Document
Foghorn Therapeutics Provides Third Quarter 2025 Financial and Corporate Update
Ongoing FHD-909 (LY4050784) Phase 1 dose escalation trial in SMARCA4(BRG1)-mutated cancer remains on track with non-small cell lung cancer (NSCLC) as the primary target population
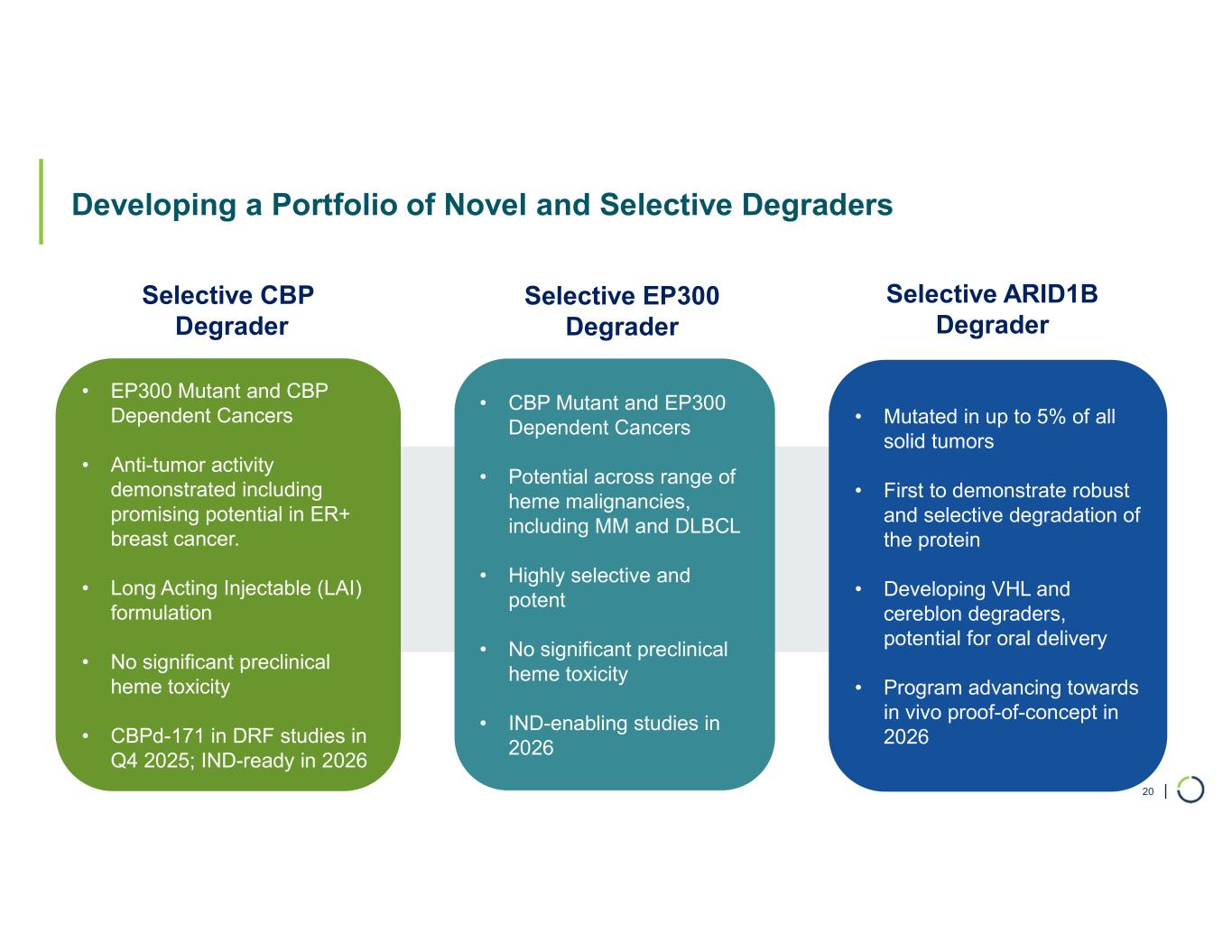
Selective CBP degrader entered non-GLP toxicology studies in Q4 2025 with potential in EP300-mutant cancers and ER+ breast cancer; IND-ready in 2026
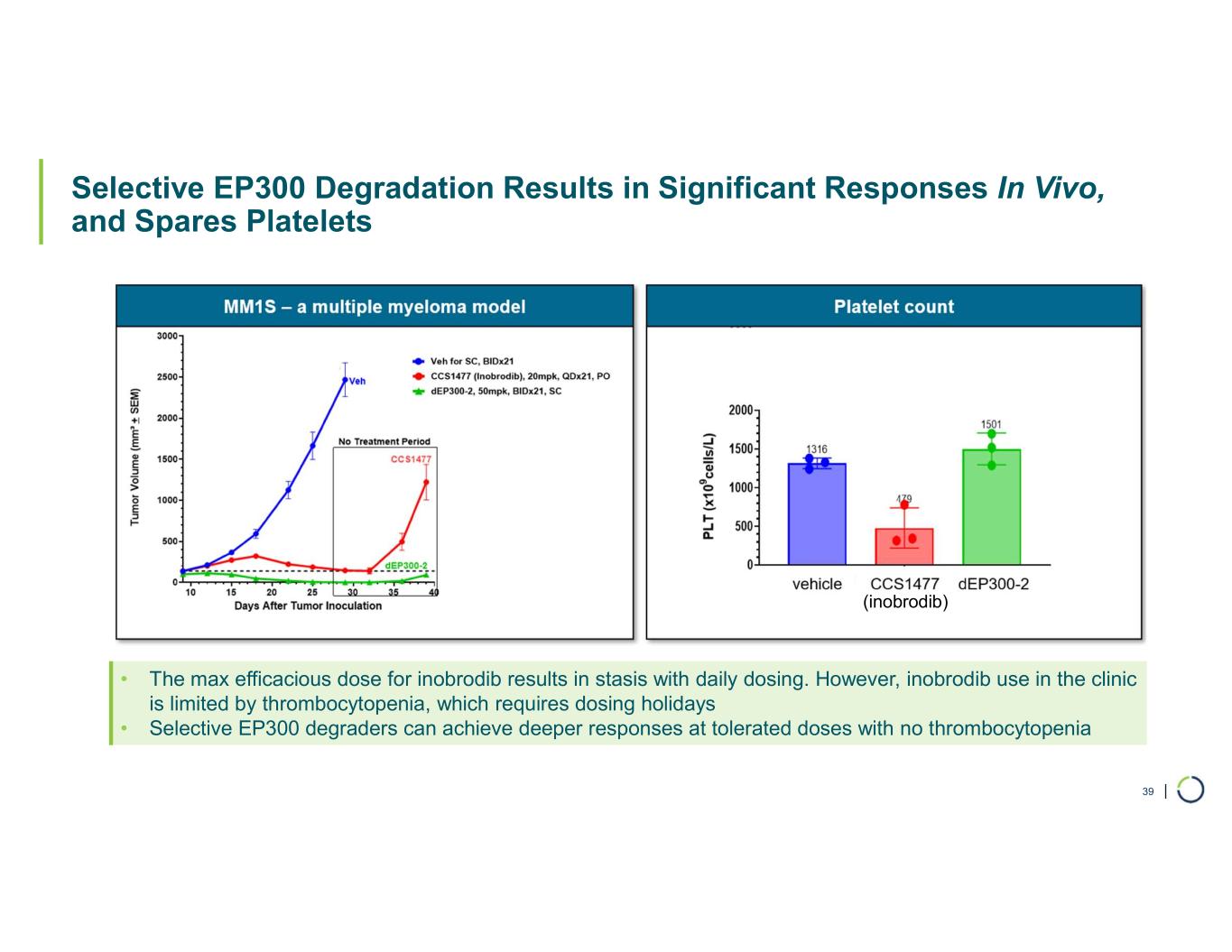
Robust preclinical anti-tumor activity and favorable tolerability across hematological malignancies differentiate novel, Selective EP300 degrader from dual CBP/EP300 approaches
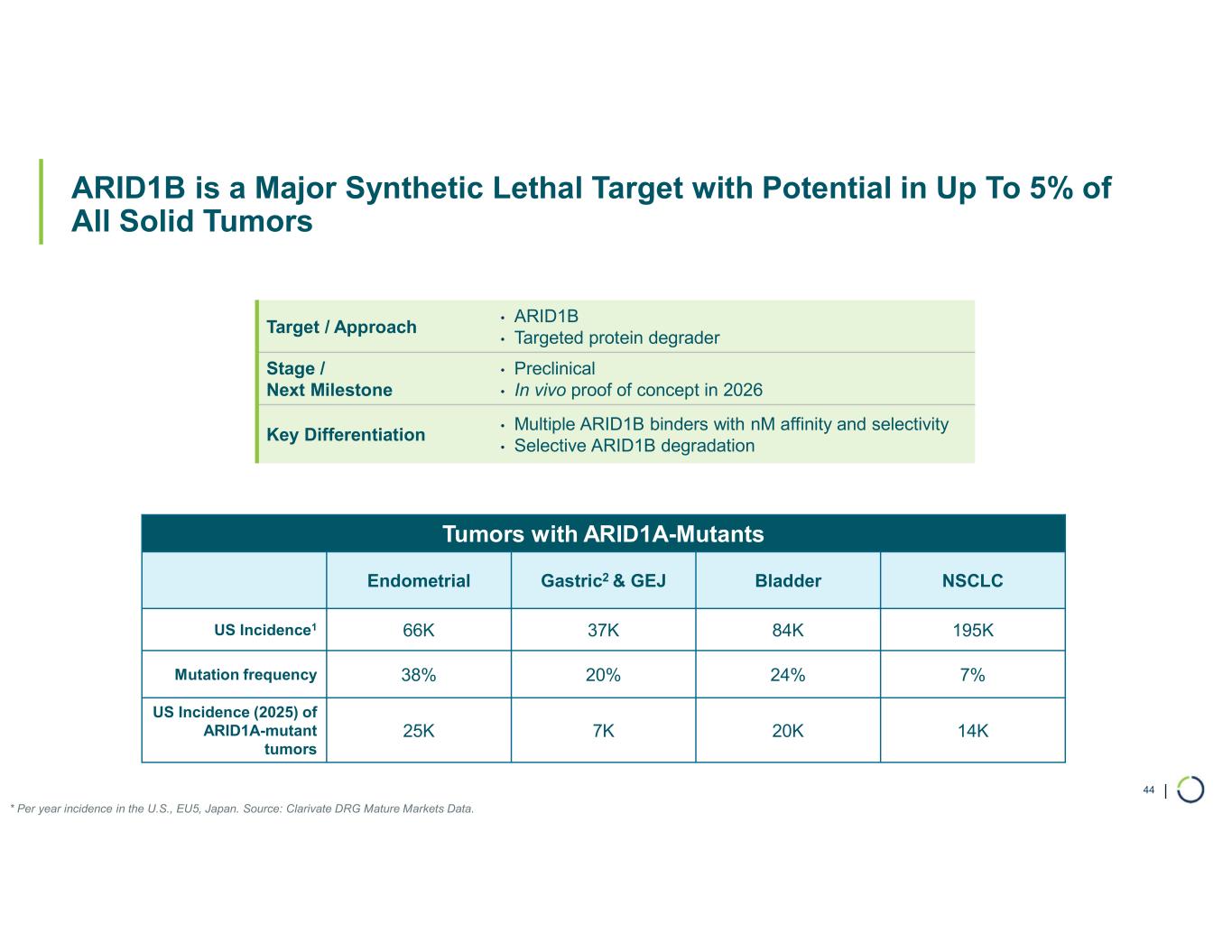
Selective ARID1B degrader advancing towards in vivo proof of concept in 2026 with relevance in up to 5% of solid tumors
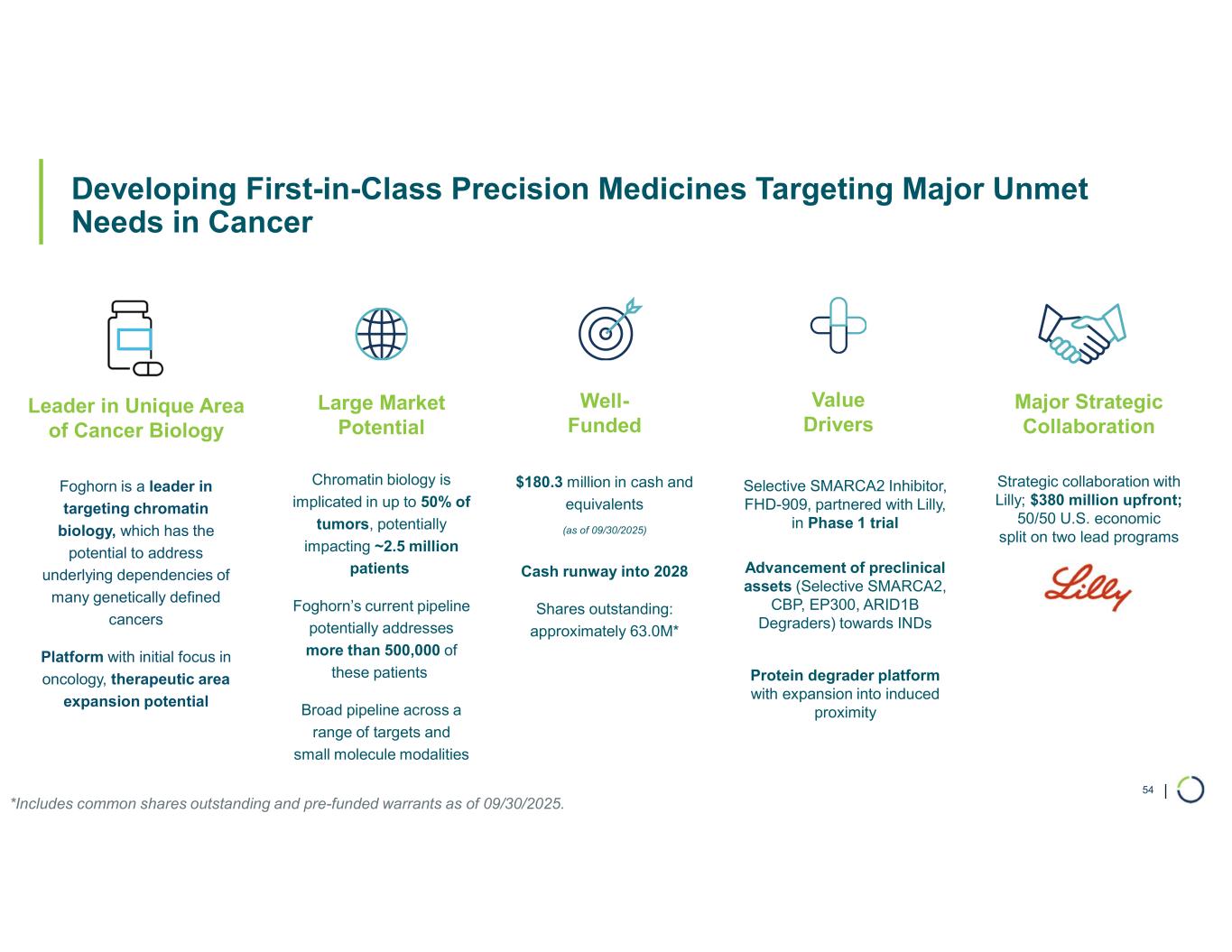
Strong balance sheet with cash, cash equivalents, and marketable securities of
$180.3 million as of September 30, 2025; cash runway into 2028
CAMBRIDGE, Mass. -- (GLOBE NEWSWIRE) – November 5, 2025 -- Foghorn® Therapeutics Inc. (Nasdaq: FHTX), a clinical-stage biotechnology company pioneering a new class of medicines that treat serious diseases by correcting abnormal gene expression, today provided a financial and corporate update in conjunction with the Company’s 10-Q filing for the quarter ended September 30, 2025. The Company also announced that Chief Financial Officer, Kristian Humer, will be departing to pursue another opportunity.
