Document
NEWS RELEASE
AbCellera Reports Full Year 2024 Business Results
2/27/2025
VANCOUVER, British Columbia--(BUSINESS WIRE)-- AbCellera (Nasdaq: ABCL) today announced financial results for the full year 2024. All financial information in this press release is reported in U.S. dollars, unless otherwise indicated.
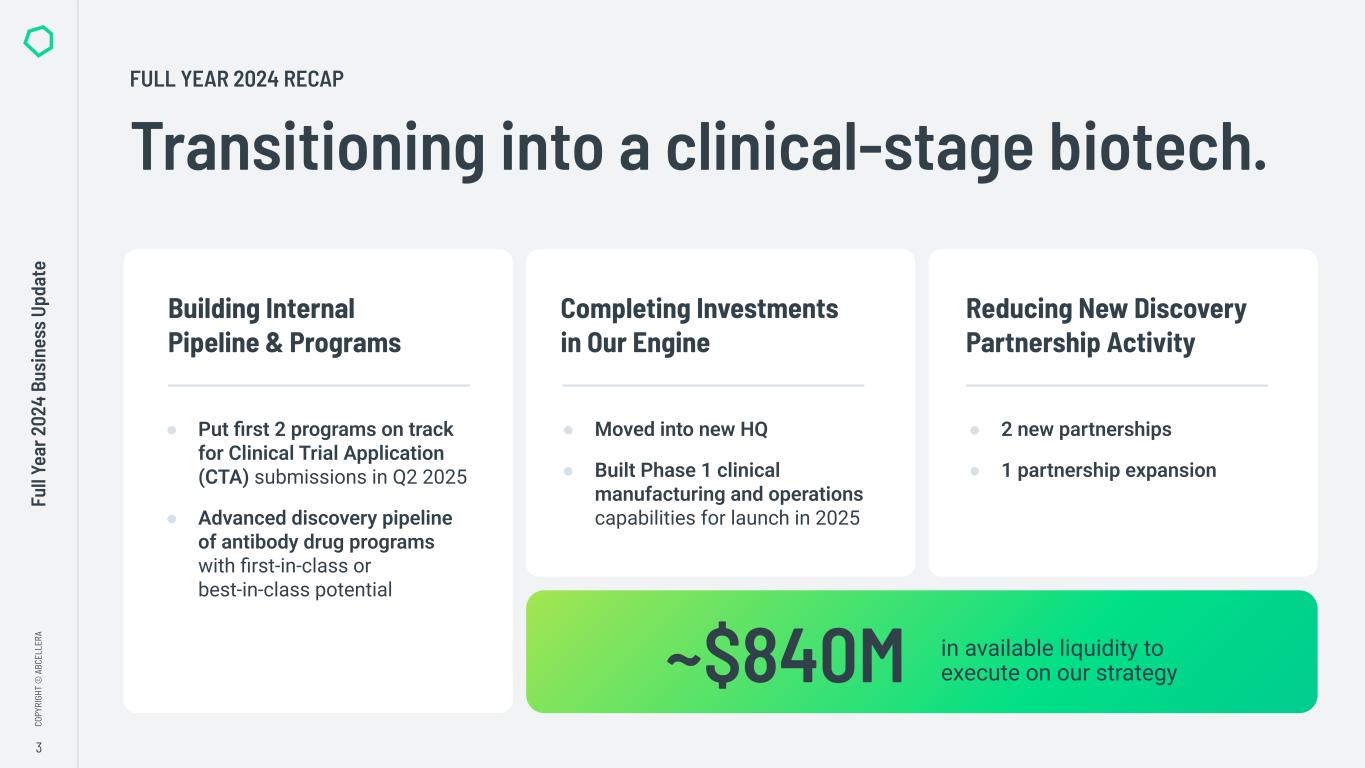
