
PRESENTED BY: Tumor Treating Fields (TTFields) Therapy with Standard of Care (SOC) in Metastatic Non-Small Cell Lung Cancer (mNSCLC) After Platinum-based Therapies: Randomized, Phase 3 LUNAR Study Ticiana Leal, MD, Winship Cancer Institute - Emory University Ticiana Leal1, Rupesh Kotecha2, Rodryg Ramlau3, Li Zhang4, Janusz Milanowski5, Manuel Cobo6, Jaromir Roubec7, Lubos Petruzelka8, Libor Havel9, Sujith Kalmadi10, Jeffrey Ward11, Zoran Andric12, Thierry Berghmans13, David E. Gerber14, Goetz Kloecker15, Rajiv Panikkar16, Joachim Aerts17, Angelo Delmonte18, Miklos Pless19, Richard Greil20, Christian Rolfo21, Wallace Akerley22, Michael Eaton23, Mussawar Iqbal24, and Corey Langer25; on behalf of the LUNAR study investigators 1Winship Cancer Institute at Emory University, Atlanta, GA, USA; 2Miami Cancer Institute, Baptist Health South Florida, Miami, FL, USA; 3Rodryg Ramlau, Poznan University of Medical Sciences, Poznan, Poland; 4Sun Yat-sen University Cancer Center (SYSUCC), Guangzhou, China; 5Medical University of Lublin, Lublin, Poland; 6Medical Oncology Intercenter Unit, Regional and Virgen de la Victoria University Hospitals, IBIMA, Málaga, Spain; 7Nemocnice AGEL Ostrava-Vítkovice, Ostrava, Czech Republic; 8General University Hospital in Prague, Prague, Czech Republic; 9Thomayer Hospital, Prague, Czech Republic; 10Ironwood Cancer & Research Centers, Chandler, AZ, USA; 11Washington University School of Medicine, St. Louis, MO, USA; 12Clinical Hospital Centre Bezanijska Kosa, Belgrade, Serbia; 13Jules Bordet Institute, Hôpitaux Universitaires de Bruxelles, Université Libre de Bruxelles, Brussels, Belgium; 14Harold C. Simmons Comprehensive Cancer Center, UT Texas Southwestern Medical Center, Dallas, TX, USA; 15University of Louisville, Louisville, KY, USA; 16Geisinger Cancer Institute, Danville, PA, USA; 17Erasmus University Medical Center, Erasmus MC Cancer Institute, Rotterdam, The Netherlands; 18IRCCS Istituto Romagnolo per lo Studio dei Tumori "Dino Amadori" (IRST), Meldola, Italy, Meldola, Italy; 19Kantonsspital Winterthur, Winterthur, Switzerland; 20Salzburg Cancer Research Institute-Center for Clinical Cancer and Immunology Trials (SCRI-CCCIT); Paracelsus Medical University Salzburg; Cancer Cluster, Salzburg, Austria; 21Center for Thoracic Oncology, Tisch Cancer Institute at Icahn School of Medicine, Mount Sinai, New York, NY, USA; 22Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA; 23St Francis Hospital, Indianapolis, IN, USA; 24College of Medicine, University of Saskatchewan, Saskatoon, Canada; 25Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA

PRESENTED BY: Background • Metastatic NSCLC remains largely incurable • Platinum-based chemotherapy with immune checkpoint inhibitors (ICIs) are standard first line therapy for metastatic NSCLC lacking actionable driver mutations1–3 • Unfortunately, most patients develop disease progression4–7 and 5-year survival is only 9%8 • Treatment options that extend survival beyond progression are limited • Current approaches in the second line include chemotherapy regimens, mainly docetaxel (DTX) with or without ramucirumab, or ICI (if eligible)2 • Phase 3 studies demonstrated that ICIs (OS of 10–14 months) are superior to DTX (OS of 8–9 months) for NSCLC progressing on platinum therapy9–11 • Unmet need remains high for new, well-tolerated and effective options for second-line treatment and beyond DTX, docetaxel; ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; OS, overall survival. 1. Hendriks LE et al. Ann Oncol. 2023;34(4):358–376; 2. National Comprehensive Cancer Network. Version 3. 2022; 3. Owen DH et al. J Clin Oncol. 2023;41(5):e1–e9; 4. de Castro G et al. J Clin Oncol. 2023;41(11):1986–1991; 5. Reck M et al. J Clin Oncol. 2021;39(21):2339–2349; 6. Brahmer JR et al. J Clin Oncol. 2023;41(6):1200–1212; 7. Herbst RS et al. N Engl J Med. 2020;383(14):1328–1339; 8. www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics; 9. Herbst RS et al. J Thorac Oncol. 2021;16(10):1718–1732. 10. Borghaei H et al. J Clin Oncol. 2021;39(7):723–733. 11. Rittmeyer A et al. Lancet. 2017;389(10066):255–265.
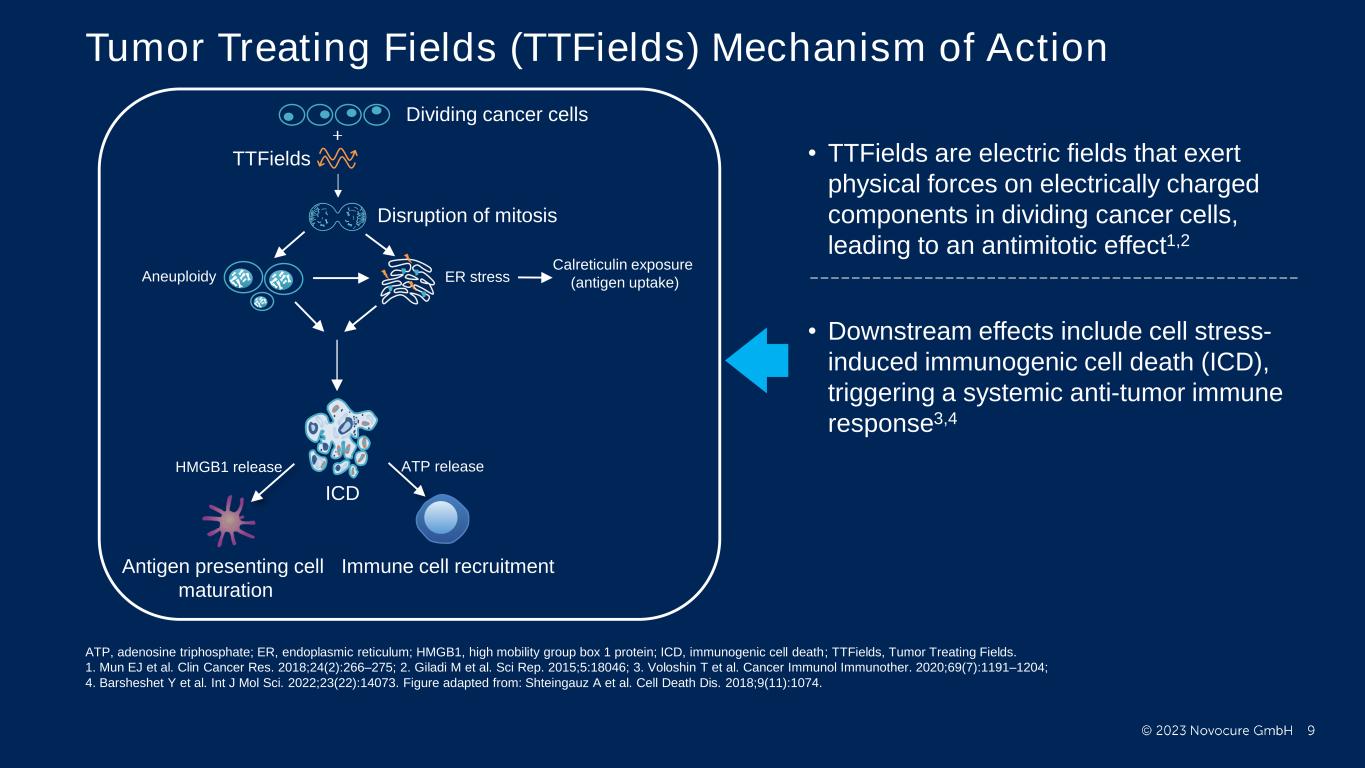
PRESENTED BY: Tumor Treating Fields (TTFields) Mechanism of Action Dividing cancer cells TTFields Aneuploidy ER stress ICD Immune cell recruitmentAntigen presenting cell maturation HMGB1 release ATP release Calreticulin exposure (antigen uptake) Disruption of mitosis • TTFields are electric fields that exert physical forces on electrically charged components in dividing cancer cells, leading to an antimitotic effect1,2 • Downstream effects include cell stress- induced immunogenic cell death (ICD), triggering a systemic anti-tumor immune response3,4 ATP, adenosine triphosphate; ER, endoplasmic reticulum; HMGB1, high mobility group box 1 protein; ICD, immunogenic cell death; TTFields, Tumor Treating Fields. 1. Mun EJ et al. Clin Cancer Res. 2018;24(2):266–275; 2. Giladi M et al. Sci Rep. 2015;5:18046; 3. Voloshin T et al. Cancer Immunol Immunother. 2020;69(7):1191–1204; 4. Barsheshet Y et al. Int J Mol Sci. 2022;23(22):14073. Figure adapted from: Shteingauz A et al. Cell Death Dis. 2018;9(11):1074.
