FALSE2024FY00014176630.00270.00270.00270.0027P5D0.002712/31/202412/31/202512/31/2043iso4217:USDxbrli:sharesiso4217:USDxbrli:sharesxbrli:puresnwv:segmentsnwv:warrant00014176632024-01-012024-12-3100014176632024-06-2800014176632025-03-1800014176632024-12-3100014176632023-12-310001417663us-gaap:NonrelatedPartyMember2024-12-310001417663us-gaap:NonrelatedPartyMember2023-12-310001417663us-gaap:RelatedPartyMember2024-12-310001417663us-gaap:RelatedPartyMember2023-12-310001417663us-gaap:SeriesAPreferredStockMember2023-12-310001417663us-gaap:SeriesAPreferredStockMember2024-12-310001417663us-gaap:SeriesBPreferredStockMember2023-12-310001417663us-gaap:SeriesBPreferredStockMember2024-12-310001417663us-gaap:SeriesCPreferredStockMember2023-12-310001417663us-gaap:SeriesCPreferredStockMember2024-12-310001417663us-gaap:SeriesDPreferredStockMember2023-12-310001417663us-gaap:SeriesDPreferredStockMember2024-12-3100014176632024-10-182024-10-1800014176632023-01-012023-12-310001417663us-gaap:NonrelatedPartyMember2024-01-012024-12-310001417663us-gaap:NonrelatedPartyMember2023-01-012023-12-310001417663us-gaap:RelatedPartyMember2024-01-012024-12-310001417663us-gaap:RelatedPartyMember2023-01-012023-12-310001417663us-gaap:CommonStockMember2022-12-310001417663us-gaap:AdditionalPaidInCapitalMember2022-12-310001417663us-gaap:RetainedEarningsMember2022-12-310001417663us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-3100014176632022-12-310001417663us-gaap:CommonStockMember2023-01-012023-12-310001417663us-gaap:AdditionalPaidInCapitalMember2023-01-012023-12-310001417663us-gaap:CommonStockMembersnwv:ConversionOfDebtMember2023-01-012023-12-310001417663us-gaap:AdditionalPaidInCapitalMembersnwv:ConversionOfDebtMember2023-01-012023-12-310001417663snwv:ConversionOfDebtMember2023-01-012023-12-310001417663us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310001417663us-gaap:RetainedEarningsMember2023-01-012023-12-310001417663us-gaap:CommonStockMember2023-12-310001417663us-gaap:AdditionalPaidInCapitalMember2023-12-310001417663us-gaap:RetainedEarningsMember2023-12-310001417663us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310001417663us-gaap:CommonStockMember2024-01-012024-12-310001417663us-gaap:AdditionalPaidInCapitalMember2024-01-012024-12-310001417663us-gaap:CommonStockMembersnwv:ConversionOfWarrantsMember2024-01-012024-12-310001417663us-gaap:AdditionalPaidInCapitalMembersnwv:ConversionOfWarrantsMember2024-01-012024-12-310001417663snwv:ConversionOfWarrantsMember2024-01-012024-12-310001417663us-gaap:CommonStockMembersnwv:ConversionOfDebtMember2024-01-012024-12-310001417663us-gaap:AdditionalPaidInCapitalMembersnwv:ConversionOfDebtMember2024-01-012024-12-310001417663snwv:ConversionOfDebtMember2024-01-012024-12-310001417663us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-12-310001417663us-gaap:RetainedEarningsMember2024-01-012024-12-310001417663us-gaap:CommonStockMember2024-12-310001417663us-gaap:AdditionalPaidInCapitalMember2024-12-310001417663us-gaap:RetainedEarningsMember2024-12-310001417663us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-12-310001417663us-gaap:CustomerRelationshipsMember2024-01-012024-12-310001417663us-gaap:PatentsMember2024-01-012024-12-310001417663us-gaap:TradeNamesMember2024-01-012024-12-3100014176632024-06-012024-06-300001417663us-gaap:StockOptionMember2024-01-012024-12-310001417663us-gaap:StockOptionMember2023-01-012023-12-310001417663us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-12-310001417663us-gaap:RestrictedStockUnitsRSUMember2023-01-012023-12-310001417663us-gaap:WarrantMember2024-01-012024-12-310001417663us-gaap:WarrantMember2023-01-012023-12-310001417663us-gaap:ConvertibleNotesPayableMember2024-01-012024-12-310001417663us-gaap:ConvertibleNotesPayableMember2023-01-012023-12-310001417663us-gaap:CustomerRelationshipsMember2024-12-310001417663us-gaap:CustomerRelationshipsMember2023-12-310001417663us-gaap:PatentsMember2024-12-310001417663us-gaap:PatentsMember2023-12-310001417663us-gaap:TrademarksMember2024-12-310001417663us-gaap:TrademarksMember2023-12-310001417663snwv:FactoringAgreementMember2021-06-012021-06-300001417663srt:MaximumMembersnwv:FactoringAgreementMember2021-06-300001417663snwv:FactoringAgreementMember2021-06-300001417663snwv:SeniorSecuredPromissoryNotesPayableMember2024-12-310001417663snwv:SeniorSecuredPromissoryNotesPayableMember2023-12-310001417663snwv:SeniorSecuredPromissoryNotesPayableMember2020-08-310001417663snwv:SeniorSecuredPromissoryNotesPayableMembersnwv:SecondAmendmentToNoteAndWarrantPurchaseAndSecurityAgreementMember2022-02-280001417663snwv:SeniorSecuredPromissoryNotesPayableMember2022-02-280001417663snwv:WarrantOneMembersnwv:SeniorSecuredPromissoryNotesPayableMembersnwv:SecondAmendmentToNoteAndWarrantPurchaseAndSecurityAgreementMember2022-02-012022-02-280001417663snwv:WarrantOneMembersnwv:SeniorSecuredPromissoryNotesPayableMembersnwv:SecondAmendmentToNoteAndWarrantPurchaseAndSecurityAgreementMember2022-02-280001417663snwv:WarrantTwoMembersnwv:SeniorSecuredPromissoryNotesPayableMembersnwv:SecondAmendmentToNoteAndWarrantPurchaseAndSecurityAgreementMember2022-02-012022-02-280001417663snwv:SeniorSecuredPromissoryNotesPayableMember2024-01-012024-12-310001417663snwv:SeniorSecuredPromissoryNotesPayableMember2023-06-012023-06-300001417663snwv:SeniorSecuredPromissoryNotesPayableMember2023-07-152023-07-150001417663snwv:NoteAndWarrantPurchaseAndSecurityAgreementMembersnwv:SeniorSecuredPromissoryNotesPayableMember2024-06-300001417663snwv:ConvertiblePromissoryNoteMemberus-gaap:ConvertibleDebtMember2020-08-060001417663us-gaap:PrivatePlacementMember2024-10-1700014176632024-10-170001417663snwv:ConversionOfWarrantsMember2024-10-182024-10-180001417663snwv:SeniorSecuredPromissoryNotesPayableMember2023-01-012023-12-310001417663us-gaap:NotesPayableOtherPayablesMember2023-12-310001417663snwv:ConvertiblePromissoryNotesPayableRelatedPartiesMember2023-12-310001417663us-gaap:ConvertibleNotesPayableMember2023-12-310001417663snwv:Two022ConvertibleNotesPayableRelatedPartiesMember2023-12-310001417663snwv:AssetBackedSecuredPromissoryNotesMembersnwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2022-08-310001417663snwv:AssetBackedSecuredPromissoryNotesMembersnwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2022-11-300001417663snwv:AssetBackedSecuredPromissoryNotesMembersnwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2023-05-310001417663snwv:AssetBackedSecuredPromissoryNotesMembersnwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2024-12-310001417663snwv:AssetBackedSecuredPromissoryNotesMembersnwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2024-01-310001417663snwv:AssetBackedSecuredPromissoryNotesMembersnwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2024-06-300001417663snwv:WarrantOneMembersnwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2024-06-300001417663snwv:WarrantTwoMembersnwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2024-06-300001417663snwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2024-01-012024-12-310001417663snwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2023-01-012023-12-310001417663snwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2024-12-310001417663srt:MaximumMembersnwv:SecuritiesPurchaseAgreementAndFutureAdvanceConvertiblePromissoryNotesMember2024-12-310001417663snwv:AugustIssued2022ConvertiblePromissoryNotesMember2023-08-310001417663snwv:AugustIssued2022ConvertiblePromissoryNotesMember2023-08-012023-08-310001417663us-gaap:CommonStockMembersnwv:AugustIssued2022ConvertiblePromissoryNotesMember2023-08-012023-08-310001417663snwv:NovemberIssued2022ConvertiblePromissoryNotesMember2023-11-300001417663snwv:NovemberIssued2022ConvertiblePromissoryNotesMember2023-11-012023-11-300001417663us-gaap:CommonStockMembersnwv:NovemberIssued2022ConvertiblePromissoryNotesMember2023-11-012023-11-3000014176632024-05-3100014176632024-05-312024-05-310001417663us-gaap:CommonStockMember2024-10-182024-10-1800014176632024-10-180001417663us-gaap:RelatedPartyMemberus-gaap:NotesPayableOtherPayablesMember2024-06-300001417663snwv:CelularitySUltraMISTAssetsMemberus-gaap:NotesPayableOtherPayablesMember2020-08-012020-08-310001417663snwv:CelularitySUltraMISTAssetsMemberus-gaap:NotesPayableOtherPayablesMember2020-08-310001417663snwv:CelularitySUltraMISTAssetsMemberus-gaap:NotesPayableOtherPayablesMember2024-12-310001417663snwv:CelularitySUltraMISTAssetsMemberus-gaap:NotesPayableOtherPayablesMember2023-12-310001417663snwv:CelularitySUltraMISTAssetsMemberus-gaap:NotesPayableOtherPayablesMember2024-01-012024-12-310001417663snwv:ConvertiblePromissoryNotesPayableRelatedPartiesMember2020-08-310001417663snwv:ConvertiblePromissoryNotesPayableRelatedPartiesMember2024-12-310001417663snwv:ConvertiblePromissoryNotesPayableRelatedPartiesMember2024-01-012024-12-310001417663snwv:AssetBackedSecuredPromissoryNotesMember2023-07-310001417663snwv:AssetBackedSecuredPromissoryNotesMember2023-07-012023-07-310001417663snwv:AssetBackedSecuredPromissoryNotesMember2024-01-012024-12-310001417663snwv:AssetBackedSecuredPromissoryNotesMemberus-gaap:NonrelatedPartyMember2023-12-310001417663snwv:AssetBackedSecuredPromissoryNotesMemberus-gaap:RelatedPartyMember2023-12-310001417663snwv:AssetBackedSecuredPromissoryNotesMember2023-12-310001417663us-gaap:FairValueMeasurementsRecurringMemberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2024-12-310001417663us-gaap:FairValueInputsLevel1Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001417663us-gaap:FairValueInputsLevel2Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001417663us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001417663us-gaap:FairValueMeasurementsRecurringMember2024-12-310001417663us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001417663us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001417663us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001417663us-gaap:FairValueMeasurementsRecurringMemberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2023-12-310001417663us-gaap:FairValueInputsLevel1Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001417663us-gaap:FairValueInputsLevel2Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001417663us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001417663us-gaap:FairValueMeasurementsRecurringMembersnwv:EmbeddedConversionOptionMember2023-12-310001417663us-gaap:FairValueInputsLevel1Membersnwv:EmbeddedConversionOptionMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001417663us-gaap:FairValueInputsLevel2Membersnwv:EmbeddedConversionOptionMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001417663us-gaap:FairValueInputsLevel3Membersnwv:EmbeddedConversionOptionMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001417663us-gaap:FairValueMeasurementsRecurringMember2023-12-310001417663us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001417663us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001417663us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001417663snwv:A0.04WarrantsMembersnwv:MeasurementInputExchangeRatioMember2024-12-310001417663snwv:A0.04WarrantsMember2024-12-310001417663snwv:A0.067WarrantsMembersnwv:MeasurementInputExchangeRatioMember2024-12-310001417663snwv:A0.067WarrantsMember2024-12-310001417663snwv:MeasurementInputExchangeRatioMember2023-12-310001417663us-gaap:MeasurementInputExpectedTermMemberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2024-12-310001417663us-gaap:MeasurementInputPriceVolatilityMemberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2024-12-310001417663us-gaap:MeasurementInputSharePriceMemberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2024-12-310001417663us-gaap:MeasurementInputRiskFreeInterestRateMemberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2024-12-310001417663us-gaap:MeasurementInputExpectedDividendRateMemberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2024-12-310001417663us-gaap:FairValueInputsLevel3Member2022-12-310001417663us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2022-12-310001417663us-gaap:FairValueInputsLevel3Member2023-01-012023-12-310001417663us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2023-01-012023-12-310001417663us-gaap:FairValueInputsLevel3Member2023-12-310001417663us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2023-12-310001417663us-gaap:FairValueInputsLevel3Member2024-01-012024-12-310001417663us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2024-01-012024-12-310001417663us-gaap:FairValueInputsLevel3Member2024-12-310001417663us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2024-12-310001417663us-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2023-12-310001417663snwv:EmbeddedConversionOptionMember2022-12-310001417663snwv:EmbeddedConversionOptionMember2023-01-012023-12-310001417663snwv:EmbeddedConversionOptionMember2023-12-310001417663snwv:EmbeddedConversionOptionMember2024-01-012024-12-310001417663snwv:EmbeddedConversionOptionMember2024-12-3100014176632022-01-012022-12-3100014176632022-11-300001417663us-gaap:PrivatePlacementMember2024-10-182024-10-180001417663snwv:VendorAMemberus-gaap:SupplierConcentrationRiskMemberus-gaap:CostOfGoodsTotalMember2024-01-012024-12-310001417663snwv:VendorAMemberus-gaap:SupplierConcentrationRiskMemberus-gaap:CostOfGoodsTotalMember2023-01-012023-12-310001417663snwv:VendorBMemberus-gaap:SupplierConcentrationRiskMemberus-gaap:CostOfGoodsTotalMember2024-01-012024-12-310001417663snwv:VendorBMemberus-gaap:SupplierConcentrationRiskMemberus-gaap:CostOfGoodsTotalMember2023-01-012023-12-310001417663country:USsnwv:ConsumablesAndPartsMember2024-01-012024-12-310001417663us-gaap:NonUsMembersnwv:ConsumablesAndPartsMember2024-01-012024-12-310001417663snwv:ConsumablesAndPartsMember2024-01-012024-12-310001417663country:USsnwv:ConsumablesAndPartsMember2023-01-012023-12-310001417663us-gaap:NonUsMembersnwv:ConsumablesAndPartsMember2023-01-012023-12-310001417663snwv:ConsumablesAndPartsMember2023-01-012023-12-310001417663country:USsnwv:SystemRevenueMember2024-01-012024-12-310001417663us-gaap:NonUsMembersnwv:SystemRevenueMember2024-01-012024-12-310001417663snwv:SystemRevenueMember2024-01-012024-12-310001417663country:USsnwv:SystemRevenueMember2023-01-012023-12-310001417663us-gaap:NonUsMembersnwv:SystemRevenueMember2023-01-012023-12-310001417663snwv:SystemRevenueMember2023-01-012023-12-310001417663country:USsnwv:LicenseFeesAndOtherMember2024-01-012024-12-310001417663us-gaap:NonUsMembersnwv:LicenseFeesAndOtherMember2024-01-012024-12-310001417663snwv:LicenseFeesAndOtherMember2024-01-012024-12-310001417663country:USsnwv:LicenseFeesAndOtherMember2023-01-012023-12-310001417663us-gaap:NonUsMembersnwv:LicenseFeesAndOtherMember2023-01-012023-12-310001417663snwv:LicenseFeesAndOtherMember2023-01-012023-12-310001417663country:US2024-01-012024-12-310001417663us-gaap:NonUsMember2024-01-012024-12-310001417663country:US2023-01-012023-12-310001417663us-gaap:NonUsMember2023-01-012023-12-310001417663us-gaap:EmployeeStockOptionMembersnwv:A2024EquityIncentivePlanMember2024-10-220001417663us-gaap:EmployeeStockOptionMembersnwv:A2024EquityIncentivePlanMember2024-01-012024-12-310001417663us-gaap:GeneralAndAdministrativeExpenseMember2024-01-012024-12-310001417663us-gaap:EmployeeStockOptionMember2024-01-012024-12-310001417663us-gaap:RestrictedStockUnitsRSUMember2023-12-310001417663us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-12-310001417663us-gaap:RestrictedStockUnitsRSUMember2024-12-310001417663us-gaap:DomesticCountryMember2024-01-012024-12-310001417663us-gaap:ForeignCountryMember2024-01-012024-12-310001417663snwv:A2005Through2017Member2024-12-310001417663snwv:A2018Through2024Member2024-12-310001417663snwv:A2005Through2044Member2024-12-310001417663us-gaap:EarliestTaxYearMember2024-01-012024-12-310001417663us-gaap:LatestTaxYearMember2024-01-012024-12-310001417663snwv:SEPAcquisitionCorpMember2024-02-280001417663snwv:CelularityMember2024-06-012024-06-300001417663snwv:ReportableSegmentMember2024-01-012024-12-310001417663snwv:ReportableSegmentMember2023-01-012023-12-310001417663snwv:LicenseAndOptionAgreementMember2024-01-012024-09-300001417663snwv:LicenseAndOptionAgreementMember2024-03-3100014176632024-10-012024-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
|
|
|
|
|
|
| x |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2024
or
|
|
|
|
|
|
| o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___________________to____________________.
Commission File Number: 000-52985
SANUWAVE Health, Inc.
(Exact Name of Registrant as Specified in Charter)
|
|
|
|
|
|
|
|
|
| Nevada |
|
20-1176000 |
| (State or Other Jurisdiction of Incorporation) |
|
(I.R.S. Employer Identification No.) |
|
|
|
|
|
|
|
|
|
|
11495 Valley View Road
Eden Prairie, MN
|
|
55344 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
(952) 656-1029
Registrant’s Telephone Number, Including Area Code
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
| Common Stock, par value $0.001 per share |
SNWV |
The Nasdaq Stock Market, LLC |
Securities registered pursuant to Section 12(g) of the Act:
None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such file). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer” “smaller reporting company” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
Large accelerated filer o |
Accelerated filer o |
|
|
Non-accelerated filer x |
Smaller reporting company x |
|
|
|
Emerging growth company o |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. o
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrants’ executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark whether registrant is a shell company (as defined in Rule 12b-2 of the Act). YES o NO x
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant (assuming, for purposes of this calculation only, that the registrant’s directors, executive officers and greater than 10% stockholders are affiliates of the registrant), based upon the closing sale price of the registrant’s common stock on June 28, 2024, the last business day of the registrant’s most recently completed second fiscal quarter, was $13.6 million.
As of March 18, 2025, there were issued and outstanding 8,548,473 shares of the registrant’s common stock.
SANUWAVE Health, Inc.
Table of Contents
PART I
Special Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K of SANUWAVE Health, Inc. and its subsidiaries (“Sanuwave” or the “Company”) contains forward-looking statements. All statements in this Annual Report on Form 10-K, including those made by the management of the Company, other than statements of historical fact, are forward-looking statements. Examples of forward-looking statements include statements regarding: results of operations, liquidity, and operations, restrictions and new regulations on our operations and processes, including the execution of clinical trials; the Company’s future financial results, operating results, and projected costs; market acceptance of and demand for UltraMIST® and PACE®; success of future business development and acquisition activities; management’s plans and objectives for future operations; industry trends; regulatory actions that could adversely affect the price of or demand for our approved products; our intellectual property portfolio; our business, marketing and manufacturing capacity and strategy; estimates regarding our capital requirements, the anticipated timing of the need for additional funds, and our expectations regarding future capital-raising transactions, including through investments by strategic partners for market opportunities, which may include strategic partnerships or licensing agreements, or raising capital through the conversion of outstanding warrants or issuances of securities; product liability claims; economic conditions that could adversely affect the level of demand for or the cost of our products; timing of clinical studies and any eventual U.S. Food and Drug Administration ("FDA") approval of new products and new uses of our current products; financial markets; the competitive environment; supplier and customer disputes; and our plans to remediate our material weaknesses in our disclosure controls and procedures and our internal control over financial reporting. These forward-looking statements are based on management’s estimates, projections and assumptions as of the date hereof and include the assumptions that underlie such statements. Forward-looking statements may contain words such as “may,” “will,” “should,” “could,” “would,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “potential” and “continue,” the negative of these terms, or other comparable terminology. Any expectations based on these forward-looking statements are subject to risks and uncertainties and other important factors, including those discussed in this report, including the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Other risks and uncertainties are and will be disclosed in the Company’s subsequent filings with the U.S. Securities and Exchange Commission (the “SEC”). These and many other factors could affect the Company’s future financial condition and operating results and cause actual results to differ materially from expectations based on forward-looking statements made in this document or elsewhere by the Company or on its behalf. Except to the extent required by law, the Company does not undertake, and expressly disclaims, any duty, or obligation to revise or update any forward-looking statements whether as a result of new information, future events, changes in assumptions or otherwise.
Except as otherwise indicated by the context, references in this Annual Report on Form 10-K to “we,” “us” and “our” are to the consolidated business of the Company.
Item 1. BUSINESS
Overview
Sanuwave is a medical device company providing directed energy products into the wound care space. Our mission is to improve patient lives and outcomes by developing and marketing effective, easy to use products to decrease wound burden, lessen healing times, and reduce patient pain.
Our focus is regenerative medicine utilizing noninvasive ultrasound or shockwaves to produce a biological response promoting the repair and regeneration of tissue, musculoskeletal, and vascular structures. The Company’s patented and FDA cleared products include the UltraMIST® system (UM) and the PACE® family of products, both of which are used to treat a variety of acute and chronic wounds. These products are backed by an intellectual property (“IP”) portfolio of over 165 patents.
In the year ended December 31, 2024, we had total revenues of $32.6 million, a 60% increase from revenues of $20.4 million in the year ended December 31, 2023. UltraMIST systems and consumables represented approximately 98% of 2024 revenues versus 90% of revenues in 2023.
Our Products and Services:
The Company currently markets directed energy products using both ultrasound and high energy acoustic shockwaves.
UltraMIST
The UltraMIST system delivers low frequency, non-thermal ultrasound to target tissues using a fluid mist to transmit energy in a non-contact and pain free fashion. This energy penetrates deep into wound beds to promote healing while reducing inflammation, killing bacteria and biofilms, and increasing the growth of blood vessels in the wound and peri-wound.
This proprietary technology has been shown to speed healing and reduce reported pain, and it has been cleared by the FDA for wound healing and debridement for a variety of acute and chronic wounds including:
•diabetic foot ulcers,
•venous leg ulcers,
•split thickness wounds/skin grafts,
•deep tissue pressure injuries,
•surgical wounds, and
•many more wound types.
The UltraMIST System is cleared for marketing in the U.S. by the FDA (K140782) and has CMS schedule one reimbursement under code 97610. The UltraMIST system treatment must be administered by a healthcare professional.
The UltraMIST system is highly portable and is used in hospitals, physician’s offices, wound centers, nursing homes, and skilled nursing facilities, and by mobile wound care providers serving patient homes. Treatment may be provided by a doctor, nurse, nurse practitioner, or physical therapist.
Treating chronic wounds is a difficult and time-consuming process. Current modalities for wound management typically involve the use of ointments and liquid solutions, specialized bandages, topical skin substitutes, negative pressure, or hyperbaric oxygen. Despite this, many patients are left with chronic wounds resistant to healing.
Sanuwave’s Energy First™ protocol is at the forefront of improving the standard of care for advanced and chronic wounds. Our solutions help expedite the healing process at a cellular level, a better and simpler alternative that can lead to improved patient outcomes and enhanced quality of life.
As the UltraMIST device never touches the wound, the treatment is painless and patients report a significant reduction in pain post treatment. Significant clinical research demonstrates reductions in healing time, patient pain, and other indicators of patient healing (for more clinical information, please visit our website: https://sanuwave.com/clinical). The UltraMIST system is in use with many top hospitals and wound care providers across the United States. Typical treatment time is 6 minutes (and ranges from 3 to 20 minutes).
UltraMIST is sold using a simple “razor/razor blade” model. Customers purchase an UltraMIST system and then each treatment utilizes a sterile, single use applicator sold in cases of 12. UM consumables revenues constitute the majority of Sanuwave revenue amounting to approximately 61% of total revenues in 2024 and are expected to remain the largest revenue stream for the Company. Over 1,000 UM systems were in the field as of December 31, 2024.
PACE
The PACE systems use acoustic pressure shockwaves generated by the Company’s Pulsed Acoustic Cellular Expression (PACE) technology to converge at precise selected targets to produce an extremely short duration compression burst and are used in both wound and orthopedic applications under the brand names dermaPACE, Profile, and orthoPACE.
The PACE systems are marketed in the U.S., and the European Union (CE Mark).
Market
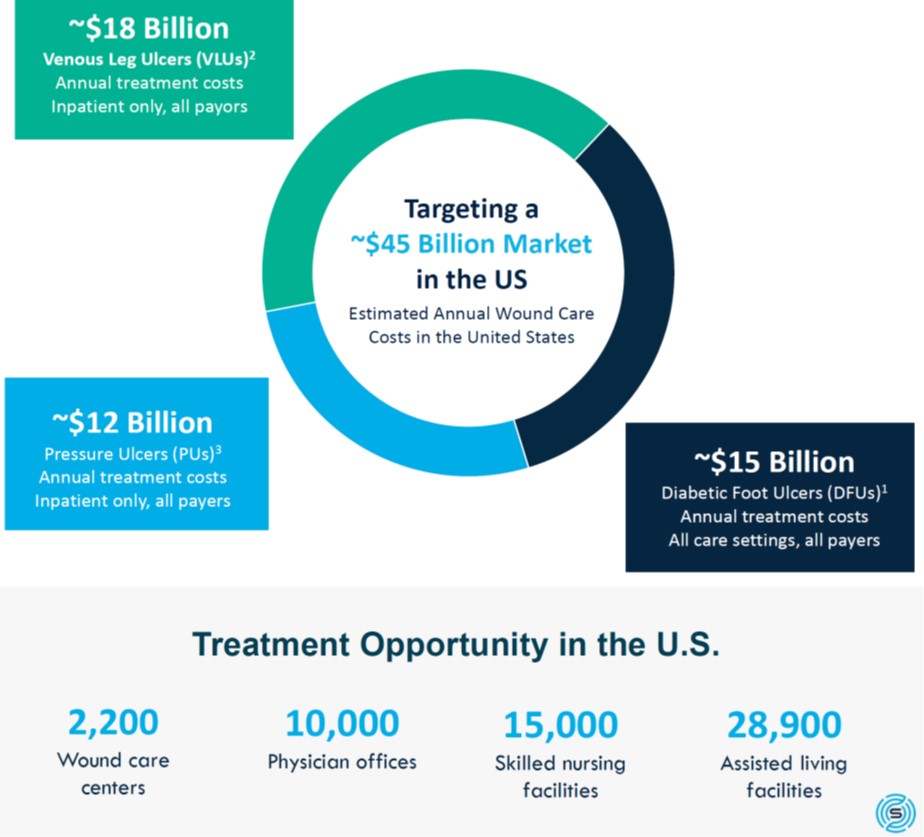
The wound care market exceeds $45 billion per year in the United States and is spread among a number of key categories:
1) Rice et al. Diabetes Care 2014;37:651-658.
2) Rice et al. J Med Econ. 2014;17-(5): 347-356.
3) National Pressure Ulcer Advisory Panel (NPUAP).
The Centers for Medicare and Medicaid Services (the "CMS") have increasingly classified regenerative technology as medically necessary. With an aging population and high incidence of obesity, diabetes, cancers, and autoimmune disorders, the Company believes this market is likely to continue to expand.
Strategy
The Company plans to focus on building a direct sales force and distribution network to market the UltraMIST product in the United States and to assess potential expansion abroad. The Company sees two broad trends favoring UM adoption:
transition to evidence-based medicine in wound care and “care to the edge”, as care is being directed away from hospital settings in an effort to treat patients where they are to increase ease of care and to reduce risks of nosocomial infection.
Reimbursement is being restructured around efficacy and cost effectiveness as payors, physicians, and patients seek better outcomes for less money.
The Company believes that both trends are favorable to Sanuwave.
We aim to change the wound care space by bringing cost effective, easy to use technology to market, and putting it into the hands of providers who can easily adopt it as well as integrate it into their workflow and patient treatment plans. To generate broad adoption, a technology needs to be more than just effective, it needs to be both user and patient friendly.
Traditionally, many patients have been resistant to seek wound care because treatments are long in duration, difficult, involve cumbersome medical devices that must be worn long term, and are frequently painful. The Company seeks to overcome such patient and provider objections in order to expand access to high quality, modern wound care and to get more patients seen and seen earlier. By lowering the bar of “willing to seek treatment”, Sanuwave seeks to engage with patients and wounds before they become severe.
By providing an effective, pain free system with short treatment times that practitioners can learn to use via video conference and for which nationwide CMS reimbursement is already available, the Company seeks to appeal to the “three P’s.”
•Patients want to get better sooner and to experience less pain.
•Physicians want patients to get better but also need to be able to integrate care into their practice flow and economic models.
•Payors want to see patients get better faster and to receive early treatment often outside of hospital settings as such treatments save money.
When the needs of Patients, Physicians, and Payors are aligned, markets are ready adopters of products and markets are ready for change.
The Company seeks to move wound care from a more “transactional” mindset to a consultative one, helping practitioners work effective wound treatment into the practice flows and patients' lives. No one wants to live with chronic wounds. We do not believe they should have to.
Sales, Marketing and Distribution
The Company sells systems through a combination of direct sales representatives and independent distributors. The systems are used in hospitals, clinics, and alternate care facilities. Our primary sales are in the Unites States.
Manufacturing
The Company has developed a network of suppliers, manufacturers, and contract service providers to provide sufficient quantities of our products.
The Company is party to a manufacturing supply agreement with Nortech in Wayzata, MN and Biomerics in Salt Lake City UT, covering the generator and treatment wand components of our products. Our generators and treatment wands are manufactured in accordance with applicable quality standards and applicable industry and regulatory standards. In addition, the Company performs the final product testing for generators and treatment wands internally.
The Company is party to a manufacturing supply agreement with Dynamic Group in Ramsey, MN, covering the applicator component of our products. Our applicators are manufactured in accordance with applicable quality standards and applicable industry and regulatory standards. Dynamic Group produces the applicators and applicator kits for our products.
Our facility in Eden Prairie, MN consists of 8,199 square feet and provides office, product development, quality control, and warehouse space. It is an FDA-registered facility and is International Organization for Standardization (ISO) 13485:2016 certified.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our products, technology, and know-how, to operate without infringing on the proprietary rights of others and to prevent others from infringing upon our proprietary rights. The Company seeks to protect our proprietary position by, among other methods, filing United States and selected foreign patent applications and United States and selected foreign trademark applications related to our proprietary technology, inventions, products, and improvements that are important to the development of our business. Effective trademark, service mark, copyright, patent, and trade secret protection may not be available in every country in which our products are made available. The protection of our intellectual property may require the expenditure of significant financial and managerial resources.
Patents
The Company considers the protection afforded by patents important to our business. The Company intends to seek and maintain patent protection in the United States and select foreign countries, where deemed appropriate for products that the Company develops. As of December 31, 2024, Sanuwave held more than 140 issued or pending patents worldwide that cover various aspects of the Company’s technology. In general, our patents are effective, ranging from 6 months to 16 years. There are no assurances that any patents will result from our patent applications, or that any patents that may be issued will protect our intellectual property, or that any issued patents or pending applications will not be successfully challenged, including as to ownership and/or validity, by third parties. In addition, if the Company does not avoid infringement of the intellectual property rights of others, the Company may have to seek a license to sell our products, defend an infringement action or challenge the validity of intellectual property in court. Any current or future challenges to our patent rights, or challenges by us to the patent rights of others, could be expensive and time consuming.
The Company believes that our owned and licensed patent rights provide a competitive advantage with respect to others that might seek to utilize certain of our apparatuses and methods incorporating low frequency and non-contact ultrasound and extracorporeal acoustic pressure shockwave technologies that the Company has patented. However, the Company does not hold patent rights that cover all of our products, product components, or methods that utilize our products. The Company also has not conducted a competitive analysis or valuation with respect to our issued and pending patent portfolio in relation to our current products and/or competitor products.
In August 2005, we entered into a license agreement with HealthTronics Inc. (“HealthTronics”) in connection with our acquisition of certain assets and intellectual property relating to orthopedic, tendinopathy, skin wounds, cardiac, dental, neural medical conditions and to all conditions in animals (the “Ortho Field”) from HealthTronics. The majority of the intellectual property licensed from HealthTronics was associated with the construction of shockwave devices, indications for orthopedic treatments, and wound care. These patents and patent applications have either expired or were not pursued in our portfolio.
Under our license to HealthTronics, Inc., we reserved exclusive rights in our purchased portfolio as to the Ortho Field. HealthTronics received field-exclusive and sublicensable rights under the purchased portfolio as to (1) certain HealthTronics lithotripsy devices in all fields other than the Ortho Field, and (2) all products in the treatment of renal, ureteral, gall stones and other urological conditions (the “Litho Field”).
HealthTronics also received non-exclusive and non-sublicensable rights in the purchased portfolio as to any products in all fields other than the Ortho Field and Litho Field.
Pursuant to mutual amendment and other assignment-back rights under the patent license agreement with HealthTronics, we are also a licensee of certain patents and patent applications that have been assigned to HealthTronics. We received a perpetual, non-exclusive and royalty-free license to nine issued foreign patents. Our non-exclusive license is subject to HealthTronics’ sole discretion to further maintain any of the patents and pending applications assigned back to HealthTronics.
In August 2020, we entered into an asset purchase agreement with Celularity Inc. (“Celularity”), pursuant to which we acquired all of Celularity’s assets related to the MIST Therapy System and UltraMIST System, including all intellectual property and trademarks related to MIST and UltraMIST. These assets are for use in low frequency and non-contact ultrasound to treat wounds.
In August 2020, we also entered into a License and Marketing Agreement with Celularity, pursuant to which we were granted an exclusive, royalty-bearing license to commercialize Biovance, a minimally processed human amniotic membrane, and Interfyl, a human connective tissue matrix, for the care and treatment of acute and chronic wounds performed in an operating room setting for worldwide commercialization, excluding the Asia Pacific region.
