FALSE0000353278FY2024truexbrli:sharesiso4217:DKKiso4217:DKKxbrli:sharesxbrli:purenvo:wholesalernvo:segmentnvo:commercial_unitnvo:employeenvo:sitenvo:voteiso4217:DKKiso4217:USDiso4217:DKKiso4217:CNYiso4217:DKKiso4217:JPYiso4217:DKKiso4217:CADiso4217:DKKiso4217:GBPiso4217:EURnvo:trancheutr:Miso4217:USD00003532782024-01-012024-12-310000353278dei:BusinessContactMember2024-01-012024-12-310000353278nvo:BShareCapitalMember2024-01-012024-12-310000353278nvo:AmericanDepositaryReceiptsMember2024-01-012024-12-310000353278nvo:AShareCapitalMember2024-12-310000353278nvo:BShareCapitalMember2024-12-3100003532782023-01-012023-12-3100003532782022-01-012022-12-3100003532782023-12-3100003532782022-12-3100003532782021-12-3100003532782024-12-310000353278ifrs-full:IssuedCapitalMember2023-12-310000353278ifrs-full:TreasurySharesMember2023-12-310000353278ifrs-full:RetainedEarningsMember2023-12-310000353278ifrs-full:OtherReservesMember2023-12-310000353278ifrs-full:IssuedCapitalMember2022-12-310000353278ifrs-full:TreasurySharesMember2022-12-310000353278ifrs-full:RetainedEarningsMember2022-12-310000353278ifrs-full:OtherReservesMember2022-12-310000353278ifrs-full:IssuedCapitalMember2021-12-310000353278ifrs-full:TreasurySharesMember2021-12-310000353278ifrs-full:RetainedEarningsMember2021-12-310000353278ifrs-full:OtherReservesMember2021-12-310000353278ifrs-full:RetainedEarningsMember2024-01-012024-12-310000353278ifrs-full:RetainedEarningsMember2023-01-012023-12-310000353278ifrs-full:RetainedEarningsMember2022-01-012022-12-310000353278ifrs-full:OtherReservesMember2024-01-012024-12-310000353278ifrs-full:OtherReservesMember2023-01-012023-12-310000353278ifrs-full:OtherReservesMember2022-01-012022-12-310000353278ifrs-full:TreasurySharesMember2024-01-012024-12-310000353278ifrs-full:TreasurySharesMember2023-01-012023-12-310000353278ifrs-full:TreasurySharesMember2022-01-012022-12-310000353278ifrs-full:IssuedCapitalMember2024-01-012024-12-310000353278ifrs-full:IssuedCapitalMember2023-01-012023-12-310000353278ifrs-full:IssuedCapitalMember2022-01-012022-12-310000353278ifrs-full:IssuedCapitalMember2024-12-310000353278ifrs-full:TreasurySharesMember2024-12-310000353278ifrs-full:RetainedEarningsMember2024-12-310000353278ifrs-full:OtherReservesMember2024-12-310000353278country:USnvo:USManagedCareAndMedicareMember2024-01-012024-12-310000353278country:USnvo:USManagedCareAndMedicareMember2023-01-012023-12-310000353278country:USnvo:USManagedCareAndMedicareMember2022-01-012022-12-310000353278country:USnvo:USWholesalerMember2024-01-012024-12-310000353278country:USnvo:USWholesalerMember2023-01-012023-12-310000353278country:USnvo:USWholesalerMember2022-01-012022-12-310000353278country:USnvo:USMedicaidMember2024-01-012024-12-310000353278country:USnvo:USMedicaidMember2023-01-012023-12-310000353278country:USnvo:USMedicaidMember2022-01-012022-12-310000353278country:USnvo:OtherCustomersMember2024-01-012024-12-310000353278country:USnvo:OtherCustomersMember2023-01-012023-12-310000353278country:USnvo:OtherCustomersMember2022-01-012022-12-310000353278nvo:NonNorthAmericaMembernvo:OtherCustomersMember2024-01-012024-12-310000353278nvo:NonNorthAmericaMembernvo:OtherCustomersMember2023-01-012023-12-310000353278nvo:NonNorthAmericaMembernvo:OtherCustomersMember2022-01-012022-12-310000353278nvo:RebatesProvisionMember2023-12-310000353278nvo:RebatesProvisionMember2022-12-310000353278nvo:RebatesProvisionMember2021-12-310000353278nvo:RebatesProvisionMember2024-01-012024-12-310000353278nvo:RebatesProvisionMember2023-01-012023-12-310000353278nvo:RebatesProvisionMember2022-01-012022-12-310000353278nvo:RebatesProvisionMember2024-12-310000353278srt:NorthAmericaMember2024-01-012024-12-310000353278srt:NorthAmericaMember2023-01-012023-12-310000353278srt:NorthAmericaMember2022-01-012022-12-310000353278ifrs-full:NongovernmentCustomersMembercountry:USnvo:USWholesalerMember2024-01-012024-12-310000353278ifrs-full:GovernmentCustomersMembercountry:USnvo:USMedicaidMember2024-01-012024-12-310000353278nvo:WholesalerOneMember2024-01-012024-12-310000353278nvo:WholesalerTwoMember2024-01-012024-12-310000353278nvo:WholesalerThreeMember2024-01-012024-12-310000353278nvo:WholesalerOneMember2023-01-012023-12-310000353278nvo:WholesalerTwoMember2023-01-012023-12-310000353278nvo:WholesalerThreeMember2023-01-012023-12-310000353278nvo:WholesalerOneMember2022-01-012022-12-310000353278nvo:WholesalerTwoMember2022-01-012022-12-310000353278nvo:WholesalerThreeMember2022-01-012022-12-310000353278ifrs-full:OperatingSegmentsMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278ifrs-full:OperatingSegmentsMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278ifrs-full:OperatingSegmentsMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278ifrs-full:OperatingSegmentsMembernvo:RareDiseaseMember2024-01-012024-12-310000353278ifrs-full:OperatingSegmentsMembernvo:RareDiseaseMember2023-01-012023-12-310000353278ifrs-full:OperatingSegmentsMembernvo:RareDiseaseMember2022-01-012022-12-310000353278country:US2024-01-012024-12-310000353278ifrs-full:ForeignCountriesMember2024-01-012024-12-310000353278ifrs-full:ForeignCountriesMember2023-01-012023-12-310000353278ifrs-full:CountryOfDomicileMember2024-12-310000353278ifrs-full:CountryOfDomicileMember2023-12-310000353278country:US2024-12-310000353278country:US2023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:OzempicMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:OzempicMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:OzempicMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:OzempicMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:OzempicMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:OzempicMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:OzempicMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:OzempicMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:OzempicMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:OzempicMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:OzempicMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:OzempicMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:OzempicMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:OzempicMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:OzempicMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:OzempicMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:OzempicMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:OzempicMember2022-01-012022-12-310000353278nvo:OzempicMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:OzempicMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:OzempicMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:RybelsusMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:RybelsusMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:RybelsusMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:RybelsusMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:RybelsusMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:RybelsusMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:RybelsusMember2022-01-012022-12-310000353278nvo:RybelsusMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:RybelsusMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:RybelsusMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:VictozaMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:VictozaMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:VictozaMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:VictozaMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:VictozaMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:VictozaMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:VictozaMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:VictozaMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:VictozaMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:VictozaMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:VictozaMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:VictozaMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:VictozaMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:VictozaMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:VictozaMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:VictozaMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:VictozaMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:VictozaMember2022-01-012022-12-310000353278nvo:VictozaMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:VictozaMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:VictozaMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalGLP1Member2022-01-012022-12-310000353278nvo:TotalGLP1Membernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:TotalGLP1Membernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:TotalGLP1Membernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:LongActingInsulinMember2022-01-012022-12-310000353278nvo:LongActingInsulinMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:LongActingInsulinMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:LongActingInsulinMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:AwigliMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:AwigliMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:AwigliMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:AwigliMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:AwigliMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:AwigliMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:AwigliMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:AwigliMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:AwigliMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:AwigliMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:AwigliMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:AwigliMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:AwigliMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:AwigliMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:AwigliMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:AwigliMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:AwigliMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:AwigliMember2022-01-012022-12-310000353278nvo:AwigliMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:AwigliMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:AwigliMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TresibaMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TresibaMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TresibaMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TresibaMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TresibaMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TresibaMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TresibaMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TresibaMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TresibaMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TresibaMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TresibaMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TresibaMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TresibaMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TresibaMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TresibaMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TresibaMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TresibaMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TresibaMember2022-01-012022-12-310000353278nvo:TresibaMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:TresibaMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:TresibaMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:XultophyMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:XultophyMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:XultophyMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:XultophyMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:XultophyMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:XultophyMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:XultophyMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:XultophyMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:XultophyMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:XultophyMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:XultophyMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:XultophyMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:XultophyMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:XultophyMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:XultophyMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:XultophyMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:XultophyMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:XultophyMember2022-01-012022-12-310000353278nvo:XultophyMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:XultophyMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:XultophyMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:LevemirMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:LevemirMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:LevemirMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:LevemirMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:LevemirMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:LevemirMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:LevemirMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:LevemirMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:LevemirMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:LevemirMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:LevemirMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:LevemirMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:LevemirMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:LevemirMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:LevemirMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:LevemirMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:LevemirMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:LevemirMember2022-01-012022-12-310000353278nvo:LevemirMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:LevemirMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:LevemirMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:PremixInsulinMember2022-01-012022-12-310000353278nvo:PremixInsulinMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:PremixInsulinMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:PremixInsulinMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:RyzodegMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:RyzodegMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:RyzodegMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:RyzodegMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:RyzodegMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:RyzodegMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:RyzodegMember2022-01-012022-12-310000353278nvo:RyzodegMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:RyzodegMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:RyzodegMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:NovoMixAndNovoLogMixMember2022-01-012022-12-310000353278nvo:NovoMixAndNovoLogMixMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:NovoMixAndNovoLogMixMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:NovoMixAndNovoLogMixMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:FastActingInsulinMember2022-01-012022-12-310000353278nvo:FastActingInsulinMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:FastActingInsulinMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:FastActingInsulinMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:FiaspMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:FiaspMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:FiaspMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:FiaspMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:FiaspMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:FiaspMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:FiaspMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:FiaspMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:FiaspMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:FiaspMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:FiaspMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:FiaspMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:FiaspMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:FiaspMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:FiaspMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:FiaspMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:FiaspMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:FiaspMember2022-01-012022-12-310000353278nvo:FiaspMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:FiaspMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:FiaspMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:NovoRapidAndNovoLogMember2022-01-012022-12-310000353278nvo:NovoRapidAndNovoLogMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:NovoRapidAndNovoLogMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:NovoRapidAndNovoLogMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:HumanInsulinMember2022-01-012022-12-310000353278nvo:HumanInsulinMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:HumanInsulinMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:HumanInsulinMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalInsulinMember2022-01-012022-12-310000353278nvo:TotalInsulinMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:TotalInsulinMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:TotalInsulinMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:OtherDiabetesCareMember2022-01-012022-12-310000353278nvo:OtherDiabetesCareMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:OtherDiabetesCareMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:OtherDiabetesCareMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalDiabetesCareMember2022-01-012022-12-310000353278nvo:TotalDiabetesCareMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:TotalDiabetesCareMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:TotalDiabetesCareMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:WegovyMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:WegovyMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:WegovyMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:WegovyMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:WegovyMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:WegovyMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:WegovyMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:WegovyMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:WegovyMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:WegovyMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:WegovyMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:WegovyMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:WegovyMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:WegovyMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:WegovyMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:WegovyMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:WegovyMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:WegovyMember2022-01-012022-12-310000353278nvo:WegovyMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:WegovyMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:WegovyMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:SaxendaMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:SaxendaMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:SaxendaMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:SaxendaMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:SaxendaMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:SaxendaMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:SaxendaMember2022-01-012022-12-310000353278nvo:SaxendaMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:SaxendaMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:SaxendaMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMembernvo:TotalObesityCareMember2022-01-012022-12-310000353278nvo:TotalObesityCareMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:TotalObesityCareMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:TotalObesityCareMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278country:CNnvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278country:CNnvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278country:CNnvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278country:USnvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278country:USnvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278country:USnvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:DiabetesAndObesityCareMember2024-01-012024-12-310000353278nvo:DiabetesAndObesityCareMember2023-01-012023-12-310000353278nvo:DiabetesAndObesityCareMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:RareBloodDisordersMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:RareBloodDisordersMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:RareBloodDisordersMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:RareBloodDisordersMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:RareBloodDisordersMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:RareBloodDisordersMember2022-01-012022-12-310000353278country:CNnvo:RareDiseaseMembernvo:RareBloodDisordersMember2024-01-012024-12-310000353278country:CNnvo:RareDiseaseMembernvo:RareBloodDisordersMember2023-01-012023-12-310000353278country:CNnvo:RareDiseaseMembernvo:RareBloodDisordersMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:RareBloodDisordersMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:RareBloodDisordersMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:RareBloodDisordersMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:RareBloodDisordersMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:RareBloodDisordersMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:RareBloodDisordersMember2022-01-012022-12-310000353278country:USnvo:RareDiseaseMembernvo:RareBloodDisordersMember2024-01-012024-12-310000353278country:USnvo:RareDiseaseMembernvo:RareBloodDisordersMember2023-01-012023-12-310000353278country:USnvo:RareDiseaseMembernvo:RareBloodDisordersMember2022-01-012022-12-310000353278nvo:RareBloodDisordersMembernvo:RareDiseaseMember2024-01-012024-12-310000353278nvo:RareBloodDisordersMembernvo:RareDiseaseMember2023-01-012023-12-310000353278nvo:RareBloodDisordersMembernvo:RareDiseaseMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:HaemophiliaAMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:HaemophiliaAMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:HaemophiliaAMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:HaemophiliaAMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:HaemophiliaAMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:HaemophiliaAMember2022-01-012022-12-310000353278country:CNnvo:RareDiseaseMembernvo:HaemophiliaAMember2024-01-012024-12-310000353278country:CNnvo:RareDiseaseMembernvo:HaemophiliaAMember2023-01-012023-12-310000353278country:CNnvo:RareDiseaseMembernvo:HaemophiliaAMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:HaemophiliaAMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:HaemophiliaAMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:HaemophiliaAMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:HaemophiliaAMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:HaemophiliaAMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:HaemophiliaAMember2022-01-012022-12-310000353278country:USnvo:RareDiseaseMembernvo:HaemophiliaAMember2024-01-012024-12-310000353278country:USnvo:RareDiseaseMembernvo:HaemophiliaAMember2023-01-012023-12-310000353278country:USnvo:RareDiseaseMembernvo:HaemophiliaAMember2022-01-012022-12-310000353278nvo:HaemophiliaAMembernvo:RareDiseaseMember2024-01-012024-12-310000353278nvo:HaemophiliaAMembernvo:RareDiseaseMember2023-01-012023-12-310000353278nvo:HaemophiliaAMembernvo:RareDiseaseMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:HaemophiliaBMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:HaemophiliaBMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:HaemophiliaBMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:HaemophiliaBMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:HaemophiliaBMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:HaemophiliaBMember2022-01-012022-12-310000353278country:CNnvo:RareDiseaseMembernvo:HaemophiliaBMember2024-01-012024-12-310000353278country:CNnvo:RareDiseaseMembernvo:HaemophiliaBMember2023-01-012023-12-310000353278country:CNnvo:RareDiseaseMembernvo:HaemophiliaBMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:HaemophiliaBMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:HaemophiliaBMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:HaemophiliaBMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:HaemophiliaBMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:HaemophiliaBMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:HaemophiliaBMember2022-01-012022-12-310000353278country:USnvo:RareDiseaseMembernvo:HaemophiliaBMember2024-01-012024-12-310000353278country:USnvo:RareDiseaseMembernvo:HaemophiliaBMember2023-01-012023-12-310000353278country:USnvo:RareDiseaseMembernvo:HaemophiliaBMember2022-01-012022-12-310000353278nvo:HaemophiliaBMembernvo:RareDiseaseMember2024-01-012024-12-310000353278nvo:HaemophiliaBMembernvo:RareDiseaseMember2023-01-012023-12-310000353278nvo:HaemophiliaBMembernvo:RareDiseaseMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:NovoSevenMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:NovoSevenMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:NovoSevenMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:NovoSevenMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:NovoSevenMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:NovoSevenMember2022-01-012022-12-310000353278country:CNnvo:RareDiseaseMembernvo:NovoSevenMember2024-01-012024-12-310000353278country:CNnvo:RareDiseaseMembernvo:NovoSevenMember2023-01-012023-12-310000353278country:CNnvo:RareDiseaseMembernvo:NovoSevenMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:NovoSevenMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:NovoSevenMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:NovoSevenMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:NovoSevenMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:NovoSevenMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:NovoSevenMember2022-01-012022-12-310000353278country:USnvo:RareDiseaseMembernvo:NovoSevenMember2024-01-012024-12-310000353278country:USnvo:RareDiseaseMembernvo:NovoSevenMember2023-01-012023-12-310000353278country:USnvo:RareDiseaseMembernvo:NovoSevenMember2022-01-012022-12-310000353278nvo:NovoSevenMembernvo:RareDiseaseMember2024-01-012024-12-310000353278nvo:NovoSevenMembernvo:RareDiseaseMember2023-01-012023-12-310000353278nvo:NovoSevenMembernvo:RareDiseaseMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2022-01-012022-12-310000353278country:CNnvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2024-01-012024-12-310000353278country:CNnvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2023-01-012023-12-310000353278country:CNnvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2022-01-012022-12-310000353278country:USnvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2024-01-012024-12-310000353278country:USnvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2023-01-012023-12-310000353278country:USnvo:RareDiseaseMembernvo:RareEndocrineDisordersMember2022-01-012022-12-310000353278nvo:RareEndocrineDisordersMembernvo:RareDiseaseMember2024-01-012024-12-310000353278nvo:RareEndocrineDisordersMembernvo:RareDiseaseMember2023-01-012023-12-310000353278nvo:RareEndocrineDisordersMembernvo:RareDiseaseMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2022-01-012022-12-310000353278country:CNnvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2024-01-012024-12-310000353278country:CNnvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2023-01-012023-12-310000353278country:CNnvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2022-01-012022-12-310000353278country:USnvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2024-01-012024-12-310000353278country:USnvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2023-01-012023-12-310000353278country:USnvo:RareDiseaseMembernvo:OtherBiopharmProductsMember2022-01-012022-12-310000353278nvo:OtherBiopharmProductsMembernvo:RareDiseaseMember2024-01-012024-12-310000353278nvo:OtherBiopharmProductsMembernvo:RareDiseaseMember2023-01-012023-12-310000353278nvo:OtherBiopharmProductsMembernvo:RareDiseaseMember2022-01-012022-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMember2024-01-012024-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMember2023-01-012023-12-310000353278nvo:InternationalOperationsMembernvo:RareDiseaseMember2022-01-012022-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMember2024-01-012024-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMember2023-01-012023-12-310000353278nvo:EMEA1Membernvo:RareDiseaseMember2022-01-012022-12-310000353278country:CNnvo:RareDiseaseMember2024-01-012024-12-310000353278country:CNnvo:RareDiseaseMember2023-01-012023-12-310000353278country:CNnvo:RareDiseaseMember2022-01-012022-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMember2024-01-012024-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMember2023-01-012023-12-310000353278nvo:RestOfWorldMembernvo:RareDiseaseMember2022-01-012022-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMember2024-01-012024-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMember2023-01-012023-12-310000353278srt:NorthAmericaMembernvo:RareDiseaseMember2022-01-012022-12-310000353278country:USnvo:RareDiseaseMember2024-01-012024-12-310000353278country:USnvo:RareDiseaseMember2023-01-012023-12-310000353278country:USnvo:RareDiseaseMember2022-01-012022-12-310000353278nvo:RareDiseaseMember2024-01-012024-12-310000353278nvo:RareDiseaseMember2023-01-012023-12-310000353278nvo:RareDiseaseMember2022-01-012022-12-310000353278nvo:InternationalOperationsMember2024-01-012024-12-310000353278nvo:InternationalOperationsMember2023-01-012023-12-310000353278nvo:InternationalOperationsMember2022-01-012022-12-310000353278nvo:EMEA1Member2024-01-012024-12-310000353278nvo:EMEA1Member2023-01-012023-12-310000353278nvo:EMEA1Member2022-01-012022-12-310000353278country:CN2024-01-012024-12-310000353278country:CN2023-01-012023-12-310000353278country:CN2022-01-012022-12-310000353278nvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:RestOfWorldMember2023-01-012023-12-310000353278nvo:RestOfWorldMember2022-01-012022-12-310000353278country:US2023-01-012023-12-310000353278country:US2022-01-012022-12-310000353278nvo:ResearchAndDevelopmentExpense1Member2024-01-012024-12-310000353278nvo:ResearchAndDevelopmentExpense1Member2023-01-012023-12-310000353278nvo:ResearchAndDevelopmentExpense1Member2022-01-012022-12-310000353278ifrs-full:CostOfSalesMember2024-01-012024-12-310000353278ifrs-full:CostOfSalesMember2023-01-012023-12-310000353278ifrs-full:CostOfSalesMember2022-01-012022-12-310000353278nvo:SalesAndDistributionCostsMember2024-01-012024-12-310000353278nvo:SalesAndDistributionCostsMember2023-01-012023-12-310000353278nvo:SalesAndDistributionCostsMember2022-01-012022-12-310000353278nvo:AdministrativeCostsMember2024-01-012024-12-310000353278nvo:AdministrativeCostsMember2023-01-012023-12-310000353278nvo:AdministrativeCostsMember2022-01-012022-12-310000353278nvo:OtherOperatingIncomeAndExpensesMember2024-01-012024-12-310000353278nvo:OtherOperatingIncomeAndExpensesMember2023-01-012023-12-310000353278nvo:OtherOperatingIncomeAndExpensesMember2022-01-012022-12-310000353278nvo:BeforeOffsetAmountMembernvo:PropertyPlantAndEquipmentRelatedTemporaryDifferencesMember2023-12-310000353278nvo:BeforeOffsetAmountMembernvo:IntangibleAssetRelatedTemporaryDifferencesMember2023-12-310000353278nvo:BeforeOffsetAmountMembernvo:InventoryRelatedTemporaryDifferencesMember2023-12-310000353278nvo:BeforeOffsetAmountMembernvo:ProvisionsAndOtherLiabilitiesRelatedTemporaryDifferencesMember2023-12-310000353278nvo:BeforeOffsetAmountMembernvo:OtherTemporaryDifferencesAndUnusedTaxLossesMember2023-12-310000353278nvo:OffsetAmountMember2023-12-310000353278nvo:BeforeOffsetAmountMembernvo:PropertyPlantAndEquipmentRelatedTemporaryDifferencesMember2024-01-012024-12-310000353278nvo:BeforeOffsetAmountMembernvo:IntangibleAssetRelatedTemporaryDifferencesMember2024-01-012024-12-310000353278nvo:BeforeOffsetAmountMembernvo:InventoryRelatedTemporaryDifferencesMember2024-01-012024-12-310000353278nvo:BeforeOffsetAmountMembernvo:ProvisionsAndOtherLiabilitiesRelatedTemporaryDifferencesMember2024-01-012024-12-310000353278nvo:BeforeOffsetAmountMembernvo:OtherTemporaryDifferencesAndUnusedTaxLossesMember2024-01-012024-12-310000353278nvo:BeforeOffsetAmountMembernvo:PropertyPlantAndEquipmentRelatedTemporaryDifferencesMember2024-12-310000353278nvo:BeforeOffsetAmountMembernvo:IntangibleAssetRelatedTemporaryDifferencesMember2024-12-310000353278nvo:BeforeOffsetAmountMembernvo:InventoryRelatedTemporaryDifferencesMember2024-12-310000353278nvo:BeforeOffsetAmountMembernvo:ProvisionsAndOtherLiabilitiesRelatedTemporaryDifferencesMember2024-12-310000353278nvo:BeforeOffsetAmountMembernvo:OtherTemporaryDifferencesAndUnusedTaxLossesMember2024-12-310000353278nvo:OffsetAmountMember2024-12-310000353278nvo:BeforeOffsetAmountMembernvo:PropertyPlantAndEquipmentRelatedTemporaryDifferencesMember2022-12-310000353278nvo:BeforeOffsetAmountMembernvo:IntangibleAssetRelatedTemporaryDifferencesMember2022-12-310000353278nvo:BeforeOffsetAmountMembernvo:InventoryRelatedTemporaryDifferencesMember2022-12-310000353278nvo:BeforeOffsetAmountMembernvo:ProvisionsAndOtherLiabilitiesRelatedTemporaryDifferencesMember2022-12-310000353278nvo:BeforeOffsetAmountMembernvo:OtherTemporaryDifferencesAndUnusedTaxLossesMember2022-12-310000353278nvo:OffsetAmountMember2022-12-310000353278nvo:BeforeOffsetAmountMembernvo:PropertyPlantAndEquipmentRelatedTemporaryDifferencesMember2023-01-012023-12-310000353278nvo:BeforeOffsetAmountMembernvo:IntangibleAssetRelatedTemporaryDifferencesMember2023-01-012023-12-310000353278nvo:BeforeOffsetAmountMembernvo:InventoryRelatedTemporaryDifferencesMember2023-01-012023-12-310000353278nvo:BeforeOffsetAmountMembernvo:ProvisionsAndOtherLiabilitiesRelatedTemporaryDifferencesMember2023-01-012023-12-310000353278nvo:BeforeOffsetAmountMembernvo:OtherTemporaryDifferencesAndUnusedTaxLossesMember2023-01-012023-12-310000353278nvo:SellingExpenseAndDistributionCostsMember2024-01-012024-12-310000353278nvo:SellingExpenseAndDistributionCostsMember2023-01-012023-12-310000353278nvo:SellingExpenseAndDistributionCostsMember2022-01-012022-12-310000353278nvo:AdministrativeExpenseMember2024-01-012024-12-310000353278nvo:AdministrativeExpenseMember2023-01-012023-12-310000353278nvo:AdministrativeExpenseMember2022-01-012022-12-310000353278nvo:OtherOperatingIncomeExpense1Member2024-01-012024-12-310000353278nvo:OtherOperatingIncomeExpense1Member2023-01-012023-12-310000353278nvo:OtherOperatingIncomeExpense1Member2022-01-012022-12-310000353278ifrs-full:GrossCarryingAmountMember2023-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:IntellectualPropertyRightsMember2023-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:SoftwareAndOtherIntangiblesMember2023-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:BusinessCombinationsMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:IntellectualPropertyRightsMemberifrs-full:BusinessCombinationsMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:SoftwareAndOtherIntangiblesMemberifrs-full:BusinessCombinationsMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:IntellectualPropertyRightsMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:SoftwareAndOtherIntangiblesMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMember2024-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:IntellectualPropertyRightsMember2024-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:SoftwareAndOtherIntangiblesMember2024-12-310000353278ifrs-full:AccumulatedImpairmentMember2023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:IntellectualPropertyRightsMember2023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:SoftwareAndOtherIntangiblesMember2023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:IntellectualPropertyRightsMember2024-01-012024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:SoftwareAndOtherIntangiblesMember2024-01-012024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2024-01-012024-12-310000353278ifrs-full:AccumulatedImpairmentMember2024-01-012024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:IntellectualPropertyRightsMember2024-01-012024-12-310000353278ifrs-full:AccumulatedImpairmentMember2024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:IntellectualPropertyRightsMember2024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:SoftwareAndOtherIntangiblesMember2024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2024-12-310000353278nvo:IntellectualPropertyRightsMember2024-12-310000353278nvo:SoftwareAndOtherIntangiblesMember2024-12-310000353278ifrs-full:GrossCarryingAmountMember2022-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:IntellectualPropertyRightsMember2022-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:SoftwareAndOtherIntangiblesMember2022-12-310000353278ifrs-full:GrossCarryingAmountMember2023-01-012023-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:IntellectualPropertyRightsMember2023-01-012023-12-310000353278ifrs-full:GrossCarryingAmountMembernvo:SoftwareAndOtherIntangiblesMember2023-01-012023-12-310000353278ifrs-full:AccumulatedImpairmentMember2022-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:IntellectualPropertyRightsMember2022-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:SoftwareAndOtherIntangiblesMember2022-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2022-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:IntellectualPropertyRightsMember2023-01-012023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:SoftwareAndOtherIntangiblesMember2023-01-012023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2023-01-012023-12-310000353278ifrs-full:AccumulatedImpairmentMember2023-01-012023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembernvo:IntellectualPropertyRightsMember2023-01-012023-12-310000353278nvo:IntellectualPropertyRightsMember2023-12-310000353278nvo:SoftwareAndOtherIntangiblesMember2023-12-310000353278nvo:TechnicalKnowHowMember2024-12-310000353278nvo:TechnicalKnowHowMember2023-12-310000353278nvo:TechnicalKnowHowMember2024-01-012024-12-310000353278nvo:CatalentIncMember2024-12-180000353278nvo:IntellectualPropertyLicensesMember2024-12-310000353278nvo:IntellectualPropertyLicensesMember2023-12-310000353278nvo:IntellectualPropertyLicensesMember2024-01-012024-12-310000353278nvo:IntellectualPropertyLicensesMember2023-01-012023-12-310000353278nvo:RNAiTechnologyPlatformMember2024-12-310000353278nvo:RNAiTechnologyPlatformMember2023-12-310000353278nvo:RNAiTechnologyPlatformMember2024-01-012024-12-310000353278nvo:RNAiTechnologyPlatformMember2023-01-012023-12-310000353278nvo:IntellectualPropertyRightsMember2024-12-310000353278nvo:IntellectualPropertyRightsMember2023-12-310000353278nvo:OcedurenoneMembernvo:IntellectualPropertyRightsMember2024-01-012024-12-310000353278nvo:DiabetesAndObesityCareMember2024-12-310000353278nvo:DiabetesAndObesityCareMember2023-12-310000353278nvo:RareDiseaseMember2024-12-310000353278nvo:RareDiseaseMember2023-12-310000353278nvo:IntellectualPropertyRightsMemberifrs-full:TopOfRangeMember2024-01-012024-12-310000353278nvo:TechnicalKnowHowMemberifrs-full:TopOfRangeMember2024-01-012024-12-310000353278nvo:SoftwareAndOtherIntangiblesMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310000353278nvo:SoftwareAndOtherIntangiblesMemberifrs-full:TopOfRangeMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:LandAndBuildingsMember2023-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:MachineryMember2023-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:OtherPropertyPlantAndEquipmentMember2023-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:ConstructionInProgressMember2023-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:LandAndBuildingsMemberifrs-full:BusinessCombinationsMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:MachineryMemberifrs-full:BusinessCombinationsMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:OtherPropertyPlantAndEquipmentMemberifrs-full:BusinessCombinationsMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:ConstructionInProgressMemberifrs-full:BusinessCombinationsMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:LandAndBuildingsMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:MachineryMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:OtherPropertyPlantAndEquipmentMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:ConstructionInProgressMember2024-01-012024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:LandAndBuildingsMember2024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:MachineryMember2024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:OtherPropertyPlantAndEquipmentMember2024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:ConstructionInProgressMember2024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:LandAndBuildingsMember2023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:MachineryMember2023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:OtherPropertyPlantAndEquipmentMember2023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:ConstructionInProgressMember2023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:LandAndBuildingsMember2024-01-012024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:MachineryMember2024-01-012024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:OtherPropertyPlantAndEquipmentMember2024-01-012024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:ConstructionInProgressMember2024-01-012024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:LandAndBuildingsMember2024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:MachineryMember2024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:OtherPropertyPlantAndEquipmentMember2024-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:ConstructionInProgressMember2024-12-310000353278ifrs-full:LandAndBuildingsMember2024-12-310000353278ifrs-full:MachineryMember2024-12-310000353278ifrs-full:OtherPropertyPlantAndEquipmentMember2024-12-310000353278ifrs-full:ConstructionInProgressMember2024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:LandAndBuildingsMember2022-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:MachineryMember2022-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:OtherPropertyPlantAndEquipmentMember2022-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:ConstructionInProgressMember2022-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:LandAndBuildingsMember2023-01-012023-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:MachineryMember2023-01-012023-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:OtherPropertyPlantAndEquipmentMember2023-01-012023-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:ConstructionInProgressMember2023-01-012023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:LandAndBuildingsMember2022-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:MachineryMember2022-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:OtherPropertyPlantAndEquipmentMember2022-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:ConstructionInProgressMember2022-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:LandAndBuildingsMember2023-01-012023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:MachineryMember2023-01-012023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:OtherPropertyPlantAndEquipmentMember2023-01-012023-12-310000353278ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:ConstructionInProgressMember2023-01-012023-12-310000353278ifrs-full:LandAndBuildingsMember2023-12-310000353278ifrs-full:MachineryMember2023-12-310000353278ifrs-full:OtherPropertyPlantAndEquipmentMember2023-12-310000353278ifrs-full:ConstructionInProgressMember2023-12-310000353278ifrs-full:RightofuseAssetsMember2024-01-012024-12-310000353