
October 30, 2025 Virtual Investor Event Pipeline Updates for Selective ARID1B, Selective CBP and Selective EP300 Degrader Programs Exhibit 99.1

| Forward Looking Statements 2 This presentation contains forward-looking statements that are based on management's beliefs and assumptions and on information currently available to management. All statements other than statements of historical facts contained in this presentation are forward- looking statements. In some cases, you can identify forward-looking statements by terms such as “could,” “may,” “might,” “will,” “likely,” “anticipates,” “intends,” “plans,” “seeks,” “believes,” “estimates,” “expects,” “continues,” “projects” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements include, but are not limited to, statements concerning: the potential outcomes from our collaboration agreement with Lilly; the initiation, timing, progress and results of our research and development programs and pre-clinical studies, including our Selective ARID1B degrader program and Selective CBP and Selective EP300 degrader programs, and clinical trials, including our Phase 1 dose escalation trial of FHD-909 with Lilly; our ability to advance product candidates that we may develop and to successfully complete preclinical and clinical studies; our ability to leverage our initial programs to develop additional product candidates using our Gene Traffic Control Platform®; the impact of exogeneous factors, including macroeconomic and geopolitical circumstances, on our and our collaborators’ business operations, including our research and development programs and pre-clinical studies; developments related to our competitors and our industry; our ability to expand the target populations of our programs and the availability of patients for clinical testing; our ability to obtain regulatory approval for FHD-909 and any future product candidates from the FDA and other regulatory authorities; our ability to identify and enter into future license agreements and collaborations; our ability to continue to rely on our CDMOs and CROs for our manufacturing and research needs; regulatory developments in the United States and foreign countries; our ability to attract and retain key scientific and management personnel; the scope of protection we are able to establish, maintain and enforce for intellectual property rights covering FHD-909, our future products and our Gene Traffic Control Platform; and our use of proceeds from capital-raising transactions, estimates of our expenses, capital requirements, and needs for additional financing. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Additional important factors to be considered in connection with forward-looking statements are described in the Company's filings with the Securities and Exchange Commission, including withing the section entitled "Risk Factors" in the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2024. Any forward-looking statements represent the Company’s views only as of the date of this presentation and should not be relied upon as representing its views as of any subsequent date. The Company explicitly disclaims any obligation to update any forward-looking statements. The Company’s business is subject to substantial risks and uncertainties.