Under the terms of the agreement, Celularity was to provide Biovance and Interfyl product to us for commercialization in exchange for a quarterly license fee payment. In May 2021, we received notification of non-compliance with the terms of the agreement due to alleged non-payment of the quarterly license fee. Pursuant to the notification, we ceased commercialization of the licensed products and have not resumed commercialization.
Trademarks
Since other products on the market compete with our products, the Company believes that our product brand names are an important factor in establishing and maintaining brand recognition.
The Company has the following trademark registrations: SANUWAVE® (United States, European Community, Canada, Japan, Switzerland, United Kingdom, Taiwan and under the Madrid Protocol), dermaPACE® (United States, European Community, Japan, South Korea, Switzerland, Taiwan, Canada, China, Brazil, and under the Madrid Protocol), angioPACE® (European Community and United Kingdom), PACE® - Pulsed Acoustic Cellular Expression (United States, European Community, China, Hong Kong, Singapore, Switzerland, United Kingdom Taiwan, and Canada), orthoPACE® (United States, United Kingdom, and European Community), DAP® - Diffused Acoustic Pressure (United States, United Kingdom, and European Community), Profile® (United States, European Community, and United Kingdom), Energy First® (United States), Healing Today, Curing Tomorrow® (United States), and UltraMIST® (United States).
Through the acquisition of UltraMIST®/MIST assets from Celularity Inc., the Company is the owner of the Celleration® (United States, Australia, Europe Community, and Japan), Proven Healing® (Madrid Protocol, European Community, and United Kingdom), MIST Ultrasound Healing Therapy & Design® (United States), MIST® (United States), MIST Therapy® (United States), and MIST & Design® (United States) registered trademarks.
The Company also maintains trademark registrations for: OssaTron® (United States), evoPACE® (United Kingdom), Evotron® (Switzerland), and Orthotripsy® (United States). The Company phased out the OssaTrode® (United States, Germany and Switzerland), Equitron® (United States and Switzerland), Reflectron® (Germany and Switzerland), Reflectrode® (Germany and Switzerland), OSWT® (Switzerland), Evotrode® (United States, Germany and Switzerland), and evoPACE® (Canada, Australia, European Community and Switzerland) trademarks, due to the fact that OssaTrode®, Equitron®, Reflectron®, Reflectrode®, OSWT®, Evotrode®, products are no longer available for sale in any market and evoPACE® is a product that is not commercialized.
Competition
The Company believes the advanced wound care market can benefit from our technology which up-regulates the biological factors that promote wound healing. Current medical technologies developed by Acelity L.P. Inc, (formerly Kinetic Concepts, Inc., now owned by 3M), Organogenesis, Inc., Smith & Nephew plc, Derma Sciences, Inc., MiMedx Group, Inc., Osiris Therapeutics, Inc. (now owned by Smith & Nephew), Molnlycke Health Care, Systagenix Wound Management (US), Inc. (now owned by Scapa Group Ltd) and SoftWave Tissue Regeneration Technologies manage wounds, but, in our opinion, do not provide the value proposition to the patients and care givers like our PACE technology has the potential to do. The leading medical device serving this market is the Vacuum Assisted Closure (“V.A.C.”) System marketed by Kinetic Concepts Inc. The V.A.C. is a negative pressure wound therapy device that applies suction to debride and manage wounds.
There are also several companies that market extracorporeal shockwave device products targeting lithotripsy and orthopedic markets, including Dornier MedTech, Storz Medical AG, Electro Medical Systems (EMS) S.A., SoftWave Tissue Regeneration Technologies, and CellSonic Medical, which could ultimately pursue the wound care market. Nevertheless, the Company believes that the PACE systems have a competitive advantage over all of these existing technologies by achieving wound closure by means of a minimally invasive process through innate biological response to PACE technology.
Regarding the companies that use low frequency ultrasound that creates a pressure wave producing micro-strains due to mechanical forces that deform cell membrane and therefore promote healing, there are technologies developed by Arobella Medical LLC, NanoVibronix, Chattanooga, and EDAP TMS to manage wound care. However, these treatment devices or medical systems are different in design and mode of application of the ultrasound when compared to Sanuwave’s UltraMIST. The Company believes that UltraMIST has a competitive advantage over all of these existing technologies, due to broad medical indications, simplicity of use, wound healing results and the tolerability of the treatment by the patients, especially for painful wounds.
Regulatory Matters
Food and Drug Administration (FDA) Regulation
Each of our products must be approved or cleared by the FDA before it is marketed in the United States. Before and after approval or clearance in the United States, our products are subject to extensive regulation by the FDA under the Federal Food, Drug, and Cosmetic Act and/or the Public Health Service Act, as well as by other regulatory bodies. FDA regulations govern, among other things, the development, testing, manufacturing, labeling, safety, storage, record-keeping, market clearance or approval, advertising and promotion, import and export, marketing and sales, and distribution of medical devices and pharmaceutical products.
In the United States, the FDA subjects medical products to rigorous review. If the Company does not comply with applicable requirements, the Company may be fined, the government may refuse to approve our marketing applications or to allow us to manufacture or market our products, and the Company may be criminally prosecuted. Failure to comply with the law could result in, among other things, warning letters, civil penalties, delays in approving or refusal to approve a product candidate, product recall, product seizure, interruption of production, operating restrictions, suspension or withdrawal of product approval, injunctions, or criminal prosecution.
The FDA has determined that our technology and products constitute “medical devices.” The FDA determines what center or centers within the FDA will review the product and its indication for use and determines under what legal authority the product will be reviewed. For the current indications, our products are being reviewed by the Center for Devices and Radiological Health. However, the Company cannot be sure that the FDA will not select a different center and/or legal authority for one or more of our other product candidates, in which case the governmental review requirements could vary in some respects.
FDA Approval or Clearance of Medical Devices
In the United States, medical devices are subject to varying degrees of regulatory control and are classified in one of three classes depending on the extent of controls the FDA determines are necessary to reasonably ensure their safety and efficacy:
•Class I: general controls, such as labeling and adherence to quality system regulations;
•Class II: special controls, pre-market notification (510(k)), specific controls such as performance standards, patient registries, and post market surveillance, and additional controls such as labeling and adherence to quality system regulations; and
•Class III: special controls and approval of a pre-market approval (PMA) application.
Each of our products require FDA authorization prior to marketing, by means of either a 510(k) clearance or a PMA approval. To request marketing authorization by means of a 510(k) clearance, the Company must submit a pre-market notification demonstrating that the proposed device is substantially equivalent to another legally marketed medical device, has the same intended use, and is as safe and effective as a legally marketed device and does not raise different questions of safety and effectiveness than does a legally marketed device. 510(k) submissions generally include, among other things, a description of the device and its manufacturing, device labeling, medical devices to which the device is substantially equivalent, safety and biocompatibility information, and the results of performance testing. In some cases, a 510(k) submission must include data from human clinical studies. Marketing may commence only when the FDA issues a clearance letter finding substantial equivalence. After a device receives 510(k) clearance, any product modification that could significantly affect the safety or effectiveness of the product, or that would constitute a significant change in intended use, requires a new 510(k) clearance or, if the device would no longer be substantially equivalent, would require a PMA. If the FDA determines that the product does not qualify for 510(k) clearance, then a company must submit, and the FDA must approve, a PMA before marketing can begin.
A PMA application must provide a demonstration of safety and effectiveness, which generally requires extensive pre-clinical and clinical trial data. Information about the device and its components, device design, manufacturing, and labeling, among other information, must also be included in the PMA. As part of the PMA review, the FDA will inspect the manufacturer’s facilities for compliance with quality system regulation requirements, which govern testing, control, documentation, and other aspects of quality assurance with respect to manufacturing. The PMA approval can include post-approval conditions, including, among other things, restrictions on labeling, promotion, sale and distribution, or requirements to do additional clinical studies post-approval. Even after approval of a PMA, a new PMA or PMA supplement is required to authorize certain modifications to the device, its labeling, or its manufacturing process. Supplements to a PMA often require the submission of the same type of information required for an original PMA, except that the supplement is generally limited to that information needed to support the proposed change from the product covered by the original PMA.
Obtaining medical device clearance, approval, or licensing in the United States or abroad can be an expensive process. International fee structures vary from minimal to substantial, depending on the country. In addition, the Company is subject to annual establishment registration fees in the United States and abroad. Device licenses require periodic renewal with associated fees as well. Currently, the Company is registered as a Small Business Manufacturer with the FDA and as such is subject to reduced fees. If, in the future, our revenues exceed a certain annual threshold limit, the Company may not qualify for the Small Business Manufacturer reduced fee amounts and will be required to pay full fee amounts.
Post-Approval Regulation of Medical Devices
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
•the FDA quality systems regulation, which governs, among other things, how manufacturers design, test, manufacture, exercise quality control over, and document manufacturing of their products;
•labeling and claims regulations, which prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling;
•the Medical Device Reporting regulation, which requires reporting to the FDA of certain adverse experiences associated with use of the product; and
•post market surveillance, including documentation of clinical experience and follow-on, confirmatory studies.
The Company continues to be subject to inspection by the FDA to determine our compliance with regulatory requirements, as are our suppliers, contract manufacturers, and contract testing laboratories.
International sales of medical devices manufactured in the United States that are not approved or cleared by the FDA are subject to FDA export requirements. Exported devices are subject to the regulatory requirements of each country to which the device is exported. Exported devices may also fall under the jurisdiction of the United States Department of Commerce/Bureau of Industry and Security and compliance with export regulations may be required for certain countries.
Manufacturing Certifications
The Medical Device Single Audit Program (MDSAP)
MDSAP allows a single regulatory audit of a medical device manufacturer’s quality management system to satisfy the requirements of multiple regulatory authorities (RAs). Five RAs including: The Australian Therapeutic Goods Administration (TGA), Brazil’s Agência Nacional de Vigilância Sanitária (ANVISA), Health Canada, MHLW/PMDA (Japan), and the FDA participated in a three-year MDSAP Pilot which concluded in December 2016. These RAs have continued to participate in MDSAP since the program moved into its operational phase starting January 2017, with Health Canada making a full transition from the Canadian Medical Devices Conformity Assessment System (CMDCAS) to MDSAP in January of 2019.
MDSAP uses recognized third-party auditors – auditing organizations (AOs) – to conduct a single quality management system audit that satisfies the requirements of multiple regulatory authorities. Manufacturers only needed to comply with the regulations from the jurisdictions where they sell their products. The MDSAP certificate indicates that a manufacturer complies with the regulatory requirements for the markets defined in the certificate. The certificate does not represent marketing authorization, nor does it require any regulatory authority to issue a marketing authorization or endorsement to the device manufacturer.
The Company has been certified to the MDSAP requirements for the U.S., most recently successfully completing a MDSAP surveillance audit in September 2024. Audit to additional countries will be conducted if expansion to those markets is considered. This certificate is valid for three years. Annual surveillance audits are required to maintain this certification. A full recertification will be conducted in 2025.
Manufacturing cGMP Requirements
Manufacturers of medical devices are required to comply with FDA manufacturing requirements contained in the FDA’s current Good Manufacturing Practices (cGMP) set forth in the quality system regulations promulgated under section 520 of the Federal Food, Drug and Cosmetic Act. cGMP regulations require, among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation. The manufacturing facility for our products must meet cGMP requirements to the satisfaction of the FDA pursuant to a pre-PMA approval inspection before the Company can use it. The Company and some of our third-party service providers are also subject to periodic inspections of facilities by the FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations. Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations, and civil and criminal penalties. Adverse experiences with the product must be reported to the FDA and could result in the imposition of marketing restrictions through labeling changes or in product withdrawal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following the approval.
International Regulation
We are subject to regulations and product registration requirements in many foreign countries in which we may sell our products, including in the areas of product standards, packaging requirements, labeling requirements, import and export restrictions and tariff regulations, duties and tax requirements. The time required to obtain clearance required by foreign countries may be longer or shorter than that required for FDA clearance, and requirements for licensing a product in a foreign country may differ significantly from FDA requirements.
United States Anti-Kickback and False Claims Laws
In the United States, there are Federal and state anti-kickback laws that prohibit the payment or receipt of kickbacks, bribes or other remuneration intended to induce the purchase or recommendation of healthcare products and services. Violations of these laws can lead to civil and criminal penalties, including exclusion from participation in Federal healthcare programs. These laws are potentially applicable to manufacturers of products regulated by the FDA as medical devices, such as us, and hospitals, physicians, and other potential purchasers of such products. Other provisions of Federal and state laws provide civil and criminal penalties for presenting, or causing to be presented, to third-party payors for reimbursement, claims that are false or fraudulent, or which are for items or services that were not provided as claimed. In addition, certain states have implemented regulations requiring medical device and pharmaceutical companies to report all gifts and payments over $50 to medical practitioners. Although we intend to structure our future business relationships with clinical investigators and purchasers of our products to comply with these and other applicable laws, it is possible that some of our business practices in the future could be subject to scrutiny and challenge by Federal or state enforcement officials under these laws.
Third Party Reimbursement
We anticipate that sales volumes and prices of the products we commercialize will depend in large part on the availability of coverage and reimbursement from third party payors. Third party payors include governmental programs such as Medicare and Medicaid, private insurance plans, and workers’ compensation plans. Even though a new product may have been approved or cleared by the FDA for commercial distribution, we may find limited demand for the device until adequate history of reimbursement has been obtained from governmental and private third-party payors.
The CPT code for UltraMIST is 97610. This Category 1 code describes a system used in wound care that uses low frequency ultrasonic energy to atomize a liquid and deliver continuous low frequency ultrasound to the wound bed. The CPT codes for the dermaPACE System using extracorporeal shock wave technology to treat diabetic foot ulcers are 0512T and 0513T. The codes 0512T and 0513T are for extracorporeal shock wave for integumentary wound healing, including topical application and dressing and high energy extracorporeal shockwave therapy for integumentary wound healing. While these are Category 3 codes because the dermaPACE System is considered experimental by the CMS, this designation does not preclude billing and obtaining payment. Instead, claims are reviewed on an individual basis.
In international markets, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific product lines and procedures. There can be no assurance that procedures using our products will be considered medically reasonable and necessary for a specific indication, that our products will be considered cost-effective by third-party payors, that an adequate level of reimbursement will be available or that the third-party payors’ reimbursement policies will not adversely affect our ability to sell our products profitably.
We believe that the overall escalating costs of medical products and services has led to, and will continue to lead to, increased pressures on the healthcare industry to reduce the costs of products and services. In addition, recent healthcare reform measures, as well as legislative and regulatory initiatives at the Federal and state levels, create significant additional uncertainties. There can be no assurance that third party coverage and reimbursement will be available or adequate, or that future legislation, regulation, or reimbursement policies of third-party payors will not adversely affect the demand for our products or our ability to sell these products on a profitable basis. The unavailability or inadequacy of third-party payor coverage or reimbursement would have a material adverse effect on our business, operating results and financial condition.
Confidentiality and Security of Personal Health Information and Sensitive Personal Information
The Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act ("HITECH"), and their implementing regulations (collectively referred to as "HIPAA"), contains provisions that protect individually identifiable health information from unauthorized use or disclosure by covered entities and their business associates. The Office for Civil Rights of the U.S. Department of Health and Human Services ("HHS"), the agency responsible for enforcing HIPAA, has published regulations to address the privacy (the “Privacy Rule”) and security (the “Security Rule”) of protected health information ("PHI"). HIPAA also requires that all providers who transmit claims for health care goods or services electronically utilize standard transaction and data sets and to standardize national provider identification codes. In addition, the American Recovery and Reinvestment Act enacted the HITECH Act, which extends the scope of HIPAA to permit enforcement against business associates for a violation, establishes new requirements to notify the Office for Civil Rights of HHS of a breach of HIPAA, and allows the Attorneys General of the states to bring actions to enforce violations of HIPAA.
We anticipate that, as we expand our business, we may in the future be a covered entity under HIPAA. We have adopted policies and procedures to comply with the Privacy Rule, the Security Rule and the HIPAA statute as such regulations become applicable to our business. We currently don’t capture patient data through our PACE system.
In addition to HIPAA, many states have laws that govern the processing, collection, use, disclosure, transfer, storage, disposal and protection of health-related and other sensitive and personal information. These state law protections are different and, in some cases, may be more stringent, broader in scope, or offer greater individual rights with respect to sensitive health information than HIPAA. These laws are evolving rapidly and may differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Such laws and regulations will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our future customers and strategic partners. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. By way of example, the California Consumer Privacy Act, as amended by the California Privacy Rights Act, (“CCPA”) gives California residents individual privacy rights to access and delete their personal information, opt out of certain personal information sharing, limit the use of their sensitive personal information, and receive detailed information about how their personal information is used. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches. The CCPA also established a new California agency, the California Privacy Protection Agency, which is authorized to issue new substantive regulations and has independent enforcement power alongside the California Attorney General. These additional rights and the establishment of an agency with independent enforcement powers are expected to increase data breach litigation and government enforcement activity in California. Comprehensive privacy legislation similar to the CCPA has been adopted in other U.S. states including Colorado, Connecticut, Kentucky, Maryland, Minnesota, Montana, New Jersey, New Hampshire, Nevada, Oregon, Rhode Island, Tennessee, Texas, Utah, and Virginia. In the event that we are subject to or affected by HIPAA, the CCPA, or other domestic privacy and data protection laws, any liability from failure to comply with the requirements of these laws could adversely affect our financial condition.
In addition to the state comprehensive data privacy laws, recent years have brought substantial changes to the federal and state treatment of non-HIPAA consumer health information. At the federal level, the FTC brought three enforcement actions in 2023 against a range of companies that handle electronic health information relating to collection and disclosure of non-HIPAA covered consume health information under Section 5 of the FTC Act, two of which included allegations made under the FTC’s Health Breach Notification Rule (“HBNR”). The FTC’s focus on health information continued in 2024 with changes to the HBNR that clarified its scope and emphasized applicability to non-HIPAA health care providers as well as three additional enforcement actions against companies for their use of health information for advertising purposes. On the state level, Washington and Nevada have adopted significant new legislation addressing businesses treatment of consumer health information and Connecticut added more stringent protections for health information to its existing comprehensive state privacy law. In both Washington and Nevada’s laws, there are restrictive provisions limiting collection and disclosure of consumer health information, and Washington’s law provides a separate private right of action for violations.
We intend to adopt policies and procedures to ensure material compliance with state laws regarding the confidentiality of health information as such laws become applicable to us and to monitor and comply with new or changing state laws on an ongoing basis.
Environmental and Occupational Safety and Health Regulations
Our operations are subject to extensive Federal, state, provincial and municipal environmental statutes, regulations and policies, including those promulgated by the Occupational Safety and Health Administration, the United States Environmental Protection Agency, Environment and Climate Change Canada, Alberta Environment and Protected Areas, the Department of Health Services, and the Air Quality Management District, that govern activities and operations that may have adverse environmental effects such as discharges into air and water, as well as handling and disposal practices for solid and hazardous wastes. Some of these statutes and regulations impose strict liability for the costs of cleaning up, and for damages resulting from, sites of spills, disposals, or other releases of contaminants, hazardous substances and other materials and for the investigation and remediation of environmental contamination at properties leased or operated by us and at off-site locations where we have arranged for the disposal of hazardous substances. In addition, we may be subject to claims and lawsuits brought by private parties seeking damages and other remedies with respect to similar matters. We have not to date needed to make material expenditures to comply with current environmental statutes, regulations and policies. However, we cannot predict the impact and costs those possible future statutes, regulations and policies will have on our business.
Employees
As of December 31, 2024, we had a total of 46 full time employees in the United States. Of these, seven were engaged in research and development which also includes clinical, regulatory, and quality. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We believe our relationship with our employees is good.
Corporate Information
We were formed as a Nevada corporation in 2004. Our corporate headquarters address is 11495 Valley View Road, Eden Prairie, MN 55344, and our main telephone number is (800) 545-8810 or (952) 656-1029.
Available Information
We maintain a website at www.sanuwave.com. We make available on our website, free of charge, our periodic reports and registration statements filed with the SEC, including our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Sections 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). We make these reports available through our website as soon as reasonably practicable after we electronically file such reports with, or furnish such reports to, the SEC. Our internet site and the information contained on or connected to that site are not incorporated by reference into this Annual Report on Form 10-K. The SEC also maintains a website at www.sec.gov that contains reports, proxy statements and other information regarding issuers that file electronically with the SEC.
Item 1A. RISK FACTORS
Investing in our common stock involves a high degree of risk. You should carefully consider the following risk factors and all other information contained in this Annual Report on Form 10-K, including the consolidated financial statements and the related notes, before purchasing our common stock. If any of the following risks actually occur, they may materially harm our business and our financial condition and results of operations. In any such event, the market price of our common stock could decline, and you could lose all or part of your investment.
Risks Related to our Business
Our recurring losses from operations and dependency upon future issuances of equity or other financing to fund ongoing operations have raised substantial doubt as to our ability to continue as a going concern. We will be required to raise additional funds to finance our operations and remain a going concern; we may not be able to do so, and/or the terms of any financings may not be advantageous to us.
The continuation of our business is dependent upon raising additional capital. We expect to devote substantial resources for the commercialization of UltraMIST which will require additional capital resources. We incurred a net loss of $31.4 million and $25.8 million for the years ended December 31, 2024, and 2023, respectively. The operating losses and the current portion of our Senior Secured Debt indicate substantial doubt about the Company’s ability to continue as a going concern for a period of at least twelve months from the filing of this Annual Report on Form 10-K.
Management’s plans are to obtain additional capital in 2025. The Company could obtain additional capital through the issuance of common or preferred stock, securities convertible into common stock, or secured or unsecured debt. These possibilities, to the extent available, may be on terms that result in significant dilution to the Company’s existing stockholders. In addition, there can be no assurances that the Company’s plans to obtain additional capital will be successful on the terms or timeline it expects, or at all. If these efforts are unsuccessful, the Company may be required to significantly curtail or discontinue operations or, if available, obtain funds through financing transactions with unfavorable terms.
The accompanying consolidated financial statements have been prepared in conformity with U.S. GAAP, which contemplate continuation of the Company as a going concern and the realization of assets and satisfaction of liabilities in the normal course of business. The carrying amounts of assets and liabilities presented in the consolidated financial statements do not necessarily purport to represent realizable or settlement values. The consolidated financial statements do not include any adjustment that might result from the outcome of this uncertainty. The Company’s consolidated financial statements do not include any adjustments relating to the recoverability of assets and classification of assets and liabilities that might be necessary should the Company be unable to continue as a going concern.
We have identified material weaknesses in our internal control over financial reporting. If we are unable to remediate these material weaknesses, or if we identify additional material weaknesses in the future or otherwise fail to maintain effective internal control over financial reporting or disclosure controls and procedures, it may result in material misstatements of our consolidated financial statements or cause us to fail to meet our periodic reporting obligations, which may adversely affect our business, financial condition, and results of operations.
We have identified material weaknesses in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. These material weaknesses are as follows:
•The Company lacked expertise and resources to analyze and properly apply U.S. GAAP to complex and non-routine transactions such as complex financial instruments and derivatives and complex sales distributing agreements with select vendors.
•A lack of internal resources to analyze and properly apply U.S. GAAP to account for financial instruments included in service agreements with select vendors.
•Failure to implement controls around the following accounting processes: Equity, Financial Reporting, Accounts Payable, Expenses, Revenue, Accounts Receivable, Tax, Cash, Debt, Fixed Assets, Inventory, Commissions, Entity-Level, Human Resources/Payroll, and IT processes: change management, operations, and access security. As such, we believe that accounting and IT processes and procedures need to be tested for operating effectiveness.
We are taking certain measures to remediate these material weaknesses described above as discussed further in Part II, Item 9A of this Annual Report on Form 10-K; however, such material weaknesses had not been remediated as of December 31, 2024. In addition, due to the material weaknesses in internal control over financial reporting, we have also determined that our disclosure controls and procedures were ineffective as of December 31, 2024. The material weaknesses will not be considered remediated until management completes the design and implementation of the measures described above and the controls operate for a sufficient period of time and management has concluded, through testing, that these controls are effective.
There can be no assurance as to when the material weaknesses will be remediated. At this time, we cannot provide an estimate of costs expected to be incurred in connection with implementing this remediation plan; however, these remediation measures will be time consuming, will result in us incurring significant costs, and will place significant demands on our financial and operational resources.
We cannot assure that the measures we have taken to date and may take in the future will be sufficient to remediate the control deficiencies that led to our material weaknesses in internal control over financial reporting or that they will prevent or avoid potential future material weaknesses to be identified in the future. The effectiveness of our internal control over financial reporting is subject to various inherent limitations, including cost limitations, judgments used in decision making, assumptions about the likelihood of future events, the possibility of human error and the risk of fraud. Any failure to design, implement and maintain effective internal control over financial reporting and effective disclosure controls and procedures, or any difficulties encountered in their implementation or improvement, may result in additional material misstatements of our consolidated financial statements, or cause us to fail to meet our periodic reporting obligations, which may adversely affect our business, financial condition and results of operations.
If we are unable to successfully raise additional capital, our viability may be threatened; however, if we do raise additional capital, your percentage ownership as a stockholder could decrease and constraints could be placed on the operations of our business.
We have experienced negative operating cash flows since our inception and have funded our operations primarily from proceeds received from sales of our capital stock, the issuance of promissory notes and convertible promissory notes, the issuance of notes payable to related parties, and product sales. We will seek to obtain additional funds in the future either through equity or debt financings or through strategic alliances with third parties, either alone or in combination with equity financings. These financings could result in substantial dilution to the holders of our common stock or require contractual or other restrictions on our operations or on alternative business opportunities that may be available to us. If we can raise additional funds by issuing debt securities, these debt securities could impose significant additional restrictions on our operations. Any such required financing may not be available in amounts or on terms acceptable to us, and the failure to procure such required financing could have a material adverse effect on our business, financial condition, and results of operations, or threaten our ability to continue as a going concern.
A variety of factors could impact our need to raise additional capital, the timing of any required financing and the amount of such financings. Factors that may cause our future capital requirements to be greater than anticipated or could accelerate our need for funds include, without limitation:
•unanticipated expenditures in research and development or manufacturing activities;
•unanticipated expenditures in the acquisition and defense of intellectual property rights;
•the failure to develop strategic alliances for the marketing of some of our products;
•unforeseen changes in healthcare reimbursement for procedures using any of our approved products;
•inability to train a sufficient number of physicians to create a demand for any of our approved products;
•lack of financial resources to adequately support our operations;
•difficulties in maintaining commercial scale manufacturing capacity and capability;
•unforeseen problems with our third-party manufacturers, service providers or specialty suppliers of certain raw materials;
•unanticipated difficulties in operating in international markets;
•unanticipated financial resources needed to respond to technological changes and increased competition;
•unforeseen problems in attracting and retaining qualified personnel;
•the impact of changes in U.S. health care law and policy on our operations;
•enactment of new legislation or administrative regulations;
•the application to our business of new court decisions and regulatory interpretations;
•claims that might be brought in excess of our insurance coverage;
•delays in timing of receipt of required regulatory approvals;
•the failure to comply with regulatory guidelines; and
•the uncertainty in industry demand and patient wellness behavior.
In addition, although we have no present commitments or understandings to do so, we may seek to expand our operations and product line through acquisitions. Any acquisition would likely increase our capital requirements.
The medical device/therapeutic product industries are highly competitive and subject to rapid technological change. If our competitors are better able to develop and market products that are safer and more effective than any products we may develop, our commercial opportunities will be reduced or eliminated.
Our success depends, in part, upon our ability to maintain a competitive position in the development of technologies and products. We face competition from established medical device, pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies, and private and public research institutions in the United States and abroad. Many of our principal competitors have significantly greater financial resources and expertise than we do in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements, or mergers with, or acquisitions by, large and established companies, or through the development of novel products and technologies. The industry in which we operate has undergone, and we expect it to continue to undergo, rapid and significant technological change, and we expect competition to intensify as technological advances are made.
Many of our product component materials are only produced by a single supplier for such product component. If we are unable to obtain product component materials and other products from our suppliers that we depend on for our operations, or find suitable replacement suppliers, our ability to deliver our products to market will likely be impeded, which could have a material adverse effect on us.
We depend on suppliers for product component materials and other components that are subject to stringent regulatory requirements. Many of our product component materials are only produced by a single supplier for such product components. While we believe that alternative manufacturers and suppliers offering similar components are available on an as-needed basis and could be engaged in a reasonable period of time, there can be no assurance that the loss of these suppliers will not result in a disruption to our production. Our suppliers may encounter problems during manufacturing due to a variety of reasons, including failure to follow specific protocols and procedures, failure to comply with applicable regulations, equipment malfunction and environmental factors. Certain of our suppliers must be approved by regulatory authorities, which could delay our efforts to establish additional or replacement suppliers for these materials.
If we are unable to secure, on a timely basis, sufficient quantities of the materials we depend on to manufacture our products, if we encounter delays or contractual or other difficulties in our relationships with these suppliers, or if we cannot find replacement suppliers at an acceptable cost, the manufacturing of our products may be disrupted, which could increase our costs and have a material adverse effect on our business and results of operations.
The loss of our key management would likely hinder our ability to execute our business plan.
As a small company with less than 50 employees, our success depends on the continuing contributions of our management team and qualified personnel. Turnover, transitions or other disruptions in our management team and personnel could make it more difficult to successfully operate our business and achieve our business goals and could adversely affect our results of operation and financial condition. Our success depends in large part on our ability to attract and retain highly qualified personnel. We face intense competition in our hiring efforts from other pharmaceutical, biotechnology and medical device companies, as well as from universities and nonprofit research organizations, and we may have to pay higher salaries to attract and retain qualified personnel. The loss of one or more of these individuals, or our inability to attract additional qualified personnel, could substantially impair our ability to implement our business plan.
We face an inherent risk of liability if the use or misuse of our products results in personal injury or death.
The sale of products may expose us to product liability claims which could result in financial loss. Our clinical and commercial product liability insurance coverage may not be sufficient to cover claims that may be made against us. In addition, we may not be able to maintain insurance coverage at a reasonable cost, or in sufficient amounts or scope, to protect us against losses. Any claims against us, regardless of their merit, could severely harm our financial condition, strain our management team and other resources, and adversely impact or eliminate the prospects for commercialization of the product candidate, or sale of the product, that is the subject of any such claim. Although we do not promote any off-label use, off-label uses of products are common, and the FDA does not regulate a physician’s choice of treatment. Off-label uses of any of our products may subject us to additional liability.
We are dependent on information technology and our systems and infrastructure face certain risks, including from cybersecurity breaches and data leakage.
We rely to a large extent upon sophisticated information technology systems to operate our businesses, some of which are managed, hosted, provided and/or used by third parties or their vendors. We collect, store, and transmit large amounts of confidential information, and we deploy and operate an array of technical and procedural controls to maintain the confidentiality and integrity of such confidential information. A significant breakdown, invasion, corruption, destruction or interruption of critical information technology systems or infrastructure, by our workforce, others with authorized access to our systems or unauthorized persons could negatively impact our operations. The ever-increasing use and evolution of technology, including cloud-based computing, creates opportunities for the unintentional dissemination or intentional destruction of confidential information stored in our or our third-party providers’ systems, portable media, or storage devices. We could also experience, and in some cases have experienced in the past, a business interruption, theft of confidential information, financial theft, or reputational damage from industrial espionage attacks, malware, spoofing or other cyber-attacks, which may compromise our system infrastructure, lead to data leakage, either internally or at our third-party providers, or materially adversely impact our financial condition.
We have previously disclosed that we have experienced cybersecurity breaches from email spoofing. While we have invested in the protection of data and information technology, there can be no assurance that our efforts will prevent service interruptions or security breaches. Any such interruption or breach of our systems could adversely affect our business operations and/or result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal, business, and reputational harm to us.
If we are unable to expand, manage and maintain our direct sales and marketing organizations, we may not be able to generate anticipated revenue.
Building the requisite sales, marketing and distribution capabilities to successfully market and sell our products continues to be expensive and time-consuming and requires significant attention from our leadership team to manage. Any failure or delay in the expansion of our sales, marketing or distribution capabilities would adversely impact the commercialization of our products. Additionally, we may choose to collaborate, either globally or on a territory-by-territory basis, with third parties on the commercialization of our products. If we are unable to enter into arrangements with such third parties on acceptable terms or at all, we may not be able to successfully commercialize our products.
If we fail to properly manage our growth effectively, our business could suffer.
We intend to continue to grow and may experience periods of rapid growth and expansion, which could place a significant additional strain on our limited personnel, information technology systems and other resources including but not limited to procuring sufficient office, warehouse, and production space to successfully operate our business and the inherent difficulties arising from acquiring, managing, or moving between such operating spaces. In particular, the hiring of our direct sales force requires significant management, financial and other supporting resources. Any failure by us to manage our growth effectively could have an adverse effect on our ability to achieve our development and commercialization goals.
To achieve our revenue goals, we must continue to successfully increase manufacturing output to meet expected customer demand. We may experience difficulties with manufacturing yields, quality control, component supply and shortages of qualified personnel, such as skilled operators who can assemble our product, among other problems. Any of these problems could result in delays in product availability and increases in expenses. Any such delay or increased expense could adversely affect our ability to generate revenue.
Future growth will continue to impose significant added responsibilities on management, including the need to identify, recruit, train and integrate additional employees. In addition, rapid and significant growth will place a strain on our administrative and operational infrastructure.
In order to manage our operations and growth, we will need to continue to improve our operational and management controls, reporting and information technology systems and financial internal control procedures. If we are unable to manage our growth effectively, it may be difficult for us to execute our business strategy and our operating results and may have an adverse effect on our business, financial condition, and results of operations.
We generate a portion of our revenue internationally and are subject to various risks relating to our international activities, which could adversely affect our operating results.
On an annual basis, less than one percent of our revenue comes from international sources. While we have no current plan to materially expand our international operations, there can be no assurance we will not pursue such an expansion in the future. Engaging in international business involves several difficulties and risks, including, but not limited to, the following:
•required compliance with existing and changing foreign healthcare and other regulatory requirements and laws, such as those relating to patient privacy or handling of bio-hazardous waste;
•required compliance with anti-bribery laws, data privacy requirements, labor laws and anti-competition regulations;
•export or import restrictions;
•political and economic instability,
•foreign exchange controls; and
•difficulties protecting or procuring intellectual property rights.