278ifrs-full:RightofuseAssetsMember2023-01-012023-12-310000353278ifrs-full:RightofuseAssetsMember2022-01-012022-12-310000353278ifrs-full:BuildingsMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310000353278ifrs-full:BuildingsMemberifrs-full:TopOfRangeMember2024-01-012024-12-310000353278ifrs-full:MachineryMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310000353278ifrs-full:MachineryMemberifrs-full:TopOfRangeMember2024-01-012024-12-310000353278ifrs-full:OtherPropertyPlantAndEquipmentMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310000353278ifrs-full:OtherPropertyPlantAndEquipmentMemberifrs-full:TopOfRangeMember2024-01-012024-12-310000353278ifrs-full:CurrentMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2024-12-310000353278ifrs-full:CurrentMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2024-12-310000353278ifrs-full:CurrentMemberifrs-full:TradeReceivablesMember2024-12-310000353278nvo:LaterthanonedayandnotlaterthanthreemonthsMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2024-12-310000353278nvo:LaterthanonedayandnotlaterthanthreemonthsMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2024-12-310000353278nvo:LaterthanonedayandnotlaterthanthreemonthsMemberifrs-full:TradeReceivablesMember2024-12-310000353278ifrs-full:LaterThanThreeMonthsAndNotLaterThanSixMonthsMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2024-12-310000353278ifrs-full:LaterThanThreeMonthsAndNotLaterThanSixMonthsMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2024-12-310000353278ifrs-full:LaterThanThreeMonthsAndNotLaterThanSixMonthsMemberifrs-full:TradeReceivablesMember2024-12-310000353278nvo:LaterthansixmonthsandnotlaterthanninemonthsMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2024-12-310000353278nvo:LaterthansixmonthsandnotlaterthanninemonthsMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2024-12-310000353278nvo:LaterthansixmonthsandnotlaterthanninemonthsMemberifrs-full:TradeReceivablesMember2024-12-310000353278nvo:LaterthanninemonthsandnotlaterthanoneyearMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2024-12-310000353278nvo:LaterthanninemonthsandnotlaterthanoneyearMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2024-12-310000353278nvo:LaterthanninemonthsandnotlaterthanoneyearMemberifrs-full:TradeReceivablesMember2024-12-310000353278ifrs-full:LaterThanOneYearMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2024-12-310000353278ifrs-full:LaterThanOneYearMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2024-12-310000353278ifrs-full:LaterThanOneYearMemberifrs-full:TradeReceivablesMember2024-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:TradeReceivablesMember2024-12-310000353278ifrs-full:AccumulatedImpairmentMemberifrs-full:TradeReceivablesMember2024-12-310000353278ifrs-full:TradeReceivablesMember2024-12-310000353278ifrs-full:CurrentMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2023-12-310000353278ifrs-full:CurrentMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2023-12-310000353278ifrs-full:CurrentMemberifrs-full:TradeReceivablesMember2023-12-310000353278nvo:LaterthanonedayandnotlaterthanthreemonthsMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2023-12-310000353278nvo:LaterthanonedayandnotlaterthanthreemonthsMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2023-12-310000353278nvo:LaterthanonedayandnotlaterthanthreemonthsMemberifrs-full:TradeReceivablesMember2023-12-310000353278ifrs-full:LaterThanThreeMonthsAndNotLaterThanSixMonthsMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2023-12-310000353278ifrs-full:LaterThanThreeMonthsAndNotLaterThanSixMonthsMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2023-12-310000353278ifrs-full:LaterThanThreeMonthsAndNotLaterThanSixMonthsMemberifrs-full:TradeReceivablesMember2023-12-310000353278nvo:LaterthansixmonthsandnotlaterthanninemonthsMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2023-12-310000353278nvo:LaterthansixmonthsandnotlaterthanninemonthsMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2023-12-310000353278nvo:LaterthansixmonthsandnotlaterthanninemonthsMemberifrs-full:TradeReceivablesMember2023-12-310000353278nvo:LaterthanninemonthsandnotlaterthanoneyearMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2023-12-310000353278nvo:LaterthanninemonthsandnotlaterthanoneyearMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2023-12-310000353278nvo:LaterthanninemonthsandnotlaterthanoneyearMemberifrs-full:TradeReceivablesMember2023-12-310000353278ifrs-full:LaterThanOneYearMemberifrs-full:TradeReceivablesMemberifrs-full:GrossCarryingAmountMember2023-12-310000353278ifrs-full:LaterThanOneYearMemberifrs-full:TradeReceivablesMemberifrs-full:AccumulatedImpairmentMember2023-12-310000353278ifrs-full:LaterThanOneYearMemberifrs-full:TradeReceivablesMember2023-12-310000353278ifrs-full:GrossCarryingAmountMemberifrs-full:TradeReceivablesMember2023-12-310000353278ifrs-full:AccumulatedImpairmentMemberifrs-full:TradeReceivablesMember2023-12-310000353278ifrs-full:TradeReceivablesMember2023-12-310000353278ifrs-full:AccumulatedImpairmentMemberifrs-full:TradeReceivablesMember2022-12-310000353278ifrs-full:AccumulatedImpairmentMemberifrs-full:TradeReceivablesMember2024-01-012024-12-310000353278ifrs-full:AccumulatedImpairmentMemberifrs-full:TradeReceivablesMember2023-01-012023-12-310000353278ifrs-full:LegalProceedingsProvisionMember2023-12-310000353278ifrs-full:RefundsProvisionMember2023-12-310000353278ifrs-full:MiscellaneousOtherProvisionsMember2023-12-310000353278ifrs-full:LegalProceedingsProvisionMember2024-01-012024-12-310000353278ifrs-full:RefundsProvisionMember2024-01-012024-12-310000353278ifrs-full:MiscellaneousOtherProvisionsMember2024-01-012024-12-310000353278ifrs-full:LegalProceedingsProvisionMember2024-12-310000353278ifrs-full:RefundsProvisionMember2024-12-310000353278ifrs-full:MiscellaneousOtherProvisionsMember2024-12-3100003532782024-09-130000353278nvo:BShareCapitalMemberifrs-full:IssuedCapitalMember2023-09-120000353278nvo:BShareCapitalMemberifrs-full:IssuedCapitalMember2023-09-130000353278nvo:AShareCapitalMember2022-12-310000353278nvo:BShareCapitalMember2022-12-310000353278ifrs-full:IssuedCapitalMember2022-12-310000353278ifrs-full:TreasurySharesMember2022-12-310000353278nvo:AShareCapitalMember2023-01-012023-12-310000353278nvo:BShareCapitalMember2023-01-012023-12-310000353278ifrs-full:IssuedCapitalMember2023-01-012023-12-310000353278ifrs-full:TreasurySharesMember2023-01-012023-12-310000353278nvo:AShareCapitalMember2023-12-310000353278nvo:BShareCapitalMember2023-12-310000353278ifrs-full:IssuedCapitalMember2023-12-310000353278ifrs-full:TreasurySharesMember2023-12-310000353278nvo:AShareCapitalMember2024-01-012024-12-310000353278ifrs-full:IssuedCapitalMember2024-01-012024-12-310000353278ifrs-full:TreasurySharesMember2024-01-012024-12-310000353278ifrs-full:IssuedCapitalMember2024-12-310000353278ifrs-full:TreasurySharesMember2024-12-310000353278nvo:AShareCapitalMemberifrs-full:IssuedCapitalMember2024-12-310000353278nvo:AShareCapitalMemberifrs-full:IssuedCapitalMember2024-01-012024-12-310000353278nvo:BShareCapitalMemberifrs-full:IssuedCapitalMember2024-12-310000353278nvo:AShareCapitalMemberifrs-full:IssuedCapitalMember2023-01-012023-12-310000353278nvo:BShareCapitalMemberifrs-full:IssuedCapitalMember2023-12-310000353278nvo:AShareCapitalMemberifrs-full:IssuedCapitalMember2022-01-012022-12-310000353278nvo:BShareCapitalMember2022-01-012022-12-310000353278nvo:BShareCapitalMemberifrs-full:IssuedCapitalMember2022-12-310000353278nvo:TreasurySharesMarketValueMember2024-12-310000353278nvo:TreasurySharesMarketValueMember2023-12-310000353278nvo:A2022ShareRepurchaseProgrammeMember2024-12-310000353278nvo:ShareRepurchaseProgrammeOfNovoNordiskBSharesMember2024-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfExchangeDifferencesOnTranslationMember2021-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfCashFlowHedgesMember2021-12-310000353278ifrs-full:OtherReservesMemberifrs-full:MiscellaneousOtherReservesMember2021-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfExchangeDifferencesOnTranslationMember2022-01-012022-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfCashFlowHedgesMember2022-01-012022-12-310000353278ifrs-full:OtherReservesMemberifrs-full:MiscellaneousOtherReservesMember2022-01-012022-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfExchangeDifferencesOnTranslationMember2022-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfCashFlowHedgesMember2022-12-310000353278ifrs-full:OtherReservesMemberifrs-full:MiscellaneousOtherReservesMember2022-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfExchangeDifferencesOnTranslationMember2023-01-012023-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfCashFlowHedgesMember2023-01-012023-12-310000353278ifrs-full:OtherReservesMemberifrs-full:MiscellaneousOtherReservesMember2023-01-012023-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfExchangeDifferencesOnTranslationMember2023-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfCashFlowHedgesMember2023-12-310000353278ifrs-full:OtherReservesMemberifrs-full:MiscellaneousOtherReservesMember2023-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfExchangeDifferencesOnTranslationMember2024-01-012024-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfCashFlowHedgesMember2024-01-012024-12-310000353278ifrs-full:OtherReservesMemberifrs-full:MiscellaneousOtherReservesMember2024-01-012024-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfExchangeDifferencesOnTranslationMember2024-12-310000353278ifrs-full:OtherReservesMemberifrs-full:ReserveOfCashFlowHedgesMember2024-12-310000353278ifrs-full:OtherReservesMemberifrs-full:MiscellaneousOtherReservesMember2024-12-310000353278ifrs-full:TopOfRangeMember2024-01-012024-12-310000353278currency:USDifrs-full:CurrencyRiskMember2024-01-012024-12-310000353278currency:CNYifrs-full:CurrencyRiskMember2024-01-012024-12-310000353278currency:CADifrs-full:CurrencyRiskMember2024-01-012024-12-310000353278currency:JPYifrs-full:CurrencyRiskMember2024-01-012024-12-310000353278currency:BRLifrs-full:CurrencyRiskMember2024-01-012024-12-310000353278currency:USDifrs-full:CurrencyRiskMember2023-01-012023-12-310000353278currency:CNYifrs-full:CurrencyRiskMember2023-01-012023-12-310000353278currency:CADifrs-full:CurrencyRiskMember2023-01-012023-12-310000353278currency:JPYifrs-full:CurrencyRiskMember2023-01-012023-12-310000353278currency:BRLifrs-full:CurrencyRiskMember2023-01-012023-12-310000353278currency:USDifrs-full:CurrencyRiskMember2022-01-012022-12-310000353278currency:CNYifrs-full:CurrencyRiskMember2022-01-012022-12-310000353278currency:CADifrs-full:CurrencyRiskMember2022-01-012022-12-310000353278currency:JPYifrs-full:CurrencyRiskMember2022-01-012022-12-310000353278currency:BRLifrs-full:CurrencyRiskMember2022-01-012022-12-310000353278currency:USDifrs-full:CurrencyRiskMember2024-12-310000353278currency:CNYifrs-full:CurrencyRiskMember2024-12-310000353278currency:CADifrs-full:CurrencyRiskMember2024-12-310000353278currency:JPYifrs-full:CurrencyRiskMember2024-12-310000353278currency:BRLifrs-full:CurrencyRiskMember2024-12-310000353278currency:USDifrs-full:CurrencyRiskMember2023-12-310000353278currency:CNYifrs-full:CurrencyRiskMember2023-12-310000353278currency:CADifrs-full:CurrencyRiskMember2023-12-310000353278currency:JPYifrs-full:CurrencyRiskMember2023-12-310000353278currency:BRLifrs-full:CurrencyRiskMember2023-12-310000353278currency:USDifrs-full:CurrencyRiskMember2022-12-310000353278currency:CNYifrs-full:CurrencyRiskMember2022-12-310000353278currency:CADifrs-full:CurrencyRiskMember2022-12-310000353278currency:JPYifrs-full:CurrencyRiskMember2022-12-310000353278currency:BRLifrs-full:CurrencyRiskMember2022-12-310000353278nvo:EffectOf5IncreaseInAllOtherCurrenciesMemberifrs-full:CurrencyRiskMember2024-01-012024-12-310000353278nvo:EffectOf5IncreaseInAllOtherCurrenciesMemberifrs-full:CurrencyRiskMember2023-01-012023-12-310000353278nvo:EffectOf5IncreaseInUSDMemberifrs-full:CurrencyRiskMember2024-01-012024-12-310000353278nvo:EffectOf5IncreaseInUSDMemberifrs-full:CurrencyRiskMember2023-01-012023-12-310000353278ifrs-full:CurrencyRiskMember2024-01-012024-12-310000353278currency:USD2024-01-012024-12-310000353278currency:CNY2024-01-012024-12-310000353278currency:CAD2024-01-012024-12-310000353278currency:JPY2024-01-012024-12-310000353278currency:BRL2024-01-012024-12-310000353278currency:USD2023-01-012023-12-310000353278currency:CNY2023-01-012023-12-310000353278currency:CAD2023-01-012023-12-310000353278currency:JPY2023-01-012023-12-310000353278currency:BRL2023-01-012023-12-310000353278ifrs-full:CashFlowHedgesMembercurrency:USDifrs-full:ForwardContractMember2024-12-310000353278ifrs-full:CashFlowHedgesMembercurrency:USDifrs-full:ForwardContractMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchAAARangeMembernvo:CashAtBankMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchAAARangeMemberifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchAAARangeMemberifrs-full:DerivativesMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchAAARangeMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchAARangeMembernvo:CashAtBankMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchAARangeMemberifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchAARangeMemberifrs-full:DerivativesMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchAARangeMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchARangeMembernvo:CashAtBankMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchARangeMemberifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchARangeMemberifrs-full:DerivativesMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchARangeMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchBBBRangeMembernvo:CashAtBankMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchBBBRangeMemberifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchBBBRangeMemberifrs-full:DerivativesMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchBBBRangeMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchNotRatedOrBelowBBBRangeMembernvo:CashAtBankMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchNotRatedOrBelowBBBRangeMemberifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchNotRatedOrBelowBBBRangeMemberifrs-full:DerivativesMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchNotRatedOrBelowBBBRangeMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:CashAtBankMemberifrs-full:CreditRiskMember2024-12-310000353278ifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2024-12-310000353278ifrs-full:DerivativesMemberifrs-full:CreditRiskMember2024-12-310000353278ifrs-full:CreditRiskMember2024-12-310000353278nvo:StandardPoorsMoodysAndFitchAAARangeMembernvo:CashAtBankMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchAAARangeMemberifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchAAARangeMemberifrs-full:DerivativesMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchAAARangeMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchAARangeMembernvo:CashAtBankMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchAARangeMemberifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchAARangeMemberifrs-full:DerivativesMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchAARangeMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchARangeMembernvo:CashAtBankMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchARangeMemberifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchARangeMemberifrs-full:DerivativesMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchARangeMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchBBBRangeMembernvo:CashAtBankMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchBBBRangeMemberifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchBBBRangeMemberifrs-full:DerivativesMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchBBBRangeMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchNotRatedOrBelowBBBRangeMembernvo:CashAtBankMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchNotRatedOrBelowBBBRangeMemberifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchNotRatedOrBelowBBBRangeMemberifrs-full:DerivativesMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:StandardPoorsMoodysAndFitchNotRatedOrBelowBBBRangeMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:CashAtBankMemberifrs-full:CreditRiskMember2023-12-310000353278ifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:CreditRiskMember2023-12-310000353278ifrs-full:DerivativesMemberifrs-full:CreditRiskMember2023-12-310000353278ifrs-full:CreditRiskMember2023-12-310000353278nvo:FinancialCounterpartiesMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:FinancialCounterpartiesMemberifrs-full:CreditRiskMember2023-12-310000353278nvo:TradeReceivablesOtherReceivablesLessPrepaymentsAndVATAndOtherFinancialAssetsMemberifrs-full:CreditRiskMember2024-12-310000353278nvo:TradeReceivablesOtherReceivablesLessPrepaymentsAndVATAndOtherFinancialAssetsMemberifrs-full:CreditRiskMember2023-12-310000353278country:USifrs-full:FactoringOfReceivablesMemberifrs-full:LaterThanOneYearMember2024-12-310000353278country:USifrs-full:FactoringOfReceivablesMemberifrs-full:LaterThanOneYearMember2023-12-310000353278country:USifrs-full:FactoringOfReceivablesMemberifrs-full:LaterThanOneYearMember2022-12-310000353278country:JPifrs-full:FactoringOfReceivablesMemberifrs-full:LaterThanOneYearMember2024-12-310000353278country:JPifrs-full:FactoringOfReceivablesMemberifrs-full:LaterThanOneYearMember2023-12-310000353278country:JPifrs-full:FactoringOfReceivablesMemberifrs-full:LaterThanOneYearMember2022-12-310000353278nvo:EURUndrawnCommittedCreditFacilityMember2024-12-310000353278nvo:EURUndrawnCommittedCreditFacilityMember2023-12-310000353278ifrs-full:CashFlowHedgesMembernvo:CNHCADAndJPYMemberifrs-full:ForwardContractMember2024-12-310000353278ifrs-full:CashFlowHedgesMembernvo:CNHCADAndJPYMemberifrs-full:ForwardContractMember2023-12-310000353278ifrs-full:ForwardContractMemberifrs-full:CashFlowHedgesMember2024-12-310000353278ifrs-full:ForwardContractMemberifrs-full:CashFlowHedgesMember2023-12-310000353278ifrs-full:FairValueHedgesMembercurrency:USDifrs-full:ForwardContractMember2024-12-310000353278ifrs-full:FairValueHedgesMembercurrency:USDifrs-full:ForwardContractMember2023-12-310000353278ifrs-full:FairValueHedgesMembernvo:CNHCADEURAndOthersMemberifrs-full:ForwardContractMember2024-12-310000353278ifrs-full:FairValueHedgesMembernvo:CNHCADEURAndOthersMemberifrs-full:ForwardContractMember2023-12-310000353278ifrs-full:ForwardContractMemberifrs-full:FairValueHedgesMember2024-12-310000353278ifrs-full:ForwardContractMemberifrs-full:FairValueHedgesMember2023-12-310000353278ifrs-full:DerivativesMember2024-01-012024-12-310000353278ifrs-full:DerivativesMember2024-01-012024-12-310000353278ifrs-full:DerivativesMember2023-01-012023-12-310000353278ifrs-full:DerivativesMember2023-01-012023-12-310000353278ifrs-full:OtherIntangibleAssetsMemberifrs-full:CashFlowHedgesMember2024-01-012024-12-310000353278ifrs-full:CashFlowHedgesMember2024-12-310000353278ifrs-full:LeaseLiabilitiesMember2023-12-310000353278ifrs-full:LeaseLiabilitiesMember2024-01-012024-12-310000353278ifrs-full:LeaseLiabilitiesMember2024-12-310000353278nvo:IssuedBondsMember2023-12-310000353278nvo:IssuedBondsMember2024-01-012024-12-310000353278nvo:IssuedBondsMember2024-12-310000353278nvo:Loans1Member2023-12-310000353278nvo:Loans1Member2024-01-012024-12-310000353278nvo:Loans1Member2024-12-310000353278nvo:CommercialPapersMember2023-12-310000353278nvo:CommercialPapersMember2024-01-012024-12-310000353278nvo:CommercialPapersMember2024-12-310000353278nvo:BankOverdrafts1Member2023-12-310000353278nvo:BankOverdrafts1Member2024-01-012024-12-310000353278nvo:BankOverdrafts1Member2024-12-310000353278ifrs-full:LeaseLiabilitiesMember2022-12-310000353278ifrs-full:LeaseLiabilitiesMember2023-01-012023-12-310000353278nvo:IssuedBondsMember2022-12-310000353278nvo:IssuedBondsMember2023-01-012023-12-310000353278nvo:BankOverdrafts1Member2022-12-310000353278nvo:BankOverdrafts1Member2023-01-012023-12-310000353278nvo:Eurobond0750FixedMaturingMarch2025Member2024-12-310000353278nvo:Eurobond3.375FixedMaturingMay2026Member2024-12-310000353278nvo:Eurobond1.125FixedMaturingSep2027Member2024-12-310000353278nvo:Eurobond0.125FixedMaturingJun2028Member2024-12-310000353278nvo:Eurobond3.125FixedMaturingJan2029Member2024-12-310000353278nvo:Eurobond1.375FixedMaturingMar2030Member2024-12-310000353278nvo:Eurobond3.250FixedMaturingJan2031Member2024-12-310000353278nvo:Eurobond3.375FixedMaturingMay2034Member2024-12-310000353278nvo:SaleAndRepurchaseAgreementsOfMarketableSecuritiesREPOMember2024-12-310000353278ifrs-full:NotLaterThanOneYearMember2024-12-310000353278ifrs-full:NotLaterThanOneYearMembernvo:Loans1Member2024-12-310000353278ifrs-full:NotLaterThanOneYearMembernvo:CommercialPapersMember2024-12-310000353278ifrs-full:NotLaterThanOneYearMembernvo:BankOverdrafts1Member2024-12-310000353278ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMember2024-12-310000353278ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMembernvo:Loans1Member2024-12-310000353278ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMembernvo:CommercialPapersMember2024-12-310000353278ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMembernvo:BankOverdrafts1Member2024-12-310000353278ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMember2024-12-310000353278ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMembernvo:Loans1Member2024-12-310000353278ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMembernvo:CommercialPapersMember2024-12-310000353278ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMembernvo:BankOverdrafts1Member2024-12-310000353278ifrs-full:LaterThanFiveYearsMember2024-12-310000353278ifrs-full:LaterThanFiveYearsMembernvo:Loans1Member2024-12-310000353278ifrs-full:LaterThanFiveYearsMembernvo:CommercialPapersMember2024-12-310000353278ifrs-full:LaterThanFiveYearsMembernvo:BankOverdrafts1Member2024-12-310000353278nvo:Loans1Member2024-12-310000353278nvo:CommercialPapersMember2024-12-310000353278nvo:BankOverdrafts1Member2024-12-310000353278ifrs-full:NotLaterThanOneYearMember2023-12-310000353278ifrs-full:NotLaterThanOneYearMembernvo:Loans1Member2023-12-310000353278ifrs-full:NotLaterThanOneYearMembernvo:CommercialPapersMember2023-12-310000353278ifrs-full:NotLaterThanOneYearMembernvo:BankOverdrafts1Member2023-12-310000353278ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMember2023-12-310000353278ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMembernvo:Loans1Member2023-12-310000353278ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMembernvo:CommercialPapersMember2023-12-310000353278ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMembernvo:BankOverdrafts1Member2023-12-310000353278ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMember2023-12-310000353278ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMembernvo:Loans1Member2023-12-310000353278ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMembernvo:CommercialPapersMember2023-12-310000353278ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMembernvo:BankOverdrafts1Member2023-12-310000353278ifrs-full:LaterThanFiveYearsMember2023-12-310000353278ifrs-full:LaterThanFiveYearsMembernvo:Loans1Member2023-12-310000353278ifrs-full:LaterThanFiveYearsMembernvo:CommercialPapersMember2023-12-310000353278ifrs-full:LaterThanFiveYearsMembernvo:BankOverdrafts1Member2023-12-310000353278nvo:Loans1Member2023-12-310000353278nvo:CommercialPapersMember2023-12-310000353278nvo:BankOverdrafts1Member2023-12-310000353278nvo:OtherFinancialAssetsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMember2024-12-310000353278nvo:OtherFinancialAssetsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMember2023-12-310000353278ifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMember2024-12-310000353278ifrs-full:FinancialAssetsAvailableforsaleCategoryMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMember2023-12-310000353278ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMember2024-12-310000353278ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMember2023-12-310000353278ifrs-full:DerivativesMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMember2024-12-310000353278ifrs-full:DerivativesMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMember2023-12-310000353278nvo:DerivativesHedgingInstrumentsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMember2024-12-310000353278nvo:DerivativesHedgingInstrumentsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMember2023-12-310000353278nvo:OtherFinancialAssetsMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2024-12-310000353278nvo:OtherFinancialAssetsMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2023-12-310000353278ifrs-full:TradeReceivablesMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2024-12-310000353278ifrs-full:TradeReceivablesMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2023-12-310000353278nvo:OtherReceivablesMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2024-12-310000353278nvo:OtherReceivablesMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2023-12-310000353278nvo:PrepaymentsAndVATReceivablesMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2024-12-310000353278nvo:PrepaymentsAndVATReceivablesMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2023-12-310000353278nvo:CashAtBankMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2024-12-310000353278nvo:CashAtBankMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2023-12-310000353278ifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2024-12-310000353278ifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2023-12-310000353278ifrs-full:TradeReceivablesMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2024-12-310000353278ifrs-full:TradeReceivablesMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310000353278ifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2024-12-310000353278ifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310000353278ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMemberifrs-full:DerivativesMember2024-12-310000353278ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMemberifrs-full:DerivativesMember2023-12-310000353278ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembernvo:DerivativesHedgingInstrumentsMember2024-12-310000353278ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembernvo:DerivativesHedgingInstrumentsMember2023-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:BorrowingsNoncurrentMember2024-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:BorrowingsNoncurrentMember2023-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:BorrowingsCurrentMember2024-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:BorrowingsCurrentMember2023-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:TradePayablesMember2024-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:TradePayablesMember2023-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:OtherNonCurrentLiabilitiesMember2024-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:OtherNonCurrentLiabilitiesMember2023-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:OtherLiabilities1Member2024-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:OtherLiabilities1Member2023-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:VATAndDutiesPayableMember2024-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembernvo:VATAndDutiesPayableMember2023-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2024-12-310000353278ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2023-12-310000353278ifrs-full:Level1OfFairValueHierarchyMember2024-12-310000353278ifrs-full:Level1OfFairValueHierarchyMember2023-12-310000353278ifrs-full:Level2OfFairValueHierarchyMember2024-12-310000353278ifrs-full:Level2OfFairValueHierarchyMember2023-12-310000353278ifrs-full:Level3OfFairValueHierarchyMember2024-12-310000353278ifrs-full:Level3OfFairValueHierarchyMember2023-12-310000353278nvo:MarketableSecuritiesMember2024-01-012024-12-310000353278nvo:MarketableSecuritiesMember2023-01-012023-12-310000353278nvo:MarketableSecuritiesMember2022-01-012022-12-310000353278nvo:RestrictedStockUnitsToEmployeesMember2024-01-012024-12-310000353278nvo:RestrictedStockUnitsToEmployeesMember2023-01-012023-12-310000353278nvo:RestrictedStockUnitsToEmployeesMember2022-01-012022-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementBoardMember2024-01-012024-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementBoardMember2023-01-012023-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementBoardMember2022-01-012022-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementGroupBelowManagementBoardMember2024-01-012024-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementGroupBelowManagementBoardMember2023-01-012023-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementGroupBelowManagementBoardMember2022-01-012022-12-310000353278nvo:SharesAllocatedtoIndividualEmployeesMember2024-01-012024-12-310000353278nvo:SharesAllocatedtoIndividualEmployeesMember2023-01-012023-12-310000353278nvo:SharesAllocatedtoIndividualEmployeesMember2022-01-012022-12-310000353278nvo:RestrictedStockUnitsToEmployeesMember2023-02-010000353278nvo:CEOMember2024-01-012024-12-310000353278srt:ExecutiveVicePresidentMember2024-01-012024-12-310000353278nvo:SeniorVicePresidentMember2024-01-012024-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementBoardMembernvo:AwardDatePeriodSevenMember2024-01-012024-12-310000353278nvo:ExecutiveManagementMembernvo:LongTermShareBasedIncentiveProgrammeManagementBoardMember2024-01-012024-12-310000353278nvo:ManagementBoardMembernvo:LongTermShareBasedIncentiveProgrammeManagementBoardMember2024-01-012024-12-310000353278nvo:RestrictedStockUnitsToEmployeesMembernvo:AwardDatePeriodSixMember2023-01-012023-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementBoardMembernvo:AwardDatePeriodSixMember2023-01-012023-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementBoardMembernvo:AwardDatePeriodFiveMember2022-01-012022-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementGroupBelowManagementBoardMembernvo:AwardDatePeriodSevenMember2024-01-012024-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementGroupBelowManagementBoardMembernvo:AwardDatePeriodSixMember2023-01-012023-12-310000353278nvo:LongTermShareBasedIncentiveProgrammeManagementGroupBelowManagementBoardMembernvo:AwardDatePeriodFiveMember2022-01-012022-12-310000353278nvo:SharesAllocatedtoIndividualEmployeesMembernvo:AwardDatePeriodSevenMember2024-01-012024-12-310000353278nvo:SharesAllocatedtoIndividualEmployeesMembernvo:AwardDatePeriodSixMember2023-01-012023-12-310000353278nvo:SharesAllocatedtoIndividualEmployeesMembernvo:AwardDatePeriodFiveMember2022-01-012022-12-310000353278ifrs-full:LaterThanOneYearMember2024-12-310000353278ifrs-full:LaterThanOneYearMember2023-12-310000353278nvo:CatalentMember2024-12-310000353278nvo:FormaTherapeuticsHoldingsIncMember2024-12-310000353278nvo:CatalentIncMemberifrs-full:CostOfSalesMember2024-12-180000353278nvo:CatalentIncMember2024-12-182024-12-180000353278nvo:OtherAcquisitionsMember2024-12-310000353278nvo:NovoHoldingsASMemberifrs-full:ParentMember2024-01-012024-12-310000353278nvo:NovoHoldingsASMemberifrs-full:ParentMember2023-01-012023-12-310000353278nvo:NovoHoldingsASMemberifrs-full:ParentMember2022-01-012022-12-310000353278ifrs-full:AssociatesMembernvo:NovonesisGroupMember2024-01-012024-12-310000353278ifrs-full:AssociatesMembernvo:NovonesisGroupMember2023-01-012023-12-310000353278ifrs-full:AssociatesMembernvo:NovonesisGroupMember2022-01-012022-12-310000353278ifrs-full:AssociatesMembernvo:AltasciencesGroupMember2024-01-012024-12-310000353278ifrs-full:AssociatesMembernvo:AltasciencesGroupMember2023-01-012023-12-310000353278ifrs-full:AssociatesMembernvo:AltasciencesGroupMember2022-01-012022-12-310000353278ifrs-full:AssociatesMembernvo:OtherSubsidiariesMember2024-01-012024-12-310000353278ifrs-full:AssociatesMembernvo:OtherSubsidiariesMember2023-01-012023-12-310000353278ifrs-full:AssociatesMembernvo:OtherSubsidiariesMember2022-01-012022-12-310000353278ifrs-full:AssociatesMembernvo:NNITGroupMember2024-01-012024-12-310000353278ifrs-full:AssociatesMembernvo:NNITGroupMember2023-01-012023-12-310000353278ifrs-full:AssociatesMembernvo:NNITGroupMember2022-01-012022-12-310000353278nvo:BShareCapitalMembernvo:NovoHoldingsASMemberifrs-full:ParentMember2024-01-012024-12-310000353278nvo:ExecutiveManagementMember2024-01-012024-12-310000353278nvo:ExecutiveManagementMember2023-01-012023-12-310000353278nvo:ExecutiveManagementMember2022-01-012022-12-310000353278nvo:BoardOfDirectorsMember2024-01-012024-12-310000353278nvo:BoardOfDirectorsMember2023-01-012023-12-310000353278nvo:BoardOfDirectorsMember2022-01-012022-12-310000353278nvo:RegisteredExecutiveManagementMember2024-01-012024-12-310000353278nvo:RegisteredExecutiveManagementMember2023-01-012023-12-310000353278nvo:RegisteredExecutiveManagementMember2022-01-012022-12-310000353278nvo:InversagoPharmaIncCanadaMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:NovoNordiskCanadaIncMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:NovoNordiskNorthAmericaOperationsASDenmarkMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:NovoNordiskIncUSMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaceuticalIndustriesLPMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmatechUSIncMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaIncMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:NNCorporateDevelopmentUSIncMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:NNResearchDevelopmentUSIncMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:NovoNordiskUSBioProductionIncMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:NovoNordiskUSHoldingsIncMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:DicernaPharmaceuticalsIncMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:EmisphereTechnologiesIncMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:FormaTherapeuticsIncUSMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:CatalentIndianaLLCUSMembersrt:NorthAmericaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaceuticalsASDenmarkMembernvo:InternationalMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaOperationsASMembernvo:InternationalMember2024-01-012024-12-310000353278nvo:NovoNordiskRegionAAMEOandLATAMASMembernvo:InternationalMember2024-01-012024-12-310000353278nvo:NovoNordiskRegionEuropeASMembernvo:InternationalMember2024-01-012024-12-310000353278nvo:NovoNordiskRegionJapanKoreaASMembernvo:InternationalMember2024-01-012024-12-310000353278nvo:AldaphSpAMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaGmbHMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:S.A.NovoNordiskPharmaN.V.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:CatalentBelgiumS.ABelgiumMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmad.o.o.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaEADMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskHrvatskad.o.o.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordisks.r.o.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskProductionCzechS.r.oCzechMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskDenmarkASDenmarkMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmatechASMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskEgyptLLCMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskEgyptPharmaceuticalsLtdEgyptMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskEstoniaOUEstoniaMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskFarmaOYFinlandMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:BiocorpProductionSAFranceMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskFranceMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskProductionSASMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaGmbHGermanyMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:CardiorPharmaceuticalsGmbHGermanyMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskHellasEpe.GreeceMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskHungriaKft.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskLimitedIrelandMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskProductionIrelandLtdMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskLtdIsraelMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskS.P.A.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:CatalentAnagniS.R.LItalyMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskKazakhstanLLPKazakhstanMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskKenyaLtd.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskLatviaSIALatviaMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaSARLMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:UABNovoNordiskPharmaMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskFarmadooelMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaSASMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskB.V.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskFinanceNetherlandsBVMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaLimitedNigeriaMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskNorwayASNorwayMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaceuticalServicesSp.z.o.o.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaSpzooPolandMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskComrciodeProdutosFarmacuticosLda.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskFarmaS.R.L.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskLimitedLiabilityCompanyRussiaMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskProductionSupportLLCMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskSaudiForTradingMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmad.o.o.BelgradeSerbiaMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskSlovakias.r.o.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskd.o.o.SloveniaMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPtyLimitedSouthAfricaMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaS.A.SpainMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskScandinaviaABSwedenMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskHealthCareAGSwitzerlandMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaAGSwitzerlandMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskTunisieSARLMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskSaglikrnleriTic.Ltd.Sti.Membernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskUkraineLLCUkraineMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaGulfFZEMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskLimitedUnitedKingdomMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:ZiyloLimitedUnitedKingdomMembernvo:EuropeTheMiddleEastAndAfricaMember2024-01-012024-12-310000353278nvo:NovoNordiskChinaPharmaceuticalsCo.LtdMembercountry:CN2024-01-012024-12-310000353278nvo:NovoNordiskShanghaiPharmaTradingCo.LtdMembercountry:CN2024-01-012024-12-310000353278nvo:NovoNordiskRegionChinaASDenmarkMembercountry:CN2024-01-012024-12-310000353278nvo:NovoNordiskHongKongLimitedMembercountry:CN2024-01-012024-12-310000353278nvo:NovoNordiskPharmaTaiwanLtd.Membercountry:CN2024-01-012024-12-310000353278nvo:BeijingNovoNordiskPharmaceuticalsScienceTechnologyCo.Ltd.Membercountry:CN2024-01-012024-12-310000353278nvo:NovoNordiskPharmaArgentinaS.A.Membernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaceuticalsPty.Ltd.Membernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaPrivateLimitedMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskProduoFarmacuticadoBrasilLtdaMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskFarmacuticadoBrasilLtdaMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskFarmacuticaLimitadaMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskColombiaSASMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskIndiaPrivateLimitedMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskServiceCentreIndiaPvt.LtdMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:PT.NovoNordiskIndonesiaMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskParsCo.PJSMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaLtdMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaMalaysiaSdnBhdMalaysiaMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaOperationsBusinessAreaSdnMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskMexicoS.A.deC.V.Membernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskServiceCentreMexicoSociedadAnonimMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaceuticalsLtd.NewZealandMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaPrivateLimitedPakistanMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPanamaS.A.Membernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPeruS.A.C.Membernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaceuticalsPhilippinesInc.Membernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaSingaporePteLtd.Membernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaKoreaLtdMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskLankaPVTLtdSriLankaMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskPharmaThailandLtd.Membernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NovoNordiskVietnamLtdVietnamMembernvo:RestOfWorldMember2024-01-012024-12-310000353278nvo:NNEASDenmarkMembernvo:OtherSubsidiariesAndAssociatedCompaniesMember2024-01-012024-12-310000353278nvo:OtherSubsidiariesAndAssociatedCompaniesMembernvo:NNITASDenmarkMember2024-01-012024-12-310000353278nvo:OtherSubsidiariesAndAssociatedCompaniesMembernvo:ChurchillStatesideSolarFundXIVLLCMember2024-01-012024-12-31
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
(Mark One)
|
|
|
|
|
|
|
|
|
| o |
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
OR |
| x |
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
For the fiscal year ended December 31, 2024 |
|
|
OR |
| o |
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
OR |
| o |
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number: 333-82318
NOVO NORDISK A/S
(Exact name of Registrant as specified in its charter)
|
|
|
|
|
|
| Not applicable |
The Kingdom of Denmark |
| (Translation of Registrant’s name into English) |
(Jurisdiction of incorporation or organization) |
Novo Alle 1
DK-2880 Bagsværd
Denmark
(Address of principal executive offices)
Karsten Munk Knudsen
Executive vice president and chief financial officer
Tel: +45 4444 8888
E-mail: kmkn@novonordisk.com
Novo Alle 1, DK-2880 Bagsværd, Denmark
(Name, Telephone, E-mail and Address of Company Contact Person)
|
|
|
|
|
|
|
|
|
|
|
|
| Securities registered or to be registered pursuant to Section 12(b) of the Act: |
|
| Title of each class: |
Trading Symbol(s): |
Name of each exchange on which registered: |
|
B shares, nominal value DKK 0.10 each |
|
New York Stock Exchange* |
|
| American Depositary Receipts, each representing one B Share |
NVO |
New York Stock Exchange |
|
| * Not for trading, but only in connection with the registration of American Depositary Receipts, pursuant to the requirements of the Securities and Exchange Commission. |
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the Annual Report:
|
|
|
|
|
|
A shares, nominal value DKK 0.10 each: |
1,074,872,000 |
|
B shares, nominal value DKK 0.10 each: |
3,390,128,000 |
|
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes x No o
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes o No x
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer”, “accelerated filer”, and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer x Accelerated filer o Non-accelerated filer o Emerging growth company o
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.x
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. x
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filling:
U.S. GAAP o International Financial Reporting Standards as issued by the International Accounting Standards Board x Other o
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow:
Item 17 o Item 18 o
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No x
TABLE OF CONTENTS
1
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
INTRODUCTION
In this Form 20-F the terms ‘the Company’, ‘Novo Nordisk’ and ‘the Group’ refer to the parent company Novo Nordisk A/S together with its consolidated subsidiaries. The term ‘Novo Nordisk A/S’ is used when addressing matters specifically related to this legal entity.