| Foghorn Conference Call Agenda Opening Remarks Welcome Selective ARID1B Degrader Selective CBP Degrader Selective EP300 Degrader Karin Hellsvik Vice President Investor Relations Adrian Gottschalk President and CEO Alfonso Quintás-Cardama, MD Chief Medical Officer Steve Bellon, PhD Chief Scientific Officer 3 Q&A Steve Bellon, PhD Chief Scientific Officer Adrian Gottschalk President and CEO Alfonso Quintás-Cardama, MD Chief Medical Officer Kristian Humer Chief Financial Officer Karin Hellsvik Vice President Investor Relations
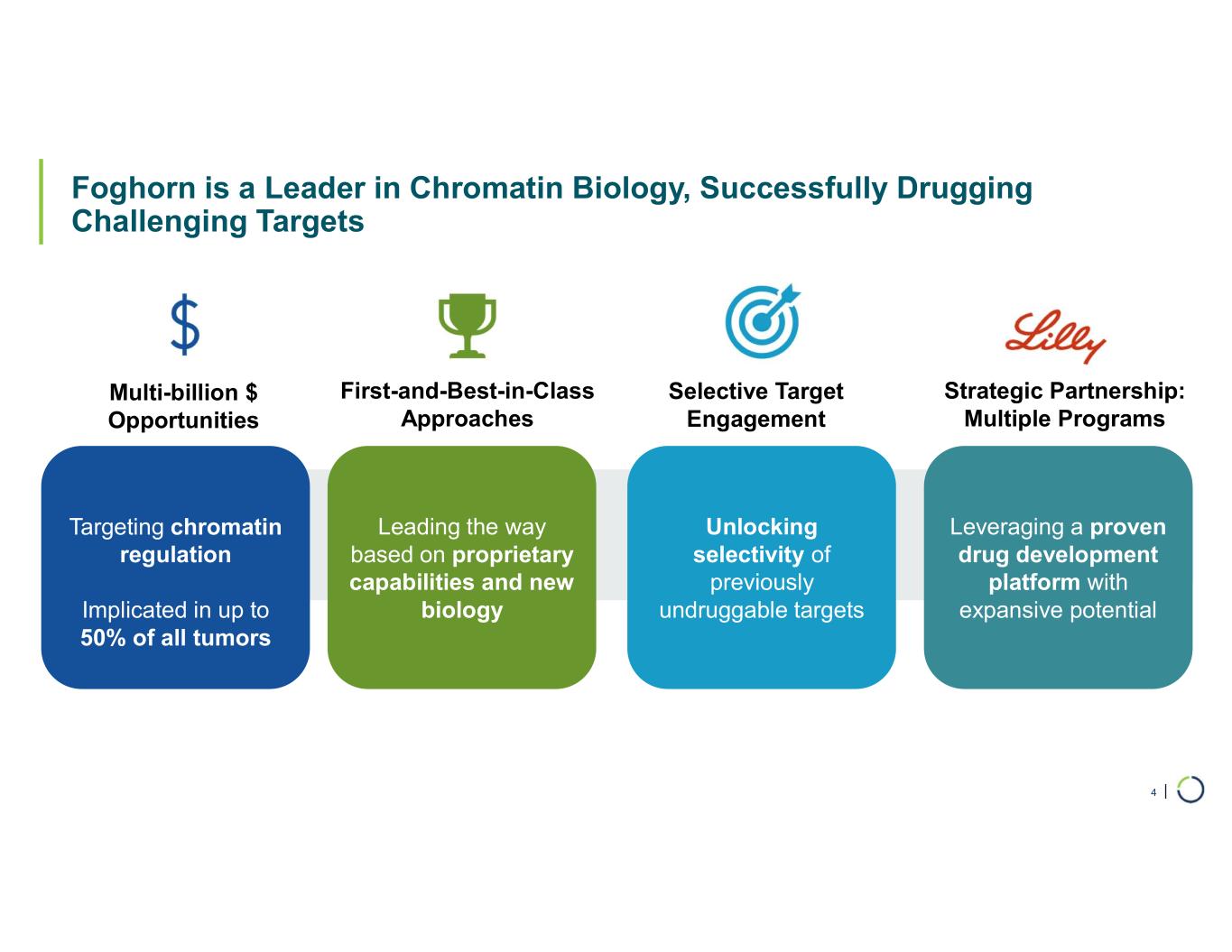
| Foghorn is a Leader in Chromatin Biology, Successfully Drugging Challenging Targets 4 Targeting chromatin regulation Implicated in up to 50% of all tumors Multi-billion $ Opportunities Leading the way based on proprietary capabilities and new biology First-and-Best-in-Class Approaches Unlocking selectivity of previously undruggable targets Selective Target Engagement Leveraging a proven drug development platform with expansive potential Strategic Partnership: Multiple Programs
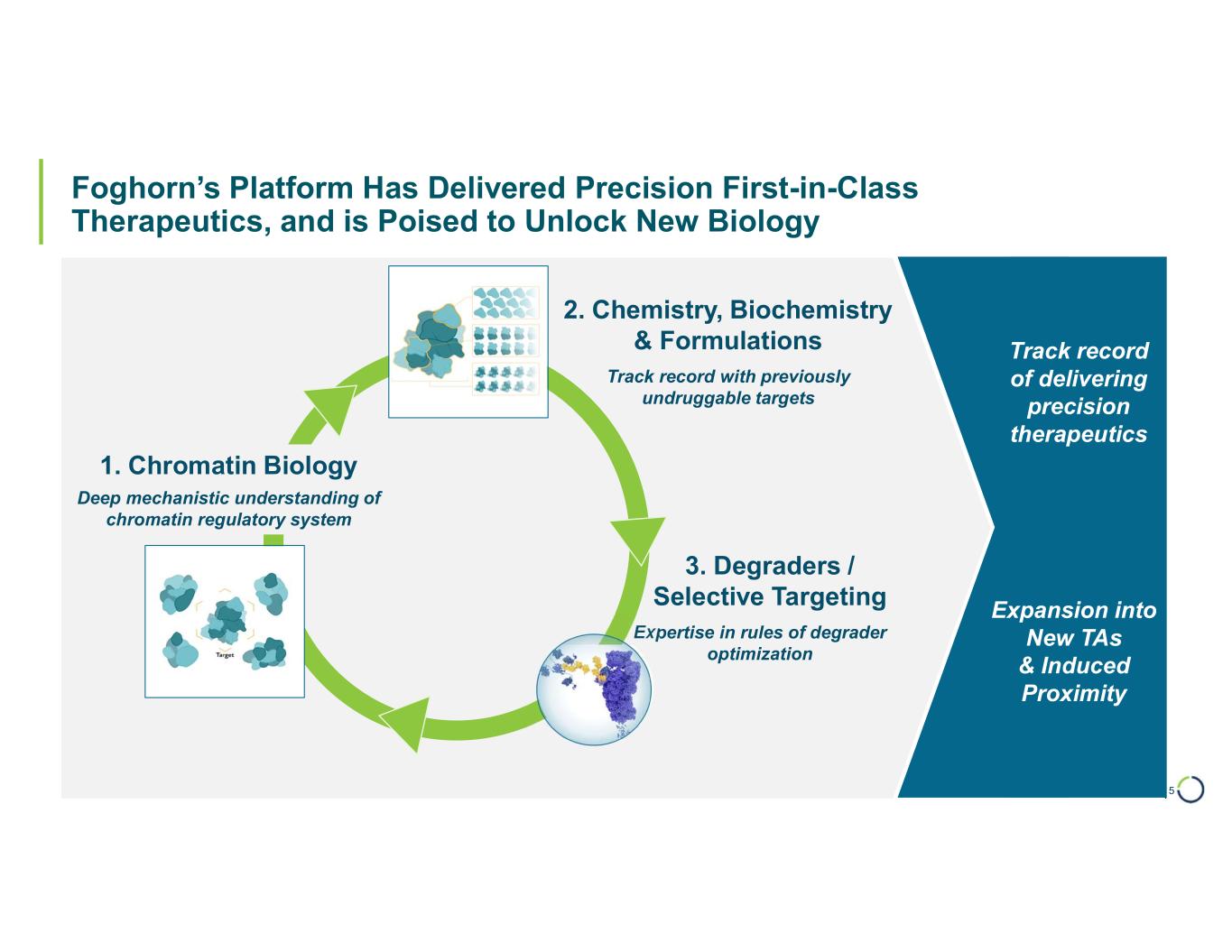
| Foghorn’s Platform Has Delivered Precision First-in-Class Therapeutics, and is Poised to Unlock New Biology 5 1. Chromatin Biology 2. Chemistry, Biochemistry & Formulations 3. Degraders / Selective Targeting Deep mechanistic understanding of chromatin regulatory system Expertise in rules of degrader optimization Track record with previously undruggable targets Expansion into New TAs & Induced Proximity Track record of delivering precision therapeutics
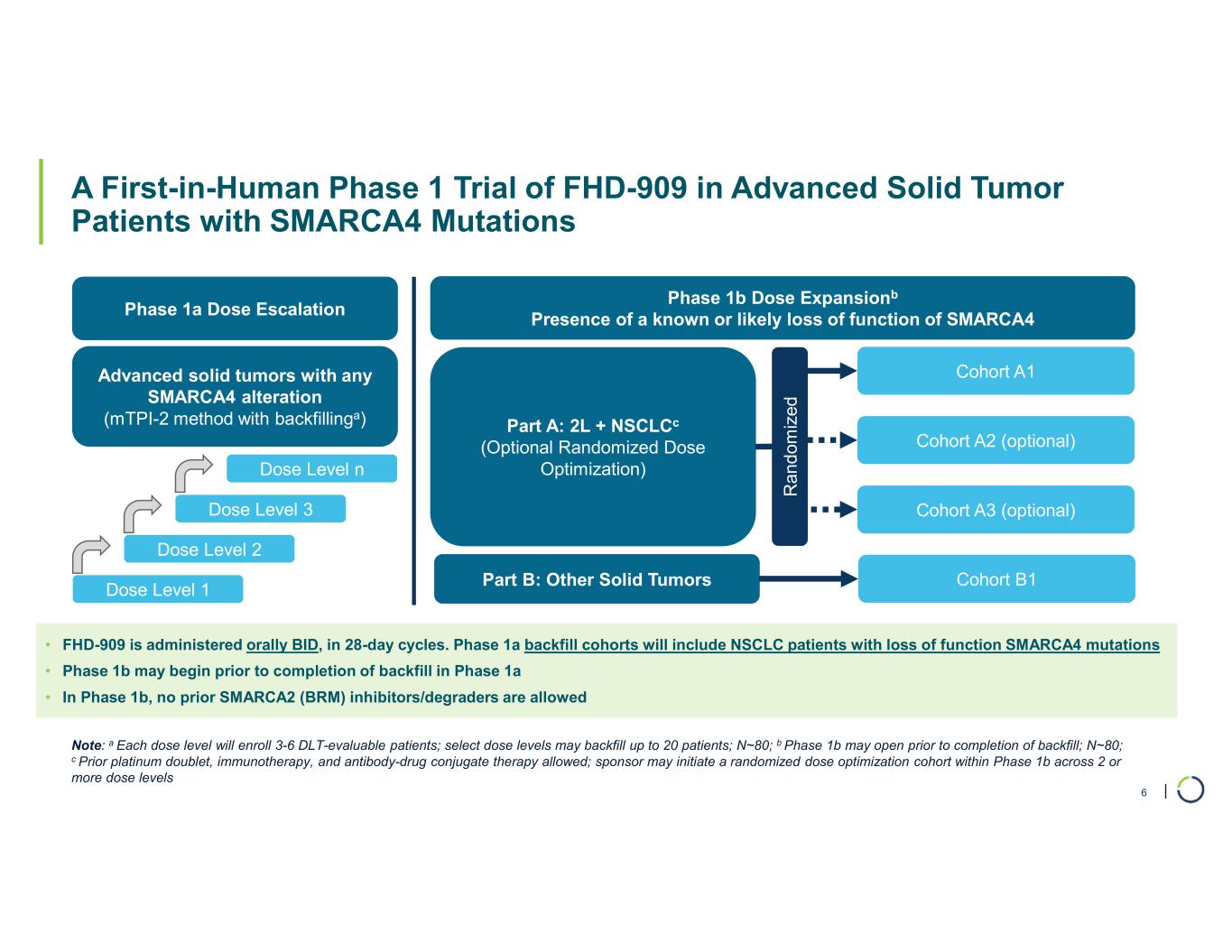
| A First-in-Human Phase 1 Trial of FHD-909 in Advanced Solid Tumor Patients with SMARCA4 Mutations Phase 1a Dose Escalation Advanced solid tumors with any SMARCA4 alteration (mTPI-2 method with backfillinga) Dose Level 1 Dose Level 2 Dose Level 3 Dose Level n Phase 1b Dose Expansionb Presence of a known or likely loss of function of SMARCA4 Part A: 2L + NSCLCc (Optional Randomized Dose Optimization) Part B: Other Solid Tumors Cohort A1 Cohort A2 (optional) Cohort A3 (optional) R an d om iz ed Cohort B1 • FHD-909 is administered orally BID, in 28-day cycles. Phase 1a backfill cohorts will include NSCLC patients with loss of function SMARCA4 mutations • Phase 1b may begin prior to completion of backfill in Phase 1a • In Phase 1b, no prior SMARCA2 (BRM) inhibitors/degraders are allowed Note: a Each dose level will enroll 3-6 DLT-evaluable patients; select dose levels may backfill up to 20 patients; N~80; b Phase 1b may open prior to completion of backfill; N~80; c Prior platinum doublet, immunotherapy, and antibody-drug conjugate therapy allowed; sponsor may initiate a randomized dose optimization cohort within Phase 1b across 2 or more dose levels 6
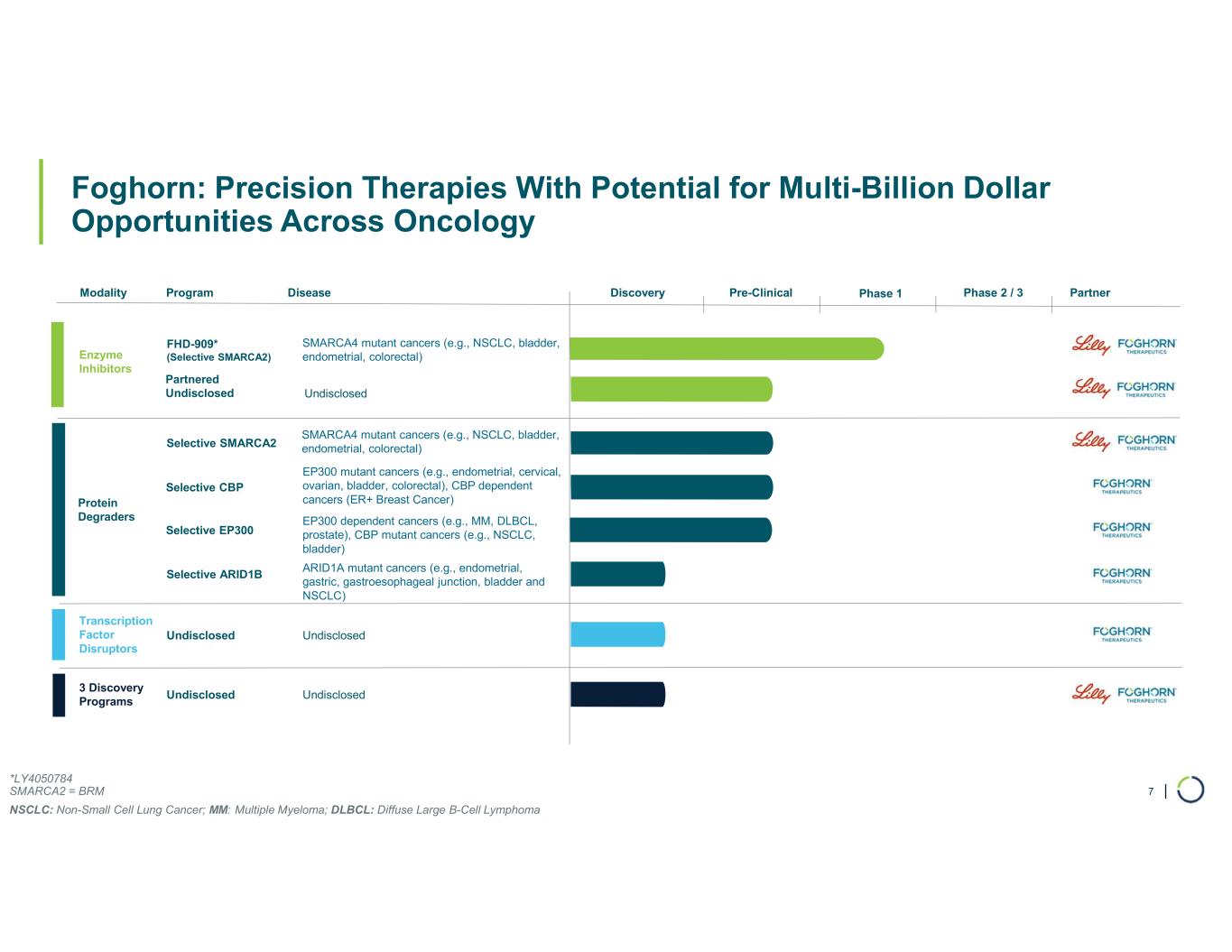
| Foghorn: Precision Therapies With Potential for Multi-Billion Dollar Opportunities Across Oncology 7 Modality Program Phase 1 Enzyme Inhibitors Transcription Factor Disruptors Undisclosed Protein Degraders Partner FHD-909* (Selective SMARCA2) 3 Discovery Programs Undisclosed Partnered Undisclosed Disease SMARCA4 mutant cancers (e.g., NSCLC, bladder, endometrial, colorectal) Selective SMARCA2 SMARCA4 mutant cancers (e.g., NSCLC, bladder, endometrial, colorectal) Selective ARID1B ARID1A mutant cancers (e.g., endometrial, gastric, gastroesophageal junction, bladder and NSCLC) Selective CBP EP300 mutant cancers (e.g., endometrial, cervical, ovarian, bladder, colorectal), CBP dependent cancers (ER+ Breast Cancer) Selective EP300 EP300 dependent cancers (e.g., MM, DLBCL, prostate), CBP mutant cancers (e.g., NSCLC, bladder) Undisclosed Undisclosed Undisclosed Pre-Clinical Phase 2 / 3Discovery *LY4050784 SMARCA2 = BRM NSCLC: Non-Small Cell Lung Cancer; MM: Multiple Myeloma; DLBCL: Diffuse Large B-Cell Lymphoma