With respect to our international operations, our results of operations and cash flows are subject to fluctuations due to changes in foreign currency exchange rates. Our expenses are generally denominated in the currencies in which our operations are located, which is in the United States. If the value of the U.S. dollar increases relative to foreign currencies in the future, in the absence of a corresponding change in local currency prices, our future revenue could be adversely affected as we convert future revenue from local currencies to U.S. dollars.
Provisions in our Articles of Incorporation, Bylaws and Nevada law might decrease the chances of an acquisition.
Provisions of our Articles of Incorporation and Bylaws and applicable provisions of Nevada law may delay or discourage transactions involving an actual or potential change in control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests. Some of the following provisions in our Articles of Incorporation or Bylaws that may decrease our attractiveness to be acquired are:
•advance notice of business to be brought is required for a meeting of our stockholders;
•the affirmative vote of the holders of at least sixty-six and two-thirds percent of the Company’s outstanding voting power is required for stockholders to amend our Bylaws;
•stockholders are prohibited from requesting or calling a special meeting of stockholders;
•no cumulative voting rights for the holders of common stock in the election of directors; and
•vacancies in the board of directors may be filled by a majority vote of the directors then in office or by a sole remaining director, in either case though less than a quorum.
In addition, Section 78.438 of the Nevada Revised Statutes prohibits a publicly-held Nevada corporation from engaging in a business combination with an interested stockholder (generally defined as a person which together with its affiliates owns, or within the last three years has owned, 10% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder) unless the business combination is approved in a prescribed manner. The existence of the foregoing provisions and other potential anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our common stock. They could also deter potential acquirers of our Company, thereby reducing the likelihood that you could receive a premium for your common stock in an acquisition.
Regulatory Risks
We are subject to extensive governmental regulation, including the FDA.
We and our products, our suppliers, and our contract manufacturers are subject to extensive regulation by governmental authorities in the United States and other countries. Failure to comply with applicable requirements could result in, among other things, any of the following actions:
•warning letters,
•fines and other monetary penalties,
•unanticipated expenditures,
•product recall or seizure,
•interruption of manufacturing,
•operating restrictions,
•injunctions, and
•criminal prosecutions.
In addition to the approval and clearance requirements, numerous other regulatory requirements apply to us and our products, our suppliers and contract manufacturers. These include requirements related to the following:
•testing,
•manufacturing,
•quality control,
•labeling,
•advertising,
•promotion,
•distribution,
•export,
•reporting to the FDA certain adverse experiences associated with the use of the products, and
•obtaining additional approvals or clearances for certain modifications to the products or their labeling or claims.
We are also subject to inspection by the FDA and other international regulatory bodies to determine our compliance with regulatory requirements, as are our suppliers and contract manufacturers, and we cannot be sure that the FDA and other international regulatory bodies will not identify compliance issues that may disrupt production or distribution or require substantial resources to correct.
The FDA’s requirements and international regulatory body requirements may change, and additional regulations may be promulgated that could affect us, our products, and our suppliers and contract manufacturers. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action. There can be no assurance that we will not be required to incur significant costs to comply with such laws and regulations in the future, or that such laws or regulations will not have a material adverse effect upon our business.
Regulatory approval of our products may be withdrawn at any time.
After regulatory approval has been obtained for medical device products, the product and the manufacturer are subject to continual review, including the review of adverse experiences and clinical results that are reported after our products are made available to patients, and there can be no assurance that such approval will not be withdrawn or restricted.
Regulators may also subject approvals to restrictions or conditions or impose post-approval obligations on the holders of these approvals, and the regulatory status of such products may be jeopardized if such obligations are not fulfilled. If post-approval studies are required, such studies may involve significant time and expense.
The manufacturing facilities we use to make any of our products will also be subject to periodic review and inspection by the FDA or other regulatory authorities, as applicable. The discovery of any new or previously unknown problems with the product or facility may result in restrictions on the product or facility, including withdrawal of the product from the market. We will continue to be subject to the FDA or other regulatory authority requirements, as applicable, governing the labeling, packaging, storage, advertising, promotion, recordkeeping, and submission of safety and other post-market information for all of our products, even those that the FDA or other regulatory authority, as applicable, had approved. If we fail to comply with applicable continuing regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approval, product recalls and seizures, operating restrictions and other adverse consequences.
If we fail to obtain an adequate level of reimbursement for our approved products by third party payors, there may be no commercially viable markets for our approved products, or the markets may be much smaller than expected.
The availability and levels of reimbursement by governmental and other third-party payors affect the market for our approved products. The efficacy, safety, performance, and cost-effectiveness of our products, and of any competing products will determine the availability and level of reimbursement. Reimbursement and healthcare payment systems in international markets vary significantly by country and include both government sponsored healthcare and private insurance. To obtain reimbursement or pricing approval in some countries, we may be required to produce clinical data, which may involve one or more clinical trials, that compares the cost-effectiveness of our approved products to other available therapies. We may not obtain international reimbursement or pricing approvals in a timely manner, if at all. Our failure to receive international reimbursement or pricing approvals would negatively impact market acceptance of our approved products in the international markets in which those pricing approvals are sought.
We believe that, in the future, reimbursement for any of our products may be subject to increased restrictions both in the United States and in international markets. Future legislation, regulation or reimbursement policies of third-party payors may adversely affect the demand for our products currently under development and limit our ability to sell our products on a profitable basis. In addition, third-party payors continually attempt to contain or reduce the costs of healthcare by challenging the prices charged for healthcare products and services. If reimbursement for our approved products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, market acceptance of our approved products would be impaired and our future revenues, if any, would be adversely affected.
Failure to obtain regulatory approval in foreign jurisdictions will prevent us from marketing our products abroad.
International sales of our products that we commercialize are subject to the regulatory requirements of each country in which the products are sold. Accordingly, the introduction of our products in markets outside the United States will be subject to regulatory approvals in those jurisdictions. The regulatory review process varies from country to country. Many countries impose product standards, packaging, and labeling requirements, and import restrictions on medical devices. In addition, each country has its own tariff regulations, duties, and tax requirements. The approval by foreign government authorities is unpredictable and uncertain and can be expensive. Our ability to market our approved products could be substantially limited due to delays in receipt of, or failure to receive, the necessary approvals or clearances.
Prior to marketing our products in any country outside the United States, we must obtain marketing approval in that country. Approval and other regulatory requirements vary by jurisdiction and differ from the United States’ requirements. We may be required to perform additional pre-clinical or clinical studies even if FDA approval has been obtained.
Uncertainty surrounding and future changes to healthcare law in the United States may have a material adverse effect on us.
The healthcare regulatory environment in the United States is currently subject to significant uncertainty and the industry may in the future continue to experience fundamental change because of regulatory reform. From time to time, legislation is drafted and introduced in the United States Congress that could significantly change the statutory provisions governing the clearance or approval, manufacture, marketing, and pricing of medical devices. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. We could experience an adverse impact on our operating results due to such changes, including increased pricing pressure in these markets.
Governments, hospitals, and other third-party payers also could reduce the amount of approved reimbursement for our products or deny coverage altogether. Reductions in reimbursement levels or coverage or other cost-containment measures could adversely affect our future operating results.
If we fail to comply with the United States Federal Anti-Kickback Statute, False Claims Act, and similar state laws, we could be subject to criminal and civil penalties and exclusion from the Medicare and Medicaid programs, which would have a material adverse effect on our business and results of operations.
A provision of the Social Security Act, commonly referred to as the Federal Anti-Kickback Statute, prohibits the offer, payment, solicitation, or receipt of any form of remuneration in return for referring, ordering, leasing, purchasing or arranging for, or recommending the ordering, purchasing or leasing of, items or services payable by Medicare, Medicaid or any other Federal healthcare program. The Federal Anti-Kickback Statute is very broad in scope and many of its provisions have not been uniformly or definitively interpreted by existing case law or regulations. In addition, most of the states have adopted laws like the Federal Anti-Kickback Statute, and some of these laws are even broader than the Federal Anti-Kickback Statute in that their prohibitions are not limited to items or services paid for by Federal healthcare programs, but instead apply regardless of the source of payment. Violations of the Federal Anti-Kickback Statute may result in substantial civil or criminal penalties and exclusion from participation in Federal healthcare programs.
Our operations may also implicate the False Claims Act. If we fail to comply with Federal and state documentation, coding, and billing rules, we could be subject to liability under the Federal False Claims Act, including criminal and/or civil penalties, loss of licenses and exclusion from the Medicare and Medicaid programs. The False Claims Act prohibits individuals and companies from knowingly submitting false claims for payments to, or improperly retaining overpayments from, the government.
Our financial relationships with healthcare providers and others who provide products or services to Federal healthcare program beneficiaries are potentially governed by the Federal Anti-Kickback Statute, False Claims Act, and similar state laws. We cannot be certain that we will not be subject to investigations or litigation alleging violations of these laws, which could be time-consuming and costly to us and could divert management’s attention from operating our business, which in turn could have a material adverse effect on our business. In addition, if our arrangements were found to violate the Federal Anti-Kickback Statute, False Claims Act or similar state laws, the consequences of such violations would likely have a material adverse effect on our business, results of operations and financial condition.
Failure to comply with the HIPAA and state-specific privacy laws, as such rules become applicable to our business, may increase our operational costs.
The HIPAA privacy and security regulations establish comprehensive Federal standards with respect to the uses and disclosures of PHI by certain entities, including health plans and health care providers, and set standards to protect the confidentiality, integrity, and availability of electronic PHI. The regulations establish a complex regulatory framework on a variety of subjects, including, for example: the circumstances under which uses and disclosures of PHI are permitted or required without a specific authorization by the patient; a patient’s right to access, amend and receive an accounting of certain disclosures of PHI; the content of notices of privacy practices describing how PHI is used and disclosed and individuals’ rights with respect to their PHI; and implementation of administrative, technical and physical safeguards to protect privacy and security of PHI. We anticipate that, as we expand our business, we will in the future be a covered entity under HIPAA. There can be no assurance that our policies and procedures will be adequate or will prevent all incidents of non-compliance with such regulations.
The HITECH Act and its implementing regulations also require healthcare providers to notify affected individuals, the Secretary of the U.S. Department of Health and Human Services, and in some cases, the media, when PHI has been breached as defined under and following the requirements of HIPAA. Many states have similar breach notification laws. In the event of a breach, to the extent such regulations are applicable to our business, we could incur operational and financial costs related to remediation as well as preparation and delivery of the notices, which costs could be substantial. Additionally, HIPAA, the HITECH Act, and their implementing regulations provide for significant civil fines, criminal penalties, and other sanctions for failure to comply with the privacy, security, and breach notification rules, including for wrongful or impermissible use or disclosure of PHI. Although the HIPAA statute and regulations do not expressly provide for a private right of action for damages, private parties may also seek damages under state laws for the wrongful or impermissible use or disclosure of confidential health information or other private personal information. Additionally, amendments to HIPAA provide that the state attorneys general may bring an action against a covered entity for a violation of HIPAA. As we expand our business such that Federal laws regarding PHI and privacy apply to our operations, any noncompliance with such regulations could have a material adverse effect on our business, results of operations and financial condition.
In addition to our obligations under HIPAA, many states have laws that govern the processing, collection, use, disclosure, transfer, storage, disposal and protection of health-related and other sensitive and personal information. These state law protections are different and, in some cases, may be more stringent, broader in scope, or offer greater individual rights with respect to sensitive health information than HIPAA. These laws are evolving rapidly and may differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Such laws and regulations will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our future customers and strategic partners. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. In addition to the state comprehensive data privacy laws, recent years have brought substantial changes to the federal and state treatment of non-HIPAA consumer health information. At the federal level, the FTC brought three enforcement actions in 2023 against a range of companies that handle electronic health information relating to collection and disclosure of non-HIPAA covered consume health information under Section 5 of the FTC Act, two of which included allegations made under the FTC’s HBNR. The FTC’s focus on health information continued in 2024 with changes to the HBNR that clarified its scope and emphasized applicability to non-HIPAA health care providers as well as three additional enforcement actions against companies for their use of health information for advertising purposes. On the state level, Washington and Nevada have adopted significant new legislation addressing businesses treatment of consumer health information, and Connecticut added more stringent protections for health information to its existing comprehensive state privacy law. In both Washington and Nevada’s laws, there are restrictive provisions limiting collection and disclosure of consumer health information, and Washington’s law provides a separate private right of action for violations. Any noncompliance with applicable laws or regulations could have a material adverse effect on our business, results of operations and financial condition.
We face periodic reviews and billing audits from governmental and private payors, and these audits could have adverse results that may negatively impact our business.
As a result of our participation in the Medicare and Medicaid programs, we are subject to various governmental reviews and audits to verify our compliance with these programs and applicable laws and regulations. We also are subject to audits under various government programs in which third-party firms engaged by the CMS conduct extensive reviews of claims data and medical and other records to identify potential improper payments under the Medicare program. Private pay sources also reserve the right to conduct audits. If billing errors are identified in the sample of reviewed claims, the billing error can be extrapolated to all claims filed, which could result in a larger overpayment than originally identified in the sample of reviewed claims. Our costs to respond to and defend reviews and audits may be significant and could have a material adverse effect on our business, financial condition, results of operations and cash flows. Moreover, an adverse review or audit could result in:
•required refunding or retroactive adjustment of amounts we have been paid by governmental or private payors;
•state or Federal agencies imposing fines, penalties and other sanctions on us;
•loss of our right to participate in the Medicare program, state programs, or one or more private payor networks; or
•damage to our business and reputation in various markets.
Any one of these results could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Product quality or performance issues may be discovered through ongoing regulation by the FDA and by comparable international agencies, as well as through our internal standard quality process.
The medical device industry is subject to substantial regulation by the FDA and by comparable international agencies. In addition to requiring clearance or approval to market new or improved devices, we are subject to ongoing regulation as a device manufacturer. Governmental regulations cover many aspects of our operations, including quality systems, marketing and device reporting. As a result, we continually collect and analyze information about our product quality and product performance through field observations, customer feedback and other quality metrics. If we fail to comply with applicable regulations or if post market safety issues arise, we could be subject to enforcement sanctions, our promotional practices may be restricted, and our marketed products could be subject to recall or otherwise impacted. Each of these potential actions could result in a material adverse effect on our business, operating results and financial condition.
The use of hazardous materials in our operations may subject us to environmental claims or liability.
We conduct research and development and manufacturing operations in our facility. Our research and development process may, at times, involve the controlled use of hazardous materials and chemicals. We may conduct experiments in which we may use small quantities of chemicals, including those that are corrosive, toxic, and flammable. The risk of accidental injury or contamination from these materials cannot be eliminated. We do not maintain a separate insurance policy for these types of risks. In the event of an accident or environmental discharge or contamination, we may be held liable for any resulting damages, and any liability could exceed our resources. We are subject to Federal, state, and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations could be significant.
Risks Related to Intellectual Property
The protection of our intellectual property is critical to our success, and any failure on our part to adequately protect those rights could materially adversely affect our business.
Our commercial success depends to a significant degree on our ability to:
•obtain and/or maintain protection for our products under the patent laws of the United States and other countries;
•defend and enforce our patents once obtained;
•obtain and/or maintain appropriate licenses to patents, patent applications or other proprietary rights held by others with respect to our technology, both in the United States and other countries;
•maintain trade secrets and other intellectual property rights relating to our products; and
•operate without infringing upon the patents, trademarks, copyrights, and proprietary rights of third parties.
The degree of intellectual property protection for our technology is uncertain, and only limited intellectual property protection may be available for our products, which may prevent us from gaining or keeping any competitive advantage against our competitors. Although we believe the patents that we own or license, and the patent applications that we own, generally provide us a competitive advantage, the patent positions of biotechnology, biopharmaceutical and medical device companies are generally highly uncertain, involve complex legal and factual questions and have been the subject of much litigation. Neither the United States Patent & Trademark Office nor the courts have a consistent policy regarding the breadth of claims allowed or the degree of protection afforded under many biotechnology patents. Even if issued, patents may be challenged, narrowed, invalidated, or circumvented, which could limit our ability to stop competitors from marketing similar products or limit the length of term of patent protection we may have for our products. Further, a court or other government agency could interpret our patents in a way such that the patents do not adequately cover our current or future products. Changes in either patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property or narrow the scope of our patent protection.
We also rely upon trade secrets and unpatented proprietary know-how and continuing technological innovation in developing our products, especially where we do not believe patent protection is appropriate or obtainable. We seek to protect this intellectual property, in part, by generally requiring our employees, consultants, and current and prospective business partners to enter into confidentiality agreements in connection with their employment, consulting or advisory relationships with us, where appropriate. We also require our employees, consultants, researchers, and advisors who we expect to work on our products to agree to disclose and assign to us all inventions conceived during the workday, developed using our property or which relate to our business. We may lack the financial or other resources to successfully monitor and detect, or to enforce our rights in respect of, infringement or breaches of these confidentiality agreements. In the case of any such undetected or unchallenged infringements or breaches, these confidentiality agreements may not provide us with meaningful protection of our trade secrets and unpatented proprietary know-how or adequate remedies. In addition, others may independently develop technology that is similar or equivalent to our trade secrets or know-how. If any of our trade secrets, unpatented know-how or other confidential or proprietary information is divulged to third parties, including our competitors, our competitive position in the marketplace could be harmed and our ability to sell our products successfully could be severely compromised. Enforcing a claim that a party illegally obtained and is using trade secrets that have been licensed to us or that we own is also difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. Costly and time-consuming litigation could be necessary to seek to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could have a material adverse effect on our business. Moreover, some of our academic institution licensees, evaluators, collaborators, and scientific advisors have rights to publish data and information to which we have rights. If we cannot maintain the confidentiality of our technologies and other confidential information in connection with our collaborations, our ability to protect our proprietary information or obtain patent protection in the future may be impaired, which could have a material adverse effect on our business.
Accordingly, we may fail to secure meaningful patent protection relating to any of our existing or future products or discoveries despite the expenditure of considerable resources. Further, there may be widespread patent infringement in countries in which we may seek patent protection, including countries in Europe and Asia, which may instigate expensive and time-consuming litigation that could adversely affect the scope of our patent protection. In addition, others may attempt to commercialize products similar to our products in countries where we do not have adequate patent protection. Failure to obtain adequate patent protection for our products, or the failure by particular countries to enforce patent laws or allow prosecution for alleged patent infringement, may impair our ability to be competitive. The availability of infringing products in markets where we have patent protection, or the availability of competing products in markets where we do not have adequate patent protection, could erode the market for our products, negatively impact the prices we can charge for our products, and harm our reputation if infringing or competing products are manufactured to inferior standards.
Patent applications owned by us or licensed to us may not result in issued patents, and our competitors may commercialize the discoveries we attempt to patent.
The patent applications that we own and that have been licensed to us, and any future patent applications that we may own or that may be licensed to us, may not result in the issuance of any patents. The standards that the United States Patent & Trademark Office and foreign patent agencies use to grant patents are not always applied predictably or uniformly and can change. Consequently, we cannot be certain as to the type and scope of patent claims to which we may in the future be entitled under our license agreements or that may be issued to us. These applications may not be sufficient to meet the statutory requirements for patentability and, therefore, may not result in enforceable patents covering the products we want to commercialize. Further, patent applications in the United States that are not filed in other countries may not be published or generally are not published until at least 18 months after they are first filed, and patent applications in certain foreign countries generally are not published until many months after they are filed. Scientific and patent publication often occurs long after the date of the scientific developments disclosed in those publications. As a result, we cannot be certain that we will be the first creator of inventions covered by our patents or applications, or the first to file such patent applications. As a result, our issued patents and our patent applications could become subject to challenge by third parties that created such inventions or filed patent applications before us or our licensors, resulting in, among other things, interference proceedings in the United States Patent & Trademark Office to determine priority of discovery or invention. Interference proceedings, if resolved adversely to us, could result in the loss of or significant limitations on patent protection for our products or technologies. Even in the absence of interference proceedings, patent applications now pending or in the future filed by third parties may prevail over the patent applications that may be owned by us or licensed to us or that we may file in the future, or may result in patents that issue alongside patents issued to us or our licensors or that may be issued or licensed to us in the future, leading to uncertainty over the scope of the patents owned by us or licensed to us or that may in the future be owned by us or impede our freedom to practice the claimed inventions.
Our patents may not be valid or enforceable and may be challenged by third parties.
We cannot assure you that the patents that have been issued or licensed to us would be held valid by a court or administrative body or that we would be able to successfully enforce our patents against infringers, including our competitors. The issuance of a patent is not conclusive as to its validity or enforceability, and the validity and enforceability of a patent is susceptible to challenge on numerous legal grounds, including the possibility of reexamination proceedings brought by third parties in the United States Patent & Trademark Office against issued patents and similar validity challenges under foreign patent laws. Challenges raised in patent infringement litigation brought by us or against us may result in determinations that patents that have been issued to us or licensed to us or any patents that may be issued to us or our licensors in the future are invalid, unenforceable or otherwise subject to limitations. In the event of any such determinations, third parties may be able to use the discoveries or technologies claimed in these patents without paying licensing fees or royalties to us, which could significantly diminish the value of our intellectual property and our competitive advantage. Even if our patents are held to be enforceable, others may be able to design around our patents or develop products similar to our products that are not within the scope of any of our patents.
In addition, enforcing the patents that we own or license and any patents that may be issued to us in the future against third parties may require significant expenditures regardless of the outcome of such efforts. Our inability to enforce our patents against infringers and competitors may impair our ability to be competitive and could have a material adverse effect on our business.
Issued patents and patent licenses may not provide us with any competitive advantage or provide meaningful protection against competitors.
The discoveries or technologies covered by issued patents we own or license may not have any value or provide us with a competitive advantage, and many of these discoveries or technologies may not be applicable to our products at all. We have devoted limited resources to identifying competing technologies that may have a competitive advantage relative to ours, especially those competing technologies that are not perceived as infringing on our intellectual property rights. In addition, the standards that courts use to interpret and enforce patent rights are not always applied predictably or uniformly and can change, particularly as new technologies develop. Consequently, we cannot be certain as to how much protection, if any, will be afforded by these patents with respect to our products if we, our licensees or our licensors attempt to enforce these patent rights and those rights are challenged in court.
The existence of third-party patent applications and patents could significantly limit our ability to obtain meaningful patent protection. If patents containing competitive or conflicting claims are issued to third parties, we may be enjoined from pursuing research, development or commercialization of product candidates or may be required to obtain licenses, if available, to these patents or to develop or obtain alternative technology. If another party controls patents or patent applications covering our product candidates, we may not be able to obtain the rights we need to those patents or patent applications in order to commercialize our product candidates or we may be required to pay royalties, which could be substantial, to obtain licenses to use those patents or patent applications.
In addition, issued patents may not provide commercially meaningful protection against competitors. Other parties may seek and/or be able to duplicate, design around or independently develop products having effects similar or identical to our patented products that are not within the scope of our patents.
Limitations on patent protection in some countries outside the United States, and the differences in what constitutes patentable subject matter in these countries, may limit the protection we have under patents issued outside of the United States. We do not have patent protection for our product candidates in several of our target markets. The failure to obtain adequate patent protection for our products in any country would impair our ability to be commercially competitive in that country.
The ability to market the products we develop is subject to the intellectual property rights of third parties.
The biotechnology, biopharmaceutical and medical device industries are characterized by many patents and patent filings and frequent litigation based on allegations of patent infringement. Competitors may have filed patent applications or have been issued patents and may obtain additional patents and proprietary rights related to products or processes that compete with or are similar to ours. We may not be aware of all the patents potentially adverse to our interests that may have been issued to others. Because patent applications can take many years to issue, there may be currently pending applications, unknown to us, which may later result in issued patents that our products or proprietary technologies may infringe. Third parties may claim that our products or related technologies infringe their patents or may claim that the products of our suppliers, manufacturers or contract service providers that produce our devices infringe on their intellectual property. Further, we, our licensees, or our licensors, may need to participate in interference, opposition, protest, reexamination or other potentially adverse proceedings in the United States Patent & Trademark Office or in similar agencies of foreign governments with regards to our patents, patent applications, and intellectual property rights. In addition, we, our licensees, or our licensors may need to initiate suits to protect our intellectual property rights.
Litigation or any other proceeding relating to intellectual property rights, even if resolved in our favor, may cause us to incur significant expenses, divert the attention of our management and key personnel from other business concerns and, in certain cases, result in substantial additional expenses to license technologies from third parties. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. An unfavorable outcome in any patent infringement suit or other adverse intellectual property proceeding could require us to pay substantial damages, including possible treble damages and attorneys’ fees, cease using our technology or developing or marketing our products, or require us to seek licenses, if available, of the disputed rights from other parties and potentially make significant payments to those parties. There is no guarantee that any prevailing party would offer us a license or that we could acquire any license made available to us on commercially acceptable terms. Even if we can obtain rights to a third party’s patented intellectual property, those rights may be nonexclusive and, therefore, our competitors may obtain access to the same intellectual property. Ultimately, we may be unable to commercialize our products or may have to cease some of our business operations because of patent infringement claims, which could materially harm our business.
We cannot guarantee that our products or technologies will not conflict with the intellectual property rights of others.
If we need to redesign our products to avoid third party patents, we may suffer significant regulatory delays associated with conducting additional clinical studies or submitting technical, clinical, manufacturing, or other information related to any redesigned product and, ultimately, in obtaining regulatory approval. Further, any such redesigns may result in less effective and/or less commercially desirable products if the redesigns are possible at all.
Additionally, any involvement in litigation in which we, or our licensees or our licensors, are accused of infringement may result in negative publicity about us or our products, injure our relations with any then-current or prospective customers and marketing partners, and cause delays in the commercialization of our products.
Risks Related to our Common Stock
Our stock price is volatile.
The market price of our common stock is volatile and could fluctuate widely in response to various factors, many of which are beyond our control, including the following:
•our ability to obtain additional financing and, if available, the terms and conditions of the financing;
•changes in our industry;
•additions or departures of key personnel;
•sales of our common stock;
•our ability to execute our business plan;
•operating results that fall below expectations;
•period-to-period fluctuations in our operating results;
•new regulatory requirements and changes in the existing regulatory environment; and
•general economic conditions and other external factors.
In addition, the securities markets have from time-to-time experienced significant price and volume fluctuations that are unrelated to the operating performance of companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
We have not paid dividends in the past and do not expect to pay dividends in the future. Any return on investment may be limited to the value of our common stock.
We have never paid cash dividends on our common stock and do not anticipate doing so in the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition and other business and economic factors affecting us at such time as our board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
The rights of the holders of common stock may be impaired by the potential issuance of preferred stock.
Our board of directors has the right, without stockholder approval, to issue preferred stock with voting, dividend, conversion, liquidation, or other rights which could adversely affect the voting power and equity interest of the holders of common stock, which could be issued with the right to more than one vote per share, and could be utilized as a method of discouraging, delaying or preventing a change of control. The possible negative impact on takeover attempts could adversely affect the price of our common stock.
We have not sought an advisory stockholder vote to approve the compensation of our named executive officers.
Rule 14a-21 under the Exchange Act requires us to seek a separate stockholder advisory vote at our annual meeting at which directors are elected to approve the compensation of our named executive officers, not less frequently than once every three years (say-on-pay vote), and, at least once every six years, to seek a separate stockholder advisory vote on the frequency with which we will submit advisory say-on-pay votes to our stockholders (say-on-frequency vote). We have not submitted to our stockholders a say-on-pay vote to approve an advisory resolution regarding our compensation program for our named executive officers, or a say-on-frequency vote. Consequently, the board of directors has not considered the outcome of our say-on-pay vote results when determining future compensation policies and pay levels for our named executive officers.
Item 1B. UNRESOLVED STAFF COMMENTS
None.
Item 1C. CYBERSECURITY
Our management and Board of Directors (the “Board”) recognize the importance of maintaining the security and resiliency of our cybersecurity environment to deliver on the expectations of our customers, business partners, employees, and investors. The Board is involved in our risk management practices. Overall, the purpose of our information security program is to protect the confidentiality, integrity and availability of our systems and data, along with the safe operation of our systems.
Technical safeguards
We deploy technical safeguards that are designed to protect our systems from cybersecurity threats, including firewalls, anti-malware software, and authentication and authorization controls.
Security awareness and training
We provide ongoing security awareness and training to educate internal users on how to identify and report potential issues. Phishing emails are discussed on a regular basis with employees to ensure proper protocols are followed. We also provide periodic updates to employees on emerging cybersecurity trends and ways to protect themselves and the Company.
Governance of Cybersecurity Risks
The Audit Committee of the Board has the primary responsibility for oversight, review and discussion with management of the Company’s information technology, data security, business continuity, artificial intelligence and cybersecurity-related risk exposures and threats; the potential impact of those risk exposures and threats on the Company’s business, operations and reputation; and the processes management has established to assess, manage, monitor and mitigate such risk exposures and threats. The Company’s Chief Executive Officer, President, and Chief Financial Officer are responsible for assessing and managing cybersecurity risks. The Company’s management periodically reports on cybersecurity issues and presents information to our Audit Committee as well as our full Board, as appropriate, on cybersecurity matters.
Upon verifying that a cybersecurity incident has occurred or is occurring, the Chief Executive Officer, President and Chief Financial Officer will promptly conduct a preliminary assessment of the severity level of the cybersecurity incident. Following this assessment, the Chief Executive Officer will determine whether to report the cybersecurity incident to the Audit Committee, who will then report such cybersecurity incident to the Board as the chair deems appropriate.
Material Impact of Cybersecurity Risks
We have not experienced a material information security breach incident, and we are not aware of any cybersecurity risks that are reasonably likely to materially affect our business. However, future incidents could have a material impact on our business strategy, results of operations or financial condition. For additional discussion of the risks posed by cybersecurity threats, see “Item 1A. Risk Factors— Risks to our Business— We are dependent on information technology and our systems and infrastructure face certain risks, including from cybersecurity breaches and data leakage.”
Item 2. PROPERTIES
Our primary corporate and operations office is a leased facility in Eden Prairie, Minnesota, consisting of 8,199 square feet of space under a lease which expires on August 31, 2025. Under the terms of the lease, we pay monthly rent, subject to a 2.5% adjustment on an annual basis.
We also have a research and development office in a leased facility in Alpharetta, Georgia, consisting of 4,332 square feet of space under a lease that expires in July 2027.
Item 3. LEGAL PROCEEDINGS
In the ordinary course of business, the Company from time to time becomes involved in various legal proceedings involving a variety of matters. We do not believe there are any pending legal proceedings that will have a material adverse effect on our business, consolidated financial position, results of operations, or cash flows. However, the outcome of such legal matters is inherently unpredictable and subject to significant uncertainties. The Company expenses legal fees in the period in which they are incurred.
There are no material proceedings known to us to be contemplated by any governmental authority.
There are no material proceedings known to us, pending, or contemplated, in which any of our directors, officers or affiliates or any of our principal security holders, or any associate of any of the foregoing, is a party or has an interest adverse to us.
Item 4. MINE SAFETY DISCLOSURE
Not applicable.
PART II
Item 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
The Company’s common stock is quoted on the Nasdaq Global Market under the symbol “SNWV”.
Holders of Common Stock
As of March 18, 2025, there were 8,548,473 shares of common stock outstanding and approximately 204 holders of record of the Company’s common stock.
Unregistered Sales of Equity Securities
On October 18, 2024, the Company issued 14,359 restricted stock units (“RSUs”) to FNK IR LLC as compensation for investor relations services. These RSUs vested in substantially equal installments on each of October 18, 2024, October 31, 2024, November 30, 2024, December 31, 2024, January 31, 2025 and February 28, 2025. The Company relied upon the exemption from registration provided by Section 4(a)(2) of the Securities Act of 1933, as amended, for transactions not involving a public offering.
Dividends
The Company did not pay a cash dividend in 2024 or 2023. The Company intends to retain future earnings, if any, to finance the expansion of its business. The Company does not anticipate paying any cash dividends in the foreseeable future.
Item 6. [Reserved]
Not applicable.
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations provides information management believes to be relevant to understanding the financial condition and results of operations of the Company. The discussion focuses on our financial results of operations for the years ended December 31, 2024 and 2023. You should read this discussion and analysis in conjunction with our consolidated financial statements and related notes thereto for the years ended December 31, 2024, and 2023, which are presented within Part II, Item 8. "Financial Statements and Supplementary Data" in this Annual Report on Form 10-K. Amounts reported in thousands within this annual report are computed based on the amounts in thousands, and therefore, the sum of the components may not equal the total amount reported in thousands due to rounding.
Executive Summary
We realized significant revenue growth during the year ended December 31, 2024, with a 60% growth in revenue to $32.6 million for the year ended December 31, 2024, as compared to $20.4 million in 2023. Gross margins also increased to 75% from 70% in 2023. As the Company continues to focus on profitable growth, we have also increased our operating income by 1103% to $5.4 million for the year ended December 31, 2024, compared to an operating loss of $0.5 million for the year ended December 31, 2023.
Net loss for the year ended December 31, 2024, was $31.4 million, or $7.03 per basic and diluted share, compared to a net loss of $25.8 million, or $12.19 per basic and diluted share, for the year ended December 31, 2023, an increase of $5.6 million, which was largely driven by a non-cash change in the fair value of derivatives. We believe these improvements set the stage for additional growth as we head into 2025.
Recent Developments
On March 7, 2025, our common stock began trading on The Nasdaq Global Market under the ticker symbol “SNWV.”
Non-GAAP Financial Measures
Throughout this Management’s Discussion and Analysis of Financial Condition and Results of Operations, we present certain financial measures that facilitate management’s review of the operational performance of the Company and as a basis for strategic planning; however, such financial measures are not presented in our financial statements prepared in accordance with accounting principles generally accepted in the United States (“U.S.”) (“U.S. GAAP”). These financial measures are considered “non-GAAP financial measures” and are intended to supplement, and should not be considered as superior to, or a replacement for, financial measures presented in accordance with U.S. GAAP.
