Document
Exhibit 99.1
C4 Therapeutics Presents Cemsidomide Phase 1 Multiple Myeloma Data Supporting Potential Best-in-Class Profile at the International Myeloma Society Annual Meeting
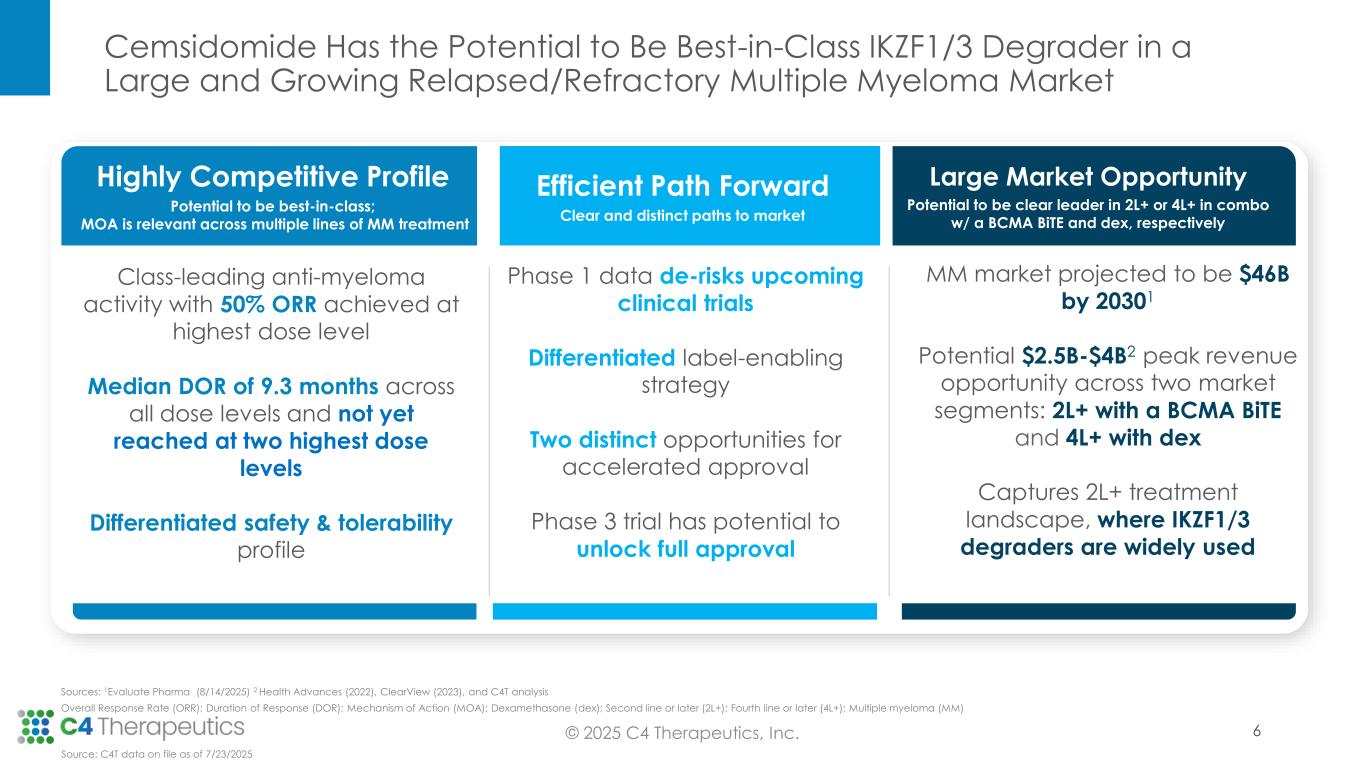
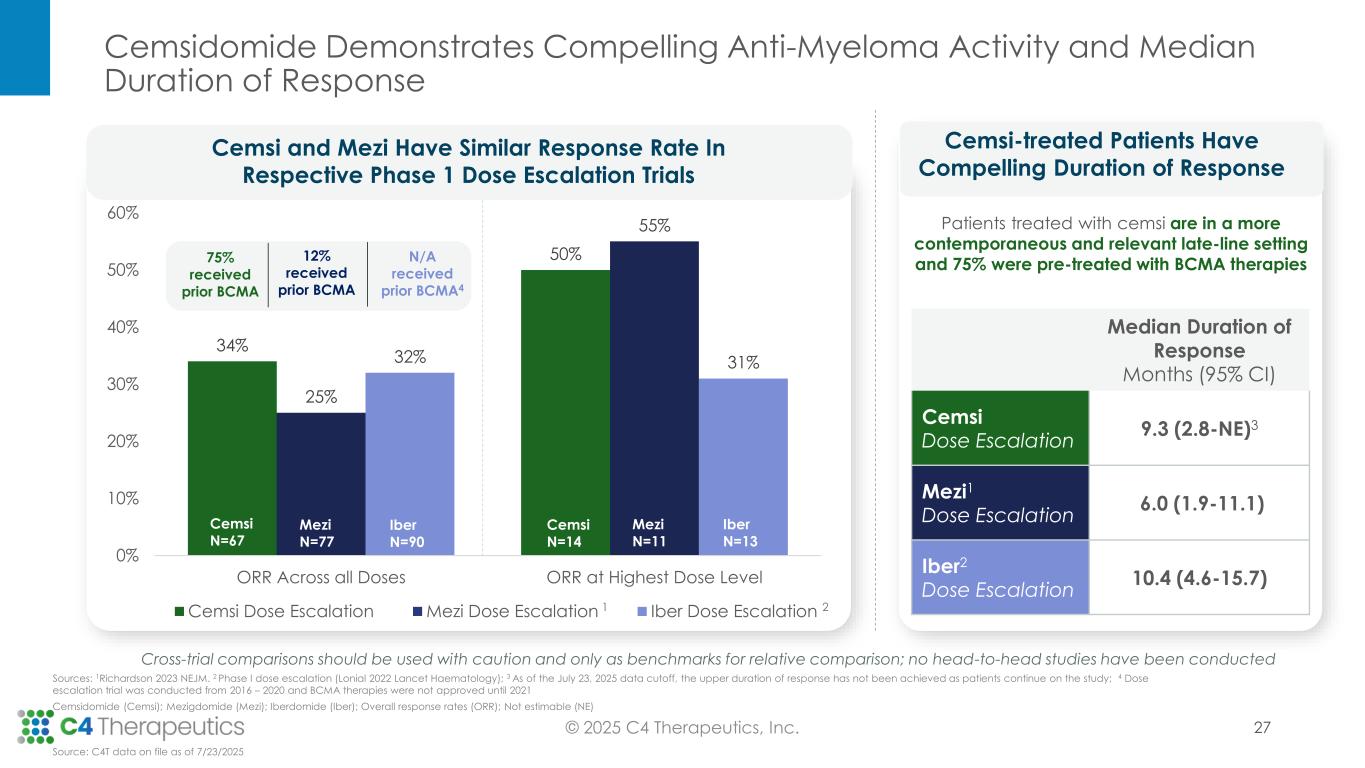
Cemsidomide in Combination With Dexamethasone Achieved a 50% Overall Response Rate (ORR) at the Highest Dose Level (100 µg) and a 40% ORR at the 75 µg Dose Level in a Heavily Pre-Treated Relapsed/Refractory Multiple Myeloma Patient Population
Responses Across Dose Levels With Median Duration of Response of 9.3 Months as of the Data Cut-off Date; Median Duration of Response Not Yet Reached at Two Highest Doses
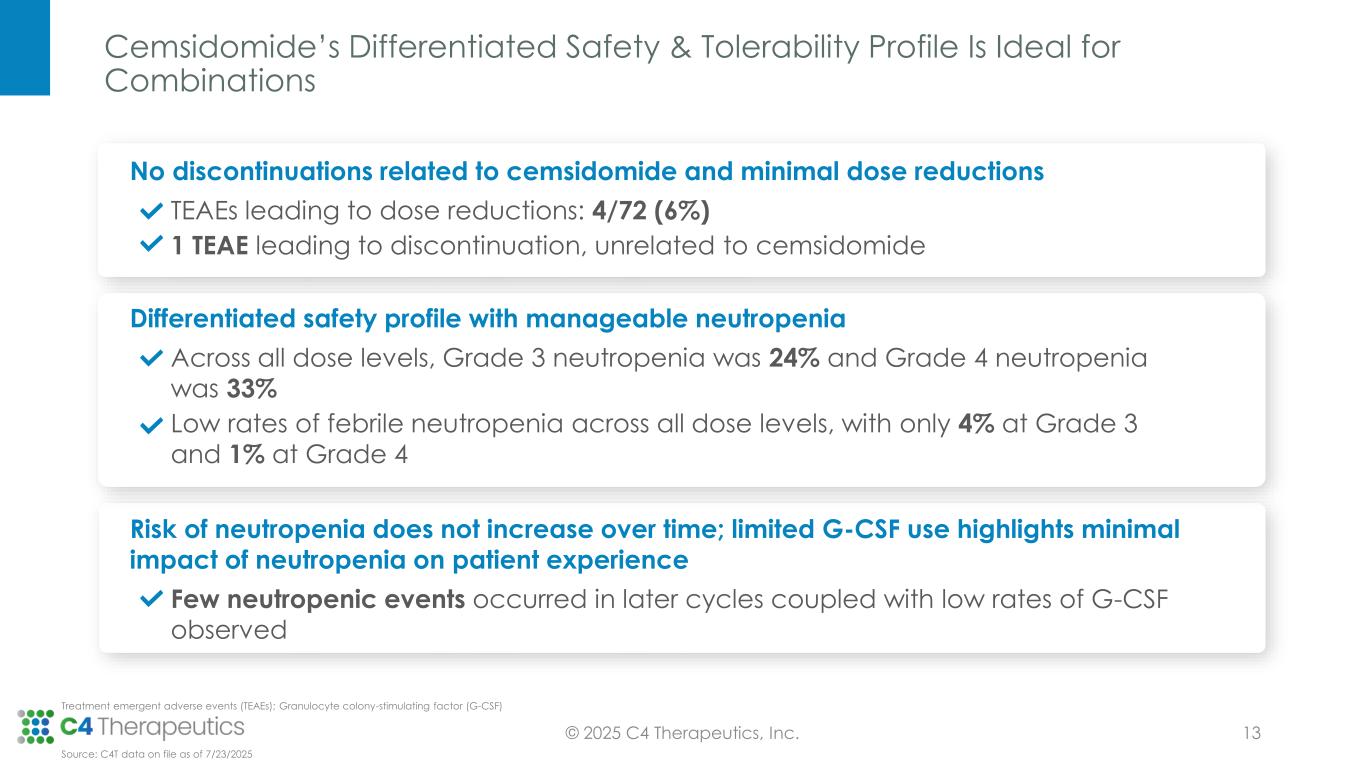
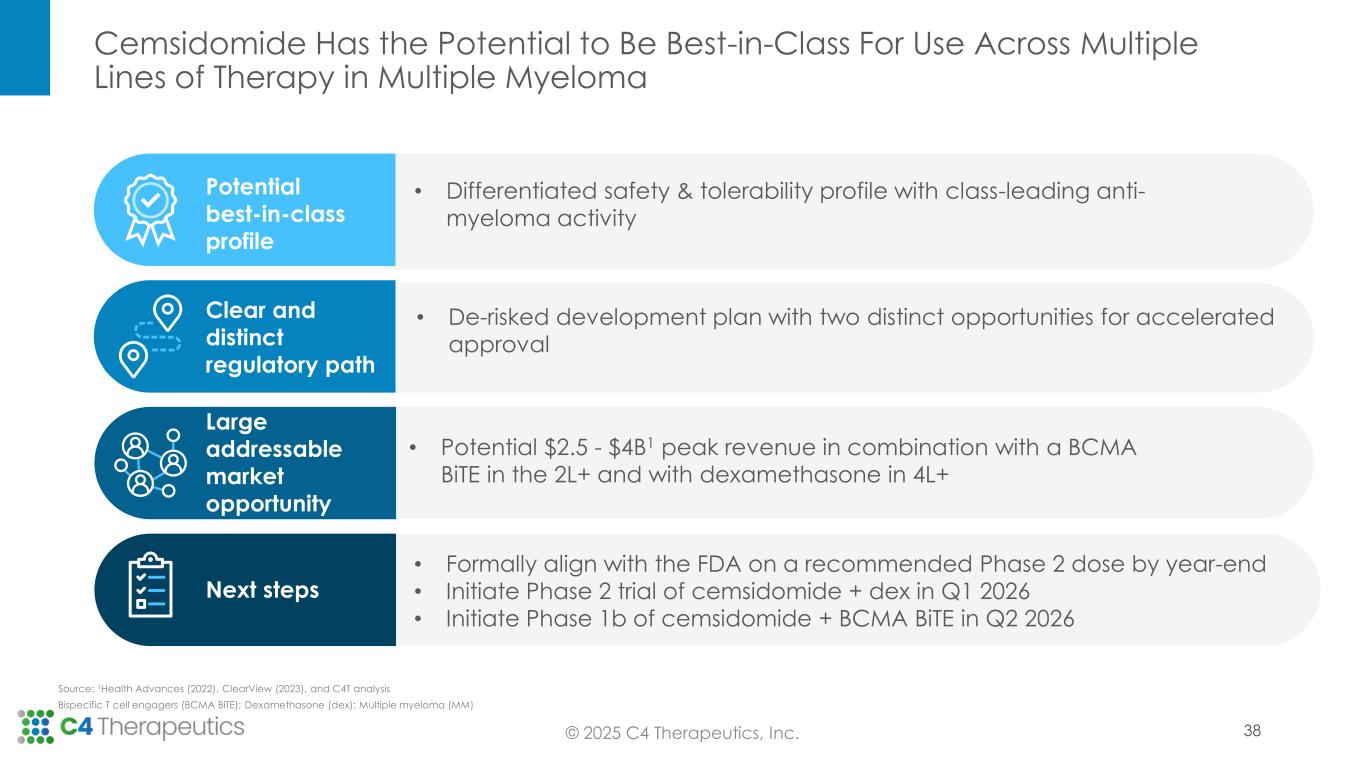
No Discontinuations Related to Cemsidomide and Few Dose Reductions Support a Safety Profile That May Be Ideal for Combination Regimens
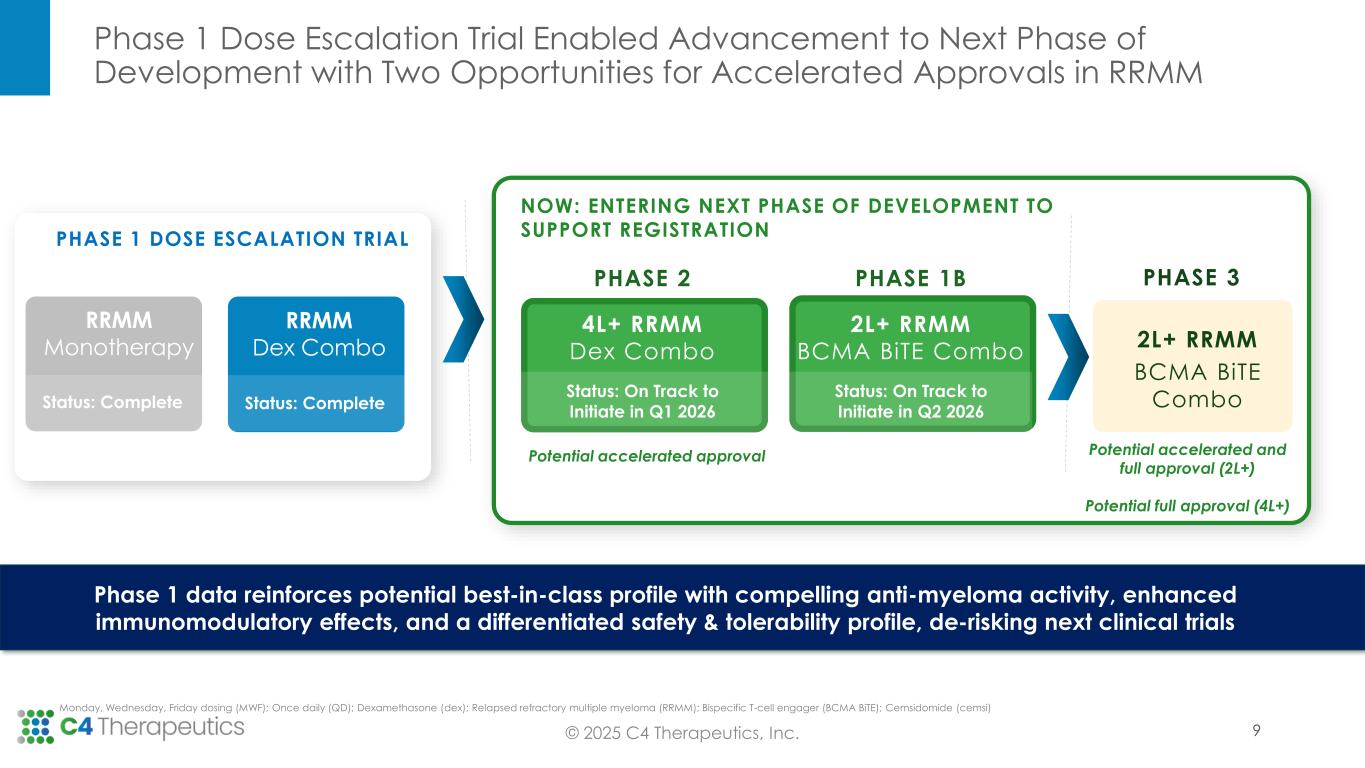
C4T to Pursue Differentiated Development Strategy With Two Distinct Opportunities for Accelerated Approval in Second Line and Later
C4T to Host Webcast Today at 3 pm ET; Webcast Link Available Here
WATERTOWN, Mass., Sept. 20, 2025 (GLOBE NEWSWIRE) -- C4 Therapeutics, Inc. (C4T) (Nasdaq: CCCC), a clinical-stage biopharmaceutical company dedicated to advancing targeted protein degradation science, today presented data from the Phase 1 clinical trial of cemsidomide, an orally bioavailable IKZF1/3 degrader, in combination with dexamethasone for the treatment of relapsed/refractory multiple myeloma (RRMM) in an oral presentation at the International