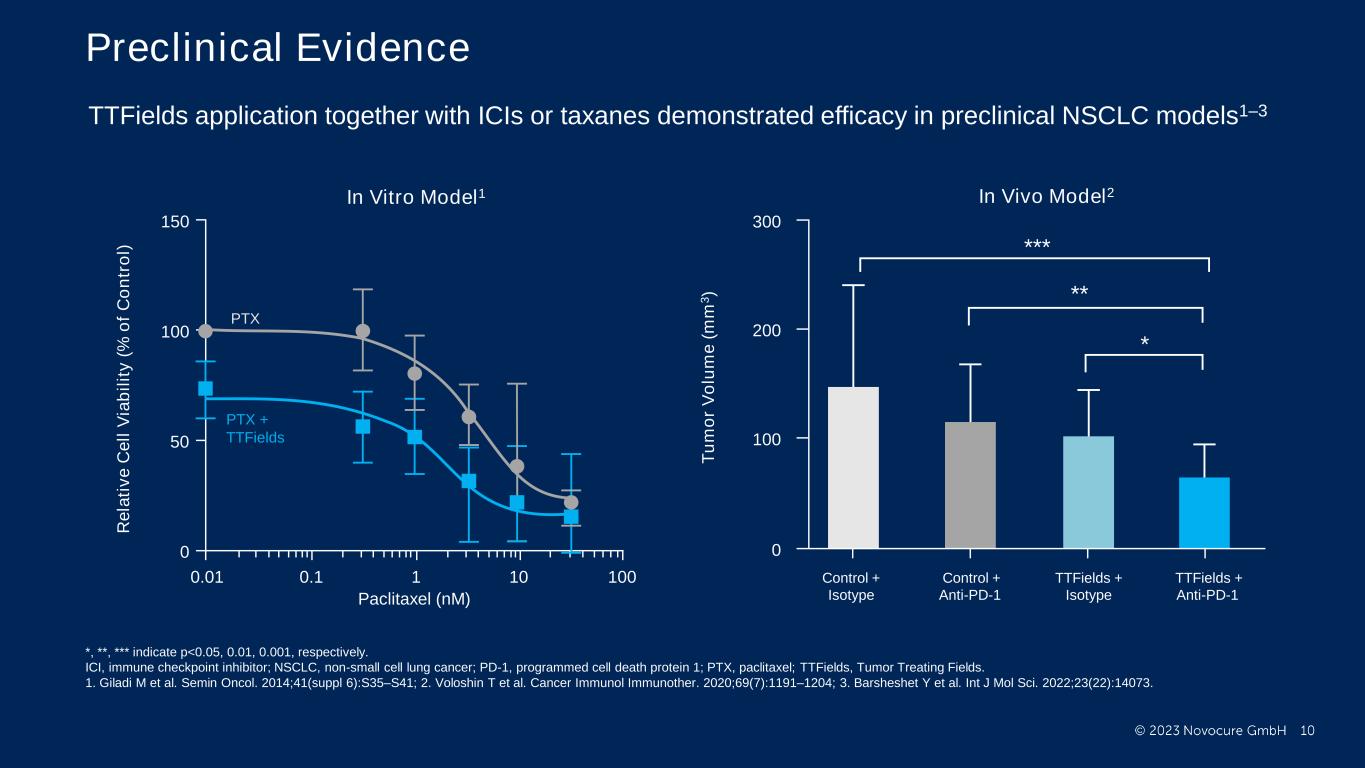
PRESENTED BY: Preclinical Evidence Ticiana Leal, MD, Winship Cancer Institute - Emory University PTX PTX + TTFields In Vitro Model1 150 100 50 0 0.01 0.1 1 10010 Paclitaxel (nM) R e la ti v e C e ll V ia b il it y ( % o f C o n tr o l) 300 0 200 100 Control + Isotype Control + Anti-PD-1 TTFields + Isotype TTFields + Anti-PD-1 * ** *** T u m o r V o lu m e ( m m 3 ) In Vivo Model2 TTFields application together with ICIs or taxanes demonstrated efficacy in preclinical NSCLC models1–3 *, **, *** indicate p<0.05, 0.01, 0.001, respectively. ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; PTX, paclitaxel; TTFields, Tumor Treating Fields. 1. Giladi M et al. Semin Oncol. 2014;41(suppl 6):S35–S41; 2. Voloshin T et al. Cancer Immunol Immunother. 2020;69(7):1191–1204; 3. Barsheshet Y et al. Int J Mol Sci. 2022;23(22):14073.

PRESENTED BY: TTFields Therapy • Noninvasive anticancer treatment modality • Delivered locoregionally to the chest by a wearable medical device and 2 pairs of arrays (adhesive bandages with biocompatible insulated ceramic discs covered by hydrogel)1 • Delivered to the patient’s home with 24/7 phone support by a device technician; continuous use (~18 h/day) • FDA-approved* for glioblastoma and malignant pleural mesothelioma2–4 • Pilot study demonstrated safety and feasibility of TTFields therapy with pemetrexed in advanced NSCLC5 Ticiana Leal, MD, Winship Cancer Institute - Emory University TTFields Device Array Placement *TTFields for glioblastoma was approved via the Premarket Approval (PMA) pathway. TTFields for malignant pleural mesothelioma was approved via the Humanitarian Device Exemption (HDE) pathway. NSCLC, non-small cell lung cancer; TTFields, Tumor Treating Fields. Image shows an actor. Used with permission from Novocure GmbH. 1. Novocure. NovoTTF™-100L system: instructions for use for unresectable pleural malignant mesothelioma; 2. Stupp R et al. Eur J Cancer. 2012;48(14):2192–2202; 3. Stupp R et al. JAMA. 2017;318(23):2306–2316; 4. Ceresoli GL et al. Lancet Oncol. 2019;20(12):1702–1709; 5. Pless M et al. Lung Cancer. 2013;81(3):445–450.