The Company uses Earnings Before Interest, Taxes, Depreciation and Amortization (“EBITDA”) and Adjusted EBITDA to assess its operating performance. Adjusted EBITDA is Earnings before Interest, Taxes, Depreciation and Amortization adjusted for the change in fair value of derivatives and any significant non-cash or non-recurring infrequent charges. EBITDA and Adjusted EBITDA should not be considered as alternatives to net loss as a measure of financial performance or any other performance measure derived in accordance with U.S. GAAP, and they should not be construed as an inference that our future results will be unaffected by unusual or infrequent items. These non-GAAP financial measures are presented in a consistent manner for each period, unless otherwise disclosed. The Company uses these measures for the purpose of evaluating its historical and prospective financial performance, as well as its performance relative to competitors. These measures also help the Company to make operational and strategic decisions. The Company believes that providing this information to investors, in addition to U.S. GAAP measures, allows them to see the Company’s results through the eyes of management, and to better understand its historical and future financial performance. These non-GAAP financial measures are also frequently used by analysts, investors, and other interested parties to evaluate companies in our industry, when considered alongside other U.S. GAAP measures.
EBITDA and Adjusted EBITDA have their limitations as analytical tools, and you should not consider them in isolation or as a substitute for analysis of our results as reported under U.S. GAAP. Some of these limitations are that EBITDA and Adjusted EBITDA:
•Do not reflect every expenditure, future requirements for capital expenditures or contractual commitments.
•Do not reflect all changes in our working capital needs.
•Do not reflect interest expense, or the amount necessary to service our outstanding debt.
As presented in the GAAP to Non-GAAP Reconciliations section below, our non-GAAP financial measures exclude the impact of certain charges that contribute to our net loss.
|
|
|
|
|
|
|
|
|
|
|
|
|
For the year ended |
| (in thousands) |
2024 |
|
2023 |
|
|
|
|
| Net (Loss) Income |
$ |
(31,372) |
|
|
$ |
(25,807) |
|
| Non-GAAP Adjustments: |
|
|
|
| Interest expense |
13,637 |
|
|
15,623 |
|
| Depreciation and amortization |
1,145 |
|
|
1,028 |
|
| EBITDA |
$ |
(16,590) |
|
|
$ |
(9,156) |
|
|
|
|
|
| Non-GAAP Adjustments for Adjusted EBITDA: |
|
|
|
| Change in fair value of derivative liabilities |
31,413 |
|
|
9,621 |
|
| Other non-cash or infrequent charges: |
|
|
|
| Gain on extinguishment of debt |
(6,326) |
|
|
- |
|
| Severance agreement and legal settlement |
741 |
|
|
- |
|
| Release of historical accrued expenses |
(1,547) |
|
|
(1,866) |
|
| Stock-based compensation |
1,514 |
|
|
- |
|
| Shares issued for services |
- |
|
|
224 |
|
| License and option agreement |
(2,500) |
|
|
- |
|
| Prepaid legal fees expensed from termination of Merger Agreement |
457 |
|
|
- |
|
| Adjusted EBITDA |
$ |
7,162 |
|
|
$ |
(1,177) |
|
Results of Operations
The following table sets forth our consolidated statement of operations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
Change |
| (in thousands) |
2024 |
|
2023 |
|
$ |
|
% |
| Revenue |
$ |
32,634 |
|
|
$ |
20,398 |
|
|
$ |
12,236 |
|
|
60 |
% |
| Cost of revenue |
8,084 |
|
|
6,035 |
|
|
2,049 |
|
|
34 |
% |
| Gross margin |
24,550 |
|
|
14,363 |
|
|
10,187 |
|
|
71 |
% |
| Gross margin % |
75 |
% |
|
70 |
% |
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
| General and administrative |
11,348 |
|
|
8,674 |
|
|
2,674 |
|
|
31 |
% |
| Selling and marketing |
6,323 |
|
|
4,898 |
|
|
1,425 |
|
|
29 |
% |
| Research and development |
673 |
|
|
579 |
|
|
94 |
|
|
16 |
% |
| Depreciation and amortization |
789 |
|
|
752 |
|
|
37 |
|
|
5 |
% |
| Operating income (loss) |
5,417 |
|
|
(540) |
|
|
5,957 |
|
|
1103 |
% |
| Other expense, net |
(36,762) |
|
|
(25,263) |
|
|
(11,499) |
|
|
46% |
| Income tax expense |
27 |
|
|
4 |
|
|
23 |
|
|
575 |
% |
| Net loss |
$ |
(31,372) |
|
|
$ |
(25,807) |
|
|
$ |
(5,565) |
|
|
22 |
% |
Revenue
Revenues for the year ended December 31, 2024 were $32.6 million, compared to $20.4 million for 2023, an increase of $12.2 million or 60%. The increase in net sales was primarily driven by the growth in quantity of UltraMIST® disposables and systems sold.
The quantity of UltraMIST® disposables sold increased by 37% in 2024 as compared to 2023. The quantity of UltraMIST® systems sold increased by 77% in 2024 as compared to 2023. Pricing of the UltraMIST® system and disposables also showed growth in 2024 as compared to 2023; disposables average selling price increased 21% in 2024, and systems average selling price increased 10% in 2024. Revenue from UltraMIST® totaled over 98% of total revenue in 2024 and 90% in 2023.
Cost of Revenue
Cost of revenues for the year ended December 31, 2024 was $8.1 million, compared to $6.0 million for 2023. Gross profit as a percentage of revenues was 75% for the year ended December 31, 2024, compared to 70% for the same period in 2023. This increase in gross margin was largely driven by increased pricing on our UltraMIST systems and applicators.
General and Administrative
General and administrative expenses for the year ended December 31, 2024 were $11.3 million as compared to $8.7 million for 2023, an increase of $2.7 million, or 31%. The increase in 2024 as compared to 2023 was primarily due to increased headcount, severance and legal settlement expenses, and non-cash charges for stock-based compensation expense.
Selling and Marketing
Selling and marketing expenses for the year ended December 31, 2024 were $6.3 million as compared to $4.9 million for 2023, an increase of $1.4 million, or 29%. The year-over-year increase in sales and marketing expenses in 2024 was largely driven by increased commission expenses due to increased sales.
Research and Development
Research and development expenses for the year ended December 31, 2024 were $0.7 million, compared to $0.6 million for 2023. The research and development costs in 2024 remained approximately consistent with the costs in 2023.
Other Income (Expense), net
Other expense, net consists of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the years ended December 31, |
|
Change |
|
2024 |
|
2023 |
|
$ |
|
% |
|
|
|
|
|
|
|
|
| Interest expense |
$ |
(13,637) |
|
|
$ |
(15,623) |
|
|
$ |
1,986 |
|
|
(13 |
%) |
| Change in fair value of derivatives |
(31,413) |
|
|
(9,621) |
|
|
(21,792) |
|
|
nm |
| Gain on extinguishment of debt |
6,326 |
|
|
- |
|
|
6,326 |
|
|
nm |
| Other expense |
(893) |
|
|
(19) |
|
|
(874) |
|
|
nm |
| Other income |
2,855 |
|
|
- |
|
|
2,855 |
|
|
nm |
| Other expense, net |
$ |
(36,762) |
|
|
$ |
(25,263) |
|
|
$ |
(11,499) |
|
|
nm |
| nm - not meaningful |
|
|
|
|
|
|
|
Other expenses totaled $36.8 million for the year ended December 31, 2024, as compared $25.3 million for 2023, an increase of $11.5 million. The increase was primarily driven by an increased loss from the change in the fair value of derivative liability of $21.8 million, partially offset by a gain on the extinguishment of debt of $6.3 million and other income of $2.5 million from a license and option agreement. The change in fair value of the derivative liability mainly relates to warrants issued during 2024, 2023, and 2022 with the convertible debt. That convertible debt and associated warrants were converted to common stock in October 2024, as further discussed in Note 16 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data”. The gain on extinguishment of debt was mainly due to the settlement of outstanding notes to Celularity and HealthTronics, as further discussed in Note 10 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data”.
Liquidity and Capital Resources
Since inception, we have incurred losses from operations each year. As of December 31, 2024, we had an accumulated deficit of $251 million. Historically, our operations have primarily been funded from the sale of capital stock, notes payable, and convertible debt securities.
See Notes 1, 10, 11, 15, and 16, to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for additional information regarding the Convertible Promissory Notes, Senior Secured Note, Reverse Stock Split, and the October 2024 transaction.
The following table presents summarized cash flow information:
|
|
|
|
|
|
|
|
|
|
|
|
|
For the period ended December 31, |
| (in thousands) |
2024 |
|
2023 |
| Cash flows provided by (used in) operating activities |
$ |
2,455 |
|
|
$ |
(4,538) |
|
| Cash flows (used in) provided by investing activities |
$ |
(490) |
|
|
$ |
21 |
|
| Cash flows provided by financing activities |
$ |
6,354 |
|
|
$ |
5,211 |
|
Cash Flows from Operating Activities
We have improved our cash flow from operations in 2024 as compared to 2023, which was driven by increased emphasis on improved cash management and operating expense management. Additional volatility in adjustments of cash flows from operations is the change in fair value of derivative liabilities connected to our convertible debt and warrants issued. The Company recognized a loss on these liabilities of $31.4 million for the year ended December 31, 2024, as compared to a loss of $9.6 million for the year ended December 31, 2023.
Cash Flows Provided by Financing Activities
Cash flows provided by financing activities increased while also paying off outstanding debt. For the year ended December 31, 2024, we received proceeds of $12.1 million from the issuance of the convertible promissory notes, sales of common stock, and proceeds from promissory note payable as compared to $6.0 million for the year ended December 31, 2023. In 2024, we paid off outstanding debt owed to Celularity, HealthTronics, and our factoring line of credit for a total of $5.0 million.
Going Concern
The Company has incurred recurring operating losses in prior years, has negative working capital, and the Senior Secured Note becomes due in September 2025, which raises substantial doubt about our ability to continue as a going concern for a period of 12 months from the filing of the Form 10-K.
During the current fiscal year, the Company has achieved operating income, reflecting a significant improvement in its financial performance. The Company is addressing its financial obligations, including the significant portion of debt that is coming due in September 2025. Management is actively engaged in discussions with lenders and financial institutions to refinance this debt, which will extend the maturity of the debt and provide additional liquidity to support ongoing operations and strategic initiatives.
Although no assurances can be given that our plans to obtain refinancing will be successful or on the terms or timeline we expect, or at all, management believes that the actions taken to date, along with the planned initiatives, will enable the Company to meet its obligations as they become due and to continue as a going concern.. If these efforts are unsuccessful, we may be required to significantly curtail or discontinue operations or obtain funds through financing transactions with unfavorable terms.
See Note 2 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for additional information on our ability to continue as a going concern.
Critical Accounting Estimates
We consider an accounting estimate to be critical if: (1) the accounting estimate requires us to make assumptions about matters that were highly uncertain at the time the accounting estimate was made, and (2) changes in the estimate are reasonably likely to occur from period to period, or use of different estimates that we reasonably could have used in the current period, would have a material impact on our financial condition or results of operations.
Management has discussed the development and selection of these critical accounting estimates with the Audit Committee of our Board of Directors. In addition, there are other items within our consolidated financial statements that require estimation, but are not deemed critical as defined above. Changes in estimates used in these and other items could have a material impact on our consolidated financial statements.
We have used various accounting policies to prepare the consolidated financial statements in accordance with U.S. GAAP. Our significant accounting policies are disclosed in Note 3 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
The preparation of the consolidated financial statements, in conformity with U.S. GAAP, requires us to use judgment in making estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, and expenses. These estimates reflect our best judgment about economic and market conditions and the potential effects on the valuation and/or carrying value of assets and liabilities based upon relevant information available. We base our estimates on historical experience and on various assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources.
The following accounting estimates are deemed critical:
Litigation Contingencies
We may be involved in legal actions involving product liability, intellectual property and commercial disputes, tax disputes, and governmental proceedings and investigations. The outcomes of these legal actions are not completely within our control and may not be known for prolonged periods of time. In some actions, the enforcement agencies or private claimants seek damages that could require significant expenditures or result in lost revenues or limit our ability to conduct business in the applicable jurisdictions. Estimating probable losses from our litigation and governmental proceedings is inherently difficult, particularly when the matters are in early procedural stages, with incomplete scientific facts or legal discovery; involve unsubstantiated or indeterminate claims for damages; potentially involve penalties, fines, or punitive damages; or could result in a change in business practice. The Company records a liability in the consolidated financial statements for loss contingencies when a loss is known or considered probable, and the amount may be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and may be reasonably estimated, the estimated loss or range of loss is disclosed. The Company has reserved approximately $150 thousand for unasserted claims. Our significant legal proceedings are discussed in Note 21 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Derivative Liabilities from Warrants
The Company determined that certain warrants qualified as derivative financial instruments. Various valuation models were used to estimate the fair value of these derivative financial instruments that are classified as derivative liabilities on the consolidated balance sheets. The models include subjective input assumptions that can materially affect the fair value estimates and as such are subject to uncertainty. The Company's volatility is the most significant assumption and changes over time with the market. Our significant input assumptions are discussed in Note 13 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Recently Issued Accounting Standards
Information regarding new accounting pronouncements is included in Note 3 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a “smaller reporting company” as defined by Item 10 of Regulation S-K, we are not required to provide the information required under this item.
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
|
|
|
|
|
Page |
| Consolidated Financial Statements |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors of
SANUWAVE Health, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of SANUWAVE Health, Inc. (the “Company”) and Subsidiaries as of December 31, 2024 and 2023, the related consolidated statements of comprehensive loss, stockholders’ deficit, and cash flows for each of the two years in the period ended December 31, 2024, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2024, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 2, the Company has incurred recurring losses, has negative working capital, and needs to refinance its debt to meet its obligations and sustain its operations. These conditions raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Valuation of Financial Instruments (Warrant Liability)
Critical Audit Matter Description
As described in Note 13 to the consolidated financial statements, the Company has entered into warrant agreements accounted for as a liability and recorded at fair value. The fair value of the warrant liability was valued using valuation techniques and key inputs as described in the footnotes.
The principal considerations for our determination that the valuation of the warrant liability is a critical audit matter were the significant judgments made by management in determining the fair value of the warrant liability. This in turn led to a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions. The audit also involved the use of professionals with specialized skill and knowledge.
How the Critical Audit Matter was Addressed in the Audit
Our audit procedures related to the valuation of the financial instruments included the following, among others:
▪We obtained an understanding of the design of the Company's controls over valuation of financial instruments, including controls over management's review of the valuation models, and the significant assumptions used in determining the fair value of the financial instruments.
▪With assistance of our valuation specialists, we audited the fair value of the warrant liability, valuation methodology, and key assumptions used in determining the fair value of the warrant liability by:
a.Evaluating the appropriateness of the valuation models and techniques used in determining the fair value;
b.Assessing the reasonableness of the significant valuation inputs, including the probability weighted expected value considering the merger agreement with SEPA Acquisition Corp. (“SEPA”), the risk adjusted expected exchange ratio, the value of SEPA’s Class A common stock, the expected timing of the closing of the merger, and the probability of the merger closing;
c.Assessing the reasonableness of the significant valuation inputs, including the probability weighted expected value considering the reverse stock split of the Company’s common stock (the “Reverse Stock Split”), the expected exchange ratio, the value of the Company’s common stock, the expected timing of the effectuating of the Reverse Stock Split, and the probability of the Reverse Stock Split occurring;
d.Assessing that the significant valuation assumption inputs in the Black Scholes valuation model of the implied volatility are consistent with those that would be used by market participants through the testing of source information; and
e.Checking the mathematical accuracy of the calculation, developing independent estimates, and comparing to those selected by management, where applicable, and recalculating management’s fair value, verifying it was reasonable.
▪We audited the completeness and accuracy of the underlying data supporting the significant valuation assumption inputs.
/s/ Marcum LLP
Marcum LLP
We have served as the Company’s auditor since 2018.
New York, NY
March 20, 2025
SANUWAVE HEALTH, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
December 31, 2024 and 2023
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands, except share data) |
2024 |
|
2023 |
| ASSETS |
|
|
|
| Current Assets: |
|
|
|
| Cash and cash equivalents |
$ |
10,237 |
|
|
$ |
1,797 |
|
Accounts receivable, net of allowance of $1,147 and $1,237, respectively |
3,329 |
|
|
3,314 |
|
| Inventory |
4,149 |
|
|
2,951 |
|
| Prepaid expenses and other current assets |
682 |
|
|
1,722 |
|
| Total Current Assets |
18,397 |
|
|
9,784 |
|
| Non-Current Assets: |
|
|
|
| Property, equipment and right of use assets, net |
732 |
|
|
938 |
|
| Intangible assets, net |
3,730 |
|
|
4,434 |
|
| Goodwill |
7,260 |
|
|
7,260 |
|
| Total Non-Current Assets |
11,722 |
|
|
12,632 |
|
| |
|
|
|
| Total Assets |
$ |
30,119 |
|
|
$ |
22,416 |
|
| |
|
|
|
| LIABILITIES |
|
|
|
| Current Liabilities: |
|
|
|
| Senior secured debt |
$ |
25,305 |
|
|
$ |
18,278 |
|
| Convertible promissory notes payable |
- |
|
|
5,404 |
|
| Convertible promissory notes payable, related parties |
- |
|
|
1,705 |
|
| Asset-backed secured promissory notes payable |
- |
|
|
3,117 |
|
| Asset-backed secured promissory notes payable, related parties |
- |
|
|
1,458 |
|
| Accounts payable |
3,728 |
|
|
5,705 |
|
| Accrued expenses |
4,678 |
|
|
5,999 |
|
| Factoring liabilities |
- |
|
|
1,490 |
|
| Warrant liability |
8,107 |
|
|
14,447 |
|
| Accrued interest |
- |
|
|
5,444 |
|
| Accrued interest, related parties |
- |
|
|
669 |
|
| Current portion of contract liabilities |
193 |
|
|
92 |
|
| Other |
334 |
|
|
947 |
|
| Total Current Liabilities |
42,345 |
|
|
64,755 |
|
| Non-Current Liabilities: |
|
|
|
| Lease liabilities, less current portion |
191 |
|
|
492 |
|
| Contract liabilities, less current portion |
300 |
|
|
347 |
|
| Total Non-Current Liabilities |
491 |
|
|
839 |
|
| Total Liabilities |
$ |
42,836 |
|
|
$ |
65,594 |
|
|
|
|
|
| Commitments and Contingencies (Footnote 21) |
|
|
|
| |
|
|
|
| STOCKHOLDERS’ DEFICIT |
|
|
|
Preferred stock, par value $0.001, 5,000,000 shares authorized, 6,175 Series A, 293 Series B, 90 Series C, and 8 Series D designated shares, respectively; no shares issues and outstanding at 2024 and 2023 |
$ |
- |
|
|
$ |
- |
|
Common stock, par value $0.001, 2,500,000,000 shares authorized, 8,543,686 and 3,041,492 issued and outstanding at 2024 and 2023, respectively * |
9 |
|
|
3 |
|
| Additional paid-in capital |
238,685 |
|
|
176,979 |
|
| Accumulated deficit |
(251,421) |
|
|
(220,049) |
|
| Accumulated other comprehensive loss |
10 |
|
|
(111) |
|
| Total Stockholders’ Deficit |
(12,717) |
|
|
(43,178) |
|
| Total Liabilities and Stockholders’ Deficit |
$ |
30,119 |
|
|
$ |
22,416 |
|
* Reflects a one-for-three hundred seventy-five (1:375) reverse stock split of the outstanding shares of the Company's common stock effected on October 18, 2024.
The accompanying notes to consolidated financial statements are an integral part of these financial statements.
SANUWAVE HEALTH, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
Years ended December 31, 2024 and 2023
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands, except share and per share data) |
2024 |
|
2023 |
| Revenue |
$ |
32,634 |
|
|
$ |
20,398 |
|
| Cost of revenues |
8,084 |
|
|
6,035 |
|
| Gross Margin |
24,550 |
|
|
14,363 |
|
| |
|
|
|
| Operating Expenses: |
|
|
|
| General and administrative |
11,348 |
|
|
8,674 |
|
| Selling and marketing |
6,323 |
|
|
4,898 |
|
| Research and development |
673 |
|
|
579 |
|
| Depreciation and amortization |
789 |
|
|
752 |
|
| Total Operating Expenses |
19,133 |
|
|
14,903 |
|
| |
|
|
|
| Operating Income (Loss) |
5,417 |
|
|
(540) |
|
| |
|
|
|
| Other Income (Expense) |
|
|
|
| Interest expense |
(12,423) |
|
|
(12,946) |
|
| Interest expense, related party |
(1,214) |
|
|
(2,677) |
|
| Change in fair value of derivative liabilities |
(31,413) |
|
|
(9,621) |
|
|
|
|
|
| Gain on extinguishment of debt |
6,326 |
|
|
- |
|
| Other expense |
(893) |
|
|
(19) |
|
| Other income |
2,855 |
|
|
- |
|
| Total Other Expense |
(36,762) |
|
|
(25,263) |
|
| |
|
|
|
| Net Loss Before Income Taxes |
(31,345) |
|
|
(25,803) |
|
| |
|
|
|
| Income tax expense |
27 |
|
|
4 |
|
| |
|
|
|
| Net Loss |
$ |
(31,372) |
|
|
$ |
(25,807) |
|
| |
|
|
|
| Other Comprehensive Loss |
|
|
|
| Foreign currency translation adjustments |
121 |
|
|
(44) |
|
| Total Comprehensive Loss |
$ |
(31,251) |
|
|
$ |
(25,851) |
|
|
|
|
|
| Net Loss per share: |
|
|
|
| Basic and Diluted * |
$ |
(7.03) |
|
|
$ |
(12.19) |
|
| Weighted average shares outstanding: |
|
|
|
| Basic and Diluted * |
4,462,883 |
|
2,116,936 |
* Reflects a one-for-three hundred seventy-five (1:375) reverse stock split of the outstanding shares of the Company's common stock effected on October 18, 2024.
The accompanying notes to consolidated financial statements are an integral part of these financial statements.
SANUWAVE HEALTH, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
(In thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
|
|
|
|
|
|
|
|
|
Number of Shares
Issued and Outstanding |
|
Par Value |
|
Additional Paid-
in Capital |
|
Accumulated
Deficit |
|
Accumulated Other
Comprehensive
Loss |
|
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balances as of December 31, 2022 |
|
1,463,300 |
|
$ |
1 |
|
|
$ |
153,298 |
|
|
$ |
(194,242) |
|
|
$ |
(67) |
|
|
$ |
(41,010) |
|
| Shares issued for services |
|
34,400 |
|
1 |
|
|
526 |
|
|
- |
|
|
- |
|
|
527 |
|
| Shares issued for settlement of debt |
|
1,543,792 |
|
1 |
|
|
23,155 |
|
|
- |
|
|
- |
|
|
23,156 |
|
| Foreign currency translation adjustment |
|
- |
|
- |
|
|
- |
|
|
- |
|
|
(44) |
|
|
(44) |
|
| Net loss |
|
- |
|
- |
|
|
- |
|
|
(25,807) |
|
|
- |
|
|
(25,807) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balances as of December 31, 2023 |
|
3,041,492 |
|
$ |
3 |
|
|
$ |
176,979 |
|
|
$ |
(220,049) |
|
|
$ |
(111) |
|
|
$ |
(43,178) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sale of common stock |
|
1,248,489 |
|
1 |
|
|
10,299 |
|
|
- |
|
|
- |
|
|
10,300 |
|
| Shares issued for settlement of warrants |
|
3,558,396 |
|
4 |
|
|
41,380 |
|
|
- |
|
|
- |
|
|
41,384 |
|
| Shares issued for settlement of debt |
|
685,737 |
|
1 |
|
|
8,513 |
|
|
- |
|
|
- |
|
|
8,514 |
|
| Stock-based compensation |
|
9,572 |
|
- |
|
|
1,514 |
|
|
- |
|
|
- |
|
|
1,514 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Foreign currency translation adjustment |
|
- |
|
- |
|
|
- |
|
|
- |
|
|
121 |
|
|
121 |
|
| Net loss |
|
- |
|
- |
|
|
- |
|
|
(31,372) |
|
|
- |
|
|
(31,372) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance as of December 31, 2024 |
|
8,543,686 |
|
$ |
9 |
|
|
$ |
238,685 |
|
|
$ |
(251,421) |
|
|
$ |
10 |
|
|
$ |
(12,717) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Reflects a one-for-three hundred seventy-five (1:375) reverse stock split of the outstanding shares of the Company's common stock effected on October 18, 2024.
The accompanying notes to consolidated financial statements are an integral part of these financial statements.
SANUWAVE HEALTH, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
Years ended December 31, 2024 and 2023
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
2024 |
|
2023 |
| Cash Flows - Operating Activities: |
|
|
|
| Net loss |
$ |
(31,372) |
|
|
$ |
(25,807) |
|
| Adjustments to reconcile net loss to net cash used by operating activities |
|
|
|
| Stock-based compensation |
1,514 |
|
|
- |
|
| Depreciation and amortization |
1,145 |
|
|
1,028 |
|
| Reserve for credit losses |
77 |
|
|
781 |
|
| Shares issued for services |
- |
|
|
224 |
|
| Gain on extinguishment of debt |
(6,326) |
|
|
- |
|
| Income tax expense |
- |
|
|
4 |
|
| Change in fair value of derivative liabilities |
31,413 |
|
|
9,621 |
|
|
|
|
|
| Amortization of debt issuance and debt discounts |
5,520 |
|
|
6,911 |
|
| Accrued interest and accrued interest, related parties |
3,387 |
|
|
6,306 |
|
| Changes in operating assets and liabilities |
|
|
|
| Accounts receivable |
(486) |
|
|
(53) |
|
| Inventory |
(1,198) |
|
|
(2,800) |
|
| Prepaid expenses and other assets |
(79) |
|
|
(206) |
|
| Accounts payable |
(1,422) |
|
|
1,546 |
|
| Accrued expenses and contract liabilities |
282 |
|
|
(2,093) |
|
| Net Cash Provided by (Used in) Operating Activities |
2,455 |
|
|
(4,538) |
|
|
|
|
|
| Cash Flows - Investing Activities |
|
|
|
| Proceeds from sale of property and equipment |
- |
|
|
21 |
|
| Purchases of property and equipment |
(490) |
|
|
- |
|
| Net Cash Flows (Used in) Provided by Investing Activities |
(490) |
|
|
21 |
|
|
|
|
|
| Cash Flows - Financing Activities |
|
|
|
| Proceeds from convertible promissory notes |
1,300 |
|
|
3,026 |
|
| Payment of note payable |
(3,548) |
|
|
- |
|
| Proceeds from asset-backed secured promissory notes payable |
- |
|
|
2,994 |
|
| Proceeds from secured promissory notes payable, related party |
500 |
|
|
- |
|
| Payments to secured promissory notes payable, related party |
(500) |
|
|
- |
|
|
|
|
|
| Payments to factoring |
(1,490) |
|
|
(639) |
|
|
|
|
|
| Proceeds from sale of common stock |
10,300 |
|
|
- |
|
|
|
|
|
| Principal payments on finance leases |
(208) |
|
|
(170) |
|
| Net Cash Flows Provided by Financing Activities |
6,354 |
|
|
5,211 |
|
| |
|
|
|
| Effect of Exchange Rates on Cash |
121 |
|
|
(50) |
|
| |
|
|
|
| Net Change in Cash During Period |
8,440 |
|
|
644 |
|
| |
|
|
|
| Cash at Beginning of Period |
1,797 |
|
|
1,153 |
|
| Cash at End of Period |
$ |
10,237 |
|
|
$ |
1,797 |
|
| |
|
|
|
| Supplemental Information: |
|
|
|
| Cash paid for interest |
$ |
4,311 |
|
|
$ |
1,958 |
|
| Non-Cash Investing and Financing Activities: |
|
|
|
| Shares issued for settlement of debt |
$ |
8,513 |
|
|
$ |
- |
|
| Write off deferred merger costs |
1,225 |
|
|
- |
|
| Warrants issued in conjunction with senior secured promissory note payable and convertible promissory notes payable |
3,557 |
|
|
1,682 |
|
| Conversion of warrants to common stock |
41,380 |
|
|
- |
|
| Conversion of convertible notes payable and accrued interest to common stock |
- |
|
|
23,156 |
|
| Embedded conversion feature on convertible debt |
- |
|
|
835 |
|
| Common shares issued for advisory shares |
- |
|
|
302 |
|
| Capitalize default interest into senior secured debt |
3,850 |
|
|
- |
|
| Conversion of asset-based secured promissory notes to convertible promissory notes |
4,584 |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes to consolidated financial statements are an integral part of these financial statements.
SANUWAVE HEALTH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2024 and 2023
1. Nature of the Business and Basis of Presentation
SANUWAVE Health, Inc. and Subsidiaries (“Sanuwave” or the “Company”) is focused on the commercialization of its patented regenerative medicine utilizing noninvasive ultrasound or shockwaves to produce a biological response promoting the repair and regeneration of tissue, musculoskeletal, and vascular structures.
Basis of Presentation - The accompanying consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and with the instructions to for 10-K and Regulation S-X. The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All significant intercompany accounts and transactions have been eliminated.
The functional currencies of the Company’s foreign operations are their local currencies. The financial statements of the Company’s foreign subsidiary have been translated into United States dollars. All balance sheet accounts have been translated using the exchange rates in effect at the balance sheet date. Income statement amounts have been translated using the average exchange rate for the year. Translation adjustments are reported in other comprehensive loss in the consolidated statements of comprehensive loss and as cumulative translation adjustments in accumulated other comprehensive loss in the consolidated balance sheets.
Reverse Stock Split - All share numbers, including the number of shares underlying warrants, options and convertible debt, and per share amounts presented in these consolidated financial statements, including these footnotes, reflect a one-for-three hundred seventy five (1:375) reverse stock split of the outstanding shares of the Company's common stock effected on October 18, 2024 (the "Reverse Stock Split"). The Company's authorized shares of common and preferred stock along with the par value did not change. No fractional shares were issued as a result of the reverse stock split. Any fractional shares that would have resulted from the reverse stock split were settled in cash. The reverse stock split affected all common stockholders uniformly and did not alter any stockholder's percentage interest in the Company's common stock, except to the extent that the reverse stock split resulted in some stockholders experiencing an adjustment of a fractional share as described above.
Reclassification - Certain accounts in the prior period consolidated statement of cash flows have been reclassified to conform to the presentation of the current year consolidated financial statements. These reclassifications had no effect on the previously reported operating results.
2. Going Concern
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has incurred recurring operating losses in prior years, has negative working capital, and its Senior Secured Note becomes due in September 2025, which raises substantial doubt as to its ability to continue as a going concern for a period of 12 months from the filing of the Form 10-K.
During the current fiscal year, the Company has achieved operating income for the year ended 2024, reflecting a significant improvement in its financial performance. This turnaround is attributable to several strategic initiatives, primarily revenue growth initiatives and a capital raise from the Private Placement, which have positively impacted the Company's operations.
Despite the positive operating income in the current year, the Company continues to monitor its financial position closely.
In addition to the operational improvements, the Company is addressing its financial obligations, including the significant portion of debt that is coming due in September 2025. Management is actively engaged in discussions with lenders and financial institutions to refinance this debt, which will extend the maturity of the debt and provide additional liquidity to support ongoing operations and strategic initiatives.
Management acknowledges that it must continue to execute its strategic initiatives effectively to ensure long-term financial stability.
The carrying amounts of assets and liabilities presented in the consolidated financial statements do not necessarily purport to represent realizable or settlement values. The consolidated financial statements do not include any adjustment that might result from the outcome of this uncertainty. Our consolidated financial statements do not include any adjustments relating to the recoverability of assets and classification of assets and liabilities that might be necessary should we be unable to continue as a going concern.
3. Summary of Significant Accounting Policies
The significant accounting policies followed by the Company are summarized below:
Estimates - These consolidated financial statements have been prepared in accordance with U.S. GAAP. Because a precise determination of assets and liabilities, and correspondingly revenues and expenses, depend on future events, the preparation of consolidated financial statements for any period necessarily involves the use of estimates and assumptions. Actual amounts may differ from these estimates. These consolidated financial statements have, in management’s opinion, been properly prepared within reasonable limits of materiality and within the framework of the accounting policies summarized herein.
Significant estimates include the recording of allowances for credit losses, the net realizable value of inventory, fair value of goodwill and other intangible assets, the determination of the valuation allowances for deferred taxes, litigation contingencies, estimated fair value of stock-based compensation, and the estimated fair value of embedded financial instruments, including warrants and embedded conversion options.
Cash and cash equivalents - Cash and cash equivalents consist of cash on hand and demand deposits. These investments are highly liquid and readily convertible to known amounts of cash with insignificant risk of changes in value. The Company classifies all such highly liquid instruments that are not restricted as cash equivalents. Cash equivalents are stated at cost, which approximates fair value due to their short-term nature. For purposes of the statement of cash flows, the Company considers cash and cash equivalents to include cash on hand and demand deposits.
Accounts receivable - Accounts receivable are stated at the amount management expects to collect from outstanding balances. The Company maintains an allowance for credit losses to provide for the estimated amount of receivables that will not be fully collected. The allowance is based on the assessment of the following factors: customer creditworthiness; historical payment experience; age of outstanding receivables. Management routinely assesses the financial strength of its customers and, consequently, believes accounts receivable are stated at the net realizable value and credit risk exposure is limited.
Allowance for credit losses roll forward - The roll-forward of the allowance for credit losses is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
|
2024 |
|
2023 |
| Allowance for credit losses, January 1 |
|
$ |
1.2 |
|
|
$ |
1.0 |
|
| Provision for credit losses |
|
0.3 |
|
0.8 |
| Write-offs |
|
(0.4) |
|
|
(0.6) |
|
| Allowance for credit losses, December 31 |
|
$ |
1.1 |
|
|
$ |
1.2 |
|
Property, plant, and equipment (PPE) - PPE is initially recorded at cost, which includes all expenditures directly attributable to bringing the asset into working condition for its intended use. Subsequent to initial recognition, PPE is carried at cost less accumulated depreciation and any impairment losses. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, with no residual value for most assets. The estimated useful lives and residual values of assets are reviewed periodically and adjusted if necessary. Major improvements and enhancements that extend the useful life of PPE are capitalized, while routine repairs and maintenance are expensed as incurred.
