Document
Exhibit 99.1
Spyre Therapeutics Reports Third Quarter 2025 Financial Results and Provides Corporate Update
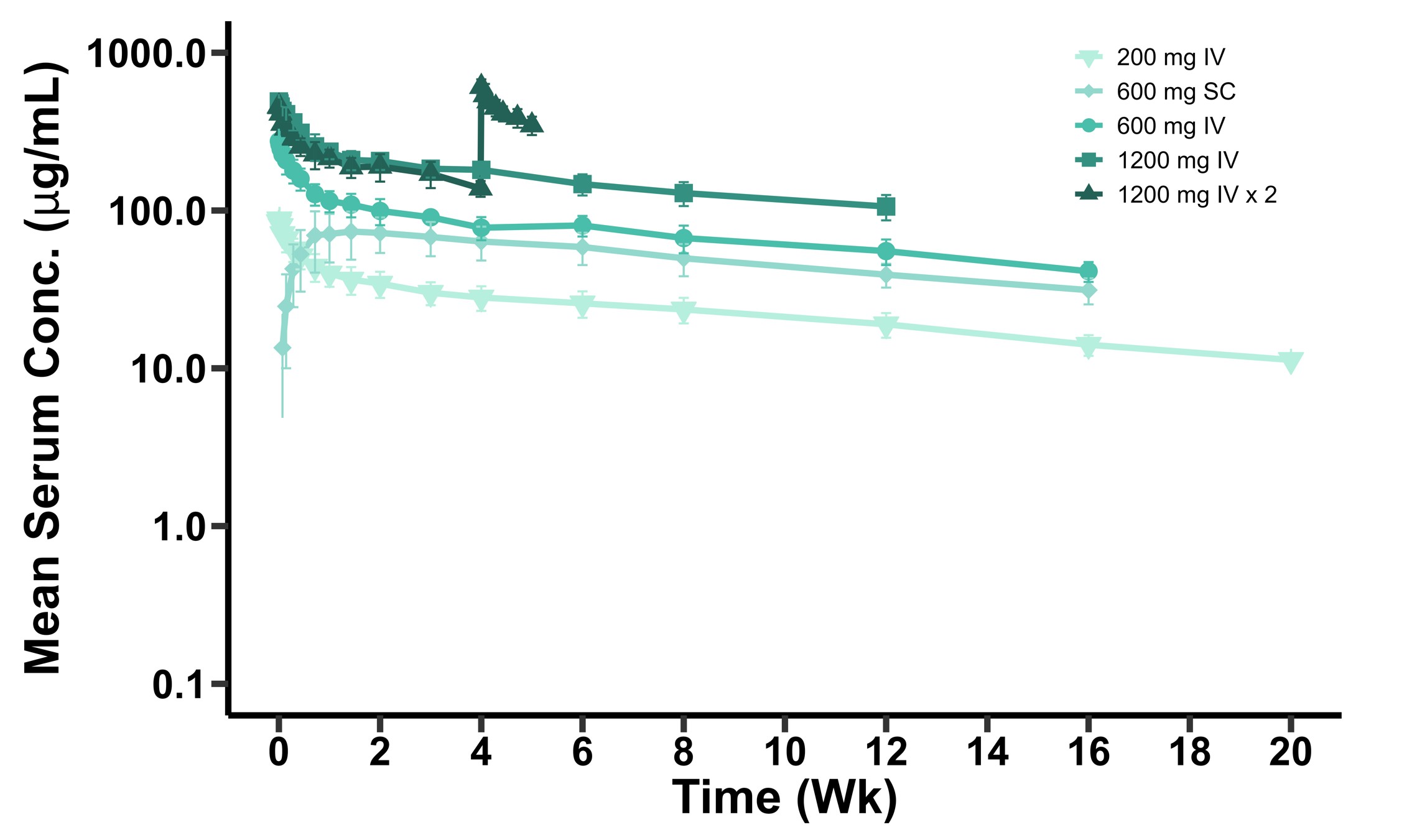
Reported positive interim Phase 1 results for SPY003, a next-generation anti-IL-23 antibody, demonstrating the molecule was well-tolerated and exhibited an ~85-day half-life supporting quarterly or twice annual maintenance dosing
Initiated Phase 2 SKYWAY basket study of SPY072 evaluating TL1A inhibition in rheumatoid arthritis ("RA"), psoriatic arthritis ("PsA"), and axial spondyloarthritis ("axSpA")
On track for 6 proof-of-concept readouts in 2026 across the SKYLINE and SKYWAY Phase 2 trials
Further strengthened balance sheet with $316 million gross proceeds from an underwritten public offering of common stock
$783 million of pro forma cash, cash equivalents, and marketable securities as of September 30, 2025, with expected runway into the second half of 2028
Waltham, Mass, November 4, 2025 (GLOBE NEWSWIRE) - Spyre Therapeutics, Inc. ("Spyre" or the "Company") (NASDAQ:SYRE), a clinical-stage biotechnology company pioneering long-acting antibodies and antibody combinations to redefine the standard of care for inflammatory bowel disease (“IBD”) and rheumatic diseases, today announced its third quarter 2025 financial results and provided program and corporate updates.
“We have now reported positive Phase 1 data for each of our four investigational antibodies, fully unlocking the breadth of our innovative Phase 2 trials evaluating product candidates with potential indication-leading profiles in diseases that together impact more than five million Americans," said Cameron Turtle, DPhil, Chief Executive Officer of Spyre. “With optimized monotherapies and uniquely differentiated combination therapies in IBD, and a potential first- and best-in-class anti-TL1A therapy in rheumatic diseases, our portfolio could redefine the standard of care in indications totaling over $60B in annual revenue. We anticipate 2026 will be a transformational year for the company as we expect to unveil six Phase 2 proof-of-concept readouts in these indications. With our world-class development organization, de-risked biology, and a recently-strengthened balance sheet, we are poised to deliver meaningful value for patients and shareholders alike.”