Pursuant to Rule 12b-23(a) of the Securities Exchange Act of 1934, as amended, certain information for the 2024 Form 20-F of Novo Nordisk A/S set out herein is being incorporated by reference from the Company's statutory Annual Report 2024, including the consolidated financial statements of Novo Nordisk A/S (hereafter the “Annual Report 2024”) and the Company’s Remuneration Report 2024 as specified elsewhere in this Form 20-F (with the exception of the items and pages so specified, the Annual Report 2024 and Remuneration Report 2024 are not deemed to be filed as part of this Form 20-F). Therefore, the information in this Form 20-F should be read in conjunction with the Annual Report 2024 and the Remuneration Report 2024 (see Exhibits 15.1 and 15.3, respectively)
Forward-looking statements
The information set forth in this Form 20-F and in the items and pages so specified as incorporated herein by reference to Novo Nordisk's statutory Annual Report 2024, contains certain forward-looking statements as the term is defined in the U.S. Private Securities Litigation Reform Act of 1995.
Forward-looking statements can be identified by the fact that they do not relate to historical or current facts and include guidance. Words such as ‘believe’, ‘expect’, ‘may’, ‘will’, ‘plan’, ‘strategy’, 'transition plan', ‘prospect’, ‘foresee’, ‘estimate’, ‘project’, ‘anticipate’, ‘can’, ‘intend’, ‘target’ and other words and terms of similar meaning in connection with any discussion of future operating, financial or sustainability performance identify forward-looking statements. Examples of such forward-looking statements include, but are not limited to:
•Statements of targets, future guidance, (transition) plans, objectives or goals for future operations, including those related to operating, financial and sustainability matters, Novo Nordisk’s products, product research, product development, product introductions and product approvals as well as cooperation in relation thereto;
•Statements containing projections of or targets for revenues, costs, income (or loss), earnings per share, capital expenditures, dividends, capital structure, net financials and other financial measures;
•Statements regarding future economic performance, future actions and outcome of contingencies such as legal proceedings; and
•Statements regarding the assumptions underlying or relating to such statements.
These statements are based on current plans, estimates, opinions, views and projections. Although Novo Nordisk believes that the expectation reflected in such forward-looking statements are reasonable, there can be no assurance that such expectation will prove to be correct. By their very nature, forward-looking statements involve risks, uncertainties, and assumptions, both general and specific, and actual results may differ materially from those contemplated, expressed or implied by any forward-looking statement.
Factors that may affect future results include, but are not limited to, global as well as local political, economic and environmental conditions, such as interest rate and currency exchange rate fluctuations or climate change, delay or failure of projects related to research and/or development, unplanned loss of patents, interruptions of supplies and production, including as a result of interruptions or delays affecting supply chains on which Novo Nordisk relies, shortages of supplies, including energy supplies, product recalls, unexpected contract breaches or terminations, government-mandated or market-driven price decreases for Novo Nordisk’s products, introduction of competing products, reliance on information technology including the risk of cybersecurity breaches, Novo Nordisk’s ability to successfully market current and new products, exposure to product liability and legal proceedings and investigations, changes in governmental laws and related interpretation thereof, including on reimbursement, intellectual property protection and regulatory controls on testing, approval, manufacturing and marketing, and taxation changes, including changes in tariffs and duties, perceived or actual failure to adhere to ethical marketing practices, investments in and divestitures of domestic and foreign companies, unexpected growth in costs and expenses, strikes and other labour market disputes, failure to recruit and retain the right employees, failure to maintain a culture of compliance, epidemics, pandemics or other public health crises, effects of domestic or international crises, civil unrest, war or other conflict, and factors related to the foregoing matters and other factors not specifically identified herein.
For an overview of some, but not all, of the risks that could adversely affect Novo Nordisk's results or the accuracy of forward-looking statements in this document, reference is made to the overview of risk factors in ‘Risks’ on pages 38-40 of our Annual Report 2024.
Unless required by law, Novo Nordisk has no duty and undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
Enforceability of civil liabilities
The Company is a Danish corporation and a majority of its directors and officers, as well as certain experts named herein, are non-residents of the United States. A substantial portion of the assets of Novo Nordisk A/S, its subsidiaries and such persons are located outside the United States. As a result, it may be difficult for shareholders of the Company to effect service within the United States upon directors, officers and experts who are not residents of the United States or to enforce judgments in the United States. In addition, there can be no assurance as to the enforceability in Denmark against the Company or its respective directors, officers and experts who are not residents of the United States, or in actions for enforcement of judgments of United States courts, of liabilities predicated solely upon the federal securities laws of the United States.
2
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 1 IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISORS |
PART I
ITEM 1 IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable.
ITEM 2 OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
ITEM 3 KEY INFORMATION
A. [RESERVED]
B. CAPITALIZATION AND INDEBTEDNESS
Not applicable.
C. REASONS FOR THE OFFER AND USE OF PROCEEDS
Not applicable.
D. RISK FACTORS
For information on risk factors, reference is made to ‘Risks’ on pages 38-40 of our Annual Report 2024, excluding the section ‘Mitigating actions’ on page 40. Outlined in greater detail below, we are subject to cybersecurity risks and the risk related to climate change.
The potential risk on our business as a result of cybersecurity breaches
We rely on our IT systems to protect our intellectual property, business confidential information, and personal data. Therefore, disruption as a result of cybersecurity breaches could negatively impact the Company’s business and operations or financial results.
IT systems act as a backbone for the Company. They support processes in research & development, manufacturing, sales and supply, and business administration. As we are a global company, the size and complexity of our IT systems are significant, and our IT infrastructure and networks are spread across the geographic regions in which we operate. The dedicated cybersecurity teams who operate our global IT security infrastructure may be unable to respond sufficiently to the threats facing us or may fail to prevent service interruptions or security breaches resulting from attacks by malicious third parties. Many of these cyber threats have the potential to cause significant downtime of critical IT systems or the unintended disclosure of confidential information and personal data. Although we have not previously experienced material losses as a result of such incidents, we cannot guarantee that we will be able to prevent similar incidents from occurring or adversely affecting our business in the future.
We are subject to data privacy regulation in the EU (including the General Data Protection Regulation) and to privacy laws in many other jurisdictions where we do business that impose obligations and restrictions on the collection and use of personal data. In the ordinary course of the Company’s business, it collects and stores personal data (including sensitive personal data) of patients, health care professionals, employees and other third parties.
Many third-party vendors provide support services in relation to our business processes and require access to sensitive information (including personal data) in the course of their work. Such vendors could themselves be susceptible to cybersecurity or personal data breaches. Any unauthorized access, disclosure, or other loss of personal data could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, and significant regulatory penalties, disrupt the Company’s operations and damage the Company’s reputation.
The potential risk on our business as a result of failure to meet regulatory or ethical expectations on environmental impact, including climate change
Climate change has global implications and poses a significant threat to human health and development. Companies are increasingly expected to behave in a responsible manner on a variety of environmental matters, by governmental and regulatory authorities, counterparties, such as vendors and suppliers, customers and investors. In particular, we recognize the environmental issues related to the pharmaceutical industry.
3
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
At Novo Nordisk, climate-related risks are identified and assessed through our risk management process. The risk assessment includes a natural hazards risk rating of supplier locations, provided by external insurance companies. The risk rating is related to various parameters, including natural events such as flooding, earthquakes, high-speed winds, tornados, hail storms, and lightning. The risk assessment serves to provide input for risk mitigation, and consequently prioritize actions to prevent or minimize the impact of supply disruptions on manufacturing.
The Company’s main production facilities are located in Denmark, where the risk of natural events is assessed lower; however, the Company also has other production facilities in countries that are at greater risk of natural disasters. For example, our production facility in Koriyama, Japan is exposed to a higher risk of earthquakes, our production facility in Tianjin, China is located in an area prone to storm surges due to rising sea levels and our production facilities in North Carolina, United States and Bloomington, Indiana, United States are exposed to a higher risk of tornadoes and subsequent rainfall and lightening.
In addition, availability of high-quality water is essential for the production of diabetes and biopharmaceutical products and hence the Company’s operations. The Company has production facilities in countries with high water stress levels, such as China, the United States (Clayton and Durham, North Carolina), Iran and Algeria (Blida).
Despite our commitment to identify climate-related risks, we could be unable to meet our environmental objectives in an efficient and timely manner, or at all. Our CO2e emissions continue to rise as the company grows, in particular within scope 3 emissions, which account for approximately 95% of our emissions.
To mitigate our contribution to climate change, Novo Nordisk is committed to reaching net zero emissions across scope 1, scope 2 (market-based) and scope 3 emissions by 2045. In addition, we have targets of zero scope 1 and scope 2 (market-based) CO2e emissions by 2030 and a new target of 33% absolute reduction of scope 3 emissions by 2033 compared to our base-year of 2024 (covering two-thirds of our scope 3 emissions). Key decarbonization levers include converting to lower carbon materials, requiring that our tier 1 suppliers convert to renewable energy, and lowering the emissions from our distribution.
Factors that may inhibit our ability to reach these targets or failure to maximize our environmental sustainability credentials could expose us to increased regulatory risk and put us at a commercial disadvantage relative to our peers. This could result in a material adverse effect on our business, financial condition, results of operations and prospects and lead to reputational damage.
ITEM 4 INFORMATION ON THE COMPANY
A. HISTORY AND DEVELOPMENT OF THE COMPANY
Novo Nordisk A/S was formed in 1989 by a merger of two Danish companies, Nordisk Gentofte A/S and Novo Industri A/S. Novo Industri A/S was the continuing company and its name was changed to Novo Nordisk A/S. The business activities of Nordisk Gentofte were established in 1923 by August Krogh, H. C. Hagedorn and A. Kongsted, and the business activities of Novo Industri A/S were established in 1925 by Harald and Thorvald Pedersen. From the beginning, the business of both companies was the production and sale of insulin for the treatment of diabetes.
Novo Nordisk’s B shares are listed on Nasdaq Copenhagen (NOVO-B). Its ADRs are listed on the New York Stock Exchange (NVO).
|
|
|
|
|
|
|
|
|
| Legal name: |
|
Novo Nordisk A/S |
| Commercial name: |
|
Novo Nordisk |
| Date of incorporation: |
|
November 28, 1931 |
| Legal form of the Company: |
|
A Danish public limited liability company |
| Legislation under which the Company operates: |
|
Danish law |
| Country of incorporation: |
|
Denmark |
Reference is made to ‘More information', on page 146 of our Annual Report 2024 for information on domicile.
Important events in 2024
Reference is made to ‘Introducing Novo Nordisk’, pages 4-10 and ‘2024 performance and 2025 outlook’, pages 32-37 of our Annual Report 2024 for a description of important events in 2024.
4
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 4 INFORMATION ON THE COMPANY |
Capital expenditure in 2024, 2023 and 2022
For capital expenditure in 2024, 2023 and 2022, reference is made to the section entitled ‘Cash flow and capital allocation’ on page 34 of our Annual Report 2024. No significant divestments took place in the period from 2022–2024.
For capital expenditures expected in 2025, reference is made to pages 34-35 in the subsection ‘2025 outlook’ in our Annual Report 2024. Such expenditures are expected to be financed with cash flow from operating activities.
Public takeover offers in respect of the Company’s shares
No such offers occurred during 2024 or 2025 to date.
B. BUSINESS OVERVIEW
Reference is made to the sections 'Key figures' on page 7, 'Purpose and strategy' on page 8 and ‘Strategic Aspirations’ on pages 11-31 of our Annual Report 2024.
Novo Nordisk is a global healthcare company and a world leader in Diabetes and Obesity care. The Company manufactures and markets pharmaceutical products and services that make a significant difference to patients, the medical profession and society. Headquartered in Denmark, Novo Nordisk employs more than 75,000 employees in 80 countries, and markets its products in approximately 170 countries.
The Company has a broad product portfolio across Diabetes and Obesity care and Rare disease, including a portfolio of glucagon-like-peptide-1 (GLP-1) receptor agonists for the treatment of diabetes and obesity, modern insulins and human insulins. During 2024, there has been continued strong growth across therapy areas and geographic areas in which Novo Nordisk operates.
High demand during 2024 has led to periodic supply constraints for certain products in some markets, including the leading product by sales, Ozempic® for the treatment of type 2 diabetes1. The Company also markets two drugs - Saxenda® and Wegovy® - for the treatment of obesity. In its fourth year after launch the GLP-1 product, Wegovy® revenue, grew 86% to DKK 58 billion. Further, Novo Nordisk has a Rare disease portfolio consisting mainly of growth hormone and haemophilia products.
On December 18, 2024, Novo Nordisk acquired three fill-finish sites from Novo Holdings A/S in connection with a transaction where Novo Holdings A/S acquired Catalent, Inc. (“Catalent”), a global contract development and manufacturing organisation. The acquisition of the fill-finish sites is aligned with Novo Nordisk’s strategy of reaching more people living with diabetes and obesity with current and future treatments. It is expected to enable an expansion of the manufacturing capacity and provide future optionality and flexibility for Novo Nordisk’s existing supply network. The acquisition is expected to gradually increase Novo Nordisk's filling and finish capacity. The purchase price of the three sites totalled USD 11.7 billion, which was mainly debt-financed.
Segment information
Novo Nordisk is engaged in the discovery, development, manufacturing and marketing of pharmaceutical products and has two business segments: (i) Diabetes and Obesity care and (ii) Rare disease. Reference is made to Note 2.2 ‘Segment information’ in the consolidated financial statements in our Annual Report 2024.
Seasonality
Sales of individual products in individual markets may be subject to fluctuations from quarter to quarter. However, the Company’s consolidated operating results have not been subject to significant seasonality.
Raw materials
The impact on the overall profitability of Novo Nordisk from variations in raw material prices is unlikely to be significant. Currently, there is no raw material supply shortage that is expected to significantly impact the Company’s ability to supply any significant market. Regarding the 2024 supply constraints, reference is made to page 26 of the Annual Report 2024. The supply situation has significantly improved for 2025.
Market and competition
Novo Nordisk’s insulin and other pharmaceutical products are marketed and distributed through subsidiaries, distributors and independent agents each responsible for specific geographic areas. The Company’s financial reporting is divided into: EMEA (covering Europe, the Middle East and Africa), Region China (covering Mainland China, Hong Kong and Taiwan), Rest of World (covering all other
1 Product indications described in this Form 20-F are composite summaries of the major indications approved in the product’s principal markets. Not all indications are necessarily available in each of the markets in which the products are approved. The summaries presented herein for the purpose of financial reporting do not substitute for careful consideration of the full labelling approved in each market.
5
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 4 INFORMATION ON THE COMPANY |
countries except for North America) and North America (covering the United States and Canada). For 2024, the Company's most important markets in terms of sales were the United States, China, Canada, Japan, and the major European countries.
Due to the increasing number of people with diabetes, the global pharmaceutical market for treatment of diabetes continues to grow. Several of the major international pharmaceutical companies have entered the diabetes market, specifically in the area of oral products for the treatment of type 2 diabetes. In the global diabetes market, Novo Nordisk and Eli Lilly are the most significant companies measured by market share.
The market for anti-obesity medications, primarily GLP-1s, continues to grow and expand, driven by innovative treatments coming to the market and the significant unmet medical need for safe and efficacious treatment options. Novo Nordisk and Eli Lilly are the most significant companies measured by market share, but several major international pharmaceutical companies and smaller biotech companies have anti-obesity medications under development.
The use of GLP-1 as a treatment option for people with type 2 diabetes has continued to increase resulting in significant growth of the GLP-1 market. Novo Nordisk and Eli Lilly are the most significant companies in the global GLP-1 market measured by market share.
In February 2018, Novo Nordisk launched the once-weekly GLP-1 product, Ozempic®, for the treatment of adults with type 2 diabetes in the United States and Canada. Since then, Ozempic® has become a market leading product and the Company's best performing product by sales, with global sales of more than DKK 120 billion in 2024.
The global branded obesity market almost doubled by volume in 2024. Wegovy® has been launched in the United States and more than 15 other countries outside the United States.
Market conditions within the pharmaceutical industry continue to change, including efforts by both private and governmental entities to reduce or control costs generally and in specific therapeutic areas. Most of the countries in which Novo Nordisk sells insulin and GLP-1 subsidize or control pricing. In most markets insulin and GLP-1 products are prescription drugs.
In recent years, there has been a general trend in the United States of payers managing the cost of diabetes care to exert pressure on the price of Novo Nordisk’s and competitors' products. In spite of this external pressure, Novo Nordisk has maintained a leading position in the overall diabetes care market through the quality and innovation-driven value of the Company’s Diabetes care products. In the United States, pharmacy benefit managers and managed care organizations have continued to leverage their increasing size and control to demand higher rebates which has impacted the net realized prices. Furthermore, competition has intensified, including the authorization of the first interchangeable insulin in 2021, contributing to a downward pressure on manufacturers' net prices.
Patents
To maintain and expand competitiveness, Novo Nordisk strives for the strongest possible protection for those inventions that are created during the development of new products. Novo Nordisk anticipates that the expiration of certain patents could impact sales within the coming years. However, through continued investments in research and development, Novo Nordisk strives to bring novel and innovative products to the market and thereby sustain strong patent protection in the future, as new generations of products replace currently marketed products.
For patent information on all Novo Nordisk’s marketed products, reference is made to the section ‘Patent status for products with marketing authorisation' on page 25 in our Annual Report 2024.
For key products with recent patent expiration or with patent expiration occurring within the coming years, geographic sales splits are provided and factors that may influence the potential impact of competitive product launches are discussed.
Sales of key products with recent or upcoming patent expiration:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total sales in 2024 (in DKK
million) |
|
North America Operations |
|
Hereof |
|
International
Operations |
|
Hereof |
| Product |
|
|
|
USA |
|
|
EMEA |
|
Region China |
|
Rest of World |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Victoza® |
|
5,482 |
|
|
1,796 |
|
|
1,699 |
|
|
3,686 |
|
|
1,422 |
|
|
975 |
|
|
1,289 |
|
Saxenda® |
|
6,940 |
|
|
1,377 |
|
|
777 |
|
|
5,563 |
|
|
2,920 |
|
|
102 |
|
|
2,541 |
|
6
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 4 INFORMATION ON THE COMPANY |
Patent situation of key Diabetes and Obesity care products
Today, biosimilar and/or interchangeable versions of insulin can be approved in the United States via the 351(k) pathway. In the EU, a biosimilar pathway and guidelines are available for insulins, and the guideline for biosimilar products issued in Japan is also relevant for insulin. A biosimilar to NovoRapid®/NovoLog® produced by a competitor was launched in 2020. An interchangeable biosimilar for NovoRapid®/NovoLog® produced by a competitor was approved in July 2021. Furthermore, biosimilar insulins are being developed in China by local competitors.
The total sales of Victoza® were DKK 5,482 million in 2024 (DKK 8,664 million in 2023). The compound patents for Victoza® have expired. In Japan, the drug compound patent expired in 2022; in the U.S. and Germany, the drug compound patent expired in 2023. The drug compound patent expired in China in 2017 and in 2023 a biosimilar version of Victoza® was approved in China.
Novo Nordisk has received notifications from several manufacturers that they have filed Abbreviated New Drug Applications (ANDAs) for generic versions of Victoza®, Saxenda®, Ozempic®, Wegovy®, and Rybelsus®, respectively. The ANDAs contain Paragraph IV certifications to obtain approval to engage in the commercial manufacture, use or sale of such products before the expiration of some or all of the patents currently listed for those products in the Orange Book. Novo Nordisk filed complaints for patent infringement against these manufacturers.
Novo Nordisk has entered into settlement agreements with several manufacturers that have filed ANDAs for Victoza®. Consequently, these manufacturers were licensed to launch a generic version of Victoza® as of June 22, 2024. Teva launched an authorized generic version of Victoza® in June, 2024, and Hikma Pharmaceuticals PLC launched its generic liraglutide product in December 2024. Moreover, Novo Nordisk has entered into settlement agreements regarding the U.S. patent litigation matters for Saxenda®. Novo Nordisk has now also entered into settlement agreements with Alvogen Inc. (Alvogen), Rio Biopharmaceuticals Inc. (Rio), Sun Pharmaceutical Industries Limited (Sun), Dr. Reddy’s Laboratories, Ltd. (DRL), Mylan Pharmaceuticals Inc. (Mylan), Zydus Pharmaceuticals Inc. (Zydus) and Apotex Inc. (Apotex) regarding the U.S. patent litigation for Ozempic®. All terms of the agreements are confidential. All agreements are subject to review by the U.S. Federal Trade Commission and the U.S. Department of Justice.
In March 2023, Mylan filed an IPR challenging the validity of a patent which claims a method of treating type 2 diabetes using 1 mg of semaglutide, and the Patent Trial and Appeal Board instituted an IPR proceeding. After the institution decision, Sun, DRL, and Apotex moved to join the IPR and those motions were granted. In October 2024, Novo Nordisk settled with Mylan, Sun, DRL, and Apotex prior to the IPR hearing. All terms of the agreements are confidential. All agreements are subject to review by the U.S. Federal Trade Commission and the U.S. Department of Justice.
In China, the semaglutide compound patent was subject to invalidation actions and was upheld by the Beijing IP Court in November 2023. This decision has been appealed to the Supreme People's Court where the case is currently pending.
Novo Nordisk will continue to defend its intellectual property associated with liraglutide and semaglutide, including through litigation.
The total sales of obesity care products (Saxenda® and Wegovy®) were DKK 65,146 million in 2024 (DKK 41,632 million in 2023), of which the majority of the sales comes from Wegovy®. The drug compound patent for Saxenda® (liraglutide) has expired in all countries.
Compound patent expiry in the U.S. for the semaglutide branded products - Ozempic®, Rybelsus®, and Wegovy® - is 2032. For additional information, reference is made to the section 'Patent status for products with marketing authorisation' on page 25 of our Annual Report 2024.
Impact of regulation
As a pharmaceutical company, Novo Nordisk depends on government approvals related to production, development, marketing and reimbursement of its products. Important regulatory bodies include the U.S. Food and Drug Administration, the European Medicines Agency, China's National Medical Products Administration and the Japanese Ministry of Health, Labour and Welfare. Treatment guidelines from non-governmental organizations such as the European Association for the Study of Diabetes and the American Diabetes Association may also impact the Company.
Disclosure pursuant to Section 219 of the Iran Threat Reduction and Syria Human Rights Act of 2012
Pursuant to Section 13(r) of the Securities Exchange Act of 1934 "("Section 13("r)"), Novo Nordisk is obliged to disclose if, during 2024, it or any of its affiliates have engaged in certain Iran-related activities or transactions with persons designated under Executive Order 13224 or Executive Order 13382 dealt with the Government of Iran (“GOI”). Novo Nordisk conducts limited business relating to pharmaceutical products and devices within the Diabetes care and Rare disease business segments in Iran, which is permitted under
7
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 4 INFORMATION ON THE COMPANY |
the U.S. sanctions against Iran. Set forth below is a description of the activities and transactions by Novo Nordisk’s subsidiaries that are required to be disclosed pursuant to Section 13(r). Novo Nordisk’s U.S. subsidiaries and U.S. person employees are not involved in any of Novo Nordisk’s activities in Iran. However, the United States maintains broad exceptions that permit the commercial sale and export of medicine and medical devices to Iran or the Government of Iran. Similar exceptions, like those encompassed in section 11 of Executive Order 13902, are also in place for the manufacturing of medicine and medical devices for use in Iran.
Novo Nordisk Pars (“NN Pars”), a wholly-owned subsidiary of Novo Nordisk A/S located in Iran, contracts with a number of companies that may be owned or controlled by the GOI to distribute its products. NN Pars also sponsors educational programs and congresses organized by GOI-controlled medical universities, and hosts and/or engages as scientific delegates or lecturers/speakers health care professionals employed by these medical universities at similar programs in Iran and other locations. Additionally, NN Pars makes donations to GOI-controlled public health organizations focusing on diabetes awareness and policy. NN Pars receives payments from, and makes payments to, Iranian banks (some of which may be GOI-owned or controlled) relating to the sales of pharmaceutical products and devices. NN Pars makes payments incidental to its ordinary business activities to Iranian government entities and entities that are or may be GOI-owned or controlled, such as taxes, customs fees, insurance, product registration fees and telecommunications services expenses.
In 2016, NN Pars purchased land from a GOI-owned or controlled holding company in order to construct a manufacturing facility in Iran. The facility opened and officially started production in August 2020 and is being used for assembly and packaging of insulin pens for use in Iran. NN Pars purchases utility services from a GOI-owned or controlled entity.
Novo Nordisk’s gross revenue related to transactions with GOI-owned or controlled entities in 2024 was not in excess of 1% of Group sales. Novo Nordisk does not allocate its net profit on a country-by-country or activity-by-activity basis, other than as set forth in Novo Nordisk’s consolidated financial statements prepared in accordance with IFRS® Accounting Standards as issued by the International Accounting Standards Board (IASB); however, Novo Nordisk estimates that its net profit attributable to the transactions with the GOI discussed above would not exceed a de minimis percentage of the Group’s total net profit in 2024.
The purpose of Novo Nordisk’s Iran-related activities is to provide access to important and essential pharmaceutical products such as insulin and haemophilia products to patients in Iran, and to improve the healthcare of the Iranian people in accordance with Novo Nordisk’s access to care strategy. For that purpose, and because Novo Nordisk has determined that its activities comply with all applicable laws, Novo Nordisk intends to continue these activities (including local production of these products in Iran).
C. ORGANIZATIONAL STRUCTURE
For information regarding the organizational structure and securities exchange listings of Novo Nordisk A/S, the controlling shareholder Novo Holdings A/S and the Novo Nordisk Foundation and the ownership structure of Novo Nordisk A/S, reference is made to the sections ‘Corporate Governance’ on pages 16 and ‘Shares and capital structure’ on pages 36-37 of our Annual Report 2024.
Companies in the Novo Nordisk Group are listed in the section ‘Companies in the Novo Nordisk Group’ on page 133 of our Annual Report 2024.
D. PROPERTY, PLANTS AND EQUIPMENT
The Company has its headquarters in Bagsværd, Denmark, where it occupies a number of buildings.
Sales growth in 2024 has resulted in periodic supply constraints and related drug shortage notifications across a number of products and geographies.
Following higher than expected volume growth in recent years, including GLP-1-based products such as Ozempic® and Wegovy®, combined with the expectation of continued volume growth and capacity limitations at some manufacturing sites, the 2025 outlook also reflects expected continued periodic supply constraints and related drug shortage notifications across a number of products and geographies. Novo Nordisk is investing in internal and external capacity to increase supply both short and long term.
The supply capacity is gradually increased, including the capacity for meeting growing demand in the future for our main products, Awiqli®, Fiasp®, Levemir®, Norditropin® NovoLog®/ NovoRapid®, NovoLog Mix®/ NovoMix®, NovoSeven®, Ozempic®, Rybelsus®, Ryzodeg®, Saxenda®, Tresiba®, Victoza®, Wegovy® and Xultophy®. Reference is made to the sections ‘Capital expenditures in 2024, 2023 and 2022’ under Item 4 for more information about the current expansion programs. For the nature of the Company’s property, plant and equipment, as of December 31, 2024 and 2023, reference is made to Note 3.2 ‘Property, plant and equipment’ in the consolidated financial statements in our Annual Report 2024.
8
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 4 INFORMATION ON THE COMPANY |
The major production facilities owned by the Company are located at a number of sites in Denmark, and internationally in the United States, France, China and Brazil. There are no material encumbrances on the properties; however, the facilities in Tianjin, China are constructed on land where the remaining term of the leases is 29 and 33 years.
Active pharmaceutical ingredient (API) production is located in Denmark, primarily in Kalundborg and with secondary locations in Hillerød and Gentofte, both in Denmark, as well as in New Hampshire and North Carolina, United States, respectively.
The following table sets forth certain information regarding our major production sites.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| MAJOR PRODUCTION FACILITIES |
|
Size of production area
(square meters) |
|
Major Production Activities |
| Kalundborg, Denmark |
|
168,300 |
|
Active pharmaceutical ingredients for diabetes and obesity as well as products for Diabetes care |
|
|
|
|
Active pharmaceutical ingredients for haemophilia. |
|
|
|
|
Products for Rare disease |
|
|
|
|
|
| Hillerød, Denmark |
|
156,900 |
|
Durable devices and components for disposable devices |
|
|
|
|
Products for diabetes and obesity |
|
|
|
|
Active pharmaceutical ingredients for haemophilia |
|
|
|
|
|
| Bagsværd, Denmark |
|
111,200 |
|
Products for diabetes and obesity |
|
|
|
|
|
| Clayton, North Carolina, United States |
|
89,000 |
|
Active pharmaceutical ingredients for diabetes and obesity (purification) |
|
|
|
|
Products for diabetes and obesity |
|
|
|
|
|
| Gentofte, Denmark |
|
70,800 |
|
Active pharmaceutical ingredients for glucagon and growth hormone therapy |
|
|
|
|
Products for growth hormone therapy, glucagon and haemophilia |
|
|
|
|
|
| Tianjin, China |
|
67,200 |
|
Products for diabetes |
|
|
|
|
Production of durable devices |
|
|
|
|
|
| Måløv, Denmark |
|
60,900 |
|
Products for hormone replacement therapy |
|
|
|
|
Products for oral antidiabetic treatment |
|
|
|
|
Products for oral diabetes treatment |
|
|
|
|
|
| Chartres, France |
|
58,700 |
|
Products for diabetes |
|
|
|
|
|
| Montes Claros, Brazil |
|
56,200 |
|
Products for diabetes |
|
|
|
|
Gel production for active pharmaceutical ingredients |
|
|
|
|
|
| Anagni, Italy |
|
40,400 |
|
Products for diabetes and obesity |
|
|
|
|
Contract manufacturing organization (CMO) related activities |
|
|
|
|
|
| Brussels, Belgium |
|
18,000 |
|
Products for diabetes and obesity |
|
|
|
|
CMO related activities |
|
|
|
|
|
| Bloomington, Indiana, United States |
|
13,100 |
|
Products for diabetes and obesity |
|
|
|
|
CMO related activities |
|
|
|
|
|
In December 2021, the Company announced the investment in construction of a new purification facility and a new recovery facility as well as rebuilding of one existing fermentation facility at the production site in Kalundborg, Denmark. The investment will establish additional capacity for manufacturing active pharmaceutical ingredients. The facilities are expected to increase the production area with approximately 59,900 square meters. The facilities are expected to be operational during 2027 and the expected amount of expenditures is DKK 19.5 billion with realized spend of DKK 16.5 billion as of December 31, 2024. The facilities will be financed by cash flow from operating activities.
In June 2022, the Company announced its investment in an expansion of an existing facility at the production site in Hjørring, Denmark. The investment will increase the capacity for production of NovoFine® Plus needles and is expected to increase the production area by 5,900 square meters. The expansion is expected to be finalized during 2025. The expected amount of expenditures is approximately DKK 550 million with realized spend of DKK 473 million as of December 31, 2024. The expansion will be financed by cash flow from operating activities.
In November 2022, the Company announced its investment in the expansion of its clinical manufacturing facilities in Bagsværd, Denmark. The investment will establish additional capacity in R&D for the manufacturing of active pharmaceutical ingredients to supply the Company’s global clinical trials. The expansion is expected to increase the production area with 7,000 square meters and it is expected to be finalized in 2026. The expected amount of expenditures is DKK 7.4 billion with realized spend of DKK 7.0 billion as of December 31, 2024. The expansion will be financed by cash flow from operating activities.
9
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 4 INFORMATION ON THE COMPANY |
In June 2023, the Company announced its investment in expanding an existing API production facility in Hillerød, Denmark. The facility is expected to be operational during 2028 and its production area expected to be 65,000 square meters. The expected amount of expenditures for this facility is approximately DKK 15.9 billion with realized spend of DKK 6.2 billion as of December 31, 2024. The facility will be financed by cash flow from operating activities.
In November 2023, the Company announced its investment in the expansion of its API production facility in Kalundborg, Denmark. The facility is expected to be fully operational during 2029 and its production area expected to be 170,000 square meters. The expected amount of expenditures for this facility is approximately DKK 42.4 billion with realized spend of DKK 11.0 billion as of December 31, 2024. The facility will be financed by cash flow from operating activities.