Inventory - Inventory consists of purchased medical equipment and parts and is stated at the lower of average cost, which is valued using the first in, first out (“FIFO”) method, or net realizable value less allowance for selling and distribution expenses. The Company analyzes its inventory levels and writes down inventory that has, or is expected to, become obsolete.
Goodwill - Goodwill represents the excess of the purchase price over the fair value of assets acquired and liabilities assumed. The Company accounts for goodwill under Accounting Standards Codification (ASC) Topic 350, Intangibles-Goodwill and Other.
The Company tests goodwill for impairment annually, or more frequently whenever events or circumstances indicate impairment may exist. Goodwill is stated at cost less impairment losses. The Company completes its goodwill impairment test annually in the fourth quarter. The Company performed a qualitative evaluation at the reporting unit level and determined there was no goodwill impairment as of December 31, 2024, and 2023.
Intangible assets - Intangible assets arising from the Company’s acquisition are amortized on a straight‑line basis over the estimated useful life of each asset. Customer relationships have a useful life of seven years. Patents and tradenames have a useful life of nineteen years.
Impairment of long-lived assets - The Company annually reviews long-lived assets for impairment whenever facts and circumstances indicate that the carrying amounts of the assets may not be recoverable. An impairment loss is recognized only if the carrying amount of the asset is not recoverable and exceeds its fair value. Recoverability of assets to be held and used is measured by comparing the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the asset’s carrying value is not recoverable, an impairment charge is recognized for the amount by which the carrying amount of the asset exceeds its fair value. The Company determines fair value by using a combination of comparable market values and discounted cash flows, as appropriate. There were no impairments in 2024 and 2023.
Leases - The Company determines whether an arrangement is a lease at inception. When lease arrangements include lease and non-lease components, the Company accounts for lease and non-lease components (e.g. common area maintenance) separately based on their relative standalone prices.
For leases where the Company is the lessee, Right of Use (“ROU”) assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent an obligation to make lease payments arising from the lease. ROU assets and lease liabilities are recognized at the lease commencement date based on the present value of lease payments over the lease term. As the Company’s leases did not provide an implicit interest rate, the Company used the equivalent borrowing rate for a secured financing with the term of equal to the remaining life of the lease at inception.
Any lease arrangements with an initial term of 12 months or less are not recorded on our consolidated balance sheets, and the Company recognizes lease costs for these lease arrangements on a straight-line basis over the lease term. In the event a lease arrangement would provide us with options to exercise one or more renewal terms or to terminate the lease arrangement, we would include these options when we are reasonably certain to exercise them in the lease term used to establish ROU assets and lease liabilities. None of our lease agreements include an option to purchase the leased asset, residual value guarantees, or material restrictive covenants.
The Company has other lease arrangements that are adjusted periodically based on an inflation index or rate. The future variability of these payments and adjustments are unknown, and therefore they are not included as minimum lease payments used to determine ROU assets and lease liabilities. Variable rental payments are recognized in the period in which the obligation is incurred.
Fair value of financial instruments - The carrying values of accounts payable, and other short-term obligations approximate their fair values, because of the short-term maturities of these instruments.
The Company utilizes the guidance of ASC Topic 820-10, Fair Value Measurements (“ASC 820-10”), which defines fair value, establishes a framework for measuring fair value and requires disclosures about fair value measurements. The framework that is set forth in this standard is applicable to the fair value measurements where it is permitted or required under other accounting pronouncements.
The ASC 820-10 hierarchy ranks the quality and reliability of inputs, or assumptions, used in the determination of fair value and requires financial assets and liabilities carried at fair value to be classified and disclosed in one of the following three categories:
Level 1 – Observable inputs that reflect quoted prices (unadjusted) in active markets for identical assets and liabilities:
Level 2 – Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly: and
Level 3 – Unobservable inputs that are not corroborated by market data, therefore requiring the Company to develop its own assumptions.
Convertible promissory notes – The Company evaluates its convertible instruments to determine if those contracts, or embedded components of those contracts, qualify as derivative financial instruments to be separately accounted for in accordance with ASC Topic 815 “Derivatives and Hedging” (“ASC 815”). The accounting treatment of derivative financial instruments requires that the Company record embedded conversion options and any related freestanding instruments at their fair values as of the inception date of the agreement and at fair value as of each subsequent balance sheet date. Any change in fair value is recorded as non-operating, non-cash income or expense for each reporting period at each balance sheet date. Conversion options are recorded as a discount to the host instrument and are amortized as amortization of debt discount on the consolidated statements of comprehensive loss over the life of the underlying instrument. The Company reassesses the classification of its derivative instruments at each balance sheet date. If the classification changes because of events during the period, the contract is reclassified as of the date of the event that caused the reclassification.
Debt discount – The Company records a debt discount related to warrants issued with debt at fair value and recognizes the cost using the straight-line method, which approximates the effective interest method, over the term of the related debt as interest expense, which is reported in the Other Income (Expense) section in our consolidated statements of comprehensive loss. This debt discount is reported as a reduction of the related debt liability.
Contract liabilities – Device product sales are bundled with an initial one-year warranty and the Company offers a separately priced multi-year warranty. Because the warranty represents an obligation, revenue is deferred as a contract liability and recognized over the time that the Company satisfies its performance obligations, which is generally the warranty term.
Segment reporting - The Company operates as a single reporting entity and has determined that it has one reportable segment. This conclusion is based on the fact that the Company's chief operating decision maker (“CODM”), who is the Chief Executive Officer (“CEO”), reviews the financial information and makes decisions about resource allocation and performance assessment on a consolidated basis. The Company's operations are managed and evaluated as a single business unit, and the nature of the products and services, production processes, and customer base are similar across the entire organization.
The Company is engaged in the business of designing and selling medical devices. The products and services offered by the Company are integrated and interrelated, and the Company does not have discrete financial information for different business lines that would qualify as separate operating segments.
All significant accounting policies, including those related to revenue recognition, inventory valuation, property, plant, and equipment, and other key areas, are applied consistently across the entire organization.
Revenue recognition - The core principle of ASC Topic 606 “Revenue from Contracts with Customers” (“ASC 606”) requires that an entity recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The Company allocates the transaction price to all contractual performance obligations included in the contract. If a contract has more than one performance obligation, we allocate the transaction price to each performance obligation based on standalone selling price, which depicts the amount of consideration we expect to be entitled in exchange for satisfying each performance obligation. The Company recognizes revenue primarily from the following types of contracts:
System Sales, Consumables and Part Sales - System sales, accessory and part sales include devices and applicators (new and refurbished). Performance obligations are satisfied at the point in time when the customer obtains control of the goods, which is generally at the point in time that the product is shipped.
Licensing Fees - Licensing transactions include distribution licenses and intellectual property licenses. Licensing revenue is recognized as the Company satisfies its performance obligations, which may vary with the terms of the licensing agreement.
Other Revenue - Other revenue primarily includes warranties, repairs, and billed freight. The Company allocates the device sales price to the product and the embedded warranty by reference to the stand-alone extended warranty price. Warranty revenue is recognized over the time that the Company satisfies its performance obligations, which is generally the warranty term. Repairs (parts and labor) and billed freight revenue are recognized at the point in time that the service is performed, or the product is shipped, respectively.
Shipping and handling costs - Shipping charges billed to customers are included in revenues. Shipping and handling costs incurred have been recorded in cost of goods sold totaling $482 thousand and $484 thousand for the years ended December 31, 2024, and 2023, respectively.
Research and development - Research and development costs are expensed as incurred. Research and development costs include costs of research, engineering, and technical activities to develop a new product, researching an expanded product use or making significant improvements to existing products, including the costs of clinical development.
Stock-based compensation - The Company uses the fair value method of accounting for its employee stock option program. Stock-based compensation expense for all stock-based payment awards is based on the estimated fair value of the award measured on the grant date. The Company recognizes the estimated fair value of the award as compensation cost on a straight-line basis over the requisite service period of the award, which is generally the option vesting term. The Company generally issues new shares of common stock to satisfy option and warrant exercises.
The expected life of options granted represents the period of time that options granted are expected to be outstanding and are derived from the contractual terms of the options granted calculated under the simplified method. The risk-free rate for periods within the contractual life of the option is based on the United States Treasury yield curve in effect at the time of the grant. The expected volatility is based on the average volatility of the common stock of the Company’s peer group. The expected dividend yield is based on our historical dividend experience, however, since our inception, we have not declared dividends. Forfeitures are recognized as they occur.
Comprehensive income (loss) - Comprehensive income (loss) results from the translation of the Company’s foreign entity’s financial statements from their functional currency to U.S. dollars for consolidation in the accompanying consolidated financial statements.
Deferred offering costs - Deferred stock offering costs represent amounts paid for legal, consulting, and other offering expenses directly attributable to the offering of securities in conjunction with the recapitalization under the Merger Agreement (as defined in Note 4), and are deferred and charged against the gross proceeds of the offering. In the event of a significant delay or cancellation of a planned offering of securities, all the costs would be expensed. In June 2024, the Company terminated the Merger Agreement and the deferred offering costs of $0.5 million were expensed.
New accounting pronouncements
ASU 2024-03, Disaggregation of Income Statement Expenses
In November 2024, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2024-03, Income Statement - Reporting Comprehensive Income (Topic 220): Expense Disaggregation Disclosures to improve the disclosures about a public entity’s expenses and provide more detailed information about the types of expenses in commonly presented expense captions such as inventory purchases, employee compensation, depreciation and intangible asset amortization. The disclosure requirements must be applied retrospectively to all prior periods presented in the financial statements. The effective date for the standard is for fiscal years beginning after December 15, 2026 and interim periods within fiscal years beginning after December 15, 2027, with early adoption permitted. The Company is currently evaluating the effects adoption of this guidance will have on the consolidated financial statements.
ASU 2023-09, Improvements to Income Tax Disclosures
In December 2023, the FASB issued ASC Update No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. Update No. 2023-09 aims to enhance the transparency and decision usefulness of income tax disclosures. Update No. 2023-09 modifies the rules on income tax disclosures to require entities to disclose (1) specific categories in the rate reconciliation, (2) the income or loss from continuing operations before income tax expense or benefit (separated between domestic and foreign) and (3) income tax expense or benefit from continuing operations (separated by federal, state, and foreign). ASU 2023-09 also requires entities to disclose their income tax payments to international, federal, state and local jurisdictions, among other changes. Update No. 2023-09 is effective for fiscal years beginning after December 15, 2024. We expect to adopt Update No. 2023-09 prospectively. We are currently evaluating the potential impact of adopting this new guidance on our consolidated financial statements and related disclosures.
Recently adopted accounting pronouncements
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, requiring public entities to disclose information about their reportable segments’ significant expenses and other segment items on an interim and annual basis. Public entities with a single reportable segment are required to apply the disclosure requirements in ASU 2023-07, as well as all existing segment disclosures and reconciliation requirements in ASC 280 on an interim and annual basis. The Company adopted ASU 2023-07 during the year ended December 31, 2024 and was retroactively applied to December 31, 2023. See Note 22 for further detail.
4. Merger Agreement
On August 23, 2023, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with SEP Acquisition Corp., a Delaware corporation (“SEPA”), and SEP Acquisition Holdings Inc., a Nevada corporation and a wholly owned subsidiary of SEPA. Pursuant to the terms of the Merger Agreement, a business combination between the Company and SEPA was to be effected.
On June 25, 2024, the Company delivered a notice to SEPA terminating the Merger Agreement.
5. Loss per Share
Basic net loss per share is calculated by dividing the net loss attributable to common stockholders by the weighted average number of shares outstanding for the years ended December 31, 2024, and 2023. The weighted average of number of shares outstanding includes outstanding common stock and shares issuable for nominal consideration as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
December 31, 2024 |
|
December 31, 2023 |
| Common shares |
4,463 |
|
2,059 |
| Common shares issuable assuming exercise of nominally priced warrants and options |
- |
|
58 |
| Weighted Average Shares Outstanding |
4,463 |
|
2,117 |
Diluted net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted average number of shares of common stock and dilutive common stock equivalents outstanding. To the extent that securities are “anti-dilutive,” they are excluded from the calculation of diluted net loss per share. As a result of the net loss for the years ended December 31, 2024, and 2023, all potentially dilutive shares in such periods were anti-dilutive and therefore excluded from the computation of diluted net loss per share. Anti-dilutive equity securities consist of the following:
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
December 31, 2024 |
|
December 31, 2023 |
| Common stock options |
1,137 |
|
44 |
| Restricted stock units- unvested |
5 |
|
- |
| Common stock purchase warrants |
53 |
|
3,200 |
| Convertible notes payable, including interest |
- |
|
|
431 |
|
1,195 |
|
3,675 |
6. Inventory
Inventory consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
December 31, 2024 |
|
December 31, 2023 |
| Finished goods |
$ |
386 |
|
|
$ |
416 |
|
| Parts and accessories |
3,763 |
|
|
2,535 |
|
| Total Inventory |
$ |
4,149 |
|
|
$ |
2,951 |
|
7. Intangible Assets
Carrying value of intangible assets consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2024 |
|
December 31, 2023 |
|
Weighted-
Average Useful
Life (in years) |
| (in thousands) |
Gross |
|
Accumulated
Amortization |
|
Gross |
|
Accumulated
Amortization |
|
| Definite-lived Intangibles |
|
|
|
|
|
|
|
|
|
| Customer relationships |
$ |
3,820 |
|
|
$ |
(2,400) |
|
|
$ |
3,820 |
|
|
$ |
(1,854) |
|
|
2.7 |
| Patent |
2,312 |
|
|
(535) |
|
|
2,312 |
|
|
(413) |
|
|
14.7 |
| Tradenames |
693 |
|
|
(160) |
|
|
693 |
|
|
(124) |
|
|
14.7 |
| Intangible Assets |
$ |
6,825 |
|
|
$ |
(3,095) |
|
|
$ |
6,825 |
|
|
$ |
(2,391) |
|
|
10.1 |
Amortization expense for each of the years ended December 31, 2024, and 2023 totaled $704 thousand. Future amortization expense is expected to be the following (dollars in thousands):
|
|
|
|
|
|
|
|
|
| Year ended December 31, |
|
Amortization |
| 2025 |
|
704 |
|
| 2026 |
|
704 |
|
| 2027 |
|
487 |
|
| 2028 |
|
158 |
|
| 2029 |
|
158 |
|
| Thereafter |
|
1,519 |
|
8. Accrued Expenses
Accrued expenses consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
December 31, 2024 |
|
December 31, 2023 |
| Registration penalties |
$ |
1,583 |
|
|
$ |
1,583 |
|
| License fees |
- |
|
|
892 |
|
| Board of directors fees |
249 |
|
|
942 |
|
| Employee compensation |
2,232 |
|
|
2,298 |
|
| Other |
614 |
|
|
284 |
|
| Total Accrued Expenses |
$ |
4,678 |
|
|
$ |
5,999 |
|
9. Factoring Liabilities
In June 2021, the Company entered into a factoring agreement with an unrelated third party, pursuant to which the Company may sell certain of its accounts receivables for 86.25% of the value of the receivable. Advances available under the facility are capped at the lesser of $3.0 million or a formula amount, as defined in the agreement. Interest on advances is assessed at a fixed amount upon funding, which is equivalent to an annualized rate of 15.0% for the first 30 days, and daily thereafter at an annualized rate of 14.4%. The agreement’s term is one month and automatically renews for additional one-month periods, unless either party provides 30 days’ notice of termination. The accounts receivable is sold with recourse back to the Company, therefore, the Company accounts for the arrangement as traditional financing. The factoring asset is shown within Accounts receivable on the Company's consolidated balance sheets.
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
December 31, 2024 |
|
December 31, 2023 |
| Receivables transferred |
$ |
16 |
|
|
$ |
(1,794) |
|
| Reserve amount held |
220 |
|
|
304 |
|
| Factoring asset (liability) |
$ |
236 |
|
|
$ |
(1,490) |
|
10. Senior Secured Debt
The following table summarizes outstanding senior secured debt:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
December 31, 2024 |
|
December 31, 2023 |
| (In thousands) |
Principal |
|
Debt
Discount |
|
Carrying
Value |
|
Principal |
|
Debt
Discount |
|
Carrying
Value |
| Senior secured debt |
$ |
26,898 |
|
|
$ |
(1,593) |
|
|
$ |
25,305 |
|
|
$ |
21,562 |
|
|
$ |
(3,284) |
|
|
$ |
18,278 |
|
Senior secured promissory note payable (“Senior Secured Note”) – In August 2020, the Company entered into a Note and Warrant Purchase and Security Agreement (the “NWPSA”). In accordance with the NWPSA, the Company issued a $15 million Senior Secured Promissory Note Payable (the “Senior Secured Note”) and a warrant exercisable into shares of the Company’s common stock in exchange for cash to support operations, repay outstanding debt and close on the acquisition of the UltraMIST assets from Celularity Inc. (Celularity) among other transactions.
In February 2022, the Company entered into a Second Amendment to Note and Warrant Purchase and Security Agreement (the “Second NWPSA”) for $3.0 million, for a total of $18.0 million outstanding. Along with the issuance of the note, the Company also issued warrants to purchase 43,200 shares of common stock with an exercise price of $67.50 and 54,933 shares of common stock.
Interest is charged at the greater of the prime rate or 3% plus 9%, paid quarterly. The principal increases at a rate of 3% of the outstanding principal balance (PIK interest) on each quarterly interest payment date. The original maturity date of the Senior Secured Note is September 20, 2025, and it can be prepaid.
In June 2022, the Company entered into the Third Amendment to the Note and Warrant Purchase and Security Agreement (the “Third NWPSA”). The Third NWPSA provides for (i) the extension of the agent’s and holder’s forbearance of exercising its remedies arising from Existing Defaults (as defined in the NWPSA) to the earlier of (x) the occurrence of an Event of Default (as defined in the NWPSA) or (y) August 30, 2022, and (ii) the extension to file a registration statement with the Securities and Exchange Commission to register the resale of the Advisor Shares (as defined in the NWPSA) no later than August 30, 2022.
In June 2023, the Company entered into a Fourth Amendment to the NWPSA, which provides the Company an extension of the holder forbearing from exercising the remedies arising from the existing defaults to the earlier of the occurrence of an event of default and December 31, 2024. The amendment also added a consent fee of 2% of the original principal amount of the NWPSA, payable in cash at maturity, accounted for as additional debt issuance costs. The amendment also defers interest that would otherwise have been due on June 30, 2023, and September 30, 2023. The interest will instead be compounded and added to the principal amount of the notes and bear interest at a rate of 20.25% per annum. The amendment also requires the Company to complete an equity financing that results in gross cash proceeds of at least $2.5 million by July 15, 2023. This financing successfully closed on July 21, 2023.
In March 2024, the Company entered into a Consent, Limited Waiver and Fifth Amendment to Note and Warrant Purchase Agreement (the “Fifth Amendment”). The Fifth Amendment provides (i) consent to enter into a License and Option Agreement and consummation of the License and Option Transaction (ii) a waiver of any event of default that may occur under the NWPSA, because of the License and Option Agreement or License and Option Transaction and (iii) amended the NWPSA to release certain patents from the collateral. The Fifth Amendment also provides for a forbearance of exercising remedies in connection with certain existing events of default under the NWPSA until the earlier of (x) the occurrence of another event of default under the NWPSA and (y) April 30, 2024. During the forbearance period, the outstanding obligations under the NWPSA continue to accrue interest at the default rate.
On July 15, 2024, the Company entered into the Sixth Amendment to Note and Warrant Purchase and Security Agreement (the “Sixth Amendment”), which was a modification of debt. The Sixth Amendment added, as of June 30, 2024, a consent fee of $0.7 million to the principal amount of the Senior Secured Note issued pursuant to the NWPSA. On and after April 1, 2024, for each fiscal quarter during which any interest is payable in cash, deferred interest and default interest shall be calculated based on the principal amount of the Senior Secured Note as of the beginning of the quarter and shall include any default interest accrued to date. The Sixth Amendment also provides for a forbearance of exercising remedies in connection with certain existing events of default under the NWPSA until the earlier of (x) the occurrence of another event of default under the NWPSA or (y) December 31, 2024. During the forbearance period, the outstanding obligations under the NWPSA accrued interest at the default rate.
On October 17, 2024, the Company entered into a Consent and Limited Waiver to Note and Warrant Purchase and Security Agreement (the “Consent and Limited Waiver”) with the noteholders party thereto (the “Holders”) and NH Expansion Credit Fund Holdings LP, as agent (the “Agent”). The Agent and the Holders agreed to continue to forbear upon exercising remedies in connection with certain existing events of default under the NWPSA until the earlier of (x) the occurrence of an event of default and (y) December 31, 2024. The Consent and Limited Waiver also consents to the repayment in full of amounts owed to HealthTronics, Inc. (“HealthTronics”) pursuant to the Convertible Promissory Note, dated as of August 6, 2020, by and between the Company and HealthTronics, in the original principal amount of approximately $1.4 million; gross proceeds from the sale of common stock of at least $9.0 million in a private placement (for further details on this private placement transaction, see Note 16) and the Reverse Stock Split if the Company has at least $5.0 million of liquidity following such transactions, which is required pursuant to the minimum liquidity covenant in the NWPSA. The Agent and the Holders have also agreed that any existing event of default that exists due to the Company’s failure to meet the minimum liquidity covenant will be waived if the conditions set forth in the Consent and Limited Waiver, including at least $5.0 million of liquidity following such transactions, are met. As a condition to the effectiveness of the Consent and Limited Waiver, the Agent exercised, on a cashless basis, all warrants issued by the Company to the Agent in exchange for the issuance of 146,302 shares of the Company’s common stock following the Reverse Stock Split.
As of December 31, 2024, the Company is no longer in default and is in compliance with all covenants in the Senior Secured Note.
The debt issuance costs, and debt discount related to the Senior Secured Note were capitalized as a reduction in the principal amount and are being amortized to interest expense over the life of the Senior Secured Note.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Twelve Months Ended |
| (In millions) |
|
December 31, 2024 |
|
December 31, 2023 |
| Amortization of debt issuance costs and debt discount, included in interest expense |
|
$ |
2.1 |
|
|
$ |
1.5 |
|
| Interest expense |
|
$ |
8.1 |
|
|
$ |
6.9 |
|
Accrued interest related to the Senior Secured Note was $0.0 million and $3.2 million on December 31, 2024, and December 31, 2023, respectively. Interest expense on the Senior Secured Note totaled $8.1 million and $6.9 million for the years ended December 31, 2024, and 2023, respectively.
11. Promissory Notes Payable
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
| (In thousands, except conversion price) |
Conversion
Price |
|
Principal |
|
Debt
Discount |
|
Conversion
Option |
|
Carrying
Value |
| Acquisition convertible promissory note, in default |
$ |
37.50 |
|
|
$ |
4,000 |
|
|
- |
|
|
- |
|
|
$ |
4,000 |
|
| Convertible promissory note payable, related parties, in default |
$ |
37.50 |
|
|
1,373 |
|
|
- |
|
|
- |
|
|
1,373 |
|
| Convertible notes payable |
$ |
15.00 |
|
|
2,639 |
|
|
(1,235) |
|
|
- |
|
|
1,404 |
|
| 2022 Convertible notes payable, related parties |
$ |
15.00 |
|
|
450 |
|
|
(118) |
|
|
- |
|
|
332 |
|
| Total Convertible Promissory Notes |
|
|
$ |
8,462 |
|
|
$ |
(1,353) |
|
|
$ |
- |
|
|
$ |
7,109 |
|
Convertible Notes Payable and Convertible Notes Payable, Related Parties - In August 2022, November 2022, May 2023, December 2023, January 2024, and June 2024, the Company entered into Securities Purchase Agreements (the “Purchase Agreements”) for the sale in a private placement of (i) Future Advance Convertible Promissory Notes (the “Notes”) in an aggregate principal amount of $16.2 million in August 2022, $4.0 million in November 2022, $1.2 million in May 2023, $1.9 million in December 2023, $4.6 million in January 2024 related to the conversion of the Asset-Backed Secured Promissory Notes (described in Note 12), and $1.3 million in June 2024 (ii) Common Stock Purchase Warrants to purchase an additional 1.9 million shares of common stock with an exercise price of $25.13 per share and (iii) Common Stock Purchase Warrants to purchase an additional 1.9 million shares of common stock with an exercise price of $15.00 per share. Interest expense for the years ended December 31, 2024 and 2023, totaled $5.0 million and $6.4 million, respectively.
Pursuant to the Notes, the Company promised to pay in cash and/or in shares of common stock, at a conversion price of $15 (the “Conversion Price”), the principal amount and interest at a rate of 15% per annum on any outstanding principal. The Conversion Price of the Notes is subject to adjustment, including if the Company issues or sells shares of common stock for a price per share less than the Conversion Price of the Notes or if the Company lists its shares of common stock on The Nasdaq Capital Market and the average volume weighted average price of such common stock for the five trading days preceding such listing is less than $15 per share; provided, however, that the Conversion Price shall never by less than $3.75. The Notes contain customary events of default and covenants, including limitations on incurrences of indebtedness and liens. The Notes have a term of 12 months from the date of issue.
In August 2023 and November 2023, the Company utilized its election to convert the August and November issued Notes into shares of common stock upon the Notes’ maturity. The August Notes totaling $16.2 million in principal and $2.4 million in interest were converted to 1,238,508 shares of common stock. The November Notes totaling $4.0 million in principal and $0.6 million in interest were converted to 305,282 shares of common stock.
In May 2024, the Company utilized its election to convert the May Notes into shares of common stock upon the Notes' maturity. The May Notes totaling $1.2 million in principal and $0.2 million interest were converted to 0.1 million shares of common stock.
All remaining outstanding convertible notes payable and convertible notes payable related party converted on October 18, 2024 to 591,802 shares of common stock. The outstanding principal and interest converted totaled $8.9 million. The Company recognized a $0.3 million gain on conversion of the Notes.
Promissory note payable, related parties - In June 2024 the Company entered into a $0.5 million promissory note with a related party. Interest was accrued at 12% with an original maturity date of December 3, 2024. The Note was paid in full with accrued interest in October 2024.
Acquisition Convertible promissory notes payable - In August 2020, the Company entered into an asset purchase agreement with Celularity to acquire Celularity’s UltraMIST assets. A portion of the aggregate consideration of $24 million paid for the assets included the issuance of a promissory note to Celularity in the principal amount of $4 million (the “Seller Note”). The Seller Note matured on August 6, 2021, and was not repaid. The Company’s failure to pay the outstanding principal balance when due constituted an event of default under the terms of the Seller Note and, accordingly, it began accruing additional interest of 5.0% in addition to the 12.0% initial rate, as of the date of the default. As of December 31, 2024, and 2023, the Seller Note had outstanding accrued interest of $0 and $1.5 million, respectively. This Seller Note was settled in 2024 for a cash payment of $2.2 million. See Note 21 for additional disclosures regarding the settlement of the Seller Note.
The Company evaluated embedded conversion features within the convertible promissory note and determined that the conversion feature does not require to be bifurcated. Upon adoption of ASU 2020-06 effective January 1, 2021, the convertible promissory note is accounted for as a single liability due to the elimination of the beneficial conversion feature accounting model.
Convertible promissory notes payable, related party - In August 2020, the Company issued a convertible promissory note payable in the amount of $1.4 million. The note matured on August 6, 2021, and was not repaid was in default. As of December 31, 2024, and 2023, the note had outstanding accrued interest of $0 and $444 thousand, respectively.
The convertible promissory note was settled in 2024 for a cash payment of $1.4 million, which resulted in a gain on the extinguishment of debt of $0.8 million and a reduction in accrued interest of $0.8 million.
12. Asset-Backed Secured Promissory Notes
In July 2023, the Company issued Asset-Backed Secured Promissory Notes (the “ABS Promissory Notes”) in an aggregate principal amount of $4.6 million to certain accredited investors (the “Purchasers”) at an original issue discount of 33.33%. The ABS Promissory Notes bear an interest rate of 0% per annum and mature on January 21, 2024 (the “Maturity Date”). The Company received total proceeds of approximately $3.0 million. The Company entered into a Security Agreement providing for a continuing and unconditional security interest in any and all property of the Company. This security interest is subordinate to the Senior Secured Debt described in Note 10. Interest expense for the year ended December 31, 2024, totaled $0.1 million.
The Company and the Purchasers also entered into a side letter pursuant to which the parties agreed that upon the Maturity Date, or upon a fundamental transaction as defined by the ABS Promissory Notes, the Company will issue each Purchaser a Future Advance Convertible Promissory Note with the same principal amount as the principal amount of such Purchasers’ ABS Promissory Notes, plus any accrued and unpaid interest and two Common Stock Purchase Warrants, substantially in the forms of the Notes and Common Stock Purchase Warrants disclosed in Note 11.
On January 21, 2024, pursuant to the side letter, the Company issued each Purchaser a Convertible Note Payable with the same principal amount as the principal amount of such Purchasers’ ABS Promissory Notes. Pursuance to this side letter the ABS Promissory Notes converted to convertible promissory notes, as described in Note 11. The Company recorded a net loss on extinguishment of debt totaling $0.1 million for the twelve months ended December 31, 2024.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
| (In thousands) |
|
Principal |
|
Debt
Discount |
|
Embedded
Derivative |
|
Carrying
Value |
| ABS promissory notes |
|
$ |
3,122 |
|
|
$ |
(53) |
|
|
$ |
48 |
|
|
$ |
3,117 |
|
| ABS promissory notes, related parties |
|
1,462 |
|
|
(49) |
|
|
45 |
|
|
1,458 |
|
| Total ABS Promissory Notes |
|
$ |
4,584 |
|
|
$ |
(102) |
|
|
$ |
93 |
|
|
$ |
4,575 |
|
13. Fair Value Measurements
The Company uses various inputs to measure the outstanding warrants and certain embedded conversion features associated with convertible debt on a recurring basis to determine the fair value of the liabilities. The following table classifies the Company’s liabilities measured at fair value on a recurring basis into the fair value hierarchy:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value measurement at December 31, 2024 |
| (in thousands) |
Fair value |
|
Quoted
prices in
active
markets
(Level 1) |
|
Significant
other
observable
inputs
(Level 2) |
|
Significant
unobservable
inputs
(Level 3) |
| Warrant liability |
$ |
8,107 |
|
|
- |
|
|
- |
|
|
$ |
8,107 |
|
|
|
|
|
|
|
|
|
| Total Fair Value |
$ |
8,107 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
8,107 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value measurement at December 31, 2023 |
| (in thousands) |
Fair value |
|
Quoted
prices in
active
markets
(Level 1) |
|
Significant
other
observable
inputs
(Level 2) |
|
Significant
unobservable
inputs
(Level 3) |
| Warrant liability |
$ |
14,447 |
|
|
- |
|
|
- |
|
|
$ |
14,447 |
|
| Conversion option |
93 |
|
|
- |
|
|
- |
|
|
93 |
|
| Total Fair Value |
$ |
14,540 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
14,540 |
|
There were no transfers between Level 1, 2, or 3, during the years ended December 31, 2024, and 2023. Both observable and unobservable inputs were used to determine fair value of the positions that the Company classified within the Level 3 category. Unrealized gains and losses associated with the liabilities within the Level 3 category include changes in fair value that were attributable to both observable and unobservable inputs.
Warrant Liability
The Company's liability classified warrants as of December 31, 2024 were valued using the Black Scholes valuation model.
The Company’s initial valuation of warrant liability from the June 2024 financing, were valued using a probability weighted expected value considering the proposed reverse stock split of the Company's common stock (the "Reverse Stock Split") that would effectuate the exchange of Notes and Common Stock Purchase Warrants for shares of the Company's common stock (the "Note and Warrant Exchange"), and the previous Black Scholes valuation model, with significant value stemming from the Note and Warrant Exchange. Significant inputs under the Note and Warrant Exchange included the expected exchange ratio of 0.90 for $15.00 warrants and 0.85 for $25.13 warrants, the value of the Company’s common stock, the expected timing of the Reverse Stock Split effectuating, and the probability of the Note and Warrant Exchange occurring (90% probability).
The Company’s liability classified warrants as of December 31, 2023 and the value of initial warrant liability from the conversion of the ABS Promissory Notes, were valued using a probability weighted expected value considering the Merger Agreement and the previous Black Scholes valuation model, with significant value stemming from the Merger Agreement. Significant inputs under the Merger Agreement valuation included the expected exchange ratio 0.003, the value of SEPA’s Class A Common Stock, the expected timing of the closing of the Merger, and the then-current probability of the Merger closing.
Significant Black Scholes valuation model inputs related to the Company’s warrants are listed below:
|
|
|
|
|
|
|
|
|
December 31, 2024 |
|
|
| Weighted average expected life in years |
0.85 |
|
|
| Weighted average volatility |
91% |
|
|
| Value of underlying shares |
$2.05 |
|
|
| Weighted average risk free interest rate |
4.10% |
|
|
| Expected dividend yield |
0% |
|
|
A summary of the Level 3 warrant activity is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands, except per share data) |
Warrants
Outstanding |
|
Fair Value
per Share |
|
Warrant Liability
Fair Value |
| Balance December 31, 2022 |
2,845 |
|
$ |
0.50 |
|
|
$ |
1,416 |
|
|
|
|
|
|
|
| Issuance of warrants classified as liabilities |
412 |
|
4.02 |
|
|
1,655 |
|
| Change in fair value |
- |
|
- |
|
|
11,376 |
|
| Balance December 31, 2023 |
3,257 |
|
$ |
4.44 |
|
|
$ |
14,447 |
|
| Issuance of warrants classified as liabilities |
781 |
|
4.55 |
|
|
3,557 |
|
| Exercised |
(102) |
|
6.01 |
|
|
(613) |
|
| Converted to equity |
(3,883) |
|
10.50 |
|
|
(40,772) |
|
| Change in fair value |
- |
|
- |
|
|
31,488 |
|
| Balance December 31, 2024 |
53 |
|
$ |
152.96 |
|
|
$ |
8,107 |
|
Embedded Conversion Option
Certain convertible notes include a conversion option that meets the definition of a derivative liability and, accordingly, is required to be bifurcated. The fair value for the conversion option liability of the June 2024 transaction was valued using a probability weighted expected value considering the proposed Reverse Stock Split of the Company's common stock that would effectuate the Note and Warrant Exchange, and the previous Black Scholes valuation model, with significant value stemming from the Note and Warrant Exchange.