Myeloma Society (IMS) Annual Meeting. With enrollment in the Phase 1 trial complete, data continue to show cemsidomide’s differentiated safety and tolerability profile and potentially class-leading anti-myeloma activity, supporting clear development paths for second line and later patient populations.
“Cemsidomide’s clinical trial results to date have shown compelling anti-myeloma activity, a differentiated safety and tolerability profile and immunomodulatory effects across all dose levels, which have allowed us to create a derisked development plan that we are prepared to rapidly execute to potentially bring cemsidomide to patients, caregivers and hematologist-oncologists,” said Len Reyno, M.D., chief medical officer of C4 Therapeutics. “As we prepare to initiate the Phase 2 study in Q1 2026 to evaluate cemsidomide in combination with dexamethasone and the Phase 1b study in Q2 2026 to evaluate cemsidomide and dexamethasone in combination with a BCMA BiTE—both development pathways that have the potential for accelerated approval—we are excited to further differentiate cemsidomide as the IKZF1/3 degrader of choice among approved medicines in this class, which are used across lines of therapy and in various combination regimens. We look forward to generating data in the future that further demonstrates cemsidomide’s potential to become a class-leading IKZF1/3 degrader across the growing populations of relapsed/refractory multiple myeloma patients.”
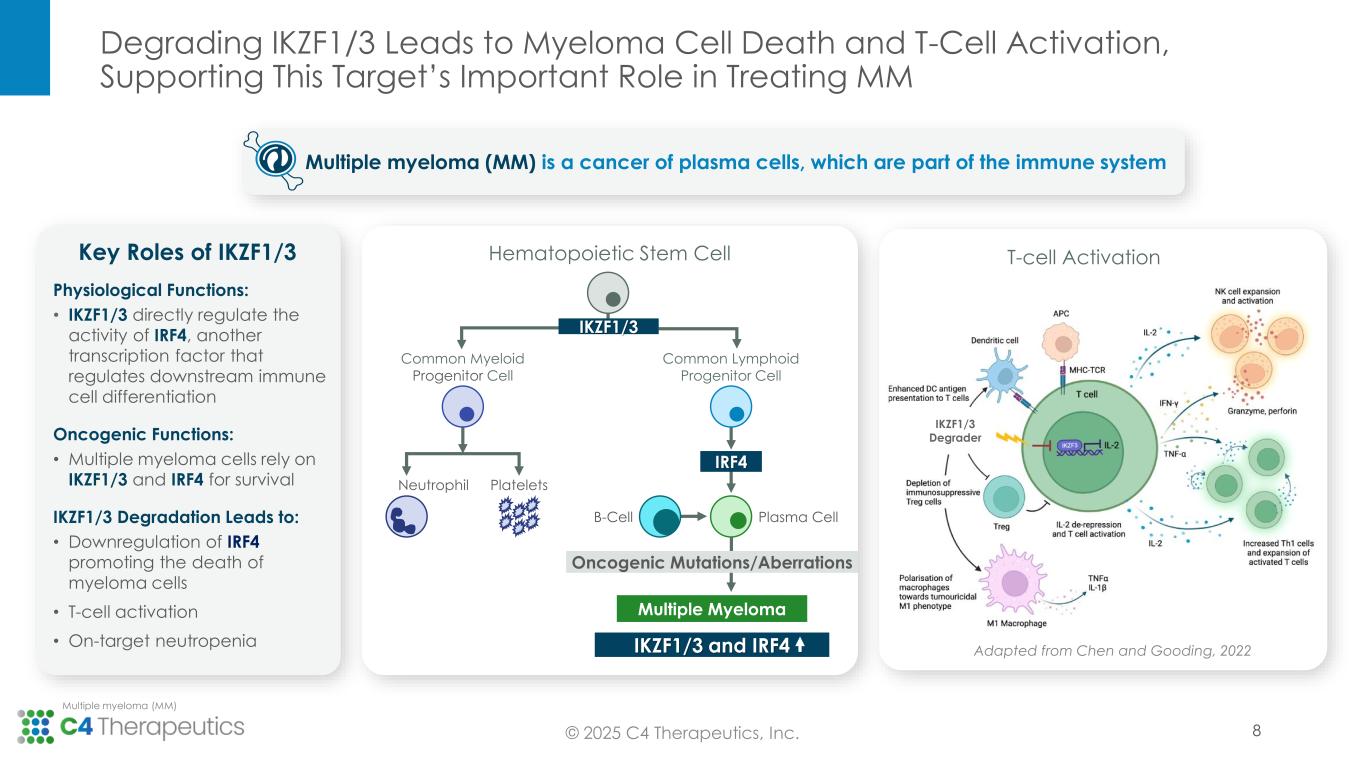
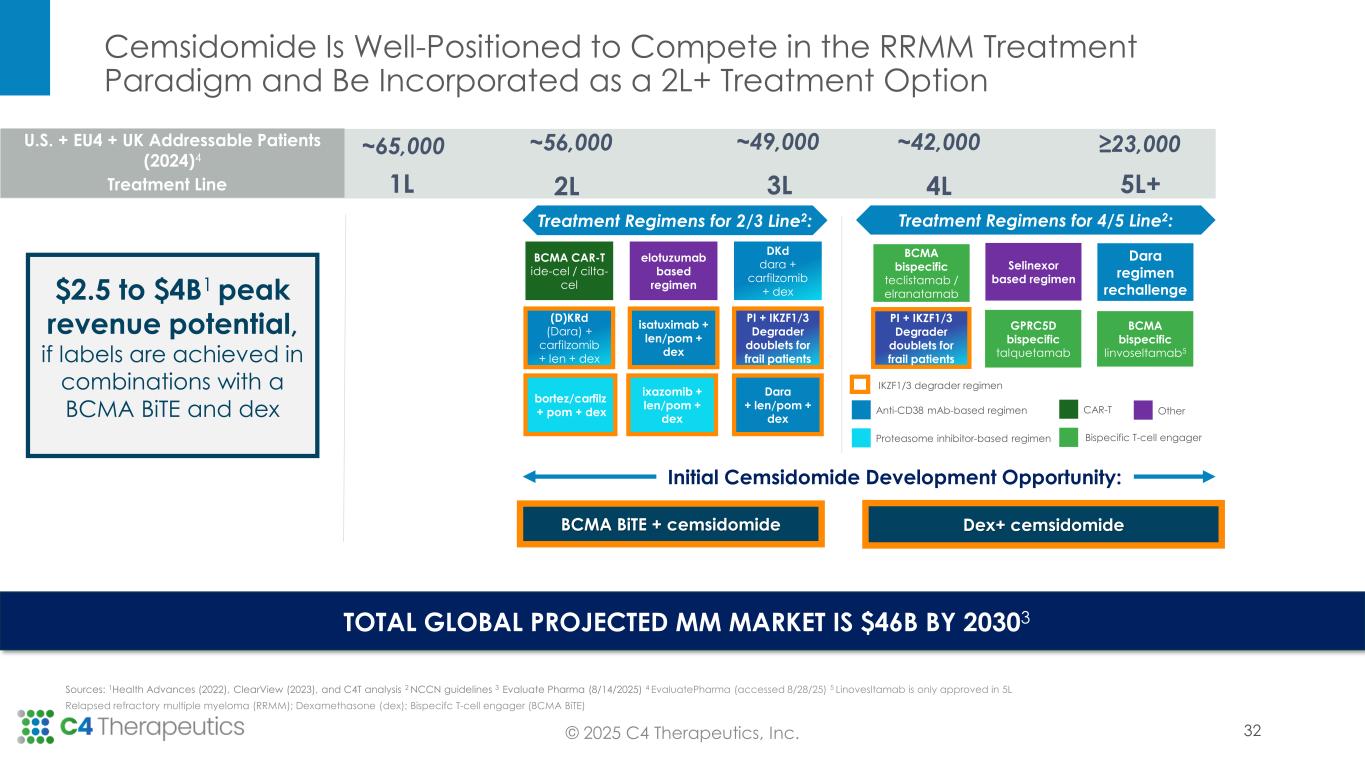
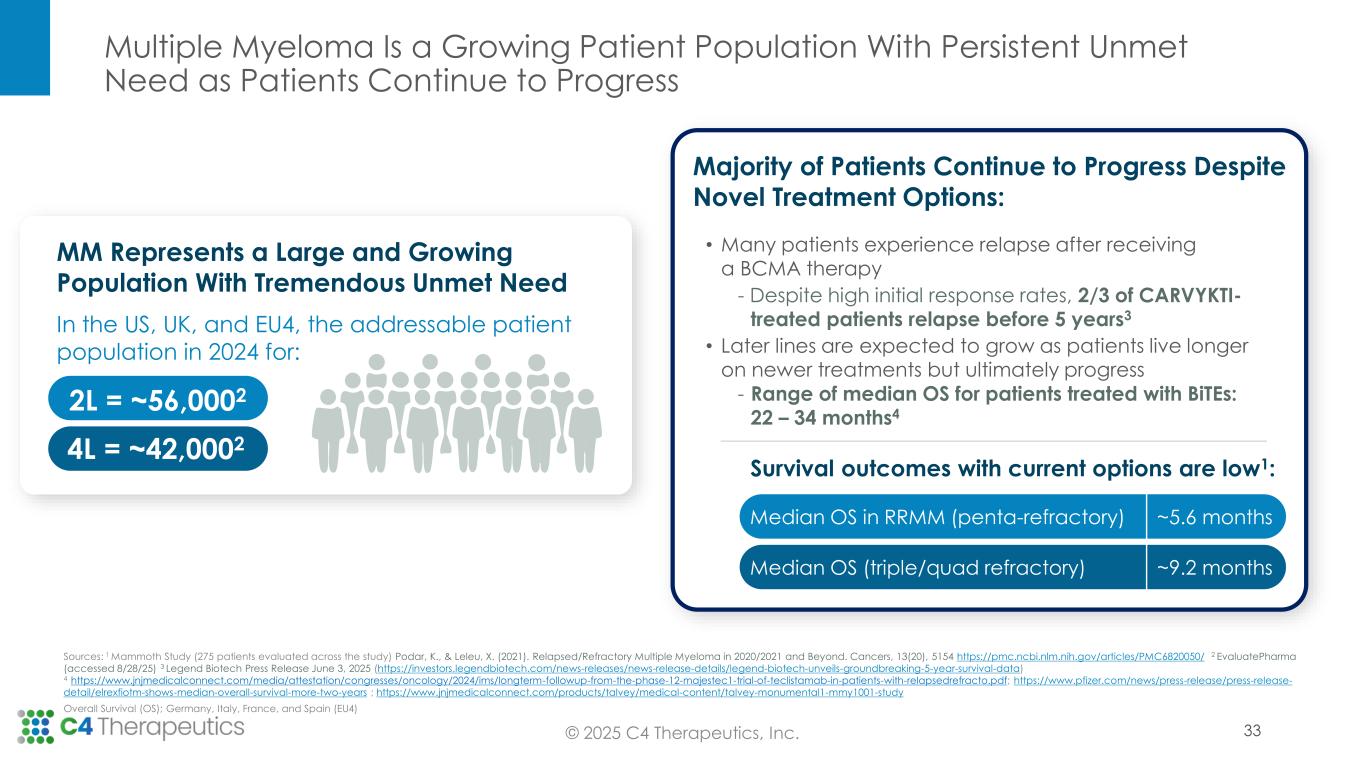