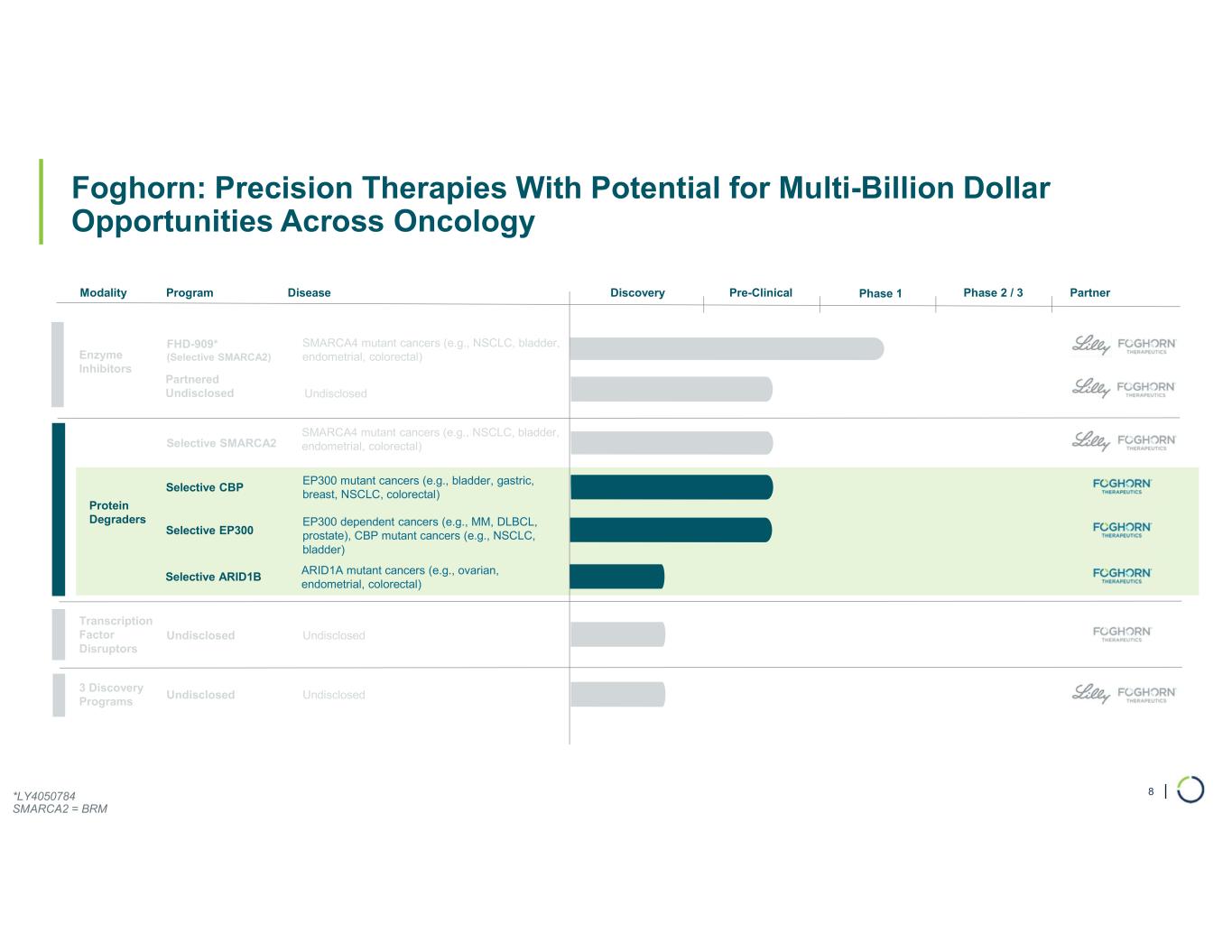
| Foghorn: Precision Therapies With Potential for Multi-Billion Dollar Opportunities Across Oncology 8 Modality Program Phase 1 Enzyme Inhibitors Transcription Factor Disruptors Undisclosed Partner FHD-909* (Selective SMARCA2) 3 Discovery Programs Undisclosed Partnered Undisclosed Disease SMARCA4 mutant cancers (e.g., NSCLC, bladder, endometrial, colorectal) Selective SMARCA2 SMARCA4 mutant cancers (e.g., NSCLC, bladder, endometrial, colorectal) Selective CBP EP300 mutant cancers (e.g., bladder, gastric, breast, NSCLC, colorectal) Undisclosed Undisclosed Undisclosed Pre-Clinical Phase 2 / 3Discovery *LY4050784 SMARCA2 = BRM Selective EP300 EP300 dependent cancers (e.g., MM, DLBCL, prostate), CBP mutant cancers (e.g., NSCLC, bladder) Selective ARID1B ARID1A mutant cancers (e.g., ovarian, endometrial, colorectal) Protein Degraders
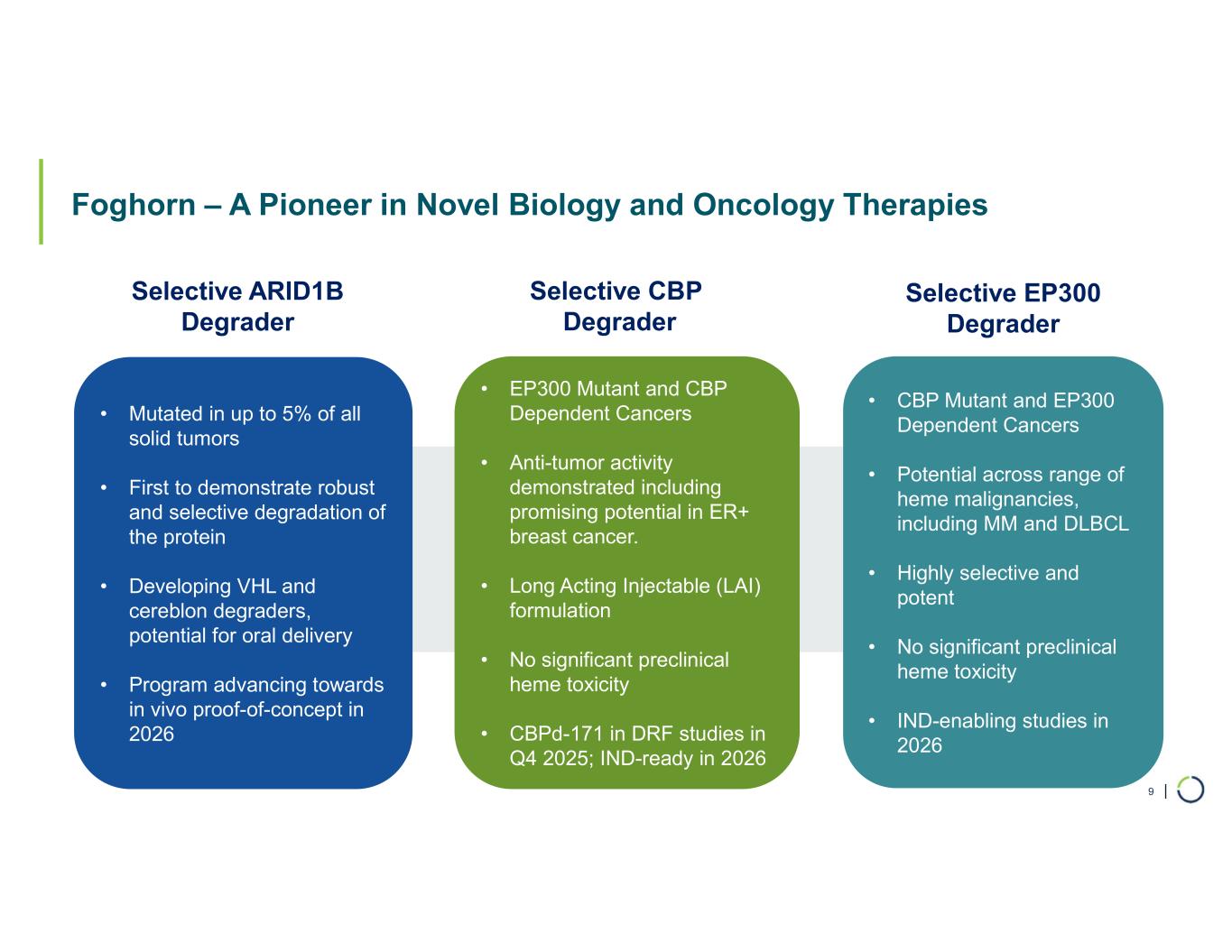
| Foghorn – A Pioneer in Novel Biology and Oncology Therapies 9 Selective ARID1B Degrader Selective CBP Degrader • Mutated in up to 5% of all solid tumors • First to demonstrate robust and selective degradation of the protein • Developing VHL and cereblon degraders, potential for oral delivery • Program advancing towards in vivo proof-of-concept in 2026 • EP300 Mutant and CBP Dependent Cancers • Anti-tumor activity demonstrated including promising potential in ER+ breast cancer. • Long Acting Injectable (LAI) formulation • No significant preclinical heme toxicity • CBPd-171 in DRF studies in Q4 2025; IND-ready in 2026 • CBP Mutant and EP300 Dependent Cancers • Potential across range of heme malignancies, including MM and DLBCL • Highly selective and potent • No significant preclinical heme toxicity • IND-enabling studies in 2026 Selective EP300 Degrader

Foghorn’s Platform Demonstrates Significant Progress Across Degrader Portfolio • Selective ARID1B Degrader • Selective CBP Degrader • Selective EP300 Degrader Steve Bellon, PhD Chief Scientific Officer
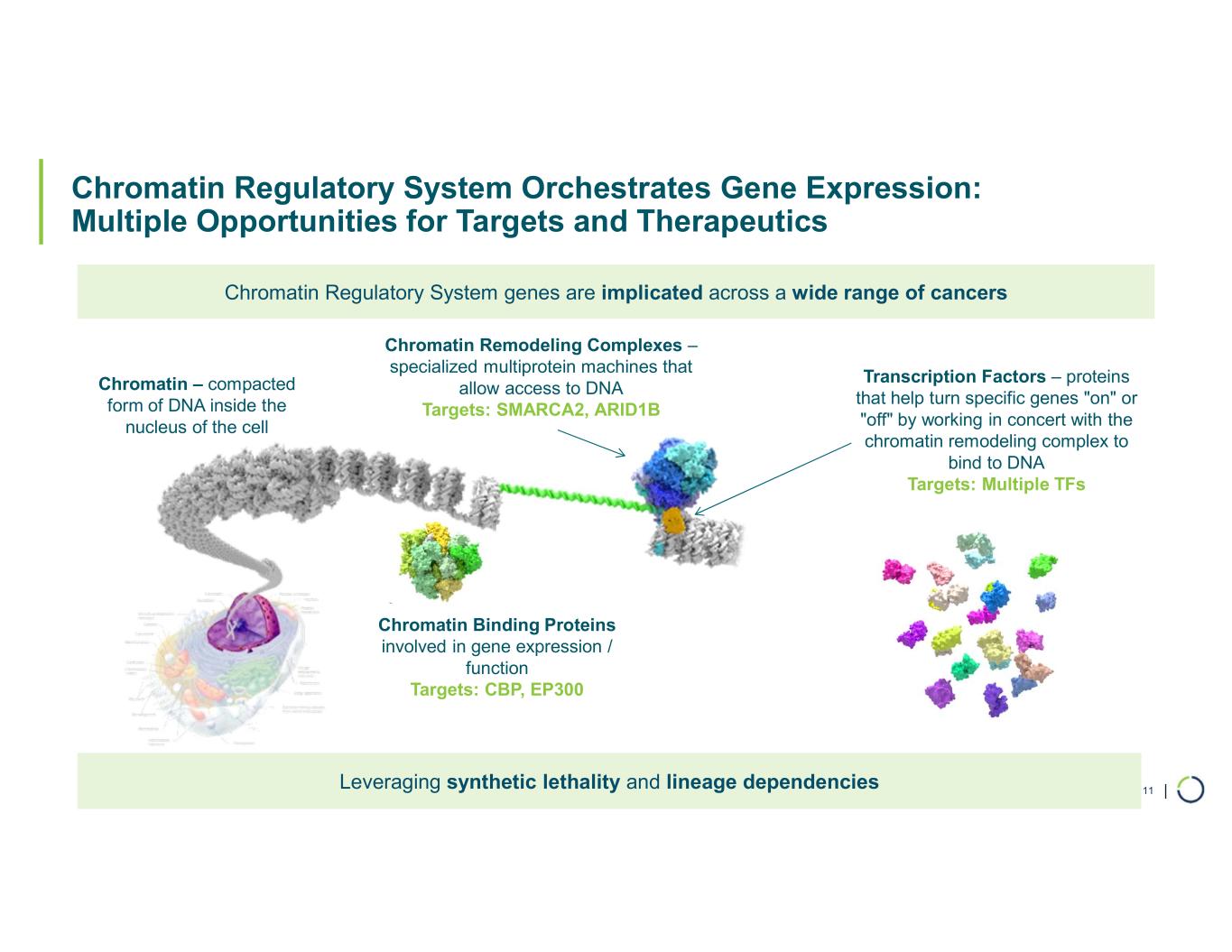
| Chromatin Regulatory System Orchestrates Gene Expression: Multiple Opportunities for Targets and Therapeutics 11 Chromatin – compacted form of DNA inside the nucleus of the cell Chromatin Remodeling Complexes – specialized multiprotein machines that allow access to DNA Targets: SMARCA2, ARID1B Transcription Factors – proteins that help turn specific genes "on" or "off" by working in concert with the chromatin remodeling complex to bind to DNA Targets: Multiple TFs Chromatin Binding Proteins involved in gene expression / function Targets: CBP, EP300 Chromatin Regulatory System genes are implicated across a wide range of cancers Leveraging synthetic lethality and lineage dependencies
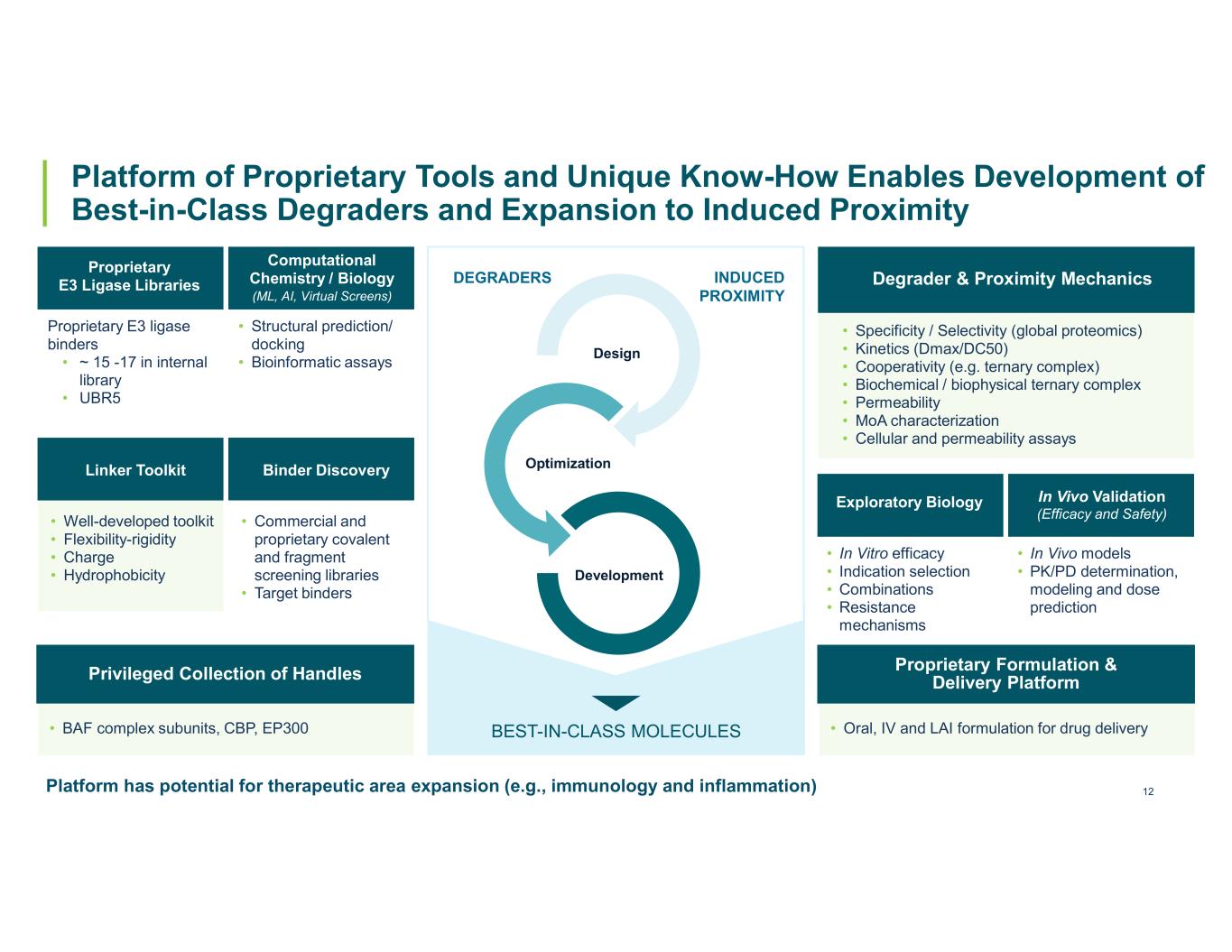
12 Platform of Proprietary Tools and Unique Know-How Enables Development of Best-in-Class Degraders and Expansion to Induced Proximity DEGRADERS INDUCED PROXIMITY Design Optimization Development BEST-IN-CLASS MOLECULES Proprietary E3 ligase binders • ~ 15 -17 in internal library • UBR5 • Well-developed toolkit • Flexibility-rigidity • Charge • Hydrophobicity Proprietary E3 Ligase Libraries Linker Toolkit • Structural prediction/ docking • Bioinformatic assays Computational Chemistry / Biology (ML, AI, Virtual Screens) • BAF complex subunits, CBP, EP300 Privileged Collection of Handles • Commercial and proprietary covalent and fragment screening libraries • Target binders Binder