceeds |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Total |
|
|||||||||||||||
Balance, June 30, 2022 |
|
|
- |
|
|
|
8,100,000 |
|
|
$ | 13,500,000 |
|
|
|
900,000 |
|
|
|
|
|
$ | 1,500,000 |
|
|
$ | (15,000,000 | ) |
|
|
4,030 |
|
|
$ | 40 |
|
|
|
9,270,947 |
|
|
$ | 92,709 |
|
|
$ | 404,168,822 |
|
|
$ | (387,993,696 | ) |
|
$ | 16,267,875 |
|
|
Stock-based compensation |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
84,062 |
|
|
|
841 |
|
|
|
1,673,496 |
|
|
|
|
|
|
|
1,674,337 |
|
|
Withholding taxes related to restricted stock units |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(20,468 | ) |
|
|
(205 | ) |
|
|
(145,857 | ) |
|
|
- |
|
|
|
(146,062 | ) | |
Redemption of convertible series B & series C preferred stock |
|
|
- |
|
|
|
(8,100,000 | ) |
|
|
(13,500,000 | ) |
|
|
(900,000 | ) |
|
|
|
|
|
(1,500,000 | ) |
|
|
15,000,000 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Sale of common stock and warrants, net of costs |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,524,034 |
|
|
|
15,240 |
|
|
|
1,184,149 |
|
|
|
- |
|
|
|
1,199,389 |
|
|
Conversion of liability classified warrants |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,324,653 |
|
|
|
|
|
|
|
3,324,653 |
|
|
Warrant exercises |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
798,182 |
|
|
|
7,982 |
|
|
|
(7,904 | ) |
|
|
- |
|
|
|
78 |
|
|
Reverse stock split fractional shares |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(43 | ) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Reclassification of contingently redeemable warrants |
|
|
263,400 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(263,400 | ) |
|
|
- |
|
|
|
(263,400 | ) | |
Net loss |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(24,036,741 | ) |
|
|
(24,036,741 | ) | |
Balance, June 30, 2023 |
|
|
263,400 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
4,030 |
|
|
|
40 |
|
|
|
11,656,714 |
|
|
|
116,567 |
|
|
|
409,933,959 |
|
|
|
(412,030,437 | ) |
|
|
(1,979,871 | ) | |
Stock-based compensation |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
98,372 |
|
|
|
984 |
|
|
|
1,871,714 |
|
|
|
- |
|
|
|
1,872,698 |
|
|
Withholding taxes related to restricted stock units |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(25,467 | ) |
|
|
(255 | ) |
|
|
(56,146 | ) |
|
|
- |
|
|
|
(56,401 | ) | |
Sale of common stock, net of costs |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
2,048,530 |
|
|
|
20,485 |
|
|
|
9,642,990 |
|
|
|
- |
|
|
|
9,663,475 |
|
|
Conversion of liability classified warrants |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
11,423,203 |
|
|
|
- |
|
|
|
11,423,203 |
|
|
Conversion of liability classified warrants upon warrant exercise |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
2,358,491 |
|
|
|
23,585 |
|
|
|
2,366,318 |
|
|
|
- |
|
|
|
2,389,903 |
|
|
Warrant exercises |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
- |
|
|
|
3,233,277 |
|
|
|
32,333 |
|
|
|
6,015,876 |
|
|
|
- |
|
|
|
6,048,209 |
|
|
Shares held in abeyance |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(1,443,277 |
) |
|
|
(14,433 |
) |
|
|
14,433 |
|
|
|
- |
|
|
|
- |
|
|
Reclassification of contingently redeemable warrants |
|
|
(263,400 | ) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
263,400 |
|
|
|
- |
|
|
|
263,400 |
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(29,736,113 | ) |
|
|
(29,736,113 | ) | |
Balance June 30, 2024 |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
4,030 |
|
|
$ | 40 |
|
|
|
17,926,460 |
|
|
$ | 179,266 |
|
|
$ | 441,475,747 |
|
|
$ | (441,766,550 | ) |
|
$ | (111,497 | ) |
The accompanying notes are an integral part of these consolidated financial statements
| 50 |
| Table of Contents |
PALATIN TECHNOLOGIES, INC. | ||||||||
and Subsidiary | ||||||||
Consolidated Statements of Cash Flows | ||||||||
|
|
|
|
|
|
|
||
|
|
Year Ended June 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
||
Net loss |
|
$ | (29,736,113 | ) |
|
$ | (24,036,741 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
339,075 |
|
|
|
288,331 |
|
Decrease in right-of-use asset |
|
|
348,780 |
|
|
|
371,339 |
|
Unrealized foreign currency transaction gain (loss) |
|
|
(59,753 | ) |
|
|
429,971 |
|
Stock-based compensation |
|
|
1,872,698 |
|
|
|
1,674,337 |
|
Change in fair value of liability classified warrants |
|
|
6,962,562 |
|
|
|
(4,620,911 | ) |
Gain on sale of Vyleesi |
|
|
(7,781,844 | ) |
|
|
- |
|
Gain on purchase commitment |
|
|
- |
|
|
|
(1,027,322 | ) |
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
2,915,760 |
|
|
|
(1,135,740 | ) |
Prepaid expenses and other assets |
|
|
1,218,846 |
|
|
|
35,173 |
|
Inventories |
|
|
(1,154,355 | ) |
|
|
418,471 |
|
Accounts payable |
|
|
(201,598 | ) |
|
|
1,109,541 |
|
Accrued expenses |
|
|
(2,367,651 | ) |
|
|
(364,157 | ) |
Operating lease liabilities |
|
|
(354,051 | ) |
|
|
(371,122 | ) |
Other liabilities |
|
|
(3,463,797 | ) |
|
|
(2,042,516 | ) |
Net cash used in operating activities |
|
|
(31,461,441 | ) |
|
|
(29,271,346 | ) |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
Maturity of marketable securities |
|
|
2,992,890 |
|
|
|
- |
|
Purchase of marketable securities |
|
|
|
|
|
|
(2,992,890 | ) |
Proceeds from sale of Vyleesi |
|
|
9,500,000 |
|
|
|
- |
|
Purchases of property and equipment |
|
|
(42,526 | ) |
|
|
(433,927 | ) |
Net cash provided by (used in) investing activities |
|
|
12,450,364 |
|
|
|
(3,426,817 | ) |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
Payment of withholding taxes related to restricted stock units |
|
|
(56,401 | ) |
|
|
(146,062 | ) |
Proceeds from the sale of common stock and warrants, net |
|
|
14,666,042 |
|
|
|
10,995,497 |
|
Payment of finance lease obligations |
|
|
(106,392 | ) |
|
|
(100,922 | ) |
Proceeds from exercise of warrants |
|
|
6,045,642 |
|
|
|
78 |
|
Net cash provided by financing activities |
|
|
20,548,891 |
|
|
|
10,748,591 |
|
|
|
|
|
|
|
|
|
|
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS |
|
|
1,537,814 |
|
|
|
(21,949,572 | ) |
|
|
|
|
|
|
|
|
|
CASH AND CASH EQUIVALENTS, beginning of period |
|
|
7,989,582 |
|
|
|
29,939,154 |
|
|
|
|
|
|
|
|
|
|
CASH AND CASH EQUIVALENTS, end of period |
|
$ | 9,527,396 |
|
|
$ | 7,989,582 |
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
|
Cash paid for interest |
|
$ | 17,114 |
|
|
$ | 20,013 |
|
Conversion of liability classified warrants |
|
|
11,423,203 |
|
|
|
- |
|
Conversion of liability classified warrants upon warrant exercise |
|
|
2,389,903 |
|
|
|
- |
|
The accompanying notes are an integral part of these consolidated financial statements
| 51 |
| Table of Contents |
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(1) ORGANIZATION
Nature of Business- Palatin Technologies, Inc. (“Palatin” or the “Company”) is a biopharmaceutical company developing first-in-class medicines based on molecules that modulate the activity of the melanocortin receptor system. The Company’s product candidates are targeted, receptor-specific therapeutics for the treatment of diseases with significant unmet medical need and commercial potential.
Melanocortin Receptor System. The melanocortin receptor system has effects on food intake, metabolism, sexual function, inflammation, and immune system responses. There are five melanocortin receptors, MC1r through MC5r. Modulation of these receptors, through use of receptor-specific agonists, which activate receptor function, or receptor-specific antagonists, which block receptor function, can have significant pharmacological effects.
The Company’s prior commercial product, Vyleesi®, was approved by the U.S. Food and Drug Administration (“FDA”) in June 2019 for the treatment of hypoactive sexual desire disorder (“HSDD”) in premenopausal women. As disclosed in Note 4, this product was acquired by Cosette Pharmaceuticals, Inc. (“Cosette”) on December 19, 2023.
Our new product development activities focus primarily on use of bremelanotide, or other MC4r agonists, with tirzepatide, a GLP-1 agonist for treatment of obesity, which entered Phase 2 in the second quarter of calendar year 2024, and a co-formulation of bremelanotide with a PDE5i for treatment of erectile dysfunction in patients that do not respond to PDE5i monotherapy.
The Company is also developing, dependent on resources for development activities, MC1r agonist products, with potential to treat inflammatory and autoimmune diseases, such as dry eye disease, which is also known as keratoconjunctivitis sicca, uveitis, diabetic retinopathy, and inflammatory bowel disease. The Company believes that the MC1r agonist peptides in development have broad anti-inflammatory effects and appear to utilize mechanisms engaged by the endogenous melanocortin system in regulation of the immune system and resolution of inflammatory responses. The Company is also developing peptides that are active at more than one melanocortin receptor, and MC4r peptide and small molecule agonists with potential utility in obesity and metabolic-related disorders, including rare disease and orphan indications.
Business Risks and Liquidity –The Company has incurred operating losses and negative cash flows from operations since inception and will need additional funding to complete its planned product development efforts. As shown in the accompanying consolidated financial statements, the Company had an accumulated deficit as of June 30, 2024 of $441,766,550 and a net loss for the year ended June 30, 2024 of $29,736,113. The Company anticipates incurring significant expenses in the future as a result of spending on its development programs and will require substantial additional financing or revenues to continue to fund its planned activities. To achieve sustained profitability, if ever, the Company, alone or with others, must successfully develop and commercialize its technologies and proposed products, conduct successful preclinical studies and clinical trials, obtain required regulatory approvals, and successfully manufacture and market such technologies and proposed products. The time required to reach sustained profitability is highly uncertain, and the Company may never be able to achieve profitability on a sustained basis, if at all.
As of June 30, 2024, the Company’s cash and cash equivalents were $9,527,396 and current liabilities were $9,657,681. Management intends to utilize existing capital resources for general corporate purposes and working capital, including clinical development of the Company’s MC1r and MC4r programs, and development of other portfolio products.
The Company follows the provisions of Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 205-40, Presentation of Financial Statements — Going Concern, which requires management to assess the Company’s ability to continue as a going concern for one year after the date the consolidated financial statements are issued. While the Company has raised funding in the past, the ability to raise funding in future periods is not considered probable, as defined under the accounting standards. As such, under the requirements of ASC 205-40, management may not consider the potential for future funding in their assessment of the Company’s ability to meet its obligations for the next year.
| 52 |
| Table of Contents |
Based on our available cash and cash equivalents as of June 30, 2024, management has concluded that substantial doubt exists about the Company’s ability to continue as a going concern for one year from the date these consolidated financial statements are issued. The Company is evaluating strategies to obtain additional funding for future operations which include but are not limited to obtaining equity financing, issuing debt, or reducing planned expenses. A failure to raise additional funding or to effectively implement cost reductions could harm the Company’s business, results of operations, and future prospects. If the Company is not able to secure adequate additional funding in future periods, the Company would be forced to make additional reductions in certain expenditures. This may include liquidating assets and suspending or curtailing planned programs. The Company may also have to delay, reduce the scope of, suspend, or eliminate one or more research and development programs or its commercialization efforts or pursue a strategic transaction. If the Company is unable to raise capital when needed or enter into a strategic transaction, then the Company may be required to cease operations, which could cause its stockholders to lose all or part of their investment. The consolidated financial statements have been prepared assuming the Company will continue as a going concern, which contemplates the continuity of operations, the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. Assuming no additional funding and based on its current operating and development plans, the Company expects that existing cash and cash equivalents as of the date of this filing will be sufficient to fund currently anticipated operating expenses through the second half of calendar year 2024.
The Company may receive contingent, sales-based milestone payments of up to $159,000,000 on sales of Vyleesi by Cosette Pharmaceuticals, Inc. (“Cosette”) and its licensees.
Concentrations – Concentrations in the Company’s assets and operations subject it to certain related risks. Financial instruments that subject the Company to concentrations of credit risk primarily consist of cash, cash equivalents, and accounts receivable. The Company’s cash and cash equivalents are primarily invested in one investment account sponsored by a large financial institution.
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation – The consolidated financial statements include the accounts of the Company and its wholly-owned inactive subsidiary. All intercompany accounts and transactions have been eliminated in consolidation.
Revision of Previously Issued Financial Statements - The Company has revised certain prior period amounts on the consolidated financial statements to correct a misstatement with respect to improperly classifying warrants as equity instead of as a warrant liability that is adjusted to the income statement each quarter to reflect changes in the fair value of the warrants, under the guidance of ASC 815-40, Contracts in Entity’s Own Equity. The Company recorded an adjustment to record a liability for the warrants of $1,850,544 million as of June 30, 2023, and adjusted contingently redeemable warrants for $263,400, decreased additional paid-in capital for $5,619,090 and increased accumulated deficit for $3,505,146.
The Company also recorded a gain of $4,620,911 as a result in the change in fair value of the warrant liabilities for the year ended June 30, 2023. The Company recorded $1,115,765 of offering expenses for the year ended June 30, 2023. As a result of these adjustments, the cash flow from operations decreased by $852,345 and cash flows from financing activities increased by $852,345 for the year ended June 30, 2023.
Use of Estimates – The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash, Cash Equivalents – Cash and cash equivalents include cash on hand, cash in banks, and all highly liquid investments with a purchased maturity of less than three months. Cash equivalents consist of $9,089,113 in a money market accounts and $5,789,218 in money market and treasury bills at June 30, 2024 and 2023, respectively.
Marketable Securities - The Company’s marketable securities consist of debt securities with original maturities of greater than 90 days that are classified as available for sale securities.
Fair Value of Financial Instruments – The Company’s financial instruments consist primarily of cash equivalents, marketable securities, accounts receivable, and accounts payable. Management believes that the carrying values of cash equivalents, accounts receivable, and accounts payable are representative of their respective fair values based on the short-term nature of these instruments.
| 53 |
| Table of Contents |
Credit Risk – Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash, cash equivalents, and accounts receivable. Total cash and cash equivalent balances have exceeded balances insured by the Federal Depository Insurance Company. Currently, product revenues and related accounts receivable are generated primarily from one specialty pharmacy.
Trade Accounts Receivable - Trade accounts receivable are amounts owed to the Company by its customers for product that has been delivered. The trade accounts receivable is recorded at the invoice amount, less prompt pay and other discounts, chargebacks, and an allowance for credit losses, if any. Credit losses have not been significant to date.
Inventories– Inventory is stated at the lower of cost or net realizable value, with cost being determined on a first-in, first-out basis.
On a quarterly basis, the Company reviews inventory levels to determine whether any obsolete, expired, or excess inventory exists. If any inventory is expected to expire prior to being sold, has a cost basis in excess of its net realizable value, is in excess of expected sales requirements as determined by internal sales forecasts, or fails to meet commercial sale specifications, the inventory is written down through a charge to operating expenses. Inventory consisting of Vyleesi has a shelf-life of three years from the date of manufacture.
Property and Equipment – Property and equipment consists of office and laboratory equipment, office furniture, and leasehold improvements and includes assets acquired under finance leases. Property and equipment are recorded at cost. Depreciation is recognized using the straight-line method over the estimated useful lives of the related assets, generally five years for laboratory and computer equipment, seven years for office furniture and equipment, and the lesser of the term of the lease or the useful life for leasehold improvements. Amortization of assets acquired under finance leases is included in depreciation expense. Maintenance and repairs are expensed as incurred while expenditures that extend the useful life of an asset are capitalized.
Impairment of Long-Lived Assets – The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. To determine recoverability of a long-lived asset, management evaluates whether the estimated future undiscounted net cash flows from the asset are less than its carrying amount. If impairment is indicated, the long-lived asset would be written down to fair value. Fair value is determined by an evaluation of available price information at which assets could be bought or sold, including quoted market prices, if available, or the present value of the estimated future cash flows based on reasonable and supportable assumptions.
Leases - At lease inception, the Company determines whether an arrangement is or contains a lease. Operating leases are included in operating lease right-of-use (“ROU”) assets, short-term operating lease liabilities, and long-term operating lease liabilities in the consolidated financial statements. Finance leases are included in property and equipment for ROU assets, short-term finance lease liabilities, and long-term finance lease liabilities in the consolidated financial statements. ROU assets represent the Company’s right to use leased assets over the term of the lease. Lease liabilities represent the Company’s contractual obligation to make lease payments over the lease term. ROU assets and lease liabilities are recognized at the commencement date. The lease liability is measured as the present value of the lease payments over the lease term. The Company uses the rate implicit in the lease if it is determinable. When the rate implicit in the lease is not determinable, the Company uses an estimate based on a hypothetical rate provided by a third party as the Company currently does not have issued debt. Lease terms may include renewal or extension options to the extent they are reasonably certain to be exercised. The assessment of whether renewal or extension options are reasonably certain to be exercised is made at lease commencement. Factors considered in determining whether an option is reasonably certain of exercise include, but are not limited to, the value of any leasehold improvements, the value of renewal rates compared to market rates, and the presence of factors that would cause incremental costs to the Company if the option were not exercised.
The ROU asset is initially measured at cost, which comprises the initial amount of the lease liability adjusted for lease payments made at or before the lease commencement date, plus any initial direct costs incurred less any lease incentives received. For operating leases, the ROU asset is subsequently measured throughout the lease term at the carrying amount of the lease liability, plus initial direct costs, plus (minus) any prepaid (accrued) lease payments, less the unamortized balance of lease incentives received. Lease expense for lease payments is recognized on a straight-line basis over the lease term. For finance leases, the ROU asset is subsequently amortized using the straight-line method from the lease commencement date to the earlier of the end of its useful life or the end of the lease term unless the lease transfers ownership of the underlying asset to the Company or the Company is reasonably certain to exercise an option to purchase the underlying asset. In those cases, the ROU asset is amortized over the useful life of the underlying asset. Amortization of the ROU asset is recognized and presented as an operating expense separately from interest expense on the lease liability.
| 54 |
| Table of Contents |
The Company has elected not to recognize an ROU asset and obligation for leases with an initial term of twelve months or less. The expense associated with short-term leases is included in selling, general and administrative expense in the statements of operations. To the extent a lease arrangement includes both lease and non-lease components, the Company has elected to account for the components as a single lease component.
Revenue Recognition – The Company recognizes product revenues in accordance with FASB ASC Topic 606, Revenue from Contracts with Customers. The provisions of ASC Topic 606 require the following steps to determine revenue recognition: (1) Identify the contract(s) with a customer; (2) Identify the performance obligations in the contract; (3) Determine the transaction price; (4) Allocate the transaction price to the performance obligations in the contract; and (5) Recognize revenue when (or as) the entity satisfies a performance obligation.
In accordance with ASC Topic 606, the Company recognizes product revenue when its performance obligation is satisfied by transferring control of the product to a customer. Per the Company’s contracts with customers, control of the product is transferred upon the conveyance of title, which occurs when the product is sold to and received by a customer. Trade accounts receivable due to the Company from contracts with its customers are stated separately in the consolidated balance sheet, net of various allowances as described in the Trade Accounts Receivable policy above.
Product revenues consist of sales of Vyleesi in the United States. Prior to selling the Vyleesi product to Cosette in December 2023, the Company sold Vyleesi to specialty pharmacies at the wholesale acquisition cost and payment is currently made within approximately 30 days. In addition to distribution agreements with customers, the Company enters into arrangements with healthcare payers that provide for privately negotiated rebates, chargebacks, and discounts with respect to the purchase of the Company’s products.
The Company records product revenues net of allowances for direct and indirect fees, discounts, co-pay assistance programs, estimated chargebacks and rebates. Product sales are also subject to return rights, which have not been significant to date.
Gross product sales offset by product sales allowances for the years ended June 30, 2024 and 2023 are as follows:
|
|
Year Ended June 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
|
|
|
|
|
|
|
||
Gross product sales |
|
|
8,875,153 |
|
|
$ | 12,460,140 |
|
Product sales allowances and accruals |
|
|
(4,385,063 | ) |
|
|
(7,609,462 | ) |
Net sales |
|
$ | 4,490,090 |
|
|
$ | 4,850,678 |
|
For licenses of intellectual property, the Company assesses at contract inception whether the intellectual property is distinct from other performance obligations identified in the arrangement. If the licensing of intellectual property is determined to be distinct, revenue is recognized for nonrefundable, upfront license fees when the license is transferred to the customer and the customer can use and benefit from the license. If the licensing of intellectual property is determined not to be distinct, then the license is bundled with other promises in the arrangement into one performance obligation. The Company needs to determine if the bundled performance obligation is satisfied over time or at a point in time. If the Company concludes that the nonrefundable, upfront license fees will be recognized over time, the Company will need to assess the appropriate method of measuring proportional performance.
Regulatory milestone payments are excluded from the transaction price due to the inability to estimate the probability of reversal. Revenue relating to achievement of these milestones is recognized in the period in which the milestone is achieved.
Sales-based royalty and milestone payments resulting from customer contracts solely or predominately for the license of intellectual property will only be recognized upon occurrence of the underlying sale or achievement of the sales milestone in the future and such sales-based royalties and milestone payments will be recognized in the same period earned.
