UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
(Mark One)
☐ |
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2023
OR
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _________ to
OR
☐ |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report ____________________
Commission file number: 001-39152
FSD Pharma Inc. |
(Exact name of Registrant as specified in its charter) |
Ontario, Canada
(Jurisdiction of incorporation or organization)
199 Bay St., Suite 4000
Toronto, Ontario M5L 1A9, Canada
(Address of principal executive offices)
Zeeshan Saeed, Founder, Chief Executive Officer, President and Executive Co-Chairman of the Board
FSD Pharma Inc.
199 Bay St., Suite 4000
Toronto, Ontario M5L 1A9, Canada
Telephone: (416) 854-8884
Email: zsaeed@fsdpharma.com
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Class B Subordinate Voting Shares, no par value |
|
HUGE |
|
The Nasdaq Stock Market LLC |
Securities registered or to be registered pursuant to Section 12(g) of the Act. None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act. None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
Class A Multiple Voting Shares, no par value: 72 shares outstanding as of December 31, 2023
Class B Subordinate Voting Shares, no par value: 39,376,723 shares outstanding as of December 31, 2023
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐ No ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
Non-accelerated filer |
☒ |
Emerging growth company |
☒ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP ☐ |
International Financial Reporting Standards as issued by the International Accounting Standards Board ☒ |
Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☒
TABLE OF CONTENTS
6 |
||
|
|
|
6 |
||
|
|
|
9 |
||
|
|
|
10 |
||
|
|
|
|
11 |
|
|
|
|
11 |
||
|
|
|
11 |
||
|
|
|
11 |
||
|
|
|
11 |
||
11 |
||
11 |
||
11 |
||
|
|
|
29 |
||
|
|
|
29 |
||
34 |
||
46 |
||
48 |
||
|
|
|
48 |
||
|
|
|
48 |
||
|
|
|
48 |
||
48 |
||
48 |
||
48 |
||
48 |
||
|
|
|
48 |
||
|
|
|
48 |
||
51 |
||
59 |
||
65 |
||
65 |
||
| 3 |
| Table of Contents |
65 |
||
|
|
|
65 |
||
67 |
||
68 |
||
|
|
|
68 |
||
|
|
|
68 |
||
71 |
||
|
|
|
71 |
||
|
|
|
|
||
71 |
||
71 |
||
71 |
||
71 |
||
71 |
||
|
|
|
72 |
||
|
|
|
72 |
||
72 |
||
72 |
||
72 |
||
73 |
||
79 |
||
79 |
||
79 |
||
79 |
||
79 |
||
|
|
|
|
||
|
|
79 |
79 |
||
|
|
|
79 |
||
79 |
||
79 |
||
79 |
||
|
|
|
|
|
|
|
|
|
80 |
| 4 |
| Table of Contents |
Material Modifications to the Rights of Security Holders and Use of Proceeds. |
80 |
|
|
|
|
80 |
||
80 |
||
|
|
|
82 |
||
|
|
|
82 |
||
Management’s Annual Report on Internal Control over Financial Reporting |
82 |
|
82 |
||
82 |
||
|
|
|
82 |
||
|
|
|
82 |
||
|
|
|
83 |
||
|
|
|
83 |
||
|
|
|
83 |
||
|
|
|
Purchases of Equity Securities by the Issuer and Affiliated Purchasers. |
83 |
|
|
|
|
85 |
||
|
|
|
86 |
||
|
|
|
86 |
||
|
|
|
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections. |
86 |
|
|
|
|
86 |
||
|
|
|
86 |
||
|
|
|
|
||
|
|
|
88 |
||
|
|
|
88 |
||
|
|
|
88 |
| 5 |
| Table of Contents |
INTRODUCTION
Unless otherwise noted or the context otherwise requires, all references in this Annual Report on Form 20-F (this “Annual Report”), or this Annual Report, to “FSD,” “FSD Pharma,” “Company,” “Corporation,” “we,” “us” and “our” refer to FSD Pharma Inc., a corporation formed under the Business Corporations Act (Ontario) (the “OBCA”) and the direct or indirect subsidiary entities of FSD and any partnership interests held by FSD Pharma and its subsidiary entities, including Lucid Psycheceuticals Inc. (“Lucid”), FSD BioSciences Inc. (“FSD Biosciences”), FV Pharma Inc. (“FV Pharma”), Prismic Pharmaceuticals, Inc. (“Prismic”), FSD Strategic Investments Inc. (“FSD Strategic Investments”), FSD Pharma Australia Pty Ltd. (“FSD Australia”) and Celly Nutrition Corp. (“Celly Nu”).
Our fiscal year ends on December 31. This Annual Report includes our audited consolidated financial statements as of December 31, 2023 and 2022 and for the years ended December 31, 2023 and 2022, (the “2023 Annual Financial Statements”) which are prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board. None of our financial statements were prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”).
Except where expressly indicated otherwise, our financial information is presented in U.S. dollars. All references in this Annual Report to “$” or “US$” mean United States of American (“U.S.” or “United States”) dollars, and all references in this Annual Report to “C$” mean Canadian dollars. For the convenience of the reader, in this Annual Report, unless otherwise indicated, translations from Canadian dollars into U.S. dollars were made at the rate of US$1.00 to C$1.3497, which is the average rate for the 2023 fiscal year, (2022 average rate: US$1.00=C$1.3013). Such U.S. dollar amounts are not necessarily indicative of the amounts of U.S. dollars that could actually have been purchased upon exchange of Canadian dollars at the dates indicated.
We have made rounding adjustments to some of the figures included in this Annual Report. Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that preceded them.
This Annual Report includes registered and unregistered trademarks such as “Unbuzzd,” and “ALCOHOLDEATH,” which are protected under applicable intellectual property laws and are the property of the Company. Solely for convenience, our trademarks referred to in this Annual Report and in other publicly filed documents may appear without the ® or ™ symbol, but such references are not intended to indicate, in any way, that we will not assert our rights to the fullest extent under applicable law. All other trademarks used in this Annual Report are the property of their respective owners. For more information, please see “Item 4. Information on the Company. – B. Business Overview – Intellectual Property”.
We are incorporated under the laws of Ontario, Canada. Substantially all of our assets are located outside the United States. In addition, several of our directors and officers are nationals and/or residents of countries other than the United States, and all or a substantial portion of such directors’ and officers’ assets may be located outside the United States. As a result, it may be difficult for investors to effect service of process within the United States upon us or our officers or directors or to enforce against us or them judgments obtained in United States courts, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state thereof. In addition, investors should not assume that the courts of Canada (i) would enforce judgments of United States courts obtained in actions against us, our officers or directors, or other said persons, predicated upon the civil liability provisions of the U.S. federal securities laws or other laws of the United States or (ii) would enforce, in original actions, liabilities against us or such directors, officers or experts predicated upon the United States federal securities laws or any securities or other laws of any state or jurisdiction of the United States.
In addition, there is doubt as to the applicability of the civil liability provisions of the United States federal securities law to original actions instituted in Canada. It may be difficult for an investor, or any other person or entity, to assert United States securities laws claims in original actions instituted in Canada.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report contains statements that constitute forward-looking statements. All statements other than statements of historical facts contained in this Annual Report, including statements regarding our future results of operations and financial position, business strategy, product candidates, product pipeline, ongoing and planned clinical studies, including those of our collaboration partners, regulatory approvals, research and development (“R&D”) costs, timing and likelihood of success, as well as plans and objectives of management for future operations, are forward-looking statements. Many of the forward-looking statements contained in this Annual Report are often, but not always, identified by words or phrases such as “hope”, “would”, “seek”, “anticipate”, “believe”, “expect”, “plan”, “continue”, “estimate”, “will”, “predict”, “intend”, “forecast”, “future”, “target”, “project”, “capacity”, “could”, “should”, “might”, “focus”, “proposed”, “scheduled”, “outlook”, “potential”, “may” or similar expressions and includes suggestions of future outcomes, including, but not limited to statements about:
|
· |
discussions concerning the Company’s exploration of near-term funding strategies; |
|
· |
the Company’s plans to advance the R&D of its Product Candidates (as defined herein) to commercialization through studies and clinical trials, including anticipated timing and associated costs; |
|
· |
the application and the costs associated with such planned trials, and the Company’s ability to obtain required funding and the terms and timing thereof; |
|
· |
the expansion of our product offering(s); |
|
· |
our business objectives and the expected impacts of previously announced acquisitions and developments; |
|
· |
the U.S. Food and Drug Administration (“FDA”) and Health Canada, or comparable regulatory authority, application process and any review thereof and its effects on our business objectives. |
| 6 |
| Table of Contents |
Readers are cautioned not to place undue reliance on Forward-Looking Statements as the Company’s actual results may differ materially and adversely from those expressed or implied.
The Corporation has made certain assumptions with respect to the Forward-Looking Statements regarding, among other things:
|
· |
the Corporation’s ability to generate sufficient cash flow from operations and obtain financing, if needed, on acceptable terms or at all; |
|
· |
the general economic, financial market, regulatory and political conditions in which the Corporation operates; |
|
· |
the interest of potential purchasers in the Product Candidates; |
|
· |
anticipated and unanticipated costs; the government regulation of the Corporation’s activities and Product Candidates; |
|
· |
the timely receipt of any required regulatory approvals; |
|
· |
the Corporation’s ability to obtain qualified staff, equipment and services in a timely and cost efficient manner; |
|
· |
the Corporation’s ability to conduct operations in a safe, efficient and effective manner; and |
|
· |
the Corporation’s expansion plans and timeframe for completion of such plans. |
Although the Corporation believes that the expectations and assumptions on which the Forward-Looking Statements are based are reasonable, undue reliance should not be placed on the Forward-Looking Statements, because no assurance can be given that such statements will prove to be correct.
Forward-looking statements appear in a number of places in this Annual Report and include, but are not limited to, statements regarding our intent, belief, or current expectations. Forward-looking statements are based on our management’s beliefs and assumptions, and on information currently available to our management. Such statements are subject to risks and uncertainties, and actual results may differ materially from those expressed or implied in the forward-looking statements due to various factors, including, but not limited to, those identified under “Item 3. Key information - D. Risk Factors” in this Annual Report. These risks and uncertainties include multiple factors:
|
· |
the success of our clinical studies, our ability to obtain and maintain regulatory approval and to commercialize our Product Candidates, which include Lucid-21-302 (“Lucid-MS”), for the treatment of multiple sclerosis (“MS”) and our product for alcohol misuse in the healthcare area; |
|
· |
the ability of our licensing partner, Celly Nu to commercialize, sell and distribute Unbuzzd™, a functional beverage product that seeks to provide relief from inebriation and accelerate alcohol metabolism in the consumer market; |
|
· |
the ability of our competitors to discover, develop or commercialize competing products to Unbuzzd™, Lucid-MS or other product candidates before or more successfully than we do; |
|
· |
our plans to research, develop and commercialize our Product Candidates; |
|
· |
the identification of serious adverse, undesirable, or unacceptable side effects related to our Product Candidates; |
|
· |
our ability to maintain our current strategic relationships with Celly Nu, our licensing partner to commercialize Unbuzzd™; |
|
· |
our ability to protect and maintain our, and not infringe on third parties’, intellectual property rights throughout the world; |
|
· |
our ability to raise capital when needed in order to continue our product development programs and commercialization efforts; |
|
· |
our ability to attract and retain qualified employees and key personnel; |
|
· |
the acceptance by the FDA and applicable foreign regulatory authorities of data from studies for Lucid-MS that we and our collaboration partners conduct within and outside the U.S. now and in the future; |
|
· |
our foreign private issuer status, the loss of which would require us to comply with the Exchange Act of 1934’s, as amended (the “Exchange Act”) domestic reporting regime, and cause us to incur significant legal, accounting, and other expenses; |
|
· |
our incorporation in Ontario, the laws of which govern our corporate affairs and may differ from those applicable to companies incorporated in the U.S.; |
|
· |
the limited operating history of the Company and history of losses, and anticipated significant losses for the foreseeable future incurred to pursue commercialization of the Product Candidates; |
|
· |
the Company’s inability to file investigational new drug applications (“INDs”) or clinical trial application (“CTAs”) on timelines it reasonably anticipates, if at all; |
|
· |
the Company’s ability to identify, license or discover additional product candidates; |
|
· |
the Product Candidates being in the preclinical development stage; |
|
· |
the Company’s reliance on its Product Candidates; |
|
· |
the Company’s ability to successfully develop new commercialized products or find a market for their sale; |
|
· |
the impact of any future recall of the Company’s products; |
|
· |
the Company’s ability to promote and sustain its products, including any restrictions or constraints on marketing practices under the regulatory framework in which the Company operates; |
|
· |
failure to achieve the degree of market acceptance and demand for our products or Product Candidates by physicians, patients, healthcare payors, and others in the medical community which are necessary for commercial success, including due to the possibility that alternative, superior treatments may be available prior to the approval and commercialization of Product Candidates, should such approval be received at all; |
| 7 |
| Table of Contents |
|
· |
failure of clinical trials to demonstrate substantial evidence of the safety and/or effectiveness of Product Candidates, which could prevent, delay or limit the scope of regulatory approval and commercialization, including from difficulties encountered in enrolling patients in clinical trials, and reliance on third parties to conduct our clinical trials and some aspects of our research and preclinical testing, or results from future clinical testing which may demonstrate opposing evidence and draw negative conclusions regarding the effectiveness of any Product Candidate, including the effectiveness of Lucid-MS as a treatment for MS; |
|
· |
results of earlier studies or clinical trials not being predictive of future clinical trials and initial studies or clinical trials not establishing an adequate safety or efficacy profile for the Product Candidates to justify proceeding to advanced clinical trials or an application for regulatory approval; |
|
· |
potential side effects, adverse events or other properties or safety risks of the Product Candidates, which could delay or halt their clinical development, prevent their regulatory approval, cause suspension or discontinuance of clinical trials, abandonment of a Product Candidate, limit their commercial potential, if approved, or result in other negative consequences; |
|
· |
preliminary, interim data obtained from the Company’s clinical trials that it may announce or publish from time to time may not be indicative of future scientific observations or conclusions as more patient data becomes available, further analyses are conducted, and as the data becomes subject to subsequent audit and verification procedures; |
|
· |
inability to establish sales and marketing capabilities, or enter into agreements with third parties, to sell and market any Product Candidates that the Company may develop; |
|
· |
the ability to provide the capital required for research, product development, operations and marketing; |
|
· |
violations of laws and regulations resulting in repercussions; |
|
· |
risks inherent in an pharmaceutical business and the development and commercialization of pharmaceutical products, including the inability to accurately predict timing or amounts of expenses, requirements of regulatory authorities, and completion of clinical studies on anticipated timelines, which may encounter substantial delays or may not be able to be completed at all; |
|
· |
delays in clinical trials; |
|
· |
the Company’s inability to attain or maintain the regulatory approvals it needs in any jurisdiction to commercialize, distribute or sell any Product Candidate or other pharmaceutical products; |
|
· |
failure of counterparties to perform contractual obligations; |
|
· |
changes, whether anticipated or not, in laws, regulations and guidelines that may result in significant compliance costs for the Company, including in relation to restrictions on branding and advertising, regulation of distribution and excise taxes; |
|
· |
uncertainty associated with insurance coverage and reimbursement status for newly-approved pharmaceutical products, which could result in Product Candidates becoming subject to unfavorable pricing regulations, third-party coverage and reimbursement practices, or healthcare reform initiatives, including legislative measures aimed at reducing healthcare costs; |
|
· |
the effect that any public health crises, such as pandemics or epidemics may have on the Company’s business; |
|
· |
the price of our securities may be volatile due to a variety of factors, including volatility in the capital markets generally, geopolitical events, public health emergencies, macro economic pressures and natural disasters; |
|
· |
the inability to obtain required additional financing on terms favourable to the Corporation or at all; |
|
· | the Company’s anticipated negative cash flow from operations and non-profitability for the foreseeable future; |
|
· |
the issuances of equity securities and the conversion of outstanding securities to Class B subordinate voting shares in the capital of the Company (the “Class B Shares”); |
|
· |
the Company’s dual class share structure; |
|
· |
the market price of the Class B Shares possibly being subject to wide price fluctuations; |
|
· |
whether an active trading market for the Class B Shares is sustained; |
|
· |
the Company’s ability to maintain compliance with Nasdaq Stock Market LLC’s (“Nasdaq”) rules for continued listing on the Nasdaq; |
|
· |
the Company’s ability to identify and execute future acquisitions or dispositions effectively, including the ability to successfully manage the impacts of such transactions on its operations; |
|
· |
lack of dividends, and reinvestment of retained earnings, if any, into the Company’s business; |
|
· |
the Company’s reliance on management, key persons and skilled personnel; |
|
· |
reliance on contract manufacturing facilities; |
|
· |
manufacturing problems that could result in delay of the Company’s development or commercialization programs; |
|
· |
the Company’s expected minimal environmental impacts; insurance and uninsured risks; |
|
· |
claims from suppliers; conflicts of interest between the Company and its directors and officers; |
|
· |
the Company’s ability to manage its growth effectively; |
|
· |
the Company’s ability to realize production targets; |
|
· |
supply chain interruptions and the ability to maintain required supplies of, equipment, parts and components; |
|
· |
the Company’s ability to successfully implement and maintain adequate internal controls over financial reporting or disclosure controls and procedures; |
|
· |
results of litigation; |
|
· |
the dependence of the Company’s operations, in part, on the maintenance and protection of its information technology systems, and the information technology systems of its third-party research institution collaborators, contract research organizations (“CROs”) or other contractors or consultants, which could face cyber-attacks; |
|
· |
failure to execute definitive agreements with entities in which the Company has entered into letters of intent or memoranda of understanding; |
|
· |
unfavorable publicity or consumer perception towards the Product Candidates; |
| 8 |
| Table of Contents |
|
· |
reputational risks to third parties with whom the Company does business; failure to comply with laws and regulations; |
|
· |
the Company’s reliance on its own market research and forecasts; |
|
· |
competition from other technologies and pharmaceutical products, including from synthetic production, new manufacturing processes and new technologies, and expected significant competition from other companies with similar businesses, and significant competition in an environment of rapid technological and scientific change; |
|
· |
the Company’s ability to safely, securely, efficiently and cost-effectively transport our products to consumers; |
|
· |
liability arising from any fraudulent or illegal activity, or other misconduct or improper activities that the Company’s directors, officers, employees, contractors, consultants, commercial partners or vendors may engage in, including noncompliance with regulatory standards and requirements; |
|
· |
unforeseen claims made against the Company, including product liability claims or regulatory actions; |
|
· |
reliance on single-source suppliers, including single-source suppliers for the acquisition of the drug substance and drug product for any of the Product Candidates; |
|
· |
inability to obtain or maintain sufficient intellectual property protection for the Product Candidates; |
|
· |
third-party claims of intellectual property infringement; |
|
· |
patent terms being insufficient to protect competitive position on Product Candidates; |
|
· |
inability to obtain patent term extensions or non-patent exclusivity; |
|
· |
inability to protect the confidentiality of trade secrets; |
|
· |
inability to protect trademarks and trade names; |
|
· |
filing of claims challenging the inventorship of the Company’s patents and other intellectual property; |
|
· |
invalidity or unenforceability of patents, including legal challenges to patents covering any of the Product Candidates; |
|
· |
claims regarding wrongfully used or disclosed confidential information of third parties; |
|
· |
risks related to the Company’s investment in Celly Nu, including the ability of Celly Nu to commercialize the exclusive rights to the recreational applications for the Company’s alcohol misuse technology for rapid alcohol detoxification; |
|
· |
inability to protect property rights around the world; the impact of general economic conditions on the Company’s mortgage investment activities; |
|
· |
risks related to the Company’s status as a foreign private issuer; |
|
· |
the Company taking advantage of reduced disclosure requirements applicable to emerging growth companies; |
|
· |
the Company’s classification as a “passive foreign investment company”; |
|
· |
that the Company’s international business operations, including expansion to new jurisdictions, could expose it to regulatory risks or factors beyond our control such as currency exchange rates and changes in governmental policy; |
|
· |
risks related to expansion of international operations; |
|
· |
the Company’s ability to produce and sell products in, and export products to, other jurisdictions within and outside of Canada and the United States, which is dependent on compliance with additional regulatory or other requirements; |
|
· |
regulatory regimes of locations for clinical trials outside of Canada and the United States; |
|
· |
failure to obtain approval to commercialize Product Candidates outside of Canada and the United States; |
|
· |
if clinical trials are conducted for Product Candidates outside of Canada and the United States, FDA, Health Canada and comparable regulatory authorities may not accept data from such trials, or the scope of such approvals from regulatory authorities may be limited; |
|
· |
other factors beyond the Company’s control; |
|
· |
the other risk factors discussed under “Item 3. Key information - D. Risk Factors”. |
These forward-looking statements are applicable only as of the date of this Annual Report, and are subject to a number of risks, uncertainties and assumptions described under the sections in this Annual Report entitled “Item 3. Key information - D. Risk Factors” and “Item 5. Operating and Financial Review and Prospects” and elsewhere in this Annual Report. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
MARKET AND INDUSTRY DATA
This Annual Report includes market and industry data that has been obtained from third party sources, including industry publications. The Company believes that its industry data is accurate and that its estimates and assumptions are reasonable, but there is no assurance as to the accuracy or completeness of this data. Third party sources generally state that the information contained therein has been obtained from sources believed to be reliable, but there is no assurance as to the accuracy or completeness of included information. Although the data is believed to be reliable, the Company has not independently verified any of the data from third party sources referred to in this Annual Report or ascertained the underlying economic assumptions relied upon by such sources.
| 9 |
| Table of Contents |
SUMMARY RISK FACTORS
Our business is subject to a number of risks and uncertainties, including those risks discussed at length in the section below titled “Risk Item 3. Key information - D. Risk Factors”. These risks include, among others, the following:
RISKS RELATED TO OUR PRODUCT CANDIDATES
|
· |
We have a limited operating history and funding, which may make it difficult to evaluate Lucid-MS, our products for alcohol misuse and their product development, product prospects and overall likelihood of success; |
|
· |
Our drug product candidate, Lucid-MS or our products relating to alcohol misuse, may not receive regulatory approval from Health Canada or the FDA, in a timely manner, if at all, or may receive regulatory approval on limiting terms; |
|
· |
We are relying on Celly Nu, our licensing partner, to develop and promote Unbuzzd™, an alcohol misuse product for the retail market; |
|
· |
The Company may be unable to raise the capital necessary for it to execute its strategy on favorable terms or at all; and |
|
· |
Drug development is a highly uncertain undertaking and involves a substantial degree of risk. |
RISKS RELATED TO THE PHARMACEUTICAL BUSINESS
|
· |
We rely on the UHN License (as defined herein) to use for pharmaceutical purposes certain patents and other intellectual property rights associated with Lucid-MS; |
|
· |
We rely on the Epitech License Agreement and the UHN License to use for pharmaceutical purposes certain patents and other intellectual property rights associated with FSD-PEA and Lucid-MS; |
|
· |
Even if Lucid-MS receives regulatory approval, we may nonetheless fail to achieve the degree of market acceptance of Lucid-MS by physicians, patients, healthcare payors, and others in the medical community necessary for commercial success; |
|
· |
We face significant competition for our Lucid-MS drug, and there is a possibility that our competitors may achieve regulatory approval for an effective treatment for MS before us or develop therapies that are safer, more advanced, or more effective than ours; |
|
· |
Psychedelic or psychedelic-inspired drugs may never be approved as medicines or other therapeutic applications, and violations of applicable laws and regulations, or changes in the regulatory or political discourse with respect to psychedelic or psychedelic-inspired drugs, could result in repercussions; |
|
· |
The loss of single-source suppliers, or their failure to supply us with the drug substance or drug product, could materially and adversely affect our business; |
|
· |
We currently rely on, and expect to continue to rely on, third parties to conduct drug trials and aspects of our research and preclinical testing for Lucid-MS, our products relating to alcohol misuse and other possible drug candidates; |
|
· |
We, our service providers, or any third-party manufacturers may fail to comply with regulatory requirements which could subject us to enforcement actions; and |
|
· |
The FDA, Health Canada or other comparable regulatory authorities may not accept data from trials conducted in foreign jurisdictions. |
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
|
· |
We may be unable to obtain and maintain sufficient intellectual property protection for our Product Candidates; |
|
· |
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts; and |
|
· |
If we are unable to adequately protect the confidentiality of our trade secrets, our trademarks or trade names, our business may be adversely affected. |
GENERAL CORPORATE RISKS
|
· |
Macroeconomic pressures in the markets in which we operate, including, but not limited to, the lasting effects of the COVID-19 pandemic, epidemic, or outbreak of an infectious disease, inflation, stagflation, supply chain and interest rate pressures, foreign currency exchange rate fluctuations, the ongoing conflict between Russia and Ukraine and political developments in Hong Kong and Taiwan, natural disasters and other macroeconomic and geopolitical events may materially and adversely affect our business and financial results and could cause a disruption to the development of our Product Candidates; |
|
· |
The Company operates in a highly regulated industry and is subject to a wide range of federal, state, and local laws, rules, and regulations, including FDA and Health Canada regulatory requirements and laws pertaining to fraud and abuse in healthcare, that affect nearly all aspects of our operations. Failure to comply with these laws, rules, and regulations, or to obtain and maintain required licenses, could subject the Company to enforcement actions, including substantial civil and criminal penalties, and might require us to recall or withdraw a product from the market or cease operations, which could materially and adversely affect our business, financial condition, and results of operations; |
|
· |
Any significant interruption in the supply chain for key inputs could materially impact the Company’s business; |
|
· |
Future sales or issuances of equity securities and the conversion of outstanding securities to Class B Shares could decrease the value of the Class B Shares and dilute investors’ voting power; |
|
· |
The Company’s dual class structure has the effect of concentrating voting control and the ability to influence corporate matters with a limited number of holders of Class A multiple voting shares in the capital of the Company (the “Class A Shares”); |
|
· |
A decline in general economic conditions may impact the viability and success of our mortgage investment activities; |
|
· |
We may lose our status as a foreign private issuer; |
|
· |
There can be no assurance that we will be able to comply with the continued listing standards of the Nasdaq and/or Canadian Securities Exchange (the “CSE”); |
|
· |
The Company is currently party to several legal proceedings and may become a party to potential future litigation; and |
|
· |
We are a passive foreign investment company for U.S. federal income tax purposes, which may result in adverse U.S. federal income tax consequences for U.S. Holders of our Class B Shares. |
| 10 |
| Table of Contents |
PART I
Item 1. Identity of Directors, Senior Management and Advisers.
A. Directors and Senior Management
Not applicable.
B. Advisers
Not applicable.
C. Auditors
Not applicable.
Item 2. Offer Statistics and Expected Timetable.
Not applicable.
Item 3. Key Information
A. [Reserved]
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
An investment in securities of the Company should only be made by persons who can afford a significant or total loss of their investment. We are exposed to a number of risks through the pursuit of our business objectives. The following risks and uncertainties identified below are those we believe may, individually or in combination with other risks and uncertainties, have a material impact on our business, but these are not the only risks and uncertainties we face. Additional risks and uncertainties not presently known to us, or risks that we currently deem immaterial, may also impair our business operations. If any of the following risks, or any other risks and uncertainties that we have not yet identified or that we currently consider not to be material, actually occur, or become material risks, our business, financial condition, results of operations and cash flows, and consequently the price of the Class B Shares, could be materially and adversely affected.
The risks discussed below also include Forward-Looking Statements and our actual results may differ substantially from those discussed in these Forward-Looking Statements. See “Cautionary Note Regarding Forward-Looking Statements” in this Annual Report.
Risks relating to our Product Candidates
Drug development is highly uncertain undertaking and involves a substantial degree of risk. We have no product sales, which, together with our limited operating history, makes it difficult to evaluate our business and assess our future viability.
Pharmaceutical and biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. We are a biotechnology corporation with a limited operating history. We have no pharmaceutical products approved for commercial sale and have not generated any revenue from pharmaceutical product sales. We are currently focused on developing Lucid-MS, a patented new chemical entity targeting the treatment of MS. The effectiveness of Lucid-MS is not yet known. We continue to incur significant research and development and other expenses related to clinical trials and other operating expenses, ongoing operations and expect to incur losses for the foreseeable future. We anticipate these losses will increase and that we will not generate any revenue from product sales of Lucid-MS unless and until after we have successfully completed clinical development and received regulatory approval, for the commercial sale of this product.
We may never be able to develop or commercialize Lucid-MS or any other drug candidate or achieve profitability. Revenue from the sale of Lucid-MS, if regulatory approval is obtained, will be dependent, in part, upon the size of the markets in the territories for which we obtain regulatory approval, the accepted price for the product, the ability to obtain reimbursement at any price and whether we own the commercial rights for that territory, as well as the efficiency and availability of any comparable products. Our growth strategy depends on our ability to generate revenue. In addition, if the number of addressable patients is less than anticipated, the indication approved by regulatory authorities is narrower than expected, or the reasonably accepted population for treatment is narrowed by competition, physician choice or treatment guidelines, we may not generate significant revenue from sales of Lucid-MS or any other drug product, even if approved. Even if we are able to generate revenue from the sale of Lucid-MS, we may not become profitable and may need to obtain additional funding to continue operations. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our failure to achieve sustained profitability would depress our value and could impair our ability to raise capital, expand our business, diversify our research and development pipeline, market Lucid-MS and any other product candidates that we may identify and pursue or continue our operations.
| 11 |
| Table of Contents |
Our future success is dependent on the regulatory approval and commercialization of our Product Candidates.
We do not have any products that have gained regulatory approval. As a result, our ability to finance our operations and generate revenue, are substantially dependent on our ability to obtain regulatory approval for, and, if approved, to successfully commercialize our product candidates in a timely manner. We cannot commercialize our other product candidates in Canada or the U.S. without first obtaining regulatory approval for each product from Health Canada or the FDA; similarly, we cannot commercialize any product candidates outside of the U.S. or Canada without obtaining regulatory approval from comparable foreign regulatory authorities, including the European Medicines Agency (the “EMA”). The FDA review process typically takes years to complete and approval is never guaranteed. Before obtaining regulatory approvals for the commercial sale of Lucid-MS or any of our potential product candidates for a target indication, we must demonstrate with substantial evidence gathered in preclinical and well-controlled clinical studies, with respect to approval in Canada and in the U.S. to the satisfaction of Health Canada and the FDA, and in Europe, to the satisfaction of the EMA, that the product candidate is safe and effective for use for that target indication; and that the manufacturing facilities, processes and controls are adequate. Obtaining regulatory approval for marketing of Lucid-MS or future product candidates in one country does not ensure we will be able to obtain regulatory approval in other countries. A failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in other countries.
Even if Lucid-MS or any of our other product candidates were to successfully obtain approval from Health Canada or the FDA or comparable foreign regulatory authorities, any approval might contain significant limitations related to use restrictions for specified age groups, warnings, precautions, or contraindications, or may be subject to burdensome post-approval studies or risk management requirements. If we are unable to obtain regulatory approval for our Product Candidates in one or more jurisdictions, or any approval contains significant limitations, we may not be able to obtain sufficient funding or generate sufficient revenue to continue the development of any of our other Product Candidates that we are developing or may discover, in-license, develop or acquire in the future. Also, any regulatory approval of our Product Candidates, once obtained, may be withdrawn. Furthermore, even if we obtain regulatory approval for any of our Product Candidates, their commercial success will depend on a number of factors, including the following:
|
· |
development of a commercial organization within the Company or establishment of a commercial collaboration with a commercial infrastructure; |
|
· |
establishment of commercially viable pricing and obtaining approval for adequate reimbursement from third-party and government payers; |
|
· |
our ability to manufacture quantities of our Product Candidates using commercially satisfactory processes and at a scale sufficient to meet anticipated demand and enable us to reduce our cost of manufacturing; |
|
· |
our success in educating physicians and patients about the benefits, administration, and use of our Product Candidates; |
|
· |
the availability, perceived advantages, relative cost, relative safety, and relative efficacy of alternative and competing treatments; |
|
· |
the effectiveness of our own or our potential strategic collaborators’ marketing, sales and distribution strategy and operations; |
|
· |
acceptance as a safe and effective therapy by patients and the medical community; and |
|
· |
· a continued acceptable safety profile following approval. |
Many of these factors are beyond our control. If we are unable to successfully commercialize our Product Candidates, we may not be able to earn sufficient revenues to continue our business.
We our relying on Celly Nu, our licensing partner, to promote our Unbuzzd™ brand and if we fail to maintain a good relationship with Celly Nu our business, financial condition and results of operations could be adversely affected.
On July 31, 2023, we entered into the Celly Nu IP License Agreement (as defined herein). Pursuant to the Celly Nu IP License Agreement, we are relying on Celly Nu to promote, commercialize and distribute Unbuzzd™ to the consumer market. Although we can maintain control over Celly Nu’s products and content to a certain degree through contractual provisions in the licensing agreement, we have limited control over its marketing and commercialization strategy.
The viability of the Celly Nu IP License Agreement depends on our ability to establish and maintain good relationship with Celly Nu. The value of our Unbuzzd™ brand and the rapport that we maintain with Celly Nu is an important factor for the success of this relationship. If we are unable to maintain a good relationship with Celly Nu, it could have a material adverse effect on our results of operations. Our license agreements require us and Celly Nu to comply with operational and performance conditions that are subject to interpretation and could result in disagreements. At any given time, we could have a dispute with Celly Nu regarding the interpretation of a provision in the Celly Nu IP License Agreement. An adverse result in any such dispute could materially adversely impact our results of operations and business.
For more information, please see “Item 4. Information on the Company. - A. History and Development of the Company - Overview and History”.
| 12 |
| Table of Contents |
We rely on the UHN License to use for pharmaceutical purposes certain patents and other intellectual property rights associated with Lucid-MS.
One of our principal assets is the UHN License, which provides us with an exclusive, multi-jurisdictional license to use certain patents and other intellectual property rights associated with Lucid-MS, which is owned by the University Health Network (“UHN”). We are obligated to make milestone payments and royalties to UHN under the UHN License Agreement, which may limit our future profitability and our ability to enter into marketing partnership agreements. If we materially breach any of the terms of the UHN License Agreement (and fail to cure such breach with the specified time, to the extent a cure period is available for such breach), UHN, could terminate such agreement. If we were to lose or otherwise be unable to maintain the UHN License on acceptable terms, or find that it is necessary or appropriate to secure new licenses from other third parties, we may not be able to market Lucid-MS, and our current business model and plan would be impaired, which would have a material adverse effect on our business, operating results, and financial condition.
After receiving regulatory approvals, Lucid-MS may fail to achieve a sufficient degree of market acceptance by physicians, patients, healthcare payors, and others in the medical community.
The commercial success of Lucid-MS or other drug candidates that we develop, after receiving required regulatory approvals, will depend on their degree of market acceptance by physicians, patients, third-party payors, and others in the medical community. The degree of market acceptance of Lucid-MS will depend on a number of factors, including (i) the availability of alternative, superior treatments for a MS prior to the approval and commercialization Lucid-MS for such treatment; (ii) the efficacy and safety of Lucid-MS, including side effects or unexpected characteristics; (iii) the ability to offer Lucid-MS for sale at competitive prices; (iv) the ability to manufacture Lucid-MS in sufficient quantities and to offer appropriate patient access programs, such as co-pay assistance; (v) convenience and ease of dosing and administration compared to alternative treatments; (vi) the clinical indications for which Lucid-MS is approved by the FDA or Health Canada, if it approved at all, or comparable regulatory agencies; (vii) product labeling or product insert requirements of the FDA, Health Canada or other comparable regulatory authorities, including any limitations, contraindications or warnings contained in a product’s approved labeling; (viii) restrictions on how Lucid-MS is distributed; (ix) publicity concerning Lucid-MS or competing products and treatments; (x) the strength of marketing and distribution support; (xi) favorable third-party coverage and sufficient reimbursement; and (xii) the prevalence and severity of any side effects or adverse effects.
Sales of pharmaceutical products, such as Lucid-MS if and when it is approved by regulatory authorities, will depend on the willingness of physicians to prescribe the treatment, which is likely to be based on a determination by these physicians that the products are safe, therapeutically effective and cost effective. In addition, the inclusion or exclusion of products from treatment guidelines established by various physician groups and the viewpoints of influential physicians can affect the willingness of other physicians to prescribe the treatment. We cannot predict whether physicians, physicians’ organizations, hospitals, other healthcare providers, government agencies or private insurers will determine that Lucid-MS is safe, therapeutically effective and cost effective as compared with competing treatments. If Lucid-MS does not achieve adequate levels of acceptance, we may not generate significant product revenue, and we may not become profitable.
We face significant competition for our Lucid-MS drug and there is a possibility that our competitors may develop therapies that are safer, more advanced, or more effective than ours from MS.
The development and commercialization of new drug products is highly competitive. We face competition with respect to Lucid-MS for the treatment of MS from major pharmaceutical companies, specialty pharmaceutical companies, and biotechnology companies world-wide. Potential competitors also include academic institutions, government agencies, and other public and private research organizations that conduct research, seek patent protection, and establish collaborative arrangements for research, development, manufacturing, and commercialization. Even if we are successful in achieving regulatory approval to commercialize Lucid-MS ahead of our competitors, our future pharmaceutical products may face direct competition from generic and other follow-on drug products.
More established companies may have a competitive advantage over us due to their greater size, cash flows and institutional experience. Compared to us, many of our competitors may have significantly greater financial, technical and human resources. As a result of these factors, our competitors may obtain regulatory approval of their products before we do, which will limit our ability to develop or commercialize any of our Product Candidates. In addition, many companies are developing new therapeutics to supplant or expand upon the standard of care for a number of diseases, as a result, we cannot predict what the standard of care will be as our Product Candidates progress through clinical development.
Interim, “top-line,” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data becomes available or as additional analyses are conducted, and as the data are subject to audit and verification procedures, that could result in material changes in the final data.
From time to time, we may publish interim, “top-line,” or preliminary data from our clinical studies. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Preliminary or “top-line” data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim and preliminary data should be viewed with caution until the final data are available. Material adverse changes between preliminary, “top-line,” or interim data and final data could significantly harm our business prospects.
| 13 |
| Table of Contents |
We expect to rely on third parties to conduct product candidate drug trials and aspects of our research and preclinical testing.
We currently rely and expect to continue to rely on third parties, such as CROs, clinical data management organizations, medical institutions, and clinical investigators, to conduct some aspects of research and preclinical testing and clinical trials. Any of these third parties may terminate their engagements with us or be unable to fulfill their contractual obligations. If any of our relationships with these third parties terminate, we may not be able to enter into arrangements with alternative third parties on commercially reasonable terms, or at all. If we need to enter into alternative arrangements, it could delay our development activities.
Our reliance on these third parties for research and development activities reduces control over these activities but does not relieve us of our responsibilities. For example, we remain responsible for ensuring that product candidate drug trials are each conducted in accordance with the general investigational plan and protocols for each trial and applicable legal, regulatory, and scientific standards, and our reliance on third parties does not relieve us of our regulatory responsibilities. In addition, the FDA, Health Canada, and other comparable regulatory authorities require compliance with good clinical practices for conducting, recording, and reporting the results of clinical trials to assure that data and reported results are credible, reproducible and accurate and that the rights, integrity, and confidentiality of trial participants are protected. Regulatory authorities enforce these good clinical practices through periodic inspections of trial sponsors, principal investigators, and trial sites. If we or any of these third parties fail to comply with applicable good clinical practice regulations, some or all of the clinical data generated in any product candidate drug trials may be deemed unreliable and the FDA, Health Canada or other comparable regulatory authorities may reject our marketing applications or require us to perform additional nonclinical or clinical trials or to enroll additional patients before approving our marketing applications. We cannot be certain that, upon inspection, such regulatory authorities will determine that any product candidate drug trial complies with the good clinical practice regulations. For any violations of laws and regulations during the conduct of clinical trials, we could be subject to untitled and warning letters or enforcement action that may include civil penalties and criminal prosecution. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database within certain timeframes. Failure to do so can result in fines, adverse publicity, and civil and criminal sanctions.
If these third parties do not successfully carry out their contractual duties, meet expected deadlines, or conduct clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for a product candidate and will not be able to, or may be delayed in our efforts to, successfully commercialize product candidates. Our failure or the failure of these third parties to comply applicable regulatory requirements or our stated protocols could also subject us to enforcement action.
We also expect to rely on other third parties to store and distribute drug supplies for product candidate drug trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of any product candidates we may develop or commercialization of our medicines or other therapeutic applications, producing additional losses and depriving us of potential product revenue.
Results of earlier studies or clinical trials may not be predictive of future clinical trial results and may not justify proceeding to advanced clinical trials or an application for regulatory approval.
Success in preclinical testing and early clinical trials does not ensure that later clinical trials will generate adequate data to demonstrate the efficacy and safety of an investigational drug. A number of companies in the pharmaceutical and biotechnology industries, including those with greater resources and experience, have suffered significant setbacks in clinical trials, even after seeing promising results in earlier clinical trials. We do not know whether the clinical trials we are conducting, or may conduct, will demonstrate adequate efficacy and safety to result in regulatory approval to market any of our product candidates in any particular jurisdiction. Even if we believe that we have adequate data to support an application for regulatory approval to market our product candidates, the FDA or other comparable foreign regulatory authorities may not agree and could require us to conduct additional research studies, including late-stage clinical trials. If late-stage clinical trials do not produce favorable results, our ability to achieve regulatory approval for any of our product candidates may be adversely impacted.
Product candidates could be associated with side effects which could delay or halt clinical development, prevent regulatory approval, or result in other significant negative consequences.
As is the case with pharmaceuticals generally, it is likely that there may be side effects associated with Lucid-MS or our other drug product candidates. If Lucid-MS or our other drug product candidates are associated with undesirable side effects in preclinical studies or clinical trials or have characteristics that are unexpected, we may elect to abandon their development or limit their development to more narrow uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective, which may limit the commercial expectations for this product candidate if approved. We may also be required to modify or terminate our study plans based on findings in our preclinical studies or clinical trials.
Additionally, adverse developments in clinical trials of pharmaceutical and biopharmaceutical products conducted by others may cause the FDA, Health Canada, or other regulatory oversight bodies to suspend or terminate our clinical trials or to change the requirements for approval of Lucid-MS or our other drug product candidates.
Additionally, if we or others later identify undesirable side effects caused by Lucid-MS or our other drug product candidates once approved, several potentially significant negative consequences could result, including: (i) regulatory authorities may suspend or withdraw approvals of such product candidate; (ii) we may be required to change the way a product candidate is administered or conduct additional clinical trials; (iii) we may be required to include additional warnings on a product candidate’s labeling or the product candidate may be subject to restrictive distribution requirements; (iv) we could be sued and held liable for harm caused to patients; and (v) our reputation may suffer. Any of these occurrences may harm our business, financial condition, and prospects significantly.
In addition to side effects caused by the product candidate, the administration process or related procedures also can cause adverse side effects. If any such adverse events occur, our clinical trials could be suspended or terminated. If we are unable to demonstrate that any adverse events were caused by the administration process or related procedures, the FDA, Health Canada, or other regulatory authorities could order us to cease further development of, or deny approval of, a product candidate for any or all targeted indications. Even if we can demonstrate that all future serious adverse events are not product-related, such occurrences could affect patient recruitment or the ability of enrolled patients to complete the trial. Moreover, if we elect, or are required, to not initiate, delay, suspend or terminate any future clinical trial of Lucid-MS or any of our product candidates, the commercial prospects of Lucid-MS or such other product candidates may be harmed and our ability to generate product revenues from Lucid-MS or any of these other product candidates may be delayed or eliminated. Any of these occurrences may harm our ability to develop other product candidates, and may harm our business, financial condition, and prospects significantly.
| 14 |
| Table of Contents |
The Company may not be successful in its efforts to identify, license or discover additional product candidates.
Although a substantial amount of the Company’s effort will focus on the continued research and pre‐clinical and clinical testing, potential approval and commercialization of its Product Candidates, the success of its business also depends in part upon its ability to identify, license or discover additional product candidates. The Company’s research programs or licensing efforts may fail to yield additional product candidates for clinical development for a number of reasons, including but not limited to the following: (i) the Company’s research or business development methodology or search criteria and process may be unsuccessful in identifying potential product candidates; (ii) the Company may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates; (iii) the Company’s product candidates may not succeed in pre‐clinical or clinical testing; (iv) the Company’s product candidates may be shown to have harmful side effects or may have other characteristics that may make the product candidates unmarketable or unlikely to receive marketing approval; (v) competitors may develop alternatives that render the Company’s product candidates obsolete or less attractive; (vi) product candidates the Company develops may be covered by third parties’ patents or other exclusive rights; (vii) the market for a product candidate may change during the Company’s program such that the further development of a product candidate may become undesirable; (viii) a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and (ix) a product candidate may not be accepted as safe and effective by patients, the medical community or third‐party payors.
If any of these events occurs, the Company may be forced to abandon its development efforts to identify, license or discover additional product candidates, which could have a material adverse effect on its business, prospects, results of operations and financial condition and could potentially cause the Company to cease operations. Research programs to identify new product candidates require substantial technical, financial, and human resources. The Company may focus its efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
The FDA, Health Canada or other comparable regulatory authorities may not accept data from trials conducted in foreign jurisdictions.
Obtaining regulatory approval in one country does not mean that regulatory approval will be obtained in any other country. We intend on submitting our initial regulatory approvals for Lucid-MS in the U.S. and Canada. Approval processes vary among countries and can involve additional product testing and validation and additional or different administrative review periods, including additional preclinical studies or clinical trials, as data from clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States and Canada, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
Non-U.S. and non-Canadian regulatory approval processes may include all of the risks associated with obtaining FDA or Health Canada approval, as well as additional risks. We do not have any product candidates approved for sale in any jurisdiction, including international markets, and we do not have experience in obtaining regulatory approval in international markets. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approval in international markets is delayed, our target market will be reduced and our ability to realize the full market potential of our Product Candidates will be harmed. In addition, if we conduct trials outside of the U.S. or Canada, the FDA or Health Canada, as applicable, may not accept the data from such trials and may require additional trials, which could be costly and time-consuming and delay aspects of our business plan.
Our suppliers could experience manufacturing problems that result in delays in our development or commercialization programs or otherwise harm our business.
Our contract manufacturing organization (“CMO”) must employ multiple steps to control the manufacturing process to assure that the process is reproducible and the product candidate is made strictly and consistently in compliance with the process. Problems with the manufacturing process, even minor deviations from the normal process, could result in product defects or manufacturing failures that result in lot failures, product recalls, product liability claims or insufficient inventory to conduct clinical trials or supply commercial markets. Furthermore, all entities involved in the preparation of product candidates for clinical trials or commercial sale, including our existing CMOs for all of our Product Candidates, are subject to extensive regulation. Components of a finished therapeutic products approved for commercial sale or used in certain clinical trials must be manufactured in accordance with good manufacturing practices (“GMP”), or similar regulatory requirements outside the United States and Canada. Our failure, or the failure of third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, suspension of production, seizures or recalls of Product Candidates or marketed drugs, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect clinical or commercial supplies of our Product Candidates and increase our costs. Consequently, there may be a material adverse effect on the business, results of operations, financial condition, and prospects of the Company.
In addition, the FDA, Health Canada, and other regulatory authorities may require us to submit samples of any lot of any approved Product Candidates together with the protocols showing the results of applicable tests at any time. Under some circumstances, the FDA, Health Canada, or other regulatory authorities may require that we not distribute a lot until the agency authorizes its release. Slight deviations in the manufacturing process, including those affecting quality attributes and stability, may result in unacceptable changes in the product that could result in lot failures or product recalls. Lot failures or product recalls could cause us to delay product launches or clinical trials, which could be costly to us and otherwise harm our business, results of operations, financial condition, and prospects.
| 15 |
| Table of Contents |
Our CMOs also may encounter problems hiring and retaining the experienced scientific, quality assurance, quality-control and manufacturing personnel needed to operate our manufacturing processes, which could result in delays in production or difficulties in maintaining compliance with applicable regulatory requirements.
Any problems in our CMOs’ manufacturing process or facilities could result in delays or cancellations of planned clinical trials, failures in satisfying ongoing regulatory obligations (before and after regulatory approval for a product candidate is obtained) and increased costs. Such problems could also make us a less attractive collaborator for potential partners, including larger biotechnology companies and academic research institutions, which could limit access to additional attractive development programs. Problems in our manufacturing process could restrict our ability to meet potential future market demand for products.
Lucid-MS, after it is approved, will be subject to extensive post-approval regulation.
After a product is approved, numerous post-approval requirements apply. Among other things, the holder of an approved NDA is subject to periodic and other FDA monitoring and reporting obligations, including obligations to monitor and report adverse events and instances of the failure of a product to meet the specifications in the NDA. Application holders must submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling, or manufacturing process. Application holders must also submit advertising and other promotional material to the FDA and report on ongoing clinical studies.
Depending on the circumstances, failure to meet these post-approval requirements can result in criminal prosecution, fines, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of pre-marketing product approvals, or refusal to allow us to enter into supply contracts, including government contracts. In addition, even if we comply with FDA and other requirements, new information regarding the safety or effectiveness of a product could lead the FDA to modify or withdraw product approval. Similar laws in other jurisdictions would also apply.
After our Product Candidates are commercialized, they may be subject to recalls for a variety of reasons, which could require the Company to expend significant management and capital resources.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. If any of the Company’s approved and commercialized Product Candidates are recalled due to an alleged product defect or for any other reason, the Company could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. The Company may lose a significant amount of sales made on such products and may not be able to replace those sales at an acceptable margin or at all. In addition, a product recall may require significant management attention. Although the Company has detailed procedures in place for testing its products, there can be no assurance that any quality, potency, or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. Additionally, if one of the Company’s significant brands were subject to recall, the image of that brand and the Company could be harmed. A recall for any of the foregoing reasons could lead to decreased demand for the Company’s products and could have a material adverse effect on the results of the operations and financial condition of the Company. Additionally, product recalls may lead to increased scrutiny of the Company’s operations by the FDA, Health Canada or other regulatory agencies, requiring further management attention and potential legal fees and other expenses.
If approved, Lucid-MS may face competition from generic drugs approved through an abbreviated regulatory pathway.
The Drug Price Competition and Patent Term Restoration Act of 1984, otherwise known as the Hatch-Waxman Amendments to the Federal Food, Drug, and Cosmetic Act (the “FDC Act”), authorized the FDA to approve generic drugs that are the same as drugs previously approved for marketing under the new drug application (“NDA”) provisions of the statute pursuant to an abbreviated new drug application (“ANDA”). An ANDA relies on the preclinical and clinical testing conducted for a previously approved reference listed drug (“RLD”), and must demonstrate to the FDA that the generic drug product is identical to the RLD with respect to the active ingredients, the route of administration, the dosage form, and the strength of the drug and also that it is “bioequivalent” to the RLD. The FDA is prohibited by statute from approving an ANDA when certain marketing or data exclusivity protections apply to the RLD. If any such competitor or third party is able to demonstrate bioequivalence without infringing our patents, then this competitor or third party may then be able to introduce a competing generic product onto the market. The Hatch-Waxman Amendments also enacted the 505(b)(2) NDA pathway, which permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. A Section 505(b)(2) applicant may eliminate the need to conduct certain preclinical or clinical studies, if it can establish that reliance on studies conducted for a previously approved product is scientifically appropriate.
Market exclusivity provisions authorized under the FDC Act can delay the submission or the approval of certain marketing applications. The FDC Act provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to obtain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not approve or even accept for review an ANDA or an NDA submitted under Section 505(b)(2) by another company for another drug based on the same active moiety, regardless of whether the drug is intended for the same indication as the original innovative drug or for another indication, where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement to one of the patents listed with the FDA by the innovator NDA holder.
| 16 |
| Table of Contents |
The FDC Act also provides three years of marketing exclusivity for an NDA, or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example new indications, dosages, or strengths of an existing drug. This three-year exclusivity covers only the modification for which the drug received approval on the basis of the new clinical investigations and does not prohibit the FDA from approving ANDAs or 505(b)(2) NDAs for drugs containing the active agent for the original indication or condition of use. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to any preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
If competitors are able to obtain marketing approval for generic drugs referencing our products, our products may become subject to competition from such generic drugs. The availability of competitive generic products could limit the demand, and the price we are able to charge, for any products that we may develop and commercialize.
Product liability lawsuits against us could cause us to incur substantial liabilities and could limit commercialization of any product candidates that we may develop.
We face an inherent risk of product liability exposure related to the testing of product candidates in human clinical trials and will face an even greater risk if we commercially sell any medicines or other therapeutic applications that we may develop. If we cannot successfully defend ourselves against claims that our Product Candidates, medicines, or other therapeutic applications caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in: (i) decreased demand for any product candidates, medicines or other therapeutic applications that we may develop; (ii) injury to our reputation and significant negative media attention; (iii) withdrawal of clinical trial participants; (iv) significant costs to defend the related litigation; (v) substantial monetary awards to trial participants or patients; (vi) loss of revenue; and (vii) the inability to commercialize our Product Candidates.
Although we intend to maintain product liability insurance, including coverage for clinical trials that we plan to sponsor, it may not be adequate to cover all liabilities that we may incur. We anticipate that we will need to increase our insurance coverage as we commence additional clinical trials and if we successfully commercialize any Product Candidates. The market for insurance coverage is increasingly expensive, and the costs of insurance coverage will increase as our clinical programs increase in size. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
We may become liable for uninsured or uninsurable risk.
The Company may become subject to liability for risks which are uninsurable or against which the Company may opt out of insuring due to the high cost of insurance premiums or other factors. The payment of any such liabilities would reduce the funds available for usual business activities. Payment of liabilities for which insurance is not carried may have a material adverse effect on the Company’s financial position and operations.
Our employees, directors, independent contractors, consultants, commercial partners, and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of fraud, misconduct or other illegal activity by our employees, directors, independent contractors, consultants, commercial partners, and vendors. Misconduct by these parties could include intentional, reckless and negligent conduct that fails to: (i) comply with the requirements of the FDA, Health Canada and other comparable regulatory authorities; (ii) provide true, complete and accurate information to the FDA, Health Canada and other comparable regulatory authorities; (iii) comply with manufacturing standards we have established; (iv) comply with healthcare fraud and abuse laws and similar other fraudulent misconduct laws in the United States or Canada; or (v) report financial information or data accurately or to disclose unauthorized activities appropriately. If we obtain approval of our Product Candidates from the FDA, Health Canada or other comparable regulatory authorities and begin commercializing those products in the United States, Canada or other countries, our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. In particular, research, sales, marketing, education, and other business arrangements in the healthcare industry are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, educating, marketing and promotion, sales and commission, certain customer incentive programs and other business arrangements generally. Activities subject to these laws and regulations also involve the improper use of information obtained in the course of patient recruitment for clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation. The board of directors of the Company (the “Board”) has adopted a Code of Conduct and Ethics (the “Code”) which provides guidelines surrounding, among other items, compliance with applicable laws, conflicts of interest, certain opportunities, confidentiality and disclosure, employment practices, and use of company property and resources. However, it is not always possible to identify and deter misconduct by employees, directors and third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws and regulations. If any such actions or lawsuits are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions or lawsuits could have a significant impact on our business, including the imposition of significant fines or other sanctions.
| 17 |
| Table of Contents |
We may be unable to establish sufficient sales and marketing capabilities or enter into agreements with third parties to sell and market any product candidates in a compliant manner even if regulatory approvals are obtained.
We do not currently have a comprehensive infrastructure for the sales, marketing, and distribution of pharmaceutical drug products. The cost of establishing and maintaining such an infrastructure may exceed the cost-effectiveness of doing so. In order to market any products that may be approved by the FDA and comparable foreign regulatory authorities, we must build our sales, marketing, managerial and other nontechnical capabilities or make arrangements with third parties to perform these services for which we would incur substantial costs. If we are unable to establish adequate sales, marketing, and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and may not sustain profitability. We will be competing with many companies that have extensive and well-funded sales and marketing operations. Without an internal commercial organization or the support of a third party to perform sales and marketing functions, or a combination of both, we may be unable to compete successfully against more established companies.
Our Product Candidates may become subject to unfavorable pricing regulations, third-party coverage and reimbursement practices, or healthcare reform initiatives, which would harm our business.
The regulations that govern marketing approvals, pricing, coverage, and reimbursement for new drugs vary widely from country to country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more Product Candidates, even if any Product Candidates we may develop obtain marketing approval.
Our ability to successfully commercialize our Product Candidates also will depend in part on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers, and other organizations. If coverage and adequate reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our Product Candidates. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment.
In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medicines, but monitor and control corporation profits. Additional price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our Product Candidates.
Risks Related to our Intellectual Property
We may be unable to obtain and maintain sufficient intellectual property protection for our Product Candidates.
As is the case with pharmaceutical companies and other biotechnology companies, our success depends in large part on our ability to obtain and maintain protection of the intellectual property we may own solely and jointly with others, particularly patents, in the United States, Canada and other countries with respect to our Product Candidates and technology. We seek to protect our proprietary position by filing patent applications in the United States, Canada and in other countries related to the Product Candidates or other product candidates that we may identify. On April 24, 2023, the Company filed a provisional patent application with the United States Patent and Trademark Office (“USPTO”) with respect to the Company’s alcohol misuse treatment technology. We have an exclusive license from UHN to use patents and other intellectual property that is used in our Lucid-MS compound.
Obtaining and enforcing pharmaceutical and biopharmaceutical patents is costly, time consuming and complex, and we or our licensors may not be able to file and prosecute all necessary or desirable patent applications, or maintain, enforce, and license any patents that may issue from such patent applications, at a reasonable cost or in a timely manner, if at all. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. We may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the rights to patents licensed to third parties. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal, technological, and factual questions and has in recent years been the subject of much litigation. In addition, the laws of certain countries may not protect our rights to the same extent as the laws of other countries, including the United States and Canada, and vice versa. Further, we may not be aware of all third-party intellectual property rights potentially relating to our Product Candidates. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States, Canada and other jurisdictions are typically not published until 18 months after filing or, in some cases, not at all. Therefore, we cannot know with certainty whether our licensors were the first to make the inventions claimed in our licensed patents, or that our licensors were the first to file for patent protection of such inventions. Furthermore, the scope of a patent claim is determined by an interpretation of the law, the written disclosure in a patent and the patent’s prosecution history and can involve other factors such as expert opinion. Our analysis of these issues, including interpreting the relevance or the scope of claims in a patent or a pending application, determining applicability of such claims to our proprietary technologies or Product Candidates, predicting whether a third party’s pending patent application will issue with claims of relevant scope, and determining the expiration date of any patent in the United States, Canada or abroad that we consider relevant may be incorrect, which may negatively impact our ability to develop and market our Product Candidates. We do not always conduct independent reviews of pending patent applications of and patents issued to third parties. As a result, the issuance, scope, validity, enforceability, and commercial value of our patent rights, including licensed patent rights, are highly uncertain. Our future patent applications may not result in patents being issued that protect our Product Candidates, in whole or in part, or which effectively prevent others from commercializing competitive product candidates. Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our licensed patents by developing similar or alternative product candidates in a non-infringing manner.
| 18 |
| Table of Contents |
Our licensors’ ability to enforce patent rights also depends on our licensors’ ability to detect infringement. It may be difficult to detect infringers who do not advertise the components or methods that are used in connection with their products and services. Moreover, it may be difficult or impossible to obtain evidence of infringement in a competitor’s or potential competitor’s product or service. We, along with our licensors, may not prevail in any lawsuits that we initiate and the damages or other remedies awarded if we were to prevail may not be commercially meaningful. If we initiate lawsuits to protect or enforce our licensed patents, or litigate against third-party claims, such proceedings would be expensive and would divert the attention of our management and technical personnel. Such proceedings could also provoke third parties to assert claims against us, including that some or all of the claims in one or more of our or our licensed patents are invalid or otherwise unenforceable.
Moreover, we may be subject to a third-party pre-issuance submission of prior art to the USPTO, or become involved in opposition, derivation, re-examination, inter partes review, post-grant review or interference proceedings challenging our or our licensors’ patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our licensed patents, allow third parties to commercialize our Product Candidates and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize drugs without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our licensed patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
In addition, the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our licensed patents may be challenged in the courts or patent offices in the United States, Canada and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated, or held unenforceable, in whole or in part, which could limit our licensors’ abilities to stop others from using or commercializing similar or identical product candidates to ours, or limit the duration of the patent protection of our Product Candidates.
Filing, prosecuting, and defending the licensed patents on our Product Candidates in all countries throughout the world would be prohibitively expensive. Additionally, the laws of some other countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we and our licensors may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained licensed patent protection to develop their own products and may also export infringing products to territories where we have patent protection, but enforcement is not as strong as that in the U.S. or Canada. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio, including licensed patents, may not provide us with sufficient rights to exclude others from commercializing drugs similar or identical to ours.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. However, our research, development and commercialization activities may be subject to claims that we infringe or otherwise violate patents or other intellectual property rights owned or controlled by third parties. In May 2023, GBB Drink Lab, Inc. (“GBB”) filed a lawsuit against the Company alleging a material breach of a mutual nondisclosure agreement and trade secret misappropriation in the U.S. District Court for the Southern District of Florida. This lawsuit is ongoing. For more information, please see “Item 8. Financial Information - A. Consolidated Statements and Other Financial Information – Legal Proceedings”.
There is a substantial amount of litigation, both within and outside the U.S. and Canada, involving patent, trade secret and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions and inter partes re-examination proceedings. Numerous U.S. and international issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are pursuing development candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our Product Candidates may be subject to claims of infringement of the patent rights of third parties.
Other third parties may assert that we are employing their proprietary technology without authorization. There may be other third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of the Product Candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that the Product Candidates or other product candidates that we may identify may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of the Product Candidates or other product candidates that we may identify, any molecules formed during the manufacturing process or any final product itself, the holders of any such patents may be able to block our ability to commercialize such product candidate unless we obtained a license under the applicable patents, or until such patents expire.
Similarly, if any third-party patents were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, including combination therapy, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product candidate unless we obtained a license or until such patent expires. In either case, such a license may not be available on commercially reasonable terms or at all, or it may be non-exclusive, which could result in our competitors gaining access to the same intellectual property.
| 19 |
| Table of Contents |
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize the Product Candidates or other product candidates that we may identify. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing Product Candidates, or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
Parties making claims against us, may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of our confidential information could be compromised by disclosure. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have material adverse effect on ability to raise additional funds or otherwise have a material adverse effect on our business, results of operations, financial condition, and prospects.
If our licensors are not able to obtain patent term extension or non-patent exclusivity in the United States under the Hatch-Waxman Act and in other countries under similar legislation, thereby potentially extending the marketing exclusivity term of our product candidates, our business may be materially harmed.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional or international patent application filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited.
Depending upon the timing, duration, and specifics of FDA marketing approval of our Product Candidates, one of the U.S. patents covering each of such Product Candidates or the use thereof may be eligible for up to five years of patent term extension under the Hatch-Waxman Act. The Hatch-Waxman Act allows a maximum of one patent to be extended per FDA approved product as compensation for the patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only those claims covering such approved drug product, a method for using it or a method for manufacturing it may be extended.
Patent term extension also may be available in certain other countries upon regulatory approval of our Product Candidates. Nevertheless, our licensors may not be granted patent term extension either in the United States, Canada or in any other country because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the term of extension, as well as the scope of patent protection during any such extension, afforded by the governmental authority could be less than requested.
If our licensors are unable to obtain patent term extension or restoration, or the term of any such extension is less than we request, the period during which we will have the right to exclusively market our product may be shortened and our competitors may obtain approval of competing products following the patent expiration sooner, and our revenue could be reduced, possibly materially.
It is possible that our licensors will not obtain patent term extension under the Hatch-Waxman Act for a U.S. patent covering a Product Candidate even where that patent is eligible for patent term extension, or if we obtain such an extension, it may be for a shorter period than we had sought. Further, for certain of our licensed patents, we do not have the right to control prosecution, including filing with the USPTO, a petition for patent term extension under the Hatch-Waxman Act. Thus, if one of our licensed patents is eligible for patent term extension under the Hatch-Waxman Act, we may not be able to control whether a petition to obtain a patent term extension is filed, or obtained, from the USPTO.
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected and our business would be harmed.
We seek to protect our confidential proprietary information, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and collaborators. These agreements are designed to protect our proprietary information. However, we cannot be certain that such agreements have been entered into with all relevant parties, and we cannot be certain that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. For example, any of these parties may breach the agreements and disclose proprietary information, including trade secrets, and we may not be able to obtain adequate remedies for such breaches. We also seek to preserve the integrity and confidentiality of our confidential proprietary information by maintaining physical security of our premises and physical and electronic security of our information technology systems, but it is possible that these security measures could be breached. If any of our confidential proprietary information were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such competitor from using that technology or information to compete with us, which could harm our competitive position.
| 20 |
| Table of Contents |
Unauthorized parties may also attempt to copy or reverse engineer certain aspects of our Product Candidates that we consider proprietary. We may not be able to obtain adequate remedies in the event of such unauthorized use. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, some courts are less willing or unwilling to protect trade secrets. Trade secrets will also over time be disseminated within the industry through independent development, the publication of journal articles and the movement of personnel skilled in the art from corporation to corporation or academic to industry scientific positions. Though our agreements with third parties typically restrict the ability of our advisors, employees, collaborators, licensors, suppliers, third-party contractors, and consultants to publish data potentially relating to our trade secrets, our agreements may contain certain limited publication rights.
In addition, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such competitor from using that technology or information to compete with us, which could harm our competitive position. Despite employing the contractual and other security precautions described above, the need to share trade secrets increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. If any of these events occurs or if we otherwise lose protection for our trade secrets, the value of this information may be greatly reduced and our competitive position, business, results of operations, financial condition and prospects would be harmed.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented, or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential collaborators or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. We may license our trademarks and trade names to third parties, such as distributors. Though these license agreements may provide guidelines for how our trademarks and trade names may be used, a breach of these agreements or misuse of our trademarks and tradenames by our licensees may jeopardize our rights in or diminish the goodwill associated with our trademarks and trade names. Our efforts to enforce or protect our proprietary rights related to trademarks, trade names, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect our competitive position, business, results of operations, financial condition, and prospects.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
Our agreements with employees and our personnel policies provide that any inventions conceived by an individual in the course of rendering services to us shall be our exclusive property. Although our policy is to have all such individuals complete these agreements, we may not obtain these agreements in all circumstances, and individuals with whom we have these agreements may not comply with their terms. The assignment of intellectual property may not be automatic upon the creation of an invention and despite such agreement, such inventions may become assigned to third parties. In the event of unauthorized use or disclosure of our trade secrets or proprietary information, these agreements, even if obtained, may not provide meaningful protection, particularly for our trade secrets or other confidential information.
We or our licensors may be subject to claims that former employees, collaborators or other third parties have an interest in our owned or licensed patents, trade secrets, or other intellectual property as an inventor or co-inventor. For example, we or our licensors may have inventorship disputes arise from conflicting obligations of employees, consultants or others who are involved in developing our Product Candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or our licensors’ ownership of our owned or licensed patents, trade secrets or other intellectual property. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property that is important to our Product Candidates. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Any of the foregoing could have a material adverse effect on our competitive position, business, results of operations, financial condition, and prospects.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology and pharmaceutical industry, we employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants, and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee’s former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Risks relating to our Psychedelic Products
Due to funding issues, we made a decision to place all research relating to Lucid-PSYCH on hold in April 2023. If and when we resume this research, we will encounter the following risks with respect to our Lucid-PSYCH drug.
The loss of single-source suppliers, or their failure to supply us with the drug substance or drug product, could materially and adversely affect our business.
When we were actively researching Lucid-PSYCH, we relied upon a single-source supplier for the supply of drug substances and products for this compound. Although we believe that there are alternate sources of supply that could satisfy our clinical and commercial requirements, we cannot assure you that identifying alternate sources and establishing relationships with such sources would not result in significant delay in the development of the Lucid-PSYCH Product Candidate.
Our dependence on a single-source supplier exposes us to certain risks, that may materially impact our ability to progress our business, including (i) our supplier may cease or reduce production or deliveries, raise prices or renegotiate terms; (ii) delays caused by supply issues which may harm our reputation; and (iii) our single-source supplier or CMOs may experience significant business challenges, disruption or failures due to issues such as financial difficulties or bankruptcy, issues relating to regulatory or quality compliance issues, or other legal or reputational issues.
Additionally, we may not be able to enter into supply arrangements with alternative suppliers on commercially reasonable terms, or at all. A delay in the development of a Product Candidate or having to enter into a new agreement with a different third party on less favorable terms than we have with our current suppliers could have a material adverse impact upon on our business.
Psychedelic or psychedelic-inspired drugs may never be approved as medicines or other therapeutic applications and violations of applicable laws and regulations could result in repercussions.
In the United States, certain psychedelic drugs are classified as Schedule I drugs under the CSA (21 U.S.C. § 811) and the Controlled Substances Import and Export Act and as such, medical and recreational use is illegal under the U.S. federal laws.
| 21 |
| Table of Contents |
In Canada, certain substances are classified as controlled substances and are listed on Schedule III of the Controlled Drugs and Substances Act (Canada) (“CDSA”) and are also listed under the Schedule to Part J to the Food and Drug Regulations, which results in very restricted use as substances listed under Part J can generally only be used for research or clinical testing under limited circumstances. There is no guarantee that psychedelic drugs will ever be approved as medicines or other therapeutic applications in any jurisdiction in which the Company operates.
The Company's programs for Lucid-PSYCH involved controlled drugs and were conducted in strict compliance with the laws and regulations regarding the production, storage, and use of such drugs. Although the Company put a temporary hold on all research and development related to controlled drugs, if it begins research again, it will be subject to these risks. As such, all facilities engaged with such substances by or on behalf of the Company do so under current licenses and permits issued by appropriate governmental agencies. Unforeseen delays to the drug substance and drug product manufacture and supply chain may occur due to delays, errors, or other unforeseen problems with the permitting and quota process.
The failure of the Company to maintain compliance with applicable federal, state, or provincial requirements, or the loss or diversion of controlled substances, can result in significant enforcement actions. The Drug Enforcement Administration (“DEA”) and/or state authorities could seek civil penalties, refuse to renew registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to civil and criminal prosecutions, fines, penalties, and forfeitures. Overall, a violation of any laws and regulations in the jurisdictions in which the Company operates could result in significant fines, penalties, administrative sanctions, convictions, or settlements arising from civil proceedings initiated by either government entities in the jurisdictions in which the Company operates, or by private citizens, or through criminal charges. The loss of the necessary licenses, permits or exemptions, including the loss of access to licensed facilities, for use of controlled drugs could have an adverse effect on the Company's operations.
Regulatory or political change with respect to psychedelic-inspired drugs could occur.
When the Company begins actively researching psychedelic drugs, its success will depend in part, on the legality of the use of psychedelic-inspired drugs for the treatment of neuropsychiatric disorders and the acceptance of such use in the medical community. The political environment surrounding the psychedelics industry in general can be volatile and a shift in the regulatory or political realm could occur and have a drastic impact on the use of psychedelics as a whole, adversely impacting the Company's ability to successfully operate or grow its business. Furthermore, failure to follow applicable regulatory requirements will have a materially negative impact on the business of the Company.
General Corporate Risks
Macroeconomic pressures in the markets in which we operate, including, but not limited to, the lasting effects of the COVID-19 pandemic, political developments, geopolitical unrest or other conflicts or natural disasters in foreign nations, including the ongoing conflict between Russia and Ukraine, political developments in Hong Kong and Taiwan, and inflationary pressures may alter the ways in which we conduct our business operations and manage our financial capacities.
To varying degrees, the ways in which we conduct our business operations and manage our financial capacities are influenced by macroeconomic conditions that affect companies directly involved in or providing services related to the drug and biological product development. For example, real GDP growth, business and investor confidence, the lasting effects of COVID-19 pandemic, inflation, employment levels, oil prices, interest rates, tax rates, availability of consumer and business financing, housing market conditions, foreign currency exchange rate fluctuations, costs for items such as fuel and food and other macroeconomic trends can adversely affect not only our decisions and ability to engage in research and development and clinical trials, but also those of our management, employees, third-party contractors, manufacturers and suppliers, competitors, Shareholders and regulatory authorities. The ongoing military conflict between Russia and Ukraine and other geopolitical and social unrest has created extreme volatility in the global capital markets and is expected to have further global economic consequences, including disruptions of the global supply chain and energy markets. Any such volatility and disruptions may adversely affect our business or the third parties on whom we rely. If the equity and credit markets deteriorate, including as a result of political unrest, natural disasters or war, it may make any necessary debt or equity financing more difficult to obtain in a timely manner or on favorable terms, more costly or more dilutive. Increased inflation rates can adversely affect us by increasing our costs, including labor and employee benefit costs. In addition, higher inflation and macro turmoil and uncertainty could also adversely affect our customers, which could reduce demand for our products.
Economic uncertainty may adversely affect our access to capital, cost of capital and ability to execute our business plan as scheduled.
Generally, worldwide economic conditions remain uncertain. Access to capital markets is critical to our ability to operate. Traditionally, biotechnology companies have funded their research and development expenditures through raising capital in the equity markets. Declines and uncertainties in these markets in the past have severely restricted raising new capital and have affected companies’ ability to continue to expand or fund existing research and development efforts. We require significant capital for research and development for our product candidates and clinical trials. The general economic and capital market conditions, both in the U.S. and worldwide, have been volatile and at times have adversely affected our access to capital and increased the cost of capital. For example, the ongoing military conflict between Russia and Ukraine, the possibility of a wider European or global conflict, global sanctions imposed in response thereto and the possibility of a global energy crisis resulting therefrom, has created extreme volatility in the global capital markets and is expected to have further global economic consequences, including disruptions of the global supply chain and energy markets. Any such volatility and disruptions may adversely affect our business or the third parties on whom we rely. If global capital markets deteriorate, including as a result of political unrest or war, it may make any necessary financing more difficult to obtain in a timely manner or on favorable terms, more costly or more dilutive. If economic conditions become worse, our future cost of equity or debt capital and access to the capital markets could be adversely affected. If we are unable to access the capital markets on favorable terms, our ability to execute our business plan as scheduled would be compromised. Moreover, we rely and intend to rely on third parties, including clinical research organizations, contract manufacturing organizations and other important vendors and consultants. Global economic conditions may result in a disruption or delay in the performance of our third-party contractors and suppliers. If such third parties are unable to adequately satisfy their contractual commitments to us in a timely manner, our business could be adversely affected.
The Company’s limited operating history makes it difficult to evaluate its current business and future prospects and the Company may never be able to generate sufficient revenue to be profitable.
The Company’s limited operating history makes it difficult to evaluate its current business and future prospectus. The Company has never generated any material amount of revenue and has not generated any revenue from its bio-tech business. We have incurred significant losses since our inception, and we anticipate that we will continue to incur significant losses and will not be profitable or generate positive cash flow from operating activities for the foreseeable future. In addition, the Company expects to continue to increase operating expenses as it implements initiatives to continue to grow its business and pursue the commercialization of its Product Candidates. If the Company does not generate sufficient revenue to offset these expected increases in costs and operating expenses, it will not be profitable. The Company cannot predict when it will generate any revenue, or when or if it will become profitable or generate positive cash flow from operating activities, if at all.
In general, the Company is subject to many of the risks common to early-stage enterprises, including undercapitalization, cash shortages, limitations with respect to personnel, financial, and other resources and lack of revenues. There is no assurance that the Company will be successful in achieving a return on the Company’s shareholders’ (“Shareholders”) investment and the likelihood of success must be considered in light of the early stage of our operations.
Future transfers by holders of Class A Shares to arm’s length parties or other than to permitted holders will generally result in those shares converting to Class B Shares, which will have the effect, over time, of increasing the relative voting power of those holders of Class A Shares who retain their shares. Such holders could, in the future, control a significant percentage of the combined voting power of Class A Shares and Class B Shares.
Each of the Company’s directors and officers owes a fiduciary duty to the Company and must act honestly and in good faith with a view to the best interests of Company. However, any director and/or officer that is a Shareholder, even a controlling Shareholder, is entitled to vote its shares in its own interests, which may not always be in the interests of the Shareholders generally. The inability of the Class B Shares to control the matters affecting the Company, combined with the ability of holders of Class A Shares to control matters affecting the Company and to take actions that the holders of Class B Shares may not view as beneficial, may adversely affect the market price of the Class B Shares.
| 22 |
| Table of Contents |
Dilution of the percentage ownership of the Shareholders
Future sales and issuances of the Company’s Class B Shares or rights to purchase Class B Shares, including pursuant to the Company’s equity incentive plans, could result in additional dilution of the percentage ownership of the Shareholders and could cause the Company’s stock price to fall. The Company expects that significant additional capital may be needed in the future to continue its planned operations, including conducting clinical trials, commercialization efforts, expanded research and development activities, potential acquisitions, in licenses, or collaborations and costs associated with operating a public company. To raise capital, the Company may sell Class B Shares, convertible securities, or other equity securities in one or more transactions at prices and in a manner it determines from time to time. If the Company sells Class B Shares, convertible securities, or other equity securities in more than one transaction, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to the Shareholders, and new investors could gain rights, preferences, and privileges senior to the holders of our Class B Shares, including Class B Shares sold in this offering upon exercise of Class B Share purchase warrants.
Failure to comply with laws and regulations could have a material adverse effect on the Company's business.
We are subject to complex laws, rules and regulations affecting our domestic and international operations in Canada, the United States and Australia relating to numerous topics, including the research and development of our pharmaceutical drugs, health care and data privacy laws, labor and employment and regulatory requirements of the CSE and Nasdaq. In addition, we are required to comply with certain U.S. Securities Exchange Commission (the “SEC”) and other legal requirements affecting public companies. Compliance with, and monitoring of, applicable laws and regulations may be difficult, time consuming and costly. Those laws and regulations and their interpretation and application may also change from time to time and those changes could have a material adverse effect on our business, investments, and results of operations. In addition, a failure to comply with applicable laws or regulations, as interpreted and applied, could have a material adverse effect on our business, including our ability to negotiate and complete our initial acquisitions, and our future results.
Although, to our knowledge, we are currently in material compliance with all applicable laws, regulations and guidelines in such jurisdictions, no assurance can be given that new laws, regulations, and guidelines will not be enacted or that existing laws, regulations, and guidelines will not be interpreted or applied in a manner which could limit or curtail our operations in such jurisdictions.
On April 17, 2023, FSD Strategic Investments entered into the CEO Mortgage Loan (as defined herein). Although the Company believes the CEO Mortgage Loan complies with the exemption contained in Section 13(k) of the Exchange Act, there can be no assurances that it complies with these regulations. There is limited regulatory guidance or legislative history as to the scope of this exemption. If the CEO Mortgage Loan does not comply, the Company could be subject to fines, penalties and/or other regulatory actions, which would have an adverse effect on our business, financial condition, and results of operations. Furthermore, amendments to current laws, regulations and guidelines, more stringent implementation, or enforcement thereof or other unanticipated events, are beyond our control and could require extensive changes to our operations, which in turn may also result in a material adverse effect on our business, financial condition, and results of operations.
For more information, see “Item 7. Major Shareholders and Related Party Transactions - B. Related Party Transactions.”
Any significant interruption in the supply chain for key inputs could materially impact the Company’s business.
Our business is dependent on a number of key inputs and their related costs including raw materials and supplies, as well as electricity, water, and other local utilities. The ability of the Company to research and develop pharmaceutical products is dependent upon, among other things, sufficient access to timely delivery of equipment, parts, and components at reasonable costs. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs could materially impact our business, financial condition, and operating results. Any inability to secure required supplies and services or to do so on appropriate terms could have a material adverse impact on our business, financial condition, and operating results.
The Company may be unable to raise the capital necessary for it to execute its strategy on favorable terms or at all.
There is no guarantee that the Company will be able to execute on its strategy. Developing Lucid-MS, a biopharmaceutical products, and products for alcohol misuse, is expensive and time-consuming, and we expect to require substantial additional capital to conduct research, preclinical testing and human studies, to potentially establish pilot scale and commercial scale manufacturing processes and facilities, and to establish and develop quality control, regulatory, marketing, sales and administrative capabilities to support our existing programs and pursue potential additional programs. We are or may in the future also be responsible for the payments to third parties of expenses that may include milestone payments, license maintenance fees and royalties, including in the case of certain of our agreements with academic institutions or other companies from whom intellectual property rights underlying their respective programs have been licensed or acquired. Because the outcome of any preclinical or clinical development and regulatory approval process for Lucid-MS and other product candidates that we may develop in the future is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development, regulatory approval process and commercialization of any product candidates we may identify.
| 23 |
| Table of Contents |
Our future funding requirements for the development of pharmaceutical products will depend on many factors, including, but not limited to: (i) the time and cost necessary to complete planned clinical trials to pursue regulatory approvals for our Lucid-MS and any other drug candidates, and to conduct post-marketing studies that could be required by regulatory authorities; (ii) the progress, timing, scope and costs of our nonclinical studies, preclinical studies, clinical trials and other related activities, including the ability to enroll patients in a timely manner for planned clinical trials described in this Annual Report and potential future clinical trials; (iii) the costs of obtaining clinical and commercial supplies of raw materials and drug products for Lucid-MS and other product candidates; (iv) our ability to successfully identify and negotiate acceptable terms for third-party supply and contract manufacturing agreements with CMO’s; (v) our ability to successfully commercialize our Product Candidates, either directly or through licensing agreements, ;(vi) the manufacturing, selling and marketing costs associated with our Product Candidates, including the cost and timing of expanding our internal sales and marketing capabilities or entering into strategic collaborations with third parties to leverage or access these capabilities; (vii) the amount and timing of sales and other revenues from our Product Candidates, if any are approved, including the sales price and the availability of adequate third-party reimbursement; (viii) the cash requirements of any future acquisitions or discovery of product candidates; (ix) the time and cost necessary to respond to technological, market, regulatory or political developments; (x) the costs of acquiring, licensing or investing in intellectual property rights (including the protection of such rights), products, product candidates and businesses; and (xi) our ability to attract, hire and retain qualified personnel.
Additional funds may not be available when we need them, on terms that are acceptable, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, or terminate one or more research or development programs or the commercialization of any Product Candidates or be unable to expand operations or otherwise capitalize on business opportunities, as desired, which could materially affect our business, results of operations, financial condition, and prospects.
In addition, the continued development of the Company’s pharmaceutical operations will require significant additional financing over several years. The failure to raise such capital could result in the delay or indefinite postponement of current business strategy or the Company ceasing to carry on business. There can be no assurance that additional capital or other types of financing will be available if needed or that, if available, the terms of such financing will be favorable to the Company, at times for reasons beyond the Company’s control. For example, economic downturns or uncertain market conditions, whether affecting the economy in general or the pharmaceutical industry in particular, could adversely impact the Company’s ability to raise capital through equity or debt financing. In addition, any further issuances of equity securities could have a significant dilutive effect on the holders of Class B Shares.
In addition, from time to time, the Company may enter into transactions to acquire assets or the shares of other companies. These transactions may be financed wholly or partially with debt, which may temporarily increase the Company’s debt levels above industry standards. Any debt financing secured in the future could involve restrictive covenants relating to capital raising activities and other financial and operational matters, which may make it more difficult for the Company to obtain additional capital and to pursue business opportunities, including potential acquisitions.
Future sales or issuances of equity securities and the conversion of outstanding securities to Class B Shares could decrease the value of the Class B Shares and dilute investors’ voting power.
The Company may sell additional equity securities in future offerings, including through the sale of securities convertible into equity securities, to finance operations, acquisitions or projects, and issue additional Class B Shares, which may result in dilution.
The Board has the authority to authorize certain offers and sales of additional securities without the vote of, or prior notice to, shareholders. Based on the need for additional capital to fund expected expenditures and growth, it is likely that the Company will issue additional securities to provide such capital. Such additional issuances may involve the issuance of a significant number of Class B Shares.
Sales of substantial amounts of the Company’s securities, or the availability of such securities for sale, as well as the issuance of substantial amounts of the Class B Shares upon conversion of outstanding convertible, exercisable or exchangeable securities, could adversely affect the prevailing market prices for the Company’s securities and dilute investors’ earnings per share. A decline in the market prices of the Company’s securities could impair its ability to raise additional capital through the sale of securities should the Company desire to do so.
The success of the Company is dependent upon its senior management and key personnel and ability to hire skilled personnel.
Another risk associated with the production and sale of pharmaceutical products is the loss of important personnel. The success of the Company will be dependent upon the ability, expertise, judgment, discretion and good faith of its senior management and key personnel. While employment agreements are customarily used as a primary method of retaining the services of key employees, these agreements cannot assure the continued services of such employees. While, as of the date of this Annual Report, the Company does not anticipate any senior management turnover in the near term, there is no guarantee that the Company will be able to retain its senior management going forward. If key personnel depart, including Zeeshan Saeed, Anthony Durkacz, Nathan Coyle, Dr. Lakshmi Kotra or Donal Carroll, the Company may not be able to find appropriate replacements on a timely basis.
Furthermore, each of our executive officers may terminate their employment with us at any time. We do not maintain “key person” insurance for any of our executives or employees. Recruiting and retaining qualified scientific and clinical personnel and, if any of our Product Candidates are commercialized, sales and marketing personnel, will be critical to our success. The loss of the services of key personnel as well as the diversion of management’s and the Board’s attention to replace the services of such individuals, could have a material adverse effect on the Company’s business, operating results, or financial condition.
| 24 |
| Table of Contents |
In addition, the Company’s future success depends on its continuing ability to attract, develop, motivate, and retain highly qualified and skilled employees. Due to the specialized scientific and managerial nature of our business, the Company relies heavily on its ability to attract and retain qualified scientific, technical, and managerial personnel. In particular, specialized knowledge with respect to research and clinical development is important to the pharmaceutical industry. Qualified individuals are in high demand, and the Company may incur significant costs to attract and retain them, if it is able to hire them at all. If we are unable to identify, attract, hire, and retain qualified personnel in the future, such inability could have a material adverse effect on our business, operating results, and financial condition.
The Company’s dual class structure has the effect of concentrating voting control and the ability to influence corporate matters with a limited number of holders of Class A Shares.
The Company’s dual class structure has the effect of concentrating voting control for holders of Class A Shares and the ability to influence corporate matters with those Shareholders. Currently, there are 72 outstanding Class A Shares issued and outstanding. Class A Shares have 276,660 votes per share and Class B Shares have one vote per share. As of March 28, 2024, Shareholders who hold Class A Shares together hold approximately 33.6% of the voting power of the Company’s outstanding voting shares and therefore have significant influence over management and affairs of the Company and over all matters requiring Shareholder approval.
In addition, because of the voting ratio between Class A Shares and Class B Shares, the holders of Class A Shares collectively continue to control a majority of the combined voting power of the voting shares even where the Class A Shares represent a substantially reduced percentage of the total outstanding shares. The different voting rights could diminish the value of the Class B Shares to the extent that investors or any potential future purchasers of the Class B Shares attribute value to the superior voting or other rights of the Class A Shares. Other than as required by applicable law, holders of the Class B Shares will only have a right to vote, as a class, in limited circumstances as described in its constating documents.
The concentrated voting control of holders of Class A Shares limits the ability of holders of Class B Shares to influence corporate matters and all matters requiring Shareholder approval, including the election of directors as well as with respect to decisions regarding amendment of the Company’s share capital, creating and issuing additional classes of shares, making significant acquisitions, selling significant assets or parts of our business, merging with other companies and undertaking other significant transactions.
As a result, holders of Class A Shares have the ability to control substantially all matters affecting us and actions may be taken that our holders of Class B Shares may not view as beneficial. The market price of the Class B Shares could be adversely affected due to the significant influence and voting power of the holders of Class A Shares. Additionally, the significant voting interest of holders of Class A Shares may discourage transactions involving a change of control, including transactions in which an investor, as a holder of the Class B Shares, might otherwise receive a premium for the Class B Shares over the then-current market price, or discourage competing proposals if a going private transaction is proposed by one or more holders of Class A Shares.
The market price of the Class B Shares may be subject to wide price fluctuations.
The market price of the Class B Shares may be subject to wide fluctuations in response to many factors, including variations in the operating results of the Company and its subsidiaries, divergence in financial results from analysts’ expectations, changes in earnings estimates by stock market analysts, changes in the business prospects for the Company, general economic conditions, legislative changes, and other events and factors outside of the Company’s control. In addition, stock markets have from time to time experienced extreme price and volume fluctuations, which, as well as general economic and political conditions, could adversely affect the market price for the Class B Shares.
There is no assurance of an active or liquid market.
No assurance can be given that an active or liquid trading market for the Class B Shares will be sustained. If an active or liquid market for the Class B Shares fails to be sustained, the prices at which such securities trade may be adversely affected. Whether or not the Class B Shares will trade at lower prices depends on many factors, including the liquidity of the Class B Shares, prevailing interest rates, the markets for similar securities, general economic conditions and the Company’s financial condition, historic financial and operating performance, and future prospects.
The Company may be unable to manage its growth, including capacity constraints and pressure on its internal systems and controls.
The Company may be subject to growth-related risks including capacity constraints and pressure on its internal systems and controls. The ability of the Company to manage growth effectively will require it to continue to implement and improve its operational and financial systems and to expand, train and manage its employee base. The inability of the Company to deal with this growth may have a material adverse effect on the Company’s business, results of operations, financial condition and prospects.
Management may not be able to successfully implement and maintain adequate internal controls over financial reporting or disclosure controls and procedures.
Effective internal controls are necessary for the Company to provide reliable financial reports and to help prevent fraud. Although the Company has undertaken a number of procedures and has implemented a number of safeguards, in each case, in order to help ensure the reliability of its financial reports, including those imposed on the Company under applicable securities laws, the Company cannot be certain that such measures will ensure that the Company will maintain adequate control over financial processes and reporting. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm the Company’s results of operations, or cause it to fail to meet its reporting obligations.
| 25 |
| Table of Contents |
Effective systems of internal control over financial reporting and disclosure are critical to the operation of a public corporation. However, we do not expect that our disclosure controls and procedures or internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints and the benefits of such controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Due to the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected in a timely manner or at all. If we cannot provide reliable financial reports or prevent fraud, our reputation and operating results could be materially adversely affected, which could cause investors to lose confidence in us and our reported financial information, which in turn could result in a reduction in the value of the Class B Shares.
A decline in general economic conditions may impact the viability and success of our mortgage investment activities.
FSD Strategic Investments has made and intends on continuing to make investments in loans that are secured by first or second collateral mortgages on residential real estate in the Greater Toronto Area. A decline in general economic conditions could adversely impact the ability of borrowers to service their loans and could cause default rates to increase. This could have a material adverse effect on FSD Strategic Investments’ financial condition and results of operations.
A decline in property values could adversely affect the value of the security on mortgages held by FSD Strategic Investments, thereby reducing the ability to liquidate properties held by defaulting borrowers at favorable prices.
The profits earned on mortgages depend, in part, on the spread between mortgage rates and capital market funding rates and any fee income derived therefrom. FSD Strategic Investments’ mortgage portfolios include assets whose value can fluctuate because of changing interest rates and economic and market conditions. In addition, some of these assets could be difficult to sell at any given time. Changes in interest rates and other market factors such as stock market prices and demographics could affect the preferences of its customers for different types of loan products and adversely impact our profitability. A reduction in positive spreads between mortgage rates and capital market funding rates could have a material adverse effect on FSD Strategic Investments’ financial condition and results of operations.
Investments in mortgages are relatively non-liquid assets. The nature of the assets held by FSD Strategic Investments may inhibit its ability to quickly respond to changes in broader economic or investment conditions. If the value of the properties underlying FSD Strategic Investments’ mortgages begin to deteriorate, it will be difficult for FSD Strategic Investments to liquidate certain assets in response to these changes. The liquidity profile of FSD Strategic Investments’ mortgages can create challenges for it to manage its risk exposure. Reduced asset liquidity may restrict FSD Strategic Investments’ ability to sell assets for cash without taking significant losses, which may result in a material adverse effect on FSD Strategic Investments’ financial condition and results of operations.
Risks related to our status as a foreign private issuer.
As a “foreign private issuer” under the rules and regulations of the SEC, we are permitted to, and will, file less or different information with the SEC than a company incorporated in the United States or otherwise subject to these rules, and will follow certain home country corporate governance practices in lieu of certain Nasdaq requirements applicable to U.S. issuers.
The Company is considered a “foreign private issuer” under the Exchange Act and is therefore exempt from certain rules under the Exchange Act. For example, we are not required to file current reports on Form 8-K or quarterly reports on Form 10-Q, we are exempt from the U.S. proxy rules which impose certain disclosure and procedural requirements for U.S. proxy solicitations and we will not be required to file financial statements prepared in accordance with or reconciled to U.S. GAAP so long as our financial statements are prepared in accordance with IFRS as issued by the International Accounting Standards Board. We are not required to comply with Regulation FD, which imposes restrictions on the selective disclosure of material information to shareholders, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions of Section 16 of the Exchange Act. In addition, we are not required to file periodic reports and financial statements with the SEC as frequently or within the same time frames as U.S. companies with securities registered under the Exchange Act. Accordingly, holders of the Company’s securities may receive less or different information about the Company than they may receive with respect to public companies incorporated in the United States.
In addition, as a “foreign private issuer” whose common shares are listed on Nasdaq, we are permitted to follow certain home country corporate governance practices in lieu of certain Nasdaq requirements, including those related to: shareholder approval for certain dilutive events under Nasdaq Marketplace Rule 5635, quorum requirements for shareholder meetings under Nasdaq Marketplace Rule 5620(c), certain independence requirements of certain committees of our Board under Nasdaq Marketplace Rule 5605 and proxy delivery requirements under Nasdaq Marketplace Rule 5620(b). Accordingly, the Company has opted to follow certain corporate governance practices required by its home country under the CSE, Canadian federal and provincial corporate and securities laws and the Company’s Articles, as applicable. See “Item 16G. Corporate Governance” for more details related to the differences between our home country requirements and Nasdaq requirements.
We could lose our status as a “foreign private issuer” under current SEC rules and regulations if more than 50% of our outstanding voting securities become directly or indirectly held of record by U.S. holders and one of the following is true: (i) the majority of our directors or executive officers are U.S. citizens or residents; (ii) more than 50% of our assets are located in the United States; or (iii) our business is administered principally in the United States. If we lose our status as a foreign private issuer in the future, we will no longer be exempt from the rules described above and, among other things, will be required to file periodic reports and annual and quarterly financial statements as if we were a company incorporated in the United States (including preparation of financial statements in accordance with U.S. GAAP). If this were to happen, we would likely incur substantial costs in fulfilling these additional regulatory requirements and members of our management would likely have to divert time and resources from other responsibilities to ensuring these additional regulatory requirements are fulfilled.
| 26 |
| Table of Contents |
There can be no assurance that we will be able to comply with the continued listing standards of Nasdaq and/or CSE.
Our Class B Shares are listed on Nasdaq and CSE. There can be no assurance that we will continue to meet Nasdaq and/or CSE’s listing standards. On September 27, 2022, we received a letter from the listing qualifications department staff of Nasdaq notifying us that the Company is not in compliance with the minimum bid price requirement set forth in Nasdaq’s rules for continued listing on the Nasdaq Capital Market. While we have since regained compliance with Nasdaq’s minimum bid price requirement, there can be no guarantee that we will be able to maintain such compliance in the future. If we lose our ability to maintain compliance with Nasdaq and/or the CSE’s continued listing rules, we and our Shareholders could face significant material adverse consequences, including:
|
· |
a limited availability of market quotations for our securities; |
|
· |
reduced liquidity for our securities; |
|
· |
a determination that our Class B Shares is a “penny stock,” in the U.S. which will require brokers trading in our Class B Shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our securities; |
|
· |
a limited amount of news and analyst coverage; and |
|
· |
decreased ability to issue additional securities or obtain additional financing in the future. |
As an “emerging growth company,” the Company cannot be certain if the reduced disclosure and governance requirements applicable to “emerging growth companies” will make its shares less attractive to investors.
As an “emerging growth company,” the Company may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies, including not being required to obtain an assessment of the effectiveness of its internal controls over financial reporting from its independent registered public accounting firm pursuant to Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. In addition, the U.S. Jumpstart Our Business Startups Act (the “JOBS Act”) provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards, which the Company has elected to do.
We cannot predict if investors will find our shares less attractive because we will rely on these exemptions. If some investors find our shares less attractive as a result, there may be a less active market for our shares, our share price may be more volatile and the price at which our securities trade could be less than if we did not use these exemptions.
We expect to incur costs related to our internal control over financial reporting in the upcoming years to further improve our internal control environment. If we identify deficiencies in our internal controls over financial reporting or if we are unable to comply with the requirements applicable to us as a public company, including the requirements of Section 404 of the Sarbanes-Oxley Act, in a timely manner, we may be unable to accurately report our financial results, or report them within the timeframes required by the SEC. If this occurs, we also could become subject to sanctions or investigations by the SEC or other regulatory authorities. In addition, if we are unable to assert that our internal controls over financial reporting are effective, or if our independent registered public accounting firm is unable to express an opinion as to the effectiveness of our internal control over financial reporting, or express an adverse opinion, investors may lose confidence in the accuracy and completeness of our financial reports, we may face restricted access to the capital markets and our share price may be adversely affected.
We may not be able to successfully identify and execute future acquisitions or dispositions or to successfully manage the impacts of such transactions on our operations.
The Company has made and may continue to pursue acquisition opportunities to advance its strategic plan. The successful integration of an acquired business typically requires the management of the pre-acquisition business strategy, including the retention and addition of senior management, customers, realization of identified synergies, retention of key staff and the development of a common corporate culture. Achieving the benefits of acquisitions depends in part on successfully consolidating functions and integrating operations and procedures in a timely and efficient manner, as well as the ability to realize anticipated growth opportunities and synergies from newly formed partnerships. Any failure to integrate an acquired business or realize the anticipated benefits of new partnerships may have a material adverse effect on the Company’s business, results of operations, financial condition, and prospects, including its future prospects for acquisitions or partnerships. There is no assurance that the Company will be able to successfully integrate an acquired business in order to maximize or realize the benefits associated with an acquisition.
In addition, from time to time the Company enters into letters of intent and memoranda of understanding with respect to which definitive agreements have not yet been, but are expected to be, executed. The Company may not be able to perform under these contracts as a result of operational or other breaches or due to events beyond its control, and the Company may not be able to ultimately execute a definitive agreement in cases where one does not currently exist.
| 27 |
| Table of Contents |
Any expansion of our international operations will result in increased operational, regulatory, and other risks.
We established an Australian subsidiary in November 2022 and may in the future expand into other geographic areas, which could increase our operational, regulatory, compliance, reputational and foreign exchange rate risks. The failure of our operating infrastructure to support such expansion could result in operational failures and regulatory fines or sanctions.
The Company is currently party to several legal proceedings and may become a party to potential future litigation.
The Company is currently party to a number of proceedings; see “Item 8. Financial Information - A. Consolidated Statements and Other Financial Information – Legal Proceedings”. Such litigation could be costly and time-consuming and could divert the attention of management and other key personnel from the Company’s business and operations. The complexity of any such claims and the inherent uncertainty of commercial, employment and other litigation increases these risks. In recognition of these considerations, the Company could suffer significant litigation expenses in defending any of these claims and may enter into settlement agreements.
The Company may also become party to additional litigation in the future, including class action lawsuits, securities litigation and anti-trust and anti-competitive actions, which could adversely affect its business. Should any litigation in which the Company becomes involved be determined against the Company, such a decision could adversely affect the Company’s ability to continue operating and the market price for Company’s Class B Shares and could result in the use of significant resources. Even if the Company is involved in litigation and wins, litigation can redirect significant corporate resources and management attention.
Conflicts of interest may arise between the Company and its directors and officers as a result of other business activities undertaken by such individuals.
Certain directors and officers of the Company are, and may in the future become, directors and officers of other entities, or are otherwise engaged, and will continue to be engaged, in activities that may put them in conflict with the business strategy of the Company. In particular, certain directors and officers of the Company serve as directors or officers of entities that may compete with or have conflicting interests with the Company.
The Company’s directors and the officers are required to act honestly and in good faith with a view to its best interests. However, in conflict of interest situations, the Company’s directors and officers may owe the same duty to another corporation and will need to balance their competing interests with their duties to the Company. Circumstances (including with respect to future corporate opportunities) may arise that may be resolved in a manner that is unfavorable to the Company. These business interests could require the investment of significant time and attention by our executive officers and directors. In some cases, our executive officers and directors may have fiduciary obligations associated with business interests that interfere with their ability to devote time to our business and affairs, which could adversely affect our operations.
The Company does not anticipate paying dividends in the near future.
Effective November 29, 2023, the Corporation completed the Plan of Arrangement, which included the distribution of the Celly Nu Shares to the FSD Pharma Securityholders. For more information, please see “Item 4. Information on the Company. - A. History and Development of the Company - Significant Developments in Fiscal 2023 through to March 28, 2024”.
The Company does not anticipate paying cash or stock dividends in the near future. The Company expects to retain earnings to finance the development and enhancement of its Product Candidates and to otherwise reinvest in the Company’s business. Any decision to declare and pay dividends in the future will be made at the discretion of the Board and will depend on, among other things, financial results, cash requirements, contractual restrictions, and other factors that the Board may deem relevant. As a result, investors may not receive any return on their investment in Class B Shares unless they sell them for a share price that is greater than that at which such investors purchased them.
The Company’s operations depend, in part, on the maintenance and protection of its information technology systems and the information technology systems of its third-party research institution collaborators, CROs or other contractors or consultants, which could face cyber-attacks that cause material losses to our business.
We have entered into agreements with third parties for hardware, software, telecommunications, and other information technology (“IT”) services in connection with our operations. Our operations depend, in part, on how well we, our CROs, other contractors, consultants and our suppliers protect networks, equipment, IT systems and software against damage from a number of threats, including, but not limited to, cable cuts, damage to physical plants, natural disasters, terrorism, fire, power loss, hacking, computer viruses, vandalism and theft. Our operations also depend on the timely maintenance, upgrade and replacement of networks, equipment, IT systems and software, as well as pre-emptive expenses to mitigate the risks of failures. Any of these and other events could result in information system failures, delays and/or increase in capital expenses. The failure of information systems or a component of information systems could, depending on the nature of any such failure, adversely impact our reputation and results of operations.
For example, the loss of, or damage to, clinical trial data from completed, ongoing or future preclinical or clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely or expect to rely on third parties for research and development, the manufacture and supply of drug product and to conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or systems, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our Product Candidates could be delayed.
| 28 |
| Table of Contents |
Certain data breaches must also be reported to affected individuals and certain regulatory bodies, and in some cases may be required to be publicly disclosed under U.S. federal and state law, federal and provincial data protection legislation in Canada and the requirements of other jurisdictions, and financial or other penalties may also apply.
Cyber incidents can result from deliberate attacks or unintentional events. Cyber-attacks could result in any person gaining unauthorized access to digital systems for purposes of misappropriating assets or sensitive information, including personally identifiable information, corrupting data, or causing operational disruption. Cyber-attacks could also result in important remediation costs, increased cyber security costs, lost revenues due to a disruption of activities, litigation and reputational harm affecting customer and investor confidence, which could materially adversely affect our business and financial results.
We have not experienced any material losses to date relating to cyber-attacks or other information security breaches, but there can be no assurance that we will not incur such losses in the future, which could be in excess of any available insurance and could materially adversely affect our business and financial results. Our risk and exposure to these matters cannot be fully mitigated because of, among other things, the evolving nature of these threats. As a result, cyber security and the continued development and enhancement of controls, processes and practices designed to protect systems, computers, software, data and networks from attack, damage or unauthorized access is a priority. As cyber threats continue to evolve, we may be required to expend additional resources to continue to modify or enhance protective measures or to investigate and remediate any security vulnerabilities.
We may be a passive foreign investment company, which may result in adverse U.S. federal income tax consequences for holders of our Class B Shares who are U.S. taxpayers.
Generally, if for any taxable year 75% or more of our gross income is passive income, or 50% or more of the average quarterly value of our assets are held for the production of, or produce, passive income, we would be characterized as a passive foreign investment company, or “passive foreign investment company” (“PFIC”), for U.S. federal income tax purposes. The determination of whether we are a PFIC is a fact-intensive determination made on an annual basis applying principles and methodologies that in some circumstances are unclear and subject to varying interpretation, and the Company’s PFIC status will depend among other things upon changes in the composition and relative value of its gross receipts and assets. We believe that we were a PFIC for the year ended December 31, 2023. In addition, although PFIC status is determined on an annual basis and generally cannot be determined until the end of the taxable year, we believe that we may be considered a PFIC for the current taxable year. Because we may continue to hold a substantial amount of cash and cash equivalents, and because the market value of the Company’s assets (including for this purpose goodwill) may be measured in large part by the market price of our shares, which is likely to fluctuate, no assurance can be given that the Company will not also be a PFIC in any future taxable year. If we are characterized as a PFIC, our shareholders who are U.S. taxpayers may suffer adverse tax consequences, including the treatment of gains realized on the sale of our Class B Shares as ordinary income, rather than as capital gain, the loss of the preferential rate applicable to dividends received on our Class B Shares by individuals who are U.S. taxpayers, and the addition of interest charges to the tax on such gains and certain distributions. For more information, please see “Item 10. Additional Information - E. Taxation - Certain Material U.S. Federal Income Tax Considerations”.
Item 4. Information on the Company.
A. History and Development of the Company
Overview and History
We were incorporated in 1998 under the OBCA under the name of Century Financial Group, Inc. On May 24, 2018, pursuant to Articles of Amendment, the Company changed its name to “FSD Pharma Inc.” From May 2018 to March 2020, the focus of the Company’s business was the cultivation, processing and sale of medical cannabis; in March 2020, however, the Company pivoted its focus to pharmaceuticals and biotechnology.
The Company is building a portfolio of innovative assets and biotech solutions for the treatment of challenging neurodegenerative and metabolic disorders and alcohol misuse disorders with drug candidates (“Product Candidates”) in different stages of development. We are currently focused on the research and development of our lead compound, Lucid-MS, a patented new chemical entity shown to prevent and reverse myelin degradation, the underlying mechanism of multiple sclerosis, in preclinical models. The Company is also focused on the research and development of novel formulations for the treatment for alcohol misuse. In addition, the Company maintains a portfolio of strategic investments through its wholly owned subsidiary, FSD Strategic Investments, which represent loans secured by residential properties.
Alcohol Misuse Disorder Product Candidates
With respect to the Product Candidates for alcohol misuse disorders, the Corporation sees two distinct routes where this segment can be developed, (i) recreational retail and (ii) healthcare; Celly Nu, through the Celly Nu IP License Agreement will be focusing on the recreational retail sector and the Corporation will be focusing on the healthcare sector as further outlined below:
(i) Alcohol Misuse: Retail Product (known as “Unbuzzd™”)
| 29 |
| Table of Contents |
A consumer recreational beverage product that will be sold via retail distribution. On July 31, 2023, the Corporation entered into a definitive exclusive intellectual property license agreement (the “Celly Nu IP License Agreement”) with Celly Nu and Lucid, which granted Celly Nu the exclusive rights to the recreational applications for the Corporation’s alcohol misuse technology for rapid alcohol detoxification, and the rights to the trademarks, Unbuzzd™ and ALCOHOLDEATH™, in exchange for securities in Celly Nu and a royalty on any product sales, so that Celly Nu could fund research and development, market and sell Unbuzzd™. As part of the Celly Nu IP License Agreement, the Corporation loaned Celly Nu C$1,000,000 on a secured basis with a term of 3 years, which bears interest at a rate of 10% per annum, payable on each anniversary, to assist Celly Nu in achieving that goal; however, the Corporation does not have an obligation to fund Celly Nu in the future (the “Celly Nu Loan Agreement”). The Celly Nu loan was secured by all of Celly Nu’s collateral (the “Celly Nu Security Agreement”).
Pursuant to the Celly Nu IP License Agreement, the Corporation will receive a 7% royalty on revenue from Celly Nu, until total royalties in the amount of C$250,000,000 have been paid to the Corporation, at which point the royalty rate is reduced to 3%. In addition, Celly Nu issued the Corporation 100,000,000 Celly Nu Shares (as defined below) as a licence fee and issued the Corporation an anti-dilution warrant, entitling the Corporation to exercise the warrant at any time, in whole or in part, for a period of five years from the date of issuance to increase their holdings in Celly Nu to 25% for nominal consideration. Upon completion of the transaction with Celly Nu, the Corporation held approximately 34.66% of the issued and outstanding Celly Nu Shares on a non-diluted basis.
The Corporation will retain all rights to medical and pharmaceutical applications under its umbrella to further develop the franchise as part of its portfolio.
Effective November 29, 2023, the Corporation completed the Plan of Arrangement (as defined herein). Upon completion of the Plan of Arrangement, the Corporation continues to hold 154,287,471 Celly Nu Shares, which represents approximately 26.15% of the issued and outstanding Celly Nu Shares on a non-diluted basis. For more information, please see “Item 4. Information on the Company. - A. History and Development of the Company - Significant Developments in Fiscal 2023 through to March 28, 2024”.
The Plan of Arrangement has not had, and does not expect to have, any impact on the development of the retail product, Unbuzzd™.
The Corporation’s continued operations are not dependent on the development of Unbuzzd™.
(ii) Alcohol Misuse: Healthcare Product (the “Healthcare Product”)
The Healthcare Product has the potential to assist emergency room physicians and their medical staff with the abundance of intoxicated patients they receive as these patients are utilizing critical resources (i.e. the physicians and their medical staff) whose time can be used for more urgent and critical needs. The Corporation did not license the intellectual property with respect to the Product Candidate for the Healthcare Product to Celly Nu and will be conducting further research and development, including clinical trials, into the viability of the Healthcare Product. Although any research and development conducted by the Corporation on the Healthcare Product could be shared with, and may assist, Celly Nu in developing Unbuzzd™, the Corporation has no obligation, pursuant to the Celly Nu IP License Agreement, to share such information with Celly Nu.
The viability, development and advancement of the Healthcare Product is dependent on the Corporation obtaining requisite funding, in the amount of approximately US$10,998,811, for the Corporation to complete further research and development. The Corporation, through its initial research, has discovered that there is significant demand in the market for this type of product, an opportunity for them to capture market share and believes that if it were able to develop and sell the Healthcare Product, it would bring immense value to its shareholders. If the requisition financing is not obtained, the Corporation will be unable to develop the Healthcare Product.
The Corporation’s continued operations are not dependent on the Healthcare Product’s development.
Lucid-MS
Through Lucid, the Corporation is also currently focused on the research and development of its Lucid-MS compound. Lucid-MS is a patented new chemical entity shown to prevent and reverse myelin degradation, the underlying mechanism of MS, in preclinical models. On April 17, 2023, the Corporation announced the completion of its first-in-human dosing of Lucid-MS in the Corporation’s Phase 1 clinical trial. On May 10, 2023, the Corporation announced the completion of dosing for the first cohort of patients in the Phase 1 clinical trial of Lucid-MS.
FSD-PEA
On June 2, 2023, the Corporation terminated any further clinical development of its proprietary ultra-micronized palmitoylethanolamide (“FSD-PEA”) (also known as “FSD201”) formulation which was being developed for the treatment of inflammatory diseases. The Corporation’s team of internal medical experts conducted a profitability assessment of FSD-PEA and ultimately determined that the FSD-PEA molecule was not profitable compared against the currently available products in the market and it would not be possible to cover the Corporation’s manufacturing and research and development investments at a price that would be accepted in the market.
| 30 |
| Table of Contents |
Lucid-PSYCH
Additionally, management made the decision to put the research and development activities associated with Lucid-PSYCH (formerly Lucid-201) on hold during June 2023. This decision was made based on the cumulative cash requirements to advance the research and development of the Corporation’s portfolio of compounds. Due to cash flow prioritization strategies, management elected to prioritize the Lucid-MS compound and its alcohol misuse treatment products. The Corporation has not recognized an amount specific to Lucid-PSYCH. When the Corporation acquired Lucid, it recognized an intangible asset consisting of the world-wide exclusive license agreement with the University Health Network (the “UHN License”) for the exclusive rights to the novel Lucid-MS compound and the U.S. patent for the Lucid-MS compound covered by the UHN license. Lucid-PSYCH was not covered by the license agreement and did not have any patent protection.
Prismic
The Corporation does not operate through Prismic, however Prismic holds the right to receive certain payments based on net sales of certain products from the Corporation pursuant to an assignment agreement between Prismic and the Corporation.
FSD Strategic Investments
Through FSD Strategic Investments, the Corporation is involved in the issuance of loans secured by residential or commercial property.
The Company’s Class B Shares trades on the CSE and Nasdaq under the symbol “HUGE.”
The Company’s principal office is located at 199 Bay Street, Suite 4000, Toronto, Ontario M5L 1A9 and its telephone number is 416-854-8884. As at the date of this Annual Report, the Company is a reporting issuer in each of the provinces of Canada. The Company’s registrar and transfer agent is Marrelli Trust Company Limited. The Company’s agent for service in the United States is CT Company, 28 Liberty Street, New York, New York 10005.
For a description of our principal capital expenditures, principal acquisitions and divestitures for the three years ended December 31, 2023 and for those currently in progress, see. “Item 4. Information on the Company - A. History and Development of the Company” and “Item 4. Information on the Company - B. Business Overview” and “Item 5. - Operating and Financial Review and Prospects”.
The SEC maintains an internet site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC, which can be found at http://www.sec.gov. Our internet address is www.fsdpharma.com. The information contained on our website is not incorporated by reference and does not form part of this Annual Report.
Significant Developments in Fiscal 2023 through to March 28, 2024
2023 Normal Course Issuer Bid
On January 12, 2023, the Board authorized a normal course issuer bid pursuant to which the Company was able to repurchase for cancellation up to 1,925,210 Class B Shares, being approximately 5% of the Company’s issued and outstanding Class B Shares as of January 12, 2023, over a 12-month period (the “2023 NCIB”). The 2023 NCIB commenced on January 18, 2023 and was terminated on January 12, 2024. Under the 2023 NCIB, the Company repurchased for cancellation 1,904,700 Class B Shares at an average price of approximately C$2.11 per Class B Shares. All Class B Shares were repurchased through the facilities of the CSE at the prevailing market price on the CSE at the time of repurchase.
Issuance of Warrants
On February 13, 2023, the Company issued warrants to purchase 500,000 Class B Shares to Jason Gold and warrants to purchase 300,000 Class B Shares to Pillow Hog Ventures Inc. in exchange for consulting services provided to the Company. The warrants vested on issuance and expire on March 30, 2024, with an exercise price ranging from US$1.50 to US$4.50.
On February 13, 2023, the Company issued warrants to purchase 500,000 Class B Shares to Zapability LLC in exchange for consulting services provided to the Company. Each tranche of warrants expires 12 months from the first day it vested, with the final tranche expiring on February 15, 2026. The warrants have an exercise price ranging from US$1.85 to US$8.00.
On February 27, 2023, the Company issued warrants to purchase 1,000,000 Class B Shares to Kevin Harrington in exchange for consulting services provided to the Company. The warrants vested on issuance and expire on February 27, 2026, with an exercise price ranging from US$1.75 USD to US$8.00.
| 31 |
| Table of Contents |
On March 24, 2023, the Company issued warrants to purchase 1,000,000 Class B Shares to Gerard David in exchange for consulting services provided to the Company. The Warrants on issuance and expire on March 24, 2026, with an exercise price ranging from US$1.75 to US$8.00.
Discontinuation of Research on Lucid-PSYCH and PEA
On June 2, 2023, the Corporation terminated any further clinical development of FSD-PEA
Interest-Bearing Mortgage Loan to CEO
On April 17, 2023, FSD Strategic Investments entered into a secured loan agreement with the CEO for C$1,200,000, with monthly payments of C$6,000 based on an annual interest rate of 6% and a blended rate of 7% (the “CEO Mortgage Loan”). The business purpose of the CEO Mortgage Loan was a treasury function to earn a rate of return on excess capital held. For more information, see “Item 7. Major Shareholders and Related Party Transactions - B. Related Party Transactions.”
Change in Board and Management
On July 4, 2023, the Company announced the appointment of Mr. Zeeshan Saeed as CEO of the Company, to succeed Mr. Anthony Durkacz, who served as interim CEO of the Company since July 2021.
At the annual general and special meeting of the Shareholders held on June 29, 2023, Messrs. Michael Zapolin and Dr. Eric Hoskins were elected as directors of the Company.
On January 24, 2024, the Company appointed Dr. Sanjiv Chopra, MD to the Board to replace Nitin Kaushal.
Celly Nu IP License Agreement
On July 31, 2023, the Company entered into the Celly Nu IP License Agreement, Celly Nu Loan Agreement and Celly Nu Licensing Agreement. For more information, please see “Item 4. Information on the Company. - A. History and Development of the Company - Overview and History”.
Plan of Arrangement
On April 11, 2023, the Corporation announced its intention to complete a spin-out transaction via a statutory plan of arrangement and to hold a regarding the same at its upcoming meeting of shareholders. The Corporation ultimately made the decision to defer the spin-out transaction and did not ask its shareholders to approve the transaction at its annual general and special meeting of shareholders held on June 29, 2023.
On October 5, 2023, the Corporation announced that it had entered into a definitive arrangement agreement with Celly Nu dated October 4, 2023 (the “Arrangement Agreement”) with respect to the distribution of a portion of the Corporation’s shareholdings of Celly Nu to the FSD Pharma Securityholders (as defined herein).
Pursuant to the Arrangement Agreement, the Corporation had FSD Pharma Securityholders pass a special resolution at the special meeting of shareholders held on November 20, 2023 to approve a statutory plan of arrangement under section 182 of the OBCA (the “Plan of Arrangement”), which involved (i) an amendment to the capital structure of the Corporation (the “Share Capital Amendment”); and (ii) the distribution of a portion of the common shares in the capital of Celly Nu (“Celly Nu Shares”) to the holders of the Corporation’s Class B Shares, class A multiple voting shares (“Class A Shares”), and outstanding warrants exercisable for the purchase of Class B Shares, provided the applicable warrant certificate entitles the holder thereof to receive distributions substantially similar to those received by the holders of Class B Shares (“FSD Pharma Distribution Warrants” and together with Class A Shares and Class B Shares, the “FSD Pharma Securities”). The Shareholders and the holders of FSD Pharma Distribution Warrants (collectively, the “FSD Pharma Securityholders”) would each receive one Celly Nu Share for each Class A Share, Class B Share or FSD Pharma Distribution Warrant held.
On November 24, 2023, the Corporation received a final order from the Ontario Superior Court of Justice (Commercial List) approving the Plan of Arrangement.
The record date of the Plan of Arrangement was set at November 28, 2023 (the “Record Date”), and the ex-dividend date was set at November 27, 2023.
Effective November 29, 2023, the Corporation completed the Plan of Arrangement. Holders of FSD Pharma Securities received one Celly Nu Share for each Class A Share, Class B Share, or FSD Pharma Distribution Warrant held. FSD Pharma Securityholders also received new Class A Shares, new Class B Shares, and new FSD Pharma Distribution Warrants (“New FSD Pharma Securities”) in exchange for their Class A Shares, Class B Shares, and FSD Pharma Distribution Warrants (the “Share Exchange”). Pursuant to the Share Exchange and in accordance with the terms of the Arrangement Agreement, 24 Class A Shares were exchanged to 24 new Class B Shares. Following the closing of the Plan of Arrangement, the Corporation had 48 new Class A Shares, 39,376,723 new Class B Shares, and 6,335,758 new FSD Pharma Distribution Warrants issued and outstanding. Further details concerning the Share Exchange are set forth in the Special Meeting Circular.
| 32 |
| Table of Contents |
All Celly Nu Shares distributed to FSD Pharma Securityholders pursuant to the Plan of Arrangement are subject to restrictions on resale, and may not be transferred until May 31, 2024, provided that, Celly Nu may, in its sole discretion, waive such restrictions, in whole or in part.
The New CUSIP and ISIN numbers for Class B Shares following the completion of the Plan of Arrangement are 35954B404 and CA35954B4047, respectively, the New CUSIP and ISIN numbers for Class A Shares following the completion of the Plan of Arrangement are 35954B305 and CA35954B3056, respectively and the Celly Nu Shares distributed pursuant to the Plan of Arrangement have CUSIP and ISIN numbers of 150965200 and CA1509652006, respectively.
The Plan of Arrangement resulted in an aggregate of 45,712,529 Celly Nu Shares being distributed to the FSD Pharma Securityholders and an aggregate of 154,287,471 Celly Nu Shares retained by the Corporation, which represents approximately 26.15% of the issued and outstanding Celly Nu Shares on a non-diluted basis.
For more information regarding the Plan of Arrangement, please see the Special Meeting Circular and Arrangement Agreement, each of which is available under the Corporation’s profile on System for Electronic Document Analysis and Retrieval plus (“SEDAR+”) at www.sedarplus.ca and on EDGAR at www.sec.gov.
The Plan of Arrangement was considered a “business combination” pursuant to Multilateral Instrument 61-101 – Protection of Minority Security Holders in Special Transactions (“MI 61-101”) since (i) the FSD Pharma Securityholders’ interest in the FSD Pharma Securities may have been terminated without their consent as a result of the Share Capital Amendment; and (ii) Michael (Zappy) Zapolin (“Zapolin”), a director of the Corporation and therefore a “related party” under MI 61-101 was party to a “connected transaction” to the Plan of Arrangement. The Plan of Arrangement and subscription by Zapolin for 28,800,000 Celly Nu Shares on August 1, 2023 was a “connected transaction” (the “Related Party Purchase”). Both transactions involved Celly Nu as a common party and the Plan of Arrangement and Related Party Purchase were arguably negotiated at approximately the same time. At the time, Zapolin owned, directly or indirectly, nil Class B Shares, nil Class A Shares, nil FSD Pharma Distribution Warrants, and 500,000 warrants, each exercisable for the purchase of one Class B Share. Any FSD Pharma Securities held by Zapolin were treated in the same fashion under the Plan of Arrangement as the FSD Pharma Securities held by every other FSD Pharma Securityholder.
The Plan of Arrangement was not a “related party transaction” pursuant to MI 61-101 as a result of it being a “business combination” pursuant to MI 61-101.
The Plan of Arrangement did not have a material impact or represent a material change on the Corporation’s financial performance and condition.
Settlement of Lawsuit with Syneos Health
On August 2, 2023, the Corporation entered into a settlement agreement (the “Settlement Agreement”) with Syneos Health, LLC and Syneos Health UK Limited (collectively, the “Syneos”), whereby it was agreed that, among other things, the Corporation agreed to pay Syneos the amount of US$100,000 within five days of the execution of the Settlement Agreement and upon receipt by Syneos of such settlement payment, Syneos shall waive, release and forgive the Corporation’s payment of (i) the different between the settlement payment and the damages payment (i.e. US$1,607,831) and (ii) interest on the damages payment ordered by the award, and any other amounts that were or could have been sought in the arbitration. Pursuant to the Settlement Agreement, Syneos also agreed to withdraw its recognition application that was filed on June 30, 2023. Payment was made by the Corporation on August 4, 2023 and pursuant to the terms of the Settlement Agreement, the matter was concluded in its entirety.
Investigation of Market Activity
On July 10, 2023, the Corporation announced that it had retained Christian Attar Law, a regional litigation firm located in Houston, Texas, to co-lead, along with New York City law firm, Warshaw Burstein, LLP, an investigation of any potential naked short selling or other market manipulation of the Corporation’s securities. The Corporation has been advised that Christian Attar Law has completed their preliminary assessment of the predatory short selling and they anticipate that they will recommend to move the matter forward to the next stage but have yet to make a formal decision. The Corporation will provide updates in the event of any material progress on the investigation.
On November 22, 2023, the Corporation was provided an update from its United States counsel in connection with the possible naked short selling and market manipulation case, counsel informed the Corporation on a phone call that they plan to file a motion in the coming year. Although initially expected to be filed in February or March 2024, the Corporation is still in the information gathering process and expect to provide an update on the status of the potential naked short selling or other market manipulation in the coming months.
December Class A Share Private Placement
Effective December 4, 2023, the Corporation closed a non-brokered private placement of Class A Shares for gross proceeds of C$45.60 through the issuance of 24 Class A Shares at a price of C$1.90 per Class A Share (the “December Class A Private Placement”). All securities issued pursuant to the December Class A Private Placement were subject to a statutory hold period of four months plus a day from issuance in accordance with applicable securities laws of Canada. The Corporation intends to use the proceeds of the December Class A Private Placement for general working capital purposes. For more information, see “Item 7. Major Shareholders and Related Party Transactions - B. Related Party Transactions.”
| 33 |
| Table of Contents |
Dr. Raza Bokhari
For an update on the status of our outstanding lawsuits with Dr. Raz Bokhari in 2023, please see “Item 8. Financial Statements – Legal Proceedings.”
Shelf Prospectuses
In order to replace its prior base shelf prospectus that expired, effective December 22, 2023, the Company filed and obtained a receipt for its final short form base shelf prospectus dated December 22, 2023 (the “Canadian Prospectus”) to provide the Company with the flexibility to take advantage of financing opportunities and favourable market conditions, if and when needed, during the 25-month period that the Prospectus remains effective (the “Effective Period”). The Canadian Prospectus has been filed in each of the provinces and territories in Canada. The Canadian Prospectus enables the Company to offer, issue and sell, from time to time: Class B Shares, subscription receipts, warrants and units, or any combination thereof (collectively, the “Prospectus Securities”) for up to an aggregate offering amount of US$50,000,000, in one or more transactions during the Effective Period. Should the Company decide to offer Securities during the Effective Period, the specific terms, including the use of proceeds from any offering of Securities, will be set forth in one or more related prospectus supplements to the Canadian Prospectus.
Effective December 22, 2023, the Company also filed a registration statement on Form F-3 (File No. 333-276264) filed under the Securities Act with the SEC and declared effective on January 4, 2024 (the “Registration Statement”) containing a base shelf prospectus with the SEC (the “U.S. Base Prospectus”). The Registration Statement also qualifies the offer, issue and sale, from time to time of Securities up to an aggregate amount of US$50,000,000, subject to limitations, as applicable, under Form F-3. The Registration Statement is available for use by the Company until January 4, 2027. The terms of any Securities to be offered under the U.S. Base Prospectus will be specified in a prospectus supplement, which will be filed with the SEC in connection with any such offer.
ATM Offering
Effective February 16, 2024, the Company entered into an at-the-market offering agreement (the “ATM Agreement”) with H.C. Wainwright & Co., LLC (“Wainwright”), pursuant to which the Company, at its discretion, may offer and sell, from time to time, through Wainwright as sales agent, Class B Shares, having an aggregate offering price of up to US$11,154,232 (the “ATM Offering”). A cash commission of 3.0% on the aggregate gross proceeds raised under the ATM Offering will be paid to Wainwright in connection with its services. The ATM Offering was made in the United States pursuant to the Registration Statement and the prospectus supplement dated February 16, 2024 (“Prospectus Supplement”, together with U.S. Base Prospectus, the “U.S. Prospectus”) filed with the SEC.
Sales of the Class B Shares under the U.S. Prospectus will be made in transactions that are deemed to be "at-the-market" offering as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended, (the “Securities Act”) including sales made directly on or through the Nasdaq. The Class B Shares will be distributed at the prevailing market prices at the time of each sale. As a result, prices may vary as between purchasers and during the period of distribution. No Class B Shares in the ATM Offering will be sold on the CSE or any other trading market in Canada.
The volume and timing of sales, if any, will be determined at the sole discretion of the Corporation’s management and in accordance with the terms of the ATM Agreement. If the Company chooses to sell Class B Shares under the ATM Offering, the Company intends to use the net proceeds of the ATM Offering (i) to fund our various clinical studies, trials and development programs, (ii) to fund research and development, and (iii) for general corporate purposes and working capital.
B. Business Overview
For more information, please see “Item 4. Information on the Company. - A. History and Development of the Company - Overview and History”.
The Corporation currently has two (2) significant programs, which are focused on the development of treatments for challenging neurodegenerative, inflammatory, and metabolic disorders. They are:
|
1. |
Lucid-MS: A potential treatment for Multiple Sclerosis with the lead candidate, Lucid-21-302; and |
|
2. |
Novel Treatments for Alcohol Misuse, and related conditions. |
| 34 |
| Table of Contents |
All programs are clinical stage programs, with significant benefits to help patients, if successfully approved for clinical use. With respect to the Product Candidates for alcohol misuse disorders, the Corporation sees two distinct routes where this segment can be developed, (i) recreational retail and (ii) healthcare; Celly Nu, through the Celly Nu IP License Agreement will be focusing on the recreational retail sector and the Corporation will be focusing on the healthcare sector. Below, a brief summary on each program is provided and their corresponding status along with any partnership/licensing activities.
Lucid-MS
This program is focused on the development of novel drugs for MS. Progressive MS has no standard of care, and almost all available drugs are immunomodulatory, and do not address the neurodegeneration in the patients. The Corporation believes it has a solution that can significantly change the course of neurodegenerative decline in MS patients. The Corporation, through the acquisition of Lucid, acquired the multiple sclerosis program with the lead candidate, Lucid-MS (development code, Lucid-21-302). Lucid-21-302 exhibits moderate inhibition profile against peptidyl arginine deiminase (“PAD”) 2 and PAD 4 isozymes. There is strong evidence that hypercitrullination of myelin, mediated by increased activities of PAD 2 and potentially PAD 4, may contribute to demyelination and multiple sclerosis pathogenesis through two mechanisms: (1) destabilizing myelin integrity on neuronal axons, leading to demyelination and degeneration, and (2) generating antigenic neoepitopes, leading to immune activation. Lucid-21-302 reduced hypercitrullination, prevented demyelination and helped remyelination in various non-clinical animal models of multiple sclerosis, including functional recovery in the animals. Lucid-MS is being developed as a first-in-class, non-immunomodulatory drug for the treatment of progressive multiple sclerosis. Current data from preclinical and clinical development suggests that Lucid-21-302 could achieve the therapeutic dose to launch a proof-of-concept human study in a small number of patients (Phase 2a PoC clinical trial). This trial will pave way for a larger Phase 2b study, with appropriate biomarkers and endpoints for the treatment of progressive MS with multiple clinical sites. The Corporation has actively been planning a potential phase-2 clinical trial. The current patent on Lucid-21-302 are effective until 2036 (US10716791B2) and was licensed from University Health Network (Toronto, Canada) exclusively for development and commercialization.
Clinical Trials
On January 17, 2023, the Company submitted the CTA for a planned Phase 1 clinical trial for Lucid-MS to Health Canada. In February 2023, the Company received regulatory clearance from Health Canada to proceed with the Company’s Phase 1 clinical trial of Lucid-MS in Canada. On May 10, 2023, the Company announced the completion of dosing the first cohort of patients in the Phase 1 clinical trial of Lucid-MS.
On July 10, 2023, the Company received a “no objection letter” for a Phase 1 Lucid-MS clinical trial for the submission Clinical Trail Application, which was acknowledged on June 12, 2023. On July 19, 2023, the Company submitted a request for pre-IND meeting to the FDA, which was acknowledged August 3, 2023, and a response was received on September 21, 2023. On August 25, 2023, the Company received a “no objection letter” for the Clinical Trail Application, which was acknowledged on July 31, 2023. On September 18, 2023, the completion of study notification (after completion of five cohorts) was submitted to Health Canada. On October 2, 2023, the Company submitted a provisional patent application to the USPTO was submitted on the clinical formulation containing Lucid-MS.
The Company presented the results from its first-in-human Phase 1 study of Lucid-MS at the America’s Committee meeting for the treatment and research in multiple sclerosis in February 2024. This presentation detailed the final results including adverse events profile of Lucid-21-302 in the single-ascending dose studies. The study concluded that Lucid-21-302 is safe and well-tolerated in the dose range of 50-300 mg p.o. administered once, with no difference in pharmacokinetics between the fed and fasted states. There were no serious adverse effects, and most adverse effects (7/12) in participants receiving Lucid-21-302 were characterized as unlikely related or unrelated to study drug. In the dose range 50-300 mg, drug exposure was proportional to dose of the drug. It also demonstrated good oral absorption with ‘area under the curve’ at 300mg comparable to ‘area under the curve’ in mouse efficacy studies. Based on its internal review of the Phase 1 data, the Company believes that the positive results warrant moving to a Phase 2 clinical trial.
Phase 2 Clinical Study
Based on the positive results that the Phase-1 study yielded, the Board, on recommendation from the advisory committee, resolved to proceed with completing a Phase II MS-Study on Lucid-MS with the goal of getting the Lucid-MS to commercialization. The Company has determined that in order to get to commercialization it will cost approximately US$30,655,469.
As Lucid-MS advances from Phase-1 to Phase-2, milestone-driven investigations are planned to expedite late-stage clinical development, aligned with our chronic toxicology program. This synergy ensures enabling data for regulatory submissions for the next clinical phases, such as Phase-1b multiple ascending dose (“MAD”) cohorts, Phase-2a, and Phase-2b. To initiate Phase-2, we require data from MAD cohorts, at least three months of toxicology data, and any additional data requested by regulatory authorities. Long-term toxicology data is crucial for dosing extending up to six months or more, reflecting Lucid-MS’s potential as a chronic treatment or disease-modifying therapy for MS patients. The Lucid-MS program’s ultimate goal is to conduct regulatory clinical studies, investigating its potential as a non-immunomodulatory drug to halt disease progression and neurodegeneration in multiple sclerosis.
| 35 |
| Table of Contents |
The Company’s innovative clinical development program targets multiple sclerosis, aiming to create a groundbreaking treatment. The Company has engaged thought leaders and conducted internal discussions on regulatory guidance to design an efficient, cost-effective program spanning chemistry, product development, and data acquisition for clinical stages. The Company’s clinical trials are moving forward in Australia.
Novel Treatments for Alcohol Misuse and Related Conditions
Excess alcohol consumption (alcohol misuse or mild acute alcohol intoxication) is clinically harmful that typically follows the ingestion of excess amount of alcohol. Clinical symptoms and manifestations are heterogeneous, and can have behavioral, cardiac, gastrointestinal, pulmonary, neurological, and metabolic effects. The Company is focused on treatments to reverse inebriation and to assist accelerating alcohol metabolism in people who consumed excess alcohol, reaching blood alcohol levels around/slightly above the legal limits in various countries. Available options for the emergency response doctors and nurses are to provide a vitamin intravenous drip or let alcohol “wear off”, until medical professionals can tend to those individuals who are inebriated, occupying expensive resources in the emergency room. The Company also identified that excess alcohol consumption is a problem in the general society (consumer market), and the commonly available remedies mostly fall in the category of “hangover remedies”. Thus, there is a great need for immediate treatments that can address the challenges when one consumes excess alcohol.
Medical and R&D teams at Lucid identified several natural ingredients that are dietary supplements that can function as alcohol metabolism accelerants and enhance mental alertness; the team developed several formulations that will help enhance mental alertness, replenish cofactors, and may accelerate the rate of alcohol metabolism. This formulation may be useful in treating intoxicated individuals who wish to speed up their recovery from the effects of alcohol as well as for the treatment of intoxicated patients entering emergency departments in the hospitals. The Company will continue its R&D program and develop products for use in emergency departments and other healthcare settings. Regulatory activity in the United States, and other markets globally will be continued, aligned with the R&D and potential clinical trials (as needed) for commercialization, marketing, and distribution.
As of March 28, 2024, the Company has registered five trademarks with the Canadian Intellectual Property Office (Registrar of Trademarks) (“CIPO”) and the USPTO and 17 trademarks with the CIPO, relating to novel treatments for alcohol misuse and related conditions, including Unbuzzd™ and ALCOHOLDEATH™, which were licensed to Celly Nu pursuant to the Celly Nu IP License Agreement.
On April 24, 2023, the Company filed a provisional patent application with the USPTO with respect to the Company’s alcohol misuse treatment technology, which was licensed to Celly Nu under the Celly Nu IP License Agreement for retail use.
The Company sees two distinct routes where this segment can be developed, (i) recreational retail and (ii) healthcare. Celly Nu, through the Celly Nu IP License Agreement will be focusing on the recreational retail sector and the Company will be focusing on the healthcare sector.
Product Development on Hold or Discontinued