Development Pipeline Overview and Update
The Company is pioneering long-acting antibodies and antibody combinations to redefine the standard of care in IBD and rheumatic diseases. IBD is a chronic condition characterized by inflammation within the gastrointestinal tract, including two main disorders: ulcerative colitis ("UC") and Crohn's disease ("CD"). In the United States, it is estimated that approximately 2.4 million individuals are diagnosed with IBD. RA, PsA, and axSpA are chronic inflammatory autoimmune conditions primarily characterized by pain, stiffness, and swelling of the joints, as well as impacts on the spine and skin. Together, these rheumatic conditions affect more than three million individuals in the U.S. Existing therapies for these diseases today generally offer incomplete efficacy, meaningful safety warnings, and inconvenient dosing profiles.
Each of the Company's monotherapy programs in IBD target validated mechanisms with the potential for safe and effective treatment of UC and CD with infrequent dosing as a monotherapy or in rational combinations. The Company is also studying its anti-TL1A program as a monotherapy in indications outside IBD, including RA, PsA, and axSpA.
SPY001 – a highly potent and selective investigational monoclonal antibody targeting α4β7, engineered with half-life extension technology and formulated at high concentration with the goal of maximizing efficacy and enabling infrequent, subcutaneous maintenance dosing.
•In May 2025, extended follow up data were presented at Digestive Disease Week ("DDW") 2025 from the Phase 1 healthy volunteer trial, demonstrating a favorable safety profile across all dose groups, a meaningfully differentiated pharmacokinetic ("PK") profile supporting potential Q3M or Q6M maintenance dosing, and rapid and complete saturation of α4β7 receptors beyond six months with a single dose of 600mg.
•Based on these interim results, SPY001 was advanced into the SKYLINE Phase 2 platform trial, which initiated in May 2025.