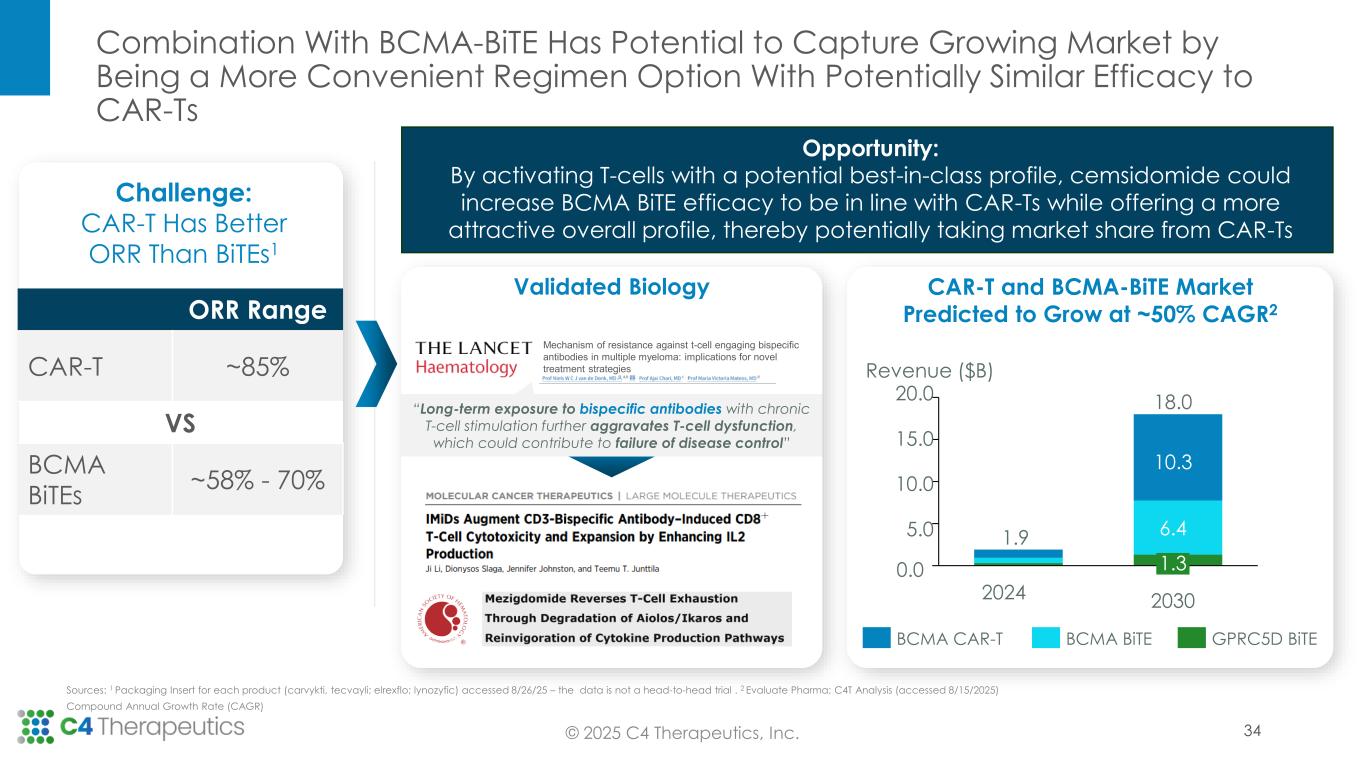
Approved IKZF1/3 degraders remain backbone therapy across lines of multiple myeloma treatment, even as novel therapeutic approaches enter the treatment landscape. Recent advances in treatment, including immune-directed therapies, are not cures and the majority of patients ultimately relapse, creating a need for new medicines targeted at these heavily pretreated patients. This need for therapeutic options in later lines of therapy, which continue to incorporate IKZF1/3 degradation into the treatment regimen to promote myeloma cell death and T-cell activation, is expected to grow as patients live longer on newer treatments but still ultimately progress.
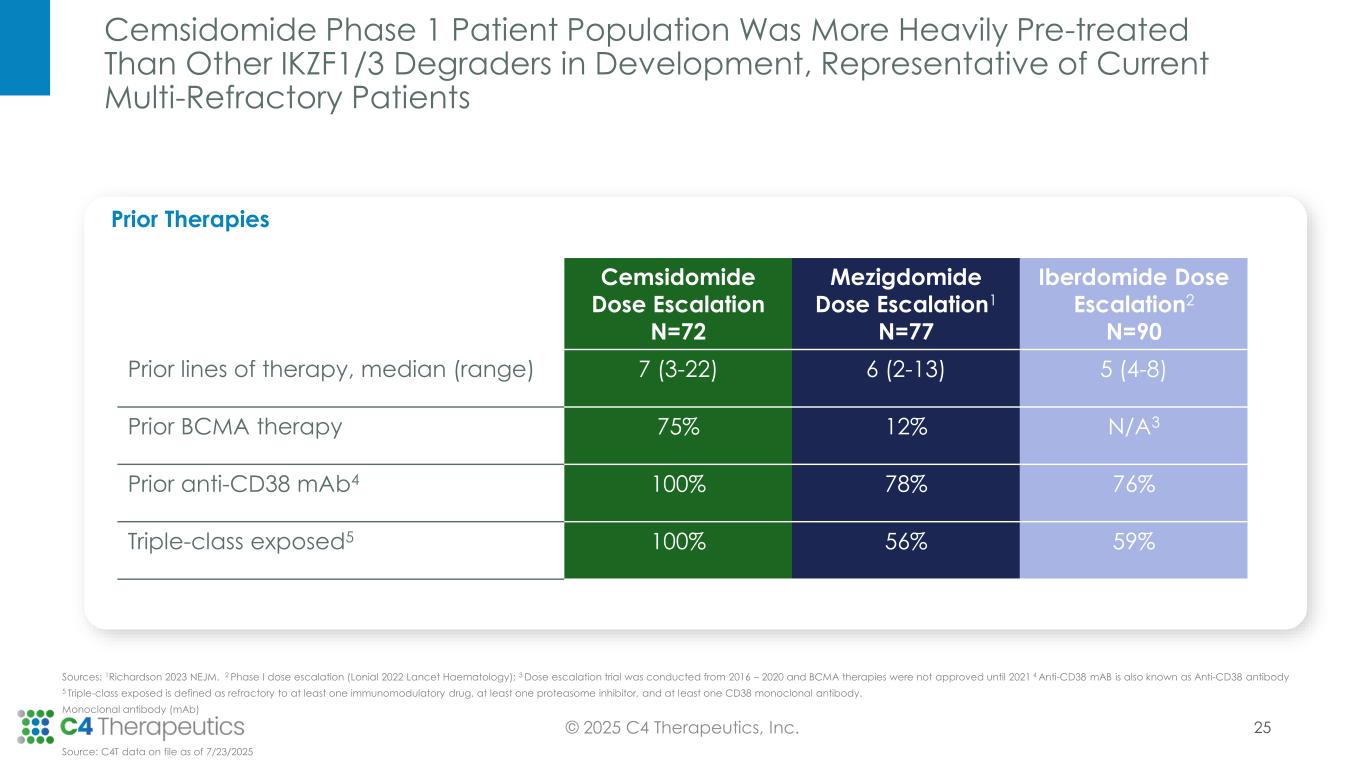
“The clinical data presented today for this potent Cereblon-based IKZF1/3 degrader shows a potentially class-leading safety profile and impressive evidence of anti-myeloma activity in a population of patients with extensive prior therapies—including 75% of patients who have progressed despite having received prior immune-based therapies, including BiTEs or CAR-Ts,” said Binod Dhakal, M.D., M.S., associate professor of medicine, Medical College of Wisconsin, Division of Hematology. “Cemsidomide in combination with dexamethasone is well positioned both as a potential therapeutic option for patients with multi-refractory disease, and as the potential combination regimen of choice with immune-directed therapies due to its ability to enhance the immune response and add additional direct anti-myeloma effects via IKZF1/3 degradation.”