In June 2023, the Company terminated any further clinical development of its proprietary ultra-micronized FSD-PEA formulation for the treatment of inflammatory diseases. The Company’s team of internal medical experts conducted a profitability assessment of FSD-PEA and ultimately determined that the FSD-PEA molecule was not profitable compared against the currently available products in the market and it would not be possible to cover the Company’s manufacturing and research and development investments at a price that would be accepted in the market.
Additionally, management made the decision to put the research and development activities associated with Lucid-PSYCH (formerly Lucid-201) on hold during June 2023. This decision was made based on the cumulative cash requirements to advance the research and development of the Company’s portfolio of compounds. Due to cash flow prioritization strategies, management elected to prioritize the Lucid-MS compound and its alcohol misuse treatment products.
Milestones for Future Development
In light of the above, the Company has determined that it will prioritize the development of its viable assets, utilizing the limited in-house resources available, to maximize the chances of their successful commercialization. Therefore, the Company resolved to push forward with the development of (i) Lucid-MS and (ii) the Healthcare Product, (ii) license Unbuzzd™ to Celly and (iv), pause the development of Lucid-PSYCH and terminate the development of FSD201.
The Company’s adaptable trial planning integrates insights from clinical and non-clinical studies, regulatory guidance, and market dynamics. Our adjusted timelines, especially in 2024 and 2025, are influenced by key market regulations, ensuring efficiency and cost-effectiveness across chemistry, product development, and data acquisition.
The Company’s overarching strategy allows it to conduct studies efficiently in terms of time and cost, considering that toxicology and clinical studies can span several months to several years. It also provides flexibility to adjust or explore alternative pathways cost-effectively in case of unexpected toxicities or efficacy issues.
The table below sets forth the status of these milestones as of March 28, 2024, the estimated costs and estimated timeframe for completion thereof. The following are “forward-looking statements” and as such, there is no guarantee that such milestones will be achieved on the timelines indicated or at all. Forward-looking statements are based on management’s current expectations and are subject to a number of risks, uncertainties, and assumptions. See “Caution Regarding Forward-Looking Statements” and “Item 3. Key information - D. Risk Factors” on the Company’s ability to achieve certain of its objectives and milestones, which are contingent upon raising additional financing.
| 36 |
| Table of Contents |
Objective |
Milestone(1)(2) |
Estimated Cost
|
Estimated Timeframe for Completion(3)(4) |
Notes |
1. MAD Cohorts | ||||
|
|
Regulatory Agency Approval
|
US$601,742 |
Q2, 2024
|
These studies are listed separately here; earlier they were a part of Clinical Studies below. Data from these studies will feed into Phase-2 clinical study designs. Timeframe is modified accordingly.
|
|
Site Pass Through Costs
|
US$730,413 |
Q2-Q4, 2024 |
||
|
First Participants In
|
US$376,012
|
Q2, 2024
|
||
|
Last Participant In
|
US$376,012
|
Q3, 2024
|
||
|
Completion of Report
|
US$150,282
|
Q1, 2025
|
||
|
|
Sub-total
|
US$2,234,461
|
|
|
|
2. Chronic Toxicity to initiate phase-2 (3-month study) | ||||
|
|
Study design for 2-species toxicity trial
|
US$37,158
|
Q2, 2024
|
These studies will be completed prior to Phase-2 initiation, and additional drug substances will be required. Proposed timeframe permits these activities.
|
|
First interim report
|
US$260,107
|
Q3, 2024
|
||
|
Second interim report
|
US$260,107
|
Q3, 2024
|
||
|
Final Report
|
US$185,791
|
Q4, 2024
|
||
|
|
Sub-total |
US$743,163 |
|
|
|
3. Lucid-MS Program | ||||
|
Non-clinical studies |
|
|||
|
Phase 2 enabling pharmacology studies
|
US$111,474 |
Q3, 2024 |
These non-clinical studies will be launched a few months ahead of Phase-2 studies such that continuous safety data from the non-clinical studies will advance Phase-2 dosing for chronic treatment. Reproductive toxicology and autoradiography will be required for an NDA or for Phase 3 trial application submission. |
|
|
Chronic tox studies to complete phase-2 (2 species, up to 9 months)
|
US$1,168,582 |
Q4, 2025 |
||
|
Reproductive toxicology and autoradiography
|
US$1,857,907 |
Q3, 2026 |
||
|
Drug Substance and Product Manufacturing |
Synthesis of non-GMP drug substance for chronic toxicology studies
|
US$779,500 |
Q3, 2024 |
Proposed timeline of Q1, 2024 is required to obtain the drug substance in time for toxicology studies (3 months and 9 months). |
|
Development of clinical and non-clinical Formulations
|
US$334,423 |
Q4, 2024-Q1, 2025 |
This development is required for launching chronic toxicity and Phase-2 clinical studies; thus, the time frame is adjusted to fit those milestones. |
|
|
Drug Substance for Phase 2 studies
|
US$1,114,744 |
Q1-Q2, 2025 |
Manufacturing of the drug substance for launching Phase-2 study; time frame aligns with two quarters prior to the initiation of any Phase-2 activity. |
|
|
Drug Product for Phase 2 studies
|
US$445,898 |
Q2-Q3, 2025 |
Manufacturing of the drug substance for launching Phase-2 study; time frame aligns with one quarter prior to the initiation of any Phase-2 activity. |
|
| 37 |
| Table of Contents |
|
Clinical Studies
|
2nd Phase 2a clinical trial site and CRO identification and deposits |
US$966,112 |
Q4, 2024-Q3, 2025 |
The time frame includes submission of regulatory files, discussions and approvals from the regulator, identification of potential clinical sites and contracts negotiations. The time frame is scheduled after completing 3-month chronic toxicology, development of clinical formulation and other Phase-2 enabling studies. |
|
Phase 2a proof of concept (“PoC”) clinical trial (launch, biomarkers, labs, clinical site, regulatory and other activities) |
US$4,458,977 |
Q3, 2025-Q4, 2026 |
This time frame is after the above line item, to conduct the clinical trial. |
|
|
Phase-2b clinical trial (launch, biomarkers, biostats, labs, clinical sites, regulatory and other activities) |
US$14,863,258 |
Q3, 2025-Q4, 2026 |
Will be initiated after Phase 2a PoC, or can be in place of Phase 2a PoC, depending on market/regulatory strategy |
|
|
Regulatory, licensing, and other support costs
|
US FDA/Health Canada/UK MHRA regulatory activities, patents maintenance/new filings, patent licensing costs. |
US$3,715,815 |
Q4, 2024-Q4, 2026 |
These are continuous activities for patents maintenance, licensing costs (to UHN), regulatory filings for early market access among others. Milestones will be based on each activity undertaken, and success of regulatory reviews. Each major milestone calls for a milestone payment to UHN. |
|
|
Sub-total |
US$29,816,690 |
|
|
|
4.Alcohol Misuse Treatments Program: Healthcare Product | ||||
|
Non-clinical activities |
In vitro and in vivo toxicology studies and dose range for the oral liquid formulation |
US$743,163 |
Q4, 2024-Q1, 2025 |
These non-clinical activities will be undertaken as a part of our R&D program for new formulations in late 2024, that will serve the development of hospital and consumer products. Current focus is on licensed activities for consumer market, in early 2024. |
|
In vitro and in vivo toxicology studies and dose range for the intravenous formulation |
US$2,229,489
|
Q4, 2024-Q2, 2025 |
These studies are aligned with the above line item for hospital product development. |
|
|
Drug Substance and Product Manufacturing |
Oral liquid formulation development |
US$743,163 |
Q1-Q3, 2025 |
GMP R&D manufacturing of oral liquid formulation for hospital line product; aligned with completion of non-clinical activities above. |
|
Intravenous formulation development |
US$1,114,744 |
Q1, 2025-Q1, 2026 |
GMP R&D manufacturing of intravenous formulation for hospital line product; aligned with completion of non-clinical activities above, and after the oral formulation development, in the above line item. |
|
|
Oral liquid formulation manufacturing for clinical study |
US$371,581
|
Q3, 2025
|
Manufacturing of clinical trial material (liquid oral), will commence after R&D during Q1-Q3, 2025
|
|
|
GMP Sterile formulation manufacturing for clinical studies |
US$1,114,744 |
Q1-Q2, 2026 |
Manufacturing of clinical trial material (intravenous), will commence after R&D during Q1, 2025-Q1, 2026 |
|
| 38 |
| Table of Contents |
|
Clinical Studies |
Clinical study with one oral formulation |
US$1,114,744 |
Q4, 2025-Q2, 2026 |
Clinical study using novel oral formulation is scheduled after the completion of toxicology and clinical trial materials manufacturing. |
|
Clinical study with one intravenous formulation for regulatory submission |
US$1,857,907 |
Q3-Q4, 2026 |
Clinical study using novel intravenous formulation is scheduled after the completion of toxicology and intravenous clinical trial materials manufacturing. |
|
|
Regulatory, IP and other support costs |
Regulatory activities and submissions in the USA and Canada |
US$222,949 |
Q4, 2024-Q4, 2026 |
These are continuous activities for patents maintenance, licensing, regulatory filings for market access among others. Milestones will be based on each activity undertaken, and success of regulatory reviews. |
|
Marketing and related activities |
Medical education, pre-launch, and partnership activities |
US$1,486,326 |
Q4, 2025-Q4, 2026 |
As the clinical studies commence, marketing, outreach and partnership activities will be undertaken; the time frame is based on the clinical studies scheduling above, and the anticipated prior work for late-stage marketing and potential pre-launch for the products. |
|
|
Sub-total |
US$10,998,810 |
|
|
|
Operations |
Team members salaries, benefits, external consultants, and key opinion leaders |
US$4,087,396 |
Q4, 2024-Q4, 2026 |
These costs include additional personnel will be required for all planned clinical drug development, toxicology, project management and regulatory affairs, for all programs. |
|
Information technology, legal, telecommunications, facilities infrastructure, travel, shipping/logistics |
US$2,229,489 |
Q4, 2024-Q4, 2026 |
Planned programs will incur indirect costs in order to support the R&D and clinical activities. |
|
|
Sub-total |
US$6,316,885 |
|
|
Notes:
(1) |
There may be circumstances where, for sound business reasons, the Company’s reallocates the funds or determines not to proceed with a milestone. |
(2) |
Subject to receipt of all necessary approvals, including any approvals required by the academic and scientific organizations with which the Company is working. |
(3) |
The total expenditure may be incurred by the Company after the relevant quarter that is indicated as the target timeframe for completion. |
(4) |
Based on a calendar year end. |
The materials factors or assumptions used to develop the estimated costs disclosed above are included in the “Cautionary Note Regarding Forward-Looking Statements” section above. The actual amount that the Company spends in connection with each of the intended milestones will depend on several factors, including those listed under “Item 3. Key information - D. Risk Factors” in or incorporated by reference in this Annual Report or unforeseen events. While the Company believes it has the skills and resources necessary to accomplish these business objectives, there is no guarantee that the Company will be able to do so within the timeframes indicated above, or at all. The Company will rely on third-party opinions evaluating novelty and patentability of its drug compounds, as well as data generated by tests performed by third parties indicating there is preclinical evidence of improved efficacy or safety profiles compared to currently known treatments for challenging neurodegenerative, inflammatory, and metabolic disorders based on scientifically sound preclinical studies. These tests are ongoing. While the Company believes its approach mitigates many risks associated with the challenges of obtaining regulatory approval for certain difficult to treat indications, the development of potential drugs for treatment of challenging neurodegenerative, inflammatory, and metabolic disorders involves a high degree of risk and uncertainty. The Company is committed to funding research it believes is essential for advancing the study of drugs to treat these conditions.
Research and Development
As at the date of this Annual Report, the Corporation has not generated any revenue from the sale of pharmaceutical drugs or other products. The Corporation is focused on development of pharmaceutical drugs and other products, through the research and development of novel chemical compounds and delivery mechanisms and the study of such compounds in preclinical studies. The Corporation’s preclinical studies are conducted via the various CROs and contract manufacturers it has engaged, including Ingenu CRO (a Cannvalate Pty Ltd Company) (“Ingenu”), BioPharma Services Inc. (“BioPharma”), and Vibrant Pharma Inc. (“Vibrant Pharma”). Each of Ingenu, BioPharma, and Vibrant Pharma are CROs that, in the ordinary course of the Corporation’s business, have entered into service agreements with the Corporation to provide services related to the Corporation’s preclinical studies and/or the manufacture of its various chemical compounds. The Corporation is not dependent on third party contracts. Although each of the CROs will be involved in the synthesis, or testing thereof, for the Corporation, none of these agreements allows for the various CROs to utilize any of the Corporation’s intellectual property, including its patents, formulae, trade secrets, or processes, for their own purposes. The pharmaceutical industry is a competitive and, in the event that one, or all, of these contractual relationships become unsatisfactory, the Corporation does not anticipate having difficulty retaining other services providers to perform similar services. The Corporation does not anticipate generating any revenue from any of these, or any other, service agreements.
| 39 |
| Table of Contents |
The Corporation anticipates growing its pipeline of pharmaceutical drugs and other products through its research, development, proprietary discovery programs, mergers and acquisitions, joint ventures and collaborative development agreements. The Corporation has sought protection for the intellectual property rights generated by its research and development activities through patent applications and as trade secrets. The Corporation anticipates that as these programs mature it will file additional patent applications and details about these programs will be disclosed at such time. The Corporation further anticipates that existing patent applications will result in successful patent grants by the respective intellectual property regulators of each jurisdiction in which the Corporation has submitted such applications.
The Corporation’s research and development activities (including such activities conducted by third party contractors) are conducted in strict compliance with the regulations of federal, state, local and regulatory agencies in Canada, Australia and the United States. These regulatory authorities regulate, among other things, the research, manufacture, promotion and distribution of drugs in specific jurisdictions under applicable laws and regulations.
See “Item 4. Information on the Company. – B. Business Overview - Milestones for Future Development” for further information on the Corporation’s objectives and milestones.
Intellectual Property
The following tables set forth the status for each patent applicable to the Company’s current and anticipated business for Lucid-MS and Celly Nu’s activities:
Title |
Jurisdiction of Filing |
Application Number |
Filing Date/Patent Date/Priority Date |
Status |
Program |
Inhibitors of Peptidyl Arginine Deiminase (PAD) Enzymes and Uses Thereof |
United States Patent and Trademark Office |
Appl. No.: 15/753,208 Patent No.: US10,716,791 B2 |
Filing Date: 2016-08-15 Patent Date: 2020-07-21 |
Exclusive license from University Health Network (Toronto) |
Lucid-MS |
Inhibitors of Peptidyl Arginine Deiminase (PAD) Enzymes and Uses Thereof |
European Patent Office |
Appl. No.: 22187901.8 |
Filing Date: 2016-08-15 Priority Date: 2016-08-15 |
Exclusive license from University Health Network (Toronto) |
Lucid-MS |
Methods and Compositions Comprising a 5-HT Receptor Antagonist |
United States Patent and Trademark Office |
Appl. No.: 63/454,587 |
Filing Date: 2023-03-24 |
Provisional patent application (PAT 114860P-2) |
Lucid-PSYCH(1) |
An Ingestible Formulation and Uses Thereof |
United States Patent and Trademark Office |
Appl. No.: 63/497,772 |
Filing Date: 2023-04-24 |
Provisional patent application (PAT 114119P-2) |
Licensed to Celly Nu |
Note:
(1) |
The Company has put any future work programs relating to Lucid-PSYCH on hold. |
| 40 |
| Table of Contents |
The Company’s wholly owned subsidiary, Lucid, has filed pending applications for the trademarks set forth in the table below with the Innovation, Science and Economic Development Canada – CIPO:
Applicant |
Filing Date |
Reference Number |
File Number |
Trademark Details |
Trademark Type |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150984-1 |
2243755 |
REKVRY |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150985-1 |
2243758 |
DETOXIQ |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150986-1 |
2243760 |
RESOBER |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150958-1 |
2243743 |
ALCOHOLDEATH(1) |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150987-1 |
2243761 |
Unbuzzd(1) |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150959-1 |
2243741 |
DRUNQUELL |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150957-1 |
2243742 |
FRESHKA |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150956-1 |
2243736 |
FRESHA |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150955-1 |
224374 |
ALKACLEAR |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150954-1 |
224379 |
LOWBAC |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150953-1 |
2243740 |
SOBRY |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150952-1 |
2243744 |
BACLEAR |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 15951-1 |
2243737 |
READY IN 1 |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150950-1 |
2243735 |
QLARITY |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150949-1 |
2243738 |
WAKEAID |
Standard Characters |
Note:
(1) |
Licensed to Celly Nu. |
The Company’s wholly owned subsidiary, Lucid, has filed pending trademark applications for the marks in the table below with the Innovation, Science and Economic Development Canada – CIPO:
Applicant |
Filing Date |
Reference Number |
File Number |
Trademark Details |
Trademark Type |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150940-1 |
2243726 |
EVERYONE MAY NEED A LITTLE IN THEIR LIFE |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150825-1 |
2243752 |
THE RITUAL AFTER THE LAST CALL |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150824-1 |
2243750 |
THE PROTOCOL AFTER THE LAST CALL |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150823-1 |
2243749 |
HELPS REDUCE BUZZ |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150822-1 |
2243733 |
A RESPONSIBLE AFTER DRINK |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150821-1 |
2243732 |
THROUGH SCIENCE FIND |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150820-1 |
2243730 |
THROUGH SCIENCE, FIND CLARITY |
Standard Characters |
Lucid PsycheCeuticals Inc. |
March 7, 2023 |
TM 150819-1 |
2243729 |
EVERYONE MAY NEED A LITTLE |
Standard Characters |
As the Company generates new data it will continue to file or acquire additional patent applications through the Company’s development program.
Regulatory Environment
The Corporation is currently focused on obtaining regulatory approvals in the United States, Canada and Australia for the drug candidates it is developing through FSD BioSciences, Lucid and FSD Pharma Australia. In the future, the Corporation may consider seeking approvals for these drug candidates in other countries. The following is a summary of the FDA, Health Canada and the Australian Therapeutics Goods Administration (“TGA”) approval process that the Corporation and/or its related entities are undertaking with each of the Product Candidates in the United States, Canada and Australia. Assuming the Corporation is successful in obtaining the requisite approvals from the FDA, TGA or Health Canada (together the “Regulatory Approvals”) pursuant to the process set out below, it may decide to seek comparable approvals in other countries, which would be subject to different and additional regulatory requirements. Obtaining Regulatory Approval often takes several years, involves the expenditure of substantial resources, and depends on a number of factors, including the severity of the disease in question, the availability of alternative treatments, and the risks and benefits demonstrated in clinical trials.
| 41 |
| Table of Contents |
The Corporation will be subject to extensive regulations while it focuses on gaining Regulatory Approvals for treatments it is developing with each of the Product Candidates. The United States Food, Drug and Cosmetic Act of 1938, as amended, Public Health Service Act (United States), Therapeutic Goods Act 1989 (Cth) (Australia), and other federal, provincial and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labelling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical product candidates for their respective jurisdictions. Failure to comply with applicable regulatory requirements may subject the Corporation to a variety of administrative or judicial sanctions, such as application refusals, warning or untitled letters, product candidate recalls, product candidate seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
Pharmaceutical product candidate development in the United States, Canada and Australia typically involves preclinical laboratory and animal tests, followed by a submission to commence clinical testing to, as applicable:
|
(a) |
the FDA for the United States (an IND); |
|
(b) |
Health Canada for Canada (a CTA)); or |
|
(c) |
in Australia, (i) where the Clinical Trial Notification (“CTN”) process is utilized, to a Human Research Ethics Committee, or (ii) where the Australian CTA process is utilized, to the TGA. |
If:
|
(a) |
there are no comments from the FDA within 30 days after the submission of the application in the United States; |
|
(b) |
a “no objection letter” is received from Health Canada; or |
|
(c) |
in Australia, (i) where the CTN process is utilized, the applicable Human Research Ethics Committee provides its approval and the TGA is notified by way of the due submission of a CTN, or (ii) where the Australian CTA process is utilized, the TGA provides its approval, |
then clinical trials for the drug may commence in the respective jurisdiction assuming all other requirements are met (such as institution review board approval, informed consents and any additional approvals related to the use of controlled substances). The satisfaction of pre-market approval requirements typically takes many years. The actual time required may vary substantially based upon the type, complexity and novelty of the product candidate or the diseases a product candidate targets.
Before testing any compound in human patients in the U.S., Canada or Australia, a company must generate extensive preclinical data. Preclinical testing generally includes laboratory evaluation of product chemistry and formulation, as well as toxicological and pharmacological studies in several animal species to assess the toxicity and dosing of the product candidate and its potential safety and efficacy. The conduct of the preclinical tests must comply with government regulations and requirements, including good laboratory practices. For example, in the U.S., certain animal studies must be performed in compliance with the FDA’s Good Laboratory Practice regulations and the U.S. Department of Agriculture’s Animal Welfare Act.
A Regulatory Approval must be in effect before human clinical trials may commence in the U.S., Canada or Australia, respectively. The results of preclinical testing and any previous human experience with the investigational drug are submitted to the FDA, Health Canada or TGA as part of the Regulatory Approval process in each jurisdiction, along with other information, including information about product candidate chemistry, manufacturing and controls, information about the study investigator, and a proposed clinical trial protocol.
There can be regulatory barriers to obtaining an effective Regulatory Approval based on FDA’s, Health Canada’s or TGA’s respective review of the investigative drug and, where applicable, its classification as a known controlled substance.
Clinical trials involve the administration of the product candidate that is the subject of the Regulatory Approval to healthy volunteers or study participants with the disease or condition being studied under the supervision of a qualified investigator. Clinical trials to support a NDA for marketing approval are typically conducted in three sequential phases, but the phases may overlap.
There is a process under which clinical trials may begin and involve the administration of the product candidate that is the subject of the Regulatory Approval to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (i) in compliance with applicable government regulations, (ii) in compliance with Good Clinical Practice, an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors, and (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on patients and subsequent protocol amendments must be submitted to the FDA, Health Canada and/or TGA as part of the Regulatory Approval process, as applicable.
The FDA, Health Canada or TGA may order the temporary, or permanent, discontinuation of a clinical trial at any time or impose other sanctions if it believes that the clinical trial either is not being conducted in accordance with applicable regulatory requirements or presents an unacceptable risk to the clinical trial patients. The trial protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board (“IRB”) for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements or may impose other conditions.
If the trials for any of its Product Candidates are successful, the Corporation may pursue additional trials as required and may ultimately pursue a NDA, which may involve applying for additional Regulatory Approvals required to market the Corporation’s synthetic treatments in the United States or in other jurisdictions. There is no assurance that the Corporation will be successful in receiving the required approvals, and the clinical trials are subject to numerous risks.
See “Caution Regarding Forward-Looking Statements” and “Item 3. Key information - D. Risk Factors” in this Annual Report.
| 42 |
| Table of Contents |
New Drug Application and New Drug Submission Process
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, nonclinical studies and clinical trials are submitted to the FDA as part of a NDA requesting approval to market the product for one or more indications. The application must include the results of all preclinical, clinical, and other testing and a compilation of data relating to the product’s pharmacology and chemistry, manufacture, and controls. Under the Prescription Drug User Fee Act, a substantial application user fee is required for most NDAs, and the applicant under an approved NDA is also subject to an annual program fee for each prescription product.
After evaluating the NDA, the FDA issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission. Substantial additional testing or information may be required in order for the FDA to reconsider the application. If regulatory approval of a product is granted, such approval will be granted for particular indications and may entail limitations on the indicated uses for which such product may be marketed. Additionally, as a condition of approval, the FDA may impose restrictions that could affect the commercial success of a drug or require post-approval commitments, including the completion within a specified time period of additional clinical studies, which often are referred to as “Phase 4” or “post-marketing” studies. For example, as a condition of approval, the FDA may require a risk evaluation and mitigation strategy (“REMS”) to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use, such as special training or certification for prescribing or dispensing. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy.
Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing. Once an NDA is approved, a product will be subject to certain post-approval requirements, including, among other things, requirements related to record-keeping, providing the FDA with updated safety information, product sampling and distribution, and promotion and advertising. Post-approval modifications to the drug, such as changes in indications, labeling, or manufacturing processes or facilities, may require a sponsor to develop additional data or conduct additional preclinical studies or clinical trials, to be submitted in a new or supplemental NDA, which would require FDA approval.
Similarly, Health Canada (in Canada) and the TGA (in Australia) regulates, among other things, the research, development, testing, manufacture, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, post-approval monitoring, marketing and import and export of pharmaceutical products. Drug approval laws require licensing of manufacturing facilities, carefully controlled research and testing of products, and government review and approval of experimental results prior to giving approval to sell drug products.
The process required by the applicable regulatory authorities before prescription drug product candidates can be marketed in Canada or Australia requires:
|
(a) |
in the case of Canada, the submission of a new drug submission (“NDS”) to Health Canada; or |
|
|
|
|
(b) |
in the case of Australia, an application for registration in the Australian Register of Therapeutic Goods (“ARTG”), the NDS, and application for registration on the ARTG collectively referred to as “New Drug Application”. |
Health Canada and/or TGA, as the case may be, must review and approve the relevant “New Drug Application”. Health Canada must also and issue a notice of compliance and both Health Canada and TGA must issue a drug identification number prior to any commercial marketing, sale, or shipment of the drug.
Even if Health Canada approves a NDS or TGA registers the drug on the ARTG, the relevant regulatory authority may limit the approved indications for use of the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies be conducted to further assess a drug’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms.
The regulatory review process of a drug application in each of the U.S., Canada and Australia includes the satisfactory completion of an inspection of the manufacturing facility or facilities where the product is produced (or other evidence acceptable to the regulator) to ensure that the facilities are in compliance with current GMP (“cGMP”) requirements and are adequate to assure consistent production of the product within required specifications.
The FDA, Health Canada and TGA also conduct regular, periodic visits to re-inspect equipment, facilities, and processes following the initial approval of a product. Failure to comply with applicable cGMP requirements and other conditions of product approval may lead the regulatory authority to take enforcement action or seek sanctions, including fines, issuance of warning letters, civil penalties, injunctions, suspension of manufacturing operations, operating restrictions, withdrawal of approval, seizure or recall of products, and criminal prosecution.
Controlled Substances - United States
In June 2023, the Company decided to stop any development efforts for Lucid-PSYCH, which is considered a controlled-substance in the United States, Canada, and Australia. If and when the Company continues with the development of Lucid- PSYCH for psycho functions, it will need to comply with the controlled substances laws in various jurisdictions.
Drugs and other substances that are determined to have a potential for abuse are also regulated under the United States Comprehensive Drug Abuse Prevention and Control Act of 1970, as amended, also known as the Controlled Substances Act (the "CSA") and its implementing regulations, as "controlled substances." The CSA establishes a closed chain of distribution for entities handling controlled substances, which include researchers, manufacturers, distributors, pharmacies and physicians, importers, and exporters. The CSA and regulations enforced by the DEA impose registration, security, quotas inventory, recordkeeping, reporting, storage, manufacturing, distribution, importation, exportation, and other requirements on entities handling controlled substances. Practitioners such as pharmacies and physicians, as well as other types of entities that handle controlled substances, such as researchers and analytical laboratories, are also subject to DEA registration and other requirements related to controlled substances.
| 43 |
| Table of Contents |
The CSA categorizes controlled substances into one of five schedules - Schedule I, II, III, IV, or V - depending on the potential for abuse and physical or psychological dependence. Schedule I substances by definition have a high potential for abuse, have no currently accepted medical use in treatment in the United States, and lack accepted safety for use under medical supervision. They may not be marketed or sold for dispensing to patients in the United States. Certain "hallucinogens" or psychedelic drugs are currently regulated as Schedule I controlled substances, as is any substance that includes any of a Schedule I substance's salts, isomers (e.g., optical, position, and geometric isomers), or salts of isomers, whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical. Pharmaceutical products having a currently accepted medical use and that are otherwise approved for marketing may be listed as Schedule II, III, IV, or V substances, with Schedule II substances presenting the highest potential for abuse and physical or psychological dependence, and Schedule V substances presenting the lowest relative potential for abuse and dependence.
Whether a new drug or substance is ultimately controlled or not is a fact specific determination that the DEA makes based on the input of the Department of Health and Human Services (including the FDA), which provides scientific and medical findings and recommendations to the DEA. During the FDA approval process, the FDA will generally conduct an abuse potential evaluation of any substance that could have an effect on the central nervous system. If FDA finds that a new drug or substance may have an abuse potential that would require the drug to be controlled, FDA notifies the DEA and provides information/recommendation to the DEA on its scheduling. The DEA must conduct notice and comment rulemaking to propose scheduling of a new substance. If a drug being approved contains a substance already controlled under the CSA, that drug will generally be controlled in the same schedule absent findings or recommendations that it should be placed in another schedule.
Lucid-PSYCH is a Schedule I listed substance under the CSA. Its use in the United States is highly restricted under Federal law, even though there have been a few state and local laws seeking to loosen restrictions. A facility that seeks to manufacture, distribute, import, or export any Schedule I controlled substance must register with the DEA. The DEA registration is specific to the particular location, activity, and controlled substance. A DEA registered facility must maintain records documenting all activities, including the manufacture, receipt, and distribution, of controlled substances. The import or export of a Schedule I substance requires a permit and may need to comply with international drug control treaties as well as DEA requirements.
Any Schedule I drug or substance approved by the FDA must be rescheduled (or descheduled) to another schedule before it can be commercially marketed in the United States. Rescheduling or descheduling a Schedule I substance to another schedule is dependent on FDA approval and FDA recommendation as to the appropriate schedule. Any rescheduling or descheduling action requires the DEA to conduct notice and comment rulemaking. Such action will be subject to public comment and requests for hearing which could affect the scheduling of these substances.
Controlled Substances – Canada
A controlled substance is a type of drug that the Government of Canada has categorized as having a higher-than-average potential for abuse or addiction and is listed in one of the schedules (I to V) of the CDSA. Lucid-PSYCH is a controlled substance in Canada. The possession, sale or distribution of controlled substances is prohibited unless specifically permitted by the government.
Under section 56 of the CDSA the Minister of Health may exempt a person or a class of persons or any controlled substance or class thereof from the application of all or any provision of the CDSA or regulations if necessary for a medical or a scientific purpose or is otherwise in the public interest. Researchers requiring a controlled substance for research, including clinical trials, must receive an exemption under the CDSA, which can permit the importation, possession and/or use of a specified quantity of the controlled substance for a specified purpose. The Minister of Health can impose any terms and conditions that the Minister considers necessary in respect of the exemption. Through agreements with third parties, the Company has access to facilities that have experience and licenses required to handle Controlled Substances listed under the CDSA. Similar to the United States, in Canada certain scheduled substances would require reclassification to a different schedule in order to permit commercial marketing.
Controlled Substances – Australia
Like in the United States and Canada, a controlled drug is a type of drug that the Australian Government has categorized as having a higher-than-average potential for abuse or addiction and is listed in one of the schedules (1 to 10) of the Poisons Standard.
Substances with therapeutic uses are generally contained in Schedules 2, 3, 4 and 8 with progression through these Schedules signifying increasingly restrictive regulatory controls. Schedule 9 details substances that should be available only for teaching, training, medical or scientific research including clinical trials conducted with the approval of Commonwealth and/or State and Territory health authorities.
Lucid-PSYCH is currently a prohibited drug in Australia, meaning its supply is largely limited to clinical trials.
FSD Strategic Investments
The Company’s Strategic Investment segment generates interest income earned on a portfolio of finance receivables, which represent loans secured by residential or commercial property, with FSD Strategic Investments having a first collateral mortgage on the secured property for a sum equal to the interest payments plus the principal amount. FSD Strategic Investments earns interest through fixed rate lending arrangements, which includes faith-based loans, that have an average term to maturity of two years from the date of issuance.
| 44 |
| Table of Contents |
Strategic Investments has historically not incurred any significant operating expenditures as the loans are arranged through a third-party financing intermediary, with the borrower being responsible for covering all administrative related costs.
The Board has developed criteria for making investments decisions, as follows: i) the maximum loan-to-value ratio is 55%; ii) the maximum dollar value for any given secured loan is not to exceed C$1,200,000; and iii) the residential property must be located in the Greater Toronto Area. Before issuing a secured loan, the Company undertakes extensive due diligence to ensure that adequate care is exercised in the funding of mortgage or loan transactions, including checking personal identification, verifying title documents, attending the property, or conducting an on-site appraisal to satisfy as to the value of the property, and reviewing application and supporting documentation with legal counsel.
As at December 31, 2023, the Company has a finance receivable balance of $8,095,354 and minimum contractual payments receivable at the end of the loan terms totaling $8,527,569. The loans will begin to mature in the second quarter of fiscal 2024.
Other Significant Operations and Principal Activities – Fiscal 2021 and 2022
2021 and 2022 At-The-Market Financings
Between July 2020 and February 2021, the Company issued and sold Class B Shares for gross proceeds of approximately US$20,000,000 under an equity distribution agreement dated July 10, 2020 entered into with A.G.P./Alliance Global Partners (the “Sales Agent”), reaching the maximum amount allowed under such agreement. On February 11, 2021, the Company entered into a new equity distribution agreement (the “2021 Equity Distribution Agreement”) with the Sales Agent and issued and sold Class B Shares for gross proceeds of US$18,167,511 between February 11, 2021 and March 12, 2021. Sales of Class B Shares under the equity distribution agreements were made through "at-the-market" offerings as defined in Rule 415(a)(4) promulgated under the Securities Act including sales made directly on or through the Nasdaq. As a result, prices may varied as between purchasers and during the period of distribution. No offers or sales of Class B Shares were made in Canada on the CSE or other trading markets in Canada.
Licensing Agreement with University Health Network
Prior to its acquisition by the Company, Lucid entered into a licensing agreement dated May 19, 2021 (the “UHN License Agreement”) with the UHN. Under the terms of the UHN License Agreement, the Company pays an annual license maintenance fee of C$100,000 to UHN until the first commercial sale of a product utilizing the intellectual property licensed to the Company under the UHN License Agreement, including Lucid-MS, is made. In addition, the Company is committed to making milestone payments totaling up to C$12,500,000 to UHN if all product development and regulatory milestones are met. Furthermore, the Company will also pay revenue milestone payments and royalties if revenue milestones from commercial sales are achieved as well as a percentage of sublicensing revenue received by the Company under any sublicense. Milestones can be extended by mutual agreement. Unless otherwise terminated in accordance with its terms, the UHN License Agreement will remain in force until the expiration of the last valid claim under the last licensed patent covering the products licensed from UHN.
Lucid Acquisition
On August 25, 2021, the Company entered into a definitive agreement (the “Master Agreement”) to acquire 100% of the issued and outstanding shares of Lucid, an early-stage Canadian-based specialty biotechnology company focused on the development of therapies to treat critical neurodegenerative diseases, for total consideration of 4,502,392 Class B Shares, 161,091 Options and 112,162 warrants to purchase Class B Shares (the “Lucid Acquisition”). 304,880 Class B Shares and all of the warrants issued as part of the consideration for the Lucid Acquisition were issued to First Republic Capital Corporation, a company controlled by Anthony Durkacz, (“First Republic”) the CEO of the Company, in exchange for securities of Lucid held by First Republic prior to the completion of the Lucid Acquisition.
On September 13, 2021, shareholder approval for the Lucid Acquisition was obtained at a special meeting of Lucid shareholders. On September 21, 2021, the transaction was completed by way of a three-cornered amalgamation between Lucid, the Company and a wholly owned subsidiary of the Company pursuant to an amalgamation agreement dated September 20, 2021, entered into among the Company, Lucid and a wholly owned subsidiary of the Company in respect of the Lucid Acquisition. The Lucid Acquisition involved the issuance of approximately 4.5 million Class B Shares as the acquisition consideration, at a deemed price of approximately US$1.56 per Class B Share. Additionally, all of the outstanding Lucid stock options and warrants became exercisable into Class B Shares, with the number and exercise price of such securities adjusted in accordance with the transaction’s exchange ratio. In connection with the closing of the Lucid Acquisition, Dr. Lakshmi Kotra, maintained his position as Lucid’s CEO.
Covar Agreement
On October 1, 2021, the Company entered into an agreement with Covar Pharmaceuticals Inc. (“Covar”), a contract development and manufacturing services organization, to commence work on providing research quantities of the Company’s drug candidate, Lucid-PSYCH, on an exclusive basis for further clinical evaluation (the “Covar Agreement”). Lucid-PSYCH is a psychoactive compound that is being researched by the Company through Lucid in connection with the treatment of major depressive disorder. Covar’s research and development facility is licensed to handle psychoactive compounds such as Lucid-PSYCH, which are “controlled substances” listed under the Controlled Drugs and Substances Act (Canada).
| 45 |
| Table of Contents |
2022 Normal Course Issuer Bid
On December 21, 2021, the Board authorized a normal course issuer bid pursuant to which the Company was able to repurchase for cancellation up to 2,000,000 Class B Shares, being approximately 5% of the Company’s issued and outstanding Class B Shares as of December 21, 2021, over a 12-month period (the “2022 NCIB”). The 2022 NCIB commenced on January 4, 2022, and was terminated on December 21, 2022. Under the 2022 NCIB, the Company repurchased for cancellation 1,999,800 Class B Shares at an average price of approximately C$1.20 per Class B Shares. All Class B Shares were repurchased through the facilities of the CSE at the prevailing market price on the CSE at the time of repurchase.
Sale of FV Pharma’s Facility
On May 6, 2022, the Company decided to focus its efforts and resources on the pharmaceutical business and initiated the process to exit the medical cannabis industry. In connection with that decision, the Company sold FV Pharma’s facility located at 520 William Street, Cobourg, Ontario, K9A 3A5 (and the 64-acre property on which the facility was located for total consideration of $12,730,942 (C$16,400,000).
Specialized Knowledge and Personnel
The Board and executive officers of the Company, led by Zeeshan Saeed, as CEO and Co-Chairman, Anthony Durkacz, Co-Chairman, and Dr. Lakshmi P. Kotra, as CEO of Lucid, have a wide combination of the skills, knowledge and experience that are necessary for the successful advancement of the Company’s business plan. Our future growth and success depend on our ability to recruit, retain, manage, and motivate our qualified employees. The inability to hire or retain experienced personnel in the pharmaceutical field could adversely affect our ability to execute our business plan and harm our operating results. Due to the specialized scientific and managerial nature of our business, we rely heavily on our ability to attract and retain qualified scientific, technical and managerial personnel. The competition for qualified personnel in the pharmaceutical field is intense. Due to this intense competition, we may be unable to continue to attract and retain qualified personnel necessary for the development of our business or to recruit suitable replacement personnel.
Competitive Conditions
The pharmaceutical industry market for MS drugs is highly competitive and subject to rapid change. The industry continues to expand and evolve as an increasing number of competitors and potential competitors enter the market. There are few approved therapies for progressive multiple sclerosis such as ocrelizumab by Roche and siponimod by Novartis), all of which are immunomodulatory drugs. Bruton's Tyrosine Kinase Inhibitors are also being investigated as therapies for progressive MS in Phase 3 clinical trials; for example fenebrutinib by Roche (NCT04544449) and tolebrutinib by Sanofi (NCT04458051. Kyverna Therapeutics recently announced their Phase-2 clinical trial on the cell-based therapy candidate KYV-101, in people with treatment-resistant progressive multiple sclerosis (MS). Immunic Therapeutics is developing vidofludimus as a potential treatment for all MS types; the Phase 2 CALLIPER clinical trial (NCT05054140) is testing it against a placebo specifically in people with progressive forms of the disease. Tiziana Life Sciences is developing an antibody, foralumab that is designed to reduce inflammation in the brain and spinal cord by blocking CD3, a protein found on the surface of T-cells. This type of immune cells is involved in MS progression. All known drugs and those under development are immunomodulatory, several are biologics and do not directly address demyelination, which is the hallmark feature in MS, to the best of our knowledge. Even if Lucid-MS is approved, it will compete with product offerings from large and well-established companies that have greater marketing and sales experience and capabilities than we have or our third-party research collaborators. Other companies with greater resources than us may announce similar plans in the future.
However, we believe that Lucid-MS will be superior to the other MS products for individuals with progressive because based on our current clinical work, it appears to restore myeline growth and prevents degration.
Environmental Matters
The Company expects the financial and operational effects of environmental protection requirements on its capital expenditures, profit, and competitive position in the current and future financial years to be minimal.
Employees
As at December 31, 2023, the Company directly employed eight full-time employees and one part-time employee. All of these employees are based in the Toronto, Ontario area. The Company believes its relationship with its employees, consultants and contractors is good. None of the Company’s employees are represented by a labour union or subject to a collective bargaining agreement.
Reorganizations
The Company has not completed any material reorganization within the three most recently completed financial years, except for the reorganization that occurred pursuant to the Plan of Arrangement.
C. Organizational Structure
As at the date of this Annual Report, the Company has seven subsidiaries, Lucid, FSD BioSciences, Prismic, FSD Strategic Investments, FSD Australia, FV Pharma and Celly Nu. The corporate chart of the Company including the Company’s subsidiaries, together with the jurisdiction of incorporation of the Company and its subsidiary and the percentage of voting securities beneficially owned, controlled, or directed, directly or indirectly, by the Company is as follows:
| 46 |
| Table of Contents |
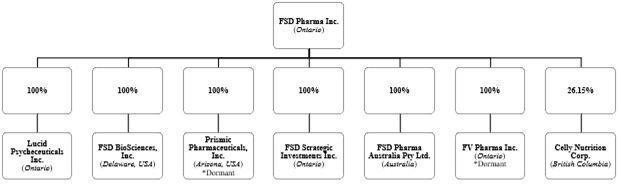
The Company has 4 active subsidiaries, which are:
Lucid
Lucid is a focused on the development of therapies to treat critical neurodegenerative diseases. Lucid is currently focused on research and development of Lucid-MS, which is a molecular compound identified for potential treatment of MS. Lucid conducted research on LUCID-Psych, which was being considered as a treatment for major depressive orders, which research is currently on hold due to funding concerns.
Celly Nu
Celly Nu. Is focused on the commercialization of Unbuzzd™. The Company has entered into the Celly Nu IP License with Celly Nu in June 2023.
FSD Strategic Investments
FSD Strategic Investments was incorporated in Ontario on May 13, 2022. During Fiscal 2022, the Company invested approximately C$7 million dollars in loans secured by real property primarily located in the Greater Toronto Area. These loans are considered to be highly collateralized as they are issued up to 55% of the appraised value of the secured property. FSD Strategic Investments is focused on generating returns and cashflow through the issuance of loans secured by residential or commercial property.
FSD Australia
On November 24, 2022, the Company incorporated a new Australian subsidiary, FSD Australia, to facilitate its development of Lucid-PSYCH and other assets. The registered and head office of FSD Australia is Level 7 330 Collins Street, Melbourne VIC, 3000. FSD Australia was established to facilitate the Company’s development of Lucid-PSYCH by running Australian clinical trials in respect of Lucid-PSYCH, and potentially other assets. Subject to satisfaction of relevant eligibility criteria, FSD Australia may be entitled to claim the Australian research and development tax incentive for eligible expenditure it incurs on eligible research and development activities.
Inactive Subsidiaries
FV Pharma
The Company suspended all activities by FV Pharma Inc, which had engaged in the cannabis business, as of September 2020 and in May 2022, substantially all of the assets of FV Pharma were sold. FV Pharma has accumulated historic tax losses and continues to exist as an entity wholly owned by the Company. Upon termination of FSD-PEA, Prismic did not have any assets or remaining liabilities other than outstanding notes payable which were assumed on the acquisition of Prismic and are classified as current liabilities of the Company. Prismic has accumulated historic tax losses and continues to exist as an entity wholly owned by the Company. For more information, please see “Item 4. Information on the Company. - A. History and Development of the Company - Other Significant Operations and Principal Activities – Fiscal 2021 and 2022”.
FSD BioSciences
FSD BioSciences was focused on the research and development of FSD-PEA (also known as FSD-201), an ultra-micronized PEA. On June 2, 2023, the Company terminated any further clinical development of FSD-PEA formulation for the treatment of inflammatory diseases.
Prismic
The Company does not operate through Prismic; however, Prismic holds the right to receive certain payments based on net sales of certain products under an amended and restated licensing agreement between Epitech Group SpA dated dated January 8, 2020, as amended, (the “Epitech License Agreement”) and the Company pursuant to an assignment agreement between Prismic and the Company (the “Prismic Assignment Agreement”). On June 2, 2023, the Company terminated any further clinical development of FSD-PEA formulation for the treatment of inflammatory diseases.
| 47 |
| Table of Contents |
Property, Plants and Equipment
The Company’s current operating plan does not include building infrastructure. The Company operates from its head office located in Toronto, Ontario, Canada. The Toronto office space costs approximately C$163,093 per annum (excluding operating costs and taxes) and is rented on a fixed term, ending on August 31, 2024. The Company believes that its current facilities are adequate to meet its ongoing needs and that, if the Company requires additional space, it will be able to obtain additional facilities on commercially reasonable terms.
Item 4A. Unresolved Staff Comments.
Not applicable.
Item 5. Operating and Financial Review and Prospects
A. Operating Results
See the Company’s Management’s Discussion and Analysis of Financial Condition and Results of Operations for the three months ended and fiscal years ended December 31, 2023 and 2022 (the “2023 Annual MD&A”) attached hereto as Exhibit 15.1.
B. Liquidity and Capital Resources
See the 2023 Annual MD&A attached hereto as Exhibit 15.1.
C. Research and Development, Patents and Licenses, etc.
For a discussion of our research and development activities, see “Item 4. Information on the Company. – B, Business Overview - Products and Sales” and the 2023 Annual MD&A attached hereto as Exhibit 15.1.
D. Trend Information
Other than as disclosed elsewhere in this Annual Report, we are not aware of any trends, uncertainties, demands, commitments, or events for the period from January 1, 2023 to December 31, 2023 that are reasonably likely to have a material adverse effect on our net revenues, income from continuing operations, profitability, liquidity or capital resources, or that would cause our reported financial information not necessarily to be indicative of future operating results or financial condition. For a discussion of trends, see “Item 4.B.-Business Overview” and the 20223 Annual MD&A attached hereto as Exhibit 15.1.
E. Critical Accounting Estimates
See Notes 2 and 3 to our 2023 Annual Financial Statements in Item 18.
Item 6. Directors, Senior Management and Employees
A. Directors and Senior Management.
The following table sets forth certain information with respect to our executive officers and directors as of March 28, 2024:
Name |
|
Age |
|
Position(s) with the Company |
|
Other Directorships |
|
Date of Initial Appointment |
Anthony Durkacz |
|
48 |
|
Co-Executive Chairman and Director |
|
Stock Trend Capital Inc. |
|
May 18, 2018 |
Zeeshan Saeed |
|
54 |
|
CEO, President, Co-Executive Chairman and Director |
|
Celly Nu |
|
May 24, 2018(1) |
Nathan Coyle |
|
43 |
|
Chief Financial Officer (“CFO”) |
|
N/A |
|
May 5, 2021 |
Donal Carroll |
|
48 |
|
Chief Operating Officer (“COO”) |
|
Bird River Resources Inc.; The Hast Corporation (formerly, Senternet Phi Gamma Inc.) |
|
May 18, 2018(3) |
Dr. Lakshmi P. Kotra |
|
53 |
|
Director, CEO of Lucid, President of FSD Biosciences and CEO of FSD Australia |
|
Celly Nu |
|
November 25, 2023 |
Adnan Bashir |
|
54 |
|
Director |
|
N/A |
|
June 1, 2021 |
Dr. Eric Hoskins |
|
63 |
|
Director |
|
Celly Nu; Cybin Inc.; Think Research Corporation (formerly, AIM4 Ventures Inc.) |
|
June 29, 2023 |
Dr. Sanjiv Chopra (2) |
|
74 |
|
Director |
|
N/A |
|
January 29, 2024 |
Michael (Zappy) Zapolin |
|
57 |
|
Director |
|
N/A |
|
June 29, 2023 |
| 48 |
| Table of Contents |
Notes:
(1) |
Mr. Saeed departed from his position as President and director of the Company effective January 25, 2021 but was re-elected as a director of the Company on May 14, 2021 and re-appointed as President of the Company on July 27, 2021. |
(2) |
Dr. Sanjiv Chopra replaced Nitin Kaushal on the Board effective January 29, 2024. |
(3) |
Mr. Carroll was initially appointed to the Board on May 18, 2018 until August 2018. On July 27, 2018, he was appointed interim CFO and on January 2, 2020, he became the permanent CFO until May 4, 2021. On May 14, 2021, he once again was a Board member until January 29, 2024. In August 2021, he became COO. |
Term of Office
Each director is to serve until his or her successor is elected and qualified or until his death, resignation, or removal. Our Board appoints our officers and each officer is to serve until his successor is appointed and qualified or until his or her death, resignation, or removal.
Executive Officers
Anthony Durkacz
Mr. Durkacz served as the Company’s interim CEO from July 2021 to July 2023, and has served as its Executive Co-Chairman since May 2021 and has served as a member of the Board since June 2018. Mr. Durkacz is also a director and the Executive Vice-President of First Republic and has served in those roles since 2014. In addition, Mr. Durkacz is the Chairman of World Class Extractions Inc. (CSE: PUMP; OTCQB: WCEXF) and has served in that role since 2018. Prior to co-founding the Company, from January 2013 to December 2013, Mr. Durkacz was President of Capital Ideas Investor Relations. He previously served as the CFO and a director of Snipp Interactive Inc. (TSXV: SPN.V), a global marketing solutions company that provides a modular software-as-a-service technology suite from January 2011 to January 2013. Mr. Durkacz was instrumental in the financing and public listing of Snipp Interactive Inc. with operations in Canada, the United States, Mexico, and India. From 2006 to 2009, he served as COO and CFO of MKU Canada Inc. and engaged in mergers and acquisitions of companies around the world. From 2002 to 2006, Mr. Durkacz served as the CFO and a director of Astris Energi Inc., a dual-listed public company in the United States and Canada which was acquired by an international conglomerate. Mr. Durkacz began his career at TD Securities on the capital markets trading floor. He holds an Honours Bachelor of Business Administration degree from Brock University with a major in both Accounting and Finance.
Zeeshan Saeed
Mr. Saeed, a co-founder of the Company, has served as the Company’s President and Executive Co-Chairman since May 2021. He became the Company’s CEO on July 4, 2023. Previously, he served as President of the Company from May 2019 to January 2021 and as a director from May 2018 to January 2021. From December 2017 to May 2019, Mr. Saeed served as Executive Vice President of FV Pharma, a subsidiary of the Company and a former licensed producer of cannabis in Canada under the Cannabis Act (Canada). From October 2013 to December 2017, he provided consulting services to FV Pharma from April 2003 to December 2017, Mr. Saeed served as President of ZZ Telecommunications Inc., a long-distance telecommunications common carrier. Mr. Saeed was the founder and CEO of Platinum Telecommunications Inc. from 2011 to 2013. He has a Bachelor of Science in Mechanical Engineering from the University of Engineering and Technology Lahore.
Nathan Coyle
Nathan Coyle has served as the Company’s CFO since May 2021. He previously served as the Company’s Corporate Controller from January 2020 to May 2021 and as Controller of Chem-Ecol Ltd. from July 2013 to January 2020. From July 2013 to January 2020, Mr. Coyle worked with Turtle Holdings Limited, a family investment company, implementing corporate strategies to maximize growth. From 2005 to 2013, Mr. Coyle was with Illinois Tool Works, where he was a key player in restructuring the organization, shaping the growth, and streamlining businesses within his industrial packaging segment. Mr. Coyle’s involvement in multiple mergers and acquisitions and integrating those organizations was key to company growth. Mr. Coyle holds a Bachelor of Business Administration with honours from Brock University and is a Chartered Professional Accountant.
Dr. Lakshmi P. Kotra
Dr. Lakshmi P. Kotra has served as CEO of Lucid since September 2020, which he co-founded in 2020 and has served as a director on the Board since November 2022. Dr. Kotra received his Ph.D. in Pharmacy (Medicinal Chemistry) from the University of Georgia under Prof. David Chu’s supervision, and completed postdoctoral training at Wayne State University under Prof. Shahriar Mobashery’s supervision. He joined the Faculty of Pharmacy, University of Toronto in 2000, and University Health Network in 2006, where he led a research group and drug discovery program with multiple portfolios. An academic entrepreneur, Dr. Kotra has contributed to a number of important drug discovery and development projects, including metabolic disorders, neurodegenerative and immunological disorders, anti-HIV drugs, antibacterials, and antimalarials. He has authored/co-authored over 130 publications and delivered over 140 scientific talks internationally. Dr. Kotra is the recipient of several awards for his accomplishments, including the Julia Levy Award in 2021 from the Society of Chemical Industry (SCI) Canada in recognition of his substantial contribution to the successful commercialization of innovation in Canada in the field of biomedical science and engineering. In addition to Lucid, he co-founded WinSanTor Biosciences, a San Diego, CA-based company developing treatments for peripheral neuropathies, and CannScience Innovations focused on medical cannabis and cannabinoids. Dr. Kotra has been serving the Company as Chief Executive Officer of its wholly owned subsidiary, Lucid, since the completion of the Company’s acquisition of Lucid in September 2021.
| 49 |
| Table of Contents |
Donal Carroll
Mr. Carroll joined the Company as interim CFO in 2018 and was appointed to the position on a permanent basis in December 2019, where he served until May 2021. Mr. Carroll was appointed as COO of the Company on August 15, 2021. Mr. Carroll has also served as a director on the Company’s Board from May 2018 to July 2018 and from May 2021 to January 2024. Mr. Carroll has 20 years of corporate finance leadership and public company experience, as well as experience in syndicate investing both in equity and debt securities. From June 2005 to January 2008, he served as an Accounting Supervisor with Alberto Culver (now Unilever (NYSE:UL)), from February 2008 to October 2013, Mr. Carroll has served as Controller with Videojet Technologies, and from October 2013 to July 2017, he served as a Corporate Controller with Cardinal Meats, where he was instrumental in major restructuring activities, mergers and acquisitions and the implementations of new internal controls and ERP systems. Mr. Carroll has been a Director of Bird River Resources Inc. since August 2019 and was a Director of Climb Credit Inc. He holds a CPA-CMA designation as well as a Bachelor of Commerce degree from University College Dublin.
Non-Employee Directors
Adnan Bashir
Mr. Bashir has over 14 years of experience in strategic management and operations. He is the founder and President of 58Northwest Inc., a management consulting and marketing services company, and has held the role since 2018. From 2005-2018, Mr. Bashir was General Manager for Al Batha Group, a diversified business conglomerate based in Dubai, UAE. Mr. Bashir was responsible for overseeing the management and operations of 4 companies within the group and was instrumental in acquiring and developing new businesses and partners from Europe, the US and China. During his tenure at Al Batha Group, Mr. Bashir gathered extensive experience in executing turnaround strategies, transforming weak businesses into sustainable and profitable ones and implementing new technologies. Mr. Bashir holds a Bachelor of Science Degree in Mechanical Engineering from University of Engineering and Technology Lahore and has completed extensive executive education, including in strategic management, audit, sales management, and technical management.
Dr. Eric Hoskins
Dr. Eric Hoskins is a medical doctor and public health expert with more than 30 years’ experience in healthcare, public policy, economic development, and international trade. Dr. Hoskins recently served as the Chair of the Federal Advisory Council on the Implementation of National Pharmacare.
He previously served as president of War Child Canada and was awarded the Order of Canada in 2007 for his humanitarian work. During Dr. Hoskins’ nearly 10 years as a member of provincial parliament in Ontario, he held several cabinet positions including Minister of Health and Long-Term Care; Economic Development, Trade and Employment; Children and Youth Services; as well as Citizenship and Immigration. As a tireless health advocate, Dr. Hoskins has many years of experience creating and delivering health programs in Africa and the Middle East.
Dr. Sanjiv Chopra
Sanjiv Chopra, MD, is Professor of Medicine and served as Faculty Dean for Continuing Medical Education at Harvard Medical School for 12 years. He serves as a Marshall Wolf Distinguished Clinician Educator Brigham and Women’s Hospital.
Dr. Chopra has more than 170 publications and ten books to his credit. Dr. Chopra is Editor-in-Chief of the Hepatology Section of UpToDate, the most widely used electronic textbook in the world subscribed to by more than 1.5 million physicians in 195 countries.
He is a sought after inspirational speaker across the United States and abroad, addressing diverse audiences on topics related to medicine, leadership, happiness, and living with purpose.
Michael (Zappy) Zapolin
Zappy Zapolin is a well-known futurist, psychedelic concierge to the stars, and award-winning filmmaker who is dedicated to expanding human consciousness.
As the youngest Vice President in the history of investment bank Bear Stearns, Zappy is a frequent commentator on investment opportunities in the biotech and emerging psychedelic industry.
| 50 |
| Table of Contents |
Certain Proceedings involving Directors
Mr. Durkacz has been serving as director of FSD since June 18, 2018. On March 5, 2021, FSD was subject to a court order with respect to the 2021 Annual and Special Meeting which, among other things, prohibited the Company’s then CEO and directors, other than Mr. Durkacz, from voting certain of their shares at the 2021 Annual and Special Meeting. On April 9, 2021, the Court ordered an injunction restraining the Company’s then CEO and former directors, other than Mr. Durkacz, from authorizing or undertaking any transaction by FSD other than in the ordinary course of business, issuing any Class B Shares or authorizing the payment of any form of compensation to such former CEO and directors prior to the 2021 Annual and Special Meeting.
Family Relationships
There are no family relationships among any of our executive officers or directors.
Special Arrangements
Not applicable.
B. Compensation.
Executive Compensation
The purpose of this Compensation Discussion and Analysis is to provide information about the Company’s philosophy, objectives, and processes regarding executive compensation. This disclosure is intended to communicate compensation provided to: (i) the CEO; (ii) the CFO; (iii) each of the three most highly compensated executive officers of the Company, including any of its subsidiaries, or the three most highly compensated individuals acting in a similar capacity, other than the CEO and CFO, as at the end of the most recently completed financial year whose total compensation was, individually, more than C$150,000; and (iv) each individual who would be a NEO under paragraph (c) but for the fact that the individual was neither an executive officer of the Company or its subsidiaries, nor acting in a similar capacity, as at December 31, 2023, (collectively, the “NEOs”) and (v) the directors of the Company.
During the year ended December 31, 2023, the NEOs of the Company were as follows:
|
1) |
Zeeshan Saeed, CEO and Co-Executive Chairman, and Director of the Company; |
|
2) |
Anthony Durkacz, Co-Executive Chairman, and Director of the Company; |
|
3) |
Nathan Coyle, CFO of the Company; |
|
4) |
Donal Carroll, COO of the Company; and |
|
5) |
Dr. Lakshmi Kotra, Director of the Company, CEO of Lucid, President of FSD Biosiences, and CEO FSD Australia. |
The description of the Company’s compensation philosophy and objectives and the elements of such compensation for the year ended December 31, 2023 is set forth below:
Compensation Philosophy and Objectives
The executive compensation program adopted by the Company and applied to its executive officers is designed to attract and retain qualified and experienced executives who will contribute to the success of the Company. The executive compensation program attempts to ensure that the compensation of the senior executive officers provides a competitive base compensation package and a strong link between corporate performance and compensation. Senior executive officers are motivated through the program to enhance long-term shareholder value and rewarded for their yearly individual contribution in the context of overall annual corporate performance.
Elements of Compensation
The executive compensation program during the year, ended December 31, 2023 consisted of three principal components: (i) base compensation; (ii) potential annual incentive award; (iii) Options to Class B Shares (“Options”); (iv) restricted share units (“RSUs”); and (v) performance share units (“PSUs”). Options, RSUs, and PSUs are granted pursuant to the Company’s equity incentive plan (the “Equity Incentive Plan”) which replaced the Company’s rolling stock option plan (the “Stock Option Plan”). For the year ended December 31, 2023, all executive compensation was determined and administered by the Board based on recommendations by the compensation, nominating and governance committee of the Company (“Compensation, Nominating and Governance Committee”).
Compensation Governance
The Compensation, Nominating and Governance Committee is currently comprised of three directors, Zeeshan Saeed (Chair), Michael (Zappy) Zapolin, and Adnan Bashir. Zeeshan Saeed is not an independent director, and Michael (Zappy) Zapolin, and Adnan Bashir are independent directors.
The primary goal of the Company’s executive compensation program is to attract and retain the key executives necessary for the Company’s long term success, to encourage executives to further the development of the Company and its operations, and to motivate top quality and experienced executives.
| 51 |
| Table of Contents |
The Compensation, Nominating and Governance Committee has been tasked with establishing an executive compensation program, which, prior to May 16, 2022, included equity compensation by way of share awards and Options granted under the Stock Option Plan. As of May 16, 2022, the executive compensation program includes equity compensation by way of share awards, RSUs, PSUs and Options granted under the Equity Incentive Plan.
The Compensation, Nominating and Governance Committee reviews the adequacy of remuneration for the executive officers by evaluating their performance in light of the Company’s goals and objectives, the bonus opportunities contained in their employment agreements, and by comparing the performance of the Company with other reporting issuers of similar size in the same industry.
The Board is of the view that all elements of the total program should be considered, rather than any single element. As such, the Company does not use fixed criteria in determining the mix of compensation and instead determines compensation based on a contextual analysis of the Company. While the Company does not have a formally established peer group in determining compensation, the Compensation, Nominating and Governance Committee will on occasion reference other comparable publicly traded Canadian companies to align its compensation practices with market practice.
The terms of any proposed compensation for the directors of the Company who are not also officers of the Company (including any Options, RSUs and PSUs to be granted) will be determined by the Compensation, Nominating and Governance Committee. The compensation program is designed to provide income certainty, to attract and retain executives and to provide incentives for the achievement of both short-term and long-term objectives of the Company.
Compensation Process
The Compensation, Nominating and Governance Committee, through discussion without any formal objectives, criteria, or analysis, determines the compensation of the Company’s executive officers. The Compensation, Nominating and Governance Committee has no formal criteria or goals tied to total compensation or any significant element of total compensation. The Board, through the Compensation, Nominating and Governance Committee, is responsible for determining all forms of compensation, including share-based compensation and long-term incentives in the form of Options, RSUs and PSUs to be granted to the Company’s executive officers and directors, and for reviewing the recommendations respecting compensation of other officers of the Company from time-to-time, to ensure such arrangements reflect the responsibilities and risks associated with each position. The Compensation, Nominating and Governance Committee determines compensation by considering: (i) recruiting and retaining executives critical to the Company’s success and the enhancement of shareholder value; (ii) providing fair and competitive compensation; (iii) balancing the interests of management and the Shareholders; and (iv) rewarding performance, both on an individual basis and with respect to the Company’s operations in general.
Annual Incentive Awards
Annual incentive awards are designed to motivate NEOs to achieve the Company’s short-term corporate goals, and rewards individual and overall performance. Annual incentives are based on objective, identifiable measures set at the beginning of each financial year at the discretion of the Compensation, Nominating and Governance Committee, which may vary from year to year and incentive payments are expected to be determined by the Board on the recommendation of the Compensation, Nominating and Governance Committee.
Option Awards
Long-term incentives in the form of Options are intended to align the interests of the Company’s directors and its executive officers with those of its Shareholders, to provide a long-term incentive that rewards these individuals for their contribution to the creation of Shareholder value, and to reduce the cash compensation the Company would otherwise pay. The Equity Incentive Plan is administered by the Board. While the Company does not have a formally established peer group in determining compensation, in considering the number of Options to be granted to the NEOs, reference is made to the number of Options granted to officers of other comparable publicly traded Canadian companies. The Compensation, Nominating and Governance Committee also considers previous grants of equity incentives and the overall number of equity incentives that are outstanding relative to the number of outstanding Shares in determining whether to make any new grants of Options and the size and terms of any such grants, as well as the level of effort, time, responsibility, ability, experience, and level of commitment of the executive officer in determining the level of Option compensation.
Share Unit Awards
The Equity Incentive Plan provides that Eligible Persons (as defined in the Equity Incentive Plan) may be allocated share units in the form of RSUs or PSUs (collectively, “Share Units”), which represent the right to receive an equivalent number of Class B Shares or the Market Price (as defined in the Equity Incentive Plan) in cash on the vesting date. The issuance of Class B Shares may be subject to vesting requirements similar to those described above with respect to the exercise of Options, including such time- or performance-based conditions as may be determined from time to time by the Board in its discretion.
The Equity Incentive Plan provides for the express designation of Share Units as either RSUs, which have time-based vesting conditions, or PSUs, which have performance-based vesting conditions over a specified period. The Equity Incentive Plan provides that if Share Units are scheduled to settle during a blackout period, such settlement shall be postponed until the trading day following the date on which the blackout period ends (or as soon as practicable thereafter, and in any event, within 10 business days following the end of the blackout period), and the Market Price of any RSUs or PSUs settled in cash will be determined as of the trading day immediately prior to the settlement date.
| 52 |
| Table of Contents |
The Compensation, Nominating and Governance Committee also considers previous grants of equity incentives and the overall number of equity incentives that are outstanding relative to the number of outstanding Shares in determining whether to make any new grants of Share Units and the size and terms of any such grants, as well as the level of effort, time, responsibility, ability, experience, and level of commitment of the executive officer in determining the level of Share Unit compensation.
Insider Trading and Blackout Period Policy
All of the Company’s executives, other employees and directors are subject to the Company’s Insider Trading and Blackout Period Policy, which prohibits trading in the Company’s securities while in possession of material undisclosed information about the Company. Under this policy, such individuals are also prohibited from entering into hedging transactions involving securities of the Company, such as short sales, puts and calls. Furthermore, subject to certain limited exceptions, the Company permits executives, including the NEOs, to trade in the Company’s securities only during prescribed trading windows.
Risk Analysis
The Board and Compensation, Nominating and Governance Committee considered risks associated with executive compensation and do not believe that the Company’s executive compensation policies and practices encourage its executive officers to take inappropriate or excessive risks. Aside from a fixed base salary, NEOs are compensated through the granting of awards, which are compensation that is both “at risk” and associated with long-term value creation. The value of such compensation is dependent upon Shareholder return over award vesting periods which reduces the incentive for executives to take inappropriate or excessive risks as their long-term compensation is at risk.
Performance graph
The following performance graph compares the total cumulative return to a Shareholder who invested C$100 in Shares on January 1, 2018, assuming reinvestment of dividends, with the cumulative total return on the S&P/TSX Composite Total Return Index and SPDR S&P Biotech ETF for each year following January 1, 2018. The performance of the Shares as set out in the graph below does not necessarily indicate future performance.
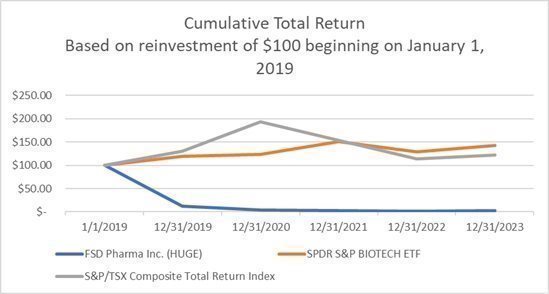
| 53 |
| Table of Contents |
|
|
December 31, 2019 (C$) |
|
|
December 31, 2020 (C$) |
|
|
December 31, 2021 (C$) |
|
|
December 31, 2022 (C$) |
|
|
December 31, 2023 (C$) |
|
|||||
FSD Pharma Inc. |
|
|
11.85 |
|
|
|
3.40 |
|
|
|
2.23 |
|
|
|
1.72 |
|
|
|
2.01 |
|
|
SPDR S&P Biotech ETF (NYSEARCA: XBI) |
|
|
118.93 |
|
|
|
123.96 |
|
|
|
151.55 |
|
|
|
129.57 |
|
|
|
143.46 |
|
|
S&P/TSX Composite Total Return Index |
|
|
130.54 |
|
|
|
193.22 |
|
|
|
153.66 |
|
|
|
113.92 |
|
|
|
122.55 |
|
The Company is of the view that compensation levels for the directors and executive officers cannot and should not be directly compared to year-over-year relative market performance. Market performance is impacted by a number of external factors beyond the control of management and an increase or decrease in the market price and thus should not be a determining factor in establishing the annual compensation of the Company’s directors and executive officers. The stock price directly impacts the benefits enjoyed by the directors and executive officers from the Stock Option Plan and Equity Incentive Plan. As a result, the trend shown in the above graph does not necessarily correspond to the Company’s compensation to its directors and executive officers for the same period.
The Company’s compensation package is based on competitive compensation trends and the value of the services provided and is designed to attract and retain top quality personnel for the longer term in order to manage and grow the business through both adverse and favorable economic cycles. These factors may not yield immediate results in stock price.
Summary Compensation Table
|
Name and Principal Position |
Year |
Salary (US$) |
Share-Based Awards(1) (US$) |
Option Based Awards(2) (US$) |
Non-Equity Incentive Plan Compensation (US$) |
Pension Value |
All Other Compensation(3) (US$) |
Total Compensation (US$) |
|
|
Annual incentive plans |
Long-term incentive plans |
||||||||
|
Anthony Durkacz(4) Co-Executive Chairman and Director
|
2023 |
222,625 |
Nil |
377,248 |
Nil |
Nil |
Nil |
599,873 |
|
2022 |
239,269 |
316,834(5) |
Nil |
Nil |
Nil |
136,710(6) |
692,813 |
||
2021 |
103,703 |
Nil |
777,798 |
Nil |
Nil |
Nil |
881,501 |
||
|
Nathan Coyle(7) CFO |
2023 |
178,208 |
Nil |
Nil |
Nil |
Nil |
Nil |
178,208 |
|
2022 |
191,891 |
Nil |
Nil |
Nil |
Nil |
Nil |
191,891 |
||
2021 |
189,186 |
Nil |
46,586 |
15,734 |
Nil |
Nil |
251,506 |
||
|
Zeeshan Saeed(8) CEO& Co-Executive Chairman |
2023 |
222625 |
Nil |
377,248 |
Nil |
Nil |
Nil |
599,873 |
|
2022 |
239,269 |
398,010 (5) |
Nil |
Nil |
Nil |
136,710 (6) |
773,989 |
||
2021 |
225,271 |
Nil |
777,798 |
Nil |
Nil |
Nil |
1,003,069 |
||
|
Donal Carroll(9) COO |
2023 |
225,625 |
Nil |
377,248 |
Nil |
Nil |
Nil |
602,873 |
|
2022 |
239,269 |
321,543(5) |
34,519 |
Nil |
Nil |
136,710 (6) |
732,041 |
||
2021 |
351,406 |
Nil. |
777,798 |
78,670 |
Nil |
4,779(6) |
1,212,653 |
||
|
Dr. Lakshmi Kotra Director, CEO of Lucid, President of FSD Biosiences, and CEO FSD Australia |
2023 |
144,724 |
Nil. |
377,248 |
Nil |
Nil |
Nil |
521,972 |
|
2022 |
331,510(10) |
80,212(5)(10) |
Nil |
Nil |
Nil |
Nil |
411,722 |
||
2021 |
44,430(10) |
Nil |
172,799 |
Nil |
Nil |
Nil |
217,228.96 |
||
| 54 |
| Table of Contents |
Notes:
(1) |
“Share-based Award” means an award of Class B Shares and includes PSUs and RSUs. The dollar amount disclosed is based on the closing price per Class B Share at the date of each grant. |
(2) |
“Option-based Award” means an award of Options under the Stock Option Plan or the Equity Incentive Plan. This does not represent cash paid to the NEO. This figure is based on the grant date fair value of such Options. The grant date fair value was determined in accordance with International Financial Reporting Standards. This methodology was chosen in order to be consistent with the accounting fair value used by the Company in its financial statements, and the Black-Scholes option pricing model is a commonly used methodology for valuing options which provides an objective and reasonable estimate of fair value. Calculating the value of stock options using the Black-Scholes option pricing model is very different from a simple “in-the-money” value calculation. Accordingly, caution must be exercised in comparing grant date fair value amounts with cash compensation or an in-the-money option value calculation. |
(3) |
Includes Company-paid health and life insurance benefits and car allowances for all NEOs. |
(4) |
Mr. Durkacz has been a director of the Company since June 18, 2018 and was appointed as interim CEO of the Company on July 27, 2021. |
(5) |
This figure represents PSUs granted under the Equity Incentive Plan. This does not represent cash paid to the NEO. This figure is based on the incremental grant date fair value of such PSUs. The grant date fair value was determined in accordance with International Financial Reporting Standards. The incremental fair value is the difference between the fair value of the PSUs based on the share price on the grant date and the fair value of the share options cancelled as measured on date of modification. This methodology was chosen in order to be consistent with the accounting fair value used by the Company in its financial statements. For further details on the valuation of PSUs, see Note 16 to the 2023 Annual Financial Statements, starting at page F-1. |
(6) |
These amounts represent cash bonuses paid to the NEO. |
(7) |
Mr. Coyle was appointed as the CFO of the Company on May 4, 2021, initially on interim and then on a permanent basis. |
(8) |
Mr. Saeed departed from his position as President and director of the Company effective January 25, 2021 but was re- elected as a director of the Company on May 14, 2021 and re-appointed as President of the Company on July 27, 2021. |
(9) |
Mr. Carroll resigned as CFO of the Company on May 4, 2021 and was elected as a director of the Company at the 2021 Meeting on May 14, 2021. Mr. Carroll was appointed as COO of the Company on August 15, 2021. |
(10) |
Dr. Kotra is not paid a salary by the Company, but was paid a consulting fee of $331,510.00 for the year ended December 31, 2022 and $44,430.00 for the year ended December 31, 2022. Dr. Kotra’s PSU awards for the year ended December 31, 2022 includes 51,500 PSUs issued to ILace Therapeutics International Inc., a corporation controlled by Dr. Kotra. |
Outstanding Option-Based and Share-Based Awards
The following table sets forth information with respect to Options, RSUs and PSUs held by the NEOs which were outstanding as of December 31, 2023:
|
|
Option-based Awards |
|
Share-based Awards |
|
|||||||||||||||||
Name |
|
Number of securities underlying unexercised options (#) (b) |
|
|
Option exercise price ($) (c) |
|
|
Option expiration date (d) |
|
Value of unexercised in-the-money options ($) (e) |
|
Number of shares or units of shares that have not vested (#) (f) |
|
Market or payout value of share- based awards that have not vested ($) (g) |
|
|
Market or payout value of vested share-based awards not paid out or distributed ($) (h) |
|
||||
|
Zeeshan Saeed CEO, Co-Executive Chairman, and Director |
|
|
500,000 |
|
|
|
1.30 |
|
|
2028-01-25 |
|
Nil |
|
Nil |
|
Nil |
|
|
|
596,080 |
|
|
Nathan Coyle |
|
|
5,000 |
|
|
|
2.88 |
|
|
2024-03-18 |
|
Nil |
|
Nil |
|
N/A |
|
|
|
N/A |
|
|
CFO |
|
|
30,000 |
|
|
|
1.73 |
|
|
2024-06-01 |
|
Nil |
|
Nil |
|
N/A |
|
|
|
N/A |
|
|
|
Anthony Durkacz Co-Executive Chairman & Director |
|
|
500,000 |
|
|
|
1.30 |
|
|
2028-01-25 |
|
Nil |
|
Nil |
|
Nil |
|
|
|
596,080 |
|
|
|
Lakshmi Kotra Director, CEO of Lucid, President of FSD Biosiences, and CEO FSD Australia |
|
|
500,000 |
|
|
|
1.30 |
|
|
2028-01-25 |
|
Nil |
|
Nil |
|
Nil |
|
|
|
184,744 |
|
|
|
Donal Carroll COO |
|
|
500,000 |
|
|
|
1.30 |
|
|
2028-01-25 |
|
Nil |
|
Nil |
|
Nil |
|
|
|
600,596 |
|
|
| 55 |
| Table of Contents |
Incentive plan awards – value vested or earned during the year
|
Name (a) |
|
Option-based awards – Value vested during the year ($) (b) |
|
|
Share-based awards – Value vested during the year ($) (c) |
|
Non-equity incentive plan compensation – Value earned during the year ($) (d) |
||
|
Zeeshan Saeed CEO, Co-Executive Chairman, and Director |
|
377,248 |
|
|
Nil |
|
Nil |
||
|
Nathan Coyle CFO |
|
Nil |
|
|
Nil |
|
Nil |
||
|
Anthony Durkacz Co-Executive Chairman & Director |
|
377,248 |
|
|
Nil |
|
Nil |
||
|
Lakshmi Kotra Director, CEO of Lucid, President of FSD Biosiences, and CEO FSD Australia |
|
377,248 |
|
|
Nil |
|
Nil |
||
|
Donal Carroll COO |
|
377,248 |
|
|
Nil |
|
Nil |
||
Stock option plans and other incentive plans
Equity Incentive Plan
On May 16, 2022, the Board adopted the Equity Incentive Plan, which was effective on June 29, 2023, upon the Company receiving shareholder approval at the annual general and special meeting. The Equity Incentive Plan replaced the Stock Option Plan.
Purpose of the Equity Incentive Plan
The principal purposes of the Equity Incentive Plan is to: (i) promote a further alignment of interests between officers, employees and other eligible service providers and the Shareholders, (ii) associate a portion of the compensation payable to officers, employees and other eligible service providers with the returns achieved by the Shareholders, and (iii) attract and retain officers, employees and other eligible service providers with the knowledge, experience and expertise required by the Company.
The Equity Incentive Plan contains provisions applicable to all grants of Options, RSUs, and PSUs (collectively, “Awards”) in the capital of the Company, to Eligible Persons (as defined in the Equity Incentive Plan).
Equity Incentive Plan Maximum, Limits and Vesting Restrictions
| 56 |
| Table of Contents |
The Equity Incentive Plan provides that: (i) the aggregate number of Class B Shares that may be issued pursuant to the Equity Incentive Plan, together with all other security-based compensation arrangements of the Company, shall be equal to 10% of the issued and outstanding Class B, from time to time; (ii) the aggregate number of Class B Shares reserved for issuance pursuant to the Equity Incentive Plan to any one participant, together with all other security-based compensation arrangements of the Company, must not exceed 5% of the aggregate issued and outstanding Class B Shares; (iii) the maximum number of Class B Shares (a) issued to Insiders (as defined in the Equity Incentive Plan) within any one year period, and (b) issuable to Insiders, at any time, under the Equity Incentive Plan or when combined with all other security-based compensation arrangements of the Company, shall not exceed 10% of the number of the aggregate issued and outstanding Class B Shares; and (iv) the maximum number of Class B Shares issued within any 12-month period to an individual Employed (as defined in the Equity Incentive Plan) by the Company or any of its subsidiaries, or a service provider engaged in investor relations activities must not exceed 1% of the issued and outstanding Class B Shares. Unless otherwise designated by the Board in the applicable grant agreement, 25% of the Options granted under the Equity Incentive Plan shall vest and become exercisable on the first anniversary of the grant date and the remaining 75% of the Options shall vest and become exercisable in equal quarterly installments beginning on the 15-month anniversary of the grant date and ending on the four-year anniversary of the grant date.
As at December 31, 2023 the Company had issued an aggregate total of 2,460,615 Awards outstanding under the Equity Incentive Plan, comprised of 2,460,615 Options, Nil RSUs, Nil PSUs and a total of 1,477,057 Class B Shares remained authorized for issuance under the Equity Incentive Plan.
The Equity Incentive Plan is administered by the Board, which has full and complete discretionary authority with respect to the granting of all awards thereunder. The Board may, subject to applicable law and certain restrictions, delegate its powers, rights, and duties under the Equity Incentive Plan to a committee of the Board, a person or persons, as it may determine, from time to time, on terms and conditions as it may determine. Awards may be granted under the Equity Incentive Plan to such service providers of the Company and its subsidiaries, if any, as the Board may from time to time designate. With respect to Options, exercise prices will be determined by the Board but will, in no event, be less than the market price of the Class B Shares on the grant date or the lowest price permitted by the policies of any stock exchange on which the Class B Shares may be listed. Generally, all Options granted under the Equity Incentive Plan will expire not later than the date that is ten years from the date that such Options are granted.
With respect to RSUs and PSUs, the number of such awards granted shall be determined by dividing the grant value for such grant, as determined by the Board, by the market price of a subordinate voting share as at the grant date (or otherwise determined by the Board). Settlement of RSUs and PSUs shall be made by the issuance of one Class B Share for each RSU or PSU then being settled, a cash payment equal to the market price of one subordinate voting share on the vesting date of the RSUs or PSUs being settled in cash or a combination of Class B Shares and cash, all as determined by the Board in its discretion, or as specified in applicable grant agreement. Subject to certain exceptions, any awards granted under the Equity Incentive Plan are not transferable or assignable other than by testamentary disposition or pursuant to the laws of intestate succession.
Stock Option Plan
The Stock Option Plan was replaced by the Equity Incentive Plan. A summary of the material terms of the Stock Option Plan can be found in the management information circular dated May 19, 2023. There are no predecessor Option outstanding under the Stock Option Plan.
Pension Disclosure
The Company established a 401(k) plan on January 31, 2021, but the plan was terminated on December 31, 2021. The Company currently does not have any pension plans that provide for payments or benefits at, following, or in connection with retirement.
Termination and Change of Control
The Company has entered into executive employment agreements with each of the NEOs (the “Executive Agreements”) other than Nathan Coyle. Each Executive Agreement provides for the NEO’s annual base salary, vacation entitlement and benefits.
The following is a description of material provisions of the Executive Agreements as they relate to termination and change of control.
Anthony Durkacz (Co-Executive Chairman and Director)
Mr. Durkacz has an executive employment agreement with the Company. In the event of both a change of control transaction and Mr. Durkacz ceasing to be employed by the Company for any reason, all outstanding unvested Options held as of the date Mr. Durkacz ceases to be employed by the Company shall immediately vest and remain outstanding and exercisable for a period of five years from that date. All vested Options held by Mr. Durkacz that are outstanding on that date shall remain outstanding and be exercisable for five years following the applicable vesting date of the Options. Additionally, in the event of any change of control transaction, the expiry date of each Option issued to Mr. Durkacz prior to the date, and during the term, of his Executive Agreement shall be the date that is five years from the applicable vesting date of such Option. In the event Mr. Durkacz’s employment is terminated as a result of a change of control transaction, he would be entitled to receive C$50,000.
Zeeshan Saeed (CEO, Co-Executive Chairman and Director)
Mr. Saeed has an executive employment agreement with the Company. In the event the Company terminates Mr. Saeed’s employment without cause or in the event of any termination of the agreement by either party following a change of control, the Company will provide Mr. Saeed with a cash payment in an amount equal to 24-months (2 years) compensation, being the sum of: (i) base salary, (ii) the applicable target bonus, and (iii) the cash value of any stock grants provided in the last 12-months. In the event of a change of control transaction and Mr. Saeed ceasing to be employed by the Company for any reason, all outstanding unvested Options held by Mr. Saeed as of the date that he ceases being employed by the Company shall immediately vest and shall remain outstanding and be exercisable for five years following that date. All vested Options held by Mr. Saeed that are outstanding on that date will remain outstanding and be exercisable for five years following the applicable vesting date of the Options. Additionally, in the event of any change of control transaction, the expiry date of each Option issued to Mr. Saeed prior to the date, and during the term, of his Executive Agreement shall be the date that is five years from the applicable vesting date of such Option. In the event Mr. Saeed’s employment is terminated without cause or as a result of a change of control transaction, he would be entitled to receive C$600,000.
| 57 |
| Table of Contents |
Donal Carroll (COO)
Mr. Carroll has an executive employment agreement with the Company. In the event the Company terminates Mr. Carroll’s employment without cause or in the event of any termination of the agreement by either party following a change of control, the Company shall pay Mr. Carroll a cash payment in an amount equal to 24 months (two years) compensation, being the sum of: (i) base salary, (ii) the applicable target bonus, and (iii) the cash value of any stock grants provided in the last 12-months. In the event of a change of control transaction and Mr. Carroll ceasing to be employed by the Company for any reason, all outstanding unvested Options, as of the date Mr. Carroll ceases to be employed by the Company shall immediately vest and shall remain outstanding and be exercisable for a period of five years following that date. All vested Options held by Mr. Carroll that are outstanding as of that date shall remain outstanding and be exercisable for five years following the applicable vesting date of the Options. Additionally, in the event of any change of control transaction, the expiry date of each Option issued to Mr. Carroll prior to the date, and during the term, of his Executive Agreement shall be the date that is five years from the applicable vesting date of such Option. In the event Mr. Carroll’s employment is terminated without cause or as a result of a change of control transaction, he would be entitled to receive C$600,000.
Nathan Coyle (CFO)
Mr. Coyle has an executive employment agreement with the Company. In the event the Company terminates Mr. Coyle’s employment without cause or in the event of any termination of the agreement by either party following a change of control, the Company shall pay Mr. Coyle a cash payment in an amount equal to one month’s salary per year of employment up to a maximum of 12 months and no less than four months compensation. In the event of a change of control transaction and Mr. Coyle ceasing to be employed by the Company for any reason, the expiry date of each Option issued to Mr. Coyle prior to the date, and during the term, of his Executive Agreement shall be the date that is five years from the applicable vesting date of such Option.
Liability Insurance of Directors and Officers
The Company has directors’ and officers’ liability insurance coverage for losses to the Company if the Company is required to reimburse directors and officers, where permitted, and for direct indemnity of directors and officers where corporate reimbursement is not permitted by law. This insurance protects the Company against liability (including costs), subject to standard policy exclusions, which may be incurred by directors and/or officers acting in such capacity for the Company. All directors and officers are covered by the policy and the amount of insurance applies collectively to all. The annual cost for this insurance during the year ended December 31, 2023 was C$108,000 per annum.
Indemnification
Amended and Restated By-Law No. 1 provides for indemnification of each of the Company’s directors, former directors, officers, former officers, respective heirs and legal representatives of directors and officers and each individual who acts or acted at the Company’s request as a director or officer of a body corporate or an individual acting in a similar capacity of another entity (each, an “Indemnified Person”) against all costs, charges and expenses reasonably incurred in respect of any civil, criminal or administrative, investigative or other proceeding to which that Indemnified Person is made a party by reason of being or having been at the relevant time a director or officer of the Company or of a body corporate of which the Company is or was a shareholder or creditor, or by reason of having acted in a similar capacity for any other entity that is or was at the relevant time directly or indirectly owned or controlled by the Company, as required by the OBCA.
The Company has entered into indemnity agreements with each director and officer providing that if such director or officer is or was involved in any threatened, pending or completed proceeding by reason of the fact that such director or officer is or was a director or officer of the Company or is or was serving at the Company’s request as a director or officer of another entity, such director or officer will be indemnified and held harmless by the Company to the fullest extent authorized by and in the manner set forth in the Amended and Restated By-Law No.1 and OBCA against all expense, liability and loss reasonably incurred or suffered by such director or officer in connection therewith. Under such indemnity agreements, to the fullest extent allowable under applicable law, the Company shall also indemnify against any costs actually and reasonably paid or incurred by a director or officer in connection with any action or proceeding by such director or officer for (i) indemnification or reimbursement of any costs, or payment of any cost advance, by the Company under any provision of the agreements, or under any other agreement or provision of our constating documents and (ii) recovery under any directors’ and officers’ liability insurance policies maintained by the Company, regardless of whether the director or officer ultimately is determined to be entitled to such indemnification or insurance recovery, as the case may be.
A new Board was elected on June 29, 2023 at the annual general and special meeting of shareholders. The following describes the director compensation program that was in effect for the portion of the year ended after the 2023 Meeting. A cash retainer of C$50,000 per year is paid in monthly installments in arrears. In addition, C$10,000 per year is paid to the Chair of the audit committee of the Company (the “Audit Committee”) and C$20,000 per year is paid to the Chairs of each of the Compensation, Nominating and Governance Committee and the Disclosure Committee, paid in monthly installments in arrears in each case. No additional fees are paid to the members for attending the meetings of our Board and meetings of our standing committees. Under the director compensation program for the year ended December 31, 2023, newly appointed directors were granted stock options that vested immediately.
| 58 |
| Table of Contents |
Director Compensation Table
The following table sets forth information concerning compensation accrued or paid to the Company’s non-employee directors, other than NEOs whose compensation is reported in the Summary Compensation Table above, during the year ended December 31, 2023:
Name(1) |
|
Fees earned ($) (b) |
|
|
Share-based awards ($) (c) |
|
Option-based awards ($) (d) |
|
Non-equity incentive plan compensation ($) (e) |
|
|
Pension value ($) (f) |
|
All other compensation ($) (g) |
|
|
Total ($) (h) |
|
||||
Michael (Zappy) Zapolin |
|
|
26,000 |
|
|
Nil |
|
Nil |
|
Nil |
|
|
Nil |
|
100,000 |
|
|
|
126,000 |
|
||
Adnan Bashir |
|
|
44,525 |
|
|
Nil |
|
Nil |
|
|
94,848 |
|
|
Nil |
|
Nil |
|
|
|
139,373 |
|
|
Nitin Kaushal |
|
|
56,599 |
|
|
Nil |
|
Nil |
|
|
94,848 |
|
|
Nil |
|
Nil |
|
|
|
151,447 |
|
|
Dr. Eric Hoskins |
|
|
- |
|
|
Nil |
|
Nil |
|
Nil |
|
|
Nil |
|
Nil |
|
|
|
- |
|
||
Lawrence (Larry) Latowsky(2) |
|
|
25,992 |
|
|
Nil |
|
Nil |
|
|
94,848 |
|
|
Nil |
|
Nil |
|
|
|
120,840 |
|
|
Joseph L. Romano(3) |
|
|
22,023 |
|
|
Nil |
|
Nil |
|
Nil |
|
|
Nil |
|
Nil |
|
|
|
22,023 |
|
||
Notes:
(1) |
The relevant disclosure pertaining to directors who are NEOs (meaning, Zeeshan Saeed and Anthony Durkacz) are reflected in the summary compensation table set forth above. |
(2) |
Lawrence (Larry) Latowsky resigned from the Board effective June 29, 2023. |
(3) |
Joseph L. Romano resigned from the Board effective June 29, 2023. |
Director Compensation - Share-based Awards, Option-Based Awards and Non-Equity Incentive Plan Compensation
Name |
|
Option-based awards – Value vested during the year ($) |
|
Share-based awards – Value vested during the year ($) |
|
Non-equity incentive plan compensation – Value earned during the year ($) |
|
|
Michael (Zappy) Zapolin |
|
Nil |
|
Nil |
|
Nil |
|
|
Adnan Bashir |
|
Nil |
|
Nil |
|
|
94,848 |
|
Nitin Kaushal |
|
Nil |
|
Nil |
|
|
94,848 |
|
Dr. Eric Hoskins |
|
Nil |
|
Nil |
|
Nil |
|
|
Lawrence (Larry) Latowsky |
|
Nil |
|
Nil |
|
|
94,848 |
|
Joseph L. Romano |
|
Nil |
|
Nil |
|
Nil |
|
|
C. Board Practices
Our Board is responsible for our stewardship and strategic direction. It does not actively manage but rather supervises the management of our business and affairs to ensure a consistent focus on increasing shareholder value. In exercising their powers and discharging their duties, our directors shall (a) act honestly and in good faith with a view to the best interests of the Company; and (b) exercise the care, diligence, and skill that a reasonably prudent person would exercise in comparable circumstances.
Board
Our Board currently consists of seven members. The Board is responsible for the stewardship of the Company and for the supervision of management to protect Shareholder interests. The Board oversees the development of the Company’s strategic plan and the ability of management to continue to deliver on the corporate objectives.
The independent judgment of the Board in carrying out its responsibilities is the responsibility of all directors. The Board facilitates independent supervision of management through meetings of the Board and through frequent informal discussions among independent members of the Board and management. In addition, the Board has free access to the Company’s external auditors, external legal counsel and to the Company’s officers.
The Board is responsible for assessing the effectiveness of the Board as a whole, the committees of the Board and the contribution of individual directors. Each Board member has considerable experience in the guidance and management of public companies and the Board has found this has been sufficient to meet the needs of the Company to date.
Composition and Independence of the Board
The Board is currently comprised of seven directors: Anthony Durkacz, Zeeshan Saeed, Dr. Sanjiv Chopra, Dr. Eric Hoskins, Adnan Bashir, Dr. Lakshmi P. Kotra and Michael (Zappy) Zapolin.
| 59 |
| Table of Contents |
The Board has determined that four of the seven directors, namely Dr. Sanjiv Chopra, Dr. Eric Hoskins, Adnan Bashir, and Michael (Zappy) Zapolin, have no material relationship with the Company, either directly or indirectly, which could, in the view of the Board, be reasonably expected to interfere with the exercise of such individual’s independent judgment, and are “independent” (as determined under Rule 5605(a)(2) of the Nasdaq Stock Market Rules and as such term is defined in National Instrument 52-110 - Audit Committees (“NI 52-110”)).
Anthony Durkacz, Zeeshan Saeed and Dr. Lakshmi Kotra are not “independent” (as determined under Rule 5605(a)(2) of the Nasdaq Stock Market Rules and as such term is defined in NI 52-110), as Anthony Durkacz is the Co-Executive Chairman of the Company and a holder, through a corporation he owns and controls, of Class A Shares, Zeeshan Saeed is the CEO and Co-Executive Chairman of the Company and has an interest in Class A Shares held in a trust for his economic benefit and Dr. Lakshmi Kotra is the CEO of Lucid, President of FSD Biosciences and CEO of FSD Australia.
Board Meetings
Although the independent directors do not hold regularly scheduled meetings at which non-independent directors and members of management are not in attendance, the Board has adopted the practice of following each meeting with an independent directors’ discussion. The Board ensures open and candid discussion among its independent directors by continuously monitoring situations where a conflict of interest or perceived conflict of interest with respect to a director may exist. In cases where such a conflict of interest or perceived conflict of interest is identified, it is addressed in accordance with the Business Corporations Act (Ontario) and the Board Mandate. The Board may determine that it is appropriate to hold an in-camera session excluding a director with a conflict of interest or perceived conflict of interest, or such director may consider that it is appropriate to recuse him or herself from considering and voting with respect to the matter under consideration.
The Co-Executive Chairmen of the Board, Anthony Durkacz and Zeeshan Saeed, are non-independent directors. The independent chair of the Audit Committee, Dr. Eric Hoskins, provides leadership for its independent directors. Messrs. Durkacz, Saeed, and Hoskins are responsible for encouraging open and candid discussion among the independent directors, as discussed above, as well as facilitating Board meetings.
Messrs. Durkacz, Saeed, and Hoskins collaborate when setting the agenda of Board meetings and both work with the broader Board to promote good governance and ethics in the decision-making process of the Board.
Considering the guidelines contained in National Policy 58-201 – Corporate Governance Guidelines, the Board convenes meetings, as deemed necessary, of the independent directors at which non-independent directors and members of the management are not in attendance. The Board is of the opinion that no formal leadership of independent directors is required given the size of the Board and the ability of the independent directors to convene meetings of independent directors.
The attendance record of each of the Company’s directors for Board meetings and committee meetings held during the year ended December 31, 2023 was as follows:
Name |
Board Meetings Attended/Held |
Audit Committee Meetings Attended/Held |
Compensation, Nominating and Governance Committee Meetings Attended/Held |
Disclosure Committee Meetings Attended/Held |
Anthony Durkacz |
7/7 |
- |
- |
1/1 |
Zeeshan Saeed |
7/7 |
- |
1/1 |
- |
Donal Carroll(1) |
4/4 |
- |
- |
- |
Adnan Bashir |
7/7 |
4/4 |
1/1 |
1/1 |
Nitin Kaushal |
7/7 |
4/4 |
- |
- |
Lawrence (Larry) Latowsky(1) |
4/4 |
2/2 |
1/1 |
1/1 |
Joseph Romano(1) |
4/4 |
- |
- |
- |
Dr. Lakshmi P. Kotra |
7/7 |
- |
- |
- |
Dr. Eric Hoskins(1) |
3/3 |
- |
- |
- |
Michael (Zappy) Zapolin(1) |
3/3 |
2/2 |
- |
- |
| 60 |
| Table of Contents |
Notes:
(1) |
On May 25, 2023, Donal Carroll resigned from the Board and Dr. Eric Hoskins was appointed. At the annual general and special meeting of the Company’s shareholders held on June 29, 2023, Michael (Zappy) Zapolin and Dr. Eric Hoskins were elected as directors of the Company to replace Joseph Romano and Lawrence (Larry) Latowsky. The numbers in these rows reflect attendance with respect to Board and committee meetings held in the period in 2023 during which such individual was a member of the Board. |
Board Mandate
The duties and responsibilities of the directors of the Board are to supervise the management of the business and affairs of the Company; and to act in the best interests of the Company. In discharging its mandate, the directors of the Company are responsible for the oversight and review of the development of, among other things, the following matters:
|
· |
the strategic direction of the Company; |
|
· |
identifying the principal business risks of the Company and ensuring that procedures and people are in place to appropriately manage these risks; |
|
· |
succession planning, including appointing, training, and monitoring senior management; |
|
· |
a communications policy for the Company to facilitate communications with investors and other interested parties; and |
|
· |
the integrity of the internal controls and procedures (including adequate management information systems and the oversight of the testing of internal controls) within the Company. |
The Board also has the mandate to assess the effectiveness of the Board as a whole, its committees and the contributions of individual directors. The Board discharges its responsibilities and obligations either directly or through its committees, currently consisting of the Audit Committee, Compensation, Nominating and Governance Committee and the Disclosure Committee.
Position Descriptions
There are currently no position descriptions for the Co-Chairmen of the Corporation and the Chair of each committee. The persons acting as chairs of Board committees have the experience and expertise necessary to assess the role they must play in the context of a public company. The Chairmen and the Chair of each committee presides over all meetings (of the Board or committee, as applicable), participates in the development of meeting calendars and agenda, and ensures the orderly and efficient use of time in the meetings. Each committee chair reports to the Board on a regular basis. The primary role for each is to lead the Board and its committees in fulfilling the duties set out in their charters and/or mandates.
There are also no position descriptions in place for each of the CEO, CFO or COO, respectively. Their roles and responsibilities are delineated through the involvement of the Board in the Corporation’s affairs and the ongoing formal and informal communication between the Board and the CEO, CFO and COO, respectively.
Feedback Policy
There are no formal measures in place for receiving feedback from Shareholders.
Orientation and Continuing Education
New directors are provided orientations which include meetings with management on business directions, operational issues, and financial aspects of the Company.
The Compensation, Nominating and Governance Committee ensures that new directors receive orientation materials describing the Company’s business and its corporate governance policies and procedures. New directors will have meetings with the Co-Executive Chairmen of the Board and CEO, and with the CFO, and are expected to visit the Company’s principal offices. The Compensation, Nominating and Governance Committee is responsible for confirming that procedures are in place and resources are made available to provide directors with appropriate continuing education opportunities.
Management updates the Board on a regular basis regarding the business and activities of the Company to ensure that the directors have the necessary knowledge to meet their obligations as directors. Directors are encouraged to communicate with management, the auditors and the Company’s legal counsel to keep themselves current with the Company’s business. Directors are also provided with full access to the Company’s records.
Ethical Business Conduct
All Board members and employees are committed to maintaining the highest standards of integrity and ethical business conduct in the management of the Company and their interaction with all key Shareholders. These standards can only be achieved by the Company by adhering to the values and principles of conduct.
The Company expects all Board members and employees to conduct themselves in an ethical and law-abiding manner, in all areas, including but not limited to conflicts of interest and the protection and proper use of corporate assets, information and opportunities.
The Board has adopted the Code, which provides guidelines surrounding, among other items, compliance with applicable laws, conflicts of interest, certain opportunities, confidentiality and disclosure, employment practices, and use of company property and resources. The Code is available on the Company’s SEDAR+ profile accessible at www.sedarplus.ca and the Company’s website, https://fsdpharma.com/for-investors/.
| 61 |
| Table of Contents |
The Board has delegated responsibility for monitoring compliance with the Code and for investigating and enforcing matters related to the Code to management, who will report breaches of the Code to the Company’s general counsel or human resources.
Directors are required by applicable law and the Code to promptly disclose any potential conflict of interest that may arise. If a director has a material interest in an agreement or transaction, applicable law, the Code, and principles of sound corporate governance require them to declare the interest in writing or request to have such interest entered in the minutes of meetings of directors and, where required by applicable law, abstain from voting with respect to the agreement or transaction.
Conflicts of Interest
When faced with a conflict, it is required that business judgment of responsible persons, uninfluenced by considerations other than the best interests of the Company, will be exercised in compliance with the guidelines set out in the Code. Pursuant to the OBCA, any officer or director of the Company with a conflict of interest must disclose the nature and extent of such conflict to the Board and recuse themselves from a matter that materially conflicts with that individual’s duty as a director or senior officer of the Company.
Protection and Proper Use of Corporate Assets, Information and Opportunities
Confidential information is not to be used for any purposes other than those of the Company. This requirement of confidentiality extends beyond the duty not to discuss private information, whether about the Company and/or its management and also applies to any asset of the Company, including trade secrets, patient, supplier or customer lists, business plans, computer software, company records and other proprietary information. The Code adopted by the Board provides for certain specific guidelines around the duty of confidentiality of employees, officers, and directors of the Company.
In the situation of contracts with third parties such as suppliers and service providers, management is to share only that information which is needed to satisfy the conditions of the contract and only to those individuals who need to know.
The duty of confidentiality applies to all Board members and employees even after leaving the Company regardless of the reason for departing.
Compliance with Laws, Rules, and Regulations
It is required that the Company is in compliance with all legislation applicable to the Company’s business operations, including but not restricted to the laws of the Province of Ontario, all Canadian provincial laws and legislation, and any other similar legislation in jurisdictions where the Company operates.
All Board members and employees have a duty to know, understand and comply with any specific legislation pertaining to the business of the Company and any legislation applicable to their duties and responsibilities.
The Board has adopted the Code which provides guidelines surrounding, among other items, compliance with applicable laws, conflicts of interest, certain opportunities, confidentiality and disclosure, employment practices, and use of company property and resources.
Board Committees
Audit Committee
The Board is responsible for reviewing and approving the unaudited interim financial statements, and annual audited financial statements, together with other financial information of the Company and for ensuring that Management fulfills its financial reporting responsibilities. The Audit Committee assists the Board in fulfilling this responsibility. The Audit Committee meets with management to review the financial reporting process, unaudited interim financial statements, and annual audited financial statements, together with other financial information of the Company. The Audit Committee reports its findings to the Board for its consideration in approving the unaudited interim financial statements, and annual audited financial statements, together with other financial information of the Company for issuance to the Shareholders.
The Audit Committee meets regularly on at least a quarterly basis. The members of the Audit Committee do not have fixed terms and are appointed and replaced from time to time by resolution of the Board.
Pursuant to NI 52-110, the Audit Committee is required to have a charter, which is incorporated by reference hereto as Exhibit 99.1.
Composition of the Audit Committee
| 62 |
| Table of Contents |
As of the date of this Annual Report, the following are the members of the Audit Committee:
Name |
|
Independent(1) |
|
Financially Literate(2) |
Dr. Eric Hoskins |
|
Yes |
|
Yes |
Michael (Zappy) Zapolin |
|
Yes |
|
Yes |
Adnan Bashir |
|
Yes |
|
Yes |
Notes:
|
1. |
Within the meaning of subsection 1.4 of NI 52-110 and as determined under Exchange Act Rule 10A-3 and Rule 5605(a)(2) of the Nasdaq Stock Market Rules. |
|
2. |
Within the meaning of subsection 1.6 of NI 52-110. |
Relevant Education and Experience
The Board believes that the composition of the Audit Committee reflects financial literacy and expertise. Currently, all members of the Audit Committee have been determined by the Board to be “independent” and “financially literate” (as determined under Rule 5605(a)(2) of the Nasdaq Stock Market Rules and as such terms are defined under NI 52-110). The Board has made these determinations based on the education as well as breadth and depth of experience of each member of the Audit Committee.
All the members of the Audit Committee have the education and/or practical experience required to understand and evaluate financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to the breadth and complexity of issues that can reasonably be expected to be raised by the Company’s financial statements. For more information related to the experience of each of the members of the Audit Committee, see “Item 6.A. Directors and Senior Management”.
Audit Committee Oversight
At no time since the commencement of the Company’s most recently completed fiscal year was a recommendation of the Audit Committee to nominate or compensate an external auditor not adopted by the Board.
Reliance on Certain Exemptions
At no time since the commencement of the Company’s most recently completed fiscal year has the Company relied on an exemption from the provisions of NI 52-110.
Pre-Approval Policies and Procedures
The Audit Committee pre-approves all audit services to be provided to the Company by its independent auditors. Non-audit services that are prohibited to be provided to the Company by its independent auditors may not be pre-approved. In addition, prior to the granting of any pre-approval, the Audit Committee must be satisfied that the performance of the services in question will not compromise the independence of the independent auditors. All non-audit services performed by the Company’s auditor for the fiscal year ended December 31, 2023 were pre-approved by the Audit Committee. No non-audit services were approved pursuant to the de minimis exemption to the pre-approval requirement set forth in Rule 2-01(c)(7)(i)(C) of Regulation S-X.
External Auditor Service Fees
Please see, “Item 16.C. Principal Accountant Fees and Services”.
Compensation, Nominating and Governance Committee
Nomination of Directors
The Compensation, Nominating and Governance Committee is currently comprised of three directors of the Company: Dr. Eric Hoskins (Chair), Zeeshan Saeed and Adnan Bashir and. Dr. Eric Hoskins and Adnan Bashir are considered to be “independent” (as determined under Rule 5605(a)(2) of the Nasdaq Stock Market Rules and as such term is defined under NI 52-110).
The Compensation, Nominating and Governance Committee is responsible for recommending to the Board a list of candidates for nomination for election to the Board at each annual meeting of Shareholders. In addition, as the need arises, it will identify and recommend to the Board new candidates for Board membership. The Compensation, Nominating and Governance Committee selects potential directors with the desired background and qualifications, taking into account the needs of the Board at the time. In making its recommendations, the Compensation, Nominating and Governance Committee considers whether each candidate is or would be “independent” and “financially literate” (as determined under Rule 5605(a)(2) of the Nasdaq Stock Market Rules and as such terms are defined under NI 52-110) and possesses the competencies and skills that: (i) are considered to be necessary for the Board, as a whole, to possess; (ii) are considered to be necessary for each existing director to possess; and (iii) each new nominee will bring to the boardroom. The Compensation, Nominating and Governance Committee also considers whether or not each new nominee can devote sufficient time and resources to his or her duties as a Board member.
The Compensation, Nominating and Governance Committee is also responsible for examining the size of the Board and recommending to the Board a size that facilitates effective decision making, reviewing the overall composition of the Board, taking into consideration factors such as business experience, areas of expertise and competency of each director and evaluating whether the necessary and appropriate committees exist to support the work of the Board.
| 63 |
| Table of Contents |
Compensation
The Compensation, Nominating and Governance Committee is also responsible for determining the compensation for the directors and the executive officers. The Compensation, Nominating and Governance Committee reviews the adequacy of remuneration for the executive officers by evaluating their performance in light of the Company’s goals and objectives, and by comparing with other reporting issuers of similar size in the same industry.
The Compensation, Nominating and Governance Committee also periodically reviews the adequacy and form of directors’ compensation and recommends to the Board a compensation model that appropriately compensates directors for the responsibilities and risks involved in being a director and a member of one or more committees, as applicable. The Compensation, Nominating and Governance Committee is also responsible for reviewing the executive compensation disclosure before the Company discloses this information publicly.
The Compensation, Nominating and Governance Committee is also responsible for: (i) ensuring that the mission and strategic direction of the Company is reviewed annually; (ii) ensuring that the Board and each of its committees carry out its functions in accordance with due process; (iii) assessing the effectiveness of the Board as a whole, each committee of the Board, and the contribution of each individual director; (iv) identifying, recruiting, endorsing, appointing, and orienting new directors; (v) reviewing and making compensation related recommendations and determinations regarding senior executives and directors; and (vi) the Company’s human resources and compensation policies and processes.
Governance
The Compensation, Nominating and Governance Committee is also responsible for, among other things: (i) assisting the Company and the Board in fulfilling their respective corporate governance responsibilities under applicable securities laws, instruments, rules and mandatory policies and regulatory requirements; (ii) promoting a culture of integrity throughout the Company; and (iii) developing the Company’s approach to governance issues and establishing sound corporate governance practices that are in the interests of shareholders and that contribute to effective and efficient decision-making.
Disclosure Committee
The Disclosure Committee is currently comprised of three directors of the Company: Michael (Zappy) Zapolin (Chair), Anthony Durkacz and Adnan Bashir. Michael (Zappy) Zapolin and Adnan Bashir are considered to be “independent” (as determined under Rule 5605(a)(2) of the Nasdaq Stock Market Rules and as such term is defined under NI 52-110).
The Disclosure Committee is responsible for, among other things, ensuring that the Company complies with its timely continuous disclosure obligations, overseeing and monitoring compliance with the Company’s disclosure policies, guidelines, and procedures, promoting effective communication and preserving confidentiality of material information.
Assessments
The Board and its individual directors are assessed on an informal basis continually as to their effectiveness and contribution. The Board encourages discussion among members as to evaluation of its effectiveness as a whole, of each individual director and each of its committees. The Board does not consider that formal assessments would be useful at this stage of the Company’s development. To assist in its review, the Board may conduct informal surveys of its directors. In addition, the Board works closely with management and, accordingly, are in a position to assess individual director’s performance on an ongoing basis.
Director Term Limits and Other Mechanisms of Board Renewal
The Board has not adopted a term limit for directors and, as part of the Board’s assessment process, the Board considers the benefit of renewal among directors in the context of the needs of the Board from time to time. In light of the nature of the industry in which the Company operates, the Board does not believe that adopting a term limit for directors is necessary or appropriate at this time.
Policies Regarding the Representation of Women on the Board
The Company does not have a written policy relating to the identification and nomination of women directors. When considering and recommending qualified director nominees, the Compensation, Nominating and Governance Committee evaluates all candidates on their skills and experience in the context of what the Board as a whole requires to be effective, taking the background and diversity, including gender, of all directors and nominees into consideration.
| 64 |
| Table of Contents |
Consideration of the Representation of Women in the Director Identification and Selection Process
The Compensation, Nominating and Governance Committee goes through a rigorous process when considering a director nominee, including an evaluation of the skills and experience of the current directors, determining the gaps in skills and experience that exist and finding potential candidates to fill those gaps and round out the skills and experience of the Board as a whole. Diversity (including the representation of women on the Board and in executive officer positions) is a factor considered in determining the optimal composition of the Board. The final recommendation for nomination or appointment to the Board has been based on the best combination of skills and experience for the position, with due regard for the benefits of diversity on the Board.
Consideration Given to the Representation of Women in Executive Officer Appointments
The Board encourages the consideration of women who have the necessary skills, knowledge, experience, and character when considering new potential candidates for executive officer positions.
Issuer’s Targets Regarding the Representation of Women on the Board and in Executive Officer Positions
The Board does not have specific targets in respect of appointing women to the Board and in respect of executive officer appointments.
Number of Women on the Board and in Executive Officer Positions
As of the date of this Annual Report there are no women on the Board or in executive officer positions (0%).
D. Employees
As of December 31, 2023, we had 8 full-time employees. None of our employees are represented by collective bargaining agreements. We believe that we maintain good relations with our employees. At each date shown, we had the following number of full-time employees, broken out by function.
|
|
December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Function: |
|
|
|
|
|
|
||||||
Research and development |
|
|
3 |
|
|
|
12 |
|
|
|
4 |
|
General and administrative |
|
|
5 |
|
|
|
5 |
|
|
|
6 |
|
Total |
|
|
8 |
|
|
|
17 |
|
|
|
10 |
|
E. Share Ownership.
For information regarding the share ownership of our directors and executive officers, see “Item 6.B.-Compensation” and “Item 7.A.-Major Shareholders.”
F. Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation.
Not applicable.
Item 7. Major Shareholders and Related Party Transactions.
A. Major Shareholders
The following table provides information with respect to the beneficial ownership of our Class A Shares and Class B Shares as of March 28, 2024:
|
· |
each person, or group of affiliated persons, known by us to beneficially own five percent (5%) or more of any class of our shares; |
|
· |
each of our NEOs; |
|
· |
each of our directors; and |
|
· |
all of our directors and executive officers as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and include shares that can be acquired within 60 days of March 28, 2024. The percentage ownership information shown in the table is based upon 72 Class A Shares and 40,429,569 Class B Shares outstanding as of March 28, 2024. Each Class A Share is convertible into one Class B Share at the option of the Class A Share holder. Class A Shares have 276,660 votes per share and Class B Shares have one vote per share. For further information regarding the voting rights of the Class A Shares and the Class B Shares, see Exhibit 2, “Description of Securities”.
Except as otherwise indicated, all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. The information is not necessarily indicative of beneficial ownership for any other purpose.
| 65 |
| Table of Contents |
In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we deemed outstanding shares subject to Options and Warrants held by that person that are immediately exercisable or exercisable within 60 days of March 28, 2024. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person. The information in the table below is based on information known to us or ascertained by us from public filings made by the shareholders. Except as otherwise indicated, addresses of the directors, executive officers and named beneficial owners are in the care of FSD at 199 Bay Street, Suite 4000, Toronto, Ontario M5L 1A9.
For additional information regarding the Options reported in the following table, see “Item 6. Directors, Senior Management and Employees - B. Compensation”.
|
|
Class A Shares(1) |
|
Class B Shares |
|
Total Voting Power |
|||||||||
Name of Beneficial Owner |
|
Number |
|
|
Percent |
|
Number |
|
|
Percent |
|
Percent |
|||
|
|
|
|
|
|
|
|
||||||||
Five Percent or Greater Holders: |
|
|
|
|
|
|
|
||||||||
Rehan Saeed(3) |
|
|
36 |
|
|
50% |
|
|
731,086 |
|
|
1.8% |
|
17.7% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Current Directors and Named Executive Officers: |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Anthony Durkacz Co-Executive Chairman and Director(4) |
|
|
36 |
|
|
50% |
|
|
1,623,165 |
|
|
4.0% |
|
19.2% |
|
|
Zeeshan Saeed CEO, President, Co-Executive Chairman and Director(5) |
|
|
36 |
|
|
50% |
|
|
2,741,146 |
|
|
6.8% |
|
21.0% |
|
|
Nathan Coyle CFO(6) |
|
|
|
|
0% |
|
|
32,500 |
|
|
0.1% |
|
0.1% |
||
|
Donal Carroll COO(7) |
|
|
|
|
0% |
|
|
973,268 |
|
|
2.4% |
|
1.6% |
||
|
Adnan Bashir Director(8) |
|
|
|
|
0% |
|
|
9,393 |
|
|
0.0% |
|
0.0% |
||
|
Dr. Eric Hoskins Director |
|
|
|
|
0% |
|
|
- |
|
|
0.0% |
|
0.0% |
||
|
Dr. Sanjiv Chopra Director |
|
|
|
|
0% |
|
|
- |
|
|
0.0% |
|
0.0% |
||
|
Michael (Zappy) Zapolin Director |
|
|
|
|
0% |
|
|
- |
|
|
0.0% |
|
0.0% |
||
|
Dr. Lakshmi P. Kotra Director, CEO of Lucid, President of FSD Biosciences and CEO of FSD Australia (9) |
|
|
|
|
0% |
|
|
1,922,197 |
|
|
4.8% |
|
3.2% |
||
All current directors and executive officers (9 individuals) |
|
|
72 |
|
|
100% |
|
|
7,301,669 |
|
|
18.1% |
|
45.1% |
|
Notes:
(1) |
Class A Shares have 276,660 votes per share. |
(2) |
The reported number of Class A Shares consists of shares held by Mr. Bokhari. The reported number of Class B Shares consists of 24 Class B Shares issuable upon conversion of Class A Shares. |
(3) |
The reported number of Class A Shares consists of shares held by the Xorax Family Trust (“Xorax”), as to which Mr. Rehan Saeed, the trustee of Xorax, has shared voting and dispositive power (and which such Class A Shares are held for the benefit of Mr. Zeeshan Saeed). The reported number of Class B Shares consists of (a) 40,055 outstanding Class B Shares held by Mr. Rehan Saeed, and (b) the following shares as to which Mr. Rehan Saeed has shared voting and dispositive power: (i) 441,031 outstanding Class B Shares held by Xorax, (ii) 24 Class B Shares issuable upon conversion of Class A Shares held by Xorax, (iii) 100,000 Class B Shares issuable upon exercise of outstanding Warrants held by Legacy Family Trust (“Legacy”), of which Mr. Rehan Saeed is the trustee, exercisable within 60 days of March 28, 2024 and (iv) 150,000 outstanding Class B Shares held by Legacy. |
(4) |
The reported number of Class A Shares consists of shares held by Fortius Research and Trading Corporation (“Fortius”), as to which Mr. Durkacz, who controls Fortius, has shared voting and dispositive power. The reported number of Class B Shares consists of (a) the following shares as to which Mr. Durkacz has sole voting and dispositive power: (i) 975,122 outstanding Class B Shares and (ii) 500,000 Class B Shares issuable upon exercise of outstanding Options exercisable within 60 days of March 28, 2024 and (b) the following shares as to which Mr. Durkacz has shared voting and dispositive power: (i) 106,043 outstanding Class B Shares held by Fortius, (ii) 24 Class B Shares issuable upon conversion of Class A Shares held by Fortius and (iii) 42,000 outstanding Class B Shares held by Jacqueline Burns, the spouse of Mr. Durkacz. The reported number of Class B Shares does not include 373,671 outstanding Class B Shares held by First Republic, of which Mr. Durkacz is a director, Executive Vice President and majority shareholder. Mr. Durkacz does not have or share voting or investment power over the Class B Shares held by First Republic. |
| 66 |
| Table of Contents |
(5) |
The reported number of Class A Shares consists of 36 Class A Shares held by Xorax, of which Mr. Zeeshan Saeed has shared voting and dispositive power. The reported number of Class B Shares consists of the following shares as to which Mr. Zeeshan Saeed (a) has sole voting and dispositive power: (i) 1,800,115 outstanding Class B Shares and (ii) 500,000 Class B Shares issuable upon exercise of outstanding Options exercisable within 60 days of March 28, 2024 and (b) has shared voting and dispositive power: (i) 36 Class B Shares issuable upon conversion of Class A Shares held by Xorax and (ii) 441,031 outstanding Class B Shares held by Xorax. |
(6) |
The reported number of Class B Shares consists of the following shares as to which Mr. Nathan Coyle has sole voting and dispositive power: (a) 2,500 outstanding Class B Shares and (b) 35,000 Class B Shares issuable upon exercise of outstanding Options exercisable within 60 days of March 28, 2024 as to which Mr. Nathan Coyle has sole voting and dispositive power. |
(7) |
The reported number of Class B Shares consists of (a) 971,268 outstanding Class B Shares and (b) 500,000 Class B Shares issuable upon exercise of outstanding Options exercisable within 60 days of March 28, 2024. |
(8) |
The reported number of Class B Shares consists of (a) 9,200 outstanding Class B Shares as to which Mr. Adnan Bashir has sole voting and dispositive power, (b) 98 outstanding Class B Shares held by 58 Northwest, a corporation controlled by Mr. Bashir, and (c) 95 outstanding Class B Shares held by TFSA, a corporation controlled by Mr. Bashir. |
(9) |
The reported number of Class B Shares consists of the following shares as to which Dr. Lakshmi Kotra has sole voting and dispositive power: (a) 515,797 outstanding Class B Shares, (b) 865,200 outstanding Class B Shares held by ILace Therapeutics International Inc., a corporation controlled by Dr. Kotra, (c) 41,200 outstanding Class B Shares held by Kotra Trust, a trust of which Dr. Kotra is the trustee, and (d) 500,000 Class B Shares issuable upon exercise of outstanding Options exercisable within 60 days of March 28, 2024. |
|
(12)
|
As noted above in Notes 3 and 5 to this chart, the 36 Class A Shares and 441,031 of the Class B Shares reported under Mr. Rehan Saeed and Mr. Zeeshan Saeed’s shareholdings represent the same Class A Shares and Class B Shares, which are held by Xorax, of which Mr. Rehan Saeed and Mr. Zeeshan Saeed have shared voting and dispositive power. |
As of March 28, 2024, we estimate that approximately 53.7% of our outstanding Class B Shares, which equates to 21,720,976 Class B Shares, were held in the United States by 34 holders of record. This represents 36% of the voting control of the Company. The number of holders of record does not include beneficial owners whose Class B Shares are held in street name by brokers and other nominees. The number of holders of record also does not include holders whose shares may be held in trust by other entities.
B. Related Party Transactions
Since January 1, 2023, we have engaged in the following transactions with our related parties. For this purpose, our related parties include (a) enterprises that directly or indirectly control or are controlled by, or are under common control with, us; (b) our associates; (c) shareholders beneficially owning 10% or more of our voting power and other individuals with significant influence over us, and close members of any such individual’s family; (d) our directors and executive officers, and close members of their families; and (e) enterprises in which a substantial interest in the voting power is owned, directly or indirectly, by any person described in (c) or (d) or over which such a person is able to exercise significant influence. Our related parties include enterprises owned by directors or major shareholders and enterprises that have a member of key management in common with us. All of the transactions have been reviewed and approved by our Board or another independent committee of the Board.
Transactions with Celly Nu
For more information on the Celly Nu IP License Agreement, Celly Nu Loan Agreement, Celly Nu Security Agreement and Plan of Arrangement, please see “Item 4. Information on the Company. A. History and Development of the Company Overview and History”.
For accounting purposes, the Company determined that it obtained control of Celly Nu on July 31, 2023, and control was maintained at all times from July 31, 2023, through December 31, 2023. Celly Nu is significantly dependent on the Company as a result of the IP License Agreement and Celly Nu Loan Agreement. The Company concluded it has control of Celly Nu, as the Company, together with persons or entities considered to be de facto agents of the Company, hold a combined 52.05% of the voting rights of Celly Nu as of December 31, 2023. In addition, key management personnel of the Company hold three of the four board of director positions of Celly Nu. The assessment of control is performed on a continuous basis.
Mortgage Loan to CEO
On April 17, 2023, FSD Strategic Investments entered into the CEO Mortgage Loan with the CEO. The CEO Mortgage Loan matures on April 26, 2025, and is part of FSD Strategic Investments’ portfolio of loans. As of December 31, 2023, the amount due on the CEO Mortgage Loan was C$1,200,000. The business purpose of the CEO Mortgage Loan was a treasury function to earn a rate of return on excess capital held.
FSD Strategic Investments secures each mortgage loan with collateral in the residential property at least equal to 55% of the value of the loan. In the case of the CEO Mortgage Loan, although all the other loans under FSD Strategic Investments’ portfolio of loans prior to this one were a first charge mortgage on the underlying residential property, the CEO Mortgage Loan was a second charge mortgage. FSD Strategic Investments determined that because the CEO Mortgage Loan met its strict underwriting requirements and had an undertaking requirement (as described herein), it would take a second charge on the residential property. The CEO’s residential property had a Home Equity Line of Credit (“HELOC”), of which no funds have been withdrawn against the property. FSD Strategic Investments viewed this as appropriate security as the HELOC had a zero balance and the CEO undertook to FSD Strategic Investment that the HELOC would only be used by the CEO to pay the CEO Mortgage Loan. FSD Strategic Investments determined that this undertaking by the CEO to use the HELOC funds for payment of the CO Mortgage Loan provided sufficient security under the CEO Mortgage Loan. In addition, the CEO met the underwriting requirements of FSD Strategic Investments.
| 67 |
| Table of Contents |
The CEO Mortgage Loan was made to the CEO on the same terms and conditions that a member of the general public would receive, based on the underwriting requirements of FSD Strategic Investments. In determining the interest rate for each borrower under its portfolio of loans, FSD Strategic Investments took the following criteria into consideration: (i) their credit score; (ii) employment status; (iii) salary; (iv) property location; (v) equity on the property; and (vi) the Bank of Canada rate at the time of the mortgage. In the case of the CEO Mortgage Loan, although all the other loans under FSD Strategic Investments’ portfolio of loans, are a first charge mortgage on the underlying residential property, his was a second charge. The Company determined that because the CEO Mortgage Loan was made to an individual with a salary that is more than double that of the other borrowers, with a very clear picture of longevity, the exception was made as the first charge on his residential property was for a HELOC which no funds have been withdrawn against. The Company viewed this as appropriate security as the HELOC had a zero balance and the borrower undertook to the Company that the HELOC will not be used unless for payment towards the Company’s second mortgage.
Before the Board approved the Mortgage Loan, FSD Strategic Investments sought the advice of counsel to guide the Board with its decision in the CEO Mortgage Loan transaction. Under Section 13(k) of the Exchange Act, an issuer, who is in the consumer credit business, can provide loans to its executive officers and directors that (i) are made in the ordinary course of the issuer’s consumer credit business, (ii) are of a type that is generally made available by the issuer to the public and (iii) made on market terms that are no more favorable than those offered by the issuer to the general public. “Consumer credit” generally includes credit extended to an individual primarily for personal, family or household purposes. A standard loan made to an individual to purchase his or her primary residence should normally fall within the definition of consumer credit.
The Company and FSD Strategic Investments believe that the CEO Mortgage Loan complies with this exemption in that FSD Strategic Investments is in the business or providing mortgage loans and the CEO Mortgage Loan was made in the ordinary course of business, is of a type generally made available by it to the public and is made on market terms to the CEO that are no more favorable than those offered by it to the general public. However, there is limited regulatory and legislative guidance regarding the scope of this exemption and FSD Strategic Investments cannot provide assurances that it in full compliance with United States laws. Any failure to comply with any laws and regulations, may adversely affect its business, and results of operations” See “Item 3. Key information - D. Risk Factors Failure to comply with laws and regulations could have a material adverse effect on the Company's business”.
With respect to Canadian law, in completing the CEO Mortgage Loan, the Company relied on exemptions from the formal valuation and minority approval requirements of MI 61-101 on the basis that at the time the CEO Mortgage Loan was agreed to, neither the fair market value of the CEO Mortgage Loan, nor the fair market value of the consideration for the CEO Mortgage Loan, insofar as it involved the interested party, exceeded 25% of the Company’s market capitalization, as determined in accordance with MI 61-101.
December Class A Share Private Placement
Effective December 4, 2023, the Corporation closed the December Class A Private Placement.
Xorax Family Trust, a trust of which Zeeshan Saeed, the CEO and Co-Chairman of FSD Pharma is a beneficiary, and Fortius Research and Trading Corp., a corporation of which Anthony Durkacz, a director of FSD is a director, purchased all of the Class A Shares issued pursuant to the December Class A Private Placement. The participation by such insiders is considered a “related-party transaction” within the meaning of MI 61-101. The Corporation relied on exemptions from the formal valuation and minority shareholder approval requirements of MI 61-101 contained in respectively, sections 5.5(a) and 5.7(1)(a) of MI 61-101 in respect of related party participation in the December Class A Private Placement as neither the fair market value (as determined under MI 61-101) of the subject matter of, nor the fair market value of the consideration for, the transaction, insofar as it involved the related parties, exceeded 25% of the Corporation’s market capitalization (as determined under MI 61-101). The Corporation did not file a material change report more than 21 days before the expected closing of the December Class A Private Placement because the details of the participation therein by related parties to the Corporation were not settled until shortly prior to the closing, and the Corporation wished to close on an expedited basis for business reasons.
Directors and Officers Liability Insurance
We maintain directors’ and officers’ liability insurance policies for the liability of our directors and officers arising out of the performance of their duties and for our liability arising out of securities claims. The policies provide coverage in respect of a maximum total liability of C$3,000,000, and includes specific exclusions described in the policy.
D&O Indemnification Agreements
See “Item 6.B. Compensation - Indemnification” for details.
Lucid Acquisition
See “Item 4.A. History and Development of the Company-General Development of the Business - Three Year History-Lucid Acquisition” for further details.
C. Interests of Experts and Counsel
Not applicable.
Item 8. Financial Information
A. Consolidated Statements and Other Financial Information
Consolidated Financial Statements
Our 2023 Annual Financial Statements are appended at the end of this Annual Report, starting at page F-1, and incorporated herein by reference.
Dividend Distribution Policy
In connection with the Plan of Distribution, the Company distributed an aggregate of approximately 45,712,529 Celly Nu Shares to its shareholders. As of December 31, 2023, the Company owns 26.15% Celly Nu Shares on a non-diluted basis.
| 68 |
| Table of Contents |
Except for the Celly Nu shares distributed in the Plan of Arrangement, the Company has not paid any dividends or distributions on its outstanding Class B Shares, and we have no current intention to declare dividends on our Class B Shares in the foreseeable future. Any decision to pay dividends on our Class B Shares in the future will be at the discretion of our Board and will depend on, among other things, our results of operations, current and anticipated cash requirements and surplus, financial condition, any future contractual restrictions and financing agreement covenants, solvency tests imposed by corporate law and other factors that our Board may deem relevant.
Legal Proceedings
The Company is engaged in certain legal proceedings, as further described below. Litigation has been, and is expected to be, costly and time-consuming and could divert the attention of management and key personnel from our business operations. Although we intend to vigorously defend ourselves against any pending claims, and future claims that may occur, we cannot assure that we will succeed in defending any of these claims and that the judgments will not be upheld against us. If we are unsuccessful in our defense of these claims or unable to settle the claims in a manner satisfactory to us, we may be faced with outcomes that could have a material adverse effect on the Company and its financial condition. Except as otherwise disclosed below, there are no material outstanding legal proceedings or regulatory actions to which the Company is party, nor, to Company’s knowledge, are there any such proceedings or actions contemplated.
GBB Drink Lab Litigation
On May 12, 2023, the Corporation announced receipt of a lawsuit filed in the United States District Court for the Southern District of Florida (the “U.S. District Court”) by GBB against the Corporation, alleging breach of a mutual non-disclosure agreement and misappropriation of trade secrets, valued, as of August 30, 2022 (prior to the misappropriation and material breach) at US$53,047,000. The Corporation believes the allegations are without merit and intends to defend itself in the lawsuit. On June 23, 2023, the Corporation filed a motion to dismiss the complaint. On July 3, 2023, GBB responded in opposition to the Corporation’s motion to dismiss the complaint. The Motion to Dismiss the Amended Complaint filed on June 23, 2023 has been fully briefed and is awaiting adjudication by the U.S. District Court. In the meantime, on August 24, 2023, the parties filed a proposed joint scheduling report with the U.S. District Court, which set forth various deadlines that would govern this action. Under the proposed joint schedule, which still needs to be approved by the U.S. District Court, the case would be trial-ready by November 30, 2024.
Dr. Raza Bokhari
Following the contested meeting held on, the former CEO, Dr. Raza Bokhari commenced five actions against the Company and management, which resulted in counterclaims and additional unexpected legal and operating expenses. As at March 28, 2024, the status of the matters is as follows:
Wrongful Dismissal Arbitration
On July 15, 2021, the Corporation’s former CEO, Dr. Raza Bokhari, filed an arbitration notice seeking C$30.2 million for breach of contract, severance, and damages, along with C$500,000 for punitive damages and legal fees. Dr. Bokhari had been placed on administrative leave after the May 14, 2021 shareholder meeting and was terminated for cause on July 27, 2021, following an investigation by a special committee of the Board. The Company defended the arbitration and counterclaimed against Dr. Bokhari for reimbursement of expenses he directed the Company to pay to himself, as well as losses the Company sustained as a result of Dr. Bokhari’s decision to authorize a series of dilutive share issuances.
The arbitration concluded in August 2022. In its 174 page arbitral award, the arbitrator dismissed Dr. Bokhari’s claims in their entirety. The arbitrator also ordered Dr. Bokhari to repay certain monies to the Company in respect of the Company’s counterclaim, while also awarding the Company its costs of the arbitration which he subsequently fixed at approximately C$2.8 million, plus interest.
On December 9, 2022, Dr. Bokhari sought to set aside the award, citing unfair treatment and inadequate reasoning. On October 4, 2023, the Company announced that the Ontario Superior Court of Justice had dismissed Dr. Bokhari’s motion to set aside the arbitration award. Dr. Bokhari was required to put up C$150,000 as security for costs before the motion was heard, which he has forfeited. In addition, Dr. Bokhari was ordered to pay C$175,000 to cover the Company’s legal costs for his failed set aside motion.
On October 13, 2023, Dr. Bokhari served notices of motion on the Company for leave to appeal the set aside and enforcement orders issued by the Ontario Superior Court of Justice’s on October 4, 2023. On December 1, 2023, the Company filed a petition to confirm the arbitration award in the United States District Court for Eastern District of Pennsylvania. On December 15, 2023, the Company submitted a responding party’s factum to the Court of Appeal for Ontario. On February 6, 2024, the Company announced that the Ontario Superior Court of Justice affirmed judgment and awarded an additional C$5,000 in costs in light of Dr. Bokhari’s failed motion for leave to appeal. As of the date hereof, the litigation is ongoing.
| 69 |
| Table of Contents |
Restraining Order and Class B Share Cancellation Application
On January 21, 2021 and February 10, 2021, the Board authorized the issuance of an aggregate of 1,349,765 Class B Shares as share based awards to certain directors and officers of the Corporation, including Dr. Bokhari. Upon determining that 1,198,146 of these Class B Shares (the “Contested Shares”) had been inappropriately issued contrary to applicable laws, the Board resolved to cancel the Contested Shares on June 1, 2021, and later directed the Corporation’s transfer agent to cancel and return the Contested Shares to treasury. On July 2, 2021, Dr. Bokhari, filed an action against the Corporation seeking to prevent the Corporation from cancelling his portion of the Contested States. The motion was heard and denied on July 27, 2021. On July 21, 2021, the Corporation commenced a legal proceeding against Dr. Bokhari, former members of the Board, including James Datin, Robert Ciaruffoli, Stephen Buyer and Gerald Goldberg, Dr. Bokhari’s brokerages’ Haywood Securities Inc. and Haywood Securities (US) Inc., and the Corporation’s transfer agent. The Corporation made an application before the Superior Court stating that the Contested Shares were issued contrary to section 23(2) of the OBCA and validly cancelled by resolution of the Board passed on June 1, 2021. The Corporation was able to reach an agreement with all of the former directors other than Dr. Bokhari under which they did not oppose the Corporation’s application and agreed to be bound by the decision in the application, and the Corporation agreed not to seek costs against them. Neither the Corporation’s transfer agent nor any of Dr. Bokhari’s brokerages took any position on the application. On March 8, 2022, the court issued a mixed decision in the application, permitting the Contested Share grant to Dr. Bokhari until the date of his termination but cancelling 504,888 Contested Shares relating to services that were to be provided after the date of termination.
Bokhari v. FSD Pharma Inc. Et al.
On July 2, 2021, Dr Bokhari filed an action against the Corporation, FSD BioSciences, Anthony Durkacz and Zeeshan Saeed. The case was placed in civil suspense pending resolution of arbitration. Therefore, no further activity will occur in this case unless and until the aforementioned arbitration concludes. As of the date hereof, the litigation is ongoing.
Bokhari Wrongful Means Action
In June 2023, Dr. Bokhari commenced an action against the Company and FSD Biosciences by way of notice of action issued out of the Ontario Superior Court of Justice in Toronto, Canada. He subsequently filed a statement of claim, of July 7, 2023 and served the notice of action and statement of claim on a former director of the Company on December 19, 2023. The action seeks USD $1.5 million in damages for intentional interference with economic relations, misrepresentation, negligence, and other causes of action to be specified in a statement of claim. We delivered a notice of intent to defend in the action on January 5, 2024, but thus far not been required to provide a statement of defense. We believe these claims are without merit.
Bokhari Employment Claim
By way of notice of action issued on May 11, 2023, Dr. Bokhari commenced an action for damages for breach of contract against the Company in the Ontario Court of Justice in Toronto Canada. He subsequently filed a statement of claim in which he specified the claim as a claim for USD $30.2 million in damages on the basis that the Company breached his employment agreement by not providing him notice of default before terminating his employment. On November 10, 2023, the last day on which he could do so, Dr. Bokhari served the notice of action and statement of claim on an FSD director. We served a notice of intent to defend in this action on November 22, 2023 but have not been required to serve a statement of defense. We note that to the extent he wished to advance this claim, it is a claim that Dr. Bokhari should have advanced in the employment arbitration, but did not do so As such, and bearing in mind the decision the arbitrator reached in that proceeding in our view, we believe that this claim has no merit.
Cunningham Assessment Application
By notice of application dated September 26, 2023, Dr. Bokhari applied to the Ontario Superior Court of Justice for an order directing an assessment of the accounts/billing rendered by former Justice Douglas Cunningham as arbitrator in the Wrongful Dismissal Arbitration noted above. In late January 2024, Dr. Bokhari served Arbitrator Cunningham with the notice of application and a supporting affidavit sworn January 24, 2024. Under the terms of the retainer agreement between FSD, Dr. Bokhari and Arbitrator Cunningham, FSD is jointly and severally liable for any costs Arbitrator Cunningham might incur as a result of this proceeding. The liability exposure that FSD could have in this matter is approximately C$182,777.50, which represents half of the arbitration fees, plus any costs in defending the arbitrator To protect its interest, FSD has instructed its legal counsel to move to have FSD joined to the proceeding. We believe this claim is without merit.
Bokhari Indemnification Application
On November 12, 2021, Dr. Bokhari commenced an application in the Ontario Superior Court of Justice, Commercial List, seeking an order appointing an arbitrator to arbitrate his claim to be entitled to indemnification of his legal expenses associated with the litigation he commenced against FSD or in which he was named as a party by FSD. FSD denied the validity of the underlying indemnification agreement and therefore opposed the application. In April 2022, the parties agreed to allow Dr. Bokhari to adjourn the application indefinitely. Last year, Dr. Bokhari retained new counsel who indicated that it intended to pursue the application. To date that new counsel has taken no steps to do so. We believe this claim is without merit.
On April 6, 2022, Dr. Bokhari commenced an application in the Superior Court seeking an order appointing an arbitrator to arbitrate his claim to be entitled to indemnification of his legal expenses associated with the litigation he has commenced against the Corporation or in which he has been named as a defendant against the Corporation. The Corporation denied the validity of the underlying indemnification agreement and opposed the application. In April 2022, the parties agreed to adjourn the application without setting a new hearing date. As of the date hereof, the litigation is ongoing.
| 70 |
| Table of Contents |
FSD Petition against Raza Bokhari to Confirm Arbitration Award
On December 1, 2023, the Company filed a Petition to Confirm Arbitration (the “Petition”), in the Eastern District of Pennsylvania, which seeks to (a) confirm the four awards entered in an arbitration in Ontario, Canada, in favor of the Company and against former CEO Raza Bokhari and (b) enter final judgment against Bokhari in an amount in excess of C$3,000,000. The petition was filed in the U.S. District Court for the Eastern District of Pennsylvania. Bokhari filed a response on February 9, 2024. The Company filed a response thereafter and the litigation is ongoing.
Parkway Clinical Laboratories
On July 8, 2021, Parkway Clinical Laboratories, a company wholly owned by Dr. Bokhari, filed an action against the Company, which was subsequently settled following a conference between the parties on October 20, 2021.
On July 20, 2021, a shareholder of the Corporation filed a claim in the Delaware Chancery Court against the Corporation and its directors and officers seeking to remedy harm they believe the directors and officers of the Corporation have caused by their actions. The shareholder has filed the claim on count of breach of fiduciary duties and corporate waste against the directors and officers with no dollar amount being claimed. On September 13, 2021, the Corporation filed a motion to dismiss in its entirety and the motion was heard on February 8, 2022. The claim was dismissed by the court May 6, 2022.
Employment Litigation
From time to time, the Company is involved with employment litigation, in the normal course of its business.
Subsequent to the resignation of two former FSD BioSciences employees (the “Former Employees”), on July 9, 2021, the Former Employees filed a joint claim against the Corporation. On September 17, 2021, the Corporation filed a motion to dismiss the claim in its entirety. On December 13, 2021, the Court granted the Corporation’s motion and dismissed the case. One of the Former Employee’s claims was dismissed with prejudice while the other was not.
On July 18, 2023, Kevin Cassidy, a former employee sued the Company and Lucid and the Company in the Ontario Superior Court of Justice. The claim is for wrongful dismissal and the amounts claimed are C$497,000, plus additional damages, which have not been quantified, plus interest and costs. Pleadings have been exchanged and, on January 26, 2024, Cassidy’s counsel served a motion for summary judgment. The hearing date for the summary judgment motion has not been scheduled as the parties are currently in the process of discussing a timetable for all necessary steps which must be completed prior to the hearing. Once all such steps are completed, the parties will attend at court to schedule the hearing date. It is premature to estimate the likely outcome of this claim.
On October 12, 2023, Dr. Sima Salahshor, a former employee sued the Company and Lucid in the Ontario Superior Court of Justice. The claim is for wrongful dismissal and the amounts claimed are C$97,500, plus other damages, which have not been quantified, plus interest and costs. The claim was served. This matter is at the pleadings stage and we expect that the defense will be served shortly. It is premature to estimate the likely outcome of this claim.
B. Significant Changes
A discussion of the significant changes in our business can be found under “Item 4. Information on the Company—A. History and Development of the Company” and “Item 4. Information on the Company—B. Business Overview.”
Item 9. The Offer and Listing
A. Offer and Listing Details
No offering is made by this Annual Report. The Company’s Class B Shares commenced trading on the CSE on May 29, 2018 under the symbol “HUGE”. Prior to the CSE listing there was no public trading in any securities of the Company. The Class B Shares commenced trading on the Nasdaq in the United States on January 9, 2020 under the symbol “HUGE”. The Company’s Class B Shares are listed on the Frankfurt Stock Exchange under the symbol “0K9A”. Trading on the FSE market is minimal.
B. Plan of Distribution
Not applicable.
C. Markets
See Item 9.A “Offer and listing details.”
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
| 71 |
| Table of Contents |
Item 10. Additional Information
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
See Exhibit 2.1 to this Annual Report on Form 20-F for a summary of certain material provisions of our articles of incorporation, as amended; bylaws, as amended; and certain related sections of the OBCA. See Exhibit 1.1 to this Annual Report on Form 20-F for our articles of incorporation, as amended, and Exhibit 1.2 for our bylaws, as amended.
C. Material Contracts.
Except as set forth below, the material terms of our material contracts are described elsewhere in this Annual Report. Below is a a summary of certain material contracts, together with references to the relevant sections of this Annual Report where the material terms of such contracts are described. Please also see Item 19. Exhibits for a complete summary of all material contracts.
The summaries provided below and elsewhere in this Annual Report are not meant to be exhaustive and are qualified in their entirety by the full text of the relevant agreements, copies of which are filed as exhibits to this Annual Report.
Celly Nu IP License Agreement, Celly Nu Loan Agreement, Celly Nu Security Agreement and Plan of Arrangement
For more information on the Celly Nu IP License Agreement, Celly Nu Loan Agreement, Celly Nu Security Agreement and Plan of Arrangement, please see “Item 4. Information on the Company. - A. History and Development of the Company Overview and History”.
Coattail Agreement
In accordance with the rules of the CSE designed to ensure that, in the event of a take-over bid, the holders of Class B Shares will be entitled to participate on an equal footing with holders of Class A Shares, the holders of not less than 80% of the outstanding Class A Shares have entered into the coattail agreement dated May 24, 2018 among the Company, Computershare Investor Services Inc., the Company’s previous registrar and transfer agent of the Company, and certain of the shareholders holding at least 80% of the Class A Shares. The Coattail Agreement contains provisions customary for dual class, publicly traded Ontario corporations designed to prevent transactions that otherwise would deprive the holders of Class B Shares of rights under the take-over bid provisions of applicable Canadian securities legislation to which they would have been entitled if the Class A Shares had been Class B Shares.
See Exhibit 2.1, “Description of Securities,” for details.
UHN License Agreement
For more information, please see “Item 4. Information on the Company. - A. History and Development of the Company - Other Significant Operations and Principal Activities – Fiscal 2021 and 2022”.
Epitech License Agreement and Prismic Assignment Agreement
For more information, please see “Item 4. Information on the Company. – C. Organizational Structure” and Exhibits 4.9, 4.10 and 4.11..
ATM Agreement
For more information, please see “Item 4. Information on the Company. - A. History and Development of the Company - Significant Developments in Fiscal 2023 through to March 28, 2024”.
Lucid Amalgamation Agreement and Master Agreement
The Lucid Amalgamation Agreement is the amalgamation agreement dated September 20, 2021, entered into among the Company, Lucid and a wholly owned subsidiary of the Company (“Subco”) in connection with the Lucid Acquisition. Pursuant to the Lucid Amalgamation Agreement, Lucid Psycheceuticals Inc. and Subco agreed to amalgamate into Lucid.
For more information, please see “Item 4. Information on the Company. - A. History and Development of the Company - Other Significant Operations and Principal Activities – Fiscal 2021 and 2022”.
D. Exchange Controls
The Company was formed under and subject to the laws of the Province of Ontario, Canada. Subject to the next paragraph and the disclosure under “Exhibit 2.1 - Description of Securities-Competition Act” and “Exhibit 2.1 - Description of Securities - Investment Canada Act” below, there is no law or governmental decree or regulation in Canada that restricts the export or import of capital, or affects the payment of dividends or interest or other amounts to a non-resident holder of Class B Shares, other than withholding tax requirements.
| 72 |
| Table of Contents |
There is no limitation imposed by Canadian law or by the charter or other constituent documents of the Company on the right of a non-resident to hold or vote Class B Shares of the Company. However, the Competition Act (Canada) and the Investment Canada Act (Canada) have rules regarding certain acquisitions of shares by certain persons, including non-residents, along with other requirements under that legislation.
See “Item 10. Additional Information - E. Taxation” for additional information regarding the material U.S. and Canadian federal income tax consequences relating to the ownership and disposition of our Class B Shares by U.S. Holders (as defined therein).
E. Taxation
Certain Material U.S. Federal Income Tax Considerations
The following is a general summary of certain material U.S. federal income tax considerations applicable to a U.S. Holder (as defined below) arising from and relating to the acquisition, ownership, and disposition of Class B Shares acquired pursuant to the offering.
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder arising from and relating to the acquisition, ownership, and disposition of Class B Shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder, including, without limitation, specific tax consequences to a U.S. Holder under an applicable income tax treaty. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. This summary does not address the U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences to U.S. Holders of the acquisition, ownership, and disposition of Class B Shares. In addition, except as specifically set forth below, this summary does not discuss applicable tax reporting requirements. Each prospective U.S. Holder is urged to consult its own tax advisor regarding the U.S. federal income, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership, and disposition of Class B Shares.
No ruling from the Internal Revenue Service (the “IRS”) has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Class B Shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities on which this summary are based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the conclusions described in this summary.
Scope of this Summary
This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed), published rulings of the IRS, published administrative positions of the IRS, the Convention Between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the “Canada-U.S. Tax Convention”), and U.S. court decisions that are applicable, and, in each case, as in effect, as of the date of this document. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied retroactively. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation.
For purposes of this summary, the term “U.S. Holder” means a beneficial owner of Class B Shares acquired pursuant to the offering that is for U.S. federal income tax purposes:
|
· |
an individual who is a citizen or resident of the United States; |
|
· |
a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) organized under the laws of the United States, any state thereof or the District of Columbia; |
|
· |
an estate whose income is subject to U.S. federal income taxation regardless of its source; or |
|
· |
a trust that (i) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons for all substantial decisions or (ii) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
For purposes of this summary, the term “U.S. Holder” means a beneficial owner of Class B Shares acquired pursuant to the offering that is for U.S. federal income tax purposes:
| 73 |
| Table of Contents |
This summary does not address the U.S. federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to, U.S. Holders that: (a) are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) are broker-dealers, dealers, or traders in securities or currencies that elect to apply a mark-to-market accounting method; (d) have a “functional currency” other than the U.S. dollar; (e) own Class B Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other arrangement involving more than one position; (f) acquire Class B Shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) hold Class B Shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); (h) are required to accelerate the recognition of any item of gross income with respect to Class B Shares as a result of such income being recognized on an applicable financial statement; or (i) own, have owned or will own (directly, indirectly, or by attribution) 10% or more of the total combined voting power or value of the outstanding shares of the Company. This summary also does not address the U.S. federal income tax considerations applicable to U.S. Holders who are: (a) U.S. expatriates or former long-term residents of the U.S.; (b) persons that have been, are, or will be a resident or deemed to be a resident in Canada for purposes of the Tax Act; (c) persons that use or hold, will use or hold, or that are or will be deemed to use or hold Class B Shares in connection with carrying on a business in Canada; (d) persons whose Class B Shares constitute “taxable Canadian property” under the Tax Act; or (e) persons that have a permanent establishment in Canada for the purposes of the Canada-U.S. Tax Convention. U.S. Holders that are subject to special provisions under the Code, including, but not limited to, U.S. Holders described immediately above, should consult their own tax advisor regarding the U.S. federal income, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership, and disposition of Class B Shares.
If an entity or arrangement that is classified as a partnership (or other “pass-through” entity) for U.S. federal income tax purposes holds Class B Shares, the U.S. federal income tax consequences to such entity and the partners (or other owners) of such entity generally will depend on the activities of the entity and the status of such partners (or owners). This summary does not address the tax consequences to any such partner (or owner). Partners (or other owners) of entities or arrangements that are classified as partnerships or as “pass-through” entities for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership, and disposition of Class B Shares.
Ownership and Disposition of Class B Shares
The following discussion is subject in its entirety to the rules described below under the heading “Passive Foreign Investment Company Rules”.
Taxation of Distributions
A U.S. Holder that receives a distribution, including a constructive distribution, with respect to a Class B Share will be required to include the amount of such distribution in gross income as a dividend (without reduction for any foreign income tax withheld from such distribution) to the extent of the current or accumulated “earnings and profits” of the Company, as computed for U.S. federal income tax purposes. To the extent that a distribution exceeds the current and accumulated “earnings and profits” of the Company, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in the Class B Shares and thereafter capital gain to the extent of the excess over the U.S. Holder’s tax basis, Capital gain will be taxed in the manner described below at “Sale or Other Taxable Disposition of Class B Shares”. The Company may not maintain the calculations of its earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder may have to assume that any distribution by the Company with respect to the Class B Shares will constitute dividend income. Dividends received on Class B Shares by corporate U.S. Holders generally will not be eligible for the “dividends received deduction”. Subject to applicable limitations and provided the Company is eligible for the benefits of the Canada-U.S. Tax Convention or the Class B Shares are readily tradable on a United States securities market, dividends paid by the Company to non-corporate U.S. Holders, including individuals, generally will be eligible for the preferential tax rates applicable to long-term capital gains for dividends, provided certain holding period and other conditions are satisfied, including that the Company not be classified as a PFIC in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of such rules.
Sale or Other Taxable Disposition of Class B Shares
A U.S. Holder will recognize gain or loss on the sale or other taxable disposition of Class B Shares in an amount equal to the difference, if any, between (a) the amount of cash plus the fair market value of any property received and (b) such U.S. Holder’s tax basis in such Class B Shares sold or otherwise disposed of. Any such gain or loss generally will be capital gain or loss, which will be long-term capital gain or loss if, at the time of the sale or other disposition, such Class B Shares are held for more than one year.
Preferential tax rates apply to long-term capital gains of a U.S. Holder that is an individual, estate, or trust. Deductions for capital losses are subject to significant limitations under the Code.
Passive Foreign Investment Company Rules
If the Company were to constitute a PFIC for any year during a U.S. Holder’s holding period, then certain potentially adverse rules would affect the U.S. federal income tax consequences to a U.S. Holder resulting from the acquisition, ownership, and disposition of Class B Shares. The Company believes that it was a PFIC for the prior tax year ended December 31, 2023, and based on current business plans and financial expectations, the Company expects to be a PFIC for the current tax year. If the Company is a PFIC in the taxable year in which a U.S. Holder first invests in the Company, the adverse rules described below will apply indefinitely unless the Company no longer is a PFIC in a subsequent taxable year and the U.S. Holder makes a timely “purging election” as described below. No opinion of legal counsel or ruling from the Internal Revenue Service (“IRS”) concerning the status of the Company as a PFIC has been obtained or is currently planned to be requested. However, PFIC classification is fundamentally factual in nature, generally cannot be determined until the close of the tax year in question, and is determined annually. In addition, the analysis depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations.
| 74 |
| Table of Contents |
General PFIC Rules
In any year in which the Company is classified as a PFIC, a U.S. Holder will be required to file an annual report with the IRS containing such information as Treasury Regulations and/or other IRS guidance may require. In addition to penalties, a failure to satisfy such reporting requirements may result in an extension of the time period during which the IRS can assess a tax. U.S. Holders should consult their own tax advisors regarding the requirements of filing such information returns under these rules, including the requirement to file an IRS Form 8621 annually.
The Company generally will be a PFIC if, after the application of certain “look-through” rules with respect to subsidiaries in which the Company holds at least 25% of the value of such subsidiary, for a tax year, (a) 75% or more of the gross income of the Company for such tax year is passive income (the “income test”) or (b) 50% or more of the value of the Company’s assets either produce passive income or are held for the production of passive income (the “asset test”), based on the quarterly average of the fair market value of such assets. “Gross income” generally includes all sales revenues less the cost of goods sold, plus income from investments and from incidental or outside operations or sources, and “passive income” generally includes, for example, dividends, interest, certain rents and royalties, certain gains from the sale of stock and securities, and certain gains from commodities transactions. Active business gains arising from the sale of commodities generally are excluded from passive income if substantially all of a foreign corporation’s commodities are stock in trade or inventory, depreciable property used in a trade or business or supplies regularly used or consumed in the ordinary course of its trade or business, and certain other requirements are satisfied.
If the Company were a PFIC in any tax year during which a U.S. Holder held Class B Shares, and subject to a U.S. Holder making a “QEF Election” or “Mark-to-Market Election” as described below, such holder generally would be subject to special rules with respect to “excess distributions” made by the Company on the Class B Shares and with respect to gain from the disposition of Class B Shares. An “excess distribution” generally is defined as the excess of distributions with respect to the Class B Shares received by a U.S Holder in any tax year over 125% of the average annual distributions such U.S. Holder has received from the Company during the shorter of the three preceding tax years, or such U.S. Holder’s holding period for the Class B Shares. Generally, a U.S. Holder would be required to allocate any excess distribution or gain from the disposition of the Class B Shares ratably over its holding period for the Class B Shares. Such amounts allocated to the year of the disposition or excess distribution would be taxed as ordinary income, and the preferential tax rates applicable to capital gains or dividends received on our Class B shares would not be available. In addition, amounts allocated to prior tax years would be taxed as ordinary income at the highest tax rate in effect for each such year and an interest charge would apply at a rate applicable to underpayments. These adverse tax consequences would not apply to a pension or profit-sharing trust or other tax-exempt organization that did not borrow funds or otherwise utilize leverage in connection with its acquisition of Class B Shares. In addition, if a non-electing U.S. Holder who is an individual dies while owning our Class B Shares, such U.S. Holder’s successor generally would not receive a step-up in tax basis with respect to such Class B Shares, but instead would have a tax basis equal to the lower of the fair market value of such Class B Shares or the decedent’s tax basis in such Class B Shares.