Discovery Platform has potential for therapeutic area expansion (e.g., immunology and inflammation) • Specificity / Selectivity (global proteomics) • Kinetics (Dmax/DC50) • Cooperativity (e.g. ternary complex) • Biochemical / biophysical ternary complex • Permeability • MoA characterization • Cellular and permeability assays Degrader & Proximity Mechanics • In Vitro efficacy • Indication selection • Combinations • Resistance mechanisms Exploratory Biology • In Vivo models • PK/PD determination, modeling and dose prediction In Vivo Validation (Efficacy and Safety) • Oral, IV and LAI formulation for drug delivery Proprietary Formulation & Delivery Platform
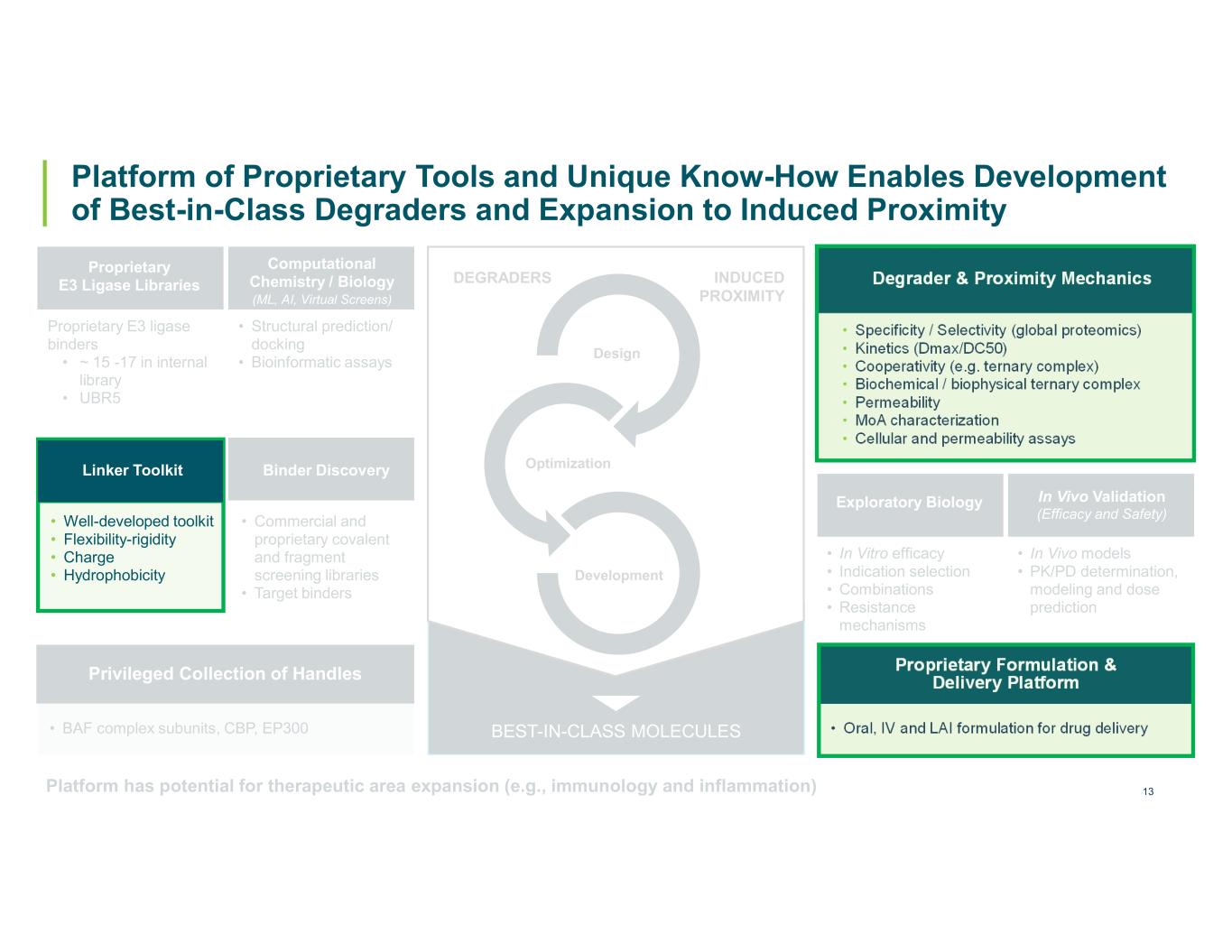
13 Platform of Proprietary Tools and Unique Know-How Enables Development of Best-in-Class Degraders and Expansion to Induced Proximity DEGRADERS INDUCED PROXIMITY Design Optimization Development BEST-IN-CLASS MOLECULES Proprietary E3 ligase binders • ~ 15 -17 in internal library • UBR5 • Well-developed toolkit • Flexibility-rigidity • Charge • Hydrophobicity Proprietary E3 Ligase Libraries Linker Toolkit • Structural prediction/ docking • Bioinformatic assays Computational Chemistry / Biology (ML, AI, Virtual Screens) • BAF complex subunits, CBP, EP300 Privileged Collection of Handles • Commercial and proprietary covalent and fragment screening libraries • Target binders Binder Discovery Platform has potential for therapeutic area expansion (e.g., immunology and inflammation) • Specificity / Selectivity (global proteomics) • Kinetics (Dmax/DC50) • Cooperativity (e.g. ternary complex) • Biochemical / biophysical ternary complex • Permeability • MoA characterization • Cellular and permeability assays Degrader & Proximity Mechanics • In Vitro efficacy • Indication selection • Combinations • Resistance mechanisms Exploratory Biology • In Vivo models • PK/PD determination, modeling and dose prediction In Vivo Validation (Efficacy and Safety) • Oral, IV and LAI formulation for drug delivery Proprietary Formulation & Delivery Platform
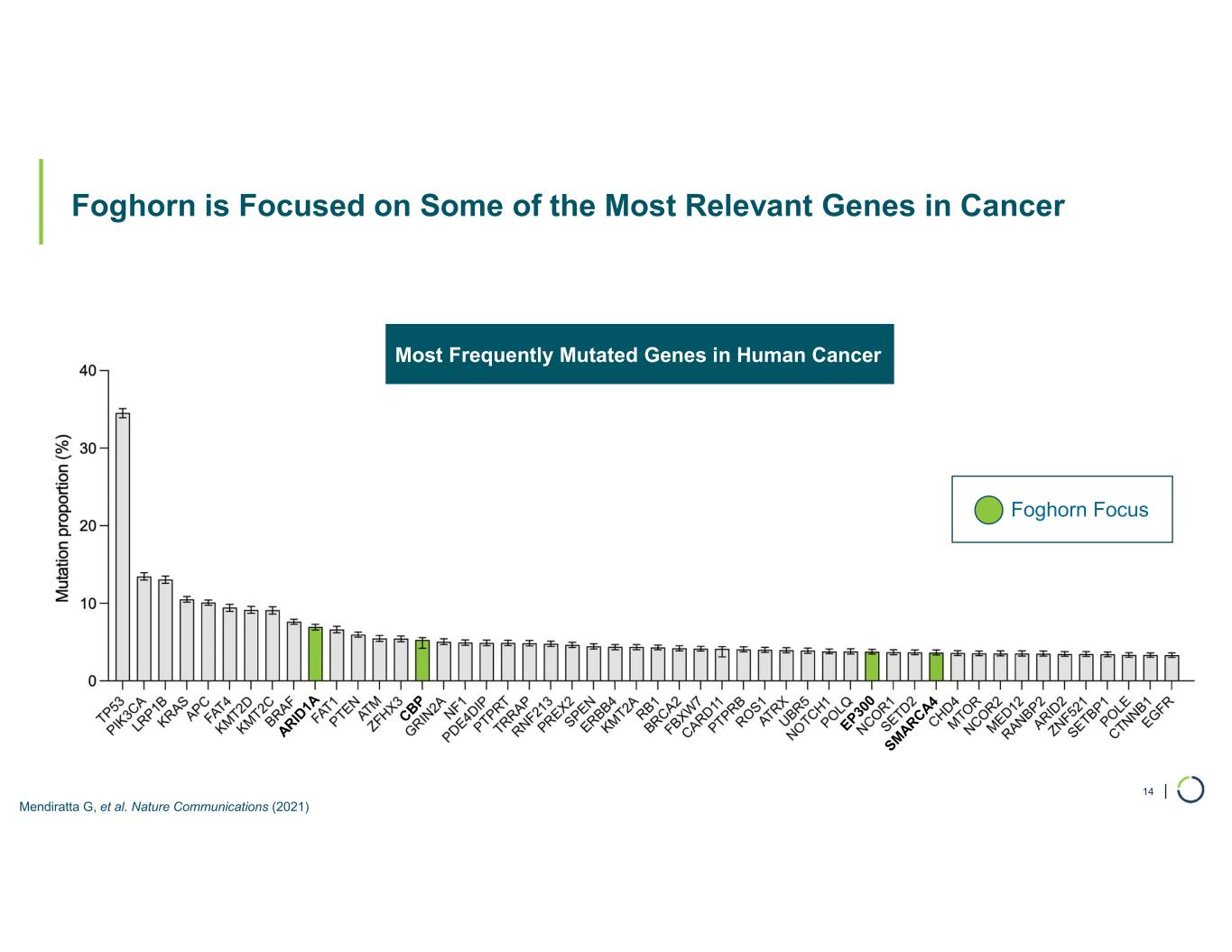
| Foghorn is Focused on Some of the Most Relevant Genes in Cancer 14 Mendiratta G, et al. Nature Communications (2021) Foghorn Focus Most Frequently Mutated Genes in Human Cancer Foghorn Focus
Alfonso Quintás-Cardama, MD Chief Medical Officer Selective ARID1B Degrader • Selective degradation achieved • VHL and cereblon based degraders • Modulation of ARID target genes achieved • In vivo proof-of-concept in 2026 Steve Bellon, PhD Chief Scientific Officer
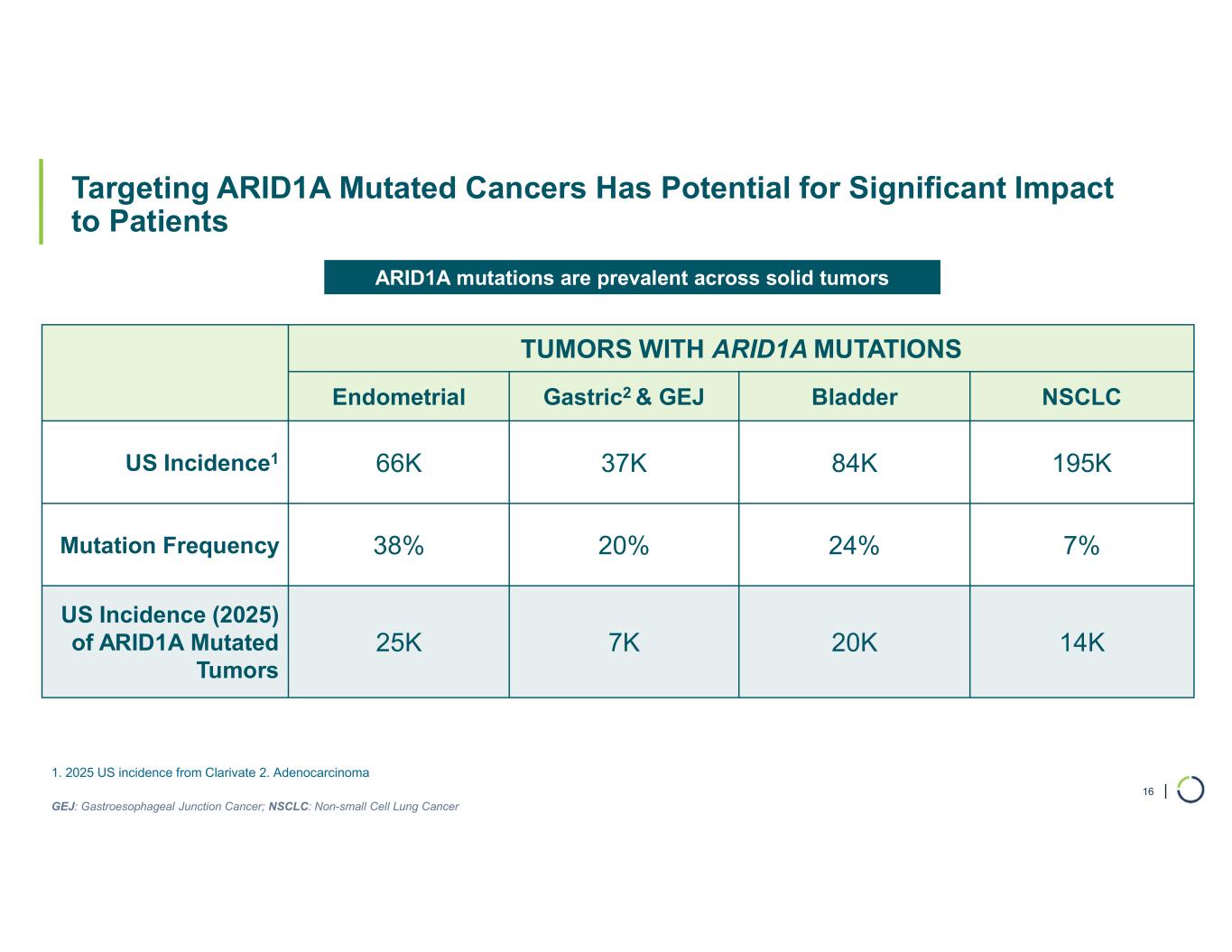
| Targeting ARID1A Mutated Cancers Has Potential for Significant Impact to Patients 16 TUMORS WITH ARID1A MUTATIONS NSCLCBladderGastric2 & GEJEndometrial 195K84K37K66KUS Incidence1 7%24%20%38%Mutation Frequency 14K20K7K25K US Incidence (2025) of ARID1A Mutated Tumors ARID1A mutations are prevalent across solid tumors 1. 2025 US incidence from Clarivate 2. Adenocarcinoma GEJ: Gastroesophageal Junction Cancer; NSCLC: Non-small Cell Lung Cancer
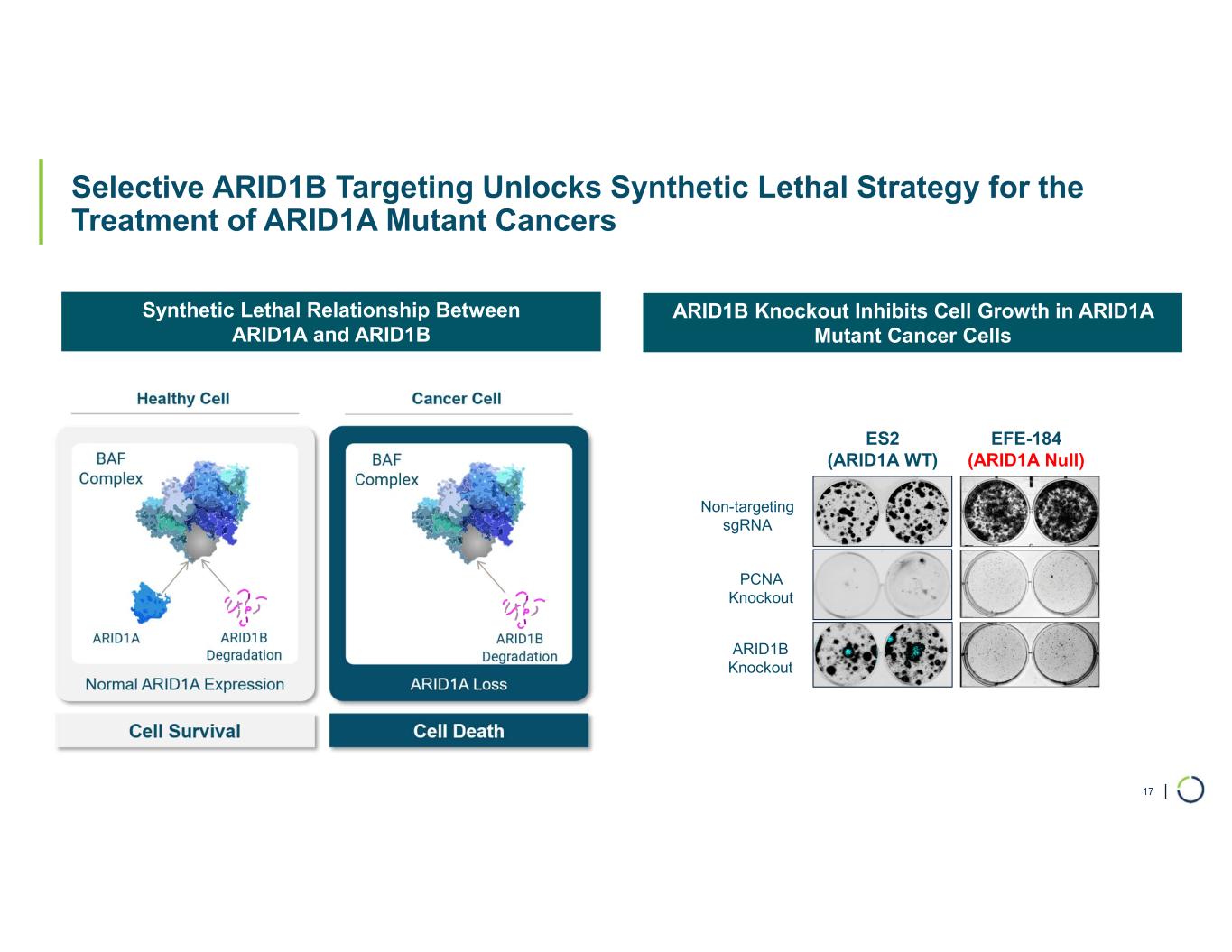
| Selective ARID1B Targeting Unlocks Synthetic Lethal Strategy for the Treatment of ARID1A Mutant Cancers 17 Synthetic Lethal Relationship Between ARID1A and ARID1B ARID1B Knockout Inhibits Cell Growth in ARID1A Mutant Cancer Cells EFE-184 (ARID1A Null) Non-targeting sgRNA ES2 (ARID1A WT) PCNA Knockout ARID1B Knockout
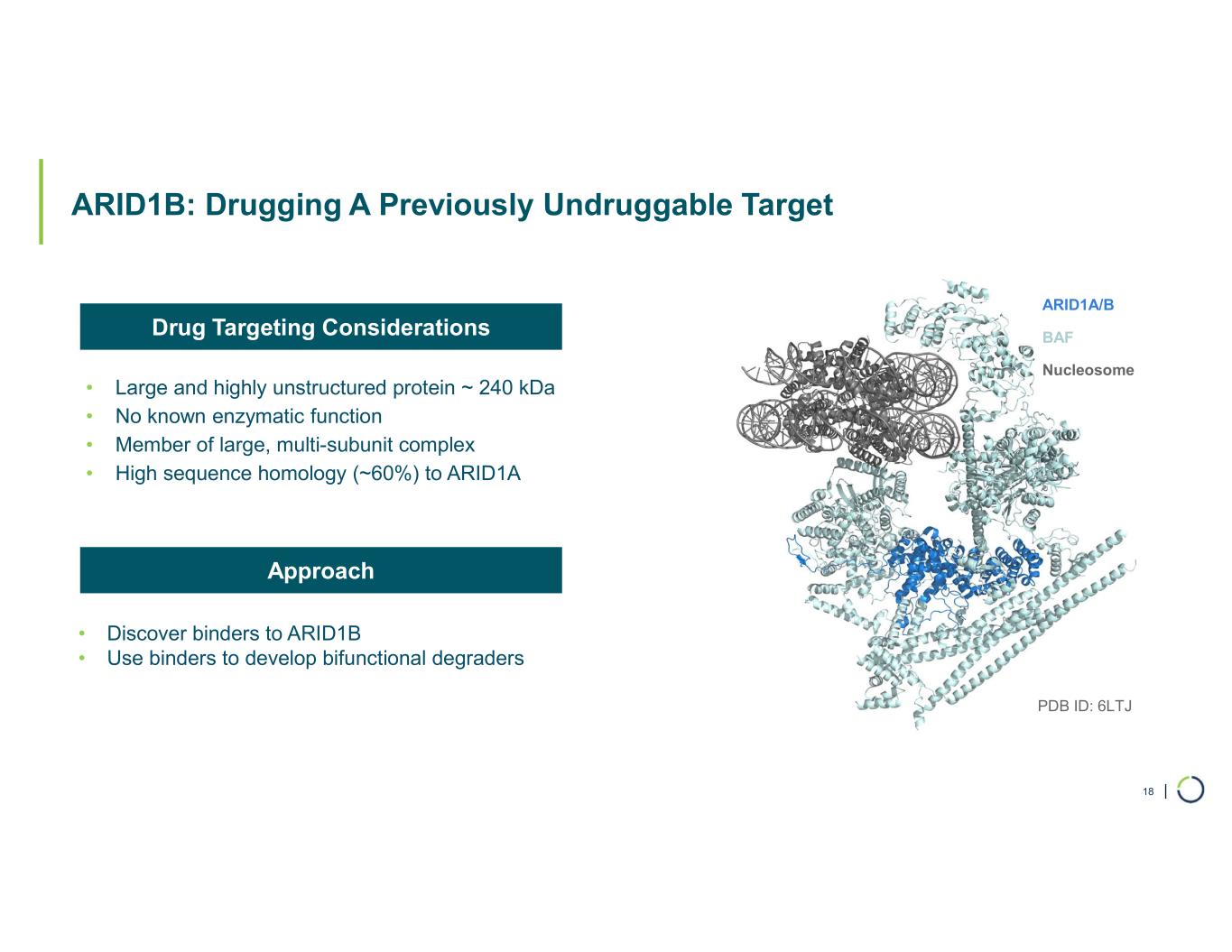