SPY002 and SPY072 – two highly potent and selective, investigational anti-TL1A monoclonal antibodies, engineered with half-life extension technology and formulated at high concentration with the goal of maximizing efficacy and enabling infrequent, subcutaneous maintenance dosing. The Company believes TL1A has emerged as one of the most promising targets in IBD and broader immunology indications. SPY002 is being evaluated for the treatment of IBD in the SKYLINE study and SPY072 is being evaluated for the treatment of rheumatic diseases in the SKYWAY study.
•In June 2025, interim healthy volunteer data from two Phase 1 trials (one for SPY002 and one for SPY072) were presented, demonstrating favorable safety profiles, meaningfully differentiated PK profiles supporting potential Q3M or Q6M maintenance dosing, and complete suppression of free TL1A through up to 20 weeks at single 100mg doses. Longer-term data from these Phase 1 trials were presented at recent medical meetings, providing further support for these potential best-in-class profiles.
•Based on these interim results, SPY002 was advanced to the SKYLINE Phase 2 platform trial, and SPY072 was advanced to the SKYWAY Phase 2 basket trial, which initiated in September 2025.
SPY003 – a highly potent and selective investigational monoclonal antibody targeting the p19 subunit of IL-23, engineered with half-life extension technology and formulated at high concentration with the goal of maximizing efficacy and enabling infrequent, subcutaneous maintenance dosing.
•In November 2025, interim healthy volunteer data from a Phase 1 trial were disclosed, demonstrating that SPY003 exhibited a favorable safety profile and a half-life of ~85 days, supporting potential Q3M or Q6M maintenance dosing.
•Based on these interim results, SPY003 is expected to advance to the SKYLINE Phase 2 platform trial.
Rational Combinations – the Company plans to investigate combinations of our proprietary antibodies in nonclinical studies and clinical trials in order to evaluate whether combinations can potentially lead to best-in-class efficacy in IBD, with less frequent dosing.
•In February and May 2025, preclinical data for SPY120 were presented at medical meetings, demonstrating that the combined inhibition of TL1A and α4β7 is superior to either monotherapy in mouse models of colitis and that the PK profiles of SPY001 and SPY002 were similar in non-human primates whether dosed as monotherapy or in combination, while also demonstrating no drug effects on PK.
•Preclinical data for SPY130 and SPY230 have demonstrated enhanced efficacy and pharmacodynamics with SPY003 in combination with SPY001 and with SPY002.
•The Company expects to include each of its rational combinations in Part B of the SKYLINE trial.
SKYLINE Phase 2 Platform Trial - in May 2025, the Company initiated a Phase 2 induction and maintenance platform trial of SPY001, SPY002, SPY003, as well as pairwise combinations thereof (six active investigational agents in total) in patients with moderately to severely active UC. The trial consists of two parts:
•Part A: Open-label assessment of the safety and preliminary efficacy of a single dose level of each investigational monotherapy, with induction data expected in 2026.
•Part B: Randomized and placebo-controlled assessment of the safety and efficacy of investigational monotherapies (two dose levels) and combinations, with induction data expected in 2027.
SKYLINE is currently enrolling subjects into the SPY001 and SPY002 arms of Part A, with SPY003 expected to begin enrolling in the coming months. Part B is expected to begin enrolling after Part A completes enrollment.
SKYWAY Phase 2 Basket Trial in Rheumatic Diseases (RA, PsA, axSpA) - in September 2025, the Company initiated a Phase 2 randomized and placebo-controlled basket trial of SPY072 in patients with moderately to severely active RA, PsA, or axSpA. The trial consists of three sub-studies, each expected to provide proof-of-concept data in 2026:
•RA sub-study: Double-blind, placebo-controlled safety and efficacy study of two dose levels of SPY072 at Week 12.
•PsA sub-study: Double-blind, placebo-controlled safety and efficacy study of a single dose level of SPY072 at Week 16.
•axSpA sub-study: Double-blind, placebo-controlled safety and efficacy study of a single dose level of SPY072 at Week 16.
