UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): August 5, 2025
Cocrystal Pharma, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-38418 | 35-2528215 | ||
|
(State or other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
19805 N. Creek Parkway Bothell, WA |
98011 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (877) 262-7123
(Former name or former address, if changed since last report.): n/a
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Stock | COCP | The Nasdaq Stock Market LLC (The Nasdaq Capital Market) |
Item 7.01 Regulation FD Disclosure.
On August 5, 2025, Cocrystal Pharma, Inc. (the “Company”) issued a press release announcing the presentation of safety and tolerability data from a randomized, double-blinded, placebo-controlled Phase 1 study with its oral, direct-acting pan-viral inhibitor CDI-988 at the 2025 Military Health System Research Symposium (MHSRS), being held August 4-7, 2025 in Kissimmee, Florida. A copy of the press release is being furnished as Exhibit 99.1, and a copy of the slide presentation is being furnished as Exhibit 99.2.
The information in this Item 7.01 (including Exhibits 99.1 and 99.2) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities under such section, and shall not be deemed to be incorporated by reference into any filing of the Company under the Securities Act of 1933 or the Exchange Act.
Item 9.01 Financial Statements and Exhibits
(d) Exhibits
| Exhibit | Description | |
| 99.1 | Press Release dated August 5, 2025 | |
| 99.2 | Presentation dated August 5, 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Cocrystal Pharma, Inc. | ||
| Date: August 5, 2025 | By: | /s/ James Martin |
| Name: | James Martin | |
| Title: | Chief Financial Officer and Co-Chief Executive Officer | |
Exhibit 99.1

Cocrystal Pharma Presents Phase 1 Results for Pan-Viral Inhibitor CDI-988 at Department of Defense Medical Conference
| ● | All CDI-988 doses, ranging from 100 mg to 1200 mg, in the Phase 1 study were well tolerated | |
| ● | Company expects to initiate Phase 1b study with CDI-988 in norovirus-infected healthy subjects later this year | |
| ● | Lack of approved norovirus treatments or vaccines creates critical unmet medical need |
BOTHELL, Wash. (August 5, 2025) – Cocrystal Pharma, Inc. (Nasdaq: COCP) announces the presentation of favorable safety and tolerability data from a randomized, double-blinded, placebo-controlled Phase 1 study with its oral, direct-acting pan-viral inhibitor CDI-988 at the 2025 Military Health System Research Symposium (MHSRS), being held August 4-7 in Kissimmee, Florida. The results support Cocrystal’s continued clinical development of CDI-988 as a potential norovirus prophylaxis and treatment.
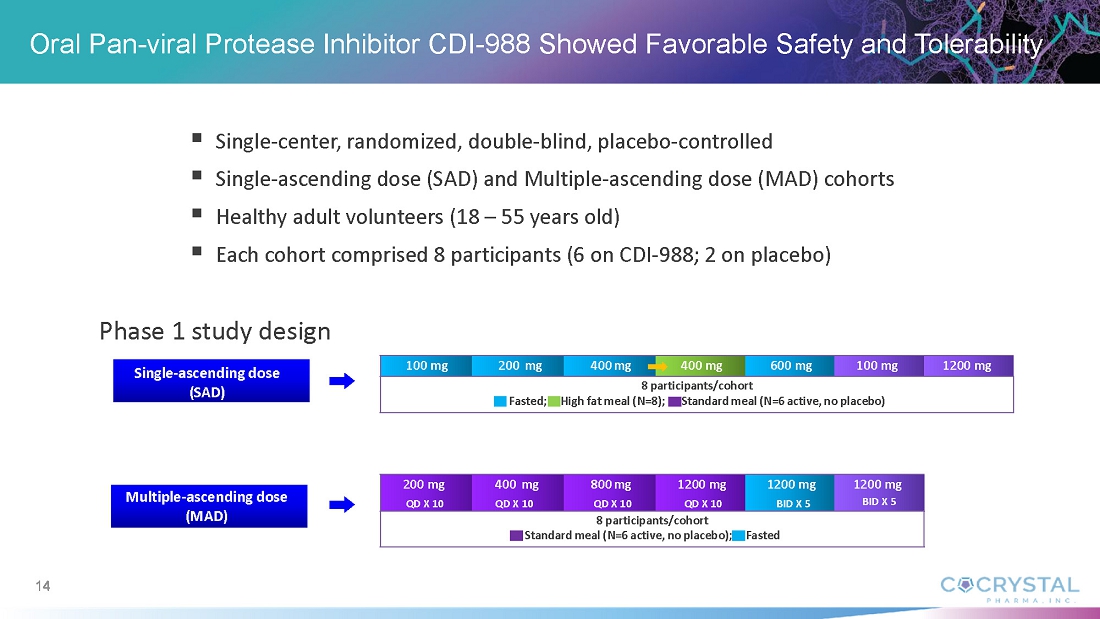
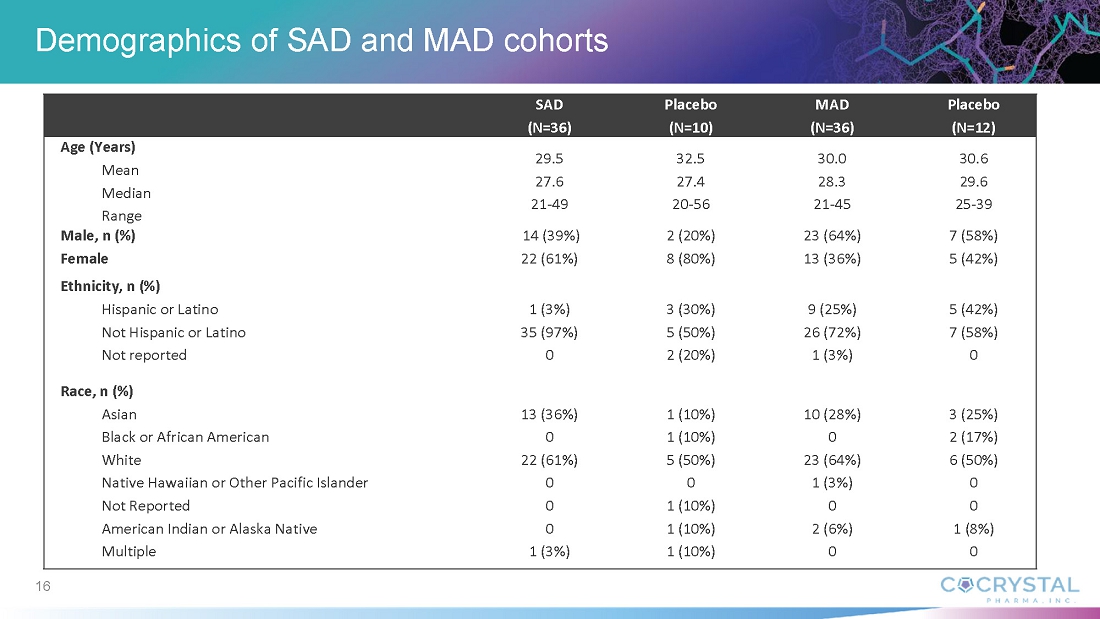
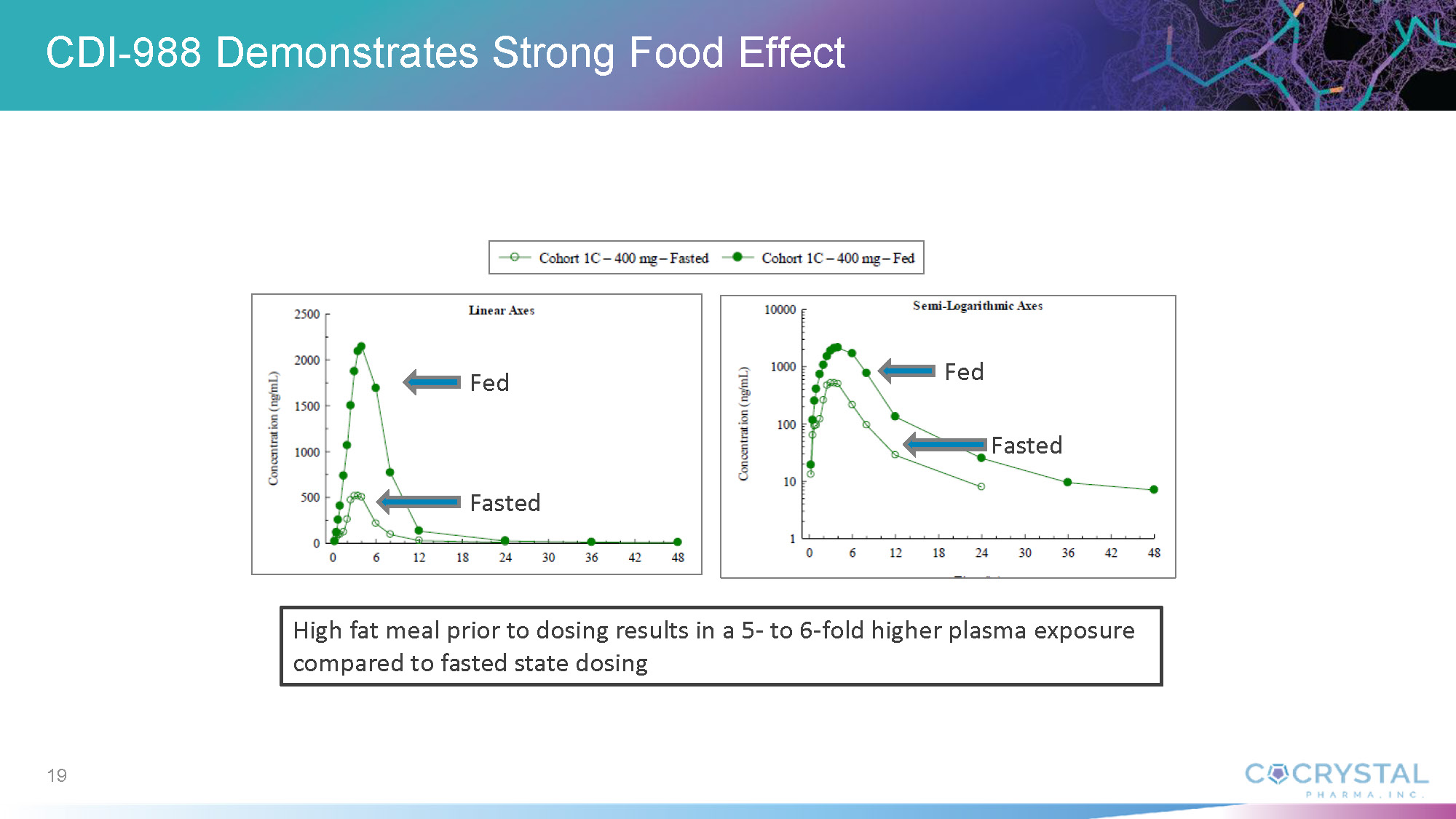
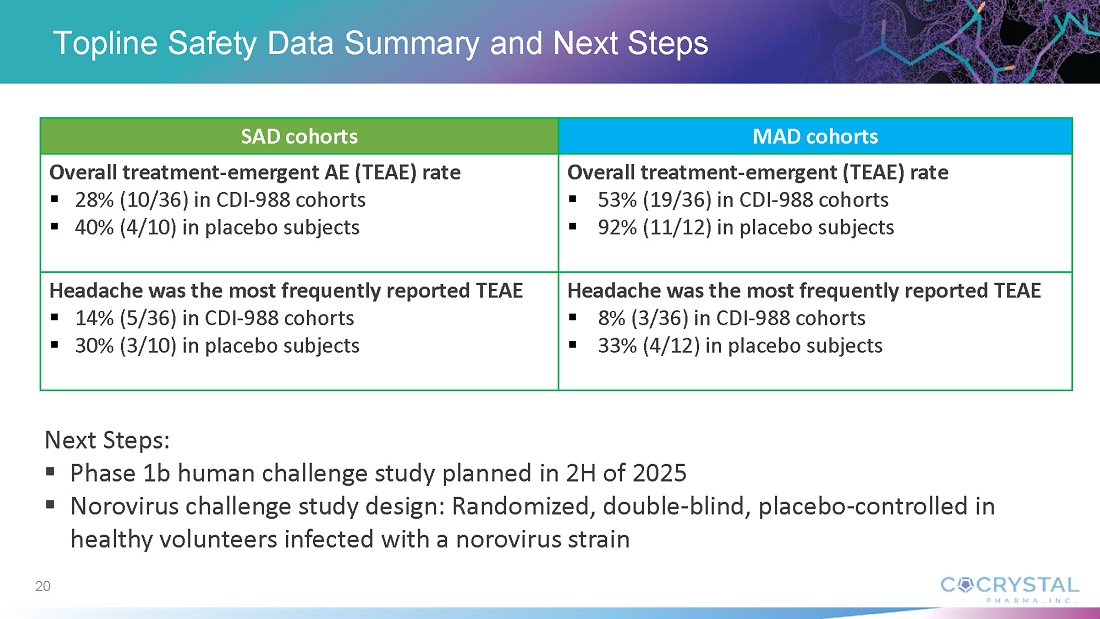