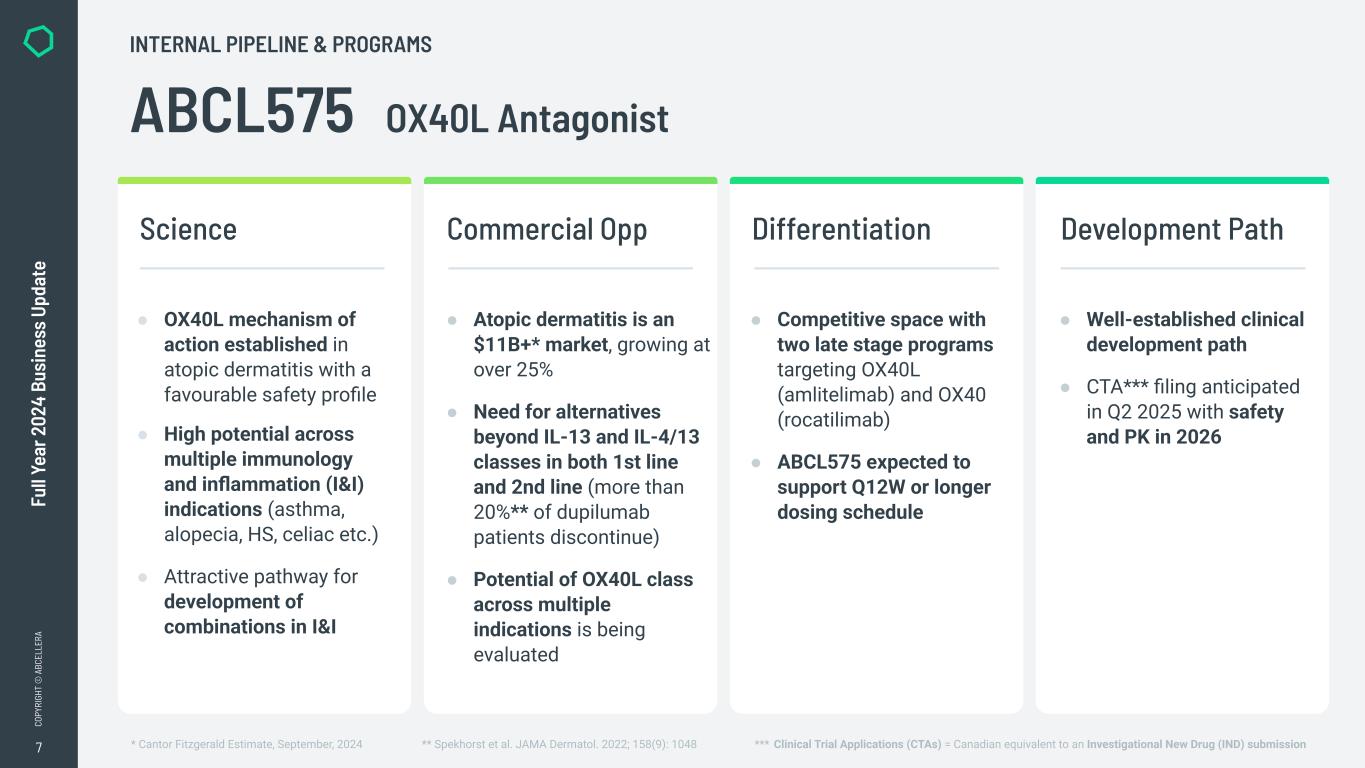
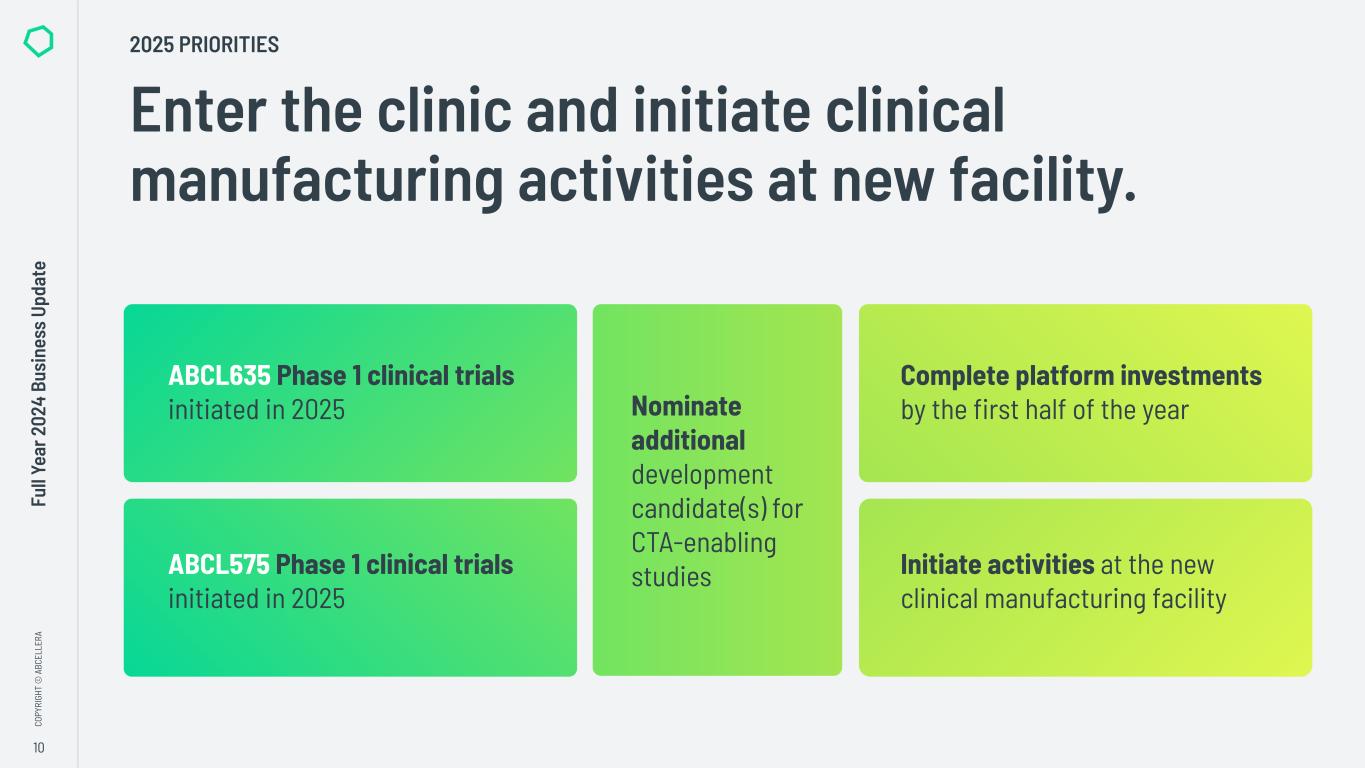
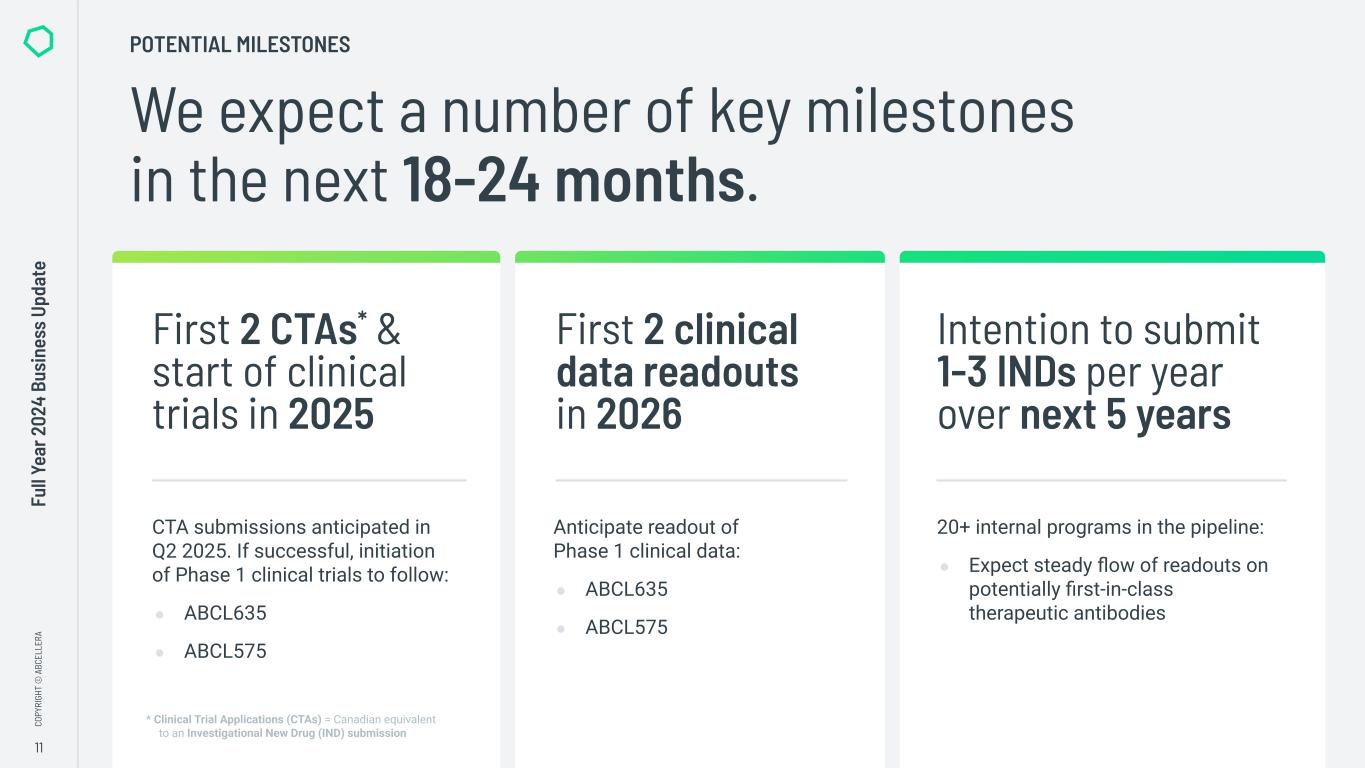
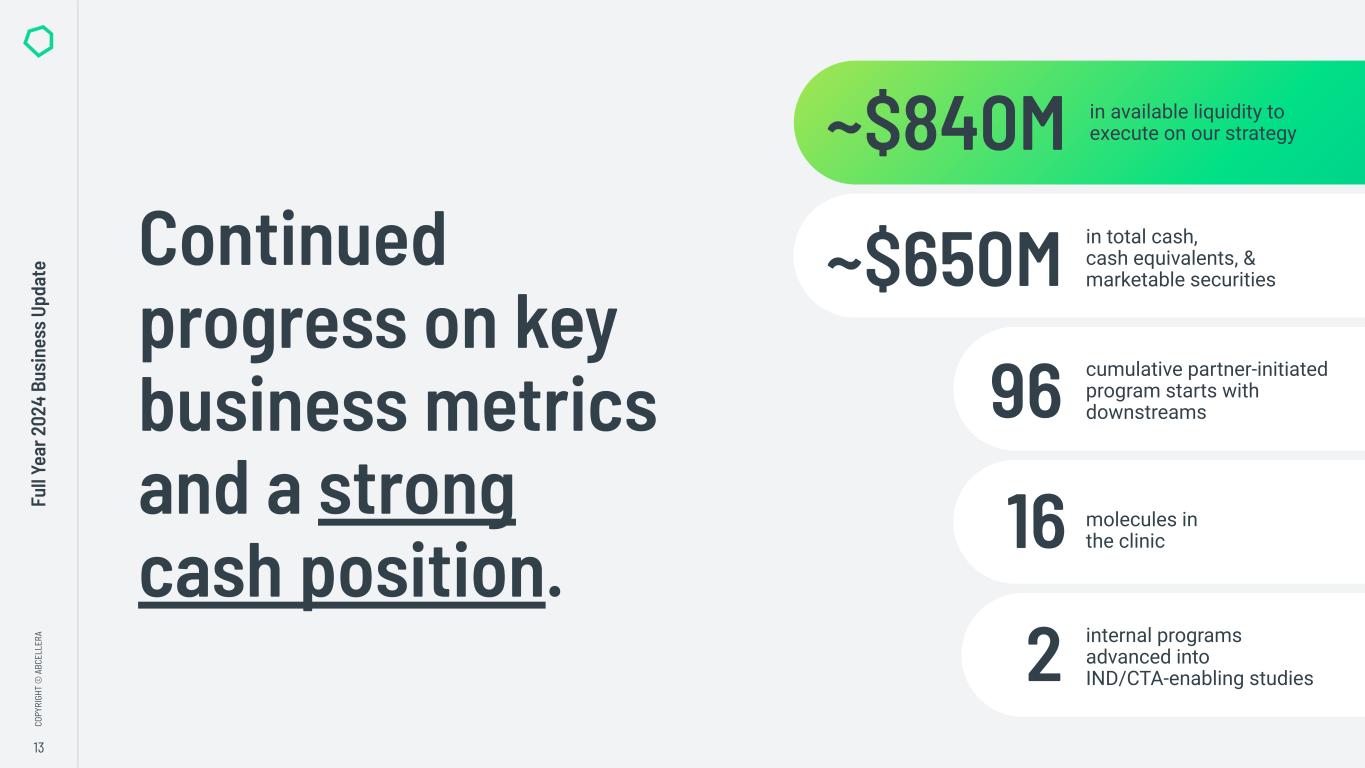
“In 2024 we made significant progress in transitioning from a platform company to a clinical-stage biotech, including advancing our internal pipeline and completing significant investments in our capabilities. We also maintained our strong cash position, closing the year with over $800 million in available liquidity to execute on our strategy,” said Carl Hansen, Ph.D., founder and CEO of AbCellera. “As a result, we enter 2025 on track to initiate Phase 1 clinical trials for our first two programs, ABCL635 and ABCL575, and to start activities in our new clinical manufacturing facility.”