PRESENTED BY: LUNAR Phase 3 Study Design Key eligibility criteria • ≥22 years of age • Metastatic NSCLC • Progression on/after platinum-based therapy • ECOG PS 0–2 Randomized (1:1) TTFields therapy* and SOC (Investigator’s choice ICI† or docetaxel) SOC (Investigator’s choice ICI† or docetaxel) Baseline evaluation (incl. MRI) N=276 Data cut-off: November 26, 2022 Study sites: 124 in 17 countries (North America, Europe, Asia) Objective: To evaluate safety and efficacy of TTFields therapy with standard of care (SOC) compared to SOC alone in metastatic NSCLC progressing on or after platinum-based therapy Q6W follow-up until progression Q6W follow-up until progression Survival follow-up 3 post- progression follow-up visits Stratified by region, SOC treatment, and histology *150 kHz; ≥18 h/day; †pembrolizumab, nivolumab, or atezolizumab. ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; MRI, magnetic resonance imaging; NSCLC, non-small cell lung cancer; Q6W, every 6 weeks; SOC, standard of care; TTFields, Tumor Treating Fields.
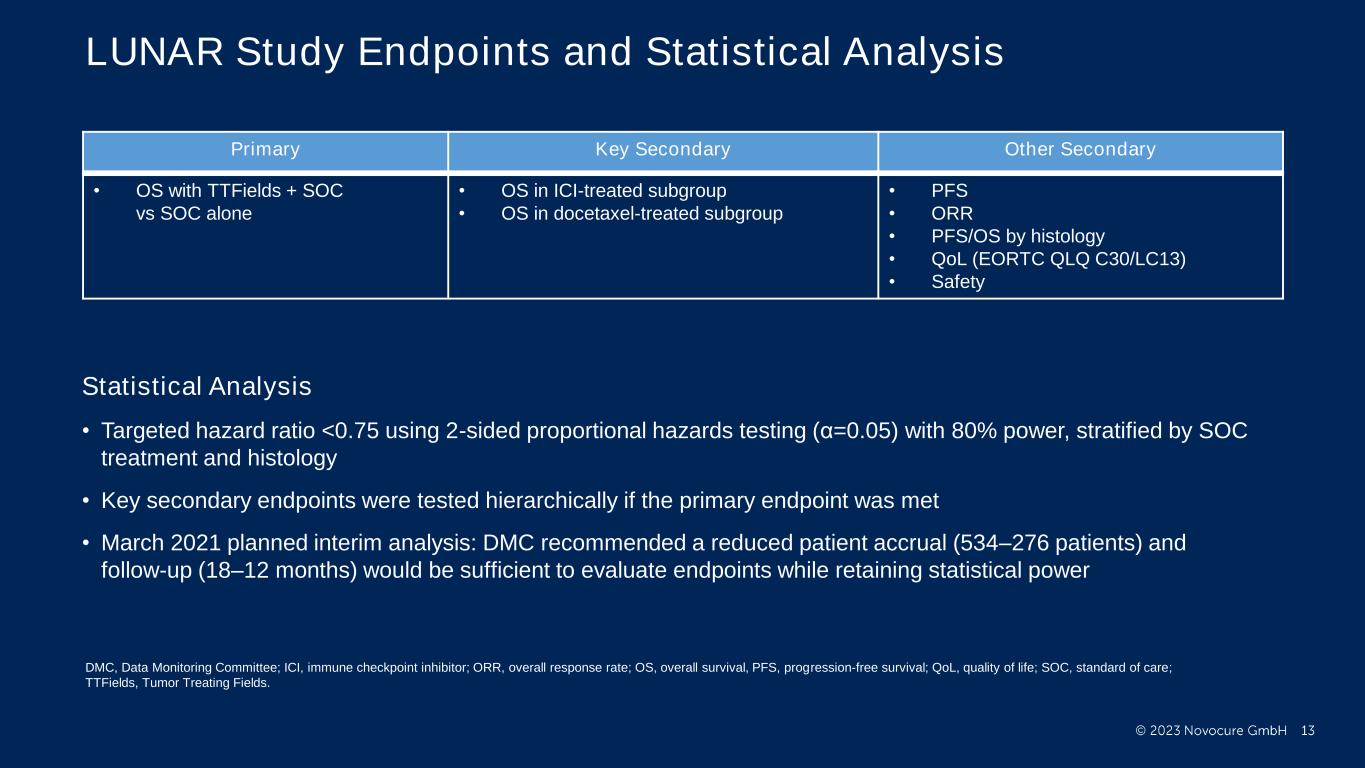
PRESENTED BY: LUNAR Study Endpoints and Statistical Analysis Statistical Analysis • Targeted hazard ratio <0.75 using 2-sided proportional hazards testing (α=0.05) with 80% power, stratified by SOC treatment and histology • Key secondary endpoints were tested hierarchically if the primary endpoint was met • March 2021 planned interim analysis: DMC recommended a reduced patient accrual (534–276 patients) and follow-up (18–12 months) would be sufficient to evaluate endpoints while retaining statistical power Primary Key Secondary Other Secondary • OS with TTFields + SOC vs SOC alone • OS in ICI-treated subgroup • OS in docetaxel-treated subgroup • PFS • ORR • PFS/OS by histology • QoL (EORTC QLQ C30/LC13) • Safety DMC, Data Monitoring Committee; ICI, immune checkpoint inhibitor; ORR, overall response rate; OS, overall survival, PFS, progression-free survival; QoL, quality of life; SOC, standard of care; TTFields, Tumor Treating Fields.