In An Oral Pan-viral Protease Inhibitor for the Prevention and Treatment of Norovirus and Coronavirus Infections: Mechanism of Action and Phase 1 Study Results, Sam Lee, Ph.D., Cocrystal President and co-CEO, discussed findings from the CDI-988 Phase 1 single-ascending (SAD) and multiple-ascending (MAD) cohorts. Data indicate that all doses, ranging from 100 mg to 1200 mg, were well tolerated. Overall treatment-emergent adverse events among CDI-988 subjects were 28% (10/36) compared with 40% (4/10) among placebo subjects for the SAD cohorts, and 53% (19/36) and 92% (11/12), respectively, for the MAD cohorts. Headache was the most common adverse event. All subjects in the SAD cohorts and all but one in the MAD cohorts completed the study. No severe treatment-emergent adverse events, no clinically relevant ECG changes and no clinically significant pathology results were reported from the CDI-988 Phase 1 single-ascending (SAD) and multiple-ascending (MAD) cohorts.
“Consistent with interim results from the Phase 1 study, CDI-988 was well-tolerated with a favorable safety profile across all dose levels tested in this study,” said Dr. Lee. “Our plan to continue CDI-988’s clinical development for norovirus is particularly relevant for the military, where this highly transmissible pathogen poses significant operational and economic risks. In confined settings such as naval vessels and military installations, norovirus can rapidly spread, causing debilitating gastrointestinal symptoms that could compromise mission readiness.