| ARID1B: Drugging A Previously Undruggable Target • Large and highly unstructured protein ~ 240 kDa • No known enzymatic function • Member of large, multi-subunit complex • High sequence homology (~60%) to ARID1A 18 ARID1A/B BAF Nucleosome PDB ID: 6LTJ • Discover binders to ARID1B • Use binders to develop bifunctional degraders Drug Targeting Considerations Approach
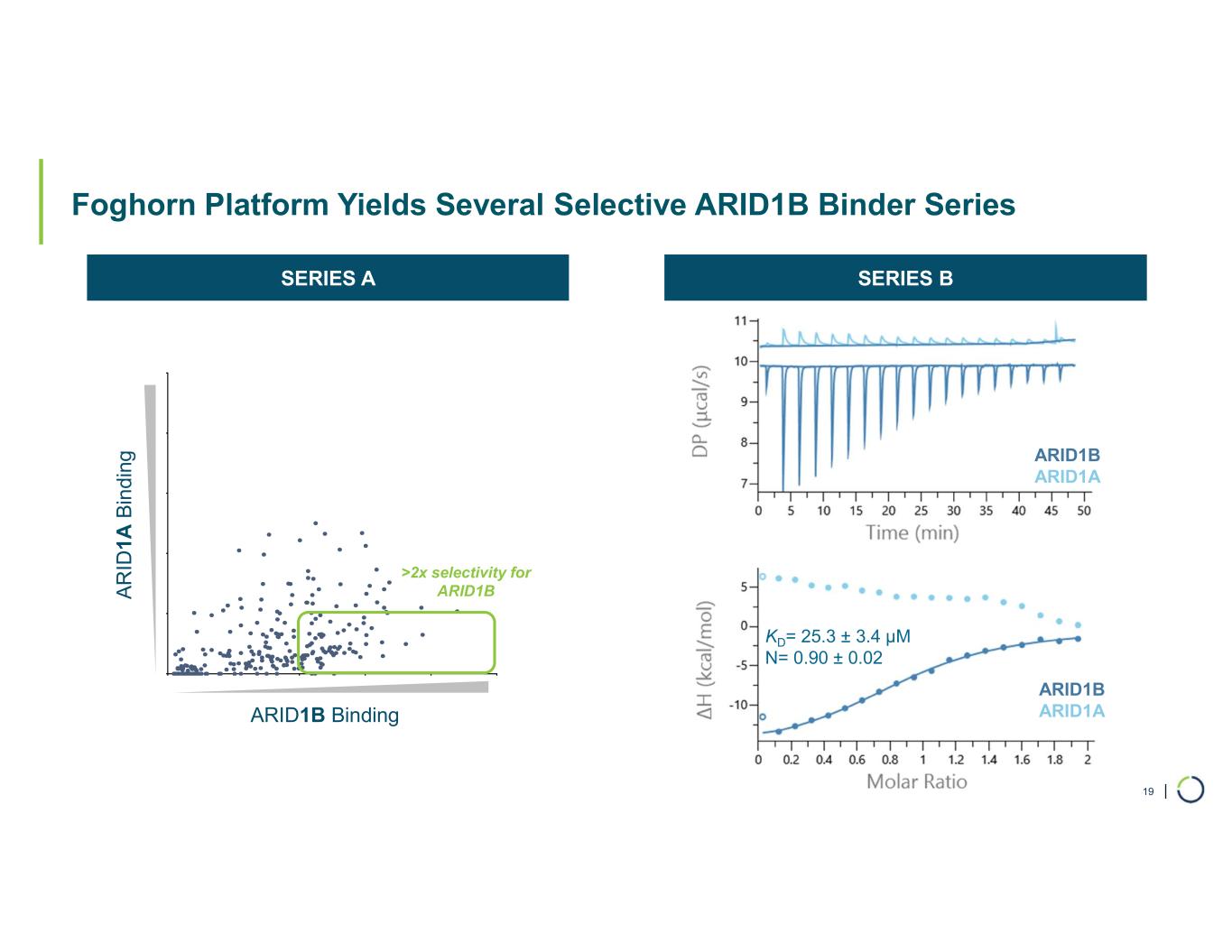
| Foghorn Platform Yields Several Selective ARID1B Binder Series 19 ARID1B ARID1A KD= 25.3 ± 3.4 µM N= 0.90 ± 0.02 ARID1B ARID1A >2x selectivity for ARID1B ARID1B Binding A R ID 1A B in di ng SERIES A SERIES B
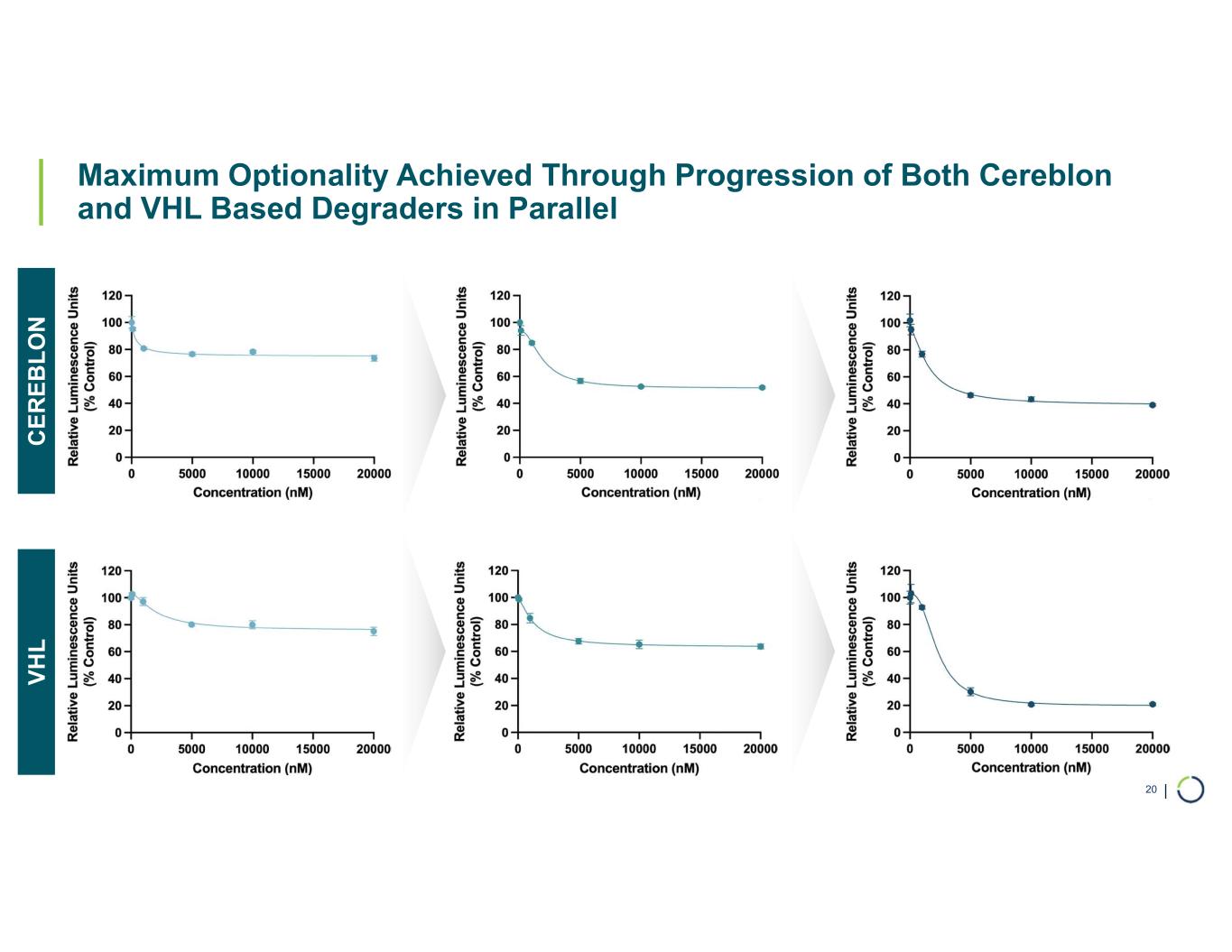
| Maximum Optionality Achieved Through Progression of Both Cereblon and VHL Based Degraders in Parallel 20 C E R E B L O N V H L
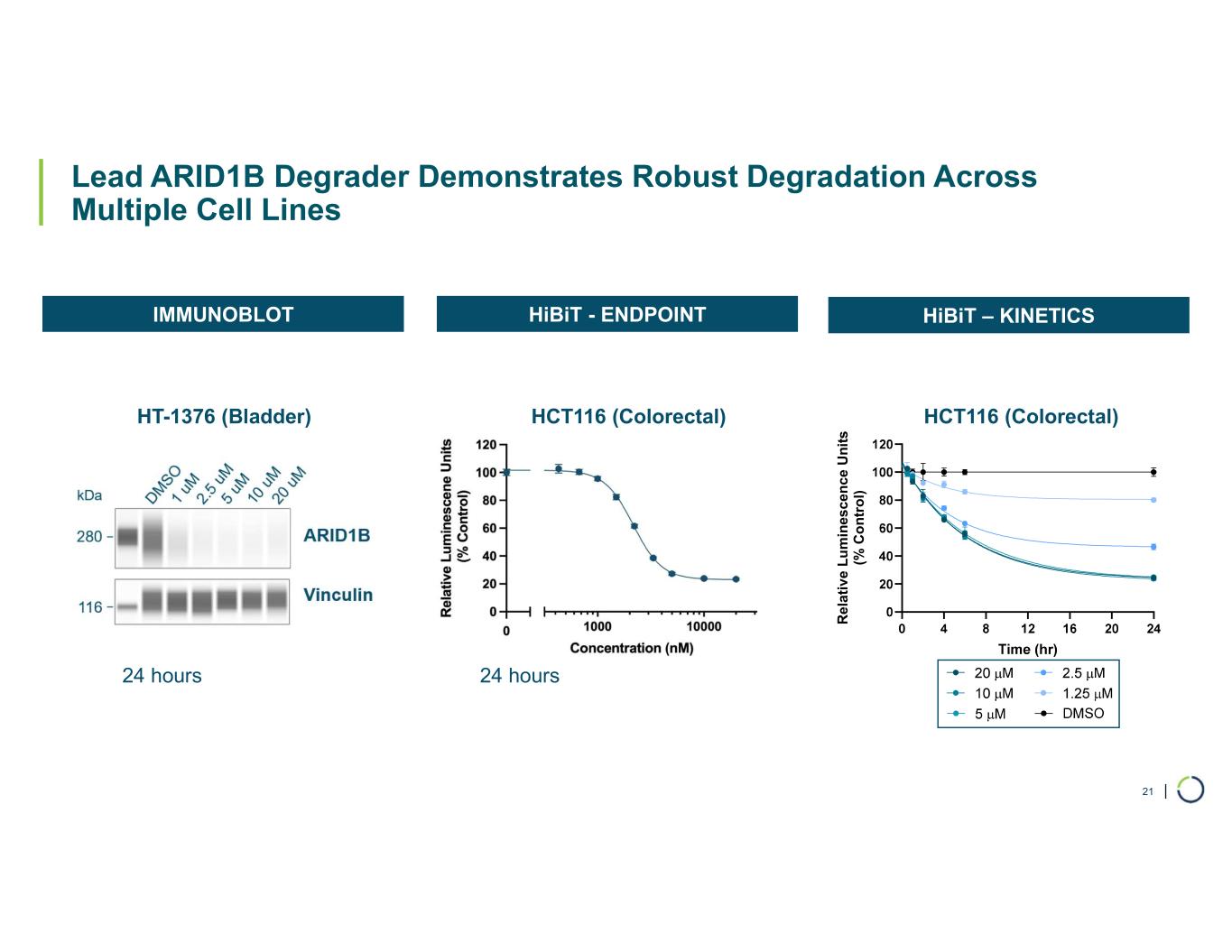
| Lead ARID1B Degrader Demonstrates Robust Degradation Across Multiple Cell Lines 21 IMMUNOBLOT HiBiT - ENDPOINT HCT116 (Colorectal)HT-1376 (Bladder) HiBiT – KINETICS HCT116 (Colorectal) 24 hours 24 hours R e la ti v e L u m in es c e n c e U n it s (% C o n tr o l)
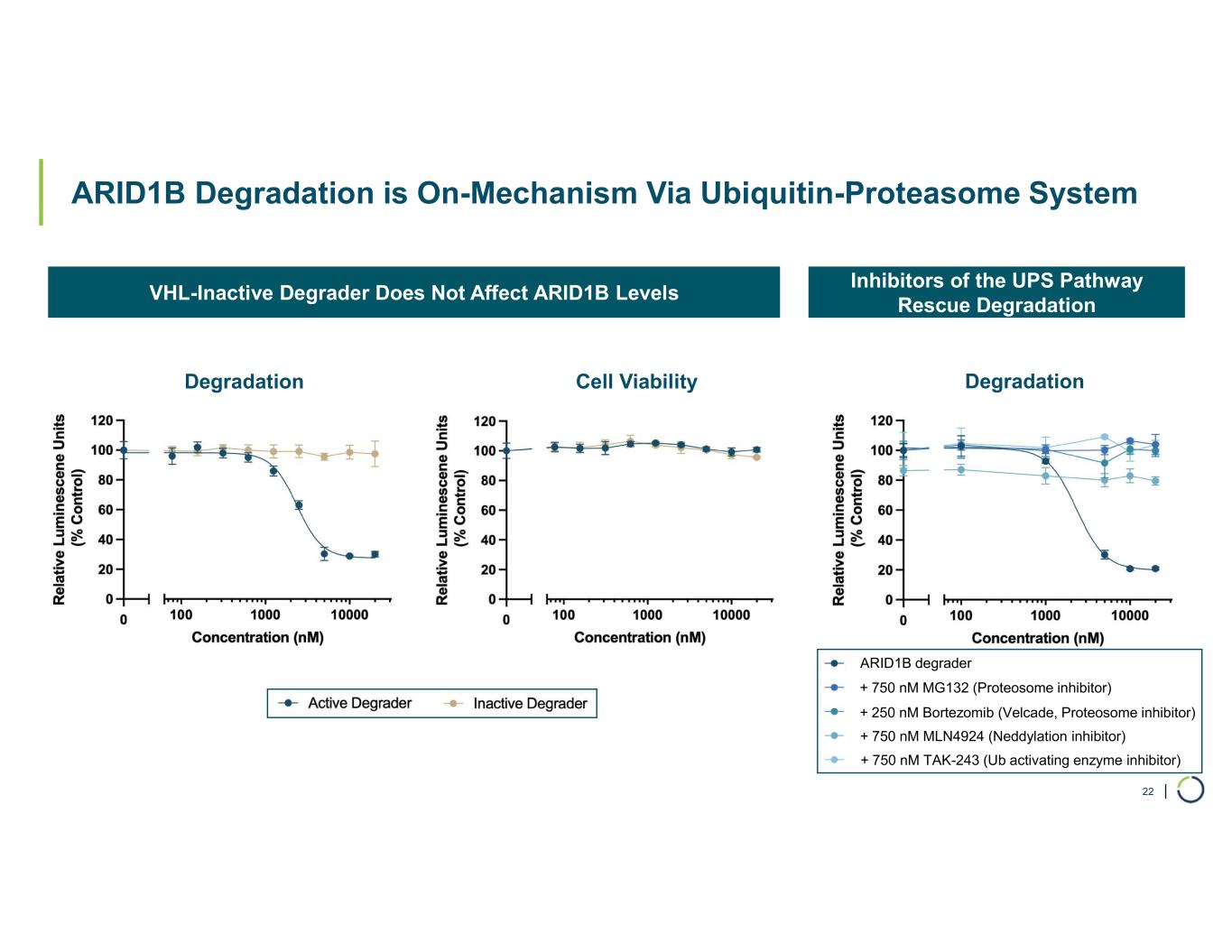
| ARID1B Degradation is On-Mechanism Via Ubiquitin-Proteasome System 22 Inhibitors of the UPS Pathway Rescue Degradation VHL-Inactive Degrader Does Not Affect ARID1B Levels Degradation Cell Viability Degradation + 750 nM MG132 (Proteosome inhibitor) + 250 nM Bortezomib (Velcade, Proteosome inhibitor) + 750 nM MLN4924 (Neddylation inhibitor) + 750 nM TAK-243 (Ub activating enzyme inhibitor) ARID1B degrader
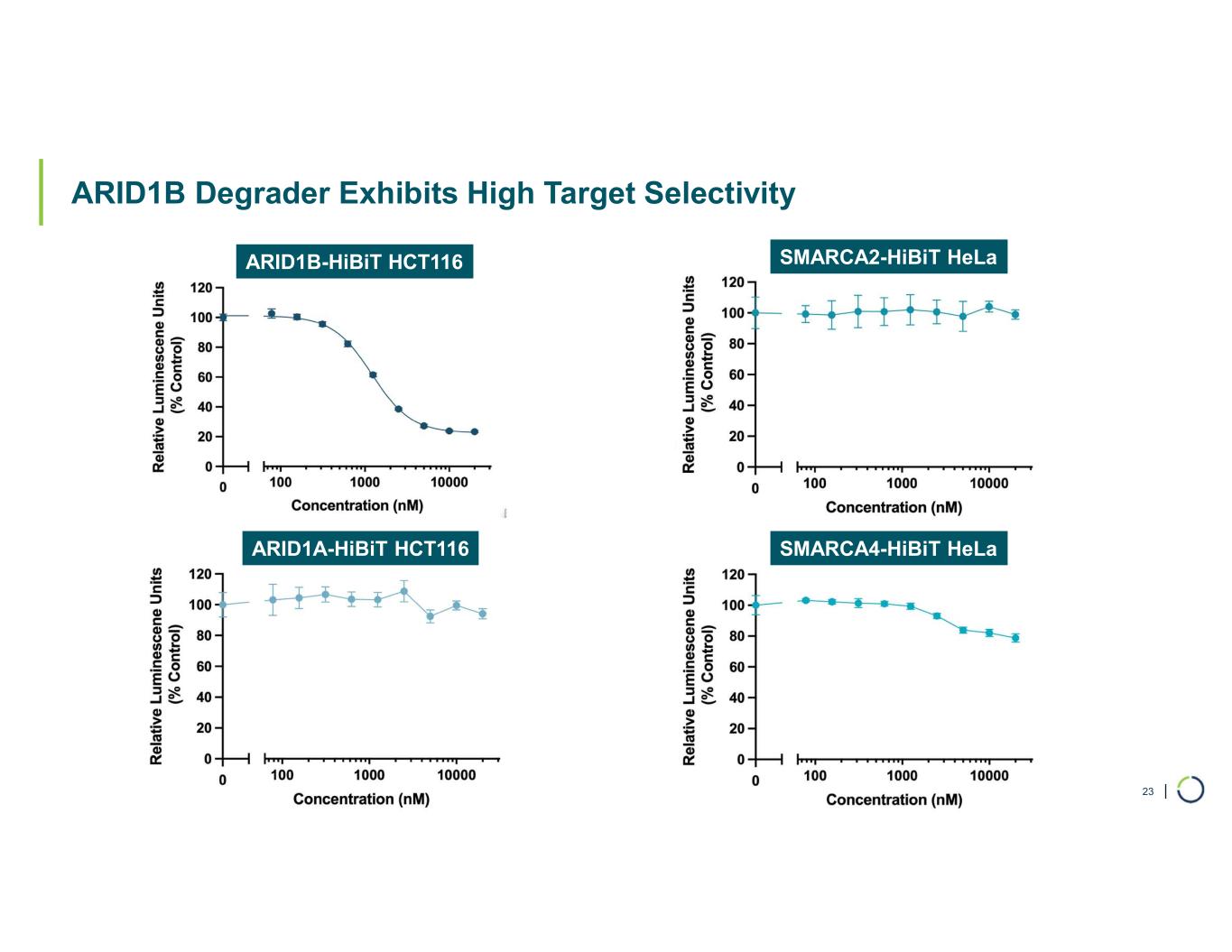
| ARID1B Degrader Exhibits High Target Selectivity 23 ARID1B-HiBiT HCT116 ARID1A-HiBiT HCT116 SMARCA2-HiBiT HeLa SMARCA4-HiBiT HeLa
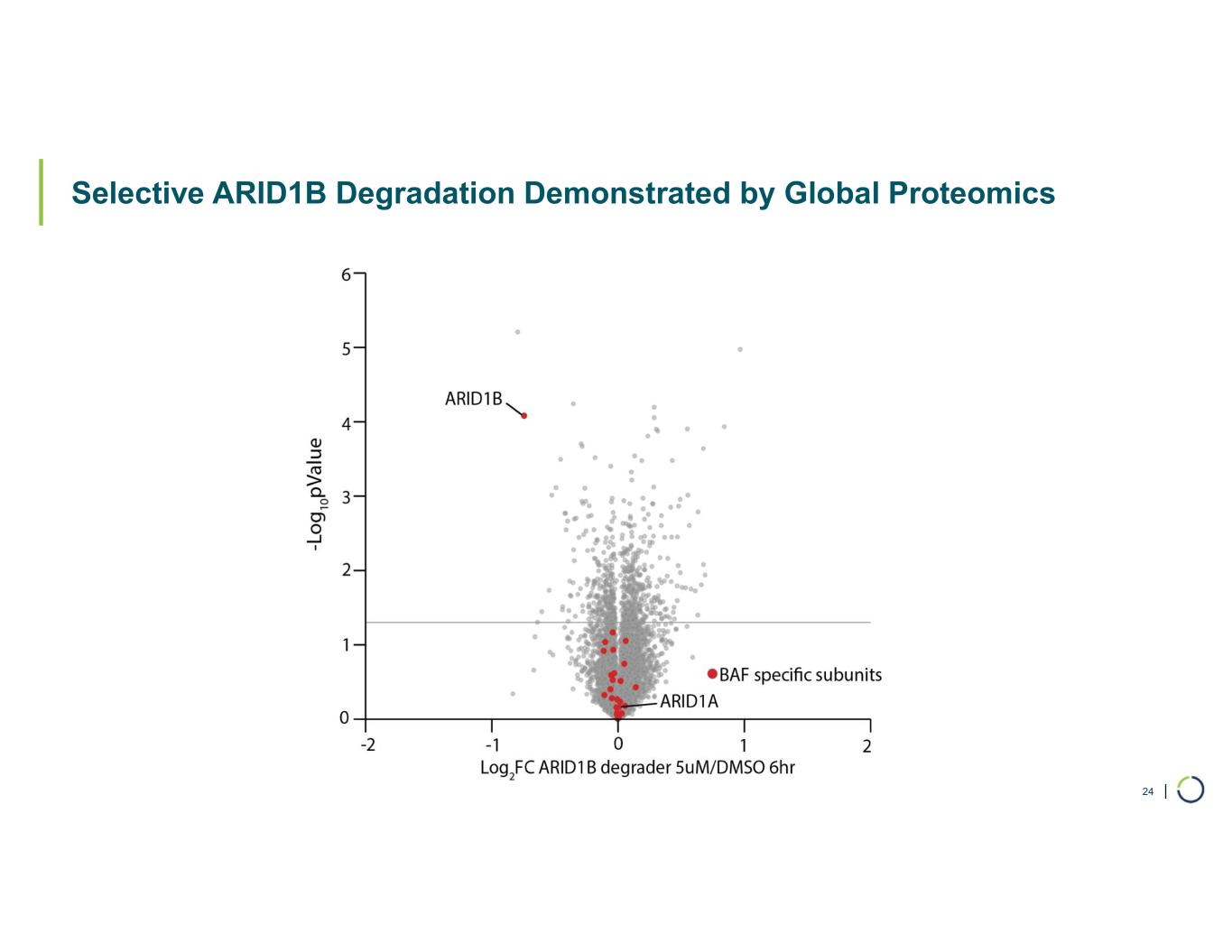
| Selective ARID1B Degradation Demonstrated by Global Proteomics 24
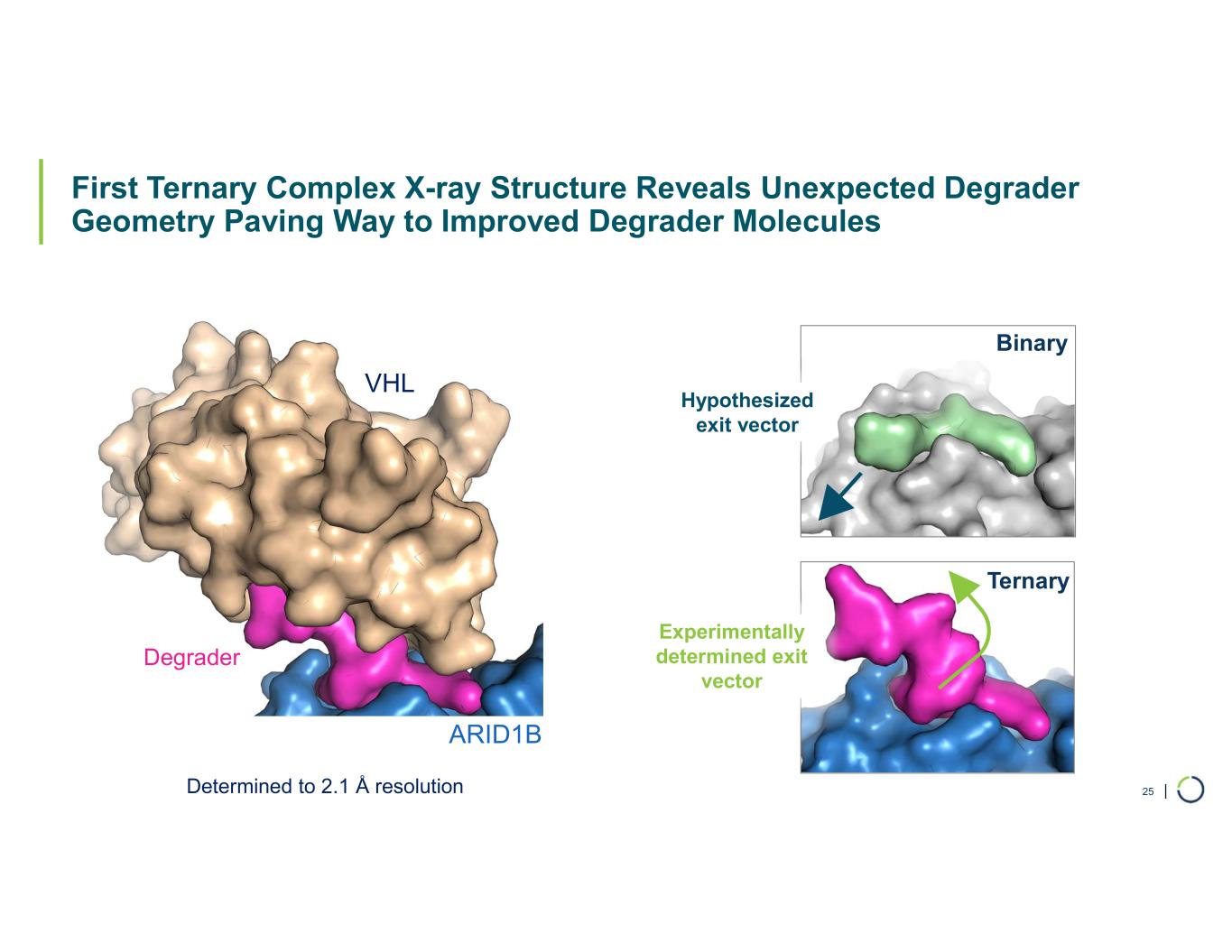
| First Ternary Complex X-ray Structure Reveals Unexpected Degrader Geometry Paving Way to Improved Degrader Molecules 25 Hypothesized exit vector Binary Ternary Determined to 2.1 Å resolution Experimentally determined exit vector VHL Degrader ARID1B
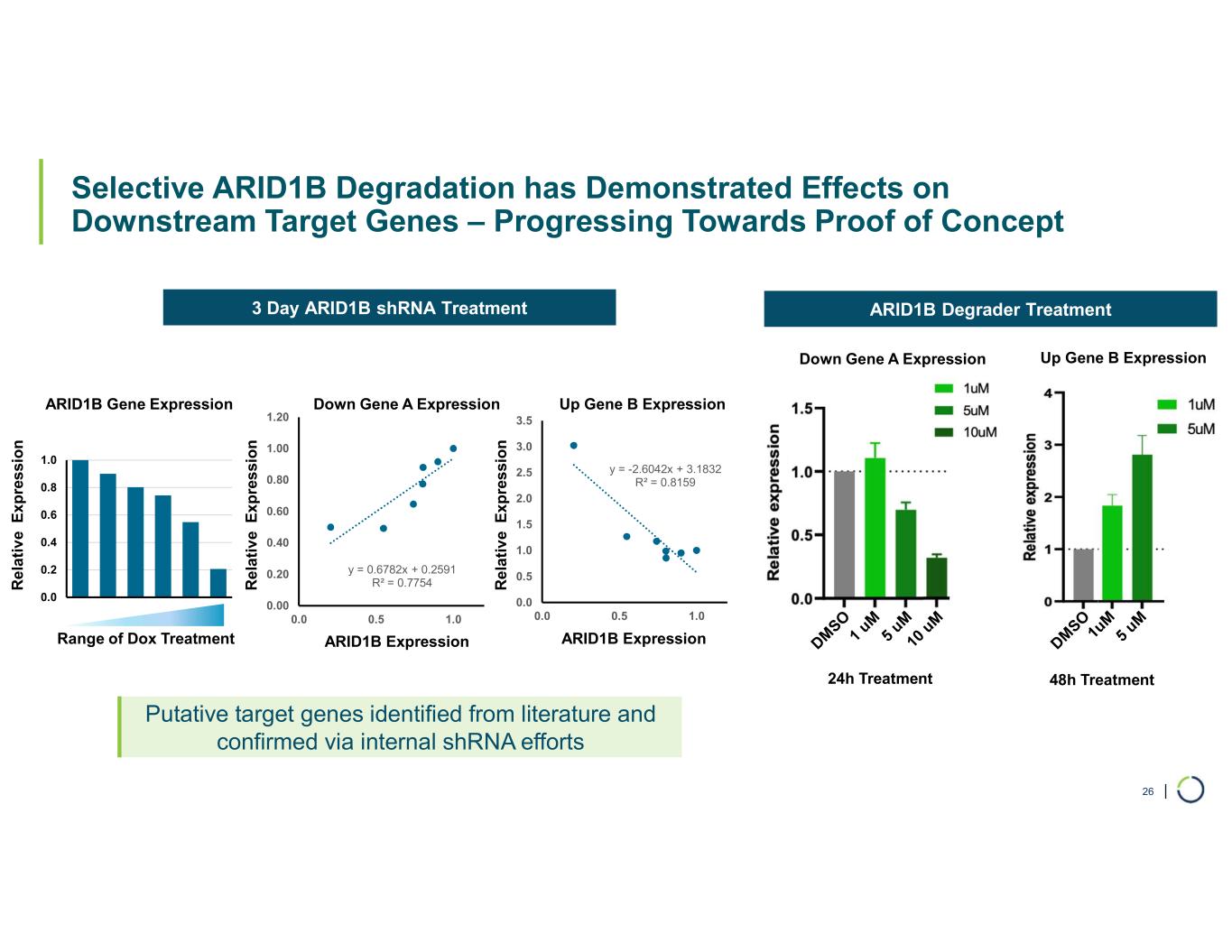
| 0.0 0.2 0.4 0.6 0.8 1.0 1 2 3 4 5 6 Selective ARID1B Degradation has Demonstrated Effects on Downstream Target Genes – Progressing Towards Proof of Concept 26 3 Day ARID1B shRNA Treatment ARID1B Degrader Treatment y = 0.6782x + 0.2591 R² = 0.7754 0.00 0.20 0.40 0.60 0.80 1.00 1.20 0.0 0.5 1.0 ARID1B expression y = -2.6042x + 3.1832 R² = 0.8159 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 0.0 0.5 1.0 ARID1B expression ARID1B Gene Expression ARID1B Expression ARID1B ExpressionRange of Dox Treatment R e la ti v e E x p re s si o n R e la ti v e E x p re s si o n R e la ti v e E x p re s si o n Down Gene A Expression Up Gene B Expression 24h Treatment 48h Treatment Down Gene A Expression Up Gene B Expression Putative target genes identified from literature and confirmed via internal shRNA efforts

Alfonso Quintás-Cardama, MD Chief Medical Officer Selective CBP Degrader • Application in EP300 mutant cancers – synthetic lethal • Potential application in ER+ breast cancer – Lineage dependency on CBP • Candidate molecule CBPd-171 advancing to DRF toxicology studies Q4 • IND-ready in 2026 Steve Bellon, PhD Chief Scientific Officer
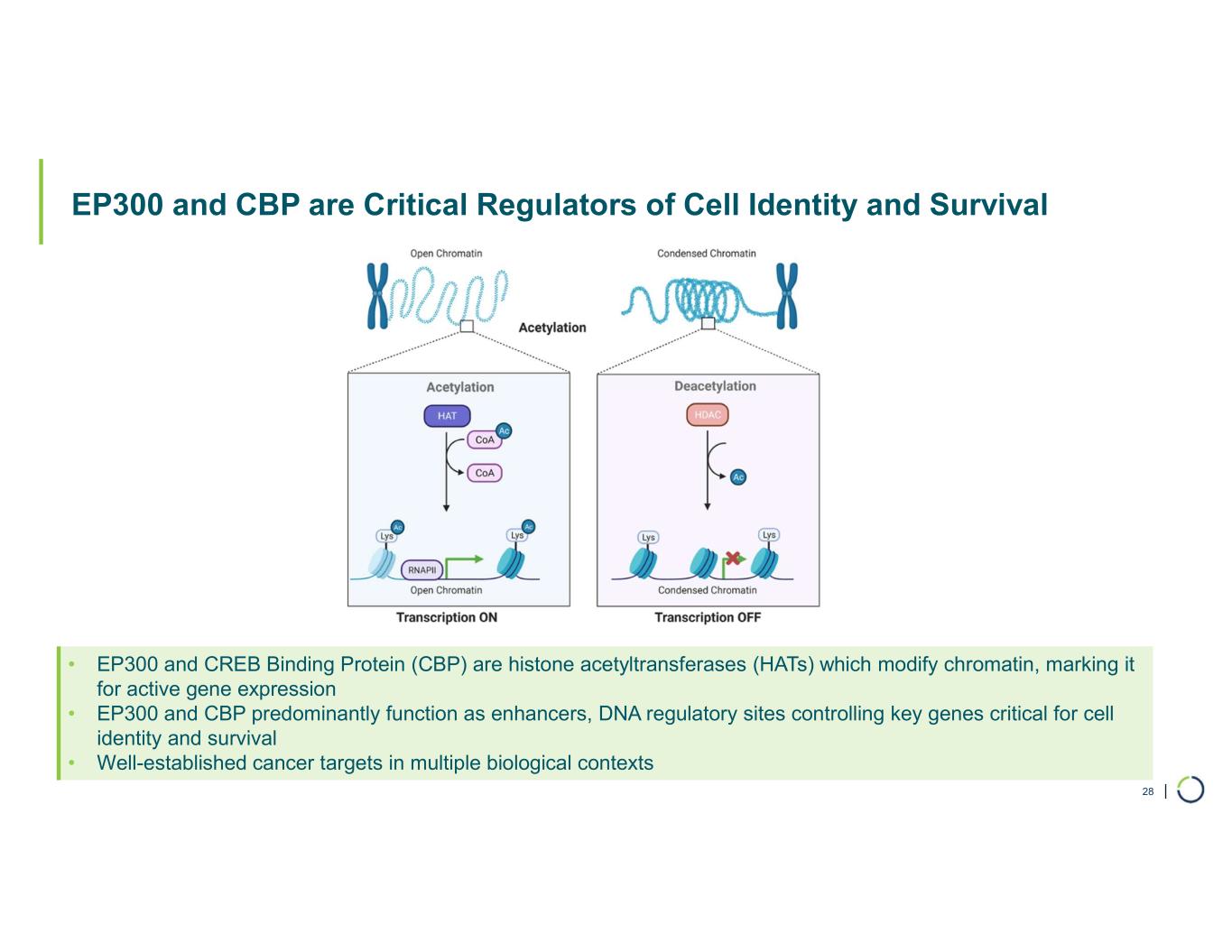
| EP300 and CBP are Critical Regulators of Cell Identity and Survival 28 • EP300 and CREB Binding Protein (CBP) are histone acetyltransferases (HATs) which modify chromatin, marking it for active gene expression • EP300 and CBP predominantly function as enhancers, DNA regulatory sites controlling key genes critical for cell identity and survival • Well-established cancer targets in multiple biological contexts