FY 2024 Business Summary
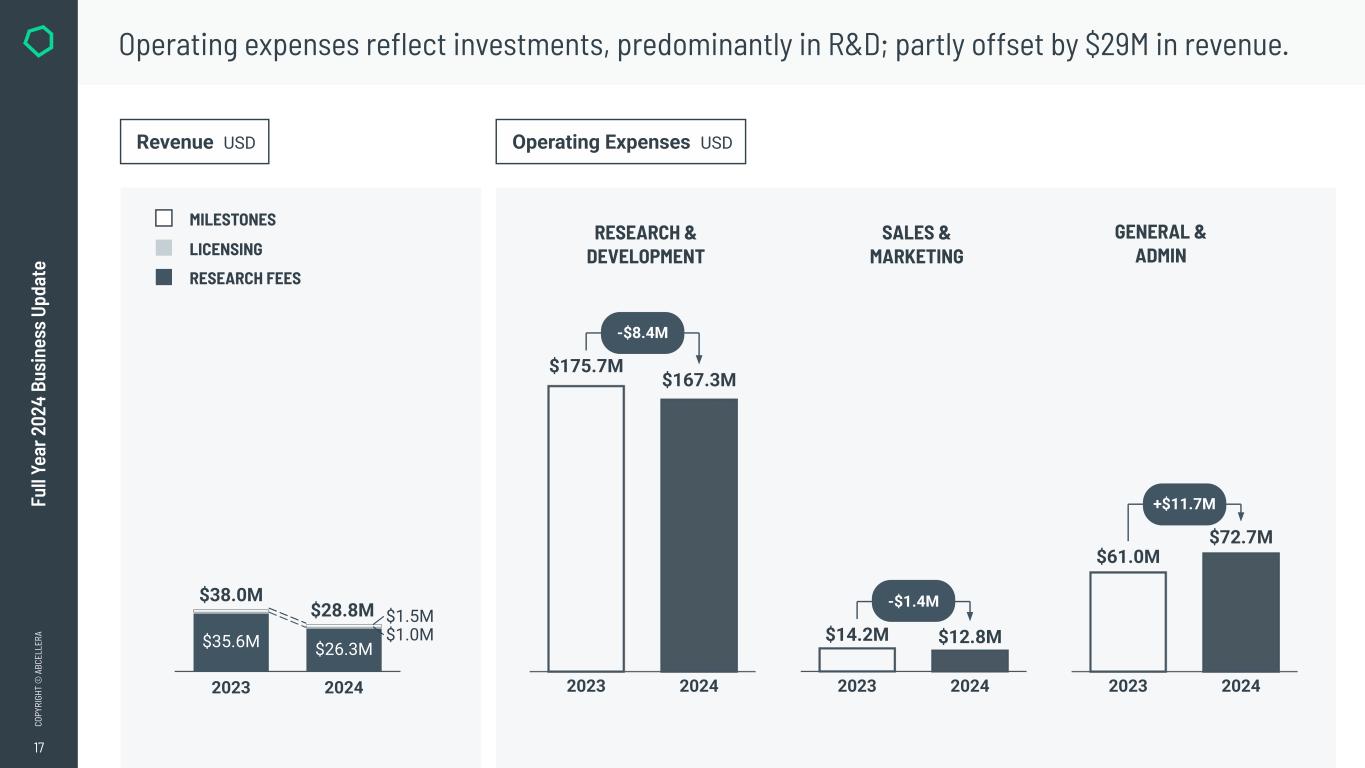
●Earned $28.8 million in total revenue.
●Generated a net loss of $162.9 million, compared to net loss of $146.4 million in 2023.