Significant inputs under the Note and Warrant Exchange included the value of the Company’s common stock, the expected timing of the Reverse Stock Split effectuating, and the probability of the Note and Warrant Exchange occurring (90% probability).
The Company’s liability classified warrants as of December 31, 2023 and the value of initial warrant liability from the conversion of the ABS Promissory Notes, were valued using a probability weighted expected value considering the Merger Agreement and the previous Black Scholes valuation model, with significant value stemming from the Merger Agreement. Significant inputs under the Merger Agreement valuation included the expected exchange ratio 0.003, the value of SEPA’s Class A Common Stock, the expected timing of the closing of the Merger (estimated by February 29, 2024), and the probability of the then-current Merger closing (90% probability).
In October 2024, the Company exchanged all outstanding convertible notes for Common Stock as part of the Note and Warrant Exchange, see Note 11.
A summary of the conversion option liability activity is as follows:
|
|
|
|
|
|
| (in thousands) |
Conversion
Liability |
| Balance December 31, 2022 |
$ |
2,340 |
|
| Issuance of Convertible Notes |
(519) |
|
|
|
| Change in fair value |
(1,728) |
|
| Balance December 31, 2023 |
$ |
93 |
|
| Issuance of Convertible Notes |
8 |
|
| Settlement of convertible notes |
(26) |
|
| Change in fair value |
(75) |
|
| Balance December 31, 2024 |
$ |
- |
|
14. Contract Liabilities
During the years ended December 31, 2024, and 2023, the Company recognized revenue related to these contract liabilities of $95 thousand and $60 thousand, respectively, that were included in the beginning contract liability balances for each of those periods.
The following table summarizes the changes in contract liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
| (in thousands) |
2024 |
|
2023 |
| Beginning balance |
$ |
439 |
|
|
$ |
290 |
|
| New service agreements |
149 |
|
|
209 |
|
| Revenue recognized |
(95) |
|
|
(60) |
|
| Total Contract Liabilities |
$ |
493 |
|
|
$ |
439 |
|
15. Common Stock Purchase Warrants
A summary of the warrant activity is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands, except per share data) |
Warrants |
|
Weighted
Average
Exercise Price |
|
Weighted
Average
Remaining
Life (years) |
| Warrants at December 31, 2022 |
3,221 |
|
|
$ |
26.25 |
|
|
3.55 |
| Issuances |
412 |
|
|
22.50 |
|
|
|
|
|
|
|
|
|
| Forfeited or expired |
(376) |
|
|
- |
|
|
|
| Outstanding at December 31, 2023 |
3,257 |
|
|
$ |
22.50 |
|
|
4.01 |
| Issuances |
781 |
|
|
20.06 |
|
|
|
| Exercised |
(107) |
|
|
29.45 |
|
|
|
| Converted |
(3,883) |
|
|
20.06 |
|
|
|
| Forfeited or expired |
5 |
|
|
- |
|
|
|
| Outstanding at December 31, 2024 |
53 |
|
|
$ |
4.05 |
|
|
0.86 |
16. Common Stock
In December 2022, the Company’s stockholders approved an amendment to the Company’s Articles of Incorporation to increase the number of authorized shares of common stock from 800,000,000 to 2,500,000,000. In January 2023, the Company filed the amendment to the Articles of Incorporation with the state of Nevada to affect the increase in authorized shares.
Private Investment in Public Equity (PIPE) Transaction
On October 16, 2024, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with the purchasers (the “Purchasers”), for the private placement (the “Private Placement”) of approximately 1.3 million shares (the “Shares”) of common stock at a purchase price of $8.25 per share, in each case, after adjustment to reflect the Reverse Stock Split. The Private Placement closed on October 18, 2024, and aggregate gross proceeds were approximately $10.3 million, before deducting $0.1 million offering expenses.
The Company used the net proceeds from the Private Placement to pay all amounts owed pursuant to the Consent and Limited Waiver and that certain letter agreement, dated as of August 8, 2024, between the Company and HealthTronics with respect to the HealthTronics Note, and intends to use the remaining net proceeds for working capital and general corporate purposes, which may include the repayment of other indebtedness.
In addition, on October 16, 2024, the Company and the Purchasers entered into a registration rights agreement (the “Registration Rights Agreement”), pursuant to which the Company agreed to file the registration statement with the SEC on or before December 17, 2024 (subject to certain exceptions) for purposes of registering the resale of the Shares, to use its commercially reasonable efforts to have such registration statement declared effective within the time period set forth in the Registration Rights Agreement, and to keep the registration statement effective until the date that all Placement Shares have been sold, thereunder or pursuant to Rule 144.
17. Concentration of Credit Risk and Limited Suppliers
Major customers are defined as customers whose accounts receivable, or sales individually consist of more than ten percent of total trade receivable or total sales, respectively. There were no accounts receivable concentrations on December 31, 2024, or 2023.
Cash equivalents are financial instruments that potentially subject the Company to concentration of credit risk. As of December 31, 2024, the Company's cash equivalent securities were largely comprised of money market funds. The Company’s cash accounts are insured by the Federal Deposit Insurance Corporation (“FDIC”) up to $250,000 per financial institution in the United States.
The Company currently purchases most of its product component materials from single suppliers and the loss of any of these suppliers could result in a disruption in our production. The percentage of purchases from major vendors of the Company that exceeded ten percent of total purchases were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
2024 |
|
2023 |
| Purchases: |
|
|
|
| Vendor A |
26 |
% |
|
19 |
% |
| Vendor B |
0 |
% |
|
19 |
% |
18. Revenue
The disaggregation of revenue is based on type and geographical region. The following table presents revenue from contracts with customers:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Year ended December 31, 2024 |
|
Year ended December 31, 2023 |
| |
United States |
|
International |
|
Total |
|
United States |
|
International |
|
Total |
| Consumables and parts revenue |
$ |
21,024 |
|
|
$ |
112 |
|
|
$ |
21,136 |
|
|
$ |
13,143 |
|
|
$ |
79 |
|
|
$ |
13,222 |
|
| System revenue |
11,072 |
|
|
83 |
|
|
11,155 |
|
|
5,841 |
|
|
116 |
|
|
5,957 |
|
| License fees and other |
- |
|
|
40 |
|
|
40 |
|
|
41 |
|
|
35 |
|
|
76 |
|
| Product Revenue |
$ |
32,096 |
|
|
$ |
235 |
|
|
$ |
32,331 |
|
|
$ |
19,025 |
|
|
$ |
230 |
|
|
$ |
19,255 |
|
| Rental Income |
303 |
|
|
- |
|
|
303 |
|
|
1,143 |
|
|
- |
|
|
1,143 |
|
| Total Revenue |
$ |
32,399 |
|
|
$ |
235 |
|
|
$ |
32,634 |
|
|
$ |
20,168 |
|
|
$ |
230 |
|
|
$ |
20,398 |
|
19. Stock-Based Compensation
On October 22, 2024, the Company granted stock options under the Company’s 2024 Equity Incentive Plan (the “2024 Plan”). The 2024 Plan authorizes the issuance of 1,376,556 shares. Stock options issued under the 2024 Plan generally vest over a three-year period, expire 10 years after the grant date, and are forfeited upon separation from the Company. Shares vest over a 3 year period.
On November 1, 2010, the Company approved the Amended and Restated 2006 Stock Incentive Plan of SANUWAVE Health, Inc. effective as of January 1, 2010 (the “Stock Incentive Plan”). Upon the approval of the 2024 Plan by the Company's stockholders, no further awards will be made under the Stock Incentive Plan.
The Stock Incentive Plan permitted grants of awards to selected employees, directors, and advisors of the Company in the form of restricted stock or options to purchase shares of common stock. Options granted may include non-statutory options as well as qualified incentive stock options. The Stock Incentive Plan is administered by the board of directors of the Company. The Stock Incentive Plan gives broad powers to the board of directors of the Company to administer and interpret the form and conditions of each option.
The following table presents stock compensation expense recognized by the Company for the year ended December 31, 2024. Total unrecognized compensation cost related to equity awards as of December 31, 2024 was $8.3 million and is expected to be recognized over the next 3 years. The Company recognizes compensation expense on a straight-line basis over the requisite service period, net of actual forfeitures.
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| (in thousands) |
|
2024 |
| General and administrative |
|
$ |
1,514 |
|
| Total expense |
|
$ |
1,514 |
|
The following table presents a summary of stock options award activity during the year ended December 31, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Options (in thousands) |
|
Weighted Average Exercise Price |
|
Weighted Average Remaining Contractual Term (Years) |
|
Aggregate Intrinsic Value (in thousands) |
| Outstanding |
43 |
|
|
$ |
51.55 |
|
|
3.4 |
|
$ |
- |
|
| Granted |
1,119 |
|
|
|
|
|
|
|
| Exercised |
- |
|
|
|
|
|
|
|
| Forfeited |
(25) |
|
|
|
|
|
|
|
| Outstanding, December 31, 2024 |
1,137 |
|
|
$ |
15.97 |
|
|
9.5 |
|
$ |
9,305 |
|
| Exercisable, December 31, 2024 |
117 |
|
|
$ |
28.71 |
|
|
6.8 |
|
$ |
641 |
|
Valuation Information for Stock-Based Compensation
The fair value of each stock option award during the year ended December 31, 2024 was based on the closing price of the Company's common stock on the date of the grant. Expected volatility was based on 100% of the historical realized volatilities of peer companies. The risk-free interest rate was based on the implied yield for U.S. Treasury zero-coupon issue with the remaining term equal to the expected term. The expected holding period was calculated using the simplified method. No dividend was assumed as the Company does not pay regular dividends on its common stock and does not anticipate paying any dividends in the foreseeable future. The Company's policy is to recognize forfeitures as they occur.
The weighted average assumptions used in the Black-Scholes option pricing model in valuing stock options granted in the year ended December 31, 2024 are summarized in the table below:
|
|
|
|
|
|
|
2024 |
| Fair value at grant date |
$14.54 |
| Expected volatility |
63% |
| Risk-free interest rate |
4% |
| Expected holding period, in years |
6.05 |
| Dividend yield |
0% |
The following table presents a summary of restricted stock unit activity during the year ended December 31, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Options (in thousands) |
|
Weighted Average Grant Date Fair Price |
| Unvested |
5 |
|
|
$ |
13.03 |
|
| Vested |
9 |
|
|
13.03 |
| Forfeited |
- |
|
|
|
| Total December 31, 2024 |
14 |
|
|
$ |
13.03 |
|
20. Income Taxes
The Company files income tax returns in the United States Federal jurisdiction and various state and foreign jurisdictions. The Company is subject to United States Federal and state income tax examinations by tax authorities for any years that have net operating losses open until the net operating losses are used.
The components of the net loss before income taxes are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| (In thousands) |
2024 |
|
2023 |
| Domestic |
$ |
(31,319) |
|
|
$ |
(25,783) |
|
| Foreign |
(26) |
|
|
(20) |
|
| Net loss before income taxes |
$ |
(31,345) |
|
|
$ |
(25,803) |
|
In accordance with ASC Topic 740, Income Taxes (“ASC 740”), the Company accounts for income taxes utilizing the asset and liability method. Deferred tax assets and liabilities are determined based on differences between the financial reporting and tax basis of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is provided for the deferred tax assets, including loss carryforwards, when it is more likely than not that some portion or all a deferred tax asset will not be realized.
The income tax provision (benefit) from continuing operations consists of the following:
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
December 31, 2024 |
|
December 31, 2023 |
| Current: |
|
|
|
| Federal |
$ |
- |
|
|
$ |
- |
|
| State |
27 |
|
|
4 |
|
| Foreign |
- |
|
|
- |
|
| Current Tax Provision |
$ |
27 |
|
|
$ |
4 |
|
| Deferred: |
|
|
|
| Federal |
$ |
(1,157) |
|
|
$ |
(3,564) |
|
| State |
(328) |
|
|
(459) |
|
| Foreign |
(10) |
|
|
(3) |
|
| Change in valuation allowance |
1,495 |
|
|
4,026 |
|
| Deferred Tax Provision |
$ |
- |
|
|
$ |
- |
|
As of December 31, 2024, and 2023, the Company did not have any undistributed earnings of our foreign subsidiaries. As a result, no additional income or withholding taxes have been provided for. The Company does not anticipate any impacts of the global intangible low taxed income (“GILTI”) and base erosion anti-abuse tax (“BEAT”) and as such, the Company has not recorded any impact associated with either GILTI or BEAT.
The income tax provision (benefit) amounts differ from the amounts computed by applying the United States Federal statutory income tax rate of 21% for the years ended December 31, 2024, and 2023. Adjustments to determine income tax expense are as follow:
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
Years ended December 31, |
|
2024 |
|
2023 |
| Tax benefit at statutory rate |
$ |
(6,582) |
|
|
$ |
(5,485) |
|
| Increase (reduction) in income taxes resulting from: |
|
|
|
| State income tax benefits, net of federal benefit |
28 |
|
|
(307) |
|
| Non-deductible gain on warrant adjustment valuation |
6,597 |
|
|
2,102 |
|
| Change in valuation allowance |
(1,495) |
|
|
4,026 |
|
| Registration penalties |
- |
|
|
- |
|
| Accrual to Return |
1,264 |
|
|
- |
|
| Other |
215 |
|
|
(332) |
|
| Income Tax Expense |
$ |
27 |
|
|
$ |
4 |
|
The tax effects of temporary differences that give rise to the deferred tax assets are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
December 31, 2024 |
|
December 31, 2023 |
| Deferred Tax Assets |
|
|
|
| Net operating loss carryforwards |
$ |
41,534 |
|
|
$ |
42,484 |
|
| Net operating loss carryforwards - foreign |
19 |
|
|
27 |
|
| Excess of tax basis over book value of property and equipment |
53 |
|
|
70 |
|
| Excess of tax basis over book value of intangible assets |
961 |
|
|
1,162 |
|
| Lease liability |
110 |
|
|
192 |
|
| Stock-based compensation |
1,668 |
|
|
1,495 |
|
| Accrued employee compensation |
212 |
|
|
338 |
|
| Capitalized equity costs |
- |
|
|
235 |
|
| Capitalized research and development |
340 |
|
|
1,273 |
|
| Net change in reserve accounts |
819 |
|
|
- |
|
| Gross deferred tax asset |
45,716 |
|
|
47,276 |
|
| Valuation Allowance |
(45,601) |
|
|
(47,096) |
|
| Net Deferred Tax Asset |
115 |
|
|
180 |
|
| Deferred Tax Liabilities |
|
|
|
| Right-of-use asset |
(95) |
|
|
(180) |
|
| Reserve for credit losses |
(20) |
|
|
- |
|
| Gross deferred tax liability |
(115) |
|
|
(180) |
|
| TOTAL |
$ |
- |
|
|
$ |
- |
|
The Tax Cuts and Jobs Act (“TCJA”) requires taxpayers to capitalize and amortize research and development (“R&D”) expenditures under section 174 for tax years beginning after December 31, 2021. This rule became effective for the Company during 2022 and resulted in capitalized R&D costs of $2.2 million as of December 31, 2024. The Company will amortize these costs for tax purposes over five years for R&D performed in the U.S. and over 15 years for R&D performed outside the U.S. In 2024, all R&D was performed in the U.S.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. In assessing the realization of deferred tax assets, management considers, whether it is “more likely than not”, that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which temporary differences representing net future deductible amounts become deductible.
ASC 740 requires that a valuation allowance be established when it is “more likely than not” that all, or a portion of, deferred tax assets will not be realized. A review of all available positive and negative evidence needs to be considered, including the scheduled reversal of deferred tax liabilities, projected future taxable income, and tax planning strategies.
After consideration of all the information available, management believes that uncertainty exists with respect to future realization of its deferred tax assets and has, therefore, established a full valuation allowance as of December 31, 2024, and 2023.
The Company’s ability to use its net operating loss carryforwards could be limited and subject to annual limitations. Since a full analysis under Section 382 of the Internal Revenue Code has not been performed, the Company may realize a “more than 50% change in ownership” which could limit its ability to use its net operating loss carryforwards accumulated to date to reduce future taxable income and tax liabilities. Additionally, because United States tax laws limit the time during which net operating loss carryforwards may be applied against future taxable income and tax liabilities, the Company may not be able to take advantage of all or portions of its net operating loss carryforwards for Federal income tax purposes.
The Federal net operating loss carryforwards of approximately $77.9 million from years ended December 31, 2005, through December 31, 2017, will begin to expire in 2025. The Federal net operating loss carryforward for the years ended December 31, 2018, through 2024 of approximately $95.9 million will not expire. The state net operating loss carryforwards of approximately $73.9 million from years ending December 31, 2005, through December 31, 2024, will expire at various dates through 2044.
A provision of ASC 740 specifies that companies are to account for uncertainties in income tax reporting, and prescribes a methodology for recognizing, reversing, and measuring the tax benefits of a tax position taken, or expected to be taken, in a tax return. ASC 740 requires the evaluation of tax positions taken or expected to be taken while preparing the Company’s tax returns to determine whether the tax positions would “more-likely-than-not” be sustained if challenged by the applicable tax authority. Tax positions not deemed to meet the more-likely-than-not threshold would be recorded as a tax benefit or expense in the current year. Management has evaluated and concluded that there were no material uncertain tax positions requiring recognition in the Company’s consolidated financial statements as of December 31, 2024, and 2023. The Company does not expect any significant changes in the unrecognized tax benefits within twelve months of the reporting date.
The Company will recognize in income tax expense, interest and penalties related to income tax matters. For the years ended December 31, 2024, and 2023, the Company did not have any amounts recorded for interest and penalties.
The Company is subject to taxation in the United States, various state jurisdictions and Switzerland. The Company is subject to income tax examination by U.S. and state tax authorities for the year ended December 31, 2021 and forward and foreign tax authorities for the year ended December 31, 2020 and forward. However, to the extent allowed by law, the taxing authorities may have the right to examine prior periods where net operating losses were generated and carried forward, and make adjustments up to the amount of the net operating losses utilized in open tax years.
21. Commitments and Contingencies
Litigation
In the ordinary course of business, the Company from time to time becomes involved in various legal proceedings involving a variety of matters. The Company does not believe there are any pending legal proceedings that will have a material adverse effect on the Company’s business, consolidated financial position, results of operations, or cash flows. However, the outcome of such legal matters is inherently unpredictable and subject to significant uncertainties. The Companies expenses legal fees in the period in which they are occurred.
In February 2024, the Company entered into a termination agreement with an advisor to agree on termination fees owed with respect to a previous engagement agreement. The Company agreed to a contingent payment of $0.7 million upon the closure of the Merger as disclosed in Note 4. Upon the Company's termination of the Merger Agreement in June 2024, the related contingent consideration liability was derecognized.
Acquisition Dispute
In May 2022, the Company received notification alleging that it is not in compliance with the license agreement with Celularity entered in connection with the acquisition of the UltraMIST assets. The Company has settled this dispute in June 2024 for a cash payment of $2.2 million, which resulted in the removal of $4.0 million in convertible promissory note payable, $2.4 million in accrued interest, $0.9 million of accrued expenses, $0.5 million of other liabilities, and $0.4 million in accounts receivable, which resulted in the recognition of a gain on extinguishment of debt of $5.3 million.
Lease Commitments
As of December 31, 2024, the maturities of the Company’s operating and financing leases, which have initial or remaining lease terms more than one year, consist of the following:
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
Operating
Leases |
|
Finance
Leases |
| Year ended December 31, |
|
|
|
| 2025 |
$ |
122 |
|
|
$ |
179 |
|
| 2026 |
67 |
|
|
44 |
|
| 2027 |
59 |
|
|
- |
|
|
|
|
|
|
|
|
|
| Total Lease Payments |
$ |
248 |
|
|
$ |
223 |
|
22. Segment Information
The Company operates in one reportable segment engaged in the design and sale of medical devices.
The accounting policies of the one reportable segment are the same as those described in the summary of significant accounting policies. The CODM assesses performance for the reportable segment and decides how to allocate resources primarily based on gross profit that is also reported on the consolidated statements of comprehensive loss. The measure of segment assets is reported on the consolidated balance sheets as total consolidated assets.
The CODM uses gross profit to evaluate income generated from segment assets (return on assets) in deciding whether to reinvest profits or to apply them to other parts of the entity.
Net (loss) income is used to monitor budget versus actual results. The CODM also uses net (loss) income in competitive analysis by benchmarking to competitors. The competitive analysis along with the monitoring of budgeted versus actual results are used in assessing performance of the segment and in establishing management’s compensation.
Management has determined that Morgan Frank, CEO, is the CODM.
The following table sets forth our consolidated statement of operations used by the CODM:
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
| (in thousands) |
2024 |
|
2023 |
| Revenue |
$ |
32,634 |
|
|
$ |
20,398 |
|
| Cost of revenue |
8,084 |
|
|
6,035 |
|
| Gross margin |
24,550 |
|
|
14,363 |
|
| Operating expenses: |
|
|
|
| General and administrative |
11,348 |
|
|
8,674 |
|
| Selling and marketing |
6,323 |
|
|
4,898 |
|
| Research and development |
673 |
|
|
579 |
|
| Depreciation and amortization |
789 |
|
|
752 |
|
| Operating income (loss) |
5,417 |
|
|
(540) |
|
| Other expense, net |
(36,762) |
|
|
(25,263) |
|
| Income tax expense |
27 |
|
|
4 |
|
| Net loss |
$ |
(31,372) |
|
|
$ |
(25,807) |
|
23. License and Option Agreement
In March 2024, the Company entered into an exclusive license and option agreement (the "Patent License") with a third party licensee in connection with a portfolio of patents related to the field of intravascular shockwave applications. The Company received a one-time payment of $2.5 million related to this Patent License, which was recorded in other income during the nine months ended September 30, 2024. The Company granted the Licensee an exclusive license to the patents and an option to acquire the patents for an additional one-time payment in the mid single-digit millions of dollars for a period of 3 years following the effective date of the Agreement. Upon acquisition of the patents, the Licensee will distribute any resulting proceeds to the Company, including but not limited to any royalties, license fees, settlement payments, or other proceeds generated from the licensing or assertion of the patents, in accordance with a revenue sharing agreement. If the Licensee does not exercise its option to acquire the Patents during a specified option period of 3 years from the effective date of the Agreement, the license terminates, a supplement license fee is owed, and all rights revert back to the Company.
Contingent Consideration - The Company considers such royalties, license fees, settlement payments, or other proceeds as variable or contingent consideration. The Company determined that the amount of variable consideration would be constrained until the period the uncertainty related to the consideration is relieved.
24. Subsequent Event
On March 3, 2025, the Company received a letter from the Listing Qualifications Staff of the Nasdaq Stock Market LLC ('Nasdaq") approving the uplisting of the Company's common stock. Trading of the Company's common stock on Nasdaq commenced on March 7, 2025 under the ticker symbol "SNWV".
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None
Item 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures, as defined in Rule 13a-15(e) and 15d-15(e) promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are designed to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the Company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
We carried out an evaluation under the supervision and with the participation of our management, including our Chief Executive Officer (principal executive officer) and Chief Financial Officer (principal financial officer and accounting officer), of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2024. Based on this evaluation, the Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were not operating effectively as of December 31, 2024.
Management’s Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) for the Company. The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance of achieving their control objectives.
Management, with the participation of the Chief Executive Officer (principal executive officer) and the Chief Financial Officer (principal financial and accounting officer), evaluated the effectiveness of the Company’s internal control over financial reporting as of December 31, 2024. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control — Integrated Framework (2013).
As of December 31, 2024, the Company identified the following material weaknesses:
1.The Company lacked expertise and resources to analyze and properly apply U.S. GAAP to complex and non-routine transactions such as complex financial instruments and derivatives and complex sales distributing agreements with select vendors.
2.The Company lacked of internal resources to analyze and properly apply U.S. GAAP to account for financial instruments included in service agreements with select vendors.
3.The Company has failed to implement controls around the following accounting processes: Equity, Financial Reporting, Accounts Payable, Expenses, Revenue, Accounts Receivable, Tax, Cash, Debt, Fixed Assets, Inventory, Commissions, Entity-Level, Human Resources/Payroll, and IT processes: change management, operations, access security. As such, we believe that accounting and IT processes and procedures need to be tested for operating effectiveness.
As a result, management concluded that its internal control over financial reporting was not effective as of December 31, 2024.
Remediation Plan
Management is fully committed to addressing the material weaknesses in our internal controls and has implemented several key initiatives to strengthen them. To that end, the Company has continued to implement and enhance appropriate internal controls and contracted with CliftonLarsonAllen LLP starting in the third quarter 2023 to conduct a valuation engagement. This engagement aims to provide an estimated conclusion of value and a summary, restricted appraisal report to assist management in determining the fair value of warrants to purchase the Company’s stock, embedded derivatives, and other interests.
We plan to remediate and implement these controls for high-risk processes throughout 2025. We hired a highly experienced Director of Internal Audit to review, adjust, as needed, and test our internal controls and ensure they function as intended. We recently deployed Governance, Risk, and Compliance software from Workiva to support these efforts. Furthermore, we plan to hire additional personnel and segregate certain duties to enhance our control activities and effectively mitigate risks. Until we fully remediate the material weaknesses, we will perform additional analyses and procedures to ensure our consolidated financial statements comply with U.S. GAAP. We remain vigilant in identifying any additional material weaknesses that may arise and are prepared to adjust our remediation strategies as needed.
We are collaborating with an external vendor to enhance our IT general controls over our enterprise resource planning system. This initiative aims to establish a robust framework for executing IT general controls, addressing weaknesses in our internal controls, and providing a solid framework for future testing.
The existence of any material weakness or significant deficiency requires management to devote significant time and incur significant expense to remediate any such material weaknesses or significant deficiencies, and management may not be able to remediate any such material weaknesses or significant deficiencies in a timely manner. The existence of any material weakness in our internal control over financial reporting could also result in errors in our financial statements that could require us to restate our financial statements, cause us to fail to meet our reporting obligations, and cause shareholders to lose confidence in our reported financial information, all of which could materially and adversely affect our business and stock price.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2024, that materially affect, or are reasonably likely to materially affect, our internal control over financial reporting, except as disclosed in “Remediation Plan” above.
Item 9B. OTHER INFORMATION
During the three months ended December 31, 2024, none of our directors or officers (as defined in Rule 16a-1(f) of the Exchange Act) adopted or terminated any contract, instruction, or written plan for the purchase or sale of our securities that was intended to satisfy the affirmative defense conditions of Rule 10b5-1(c) of the Exchange Act or any non-Rule 10b5-1 trading arrangement (as defined in Item 408(c) of Regulation S-K).
Item 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
MANAGEMENT
The following section sets forth the name, age, and business experience of the Company’s executive officers and directors.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
|
Age |
|
Position Held |
| Morgan Frank |
|
53 |
|
Chief Executive Officer, Chairman of the Board |
| Peter Sorensen |
|
32 |
|
Chief Financial Officer |
| Peter Stegagno |
|
65 |
|
Chief Operating Officer |
| Iulian Cioanta, PhD |
|
62 |
|
Chief Science and Technology Officer |
| Andrew Walko |
|
41 |
|
President |
| Tim Wern |
|
57 |
|
Executive Vice President of Sales |
| Nanci Gilmore |
|
53 |
|
Chief Commercial Officer |
| A. Michael Stolarski |
|
54 |
|
Director |
| Jeff Blizard |
|
56 |
|
Director |
| Ian Miller |
|
49 |
|
Director |
| James Tyler |
|
67 |
|
Director |
Morgan Frank joined the board as Chairman in August 2022 and was appointed Chief Executive Officer in May 2023. Mr. Frank is a founder and principal at Manchester Explorer Fund (19 years) and at Manchester Explorer Ltd (Cayman), two life science focused public equity hedge funds specializing in hands-on microcap growth and development companies. He has over 30 years of experience in investing, capital markets, corporate strategy, corporate finance, corporate restarts, and intellectual property. Mr. Frank was formerly a principal at First Principles Group, a firm focused on corporate restarts and a portfolio manager for technology and venture capital at Hollis Capital, a San Francisco hedge fund. He also sits on the board of Modular Medical, Inc. (MODD), a development stage company focused on next generation insulin delivery. Mr. Frank has degrees in economics and political science from Brown University.
Peter Sorensen joined the Company as Chief Financial Officer in April 2024 and brings over a decade of finance experience including in the medical device industry since 2017. Peter was most recently the Vice President of Finance and Human Resources at Endogenex, Inc., a venture-backed medical device company focused on the treatment of type-2 diabetes. Prior to Endogenex, he spent time at LivaNova PLC in the new ventures group with the Transcatheter Mitral Valve Replacement and Vagus Nerve Stimulation for Heart Failure divisions. He also spent time in consulting at eCapital Advisors implementing FP&A solutions for large public and private companies. Mr. Sorensen brings strong finance, forecasting, analysis, and capital markets experience as well as abilities in software, process automation, and human resources to Sanuwave. Mr. Sorensen earned his bachelor’s degree from Bethel University and his Master of Business Administration from St. Cloud State University.
Peter Stegagno joined the Company as Vice President, Operations in March 2006 and was named Chief Operating Officer in 2018. Mr. Stegagno brings to the Company significant experience in the medical device market encompassing manufacturing, design and development, quality assurance and international and domestic regulatory affairs. He most recently served as Vice President of Quality and Regulatory Affairs for Elekta, and other medical device companies including Genzyme Biosurgery. Before focusing on the medical field, Mr. Stegagno enjoyed a successful career encompassing production roles in the space industry, including avionics guidance systems for military applications and control computers for the space shuttle. Mr. Stegagno graduated from Tufts University with a Bachelor of Science degree in Chemical Engineering.
Iulian Cioanta, PhD joined the Company in June 2007 as Research and Development Director. In 2009, he was promoted to Vice President of Research and Development and in 2018 to Chief Science and Technology Officer. Before joining the Company, Dr. Cioanta served as Business Unit Manager with Cordis Endovascular, a Johnson & Johnson company. Prior to that, Dr. Cioanta worked as Director of Development Engineering with Kensey Nash Corporation, Research Manager at ArgoMed Inc. and Project Manager and Scientist with the Institute for the Design of Research Apparatus. Dr. Cioanta also worked in academia at Polytechnic University of Bucharest in Romania, Leicester University in the United Kingdom and Duke University in the United States.
Dr. Cioanta received a Master of Science degree in Mechanical Engineering and Technology from the Polytechnic University of Bucharest and he earned his PhD degree in Biomedical Engineering from Duke University in the field of extracorporeal shockwave lithotripsy.
Andrew Walko joined the Company as President in July 2023. Mr. Walko brings deep experience in contract manufacturing, supply chain management, medical device production, and logistics from his previous roles as President at Biomerics, LLC (medical device contract manufacturing) from August 2021 to April 2023; at Minnetronix, Inc. (medical device design and manufacturing), including as Director of Manufacturing (Operations) from March 2020 to July 2021 and Senior Manager, Manufacturing (Operations) from June 2018 to February 2020; and at Integer Holdings Corporation. Prior to this, he served as Operations and Logistics Manager for the U.S. Army both at home and overseas. He earned his MBA from the University of Minnesota and Bachelor of Science from West Virginia University.
Tim Wern joined the Company as Executive Vice President of Sales for the U.S. Wound business in January 2025. He brings over 20 years of experience in sales leadership within the medical device industry.Tim has held progressive leadership roles at pioneering companies in healthcare, including HeartWare (acquired by Medtronic), Abiomed (acquired by Johnson & Johnson), and Ceevra Inc. His extensive background includes leading high-performing sales teams and successfully launching cutting-edge medical devices in the U.S. and Canadian markets.Tim is known for his passion for building strong, results-driven teams and his dedication to delivering impactful results. He takes pride in fostering a collaborative environment where individuals can thrive, which has led to consistent growth and innovative strategies that connect sales efforts to company goals. His expertise spans sales management, strategic planning, and launching transformative technologies that improve patient outcomes. Tim earned a Bachelor of Science in Economics from Cornell University.
Nanci Gilmore joined the Company as Vice President of Commercial Operations in February 2023 and was named Chief Commercial Officer in June 2024. Ms. Gilmore has over 25 years of progressive experience in the medical device industry, specifically focusing on start-up, rapid-growth enterprises. Ms. Gilmore’s passion and commitment to bringing innovative technologies to clinicians, with a patient-centric mindset, results in high-yield, consumer program development and implementation. Ms. Gilmore’s diverse experience spans multiple medical specialties, representing organizations including Entellus Medical, SenoRx, Echosens, and most recently, THINK Surgical. Ms. Gilmore’s unique skill set will vitalize Sanuwave’s customer relations and strategic growth. Ms. Gilmore earned her bachelor’s degree from Clark University and her master’s from the University of North Carolina at Chapel Hill.
Michael Stolarski joined the Company as a member of the Board of Directors in April 2016. Mr. Stolarski founded Premier Shockwave, Inc. in October 2008 and has since served as its President & CEO. From 2005 to 2008, Mr. Stolarski was the Vice President of Business Development and, previously, Acting CFO of Sanuwave, Inc. From 2001 to 2005, he was the President – Orthopedic Division and Vice President of Finance for HealthTronics Surgical Services, Inc. From 1994 to 2001, he was the CFO and Controller of the Lithotripsy Division, Internal Auditor, and Paralegal of Integrated Health Services, Inc. Mr. Stolarski brings to our board an in-depth understanding of the orthopedic and podiatric shock wave market. In addition to being a Certified Public Accountant in the state of Maryland (inactive), he holds a M.S. in Finance from Loyola College, Baltimore and a B.S. in Accounting and a B.S. in Finance from the University of Maryland, College Park.
Jeff Blizard joined the Board as a Director in April 2022. Mr. Blizard is the Senior Director of Sales at Abiomed, where he led sales of Impella in the surgical market bringing it from $16 million to $150 million in 6 years. Mr. Blizard brings a strong knowledge of capital equipment and sales leadership specific to the medical industry. Throughout his career, Mr. Blizard has shown strength in business and market development.