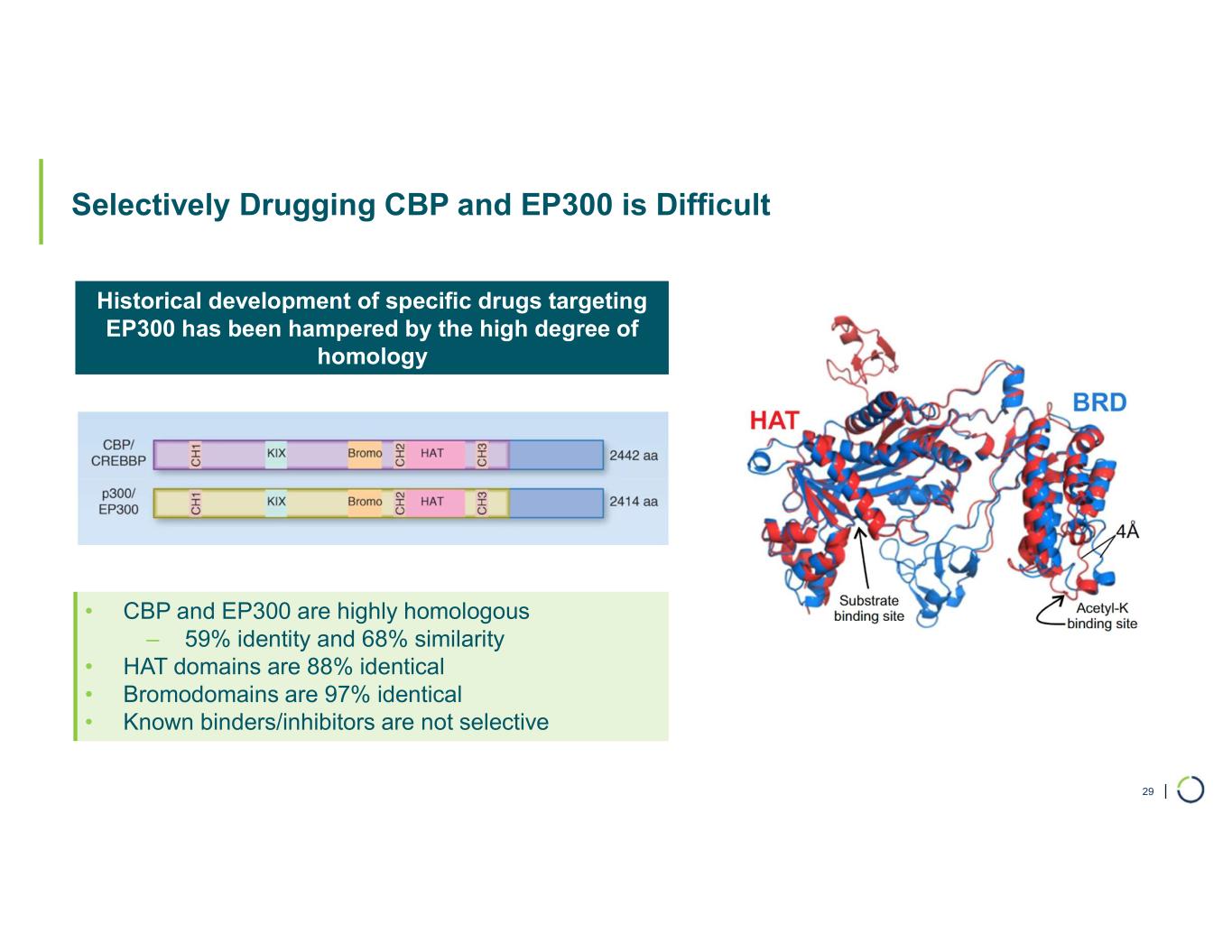
| Selectively Drugging CBP and EP300 is Difficult 29 Historical development of specific drugs targeting EP300 has been hampered by the high degree of homology • CBP and EP300 are highly homologous ‒ 59% identity and 68% similarity • HAT domains are 88% identical • Bromodomains are 97% identical • Known binders/inhibitors are not selective
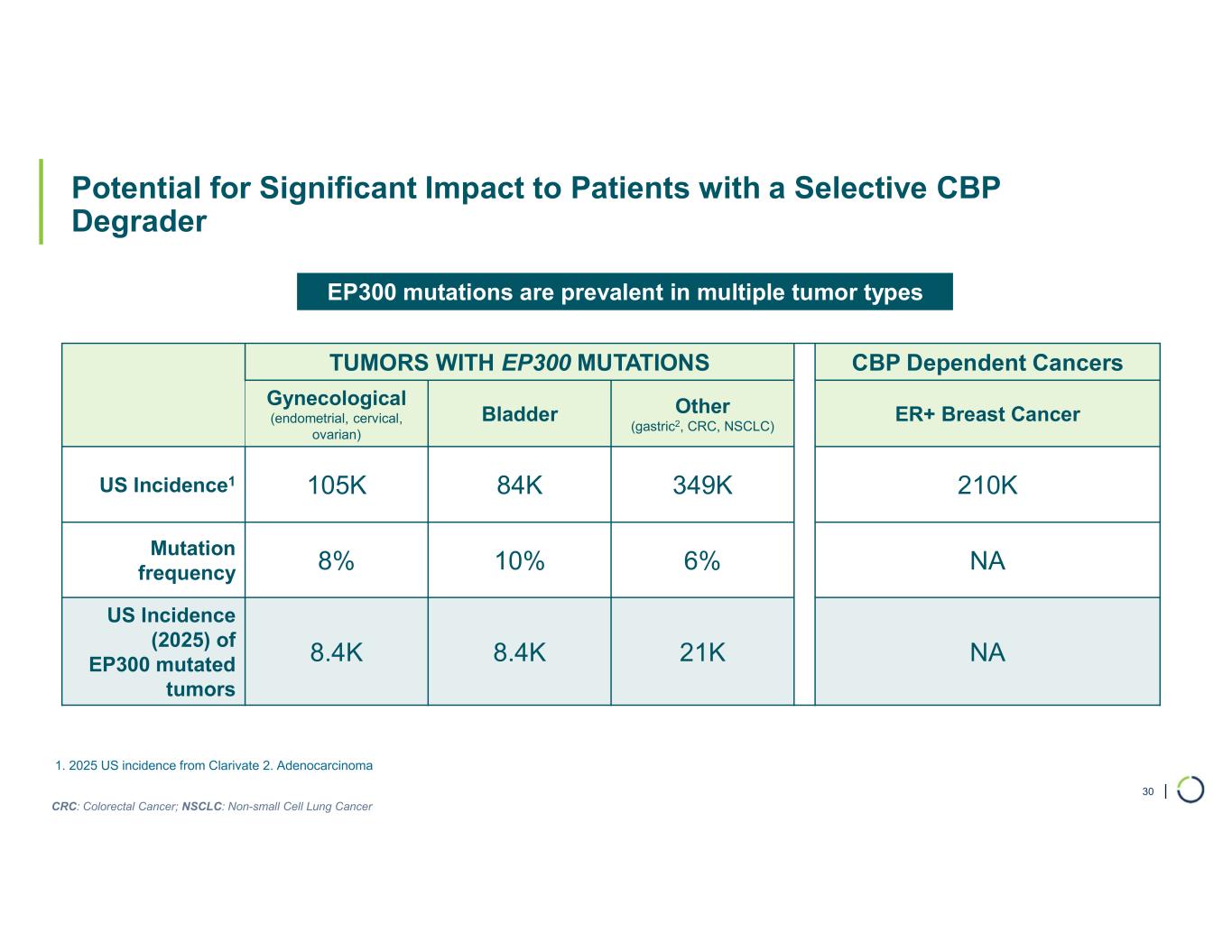
| Potential for Significant Impact to Patients with a Selective CBP Degrader 30 CBP Dependent CancersTUMORS WITH EP300 MUTATIONS ER+ Breast CancerOther (gastric2, CRC, NSCLC) Bladder Gynecological (endometrial, cervical, ovarian) 210K349K84K105KUS Incidence1 NA6%10%8% Mutation frequency NA21K8.4K8.4K US Incidence (2025) of EP300 mutated tumors EP300 mutations are prevalent in multiple tumor types 1. 2025 US incidence from Clarivate 2. Adenocarcinoma CRC: Colorectal Cancer; NSCLC: Non-small Cell Lung Cancer
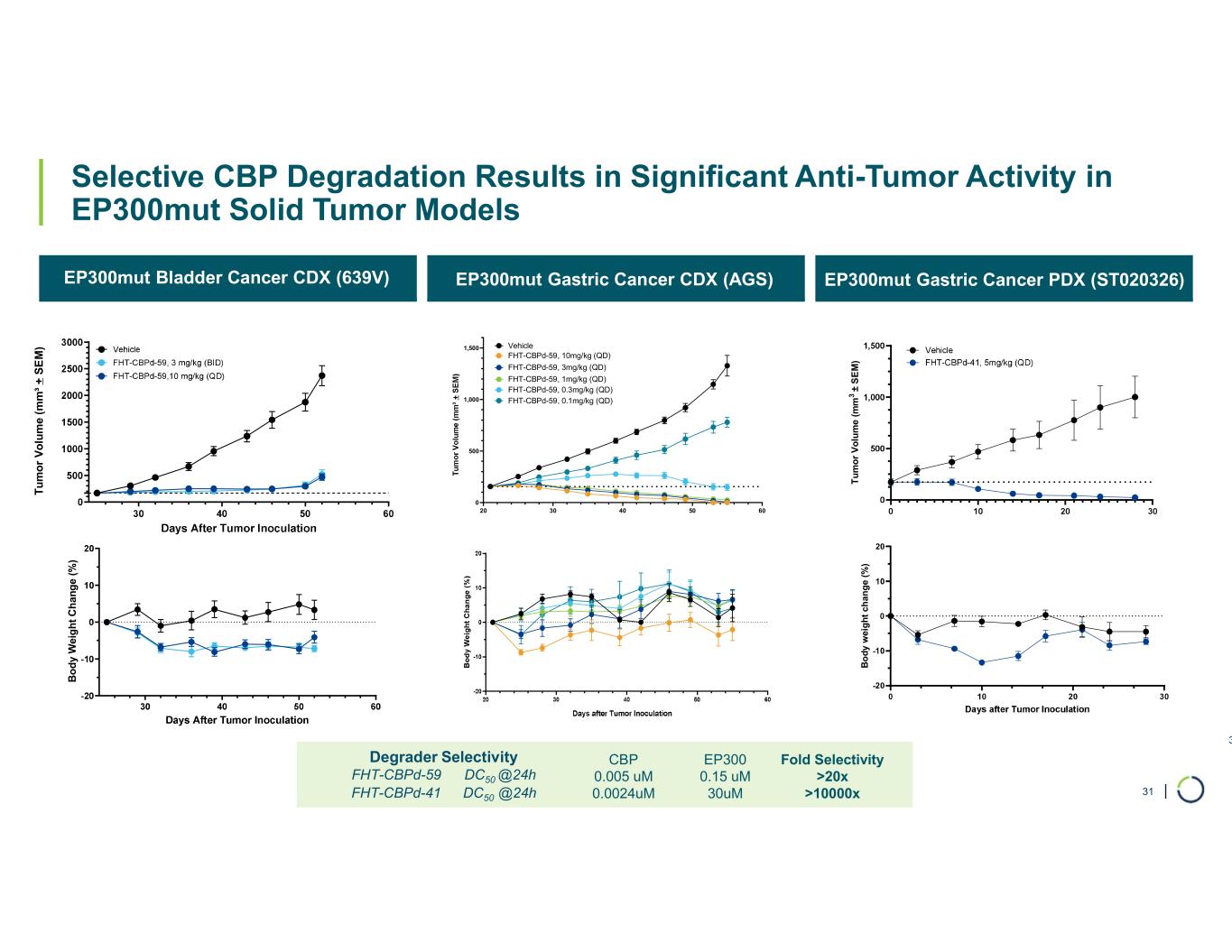
| Selective CBP Degradation Results in Significant Anti-Tumor Activity in EP300mut Solid Tumor Models 31 EP300mut Bladder Cancer CDX (639V) EP300mut Gastric Cancer CDX (AGS) EP300mut Gastric Cancer PDX (ST020326) T u m o r V o lu m e (m m ³ + S E M ) B o d y W ei g h t C h an g e (% ) 20 30 40 50 60 0 500 1,000 1,500 Vehicle FHT-CBPd-59, 10mg/kg (QD) FHT-CBPd-59, 3mg/kg (QD) FHT-CBPd-59, 1mg/kg (QD) FHT-CBPd-59, 0.3mg/kg (QD) FHT-CBPd-59, 0.1mg/kg (QD) T u m o r V o lu m e ( m m 3 ± S E M ) B o d y w ei g h t ch an g e (% ) Fold Selectivity >20x >10000x EP300 0.15 uM 30uM CBP 0.005 uM 0.0024uM Degrader Selectivity FHT-CBPd-59 DC50 @24h FHT-CBPd-41 DC50 @24h 31
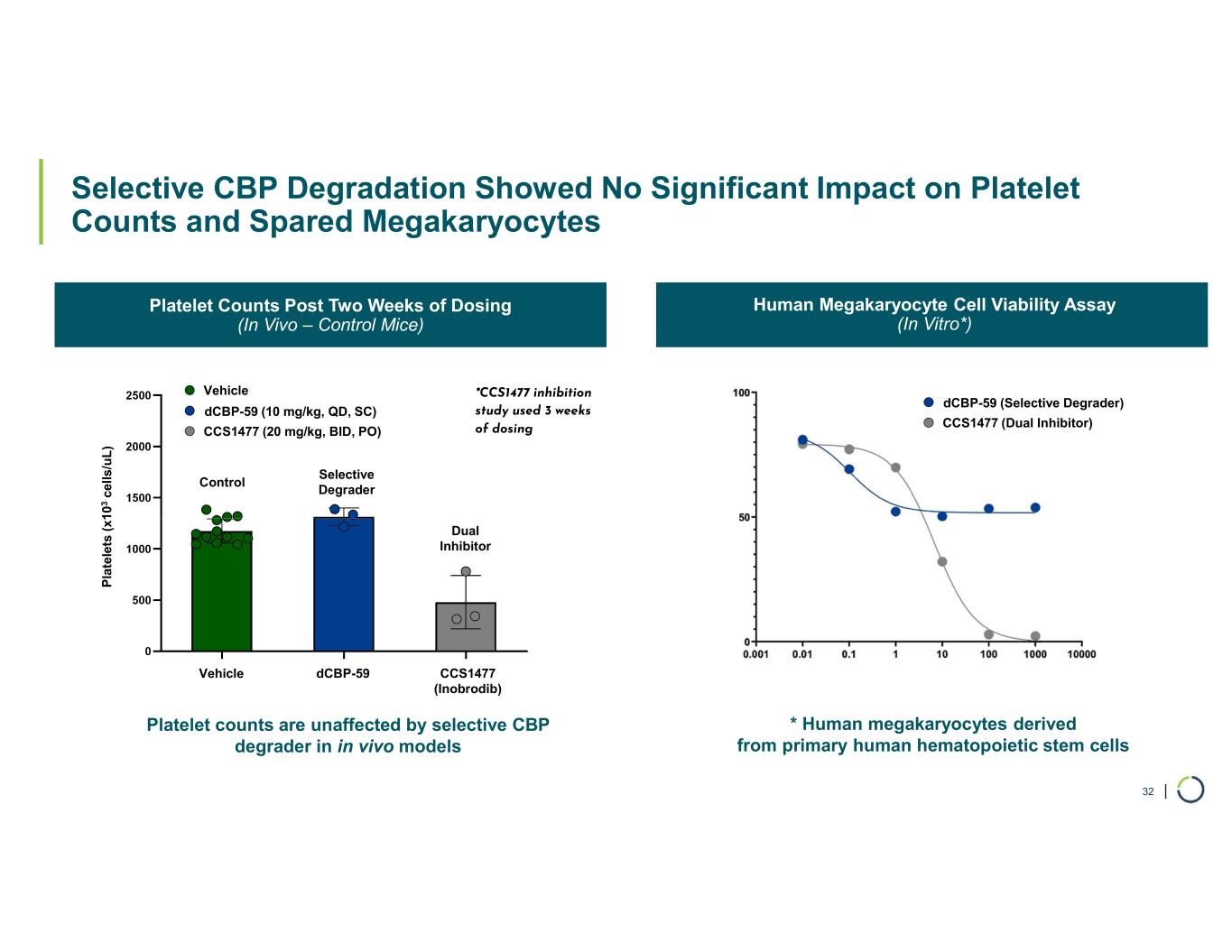
| Selective CBP Degradation Showed No Significant Impact on Platelet Counts and Spared Megakaryocytes 32 Platelet Counts Post Two Weeks of Dosing (In Vivo – Control Mice) Human Megakaryocyte Cell Viability Assay (In Vitro*) dCBP-59 CCS1477 (Inobrodib) Vehicle dCBP-59 (10 mg/kg, QD, SC) Vehicle CCS1477 (20 mg/kg, BID, PO) Dual Inhibitor Selective Degrader Control P la te le ts ( x1 03 ce lls /u L ) *CCS1477 inhibition study used 3 weeks of dosing dCBP-59 (Selective Degrader) CCS1477 (Dual Inhibitor) Platelet counts are unaffected by selective CBP degrader in in vivo models * Human megakaryocytes derived from primary human hematopoietic stem cells
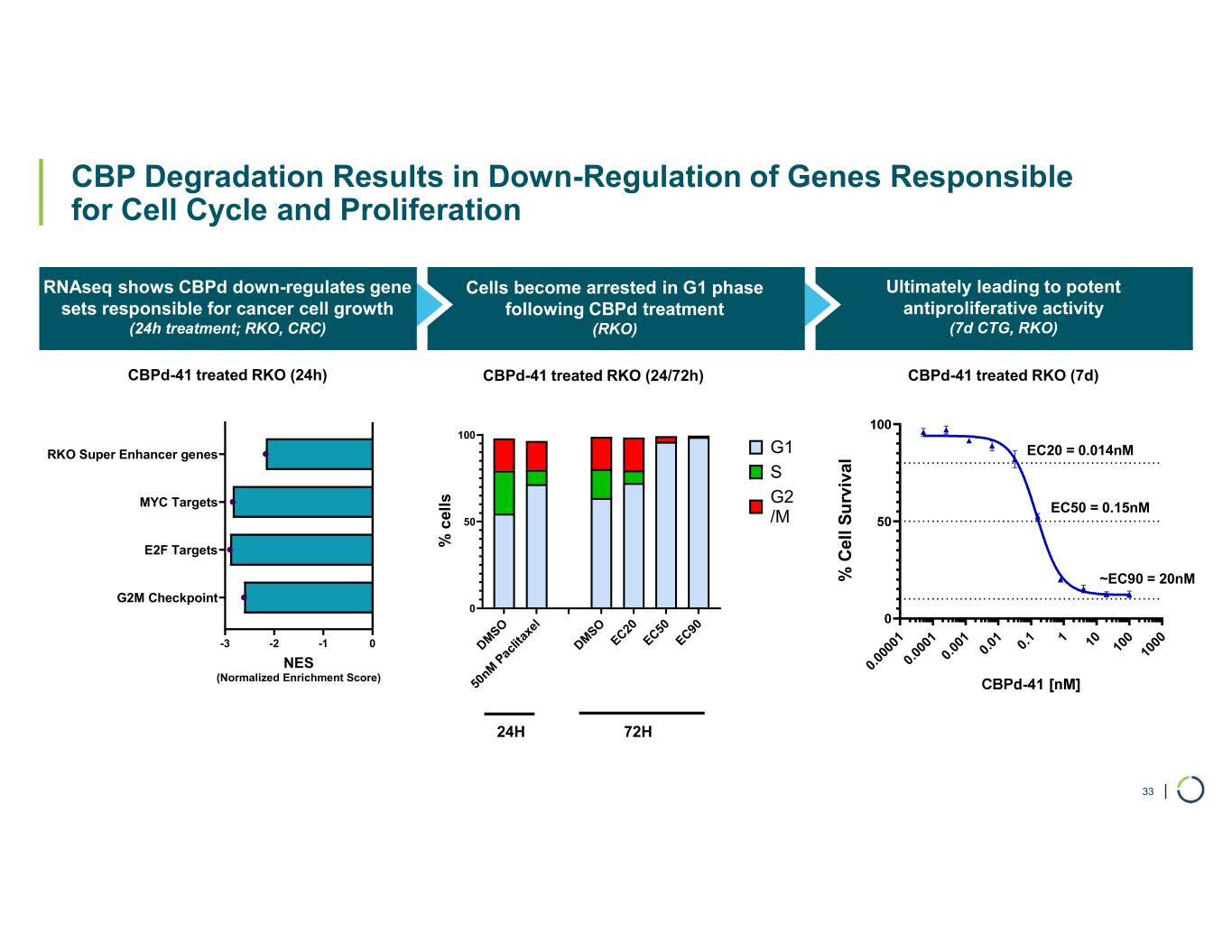
| CBP Degradation Results in Down-Regulation of Genes Responsible for Cell Cycle and Proliferation CBP RNAseq shows CBPd down-regulates gene sets responsible for cancer cell growth (24h treatment; RKO, CRC) Cells become arrested in G1 phase following CBPd treatment (RKO) Ultimately leading to potent antiproliferative activity (7d CTG, RKO) 33 -3 -2 -1 0 G2M Checkpoint E2F Targets MYC Targets RKO Super Enhancer genes NES (Normalized Enrichment Score) DM SO 50 nM P ac lit ax el DM SO EC20 EC50 EC90 0 50 100 % c el ls G1 S G2 /M 0. 00 00 1 0. 00 01 0. 00 1 0. 01 0. 1 1 10 10 0 10 00 % C el l S u rv iv al EC20 = 0.014nM EC50 = 0.15nM ~EC90 = 20nM CBPd-41 treated RKO (24h) CBPd-41 treated RKO (24/72h) 24H 72H CBPd-41 treated RKO (7d)
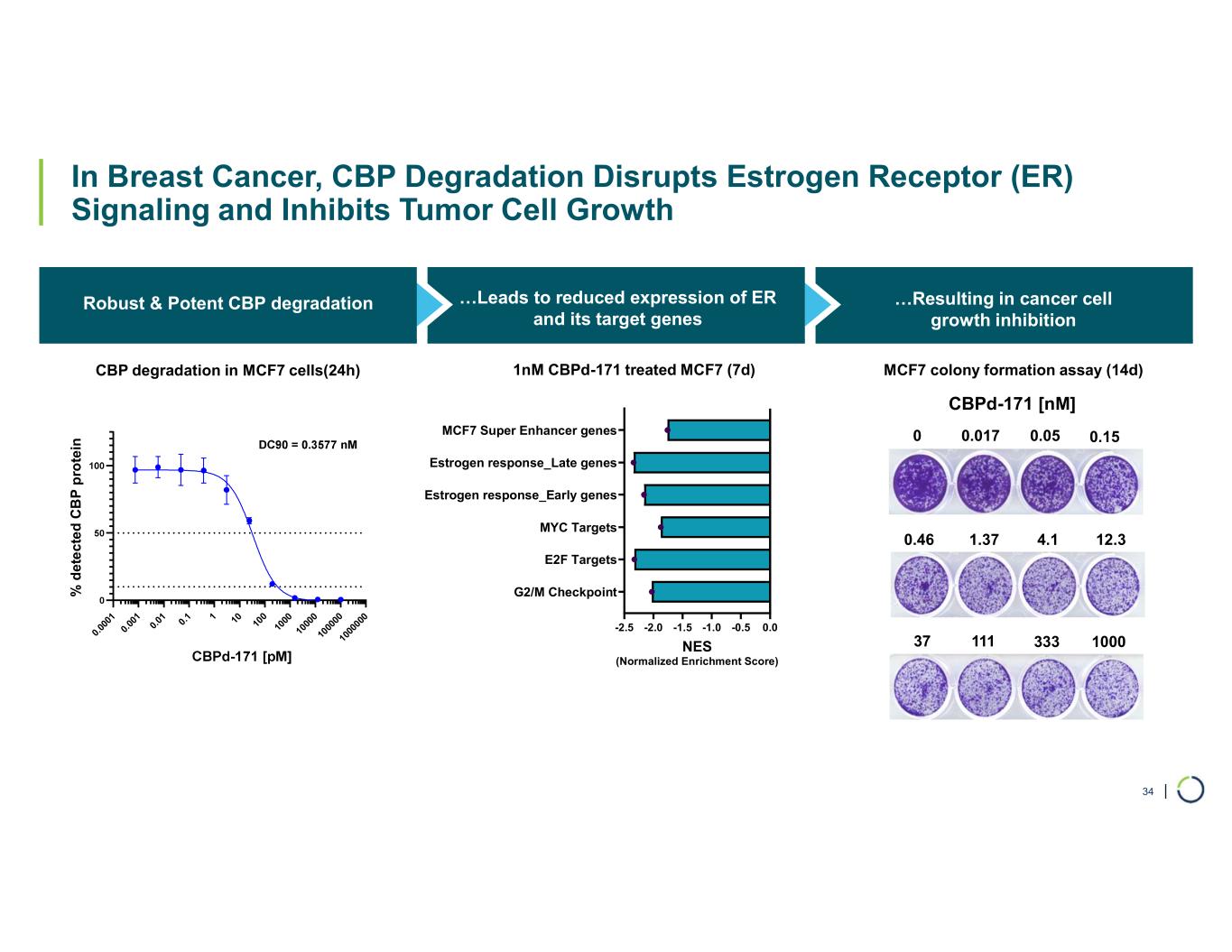