Phase 1 Results
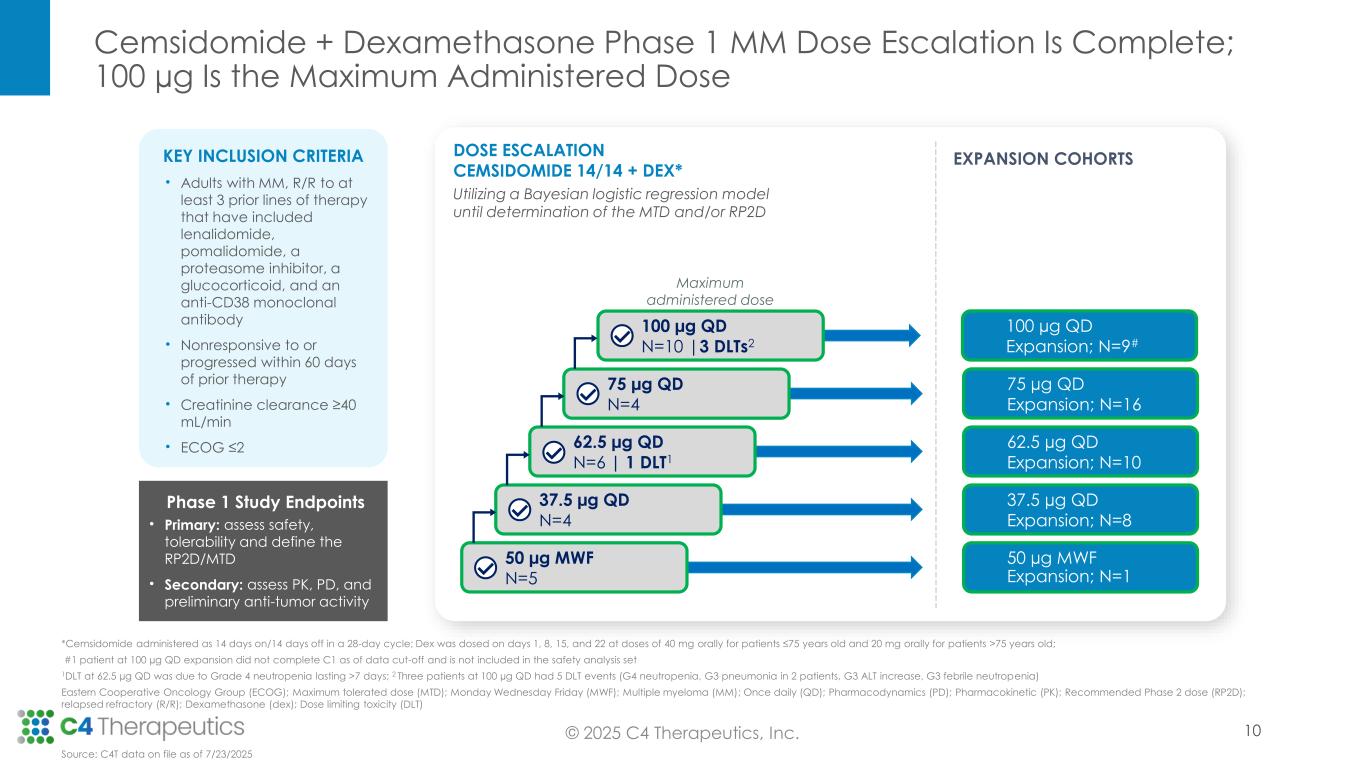
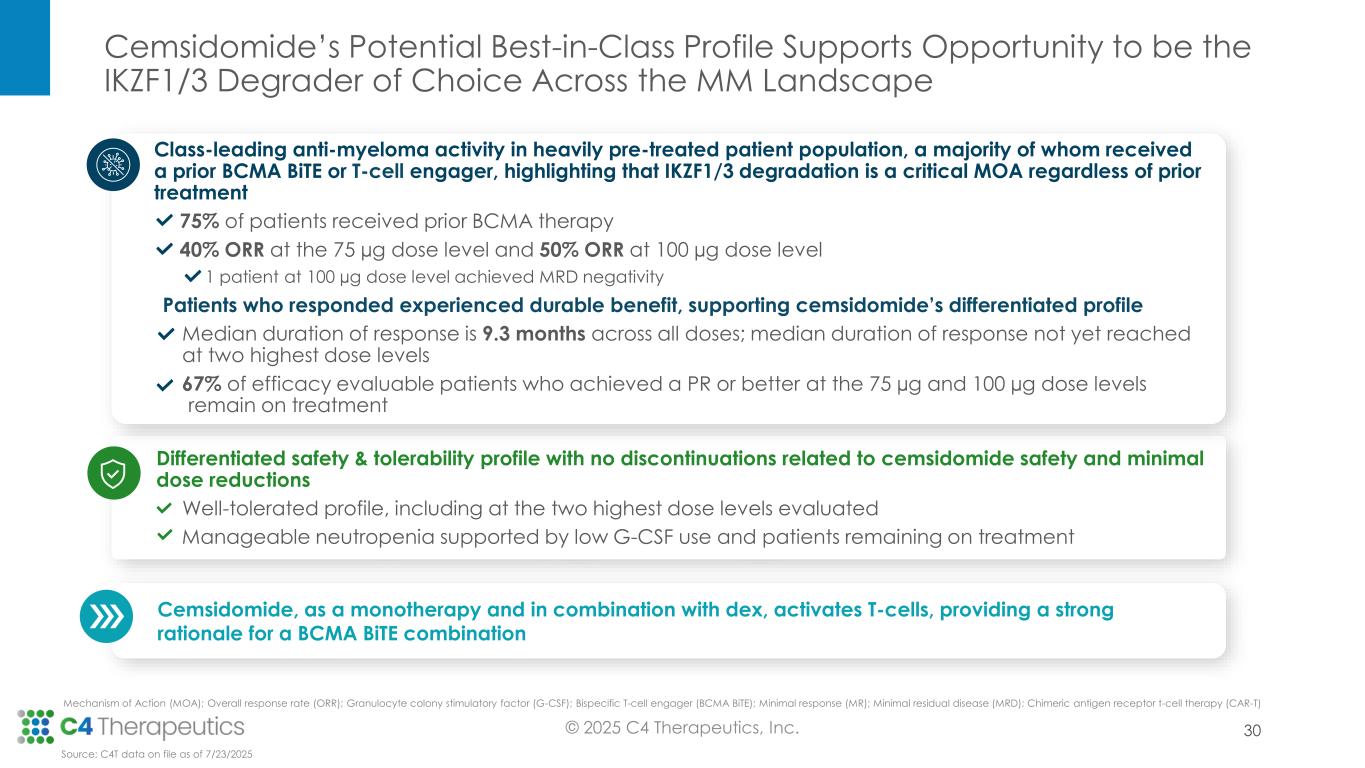
At the IMS Annual Meeting, C4T presented data from the Phase 1 dose escalation trial, for which enrollment is now complete. These data demonstrate cemsidomide’s potential to have a class-leading profile based on both its anti-myeloma activity and safety and tolerability profile, which positions the investigational medicine to become the IKZF1/3 degrader of choice across lines of therapy.
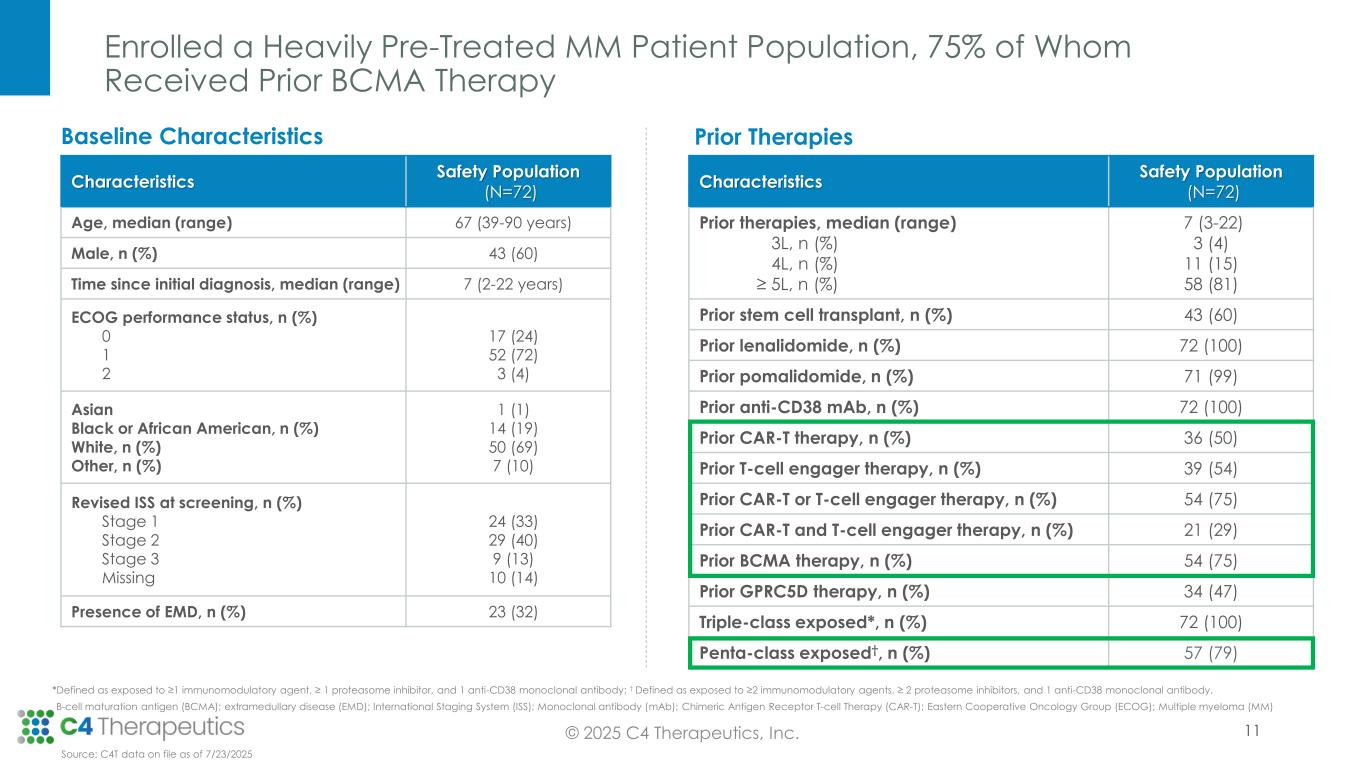
As of the July 23, 2025 data cutoff, a total of 72 patients received cemsidomide in combination with dexamethasone across five dose levels (50 µg dosed Monday, Wednesday, Friday [MWF]; 37.5 µg dosed once daily [QD]; 62.5 µg QD; 75 µg QD; 100 µg QD). The trial enrolled a heavily pretreated relapsed/refractory patient population that had received a median of seven prior therapies. Fifty-four patients (75%) received prior BCMA-targeted therapy, and 54 patients (75%) received prior CAR-T or T-cell engager therapy.
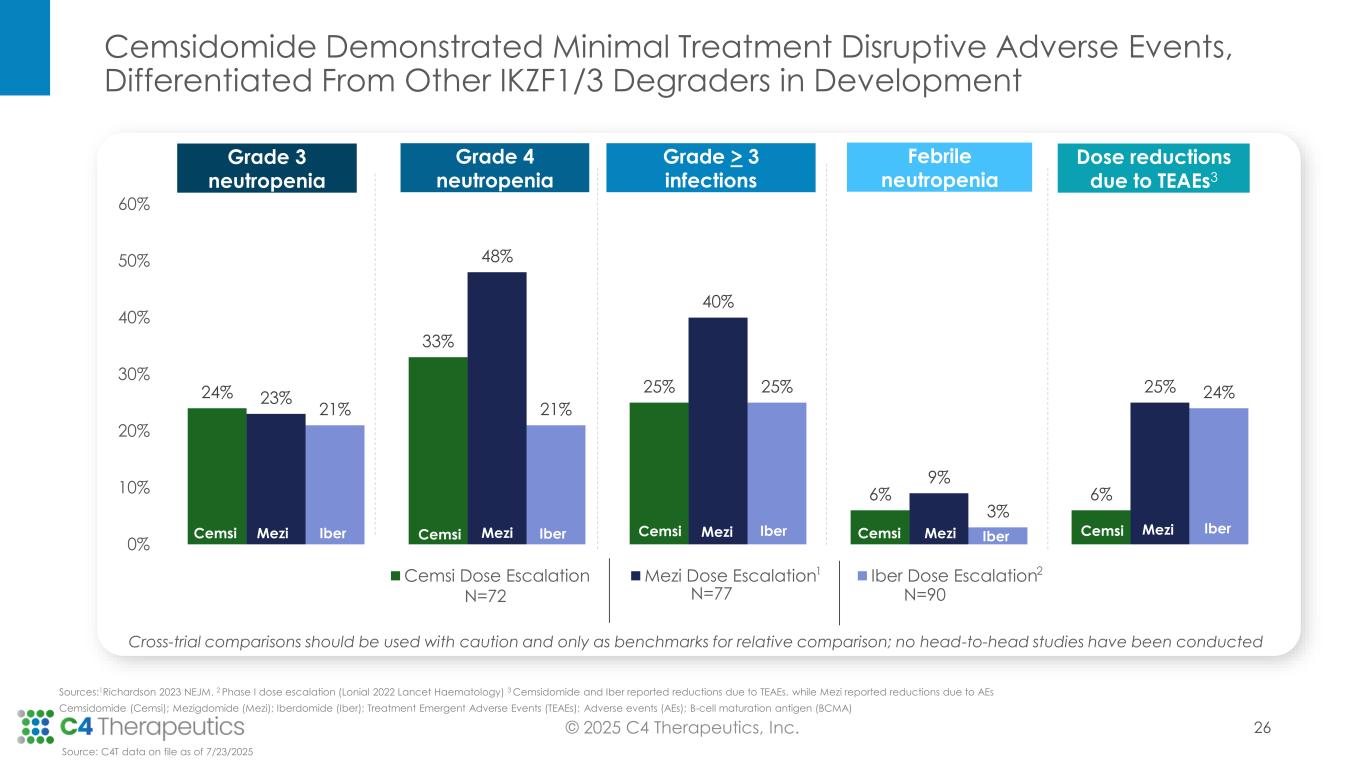
Safety: Cemsidomide in combination with dexamethasone was generally well tolerated over the range of doses tested.