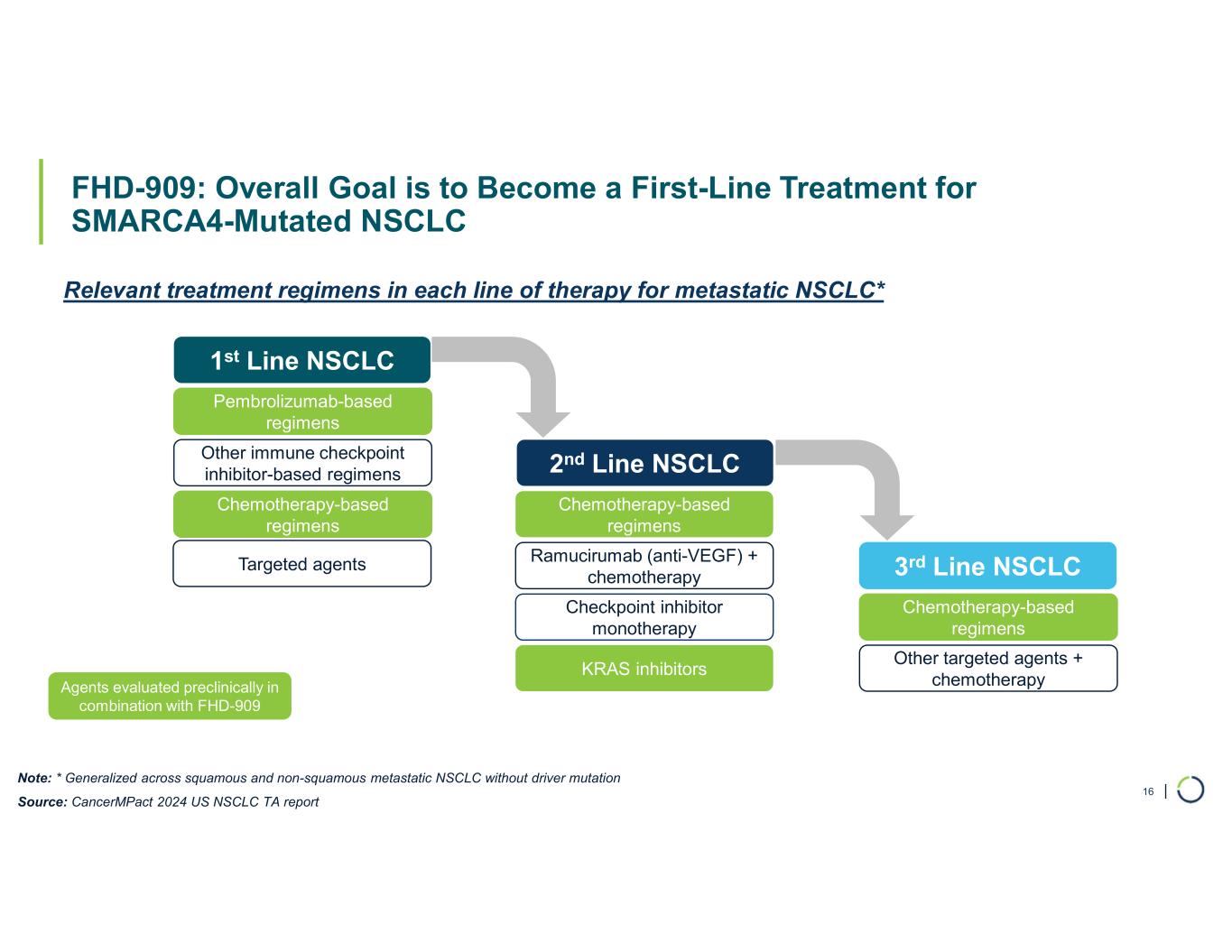
“This quarter marked continued execution across our portfolio, reinforcing our leadership in developing novel precision therapies with broad applicability across cancers,” said Adrian Gottschalk, President and Chief Executive Officer of Foghorn. “FHD-909, in collaboration with Lilly, is advancing in a Phase 1 dose escalation trial for the treatment of SMARCA4-mutated cancers with a focus on NSCLC. Based on our preclinical monotherapy and combination data, we are enthusiastic about the development of FHD-909 with the goal of developing it as a front-line therapy in NSCLC. Our strategic collaboration with Lilly provides the necessary strategic and financial resources to develop FHD-909.”
Mr. Gottschalk continued, “Momentum is strong for our wholly-owned, first-in-class selective degrader programs targeting CBP, EP300 and ARID1B with program updates highlighted during our recent virtual investor event. Our Selective CBP degrader, with potential in ER+ breast cancer, entered non-GLP toxicology studies in Q4 2025 and is advancing towards IND in 2026, and our Selective EP300 degrader continues to show broad spectrum efficacy across hematological malignancies and favorable tolerability in preclinical studies, differentiating it from dual CBP/EP300 approaches. Our Selective ARID1B degrader is progressing towards in vivo proof-of-concept in 2026 with potential in up to 5% of all solid tumors including endometrial, gastric, gastroesophageal junction, bladder