In November 2023, the Company announced the investment in an expansion of an existing facility at the production site in Chartres, France. The investment will significantly increase the capacity of the manufacturing site, adding aseptic production and finished production processes and an extension of the current Quality Control Laboratory. The facility is expected to be gradually finalized from 2026 to 2028 and its production area expected to be 51,100 square meters. The expected amount of expenditures for this facility is approximately DKK 16.9 billion with realized spend of DKK 3.8 billion as of December 31, 2024. The facility will be financed by cash flow from operating activities.
In March 2024, the Company announced the investment in an expansion of an existing facility at the production site in Tianjin, China. The investment will significantly increase the capacity of the manufacturing site, adding aseptic production. The facility is expected to be fully operational during 2028 and its production area expected to be 25,000 square meters. The expected amount of expenditures for this facility is approximately DKK 4.1 billion with realized spend of DKK 1.1 billion as of December 31, 2024. The facility will be financed by cash flow from operating activities.
In June 2024, the Company announced the investment in an expansion of an existing facility at the production site in the U.S. in Clayton, North Carolina. The investment will significantly increase the capacity of the manufacturing site, adding aseptic production and finished production processes. The facility is expected to be fully operational during 2029 and its production area is expected to be 130,000 square meters. The expected amount of expenditures for this facility is approximately DKK 27.0 billion with realized spend of DKK 5.1 billion as of December 31, 2024. The facility will be financed by cash flow from operating activities.
In November 2024, the Company announced the investment in an expansion of an existing facility at the production site in Hillerød, Denmark. The investment will significantly increase the capacity of QC facilities. The facility is expected to be fully operational during 2027 and its production area is expected to be 53,000 square meters. The expected amount of expenditures for this facility is approximately DKK 2.9 billion with realized spend of DKK 0.3 billion as of December 31, 2024. The facility will be financed by cash flow from operating activities.
In December 2024, the Company announced the investment of a newly established facility in Odense, Denmark. The investment will significantly increase the capacity of the manufacturing site, adding aseptic production and finished production processes. The facility is expected to be fully operational during 2027 and its production area is expected to be 40,000 square meters. The expected amount of expenditures for this facility is approximately DKK 8.5 billion with realized spend of DKK 1.2 billion as of December 31, 2024. The facility will be financed by cash flow from operating activities.
ITEM 4A UNRESOLVED STAFF COMMENTS
None.
ITEM 5 OPERATING AND FINANCIAL REVIEW AND PROSPECTS
New accounting pronouncements
Reference is made to Note 1.2 ‘Changes in accounting policies and disclosures’ in the consolidated financial statements in our Annual Report 2024.
A. OPERATING RESULTS
Reference is made to the section ‘Forward-looking statements’ on page 35-36 of our Annual Report 2024 and the discussion under the caption ‘Risk factors’ under Item 3 of this Form 20-F. Further reference is made to ‘Risks’ on pages 38-40 of our Annual Report 2024.
The information in this section is based on our Annual Report 2024 and should be read in conjunction with such report. The analysis and discussion included in such report is primarily based on the Company's consolidated financial statements which are prepared in accordance with IFRS Accounting Standards as issued by the International Accounting Standards Board.
10
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 5 OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
2024 compared with 2023
The following portions of our Annual Report 2024 constitute the Board of Directors’ and Executive Management’s discussion and analysis of results of operations (incorporated herein by reference): ‘Introducing Novo Nordisk’ (pages 4-10) and ‘2024 performance and 2025 outlook’ (pages 32-37).
2023 compared with 2022
For a discussion of our results of operations for 2023 compared with 2022, see ‘Item 5.A. Operating Results, 2023 Compared with 2022‘ included in our 2023 Annual Report on Form 20-F (File No. 333-82318) filed with the SEC on January 31, 2024 (hereafter "Annual Report 2023").
Segment information
Reference is made to Note 2.2 ‘Segment information’ in the consolidated financial statements in our Annual Report 2024 for details on segmented results.
Sales in Russia and Ukraine constituted less than 1% of Novo Nordisk's global sales in 2024. Novo Nordisk's factory in Russia is still operating to supply insulin to patients in Russia only. While Novo Nordisk maintains supply of medicine in Russia to ensure that more than 700,000 patients can continue their treatment with essential medication. Novo Nordisk has ceased filing for marketing authorizations of new medication and has suspended further clinical investments in Russia. Novo Nordisk has to the extent possible continued supply of medicines in Ukraine and Novo Nordisk medicines are currently available in more than 90% of Ukraine.
Foreign currencies
Reference is made to Note 4.4 ‘Financial risks’ in the consolidated financial statements in our Annual Report 2024 and for further description of foreign currency exposure and hedging activities, reference is made to the description of financial instruments in Note 4.5 ‘Derivative financial instruments’ in the consolidated financial statements in our Annual Report 2024.
Governmental policies
Please refer to pages 11-37 ‘Strategic Aspirations’ of our Annual Report 2024 and Item 4 hereof.
Off-balance sheet arrangements
Reference is made to Note 4.4 ‘Financial risks’ in the consolidated financial statements and Note 5.2 ‘Commitments’ in the consolidated financial statements in our Annual Report 2024.
B. LIQUIDITY AND CAPITAL RESOURCES
Novo Nordisk maintains a centralized approach to the management of the Group’s financial risks. The overall objectives and policies for Novo Nordisk’s financial risk management are outlined in the Novo Nordisk Treasury Policy, which is approved by the Board of Directors. The Treasury Policy governs the Group’s use of financial instruments. For further information, reference is made to Item 11.
Financial resources
Reference is made to ‘Cash flow statement’ on page 103 and ‘Balance sheet’ on page 104 of our Annual Report 2024. In addition, Novo Nordisk has obtained a credit rating from two independent external rating agencies.
Novo Nordisk believes its financial resources are sufficient to meet its requirements for at least the next 12 months.
Cash flow in 2024, 2023 and 2022
Reference is made to ’Cash flow statement' on page 103 of our Annual Report 2024.
The most significant source of cash flow from operating activities is sales of Diabetes and Obesity care and Rare disease products. Generally, other factors that affect operating earnings, such as pricing, volume, product mix, costs and exchange rates, also have an impact on realized cash flow from operating activities. Except as disclosed in Note 4.8 'Financial assets and liabilities' in the consolidated financial statements in our Annual Report 2024, there are no material restrictions on the ability of subsidiaries with material cash amounts to transfer funds to the parent company, Novo Nordisk A/S.
Trade receivable program
Trade receivable program, as of December 31, 2024, 2023 and 2022, respectively, are shown in Note 4.4 ‘Financial risks’ in the consolidated financial statements in our Annual Report 2024.
11
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 5 OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
Debt financing
Reference is made to ‘Balance sheet’ on page 104 and to Note 4.6 ‘Borrowings’ in the consolidated financial statements in our Annual Report 2024 for information on Current and Non-current debt.
Derivative financial instruments
Novo Nordisk only hedges commercial exposures, including selected business development activities (mergers and acquisitions), and consequently does not enter into derivative transactions for trading or speculative purposes. Currency hedging is done with foreign exchange forwards and foreign exchange options. Reference is made to Note 4.4 ‘Financial risks’ and Note 4.5 ‘Derivative financial instruments’ in the consolidated financial statements in our Annual Report 2024 for further information on financial instruments including currency exposure.
Commitments for capital expenditure etc.
Contractual obligations for capital expenditure and other contingent liabilities as of December 31, 2024 and 2023, respectively, are shown in Note 5.2 ‘Commitments’ in the consolidated financial statements in our Annual Report 2024.
The Executive Management of the Group believes that the obligations are covered by the Group’s financial resources as well as expected future cash flows from operating activities.
C. RESEARCH AND DEVELOPMENT, PATENTS AND LICENSES, ETC.
Novo Nordisk research and development is mainly focused on:
–Insulins, GLP-1s and other therapeutic compounds for diabetes treatment
–GLP-1s, combinations and other modes of action for obesity care
–Blood-clotting factors and other modes of action for treatment of haemophilia and other rare blood disorders
–Human growth hormone and other modes of action for treatment of growth disorders and other rare endocrine disorders
–New indications with existing assets and other modes of action for treatment of cardiovascular diseases, MASH and other serious chronic diseases
–Research technology platforms including cell therapy and RNAi for treatment of diseases within Diabetes, Obesity, Rare disease and Cardiovascular & Emerging Therapy Areas
The research activities mainly utilise biotechnological methods based on advanced protein chemistry and protein engineering. These methods have played a key role in the development of the production technology used to manufacture insulin, GLP-1, recombinant blood-clotting factors and human growth hormone. Research activities further utilise technology platforms including stem cells, gene therapy and RNAi therapies. Research and development activities are carried out by Novo Nordisk's research and development centres, mainly in Denmark, the United States (U.S.), the United Kingdom and China. Clinical trials are carried out all over the world.
Novo Nordisk also enters into partnerships and licence agreements. Reference is made to Note 2.3 ‘Research and development costs’ in the consolidated financial statements in our Annual Report 2024 for research and development costs in 2024, 2023 and 2022, respectively. Novo Nordisk’s research and development organization is comprising more than 10,000 employees as of December 31, 2024.
Research costs comprise the early stages of the drug development cycle from the initial drug discovery until the drug is ready for administration to humans. The activities initially focus on identifying a single drug candidate with a profile that will support a decision to initiate development activities. Before selection of the final drug candidate, it is tested in animals to gather efficacy, toxicity and pharmacokinetic information. Development costs are incurred from the start of phase 1, when the drug is administered to humans for the first time; these are the projects captured in the ’Pipeline overview’ (page 23 of our Annual Report 2024). The final product is developed, and subsequent clinical trials (phases 2 and 3) are conducted to further test the drug in humans, using the results from these trials to attempt to obtain marketing authorization, permitting Novo Nordisk to market and sell the developed products. Historically, Novo Nordisk has spent approximately 70-80% of total research and development expenditures on clinical development activities, and approximately 20-30% on research activities. The split between research and development will fluctuate in individual years depending on the composition of the clinical development portfolio.
In general, Novo Nordisk expects that growth in research and development spending will follow a trend in line with or slightly above sales growth indicating that the research and development cost to sales ratio is expected to gradually increase in the foreseeable future. Thus, Novo Nordisk currently expects to modestly expand upon the current expenditure level of around 14-16% of sales in research and development activities going forward. Increased early and late-stage clinical trial activities across all therapy areas as well as increased business development activities are driving costs.
12
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 5 OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
Novo Nordisk has multiple phase 3 programs currently in progress, see the below table for the full list.
The following Novo Nordisk compounds are currently in phase 3 development or have recently been filed for regulatory approval:
|
|
|
|
|
|
|
|
|
| COMPOUND / BRAND NAME / INDICATION |
Year entered into phase 3 or filed with the regulatory authorities |
Patent expiration |
|
|
|
|
|
|
| Concizumab (NN7415) / Haemophilia A and B with or without inhibitors |
Regulatory submission for some indications ongoing |
20341 |
|
|
|
|
|
|
|
|
|
| Insulin Icodec (NN1436) / Once-weekly basal insulin analogue |
Regulatory submission in 2023 |
20372 |
|
|
|
| Semaglutide (oral) 25 mg and 50 mg (NN9924) / Diabetes |
Phase 3 completed in 2023 |
2032 |
|
|
|
Semaglutide (oral) 25 mg and 50 mg / Obesity |
Phase 3 completed in 2024 |
2032 |
|
|
|
| Cagrisema (NN9388)/ Diabetes |
Phase 3 initiated in 2023 |
2037 |
|
|
|
Cagrisema (NN9838)/ Obesity |
Phase 3 initiated in 2022 |
2037 |
|
|
|
| IcoSema (NN1535) / A combination of GLP-1 semaglutide and insulin icodec |
Regulatory submission in 2024 |
20372 |
|
|
|
| Etavopivat / Second generation selective, small molecule PKR-activator intended for once-daily oral administration in sickle cell disease |
Phase 3 initiated in 2022 |
20393 |
|
|
|
| Mim8 (NN7769) |
Phase 3 initiated in 2021 |
20404 |
|
|
|
| Semaglutide in MASH (NN9931) |
Phase 3 completed in 2024 |
2032 |
|
|
|
| Semaglutide in Alzheimer's (NN6535) |
Phase 3 initiated in 2021 |
2032 |
| Ziltivekimab (NN6018) / Cardiovascular disease |
|
|
| Ziltivekimab (NN6018) / Cardiovascular disease |
Phase 3 initiated in 2021 |
20355 |
|
|
|
|
|
|
1 Current estimate United States. Key EU markets estimate 2035, Japan expiry 2034
2 Current estimate of regulatory data protection in the United States. Key EU markets and Japan estimate 2034
3 Current estimate, United States. Key EU markets and Japan estimated in 2038
4 Current estimate, United States. Key EU markets estimate 2041 and Japan estimated in 2044
5 Current estimate, United States. Key EU markets and Japan estimate 2032. In addition to patents, the product is eligible for Regulatory Data Protection, i.e. 10 years from market authorization in the EU and 12 years from market authorization in the U.S.
In determining whether or not any project or group of related projects is significant, we consider the following qualitative and quantitative criteria:
•Assessment of the unmet medical need targeted with the specific project;
•The inherent project risk including the risk of safety issues, unsatisfactory tolerability profile, limitations on the efficacy of the compound;
•Timeline for completing the clinical testing and submitting an application for approval to regulatory authorities;
•Regulatory authorities’ position towards approval and drug label;
•Changes in competitive landscape during the development and approval cycle including competing drugs being developed by others;
•Changes in medical practice during the development period;
•Position of payers, the medical society and patients towards treatment with the drug and price of the drug;
•Expected uptake in market following launch; and
•Expected net present value of the project.
In assessing the criteria listed above, we refer to ‘Risks’ on pages 38-40 in our Annual Report 2024. It is important to note that due to the risks and uncertainties involved in progressing through pre-clinical development and clinical trials, and the time and cost involved in obtaining regulatory approvals, we cannot reasonably estimate the nature, timing, completion dates and costs of the efforts necessary to complete the development. The nature of Novo Nordisk’s development activities is such that a compound must first be
13
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 5 OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
proven to work by means of multiple clinical trials, which may require treatment of thousands of patients and could take years to complete. Even if initial results of preclinical studies or clinical trial results are promising, the Company may obtain different results that fail to show the desired levels of safety and efficacy, or Novo Nordisk may not obtain applicable regulatory approval for a variety of other reasons. The compound must be approved by either the U.S. Food and Drug Administration, the European Medicines Agency or by similar agencies around the world, each of which may have differing requirements. During each stage, there is a substantial risk that Novo Nordisk will encounter serious obstacles which will further delay us, or that the Company will not achieve its goals and, accordingly, may abandon a product in which it has invested substantial resources. Furthermore, the commercial potential of a project is dependent on the label granted by the regulatory authority upon approval. The label specifies for which indications a product is approved for, major and minor safety concerns associated with drug treatment, as well as if the drug is approved for use in combination with other types of medication. Thus a label can restrict usage substantially. Reference is made to the caption ‘Risk factors’ contained under Item 3 hereof.
Given the uncertainties related to the process of product development, during the periods presented in our 2024 Form 20-F no single project in product development was material to total R&D spend nor significant based on the qualitative and quantitative criteria. However, during the periods presented, two groups of projects were considered significant; the Diabetes and Obesity care group and the Rare disease group.
Information related to selected research and development projects can be found under ‘Research and development progress’ on page 24 of our Annual Report 2024.
D. TREND INFORMATION
For more information on commercial dynamics across Novo Nordisk therapy areas, we refer to ‘Commercial Execution’ on pages 26-31 of our Annual Report 2024.
The key drivers behind Novo Nordisk’s performance continue to be the changes in demographics globally reflecting a continuous growth in the proportion of people who live in cities (urbanization), an increasing proportion of elderly people and a growing prevalence of obesity. These trends have contributed to the significant increase in the number of people with diabetes and obesity worldwide. According to the International Diabetes Federation, the number of people with diabetes is projected to increase from 573 million today to 780 million in 2045. Additionally, there are currently more than 800 million people living with obesity. This is also expected to grow in the coming decades.
Diabetes and Obesity care is Novo Nordisk’s largest segment comprising more than 90% of sales. The epidemic growth in the number of people with diabetes and obesity and the increasing use of the GLP-1 category is driving Novo Nordisk’s growth within the Diabetes and Obesity care segment.
Payers continued to leverage their size and control to demand higher rebates, particularly in the insulin segment, but increasingly in the GLP-1 category, as well. As a result, average prices after rebates for the Novo Nordisk portfolio in 2024 in the United States declined. Ultimately, pricing pressure is expected to continue in the future, driven by: increasing rebates in the commercial segment, the effect of payer consolidation, increasing exposure to high rebate channels such as Medicare and Medicaid, as well as increasing competition.
In January 2021, Novo Nordisk changed its policy relating to 340B Drug Pricing Program (under Section 340B of the Public Health Service Act, pharmaceutical manufacturers participating in Medicaid are required to sell outpatient drugs at discounted prices to certain health care organizations that care for uninsured and low-income patients), whereby Novo Nordisk no longer provides 340B statutory discounts to certain pharmacies that contract with covered entities participating in the 340B Drug Pricing Program. Novo Nordisk’s 340B policy has been the subject of litigation in the U.S. courts. On January 30, 2023, the U.S. Court of Appeals for the Third Circuit issued a ruling holding that Novo Nordisk’s drug distribution policy was consistent with the 340B statute. On May 21, 2024, the U.S. Court of Appeals for the DC Circuit issued a ruling in a related case involving other pharmaceutical manufacturers that similarly held that their drug distribution policies were consistent with the 340B statute. However, an appeal in another related case is still pending before the U.S. Court. See Note 3.5 ‘Provisions and contingent liabilities’ in the consolidated financial statements in our Annual Report 2024.
In August 2022 the Inflation Reduction Act of 2022 was passed into law. This legislation included several healthcare reforms, which resulted in minor near-term sales impacts, but could also have medium and long-term impacts. Reference is made to Note 2.1 'Net sales and rebates' in the consolidated financial statements in our Annual Report 2024 for information on the Company's sales and rebates.
For 2025, continued pricing pressure within Diabetes and Obesity Care is expected.
14
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 5 OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
For further information on trends, reference is made to the section ‘2024 performance and 2025 outlook’ on pages 32-37 of our Annual Report 2024. Information about expectations for the financial year 2025 can be found on pages 34-35 in the subsection ‘2025 outlook’.
E. CRITICAL ACCOUNTING ESTIMATES
Reference is made to Note 1.1 ‘Material accounting policies and key accounting estimates’ in the consolidated financial statements in our Annual Report 2024.
ITEM 6 DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. DIRECTORS AND SENIOR MANAGEMENT
Reference is made to pages 42-44 of our Annual Report 2024 for name, position and period of service as director for members of the Board of Directors.
Reference is made to page 45 of our Annual Report 2024 for name, position, age and other management duties for members of Executive Management, except in respect of Doug Langa. Business experience, year of appointment and year of joining Novo Nordisk for each member of Executive Management are included below:
|
|
|
|
Lars Fruergaard Jørgensen
President and chief executive officer (CEO)
Mr Jørgensen joined Novo Nordisk in 1991 as an economist in Health Care, Economy & Planning and has over the years completed overseas postings in the Netherlands, the U.S. and Japan. In 2004 he was appointed senior vice president for IT & Corporate Development. In January 2013 he was appointed executive vice president and chief information officer assuming responsibility for IT, Quality & Corporate Development. In November 2014 he took over the responsibilities for Corporate People & Organisation and Business Assurance and became chief of staff. Mr Jørgensen was appointed president and chief executive officer in January 2017.
|
|
Maziar Mike Doustdar
Executive vice president, International Operations
Mr Doustdar joined Novo Nordisk in 1992 as an office clerk in Vienna, Austria. From 1993 through 2007 he took up various positions in finance, IT, logistics, operations and marketing, within various parts of Novo Nordisk’s emerging markets, first in Vienna and subsequently in Athens and Zurich before he was appointed general manager of Novo Nordisk Near East, based in Turkey, in 2007. In 2010 Mr Doustdar was promoted to vice president of Business Area Near East and in 2012 he re-located to Malaysia to head the Business Area Oceania South East Asia. In 2013 he was promoted to senior vice president of Novo Nordisk’s International Operations, and in April 2015 Mr Doustdar was promoted to executive vice president, continuing his responsibility for Novo Nordisk’s International Operations. In September 2016 Mike Doustdar assumed additional geographical responsibilities and was promoted to executive vice president for an expanded International Operations, leading all commercial units globally, except for the U.S. and Canada. Effective January 1, 2025, Canada was integrated into IO.
|
|
Ludovic Helfgott
Executive vice president, Rare disease
Mr Helfgott joined Novo Nordisk in April 2019 as executive vice president for Rare disease
Mr Helfgott joined Novo Nordisk from AstraZeneca, UK, where he was global vice president in charge of the company's cardiovascular, metabolism and renal global franchise. He joined AstraZeneca in 2005 in an international sales effectiveness role and has since held operational leadership roles with increasing responsibilities in Italy, Spain and at corporate headquarters. Prior to this, Mr Helfgott was with McKinsey & Company in Paris, Moscow and Brussels from 1998 to 2005.
|
|
Karsten Munk Knudsen
Executive vice president and chief financial officer (CFO)
Mr Knudsen joined Novo Nordisk in 1999 as a business analyst in NNIT A/S, previously a subsidiary of Novo Nordisk, and has since held finance positions of growing size and complexity throughout the Novo Nordisk value chain. From 2010 to 2014 Mr Knudsen was corporate vice president for Finance & IT at Novo Nordisk Inc. in the U.S. and in 2014 he was appointed senior vice president of Corporate Finance in Novo Nordisk. In February 2018 Mr Knudsen was promoted to executive vice president and chief financial officer. In 2019 Mr Knudsen assumed further responsibilities as his area was expanded to cover Finance, Legal & Procurement, followed by a further expansion in 2022 where he assumed responsibility for Global Solutions.
Effective January 1, 2025, Mr Knudsen assumed responsibility for Novo Nordisk’s corporate strategy in addition to his current responsibilities.
|
|
Martin Holst Lange
Executive vice president, Development
Mr Lange joined Novo Nordisk in 2002, as first operationally and subsequently medically responsible for several projects within Global Development. From 2006 to 2008 Mr Lange worked in Novo Nordisk Inc., USA, in the Medical Department as senior medical director. In 2008, he moved back to Denmark and became vice president, Medical & Science liraglutide, transferring in 2010 to insulin degludec in a similar position. From 2013 to 2017, he served as corporate project vice president for Insulin & Diabetes Outcomes and subsequently Insulin & Devices. In January 2018, he was appointed senior vice president for Global Development. In March 2021, Mr Lange was appointed executive vice president for Development.
From 1997 to 2002, Mr Lange did clinical work as well as clinical research of which the latter, three years at the Department of Endocrinology, National University Hospital, Denmark. Mr Lange has served on the board of directors of Beta Bionics Inc., USA.
|
15
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 6 DIRECTORS, EXECUTIVE MANAGEMENT AND EMPLOYEES |
|
|
|
|
Marcus Schindler
Executive vice president, Research & Early Development and chief scientific officer (CSO)
Mr Schindler joined Novo Nordisk in January 2018 as senior vice president for External Innovation and Strategy. From March 2018 to 2021 he was senior vice president for Global Drug Discovery and in March 2021, Mr Schindler was appointed executive vice president for Research & Early Development and chief scientific officer.
Prior to joining Novo Nordisk Mr Schindler was vice president, head of cardiovascular and metabolic diseases innovative medicines at AstraZeneca, Sweden. From 2009 to 2012, he was head of research at (OSI) Prosidion, Oxford, UK. From 2000 to 2008, he worked in various leadership roles at Boehringer Ingelheim, Germany after having started his career with Glaxo Wellcome/GSK, UK in 1997.
|
|
Camilla Sylvest
Executive vice president, Commercial Strategy & Corporate Affairs
Ms Sylvest joined Novo Nordisk in 1996 as a trainee. From 1997 to 2008 Ms Sylvest had roles in headquarters and regions within pricing, health economics, marketing and sales effectiveness. In 2003, she was appointed vice president of sales and marketing effectiveness in Region Europe. From 2008 to 2015 Ms Sylvest headed up subsidiaries and business areas of growing size and complexity in Europe and Asia and in 2013 she was also appointed corporate vice president. In August 2015 Ms Sylvest was appointed senior vice president and general manager of Novo Nordisk’s Region China. In October 2017, Ms Sylvest was promoted to executive vice president for Commercial Strategy & Corporate Affairs.
|
|
|
Henrik Wulff
Executive vice president, Product Supply, Quality & IT
Mr Wulff joined Novo Nordisk in 1998 in the logistic and planning function. From 2001 to 2008 he held different managerial roles within Novo Nordisk’s manufacturing organization, Product Supply, before being appointed senior vice president of Diabetes API in Product Supply, Denmark. In 2012 Mr Wulff was appointed senior vice president of the worldwide division Diabetes Finished Products. In 2013 he was promoted senior vice president of Product Supply globally. In April 2015 Mr Wulff was promoted executive vice president and in 2019 his area of responsibility expanded to also cover Quality Assurance, Digital Data & IT.
|
|
Tania Sabroe
Executive vice president, People & Organisation
Ms Sabroe joined Novo Nordisk in 2007 as a project manager in Media Relations. Based out of Switzerland she held several positions in Novo Nordisk’s International Operations from 2013 to 2021 within both Media Relations, Communications and People & Organisation, including the position as corporate vice president of People & Organisation, Communication & Sustainability from 2018 to 2021. In January 2022 Ms Sabroe was appointed senior vice president of People & Organisation, Centres of Excellence & Services and in March 2023 she was promoted to executive vice president for People & Organisation.
Prior to joining Novo Nordisk in 2007, Ms Sabroe held a position as a communications manager at NHS National Services Scotland, UK.
|
|
David Moore
Executive vice president, U.S. Operations & Business Development
Mr Moore first joined Novo Nordisk in 2017 as senior vice president of Marketing and later senior vice president of Commercial, both at Novo Nordisk in the U.S., until leaving the company in 2019. Mr Moore re-joined Novo Nordisk in September 2022 as senior vice president for Corporate Development and in March 2023 he was promoted to executive vice president for Corporate Development. Effective January 1, 2025, David Moore also assumed the position of executive vice president of U.S. Operations & Business Development.
Prior to joining Novo Nordisk in 2017, Mr Moore held various commercial and executive roles with Johnson & Johnson, Tranzyme Pharma, Ocera Therapeutics and Cempra Pharmaceuticals. From 2019 to 2022 Mr Moore first served as CEO of the infectious disease business at Roivant Sciences, followed by being investment partner with Gurnet Point Capital.
|
Effective January 1, 2025, Doug Langa stepped aside from his role as executive vice president of North America Operations.
As Executive Management has become a global team, all executives based in Denmark apart from the CEO and CFO were deregistered from the Danish Business Authority as members of Executive Management, or registered managers, within the meaning of the Danish Companies Act, effective December 31, 2023, to align the registration practice and to treat all team members equally, regardless of where they are based.
Novo Nordisk has a two-tier management structure consisting of the Board of Directors and Executive Management. The Board of Directors and Executive Management are separate bodies and have no overlapping members.
The Board of Directors is responsible for the overall strategic management and supervision of Novo Nordisk’s affairs and supervises the work of Executive Management. Executive Management is responsible for the day-to-day management of the Company, development and implementation of strategies and policies, the Company’s operations and organization and timely reporting to the Board of Directors and Novo Nordisk’s stakeholders.
The key roles of members of the Board of Directors and members of Executive Management outside the Company are included in our Annual Report 2024 under the section ‘Management’ on pages 41-45.
16
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 6 DIRECTORS, EXECUTIVE MANAGEMENT AND EMPLOYEES |
There are no family relationships between the Board of Directors, Executive Management or between any of the members of the Board of Directors and any member of Executive Management. No director or member of Executive Management has been elected according to an arrangement or understanding with shareholders, customers, suppliers or others. As required by the Danish Companies Act, members of board of Directors are elected by the general meeting by a simple majority vote. Members of the Board of Directors elected by the general meeting are elected for a term of one year until the next annual general meeting and may be re-elected. In addition, four employee representatives are elected for a statutory four-year term by the employees of Novo Nordisk A/S.
B. COMPENSATION
For compensation data in respect of the members of the Company’s Board of Directors, reference is made to section 2.1 'Key developments in Board remuneration in 2024', section 2.2 'Remuneration composition', section 2.4 'Board and committee fee levels 2024' and section 2.5 'Board remuneration 2024' in our Remuneration Report 2024.
For compensation data in respect of the members of the Company’s Executive Management, reference is made to section 3.1 'Key developments in executive remuneration 2024', section 3.2 'Remuneration composition', section 3.4 'Executive remuneration in 2024’, section 3.5 'Short-term incentive programme 2024', section 3.6 'Long-term incentive programme design', section 3.7 'Long-term incentive programme 2022' and section 3.8 'Long-term incentive programmes 2023 and 2024' in our Remuneration Report 2024 and Note 5.1 'Share-based payment schemes' in the consolidated financial statements in our Annual Report 2024.
C. BOARD PRACTICES
The year of election and term for each member of the Board of Directors is included on pages 42-43 of our Annual Report 2024. The year of appointment for each member of Executive Management is included in Item 6A.
The Audit Committee
The Audit Committee mainly assists the Board of Directors with the oversight of: external auditors; the internal audit function; handling complaints reported through the Compliance Hotline (the Company's whistleblower complaint system); financial and sustainability reporting (environmental, social and governance); enterprise risk management system and financial counterpart exposure; internal controls over financial and ESG reporting; business ethics compliance; information security; insurance coverage and special theme reviews.
Under Danish law, the statutory external auditor is elected by the general meeting. All shareholders as well as the Board of Directors have the right to propose external auditor candidates for election. The Audit Committee recommends to the Board of Directors the statutory external auditor to be nominated by the Board of Directors and elected by the shareholders at the annual general meeting.
As part of its oversight of external reporting, the Audit Committee perform assessments of the risk exposure of Novo Nordisk, including the impact on the financial and sustainability processes and accounting for material legal and tax issues. The Audit Committee has quarterly discussions with the chief financial officer, head of finance and compliance, the general counsel, head of group internal audit and the external auditor. The chief financial officer is charged with responsibility for the tax strategy and policy, which is endorsed by the Board of Directors.
The Audit Committee consists of five members elected by and from the Board of Directors. One member of the Audit Committee is designated as chair and one member is an employee-elected member of the Board of Directors.
In March 2024, the Board of Directors elected the following members to the Audit Committee: Laurence Debroux (member since 2019 and chair since 2021, independent), Sylvie Grégoire (member since 2015, independent), Mette Bøjer Jensen (member since 2022, employee-elected member of the Board of Directors, not independent but relies on an exemption, reference is made to item 16D in this Form 20-F), Christina Law (member since 2022, independent) and Henrik Poulsen (member since 2021, not independent but relies on an exemption, reference is made to item 16D in this Form 20-F).
The Remuneration Committee
The Remuneration Committee assists the Board of Directors with the preparation and/or oversight of: the Remuneration Policy for the members of the Board of Directors and Executive Management; the remuneration of the members of the Board of Directors and its committees; the remuneration and employment terms of Executive Management; the Remuneration Report and other reporting.
The Remuneration Committee has four members elected by and from the Board of Directors. One member of the Remuneration Committee is designated as chair and one member is an employee-elected member of the Board of Directors.
In March 2024, the Board of Directors elected the following members to the Remuneration Committee: Henrik Poulsen (member since 2022 and chair since 2023, not independent), Elisabeth Dahl Christensen (member since 2022, employee-elected member of the
17
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 6 DIRECTORS, EXECUTIVE MANAGEMENT AND EMPLOYEES |
Board of Directors, not independent), Laurence Debroux (member since 2021, independent) and Martin Mackay (member since 2021, independent).
Directors’ service contracts
Reference is made to the section ‘Corporate Governance’, page 16 of our Annual Report 2024 for the description of the termination payments for Executive Management.
D. EMPLOYEES
Reference is made to Note 2.4 'Employee costs' in the consolidated financial statements in our Annual Report 2024 regarding the total number of full-time employees in Novo Nordisk at year-end for the years 2024–2022. Employees outside Denmark as a percentage of the total number of employees for 2024 was 54% (2023: 55% and 2022: 58%).
Executive Management believes that the Company has a good relationship with its employees in general and with the labour unions of the Novo Nordisk employees.
E. SHARE OWNERSHIP
For information on the Board of Directors and Executive Management members' individual holdings of shares and restricted stock units, including shares and restricted stock units granted in the year ended December 31, 2024 and trading in shares by the Board of Directors and Executive Management in the same period, reference is made to section 2.6 'Shareholdings of Board Members' and section 3.9 'Shareholdings of Executive Management' in our Remuneration Report 2024 and Note 5.1 'Share-based payment schemes' in the consolidated financial statements in our Annual Report 2024. As of February 4, 2025, the members of the Board of Directors and Executive Management held 1,534,378 B shares, representing in the aggregate less than 1% of the beneficial ownership of the Company.
In the period from January 1, 2025 until February 4, 2025, no B shares were sold or purchased by the members of the Board of Directors or Executive Management. The internal rules on trading in Novo Nordisk securities by members of the Board of Directors and Executive Management only permit trading in the 15 calendar day period following each quarterly earnings announcement. For information on vested shares for Executive Management on February 5, 2025, reference is made to section 3.7 'Long-term incentive programme 2022’ in our Remuneration Report 2024.
For further information, reference is made to Note 5.1 'Share-based payment schemes' in the consolidated financial statements in our Annual Report 2024.
F. DISCLOSURE OF A REGISTRANT’S ACTION TO RECOVER ERRONEOUSLY AWARDED COMPENSATION
None.
ITEM 7 MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. MAJOR SHAREHOLDERS
For information on major shareholders reference is made to ‘Shares and capital structure’ on pages 36-37 of our Annual Report 2024.
Novo Nordisk Foundation (the 'Foundation') owns its shares in Novo Nordisk A/S through Novo Holdings A/S. The purpose of Novo Holdings A/S is to administer the Foundation's portfolio of securities and minority capital interests and to administer and vote on the A shares and B shares in Novo Nordisk A/S, thereby securing a satisfactory financial return for Novo Holdings A/S' sole shareholder, the Foundation.
Under the Foundation’s statutes, the Foundation is governed by a board of directors, which must be comprised of six to twelve members (of whom at least two members must have a medical or scientific background, and at least one of these two members must have a medical background). Members of the Foundation’s board of directors are typically nominated by the Foundation’s nomination committee and elected by a two-thirds vote of the board members who have themselves been previously elected pursuant to the Foundation’s statutes. Any board member can be removed as provided for in the Danish Act on Foundations (‘lov om erhvervsdrivende fonde’). In addition, employee-elected board members are elected for a statutory four-year term by the employees of the Foundation and of the subsidiaries of the Foundation. No person or entity exercises any kind of formal influence over the Foundation’s board. The Foundation’s board currently consists of ten persons.