•As of the data cutoff date, 72 patients were evaluable for safety.
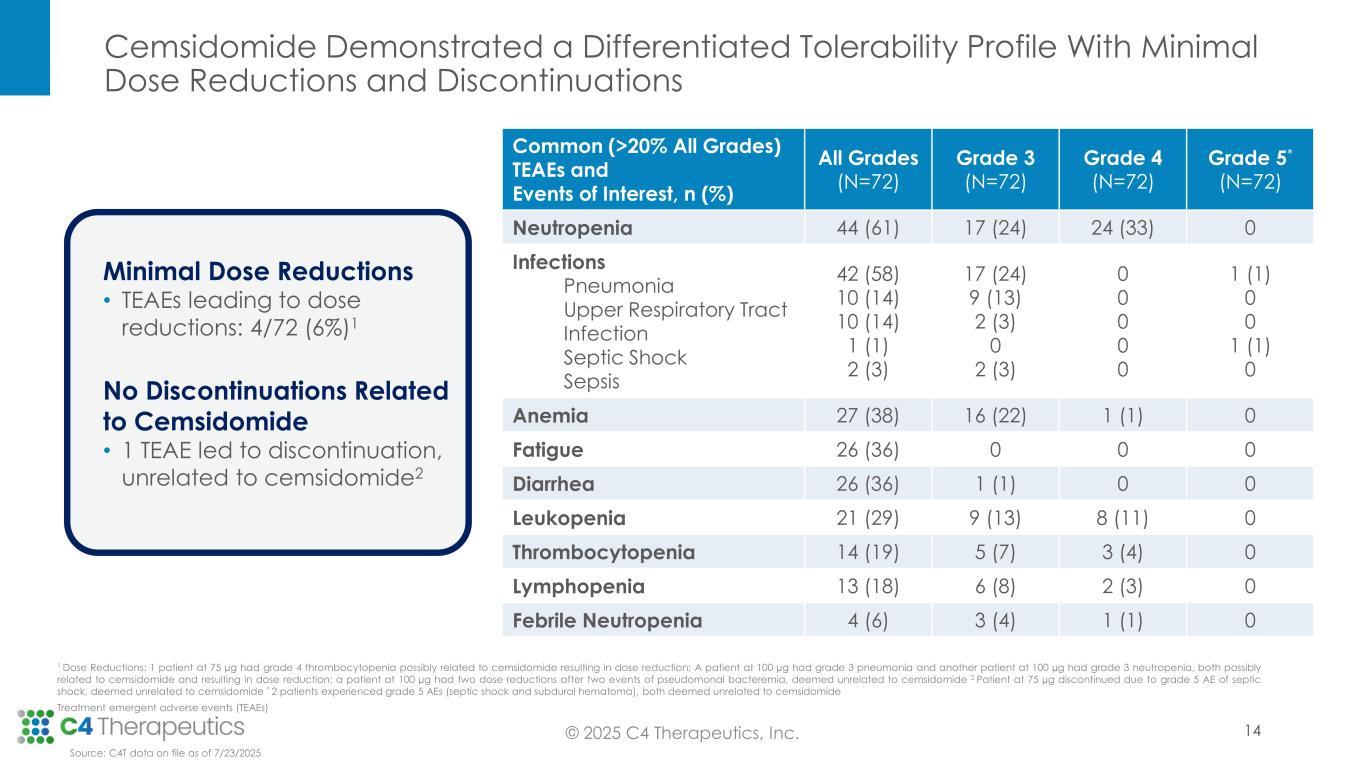
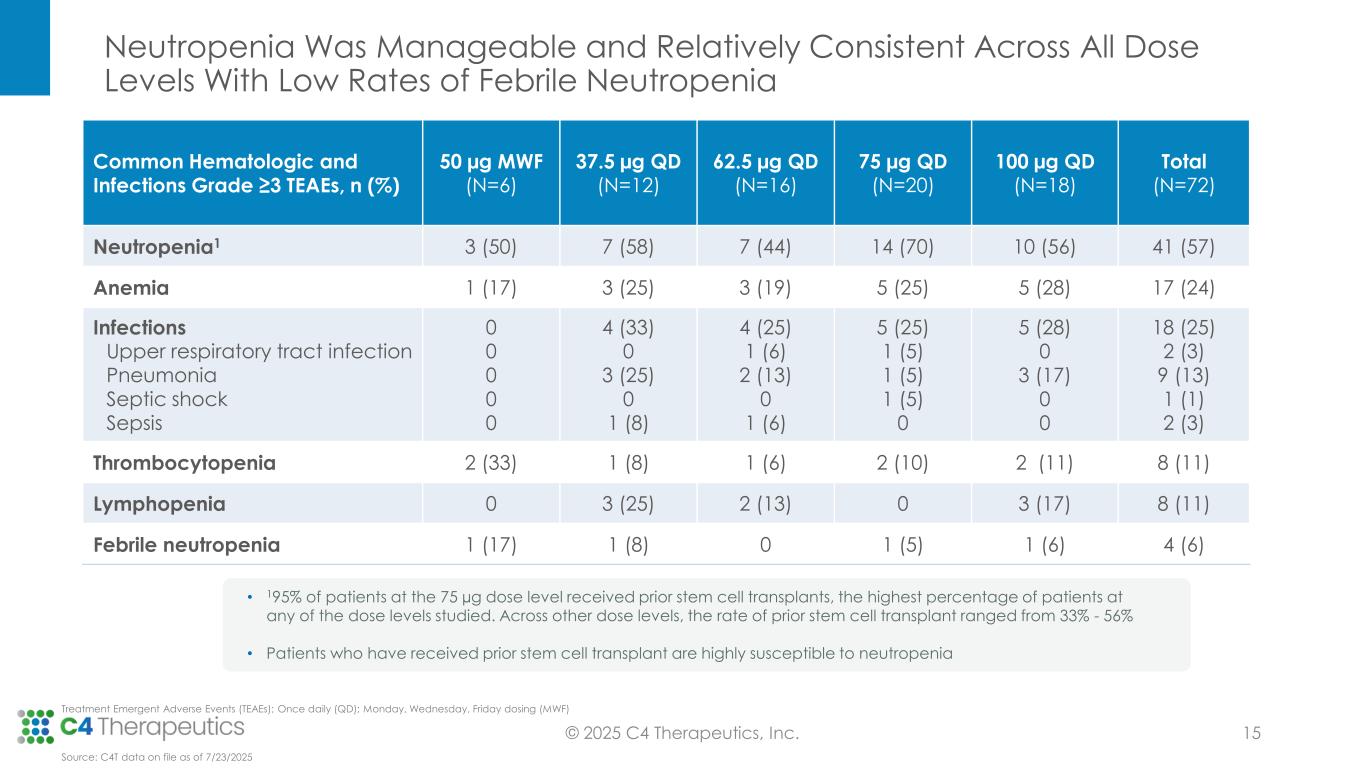
•Cemsidomide was generally well tolerated with manageable incidents of on-target neutropenia across all dose levels; there were low rates of febrile neutropenia across all dose levels: three patients (4%) at Grade 3, one patient (1%) at Grade 4 and no patients at Grade 5.
•There were low rates of thrombocytopenia across all dose levels: five patients (7%) at Grade 3, three patients (4%) at Grade 4 and no patients at Grade 5.
•All treatment emergent adverse events were manageable; there were minimal dose reductions (four patients; 6%) and no discontinuations related to cemsidomide treatment.
•The maximum administered dose is 100 µg QD.
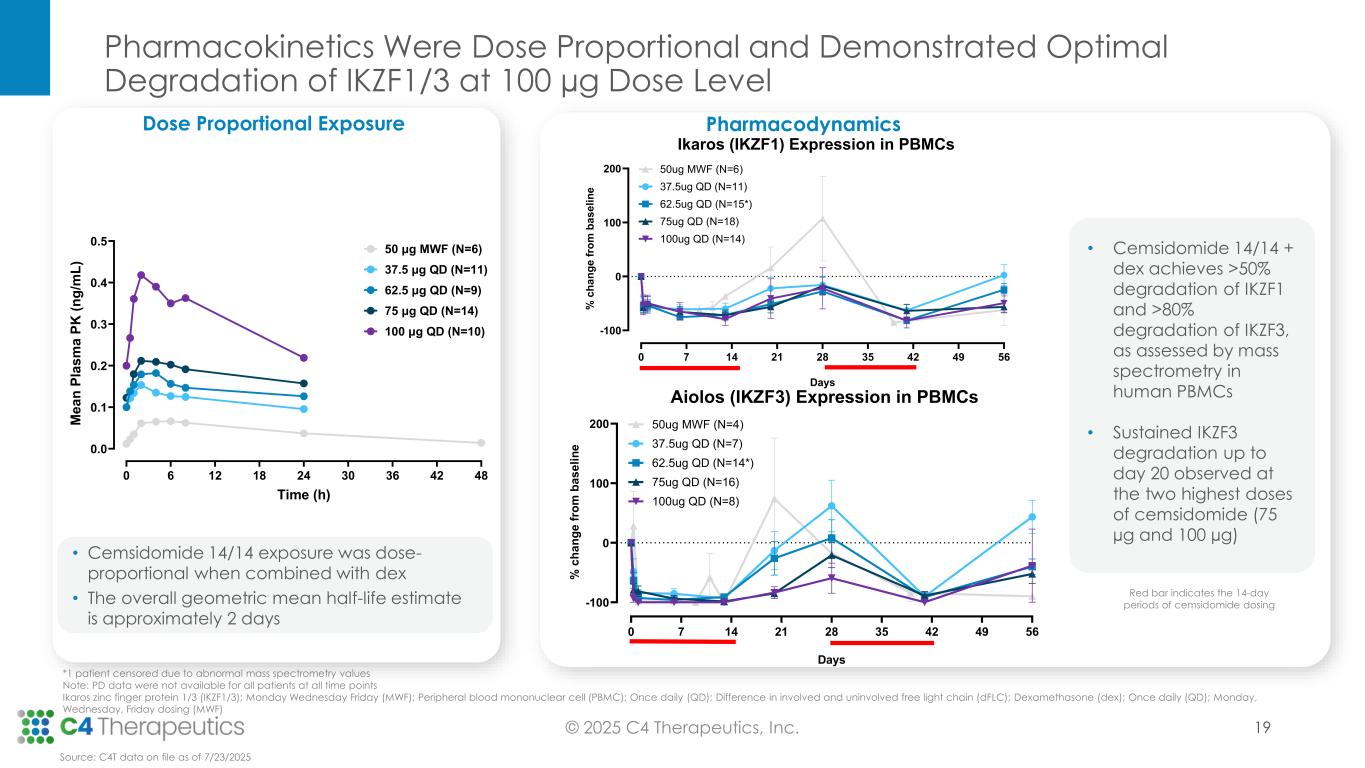
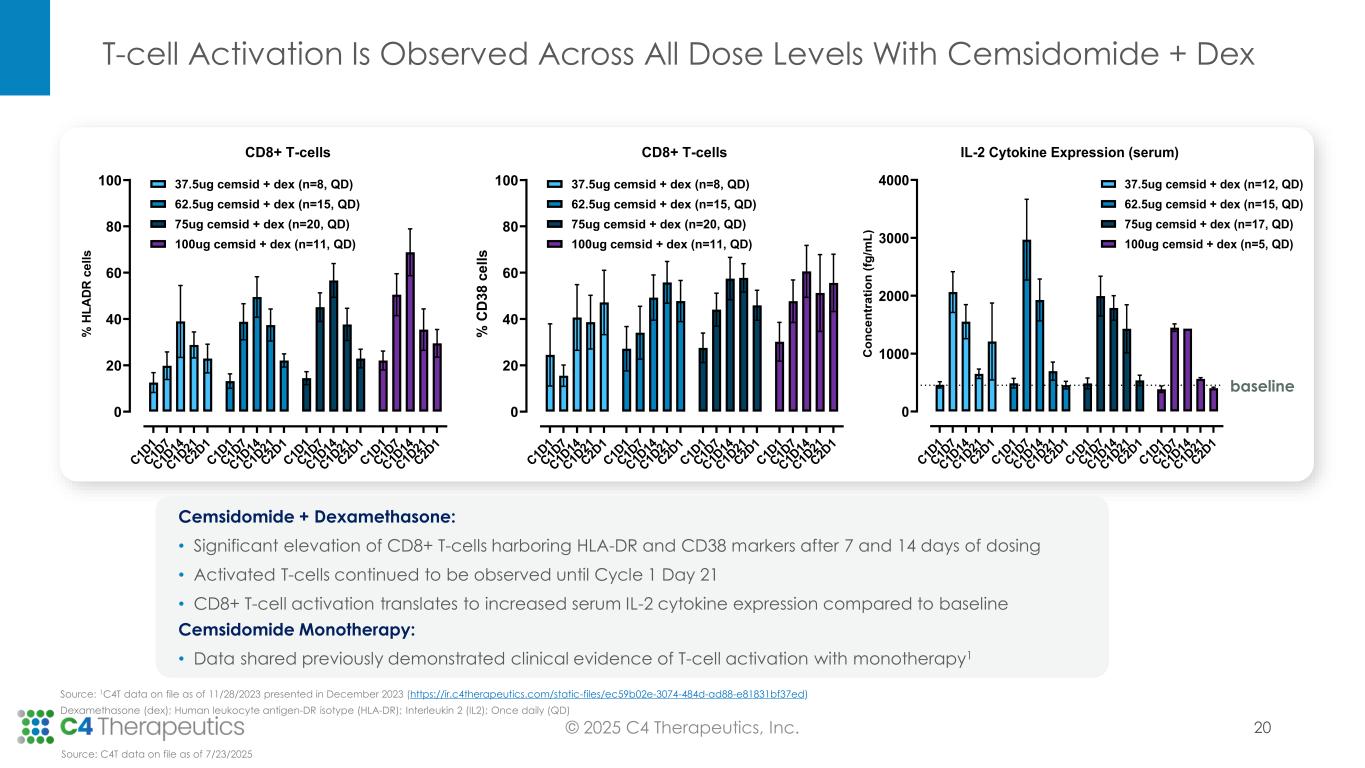
Pharmacodynamics: Cemsidomide in combination with dexamethasone leads to robust IKZF1/3 degradation and T-cell activation, reinforcing its potential to be administered with dexamethasone, and with dexamethasone in combination with a B-cell maturation antigen bispecific T-cell engager (BCMA BiTE).