| In Breast Cancer, CBP Degradation Disrupts Estrogen Receptor (ER) Signaling and Inhibits Tumor Cell Growth CBP Robust & Potent CBP degradation …Resulting in cancer cell growth inhibition 34 0. 00 01 0. 00 1 0. 01 0. 1 1 10 10 0 10 00 10 00 0 10 00 00 10 00 00 0 % d e te c te d C B P p ro te in CBP degradation in MCF7 cells(24h) …Leads to reduced expression of ER and its target genes MCF7 colony formation assay (14d) 33311137 12.34.11.370.46 0.150.050.017 1000 0 CBPd-171 [nM] -2.5 -2.0 -1.5 -1.0 -0.5 0.0 G2/M Checkpoint E2F Targets MYC Targets Estrogen response_Early genes Estrogen response_Late genes MCF7 Super Enhancer genes NES (Normalized Enrichment Score) 1nM CBPd-171 treated MCF7 (7d)
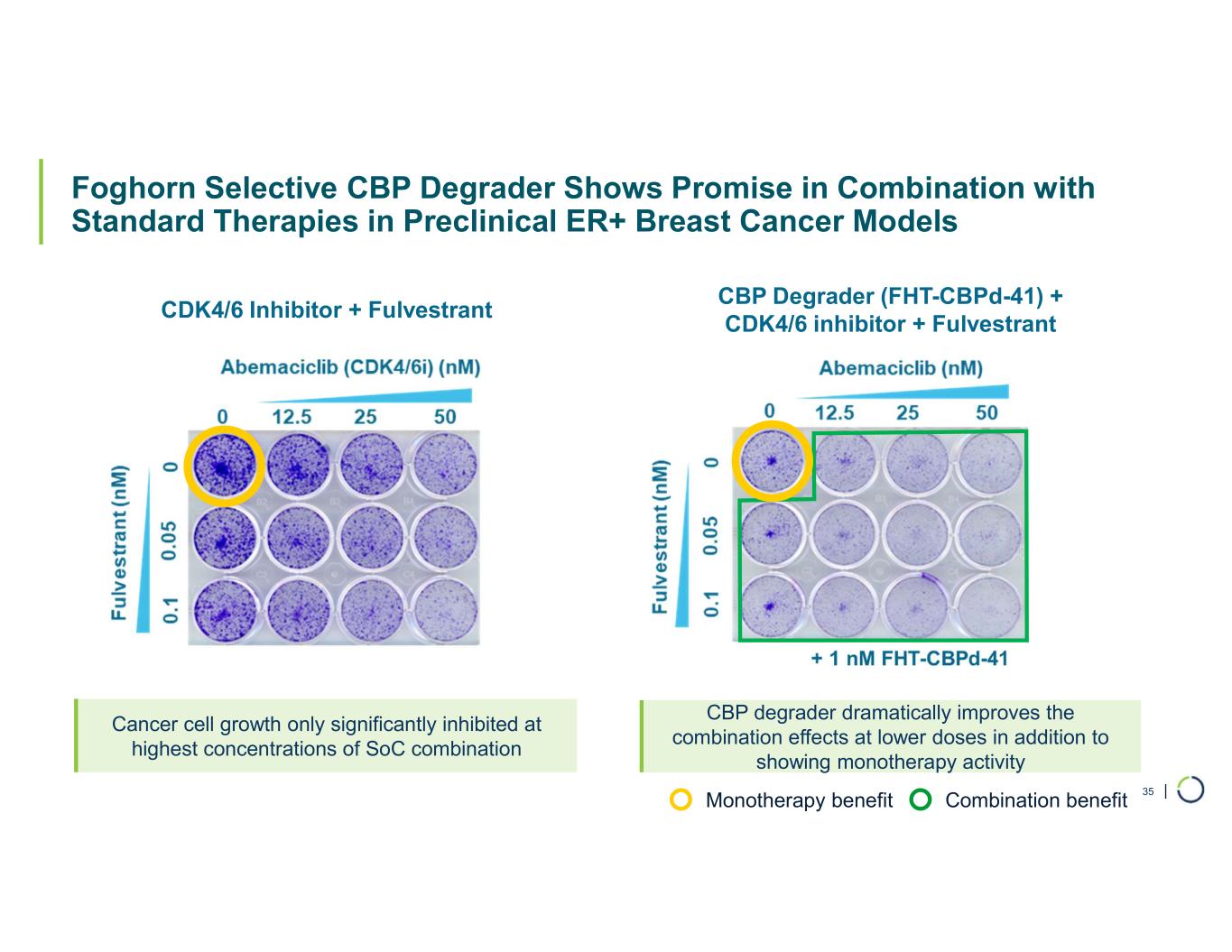
| Foghorn Selective CBP Degrader Shows Promise in Combination with Standard Therapies in Preclinical ER+ Breast Cancer Models 35 CDK4/6 Inhibitor + Fulvestrant CBP Degrader (FHT-CBPd-41) + CDK4/6 inhibitor + Fulvestrant Cancer cell growth only significantly inhibited at highest concentrations of SoC combination CBP degrader dramatically improves the combination effects at lower doses in addition to showing monotherapy activity Monotherapy benefit Combination benefit
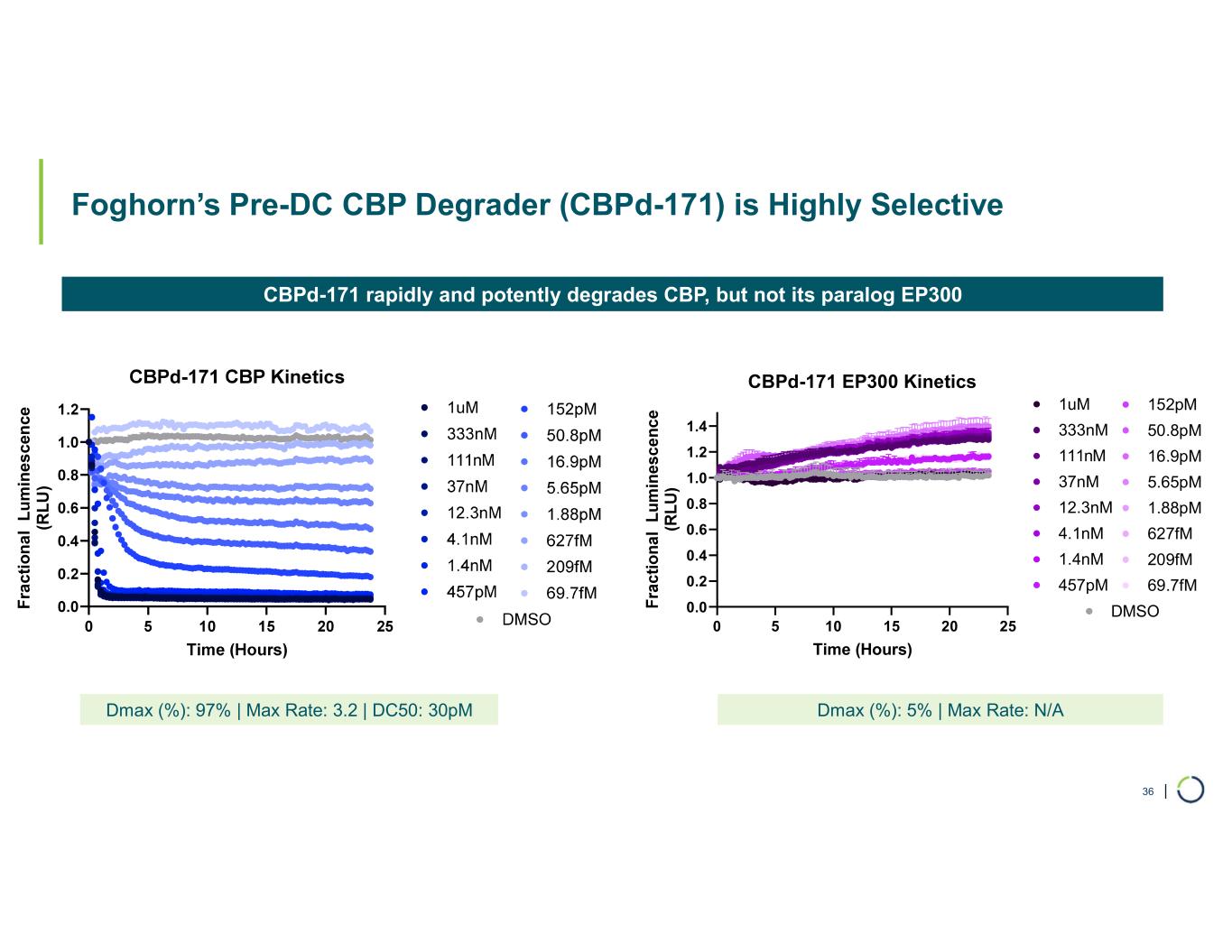
| Foghorn’s Pre-DC CBP Degrader (CBPd-171) is Highly Selective 36 CBPd-171 rapidly and potently degrades CBP, but not its paralog EP300 Dmax (%): 97% | Max Rate: 3.2 | DC50: 30pM Dmax (%): 5% | Max Rate: N/A F ra c ti o n a l L u m in e s c en ce (R L U ) 0 5 10 15 20 25 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 CBPd-171 EP300 Kinetics Time (Hours) DMSO 1uM 333nM 111nM 37nM 12.3nM 4.1nM 1.4nM 457pM 152pM 50.8pM 16.9pM 5.65pM 1.88pM 627fM 209fM 69.7fM
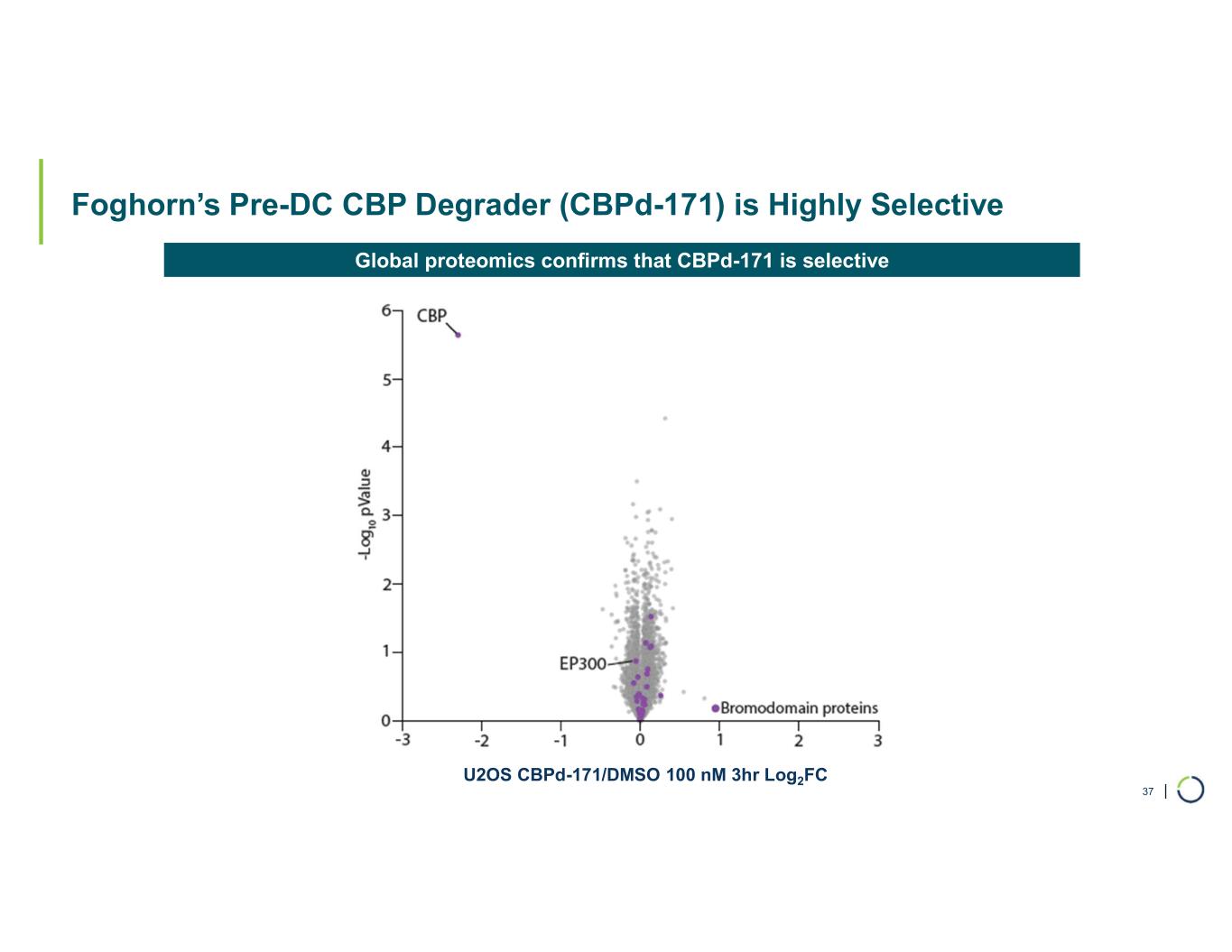
| Foghorn’s Pre-DC CBP Degrader (CBPd-171) is Highly Selective 37 U2OS CBPd-171/DMSO 100 nM 3hr Log2FC Global proteomics confirms that CBPd-171 is selective
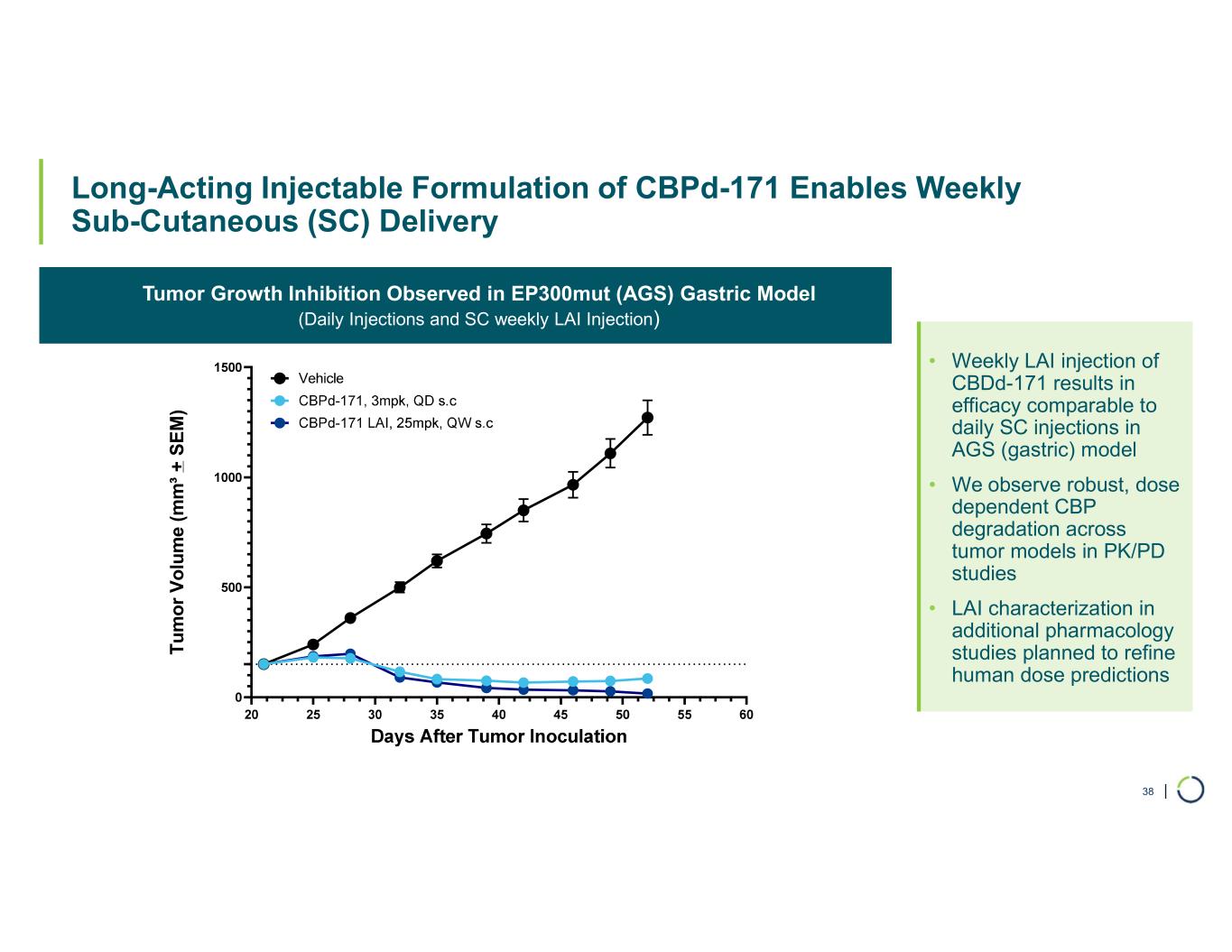
| Long-Acting Injectable Formulation of CBPd-171 Enables Weekly Sub-Cutaneous (SC) Delivery 38 Tumor Growth Inhibition Observed in EP300mut (AGS) Gastric Model (Daily Injections and SC weekly LAI Injection) • Weekly LAI injection of CBDd-171 results in efficacy comparable to daily SC injections in AGS (gastric) model • We observe robust, dose dependent CBP degradation across tumor models in PK/PD studies • LAI characterization in additional pharmacology studies planned to refine human dose predictions T u m o r V o lu m e (m m ³ + S E M )

Alfonso Quintás-Cardama, MD Chief Medical Officer Selective EP300 Program • VHL based selective degrader shows impressive efficacy in multiple myeloma without thrombocytopenia • EP300 degraders show full efficacy in IMiD resistant cell lines • Collection of advanced leads evaluated for progression into toxicology studies • IND-enabling studies in 2026 Steve Bellon, PhD Chief Scientific Officer
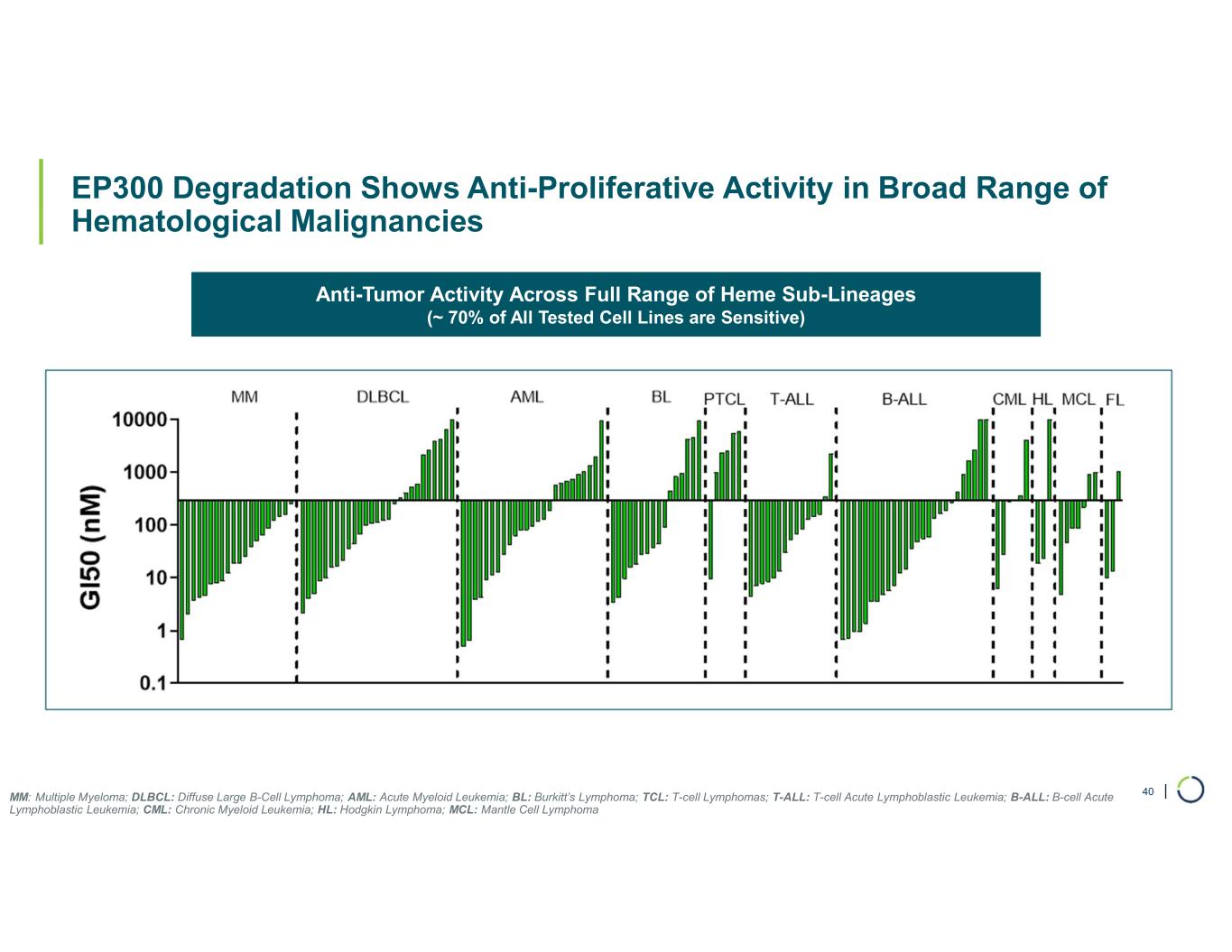
| EP300 Degradation Shows Anti-Proliferative Activity in Broad Range of Hematological Malignancies 40MM: Multiple Myeloma; DLBCL: Diffuse Large B-Cell Lymphoma; AML: Acute Myeloid Leukemia; BL: Burkitt’s Lymphoma; TCL: T-cell Lymphomas; T-ALL: T-cell Acute Lymphoblastic Leukemia; B-ALL: B-cell Acute Lymphoblastic Leukemia; CML: Chronic Myeloid Leukemia; HL: Hodgkin Lymphoma; MCL: Mantle Cell Lymphoma Anti-Tumor Activity Across Full Range of Heme Sub-Lineages (~ 70% of All Tested Cell Lines are Sensitive)
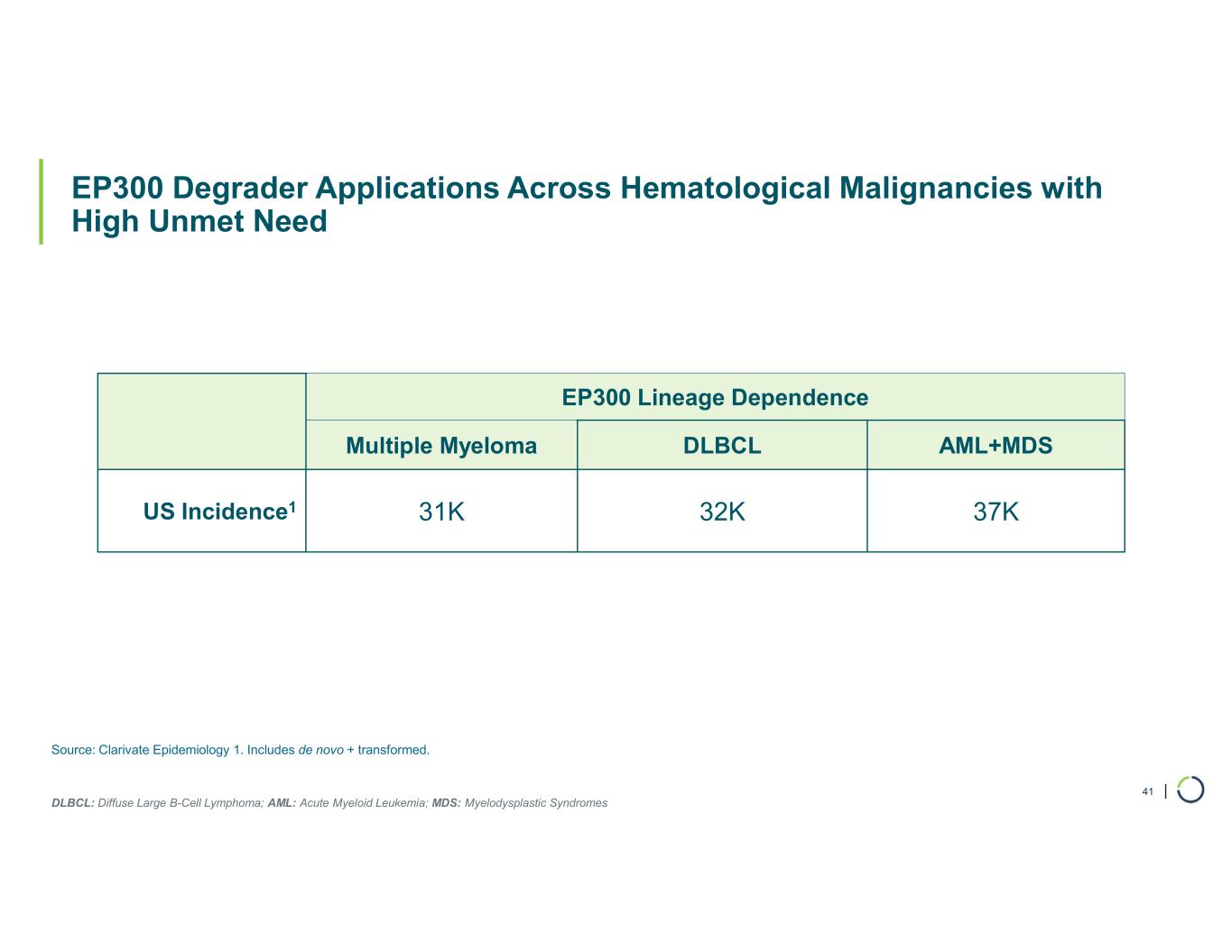
| EP300 Degrader Applications Across Hematological Malignancies with High Unmet Need 41 Source: Clarivate Epidemiology 1. Includes de novo + transformed. EP300 Lineage Dependence AML+MDSDLBCLMultiple Myeloma 37K32K31KUS Incidence1 DLBCL: Diffuse Large B-Cell Lymphoma; AML: Acute Myeloid Leukemia; MDS: Myelodysplastic Syndromes