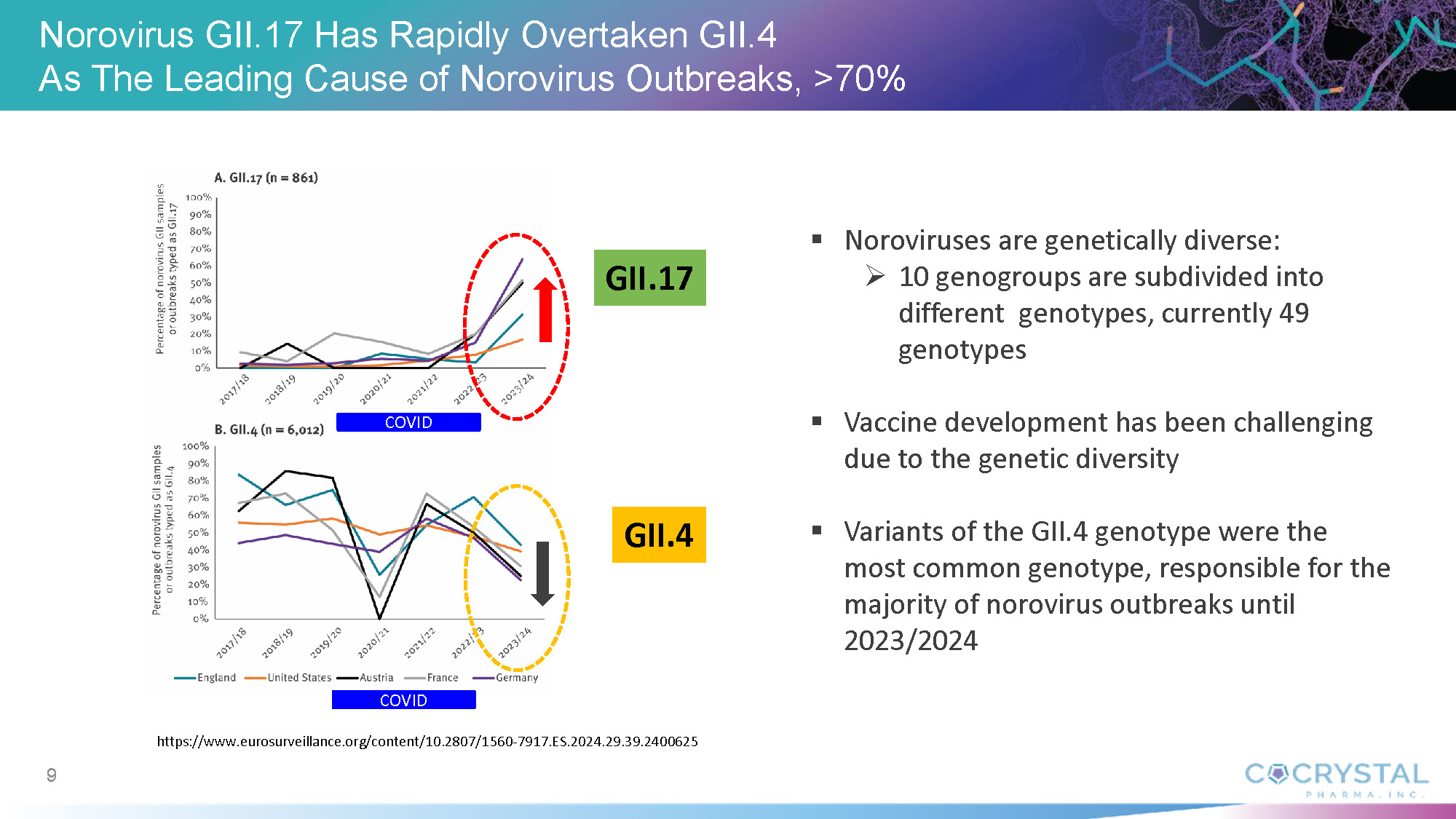
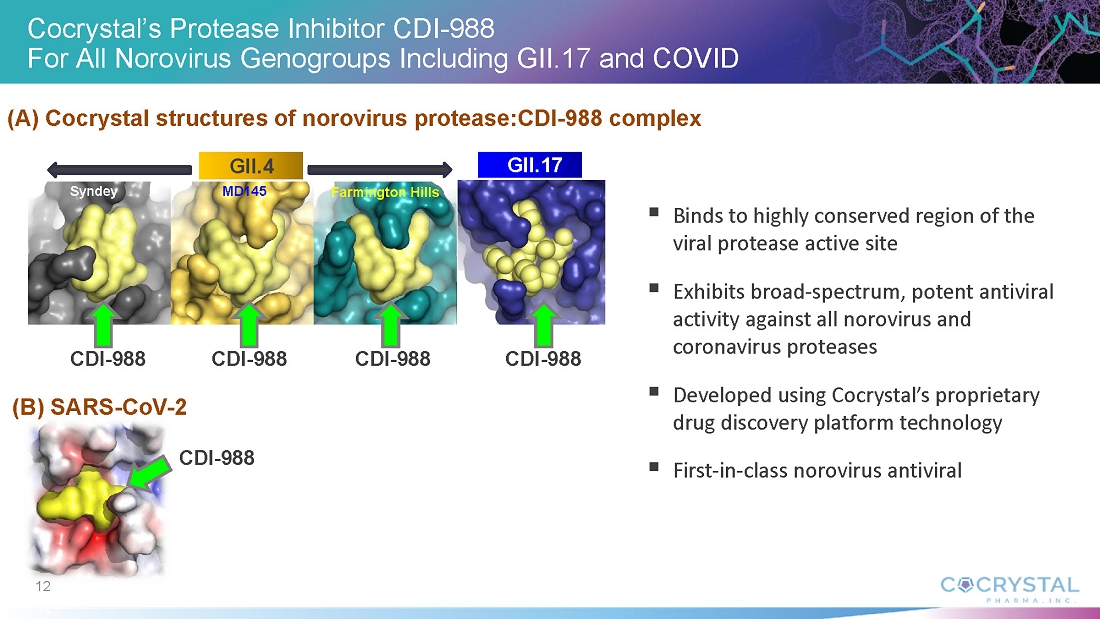
“The absence of approved norovirus treatments or vaccines creates a critical unmet medical need,” he added. “Norovirus presents significant vaccine development challenges due to its high genetic variability and mutation rate. CDI-988’s mechanism of action targeting viral replication and its broad-spectrum coverage offers a promising solution as a potential prophylactic and therapeutic intervention across all norovirus genogroups including GII.4 and GII.17. This could be a new approach to outbreak prevention and management. We expect to initiate a Phase 1b challenge study with CDI-988 in norovirus-infected healthy subjects later this year.”