•Cemsidomide achieved >50% degradation of IKZF1 and > 80% degradation of IKZF3, as assessed by mass spectrometry in human peripheral blood mononuclear cells (PBMCs).
•Across all dose levels, cemsidomide in combination with dexamethasone led to significant T-cell activation associated with an enhancement of cytokine production, including IL-2.
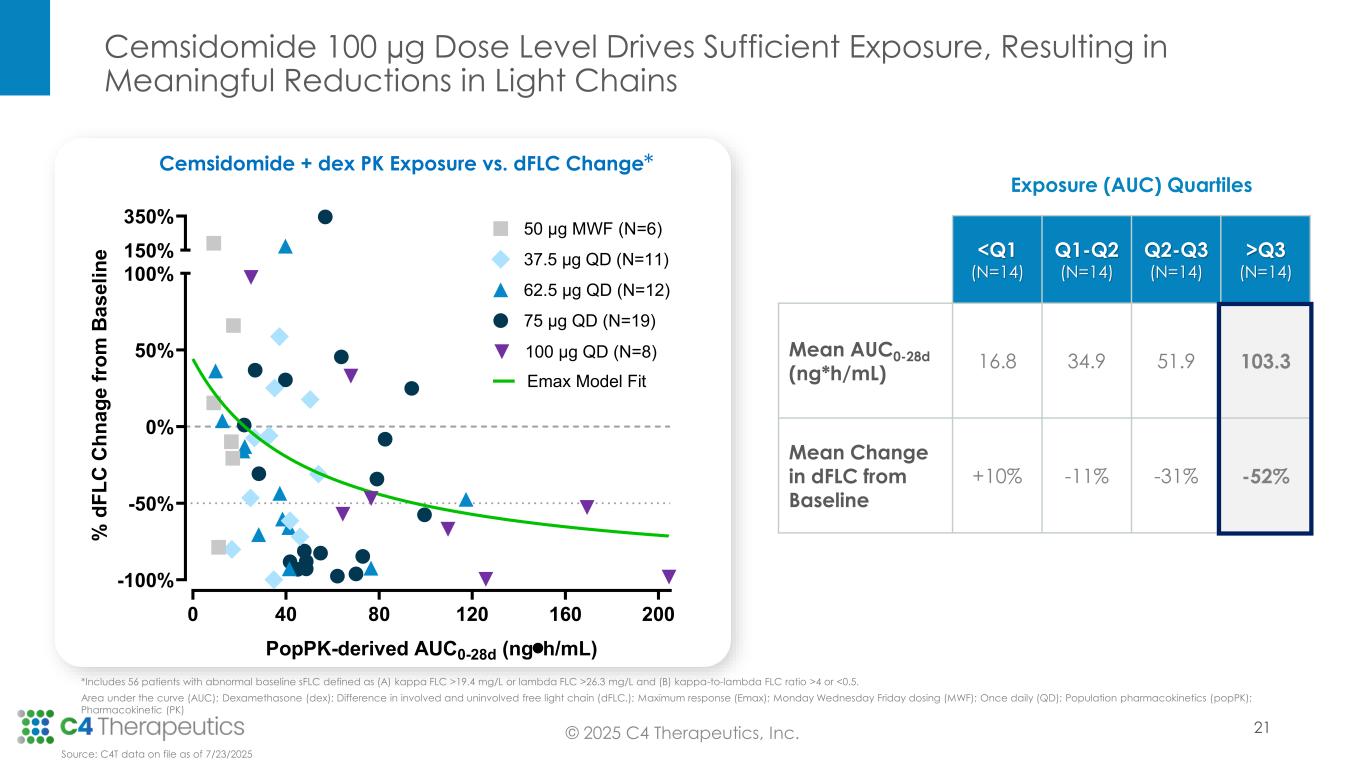
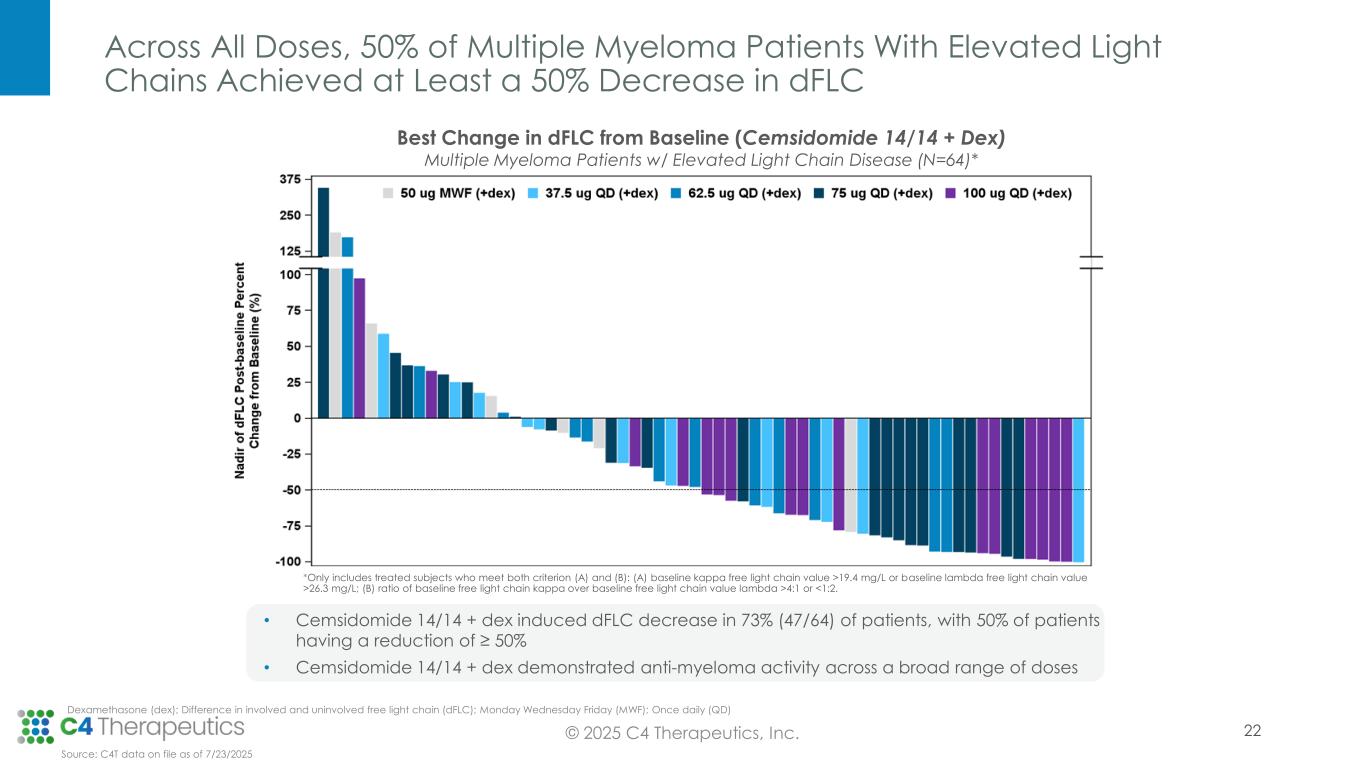
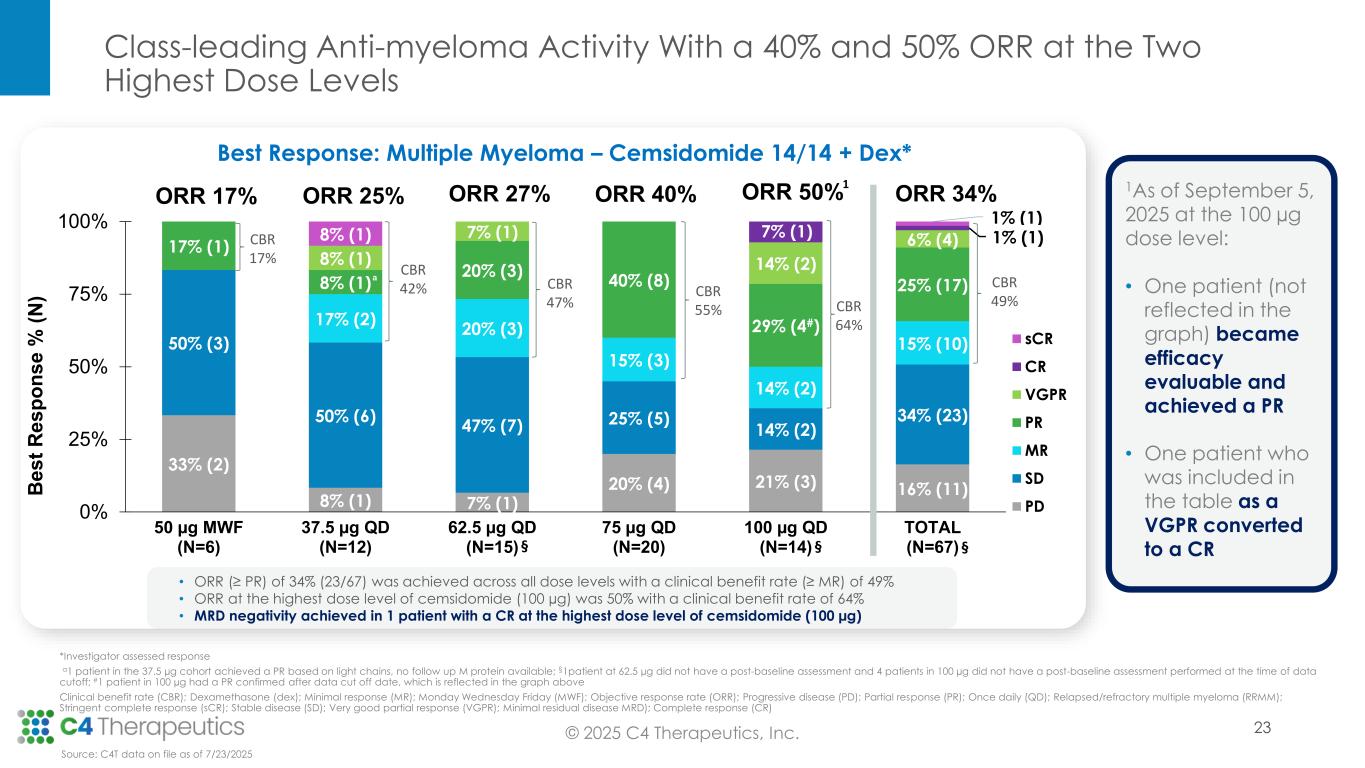
Anti-myeloma activity: Cemsidomide in combination with dexamethasone demonstrates the potential for class-leading anti-myeloma activity.
•As of the data cutoff, 67 patients were evaluable for anti-myeloma activity.