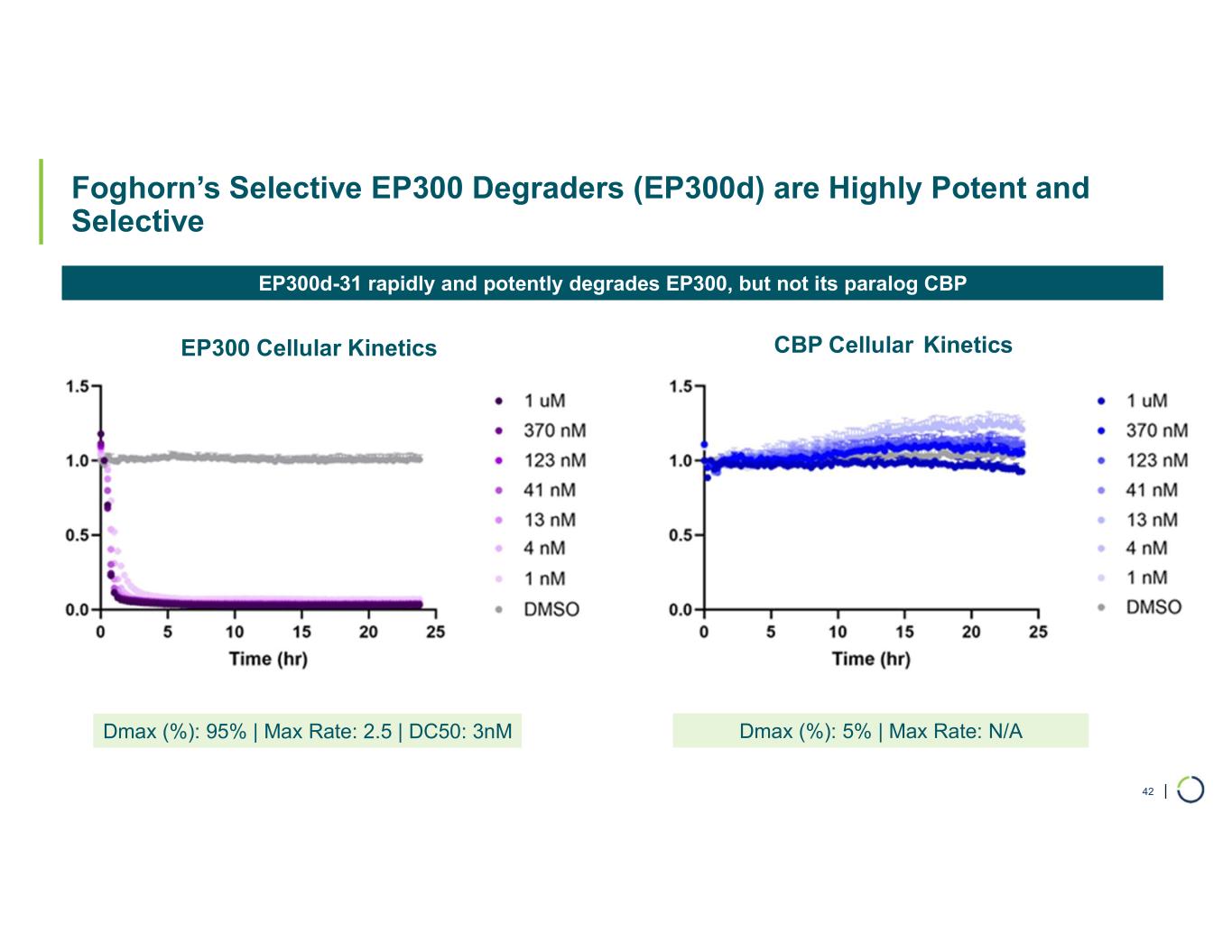
| Foghorn’s Selective EP300 Degraders (EP300d) are Highly Potent and Selective 42 Dmax (%): 95% | Max Rate: 2.5 | DC50: 3nM Dmax (%): 5% | Max Rate: N/A EP300 Cellular Kinetics CBP Cellular Kinetics EP300d-31 rapidly and potently degrades EP300, but not its paralog CBP
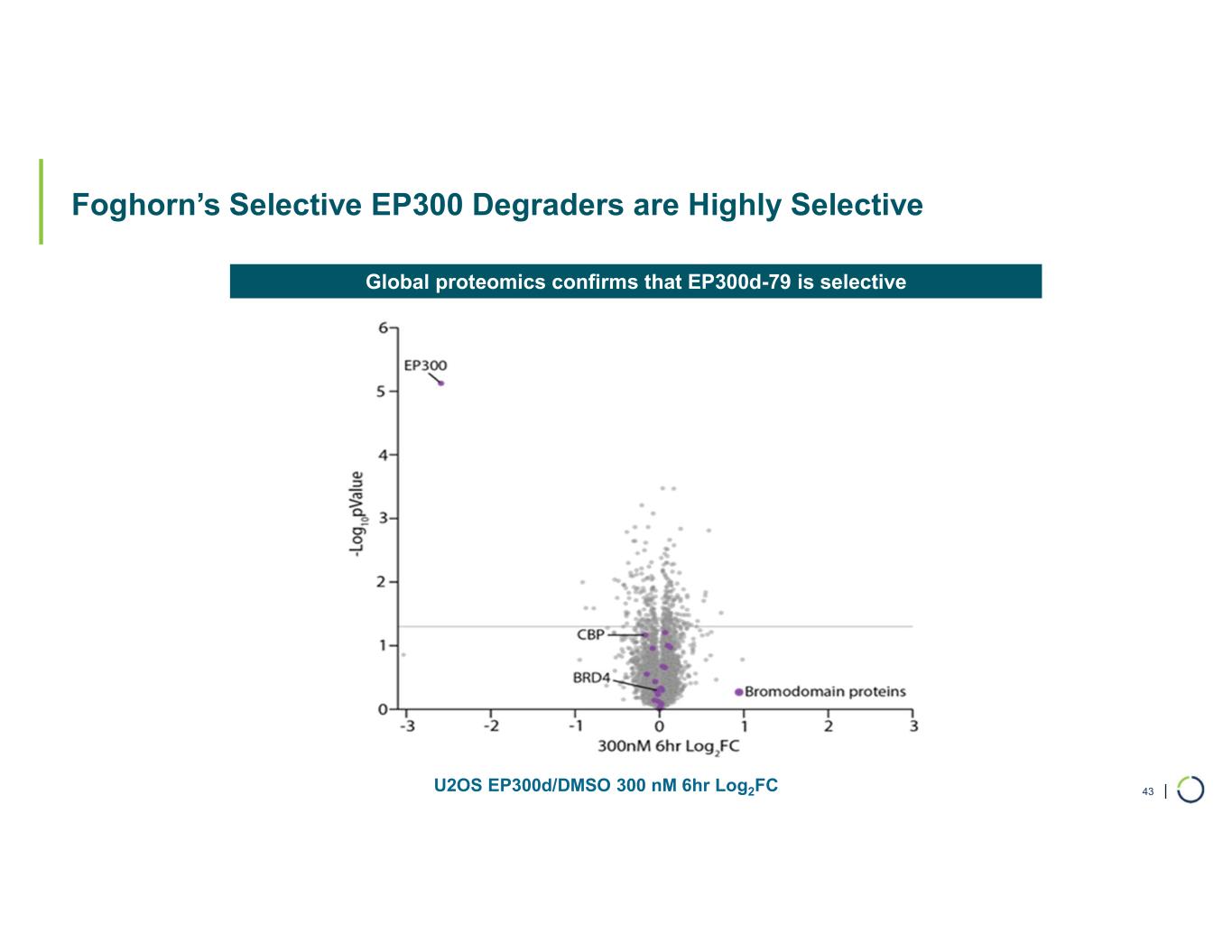
| Foghorn’s Selective EP300 Degraders are Highly Selective 43U2OS EP300d/DMSO 300 nM 6hr Log2FC Global proteomics confirms that EP300d-79 is selective
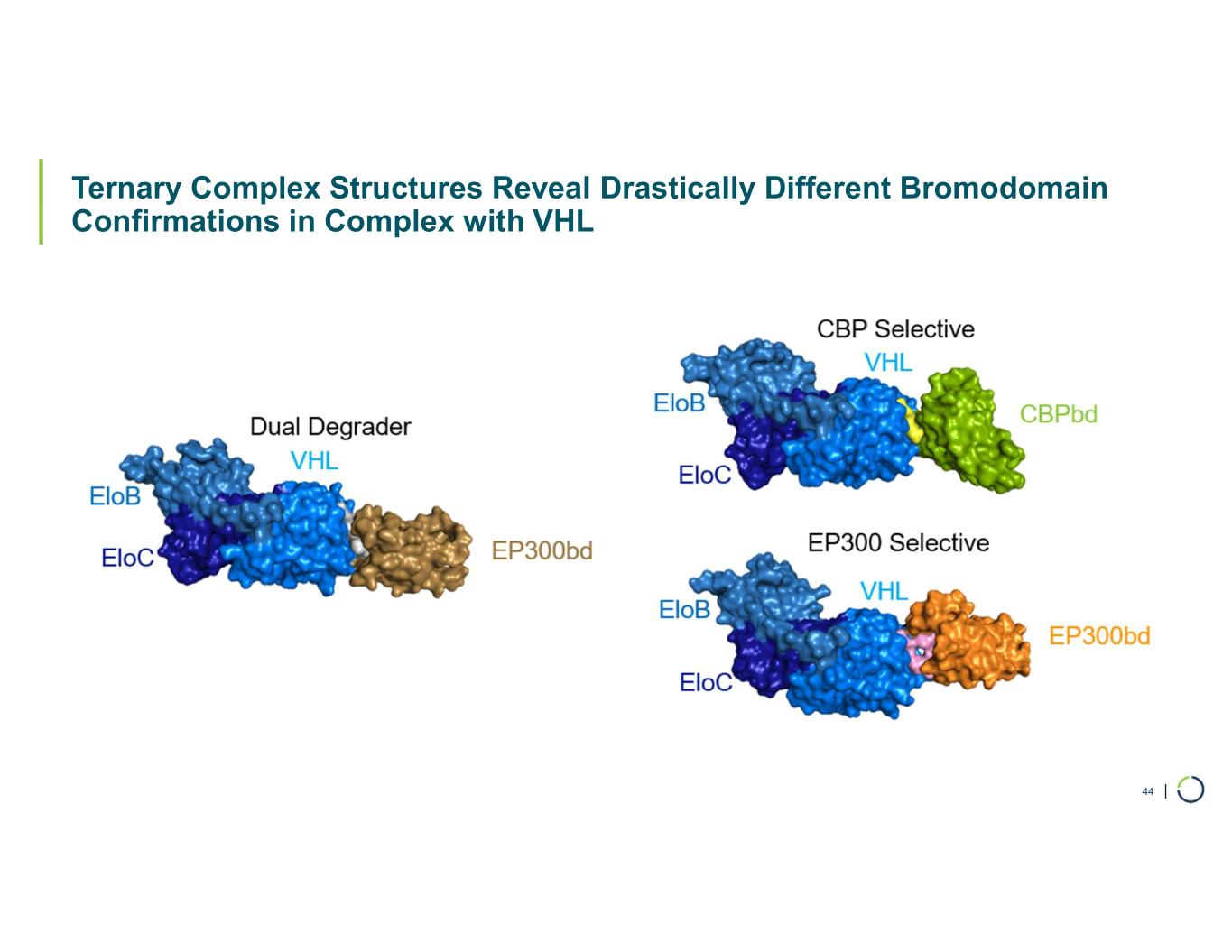
| Ternary Complex Structures Reveal Drastically Different Bromodomain Confirmations in Complex with VHL 44
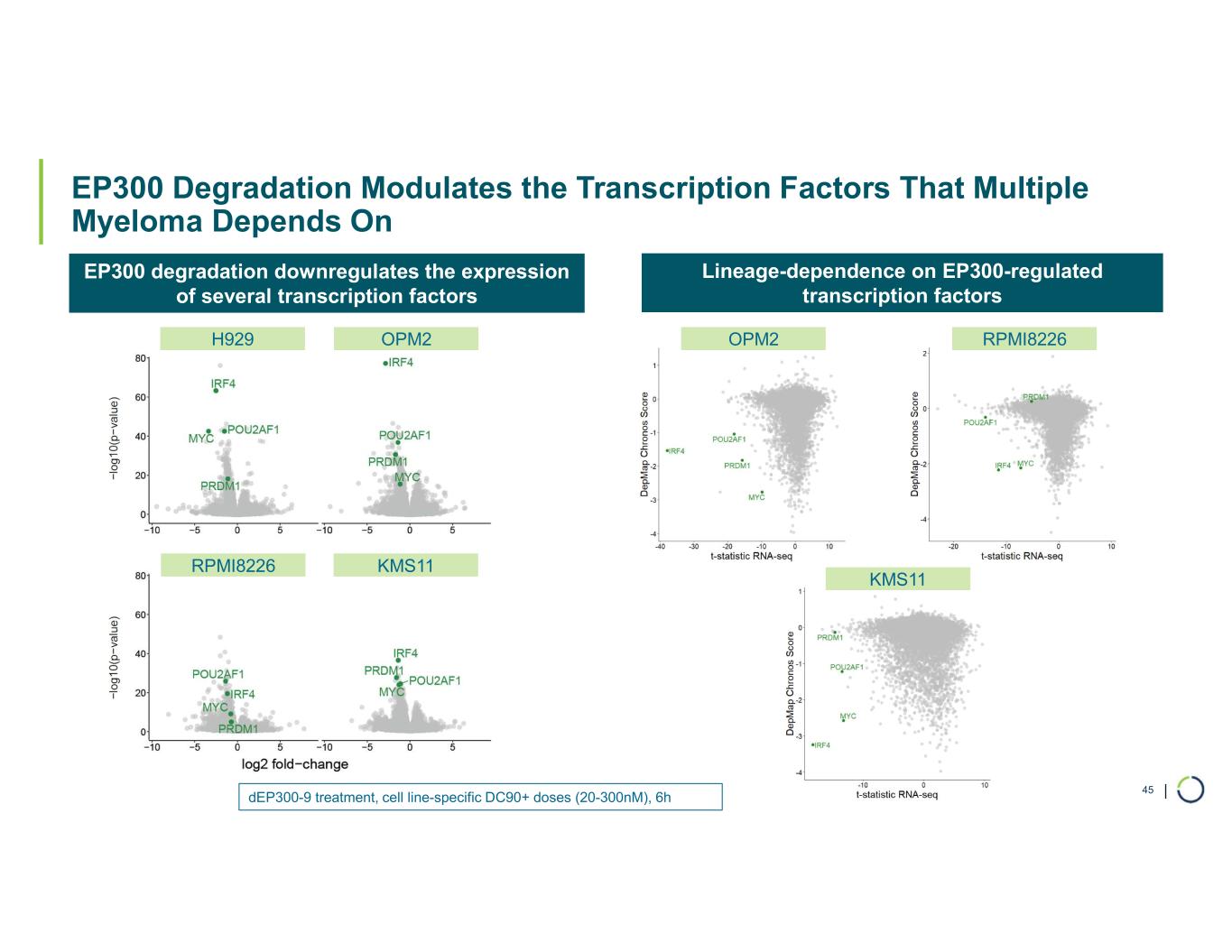
| EP300 Degradation Modulates the Transcription Factors That Multiple Myeloma Depends On 45 EP300 degradation downregulates the expression of several transcription factors H929 OPM2 Lineage-dependence on EP300-regulated transcription factors KMS11RPMI8226 OPM2 RPMI8226 KMS11 dEP300-9 treatment, cell line-specific DC90+ doses (20-300nM), 6h
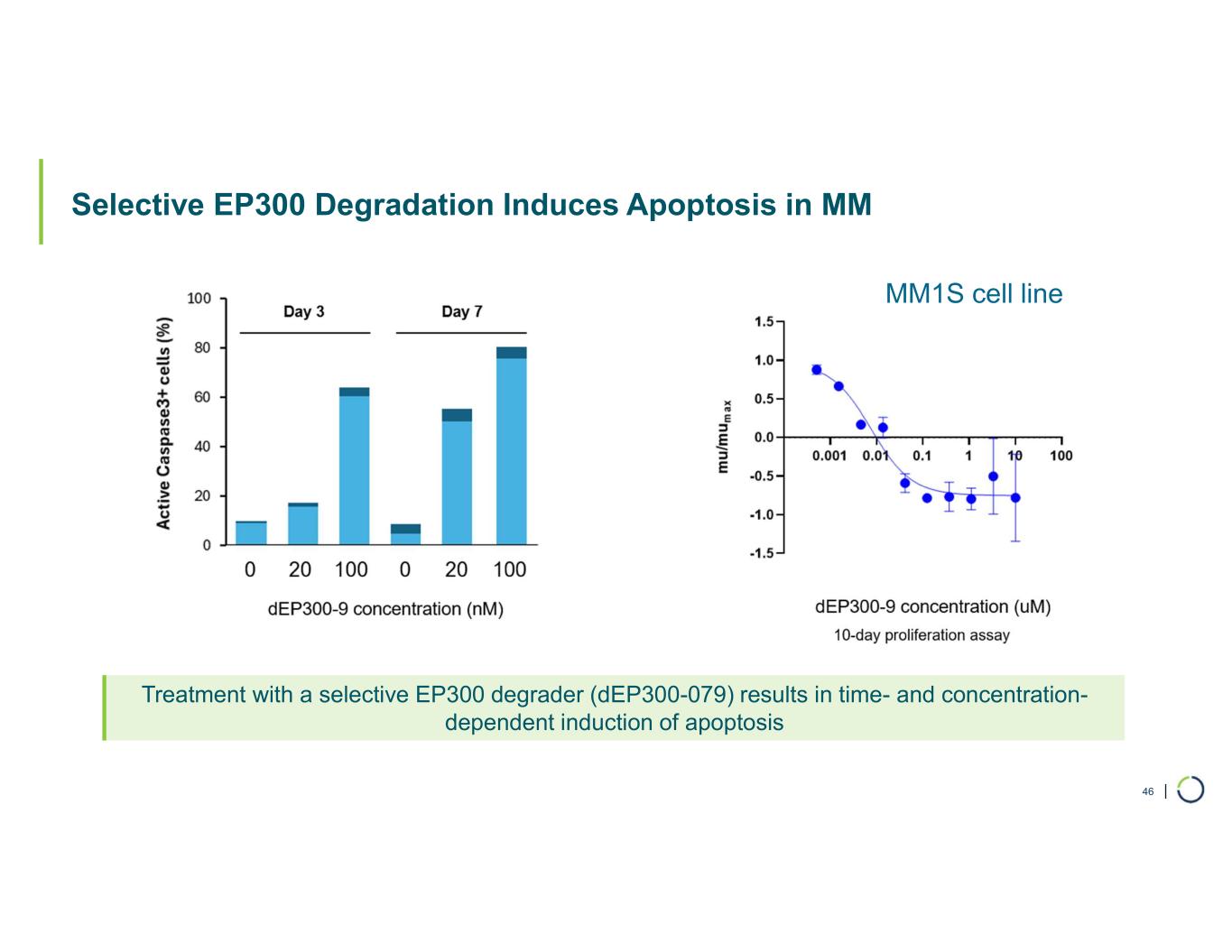
| Selective EP300 Degradation Induces Apoptosis in MM 46 Treatment with a selective EP300 degrader (dEP300-079) results in time- and concentration- dependent induction of apoptosis MM1S cell line
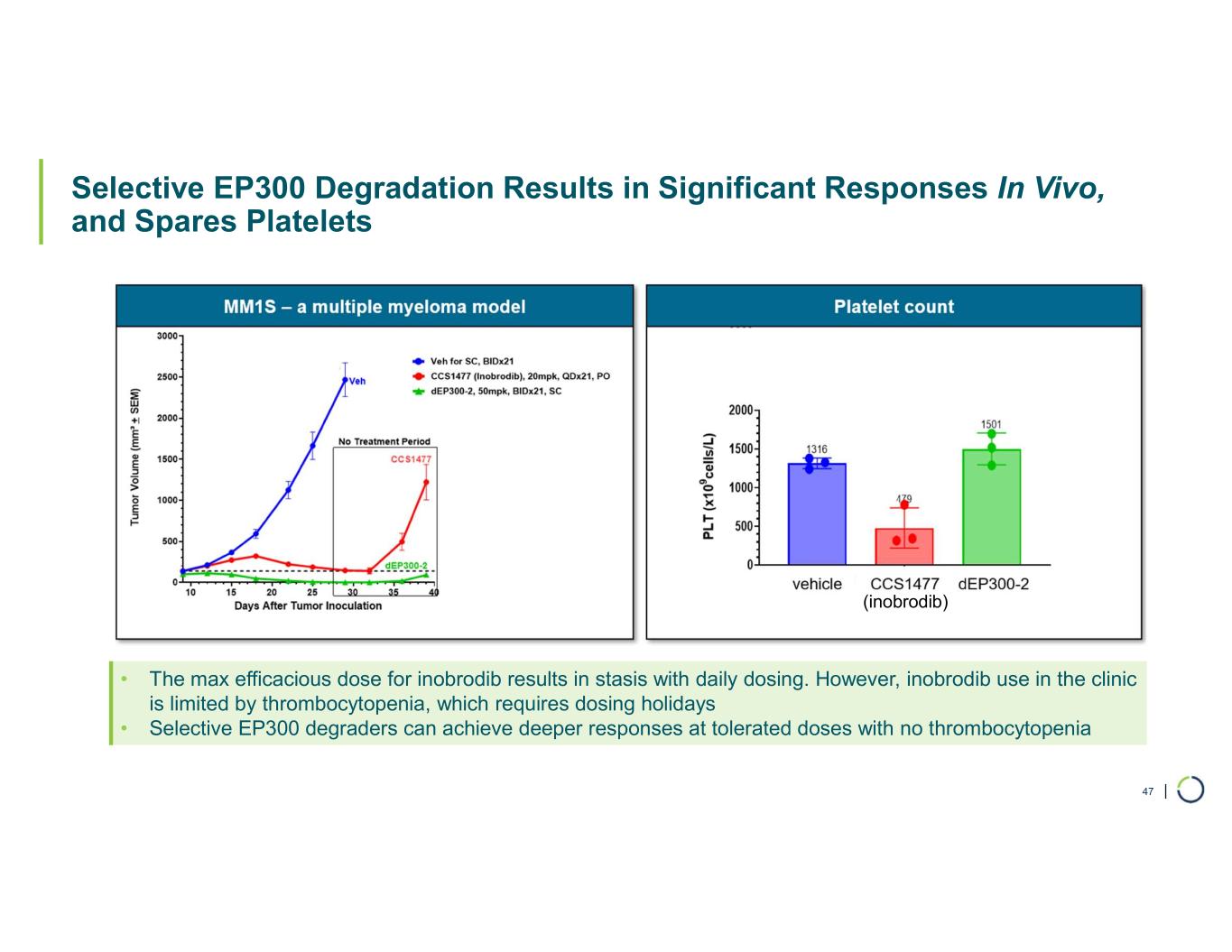
| Selective EP300 Degradation Results in Significant Responses In Vivo, and Spares Platelets 47 • The max efficacious dose for inobrodib results in stasis with daily dosing. However, inobrodib use in the clinic is limited by thrombocytopenia, which requires dosing holidays • Selective EP300 degraders can achieve deeper responses at tolerated doses with no thrombocytopenia (inobrodib)
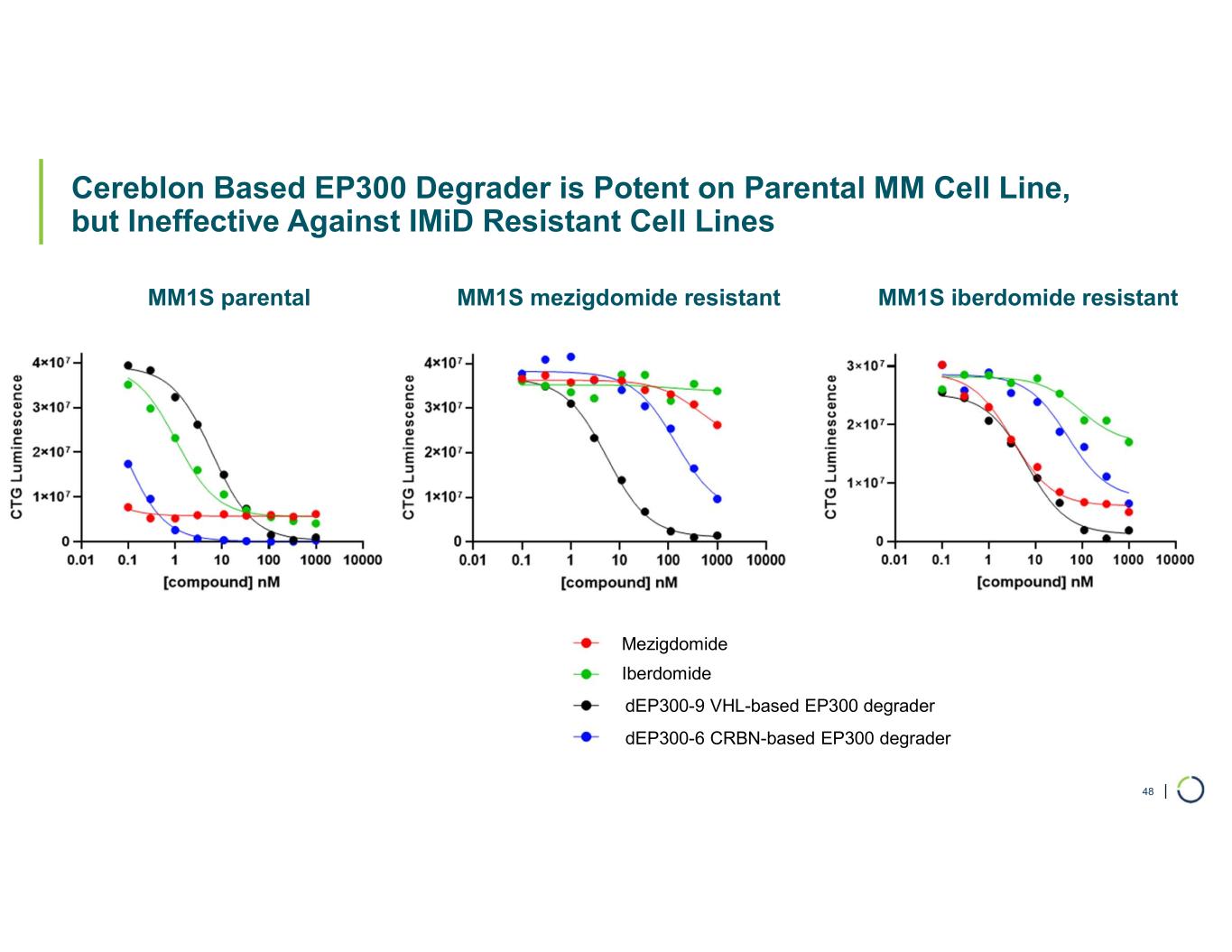
| Cereblon Based EP300 Degrader is Potent on Parental MM Cell Line, but Ineffective Against IMiD Resistant Cell Lines 48 dEP300-9 VHL-based EP300 degrader dEP300-6 CRBN-based EP300 degrader MM1S parental MM1S mezigdomide resistant MM1S iberdomide resistant Iberdomide Mezigdomide
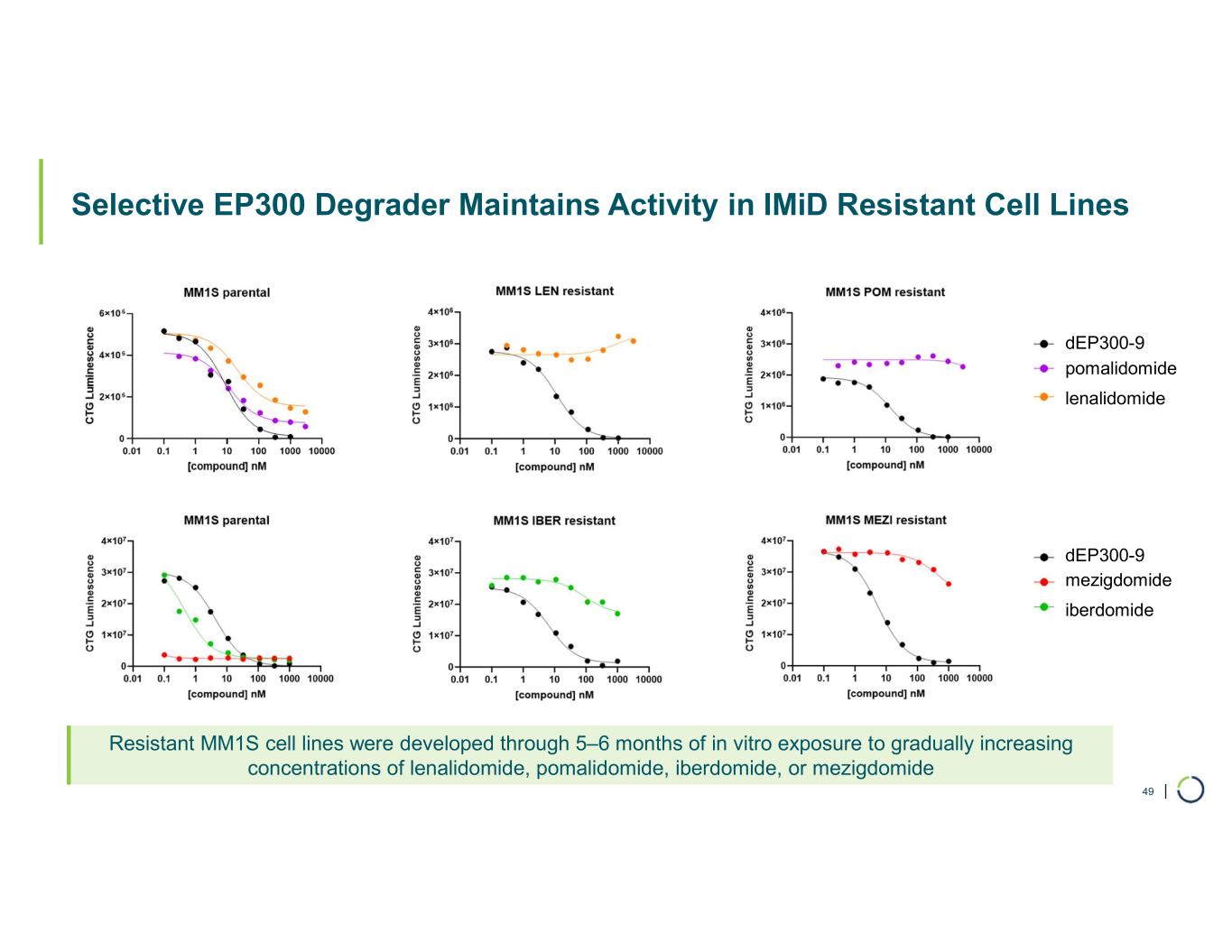
| Selective EP300 Degrader Maintains Activity in IMiD Resistant Cell Lines 49 Resistant MM1S cell lines were developed through 5–6 months of in vitro exposure to gradually increasing concentrations of lenalidomide, pomalidomide, iberdomide, or mezigdomide dEP300-9 dEP300-9 pomalidomide lenalidomide mezigdomide iberdomide
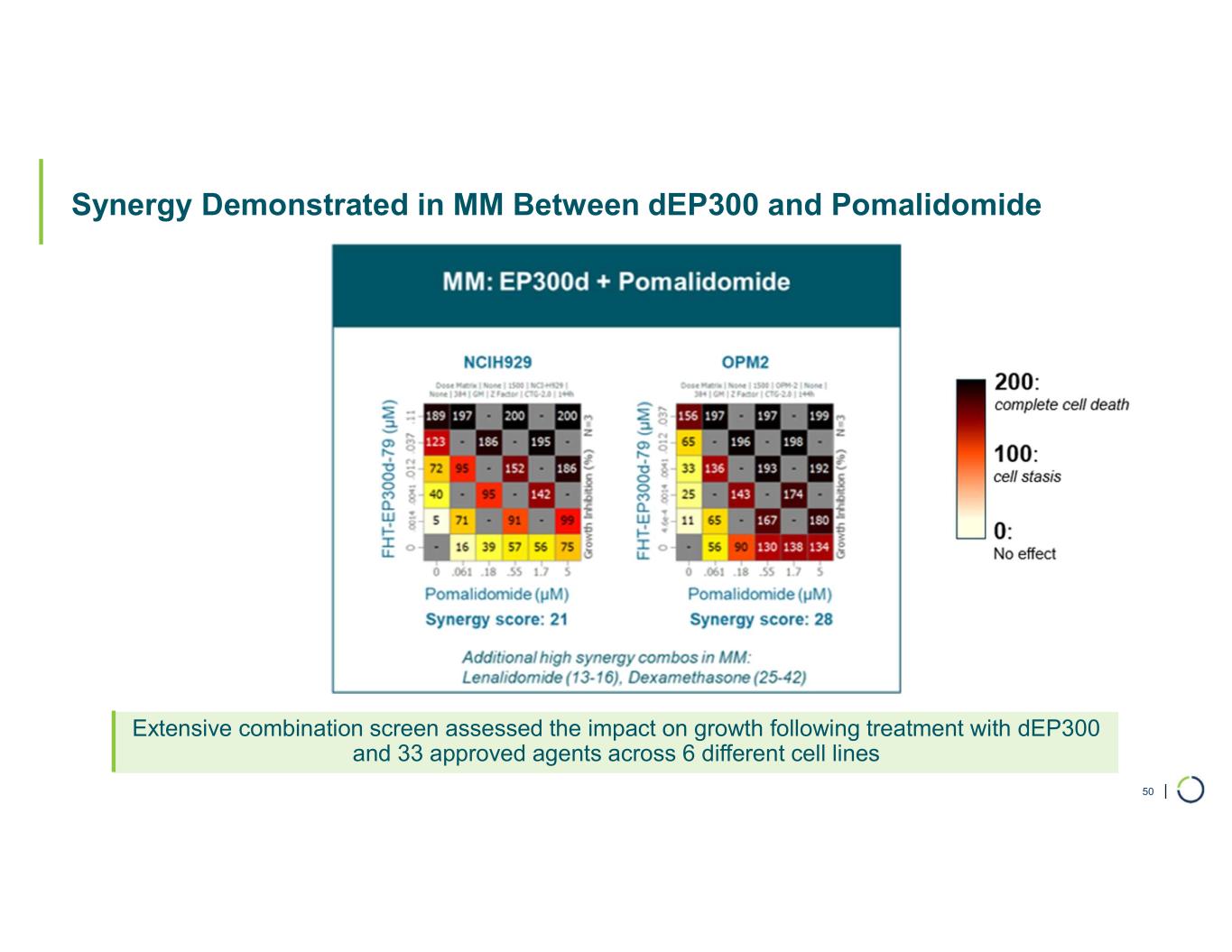
| Synergy Demonstrated in MM Between dEP300 and Pomalidomide 50 Extensive combination screen assessed the impact on growth following treatment with dEP300 and 33 approved agents across 6 different cell lines
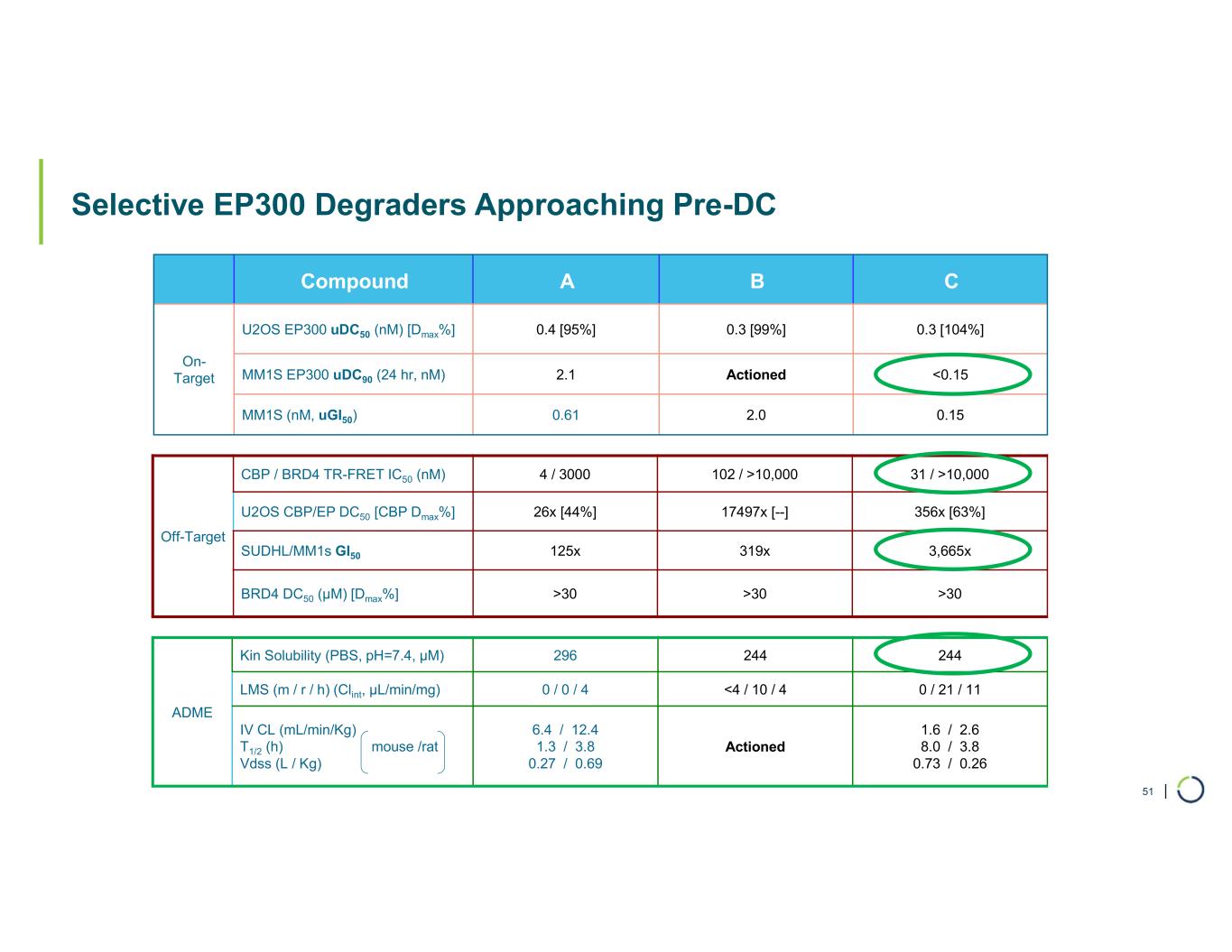
| Selective EP300 Degraders Approaching Pre-DC CBACompound 0.3 [104%]0.3 [99%]0.4 [95%]U2OS EP300 uDC50 (nM) [Dmax%] On- Target <0.15Actioned2.1MM1S EP300 uDC90 (24 hr, nM) 0.152.00.61MM1S (nM, uGI50) 31 / >10,000102 / >10,0004 / 3000CBP / BRD4 TR-FRET IC50 (nM) Off-Target 356x [63%]17497x [--]26x [44%]U2OS CBP/EP DC50 [CBP Dmax%] 3,665x319x125xSUDHL/MM1s GI50 >30>30>30BRD4 DC50 (µM) [Dmax%] 244244296Kin Solubility (PBS, pH=7.4, µM) ADME 0 / 21 / 11<4 / 10 / 40 / 0 / 4LMS (m / r / h) (Clint, µL/min/mg) 1.6 / 2.6 8.0 / 3.8 0.73 / 0.26 Actioned 6.4 / 12.4 1.3 / 3.8 0.27 / 0.69 IV CL (mL/min/Kg) T1/2 (h) mouse /rat Vdss (L / Kg) 51

Closing Remarks Adrian Gottschalk President and CEO

Question & Answers




















