Third Quarter 2025 Financial Results
Cash Position: As of September 30, 2025, Spyre had cash, cash equivalents, and marketable securities of $486.2 million. Pro forma cash of $782.7 million at September 30, 2025 also reflects $296.5 million in net proceeds from the recently closed October 2025 underwritten public offering of common stock. Net cash used in operating activities was $37.1 million for the third quarter of 2025.
Research and Development (R&D) expenses: R&D expenses totaled $45.2 million for the third quarter of 2025 and $44.7 million for the third quarter of 2024. Higher clinical trial expenses and milestone payments were offset by lower early-stage R&D activities.
General and Administrative (G&A) expenses: G&A expenses totaled $11.6 million for the third quarter of 2025 and $10.6 million for the third quarter of 2024. The increase was primarily driven by higher headcount.
Other income (expense), net: Other income totaled $45.7 million for the third quarter of 2025 versus $13.6 million of expense for the third quarter of 2024 primarily driven by changes in the fair value of the contingent value right (CVR) liability.
Net Loss: Net loss totaled $11.2 million and $69.0 million for the third quarters of 2025 and 2024, respectively.
About Spyre Therapeutics
Spyre Therapeutics is a clinical-stage biotechnology company pioneering long-acting antibodies and antibody combinations to redefine the standard of care for inflammatory bowel disease (“IBD”) and rheumatic diseases. Spyre's pipeline includes investigational extended half-life antibodies targeting α4β7, TL1A, and IL-23.
For more information, please visit http://spyre.com.
Safe Harbor / Forward Looking Statements
This press release contains "forward-looking" statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. All statements contained in this press release, other than statements of historical fact, are forward-looking statements. These forward-looking statements include statements regarding the Company's future results of operations and financial position; its business strategy, including the Company's ability to successfully develop best-in-class therapeutics for IBD or RD that meaningfully improve both efficacy and convenience compared to today's standard of care and our ability to develop first-in-class therapeutics for RD; the potential consistency of the SPY001, SPY002, SPY072 and SPY003 Phase 1 trial final data readouts with previously disclosed interim Phase 1 results; the sufficiency of the Company's funding to support the development of its assets, including expectations of cash runway extending into the second half of 2028; the length of time that the Company believes its existing cash resources will fund its operations; estimated market sizes and potential growth opportunities; its nonclinical and future clinical development activities, including the timing of data readouts for the ongoing SKYWAY-RD Phase 2 basket trial, plans for and timing of monotherapy/combination arm enrollment, cohort initiation and data readouts for the ongoing SKYLINE-UC Phase 2 platform trial and further clinical evaluation of therapeutic combinations, enrollment of clinical trials, number of data readouts expected to be delivered in 2026 and 2027 and the advancement of SPY003 to the SKYLINE-UC Phase 2 platform trial, and related regulatory feedback; further clinical evaluation of therapeutic combinations, including expectations regarding efficacy and dosing regime; the potential efficacy, tolerability, convenience, commercial viability and safety profile of its product candidates, including in combinations; the planned dosing regimen for SPY001, SPY002, SPY072 and our other product candidates, including the potential for a Q3M or Q6M dosing profile; and the potential therapeutic benefits and economic value of its product candidates as monotherapies or in combinations and their extended half-life. The words “believe,” “may,” “will,” “potentially,” “estimate,” “continue,” “anticipate,” “predict,” “target,” “intend,” “could,” “would,” “should,” “project,” “plan,” “expect,” the negatives of these terms, and similar expressions that convey uncertainty of future events or outcomes are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those arising from regulatory feedback, including potential disagreement by regulatory authorities with our clinical trial design, interpretation of data and our ongoing or planned clinical trials for our product candidates, including our plans for and timing of cohort initiation for combination arms for the ongoing SKYLINE-UC Phase 2 platform trial across different jurisdictions; the potential for final clinical data not being consistent with or different than the previously disclosed interim data for our programs; the impacts of adverse events or disappointing results in clinical trials of third parties, including our competitors developing product candidates that target similar mechanisms of action and/or indications as our product candidates; the expected or potential impact of macroeconomic conditions, including inflationary pressures, rising interest rates, general economic slowdown or a recession, changes in tariff/trade and monetary policy, volatile market conditions, financial institution instability, as well as geopolitical instability, including the ongoing military conflicts between Ukraine and Russia, conflicts in the Middle East, and geopolitical tensions between the United States and other countries, including China, on the Company's operations; the implementation of changes in law, tariffs, sanctions, export or import controls, and other government measures that could impact our business operations, including restricting international trade by the United States, China or other countries and the BIOSECURE Act or similar act if passed into law; and those risks described in the Company’s Quarterly Reports on Form 10-Q, Annual Reports on Form 10-K, as well as in other filings and reports that the Company makes from time to time with the Securities and Exchange Commission. Moreover, the Company operates in a very competitive and rapidly changing environment, and new risks emerge from time to time. It is not possible for the Company’s management to predict all risks, nor can the Company assess the impact of all factors on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements it may make.