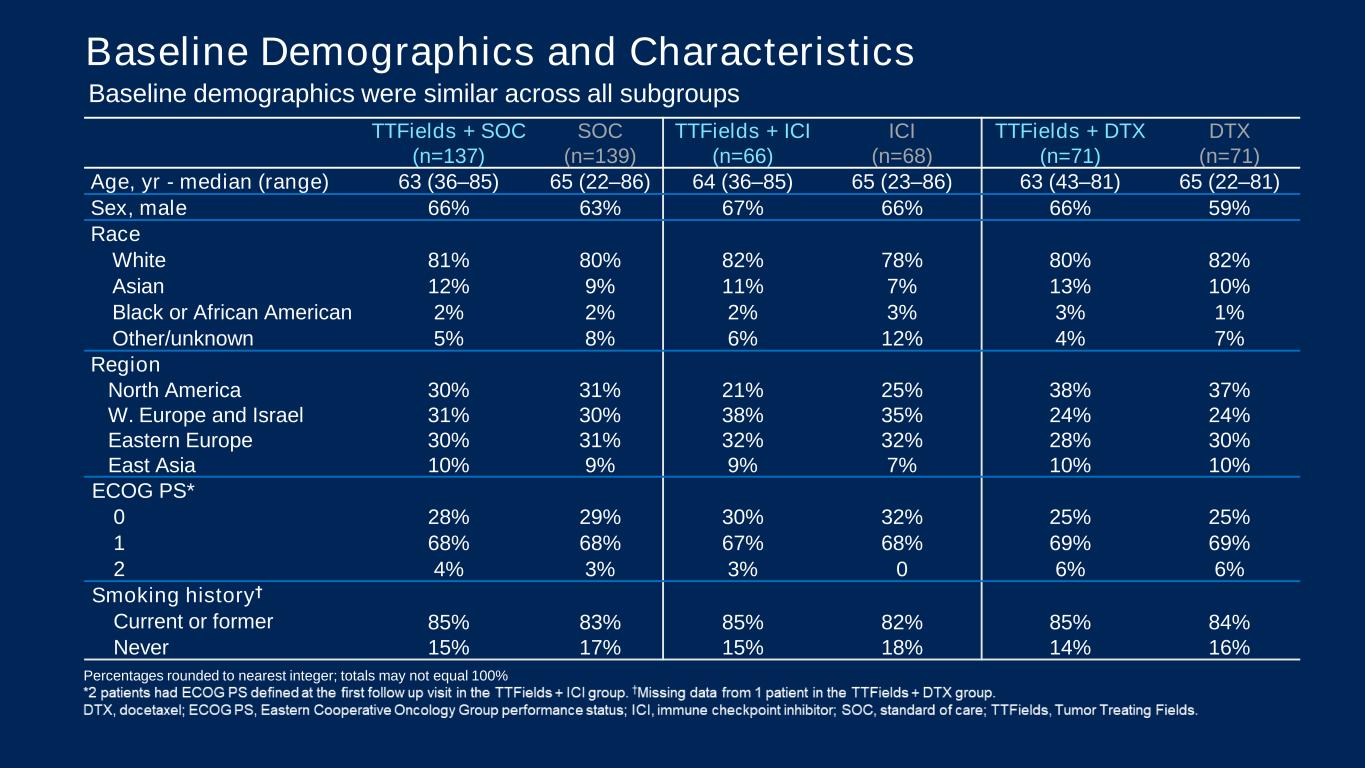
PRESENTED BY: TTFields + SOC (n=137) SOC (n=139) TTFields + ICI (n=66) ICI (n=68) TTFields + DTX (n=71) DTX (n=71) Age, yr - median (range) 63 (36–85) 65 (22–86) 64 (36–85) 65 (23–86) 63 (43–81) 65 (22–81) Sex, male 66% 63% 67% 66% 66% 59% Race White 81% 80% 82% 78% 80% 82% Asian 12% 9% 11% 7% 13% 10% Black or African American 2% 2% 2% 3% 3% 1% Other/unknown 5% 8% 6% 12% 4% 7% Region North America 30% 31% 21% 25% 38% 37% W. Europe and Israel 31% 30% 38% 35% 24% 24% Eastern Europe 30% 31% 32% 32% 28% 30% East Asia 10% 9% 9% 7% 10% 10% ECOG PS* 0 28% 29% 30% 32% 25% 25% 1 68% 68% 67% 68% 69% 69% 2 4% 3% 3% 0 6% 6% Smoking history† Current or former 85% 83% 85% 82% 85% 84% Never 15% 17% 15% 18% 14% 16% Baseline Demographics and Characteristics Baseline demographics were similar across all subgroups Percentages rounded to nearest integer; totals may not equal 100%

PRESENTED BY: Baseline Disease Characteristics TTFields + SOC (n=137) SOC (n=139) TTFields + ICI (n=66) ICI (n=68) TTFields + DTX (n=71) DTX (n=71) Histology Non-squamous/squamous 58%/42% 55%/45% 56%/44% 54%/46% 59%/41% 56%/44% PD-L1 <1% 17% 17% 18% 24% 16% 10% 1–49% 27% 29% 26% 27% 28% 31% ≥50% 7% 13% 8% 12% 7% 14% Unknown* 49% 42% 49% 38% 49% 45% Prior lines of systemic therapy** 1 89% 89% 97% 94% 82% 85% 2+ 11% 10% 3% 4% 18% 15% Prior ICI 31% 31% 2% 3% 58% 58% Best response to any prior therapy Complete response 6% 4% 6% 4% 6% 3% Partial response 23% 26% 29% 19% 18% 32% Stable disease 34% 32% 38% 31% 31% 32% Progressive disease 21% 26% 15% 29% 27% 23% Unknown 15% 13% 12% 16% 18% 10% Liver metastasis† 15% 16% 14% 12% 17% 20% CNS metastasis‡ 0 1% 0 0 0 3%
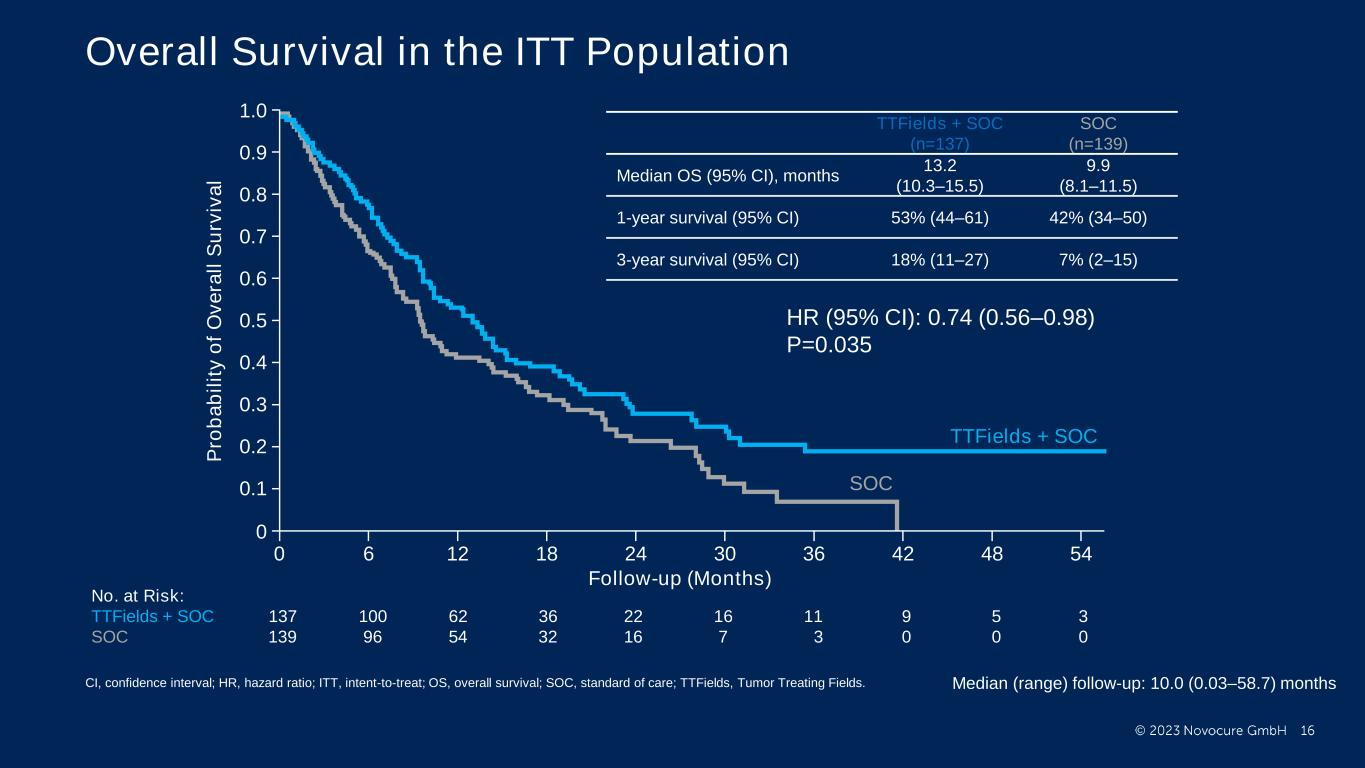
PRESENTED BY: Ticiana Leal, MD, Winship Cancer Institute - Emory University Overall Survival in the ITT Population TTFields + SOC (n=137) SOC (n=139) Median OS (95% CI), months 13.2 (10.3–15.5) 9.9 (8.1–11.5) 1-year survival (95% CI) 53% (44–61) 42% (34–50) 3-year survival (95% CI) 18% (11–27) 7% (2–15) Follow-up (Months) No. at Risk: TTFields + SOC 137 100 62 36 22 16 11 9 5 3 SOC 139 96 54 32 16 7 3 0 0 0 TTFields + SOC SOC 0 0 6 12 48 544236302418 1.0 P ro b a b il it y o f O v e ra ll S u rv iv a l 0.8 0.6 0.4 0.2 0.9 0.7 0.5 0.3 0.1 Median (range) follow-up: 10.0 (0.03–58.7) months HR (95% CI): 0.74 (0.56–0.98) P=0.035 CI, confidence interval; HR, hazard ratio; ITT, intent-to-treat; OS, overall survival; SOC, standard of care; TTFields, Tumor Treating Fields.