and non-small cell lung cancer. Backed by our strong balance sheet and a cash runway into 2028, we are focused on delivering breakthrough therapies that harness the broad therapeutic potential of protein degradation and chromatin regulation.
Finally, I want to thank Kristian for his leadership and commitment during his time at Foghorn. He has been a valued member of the team, and I wish him well in his future endeavors.”
Program Overview and Upcoming Milestones
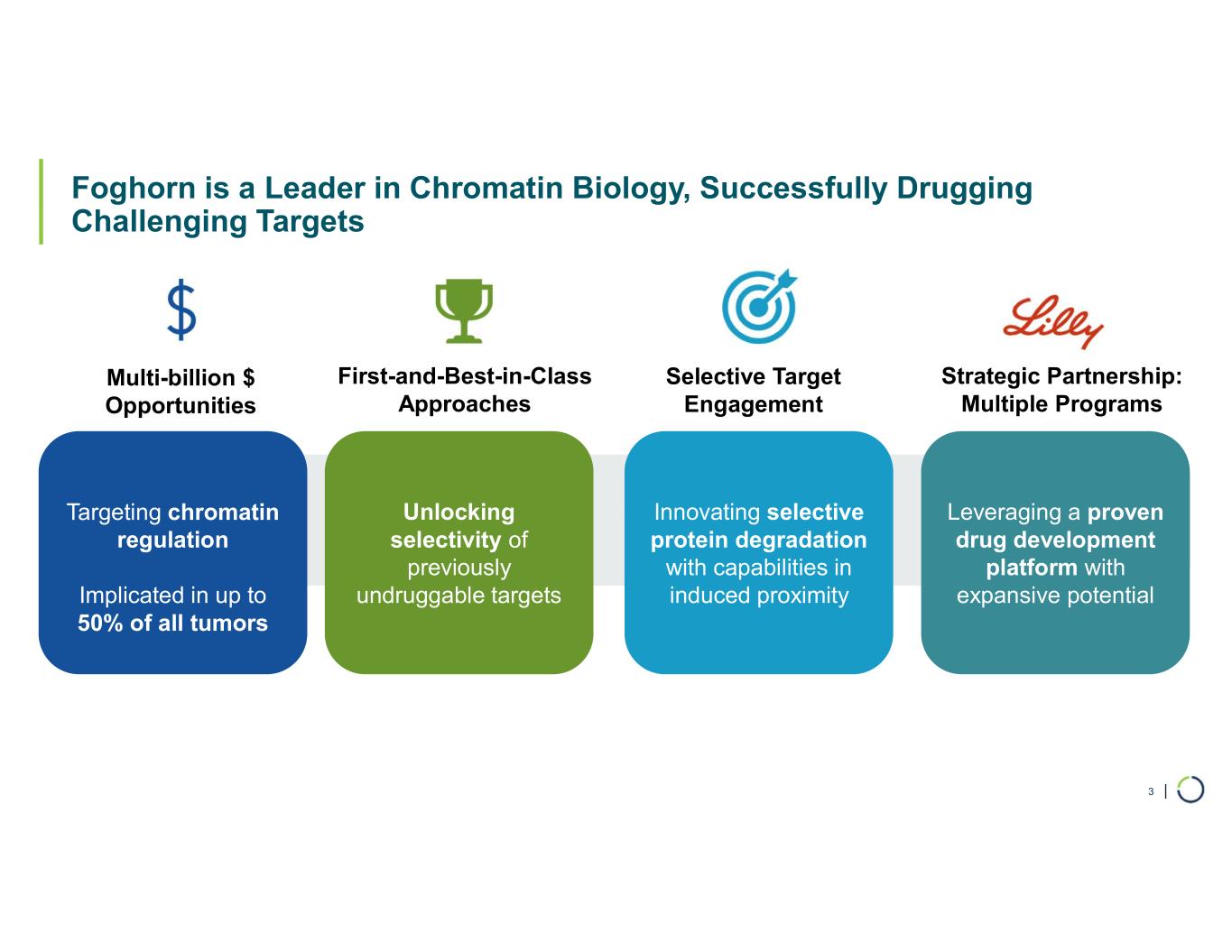
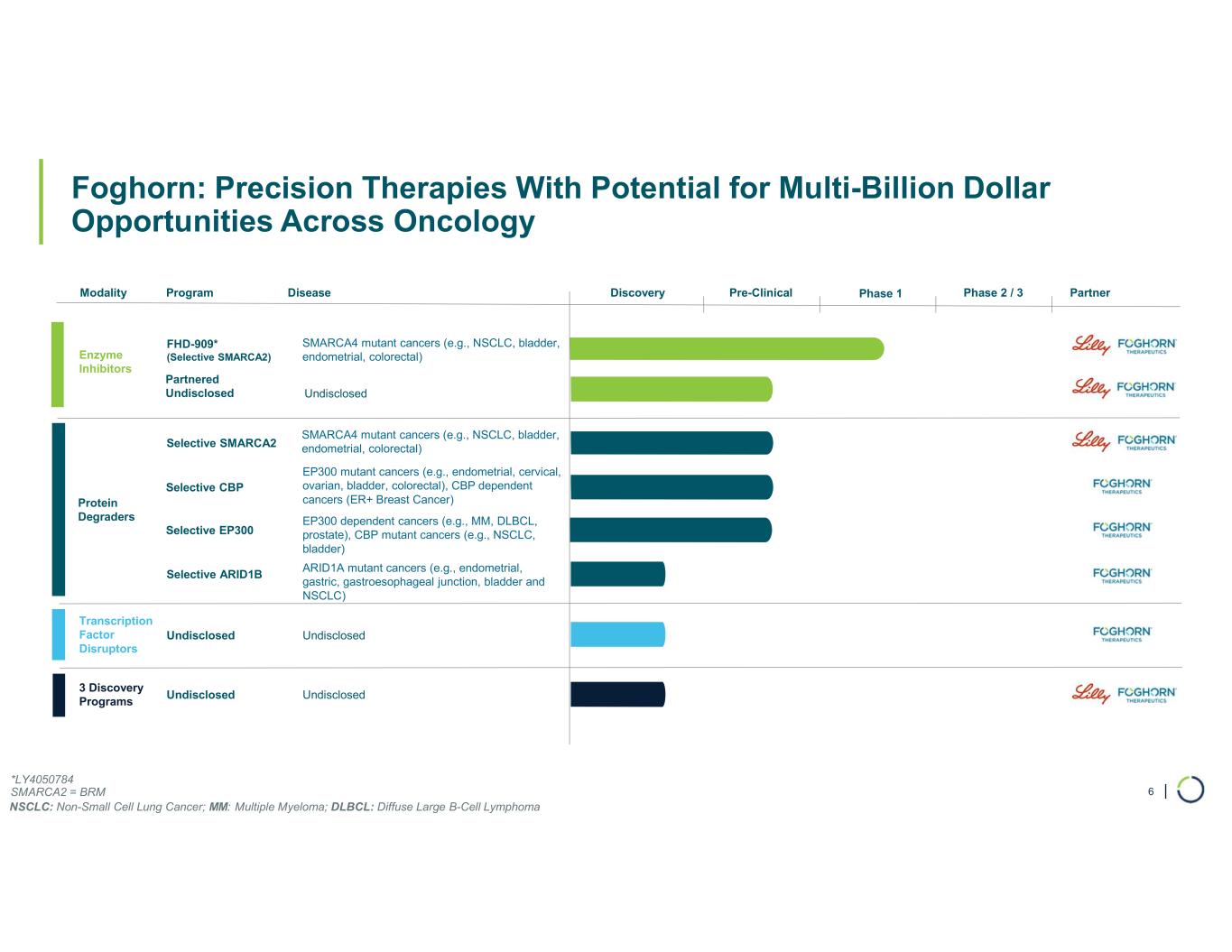
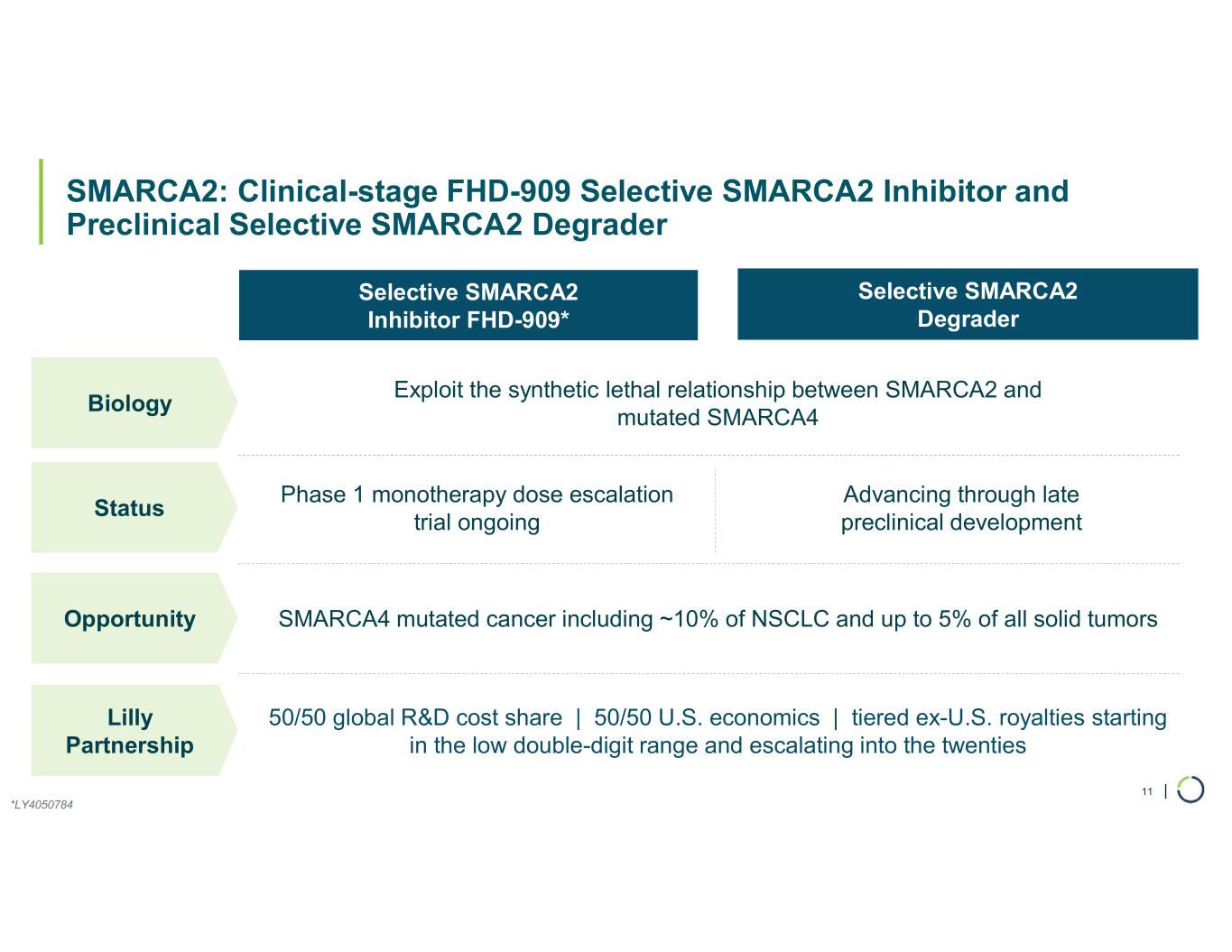
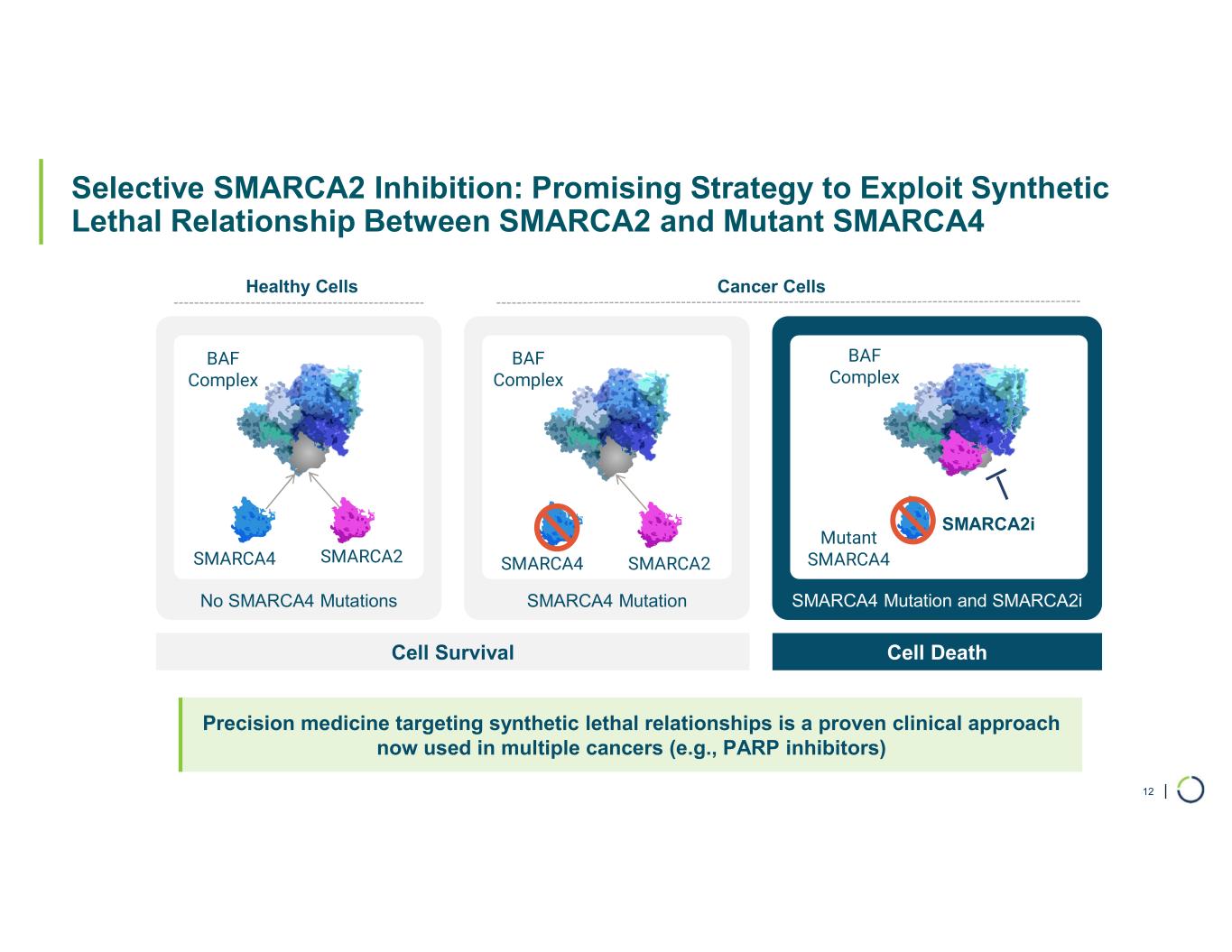
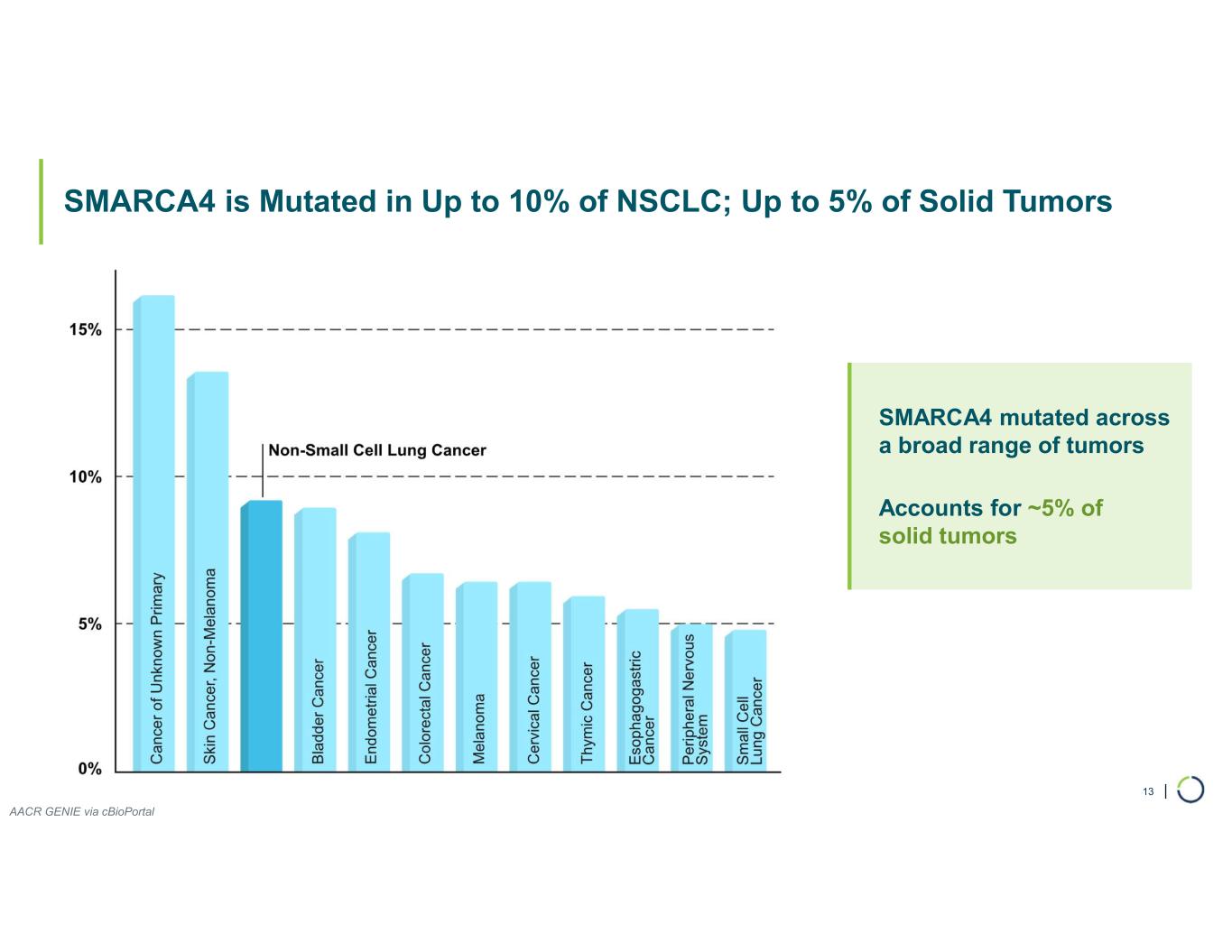
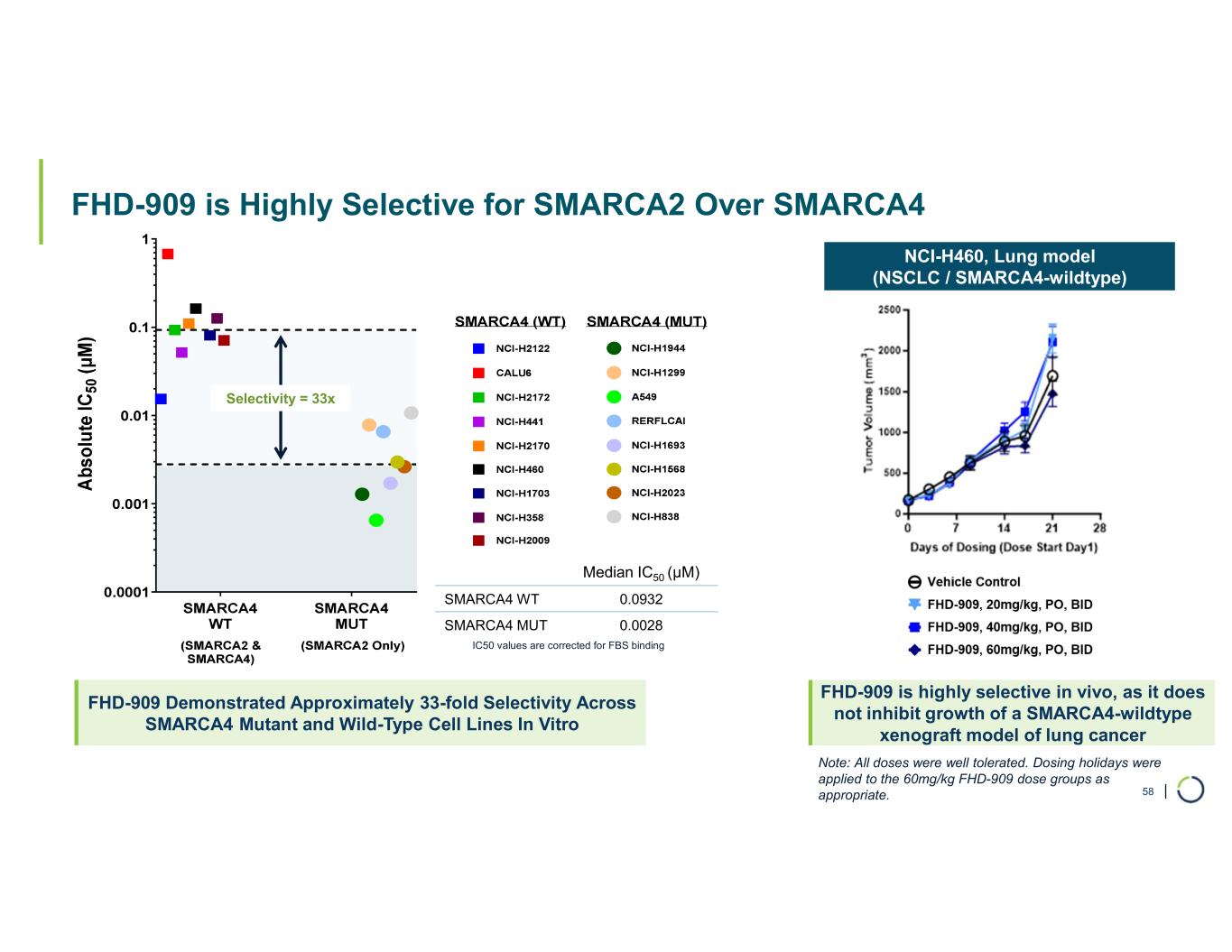
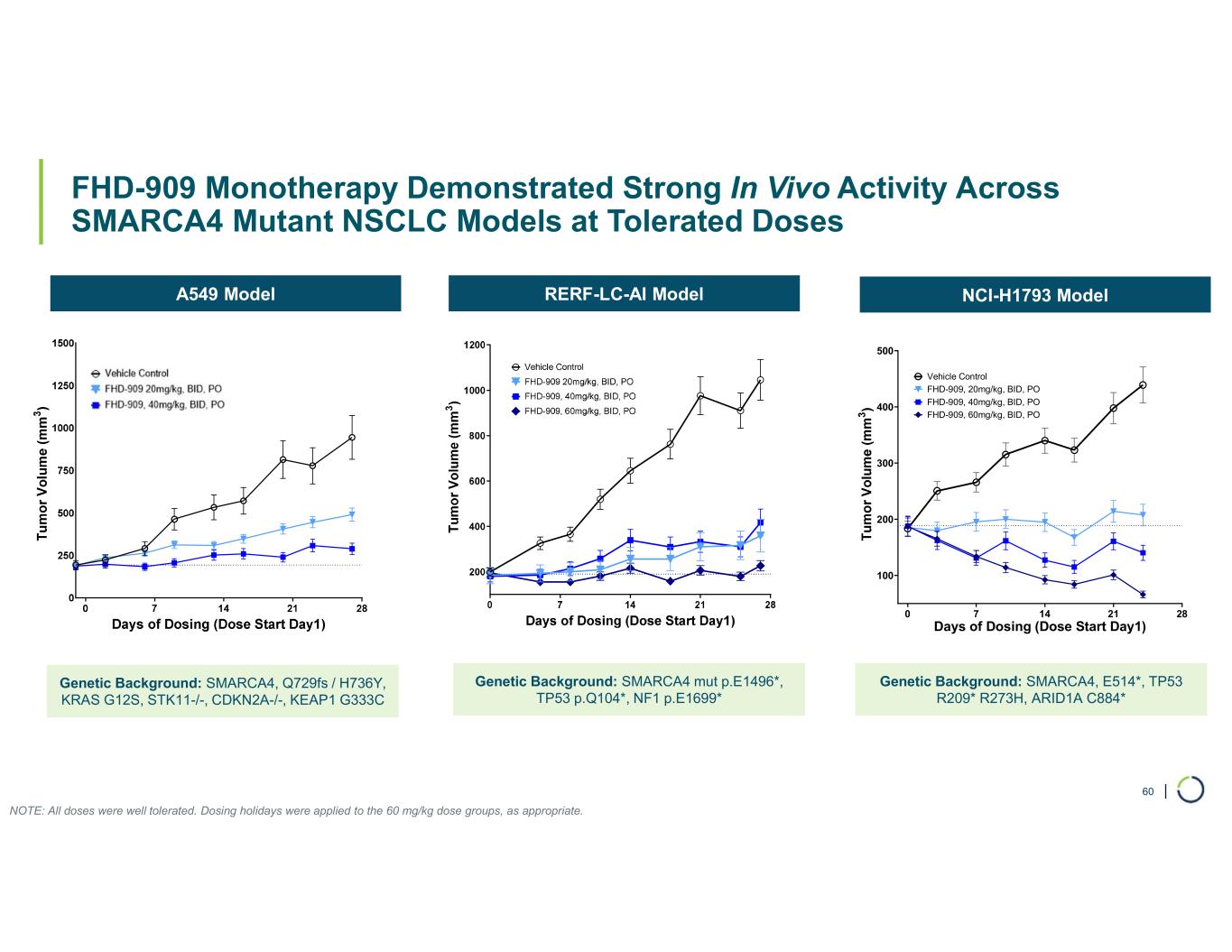
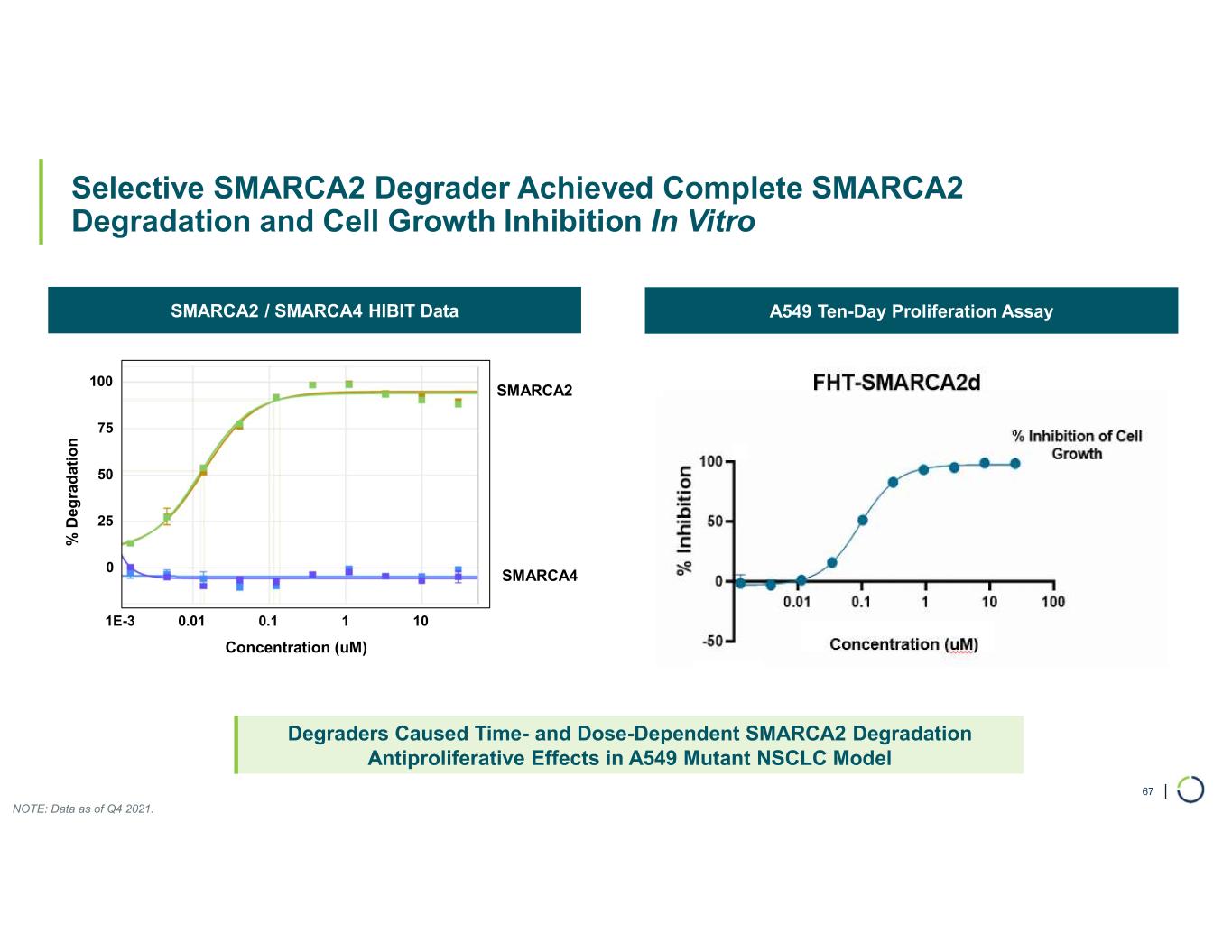
FHD-909 (LY4050784). FHD-909 is a first-in-class oral SMARCA2 selective inhibitor that has demonstrated in preclinical studies to have high selectivity over its closely-related paralog SMARCA4, two proteins that are the catalytic engines across all forms of the BAF complex. Selectively blocking SMARCA2 activity is a promising synthetic lethal strategy intended to induce tumor death while sparing healthy cells. SMARCA4 is mutated in up to 10% of NSCLC alone and implicated in a significant number of solid tumors.
•Phase 1 trial enrolling well. Enrollment in the first-in-human Phase 1 multi-center trial of FHD-909, with NSCLC as the primary target population, is progressing well and the study remains on track.