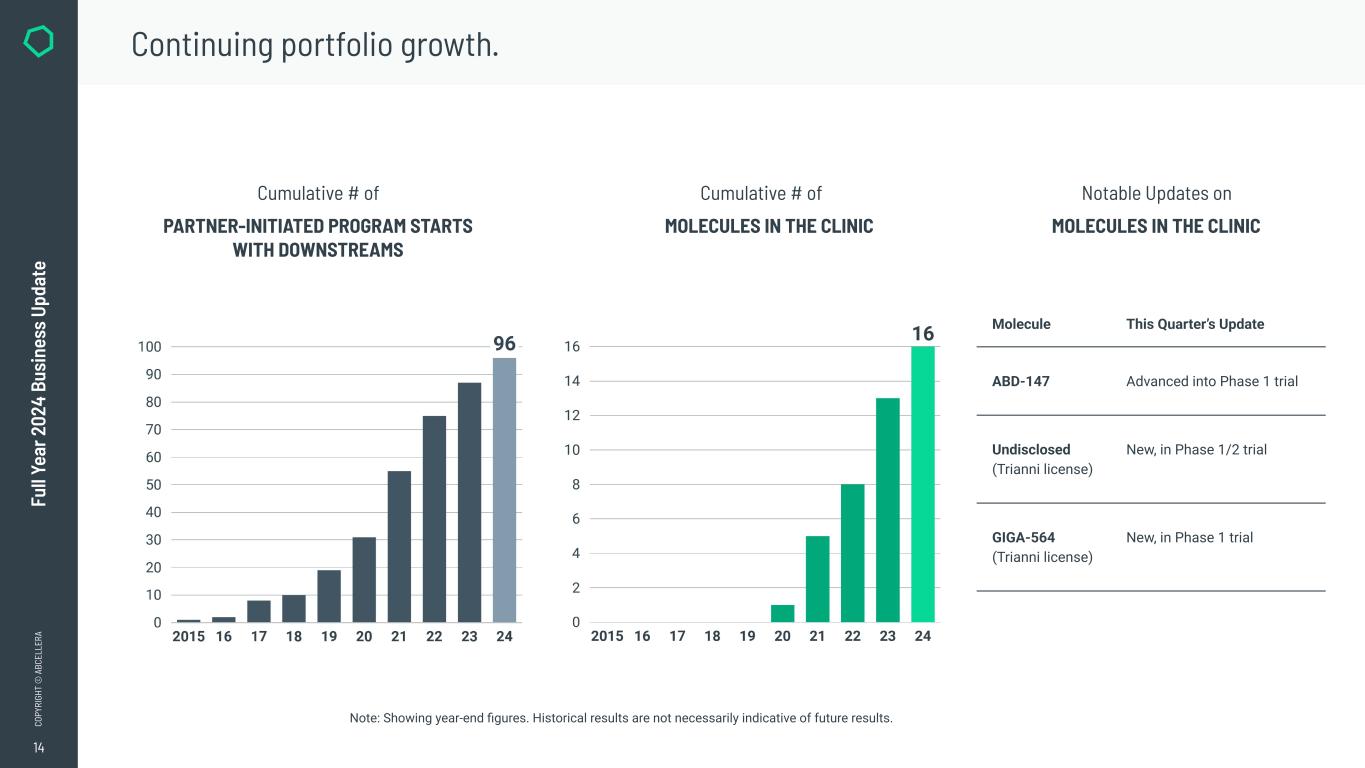
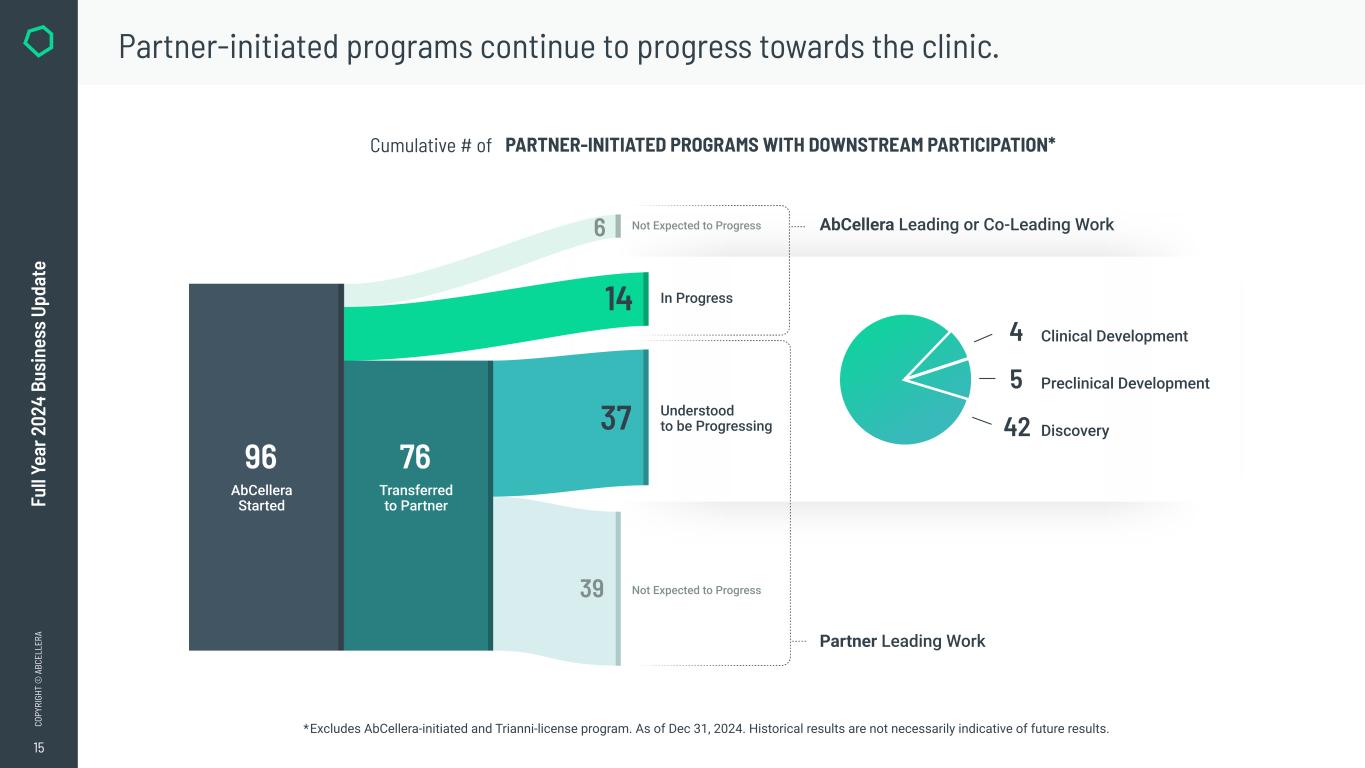
●Reached a cumulative total of 96 partner-initiated program starts with downstreams.
●Reporting the advancement of three additional molecules in the clinic, bringing the cumulative total to 16 molecules to have reached the clinic.