MHSRS is an annual educational symposium with approximately 4,000 attendees that provides a collaborative environment for military medical care providers with deployment experience, research and academic scientists, international partners and industry on research and related healthcare initiatives falling under the topic areas of combat casualty care, military operational medicine, clinical and rehabilitative medicine, information sciences, military infectious diseases and radiation health effects. More information is available here.
Pan-viral Protease Inhibitor CDI-988
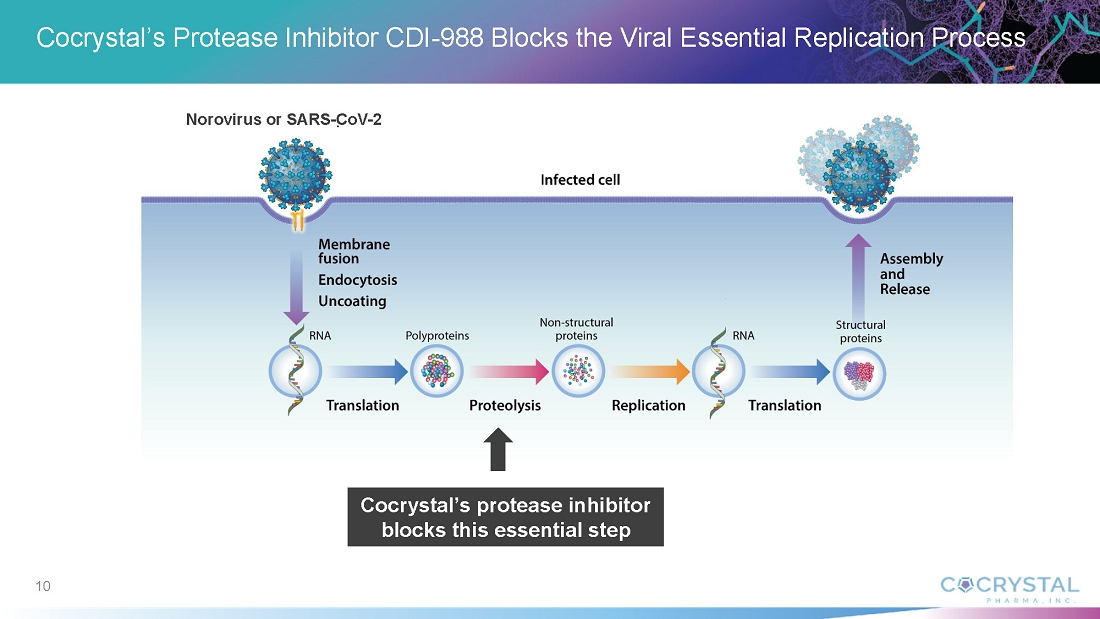
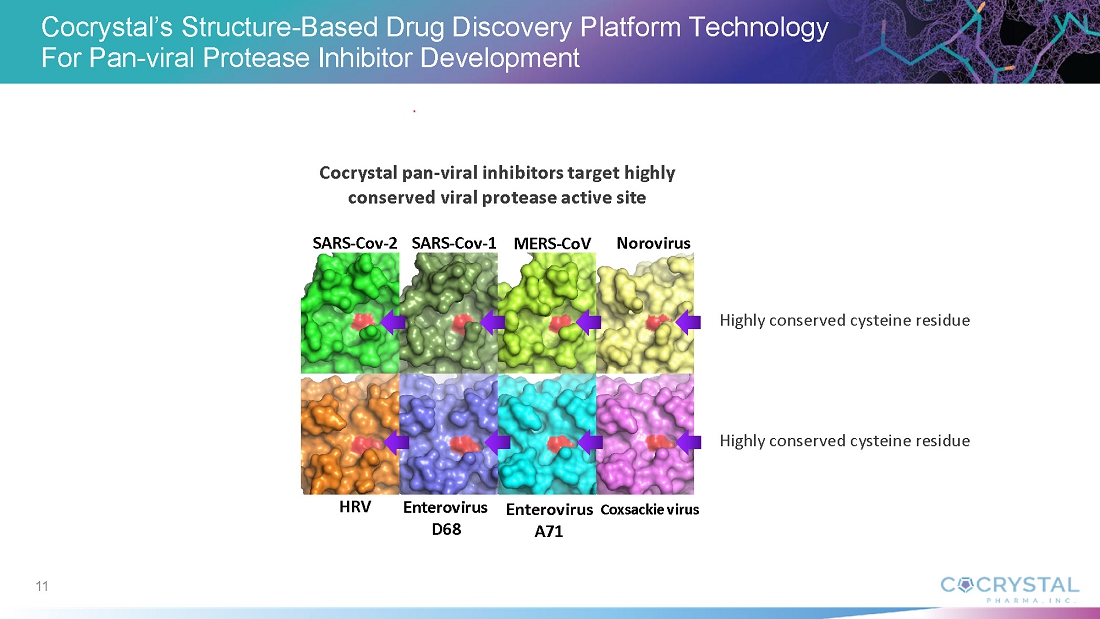
CDI-988 was designed and developed with Cocrystal’s proprietary structure-based platform technology as a broad-spectrum inhibitor to a highly conserved region in the active site of 3CL viral proteases. Based on a novel mechanism of action and superior broad-spectrum antiviral activity, CDI-988 represents a compelling first potential oral treatment for noroviruses, and for coronaviruses.