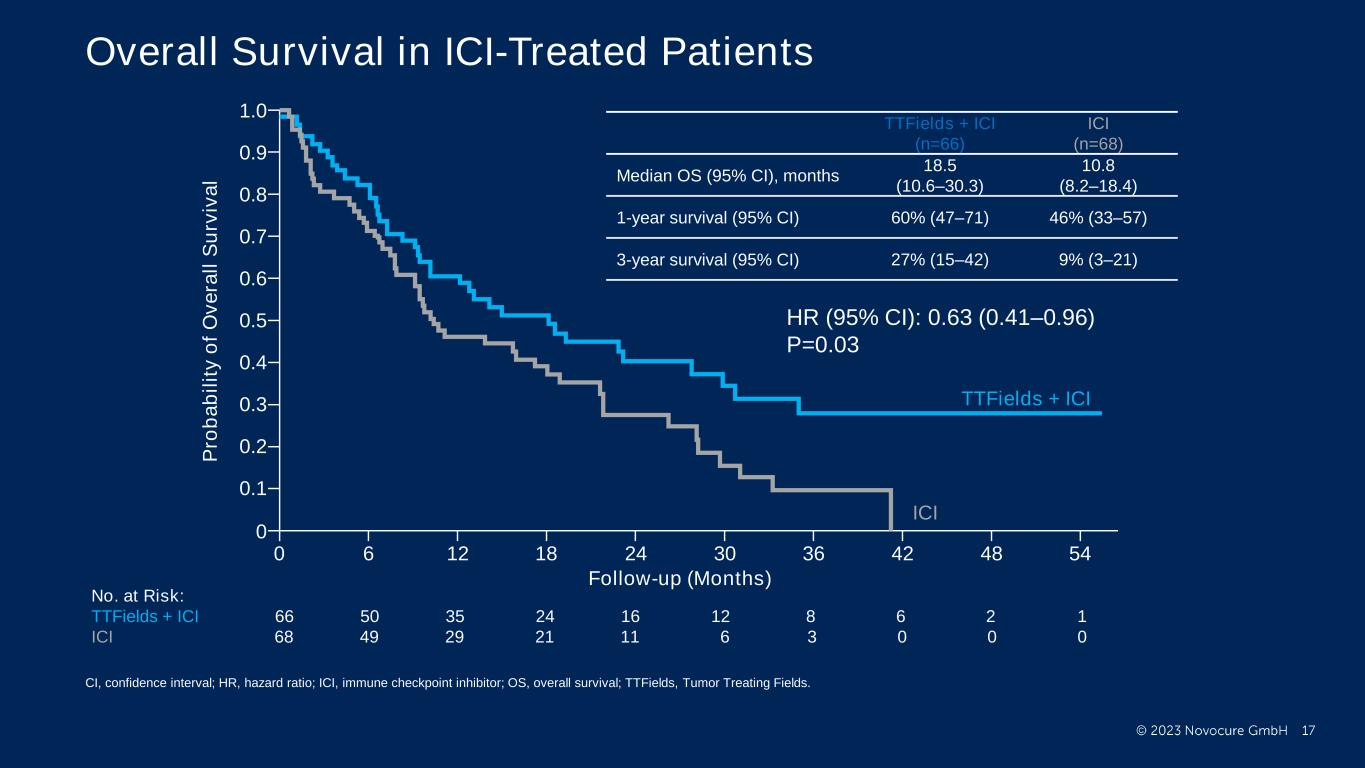
PRESENTED BY: Ticiana Leal, MD, Winship Cancer Institute - Emory University Overall Survival in ICI-Treated Patients No. at Risk: TTFields + ICI 66 50 35 24 16 12 8 6 2 1 ICI 68 49 29 21 11 6 3 0 0 0 1.0 P ro b a b il it y o f O v e ra ll S u rv iv a l 0.8 0.6 0.4 0.2 0 0.9 0.7 0.5 0.3 0.1 0 6 12 48 544236302418 TTFields + ICI ICI Follow-up (Months) TTFields + ICI (n=66) ICI (n=68) Median OS (95% CI), months 18.5 (10.6–30.3) 10.8 (8.2–18.4) 1-year survival (95% CI) 60% (47–71) 46% (33–57) 3-year survival (95% CI) 27% (15–42) 9% (3–21) HR (95% CI): 0.63 (0.41–0.96) P=0.03 CI, confidence interval; HR, hazard ratio; ICI, immune checkpoint inhibitor; OS, overall survival; TTFields, Tumor Treating Fields.

PRESENTED BY: 1.0 0.8 0.6 0.4 0.2 0 0.9 0.7 0.5 0.3 0.1 0 6 12 48 544236302418 TTFields + DTX DTX Follow-up (Months) No. at Risk: TTFields + DTX 71 50 27 12 6 4 3 3 3 2 DTX 71 47 25 11 5 1 0 0 0 0 Ticiana Leal, MD, Winship Cancer Institute - Emory University Overall Survival in DTX-Treated Patients P ro b a b il it y o f O v e ra ll S u rv iv a l TTFields + DTX (n=71) DTX (n=71) Median OS (95% CI), months 11.1 (8.2–14.1) 8.7 (6.3–11.3) 1-year survival (95% CI) 46% (33–57) 38% (27–49) 3-year survival (95% CI) 9% (3–20) 5% (0–18) HR (95% CI): 0.81 (0.55–1.19) P=0.28 CI, confidence interval; DTX, docetaxel; HR, hazard ratio; OS, overall survival; TTFields, Tumor Treating Fields.