The Company recognizes revenue for reimbursements of research and development costs under collaboration agreements as the services are performed. The Company records these reimbursements as revenue and not as a reduction of research and development expenses as the Company is the principal in the research and development activities based upon its control of such activities, which is considered part of its ordinary activities.
| 55 |
| Table of Contents |
Development milestone payments are generally due 30 business days after the milestone is achieved. Sales milestone payments are generally due 45 business days after the calendar year in which the sales milestone is achieved. Royalty payments are generally due on a quarterly basis 20 business days after being invoiced.
Research and Development Costs – The costs of research and development activities are charged to expense as incurred, including the cost of equipment for which there is no alternative future use.
Accrued Expenses – Third parties perform a significant portion of the Company’s development activities. The Company reviews the activities performed under all contracts each quarter and accrues expenses and the amount of any reimbursement to be received from its collaborators based upon the estimated amount of work completed considering milestones achieved. Estimating the value or stage of completion of certain services requires judgment based on available information. If the Company does not identify services performed for it but not billed by the service-provider, or if it underestimates or overestimates the value of services performed as of a given date, reported expenses will be understated or overstated.
Stock-Based Compensation –The Company charges to expense the fair value of stock options and other equity awards granted to employees and nonemployees for services. Compensation costs for stock-based awards with time-based vesting are determined using the quoted market price of the Company’s common stock on the grant date or for stock options, the value determined utilizing the Black-Scholes option pricing model, and are recognized on a straight-line basis, while awards containing a market condition are valued using multifactor Monte Carlo simulations and are recognized over the derived service period. Compensation costs for awards containing a performance condition are determined using the quoted price of the Company’s common stock on the grant date or for stock options, the value determined utilizing the Black Scholes option pricing model and are recognized based on the probability of achievement of the performance condition over the service period. Forfeitures are recognized as they occur.
Income Taxes – The Company and its subsidiary file consolidated federal and separate-company state income tax returns. Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of assets and liabilities and their respective tax basis and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences or operating loss and tax credit carryforwards are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that includes the enactment date. The Company has recorded and continues to maintain a full valuation allowance against its deferred tax assets based on the history of losses incurred and lack of experience projecting future product revenue and sales-based royalty and milestone payments.
Net Loss per Common Share–Basic and diluted loss per common share (“EPS”) are calculated in accordance with the provisions of FASB ASC Topic 260, Earnings per Share.
For the years ended June 30, 2024 and 2023, no additional common shares were added to the computation of diluted EPS because to do so would have been anti-dilutive. The potential number of common shares excluded from diluted EPS during the year ended June 30, 2024 and June 30, 2023 was 11,127,632 and 4,161,377 respectively.
Included in the weighted average common shares used in computing basic and diluted net loss per common share are 508,011 and 356,003 vested restricted stock units that had not been issued as of June 30, 2024 and 2023, respectively, due to a provision in the restricted stock unit agreements to delay delivery.
Translation of foreign currencies– Transactions denominated in currencies other than the Company’s functional currency (US Dollar) are recorded based on exchange rates at the time such transactions arise. Subsequent changes in exchange rates result in transaction gains and losses, which are reflected in the consolidated statements of operations as unrealized (based on the applicable period-end exchange rate) or realized upon settlement of the transactions.
(3) NEW AND RECENTLY ADOPTED ACCOUNTING PRONOUNCEMENTS
In November 2023, the FASB issued Accounting Standards Update (“ASU”) 2023-07, Segment Reporting (Topic 280) – Improvements to Reportable Segment Disclosures. This ASU requires that a public entity provide additional segment disclosures on an interim and annual basis. The amendments in this ASU should be applied retrospectively to all prior periods presented in the financial statements, unless impracticable. Upon transition, the segment expense categories and amounts disclosed in the prior periods should be based on the significant segment expense categories identified and disclosed in the period of adoption. The ASU is effective for fiscal years beginning after December 15, 2023 and interim periods within fiscal years beginning after December 15, 2024.
| 56 |
| Table of Contents |
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments – Credit Losses: Measurement of Credit Losses on Financial Instruments, which requires measurement and recognition of expected credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. This is different from the current guidance as this will require immediate recognition of estimated credit losses expected to occur over the remaining life of many financial assets. The guidance was applicable to the Company beginning July 1, 2023. The adoption of this standard did not have an impact on the Company’s consolidated financial statements.
(4) ASSET PURCHASE AGREEMENT
On December 19, 2023, the Company entered into an asset purchase agreement (the “Cosette Purchase Agreement”) with Cosette pursuant to which Cosette acquired from the Company worldwide rights to Vyleesi®.
Under the terms of the Cosette Purchase Agreement, the Company sold certain assets (the “Purchased Assets”) to Cosette, comprising the exclusive right to market and sell Vyleesi for treatment of hypoactive sexual desire disorder in women, and contracts relating manufacturing and distribution of Vyleesi. The Purchased Assets include applicable intellectual property pertaining to the marketing and sale of Vyleesi, including patents, patent applications, trademarks and copyrights. In addition, Cosette acquired records pertaining to the historical sales and distribution of Vyleesi, as well as quality control and pharmacovigilance records and other records. The Company will receive up to $171,000,000, consisting of an upfront purchase price of $9,500,000, $2,500,000 payable upon the settlement of certain purchase commitments, and sales-based milestone payments of up to $159,000,000 based on annual net sales of from $15,000,000 to $20,000,000. The closing of the transaction took place simultaneously with the signing of the Cosette Purchase Agreement. As a result of the transaction, the Company recorded gain of $7,781,844 on the sale of Vyleesi for the year ended June 30, 2024.
The Cosette Purchase Agreement includes customary representations, warranties and covenants, as well as standard mutual indemnities covering losses arising from any material breach of the Cosette Purchase Agreement or inaccuracy of representations and warranties.
The parties also entered into a transition service agreement pursuant to which the Company provided certain transition services to Cosette for a period time and the Company was reimbursed for the costs of the transition services.
The Company is also eligible to receive regulatory approval milestones associated with the previous licensing of Vyleesi to Fosun for China (see Note 6) and Kwangdong for the Republic of Korea (“Korea”) (see Note 7).
(5) MANUFACTURING SUPPLY AGREEMENTS FOR VYLEESI
The Company has transferred to Cosette its right, title and interest in contracts and agreements to manufacture Vyleesi, including manufacturing contracts with Catalent Belgium S.A. (“Catalent”), a subsidiary of Catalent Pharma Solutions, Inc., to manufacture drug product and prefilled syringes and assemble prefilled syringes into an auto-injector device; Ypsomed AG (“Ypsomed”), to manufacture the auto-injector device (the “Ypsomed Agreement”); and Lonza Ltd. (“Lonza”), to manufacture the active pharmaceutical ingredient peptide (the “Lonza Agreement”).
(6) AGREEMENT WITH FOSUN
On September 6, 2017, the Company entered into a license agreement with Shanghai Fosun Pharmaceutical Industrial Development Co. Ltd. (“Fosun”) for exclusive rights to commercialize Vyleesi in China (the “Fosun License Agreement”). Under the terms of the Fosun License Agreement, the Company received $4,500,000 in October 2017, which consisted of an upfront payment of $5,000,000 less $500,000 that was withheld in accordance with tax withholding requirements in China and recorded as an expense during the year ended June 30, 2018. The Company has agreed to assign the Fosun License Agreement to Cosette, provided that the Company retains the right to receive a $7,500,000 milestone payment upon regulatory approval in China.
(7) AGREEMENT WITH KWANGDONG
On November 21, 2017, the Company entered into a license agreement with Kwangdong Pharmaceutical Co., Ltd. (“Kwangdong”) for exclusive rights to commercialize Vyleesi in Korea (the “Kwangdong License Agreement”). Under the terms of the Kwangdong License Agreement, the Company received $417,500 in December 2017, consisting of an upfront payment of $500,000, less $82,500, which was withheld in accordance with tax withholding requirements in Korea and recorded as an expense during the year ended June 30, 2018. The Company has agreed to assign the Kwangdong License Agreement to Cosette, provided that the Company retains the right to receive a $3,000,000 milestone payment based on the first commercial sale in Korea.
| 57 |
| Table of Contents |
(8) PREPAID EXPENSES AND OTHER CURRENT ASSETS
Prepaid expenses and other current assets consist of the following:
|
|
June 30, |
|
|
June 30, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Clinical / regulatory costs |
|
$ | 23,926 |
|
|
$ | 141,512 |
|
Insurance premiums |
|
|
71,097 |
|
|
|
342,645 |
|
Vyleesi contractual advances |
|
|
- |
|
|
|
816,750 |
|
Other |
|
|
147,249 |
|
|
|
596,374 |
|
|
|
$ | 242,272 |
|
|
$ | 1,897,281 |
|
(9) FAIR VALUE MEASUREMENTS
The fair value of cash equivalents is classified using a hierarchy prioritized based on inputs. Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities. Level 2 inputs are quoted prices for similar assets and liabilities in active markets or inputs that are observable for the asset or liability, either directly or indirectly through market corroboration, for substantially the full term of the financial instrument. Level 3 inputs are unobservable inputs based on management’s own assumptions used to measure assets and liabilities at fair value. A financial asset’s or liability’s classification within the hierarchy is determined based on the lowest level input that is significant to the fair value measurement.
The following table provides the assets carried at fair value:
|
|
Carrying Value |
|
|
Quoted prices in active markets (Level 1) |
|
|
Other quoted/observable inputs (Level 2) |
|
|
Significant unobservable inputs (Level 3) |
|
||||
June 30, 2024: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cash equivalents - Money market funds |
|
$ | 9,089,113 |
|
|
$ | 9,089,113 |
|
|
$ | - |
|
|
$ | - |
|
June 30, 2023: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents - Money market funds |
|
$ | 2,808,598 |
|
|
$ | 2,808,598 |
|
|
|
- |
|
|
|
- |
|
Cash equivalents - Treasury bill |
|
|
2,980,620 |
|
|
|
2,980,620 |
|
|
|
- |
|
|
|
- |
|
Marketable securities - Treasury bill |
|
|
2,992,890 |
|
|
|
2,992,890 |
|
|
|
- |
|
|
|
- |
|
Total |
|
$ | 8,782,108 |
|
|
$ | 8,782,108 |
|
|
$ | - |
|
|
$ | - |
|
(10) INVENTORIES
Inventories consist of raw materials and finished goods related to Vyleesi. The following table summarizes the components of inventories:
|
|
June 30, |
|
|
June 30, |
|
||
|
|
2024 |
|
|
2023 |
|
||
|
|
|
|
|
|
|
||
Raw materials |
|
$ | - |
|
|
$ | 526,000 |
|
Finished goods |
|
|
- |
|
|
|
- |
|
|
|
$ | - |
|
|
$ | 526,000 |
|
(11) LEASES
The Company has operating leases for office and laboratory space, which expire on June 30, 2025 and October 31, 2026, respectively.
| 58 |
| Table of Contents |
The components of operating lease cost are as follows:
Operating lease cost |
|
Year ended June 30, 2024 |
|
|
Year ended June 30, 2023 |
|
||
Operating lease cost |
|
$ | 263,859 |
|
|
$ | 291,878 |
|
Variable lease cost |
|
|
113,708 |
|
|
|
114,441 |
|
Total operating lease cost |
|
$ | 377,567 |
|
|
$ | 406,319 |
|
The components of finance lease cost are as follows:
Finance lease cost |
|
Year ended June 30, 2024 |
|
|
Year ended June 30, 2023 |
|
||
Right-of-use asset amortization |
|
$ | 106,390 |
|
|
$ | 100,922 |
|
Interest expense |
|
|
5,507 |
|
|
|
10,975 |
|
Total finance lease cost |
|
$ | 111,897 |
|
|
$ | 111,897 |
|
Supplemental lease term and discount rate information related to leases was as follows:
|
|
June 30, 2024 |
|
|
June 30, 2023 |
|
||
Weighted-average remaining lease term (years) operating leases |
|
|
1.7 |
|
|
|
2.3 |
|
Weighted-average remaining lease term (years) finance leases |
|
|
0.4 |
|
|
|
1.4 |
|
Weighted-average discount rate operating leases |
|
|
5.50 | % |
|
|
5.50 | % |
Weighted-average discount rate finance leases |
|
|
5.29 | % |
|
|
5.29 | % |
Supplemental cash flow information related to leases was as follows:
|
|
Year ended June 30, 2024 |
|
|
Year ended June 30, 2023 |
|
||
Cash paid for the amounts included in the measurement of lease liabilities: |
|
|
|
|
|
|
||
Operating cash flows for operating leases |
|
$ | 387,910 |
|
|
$ | 406,319 |
|
Operating cash flows for finance leases |
|
|
5,507 |
|
|
|
10,975 |
|
Financing cash flows for finance leases |
|
|
106,392 |
|
|
|
100,922 |
|
|
|
$ | 499,809 |
|
|
$ | 518,216 |
|
|
|
|
|
|
|
|
|
|
Supplemental non-cash information on lease liabilities arising from obtaining right-of-use assets: |
|
|
|
|
|
|
|
|
Right-of-use assets obtained in exchange for new operating lease obligation |
|
$ | - |
|
|
$ | 368,975 |
|
The following table summarizes the maturity of the Company’s lease liabilities as of June 30, 2024:
Operating leases: |
|
|
|
|
Year Ending June 30 |
|
|
|
|
2025 |
|
$ | 398,196 |
|
2026 |
|
|
134,973 |
|
2027 |
|
|
33,894 |
|
Less imputed interest |
|
|
(22,741 | ) |
Total |
|
$ | 544,322 |
|
|
|
|
|
|
Finance leases: |
|
|
|
|
Year Ending June 30, 2025 |
|
|
46,585 |
|
Less imputed interest |
|
|
(569 | ) |
Total |
|
$ | 46,016 |
|
| 59 |
| Table of Contents |
(12) PROPERTY AND EQUIPMENT, NET
Property and equipment, net, consists of the following:
|
|
June 30, |
|
|
June 30, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Office equipment |
|
$ | 1,229,300 |
|
|
$ | 1,229,300 |
|
Laboratory equipment |
|
|
1,220,395 |
|
|
|
1,177,868 |
|
Leasehold improvements |
|
|
1,196,706 |
|
|
|
1,196,706 |
|
|
|
|
3,646,401 |
|
|
|
3,603,874 |
|
Less: Accumulated depreciation and amortization |
|
|
(3,258,040 | ) |
|
|
(2,918,964 | ) |
|
|
$ | 388,361 |
|
|
$ | 684,910 |
|
Included in property and equipment, net as of June 30, 2024 is $309,791 in equipment under finance leases and $263,777 related accumulated amortization.
(13) ACCRUED EXPENSES
Accrued expenses consist of the following:
|
|
June 30, |
|
|
June 30, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Clinical / regulatory costs |
|
$ | 1,509,797 |
|
|
$ | 2,960,126 |
|
Other research related expenses |
|
|
65,972 |
|
|
|
121,121 |
|
Professional Services |
|
|
284,215 |
|
|
|
339,258 |
|
Personnel costs |
|
|
1,771,694 |
|
|
|
1,563,847 |
|
Selling expenses |
|
|
351,485 |
|
|
|
1,266,653 |
|
Other |
|
|
201,883 |
|
|
|
260,054 |
|
|
|
$ | 4,185,046 |
|
|
$ | 6,511,059 |
|
(14) COMMITMENTS AND CONTINGENCIES
Inventory Purchases –The Company had certain supply agreements with manufacturers and suppliers, including the Catalent Agreement, Ypsomed Agreement, and Lonza Agreement, all of which have been transferred to Cosette. As a result of the sale of Vyleesi to Cosette, the Company is still required to make certain payments for the manufacture and supply of Vyleesi.
The following table summarizes the contractual obligations under the Catalent Agreement, Ypsomed Agreement, and Lonza Agreement as of June 30, 2024:
|
|
Total |
|
|
Current |
|
|
1 - 3 Years |
|
|
4 - 5 Years |
|
||||
Inventory purchase commitments |
|
$ | 2,492,600 |
|
|
$ | 1,460,300 |
|
|
$ | 1,032,300 |
|
|
$ | - |
|
As of June 30, 2024, the Company has $944,150 and $1,032,300 accrued within other current and long-term liabilities, respectively, in the consolidated balance sheet related to estimated losses for firm commitment contractual obligations under these agreements. As of June 30, 2023, $3,856,800 and $2,083,200 was accrued within other current and long-term liabilities, respectively. Losses on these firm commitment contractual obligations are recognized based upon the terms of the respective agreement and similar factors considered for the write-down of inventory, including expected sales requirements as determined by internal sales forecasts.
The commitment contractual obligation amounts above are denominated in Swiss Francs and Euros and have been translated using period end exchange rates. The Company may experience a negative impact on future earnings and equity solely as a result of future foreign currency exchange rate fluctuations.
Employment Agreements – The Company has employment agreements with two executive officers which provide a stated annual compensation amount, subject to annual increases, and annual bonus compensation in an amount to be approved by the Company’s board of directors. Each agreement allows the Company or the employee to terminate the agreement in certain circumstances. In some circumstances, early termination by the Company may result in severance pay to the employee for a period of 18 to 24 months at the salary then in effect, continuation of health insurance premiums over the severance period and immediate vesting of all stock options and restricted stock units. Termination following a change in control will result in a lump sum payment of one and one-half to two times the salary then in effect and immediate vesting of all stock options and restricted stock units.
| 60 |
| Table of Contents |
Employee Retirement Savings Plan – The Company maintains a defined contribution 401(k) plan for the benefit of its employees. The Company currently matches a portion of employee contributions to the plan. For the years ended June 30, 2024 and 2023, Company contributions were $336,164 and $294,431, respectively.
Contingencies– The Company accounts for litigation losses in accordance with ASC 450-20, Loss Contingencies. In addition, the Company is subject to other contingencies, such as product liability, arising in the ordinary course of business. Loss contingency provisions are recorded for probable losses when management is able to reasonably estimate the loss. Any outcome upon settlement that deviates from the Company’s best estimate may result in additional expense or in a reduction in expense in a future accounting period. The Company records legal expenses associated with such contingencies as incurred.
The Company is involved, from time to time, in various claims and legal proceedings arising in the ordinary course of its business. The Company is not currently a party to any such claims or proceedings that, if decided adversely to it, would either individually or in the aggregate have a material adverse effect on its business, financial condition, or results of operations.
(15) REDEEMABLE CONVERTIBLE PREFERRED STOCK, ESCROWED PROCEEDS, AND STOCKHOLDERS’ EQUITY
Series B and C Redeemable Convertible Preferred Stock – On May 11, 2022, Palatin entered into a securities purchase agreement with institutional investors, and on May 12, 2022, Palatin issued and sold 8,100,000 shares of Series B Redeemable Convertible Preferred Stock (“Series B Preferred Stock”) and 900,000 shares of Series C Redeemable Convertible Preferred Stock (“Series C Preferred Stock”). Each share of Series B Preferred Stock and Series C Preferred Stock had a purchase price of $1.67. The investors in the Series B Preferred Stock and Series C Preferred Stock also received warrants to purchase up to 66,666 shares of common stock at an exercise price of $12.50 per share, which expire 48 months following issuance. Total gross proceeds from the offering, before expenses, was $15,000,000 which was deposited in an escrow account. The escrowed proceeds were presented as a deduction to the Series B Preferred Stock and Series C Preferred Stock on the Company’s consolidated balance sheet. In November 2022, the investors provided the Company with Notices of Redemption, electing to have the Series B and Series C Preferred Stock redeemed in cash. Accordingly, the Company and investors directed the escrow agent for the escrow account to release $15,750,000 to the investors, comprising the total gross proceeds from the offering of $15,000,000 and a fee of $750,000.
Given that the fee and other costs were not refundable to the Company as of June 30, 2022, regardless of the election selected by the investors, the $750,000 fee, the fair value of the warrants ($234,443), and other costs of $150,995 were recorded as expenses within selling, general and administrative expenses during the year ended June 30, 2022.
The Company called a meeting of stockholders on June 24, 2022 to seek approval of, among other things, an amendment to its certificate of incorporation authorizing a reverse stock split. Except as otherwise required by law, holders of the Series B Preferred Stock and Series C Preferred Stock were entitled to vote only on the reverse stock split and any adjournment of the meeting relating to the reverse stock split. The Company’s common stock, outstanding Series A Preferred Stock, the Series B Preferred Stock and the Series C Preferred Stock voted as a single class on an as-if converted basis. The holders of Series B Preferred Stock had votes equal to the number of shares of common stock into which the Series B Preferred Stock is convertible. The holders of Series C Preferred Stock were entitled to 20,000 votes per share of common stock into which the Series C Preferred Stock is convertible but could only vote in the same proportion as the shares of common stock, Series A preferred stock, and Series B preferred stock were voted on the reverse stock split or any adjournment of the stockholder meeting relating thereto. The holders of the Series B Preferred Stock agreed to vote in favor of the reverse stock split, which was approved and ultimately became effective on August 30, 2022.
Series A Convertible Preferred Stock – As of June 30, 2024, 4,030 shares of Series A Convertible Preferred Stock were outstanding. Each share of Series A Convertible Preferred Stock is convertible at any time, at the option of the holder, into the number of shares of common stock equal to $100 divided by the Series A Conversion Price. As of June 30, 2024, the Series A Conversion Price was $75.45, and each share of Series A Convertible Preferred Stock is convertible into approximately 1.33 shares of common stock. The Series A Conversion Price is subject to adjustment, under certain circumstances, upon the sale or issuance of common stock for consideration per share less than either (i) the Series A Conversion Price in effect on the date of such sale or issuance, or (ii) the market price of the common stock as of the date of such sale or issuance. The Series A Conversion Price is also subject to adjustment upon the occurrence of a merger, reorganization, consolidation, reclassification, stock dividend or stock split which will result in an increase or decrease in the number of shares of common stock outstanding. Shares of Series A Convertible Preferred Stock have a preference in liquidation, including certain merger transactions, of $100 per share, or $403,000 in the aggregate as of June 30, 2024. Additionally, the Company may not pay a dividend or make any distribution to holders of any class of stock unless the Company first pays a special dividend or distribution of $100 per share to holders of the Series A Convertible Preferred Stock.
| 61 |
| Table of Contents |
Financing Transactions –On January 29, 2024, the Company entered into a securities purchase agreement (the “January 2024 Purchase Agreement”) to sell in a registered direct offering (the “January 2024 RD Offering”), an aggregate of 1,831,503 shares of common stock, of the Company. Pursuant to the January 2024 Purchase Agreement, the Company issued to the investors in the January 2024 RD Offering unregistered warrants (the “January 2024 Private Warrants”) to purchase up to 1,831,503 shares of the Company’s common stock (the “January 2024 Private Warrant Shares”) in a concurrent private placement (the “January 2024 Private Offering” and together with the January 2024 RD Offering, the “January 2024 Offering”). The shares of common stock and accompanying January 2024 Private Warrants were offered at a combined offering price of $5.46.