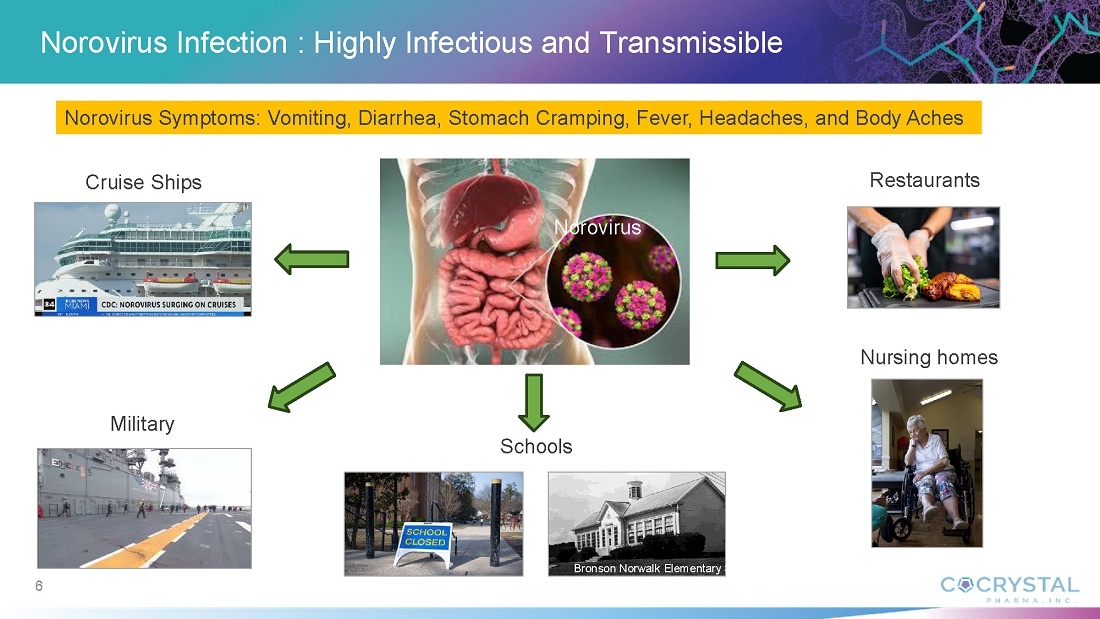
Norovirus Infection
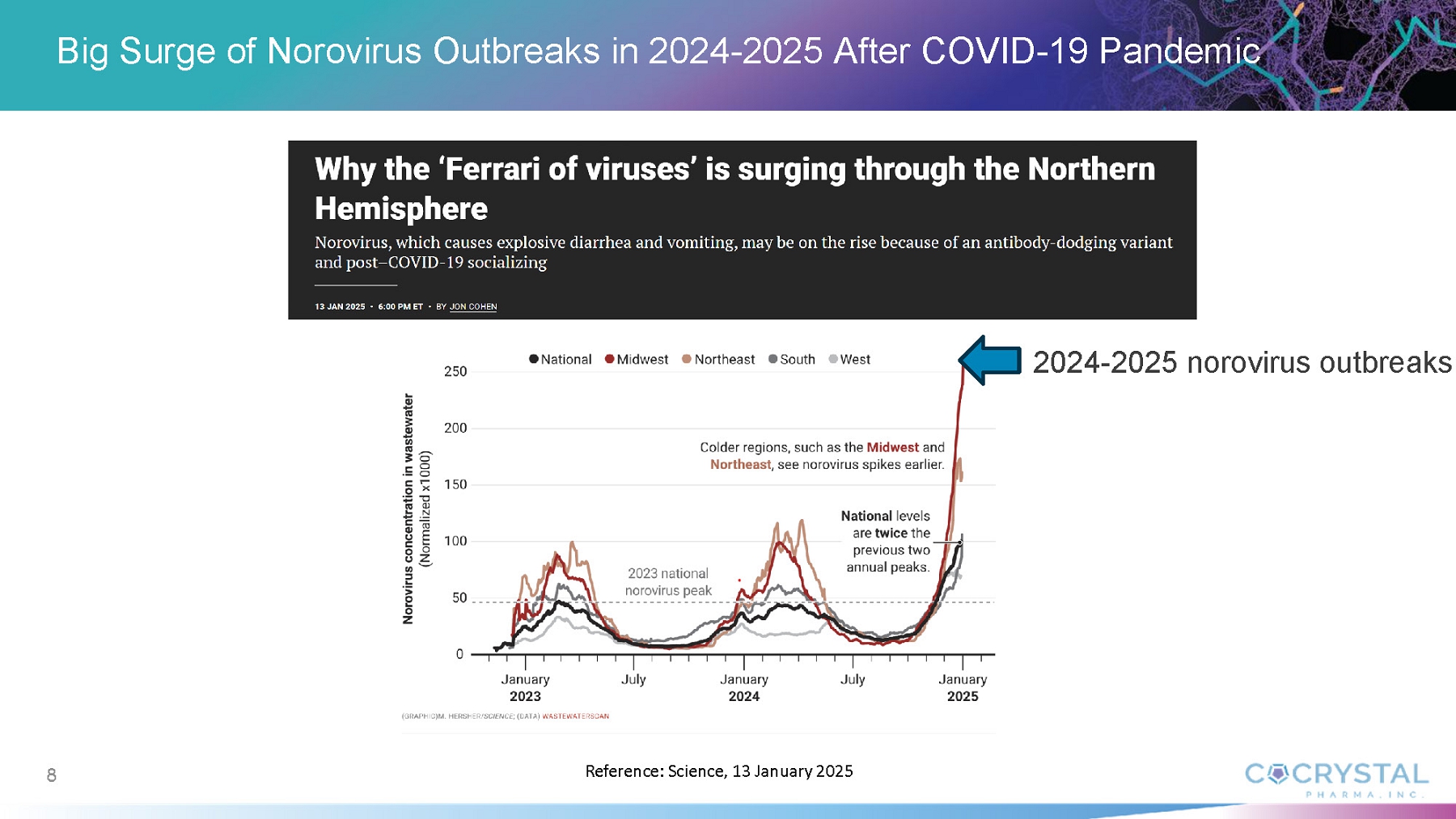
Norovirus is a common and highly contagious virus that afflicts people of all ages and causes symptoms of acute gastroenteritis including nausea, vomiting, stomach pain and diarrhea, as well as fatigue, fever and dehydration. Norovirus outbreaks occur most commonly in semi-closed communities such as hospitals, nursing homes, childcare facilities, cruise ships, schools and disaster relief sites. Norovirus infections are estimated to cost society approximately $60 billion annually worldwide.
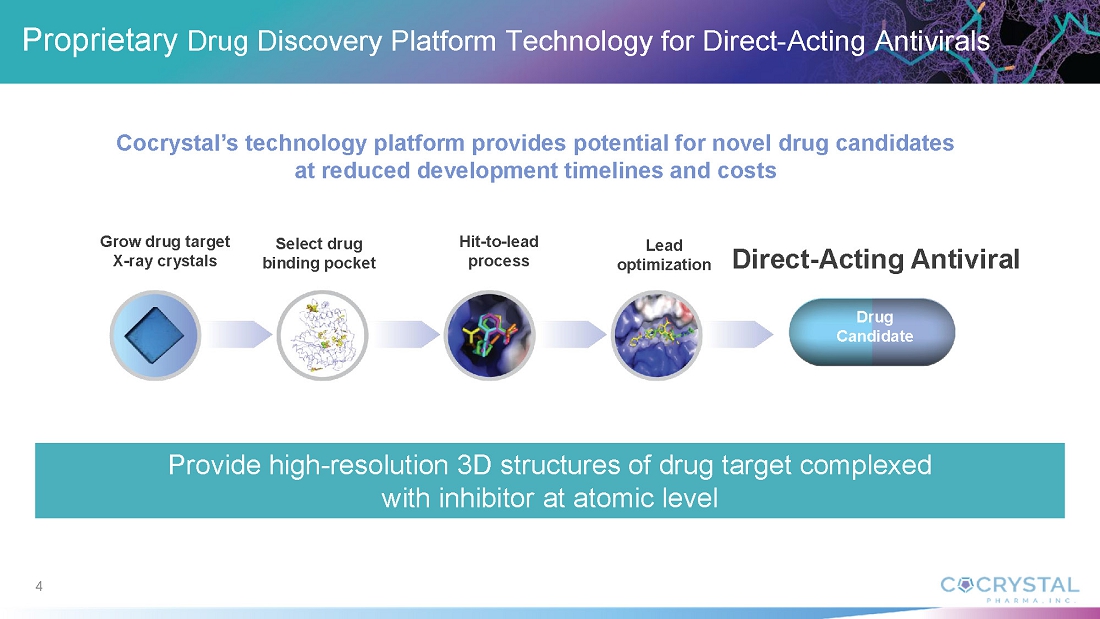
Structure-Based Drug Discovery Platform Technology
Cocrystal’s proprietary structural biology, along with its expertise in enzymology and medicinal chemistry, enable its development of novel antiviral agents. The Company’s platform provides a three-dimensional structure of inhibitor complexes at near-atomic resolution, providing immediate insight to guide Structure Activity Relationships. This helps to identify novel binding sites and allows for a rapid turnaround of structural information through highly automated X-ray data processing and refinement. The goal of this technology is to facilitate the development of novel broad-spectrum antivirals for the treatment of acute and chronic viral diseases.