PRESENTED BY: TTFields + SOC SOC 1.0 0.9 0.3 0.4 0.5 0.6 0.7 0.8 0.2 0.1 0.0 6 12 18 24 30 36 42 48 54 46 43 28 15 11 9 7 5 2 158 62 23 13 4 1 1 Months TTFields + SOC SOC No. at Risk 000SOC TTFields + SOC No. at Risk TTFields + SOC SOC 1.0 0.9 0.3 0.4 0.5 0.6 0.7 0.8 0.2 0.1 0.0 Months 6 12 18 24 30 36 42 48 54 57 34 21 11 7 4 4 3 2 50 79 77 31 19 6 212 00 0 Overall Survival by Histology Subgroups TTFields + SOC (n=79) SOC (n=77) Median OS (95% CI), months 12.6 (8.8–19.8) 9.9 (6.9–16.4) TTFields + SOC (n=58) SOC (n=62) Median OS (95% CI), months 13.9 (9.7–17.1) 10.1 (8.3–14.3) Non-squamous Squamous P ro b a b il it y o f O v e ra ll S u rv iv a l P ro b a b il it y o f O v e ra ll S u rv iv a l HR (95% CI): 0.80 (0.54–1.16) P=0.28 HR (95% CI): 0.67 (0.44–1.01) P=0.05 CI, confidence interval; HR, hazard ratio; OS, overall survival; SOC, standard of care; TTFields, Tumor Treating Fields.

PRESENTED BY: No. at risk: TTFields + SOC 137 44 17 9 SOC 139 40 21 9 Follow-up (Months) TTFields + SOC SOC 0 0 18126 1.0 P ro b a b il it y o f P ro g re s s io n -f re e S u rv iv a l 0.8 0.6 0.4 0.2 0.9 0.7 0.5 0.3 0.1 TTFields + SOC (n=137) SOC (n=139) Median PFS (95% CI), months 4.8 (4.1–5.7) 4.1 (3.1–4.6) Ticiana Leal, MD, Winship Cancer Institute - Emory University Progression-free Survival in the ITT Population HR (95% CI): 0.85 (0.67–1.11) P=0.23 PFS was defined as the time from date of randomization until date of disease progression, or death by any cause. CI, confidence interval; HR, hazard ratio; ITT, intent-to-treat; PFS, progression-free survival; SOC, standard of care; TTFields, Tumor Treating Fields.
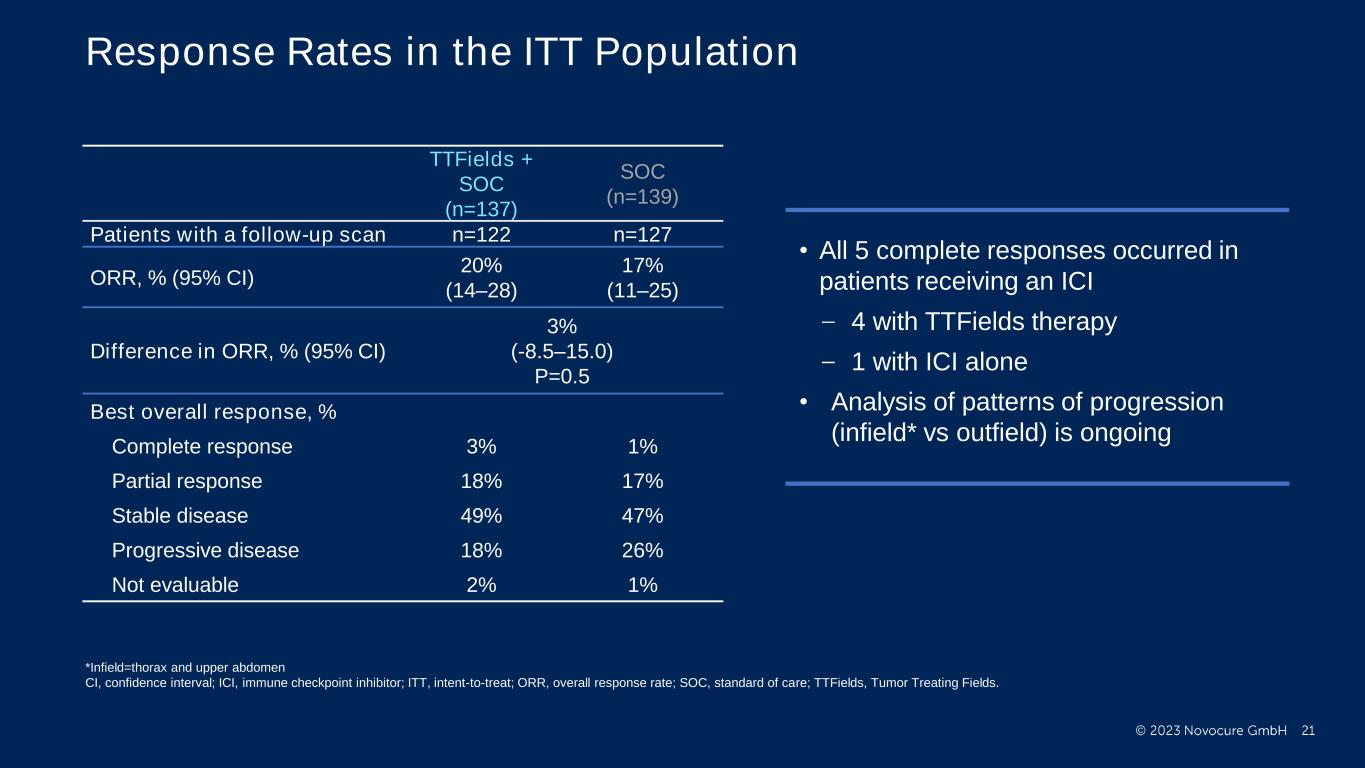
PRESENTED BY: Response Rates in the ITT Population TTFields + SOC (n=137) SOC (n=139) Patients with a follow-up scan n=122 n=127 ORR, % (95% CI) 20% (14–28) 17% (11–25) Difference in ORR, % (95% CI) 3% (-8.5–15.0) P=0.5 Best overall response, % Complete response 3% 1% Partial response 18% 17% Stable disease 49% 47% Progressive disease 18% 26% Not evaluable 2% 1% • All 5 complete responses occurred in patients receiving an ICI 4 with TTFields therapy 1 with ICI alone • Analysis of patterns of progression (infield* vs outfield) is ongoing *Infield=thorax and upper abdomen CI, confidence interval; ICI, immune checkpoint inhibitor; ITT, intent-to-treat; ORR, overall response rate; SOC, standard of care; TTFields, Tumor Treating Fields.