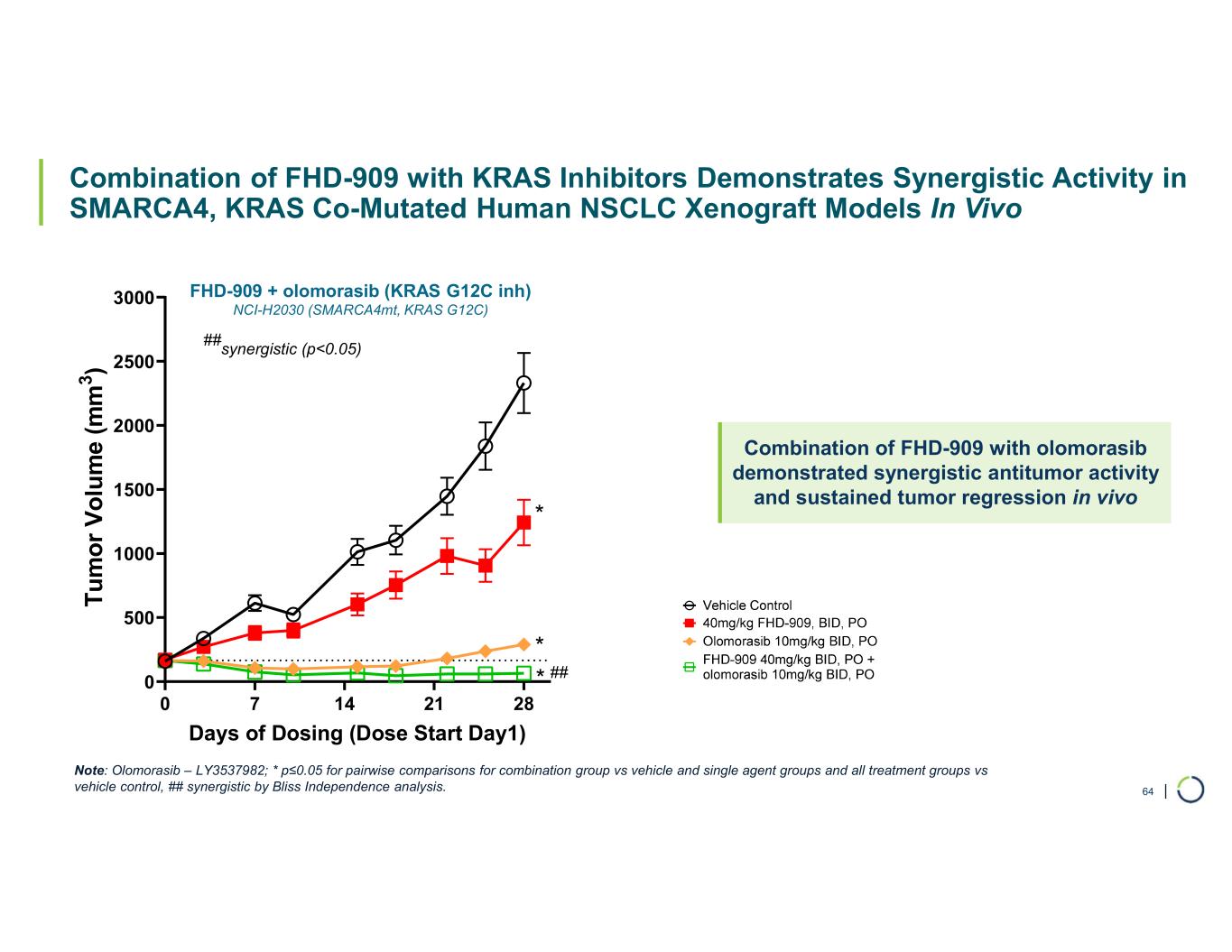
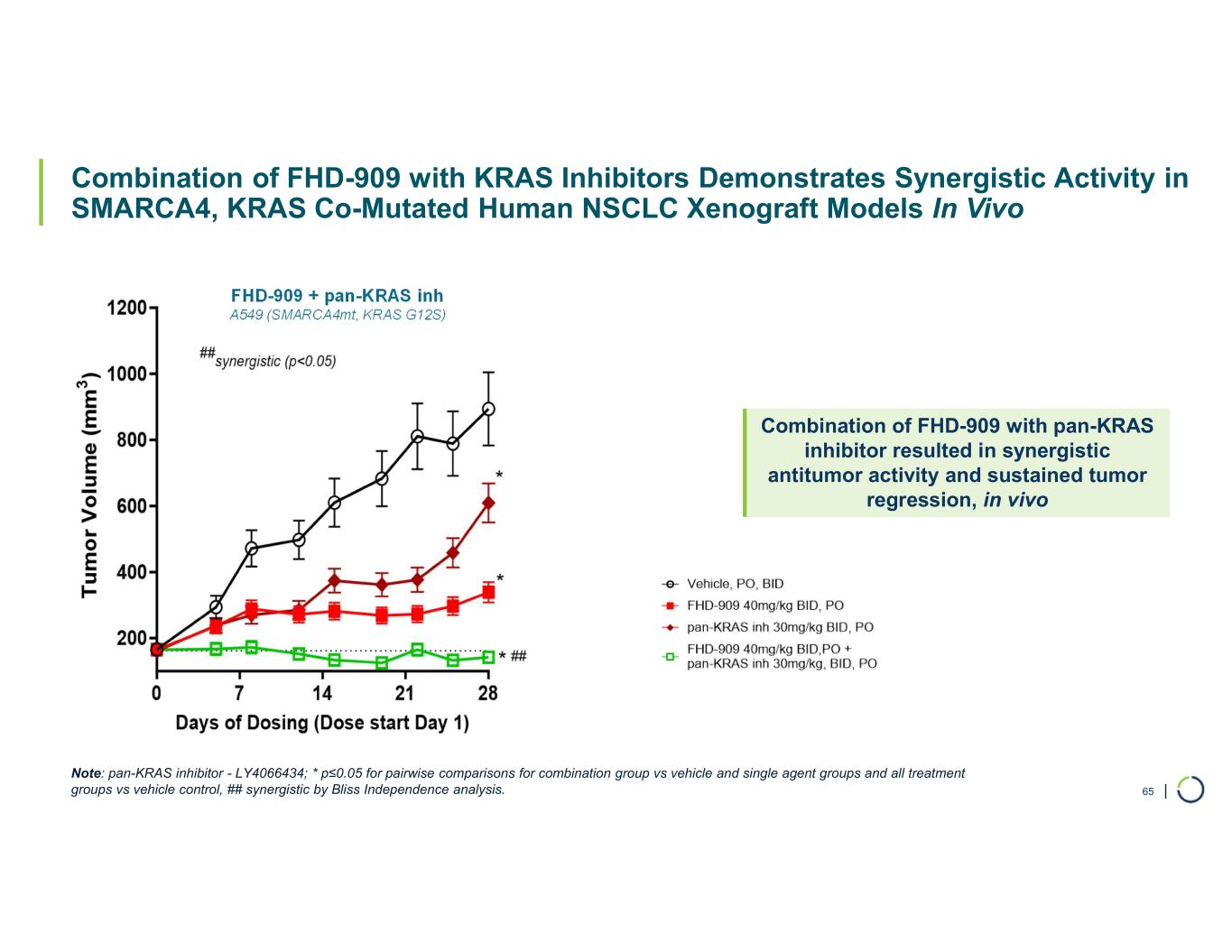
•Synergistic preclinical data of FHD-909 in combination with pembrolizumab and KRAS inhibitors. Preclinical data demonstrates enhanced anti-tumor activity of FHD-909 in combination with standard-of-care (SoC) chemotherapies, anti-PD-1 pembrolizumab and several novel KRAS inhibitors in NSCLC animal models. The combination data will inform further development plans of FHD-909.
Ongoing strategic collaboration with Lilly. Foghorn is collaborating with Lilly to develop novel oncology medicines, including a U.S. 50/50 U.S. co-development and co-commercialization agreement for its selective SMARCA2 oncology program that includes both a selective inhibitor and a selective degrader, as well as an additional undisclosed oncology target. The collaboration also includes three discovery programs.
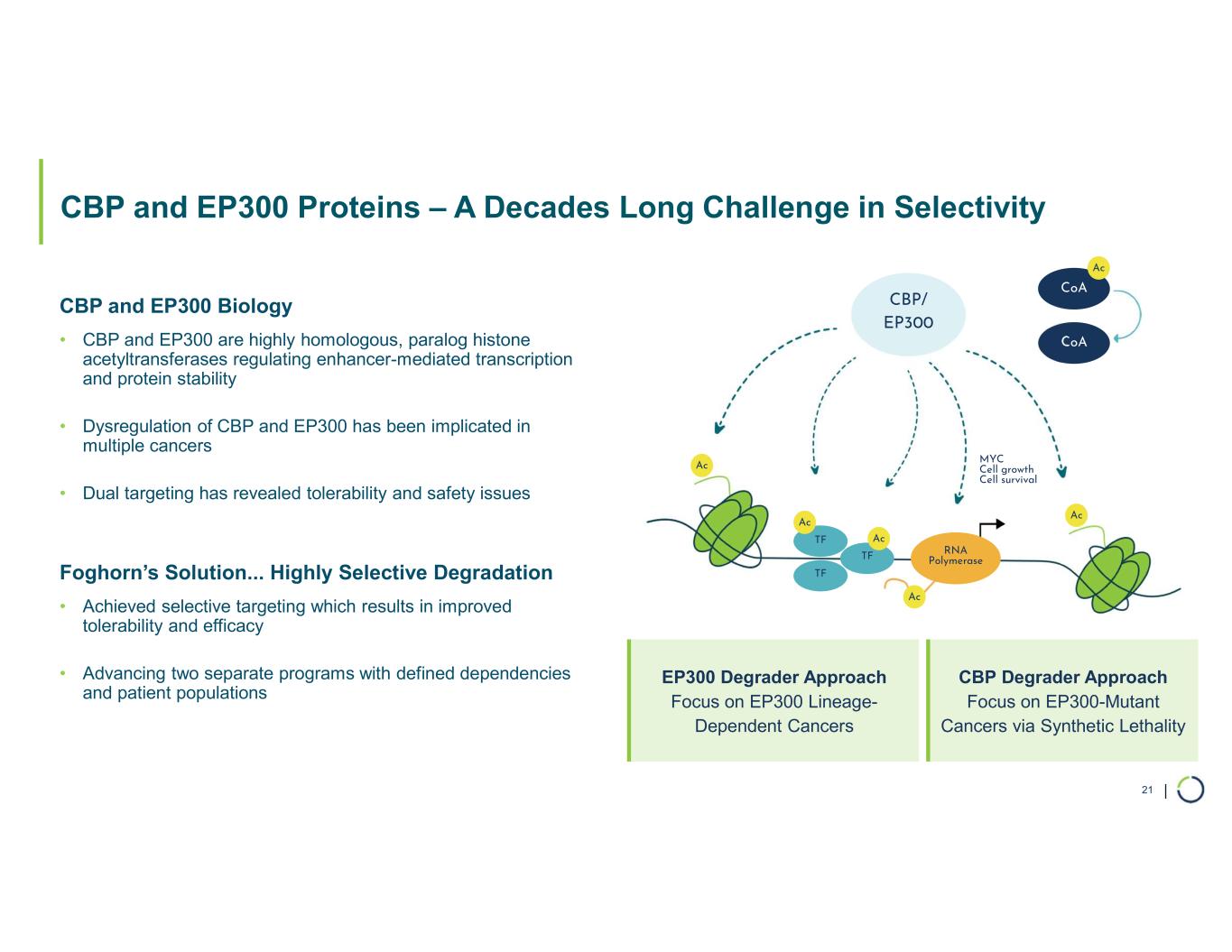
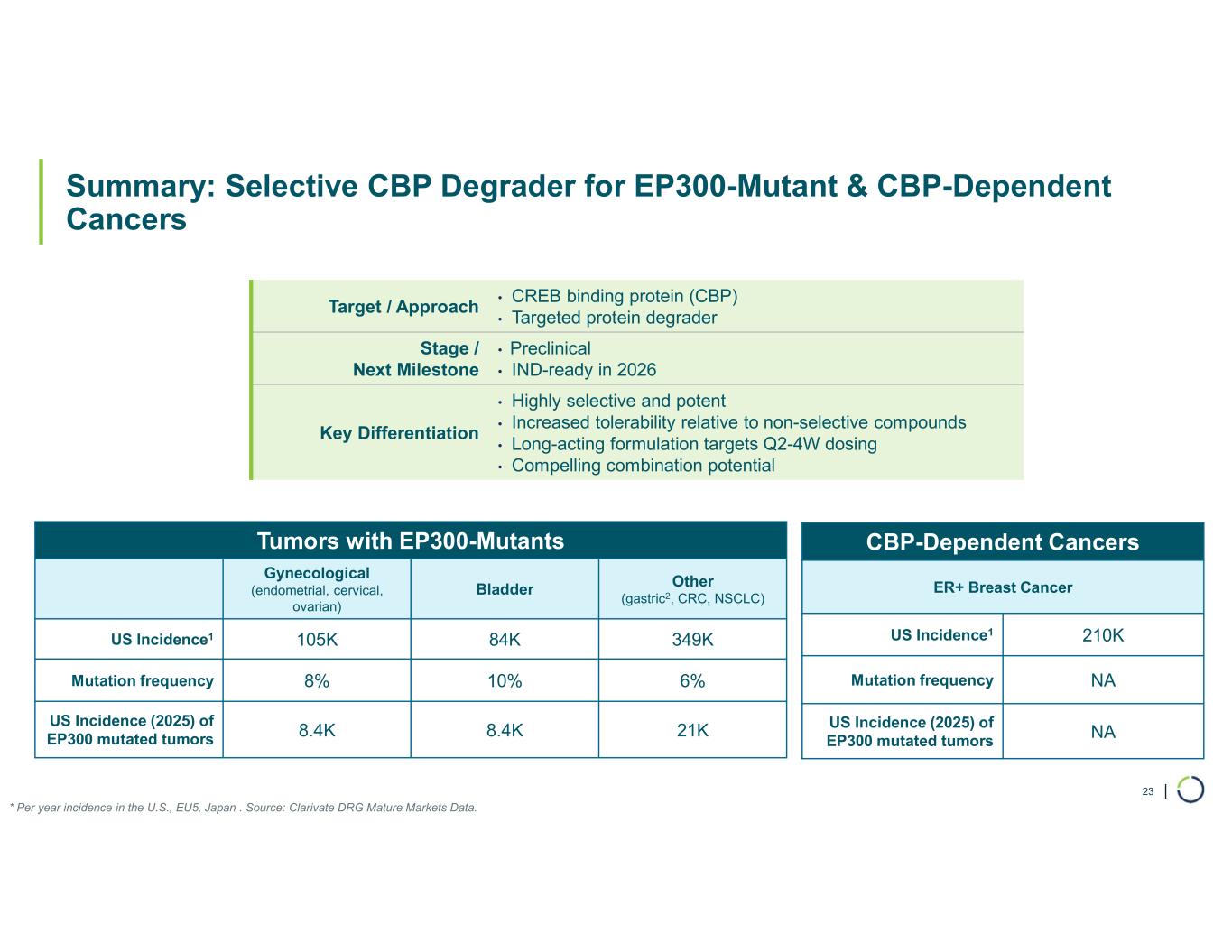
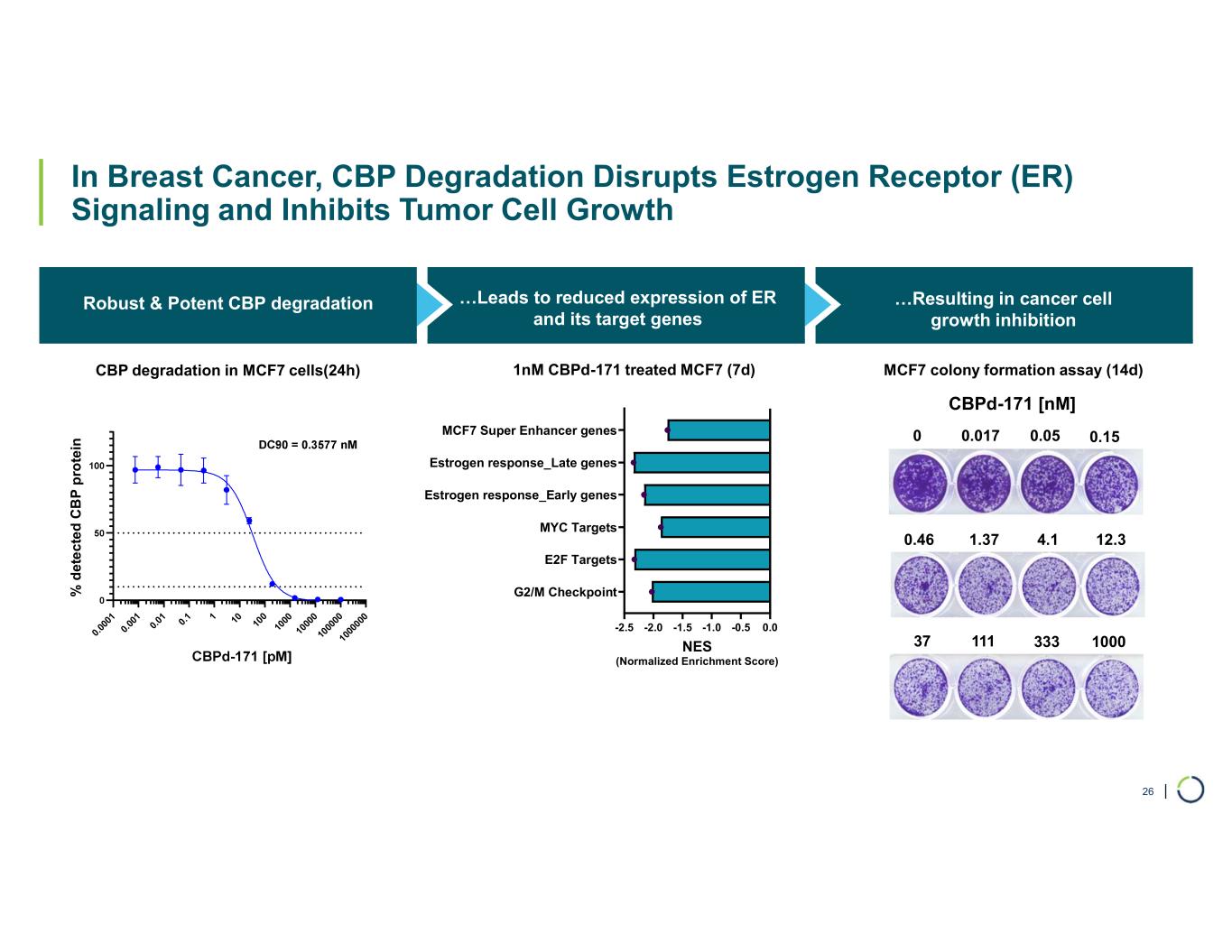
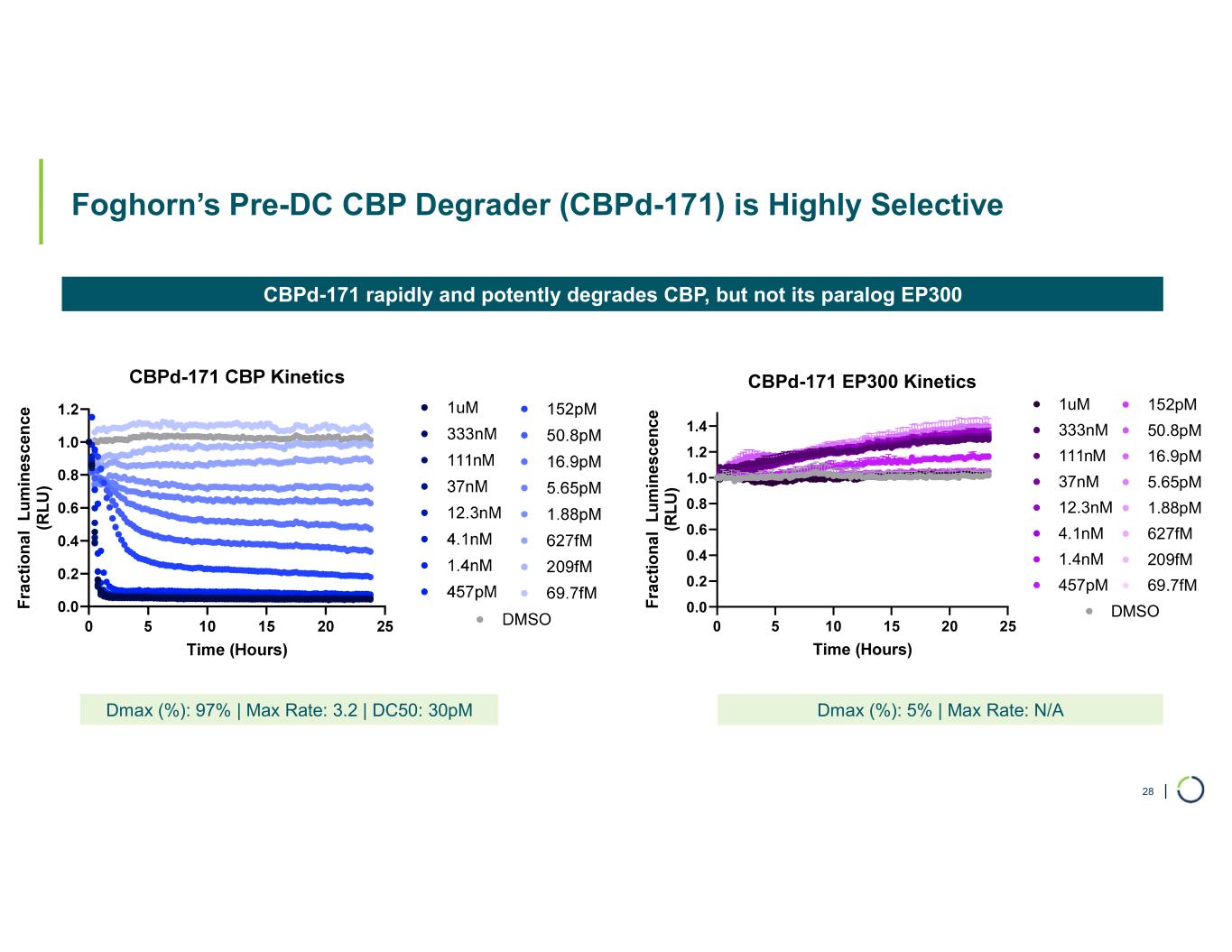
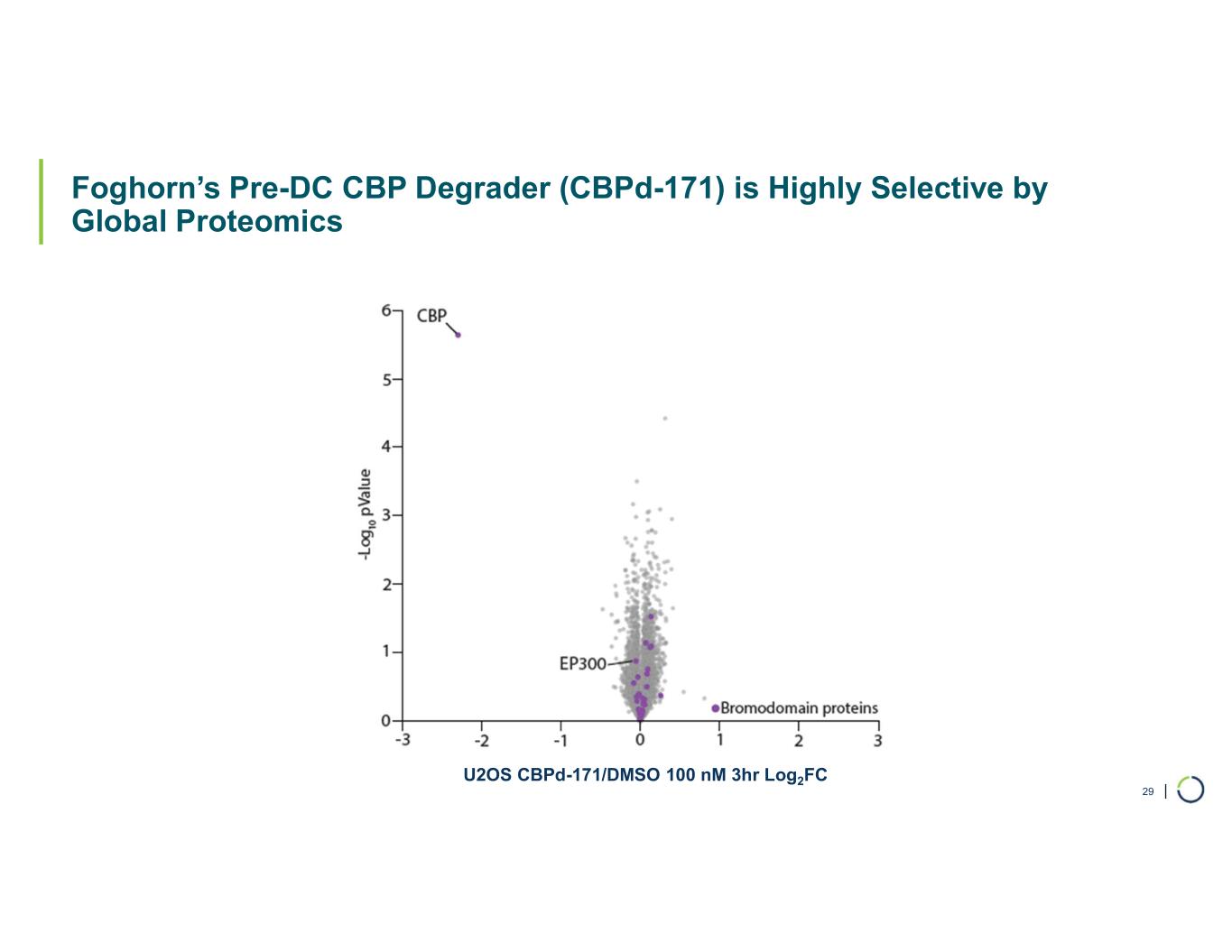