The January 2024 Private Warrants are exercisable on the six-month anniversary of the issuance date for a period of four years from the issuance date, at an exercise price equal to $5.46 per January 2024 Private Warrant Share. The January 2024 Private Warrants are exercisable for cash, or, solely during any period when a registration statement for the issuance or resale of the January 2024 Private Warrant Shares issuable upon exercise of the January 2024 Private Warrants to or by the holder of such January 2024 Private Warrants is not in effect, on a cashless basis.
The Company paid the placement agent a cash fee equal to 7.0% of the aggregate gross proceeds of the January 2024 Offering and for certain expenses and legal fees in connection with the January 2024 Offering. In addition, the Company also issued to the placement agent or its designees warrants (the “January 2024 Placement Agent Warrants”) to purchase up to 91,575 shares of the Company’s common stock (the “January 2024 Placement Agent Warrant Shares”) as part of the compensation payable to the placement agent. The January 2024 Placement Agent Warrants have substantially the same terms as the January 2024 Private Warrants, except that the January 2024 Placement Agent Warrants have an exercise price of $6.825 per share.
On March 14, 2024, the Company filed a registration statement on Form S-1 to register the January 2024 Private Warrants and the January 2024 Placement Agent Warrants, which registration statement was declared effective on March 28, 2024 and a prospectus was filed on the same date.
The gross proceeds from the January 2024 Offering totaled $10,000,006, with net proceeds from the January 2024 Offering, after deducting the placement agent fees and offering expenses, amounting to $9,224,056. The Company intends to use the net proceeds received from the January 2024 Offering for general working capital purposes.
On October 20, 2023, the Company entered into a securities purchase agreement (the “October 2023 Purchase Agreement”) with a certain institutional investor, to sell in a registered direct offering (the “October 2023 RD Offering”), an aggregate of (i) 1,325,000 shares of common stock (the “October 2023 Shares”), of the Company and (ii) pre-funded warrants (the “October 2023 Pre-Funded Warrants”) to purchase up to 1,033,491 shares of the Company’s common stock (the “October 2023 Pre-Funded Warrant Shares”). Pursuant to the October 2023 Purchase Agreement the Company also issued unregistered warrants (the “October 2023 Private Warrants”) to purchase up to 2,358,491 shares of the Company’s common stock (the “October 2023 Private Warrant Shares”) in a concurrent private placement (the “October 2023 Private Offering” and together with the October 2023 RD Offering, the “October 2023 Offering”). The October 2023 Shares and accompanying October 2023 Private Warrants were offered at a combined offering price of $2.12. The October 2023 Pre-Funded Warrants and accompanying October 2023 Private Warrants were offered at a combined offering price of $2.1199. The October 2023 Offering closed on October 24, 2023.
The October 2023 Private Warrants are exercisable on the six-month anniversary of issuance for a period of five and one-half years from the issuance date, at an exercise price equal to $2.12 per October 2023 Private Warrant Share. The October 2023 Private Warrants will be exercisable for cash, or, solely during any period when a registration statement for the issuance or resale of the October 2023 Private Warrant Shares issuable upon exercise of the October 2023 Private Warrants to or by the holder of such October 2023 Private Warrants is not in effect, on a cashless basis.
The October 2023 Pre-Funded Warrants had an exercise price of $0.0001 per October 2023 Pre-Funded Warrant Share and were exercisable upon issuance. During the three months ended December 31, 2023, the institutional investor exercised the outstanding October 2023 Pre-Funded Warrants to purchase 1,033,491 shares of the Company’s common stock.
The net proceeds from the October 2023 Offering, after deducting the placement agent fees and offering expenses, were $4,573,948.
| 62 |
| Table of Contents |
On October 31, 2022, the Company entered into a securities purchase agreement with a certain institutional investor to sell, in a registered direct offering (the “October 2022 RD Offering”), an aggregate of (i) 1,020,000 shares of the Company’s common stock, (ii) prefunded warrants (the “October 2022 Pre-Funded Warrants”) to purchase up to 798,182 shares of the Company’s common stock, and (iii) common stock warrants (the “October 2022 Common Warrants”) to purchase up to 1,818,182 shares of the Company’s common stock. Each share of common stock was offered with one accompanying October 2022 Common Warrant with a combined offering price of $5.50. Each October 2022 Pre-Funded Warrant was offered with one accompanying October 2022 Common Warrant with a combined offering price of $5.4999. The October 2022 RD Offering was completed on November 2, 2022.
The October 2022 Common Warrants have an exercise price of $5.83 per share, are exercisable beginning six months after the date of issuance and will expire five and one-half years from the date of issuance. The October 2022 Pre-Funded Warrants had an exercise price of $0.0001 per share and were exercisable upon issuance. During the year ended June 30, 2023, the institutional investor exercised the outstanding October 2022 Pre-Funded Warrants to purchase 798,182 shares of the Company’s common stock. The October 2022 Common Warrants will be exercisable for cash, or, solely during any period when a registration statement for the issuance or resale of the shares of common stock issuable upon exercise of the October 2022 Common Warrants to or by the holder of such October 2022 Common Warrants is not in effect, on a cashless basis.
The proceeds from the October RD 2022 Offering, after deducting the placement agent fees and expenses and other estimated offering expenses, were $9,109,117.
The private warrants and common warrants related to the October 2022 and October 2023 financings met the definition of a derivative instrument under ASC Subtopic 815-40 and were reported as liabilities as of June 30, 2023 since the warrants did not meet the criteria for equity classification. The Company recorded the warrants at fair value on its balance sheet with changes in the fair value of the warrants recorded as a non-cash charge or gain in the consolidated statements of operations.
The January 2024 Placement Agent Warrants were issued to non-employees in exchange for services related to the offering are accounting for in accordance ASC 718 which requires the fair value of the warrants to be recognized as an offering expense. The placement agent warrants contain certain contingent cash settlement features that are not probable of occurring and not within the control of Company, therefore the placement agent warrants are classified out of permanent equity.
On January 24, 2024, the Company and warrant holders amended the terms of warrants related to the October 2022 and October 2023 financings. As a result, all liability classified warrants were reclassified to additional paid-in capital.
On April 12, 2023, the Company entered into a new equity distribution agreement (the “2023 Equity Distribution Agreement”) with Canaccord Genuity LLC (“Canaccord”), pursuant to which the Company may, from time to time, sell shares of the Company’s common stock at market prices by methods deemed to be an “at-the-market offering” as defined in Rule 415 promulgated under the Securities Act of 1933, as amended. The 2023 Equity Distribution Agreement and related prospectus is limited to sales of up to an aggregate maximum $50.0 million of shares of the Company’s common stock. The Company pays Canaccord 3.0% of the gross proceeds as a commission.
Proceeds raised under the 2023 Equity Distribution Agreement are as follows:
|
|
Year Ended June 30, 2024 |
|
|
Year Ended June 30, 2023 |
|
||||||||||
|
|
Shares |
|
|
Proceeds |
|
|
Shares |
|
|
Proceeds |
|
||||
Gross proceeds |
|
|
217,027 |
|
|
$ | 547,803 |
|
|
|
504,034 |
|
|
$ | 1,196,739 |
|
Fees |
|
|
- |
|
|
|
(16,434 | ) |
|
|
- |
|
|
|
(35,902 | ) |
Expenses |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(126,800 | ) |
Net proceeds |
|
|
217,027 |
|
|
$ | 531,369 |
|
|
|
504,034 |
|
|
$ | 1,034,037 |
|
| 63 |
| Table of Contents |
Stock Warrants - On June 20, 2024, the Company entered into a letter agreement (the “Inducement Letter”) with a holder (the “Exercising Holder”) of outstanding common stock purchase warrants that the Company issued on November 2, 2022, and October 24, 2023 (the “Existing Warrants”). Pursuant to the Inducement Letter, the Exercising Holder agreed to exercise, for cash, Existing Warrants to purchase, in the aggregate, 3,233,277 shares of common stock in exchange for the Company’s agreement to (i) lower the exercise price to $1.88 per share for the 3,233,277 Existing Warrants being exercised pursuant to the Inducement Letter and (ii) issue to the Exercising Holder an aggregate of 4,849,915 warrants to purchase shares of common stock, comprised of Series A common stock purchase warrants to purchase 2,727,273 shares of common stock (the “Series A Warrants”) and Series B common stock purchase warrants to purchase 2,122,642 (of which 1,624,201 shares of common stock are subject to stockholder approval) shares of common stock (the “Series B Warrants” and together with the Series A Warrants, the “Inducement Warrants”). The Company received aggregate gross proceeds of $6,078,561 from the exercise of the Existing Warrants by the Exercising Holder (the “Warrant Inducement”). As part of the agreement, 1,443,277 shares were held in abeyance on behalf of the Exercising Holder. The Company intends to use the net proceeds for working capital and general corporate purposes. The incremental value of the Warrant Inducement was recorded as an offering expense against the proceeds received in additional paid-in capital.
As of June 30, 2024, the Company had outstanding warrants for shares of common stock as follows:
|
|
Shares of Common |
|
|
Exercise Price per |
|
|
Latest Expiration |
|||
Description |
|
Stock |
|
|
Share |
|
|
Date |
|||
May 2022 Warrants |
|
|
66,666 |
|
|
$ | 12.50 |
|
|
May 11, 2026 |
|
October 2022 Placement Agent Warrants |
|
|
90,909 |
|
|
$ | 6.88 |
|
|
October 31, 2027 |
|
October 2023 Private Warrants |
|
|
943,396 |
|
|
$ | 2.12 |
|
|
April 24, 2029 |
|
October 2023 Placement Agent Warrants |
|
|
117,925 |
|
|
$ | 2.65 |
|
|
October 20, 2028 |
|
January 2024 Private Warrants |
|
|
1,831,503 |
|
|
$ | 5.46 |
|
|
February 1, 2028 |
|
January 2024 Placement Agent Warrants |
|
|
91,575 |
|
|
$ | 6.83 |
|
|
February 1, 2028 |
|
June 2024 Series A Warrants |
|
|
2,727,273 |
|
|
$ | 1.88 |
|
|
June 24, 2029 |
|
June 2024 Series B Warrants |
|
|
2,122,642 |
|
|
$ | 1.88 |
|
|
June24, 2029 |
* |
|
|
|
|
|
|
|
|
|
|
|
|
* 1,624,201 shares expire on the five year anniversary following stockholder approval |
|||||||||||
Stock Plan – The Company’s 2011 Stock Incentive Plan (“2011 Stock Incentive Plan”) was approved by the Company’s stockholders at the annual meeting of stockholders held in May 2011 and amended at the annual meeting of stockholders held on June 8, 2017, June 26, 2018, June 25, 2020, June 24, 2022, June 20, 2023 and again at the annual meeting of stockholders held on June 27, 2024. The 2011 Stock Incentive Plan, as amended, provides for incentive and nonqualified stock option grants, restricted stock unit awards and other stock-based awards to employees, non-employee directors and consultants for up to 4,300,000 shares of common stock. The 2011 Stock Incentive Plan is administered under the direction of the Company’s board of directors, which may specify grant terms and recipients. Options granted by the Company generally expire ten years from the date of grant and generally vest over three to four years. The Company’s former 2005 Stock Plan was terminated and replaced by the 2011 Stock Incentive Plan, and shares of common stock that were available for grant under the 2005 Stock Plan became available for grant under the 2011 Stock Incentive Plan. No new awards can be granted under the 2005 Stock Plan, but awards granted under the 2005 Stock Plan remained outstanding in accordance with their terms. As of June 30, 2024, 206,474 shares were available for grant under the 2011 Stock Incentive Plan. The Company expects to settle option exercises under any of its plans with authorized but currently unissued shares.
| 64 |
| Table of Contents |
The following table summarizes option activity and related information for the years ended June 30, 2024 and 2023:
|
|
Number of Shares |
|
|
Weighted Average Exercise Price |
|
|
Weighted Average Remaining Term in Years |
|
|
Aggregate Intrinsic Value |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Outstanding - June 30, 2022 |
|
|
1,163,962 |
|
|
$ | 15.98 |
|
|
|
7.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
712,310 |
|
|
|
2.30 |
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
(274,440 | ) |
|
|
12.00 |
|
|
|
|
|
|
|
|
|
Exercised |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
Expired |
|
|
(51,232 | ) |
|
|
17.45 |
|
|
|
|
|
|
|
|
|
Outstanding - June 30, 2023 |
|
|
1,550,600 |
|
|
$ | 8.27 |
|
|
|
8.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
742,800 |
|
|
|
1.83 |
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
(18,988 | ) |
|
|
3.79 |
|
|
|
|
|
|
|
|
|
Exercised |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
Expired |
|
|
(10,972 | ) |
|
|
20.98 |
|
|
|
|
|
|
|
|
|
Outstanding - June 30, 2024 |
|
|
2,263,440 |
|
|
$ | 6.11 |
|
|
|
8.2 |
|
|
$ | - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at June 30, 2024 |
|
|
940,161 |
|
|
$ | 10.81 |
|
|
|
6.6 |
|
|
$ | 89,136 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expected to vest at June 30, 2024 |
|
|
1,323,279 |
|
|
$ | 2.84 |
|
|
|
9.4 |
|
|
$ | 89,136 |
|
On December 16, 2022, Carl Spana, President and CEO of the Company, and Stephen T. Wills, CFO, COO and Executive Vice President of the Company, voluntarily contributed stock options previously issued to them to purchase 143,360 and 124,220 shares, respectively, of the Company’s common stock to the 2011 Stock Incentive Plan. The stock options were forfeited and cancelled without payment of any consideration by the Company.
Stock options granted to the Company’s executive officers and employees generally vest over a 48-month period, while stock options granted to its non-employee directors vest over a 12-month period.
Included in the outstanding options in the table above are 418,945 and 88,911 unvested performance-based stock options granted to executive officers and other employees, respectively, which were granted in June 2020, 2021, 2022 and 2023. Grants in June 2021, 2022, 2023 and 2024 were 95,167, 60,566, 238,838 and 264,945, respectively. The performance-based stock options vest on annual performance criteria through the fiscal years ending June 30, 2028 relating to advancement of MC1r programs, including initiation of clinical trials and licensing of Vyleesi in additional countries or regions.
For the years ended June 30, 2024 and 2023, the fair value of option grants was estimated at the grant date using the Black-Scholes model. The Company’s weighted average assumptions for the years ended June 30, 2024 and 2023 were as follows:
|
|
Year Ended June 30, |
|
|
Year Ended June 30, |
|
||
|
|
2024 |
|
|
2023 |
|
||
|
|
|
|
|
|
|
||
Risk-free interest rate |
|
|
4.3 | % |
|
|
3.9 | % |
Volatility factor |
|
|
75.8 | % |
|
|
65.6 | % |
Dividend yield |
|
|
0 | % |
|
|
0 | % |
Expected option life (years) |
|
|
6.1 |
|
|
|
6.1 |
|
Weighted average grant date fair value |
|
$ | 0.82 |
|
|
$ | 0.99 |
|
Expected volatilities are based on the Company’s historical volatility. The expected term of options is based upon the simplified method, which represents the average of the vesting term and the contractual term. The risk-free interest rate is based on U.S. Treasury yields for securities with terms approximating the expected term of the option.
| 65 |
| Table of Contents |
For the years ended June 30, 2024 and 2023, the Company recorded stock-based compensation related to stock options of $873,633 and $794,735, respectively. As of June 30, 2024, there was $1,409,506 of unrecognized compensation cost related to unvested options, which is expected to be recognized over a weighted-average period of 2.7 years.
Restricted Stock Units –The following table summarizes restricted stock award activity for the years ended June 30, 2024 and 2023.
|
|
Year Ended June 30, |
|
|
Year Ended June 30, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Outstanding at beginning of year |
|
|
987,521 |
|
|
|
649,149 |
|
Granted |
|
|
517,800 |
|
|
|
425,750 |
|
Forfeited |
|
|
(11,667 | ) |
|
|
(3,312 | ) |
Vested |
|
|
(98,372 | ) |
|
|
(84,062 | ) |
Expirations |
|
|
(20,302 | ) |
|
|
- |
|
Fractional shares |
|
|
- |
|
|
|
(4 | ) |
Outstanding at end of year |
|
|
1,374,980 |
|
|
|
987,521 |
|
For the years ended June 30, 2024 and 2023, the Company recorded stock-based compensation related to restricted stock units of $839,335 and $616,182, respectively.
Included in outstanding restricted stock units in the table above are 508,011 vested shares that have not been issued as of June 30, 2024 due to a provision in the restricted stock unit agreements to delay delivery.
Time-based restricted stock units granted to the Company’s executive officers, employees and non-employee directors generally vest over 48 months, 48 months, and 12 months, respectively.
Included in the outstanding restricted stock units in the table above are 274,549 and 59,842 unvested performance-based restricted stock units granted to executive officers and other employees, respectively, which were granted in June 2021, 2022, 2023, and 2024. Grants in June 2021, 2022, 2023 and 2024 were 22,343, 40,707, 152,432 and 184,443 restricted stock units, respectively. The performance-based restricted stock units vest on annual performance criteria through the fiscal years ending June 30, 2026 relating to advancement of MC1r programs, including initiation of clinical trials, and licensing of Vyleesi in additional countries or regions.
In connection with the vesting of restricted share units during the years ended June 30, 2024 and 2023, the Company withheld 25,467 and 20,468, shares, respectively, with aggregate values of $56,401 and $146,062, respectively, in satisfaction of minimum tax withholding obligations.
(16) INCOME TAXES
The Company has participated in the State of New Jersey’s Technology Business Tax Certificate Transfer Program (the “Program”) sponsored by The New Jersey Economic Development Authority. The Program enables approved biotechnology companies with unused Net Operating Losses (“NOLs”) and unused research and development credits (“R&D credits”) to sell these tax benefits for at least 80% of the value of the tax benefits to unaffiliated, profitable corporate taxpayers in the State of New Jersey. The Company received final approval in December 2022 for the sale of NOLs and R&D credits that resulted in the receipt of $4,674,999 in January 2023. As a result, the Company recorded an income tax benefit for the year ended June 30, 2023.
For fiscal 2024 and 2023, the Company recorded no income tax expense as a result of the generation of operating losses that were subject to a full valuation allowance.
Deferred tax assets and liabilities are determined based on the estimated future tax effect of differences between the financial statement and tax reporting basis of assets and liabilities, as well as for, NOL carryforwards and R&D credit carryforwards, given the provisions of existing tax laws.
As of June 30, 2024, the Company had state NOL carryforwards of approximately $181,000,000, which will expire, if not utilized, between 2036 and 2043, federal NOL carryforwards of approximately $130,000,000 and federal R&D and Alternative Minimum Tax (“AMT”) credits of approximately $9,500,000, which expire, if not utilized, between 2035 and 2043, and foreign tax credits of $582,500, which expire, if not utilized, in 2028.
| 66 |
| Table of Contents |
In assessing the realizability of deferred tax assets, the Company considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income and the application of loss limitation provisions related to ownership changes. The Company assesses the available positive and negative evidence to estimate if sufficient future taxable income will be generated to use the existing deferred tax assets. The Company also considers the scheduled reversal of deferred tax liabilities (including the impact of available carryback and carryforward periods), projected future taxable income, and tax‑planning strategies in making this assessment. Based on a history of losses incurred, the Company has recognized a full valuation allowance against its deferred tax assets during the years ended June 30, 2024 and 2023. The Company’s valuation allowance increased by $11,193,000 and $5,653,000 for the years ended June 30, 2024 and 2023, respectively.
A sustained period of profitability in the Company’s operations is required before it would change its judgment regarding the need for a full valuation allowance against its net deferred tax assets. Until such time, the use of NOL carryforwards and tax credits to offset profits, if any, will reduce the overall level of deferred tax assets subject to valuation allowance.
The Tax Reform Act of 1986 (the “Tax Reform Act”) provides for limitation on the use of the Company’s NOL and R&D tax credit carryforwards following certain ownership changes (as defined by the Tax Reform Act) that could limit the Company’s ability to utilize these carryforwards. Since its inception, the Company has completed several financings and sales of common stock which has resulted in multiple ownership changes defined by Section 382 of the Tax Reform Act. Accordingly, the Company’s ability to utilize the aforementioned carryforwards are subject to limitation under Section 382.
If the Company undergoes a future ownership change or as it completes its Section 382 limitation assessments, any unutilized carryforwards that were not previously subject to a Section 382 limitation may become subject to limitation which may result in a significant limitation and loss of NOL carryforwards and R&D credits.
Additionally, U.S. tax laws limit the time during which these carryforwards may be applied against future taxes; therefore, the Company may not be able to take full advantage of these carryforwards for federal income tax purposes. Accordingly, a portion of the carryforwards may expire unutilized.