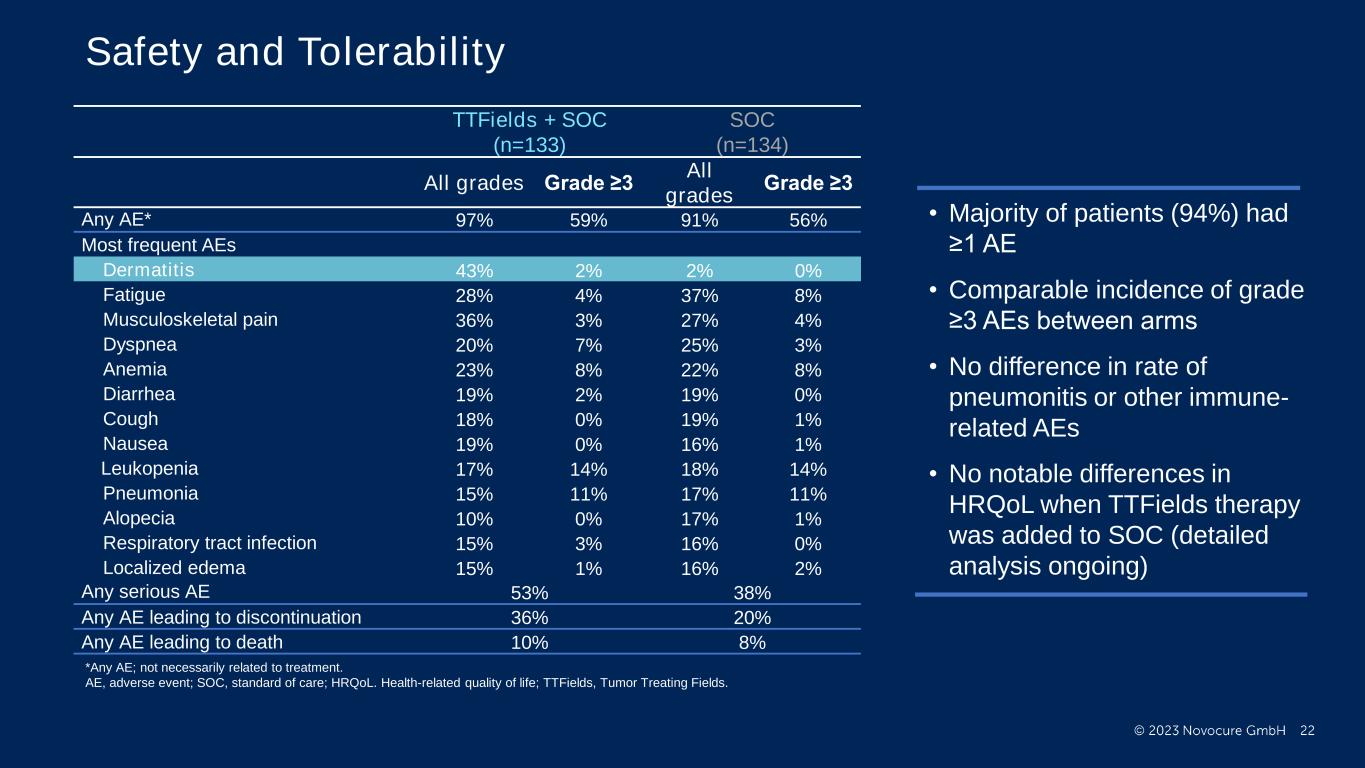
PRESENTED BY: Ticiana Leal, MD, Winship Cancer Institute - Emory University Safety and Tolerability TTFields + SOC (n=133) SOC (n=134) All grades Grade ≥3 All grades Grade ≥3 Any AE* 97% 59% 91% 56% Most frequent AEs Dermatitis 43% 2% 2% 0% Fatigue 28% 4% 37% 8% Musculoskeletal pain 36% 3% 27% 4% Dyspnea 20% 7% 25% 3% Anemia 23% 8% 22% 8% Diarrhea 19% 2% 19% 0% Cough 18% 0% 19% 1% Nausea 19% 0% 16% 1% Leukopenia 17% 14% 18% 14% Pneumonia 15% 11% 17% 11% Alopecia 10% 0% 17% 1% Respiratory tract infection 15% 3% 16% 0% Localized edema 15% 1% 16% 2% Any serious AE 53% 38% Any AE leading to discontinuation 36% 20% Any AE leading to death 10% 8% • Majority of patients (94%) had ≥1 AE • Comparable incidence of grade ≥3 AEs between arms • No difference in rate of pneumonitis or other immune- related AEs • No notable differences in HRQoL when TTFields therapy was added to SOC (detailed analysis ongoing) *Any AE; not necessarily related to treatment. AE, adverse event; SOC, standard of care; HRQoL. Health-related quality of life; TTFields, Tumor Treating Fields.
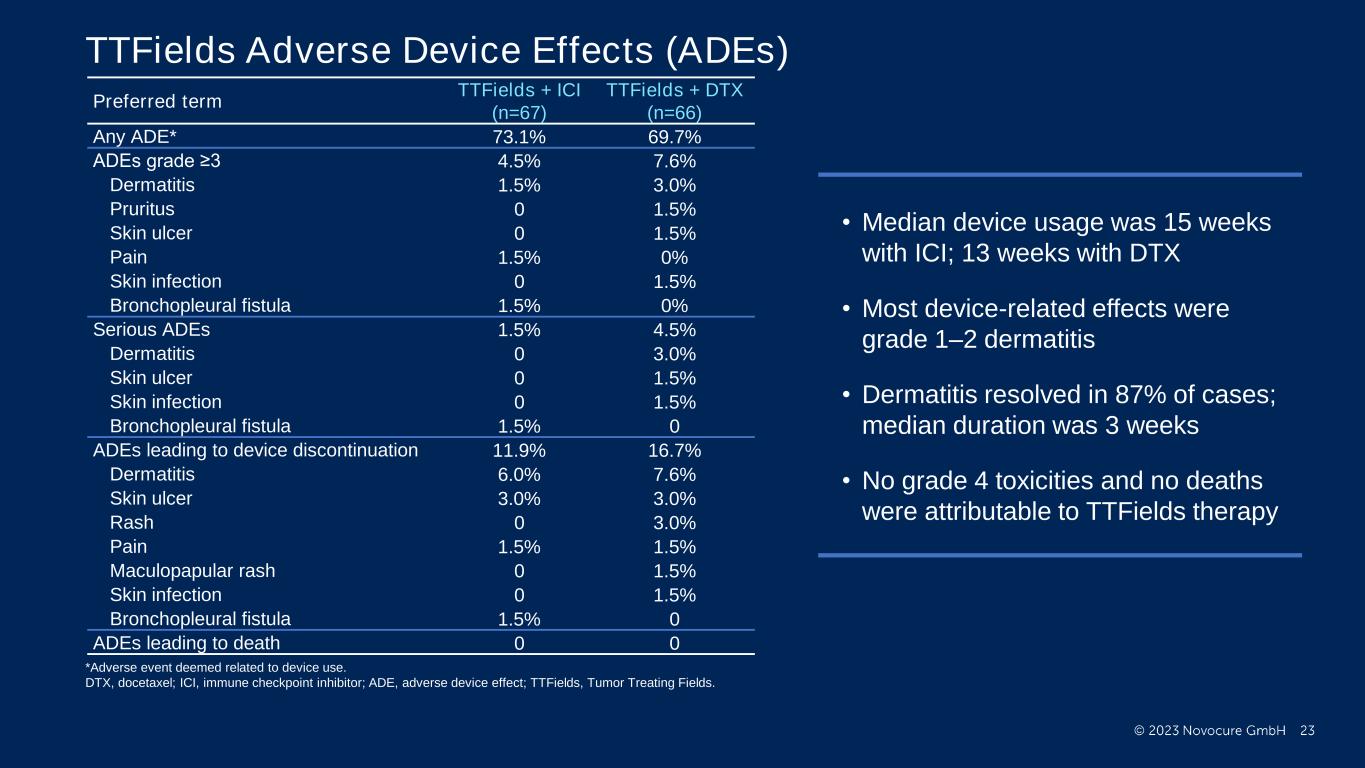
PRESENTED BY: Ticiana Leal, MD, Winship Cancer Institute - Emory University TTFields Adverse Device Effects (ADEs) Preferred term TTFields + ICI (n=67) TTFields + DTX (n=66) Any ADE* 73.1% 69.7% ADEs grade ≥3 4.5% 7.6% Dermatitis 1.5% 3.0% Pruritus 0 1.5% Skin ulcer 0 1.5% Pain 1.5% 0% Skin infection 0 1.5% Bronchopleural fistula 1.5% 0% Serious ADEs 1.5% 4.5% Dermatitis 0 3.0% Skin ulcer 0 1.5% Skin infection 0 1.5% Bronchopleural fistula 1.5% 0 ADEs leading to device discontinuation 11.9% 16.7% Dermatitis 6.0% 7.6% Skin ulcer 3.0% 3.0% Rash 0 3.0% Pain 1.5% 1.5% Maculopapular rash 0 1.5% Skin infection 0 1.5% Bronchopleural fistula 1.5% 0 ADEs leading to death 0 0 • Median device usage was 15 weeks with ICI; 13 weeks with DTX • Most device-related effects were grade 1–2 dermatitis • Dermatitis resolved in 87% of cases; median duration was 3 weeks • No grade 4 toxicities and no deaths were attributable to TTFields therapy *Adverse event deemed related to device use. DTX, docetaxel; ICI, immune checkpoint inhibitor; ADE, adverse device effect; TTFields, Tumor Treating Fields.