Key Business Metrics
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cumulative Metrics |
|
December 31, 2023 |
|
December 31, 2024 |
|
Change % |
| Partner-initiated program starts with downstreams |
|
87 |
|
96 |
|
10 |
% |
| Molecules in the clinic |
|
13 |
|
16 |
|
23 |
% |
AbCellera started discovery on an additional nine partner-initiated programs with downstreams to reach a cumulative total of 96 partner-initiated program starts with downstreams in 2024 (up from 87 on December 31, 2023). AbCellera’s partners have advanced a cumulative total of 16 molecules into the clinic (up from 13 on December 31, 2023).
Discussion of FY 2024 Financial Results
●Revenue – Total revenue was $28.8 million, compared to $38.0 million in 2023. In both periods, the majority of revenues were research fees generated by our partnerships.
●Research & Development (R&D) Expenses – R&D expenses were $167.3 million, compared to $175.7 million in 2023, reflecting underlying continued growth in program execution, platform development, and investments in internal programs.
●Sales & Marketing (S&M) Expenses – S&M expenses were $12.8 million, compared to $14.2 million in 2023.
●General & Administrative (G&A) Expenses – G&A expenses were $72.7 million, compared to $61.0 million in 2023.
●Net Loss – Net loss of $162.9 million, or $(0.55) per share on a basic and diluted basis, compared to net loss of $146.4 million, or $(0.51) per share on a basic and diluted basis, in 2023.
●Liquidity – $652.9 million of total cash, cash equivalents, and marketable securities and approximately $186 million in available non-dilutive government funding, bringing total available liquidity to approximately $840 million to execute on AbCellera's strategy.
Q4 Highlights and Financial Results
●Abdera advanced ABD-147 into a Phase 1 clinical trial. AbCellera is a founding partner in Abdera, has a low-single-digit royalty stake in Abdera’s programs, and has a mid-single-digit equity ownership position.
●Reporting the advancement of two Trianni-license molecules into the clinic.
●Started one partner-initiated program with downstreams.
●Revenue for the fourth quarter of 2024 was $5.1 million, the majority of which was research fees generated by our partnerships, representing 18% of total revenue for 2024.
●Operating expenses totaled $77.8 million in the fourth quarter, or 23% of the total for 2024, and included investments made in co-development and internal programs.
●The net loss for the fourth quarter was $34.2 million, or $(0.12) per share, on a basic and diluted basis.
Conference Call and Webcast
AbCellera will host a conference call and live webcast to discuss these results today at 2:00 p.m. Pacific Time (5:00 p.m. Eastern Time).
The live webcast of the earnings conference call can be accessed on the Events and Presentations section of AbCellera’s Investor Relations website. A replay of the webcast will be available through the same link following the conference call.
About AbCellera Biologics Inc.
AbCellera (Nasdaq: ABCL) discovers and develops antibody medicines for indications across therapeutic areas including cancer, metabolic and endocrine conditions, and autoimmune disorders. AbCellera integrates technology, data science, infrastructure, and interdisciplinary teams to solve the most challenging antibody discovery problems. AbCellera is focused on advancing an internal pipeline of first-in-class and best-in-class programs and collaborating on innovative drug development programs with partners. For more information, please visit www.abcellera.com.
Definition of Key Business Metrics
We regularly review the following key business metrics to evaluate our business, measure our performance, identify trends affecting our business, formulate financial projections, and make strategic decisions. We believe that the following metrics are important to understand our current business. These metrics may change or may be substituted for additional or different metrics as our business develops.
Partner-initiated program starts with downstreams represent the number of unique partner-initiated programs where we stand to participate financially in downstream success for which we have commenced the discovery effort. The discovery effort commences on the later of (i) the day on which we receive sufficient reagents to start discovery of antibodies against a target and (ii) the day on which the kick-off meeting for the program is held. We view this metric as an indication of the selection and initiation of projects by our partners and the resulting potential for near-term payments. Cumulatively, partner-initiated program starts with downstream participation indicate our total opportunities to earn downstream revenue from milestone fees and royalties (or royalty equivalents) in the mid- to long-term.
Molecules in the clinic represent the count of unique molecules for which an Investigational New Drug, or IND, New Animal Drug, or equivalent under other regulatory regimes, application has reached "open" status or has otherwise been approved based on an antibody that was discovered either by us or by a partner using licensed AbCellera technology. Where the date of such application approval is not known to us, the date of the first public announcement of a clinical trial will be used for the purpose of this metric. We view this metric as an indication of our near- and mid-term potential revenue from milestone fees and potential royalty payments in the long term.
AbCellera Forward-Looking Statements
This press release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. The forward-looking statements are based on management’s current beliefs and assumptions and on information currently available to management. All statements contained in this release other than statements of historical fact are forward-looking statements, including statements regarding our ability to develop, commercialize and achieve market acceptance of our current and planned products and services, our research and development efforts, and other matters regarding our business strategies, use of capital, results of operations and financial position, and plans and objectives for future operations.
In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, levels of activity, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors are described under “Risk Factors,” “Management's Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in the documents we file with the Securities and Exchange Commission from time to time. We caution you that forward-looking statements are based on a combination of facts and factors currently known by us and our projections of the future, about which we cannot be certain. As a result, the forward-looking statements may not prove to be accurate. The forward-looking statements in this press release represent our views as of the date hereof. We undertake no obligation to update any forward-looking statements for any reason, except as required by law.
Source: AbCellera Biologics Inc.
Inquiries
Media: Tiffany Chiu; media@abcellera.com, +1(236)521-6774 Partnering: Murray McCutcheon, Ph.D.; partnering@abcellera.com, +1(604)559-9005 Investor Relations: Peter Ahn; ir@abcellera.com, +1(778)729-9116 (1) Exclusive of depreciation, amortization, and impairment
AbCellera Biologics Inc.