The Company’s net deferred tax assets are as follows:
|
|
June 30, |
|
|
June 30, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Net operating loss carryforwards |
|
$ | 40,051,000 |
|
|
$ | 40,073,000 |
|
Research and development and AMT tax credits |
|
|
9,464,000 |
|
|
|
8,094,000 |
|
Foreign tax credits |
|
|
583,000 |
|
|
|
583,000 |
|
Basis differences in fixed assets and other |
|
|
12,611,000 |
|
|
|
2,766,000 |
|
|
|
|
62,709,000 |
|
|
|
51,516,000 |
|
Valuation allowance |
|
|
(62,709,000 | ) |
|
|
(51,516,000 | ) |
Net deferred tax assets |
|
$ | - |
|
|
$ | - |
|
The Company recognizes interest expense and penalties on uncertain income tax positions as a component of interest expense. No interest expense or penalties were recorded for uncertain income tax matters in fiscal 2024 or 2023. As of June 30, 2024 and 2023, the Company had no liabilities for uncertain income tax matters.
| 67 |
| Table of Contents |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures, as defined in Exchange Act Rules 13a-15(e) and 15d-15(e), as of the end of the period covered by this report. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of June 30, 2024, our disclosure controls and procedures were not effective due to a material weakness in our controls over the accounting for complex financial instruments, specifically in connection with the valuation of warrant liability. In light of this material weakness, we performed additional analysis deemed necessary to ensure that our consolidated financial statements were prepared in accordance with U.S. GAAP. Accordingly, management believes that the consolidated financial statements included in this Annual Report on Form 10-K, present fairly, in all material respects, our financial position as of June 30, 2024 and June 30, 2023, and our results of operations and cash flows for each of the years then ended. The Company will adjust prior periods as those financial statements are presented for comparative purposes in future filings. While we processes to identify and appropriately apply applicable account requirements, we are improving these processes to ensure that the nuances of the accounting standards that apply to complex financial instruments and such other significant or unusual transactions are effectively evaluated. There were no other changes in our internal control over financial reporting that occurred during our most recent fiscal quarter that materially affected, or that are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
During the Company’s fiscal quarter ended June 30, 2024, no director or officer, as defined in Rule 1a-1(f), adopted or terminated a “Rule 10b5-1 trading arrangement” or a “non-Rule 10b5-1 trading arrangement,” each as defined in Item 408 of Regulation S-K.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
| 68 |
| Table of Contents |
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Identification of Directors
The following table sets forth the names, ages, positions and committee memberships of our current directors. All directors hold office until the next annual meeting of stockholders or until their successors have been elected and qualified. All current directors were elected at our annual meeting of stockholders meeting on June 27, 2024.
Name |
Age |
Position with Palatin |
Carl Spana, Ph.D. |
62 |
Chief Executive Officer, President and a Director |
John K.A. Prendergast, Ph.D. (3) |
70 |
Director, Chairman of the Board of Directors |
Robert K. deVeer, Jr. (1) (2) |
78 |
Director |
J. Stanley Hull (1) (2) |
72 |
Director |
Alan W. Dunton, M.D. (1) (2) |
70 |
Director |
Arlene M. Morris (2) (3) |
72 |
Director |
Anthony M. Manning, Ph.D. (1) (3) |
62 |
Director |
|
(1) Member of the audit committee. (2) Member of the compensation committee. (3) Member of the nominating and corporate governance committee. | ||
CARL SPANA, Ph.D., co-founder of Palatin, has been our Chief Executive Officer and President since June 14, 2000. He has been a director of Palatin since June 1996 and has been a director of our wholly owned subsidiary, RhoMed Incorporated, since July 1995. From June 1996 through June 14, 2000, Dr. Spana served as an executive vice president of the Company and our chief technical officer. From June 1993 to June 1996, Dr. Spana was vice president of Paramount Capital Investments, LLC, a biotechnology and biopharmaceutical merchant banking firm, and of The Castle Group Ltd., a medical venture capital firm. Through his work at Paramount Capital Investments and The Castle Group, Dr. Spana co-founded and acquired several private biotechnology firms. From July 1991 to June 1993, Dr. Spana was a Research Associate at Bristol-Myers Squibb, a publicly held pharmaceutical company, where he was involved in scientific research in the field of immunology. Dr. Spana received his Ph.D. in molecular biology from The Johns Hopkins University and his B.S. in biochemistry from Rutgers University.
Dr. Spana’s qualifications for our board include his scientific expertise, leadership experience, business judgment, and industry knowledge. As a senior executive of Palatin for over twenty years, he provides in-depth knowledge of our company, our drug products under development and the competitive and corporate partnering landscape.
JOHN K.A. PRENDERGAST, Ph.D. has served as the non-executive Chairman of the board since June 14, 2000, and as a director since August 1996. While Dr. Prendergast has served as a member of the board, he does not serve, and has not served, in a management or operational role with the Company. Dr. Prendergast has been president and sole stockholder of Summercloud Bay, Inc., an independent consulting firm providing services to the biotechnology industry, since 1993. Dr. Prendergast is lead director of Scorpius Holdings, Inc. (NYSE American: SCPX), a publicly traded integrated contract development and manufacturing organization (CDMO), and a director and Executive Chairman of Recce Pharmaceuticals Ltd. (ASX: RCE), a publicly traded Australian pharmaceutical company developing a new class of anti-infective agents. He was previously a member of the board of the life science companies AVAX Technologies, Inc., Avigen, Inc. and MediciNova, Inc. From October 1991 through December 1997, Dr. Prendergast was a managing director of The Castle Group Ltd., a medical venture capital firm. Dr. Prendergast received his M.Sc. and Ph.D. from the University of New South Wales, Sydney, Australia and a C.S.S. in administration and management from Harvard University.
Dr. Prendergast brings a historical perspective to our board coupled with extensive industry experience in corporate development and finance in the life sciences field. His prior service on other publicly traded company boards provides experience relevant to good corporate governance practices.
| 69 |
| Table of Contents |
ROBERT K. deVEER, Jr. has been a director of Palatin since November 1998. Since January 1997, Mr. deVeer has been the president of deVeer Capital LLC, a private investment company. He was a director of Solutia Inc., a publicly held chemical-based materials company, until its merger with Eastman Chemical Company in July 2012. From 1995 until his retirement in 1996, Mr. deVeer served as Managing Director, Head of Industrial Group, at New York-based Lehman Brothers. From 1973 to 1995, he held increasingly responsible positions at New York-based CS First Boston, including Head of Project Finance, Head of Industrials and Head of Natural Resources. He was a managing director, member of the investment banking committee and a trustee of the First Boston Foundation. He received a B.A. in economics from Yale University and an M.B.A. in finance from Stanford Graduate School of Business.
Mr. deVeer has extensive experience in investment banking and corporate finance, including the financing of life sciences companies, and serves as the audit committee’s financial expert.
J. STANLEY HULL has been a director of Palatin since September 2005. Mr. Hull has over three decades of experience in the field of sales, marketing, and drug development. Mr. Hull joined GlaxoSmithKline, a research-based pharmaceutical company, in October 1987 and retired as Senior Vice President, Pharmaceuticals – North America in May 2010. Mr. Hull was responsible for all commercial activities including sales, marketing, sales training, and office operations. Previously, Mr. Hull served in the R&D organization of Glaxo Wellcome as Vice President and Worldwide Director of Therapeutic Development and Product Strategy – Neurology and Psychiatry. Prior to his service in the R&D organization he was Vice President of Marketing – Infectious Diseases and Gastroenterology for Glaxo Wellcome-U.S. Mr. Hull started his career in the pharmaceutical industry with SmithKline and French Laboratories in 1978. Mr. Hull received his B.S. in business administration from the University of North Carolina at Greensboro.
Mr. Hull has extensive experience in commercial operations, development, and marketing of pharmaceutical drugs and corporate alliances between pharmaceutical companies and biotechnology companies.
ALAN W. DUNTON, M.D. has been a director of Palatin since June 2011. He founded Danerius, LLC, a biotechnology consulting company, in 2006. From November 2015 through March 2018, he was senior vice president of research, development, and regulatory affairs for Purdue Pharma L.P., with responsibilities for overall research strategy and development programs. From January 2007 to March 2009, Dr. Dunton served as president and chief executive officer of Panacos Pharmaceuticals Inc. and he served as a managing director of Panacos from March 2009 to January 2011. Dr. Dunton is currently a member of the board of directors of the publicly traded companies Recce Pharmaceuticals Ltd (ASX: RCE), CorMedix Inc. (NYSE: CRMD) and Oragenics, Inc. (NYSE: OGEN). He previously served on the board of directors of the publicly traded companies Targacept, Inc., EpiCept Corporation (as Non-Executive Chairman), Adams Respiratory Therapeutics, Inc. (acquired by Reckitt Benckiser Group plc), MediciNova, Inc. and Panacos Pharmaceuticals, Inc. Dr. Dunton has served as a director or executive officer of various pharmaceutical companies, and from 1994 to 2001, Dr. Dunton was a senior executive in various capacities in the Pharmaceuticals Group of Johnson & Johnson, including president and managing director of the Janssen Research Foundation, the primary global R&D organization for Johnson & Johnson. Dr. Dunton received his M.D. degree from New York University School of Medicine, where he completed his residency in internal medicine. He also was a Fellow in Clinical Pharmacology at the New York Hospital/Cornell University Medical Center.
Dr. Dunton has extensive drug development, regulatory, and clinical research experience, having played a key role in the development of more than 20 products to regulatory approval, and also has extensive experience as an executive and officer for both large pharmaceutical companies and smaller biotechnology and biopharmaceutical companies.
ARLENE M. MORRIS has been a director of Palatin since June 2015. Since May 2015 she has served as the chief executive officer of Willow Advisors, LLC, a consultancy to biotech companies on business development, commercial development and corporate strategy. From April 2012 until May 2015, she was President and Chief Executive Officer of Syndax Pharmaceuticals, Inc., a privately held biopharmaceutical company focused on the development and commercialization of an epigenetic therapy for treatment-resistant cancers and was a member of the board of directors from May 2011 until May 2015. From 2003 to January 2011, Ms. Morris served as the President, Chief Executive Officer and a member of the board of directors of Affymax, Inc., a publicly traded biotechnology company. Ms. Morris has also held various management and executive positions at Clearview Projects, Inc., a corporate advisory firm, Coulter Pharmaceutical, Inc., a publicly traded pharmaceutical company, Scios Inc., a publicly traded biopharmaceutical company, and Johnson & Johnson, a publicly traded healthcare company. She is currently a member of the board of directors of Viridian Therapeutics, Inc. (Nasdaq: VRDN), a publicly traded therapeutic antibody company, Cogent Biosciences, Inc. (Nasdaq: COGT), a publicly traded oncology biopharmaceutical company, Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, and is a director (since February 2022) and currently chair of TC Biopharm (Holdings) PLC (Nasdaq: TCBP), a United Kingdom biopharmaceutical company. She was previously a director of Viveve Medical, Inc., a publicly traded female healthcare medical device company, until February 2023, Neovacs SA, a publicly traded French company, Biodel Inc., a publicly traded specialty pharmaceutical company, from 2015 until its merger with Albireo Limited in 2016, and Dimension Therapeutics, Inc., a publicly traded gene therapy company, until its acquisition by Ultragenyx Pharmaceutical Inc. in 2017. Ms. Morris received a B.A. in Biology and Chemistry from Carlow College.
| 70 |
| Table of Contents |
Ms. Morris has extensive experience in the biotechnology industry, including prior leadership positions, senior management, and board service, and experience as chief executive officer of companies with product candidates in phase 3 clinical trials.
ANTHONY M. MANNING, Ph.D. has been a director of Palatin since September 2017, and since July 2023 has been a director of Monte Rosa Therapeutics, Inc. (Nasdaq: GLUE), a clinical-stage biotechnology company developing novel molecular glue degrader (MGD)-based medicines. Since March 2021, Dr. Manning has been providing scientific and strategic advice to biotechnology companies as the principal of Manning Bio Worldwide LLC. From 2013 until March 2021, Dr. Manning was senior vice president of research, and since 2018 was chief scientific officer, at Momenta Pharmaceuticals, Inc., a publicly traded biopharmaceutical company developing innovative therapeutics for rare immune-related diseases which was acquired by Johnson & Johnson in October 2020. From 2011 to 2013, he was senior vice president of research and development at Aileron Therapeutics, Inc., a publicly traded biopharmaceutical company developing stapled peptide therapeutics for cancers and other diseases. From 2007 to 2011, he was vice president and head of inflammation and autoimmune diseases research at Biogen, Inc., a publicly traded biopharmaceutical company developing medicines for neurological and neurodegenerative conditions. From 2002 to 2007, he was vice president and global therapy area head for Inflammation, Autoimmunity and Transplantation Research at Roche Pharmaceuticals, the pharmaceutical division of Roche Holding AG, and from 2000 to 2002 he was vice president of Pharmacia, a global pharmaceutical company acquired by Pfizer in 2002. Dr. Manning received his Ph.D., M.Sc. and B.Sc. from the University of Otago, Dunedin, New Zealand.
Dr. Manning has extensive experience in translational research and development of new pharmaceutical products, and in pharmaceutical and biotechnology research, development, and business strategy.
The Board and Its Committees
Committees and meetings. The board of directors has an audit committee, a compensation committee, and a nominating and corporate governance committee. During the fiscal year ended June 30, 2024 (“fiscal 2024”), the board of directors met four times, the audit committee met four times, the compensation committee met two times and the nominating and corporate governance committee met two times. Each director attended at least 75% of the total number of meetings of the board of directors and committees of the board of directors on which he or she served. The independent directors meet in executive sessions at least annually, following the annual board of directors meeting. We do not have a policy requiring our directors to attend stockholder meetings. The directors did not attend the virtual annual meeting of stockholders held on June 27, 2024.
Audit committee. The audit committee reviews the engagement of the independent registered public accounting firm and reviews the independence of the independent registered public accounting firm. The audit committee also reviews the audit and non-audit fees of the independent registered public accounting firm and the adequacy of our internal control procedures. The audit committee is currently composed of four independent directors, Mr. deVeer (chair), and Dr. Dunton, Dr. Manning and Mr. Hull. The board of directors has determined that the members of the audit committee are independent, as defined in the listing standards of the NYSE American and satisfy the requirements of the NYSE American as to financial literacy and expertise. The board has determined that at least one member of the committee, Mr. deVeer, is the audit committee financial expert as defined by Item 407 of Regulation S-K. The responsibilities of the audit committee are set forth in a written charter adopted by the board of directors and updated as of October 1, 2013, a copy of which is available on our web site at www.palatin.com/investors/corporate-governance/.
Compensation committee. The compensation committee reviews and recommends to the board of directors on an annual basis employment agreements and compensation for our officers, directors, and some employees. The compensation committee is composed of Dr. Dunton (chair), Ms. Morris and Messrs. deVeer and Hull. The board has determined that the members of the compensation committee are independent, as defined in the listing standards of the NYSE American. Our Chief Executive Officer aids the compensation committee by providing annual recommendations regarding the compensation of all executive officers, other than himself. Our Chief Financial Officer supports the committee in its work by gathering, analyzing, and presenting data on our compensation arrangements and compensation in the marketplace.
| 71 |
| Table of Contents |
The responsibilities of the compensation committee are set forth in a written charter adopted by the board of directors effective October 1, 2013, a copy of which is available on our web site at www.palatin.com/investors/corporate-governance/. The compensation committee administers our 2011 Plan, under which it has delegated to an officer its authority to grant stock options to employees and to a single-member committee of the board of directors its authority to grant restricted stock units to officers and to grant options and restricted stock units to our consultants, but in either instance not to grant options or restricted stock units to themselves, any member of the board of directors or officer, or any person subject to Section 16 of the Exchange Act.
Nominating and corporate governance committee. The nominating and corporate governance committee assists the board of directors in recommending nominees for directors, and in determining the composition of committees. It also reviews, assesses, and makes recommendations to the board of directors concerning policies and guidelines for corporate governance, including relationships of the board of directors, the stockholders and management in determining our direction and performance. The responsibilities of the nominating and corporate governance committee are set forth in a written charter adopted by the board of directors and updated as of October 1, 2013, a copy of which is available on our web site at www.palatin.com/investors/corporate-governance/. The nominating and corporate governance committee is composed of Dr. Prendergast (chair), Ms. Morris and Dr. Manning, each of whom meets the independence requirements established by the NYSE American.
Duration of Office. Unless a director resigns, all directors hold office until the next annual meeting of stockholders or until their successors have been elected and qualified. Directors serve as members of committees as the board of directors determines from time to time.
Communicating With Directors
Generally, stockholders or other interested parties who have questions or concerns should contact Stephen T. Wills, Secretary, Palatin Technologies, Inc., 4B Cedar Brook Drive, Cranbury, NJ 08512. However, any stockholder or other interested party who wishes to address questions regarding our business directly to the board of directors, or any individual director, including the Chairman or non-management directors as a group, can direct questions to the members of the board of directors or a director by regular mail to the Secretary at the address above or by e-mail at boardofdirectors@palatin.com. Stockholders or other interested parties may also submit their concerns anonymously or confidentially by postal mail.
Communications are distributed to the board of directors, or to any individual directors as appropriate, depending on the facts and circumstances outlined in the communication, unless the Secretary determines that the communication is unrelated to the duties and responsibilities of the board of directors, such as product inquiries, resumes, advertisements or other promotional material. Communications that are unduly hostile, threatening, illegal or similarly unsuitable will also not be distributed to the board of directors or any director. All communications excluded from distribution will be retained and made available to any non-management director upon request.
Board Role in Risk Oversight
Our board of directors, as part of its overall responsibility to oversee the management of our business, considers risks generally when reviewing our strategic plan, financial results, business development activities, legal and regulatory matters. The board of directors satisfies this responsibility through regular reports directly from our officers responsible for oversight of particular risks. The board of directors’ risk management oversight also includes full and open communications with management to review the adequacy and functionality of the risk management processes used by management. The board of directors’ role in risk oversight has no effect on the board of directors’ leadership structure. In addition, committees of the board of directors assist in its risk oversight responsibility, including:
|
· |
The audit committee assists the board of directors in its oversight of the integrity of the financial reporting and our compliance with applicable legal and regulatory requirements. It also oversees our internal controls and compliance activities and meets privately with representatives from our independent registered public accounting firm. |
|
|
|
|
· |
The compensation committee assists the board of directors in its oversight of risk relating to compensation policies and practices. The compensation committee annually reviews our compensation policies, programs, and procedures, including the incentives they create and mitigating factors that may reduce the likelihood of excessive risk taking, to determine whether they present a significant risk to our company. |
| 72 |
| Table of Contents |
Board Leadership Structure
Since 2000, the roles of chairman of the board of directors and chief executive officer have been held by separate persons. John K.A. Prendergast, Ph.D., a non-employee director, has served as Chairman of the board of directors since June 2000. Carl Spana, Ph.D., has been our Chief Executive Officer and President since June 2000. Generally, the chairman is responsible for advising the chief executive officer, assisting in long-term strategic planning, and presiding over meetings of the board of directors, and the chief executive officer, together with our chief financial officer and chief operating officer, is responsible for leading our day-to-day performance and operations. While we do not have a written policy with respect to separation of the roles of chairman of the board of directors and chief executive officer, the board of directors believes that the existing leadership structure, with the separation of these roles, provides several important advantages, including: enhancing the accountability of the chief executive officer to the board of directors; strengthening the board of directors’ independence from management; assisting the board of directors in reaching consensus on particular strategies and policies; and facilitating robust director, board of directors, and executive officer evaluation processes.
Code of Corporate Conduct and Ethics
We have adopted a code of corporate conduct and ethics, updated as of March 8, 2021, that applies to all of our directors, officers and employees, including our Chief Executive Officer and Chief Financial Officer. You can view the code of corporate conduct and ethics at our website at www.palatin.com/investors/corporate-governance/. We will disclose any amendments to, or waivers from, provisions of the code of corporate conduct and ethics that apply to our directors, principal executive and financial officers in a current report on Form 8-K, unless the rules of the NYSE American permit website posting of any such amendments or waivers.
Executive Officers
Executive officers are appointed by the board of directors and serve at the discretion of the board of directors. Each officer holds his position until his successor is appointed and qualified. The current executive officers, each of whom hold office under employment agreements, are as follows.
|
Name
|
Age
|
Position with Palatin
|
Carl Spana, Ph.D. |
62 |
Chief Executive Officer, President and Director |
Stephen T. Wills, MST, CPA |
67 |
Chief Financial Officer, Chief Operating Officer, Executive Vice President, Secretary and Treasurer |
Additional information about Dr. Spana is included above under the heading “Identification of Directors.”
STEPHEN T. WILLS, CPA, MST currently serves as the Chief Financial Officer (since 1997), Chief Operating Officer (since 2011), Treasurer and Secretary, of Palatin. Mr. Wills has served on the board of directors of MediWound Ltd. (Nasdaq: MDWD), a biopharmaceutical company focused on treatment in the fields of severe burns, chronic and other hard to heal wounds, since April 2017, and as Chairman from October 2017 until August 2022, and starting September 2022, is the chairman of the audit committee, a member of the compensation committee and research and development committee. He also has served on the board of directors of Gamida Cell Ltd. (Nasdaq: GMDA), a leading cellular and immune therapeutics company, from March 2019 to June 2024, following a transaction with Highbridge Capital Management, LLC. Mr. Wills was chairman of the audit committee and a member of the compensation and finance committees of Gamida Cell Ltd. Mr. Wills served as the Chief Financial Officer of Cactus Acquisition Corp (Nasdaq: CCTS), a Special Purpose Acquisition Company (SPAC) from November 2021 to May 2024. Mr. Wills served on the board of directors of Amryt Pharma Plc, a biopharmaceutical company focused on developing and delivering treatments to help improve the lives of patients with rare and orphan diseases, from September 2019 through April 2023, when Amryt was acquired by Chiesi Farmaceutici. Mr. Wills served as the chairman of the audit committee and a member of the compensation, finance and compliance committees. Mr. Wills served on the board of trustees and executive committee of The Hun School of Princeton, a college preparatory day and boarding school from 2014, and as its Chairman starting June 2018, until his retirement in June 2023. Mr. Wills served on the board of directors of Caliper Corporation, a psychological assessment and talent development company, since March 2016, and as Chairman from December 2016 to December 2019, when PSI Corporation acquired Caliper. Mr. Wills served as Executive Chairman and Interim Principal Executive Officer of Derma Sciences, Inc., a provider of advanced wound care products, from December 2015 to February 2017, when Derma Sciences was acquired by Integra Lifesciences (Nasdaq: IART). Previously, Mr. Wills served on the board of directors of Derma Sciences as the lead director and chairperson of the audit committee from June 2000 to December 2015, and served as the Chief Financial Officer of Derma Sciences from 1997 to 2000. Mr. Wills served as the President and Chief Operating Officer of Wills, Owens & Baker, P.C., a public accounting firm, from 1991 to 2000. Mr. Wills, a certified public accountant, earned his Bachelor of Science in accounting from West Chester University, and a Master of Science in taxation from Temple University.
| 73 |
| Table of Contents |
Item 11. Executive Compensation.