Ian Miller joined the Board as a Director in April 2022. Mr. Miller is the Commercial Vice President of Hoogwegt US where he manages a team of traders generating more than $500 million in annual revenue by purchasing and selling in excess of 250,000 metric tons of commodities which are distributed around the globe. Mr. Miller has a Master of Business Administration from Drake University and brings over 20 years of sales leadership knowledge that will help SANUWAVE develop its non-medical verticals and growth strategies. Throughout his career, Mr. Miller has built a successful track record for business development and strategic implementation that have helped companies grow both their top and bottom lines.
James Tyler joined the Board as a Director in April 2021. Mr. Tyler is an advisory partner to Morgan Stanley Expansion Capital. Mr. Tyler has over 40 years of operations and financial leadership in various healthcare delivery models. Mr.
Tyler built a successful track record for operational excellence, specifically in the wound care industry, as COO with National Healing which later became Healogics, the nation’s leading provider of advanced wound care.
CORPORATE GOVERNANCE AND BOARD MATTERS
The Board of Directors
The Company’s current board of directors consists of five members, four of whom have been determined by the board to be “independent” as defined under the Nasdaq rules. The board of directors has determined that Mr. Frank is not independent under the applicable Nasdaq rules. During 2024, the Board held five meetings. Each incumbent director attended at least 75% of the aggregate of the total number of meetings of the Board held during the period for which he has been a director and the total number of meetings held by all committees of the Board on which he served during the periods that he served.
Board’s Leadership Structure
The Company’s board of directors elects the Company’s chief executive officer and its chairman, and each of these positions may be held by the same person or may be held by two persons. The chairman’s primary responsibilities are to manage the board and serve as the primary liaison between the board of directors and the chief executive officer, while the primary responsibility of the chief executive officer is to manage the day-to-day affairs of the Company, considering the policies and directions of the board of directors. Such an arrangement promotes more open and robust communication among the board and provides an efficient decision-making process with proper independent oversight. The Company’s board of directors, as of May 2023, with the appointment of Morgan Frank as Chief Executive Officer,, determined that it is currently in the best interest of the Company and its stockholders to combine the roles of chairman of the board and chief executive officer. Because Mr. Frank is not independent, the board of directors has designated Ian Miller to serve as our Lead Director. The Lead Director’s responsibilities include, but are not limited to: presiding over all meetings of the board of directors at which the chairman of the board of directors is not present, including any executive sessions of the independent directors; approving board of directors' meeting schedules and agendas; and acting as the liaison between the independent directors and the chief executive officer.
The Company believes, however, that there is no single leadership structure that is always the best and most effective in all circumstances. Accordingly, the board of directors retains the authority to later separate these roles if doing so would be in the best interests of the Company and its stockholders.
The Company’s board of directors is authorized to have an audit committee, a compensation committee, a nominating and corporate governance committee, and a strategy and finance committee, to assist the Company’s board of directors in discharging its responsibilities.
Board’s Role in Risk Oversight
While the Company’s management is responsible for the day-to-day management of risk to the Company, the board of directors has broad oversight responsibility for the Company’s risk management programs. The various committees of the board of directors assist the board of directors in fulfilling its oversight responsibilities in certain areas of risk. In particular, the audit committee focuses on financial and enterprise risk exposures, including internal controls, and discusses with management and the Company’s independent registered public accountants the Company’s policies with respect to risk assessment and risk management. The compensation committee is responsible for considering those risks that may be implicated by the Company’s compensation programs and reviews those risks with the Company’s board of directors and chief executive officer.
Audit Committee
The audit committee operates under a written charter adopted by the board of directors which is available on the Company’s website at www.sanuwave.com. The primary responsibility of the audit committee is to oversee the Company’s financial reporting process on behalf of the board of directors. The Audit Committee reviews and discusses with management and the independent registered public accounting firm the annual audited and quarterly financial statements (including the related disclosures under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in the annual report on Form 10-K and the quarterly reports on Form 10-Q), reviews the integrity of the financial reporting processes, both internal and external, and reviews the qualifications, performance, and independence of the registered public accounting firm. Among other things, the audit committee is also responsible for reviewing with management the effectiveness of the Company’s internal controls and disclosure controls and procedures.
The audit committee is directly responsible for the appointment, compensation, retention, and oversight of the work of the Company’s independent auditors, currently Marcum LLP, including the resolution of disagreements, if any, between management and the auditors regarding financial reporting. In addition, the audit committee is responsible for reviewing and approving any related party transaction that is required to be disclosed pursuant to Item 404 of Regulation S-K promulgated under the Exchange Act.
The current members of the Company’s audit committee are Ian Miller (Chairperson), Jeffrey Blizard, and James Tyler. Mr. Miller, Mr. Blizard, and Mr. Tyler are determined to be independent directors, pursuant to Nasdaq rules and Rule 10A-3 of the Exchange Act. Mr. Miller, who is the chair of the committee, has been determined by the board of directors to be an audit committee financial expert as defined pursuant to the rules of the SEC.
Compensation Committee
The current chair of the Company’s compensation committee is Jeffrey Blizard, who is an independent director, pursuant to the Nasdaq rules. The other current member of the compensation committee is Jim Tyler, who is also an independent director pursuant to the Nasdaq rules. Each of Messrs. Blizard and Tyler is also independent pursuant to Rule 10C-1(b)(1) promulgated under the Exchange Act and is a “Non-Employee Director” as defined in Rule 16b-3 under the Exchange Act. The primary purpose of the compensation committee is to discharge the responsibilities of the board of directors relating to compensation of the Company’s executive officers. Pursuant to the Company’s Compensation Committee Charter, the compensation committee is required to consist of at least two independent directors.
The compensation committee operates under a written charter adopted by the board of directors which is available on the Company’s website at www.sanuwave.com. Specific responsibilities of the compensation committee include reviewing and recommending approval of compensation of the Company’s named executive officers, administering the Company’s stock incentive plan, and reviewing and making recommendations to the Company’s board of directors with respect to incentive compensation and equity plans.
Nominating and Corporate Governance Committee
The current chair of the Company’s nominating and corporate governance committee is James Tyler, who is an independent director, pursuant to the Nasdaq rules. The other current members of the committee are Ian Miller, A. Michael Stolarski, and Jeffrey Blizard, who are also independent directors pursuant to the Nasdaq rules. Pursuant to the Company’s Nominating and Corporate Governance Committee Charter, the nominating and corporate governance committee is required to consist of at least two independent directors.
The nominating and corporate governance committee operates under a written charter adopted by the board of directors which is available on the Company’s website at www.sanuwave.com. Specific responsibilities of the nominating and corporate governance committee include identifying and recommending nominees for election to the Company’s board of directors; developing and recommending to the board of directors the Company’s corporate governance principles; overseeing the evaluation of the board of directors; and reviewing and approving compensation for non-employee members of the board of directors.
Strategy and Finance Committee
The current chair of the Company’s strategy and finance committee is A. Michael Stolarski. The other current members of the committee are James Tyler and Ian Miller. The strategy and finance committee operates under a written charter adopted by the board of directors which is available on the Company’s website at www.sanuwave.com. Specific responsibilities of the strategy and finance committee include identifying financial strategies to improve the Company’s balance sheet position and stockholder value.
Stockholder Communications with the Board of Directors
The board of directors has implemented a process for stockholders to send communications to the board of directors. Stockholders who wish to communicate directly with the board of directors or any director should deliver any such communications in writing to the Secretary of the Company. The Secretary will compile any communications they receive from stockholders and deliver them periodically to the board of directors or the specific directors requested. The Secretary of the Company will not screen or edit such communications but will deliver them in the form received from the stockholder.
Code of Conduct and Ethics
It is the Company’s policy to conduct its affairs in accordance with all applicable laws, rules and regulations of the jurisdictions in which it does business. The Company has adopted a code of business conduct and ethics with policies and procedures that apply to all associates (all employees are encompassed by this term, including associates who are officers) and directors, including the chief executive officer, chief financial officer, controller, and persons performing similar functions.
The Company has made the code of business conduct and ethics available on its website at www.sanuwave.com. If any substantive amendments to the code of business conduct and ethics are made or any waivers are granted, including any implicit waiver, the Company intends to disclose the nature of such amendment or waiver on its website or in a Current Report on Form 8-K.
Insider Trading Policy
We have adopted an insider trading policy governing the purchase, sale, and other disposition of our securities by directors, officers, employees and certain other covered persons, a copy of which is filed as an exhibit to this Annual Report on Form 10-K. The policy is designed to promote compliance with insider trading laws, rules, and regulations, as well as applicable listing standards. In addition, with regard to the Company’s trading in its own securities, it is the Company's policy to comply with the federal securities laws.
No Family Relationships Among Directors and Officers
There are no family relationships between any director or executive officer of the Company and any other director or executive officer of the Company.
Limitation of Directors Liability and Indemnification
The Nevada Revised Statutes authorize corporations to limit or eliminate, subject to certain conditions, the personal liability of directors to corporations and their stockholders for monetary damages for breach of their fiduciary duties. Our articles of incorporation limit the liability of our directors to the fullest extent permitted by Nevada law.
We have director and officer liability insurance to cover liabilities our directors and officers may incur in connection with their services to us, including matters arising under the Securities Act of 1933, as amended. Our articles of incorporation and bylaws also provide that we will indemnify our directors and officers who, by reason of the fact that he or she is one of our directors or officers, is involved in a legal proceeding of any nature.
There is no pending litigation or proceeding involving any of our directors, officers, employees, or agents in which indemnification will be required or permitted. We are not aware of any threatened litigation or proceeding that may result in a claim for such indemnification.
DELINQUENT SECTION 16(a) REPORTS
Section 16(a) of the Exchange Act requires our directors and executive officers, and persons who own more than 10% of our equity securities which are registered pursuant to Section 12 of the Exchange Act, to file with the SEC initial reports of ownership and reports of changes in ownership of our equity securities.
Based solely upon a review of the Forms 3, 4 and 5 (and amendments thereto) furnished to us for our fiscal year ended December 31, 2024, written representations by our directors and executive officers, and the issuances of securities in the Company’s records, we have determined that the following reports and transactions were not timely reported during our fiscal year ending December 31, 2025 to date, our fiscal year ended December 31, 2024, and prior fiscal years: five reports and eight transactions for Mr. Tyler; four reports and three transactions for Mr. Cioanta; three reports and two transactions for Mr. Blizard; three reports and four transactions for Mr. Frank; two reports and one transaction each for Ms. Gilmore, Mr. Sorensen and Mr. Wern; one report and one transaction for Mr. Hendricks; and eight reports and 63 transactions for Manchester Management PR, LLC, Manchester Management Company, LLC, Manchester Explorer, L.P., James E. Besser and Mr. Frank. The Company has also determined that the following reports and transactions have not been reported during our fiscal year ended December 31, 2024 or prior fiscal years: six reports and 13 transactions for Kevin A. Richardson, II; 11 reports and 13 transactions for Mr. Stegagno; eight reports and 15 transactions for Mr.
Miller, seven reports and 22 transactions for Mr. Stolarski and one report and zero transactions for Toni Rinow. The Company is working with all of its current executive officers and directors to disclose all remaining undisclosed transactions pursuant to Section 16(a) of the Exchange Act as soon as practicable.
Item 11. EXECUTIVE COMPENSATION
This section discusses the material components of the executive compensation program offered to our executives, and in particular to our named executive officers for 2024, who were:
•Morgan Frank, Chief Executive Officer
•Nanci Gilmore, Chief Commercial Officer
•Andrew Walko, President
Summary Compensation Table
The following table provides certain information concerning compensation earned for services rendered in all capacities by our named executive officers during the fiscal years ended December 31, 2024, and 2023.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name and Position |
Year |
|
Salary |
|
Bonus (1) |
|
Option Awards (2) |
|
All other
compensation (3)
|
|
Total |
| Morgan Frank, Chief Executive Officer |
2024 |
|
$ |
4 |
|
|
$ |
- |
|
|
1,383,842 |
|
|
$ |
- |
|
|
$ |
1,383,846 |
|
|
2023 |
|
1 |
|
|
- |
|
|
- |
|
|
100,000 |
|
|
100,001 |
|
| Andrew Walko, President |
2024 |
|
230,000 |
|
|
- |
|
|
909,230 |
|
|
- |
|
|
1,139,230 |
|
|
2023 |
|
95,833 |
|
|
- |
|
|
- |
|
|
- |
|
|
95,833 |
|
| Nanci Gilmore, Chief Commercial Officer |
2024 |
|
225,000 |
|
|
65,000 |
|
|
636,462 |
|
|
7,200 |
|
|
933,662 |
|
|
2023 |
|
212,308 |
|
|
- |
|
|
- |
|
|
6,900 |
|
|
219,208 |
|
(1)The bonus paid to Ms. Gilmore in 2024 was a discretionary bonus.
(2)The amounts reported in the "Option Awards" column represent the grant-date fair value of stock options granted to the named executive officers in 2024, calculated in accordance with FASB ASC 718. These stock options vest over a three-year period, except for $20,000 paid to Mr. Frank for 2024 board fees which vested immediately upon grant, subject to continued service with the Company. The reported values reflect the total grant-date fair value and do not correspond to the amounts the executives may ultimately realize upon vesting or exercise.
(3)All other compensation paid to Mr. Frank in 2023 includes board fees earned. 2024 board fees were changed to option awards in lieu of cash fees earned. All other compensation paid to Ms. Gilmore includes a car allowance.
2024 Named Executive Officer Compensation Plan
Base Salary
Our salaries reflect the responsibilities of each Named Executive Officer (NEO) and the competitive market for comparable professionals in our industry. Base salaries and benefits packages are fixed components of our NEO’s compensation and do not vary with Company performance.
Short-term Cash Incentives
The performance-based compensation plan reflects our pay-for-performance philosophy and directly ties short-term incentives to short-term business performance. These awards are linked to specific annual financial goals and key business initiatives for the overall Company. Annual employee bonus incentives are paid to reward the achievement of critical short-term operating, financial, and strategic goals. The annual employee bonus is calculated based on a percentage of each NEO’s salary: 50% is paid on individual performance goals, as assigned by leadership and the Board of Directors, and the remainder is paid based on Company performance measures.
2006 Stock Incentive Plan
On October 24, 2006, Sanuwave, Inc.’s board of directors adopted the 2006 Stock Incentive Plan of Sanuwave, Inc. On November 1, 2010, the Company approved the Amended and Restated 2006 Stock Incentive Plan of SANUWAVE Health, Inc. effective as of January 1, 2010 (the “2006 Plan”). The 2006 Plan permitted grants of awards to selected employees, directors, and advisors of the Company in the form of restricted stock or options to purchase shares of common stock. The 2006 Plan was administered by the board of directors of the Company. The 2006 Plan gave broad powers to the board of directors of the Company to administer and interpret the form and conditions of each option. The stock options granted under the 2006 Plan are generally non-statutory options which vested over a period of up to three years and have a maximum ten-year term. The options were granted at an exercise price equal to the fair market value of the common stock on the date of the grant.
The terms of the options granted under the 2006 Plan expire as determined by individual option agreements (or on the tenth anniversary of the grant date), unless terminated earlier, on the first to occur of the following: (1) the date on which the participant’s service with the Company is terminated by the Company for cause; (2) 60 days after the participant’s death; or (3) 60 days after the termination of the participant’s service with the Company for any reason other than cause or the participant’s death; provided that, if during any part of such 60 day period the option is not exercisable solely because of specified securities law restrictions, the option will not expire until the earlier of the expiration date or until it has been exercisable for an aggregate period of 60 days after the termination of the participant’s service with the Company. The options vested as provided for in each option agreement and the exercise prices for the options were determined by the board of directors at the time the option was granted, provided that the exercise price could in no event be less than the fair market value per share of the Company’s common stock on the grant date. In the event of any change in the common stock underlying the options, by reason of any merger or exchange of shares of common stock, the board of directors shall make such substitution or adjustment as it deems to be equitable to (1) the class and number of shares underlying such option, (2) the exercise price applicable to such option, or (3) any other affected terms of such option.
In the event of a change of control, unless specifically modified by an individual option agreement: (1) all options outstanding as of the date of such change of control will become fully vested; and (2) notwithstanding (1) above, in the event of a merger or share exchange, the board of directors may, in its sole discretion, determine that any or all options granted pursuant to the 2006 Plan will not vest on an accelerated basis if the board of directors, the surviving corporation or the acquiring corporation, as the case may be, has taken such action that in the opinion of the board of directors is equitable or appropriate to protect the rights and interests of the participants under the 2006 Plan.
2024 Equity Incentive Plan
The stockholders of the Company approved the Company’s 2024 Equity Incentive Plan (the “2024 Plan”) on August 7, 2024 (the “Effective Date”). The following shares of the Company’s common stock, are available for issuance under the 2024 Plan: (a) 1,376,556 shares of common stock (the “New Shares”), and (b) up to 42,605 shares of common stock which were subject to outstanding awards under the 2006 Plan as of the Effective Date (the “Outstanding Shares”). The Outstanding Shares will be available for future grants under the 2024 Plan to the extent that, on or after the Effective Date, such awards are forfeited, cancelled, settled, paid in cash, or expire before being exercised or settled in full. Upon stockholder approval of the 2024 Plan on the Effective Date, no new awards may be granted under the 2006 Plan.
Awards under the 2024 Plan may be granted to employees, non-employee directors and consultants of the Company and its subsidiaries in the form of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock, restricted stock units, and other equity-based or equity-related awards. The 2024 Plan is administered by the Compensation Committee of the Board.
The stock options granted under the 2024 Plan typically vest over a period of up to three years and have a maximum ten-year term. The options are granted at an exercise price equal to the fair market value of the common stock on the date of the grant.
Employment Agreement with Mr. Frank
Effective May 23, 2023, the Sanuwave board appointed Morgan Frank, as Sanuwave’s interim Chief Executive Officer. In connection with this appointment, Sanuwave and Mr. Frank entered into an Executive Employment Agreement, effective May 23, 2023 (the “Frank Employment Agreement”). Pursuant to the Frank Employment Agreement, Mr. Frank was paid a de minimis base salary of $1.00 per year, which was increased to $3,704 per year as of August 2024, may be eligible to receive an incentive bonus opportunity in accordance with certain performance criteria determined by the Sanuwave board, and is entitled to participate in Sanuwave’s employee benefit plans and programs.
Mr. Frank’s employment will be terminated upon (i) written notice of termination or resignation by either Sanuwave or Mr. Frank, respectively, for any reason, provided that Mr. Frank must provide at least 60 days’ prior notice of his resignation, or (ii) Mr. Frank’s death or disability. Moreover, during the term of his employment and for a period of one year thereafter, Mr. Frank agreed (i) not to perform services for or have any interest in any competitive business and (ii) not to solicit (a) Sanuwave’s current or former employees or independent contractors or (b) actual or prospective customers, clients, vendors, service providers, suppliers or contractors. Finally, the Frank Employment Agreement also includes customary confidentiality and non-disparagement provisions.
Offer Letter with Mr. Walko
Effective July 31, 2023, the Board of Directors of Sanuwave appointed Andrew Walko as the Company’s President. In connection with his appointment as President, the Company and Mr. Walko entered into an offer letter, dated July 20, 2023 (the “Walko Offer Letter”). Pursuant to the Walko Offer Letter, Mr. Walko (i) receives an annual base salary of $230,000, (ii) is eligible to earn an annual bonus of 25% of his base salary, based on the achievement of performance goals established by the Company, and (iii) received an option grant.
Offer Letter with Ms. Gilmore
Effective January 23, 2023, Nanci Gilmore joined the Company as Vice President Commercial Strategy. The Company and Ms. Gilmore entered into an offer letter, dated January 11, 2023 (the “Gilmore Offer Letter”). Pursuant to the Gilmore Offer Letter, Ms. Gilmore (i) receives an annual base salary of $225,000, (ii) is eligible to earn an annual bonus of 50% of her base salary, based on the achievement of performance goals established by the Company, (iii) received an option grant, and (iv) is eligible for cash severance in the amount equal to five months of base salary at the rate in effect on the termination date.
Outstanding Equity Awards at 2024 Fiscal Year End
The following table provides certain information concerning the outstanding equity awards for each named executive officer as of December 31, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
Number of
securities
underlying
unexercised
options
exercisable |
|
Number of
securities
underlying options
unexercisable |
|
Equity incentive
plan awards
number of
securities
underlying
unexercised
unearned options |
|
Exercise
price ($) |
|
Expiration
Date |
| Morgan Frank, Chief Executive Officer |
- |
|
160,000 |
|
- |
|
$ |
14.20 |
|
|
10/22/2034 |
|
1,559 |
|
- |
|
- |
|
$ |
22.76 |
|
|
12/31/2029 |
| Andrew Walko, President |
- |
|
106,667 |
|
- |
|
$ |
14.20 |
|
|
10/22/2034 |
| Nancy Gilmore, Chief Commercial Officer |
- |
|
74,667 |
|
- |
|
$ |
14.20 |
|
|
10/22/2034 |
Director Compensation Table for Fiscal Year 2024
Beginning October 2024, the director compensation plan changed from a base retainer of cash to option awards. The Company provides an additional option award for committee leadership of the Audit Committee. The Compensation Committee believes the structure aligns compensation according to the level of service contributions by each director. Mr.
Frank's compensation is described above in the Summary Compensation Table which includes his director compensation earned for 2024.
|
|
|
|
|
|
|
|
|
| Director |
Fee Earned or
paid in cash
(in thousands) |
Option Awards Earned (in thousands) (1) |
| A. Michael Stolarski |
$ |
– |
|
$ |
20 |
|
| Jeff Blizard |
$ |
– |
|
$ |
20 |
|
| Ian Miller |
$ |
– |
|
$ |
23 |
|
| James Tyler |
$ |
– |
|
$ |
20 |
|
(1) The amounts reported in the "Option Awards Earned" column represent the grant-date fair value of stock options granted to the directors in 2024, calculated in accordance with FASB ASC 718. These stock options vest immediately upon grant and are subject to continued service with the Company. The reported values reflect the total grant-date fair value and do not correspond to the amounts the directors may ultimately realize upon exercise.
Policies and Practices Related to the Grant of Certain Equity Awards
We have established processes to ensure that the timing of any stock option grants to executives is not influenced by material nonpublic information (“MNPI”), and that all grant decisions are made based on a predetermined schedule, taking into account factors like employee performance and market conditions, regardless of any upcoming announcements or events that could impact our stock price. The compensation committee carefully reviews any potential MNPI before granting options and will delay a grant if necessary to avoid any appearance of impropriety related to the timing of the award.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information, as of March 18, 2025, with respect to the beneficial ownership of the Company’s outstanding common stock by (i) any holder of more than five percent, (ii) each of the Company’s named executive officers and directors, and (iii) the Company’s directors and executive officers as a group.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Name of Beneficial Owner (1) |
|
Number of
Share
Beneficially
Owned |
|
Percent of
Shares
Outstanding (2)
|
Morgan Frank (3) |
|
1,087,668 |
|
12.7 |
% |
| Andrew Walko |
|
17,777 |
|
* |
| Nanci Gilmore |
|
14,015 |
|
* |
| A. Michael Stolarski |
|
363,087 |
|
4.2 |
% |
| James Tyler |
|
17,147 |
|
* |
| Ian Miller |
|
57,068 |
|
* |
| Jeff Blizard |
|
8,447 |
|
* |
| All Directors and Executives as a group (11 persons) |
|
1,610,342 |
|
18.5% |
| Greater than 5% Holders: |
|
|
|
|
Opaleye, L.P. (4) |
|
944,132 |
|
11.0 |
% |
| Manchester Management PR, LLC |
|
|
|
|
| Manchester Management Company, LLC |
|
|
|
|
| Manchester Explorer, L.P. |
|
|
|
|
| James E. Besser |
|
1,094,789 |
|
12.8 |
% |
Solas Capital Management, LLC (5) |
|
713,752 |
|
8.3 |
% |
AWM Investment Company, Inc (6) |
|
606,061 |
|
7.1 |
% |
*Denotes less than 1% beneficial ownership.
(1)Unless otherwise noted, each beneficial owner has the same address as the Company.
(2)Applicable percentage ownership is based on 8,548,473 shares of common stock outstanding as of March 18, 2025. “Beneficial ownership” includes shares for which an individual, directly or indirectly, has or shares voting or investment power, or both, and includes options, warrants and convertible promissory notes, that are exercisable within 60 days of March 18, 2025. Unless otherwise indicated, all the listed persons have sole voting and investment power over the shares listed opposite their names. Beneficial ownership as reported in the above table has been determined in accordance with Rule 13d-3 of the Exchange Act.
(3)Manchester Management PR, LLC (“Manchester”) and Manchester Management Company, LLC (“GP”) may be deemed to be the owner of 1,090,789 shares of common stock. Manchester and GP have the sole power to vote or direct the vote of 0 shares of common stock, and have the shared power to vote or direct the vote of 1,090,789 shares of common stock.
Manchester Explorer, L.P. (“Explorer”) may be deemed to be the beneficial owner of 1,006,790 shares of common stock. Explorer has the sole power to vote or direct the vote of 0 shares of common stock, and has the shared power to vote or direct the vote of 1,006,790 shares of common stock.
Mr. Besser has the sole power to vote or direct the vote of 4,000 shares of common stock, and has the shared power to vote or direct the vote of 1,090,789 shares of common stock.
Mr. Frank has the sole power to vote or direct the vote of 52,653 shares of common stock, and has the shared power to vote or direct the vote of 1,059,443 shares of common stock.
Mr. Besser is the managing member of Manchester and GP and Mr. Frank serves as a portfolio manager and as a consultant for Explorer. Manchester is the investment manager of Explorer and GP is the general partner of Explorer. The principal business address for each of Manchester, GP, Explorer and Messrs. Besser and Frank is 2 Calle Candina, #1701, San Juan, Puerto Rico, 00907.
(4)Opaleye Management Inc. (“Opaleye”) serves as investment manager to Opaleye, L.P. and as a portfolio manager for a separate managed account (the “Managed Account”) and may be deemed to indirectly beneficially own securities owned by the Managed Account. Opaleye disclaims beneficial ownership of the shares held by the Managed Account. Mr. James Silverman is the President of Opaleye. The address of Opaleye is One Boston Place, 26th Floor, Boston, MA 02108.
(5)Solas Capital Management, LLC is an investment adviser that is registered under the Investment Advisers Act of 1940. Solas Capital Management, LLC, which serves as the investment manager to two private funds ("Funds") and as sub-adviser to another private fund ("Other Fund"), which hold securities for the benefit of their investors, and Mr. Frederick Tucker Golden, as Portfolio Manager of Solas Capital Management, LLC, with the power to exercise investment and voting discretion, may be deemed to be the beneficial owner of all shares of common stock held by the Funds and by the Other Fund. Pursuant to Rule 13d-4 under the Exchange Act, each of the Funds expressly disclaims beneficial ownership over any of the securities held by the Funds and the Other Fund.
(6)AWM Investment Company, Inc., a Delaware corporation ("AWM"), is the investment adviser to Special Situations Cayman Fund, L.P., a Cayman Islands Limited Partnership ("CAYMAN"), Special Situations Fund III QP, L.P., a Delaware limited partnership ("SSFQP"), Special Situations Private Equity Fund, L.P., a Delaware limited partnership ("SSPE") and Special Situations Life Sciences Fund, L.P., a Delaware limited partnership ("SSLS"). The principal business of each fund is to invest in equity and equity-related securities and other securities of any kind or nature.
David M. Greenhouse ("Greenhouse") and Adam C. Stettner ("Stettner") are members of: SSCayman, L.L.C., a Delaware limited liability company ("SSCAY"), the general partner of CAYMAN; MGP Advisers Limited Partnership, a Delaware limited partnership ("MGP"), the general partner of SSFQP; MG Advisers, L.L.C., a New York limited liability company ("MG"), the general partner of SSPE; and LS Advisers, L.L.C., a New York limited liability company ("LS"). Greenhouse and Stettner are also controlling principals of AWM.
AWM is the investment adviser to each of the funds. As the investment adviser to the funds, AWM holds sole voting over 91,948 shares of common stock of the Company held by CAYMAN, 330,835 shares held by SSFQP, 109,091 shares held by SSPE and 74,187 shares held by SSLS. Greenhouse and Stettner are members of: SSCAY, the general partner of CAYMAN; MGP, the general partner of SSFQP; MG, the general partner of SSPE; and LS, the general partner of SSLS. Greenhouse and Stettner are also controlling principals of AWM.
Securities Authorized for Issuance Under Equity Compensation Plans
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Plan category |
|
Number of
securities to be
issued upon
exercise of
outstanding
options, warrants,
and rights (a) |
|
Weighted-
average
exercise price
of outstanding
options,
warrants and
rights (b) |
|
Number of
securities
remaining
available for
future issuance
under equity
compensation
plans (c) |
| Equity compensation plans approved by security holders |
|
1,118,658 |
|
$ |
14.55 |
|
|
257,898 |
| Equity compensation plans not approved by security holders |
|
42,605 |
|
51.55 |
|
|
- |
| Total |
|
1,161,263 |
|
$ |
15.97 |
|
|
257,898 |
Stock Incentive Plans
The stockholders of the Company approved the 2024 Plan on the Effective Date. The following shares of the Company’s common stock are available for issuance under the 2024 Plan: (a) 1,376,556 shares of common stock, and (b) up to 42,605 Outstanding Shares. The Outstanding Shares will be available for future grants under the 2024 Plan to the extent that, on or after the Effective Date, such awards are forfeited, cancelled, settled, paid in cash, or expire before being exercised or settled in full. Upon stockholder approval of the 2024 Plan on the Effective Date, no new awards may be granted under the 2006 Plan.
Awards under the 2024 Plan may be granted to employees, non-employee directors and consultants of the Company and its subsidiaries in the form of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock, restricted stock units, and other equity-based or equity-related awards. The 2024 Plan is administered by the Compensation Committee of the Board.
The stock options granted under the 2024 Plan typically vest over a period of up to three years and have a maximum ten-year term. The options are granted at an exercise price equal to the fair market value of the common stock on the date of the grant.
On November 1, 2010, the Company approved the 2006 Plan. The 2006 Plan permitted grants of awards to selected employees, directors, and advisors of the Company in the form of restricted stock or options to purchase shares of common stock. The 2006 Plan was administered by the board of directors of the Company. The 2006 Plan gave broad powers to the board of directors of the Company to administer and interpret the form and conditions of each option. The stock options granted under the 2006 Plan are generally non-statutory options which vested over a period of up to three years and have a ten-year term. The options were granted at an exercise price equal to the fair market value of the common stock on the date of the grant which was approved by the board of directors of the Company. No further awards may be made under the 2006 Plan after the effective date of the 2024 Plan.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Director Independence
Our board of directors has determined that Jeffrey Blizard, Ian Miller, James Tyler and A. Michael Stolarski qualify as independent directors based on Nasdaq's definition of “independent director.” Our board of directors has determined that our other director, Morgan Frank, does not qualify as an independent director based on Nasdaq's definition of “independent director.” There are no family relationships among any of the directors or executive officers of the Company.
Related Party Transactions
In August 2022 and November 2022, the Company entered into Purchase Agreements for the sale of Notes and Common Stock Purchase Warrants in an aggregate principal amount of $16.2 million in August and $4.0 million in November. In these transactions, James Besser, Morgan C. Frank, Chief Executive Officer and Chairman of the Board; Kevin A. Richardson, II, former Chairman of the Board and former Chief Executive Officer and Chief Strategy Officer of the Company; A. Michael Stolarski; Manchester Explorer, L.P., and Opaleye, L.P., beneficial owners of more than five percent of the Company’s common stock, purchased Notes, which were accompanied by Common Stock Purchase Warrants, with an aggregate principal amount of $400,000, $250,000, $261,780, $1,434,966, $2,500,000 and $2,900,000, respectively. Messrs. Besser and Frank share voting and dispositive power with respect to the securities acquired by Manchester Explorer, L.P. The Notes issued to each of Messrs. Richardson and Stolarski included $90,000 in principal amount for which the consideration was accrued and unpaid director fees. Certain other directors received Notes with an aggregate principal amount of $527,000 for which the consideration was accrued and unpaid director fees. These Notes along with interest were converted into shares of common stock during 2023. Additional information regarding the Notes and accompanying Common Stock Purchase Warrants issued in August 2022 and November 2022 is disclosed in Note 11 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
In May 2023 and December 2023, the Company entered into Purchase Agreements for the sale of Notes and Common Stock Purchase Warrants in an aggregate principal amount of $1.2 million and $1.8 million, respectively. In these transactions, Manchester Explorer, L.P. purchased Notes, which were accompanied by Common Stock Purchase Warrants, with an aggregate principal amount of $300,000 in May 2023 and $100,000 in December 2023. Additional information regarding the Notes and accompanying Common Stock Purchase Warrants issued in May 2023 and December 2023 is disclosed in Note 11 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
In July 2023, the Company issued Asset-Backed Secured Promissory Notes ("ABS Promissory Notes") in the aggregate principal amount of $4.6 million at an original issue discount of 33.33%. The Company and the parties to the ABS Promissory Notes entered into a side letter pursuant to which the parties agreed that upon the maturity date, the Company would issue each lender a convertible promissory note and warrants consistent with the form of the above-described Purchase Agreements for the sale of Notes and Common Stock Purchase Warrants. A. Michael Stolarski, Manchester Explorer and Opaleye, L.P, purchased ABS Promissory Notes in an aggregate principal amount of $149,993, $862,457, and $299,985, respectively. In January 2024, these ABS Promissory Notes were converted to convertible notes consistent with the Notes described above. Additional information regarding the ABS Promissory Notes is disclosed in Note 12 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
In June 2024, the Company issued a promissory note to Manchester Explorer, L.P. (“Manchester”) in an aggregate principal amount of $0.5 million (the “Promissory Note”). The Promissory Note bore interest at a rate of fifteen percent (15%) per annum and matured on December 18, 2024 (the “Maturity Date”). Prepayment of the Promissory Note by the Company was permitted in whole or in part, at any time or from time to time, without penalty, upon seven calendar days’ prior written notice. The Promissory Note was paid in full in October 2024.