Selective CBP degrader program. Foghorn's Selective CBP degrader selectively targets CBP, an acetyltransferase closely related to EP300 designed to target a synthetic relationship in EP300-mutated cancers, which includes endometrial, cervical, ovarian, bladder and colorectal cancer. Attempts to selectively drug CBP have been challenging due to the high level of similarity between the two proteins, while dual inhibition of CBP/EP300 has been associated with dose-limiting toxicities. CBP lineage dependencies are also established in several cancers, including ER+ breast cancer.
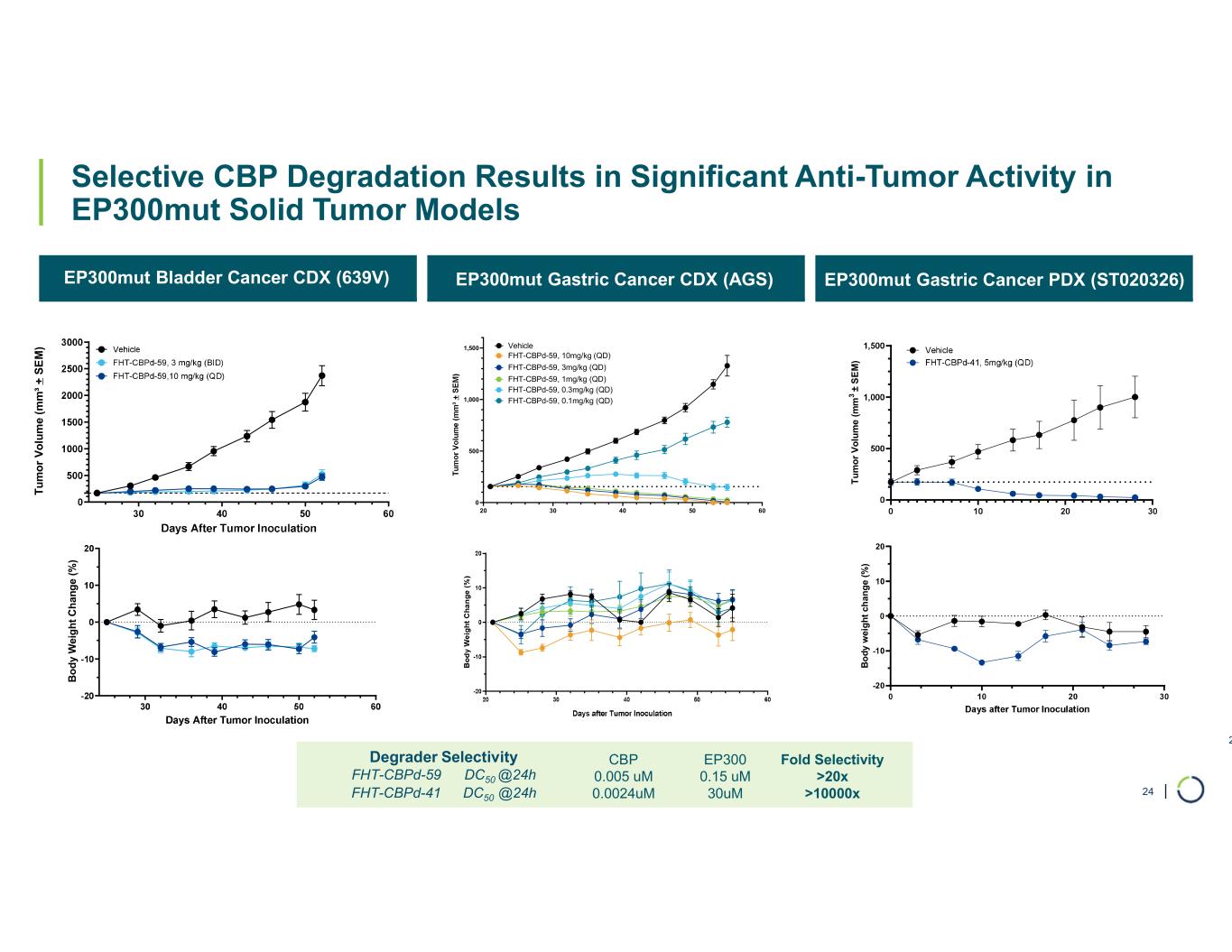
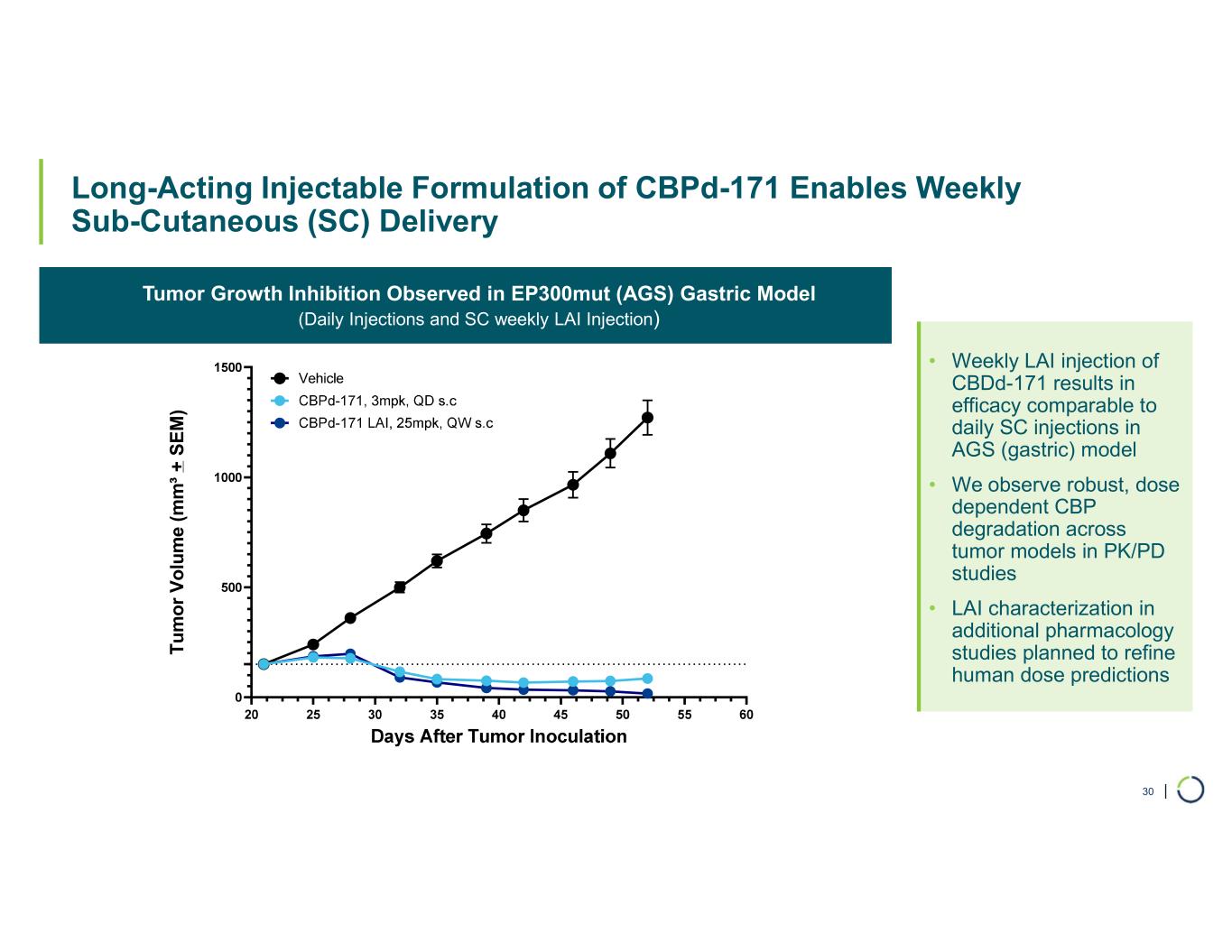
•Selective CBP degrader program, IND-ready anticipated in 2026. In October 2025, preclinical data for Selective CBP degrader with potential in EP300-mutated cancers and in ER+ breast cancer was presented during a Foghorn virtual investor event which included:
•Highly potent and selective lead candidate CBPd-171 advancing to dose range finding toxicology studies in Q4 2025
•Anti-tumor activity in EP300 mutant solid tumors and in CBP dependent cancers, including promising potential in ER+ breast cancer