Consolidated Statements of Income (Loss) and Comprehensive Income (Loss)
(All figures in U.S. dollars. Amounts are expressed in thousands except share and per share data.)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
|
|
2022 |
|
2023 |
|
2024 |
| Revenue: |
|
|
|
|
|
|
|
| Research fees |
|
|
$ |
40,802 |
|
|
$ |
35,556 |
|
|
$ |
26,284 |
|
| Licensing revenue |
|
|
696 |
|
|
969 |
|
|
1,049 |
|
| Milestone payments |
|
|
900 |
|
|
1,500 |
|
|
1,500 |
|
| Royalty revenue |
|
|
443,026 |
|
|
— |
|
|
— |
|
| Total revenue |
|
|
485,424 |
|
|
38,025 |
|
|
28,833 |
|
| Operating expenses: |
|
|
|
|
|
|
|
| Royalty fees |
|
|
66,436 |
|
|
— |
|
|
— |
|
Research and development(1) |
|
|
107,879 |
|
|
175,658 |
|
|
167,259 |
|
Sales and marketing(1) |
|
|
11,270 |
|
|
14,180 |
|
|
12,779 |
|
General and administrative(1) |
|
|
55,485 |
|
|
60,999 |
|
|
72,711 |
|
| Depreciation, amortization, and impairment |
|
|
27,843 |
|
|
24,395 |
|
|
90,850 |
|
| Total operating expenses |
|
|
268,913 |
|
|
275,232 |
|
|
343,599 |
|
| Income (loss) from operations |
|
|
216,511 |
|
|
(237,207) |
|
|
(314,766) |
|
| Other (income) expense |
|
|
|
|
|
|
|
| Interest income |
|
|
(16,079) |
|
|
(42,247) |
|
|
(38,473) |
|
| Grants and incentives |
|
|
(10,554) |
|
|
(14,155) |
|
|
(13,620) |
|
Other |
|
|
4,045 |
|
|
(6,776) |
|
|
(62,278) |
|
| Total other income |
|
|
(22,588) |
|
|
(63,178) |
|
|
(114,371) |
|
| Net earnings (loss) before income tax |
|
|
239,099 |
|
|
(174,029) |
|
|
(200,395) |
|
| Income tax (recovery) expense |
|
|
80,580 |
|
|
(27,631) |
|
|
(37,538) |
|
| Net earnings (loss) |
|
|
$ |
158,519 |
|
|
$ |
(146,398) |
|
|
$ |
(162,857) |
|
| Foreign currency translation adjustment |
|
|
(1,671) |
|
|
(329) |
|
|
(2,658) |
|
| Comprehensive income (loss) |
|
|
$ |
156,848 |
|
|
$ |
(146,727) |
|
|
$ |
(165,515) |
|
|
|
|
|
|
|
|
|
| Net earnings (loss) per share |
|
|
|
|
|
|
|
| Basic |
|
|
$ |
0.56 |
|
|
$ |
(0.51) |
|
|
$ |
(0.55) |
|
| Diluted |
|
|
$ |
0.50 |
|
|
$ |
(0.51) |
|
|
$ |
(0.55) |
|
| Weighted-average common shares outstanding |
|
|
|
|
|
|
|
| Basic |
|
|
285,056,606 |
|
289,166,486 |
|
294,327,532 |
| Diluted |
|
|
314,827,255 |
|
289,166,486 |
|
294,327,532 |
AbCellera Biologics Inc.
Consolidated Balance Sheets
(All figures in U.S. dollars. Amounts are expressed in thousands except share data.)
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
December 31, 2024 |
| Assets |
|
|
|
| Current assets: |
|
|
|
| Cash and cash equivalents |
$ |
133,320 |
|
|
$ |
156,325 |
|
| Marketable securities |
627,265 |
|
|
469,289 |
|
| Total cash, cash equivalents, and marketable securities |
760,585 |
|
|
625,614 |
|
| Accounts and accrued receivable |
30,590 |
|
|
33,616 |
|
| Restricted cash |
25,000 |
|
|
25,000 |
|
| Other current assets |
55,810 |
|
|
67,140 |
|
| Total current assets |
871,985 |
|
|
751,370 |
|
| Long-term assets: |
|
|
|
| Property and equipment, net |
287,696 |
|
|
340,429 |
|
| Intangible assets, net |
120,425 |
|
|
42,113 |
|
| Goodwill |
47,806 |
|
|
47,806 |
|
| Investments in equity accounted investees |
65,938 |
|
|
82,297 |
|
| Other long-term assets |
94,244 |
|
|
96,538 |
|
| Total long-term assets |
616,109 |
|
|
609,183 |
|
| Total assets |
$ |
1,488,094 |
|
|
$ |
1,360,553 |
|
| Liabilities and shareholders' equity |
|
|
|
| Current liabilities: |
|
|
|
| Accounts payable and other current liabilities |
$ |
49,580 |
|
|
$ |
55,004 |
|
| Contingent consideration payable |
50,475 |
|
|
8,087 |
|
|
|
|
|
|
|
|
|
| Deferred revenue |
18,958 |
|
|
13,521 |
|
| Total current liabilities |
119,013 |
|
|
76,612 |
|
| Long-term liabilities: |
|
|
|
| Operating lease liability |
71,222 |
|
|
60,743 |
|
| Deferred revenue |
8,195 |
|
|
5,700 |
|
| Deferred government contributions |
95,915 |
|
|
149,893 |
|
| Contingent consideration payable |
4,913 |
|
|
— |
|
| Deferred tax liability |
30,612 |
|
|
10,052 |
|
| Other long-term liabilities |
5,906 |
|
|
1,469 |
|
| Total long-term liabilities |
216,763 |
|
|
227,857 |
|
| Total liabilities |
335,776 |
|
|
304,469 |
|
| Commitments and contingencies |
|
|
|
| Shareholders' equity: |
|
|
|
| Common shares: no par value, unlimited authorized shares at December 31, 2023 and December 31, 2024: 290,824,970 and 295,757,002 shares issued and outstanding at December 31, 2023 and December 31, 2024, respectively |
753,199 |
|
|
777,171 |
|
| Additional paid-in capital |
121,052 |
|
|
166,361 |
|
| Accumulated other comprehensive loss |
(1,720) |
|
|
(4,378) |
|
| Accumulated earnings |
279,787 |
|
|
116,930 |
|
| Total shareholders' equity |
1,152,318 |
|
|
1,056,084 |
|
| Total liabilities and shareholders' equity |
$ |
1,488,094 |
|
|
$ |
1,360,553 |
|
AbCellera Biologics Inc.