In October 2024, the Company entered into a securities purchase agreement with the purchasers named therein (the “Purchasers”), for the private placement (the “Private Placement”) of approximately 1.3 million shares (the “Shares”) of common stock at a purchase price of $8.25 per Share. The Private Placement closed in October 2024, and aggregate gross proceeds were approximately $10.3 million, before deducting offering expenses. In this transaction, Manchester Explorer, L.P. purchased $1,500,000 of common stock. Additional information regarding the Private Placement is disclosed in Note 15 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
In October 2024, the Company issued an aggregate of 3,989,456 shares of common stock in exchange for all outstanding Notes and Common Stock Purchase Warrants issued by the Company in private placements in August 2022, November 2022, May 2023, December 2023, January 2024 and June 2024 (the “Exchange”). Pursuant to the Exchange, (i) each outstanding Note was fully accelerated to maturity (with 15% interest paid on the outstanding principal) and then converted (per the terms of the Note) into shares of common stock at $15.00 per share, (ii) each Common Stock Purchase Warrant with an exercise price of $15.00 per share was exchanged for 0.0024 shares of common stock per share subject to such Common Stock Purchase Warrant, and (iii) each common Stock Purchase Warrant with an exercise price of $25.13 per share was exchanged for approximately 0.0023 shares of common stock per share subject to such Common Stock Purchase Warrant.
Additional information regarding the Exchange is disclosed in Note 16 to the consolidated financial statements in Part II, Item 8. “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table summarizes the fees that we have paid or accrued for audit and other services provided by our principal independent registered public accounting firm, Marcum LLP:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
|
For the Year Ended December 31, |
| Fee Category |
|
2024 |
|
2023 |
| Audit fees |
|
$ |
471 |
|
|
$ |
545 |
|
| Total Fees |
|
$ |
471 |
|
|
$ |
545 |
|
For purposes of the preceding table:
•Audit fees consist of fees for the annual audit of our consolidated financial statements, the review of the interim financial statements included in our quarterly reports on Form 10-Q, and other professional services provided in connection with statutory and regulatory filings and consents related to capital markets transactions and engagements for those fiscal years.
The audit committee must pre-approve all audits and permitted non-audit services to be provided by our principal independent registered public accounting firm unless an exception to such pre-approval exists under the Exchange Act or the rules of the SEC. Each year, the board of directors approves the retention of the independent auditor to audit our consolidated financial statements, including the associated fee. At this time, the audit committee evaluates and approves other known potential engagements of the independent auditor, including the scope of audit-related services, tax services and other services proposed to be performed and the proposed fees, and approves or rejects each service, taking into account whether the services are permissible under applicable law and the possible impact of each non-audit service on the independent auditor’s independence from management.
PART IV
Item 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
1.All financial statements
The following financial statements are included in this Annual Report on Form 10-K in Item 8 of Part II:
|
|
|
|
|
|
|
Page |
| Consolidated financial statements |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.Financial statement schedules
No schedules are required because either the required information is not present or is not present in amounts sufficient to require submission of the schedule, or because the information required is included in the consolidated financial statements or the notes thereto.
The exhibits below are furnished or filed and, as applicable, are incorporated by reference herein as part of this Annual Report on Form 10-K.
|
|
|
|
|
|
|
|
|
| Exhibit No. |
|
Description |
| 2.1 |
|
|
| 2.2 |
|
|
| 2.3 |
|
|
| 2.4 |
|
|
| 3.1 |
|
|
| 3.2 |
|
|
| 3.3 |
|
|
| 3.4 |
|
|
|
|
|
|
|
|
|
|
|
| 3.5 |
|
|
| 3.6 |
|
|
| 3.7 |
|
|
| 3.8 |
|
|
| 3.9 |
|
|
| 3.10 |
|
|
| 3.11 |
|
|
| 3.12 |
|
|
| 3.13 |
|
|
| 3.14 |
|
|
| 10.1∞ |
|
|
10.2 |
|
|
10.3 |
|
|
10.4 |
|
|
10.5 |
|
|
10.6 |
|
|
10.7 |
|
|
10.8 |
|
|
10.9∞ |
|
|
10.10 |
|
|
10.11∞ |
|
|
10.12∞ |
|
|
|
|
|
|
|
|
|
|
|
| 10.13 |
|
|
| 10.14 |
|
|
| 10.15 |
|
|
| 10.16 |
|
|
10.17∞ |
|
|
| 10.18 |
|
|
| 10.19 |
|
|
10.20∞ |
|
|
| 10.21 |
|
|
| 10.22 |
|
|
| 10.23 |
|
|
10.24∞ |
|
|
10.25∞ |
|
|
10.26∞ |
|
|
10.27∞ |
|
|
| 19.1* |
|
|
| 23.1* |
|
|
| 24.1* |
|
|
|
|
Rule 13a-14(a)/15d-14(a) Certification of the Chief Executive Officer. |
|
|
|
|
|
Rule 13a-14(a)/15d-14(a) Certification of the Chief Financial Officer. |
|
|
|
|
|
Section 1350 Certification of the Chief Executive Officer. |
|
|
|
|
|
Section 1350 Certification of the Chief Financial Officer. |
|
|
|
|
|
|
|
|
|
|
|
|
| 97.1* |
|
|
|
|
|
| 101.INS |
|
XBRL Instance |
|
|
|
| 101.SCH |
|
XBRL Taxonomy Extension Schema |
|
|
|
| 101.CAL |
|
XBRL Taxonomy Extension Calculation |
|
|
|
| 101.DEF |
|
XBRL Taxonomy Extension Definition |
|
|
|
| 101.LAB |
|
XBRL Taxonomy Extension Labels |
|
|
|
| 101.PRE |
|
XBRL Taxonomy Extension Presentation |
|
|
|
| 104 |
|
Cover Page with Interactive Data File |
________________________________________________________________
∞ Indicates management contract or compensatory plan or arrangement.
*Filed herewith
# Confidential treatment has been requested as to certain portions of this exhibit, which portions have been omitted and submitted separately to the Securities and Exchange Commission.
β Confidential portions of this exhibit have been omitted as permitted by applicable regulations.
Item 16. Form 10-K Summary
The Company has elected not to include summary information.
SIGNATURES
Pursuant to the requirements of Section 13 of 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
SANUWAVE HEALTH, INC. |
|
|
|
Dated: March 20, 2025 |
By:_ |
/s/ Morgan Frank |
|
|
Name: Morgan Frank |
|
|
Title: Chief Executive Officer |
POWER OF ATTORNEY
Know all persons by these presents, that each person whose signature appears below constitutes and appoints Morgan Frank and Peter Sorensen, and each of them, as such person’s true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for such person and in such person’s name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, granting unto each said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as such person might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them or their or such person’s substitute or substitutes, may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Signatures |
|
Capacity |
|
Date |
|
|
|
|
|
|
By: /s/ Morgan Frank
Name: Morgan Frank
|
|
Chief Executive Officer and Chairman of the Board of Directors
(principal executive officer)
|
|
March 20, 2025 |
|
|
|
|
|
|
By: /s/ Peter Sorensen
Name: Peter Sorensen
|
|
Chief Financial Officer
(principal financial and accounting officer)
|
|
March 20, 2025 |
|
|
|
|
|
|
By: /s/ A. Michael Stolarski
Name: A. Michael Stolarski
|
|
Director |
|
March 20, 2025 |
|
|
|
|
|
|
By: /s/ Jeffrey Blizard
Name: Jeffrey Blizard
|
|
Director |
|
March 20, 2025 |
|
|
|
|
|
|
By: /s/ Ian Miller
Name: Ian Miller
|
|
Director |
|
March 20, 2025 |
|
|
|
|
|
|
By: /s/ James Tyler
Name: James Tyler
|
|
Director |
|
March 20, 2025 |
EX-19.1
2
ex-191snwvxinsidertradingp.htm
EX-19.1
Document
SANUWAVE HEALTH, INC.
INSIDER TRADING POLICY
Adopted: December 27, 2024
Federal and state securities laws prohibit individuals from trading in the securities of a company while they are aware of material information about that company that is not generally known or available to the public. Such trading is often referred to as “insider trading.” The purpose of this Insider Trading Policy (this “Policy”) is to prevent insider trading or allegations of insider trading, and to protect the reputation for integrity and ethical conduct of Sanuwave Health, Inc. (the “Company”).
APPLICABILITY OF POLICY
A. Material Nonpublic Information means material information (described below) that has either not been disclosed to the public generally or has been disclosed so recently that sufficient time has not yet passed to allow the information to become widely available among investors and the financial community.
B. Material Information means information about a company that would be expected to affect the investment or voting decision of a reasonable investor, or information that could reasonably be expected to have an effect on the price of that company’s securities. Examples of what might be considered material information are listed later in this Policy.
C. Covered Individuals. This Policy applies to:
1. Company Personnel. All directors, officers and employees of the Company and any subsidiary (“Company Personnel”), as well as members of their immediate families and others living in the same household.
2. Consultants and Advisors. All consultants and advisors to the Company and any subsidiaries whose work for the Company brings them into contact with material nonpublic information.
3. Related Parties. Any other person or entity, including a trust, corporation, partnership or other association, whose transactions in Company securities are directed by any person covered by paragraph C.1 or C.2 or are subject to that person’s influence or control.
The individuals and entities described in paragraphs C.1, C.2 and C.3 are referred to as “Covered Persons.”
D. Covered Companies. This Policy applies to trading in the securities of:
▪the Company; and
▪any other company with which the Company or any subsidiary is or may be doing business, such as customers, suppliers or companies with which a major transaction such as a merger, acquisition or divestiture may be or is being negotiated.
E. Covered Transactions. The securities trading that this Policy covers includes purchases and sales of common stock, options to acquire common stock and any other securities the Company may issue from time to time, such as preferred stock, warrants and convertible debentures, and purchases and sales of derivative securities relating to the Company’s stock, whether or not issued by the Company, such as exchange-traded options. Trading covered by this Policy may or may not include transactions under Company-sponsored plans as follows:
1. Stock Option Exercises. The Policy’s trading restrictions do not apply to the purchase of Company stock through the exercise of stock options granted by the Company. The trading restrictions do apply to any contemporaneous (such as a sale through a broker as part of a cashless exercise of the option) or subsequent sale of Company stock acquired through an option exercise.
2. Employee Stock Purchase Plan Purchases. The Policy’s trading restrictions do not apply to the purchase of Company stock through any Employee Stock Purchase Plan that the Company may maintain from time to time (but the Policy’s trading restrictions do apply to any election to participate in such plan, any election to change the level of participation in such plan or the sale of any shares acquired under such plan).
3. Restricted Stock/Unit and Performance Stock/Unit Awards. The Policy’s trading restrictions do not apply to the vesting of restricted stock, restricted stock units, performance stock or performance stock units, or to the exercise of a tax withholding right pursuant to which the person elects to have the Company withhold shares of stock to satisfy tax withholding requirements upon vesting. The trading restrictions do apply to any market sales of shares, such as a sale-to-cover.
4. Certain Gifts. The Policy’s trading restrictions do not apply to a bona fide gift of Company stock so long as either (i) the recipient of the gift is subject to the same trading restrictions under this Policy as are applicable to you, or (ii) you otherwise have no reason to believe that the recipient intends to sell the securities immediately or during a period when you would not be permitted to trade pursuant to the terms of this Policy.
STATEMENT OF POLICY
Insider trading involves trading at any time when the person making the purchase or sale is aware of material nonpublic information regarding the company whose securities are being traded. If you have a doubt or question about whether you are aware of or in possession of material nonpublic information concerning the Company or another company, you should contact the Company’s Chief Executive Officer.
A. No Trading on Material Nonpublic Information.
1. Company Securities. If you are a Covered Person, you must not purchase or sell any Company securities, or otherwise advise or assist any third party trading Company securities, while you are aware of material nonpublic information regarding the Company.
2. Other Companies’ Securities. If you are a Covered Person and you obtain material nonpublic information about any other publicly-held company as a result of your work on behalf of the Company or any subsidiaries, you must not trade in that company’s securities.
B. No Disclosure to Others Who Might Trade. If you are a Covered Person, you must not communicate material nonpublic information to any person who does not need that information for a legitimate business purpose, or recommend to anyone the purchase or sale of securities when you are aware of material nonpublic information about the company involved. This practice, known as “tipping,” also violates the securities laws and can result in the same civil and criminal penalties that apply to insider trading, even though you did not actually trade and did not benefit from another’s trading.
C. Protect Material Nonpublic Information. In order to reduce the possibility that material nonpublic information will be inadvertently disclosed:
•You must treat material nonpublic information as confidential, exercise the utmost caution in preserving the confidentiality of that information and should not discuss it with any other person who does not need to know it for a legitimate business purpose.
•You should refrain from discussing material nonpublic information relating to the Company or any public company in public places where such discussions can be overheard.
•If you become aware of any leak of material nonpublic information, whether inadvertent or otherwise, you should report the leak immediately to the Company’s Chief Executive Officer.
D. Specific Material Developments. From time to time, material developments known only to a limited number of Company Personnel may occur and cause the Company to impose on an appropriate group of Company Personnel additional restrictions on trading. You will be notified if you become part of such a group, and you should not disclose to others the fact that you have been so notified and that restrictions on trading have been imposed.
Exceptions for Approved 10b5-1 Plans
Transactions in Company securities that are executed pursuant to a 10b5-1 plan (or modifications thereto) approved in writing in advance by the Chief Executive Officer are not subject to prohibition on trading on the basis of material nonpublic information or the restrictions in the attached Addendum relating to the pre-clearance approval process or window periods.
Rule 10b5-1 provides an affirmative defense from insider trading liability under the federal securities laws for trading plans that meet certain requirements. In general, a 10b5-1 plan must be entered into (or modified) in good faith, not as part of a plan or scheme to evade the prohibitions of the insider trading rules and during a time when you are not aware of material nonpublic information. The plan must also provide for a cooling-off period for at least the minimum period required under, and must comply with all other applicable provisions of, Rule 10b5-1(c) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
Once the plan is adopted, you must not exercise any influence over the securities subject to the plan, including the amount of securities to be traded, the price at which they are traded or the date of the trade. The plan must either specify (including by formula) the amount, pricing and timing of the transactions in advance or delegate discretion on those matters to an independent party.
ADDITIONAL RESTRICTIONS ON CORPORATE INSIDERS
If you are a Corporate Insider (directors and officers of the Company subject to Section 16 of the Exchange Act (“Section 16”) and other officers and key employees of the Company and any subsidiaries who have been designated as Corporate Insiders by the Chief Executive Officer), you are subject to additional restrictions on trading Company securities as set out in the attached Addendum. The Company may also, from time to time, impose on all or an appropriate group of Covered Persons additional restrictions on trading Company securities when circumstances warrant. These additional restrictions will be communicated by the Chief Executive Officer.
DISCIPLINARY ACTION AND POTENTIAL CIVIL AND CRIMINAL PENALTIES
A. Disciplinary Action. Company Personnel who fail to comply with this Policy will be subject to appropriate disciplinary action, which may include ineligibility to participate in the Company’s equity incentive plans or termination of employment.
B. Civil and Criminal Penalties. The penalties for violating insider trading laws are severe. If you trade on (or tip) material nonpublic information, you are subject to civil penalties of up to three times the profit gained or loss avoided, criminal fines of up to $5,000,000 and up to 20 years imprisonment. If the Company fails to take appropriate steps to prevent insider trading, the Company and its directors, officers and other supervisory personnel may be subject to “controlling person” liability and potential civil and criminal penalties.
MATERIAL INFORMATION
There are various categories of information that are particularly sensitive and, as a general rule, will presumptively be considered material. Examples of such information include:
•Financial results or financial condition
•Projections of future earnings or losses
•Restatements of financial results or material impairments, write-offs or restructurings
•Changes in auditors
•Default under a significant financing arrangement, or financial liquidity problems
•Business plans or budgets
•Significant developments involving business relationships, including execution, modification or termination of significant agreements or orders with customers, suppliers, distributors, manufacturers or other business partners
•Product introductions, modifications, defects or recalls, significant pricing changes or other product announcements of a significant nature
•Significant developments in research and development or clinical trials, or relating to intellectual property
•Public or private securities (equity or debt) offerings
•Significant litigation exposure due to actual or threatened litigation
•Significant regulatory exposure due to actual or threatened action by state or federal regulators
•Significant corporate events, such as a pending or proposed merger, joint venture or tender offer, a significant investment, the acquisition or disposition of a significant business or asset or a change in control of the company
•Major personnel changes, such as changes in senior management or lay-offs
•Major events regarding a company’s securities (such as defaults, redemptions, stock splits, repurchase plans or changes in dividends)
QUESTIONS
Questions regarding any of the provisions or procedures of this Insider Trading Policy should be directed to the Chief Executive Officer.
ADDENDUM
Additional Requirements and Responsibilities for Corporate Insiders
A. Purpose. This Addendum supplements the Sanuwave Health, Inc. (the “Company”) Insider Trading Policy and applies to Company directors and officers as well as to key employees designated by the Chief Executive Officer. These people are subject to both the general requirements of the Insider Trading Policy as well as to additional procedures and requirements described below to help prevent inadvertent violations of federal securities laws, to avoid even the appearance of impermissible insider trading and to facilitate their compliance with certain legal requirements not applicable to Company Personnel generally.
B. Persons Covered.
1. Directors and Section 16 Officers. All provisions of this Addendum apply to the directors and officers of the Company subject to Section 16 (referred to herein as “Section 16 Officers”).
2. Other Officers and Key Employees. Designated provisions of this Addendum apply to the other officers of the Company and to designated key employees. These other officers and key employees, whose duties cause them to regularly have access to material nonpublic information about the Company, will be notified by the Chief Executive Officer that they are subject to this Addendum.
3. Related Parties. If you are covered by paragraph B.1 or B.2, then this Addendum also applies to the same extent to your immediate family members and other individuals living in your household, and to any other person or entity, including a trust, corporation, partnership or other association, whose transactions in Company securities are directed by you or are subject to your influence or control.
The individuals and entities described in paragraphs B.1, B.2 and B.3 above are collectively referred to as “Corporate Insiders.”
C. Blackout Periods for all Corporate Insiders.
1. Trading Not Permitted During Blackout Periods. If you are a Corporate Insider, you may not purchase, sell or otherwise trade Company securities during the period beginning at the end of the 15th day of the third month of each fiscal quarter and ending at the start of the second full trading day following the date of public disclosure of the financial results for that fiscal quarter.
2. Trading Outside Blackout Periods. If a Corporate Insider wishes to trade outside of a blackout period, the person may do so only if he or she is not then aware of any material nonpublic information. In addition, before Corporate Insiders may trade outside of any blackout period, they must comply with the notification and pre-clearance procedures described below.
D. Required Preclearance of Trades.
1. Notices of Intended Transaction and Requests for Approval. If you are a Corporate Insider, you may not engage in any transaction involving Company securities without first obtaining pre-clearance of that transaction from the Company’s Chief Executive Officer or other individual designated by the Company’s Chief Executive Officer or board of directors. Prior to initiating any transaction in Company securities, you must deliver to the Chief Executive Officer (or in the case of the Chief Executive Officer, the Chair of the Audit Committee) a written notice describing any intended transaction in Company securities by you during a permitted trading period (a form to request preclearance is attached as Exhibit A). Notices of intended transactions and requests for approval may be delivered by e-mail.
2. Clearance to Proceed with a Transaction. Prior to completing a transaction, a Corporate Insider must receive clearance from the Chief Executive Officer (or in the case of the Chief Executive Officer, the Chair of the Audit Committee). Clearance in response to a written request for approval will generally be valid for two business days, unless a different deadline is imposed by the Chief Executive Officer (or in the case of the Chief Executive Officer, the Chair of the Audit Committee), but a clearance may be revoked at any time without notice.
E. Additional Restrictions on Trading by Directors and Section 16 Officers.
1. Restricted Transactions. Directors and Section 16 Officers, or their designees, are also prohibited from engaging in the following transactions with respect to Company securities:
•purchasing Company securities on margin, or otherwise pledging Company securities;
•short sales of Company securities (selling securities not owned at the time of sale);
•buying or selling put or call options or other derivative securities based on Company securities;
•purchasing any financial instruments (including prepaid variable forward contracts, equity swaps, collars and exchange funds) or otherwise engaging in transactions that are designed to or have the effect of hedging or offsetting any decrease in the market value of equity securities (i) granted to the individual by the Company as part of the compensation of the individual or (ii) held, directly or indirectly, by the individual; and
•engaging in limit orders or other pre-arranged transactions that execute automatically, except for “same-day” limit orders and approved 10b5-1 plans.
2. Short-Swing Trading Restrictions. Directors and Section 16 Officers of the Company must also comply with the reporting obligations and limitations on short-swing trading transactions imposed by Section 16. Among other things, Section 16 requires directors and Section 16 Officers to pay over to the Company any profit realized from any purchase and sale (in either order) of Company securities that occur within six months of each other.
Exhibit A
FORM OF CORPORATE INSIDER REQUEST FOR APPROVAL TO TRADE
•I certify I do not have any material, non-public information;
•In connection with my proposed trade, I certify that, in making this request, I am complying with the applicable provisions of the Sanuwave Health, Inc. Insider Trading Policy;
•I understand that clearance for the transaction(s), if granted, will be valid only until the earlier of the trade occurring or two business days; and
•If I become aware of any material nonpublic information prior to the trade occurring, I will not execute the trade.
If you are submitting a 10b-5 plan, you may omit the last two bullets above.
Type of Transaction:
¨ Purchase ¨ Sale ¨ Exercise of Option ¨ Exercise of Option
and Sale of Securities
¨ Other (explain):
Securities to be Traded:
Number of shares or principal amount: _________________________________________
If You Are Buying or Selling Securities (check one):
¨ Securities held/to be held directly by me
¨ Securities held/to be held by securities holder other than me:
Print name of holder: _____________________________
Relationship of securities holder to me: _____________________________
I hereby represent that the transaction(s) referenced above will occur within the current permitted trading period of ____________________ to ____________________.
Corporate Insider Acknowledgment Form
I have read the Sanuwave Health, Inc. Insider Trading Policy and understand its contents outlined above.
Print Name:
Signature:
Date: _________________________________
EX-23.1
3
exhibit231-independentregi.htm
EX-23.1
Document
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM’S CONSENT
We consent to the incorporation by reference in the Registration Statement of SANUWAVE Health, Inc. on Form S-8 (File No. 333-170301, File No. 333-282726) and Form S-1 (File No. 333-267731, File No. 333-273060, File No. 333-283576) of our report dated March 20, 2025, which includes an explanatory paragraph as to the Company’s ability to continue as a going concern, with respect to our audits of the consolidated financial statements of SANUWAVE Health, Inc. and Subsidiaries as of December 31, 2024 and 2023 and for each of the two years in the period ended December 31, 2024, which report is included in this Annual Report on Form 10-K of SANUWAVE Health, Inc. for the year ended December 31, 2024.
|
|
|
| /s/ Marcum LLP |
|
| Marcum LLP |
| New York, NY |
| March 20, 2025 |
EX-31.1
4
sanuwavehealthinc-exx311.htm
EX-31.1
Document
EXHIBIT 31.1
CERTIFICATION PURSUANT TO RULE 13a-14(a)/15d-14(a) OF THE SECURITIES
EXCHANGE ACT OF 1934, AS ADOPTED PURSUANT TO SECTION 302 OF THE
SARBANES-OXLEY ACT OF 2002
I, Morgan Frank, certify that:
1.I have reviewed this Annual Report on Form 10-K of SANUWAVE Health, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
Date: March 20, 2025
/s/ Morgan Frank
Morgan Frank
Chief Executive Officer
(Principal Executive Officer)
EX-31.2
5
sanuwavehealthinc-exx312.htm
EX-31.2
Document
EXHIBIT 31.2
CERTIFICATION PURSUANT TO RULE 13a-14(a)/15d-14(a) OF THE SECURITIES
EXCHANGE ACT OF 1934, AS ADOPTED PURSUANT TO SECTION 302 OF THE
SARBANES-OXLEY ACT OF 2002
I, Peter Sorensen, certify that:
1.I have reviewed this Annual Report on Form 10-K of SANUWAVE Health, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
Date: March 20, 2025
/s/ Peter Sorensen
Peter Sorensen
Chief Financial Officer
(Principal Financial Officer and Principal Accounting Officer)
EX-32.1
6
sanuwavehealthinc-exx321.htm
EX-32.1
Document
EXHIBIT 32.1
CERTIFICATION PURSUANT TO 18 U.S.C.
SECTION 1350,
AS ADOPTED PURSUANT TO SECTION
906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of SANUWAVE Health, Inc. (the “Company”) on Form 10-K for the year ended December 31, 2024, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Morgan Frank, Chief Executive Officer of the Company, hereby pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
1.The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
2.The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: March 20, 2025
/s/ Morgan Frank
Morgan Frank
Chief Executive Officer
(Principal Executive Officer)
EX-32.2
7
sanuwavehealthinc-exx322.htm
EX-32.2
Document
EXHIBIT 32.2
CERTIFICATION PURSUANT TO 18 U.S.C.
SECTION 1350,
AS ADOPTED PURSUANT TO SECTION
906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of SANUWAVE Health, Inc. (the “Company”) on Form 10-K for the year ended December 31, 2024, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Peter Sorensen, Chief Financial Officer of the Company, hereby certify pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
1.The Report fully complies with the requirements of Section 13(a) or 15(d), as applicable, of the Securities Exchange Act of 1934, as amended; and
2.The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: March 20, 2025
/s/ Peter Sorensen
Peter Sorensen
Chief Financial Officer
(Principal Financial Officer and Principal Accounting Officer)
EX-97.1
8
ex-971snwvxcompensationrec.htm
EX-97.1
Document
SANUWAVE HEALTH, INC.
COMPENSATION RECOVERY POLICY
Effective March 4, 2025
Policy
The Board of Directors (the “Board”) of Sanuwave Health, Inc. (the “Company”) has adopted this Compensation Recovery Policy (this “Policy”) pursuant to Rule 10D-1 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), the Securities and Exchange Commission (the “SEC”) regulations promulgated thereunder and applicable Nasdaq Stock Market (“Nasdaq”) listing standards. Subject to and in accordance with the terms of this Policy, upon a Recoupment Event, each Covered Executive shall be obligated to return to the Company, reasonably promptly, the amount of Erroneously Awarded Compensation that was received by such Covered Executive during the Lookback Period.
Administration
This Policy will be administered by the Compensation Committee of the Board (the “Committee”). Any determinations made by the Committee will be final and binding on all affected individuals.
Definitions
“Accounting Restatement” means an accounting restatement due to the material noncompliance of the Company with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period.
“Covered Executive” means each of the Company’s current and former executive officers who is or was an “officer” of the Company within the meaning of Rule 16a-1(f) of the Exchange Act.
“Erroneously Awarded Compensation” means, with respect to each Covered Executive in connection with an Accounting Restatement, the amount of Incentive-Based Compensation received by the Covered Executive during the Lookback Period that exceeds the amount of Incentive-Based Compensation that otherwise would have been received had it been determined based on the restated amounts, computed without regard to any taxes paid. For Incentive-Based Compensation based on stock price or total shareholder return, where the amount of Erroneously Awarded Compensation is not subject to mathematical recalculation directly from the information in an Accounting Restatement: (a) the amount must be based on a reasonable estimate of the effect of the Accounting Restatement on the stock price or total shareholder return upon which the Incentive-Based Compensation was received; and (b) the Company must maintain documentation of the determination of that reasonable estimate and provide such documentation to Nasdaq.
“Financial Reporting Measures” are any measures that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and any measures derived wholly or in part from such measures. Stock price and total shareholder return are also Financial Reporting Measures. A Financial Reporting Measure need not be presented within the financial statements or included in a filing with the SEC.
“Incentive-Based Compensation” is any compensation that is granted, earned or vested based wholly or in part upon the attainment of a Financial Reporting Measure.
“Lookback Period” means the three completed fiscal years immediately preceding the Required Restatement Date and any transition period (that results from a change in the Company’s fiscal year) of less than nine months within or immediately following those three completed fiscal years.
The Company’s obligation to recover Erroneously Awarded Compensation is not dependent on if or when the restated financial statements are filed.
A “Recoupment Event” occurs when the Company is required to prepare an Accounting Restatement.
“Required Restatement Date” means the earlier to occur of: (a) the date the Company’s Board, a committee of the Board, or the officer(s) of the Company authorized to take such action if Board action is not required, concludes, or reasonably should have concluded, that the Company is required to prepare an Accounting Restatement, or (b) the date a court, regulator, or other legally authorized body directs the Company to prepare an Accounting Restatement.
“Section 409A” means Section 409A of the Internal Revenue Code and the regulations and guidance promulgated thereunder.
Amount Subject to Recovery
The Incentive-Based Compensation that is subject to recovery under this Policy includes such compensation that is received by a Covered Executive (i) on or after October 2, 2023 (even if such Incentive-Based Compensation was approved, awarded or granted prior to that date), (ii) after the individual began service as a Covered Executive, (iii) if the individual served as a Covered Executive at any time during the performance period for such Incentive-Based Compensation, and (iv) while the Company has a class of securities listed on a national securities exchange or national securities association.
The amount of Incentive-Based Compensation subject to recovery from a Covered Executive upon a Recoupment Event is the Erroneously Awarded Compensation, which amount shall be determined by the Committee in accordance with this Policy.
For purposes of this Policy, Incentive-Based Compensation is deemed “received” in the Company’s fiscal period during which the Financial Reporting Measure specified in the Incentive-Based Compensation award is attained, even if the payment or grant of the Incentive-Based Compensation occurs after the end of that period.
Recovery of Erroneously Awarded Compensation
Promptly following a Recoupment Event, the Committee will determine the amount of Erroneously Awarded Compensation for each Covered Executive, and the Company will provide each such Covered Executive with a written notice of such amount and a demand for repayment or return. Upon receipt of such notice, each affected Covered Executive shall promptly repay or return such Erroneously Awarded Compensation to the Company.
If such repayment or return is not made within a reasonable time, the Company shall recover Erroneously Awarded Compensation in a reasonable and prompt manner using any lawful method determined by the Committee; provided that recovery of any Erroneously Awarded Compensation must be made in compliance with Section 409A.
Limited Exceptions
Erroneously Awarded Compensation will be recovered in accordance with this Policy unless the Committee determines that recovery would be impracticable and one of the following conditions is met:
•the direct expense paid to a third party to assist in enforcing this Policy would exceed the amount to be recovered, provided the Company has first made a reasonable effort to recover the Erroneously Awarded Compensation, documented such reasonable attempt(s) to recover and provided that documentation to Nasdaq; or
•the recovery would likely cause a U.S. tax-qualified retirement plan, under which benefits are broadly available to employees of the Company, to fail to meet the requirements of Internal Revenue Code Sections 401(a)(13) and 411(a) and the regulations thereunder.
Reliance on any of the above exemptions will further comply with applicable listing standards, including without limitation, documenting the reason for the impracticability and providing required documentation to Nasdaq.
No Insurance or Indemnification
Neither the Company nor any of its affiliates or subsidiaries may indemnify any Covered Executive against the loss of any Erroneously Awarded Compensation (or related expenses incurred by the Covered Executive) pursuant to a recovery of Erroneously Awarded Compensation under this Policy, nor will the Company nor any of its affiliates or subsidiaries pay or reimburse a Covered Executive for any insurance premiums on any insurance policy obtained by the Covered Executive to protect against the forfeiture or recovery of any compensation pursuant to this Policy.
Interpretation
The Committee is authorized to interpret and construe this Policy and to make all determinations necessary, appropriate, or advisable for the administration of this Policy. This Policy shall be applied and interpreted in a manner that is consistent with the requirements of Rule 10D-1 and any applicable regulations, rules or standards adopted by the SEC or the rules of any national securities exchange or national securities association on which the Company’s securities are listed. In the event that this Policy does not meet the requirements of Rule 10D-1, the SEC regulations promulgated thereunder or the rules of any national securities exchange or national securities association on which the Company’s securities are listed, this Policy shall be deemed to be amended to meet such requirements.
Indemnification of Policy Administrators
Any members of the Committee who participate in the administration of this Policy shall not be personally liable for any action, determination or interpretation made with respect to this Policy and shall be fully indemnified by the Company to the fullest extent permitted under applicable law and Company governing documents and policies with respect to any such action, determination or interpretation. The foregoing shall not limit any other rights to indemnification of the members of the Committee under applicable law or Company governing documents and policies.
Amendment; Termination
The Board or the Committee may amend this Policy in its discretion and shall amend this Policy as it deems necessary to comply with the regulations adopted by the SEC under Rule 10D-1 and the rules of any national securities exchange or national securities association on which the Company’s securities are listed. The Board or the Committee may terminate this Policy at any time. Notwithstanding anything herein to the contrary, no amendment or termination of this Policy shall be effective if that amendment or termination would cause the Company to violate any federal securities laws, SEC rules or the rules of any national securities exchange or national securities association on which the Company’s securities are listed.
Other Recoupment Rights
The Board intends that this Policy will be applied to the fullest extent of the law. Any Incentive-Based Compensation provided for in an employment agreement, incentive compensation plan, policy, program or agreement, equity award, or similar plan, program or agreement shall, as a condition to the grant of any benefit thereunder, be subject to the terms of this Policy.
Any right of recoupment under this Policy is in addition to, and not in lieu of, any other remedies or rights of recoupment that may be available to the Company pursuant to the terms of any similar provision in any employment agreement, incentive compensation plan, policy, program or agreement, equity award or similar plan, program or agreement and any other legal remedies available to the Company. This Policy is in addition to any other clawback or compensation recovery, recoupment or forfeiture policy in effect or that may be adopted by the Company from time to time, or any laws, rules or listing standards applicable to the Company, including without limitation, the Company’s right to recoup compensation subject to Section 304 of the Sarbanes-Oxley Act of 2002.
Successors
This Policy shall be binding and enforceable against all Covered Executives and their beneficiaries, heirs, executors, administrators or other legal representatives.
Applicable Law
This Policy and all rights and obligations hereunder shall be governed by and construed in accordance with the law of the State of Delaware regardless of the application of rules of conflicts of laws that would apply to the laws of any other jurisdiction.
Venue
The jurisdiction and venue for any disputes arising under, or any action brought to enforce (or otherwise relating to), this Policy will be exclusively in the courts in the State of Delaware, including the Federal Courts located therein (should Federal jurisdiction exist). The parties consent to and submit to the personal jurisdiction and venue of courts of the State of Delaware and irrevocably waive any claim or argument that the courts in the State of Delaware are an inconvenient forum.