Fiscal 2024 Summary Compensation Table
The following table summarizes the compensation earned by or paid to our principal executive officer and our principal financial officer, who constitute all of our executive officers, for fiscal 2024 and fiscal 2023. We have no defined benefit or actuarial pension plan, and no deferred compensation plan.
Name and Principal Position |
|
Fiscal Year |
|
Salary ($) |
|
|
Stock awards (1) ($) |
|
|
Option awards (1) ($) |
|
|
Nonequity incentive plan compensation (2) ($) |
|
|
All other compensation (3) ($) |
|
|
Total ($) |
|
||||||
Carl Spana, Ph.D., |
|
2024 |
|
|
700,000 |
|
|
|
195,400 |
|
|
|
178,700 |
|
|
|
357,000 |
|
|
|
17,250 |
|
|
|
1,448,350 |
|
Chief Executive Officer and President |
|
2023 |
|
|
700,000 |
|
|
|
170,500 |
|
|
|
154,300 |
|
|
|
262,500 | (4) |
|
|
16,500 |
|
|
|
1,303,800 |
|
Stephen T. Wills, MST, CPA, Chief Financial Officer, |
|
2024 |
|
|
650,000 |
|
|
|
170,700 |
|
|
|
155,800 |
|
|
|
431,500 |
|
|
|
16,644 |
|
|
|
1,424,644 |
|
Chief Operating Officer and Executive Vice President |
|
2023 |
|
|
650,000 |
|
|
|
148,500 |
|
|
|
134,100 |
|
|
|
243,800 | (4) |
|
|
16,750 |
|
|
|
1,193,150 |
|
_______________
(1) Amounts in these columns represent the aggregate grant date fair value for stock awards and option awards computed using the Black-Scholes model. The aggregate fair value of the performance-based restricted stock units and performance-based stock options granted in fiscal 2024 was as follows: for Dr. Spana, $50,800 for performance-based restricted stock units and $34,200 for performance-based stock options; and for Mr. Wills, $44,400 for performance-based restricted stock units and $29,700 for performance-based stock options. The aggregate fair value of the performance-based restricted stock units and performance-based stock options granted in fiscal 2023 was as follows: for Dr. Spana, $25,900 for performance-based restricted stock units and $9,700 for performance-based stock options; and for Mr. Wills, $22,600 for performance-based restricted stock units and $8,400 for performance-based stock options. For a description of the assumptions we used to calculate these amounts, see Note 15 to the consolidated financial statements included in this Annual Report.
(2) Annual incentive and merit amounts.
(3) Consists of matching contributions to 401(k) plan.
(4) Bonus amounts for fiscal years 2023 and 2024 paid after fiscal year end but accrued as of June 30.
Base Salary
The salary for each named executive officer is based, among other factors, upon job responsibilities, level of experience, individual performance, comparisons to the salaries of executives in similar positions obtained from market surveys, and internal comparisons. The compensation committee considers changes in the base salaries of our named executive officers annually. Effective July 1, 2024, the compensation committee approved increases in base salaries to $721,000 for Dr. Spana and $670,000 for Mr. Wills.
Annual Incentive Program
We provide annual incentive opportunities to our named executive officers to promote the achievement of annual performance objectives. Each year, the compensation committee establishes the target annual incentive opportunity for each named executive officer, which is based on a percentage of his base salary.
The fiscal 2024 annual incentive bonus for the named executive officers was determined based on corporate performance and individual achievements and performance, as warranted. In determining the annual incentive bonus opportunity for executives, the executive’s annual base salary is multiplied by the target bonus percentage. The resulting amount is then multiplied by the corporate performance percentage approved by the compensation committee, which is dependent on the achievement of corporate performance goals, and also potentially adjusted upwards or downwards for individual executives based on their individual contribution toward the corporate results during the relevant year. The corporate objectives are established so that target attainment is not assured. Instead, our executives are required to demonstrate significant effort, dedication, and achievement to attain payment for performance at target or above.
| 74 |
| Table of Contents |
The following table briefly describes each category of corporate objectives, the relative weighting of each objective, and the related achievement level for fiscal 2024:
Corporate Objectives Related to: |
|
Weight |
|
|
Achievement Level |
|
|
Discretionary Adjustments |
|
|
Total Weighted Achievement |
|
||||
Vyleesi (bremelanotide) SF Program |
|
|
30.0 | % |
|
|
75.0 | % |
|
|
12.5 | % |
|
|
35.0 | % |
Vyleesi Obesity Program |
|
|
5.0 | % |
|
|
50.0 | % |
|
|
0.0 | % |
|
|
2.5 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anti-Inflammatory Programs |
|
|
25.0 | % |
|
|
40.0 | % |
|
|
0.0 | % |
|
|
10.0 | % |
Ocular Programs |
|
|
25.0 | % |
|
|
70.0 | % |
|
|
5.0 | % |
|
|
22.5 | % |
Other Corporate |
|
|
15.0 | % |
|
|
100.0 | % |
|
|
0.0 | % |
|
|
15.0 | % |
Total Payout |
|
|
|
|
|
|
|
|
|
|
|
85.0 | % | |||
For fiscal 2024, the compensation committee determined that our named executive officers achieved 85.0% of their target objectives. As a result, each named executive officer received a payout under the 2024 annual incentive program equal to 85.0% of his target annual incentive opportunity, or $357,000 for Dr. Spana and $331,500 for Mr. Wills (subject to rounding conventions). In addition to his annual incentive, the compensation committee also awarded Mr. Wills a $100,000 cash bonus in fiscal 2024 pursuant to his efforts in the sale of Vyleesi to Cosette Pharmaceuticals, Inc.
Long-Term Incentive Program
The total direct compensation levels for our named executive officers are heavily weighted to long-term incentive opportunities. This structure is intended to align executives’ interests with those of our stockholders, enhance our retention incentives and focus our executives on delivering sustainable performance over the longer term.
The design of this program has evolved over the past several years to reflect core performance metrics and an incentive structure the compensation committee believes is necessary to drive our long-term success and that reflects feedback received from investors during our stockholder engagement process.
Each year, the compensation committee establishes the target long-term incentive opportunity for each named executive officer, which is based on a percentage of his base salary. For both fiscal 2024 and fiscal 2023, the target long-term incentive opportunity for each named executive officer equaled 250% of base salary for Dr. Spana and 235% of base salary for Mr. Wills, however for fiscal 2023 and 2024, to conserve the number of available shares under the plan, the target long-term incentive opportunity for each named executive officer was reduced to 33% of target, or 83% of base salary for Dr. Spana and 78% of base salary for Mr. Wills.
On June 4, 2024, as part of our fiscal 2025 long-term incentive program, we granted 79,000 time-based restricted stock units and 79,000 performance-based restricted stock units to Dr. Spana, and 69,000 time-based restricted stock units and 69,000 performance-based restricted stock units to Mr. Wills. The time-based restricted stock units vest as to 25% of the number of shares granted at each anniversary of the date of grant. The performance-based restricted stock units vest on annual performance criteria relating to corporate objectives, including stock appreciation, advancement of development programs, and licensing of Vyleesi in additional countries or regions. The grants were subject to certification by the Chief Financial Officer that the Company’s stockholders had increased the shares reserved under the 2011 Stock Incentive Plan, and that there were no impediments to the grant of the restricted stock units. The required certification was made on July 15, 2024.
On June 4, 2024, we granted 113,500 time-based stock options to Dr. Spana and 99,000 time-based stock options to Mr. Wills, which vest as to 25% of the number of shares granted on each anniversary of the date of grant. Additionally on June 4, 2024, we granted 113,500 performance-based stock options to Dr. Spana and 99,000 performance-based stock options to Mr. Wills which vest based on annual performance criteria relating to corporate objectives, including stock appreciation and advancement of development programs,. The options have an exercise price of $1.83, the fair market value of the common stock on the date of grant, and they expire on June 4, 2034. The grants were subject to certification by the Chief Financial Officer that the Company’s stockholders had increased the shares reserved under the 2011 Stock Incentive Plan, and that there were no impediments to the grant of the options. The required certification was made on July 15, 2024.
| 75 |
| Table of Contents |
On June 20, 2023, as part of our fiscal 2024 long-term incentive program, we granted 66,000 time-based restricted stock units and 66,000 performance-based restricted stock units to Dr. Spana, and 57,500 time-based restricted stock units and 57,500 performance-based restricted stock units to Mr. Wills. The time-based restricted stock units vest as to 25% of the number of shares granted at each anniversary of the date of grant. The performance-based restricted stock units vest on annual performance criteria relating to corporate objectives, including stock appreciation, advancement of development programs, and licensing of Vyleesi in additional countries or regions.
On June 20, 2023, we granted 103,500 time-based stock options to Dr. Spana and 90,000 time-based stock options to Mr. Wills, which vest as to 25% of the number of shares granted on each anniversary of the date of grant. Additionally on June 20, 2023, we granted 103,500 performance-based stock options to Dr. Spana and 90,000 performance-based stock options to Mr. Wills which vest based on annual performance criteria relating to corporate objectives, including stock appreciation, advancement of development programs, and licensing of Vyleesi in additional countries or regions. The options have an exercise price of $2.19, the fair market value of the common stock on the business day immediately preceding the date of grant, and they expire on June 20, 2033.
Employment Agreements
Effective July 1, 2022, we entered into employment agreements with Dr. Spana and Mr. Wills which continue through June 30, 2025 unless terminated earlier. Under these agreements Dr. Spana is serving as Chief Executive Officer and President at an initial base salary of $700,000 per year and Mr. Wills is serving as Chief Financial Officer and Chief Operating Officer at an initial base salary of $650,000 per year. Each agreement also provides for:
|
· |
annual discretionary bonus compensation, in an amount to be decided by the compensation committee and approved by the board, based on achievement of yearly performance objectives; and |
|
· |
participation in all benefit programs that we establish, to the extent the executive’s position, tenure, salary, age, health and other qualifications make him eligible to participate. |
Each agreement allows us or the executive to terminate the agreement upon written notice and contains other provisions for termination by us for “cause,” or by the employee for “good reason” or due to a “change in control” (as these terms are defined in the employment agreements and set forth below). Early termination may, in some circumstances, result in severance pay at the salary then in effect, plus continuation of medical and dental benefits then in effect for a period of two years. In addition, the agreements provide that options and restricted stock units granted to these officers accelerate upon termination of employment except for voluntary resignation by the officer or termination for cause. In the event of retirement, termination by the officer for good reason, or termination by us other than for “cause”, options may be exercised until the earlier of twenty-four months following termination or expiration of the option term. Arrangements with our named executive officers in connection with a termination following a change in control are described below. Each agreement includes non-competition, non-solicitation and confidentiality covenants.
Other Compensation Practices and Policies
At our last annual meeting of stockholders on June 27, 2024, our non-binding stockholder advisory vote to approve the compensation of our named executive officers (commonly known as a “Say-on-Pay” vote) was supported by approximately 58% of the votes cast for or against advisory approval. We continue to evaluate our executive compensation program and solicit input from our largest investors. Following is a summary of our current compensation practices and policies.
|
· |
Retain an Independent Compensation Advisor. The compensation committee engaged Aon Consulting, Inc. through its Aon Rewards Solutions division (“Aon Rewards”), a nationally recognized global human resources consulting firm, as its independent compensation advisor for fiscal 2023 and fiscal 2024. Aon Rewards principally provided analysis, advice, and recommendations on named executive officers and non-employee director compensation. Our compensation peer group for named executive officer awards made in June 2023 was designed to reflect the industry and sector in which Palatin competes, as well as companies comparable to Palatin in terms of company life cycle, phase of development of potential products, market capitalization and talent market, and consists of: |
|
AcelRx Pharmaceuticals, Inc. |
|
Aldeyra Therapeutics, Inc. |
|
|
|
|
|
AIM ImmunoTech, Inc. |
|
Aptevo Therapeutics, Inc. |
|
|
|
|
|
Ardelyx, Inc. |
|
Kezar Life Sciences, Inc. |
|
|
|
|
|
Athersys, Inc. |
|
MEI Pharma, Inc. |
|
|
|
|
|
Clearside Biomedical, Inc. |
|
MeiraGTx Holdings plc |
|
|
|
|
|
Cumberland Pharmaceuticals, Inc. |
|
Paratek Pharmaceuticals, Inc. |
|
|
|
|
|
Eton Pharmaceuticals, Inc. |
|
Savara Inc. |
|
|
|
|
|
Kala Pharmaceuticals, Inc. |
|
Verastem, Inc. |
| 76 |
| Table of Contents |
|
|
We have determined to conduct a compensation peer group analysis every two years and anticipate that an independent compensation advisor will utilize a revised compensation peer group for awards to be made in June 2025 for the fiscal year ending June 30, 2026, including utilization of a compensation peer group. |
|
|
|
|
· |
Compensation at Risk. Our executive compensation program is designed so that a significant portion of compensation is “at risk” based on our performance, as well as short-term cash and long-term equity incentives to align the interests of our executive officers and stockholders. Long-term equity incentives will be no less than base salaries, with at least half of long-term equity incentives being performance-based. |
|
|
|
|
· |
Use a Pay-for-Performance Philosophy. The compensation committee employs a mixture of compensation elements designed to balance short-term goals with longer-term performance. Our executive compensation program includes these principal elements: |
|
○ |
Base salary, which targets the comparable position median salary for our peer group; |
|
|
|
|
○ |
An annual incentive compensation opportunity, with a target bonus payout of no less than 60% of base salary, depending on performance; and, |
|
|
|
|
○ |
A long-term incentive program consisting of stock option and restricted stock unit awards. In fiscal 2023, approximately 50% of all long-term incentive awards were allocated to performance-based stock options and performance-based restricted share units. |
|
· |
Maintain a Stock Ownership Policy. We adopted a stock ownership policy effective April 1, 2019, that requires our named executive officers, as well as our board members, to maintain a minimum ownership level of our common stock. As of June 30, 2024, the most recent “Determination Date” under the stock ownership policy, all board members met the target ownership level of shares of at least two times the annual retainer for board members. The named executive officers met the target ownership levels because they had met the policy guidelines previously, and no recalculation was required under the policy. Our stock ownership policy, which is on our website at www.palatin.com/investors/corporate-governance/, provides that if covered individuals meet the minimum ownership level of our common stock, a decrease in share price or increase in salary will not result in recalculation of the number of shares needed to satisfy the stock ownership policy unless the covered individual’s actual ownership levels drop below the number of shares required as of the Determination Date that he or she first satisfied the guidelines. The current named executive officers met the target ownership levels of shares as of June 30, 2022 and at all prior Determination Dates. In addition, certain time-based and performance-based restricted stock unit awards contain deferred delivery provisions providing for delivery of the common stock after the grantee’s separation from service or a defined changed in control. |
|
|
|
|
· |
Maintain a Clawback Policy. We have adopted a clawback policy allowing Palatin to recover related compensation should the board determine that compensation paid to named executive officers resulted from material noncompliance with financial reporting requirements under federal securities law. Our clawback policy is on our website at www.palatin.com/investors/corporate-governance/. |
|
|
|
|
· |
Maintain an Independent Compensation Committee. The compensation committee consists entirely of independent directors. |
|
|
|
|
· |
Annual Executive Compensation Review. The compensation committee conducts an annual review and approval of our compensation strategy, utilizing an independent compensation advisor. This review, including a peer group review, is intended to ensure that our compensation programs appropriately reward corporate growth without encouraging excessive or inappropriate risk-taking. |
|
|
|
|
· |
“Double Trigger” Feature for Acceleration of CEO and CFO/COO Equity Awards. Under employment agreements with our named executive officers, outstanding equity awards granted to our named executive officers provide that, upon a change in control of Palatin, the vesting of such awards will accelerate only in the event of a subsequent involuntary termination of employment (a “double-trigger” provision). |
| 77 |
| Table of Contents |
|
· |
No Excise Tax Gross-Ups. Prior to July 1, 2019, our employment agreements for the named executive officers provided that they were entitled to a tax gross-up for any golden parachute excise tax imposed on payments received in connection with a change in control. Most investors disfavor this type of tax gross-up benefit. In response to stockholder feedback, effective with new employment agreements for our named executive officers commencing July 1, 2019, we removed all golden parachute excise tax gross-up provisions. As a result, the Company no longer provides tax gross-ups for named executive officers or any other employees in the event they are subject to golden parachute excise taxes on payments received in connection with a change in control. |
|
|
|
|
· |
No Stock Option Re-pricing. Our 2011 Stock Incentive Plan does not permit options to purchase shares of our common stock to be repriced to a lower exercise or strike price without the approval of our stockholders. |
|
|
|
|
· |
No Dividends or Dividend Equivalents Payable on Unvested or Undelivered Equity Awards. Under our restricted share unit agreements, we do not pay dividends or dividend equivalents on unvested restricted stock unit awards or vested restricted stock unit awards subject to delayed delivery. |
|
|
|
|
· |
No Executive Retirement Plans. We do not offer pension arrangements or retirement plans or arrangements to our executive officers that are different from or in addition to those offered to our other employees. |
|
|
|
|
· |
No Special Welfare or Health Benefits. Our executive officers participate in broad-based Company-sponsored health and welfare benefit programs on the same basis as our other full-time, salaried employees. |
| 78 |
| Table of Contents |
Outstanding Equity Awards at 2024 Fiscal Year-End
The following table summarizes all of the outstanding equity-based awards granted to our named executive officers as of June 30, 2024, the end of our fiscal year.
|
|
|
Option awards (1) |
|
|
Stock awards (2) |
|
|||||||||||||||||||||||||||||||
Name |
|
Option or stock award grant date |
|
Number of securities underlying unexercised options (#) exercisable |
|
|
Number of securities underlying unexercised options (#) unexercisable |
|
|
Equity incentive plan award: number of securities underlying unexercised unearned options (#) |
|
|
Option exercise price ($) |
|
|
Option expiration date |
|
|
Number of shares or units of stock that have not vested (#) |
|
|
Market value of shares or units of stock that have not vested ($) (3) |
|
|
Equity incentive plan awards: number of unearned shares, unit or other rights that have not vested (#) |
|
|
Equity incentive plan awards: market or payout value of unearned shares, units or other rights that have not vested ($)(3) |
|
|||||||||
Carl Spana |
|
06/20/17 |
|
|
37,520 |
|
|
|
- |
|
|
|
- |
|
|
|
9.25 |
|
|
06/20/27 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/16/20 |
|
|
42,860 |
|
|
|
- |
|
|
|
- |
|
|
|
14.50 |
|
|
06/16/30 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/16/20 |
|
|
40,466 |
|
|
|
- |
|
|
|
- |
|
|
|
14.50 |
|
|
06/16/30 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/22/21 |
|
|
34,500 |
|
|
|
11,500 |
|
|
|
- |
|
|
|
13.75 |
|
|
06/22/31 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/22/21 |
|
|
31,966 |
|
|
|
- |
|
|
|
14,034 |
|
|
|
13.75 |
|
|
06/22/31 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/22/22 |
|
|
13,540 |
|
|
|
13,540 |
|
|
|
- |
|
|
|
7.25 |
|
|
06/22/32 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/22/22 |
|
|
12,143 |
|
|
|
- |
|
|
|
14,937 |
|
|
|
7.25 |
|
|
06/22/32 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/20/23 |
|
|
25,875 |
|
|
|
77,625 |
|
|
|
- |
|
|
|
2.19 |
|
|
06/20/33 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/20/23 |
|
|
21,993 |
|
|
|
- |
|
|
|
81,507 |
|
|
|
2.19 |
|
|
06/20/33 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/04/24 |
|
|
- |
|
|
|
113,500 |
|
|
|
- |
|
|
|
1.83 |
|
|
06/04/34 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/04/24 |
|
|
- |
|
|
|
- |
|
|
|
113,500 |
|
|
|
1.83 |
|
|
06/04/34 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
06/22/21 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,045 |
|
|
|
13,738 |
|
|
|
5,548 |
|
|
|
10,819 |
|
||||
|
|
06/22/22 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
9,100 |
|
|
|
17,745 |
|
|
|
10,038 |
|
|
|
19,574 |
|
||||
|
|
06/30/23 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
49,500 |
|
|
|
96,525 |
|
|
|
51,975 |
|
|
|
101,351 |
|
||||
|
|
06/04/24 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
79,000 |
|
|
|
154,050 |
|
|
|
79,000 |
|
|
|
154,050 |
|
||||
|
|
Total Stock Awards |
|
|
|
|
|
|
|
|
|
|
|
|
|
144,645 |
|
|
|
282,058 |
|
|
|
146,561 |
|
|
|
285,794 |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Stephen T. Wills |
|
06/20/17 |
|
|
34,360 |
|
|
|
- |
|
|
|
- |
|
|
|
9.25 |
|
|
06/20/27 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/16/20 |
|
|
36,920 |
|
|
|
- |
|
|
|
- |
|
|
|
14.50 |
|
|
06/16/30 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/16/20 |
|
|
34,858 |
|
|
|
- |
|
|
|
- |
|
|
|
14.50 |
|
|
06/16/30 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/22/21 |
|
|
29,820 |
|
|
|
9,940 |
|
|
|
- |
|
|
|
13.75 |
|
|
06/22/31 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/22/21 |
|
|
27,630 |
|
|
|
- |
|
|
|
12,130 |
|
|
|
13.75 |
|
|
06/22/31 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/22/22 |
|
|
11,750 |
|
|
|
11,750 |
|
|
|
- |
|
|
|
7.25 |
|
|
06/22/32 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/22/22 |
|
|
10,538 |
|
|
|
- |
|
|
|
12,962 |
|
|
|
7.25 |
|
|
06/22/32 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/20/23 |
|
|
22,500 |
|
|
|
67,500 |
|
|
|
- |
|
|
|
2.19 |
|
|
06/20/33 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/20/23 |
|
|
19,125 |
|
|
|
- |
|
|
|
70,875 |
|
|
|
2.19 |
|
|
06/20/33 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/04/24 |
|
|
- |
|
|
|
99,000 |
|
|
|
- |
|
|
|
1.83 |
|
|
06/04/34 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
06/04/24 |
|
|
- |
|
|
|
- |
|
|
|
99,000 |
|
|
|
1.83 |
|
|
06/04/34 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
06/22/21 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6,090 |
|
|
|
11,875 |
|
|
|
4,992 |
|
|
|
9,734 |
|
||||
|
|
06/22/22 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,900 |
|
|
|
15,405 |
|
|
|
8,715 |
|
|
|
16,994 |
|
||||
|
|
06/20/23 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
43,125 |
|
|
|
84,094 |
|
|
|
45,282 |
|
|
|
88,300 |
|
||||
|
|
06/04/24 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
69,000 |
|
|
|
134,550 |
|
|
|
69,000 |
|
|
|
134,550 |
|
||||
|
|
Total Stock Awards |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
126,115 |
|
|
|
245,924 |
|
|
|
127,989 |
|
|
|
249,578 |
|
|||||||
_____________
(1) |
Stock option vesting schedules: all options granted before June 22, 2021 have fully vested. Options granted on or after June 20, 2018 vest over four years with 1/4 of the shares vesting per year starting on the first anniversary of the grant date, provided that the named executive officer remains an employee; see “Termination and Change-In-Control Arrangements” below for a description of events that could accelerate vesting, except for performance-based options granted on June 22, 2021, June 22, 2022, June 20, 2023 and June 4, 2024, which vest according to the terms of the grants described above. |
|
|
(2) |
Time-based stock award vesting schedule: restricted stock units granted on June 22, 2021 as to 28,180 shares for Dr. Spana and 24,360 shares for Mr. Wills; restricted stock units granted on June 22, 2022 as to 18,200 shares to Dr. Spana and 15,800 shares for Mr. Wills; restricted stock units granted on June 20, 2023 as to 66,000 shares for Dr. Spana and 57,500 shares for Mr. Wills and restricted stock units granted on June 4, 2024 as to 79,000 shares for Dr. Spana and 69,000 shares for Mr. Wills, which vest in equal amounts over a four year period, provided that the named executive officer remains an employee. Both time-based and performance-based restricted stock unit awards prior to fiscal 2019 contain deferred delivery provisions providing for delivery of the common stock after the grantee’s separation from service or a defined change in control. See “Stock Options and Restricted Stock Unit Awards” above and “Termination and Change-In-Control Arrangements” below. |
|
|
(3) |
Calculated by multiplying the number of restricted stock units by $1.95, the closing market price of our common stock on June 28, 2024, the last trading day of our most recently completed fiscal year. |
| 79 |
| Table of Contents |
Termination and Change-In-Control Arrangements
The employment agreements, stock option agreements and restricted stock unit agreements with Dr. Spana and Mr. Wills contain the following provisions concerning severance compensation and the vesting of stock options and restricted stock units upon termination of employment or upon a change in control. The executive’s entitlement to severance, payment of health benefits and accelerated vesting of options is contingent on the executive executing a general release of claims against us.