Under Novo Holdings A/S’ statutes, Novo Holdings A/S is governed by a board of directors, which must be comprised of three to nine members elected annually by the shareholders. According to the Foundation’s statutes, its board can and shall provide for members of its own board of directors to be elected to Novo Holdings A/S’ board of directors. Novo Holdings A/S’ board of directors is currently
18
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 7 MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS |
comprised of nine members, two of whom are also members of the Foundation’s board of directors (Steen Risgaard and Lars Rebien Sørensen) and one of whom is also member of the board of directors of Novo Nordisk A/S (Henrik Poulsen). Moreover, the chief executive officer of Novo Holdings A/S (Kasim Kutay) is also a member of the Board of Directors of Novo Nordisk A/S. The chair of the Foundation’s board of directors (Lars Rebien Sørensen) serves as the chair of Novo Holdings A/S’ board of directors.
The A shares in Novo Nordisk A/S held by Novo Holdings A/S cannot be sold or be subject to any disposition so long as the Foundation exists. The dissolution of the Foundation or any change in its objectives requires a unanimous vote of the Foundation’s board of directors. Other changes in the Foundation’s statutes require approval of two-thirds of the Foundation’s board members and approval by the Danish foundation authorities. According to its statutes, the Foundation is required to maintain material influence over Novo Nordisk A/S and its majority vote in Novo Holdings A/S.
For further information reference is made to ‘Shares and capital structure’ on pages 36-37 of our Annual Report 2024.
The B shares of Novo Nordisk A/S are registered with Euronext Securities (legal name: VP Securities A/S (‘Euronext) and are not represented by certificates. Generally, Euronext does not provide the Company with information with respect to registration. However, set forth below is information as of February 4, 2025 with respect to (a) any shareholder who is known to the Company to be the owner of more than 5% of any class of Novo Nordisk A/S' securities and (b) the total amount of any class owned by Novo Nordisk A/S and its subsidiaries (treasury shares) and by the Board of Directors and Executive Management as a group:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Title of class |
|
Identity of person or group |
|
Shares owned |
|
Percent of class |
|
Percent of total
votes |
|
|
|
|
|
|
|
|
|
| A shares |
|
Novo Holdings A/S |
|
1,074,872,000 |
|
|
100.00 |
|
|
76.02 |
|
| B shares |
|
Novo Holdings A/S |
|
177,560,500 |
|
|
5.24 |
|
|
1.26 |
|
| B shares |
|
Novo Nordisk A/S and subsidiaries (treasury shares) |
|
25,947,151 |
|
* |
0.77 |
|
|
0.18 |
|
| B shares |
|
Board of Directors and Executive Management |
|
1,534,378 |
|
|
0.05 |
|
|
0.01 |
|
*) Treasury shares are included, however, voting rights of treasury shares cannot be exercised.
For information on share repurchases under the Company's share repurchase program in 2022/2023 reference is made to Note 4.2 ‘Distribution to shareholders’ in the consolidated financial statements in our Annual Report 2024. Information on the 2024/2025 share repurchase program, reference is made to ‘Shares and capital structure’ on pages 36-37 of our Annual Report 2024.
There is no complete record of all shareholders, nor of U.S. shareholders, and therefore it is not possible to give an accurate breakdown of geographical distribution of share capital nor of the number of B shareholders by country of residence. Additionally, certain of our B shares are held by brokers or other nominees and, as a result, the number of holders of record is not representative of the number of beneficial holders or of the residence of such beneficial holders.
However, based on available sources of information, as of December 31, 2024 it is estimated that share capital (including A and B share capital) was geographically distributed in the following manner: 38% Denmark, 28% North America, 3% UK, and 31% Other.
Furthermore, JPMorgan Chase Bank, N.A., our ADR Depositary, has informed us that as of December 31, 2024 the total number of ADRs outstanding was 440,748,211 representing approximately 13.82% of the issued B share capital outstanding (excluding treasury shares and shares held by Novo Holdings A/S) as at that date. All of the Company’s ADRs are held of record by the Depositary. For more information regarding our ADRs, see Item 12D below.
B. RELATED PARTY TRANSACTIONS
Related parties include the Novo Nordisk Foundation, Novo Holdings A/S, Novonesis A/S, Innate Pharma SA, Xellia Pharmaceuticals ApS, Altasciences Group, Sonion A/S (due to shared controlling shareholder, Novo Holdings A/S) and NNIT A/S being an associated company with shared controlled shareholding between Novo Holdings A/S and Novo Nordisk A/S.
In 2024 Novo Nordisk acquired three fill-finish manufacturing sites from Novo Holdings A/S in connection with a transaction where Novo Holdings A/S acquired Catalent, Inc. The purchase price of the three sites totalled USD 11.7 billion, which was mainly debt-financed.
Related party transactions in 2024, 2023 and 2022 were primarily payments for services provided between the Novo Nordisk Group and the Novonesis Group, Xellia Pharmaceuticals ApS, Altasciences Group, Sonion A/S and transactions with associated companies. The overall financial impact of these related party transactions is limited.
19
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 8 FINANCIAL INFORMATION |
Being an associated company of Novo Nordisk A/S, Churchill Stateside Solar Fund XIV, LLC ('CS Solar Fund XIV') is considered a related party. Being an associated company of Novo Holdings A/S, Unchained Labs, Inc. is considered a related party to Novo Nordisk A/S.
Novo Nordisk A/S has access to certain assets of and can purchase certain services from Novo Holdings A/S and Novonesis A/S and vice versa. All agreements relating to such assets and services are based on the list prices used for sales to third parties where such list prices exist, or the price has been set at what is regarded as market price. The material terms of these agreements are renegotiated on a regular basis.
Since December 31, 2024, there have been no further significant transactions with related parties out of the ordinary course of business. For further information reference is made to Note 5.4 ‘Related party transactions’ in the consolidated financial statements in our Annual Report 2024.
C. INTERESTS OF EXPERTS AND COUNSEL
Not applicable.
ITEM 8 FINANCIAL INFORMATION
A. CONSOLIDATED STATEMENTS AND OTHER FINANCIAL INFORMATION
The financial statements required by this item accompany this annual report in the form of our Annual Report 2024 (filed as Exhibit 15.1 to this Form 20-F).
Legal proceedings
Reference is made to Note 3.5 ‘Provisions and contingent liabilities’ in the consolidated financial statements in our Annual Report 2024.
Dividends
Reference is made to ‘Shares and capital structure’, on pages 36-37 of our Annual Report 2024.
B. SIGNIFICANT CHANGES
No significant events have occurred since the date of the annual financial statements. For description of important events and achievements in 2024, reference is made to ‘Introducing Novo Nordisk’ on pages 4-10 and ‘2024 performance and 2025 outlook’ on pages 32-37 of our Annual Report 2024.
ITEM 9 THE OFFER AND LISTING
A. OFFER AND LISTING DETAILS
The Company's B shares are listed in Denmark on Nasdaq Copenhagen, and traded under the symbol "NOVO-B". The Company's ADRs are traded on the New York Stock Exchange (the "NYSE") under the symbol "NVO". See Exhibit 2.2 to this Form 20-F for a description of the B Shares.
B. PLAN OF DISTRIBUTION
Not applicable.
C. MARKETS
Reference is made to ‘Shares and capital structure’, on pages 36-37 of our Annual Report 2024.
D. SELLING SHAREHOLDERS
Not applicable.
E. DILUTION
Not applicable.
F. EXPENSES OF THE ISSUE
Not applicable.
20
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 10 ADDITIONAL INFORMATION |
ITEM 10 ADDITIONAL INFORMATION
A. SHARE CAPITAL
Not applicable.
B. MEMORANDUM AND ARTICLES OF ASSOCIATION
See Exhibit 2.2. to this Form 20-F for a summary of certain material provisions of Novo Nordisk A/S’ Articles of Association, certain other constitutive documents and relevant Danish corporate law. See Exhibit 1.1 to this Form 20-F for a translation into English language of the Articles of Association.
C. MATERIAL CONTRACTS
There have been no material contracts outside the ordinary course of business.
D. EXCHANGE CONTROLS
Other than the Danish rules on screening of certain foreign direct investments ("FDI"), etc. in Denmark (the "Danish FDI Rules") and applicable international trade and financial sanctions as outlined below, (i) there are no governmental laws, decrees, or regulations in Denmark (including, but not limited to, foreign exchange controls) that restrict the export or import of capital, or that affect the remittance of dividends, interest or other payments to non-resident holders of the B shares or the ADRs, and (ii) there are no limitations on the right of non-resident or foreign owners to hold or vote the B shares or the ADRs imposed by the laws of Denmark or the Articles of Association of the Company.
Under the Danish FDI Rules, a screening mechanism applies to foreign direct investments in certain sensitive sectors, if the foreign investor obtains at least 10% ownership or voting rights, or equivalent control by other means. Among such sensitive sectors are companies and entities within critical infrastructure in Denmark that are necessary to maintain or restore the production, registration, distribution, and monitoring of prescription drugs. If a contemplated foreign direct investment in Novo Nordisk A/S is considered to fall within the scope of the mandatory screening mechanism, the foreign investor is required to apply for prior authorization with the Danish Business Authority. FDI filings, notifications or approvals may under certain circumstances also be required in non-Danish jurisdictions.
If a foreign investor fails to comply with the Danish FDI Rules, the Danish Business Authority may impose restrictions, inter alia, ordering to reverse the investment or to suspend the foreign investor's voting rights.
International trade and financial sanctions are continually evolving. If applicable, such international trade and financial sanctions may under certain circumstances prevent the possibility of export and import of capital, and affect the remittance of dividends, interests and other payments to the non-resident holders of the B shares or the ADRs. In addition, international trade and financial sanctions may also restrict the right of non-resident or foreign owners to acquire, transfer, hold or vote the B shares and ADRs. Failure to comply with international trade and financial sanctions can lead to criminal and civil liability.
E. TAXATION
Danish Taxation
The following summary outlines certain Danish tax consequences to U.S. Holders (as defined below):
Withholding Tax
Generally, Danish withholding tax is deducted from dividend payments to U.S. Holders at a 27% rate, the rate generally applicable to non-residents in Denmark without regard to eligibility for a reduced treaty rate. Under the current Convention between the Government of the United States of America and the Government of the Kingdom of Denmark for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to Taxes on Income (the ‘Current Convention’), the maximum rate of Danish tax that may be imposed on a dividend paid to a U.S. Holder that does not have a ‘permanent establishment’ (as defined therein) in Denmark is generally 15% and, for certain pension funds, 0% (each, the ‘Treaty Rate’). U.S. Holders eligible for the Treaty Rate may apply to the Danish tax authorities to obtain a refund to the extent that the amount withheld reflects a rate in excess of the Treaty Rate (any such amount, the ‘Excess Withholding Tax’).
Any U.S. Holders of ADRs wishing to apply for a refund of Excess Withholding Tax will have to provide a Danish Claim for Refund of Danish Dividend Tax, a properly completed U.S. Internal Revenue Service Form 6166 and additional documentation including: proof of dividend received; proof of ownership of the ADR and eligibility for the dividend received and proof that the dividend received was reduced by an amount corresponding to the Danish withholding tax. These documentation requirements may be expanded and may
21
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 10 ADDITIONAL INFORMATION |
be subject to change. Refund claims must be filed within the three-year period following the date in which the dividend was paid in Denmark.
Information on tax reclaims, how they should be filed and the requisite tax forms may be obtained from:
JPMorgan Chase Bank, N.A.
c/o Globe Tax Services, Inc.
1 New York Plaza, 34th Floor
New York, New York 10004 USA
Phone: +1 (212) 747 9100
U.S. Holders should consult their tax advisers regarding dividend withholding tax refunds.
Sale or Exchange of ADRs or B Shares
Any gain or loss realized on the sale or other disposition of ADRs or B shares by a U.S. Holder that is not either a resident of Denmark or a corporation that is doing business in Denmark is not subject to Danish taxation. In addition, any non-resident of Denmark may remove from Denmark any convertible currency representing the proceeds of the sales of ADRs or B shares in Denmark.
U.S. Taxation
The following summary outlines certain U.S. federal income tax consequences for U.S. Holders (defined below) of owning and disposing of ADRs or B shares. A ‘U.S. Holder’ is a person that, for U.S. federal income tax purposes, is a beneficial owner of ADRs or B shares that is eligible for the benefits of the Current Convention and is (i) a citizen or individual resident of the United States, (ii) a corporation, or other entity taxable as a corporation, created or organized in or under the laws of the United States or any state therein or the District of Columbia, or (iii) an estate or trust the income of which is subject to U.S. federal income taxation regardless of its source. This discussion applies only to a U.S. Holder that holds ADRs or B shares as capital assets for U.S. tax purposes and does not apply to persons that own or are deemed to own ADRs or common shares representing 10% or more of the voting power or value of Novo Nordisk. In addition, this discussion does not describe all of the tax consequences or potentially different tax consequences that may be relevant in light of the U.S. Holder’s particular circumstances, including tax consequences applicable to U.S. Holders subject to special rules, such as certain financial institutions, entities classified as partnerships for U.S. federal income tax purposes, persons subject to the provisions of the U.S. Internal Revenue Code and Treasury regulations thereunder commonly known as the Medicare contribution tax, persons subject to any minimum tax, or persons holding ADRs or B shares in connection with a trade or business conducted outside of the United States. This discussion is based, in part, on certain representations by the Depositary and assumes that each obligation under the deposit agreement will be performed in accordance with its terms. This discussion assumes that the Company is not, and will not become, a passive foreign investment company for U.S. federal income tax purposes.
For U.S. federal income tax purposes, the holders of ADRs will be treated as the beneficial owners of the underlying B shares. Accordingly, no gain or loss for U.S. federal income tax purposes will be recognized if a U.S. Holder exchanges ADRs for the underlying B shares represented by those ADRs or B shares for ADRs.
Taxation of Distributions
For U.S. federal income tax purposes, the gross amount of distributions on ADRs or B shares received by U.S. Holders, before reduction for any Danish tax withheld, generally will be included in the U.S. Holder’s income as foreign-source dividend income and will not be eligible for the dividends-received deduction generally available to U.S. corporations. The amount of any dividend income paid in Danish kroner will be the U.S. dollar amount calculated by reference to the exchange rate in effect on the date of the U.S. Holder’s, or, in the case of ADRs, the Depositary’s receipt of the dividend regardless of whether the payment is in fact converted into U.S. dollars at that time. If the dividend is converted into U.S. dollars on the date of receipt, a U.S. Holder should not be required to recognize foreign currency gain or loss in respect of the dividend income. A U.S. Holder may have foreign currency gain or loss if the dividend is converted into U.S. dollars after the date of receipt. U.S. Holders that receive a refund of Danish withholding tax after the dividend is received, as discussed above under the section ‘Danish Taxation Withholding Tax,’ may be required to recognize foreign currency gain or loss with respect to the amount of the refund. U.S. Holders should consult their tax advisers regarding whether any foreign currency gain or loss should be recognized in connection with distributions on ADRs or B shares.
Subject to applicable limitations and conditions under U.S. federal income tax law, dividends paid to certain non-corporate U.S. Holders may be taxable at favorable rates. In order to be eligible for the favorable rates, a non-corporate U.S. Holder must fulfill certain holding period and other requirements.
Subject to applicable limitations under U.S. federal income tax law, a U.S. Holder may be eligible to credit against its U.S. federal income tax liability Danish taxes withheld from dividends on ADRs or B shares at a rate not exceeding the applicable rate under the
22
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 10 ADDITIONAL INFORMATION |
Current Convention. Danish taxes withheld in excess of the applicable rate under the Current Convention will not be eligible for credit against a U.S. Holder’s federal income tax liability. The rules governing foreign tax credits are complex and, therefore, U.S. Holders should consult their tax advisers regarding the availability of foreign tax credits in their particular circumstances. Alternatively, subject to applicable limitations, U.S. Holders may elect to deduct Danish taxes withheld from dividend payments. An election to deduct non-U.S. taxes instead of claiming a foreign tax credit applies to all otherwise creditable non-U.S. taxes paid or accrued in the taxable year.
Sale or Exchange of ADRs or B Shares
A U.S. Holder will recognize capital gain or loss for U.S. federal income tax purposes on a sale or other disposition of ADRs or B shares, which will be long-term capital gain or loss if the U.S. Holder held the ADRs or B shares for more than one year. The amount of the gain or loss will equal the difference between the U.S. Holder’s tax basis in the ADRs or B shares disposed of and the amount realized on the disposition, in each case as determined in U.S. dollars. Such gain or loss will generally be U.S. source gain or loss for foreign tax credit purposes.
Information Reporting and Backup Withholding
Payments of dividends and sales proceeds that are made within the United States or through certain U.S. related financial intermediaries may be subject to information reporting and backup withholding, unless (i) the U.S. Holder is a corporation or other exempt recipient or (ii) in the case of backup withholding, the U.S. Holder provides a correct taxpayer identification number and certifies that it is not subject to backup withholding.
The amount of any backup withholding from a payment to a U.S. Holder will be allowed as a credit against the holder’s U.S. federal income tax liability and may entitle it to a refund, provided that the required information is timely furnished to the Internal Revenue Service.
Certain U.S. Holders who are individuals (and certain specified entities) may be required to report information relating to securities issued by a non-U.S. person or non U.S. accounts through which such securities are held, subject to certain exceptions (including an exception for securities held in accounts maintained by U.S. financial institutions). U.S. Holders should consult their tax advisers regarding their possible reporting obligations with respect to the ADRs or B shares.
The foregoing sections offer a general description and U.S. Holders should consult their tax advisers to determine the U.S. federal, state, local and non-U.S. tax consequences of owning and disposing of ADRs or B shares in their particular circumstances.
F. DIVIDENDS AND PAYING AGENTS
Not applicable.
G. STATEMENTS BY EXPERTS
Not applicable.
H. DOCUMENTS ON DISPLAY
Documents referred to and filed with the SEC together with this Form 20-F can be read and copied at the SEC’s public reference room located at 100 F Street, NE, Washington, DC 20549. Please call the United States Securities and Exchange Commission at 1-800-SEC-0330 for further information on the public reference rooms.
Copies of this Form 20-F as well as our Annual Report 2024, Annual Report 2023 and Remuneration Report 2024 can be downloaded from the investors page at novonordisk.com. The contents of this website are not incorporated by reference into this Form 20-F. This Form 20-F is also filed and can be viewed via EDGAR on www.sec.gov.
I. SUBSIDIARY INFORMATION
Not applicable.
ITEM 11 QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK
Financial exposure and financial risk management
For a description and discussion of the Company’s foreign exchange risk management, interest rate risk management, liquidity risk management and credit risk management, reference is made to Note 4.4 ‘Financial risks’ in the consolidated financial statements and the section ‘Risks’ on pages 38-40 of our Annual Report 2024.
23
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 12 DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
Sensitivity analysis
When conducting a sensitivity analysis, the Group assesses the change in fair value on the market-sensitive instruments following hypothetical changes in market rates and prices. The rates used to mark-to-market the instruments are market data as of December 31, 2024.
Interest rate sensitivity analysis
For information on Interest rate sensitivity analysis in the financial year of 2024, reference is made to Note 4.4 ‘Financial risks’ in the consolidated financial statements in our Annual Report 2024.
Foreign exchange sensitivity analysis
For information on Foreign exchange sensitivity analysis in the financial year of 2024, reference is made to Note 4.4 ‘Financial risks’ in the consolidated financial statements and the section ‘Risks’ on pages 38-40 of our Annual Report 2024.
ITEM 12 DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. DEBT SECURITIES
Not applicable.
B. WARRANTS AND RIGHTS
Not applicable.
C. OTHER SECURITIES
Not applicable.
D. AMERICAN DEPOSITARY SHARES
Novo Nordisk’s ADR program is administered by J.P. Morgan Depositary Receipts Group as Depositary, JPMorgan Chase Bank, N.A., 383 Madison Avenue, Floor 11, New York, United States. The ADRs are traded under the symbol "NVO" on the New York Stock Exchange and the underlying security is the Novo Nordisk B share, NOVO-B on Nasdaq Copenhagen. Each ADR represents one deposited Novo Nordisk B share. One ADR carries the same voting rights as one Novo Nordisk B share.
The Depositary distributes relevant notices, reports and proxy materials to the holders of the ADRs. When dividends are paid to shareholders, the Depositary converts the amounts into U.S. dollars and distributes the dividends to the holders of the ADRs. See Exhibit 2.1. to this Form 20-F for a description of the rights of holders of the ADRs.
The holder of an ADR may have to pay the following fees and charges related to services in connection with the ownership of the ADR up to the amounts set forth in the table below.
|
|
|
|
|
|
|
|
|
| Service |
|
Fee |
| Issuance or delivery of an ADR, surrendering of an ADR for delivery of a Novo Nordisk B share, cancellation of an ADR, including issuance, delivery, surrendering or cancellation in connection with share distributions, stock splits, rights and mergers |
|
A maximum of USD 5.00 for each 100 ADRs (or portion thereof), to be paid to the Depositary |
|
|
|
| Distribution of dividend to the holder of the ADR |
|
A maximum of USD 0.05 per ADR (or portion thereof), to be paid to the Depositary |
|
|
|
| Transfer of the Novo Nordisk B shares from the Danish custodian bank to the holder of the ADR’s account in Denmark |
|
USD 20.00 cabling fee per transfer, to be paid to the Depositary |
|
|
|
| Taxes and other governmental charges the holder of the ADR has to pay on any ADR or share underlying the ADR |
|
As necessary |
For the calendar year 2024, Novo Nordisk received a payment of USD 13,575,585 under the terms of its revenue sharing arrangement with JPMorgan Chase Bank, N.A.
24
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 13 DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES |
PART II
ITEM 13 DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
ITEM 14 MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
None
ITEM 15 CONTROLS AND PROCEDURES
Evaluation of disclosure controls and procedures
Novo Nordisk maintains disclosure controls and procedures that are designed to ensure that information required to be disclosed in reports that Novo Nordisk files or submits under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported, within the time periods specified in the rules and forms of the United States Securities and Exchange Commission (the "SEC"), and that such information is accumulated and communicated to Management of the Company, including the chief executive officer and chief financial officer, as appropriate to allow timely decisions regarding required disclosure.
Novo Nordisk Management, including the chief executive officer and chief financial officer, evaluated the Company’s disclosure controls and procedures as of December 31, 2024. Based on this evaluation, the Company’s chief executive officer and chief financial officer concluded that as of December 31, 2024, the Company’s disclosure controls and procedures were effective at the reasonable assurance level.
In designing and evaluating the disclosure controls and procedures, Management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives.
Report of Novo Nordisk Management on Internal Control over Financial Reporting
Novo Nordisk’s Management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed by, or under the supervision of, the chief executive officer and chief financial officer, and effected by the Company’s Board of Directors, Management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS Accounting Standards as issued by the International Accounting Standards Board.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Novo Nordisk Management, including the chief executive officer and chief financial officer, assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2024, using the criteria established in the Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (‘COSO’). Based on this assessment, Novo Nordisk Management, including the chief executive officer and chief financial officer, concluded that, as of December 31, 2024, the Novo Nordisk Group’s internal control over financial reporting was effective based on criteria stated in Internal Control – Integrated Framework (2013) issued by the COSO.
The Company’s 2024 acquisition of three former Catalent, Inc. fill-finish sites has been excluded from the scope of management’s assessment and conclusion on internal control over financial reporting as of December 31, 2024, as the acquisition was completed on December 18, 2024. The acquisition is included in the 2024 consolidated financial statements in our Annual Report 2024 and in the aggregate represents 17% of total assets as of December 31, 2024, and less than 1% of net profit for the year ended December 31, 2024.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2024 has been audited by Deloitte, Statsautoriseret Revisionspartnerselskab, Denmark, an independent registered public accounting firm, as stated in their report which appears on pages 33-35 of this Form 20-F.
25
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 16A AUDIT COMMITTEE FINANCIAL EXPERTS |
Changes in internal controls over financial reporting
There were no changes in the Company’s internal control over financial reporting that occurred during the year ended December 31, 2024 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
ITEM 16A AUDIT COMMITTEE FINANCIAL EXPERT
The Audit Committee is comprised of five members elected by the Board of Directors. One member is designated as chair, and two members, Laurence Debroux (the chair) and Henrik Poulsen, are designated as Audit Committee financial experts as defined by the SEC.
Three members qualify as independent as defined by the SEC and two members rely on an exemption. See item 16D below. The chair, Laurence Debroux is independent as defined by the SEC.
Reference is made to pages 42-44 of our Annual Report 2024 for the name, position and experience for the members of the Audit Committee.
ITEM 16B CODE OF ETHICS
Novo Nordisk has a vision and a set of essentials named the Novo Nordisk Way. The Novo Nordisk Way describes who Novo Nordisk as a company is, where Novo Nordisk wants to go and how its employees work. The Novo Nordisk Way is principle-based and describes corporate essentials and the required values and mindset of employees on business conduct and ethics including a number of the topics required by the Sarbanes–Oxley Act and the NYSE Listed Company Manual. In addition to the Novo Nordisk Way, a number of guidelines are in place including OneCode, which serves as a single resource for the principles that guide how Novo Nordisk operates, including business ethics. The Novo Nordisk Way and OneCode apply to all employees of Novo Nordisk including the chief executive officer and chief financial officer, as well as the Board of Directors.
The Novo Nordisk Way and OneCode may be found on our website at novonordisk.com (the contents of the website are not incorporated by reference into this Form 20-F).
ITEM 16C PRINCIPAL ACCOUNTANT FEES AND SERVICES
Reference is made to Note 5.5 ‘Fee to statutory auditors’ in the consolidated financial statements in our Annual Report 2024 regarding fees paid to our statutory auditors.
The audit opinion of Deloitte Statsautoriseret Revisionspartnerselskab (PCAOB no. 1294) is included in Item 18.
Statutory Audit Fees
Statutory audit fees consist of fees incurred for the annual audit of the Company’s Annual Report, the financial statements of the Parent Company, Novo Nordisk A/S, and financial statements of wholly-owned subsidiaries including audit of internal controls over financial reporting (Sarbanes–Oxley Act, Section 404). Also included are services that can only be provided by our auditor, such as audit services required for regulatory filings.
Audit-Related Fees
Fees for audit-related services consist of fees incurred for assurance and related services provided by the independent auditor but not restricted to those that can only be provided by the auditor signing the audit report. This includes, amongst others, the assurance provided on the Company’s Sustainability statement included in the Annual Report 2024 and also includes work concerning interpretation of financial accounting reporting standards.
Tax Fees
Fees for tax advisory services include fees incurred for tax compliance services, tax consultations and assistance in connection with tax audits and appeals and transfer pricing.
Other Fees
Fees for other services includes consultancy services pertaining to digital initiatives within Novo Nordisk’s Development function, consultancy costs associated with IT cost controlling in the Product Supply organization and other permissible services not included in the categories above.
26
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 16D EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES |
Pre-approval policies
The Audit Committee assesses and pre-approves all audit and non-audit services provided by the statutory auditors. The pre-approval includes the type of service and a fee budget. Furthermore, the Audit Committee receives a quarterly update on actual services provided and fees realized.
ITEM 16D EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Novo Nordisk’s ADRs are listed on the New York Stock Exchange, the corporate governance rules of which require a foreign private issuer such as Novo Nordisk to have an Audit Committee that satisfies the requirements of Rule 10A-3 under the U.S. Securities Exchange Act of 1934, as amended. These requirements include a requirement that the Audit Committee be composed of members that are “independent” of the issuer, as defined in the Rule, subject to certain exemptions.
Of the current five members of Novo Nordisk’s Audit Committee, three are considered independent, including the chair Laurence Debroux, and two members rely on an exemption.
Henrik Poulsen is a member of the Board of Directors of the controlling shareholder Novo Holdings A/S. Accordingly, his service on the Audit Committee is permissible pursuant to the exemption from the independence requirements provided for by paragraph (b)(1)(iv)(B) of Rule 10A-3.
Mette Bøjer Jensen is a current employee of Novo Nordisk A/S who has been elected to the Board of Directors by the employees pursuant to the Danish Companies Act (in Danish: "Selskabsloven"). The Danish Companies Act requires any limited liability company with more than 35 employees on average over a three-year period to organize a vote in which the employees are entitled to decide whether they would like employee representation on the Board of Directors. Mette Bøjer Jensen is not an executive officer of Novo Nordisk. Accordingly, her service on the Audit Committee is permissible pursuant to the exemption from the independence requirements provided for by paragraph (b)(1)(iv)(C) of Rule 10A-3.
Novo Nordisk does not believe the reliance on such exemptions would materially adversely affect the ability of the Audit Committee to act independently and to satisfy the other requirements of the Rule 10A-3.
ITEM 16E PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Number
of Shares
Purchased
(a)* |
|
Average Price
Paid per Share
in DKK (b) |
|
Total Number
of Shares Purchased
as Part of Publicly
Announced Plans or
Programs
(c) |
|
Maximum Approximate
Value of Shares that may yet be purchased under the Plans or Programs in DKK (d) |
| 2023 repurchase program |
|
|
|
|
|
|
|
|
| Status year end 2023** |
|
47,815,778 |
|
|
594.60 |
|
|
47,815,778 |
|
|
1,568,754,498 |
|
| January 1-31, 2024 |
|
2,166,000 |
|
|
724.26 |
|
|
49,981,778 |
|
|
8,789 |
|
| Total*** |
|
49,981,778 |
|
|
600.22 |
|
|
49,981,778 |
|
|
8,789 |
|
|
|
|
|
|
|
|
|
|
| 2024 repurchase program** |
|
|
|
|
|
|
|
20,000,000,000 |
|
| February 1-29, 2024 |
|
781,000 |
|
|
836.86 |
|
|
781,000 |
|
|
19,346,410,086 |
|
| March 1-31, 2024 |
|
769,500 |
|
|
890.99 |
|
|
1,550,500 |
|
|
18,660,790,574 |
|
| April 1-30, 2024 |
|
872,500 |
|
|
871.89 |
|
|
2,423,000 |
|
|
17,900,066,453 |
|
| May 1-31, 2024 |
|
6,943,795 |
|
|
864.90 |
|
|
9,366,795 |
|
|
11,894,350,842 |
|
| June 1-30, 2024 |
|
694,830 |
|
|
982.57 |
|
|
10,061,625 |
|
|
11,211,629,830 |
|
| July 1-31, 2024 |
|
882,493 |
|
|
941.45 |
|
|
10,944,118 |
|
|
10,380,808,841 |
|
| August 1-31, 2024 |
|
867,195 |
|
|
899.87 |
|
|
11,811,313 |
|
|
9,600,449,351 |
|
| September 1-30, 2024 |
|
901,871 |
|
|
881.35 |
|
|
12,713,184 |
|
|
8,805,584,138 |
|
| October 1-31, 2024 |
|
1,078,964 |
|
|
794.27 |
|
|
13,792,148 |
|
|
7,948,597,474 |
|
| November 1-30, 2024 |
|
7,634,956 |
|
|
748.90 |
|
|
21,427,104 |
|
|
2,230,780,156 |
|
| December 1-31, 2024 |
|
1,087,870 |
|
|
774.82 |
|
|
22,514,974 |
|
|
1,387,874,200 |
|
| Total |
|
22,514,974 |
|
|
826.66 |
|
|
22,514,974 |
|
|
1,387,874,200 |
|
*) All shares purchased through a publicly announced program.
**) Shares purchased under 2023 repurchase program during 2023.
***) As of January 31, 2024, Novo Nordisk had since February 1, 2023, repurchased a total of 49,981,778 B shares equal to a transaction value of DKK 30 billion. The DKK 30 billion share repurchase program first announced as DKK 28 billion on February 1, 2023, and later increased to DKK 30 billion in total, was thereby concluded.
27
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 16F CHANGE IN REGISTRANT'S CERTIFYING ACCOUNTANT |
Note to column (a) and (d)
The Board of Directors has been authorized by the annual general meeting to have the Company acquire up to 10% of the share capital at the price quoted at the time of the purchase with a deviation of up to 10%. This authorization is renewed annually at the annual general meeting. If the limit of 10% is reached, a number of shares would have to be cancelled before further purchases can be made. The cancellation of shares must be approved by the shareholders.
Under this authorization, a share repurchase program for 2023 of DKK 30 billion initiated in February 2023, was completed in January 2024. A new share repurchase program for 2024 of DKK 20 billion initiated in February 2024 was completed in January 2025. The shares have been purchased through a bank directly in the market or directly from Novo Holding A/S.
Column (a) shows shares Novo Nordisk purchased as part of our share repurchase program initiated in February 2023 (completed in January 2024) and our share repurchase program initiated in February 2024.
Notes to columns (c) and (d)
In order to maintain capital structure flexibility, the Board of Directors intends to propose at the annual general meeting on March 27, 2025, a reduction in the B share capital, by cancellation of 45 million shares (nominal value DKK 0.10) of current treasury B shares, to DKK 339,012,800. This would correspond to a 1.3% reduction of the total share capital.
ITEM 16F CHANGE IN REGISTRANT'S CERTIFYING ACCOUNTANT
Not applicable.
ITEM 16G CORPORATE GOVERNANCE
Novo Nordisk A/S is a public limited company incorporated under the laws of Denmark. Novo Nordisk’s B-shares are admitted to trading and listing on Nasdaq Copenhagen A/S. Novo Nordisk A/S is therefore subject to the Danish Corporate Governance Recommendations issued by the Danish Committee on Corporate Governance in December 2020, which are implemented by Nasdaq Copenhagen A/S in the Nordic Main Market Rulebook for Issuer of Shares.
Further, Novo Nordisk A/S has ADRs listed on NYSE and is therefore required to comply with certain U.S. securities laws and regulations, including the Sarbanes-Oxley Act, and the NYSE Corporate Governance Standards (the “NYSE Standards”) applicable to listed companies as described in the NYSE Listed Company Manual’s Section 303A. As a Foreign Private Issuer ("FPI"), Novo Nordisk A/S is permitted to follow the corporate governance practice of its home country in lieu of certain provisions of the NYSE Standards.
Novo Nordisk A/S complies with the requirements of the SEC and NYSE except that Novo Nordisk, pursuant to section 303A.00 of the NYSE Listed Company Manual, is not obliged to comply with Sections 303A.01 (majority independent directors), 303A.04 (nominating/corporate governance committee) and 303A.05 (compensation committee) of the NYSE Listed Company Manual because Novo Nordisk A/S is a “controlled company” (a listed company of which more than 50% of the voting power for the election of directors is held by an individual, a group or another company).
Moreover, Novo Nordisk A/S as a foreign private issuer is permitted to follow home country practice in lieu of sections 303A.02 (independence tests), 303A.03 (executive sessions), 303A.07 (audit committee), 303A.08 (shareholder approval of equity compensation plans), 303A.09 (corporate governance guidelines), 303A.10 (code of business conduct and ethics) and 303A.12 (a) (certification requirements).