•No significant impact on platelet counts and megakaryocytes spared with CBPd-171
•Long Acting Injectable (LAI) formulation optimized for subcutaneous injection weekly or every other week for convenient administration
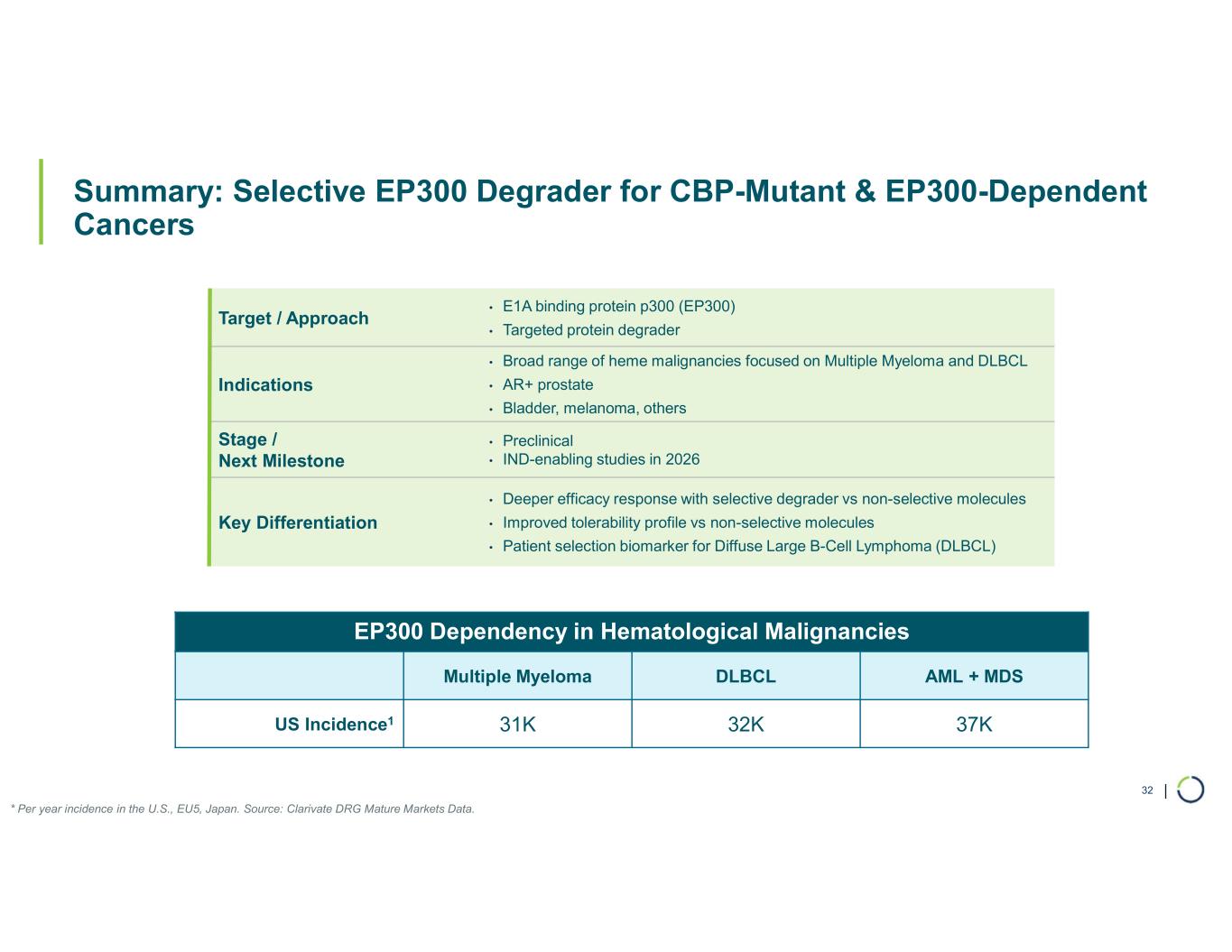
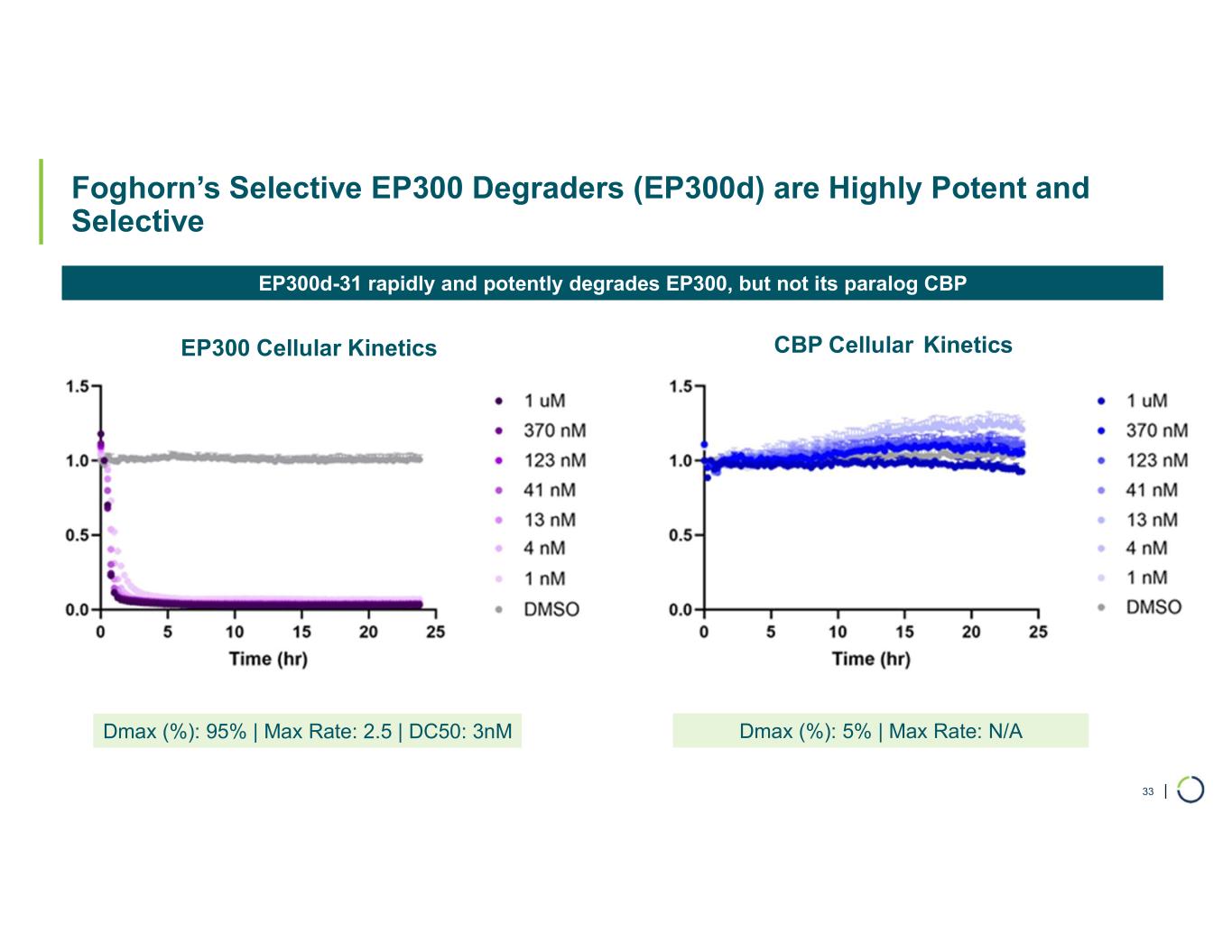
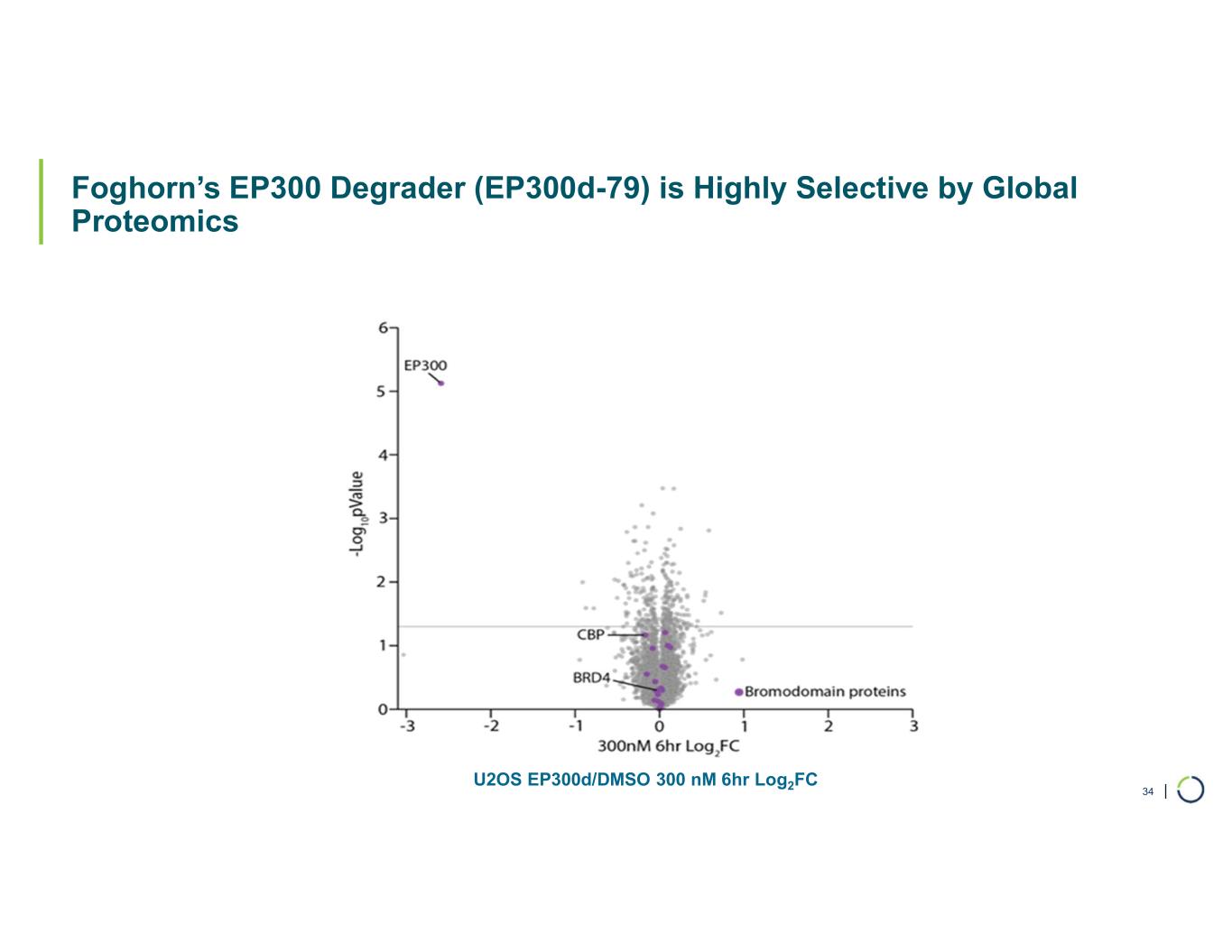
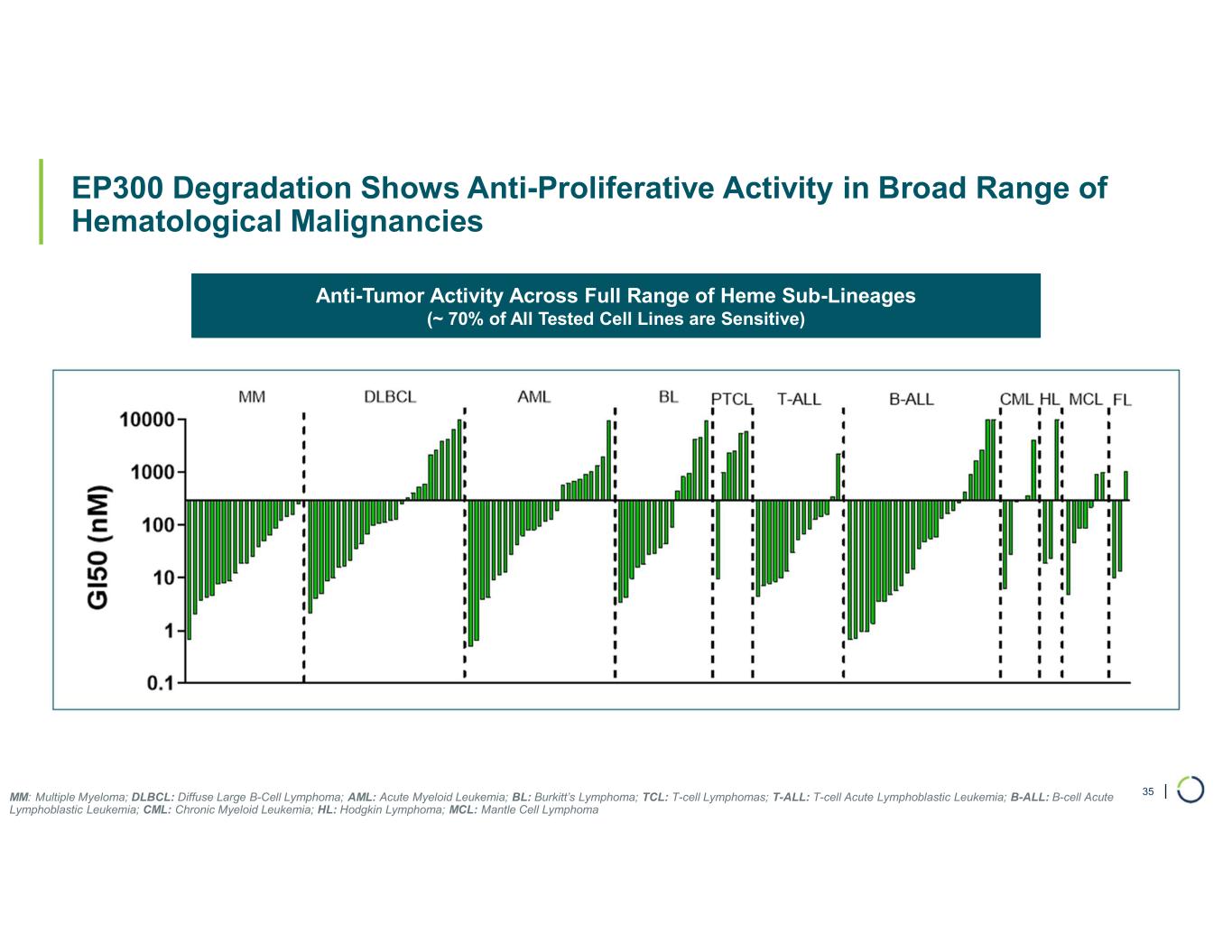
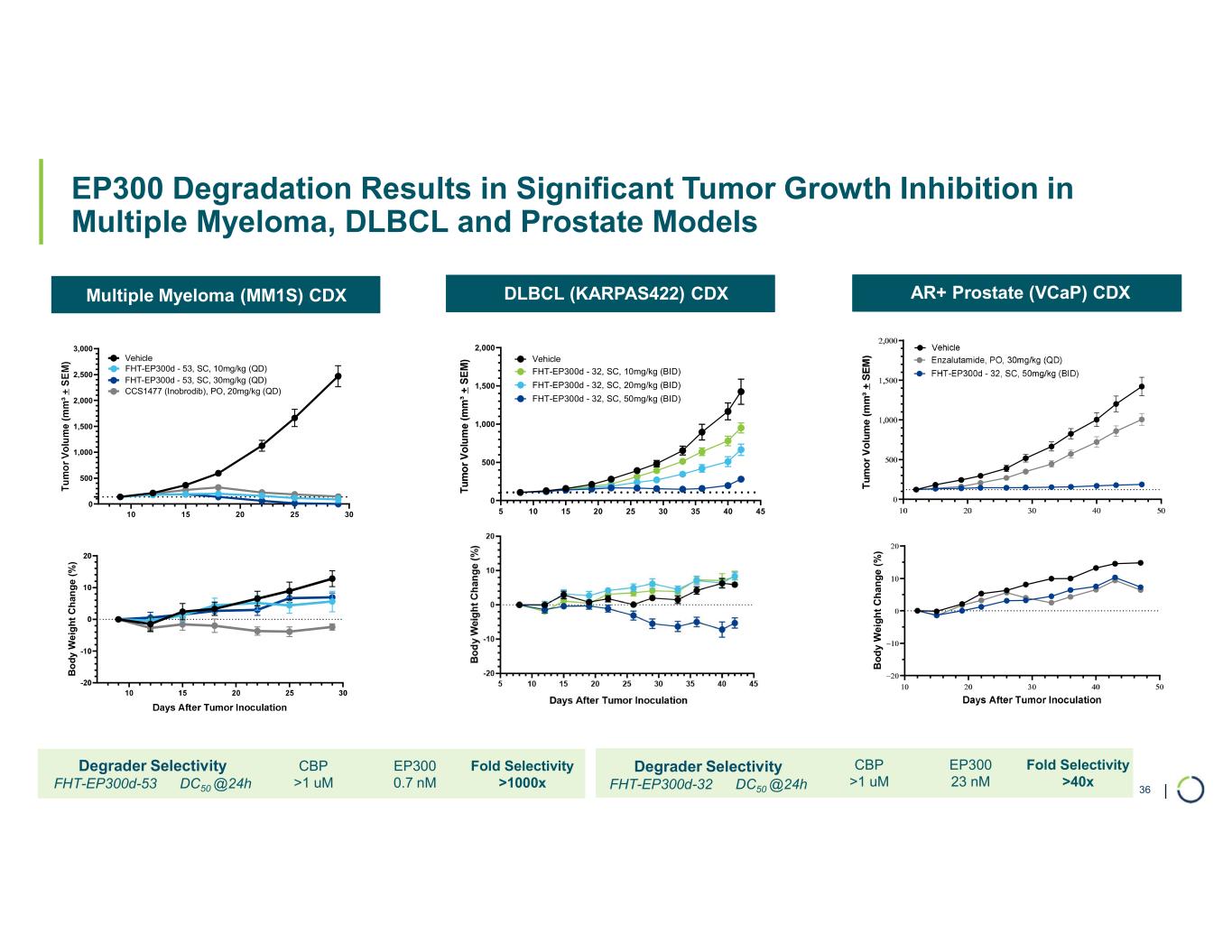
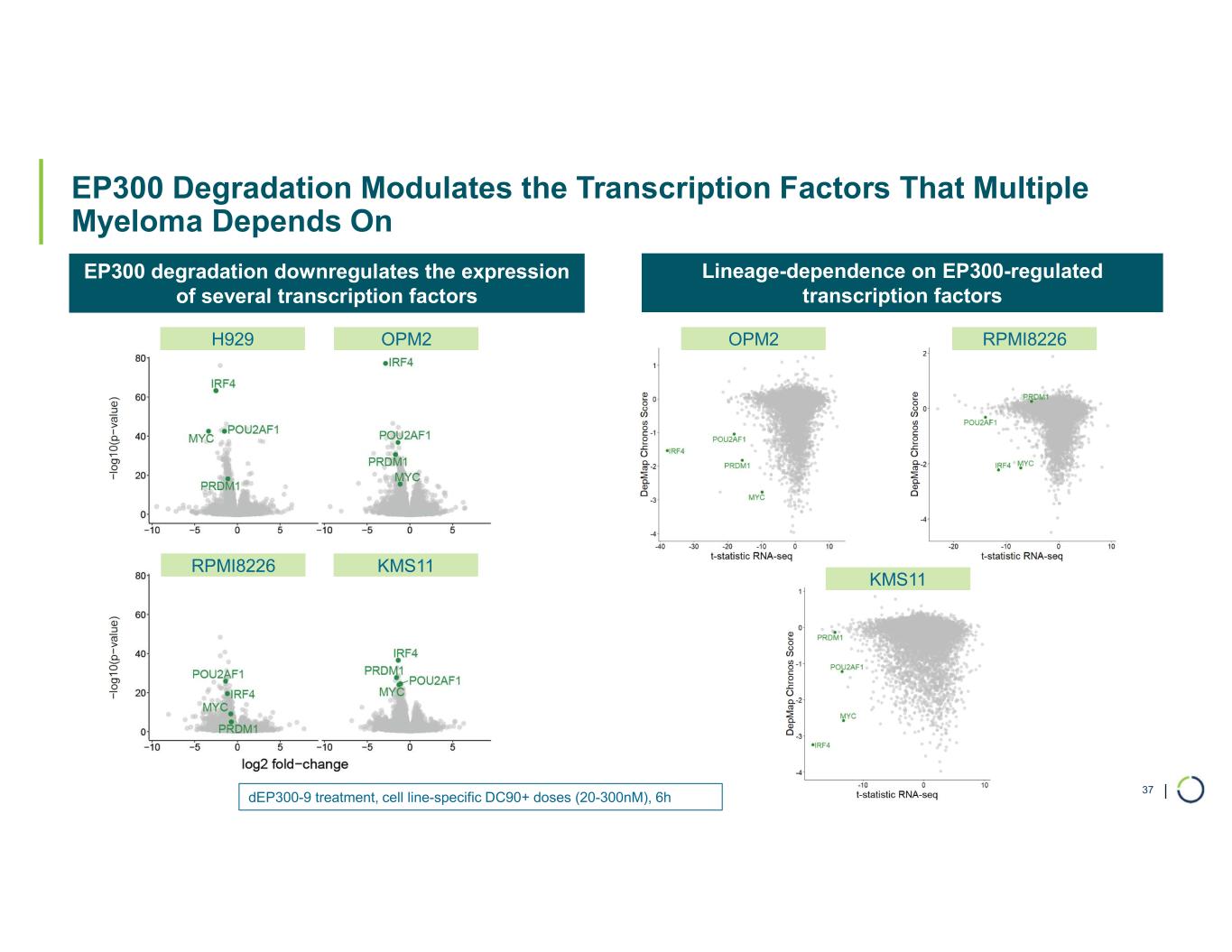
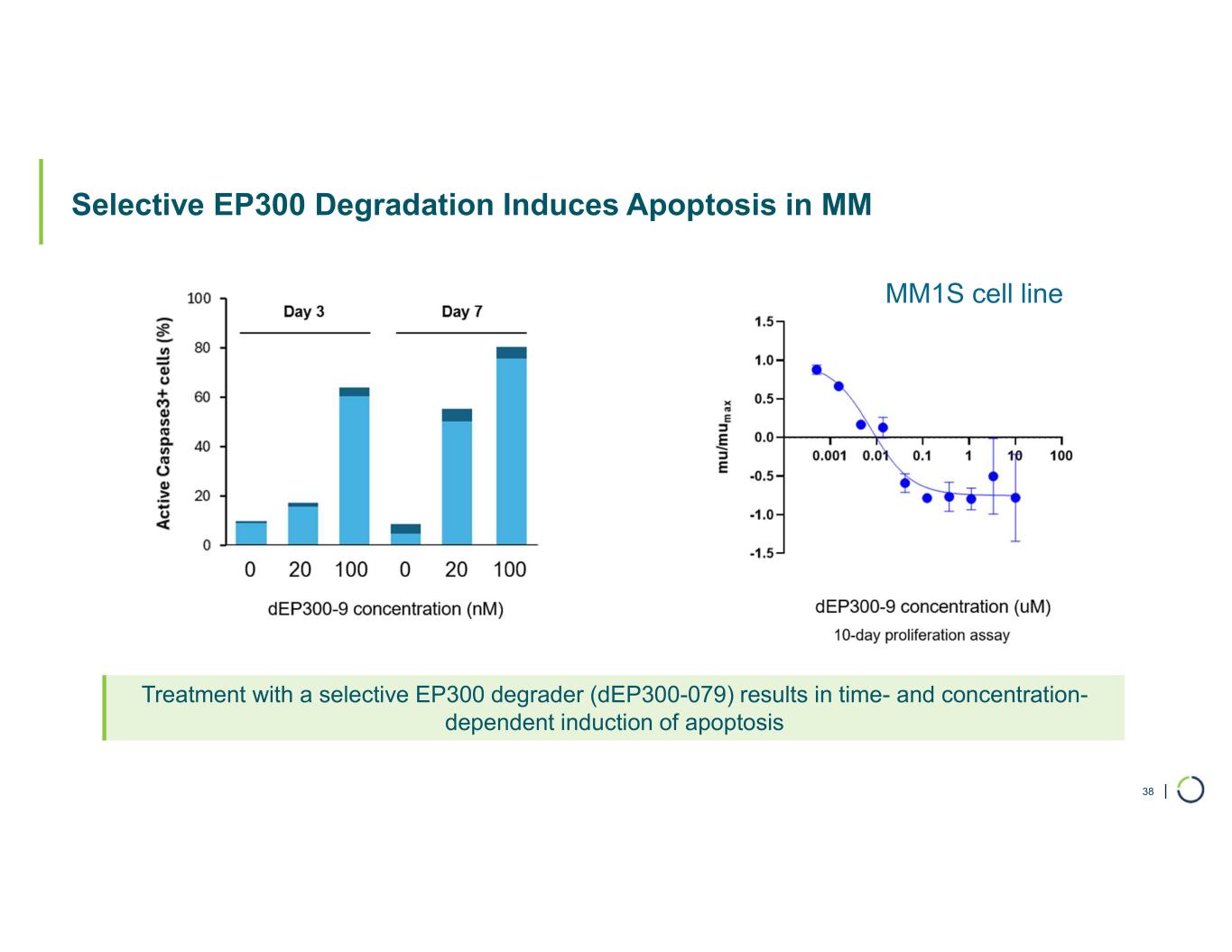
Selective EP300 degrader program. Foghorn is developing a Selective EP300 degrader for the treatment of hematological malignancies and prostate cancer. Attempts to selectively drug EP300 have been challenging due to the high level of similarity between EP300 and CBP, while dual inhibition of CBP/EP300 has been associated with dose limiting toxicities.
EP300 lineage dependencies are established in multiple myeloma (MM) and diffuse large b-cell lymphoma (DLBCL).
•Selective EP300 Degrader program, with a focus in MM and DLBCL, IND-enabling studies expected in 2026. In October 2025 Foghorn presented efficacy and safety of Selective EP300 degraders in preclinical models of hematological malignancies which included:
•Broad anti-tumor activity in over 70% of all heme sub-lineages tested
•VHL based selective degrader shows impressive efficacy in MM without hematological toxicities including thrombocytopenia
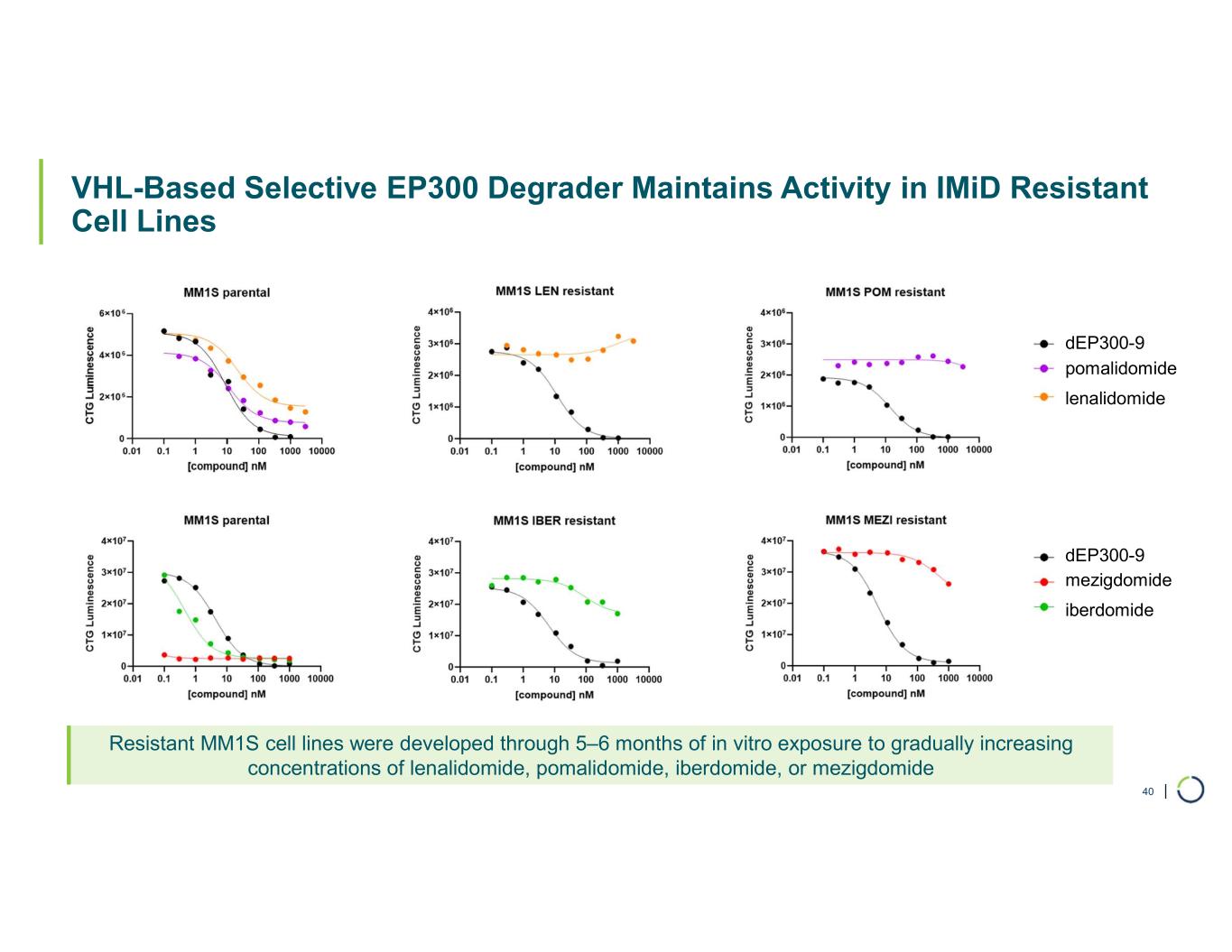
•EP300 degraders show full efficacy in IMiD-resistant MM cell lines
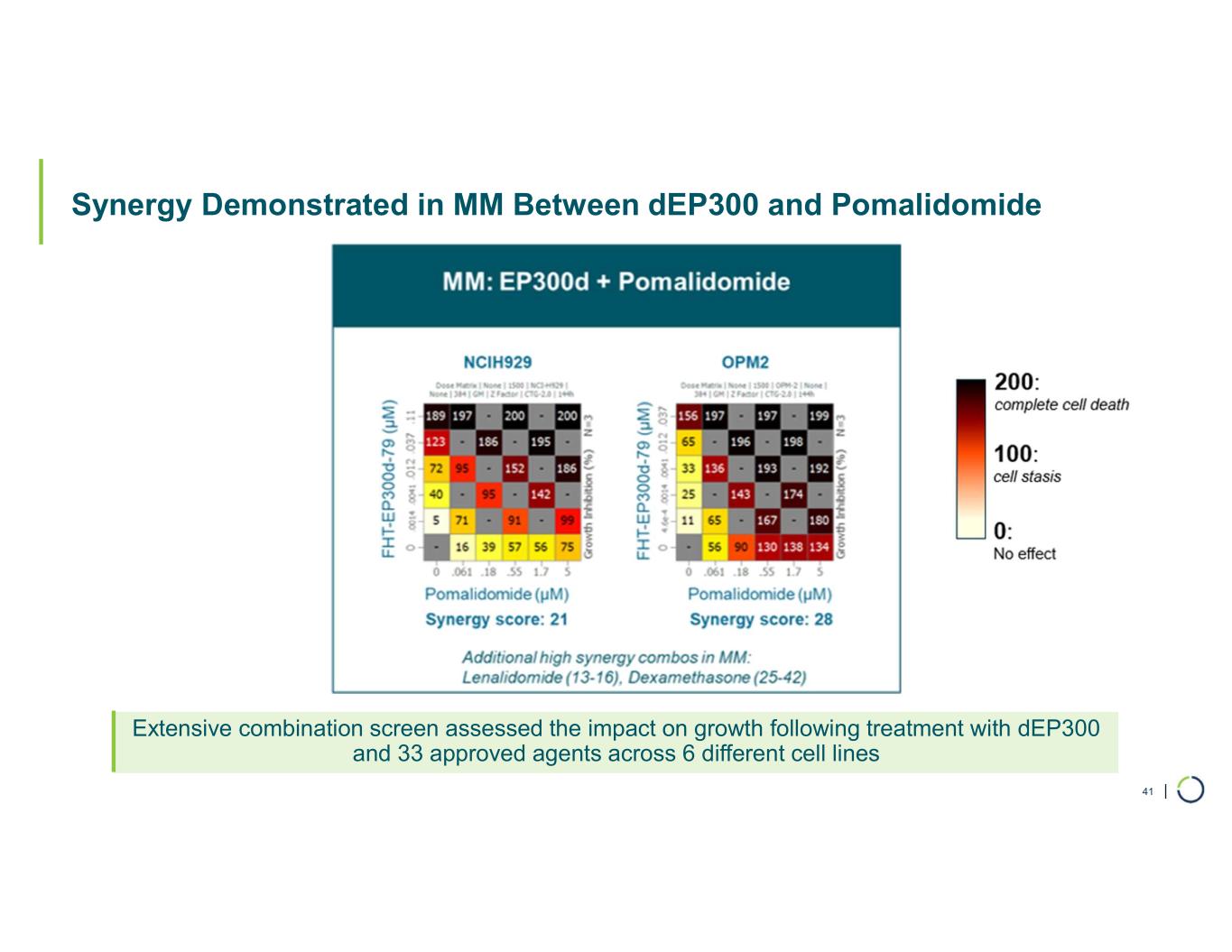
•Tolerability profile with widespread potential for combinations
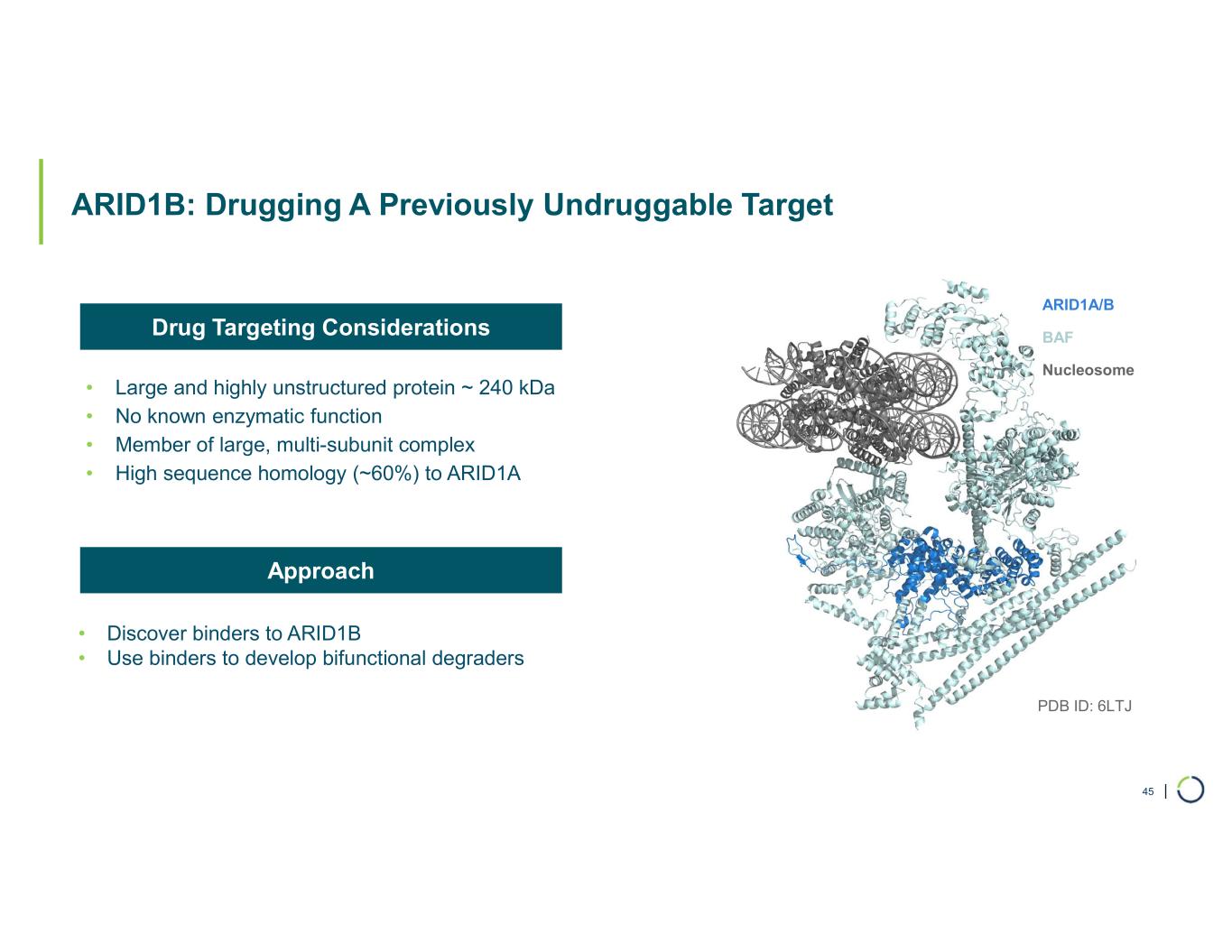
ARID1B degrader program. Foghorn's Selective ARID1B degrader selectively targets and degrades ARID1B in ARID1A-mutated cancers. ARID1A is the most mutated subunit in the BAF complex and amongst the most mutated proteins in cancer. These mutations lead to a dependency on ARID1B in several types of cancer, including endometrial, gastric, gastroesophageal junction, bladder and NSCLC. Attempts to selectively drug ARID1B have been challenging because of the high degree of similarity between ARID1A and ARID1B and the fact that ARID1B has no enzymatic activity to target. ARID1B is a major synthetic lethal target implicated in up to 5% of all solid tumors.