Termination Without Severance Compensation. Regardless of whether there has been a change in control, if we terminate employment for cause or the executive terminates employment without good reason (as those terms are defined in the employment agreement and set forth below), then the executive will receive only his accrued salary and vacation benefits through the date of termination. He may also elect to receive medical and dental benefits pursuant to COBRA for up to two years but must remit the cost of coverage to us. Under the terms of our outstanding options and restricted stock units, all unvested options and restricted stock units would terminate immediately, and vested options would be exercisable for three months after termination.
Severance Compensation After Death or Disability. In the event of the executive’s death or disability, we will provide lump sum severance pay equal to 24 months of base pay, as well as the opportunity for COBRA benefits as described above under “Termination Without Severance Compensation.”
Severance Compensation Without a Change in Control. If we terminate or fail to extend the employment agreement without cause, or the executive terminates employment with good reason, then the executive will receive as severance pay his salary then in effect, paid in a lump sum, plus medical and dental benefits at our expense, for a period of two years after the termination date. In addition, upon such event all unvested options would immediately vest and be exercisable for two years after the termination date or, if earlier, the expiration of the option term, and all unvested restricted stock units would accelerate and become fully vested.
Severance Compensation After a Change in Control. If, within one year after a change in control, we terminate employment or the executive terminates employment with good reason, then the executive will receive as severance pay 200% of his salary then in effect, paid in a lump sum, plus medical and dental benefits at our expense, for a period of two years after the termination date. We would also reimburse the executive for up to $25,000 in fees and expenses during the six months following termination, for locating employment. All unvested options would immediately vest and be exercisable for two years after the termination date or, if earlier, the expiration of the option term. All unvested restricted stock units would vest upon a change in control, without regard to whether the executive’s employment is terminated.
Option and Restricted Stock Unit Vesting Upon a Change in Control. Pursuant to the employment agreements, options and restricted stock units granted under the 2011 Stock Incentive Plan vest upon termination of the employee within twelve months following a change in control. If any options granted under the 2005 Stock Plan are to be terminated in connection with a change in control, those options will vest in full immediately before the change in control.
Definitions. Under the employment agreements, a “change in control,” “cause” and “good reason” are defined as follows:
A “change in control” occurs when:
|
(a) |
any person or entity acquires more than 50% of the voting power of our outstanding securities; |
|
|
|
|
(b) |
the individuals who, during any twelve-month period, constitute our board of directors cease to constitute at least a majority of the board of directors; |
|
|
|
|
(c) |
the consummation of a merger or consolidation; or |
|
|
|
|
(d) |
we sell substantially all our assets. |
| 80 |
| Table of Contents |
The term “cause” means:
|
(a) |
the occurrence of (i) the executive’s material breach of, or habitual neglect or failure to perform the material duties which he is required to perform under, the terms of his employment agreement; (ii) the executive’s material failure to follow the reasonable directives or policies established by or at the direction of our board of directors; or (iii) the executive’s engaging in conduct that is materially detrimental to our interests such that we sustain a material loss or injury as a result thereof, provided that the breach or failure of performance is not cured, to the extent cure is possible, within ten days of the delivery to the executive of written notice thereof; |
|
|
|
|
(b) |
the willful breach by the executive of his obligations to us with respect to confidentiality, invention and non-disclosure, non-competition or non-solicitation; or |
|
|
|
|
(c) |
the conviction of the executive of, or the entry of a pleading of guilty or nolo contendere by the executive to, any crime involving moral turpitude or any felony. |
The term “good reason” means the occurrence of any of the following, with our failure to cure such circumstances within 30 days of the delivery to us of written notice by the executive of such circumstances:
|
(a) |
any material adverse change in the executive’s duties, authority or responsibilities, which causes the executive’s position with us to become of significantly less responsibility, or assignment of duties and responsibilities inconsistent with the executive’s position; |
|
|
|
|
(b) |
a material reduction in the executive’s salary; |
|
|
|
|
(c) |
our failure to continue in effect any material compensation or benefit plan in which the executive participates, unless an equitable arrangement has been made with respect to such plan, or our failure to continue the executive’s participation therein (or in a substitute or alternative plan) on a basis not materially less favorable, both in terms of the amount of benefits provided and the level of the executive’s participation relative to other participants; |
|
|
|
|
(d) |
our failure to continue to provide the executive with benefits substantially similar to those enjoyed by the executive under any of our health and welfare insurance, retirement and other fringe-benefit plans, the taking of any action by us which would directly or indirectly materially reduce any of such benefits, or our failure to provide the executive with the number of paid vacation days to which he is entitled; or |
|
|
|
|
(e) |
the relocation of the executive to a location which is a material distance from Cranbury, New Jersey. |
Director Compensation
The following table sets forth the compensation we paid to all directors during fiscal 2024, except for Dr. Spana, whose compensation is set forth above in the Summary Compensation Table and related disclosure. Dr. Spana did not receive any separate compensation for his services as a director.
Name |
|
Fees earned or paid in cash ($) |
|
|
Stock awards ($) (1) (2) |
|
|
Option awards ($) (1) (2) |
|
|
Total ($) |
|
||||
John K.A. Prendergast, Ph.D. |
|
|
97,500 |
|
|
|
28,470 |
|
|
|
28,836 |
|
|
|
154,806 |
|
Robert K. deVeer, Jr. |
|
|
70,000 |
|
|
|
21,900 |
|
|
|
20,972 |
|
|
|
112,872 |
|
J. Stanley Hull |
|
|
60,000 |
|
|
|
21,900 |
|
|
|
20,972 |
|
|
|
102,872 |
|
Alan W. Dunton, M.D. |
|
|
70,000 |
|
|
|
21,900 |
|
|
|
20,972 |
|
|
|
112,872 |
|
Arlene Morris |
|
|
55,000 |
|
|
|
21,900 |
|
|
|
20,972 |
|
|
|
97,872 |
|
Anthony Manning, Ph.D. |
|
|
55,000 |
|
|
|
21,900 |
|
|
|
20,972 |
|
|
|
97,872 |
|
(1) |
The aggregate number of shares underlying option awards and unvested stock awards outstanding at June 30, 2024, for each director was: |
|
|
Option awards |
|
|
Stock awards |
|
||
Dr. Prendergast |
|
|
82,080 |
|
|
|
16,000 |
|
Mr. deVeer |
|
|
57,180 |
|
|
|
12,000 |
|
Mr. Hull |
|
|
57,180 |
|
|
|
12,000 |
|
Dr. Dunton |
|
|
57,180 |
|
|
|
12,000 |
|
Ms. Morris |
|
|
57,180 |
|
|
|
12,000 |
|
Dr. Manning |
|
|
54,280 |
|
|
|
12,000 |
|
(2) |
Amounts in these columns represent the aggregate grant date fair value for stock awards and option awards. For a description of the assumptions we used to calculate these amounts, see Note 15 to the consolidated financial statements included in this Annual Report. Amounts in this column include options granted on June 4, 2024 for our current fiscal year ending June 30, 2025. |
| 81 |
| Table of Contents |
Our director compensation program is designed to enhance our ability to attract and retain highly qualified directors and to align their interests with the long-term interests of our stockholders. The program includes an equity component, which is designed to align the interests of non-employee directors and stockholders, and a cash component, which is designed to compensate non-employee directors for their service on the board of directors. Directors who are employees of the Company receive no additional compensation for their service on the board of directors.
The compensation committee annually reviews compensation paid to our non-employee directors and makes recommendations for adjustments, as appropriate, to the full board of directors. As part of this annual review, the compensation committee considers the significant time commitment and skill level required by each non-employee director in serving on the board of directors and its various committees. The compensation committee seeks to maintain a market competitive director compensation program and, with the assistance of its independent compensation consultant, Aon Rewards, benchmarks our director compensation program against the peer group we use to evaluate our executive compensation program.
Non-Employee Directors’ Equity Grants. Our non-employee directors receive an annual equity grant at the board of directors meeting closest to the beginning of each fiscal year, or such other date as may be determined by the board of directors.
On June 4, 2024, we granted the Chairman of the board of directors 16,000 restricted stock units which vest on June 4, 2025 and an option to purchase 23,000 shares of common stock, and each other serving non-employee director received 12,000 restricted stock units which vest on June 4, 2025 and an option to purchase 17,000 shares of common stock. All of the options have an exercise price of $1.83 per share, the closing price of our common stock on the date of grant, vests on June 4, 2025, expire ten years from the date of grant and provide for accelerated vesting in the event of involuntary termination as a director following a change in control, with exercise permitted following accelerated vesting for up to the earlier of one year after termination or the expiration date of the option. The grants were subject to certification by the Chief Financial Officer that the Company’s stockholders had increased the shares reserved under the 2011 Stock Incentive Plan, and that there were no impediments to the grant of the restricted stock units and options. The required certification was made on July 15, 2024.
On June 20, 2023, the Chairman of the board of directors received 13,000 restricted stock units which vest on June 20, 2024 and an option to purchase 22,000 shares of common stock, and each other serving non-employee director received 10,000 restricted stock units which vest on June 20, 2024 and an option to purchase 16,000 shares of common stock. All of the options have an exercise price of $2.19 per share, the closing price of our common stock on the business day immediately preceding the date of grant, vest in twelve monthly installments beginning July 31, 2023, expire ten years from the date of grant and provide for accelerated vesting in the event of involuntary termination as a director following a change in control, with exercise permitted following accelerated vesting for up to the earlier of one year after termination or the expiration date of the option.
Non-Employee Directors’ Cash Compensation. Dr. Prendergast serves as Chairman of the board of directors and for fiscal 2024 received an annual retainer of $87,500, payable quarterly. Other non-employee directors received an annual base retainer of $40,000, payable on a quarterly basis. The chairperson of the audit committee received an additional annual retainer of $20,000, the chairperson of the compensation committee received an additional annual retainer of $20,000 and the chairperson of the corporate governance committee received an additional annual retainer of $10,000. Members of the foregoing committees, other than the non-employee Chairman, received an additional retainer of one-half the retainer payable to the committee chairperson. For the fiscal year ending June 30, 2025, Dr. Prendergast serves as Chairman of the board of directors and will receive an annual retainer of $109,500, payable quarterly. Other non-employee directors will receive an annual base retainer of $50,000, payable on a quarterly basis. The chairperson of the audit committee will receive an additional annual retainer of $20,000, the chairperson of the compensation committee will receive an additional annual retainer of $20,000 and the chairperson of the corporate governance committee will receive an additional annual retainer of $10,000. Members of the foregoing committees, other than the non-employee Chairman, receive an additional retainer of one-half the retainer payable to the committee chairperson.
| 82 |
| Table of Contents |
The board of directors also formed a program development committee, charged with reviewing new product opportunities and product development strategy. The chairperson of the program development committee receives $3,500 per day of service, and members of the committee receive $2,500 per day of service.
Non-Employee Directors’ Expenses. Non-employee directors are reimbursed for expenses incurred in performing their duties as directors, including attending all meetings of the board of directors and any committees on which they serve.
Employee Directors. Employee directors are not separately compensated for services as directors but are reimbursed for expenses incurred in performing their duties as directors, including attending all meetings of the board of directors and any committees on which they serve.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Securities Authorized for Issuance Under Equity Compensation Plans. The table below provides information on our equity compensation plans as of June 30, 2024:
|
Equity Compensation Plan Information as of June 30, 2024 | ||||||||||||
Plan category |
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
|
|
Weighted-average exercise price of outstanding options, warrants and rights |
|
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|
|||
|
|
(a) |
|
|
(b) |
|
|
(c) |
|
|||
Equity compensation plans approved by security holders |
|
|
3,638,420 | (1) |
|
$ | 6.11 | (2) |
|
|
206,474 |
|
Equity compensation plans not approved by security holders |
|
|
- |
|
|
|
- |
|
|
|
- |
|
Total |
|
|
3,638,420 |
|
|
|
|
|
|
|
206,474 |
|
(1) |
Includes 2,263,440 options and 1,374,980 restricted stock units granted under our 2011 Stock Incentive Plan. |
|
|
(2) |
The amount in column (a) for equity compensation plans approved by security holders includes 1,374,980 shares reserved for issuance on vesting of outstanding restricted stock units, granted under our 2011 Stock Incentive Plan, which vest on various dates through June 4, 2028, subject to the fulfillment of service, or performance conditions. Because no exercise price is required for issuance of shares on vesting of the restricted stock units, the weighted-average exercise price in column (b) does not take the restricted stock units into account. |
Beneficial Ownership Tables. The tables below show the beneficial stock ownership and voting power, as of September 27, 2024, of:
|
· |
each director, each of the named executive officers, and all current directors and officers as a group; and |
|
|
|
|
· |
all persons who, to our knowledge, beneficially own more than five percent of the common stock or Series A preferred stock. |
“Beneficial ownership” here means direct or indirect voting or investment power over outstanding stock and stock which a person has the right to acquire now or within 60 days after September 27, 2024. See the footnotes for more detailed explanations of the holdings. Except as noted, to our knowledge, the persons named in the tables beneficially own and have sole voting and investment power over all shares listed.
The common stock has one vote per share and the Series A preferred stock has approximately one vote per share of Series A preferred stock. Voting power is calculated on the basis of the aggregate of common stock and Series A preferred stock outstanding as of September 27, 2024, on which date 19,548,167 shares of common stock and 4,030 shares of Series A preferred stock, convertible into 5,333 shares of common stock, were outstanding.
| 83 |
| Table of Contents |
Under our Insider Trading and Securities Law Compliance Policy directors and officers may not engage in hedging, monetization or pledging transactions of our securities. None of the shares of our management and directors shown on the table below are pledged.
The address for all members of our management and directors is c/o Palatin Technologies, Inc., 4B Cedar Brook Drive, Cranbury, NJ 08512. Addresses of other beneficial owners are in the table.
MANAGEMENT:
Class |
|
Name of beneficial owner |
|
Amount and nature of beneficial ownership |
|
|
Percent of class |
|
|
Percent of total voting power |
|
|||
Common |
|
Carl Spana, Ph.D. |
|
|
522,322 | (1) |
|
|
2.6 | % |
|
* |
|
|
Common |
|
Stephen T. Wills |
|
|
462,956 | (2) |
|
|
2.3 | % |
|
* |
|
|
Common |
|
John K.A. Prendergast, Ph.D. |
|
|
106,390 | (3) |
|
* |
|
|
* |
|
||
Common |
|
Robert K. deVeer, Jr. |
|
|
66,245 | (4) |
|
* |
|
|
* |
|
||
Common |
|
J. Stanley Hull |
|
|
70,452 | (5) |
|
* |
|
|
* |
|
||
Common |
|
Alan W. Dunton, M.D. |
|
|
70,474 | (6) |
|
* |
|
|
* |
|
||
Common |
|
Arlene M. Morris |
|
|
69,760 | (7) |
|
* |
|
|
* |
|
||
Common |
|
Anthony M. Manning, Ph.D. |
|
|
61,360 | (8) |
|
* |
|
|
* |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
All current directors and executive officers as a group (eight persons) |
|
|
1,429,959 | (9) |
|
|
7.0 | % |
|
|
2.2 | % |
*Less than one percent.
(1) |
Includes 260,863 shares of common stock underlying outstanding options and 120,640 shares of common stock underlying restricted stock units, all of which shares of common stock underlying restricted stock units have vested but not been delivered under deferred delivery provisions providing for delivery after the grantee’s separation from service or a defined change in control, but does not include shares of common stock underlying outstanding options or restricted stock unit awards that have not vested and will not vest within 60 days. |
|
|
(2) |
Includes 227,501 shares of common stock underlying outstanding options and 106,540 shares of common stock underlying restricted stock units, all of which shares of common stock underlying restricted stock units have vested but not been delivered under deferred delivery provisions providing for delivery after the grantee’s separation from service or a defined change in control, but does not include shares of common stock underlying outstanding options or restricted stock unit awards that have not vested and will not vest within 60 days. |
|
|
(3) |
Includes 59,080 shares of common stock underlying outstanding options and 6,400 shares of common stock underlying restricted stock units, all of which shares of common stock underlying restricted stock units have vested but not been delivered under deferred delivery provisions providing for delivery after the grantee’s separation from service or a defined change in control, but does not include shares of common stock underlying outstanding options or restricted stock unit awards that have not vested and will not vest within 60 days. |
| 84 |
| Table of Contents |
(4) |
Includes 40,180 shares of common stock underlying outstanding options and 3,200 shares of common stock underlying restricted stock units, all of which shares of common stock underlying restricted stock units have vested but not been delivered under deferred delivery provisions providing for delivery after the grantee’s separation from service or a defined change in control, but does not include shares of common stock underlying outstanding options or restricted stock unit awards that have not vested and will not vest within 60 days. |
|
|
(5) |
Includes 40,180 shares of common stock underlying outstanding options and 3,200 shares of common stock underlying restricted stock units, all of which shares of common stock underlying restricted stock units have vested but not been delivered under deferred delivery provisions providing for delivery after the grantee’s separation from service or a defined change in control, but does not include shares of common stock underlying outstanding options or restricted stock unit awards that have not vested and will not vest within 60 days. |
|
|
(6) |
Includes 40.180 shares of common stock underlying outstanding options and 2,800 shares of common stock underlying restricted stock units, all of which shares of common stock underlying restricted stock units have vested but not been delivered under deferred delivery provisions providing for delivery after the grantee’s separation from service or a defined change in control, but does not include shares of common stock underlying outstanding options or restricted stock unit awards that have not vested and will not vest within 60 days. |
|
|
(7) |
Includes 40,180 shares of common stock underlying outstanding options and 2,000 shares of common stock underlying restricted stock units, all of which shares of common stock underlying restricted stock units have vested but not been delivered under deferred delivery provisions providing for delivery after the grantee’s separation from service or a defined change in control, but does not include shares of common stock underlying outstanding options or restricted stock unit awards that have not vested and will not vest within 60 days. |
|
|
(8) |
Includes 37,280 shares of common stock underlying outstanding options and 600 shares of common stock underlying restricted stock units, all of which shares of common stock underlying restricted stock units have vested but not been delivered under deferred delivery provisions providing for delivery after the grantee’s separation from service or a defined change in control, but does not include shares of common stock underlying outstanding options or restricted stock unit awards that have not vested and will not vest within 60 days. |
|
|
(9) |
Includes 990,824 shares of common stock underlying outstanding options and restricted stock units. |
| 85 |
| Table of Contents |
MORE THAN 5% BENEFICIAL OWNERS:
Class |
|
Name and address of beneficial owner |
|
Amount and nature of beneficial ownership (1) |
|
|
Percent of class |
|
|
Percent of total voting power |
|||
|
Series A Preferred |
|
Steven N. Ostrovsky 43 Nikki Ct. Morganville, NJ 07751 |
|
|
500 |
|
|
|
12.4 | % |
|
* |
|
|
Series A Preferred |
|
Thomas L. Cassidy IRA Rollover 38 Canaan Close New Canaan, CT 06840 |
|
|
500 |
|
|
|
12.4 | % |
|
* |
|
|
Series A Preferred |
|
Jonathan E. Rothschild 300 Mercer St., #28F New York, NY 10003 |
|
|
500 |
|
|
|
12.4 | % |
|
* |
|
|
Series A Preferred |
|
Arthur J. Nagle 19 Garden Avenue Bronxville, NY 10708 |
|
|
250 |
|
|
|
6.2 | % |
|
* |
|
|
Series A Preferred |
|
Thomas P. and Mary E. Heiser, JTWROS 10 Ridge Road Hopkinton, MA 01748 |
|
|
250 |
|
|
|
6.2 | % |
|
* |
|
|
Series A Preferred |
|
Carl F. Schwartz 31 West 87th St. New York, NY 10016 |
|
|
250 |
|
|
|
6.2 | % |
|
* |
|
|
Series A Preferred |
|
Michael J. Wrubel 3650 N. 36 Avenue, #39 Hollywood, FL 33021 |
|
|
250 |
|
|
|
6.2 | % |
|
* |
|
|
Series A Preferred |
|
Myron M. Teitelbaum, M.D. 175 Burton Lane Lawrence, NY 11559 |
|
|
250 |
|
|
|
6.2 | % |
|
* |
|
|
Series A Preferred |
|
Laura Gold Galleries Ltd. Profit Sharing Trust Park South Gallery at Carnegie Hall 154 West 57th Street, Suite 114 New York, NY 10019 |
|
|
250 |
|
|
|
6.2 | % |
|
* |
|
|
Series A Preferred |
|
Laura Gold 180 W. 58th Street New York, NY 10019 |
|
|
250 |
|
|
|
6.2 | % |
|
* |
|
|
Series A Preferred |
|
Nadji T. Richmond 20 E. Wedgewood Glen The Woodlands, TX 77381 |
|
|
230 |
|
|
|
5.7 | % |
|
* |
|
*Less than one percent.