Below is a list of practices followed by Novo Nordisk A/S as a foreign private issuer that differ from certain corporate governance requirements under the NYSE Standards:
Independence requirements
Under the NYSE Standards, listed companies must have at least a majority of independent directors and no director qualifies as “independent” unless the Board of Directors affirmatively determines that the director has no material relationship with the listed company (either directly or as a partner, shareholder or officer of an organization that has a relationship with the Company).
Under the Danish Corporate Governance Recommendations, at least half of the shareholder-elected members of the Board of Directors, i.e. excluding any employee-elected members of the Board of Directors, should be independent. Employees are entitled to be represented by half of the total number of the shareholder-elected members of the Board of Directors.
28
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 16G CORPORATE GOVERNANCE |
In accordance with the NYSE Standards, a director is not deemed independent if the director is, or has been within the last three years, an employee of the listed company, or an immediate family member is, or has been within the last three years, an executive officer, of the listed company. For the purposes of the independence standards, Section 303A.02 defines ‘listed company’ as including ‘any parent or subsidiary in a consolidated group with the listed company or such other company as is relevant to any determination under the independence standards set forth in this Section 303A.02(b)’.
In accordance with the requirements of the Danish Companies Act, four employees have been elected as members of the Board of Directors by the employees of Novo Nordisk A/S. In addition, one member of the Board of Directors serves as chief executive officer of Novo Holdings A/S. No other member of the Board of Directors or their immediate family members have within the last three years been an employee or executive of Novo Nordisk A/S or any parent or subsidiary in a consolidated group with Novo Nordisk A/S. In 2024, Novo Nordisk made a payment to Novo Holdings A/S, corresponding to a certain asset purchase, which exceeded the applicable amount under Section 303A.02(b)(v).
As permitted by the NYSE standards applicable to FPIs and in accordance with Danish law and practice, the Board of Directors generally determines whether its members qualify as independent under the Danish Corporate Governance Recommendations. The Board of Directors has also determined whether each member of the Audit Committee, qualifies as independent under Rule 10A-3 in the Securities Exchange Act. Such determination is disclosed in the Annual Report 2024. Further, the Annual Report 2024 provides detailed and individual information regarding the members of the Board of Directors, but it does not explicitly identify which Board members the Board of Directors considers independent under the NYSE Standards.
The Audit Committee
Under Section 303A.06 of the NYSE Standards, the Audit Committee of a listed company must be composed entirely of independent directors as set out in Section 303A.02 and, in the absence of an applicable exemption, Rule 10A-3(b)(1). The members of the Audit Committee are appointed at a Board meeting held immediately following the annual general meeting. The Audit Committee has five members, three of whom are considered independent under Rule 10A-3.
One Audit Committee member is a member of the board of directors of the controlling shareholder, Novo Holdings A/S and is exempt from the independence requirements provided for by paragraph (b)(1)(iv)(B) of Rule 10A-3 and one Audit Committee member is an employee-elected member of the Board of Directors and is exempt from the independence requirements provided for by paragraph (b)(1)(iv)(C) of Rule 10A-3. See Item 16D above for further details.
Further, the Audit Committee is, among other things, responsible for oversight of and reporting to the Board of Directors on the matters specified under the NYSE Standards, including those matters set out in paragraphs (b) (2), (3), (4) and (5) of Rule 10A-3, except that with respect to legal and regulatory requirements the Audit Committee’s oversight responsibility only includes oversight of compliance as such requirements relate to business ethics compliance, financial and sustainability reporting.
The Remuneration Committee
Under the NYSE Standards listed companies must have a compensation committee composed entirely of independent directors. Compensation committee members must satisfy the additional independence requirements specific to compensation committee membership set forth in section 303A.02(a)(ii). The NYSE Standards states that in affirmatively determining the independence of any director who will serve on the compensation committee of the listed company’s Board of Directors, the Board of Directors must consider all factors specifically relevant to determining whether a director has a relationship to the listed company which is material to that director’s ability to be independent from management in connection with the duties of a compensation committee member.
As a controlled company, Novo Nordisk A/S is exempt from the requirement to establish a compensation committee. The Board of Directors has, however, established a Remuneration Committee. The members of the Remuneration Committee are appointed at a Board meeting held immediately following the annual general meeting. When appointing the members, the Board of Directors considers relevant factors to determine whether any member of the Remuneration Committee has a relationship to Novo Nordisk that would materially affect the member’s ability to exercise judgment independent from management. The Danish Corporate Governance Recommendations recommend that a majority of the members of a board committee should qualify as independent. Under the Danish Corporate Governance Recommendations, half of the members of the Remuneration Committee are considered independent, as opposed to the majority as recommended. This is to allow for representation from both employee-elected member of the Board of Directors and members of the Board of Directors representing the controlling shareholder, while maintaining an operational structure comprising relatively few members. The composition of the Remuneration Committee thus deviates from the Danish Corporate Governance Recommendations with respect to the recommendation on independence in board committees. However, as Novo Nordisk A/S explains its chosen approach, Novo Nordisk A/S is considered as in compliance with the recommendation by the Danish Corporate Governance Recommendations.
29
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 16H MINE SAFETY DISCLOSURE |
The People & Governance Committee (previously the Nomination Committee)
Under the NYSE Standards listed companies must have a nominating/corporate governance committee composed entirely of independent directors. As a controlled company, Novo Nordisk A/S is exempt from the requirement. The Board of Directors has, however, established a People & Governance Committee and the members of the People & Governance Committee are appointed at a Board meeting held immediately following the annual general meeting. The Danish Corporate Governance Recommendations recommend that a majority of the members of a board committee should qualify as independent. Under the Danish Corporate Governance Recommendations, half of the members of the People & Governance Committee are considered independent, as opposed to the majority as recommended. This is to allow for representation from both employee-elected members of the Board of Directors and members of the Board of Directors representing the controlling shareholder, while maintaining an operational structure comprising relatively few members. The composition of the People & Governance Committee thus deviates from the Danish Corporate Governance Recommendations with respect to the recommendation on independence in board committees. However, as Novo Nordisk A/S explains its chosen approach, Novo Nordisk A/S is considered as in compliance with the recommendation by the Danish Corporate Governance Recommendations.
Equity-compensation plans
Under Section 303A.08 of the NYSE Standards, shareholders must be given the opportunity to vote on all equity compensation plans and material revisions thereto, with certain limited exceptions. The Remuneration Policy adopted by the annual general meeting describes remuneration of the members of the Board of Directors and Executive Management. Adjustments to the policy were most recently adopted by the annual general meeting in March 2024 to adjust the remuneration of the Board of the Directors. The Remuneration Policy applies to Board of Directors’ and Executive Management’s remuneration in relation to the calendar year 2024 onwards. All incentive programs offered to the members of Board of Directors and/or Executive Management shall comply with the framework set out in the Remuneration Policy. However, under Danish law, the practice of voting on equity compensation plans is not contemplated and accordingly, equity compensation plans are only subject to shareholder approval if they result in the issuance of new shares (and not if treasury shares are used).
Code of business conduct and ethics
Under Section 303A.10 of the NYSE Standards, listed companies must adopt and disclose a code of business conduct and ethics for directors, officers and employees, and promptly disclose any waivers of the code for directors or executive officers. As permitted by the NYSE standards applicable to FPIs and in accordance with Danish law and practice, maintains a framework of rules and guidelines, including but not limited to the Novo Nordisk Way and OneCode, which serve as the principles guiding how the company and individual employees act, and the supporting Ethics Navigator, which describe corporate values and Novo Nordisk’s expectations for the standard of business conduct and ethics expected of its directors, officers, employees and business partners acting on behalf of Novo Nordisk as Third Party Representatives. Every topic mentioned in the NYSE Listed Company Manual is either specifically addressed in this framework of rules and guidelines, or routinely included in Novo Nordisk’s employment contracts. See Item 16B. While certain topics mentioned in the NYSE Listed Company Manual are addressed in this framework of rules and guidelines, others are not specifically addressed.
CEO certification
Under Section 303A.12(a) of the NYSE Standards, each listed company's chief executive officer must certify to the NYSE each year that he or she is not aware of any violation by the listed company of NYSE Standards, qualifying the certification to the extent necessary. As permitted by the NYSE standards applicable to FPIs and in accordance with Danish law and practice (which do not contemplate such certifications), Novo Nordisk does not submit such certifications.
ITEM 16H MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I DISCLOSURES REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
ITEM 16J INSIDER TRADING POLICIES
Novo Nordisk has adopted insider trading policies and procedures governing the purchase, sale, and other dispositions of our securities by directors, senior management and covered employees designed to promote compliance with applicable insider trading laws, rules and regulations, and any listing standards applicable to Novo Nordisk. The key policies and procedures, which are filed as Exhibit 11.1 to this Form 20-F., are comprised of the following:
30
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 16I DISCLOSURES REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
•Internal Rules on Insiders' Trading in Shares and Bonds (Insider Rules)
•Internal Rules on Notification of Trading in Shares Made by Board Members and Executives (PDMR notification rules)
•Internal Rules Trading in Own Shares and Bonds
•Terms and Definitions regarding Material News and Insiders’ Trading
Novo Nordisk monitors inside information as defined under the EU Market Abuse Regulation 2014/596 (“MAR”) as part of our compliance with MAR and as part of our disclosure controls and procedures, and imposes restrictions on trading in our own securities when we have undisclosed inside information. Novo Nordisk also refrains from trading in our own securities during our regular closed periods.
ITEM 16K CYBERSECURITY
Cybersecurity risk management, strategy and governance
The cybersecurity governance and programme are defined in a charter approved by executive management, which is anchored in a risk-based approach based on industry standards to balance the level of cybersecurity against the risks to Novo Nordisk.
At Novo Nordisk, cybersecurity risk management is an integral part of our enterprise risk management framework defined in our information security framework. The framework aligns with industry best practice covering IT infrastructure, IT systems, and third-party service providers, and includes steps for identifying and assessing the severity of a cybersecurity threat, evaluating the potential business impact, implementing countermeasures and mitigation strategies, and informing executive management of material cybersecurity threats and incidents. Risks are consolidated across business areas and integrated into the enterprise risk management framework, where the likelihood and impact of cybersecurity risk scenarios are evaluated for risk treatment by executive management and reported to the Board of Directors. The cybersecurity risk management programme is validated through peer-benchmarked maturity assessments, external technical assessments of the core infrastructure, key applications and operational processes, as well as group internal audit evaluations of the cross-organisational controls implementation.
The Board of Directors has overall oversight responsibility for our risk management, and is charged with oversight of our threat landscape, posture, performance, and strategy related to cybersecurity. The Audit Committee is charged with overseeing the cybersecurity incident trends and potentially significant incidents that have been handled. Executive management is responsible for identifying, considering and assessing material cybersecurity risks on an ongoing basis, establishing processes to ensure that such potential cybersecurity risk exposures are monitored, putting in place appropriate mitigation measures and maintaining cybersecurity programmes.
Novo Nordisk cybersecurity programmes and teams are under the direction of our Chief Information Security Officer (CISO) in alignment with the strategic direction set by executive management. Novo Nordisk CISO is an experienced information security officer, who holds multiple industry certifications such as Certified Information Systems Security Professional (CISSP) and Certified Information Security Manager® (CISM). Our teams are comprised of certified and experienced information systems security professionals and information security managers.
Novo Nordisk cybersecurity teams monitor, detect, contain, respond to and report upon cybersecurity threats, events, and incidents in collaboration with specialised third-party service providers. This covers processes for handling major cybersecurity incidents, which is integrated into the corporate crisis management framework for management of large-scale cyber events. Management, including the CISO and our cybersecurity teams, regularly reports on cybersecurity to various organisational levels including submitting regular reports to the Audit Committee and Board of Directors.
In 2024, we did not identify any cybersecurity threats that have materially affected or are reasonably likely to materially affect our business strategy, results of operations, or financial condition. However, despite our efforts, we cannot eliminate all risks from cybersecurity threats, or provide assurances that we have not experienced an undetected cybersecurity incident. For more information about these risks, please see 'Risk Factors—The potential risk on our business as a result of cybersecurity breaches' under Item 3.D.
31
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 17 FINANCIAL STATEMENTS |
PART III
ITEM 17 FINANCIAL STATEMENTS
See response to Item 18.
ITEM 18 FINANCIAL STATEMENTS
The financial statements required by this item accompany this annual report in the form of our Annual Report 2024 (see Item 19).
Reconciliation of non-IFRS financial measures
In the Financial statements, Novo Nordisk discloses certain financial measures of the Group’s financial performance, financial position and cash flows that reflect adjustments to the most directly comparable measures calculated and presented in accordance with IFRS Accounting Standards as issued by the International Accounting Standards Board. The inclusion of non-IFRS measures has been expressly permitted by the Danish Business Authority and thereby exempted from the prohibition in Item 10(e)(1)(ii)(C) of Regulation S-K. However, these non-IFRS financial measures may not be defined and calculated by other companies in the same manner and may thus not be comparable with such measures.
The non-IFRS financial measures presented in our Annual Report 2024 are:
•Sales at constant exchange rates;
•Operating profit at constant exchange rates;
•'Net profit’, adjusted for 'income taxes', 'financial items', 'depreciation and amortisation' and 'impairment losses and
reversals' (EBITDA) and EBITDA at constant exchange rates;
•Return on invested capital (ROIC);
•Free cash flow; and
•Cash to earnings.
Reference is made to the section ‘Non-IFRS financial measures’ on pages 134-137 in our Annual Report 2024.
32
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 18 FINANCIAL STATEMENTS |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the shareholders and the Board of Directors of Novo Nordisk A/S
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Novo Nordisk A/S and its subsidiaries (the "Company" or “Novo Nordisk”) as of December 31, 2024 and 2023, the related consolidated income statements, statements of comprehensive income, equity statements and cash flow statements for each of the three years in the period ended December 31, 2024, and the related notes (collectively referred to as the "financial statements"). We also have audited the Company’s internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2024 in conformity with IFRS Accounting Standards as issued by the International Accounting Standards Board. Also, in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.
As described in the Report of Novo Nordisk Management on Internal Control over Financial Reporting, management excluded from its assessment the internal control over financial reporting the acquired Catalent Fill-finish Sites, which were acquired on December 18, 2024, and whose total assets represent approximately 17% and total net profit represents less than 1% of the consolidated financial statement amounts of the Company as of and for the year ended December 31, 2024. Accordingly, our audit did not include the internal control over financial reporting at Catalent Fill-finish Sites.
Basis for Opinions
The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the Report of Novo Nordisk Management on Internal Control over Financial Reporting appearing under Item 15. Our responsibility is to express an opinion on these financial statements and an opinion on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the financial statements included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures to respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
33
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 18 FINANCIAL STATEMENTS |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current-period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
US sales rebates – Refer to notes 2.1 and 3.5 to the financial statements
Critical Audit Matter Description
In the United States (US), sales rebates are paid in connection with public healthcare insurance programs, namely Medicare and Medicaid, as well as rebates to pharmacy benefit managers and managed healthcare plans. In January 2021, the Company changed its policy in the US related to the 340B Drug Pricing Program, whereby Novo Nordisk no longer provides 340B statutory discounts to certain pharmacies that contract with covered entities participating in the 340B Drug Pricing Program. Novo Nordisk has only recognized revenue related to the 340B Drug Pricing Program to the extent that it is highly probable that its inclusion will not result in a significant revenue reversal in the future. When sales are recognized, Novo Nordisk also records provisions for the expected value of the sales deductions (variable consideration) at the time the related sales are recorded.
We identified the US sales rebates, including provisions related to the 340B Drug Pricing Program, as a critical audit matter due to the significant measurement uncertainty involved in developing these provisions, as the provisions are based on legal interpretations of applicable laws and regulations, historical claims experience, payer channel mix, current contract prices, unbilled claims, claims submission time lags and inventory levels in the distribution channel. In addition, significant judgments are involved in determining whether a significant reversal in the amount of cumulative revenue recognized will not occur. This led to a high degree of auditor judgment and an increased extent of effort and subjectivity in applying procedures relating to these provisions.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to US sales rebates included the following, among others:
•We evaluated the appropriateness of the Company’s methodology used to develop their sales rebates provisions, including provisions related to the 340B Drug Pricing Program, by involving audit professionals with industry and quantitative analytics experience to assist us in performing our auditing procedures.
•We tested the effectiveness of controls relating to sales rebates, including controls over the assumptions and data used to estimate these rebates.
•We tested rebate claims processed by the Company, including evaluating those claims for consistency with the conditions and terms of the Company’s rebate arrangements.
•We tested the overall reasonableness of the accruals recorded at period end by developing an expectation for comparison to actual recorded balances.
•We evaluated the Company’s ability to estimate sales rebates accurately by considering the historical accuracy of the Company’s estimates in prior year.
Acquisition of Catalent Fill-finish Sites – Refer to notes 3.1 and 5.3 to the financial statements
Critical Audit Matter Description
On December 18, 2024, Novo Nordisk acquired fill-finish sites from Novo Holdings A/S for a purchase price of USD 11.7 billion (DKK 82.1 billion). The Company accounted for the acquisition as a business combination and, accordingly, has performed procedures to identify all assets and liabilities and allocated the purchase price to the assets acquired and liabilities assumed based on their respective estimated fair values as of the date of acquisition. Intangible assets acquired primarily included know-how. The excess of the purchase consideration over the fair value of identifiable assets acquired and liabilities assumed was recorded as goodwill.
34
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
|
|
|
| ITEM 18 FINANCIAL STATEMENTS |
We identified the recognition of a separably identifiable know-how intangible asset and the valuation approach applied in valuing such an asset as a critical audit matter due to the high level of complexity and management judgement involved. This led to a high degree of auditor judgment and an increased extent of effort in applying procedures relating to these significant estimates and judgement.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the acquisition included the following, among others:
•We assessed the appropriateness of the recognition of a separably identifiable know-how intangible asset in relation to the recognition criteria in IFRS 3, Business Combinations and IAS 38, Intangible Assets.
•Due to the complexity and significance of the matter, we also consulted with IFRS technical accounting specialists regarding the appropriateness of management's conclusion that such know-how fulfills the separability criteria in IAS 38, Intangible Assets and thus can be recognized as an intangible asset.
•With the assistance of our fair value specialists, we evaluated the appropriateness of the valuation approach and methodology used in determining the fair value of the know-how intangible asset.
•We tested the effectiveness of internal controls over the business combination.
•We assessed the knowledge, skills, abilities, and objectivity of management’s experts used in determining the appropriateness of recognition of a separable intangible asset and the determination of the appropriate method by which to value such assets and evaluated the work performed.
/s/ Deloitte Statsautoriseret Revisionspartnerselskab
Copenhagen, Denmark
February 5, 2025
We have served as the Company’s auditor since 2021.
35
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
ITEM 19 EXHIBITS
A. ANNUAL REPORT
The following pages from our Annual Report 2024 (see Exhibit 15.1) are incorporated by reference into this Form 20-F. The content of websites, scientific articles and other sources referenced on these pages are not incorporated by reference into this Form 20-F.
|
|
|
|
|
|
|
|
|
|
Page(s) in our Annual Report |
| Management Discussion and Analysis |
|
| Introducing Novo Nordisk |
4-10 |
| Strategic Aspirations |
11-37 |
| Corporate Governance |
16 |
| Pipeline overview |
23 |
| 2024 performance and 2025 outlook |
32-37 |
| Shares and capital structure |
36-37 |
| Risks |
38-40 |
| Board of Directors |
42-44 |
| Executive Management |
45 |
|
|
|
|
| Consolidated Financial Statements |
|
| Consolidated Income statement and Statement of comprehensive income for the years ended 31 December 2024, 2023 and 2022 |
102 |
| Consolidated Cash flow statement for the years ended 31 December 2024, 2023 and 2022 |
103 |
| Consolidated Balance sheet at 31 December 2024 and 2023 |
104 |
| Consolidated Equity statement at 31 December 2024, 2023 and 2022 |
105 |
| Notes to the Consolidated financial statements |
106-133 |
| Companies in the Novo Nordisk Group |
133 |
B. REMUNERATION REPORT
The following pages from our Remuneration Report 2024 (see Exhibit 15.3) are incorporated by reference into this Form 20-F. The content of websites, scientific articles and other sources referenced on these pages are not incorporated by reference into this Form 20-F.
|
|
|
|
|
|
|
|
|
|
Page(s) in the Remuneration Report |
| 2.1 Key developments in Board remuneration in 2024 |
4 |
| 2.2 Remuneration composition |
4-5 |
| 2.4 Board and committee fee levels 2024 |
6 |
| 2.5 Board remuneration 2024 |
7 |
| 2.6 Shareholdings of Board Members |
7 |
| 3.1 Key developments in executive remuneration 2024 |
8 |
| 3.2 Remuneration composition |
8-10 |
| 3.4 Executive remuneration in 2024 |
12 |
| 3.5 Short-term incentive programme 2024 |
13-15 |
| 3.7 Long-term incentive programme 2022 |
17 |
| 3.8 Long-term incentive programmes 2023 and 2024 |
18 |
| 3.9 Shareholdings of Executive Management |
19 |
36
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
C. EXHIBITS
List of exhibits:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Exhibit No. |
|
Description |
|
Method of filing |
|
|
|
|
|
|
|
Articles of Association of Novo Nordisk A/S |
|
Incorporated by reference to the Registrant’s Report furnished to the SEC on Form 6-K on March 25, 2024. |
|
|
|
|
|
|
|
Description of the rights of American Depositary Shares registered under Section 12 of the Securities Exchange Act of 1934 |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
|
|
Description of the rights of B Shares registered under Section 12 of the Securities Exchange Act of 1934 |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
|
|
Companies in the Novo Nordisk Group |
|
Incorporated by reference to page 133 of the Annual Report 2024, filed as Exhibit 15.1 to this Form 20-F 2024. |
|
|
|
|
|
|
|
Insider Trading Policies |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
|
|
Certification of Lars Fruergaard Jørgensen, president and chief executive officer of Novo Nordisk, pursuant to Section 302 of the Sarbanes–Oxley Act of 2002. |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
|
|
Certification of Karsten Munk Knudsen, executive vice president and chief financial officer of Novo Nordisk, pursuant to Section 302 of the Sarbanes–Oxley Act of 2002. |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
|
|
Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes–Oxley Act of 2002. |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
|
|
The Registrant's Annual Report for the fiscal year ended December 31, 2024. |
|
Filed together with this Form 20-F 2024. Certain of the information included within Exhibit 15.1, which is provided pursuant to Rule 12b-23(a)(3) of the Securities Exchange Act of 1934, as amended, is incorporated by reference in this Form 20-F, as specified elsewhere in this Form 20-F. With the exception of the items and pages so specified, Exhibit 15.1 is not deemed to be filed as part of this Form 20-F. |
|
|
|
|
|
|
|
Consent of independent registered public accounting firm. |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
|
|
The Registrant's Remuneration Report for the fiscal year ended December 31, 2024. |
|
Incorporated by reference to the portions of the Registrant’s Report furnished to the SEC on Form 6-K on February 5, 2025 identified in Item 19.B of this Form 20-F. |
|
|
|
|
|
|
|
Compensation Recovery Policy |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
| EX-101.SCH |
|
XBRL Taxonomy Extension Schema Document |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
| EX-101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
| EX-101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
| EX-101.LAB |
|
XBRL Taxonomy Extension Labels Linkbase Document |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
| EX-101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
Filed together with this Form 20-F 2024. |
|
|
|
|
|
37
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
SIGNATURES
The Registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
NOVO NORDISK A/S
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| /s/ Lars Fruergaard Jørgensen |
|
/s/ Karsten Munk Knudsen |
| Name: |
Lars Fruergaard Jørgensen |
|
Name: |
Karsten Munk Knudsen |
| Title: |
President and chief executive officer |
|
Title: |
Executive vice president and chief financial officer |
Bagsværd, Denmark
Dated: February 5, 2025
38
|
|
|
|
|
|
|
Novo Nordisk Form 20-F 2024 |
EX-2.1
2
exhibit21descriptionofther.htm
EX-2.1
Document
Exhibit 2.1
Description of the rights of B shares registered under Section 12 of the Securities Exchange Act of 1934
B Shares
A. OFFER AND LISTING DETAILS
Novo Nordisk A/S (the “Company”) is a limited liability company organized under the laws of Denmark and registered with the Danish Business Authority under CVR number 24256790.
The Company has a total share capital of DKK 446,500,000, divided into an A share capital of nominally DKK 107,487,200 and a B share capital of nominally DKK 339,012,800. Each A share of DKK 0.10 carries 100 votes and each B share of DKK 0.10 carries 10 votes at General Meetings of the Company.
The Company’s B shares are listed in Denmark on Nasdaq Copenhagen, and traded under the symbol "NOVO-B" and on the New York Stock Exchange (“NYSE”) as American Depository Receipts (“ADRs”), traded under the symbol "NVO". Each of the Company’s A shares and B shares has been fully paid up and is registered, in the case of the A shares, on the Company's Register of Shareholders and, in the case of the B shares, by VP Securities, a central securities depositary in Denmark.
The A shares and the B shares have the rights, preferences and restrictions described below in “-Memorandum and Articles of Association.”
B. MEMORANDUM AND ARTICLES OF ASSOCIATION
The following section summarizes certain material provisions of the Company’s Articles of Association, certain other constitutive documents and relevant Danish corporate law. For further information, see Exhibit 1.1 to this Form 20-F for a translation into the English language of the Articles of Association.
General
The Company’s objectives are to carry out research and development and to manufacture and commercialize pharmaceutical, medical and technical products and services as well as any other activity related thereto as determined by its Board of Directors. It strives to conduct its activities in a financially, environmentally and socially responsible way. The Company's objectives are set out in Article 2 of its Articles of Association.
Powers of the Board of Directors
All members of the Board of Directors have equal voting rights, and all resolutions are passed by a simple majority of votes. However, in the event of a tie, the Chair shall have the casting vote. The Board of Directors forms a quorum when at least a majority of its members is present.
According to the Danish Companies Act, no member of the Board of Directors or the Executive Management may take part in the consideration of any business involving agreements between any member of the Group and himself, legal actions brought against the individual, or any business involving agreements between any member of the Group and any third party or legal actions brought against any third party, if the individual has a major interest therein that might conflict with the Company’s interests. The Danish Companies Act also includes restrictions on the Company’s ability to grant loans or provide security to any member of the Board of Directors or anyone particularly close to such a member of the Board of Directors. The Company's ability to grant loans or provide security is subject to a number of conditions including shareholder approval or delegation of authorization to the Board of Directors by the General Meeting.
The remuneration of the Board of Directors must be approved by the Company’s shareholders at the Annual General Meeting.
Rights, restrictions and preferences attaching to the shares
If the shareholders at an Annual General Meeting approve a recommendation by the Board of Directors to pay dividends, dividends shall be distributed as follows: a priority dividend of 0.5% to the holders of A shares and then up to a dividend of 5% to the holders of B shares. Any distribution of additional dividends shall be subject to the provision that the holders of A shares shall never receive a total dividend exceeding the percentage rate of the dividend paid to the holders of B shares. A shares take priority for dividends below 0.5%. B shares take priority for dividends between 0.5% and 5%. However, in practice, A shares and B shares receive the same amount of dividends per share of DKK 0.01. Dividends on A shares shall be remitted to the shareholders at the addresses entered in the Company's Register of Shareholders as at the date of the Annual General Meeting. Dividends on B shares shall be paid with fully discharging effect for the Company through a central securities depository and an account-holding bank to shareholders registered by VP Securities at the time of payment.
The Board of Directors has been granted authority to distribute extraordinary dividends. This authority is included in the Articles of Association of the Company. Hence the Board of Directors has been granted authority to pay interim dividends without obtaining specific approval from the Annual General Meeting. Any Board resolution to pay extraordinary dividends must be accompanied by a balance sheet showing that sufficient funds are available for distribution. An authorized auditor must review the balance sheet.
1
|
|
|
|
|
|
|
Exhibit 2.1 2024 - Novo Nordisk |
Subject to the preference mechanism described above, the A shares and the B shares rank as equal in the event of a return on capital by the Company. Upon a winding-up, liquidation or otherwise, the B shares rank ahead of the A shares with regard to payment of each share’s nominal amount. All shares rank as equal in respect of further distributions from a winding-up.
Each A share of DKK 0.10 carries 100 votes and each B share of DKK 0.10 carries 10 votes at General Meetings. A shares are non-negotiable instruments whereas B shares are negotiable instruments.
The holders of A shares have a pro-rata right of first refusal with regard to any A shares sold by another shareholder. However, currently all A-shares are owned by Novo Holdings A/S and cannot be divested.
The share capital has been fully paid up and shareholders are not liable to further capital calls by the Company. No shareholder shall be obliged to have his shares redeemed in whole or in part. There is no sinking fund provision in the Articles of Association. There is no provision in the Articles of Association discriminating against any existing or prospective holder of such securities as a result of such shareholder owning a substantial number of shares. The members of the Board of Directors do not stand for reelection at staggered intervals and there is no cumulative voting arrangement.
Changes in shareholders’ rights
Changes in the rights of holders of A shares or B shares require an amendment of the Articles of Association. Unless stricter requirements are made under the Danish Companies Act for any such resolution to be passed, (i) at least 2/3 of the total number of votes in the Company shall be represented at the General Meeting, and (ii) at least 2/3 of the votes cast and of the voting share capital shall vote in favor of such a resolution. If the quorum requirement in (i) is not fulfilled, the Board of Directors shall within two weeks convene another General Meeting at which the resolution may be passed irrespective of the number of votes represented.
General Meetings
The Company’s General Meetings shall be held at a venue in the Capital Region of Denmark. The Annual General Meeting shall be held before the end of April in every year. Extraordinary General Meetings shall be held as resolved by the General Meeting or the Board of Directors, or upon the request of the auditors or shareholders representing in total at least 5% of the share capital. The Extraordinary General Meeting shall then be called not later than two weeks after receipt of such request.
General Meetings shall be called by the Board of Directors not earlier than five weeks and not later than three weeks prior to the General Meeting. The notice calling such General Meeting, stating the agenda for the meeting, shall be published on the Company’s website: novonordisk.com (the contents of this website are not incorporated by reference into this Form 20-F). The notice convening the meeting shall also be forwarded by mail or by email in writing to all shareholders entered in the Register of Owners who have so requested.
A shareholder’s right to attend and vote at a General Meeting shall be determined by the shares or ADRs which such shareholder owns at the applicable record date. The Danish record date is one week prior to the General Meeting. Any shareholder who is entitled to attend the General Meeting is required to apply for an admission card to such General Meeting no later than three days prior to the date of such General Meeting. ADR holders who wish to attend the General Meeting in Denmark should contact Investor Relations, via e-mail to IRofficer@novonordisk.com.
The shares held by each shareholder at the Danish record date shall be calculated based on the registration of the shareholder’s shares in the Register of Owners as well as any notification received by the Company with respect to registration of shares in the Register of Owners, which have not yet been entered in the Register of Owners.
Ownership restrictions
There are no limitations on the rights of non-resident or foreign owners to hold or vote the shares imposed by the laws of Denmark, the Company’s Articles of Association, or any other of its constituent documents.
Change of control
There is no provision in the Articles of Association, nor any other constituent document, that would have an effect of delaying, deferring or preventing a change in control of the Company and that would operate only with respect to a merger, acquisition or corporate restructuring involving the Company (or any of its subsidiaries). However, based on the current shareholder structure, the voting rights held by holders of A shares outlined above afford the Novo Nordisk Foundation, acting through its wholly-owned subsidiary Novo Holdings A/S, to have veto power against any change of control.
Ownership disclosure
According to the Danish Capital Markets Act and the Danish Companies Act, shareholders of the Company must notify the Danish Financial Supervisory Authority and the Company of their ownership if they own 5% or more of the voting rights or share capital. Also, shareholders must notify changes in holdings if thresholds of 5%, 10%, 15%, 20%, 25%, 50%, 90% or 100% and 1/3 and 2/3 of the voting rights or share capital are crossed.
Changes in capital
The Company’s Articles of Association do not contain conditions governing changes in the capital more stringent than those contained in the Danish Companies Act.
There are no limitations on the right of non-resident or foreign owners to hold or vote the B shares or the ADRs imposed by the laws of Denmark or the Articles of Association of the Company.
American Depositary Shares
2
|
|
|
|
|
|
|
Exhibit 2.1 2024 - Novo Nordisk |
The Company’s American Depositary Receipts (“ADR”) program is administered by J.P. Morgan Depositary Receipts Group, JPMorgan Chase Bank, N.A., 383 Madison Avenue, Floor 11, New York, NY 10179, United States (the “Depositary”). ADRs evidence American Depositary Shares (“ADSs”) issuable by the Depositary pursuant to the terms of the Amended and Restated Deposit Agreement (the “Deposit Agreement”), the form of which is attached as an exhibit to the registration statement on Form F-6 filed by the Company with the U.S. Securities and Exchange Commission (the “SEC”) on March 2, 2017 (File No. 333-192740). Each ADS currently represents one deposited Novo Nordisk B share. The ADS to share ratio is subject to amendment as provided in the form of ADR (which may give rise to fees contemplated by the form of ADR, which is attached to the Deposit Agreement). In the future, each ADS will also represent any securities, cash or other property deposited with the Depositary but which has not been distributed directly to ADS holders (together with any deposited shares, the “Deposited Securities”).
For the purposes of the following description, “Holders” refers to the registered ADS holders. ADSs may be held either directly or indirectly through a broker or other financial institution. If an ADS holder holds their ADSs directly, they will be a registered ADS holder. If an ADS is held indirectly, the relevant holder must rely on the procedures of their broker or other financial institution to assert the rights of ADS holders described below. Such holders should consult with their broker or financial institution to find out what those procedures are.
A. SHARE DIVIDENDS AND OTHER DISTRIBUTIONS
The Company may make various types of distributions with respect to its securities. To the extent practicable, the Depositary will deliver such distributions to each Holder entitled thereto, in proportion to their interests, on the record date set by the Depositary, in the following manner:
•Cash. The Depositary will distribute any U.S. dollars available to it resulting from a cash dividend or other cash distribution or the net proceeds of sales of any other distribution or portion thereof on an averaged or other practicable basis, subject to (i) appropriate adjustments for taxes withheld, (ii) such distribution being impermissible or impracticable with respect to certain Holders, and (iii) deduction of the Depositary's and/or its agents' fees and expenses in (1) converting any foreign currency to U.S. dollars by sale or in such other manner as the Depositary may determine to the extent that it determines that such conversion may be made on a reasonable basis, (2) transferring foreign currency or U.S. dollars to the United States by such means as the Depositary may determine to the extent that it determines that such transfer may be made on a reasonable basis, (3) obtaining any approval or license of any governmental authority required for such conversion or transfer, which is obtainable at a reasonable cost and within a reasonable time and (4) making any sale by public or private means in any commercially reasonable manner.