In light of these risks, uncertainties, and assumptions, the forward-looking events and circumstances discussed in this press release may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. The Company undertakes no obligation to update publicly any forward-looking statement for any reason after the date of this press release to conform these statements to actual results, to reflect changes in the Company’s expectations, or otherwise, except as required by law. You should read press release with the understanding that the Company’s actual results, levels of activity, performance, events, outcomes, and the timing of results and outcomes, and other circumstances may be materially different from what the Company expects.
Contact Information:
Media Contact
Josie Butler, 1AB
josie@1abmedia.com
Investor Contact
Eric McIntyre
eric.mcintyre@spyre.com
Spyre Therapeutics, Inc.
Consolidated Balance Sheets
(Unaudited, in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30,
2025 |
|
December 31,
2024 |
| ASSETS |
|
|
|
| CURRENT ASSETS |
|
|
|
| Cash and cash equivalents |
$ |
64,897 |
|
|
$ |
89,423 |
|
| Marketable securities |
421,301 |
|
|
513,665 |
|
|
|
|
|
| Prepaid expenses and other current assets |
18,406 |
|
|
5,386 |
|
| Total current assets |
504,604 |
|
|
608,474 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other non-current assets |
— |
|
|
10 |
|
| TOTAL ASSETS |
$ |
504,604 |
|
|
$ |
608,484 |
|
|
|
|
|
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
| CURRENT LIABILITIES |
|
|
|
| Accounts payable |
$ |
7,457 |
|
|
$ |
666 |
|
|
|
|
|
| CVR liability |
11,020 |
|
|
25,080 |
|
|
|
|
|
|
|
|
|
| Accrued and other current liabilities |
23,827 |
|
|
27,711 |
|
| Related party accounts payable |
3,970 |
|
|
603 |
|
| Total current liabilities |
46,274 |
|
|
54,060 |
|
| Non-current CVR liability |
3,230 |
|
|
36,620 |
|
|
|
|
|
|
|
|
|
| TOTAL LIABILITIES |
49,504 |
|
|
90,680 |
|
| Commitments and Contingencies |
|
|
|
|
|
|
|
| STOCKHOLDERS’ EQUITY |
|
|
|
Series A non-voting convertible preferred stock, $0.0001 par value; 1,086,341 shares authorized as of September 30, 2025 and December 31, 2024; 346,045 shares issued and outstanding as of September 30, 2025 and December 31, 2024. |
146,425 |
|
|
146,425 |
|
Series B non-voting convertible preferred stock, $0.0001 par value; 271,625 shares authorized and 16,667 shares issued and outstanding as of September 30, 2025 and December 31, 2024. |
9,395 |
|
|
9,395 |
|
Preferred stock, $0.0001 par value; 8,642,034 shares authorized as of September 30, 2025 and December 31, 2024; no shares issued and outstanding as of September 30, 2025 and December 31, 2024. |
— |
|
|
— |
|
Common stock, $0.0001 par value; 400,000,000 shares authorized as of September 30, 2025 and December 31, 2024; 60,471,793 shares and 60,257,023 shares issued and outstanding as of September 30, 2025 and December 31, 2024, respectively. |
13 |
|
|
13 |
|
| Additional paid-in capital |
1,363,581 |
|
|
1,334,223 |
|
| Accumulated other comprehensive income |
791 |
|
|
180 |
|
| Accumulated deficit |
(1,065,105) |
|
|
(972,432) |
|
| TOTAL STOCKHOLDERS’ EQUITY |
455,100 |
|
|
517,804 |
|
| TOTAL LIABILITIES, CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY |
$ |
504,604 |
|
|
$ |
608,484 |
|
Spyre Therapeutics, Inc.