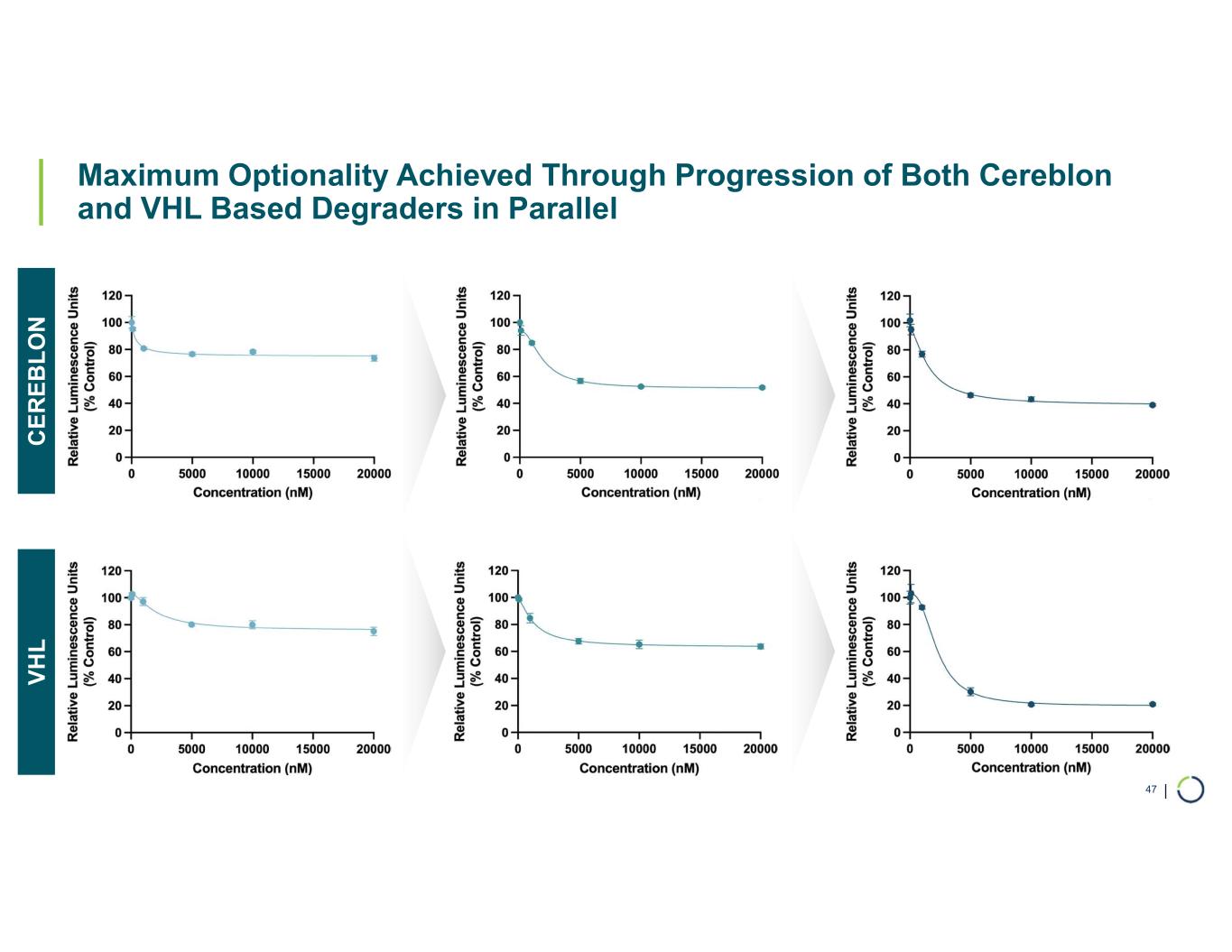
•Selective ARID1B degrader program advancing towards in vivo proof of concept in 2026. In October 2025, data for Selective ARID1B degrader was presented at the TPD and Induced Proximity Summit and during a Foghorn virtual investor event which included:
•Developed VHL and cereblon based bifunctional degraders with potential for oral delivery
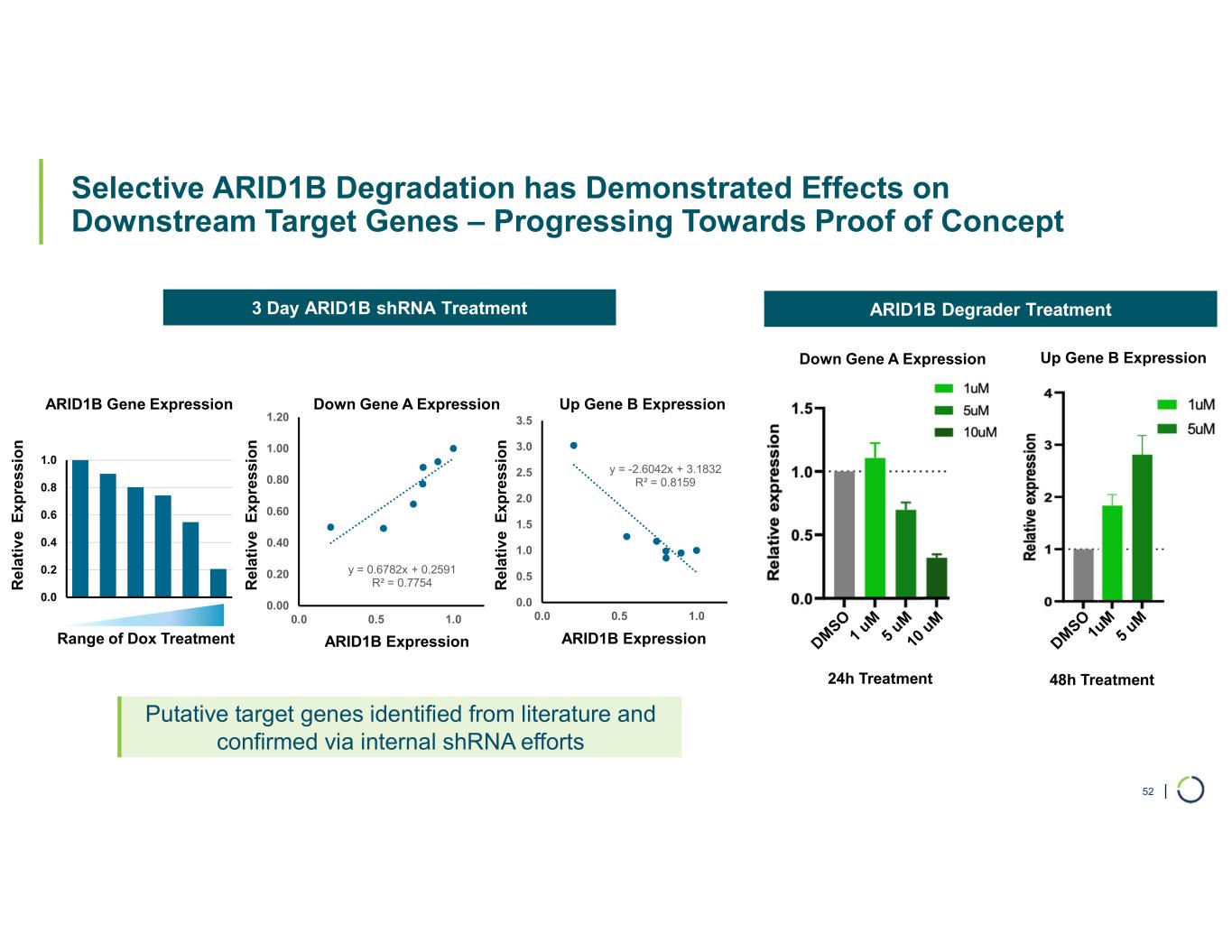
•Selective degradation of ARID1B achieved
•Modulation of downstream target genes following ARID1B degradation
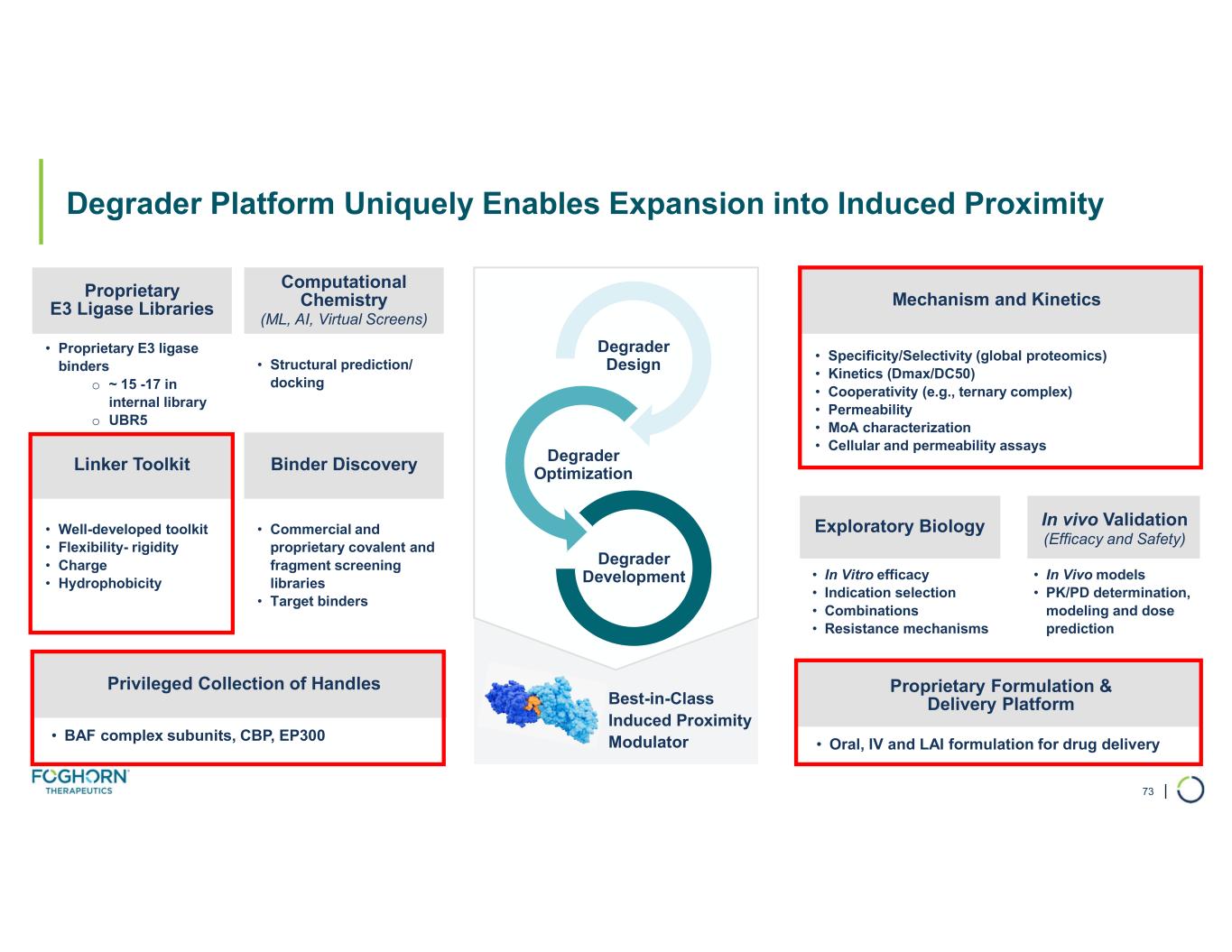
Degrader Platform. Foghorn continues to advance its degrader platform with investments in novel ligases, long-acting injectables, oral delivery, and induced proximity.
Corporate Update
Leadership. Chief Financial Officer, Kristian Humer, will be departing from the company, with his last day being November 14, 2025. A formal search for a successor has begun.
Third Quarter 2025 Financial Highlights
•Collaboration Revenue. Collaboration revenue was $8.2 million for the three months ended September 30, 2025, compared to $7.8 million for the three months ended September 30, 2024. The increase was driven by the continued advancement of programs under the Lilly Collaboration Agreement.
•Research and Development Expenses. Research and development expenses were $20.0 million for the three months ended September 30, 2025, compared to $24.7 million for the three months ended September 30, 2024. The decrease is attributed to a decrease in FHD-286 costs, decreases in personnel-related costs, early development and other research external costs and facilities and IT-related expenses, partially offset by an increase in Lilly-partnered programs.
•General and Administrative Expenses. General and administrative expenses were $6.7 million for the three months ended September 30, 2025, compared to $7.0 million for the three months ended September 30, 2024. This decrease was primarily due to lower facilities and IT related expenses.
•Net Loss. Net loss was $15.8 million for the three months ended September 30, 2025, compared to a net loss of $19.1 million for the three months ended September 30, 2024.
•Cash, Cash Equivalents, and Marketable Securities. As of September 30, 2025, the Company had $180.3 million in cash, cash equivalents, and marketable securities, providing cash runway into 2028.
About FHD-909
FHD-909 (LY4050784) is a potent, first-in-class, allosteric, and orally available small molecule that selectively inhibits the ATPase activity of SMARCA2 (BRM) over its closely related paralog SMARCA4 (BRG1), two proteins that are the catalytic engines across all forms of the BAF complex, one of the key regulators of the chromatin regulatory system. In preclinical studies, tumors with mutations in SMARCA4 rely on SMARCA2 for their survival. FHD-909 has shown significant anti-tumor activity across multiple SMARCA4-mutant lung tumor models.
About Foghorn Therapeutics
Foghorn® Therapeutics is discovering and developing a novel class of medicines targeting genetically determined dependencies within the chromatin regulatory system. Through its proprietary scalable Gene Traffic Control® platform, Foghorn is systematically studying, identifying, and validating potential drug targets within the chromatin regulatory system. The Company is developing multiple product candidates in oncology. Visit our website at www.foghorntx.com for more information on the Company, and follow us on X (formerly Twitter) and LinkedIn.
Forward-Looking Statements
This press release contains “forward-looking statements.” Forward-looking statements include statements regarding the Company’s ongoing Phase 1 trial of FHD-909 in SMARCA4-mutated cancers, pre-clinical product candidates, expected timing of regulatory filings, including INDs, clinical data, expected cash runway, expected timing of regulatory filings, and research efforts and other statements identified by words such as “could,” “may,” “might,” “will,” “likely,” “anticipates,” “intends,” “plans,” “seeks,” “believes,” “estimates,” “expects,” “continues,” “projects” and similar references to future periods. Forward-looking statements are based on our current expectations and assumptions regarding capital market conditions, our business, the economy and other future conditions. Because forward-looking statements relate to the future, by their nature, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. As a result, actual results may differ materially from those contemplated by the forward-looking statements. Important factors that could cause actual results to differ materially from those in the forward-looking statements include regional, national or global political, economic, business, competitive, market and regulatory conditions, including risks relating to our clinical trials and other factors set forth under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, as filed with the Securities and Exchange Commission. Any forward-looking statement made in this press release speaks only as of the date on which it is made.
Condensed Consolidated Balance Sheets
(In thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2025 |
|
December 31, 2024 |
| Cash, cash equivalents and marketable securities |
$ |
180,278 |
|
|
$ |
243,747 |
|
All other assets |
24,684 |
|
|
40,235 |
|
Total assets |
$ |
204,962 |
|
|
$ |
283,982 |
|
Deferred revenue, total |
$ |
258,401 |
|
|
$ |
280,063 |
|
All other liabilities |
36,219 |
|
|
49,447 |
|
Total liabilities |
$ |
294,620 |
|
|
$ |
329,510 |
|
Total stockholders’ deficit |
$ |
(89,658) |
|
|
$ |
(45,528) |
|
Total liabilities and stockholders’ deficit |
$ |
204,962 |
|
|
$ |
283,982 |
|
Condensed Consolidated Statements of Operations
(In thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
2025 |
|
2024 |
| Collaboration revenue |
$ |
8,153 |
|
|
$ |
7,808 |
|
| Operating expenses: |
|
|
|
| Research and development |
20,002 |
|
|
24,689 |
|
| General and administrative |
6,652 |
|
|
6,971 |
|
|
|
|
|
| Total operating expenses |
$ |
26,654 |
|
|
$ |
31,660 |
|
| Loss from operations |
$ |
(18,501) |
|
|
$ |
(23,852) |
|
| Total other income, net |
$ |
2,652 |
|
|
$ |
4,730 |
|
|
|
|
|
| Net loss |
$ |
(15,849) |
|
|
$ |
(19,122) |
|
| Net loss per share attributable to common stockholders—basic and diluted |
(0.25) |
|
|
(0.31) |
|
| Weighted average common shares outstanding—basic and diluted |
63,029,293 |
|
|
62,602,848 |
|
Contact:
Karin Hellsvik, Foghorn Therapeutics Inc.
khellsvik@foghorntx.com