QEF Election
The tax consequences described above upon a PFIC determination may be mitigated if a U.S. Holder makes a timely “qualified electing fund” election (a “QEF election”) with respect to its interest in the PFIC, provided the Company provides the U.S Holder with the necessary information regarding its ordinary earnings and net capital gain. Consequently, if the Company is classified as a PFIC, it would likely be advantageous for a U.S. Holder to elect to treat the investment as a “qualified electing fund” (a “QEF”) with respect to such U.S. Holder in the first year in which it holds Class B Shares. If a U.S. Holder makes a timely QEF election with respect to the Company, the electing U.S. Holder would be required in each taxable year that the Company is considered to be a PFIC to include in gross income (i) as ordinary income, the U.S. Holder’s pro rata share of the ordinary earnings of the Company and (ii) as capital gain, the U.S. Holder’s pro rata share of the net capital gain (if any) of the Company, whether or not the ordinary earnings or net capital gain are distributed. An electing U.S. Holder’s basis in its Class B Shares will be increased to reflect the amount of any taxed but undistributed income. Distributions of income that had previously been taxed will result in a corresponding reduction of basis in the Class B Shares and will not be taxed again as distributions to the U.S. Holder. Gain realized from the sale of our Class B Shares covered by a QEF election would be taxed as a capital gain and the denial of the basis step-up at death described above would not apply. Generally, a QEF election must be made by the U.S. Holder in a timely filed tax return for the first taxable year in which the U.S. Holder held our Class B Shares that includes the close of our taxable year for which we met the PFIC gross income test or asset test. A separate QEF election would need to be made for any of our subsidiaries that are classified as a PFIC. A QEF election is made on IRS Form 8621.
The U.S. federal income tax on any gain from the disposition of Class B Shares or from the receipt of Excess Distributions may be greater than the tax that would apply if a timely QEF election is made. If the Company does not provide the required information with regard to the QEF election, U.S. Holders will not be able to make a QEF election and will, subject to the discussion of the mark-to-market election below, continue to be subject to the general PFIC rules as described above. U.S. Holders are urged to consult their own tax advisors regarding the advisability and availability of making a QEF election with respect to the Company.
Mark-to-Market Election
Alternatively, if the Company were to be classified as a PFIC, a U.S. Holder could also avoid certain of the general PFIC rules described above by making a timely mark-to-market election on Form 8621 (instead of a QEF election), provided the Class B Shares are treated as regularly traded on a qualified exchange or other market within the meaning of the applicable Treasury regulations. U.S. Holders are urged to consult their own tax advisers regarding the potential availability and consequences of a mark-to-market election. A U.S. Holder who makes the mark-to-market election generally must include as ordinary income each year the increase in the fair market value of the Class B Shares and deduct from gross income the decrease in the value of such shares during each of its taxable years, but with losses limited to the amount of previously recognized net gains. The U.S. Holder’s tax basis in the Class B Shares would be adjusted to reflect any income or loss recognized as a result of the mark-to-market election. If a mark-to-market election with respect to our Class B Shares is in effect on the date of a U.S. Holder’s death, the tax basis of the Class B Shares in the hands of a U.S. Holder who acquired them from a decedent will be the lesser of the decedent’s tax basis or the fair market value of the Class B Shares. Any gain from a sale, exchange or other disposition of the Class B Shares in any taxable year in which we are a PFIC (i.e., when we meet the gross income test or asset test described above) would be treated as ordinary income and any loss from a sale, exchange or other disposition would be treated first as an ordinary loss (to the extent of any net mark-to-market gains previously included in income) and thereafter as a capital loss. If we cease to be a PFIC, any gain or loss recognized by a U.S. Holder on the sale or exchange of the Class B Shares would be classified as a capital gain or loss. The Class B Shares should be marketable stock as long as they are listed on the Nasdaq Capital Market and are regularly traded. A mark-to-market election will not apply to the Class B Shares for any taxable year during which we are not a PFIC but will remain in effect with respect to any subsequent taxable year in which we again become a PFIC. Such election will not apply to any subsidiary that we own. Accordingly, a U.S. Holder may continue to be subject to the PFIC rules with respect to any lower-tier PFICs notwithstanding the U.S. Holder’s mark-to-market election.
| 75 |
| Table of Contents |
Purging Election
If we are a PFIC at any time when a U.S. Holder holds our Class B Shares, we will generally continue to be treated as a PFIC with respect to the U.S. Holder for all succeeding years during which the U.S. Holder holds our Class B Shares even if we cease to meet the PFIC gross income test or asset test in a subsequent year. However, if we cease to meet these tests, a U.S. Holder can avoid the continuing impact of the PFIC rules by making a special election (a Purging Election) to recognize gain by making a “deemed sale” election with respect to all of the U.S. Holder’s Class B Shares and have such Class B Shares deemed to be sold at their fair market value on the last day of the last taxable year during which we were a PFIC. Under another type of purging election, the Company will be deemed to have made a distribution to the U.S. Holder of such U.S. Holder’s pro rata share of the Company’s earnings and profits as determined for U.S. federal income tax purposes. In order for the U.S. Holder to make this second election, the Company must also be determined to be a “controlled foreign corporation” as defined by the U.S. Tax Code (which may not be the case, but please see the Controlled Foreign Corporation section below). The shareholder makes a purging election under Code section 1298(b)(1) and regulations section 1.1298–3 on IRS Form 8621 attached to the shareholder’s tax return (including an amended return), or requests the consent of the IRS Commissioner to make a late election under Code section 1298(b)(1) and regulations section 1.1298–3(e) (late purging election) on Form 8621-A. In addition, for a U.S. Holder making such an election, a new holding period would be deemed to begin for our Class B Shares for purposes of the PFIC rules. After the Purging Election, the Class B Shares with respect to which the Purging Election was made will not be treated as shares in a PFIC unless we subsequently again become a PFIC.
Each U.S. person who is a shareholder of a PFIC generally must file an annual report (on IRS Form 8621) with the IRS containing certain information, and the failure to file such report could result in the imposition of penalties on such U.S. person and in the extension of the statute of limitations with respect to federal income tax returns filed by such U.S. person.
U.S. Holders should be aware that, for each tax year, if any, that the Company is a PFIC, the Company can provide no assurances that it will satisfy the record keeping requirements or make available to U.S. Holders the information such U.S. Holders require to make a QEF Election with respect to the Company or any subsidiary that also is classified as a PFIC. U.S. Holders should consult their own tax advisors regarding the potential application of the PFIC rules to the ownership and disposition of Class B Shares, and the availability of certain U.S. tax elections under the PFIC rules.
Additional Considerations
Controlled Foreign Corporation
As a result of the enactment of the Tax Cuts and JOBS Act and the repeal of Code section 958(b)(4), it is possible to accidentally create a controlled foreign corporation (“CFC”) without having a direct or indirect United States shareholder. Historically, Code section 958(b)(4) prevented stock owned by a foreign person from being attributed downward to a U.S. person (e.g., a partnership, corporation, trust, or estate) owned by such foreign person. Effective as of the last taxable year of a foreign corporation beginning before January 1, 2018, stock may be attributed downward from a person to a corporation if “50 percent or more in value of the stock in a corporation is owned, directly or indirectly by or such person.” As a result of the repeal of Code section 958(b)(4), it is thus possible to accidentally create CFCs without a direct or indirect United States shareholder. This is because, since the repeal, a U.S. corporation owned by a foreign parent corporation may be treated as constructively owning the stock of the foreign parent or even the foreign parent’s foreign subsidiaries (i.e., foreign brother-sister companies). The Company believes that certain case law along with the legislative intent of the repeal may substantiate that it is not a CFC, although it is possible that the IRS may disagree. In discussing the repeal of Code section 958(b)(4), the Senate amendment that was followed by the Conference Report provides: “[f]urthermore, the Senate Finance Committee explanation states that the provision is not intended to cause a foreign corporation to be treated as a controlled foreign corporation regarding a U.S. shareholder as a result of attribution of ownership under section 318(a)(3) to a U.S. person that is not a related person (within the meaning of section 954(d)(3)) to such U.S. shareholder as a result of the repeal of section 958(b)(4).” The Tax Court case of Nettie Miller v. Commissioner stands for the premise that reading the current rules strictly would result in an absurd result that the Company’s U.S. subsidiary (a non-Code section 958(a) shareholder) is treated as constructively owning the Company. Furthermore, in Rev. Rul. 74-605, the IRS concluded that a subsidiary could not be attributed ownership of its direct or indirect parent corporations for purposes of applying Code section 304. The IRS’s rationale is that if a subsidiary were treated as constructively owning its parent (or grandparent) under the constructive ownership rules (that is, Code section 318(a)(3)(C)), it would further be treated as owning its own stock. The IRS concluded that treating a subsidiary as owning its own stock would violate regulation section 1.318-1(b)(1), which provides that “a corporation shall not be considered to own its own stock by reason of section 318(a)(3)(C).” Thus, the Company may not be a CFC as a result of Rev. Rul. 74-605, Nettie Miller, and the legislative intent of the repeal of Code section 958(b)(4). It should be noted, however, that the rationale for the Company not being classified as a CFC may not extend to the Company’s non-U.S. subsidiaries. As such, any U.S. Holders that own 10 percent or more of the total combined voting power of all classes of stock entitled to vote of the Company, or 10 percent or more of the total value of shares of all classes of stock of the Company should consider whether the overlap rules for CFCs and PFICs apply to them pursuant to Code section 1297(d) when determining their U.S. tax obligations for the Company or any of its non-U.S. subsidiaries. U.S. Holders should consult their own tax advisors regarding the application of this rule since the attribution rules related to ownership are very complex.
| 76 |
| Table of Contents |
Additional Tax on Passive Income
Certain U.S. Holders that are individuals, estates and trusts whose income exceeds certain thresholds will be required to pay a 3.8% surtax on “net investment income” including, among other things, dividends, and net gain from disposition of property (other than property held in certain trades or businesses). U.S. Holders should consult their own tax advisors regarding the application, if any, of this tax on their ownership and disposition of Class B Shares.
Receipt of Foreign Currency
The amount of any distribution paid to a U.S. Holder in foreign currency, or on the sale, exchange, or other taxable disposition of Class B Shares, generally will be equal to the U.S. dollar value of such foreign currency based on the exchange rate applicable on the date of receipt (regardless of whether such foreign currency is converted into U.S. dollars at that time). A U.S. Holder will have a basis in the foreign currency equal to its U.S. dollar value on the date of receipt. Any U.S. Holder who converts or otherwise disposes of the foreign currency after the date of receipt may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method of tax accounting. Each U.S. Holder should consult its own U.S. tax advisor regarding the U.S. federal income tax consequences of receiving, owning, and disposing of foreign currency.
Foreign Tax Credit
Subject to the PFIC rules discussed above, a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the Class B Shares generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax. Generally, a credit will reduce a U.S. Holder’s U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income that is subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year.
Complex limitations apply to the foreign tax credit, including the general limitation that the credit cannot exceed the proportionate share of a U.S. Holder’s U.S. federal income tax liability that such U.S. Holder’s “foreign source” taxable income bears to such U.S. Holder’s worldwide taxable income. In applying this limitation, a U.S. Holder’s various items of income and deduction must be classified, under complex rules, as either “foreign source” or “U.S. source.” Generally, dividends paid by a foreign corporation should be treated as foreign source for this purpose, and gains recognized on the sale of stock of a foreign corporation by a U.S. Holder should be treated as U.S. source for this purpose, except as otherwise provided in an applicable income tax treaty, and if an election is properly made under the Code. However, the amount of a distribution with respect to the Class B Shares that is treated as a “dividend” may be lower for U.S. federal income tax purposes than it is for Canadian federal income tax purposes, resulting in a reduced foreign tax credit allowance to a U.S. Holder. In addition, this foreign tax credit limitation is calculated and applied separately with respect to specific categories of income. The foreign tax credit rules are complex, and each U.S. Holder should consult its own U.S. tax advisor regarding the foreign tax credit rules.
Backup Withholding and Information Reporting
Under U.S. federal income tax law and Treasury Regulations, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a foreign corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on individuals who are U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold amounts. The definition of specified foreign financial assets includes not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained by a financial institution, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a foreign entity. U. S. Holders may be subject to these reporting requirements unless their Class B Shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult their own tax advisors regarding the requirements of filing information returns, including the requirement to file an IRS Form 8938.
Payments made within the United States or by a U.S. payor or U.S. middleman, of dividends on, and proceeds arising from the sale or other taxable disposition of, Class B Shares will generally be subject to information reporting and backup withholding tax, at the rate of 24%, if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer identification number (generally on Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding tax, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding tax. However, certain exempt persons generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding tax rules generally will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner.
The discussion of reporting requirements set forth above is not intended to constitute a complete description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax, and under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirement. Each U.S. Holder should consult its own tax advisor regarding the information reporting and backup withholding rules.
| 77 |
| Table of Contents |
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE ACQUISITION, OWNERSHIP, AND DISPOSITION OF CLASS B SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN THEIR OWN PARTICULAR CIRCUMSTANCES.
Certain Canadian Federal Income Tax Considerations
The following summary describes, as of the date hereof, the material Canadian federal income tax considerations generally applicable to a Shareholder who is a beneficial owner of our Class B Shares and who, at all relevant times, for the purposes of the application of the Income Tax Act (Canada) and the Income Tax Regulations (collectively, the “Canadian Tax Act”), (1) is not, and is not deemed to be, resident in Canada for purposes of the Canadian Tax Act and any applicable income tax treaty or convention; (2) deals at arm’s length with us; (3) is not affiliated with us; (4) does not use or hold, and is not deemed to use or hold, Class B Shares in a business or part of a business carried on in Canada; (5) has not entered into, with respect to the Class B Shares, a “derivative forward agreement”, as that term is defined in the Canadian Tax Act and (6) holds the Class B Shares as capital property (a “Non-Canadian Holder”). This summary does not apply to a Non-Canadian Holder that is an insurer carrying on an insurance business in Canada and elsewhere or an “authorized foreign bank”, as that term is defined in the Canadian Tax Act. Such Non-Canadian Holders should consult their own tax advisors for advice having regards to their particular circumstances.
This summary is based on the current provisions of the Canadian Tax Act and the Canada-United States Tax Convention (1980), as amended (the “Canada-U.S. Tax Treaty”), and an understanding of the current administrative policies and assessing practices of the Canada Revenue Agency published in writing prior to the date hereof. It takes into account all specific proposals to amend the Canadian Tax Act and the Canada-U.S. Tax Treaty, publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Proposed Amendments”) and assumes that all Proposed Amendments will be enacted in the form proposed. However, no assurances can be given that the Proposed Amendments will be enacted as proposed, or at all. This summary does not otherwise take into account or anticipate any changes in law or administrative policy or assessing practice whether by legislative, regulatory, administrative or judicial decision or action nor does it take into account tax legislation or considerations of any province, territory or foreign jurisdiction, which may differ from those discussed herein.
This summary is of a general nature only and is not, and is not intended to be, legal or tax advice to any particular shareholder, and no representations with respect to the income tax consequences to any particular shareholder are made. This summary is not exhaustive of all Canadian federal income tax considerations. Accordingly, you should consult your own tax advisor with respect to your particular circumstances.
Generally, for purposes of the Canadian Tax Act, all amounts relating to the acquisition, holding or disposition of the Class B Shares must be converted into Canadian dollars based on the exchange rate quoted by the Bank of Canada on the date such amount arose or such other rate of exchange as is acceptable to the Minister of National Revenue (Canada).
Dividends
Dividends paid or credited on the Class B Shares or deemed to be paid or credited on the Class B Shares to a Non-Canadian Holder will be subject to Canadian withholding tax at the rate of 25% on the gross amount of the dividend, subject to any reduction in the rate of withholding to which the Non-Canadian Holder is entitled under any applicable income tax treaty or convention between Canada and the country in which the Non-Canadian Holder is resident. For example, under the Canada-U.S. Tax Treaty, where dividends on the Class B Shares are considered to be paid to or derived by a Non-Canadian Holder that is a beneficial owner of the dividends and is a U.S. resident for the purposes of, and is entitled to the full benefits of, the Canada-U.S. Tax Treaty, the applicable rate of Canadian withholding tax is generally reduced to 15%. We will be required to withhold the applicable withholding tax from any dividend and remit it to the Canadian government for the Non-Canadian Holder’s account. Non-Canadian Holders are urged to consult their own advisors to determine their entitlement to relief under an applicable income tax treaty or convention.
Dispositions
A Non-Canadian Holder will not be subject to tax under the Canadian Tax Act on any capital gain realized on a disposition or deemed disposition of a Class B Share, unless the Class B Share is “taxable Canadian property” to the Non-Canadian Holder for purposes of the Canadian Tax Act at the time of disposition and the Non-Canadian Holder is not entitled to relief under an applicable income tax treaty or convention between Canada and the country in which the Non-Canadian Holder is resident.
Generally, the Class B Shares will not constitute “taxable Canadian property” to a Non-Canadian Holder at a particular time provided that the Class B Shares are listed at that time on a “designated stock exchange” (as defined in the Canadian Tax Act), which currently includes the CSE and the Nasdaq, unless at any particular time during the 60-month period that ends at that time the following two conditions are met concurrently:
|
· |
at least 25% of the issued shares of any class or series of our capital stock was owned by or belonged to any combination of (a) the Non-Canadian Holder, (b) persons with whom the Non-Canadian Holder does not deal at arm’s length for purposes of the Canadian Tax Act, and (c) partnerships in which the Non-Canadian Holder or a person described in (b) holds a membership interest directly or indirectly through one or more partnerships, and |
|
· |
more than 50% of the fair market value of the Class B Shares was derived, directly or indirectly, from one or any combination of: (i) real or immoveable property situated in Canada, (ii) “Canadian resource properties” (as that term is defined in the Canadian Tax Act), (iii) “timber resource properties” (as that term is defined in the Canadian Tax Act) or (iv) options in respect of, or interests in, or for civil law rights in, a property described in any of the foregoing whether or not the property exists. |
| 78 |
| Table of Contents |
Notwithstanding the foregoing, in certain circumstances, Class B Shares could be deemed to be “taxable Canadian property” to a Non-Canadian Holder. Non-Canadian Holders whose Class B Shares are, or may constitute, “taxable Canadian property” should consult their own tax advisors.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We are subject to the information reporting requirements of the Exchange Act applicable to foreign private issuers and under those requirements will file reports with the SEC. Those reports may be inspected without charge at the locations described below. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. Nevertheless, we will file with the SEC an Annual Report on Form 20-F containing financial statements that have been examined and reported on, with and opinion expressed by an independent registered public accounting firm.
We maintain a corporate website at www.fsdpharma.com. We intend to post our Annual Report on Form 20-F on our website promptly following its filing with the SEC. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report. We have included our website address in this Annual Report solely as an inactive textual reference.
The SEC maintains a website (www.sec.gov) that contains reports, proxy and information statements and other information regarding registrants, such as us, that file electronically with the SEC.
This Annual Report, copies of our financial statements and other continuous disclosure documents required under the Securities Act (Ontario) are available for viewing on the Company’s SEDAR+ profile accessible at www.sedarplus.ca. All of the documents referred to are in English.
With respect to references made in this Annual Report to any contract or other document of our company, such references are not necessarily complete, and you should refer to the exhibits attached or incorporated by reference to this Annual Report for copies of the actual contract or document.
I. Subsidiary Information
Not applicable.
J. Annual Report to Security Holders
Not applicable.
Item 11. Quantitative and Qualitative Disclosures About Market Risk.
Not applicable.
Item 12. Description of Securities Other than Equity Securities
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
D. American Depositary Shares
Not applicable.
| 79 |
| Table of Contents |
PART II
Item 13. Defaults, Dividend Arrearages and Delinquencies
Not applicable.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds.
A.-D.
Not applicable.
E. Use of Proceeds
Use of Proceeds Reconciliation from 2020 Prospectuses
2020 Prospectuses(1)(2)(3) | |||
|
Allocated |
Spent to Date |
Difference |
Acquisitions(4)(5) |
US$5,000,000 |
|
US$5,000,000 |
|
Investments(6) Strategic Investments |
US$6,000,000
|
US$7,720,190 |
US$6,000,000 (US$7,720,190) |
|
Capital Expenditures (Research and Development)(5) FSD201 Lucid-MS Lucid-Psych Alcohol Misuse Segment (UNBUZZED and the Healthcare Product) |
US$35,000,000
|
US$10,578,381 US$4,957,100 US$2,383,733 US$1,544,210 |
US$35,000,000 (US$10,578,381) (US$4,957,100) (US$2,383,733) (US$1,544,210) |
|
Working Capital and General Corporate((7)(8)9)(10)(11) Legal Expenses, Litigation and Contested Meeting Operating Expenses |
US$10,000,000
|
US$11,681,072 US$10,416,756 |
US$10,000,000 (US$11,681,072) (US$10,416,756) |
|
Strategic Initiatives(12) Share Buyback 2022 Share Buyback 2023 |
|
US$1,730,255 US$2,895,487 |
(US$1,730,255) (US$2,895,487) |
Total: |
US$56,000,000 |
US$53,907,184 |
US$2,092,816 |
Notes:
1. |
Pursuant to the Company’s previous shelf registration statement dated on February 8, 2020 filed on Form F-10, SEC File No. 333- 236780, which became effective on June 17, 2020 (as amended, the "2020 Registration Statement") and the Company’s short form base shelf prospectus dated June 16, 2020 (the “2020 Base Shelf Prospectus” and together with the 2020 Registration Statement, the “2020 Prospectus”), the Corporation planned to distribute up to an aggregate initial offering price of US$100,000,000 (or the equivalent in other currencies or currency units based on the applicable exchange rate at the time of the offering) of its securities. |
||||||||
2. |
Pursuant to the 2020 Prospectuses, the Corporation allocated anticipated proceeds for the development of FSD201, future acquisitions and investments, to financial capital expenditures and for working capital and general corporate purposes. |
||||||||
3. |
Under the 2020 Prospectuses, the Corporation, completed four raises totaling US$59,453,014. |
||||||||
i. |
An at-the-market offering of up to US$19,976,512 pursuant to a prospectus supplement dated July 10, 2020 (the “First ATM”). Under the First ATM, the Corporation raised US$19,976,512 through the issuance of 7,412,574 Class B Shares at an average price of US$2.69 per Class B Share. For more information, please see “Item 4. Information on the Company. - A. History and Development of the Company - Other Significant Operations and Principal Activities – Fiscal 2021 and 2022”. |
||||||||
ii. |
An offering of up to US$9,999,996 pursuant to a prospectus supplement dated July 31, 2020 (the “July 2020 Offering”). Under the July 2020 Offering, the Corporation raised US$9,999,996 by issuing a total of 2,762,430 Class B Shares at a price of US$3.62 per Class B Share and warrants to purchase 1,381,215 Class B Shares. Each warrant had an exercise price of US$4.26 per Class B Share and expires five years from the date of issuance. |
||||||||
iii. |
An offering of up to US$9,499,994 pursuant to a prospectus supplement dated October 16, 2020 (the “October 2020 Offering”). Under the October 2020 Offering, the Corporation raised US$9,499,994 by issuing a total of 4,318,179 Class B Shares at a price of US$2.20 per Class B Share and warrants to purchase 3,454,543 Class B Shares. Each warrant had an exercise price of US$2.60 per Class B Share and expires five years from the date of issuance. |
||||||||
iv. |
An at-the-market offering of up to US$19,994,712 pursuant to a prospectus supplement dated February 11, 2021 (the “Second ATM”). Under the Second ATM, the Corporation raised US$19,994,713 through the issuance of 8,124,136 Class B Shares at an average price of US$2.46. For more information, please see “Item 4. Information on the Company. - A. History and Development of the Company - Other Significant Operations and Principal Activities – Fiscal 2021 and 2022”. |
||||||||
4. |
On September 21, 2021, the Corporation acquired Lucid, which was satisfied in Class B Shares. This transaction did not require a cash outlay and thus the Corporation repurposed the funds towards two share repurchase programs, as further outlined in Note 12 below. |
||||||||
5. |
Includes funds allocated to complete one or more strategic corporate transactions (such as acquisitions) intended to accelerate the growth and development of the Corporation’s businesses, which funds remain subject to reallocation by the Corporation towards budgets and expenditures which are identified and/or otherwise unidentified or unforeseen, as at the relevant date. |
||||||||
6. |
During the effectiveness of the 2020 Prospectuses, the Corporation explored multiple investment opportunities, but none of them met the investment criteria of the Corporation and the Corporation repurposed the funds towards secured loans with an average maturity of two years from the date of issuance that earn fees at fixed rates. |
||||||||
7. |
Includes (i) funds allocated for the payment of (a) general corporate and administrative expenses, (b) ongoing legal fees and other professional and consulting fees, and salaries, and (c) ongoing costs associated with being a reporting issuer, and (ii) funds generally reserved for (x) allocation towards future expenditures which are otherwise unidentified or unforeseen as of the relevant date, and (y) re-allocation towards the budgets allocated for identified purposes as of the relevant date. |
||||||||
| 80 |
| Table of Contents |
8. |
On January 4, 2021, certain shareholders of the Corporation (the “Requisitioning Shareholders”) requisitioned a meeting of shareholders of the Corporation in compliance with section 105 of the OBCA (the “Contested Meeting”). On January 21, 2021, the Board called the Contested Meeting to be held on June 29, 2021; however, the Requisitioning Shareholders filed an application with the Commercial List of the Superior Court of Justice (the “Superior Court”) to, among other things, have the date of the Contested Meeting accelerated. This led to a series of appearances before the Superior Cout and subsequently the divisional court. On March 30, 2021, the Corporation commenced an action against the Requisition Shareholders alleging that their dissident circular was mislead and deficient, however, this claim was dismissed in its entirety by the Superior Court on May 10, 2021. In addition, the Requisitioning Shareholders filed an additional statement of claim on April 6, 2021, against the Corporation and Board stating that, among other things, their conduct against shareholders, including the Requisition Shareholders, was oppressive (the “Oppression Claim”). The Oppression Claim has been dormant since the conclusion of the Contested Meeting. In April 2021, Mr. Durkacz brought a motion before the Superior Court seeking various relief in relation to the conduct of the Contested Meeting, which was partially granted by the Superior Court on May 10, 2021. At the Contest Meeting, held on May 14, 2021, the previous Board members were relieved of there duties. The Contested Meeting and associated events resulted in additional unexpected legal and operating expenses of C$3,113,170 (approximately US$2,338,795, converted at a price of C$1.00:US$0.7513 based on the Bank of Canada exchange rate as of December 21, 2023). |
|
9. |
Following the Contested Meeting, the former CEO, Dr. Raza Bokhari commenced five actions against the Corporation and management, which resulted in counterclaims and additional unexpected legal and operating expenses of C$7,402,079 (approximately US$5,560,874, converted at a price of C$1.00:US$0.7513 based on the Bank of Canada exchange rate as of December 21, 2023): |
i. |
Dr. Raza Bokhari: On July 15, 2021, the Corporation’s former CEO, Dr. Raza Bokhari, filed an arbitration notice seeking C$30.2 million for breach of contract, severance, and damages, along with C$500,000 for punitive damages and legal fees. Dr. Bokhari had been placed on administrative leave after the May 14, 2021 shareholder meeting and was terminated for cause on July 27, 2021, following an investigation by a special committee. The arbitration concluded in August 2022 with Dr. Raza Bokhari’s claims dismissed. Dr. Bokhari was also ordered to repay certain funds to the Corporation and cover arbitration costs in the amount of approximately C$2.8M plus interest. On December 9, 2022, Dr. Bokhari sought to set aside the award, citing unfair treatment and inadequate reasoning. On October 4, 2023, the Corporation announced that the Ontario Superior Court of Justice had dismissed Dr. Bokhari’s motion to set aside the arbitration award. Dr. Bokhari was required to put up C$150,000 as security for costs before the motion was heard, which he has forfeited. In addition, Dr. Bokhari was ordered to pay C$175,000 to cover the Corporation’s legals costs for his failed set aside motion. On December 1, 2023, the Corporation filed a petition to confirm the arbitration award in the United States District Court for Eastern District of Pennsylvania. On October 13, 2023, Dr. Bokhari served notices of motion on the Corporation for leave to appeal the set aside and enforcement orders issued by the Ontario Superior Court of Justice’s on October 4, 2023. On December 15, 2023, the Corporation submitted a responding party’s factum to the Court of Appeal for Ontario. As of the date hereof, the litigation is ongoing. |
||||||||
ii. |
Parkway Clinical Laboratories: On July 8, 2021, Parkway Clinical Laboratories, a company wholly owned by Dr. Bokhari, filed an action against the Corporation, which was subsequently settled following a conference between the parties on October 20, 2021. |
||||||||
iii. |
Bokhari v. FSD Pharma Inc. Et al.: On July 2, 2021, Dr Bokhari filed an action against the Corporation, FSD BioSciences, Anthony Durkacz and Zeeshan Saeed. The case was placed in civil suspense pending resolution of arbitration. Therefore, no further activity will occur in this case unless and until the aforementioned arbitration concludes. As of the date hereof, the litigation is ongoing. |
||||||||
iv. |
Restraining Order and Class B Share Cancellation Application: On January 21, 2021 and February 10, 2021, the Board authorized the issuance of an aggregate of 1,349,765 Class B Shares as share based awards to certain directors and officers of the Corporation, including Dr. Bokhari. Upon determining that the Contested Shares had been inappropriately issued contrary to applicable laws, the Board resolved to cancel the Contested Shares on June 1, 2021, and later directed the Corporation’s transfer agent to cancel and return the Contested Shares to treasury. On July 2, 2021, Dr. Bokhari, filed an action against the Corporation seeking to prevent the Corporation from cancelling his portion of the Contested States. The motion was heard and denied on July 27, 2021. On July 21, 2021, the Corporation commenced a legal proceeding against Dr. Bokhari, former members of the Board, including James Datin, Robert Ciaruffoli, Stephen Buyer and Gerald Goldberg, Dr. Bokhari’s brokerages’ Haywood Securities Inc. and Haywood Securities (US) Inc., and the Corporation’s transfer agent. The Corporation made an application before the Superior Court stating that the Contested Shares were issued contrary to section 23(2) of the OBCA and validly cancelled by resolution of the Board passed on June 1, 2021. The Corporation was able to reach an agreement with all of the former directors other than Dr. Bokhari under which they did not oppose the Corporation’s application and agreed to be bound by the decision in the application, and the Corporation agreed not to seek costs against them. Neither the Corporation’s transfer agent nor any of Dr. Bokhari’s brokerages took any position on the application. On March 8, 2022, the court issued a mixed decision in the application, permitting the Contested Share grant to Dr. Bokhari until the date of his termination but cancelling 504,888 Contested Shares relating to services that were to be provided after the date of termination. |
||||||||
v. |
Indemnity Application: On April 6, 2022, Dr. Bokhari commenced an application in the Superior Court seeking an order appointing an arbitrator to arbitrate his claim to be entitled to indemnification of his legal expenses associated with the litigation he has commenced against the Corporation or in which he has been named as a defendant against the Corporation. The Corporation denied the validity of the underlying indemnification agreement and opposed the application. In April 2022, the parties agreed to adjourn the application without setting a new hearing date. As of the date hereof, the litigation is ongoing. |
||||||||
|
10. |
On July 20, 2021, a shareholder of the Corporation filed a claim in the Delaware Chancery Court against the Corporation and its directors and officers seeking to remedy harm they believe the directors and officers of the Corporation have caused by their actions. The shareholder filed the claim on count of breach of fiduciary duties and corporate waste against the directors and officers with no dollar amount being claimed. On September 13, 2021, the Corporation filed a motion to dismiss in its entirety and the motion was heard on February 8, 2022. The claim was dismissed by the court May 6, 2022. |
|
11. |
FSD BioSciences Employees: Subsequent to the resignation of the Former Employees, on July 9, 2021, the Former Employees filed a joint claim against the Corporation. On September 17, 2021, the Corporation filed a motion to dismiss the claim in its entirety. On December 13, 2021, the Court granted the Corporation’s motion and dismissed the case. One of the Former Employee’s claims was dismissed with prejudice while the other was not. |
|
12. |
As the Corporation had excess proceeds from the 2020 Prospectuses and did not identify any strategic acquisitions and/or investment opportunities, the Corporation conducted two share repurchase programs, one beginning in December 2021 and the other in January 2023, as the Board determined that the market price of the Class B Shares was undervalued, and these repurchases would strategically return value to shareholders. |
In all of these offerings, the Company used the proceeds in a manner consistent with that set forth in the applicable prospectus supplements. None of the net proceeds was paid to any of our directors or officers or their associates, or any 10% stockholder or any affiliate of ours.
| 81 |
| Table of Contents |
For more information on the legal proceedings, please see “Item 8. Financial Information - A. Consolidated Statements and Other Financial Information – Legal Proceedings”.
Item 15. Controls and Procedures
A. Disclosure Controls and Procedures
We maintain disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, which are designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and that such information is accumulated and communicated to our management, including our CEO and CFO, as appropriate to allow timely decisions regarding required disclosure.
Under the supervision and with the participation of our CEO and CFO, our management has evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2023, the end of the period covered by this annual report. Based on that evaluation, our CEO and CFO concluded that our disclosure controls and procedures were effective as of December 31, 2023.
The effectiveness of our disclosure controls and procedures and our internal control over financial reporting is subject to various inherent limitations, including cost limitations, judgments used in decision making, assumptions about the likelihood of future events, the soundness of our systems, the possibility of human error, and the risk of fraud. Moreover, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions and the risk that the degree of compliance with policies or procedures may deteriorate over time. Because of these limitations, there can be no assurance that any system of disclosure controls and procedures or internal control over financial reporting will be successful in preventing all errors or fraud or in making all material information known in a timely manner to the appropriate levels of management.
B. Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting is the process designed by and under the supervision of our CEO and CFO to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our financial statements for external reporting in accordance with accounting principles generally accepted in the United States of America. Management has evaluated the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013).
Under the supervision and with the participation of our CEO and CFO, our management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2023 and concluded that it was effective.
C. Attestation Report of the Registered Public Accounting Firm
Not applicable.
D. Changes in Internal Control Over Financial Reporting
Under the supervision and with the participation of our CEO and CFO, our management has evaluated changes in our internal control over financial reporting that occurred during the period covered by this Annual Report. Based on that evaluation, our CEO and CFO did not identify any change in our internal control over financial reporting that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 16. [Reserved.]
Item 16A. Audit Committee Financial Expert.
Our Board has determined that Nitin Kaushal, the Chair of the Audit Committee of our Board, is an “audit committee financial expert” as defined by SEC rules and has the requisite financial sophistication under the listing standards of the Nasdaq Stock Market. Mr. Kaushal meets the standards of independence applicable to audit committees under Rule 10A-3 under the Exchange Act and under the listing standards of the Nasdaq Stock Market.
| 82 |
| Table of Contents |
Item 16B. Code of Ethics.
We have adopted the Code that is applicable to all of our directors, executive officers, and employees, including our principal executive officer, principal financial officer, principal accounting officer and controller. A copy of the Code is available on our website at www.fsdpharma.com.
During 2023, except for the CEO Mortgage Loan, no provision of the Code applicable to our principal executive officer, principal financial officer, principal accounting officer or controller was amended (other than technical, administrative, or other non-substantive amendments), nor did we grant any waiver (including an implicit waiver) of any provision of the Code to any such officer.
We intend to disclose any amendments to the Code applicable to our principal executive officer, principal financial officer, principal accounting officer, controller or persons performing similar functions (other than technical, administrative, or other non-substantive amendments) and any waiver of the Code for any such persons on our website to the extent required by the rules and regulations of the SEC, including the instructions to Item 16B of Form 20-F. Any waiver of the Code for these covered persons may be made only by the Board or the Chief Executive Officer and will be disclosed promptly to stockholders and others, as required by applicable law. The Company must disclose changes to and wavers of the Code in accordance with applicable law.
Item 16C. Principal Accountant Fees and Services.
MNP LLP, auditors of the Company since November 29, 2019, served as our independent registered public accounting firm for the years ended December 31, 2023, 2022 and 2021. The following table provides a summary of the fees for professional services rendered by MNP for the years ended December 31, 2023 and 2022:
Auditors’ Fees
The following table sets forth the fees billed by the Company’s auditor during the years ended December 31, 2023 and December 31, 2022:
Fee |
|
2023 |
|
|
2022 |
|||
Audit Fees(1) |
|
C$ | 380,138 |
|
|
C$ |
386,599 |
|
Audit-Related Fees(2) |
|
C$ | 57,432 |
|
|
|
Nil |
|
Tax Fees(3) |
|
C$ | 63,990.28 |
|
|
C$ |
45,176 |
|
All Other Fees(4) |
|
$ | 57,432 |
|
|
Nil |
||
Total |
|
$ | 558,503 |
|
|
C$ | 431,775 |
|
Notes:
(1) |
“Audit Fees” include fees necessary to perform the annual audit and quarterly reviews of the Company’s consolidated financial statements. Audit Fees include fees for review of tax provisions and for accounting consultations on matters reflected in the financial statements. Audit Fees also include audit or other attest services required by legislation or regulation, such as comfort letters, consents, reviews of securities filings and statutory audits. |
(2) |
“Audit-Related Fees” include services that are traditionally performed by the auditor. These audit-related services include employee benefit audits, due diligence assistance, accounting consultations on proposed transactions, internal control reviews and audit or attest services not required by legislation or regulation. |
(3) |
“Tax Fees” include fees for all tax services other than those included in “Audit Fees” and “Audit-Related Fees”. This category includes fees for tax compliance, tax planning and tax advice. Tax planning and tax advice includes assistance with tax audits and appeals, tax advice related to mergers and acquisitions, and requests for rulings or technical advice from tax authorities. |
(4) |
“All Other Fees” include all other non-audit services. |
All permissible categories of non-audit services require pre-approval by the Audit Committee, subject to certain statutory exemptions.
Item 16D. Exemptions from the Listing Standards for Audit Committees.
Not applicable.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers.
On January 12, 2023, the Board authorized the 2023 NCIB, pursuant to which the Company was able to repurchase for cancellation up to 1,925,210 Class B Shares, being approximately 5% of the Company’s issued and outstanding Class B Shares as of January 12, 2023, over a 12-month period. The 2023 NCIB commenced on January 18, 2023 and was terminated on January 12, 2024. Under the 2023 NCIB, the Company repurchased for cancellation 1,904,700 Class B Shares at an average price of approximately C$2.11 per Class B Shares. All Class B Shares were repurchased through the facilities of the CSE at the prevailing market price on the CSE at the time of repurchase.
| 83 |
| Table of Contents |
The following table specifies the number of shares purchased by the Company under the 2023 NCIB:
Period |
|
Total Number of Shares Purchased |
|
|
Average Price Paid Per Share (C$) |
|
|
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs |
|
|
Maximum Number of Shares that May Yet Be Purchased Under the Plans or Programs |
|
||||
|
|
|
|
|
|
|
|
|
||||||||
|
Month #1 (January 1, 2023 - January 31, 2023) |
|
|
289,600 |
|
|
|
1.39 |
|
|
|
289,600 |
|
|
|
1,635,610 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Month #2 (February 1, 2023 - February 28, 2023) |
|
|
1,328,700 |
|
|
|
2.27 |
|
|
|
1,618,300 |
|
|
|
306,910 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Month #3 (March 1, 2023 - March 31, 2023) |
|
|
286,400 |
|
|
|
2.10 |
|
|
|
1,904,700 |
|
|
|
20,510 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Month #4 (April 1, 2023 - April 30, 2023) |
|
|
- |
|
|
|
- |
|
|
|
1,904,700 |
|
|
|
20,510 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Month #5 (May 1, 2023 - May 31, 2023) |
|
|
- |
|
|
|
- |
|
|
|
1,904,700 |
|
|
|
20,510 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Month #6 (June 1, 2023 - June 30, 2023) |
|
|
- |
|
|
|
- |
|
|
|
1,904,700 |
|
|
|
20,510 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Month #7 (July 1, 2023 - July 31, 2023) |
|
|
- |
|
|
|
- |
|
|
|
1,904,700 |
|
|
|
20,510 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Month #8 (August 1, 2023 - August 31, 2023) |
|
|
- |
|
|
|
- |
|
|
|
1,904,700 |
|
|
|
20,510 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Month #9 (September 1, 2023 - September 30, 2023) |
|
|
- |
|
|
|
- |
|
|
|
1,904,700 |
|
|
|
20,510 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Month #10 (October 1, 2023 - October 31, 2023) |
|
|
- |
|
|
|
- |
|
|
|
1,904,700 |
|
|
|
20,510 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Month #11 November 1, 2023 - November 30, 2023 |
|
|
- |
|
|
|
- |
|
|
|
1,904,700 |
|
|
|
20,510 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Month #12 (December 1, 2023 - December 31, 2023) |
|
|
- |
|
|
|
- |
|
|
|
1,904,700 |
|
|
|
20,510 |
|
| 84 |
| Table of Contents |
Item 16F. Change in Registrant’s Certifying Accountant.
Not applicable.
Item 16G. Corporate Governance.
The Company is a foreign private issuer and its Class B Shares are listed on Nasdaq. Nasdaq Marketplace Rule 5615(a)(3) permits a foreign private issuer to follow its home country practices in lieu of most of the requirements of the 5600 Series of the Nasdaq Marketplace Rules. In order to claim such an exemption, the Company must disclose the significant differences between its corporate governance practices and those required to be followed by U.S. domestic issuers under Nasdaq’s corporate governance requirements. Set forth below is a brief summary of such differences:
Independent Director Requirements
Nasdaq Marketplace Rule 5605(b)(1) requires a majority of the board of directors of each issuer to be comprised of independent directors, as that term is defined under Rule 5605(a)(2). The Company has a majority of independent directors and follows the Nasdaq Marketplace Rule and complies with the applicable CSE rules and applicable Canadian and Ontario corporate and securities regulatory requirements.
Shareholder Approval Requirements
Nasdaq Marketplace Rule 5635 requires each issuer to obtain shareholder approval prior to certain dilutive events, including a transaction other than a public offering involving the sale of 20% or more of the issuer’s outstanding shares of common stock prior to the transaction for less than the greater of book or market value of the stock. The Company does not follow this Nasdaq Marketplace Rule. Instead, and in accordance with the Nasdaq exemption, the Company complies with Ontario corporate and securities laws, which do not require shareholder approval for dilutive events unless the Company were to dispose of all or substantially all of its undertaking. In addition, the Company follows the CSE policies which require shareholder approval on the occurrence of a “fundamental change,” defined by the policies of the CSE to be a “major acquisition” (whereby for the next 12-month period at least 50% of the issuer’s assets will be comprised of, or anticipated revenues are expected to be derived from, the assets, properties, businesses or other interests that are the subject of the major acquisition) accompanied or preceded by a “change of control.” In such context, a “change of control” would include the distribution of a number of equity securities of the issuer equal to or greater than 100% of the number outstanding prior to the transaction, as well as a substantial change of management or the board of directors of the issuer.
In addition, Nasdaq Marketplace Rule 5635 requires shareholder approval of most equity compensation plans and material revisions to such plans. We do not follow this Nasdaq Marketplace Rule. Instead, and in accordance with the Nasdaq exemption, we comply with Ontario corporate and securities laws, which do not require shareholder approval of equity compensation plans. In addition, the Company intends to follow the CSE policies and certain provisions of Canadian securities laws which require limitations on the number of equity compensation securities that can be distributed to persons performing investor relations services to 1% of the issued and outstanding amount of listed securities in a 12-month period, and further limit the number of equity compensation securities that can be distributed to a director, officer or a related entity of the issuer, or an associate thereof (each a “related person”), on a fully diluted basis to not exceed 5% of the outstanding securities of the issuer, or collectively to related persons exceeds 10% of the outstanding securities of the issuer.
Quorum Requirement
Nasdaq Marketplace Rule 5620(c) requires that each company that is not a limited partnership shall provide for a quorum as specified in its by-laws for any meeting of holders of common stock; provided, however, that in no case shall such quorum be less than 33-1/3% of the outstanding shares of the Company’s common voting stock. The Company does not presently follow this Nasdaq Marketplace Rule. Instead, the Company complies with Ontario corporate and securities laws and its by-laws which do not require a quorum of no less than 33-1/3% of the outstanding shares of the Company’s common voting stock and provides that the quorum for the transaction of business at a meeting of shareholders is at least two voting persons holding or representing, in the aggregate, not less than 10% of the issued and outstanding shares of the applicable class.
| 85 |
| Table of Contents |
Independent Director Oversight of Executive Compensation and Board Nominations
Nasdaq’s Marketplace Rule 5605(d) requires independent director oversight of executive officer compensation arrangements by approval of such compensation by a committee comprised solely of independent directors, and Marketplace Rule 5605(e) requires similar oversight with respect to the process of selecting nominees to the board or oversight by a majority of the independent directors. Under the exemption available to foreign private issuers under Rule 5615(a)(3), the Company is not required to comply with Nasdaq Marketplace Rules 5605(d) or 5605(e). Instead, and in accordance with the Nasdaq exemption, the Company complies with the applicable CSE rules and applicable Canadian corporate and securities regulatory requirements.
Proxy Delivery Requirements
Nasdaq Marketplace Rule 5620(b) requires that a listed company that is not a limited partnership to solicit proxies and provide proxy statements for all meetings of shareholders, and also provide copies of such proxy solicitation materials to Nasdaq. The Company is a “foreign private issuer” as defined in Rule 3b-4 under the Exchange Act, and the equity securities of the Company are accordingly exempt from the proxy rules set forth in Sections 14(a), 14(b), 14(c) and 14(f) of the Exchange Act. The Company solicits proxies in accordance with applicable rules and regulations in Canada.
Item 16H. Mine Safety Disclosure
Not applicable.
Item 16I. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
Item 16J. Insider Trading Policies
This disclosure item does not yet apply to the Company.
Item 16K. Cybersecurity.
We have established policies and processes for assessing, identifying, and managing risks from cybersecurity threats, and have integrated these processes into our overall risk management systems and processes. We routinely assess risks from cybersecurity threats, including any potential unauthorized occurrence on or conducted through our information systems that may result in adverse effects on the confidentiality, integrity, or availability of our information systems or any information residing therein.
We conduct periodic risk assessments to identify cybersecurity threats, as well as assessments in the event of a material change in our business practices that may affect information systems that are vulnerable to such cybersecurity threats. These risk assessments include identification of reasonably foreseeable internal and external risks, the likelihood and potential damage that could result from such risks, and the sufficiency of existing policies, procedures, systems, and safeguards in place to manage such risks.
Following these risk assessments, we evaluate whether and how to re-design, implement, and maintain reasonable safeguards to minimize identified risks; reasonably address any identified gaps in existing safeguards; and regularly monitor the effectiveness of our safeguards. We devote significant resources and designate high-level personnel, including our CFO, who reports to our Board, to manage the risk assessment and mitigation process.
As part of our overall risk management system, we monitor our safeguards and train our employees on these safeguards. Personnel at all levels and departments are made aware of our cybersecurity policies through trainings integrated into new employee onboarding processes and annual employee re-training.
We engage consultants, experts, or other third parties in connection with our risk assessment processes. These third parties assist us in designing and implementing our cybersecurity policies and procedures, as well as in monitoring and testing our safeguards.
We require each third-party service provider who may have access to our systems and/or our sensitive data to confirm that it has the ability to implement and maintain appropriate security measures, consistent with all applicable laws, to implement and maintain reasonable security measures in connection with their work with us, and to promptly report any suspected breach of its security measures that may affect our company.
We have not experienced any cybersecurity incidents that have been determined to be material in the past, however, like other life sciences technology companies, we have experienced cybersecurity incidents and may continue to experience them in the future. For additional information regarding whether any risks from cybersecurity threats, including as a result of any previous cybersecurity incidents, materially affected or are reasonably likely to materially affect our company, including our business strategy, results of operations, or financial condition, please refer to “Item 3. Key information - D. Risk Factors” in this Annual Report on Form 20-F.
| 86 |
| Table of Contents |
Governance
One of the key functions of our Board is informed oversight of our risk management process, including risks from cybersecurity threats. Our Board is responsible for monitoring and assessing strategic risk exposure, and our executive officers are responsible for the day-to-day management of the material risks we face. Our Board administers its cybersecurity risk oversight function directly as a whole, as well as through the audit committee.
Our CFO and our management committee on cybersecurity, and outside consultants, who collectively possess significant experience in evaluating, managing, and mitigating security and other risks, including cybersecurity risks, are primarily responsible to assess and manage our material risks from cybersecurity threats.
Our CFO and our management committee on cybersecurity oversee our cybersecurity policies and processes, including those described in “Risk Management and Strategy” above. The processes by which our CFO and representatives from our management committee on cybersecurity are informed about and monitor the prevention, detection, mitigation, and remediation of cybersecurity incidents includes the following:
|
· |
monitoring of Company computer and information systems for potential malware, ransomware and other malicious activity, and remediation of identified issues, including mitigation of identified risks and containment and elimination of any malicious software; |
|
|
|
|
· |
mandatory cybersecurity training as part of new employee onboarding along with required annual employee cybersecurity re-training; |
|
|
|
|
· |
monitoring of systems and network infrastructure by security information and event management application; |
|
|
|
|
· |
prompt incident reporting directly to the Board; and |
|
|
|
|
· |
escalation to the Company’s Audit Committee and Board as warranted based upon the nature of the identified issue. |
Our CFO and/or representatives from our management committee on cybersecurity provide periodic briefings to the audit committee regarding our company’s cybersecurity risks and activities, including any recent cybersecurity incidents and related responses, cybersecurity systems testing, activities of third parties, and the like. Our Audit Committee provides regular updates to the Board on such reports.
| 87 |
| Table of Contents |
PART III
Item 17. Financial Statements
Not applicable.
Item 18. Financial Statements
See pages F-1 through F-44 appearing at the end of this Annual Report on Form 20-F following the signature page.
Item 19. Exhibits.
2.1* |
Description of Securities |
| 88 |
| Table of Contents |
101.1* |
Interactive Data File. |
101.INS* |
Inline XBRL Instance Document–the instance document does not appear in the Interactive Data File as its XBRL tags are embedded within the Inline XBRL document. |
101.SCH* |
Inline XBRL Taxonomy Extension Schema Document. |
101.CAL* |
Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
101.DEF* |
Inline XBRL Taxonomy Extension Definition Linkbase Document. |
101 LAB* |
Inline XBRL Taxonomy Extension Label Linkbase Document |
101 PRE* |
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
101 |
LAB* Inline XBRL Taxonomy Extension Label Linkebase Document |
101 |
PRE Inline XBRL Taxonomy Exension Presentation Linkbase Document |
104 |
Cover Page Interactive Data File (embedded within the Inline XBRL and contained in Exhibit 101) |
* Filed herewith.
** Furnished herewith.
† Indicates a management contract or any compensatory plan, contract, or arrangement.
| 89 |
| Table of Contents |
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
|
FSD Pharma Inc. |
|
|
|
|
|
|
|
By: |
/s/ Zeeshan Saeed |
|
|
Name: |
Zeeshan Saeed |
|
|
Title: |
Chief Executive Officer |
|
|
|
|
|
|
Date: |
April 1, 2024 |
|
87 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM |
To the Board of Directors and Shareholders of FSD Pharma Inc. Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of financial position of FSD Pharma Inc. (the “Company”) as of December 31, 2023 and 2022 and the related consolidated statements of loss and comprehensive loss, changes in shareholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”).
In our opinion, the consolidated financial statements present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2023 and 2022, and the results of its consolidated operations and its consolidated cash flows for each of the years in the three-year period ended December 31, 2023, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Material Uncertainty Related to Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has an accumulated deficit and has suffered a net loss that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Chartered Professional Accountants Licensed Public Accountants
We have served as the Company’s auditor since 2019
PCAOB ID:1930
Mississauga, Canada
March 28, 2024
MNP LLP |
|
|
Suite 900, 50 Burnhamthorpe Road W, Mississauga ON, L5B 3C2 |
|
T: 416.626.6000 F: 416.626.8650 |
|
|
|
MNP.ca |
| F-1 |
FSD Pharma Inc.
Consolidated financial statements
For the years ended December 31, 2023, 2022 and 2021
(expressed in United States dollars, except per share amounts)
| F-2 |
FSD PHARMA INC. |
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|||||||||||||
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION | |||||||||||||||||||
[expressed in United States dollars] | |||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||
As at |
|
|
|
December 31, |
|
|
December 31, |
|
|||||||||||
|
|
|
|
2023 |
|
|
2022 |
|
|||||||||||
|
|
Notes |
|
|
$ |
|
|
$ |
|
||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||
ASSETS |
|
|
|
|
|
|
|
|
|
||||||||||
Current assets |
|
|
|
|
|
|
|
|
|
||||||||||
Cash and cash equivalents |
|
|
|
|
|
2,757,040 |
|
|
|
16,980,472 |
|
||||||||
Other receivables |
|
|
6 |
|
|
|
228,764 |
|
|
|
374,377 |
|
|||||||
Prepaid expenses and deposits |
|
|
7 |
|
|
|
155,413 |
|
|
|
472,137 |
|
|||||||
Investments |
|
|
9 |
|
|
|
756,100 |
|
|
|
— |
|
|||||||
Finance receivables, net |
|
|
8 |
|
|
|
7,187,988 |
|
|
|
— |
|
|||||||
Net investment in lease |
|
|
|
|
|
|
— |
|
|
|
23,188 |
|
|||||||
|
|
|
|
|
|
|
11,085,305 |
|
|
|
17,850,174 |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Non-current assets |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Equipment, net |
|
|
|
|
|
|
87,583 |
|
|
|
105,729 |
|
|||||||
Investments |
|
|
9 |
|
|
|
6,049 |
|
|
|
827,612 |
|
|||||||
Right-of-use asset, net |
|
|
|
|
|
|
32,838 |
|
|
|
155,196 |
|
|||||||
Finance receivables, net |
|
|
8 |
|
|
|
907,366 |
|
|
|
7,431,656 |
|
|||||||
Intangible assets, net |
|
|
10 |
|
|
|
5,355,687 |
|
|
|
12,040,289 |
|
|||||||
|
|
|
|
|
|
|
17,474,828 |
|
|
|
38,410,656 |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Current liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Trade and other payables |
|
|
11 |
|
|
|
4,195,029 |
|
|
|
7,108,419 |
|
|||||||
Lease obligations |
|
|
|
|
|
|
38,650 |
|
|
|
177,870 |
|
|||||||
Warrants liability |
|
|
12 |
|
|
|
31,338 |
|
|
|
243,594 |
|
|||||||
Notes payable |
|
|
|
|
|
|
300,549 |
|
|
|
300,549 |
|
|||||||
|
|
|
|
|
|
|
4,565,566 |
|
|
|
7,830,432 |
|
|||||||
Non-current liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Lease obligations |
|
|
|
|
|
|
— |
|
|
|
38,004 |
|
|||||||
|
|
|
|
|
|
|
4,565,566 |
|
|
|
7,868,436 |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Class A share capital |
|
|
13 |
|
|
|
151,622 |
|
|
|
151,588 |
|
|||||||
Class B share capital |
|
|
13 |
|
|
|
137,626,863 |
|
|
|
143,258,972 |
|
|||||||
Warrants |
|
|
13 |
|
|
|
2,723,356 |
|
|
|
2,142,400 |
|
|||||||
Contributed surplus |
|
|
|
|
|
|
30,225,741 |
|
|
|
28,500,924 |
|
|||||||
Foreign exchange translation reserve |
|
|
|
|
|
|
417,341 |
|
|
|
652,601 |
|
|||||||
Accumulated deficit |
|
|
|
|
|
|
(157,908,160 | ) |
|
|
(144,164,265 | ) | |||||||
Equity attributable to shareholders of the Company |
|
|
|
|
|
|
13,236,763 |
|
|
|
30,542,220 |
|
|||||||
Non-controlling interests |
|
|
15 |
|
|
|
(327,501 | ) |
|
|
— |
|
|||||||
|
|
|
|
|
|
|
12,909,262 |
|
|
|
30,542,220 |
|
|||||||
|
|
|
|
|
|
|
17,474,828 |
|
|
|
38,410,656 |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Going concern uncertainty |
|
|
1 |
|
|
|
|
|
|
|
|
|
|||||||
Commitments and contingencies |
|
|
20 |
|
|
|
|
|
|
|
|
|
|||||||
Subsequent events |
|
|
24 |
|
|
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements. |
|
|
|
|
|
On behalf of the Board: |
|
|
|
|
|
|
|
"Signed" |
"Signed" |
|
|
Director - Zeeshan Saeed |
Director - Eric Hoskins |
|
|
| F-3 |
FSD PHARMA INC. |
|
|
|
|
|
|
|
|
|
|
|
CONSOLIDATED STATEMENTS OF LOSS AND COMPREHENSIVE LOSS | |||||
[expressed in United States dollars, except number of shares] | |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
For the years ended December 31, |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||||
|
|
Notes |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
||||
General and administrative |
|
|
17 |
|
|
|
9,032,724 |
|
|
|
14,450,094 |
|
|
|
15,926,103 |
|
External research and development fees |
|
|
|
|
|
|
3,859,178 |
|
|
|
6,910,844 |
|
|
|
6,328,104 |
|
Share-based payments |
|
|
14 |
|
|
|
3,835,475 |
|
|
|
1,531,258 |
|
|
|
7,443,930 |
|
Depreciation and amortization |
|
|
10 |
|
|
|
2,506,316 |
|
|
|
4,537,415 |
|
|
|
4,045,523 |
|
Impairment loss |
|
|
10 |
|
|
|
4,555,805 |
|
|
|
— |
|
|
|
— |
|
Total operating expenses |
|
|
|
|
|
|
23,789,498 |
|
|
|
27,429,611 |
|
|
|
33,743,660 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from continuing operations |
|
|
|
|
|
|
(23,789,498 | ) |
|
|
(27,429,611 | ) |
|
|
(33,743,660 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
18 |
|
|
|
(786,363 | ) |
|
|
(367,735 | ) |
|
|
(1,292 | ) |
Finance expense, net |
|
|
|
|
|
|
299 |
|
|
|
48,822 |
|
|
|
69,404 |
|
Gain on remeasurement of financial liability |
|
|
20 |
|
|
|
(4,939,015 | ) |
|
|
(119,453 | ) |
|
|
(49,792 | ) |
Gain on change in fair value of derivative liability |
|
|
12 |
|
|
|
(212,256 | ) |
|
|
(521,809 | ) |
|
|
(682,507 | ) |
Loss on changes in fair value of investments |
|
|
9 |
|
|
|
378,425 |
|
|
|
234,226 |
|
|
|
858,483 |
|
Net loss from continuing operations |
|
|
|
|
|
|
(18,230,588 | ) |
|
|
(26,703,662 | ) |
|
|
(33,937,956 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) from discontinued operations |
|
|
5 |
|
|
|
— |
|
|
|
3,096,834 |
|
|
|
(1,347,473 | ) |
Net loss |
|
|
|
|
|
|
(18,230,588 | ) |
|
|
(23,606,828 | ) |
|
|
(35,285,429 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Items that may be subsequently reclassified to loss: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exchange (loss) gain on translation of foreign operations |
|
|
|
|
|
|
(235,260 | ) |
|
|
412,989 |
|
|
|
31,815 |
|
Comprehensive loss |
|
|
|
|
|
|
(18,465,848 | ) |
|
|
(23,193,839 | ) |
|
|
(35,253,614 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity owners of the Company |
|
|
|
|
|
|
(17,902,179 | ) |
|
|
(23,606,828 | ) |
|
|
(35,285,429 | ) |
Non-controlling interests |
|
|
15 |
|
|
|
(328,409 | ) |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
(18,230,588 | ) |
|
|
(23,606,828 | ) |
|
|
(35,285,429 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted - continuing operations |
|
|
16 |
|
|
|
(0.46 | ) |
|
|
(0.69 | ) |
|
|
(0.97 | ) |
Basic and diluted - discontinued operations |
|
|
16 |
|
|
|
— |
|
|
|
0.08 |
|
|
|
(0.04 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares outstanding – basic and diluted |
|
|
16 |
|
|
|
39,588,663 |
|
|
|
38,732,381 |
|
|
|
34,945,210 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| F-4 |
FSD PHARMA INC. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS' EQUITY | ||||||||||||||||||||||||||||||||||||||||||||
For the years ended December 31, 2023, 2022 and 2021 | ||||||||||||||||||||||||||||||||||||||||||||
[expressed in United States dollars, except number of shares] | ||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
|
|
Class A shares |
|
|
Class B shares |
|
|
Warrants |
|
|
Contributed surplus |
|
|
Non-controlling interests |
|
|
Foreign exchange translation reserve |
|
|
Accumulated deficit |
|
|
Total |
|
||||||||||||||||||||
|
|
# |
|
|
$ |
|
|
# |
|
|
$ |
|
|
# |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|||||||||||
Balance, December 31, 2020 |
|
|
72 |
|
|
|
151,588 |
|
|
|
19,161,620 |
|
|
|
103,056,538 |
|
|
|
6,749,109 |
|
|
|
4,968,958 |
|
|
|
18,792,590 |
|
|
|
— |
|
|
|
207,797 |
|
|
|
(90,868,888 | ) |
|
|
36,308,583 |
|
Shares issued [note 13] |
|
|
— |
|
|
|
— |
|
|
|
15,480,462 |
|
|
|
38,341,407 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
38,341,407 |
|
Share-based payments [note 14] |
|
|
— |
|
|
|
— |
|
|
|
1,462,558 |
|
|
|
3,751,412 |
|
|
|
100,000 |
|
|
|
98,513 |
|
|
|
3,594,006 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
7,443,931 |
|
Share cancellation [note 13] |
|
|
— |
|
|
|
— |
|
|
|
(156,278 | ) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Lucid acquisition [note 4] |
|
|
— |
|
|
|
— |
|
|
|
4,502,392 |
|
|
|
7,023,732 |
|
|
|
112,162 |
|
|
|
70,563 |
|
|
|
196,436 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
7,290,731 |
|
Warrants expired |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,476 | ) |
|
|
(617 | ) |
|
|
617 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Comprehensive loss for the year |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
31,815 |
|
|
|
(35,285,429 | ) |
|
|
(35,253,614 | ) |
Balance, December 31, 2021 |
|
|
72 |
|
|
|
151,588 |
|
|
|
40,450,754 |
|
|
|
152,173,089 |
|
|
|
6,956,795 |
|
|
|
5,137,417 |
|
|
|
22,583,649 |
|
|
|
— |
|
|
|
239,612 |
|
|
|
(126,154,317 | ) |
|
|
54,131,038 |
|
Share repurchase [note 13] |
|
|
— |
|
|
|
— |
|
|
|
(1,999,800 | ) |
|
|
(7,523,117 | ) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5,596,880 |
|
|
|
(1,926,237 | ) |
Share-based payments [note 14] |
|
|
— |
|
|
|
— |
|
|
|
158,144 |
|
|
|
169,500 |
|
|
|
— |
|
|
|
— |
|
|
|
1,361,758 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,531,258 |
|
Share cancellation [note 13] |
|
|
— |
|
|
|
— |
|
|
|
(504,888 | ) |
|
|
(1,752,090 | ) |
|
|
— |
|
|
|
— |
|
|
|
1,752,090 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Warrants expired [note 13] |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(474,702 | ) |
|
|
(2,995,017 | ) |
|
|
2,995,017 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
PSUs converted to shares [note 14] |
|
|
— |
|
|
|
— |
|
|
|
400,000 |
|
|
|
191,590 |
|
|
|
— |
|
|
|
— |
|
|
|
(191,590 | ) |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Comprehensive loss for the year |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
412,989 |
|
|
|
(23,606,828 | ) |
|
|
(23,193,839 | ) |
Balance, December 31, 2022 |
|
|
72 |
|
|
|
151,588 |
|
|
|
38,504,210 |
|
|
|
143,258,972 |
|
|
|
6,482,093 |
|
|
|
2,142,400 |
|
|
|
28,500,924 |
|
|
|
— |
|
|
|
652,601 |
|
|
|
(144,164,265 | ) |
|
|
30,542,220 |
|
Initial recognition of non-controlling interests |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(24,467 | ) |
|
|
— |
|
|
|
(40,583 | ) |
|
|
(65,050 | ) |
Plan of arrangement [note 13] |
|
|
— |
|
|
|
34 |
|
|
|
23 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
8,673 |
|
|
|
— |
|
|
|
(8,673 | ) |
|
|
34 |
|
Share repurchase [note 13] |
|
|
— |
|
|
|
— |
|
|
|
(1,904,700 | ) |
|
|
(7,165,356 | ) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,207,540 |
|
|
|
(2,957,816 | ) |
Share-based payments [note 14] |
|
|
— |
|
|
|
— |
|
|
|
36,086 |
|
|
|
36,000 |
|
|
|
— |
|
|
|
— |
|
|
|
2,410,010 |
|
|
|
16,702 |
|
|
|
— |
|
|
|
— |
|
|
|
2,462,712 |
|
Share options exercised [note 13] |
|
|
— |
|
|
|
— |
|
|
|
21,000 |
|
|
|
33,247 |
|
|
|
— |
|
|
|
— |
|
|
|
(13,000 | ) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
20,247 |
|
PSUs converted to shares [note 14] |
|
|
— |
|
|
|
— |
|
|
|
2,720,104 |
|
|
|
1,464,000 |
|
|
|
— |
|
|
|
— |
|
|
|
(1,464,000 | ) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Warrants issued [note 13] |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,975,000 |
|
|
|
1,372,763 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,372,763 |
|
Warrants expired [note 13] |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(133,050 | ) |
|
|
(791,807 | ) |
|
|
791,807 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Comprehensive loss for the year |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(328,409 | ) |
|
|
(235,260 | ) |
|
|
(17,902,179 | ) |
|
|
(18,465,848 | ) |
Balance, December 31, 2023 |
|
|
72 |
|
|
|
151,622 |
|
|
|
39,376,723 |
|
|
|
137,626,863 |
|
|
|
10,324,043 |
|
|
|
2,723,356 |
|
|
|
30,225,741 |
|
|
|
(327,501 | ) |
|
|
417,341 |
|
|
|
(157,908,160 | ) |
|
|
12,909,262 |
|
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
FSD PHARMA INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the years ended December 31, 2023, 2022 and 2021
[expressed in United States dollars]
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Operating activities |
|
|
|
|
|
|
|
|
|
|||
Net loss from continuing operations |
|
|
(18,230,588 | ) |
|
|
(26,703,662 | ) |
|
|
(33,937,956 | ) |
Add (deduct) items not affecting cash |
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
2,506,316 |
|
|
|
4,534,586 |
|
|
|
4,045,523 |
|
Interest expense |
|
|
24,288 |
|
|
|
63,411 |
|
|
|
69,404 |
|
Share-based payments |
|
|
3,835,475 |
|
|
|
1,531,258 |
|
|
|
7,443,930 |
|
Change in fair value of investments |
|
|
378,425 |
|
|
|
234,226 |
|
|
|
858,483 |
|
Change in fair value of derivative liability |
|
|
(212,256 | ) |
|
|
(521,809 | ) |
|
|
(682,507 | ) |
Unrealized foreign exchange (gain) loss |
|
|
(383,514 | ) |
|
|
934,100 |
|
|
|
— |
|
Gain on remeasurement of financial liability |
|
|
(4,939,015 | ) |
|
|
(119,453 | ) |
|
|
(49,792 | ) |
Impairment loss |
|
|
4,555,805 |
|
|
|
— |
|
|
|
— |
|
Gain on net investment in lease |
|
|
— |
|
|
|
(22,619 | ) |
|
|
— |
|
Changes in non-cash working capital balances |
|
|
|
|
|
|
|
|
|
|
|
|
Finance receivables |
|
|
(663,698 | ) |
|
|
(7,431,656 | ) |
|
|
— |
|
Other receivables |
|
|
159,585 |
|
|
|
215,175 |
|
|
|
(106,880 | ) |
Prepaid expenses and deposits |
|
|
316,724 |
|
|
|
795,930 |
|
|
|
(609,153 | ) |
Note receivable |
|
|
(224,610 | ) |
|
|
— |
|
|
|
— |
|
Trade and other payables |
|
|
2,049,799 |
|
|
|
(699,778 | ) |
|
|
3,604,766 |
|
Cash used in continuing operating activities |
|
|
(10,827,264 | ) |
|
|
(27,190,291 | ) |
|
|
(19,364,182 | ) |
Cash used in discontinued operating activities |
|
|
— |
|
|
|
(1,142,982 | ) |
|
|
(1,382,041 | ) |
Cash used in operating activities |
|
|
(10,827,264 | ) |
|
|
(28,333,273 | ) |
|
|
(20,746,223 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Investing activities |
|
|
|
|
|
|
|
|
|
|
|
|
Cash acquired from acquisition of Lucid Psycheceuticals Inc. |
|
|
— |
|
|
|
— |
|
|
|
768,964 |
|
Purchase of investments |
|
|
(744,500 | ) |
|
|
(401,612 | ) |
|
|
— |
|
Purchase of equipment |
|
|
— |
|
|
|
(113,958 | ) |
|
|
— |
|
Additions to intangible assets |
|
|
— |
|
|
|
(250,000 | ) |
|
|
(500,000 | ) |
Net cash upon control of subsidiary |
|
|
31,783 |
|
|
|
— |
|
|
|
— |
|
Proceeds from sale of investments |
|
|
443,138 |
|
|
|
158,036 |
|
|
|
— |
|
Cash (used in) provided by continuing investing activities |
|
|
(269,579 | ) |
|
|
(607,534 | ) |
|
|
268,964 |
|
Cash provided by discontinued investing activities |
|
|
— |
|
|
|
12,730,942 |
|
|
|
— |
|
Cash (used in) provided by investing activities |
|
|
(269,579 | ) |
|
|
12,123,408 |
|
|
|
268,964 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
Share repurchase |
|
|
(2,957,816 | ) |
|
|
(1,926,237 | ) |
|
|
— |
|
Proceeds from issuance of shares, net |
|
|
34 |
|
|
|
— |
|
|
|
38,341,407 |
|
Repayment of notes payable |
|
|
— |
|
|
|
— |
|
|
|
(71,759 | ) |
Payment of lease obligation |
|
|
(189,054 | ) |
|
|
(143,071 | ) |
|
|
(57,566 | ) |
Share options exercised |
|
|
20,247 |
|
|
|
— |
|
|
|
— |
|
Cash (used in) provided by continuing financing activities |
|
|
(3,126,589 | ) |
|
|
(2,069,308 | ) |
|
|
38,212,082 |
|
Cash (used in) provided by financing activities |
|
|
(3,126,589 | ) |
|
|
(2,069,308 | ) |
|
|
38,212,082 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net decrease |
|
|
(14,223,432 | ) |
|
|
(18,279,173 | ) |
|
|
17,734,823 |
|
Cash and cash equivalents, beginning of the year |
|
|
16,980,472 |
|
|
|
35,259,645 |
|
|
|
17,524,822 |
|
Cash and cash equivalents, end of the year |
|
|
2,757,040 |
|
|
|
16,980,472 |
|
|
|
35,259,645 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements. |
|
|
|
|
|
|
|
|
|
|
|
|
| F-6 |
FSD PHARMA INC.
Notes to the consolidated financial statements
(expressed in United States dollars)
December 31, 2023 and 2022
1. Nature of business
FSD Pharma Inc. (“FSD” or the “Company”) is a biopharmaceutical company dedicated to building a portfolio of innovative assets and biotech solutions for the treatment of challenging neurodegenerative, inflammatory and metabolic disorders and alcohol misuse disorders with drug candidates in different stages of development. Through its wholly-owned subsidiary, Lucid Psycheceuticals Inc. (“Lucid”), FSD is focused on the research and development of its lead compound, Lucid-MS (formerly Lucid-21-302) (“Lucid-MS”). Lucid-MS is a patented new chemical entity shown to prevent and reverse myelin degradation, the underlying mechanism of multiple sclerosis, in preclinical models. FSD is also focused on the research and development of a treatment for alcohol misuse for application in hospitals and other medical practices. FSD maintains a portfolio of strategic investments through its wholly-owned subsidiary, FSD Strategic Investments Inc., which represent loans secured by residential property.
The Company’s registered office is located at 199 Bay Street, Suite 4000, Toronto, Ontario, M5L 1A9. The Company’s shares are listed on the Nasdaq Capital Market and on the Canadian Securities Exchange under the symbol “HUGE”.
On July 31, 2023, the Company entered into an exclusive intellectual property license agreement (the “License Agreement”) with Celly Nutrition Corp. (“Celly”). The License Agreement provides Celly access to proprietary information for the purposes of consumer product development and marketing. The License Agreement grants Celly the rights to a proprietary formulation of natural ingredients, vitamins, and minerals to help with liver and brain function for the purposes of potentially quickly relieving from the effects of alcohol consumption, such as inebriation, and restoring normal lifestyle. The License Agreement also grants Celly rights to certain trademarks. In exchange, FSD received 200,000,000 common shares in the capital of Celly following a 2:1 share-split. The Company also received an anti-dilution Warrant Certificate that entitles FSD to purchase up to 25% of the common shares deemed outstanding less the 200,000,000 common shares issued under the License Agreement and from time to time as a result of any partial exercise under the anti-dilution Warrant Certificate. FSD Pharma is also entitled to certain license fees and royalties under the License Agreement. Through the License Agreement, FSD acquired 34.66% of Celly. On July 31, 2023, the Company and Celly entered into a loan agreement for gross proceeds of C$1,000,000. The loan was funded on August 1, 2023, and accrues interest at a rate of 10% per annum. Interest is payable annually and the loan matures on July 31, 2026. In November 2023, through the Plan of Arrangement the Company distributed 45,712,529 of its 200,000,000 shares of Celly to its shareholders. The consolidated financial statements incorporate the assets and liabilities of Celly as of December 31, 2023, and the results of operations and cash flows for the period commencing on July 31, 2023, being the date on which FSD obtained control of Celly (Note 2(e)).
Going concern uncertainty
The consolidated financial statements (“financial statements”) of the Company for the years ended December 31, 2023, 2022 and 2021, have been prepared on the basis of accounting principles applicable to a going concern, which assumes that the Company will continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities and commitments in the normal course of operations. These financial statements do not include any adjustments to the amounts and classification of assets and liabilities that would be necessary should the Company be unable to continue as a going concern. Such adjustments could be material.
The Company is in the preliminary stages of its planned operations and has not yet determined whether its processes and business plans are economically viable. The continued operations of the Company and the recoverability of amounts shown for intangible assets are dependent upon the ability of the Company to obtain sufficient financing to complete the research and development program of Lucid-MS. As well as fund the research and development of a treatment for alcohol misuse for application in hospitals and other medical practices.
| F-7 |
FSD PHARMA INC.
Notes to the consolidated financial statements
(expressed in United States dollars)
December 31, 2023 and 2022
As at December 31, 2023, the Company has an accumulated deficit of $157.9 million, a net loss of $18.2 million and a working capital surplus of $6.5 million. Whether, and when, the Company can attain profitability and positive cash flows from operations is subject to material uncertainty. The application of the going concern assumption is dependent upon the Company’s ability to generate future profitable operations and obtain necessary financing to do so. The Company will need to raise additional capital in order to fund its planned operations and meet its obligations. While the Company has been successful in obtaining financing to date and believes it will be able to obtain sufficient funds in the future and ultimately achieve profitability and positive cash flows from operations, there can be no assurance that the Company will achieve profitability and be able to do so on terms favourable for the Company. The above events and conditions indicate there is a material uncertainty that casts significant doubt about the Company’s ability to continue as a going concern.
Subsidiaries
The Company has the following subsidiaries:
|
|
|
Ownership percentage as at |
|
||||||||||
Entity Name |
|
Country |
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
|
|
|
|
% |
|
|
% |
|
|
% |
|
|||
FSD Biosciences Inc. |
|
USA |
|
|
100.00 |
|
|
|
100.00 |
|
|
|
100.00 |
|
Prismic Pharmaceuticals Inc. |
|
USA |
|
|
100.00 |
|
|
|
100.00 |
|
|
|
100.00 |
|
FV Pharma Inc. |
|
Canada |
|
|
100.00 |
|
|
|
100.00 |
|
|
|
100.00 |
|
Lucid Psycheceuticals Inc. |
|
Canada |
|
|
100.00 |
|
|
|
100.00 |
|
|
|
100.00 |
|
FSD Strategic Investments Inc. |
|
Canada |
|
|
100.00 |
|
|
|
100.00 |
|
|
|
— |
|
FSD Pharma Australia Pty Ltd |
|
Australia |
|
|
100.00 |
|
|
|
100.00 |
|
|
|
— |
|
Celly Nutrition Corp. |
|
Canada |
|
|
26.15 |
|
|
|
— |
|
|
|
— |
|
2. Basis of presentation
[a] Statement of compliance
These financial statements have been prepared by management in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”). The policies set out below have been consistently applied to all periods presented, unless otherwise noted.
These financial statements were approved and authorized for issuance by the Board of Directors (the “Board”) of the Company on March 26, 2024.
[b] Basis of measurement
These financial statements have been prepared on a historical cost basis, except for certain financial instruments which are measured at fair value. Historical costs are generally based upon the fair value of the consideration given in exchange for goods and services received.
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date, regardless of whether that price is directly observable or estimated using another valuation technique. In estimating the fair value of an asset or a liability, the Company takes into account the characteristics of the asset or liability if market participants would take those characteristics into account when pricing the asset or liability at the measurement date. Fair value for measurement and/or disclosure purposes in these financial statements is determined on such a basis, except for share-based payment transactions that are within the scope of IFRS 2, Share-based Payment (“IFRS 2”) and measurements that have some similarities to fair value, but are not fair value, such as value in use in IAS 36, Impairment of Assets (“IAS 36”).
| F-8 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
[c] Basis of presentation
The accompanying financial statements include the accounts of FSD and its subsidiaries. The financial statements incorporate the assets and liabilities of the Company and its subsidiaries as at December 31, 2023 and 2022 and the results for the Company and its subsidiaries for the years ended December 31, 2023, 2022 and 2021.
Subsidiaries are those entities over which the Company has control. The Company controls an entity when it is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the Company. All intra-entity assets and liabilities, revenues, expenses and cash flows relating to transactions between subsidiaries of the Company are eliminated in full on consolidation.
Non-controlling interests (“NCI”) represent ownership interests in consolidated subsidiaries by parties that are not shareholders of the Company. They are shown as a component of total equity in the consolidated statements of financial position, and the share of income (loss) attributable to non-controlling interests is shown as a component of net income (loss) in the consolidated statements of loss and comprehensive loss. Changes in the parent company’s ownership that do not result in a loss of control are accounted for as equity transactions.
[d] Functional currency and presentation currency
The financial statements of each company within the consolidated group are measured using their functional currency, which is the currency of the primary economic environment in which an entity operates. The Company’s functional currency is the United States dollar and the functional currencies of its subsidiaries are as follows:
|
FSD Biosciences Inc. |
United States Dollar |
|
Prismic Pharmaceuticals Inc. |
United States Dollar |
|
FV Pharma Inc. |
Canadian Dollar |
|
Lucid Psycheceuticals Inc. |
Canadian Dollar |
|
FSD Strategic Investments Inc. |
Canadian Dollar |
|
FSD Pharma Australia Pty Ltd |
Australian Dollar |
|
Celly Nutrition Corp. |
Canadian Dollar |
[e] Use of estimates and judgments
The preparation of these financial statements in conformity with IFRS requires management to make estimates, judgments and assumptions that affect the application of accounting policies and the reported amounts of assets and liabilities as at the date of the financial statements and the reported amounts of revenue and expenses during the reporting periods. Actual results could differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that the Company may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period, or in the period of the revision and future periods if the revision affects both current and future periods.
| F-9 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The following are the critical judgments, apart from those involving estimations, that management has made in the process of applying the Company’s accounting policies and that have the most significant effect on the amounts recognized in the financial statements:
[i] Going concern
At each reporting period, management assesses the basis of preparation of the financial statements. These financial statements have been prepared on a going concern basis in accordance with IFRS. The going concern basis of presentation assumes that the Company will continue its operations for the foreseeable future and be able to realize its assets and discharge its liabilities and commitments in the normal course of business.
[ii] Contingencies
From time to time, the Company is named as a party to claims or involved in proceedings, including legal, regulatory and tax related, in the ordinary course of its business. While the outcome of these matters may not be estimable at the reporting date, the Company makes provisions, where possible, for the estimated outcome of such claims or proceedings. Should a loss result from the resolution of any claims or proceedings that differs from these estimates, the difference will be accounted for as a charge to profit or loss in that period. The actual results may vary and may cause significant adjustments.
[iii] Intangible assets
The Company employs significant estimates to determine the estimated useful lives of intangible assets, considering the nature of the asset, contractual rights, expected use and review of asset useful lives. The Company reviews amortization methods and useful lives annually or when circumstances change and adjusts its amortization methods and assumptions prospectively.
The Company reviews intangible assets for impairment annually or when impairment indicators exist. If the recoverable amount of the respective intangible asset is less than its carrying amount, it is considered to be impaired. In the process of measuring the recoverable amount, management makes assumptions about future events and circumstances. The actual results may vary and may cause significant adjustments.
[iv] Valuation of share-based payments and warrants
Management measures the costs for share-based payments and warrants, including certain warrant liabilities, using market-based option valuation techniques. Assumptions are made and estimates are used in applying the valuation techniques. These include estimating the future volatility of the share price, expected dividend yield, expected term, expected risk-free interest rate and the rate of forfeiture. For performance share units (“PSUs”), management is required to estimate when the vesting conditions will be met. Such estimates and assumptions are inherently uncertain. Changes in these assumptions affect the fair value estimates of share-based payments, warrants and warrant liabilities.
[v] Allowance for credit losses
Judgment is required as to the timing of establishing an allowance for credit losses and to estimate the amount of expected credit losses taking into consideration counterparty creditworthiness, the fair value of underlying collateral, current and future economic trends, the expected residual value of the underlying assets and past experience.
[vi] Valuation of investments
The Company holds investments that have do not have quoted prices in active markets. In determining the fair value of investments, management is required to make certain estimates and assumptions regarding the fair value as of the reporting date. Assumptions are made and estimates are used in applying the valuation techniques to determine fair value. These include observable inputs other than quoted prices in active markets. Such investments are classified as Level 2 within the fair value hierarchy. The value at which the Company could ultimately realize upon disposition of these investments may differ from their carrying value and such differences could be material.
| F-10 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The financial information of private companies may not always be available, or such information may be insufficient or unreliable for valuation purposes. In determining the fair value of shares held in private company investments, management is required to make certain estimates and assumptions regarding the fair value as of the reporting date. Assumptions are made and estimates are used in applying the valuation techniques to determine fair value. These include the most recently available financial statements of the investee, price for most recently completed financing, as well as closely comparable public companies and general market and economic conditions. Such investments are classified as Level 3 within the fair value hierarchy. The value at which the Company could ultimately realize upon disposition of these investments may differ from their carrying value and such differences could be material.
[vii] Functional currency
The Company and its subsidiaries are required to determine their functional currencies based on the primary economic environment in which each entity operates. In order to do that, management has to analyze several factors, including which currency mainly influences the cost of undertaking the business activities, in which currency the entity has received financing, and in which currency it keeps its receipts from operating activities. Management uses its judgment to determine which factors are most important when the above indicators are mixed and the functional currency is not obvious.
[viii] Disclosure of interests in other entities
To assess the investment in Celly, judgment was required to determine if the Company has significant influence or control of Celly. The Company considered the relevant guidance in IFRS 10 – Consolidated Financial Statements, IAS 24 – Related Party Disclosures and IAS – 28 Investments in Associates and Joint Ventures.
Judgment is applied in determining when the Company controls an investment even if the Company holds less than a majority of the investee’s voting rights (the existence of de facto control). The Company concluded it has control of Celly even though the Company only holds 26.15% of the voting rights as of December 31, 2023. The Company concluded it has control of Celly as the Company, together with persons or entities considered to be de facto agents of the Company, holds a combined 52.05% of the voting rights of Celly. In addition, key management personnel of the Company hold three of the four board of director positions of Celly. The assessment of control is performed on a continuous basis. The Company determined that it obtained control of Celly on July 31, 2023, and control was maintained at all times from July 31, 2023, through December 31, 2023. Celly is significantly dependent on the Company as a result of the License Agreement and the loan. The NCI component of Celly is included as a separate component in equity (Note 15).
3. Material accounting policies
[a] Equipment
Equipment is measured at cost less accumulated depreciation and impairment losses. The cost of an item of equipment includes expenditures that are directly attributable to the acquisition or construction of the asset.
Depreciation is based on the estimated useful lives of the assets provided as follows:
|
Computer equipment |
3 years |
|
Furniture and fixtures Lease improvements |
3 – 10 years Over the term of the lease |
An item of equipment and any significant part initially recognized are derecognized upon disposal or when no future economic benefits are expected from their use or disposal. Any gain or loss arising on derecognition of the asset (calculated as the difference between the net disposal proceeds and the carrying amount of the asset) is included in the consolidated statements of loss and comprehensive loss when the asset is derecognized. The assets’ residual values, useful lives and methods of depreciation and the depreciation charge are adjusted prospectively, if appropriate.
| F-11 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
[b] Intangible Assets
Intangible assets are recorded at cost less accumulated amortization and accumulated impairment losses. Amortization is recognized in the consolidated statements of loss and comprehensive loss on a straight-line basis over the useful life, as follows:
|
Intellectual Property |
5 – 15 years |
Expenditures on internally generated intangible assets during the research phase are expensed as incurred. Expenditures on internally generated intangible assets during the development phase, which comprise deferred development costs, are initially capitalized and recognized in the consolidated balance sheet if they meet the recognition criteria. Subsequent to initial recognition, deferred development costs are accounted for at cost less accumulated amortization and are amortized on a straight-line basis over an estimated useful life beginning once the deferred development costs are used in commercial production.
[c] Foreign Currency Transactions
Foreign currency transactions are translated into functional currencies at exchange rates in effect on the date of the transactions. At the end of each reporting period, monetary assets and liabilities denominated in foreign currencies are translated into functional currencies at the foreign exchange rate applicable at that period-end date. Non-monetary assets and liabilities that are measured in terms of historical cost in a foreign currency are translated using the exchange rate at the date of the transaction. Realized and unrealized exchange gains and losses are recognized in the consolidated statements of loss and comprehensive loss.
On consolidation, assets and liabilities of operations with a functional currency other than United States dollar are translated into United States dollar at period end foreign currency rates. Expenses of such operations are translated into the United States dollar at average rates for the period. Foreign currency translation gains and losses are recognized in other comprehensive income. The relevant amount in cumulative foreign currency translation adjustment is reclassified into earnings upon disposition of a foreign operation.
[d] Financial Instruments
Financial assets and financial liabilities are recognized when the Company becomes a party to the contractual provisions of the instruments.
Financial assets and financial liabilities are initially measured at fair value. Transaction costs that are directly attributable to the acquisition or issue of financial assets and financial liabilities (other than financial assets and financial liabilities at fair value through profit or loss) are added to or deducted from the fair value of the financial assets or financial liabilities, as appropriate, on initial recognition. Transaction costs directly attributable to the acquisition of financial assets or financial liabilities at fair value through profit or loss are recognized immediately in the consolidated statements of loss and comprehensive loss.
· |
Financial assets
On initial recognition, a financial asset is classified as measured at amortized cost, fair value through other comprehensive income (‘‘FVOCI’’), or fair value through profit and loss (‘‘FVTPL’’). The classification of financial assets is based on the business model in which a financial asset is managed and its contractual cash flow characteristics. |
| F-12 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
|
A financial asset is measured at amortized cost if it meets both of the following conditions and is not designated as at FVTPL: |
|
|
|
|
|
· |
It is held within a business model whose objective is to hold assets to collect contractual cash flows; and |
|
|
|
|
· |
Its contractual terms give rise on specified dates to cash flows that are solely payments of principal and interest on the principal amount outstanding. |
|
|
|
|
A financial asset (unless it is a trade receivable without a significant financing component that is initially measured at the transaction price) is initially measured at fair value plus, for an item not at FVTPL, transaction costs that are directly attributable to its acquisition.
The following accounting policies apply to the subsequent measurement of financial assets. |
|
Financial assets at FVTPL |
Subsequently measured at fair value. Net gains and losses, including any interest or dividend income, are recognized in the consolidated statements of loss and comprehensive loss. |
Financial assets at amortized cost |
Subsequently measured at amortized cost using the effective interest method, less any impairment losses. Interest income, foreign exchange gains and losses and impairment losses are recognized in profit or loss. Any gain or loss on derecognition is recognized in consolidated statements of loss and comprehensive loss. |
· |
Financial liabilities |
|
The Company initially recognizes financial liabilities at fair value on the date at which the Company becomes a party to the contractual provisions of the instrument.
The Company classifies its financial liabilities as either financial liabilities at FVTPL or amortized cost.
Subsequent to initial recognition, other liabilities are measured at amortized cost using the effective interest method. Financial liabilities at FVTPL are stated at fair value with changes being recognized in consolidated statements of loss and comprehensive loss.
The Company derecognizes a financial liability when its contractual obligations are discharged or cancelled or expire. |
· |
Financial liabilities and equity instruments |
|
· |
Classification as debt or equity |
|
|
Debt and equity instruments issued by the Company are classified as either financial liabilities or as equity in accordance with the substance of the contractual arrangements and the definitions of a financial liability and an equity instrument. The Company does not reclassify financial liabilities or equity after initial recognition due to a change in circumstance. |
|
|
|
|
· |
Equity instruments |
|
|
|
|
|
An equity instrument is any contract that evidences a residual interest in the assets of an entity after deducting all of its liabilities. Equity instruments issued by a group entity are recognized at the proceeds received, net of direct issue costs.
Repurchase of the Company’s own equity instruments is recognized and deducted directly in equity. No gain or loss is recognized in the consolidated statements of loss and comprehensive loss on the purchase, sale, issue or cancellation of the Company’s own equity instruments. |
| F-13 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
· |
Classification of financial instruments |
|
The Company classifies its financial assets and liabilities depending on the purpose for which the financial instruments were acquired, their characteristics and management intent as outlined below: |
|
Cash and cash equivalent |
Amortized cost |
|
Other receivables |
Amortized cost |
|
Investments |
Fair value through profit or loss |
|
Finance receivables |
Amortized cost |
|
Trade and other payables |
Amortized cost |
|
Warrants liability |
Fair value through profit or loss |
|
Notes payable |
Amortized cost |
· |
Impairment of financial assets |
|
· |
Other receivables |
|
|
An expected credit loss (“ECL”) model applies to financial assets measured at amortized cost. The Company’s financial assets measured at amortized cost and subject to the ECL model consist primarily of finance and other receivables. The Company applies the simplified approach to impairment for finance and other receivables by recognizing a loss allowance based on lifetime expected losses at each reporting date taking into considerations historical credit loss experience and financial factors specific to the debtors and general economic conditions. The Company has assessed the impairment of its finance and other receivables using the expected credit loss model. |
|
· |
Finance receivables |
|
|
Finance receivables are a financial asset initially recognized at fair value and are subsequently carried at amortized cost using the effective interest method. The Company’s business model is to hold these receivables to collect contractual cash flows that represent solely payments of principal and interest. Finance receivables are assessed for impairment at the end of each reporting period in accordance with IFRS 9 as outlined below.
The ECL model is based on the credit losses expected to arise over the life of the assets, unless there has been no significant increase in credit risk since origination, in which case the allowance is based on the 12 months’ expected credit loss. The ECL model uses a three-stage impairment approach based on changes in the credit risk of the finance receivable since initial recognition. The three stages are as follows:
Stage 1– Finance receivables that have not experienced a significant increase in credit risk since initial recognition.
Stage 2– Finance receivables that have experienced a significant increase in credit risk since initial recognition.
Stage 3 – Finance receivables for which there is objective evidence of impairment. |
| F-14 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The Company considers a number of factors when assessing if there has been a significant increase in credit risk, including the number of days past due, changes in the financial condition of the borrower, responsiveness of the borrower and other borrower specific information that may be available, without consideration of collateral.
In determining its estimation of the ECL allowances, the Company also considers past events, current market conditions including interest rates, real estate market statistics, and supportable forward-looking information, including macro-economic factors, such as housing price and interest rate forecasts.
The ECL model requires the recognition of credit losses equal to 12-month ECLs for Stage 1 and recognition of lifetime expected credit losses for Stage 2 and 3. The 12-month ECLS are lifetime ECLS that are expected to occur within 12 months after the reporting date. The lifetime ECLs represent the expected loss in value due to possible default events over the life of a mortgage receivable weighted by the likelihood of a loss. Three factors are primarily used to measure ECLs: probability of default (PD), loss given default (LGD) and exposure at default (EAD).
[e] Impairment of long-lived assets
Long-lived assets, including equipment and intangible assets are tested for impairment at each reporting date when there are indicators of impairment or whenever events or changes in circumstances indicate that the carrying amount of an asset exceeds its recoverable amount. Intangible assets with an indefinite useful life are tested for impairment at least annually in the fourth quarter and whenever there is an indication that the asset may be impaired. The Company has no indefinite life intangible assets.
For the purpose of impairment testing, assets that cannot be tested individually are grouped together into the smallest group of assets that generates cash inflows from continuing use that are largely independent of the cash inflows of other assets or groups of assets (the cash-generating unit, or “CGU”). The recoverable amount of an asset or a CGU is the higher of its fair value less costs to sell and its value in use. If the carrying amount of an asset exceeds its recoverable amount, an impairment charge is recognized immediately in net loss equal to the amount by which the carrying amount exceeds the recoverable amount. Where an impairment loss subsequently reverses, the carrying amount of the asset is increased to the lesser of the revised estimate of the recoverable amount, and the carrying amount that would have been recorded had no impairment loss been recognized previously.
[f] Income Taxes
Income tax expense comprises current and deferred tax. Current tax and deferred tax are recognized in net profit or loss except to the extent that it relates to a business combination or items recognized directly in equity or in other comprehensive loss.
Current income taxes are recognized for the estimated income taxes payable or receivable on taxable income or loss for the current year and any adjustment to income taxes payable in respect of previous years. Current income taxes are determined using tax rates and tax laws that have been enacted or substantively enacted by the year-end date.
Deferred tax assets and liabilities are recognized where the carrying amount of an asset or liability differs from its tax base, except for taxable temporary differences arising on the initial recognition of goodwill and temporary differences arising on the initial recognition of an asset or liability in a transaction which is not a business combination and at the time of the transaction affects neither accounting nor taxable profit or loss.
Recognition of deferred tax assets for unused tax losses, tax credits and deductible temporary differences is restricted to those instances where it is probable that future taxable profit will be available against which the deferred tax asset can be utilized. At the end of each reporting period, the Company reassesses unrecognized deferred tax assets. The Company recognizes a previously unrecognized deferred tax asset to the extent that it has become probable that future taxable profit will allow the deferred tax asset to be recovered.
| F-15 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
[g] Share-based Compensation
Share options and warrants awarded to non-employees are accounted for using the fair value of the instrument awarded or service provided, whichever is considered more reliable. Share options, PSUs and warrants awarded to employees are accounted for using the fair value method. The fair value of the share options, PSUs and warrants granted are recognized as an expense on a proportionate basis consistent with the vesting features of each tranche of the grant. The fair value of share options and warrants are calculated using the Black-Scholes option pricing model with assumptions applicable at the date of grant. The fair value of PSUs is calculated using market share prices at the date of grant.
[h] Net Loss per Share
Net loss per share is calculated based on the loss for the financial year and the weighted average number of common shares outstanding during the year. Diluted net loss per share is calculated using the loss for the financial year adjusted for the effect of any dilutive instruments and the weighted average diluted number of shares (ignoring any potential issue of common shares that would be anti-dilutive) during the year.
[i] External research and development
External research and development costs are expensed in the periods in which they are incurred, with the exception of development costs for new products with proven technical feasibility and for which a defined future market exists. Such development costs are capitalized in accordance with the Company’s policy for intangible assets. The Company’s external research and development costs relate primarily to third-party contract research organizations.
[j] Discontinued operations
Discontinued operations are reported when a component of the Company, representing a separate major line of business or area of operations with clearly distinguishable cash flows, has been disposed of or is held for sale. Classification as a discontinued operation occurs upon disposal or when the operation meets the criteria to be classified as held for sale, if earlier. Discontinued operations are reported as a separate element of net income or loss on the consolidated statements of loss and comprehensive loss for both the current and comparative periods. When a disposal group is classified as held for sale, assets and liabilities are aggregated and presented as separate line items, respectively, on the consolidated statements of financial position. Comparative periods are not restated on the consolidated statements of financial position. Assets held for sale are not depreciated and are measured at the lower of carrying value and fair value less costs to sell.
New standards, amendments and interpretations recently adopted by the Company
IAS 1, Presentation of financial statements (“IAS 1”)
In January 2020, the IASB issued Classification of Liabilities as Current or Non-current (Amendments to IAS 1). The amendments aim to promote consistency in applying the requirements by helping companies determine whether, in the consolidated statements of financial position, debt and other liabilities with an uncertain settlement date should be classified as current (due or potentially due to be settled within one year) or non-current. The amendments include clarifying the classification requirements for debt a company might settle by converting it into equity.
The amendments are effective for annual reporting periods beginning on or after January 1, 2024, with earlier application permitted. The Company early adopted these amendments effective January 1, 2023. The impact of adopting these amendments on the Company’s financial statements was not material.
| F-16 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
IAS 8, Accounting Policies, Changes in Accounting Estimates and Errors (“IAS 8”)
In February 2021, the IASB issued Definition of Accounting Estimates, which amends IAS 8. The amendment clarifies how to distinguish changes in accounting policies from changes in accounting estimates. Under the new definition, accounting estimates are “monetary amounts in financial statements that are subject to measurement uncertainty”. The amendment provides clarification to help entities to distinguish between accounting policies and accounting estimates.
The amendments are effective for annual periods beginning on or after January 1, 2023. The impact of adopting these amendments on the Company’s financial statements was not material.
IAS 12, Income Taxes (“IAS 12”)
In May 2021, the IASB issued Deferred Tax related to Assets and Liabilities arising from a single transaction (Amendments to IAS 12). The amendment narrows the scope of the initial recognition exemption so that it does not apply to transactions that give rise to equal taxable and deductible temporary differences. As a result, companies will need to recognize a deferred tax asset and deferred tax liability for temporary differences arising on initial recognition of transactions such as leases and decommissioning obligations.
The amendments are effective for annual reporting periods beginning on or after January 1, 2023 and are to be applied retrospectively. The impact of adopting these amendments on the Company’s financial statements was not material.
New standards, amendments and interpretations not yet adopted by the Company
IFRS 16 – Leases (“IFRS 16”)
In September 2022, the IASB issued amendments to IFRS 16, Leases, which add to requirements explaining how a company accounts for a sale and leaseback after the date of the transaction.
The amendments are effective for annual reporting periods beginning on or after January 1, 2024. Earlier application is permitted.
All other IFRSs and amendments issued but not yet effective have been assessed by the Company and are not expected to have a material impact on the financial statements.
4. Acquisition of Lucid
On September 21, 2021, the Company acquired all of the issued and outstanding common shares of Lucid, an early-stage Canadian-based specialty pharmaceutical company focused on the development of therapies to treat critical neurodegenerative diseases, for total consideration of $7,290,731. The acquisition is part of the Company’s strategy of building a portfolio of biotech assets.
Prior to the acquisition, the Company’s Executive Co-Chairman of the Board beneficially held approximately 4.5% ownership interest in Lucid through an entity related to this individual.
It was determined that the acquisition of Lucid did not qualify as a business combination in accordance with IFRS 3, and therefore it was accounted for as an asset acquisition. The individual identifiable assets acquired and liabilities assumed were identified. The purchase consideration was first allocated to the fair values of the acquired cash and cash equivalents, other receivables, prepaid expenses and deposits and trade and other payables, as their carrying values were determined to equal their fair values. The remaining purchase price was allocated to the acquired intangible assets.
| F-17 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The total consideration for the purchase of Lucid was $7,290,731. The purchase consideration consisted of $7,023,732 of Class B shares, $196,436 of share options and $70,563 of warrants. 304,880 Class B shares and all of the warrants were issued to an entity related to the interim CEO and Executive Co-Chairman of the Board. The fair value of the Class B shares was determined based on a total of 4,502,392 shares issued and a fair value of $1.56 per share, which reflects the share price on the date of acquisition. The fair value of the 161,091 share options and 112,162 warrants issued as part of the consideration were determined using the Black-Scholes options pricing model with the following assumptions:
|
|
Warrants |
|
|
Share Options |
|
||
Grant date share price |
|
$ | 1.56 |
|
|
$ | 1.56 |
|
Exercise Price |
|
$ | 0.96 - $1.93 |
|
|
$ | 1.35 - $2.31 |
|
Expected dividend yield |
|
|
— |
|
|
|
— |
|
Risk free interest rate |
|
|
0.43 | % |
|
0.43% - 0.79% |
|
|
Expected life (years) |
|
1.19 - 1.28 |
|
|
2.23 - 4.28 |
|
||
Annualized volatility |
|
|
88 | % |
|
|
124 | % |
The allocation of the total consideration to the fair value of the identifiable assets acquired and liabilities assumed as at the date of the acquisition was as follows:
|
|
Fair value recognized on acquisition |
|
|
|
|
$ |
|
|
Cash and cash equivalents |
|
|
768,964 |
|
Other receivables |
|
|
271,564 |
|
Prepaid expenses and deposits |
|
|
167,776 |
|
Intangible assets |
|
|
6,186,251 |
|
Trade and other payables |
|
|
(103,824 | ) |
|
|
|
7,290,731 |
|
The Company also capitalized $128,320 of acquisition related costs to the acquired intellectual property (Note 10).
5. Discontinued operations
In March 2020, the Company decided to focus its efforts and resources on the pharmaceutical business and initiated the process to exit the medical cannabis industry and sell FV Pharma’s facility located at 520 William Street, Cobourg, Ontario, K9A 3A5 (the “Facility”) and the 64-acre property on which the Facility is located (the “Facility Property”). On May 6, 2022, the Company closed the sale of the Facility and the Facility Property for total consideration of $12,730,942 (C$16,400,000). The Company recognized a gain of $4,249,582 on the sale of the Facility and the Facility Property and incurred selling expenses of $616,002 for the year ended December 31, 2022.
Results of operations related to the Disposal Group are reported as discontinued operations for the years ended December 31, 2022 and 2021.
| F-18 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
Net income (loss) from discontinued operations for the years ended December 31, 2022 and 2021 is comprised of the following:
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
For the years ended December 31, |
|
||||||
|
|
Notes |
|
|
2022 $ |
|
|
2021 $ |
|
|||
Expenses |
|
|
|
|
|
|
|
|
|
|||
General and administrative |
|
|
17 |
|
|
|
1,185,600 |
|
|
|
1,412,392 |
|
Total operating expenses |
|
|
|
|
|
|
1,185,600 |
|
|
|
1,412,392 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from discontinued operations |
|
|
|
|
|
|
(1,185,600 | ) |
|
|
(1,412,392 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income |
|
|
|
|
|
|
(32,852 | ) |
|
|
(64,919 | ) |
Gain on sale of property and plant |
|
|
|
|
|
|
(4,249,582 | ) |
|
|
— |
|
Net income (loss) from discontinued operations |
|
|
|
|
|
|
3,096,834 |
|
|
|
(1,347,473 | ) |
Cash flows from discontinued operations for the year ended December 31, 2022 and 2021 is comprised of the following:
|
|
For the years ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
|
|
$ |
|
|
$ |
|
||
Operating activities |
|
|
|
|
|
|
||
Net income (loss) from discontinued operations |
|
|
3,096,834 |
|
|
|
(1,347,473 | ) |
Add (deduct) items not affecting cash |
|
|
|
|
|
|
|
|
Changes in non-cash working capital balances |
|
|
|
|
|
|
|
|
Gain on sale of property and plant |
|
|
(4,249,582 | ) |
|
|
— |
|
Other receivables |
|
|
(88,588 | ) |
|
|
38,822 |
|
Prepaid expenses and deposits |
|
|
98,354 |
|
|
|
(20,091 | ) |
Trade and other payables |
|
|
— |
|
|
|
(53,299 | ) |
Cash used in operating activities |
|
|
(1,142,982 | ) |
|
|
(1,382,041 | ) |
|
|
|
|
|
|
|
|
|
Proceeds from sale of property and plant, net |
|
|
12,730,942 |
|
|
|
— |
|
Cash provided by investing activities |
|
|
12,730,942 |
|
|
|
— |
|
There were no discontinued operations for the year ended December 31, 2023.
6. Other receivables
The Company’s other receivables are comprised of the following:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
|
|
$ |
|
|
$ |
|
||
Sales tax recoverable |
|
|
209,550 |
|
|
|
279,333 |
|
Interest receivable |
|
|
15,511 |
|
|
|
95,044 |
|
Other receivables |
|
|
3,703 |
|
|
|
— |
|
|
|
|
228,764 |
|
|
|
374,377 |
|
| F-19 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
7. Prepaid expenses and deposits
The Company’s prepaid expenses and deposits include the following:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
|
|
$ |
|
|
$ |
|
||
Research and development |
|
|
30,705 |
|
|
|
308,502 |
|
Insurance |
|
|
60,999 |
|
|
|
95,697 |
|
Other prepaids and deposits |
|
|
63,709 |
|
|
|
67,938 |
|
|
|
|
155,413 |
|
|
|
472,137 |
|
8. Finance receivables
Finance receivables consist of secured loan receivables measured at amortized cost, net of allowance for expected credit losses.
Finance receivables as at December 31, 2023 are as follows:
|
|
|
$ |
|
Balance – January 1, 2022 |
|
|
— |
|
Additions |
|
|
7,805,825 |
|
Add: Interest income |
|
|
183,037 |
|
Less: Interest payments |
|
|
(149,906 | ) |
Less: Principal payments |
|
|
(203,077 | ) |
Effects of foreign exchange |
|
|
(204,223 | ) |
Balance – December 31, 2022 |
|
|
7,431,656 |
|
Additions |
|
|
1,021,489 |
|
Add: Interest income |
|
|
568,919 |
|
Less: Interest payments |
|
|
(597,986 | ) |
Less: Principal payments |
|
|
(526,107 | ) |
Effects of foreign exchange |
|
|
197,383 |
|
Balance – December 31, 2023 |
|
|
8,095,354 |
|
Current |
|
|
7,187,988 |
|
Non-current |
|
|
907,366 |
|
Balance – December 31, 2023 |
|
|
8,095,354 |
|
Allowances for expected credit losses as at December 31, 2023, were $nil. Finance receivables earn fees at fixed rates and have an average term to maturity of two years from the date of issuance. The loans are secured by residential property with a first or second collateral mortgage on the secured property, except for the loan issued to a related party (Note 21). Loans are issued up to 55% of the initial appraised value of the secured property at the time of issuance.
| F-20 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
Finance receivables include the following:
|
|
December 31, 2023 |
|
|
|
|
$ |
|
|
Minimum payments receivable |
|
|
8,527,569 |
|
Unearned income |
|
|
(432,215 | ) |
Net investment |
|
|
8,095,354 |
|
Allowance for credit losses |
|
|
— |
|
Finance receivables, net |
|
|
8,095,354 |
|
As at December 31, 2023, all loans were classified as stage 1 and there were no changes between stages during the year.
9. Investments
The following tables outline changes in investments during the periods:
Entity |
|
Instrument |
|
Note |
|
Balance at December 31, 2022 |
|
|
Proceeds from sale |
|
|
Additions |
|
|
Change in fair value through profit or loss |
|
|
Effects of foreign exchange |
|
|
Balance at December 31, 2023 |
|
||||||
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|||||||||
Solarvest BioEnergy Inc. |
|
Shares |
|
(i) |
|
|
221,490 |
|
|
|
— |
|
|
|
— |
|
|
|
(221,490 | ) |
|
|
|
|
|
— |
|
|
Solarvest BioEnergy Inc. |
|
Convertible debenture |
|
(i) |
|
|
177,192 |
|
|
|
— |
|
|
|
— |
|
|
|
(177,192 | ) |
|
|
— |
|
|
|
— |
|
A2ZCryptoCap Inc. |
|
Shares |
|
(ii) |
|
|
10,632 |
|
|
|
— |
|
|
|
— |
|
|
|
(4,583 | ) |
|
|
— |
|
|
|
6,049 |
|
Lions Bay Fund |
|
Shares |
|
(iii) |
|
|
418,298 |
|
|
|
443,138 |
|
|
|
— |
|
|
|
24,840 |
|
|
|
— |
|
|
|
— |
|
Royal Bank of Canada |
|
Guaranteed Investment Certificate |
|
(iv) |
|
|
— |
|
|
|
— |
|
|
|
744,500 |
|
|
|
— |
|
|
|
11,600 |
|
|
|
756,100 |
|
|
|
|
|
|
|
|
827,612 |
|
|
|
443,138 |
|
|
|
744,500 |
|
|
|
(378,425 | ) |
|
|
11,600 |
|
|
|
762,149 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current |
|
|
|
|
|
|
|
756,100 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Current |
|
|
|
|
|
|
|
6,049 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
762,149 |
|
Entity |
|
Instrument |
|
Note |
|
Balance at December 31, 2021 |
|
|
Proceeds from sale |
|
|
Additions |
|
|
Change in fair value through profit or loss |
|
|
Balance at December 31, 2022 |
|
|||||
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||||||
True Pharma Strip Inc. |
|
Shares |
|
|
|
|
197 |
|
|
|
197 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
HUGE Shops |
|
Shares |
|
|
|
|
157,760 |
|
|
|
157,760 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
SciCann Therapeutics |
|
Shares |
|
|
|
|
79 |
|
|
|
79 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Solarvest BioEnergy Inc. |
|
Shares |
|
(i) |
|
|
366,792 |
|
|
|
— |
|
|
|
— |
|
|
|
(145,302 | ) |
|
|
221,490 |
|
Solarvest BioEnergy Inc. |
|
Convertible debenture |
|
(i) |
|
|
293,434 |
|
|
|
— |
|
|
|
— |
|
|
|
(116,242 | ) |
|
|
177,192 |
|
A2ZCryptoCap Inc. |
|
Shares |
|
(ii) |
|
|
— |
|
|
|
— |
|
|
|
6,162 |
|
|
|
4,470 |
|
|
|
10,632 |
|
Lions Bay Fund |
|
Shares |
|
(iii) |
|
|
— |
|
|
|
— |
|
|
395450 |
|
|
|
22,848 |
|
|
|
418,298 |
|
|
|
|
|
|
|
|
|
818,262 |
|
|
|
158,036 |
|
|
401,612 |
|
|
|
(234,226 | ) |
|
|
827,612 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Current |
|
|
|
827,612 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
827,612 |
|
(i) Solarvest BioEnergy Inc. (“Solarvest”)
The Company holds 3,000,000 common shares of Solarvest and a convertible debenture with a principal amount of C$2,400,000 maturing on May 31, 2024. The convertible debenture can be converted into common shares of Solarvest at a price of $1.00 per share.
As at December 31, 2023, the fair value of the shares was determined to be $nil given the halt in trading of Solarvest’s shares as a result of the entity failing to maintain a transfer agent and due to the significant financial and operational challenges being faced by the entity. The fair value of the convertible debenture was determined to be $nil as well. The shares have been classified as level 1 within the fair value hierarchy – quoted market price, and the convertible debenture has been classified as level 2 – valuation technique with observable market inputs.
| F-21 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
As at December 31, 2022, the fair value of the shares was determined based on the quoted market price of the shares of C$0.10 per share. The fair value of the convertible debenture is calculated as the fair value of the shares if the debenture were converted at the Solarvest share price of C$0.10 as at December 31, 2022. The shares have been classified as level 1 within the fair value hierarchy – quoted market price, and the convertible debenture has been classified as level 2 – valuation technique with observable market inputs.
(ii) A2ZCryptoCap Inc. (“A2Z”)
On June 23, 2022, the Company acquired 80,000 shares of A2Z for C$0.10 per share. As at December 31, 2023, the fair value of the shares was determined based on the quoted market price of the shares of C$0.10 per share (December 31, 2022 – C$0.18). The shares have been classified as level 1 within the fair value hierarchy – quoted market price.
(iii) Lions Bay Fund (“Fund”)
During the year ended December 31, 2022, the Company invested $395,450 into the Fund. The investment was sold for proceeds of $443,138. The Company recognized a gain of $24,840 on the sale of the Fund during the year ended December 31, 2023.
(iv) On August 9, 2023, the Company purchased a Guaranteed Investment Certificate (“GIC”) in the amount of $744,500 from Royal Bank of Canada (“RBC”) with a maturity date of August 9, 2024. The GIC pays variable interest based on RBC’s Prime Interest Rate minus 2.00%. The GIC has been classified as level 2 – valuation technique with observable market inputs.
10. Intangible assets
Intangible assets as at December 31, 2023 are as follows:
|
|
Innovet |
|
|
Prismic |
|
|
Lucid |
|
|
Total |
|
||||
Cost |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
As at December 31, 2021 |
|
|
500,000 |
|
|
|
19,201,493 |
|
|
|
6,314,571 |
|
|
|
26,016,064 |
|
Additions |
|
|
250,000 |
|
|
|
— |
|
|
|
— |
|
|
|
250,000 |
|
As at December 31, 2022 |
|
|
750,000 |
|
|
|
19,201,493 |
|
|
|
6,314,571 |
|
|
|
26,266,064 |
|
Impairment |
|
|
(750,000 | ) |
|
|
(19,201,493 | ) |
|
|
— |
|
|
|
(19,951,493 | ) |
As at December 31, 2023 |
|
|
— |
|
|
|
— |
|
|
|
6,314,571 |
|
|
|
6,314,571 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated amortization |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As at December 31, 2021 |
|
|
79,409 |
|
|
|
9,617,361 |
|
|
|
117,555 |
|
|
|
9,814,325 |
|
Amortization |
|
|
150,524 |
|
|
|
3,840,261 |
|
|
|
420,665 |
|
|
|
4,411,450 |
|
As at December 31, 2022 |
|
|
229,933 |
|
|
|
13,457,622 |
|
|
|
538,220 |
|
|
|
14,225,775 |
|
Amortization |
|
|
39,971 |
|
|
|
1,904,348 |
|
|
|
420,664 |
|
|
|
2,364,983 |
|
Impairment |
|
|
(269,904 | ) |
|
|
(15,361,970 | ) |
|
|
— |
|
|
|
(15,631,874 | ) |
As at December 31, 2023 |
|
|
— |
|
|
|
— |
|
|
|
958,884 |
|
|
|
958,884 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net book value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As at December 31, 2022 |
|
|
520,067 |
|
|
|
5,743,871 |
|
|
|
5,776,351 |
|
|
|
12,040,289 |
|
As at December 31, 2023 |
|
|
— |
|
|
|
— |
|
|
|
5,355,687 |
|
|
|
5,355,687 |
|
During the year ended December 31, 2023, the Company recognized an impairment loss of $480,096 in the statements of loss and comprehensive loss related to the Innovet License Agreement as the Company made a strategic decision to no longer pursue the development of ultra-micro PEA for veterinary purposes.
During the year ended December 31, 2023, the Company recognized an impairment loss of $3,839,523 in the statements of loss and comprehensive loss related to licensed compound ultra-micro PEA (“FSD-201”) acquired through the acquisition of Prismic as the Company made a strategic decision to no longer pursue the development of FSD-201.
| F-22 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The Company’s intangible asset for Lucid represents the license agreement with the University Health Network giving the Company world-wide exclusive rights to the Lucid-MS compound and related patents.
11. Trade and other payables
Trade and other payables consist of the following:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
|
|
$ |
|
|
$ |
|
||
Trade payables |
|
|
3,240,658 |
|
|
|
2,760,002 |
|
Accrued liabilities (i) |
|
|
954,371 |
|
|
|
4,348,417 |
|
|
|
|
4,195,029 |
|
|
|
7,108,419 |
|
(i) Accrued liabilities consist of the following:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
|
|
$ |
|
|
$ |
|
||
External research and development fees |
|
|
— |
|
|
|
3,531,996 |
|
Operational expenses |
|
|
71,953 |
|
|
|
92,783 |
|
Professional and other fees |
|
|
473,225 |
|
|
|
314,445 |
|
Accrued interest |
|
|
409,193 |
|
|
|
409,193 |
|
|
|
|
954,371 |
|
|
|
4,348,417 |
|
12. Warrants Liability
In August 2020, the Company issued 2,762,430 Class B shares and 1,381,215 warrants to purchase Class B shares for total cash proceeds of $9,999,997. Each warrant is exercisable to purchase one Class B share of the Company at an exercise price of $4.26 per share and expire five years from the date of issuance. The fair value of these warrants is classified as Level 2 in the fair value hierarchy.
On initial recognition the Company determined that these warrants did not meet the IFRS definition of equity due to the exercise price being denominated in United States dollar, which was not the functional currency of the Company at the time resulting in variability in exercise price. The change in functional currency on October 1, 2020, was determined to be a change in circumstance and, as such, the Company has made an accounting policy choice to continue to recognize the warrants as a financial liability classified at fair value through profit or loss.
The fair value of the warrants liability as at December 31, 2023, was $31,338 (December 31, 2022 – $243,594) resulting in a gain on change in fair value of $212,256 for the year ended December 31, 2023 (December 31, 2022 – $521,809).
The fair value was determined using the Black-Scholes option pricing model and the following assumptions:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||
Share price |
|
$ | 0.92 |
|
|
$ | 0.79 |
|
Exercise price |
|
$ | 4.26 |
|
|
$ | 4.26 |
|
Expected dividend yield |
|
|
— |
|
|
|
— |
|
Risk free interest rate |
|
|
3.91 | % |
|
|
4.07 | % |
Expected life |
|
|
1.60 |
|
|
|
2.60 |
|
Expected volatility |
|
|
66 | % |
|
|
96 | % |
| F-23 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
13. Share capital
[a] Authorized
The Company is authorized to issue an unlimited number of Class A multiple voting shares (“Class A shares”) and an unlimited number of Class B subordinate voting shares (“Class B shares”), all without par value. All shares are ranked equally with regard to the Company’s residual assets.
The holders of Class A shares are entitled to 276,660 votes per Class A share held. Class A shares are held by the Chief Executive Officer (“CEO”), President, Executive Co-Chairman of the Board and the Director and Executive Co-Chairman of the Board. The holders of Class B shares are entitled to one (1) vote per share held.
[b] Issued and outstanding
Reconciliation of the Company’s share capital is as follows:
|
|
Class A shares |
|
|
Class B shares |
|
|
Warrants |
|
|||||||||||||||
|
|
# |
|
|
$ |
|
|
# |
|
|
$ |
|
|
# |
|
|
$ |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Balance, December 31, 2020 |
|
|
72 |
|
|
|
151,588 |
|
|
|
19,161,620 |
|
|
|
103,056,538 |
|
|
|
6,749,109 |
|
|
|
4,968,958 |
|
Shares issued [a] |
|
|
— |
|
|
|
— |
|
|
|
15,480,462 |
|
|
|
38,341,407 |
|
|
|
— |
|
|
|
— |
|
Share-based payments [b] |
|
|
— |
|
|
|
— |
|
|
|
1,462,558 |
|
|
|
3,751,412 |
|
|
|
100,000 |
|
|
|
98,513 |
|
Share cancellation [b] |
|
|
— |
|
|
|
— |
|
|
|
(156,278 | ) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Lucid acquisition [c] |
|
|
— |
|
|
|
— |
|
|
|
4,502,392 |
|
|
|
7,023,732 |
|
|
|
112,162 |
|
|
|
70,563 |
|
Warrants expired |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,476 | ) |
|
|
(617 | ) |
Balance, December 31, 2021 |
|
|
72 |
|
|
|
151,588 |
|
|
|
40,450,754 |
|
|
|
152,173,089 |
|
|
|
6,956,795 |
|
|
|
5,137,417 |
|
Share repurchase [d] |
|
|
— |
|
|
|
— |
|
|
|
(1,999,800 | ) |
|
|
(7,523,117 | ) |
|
|
— |
|
|
|
— |
|
Share-based payments [e] |
|
|
— |
|
|
|
— |
|
|
|
158,144 |
|
|
|
169,500 |
|
|
|
— |
|
|
|
— |
|
Share cancellation [f] |
|
|
— |
|
|
|
— |
|
|
|
(504,888 | ) |
|
|
(1,752,090 | ) |
|
|
— |
|
|
|
— |
|
PSU converted to shares |
|
|
— |
|
|
|
— |
|
|
|
400,000 |
|
|
|
191,590 |
|
|
|
— |
|
|
|
— |
|
Warrants expired |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(474,702 | ) |
|
|
(2,995,017 | ) |
Balance, December 31, 2022 |
|
|
72 |
|
|
|
151,588 |
|
|
|
38,504,210 |
|
|
|
143,258,972 |
|
|
|
6,482,093 |
|
|
|
2,142,400 |
|
Plan of arrangement [k] |
|
|
— |
|
|
|
34 |
|
|
|
23 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Share repurchase [g] |
|
|
— |
|
|
|
— |
|
|
|
(1,904,700 | ) |
|
|
(7,165,356 | ) |
|
|
— |
|
|
|
— |
|
Share-based payments [j] |
|
|
— |
|
|
|
— |
|
|
|
36,086 |
|
|
|
36,000 |
|
|
|
— |
|
|
|
— |
|
Share options exercised [i] |
|
|
— |
|
|
|
— |
|
|
|
21,000 |
|
|
|
33,247 |
|
|
|
— |
|
|
|
— |
|
PSU converted to shares |
|
|
— |
|
|
|
— |
|
|
|
2,720,104 |
|
|
|
1,464,000 |
|
|
|
— |
|
|
|
— |
|
Warrants issued [h] |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,975,000 |
|
|
|
1,372,763 |
|
Warrants expired |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(133,050 | ) |
|
|
(791,807 | ) |
Balance, December 31, 2023 |
|
|
72 |
|
|
|
151,622 |
|
|
|
39,376,723 |
|
|
|
137,626,863 |
|
|
|
10,324,043 |
|
|
|
2,723,356 |
|
Activity during the period from December 31, 2020 to December 31, 2021:
|
[a] |
During the year ended December 31, 2021, the Company issued 15,480,462 Class B shares through the Equity Distribution Agreements with A.G.P/Alliance Global Partners for gross proceeds of $39,765,474. The Company incurred transaction fees of $1,424,067. |
|
|
|
|
[b] |
On February 17, 2021, the Company issued 1,349,764 Class B shares to certain officers and members of the Board as share-based compensation with a fair value of $3,576,875 based on a share-price of $2.65 on the day of issuance. |
|
|
On July 26, 2021, the Company issued 100,000 warrants to a related party with a fair value of $98,513. Each warrant is exercisable to purchase one Class B share of the Company. The fair value was determined using the Black-Scholes option pricing model and the following assumptions: exercise price of $1.99, underlying share price of $1.63, risk-free interest rate of 0.46% and annualized volatility of 129%. |
| F-24 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
|
|
During the year ended December 31, 2021, the Company issued 112,794 Class B shares for services received during the period with a fair value of $174,537. The Company determined the fair value of the services received could not be measured reliably and determined fair value based on the underlying share price on the date of issuance. |
|
|
|
|
[c] |
On September 21, 2021, the Company issued 4,502,392 Class B shares and 112,162 warrants as part of the Lucid acquisition (Note 4). |
|
|
|
Activity during the period from December 31, 2021 to December 31, 2022: | ||
|
|
|
|
[d] |
During the year ended December 31, 2022, the Company repurchased and cancelled 1,999,800 Class B Common Shares at prevailing market prices as part of its share repurchase program. |
|
|
|
|
[e] |
During the year ended December 31, 2022, the Company issued 107,144 Class B shares for services received during the period with a fair value of $120,000. The fair value was based on services received. During the year ended December 31, 2022, the Company issued 51,000 Class B shares for services received during the period with a fair value of $49,500. The Company determined the fair value of the services received could not be measured reliably and determined fair value based on the underlying share price on the date of issuance. |
|
|
|
|
[f] |
On March 29, 2022, the Company cancelled 504,888 Class B shares previously held by the former CEO following a court decision with respect to the shares issued in February 2021. |
|
|
|
Activity during the period from December 31, 2022 to December 31, 2023: | ||
|
|
|
|
[g] |
During the year ended December 31, 2023, the Company repurchased and canceled 1,904,700 Class B Common Shares at prevailing market prices as part of its share repurchase program. |
|
|
|
|
[h] |
During the year ended December 31, 2023, the Company issued 3,975,000 warrants for consulting services with a fair value of $1,384,970. The Company recognized $1,372,763 as expense during the year ended December 31, 2023, with the remaining $12,206 to be recognized over the vesting period of certain warrants. The Company determined the fair value of the services received could not be measured reliably and determined the fair value using the Black-Scholes model. |
|
|
|
|
[i] |
During the year ended December 31, 2023, the Company issued 21,000 Class B shares upon the exercise of 21,000 share options with an exercise price of C$1.30. |
|
|
|
|
[j] |
During the year ended December 31, 2023, the Company issued 36,086 Class B shares for services received during the period with a fair value of $36,000. |
|
|
|
|
[k] |
In November 2023, the Company completed the Plan of Arrangement reorganization. The Company cancelled all 72 Class A Shares of the Company and reissued 24 new Class B shares and 48 new Class A Shares. The Company cancelled all 39,376,699 Class B shares outstanding and reissued 39,376,698 new Class B shares. There was 1 previously issued Class B share that was removed due to an administrative adjustment. The Company also cancelled and reissued 6,335,758 FSD Pharma New Distribution Warrants. Each holder of the Company’s Class A shares, Class B shares and the FSD Pharma New Distribution Warrants was distributed a share of Celly from the Company for each Class A share, Class B share and New Distribution Warrant held. As a result, the Company issued 45,712,529 shares of Celly which was recognized as a deemed dividend of $8,673 with a corresponding adjustment to NCI. |
|
|
The Company issued 24 Class A shares through a private placement for total proceeds of $34. 12 Class A shares were issued to the CEO, President, and Co-Chairman of the Board and 12 Class A Share were issued to the Director and Co-Chairman of the Board. |
| F-25 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The changes in the number of warrants outstanding during the year ended December 31, 2023, 2022 and 2021 were as follows:
|
|
Number of warrants |
|
|
Weighted average exercise price |
|
||
|
|
# |
|
|
C$ |
|
||
Outstanding as at December 31, 2020 |
|
|
6,749,109 |
|
|
|
5.62 |
|
Issued |
|
|
212,162 |
|
|
|
1.93 |
|
Expired |
|
|
(4,476 | ) |
|
|
5.43 |
|
Outstanding as at December 31, 2021 |
|
|
6,956,795 |
|
|
|
5.50 |
|
Expired |
|
|
(474,702 | ) |
|
|
8.54 |
|
Outstanding as at December 31, 2022 |
|
|
6,482,093 |
|
|
|
5.48 |
|
Issued |
|
|
3,975,000 |
|
|
|
4.55 |
|
Expired |
|
|
(133,050 | ) |
|
|
4.01 |
|
Outstanding as at December 31, 2023 |
|
|
10,324,043 |
|
|
|
5.05 |
|
Measurement of fair values
The fair value of the warrants issued during the year ended December 31, 2023 and 2021, were estimated at the date of grant using the Black-Scholes option pricing model with the following inputs:
|
|
2023 |
|
|
2021 |
|
||
Grant date share price |
|
C$1.44 - C$2.29 |
|
|
C$2.00 - C$2.04 |
|
||
Exercise price |
|
C$1.50 - C$10.82 |
|
|
C$1.53 - C$2.50 |
|
||
Expected dividend yield |
|
|
— |
|
|
|
— |
|
Risk free interest rate |
|
3.08% - 4.26% |
|
|
0.43% - 0.46% |
|
||
Expected life |
|
0.75 - 5 years |
|
|
1.19 - 2 years |
|
||
Expected volatility |
|
64% - 109% |
|
|
88% - 129% |
|
||
There were no warrants granted during the year ended December 31, 2022.
| F-26 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The following table is a summary of the Company’s warrants outstanding as at December 31, 2023:
|
|
|
|
Exercise price |
|
|
Number outstanding |
|
||||
Expiry Date |
|
|
|
C$ |
|
|
# |
|
||||
March 14, 2024 |
|
|
(i) |
|
|
2.45 |
|
|
|
200,000 |
|
|
March 14, 2024 |
|
|
(i) |
|
|
5.63 |
|
|
|
100,000 |
|
|
March 14, 2024 |
|
|
(i) |
|
|
10.58 |
|
|
|
200,000 |
|
|
March 30, 2024 |
|
|
(i) |
|
|
1.98 |
|
|
|
300,000 |
|
|
March 30, 2024 |
|
|
(i) |
|
|
3.97 |
|
|
|
250,000 |
|
|
March 30, 2024 |
|
|
(i) |
|
|
5.95 |
|
|
|
250,000 |
|
|
May 24, 2024 |
|
|
(i) |
|
|
1.98 |
|
|
|
50,000 |
|
|
February 27, 2025 |
|
|
(i) |
|
|
2.31 |
|
|
|
400,000 |
|
|
February 27, 2025 |
|
|
(i) |
|
|
5.29 |
|
|
|
400,000 |
|
|
February 27, 2025 |
|
|
(i) |
|
|
10.58 |
|
|
|
200,000 |
|
|
March 15, 2025 |
|
|
|
|
|
|
1.50 |
|
|
|
37,500 |
|
March 15, 2025 |
|
|
|
|
|
|
3.00 |
|
|
|
37,500 |
|
March 23, 2025 |
|
|
|
|
|
|
1.50 |
|
|
|
50,000 |
|
March 24, 2025 |
|
|
(i) |
|
|
2.31 |
|
|
|
400,000 |
|
|
March 24, 2025 |
|
|
(i) |
|
|
5.29 |
|
|
|
400,000 |
|
|
March 24, 2025 |
|
|
(i) |
|
|
10.58 |
|
|
|
200,000 |
|
|
May 4, 2025 |
|
|
|
|
|
|
26.73 |
|
|
|
3,730 |
|
May 10, 2025 |
|
|
|
|
|
|
26.73 |
|
|
|
1,865 |
|
May 17, 2025 |
|
|
|
|
|
|
26.73 |
|
|
|
3,730 |
|
May 31, 2025 |
|
|
|
|
|
|
26.73 |
|
|
|
1,865 |
|
June 8, 2025 |
|
|
|
|
|
|
9.65 |
|
|
|
1,500,000 |
|
August 6, 2025 |
|
|
(i) |
|
|
5.63 |
|
|
|
1,381,215 |
|
|
October 20, 2025 |
|
|
(i) |
|
|
3.44 |
|
|
|
3,454,543 |
|
|
January 16, 2026 |
|
|
|
|
|
|
26.73 |
|
|
|
1,722 |
|
January 20, 2026 |
|
|
|
|
|
|
26.73 |
|
|
|
373 |
|
May 15, 2028 |
|
|
|
|
|
|
1.50 |
|
|
|
500,000 |
|
|
|
|
|
|
|
|
5.05 |
|
|
|
10,324,043 |
|
(i) Warrants were issued in US$
| F-27 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The following table is a summary of the Company’s warrants outstanding as at December 31, 2022:
|
|
|
|
Exercise price |
|
|
Number outstanding |
|
||||
Expiry Date |
|
|
|
C$ |
|
|
# |
|
||||
May 20, 2023 |
|
|
|
|
|
16.08 |
|
|
|
7,311 |
|
|
June 23, 2023 |
|
|
|
|
|
2.50 |
|
|
|
100,000 |
|
|
July 24, 2023 |
|
|
|
|
|
13.07 |
|
|
|
3,357 |
|
|
September 11, 2023 |
|
|
|
|
|
5.43 |
|
|
|
22,382 |
|
|
May 4, 2025 |
|
|
|
|
|
26.73 |
|
|
|
3,730 |
|
|
May 10, 2025 |
|
|
|
|
|
26.73 |
|
|
|
1,865 |
|
|
May 17, 2025 |
|
|
|
|
|
26.73 |
|
|
|
3,730 |
|
|
May 31, 2025 |
|
|
|
|
|
26.73 |
|
|
|
1,865 |
|
|
June 8, 2025 |
|
|
|
|
|
9.65 |
|
|
|
1,500,000 |
|
|
August 6, 2025 |
|
|
(i) |
|
|
5.77 |
|
|
|
1,381,215 |
|
|
October 20, 2025 |
|
|
(i) |
|
|
3.52 |
|
|
|
3,454,543 |
|
|
January 16, 2026 |
|
|
|
|
|
|
26.73 |
|
|
|
1,722 |
|
January 20, 2026 |
|
|
|
|
|
|
26.73 |
|
|
|
373 |
|
|
|
|
|
|
|
|
5.48 |
|
|
|
6,482,093 |
|
(i) Warrants were issued in US$
The following table is a summary of the Company’s warrants outstanding as at December 31, 2021:
|
|
|
|
Exercise price |
|
|
Number outstanding |
|
||||
Expiry Date |
|
|
|
C$ |
|
|
# |
|
||||
May 24, 2022 |
|
|
|
|
|
18.09 |
|
|
|
163,535 |
|
|
September 15, 2022 |
|
|
|
|
|
4.42 |
|
|
|
199,005 |
|
|
November 30, 2022 |
|
|
|
|
|
1.21 |
|
|
|
46,242 |
|
|
December 31, 2022 |
|
|
|
|
|
2.43 |
|
|
|
65,920 |
|
|
May 20, 2023 |
|
|
|
|
|
16.08 |
|
|
|
7,311 |
|
|
June 23, 2023 |
|
|
|
|
|
2.50 |
|
|
|
100,000 |
|
|
July 24, 2023 |
|
|
|
|
|
13.07 |
|
|
|
3,357 |
|
|
September 11, 2023 |
|
|
|
|
|
5.43 |
|
|
|
22,382 |
|
|
May 4, 2025 |
|
|
|
|
|
26.73 |
|
|
|
3,730 |
|
|
May 10, 2025 |
|
|
|
|
|
26.73 |
|
|
|
1,865 |
|
|
May 17, 2025 |
|
|
|
|
|
26.73 |
|
|
|
3,730 |
|
|
May 31, 2025 |
|
|
|
|
|
26.73 |
|
|
|
1,865 |
|
|
June 8, 2025 |
|
|
|
|
|
9.65 |
|
|
|
1,500,000 |
|
|
August 6, 2025 |
|
|
(i) |
|
|
5.40 |
|
|
|
1,381,215 |
|
|
October 20, 2025 |
|
|
(i) |
|
|
3.30 |
|
|
|
3,454,543 |
|
|
January 16, 2026 |
|
|
|
|
|
|
26.73 |
|
|
|
1,722 |
|
January 30, 2026 |
|
|
|
|
|
|
26.73 |
|
|
|
373 |
|
|
|
|
|
|
|
|
5.50 |
|
|
|
6,956,795 |
|
(i) Warrants were issued in US$
| F-28 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
14. Share-based compensation
The Company has established a share option plan (the “Option Plan”) for directors, officers, employees and consultants of the Company. The Company’s Board determines, among other things, the eligibility of individuals to participate in the Option Plan, the term and vesting periods, and the exercise price of options granted to individuals under the Option Plan.
Each share option converts into one common share of the Company on exercise. No amounts are paid or payable by the individual on receipt of the option. The options carry neither rights to dividends nor voting rights. Options may be exercised at any time from the date of vesting to the date of their expiry.
[i] Share-based payment arrangements
During the year ended December 31, 2023, the Company granted 2,488,000 (2022 – 60,000 and 2021 – 2,841,086) share options to directors, officers, employees and consultants of the Company.
The change in the number of share options outstanding during the year ended December 31, 2023, were as follows:
|
|
Number of options |
|
|
Weighted average exercise price |
|
||
|
|
# |
|
|
C$ |
|
||
Outstanding as at December 31, 2022 |
|
|
418,529 |
|
|
|
3.71 |
|
Granted |
|
|
2,488,000 |
|
|
|
1.52 |
|
Forfeited |
|
|
(123,500 | ) |
|
|
2.09 |
|
Exercised |
|
|
(21,000 | ) |
|
|
1.30 |
|
Expired |
|
|
(301,414 | ) |
|
|
4.03 |
|
Outstanding as at December 31, 2023 |
|
|
2,460,615 |
|
|
|
1.56 |
|
Exercisable as at December 31, 2023 |
|
|
2,396,863 |
|
|
|
1.54 |
|
The change in the number of share options outstanding during the year ended December 31, 2022, were as follows:
|
|
Number of options |
|
|
Weighted average exercise price |
|
||
|
|
# |
|
|
C$ |
|
||
Outstanding as at December 31, 2021 |
|
|
3,224,859 |
|
|
|
2.75 |
|
Granted |
|
|
60,000 |
|
|
|
1.30 |
|
Forfeited |
|
|
(4,000 | ) |
|
|
3.75 |
|
Expired |
|
|
(42,226 | ) |
|
|
3.71 |
|
Cancelled |
|
|
(2,820,104 | ) |
|
|
2.56 |
|
Outstanding as at December 31, 2022 |
|
|
418,529 |
|
|
|
3.71 |
|
Exercisable as at December 31, 2022 |
|
|
416,271 |
|
|
|
3.71 |
|
The change in the number of share options outstanding during the year ended December 31, 2021, were as follows:
|
|
Number of options |
|
|
Weighted average exercise price |
|
||
|
|
# |
|
|
C$ |
|
||
Outstanding as at December 31, 2020 |
|
|
1,693,063 |
|
|
|
6.11 |
|
Granted |
|
|
2,841,086 |
|
|
|
2.26 |
|
Forfeited |
|
|
(47,500 | ) |
|
|
4.83 |
|
Expired |
|
|
(953,803 | ) |
|
|
4.87 |
|
Cancelled |
|
|
(307,987 | ) |
|
|
9.85 |
|
Outstanding as at December 31, 2021 |
|
|
3,224,859 |
|
|
|
2.75 |
|
Exercisable as at December 31, 2021 |
|
|
3,197,601 |
|
|
|
2.72 |
|
| F-29 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
During the year ended December 31, 2023, 301,414 share options (2022 – 42,226 and 2021 – 953,803) related to former officers and employees who are no longer with the Company expired. Individuals who are no longer with the Company have 30 days after their last day to exercise any vested share options. Vested options that remain unexercised after 30 days expire.
During the year ended December 31, 2022, the Company cancelled 2,820,104 share options issued to officers and consultants of the Company and issued 2,820,104 replacement performance share units.
Measurement of fair values
The fair value of share options granted during the year ended December 31, 2023, 2022 and 2021, were estimated at the date of grant using the Black-Scholes option pricing model with the following inputs:
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Grant date share price |
|
C$1.28 - C$2.48 |
|
|
C$1.19 |
|
|
C$1.96 — C$2.85 |
|
|||
Exercise price |
|
C$1.30 - C$2.45 |
|
|
C$1.30 |
|
|
C$1.70 — C$4.25 |
|
|||
Expected dividend yield |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Risk free interest rate |
|
2.91% - 3.99% |
|
|
|
2.87 | % |
|
0.34% — 1.10% |
|
||
Expected life |
|
3 - 5 years |
|
|
3 years |
|
|
2 — 6 years |
|
|||
Expected volatility |
|
95% - 110% |
|
|
|
112 | % |
|
116% — 132% |
|
||
Expected volatility was estimated by using the annualized historical volatility of the Company. The expected option life represents the period of time that options granted are expected to be outstanding. The risk-free interest rate is based on Canadian government bonds with a remaining term equal to the expected life of the options.
The following table is a summary of the Company’s share options outstanding as at December 31, 2023:
Options outstanding |
|
|
Options exercisable |
|
||||||||||||||
Exercise price |
|
|
Number outstanding |
|
|
Weighted average remaining contractual life [years] |
|
|
Exercise price |
|
|
Number exercisable |
|
|||||
C$ |
|
|
# |
|
|
# |
|
|
C$ |
|
|
# |
|
|||||
|
1.30 |
|
|
|
2,000,000 |
|
|
|
4.07 |
|
|
|
1.30 |
|
|
|
2,000,000 |
|
|
1.70 |
|
|
|
67,980 |
|
|
|
1.87 |
|
|
|
1.70 |
|
|
|
67,980 |
|
|
2.25 |
|
|
|
50,002 |
|
|
|
0.42 |
|
|
|
2.25 |
|
|
|
50,000 |
|
|
2.31 |
|
|
|
15,000 |
|
|
|
2.16 |
|
|
|
2.31 |
|
|
|
15,000 |
|
|
2.31 |
|
|
|
15,000 |
|
|
|
2.23 |
|
|
|
2.31 |
|
|
|
15,000 |
|
|
2.45 |
|
|
|
294,000 |
|
|
|
2.15 |
|
|
|
2.45 |
|
|
|
231,500 |
|
|
2.91 |
|
|
|
5,150 |
|
|
|
2.00 |
|
|
|
2.91 |
|
|
|
5,150 |
|
|
3.75 |
|
|
|
5,000 |
|
|
|
0.21 |
|
|
|
3.75 |
|
|
|
5,000 |
|
|
3.86 |
|
|
|
5,000 |
|
|
|
2.86 |
|
|
|
3.86 |
|
|
|
3,750 |
|
|
50.25 |
|
|
|
3,483 |
|
|
|
0.28 |
|
|
|
50.25 |
|
|
|
3,483 |
|
|
1.56 |
|
|
|
2,460,615 |
|
|
|
3.66 |
|
|
|
1.54 |
|
|
|
2,396,863 |
|
| F-30 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The following table is a summary of the Company’s share options outstanding as at December 31, 2022:
Options outstanding |
|
|
Options exercisable |
|
||||||||||||||
Exercise price |
|
|
Number outstanding |
|
|
Weighted average remaining contractual life [years] |
|
|
Exercise price |
|
|
Number exercisable |
|
|||||
C$ |
|
|
# |
|
|
# |
|
|
C$ |
|
|
# |
|
|||||
|
1.30 |
|
|
|
60,000 |
|
|
|
2.58 |
|
|
|
1.30 |
|
|
|
60,000 |
|
|
1.70 |
|
|
|
103,453 |
|
|
|
2.21 |
|
|
|
1.70 |
|
|
|
103,453 |
|
|
2.25 |
|
|
|
168,898 |
|
|
|
1.04 |
|
|
|
2.25 |
|
|
|
168,898 |
|
|
2.61 |
|
|
|
12,687 |
|
|
|
0.49 |
|
|
|
2.61 |
|
|
|
12,683 |
|
|
2.91 |
|
|
|
5,150 |
|
|
|
3.00 |
|
|
|
2.91 |
|
|
|
5,150 |
|
|
3.75 |
|
|
|
5,000 |
|
|
|
1.21 |
|
|
|
3.75 |
|
|
|
5,000 |
|
|
3.86 |
|
|
|
5,000 |
|
|
|
3.86 |
|
|
|
3.86 |
|
|
|
2,750 |
|
|
5.43 |
|
|
|
16,265 |
|
|
|
0.49 |
|
|
|
5.43 |
|
|
|
16,264 |
|
|
10.65 |
|
|
|
3,731 |
|
|
|
0.49 |
|
|
|
10.65 |
|
|
|
3,730 |
|
|
13.07 |
|
|
|
10,856 |
|
|
|
0.49 |
|
|
|
13.07 |
|
|
|
10,855 |
|
|
13.47 |
|
|
|
1,418 |
|
|
|
0.49 |
|
|
|
13.47 |
|
|
|
1,418 |
|
|
16.08 |
|
|
|
18,410 |
|
|
|
0.49 |
|
|
|
16.08 |
|
|
|
18,409 |
|
|
17.89 |
|
|
|
4,178 |
|
|
|
0.49 |
|
|
|
17.89 |
|
|
|
4,178 |
|
|
50.25 |
|
|
|
3,483 |
|
|
|
1.28 |
|
|
|
50.25 |
|
|
|
3,483 |
|
|
3.71 |
|
|
|
418,529 |
|
|
|
1.52 |
|
|
|
3.71 |
|
|
|
416,271 |
|
The following table is a summary of the Company’s share options outstanding as at December 31, 2021:
Options outstanding |
|
|
Options exercisable |
|
||||||||||||||
Exercise price |
|
|
Number outstanding |
|
|
Weighted average remaining contractual life [years] |
|
|
Exercise price |
|
|
Number exercisable |
|
|||||
C$ |
|
|
# |
|
|
# |
|
|
C$ |
|
|
# |
|
|||||
|
1.70 |
|
|
|
154,953 |
|
|
|
3.46 |
|
|
|
1.70 |
|
|
|
154,953 |
|
|
2.25 |
|
|
|
2,559,995 |
|
|
|
2.42 |
|
|
|
2.25 |
|
|
|
2,559,995 |
|
|
2.61 |
|
|
|
12,684 |
|
|
|
1.49 |
|
|
|
2.61 |
|
|
|
12,683 |
|
|
2.91 |
|
|
|
5,150 |
|
|
|
4.00 |
|
|
|
2.91 |
|
|
|
5,150 |
|
|
3.75 |
|
|
|
10,500 |
|
|
|
3.92 |
|
|
|
3.75 |
|
|
|
6,500 |
|
|
3.86 |
|
|
|
256,245 |
|
|
|
3.21 |
|
|
|
3.86 |
|
|
|
252,993 |
|
|
4.42 |
|
|
|
99,503 |
|
|
|
0.71 |
|
|
|
4.42 |
|
|
|
99,502 |
|
|
4.75 |
|
|
|
15,000 |
|
|
|
3.29 |
|
|
|
4.75 |
|
|
|
15,000 |
|
|
5.43 |
|
|
|
16,265 |
|
|
|
1.49 |
|
|
|
5.43 |
|
|
|
16,264 |
|
|
7.63 |
|
|
|
50,000 |
|
|
|
4.00 |
|
|
|
7.63 |
|
|
|
30,000 |
|
|
10.65 |
|
|
|
3,731 |
|
|
|
1.49 |
|
|
|
10.65 |
|
|
|
3,730 |
|
|
13.07 |
|
|
|
10,856 |
|
|
|
1.49 |
|
|
|
13.07 |
|
|
|
10,855 |
|
|
13.47 |
|
|
|
1,418 |
|
|
|
1.49 |
|
|
|
13.47 |
|
|
|
1,418 |
|
|
16.08 |
|
|
|
18,410 |
|
|
|
1.49 |
|
|
|
16.08 |
|
|
|
18,409 |
|
|
17.89 |
|
|
|
4,178 |
|
|
|
1.49 |
|
|
|
17.89 |
|
|
|
4,178 |
|
|
18.09 |
|
|
|
2,488 |
|
|
|
1.24 |
|
|
|
18.09 |
|
|
|
2,488 |
|
|
50.25 |
|
|
|
3,483 |
|
|
|
2.28 |
|
|
|
50.25 |
|
|
|
3,483 |
|
|
2.75 |
|
|
|
3,224,859 |
|
|
|
2.50 |
|
|
|
2.72 |
|
|
|
3,197,601 |
|
[ii] Performance Share Units (“PSUs”)
In May 2022, the Company established a performance share unit plan (“PSU Plan”), for directors, offers, employees and consultants of the Company. The Company’s Board determines the eligibility of individuals to participate in the PSU Plan in order to align their interests with those of the Company’s shareholders.
| F-31 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
No amounts are paid or payable by the individual on receipt of the PSUs. Each PSU converts into one common share of the Company at $nil exercise price. The Company’s PSU Plan provides that the number of common shares reserved for issuance may not exceed 10% of the aggregate number of common shares that are outstanding unless the Board has increased such limit by a Board resolution.
The change in the number of PSUs during the years ended December 31, 2023 and 2022, is as follows:
|
|
Number of PSUs |
|
|
|
|
# |
|
|
Outstanding as at December 31, 2021 |
|
|
— |
|
Granted |
|
|
2,820,104 |
|
Converted to Class B Common shares |
|
|
(400,000 | ) |
Outstanding as at December 31, 2022 |
|
|
2,420,104 |
|
Granted |
|
|
400,000 |
|
Forfeited |
|
|
(100,000 | ) |
Converted to Class B Common shares |
|
|
(2,720,104 | ) |
Outstanding as at December 31, 2023 |
|
|
— |
|
During the year ended December 31, 2023, the Company converted 2,720,104 PSUs to Class B shares. The PSUs were fully vested as of January 6, 2023, upon the filing of the MS Phase 1 IND. During the year ended December 31, 2023, 100,000 PSUs related to a former independent director who is no longer with the Company were forfeited.
The Company recognized share-based compensation for the years ended December 31, 2023, 2022 and 2021 as follows:
|
|
For the years ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Share options |
|
|
1,951,757 |
|
|
|
69,780 |
|
|
|
3,594,005 |
|
PSUs |
|
|
458,253 |
|
|
|
1,291,978 |
|
|
|
98,513 |
|
Class B Common Shares issued for services |
|
|
36,000 |
|
|
|
169,500 |
|
|
|
174,537 |
|
Class B Common Shares issued for compensation |
|
|
— |
|
|
|
— |
|
|
|
3,576,875 |
|
Warrants issued for services |
|
|
1,372,763 |
|
|
|
— |
|
|
|
— |
|
Other (i) |
|
|
16,702 |
|
|
|
— |
|
|
|
— |
|
|
|
|
3,835,475 |
|
|
|
1,531,258 |
|
|
|
7,443,930 |
|
|
(i) |
Share-based compensation related to share options and restricted share units issued by Celly and convertible into common shares of Celly. |
15. Non-controlling interests
Through the License Agreement, FSD acquired 34.66% of Celly on July 31, 2023. As of December 31, 2023, the Company has a 26.15% (2022 – 0%) ownership interest in Celly through common shares held in Celly. The non-controlling interest represents the common shares of Celly not attributable to the Company.
Reconciliation of non-controlling interest is as follows:
|
|
|
$ |
|
Balance, December 31, 2022 |
|
|
— |
|
Initial recognition of non-controlling interests |
|
|
(24,467 | ) |
Share-based payments |
|
|
16,702 |
|
Deemed dividend (Note 13) |
|
|
8,673 |
|
Comprehensive loss for the period |
|
|
(328,409 | ) |
Balance, December 31, 2023 |
|
|
(327,501 | ) |
| F-32 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
16. Loss per share
Net loss per common share represents net loss attributable to common shareholders divided by the weighted average number of common shares outstanding during the year.
For all the periods presented, diluted loss per share equals basic loss per share due to the anti-dilutive effect of warrants, share options and PSUs. The outstanding number and type of securities that could potentially dilute basic net loss per share in the future but would have decreased the loss per share (anti-dilutive) for the years ended December 31, 2023, 2022 and 2021 are as follows:
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
|||
|
|
# |
|
|
# |
|
|
# |
|
|||
Warrants |
|
|
10,324,043 |
|
|
|
6,482,093 |
|
|
|
6,956,795 |
|
Share Options |
|
|
2,460,615 |
|
|
|
418,529 |
|
|
|
3,224,859 |
|
PSUs |
|
|
— |
|
|
|
2,420,104 |
|
|
|
— |
|
|
|
|
12,784,658 |
|
|
|
9,320,726 |
|
|
|
10,181,654 |
|
17. General and administrative
Components of general and administrative expenses for the years ended December 31, 2023, 2022 and 2021 were as follows:
|
|
For thee years ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Professional fees |
|
|
3,248,233 |
|
|
|
5,208,356 |
|
|
|
6,256,165 |
|
General office, insurance and administration expenditures |
|
|
2,294,476 |
|
|
|
2,838,303 |
|
|
|
3,479,801 |
|
Consulting fees |
|
|
1,305,434 |
|
|
|
1,452,070 |
|
|
|
2,196,812 |
|
Salaries, wages and benefits |
|
|
1,855,087 |
|
|
|
2,798,074 |
|
|
|
2,856,887 |
|
Investor relations |
|
|
665,915 |
|
|
|
1,495,695 |
|
|
|
1,642,653 |
|
Building and facility costs |
|
|
— |
|
|
|
519,954 |
|
|
|
759,590 |
|
Foreign exchange (gain) loss |
|
|
(336,421 | ) |
|
|
1,323,242 |
|
|
|
146,587 |
|
|
|
|
9,032,724 |
|
|
|
15,635,694 |
|
|
|
17,338,495 |
|
Allocated to: |
|
|
|
|
|
|
|
|
|
|
|
|
Continuing operations |
|
|
9,032,724 |
|
|
|
14,450,094 |
|
|
|
15,926,103 |
|
Discontinued operations |
|
|
— |
|
|
|
1,185,600 |
|
|
|
1,412,392 |
|
18. Segment information
Reportable segments are reported in a manner consistent with the internal reporting provided to the chief operating decision maker, with appropriate aggregation. The chief operating decision maker is the CEO who is responsible for allocating resources, assessing the performance of the reportable segment and making key strategic decisions. The Company operates in two segments: Biopharmaceutical and Strategic Investments.
The Company’s Biopharmaceutical segment is focused on furthering the research and development of the Company’s drug candidates and the development of a treatment for alcohol misuse for application in hospitals and other medical practices. The Biopharmaceutical segment primarily earns interest income on excess cash on hand invested in short-term guaranteed investment certificates.
| F-33 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The Company’s Strategic Investments segment is focused on generating returns and cashflow through the issuance of loans secured by residential property, with FSD Strategic Investments having a first or second collateral mortgage on the secured property.
The following tables summarize the Company’s total current and non-current assets and current and non-current liabilities as of December 31, 2023 and 2022, on a segmented basis:
|
|
As at December 31, 2023 |
|
|||||||||
|
|
Biopharmaceutical |
|
|
Strategic Investments |
|
|
Consolidated |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Current assets |
|
|
4,516,910 |
|
|
|
7,187,988 |
|
|
|
11,704,898 |
|
Non-current assets |
|
|
5,482,157 |
|
|
|
907,366 |
|
|
|
6,389,523 |
|
Current liabilities |
|
|
4,565,566 |
|
|
|
619,593 |
|
|
|
5,185,159 |
|
Non-current liabilities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
As at December 31, 2022 |
|
|||||||||
|
|
Biopharmaceutical |
|
|
Strategic Investments |
|
|
Consolidated |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Current assets |
|
|
18,087,292 |
|
|
|
— |
|
|
|
18,087,292 |
|
Non-current assets |
|
|
13,128,826 |
|
|
|
7,431,656 |
|
|
|
20,560,482 |
|
Current liabilities |
|
|
7,830,432 |
|
|
|
237,118 |
|
|
|
8,067,550 |
|
Non-current liabilities |
|
|
38,004 |
|
|
|
— |
|
|
|
38,004 |
|
The following tables summarize the Company’s interest income, total operating expenses, and net loss for the years ended December 31, 2023 and 2022 on a segmented basis:
|
|
For the year ended December 31, 2023 |
|
|||||||||
|
|
Biopharmaceutical |
|
|
Strategic Investments |
|
|
Consolidated |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Interest income |
|
|
(675,731 | ) |
|
|
(110,632 | ) |
|
|
(786,363 | ) |
Total operating expenses |
|
|
23,169,675 |
|
|
|
619,823 |
|
|
|
23,789,498 |
|
Net loss |
|
|
(18,204,886 | ) |
|
|
(25,702 | ) |
|
|
(18,230,588 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, 2022 |
|
|||||||||
|
|
Biopharmaceutical |
|
|
Strategic Investments |
|
|
Consolidated |
|
|||
|
|
|
$ |
|
|
$ |
|
|
$ |
|
||
Interest income |
|
|
(344,294 | ) |
|
|
(23,441 | ) |
|
|
(367,735 | ) |
Total operating expenses |
|
|
26,798,301 |
|
|
|
631,310 |
|
|
|
27,429,611 |
|
Net loss |
|
|
(23,541,030 | ) |
|
|
(65,798 | ) |
|
|
(23,606,828 | ) |
| F-34 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
19. Income taxes
The reconciliation of income tax expense for the years ended December 31, 2023, 2022 and 2021 consists of the following:
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Loss from continuing operations before income taxes |
|
|
(18,230,588 | ) |
|
|
(26,703,662 | ) |
|
|
(33,937,956 | ) |
Statutory federal and provincial tax rate |
|
|
26.50 | % |
|
|
26.50 | % |
|
|
26.50 | % |
Income tax recovery at the statutory tax rate |
|
|
(4,831,106 | ) |
|
|
(7,076,470 | ) |
|
|
(8,993,558 | ) |
Permanent differences |
|
|
2,557,822 |
|
|
|
1,639,590 |
|
|
|
3,758,401 |
|
Book to filing adjustments |
|
|
(119,668 | ) |
|
|
438,255 |
|
|
|
75,474 |
|
Share issuance cost booked directly to equity |
|
|
— |
|
|
|
— |
|
|
|
(377,378 | ) |
Impact of tax rate changes |
|
|
(42,277 | ) |
|
|
— |
|
|
|
— |
|
Foreign exchange |
|
|
(582,404 | ) |
|
|
1,044,135 |
|
|
|
(120 | ) |
Change in tax benefits not recognized |
|
|
3,017,633 |
|
|
|
3,954,490 |
|
|
|
5,537,181 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Deferred tax assets have not been recognized in respect of the following temporary differences as at December 31, 2023 and 2022:
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
|
$ |
|
|
$ |
|
|
$ |
|
||
Non-capital losses - Canada |
|
|
88,880,329 |
|
|
|
77,271,986 |
|
|
|
63,216,617 |
|
Net-operating loss - US |
|
|
5,073,156 |
|
|
|
5,120,395 |
|
|
|
5,111,610 |
|
Unrealized foreign exchange loss |
|
|
— |
|
|
|
94,733 |
|
|
|
94,733 |
|
Share-issuance costs |
|
|
1,046,315 |
|
|
|
2,045,027 |
|
|
|
3,349,261 |
|
Capital losses carried forward |
|
|
3,534,651 |
|
|
|
— |
|
|
|
— |
|
Other investments |
|
|
2,528,001 |
|
|
|
5,542,253 |
|
|
|
5,308,027 |
|
IFRS 16 |
|
|
5,814 |
|
|
|
37,439 |
|
|
|
87,050 |
|
Property, plant and equipment |
|
|
849,854 |
|
|
|
324,798 |
|
|
|
167,653 |
|
Total |
|
|
101,918,120 |
|
|
|
90,436,631 |
|
|
|
77,334,951 |
|
The Company’s Canadian non-capital income tax losses expire as follows:
|
|
|
$ |
|
2038 |
|
|
6,203,680 |
|
2039 |
|
|
10,989,236 |
|
2040 |
|
|
22,067,467 |
|
2041 |
|
|
19,735,799 |
|
Thereafter |
|
|
29,884,147 |
|
|
|
|
88,880,329 |
|
The company has cumulative US federal net operating loss carryforwards of approximately $5.07 million which will start to expire in 2026. Utilization of net operating loss carryforwards may be subject to limitations in the event of a change in ownership pursuant to United States Internal Revenue Code (“IRC”) § 382, and similar state provisions. As a result of the acquisition of Prismic on June 28, 2019, the preacquisition net operating loss carryforwards of approximately $4.93 million could be subject to IRC § 382 limitation as the acquisition could constitute a change of ownership.
| F-35 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
20. Commitments and contingencies
Commitments
Lucid-MS Agreement
The Company has entered into a license agreement that governs the Lucid-MS compound. Under the terms of the agreement, the Company shall pay a yearly license maintenance fee of C$100,000 until the first commercial sale of a product is made.
Under the agreement the Company is committed to minimum milestone payments of $nil and maximum milestone payments of C$12,500,000 if all product development and regulatory milestones are met. Furthermore, the Company is also responsible to pay revenue milestone payments and royalties if revenue milestones from commercial sales are achieved. Milestones can be extended by mutual agreement. No payments have been made to date related to these milestones.
Contingencies
Legal Matters
From time to time, the Company is named as a party to claims or involved in proceedings, including legal, regulatory and tax related, in the ordinary course of its business. While the outcome of these matters may not be estimable at the reporting date, the Company makes provisions, where possible, for the estimated outcome of such claims or proceedings. Should a loss result from the resolution of any claims or proceedings that differs from these estimates, the difference will be accounted for as a charge to the consolidated statements of loss and comprehensive loss in that period.
Contract Research Organization (“CRO”) Dispute
The Company was involved in arbitration proceedings with a CRO regarding amounts claimed to be owed to the CRO by the Company. The CRO was claiming it is owed amounts outstanding for work on clinical trials in the United States.
In November 2022, evidentiary hearings were held in New York. The parties submitted post-hearing briefs in December 2022. On May 19, 2023, an arbitrator arrived at a non-binding decision that both parties breached the agreements and awarded the CRO $1.7 million plus interest on past due amounts. On June 30, 2023, the CRO filed a motion to make the May 19, 2023 award recognized and enforceable in Ontario, Canada.
On August 2, 2023, the Company entered into a settlement agreement with the CRO for $100,000. The Company paid the settlement amount during the three months ended September 30, 2023. The Company derecognized all amounts previously recorded in trade and other payables on the statements of financial position as of September 30, 2023. This resulted in a gain on remeasurement of financial liability recognized in the statements of loss and comprehensive loss.
As at December 31, 2023, all matters have been resolved.
Raza Bokhari
On July 15, 2021, the Company’s former CEO, Raza Bokhari, filed a notice of arbitration seeking relief and support for breach of contract and severance and damages in the amount of $30,200,000, for aggravated and punitive damages in the amount of $500,000 and legal fees and disbursements associated with the arbitration.
Raza Bokhari was placed on administrative leave from his role as the Company’s Chief Executive Officer following the Company’s annual general and special meeting of shareholders on May 14, 2021, pending the outcome of an investigation of various concerns by a Special Committee comprised of independent directors using independent legal counsel. Upon the recommendation of the Special Committee, Raza Bokhari’s employment was terminated for cause by the Company’s board on July 27, 2021.
| F-36 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The Company disputed the allegations and counterclaimed against Raza Bokhari for losses sustained as a result of his alleged breaches of his duties to the Corporation. The arbitration hearing concluded in August 2022 and the arbitrator issued his decision in November 2022. Raza Bokhari’s claim for USD $30.2 million was dismissed in its entirety along with his claim that he had been wrongfully dismissed. The arbitrator ordered that Raza Bokhari repay certain monies to FSD Pharma, while also holding him responsible for FSD Pharma’s costs of the arbitration.
On December 9, 2022, Raza Bokhari filed an application in the Ontario Superior Court seeking to set aside the arbitral award of the court on the grounds that he was not treated equally and fairly and the arbitrator’s written award provided inadequate reasons for his decision.
On December 20, 2022, the Company’s legal counsel wrote to the Commercial List of the Ontario Superior Court of Justice seeking to transfer the application from the Civil List to the Commercial List. The request was granted on January 12, 2023.
On April 28, 2023, the court ordered the case to be heard at the Commercial List on September 27, 2023.
On September 27 and 28, 2023, the application to set aside the award and cost of ground of unfairness was dismissed. As Raza Bokhari lost the set aside application, the court ordered Raza Bokhari to pay the Company C$165,000 to cover the Company’s legal expenses.
On October 13, 2023, Raza Bokhari filed a “Notice of Motion for Leave to Appeal” with the Court of Appeal for Ontario.
On December 15, 2023, the Company submitted a responding party’s factum to the Court of Appeal for Ontario.
On February 6, 2024, the Ontario Superior Court of Justice affirmed judgment and awarded an additional C$5,000 in costs in light of Raza Bokhari’s failed motion for leave to appeal. As of the date hereof, the litigation is ongoing.
GBB Drink Lab, Inc.
GBB Drink Lab, Inc. (“GBB”) has filed a complaint with the United States District Court of Southern District of Florida, Fort Lauderdale Division against FSD Biosciences, Inc. and FSD Pharma, Inc. claiming a material breach of a mutual non-disclosure agreement and misappropriation of trade secrets, which GBB claims has and continues to cause irreparable harm, valued, as of August 30, 2022 (prior to the misappropriation and material breach) at $53,047,000. On June 23, 2023, the Company filed a motion to dismiss the complaint. On July 3, 2023, GBB responded in opposition to the Company’s motion to dismiss the complaint. On August 24, 2023, the parties filed a proposed joint scheduling report with the U.S. District Court, which set forth various deadlines that would govern this action. Under the proposed joint schedule, which still needs to be approved by the U.S. District Court, the case would be trial-ready by November 30, 2024.
The ultimate outcome of the matter cannot be determined at this time.
21. Related party transactions
Key management personnel are those persons having the authority and responsibility for planning, directing and controlling activities of the entity, directly or indirectly.
Transactions with key management and directors comprised the following:
|
a) |
In fiscal 2023, the Company paid independent directors’ compensation of C$60,000, with the chair of the audit committee receiving an additional C$20,000 and the chair of the compensation committee receiving an additional C$10,000. Director’s compensation for the year ended December 31, 2023, was $175,140 (2022 – $215,104 and 2021 – $757,690). |
| F-37 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
|
b) |
During the year ended December 31, 2023, the Company granted 400,000 (2022 – 2,820,104 and 2021 – nil) PSUs to independent members of the Board. As at December 31, 2023, the PSUs had fully vested upon the filing of the MS Phase 1 IND on January 6, 2023 and were settled with the issuance of Class B shares. |
|
|
|
|
c) |
During the year ended December 31, 2023, the Company granted the previous interim CEO, the current CEO, the Chief Operating Officer (“COO”) and the CEO of Lucid, 500,000 (2022 – nil and 2021 – nil) share options each with an exercise price of C$1.30 and an expiry date of January 25, 2028. All options were fully vested on grant. Each share option can be exercised to acquire one Class B share. |
|
|
|
|
d) |
During the year ended December 31, 2023, the Company entered into a secured loan agreement with the CEO, President, and Executive Co-Chairman of the Board in the amount of C$1,200,000, with monthly payments of C$6,000 based on an annual interest rate of 6%. The loan matures on April 26, 2025, and is part of FSD Strategic Investments’ portfolio of loans. The loan is secured by a second charge mortgage on underlying residential property. |
|
|
|
|
e) |
During the year ended December 31, 2023, the Company issued 1,000,000 warrants for consulting services to certain independent members of the Board with a fair value of $533,206, prior to them joining the Board. The Company determined the fair value of the services received could not be measured reliably and determined the fair value using the Black-Scholes model. |
|
|
|
|
f) |
In November 2023, the Company issued 24 Class A shares through a private placement for proceeds of $34. 12 Class A shares were issued to the CEO, President, and Executive Co-Chairman of the Board and 12 Class A Share were issued to the Director and Executive Co-Chairman of the Board. |
|
|
|
|
g) |
In February 2021, as compensation, the Company issued 1,349,764 shares with a fair value of $3,576,875 to former CEO, Raza Bokhari, in his capacity as Board Chair and Chief Executive Officer, and to certain other directors. Of the 1,349,764 shares issued, 1,173,709, with a fair value of $3,110,330, were issued to Raza Bokhari and 176,055 shares, with a fair value of $466,545, were issued to other directors. In June 2021, 156,278 of the shares issued to directors in February 2021 were cancelled. On March 8, 2022, following litigation with respect to certain of the shares issued to Raza Bokhari in February 2021, the court issued a decision, permitting the part of the share grant to Raza Bokhari until the date of his termination (being 536,979 Class B shares) but cancelling the shares relating to services that were to be provided after the date of termination (being 504,888 Class B shares). The shares were cancelled on March 29, 2022. |
|
|
|
|
h) |
For the year ended December 31, 2023, the Company paid expenses of $nil (2022 – $nil and 2021 – $262,834) to a company owned by the former CEO. |
|
|
|
|
i) |
For the year ended December 31, 2023, the Company reimbursed $145,081 (2022 – $41,596 and 2021 – $528,872) to a related party of the CEO, President, and Executive Co-Chairman of the Board for legal expenses. |
|
|
|
|
j) |
During the year ended December 31, 2021, the Company reimbursed certain directors C$1,334,158 for expenses incurred in relation to requisitioning, calling and holding the shareholders’ meeting. |
| F-38 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
Key management personnel compensation during the years ended December 31, 2023, 2022 and 2021 is comprised of:
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Salaries, benefits, bonuses and consulting fees |
|
|
1,395,096 |
|
|
|
1,839,441 |
|
|
|
2,075,893 |
|
Share-based payments |
|
|
1,980,732 |
|
|
|
1,345,952 |
|
|
|
6,881,641 |
|
Total |
|
|
3,375,828 |
|
|
|
3,185,393 |
|
|
|
8,957,534 |
|
As at December 31, 2023, the Company owes an executive officer $140,012, for legal fees incurred by the Company and paid by the executive officer on behalf of the Company. The amount owed is recorded within trade and other payables.
22. Capital Management
The Company’s capital management objectives are to maintain financial flexibility in order to complete the research and development of a proprietary formulation of natural ingredients, vitamins, and minerals to help with liver and brain function for the purposes of quickly relieving individuals from the effects of alcohol consumption.
The Company defines capital as the aggregate of its capital stock and borrowings.
As at December 31, 2023, the Company’s Share Capital was $137,778,485 (2022 – $143,410,560). The Company does not have any long-term debt. Outstanding notes payable were assumed on acquisition of Prismic and are due on demand.
The Company manages its capital structure in accordance with changes in economic conditions. In order to maintain or adjust its capital structure, the Company may elect to issue or repay financial liabilities, issue shares, repurchase shares or undertake any other activities as deemed appropriate under the specific circumstances. The Company is not subject to any externally imposed capital requirements.
23. Financial Instruments and Risk Management
Credit risk
Credit risk is the risk of financial loss to the Company if a customer or counterparty to a financial instrument fails to meet its contractual obligations and arises principally from deposits with banks and outstanding other receivables and finance receivables. The Company trades only with recognized, creditworthy third parties.
The Company does not hold any collateral as security for its outstanding finance receivables but mitigates this risk by dealing only with, what management believes to be, financially sound counterparties and, accordingly, does not anticipate significant loss for non-performance. The loans are secured by real estate properties and the Company is granted a first or second collateral charge mortgage on the properties for a sum equal to the interest payments plus the principal amount. The Company performs assessments on factors such as: timing of payments, loan to value, communications with the borrower and external macro factors such as interest rates and economic conditions to mitigate risks.
Liquidity risk
Liquidity risk is the risk the Company will not be able to meet its financial obligations as they come due. The Company’s exposure to liquidity risk is dependent on the Company’s ability to raise additional financing to meet its commitments and sustain operations. The Company mitigates liquidity risk by management of working capital, cash flows, the issuance of share capital and if desired, the issuance of debt. The Company’s trade and other payables and notes payables are all due within twelve months from the date of these financial statements.
| F-39 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
If unanticipated events occur that impact the Company’s ability to carry out the planned clinical trials, the Company may need to take additional measures to increase its liquidity and capital resources, including issuing debt or additional equity financing or strategically altering the business forecast and plan. In this case, there is no guarantee that the Company will obtain satisfactory financing terms or adequate financing. Failure to obtain adequate financing on satisfactory terms could have a material adverse effect on the Company’s results of operations or financial condition.
Market risk
Market risk is the risk the fair value or future cash flows of a financial instrument will fluctuate because of changes in market prices. Market risk comprises three types of risk: foreign currency risk, interest rate risk and other price risk.
· |
Foreign currency risk |
|
|
|
Foreign currency risk arises on financial instruments that are denominated in a currency other than the functional currency in which they are measured. The Company’s primary exposure with respect to foreign currencies is from Canadian dollar denominated cash, investments and trade and other payables. A 1% change in the foreign exchange rates would not result in any significant impact to the financial statements. |
|
|
· |
Interest rate risk |
|
|
|
Interest rate risk is the risk the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The Company does not have any material long-term borrowings outstanding subject to variable interest rates. Therefore, the Company is not exposed to interest rate risk as at December 31, 2023. |
|
|
· |
Other price risk |
|
|
|
Other price risk is the risk the fair value or future cash flows of a financial instrument will fluctuate because of changes in market prices (other than those arising from interest rate risk or currency risk), whether those changes are caused by factors specific to the individual financial instrument or its issuer, or factors affecting all similar financial instruments traded in the market. The Company is not exposed to other price risk as at December 31, 2023. |
Fair values
The carrying values of cash, other receivables, trade and other payables and notes payable approximate fair values due to the short-term nature of these items or they are being carried at fair value or, for notes payable, interest payables are close to the current market rates. The risk of material change in fair value is not considered to be significant. The Company does not use derivative financial instruments to manage this risk.
Financial instruments recorded at fair value on the consolidated statement of financial position are classified using a fair value hierarchy that reflects the significance of the inputs used in making the measurements. The Company categorizes its fair value measurements according to a three-level hierarchy. The hierarchy prioritizes the inputs used by the Company’s valuation techniques. A level is assigned to each fair value measurement based on the lowest-level input significant to the fair value measurement in its entirety. The three levels of the fair value hierarchy are defined as follows:
|
· |