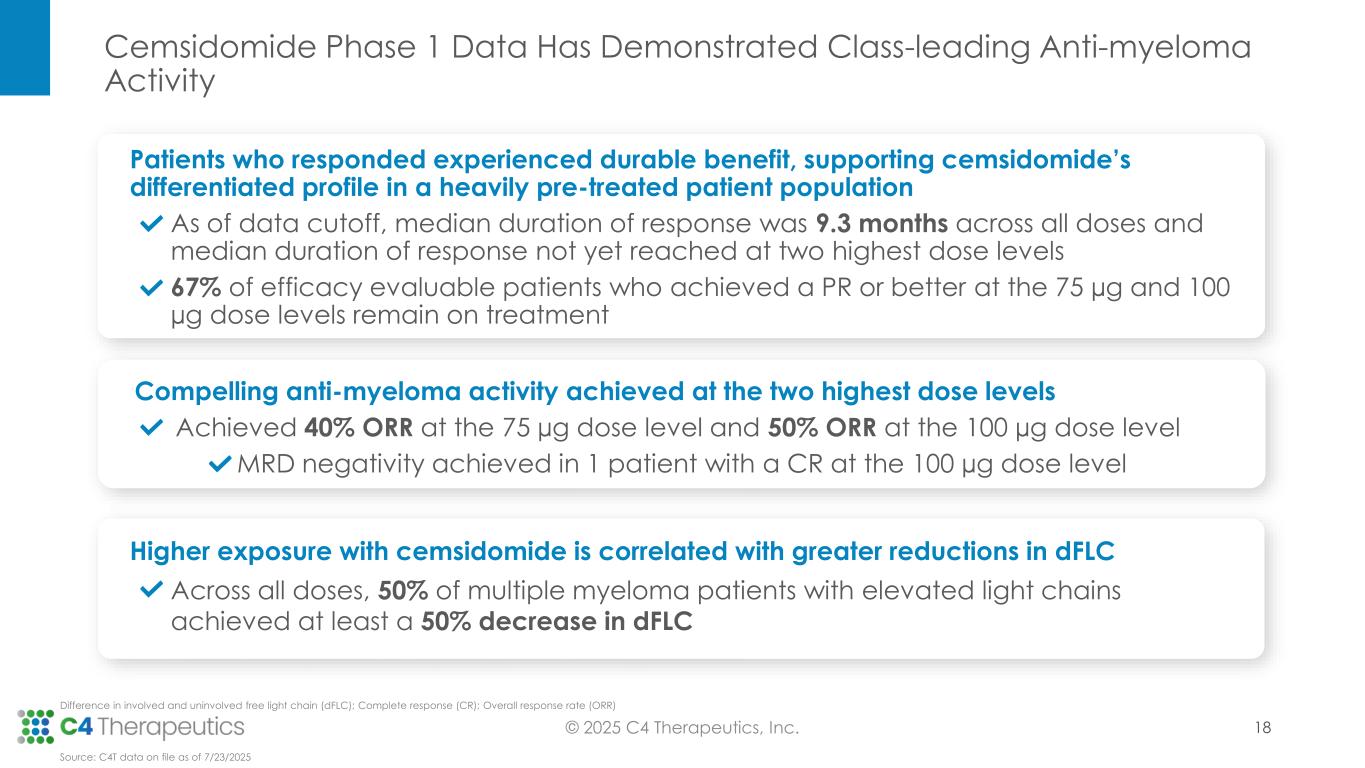
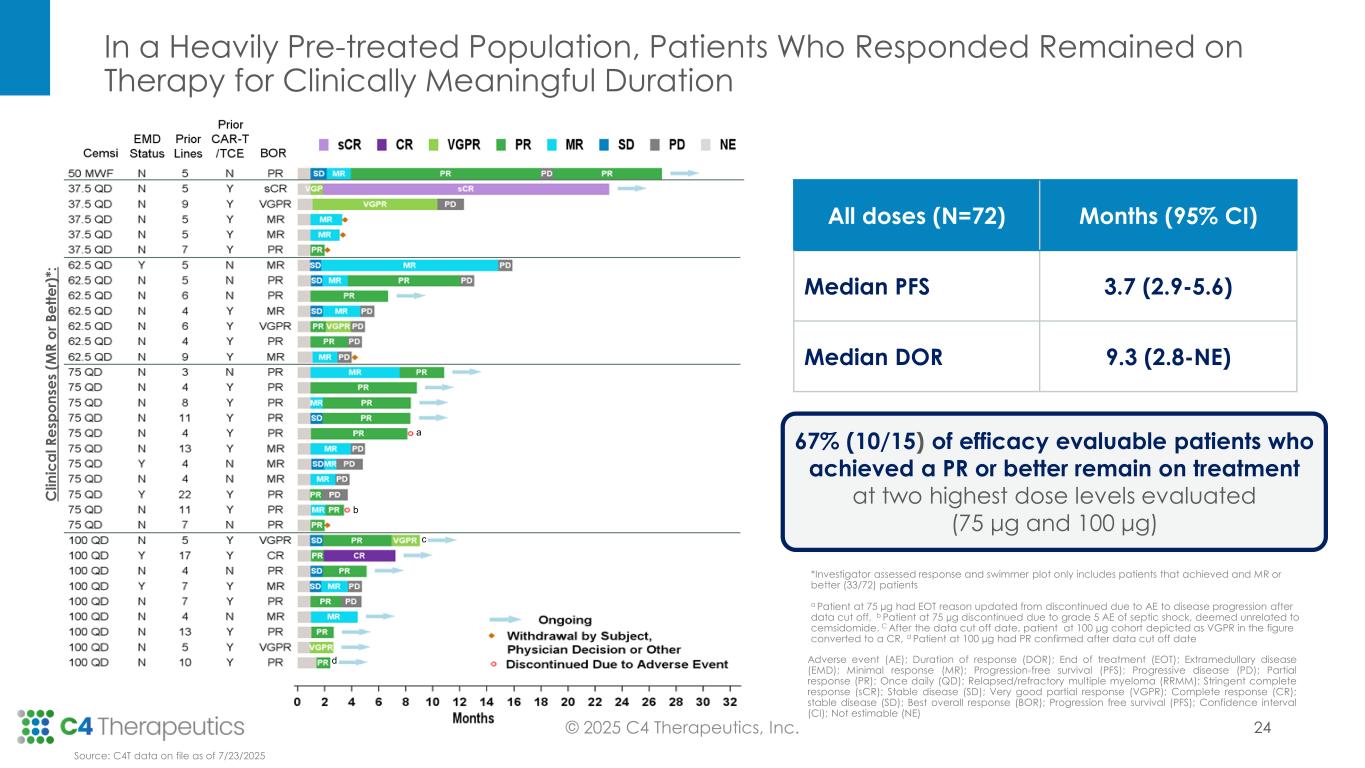
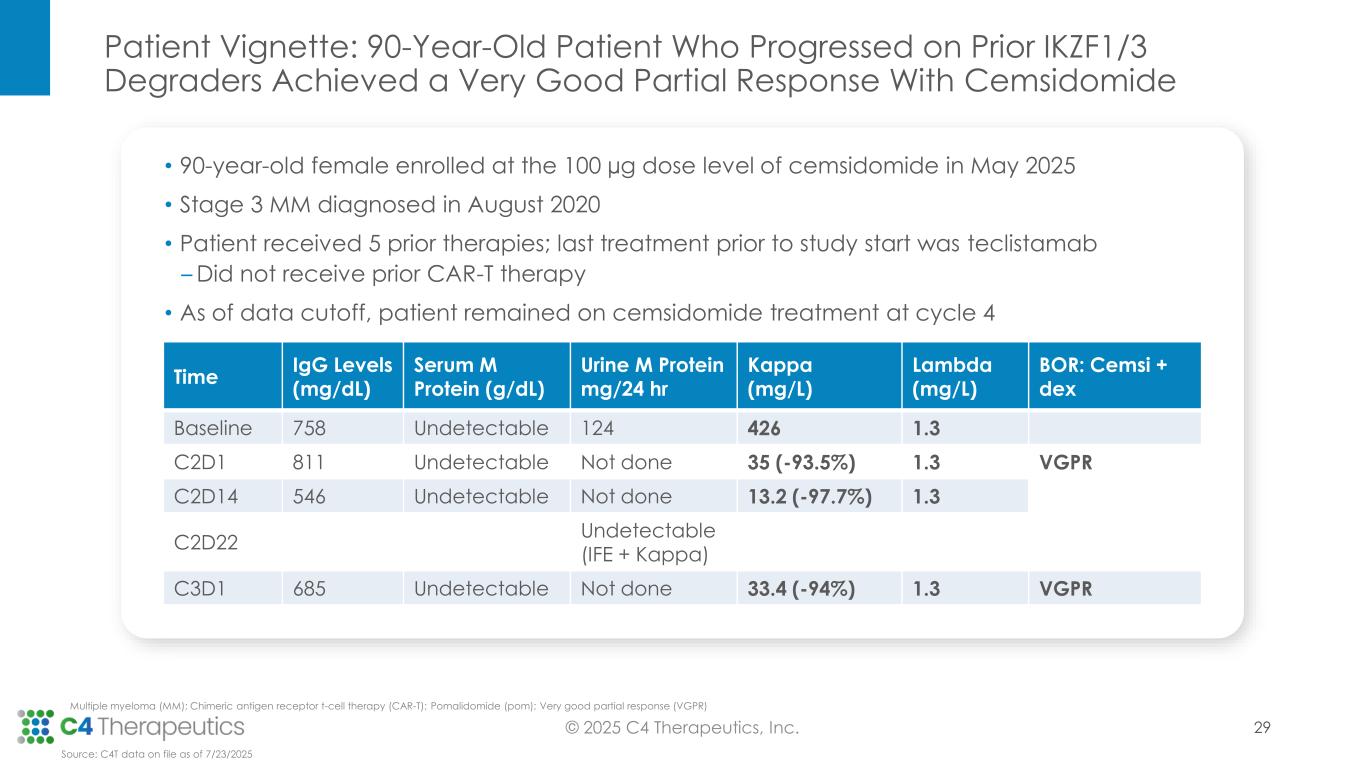
•Across all dose levels, 23 patients (34%) achieved a partial response (PR) or better, with a median duration of response of 9.3 months.
•At the 100 μg dose level, seven patients (50%) achieved a PR or better.
◦One patient achieved a minimal residual disease (MRD) negative complete response.
◦After the data cutoff and as of September 5, 2025, one patient who had achieved a very good partial response (VGPR) converted to a complete response (CR).
◦After the data cutoff and as of September 5, 2025, one patient who became efficacy evaluable achieved a PR; this PR is not included in the ORR reported above.
•At the 75 μg dose level, eight patients (40%) achieved a PR or better.
•Ten of the 15 efficacy evaluable patients (67%) who achieved a PR or better at the 75 µg and 100 µg dose levels remain on treatment; median duration of response has not yet been reached at 100 μg and 75 μg.
Cemsidomide’s Regulatory Path
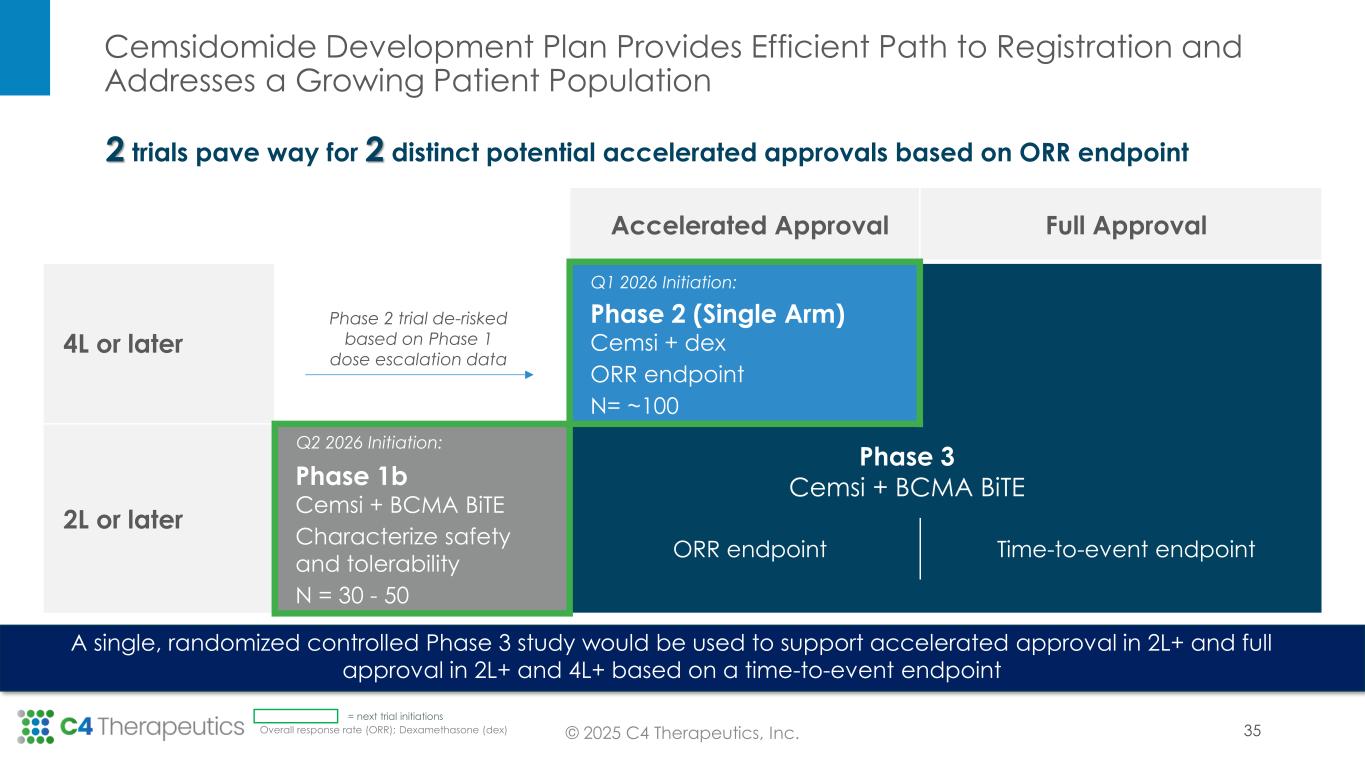
Based on the Phase 1 trial results supporting cemsidomide’s differentiated safety and anti-myeloma activity, as well as insights gathered in the June 2025 Type C Meeting with the U.S. Food & Drug Administration (FDA), C4T plans to advance cemsidomide through two clinical trials that will position the investigational medicine for two distinct potential accelerated approvals.