•Shares. In the case of a distribution in shares, the Depositary will issue the number of ADSs representing such shares. Only whole ADSs will be issued. Any shares which would result in fractional ADSs will be sold and the net proceeds will be distributed in the same manner as cash to the ADR holders entitled thereto.
•Rights. The Depositary will distribute warrants or other instruments in the discretion of the Depositary representing rights to acquire additional ADRs in respect of any rights to subscribe for additional shares or rights of any nature available to the Depositary as a result of a distribution on Deposited Securities, to the extent that the Company timely furnishes to the Depositary evidence satisfactory to the Depositary that the Depositary may lawfully distribute the same (the Company has no obligation to so furnish such evidence). However, to the extent the Company does not timely furnish such evidence, the Depositary may:
i. sell such rights if practicable and distribute the net proceeds from the sale of such rights in the same manner as cash; or
ii. if it is not practicable to sell the rights by reason of the non-transferability of such rights, limited markets therefor, their short duration or otherwise, do nothing (and allow such rights to lapse).
•Other Distributions. In the case of a distribution of securities or property other than those described above, the Depositary may either (i) distribute such securities or property in any manner it deems equitable and practicable or (ii) to the extent the Depositary deems distribution of such securities or property not to be equitable and practicable, sell such securities or property and distribute the net proceeds in the same way it distributes cash.
To the extent that any of the Deposited Securities is not or shall not be entitled, by reason of its date of issuance, or otherwise, to receive the full amount of any cash dividend, distribution, or net proceeds of sales as contemplated by clause (a) of the prior paragraph, the Depositary shall make appropriate adjustments in the amounts distributed to the Holders issued in respect of such Deposited Securities. To the extent the Company or the Depositary shall be required to withhold and does withhold from any cash dividend, distribution or net proceeds from sales in respect of any Deposited Securities an amount on account of taxes, the amount distributed on the ADSs issued in respect of such Deposited Securities shall be reduced accordingly.
To the extent the Depositary determines in its discretion that it would not be permitted by applicable law, rule or regulation, or it would not otherwise be practicable, to convert foreign currency into U.S. dollars and/or distribute such U.S. dollars to any or all of the Holders entitled thereto, the Depositary may in its discretion distribute some or all of the foreign currency received by the Depositary as it deems permissible and practicable to, or retain and hold such foreign currency uninvested and without liability for interest thereon for the respective accounts of, the Holders entitled to receive the same.
To the extent that the Depositary determines in its discretion that any distribution pursuant to the above would not be permissible by applicable law, rule or regulation, or is not otherwise practicable with respect to any or all Holders, the Depositary may in its discretion make such distribution as it so deems permissible and practicable, including the distribution of some or all of any cash, foreign currency, securities or other property (or appropriate documents evidencing the right to receive some or all of any such cash, foreign currency, securities or other property), and/or the Depositary may retain and hold some or all of such cash, foreign currency, securities or other property as Deposited Securities with respect to the applicable Holders’ ADRs (without liability for interest thereon or the investment thereof).
3
|
|
|
|
|
|
|
Exhibit 2.1 2024 - Novo Nordisk |
To the extent the Depositary retains and holds any cash, foreign currency, securities or other property, any and all fees, charges and expenses related to, or arising from, the holding thereof (including, but not limited to those provided in any ADR) shall be paid from such cash, foreign currency, securities or other property, or the net proceeds from the sale thereof, thereby reducing the amount so held.
The Depositary is not responsible if it fails to determine that any distribution or action is lawful or reasonably practicable.
There can be no assurance that the Depositary will be able to convert any currency at a specified exchange rate or sell any property, rights, shares or other securities at a specified price, nor that any of such transactions can be completed within a specified time period.
The Depositary reserves the right to utilize a division, branch or affiliate of the Depositary to direct, manage and/or execute any public and/or private sale of securities and/or property under the Deposit Agreement. Such division, branch and/or affiliate may charge the Depositary a fee in connection with such sales, which fee is considered an expense of the Depositary. All purchases and sales of securities will be handled by the Depositary in accordance with its then current policies, which are currently set forth on the “Disclosures” page (or successor page) of ADR.com, the location and contents of which the Depositary shall be solely responsible for.
B. ISSUANCE, WITHDRAWAL AND CANCELLATION
Upon each deposit of shares or evidence of rights to receive shares, receipt of related delivery documentation and compliance with the other provisions of the Deposit Agreement, including the payment of the fees and charges of the Depositary and any taxes or other fees or charges owing, the Depositary will issue an ADR or ADRs in the name or upon the order of the person entitled thereto evidencing the number of ADSs to which such person is entitled. Shares deposited must be accompanied by certain delivery documentation and shall, at the time of such deposit, be registered in the name of JPMorgan Chase Bank, N.A., as depositary for the benefit of holders of ADRs or in such other name as the Depositary shall direct. All of the ADSs issued will, unless specifically requested to the contrary, be part of the Depositary's direct registration system, and a registered holder will receive periodic statements from the Depositary which will show the number of ADSs registered in such holder's name. An ADR holder can request that the ADSs not be held through the Depositary's direct registration system and that a certificated ADR be issued.
In its capacity as Depositary, the Depositary shall not lend shares or ADSs.
Upon surrender for cancellation of (i) a certificated ADR in form satisfactory to the Depositary at the Depositary’s office or (ii) proper instructions and documentation in the case of a direct registration ADR, the Depositary will, upon payment of certain applicable fees, charges and taxes, deliver the underlying shares to the Holder or upon the Holder’s written order. Delivery of deposited securities in certificated form will be made at the custodian's office. At the Holder’s risk, expense and request, the Depositary may deliver deposited securities at such other place as the Holder may request.
C. TRANSFER
ADRs are transferable on the ADR register and may be split into other ADRs or combined with other ADRs into one ADR, evidencing the aggregate number of ADSs surrendered for split-up or combination, by the Holder thereof or by duly authorized attorney upon surrender of the relevant ADR upon delivery to the Depositary of proper instruments of transfer and duly stamped as may be required by applicable law; provided that the Depositary may close the ADR register at any time or from time to time when deemed expedient by it or, in the case of the issuance book portion of the ADR register, when reasonably requested by the Company solely in order to enable the Company to comply with applicable law. At the request of a Holder, the Depositary shall, for the purpose of substituting a certificated ADR with a direct registration ADR, or vice versa, execute and deliver a certificated ADR or a direct registration ADR, as the case may be, for any authorized number of ADSs requested, evidencing the same aggregate number of ADSs as those evidenced by the certificated ADR or direct registration ADR, as the case may be, substituted.
D. RECORD DATES
The Depositary may fix a record date (which, to the extent applicable, shall be as near as practicable to any corresponding record dates set by the Company) for the determination of the Holders who shall be entitled or obligated:
•to receive any distribution on or in respect of Deposited Securities;
•to give instructions for the exercise of any voting rights;
•to pay the fee assessed by the Depositary for administration of the ADR program and for any expenses as provided for in the ADR;
•to receive any notice or to act in respect of other matters, all subject to the provisions of the Deposit Agreement.
E. VOTING RIGHTS
As soon as practicable after receipt of notice of any meeting at which the holders of Shares are entitled to vote, or of solicitation of consents or proxies from holders of shares or Deposited Securities, the Depositary shall fix the ADS record date provided that if the Depositary receives a written request from the Company in a timely manner and at least thirty (30 days) prior to the date of such vote or meeting, the Depositary shall, at the Company’s expense, distribute to Holders a notice stating (i) such information as contained in such notice and any solicitation materials, (ii) that each Holder on the record date set by the Depositary will, subject to any applicable provisions of the laws of Denmark, be entitled to instruct the Depositary as to the exercise of the voting rights, if any, pertaining to the Deposited Securities represented by the ADSs evidenced by such Holder's ADRs and (iii) the manner in which such instructions may be given or deemed as provided below, including instructions for giving a discretionary proxy to a person designated by the Company.
4
|
|
|
|
|
|
|
Exhibit 2.1 2024 - Novo Nordisk |
Each Holder shall be solely responsible for the forwarding of Voting Notices to the beneficial owners of ADSs registered in such Holder’s name. Following actual receipt by the ADR department responsible for proxies and voting of Holders’ instructions (including, without limitation, instructions of any entity or entities acting on behalf of the nominee for DTC), the Depositary shall, in the manner and on or before the date established by the Depositary for such purpose, endeavor to vote or cause to be voted the Deposited Securities represented by the ADSs evidenced by such Holders' ADRs in accordance with such instructions. The Depositary will not itself exercise any voting discretion in respect of any Deposited Securities. If the Depositary timely receives voting instructions from a Holder which fail to specify the manner in which the Depositary is to vote the Deposited Securities represented by such Holder's ADSs, the Depositary will deem such Holder (unless otherwise specified in the notice distributed to Holders) to have instructed the Depositary to vote in favor of the items set forth in such voting instructions. Deposited Securities represented by ADSs for which no timely voting instructions are received by the Depositary from the Holder shall not be voted.
There is no guarantee that Holders generally or any Holder in particular will receive the notice described above with sufficient time to enable such Holder to return any voting instructions to the Depositary in a timely manner.
Notwithstanding anything contained in the Deposit Agreement or any ADR, the Depositary may, to the extent not prohibited by law or regulations, or by the requirement of the stock exchange on which the ADSs are listed, in lieu of distribution of the materials provided to the Depositary in connection with any meeting of, or solicitation of consents or proxies from, Holders, distribute to the Holders a notice that provides Holders with, or otherwise publicizes to Holders, instructions on how to retrieve such materials or receive such materials upon request (i.e., by reference to a website containing the materials for retrieval or a contact for requesting copies of the materials). Holders are strongly encouraged to forward their voting instructions as soon as possible. Voting instructions will not be deemed received until such time as the ADR department responsible for proxies and voting has received such instructions, notwithstanding that such instructions may have been physically received by the Depositary prior to such time.
F. REPORTS AND OTHER COMMUNICATIONS
The Depositary will make available for inspection by Holders at the offices of the Depositary and of the custodian the Deposit Agreement, the provisions of or governing Deposited Securities, and any written communications from the Company, which are both received by the custodian or its nominee as a holder of Deposited Securities and made generally available to the holders of Deposited Securities.
Additionally, the Depositary will distribute copies of such communications (or English translations or summaries thereof) to Holders when furnished by the Company.
G. FEES AND EXPENSES
The Depositary may charge, and collect from, (i) each person to whom ADSs are issued, including, without limitation, issuances against deposits of shares, issuances in respect of share distributions, rights and other distributions, issuances pursuant to a stock dividend or stock split declared by the Company or issuances pursuant to a merger, exchange of securities or any other transaction or event affecting the ADSs or Deposited Securities, and (ii) each person surrendering ADSs for withdrawal of Deposited Securities or whose ADSs are cancelled or reduced for any other reason, a fee of up to U.S. $5.00 for each 100 ADSs (or portion thereof) issued, delivered, reduced, cancelled or surrendered, or upon which a share distribution or elective distribution is made or offered (as the case may be). The Depositary may sell (by public or private sale) sufficient securities and property received in respect of a share distribution, rights and/or other distribution prior to such deposit to pay such charge.
The following additional fees, charges and expenses shall also be incurred by the Holders, the beneficial owners, by any party depositing or withdrawing shares or by any party surrendering ADSs and/or to whom ADSs are issued (including, without limitation, issuances pursuant to a stock dividend or stock split declared by the Company or an exchange of stock regarding the ADSs or the Deposited Securities or a distribution of ADSs), whichever is applicable:
•a fee of U.S. $0.05 or less per ADS for any cash distribution made, or for any elective cash/stock dividend offered, pursuant to the Deposit Agreement;
•a fee of up to U.S.$0.05 per ADS held for the direct or indirect distribution of securities (other than ADSs or rights to purchase additional ADSs or the net cash proceeds from the public or private sale of any such securities, regardless of whether any such distribution and/or sale is made by, for, or received from, or (in each case) on behalf of, the Depositary, the Company and/or any third party (which fee may be assessed against Holders as of a record date set by the Depositary),
•an aggregate fee of up to U.S. $0.05 per ADS per calendar year (or portion thereof) for services performed by the Depositary in administering the ADRs (which fee may be charged on a periodic basis during each calendar year and shall be assessed against Holders as of the record date or record dates set by the Depositary during each calendar year and shall be payable at the sole discretion of the Depositary by billing such Holders or by deducting such charge from one or more cash dividends or other cash distributions;
•an amount for the reimbursement of such charges and expenses as are incurred by the Depositary and/or any of its agents (including, without limitation, the custodian as well as charges and expenses incurred on behalf of Holders in connection with compliance with foreign exchange control regulations or any law or regulation relating to foreign investment) in connection with the servicing of the shares or other Deposited Securities, the sale of securities (including, without limitation, deposited securities), the delivery of Deposited Securities or otherwise in connection with the Depositary's or its custodian's compliance with applicable law, rule or regulation (which charges and expenses may be assessed on a proportionate basis against Holders as of the record date or dates set by the Depositary and shall be payable at the sole discretion of the Depositary by billing such Holders or by deducting such charge or expense from one or more cash dividends or other cash distributions); and
•a fee for the distribution of securities or the sale of securities in connection with a distribution, such fee being in an amount equal to the fee for the execution and delivery of ADSs which would have been charged as a result of the deposit
5
|
|
|
|
|
|
|
Exhibit 2.1 2024 - Novo Nordisk |
of such securities (treating all such securities as if they were shares) but which securities or the net cash proceeds from the sale thereof are instead distributed by the Depositary to those Holders entitled thereto;
The Company will pay all other fees, charges and expenses of the Depositary and any agent of the Depositary (except the custodian) pursuant to agreements from time to time between the Company and the Depositary, except:
•stock transfer or other taxes and other governmental charges (which are payable by Holders or persons depositing shares);
•a transaction fee per cancellation request (including any cancellation request made through SWIFT, facsimile transmission or any other method of communication) as disclosed on the "Disclosures" page (or successor page) of www.adr.com (as updated by the Depositary from time to time, "ADR.com") and any applicable delivery expenses (which are payable by such persons or Holders); and
•transfer or registration expenses for the registration of transfer of Deposited Securities on any applicable register in connection with the deposit or withdrawal of Deposited Securities (which are payable by persons depositing shares or Holders withdrawing Deposited Securities).
To facilitate the administration of various depositary receipt transactions, including disbursement of dividends or other cash distributions and other corporate actions, the Depositary may engage the foreign exchange desk within JPMorgan Chase Bank, N.A. (the “Bank”) and/or its affiliates in order to enter into spot foreign exchange transactions to convert foreign currency into U.S. dollars (“FX Transactions”). For certain currencies, FX Transactions are entered into with the Bank or an affiliate, as the case may be, acting in a principal capacity. For other currencies, FX Transactions are routed directly to and managed by an unaffiliated local custodian (or other third-party local liquidity provider), and neither the Bank nor any of its affiliates is a party to such FX Transactions.
The foreign exchange rate applied to an FX Transaction will be either (i) a published benchmark rate, or (ii) a rate determined by a third-party local liquidity provider, in each case plus or minus a spread, as applicable. The Depositary will disclose which foreign exchange rate and spread, if any, apply to such currency on the "Disclosures" page (or successor page) of ADR.com. Such applicable foreign exchange rate and spread may (and neither the Depositary, the Bank nor any of their affiliates is under any obligation to ensure that such rate does not) differ from rates and spreads at which comparable transactions are entered into with other customers or the range of foreign exchange rates and spreads at which the Bank or any of its affiliates enters into foreign exchange transactions in the relevant currency pair on the date of the FX Transaction. Additionally, the timing of execution of an FX Transaction varies according to local market dynamics, which may include regulatory requirements, market hours and liquidity in the foreign exchange market or other factors. Furthermore, the Bank and its affiliates may manage the associated risks of their position in the market in a manner they deem appropriate without regard to the impact of such activities on the Company, the Depositary, Holders or beneficial owners. The spread applied does not reflect any gains or losses that may be earned or incurred by the Bank and its affiliates as a result of risk management or other hedging related activity.
Notwithstanding the foregoing, to the extent the Company provides U.S. dollars to the Depositary, neither the Bank nor any of its affiliates will execute an FX Transaction as set forth herein. In such case, the Depositary will distribute the U.S. dollars received from the Company. Further details relating to the applicable foreign exchange rate, the applicable spread and the execution of FX Transactions will be provided by the Depositary on ADR.com. The Company, Holders and beneficial owners each acknowledge and agree that the terms applicable to FX Transactions disclosed from time to time on ADR.com will apply to any FX Transaction executed pursuant to the Deposit Agreement.
The right of the Depositary to charge and receive payment of fees, charges and expenses survives the termination of the Deposit Agreement, and shall extend for those fees, charges and expenses incurred prior to the effectiveness of any resignation or removal of the Depositary.
The Depositary anticipates reimbursing the Company for certain expenses incurred by the Company that are related to the establishment and maintenance of the ADR program upon such terms and conditions as the Company and the Depositary may agree from time to time. The Depositary may make available to the Company a set amount or a portion of the depositary fees charged in respect of the ADR program or otherwise upon such terms and conditions as the Company and the Depositary may agree from time to time. The Depositary may agree to reduce or waive certain fees, charges and expenses, including, without limitation, those described herein that would normally be charged on ADSs issued to or at the direction of, or otherwise held by, the Company and/or certain Holders and beneficial owners and holders and beneficial owners of shares of the Company.
H. TAXES
Holders must pay any tax or other governmental charge payable by the custodian or the Depositary on any ADS or ADR, Deposited Security or distribution. If any tax or other governmental charges (including any penalties and/or interest) shall become payable by or on behalf of the custodian or the Depositary with respect to any ADR, any Deposited Securities represented by the ADSs evidenced thereby or any distribution thereon, such tax or other governmental charge shall be paid by the Holder hereof to the Depositary and by holding, or having held, an ADR the Holder and all prior Holders thereof jointly and severally agree to indemnify, defend and save harmless each of the Depositary and its agents in respect thereof. The Depositary may also refuse to effect any registration, registration of transfer, split-up or combination of any ADR, or any withdrawal of Deposited Securities, until such payment is made.
If the Depositary determines that any distribution in property other than cash (including shares or rights) on Deposited Securities is subject to any tax that the Depositary or the custodian is obligated to withhold, the Depositary may dispose of all or a portion of such property in such amounts and in such manner as the Depositary deems necessary and practicable to pay such taxes, by public or private sale, and the Depositary shall distribute the net proceeds of any such sale or the balance of any such property after deduction of such taxes to the Holders entitled thereto.
Holders of any ADR or any interest therein, agree to indemnify the Company, the Depositary, the custodian and any of their respective directors, employees, agents and affiliates against, and hold each of them harmless from, any claims by any
6
|
|
|
|
|
|
|
Exhibit 2.1 2024 - Novo Nordisk |
governmental authority with respect to taxes, additions to tax, penalties or interest arising out of any refund of taxes, reduced rate of withholding at source or other tax benefit obtained.
I. RECLASSIFICATIONS, RECAPITALIZATIONS AND MERGERS
In the event of changes affecting the Deposited Securities, including (i) any change in par value, split-up, consolidation, cancellation or other reclassification of Deposited Securities, (ii) any distributions of shares or other property not made to Holders or (iii) any recapitalization, reorganization, merger, consolidation, liquidation, receivership, bankruptcy or sale of all or substantially all the assets of the Company, then the Depositary may in its discretion:
•amend the form of ADR;
•distribute additional or amended ADRs;
•distribute cash, securities or other property received by it in connection with such actions;
•sell any securities or property received and distribute the proceed as cash; or
•choose to do none of the above.
If the depositary chooses not to take any of the above actions, any of the cash, securities or other property it receives will constitute a part of the Deposited Securities and each ADS will then represent a proportionate interest in such property.
J. AMENDMENT AND TERMINATION
The ADRs and the Deposit Agreement may be amended by the Company and the Depositary, provided that any amendment that imposes or increases any fees, charges or expenses (other than stock transfer or other taxes and other governmental charges, transfer or registration fees, the transaction fee per cancellation request (including any cancellation request made through SWIFT, facsimile transmission, or any other method of communication, applicable delivery expenses, or other such fees, charges or expenses,) or that shall otherwise prejudice any substantial existing right of Holders or beneficial owners, shall become effective thirty (30) days after notice of such amendment shall have been given to the Holders. If an ADR holder continues to hold an ADR or ADRs after being so notified, such ADR holder shall be deemed, by continuing to hold such ADR, to consent and agree to such amendment and to be bound by the Deposit Agreement as amended thereby. In no event shall any amendment impair the right of any ADR holder to surrender such ADR and receive the Deposited Securities represented thereby, except in order to comply with mandatory provisions of applicable law.
Any amendments or supplements which (i) are reasonably necessary (as agreed by the Company and the Depositary) in order for (a) the ADSs to be registered on Form F-6 under the Securities Act of 1933 or (b) the ADSs or shares to be traded solely in electronic book-entry form and (ii) do not in either such case impose or increase any fees or charges to be borne by Holders, shall be deemed not to prejudice any substantial rights of Holders. Notwithstanding the foregoing, if any governmental body or regulatory body should adopt new laws, rules or regulations which would require amendment or supplement of the Deposit Agreement or the form of ADR to ensure compliance therewith, we and the Depositary may amend or supplement the Deposit Agreement and the ADR at any time in accordance with such changed laws, rules or regulations. Such amendment or supplement to the Deposit Agreement in such circumstances may become effective before a notice of such amendment or supplement is given to Holders or within any other period of time as required for compliance.
Notice of any amendment to the Deposit Agreement or form of ADRs shall not need to describe in detail the specific amendments effectuated thereby, and failure to describe the specific amendments in any such notice shall not render such notice invalid, provided, however, that, in each such case, the notice given to the Holders identifies a means for Holders to retrieve or receive the text of such amendment (i.e., upon retrieval from the SEC's website or upon request from the Depositary).
The Depositary may, and shall at the Company’s written direction, terminate the Deposit Agreement and the ADRs by mailing notice of such termination to the Holders at least 30 days prior to the date fixed in such notice for such termination; provided, however, if the Depositary shall have (i) resigned as depositary under the Deposit Agreement, notice of such termination by the Depositary shall not be provided to Holders unless a successor depositary shall not be operating under the Deposit Agreement within 45 days of the date of such resignation, and (ii) been removed as depositary under the Deposit Agreement, notice of such termination by the Depositary shall not be provided to Holders unless a successor depositary shall not be operating under the Deposit Agreement on the 90th day after the Company’s notice of removal was first provided to the Depositary. Notwithstanding anything to the contrary herein, the Depositary may terminate the Deposit Agreement (a) without notice to the Company, but subject to giving 30 days' notice to the Holders, under the following circumstances: (w) in the event of the Company's bankruptcy or insolvency, (x) if the Shares cease to be listed on an internationally recognized stock exchange, (y) if the Company effects (or will effect) a redemption of all or substantially all of the Deposited Securities, or a cash or share distribution representing a return of all or substantially all of the value of the Deposited Securities, or (z) there occurs a merger, consolidation, sale of assets or other transaction as a result of which securities or other property are delivered in exchange for or in lieu of Deposited Securities, and (b) immediately without prior notice to the Company, any Holder or beneficial owner or any other person if required by any law, rule or regulation or any governmental authority or body, or the Depositary would be subject to liability under or pursuant to any law, rule or regulation, or by any governmental authority or body, in each case as determined by the Depositary in its reasonable discretion.
After the date so fixed for termination, the Depositary and its agents will perform no further acts under the Deposit Agreement or the ADRs, except to receive and hold (or sell) distributions on Deposited Securities and deliver Deposited Securities being withdrawn. As soon as practicable after the expiration of six months from the date so fixed for termination, the Depositary shall sell the Deposited Securities and shall thereafter (as long as it may lawfully do so) hold in a segregated account the net proceeds of such sales, together with any other cash then held by it under the Deposit Agreement, without liability for interest, in trust for the pro rata benefit of the Holders not theretofore surrendered. After making such sales, the Depositary shall be discharged from all obligations in respect of the Deposit Agreement and the ADRs, except to account for such net proceeds and other cash.
7
|
|
|
|
|
|
|
Exhibit 2.1 2024 - Novo Nordisk |
K. LIMITATIONS ON OBLIGATIONS AND LIABILITY
Prior to the issue, registration, registration of transfer, split-up, combination, or cancellation of any ADR, the delivery of any distribution in respect thereof, or the withdrawal of any Deposited Securities, and from time to time in the case of the production of proofs as described below, the Company, the Depositary or the custodian may require:
•payment with respect thereto of (i) any stock transfer or other tax or other governmental charge, (ii) any stock transfer or registration fees in effect for the registration of transfers of shares or other Deposited Securities upon any applicable register and (iii) any applicable fees and expenses described in the Deposit Agreement;
•the production of proof satisfactory to it of (i) the identity of any signatory and genuineness of any signature and (ii) such other information, including without limitation, information as to citizenship, residence, exchange control approval, beneficial ownership of any securities, compliance with applicable law, regulations, provisions of or governing Deposited Securities and terms of the Deposit Agreement and the ADRs, as it may deem necessary or proper; and
•compliance with such regulations as the Depositary may establish consistent with the Deposit Agreement.
The issuance of ADRs, the acceptance of deposits of shares, the registration, registration of transfer, split-up or combination of ADRs or the withdrawal of Deposited Securities may be suspended, generally or in particular instances, when the ADR register or any register for Deposited Securities is closed or when any such action is deemed advisable by the Depositary.
The Deposit Agreement expressly limits the obligations and liability of the Depositary, the Company and their respective agents, provided, however, that no disclaimer of liability under the Securities Act of 1933 is intended by any of the limitations of liabilities provisions of the Deposit Agreement. The Deposit Agreement provides that the Depositary, the Company and each of their respective directors, officers, employees, agents and affiliates will:
•incur or assume no liability (including, without limitation, to Holders or beneficial owners) (A) if any present or future law, rule, regulation, fiat, order or decree of Denmark, the United States or any other country or jurisdiction, or of any governmental or regulatory authority or any securities exchange or market or automated quotation system, the provisions of or governing any Deposited Securities, any present or future provision of the Company’s Articles of Association, any act of God, war, terrorism, epidemic, pandemic, nationalization, expropriation, currency restrictions, extraordinary market conditions, work stoppage, strike, civil unrest, revolutions, rebellions, explosions, cyber, ransomware or malware attack, computer failure or circumstance beyond the Company’s, the Depositary's or their respective agents' direct and immediate control shall prevent or delay, or shall cause any of them to be subject to any civil or criminal penalty in connection with, any act which the Deposit Agreement or the ADRs provide shall be done or performed by the Company, the Depositary or their respective agents (including, without limitation, voting), or (B) by reason of any non-performance or delay, caused as aforesaid, in the performance of any act or things which by the terms of the Deposit Agreement it is provided shall or may be done or performed or any exercise or failure to exercise any discretion given it in the Deposit Agreement or the ADRs (including, without limitation, any failure to determine that any distribution or action may be lawful or reasonably practicable);
•incur or assume no liability (including, without limitation, to Holders or beneficial owners) except to perform its obligations to the extent they are specifically set forth in the Deposit Agreement and the ADRs without gross negligence or willful misconduct and the Depositary shall not be a fiduciary or have any fiduciary duty to Holders or beneficial owners;
•in the case of the Depositary and its agents, be under no obligation to appear in, prosecute or defend any action, suit or other proceeding in respect of any Deposited Securities, the ADSs or the ADRs;
•In the case of the Company and its agents, be under no obligation to appear in, prosecute or defend any action, suit or other proceeding in respect of any Deposited Securities, the ADSs or the ADRs, which in the Company’s or its agent’s opinion, as the case may be, may involve it in expense or liability, unless indemnity satisfactory to it against all expense (including fees and disbursements of counsel) and liability be furnished as often as may be required; and
•not be liable (including, without limitation, to Holders or beneficial owners) for any action or inaction by it in reliance upon the advice of or information from any legal counsel, any accountants, any person presenting shares for deposit, any Holder, or any other person believed by it to be competent to give such advice or information and/or, in the case of the Depositary, the Company.
Furthermore, the Depositary shall not be responsible for, and shall incur no liability in connection with or arising from, the insolvency of any custodian that is not a branch or affiliate of the Depositary. Notwithstanding anything to the contrary contained in the Deposit Agreement or any ADRs, the Depositary shall not be responsible for, and shall incur no liability in connection with or arising from, any act or omission to act on the part of the custodian except to the extent that any Holder has incurred liability directly as a result of the custodian having (i) committed fraud or willful misconduct in the provision of custodial services to the Depositary or (ii) failed to use reasonable care in the provision of custodial services to the Depositary as determined in accordance with the standards prevailing in the jurisdiction in which the custodian is located. The Depositary shall not be liable for the acts or omissions made by, or the insolvency of, any securities depository, clearing agency or settlement system. The Depositary, its agents and the Company may rely and shall be protected in acting upon any written notice, request, direction, instruction or document believed by them to be genuine and to have been signed, presented or given by the proper party or parties. The Depositary shall be under no obligation to inform Holders or beneficial owners about the requirements of the laws, rules or regulations or any changes therein or thereto of Denmark, the United States or any other country or jurisdiction or of any governmental or regulatory authority or any securities exchange or market or automated quotation system. Neither the Depositary nor its agents will be responsible for any failure to carry out any instructions to vote any of the Deposited Securities, for the manner in which voting instructions are given or deemed to be given, including instructions to give a discretionary proxy to a person designated by the Company, for the manner in which any vote is cast, including, without limitation, any vote cast by a person to whom the Depositary is instructed to grant a discretionary proxy or deemed to have been instructed to grant a discretionary proxy or for the effect of any such vote. The Depositary shall endeavor to effect any sale of securities or other
8
|
|
|
|
|
|
|
Exhibit 2.1 2024 - Novo Nordisk |
property and any conversion of currency, securities or other property, in accordance with the Depositary's normal practices and procedures under the circumstances applicable to such sale or conversion, but shall have no liability (in the absence of its own willful default or gross negligence or that of its agents, officers, directors or employees) with respect to the terms of any such sale or conversion, including the price at which such sale or conversion is effected, or if such sale or conversion shall not be practicable, or shall not be believed, deemed or determined to be practicable by the Depositary. Specifically, the Depositary shall not have any liability for the price received in connection with any public or private sale of securities (including, without limitation, for any sale made at a nominal price), the timing thereof or any delay in action or omission to act nor shall it be responsible for any error or delay in action, omission to act, default or negligence on the part of the party so retained in connection with any such sale or proposed sale.
The Depositary shall not incur any liability in connection with or arising from any failure, inability or refusal by the Company or any other party, including any share registrar, transfer agent or other agent appointed by the Company, the Depositary or any other party, to process any transfer, delivery or distribution of cash, shares, other securities or other property, including without limitation upon the termination of the Deposit Agreement, or otherwise to comply with any provisions of the Deposit Agreement that are applicable to it.
The Depositary may rely upon instructions from the Company or its counsel in respect of any approval or license required for any currency conversion, transfer or distribution. The Depositary and its agents may own and deal in any class of securities of the Company and its affiliates and in ADRs. Notwithstanding anything to the contrary set forth in the Deposit Agreement or any ADR, the Depositary and its agents may fully respond to any and all demands or requests for information maintained by or on its behalf in connection with the Deposit Agreement, any Holder or any ADRs to the extent such information is requested or required by or pursuant to any lawful authority, including without limitation laws, rules, regulations, administrative or judicial process, banking, securities or other regulators.
None of the Depositary, the custodian or the Company, or any of their respective directors, officers, employees, agents or affiliates shall be liable for the failure by any Holder or beneficial owner to obtain the benefits of credits or refunds of non-U.S. tax paid against such Holder’s or beneficial owner’s income tax liability. The Depositary is under no obligation to provide the Holders and beneficial owners, or any of them, with any information about the tax status of the Company. None of the Depositary, the custodian or the Company, or any of their respective directors, officers, employees, agents and affiliates, shall incur any liability for any tax or tax consequences that may be incurred by Holders or beneficial owners on account of their ownership of the ADRs or ADSs.
The Depositary shall not incur any liability for the content of any information submitted to it by or on behalf of the Company for distribution to the Holders or for any inaccuracy of any translation thereof, for any investment risk associated with acquiring an interest in the Deposited Securities, for the validity or worth of the Deposited Securities, for the creditworthiness of any third party, for allowing any rights to lapse upon the terms of the Deposit Agreement or for the failure or timeliness of any notice from the Company.
Notwithstanding anything herein or in the Deposit Agreement to the contrary, the Depositary and the custodian(s) may use third-party delivery services and providers of information regarding matters such as, but not limited to, pricing, proxy voting, corporate actions, class action litigation and other services in connection herewith and the Deposit Agreement, and use local agents to provide services such as, but not limited to, attendance at any meetings of security holders of issuers. Although the Depositary and the custodian will use reasonable care (and cause their agents to use reasonable care) in the selection and retention of such third-party providers and local agents, they will not be responsible for any errors or omissions made by them in providing the relevant information or services.
The Depositary shall not be liable for any acts or omissions made by a successor depositary whether in connection with a previous act or omission of the Depositary or in connection with any matter arising wholly after the removal or resignation of the Depositary.
Notwithstanding any other provision of the Deposit Agreement or the ADR to the contrary, neither the Company nor the Depositary, nor any of their agents, shall be liable to the other for any indirect, special, punitive or consequential damages (collectively "Special Damages") except (i) to the extent such Special Damages arise from the gross negligence or willful misconduct of the party from whom indemnification is sought or (ii) to the extent Special Damages arise from or out of a claim brought by a third party (including, without limitation, Holders) against the Depositary or its agents, except to the extent such Special Damages arise out of the gross negligence or willful misconduct of the party seeking indemnification.
No provision of the Deposit Agreement or the ADR is intended to constitute a waiver or limitation of any rights which Holders or beneficial owners may have under the Securities Act of 1933 or the Securities Exchange Act of 1934, to the extent applicable.
I. DISCLOSURE OF INTEREST IN ADSs
To the extent that the provisions of or governing any Deposited Securities may require disclosure of or impose limits on beneficial or other ownership of Deposited Securities, other shares and other securities and may provide for blocking transfer, voting or other rights to enforce such disclosure or limits, Holders and all persons holding ADRs agree to comply with all such disclosure requirements and ownership limitations and to comply with any reasonable instructions the Company may provide in respect thereof. The Company reserves the right to instruct Holders to deliver their ADSs for cancellation and withdrawal of the Deposited Securities so as to permit the Company to deal with the Holder directly as a holder of shares and Holders agree to comply with such instructions. The Depositary agrees to cooperate with the Company in its efforts to inform Holders of the Company's exercise of its rights under this paragraph and agrees to consult with, and provide reasonable assistance without risk, liability or expense on the part of the Depositary, to the Company on the manner or manners in which it may enforce such rights with respect to any Holder. The Company may from time to time request Holders or beneficial owners of an interest in ADRs to provide information as to the capacity in which such Holders own or owned ADRs and regarding the identity of any other persons then or previously having a beneficial interest in such ADRs and the nature of such interest and various other matters. Each Holder agrees to provide any information requested by the Company or the Depositary pursuant to the Deposit Agreement.