Level 1 – Unadjusted quoted prices as at the measurement date for identical assets or liabilities in active markets. |
|
|
|
|
· |
Level 2 – Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data. |
|
|
|
|
· |
Level 3 – Significant unobservable inputs that are supported by little or no market activity. The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. |
| F-40 |
|
FSD PHARMA INC. Notes to the consolidated financial statements (expressed in United States dollars) December 31, 2023 and 2022 |
The fair value hierarchy requires the use of observable market inputs whenever such inputs exist. A financial instrument is classified to the lowest level of the hierarchy for which a significant input has been considered in measuring fair value. During the year, there were no transfers of amounts between levels.
24. Subsequent events
On January 24, 2024, the Company entered into an agreement with SBS Intl Group LLC. (“SBS”) to assist the Company in enhancing its market awareness and foster productive, continuing dialogues with shareholders and other market participants. The agreement grants SBS 100,000 share options with an exercise price of $1.05 and expiry date of January 24, 2026. Per the agreement 19,000 share options vest on the 45th day following the date of grant and 9,000 share options vest on a monthly basis starting in the fourth month following the date of grant.
On January 2024, the Company entered into an agreement with Draper, Inc. (“Draper”) and Carriage House Capital, Corp. (“Carriage House”) to assist the Company in enhancing its market awareness and foster productive, continuing dialogues with shareholders and other market participants. The agreement grants Draper and Carriage 350,000 share options each with the exercise price of $1.05 and expiry date of January 24, 2026. Per the agreement 150,000 share options vest on the 45th day and 61,111 share options vest on a monthly basis starting in the fourth month following the date of grant.
On February 23, 2024, the Company entered into a settlement agreement to issue 70,000 Class B shares to settle $81,900 of trade and other payables.
On February 23, 2024, the Company entered into a settlement agreement to issue 475,000 Class B shares to settle $836,309 of trade and other payables.
On February 23, 2024, the Company granted 55,000 RSUs to advisors of the Company for services provided. The RSUs vested immediately upon grant.
On March 26, 2024, the Board approved an amendment to the loan agreement with Celly, to increase the loan amount from C$1,000,000 to C$1,300,000. The amendment provides the Company the right to convert any loan amount outstanding including interest into Common Shares of Celly at $0.03 per share upon the occurrence of an event of default.
Subsequent to December 31, 2023, the Company entered into an at-the-market offering agreement (the “ATM Agreement”) with H.C Wainwright & Co., LLC to sell Class B shares, having an aggregate offering price up to $11,154,232.
| F-41 |
EXHIBIT 1.2
|
|
AMENDED AND RESTATED BY-LAW NUMBER 1
|
|
A BY-LAW RELATING GENERALLY TO THE REGULATION OF THE BUSINESS AND AFFAIRS OF
FSD PHARMA INC.
TABLE OF CONTENTS
|
|
| Page |
|
|
|
|
|
|
|
|
| ARTICLE 1 INTERPRETATION |
| 1 |
|
|
| 1.1 | Definitions |
| 1 |
|
| 1.2 | Number, Gender and Headings |
| 2 |
|
| 1.3 | By-Law Subordinate to Other Documents |
| 2 |
|
| 1.4 | Computation of Time |
| 2 |
|
|
|
|
|
|
|
| ARTICLE 2 DIRECTORS |
| 2 |
|
|
| 2.1 | Notice of Meeting |
| 2 |
|
| 2.2 | Waiver of Notice |
| 3 |
|
| 2.3 | No Notice to Newly Appointed Director |
| 3 |
|
| 2.4 | Meetings Without Notice |
| 3 |
|
| 2.5 | Adjourned Meeting |
| 3 |
|
| 2.6 | Regular Meetings |
| 3 |
|
| 2.7 | Place of Meeting |
| 3 |
|
| 2.8 | Quorum for Board Meetings |
| 3 |
|
| 2.9 | Chairman of Board Meetings |
| 3 |
|
| 2.10 | Votes at Board Meetings |
| 4 |
|
| 2.11 | Meeting by Telephonic or Electronic Means |
| 4 |
|
|
|
|
|
|
|
| ARTICLE 3 MEETINGS OF SHAREHOLDERS |
| 4 |
|
|
| 3.1 | Notice of Shareholders’ Meetings |
| 4 |
|
| 3.2 | Quorum at Meetings of Shareholders |
| 4 |
|
| 3.3 | Chairman’s Vote |
| 4 |
|
| 3.4 | Voting |
| 4 |
|
| 3.5 | Chairman, Secretary and Scrutineers |
| 5 |
|
| 3.6 | Who May Attend Shareholders’ Meeting |
| 5 |
|
| 3.7 | Meeting by Telephonic or Electronic Means |
| 5 |
|
|
|
|
|
|
|
| ARTICLE 4 SECURITY CERTIFICATES, PAYMENTS |
| 5 |
|
|
| 4.1 | Certificates |
| 5 |
|
| 4.2 | Cheques |
| 5 |
|
| 4.3 | Cheques to Joint Shareholders |
| 6 |
|
| 4.4 | Non-Receipt of Cheques |
| 6 |
|
| 4.5 | Currency of Dividends |
| 6 |
|
|
|
|
|
|
|
| ARTICLE 5 SIGNATORIES, INFORMATION |
| 6 |
|
|
| 5.1 | Signatories |
| 6 |
|
| 5.2 | Facsimile or Electronic Signatures |
| 6 |
|
| 5.3 | Voting Rights in other Corporations |
| 6 |
|
| 5.4 | Restriction on Information Disclosed |
| 7 |
|
|
|
|
|
|
|
| ARTICLE 6 PROTECTION AND INDEMNITY |
| 7 |
|
|
| 6.1 | Transactions with the Corporation |
| 7 |
|
| 6.2 | Limitation of Liability |
| 8 |
|
| 6.3 | Indemnity of Directors and Officers |
| 8 |
|
| 6.4 | Advances by the Corporation |
| 8 |
|
| 6.5 | Indemnities Not Limiting |
| 8 |
|
| 6.6 | Insurance |
| 8 |
|
|
|
|
|
|
|
| ARTICLE 7 NOTICES |
| 9 |
|
|
| 7.1 | Procedure for Sending Notices |
| 9 |
|
| 7.2 | Notices to Successors in Title |
| 9 |
|
| 7.3 | Notice to Joint Shareholders |
| 9 |
|
| 7.4 | Signatures on Notices |
| 9 |
|
| 7.5 | Omission of Notice Does Not Invalidate Actions |
| 9 |
|
| 7.6 | Waiver of Notice |
| 9 |
|
|
|
|
|
|
|
| ARTICLE 8 REPEAL OF FORMER BY-LAWS |
| 9 |
|
|
| 8.1 | Former By-Laws Repealed |
| 9 |
|
| - i - |
ARTICLE 1
INTERPRETATION
1.1 Definitions
In this by-law:
“Act” means the Business Corporations Act (Ontario) and the regulations enacted pursuant to it and any statute and regulations that may be substituted for them, as amended or re-enacted from time to time;
“articles” means the articles, as that term is defined in the Act, of the Corporation; “auditor” means the auditor of the Corporation;
“board” means the board of directors of the Corporation; “by-law” means a by-law of the Corporation; “Corporation” means FSD Pharma Inc.;
“director” means a director of the Corporation; “Indemnified Person” means
|
| (a) | each director and former director of the Corporation; |
|
|
|
|
|
| (b) | each officer and former officer of the Corporation; |
|
|
|
|
|
| (c) | each individual who acts or acted at the Corporation’s request as a director or officer of a body corporate or an individual acting in a similar capacity of another entity; and |
|
|
|
|
|
| (d) | the respective heirs and legal representatives of each of the persons designated in the preceding paragraphs (a) through (b). |
“number of directors” means the number of directors provided for in the articles or, where a minimum and maximum number of directors is provided for in the articles, the number of directors determined by a special resolution or resolution of the board where it is empowered by special resolution to determine the number of directors.
“officer” means an officer of the Corporation, and reference to any specific officer is to the individual holding that office of the Corporation;
“person” means an individual, body corporate, partnership, joint venture, trust, unincorporated organization, association, the Crown or any agency or instrumentality thereof, or any entity recognized by law;
“proxyholder” means an individual holding a valid proxy for a shareholder; “resident Canadian” has the meaning ascribed to that phrase in the Act;
- 2 -
“shareholder” means a shareholder of the Corporation;
“telephonic or electronic means” means telephone calls or messages, facsimile messages, electronic mail, transmission of data or information through automated touch- tone telephone systems, transmission of data or information through computer networks, any other similar means or any other means prescribed by the Act; and
“voting person” means, in respect of a meeting of shareholders, an individual who is either a shareholder entitled to vote at that meeting, a duly authorized representative of a shareholder entitled to vote at the meeting or a proxyholder entitled to vote at that meeting.
1.2 Number, Gender, and Headings
In this by-law, words in the singular include the plural and vice-versa and words in one gender include all genders. The insertion of headings in this by-law and its division into articles, sections and other subdivisions are for convenience of reference only, and shall not affect the interpretation of this by-law.
1.3 By-Law Subordinate to Other Documents
This by-law is subordinate to, and should be read in conjunction with, the Act, the articles and any unanimous shareholder agreement of the Corporation.
1.4 Computation of Time
The computation of time and any period of days shall be determined in accordance with the Act.
ARTICLE 2
DIRECTORS
2.1 Notice of Meeting
Any director may call a meeting of the board by petitioning and providing the chairman and secretary with written notice stating the business proposed to be conducted and the proposed timing of such meeting. The chairman shall thereupon direct the secretary to issue notice to the directors establishing a place, date and time for such meeting, having regard to the directors’ availability and ability to achieve quorum, the urgency of the business proposed to be conducted, and any regularly scheduled board meeting already called pursuant to Section 2.6 at which such business could alternatively be conducted in a timely manner. The secretary shall at the direction of the chairman or other director petitioning the meeting deliver any additional materials corresponding to the agenda items set out in the notice of meeting that may be necessary or advisable to allow the directors to make an informed decision with respect to the business put before the meeting.
The board also may appoint, by resolution, dates, time and places for meetings of the board. A copy of any such resolution shall be sent to each director forthwith after being passed, but no other notice is required for any such meeting.
These powers are in addition to the powers of a majority of those representing a quorum of the board to call a meeting in accordance with Section 126(8) of the Act.
- 3 -
2.2 Waiver of Notice
A director may in any manner and at any time waive notice of or otherwise consent to a meeting of the board, including by sending an electronic document to that effect. Attendance of a director at a meeting of the board shall constitute a waiver of notice of that meeting, except where a director attends for the express purpose of objecting to the transaction of any business on the grounds that the meeting has not been properly called.
2.3 No Notice to Newly Appointed Director
An individual need not be given notice of the meeting at which that individual is appointed by the other directors to fill a vacancy on the board, if that individual is present at that meeting.
2.4 Meetings Without Notice
A meeting of the board may be held without notice immediately following any annual meeting of shareholders.
2.5 Adjourned Meeting
Notice of an adjourned meeting of the board is not required if the time and place of the adjourned meeting is announced at the original meeting.
2.6 Regular Meetings
The board may appoint a day or days in any month or months for regular meetings of the board at a place and hour to be named. A copy of any resolution of the board fixing the place and time of such regular meetings shall be sent to each director forthwith after being passed, but no other notice shall be required for any such regular meeting except where the Act requires the purpose thereof or the nature of the business to be transacted to be specified.
2.7 Place of Meeting
A meeting of the board may be held at any place within or outside Ontario, and no such meeting need be held at a place within Canada.
2.8 Quorum for Board Meetings
The quorum for the transaction of business at any meeting of the board shall consist of a majority of the directors. If, however, the Corporation has fewer than three directors, all directors must be present at any meeting of the board to constitute a quorum.
2.9 Chairman of Board Meetings
The chairman of any meeting of the board shall be a director and the Chairman of the Board, and if a Chairman of the Board has not been appointed, the directors present at the meeting shall choose a director to preside as chairman of the meeting.
- 4 -
2.10 Votes at Board Meetings
Each director present at a meeting of the board shall have 1 vote on each motion arising. Motions arising at meetings of the board shall be decided by a majority vote. The chairman of the meeting shall have a second or casting vote in the event of a tied vote on a matter before the board of directors.
2.11 Meeting by Telephonic or Electronic Means
A meeting of the board or of a committee of the board may be held by telephonic or electronic means or other communication facility that permits all persons participating in the meeting to communicate with each other simultaneously and instantaneously. A director who, through those means, votes at such a meeting or establishes a communications link to such a meeting shall be deemed for the purposes of the Act to be present at the meeting.
ARTICLE 3
MEETINGS OF SHAREHOLDERS
3.1 Notice of Shareholders’ Meetings
The board may call a meeting of shareholders by causing notice of the date, time and place of the meeting to be sent to each shareholder entitled to vote at the meeting, each director and the auditor. Such notice shall be sent no less than 21 days and no more than 50 days before the meeting, if the Corporation is an offering corporation (as defined in the Act), or no less than 10 days and no more than 50 days before the meeting if the Corporation is not an offering corporation.
3.2 Quorum at Meetings of Shareholders
A quorum for the transaction of business at any meeting of shareholders shall be 2 voting persons holding or representing in the aggregate not less than 10% of the issued and outstanding shares of the Corporation, or of the class or classes respectively (if there is more than one class of shares outstanding for the time being). Notwithstanding the foregoing, if the Corporation has only one shareholder, or only one shareholder of any class or series of shares, the shareholder present in person or by proxy constitutes a meeting and a quorum for such meeting.
3.3 Chairman’s Vote
The chairman of any meeting of shareholders shall not have a second or casting vote.
3.4 Voting
Unless the chairman of a meeting of shareholders directs a ballot, or a voting person demands one, each motion shall be voted upon by a show of hands. Each voting person has 1 vote in a vote by show of hands. Whenever a vote by show of hands shall have been taken upon a question, unless a ballot thereon is so required or demanded, a declaration by the chairman of the meeting as to the result of the vote upon the question and an entry to that effect in the minutes of the meeting shall be prima facie evidence of the fact without proof of the number or proportion of the votes recorded in favour of or against any resolution or other proceeding in respect of such question, and the result of the vote so taken shall be the decision of the shareholders upon such question. A ballot may be directed or demanded either before or after a vote by show of hands. A demand for a ballot may be withdrawn at any time prior to the taking of the ballot. If a ballot is taken, a prior vote by show of hands has no effect.
- 5 -
3.5 Chairman, Secretary and Scrutineers
The chairman of any meeting of shareholders shall be the first mentioned of such of the following officers as have been appointed and who is present at the meeting and willing to serve: chairman of the board, managing director, or chief executive officer, president or a vice-president who is a shareholder. If no such officer is present within 15 minutes from the time fixed for holding the meeting, the persons present and entitled to vote shall choose one of their number to be chairman. If the secretary of the Corporation is absent, the chairman shall appoint some person, who need not be a shareholder, to act as secretary of the meeting. If desired, one or more scrutineers, who need not be shareholders, may be appointed by a resolution or by the chairman with the consent of the meeting.
3.6 Who May Attend Shareholders’ Meeting
The only persons entitled to attend a meeting of shareholders are voting persons, the directors, the auditor and, if any, the chairman, the managing director and the chief executive officer, as well as others permitted by the chairman of the meeting.
3.7 Meeting by Telephonic or Electronic Means
A meeting of the shareholders may be held by telephonic or electronic means and a shareholder who, through those means, votes at the meeting or establishes a communications link to the meeting shall be deemed for the purposes of the Act to be present at the meeting.
ARTICLE 4
SECURITY CERTIFICATES, PAYMENTS
4.1 Certificates
|
| (a) | Subject to Section 4.1(b), security certificates shall be in such form as the board may approve or the Corporation adopt. The president or the board may order the cancellation of any security certificate that has become defaced and the issuance of a replacement certificate for it when the defaced certificate is delivered to the Corporation or to a transfer agent or branch transfer agent of the Corporation. |
|
|
|
|
|
| (b) | Unless otherwise provided in the articles, the board may provide by resolution that any or all classes and series of shares or other securities shall be uncertificated securities, provided that such resolution shall not apply to securities represented by a certificate until such certificate is surrendered to the Corporation. |
4.2 Cheques
Any amount payable in cash to shareholders (including dividends payable in cash) may be paid by cheque drawn on any of the Corporation’s bankers to the order of each registered holder of shares of the class or series in respect of which such amount is to be paid or, paid by electronic funds transfer to the bank account designated by the registered holder, unless such holder otherwise directs. Cheques may be sent by delivery or first class mail to such registered holder at that holder’s address appearing on the register of shareholders, unless that holder otherwise directs in writing. By sending a cheque, as provided in this by-law, or the electronic funds transfer as aforesaid, in the amount of the dividend less any tax that the Corporation is required to withhold, the Corporation discharges its liability to pay the amount of that dividend, unless the cheque is not paid on due presentation.
- 6 -
4.3 Cheques to Joint Shareholders
Cheques payable to joint shareholders shall be made payable to the order of all such joint shareholders unless such joint shareholders direct otherwise. Such cheques may be sent to the joint shareholders at the address appearing on the register of shareholders in respect of that joint holding, to the first address so appearing if there is more than one, or to such other address as those joint shareholders direct in writing.
4.4 Non-Receipt of Cheques
The Corporation shall issue a replacement cheque in the same amount to any person who does not receive a cheque sent as provided in this by-law, if that person has satisfied the conditions regarding indemnity, evidence of non-receipt and title set by the board from time to time, either generally or for that particular case.
4.5 Currency of Dividends
Dividends or other distributions payable in cash may be paid to some shareholders in Canadian currency and to other shareholders in equivalent amounts of a currency or currencies other than Canadian currency. The board may declare dividends or other distributions in any currency or in alternative currencies and make such provisions as it deems advisable for the payment of such dividends or other distributions.
ARTICLE 5
SIGNATORIES, INFORMATION
5.1 Signatories
Except for documents executed in the usual and ordinary course of the Corporation’s business, which may be signed by any officer or employee of the Corporation acting within the scope of his or her authority, the following are the only persons authorized to sign any document on behalf of the Corporation:
|
| (a) | any individual appointed by resolution of the board to sign the specific document, that type of document or documents generally on behalf of the Corporation; or |
|
|
|
|
|
| (b) | any director or any officer appointed to office by the board. |
Any document so signed may, but need not, have the corporate seal of the Corporation applied, if there is one.
5.2 Facsimile or Electronic Signatures
The signature of any individual authorized to sign on behalf of the Corporation may, if specifically authorized by resolution of the board, be written, printed, stamped, engraved, lithographed or otherwise mechanically reproduced or may be an electronic signature. Anything so signed shall be as valid as if it had been signed manually, even if that individual has ceased to hold office when anything so signed is issued or delivered, until revoked by resolution of the board.
5.3 Voting Rights in other Corporations
All securities carrying voting rights of any other corporation held from time to time by the Corporation may be voted at any and all meetings of shareholders, bond holders, debenture holders or holders of other securities (as the case may be) of such other corporation and in such manner as the board may from time to time determine. Any person or persons authorized to sign on behalf of the Corporation may also from time to time execute and deliver for and on behalf of the Corporation proxies and/or arrange for the issuance of voting certificates and/or other evidence of the right to vote in such names as they may determine.
- 7 -
5.4 Restriction on Information Disclosed
Except as required by the Act or authorized by the board, no shareholder is entitled by virtue of being a shareholder to disclosure of any information, document or records respecting the Corporation or its business.
PROTECTION AND INDEMNITY
6.1 Transactions with the Corporation
A director or officer of the Corporation who is:
|
| (a) | a party to a material contract or transaction or proposed material contract or proposed transaction with the Corporation; or |
|
|
|
|
|
| (b) | a director or an officer of, or has a material interest in, any person who is a party to a material contract or transaction or proposed material contract or proposed transaction with the Corporation. |
shall, at the time and in the manner provided in the Act, disclose in writing to the Corporation or request to have entered in the minutes of meetings of directors, the nature and extent of his or her interest. Except as provided in the Act, no such director of the Corporation shall attend any part of a meeting of directors during which the contract or transaction is discussed, and no such director shall vote on any resolution to approve such contract or transaction.
If a material contract is made or a material transaction is entered into between the Corporation and one or more of its directors or officers, or between the Corporation and another person of which a director or officer of the Corporation is a director or officer or in which he or she has a material interest, the director or officer shall not be accountable to the Corporation or its shareholders for any profit or gain realized from the contract or transaction, and the contract shall not be void or voidable, by reason only of that relationship or by reason only that such director is present at or is counted to determine the presence of a quorum at the meeting of directors that authorized the contract or transaction, if (a) the director or officer disclosed his or her interest in accordance with the Act, and (b) the contract or transaction was reasonable and fair to the Corporation at the time it was approved.
Even if the foregoing conditions are not met, a director or officer, acting honestly and in good faith, shall not be accountable to the Corporation or to its shareholders for any profit or gain realized from any such contract or transaction, by reason only of his or her holding the office of director or officer, and the contract or transaction, if it was reasonable and fair to the Corporation at the time it was approved, shall not be by reason only of the director’s or officer’s interest therein void or voidable, where (a) the contract or transaction is confirmed or approved by special resolution at a meeting of the shareholders duly called for that purpose, and (b) the nature and extent of the director’s or officer’s interest in the contract or transaction are disclosed in reasonable detail in the notice calling the meeting or in the information circular.
- 8 -
6.2 Limitation of Liability
Subject to the Act, no director or officer of the Corporation shall be liable for the acts or omissions of any other director, officer, employee or agent of the Corporation, or for any costs, charges or expenses of the Corporation resulting from any deficiency of title to any property acquired for or on behalf of the Corporation, or for the insufficiency of any security in or upon which any of the moneys of the Corporation shall be invested, or for any loss or damage arising from bankruptcy or insolvency, or in respect of any tortious acts of or relating to the Corporation or any other director, officer, employee or agent of the Corporation, or for any loss occasioned by an error of judgment or oversight on the part of any other director, officer, employee or agent of the Corporation, or for any other costs, charges or expenses of the Corporation occurring in connection with the execution of the duties of the director or officer, unless such costs, charges or expenses are incurred as a result of such person’s own wilful neglect, default or negligence. Nothing in this by-law, however, shall relieve any director or officer from the duty to act in accordance with the Act or from liability for any breach of the Act.
6.3 Indemnity of Directors and Officers
As required or permitted by the Act, the Corporation shall indemnify each Indemnified Person against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, which that Indemnified Person reasonably incurs in respect of any civil, criminal or administrative, investigative or other proceeding to which that Indemnified Person is made a party by reason of being or having been at the relevant time a director or officer of the Corporation or of a body corporate of which the Corporation is or was a shareholder or creditor, or by reason of having acted in a similar capacity for any other entity that is or was at the relevant time directly or indirectly owned or controlled by the Corporation, if:
|
| (a) | the Indemnified Person acted honestly and in good faith with a view to the best interests of the Corporation; and |
|
|
|
|
|
| (b) | in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, the Indemnified Person had reasonable grounds for believing that the conduct was lawful. |
6.4 Advances by the Corporation
The Corporation shall advance monies to an Indemnified Person for the costs, charges and expenses of a proceeding referred to in Section 6.3 provided the Indemnified person shall repay such monies if the Indemnified person does not fulfil the conditions set out in Subsections 6.3(a) and (b).
6.5 Indemnities Not Limiting
The provisions of this Article 6 shall be in addition to and not in substitution for any rights, immunities and protections to which an Indemnified Person is otherwise entitled under the Act or as the law may permit or require.
6.6 Insurance
Subject to the Act, the Corporation may purchase and maintain such insurance for the benefit of any individual referred to in Section 6.3 as the board may determine.
- 9 -
ARTICLE 7
NOTICES
7.1 Procedure for Sending Notices
Notice shall be deemed to have been sufficiently sent if sent in writing to the address of the addressee on the books of the Corporation and delivered in person, sent by prepaid first class mail or sent by any telephonic or electronic means of sending messages. Notice shall not be sent by mail if there is any general interruption of postal services in the municipality in which or to which it is mailed. Each notice so sent shall be deemed to have been received on the day it was delivered or sent by electronic means or on the fifth day after it was mailed.
7.2 Notices to Successors in Title
Notice to a shareholder is sufficient notice to each successor in title to that shareholder until the name and address of that successor have been entered on the Corporation’s share register.
7.3 Notice to Joint Shareholders
Notice to one joint shareholder is sufficient notice to all of them. Such notice shall be addressed to all such joint shareholders and sent to the address for them on the Corporation’s register of shareholders, or to the first such address if there is more than one.
7.4 Signatures on Notices
The signature on any notice or other communication or document to be sent by the Corporation may be written, printed, stamped, engraved, lithographed or otherwise mechanically reproduced or may be an electronic signature.
7.5 Omission of Notice Does Not Invalidate Actions
All actions taken at a meeting in respect of which a notice has been sent shall be valid even if:
|
| (a) | by accident, notice was not sent to any person; |
|
|
|
|
|
| (b) | notice was not received by any person; or |
|
|
|
|
|
| (c) | there was an error in a notice that did not affect the substance of that notice. |
7.6 Waiver of Notice
Any person entitled to notice under the Act, the articles or the by-laws may waive that notice. Waiver, either before or after the event referred to in the notice, shall cure any default in sending that notice.
ARTICLE 8
REPEAL OF FORMER BY-LAWS
8.1 Former By-Laws Repealed
Upon this by-law coming into force, By-Law Number 1 of the Corporation that is in effect at the time this by-law becomes effective is repealed provided that such repeal shall not affect the previous operation of such by-law so repealed or affect the validity of any act or right, privilege, obligation or liability acquired or incurred under the validity of any contract or agreement made pursuant to any such by-law prior to its repeal. All officers and provisions of this by-law and all resolutions of the shareholders or of the directors with continuing effect passed under such repealed by-law shall continue good and valid except to the extent inconsistent with this by-law and until amended or repealed.
MADE by the board on the 21st day of January, 2021.
SCHEDULE “A”
ADVANCE NOTICE BY-LAW
BY-LAW NO. 2 – ADVANCE NOTICE BY-LAW
A by-law relating generally to the advance notice requirements for the nomination of directors of FSD PHARMA INC. (the “Corporation”) is hereby made as follows:
INTRODUCTION
The purpose of this advance notice by-law (the “Advance Notice By-law”) is to establish the conditions and framework under which holders of class A multiple voting shares and class B subordinate voting shares of the Corporation may exercise their right to submit director nominations by fixing a deadline by which such nominations must be submitted by a shareholder to the Corporation prior to any annual or special meeting of shareholders, and sets forth the information that a shareholder must include in the notice to the Corporation for the notice to be in proper form.
It is the position of the Corporation that this Advance Notice By-law is beneficial to shareholders and other stakeholders of the Corporation in that it helps to: (i) facilitate an orderly and efficient annual general meeting or, where the need arises, special meeting, process; (ii) ensure that all shareholders receive adequate notice of the director nominations and sufficient information regarding all director nominees; and (iii) allow shareholders to register an informed vote after having been afforded reasonable time for appropriate deliberation. This Advance Notice By-law is intended to further these objectives.
NOMINATION OF DIRECTORS
Subject only to the Business Corporations Act (Ontario) (the “Act”) and the articles of the Corporation, only persons who are nominated in accordance with the following procedures shall be eligible for election as directors of the Corporation. For greater certainty, this Advance Notice By-law does not apply to (i) the appointment, by the board, of a director to fill a vacancy on the board or (ii) the appointment, by the board, of a director between annual meetings of the shareholders of the Corporation in accordance with the articles of the Corporation. Nominations of persons for election to the board may be made at any annual meeting of shareholders, or at any special meeting of shareholders if one of the purposes for which the special meeting was called was the election of directors. Such nominations may be accepted only if made in the following manner:
(a) by or at the direction of the board or an authorized officer of the Corporation, including pursuant to a notice of meeting;
(b) by or at the direction or request of one or more shareholders of the Corporation pursuant to a proposal made in accordance with the provisions of the Act, or a requisition of meeting of the shareholders of the Corporation made in accordance with the provisions of the Act; or
(c) by any person (a “Nominating Shareholder”): (i) who, at the close of business on the date of the giving of the notice provided below in this Advance Notice By-law and on the record date for notice of such meeting, is entered in the securities register of the Corporation as a holder of one or more shares carrying the right to vote at such meeting, or who beneficially owns shares that are entitled to be voted at such meeting and who establishes to the satisfaction of the chair of the meeting such beneficial ownership; and (ii) who complies with the notice and other procedures set out below in this Advance Notice By-law.
In addition to any other applicable requirements, for a nomination made by a Nominating Shareholder to be accepted, such Nominating Shareholder must have given timely notice thereof in proper written form to the Secretary of the Corporation at the head office of the Corporation in accordance with this Advance Notice By-law.
To be timely, a Nominating Shareholder’s notice to the Secretary of the Corporation must be given:
(a) in the case of an annual meeting of shareholders, not less than 30, nor more than 60, days prior to the date of the annual meeting of shareholders; provided, however, that in the event the annual meeting of shareholders is to be held on a date that is less than 50 days after the date (the “Notice Date”) on which the first public announcement of the date of the annual meeting was made, notice by the Nominating Shareholder must be given not later than the close of business on the 10th day following the Notice Date; and
(b) in the case of a special meeting (which is not also an annual meeting) of shareholders called for the purpose of electing directors (whether or not called for other purposes), not later than the close of business on the 15th day following the day on which the first public announcement of the date of the special meeting of shareholders was made. In no event shall any adjournment or postponement of a meeting of shareholders or the announcement thereof commence a new time period for the giving of a Nominating Shareholder’s notice as described above.
To be in proper written form, a Nominating Shareholder’s notice to the Secretary of the Corporation must set out:
(a) as to each person whom the Nominating Shareholder proposes to nominate for election as a director (i) the name, age, business address and residential address of the person, (ii) the principal occupation, business or employment of the person for the most recent five years including, without limitation, the name and principal business of any company in which any such employment is carried on, (iii) the number of securities of each class of voting securities of the Corporation or any of its subsidiaries beneficially owned, or controlled or directed, directly or indirectly, by the person as of the record date for the meeting of shareholders (if such date shall then have been made publicly available and shall have occurred) and as of the date of such notice, (iv) any other information relating to the person that would be required to be disclosed in a dissident’s proxy circular in connection with solicitations of proxies for the election of directors pursuant to the Act and Applicable Securities Laws, and (v) a duly completed personal information form in the form prescribed by the principal stock exchange on which the securities of the Corporation are listed for trading, if any; and
(b) as to the Nominating Shareholder giving the notice, any proxy, contract, arrangement, understanding or relationship pursuant to which such Nominating Shareholder has a right to vote any securities of the Corporation and any other information relating to such Nominating Shareholder that would be required to be made in a dissident’s proxy circular in connection with solicitations of proxies for election of directors pursuant to the Act and Applicable Securities Laws.
Such notice must be accompanied by the written consent of each nominee to being named as a nominee and to serve as a director, if elected. The Corporation may require any proposed nominee to furnish such other information as the Corporation may request to determine the eligibility of such proposed nominee to serve as an independent director of the Corporation or that could be material to a reasonable shareholder’s understanding of the independence, or lack thereof, of such proposed nominee.
No person shall be eligible for election as a director of the Corporation unless nominated in accordance with the provisions of this Advance Notice By-law; provided, however that nothing in this Advance Notice
By-law shall be deemed to preclude discussions by a shareholder (as distinct from the nomination of directors) at a meeting of shareholders of any matter in respect of which such shareholder would have been entitled to submit a proposal pursuant to the provisions of the Act. The chair of the meeting shall have the power and duty to determine whether a nomination was made in accordance with the procedures set out in the foregoing provisions and, if any proposed nomination is not in compliance with such foregoing provisions, to declare that such nomination is defective and cannot be accepted.
For purposes of this Advance Notice By-law:
“public announcement” shall mean disclosure in a news release disseminated through a national news service in Canada, or in a communication otherwise provided (through electronic means or otherwise) to all shareholders; and
“Applicable Securities Laws” means the applicable securities legislation of each relevant province and territory of Canada, as amended from time to time, the rules, regulations and forms made or promulgated under any such statute and the published national instruments, multilateral instruments, policies, bulletins and notices of the securities commissions and similar regulatory authorities of each relevant province and territory of Canada.
Notwithstanding anything to the contrary in the by-laws, notice given to the Secretary of the Corporation pursuant to this Advance Notice By-law may only be given by personal delivery, facsimile transmission or by email (at such email address as stipulated from time to time by the Secretary of the Corporation for the purposes of this notice), and shall be deemed to have been given and made only at the time it is served by personal delivery, email (at the address as aforesaid) or sent by facsimile transmission (provided that receipt of confirmation of such transmission has been received) to the Secretary of the Corporation at the address of the head office of the Corporation; provided that if such delivery or electronic communication is made on a day that is not a business day or later than 5:00 p.m. (local time at the head office of the Corporation) on a day that is a business day, then such delivery or electronic communication shall be deemed to have been made on the first subsequent day that is a business day.
Notwithstanding the foregoing, the board may, in its sole discretion, waive any requirement in this Advance Notice By-law.
EXHIBIT 2.1
DESCRIPTION OF SECURITIES
Except as otherwise stated, the information in this Description of Securities is provided as of the date of the Annual Report.
The Corporation's authorized share capital consists of an unlimited number of Class A Shares and an unlimited number of Class B Shares, each with no par value. As of March 28, 2024, there were 72 Class A Shares issued and outstanding and 40,425,569 Class B Shares issued and outstanding. Neither the Class A Shares nor the Class B Shares are bearer shares; instead, the Corporation maintains a register of the holders of the Class A Shares and the Class B Shares and engages a transfer agent and registrar to process transfers of shares and maintain the register. The Class B Shares are registered under Section 12(b) of the Exchange Act and trade on the Canadian Securities Exchange and Nasdaq Capital Market under the symbol “HUGE”. The Class B Shares are also listed and posted for trading on the Börse Frankfurt, or Frankfurt Stock Exchange, under “WKN: A2JM6M” and the trading symbol “0K9A”. Prior to the CSE listing, there was no public trading in any securities of the Corporation. As of March28, 2024, shareholders who hold Class A Shares together hold approximately 33.6% of the voting power of the Company’s outstanding voting and therefore have significant influence over management and affairs of the Company and over all matters requiring Shareholder approval.
The following is a summary of the rights, privileges, restrictions and conditions attached to the Class A Shares and Class B Shares.
Voting Rights
Except as otherwise prescribed by the Business Corporations Act, (Ontario) (the “OBCA”), at a meeting of the Shareholders, each Class B Share entitles the holder thereof to one vote, and each Class A Share entitles the holder thereof to 276,660 votes on all matters.
Rank
The Class A Shares and Class B Shares rank pari passu with respect to the payment of dividends, return of capital and distribution of assets in the event of the liquidation, dissolution or winding up of the Corporation. In the event of the liquidation, dissolution or winding-up of the Corporation or any other distribution of its assets among its shareholders for the purpose of winding-up its affairs, whether voluntarily or involuntarily, the holders of Class A Shares and the holders of Class B Shares are entitled to participate equally, share for share, subject always to the rights of the holders of any class of shares ranking senior to the Class A Shares and the Class B Shares, in the remaining property and assets of the Corporation available for distribution to Shareholders, without preference or distinction among or between the Class A Shares and the Class B Shares.
| 1 |
Dividends
Holders of Class A Shares and Class B Shares are entitled to receive, subject always to the rights of the holders of any class of shares ranking senior to the Class A Shares and Class B Shares, dividends out of the assets of the Corporation legally available for the payment of dividends at such times and in such amount and form as the Board may from time to time determine, and the Corporation will pay dividends thereon on a pari passu basis, if, as and when declared by the Board.
Conversion
The Class B Shares are not convertible into any other class of shares. Each outstanding Class A Share may, at any time at the option of the holder, be converted into one Class B Share. Upon the first date that any Class A Share is held other than by a permitted holder, the permitted holder which held such Class A Share until such date, without any further action, shall automatically be deemed to have exercised his, her or its rights to convert such Class A Share into a fully paid and non-assessable Class B Share.
Future transfers by holders of Class A Shares to arm's length parties or other than to permitted holders will generally result in those shares converting to Class B Shares, which will have the effect, over time, of increasing the relative voting power of those holders of Class A Shares who retain their shares. Such holders could, in the future, control a significant percentage of the combined voting power of Class A Shares and Class B Shares.
Subdivision or Consolidation
No subdivision or consolidation of the Class A Shares or the Class B Shares may be carried out unless, at the same time, the Class A Shares or the Class B Shares, as the case may be, are subdivided or consolidated in the same manner and on the same basis.
On October 16, 2019, the Corporation completed the Consolidation of all of its issued and outstanding Class A Shares and Class B Shares. Pursuant to the Consolidation, all of the issued and outstanding Class A Shares and Class B Shares were consolidated on the basis of one post-Consolidation share for every 201 pre-Consolidation shares of each class.
Change of Control Transactions
The holders of Class B Shares are entitled to participate on an equal basis with holders of Class A Shares in the event of a "“Change of Control Transaction"” requiring approval of the holders of Class A Shares and Class B Shares under the OBCA, unless different treatment of the shares of each such class is approved by a majority of the votes cast by the holders of outstanding Class A Shares and by a majority of the votes cast by the holders of outstanding Class B Shares, each voting separately as a class.
| 2 |
Proposals to Amend the Articles of Amendment
Neither the holders of the Class A Shares nor the holders of the Class B Shares are entitled to vote separately as a class upon a proposal to amend the Articles of Amendment in the case of an amendment referred to in paragraph (a) or (e) of subsection 170(1) of the OBCA.
Neither the holders of the Class A Shares nor the holders of the Class B Shares shall be entitled to vote separately as a class upon a proposal to amend the Articles of Amendment in the case of an amendment referred to in paragraph (b) of subsection 170(1) of the OBCA unless such exchange, reclassification or cancellation: (a) affects only the holders of that class; or (b) affects the holders of Class A Shares and Class B Shares differently, on a per share basis, and such holders are not otherwise entitled to vote separately as a class under any applicable law in respect of such exchange, reclassification or cancellation.
Take-Over Bid Protection
Under applicable Canadian law, an offer to purchase Class A Shares would not necessarily require that an offer be made to purchase Class B Shares. In accordance with the rules of the CSE designed to ensure that, in the event of a take-over bid, the holders of Class B Shares will be entitled to participate on an equal footing with holders of Class A Shares, the holders of not less than 80% of the outstanding Class A Shares have entered into the Coattail Agreement. The Coattail Agreement contains provisions customary for dual class, publicly-traded Ontario corporations designed to prevent transactions that otherwise would deprive the holders of Class B Shares of rights under the take-over bid provisions of applicable Canadian securities legislation to which they would have been entitled if the Class A Shares had been Class B Shares.
The undertakings in the Coattail Agreement do not apply to prevent a sale of Class A Shares by a holder of Class A Shares party to the Coattail Agreement if concurrently an offer is made to purchase Class B Shares that:
(a) offers a price per Class B Share at least as high as the highest price per share paid or required to be paid pursuant to the take-over bid for the Class A Shares;
(b) provides that the percentage of outstanding Class B Shares to be taken up (exclusive of shares owned immediately prior to the offer by the offeror or persons acting jointly or in concert with the offeror) is at least as high as the percentage of outstanding Class A Shares to be sold (exclusive of Class A Shares owned immediately prior to the offer by the offeror and persons acting jointly or in concert with the offeror);
| 3 |
(c) has no condition attached other than the right not to take up and pay for Class B Shares tendered if no shares are purchased pursuant to the offer for Class A Shares; and
(d) is in all other material respects identical to the offer for Class A Shares.
In addition, the Coattail Agreement does not prevent the sale of Class A Shares by a holder thereof to a permitted holder, provided such sale does not or would not constitute a take-over bid or, if so, is exempt or would be exempt from the formal bid requirements (as defined in applicable securities legislation). The conversion of Class A Shares into Class B Shares shall not, in or of itself, constitute a sale of Class A Shares for the purposes of the Coattail Agreement.
Under the Coattail Agreement, any sale of Class A Shares (including a transfer to a pledgee as security) by a holder of Class A Shares party to the Coattail Agreement is conditional upon the transferee or pledgee becoming a party to the Coattail Agreement, to the extent such transferred Class A Shares are not automatically converted into Class B Shares in accordance with the Articles of Amendment.
The Coattail Agreement contains provisions for authorizing action by the trustee to enforce the rights under the Coattail Agreement on behalf of the holders of the Class B Shares. The obligation of the trustee to take such action will be conditional on the Corporation or holders of the Class B Shares providing such funds and indemnity as the trustee may require. No holder of Class B Shares has the right, other than through the trustee, to institute any action or proceeding or to exercise any other remedy to enforce any rights arising under the Coattail Agreement unless the trustee fails to act on a request authorized by holders of not less than 10% of the outstanding Class B Shares and reasonable funds and indemnity have been provided to the trustee.
The Coattail Agreement may not be amended, and no provision thereof may be waived, unless, prior to giving effect to such amendment or waiver, the following have been obtained: (a) the consent of the CSE and any other applicable securities regulatory authority in Canada and (b) the approval of at least 662/3% of the votes cast by holders of Class B Shares represented at a meeting duly called for the purpose of considering such amendment or waiver, excluding votes attached to Class B Shares held directly or indirectly by holders of Class A Shares, their affiliates and related parties and any persons who have an agreement to purchase Class A Shares on terms which would constitute a sale for purposes of the Coattail Agreement other than as permitted thereby.
No provision of the Coattail Agreement limits the rights of any holders of Class B Shares under applicable law.
At the annual and special meeting of Shareholders of the Corporation held December 16, 2019, the Shareholders approved an amendment to the Articles to authorize certain transfers of Class A Shares. The Shareholders approved an amendment to permit the holders of Class A Shares to complete transfers of Class A Shares to a director, executive officer or founder of the Corporation, such that a founder who is no longer actively involved in the business and affairs of the Corporation could transfer that founder's Class A Shares to those individuals who remain active.
| 4 |
Competition Act
Limitations on the ability to acquire and hold our Class B Shares may be imposed by the Competition Act (Canada). This legislation establishes a pre-merger notification regime for certain types of merger transactions that exceed certain statutory shareholding and financial thresholds. Transactions that are subject to notification cannot be closed until the required materials are filed and the applicable statutory waiting period has expired or been waived by the Commissioner of Competition (the “Commissioner”). Further, the Competition Act (Canada) permits the Commissioner to review any acquisition of control over or of a significant interest in us, whether or not it is subject to mandatory notification. This legislation grants the Commissioner jurisdiction, for up to one year following completion of an acquisition, to challenge this type of acquisition before the Canadian Competition Tribunal if the Commissioner believes it would, or would be likely to, prevent or lessen competition substantially in any market in Canada.
Investment Canada Act
The following discussion summarizes the principal features of the Investment Canada Act (Canada) for a non-resident who proposes to acquire Class B Shares. The discussion is general only; it is not a substitute for independent legal advice from an investor's own advisor; and it does not anticipate statutory or regulatory amendments.
Except as provided in the Investment Canada Act (Canada), there are no limitations specific to the rights of non-Canadians to hold or vote the Common Shares under the laws of Canada or the Province of British Columbia or in the Company’s constating documents.
There are no limitations under the laws of Canada or in the organizing documents of the Company on the right of foreigners to hold or vote securities of the Company, except that the Investment Canada Act (Canada)may require that a “non-Canadian” not acquire “control”of the Company without prior review and approval by the Minister of Innovation, Science and Economic Development, where applicable thresholds are exceeded. The acquisition of one-third or more of the voting shares of the Company would give rise a rebuttable presumption of an acquisition of control, and the acquisition of more than fifty percent of the voting shares of the Company would be deemed to be an acquisition of control. In addition, the Investment Canada Act provides the Canadian government with broad discretionary powers in relation to national security to review and potentially prohibit, condition or require the divestiture of, any investment in the Company by a non-Canadian, including non-control level investments. “Non-Canadian”generally means an individual who is neither a Canadian citizen nor a permanent resident of Canada within the meaning of the Immigration and Refugee Protection Act (Canada) who has been ordinarily resident in Canada for not more than one year after the time at which he or she first became eligible to apply for Canadian citizenship, or a corporation, partnership, trust or joint venture that is ultimately controlled by non-Canadians.
| 5 |
In 2009, amendments were enacted to the Investment Canada Act (Canada) concerning investments that may be considered injurious to national security. If the Minister of Innovation, Science and Industry has reasonable grounds to believe that an investment by a non-Canadian “could be injurious to national security,” the Minister of Innovation, Science and Industry may send the non-Canadian a notice indicating that an order for review of the investment may be made. The review of an investment on the grounds of national security may occur whether or not an investment is otherwise subject to review on the basis of net benefit to Canada or otherwise subject to notification under the Investment Canada Act (Canada). The Minister of Innovation, Science and Industry has published guidelines that provide an open-ended list of factors that may be considered in determining whether an investment may be “injurious to national security”. These include the potential effects of the investment on the transfer of sensitive technology (including biotechnology) that may have military, intelligence, or dual military/civilian applications.
Certain transactions, except those to which the national security provisions of the Investment Canada Act (Canada) may apply, relating to Class B Shares are exempt from the Investment Canada Act (Canada), including:
(a) acquisition of Class B Shares by a person in the ordinary course of that person's business as a trader or dealer in securities,
(b) acquisition of control of the Corporation in connection with the realization of security granted for a loan or other financial assistance and not for a purpose related to the provisions on the Investment Canada Act (Canada), and
(c) acquisition of control of the Corporation by reason of an amalgamation, merger, consolidation or corporate reorganization following which the ultimate direct or indirect control in fact of the Corporation, through the ownership of Class B Shares, remained unchanged.
See “Item 10.E.-Taxation” for additional information regarding the material U.S. and Canadian federal income tax consequences relating to the ownership and disposition of our Class B Shares by Non-Canadian Holders (as defined therein).
Any of these provisions may discourage a potential acquirer from proposing or completing a transaction that may have otherwise presented a premium to our Shareholders. We cannot predict whether investors will find the Corporation and our Class B Shares less attractive because we are governed by Canadian laws.
| 6 |
EXHIBIT 4.2
ARRANGEMENTAGREEMENT
THIS ARRANGEMENT AGREEMENT (“Agreement”)
is made as of the 4th day of October, 2023.
BETWEEN:
FSD PHARMA INC., a corporation incorporated pursuant to the laws of the Province of Ontario, Canada.
(“FSD Pharma” or “Corporation”)
- and -
CELLY NUTRITION CORP., a corporation incorporated pursuant to the laws of the Province of British Columbia, Canada.
(“Celly Nu”)
WHEREAS, pursuant to this Agreement, FSD Pharma and Celly Nu have agreed to proceed with a reorganization transaction by way of statutory plan of Arrangement under the provisions of the OBCA, whereby, among other things, FSD Pharma will undertake a reorganization transaction on the terms and conditions set out in this Agreement and the Plan of Arrangement annexed hereto as Schedule “A”;
AND WHEREAS the board of directors of FSD Pharma has determined that the consideration to be received by the holders of Class A Shares, Class B Shares, and FSD Pharma Distribution Warrants is fair to such FSD Pharma Securityholders and that the Arrangement is in the best interests of the Corporation;
NOW THEREFORE THIS AGREEMENT WITNESSES that, in consideration of the premises and the respective covenants and agreements herein contained, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged by each of the parties hereto, the parties hereto do hereby covenant and agree as follows:
ARTICLE 1
INTERPRETATION
Section 1.1 Definitions
In this Agreement, including the recitals hereto, unless there is something in the subject matter or context inconsistent therewith, the following capitalized words and terms shall have the following meanings:
“1940 Act” means the United States Investment Company Act of 1940, as amended, and the rules and regulations promulgated from time to time thereunder.
“Affiliate” means an affiliate as defined in the Securities Act.
“Agreement” means this arrangement agreement, including the Schedules attached hereto, as may be supplemented or amended from time to time.
| 1 |
“Arrangement” means the arrangement under Section 182 of the OBCA on the terms and subject to the conditions set out in the Plan of Arrangement, subject to any amendments or variations thereto made in accordance with this Agreement or the Plan of Arrangement or made at the direction of the Court in the Final Order with the consent of FSD Pharma.
“Arrangement Resolution” means the special resolution of the FSD Pharma Securityholders in respect of the Arrangement to be considered at the Meeting, substantially in the form of Schedule “A” hereto.
“Articles of Arrangement” means the articles of arrangement of the Corporation in respect of the Arrangement, to be filed with the OBCA Director pursuant to Section 183(1) of the OBCA after the Final Order is made, which shall include the Plan of Arrangement.
“Authority” means any: (i) multinational, federal, provincial, state, municipal, local or foreign governmental or public department, court, or commission, domestic or foreign; (ii) subdivision or authority of any of the foregoing; or (iii) quasi-governmental or self-regulatory organization exercising any regulatory, expropriation or taxing authority under or for the account of its members or any of the above.
“Board of Directors” means the duly appointed board of directors of FSD Pharma or Celly Nu, as applicable.
“Business Day” means a day, other than a Saturday, Sunday or statutory holiday, when banks are generally open in the City of Toronto, Ontario for the transaction of banking business.
“Circular” means the management information circular of FSD Pharma to be prepared and sent to the FSD Pharma Securityholders, FSD Pharma Non-Distribution Warrantholders and FSD Pharma Optionholders in connection with the Meeting, containing among other things, disclosure in respect of the Arrangement and prospectus level disclosure in respect of Celly Nu following completion of the Arrangement, together with all appendices, distributed by FSD Pharma to the FSD Pharma Securityholders in connection with the Meeting and filed with such Authorities in Canada as are required by Section 2.6 of this Agreement, or otherwise as required by applicable Law.
“Class A Shares” means the class A multiple voting shares of FSD Pharma.
“Class B Shares” means the class B subordinate voting shares of FSD Pharma.
“Court” means the Ontario Superior Court of Justice (Commercial List).
“Celly Nu Shares” means the common shares in the capital of Celly Nu.
“Celly Nu Restricted Stock Units” means the restricted stock units that are issued and outstanding in the capital of Celly Nu.
“Dissent Right” has the meaning attributed to that term in Section 3.1 in the Plan of Arrangement.
“Effective Date” means the date shown on the Certificate of Arrangement issued by the Director.
“Effective Time” means 12:01 a.m. (Toronto) on the Effective Date.
| 2 |
“Encumbrance” means any encumbrance, lien, charge, hypothec, pledge, mortgage, title retention agreement, security interest of any nature, prior claim, adverse claim, exception, reservation, restrictive covenant, agreement, easement, lease, license, right of occupation, option, right of use, right of first refusal, right of pre-emption, privilege or any matter capable of registration against title or any contract to create any of the foregoing.
“Fairness Opinion” means the opinion of Intellectual Capital Corporation delivered to the Board of Directors of FSD Pharma in connection with the Plan of Arrangement.
“Final Order” means the final order of the Court pursuant to Section 182(5) of the OBCA, after being informed of the intention to rely upon the exemption from registration under the U.S. Securities Act provided by Section 3(a)(10) thereunder in connection with the issuance of securities of FSD Pharma and Celly Nu to FSD Pharma Securityholders in the United States, approving the Plan of Arrangement as such order may be amended by the Court (with the consent of the Parties, acting reasonably) at any time prior to the Effective Date or, if appealed, then, unless such appeal is withdrawn or denied, as affirmed or as amended (with the consent of the Parties, acting reasonably) on appeal.
“FSD Pharma Optionholders” means a holder of FSD Pharma Options.
“FSD Pharma Options” means outstanding options to acquire FSD Pharma Shares, each of which is exercisable for the purchase of one Class B Share.
“FSD Pharma Securities” means, collectively, the Class A Shares, Class B Shares and FSD Pharma Distribution Warrants.
“FSD Pharma Securityholders” means collectively FSD Pharma Shareholders and FSD Pharma Distribution Warrantholders.
“FSD Pharma Shareholders” means collectively the holders of Class A Shares and Class B Shares, at the applicable time.
“FSD Pharma Shares” means collectively Class A Shares and Class B Shares.
“FSD Pharma Distribution Warrantholders” means the holders of FSD Pharma Distribution Warrants.
“FSD Pharma Distribution Warrants” means outstanding warrants of FSD Pharma, each of which is exercisable for the purchase of one Class B Share, and which includes a provision in its applicable warrant certificate that is substantially in the form attached as Schedule “B” hereto or with the same substantive effective.
“FSD Pharma Non-Distribution Warrantholders” means the holders of FSD Pharma Non-Distribution Warrants.
“FSD Pharma Non-Distribution Warrants” means outstanding warrants of FSD Pharma, each of which is exercisable for the purchase of one Class B Share, and which does not include a provision in its applicable warrant certificate that is substantially in the form attached as Schedule “B” hereto or with the same substantive effect.
“Interim Order” means the order made after application to the Court pursuant to Section 182 of the OBCA, providing for, among other things, the calling and holding of the Meeting, as such order may be amended, supplemented or varied by the Court (with the consent of the Parties, acting reasonably).
| 3 |
“Laws” means all laws, by-laws, statutes, rules, regulations, principles of law, orders, ordinances, protocols, codes, guidelines, policies, notices, directions and judgments or other requirements and the terms and conditions of any grant of approval, permission, authority or license of any Authority, to the extent each of the foregoing have the force of law, and the term “applicable” with respect to such laws and in a context that refers to one or more Parties, means such laws as are applicable to such Party or its business, undertaking, property or securities and emanate from a Person having jurisdiction over the Party or Parties or its or their business, undertaking, property or securities.
“Meeting” means the special meeting of FSD Pharma Securityholders and any adjournment(s) or postponement(s) thereof, to be called and held in accordance with the Interim Order to consider and to vote on Arrangement Resolution and any other matters set out in the Notice of Meeting.
“OBCA” means the Business Corporations Act (Ontario) and the regulations made thereunder, as promulgated or amended from time to time.
“OBCA Director” means the Director appointed pursuant to Section 278 of the OBCA.
“NI 45-106” means National Instrument 45-106 – Prospectus Exemptions as amended from time to time.
“Notice of Meeting” means the notice of the Meeting to be sent to the FSD Pharma Securityholders, FSD Pharma Optionholders and FSD Pharma Non-Distribution Warrants, which notice will accompany the Circular.
“Outside Date” means December 31, 2023 or such other later date as may be agreed to in writing by the Parties.
“Parties” means, collectively, FSD Pharma and Celly Nu, and “Party” means any one of them.
“Person” or “person” means and includes an individual, sole proprietorship, partnership, unincorporated association, unincorporated syndicate, unincorporated organization, trust, body corporate, trustee, executor, administrator or other legal representative and the Crown or any agency or instrumentality thereof.
“Plan of Arrangement” means the plan of arrangement in substantially the form of the plan of arrangement which is attached as Schedule “A” hereto and any amendments or variations thereto made in accordance with this Agreement, the Plan of Arrangement or upon the direction of the Court in the Final Order with the consent of FSD Pharma.
“Representative” means any director, officer, employee, agent, advisor or consultant of any Party.
“Section 3(a)(10) Exemption” means the exemption from the registration requirements of the U.S. Securities Act provided by Section 3(a)(10) thereof.
“Securities Act” means the Securities Act (Ontario).
“Securities Legislation” means the Securities Act and the equivalent law in the other applicable provinces and territories of Canada, and the published policies, instruments, rules, judgments, orders and decisions of any Authority administering those statutes.
“SEDAR+” means System for Electronic Document and Retrieval.
| 4 |
“Tax Act” means the Income Tax Act (Canada).
“U.S. Exchange Act” means the United States Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated from time to time thereunder.
“U.S. Securities Act” means the United States Securities Act of 1933, as amended, and the rules and regulations promulgated from time to time thereunder.
Section 1.2 Interpretation Not Affected by Headings
The division of this Agreement into articles, sections, paragraphs and other portions and the insertion of headings are for convenience of reference only and shall not affect the construction or interpretation of this Agreement. The terms “this Agreement”, “hereof”, “hereunder” and similar expressions refer to this Agreement (including the Schedules and appendices hereto) as a whole and not to any particular article, section, paragraph or other portion hereof and include any agreement, document or instrument supplementary or ancillary hereto. Unless something in the subject matter or context is inconsistent therewith, all references herein to articles, sections, paragraphs and other portions are to articles, sections, paragraphs and other portions of this Agreement.
Section 1.3 Construction
In this Agreement, unless something in the context is inconsistent therewith:
|
| (a) | the words “include” or “including” when following any general term or statement are not to be construed as limiting the general term or statement to the specific items or matters set forth or to similar items or matters, but rather as permitting it to refer to all other items or matters that could reasonably fall within its broadest possible scope; |
|
|
|
|
|
| (b) | a reference to time or date is to the time or date in Toronto, Ontario, unless specifically indicated otherwise; |
|
|
|
|
|
| (c) | a word importing the masculine gender includes the feminine gender or neuter and a word importing the singular includes the plural and vice versa; |
|
|
|
|
|
| (d) | a reference to “approval”, “authorization”, “consent”, “designation” or “notice” means written approval, authorization, consent, designation or notice unless specifically indicated otherwise; |
|
|
|
|
|
| (e) | unless otherwise stated, all accounting terms used in this Agreement shall have the meanings attributable thereto under International Financial Reporting Standards and all determinations of an accounting nature shall be made in a manner consistent with International Financial Reporting Standards; and |
|
|
|
|
|
| (f) | a reference to a statute or code includes every rule and regulation made pursuant thereto, all amendments to the statute or code or to any such regulation in force from time to time, and any statute, code or regulation which supplements or supersedes such statute, code, rule or regulation. |
| 5 |
Section 1.4 Date for Any Action
In the event that any date on which any action is required to be taken hereunder by either of the parties hereto is not a Business Day in the place where the action is required to be taken, such action shall be required to be taken on the next succeeding day which is a Business Day at such place, unless otherwise agreed to by the parties hereto.
Section 1.5 Currency
All amounts of money which are referred to in this Agreement are expressed in lawful money of Canada unless otherwise specified.
Section 1.6 Schedules
The following Schedules are annexed to this Agreement and are incorporated by reference to this Agreement and form a part hereof:
Schedule “A” – Plan of Arrangement
Schedule “B” – Arrangement Resolution
Section 1.7 Entire Agreement
This Agreement, together with the Schedules, agreements and other documents herein or therein referred to, constitute the entire agreement between the parties hereto pertaining to the subject manner hereof and supersedes all prior agreements, understandings, negotiations and discussions, whether oral or written, between the parties hereto with respect to the subject matter hereof.
ARTICLE 2
THE ARRANGEMENT
Section 2.1 Arrangement
FSD Pharma and Celly Nu agree to effect the Arrangement on the terms and subject to the conditions contained in this Agreement and on the terms set forth in the Plan of Arrangement.
Section 2.2 Commitment to Effect Arrangement
Subject to the satisfaction of the terms and conditions contained in this Agreement, FSD Pharma and Celly Nu shall each use all reasonable efforts and do all things reasonably required to cause the Arrangement to become effective as soon as reasonably practicable and to cause the transactions contemplated by the Plan of Arrangement and this Agreement to be completed in accordance with their terms.
Section 2.3 Effective Date and Effective Time
The Arrangement shall become effective on the Effective Date and the steps to be carried out pursuant to the Plan of Arrangement will become effective commencing at the Effective Time immediately after one another in the sequence set out therein or as otherwise specified in the Plan of Arrangement.
| 6 |
Section 2.4 Implementation Steps
|
| (a) | FSD Pharma covenants and agrees that, subject to the terms of this Agreement, it will promptly: |
|
| (i) | apply to the Court pursuant to section 182 of the OBCA and prepare, file, and diligently pursue an application for an Interim Order; |
|
|
|
|
|
| (ii) | proceed with such application and diligently pursue obtaining the Interim Order, including submission to the Court of the materials that would be submitted to FSD Pharma Securityholders, FSD Pharma Optionholders and FSD Pharma Non-Distribution Warrants, including without limitation the Circular, in connection with the Meeting; |
|
|
|
|
|
| (iii) | lawfully convene and hold the Meeting in accordance with the Interim Order, FSD Pharma's articles and applicable Laws, as soon as reasonably practicable after the Interim Order is issued, for the purpose of, among other things, having the FSD Pharma Securityholders consider the Arrangement Resolution; |
|
|
|
|
|
| (iv) | take all other actions that are reasonably necessary or desirable to obtain the approval of the Arrangement; |
|
|
|
|
|
| (v) | subject to obtaining such approvals as are required by the Interim Order, as soon as reasonably practicable after the Meeting, make an application to the Court for the Final Order; |
|
|
|
|
|
| (vi) | proceed with such application and diligently pursue obtaining the Final Order; and |
|
|
|
|
|
| (vii) | subject to: (i) obtaining the Final Order; and (ii) the satisfaction or waiver (subject to applicable Laws) of each of the conditions set forth in Article 5 (excluding conditions that by their terms cannot be satisfied until the Effective Date, but subject to the satisfaction, or when permitted, waiver of those conditions as of the Effective Date), as soon as reasonably practicable thereafter, take all steps necessary or desirable to give effect to the Arrangement. |
|
| (b) | Celly Nu covenants and agrees that, subject to the terms of this Agreement, it shall promptly: |
|
| (i) | cooperate and assist FSD Pharma in seeking the Interim Order and the Final Order; and |
|
|
|
|
|
| (ii) | subject to: (i) obtaining the Final Order; and (ii) the satisfaction or waiver (subject to applicable Laws) of each of the conditions set forth in Article 5 (excluding conditions that by their terms cannot be satisfied until the Effective Date, but subject to the satisfaction, or when permitted, waiver of those conditions as of the Effective Date), as soon as reasonably practicable thereafter, take all steps and actions necessary or desirable to give effect to the Arrangement. |
| 7 |
Section 2.5 Interim Order
The application referred to in Section 2.4(a)(i) shall, unless FSD Pharma and Celly Nu agree otherwise, include a request that the Interim Order provide, among other things:
|
| (a) | that the securities of FSD Pharma for which holders shall be entitled to receive notice of and vote on the Arrangement Resolution at the Meeting shall be the holders of Class B Shares, Class A Shares and FSD Pharma Distribution Warrants; |
|
|
|
|
|
| (b) | for a record date, for the purposes of determining the FSD Pharma Securityholders entitled to receive notice of and vote at the Meeting; |
|
|
|
|
|
| (c) | that the Meeting may be adjourned or postponed from time to time by FSD Pharma without the need for additional approval by the Court; |
|
|
|
|
|
| (d) | that, except as required by Law or subsequently ordered by the Court, the record date, for the FSD Pharma Securityholders entitled to receive notice of and to vote at the Meeting will not change in respect of or as a consequence of any adjournment or postponement of the Meeting; |
|
|
|
|
|
| (e) | the FSD Pharma Securityholders shall be entitled to vote on the Arrangement Resolution, with each FSD Pharma Securityholder being entitled to one vote for each Class B Share held by such holder, 276,660 votes for each Class A Share held by such holder, and one vote for each FSD Pharma Distribution Warrant held by such holder, and provided that the holders of Class B Shares and FSD Pharma Distribution Warrants will vote together as a class, and the holders of Class A Shares will vote separately as a class, in each case such vote to be conducted by ballot; |
|
|
|
|
|
| (f) | the requisite majority for the approval of the Arrangement Resolution shall be two- thirds of the votes cast by the holders of (i) Class B Shares and FSD Pharma Distribution Warrants, voting together as a class, and (ii) Class Shares, voting separately as a class, and in each case present in person or by proxy at the Meeting; |
|
|
|
|
|
| (g) | that in all other respects, the terms, conditions and restrictions of FSD Pharma's constating documents, including quorum requirements with respect to meeting of FSD Pharma Securityholders and other matters, shall apply with respect to the Meeting; |
|
|
|
|
|
| (h) | for the grant of the Dissent Rights to the FSD Pharma Shareholders who are registered holders of Class A Shares or Class B Shares, as set forth in the Plan of Arrangement; |
|
|
|
|
|
| (i) | for the notice requirements with respect to the presentation of the application to the Court for the Final Order; and |
|
|
|
|
|
| (j) | for such other matters as FSD Pharma may reasonably require, subject to obtaining the prior consent of Celly Nu, such consent not to be unreasonably conditioned, withheld or delayed, and subject to the approval of the Court. |
| 8 |
Section 2.6 Information Circular and Meeting
Subject to Article 6, as promptly as practical following the execution of this Agreement and in compliance with the Interim Order and applicable Laws,
|
| (a) | FSD Pharma shall: |
|
| (i) | prepare the Circular together with any other documents required by the OBCA or any other applicable Laws in connection with the approval of, among other things, the Arrangement Resolution by the FSD Pharma Securityholders at the Meeting; |
|
|
(ii)
|
subject to the Interim Order, cause the notice of the Meeting and the Circular to be: (A) sent to the FSD Pharma Securityholders, FSD Pharma Optionholders and FSD Pharma Non-Distribution Warrants in compliance with the OBCA, FSD Pharma's articles and the timing requirements (as may be abridged by FSD Pharma) contemplated by National Instrument 54-101 – Communication with Beneficial Owners of Securities of a Reporting Issuer; and (B) filed with one or more Authorities as required by the Interim Order and applicable Laws, including on SEDAR+ for the benefit of the public and the Canadian securities regulatory authorities, pursuant to and in accordance with the Interim Order and applicable Securities Legislation;
|
|
| (iii)
| ensure that the Circular complies in all material respects of the Law, does not contain a misrepresentation other than with respect to any written information that is furnished by or on behalf of Celly Nu for inclusion in the Circular and provides the FSD Pharma Securityholders with sufficient information to permit them to form a reasoned judgment concerning the matters to be placed before the Meeting. Without limiting the generality of the foregoing, but subject to the terms of this Agreement, the Circular shall include: (A) a summary and a copy of the Fairness Opinion; (B) a statement that the Board has received the Fairness Opinion and has, after receiving advice from its financial advisor and outside legal counsel: |
|
| i. | determined that the Consideration to be received by the FSD Pharma Securityholders pursuant to the Arrangement is fair to the FSD Pharma Securityholders and the Arrangement is in the best interests of the Corporation; |
|
| ii. | recommends that the FSD Pharma Securityholders vote in favour of the Arrangement Resolution (the “Board Recommendation”); and |
|
| (b) | Celly Nu shall cooperate in the preparation, filing and mailing of the Circular and shall provide the Corporation with all necessary information concerning its business that is required by Law to be included in the Circular or other related documents and ensure that such information does not contain a misrepresentation concerning Celly Nu or the Celly Nu Shares. Celly Nu shall use commercially reasonable efforts to obtain any necessary consents from any of its auditors and any other advisors to the use of any financial, technical, or other expert information required to be included in the Circular and to the identification in the Circular of each such advisor. |
|
|
(c) |
FSD Pharma and Celly Nu shall cooperate with each other in the preparation, filing and dissemination of any: (i) required supplement or amendment to the Circular or such other document, as the case may be; and (ii) related news release or other document necessary or desirable in connection therewith. |
| 9 |
Section 2.7 Effecting the Arrangement and Ancillary Filings with the OBCA Director
Subject to the rights of termination contained in Section 6.2 , upon the FSD Pharma Securityholders approving the Arrangement as set out in the Interim Order, the Corporation obtaining the Final Order and the satisfaction (or waiver, if applicable) of the other conditions herein contained in favour of each of the Parties, the Parties covenant and agree to, on a date and at a time to be determined exclusively by the Corporation, file with the OBCA Director any and all documents (including, with respect to the filing to be made pursuant to subsection 183(1) of the OBCA, the Articles of Arrangement) and to exchange (to the extent not previously exchanged) such other documents as may be necessary or desirable to give effect to the Arrangement and implement the Plan of Arrangement on such date. The closing of the Arrangement will take place at the offices of Garfinkle Biderman LLP, Suite 801, 1 Adelaide Street East, Toronto, Ontario M5C 2V9 at the Effective Time, or at such other time and place as may be agreed to by the Parties.
Section 2.8 Income Tax Matters
|
| (a) | FSD Pharma and Celly Nu, as the case may be, will be entitled to deduct and withhold from any consideration otherwise payable to any FSD Pharma Securityholders under the Plan of Arrangement (including any payment to FSD Pharma Shareholders exercising Dissent Rights) such amounts as FSD Pharma or Celly Nu are permitted or required to deduct and withhold with respect to such payment under the Tax Act and the rules and regulations promulgated thereunder, or any provision of any provincial, state, local or foreign tax Law as counsel may advise is permitted or required to be so deducted and withheld by FSD Pharma or Celly Nu, as the case may be. FSD Pharma or Celly Nu, or the duly appointed agent with respect to that matter, shall be entitled to dispose of such number of Celly Nu Shares as is necessary to satisfy the withholdings contemplated herein (if any). |
|
|
|
|
|
| (b) | For the purposes of such deduction and withholding: (i) all withheld amounts shall be treated as having been paid to the person in respect of which such deduction and withholding was made on account of the obligation to make payment to such person hereunder; and (ii) such deducted or withheld amounts shall be remitted to the appropriate Authority in the time and manner permitted or required by the applicable Law by or on behalf of FSD Pharma or Celly Nu, as the case may be. |
|
|
|
|
Section 2.9 U.S. Securities Law Matters
The Parties agree that the Arrangement will be carried out with the intention that all securities of FSD Pharma and Celly Nu to be issued pursuant to the Arrangement will be issued and exchanged in accordance with the Plan of Arrangement in reliance on the exemption from the registration requirements of the U.S. Securities Act provided by Section 3(a)(10) thereof. In order to ensure the availability of the Section 3(a)(10) Exemption, the Parties agree that the Arrangement will be carried out on the following basis:
|
| (a) | the Arrangement will be subject to the approval of the Court; |
|
|
(b) |
the Court will be invited to satisfy itself and find, prior to approving the Arrangement, that the Arrangement is fair and reasonable, both procedurally and substantively, to the security holders of FSD Pharma including being provided sufficient information before it to determine the value of Arrangement Consideration Shares (as such term is defined in the Plan of Arrangement); |
| 10 |
|
| (c) | the Court will be provided a copy of the draft materials in substantially the form that would be submitted to FSD Pharma Securityholders, FSD Pharma Optionholders and FSD Pharma Non-Distribution Warrantholders in connection with the Meeting; |
|
|
(d) |
the Parties will ensure that each securityholder of FSD Pharma entitled to receive securities pursuant to the Arrangement will be given adequate notice advising such securityholder of FSD Pharma of his, her or its right to attend the hearing of the Court and provide each with sufficient information necessary for him or her to exercise that right, which notice shall be communicated to the FSD Pharma Securityholders by the issuance of a news release that shall include all appropriate details and posted on SEDAR+; |
|
|
(e) |
FSD Pharma Securityholders will be advised that the securities issued and being distributed to them in the Plan of Arrangement have not been registered under the U.S. Securities Act and will be so issued and distributed in reliance on the exemption from the registration requirements, provided by Section 3(a)(10) of the U.S. Securities Act and may be subject to restrictions on resale under the securities laws of the United States; |
|
|
(f) |
the Interim Order will specify that each shareholder of FSD Pharma will have the right to appear before the Court so long as they enter an appearance within a reasonable time; |
|
|
(g) |
the Final Order shall include statements substantially to the following effect: |
|
|
| “The terms and conditions of the Plan of Arrangement are procedurally and substantively fair to the securityholders of FSD Pharma Inc. and are hereby approved by the Court. This Order will serve as a basis of a claim to an exemption, pursuant to Section 3(a)(10) of the United States Securities Act of 1933, as amended, from the registration requirements otherwise imposed by that act, regarding the issuance of securities pursuant to the Plan of Arrangement” |
ARTICLE 3
REPRESENTATIONS AND WARRANTIES
Section 3.1 Representations and Warranties of FSD Pharma
FSD Pharma hereby represents and warrants to Celly Nu as follows:
|
| (a) | it is a “foreign private issuer” as defined in Rule 405 under the U.S. Securities Act and is not required to register as an investment company under the 1940 Act; |
|
|
|
|
|
| (b) | no class of securities of the corporation is registered or required to be registered under Section 12 of the U.S. Exchange Act, nor does the Corporation have a reporting obligation under Section 15(d) of the U.S. Exchange Act; |
|
|
|
|
|
| (c) | it is a corporation incorporated and subsisting under the laws of the province of Ontario and has full capacity and authority to enter into this Agreement and, subject to obtaining the requisite approvals and consents contemplated hereby, to perform its obligations hereunder; |
|
|
|
|
|
| (d) | neither the execution and delivery of this Agreement nor the performance of any of its covenants and obligations hereunder will constitute a material default under, or be in any material contravention or breach of: |
|
| (i) | any provision of its articles and by-laws; |
|
|
|
|
|
| (ii) | any judgment, decree, order, law, statute, rule, or regulation applicable to it; or |
|
|
|
|
|
| (iii) | any agreement or instrument to which it is a party or by which it is bound. |
| 11 |
|
| (e) | no dissolution, winding up, bankruptcy, liquidation or similar proceeding has been commenced or is pending or proposed in respect of it; |
|
|
|
|
|
| (f) | the execution and delivery of this Agreement and the completion of the transaction contemplated herein have been duly approved by its board of directors, and this Agreement constitutes a valid and binding obligation of such Party enforceable against it in accordance with its terms, subject to bankruptcy, insolvency and other laws affecting the enforcement of creditors’ rights generally and to general principles of equity and limitations upon the enforcement of indemnification for fines or penalties imposed by law; |
|
|
|
|
|
| (g) | subject to receipt of the FSD Pharma Securityholders' approval of the Arrangement and receipt of the Final Order, it has taken all corporate actions necessary to authorize the execution and delivery of this Agreement and this Agreement has been duly executed and delivered by it; and |
|
|
|
|
|
| (h) | FSD Pharma owns 200,000,000 Celly Nu Shares beneficially and of record, free and clear of all Encumbrances. |
|
|
|
|
Section 3.2 Representations and Warranties of Celly Nu
Celly Nu hereby represents and warrants to FSD Pharma as follows:
|
| (a) | it is a “foreign private issuer” as defined in Rule 405 under the U.S. Securities Act and is not required to register as an investment company under the 1940 Act; |
|
|
|
|
|
| (b) | no class of securities of the corporation is registered or required to be registered under Section 12 of the U.S. Exchange Act, nor does the corporation have a reporting obligation under Section 15(d) of the U.S. Exchange Act; |
|
|
|
|
|
| (c) | it is a corporation incorporated and subsisting under the laws of the Province of British Columbia and has full capacity and authority to enter into this Agreement and, subject to obtaining the requisite approvals and consents contemplated hereby, to perform its obligations hereunder; |
|
|
|
|
|
| (d) | it has taken all corporate action necessary to authorize the execution and delivery, and the performance of the provisions, of this Agreement and this Agreement has been duly authorized by it; |
|
|
|
|
|
| (e) | neither the execution and delivery of this Agreement nor the performance of any of its covenants and obligations hereunder will constitute a material default under, or be in any material contravention or breach of: |
|
| (i) | any provision of its articles and notice of articles; |
|
|
|
|
|
| (ii) | any judgment, decree, order, law, statute, rule or regulation applicable to it; or |
|
|
|
|
|
| (iii) | any agreement or instrument to which it is a party or by which it is bound; |
| 12 |
|
| (f) | no dissolution, winding-up, bankruptcy, liquidation or similar proceedings have been commenced or are pending or proposed in respect of it; |
|
|
|
|
|
| (g) | the execution and delivery of this Agreement and the completion of the transaction contemplated herein have been duly approved by its board of directors, and this Agreement constitutes a valid and binding obligation of such Party enforceable against it in accordance with its terms, subject to bankruptcy, insolvency and other laws affecting the enforcement of creditors’ rights generally and to general principles of equity and limitations upon the enforcement of indemnification for fines or penalties imposed by law; |
|
|
|
|
|
| (h) | Celly Nu's current issued and outstanding capital is comprised of the following: |
|
| i. | 577,000,000 Celly Nu Shares; and |
|
|
|
|
|
| ii. | 177,230,766 Celly Nu Restricted Stock Units. |
|
| (i) | other than set out in the section above there are no other securities exercisable or convertible into Celly Nu Shares; and |
|
|
|
|
|
| (j) | there are no amounts due from FSD Pharma to Celly Nu. |
Section 3.3 Survival of Representations and Warranties
The representations and warranties of each of the Parties contained herein will not survive the completion of this Arrangement and will expire and be terminated on the earlier of: (i) the termination of this Agreement in accordance with its terms; and (ii) the Effective Time.
ARTICLE 4
COVENANTS
Section 4.1 General Covenants
Each of FSD Pharma and Celly Nu will:
|
| (a) | use all commercially reasonable efforts and do all things reasonably required of it to cause the Arrangement to become effective as soon as reasonably practicable or on such date as FSD Pharma may determine; |
|
|
|
|
|
| (b) | do and perform all such acts and things, and execute and deliver all such agreements, assurances, notices and other documents and instruments as may reasonably be required to facilitate the carrying out of the intent and purpose of this Agreement including, without limitation, complying with the requirements for obtaining an exemption from the registration requirements of the U.S. Securities Act provided by Section 3(a)(10) thereunder; |
|
|
|
|
|
| (c) | use their best efforts to obtain all necessary consents, assignments, waivers and amendments to, or terminations of, any instruments and take such measures as may be appropriate to fulfill its obligations hereunder and to carry out the transactions contemplated hereby; and |
|
|
|
|
|
| (d) | cooperate with and assist each other in dealing with transitional matters relating to or arising from the Arrangement or this Agreement. |
|
|
|
|
Section 4.2 Covenants of FSD Pharma
Subject to Article 6, FSD Pharma hereby covenants and agrees with Celly Nu as follows:
|
| (a) | until the earlier of: (i) the Effective Date; and (ii) the termination of this Agreement, not perform any act or enter into any transaction which interferes or is inconsistent with the completion of the Plan of Arrangement; |
| 13 |
|
| (b) | it will make an application to the Court for the Interim Order and provide draft materials that would be submitted to the FSD Pharma Securityholders, FSD Pharma Optionholders and FSD Pharma Non-Distribution Warrantholders in connection with the Meeting, including without limitation: (i) the Circular; (ii) sufficient information before it to determine the value of Arrangement Consideration Shares (as such term is defined in the Plan of Arrangement), and (iii) any other materials required by the Court; |
|
|
(c) |
it shall in a timely and expeditious manner: (i) carry out the terms of the Interim Order; (ii) ensure that the Circular complies with National Instrument 51-102 – Continuous Disclosure Obligations and Form 51-102F5 thereunder and Multilateral Instrument 61-101 – Protection of Minority Security Holders in Special Transactions and provide FSD Pharma Securityholders with sufficient detail to permit them to form a reasoned judgment concerning the matters to be placed before them at the Meeting; (iii) file the Circular in all jurisdictions where the same is required to be filed and mail the same as ordered by the Interim Order and in accordance with all applicable laws, and solicit proxies to be voted at the Meeting in favour of the Arrangement and related matters; (iv) conduct the Meeting in accordance with the Interim Order and the constating documents of FSD Pharma, as applicable, and as otherwise required by applicable laws; (v) use commercially reasonable efforts to obtain such other consents, orders, rulings, approvals and assurances as counsel may advise are necessary or desirable in connection with the completion of the Arrangement and as contemplated by this Agreement; and (vi) use its best efforts to obtain the approval of the Arrangement Resolution; |
|
|
(d) |
it will use all reasonable efforts to cause each of the conditions precedent set out in Section 5.1 and Section 5.2 hereof to be complied with on or before the Effective Date; |
|
|
(e) |
it will not take any action on its part to divert the use of Celly Nu's available capital other than for the purposes of completing the Arrangement, preparing the Circular, conducting the Meeting, or activities that directly relate to Celly Nu's business plan; |
|
| (f) | ensure that the information set forth in the Circular relating to FSD Pharma and Celly Nu, and their respective businesses and the effect of the Plan of Arrangement thereon will be true, correct and complete in all material respects and will not contain any untrue statement of any material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein not misleading in light of the circumstances in which they are made; and |
|
|
(g) |
it shall be responsible for all costs associated with the Arrangement and the Meeting, and the preparation of the related documentation, including the Circular and all items identified in Section 2.4. |
Section 4.3 Covenants of Celly Nu
Celly Nu hereby covenants and agrees with FSD Pharma as follows:
|
| (a) | until the earlier of: (i) the Effective Date; and (ii) the termination of this Agreement, it will not perform any act or enter into any transaction which interferes or is inconsistent with the completion of the Plan of Arrangement; |
|
|
|
|
|
| (b) | it shall perform the obligations required to be performed by it, and shall enter into all agreements required to be entered into by it, under this Agreement and the Plan of Arrangement and shall do all such other acts and things as may be necessary or desirable in order to carry out and give effect to the Arrangement and related transactions as described in the Circular and, without limiting the generality of the foregoing, to the extent requested by FSD Pharma, it shall seek and cooperate with FSD Pharma in seeking (i) the Interim Order and the Final Order; and (ii) such other consents, orders, rulings, approvals and assurances as counsel may advise are necessary or desirable in connection with the completion of the Arrangement; |
| 14 |
|
| (c) | it will use all reasonable efforts to cause each of the conditions precedent set out in Section 5.1 and Section 5.2 hereof to be complied with on or before the Effective Date; |
|
|
|
|
|
| (d) | not, without limiting the generality of the foregoing covenants, until the Effective Date, except as required to effect the Plan of Arrangement or with the consent of FSD Pharma, alter or amend its constating documents as the same exist at the date of this Agreement except as specifically provided for hereunder. |
|
|
|
|
Each Party covenants and agrees to indemnify and save harmless the other Party from and against any and all liabilities, claims, demands, losses, costs, damages and expenses to which such Party or any of its Representatives may be subject or may suffer, in any way caused by, or arising, directly or indirectly, from or in consequence of any misrepresentation or alleged misrepresentation in any information included in the Circular that is provided by the other Party for the purpose of inclusion in the Circular; and any order made, or any inquiry, investigation or proceeding pursuant to any Securities Legislation, or by any Authority, based on any misrepresentation or any alleged misrepresentation in any information provided by the other Party for the purpose of inclusion in the Circular.
ARTICLE 5
CONDITIONS
Section 5.1 Mutual Conditions Precedent
The respective obligation of the parties hereto to complete the transactions contemplated by this Agreement, including the Arrangement and the obligation of each of FSD Pharma and Celly Nu to take such other action as is necessary or desirable to give effect to the Arrangement shall be subject to the satisfaction, or mutual waiver in writing, on or before the Effective Date, of the following conditions:
|
| (a) | the Interim Order shall have been granted in form and substance satisfactory to FSD Pharma and Celly Nu, acting reasonably, and such order shall not have been set aside or modified in a manner unacceptable to any of the Parties, acting reasonably, on appeal or otherwise; |
|
|
|
|
|
| (b) | the Arrangement and this Agreement, with or without amendment, shall have been approved by the directors and, if required, the shareholders of Celly Nu, to the extent required by, and in accordance with applicable Laws and the constating documents of Celly Nu; |
|
|
|
|
|
| (c) | the Arrangement Resolution, with or without amendment, shall have been approved by the required number of votes cast by FSD Pharma Securityholders at the Meeting, in accordance with the Interim Order and, subject to the Interim Order, the constating documents of FSD Pharma, applicable Laws and the requirements of any applicable regulatory authorities; |
| 15 |
|
| (d) | the Court shall have determined that the terms and conditions of the Arrangement are procedurally and substantively fair to the FSD Pharma Securityholders and the Final Order shall have been granted in the form and substance satisfactory to FSD Pharma, and shall not have been set aside or modified in a manner unacceptable to FSD Pharma, on appeal or otherwise; |
|
|
|
|
|
| (e) | the Celly Nu Shares to be issued in the United States pursuant to the Arrangement shall be issued in accordance with and exempt from registration requirements under applicable exemptions from registration under the U.S. Securities Act; |
|
|
|
|
|
| (f) | all material governmental, court, regulatory, third party and other approvals, consents, expiry of waiting periods, waivers, permits, exemptions, orders and agreements and all amendments and modifications to, and terminations of, agreements, indentures and arrangements considered by FSD Pharma to be necessary or desirable for the Arrangement to become effective shall have been obtained or received on terms that are satisfactory to FSD Pharma; |
|
|
|
|
|
| (g) | no action will have been instituted and be continuing on the Effective Date for an injunction to restrain, a declaratory judgment in respect of, or damages on account of or relating to the Arrangement and there will not be in force any order or decree restraining or enjoining the consummation of the transactions contemplated by this Agreement and no cease trading or similar order with respect to any securities of any of the parties will have been issued and remain outstanding; |
|
|
|
|
|
| (h) | none of the consents, orders, rulings, approvals or assurances required for the implementation of the Arrangement will contain terms or conditions or require undertakings or security deemed unsatisfactory or unacceptable by FSD Pharma; |
|
|
|
|
|
| (i) | no Laws, regulation or policy shall have been proposed, enacted, promulgated or applied which interferes or is inconsistent with the completion of the Plan of Arrangement, including any material change to the Tax Act and other relevant income tax Laws of Canada or the Province of Ontario, which would have a material adverse effect upon FSD Pharma Securityholders if the Plan of Arrangement is completed as set out in this Agreement; |
|
|
|
|
|
| (j) | no material fact or circumstance, including the fair market value of the Celly Nu Shares, shall have changed in a manner which would have a material adverse effect upon FSD Pharma or the FSD Pharma Securityholders if the Plan of Arrangement is completed; |
|
|
|
|
|
| (k) | the issuance of the securities under the Plan of Arrangement shall be exempt from registration under the U.S. Securities Act pursuant to the Section 3(a)(10) Exemption; |
|
|
|
|
|
| (l) | the issuance of the securities under the Plan of Arrangement shall be exempt from prospectus requirements under Securities Legislation pursuant to the Section 2.11 of NI 45-106; |
|
|
|
|
|
| (m) | holders of FSD Pharma Shares representing no more than 5% of the FSD Pharma Shares, in the aggregate, and, for greater certainty, disregarding the number of votes attached to Class A Shares and Class B Shares, shall have exercised their Dissent Rights; and |
|
|
|
|
|
| (n) | this Agreement shall not have been terminated pursuant to Section 6.2 hereof. |
| 16 |
Section 5.2 Additional Conditions to Obligations of Each Party
The obligation of each of FSD Pharma and Celly Nu to complete the contemplated Arrangement, is further subject to the condition, which may be waived by such Party without prejudice to the right of such Party hereto to rely on any other condition in favour of such Party, that subject to Section 6.1, each and every one of the covenants of the other Party hereto to be performed on or before the Effective Date pursuant to the terms of this Agreement shall have been performed by such Party and that, except as affected by the transaction contemplated by this Agreement, the representations and warranties of the other Party shall be true and correct in all material respects on the Effective Date (except for representations and warranties made as of the specified date, the accuracy of which shall be determined as at that specified date), with the same effect as if such representations and warranties had been made at, and as of, such time.
ARTICLE 6
AMENDMENT AND TERMINATION
Section 6.1 Amendment
Subject to any restrictions under the OBCA or in the Final Order, this Agreement (including the Schedules attached hereto) may, at any time and from time to time before or after the holding of the Meeting, but not later than the Effective Date, be amended by written agreement of the parties hereto without, subject to applicable Laws, further notice to, or authorization on the part of, the FSD Pharma Securityholders. Without limiting the generality of the foregoing, any such amendment may:
|
| (a) | change the time for performance of any of the obligations or acts of the parties; |
|
|
|
|
|
| (b) | waive any inaccuracies or modify any representation contained herein or in any document to be delivered pursuant hereto; |
|
|
|
|
|
| (c) | waive compliance with or modify any of the covenants herein contained or waive or modify performance of any of the obligations of the parties; or |
|
|
|
|
|
| (d) | make such alterations in this Agreement (including the Plan of Arrangement) as the parties may consider necessary or desirable in connection with the Interim Order, the Final Order or otherwise. |
|
|
|
|
Section 6.2 Termination
The parties agree that:
|
| (a) | if any condition contained in Article 5 is not satisfied at or before the Outside Date to the satisfaction of each Party, then such Party may, by notice to the other Party hereto terminate this Agreement and the obligations of the Parties hereunder (except as otherwise herein provided) but without detracting from the rights of such Party arising from any breach by any other Party but for which the condition would have been satisfied; |
|
|
|
|
|
| (b) | this Agreement may: |
|
| (i) | be terminated by the mutual agreement of the Parties hereto; |
|
|
|
|
|
| (ii) | be terminated by any Party hereto if there shall be passed any Law that makes consummation of the transactions contemplated by this Agreement illegal or otherwise prohibited; |
|
|
|
|
|
| (iii) | be terminated by any Party if the approval of the FSD Pharma Securityholders shall not have been obtained by reason of the failure to obtain the required vote on the Arrangement Resolution at the Meeting, at any time prior to the earlier of: (i) the Effective Date; and (ii) the Outside Date, by written notice to all other parties; |
| 17 |
|
| (c) | if the Effective Date does not occur on or prior to the Outside Date, then this Agreement shall automatically terminate without any further action of the Parties hereto; |
|
|
|
|
|
| (d) | if this Agreement is terminated in accordance with the foregoing provisions of this Section 6.2, no Party shall have any further liability to perform its obligations hereunder except as specifically contemplated hereby. |
|
|
|
|
Section 6.3 Effect of Termination
Upon the termination of this Agreement pursuant to Section 6.2 hereof, neither Party hereto shall have any liability or further obligation to the other Party hereto.
ARTICLE 7
MERGER AND SURVIVAL
Section 7.1 Merger of Conditions
The conditions set out in Section 5.1 and Section 5.2 hereof shall be conclusively deemed to have been satisfied or waived upon the Effective Date.
Section 7.2 Merger of Covenants
The provisions of Section 4.1, Section 4.2 and Section 4.3 hereof shall be conclusively deemed to have been satisfied in all respects upon the Effective Date.
Section 7.3 Survival of Representations and Warranties
The representations and warranties of FSD Pharma and Celly Nu contained in this Agreement shall not survive the completion of the Arrangement and shall expire and be terminated on the earlier of the Effective Time and the date on which this Agreement is terminated in accordance with its terms.
ARTICLE 8
GENERAL
Section 8.1 Notices
All notices to either of the parties hereto which may or are required to be given pursuant to any provision of this Agreement shall be given or made in writing and shall be deemed to be validly given if served personally or by email, in each case to the attention of the senior officer at the following address or at such other address as shall be specified by a party hereto by like notice:
| (a) | if to FSD Pharma: |
243 College Street, Suite 101,
Toronto, ON M5T 1R5
Attention: Zeeshan Saeed
Email: zsaeed@fsdpharma.com
| 18 |
| (b) | If to Celly Nu: |
PO Box 49290
1000-595 Burrard Street
Vancouver, BC V7X 1S8
Attention: John Duffy
Email: johnduffy@cellynutrition.com
Any notice that is delivered to such address shall be deemed to be delivered on the date of delivery if delivered on a Business Day prior to 5:00 p.m. (local time at the place of receipt) or on the next Business Day if delivered after 5:00 p.m. or on a non-Business Day. Any notice delivered by email shall be deemed to be delivered on the date of transmission.
Section 8.2 Time of the Essence
Time shall be of the essence of this Agreement.
Section 8.3 Assignment
Neither of the parties hereto may assign its rights or obligations under this Agreement or the Arrangement without the prior written consent of the other.
Section 8.4 Binding Effect
This Agreement and the Plan of Arrangement shall be binding upon and shall enure to the benefit of each of the parties hereto and the respective successors and permitted assigns thereof.
Section 8.5 Waiver
Any waiver or release of any of the provisions of this Agreement, to be effective, must be in writing executed by the party hereto granting such waiver or release.
Section 8.6 Further Assurances
Each party hereto shall, from time to time, and at all times hereafter, at the request of the other, but without further consideration, do, or cause to be done, all such other acts, and execute and deliver, or cause to be executed and delivered, all such further agreements, transfers, assurances, instruments or documents as may be reasonably required in order to fully perform and carry out the terms and intent hereof including, without limitation, the Arrangement.
Section 8.7 Governing Law
This Agreement shall be governed by, and be construed in accordance with, the laws of the Province of Ontario and the federal laws of Canada applicable therein but the reference to such laws shall not, by conflict of laws rules or otherwise, require the application of the law of any jurisdiction other than the Province of Ontario.
Each Party agrees that any action or proceeding arising out of or relating to this Agreement may be instituted in the courts of Ontario, waives any objection which it may have now or later to the venue of that action or proceeding, irrevocably submits to the exclusive jurisdiction of those courts in that action or proceeding and agrees to be bound by any judgement of those courts.
| 19 |
Section 8.8 Expenses
Other than noted herein, all expenses incurred in connection with this Agreement, the Arrangement and the transactions contemplated hereby and thereby shall be borne by FSD Pharma.
Section 8.9 Counterparts
This Agreement may be executed in one or more counterparts, by original, facsimile or pdf signature, each of which shall be deemed an original but all of which together shall constitute one and the same instrument.
Section 8.10 Severability
If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced by any rule, Law, or public policy, all other conditions and provisions of this Agreement will nevertheless remain in full force and effect so long as the economic or legal substance of the transactions contemplated by this Agreement is not affected in any manner materially adverse to any Party. Upon any determination that any term or other provision is invalid, illegal or incapable of being enforced, the Parties will negotiate in good faith to modify this Agreement so as to effect the original intent of the Parties as closely as possible in an acceptable manner to the end that the transactions contemplated by this Agreement are fulfilled to the fullest extent possible.
Section 8.11 Enurement
This Agreement will be binding upon and enure to the benefit of the Parties and their respective successors and permitted assigns from time to time.
Section 8.12 Entire Agreement
This Agreement, the Plan of Arrangement and the other agreements contemplated hereby and thereby constitute the entire agreement between the Parties pertaining to the subject matter hereof and supersedes all prior agreements, understandings, negotiations, and discussions, whether oral or written, of the parties. There are no warranties, conditions, or representations (including any that may be implied by statute) and there are no agreements in connection with such subject matter, except as specifically set forth or referred to in this Agreement or as otherwise set out in writing and delivered at the completion of the Arrangement. No reliance is placed on any warranty, representation, opinion, advice or assertion of fact made by any Party or its directors, officers, employees or agents, to any other Party or its directors, officers, employees or agents, except to the extent that the same has been reduced to writing and included as a term of this Agreement. Accordingly, there will be no liability, either in tort or in contract, assessed in relation to any such warranty, representation, opinion, advice or assertion of fact, except to the extent aforesaid.
Section 8.13 Language
The Parties to this Agreement confirm their express wish that this Agreement and all documents and agreements directly or indirectly relating thereto be drawn up in the English language. Les Parties reconnaissent leur volonté expresse que la présente Entente ainsi que tous les documents et commis s'y rattachant directement ou indirectement soient rédigés en anglaise.
[Signature page follows.]
| 20 |
IN WITNESS WHEREOF the parties hereto have executed this Agreement as of the date and year first above written.
| FSD PHARMA INC. | |||
| “Zeeshan Saeed” | |||
|
|
| Name: Zeeshan Saeed | |
| Title: CEO and Co-Executive, Chairman | |||
|
|
| CELLY NUTRITION CORP. |
|
|
|
|
|
|
|
|
| “John Duffy” |
|
|
|
| Name: John Duffy |
|
|
|
| Title: CEO |
|
|
|
|
|
|
| 21 |
SCHEDULE “A”
PLAN OF ARRANGEMENT
UNDER THE PROVISIONS OF SECTION 182
OF THE BUSINESS CORPORATIONS ACT (ONTARIO)
ARTICLE 1 INTERPRETATION
Section 1.1 Definitions
In this Plan of Arrangement, unless there is something in the subject matter or context inconsistent therewith, the following terms shall have the respective meanings set out below and grammatical variations of such terms shall have corresponding meanings:
“Arrangement Agreement” means the arrangement agreement dated as of October 4th, 2023, including the Schedules attached hereto, as may be supplemented or amended from time to time”
“Arrangement Consideration Shares” means the securities issued or distributed, as the case may be, pursuant to the Share Exchange, being FSD Pharma New Class B Shares, FSD Pharma New Class A Shares and Celly Nu Shares;
“Arrangement Resolution” means the special resolution of the FSD Pharma Securityholders in respect of the Arrangement to be considered at the Meeting;
“Arrangement” means the arrangement under Section 182 of the OBCA on the terms and subject to the conditions set out in this Plan of Arrangement, subject to any amendments or variations thereto made in accordance with the Arrangement Agreement or this Plan of Arrangement or made at the direction of the Court in the Final Order with the consent of FSD Pharma;
“BCBCA” means the Business Corporations Act (British Columbia) and the regulations made thereunder, as promulgated or amended from time to time;
“Board of Directors” means the duly appointed board of directors of the applicable company;
“Business Day” means a day, other than a Saturday, Sunday or statutory holiday, when banks are generally open in the City of Toronto, Ontario for the transaction of banking business;
“CDS” means CDS Clearing and Depository Services Inc.;
“Celly Nu” means Celly Nutrition Corp., a company incorporated pursuant to the laws of the Province of British Columbia, Canada;
“Celly Nu Shares” means the common shares in the capital of Celly Nu;
“Circular” means the management information circular of FSD Pharma to be prepared and sent to the FSD Pharma Securityholders, FSD Pharma Non-Distribution Warrantholders and FSD Pharma Optionholders in connection with the Meeting;
“Class A Shares” means the class A multiple voting shares of FSD Pharma;
“Class B Shares” means the class B subordinate voting shares of FSD Pharma;
“Consideration” means the consideration payable by FSD Pharma pursuant to Section 2.2 of this Plan of Arrangement to a person who is, immediately before the Effective Time, a FSD Pharma Securityholder;
| 22 |
“Court” means the Ontario Superior Court of Justice (Commercial List);
“Depository” means Marrelli Trust Company or such other person that may be appointed by the Parties for the purpose of receiving deposits of certificates formerly representing Class A Shares, Class B Shares and FSD Pharma Distribution Warrants;
“Dissent Rights” has the meaning set forth in Section 3.1 of the Plan of Arrangement;
“Dissenting Shareholder” means a registered FSD Pharma Shareholder who has validly exercised its Dissent Rights pursuant to Article 3 hereof and the Interim Order and the Final Order and has not withdrawn or been deemed to have withdrawn such exercise of Dissent Rights;
“Effective Date” means the date shown on the Certificate of Arrangement issued by the Director;
“Effective Time” means 12:01 a.m. (Toronto time) on the Effective Date;
“Encumbrance” means any encumbrance, lien, charge, hypothec, pledge, mortgage, title retention agreement, security interest of any nature, prior claim, adverse claim, exception, reservation, restrictive covenant, agreement, easement, lease, license, right of occupation, option, right of use, right of first refusal, right of pre-emption, privilege or any matter capable of registration against title or any contract to create any of the foregoing.
“Final Order” means the final order of the Court pursuant to Section 182 of the OBCA, after a hearing upon the fairness of the terms and conditions of the Arrangement, in a form acceptable to FSD Pharma approving the Arrangement, as such order may be amended by the Court (with the consent of FSD Pharma) at any time prior to the Effective Date or, if appealed, then, unless such appeal is withdrawn or denied, as affirmed or as amended (provided that any such amendment is acceptable to FSD Pharma) on appeal”;
“Permitted Holder” has the meaning ascribed thereto in the FSD Pharma Articles;
“FSD Pharma Articles” means all applicable articles of incorporation, articles of amalgamation, articles of amendment, and all constating documents of FSD Pharma;
“FSD Pharma New Class A Shares” has the meaning attributed to that term in Section 2.2(d)iii of this Plan of Arrangement;
“FSD Pharma New Class B Shares” has the meaning attributed to that term in Section 2.2(d)iv of this Plan of Arrangement;
“FSD Pharma New Option” means the new FSD Pharma options issued to FSD Pharma Optionholders as a result of this Plan of Arrangement, and each of which are exercisable for one FSD Pharma New Class B Share;
“FSD Pharma Securities” means, collectively, the Class A Shares, Class B Shares and FSD Pharma Distribution Warrants;
“FSD Pharma Securityholders” means collectively FSD Pharma Shareholders and FSD Pharma Distribution Warrantholders;
“FSD Pharma Shareholders” means the holders of Class Shares and Class B Shares, at the applicable time;
| 23 |
“FSD Pharma Shares” means all issued and outstanding Class A Shares and Class B Shares;
“FSD Pharma” means FSD Pharma Inc. a company incorporated pursuant to the laws of Ontario;
“FSD Pharma Distribution Warrantholders” means the holders of FSD Pharma Distribution Warrants.
“FSD Pharma Non-Distribution Warrants” means outstanding warrants of FSD Pharma, each of which is exercisable for the purchase of one Class B Share, and which does not include a provision in its applicable warrant certificate that is substantially in the form attached as Schedule “B” hereto or with the same substantive effect.
“FSD Pharma Non-Distribution Warrantholders” means the holders of FSD Pharma Non-Distribution Warrants.
“FSD Pharma Non-Distribution Warrants” means outstanding warrants of FSD Pharma, each of which is exercisable for the purchase of one Class B Share, and which does not include a provision in its applicable warrant certificate that is substantially in the form attached as Schedule “B” hereto or with the same substantive effect.
“FSD Pharma New Distribution Warrants” means the new FSD Pharma Distribution Warrants issued to FSD Pharma Distribution Warrantholders as a result of this Plan of Arrangement, and each of which are exercisable for one FSD Pharma New Class B Share;
“FSD Pharma New Non-Distribution Warrants” means the new FSD Pharma Non-Distribution Warrants issued to FSD Pharma Non-Distribution Warrantholders as a result of this Plan of Arrangement, and each of which are exercisable for one FSD Pharma New Class B Share;
“Interim Order” means the order made after application to the Court pursuant to Section 182 of the OBCA, providing for, among other things, the calling and holding of the Meeting, as such order may be amended, supplemented or varied by the Court (with the consent of the Parties, acting reasonably);
“Letter of Transmittal” means the letter of transmittal enclosed with the Circular sent in connection with the Meeting pursuant to which, among other things, registered FSD Pharma Securityholders are required to deliver certificates representing Class B Shares, Class A Shares and FSD Pharma Distribution Warrants, in order to receive the Consideration to which they are entitled;
“Meeting” means the special meeting of FSD Pharma Securityholders and any adjournment(s) or postponement(s) thereof, to be called and held in accordance with the Interim Order to consider and to vote on the Arrangement Resolution and any other matters set out in the Notice of Meeting;
“Notice of Meeting” means the notice of the Meeting to be sent to the FSD Pharma Securityholders, FSD Pharma Optionholders and FSD Non-Distribution Warrantholders, which notice will accompany the Circular;
“OBCA” means the Business Corporations Act (Ontario) and the regulations made thereunder, as promulgated or amended from time to time;
“Parties” means, collectively, FSD Pharma and, and “Party” means any one of them;
“Person” or “person” means and includes an individual, sole proprietorship, partnership, unincorporated association, unincorporated syndicate, unincorporated organization, trust, body corporate, trustee, executor, administrator or other legal representative and the Crown or any agency or instrumentality thereof;
| 24 |
“Plan of Arrangement” means this plan of arrangement and any amendments or variations thereto made in accordance with this Agreement, the Plan of Arrangement or upon the direction of the Court in the Final Order with the consent of FSD Pharma;
“Round Down Provision” has the meaning attributed to that term in Section 2.3 of this Plan of Arrangement;
“Share Exchange” has the meaning attributed to that term in Section 2.2(e) of this Plan of Arrangement;
“Tax Act” means the Income Tax Act (Canada) and the regulations made thereunder, as promulgated or amended from time to time; and
“Transfer Agent” means Marrelli Trust Company, or such other trust company or transfer agent as may be designated by FSD Pharma.
“US Tax Code” means the United States Internal Revenue Code of 1986 and the regulations made thereunder, as promulgated or amended from time to time; and
In addition, words and phrases used herein and defined in the OBCA and not otherwise defined herein or in the Arrangement Agreement shall have the same meaning herein as in the OBCA unless the context otherwise requires.
Section 1.2 Sections and Headings
The division of this Plan of Arrangement into articles and sections and the insertion of headings are for convenience of reference only and shall not affect the construction or interpretation of this Plan of Arrangement. Unless reference is specifically made to some other document or instrument, all references herein to articles and sections are to articles and sections of this Plan of Arrangement.
Section 1.3 Number, Gender and Persons
In this Plan of Arrangement, unless otherwise expressly stated or the context otherwise requires, words importing the singular number shall include the plural and vice versa, and words importing gender shall include all genders.
Section 1.4 Statutory References
Any reference in this Plan of Arrangement to a statute includes all regulations made thereunder, all amendments to such statute or regulation in force from time to time and any statute or regulation that supplements or supersedes such statute or regulation.
Section 1.5 Currency
Unless otherwise stated all references in this Plan of Arrangement to sums of money are expressed in lawful money of Canada.
Section 1.6 Business Day
In the event that the date on which any action is required to be taken hereunder by either of the parties is not a Business Day in the place where the action is required to be taken, such action shall be required to be taken on the next succeeding day which is a Business Day in such place.
| 25 |
Section 1.7 Governing Law
This Plan of Arrangement shall be governed by and construed in accordance with the laws of the Province of Ontario and the federal laws of Canada applicable therein.
Section 1.8 Binding Effect
This Plan of Arrangement will become effective at, and be binding at and after, the Effective Time on: FSD Pharma and all registered and beneficial FSD Pharma Securityholders, all Dissenting Shareholders, and all FSD Pharma Distribution Warrantholders. This Plan of Arrangement may be withdrawn prior to the occurrence of any of the events in Section 2.2 in accordance with the terms of the Arrangement Agreement.
ARTICLE 2
ARRANGEMENT
Section 2.1 Effect of Plan of Arrangement
This Plan of Arrangement and the Arrangement shall become effective at the Effective Time, and shall be binding on FSD Pharma, Celly Nu, all FSD Pharma Securityholders, including all Dissenting Shareholders, the Transfer Agent, the Depositary, and all other Persons, at and after the Effective Time, without any further act or formality required on the part of any Person.
Section 2.2 Arrangement
Commencing at the Effective Time, each of the events set out below shall occur and shall be deemed to occur in the following sequence or as otherwise provided below or herein, without any further act or formality on the part of any Person, in each case, unless specifically provided otherwise in this Section 2.2 effective as at two-minute intervals starting at the Effective Time:
|
| (a) | Each FSD Pharma Share held by a Dissenting Shareholder shall be deemed to be transferred by the holder thereof, without any further act or formality on its part, free and clear of all Encumbrances, to FSD Pharma for cancellation and shall be cancelled; |
|
|
|
|
|
| (b) | such Dissenting Shareholder shall cease to be the holder of such FSD Pharma Shares and to have any rights as a FSD Pharma Shareholder other than the right to be paid fair value for such FSD Pharma Shareholder by FSD Pharma in accordance with Article 3; |
|
|
|
|
|
| (c) | the name of such Dissenting Shareholder shall be removed from FSD Pharma's register of FSD Pharma Shares as a holder of FSD Pharma Shares; |
|
|
|
|
|
| (d) | The articles of FSD Pharma shall be amended to provide that the authorized share structure of FSD Pharma shall be reorganized and altered by: |
|
| i. | changing the identifying name of the issued and unissued Class A Shares from “Class A Multiple Voting Shares” to “Multiple Voting Shares” and amending the rights, privileges, restrictions and conditions attaching to those shares to require FSD Pharma to provide a notice of time and place of any meeting of shareholders to be sent at least 22 days and not more than 60 days to shareholders thereof; |
| 26 |
|
| ii. | changing the identifying name of the issued and unissued Class B Shares from “Class B Subordinate Voting Shares” to “Subordinate Voting Shares” and amending the rights, privileges, restrictions and conditions attaching to those shares to require FSD Pharma to provide a notice of time and place of any meeting of shareholders to be sent at least 22 days and not more than 60 days to shareholders thereof; |
|
|
|
|
|
| iii. | creating a new class of shares without par value, with no maximum number of shares and with the identifying name “Reorganization Multiple Voting Shares” having the rights, privileges, restrictions, and conditions identical to the Class A Shares, as more particularly described in the articles of FSD Pharma, prior to the amendments described in paragraph (d)(i) above (the “FSD Pharma New Class A Shares”); and |
|
|
|
|
|
| iv. | creating a new class of shares without par value, with no maximum number and with and with the identifying name “Reorganization Subordinate Voting Shares” having the rights, privileges, restrictions and conditions identical to the Class B Shares, as more particularly described in the articles of FSD Pharma, prior to the amendments described in paragraph (d)(ii) above (the “FSD Pharma New Class B Shares”). |
|
| (e) | FSD Pharma shall reorganize its capital within the meaning of Section 86 of the Tax Act such that each FSD Pharma Shareholder (for the avoidance of doubt, excluding any FSD Pharma Shares surrendered and cancelled in accordance with Section 2.2(a) shall dispose of all of the FSD Pharma Shareholder's securities to FSD Pharma and in consideration and exchange therefor (“Consideration”),FSD Pharma shall: |
|
| i. | with respect to the holders of Class B Shares: |
|
| a) | issue that number of FSD Pharma New Class B Shares as is equal to the number of Class B Shares previously held by each such holder; |
|
|
|
|
|
| b) | distribute a number of Celly Nu Shares equal to the number of FSD Pharma New Class B Shares held, in accordance with the provisions of Article 4 of the Plan of Arrangement as of the Effective Date |
|
| ii. | with respect to the holders of Class A Shares: |
|
|
| a) | issue (i) to any holder of a Class A Share that is a Permitted Holder at the Effective Time, that number of FSD Pharma New Class A Shares as is equal to the number of Class A Shares previously held by each such holder; or (ii) to any holder of a Class A Share that is not a Permitted Holder at the Effective Time, at the discretion of the Board of Directors of FSD Pharma, either (x) that number of FSD Pharma New Class A Shares as is equal to the number of Class A Shares previously held by each such holder; or (y) that number of FSD Pharma New Class B Shares as is equal to the number of Class A Shares previously held by each such holder; and |
|
|
|
|
|
|
|
| b) | distribute a number of Celly Nu Shares equal to the number of FSD Pharma New Class A Shares held, in accordance with the provisions of Article 4 of the Plan of Arrangement as of the Effective Date;
|
|
| (collectively, the “Share Exchange”), and, in connection with the Share Exchange: |
||
| 27 |
|
| i. | the name of each FSD Pharma Shareholder shall be removed from the shareholder register and added to the shareholder register for the FSD Pharma New Class B Shares and FSD Pharma New Class A Shares, respectively, and Celly Nu Shares as the holder of the number of FSD Pharma New Class B Shares, FSD Pharma New Class A Shares and Celly Nu Shares, respectively, received pursuant to the Share Exchange; |
|
|
|
|
|
| ii. | all issued and outstanding Class B Shares and Class A Shares shall be cancelled and the capital in respect of such securities shall be reduced to nil; |
|
|
|
|
|
| iii. | the number of Celly Nu Shares previously held by FSD Pharma and distributed pursuant to the Share Exchange shall be removed from Celly Nu's register of holders of Celly Nu Shares; and |
|
| (f) | The authorized share structure of FSD Pharma shall be reorganized and altered by: |
|
| i. | eliminating the Class B Shares from the authorized share structure of FSD Pharma; |
|
|
|
|
|
| ii. | eliminating the Class A Shares from the authorized share structure of FSD Pharma; |
|
|
|
|
|
| iii. | changing the identifying name of the issued and unissued FSD Pharma New Class B Shares from “Reorganization Subordinate Voting Shares” to “Class B Subordinate Voting Shares”; and |
|
|
|
|
|
| iv. | changing the identifying name of the issued and unissued FSD Pharma New Class A Shares from “Reorganization Multiple Voting Shares” to “Class A Multiple Voting Shares”. |
|
| (g) | Each FSD Pharma Option outstanding before the Effective Time will be deemed to be exchanged for: |
|
| i. | one FSD Pharma New Option, with each FSD Pharma New Option having an exercise price equal to the original exercise price for the FSD Pharma Option being exchanged. |
|
| (h) | Each FSD Pharma Distribution Warrant outstanding before the Effective Time will be deemed to be exchanged for: |
|
| i. | one FSD Pharma New Distribution Warrant with each FSD Pharma New Distribution Warrant having an exercise price equal to the original exercise price for the FSD Pharma Distribution Warrant being exchanged; and |
|
|
|
|
|
| ii. | one Celly Nu Share. |
|
| (i) | Each FSD Pharma Non-Distribution Warrant outstanding before the Effective Time will be deemed to be exchanged for: |
|
| i. | one FSD Pharma New Non-Distribution Warrant with each FSD Pharma New Non-Distribution Warrant having an exercise price equal to the original exercise price for the FSD Pharma Non-Distribution Warrant being exchanged. |
| 28 |
Section 2.3 No Fractional Celly Nu Shares
No fractional Celly Nu Shares shall be distributed by FSD Pharma to FSD Pharma Securityholders. If FSD Pharma would otherwise be required to distribute to FSD Pharma Securityholder an aggregate number of Celly Nu Shares that is not a round number, then the number of Celly Nu Shares, distributable to that FSD Pharma Securityholder shall be rounded down to the next lesser whole number (the “Round Down Provision”) and that FSD Pharma Securityholder shall not receive any compensation in respect thereof. In calculating such fractional interests, all Class B Shares, all Class A Shares and all FSD Pharma Distribution Warrants registered in the name of or beneficially held by such FSD Pharma Securityholder or their nominee shall be aggregated. Notwithstanding the foregoing, if the Round Down Provision would otherwise result in the number of Celly Nu Shares distributable to a particular FSD Pharma Securityholder being rounded down from one to nil, then the Round Down Provision shall not apply and FSD Pharma shall distribute one Celly Nu Share, to that FSD Pharma Securityholder.
Section 2.4 Extinction of Rights
Any instrument or certificate which immediately prior to the Effective Time represented outstanding FSD Pharma Securities that were exchanged pursuant to Section 2.2(e) and Section 2.2(h) or an affidavit of loss and bond or other indemnity pursuant to Section 4.3, shall, on or prior to the sixth (6th) anniversary of the Effective Date, cease to represent a claim or interest of any kind or nature against FSD Pharma. On such date, the aggregate FSD Pharma New Class B Shares or FSD Pharma New Class A Shares, as applicable, to which the former FSD Pharma Securityholder referred to in the preceding sentence was ultimately entitled shall be deemed to have been surrendered for no consideration to FSD Pharma and shall be returned to FSD Pharma by the Depositary. None of FSD Pharma, Celly Nu or the Depositary shall be liable to any person in respect of any amount for FSD Pharma New Class B Shares, FSD Pharma New Class A Shares or Celly Nu Shares, delivered to a public official pursuant to any applicable abandoned property, escheat or similar law.
Section 2.5 Withholding
|
| (a) | FSD Pharma and Celly Nu, as the case may be, will be entitled to deduct and withhold from any Consideration otherwise payable to any FSD Pharma Securityholder under this Plan of Arrangement (including any payment to FSD Pharma Shareholders exercising Dissent Rights) such amounts as FSD Pharma or Celly Nu are permitted or required to deduct and withhold with respect to such payment under the Tax Act and the rules and regulations promulgated thereunder, or any provision of any provincial, state, local or foreign tax Law as counsel may advise is permitted or required to be so deducted and withheld by FSD Pharma or Celly Nu, as the case may be. FSD Pharma, Celly Nu, the Transfer Agent or the duly appointed party on behalf of thereof, shall be entitled to dispose of such number of Celly Nu Shares as is necessary to satisfy the withholdings contemplated herein. |
|
|
|
|
|
| (b) | For the purposes of such deduction and withholding: (i) all withheld amounts shall be treated as having been paid to the person in respect of which such deduction and withholding was made on account of the obligation to make payment to such person hereunder; and (ii) such deducted or withheld amounts shall be remitted to the appropriate Authority in the time and manner permitted or required by the applicable Law by or on behalf of FSD Pharma or Celly Nu, as the case may be. |
|
|
|
|
|
| (c) | FSD Pharma, Celly Nu and the Transfer Agent shall be entitled to deduct and withhold from any amount otherwise payable to any FSD Pharma Securityholder, as applicable, such amounts as FSD Pharma, Celly Nu, or the Transfer Agent is required or permitted to deduct and withhold with respect to such payment under the Tax Act or US Tax Code, or any provision of any applicable federal, provincial, state, local or foreign tax law or treaty, in each case, as amended. FSD Pharma, Celly Nu or the Transfer Agent shall be entitled to dispose of such number of Celly Nu Shares as is necessary to satisfy the withholdings contemplated herein. To the extent that amounts are so withheld, such withheld amounts shall be treated for all purposes hereof as having been paid to the FSD Pharma Securityholder, as applicable, in respect of which such deduction and withholding was made, provided that such withheld amounts are actually remitted to the appropriate taxing authority. |
| 29 |
Section 2.6 Post-Effective Date Procedures
Subject to the provisions of Article 4 hereof, and upon return of a properly completed Letter of Transmittal by a registered former FSD Pharma Securityholder together with certificates, if any, which, immediately prior to the Effective Date, represented Class A Shares, Class B Shares or FSD Pharma Distribution Warrants, as the case may be and such other documents as the Depositary may require, former FSD Pharma Securityholders shall be entitled to receive delivery of certificates representing the Arrangement Consideration Shares to which they are entitled pursuant to Section 2.2.
Section 2.7 Deemed Fully Paid and Non-Assessable Shares
All Arrangement Consideration Shares issued or distributed pursuant hereto, as the case may be, shall be deemed to be validly issued and outstanding as fully paid and non-assessable shares for all purposes of the OBCA or the BCBCA, as applicable.
ARTICLE 3
RIGHTS OF DISSENT
Section 3.1 Rights of Dissent
Pursuant to the Interim Order, registered holders of Class B Shares , Class A Shares and FSD Pharma Distribution Warrants may exercise rights of dissent (the “Dissent Rights”) pursuant to and in the manner set forth in Section 185 OBCA, as modified by this Article 3, the Interim Order and the Final Order, with respect to Class B Shares and Class A Shares in connection with the Arrangement, provided that the written notice setting forth the objection of such registered FSD Pharma Securityholder to the Arrangement Resolution must be received by FSD Pharma not later than 5:00p.m. (Toronto time) on the Business Day that is two Business Days immediately preceding the Meeting or any date to which the Meeting may be postponed or adjourned. Each Dissenting Shareholder who duly exercises its Dissent Rights in accordance with this Section 3.1, shall be deemed to have transferred all FSD Pharma Shares held by such Dissenting Shareholder and in respect of which Dissent Rights have been validly exercised, to FSD Pharma, free and clear of all Encumbrances, as provided in Section 2.2(a) and if such Dissenting Shareholder:
|
| (a) | is ultimately entitled to be paid fair value for its Class B Shares or Class A Shares, such Dissenting Shareholder: (i) shall be deemed not to have participated in the transactions in Article 2 (other than Section 2.2(a)); (ii) will be entitled to be paid the fair value of such Class B Shares or Class A Shares by FSD Pharma, which fair value, notwithstanding anything to the contrary contained in section 185 of the OBCA, shall be determined as of the close of business on the Business Day immediately preceding the date on which the Arrangement Resolution was adopted; and (iii) will not be entitled to any other payment or consideration, including any payment that would be payable under the Arrangement if such Dissenting Shareholder had not exercised its Dissent Rights in respect of such Class B Shares or Class A Shares; or |
|
|
|
|
|
| (b) | ultimately is not entitled, for any reason, to be paid fair value for such Class B Shares or Class A Shares, such Dissenting Shareholder shall be deemed to have participated in the Arrangement on the same basis as a non‐dissenting holder of Class B Shares or Class A Shares and shall be entitled to receive only the Consideration contemplated by Section 2.2(e) that such Dissenting Shareholder would have received pursuant to the Arrangement if such Dissenting Shareholder had not exercised its Dissent Rights. |
| 30 |
Section 3.2 Recognition of Dissenting Shareholders
In no circumstances shall FSD Pharma or any other Person be required to recognize a Person exercising Dissent Rights unless such Person is a registered holder of those Class B Shares or Class A Shares, in respect of which such Dissent Rights are sought to be exercised. For greater certainty, in no case shall FSD Pharma or any other Person be required to recognize any Dissenting Shareholder as a holder of Class B Shares or Class A Shares in respect of which Dissent Rights have been validly exercised after the completion of the transfer under Section 2.2 (a) and the name of such Dissenting Shareholder shall be removed from the register of FSD Pharma Securityholders as to those FSD Pharma Shares in respect of which Dissent Rights have been validly exercised at the same time as the event described in Section 2.2(a) occurs.
ARTICLE 4
CERTIFICATES
Section 4.1 Delivery of Securities
As soon as practicable following the Effective Date, FSD Pharma and Celly Nu, as applicable, will forward or cause to be forwarded by the Transfer Agent or otherwise, by registered mail postage prepaid) or hand delivery to FSD Pharma Securityholders as of the Effective Date at the address specified in the register of FSD Pharma Securityholders, certificates representing the number of Celly Nu Shares to be delivered to such FSD Pharma Securityholders pursuant to the Arrangement. The Parties agree to use their reasonable efforts to deliver the Celly Nu Shares in the form of direct registration statements issued by the Transfer Agent, rather than physical certificates if practicable without undue financial expense.
Section 4.2 Payment of Consideration
|
| (a) | Following receipt of the Final Order and prior to the Effective Date, the Parties shall deliver or arrange to be delivered to the Depositary the certificates representing Celly Nu Shares required to be distributed to the FSD Pharma Securityholders in accordance with Section 4.2(a) hereof, which certificates shall be held by the Depositary as agent and nominee for such FSD Pharma Securityholders for distribution to such FSD Pharma Securityholders in accordance with the provisions hereof. Following receipt of the Final Order and prior to the Effective Date, FSD Pharma shall deliver or arrange to be delivered to the Depositary an irrevocable treasury order directing the Depository to issue the certificates representing the FSD Pharma New Class B Shares, FSD Pharma New Class A Shares and FSD Pharma New Distribution Warrants required to be issued to the FSD Pharma Securityholders in accordance with Section 4.2(a) hereof, which certificates shall be held by the Depositary as agent and nominee for such former FSD Pharma Securityholders for distribution to such former FSD Pharma Securityholders in accordance with the provisions hereof. |
| 31 |
|
| (b) | Subject to surrender to the Depositary for cancellation of a certificate which immediately prior to the Effective Time represented outstanding Class B Shares, Class A Shares or FSD Pharma Distribution Warrants together with a duly completed and executed Letter of Transmittal and such additional documents and instruments as the Depositary may reasonably require, following the Effective Time the holder of such surrendered certificate shall be entitled to receive in exchange therefor, and the Depositary shall deliver to such holder, the Consideration which such holder has the right to receive under this Plan of Arrangement, less any amounts withheld pursuant to Section 2.5, and any certificate so surrendered shall forthwith be cancelled. |
|
|
|
|
|
| (c) | Until surrendered as contemplated by Section 4.2(a), each certificate that immediately prior to the Effective Time represented a Class B Share, Class A Share or FSD Pharma Distribution Warrant shall be deemed after the Effective Time to represent only the right to receive, upon such surrender, the Consideration to which the holder thereof is entitled in lieu of such certificate as contemplated by Section 2.4 and this Section 4.2, less any amounts withheld pursuant to Section 2.5. Any such certificate formerly representing Class B Shares, Class A Shares or FSD Pharma Distribution Warrants not duly surrendered on or before the sixth anniversary of the Effective Date shall: |
|
| (i) | cease to represent a claim by, or interest of, any former FSD Pharma Securityholder of any kind or nature against or in FSD Pharma or Celly Nu (or any successor to any of the foregoing); and |
|
|
|
|
|
| (ii) | be deemed to have been surrendered to FSD Pharma and shall be cancelled. |
|
| (d) | No holder of a Class B Share or Class A Share nor any FSD Pharma Distribution Warrantholders shall be entitled to receive any consideration with respect to such securities other than the Consideration to which such holder is entitled in accordance with Section 2.2(e)i and Section 2.2(e)ii and this Section 4.2 and, for greater certainty, no such holder will be entitled to receive any interest, dividends, premium or other payment in connection therewith. |
|
|
|
|
In the event any certificate which immediately prior to the Effective Time represented one or more outstanding Class B Shares, Class A Shares, or FSD Pharma Distribution Warrants that are ultimately entitled to Consideration pursuant to Section 2.2(e) and Section 2.2(h) shall have been lost, stolen or destroyed, upon the making of an affidavit or statutory declaration of that fact by the person claiming such certificate to be lost, stolen or destroyed and who was listed immediately prior to the Effective Time as the registered holder thereof on the securities registers maintained by or on behalf of FSD Pharma, the Depositary will deliver in exchange for such lost, stolen or destroyed certificate, certificates representing the Consideration that such holder is entitled to receive in exchange for such lost, stolen or destroyed certificate, provided the holder to whom the Consideration is to be delivered shall, as a condition precedent to the delivery, give a bond satisfactory to FSD Pharma and the Depositary (acting reasonably) in such sum as FSD Pharma and the Depositary may direct, or otherwise indemnify FSD Pharma and the Depositary in a manner satisfactory to FSD Pharma and the Depositary, acting reasonably, against any claim that may be made against FSD Pharma or the Depositary with respect to the certificate alleged to have been lost, stolen or destroyed.
| 32 |
Section 4.4 Distributions with Respect to Unsurrendered Certificates
No dividend or other distribution declared or paid with a record date after the Effective Time with respect to Arrangement Consideration Shares shall be delivered to the holder of any certificate formerly representing Class B Shares, Class A Shares or FSD Pharma Distribution Warrants, respectively, unless and until the holder of such certificate shall have complied with the provisions of Section 4.2. Subject to applicable Law and to Section 4.2 at the time of such compliance, there shall, in addition to the delivery of the Consideration to which such holder is thereby entitled, be delivered to such holder, without interest, the amount of any dividend or other distribution declared or made after the Effective Time with respect to the Arrangement Consideration Shares to which such holder is entitled in respect of such holder's Consideration.
Section 4.5 Paramountcy
From and after the Effective Time: (a) this Plan of Arrangement shall take precedence and priority over any and all FSD Pharma Securities issued or outstanding at or prior to the Effective Time, (b) the rights and obligations of the FSD Pharma Securityholders and of FSD Pharma, Depositary, Transfer Agent and any transfer agent or other depositary, in relation to the FSD Pharma Securities and the Arrangement Consideration Shares shall be solely as provided for in this Plan of Arrangement, and (c) all actions, causes of action, claims or proceedings (actual or contingent and whether or not previously asserted) based on or in any way relating to any FSD Pharma Securities shall be deemed to have been settled, compromised, released and determined without liability except as set forth in this Plan of Arrangement.
Section 4.6 U.S. Securities Laws Exemption
Notwithstanding any provision herein to the contrary, the Parties agree that this Plan of Arrangement will be carried out with the intention that all of the Arrangement Consideration Shares constituting the Consideration issued pursuant to this Plan of Arrangement will be issued in reliance on the exemption from the registration requirements of the U.S. Securities Act as provided by Section 3(a)(10) thereof.
ARTICLE 5
AMENDMENTS
Section 5.1 Right to Amend
FSD Pharma reserves the right to amend, modify or supplement (or do all of the foregoing) this Plan of Arrangement from time to time and at any time prior to the Effective Date provided that any such amendment, modification and/or supplement must be contained in a written document that is:
|
| (a) | approved by Celly Nu; |
|
|
|
|
|
| (b) | filed with the Court and, if made following the Meeting, approved by the Court; and |
|
|
|
|
|
| (c) | communicated to FSD Pharma Securityholders if and as required by the Court (if so required). |
Section 5.2 Amendment Made Prior to or at the Meeting
Any amendment, modification or supplement to this Plan of Arrangement may be proposed by FSD Pharma at any time prior to or at the Meeting, with or without any other prior notice or communication (except to the extent required by the Court), and if so proposed and accepted by the persons voting at the Meeting (other than as may be required under the Interim Order), shall become part of this Plan of Arrangement for all purposes.
| 33 |
Section 5.3 Amendment After the Meeting
Any amendment, modification or supplement to this Plan of Arrangement which is approved by the Court following the Meeting shall be effective only:
|
| (a) | if it is consented to by FSD Pharma and Celly Nu; and |
|
|
|
|
|
| (b) | if required by the Court or applicable law, it is consented to by the FSD Pharma Securityholders, as applicable, voting in the manner directed by the Court. |
|
|
|
|
Section 5.4 Amendment After the Effective Date
Any amendment, modification or supplement to this Plan of Arrangement may be made following the Effective Date unilaterally by FSD Pharma, provided that it concerns a matter which, in the reasonable opinion of FSD Pharma, is of an administrative or ministerial nature required to better give effect to the implementation of this Plan of Arrangement and is not adverse to the financial or economic interest of any holder of FSD Pharma Securities or Celly Nu Shares and such amendments, modifications or supplements to the Plan of Arrangement need not be filed with Court or communicated to the FSD Pharma Securityholders.
ARTICLE 6 FURTHER ASSURANCES
Section 6.1 Further Assurances
Notwithstanding that the transactions and events set out herein shall occur and be deemed to occur at the time and in the manner set out in this Plan of Arrangement without any further act or formality, FSD Pharma and Celly Nu shall make, do and execute, or cause to be made, done or executed, all such further acts, deeds, agreements, transfers, assurances, instruments or documents as may reasonably be required by any of them in order to further document or evidence any of the transactions or events set out herein.
ARTICLE 7
TERMINATION
Section 7.1 Termination
Notwithstanding any prior approvals by the Court or by the FSD Pharma Securityholders, the Board of Directors of FSD Pharma may decide not to proceed with the Arrangement and to revoke the Arrangement Resolution adopted at the Meeting without further approval of the Court or the FSD Pharma Securityholders.
| 34 |
SCHEDULE “A”
ARRANGEMENT RESOLUTION
BE IT RESOLVED AS A SPECIAL RESOLUTION OF THE FSD PHARMA SECURITYHOLDERS THAT:
|
| 1. | The arrangement (the “Arrangement”) under Section 182 of the Business Corporations Act (Ontario) (the “OBCA”) involving FSD Pharma Inc., a corporation existing under the laws of the province of Ontario (“FSD Pharma”), its shareholders and warrantholders and Celly Nutrition Corp., a corporation existing under the laws of the Province of British Columbia (“Celly Nu”), all as more particularly described and set forth in the management information circular (the “Circular”) of FSD Pharma accompanying the notice of meeting (as the Arrangement may be, or may have been, modified or amended in accordance with its terms), is hereby authorized, approved and adopted. |
|
|
2. |
The plan of arrangement (the “Plan of Arrangement”) implementing the Arrangement, the full text of which is set out in Schedule “A” of the Circular (as the Plan of Arrangement may be, or may have been, modified or amended in accordance with its terms), is hereby authorized, approved and adopted. |
|
|
3. |
The arrangement agreement (the “Arrangement Agreement”) between FSD Pharma and Celly Nu and all the transactions contemplated therein, the actions of the directors of FSD Pharma in approving the Arrangement and the actions of the directors and officers of FSD Pharma in executing and delivering the Arrangement Agreement and any amendments thereto are hereby ratified and approved. |
|
|
4. |
Notwithstanding that this resolution has been passed (and the Arrangement approved) by the FSD Pharma Securityholders or that the Arrangement has been approved by the Ontario Superior Court of Justice, the directors of FSD Pharma are hereby authorized and empowered, without further notice to, or approval of, the FSD Pharma Securityholders: |
| 35 |
|
|
(a) |
to amend the Arrangement Agreement or the Plan of Arrangement to the extent permitted by the Arrangement Agreement or the Plan of Arrangement; or |
|
| (b) | subject to the terms of the Arrangement Agreement, not to proceed with the Arrangement; |
|
|
|
|
|
| in each case without further approval of the securityholders of FSD Pharma. |
|
|
| 5. | FSD Pharma is hereby authorized to apply for a final order from the Ontario Superior Court of Justice to approve the Arrangement on the terms set forth in the Arrangement Agreement and the Plan of Arrangement (as they may be, or may have been, modified, supplemented, or amended in accordance with their respective terms). |
|
|
|
|
|
| 6. | Any director or officer of FSD Pharma is hereby authorized and directed, for and on behalf and in the name of FSD Pharma, to execute and deliver, whether under the corporate seal of FSD Pharma or otherwise, all such deeds, instruments, assurances, agreements, forms, waivers, notices, certificates, confirmations and other documents and to do or cause to be done all such other acts and things as in the opinion of such director or officer may be necessary, desirable or useful for the purpose of giving effect to these resolutions, the Arrangement Agreement and the completion of the Plan of Arrangement in accordance with the terms of the Arrangement Agreement, including: |
|
| (a) | all actions required to be taken by or on behalf of FSD Pharma, and all necessary filings and obtaining the necessary approvals, consents, and acceptances of appropriate regulatory authorities; and |
|
|
(b) |
the signing of the certificates, consents and other documents or declarations required under the Arrangement Agreement or otherwise to be entered into by FSD Pharma, |
|
|
|
|
|
| such determination to be conclusively evidenced by the execution and delivery of such document, agreement, or instrument or the doing of any such act or thing. |
|
| 36 |
SCHEDULE “B”
FSD PHARMA DISTRIBUTION WARRANTS PROVISION
Pro Rata Distributions. During such time as this Warrant is outstanding, if the Company shall declare or make any dividend or other distribution of its assets (or rights to acquire its assets) to holders of Class B Shares, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction) (a “Distribution”), at any time after the issuance of this Warrant, then, in each such case the Holder shall be entitled to participate in such Distribution to the same extent that the Holder would have participated therein if the Holder had held the number of Class B Shares acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date of which a record is taken for such Distribution, or, if no such record is taken, the date as of which the record holders of Class B Shares are to be determined for the participation in such Distribution (provided, however, to the extent that the Holder’s right to participate in any such Distribution would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Distribution to such extent (or in the beneficial ownership of any Class B Shares as a result of such Distribution to such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
| 37 |
EXHIBIT 4.3
EXCLUSIVE INTELLECTUAL PROPERTY LICENSE AGREEMENT
THIS AGREEMENT is made on July 31, 2023,
| BETWEEN
| FSD PHARMA INC., a corporation existing pursuant to the laws of the Province of Ontario and having an office located at [redacted for confidentiality purposes] (“FSD”)
|
| AND
| CELLY NUTRITION CORP., a corporation existing pursuant to the laws of the Province of British Columbia and having an office located at [redacted for confidentiality purposes] (the “Licensee”)
|
| AND
| Lucid PsycheCeuticals Inc., a corporation existing pursuant to the laws of the Province of Ontario and having an office located at [redacted for confidentiality purposes] (“Lucid” and together with FSD, “Licensor”) |
RECITALS:
| A. | Licensor is in the business of, among other things, granting licences to the registered trademarks “ALCOHOLDEATH™” and “UNBUZZD™” in the dietary supplement and natural health product industry for the recreational market, as registered, licensed or retained by common law by FSD’s wholly owned subsidiary, Lucid, and including without limitation, pursuant to the intellectual property registrations listed on Schedule “A” hereto (the “Licensed IP”). |
|
|
|
| B. | The Licensee is entering the business of producing, marketing and selling dietary supplement and natural health products for recreational use worldwide (the “Territory”). |
|
|
|
| C. | Licensor is the sole and exclusive owner of the Licensed IP; |
|
|
|
| D. | The Licensee has requested that Licensor grant it an exclusive licence to utilize the Licensed IP and Commercialize the Licensed Products (as defined below) and the Licensed IP in the Territory in connection with the Business (as defined below). |
|
|
|
| E. | Licensor is willing to grant, and the Licensee is willing to accept, an exclusive licence to the Licensed IP for the producing, processing, packaging, marketing and sales of recreational products, as proposed by Licensee and approved in writing by Licensor, in its sole discretion, acting reasonably and in good faith, and distributed, marketed and sold by Licensee exclusively in the Territory (as applicable) and exclusively under the Licensed IP (the “Licensed Products”). |
NOW IT IS AGREED, in consideration of the respective covenants and agreements herein contained and other good and valuable consideration (the receipt and sufficiency of which are hereby acknowledged), as follows:
| 1 | DEFINITIONS AND INTERPRETATION |
|
|
|
| 1.1 | Definitions |
In this Agreement, unless there is something in the context or subject matter inconsistent therewith, the following defined terms have the meanings hereinafter set out:
"Affiliate" has the meaning given in the Corporations Act.
“Agreement” means this exclusive intellectual property license agreement, including all schedules, exhibits and all amendments or restatements, as permitted.
“Anti-Dilution Warrant Certificate” means the warrant certificate containing certain anti-dilution provisions, substantially in the form attached hereto as Schedule “B”;
“Associate” has the meaning given to that term in the Corporations Act and also includes any individual who, or any corporation or other form of business organisation which, in any country directly or indirectly (including through intermediaries), is Controlled by, or is under common Control with, or Controls, a party.
| 1 |
“Board” means the board of directors of the Licensee.
“Business” means the business of producing, processing, packaging, marketing and sales of recreational products worldwide, from time to time.
“Business Day” means a day on which the banks are open for business in Toronto, Ontario other than a Saturday, Sunday or public holiday in Toronto, Ontario.
“Change of Control” means, with respect to a Party, directly or indirectly (i) an acquisition of such Party by another entity by means of any transaction or series of related transactions (including, without limitation, any reorganization, merger or consolidation but excluding any merger effected exclusively for the purpose of changing the domicile of such Party), or (ii) a sale of all or substantially all of the assets of such Party, so long as in either case such Party’s equity holders of record immediately prior to such event will, immediately thereafter, hold less than fifty percent (50%) of the voting power of the surviving or acquiring entity.
“Claim” includes a claim, notice, demand, action, proceeding, litigation, investigation, judgment, damage, Loss, cost, expense or liability however arising, whether present, unascertained, immediate, future or contingent, whether based in contract, tort or statute and whether involving a third party or a party to this Agreement.
“Commercialize” means to:
|
| (a) | in relation to a patented product, to use, make, manufacture, have made or manufactured, sell, advertise, promote, distribute, hire or otherwise dispose of the product, or to keep if for the purpose of doing any of those things; |
|
|
|
|
|
| (b) | in relation to a patented method or process, to use the method or process or do any act referred to above in respect of a product resulting from such use; |
|
|
|
|
|
| (c) | in relation to an article the subject of a registered design, to exercise any of the rights exclusive to the owner of the registered design; |
|
|
|
|
|
| (d) | in relation to any work or other subject matter the subject of copyright, to exercise any of the rights exclusive to the owner of the copyright; and |
|
|
|
|
|
| (e) | exercise any other Intellectual Property Rights exclusive to Licensor with respect to the Licensed IP, |
and “Commercialization” has a referable meaning.
“Commercialization Revenue” means the total gross invoiced prices (or if no invoice is generated for a sale, the total sales price) of Licensed Products sold by the Licensee or its Associates, less the sum of the following actual and customary deductions where applicable and separately listed:
|
| (a) | cash, trade or quantity discounts; |
|
|
|
|
|
| (b) | sales, use, tariff, import/export duties or other taxes or levies (other than income tax) imposed on sales; |
|
|
|
|
|
| (c) | credits to customers because of rejections or returns; |
|
|
|
|
|
| (d) | markdown support for discontinued items; and |
|
|
|
|
|
| (e) | trade funding and retailer charges including slotting fees, listing fees, category line review fees, coop funding, supplier merchandising allowances, administrative fees, new item fees, promotion scan backs, promotion advertising fees; |
and includes the fair market value of all other consideration received by the Licensee or its Associates in respect of Licensed Products, whether such consideration is in cash, payment in kind, exchange or other form. For the purpose of transfers between the Licensee and its Associates the amount will be the greater of the amount payable by the Affiliate to the Licensee (if any) and the amount the Affiliate sells the Licensed Products upon an ultimate sale to a third party.
| 2 |
“Confidential Information” means all information that a party receives or otherwise becomes aware of and that the party ought reasonably know is regarded as confidential by the Disclosing Party, and includes any information in relation to a party's business, operations, dealings, employees, students, contractors, finances, policies, plans, inventions, discoveries, research, technology or Intellectual Property Rights, irrespective of whether that information was disclosed before, on or after the date of this Agreement.
“Control” has the meaning given in the Corporations Act.
“Corporations Act” means the Business Corporations Act (Ontario);
“Disclosing Party” means a party that is disclosing Confidential Information or of whose Confidential Information another party becomes aware.
“Dispute” means a dispute, controversy or Claim arising out of, relating to or in connection with this Agreement or the Licence, including any question regarding the breach or termination of this Agreement or the Licence.
“Effective Date” means the date of this Agreement.
“Financial Year” means the financial year of the Licensee, being the financial year ending July 31.
“Go-Public Transaction” means (i) a transaction with a capital pool corporation or other entity that is a reporting issuer in at least one jurisdiction of Canada by way of plan of arrangement, amalgamation, reverse take-over, qualifying transaction, or any other business combination or similar transaction pursuant to which securities of the Licensee (or securities of the resulting issuer) are listed on a recognized stock exchange in Canada or the United States, (ii) an initial public offering or other similar transaction (including a privately placed offering followed by a listing of securities) and listing of securities of the Licensee on a recognized stock exchange in Canada or the United States, or (iii) an acquisition, whether by merger, amalgamation, plan of arrangement, share purchase or other similar transaction by a person, or group of persons, of all of the outstanding securities of the Licensee.
“Government Agency” means any government, governmental, semi-governmental, administrative, fiscal or judicial body department, commission, authority, tribunal, agency or entity (including those constituted or formed under any statute) where the department, entity, agency, authority, commission, corporation or body is subject to the control or direction of any government in a country where the relevant party to this Agreement operates.
“Gross Profits” means, for each Financial Year or other period, an amount equal to the Licensee’s total income (or loss) for such year or period, determined in accordance with generally accepted accounting principles.
“Improvements” means any improvement, variation, modification, adaptation or further development of the Licensed IP developed by the Licensee or Licensor, or both.
“Insolvency Event” means the occurrence of any one or more of the following events regarding any party to this Agreement:
|
| (a) | a meeting has been convened, resolution proposed, petition presented or order made for the winding up of that party; |
|
|
|
|
|
| (b) | a receiver, receiver and manager, provisional liquidator, liquidator, or other officer of the Court, or other person of similar function has been appointed regarding all or any material asset of the party; |
|
|
|
|
|
| (c) | a security holder, mortgagee or chargee has taken attempted or indicated an intention to exercise its rights under any security of which the party is the security provider, mortgagor or chargor; or |
|
|
|
|
|
| (d) | an event has taken place with respect to the party which would make, or deem it to be, insolvent under any law applicable to it. |
| 3 |
“Intellectual Property Rights” means all present and future rights conferred by statute, common law or equity in any jurisdiction in or in relation to any registered or unregistered industrial or intellectual property rights anywhere in the world including any rights in respect of:
|
| (a) | patents or rights concerning any discovery, invention, process, process improvement, procedure, manufacturing method, technique or information regarding the chemical or genetic composition of materials (whether patentable or not); |
|
|
|
|
|
| (b) | confidential information and any right to have such information kept confidential; |
|
|
|
|
|
| (c) | trademarks, business names or trading styles (whether registered or not); |
|
|
|
|
|
| (d) | copyright material and similar or neighbouring rights; |
|
|
|
|
|
| (e) | data or databases; |
|
|
|
|
|
| (f) | registered or registrable designs; |
|
|
|
|
|
| (g) | plant breeder rights or other proprietary information concerning genetic or biological material or engineering processes; and |
|
|
|
|
|
| (h) | eligible layouts or protectable computer programs; and |
|
|
|
|
|
| (i) | other results of intellectual activity in the industrial, commercial, scientific, or literary or artistic fields, as well as any right to seek registration of, or to take action for infringement of, any such rights. |
“knowledge of” (or similar phrases) means, (i) with respect to Licensor, the actual knowledge of its directors and officers, after reasonable investigation and due enquiry, or (ii) with respect to Licensee, the actual knowledge of its directors and officers, after reasonable investigation and due enquiry;
“Licence” means the licence granted in section 2.
“License Fee” has the meaning given to that term in section 5.
“Licensed IP” means all of Licensor's Intellectual Property Rights relating to the Licensed Products under development, specifically in relation to the licensed trademarks “ALCOHOLDEATH™” and “UNBUZZD™, including any information in connection with the trademark applications, label designs, manufacturing, marketing and sales of the Licensed Products, for the consumer market in the Territory, as further described in Schedule “A”.
“Licensed IP Purchase Proposal” has the meaning given to that term in section 7.
“Licensed Product” means any consumable recreational health supplement or product that utilizes or is derived from the Licensed IP that Licensor has authorized Licensee to cause to have produced, marketed and sold in the Territory, pursuant to and in accordance with this Agreement, and shall not include Wholesale Apparel & Promotional Material.
“Litigation Matter” means the ongoing litigation matter involving FSD, as further set forth in Schedule “C” hereto.
“Loan Agreement” means the loan agreement between the Licensee and Licensor pursuant to which the Licensee is loaned $1,000,000 in aggregate with such monies to be used as working capital.
“Loan Repayment Date” means the date that is the 3rd anniversary of the “Commencement Date” (as that term is defined in the Loan Agreement.
“Loss” includes any loss, damage, cost, charge, liability (including Tax liability) or expense (including legal costs and expenses).
“Moral Rights” means rights of integrity, rights of attribution and other rights of an analogous nature which may now exist or which may exist in the future under the Copyright Act (R.S.C., 1985, c. C-42) or under the law of a country other than Canada.
| 4 |
“Quarter” means a three-month period ending on March 31, June 30, September 30 and December 31.
“Recipient” means a party receiving or that has received Confidential Information from a Disclosing Party.
“Royalty” means an amount equal to 7% of the Commercialization Revenue generated by the Licensee each Quarter during the Term until a total amount of Royalty in the amount of $250,000,000 has been paid to Licensor at which point the rate is reduced to 3%.
“Tax” means all forms of taxes, duties, imposts, charges, withholdings, rates, levies or other governmental impositions of whatever nature and by whatever authority imposed, assessed or charged together with all costs, charges, interest, penalties, fines, expenses and other additional statutory charges, incidental or related to the imposition.
“Term” means the term of this Agreement as described in section 20.1.
“Third Party Licensor” means any third party from which Licensor has received a license or sublicense for the right to Commercialize the Licensed IP.
“UNBUZZD™ Intellectual Property Rights” has the meaning given to that term in section 7.
“Wholesale Apparel & Promotional Material” means any products, other than Licensed Products, approved by Licensor and bearing the Licensed IP, sold or physically displayed by Licensee, including, but not limited to, all merchandise related to the Licensed Products (e.g., clothing, hats, accessories, etc.), physical marketing collateral, event sponsorship collateral, brand ambassador content, celebrity endorse material, advertising across all forms of media (including social media and other forms of digital media).
| 1.2 | INTERPRETATION |
In this Agreement, unless the context otherwise requires:
|
| (a) | a reference to: |
|
| (i) | the singular includes the plural and the plural includes the singular; |
|
|
|
|
|
| (ii) | words importing gender includes all genders; |
|
|
|
|
|
| (iii) | a recital, section, schedule or annexure is a reference to a section of or recital, schedule or annexure to this Agreement and references to this Agreement include any recital, schedule or annexure; |
|
|
|
|
|
| (iv) | any contract (including this Agreement) or other instrument includes any variation or replacement of it and as it may be assigned or novated; |
|
|
|
|
|
| (v) | a statute, ordinance, code or other law includes subordinate legislation (including regulations) and other instruments under it and consolidations, amendments, re-enactments or replacements of any of them; |
|
|
|
|
|
| (vi) | a person or entity includes an individual, a firm, a body corporate, a trust, an unincorporated association or an authority; |
|
|
|
|
|
| (vii) | a person includes their legal personal representatives (including executors), administrators, successors, substitutes (including by way of novation) and permitted assigns; |
|
|
|
|
|
| (viii) | a group of persons is a reference to any two or more of them taken together and to each of them individually; |
|
|
|
|
|
| (ix) | an entity which has been reconstituted or merged means the body as reconstituted or merged, and to an entity which has ceased to exist where its functions have been substantially taken over by another body, means that other body; |
|
|
|
|
|
| (x) | a reference to a day or a month means a calendar day or calendar month; |
| 5 |
|
| (b) | unless expressly stated, no party enters into this Agreement as agent for any other person (or otherwise on their behalf or for their benefit); |
|
|
|
|
|
| (c) | the meaning of any general language is not restricted by any accompanying example, and the words ‘includes’, ‘including’, ‘such as’, ‘for example’ or similar words are not words of limitation; |
|
|
|
|
|
| (d) | the words ‘costs’ and ‘expenses’ include reasonable charges, expenses and legal costs on a full indemnity basis; |
|
|
|
|
|
| (e) | headings and the table of contents are for convenience only and do not form part of this Agreement or affect its interpretation; |
|
|
|
|
|
| (f) | if a period of time is specified and dates from a given day or the day of an act or event, it is to be calculated exclusive of that day; |
|
|
|
|
|
| (g) | the time between two days, acts or events includes the day of occurrence or performance of the second but not the first day act or event; |
|
|
|
|
|
| (h) | if the last day for doing an act is not a Business Day, the act must be done instead on the next Business Day; and |
|
|
|
|
|
| (i) | a provision of this Agreement must not be construed to the disadvantage of a party merely because that party was responsible for the preparation of this Agreement or the inclusion of the provision in this Agreement. |
| 2 | GRANT OF LICENCE |
| 2.1 | From the Effective Date, Licensor grants the Licensee an exclusive, irrevocable, sublicensable right, licence and privilege to use and Commercialize the Licensed IP (including the goodwill associated therewith) in the Territory in or in association with the Business, specifically for the manufacture, marketing, sale and distribution of the Licensed Products within the Territory for the duration of the Term. |
|
|
|
| 2.2 | The Licensee must not Commercialize the Licensed IP other than in accordance with this Agreement. |
|
|
|
| 2.3 | The Licensee agrees not to: |
|
|
|
|
| (a) | use the Licensed IP for any purpose or in any manner that exceeds the scope of the Business and the rights of the licence granted to the Licensee hereunder; |
|
|
|
|
|
| (b) | use and the Licensed IP outside of the Territory; or |
|
|
|
|
|
| (c) | supply Licensed Products for use, sale, distribution, or resupply outside of the Territory. |
| 2.4 | Licensor agrees to: |
|
| (a) | make all Licensed IP available to the Licensee in accordance with this Agreement; |
|
|
|
|
|
| (b) | provide training and instruction for the use of the Licensed IP to select officers, employees, contractors and consultants of the Licensee, as reasonably required by the Licensee for the operation of the Business and in a manner consistent with the commercially sensitive nature of the Licensed IP; |
|
|
|
|
|
| (c) | obtain and maintain intellectual property protections for the Licensed IP in the Territory; and |
|
|
|
|
|
| (d) | keep confidential the Licensed IP and ensure that it does not disclose the Licensed IP to any person in the Territory other than the Licensee or its designees, unless otherwise compelled by any applicable law. |
| 6 |
| 2.5 | Licensor will, if requested by the Licensee, procure that its employees consent to the infringement of any Moral Rights subsisting with respect to the Licensed IP, other than the right not to have work falsely attributed. |
|
|
|
| 3 | EXCLUSIVITY |
|
|
|
| 3.1 | The parties acknowledge and confirm that, subject always to sections 2 and 4, the Licensee shall not grant any other person any right or licence to Commercialize the Licensed IP in the Territory in connection with: |
|
| (a) | any good, service, or product which is the same or substantially similar to the Licensed Products; |
|
|
|
|
|
| (b) | any good, service, or product which is prohibited by applicable laws or in respect of which such activities are prohibited by applicable laws; or |
|
|
|
|
|
| (c) | any good, service, or product, in any manner, without all necessary licenses, permits and authorizations required under applicable laws (for greater certainty, including though channels not authorized by applicable laws (i.e., the “black market”). |
| 3.2 | Nothing herein restricts Licensor's use of the Licensed IP outside the Territory. |
|
|
|
| 4 | SUB-LICENCING |
|
| (a) | The Licensee may sub-license the Licensed IP in the Territory only in accordance with this Agreement provided the Licensee provides prior written notice to Licensor. |
|
|
|
|
|
| (b) | Any sub-licence agreement must not grant any rights to the sub-licensee which are inconsistent with the scope of the rights granted by this Agreement and must impose: |
|
| (i) | terms on the sub-licensee that are consistent with the terms set out in this Agreement; |
|
|
|
|
|
| (ii) | confidentiality obligations on the sub-licensee which are no less onerous than the confidentiality obligations set out in section 18 of this Agreement; |
|
| (c) | The Licensee must provide Licensor with a full copy of the executed sub-licence between the Licensee and any sub-licensee within fourteen (14) days after execution of such agreements. Licensor agrees not to disclose the terms of such agreements to a third party unless required by law. |
|
|
|
|
|
| (d) | Any sub-licences granted by the Licensee under this section 4 will automatically terminate upon the termination of this Agreement. |
| 5 | LICENSE FEE AND ROYALTY |
|
|
|
| 5.1 | The Licensee agrees to pay to Licensor a license fee (the “License Fee”) on and from the Effective Date, payable as follows: |
|
| (a) | The issuance of 100,000,0000 common shares in the capital of the Licensee on the Effective Date, to be registered and delivered as directed by Licensor in writing; and |
|
|
|
|
|
| (b) | The issuance of the executed Anti-Dilution Warrant Certificate, to be registered and delivered as directed by Licensor in writing. |
| 5.2 | The Licensee agrees to pay to Licensor the Royalty on and from the date the Licensee commences manufacture or procures the manufacture of the Licensed Products in the Territory using the Licensed IP until the termination of this Agreement in accordance with section 20. |
|
|
|
| 5.3 | Royalties are to be paid by the Licensee within twenty-five (25) Business Days after the end of each Quarter and in each case. |
| 7 |
| 5.4 | For the avoidance of doubt, no Royalty will be payable unless and until the Licensee commences manufacture or procures the manufacture of the Licensed Products in the Territory using the Licensed IP. |
|
|
|
| 5.5 | The parties agree that the Royalty will be calculated on the basis of Commercialization Revenue, determined through end of Financial Year reconciliations provided by the Licensee and approved by the Board. |
|
|
|
| 5.6 | No Royalty will be payable for the use of Licensed IP for Wholesale Apparel & Promotional Material. The Licensee is granted the right to utilize the Licensed IP solely for the purpose of creating and distributing Wholesale Apparel & Promotional Material, including but not limited to advertisements, marketing campaigns, press releases, and social media content, without incurring any financial obligations towards the Licensor in the form of Royalties or other related fees. |
|
|
|
| 6 | REVENUE REPORTS |
|
|
|
| 6.1 | Each payment of License Fees and Royalties to Licensor must be accompanied by a report that sets out: |
|
| (a) | the basis upon which Commercialization Revenue was calculated by the Licensee including the number of units of each particular Licensed Product sold; |
|
|
|
|
|
| (b) | the basis upon which the License Fees and Royalties were calculated by the Licensee; |
|
|
|
|
|
| (c) | the License Fees and Royalties payable by the Licensee to Licensor for the relevant Quarter; and |
|
|
|
|
|
| (d) | any other additional details as may reasonably required by Licensor from time to time. |
| 6.2 | All royalty payments are to be made in Canadian dollars, to the bank account nominated by Licensor. Licensor must bear any currency conversion charges and bank charges incurred by the Licensee. |
|
|
|
| 7 | RIGHT OF FIRST OFFER AND CHANGE OF CONTROL |
|
|
|
| 7.1 | FSD shall retain the right to make a bona fide offer to the Licensee with respect to the repurchase of the Licensed IP. In the event that the FSD wishes to proceed with purchasing the Licensed IP, FSD shall give notice in writing to Licensee stating the terms, conditions and purchase price of such purchase proposal (the “IP Purchase Proposal”), and Licensee shall offer to FSD the exclusive right and opportunity to negotiate a potential purchase of the Licensed IP on mutually acceptable terms between FSD and the Licensee, acting reasonably. |
|
|
|
| 7.2 | FSD shall grant Licensee the right to purchase the Intellectual Property Rights with respect to the UNUZZD™ trademark (the “UNBUZZD™ Intellectual Property Rights”), on terms, conditions, and at a purchase price to be negotiation between FSD and the Licensee at such time, in the event that the Board votes to approve of a Licensee Change of Control or a Go-Public Transaction. In the event of a Change of Control of the Licensee or the consummation of a Go-Public Transaction by the Licensee, the Licensee’s obligation to pay the Royalty in respect of the Licensed IP will survive at a reduced rate of 3%, until a total amount of Royalty in the amount of $250,000,000 has been paid to Licensor. In the event of a Change of Control of the Licensee, the Licensee’s Board may negotiate with FSD to reduce or eliminate the Royalty. |
|
|
|
| 7.3 | In the event of a Change of Control in respect of FSD, all right, title and interest in and to the UNBUZZD™ Intellectual Property Rights shall be transferred to the Licensee, and the Licensor will use its commercially reasonable efforts to assist with the transfer of those UNBUZZD™ Intellectual Property Rights to the Licensee. |