Consolidated Statement of Cash Flows
(Expressed in thousands of U.S. dollars.)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2022 |
|
December 31, 2023 |
|
December 31, 2024 |
| Cash flows from operating activities: |
|
|
|
|
|
|
| Net earnings (loss) |
|
$ |
158,519 |
|
|
$ |
(146,398) |
|
|
$ |
(162,857) |
|
| Cash flows from operating activities: |
|
|
|
|
|
|
| Depreciation of property and equipment |
|
8,953 |
|
|
12,758 |
|
|
12,537 |
|
| Amortization and impairment of intangible assets |
|
18,890 |
|
|
11,637 |
|
|
78,312 |
|
| Amortization of operating lease right-of-use assets |
|
5,259 |
|
|
6,499 |
|
|
6,149 |
|
| Stock-based compensation |
|
49,481 |
|
|
64,183 |
|
|
67,581 |
|
| Fair value (gain) loss on contingent consideration and investments |
|
3,091 |
|
|
(8,018) |
|
|
(64,727) |
|
| Other |
|
3,342 |
|
|
2,237 |
|
|
(19,708) |
|
| Changes in operating assets and liabilities: |
|
|
|
|
|
|
| Research fee and grant receivable |
|
(22,715) |
|
|
(45,933) |
|
|
(75,119) |
|
| Accrued royalties receivable |
|
129,171 |
|
|
9,273 |
|
|
— |
|
| Income taxes (payable) receivable |
|
(88,609) |
|
|
30,464 |
|
|
6,651 |
|
| Accounts payable and accrued liabilities |
|
(2,094) |
|
|
(15,104) |
|
|
10,635 |
|
| Deferred revenue |
|
6,183 |
|
|
(13,976) |
|
|
(7,931) |
|
| Deferred grant income |
|
9,264 |
|
|
39,521 |
|
|
33,967 |
|
| Other assets |
|
(1,375) |
|
|
8,980 |
|
|
5,954 |
|
| Net cash provided by (used in) operating activities |
|
277,360 |
|
|
(43,877) |
|
|
(108,556) |
|
| Cash flows from investing activities: |
|
|
|
|
|
|
| Purchases of property and equipment |
|
(70,660) |
|
|
(76,947) |
|
|
(78,396) |
|
| Purchase of intangible assets |
|
(2,000) |
|
|
(560) |
|
|
— |
|
| Purchase of marketable securities |
|
(763,982) |
|
|
(1,021,510) |
|
|
(765,086) |
|
| Proceeds from marketable securities |
|
510,631 |
|
|
910,937 |
|
|
937,882 |
|
| Receipt of grant funding |
|
16,434 |
|
|
25,311 |
|
|
35,708 |
|
| Investment in and loans to equity accounted investees |
|
(25,679) |
|
|
(13,690) |
|
|
(19,626) |
|
| Long-term investments and other assets |
|
(17,369) |
|
|
(44,649) |
|
|
10,927 |
|
| Net cash provided by (used in) investing activities |
|
(352,625) |
|
|
(221,108) |
|
|
121,409 |
|
| Cash flows from financing activities: |
|
|
|
|
|
|
| Payment of liability for in-licensing agreement and other |
|
(4,383) |
|
|
(1,234) |
|
|
(729) |
|
| Proceeds from long-term liabilities and exercise of stock options |
|
2,755 |
|
|
11,590 |
|
|
13,498 |
|
| Net cash provided by (used in) financing activities |
|
(1,628) |
|
|
10,356 |
|
|
12,769 |
|
| Effect of exchange rate changes on cash and cash equivalents |
|
(9,599) |
|
|
589 |
|
|
(2,617) |
|
| Increase (decrease) in cash and cash equivalents |
|
(86,492) |
|
|
(254,040) |
|
|
23,005 |
|
| Cash and cash equivalents and restricted cash, beginning of period |
|
501,142 |
|
|
414,650 |
|
|
160,610 |
|
| Cash and cash equivalents and restricted cash, end of period |
|
$ |
414,650 |
|
|
$ |
160,610 |
|
|
$ |
183,615 |
|
| Restricted cash included in other assets |
|
3,115 |
|
|
2,290 |
|
|
2,290 |
|
| Total cash, cash equivalents, and restricted cash shown on the balance sheet |
|
$ |
411,535 |
|
|
$ |
158,320 |
|
|
$ |
181,325 |
|
| Supplemental disclosure of non-cash investing and financing activities |
|
|
|
|
|
|
| Property and equipment in accounts payable |
|
5,868 |
|
|
13,625 |
|
|
12,767 |
|
| Right-of-use assets obtained in exchange for operating lease obligation |
|
50,694 |
|
|
1,199 |
|
|
1,898 |
|