Consolidated Statements of Operations
(Unaudited, in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
Nine Months Ended
September 30, |
|
2025 |
|
2024 |
|
2025 |
|
2024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
Research and development (1) |
45,247 |
|
|
44,744 |
|
|
127,015 |
|
|
112,308 |
|
General and administrative (2) |
11,641 |
|
|
10,648 |
|
|
35,375 |
|
|
35,005 |
|
|
|
|
|
|
|
|
|
| Gain on sale of in-process research and development asset |
— |
|
|
— |
|
|
(10,000) |
|
|
— |
|
| Total operating expenses |
56,888 |
|
|
55,392 |
|
|
152,390 |
|
|
147,313 |
|
| Loss from operations |
(56,888) |
|
|
(55,392) |
|
|
(152,390) |
|
|
(147,313) |
|
|
|
|
|
|
|
|
|
| Other income (expense): |
|
|
|
|
|
|
|
| Interest income |
5,379 |
|
|
5,184 |
|
|
17,746 |
|
|
15,536 |
|
|
|
|
|
|
|
|
|
| Other income (expense), net |
40,326 |
|
|
(18,802) |
|
|
41,956 |
|
|
(19,895) |
|
| Total other income (expense) |
45,705 |
|
|
(13,618) |
|
|
59,702 |
|
|
(4,359) |
|
| Loss before income tax expense |
(11,183) |
|
|
(69,010) |
|
|
(92,688) |
|
|
(151,672) |
|
| Income tax (expense) benefit |
— |
|
|
(18) |
|
|
15 |
|
|
(50) |
|
| Net loss |
$ |
(11,183) |
|
|
$ |
(69,028) |
|
|
$ |
(92,673) |
|
|
$ |
(151,722) |
|
|
|
|
|
|
|
|
|
| Net loss per share, basic and diluted, Series A Preferred Stock |
$ |
(5.97) |
|
|
$ |
(42.22) |
|
|
$ |
(49.53) |
|
|
$ |
(95.68) |
|
| Weighted-average Series A non-voting convertible preferred stock outstanding, basic and diluted |
346,045 |
|
346,045 |
|
346,045 |
|
383,903 |
|
|
|
|
|
|
|
|
| Net loss per share, basic and diluted, Series B Preferred Stock |
$ |
(5.97) |
|
|
$ |
(42.24) |
|
|
$ |
(49.53) |
|
|
$ |
(95.68) |
|
| Weighted-average Series B non-voting convertible preferred stock outstanding, basic and diluted |
16,667 |
|
16,667 |
|
16,667 |
|
95,158 |
|
|
|
|
|
|
|
|
| Net loss per share, basic and diluted, common |
$ |
(0.15) |
|
|
$ |
(1.06) |
|
|
$ |
(1.24) |
|
|
$ |
(2.39) |
|
Weighted-average common stock outstanding, basic and diluted |
60,414,223 |
|
50,889,443 |
|
60,338,541 |
|
44,263,746 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)Includes $7.0 million and $9.6 million in related party expenses for the three and nine months ended September 30, 2025, respectively, and $7.7 million and $34.2 million related party expenses for the three and nine months ended September 30, 2024, respectively.
(2)Includes related party expenses of $0.3 million for the three months ended September 30, 2025 and 2024, and $0.8 million for the nine months ended September 30, 2025 and 2024.