(1) Unless otherwise indicated by footnote, all share amounts represent outstanding shares of the class indicated, and all beneficial owners listed have, to our knowledge, sole voting and dispositive power over the shares listed.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The board of directors has determined that all the directors except for Dr. Spana (our Chief Executive Officer and President) are independent directors, as defined in the listing standards of the NYSE American.
As a condition of employment, we require all employees to disclose in writing actual or potential conflicts of interest, including related party transactions. Our code of corporate conduct and ethics, which applies to employees, officers and directors, requires that the audit committee review and approve related party transactions. Since July 1, 2022, there have been no transactions or proposed transactions in which we were or are to be a participant, in which any related person had or will have a direct or indirect material interest.
Item 14. Principal Accountant Fees and Services.
KPMG LLP (“KPMG”), Philadelphia, PA, Auditor Firm ID: 185, served as our independent registered public accounting firm for fiscal 2024 and fiscal 2023.
Audit Fees. For fiscal 2024, fees for professional services rendered for the audit of our annual consolidated financial statements and review of our consolidated financial statements in our Forms 10-Q and services provided in connection with regulatory filings and comfort letters were $504,000.
For fiscal 2023, fees for professional services rendered for the audit of our annual consolidated financial statements and review of our consolidated financial statements in our Forms 10-Q and services provided in connection with regulatory filings and comfort letters were $491,500.
Audit-Related Fees. For fiscal 2024 and fiscal 2023, KPMG did not perform or bill us for any audit-related services.
Tax Fees. For fiscal 2024, KPMG billed us $70,662 for professional services rendered for tax compliance services. For fiscal 2023, KPMG billed us $26,600 for professional services rendered for tax compliance services.
All Other Fees. KPMG did not perform or bill us for any services other than those described above for fiscal 2024 and fiscal 2023.
| 86 |
| Table of Contents |
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Auditors. Consistent with SEC policies regarding auditor independence, the audit committee has responsibility for appointing, setting compensation for and overseeing the work of the independent registered public accounting firm. In recognition of this responsibility, the audit committee has established a policy to pre-approve all audit and permissible non-audit services provided by the independent registered public accounting firm.
The audit committee pre-approves fees for each category of service. The fees are budgeted and the audit committee requires the independent registered public accounting firm and management to report actual fees versus the budget periodically throughout the year by category of service. During the year, circumstances may arise when it may become necessary to engage the independent registered public accounting firm for additional services not contemplated in the original pre-approval. In those instances, the audit committee requires specific pre-approval before engaging the independent registered public accounting firm.
The audit committee may delegate pre-approval authority to one or more of its members. The member to whom such authority is delegated must report, for informational purposes only, any pre-approval decisions to the audit committee at its next scheduled meeting.
| 87 |
| Table of Contents |
PART IV
Item 15. Exhibit and Financial Statement Schedules.
(a) Documents filed as part of the report:
1. Financial statements: The following consolidated financial statements are filed as a part of this report under Item 8 – Financial Statements and Supplementary Data:
— Report of Independent Registered Public Accounting Firm
— Consolidated Balance Sheets
— Consolidated Statements of Operations
— Consolidated Statements of Changes in Redeemable Convertible Preferred Stock and Stockholders’ Equity
— Consolidated Statements of Cash Flows
— Notes to Consolidated Financial Statements
2. Financial statement schedules: None.
3. List of Exhibits
The following exhibits are incorporated by reference or filed as part of this report:
|
Exhibit Number |
Description |
Filed Herewith |
Form |
Filing Date |
SEC File No. |
Restated Certificate of Incorporation of Palatin Technologies, Inc., as amended. |
10-K |
September 27, 2013 |
001-15543 |
||
8-K |
September 17, 2021 |
001-15543 |
|||
3.3 |
Certificate of Decrease of Series A Convertible Preferred Stock |
|
10-Q |
May 16, 2022 |
001-15543 |
|
8-K |
August 31, 2022 |
001-15543 |
||
|
8-K |
June 21, 2024 |
001-15543 |
||
|
8-K |
June 21, 2024 |
001-15543 |
||
|
8-K |
February 1, 2024 |
001-15543 |
||
|
8-K |
February 1, 2024 |
001-15543 |
||
|
10-Q |
February 14, 2024 |
001-15543 |
||
|
10-Q |
February 14, 2024 |
001-15543 |
||
|
8-K |
October 24, 2023 |
001-15543 |
||
8-K |
October 24, 2023 |
001-15543 |
| 88 |
| Table of Contents |
|
Exhibit Number |
Description |
Filed Herewith |
Form |
Filing Date |
SEC File No. |
8-K |
October 24, 2023 |
001-15543 |
|||
10-Q |
May 16, 2022 |
001-15543 |
|||
10-Q |
May 16, 2022 |
001-15543 |
|||
8-K |
November 2, 2022 |
001-15543 |
|||
|
8-K |
November 2, 2022 |
001-15543 |
||
8-K |
November 2, 2022 |
001-15543 |
|||
|
8-K |
July 6, 2012 |
001-15543 |
||
|
8-K |
July 6, 2012 |
001-15543 |
||
|
8-K |
December 30, 2014 |
001-15543 |
||
|
8-K |
December 30, 2014 |
001-15543 |
||
8-K |
July 7, 2015 |
001-15543 |
|||
8-K |
July 7, 2015 |
001-15543 |
|||
8-K |
July 7, 2015 |
001-15543 |
|||
8-K |
August 2, 2016 |
001-15543 |
|||
8-K |
August 2, 2016 |
001-15543 |
|||
8-K |
December 1, 2016 |
001-15543 |
|||
10-K |
September 12, 2019 |
001-15543 |
|||
10-K |
September 28, 2009 |
001-15543 |
|||
10.2† |
Form of Option Certificate (Incentive Option) Under the 2005 Stock Plan. |
8-K |
September 21, 2011 |
001-15543 |
|
10.3† |
Form of Incentive Stock Option Under the 2005 Stock Plan. |
8-K |
September 21, 2011 |
001-15543 |
|
10.4† |
Form of Opinion Certificate (Non-Qualified Opinion) Under the 2005 Stock Plan. |
8-K |
September 21, 2011 |
001-15543 |
|
10.5† |
Form of Non-Qualified Stock Option Agreement Under the 2005 Stock Plan. |
8-K |
September 21, 2011 |
001-15543 |
|
10-Q |
February 8, 2008 |
001-15543 |
|||
10-Q |
May 15, 2009 |
001-15543 |
| 89 |
| Table of Contents |
|
Exhibit Number |
Description |
Filed Herewith |
Form |
Filing Date |
SEC File No. |
|
10-Q |
May 14, 2008 |
001-15543 |
||
|
10-Q |
May 14, 2008 |
001-15543 |
||
Form of Amended Option Certificate (Incentive Option) Under the 2005 Stock Plan. |
|
10-Q |
May 14, 2008 |
001-15543 |
|
10.11† |
2011 Stock Incentive Plan, as amended, restated and adopted by the stockholders on June 20, 2023. |
X |
|
|
|
Form of Restricted Share Unit Agreement Under the 2011 Stock Incentive Plan. |
|
10-Q |
May 13, 2011 |
001-15543 |
|
Form of Nonqualified Stock Option Agreement under the 2011 Stock Incentive Plan. |
|
10-Q |
May 13, 2011 |
001-15543 |
|
Form of Incentive Stock Option Agreement under the 2011 Stock Incentive Plan. |
|
10-Q |
May 13, 2011 |
001-15543 |
|
Form of Restricted Share Unit Agreement under the 2011 Stock Incentive Plan. |
|
8-K |
December 11, 2015 |
001-15543 |
|
Form of Performance-Based Restricted Share Unit Agreement under the 2011 Stock Incentive Plan. |
|
8-K |
December 11, 2015 |
001-15543 |
|
|
8-K |
December 11, 2015 |
001-15543 |
||
Amended form of Restricted Share Unit Agreement under the 2011 Stock Incentive Plan. |
|
10-Q |
February 12, 2016 |
001-15543 |
|
|
10-Q |
February 12, 2016 |
001-15543 |
||
|
10-Q |
February 12, 2016 |
001-15543 |
||
|
S-3 |
August 17, 2018 |
333-226905 |
||
|
8-K |
July 7, 2015 |
001-15543 |
| 90 |
| Table of Contents |
|
Exhibit Number |
Description |
Filed Herewith |
Form |
Filing Date |
SEC File No. |
|
10-Q |
November 16, 2020 |
001-15543 |
||
|
10-Q |
November 16, 2020 |
001-15543 |
||
|
10-Q |
February 10, 2017 |
001-15543 |
||
|
10-Q |
November 13, 2017 |
001-15543 |
||
|
8-K |
June 24, 2022 |
001-15543 |
||
|
8-K |
June 24, 2022 |
001-15543 |
||
|
8-K |
July 27, 2020 |
001-15543 |
||
|
10-K |
September 25, 2020 |
001-15543 |
||
|
10-K |
September 25, 2020 |
001-15543 |
||
|
10-Q |
November 16, 2020 |
001-15543 |
||
|
10-Q |
November 16, 2020 |
001-15543 |
| 91 |
| Table of Contents |
|
Exhibit Number |
Description |
Filed Herewith |
Form |
Filing Date |
SEC File No. |
|
8-K |
April 12, 2023 |
001-15543 |
||
|
8-K |
October 24, 2023 |
001-15543 |
||
|
10-Q |
February 14, 2024 |
001-15543 |
||
|
8-K |
February 1, 2024 |
001-15543 |
||
|
8-K |
June 21, 2024 |
001-15543 |
||
Palatin Technologies, Inc. Insider Trading and Securities Law Compliance Policy. |
X |
|
|
|
|
X |
|
|
|
||
X |
|
|
|
||
X |
|
|
|
||
X |
|
|
|
||
X |
|
|
|
||
X |
|
|
|
||
Palatin Technologies, Inc. Compensation Recovery Policy (Clawback Policy). |
X |
|
|
|
|
101.INS |
Inline XBRL Instance Document. |
X |
|
|
|
101.SCH |
Inline XBRL Taxonomy Extension Schema Document. |
X |
|
|
|
101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
X |
|
|
|
101.LAB |
Inline XBRL Taxonomy Extension Label Linkbase Document. |
X |
|
|
|
101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
X |
|
|
|
101.DEF |
Inline XBRL Taxonomy Extension Definition Linkbase Document. |
X |
|
|
|
104 |
Cover Page Interactive Data File (Formatted as Inline XBRL and contained in Exhibit 101). |
X |
|
|
|
† |
Management contract or compensatory plan or arrangement. |
†† |
Confidential treatment granted as to certain portions of the exhibit, which portions are omitted and filed separately with the SEC. |
††† |
Portions of the exhibit are omitted pursuant to Regulation S-K Item 601(b)(10). Palatin agrees to furnish to the U.S. Securities and Exchange Commission a copy of any omitted schedule and/or exhibit upon request. The confidential portions of this exhibit were omitted by means of marking such portions with asterisks because the identified confidential portions (i) are not material and (ii) would be competitively harmful if publicly disclosed. |
§ |
In accordance with Item 601(b)(32)(ii) of Regulation S-K and SEC Release Nos. 33-8238 and 34-47986, Final Rule: Management’s Reports on Internal Control Over Financial Reporting and Certification of Disclosure in Exchange Act Periodic Reports, the certifications furnished in Exhibit 32.1 and 32.2 hereto is deemed to accompany this Annual Report on Form 10-K and will not be deemed “filed” for purposes of Section 18 of the Exchange Act. Such certifications will not be deemed to be incorporated by reference into any filing under the Securities Act or the Exchange Act, except to the extent that the registrant specifically incorporates them by reference. |
Item 16. Form 10-K Summary.
None
| 92 |
| Table of Contents |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
PALATIN TECHNOLOGIES, INC.
By: |
/s/ Carl Spana |
|
|
Carl Spana, Ph.D. |
|
|
President and Chief Executive Officer |
|
|
(principal executive officer) |
|
Date: September 30, 2024
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature |
|
Title |
|
Date |
|
|
|
|
|
/s/ Carl Spana |
|
President, Chief Executive Officer and Director |
|
September 30, 2024 |
Carl Spana |
|
(principal executive officer) |
|
|
|
|
|
|
|
/s/ Stephen T. Wills |
|
Executive Vice President, Chief Financial Officer |
|
September 30, 2024 |
Stephen T. Wills |
|
and Chief Operating Officer (principal financial and accounting officer) |
|
|
|
|
|
|
|
/s/ John K. A. Prendergast |
|
Chairman and Director |
|
September 30, 2024 |
John K. A. Prendergast |
|
|
|
|
|
|
|
|
|
/s/ Robert K. deVeer, Jr. |
|
Director |
|
September 30, 2024 |
Robert K. deVeer, Jr. |
|
|
|
|
|
|
|
|
|
/s/ J. Stanley Hull |
|
Director |
|
September 30, 2024 |
J. Stanley Hull |
|
|
|
|
|
|
|
|
|
/s/ Alan W. Dunton |
|
Director |
|
September 30, 2024 |
Alan W. Dunton |
|
|
|
|
|
|
|
|
|
/s/ Arlene M. Morris |
|
Director |
|
September 30, 2024 |
Arlene M. Morris |
|
|
|
|
|
|
|
|
|
/s/ Anthony M. Manning |
|
Director |
|
September 30, 2024 |
Anthony M. Manning |
|
|
|
|
| 93 |
EXHIBIT 19
INSIDER TRADING AND SECURITIES LAW COMPLIANCE POLICY OF
PALATIN TECHNOLOGIES, INC.
Effective October 1, 2013
I. BACKGROUND
The Board of Directors of Palatin Technologies, Inc. has adopted this Insider Trading and Securities Law Compliance Policy relating to the trading of Palatin securities. The terms “Palatin,” “we,” “us,” and “our” mean Palatin Technologies, Inc. and all of its subsidiaries.
Effective as of October 1, 2013, this Insider Trading and Securities Law Compliance Policy replaces all prior policies relating to the same subject matter, including our Securities Transactions Insider Trading Policy.
Federal and state securities laws prohibit the purchase or sale of a company’s securities by persons who are aware of material information about that company that is not generally known or available to the public. These laws also prohibit persons who are aware of such material nonpublic information from disclosing this information to others who may trade. Companies and their controlling persons are also subject to liability if they fail to take reasonable steps to prevent insider trading by company personnel.
It is important that you understand the breadth of activities that constitute illegal insider trading and the resulting consequences, which can be severe. This policy is applicable to all trading of our securities. Both the Securities and Exchange Commission (the “SEC”) and the NYSE MKT LLC stock market investigate and are very effective at detecting insider trading. The SEC and U.S. Attorneys pursue insider trading violations vigorously. Cases have been successfully prosecuted against trading by employees through foreign accounts, trading by family members and friends and trading involving only a small number of shares.
This policy is designed to prevent insider trading or allegations of insider trading and protect our reputation for integrity and ethical conduct. It is your obligation to understand and comply with this policy. Should you have any questions regarding this policy, please contact our Chief Financial Officer.
II. BASIC POLICY / ADDITIONAL RESTRICTIONS ON RESTRICTED PERSONS
No director, officer, employee, consultant or agent (or their family members, members of their household or controlled entities) of Palatin and its affiliates may trade on the basis of material nonpublic information or engage in any other action to take advantage of, or pass on to others, that information. To avoid even the appearance of impropriety, additional restrictions on trading our securities apply to our directors, executive officers and certain other persons identified by us from time to time and who have been notified that they have been so identified (collectively with our directors and executive officers, “Restricted Persons”). See “Regular Blackout Periods,” “Special Blackout Periods” and “Pre-Clearance Provisions Applicable to Restricted Persons.” For the purposes of this policy, the term “Restricted Persons” shall also include our Chief Legal Officer and Chief Medical Officer, and any person, regardless of title or job description, who directly supports and reports to our Chief Executive Officer, Chief Financial Officer, Chief Legal Officer and Chief Medical Officer. All employees of Palatin should assume they are Restricted Persons for the purpose of this policy unless otherwise advised in writing by the Chief Financial Officer.
III. SCOPE OF POLICY
Persons. This policy applies to directors, officers, employees, consultants and agents of Palatin and its affiliates. The same restrictions that apply to you apply to your family members who reside with you, anyone else who lives in your household and any family members who do not live in your household but whose transactions in our securities are directed by you or are subject to your influence or control (such as parents or children who consult with you before they trade in securities). The same restrictions also apply to entities, including any corporations, partnerships, trusts and other entities, controlled by a person covered by this policy. You are responsible for making sure that the purchase or sale of any security covered by this policy by any such person or entity complies with this policy.
Transactions. The trading covered by this policy includes purchases and sales of our common stock (including initial elections, changes in elections or reallocation of funds relating to 401(k) plan accounts), derivative securities relating to our common stock (such as options for our common stock, put and call options and convertible debentures), preferred stock, warrants and debt securities (debentures, bonds and notes). Loans, pledges, hedging, gifts, charitable donations and other contributions of our securities are also subject to this policy.
Transactions in Other Companies. In addition, it is the policy of Palatin that no director, officer, employee, consultant or agent (or their family members, members of their household or controlled entities) of Palatin and its affiliates who, in the course of working for Palatin, learn of material nonpublic information about a company with which Palatin does business, may trade in that company’s securities until the information becomes public or is no longer material.
IV. DEFINITION OF MATERIAL NONPUBLIC INFORMATION
Material Information. Information is material if there is a substantial likelihood that a reasonable investor would consider it important in deciding whether to buy, hold or sell a security. Any information that could reasonably be expected to affect the price of the security is material. Common examples of material information are:
|
| · | projections of future earnings or losses or other earnings guidance; |
|
|
|
|
|
| · | earnings or losses that are inconsistent with the consensus expectations of the investment community or any earnings guidance released by Palatin; |
|
|
|
|
|
| · | a pending or proposed merger, acquisition or tender offer or an acquisition or disposition of significant assets; |
|
|
|
|
|
| · | a pending or proposed joint venture, license or collaborative agreement relating to any product under development by Palatin; |
| 2 |
|
| · | results of clinical trials of any product under development by Palatin; |
|
|
|
|
|
| · | significant regulatory actions by the U.S. Food and Drug Administration on any product under development by Palatin; |
|
|
|
|
|
| · | a change in management; |
|
|
|
|
|
| · | major events regarding our securities, including the declaration of a stock split or the offering of additional securities; |
|
|
|
|
|
| · | financial liquidity problems; |
|
|
|
|
|
| · | a change in auditors or notification that the auditor’s reports may no longer be relied upon; |
|
|
|
|
|
| · | actual or threatened major litigation, or a significant development with respect to such litigation; and |
|
|
|
|
|
| · | new major contracts, collaborations, orders, suppliers, customers or finance sources, or the loss thereof. |
This list is not exhaustive; other types of information may also be material. Both positive and negative information can be material. Because trading that receives scrutiny will be evaluated after the fact with the benefit of hindsight, questions concerning the materiality of particular information should be resolved in favor of materiality.
Nonpublic Information. Nonpublic information is information that is not generally known or available to the public. One common misconception is that material information loses its “nonpublic” status as soon as a press release is issued disclosing the information. In fact, information is considered to be available to the public only when it has been released broadly to the marketplace (such as by a press release or an SEC filing) and the investing public has had time to absorb the information fully. As a general rule, after nonpublic information is publicly disseminated, two full trading days must elapse before such information loses its status as nonpublic information.