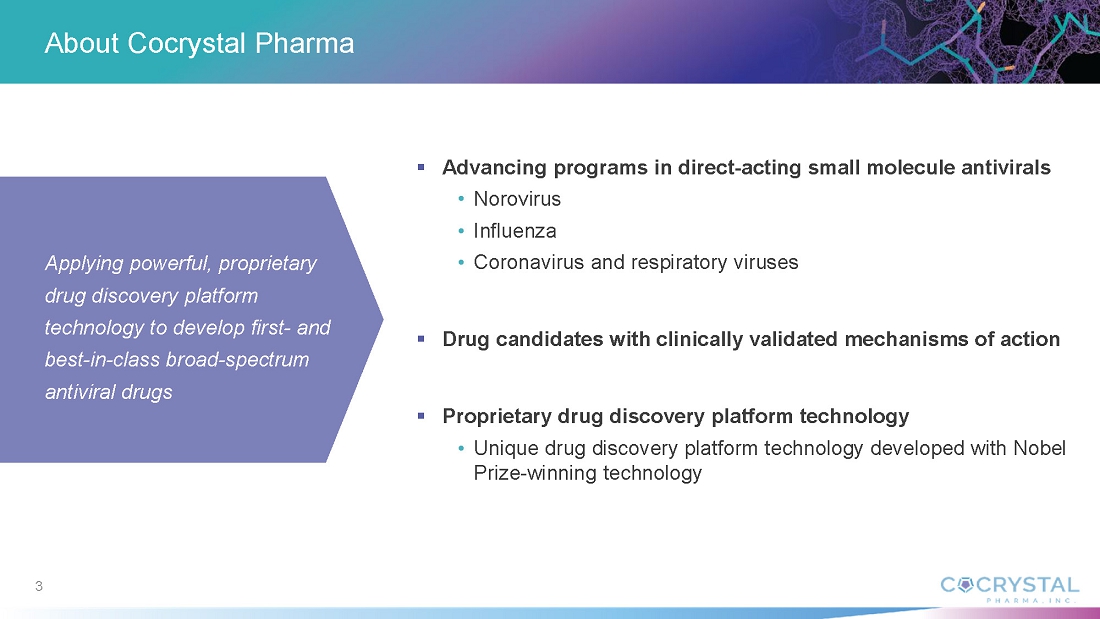
About Cocrystal Pharma, Inc.
Cocrystal Pharma, Inc. is a clinical-stage biotechnology company discovering and developing novel antiviral therapeutics that target the replication process of influenza viruses, coronaviruses (including SARS-CoV-2), noroviruses and hepatitis C viruses. Cocrystal employs unique structure-based technologies and Nobel Prize-winning expertise to create antiviral drugs. For further information about Cocrystal, please visit www.cocrystalpharma.com.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements regarding the potential efficacy of CDI-988 as a potential breakthrough for norovirus prophylaxis and treatment, and the potential characteristics of and market for such product candidate and the Company’s plan to initiate a Phase 1b study in 2025. The words “believe,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “could,” “target,” “potential,” “is likely,” “will,” “expect” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events. Some or all of the events anticipated by these forward-looking statements may not occur. Important factors that could cause actual results to differ from those in the forward-looking statements include, but are not limited to, our need for additional capital to fund our operations over the next 12 months, risks relating to our ability to obtain regulatory approval for and proceed with clinical trials including recruiting volunteers and procuring materials for such studies by our clinical research organizations and vendors, the results of such studies, our and our collaboration partners’ technology and software performing as expected, general risks arising from clinical studies, receipt of regulatory approvals, regulatory changes, and potential development of effective treatments and/or vaccines by competitors, potential mutations in a virus we are targeting that may result in variants that are resistant to a product candidate we develop, the impact of the Trump Administration’s policies and actions on regulation affecting the FDA and other healthcare agencies and potential staffing issues resulting therefrom, as well as other government actions such as tariffs which may cause delays or force us to incur additional costs to proceed without development programs. Further information on our risk factors is contained in our filings with the SEC, including our Annual Report on Form 10-K for the year ended December 31, 2024. Any forward-looking statement made by us herein speaks only as of the date on which it is made. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by law.
Investor Contact:
Alliance Advisors IR
Jody Cain
310-691-7100
jcain@allianceadvisors.com
# # #
|
|
Exhibit 99.2