| 8 |
| 8 | RECORDS, INSPECTION AND REPORTING |
|
|
|
| 8.1 | The Licensee agrees to keep true and accurate records in relation to all matters connected with the Licence, this Agreement and the Commercialization of the Licensed IP, including all records and accounts (maintained in accordance with generally accepted accounting standards) relevant to: |
|
| (a) | prosecution and maintenance of the registration of any Licensed IP and any other Intellectual Property Rights subsisting with respect to the Licensed IP; |
|
|
|
|
|
| (b) | the Licensee's progress in Commercialising the Licensed IP; |
|
|
|
|
|
| (c) | the Licensee's development and business plans in respect of the Licensed IP; |
|
|
|
|
|
| (d) | exploitation of the Licensed IP by the Licensee and distributors; |
|
|
|
|
|
| (e) | Licensed Products created or supplied by the Licensee; |
|
|
|
|
|
| (f) | Commercialization Revenue; |
|
|
|
|
|
| (g) | royalties payable to Licensor; |
|
|
|
|
|
| (h) | other Commercialization agreements; and |
|
|
|
|
|
| (i) | any other matter which Licensor reasonably requires the Licensee to maintain records of. |
| 8.2 | Subject to receipt of not less than five (5) Business Days prior written notice, the Licensee agrees to make the records referred to in section 8.1 available electronically for Licensor (or its nominee). |
|
|
|
| 8.3 | If after a review of the Licensee's records, Licensor identifies a shortfall in the amount paid to it by the Licensee pursuant to this Agreement, the Licensee must pay the shortfall within thirty (30) days of receiving a written request by Licensor (such notice must include the calculations and working papers which identified the shortfall). If the shortfall paid for a particular period is more than 5% of the total amount payable to Licensor for that period, then the Licensee will also pay Licensor's reasonable costs and expenses in undertaking the review. |
|
|
|
| 8.4 | Within fourteen (14) days of request by Licensor, the Licensee must provide Licensor with a written report as to: |
|
| (a) | any of the matters in relation to which the Licensee is required to maintain records in accordance with section 8.1; |
|
|
|
|
|
| (b) | the status of any registrable Intellectual Property Rights in relation to the Licensed IP; and |
|
|
|
|
|
| (c) | any other matter reasonably requested by Licensor. |
| 8.5 | Within sixty (60) days of the Financial Year end, the Licensee must provide to Licensor an annual statement containing: |
|
| (a) | a description of all Licensed Products sold, delivered or otherwise disposed of, during that period; and |
|
|
|
|
|
| (b) | a detailed summary of Commercialization Revenue during that period showing all revenue received and all deductions applied in the calculation of the Commercialization Revenue. |
| 9 | LICENSEE'S OBLIGATIONS |
|
|
|
| 9.1 | The Licensee agrees to use its commercially reasonable efforts to Commercialize the Licensed IP and maximise distribution of the Licensed Products in the Territory during the Term. Further to this obligation, the Licensee agrees to: |
|
| (a) | use its commercially reasonable efforts to sell and market Licensed Products in the Territory; |
| 9 |
|
| (b) | use its commercially reasonable efforts to meet market demand for the Licensed Products in the Territory, including, but not limited to, ensuring the Licensed Product inventory remains sufficiently supplied at all times to meet forecasted demand (pursuant to metrics to be agreed upon by the parties) and Licensor will use its commercially reasonable best efforts to review, in a timely manner, the requests of Licensee which could reasonably have an impact on Licensee fulfilling its obligations hereunder. The parties would work together in good faith to have at least one (1) Licensed Product (available in multiple flavours) ready for sale as soon as possible following the execution of the Licensing Agreement, but in no event later than the date to be agreed upon by the parties in the Licensing Agreement. |
|
|
|
|
|
| (c) | obtain all necessary regulatory approvals in the Territory for the Commercialization of the Licensed Products; and |
|
|
|
|
|
| (d) | not engage in any Commercialization of the Licensed IP for any other purposes other than in accordance with this Agreement. |
| 9.2 | The Licensee will Commercialize the Licensed IP and ensure that it is Commercialized: |
|
| (a) | with all due care and skill and in a good and workmanlike manner; |
|
|
|
|
|
| (b) | in a manner which meets all legal requirements and specifications of any safety, quality or other standards and all product liability laws applicable where the particular Licensed Product is to be Commercialized; and |
|
|
|
|
|
| (c) | in accordance with high professional and ethical standards of behaviour. |
| 9.3 | The Licensee will enter into a distribution agreement with FSD to grant FSD the right to distribute the Licensed Product in Canada. |
|
|
|
| 10 | MUTUAL OBLIGATIONS |
|
|
|
| 10.1 | The parties agree to use their commercially reasonable efforts as follows: |
|
| (a) | the Licensee will use its commercially reasonable efforts to meet market demand for the Licensed Products in the Territory, including, but not limited to, ensuring the Licensed Product inventory remains sufficiently supplied at all times to meet forecasted demand (pursuant to metrics to be agreed upon by the parties); |
|
|
|
|
|
| (b) | Licensor will use its commercially reasonable best efforts to review, in a timely manner, the requests of Licensee which could reasonably have an impact on Licensee fulfilling its obligations hereunder; and |
|
|
|
|
|
| (c) | the parties will use their commercially reasonable efforts to have at least one (1) Licensed Product (available in multiple flavours) ready for sale as soon as possible following the execution of this Agreement, but in no event later than twelve (12) months following the Effective Date. |
| 11 | INTELLECTUAL PROPERTY OWNERSHIP |
|
|
|
| 11.1 | The Licensee acknowledges the validity of the Licensed IP and agrees that Licensor is the sole and exclusive owner of all Intellectual Property Rights subsisting with respect to the Licensed IP and nothing in this Agreement transfers any of those Intellectual Property Rights to the Licensee. |
|
|
|
| 11.2 | The Licensee acknowledges and agrees that all right, title and interest in and to the Improvements to the Licensed IP generated by the Licensee will be owned by Licensor and will immediately vest in Licensor. |
|
|
|
| 11.3 | The Licensee must not directly or indirectly: |
|
| (a) | use the Licensed IP as part of the name of any entity or trade name, domain name, or within any prefix, suffix, or other modifying words, terms, designs, or symbols, or in any modified form that is inconsistent with the terms of this Agreement; |
| 10 |
|
| (b) | challenge Licensor's ownership of the Intellectual Property Rights subsisting with respect to the Licensed IP; |
|
|
|
|
|
| (c) | challenge any Third Party Licensor's ownership of the Intellectual Property Rights subsisting with respect to any Licensed IP; |
|
|
|
|
|
| (d) | use the Licensed IP in connection with the sale of any unauthorized product or service other than the Licensed Products; |
|
|
|
|
|
| (e) | oppose, or assist any third party to oppose, the grant of any patent or other registered right pursuant to any application in relation to the Licensed IP; |
|
|
|
|
|
| (f) | dispute, or assist any third party to dispute, the validity of any Intellectual Property Rights subsisting with respect to the Licensed IP; or |
|
|
|
|
|
| (g) | engage, or assist, intentionally or recklessly, any third party to engage, in any other conduct, directly or indirectly, that would infringe upon, harm, mislead or contest the rights of Licensor in the Licensed IP or endanger the capacity of any Licensed IP to be protected by statutory registration, or that would threaten the validity of any such registration. |
| 11.4 | Licensor will be the sole owner of any Improvements, whether created by Licensee or Licensor, and may at its discretion apply for patents and other Licensed IP Rights anywhere in the world in its own name with respect to any Improvements. |
|
|
|
| 11.5 | If requested by the Licensee, Licensor must at the Licensor's cost promptly sign all documents and provide the Licensee with any information or assistance that the Licensee requires for the purpose of taking any action pursuant to section 11.4. |
|
|
|
| 11.6 | If requested by Licensor, the Licensee must at Licensor’s cost promptly sign all documents and provide Licensor with any information or assistance that Licensor requires for the purpose of taking any action pursuant to section 11.4. |
|
|
|
| 11.7 | To the extent necessary to give effect to section 11.2, the Licensee must assign, encumbrance free, on its creation or acquisition (at Licensor's sole cost) all rights, title and interest in the Intellectual Property Rights in any Improvements to the Licensed IP generated by the Licensee. |
|
|
|
| 11.8 | The Licensee shall not apply for any registrations of the Licensed IP in the name of the Licensee. |
| 12 | INTELLECTUAL PROPERTY PROTECTION |
|
|
|
| 12.1 | The Licensor shall apply for, prosecute and maintain such patent/s or other Licensed IP Rights with respect to the Licensed IP as are commercially and legally reasonable. |
|
|
|
| 12.2 | Any patent/s or other Licensed IP Rights with respect to the Licensed IP must be filed and registered in the name of Licensor. |
|
|
|
| 12.3 | Licensor will, at the cost of the Licensor, do everything reasonably necessary to assist the Licensee to obtain registration of registrable Licensed IP. |
|
|
|
| 12.4 | Licensor agrees that it will keep the Licensee informed of all progress in relation to any applications to register patents or other Intellectual Property Rights with respect to the Licensed IP including by instructing its patent attorneys to provide Licensee with copies of all documents and correspondence relating to filing, prosecution and maintenance and any oppositions or other challenges to validity. |
|
|
|
| 12.5 | If: |
|
| (a) | Licensee requests the Licensor in writing to file any patent or other Licensed IP Right with respect to any Licensed IP, and the Licensor declines or fails to do so within thirty (30) days from the request by Licensee; or |
|
|
|
|
|
| (b) | Licensor decides that it does not wish to continue to prosecute or maintain any patent or other Licensed IP Right with respect to any Licensed IP, it must provide Licensee with notice in writing at least thirty (30) days in advance and, |
| 11 |
| then: |
||
|
|
|
|
|
| (c) | Licensee may proceed to do so solely at its own expense; and |
|
|
|
|
|
| (d) | the Intellectual Property Rights relating to that application or registration will no longer be subject to the provisions of this Agreement, and the Licensor will have no rights in relation to the same (including under the Licence). |
| 12.6 | Licensor must pay directly all fees, costs and expenses (including patent attorney and legal fees and expenses) in connection with the filing, prosecution and maintenance of any patent or other Licensed IP Right with respect to any Licensed IP, including any costs and expenses incurred in dealing with any opposition to any applications for such registrations or any challenge to the validity of such registrations. |
|
|
|
| 13 | INTELLECTUAL PROPERTY ENFORCEMENT |
|
|
|
| 13.1 | The Licensee must promptly notify Licensor with respect to any: |
|
| (a) | infringement of any Intellectual Property Rights subsisting with respect to the Licensed IP in the Territory; or |
|
|
|
|
|
| (b) | opposition or challenge to the validity of any Intellectual Property Rights relating to the Licensed IP in the Territory, |
|
|
|
|
|
| that comes to the attention of the Licensee, and provide any information requested by FSD and which Licensee has knowledge with respect to any such infringement, opposition, or challenge. |
|
| 13.2 | Licensor will, at its sole discretion and at the cost of the Licensor, take action against any third party in respect of any infringement of any Intellectual Property Rights subsisting with respect to the Licensed IP as may be commercially and legally reasonable. |
|
|
|
| 13.3 | If, upon the Licensee's notification to the Licensor of any infringement, opposition, or challenge concerning the Licensed IP, the Licensor declines to take action against the relevant third party, the Licensee reserves the right, at its own discretion and expense, to initiate action against any third party for any infringement of the Intellectual Property Rights associated with the Licensed IP, provided that such action is deemed commercially and legally reasonable. |
|
|
|
| 13.4 | Subject to section 12.5, nothing in this Agreement compels either party to institute or prosecute proceedings or take any other action in relation to any: |
|
| (a) | third party infringement of any Intellectual Property Rights subsisting with respect to the Licensed IP; |
|
|
|
|
|
| (b) | defend any opposition, claim or cross-claim asserting invalidity of any Intellectual Property Rights subsisting with respect to the Licensed IP. |
|
|
|
|
| 13.5 | The Licensee must not agree to settle any litigation, opposition or challenge where such settlement will affect the rights of Licensor, without the prior written consent of Licensor. |
|
|
|
| 14 | COMMERCIALIZATION RESPONSIBILITIES AND RISK |
|
|
|
| 14.1 | The Licensee will at its sole cost and risk be responsible for all actions required to Commercialize the Licensed IP including but not limited to any further development required to ensure that the Licensed IP is commercial ready, manufacturing, assembly, testing, promotion, sales, delivery, installation, support, and returns. Licensor may continue to conduct research and development activities in the area of alcohol intoxication, and the development of products for the treatment of alcohol intoxication and related conditions, such products potentially having multiple applications across several market segments. Licensor will discuss such developments with the Licensee as they occur, and the Licensee will be granted the right to purchase the Intellectual Property Rights associated with such developments and technologies. |
| 12 |
| 14.2 | The Licensee will be responsible for negotiating directly with any third parties that the Licensee may require for further development of the Licensed IP. |
|
|
|
| 14.3 | The Licensee must: |
|
|
|
|
| (a) | Commercialize the Licensed IP in accordance with all applicable laws and regulatory requirements; |
|
|
|
|
|
| (b) | take all necessary steps to ensure that all Licensed Products are: |
|
|
|
|
|
| (i) | safe for their intended use; and |
|
|
|
|
|
| (ii) | manufactured or provided with all due care and skill and in a good and workmanlike manner and in a manner which meets all legal requirements and specifications of any quality or other standards and all product liability laws; and |
|
|
|
|
|
| (iii) | implement and maintain appropriate quality control standards in relation to its manufacturing and distribution systems. |
|
|
|
|
| 15 | WARRANTIES AND LIABILITY |
|
|
|
| 15.1 | Each party warrants that at the Effective Date: |
|
| (a) | it has full corporate power and authority to enter into, execute and perform its obligations under this Agreement; and |
|
|
|
|
|
| (b) | the execution of this Agreement and performance of its obligations under it will not breach any other agreement or obligation to which the party is a party or by which it is bound (including any obligation of confidence). |
|
|
|
|
| 15.2 | Subject to section 15.4, FSD warrants that as at the Effective Date: |
|
|
|
|
| (a) | so far as Licensor is aware, it has all necessary rights to grant the licence set out in section 2 to the Licensee; |
|
|
|
|
|
| (b) | so far as Licensor is aware, the Licensee’s use of the Licensed IP in accordance with this Agreement will not infringe the rights of any third party; |
|
|
|
|
|
| (c) | it has not granted any licences, or entered into arrangement, that would conflict with the rights granted to the Licensee under this Agreement; |
|
|
|
|
|
| (d) | it owns or has the right to license the Licensed IP both legally and beneficially; |
|
|
|
|
|
| (e) | subject to the grant of the licence to the Licensee in this Agreement, Licensor has the exclusive right to Commercialize the Licensed IP in the Territory; |
|
|
|
|
|
| (f) | neither the Licensed IP nor the Licensed IP is not encumbered, mortgaged or charged in any way, nor subject to any lien; |
|
|
|
|
|
| (g) | neither the Licensed IP nor the Licensed IP infringes any third party Intellectual Property Rights; and |
|
|
|
|
|
| (h) | other than the Litigation Matter, there is no litigation pending (of which Licensor has been made aware) in respect of the Licensed IP to which Licensor is a party, and there is no claim or demand that has been received by Licensor from any person in relation to the Licensed IP. |
|
|
|
|
|
| (i) | none of Licensor’s directors, officers or employees have ever been convicted of any criminal offence or, to the best of the Licensor’s knowledge, materially breached any laws, regulations or industry codes relating to responsible research practices, anti-bribery and corruption, anti-money laundering or fundamental human rights including any prohibitions on child labour, slavery, forced labour and human trafficking. |
| 13 |
| 15.3 | Licensor must not during the term assign, sell or transfer ownership of the Licensed IP unless it first notifies the Licensee and procures the transferee to enter into an agreement with the Licensee pursuant to which the transferee agrees to recognise the licence granted to the Licensee on the terms of this Agreement. |
|
|
|
| 15.4 | Licensor must not during the term assign, sell or transfer its rights to the Licensed IP unless it first notifies the Licensee and procures the transferee to enter into an agreement with the Licensee pursuant to which the transferee agrees to recognise the licence granted to the Licensee on the terms of this Agreement. |
|
|
|
| 15.5 | For the purposes of section 15.2, the parties acknowledge and agree that the awareness by Licensor is based on the best actual knowledge of the members of Licensor’s directors, officers and innovation commercial team involved in the negotiation of this Agreement and the inventors of the Licensed IP. |
|
|
|
| 15.6 | The Licensee warrants that as at the Effective Date: |
|
|
|
|
| (a) | no action or proceeding is pending or is threatened against Licensee before any court, administrative agency or other tribunal which could impact upon Licensee’s right, power and authority to enter into this Agreement or to carry out its obligations hereunder; |
|
|
|
|
|
| (b) | the Licensee has sufficient expertise, skills and financial, technical and business resources to fulfil its obligations under this Agreement; and |
|
|
|
|
|
| (c) | none of the Licensee’s directors, officers or employees have ever been convicted of any criminal offence or, to the best of the Licensee’s knowledge, materially breached any laws, regulations or industry codes relating to responsible research practices, anti-bribery and corruption, anti-money laundering or fundamental human rights including any prohibitions on child labour, slavery, forced labour and human trafficking. |
|
|
|
|
|
| (d) | neither Licensor nor any of its representatives have made any representations or warranties: |
|
|
|
|
|
| (i) | concerning the utility or patentability of any of the Licensed IP; |
|
|
|
|
|
| (ii) | to the effect that the exercise of any rights in Licensed IP or Licensed IP does not infringe the rights of third parties (other than as set out in section 15.2(b)); |
|
|
|
|
|
| (iii) | in relation to the potential profits that may be realised from Exploitation of the Licensed IP; or |
|
|
|
|
|
| (iv) | in relation to the quality, performance or capabilities of the Licensed Products; |
|
|
|
|
|
| (e) | the Licensee is responsible for satisfying itself of the matters referred to in section 15.6(d); and |
|
|
|
|
|
| (f) | the Licensee accepts the risks associated with the matters referred to in section 15.6(d) and bears the sole risk of Commercialising the Licensed IP, the quality or performance of Licensed Products or Services, or the claims of third parties arising from the Commercialization of such Licensed IP, or Licensed Products. |
|
|
|
|
| 15.7 | Notwithstanding any other section of this Agreement, the parties agree that Licensor is not liable to the Licensee and excludes all liability for consequential or incidental damages, third party claims or loss of profits, revenue, goodwill or opportunities in contract, tort, under any statute or otherwise (including negligence) directly or indirectly arising from or in any way directly or indirectly related to Licensor’s gross negligence, breach of this Agreement, breach of any law, in equity, under indemnities or otherwise directly or indirectly relating to the Licensed IP or Licensed Products or the subject matter of this Agreement. |
| 14 |
| 16 | RELEASE AND INDEMNITY |
|
|
|
| 16.1 | Each of the Licensor and the Licensee release and indemnify the other Party, its respective Associates and their respective officers, employees, consultants and agents, or any one of them (“Indemnitees”), from and against all actions, Claims, proceedings and demands (including those brought by third parties), arising out of or in connection with a breach of such Party’s warranties or obligations under this Agreement or the Commercialization of the Intellectual Property Rights subsisting with respect to the Licensed IP, including by its respective Associates. |
|
|
|
| 16.2 | Each of the Licensor and the Licensee indemnify and convent the other Party to keep indemnified each of the Indemnitees against all Claims whatsoever which may be taken, suffered or made against an Indemnitee or incurred or become payable by an Indemnitee in the course of, or in connection with any enforcement proceedings relating to the Intellectual Property Rights subsisting with respect to the Licensed IP (“Enforcement Proceedings”) or other proceedings which result or arise in connection with any Enforcement Proceedings, whether the Indemnitee is a party to such proceedings or otherwise. |
|
|
|
| 16.3 | Without limiting the generality of section 16.2, the each of the Licensor and the Licensee indemnify and keep indemnified each Indemnitee in respect of all Claims incurred or arising in connection with: |
|
|
|
|
| (a) | taking advice in respect of any Enforcement Proceedings, including reviewing and obtaining advice in relation to any Court processes such as attendance at any hearings and any documentation arising in connection with any Enforcement Proceedings, such as pleadings, evidence, judgments or other documents produced or drafted which may be used in the Enforcement Proceedings, including any of these in draft form; |
|
|
|
|
|
| (b) | obtaining advice in relation to whether or not to become a party to or participate in any Enforcement Proceedings; |
|
|
|
|
|
| (c) | any personnel giving evidence or otherwise becoming involved with the Enforcement Proceedings; |
|
|
|
|
|
| (d) | assisting or being involved in any way in relation to the Enforcement Proceedings (whether as a party to those proceedings or otherwise); and |
|
|
|
|
|
| (e) | any liabilities incurred by the Indemnitees (in their discretion) in respect of any of the matters set out in this section whether the Indemnitee's actions are in relation to existing matters or potential or apprehended matters. |
|
|
|
|
| 16.4 | Licensor agrees to indemnify and hold the Licensee harmless from any costs, damages, liabilities, and expenses (including legal fees) arising out of or related to the Litigation Matter. This indemnification shall apply to any claims, suits, or actions brought against the Licensee by third parties arising out of or related to the Litigation Matter. The Licensor shall assume all responsibility for defending the Litigation Matter and shall bear all costs and damages awarded against the Licensee, provided that the Licensee promptly notifies the Licensor in writing of any claim or legal action and cooperates fully in the defence of the Litigation Matter. However, the Licensor's indemnification obligation shall not apply if the Litigation Matter arises out of the Licensee's breach of this Agreement or any unauthorized actions or conduct by the Licensee. The Licensor's indemnification obligation under this clause shall survive the termination or expiration of this Agreement. |
|
|
|
| 16.5 | The indemnities in this section 16 are continuing obligations separate and independent from the other obligations of the parties. |
|
|
|
| 16.6 | It is not necessary for an Indemnitee to incur expense or make a payment before enforcing any indemnity conferred by this section 16. |
|
|
|
| 16.7 | This section 16 operates as a deed poll in favour of, and for the benefit of, each Indemnitee which is not a party to this Agreement and may be relied upon and enforced by each Indemnitee even if that Indemnitee is not a party to this Agreement. |
| 15 |
| 17 | INSURANCE |
|
|
|
| 17.1 | The Licensee must use its commercially reasonable efforts to take out, maintain and keep current, prior to the distribution of any Licensed Products, the following insurance from an insurer approved in advance by and reasonably acceptable to Licensor: |
|
|
|
|
| (a) | a comprehensive public liability policy with a commercially reasonable limit of liability; and |
|
|
|
|
|
| (b) | a product liability policy with a commercially reasonable limit of liability for each and every event from the time that Licensed Products are first sold. |
|
|
|
|
| 17.2 | The Licensee must, upon the written request of Licensor, provide Licensor with evidence of the currency of the insurance policies referred to in this section, within fourteen (14) days of the request. |
|
|
|
| 18 | CONFIDENTIALITY AND ANNOUNCEMENTS |
|
|
|
| 18.1 | Each party must keep the Confidential Information of the other party private and confidential and only use the Confidential Information of the other party for the purpose of performing its obligations or exercising its rights under this Agreement. |
|
|
|
| 18.2 | For clarity, it is agreed that the Licensee may disclose Licensor's Confidential Information to the extent reasonably required: |
|
|
|
|
| (a) | for the purpose of filing, prosecuting or maintaining any patent, permit, registration and/or licence relating to the Licensed IP; |
|
|
|
|
|
| (b) | in the exercise of its Commercialization rights granted under this Agreement; and |
|
|
|
|
|
| (c) | to sub-licensees of the Licensee. |
|
|
|
|
| 18.3 | A party may disclose Confidential Information to its employees, officers, contractors, consultants, agents, directors and professional advisers (including lawyers, auditors and accountants) who are bound by obligations of confidence to the party. A party may not otherwise disclose any Confidential Information to any third party without the prior written consent of the Disclosing Party. |
|
|
|
| 18.4 | The obligation of confidence contemplated by this section does not apply to Confidential Information: |
|
|
|
|
| (a) | that was already known to the Recipient as at the date of disclosure; |
|
|
|
|
|
| (b) | that was in the rightful possession of the Recipient independently of any obligation of confidence; |
|
|
|
|
|
| (c) | the Recipient can show is in the public domain otherwise than by breach of this Agreement or other obligation of confidence; or |
|
|
|
|
|
| (d) | that is required to be disclosed under applicable law or the rules of any stock exchange, but only if the Recipient has given the Disclosing Party all available notice to enable the Disclosing Party to attempt to remove that requirement and the Recipient only discloses the minimum information required. |
|
|
|
|
| 18.5 | A party may at any time after the expiration or termination of this Agreement, by notice in writing to the other party, require the delivery to it of its Confidential Information and any copies in the possession or control of the Recipient. The Recipient must comply with such a notice within fourteen (14) days. Any part or copy of the Confidential Information that cannot be conveniently delivered by the Recipient to the Disclosing Party must be completely destroyed or permanently deleted, provided that the Recipient may retain copies that are stored on the Recipient's computer backup systems until they are deleted in the ordinary course. The Recipient will continue to be bound by this section with respect to such retained Confidential Information. |
|
|
|
| 18.6 | No party to this Agreement will make or permit any of its personnel to make any public announcement or communication in connection with this Agreement without previously agreeing the contents with the other party. |
|
|
|
| 16 |
| 19 | USE OF PARTY'S NAMES |
|
|
|
|
| Each party agrees that it will not use the name or trademarks of the other party in any advertising or publicity material or make any form of representation or statement which could constitute an express or implied endorsement by such other party of any Licensed Products and will not authorise others to do so, without the prior written consent of the other party. Such consent shall not be unreasonably withheld, delayed, or conditioned. Any material Licensee submits to Licensor shall be deemed approved if not disapproved in writing by Licensor within five (5) business days. If Licensor disapproves any materials, Licensor will notice Licensee of the specific reasons and provide suggested corrections and additions required to gain approval of the materials. |
|
|
|
| 20 | TERM AND TERMINATION |
|
|
|
| 20.1 | Subject to section 20.2, this Agreement and the Licence will commence on the Effective Date and will continue unless this Agreement is terminated in accordance with this section 20. |
|
|
|
| 20.2 | Either party may by notice to the other party terminate this Agreement and the Licence in the following circumstances: |
|
|
|
|
| (a) | the other party is in breach of a material obligation under this Agreement: |
|
|
|
|
|
| (i) | that is not capable of remedy; or |
|
|
|
|
|
| (ii) | and does not remedy the breach within sixty (60) days of written notice being given to the other party requiring it to remedy the breach; or |
|
|
|
|
|
| (b) | if permitted by law, the other party suffers an Insolvency Event. |
|
|
|
|
| 20.3 | This Agreement may be terminated by Licensor if, in the event of a Change of Control of the Licensee, Licensor, in its sole discretion, acting reasonably and in good faith, does not approve of the acquirer in such Change of Control receiving the benefits of this Agreement. |
|
|
|
| 20.4 | Upon the expiration or termination of this Agreement and the Licence: |
|
|
|
|
| (a) | the Licensee and any sub-licensee will cease to have any rights to Commercialize any Licensed IP except to the extent necessary to exercise the rights conferred on the Licensee under section 20.4(c); |
|
|
|
|
|
| (b) | the Licensee must pay all amounts due to Licensor under this Agreement at termination, on the earlier of the due date or within sixty (60) days of expiration or termination; and |
|
|
|
|
|
| (c) | the Licensee will, for three (3) months or such longer period as agreed between the parties, after the date of termination have the right to Commercialize all stocks of Licensed Products in its possession or control as at the date of termination. |
|
|
|
|
| 21 | LIMITATION OF LIABILITY |
|
|
|
| 21.1 | Licensor acknowledges and agrees that the Licensee has made no representations to Licensor in relation to the profits that may be generated by the Licensee as a result of the grant of the licence in section 2 or as to the volume of orders that may be placed under any supply agreements between the parties. |
|
|
|
| 21.2 | To the maximum extent permitted by law, with the exception of the warranties expressly provided in this Agreement, all warranties, guarantees, conditions, representations and undertakings either express or implied are excluded. |
|
|
|
| 22 | DISPUTES |
|
|
|
| 22.1 | A party must not commence legal proceedings with respect to any Dispute unless it has first complied with this section 22. |
| 17 |
| 22.2 | A party claiming that a Dispute has arisen must notify each other party to the Dispute giving details of the Dispute. |
|
|
|
| 22.3 | Within five (5) Business Days after a notice is given under section 22.2, each party to the Dispute (Disputant) must nominate in writing a representative authorised to settle the Dispute on its behalf. |
|
|
|
| 22.4 | During the twenty (20) Business Day period after a notice is given under section 22.3 (or longer period agreed in writing by the Disputants) (“Initial Period”) each Disputant must use its best efforts to resolve the Dispute. |
|
|
|
| 22.5 | If the Disputants are unable to resolve the Dispute within the Initial Period, they must within an additional twenty (20) Business Days, either: |
|
|
|
|
| (a) | appoint a mediator to mediate the dispute; or |
|
|
|
|
|
| (b) | if the Disputants are unable to agree on a mediator, the Disputants will approach the appropriate branch of the Resolution Institute and request that it appoints an appropriate mediator to assist with the Dispute. |
|
|
|
|
| 22.6 | The role of the mediator is to assist in negotiating a resolution of the Dispute. The mediator must not make a decision that is binding on a Disputant unless that Disputant’s representative has so agreed in writing. |
|
|
|
| 22.7 | Any information or documents prepared for the mediation and disclosed by a representative under this section: |
|
|
|
|
| (a) | must be kept confidential; and |
|
|
|
|
|
| (b) | must not be used except to attempt to settle the Dispute. |
|
|
|
|
| 22.8 | Each Disputant must bear its own costs of resolving a Dispute under this section and, unless the Disputants otherwise agree, the Disputants must bear equally the costs of any mediator engaged. |
|
|
|
| 22.9 | If in relation to a Dispute, a Disputant breaches any provision of sections 22.1 to 22.5, each other Disputant need not comply with sections 22.1 to 22.5 in relation to that Dispute. |
|
|
|
| 23 | NOTICES |
|
|
|
| 23.1 | All notices, requests, consents, claims, demands, waivers, and other communications (other than routine communications having no legal effect) shall be in writing and shall be deemed to have been given (i) when delivered by hand (with written confirmation of receipt); (ii) when received by the addressee if sent by a nationally recognized overnight courier (receipt requested); (iii) when sent, if sent by electronic mail during the recipient’s normal business hours, and if not sent during normal business hours, then on the recipient’s next Business Day, and (iv) on the third day after the date mailed, by certified or registered mail, return receipt requested, postage prepaid. Such communications must be sent to the respective parties at the following addresses: |
If to FSD:
FSD Pharma Inc.
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
If to Lucid:
Lucid PsycheCeuticals Inc.
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
| 18 |
If to the Licensee:
Celly Nutrition Corp.
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
GENERAL
| 23.2 | Relationship |
|
|
|
|
| (a) | The relationship between the parties is that of and Intellectual Property Rights licensor and licensee, and not product manufacturer and distributor and not of franchisor and franchisee. |
|
|
|
|
|
| (b) | Nothing in this Agreement will be construed or interpreted to make one party the agent, partner, joint venturer or representative of another party. |
|
|
|
|
|
| (c) | A party must not at any time, without the prior written consent of the relevant party, act as or represent that it is the agent, partner, joint venturer or representative of any other Party. |
|
|
|
|
| 23.3 | Legal Costs |
|
|
|
|
| Except as expressly stated otherwise in this Agreement, each party must pay its own legal and other costs and expenses of negotiating, preparing, executing and performing its obligations under this Agreement. |
|
|
|
| 23.4 | Governing Law and Jurisdiction |
|
|
|
|
| (a) | This agreement is governed by and is to be construed in accordance with the laws applicable in Ontario, Canada. |
|
|
|
|
|
| (b) | Each party irrevocably and unconditionally submits to the non-exclusive jurisdiction of the courts of Ontario, Canada and any courts which have jurisdiction to hear appeals from any of those courts and waives any right to object to any proceedings being brought in those courts. |
|
|
|
|
| 23.5 | Severability |
|
|
|
|
| If a provision of this Agreement is illegal or unenforceable in any relevant jurisdiction, it may be severed for the purposes of that jurisdiction without affecting the enforceability of the other provisions of this Agreement. |
|
|
|
| 23.6 | Further Steps |
|
|
|
|
| Each party must promptly do whatever any other party reasonably requires of it to give effect to this Agreement and to perform its obligations under it. |
|
|
|
| 23.7 | Consents |
|
|
|
|
| Except as expressly stated otherwise in this Agreement, a party may conditionally or unconditionally give or withhold consent to be given under this Agreement and is not obliged to give reasons for doing so. |
|
|
|
| 23.8 | Rights Cumulative |
|
|
|
|
| Except as expressly stated otherwise in this Agreement, the rights of a party under this Agreement are cumulative and are in addition to any other rights of that party. |
| 19 |
| 23.9 | Waiver and Exercise of Rights |
|
|
|
|
| (a) | A single or partial exercise or waiver by a party of a right relating to this Agreement does not prevent any other exercise of that right or the exercise of any other right. |
|
|
|
|
|
| (b) | A party is not liable for any loss, cost or expense of any other party caused or contributed to by the waiver, exercise, attempted exercise, failure to exercise or delay in the exercise of a right. |
|
|
|
|
| 23.10 | Survival |
|
|
|
|
| The provisions of sections 1, 3, 9, 12, 15, 20.3, 23 and 24 of this Agreement survive the expiry or termination of this Agreement. |
|
|
|
| 23.11 | Amendment |
|
|
|
|
| This agreement may only be varied or replaced by a written agreement executed by the parties. |
|
|
|
| 23.12 | Assignment |
|
|
|
|
| (a) | The Licensee must not assign its interest in this Agreement without the prior written consent of Licensor. |
|
|
|
|
|
| (b) | Any purported dealing in breach of this section is of no effect. |
|
|
|
|
| 23.13 | Counterparts |
|
|
|
|
| This Agreement is properly executed if each party executes this Agreement or an identical document. In the latter case, this Agreement takes effect when the separately executed documents are exchanged between the parties. Delivery of an executed counterpart of this Agreement by portable document format file (PDF file) by facsimile or other electronic method of transmission will be effective as manual delivery of an executed counterpart of this Agreement. |
|
|
|
| 23.14 | Entire Understanding |
|
|
|
|
| (a) | This Agreement and the Loan Agreement contain the entire understanding between the parties as to the subject matter of this Agreement. |
|
|
|
|
|
| (b) | All previous negotiations, understandings, representations, warranties, memoranda or commitments concerning the subject matter of this Agreement are merged in and superseded by this Agreement and are of no effect. No party is liable to any other party in respect of those matters. |
|
|
|
|
|
| (c) | No oral explanation or information provided by any party to another affects the meaning or interpretation of this Agreement or constitutes any collateral agreement, warranty or understanding between any of the Parties. |
|
|
|
|
[Signature page follows]
| 20 |
IN WITNESS OF WHICH the parties hereto have executed this Agreement as of the date first written above.
|
| FSD PHARMA INC.
|
|
|
|
| By: | /s/ “Zeeshan Saeed” |
|
|
|
| Name: Zeeshan Saeed |
|
|
|
| Title: Chief Executive Officer |
|
|
|
|
|
|
|
| CELLY NUTRITION CORP.
|
|
|
|
| By: | /s/ “Binyomin Posen” |
|
|
|
| Name: Binyomin Posen |
|
|
|
| Title: Chief Executive Officer |
|
|
| Lucid PsycheCeuticals Inc.
|
|
|
| /s/ “Lakshmi P. Kotra” |
|
|
| Name: Dr. Lakshmi P. Kotra |
|
|
| Title: Chief Executive Officer |
|
| 21 |
EXHIBIT 4.4
WARRANT
UNLESS PERMITTED UNDER SECURITIES LEGISLATION, THE HOLDER OF THIS SECURITY MUST NOT TRADE THE SECURITY OR THE SECURITIES ISSUED UPON THE EXERCISE OF THIS SECURITY BEFORE THE DATE THAT IS 4 MONTHS AND A DAY AFTER THE July 31, 2023.
EXERCISABLE FOLLOWING THE DATE HEREOF AND PRIOR TO THE EXPIRY TIME (AS DEFINED BELOW) AT WHICH TIME THESE WARRANTS SHALL EXPIRE AND BE NULL AND VOID.
| Warrant Certificate No.: W-07-2023-01 | Original Issue Date: July 31, 2023 |
|
CELLY NUTRITION CORP. |
|
(A corporation incorporated under the laws of British Columbia) |
FOR VALUE RECEIVED, CELLY NUTRITION CORP., a corporation incorporated under the laws of the Province of British Columbia (the “Corporation”), hereby certifies that FSD PHARMA INC., a corporation duly incorporated under the laws of the Province of Ontario or its registered assigns (the “Holder”) is entitled to purchase from the Corporation, at any time following the date hereof and prior to the Expiry Time (as defined herein), a number of Common Shares (as defined herein) that would result in the Holder’s aggregate holdings of Common Shares, calculated as of the Record Date, equal to (a) 25% of the Common Shares Deemed Outstanding as of the Record Date less (b) the aggregate number of Common Shares previously issued (x) under the License Agreement (as defined below); and (y) from time to time as a result of any partial exercise of this Warrant in accordance with Section 3, in each case, at an exercise price determined by the prevailing market value, which in any event, in aggregate, shall be the aggregate price of no more than CAD$1 for all Common Shares issued pursuant to this Warrant (the “Exercise Price”), all subject to the terms, conditions and adjustments set forth below in this Warrant. For clarity, in no event shall Holder’s holdings in the Corporation exceed, at any time, more than an aggregate total of 25% of the Common Shares Deemed Outstanding, pursuant to this Warrant and the License Agreement. Certain capitalized terms used herein are defined in Section 1.
This Warrant has been issued under the terms of the Exclusive Intellectual Property License Agreement, dated as of July 31, 2023 (the “License Agreement”), between the Corporation and the Holder.
1. Definitions. As used in this Warrant, the following terms have the respective meanings set forth below:
“Aggregate Exercise Price” means an amount equal to the product of (a) the number of Warrant Shares in respect of which this Warrant is then being exercised under Section 3, multiplied by (b) the Exercise Price.
“Applicable Securities Laws” means, collectively, all applicable securities laws of each of the jurisdictions in which the Warrants or Common Shares issued on exercise of the Warrants are issued or acquired, and the respective rules and regulations under the laws, together with applicable published policy statements, instruments, notices, orders and rulings of the securities regulatory authorities in the jurisdictions and all applicable rules and regulations of any exchange on which the securities are listed.
“Board” means the board of directors of the Corporation.
“Business Day” means any day, except a Saturday, Sunday or any other day on which the principal chartered banks in the City of Toronto are authorized or required to close.
| 1 |
“Common Shares” means the common shares in the capital of the Corporation and any common shares into which such Common Shares shall have been converted, exchanged or reclassified following the date hereof.
“Common Shares Deemed Outstanding” means, as of the Qualified Valuation, or if exercised prior to the Qualified Valuation, as of the Record Date, the sum of all issued and outstanding share capital of the Corporation, on a non-dilutive basis.
“Convertible Securities” means any securities (directly or indirectly) convertible into or exchangeable for Common Shares, but excluding Options.
“Corporation” has the meaning set forth in the preamble.
“Exercise Date” means, for any given exercise of this Warrant, the date on which the conditions to such exercise as set forth in Section 3 shall have been satisfied at or before 5:00 p.m., Toronto time, on a Business Day, including, without limitation, the receipt by the Corporation of the duly completed Exercise Form, the Warrant and the Aggregate Exercise Price.
“Exercise Form” has the meaning set forth in Section 3.1(a)(i).
“Exercise Period” has the meaning set forth in Section 2.
“Exercise Price” has the meaning set forth in the preamble.
“Expiry Date” means the earlier of (i) the date falling 5 years following the date hereof and (ii) immediately prior to an Exit Event (as defined in the License Agreement).
“Expiry Time” means 5:00 p.m., Toronto, Ontario time, on the Expiry Date.
“FMV” means, as of any particular date, the fair market value of the Common Shares as determined by the Board, provided that: (i) if an exercise of Warrant hereunder is conditional upon the completion of an initial public offering (“IPO”) of Common Shares, then “FMV” shall mean the offering “price to the public” specified for such Common Shares in the final prospectus in the relevant IPO (for the avoidance of doubt, before deduction of discounts, commissions, or expenses) per Common Share in such IPO; (ii) if the exercise is conditional upon the closing of a merger of the Corporation or the acquisition, sale or conveyance or other similar transaction of the Corporation’s shares or assets, then “FMV” shall mean the cash or other consideration per Common Share to be received in such transaction; and (iii) if the Common Shares are then listed or admitted to trading on an internationally recognized stock exchange and “FMV” is not being determined as described in (i) or (ii) of this definition, then “FMV” shall mean the volume-weighted average trading price of the Common Shares for the thirty (30) consecutive trading days ending immediately preceding the Exercise Date on such stock exchange on which the Common Shares are then listed.
“Holder” has the meaning set forth in the preamble.
“License Agreement” has the meaning set forth in the preamble.
“Options” means any warrants or other rights or options to subscribe for or purchase Common Shares or Convertible Securities.
“Original Issue Date” means the date on which the Warrant was issued by the Corporation under the License Agreement.
| 2 |
“Person” means any individual, sole proprietorship, partnership, limited partnership, unlimited liability company, corporation, joint venture, trust, incorporated organization or government, or department or agency thereof.
“Purchase Rights” has the meaning set forth in Section 5.
“Qualified Valuation” means if the Corporation, after the date hereof, exceeds the valuation of not less than CAD$1,000,000,000 as determined by market cap or one or more financings or transactions.
“Record Date” means the date of (a) Qualified Valuation, or (b) if earlier, the date on which the Warrant is fully exercised, the rights pertaining to all of the aforesaid shares (including registration rights, if provided in connection with such investment) to be no less favourable than those rights pertaining to any shares in the Corporation issued to the Founders (as defined in the License Agreement) upon incorporation.
“Warrant” means this Warrant and all warrants issued upon division or combination of, or in substitution for, this Warrant.
“Warrant Shares” means the common shares or other share capital of the Corporation then purchasable upon exercise of this Warrant in accordance with its terms.
“1933 Act” has the meaning set forth in Section 7.
2. Term of Warrant. Subject to the terms and conditions hereof, at any time after the date hereof and before the Expiry Time (the “Exercise Period”), the Holder of this Warrant may exercise this Warrant for all or any part of the Warrant Shares purchasable hereunder (subject to adjustment as provided herein). At the Expiry Time, all rights under the Warrants evidenced hereby, in respect of which the right of subscription and purchase herein provided for shall not theretofore have been exercised, shall expire and be of no further force and effect.
3. Exercise of Warrant.
(a) Exercise Procedure. This Warrant may be exercised from time to time on any Business Day during the Exercise Period, for all or any part of the unexercised Warrant Shares, upon:
|
| surrender of this Warrant to the Corporation at its then-principal executive offices (or an indemnification undertaking with respect to this Warrant in the case of its loss, theft or destruction), together with an Exercise Form in the form attached hereto as Exhibit A (each, an “Exercise Form”), duly completed (including specifying the number of Warrant Shares to be purchased) and executed; and | |
|
|
|
|
|
| (ii) | payment to the Corporation of the Aggregate Exercise Price in accordance with Section 3(b). |
(b) Payment of the Aggregate Exercise Price. Payment of the Aggregate Exercise Price shall be made:
|
| (i) | by delivery to the Corporation of a certified cheque or bank draft payable to the order of the Corporation or by wire transfer of immediately available funds to an account designated in writing by the Corporation, in the amount of such Aggregate Exercise Price. |
| 3 |
|
| (ii) | by instructing the Corporation to withhold a number of Warrant Shares then-issuable upon exercise of this Warrant with an aggregate FMV as of the Exercise Date equal to such Aggregate Exercise Price; |
|
|
|
|
|
| (iii) | by surrendering to the Corporation: (x) Warrant Shares previously acquired by the Holder with an aggregate FMV as of the Exercise Date equal to such Aggregate Exercise Price; or (y) other securities of the Corporation having a value as of the Exercise Date equal to the Aggregate Exercise Price (which value, in the case of debt securities, shall be the principal amount thereof, plus accrued and unpaid interest; in the case of preferred shares, shall be the liquidation value thereof, plus accumulated and unpaid dividends; and in the case of Common Shares, shall be the FMV thereof); or |
|
|
|
|
|
| (iv) | any combination of the foregoing. |
In the event of any withholding of Warrant Shares or surrender of other equity securities under Section 3(b)(ii), Section 3(b)(iii) or Section 3(b)(iv), where the number of shares whose value is equal to the Aggregate Exercise Price is not a whole number, the number of shares withheld by or surrendered to the Corporation shall be rounded up to the nearest whole share and the Corporation shall make a cash payment to the Holder (by delivery of a certified cheque or bank draft or by wire transfer of immediately available funds) based on the incremental fraction of a share being so withheld by or surrendered to the Corporation in an amount equal to the product of: (x) such incremental fraction of a share being so withheld or surrendered multiplied by (y) in the case of Common Shares, the FMV per Warrant Share as of the Exercise Date, and, in all other cases, the value thereof as of the Exercise Date determined in accordance with Section 3(b)(iii)(y).
(c) Delivery of Share Certificates. Upon receipt by the Corporation of the Exercise Form, surrender of this Warrant and payment of the Aggregate Exercise Price (in accordance with Section 3(a)), the Corporation shall, as promptly as practicable, and in any event within five (5) Business Days thereafter, execute (or cause to be executed) and deliver (or cause to be delivered) to the Holder a certificate or certificates representing the Warrant Shares issuable upon such exercise, together with cash in lieu of any fraction of a share, as provided in Section 3(d). The share certificate or certificates so delivered shall be, to the extent possible, in such denomination or denominations as the exercising Holder shall reasonably request in the Exercise Form and shall be registered in the name of the Holder or, subject to compliance with Section 6, such other Person’s name as shall be designated in the Exercise Form. This Warrant shall be deemed to have been exercised and such certificate or certificates of Warrant Shares shall be deemed to have been issued, and the Holder or any other Person so designated to be named therein shall be deemed to have become a holder of record of such Warrant Shares for all purposes, as of the Exercise Date.
(d) Fractional Shares. The Corporation shall not be required to issue a fractional Warrant Share upon exercise of any Warrant. As to any fraction of a Warrant Share that the Holder would otherwise be entitled to purchase upon such exercise, the Corporation shall pay to such Holder an amount in cash (by delivery of a certified cheque or bank draft or by wire transfer of immediately available funds) equal to the product of (i) such fraction multiplied by (ii) the FMV of one Warrant Share on the Exercise Date.
(e) Delivery of New Warrant. Unless the purchase rights represented by this Warrant shall have expired or shall have been fully exercised, the Corporation shall, at the time of delivery of the certificate or certificates representing the Warrant Shares being issued in accordance with Section 3(c), deliver to the Holder a new Warrant evidencing the rights of the Holder to purchase the unexpired and unexercised Warrant Shares called for by this Warrant. Such new Warrant shall in all other respects be identical to this Warrant.
| 4 |
(f) Valid Issuance of Warrant and Warrant Shares. With respect to the exercise of this Warrant, the Corporation hereby represents, covenants and agrees:
|
| (i) | This Warrant is, and any Warrant issued in substitution for or replacement of this Warrant shall be, upon issuance, duly authorized and validly issued. |
|
|
|
|
|
| (ii) | All Warrant Shares issuable upon the exercise of this Warrant under the terms hereof shall be, upon issuance, and the Corporation shall take all such actions as may be necessary or appropriate in order that such Warrant Shares are, validly issued, fully paid and non-assessable, and the holders of the Warrant Shares shall not be liable to the Corporation or its creditors in respect of the Warrant Shares. |
|
|
|
|
|
| (iii) | The Corporation shall take all such actions as may be necessary to ensure that all such Warrant Shares are issued without violation by the Corporation of any applicable law or governmental regulation or any requirements of each stock exchange or over-the-counter market on which the Corporation’s Common Shares or other securities constituting Warrant Shares may be listed from time to time. |
|
|
|
|
|
| (iv) | The Corporation shall use its commercially reasonable efforts to cause the Warrant Shares, immediately upon such exercise, to be listed on every stock exchange or over-the-counter market on which the Corporation’s Common Shares or other securities constituting Warrant Shares are listed at the time of such exercise at its own expense. |
|
|
|
|
|
| (v) | While any of the Warrants are outstanding, the Corporation shall comply with the securities legislation applicable to it in order that the Corporation not be in default of any requirements of such legislation and use its best efforts to do or cause to be done all things necessary to preserve and maintain its corporate existence. |
|
|
|
|
|
| (vi) | If, for any reason, other than the failure or default of the Holder, the Corporation is legally unable to issue and deliver the Common Shares or other securities as contemplated herein to the Holder upon the proper exercise by the Holder of the right to purchase any of the Common Shares purchasable upon exercise of the Warrants represented hereby, subject to Holder’s prior written approval, the Corporation may pay, at its option and in complete satisfaction of its obligations and the rights of the Holder hereunder, to the Holder, in cash, an amount equal to the difference between the Exercise Price and the FMV of such Common Shares or other securities on the date of exercise by the Holder, and upon such payment, the Corporation shall have no liability or other obligation to the Holder relating to or in respect of the Warrants. |
(g) Conditional Exercise. Notwithstanding any other provision hereof, if an exercise of any portion of this Warrant is to be made in connection with a prospectus offering or a sale of the Corporation (by an amalgamation, arrangement, sale of shares or otherwise) or an Exit Event, such exercise may at the election of the Holder be made conditional on the consummation of such transaction, in which case such exercise shall not be deemed to be effective until immediately before the consummation of such transaction.
| 5 |
4. Effect of Certain Events on Warrant Events.
(a) Adjustment to Warrant Shares upon Reorganization, Reclassification, Amalgamation, Merger or Sale. In the event of any (i) capital reorganization of the Corporation, (ii) reclassification of the shares of the Corporation (other than as a result of a stock dividend or subdivision, share split or consolidation of shares), (iii) consolidation, amalgamation, arrangement, merger or acquisition of the Corporation with or into another Person, (iv) sale or conveyance of all or substantially all of the Corporation’s properties or assets to another Person, or (v) other similar transaction, in each case, which entitles the holders of Common Shares to receive (either directly or upon subsequent liquidation) shares, securities or assets with respect to or in exchange for Common Shares, each Warrant shall, immediately after such capital reorganization, reclassification, consolidation, amalgamation, arrangement, merger or acquisition, sale or conveyance or other similar transaction, remain outstanding and shall thereafter, instead of or in addition to (as the case may be) the number of Warrant Shares then exercisable under this Warrant, be exercisable for the kind and number of shares or other securities or assets of the Corporation or of the successor Person resulting from such transaction to which the Holder would have been entitled upon such capital reorganization, reclassification, consolidation, amalgamation, arrangement, merger or acquisition, sale or conveyance or other similar transaction if the Holder had exercised this Warrant in full immediately before the time of such capital reorganization, reclassification, consolidation, amalgamation, arrangement, merger or acquisition, sale or conveyance or other similar transaction and acquired the applicable number of Warrant Shares then issuable hereunder as a result of such exercise (without taking into account any limitations or restrictions on the exercisability of this Warrant); and, in such case, appropriate adjustment (in form and substance satisfactory to the Holder) shall be made with respect to the Holder’s rights under this Warrant to ensure that the provisions of this Warrant shall thereafter be applicable, as nearly as possible, to any shares, securities or assets thereafter acquirable upon the exercise of this Warrant. The provisions of this Section 4(a) shall similarly apply to successive capital reorganizations, reclassifications, consolidations, amalgamations, arrangements, mergers or acquisitions, sales or conveyances or other similar transactions. The Corporation shall not effect any such capital reorganization, reclassification, consolidation, amalgamation, arrangement, merger or acquisition, sale or conveyance or other similar transaction unless, before the consummation thereof, the successor Person (if other than the Corporation) resulting from such capital reorganization, reclassification, consolidation, amalgamation, arrangement, merger or acquisition, sale or conveyance or other similar transaction, shall assume, by written instrument substantially similar in form and substance to this Warrant and satisfactory to the Holder, the obligation to deliver to the Holder such shares, securities or assets which, in accordance with the foregoing provisions, such Holder shall be entitled to receive upon exercise of this Warrant. Notwithstanding anything to the contrary contained herein, with respect to any corporate event or other transaction contemplated by the provisions of this Section 4(a), the Holder shall have the right to elect before the consummation of such event or transaction, to give effect to the exercise rights set out in Section 2 instead of giving effect to the provisions set out in this Section 4(a) with respect to this Warrant.
(b) Dividends and Distributions. Subject to the provisions of this Section 4(a), as applicable, if the Corporation shall, at any time or from time to time after the Original Issue Date, make or declare, or fix a record date for the determination of holders of Common Shares entitled to receive, a dividend or any other distribution payable in securities of the Corporation (other than a dividend or distribution of Common Shares, Options or Convertible Securities in respect of outstanding Common Shares), cash or other property, then, and in each such event, provision shall be made so that the Holder shall receive upon exercise of the Warrant, in addition to the number of Warrant Shares receivable thereupon, the kind and amount of securities of the Corporation, cash or other property that the Holder would have been entitled to receive had the Warrant been exercised in full into Warrant Shares on the date of such event and had the Holder thereafter, during the period from the date of such event to and including the Exercise Date, retained such securities, cash or other property receivable by them as aforesaid during such period, giving application to all adjustments called for during such period under this Section 4(b) with respect to the rights of the Holder; provided that no such provision shall be made if the Holder receives, simultaneously with the distribution to the holders of Common Shares, a dividend or other distribution of such securities, cash or other property in an amount equal to the amount of such securities, cash or other property as the Holder would have received if the Warrant had been exercised in full into Warrant Shares on the date of such event.
| 6 |
(c) Certificate as to Adjustment.
|
| (i) | As promptly as reasonably practicable following any adjustment of the Exercise Price, but in any event not later than ten (10) Business Days thereafter, the Corporation shall furnish to the Holder a certificate of an executive officer setting forth in reasonable detail such adjustment and the facts upon which it is based and certifying the calculation thereof. |
|
|
|
|
|
| (ii) | As promptly as reasonably practicable following the receipt by the Corporation of a written request by the Holder, but in any event not later than ten (10) Business Days thereafter, the Corporation shall furnish to the Holder a certificate of an executive officer certifying the Exercise Price then in effect and the number of Warrant Shares or the amount, if any, of other securities or assets then issuable upon exercise of the Warrant. |
(d) Notices. In the event:
|
| (i) | that the Corporation shall take a record of the holders of its Common Shares (or other securities at the time issuable upon exercise of the Warrant) for the purpose of entitling or enabling them to receive any dividend or other distribution, to vote at a meeting (or by resolution in writing), to receive any right to subscribe for or purchase any shares of any class or any other securities, or to receive any other security; |
|
|
|
|
|
| (ii) | of any capital reorganization of the Corporation, any reclassification of the Common Shares of the Corporation, any amalgamation or arrangement of the Corporation with or into another Person, or sale of all or substantially all of the Corporation’s assets to another Person, or an Exit Event; or |
|
|
|
|
|
| (iii) | of the voluntary or involuntary dissolution, liquidation or winding-up of the Corporation; |
then, and in each such case, the Corporation shall send or cause to be sent to the Holder at least ten (10) Business Days before the applicable record date or the applicable expected effective date, as the case may be, for the event, a written notice specifying, as the case may be, (A) the record date for such dividend, distribution, meeting or resolution, or other right or action, and a description of such dividend, distribution or other right or action to be taken at such meeting or by resolution in writing, or (B) the effective date on which such reorganization, reclassification, amalgamation, arrangement, sale, dissolution, liquidation or winding-up is proposed to take place, and the date, if any is to be fixed, as of which the books of the Corporation shall close or a record shall be taken with respect to which the holders of record of Common Shares (or such other securities at the time issuable upon exercise of the Warrant) shall be entitled to exchange their Common Shares (or such other securities) for securities or other property deliverable upon such reorganization, reclassification, amalgamation, arrangement, sale, dissolution, liquidation or winding-up, and the amount per share and character of such exchange applicable to the Warrant and the Warrant Shares.
5. Purchase Rights. In addition to any adjustments under Section 4(a), if at any time the Corporation grants, issues or sells any Common Shares, Options, Convertible Securities or rights to purchase shares, warrants, securities or other property pro rata to the registered holders of Common Shares (collectively, the “Purchase Rights”), then the Holder shall be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights that the Holder would have acquired if the Holder had held the number of Warrant Shares acquirable upon complete exercise of this Warrant immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the registered holders of Common Shares are to be determined for the grant, issue or sale of such Purchase Rights.
| 7 |
6. Transfer of Warrant. Subject to the transfer conditions referred to in the legends endorsed on this Warrant, this Warrant and all rights under it are transferable, in whole or in part, by the Holder without charge to the Holder, upon surrender of this Warrant to the Corporation at its then-principal executive offices with a properly completed and duly executed Transfer Form in the form attached hereto as Exhibit B, provided that: (a) all such transfers shall be effected in accordance with all Applicable Securities Laws and upon the prior written consent of the Corporation; and (b) after such transfer, the term “Holder” shall mean and include any transferee or assignee of the current or any future Holder. Upon such compliance, surrender and delivery, the Corporation shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees and in the denominations specified in such Transfer Form and shall issue to the assignor a new Warrant evidencing the portion of this Warrant, if any, not so assigned, and this Warrant shall promptly be cancelled.
7. No Registration. Neither the Warrants nor the Common Shares issuable upon exercise of the Warrant have been or will be registered under the United States Securities Act of 1933 (the “1933 Act”) nor under the laws of any state of the United States. Subject to certain limited exceptions, (i) Warrants may not be exercised within the United States and (ii) no Common Shares issuable upon the exercise of Warrants will be delivered to any address in the United States. The Holder acknowledges that a legend to that effect may be placed on any certificates representing the Common Shares issued on exercise of the rights represented by this Warrant. Terms used in this paragraph have the meanings given to them in Regulation S under the 1933 Act.
8. Holder Not Deemed a Shareholder; Limitations on Liability. Except as otherwise specifically provided herein (including Section 4(b)), before the issuance to the Holder of the Warrant Shares to which the Holder is then entitled to receive upon the due exercise of this Warrant, the Holder shall not be entitled to vote or receive dividends or be deemed the holder of shares of the Corporation for any purpose, nor shall anything contained in this Warrant be construed to confer upon the Holder, as such, any of the rights of a shareholder of the Corporation or any right to vote, give or withhold consent to any corporate action (whether any reorganization, issue of shares, reclassification of shares, amalgamation, arrangement, conveyance or otherwise), receive notice of meetings, receive dividends or subscription rights, or otherwise. Also, nothing contained in this Warrant shall be construed as imposing any liabilities on the Holder to purchase any securities (upon exercise of this Warrant or otherwise) or as a shareholder of the Corporation, whether such liabilities are asserted by the Corporation or by creditors of the Corporation.
9. Replacement on Loss; Division and Combination.
(a) Replacement of Warrant on Loss. Upon receipt of evidence reasonably satisfactory to the Corporation of the loss, theft, destruction or mutilation of this Warrant and upon delivery of an indemnity reasonably satisfactory to it (it being understood that a written indemnification agreement or statutory declaration of loss of the Holder shall be a sufficient indemnity) and, in case of mutilation, upon surrender of such Warrant for cancellation to the Corporation, the Corporation at its own expense shall execute and deliver to the Holder, in lieu hereof, a new Warrant of like tenor and exercisable for an equivalent number of Warrant Shares as the Warrant so lost, stolen, mutilated or destroyed; provided that, in the case of mutilation, no indemnity shall be required if this Warrant in identifiable form is surrendered to the Corporation for cancellation.
(b) Division and Combination of Warrant. Subject to compliance with the applicable provisions of this Warrant as to any transfer or other assignment that may be involved in such division or combination, this Warrant may be divided or, following any such division of this Warrant, subsequently combined with other Warrants, upon the surrender of this Warrant or Warrants to the Corporation at its then-principal executive offices, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the respective Holders or their agents or attorneys. Subject to compliance with the applicable provisions of this Warrant as to any transfer or assignment that may be involved in such division or combination, the Corporation shall at its own expense execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants so surrendered in accordance with such notice. Such new Warrant or Warrants shall be of like tenor to the surrendered Warrant or Warrants and shall be exercisable in the aggregate for an equivalent number of Warrant Shares as the Warrant or Warrants so surrendered in accordance with such notice.
| 8 |
10. No Impairment. The Corporation shall not, by amendment of its articles or by-laws, or through any reorganization, transfer of assets, amalgamation, arrangement, dissolution, issue or sale of securities, or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms to be observed or performed by it hereunder but shall at all times in good faith assist in the carrying out of all the provisions of this Warrant and in the taking of all such action as may reasonably be requested by the Holder to protect the exercise rights of the Holder against dilution or other impairment, consistent with the tenor and purpose of this Warrant.
11. Compliance with Securities Laws.
(a) Agreement to Comply with the Securities Laws; Legends. The Holder, by acceptance of this Warrant, agrees to comply in all respects with the provisions of this Section 11 and the restrictive legend requirements set forth on the face of this Warrant and further agrees that such Holder shall not offer, sell or otherwise dispose of this Warrant or any Warrant Shares to be issued upon exercise hereof except under circumstances that will not result in a violation of the Applicable Securities Laws.
|
| (i) | This Warrant shall be stamped or imprinted with a legend in substantially the following form: |
“UNLESS PERMITTED UNDER SECURITIES LEGISLATION, THE HOLDER OF THIS SECURITY MUST NOT TRADE THE SECURITY OR THE SECURITIES ISSUED UPON THE EXERCISE OF THIS SECURITY BEFORE THE DATE THAT IS 4 MONTHS AND A DAY AFTER THE JULY 31, 2023.”
|
| (ii) | Any certificate representing Common Shares issued upon the exercise of this Warrant will bear the following legend: |
“UNLESS PERMITTED UNDER SECURITIES LEGISLATION, THE HOLDER OF THIS SECURITY MUST NOT TRADE THE SECURITY OR THE SECURITIES ISSUED UPON THE EXERCISE OF THIS SECURITY BEFORE THE DATE THAT IS 4 MONTHS AND A DAY AFTER THE JULY 31, 2023.”
provided that, at any time subsequent to the date that is four months and one day after the later of (i) the date hereof, and (ii) the date the Corporation became a reporting issuer in any province or territory of Canada, any certificate representing such Common Shares may be exchanged for a certificate bearing no such legends.
(b) Representations of the Holder. In connection with the issuance of this Warrant, the Holder specifically represents, as of the date hereof, to the Corporation by acceptance of this Warrant as follows:
|
| (i) | The Holder is a resident, or if not an individual, has its head office in the jurisdiction set out next to the Holder’s name in Section 13 and such address was not created and is not solely used for the purpose of acquiring the Warrant. The Holder is subject to the Applicable Securities Laws of such jurisdiction and has and will comply with such Applicable Securities Laws in respect of the Warrant, including with respect to the transfer and resale restrictions. |
|
|
|
|
|
| (ii) | The Holder is an “accredited investor” as defined in NI 45-106 or, if the Holder is a resident of Ontario, as defined in section 73.3(1) of the Securities Act (Ontario). The Holder shall, as of the date hereof, complete, execute and deliver to the Corporation the form accredited investor certificate provided to Holder by Corporation, in the form attached hereto as Exhibit C. The information contained in the documents provided by the Holder, including the representations and warranties made therein, is complete, true and correct. The Holder agrees to furnish any additional information requested by the Corporation or any of its affiliates to assure compliance with Applicable Securities Laws in connection with the purchase and sale of the Warrant. Any information that has been furnished or that will be furnished by the Holder to evidence its status as an accredited investor is accurate and complete and does not contain any misrepresentation or material omission. |
|
|
|
|
|
| (iii) | The Holder understands and acknowledges that this Warrant and the Warrant Shares shall be subject to resale restrictions under Applicable Securities Laws. |
| 9 |
12. Warrant Register. The Corporation shall keep and properly maintain at its principal executive offices books for the registration of the Warrant and any transfers thereof. The Corporation may deem and treat the Person in whose name the Warrant is registered on such register as the Holder thereof for all purposes, and the Corporation shall not be affected by any notice to the contrary, except where the Corporation is required to take notice by statute or by order of a court of competent jurisdiction. A Holder shall be entitled to the rights evidenced by such Warrant free from all equities or rights of set-off or counterclaim between the Corporation and the original or any intermediate holder thereof and all Persons may act accordingly. The receipt by any such Holder of the Common Shares purchasable under such Warrant shall be a good discharge to the Corporation for the same, and the Corporation shall not be bound to inquire into the title of any such Holder except where the Corporation is required to take notice by statute or by order of a court of competent jurisdiction.
13. Notices. Unless otherwise specified, any notice or communication required or permitted to be delivered by the Corporation to the Holder under this Warrant Certificate (a “Communication”) may be delivered personally at, or sent by facsimile, email, courier or mail to, the latest address of the Holder recorded on the books of the Corporation. A Communication delivered personally will be deemed to have been given or made and received on the date of delivery. A Communication delivered by facsimile or email will be deemed to have been given or made and received on the date of transmission (but if transmitted on a day which is not a business day or after 5:00 p.m. (local time of the recipient), the Communication will be deemed to have been given or made and received on the next business day). A Communication delivered by courier will be deemed to have been given or made and received on the next business day. A Communication delivered by mail will be deemed to have been given or made and received on the fifth business day after the Communication is posted.
14. Cumulative Remedies. Except to the extent expressly provided in Section 8 to the contrary, the rights and remedies provided in this Warrant are cumulative and are not exclusive of, and are in addition to and not in substitution for, any other rights or remedies available at law, in equity or otherwise.
15. Equitable Relief. Each of the Corporation and the Holder acknowledges that a breach or threatened breach by such party of any of its obligations under this Warrant would give rise to irreparable harm to the other party hereto for which monetary damages would not be an adequate remedy and hereby agrees that, in the event of a breach or a threatened breach by such party of any such obligations, the other party hereto shall, in addition to any and all other rights and remedies that may be available to it in respect of such breach, be entitled to equitable relief, including a restraining order, an injunction, specific performance and any other relief that may be available from a court of competent jurisdiction.
16. Entire Agreement. This Warrant constitutes the sole and entire agreement of the parties to this Warrant with respect to the subject matter contained herein and supersedes all prior and contemporaneous understandings and agreements, both written and oral, with respect to such subject matter.
17. Successor and Assigns. This Warrant and the rights evidenced hereby shall be binding upon and shall enure to the benefit of the parties, the successors of the Corporation and the successors and permitted assigns of the Holder. Such successors or permitted assigns of the Holder shall be deemed to be a Holder for all purposes under this Warrant.
| 10 |
18. No Third-Party Beneficiaries. This Warrant is for the sole benefit of the Corporation, the Holder and their respective successors and, in the case of the Holder, permitted assigns, and nothing in this Warrant, express or implied, is intended to or shall confer upon any other Person any legal or equitable right, benefit or remedy of any nature whatsoever, under or by reason of this Warrant.
19. Headings. The headings in this Warrant are for reference only and shall not affect the interpretation of this Warrant.
20. Amendment and Modification; Waiver. Except as otherwise provided herein, this Warrant may only be amended, modified or supplemented by an agreement in writing signed by each party hereto. No waiver by the Corporation or the Holder of any of the provisions hereof shall be effective unless explicitly set forth in writing and signed by the party so waiving. No waiver by any party shall operate or be construed as a waiver in respect of any failure, breach or default not expressly identified by such written waiver, whether of a similar or different character, and whether occurring before or after that waiver. No failure to exercise, or delay in exercising, any right, remedy, power or privilege arising from this Warrant shall operate or be construed as a waiver thereof; nor shall any single or partial exercise of any right, remedy, power or privilege hereunder preclude any other or further exercise thereof or the exercise of any other right, remedy, power or privilege.
21. Severability. If any term or provision of this Warrant is invalid, illegal or unenforceable in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other term or provision of this Warrant or invalidate or render unenforceable such term or provision in any other jurisdiction.
22. Governing Law. This Warrant shall be governed by and construed in accordance with the laws of the Province of Ontario the federal laws of Canada applicable therein.
23. Forum Selection. Any and all disputes arising under this Warrant, whether as to interpretation, performance or otherwise, shall be subject to the exclusive jurisdiction of the courts of the province of Ontario, and each of the parties hereby irrevocable attorns to the exclusive jurisdiction of such courts. Service of process, summons, notice or other document by certified or registered mail to such party’s address set forth in Section 13 shall be effective service of process for any suit, action or other proceeding brought in any such court. The parties irrevocably and unconditionally waive any objection to the laying of venue of any suit, action or any proceeding in such courts and irrevocably waive and agree not to plead or claim in any such court that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum.
24. Counterparts. This Warrant may be executed in counterparts, each of which shall be deemed an original, but all of which together shall be deemed to be one and the same agreement. A signed copy of this Warrant delivered by facsimile, email or other means of electronic transmission shall be deemed to have the same legal effect as delivery of an original signed copy of this Warrant.
25. No Strict Construction. This Warrant shall be construed without regard to any presumption or rule requiring construction or interpretation against the party drafting an instrument or causing any instrument to be drafted.
[SIGNATURE PAGE FOLLOWS]
| 11 |
IN WITNESS WHEREOF, the Corporation has duly executed this Warrant on the Original Issue Date.
|
| CELLY NUTRITION CORP. |
|
|
|
|
|
|
|
|
| By: | /s/ “Binyomin Posen” |
|
|
| Name: | Binyomin Posen |
|
|
| Title: | CEO |
|
| Accepted and agreed,
|
||
| FSD PHARMA INC. |
|
|
|
|
|
|
| By: | /s/ “Zeeshan Saeed” |
|
| Name: | Zeeshan Saeed |
|
| Title: | CEO |
|
| 12 |
SCHEDULE A
SUBSCRIPTION FORM
| TO: | CELLY NUTRITION CORP. (the “Corporation”) |
Any term in this Subscription Form that is not otherwise defined has the meaning set out in the warrant certificate of which this Subscription Form forms a part (the “Warrant Certificate”).
The registered holder of the Warrants (the “Holder”), by delivery of this Subscription Form, exercises the right to subscribe for _________________ Common Shares on the terms and conditions set out in the Warrant Certificate, for an aggregate Exercise Price of $__________________.
In connection with this exercise: {check one}
| □ | 1. | the Holder certifies that (i) at the time of exercise the Holder is outside the United States and (ii) the Holder is not a U.S. Person, and the Holder is not exercising any of the Warrants on behalf of, or for the account or benefit of, a U.S. Person; or |
|
|
|
|
| □ | 2. | the Holder is delivering a written opinion of U.S. counsel, in a form acceptable to the Corporation acting reasonably, that the Common Shares issuable on exercise of the Warrants are exempt from registration requirements under the United States Securities Act and the securities laws of all applicable states of the United States. |
The Holder directs the Corporation to register the Common Shares as follows: {If any Common Shares are to be issued to a person other than the Holder, then the transfer procedures under the Warrant Certificate will apply, and the Holder must deliver a transfer form.}
Name:
Address:
The Holder directs the Corporation to deliver a certificate representing the Common Shares and, if applicable, a warrant certificate evidencing the balance of the Warrants not presently exercised, as follows: {check one}
| □ | 1. | at the office where this Subscription Form is delivered; or |
|
|
|
|
| □ | 2. | to the address for registration set out above; or |
|
|
|
|
| □ | 3. | to the following address: _____________________________________________ |
[signature page follows]
| 13 |
DATED this ____ day of , 20___.
|
|
|
|
|
| Name of Holder |
|
|
|
|
|
|
|
|
|
|
| Signature of Holder (or authorized signatory on behalf of Holder) |
|
|
|
|
|
|
|
|
|
|
| Name of authorized signatory, if applicable |
|
|
|
|
|
|
|
|
|
|
| Official capacity or title of authorized signatory, if applicable |
|
Instruction: If this Subscription Form is signed by a person in a representative capacity on behalf of the Holder, the Corporation may require the person to deliver documentation to establish the person’s authority and capacity to sign on behalf of the Holder and the Corporation may require the person’s signature to be guaranteed.
| 14 |
EXHIBIT 4.5
LOAN AGREEMENT
THIS LOAN AGREEMENT (this “Agreement”) is made as of July 31, 2023 (the “Effective Date”).
BETWEEN:
CELLY NUTRITION CORP.,
a company incorporated under the laws of the Province of British Columbia,
(the “Borrower”)
AND
FSD PHARMA INC.,
a company incorporated under the laws of the Province of Ontario
(the “Lender”)
RECITALS:
| A. | The Lender has agreed to provide the Borrower with a loan in the amount of $1,000,000 (the “Loan”), in accordance with the terms and conditions set out in this Agreement. |
|
|
|
| B. | The Borrower is entering the business of producing, marketing and selling dietary supplement and natural health products for recreational use (the “Business”). |
|
|
|
| C. | The Loan is being advanced to be used by the Borrower as working capital in connection with the Business. |
|
|
|
NOW THEREFORE in consideration for the Lender agreeing to lend money to the Borrower and the sufficiency of which the parties hereto acknowledge, and for other consideration (the receipt and sufficiency of which is acknowledged) the parties covenant and agree as follows:
ARTICLE 1
LOAN
1.1 Loan Amount
Subject to the terms and conditions set out herein, the Lender hereby promises to provide the Loan to the Borrower, in the aggregate amount of $1,000,000 (the “Loan Amount”).
1.2 Interest Rates
Interest shall accrue on the Loan Amount at a rate equal to 10% per annum (the “Interest Rate”). Interest shall be calculated on the basis of the actual number of days in the period and a year of 365 or 366 days as appropriate. Interest shall be payable annually, on the anniversary of the date hereof. In the event that anniversary date is not a business day, then the interest payment to be made on that date shall be made on the immediately following business day.
| 1 |
1.3 Repayment Terms and Maturity Date
| (a) | The Borrower may pre-pay all or any portion of the Loan Amount and unpaid interest (without payment of any prepayment charge or fee). |
|
|
|
| (b) | The Loan Amount and unpaid interest shall be payable on July 31, 2026 (the “Maturity Date”). |
|
|
|
1.4 Security
As general and continuing security to secure the due payment of the Loan Amount (and interest thereon), the Borrower shall cause the delivery to the Lender a general security agreement duly executed by the Borrower in favour of the Lender, granting to the Lender a first-priority security interest (subject to any permitted liens) on certain specified personal property assets of the Borrower.
1.5 Use of Proceeds
Unless the Lender provides its prior written consent for the Borrower to apply the proceeds of the Loan Amount for a different purpose, the Borrower shall use the proceeds of the Loan solely for working capital purposes in connection with the Business.
ARTICLE 2
CONDITIONS PRECEDENT
2.1 Conditions in Favor of the Lender
The obligation of the Lender to make the funds available under the Loan is subject to the terms and conditions of this Agreement and is conditional upon evidence being given to the Lender as to compliance with the following conditions which are for the sole benefit of the Lender and may be waived by the Lender in whole or in part:
| (a) | The representations and warranties set out in section 3 being true and correct as of the date of this Agreement. |
|
|
|
| (b) | The Lender having received the following documents (collectively the “Loan Documents”) duly executed in form and substance satisfactory to the Lender and its counsel: |
|
| (i) | this Loan Agreement, duly executed by the Borrower; |
|
|
|
|
|
| (ii) | the general security agreement contemplated in Section 1.4, duly executed by the Borrower; and |
|
|
|
|
|
| (iii) | evidence that the security interests set out in herein have been duly registered. |
| 2 |
ARTICLE 3
REPRESENTATIONS AND WARRANTIES
3.1 Borrower representations and warranties
The Borrower represents and warrants to the Lender, and acknowledges that the Lender is relying upon such representations and warranties in advancing the Loan Amount, that as of this date:
| (a) | the Borrower is duly incorporated, organized and validly existing under the Laws of its jurisdiction of incorporation and is in good standing under the Laws of each jurisdiction in which it carries on business or has assets; |
|
|
|
| (b) | the Borrower has all requisite corporate power and authority and all necessary licenses and governmental approvals required to own and operate its business as currently conducted, to borrow, to give guarantees, to give security for such borrowing/guarantees and to otherwise to perform its obligations under this Agreement and the other Loan Documents; |
|
|
|
| (c) | the Borrower has a good and marketable title to all its property and assets, free and clear of all liens and adverse rights of third parties, other than those liens which it has granted to third parties prior to the date hereof; |
|
|
|
| (d) | there are no actions, suits, investigations or proceedings, pending, or to the knowledge of the Borrower, threatened, before any court or governmental or quasi-governmental entity which may materially adversely affect the financial condition, business or operations of the Borrower; |
|
|
|
| (e) | to the knowledge of the Borrower, there are no judgments or executions against the Borrower; |
|
|
|
| (f) | all returns and reports of the Borrower required by law to be filed have been duly filed and all taxes, assessments, contributions, fees and other governmental charges (other than those presently payable without penalty and interest or those currently being contested in good faith) levied against the Borrower or any of its properties or its assets or income which are due and payable, have been paid; |
|
|
|
| (g) | all written information provided to the Lender by the Borrower in connection with the Loan, including, without limitation, information relating to the financial position of the Borrower, is, in all material respects, true, complete and accurate; and |
|
|
|
| (h) | neither the execution nor the delivery of this Agreement or any of the other Loan Documents nor the performance by the Borrower of any of its obligations hereunder or thereunder has resulted or will result in a breach of, or constitute a default under, any indenture, agreement or instrument to which it is a party or by which it is bound or be in contravention of its constating documents, by-laws or resolutions of its directors or shareholders. |
|
|
|
3.2 Survival
All representations and warranties set forth in this Article 3 and all representations and warranties contained in any certificate, financial statement or other instrument delivered by or on behalf of the Borrower pursuant to or in connection with this Agreement or any of the other Loan Documents (including any such representation, warranty or statement made in or in connection with any amendment thereto) shall constitute representations and warranties made under this Agreement.
ARTICLE 4
INTEREST PAYMENTS, CALCULATIONS AND BORROWING PROCEDURES
4.1 Interest Calculation
Interest will accrue daily on the Loan Amount from the date that the Loan Amount is advanced to the Borrower. The Borrower will be liable for and pay interest to the Lender both before and after demand, the occurrence of an Event of Default (as defined in section 5.2) and judgment at the interest rates per annum set out in this Agreement.
| 3 |
4.2 Maximum Interest Rate
| (a) | In the event that any provision of this Agreement would oblige the Borrower to make any payment of interest or any other payment which is construed by a court of competent jurisdiction to be interest in an amount or calculated at a rate which would be prohibited by law or would result in receipt by the Lender of interest at a criminal rate (as those terms are construed under the Criminal Code (Canada)), then notwithstanding that provision, that amount or rate will be deemed to have been adjusted to the maximum amount or rate of interest, as the case may be, as would not be so prohibited by law or so result in the receipt by the Lender of interest at a criminal rate, that adjustment to be effected, to the extent necessary, as follows: |
|
|
|
|
| (i) | first, by reducing the amount or rate of interest required to be paid under this Agreement; and |
|
|
|
|
|
| (ii) | second, by reducing any fees, commissions, premiums and other amounts which would constitute interest for the purposes of Section 347 of the Criminal Code (Canada); |
|
|
|
|
| (b) | If, despite giving effect to all adjustments contemplated by clause (a) of this Section, the Lender has received an amount in excess of the maximum permitted by that clause, then that excess will be applied by the Lender to the reduction of the principal balance of the Outstanding Borrowings and not to the payment of interest, or if that excessive interest exceeds that principal balance, that excess will be refunded to the Borrower. |
4.3 Payments Generally
Each payment under this Agreement will be made for value at or before 5:00 p.m. (Toronto time) on the day that payment is due, provided that, if any such day is not a business day, that payment will be deemed for all purposes of this Agreement to be due on the business day next following that day (and any such extension will be taken into account for purposes of the computation of interest and fees payable under this Agreement).
ARTICLE 5
GENERAL
5.1 Notices
All notices, instructions, or other communications required or permitted to be given by one party to another under this Agreement (each, a “Notice”) will be given in writing and delivered by personal delivery or delivery by recognized national courier, sent by facsimile transmission or delivered by registered mail, postage prepaid, or by electronic communication (including email but excluding Internet or intranet websites) addressed as follows:
| (a) | If to the Lender: |
FSD Pharma Inc.
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
| 4 |
| (b) | If to the Borrower: |
Celly Nutrition Corp.
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
[redacted for confidentiality purposes]
or at such other address or facsimile number or email address at which the addressee may from time to time notify the addressor. Any Notice properly addressed and sent by prepaid registered mail will be deemed to have been given and received on the 5th business day following the date of its mailing. Any Notice transmitted by facsimile will be deemed to have been given and received on the day in which transmission is confirmed. Notices sent to an email address will be deemed to be received on the following day, unless the sender receives notice from the intended recipient’s email server (by return email or other written acknowledgement) notifying the sender of a failure to deliver such email.
5.2 Default
| (a) | Upon the occurrence of any one or more Events of Default (as such term is defined below) the Lender may accelerate the Loan Amount and all accrued but unpaid interest to become immediately due and payable by the Borrower to the Lender. |
|
|
|
| (b) | The happening of any one of the following events unless waived by the Lender, shall constitute event of default under this Agreement (“Event of Default”) provided the Borrower fails to cure (or obtain a waiver for) such default for a period of 10 business days after the Lender provides notice of said event: |
|
| (i) | failure to pay principal or Interest when due; |
|
|
|
|
|
| (ii) | if a decree or order of a court having jurisdiction is entered adjudging the Borrower a bankrupt or insolvent under the Bankruptcy and Insolvency Act (Canada) or any other bankruptcy, insolvency or analogous laws, or issuing sequestration or process of execution against, or against any substantial part of, the property of the Borrower, or appointing a receiver of, or of any substantial part of, the property of the Borrower or ordering the winding-up or liquidation of its affairs, and any such decree or order continues unstayed and in effect for a period of 60 days; |
|
|
|
|
|
| (iii) | if the Borrower institutes proceedings to be adjudicated a bankrupt or insolvent, or consents to the institution of bankruptcy or insolvency proceedings against it under the Bankruptcy and Insolvency Act (Canada) or any other bankruptcy, insolvency or analogous laws, or consents to the filing of any such petition or to the appointment of a receiver of, or of any substantial part of, the property of the Borrower or makes a general assignment for the benefit of creditors, or admits in writing its inability to pay its debts generally as they become due; or |
|
|
|
|
|
| (iv) | if, after the date hereof, any proceedings with respect to the Borrower are taken with respect to a compromise or arrangement, with respect to creditors of the Borrower generally, under the applicable legislation of any jurisdiction. |
| 5 |
5.3 Severability
Any provision of this Agreement which is prohibited or unenforceable in any jurisdiction will not invalidate the remaining provisions of this Agreement, and any such prohibition or unenforceability in any jurisdiction will not invalidate or render unenforceable that provision in any other jurisdiction.
5.4 Governing Law and Attornment
This Agreement will be governed by and construed in accordance with the laws of the Province of Ontario and the federal laws of Canada applicable therein and will be treated in all respects as an Ontario contract. The Parties submit and attorn to the non-exclusive jurisdiction of the courts of the Province of Ontario.
5.5 Assignment
The Borrower will not be entitled to assign any of their rights under this Loan Agreement except with the prior written consent of the Lender. The Lender may assign its rights under this Loan Agreement, in whole or in part, without the prior consent, or notice to the Borrower.
5.6 Counterparts
This Agreement is properly executed if each party executes this Agreement or an identical document. In the latter case, this Agreement takes effect when the separately executed documents are exchanged between the Parties. Delivery of an executed counterpart of this Agreement by portable document format file (PDF file) by facsimile or other electronic method of transmission will be effective as manual delivery of an executed counterpart of this Agreement.
5.7 Entire Agreement and Termination of Prior Agreements
This Agreement, together with the Loan Documents, constitute the entire agreement among the Borrower, the Lender, with respect to the subject matter hereof and supersedes all prior agreements and understandings, oral or written, with respect to such subject matter. There are no representations, warranties, conditions, other agreements or acknowledgments, whether direct or collateral, express or implied, that form part of or affect this Agreement or any other Loan Document other than as expressed herein or in such other Loan Document. The execution of each Loan Document has not been induced by, nor does the Borrower rely upon or regard as material, any representations, warranties, conditions, other agreements or acknowledgments not expressly made in any Loan Document.
[Remainder of page intentionally left blank.]
| 6 |
IN WITNESS WHEREOF the parties have duly executed this Agreement as of the date first written above.
| CELLY NUTRITION CORP. |
|
|
|
|
|
|
| By: | /s/“Binyomin Posen” |
|
| Name: | Binyomin Posen |
|
| Title: | CEO |
|
|
|
|
|
| FSD PHARMA INC. |
|
|
|
|
|
|
| By: | /s/ “Zeeshan Saeed” |
|
| Name: | Zeeshan Saaed |
|
| Title: | CEO |
|
| 7 |
|
|
|
|
GENERAL SECURITY AGREEMENT
THIS GENERAL SECURITY AGREEMENT (as amended, modified, supplemented, restated or replaced from time to time, this “Agreement”), dated as of July 31, 2023, made by and between Celly Nutrition Corp., a corporation existing under the laws of the Province of British Columbia (the “Obligor”), in favor of FSD Pharma Inc. (the “Secured Party”).
WHEREAS the Obligor and the Secured Party have entered into a loan agreement dated on or about the date hereof, as the same may be amended, restated, modified or replaced from the time to time, the “Loan Agreement”).
AND WHEREAS, as a condition for funding the loan pursuant to the Loan Agreement, the Secured Party requires, among other things, that the Obligor grant to the Secured Party, a lien on and security interest in the personal property and fixtures of the Obligor described herein subject to the terms and conditions hereof.
AND WHEREAS, the Obligor will substantially benefit from the proceeds received from the loan from the Secured Party.
AND WHEREAS the Obligor has duly authorized the execution, delivery and performance of this Agreement.
NOW THEREFORE, in consideration of the mutual covenants, terms and conditions set forth herein, and for other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged by the parties hereto, and in order to induce the Secured Party to fund the loan, the Obligor agrees with the Secured Party, as follows:
1. As general and continuing security for the payment and performance of the Obligations the Obligor assigns, transfers, sets over, grants a security interest in, mortgages and charges to the Secured Party, as and by way of a fixed and specific mortgage, charge and security interest in, all of the present and after acquired personal property and all of the present and future assets, property (both real and personal) and undertaking of the Obligor and in all right, title and interest which the Obligor now has or may hereafter have in all of its assets, property and undertaking, including without limitation, all present and after acquired assets, property and undertaking of the kinds hereinafter described (collectively, the “Collateral”):
|
| (a) | All goods comprising the inventory of the Obligor, including but not limited to goods held for sale or lease or furnished or to be furnished under a contract of service or that are raw materials, work in progress or materials used or consumed in a business or profession or finished goods, including, without limitation, “inventory” as defined in the PPSA (hereinafter sometimes collectively referred to as “Inventory”). |
|
|
|
|
|
| (b) | All goods which are not inventory or consumer goods, including but not limited to furniture, fixtures, equipment, machinery, plant, tools, vehicles and other tangible personal property, including, without limitation, “equipment” as defined in the PPSA (hereinafter sometimes collectively referred to as “Equipment”). |
|
|
|
|
|
| (c) | All Computer Hardware and Software Collateral (as defined below). |
|
|
|
|
|
| (d) | All accounts, debts, demands and choses in action which are now due, owing or accruing due or which may hereafter become due, owing or accruing due to the Obligor and all claims of any kind which the Obligor now has or may hereafter have, including but not limited to claims against the Crown and claims under insurance policies (hereinafter sometimes collectively referred to together with intangibles and the Collateral described in paragraphs 1(f) and (n) as “Receivables”). |
|
|
|
|
|
| (e) | All Intellectual Property Collateral (as defined below). |
| 8 |
|
| (f) | All chattel paper. |
|
|
|
|
|
| (g) | All warehouse receipts, bills of lading and other documents of title, whether negotiable or not. |
|
|
|
|
|
| (h) | All Equity Interest Collateral (as defined below). |
|
|
|
|
|
| (i) | All financial assets. |
|
|
|
|
|
| (j) | All securities entitlements. |
|
|
|
|
|
| (k) | All investment property. |
|
|
|
|
|
| (l) | All securities accounts in the name of the Obligor, including any and all assets of whatever type or kind deposited in or credited to such securities accounts, including all financial assets, all security entitlements related to such financial assets, and all certificates and other instruments from time to time representing or evidencing the same, and all dividends, interest, distributions, cash and other property from time to time received or receivable upon or otherwise distributed or distributable in respect of or in exchange for any or all of the foregoing. |
|
|
|
|
|
| (m) | All rights, contracts (including, without limitation, rights and interests arising thereunder or subject thereto), instruments, agreements, licences, permits, consents, leases, policies, approvals, development agreements, building contracts, performance bonds, purchase orders, plans and specifications all of which may or may not be personal property but may be rights in which the Obligor has interests, all as may be amended, modified, supplemented, replaced or restated from time to time. |
|
|
|
|
|
| (n) | All rents, present or future, under any lease or agreement to lease any part of the lands of the Obligor or any building, erection, structure or facility now or hereafter constructed or located on such lands, income derived from any tenancy, use or occupation thereof and any other income and profit derived therefrom. |
|
|
|
|
|
| (o) | All intangibles, including but not limited to all money, cheques, deposit accounts, letters of credit, advances of credit and goodwill. |
|
|
|
|
|
| (p) | With respect to the property described in paragraphs 1(a) to (o) inclusive, all books, accounts, invoices, letters, papers, documents and other records in any form evidencing or relating thereto and all contracts, securities, instruments and other rights and benefits in respect thereof. |
|
|
|
|
|
| (q) | With respect to the property described in paragraphs 1(a) to (p) inclusive, all substitutions and replacements thereof and increases, additions and accessions thereto. |
|
|
|
|
|
| (r) | With respect to the property described in paragraphs 1(a) to (q) inclusive, all proceeds therefrom including personal property in any form or fixtures derived directly or indirectly from any dealing with such property or proceeds therefrom and any insurance or other payment as indemnity or compensation for loss of or damage to such property or any right to such payment, and any payment made in total or partial discharge or redemption of an intangible, chattel paper, instrument or security. |
| 9 |
The security interest created hereby shall not charge, encumber, create a lien upon or otherwise mortgage any consumer goods which the Obligor may own. In this Agreement, the words “accessions”, “account”, “chattel paper”, “consumer goods”, “document of title”, “equipment”, “goods”, “instrument”, “intangible”, “inventory” and “proceeds” shall have the same meanings as their defined meanings in the Personal Property Security Act (British Columbia), as amended, re-enacted or replaced from time to time (the “PPSA”), and the terms “certificated security”, “entitlement holder”, “entitlement order”, “financial asset”, “security”, “securities account”, “security entitlement”, “security intermediary” and “uncertificated security” whenever used herein have the meanings given to these terms in the Securities Transfer Act (British Columbia) (the “STA”) as amended, re-enacted or replaced from time to time.
The said mortgage, charge and security interest shall not extend or apply to the following:
|
| (I) | The last day of the term of any lease or any agreement therefor now held or hereafter acquired by the Obligor, but should such mortgage, charge and security interest become enforceable, the Obligor shall thereafter stand possessed of such last day and shall hold it in trust to assign the same to any Person acquiring such term or the part thereof mortgaged and charged in the course of any enforcement of the said mortgage, charge and security or any realization of the subject matter thereof. |
|
|
|
|
|
| (ii) | Any present or after-acquired agreement, right, franchise, licence or permit (for the purpose of this paragraph, the “contractual rights”) to which the Obligor is a party or of which the Obligor has the benefit to the extent that the creation of the mortgage, charge or security therein would constitute a breach of the terms of or permit any Person to terminate any of the contractual rights or otherwise constitute a breach of or violation under any existing law, statute or regulation to which the Obligor is subject, provided that all such contractual rights will be held in trust by the Obligor for the benefit of the Secured Party. Notwithstanding the foregoing, the said mortgage, charge and security interest shall apply to any proceeds of the disposition of any such contractual rights and the Obligor further agrees to hold such proceeds in trust for the Secured Party and to keep such proceeds in a segregated account for the benefit of the Secured Party. In addition, the said mortgage, charge and security interest shall extend to the contractual rights upon delivery by the Secured Party to the Obligor of written notice to such effect following the occurrence of an Event of Default. |
| 10 |
2. Unless otherwise defined herein or the context otherwise requires, capitalized terms used herein shall have the meanings provided in the Loan Agreement, and, in this Agreement:
|
| (a) | “Agreement” means this general security agreement and all renewals, substitutions, amendments and replacements hereof. The terms “Section”, “Subsection” and “Paragraph” and similar terms refer to the specified section, subsection, paragraph or other portion of this agreement, and the expressions “herein”, “hereof”, “hereto”, “above”, “below” and similar expressions used in this agreement refer and relate to the whole of this agreement and not to any part unless otherwise expressly provided. |
|
|
|
|
|
| (b) | “Applicable Law” means, in relation to any Person, property, transaction or event, all applicable provisions of: (a) statutes, laws (including the common law), rules, regulations, decrees, ordinances, codes, proclamations, treaties, declarations or orders of any Governmental Authority; (b) any consents or approvals of any Governmental Authority; and (c) any orders, decisions, advisory or interpretative opinions, injunctions, judgments, awards, decrees of, or agreements with, any Governmental Authority, in each case applicable to or binding upon such Person, property, transaction or event. |
|
|
|
|
|
| (c) | “Computer Hardware and Software Collateral” means: |
|
| (i) | all computer and other electronic data processing hardware, integrated computer systems, central processing units, memory units, display terminals, printers, features, computer elements, card readers, tape drives, hard and soft disk drives, cables, electrical supply hardware, generators, power equalizers, accessories and all peripheral devices and other related computer hardware; |
|
|
|
|
|
| (ii) | all software programs (including both source code, object code and all related applications and data files), whether now owned, licenced or leased or hereafter acquired by the Obligor, designed for use on the computers and electronic data processing hardware described in clause (i) above; |
|
|
|
|
|
| (iii) | all firmware associated therewith; |
|
|
|
|
|
| (iv) | all documentation (including flow charts, logic diagrams, manuals, guides and specifications) with respect to such hardware, software and firmware described in the preceding clauses (i) through (iii); and |
|
|
|
|
|
| (v) | all rights with respect to all of the foregoing, including, without limitation, any and all intellectual property rights, copyrights, leases, licences, options, warranties, service contracts, program services, test rights, maintenance rights, support rights, improvement rights, renewal rights and indemnifications and any substitutions, replacements, additions or model conversions of any of the foregoing. |
|
| (d) | “Control Agreement” means: |
|
| (I) | with respect to any uncertificated securities included in the Collateral, an agreement between the issuer of such uncertificated securities and another Person whereby such issuer agrees to comply with instructions that are originated by such Person in respect of such uncertificated securities, without the further consent of the Obligor; and |
|
|
|
|
|
| (ii) | with respect to any security entitlements in respect of financial assets deposited in or credited to a securities account included in the Collateral, an agreement between the securities intermediary and another Person in respect of such security entitlements pursuant to which such securities intermediary agrees to comply with any entitlement orders with respect to such security entitlements that are originated by the Secured Party, without the further consent of the Obligor. |
| 11 |
|
| (e) | “Copyright Collateral” means: |
|
| (i) | all copyrights (including without limitation copyrights for semi-conductor chip product mask works and all integrated circuit topography) of the Obligor, whether statutory or common law, registered or unregistered, now or hereafter in force throughout the world, and all applications for registration thereof, whether pending or in preparation, and all copyrights resulting from such applications; |
|
|
|
|
|
| (ii) | all extensions and renewals of any thereof; |
|
|
|
|
|
| (iii) | all copyright licences and other agreements providing the Obligor with the right to use any of the items of the type referred to in clauses (i) and (ii); |
|
|
|
|
|
| (iv) | the right to sue for past, present and future infringements of any of the Copyright Collateral referred to in clauses (i) and (ii) and, to the extent applicable, clause (iii); and |
|
|
|
|
|
| (v) | all proceeds of the foregoing, including, without limitation, licences, royalties, income, payments, claims, damages and proceeds of suit. |
|
| (f) | “Equity Interest Collateral” means all instruments, shares, stock, equity interests, warrants, bonds, Loan Agreements, Loan Agreement stock or other securities relating to the Obligor’s equity interests in each of the Guarantors, whether certificated or uncertificated. |
|
| 1. | “Events of Default” has the meaning ascribed to the term in the Loan Agreement. |
|
|
|
|
|
| (g) | “Governmental Authority” means: (a) any government, parliament or legislature, any regulatory or administrative authority, agency, commission or board and any other statute, rule or regulation making entity having jurisdiction in the relevant circumstances; (b) any Person acting within and under the authority of any of the foregoing or under a statute, rule or regulation thereof; and (c) any judicial, administrative or arbitral court, authority, tribunal or commission having jurisdiction in the relevant circumstances. |
|
|
|
|
|
| (h) | “Intellectual Property Collateral” means, collectively, the Copyright Collateral, the Patent Collateral, the Trademark Collateral and the Trade Secrets Collateral. |
|
|
|
|
|
| (i) | “Obligations” means all of the present and future indebtedness, liabilities and obligations of the Obligor of any and every kind, nature or description whatsoever (whether direct or indirect, joint or several or joint and several, absolute or contingent, matured or unmatured, in any currency, and whether as principal debtor, guarantor, surety or otherwise, including without limitation any interest that accrues thereon after or would accrue thereon but for the commencement of any case, proceeding or other action, whether voluntary or involuntary, relating to the bankruptcy, insolvency or reorganization of the Obligor, whether or not allowed or allowable as a claim in any such case, proceeding or other action) to the Secured Party (and its Affiliates) under, in connection with, relating to or with respect to the Loan Agreement, and any unpaid balance thereof. |
| 12 |
|
| (j) | “Patent Collateral” means: |
|
| (i) | all letters patent and applications for letters patent throughout the world, including all patent applications in preparation for filing anywhere in the world; |
|
|
|
|
|
| (ii) | all reissues, divisions, continuations, continuations-in-part, extensions, renewals and re-examinations of any of the items described in clause (i); |
|
|
|
|
|
| (iii) | all patent licences and other agreements providing the Obligor with the right to use any of the items of the type referred to in clauses (i) and (ii); |
|
|
|
|
|
| (iv) | the right to sue third parties for past, present or future infringements of any patent or patent application, and for breach or enforcement of any patent licence; and |
|
|
|
|
|
| (v) | all proceeds of, and rights associated with, the foregoing (including licence royalties and proceeds of infringement suits), and all rights corresponding thereto throughout the world. |
|
| (k) | “Person” means an individual, company, partnership (whether or not having separate legal personality), corporation (including a business trust), joint stock company, trust, unincorporated association, joint venture or other entity, or a government, state or political subdivision thereof. |
|
|
|
|
|
| (l) | “Trademark Collateral” means: |
|
| (i) | all trademarks, trade names, corporate names, company names, business names, fictitious business names, trade dress, service marks, logos, other source of business identifiers, prints and labels on which any of the foregoing have appeared or appear and designs (all of the foregoing items in this clause (i) being collectively called a “Trademark”), now existing anywhere in the world or hereafter adopted or acquired, whether currently in use or not, all registrations and recordings thereof and all applications in connection therewith, whether pending or in preparation for filing, including registrations, recordings and applications in the Trade-marks Branch of the Canadian Intellectual Property Office or in any office or agency of Canada or any Province thereof or any foreign country, and all reissues, extensions or renewals thereof; |
|
|
|
|
|
| (ii) | all Trademark licences and other agreements providing the Obligor with the right to use any items of the type described in clause (i); |
|
|
|
|
|
| (iii) | all of the goodwill of the business connected with the use of, and symbolized by, the items described in clause (i); |
|
|
|
|
|
| (iv) | the right to sue third parties for past, present and future infringements of any Trademark Collateral described in clauses (i) and (ii); and |
|
|
|
|
|
| (v) | all proceeds of, and rights associated with, the foregoing, including any claim by the Obligor against third parties for past, present or future infringement or dilution of any Trademark, Trademark registration or Trademark licence, or for any injury to the goodwill associated with the use of any such Trademark or for breach or enforcement of any Trademark licence and all rights corresponding thereto throughout the world. |
| 13 |
|
| (m) | “Trade Secrets Collateral” means all common law and statutory trade secrets and all other confidential or proprietary or useful information (to the extent such confidential, proprietary or useful information is protected by the Obligor against disclosure and is not readily ascertainable) and all know-how obtained by or used in or contemplated at any time for use in the business of the Obligor, including without limitation recipes and food processing know-how (all of the foregoing being collectively called a “Trade Secret”), whether or not such Trade Secret has been reduced to a writing or other tangible form, including all documents and things embodying, incorporating or referring in any way to such Trade Secret, all Trade Secret licences, and including the right to sue for and to enjoin and to collect damages for the actual or threatened misappropriation of any Trade Secret and for the breach or enforcement of any such Trade Secret licence. |
3. The fixed and specific mortgages and charges and the security interest granted under this Agreement secure payment and performance of all Obligations.
4. The Obligor hereby represents and warrants to the Secured Party as at the date of this Agreement and as at the date of the acquisition by the Obligor of Collateral (including any acquisition of Collateral after the date hereof) as follows:
|
| (a) | The Obligor is a corporation duly incorporated, organized and subsisting under the laws of its jurisdiction of incorporation with the corporate power to enter into this Agreement, this Agreement has been duly authorized by all necessary corporate action on the part of the Obligor and constitutes a legal and valid agreement binding of the Obligor, enforceable against the Obligor in accordance with its terms; the making and performance of this Agreement will not result in the breach of, constitute a default under, contravene any provision of, or result in the creation of, any lien, charge, security interest, encumbrance or any other rights of others upon any property of the Obligor pursuant to any agreement, indenture or other instrument to which the Obligor is a party or by which the Obligor or any of its property may be bound or affected. |
|
|
|
|
|
| (b) | All of the Collateral is, or when the Obligor acquires any right, title or interest therein, will be the sole property of the Obligor, free and clear of all liens, charges, security interests, encumbrances or any other rights, except as may be permitted by the Loan Agreement, and except for those permitted liens expressly consented to in writing by the Secured Party. |
|
|
|
|
|
| (c) | With respect to any material Intellectual Property Collateral: |
|
| (I) | such Intellectual Property Collateral is subsisting and has not been adjudged invalid or unenforceable, in whole or in part; |
|
|
|
|
|
| (ii) | the Obligor is the exclusive owner of the entire right, title and interest in and to such Intellectual Property Collateral owned by the Obligor and is entitled to use the Intellectual Property Collateral leased or licensed to the Obligor and, to its knowledge, no claim has been made that the use of such Intellectual Property Collateral does or may violate the asserted rights of any third party. |
| 14 |
|
| (d) | The security interest created by this Agreement, once properly perfected in accordance with Applicable Law, will be a valid first priority security interest in the Collateral, subject only to permitted liens expressly consented to in writing by the Secured Party. |
|
|
|
|
|
| (e) | The address of the Obligor’s chief executive office, principal place of business and the office where it keeps its records respecting the Receivables is that given at the end of this Agreement. |
|
|
|
|
|
| (f) | The Obligor has not granted “control” (within the meaning of such term under the STA) over any investment property forming part of the Collateral to any Person other than the Secured Party. |
|
|
|
|
|
| (g) | Except for the filings and registrations necessary to perfect the security interests created herein or otherwise provided for in the Loan Agreement, no authorization, approval or other action by, and no notice to or filing with, any governmental authority, regulatory body or any other Person is required for the grant by the Obligor of the security interest granted hereby in the Collateral or for the execution, delivery and performance of this Agreement by the Obligor. |
5. So long as any portion of the Obligations shall remain unpaid, the Obligor covenants with the Secured Party that it will comply with or perform, or cause to be complied with or performed, the following obligations:
|
| (a) | The Obligor shall maintain, use and operate the Collateral in accordance with past business practices and in accordance with the terms and conditions of the Loan Agreement. |
|
|
|
|
|
| (b) | The Obligor shall keep proper books of account with respect to the Collateral in accordance with generally accepted accounting practice. |
|
|
|
|
|
| (c) | The Obligor shall not sell, lease or otherwise dispose of the Collateral without the prior written consent of the Secured Party, except as permitted by the Loan Agreement or in the ordinary course of business. |
|
|
|
|
|
| (d) | The Obligor shall, upon reasonable request by the Secured Party, execute and deliver all such financing statements, certificates, further assignments and documents and do all such further acts and things as may be necessary and reasonably requested by the Secured Party to give effect to the intent of this Agreement. |
|
|
|
|
|
| (e) | The Obligor acknowledges that no material Collateral shall become affixed to any real property not subject to a security interest in favour of the Secured Party without the prior written consent of the Secured Party. |
|
|
|
|
|
| (f) | The Obligor will immediately, and in any event within 24 hours, notify the Secured Party if they become aware that any Person has the right to go into, collect or seize possession of the Collateral by means of execution, garnishment or other legal process. |
|
|
|
|
|
| (g) | Except with respect to goods in transit or with respect to Equipment out for repair, the Obligor shall keep all material Equipment and other tangible personal property of the Obligor either (i) in the jurisdictions in which such material Equipment or other tangible personal property are located as of the date hereof, or (ii) in jurisdictions in which all required filings have been made for the perfection of the security interests created hereby. |
|
|
|
|
|
| (h) | With respect to any Equipment or Inventory in the possession or control of any third party, upon the request of the Secured Party, acting reasonably, the Obligor shall notify such third party of the Secured Party’ security interest in such Equipment or Inventory and, upon the Secured Party’s request following the occurrence and during the continuance of an Event of Default, direct such third party to hold all such Equipment or Inventory for the Secured Party’s account and subject to the Secured Party’s instructions. |
| 15 |
|
| (i) | The Obligor shall not change the location of its chief executive office or the location of the office where it keeps its records respecting the Receivables without giving prior written notice to the Secured Party of the new location and the date upon which such change is to take effect. |
|
|
|
|
|
| (j) | Upon the reasonable request of the Secured Party, the Obligor shall deliver to the Secured Party possession of all originals of all negotiable documents, instruments and chattel paper owned or held by the Obligor evidencing an aggregate amount payable in excess of $150,000 or evidencing any right in goods in an aggregate amount exceeding $150,000 (duly endorsed in blank, if requested by the Secured Party). |
|
|
|
|
|
| (k) | If an Event of Default shall have occurred and be continuing, at the written direction of the Secured Party, all proceeds of Collateral received by the Obligor shall be delivered in kind to the Secured Party for deposit to a deposit account (the “Collateral Account”) of the Obligor maintained at the Obligor’s bank for the benefit of the Secured Party, and the Obligor shall hold all such proceeds in express trust for the benefit of the Secured Party until delivery thereof is made to the Secured Party. All amounts so held by the Secured Party or by the Obligor in trust for the benefit of the Secured Party) and all income in respect thereof will continue to be collateral security for the Obligations and will not constitute payment thereof until approved as hereinafter provided. No funds, other than proceeds of Collateral, will be deposited in the Collateral Account. |
|
|
|
|
|
| (l) | Following the Secured Party’s exercise of the remedy provided for in paragraph 5(k) hereof, the Secured Party shall have the right but not the obligation to apply any amount held in the Collateral Account to the payment of any Obligations which are due and payable or payable upon demand in such order as the Secured Party may determine in its discretion. The Secured Party may at any time transfer to the Obligor’s general demand deposit accounts any or all of the collected funds in the Collateral Account; provided, however, that any such transfer shall not be deemed to be a waiver or modification of any of the Secured Party’ rights under this paragraph 5. |
|
|
|
|
|
| (m) | The Obligor shall not, unless the Obligor shall reasonably and in good faith determine (and notice of such determination, in form and substance satisfactory to the Secured Party, shall have been delivered to the Secured Party) that any of the Intellectual Property is not material to the business of the Obligor and has negligible economic value, do any act, or omit to do any act, whereby any of the Intellectual Property may lapse or become abandoned, dedicated to the public, placed in the public domain, invalid or unenforceable, as the case may be. |
|
|
|
|
|
| (n) | The Obligor shall notify the Secured Party promptly if it knows, or has reason to believe, that any application or registration relating to any material item of the Intellectual Property Collateral may become abandoned, dedicated to the public, placed in the public domain, invalid or unenforceable, or of any materially adverse determination or development regarding the Obligor’s ownership of any of the Intellectual Property Collateral, its right to register the same or to keep and maintain and enforce the same. |
| 16 |
|
| (o) | At the reasonable request of the Secured Party, the Obligor shall execute and deliver to the Secured Party any document required to acknowledge or register or perfect the Secured Party’s interest in any part of the Intellectual Property Collateral. |
|
|
|
|
|
| (p) | The Obligor shall defend the title to the Collateral against all Persons and shall, upon reasonable demand by the Secured Party, furnish further assurance of title and execute any written instruments or do any other acts necessary to make effective the purposes and provisions of this Agreement. |
|
|
|
|
|
| (q) | The Obligor shall ensure that the representations and warranties set forth in paragraph 4 hereof will be true and correct at all times. |
6. The Obligor will maintain or cause to be maintained with reputable insurance companies insurance with respect to the Collateral against such casualties and contingencies and of such types and in such amounts as are required under the Loan Agreement.
7. The Obligor shall not create or suffer to exist any lien upon any of the Collateral to secure any indebtedness or liabilities of any Person, except for the mortgages, charges and security interest created by this Agreement and except for permitted liens expressly consented to in writing by the Secured Party.
8. Following the occurrence of an Event of Default which is continuing, (i) the Secured Party may notify any parties obligated on any of the Collateral to make any payment to the Secured Party of any amounts due or to become due thereunder and enforce collection of any of the Collateral by suit or otherwise and surrender, release, or exchange all or any part thereof, or compromise or extend or renew for any period (whether or not longer than the original period) any indebtedness thereunder or evidenced thereby, (ii) upon written request of the Secured Party, the Obligor will, at its own expense, notify any parties obligated on any of the Collateral to make any payment to the Secured Party of any amounts due or to become due thereunder, and (iii) any payment or other proceeds received by the Obligor from any party obligated on any of the Collateral shall be held by the Obligor in trust for the Secured Party and paid over to the Secured Party forthwith upon request.
9. The Obligor agrees that, forthwith upon request by the Secured Party, from time to time at its own expense, the Obligor will promptly execute and deliver all further instruments and documents, and take all further action, that may be necessary and reasonably requested by the Secured Party in order to perfect, preserve and protect any mortgages, charges and security interest created, granted or purported to be created or granted hereby or to enable the Secured Party to exercise and enforce its rights and remedies hereunder with respect to any Collateral. Without limiting the generality of the foregoing, the Obligor will:
|
| (a) | If reasonably requested by the Secured Party, mark conspicuously each chattel paper included in the Receivables and each related contract with a legend, in form and substance satisfactory to the Secured Party, indicating that such document, chattel paper or related contract is subject to the security interest granted hereby. |
|
|
|
|
|
| (b) | If reasonably requested by the Secured Party, if any Receivable shall be evidenced by a promissory note or other instrument, negotiable document or chattel paper, deliver and pledge to the Secured Party hereunder such promissory note, instrument, negotiable document or chattel paper duly endorsed and accompanied by duly executed instruments of transfer or assignment, all in form and substance satisfactory to the Secured Party. |
|
|
|
|
|
| (c) | Execute and file such financing or financing change statements, or amendments thereto (including, without limitation, any assignment of claim from or other formality under or pursuant to the Financial Administration Act (Canada) or similar provincial or territorial legislation), and such other instruments or notices, as may be necessary and reasonably requested by the Secured Party in order to perfect and preserve the security interests and other rights granted or purported to be granted to the Secured Party hereby. |
| 17 |
|
| (d) | Furnish to the Secured Party, from time to time at the Secured Party’s reasonable request, statements and schedules further identifying and describing the Collateral and such other reports in connection with the Collateral as the Secured Party may reasonably request, all in reasonable detail. |
|
|
|
|
|
| (e) | Direct the issuer of any certificated securities included in or relating to the Collateral as the Secured Party may specify in its request to register the applicable security certificate in the name of the Secured Party or such nominee as they may direct. |
|
|
|
|
|
| (f) | Direct the issuer of any uncertificated securities included in or relating to the Collateral as the Secured Party may specify in its request to register in the books and records of such issuer the Secured Party or such nominee as it may direct as the registered owner of the uncertificated security. |
|
|
|
|
|
| (g) | Direct the securities intermediary for any security entitlements in respect of financial assets deposited in or credited to a securities account included in or relating to the Collateral as the Secured Party may specify in their request to transfer any or all of the financial assets to which such security entitlements relate as the Secured Party may specify. |
Notwithstanding the foregoing, the Secured Party will be entitled, but not bound or required, to exercise any of the rights that any holder of the above may at any time have. The Secured Party will not be responsible for any loss occasioned by its exercise of such rights or by failure to exercise the same within the time limited for the exercise thereof other than any loss resulting from the gross negligence or wilful misconduct of the Secured Party.
With respect to the foregoing and the grant of the security interest hereunder, the Obligor hereby authorizes the Secured Party to file one or more financing or financing change statements, and amendments thereto, relative to all or any part of the Collateral without the signature of the Obligor where permitted by law. The Secured Party shall provide a copy of such statement to the Obligor together with details of registration thereof. A photographic or other reproduction of this Agreement or any financing statement covering the Collateral or any part thereof shall be sufficient as a financing statement where permitted by law.
10. The Obligor agrees that forthwith, upon request from time to time by the Secured Party acting reasonably, the Obligor shall give its consent in writing to:
|
| (a) | The entering into by any issuer of any uncertificated securities included in or relating to the Collateral as the Secured Party may specify in their request, of a Control Agreement with the Secured Party in respect of such uncertificated securities, which consent may be incorporated into an agreement to which such issuer, the Secured Party and the Obligor are parties. |
|
|
|
|
|
| (b) | The entering into by any securities intermediary for any security entitlements in respect of the financial assets deposited in or credited to a securities account included in or relating to the Collateral as the Secured Party may specify in their request, of a Control Agreement with the Secured Party in respect of such security entitlements which consent may be incorporated into an agreement to which such securities intermediary, the Secured Party and the Obligor are parties. |
| 18 |
11. The Obligor agrees that it shall not consent to:
|
| (a) | The entering into by any issuer of any uncertificated securities included in or relating to the Collateral of a Control Agreement in respect of such uncertificated securities with any Person other than the Secured Party or such nominee or agent as they may direct. |
|
|
|
|
|
| (b) | The entering into by any securities intermediary for any security entitlements in respect of the financial assets deposited in or credited to a securities account included in or relating to the Collateral of a Control Agreement with respect to such securities accounts or security entitlements with any Person other than the Secured Party or such nominee or agent as they may direct. |
12. Unless an Event of Default has occurred and is continuing, the Obligor may use the Collateral in any lawful manner not inconsistent with this Agreement or the Loan Agreement, and the Secured Party and their representatives shall have the right to inspect the operations of the Obligor, its books and records and the Collateral at anytime during normal business hours upon providing twenty four (24) hours’ reasonable notice to the Obligor.
13. Following the occurrence of and during the continuance of an Event of Default, the Secured Party may have any Collateral comprising instruments, shares, stock, equity interests, warrants, bonds, Loan Agreements, Loan Agreement stock or other securities, registered in its name or in the name of its nominee and will be entitled but not bound or required to exercise any of the rights that any holder of such securities may at any time have, but the Secured Party shall not be responsible for any loss occasioned by the exercise of any of such rights or by failure to exercise the same within the time limit for the exercise thereof save and except for the gross negligence or wilful misconduct of the Secured Party.
14. Upon the Obligor’s failure to perform any of its duties hereunder the Secured Party may, but shall not be obliged to, perform any or all of such duties, without waiving any rights to enforce this Agreement, and the Obligor shall pay to the Secured Party, forthwith upon written demand therefor, an amount equal to the reasonable costs, fees and expenses incurred by the Secured Party in so doing plus interest thereon from the date such costs, fees and expenses are incurred until paid at the rate or rates set out in the Loan Agreement.
15. Upon the occurrence of an Event of Default that is continuing, the security hereby granted shall immediately become enforceable and the Secured Party may, in its sole discretion, forthwith or at any time thereafter:
|
| (a) | Declare any or all of the Obligations not then due and payable to be immediately due and payable in accordance with the terms of the Loan Agreement and, in such event, such Obligations shall be forthwith due and payable to the Secured Party without presentment protest or notice of dishonour. |
|
|
|
|
|
| (b) | Commence legal action to enforce payment or performance of the Obligations. |
|
|
|
|
|
| (c) | Require the Obligor to disclose to the Secured Party the location or locations of the Collateral and the Obligor agrees to make such disclosure when so required by the Secured Party. |
| 19 |
|
| (d) | Require the Obligor, at the Obligor’s sole expense, to assemble the Collateral and deliver or make the Collateral available at a place or places designated by the Secured Party to the Obligor that is reasonably convenient for the Obligor, and the Obligor agrees to so assemble, deliver or make available the Collateral. |
|
|
|
|
|
| (e) | Enter any premises where the Collateral may be situate and take possession of the Collateral by any method permitted by law. |
|
|
|
|
|
| (f) | Repair, process, modify, complete or otherwise deal with the Collateral and prepare for the disposition of the Collateral, whether on the premises of the Obligor or otherwise and take such steps as it considers necessary to maintain, preserve or protect the Collateral. |
|
|
|
|
|
| (g) | Seize, collect, realize or dispose of the Collateral by private sale, public sale, lease, or otherwise upon such terms and conditions as the Secured Party may determine or otherwise deal with the Collateral or any part thereof in such manner, upon such terms and conditions and of such times as may seem to the Secured Party advisable. |
|
|
|
|
|
| (h) | Carry on all or any part of the business or businesses of the Obligor and may, to the exclusion of all others, enter upon, occupy and use all or any of such premises, buildings, plant, undertaking and other property of or used by the Obligor as part of or for such time and in such manner as the Secured Party see fit, free of charge, and the Secured Party shall not be liable to the Obligor for any act, omission, or negligence (other than gross negligence or wilful misconduct) in so doing or for any rent, charges, depreciation, damages or other amount in connection therewith or resulting therefrom and any sums expended by the Secured Party shall bear interest at the rate or rates set out in the Loan Agreement. |
|
|
|
|
|
| (i) | File such proofs of claim or other documents as may be necessary or desirable to have its claim lodged in any bankruptcy, winding-up, liquidation, dissolution or other proceedings (voluntary or otherwise) relating to the Obligor. |
|
|
|
|
|
| (j) | Borrow money for the purpose of carrying on the business of the Obligor or for the maintenance, preservation or protection of the Collateral and mortgage, charge, pledge or grant a security interest in the Collateral, whether or not in priority to the security created herein, to secure repayment of any money so borrowed. |
|
|
|
|
|
| (k) | Where the Collateral has been disposed of by the Secured Party as provided in paragraph 15(g), commence legal action against the Obligor for any deficiency. |
|
|
|
|
|
| (l) | Pay or discharge any Lien or claims by any Person in the Collateral and the amount so paid shall be added to the Obligations and secured hereby and shall bear interest at the highest rate of interest charged by the Secured Party at that time in respect of any of the Obligations until payment thereof. |
|
|
|
|
|
| (m) | Take any other action, suit, remedy or proceeding authorized or permitted by this Agreement, the PPSA or by law or equity. |
|
|
|
|
|
| (n) | To the extent permitted by Applicable Law, transfer any securities forming part of the Collateral into the name of the Secured Party or their nominee, with or without disclosing that the securities are subject to a security interest and cause the Secured Party or their nominee to become the entitlement holder with respect to any security entitlements forming part of the Collateral. |
|
|
|
|
|
| (o) | Sell, transfer or use any investment property included in the Collateral of which the Secured Party or their agent has “control” within the meaning of subsection 1(2) of the PPSA. |
| 20 |
16. Where required to do so by the PPSA or other Applicable Law, the Secured Party shall give to the Obligor the minimum written notice required by the PPSA or other Applicable Law of any intended disposition of the Collateral.
17. Any notice or communication to be given under this Agreement to the Obligor or the Secured Party shall be effective if given in accordance with the provisions of the Loan Agreement as to the giving of notice, and the Obligor and the Secured Party may change their respective address for notices in accordance with the said provisions.
18. If the Secured Party is entitled to exercise their rights and remedies in accordance with paragraph 15 hereof, the Secured Party may take proceedings in any court of competent jurisdiction for the appointment of a receiver (which term shall include a receiver and manager) (each herein referred to as a “Receiver”) of the Collateral or may by appointment in writing appoint any Person to be a Receiver of the Collateral and may remove any Receiver so appointed by the Secured Party and appoint another in its stead; and any such Receiver appointed by instrument in writing shall have powers of the Secured Party set out in subparagraphs 15(b) to (l), inclusive, including, without limitation, the power (i) to take possession of the Collateral, (ii) to carry on the business of the Obligor, (iii) to borrow money required for the maintenance, preservation or protection of the Collateral or for the carrying on of the business of the Obligor on the security of the Collateral in priority to the security interest created under this Agreement, and (iv) to sell, lease or otherwise dispose of the whole or any part of the Collateral at public auction, by public tender or by private sale, either for cash or upon credit, at such time and upon such terms and conditions as the Receiver may determine; provided that, to the extent permitted and in the manner prescribed by law any such Receiver shall be deemed the agent of the Obligor and the Secured Party shall not be in any way responsible for any misconduct or negligence of any such Receiver.
19. Any proceeds of any disposition of any Collateral may be applied by the Secured Party to the payment of reasonable expenses incurred in connection with retaking, holding, repairing, processing, preparing for disposition and disposing of the Collateral (including the remuneration of any Receiver appointed pursuant to paragraph 18, solicitor’s fees on a substantial indemnity basis and legal expenses and any other expenses), and any balance of such proceeds shall be applied by the Secured Party towards the payment of the Obligations in such order of application as the Secured Party may from time to time elect, subject to the provisions of the Loan Agreement. All such expenses and all amounts borrowed on the security of the Collateral under paragraphs 15 and 18 hereof shall bear interest at the rate or rates set out in the Loan Agreement. If the disposition of the Collateral fails to satisfy the Obligations and the expenses incurred by the Secured Party, the Obligor shall be liable to pay any deficiency to the Secured Party on demand.
20. Subject to Applicable Law, the Secured Party is authorized, in connection with any offer or sale of any securities forming part of the Collateral, to comply with any limitation or restriction as it may be advised by counsel is necessary to comply with Applicable Law, including compliance with procedures that may restrict the number of prospective bidders and purchasers, requiring that prospective bidders and purchasers have certain qualifications and restricting prospective bidders and purchasers to Persons who will represent and agree that they are purchasing for their own account or investment and not with a view to the distribution or resale of such securities. Subject to Applicable Law, the Secured Party will not be liable or accountable to the Obligor for any discount allowed by reason of the fact that such securities are sold in compliance with any such limitation or restriction.
| 21 |
21. The Obligor further agrees and acknowledges as follows:
|
| (a) | The Obligor shall not be discharged by any extension of time, additional advances, renewals and extensions, the taking of further security, releasing security, extinguishment of the security interest as to all or any part of the Collateral, or any other act except a release or discharge of the security interest upon the full payment of the Obligations including reasonable charges, expenses, fees, costs and interest. |
|
|
|
|
|
| (b) | Any failure by the Secured Party to exercise any right set out in this Agreement shall not constitute a waiver thereof; nothing in this Agreement or in the Obligations shall preclude any other remedy by action or otherwise for the enforcement of this Agreement or the payment in full of the Obligations. |
|
|
|
|
|
| (c) | The Secured Party may waive, in whole or in part, any breach by the Obligor of any of the provisions of this Agreement, any default by the Obligor in payment or performance of any of the Obligations or any of its rights and remedies, whether provided for herein or otherwise, provided that no such waiver shall be effective unless given by the Secured Party to the Obligor in writing. |
|
|
|
|
|
| (d) | No waiver given in accordance with paragraph 21(c) shall be a waiver of any other or subsequent breach by the Obligor of any of the provisions of this Agreement, of any other or subsequent default by the Obligor in payment or performance of any of the Obligations or any of the rights and remedies of the Secured Party, whether provided for herein or otherwise. |
|
|
|
|
|
| (e) | All rights of the Secured Party hereunder shall be assignable to the extent permitted under the Loan Agreement. |
|
|
|
|
|
| (f) | The mortgage, charge and security interest created by this Agreement is intended to attach when this Agreement is signed by the Obligor with respect to all items of Collateral in which the Obligor has rights at that moment, and shall attach to all other Collateral immediately upon the Obligor acquiring any rights therein. |
|
|
|
|
|
| (g) | Value has been given. |
22. The Obligor acknowledges having received an executed copy of this Agreement and of the financing statement registered under the PPSA evidencing the security interest created hereby.
23. The Obligor hereby irrevocably constitutes and appoints the Secured Party and each of their respective officers holding office from time to time as the true and lawful attorney of the Obligor with power of substitution in the name of the Obligor, to do any and all such acts and things or execute and deliver all such agreements, documents and instruments as the Secured Party, in their sole discretion, considers necessary or desirable to carry out the provisions and purposes of this Agreement or to exercise any of its rights and remedies hereunder, and to do all acts or things necessary to realize or collect the proceeds, including, without limitation:
|
| (a) | To ask, demand, collect, sue for, recover, compromise, receive and give a quittance and receipts for moneys due and to become due under or in respect of any of the Collateral. |
|
|
|
|
|
| (b) | To receive, endorse, and collect any drafts or other instruments, documents and chattel paper, in connection with clause (a) above. |
|
|
|
|
|
| (c) | To file any claims or take any action or institute any proceedings which the Secured Party may reasonably deem necessary or desirable for the collection of any of the Collateral or otherwise to enforce the rights of the Secured Party with respect to any of the Collateral. |
|
|
|
|
|
| (d) | To perform the affirmative obligations of the Obligor hereunder. |
| 22 |
The Obligor hereby acknowledges, consents and agrees that the power of attorney granted pursuant to this paragraph is irrevocable (until termination of the security interest hereunder) and coupled with an interest. The Obligor hereby ratifies and agrees to ratify all acts of any such attorney taken or done in accordance with this paragraph. The Secured Party agree that they shall not exercise the power of attorney granted pursuant to this paragraph 23 unless an Event of Default has occurred and is continuing.
24. The powers conferred on the Secured Party hereunder are solely to protect their interests in the Collateral and shall not impose any duty on the Secured Party to exercise any such powers. Except for reasonable care of any Collateral in its possession and the accounting for moneys actually received by it hereunder, the Secured Party shall have no duty as to any Collateral or as to the taking of any necessary steps to preserve rights against prior parties or any other rights pertaining to any Collateral.
25. Notwithstanding any other term or condition of this Agreement, this Agreement shall not relieve the Obligor or any other party to any of the Collateral from the observance or performance of any term, covenant, condition or agreement on its part to be observed or performed thereunder or from any liability to any other party or parties thereto or impose any obligation on the Secured Party to observe or perform any such term, covenant, condition or agreement to be so observed or performed, and the Obligor hereby agrees to indemnify and hold harmless the Secured Party from and against any and all losses, liabilities (including liabilities for penalties), costs and expenses which may be incurred by the Secured Party under or in respect of the Collateral and from all claims, alleged obligation or undertaking on its part to observe, perform or discharge any of the terms, covenants and agreements contained in or with respect to the Collateral. The Secured Party may, at their option, perform any term, covenant, condition or agreement on the part of the Obligor to be performed under or in respect of the Collateral (and/or enforce any of the rights of the Obligor thereunder) without thereby waiving any rights to enforce this Agreement. Nothing contained in this paragraph 25 shall be deemed to constitute the Secured Party the mortgagee in possession of the Collateral or the lessee under any lease or agreement to lease unless the Secured Party have agreed to become such mortgagee in possession or to be a lessee.
26. All rights of the Secured Party hereunder shall enure to the benefit of their respective successors and permitted assigns, provided that the Secured Party shall not be entitled to transfer or assign any of its right, title or interest in, to, or arising under this Agreement except in accordance with the provisions governing assignment contained in the Loan Agreement and all obligations of the Obligor hereunder shall bind the Obligor and its successors and assigns.
27. The Obligor acknowledges and agrees that in the event it amalgamates with any other corporation or corporations, it is the intention of the parties hereto that the security interest created hereby (i) shall extend to “Collateral” (as that term is herein defined) owned by each of the amalgamating corporations and the amalgamated corporation at the time of amalgamation and to any “Collateral” thereafter owned or acquired by the amalgamated corporation, such that the term the “Obligor” when used herein would apply to each of the amalgamating corporations and the amalgamated corporation and (ii) shall secure the “Obligations” (as that term is herein defined) of each of the amalgamating corporations and the amalgamated corporation to the Secured Party at the time of amalgamation and any “Obligations” of the amalgamated corporation to the Secured Party thereafter arising. The security interest shall attach to the additional “Collateral” at the time of amalgamation and to any “Collateral” thereafter owned or acquired by the amalgamated corporation when such becomes owned or is acquired.
| 23 |
28. This Agreement shall be governed by and construed in accordance with the laws of the Province of British Columbia and the federal laws of Canada applicable therein.
29. In the event of any conflict between the provisions hereunder and the provisions of the Loan Agreement then, notwithstanding anything contained in this Agreement, the provisions contained in the Loan Agreement shall prevail and the provisions of this Agreement will be deemed to be amended to the extent necessary to eliminate such conflict. If any act or omission of the Obligor is expressly permitted under the Loan Agreement but is expressly prohibited hereunder, such act or omission shall be permitted. If any act or omission is expressly prohibited hereunder, but the Loan Agreement does not expressly permit such act or omission, or if any act is expressly required to be performed hereunder but the Loan Agreement does not expressly relieve the Obligor from such performance, such circumstance shall not constitute a conflict between the applicable provisions hereunder and the provisions of the Loan Agreement.
30. This Agreement and the security interest, assignment and mortgage and charge granted hereby are in addition to and not in substitution for any other security now or hereafter held by the Secured Party and this Agreement is a continuing agreement and security that will remain in full force and effect until discharged by the Secured Party.
31. The Obligor will not be discharged from any of the Obligations or from this Agreement except by a release or discharge signed in writing by the Secured Party at the Obligor’s expense.
32. If any provision of this Agreement is determined to be invalid or unenforceable in whole or in part, such invalidity or unenforceability shall attach only to such provision or part thereof and the remaining part of such provision and all other provisions hereof shall continue in full force and effect.
33. This Agreement may be executed by one or more of the parties to this Agreement on any number of separate counterparts (including by telecopy or pdf), and all of said counterparts taken together shall be deemed to constitute one and the same instrument.
[Remainder of page intentionally left blank. Signature page follows.]
| 24 |
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first above written.
| CELLY NUTRITION CORP.,
By /s/ “Binyomin Posen” Name: Binyomin Posen Title: CEO
Address for Notices:
[redacted for confidentiality purposes] [redacted for confidentiality purposes]
[redacted for confidentiality purposes] [redacted for confidentiality purposes]
| FSD PHARMA INC.,
By /s/ “Zeeshan Saaed” Name: Zeeshan Saaed Title: CEO
Address for Notices:
[redacted for confidentiality purposes] [redacted for confidentiality purposes]
[redacted for confidentiality purposes] [redacted for confidentiality purposes]
|
| 25 |
EXHIBIT 8.1
Exhibit 8.1 – Subsidiaries.
| Subsidiaries |
|
| Name of Subsidiary | State or Other Jurisdiction of Incorporation |
| FSD Australia Pty Ltd. | Australia |
| FSD BioSciences, Inc. | Delaware |
| FSD Strategic Investments Inc. | Ontario, Canada |
| FY Pharma Inc. | Ontario, Canada |
| Lucid Psycheceuticals Inc. | Ontario, Canada |
| Prismic Pharmaceuticals, Inc. | Arizona |
| Celly Nutrition Corp. | British Columbia, Canada |
EXHIBIT 12.1
CERTIFICATION REQUIRED BY RULE 13a-14(a) UNDER THESECURITIES EXCHANGE ACT OF 1934
I, Zeeshan Saeed, certify that:
| 1. | I have reviewed this annual report on Form 20-F of FSD Pharma Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| 4. | The company's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
| a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
| b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
| c) | Evaluated the effectiveness of the company's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
| d) | Disclosed in this report any change in the company's internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company's internal control over financial reporting; and |
| 5. | The company's other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company's auditor and the audit committee of the company's board of directors (or persons performing the equivalent functions): |
| a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company's ability to record, process, summarize and report financial information; and |
|
| b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the company's internal control over financial reporting. |
| Date: April 1, 2024 | By: | /s/ Zeeshan Saeed |
|
| Zeeshan Saeed |
|
||
| CEO, President and Co-Executive Chairman (Principal Executive Officer) |
|
EXHIBIT 12.2
CERTIFICATION REQUIRED BY RULE 13a-14(a) UNDER THESECURITIES EXCHANGE ACT OF 1934
I, Nathan Coyle, certify that:
| 1. | I have reviewed this annual report on Form 20-F of FSD Pharma Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| 4. | The company's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
| a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
|
| b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
|
| c) | Evaluated the effectiveness of the company's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
|
| d) | Disclosed in this report any change in the company's internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company's internal control over financial reporting; and |
| 5. | The company's other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company's auditor and the audit committee of the company's board of directors (or persons performing the equivalent functions): |
| a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company's ability to record, process, summarize and report financial information; and |
|
| b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the company's internal control over financial reporting. |
| Date: April 1, 2024 | By: | /s/ Nathan Coyle |
|
| Nathan Coyle |
|
||
| Chief Financial Officer (Principal Financial Officer) |
|
EXHIBIT 13.1
CERTIFICATION PURSUANT TO
18 U.S.C. §1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report on Form 20-F of FSD Pharma Inc. (the "Company") for the period ended December 31, 2023, as filed with the Securities and Exchange Commission on the date hereof (the "Report"), I, Zeeshan Saeed, Chief Executive Officer, President and Co-Executive Chairman of the Company, certify, pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
| (1) | The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
|
| (2) | The information contained in this Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
| Date: April 1, 2024 | By: | /s/ Zeeshan Saeed |
|
| Zeeshan Saeed |
|
||
| CEO, President and Co-Executive Chairman (Principal Executive Officer) |
|
A signed original of this written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
EXHIBIT 13.2
CERTIFICATION PURSUANT TO
18 U.S.C. §1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report on Form 20-F of FSD Pharma Inc. (the "Company") for the period ended December 31, 2023, as filed with the Securities and Exchange Commission on the date hereof (the "Report"), I, Nathan Coyle, Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
| (1) | The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
|
| (2) | The information contained in this Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
| Date: April 1, 2024 | By: | /s/ Nathan Coyle |
|
| Nathan Coyle |
|
||
| Chief Financial Officer (Principal Financial Officer) |
|
A signed original of this written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
EXHIBIT 15.1
FSD PHARMA INC.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
As used in this management’s discussion and analysis of financial condition and results of operations (“MD&A”), unless the context indicates or requires otherwise, all references to the “Company”, “FSD”, “we”, “us” or “our” refer to FSD Pharma Inc., together with our subsidiaries, on a consolidated basis as constituted on December 31, 2023.
This MD&A for the three months and fiscal years ended December 31, 2023, 2022 and 2021 should be read in conjunction with the Company’s audited consolidated financial statements and the accompanying notes for the fiscal years ended December 31, 2023, 2022 and 2021 (the “financial statements”). The financial information presented in this MD&A is derived from the financial statements which have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”). All amounts are in United States dollars except where otherwise indicated.
This MD&A is dated as of March 28, 2024.
About FSD Pharma
FSD Pharma Inc. (“FSD” or the “Company”) is a biopharmaceutical company dedicated to building a portfolio of innovative assets and biotech solutions for the treatment of challenging neurodegenerative, inflammatory and metabolic disorders and alcohol misuse disorders with drug candidates in different stages of development. Through its wholly-owned subsidiary, Lucid Psycheceuticals Inc. ("Lucid"), FSD is focused on the research and development of its lead compound, Lucid-MS (formerly Lucid-21-302) ("Lucid-MS"). Lucid-MS is a patented new chemical entity shown to prevent and reverse myelin degradation, the underlying mechanism of multiple sclerosis, in preclinical models. FSD is also focused on the research and development of a treatment for alcohol misuse for application in hospitals and other medical practices. FSD maintains a portfolio of strategic investments through its wholly-owned subsidiary, FSD Strategic Investments Inc., which represent loans secured by residential property.
FORWARD-LOOKING INFORMATION
This MD&A contains forward-looking statements and forward-looking information (collectively, "forward-looking statements") within the meaning of applicable securities laws. Any statements that are contained in this MD&A that are not statements of historical fact may be deemed to be forward-looking statements. Forward-looking statements are often identified by terms such as “plans”, “expects”, “expected”, “scheduled”, “estimates”, “intends”, “anticipates”, “hopes”, “planned” or “believes”, or variations of such words and phrases, or states that certain actions, events, or results “may”, “could”, “would”, “might”, “potentially” or “will” be taken, occur or be achieved. More particularly, and without limitation, this MD&A contains forward-looking statements contained in this MD&A include statements concerning the future of FSD Pharma Inc. and are based on certain assumptions that FSD Pharma has made in respect thereof as of the date of this MD&A. FSD Pharma cannot give any assurance that such forward-looking statements will prove to have been correct.
Since forward-looking statements relate to future events and conditions, by their very nature they require making assumptions and involve inherent risks and uncertainties. The Company cautions that although it believes the expectations and material factors and assumptions reflected in these forward-looking statements are reasonable as of the date hereof, there can be no assurance that these expectations, factors and assumptions will prove to be correct, and these risks and uncertainties give rise to the possibility that actual results may differ materially from the expectations set out in the forward-looking statements. These forward-looking statements are not guarantees of future performance and are subject to a number of known and unknown risks and uncertainties including, but not limited to: the fact that the drug development efforts of both Lucid and FSD BioSciences Inc. (“FSD Biosciences”) are at a very early stage; the fact that preclinical drug development is uncertain, and the drug product candidates of Lucid and FSD BioSciences may never advance to clinical trials; the fact that results of preclinical studies and early-stage clinical trials may not be predictive of the results of later stage clinical trials; the uncertain outcome, cost, and timing of product development activities, preclinical studies and clinical trials of Lucid and FSD BioSciences; the uncertain clinical development process, including the risk that clinical trials may not have an effective design or generate positive results; the potential inability to obtain or maintain regulatory approval of the drug product candidates of Lucid and FSD BioSciences; the introduction of competing drugs that are safer, more effective or less expensive than, or otherwise superior to, the drug product candidates of Lucid and FSD BioSciences; the initiation, conduct, and completion of preclinical studies and clinical trials may be delayed, adversely affected, or impacted by COVID-19 related issues; the potential inability to obtain adequate financing; the potential inability to obtain or maintain intellectual property protection for the drug product candidates of Lucid and FSD BioSciences; and other risks. Accordingly, readers should not place undue reliance on the forward-looking statements contained in this MD&A, which speak only as of the date of this MD&A.
| 1 |
Further information regarding factors that may cause actual results to differ materially are included in the Company’s annual and other reports filed from time to time with the Canadian Securities Administrators on SEDAR+ (www.sedarplsu.ca) and with the U.S. Securities and Exchange Commission on EDGAR (www.sec.gov), including the Company’s Annual Report on Form 20-F for the fiscal year ended December 31, 2023, under the heading “Risk Factors.” This list of risk factors should not be construed as exhaustive. Readers are cautioned that events or circumstances could cause results to differ materially from those predicted, forecasted or projected. The forward-looking statements contained in this document speak only as of the date of this document. FSD Pharma does not undertake any obligation to publicly update or revise any forward-looking statements or information contained herein, except as required by applicable laws. The forward-looking statements contained in this document are expressly qualified by this cautionary statement. Additional information relating to FSD can be found on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov.
OVERVIEW
The Company was formed under and is governed by the provisions of the Business Corporations Act (Ontario) (the "OBCA") on November 1, 1998, pursuant to the amalgamation of Olympic ROM World Inc., 1305206 Ontario Company, 1305207 Ontario Inc., Century Financial Capital Group Inc. and Dunberry Graphic Associates Ltd. The Company’s registered office is located at 199 Bay Street, Suite 4000, Toronto, Ontario, M5L 1A9.
On March 15, 2018, the Company’s shareholders approved the amendments contemplated by the Articles of Amendment at the 2018 annual and special meeting of the shareholders, pursuant to which, among other things, the Company’s shareholders approved certain changes to the capital structure of the Company.
On May 24, 2018, pursuant to Articles of Amendment, the Company changed its name to "FSD Pharma Inc." and the capital structure of the Company was reorganized to create a new class of Class A shares, amend the terms of and re-designate the existing common shares as Class B subordinate voting shares (the “Class B shares”), and eliminate the existing non-voting Class A preferred shares and non-voting Class B preferred shares.
On May 29, 2018, the Class B shares commenced trading on the Canadian Securities Exchange under the trading symbol “HUGE”.
On October 16, 2019, the Company amended its articles of incorporation to complete a consolidation of all of its issued and outstanding share capital. Pursuant to the amendment, all of the issued and outstanding Class A shares and Class B shares were consolidated on the basis of one post-consolidation share for every 201 pre-consolidation shares of the Company (the “Consolidation”). Unless otherwise noted, presentation in this MD&A of the number of Class A shares, Class B shares, stock options, warrants and the issue or exercise prices and any other data related to the foregoing securities are all presented on a post-Consolidation basis.
On January 9, 2020, the Class B shares commenced trading on the NASDAQ under the trading symbol “HUGE”.
The Company operates in two segments: Biopharmaceutical and Strategic Investments. The Company’s Biopharmaceutical segment is focused on furthering the research and development of the Company’s two primary drug candidates consisting of Lucid-MS and a drug candidate to treat alcohol misuse for application in hospitals and other medical practices. The Company’s Strategic Investments segment is focused on generating returns and cashflow through the issuance of loans secured by residential real estate property, with FSD Strategic Investments (as defined below) having a first or second collateral mortgage on the secured property.
As of the date hereof, the Company currently has the following subsidiaries:
|
| (i) | FSD Biosciences Inc. (FSD Biosciences), which is wholly owned by the Company and incorporated under the laws of the State of Delaware; |
|
|
|
|
|
| (ii) | Prismic Pharmaceuticals Inc. (“Prismic”), which is wholly owned by the Company and incorporated under the laws of the State of Arizona; |
|
|
|
|
|
| (iii) | FV Pharma Inc. ("FV Pharma"), which is wholly owned by the Company and incorporated under the OBCA; |
|
|
|
|
|
| (iv) | Lucid Psycheceuticals Inc. ("Lucid"), which is wholly owned by the Company and incorporated under the OBCA; |
|
|
|
|
|
| (v) | FSD Strategic Investments Inc. (“FSD Strategic Investments”), which is wholly owned by the Company and incorporated under the OBCA; |
|
|
|
|
|
| (vi) | FSD Pharma Australia Pty Ltd. (“FSD Australia”), which is wholly owned by the Company and incorporated under the laws of Australia; and |
|
|
|
|
|
| (vii) | Celly Nutrition Corp. (“Celly”), an entity controlled by the Company and incorporated under the British Columbia Business Corporations Act. |
| 2 |
BIOPHARMACEUTICAL OPERATIONS
The Company, through its wholly owned subsidiaries, FSD Biosciences, Lucid, Prismic, and FSD Australia, is a biopharmaceutical research and development company focused on developing, over time, multiple applications of Lucid-MS and a drug candidate to treat alcohol misuse for application in hospitals and other medical practices. Lucid-MS is a patented new chemical entity that is being researched and developed by the Company through Lucid for its potential treatment of multiple sclerosis. The drug candidate to treat alcohol misuse is a proprietary formulation of natural ingredients, vitamins, and minerals to help with liver and brain function for the purposes of quickly relieving individuals from the effects of alcohol consumption.
On January 17, 2023, the Company submitted the clinical trial application for a planned Phase 1 clinical trial for Lucid-MS, a candidate for the treatment of multiple sclerosis.
On April 17, 2023, the Company completed the first-in-human sentinel dosing of Lucid-MS in the Company’s Phase I clinical trial evaluating its novel drug candidate as an orally administered treatment for multiple sclerosis.
On March 22, 2023, FSD Australia received the certificate of approval from the Alfred Ethics Committee in Australia to proceed with a Phase 1 clinical trial of Lucid-201, as a novel drug candidate for the potential treatment of Major Depressive Disorder.
On June 2, 2023, the Company terminated any further clinical development of its proprietary ultra micro-palmitoylethanolamide (“FSD-PEA”) formulation for the treatment of inflammatory diseases and put on hold any further clinical development of Lucid-PSYCH, a compound to address mental health disorders, as part of a strategic decision to focus efforts and allocate capital to the advancement of Lucid-MS and a drug candidate to treat alcohol misuse for application in hospitals and other medical practices.
On July 10, 2023, the Company received a No Objection Letter (“NOL”) for a Phase 1 Lucid-MS clinical trial for the submission Clinical Trail Application (“CTA-A”) that was acknowledged on June 12, 2023. On August 25, 2023, the Company received a NOL for the CTA-A that was acknowledged on July 31, 2023. On July 19, 2023, the Company submitted a request for pre-IND meeting to USFDA, which was acknowledged August 3, 2023, and a response was received on September 21, 2023. On September 18, 2023, the completion of study notification (after completion of five cohorts) was submitted to Health Canada.
On October 2, 2023, provisional patent application to the United States Patent and Trademark Office was submitted on the clinical formulation containing Lucid-21-302 (Lucid-MS).
On July 31, 2023, the Company entered into an exclusive intellectual property license agreement (the “License Agreement”) with Celly. The License Agreement provides Celly access to proprietary information for the purposes of consumer product development and marketing. The License Agreement grants Celly the rights to a proprietary formulation of natural ingredients, vitamins, and minerals to help with liver and brain function for the purposes of potentially quickly relieving from the effects of alcohol consumption, such as inebriation, and restoring normal lifestyle. The License Agreement also grants Celly rights to certain trademarks. In exchange, FSD received 200,000,000 common shares in the capital of Celly following a 2:1 share-split. The Company also received an anti-dilution Warrant Certificate that entitles FSD to purchase up to 25% of the common shares deemed outstanding less the 200,000,000 common shares issued under the License Agreement and from time to time as a result of any partial exercise under the anti-dilution Warrant Certificate. FSD Pharma is also entitled to certain license fees and royalties under the License Agreement. Through the License Agreement, FSD acquired 34.66% of Celly. On July 31, 2023, the Company and Celly entered into a loan agreement for gross proceeds of C$1,000,000. The loan was funded on August 1, 2023, and accrues interest at a rate of 10% per annum. Interest is payable annually and the loan matures on July 31, 2026. In November 2023, through the Plan of Arrangement the Company distributed 45,712,529 of its 200,000,000 shares of Celly to its shareholders. The consolidated financial statements incorporate the assets and liabilities of Celly as of December 31, 2023, and the results of operations and cash flows for the period commencing on July 31, 2023, being the date on which FSD obtained control of Celly.
To assess the investment in Celly, judgment was required to determine if the Company has significant influence or control of Celly. The Company considered the relevant guidance in IFRS 10 – Consolidated Financial Statements, IAS 24 – Related Party Disclosures and IAS – 28 Investments in Associates and Joint Ventures.
| 3 |
Judgment is applied in determining when the Company controls an investment even if the Company holds less than a majority of the investee’s voting rights (the existence of de facto control). The Company concluded it has control of Celly even though the Company only holds 26.15% of the voting rights as of December 31, 2023. The Company concluded it has control of Celly as the Company, together with persons or entities considered to be de facto agents of the Company, hold a combined 52.05% of the voting rights of Celly as of December 31, 2023. In addition, key management personnel of the Company hold three of the four board of director positions of Celly. The assessment of control is performed on a continuous basis. The Company determined that it obtained control of Celly on July 31, 2023, and control was maintained at all times from July 31, 2023, through December 31, 2023. Celly is significantly dependent on the Company as a result of the License Agreement and loan. The non-controlling interest (“NCI”) component of Celly is included as a separate component in equity.
Lucid-MS Agreement
On May 19, 2021, prior to its acquisition by the Company, Lucid entered into a license agreement with the University Health Network (“UHN”) that governs the world-wide licensing of certain intellectual property rights and data associated with Lucid-MS. Under the terms of the agreement, the Company shall pay a yearly license maintenance fee of C$100,000 to UHN until the first commercial sale of a product utilizing the intellectual property licensed to the Company under the agreement including Lucid-MS is made.
Under the agreement the Company is committed to minimum milestones payments of $nil and maximum milestones payments of C$12,500,000 if all product development and regulatory milestones are met.
Furthermore, the Company is also responsible to pay revenue milestone payments and royalties if revenue milestones from commercial sales are achieved. Milestones can be extended by mutual agreement.
Treatment for Alcohol Misuse
The Company is developing a product for alcohol misuse for application in hospitals and other medical practices. The compound is a proprietary formulation of natural ingredients, vitamins, and minerals to help with liver and brain function for the purposes of quickly relieving individuals from the effects of alcohol consumption.
The product has the potential to assist emergency room physicians and their medical staff with the abundance of intoxicated patients they receive as these patients are utilizing critical resources (i.e. the physicians and their medical staff) whose time can be used for more urgent and critical needs. The Company will be conducting further research and development, including clinical trials, into the viability of the product. The viability, development and advancement of the product is dependent on the Company obtaining requisite funding, in the amount of $10,998,811, to complete further research and development. The Company, through its initial research, has discovered that there is significant demand in the market for this type of product, an opportunity for them to capture market share and believes that if it were able to develop and sell the product, it would bring immense value to its shareholders. If the requisition financing is not obtained, the Company will be unable to develop the product.
STRATEGIC INVESTMENT OPERATIONS
On May 13, 2022, FSD Strategic Investments, a wholly owned subsidiary of the Company, was incorporated. FSD Strategic Investments is focused on generating returns and cashflow through the issuance of loans secured by residential property. FSD Strategic Investments earns interest through fixed rate lending arrangements that have an average term to maturity of two years from the date of issuance. The loans are secured by residential property with a first or second collateral mortgage on the secured property. Loans are issued up to 55% of the appraised value of the secured property. As at December 31, 2023, the Company has a finance receivable balance of $8,095,354 and minimum contractual payments receivable at the end of the loan terms totaling $8,527,569. The loans will begin to mature in the second quarter of fiscal 2024.
DISCONTINUED OPERATIONS
In March 2020, the Company decided to focus its efforts and resources on the pharmaceutical business and initiated the process to exit the medical cannabis industry and sell FV Pharma's facility located at 520 William Street, Cobourg, Ontario, K9A 3A5 (the "Facility") and the 64-acre property on which the Facility is located (the “Facility Property”). On May 6, 2022, the Company closed the sale of the Facility and the Facility Property for total consideration of $12,730,942 (C$16,400,000). The Company recognized a gain of $4,249,582 on the sale of the Facility and the Facility Property and incurred selling expenses of $616,002 during the year ended December 31, 2022.
| 4 |
Assets included in the sale consisted of the Facility and Facility Property. No liabilities of the Company were transferred as part of the sale. Subsequent to the sale of the Facility and the Facility Property, results of operations related to FV Pharma are reported as continued operations.
ACQUISITION OF LUCID
On September 21, 2021, the Company acquired all of the issued and outstanding common shares of Lucid, an early-stage Canadian-based specialty pharmaceutical company focused on the development of therapies to treat critical neurodegenerative diseases for total consideration of $7,290,731. In connection with the closing of the Lucid acquisition, Dr. Lakshmi Kotra, maintained his position as Lucid’s Chief Executive Officer (“CEO”).
Prior to the acquisition, the Company’s Executive Co-Chairman of the Board beneficially held approximately 4.5% ownership interest in Lucid through an entity related to this individual.
It was determined that the acquisition of Lucid did not qualify as a business combination in accordance with IFRS 3 – Business Combinations, and therefore it was accounted for as an asset acquisition. The individual identifiable assets acquired and liabilities assumed were identified. The purchase consideration was first allocated to the fair values of the acquired cash and cash equivalents, other receivables, and trade and other payables, as their carrying values was determined to equal their fair values. The remaining purchase price was allocated to the acquired intangible assets.
The total consideration for the purchase of Lucid was $7,290,731. The purchase consideration consisted of $7,023,732 of Class B shares, $196,436 of share options and $70,563 of warrants. 304,880 Class B shares and all of the warrants issued as part of the consideration for the Lucid acquisition were issued to an entity related to the interim CEO and Executive Co-chairman of the Board in exchange for securities of Lucid held by the entity prior to the completion of the Lucid acquisition. The fair value of the Class B shares was determined based on a total of 4,502,392 shares issued and a fair value of $1.56 per share, which reflects the share price on the date of acquisition. The fair value of the 161,091 share options and 112,162 warrants issued as part of the consideration were determined using the Black-Scholes options pricing model with the following assumptions:
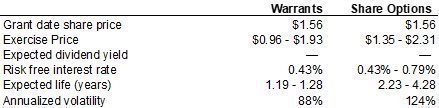
The allocation of the total consideration to the fair value of the identifiable assets acquired and liabilities assumed as at the date of the acquisition was as follows:

The Company also capitalized $128,320 of acquisition related costs to the acquired intellectual property.
| 5 |
SELECTED FINANCIAL HIGHLIGHTS
The following table presents selected financial information for the three months and years ended December 31, 2023 and 2022:

The following table presents selected financial information for the three months and years ended December 31, 2022 and 2021:
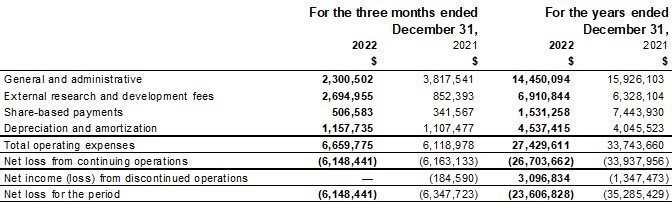
| 6 |
RESULTS OF OPERATIONS 2023
The following table outlines our consolidated statements of loss for the three months and years ended December 31, 2023 and 2022:
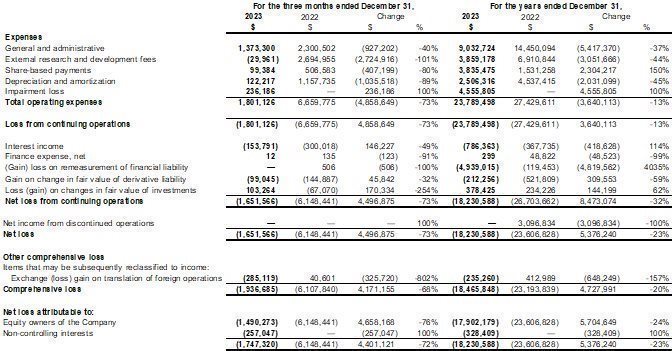
REVIEW OF OPERATIONS FOR THE THREE MONTHS AND YEARS ENDED DECEMBER 31, 2023 AND 2022
General and administrative
General and administrative expenses for the three months and years ended December 31, 2023 and 2022 are comprised of:
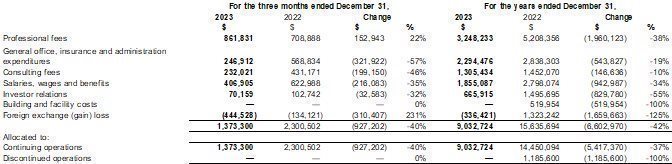
Professional fees