PRESENTED BY: Conclusions • Pivotal, phase 3 LUNAR study met its primary endpoint • TTFields therapy with SOC provided a statistically significant and clinically meaningful 3-month improvement in median OS vs SOC (HR: 0.74, P=0.035) with no added systemic toxicities Statistically significant ~8-month increase in median OS (from 10.8 to 18.5 months) was demonstrated with TTFields therapy and an ICI (HR: 0.63, P=0.030) There was a 2.4-month difference in median OS (from 8.7 to 11.1) for TTFields therapy and docetaxel vs docetaxel alone (HR: 0.81, P=0.28) • TTFields therapy should be considered part of SOC for metastatic NSCLC following progression on or after platinum-based therapy • Additional studies evaluating TTFields therapy with current SOC for first-line metastatic and locally advanced NSCLC are underway • TTFields therapy is a potentially paradigm shifting new treatment modality Ticiana Leal, MD, Winship Cancer Institute - Emory University HR, hazard ratio; ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; OS, overall survival; SOC, standard of care; TTFields; Tumor Treating Fields.

PRESENTED BY: Austria: Medical University Salzburg; Belgium: Institut Jules Bordet, Hospital AZ Sint Maarten; Canada: McGill University Health Centre, Universitaire de Sherbrooke (CIUSSS de l'Estrie - CHUS), Allan Blair Cancer Centre; Czech Republic: Thomayer Hospital, General University Hospital in Prague, Vitkovice Hospital; China: Beijing Cancer Hospital, Sun Yat-sen University Cancer Center (SYSUCC), Affiliated Cancer Hospital and Institute of Guangzhou Medical University, Henan Provincial People's Hospital, Henan Cancer Hospital, PKUCare Luzhong Hospital, Zhejiang Cancer Hospital, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine; France: Institut Bergonié, Institut de Cancérologie du Gard, Hôpital Saint-Louis; Hong Kong: Queen Mary Hospital; Hungary: Tolna County Hospital, Jasz-Nagykun-Szolnok County Hospital; Italy: IRCCS – Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST), Ospedale S.Maria delle Croci; Netherlands: St Jansdal Ziekenhuis, Erasmus MC; Poland: Non-Invasive Medicine Center, Center of Modern Therapy, Medical University of Lublin, University Hospital in Poznań; Serbia: Clinical Hospital Center, Bezanijska Kosa, Clinical Center Kragujevac; Spain: Hospital Quirón Teknon, Hospital Universitario Arnau de Vilanova, Hospital Universitario Gregorio Marañón, Hospital Universitario 12 de Octubre, Hospital Regional Universitario Carlos Haya, Hospital Universitari i Politécnic La Fe; Switzerland: KS Winterthur; United States: Central Alabama Research, Ironwood Cancer & Research Centers, St. Joseph Hospital and Medical Center, Beverly Hills Cancer Center, Compassionate Cancer Care, California Cancer Associates for Research and Excellence, Inc (cCARE), St. Joseph Heritage Healthcare, Long Beach Memorial Medical Center, Sutter Medical Group, Mercy Cancer Center, Emad Ibrahim, MD, Inc, The Oncology Institute of Hope & Innovation, McKee Medical Center, Associated Neurologists of Southern Connecticut, MedStar Washington Hospital Center, GenesisCare, Miami Cancer Institute, Mount Sinai Comprehensive Cancer Center, AdventHealth Neuro Oncology, UF Health Cancer Center at Orlando Health, South Florida Oncology & Hematology Consultants LLC, The University of Kansas Cancer Center, University of Louisville, Norton Cancer Institute, University of Illinois Hospital, Illinois CancerCare PC, Southern Illinois University School of Medicine, Franciscan Health Indianapolis, University Medical Center, Tulane Cancer Center, CHRISTUS Highland Cancer Treatment Center, Tufts Medical Center, Beth Israel Deaconess Medical Center, Central Maine Medical Center, University of Maryland, St. Joseph Mercy Hospital, Karmanos Cancer Institute, Clinical Oncology Associates, Memorial Healthcare, Regions Cancer Care Center, Saint Luke's Hospital, Washington University School of Medicine, Oncology Specialists of Charlotte, WG "Bill" Hefner VA Medical Center, Piedmont Radiation Oncology PA, Nebraska Medical Center, Oncology Hematology West, PC dba Nebraska Cancer Specialists, CHI Health Creighton University Medical Center - Bergan Mercy, New Jersey Hematology Oncology Associates, Presbyterian Cancer Care at Kaseman, OptumCare Cancer Center, Renown Regional Medical Center, Northern Westchester Hospital, New York Presbyterian/Queens Center for Research and Education, Stony Brook Cancer Center, Summa Health System, Toledo Clinic Cancer Center, Vita Medical Associates, PC, Geisinger Medical Center, University of Tennessee/Erlanger Oncology and Hematology, Texas Oncology – Amarillo, Texas Oncology – Arlington North, Christus Spohn Cancer Center, VA North Texas Health Care System, Texas Oncology – Baylor Charles A Sammons Cancer Center, UT Southwestern Medical Center, Oncology Consultants, PA, Texas Oncology – McKinney, Texas Oncology – Paris, Texas Oncology – Plano West, Texas Oncology – Waco, McClinton Cancer Center, University of Utah, Overlake Hospital Medical Center, University of Wisconsin Ticiana Leal, MD, Winship Cancer Institute - Emory University Acknowledgements Special thank you to all participating patients, their families, and clinical research teams for your commitment and contributions Statistical analysis performed by Gitit Lavy-Shahaf, PhD (Novocure) Editorial assistance from Chelsea Higgins, PhD (Novocure), Rose Goodchild, PhD and Melissa Purves, CMPP, PhD (Prime); Editorial support funded by Novocure This study was sponsored by Novocure Thank you to all participating sites around the world:
✓ ✓ ✓ • ✓ • • • • •

✓





