V. RESTRICTIONS ON PURCHASES, SALES AND TIPPING
Trading on Inside Information. You may not trade in our securities, directly or indirectly (through family members or other persons or entities), if you are aware of material nonpublic information relating to Palatin.
Tipping. You may not pass material nonpublic information on to others or recommend to anyone the purchase or sale of any securities when you are aware of such information. This practice, known as “tipping,” also violates the securities laws and can result in the same civil and criminal penalties that apply to insider trading, even though you did not trade and did not gain any benefit from another’s trading.
| 3 |
Short Sales. No director or officer may engage in any short sales of our securities (sales of securities that are not then owned), including a “sale against the box” (a sale with delayed delivery). If you are a Restricted Person other than a director or officer, you may not engage in any short sales of our securities, including a “sale against the box”, unless advance approval is obtained from the Chief Financial Officer.
Hedging and Pledging Transactions. Certain forms of hedging or monetization transactions relating to our securities (such as zero-cost collars and forward sale contracts) could involve the establishment of a short position in our securities and limit or eliminate your ability to profit from an increase in the value of our securities. No director or officer may engage in hedging, monetization or pledging transactions of our securities. If you are a Restricted Person other than a director or officer, you are prohibited from engaging in any hedging or monetization transactions involving our securities, unless advance approval is obtained from the Chief Financial Officer.
Publicly-Traded Options. If you are a Restricted Person, you may not engage in transactions in publicly-traded or other third party options relating to our securities, such as puts, calls and other derivative securities, on an exchange, in any other organized market or otherwise, unless advance approval is obtained from the Chief Financial Officer.
Limit Orders. If you are a Restricted Person, you are prohibited from placing limit orders for our securities that remain effective after the day on which they are placed (such as “good until cancelled” orders), unless advance approval is obtained from the Chief Financial Officer.
Margin Accounts. If you are a Restricted Person, you are prohibited from holding our securities in a margin account, unless advance approval is obtained from the Chief Financial Officer.
Regular Blackout Periods Applicable to Restricted Persons. In addition to the general policy prohibiting trading while in possession of material nonpublic information, all Restricted Persons, and all family members of such persons and members of their household and controlled entities, are also prohibited from purchasing or selling our securities during the period beginning one week prior to the last day of each fiscal quarter and ending two full trading days after earnings have been publicly released with respect to such quarter or fiscal year (each, a “regular blackout period”).
Special Blackout Periods. From time to time, Palatin may also prohibit our Restricted Persons and potentially a larger group of employees, consultants and agents from trading our securities because of material developments known to Palatin and not yet disclosed to the public (each, a “special blackout period”). The existence of a special blackout period will not be announced, other than to those who are aware of the event giving rise to the special blackout. If, however, a person subject to a special blackout period requests permission to trade in our securities during such period, our Chief Legal Officer and/or our Chief Financial Officer will inform the requesting person of the existence of a special blackout period, without disclosing the reason for the special blackout. Any person made aware of the existence of a special blackout period shall not disclose the existence of the blackout to any other person.
| 4 |
VI. NO SAFE HARBOR
For those persons who are subject to blackout periods, the existence of such blackouts shall not be considered a safe harbor for trading during other periods, and all of our officers, directors, other employees, consultants and agents should use good judgment at all times. For example, occasions may arise when individuals covered by this policy become aware prior to the blackout period that earnings for that quarter are likely to exceed, or fall below, market expectations to an extent that is material. In such a case, the general policy against trading on inside information would still prohibit trading even though the time period is not within the blackout period or even if you are not a Restricted Person subject to the blackout periods. If you have any questions about whether you are permitted to trade in our securities at any particular time, you should contact our Chief Financial Officer.
VII. PRE-CLEARANCE PROVISIONS APPLICABLE TO RESTRICTED PERSONS
To help prevent inadvertent violations of the federal securities laws and avoid even the appearance of trading on the basis of inside information, the following pre-clearance provisions are applicable to our Restricted Persons.
All persons subject to pre-clearance, together with their family members and other members of their household and controlled entities, shall not engage in any transaction involving our securities (including a stock plan transaction such as an option exercise, or a gift, loan, pledge, contribution to a trust, 401(k) transfer or any other transfer) without first obtaining pre-clearance of the transaction from our Chief Financial Officer. A request for pre-clearance should be submitted to our Chief Financial Officer at least two business days in advance of the proposed transaction. Our Chief Financial Officer is under no obligation to approve a trade submitted for pre-clearance and may determine not to permit the trade. If permission to engage in the transaction is denied, the person who sought pre-clearance should refrain from initiating such transaction and should not inform any other person of restriction. Our Chief Financial Officer may not trade in our securities unless our Chief Executive Officer has approved the trade in accordance with the procedures set forth in this paragraph.
In the absence or unavailability of our Chief Financial Officer, pre-clearance requests may be made to our Chief Legal Officer. All pre-clearance requests by our Chief Financial Officer shall be submitted to and reviewed by both our Chief Executive Officer and Chief Legal Officer. All requests by our Chief Executive Officer shall be submitted to and reviewed by both our Chief Financial Officer and Chief Legal Officer.
VIII. EXCEPTIONS FOR APPROVED 10B5‑1 PLANS
Trades in our securities that are executed pursuant to an approved 10b5‑1 plan are not subject to the prohibition on trading on the basis of material nonpublic information contained in this policy or the restrictions relating to pre-clearance procedures and blackout periods. Rule 10b5‑1 provides an affirmative defense from insider trading liability under the federal securities laws for trading plans that meet certain requirements. In general, a 10b5‑1 plan must be entered into before you are aware of material nonpublic information. Once the plan is adopted, you must not exercise any influence over the amount of securities to be traded, the price at which they are to be traded or the date of the trade. The plan must either specify (including by formula) the amount, pricing and timing of transactions in advance or delegate discretion on those matters to an independent third party. We require that all 10b5‑1 plans be approved in writing in advance by our Chief Financial Officer. A 10b5‑1 plan may not be adopted during a blackout period.
| 5 |
IX. POST-TERMINATION TRANSACTIONS
If you are aware of material nonpublic information when you terminate employment or services, you may not trade in our securities until that information has become public or is no longer material. In addition, if you are subject to a blackout period at the time of your termination of employment or services, the restrictions on trading in our securities will not cease to apply until the expiration of such blackout period.
X. UNAUTHORIZED DISCLOSURE
Maintaining the confidentiality of our information is essential for competitive, security and other business reasons, as well as to comply with securities laws. You should treat all information you learn about Palatin or its business plans in connection with your employment as confidential and proprietary to us. Inadvertent disclosure of confidential or inside information may expose us and you to significant risk of investigation and litigation.
The timing and nature of our disclosure of material information to outsiders is subject to legal rules, including the SEC’s Regulation FD, the breach of which could result in substantial liability to you, us and our management. Accordingly, it is important that responses to inquiries about us by the press, financial analysts, investors or others in the financial community be made on our behalf only through authorized individuals.
XI. PERSONAL RESPONSIBILITY
You should remember that the ultimate responsibility for adhering to this policy and avoiding improper trading rests with you. If you violate this policy, Palatin may take disciplinary action, including dismissal for cause.
XII. ADDITIONAL INFORMATION FOR DIRECTORS AND SECTION 16 OFFICERS
Directors, executive officers and certain other persons identified by us from time to time must also comply with the reporting obligations and limitations on short-swing transactions set forth in Section 16 of the Securities Exchange Act of 1934, as amended. The practical effect of these provisions is that directors, executive officers and such other persons who purchase and sell our securities within a six-month period must disgorge all profits to Palatin whether or not they had knowledge of any material nonpublic information. Under these provisions, and so long as certain other criteria are met, neither the receipt of an option under our option plans, nor the exercise of that option is deemed a purchase under Section 16; however, the sale of any such shares is a sale under Section 16.
| 6 |
XIII. PENALTIES FOR NONCOMPLIANCE
Civil and Criminal Penalties. Potential penalties for insider trading violations include imprisonment for a substantial term and fines of several times the amount of profits gained or losses avoided.
Controlling Person Liability. If we fail to take appropriate steps to prevent illegal insider trading, we may have “controlling person” liability for a trading violation and be subject to civil penalties of up to the greater of $1 million and three times the profit gained or loss avoided as well as a criminal penalty. The civil penalties can extend personal liability to our directors, officers and other supervisory personnel if they fail to take appropriate steps to prevent insider trading.
Palatin Sanctions. Failure to comply with this policy may also subject you to sanctions imposed by Palatin, including dismissal for cause, whether or not your failure to comply with this policy results in a violation of law. Any exceptions to this policy, if permitted, may only be granted by the Chief Financial Officer and must be provided before any activity contrary to the above requirements takes place.
XIV. ASSISTANCE
Your compliance with this policy is of the utmost importance both for you and Palatin. If you have any questions about this policy or its application to any proposed transaction, you may obtain additional guidance from our Chief Financial Officer. Do not try to resolve uncertainties on your own, as the rules relating to insider trading are often complex, not always intuitive and carry severe consequences.
|
|
|
|
|
| Carl Spana |
| Stephen T. Wills |
|
| Chief Executive Officer |
| Executive VP, CFO and COO |
|
| Date: September 30, 2013 |
| Date: September 30, 2013 |
|
| 7 |
EXHIBIT 21
SUBSIDIARIES OF THE REGISTRANT
Name Under Which
| Name of Subsidiary |
| State of Incorporation |
| Name Under Which Subsidiary Does Business |
|
|
|
|
|
|
| RhoMed Incorporated |
| New Mexico |
| RhoMed Incorporated |
EXHIBIT 23
Consent of Independent Registered Public Accounting Firm
We consent to the incorporation by reference in the registration statements (Nos. 333-275883 and 333-277934) on Form S-1, (Nos. 333-33569, 333-56605, 333-64951, 333-72873, 333-84421, 333-52024, 333-54918, 333-74990, 333-100469, 333-101764, 333-104370, 333-112908, 333-128585, 333-132369, 333-140648, 333-146392, 333-174251, 333-183837, 333-185113, 333-201821, 333-206003, 333-206047, 333-226905, and 333-262555) on Form S-3 and (Nos. 333-57079, 333-83876, 333-128854, 333-149093, 333-163158, 333-174257, 333-191467, 333-206009, 333-214618, 333-221554, 333-227354 and 333-267615) on Form S-8 of our report dated September 30, 2024, with respect to the consolidated financial statements of Palatin Technologies Inc. and subsidiary.
| /s/ KPMG LLP |
|
|
|
|
| Philadelphia, Pennsylvania |
|
| September 30, 2024 |
|
EXHIBIT 31.1
Certification of Chief Executive Officer
I, Carl Spana, certify that:
| 1. | I have reviewed this Annual Report on Form 10-K of Palatin Technologies, Inc.; |
|
|
|
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
|
|
|
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
|
|
|
| 4. | The registrant's other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
|
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiary, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
|
|
|
|
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
|
|
|
|
| (c) | Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
|
|
|
|
| (d) | Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and |
| 5. | The registrant's other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors: |
|
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and |
|
|
|
|
|
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting. |
Date: September 30, 2024
| /s/ Carl Spana |
|
| Carl Spana, President and Chief Executive Officer |
|
EXHIBIT 31.2
Certification of Chief Financial Officer
I, Stephen T. Wills, certify that:
| 1. | I have reviewed this Annual Report on Form 10-K of Palatin Technologies, Inc.; |
|
|
|
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
|
|
|
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
|
|
|
| 4. | The registrant's other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
|
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiary, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
|
|
|
|
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
|
|
|
|
| (c) | Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
|
|
|
|
| (d) | Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and |
| 5. | The registrant's other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors: |
|
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and |
|
|
|
|
|
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting. |
Date: September 30, 2024
| /s/ Stephen T. Wills |
|
| Stephen T. Wills, Executive Vice President, Chief Financial Officer and Chief Operating Officer |
|
EXHIBIT 32.1
Certification of Principal Executive Officer
Pursuant to 18 U.S.C. Section 1350
As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
I, Carl Spana, President and Chief Executive Officer of Palatin Technologies, Inc., hereby certify, to my knowledge, that the Annual Report on Form 10-K for the year ended June 30, 2024 of Palatin Technologies, Inc. (the “Form 10-K”) fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and the information contained in the Form 10-K fairly presents, in all material respects, the financial condition and results of operations of Palatin Technologies, Inc.
Dated: September 30, 2024
| /s/ Carl Spana |
|
| Carl Spana, President and Chief Executive Officer (Principal Executive Officer) |
|
EXHIBIT 32.2
Certification of Principal Financial Officer
Pursuant to 18 U.S.C. Section 1350
As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
I, Stephen T. Wills, Executive Vice President, Chief Financial Officer and Chief Operating Officer of Palatin Technologies, Inc., hereby certify, to my knowledge, that the Annual Report on Form 10-K for the year ended June 30, 2024 of Palatin Technologies, Inc. (the “Form 10-K”) fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and the information contained in the Form 10-K fairly presents, in all material respects, the financial condition and results of operations of Palatin Technologies, Inc.
Dated: September 30, 2024
| /s/ Stephen T. Wills |
|
| Stephen T. Wills, Executive Vice President, Chief Financial Officer and Chief Operating Officer (Principal Financial Officer) |
|
EXHIBIT 97
ADOPTED RESOLUTIONS:
CLAWBACK POLICY
NOW, THEREFORE, BE IT RESOLVED, that the Corporation hereby adopts a clawback policy on the following terms and conditions, effective with respect to annual incentives, time-based restricted share units, performance-based restricted share units, stock options or other performance-based compensation granted on or after April 1, 2019:
Each executive officer of the Corporation (as defined below) shall repay or forfeit, to the fullest extent permitted by law and as directed by the Board of Directors (the “Board”), the after-tax portion or all of any annual incentives, time-based restricted share units, performance-based restricted share units, stock options or other performance-based compensation received by him or her if:
|
| ■ | the payment, grant or vesting of such compensation was based on the achievement of financial results that were subsequently the subject of a restatement of the Corporation’s financial statements filed with the Securities and Exchange Commission due to material noncompliance of the Corporation with any financial reporting requirement under federal securities laws; |
|
|
|
|
|
| ■ | the executive officer would have received a smaller payment or grant, or would have vested in a smaller portion of an award, based upon the restated financial results; |
|
|
|
|
|
| ■ | the payment, grant or vesting of the award occurred during the three-year period preceding the date on which the Corporation is required to prepare the restatement; and |
|
|
|
|
|
| ■ | the Board determines in its sole discretion that it is in the best interests of the Corporation and its stockholders for the executive officer to repay or forfeit all or any portion of the compensation. |
The following types of changes to previously filed financial statements would not trigger application of this policy: (i) retrospective application of a change in accounting principle previously approved by the Corporation’s independent auditor in issuing its audit opinion (including a change from one generally accepted accounting principle to another generally accepted accounting principle when there are two or more generally accepted accounting principles that apply or when the accounting principle formerly used is no longer generally accepted, or a change in the method of applying an accounting principle); (ii) retrospective revision to reportable segment information due to a change in the structure of the Corporation’s internal organization; (iii) retrospective reclassification due to a discontinued operation; (iv) retrospective application of a change in reporting entity, such as from a reorganization of entities under common control; (v) retrospective adjustment to provisional amounts in connection with a prior business combination; and (vi) retrospective revision for stock splits.
The Board, acting solely by the independent directors as identified under the applicable exchange listing standards, shall have full and final authority to make all determinations under this policy, including without limitation whether the policy applies and if so, the after-tax amount of compensation to be repaid or forfeited by the executive officer and the method of enforcement. In determining the after-tax amount of compensation to be repaid or forfeited, the Board shall take into account its good faith estimate of the value of any tax deduction available to the executive officer in respect of such repayment or forfeiture. The Board shall have no obligation to treat executive officers uniformly and the Board may make determinations selectively among executive officers. All determinations and decisions made by the Board pursuant to the provisions of this policy shall be final, conclusive and binding on all persons, including the Corporation, its affiliates, its stockholders and employees.
Each award agreement or other document setting forth the terms and conditions of any annual incentive, time-based restricted share units, performance-based restricted share units, stock option or other performance-based award granted to an executive officer shall include a provision incorporating the requirements of this policy. Moreover, each executive officer will be required to sign a Clawback Policy Acknowledgement and Agreement in a form attached to this resolution as Exhibit A. The remedy specified in this policy shall not be exclusive and shall be in addition to every other right or remedy at law or in equity that may be available to the Corporation.
For purposes of this policy, the term “executive officer” shall mean current or former executive officers designated by the Board as defined in Rule 3b-7 under the Securities Exchange Act of 1934.
FURTHER RESOLVED, that the proper officers of the Corporation be, and they hereby are, authorized to execute and deliver all documents and to perform all actions necessary to carry fully into effect the intent and purpose of the foregoing resolution.
FURTHER RESOLVED, that all actions taken by any of the proper officers of the Corporation in connection with the foregoing resolutions prior to the date hereof, are approved, adopted, authorized, ratified and confirmed in all respects.
* * * * *
| 2 |
EXHIBIT A
CLAWBACK POLICY
ACKNOWLEDGEMENT AND AGREEMENT
This Clawback Policy Acknowledgement and Agreement (this “Agreement”) is entered into as of the ______ day of ______________, 2019, between Palatin Technologies, Inc. (the “Company”) and _______________ (“Executive”).
Recitals:
WHEREAS, Executive is an “executive officer” of the Company as defined in Rule 3b-7 under the Securities Exchange Act of 1934;
WHEREAS, the Company’s Board of Directors has adopted the Palatin Technologies, Inc. Clawback Policy, as the same may be amended from time-to-time (the “Policy”); and
WHEREAS, in consideration of, and as a condition to the receipt of, future performance-based compensation, Executive and the Company are entering into this Agreement.
Agreement:
NOW, THEREFORE, the Company and Executive hereby agree as follows:
1. Executive acknowledges receipt of the Policy, a copy of which is attached hereto as Annex A and is incorporated into this Agreement by reference. Executive has read and understands the Policy and has had the opportunity to ask questions of the Company regarding the Policy.
2. Executive hereby acknowledges and agrees that the Policy shall apply to any annual incentives, time-based restricted share units, performance-based restricted share units, stock options or other performance-based compensation granted on or after April 1, 2019 (collectively, the “Incentive Compensation”), and all such Incentive Compensation shall be subject to repayment or forfeiture under the Policy.
3. Each award agreement or other document setting forth the terms and conditions of Incentive Compensation granted to Executive shall include a provision incorporating the requirements of the Policy; provided that the Company’s failure to incorporate the Policy into any award agreement or other document shall not waive the Company’s right to enforce the Policy. In the event of any inconsistency between the provisions of the Policy and the applicable award agreement or other document setting forth the terms and conditions of any Incentive Compensation, the terms of the Policy shall govern.
4. The repayment or forfeiture of Incentive Compensation pursuant to the Policy and this Agreement shall not in any way limit or affect the Company’s right to pursue disciplinary action or dismissal, take legal action or pursue any other remedies available to the Company, including, without limitation, enforcing the forfeiture and repayment provisions under the Company’s equity incentive plan. This Agreement and the Policy shall not replace, and shall be in addition to, any rights of the Company to recover Incentive Compensation, or any other compensation, from its executive officers under applicable laws and regulations, including but not limited to the Sarbanes-Oxley Act of 2002; provided that, to the extent required by applicable law, any amount recoverable from Executive under Section 304 of the Sarbanes-Oxley Act of 2002 would be credited against any amount recoverable from Executive under the Policy.
5. To the extent that Incentive Compensation subject to repayment or forfeiture under the Policy is not immediately returned or paid to the Company or forfeited, the Company may, to the extent permitted by law, seek other remedies, including a set off of the amounts so payable to it against any amounts that may be owing from time-to-time by the Company or a subsidiary to Executive for any reason, including, without limitation, wages, future payments of Incentive Compensation, severance, or vacation pay or other benefits; provided, however, that, except to the extent permitted by Treasury Regulation Section 1.409A-3(j)(4), such offset shall not apply to amounts that are “deferred compensation” within the meaning of Section 409A of the Internal Revenue Code.
6. Executive acknowledges that Executive’s execution of this Agreement is in consideration of, and is a condition to, the receipt by Executive of awards of Incentive Compensation from the Company on and after the date hereof; provided, however, that nothing in this Agreement shall be deemed to obligate the Company to make any such awards to Executive.
7. This Agreement may be executed in two or more counterparts, and by facsimile or electronic transmission, each of which will be deemed to be an original but all of which, taken together, shall constitute one and the same Agreement.
8. No modifications, waivers or amendments of the terms of this Agreement shall be effective unless in writing and signed by the parties or their respective duly authorized agents. Notwithstanding the foregoing, the Company may amend the Policy at any time, in its sole discretion, as the Company reasonably determines to be necessary or advisable for the Policy to comply with the Dodd-Frank Wall Street Reform and Consumer Protection Act or any other rules or regulations issued by the Securities and Exchange Commission or applicable securities exchanges and Executive hereby consents to any such amendment.
9. To the extent not preempted by federal law, this Agreement shall be governed by and construed in accordance with the laws of the State of Delaware, without reference to principles of conflict of laws. Each of this Agreement and the Policy shall survive and continue in full force in accordance with its terms notwithstanding any termination of Executive’s employment with the Company and its affiliates. The provisions of this Agreement shall inure to the benefit of, and be binding upon, the successors, administrators, heirs, legal representatives and assigns of Executive, and the successors and assigns of the Company. If any provision of this Agreement is or becomes invalid, illegal or unenforceable in any jurisdiction, or would disqualify this Agreement under any law deemed applicable by the Company, such provision shall be construed or deemed amended or limited in scope to conform to applicable laws or, in the discretion of the Company, it shall be stricken and the remainder of this Agreement shall remain in full force and effect.
| 2 |
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the day and year first above written.
|
| PALATIN TECHNOLOGIES, INC. |
|
|
|
|
|
|
|
|
|
|
| By: |
|
|
| Title: |
|
|
|
|
|
|
| EXECUTIVE |
|
|
|
|
|
|
|
|
|
| 3 |