Professional fees increased from $708,888 to $861,831 or 22% and decreased from $5,208,356 to $3,248,233 or 38% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. The Company incurred approximately $515,000 of legal fees directly related to non-recurring ligation expenses during the year ended December 31, 2023, compared to approximately $1,700,000 for the year ended December 31, 2022. Professional fees fluctuate from period to period based on the nature of the transactions the Company undertakes.
| 7 |
General office, insurance and administration expenditures
General office, insurance and administration expenditures for the three months and year ended December 31, 2023 and 2022 are comprised of the following:
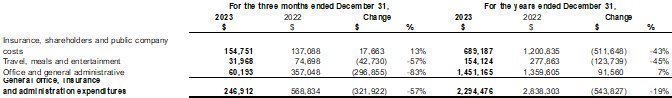
Insurance, shareholders and public company costs
Insurance, shareholders and public company costs increased from $137,088 to $154,751 or 13% and decreased from $1,200,835 to $689,187 or 43% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. These costs primarily consist of insurance and other related expenditures associated with being a publicly listed Company on the NASDAQ. For the year ended December 31, 2023, the Company was able to reduce overall insurance expenses by separately purchasing insurance policies for directors and officers from clinical trial liability insurance.
Travel, meals and entertainment
Travel, meals and entertainment expenses decreased from $74,698 to $31,968 or 57% and decreased from $277,863 to $154,124 or 45% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. Travel, meals and entertainment expenses fluctuate from period to period based on the nature of the transactions the Company undertakes.
Office and general administrative
Office and general administrative expenses decreased from $357,048 to $60,193 or 83% and increased from $1,359,605 to $1,451,165 or 7% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. Office and general administrative expenses may vary from period to period based on operational activities.
Consulting fees