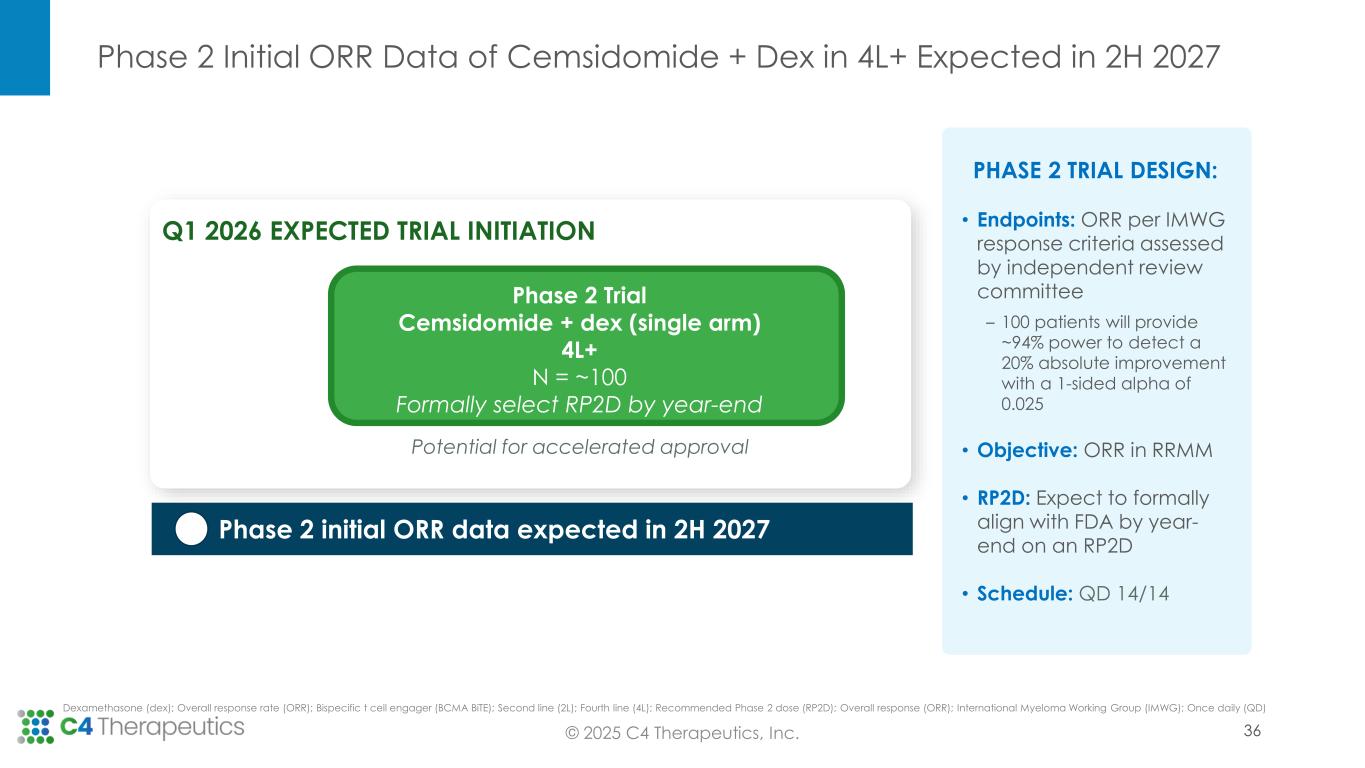
•Fourth line of therapy or later: C4T expects to initiate a Phase 2 single-arm registrational trial in the first quarter of 2026 to evaluate cemsidomide in combination with dexamethasone; initial ORR data is expected in the second half of 2027. If the data are supportive, C4T will pursue accelerated approval. In this setting, cemsidomide has the potential to provide a safe, tolerable and efficacious treatment option for highly refractory patients, including those who have received anti-BCMA therapies.
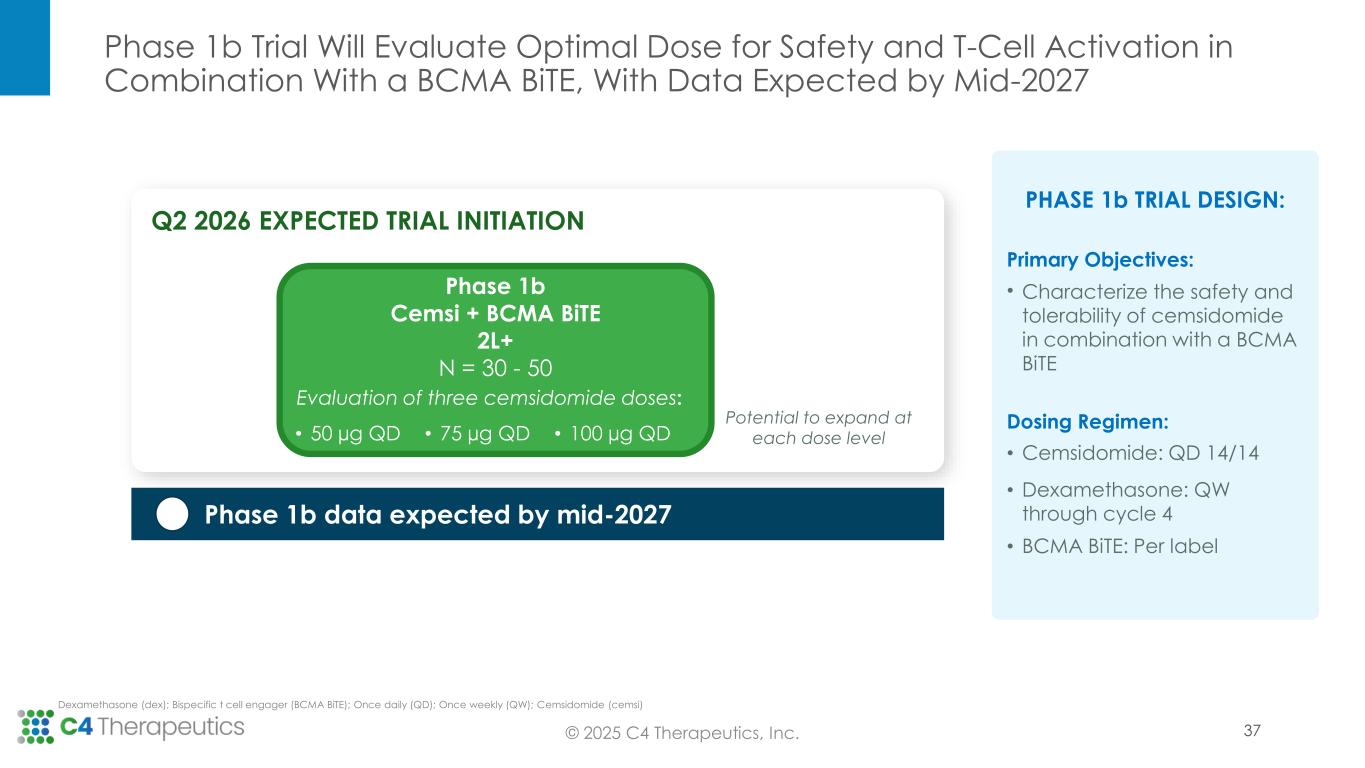
•Second line of therapy or later: C4T plans to initiate a Phase 1b trial in the second quarter of 2026 to evaluate the safety and tolerability of cemsidomide and dexamethasone in combination with a BCMA BiTE; data are expected by mid-2027. If the data are supportive, C4T will advance this combination regimen directly into a single, randomized controlled Phase 3 study. This Phase 3 study will be designed to support the full approval for both the cemsidomide and dexamethasone pathway, as described above, and the cemsidomide and dexamethasone in combination with a BCMA BiTE pathway. In preclinical studies, the combination of cemsidomide with a BCMA BiTE exhibits a strong immunomodulatory effect and enhances T-cell dependent cellular cytotoxicity of multiple myeloma cells while continuing to demonstrate anti-myeloma activity.
Expected Upcoming Milestones:
•Formally align with FDA on the recommended Phase 2 dose of cemsidomide for the Phase 2 trial by the end of 2025.
•Initiate a Phase 2 single-arm registrational trial in the first quarter of 2026 to evaluate cemsidomide in combination with dexamethasone.
•Initiate a Phase 1b trial in the second quarter of 2026 to evaluate the safety and tolerability of cemsidomide and dexamethasone in combination with a BCMA BiTE.
C4T Webcast for Analysts and Investors
C4T will host an investor webcast today, September 20, 2025, at 3 pm ET. To join the webcast, please visit this link or the “Events & Presentations” page of the Investors section on the company’s website at www.c4therapeutics.com. A replay of the webcast will be archived and available following the event.
About Cemsidomide
Cemsidomide is an investigational, orally bioavailable small-molecule degrader in clinical development for the treatment of relapsed/refractory multiple myeloma. Data from the Phase 1 trial, which has completed enrollment, show cemsidomide’s differentiated safety and tolerability profile and potentially class-leading anti-myeloma activity that support the potential for durable outcomes. Two clinical trials are planned to further evaluate cemsidomide in relapsed/refractory multiple myeloma: a Phase 2 single-arm registrational trial to evaluate cemsidomide in combination with dexamethasone, which is expected to initiate in Q1 2026; and a Phase 1b trial to evaluate the safety and tolerability of cemsidomide and dexamethasone in combination with a BCMA BiTE, which is expected to initiate in Q2 2026.
About Multiple Myeloma
Multiple myeloma (MM) is a rare blood cancer affecting plasma cells. Approximately 36,000 people in the United States are diagnosed with MM each year. Despite advances in treatment, MM remains incurable. Treatment combinations include IKZF1/3 degraders, which are established backbone therapies, across lines of therapy.
About C4 Therapeutics
C4 Therapeutics (C4T) (Nasdaq: CCCC) is a clinical-stage biopharmaceutical company dedicated to delivering on the promise of targeted protein degradation science to create a new generation of medicines that transforms patients’ lives. C4T is progressing targeted oncology programs through clinical studies and leveraging its TORPEDO® platform to efficiently design and optimize small-molecule medicines to address difficult-to-treat diseases. C4T’s degrader medicines are designed to harness the body’s natural protein recycling system to rapidly degrade disease-causing proteins, offering the potential to overcome drug resistance, drug undruggable targets and improve patient outcomes. For more information, please visit www.c4therapeutics.com.
Forward Looking Statements
This press release contains “forward-looking statements” of C4 Therapeutics, Inc., within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements may include, but may not be limited to, express or implied statements regarding our ability to develop potential therapies for patients; the safety, tolerability, design and potential efficacy of our therapeutic approaches and product candidates; the predictive capability of our TORPEDO® platform in the development of novel, selective, orally bioavailable BiDAC™ and MonoDAC™ degraders; the potential initiation, timing, design, results and advancement of our preclinical studies and clinical trials, including the potential timing for and receipt of regulatory authorization and guidance related to clinical trials and other clinical development activities including clinical trial commencement or cohort initiation; the potential for accelerated approval of our product candidates; our ability and the potential to successfully manufacture and supply our product candidates for clinical trials; our ability to replicate results achieved in our preclinical studies or clinical trials in any future studies or trials; and our ability to fund our future operations.
Any forward-looking statements in this press release are based on management’s current expectations and beliefs of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: uncertainties related to the initiation, timing, advancement and conduct of preclinical and clinical studies and other development requirements for our product candidates; the risk that any one or more of our product candidates will cost more to develop or may not be successfully developed and commercialized; the risk that our product candidates will not receive accelerated approval or that we will need to redesign our regulatory strategy; and the risk that the results of preclinical studies and/or clinical trials will or will not be predictive of results in connection with future studies or trials. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in C4 Therapeutics’ most recent Annual Report on Form 10-K and/or Quarterly Report on Form 10-Q, as filed with the Securities and Exchange Commission. All information in this press release is as of the date of the release and C4 Therapeutics undertakes no duty to update this information unless required by law.
Contacts:
Investors:
Courtney Solberg
Senior Manager, Investor Relations
CSolberg@c4therapeutics.com
Media:
Loraine Spreen
Senior Director, Corporate Communications & Patient Advocacy
LSpreen@c4therapeutics.com