Consulting fees decreased from $431,171 to $232,021 or 46% and decreased from $1,452,070 to $1,305,434 or 10% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. Consulting fees include fees paid to individuals and professional firms who provide advisory services to the Company and fluctuate from period to period based on the nature of the transactions the Company undertakes.
Salaries, wages and benefits

Salaries, wages and benefits expenses decreased from $622,988 to $406,905 or 35% and decreased from $2,798,074 to $1,855,087 or 34% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. The decrease is primarily due to a decrease in headcount for the three months and year ended December 31, 2023, compared to the equivalent periods in the prior year. The decrease in headcount was primarily attributable to the decision to terminate the research and development activities related to FSD-PEA.
Investor relations

Investor relations expenses decreased from $102,742 to $70,159 or 32% and decreased from $1,495,695 to $665,915 or 55% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. Investor relations expenses fluctuate from period to period based on the Company’s business strategy. For the three months and year ended December 31, 2022, the Company incurred significant one-time costs related to investor relations and marketing activities undertaken.
| 8 |
Building and facility costs

Building and facility costs decreased from $519,954 to $nil or 100% for the year ended December 31, 2023, compared to the equivalent period in the prior year. Such costs include property taxes, security services, repairs and maintenance expenditures and utilities. All costs related to the FV Pharma Facility and the Facility Property that were sold during the year ended December 31, 2022.
Foreign exchange (gain) loss

Foreign exchange gain increased from $134,121 to $444,528 or 231% and from a loss of $1,323,242 to gain of $336,421 or 125% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. The primary reason for the foreign exchange change was due to the change of the Canadian dollar relative to the US dollar and its impact on financial instruments denominated in the Canadian dollar.
External research and development fees

External research and development fees decreased from $2,694,955 to a recovery of $29,961 or 101% and decreased from $6,910,844 to $3,859,178 or 44% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. The decrease for the three months and year ended December 31, 2023, was primarily due to the Company terminating R&D activities relating to FSD-PEA and putting on hold R&D activities of Lucid-PSYCH. The Company recognized a recovery of external research and development fees of $29,961 for the three months ended December 31, 2023, as a result of credits received from contract research organizations that can be applied against future services.
Share-based payments

Share-based payments decreased from $506,583 to $99,384 or 80% and increased from $1,531,258 to $3,835,475 or 150% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. Share-based payments expense changes based on the variability in the number of options granted, vesting periods of the options, the number of Performance Share Units (“PSUs”) granted, vesting periods of the PSUs, number of warrants granted, vesting periods of the warrants, the grant date fair values of share-based awards, and share-based bonuses issued. The increase for the year ended December 31, 2023, is primarily related to approximately $1.9M of share options issued and vested during the period and approximately $1.3M related to warrants issued for services.
Depreciation and amortization

Depreciation and amortization decreased from $1,157,735 to $122,217 or 89% and decreased from $4,537,415 to $2,506,316 or 45% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. Depreciation and amortization is primarily related to the amortization of intellectual property. For the three months and year ended December 31, 2023, the decrease is due to the impairment of FSD-PEA and the Innovet license, resulting in lower amortization expense, as these assets were fully impaired during the year.
| 9 |
Impairment loss
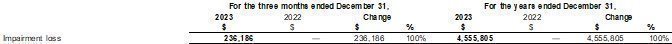
For the three months ended December 31, 2023, the Company recognized an impairment loss of $236,186 related to the note receivable as the likelihood of repayment of the note was considered remote. For the year ended December 31, 2023, the Company recognized an impairment loss of $4,319,619 related to the impairment of the Prismic intangible assets following the decision to terminate the clinical trials of FSD-PEA and impairment of the Innovet intangible asset following the decision to no longer purse the development of the ultra-micro PEA for veterinary purposes.
Interest income
Interest income decreased from $300,018 to $153,791 or 49% and increased from $367,735 to $786,363 or 114% for the three months and year ended December 31, 2023, respectively, compared to the equivalent periods in the prior year. Interest income is primarily comprised of user fees earned on finance receivables and interest earned on Guaranteed Investment Certificates (“GICs”). For the three months ended December 31, 2023, interest income is lower compared to the prior year due to lower interest income earned related to GICs in the prior year. For the year ended December 31, 2023, interest income is higher compared to the prior year due to interest income earned finance receivables.
(Gain) loss on remeasurement of financial liability
For the three months and year ended December 31, 2023, the Company recognized a gain on remeasurement of financial liabilities of $nil and $4,939,015 compared to a loss of $506 and gain of $119,953, for the three months and year ended December 31, 2022, respectively. For the year ended December 31, 2023, the gain is related to settlement reached with a Contract Research Organization. For the year ended December 31, 2022, the gain is related to settlement of outstanding accounts payable.
Gain on change in fair value of derivative liability
In August 2020, the Company issued 2,762,430 Class B shares and 1,381,215 warrants to purchase Class B shares for total cash proceeds of $9,999,997. Each warrant is exercisable to purchase one Class B share of the Company at an exercise price of $4.26 per share and expires five years from the date of issuance.
The fair value of the warrants liability as at December 31, 2023, was $31,338, resulting in a gain on change in fair value of $99,045 and $212,256 for the three months and year ended December 31, 2023.
The fair value of the warrants liability as at December 31, 2022, was $243,594, resulting in a gain on change in fair value of $144,887 and $521,809 for the three months and year ended December 31, 2022.
Loss on changes in fair value of investments
The Company has various investments accounted for at fair value through profit or loss resulting in recognition of loss or gain as the fair value fluctuates.
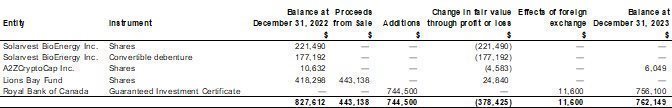
| 10 |
RESULTS OF OPERATIONS 2022
The following table outlines our consolidated statements of loss for the three months and years ended December 31, 2022 and 2021:

REVIEW OF OPERATIONS FOR THE THREE MONTHS AND YEARS ENDED DECEMBER 31, 2022 AND 2021
General and administrative
General and administrative expenses for the three months and years ended December 31, 2022 and 2021 are comprised of:
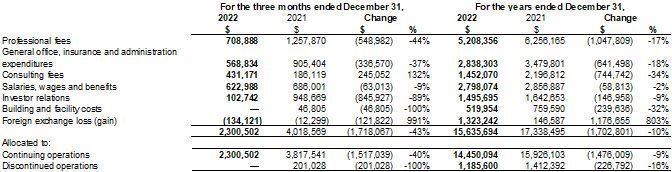
Professional fees
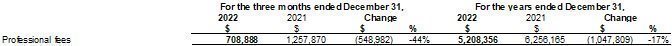
Professional fees decreased from $1,257,870 to $708,888 or 44% and decreased from $6,256,165 to $5,208,356 or 17% for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. The Company incurred approximately $1,700,000 of legal fees directly related to non-recurring litigation expenses during the year ended December 31, 2022. For the three months and year ended December 30, 2021, the Company incurred expenses related to litigation and the Company’s contested annual general and special shareholders meeting held on May 14, 2021. Professional fees fluctuate from period to period based on the nature of the transactions the Company undertakes.
| 11 |
General office, insurance and administration expenditures
General office, insurance and administration expenditures for the three months and years ended December 31, 2022 and 2021 are comprised of the following:

Insurance, shareholders and public company costs
Insurance, shareholders and public company costs decreased from $905,404 to $568,834 or 37% and decreased from $3,479,801 to $2,838,303 or 18% for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. These costs primarily consist of insurance and other related expenditures associated with being a publicly listed Company on the NASDAQ. The primary reason for the decrease for the three months and year ended December 31, 2022, compared to the equivalent periods in the prior year is a decrease in the cost of director and officers’ insurance and shareholders and public company costs.
Travel, meals and entertainment
Travel, meals and entertainment expenses increased from $70,844 to $74,698 or 5% and increased from $212,496 to $277,863 or 31% for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. Travel, meals and entertainment expenses fluctuate from period to period based on the nature of the transactions the Company undertakes.
Office and general administrative
Office and general administrative expenses increased from $273,041 to $357,048 or 31% and increased from $588,399 to $1,359,605 or 131% for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. The primary reason for the increase is due to selling expenses incurred related to the sale of the Facility and Facility Property. Office and general administrative expenses may vary from period to period based on operational activities.
Consulting fees

Consulting fees increased from $186,119 to $431,171 or 132% and decreased from $2,196,812 to $1,452,070 or 34% for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. Consulting fees include fees paid to individuals and professional firms who provide advisory services to the Company and fluctuate from period to period based on the nature of the transactions the Company undertakes.
Salaries, wages and benefits
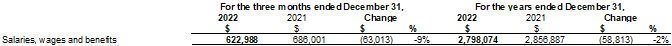
Salaries, wages and benefits expenses decreased from $686,001 to $622,988 or 9% and decreased from $2,856,887 to $2,798,074 or 2% for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. During the year ended December 31, 2022, the Company paid one-time bonus payments to the CEO, Chief Operating Officer (“COO”), President and Lucid CEO in the amount of C$180,000 each. During the year ended December 31, 2021, the Company incurred non-recurring expenses in connection with the termination of employment of employees during the period and employer health tax offset.
| 12 |
Investor relations

Investor relations expenses decreased from $948,669 to $102,742 or 89% and decreased from $1,642,653 to $1,495,695 or 9% for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. Spending on investor relations and marketing varies from period to period based on the Company’s strategy.
Building and facility costs

Building and facility costs decreased from $46,805 to $nil or 100% and decreased from $759,590 to $519,954 or 32% for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. Such costs include property taxes, security services, repairs and maintenance expenditures and utilities. The decrease was due to the sale of the Facility and Facility Property in May 2022.
Foreign exchange loss (gain)
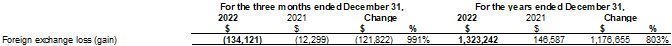
Foreign exchange loss (gain) increased from gain of $12,299 and to gain of $134,121 and increased from loss of $146,587 to loss of $1,323,242 for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. The primary reason for the foreign exchange change was due to the change of the Canadian dollar relative to the US dollar and its impact on monetary assets and liabilities denominated in the Canadian dollar.
External research and development fees

| 13 |
External research and development fees increased from $852,393 to $2,694,955 or 216% and increased from $6,328,104 to $6,910,844 or 9% for the three months and year December 31, 2022, respectively, compared to the equivalent periods in the prior year. For the three months and year ended December 31, 2022, external research and development fees were higher due costs incurred for ongoing research and development compared to the equivalent periods in the prior year, as the Company terminated Phase 2 Safety and Tolerability testing and COVID-19 study in August 2021.
Share-based payments

Share-based payments increased from $341,567 to $506,583 and decreased from $7,443,930 to $1,531,258 for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. Share-based payment expenses fluctuate based on the variability in the number of share-based awards granted, vesting periods of the awards and the grant date fair values. The decrease during the year ended December 31, 2022, compared to the equivalent period in the prior year is primarily due to a share-based bonus issued in February 2021 of $3,576,875 compared to $nil during the year ended December 31, 2022.
Depreciation and amortization

Depreciation and amortization increased from $1,107,477 to $1,157,735 or 5% and increased from $4,045,523 to $4,537,415 or 12% for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. The increase in depreciation and amortization is primarily related to intangible asset additions.
Other income
Other income increased from $nil to $300,018 or 100% and increased from $1,292 to $367,735 or 28362% for the three months and year ended December 31, 2022, respectively, compared to the equivalent periods in the prior year. The increase is primarily due to monthly interest payments received related to finance receivables and interest income earned on short term Guaranteed Investment Contracts.
| 14 |
Finance expense
For the three months and year ended December 31, 2022, finance expense was $135 and $48,822 compared to $29,205 and $69,404 for the equivalent periods in the prior year, respectively. Finance expense is primarily comprised of interest on notes payable assumed on acquisition of Prismic Pharmaceuticals in June 2019.
Loss (gain) on settlement of financial liability
For the three months and year ended December 31, 2022, the Company recognized a loss on settlement of financial liabilities of $506 and a gain of $119,453, compared to $nil and a gain of $49,792, for the three months and year ended December 31, 2021.
Loss (gain) on change in fair value of derivative liability
In August 2020, the Company issued warrants as part of a private placement that did not meet the IFRS definition of equity due to the exercise price being denominated in United States Dollar, which was not the functional currency of the Company at the time resulting in a variability in exercise price. As such, the warrants were recognized as a derivative liability with a fair value of $3,289,069 at the time of issuance.
The fair value of the warrants liability as at December 31, 2022 was $243,594 resulting in a gain on change in fair value of $521,809 for the year ended December 31, 2022. The fair value was determined using the Black-Scholes option pricing model and the following assumptions: exercise price of $4.26, the underlying share price of $0.79, risk-free interest rate of 4.07% and annualized volatility of 96%.
Loss (gain) on changes in fair value of investments
The Company has various investments accounted for at fair value through profit or loss resulting in recognition of losses or gains as the fair value fluctuates.

| 15 |
REVIEW OF DISCONTINUED OPERATIONS FOR THE YEARS ENDED DECEMBER 31, 2022 AND 2021
The following table outlines our net income (loss) from discontinued operations for the years ended December 31, 2022 and 2021:
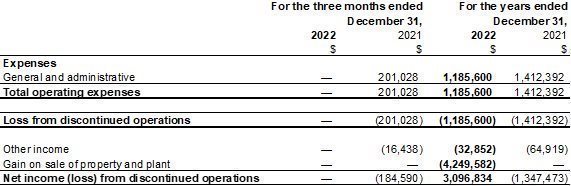
General and administrative
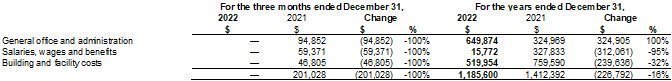
General and administrative expenses from discontinued operations decreased from $1,412,392 to $1,185,600 for the year ended December 31, 2022, compared to the equivalent periods in the prior year. On May 6, 2022, the Company closed the sale of the Facility and the Facility Property. Subsequent to sale of the Facility and the Facility Property results of operations related to FV Pharma are reported as continued operations for the year ended December 31, 2022.
SELECTED QUARTERLY INFORMATION
The following table sets forth selected unaudited quarterly statements of operations results for each of the eight quarters commencing January 1, 2022 and ending December 31, 2023. The information for each of these quarters has been prepared on the same basis as the audited annual financial statements for the year ended December 31, 2023. This data should be read in conjunction with our audited annual financial statements for the year ended December 31, 2023. These quarterly operating results are not necessarily indicative of our operating results for a full year or any future period.
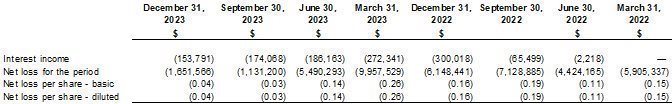
| 16 |
SEGEMENT INFORMATION
The Company’s Biopharmaceutical segment is focused on furthering the research and development of the Company’s drug candidates and the development of a treatment for alcohol misuse for application in hospitals and other medical practices. The Biopharmaceutical segment primarily earns interest income on excess cash on hand invested in short-term guaranteed investment certificates.
The Company’s Strategic Investments segment is focused on generating returns and cashflow through the issuance of loans secured by residential property, with FSD Strategic Investments having a first or second collateral mortgage on the secured property.
The following tables summarize the Company's interest income, total operating expenses, and net loss income for the years ended December 31, 2023, 2022 and 2021, on a segmented basis:

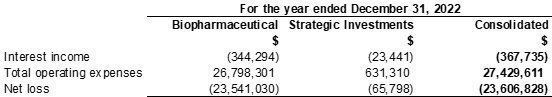
Prior to the incorporation of FSD investments the Company operated as a single segment.
| 17 |
FINANCIAL POSITION
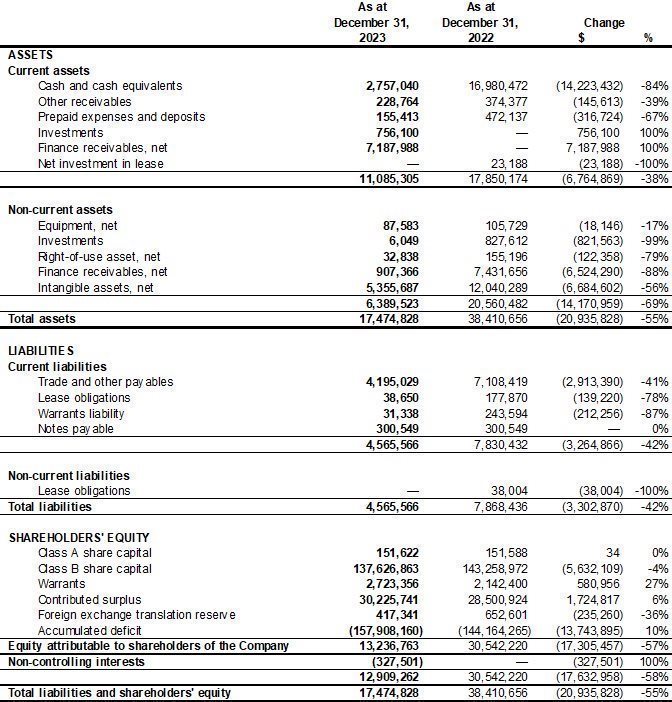
Assets
Cash and cash equivalents decreased by $14,223,432 or 84%, as a result of cash used in operating activities and financing activities during the period
Other receivables decreased by $145,613 or 39%. Other receivables primarily consist of sales taxes recoverable and interest receivable.
Prepaid expenses and deposits decreased by $316,724 or 67%, primarily related to a decrease in prepaids and deposits for planned research and development activities offset by an increase in prepaid insurance.
| 18 |
Finance receivables, current and non-current, increased by $663,698 or 9%, primarily due to new loans issued during the year offset by principal repayments.
Investments, current and non-current, decreased by $65,463 or 8%, due the to the purchase of a GIC offset by the sale of the Lions Bay Fund investment and the change in fair value of investments as a result of decreases in underlying share prices.
Intangible assets decreased by $6,684,602 or 56%, due to impairment of $4,319,619 and amortization expense of $2,364,983 incurred for the year ended December 31, 2023.
Liabilities
Trade and other payables decreased by $2,913,390 or 41%, primarily due to the reduction in trade and other payables of approximately $4.9M related to the settlement of recorded liabilities pertaining to a Contract Research Organization dispute, offset by an increase due to the timing of expenses incurred and payments made.
The fair value of the warrants liability as at December 31, 2023, was $31,338 (December 31, 2022 – $243,594) resulting in a gain on change in fair value of $212,256 for the year ended December 31, 2023 (December 31, 2022 – $521,809).
The fair value of the warrants liability as at December 31, 2023 and 2022, was determined using the Black-Scholes option pricing model and the following assumptions:

Lease obligations decreased due to lease payments made during the period.
Shareholders’ equity
|
| (i) | Shareholder’s equity decreased by $17,305,457 primarily due to: |
|
|
|
|
|
| (ii) | a decrease of $2,957,816 related to share buyback program offset by $2,462,712 for share-based payments and $20,247 for the issuance of common shares for share options exercised; |
|
|
|
|
|
| (iii) | an increase of $1,372,763 related to warrants issued during the period; |
|
|
|
|
|
| (iv) | a decrease of $235,260 related to the translation of foreign operations; and |
|
|
|
|
|
| (v) | a decrease of $18,230,588 related to net loss. |
| 19 |
Non-controlling interests
Through the License Agreement, FSD acquired 34.66% of Celly on July 31, 2023. As of December 31, 2023, the Company has a 26.15% (2022 – 0%) ownership interest in Celly through common shares held in Celly. The non-controlling interest represents the common shares of Celly not attributable to the Company.
Non-controlling interests as of December 31, 2023 was as follows:

LIQUIDITY, CAPITAL RESOURCES AND FINANCING
The general objectives of our capital management strategy are to preserve our capacity to continue operating, provide benefits to our stakeholders and provide an adequate return on investment to our shareholders by continuing to invest in our future that is commensurate with the level of operating risk we assume. We determine the total amount of capital required consistent with risk levels. This capital structure is adjusted on a timely basis depending on changes in the economic environment and risks of the underlying assets. We are not subject to any externally imposed capital requirements.
The financial statements and this MD&A have been prepared on the basis of accounting principles applicable to a going concern, which assumes that the Company will continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of operations. As at December 31, 2023, the Company has a working capital surplus, however, the Company has incurred negative cash flows and losses since inception and has generated no revenue to date. Failure to arrange adequate financing on acceptable terms and/or achieve profitability may have an adverse effect on the financial position, results of operations, cash flows and prospects of the Company. These factors indicate the existence of a material uncertainty that may cast substantial doubt about the Company’s ability to continue as going concern. The financial statements do not give effect to adjustments to assets or liabilities that would be necessary should the Company be unable to continue as a going concern. Such adjustments could be material
The financial statements and this MD&A have been prepared on the basis of accounting principles applicable to a going concern, which assumes that the Company will continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of operations. In making this assessment, management concluded that it has sufficient working capital as of December 31, 2023, in order to carry out its planned operations over the next twelve months.
| 20 |
The Company is in the preliminary stages of its planned operations and has not yet determined whether its processes and business plans are economically viable. The continuing operations of the Company are dependent upon the ability of the Company to complete the pharmaceutical research and development programs centered on the Company’s development of a treatment for alcohol misuse for application in hospitals and other medical practices and research and development of its lead compound, Lucid-MS.
As at December 31, 2023, the Company had cash of $2,757,040 representing a decrease of $14,223,432 from December 31, 2022. This decrease is primarily due to $10,827,264 of cash used in operating activities, $269,579 of cash used in investing activities and $3,126,589 of cash used in financing activities.
Cash flows for the years ended December 31, 2023 and 2022

Cash Flows Used in Operating Activities
Cash flows used in operating activities for the year ended December 31, 2023, were $10,827,264 compared to cash flows used in operating activities of $28,333,273 for the year ended December 31, 2022. The decrease in cash used in operating activities of $17,506,009 is primarily due to lower spending on general and administrative activities and external research and development.
Cash Flows (Used in) Provided by Investing Activities
Cash flows used in investing activities for the year ended December 31, 2023, were $269,579 compared to cash provided by investing activities of $12,123,408 for the year ended December 31, 2022. For the year ended December 31, 2023, the Company invested $744,500 in a Guaranteed Investment Certificate, which was offset by proceeds of $443,138 received from the sale of investments and $31,783 cash received upon the control of a subsidiary. For the year ended December 31, 2022, the Company received cash proceeds of $12,730,942 from the sale of the FV Pharma Facility and Facility Property. For the year ended December 31, 2022, the Company had cash outflows of $765,570 relating to purchases of investments, intangible assets and equipment offset by $158,036 proceeds from the sale of investments
Cash Flows Used in Financing Activities
Cash flows used in financing activities for the year ended December 31, 2023, were $3,126,589 compared to cash used in financing activities of $2,069,308 for the year ended December 31, 2022. Financing activities primarily related to the share repurchase program.
| 21 |
Cash flows for the years ended December 31, 2022 and 2021
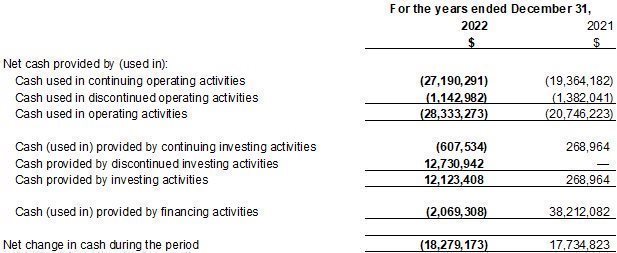
Cash Flows Used in Operating Activities
Cash flows used in continuing operating activities for the year ended December 31, 2022, were $27,190,291 compared to cash flows used in continuing operating activities of $19,364,182 for the year ended December 31, 2021. Cash flows used in discontinued operating activities for the year ended December 31, 2022, were $1,142,982 compared to cash flows used in discontinued operating activities of $1,382,041 for the year ended December 31, 2022. The increase in cash used in operating activities is primarily due to cash outflows related to finance receivables issued during the year ended December 31, 2022.
Cash Flows Provided by (Used in) Investing Activities
Cash flows provided by investing activities for the year ended December 31, 2022, were $12,123,408 compared to cash flows provided by investing activities of $268,964 for the year ended December 31, 2022. The change is primarily due to cash provided by the sale of the Facility and the Facility Property during the year ended December 31, 2022.
Cash Flows (Used in) Provided by Financing Activities
Cash flows used in financing activities for the year ended December 31, 2022, were $2,069,308 compared to cash provided by financing activities of $38,212,082 for the year ended December 31, 2021. During the year ended December 31, 2021, the Company issued shares for net proceeds of $38,341,407 offset by the repayment of $71,759 for notes payable and repayment of $57,566 for lease obligations compared to, $1,926,237 spend on share repurchases and the payment of $143,071 for lease obligations made during the year ended December 31, 2022.
| 22 |
CONTRACTUAL OBLIGATIONS
We have no significant contractual arrangements other than those noted in our financial statements.
OFF-BALANCE SHEET ARRANGEMENT
We have no off-balance sheet arrangements other than those noted in our financial statements.
USE OF PROCEEDS
The following is a reconciliation of use of proceeds from the 2020 Base Shelf Prospectus:
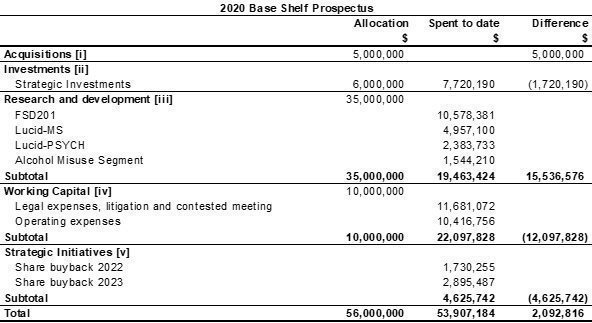
[i] Acquisitions
On September 21, 2021, the Company acquired Lucid, which was satisfied in Class B shares. This transaction did not require a cash outlay and thus the Company repurposed the funds towards two share repurchase programs.
[ii] Investments
During the effectiveness of the 2020 Base Shelf Prospectus, the Company explored multiple investment opportunities, but none of them met the investment criteria of the Company and the Cormpany repurposed the funds towards secured loans with an average maturity of two years from the date of issuance that earn fees at fixed rates.
| 23 |
[iii] Research and development
Includes funds allocated to complete one or more strategic corporate transactions (such as acquisitions) intended to accelerate the growth and development of the Company’s businesses, which funds remain subject to reallocation by the Company towards budgets and expenditures which are identified and/or otherwise unidentified or unforeseen, as at the relevant date.
[iv] Working Capital
Includes funds allocated for the payment of:
(a) general and administrative expenses;
(b) ongoing legal fees and other professional and consulting fees, and salaries; and
(c) ongoing costs associated with being a reporting issuer.
In 2021, the Company incurred an estimated $2,338,795 expenses related to the meeting of shareholders of the Company in compliance with section 105 of the OBCA (“Contested Meeting”) and associated events that resulted in additional unexpected legal and operating expenses.
Following the Contested Meeting, the former CEO, Dr. Raza Bokhari commenced five actions against the Company and management, which resulted in counterclaims and additional unexpected legal and operating expenses of approximately $5,560,874.
[v] Strategic Initiatives
As the Company had excess proceeds from the 2020 Base Shelf Prospectus and did not identify any strategic acquisitions and/or investment opportunities, the Company conducted two share repurchase programs, one beginning in December 2021 and the other in January 2023, as the Board determined that the market price of the Class B shares was undervalued, and these repurchases would strategically return value to shareholders.
Additional details are included in the Company’s’ Short Form Base Shelf Prospectus dated December 22, 2023, filed on www.sedarplus.ca.
| 24 |
The following is a breakdown of the use of proceeds for the 2023 Short Form Base Shelf Prospectus filed in December 2023.
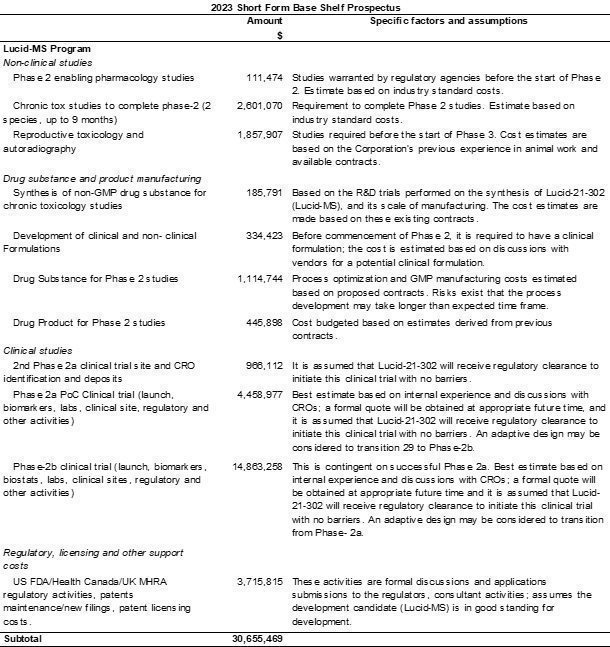
| 25 |

| 26 |

Additional details are included in the Company’s’ Short Form Base Shelf Prospectus dated December 22, 2023, filed on www.sedarplus.ca.
TRANSACTIONS WITH RELATED PARTIE
Key management personnel are those persons having the authority and responsibility for planning, directing and controlling activities of the entity, directly or indirectly.
Transactions with key management and directors comprised the following:
a) In fiscal 2023, the Company paid independent directors’ compensation of C$60,000, with the chair of the audit committee receiving an additional C$20,000 and the chair of the compensation committee receiving an additional C$10,000. Director’s compensation for the year ended December 31, 2023, was $175,140 (2022 – $215,104 and 2021 – $757,690).
b) During the year ended December 31, 2023, the Company granted 400,000 (2022 – 2,820,104 and 2021 – nil) PSUs to independent members of the Board of Directors. As at December 31, 2023, the PSUs had fully vested upon the filing of the MS Phase 1 IND on January 6, 2023 and were settled with the issuance of Class B shares.
c) During the year ended December 31, 2023, the Company granted the previous interim CEO, the current CEO, the COO and the CEO of Lucid, 500,000 (2022 – nil and 2021 – nil) share options each with an exercise price of C$1.30 and an expiry date of January 25, 2028. All options were fully vested on grant. Each share option can be exercised to acquire one Class B share.
d) During the year ended December 31, 2023, the Company entered into a secured loan agreement with the CEO, President, Executive Co-Chairman of the Board in the amount of C$1,200,000, with monthly payments of C$6,000 based on an annual interest rate of 6%. The loan matures on April 26, 2025, and is part of FSD Strategic Investments’ portfolio of loans. The loan is secured by a second charge mortgage on the underlying residential property.
e) During the year ended December 31, 2023, the Company issued 1,000,000 warrants for consulting services to certain independent members of the Board of Directors with a fair value of $533,206, prior to them joining the Board of Directors. The Company determined the fair value of the services received could not be measured reliably and determined the fair value using the Black-Scholes model.
f) In November 2023, the Company issued 24 Class A shares through a private placement for proceeds of $34. 12 Class A shares were issued to the CEO, President, and Executive Co-Chairman of the Board and 12 Class A share were issued to the Director and Executive Co-Chairman of the Board.
g) In February 2021, as compensation, the Company issued 1,349,764 shares with a fair value of $3,576,875 to former CEO, Raza Bokhari, in his capacity as Board Chair and Chief Executive Officer, and to certain other directors. Of the 1,349,764 shares issued, 1,173,709, with a fair value of $3,110,330, were issued to Raza Bokhari and 176,055 shares, with a fair value of $466,545, were issued to other directors. In June 2021, 156,278 of the shares issued to directors in February 2021 were cancelled. On March 8, 2022, following litigation with respect to certain of the shares issued to Raza Bokhari in February 2021, the court issued a decision, permitting the part of the share grant to Raza Bokhari until the date of his termination (being 536,979 Class B shares) but cancelling the shares relating to services that were to be provided after the date of termination (being 504,888 Class B shares). The shares were cancelled on March 29, 2022.
h) For the year ended December 31, 2023, the Company paid expenses of $nil (2022 – $nil and 2021 – $262,834) to a company owned by the former CEO for the year ended December 31, 2023.
| 27 |
i) For the year ended December 31, 2023, the Company reimbursed $145,081 (2022 – $41,596 and 2021 – $528,872) to a related party of the CEO, President, and Executive Co-Chairman of the Board for legal expenses.
j) During the year ended December 31, 2021, the Company reimbursed certain directors C$1,334,158 for expenses incurred in relation to requisitioning, calling, and holding the shareholders' meeting.
Key management personnel compensation during the years ended December 31, 2023 and 2022 is comprised of:
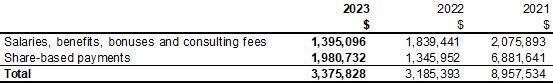
As at December 31, 2023, the Company owes an executive officer $140,012, for legal fees incurred by the Company and paid by the executive officer on behalf of the Company. The amount owed is recorded within trade and other payables.
FINANCIAL INSTRUMENTS AND OTHER INSTRUMENTS
Credit risk
Credit risk is the risk of financial loss to the Company if a customer or counterparty to a financial instrument fails to meet its contractual obligations and arises principally from deposits with banks and outstanding other receivables and finance receivables. The Company trades only with recognized, creditworthy third parties.
The Company does not hold any collateral as security for its outstanding finance receivables but mitigates this risk by dealing only with, what management believes to be, financially sound counterparties and, accordingly, does not anticipate significant loss for non-performance. The loans are secured by residential properties and the Company is granted a first or second collateral charge mortgage on the properties for a sum equal to the interest payments plus the principal amount. The Company performs assessments on factors such as: timing of payments, loan to value ratios, communications with the borrower and external macro factors such as interest rates and economic conditions to mitigate risks.
Liquidity risk
Liquidity risk is the risk the Company will not be able to meet its financial obligations as they come due. The Company’s exposure to liquidity risk is dependent on the Company’s ability to raise additional financing to meet its commitments and sustain operations. The Company mitigates liquidity risk by management of working capital, cash flows, the issuance of share capital and if desired, the issuance of debt. The Company’s trade and other payables are all due within twelve months from the date of these financial statements.
If unanticipated events occur that impact the Company’s ability to carry out the planned clinical trials, the Company may need to take additional measures to increase its liquidity and capital resources, including issuing debt or additional equity financing or strategically altering the business forecast and plan. In this case, there is no guarantee that the Company will obtain satisfactory financing terms or adequate financing. Failure to obtain adequate financing on satisfactory terms could have a material adverse effect on the Company’s results of operations or financial condition.
Market risk
Market risk is the risk the fair value or future cash flows of a financial instrument will fluctuate because of changes in market prices. Market risk comprises three types of risk: foreign currency risk, interest rate risk and other price risk.
| · | Foreign currency risk |
Foreign currency risk arises on financial instruments that are denominated in a currency other than the functional currency in which they are measured. The Company’s primary exposure with respect to foreign currencies is from Canadian dollar denominated cash and trade and other payables. A 1% change in the foreign exchange rates would not result in any significant impact to the financial statements.
| 28 |
| · | Interest rate risk |
Interest rate risk is the risk the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The Company’s finance receivables are at fixed rates and there are no material long-term borrowings outstanding. The Company is not exposed to interest rate risk as at December 31, 2023.
| · | Other price risk |
Other price risk is the risk the fair value or future cash flows of a financial instrument will fluctuate because of changes in market prices (other than those arising from interest rate risk or currency risk), whether those changes are caused by factors specific to the individual financial instrument or its issuer, or factors affecting all similar financial instruments traded in the market. The Company is not exposed to other price risk as at December 31, 2023.
Fair values
The carrying values of cash, other receivables, trade and other payables and notes payable approximate fair values due to the short-term nature of these items or they are being carried at fair value or, for notes payable, interest payables are close to the current market rates. The risk of material change in fair value is not considered to be significant. The Company does not use derivative financial instruments to manage this risk.
Financial instruments recorded at fair value on the consolidated statement of financial position are classified using a fair value hierarchy that reflects the significance of the inputs used in making the measurements. The Company categorizes its fair value measurements according to a three-level hierarchy. The hierarchy prioritizes the inputs used by the Company’s valuation techniques. A level is assigned to each fair value measurement based on the lowest-level input significant to the fair value measurement in its entirety. The three levels of the fair value hierarchy are defined as follows:
| · | Level 1 – Unadjusted quoted prices as at the measurement date for identical assets or liabilities in active markets. |
|
|
|
| · | Level 2 – Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data. |
|
|
|
| · | Level 3 – Significant unobservable inputs that are supported by little or no market activity. The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. |
The fair value hierarchy requires the use of observable market inputs whenever such inputs exist. A financial instrument is classified to the lowest level of the hierarchy for which a significant input has been considered in measuring fair value.
Private company investments measured at fair value are classified as Level 3 financial instruments. The valuation method and significant assumptions used to determine the fair value of private company investments have been disclosed in the financial statements. The Company did not hold any private company investments as of December 31, 2023. During the year, there were no transfers of amounts between levels.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Refer to Note 2 and Note 3 of the audited consolidated financial statements for the fiscal year ended December 31, 2023, for a full discussion of our critical accounting policies and estimates.
OUTSTANDING SHARE DATA
The Company is authorized to issue an unlimited number of Class A multiple voting shares ("Class A shares") and an unlimited number of Class B subordinate voting shares ("Class B shares"), all without par value. All shares are ranked equally with regards to the Company's residual assets.
The holders of Class A shares are entitled to 276,660 votes per Class A share held. Class A shares are held by the CEO, President, Co-Chairman of the Board and the Director, Co-Chairman of the Board.
| 29 |
The Company's outstanding capital was as follows as at the date of this MD&A:
| Class A shares |
|
| 72 |
|
| Class B shares |
|
| 40,116,434 |
|
| Share options |
|
| 3,260,615 |
|
| Warrants |
|
| 11,705,258 |
|
| RSUs |
|
| 55,000 |
|
SUBSEQUENT EVENTS
On January 24, 2024, the Company entered into an agreement with SBS Intl Group LLC. (“SBS”) to assist the Company in enhancing its market awareness and foster productive, continuing dialogues with shareholders and other market participants. The agreement grants SBS 100,000 share options with an exercise price of $1.05 and expiry date of January 24, 2026. Per the agreement 19,000 share options vest on the 45th day following the date of grant and 9,000 share options vest on a monthly basis starting in the fourth month following the date of grant.
On January 2024, the Company entered into an agreement with Draper, Inc. (“Draper”) and Carriage House Capital, Corp. (“Carriage House”) to assist the Company in enhancing its market awareness and foster productive, continuing dialogues with shareholders and other market participants. The agreement grants Draper and Carriage 350,000 share options each with the exercise price of $1.05 and expiry date of January 24, 2026. Per the agreement 150,000 share options vest on the 45th day and 61,111 share options vest on a monthly basis starting in the fourth month following the date of grant.
On February 23, 2024, the Company entered into a settlement agreement to issue 70,000 Class B shares to settle $81,900 of trade and other payables.
On February 23, 2024, the Company entered into a settlement agreement to issue 475,000 Class B shares to settle $836,309 of trade and other payables.
On February 23, 2024, the Company granted 55,000 RSUs to advisors of the Company for services provided. The RSUs vested immediately upon grant.
On March 26, 2024, the Board approved an amendment to the loan agreement with Celly, to increase the loan amount from C$1,000,000 to C$1,300,000. The amendment provides the Company the right to convert any loan amount outstanding including interest into Common Shares of Celly at $0.03 per share upon the occurrence of an event of default.
Subsequent to December 31, 2023, the Company entered into an at-the-market offering agreement (the “ATM Agreement”) with H.C Wainwright & Co., LLC to sell Class B shares, having an aggregate offering price up to $11,154,232.
DISCLOSURE CONTROLS AND PROCEDURES AND INTERNAL CONTROLS OVER FINANCIAL REPORTING
A. Disclosure Controls and Procedures
We maintain disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, which are designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and that such information is accumulated and communicated to our management, including our CEO and CFO, as appropriate to allow timely decisions regarding required disclosure.
Under the supervision and with the participation of our CEO and CFO, our management has evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2023, the end of the period covered by this report. Based on that evaluation, our CEO and CFO concluded that our disclosure controls and procedures were effective as of December 31, 2023.
The effectiveness of our disclosure controls and procedures and our internal control over financial reporting is subject to various inherent limitations, including cost limitations, judgments used in decision making, assumptions about the likelihood of future events, the soundness of our systems, the possibility of human error, and the risk of fraud. Moreover, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions and the risk that the degree of compliance with policies or procedures may deteriorate over time. Because of these limitations, there can be no assurance that any system of disclosure controls and procedures or internal control over financial reporting will be successful in preventing all errors or fraud or in making all material information known in a timely manner to the appropriate levels of management.
B. Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting is the process designed by and under the supervision of our CEO and CFO to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our financial statements for external reporting in accordance with accounting principles generally accepted in the United States of America. Management has evaluated the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (2013).
Under the supervision and with the participation of our CEO and CFO, our management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2023 and concluded that it was effective.
| 30 |
EXHIBIT 15.2

CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We have issued our report dated March 28, 2024 with respect to the consolidated financial statements of FSD Pharma Inc. and its subsidiaries (the “Company”) as of December 31, 2023 and 2022, and for each of the years in the three-year period ended December 31, 2023, which includes an explanatory paragraph relating to the Company’s ability to continue as a going concern, included in the Company’s Annual Report on Form 20-F of FSD Pharma Inc. for the year ended December 31, 2023, as filed with the United States Securities and Exchange Commission ("SEC"). We consent to the incorporation by reference of the aforementioned report in the Company’s Registration Statement on Form F-3 (333-276264), as amended.
We also consent to the reference to our firm under the heading “Experts” and “Transfer Agent, Registrar and Auditor” in the Registration Statement on Form F-3.
Chartered Professional Accountants Licensed Public Accountants
April 1, 2024
Mississauga, Canada
| MNP LLP Suite 900, 50 Burnhamthorpe Road W, Mississauga ON, L5B 3C2 | T: 416.626.6000 F: 416.626.8650 |
|
|
|
| MNP.ca |
|
|
EXHIBIT 97.1
FSD PHARMA INC.
CLAWBACK POLICY
This FSD Pharma Inc. Clawback Policy (this “Policy”) was approved effective as of November 28, 2023 (the “Effective Date”) by the Compensation Committee (the “Committee”) of the Board of Directors (the “Board”) of FSD Pharma Inc. (the “Company”). This Policy is adopted pursuant to and intended to comply with Rule 5608 (Recovery of Erroneously Awarded Compensation) of The Nasdaq Stock Market LLC (“Nasdaq”) so long as the Company’s securities are listed on Nasdaq.
Purpose and Policy Statement
The Company is committed to conducting business with integrity in accordance with high ethical standards and in compliance with all applicable laws, rules, and regulations. This includes the Company’s commitment to comply with all laws, rules, and regulations applicable to the presentation of the Company’s financial information to the public and to the recovery of erroneously awarded incentive-based compensation.
As a result, the Committee has adopted this Policy to provide that, in the event the Company is required to prepare an accounting restatement due to the material noncompliance of the Company with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period (each, as applicable, a “Restatement”), the Company will recover reasonably promptly the amount of any “erroneously awarded compensation” “received” by an “executive officer,” in each case as such terms are defined in this Policy, if and to the extent required by any federal or state law, rule or regulation, or rule, regulation, policy or listing standard of the Securities and Exchange Commission (“SEC”) or any securities exchange on which the Company’s securities are listed, including without limitation, Nasdaq Rule 5608 (Recovery of Erroneously Awarded Compensation).
In the event of any change in any federal or state law, rule or regulation, or rule, regulation, policy or listing standard of the SEC or any securities exchange on which the Company’s securities are listed after the Effective Date, which requires the Company to recover compensation from an executive officer, the Company will seek recovery under this Policy to the extent required by such laws, rules, regulations or listing standards.
Administration
The Committee has full power, authority, and sole and exclusive discretion to reasonably construe, interpret, and administer this Policy. The Committee will interpret this Policy consistent with Nasdaq Rule 5608 (Recovery of Erroneously Awarded Compensation) and any guidance issued thereunder, the rules and regulations of the SEC, and any other applicable laws, rules or regulations governing the mandatory recovery of compensation, as such laws, rules or regulations may change, be interpreted, or evolve from time to time. All determinations and decisions made by the Committee will be made in its reasonable discretion and will be final, conclusive, and binding on all affected individuals.
| 1 |
The term “Committee” as used in this Policy means the Compensation Committee of the Board, or in the absence of such a committee, a majority of the “independent directors” (as defined under Nasdaq Rule 5605(a)(2)) serving on the Board.
Applicability
This Policy applies to all “incentive-based compensation” “received” by a person, in each case as such terms are defined in this Policy:
|
| · | After beginning service as an “executive officer,” as such term is defined in this Policy, and who served as an executive officer at any time during the performance period for that incentive-based compensation; |
|
|
|
|
|
| · | While the Company has a class of securities listed on Nasdaq or another national securities exchange or a national securities association; and |
|
|
|
|
|
| · | During the three completed fiscal years immediately preceding the date that the Company is required to prepare the Restatement, plus any transition period (that results from a change in the Company’s fiscal year) within or immediately following those three completed fiscal years; provided, however, that a transition period between the last day of the Company’s previous fiscal year-end and the first day of its new fiscal year that comprises a period of nine to 12 months would be deemed a completed fiscal year; and provided, further, that the Company’s obligation to recover erroneously awarded compensation is not dependent on if or when the restated financial statements are filed. |
For purposes of determining the relevant recovery period, the date that the Company is required to prepare a Restatement is the earlier to occur of (i) the date the Company’s Board, a committee of the Board, or the officer or officers of the Company authorized to take such action if Board action is not required, concludes, or reasonably should have concluded, that the Company is required to prepare a Restatement; or (ii) the date a court, regulator or other legally authorized body directs the Company to prepare a Restatement.
Executive Officers Covered by Policy
This Policy covers the Company’s current and former executive officers who received erroneously awarded compensation regardless of whether the executive officer committed misconduct or contributed to the error.
The term “executive officer” as used in this Policy means the Company’s:
|
| · | president; |
|
|
|
|
|
| · | principal financial officer; |
|
|
|
|
|
| · | principal accounting officer (or if there is no such accounting officer, the controller); |
|
|
|
|
|
| · | any vice-president of the Company in charge of a principal business unit, division, or function (such as sales, administration, or finance); |
|
|
|
|
|
| · | any other officer who performs a policy-making function; or |
|
|
|
|
|
| · | any other person who performs similar policy-making functions for the Company and executive officers of the Company’s parents or subsidiaries if such individuals perform such policy-making functions for the Company. |
| 2 |
Policy-making function is not intended to include policy-making functions that are not significant.
Identification of an executive officer for purposes of this Policy would include at a minimum executive officers identified by the Company pursuant to Item 401(b) of SEC Regulation S-K.
Authority and Obligation to Recover Erroneously Awarded Compensation; Exceptions
In the event of a Restatement, the Company must reasonably promptly recover any “erroneously awarded compensation,” as such term is defined in this Policy, in compliance with this Policy, except to the extent one of the three conditions below is met and the Committee has made a determination that recovery would be impracticable.
|
| 1. | The direct expense paid to a third party to assist in enforcing this Policy would exceed the amount to be recovered and the Company has made a reasonable attempt to recover any amount of erroneously awarded compensation, has documented such reasonable attempt(s) to recover and provided that documentation to Nasdaq. |
|
|
|
|
|
| 2. | Recovery would violate home country law where that law was adopted prior to November 28, 2022, and the Company has obtained an opinion of home country counsel, acceptable to Nasdaq, that recovery would result in such a violation and has provided such opinion to Nasdaq. |
|
|
|
|
|
| 3. | Recovery would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of the Company, to fail to meet the requirements of Section 401(a)(13) or 411(a) of the U.S. Internal Revenue Code and regulations thereunder. |
Erroneously Awarded Compensation
The term “erroneously awarded compensation” as used in this Policy means that amount of “incentive-based compensation” received that exceeds the amount of “incentive-based compensation” that otherwise would have been received had it been determined based on the restated amounts, and must be computed without regard to any taxes paid.
For incentive-based compensation based on stock price or total shareholder return, where the amount of erroneously awarded compensation is not subject to mathematical recalculation directly from the information in a Restatement:
|
| · | the amount must be based on a reasonable estimate of the effect of the Restatement on the stock price or total shareholder return upon which the incentive-based compensation was received; and |
|
| · | the Company must maintain documentation of the determination of that reasonable estimate and provide such documentation to Nasdaq. |
The term “incentive-based compensation” as used in this Policy means any compensation that is granted, earned, or vested based wholly or in part upon the attainment of a financial reporting measure.
| 3 |
The term “financial reporting measures” as used in this Policy means measures that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and any measures that are derived wholly or in part from such measures. Financial reporting measures include, without limitation, stock price and total shareholder return, and may include non-GAAP financial measures. A financial reporting measure need not be presented within the Company’s financial statements or included in an SEC filing to constitute a financial reporting measure for this purpose.
Incentive-based compensation is deemed “received” as such term is used in this Policy by an executive officer in the Company’s fiscal period during which the financial reporting measure specified in the incentive-based compensation award is attained, even if the payment or grant of the incentive-based compensation occurs after the end of that period.
Notwithstanding the generality of the foregoing, “incentive-based compensation” is intended to be interpreted and construed broadly and includes with respect to any plan that takes into account incentive-based compensation (other than a tax-qualified plan) any amount contributed to a notional account based on erroneously awarded compensation and any earnings accrued to date on that notional account. Such plans include without limitation long-term disability plans, life insurance plans, supplemental executive retirement plans and other compensation, if it is based on incentive-based compensation.
For clarity and the avoidance of doubt, “incentive-based compensation” does not include the following:
|
| · | base salary (other than any base salary increase earned wholly or in part based on the attainment of a financial reporting measure, which increase is subject to recovery as incentive-based compensation hereunder); |
|
|
|
|
|
| · | bonuses paid solely at the discretion of the Committee or Board that are not paid from a “bonus pool” that is determined by satisfying a financial reporting measure performance goal; |
|
|
|
|
|
| · | bonuses paid solely upon satisfying one or more subjective standards (e.g. demonstrated leadership) and/or completion of a specified employment period; |
|
|
|
|
|
| · | non-equity incentive plan awards earned solely upon satisfying one or more strategic measures (e.g., consummating a merger or divestiture), or operational measures (e.g., completion of a project); and |
|
|
|
|
|
| · | equity awards for which the grant is not contingent upon achieving any financial reporting measure performance goal, and vesting is contingent solely upon completion of a specified employment period and/or attaining one or more non-financial reporting measures. |
| 4 |
Method of Recovery
The Committee will determine, in its reasonable discretion, the method for recovering incentive-based compensation hereunder, which may include, without limitation, any one or more of the following:
|
| · | requiring reimbursement of cash incentive-based compensation previously paid; |
|
|
|
|
|
| · | seeking recovery of any gain realized on the vesting, exercise, settlement, sale, transfer, or other disposition of any equity-based awards; |
|
|
|
|
|
| · | cancelling or rescinding some or all outstanding vested or unvested equity-based awards; |
|
|
|
|
|
| · | adjusting or withholding from unpaid compensation, deferred compensation, or other set-off; |
|
|
|
|
|
| · | cancelling or setting-off against planned future grants of equity-based awards; and/or |
|
|
|
|
|
| · | any other method required or authorized by applicable law or contract. |
Enforceability
In addition to the adoption of this Policy, the Company will take steps to implement an agreement to this Policy by all current and future executive officers. In furtherance of the foregoing, each executive officer subject to this Policy is required to sign and return to the Company the Acknowledgement Form attached hereto as Exhibit A pursuant to which such executive officer will agree to be bound by the terms and comply with this Policy.
Policy Not Exclusive
Any recovery under this Policy is in addition to, and not in lieu of, any other remedies or rights of recovery that may be available to the Company pursuant to the terms of any other clawback or recovery policy or any similar policy in any employment agreement, incentive or equity compensation plan or award or other agreement and any other legal rights or remedies available to the Company.
Notwithstanding the generality of the foregoing, to the extent that the requirements under the provisions of Section 304 of the Sarbanes-Oxley Act of 2002 are broader than the provisions in this Policy, the provisions of such law will apply to the Company’s Chief Executive Officer and Chief Financial Officer.
No Indemnification
The Company will not indemnify or agree to indemnify any executive officer or former executive officer against the loss of erroneously awarded compensation nor will the Company pay or agree to pay any insurance premium to cover the loss of erroneously awarded compensation.
| 5 |
Effective Date
This Policy is effective as of the Effective Date and applies to all incentive-based compensation received by the Company’s current and former executive officers on or after the Effective Date.
Required Disclosures
The Company will file all disclosures with respect to this Policy in accordance with the requirements of the federal securities laws, including the disclosure required by the applicable SEC filings, and will provide all required SEC and other disclosures regarding this Policy and in the event of a Restatement.
Amendment and Termination
The Committee may amend, modify, or terminate this Policy in whole or in part at any time in its sole discretion and may adopt such rules and procedures that it deems necessary or appropriate to implement this Policy or to comply with Nasdaq Rule 5608 (Recovery of Erroneously Awarded Compensation) and any other applicable laws, rules, and regulations.
Successors
This Policy shall be binding and enforceable against all current and former executive officers of the Company and their respective beneficiaries, heirs, executors, administrators, or other legal representatives.
* * * * * *
| 6 |
PETVIVO HOLDINGS, INC.
CLAWBACK POLICY
ACKNOWLEDGEMENT FORM
By signing below, the undersigned acknowledges and confirms that the undersigned has received and reviewed a copy of the PetVivo Holdings, Inc. Clawback Policy (the “Policy”).
By signing this Acknowledgement Form, the undersigned acknowledges and agrees that the undersigned is and will continue to be subject to the Policy and that the Policy will apply both during and after the undersigned’s employment with PetVivo Holdings, Inc., and its direct and indirect subsidiaries.
Further, by signing below, the undersigned agrees to abide by the terms of the Policy, including, without limitation, by returning any erroneously awarded compensation (as defined in the Policy) to PetVivo Holdings, Inc. and its direct and indirect subsidiaries to the extent required by, and in a manner permitted by the Policy.
|
| Signature: |
|
|
|
|
|
|
|
|
| Name: |
|
|
|
|
|
|
|
|
| Date: |
|
|
| 7 |