M. BOOKS OF DEPOSITARY
9
|
|
|
|
|
|
|
Exhibit 2.1 2024 - Novo Nordisk |
The Depositary or its agent will keep a register for the registration, registration of transfer, combination and split-up of ADRs, which register shall include the Depositary’s direct registration system. Registered holders of ADRs may inspect such records at the Depositary’s office at all reasonable times, but solely for the purpose of communicating with Holders in the interest of the Company or a matter relating to the Deposit Agreement.
The Depositary will maintain facilities for the delivery and receipt of ADRs.
N. APPOINTMENT
Each Holder and each beneficial owner, upon acceptance of any ADSs or ADRs (or any interest in any of them) issued in accordance with the terms and conditions of the Deposit Agreement shall be deemed for all purposes to
•be a party to and bound by the terms of the Deposit Agreement and the applicable ADR(s),
•appoint the Depositary its attorney-in-fact, with full power to delegate, to act on its behalf and to take any and all actions contemplated in the Deposit Agreement and the applicable ADR(s), to adopt any and all procedures necessary to comply with applicable law and to take such action as the Depositary in its sole discretion may deem necessary or appropriate to carry out the purposes of the Deposit Agreement and the applicable ADR(s), the taking of such actions to be the conclusive determinant of the necessity and appropriateness thereof, and
•acknowledge and agree that (i) nothing in the Deposit Agreement or any ADR shall give rise to a partnership or joint venture among the parties thereto, nor establish a fiduciary or similar relationship among such parties, (ii) the Depositary, its divisions, branches and affiliates, and their respective agents, may from time to time be in the possession of non-public information about the Company, Holders, beneficial owners and/or their respective affiliates, (iii) the Depositary and its divisions, branches and affiliates may at any time have multiple banking relationships with the Company, Holders, beneficial owners and/or the affiliates of any of them, (iv) the Depositary and its divisions, branches and affiliates may, from time to time, be engaged in transactions in which parties adverse to the Company or the Holders or beneficial owners and/or their respective affiliates may have interests, (v) nothing contained in the Deposit Agreement or any ADR(s) shall (A) preclude the Depositary or any of its divisions, branches or affiliates from engaging in any such transactions or establishing or maintaining any such relationships, or (B) obligate the Depositary or any of its divisions, branches or affiliates to disclose any such transactions or relationships or to account for any profit made or payment received in any such transactions or relationships, (vi) the Depositary shall not be deemed to have knowledge of any information held by any branch, division or affiliate of the Depositary and (vii) notice to a Holder shall be deemed, for all purposes of the Deposit Agreement and this ADR, to constitute notice to any and all beneficial owners of the ADSs evidenced by such Holder's ADRs.
For all purposes under the Deposit Agreement and this ADR, the Holder hereof shall be deemed to have all requisite authority to act on behalf of any and all beneficial owners of the ADSs evidenced by this ADR.
O. GOVERNING LAW
The Deposit Agreement and this ADR shall be governed by and construed in accordance with the laws of the State of New York.
By holding an ADR or ADS or an interest therein, Holders and beneficial owners each irrevocably agree that any legal suit, action or proceeding against or involving Holders or beneficial owners brought by the Company or the Depositary, arising out of or based upon the Deposit Agreement, the ADSs, the ADRs or the transactions contemplated therein, may only be instituted in a state or federal court in New York, New York, and by holding or owning an ADR or ADS or an interest therein each irrevocably waives any objection that or owning an ADR or it may now or hereafter have to the laying of venue of any such proceeding, and irrevocably submits to the non-exclusive jurisdiction of such courts in any such suit, action or proceeding.
By holding or owning an ADR or ADS or an interest therein, Holders and beneficial owners each also irrevocably agree that any legal suit, action or proceeding against or involving the Depositary and/or the Company brought by Holders or beneficial owners, arising out of or based upon the Deposit Agreement, the ADSs, the ADRs or the transactions contemplated therein, including, without limitation, claims under the Securities Act of 1933, may be instituted only in the United States District Court for the Southern District of New York (or in the state courts of New York County in New York if either (i) the United States District Court for the Southern District of New York lacks subject matter jurisdiction over a particular dispute or (ii) the designation of the United States District Court for the Southern District of New York as the exclusive forum for any particular dispute is, or becomes, invalid, illegal or unenforceable).
P. JURY TRIAL WAIVER
EACH PARTY TO THE DEPOSIT AGREEMENT (INCLUDING, FOR THE AVOIDANCE OF DOUBT, EACH HOLDER AND BENEFICIAL OWNER OF, AND/OR HOLDER OF INTERESTS IN ADSS OR ADRS) IRREVOCABLY WAIVES, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, ANY RIGHT IT MAY HAVE TO A TRIAL BY JURY IN ANY SUIT, ACTION OR PROCEEDING AGAINST THE DEPOSITARY AND/OR THE COMPANY DIRECTLY OR INDIRECTLY ARISING OUT OF, BASED ON OR RELATING IN ANY WAY TO THE SHARES OR OTHER DEPOSITED SECURITIES, THE ADSS OR THE ADRS, THE DEPOSIT AGREEMENT OR ANY TRANSACTION CONTEMPLATED THEREIN, OR THE BREACH THEREOF (WHETHER BASED ON CONTRACT, TORT, COMMON LAW OR ANY OTHER THEORY), INCLUDING, WITHOUT LIMITATION, ANY SUIT, ACTION, CLAIM OR PROCEEDING UNDER THE UNITED STATES FEDERAL SECURITIES LAWS. No provision of this Deposit Agreement or any ADR is intended to constitute a waiver or limitation of any rights that a Holder or any beneficial owner may have under the Securities Act of 1933 or the Securities Exchange Act of 1934, to the extent applicable.
10
|
|
|
|
|
|
|
Exhibit 2.1 2024 - Novo Nordisk |
EX-2.2
3
exhibit22descriptionofther.htm
EX-2.2
Document
Exhibit 2.2
Description of the rights of B shares registered under Section 12 of the Securities Exchange Act of 1934
A. OFFER AND LISTING DETAILS
Novo Nordisk A/S (the “Company”) is a limited liability company organized under the laws of Denmark and registered with the Danish Business Authority under CVR number 24256790.
The Company has a total share capital of DKK 446,500,000, divided into an A share capital of nominally DKK 107,487,200 and a B share capital of nominally DKK 339,012,800. Each A share of DKK 0.10 carries 100 votes and each B share of DKK 0.10 carries 10 votes at General Meetings of the Company.
The Company’s B shares are listed in Denmark on Nasdaq Copenhagen, and traded under the symbol "NOVO-B" and on the New York Stock Exchange (NYSE) as American Depository Receipts ("ADRs"), traded under the symbol "NVO". Each of the Company’s A shares and B shares has been fully paid up and is registered, in the case of the A shares, on the Company's Register of Shareholders and, in the case of the B shares, by VP Securities, a central securities depositary in Denmark.
The A shares and the B shares have the rights, preferences and restrictions described below in “Memorandum and Articles of Association.”
Novo Nordisk conducted a stock split whereby the trading unit of the Novo Nordisk B shares listed on NASDAQ Copenhagen was changed from DKK 0.20 to DKK 0.10 as of September 13, 2023. The American Depositary Receipts (ADRs) listed on the New York Stock Exchange (NYSE) were similarly split as of September 20, 2023 to ensure that the ratio of B shares to ADRs remains 1:1.
B. MEMORANDUM AND ARTICLES OF ASSOCIATION
The following section summarizes certain material provisions of the Company’s Articles of Association, certain other constitutive documents and relevant Danish corporate law. For further information, see Exhibit 1.1 to this Form 20-F for a translation into the English language of the Articles of Association.
General
The Company’s objectives are to carry out research and development and to manufacture and commercialize pharmaceutical, medical and technical products and services as well as any other activity related thereto as determined by its Board of Directors. The Company strives to conduct its activities in a financially, environmentally and socially responsible way. The Company's objectives are set out in Article 2 of its Articles of Association.
Powers of the Board of Directors
All members of the Board of Directors have equal voting rights, and all resolutions are passed by a simple majority of votes. However, in the event of a tie, the Chair shall have the casting vote. The Board of Directors forms a quorum when at least a majority of its members is present.
According to the Danish Companies Act, no member of the Board of Directors or the Executive Management may take part in the consideration of any business involving agreements between any member of the Group and himself, legal actions brought against the individual, or any business involving agreements between any member of the Group and any third party or legal actions brought against any third party, if the individual has a major interest therein that might conflict with the Company’s interests. The Danish Companies Act also includes restrictions on the Company’s ability to grant loans or provide security to any member of the Board of Directors or anyone particularly close to such a member of the Board of Directors. The Company's ability to grant loans or provide security is subject to a number of conditions including shareholder approval or delegation of authorization to the Board of Directors by the General Meeting.
The remuneration of the Board of Directors must be approved by the Company’s shareholders at the Annual General Meeting.
Rights, restrictions and preferences attaching to the shares
If the shareholders at an Annual General Meeting approve a recommendation by the Board of Directors to pay dividends, dividends shall be distributed as follows: a priority dividend of 0.5% to the holders of A shares and then up to a dividend of 5% to the holders of B shares. Any distribution of additional dividends shall be subject to the provision that the holders of A shares shall never receive a total dividend exceeding the percentage rate of the dividend paid to the holders of B shares. A shares take priority for dividends below 0.5%. B shares take priority for dividends between 0.5% and 5%. However, in practice, A shares and B shares receive the same amount of dividends per share of DKK 0.01. Dividends on A shares shall be remitted to the shareholders at the addresses entered in the Company's Register of Shareholders as at the date of the Annual General Meeting. Dividends on B shares shall be paid with fully discharging effect for the Company through a central securities depository and an account-holding bank to shareholders registered by VP Securities at the time of payment.
The Board of Directors has been granted authority to distribute extraordinary dividends. This authority is included in the Articles of Association of the Company. Hence the Board of Directors has been granted authority to pay interim dividends without obtaining specific approval from the Annual General Meeting. Any Board resolution to pay extraordinary dividends must be accompanied by a balance sheet showing that sufficient funds are available for distribution. An authorized auditor must review the balance sheet.
1
|
|
|
|
|
|
|
Exhibit 2.2 2024 - Novo Nordisk |
Subject to the preference mechanism described above, the A shares and the B shares rank as equal in the event of a return on capital by the Company. Upon a winding-up, liquidation or otherwise, the B shares rank ahead of the A shares with regard to payment of each share’s nominal amount. All shares rank as equal in respect of further distributions from a winding-up.
Each A share of DKK 0.10 carries 100 votes and each B share of DKK 0.10 carries 10 votes at General Meetings. A shares are non-negotiable instruments whereas B shares are negotiable instruments.
The holders of A shares have a pro-rata right of first refusal with regard to any A shares sold by another shareholder. However, currently all A-shares are owned by Novo Holdings A/S and cannot be divested.
The share capital has been fully paid up and shareholders are not liable to further capital calls by the Company. No shareholder shall be obliged to have his shares redeemed in whole or in part. There is no sinking fund provision in the Articles of Association. There is no provision in the Articles of Association discriminating against any existing or prospective holder of such securities as a result of such shareholder owning a substantial number of shares. The members of the Board of Directors do not stand for reelection at staggered intervals and there is no cumulative voting arrangement.
Changes in shareholders’ rights
Changes in the rights of holders of A shares or B shares require an amendment of the Articles of Association. Unless stricter requirements are made under the Danish Companies Act for any such resolution to be passed, (i) at least 2/3 of the total number of votes in the Company shall be represented at the General Meeting, and (ii) at least 2/3 of the votes cast and of the voting share capital shall vote in favor of such a resolution. If the quorum requirement in (i) is not fulfilled, the Board of Directors shall within two weeks convene another General Meeting at which the resolution may be passed irrespective of the number of votes represented.
General Meetings
The Company’s General Meetings shall be held at a venue in the Capital Region of Denmark. Provided that certain conditions are met, as described in the Articles of Association and in the Danish companies act, the Board of Directors is authorized to resolve, when it considers it appropriate, that the General Meeting is held as a partially electronic or a fully electronic General Meeting. The Annual General Meeting shall be held before the end of April in every year. Extraordinary General Meetings shall be held as resolved by the General Meeting or the Board of Directors, or upon the request of the auditors or shareholders representing in total at least 5% of the share capital. The Extraordinary General Meeting shall then be called not later than two weeks after receipt of such request.
General Meetings shall be called by the Board of Directors not earlier than five weeks and not later than three weeks prior to the General Meeting. The notice calling such General Meeting, stating the agenda for the meeting, shall be published on the Company’s website: novonordisk.com (the contents of this website are not incorporated by reference into this Form 20-F). The notice convening the meeting shall also be forwarded in writing (by mail or email at the Company's choice) to all shareholders entered in the Register of Owners who have so requested.
A shareholder’s right to attend and vote at a General Meeting shall be determined by the shares or ADRs which such shareholder owns at the applicable record date. The Danish record date is one week prior to the General Meeting. Any shareholder who is entitled to attend the General Meeting is required to apply for an admission card to such General Meeting no later than three days prior to the date of such General Meeting. ADR holders who wish to attend the General Meeting in Denmark should contact Investor Relations, via e-mail to IRofficer@novonordisk.com.
The shares held by each shareholder at the Danish record date shall be calculated based on the registration of the shareholder’s shares in the Register of Owners as well as any notification received by the Company with respect to registration of shares in the Register of Owners, which have not yet been entered in the Register of Owners.
Ownership restrictions
Other than the Danish rules on screening of certain foreign direct investments ("FDI") etc. in Denmark (the “Danish FDI Rules”) and applicable international trade and financial sanctions as outlined below, there are no limitations on the right to hold or vote the B shares or the ADRs imposed by the laws of Denmark, the Articles of Association of the Company or any other of its constituent documents.
Under the Danish FDI Rules, a screening mechanism applies to foreign direct investments in certain sensitive sectors, if the foreign investor obtains at least 10% ownership or voting rights, or equivalent control by other means. Among such sensitive sectors are companies and entities within critical infrastructure in Denmark that are necessary to maintain or restore the production, registration, distribution and monitoring of prescription drugs. If a contemplated foreign direct investment in Novo Nordisk A/S is considered to fall within the scope of the mandatory screening mechanism, the foreign investor is required to apply for prior authorization with the Danish Business Authority. If a foreign investor fails to comply with the Danish FDI Rules, the Danish Business Authority may impose restrictions, inter alia, ordering a reversal of the investment or suspending the foreign investor‘s voting rights. FDI filings, notifications or approvals may under certain circumstances also be required in non-Danish jurisdictions.
International trade and financial sanctions are continually evolving. If applicable, such international trade and financial sanctions may under certain circumstances prevent the possibility of export and import of capital, and affect the remittance of dividends, interest and other payments to the non-resident holders of the B shares or the ADRs. In addition, the international trade and financial sanctions may also restrict the rights to acquire, transfer, hold or vote the B shares and ADRs. Failure to comply with international trade and financial sanctions can lead to criminal and civil liability.
2
|
|
|
|
|
|
|
Exhibit 2.2 2024 - Novo Nordisk |
Change of control
There is no provision in the Articles of Association, nor any other constituent document, that would have an effect of delaying, deferring or preventing a change in control of the Company and that would operate only with respect to a merger, acquisition or corporate restructuring involving the Company (or any of its subsidiaries). However, based on the current shareholder structure, the voting rights held by holders of A shares outlined above afford the Novo Nordisk Foundation, acting through its wholly-owned subsidiary Novo Holdings A/S, to have veto power against any change of control.
Ownership disclosure
According to the Danish Capital Markets Act and the Danish Companies Act, shareholders of the Company must notify the Danish Financial Supervisory Authority and the Company of their ownership if they own 5% or more of the voting rights or share capital. Also, shareholders must notify changes in holdings if thresholds of 5%, 10%, 15%, 20%, 25%, 50%, 90% or 100% and 1/3 and 2/3 of the voting rights or share capital are crossed.
Changes in capital
The Company’s Articles of Association do not contain conditions governing changes in the capital more stringent than those contained in the Danish Companies Act.
3
|
|
|
|
|
|
|
Exhibit 2.2 2024 - Novo Nordisk |
EX-11.1
4
exhibit111insidertradingpo.htm
EX-11.1
Document
Internal Rules on Insiders' Trading in Share and Bonds (Insider Rules)
1.Introduction
1.1Novo Nordisk’s B shares are listed on Nasdaq Copenhagen and its ADRs are listed on NYSE, while its Bonds are listed on Euronext Dublin.
1.2The purpose of these internal rules is inter alia to ensure that no insider trading, Market Manipulation or speculation takes place.
1.3The rules apply to General Insiders and Ad Hoc Insiders (“Insiders”).
1.4Additionally, the Board of Directors and the Executive Management of Novo Nordisk (and their respective Associated Persons) are subject to a special disclosure obligation regarding transactions conducted for their own account relating to Shares and Bonds.This disclosure obligation is regulated in a separate set of internal rules.1
1.5Definitions used in these internal rules are set out in Schedule I. Examples of Material News are set out in Schedule II.
2.Prohibition against insider trading
2.1Purchase, sale or recommendation to buy or sell Shares or Bonds - for its own account or for the account of a third party, directly or indirectly - to which that information relates may not be performed by any person in possession of Material News.
2.2The prohibition against insider trading does not apply to buying or selling of Shares or Bonds where the transaction is carried out in the discharge of an obligation that has become due in good faith and not to circumvent the prohibition against insider dealing and (a) that obligation results from an order placed or an agreement concluded before the person concerned possessed Material News or (b) the transaction is carried out to satisfy a legal or regulatory obligation that arose, before the person concerned possessed Material News.
2.3In addition, the members of the Board of Directors and Executive Management may not trade in Shares during a black-out period (e.g. when changing pension provider) under US pension plans established by Novo Nordisk for its Employees (e.g. US 401k pension plan).
3.Prohibition against Market Manipulation and speculation
3.1Market Manipulation or attempts at such manipulation is in general prohibited and Insiders are also prohibited from engaging in speculative transactions involving Shares or Bonds.
4.Trading Window
4.1Insiders may only buy, sell and subscribe for Shares or Bonds for their own or any third party’s account in an open Trading Window.
4.2Regardless of whether the Trading Window is open in accordance with section 4.1, or whether permission has been granted pursuant to section 4.3, the general prohibition against insider trading applies if the person in question is in possession of Material News.
4.3Buying, selling and subscribing for Shares or Bonds may not take place outside an open Trading Window, unless the Chairman of the Disclosure Committee approves the transaction in advance and in writing. Permission will only be granted if special circumstances exist and will as a main rule only be granted in situations where a right is exercisable within a time limit on which the holder of the right has no influence, for example (i) the subscription for employee shares, (ii) exercise or sale of subscription rights, (iii) exercise of warrants, share options etc., (iv) acceptance of acquisition offers, (v) exercise of pre-emption rights or obligations, or (vi) similar special cases.
5.Prohibition on trading in shares in companies outside the Novo Nordisk Group
5.1Insiders who have information not known to the general public, obtained through their work in the Novo Nordisk Group and which, if it was made public, would be likely to have a significant effect on the price of the shares in companies outside the Novo Nordisk Group, or other confidential information, are prohibited from using such information to trade, or recommend or induce other persons to trade, shares in companies outside the Novo Nordisk Group for their own or a third party’s account.
5.2The prohibition on trading in shares in companies outside the Novo Nordisk Group applies irrespective of whether the Trading Window is open in accordance with section 4.
1 Internal Rules on Notification of Trading in Shares made by Board Members and Executives.
1
|
|
|
|
|
|
|
Exhibit 11.1 2024 - Novo Nordisk |
6.Sanctions
6.1A violation of the prohibitions set out above may be punishable by a fine or – depending on the circumstances – imprisonment.
6.2A violation of the provisions in these rules may furthermore have employment consequences in the form of a warning and, in aggravated cases, dismissal without notice, and Novo Nordisk may have to report such violation to the DFSA and/or report the Employee or member of Management to the police.
6.3In addition, a violation can result in sanctions against Novo Nordisk.
7.Schedule
7.1The ‘Terms and Definitions re Material News and Insiders’ Trading’ dated 26 October 2023 is deemed to be an integral part of these Rules. The Terms and Definitions include
•Schedule I – Terms and definitions
•Schedule II – Material News
•Schedule III – Specific disclosure requirements
•Schedule IV – Trading details to be provided by PDMRs
8.Governing Law
8.1These Rules shall be governed by and be construed in accordance with the laws of Denmark. Any dispute arising in connection with these Rules shall be submitted to the exclusive jurisdiction of the competent court in Denmark.
---o0o---
These rules have been adopted by the Disclosure Committee on 26 October 2023 with effect from 30 October 2023.
Internal Rules on Notification of Trading in Shares made by Board Members and Executives (PDMR notification rules)
1.Introduction
1.1Novo Nordisk’s B shares are listed on Nasdaq Copenhagen and its ADRs are listed on NYSE, while its Bonds are listed on Euronext Dublin.
1.2The purpose of these internal rules is to ensure that members of the Board of Directors and the Executive Management of Novo Nordisk and their Associated Persons disclose trading in Shares to the Danish Financial Supervisory Authority (“DFSA”).
1.3Board members and executives are obliged to ensure that their spouse/cohabitant and other related persons are informed about the existence of these rules and their significance as well as applicable disclosure requirements.
1.4Definitions used in these internal rules are set out in Schedule I.
2.Background
2.1Board members and executives and their Associated Persons must electronically notify the DFSA and Novo Nordisk of trading in Shares conducted for their own account. The notification must be made immediately and if special circumstances apply no later than two business days after the transaction.
2.2Board members and executives are obliged to ensure that their Associated Persons are familiar with the fact that they shall notify the DFSA and the relevant board member/executive of trading conducted for their own account relating to Shares immediately and if special circumstances apply no later than two business days after each such trading.
2.3All trades in Shares must be reported, including:
a.purchases, sales, and share loans,
b.advancements on inheritance and gifts,
c.transfers for security purposes (e.g. pledges),
d.grants, allotments or delivery of Shares or share options to executives, including Shares delivered to executives upon vesting of any such instruments, and
2
|
|
|
|
|
|
|
Exhibit 11.1 2024 - Novo Nordisk |
e.exercise of a previously allotted share option, regardless of whether this option is exercised by purchasing Shares or by balance settlement.
3.Notification procedures
3.1Novo Nordisk will arrange for notification to the DFSA on behalf of the board member/executive and their Associated Persons. Accordingly, the board member/executive must notify Novo Nordisk about trading, including trading by Associated Persons, immediately after the trading. The notification shall, to the extent possible, be received by Novo Nordisk the same day as the relevant transaction has been conducted, and if special circumstances apply in sufficient time to enable Novo Nordisk to file with the DFSA on behalf of the board member/executive and their Associated Persons within the deadline of two business days after the trading. The notification to Novo Nordisk must contain the trading details set out in Schedule IV and be directed to the mailbox “Tradereport”.
3.2By notifying Novo Nordisk of trading subject to notification to the DFSA, the board member/executive and their Associated Persons authorises Novo Nordisk to file the trading with the DFSA on behalf of the board member/executive and their Associated Persons. Each individual transaction carried out by the board member/executive and their Associated Persons must be reported to Novo Nordisk.
3.3Board members and executives and their Associated Persons shall each sign a power of attorney prepared by Corporate Legal at the first ordinary board meeting following the annual general meeting, or in connection with the first time a board member/executive gives notification to Novo Nordisk of trading, if earlier.
4.Publication of trading in Shares
4.1Notifications of trading received by Novo Nordisk from a board member/executive, including trading by Associated Persons, will be published by Novo Nordisk via Nasdaq Copenhagen as soon as possible following the receipt of the notification and simultaneously with the communication to be made to the DFSA.
5.Other reporting obligation
5.1A board member/executive shall upon election and appointment inform Novo Nordisk of the size of the board member’s/executive’s direct and indirect holding(s) of Shares. Any subsequent transactions in respect of Shares shall be reported in accordance with the provisions herein.
6.Bonds
6.1The principles referred to above shall also apply with the necessary modifications (mutatis mutandis) to trading in Bonds. If Bonds are issued by a subsidiary of Novo Nordisk, the above shall also apply with the necessary modifications (mutatis mutandis) to trading in Bonds by the management of the subsidiary.
7.Sanctions
7.1Any violation of the rules on notification and disclosure of relevant information regarding trading in Shares made by a board member/ executive and their Associated Persons is punishable by fine pursuant to MAR.
8.Schedule
8.1The ‘Terms and Definitions re Material News and Insiders’ Trading’ dated 26 October 2023 is deemed to be an integral part of these Rules. The Terms and Definitions include
•Schedule I – Terms and definitions
•Schedule II – Material News
•Schedule III – Specific disclosure requirements
•Schedule IV – Trading details to be provided by PDMRs
9.Governing Law
9.1These Rules shall be governed by and be construed in accordance with the laws of Denmark. Any dispute arising in connection with these Rules shall be submitted to the exclusive jurisdiction of the competent court in Denmark.
---o0o---
These rules have been adopted by the Disclosure Committee on 26 October 2023 with effect from 30 October 2023.
3
|
|
|
|
|
|
|
Exhibit 11.1 2024 - Novo Nordisk |
Internal Rules on Trading in Own Share and Bonds
1.Introduction
1.1Novo Nordisk’s B shares are listed on Nasdaq Copenhagen and its ADRs are listed on NYSE, while its Bonds are listed on Euronext Dublin.
1.2The purpose of these internal rules is to ensure that no insider trading and Market Manipulation by Novo Nordisk take place and to ensure that Novo Nordisk fulfils its disclosure requirements in relation to trading in its Shares and the Bonds, respectively.
1.3These internal rules shall apply to all companies in the Novo Nordisk Group.
1.4Definitions used in these internal rules are set out in Schedule I. Examples of Material News are set out in Schedule II.
2.Prohibition against insider trading and Market Manipulation
2.1Novo Nordisk is prohibited from trading in Shares and the Bonds, respectively, if the Novo Nordisk Group is in possession of Material News.
2.2The prohibition against insider trading does not apply to buying and selling Shares and the Bonds, respectively, conducted in the discharge of an obligation, provided that the obligation has become due at the time of the transaction, and where that said obligation results from an agreement concluded before the person concerned came into possession of Material News.
2.3Trading in treasury shares under buy-back programmes or trading in Shares and the Bonds, respectively, as part of the stabilisation of the price of a Share carried out in accordance with the Safe Harbour Rules is not subject to the prohibition against insider trading or Market Manipulation.
2.4Market Manipulation or attempts at such manipulation must not take place.
3.Ban on trading in Shares
3.1Regardless of whether a company within the Novo Nordisk Group would otherwise be entitled to purchase or sell Shares, such company shall not for its own or a third party’s account trade in Shares during the 15 trading days prior to publication of the Company’s interim report or announcement of annual results.
3.2The Disclosure Committee may decide to derogate from the provisions of sections 3.1 and 4.2, (i) in special cases where this may be necessary in order to avoid significant or threatening damage to the Company; (ii) in connection with general share buy-back programmes if the buy-back is not based on Material News of which disclosure has been postponed; (iii) in connection with the exercise of options programmes; or (iv) in similar cases where the time of purchase or sale has been fixed in advance. However, trading in Novo Nordisk’s Shares may never take place immediately prior to publication of Novo Nordisk’s interim reports or announcement of annual results.
3.3Despite the ban on trading in own shares, Novo Nordisk may continue a buy-back programme, if the programme complies with the Safe Harbour Rules.
4.Procedure in connection with trading in own shares
4.1Trading in own shares by the Company and its subsidiaries shall only be effected if prior consent has been given by the Disclosure Committee and the trading is not based on Material News of which disclosure has been postponed. This shall apply if Novo Nordisk for example wants to
1.Initiate a general buy-back programme.
2.Continue a general buy-back programme after ordinary suspension.
3.Continue a general buy-back programme after extraordinary suspension.
4.Grant share options.
5.Execute an employee Share programme.
6.Purchase Shares from an identified seller.
4.2A general buy-back programme, handled by one or more investment bankers, shall as far as possible only be established in the period from the publication of the preliminary announcement of the annual results and the interim reports up to and including the 15th calendar day after publication, and in any event only if the Disclosure Committee has stated that the buy-back programme is not based on Material News of which disclosure has been postponed.
5.Disclosure requirements in connection with purchase and sale of own shares
5.1If Novo Nordisk acquires or sells own shares, whereby Novo Nordisk’s holding of own shares reaches, exceeds or falls to less than 5%, 10%, 15%, 20%, 25%, 1/3, 50%, 2/3 or 90% of the voting rights or the share capital in the Company, the Company shall immediately publish an announcement describing the transaction and holdings after the transaction.
4
|
|
|
|
|
|
|
Exhibit 11.1 2024 - Novo Nordisk |
5.2When calculating Novo Nordisk’s holding of own shares, Shares that are owned by Novo Nordisk’s subsidiaries shall also be included. Acquisitions and sales include acquisitions and sales transacted by Novo Nordisk itself as well as acquisitions and sales that other persons transact in their own name but for the account of Novo Nordisk.
5.3In addition to disclosing the holding, Novo Nordisk is obliged to simultaneously notify the Danish Financial Supervisory Authority (“DFSA”) pursuant to the rules for filing of major shareholder notifications.
5.4If the purchase of own shares takes place under the Safe Harbour Rules, Novo Nordisk shall in addition give notification pursuant to such rules. Moreover, Novo Nordisk must adhere to the publication requirements issued by the US SEC.
6.Trading in Bonds
6.1In case it is contemplated to trade in Bonds, the Disclosure Committee shall sign off to ensure that it is carried out in a compliant manner.
7.Sanctions
7.1A violation of the prohibitions against insider trading and Price Manipulation as described above may be punishable by a fine or – depending on the circumstances – imprisonment.
7.2A violation of the provisions in these rules may furthermore have employment consequences in the form of a warning and, in aggravated cases, dismissal without notice, and Novo Nordisk may have to report such violation to the DFSA and/or report the Employee or member of Management to the police.
7.3In addition, a violation can result in sanctions against Novo Nordisk.
8.Schedule
8.1The ‘Terms and Definitions re Material News and Insiders’ Trading’ dated 26 October 2023 is deemed to be an integral part of these Rules. The Terms and Definitions include
•Schedule I – Terms and definitions
•Schedule II – Material News
•Schedule III – Specific disclosure requirements
•Schedule IV – Trading details to be provided by PDMRs
9.Governing Law
9.1These Rules shall be governed by and be construed in accordance with the laws of Denmark. Any dispute arising in connection with these Rules shall be submitted to the exclusive jurisdiction of the competent court in Denmark.
---o0o---
These rules have been adopted by the Disclosure Committee on 26 October 2023 with effect from 30 October 2023.
Terms and definitions re Material News and Insiders' Trading
1.Introduction
1.1Novo Nordisk’s B shares are listed on Nasdaq Copenhagen and its ADRs are listed on NYSE, while its Bonds are listed on Euronext Dublin.
1.2The purpose of this document is to have a uniform set of schedules across the internal rules relating to Material News and Insiders’ trading.
1.3Terms and Definitions are set out in Schedule I.
1.4Examples of Material News are set out in Schedule II.
5
|
|
|
|
|
|
|
Exhibit 11.1 2024 - Novo Nordisk |
1.5A non-exhaustive list of specific disclosure obligations is set out in Schedule III.
1.6Trading details to be provided by PDMRs are set out in Schedule IV.
2.Governing Law
2.1This document shall be governed by and be construed in accordance with the laws of Denmark. Any dispute arising in connection with this document shall be submitted to the exclusive jurisdiction of the competent court in Denmark.
---o0o---
This document has been adopted by the Disclosure Committee on 26 October 2023 with effect from 30 October 2023.
6
|
|
|
|
|
|
|
Exhibit 11.1 2024 - Novo Nordisk |
Schedule I - Terms and definitions
|
|
|
|
|
|
| Term |
Definition |
| ADRs (American Depository Receipts) |
Securities representing Novo Nordisk Shares listed on the New York Stock Exchange. |
| Ad Hoc Insiders |
Employees who have access to Material News on an occasional basis and are listed on Ad Hoc Insider Lists. |
| Ad Hoc Insider Lists |
Separate lists on for example a specific development project or M&A project comprising Ad Hoc Insiders who have access to Material News on the specific project. |
| Articles of Association |
Articles of Association of Novo Nordisk as in force from time to time. |
| Associated Person(s) |
Means the following persons associated with a member of the Board of Directors or Executive Management:
a.Spouse or cohabitant;
b.Children under the age of 18 years, where the relevant person is the parent holding custody;
c.Other relatives (including but not limited to brothers, sisters, parents, grandparents, children, grandchildren, cousins etc.) who have shared the same household with the relevant person for a period of at least one year on the date of the given transaction; and
d.Legal persons, trust, or partnership, the managerial responsibilities of which are discharged by a person discharging managerial responsibilities or by a person referred to in items (a), (b) or (c) above, which
i.Directly or indirectly hold the control or
ii.Hold the daily managerial responsibilities or
iii.Are set up for his/her benefit or the economic interests of the legal person are substantially equivalent to the Board member or member of the Executive Management or natural associated person.
|
| Board of Directors |
The Board of Directors of Novo Nordisk |
| Bonds |
Listed as well as non-listed debt instruments of the Novo Nordisk Group, including bonds and derivatives or other financial instruments linked there to. |
| CEO |
The Chief Executive Officer of Novo Nordisk. |
| CFO |
The Chief Financial Officer of Novo Nordisk. |
| Company |
Novo Nordisk A/S, company registration no. (CVR) 24256790, Novo Alle 1, 2880 Bagsværd, Denmark. |
| Core Team regarding Form 20-F |
The core team responsible for the due and timely submission of the Form 20-F. |
| Corporate Communications |
The corporate communication department of Novo Nordisk. |
| Corporate Legal |
The corporate legal department of Novo Nordisk. |
| Danish Companies Act |
The Danish Companies Act in force from time to time. |
| DFSA |
The Danish Financial Supervisory Authority. |
| Disclosure Committee |
A committee established by Executive Management and chaired by the CFO. The Disclosure Committee has amongst other things responsibility for considering the materiality of information and determining disclosure obligations. |
| Editorial Board |
The editorial board in respect of the annual report of Novo Nordisk. |
| Executive Management |
The executive management of Novo Nordisk. |
| Employee(s) |
Any Employee of the Novo Nordisk Group. |
| Euronext Dublin |
Euronext Dublin, Ireland |
| Form 20-F |
A form issued by the US Securities and Exchange Commission for the submission of annual reports by foreign companies with equity listed in the United States. |
| General Insiders |
Permanent Insiders and other employees with regular access to Material News who by the Disclosure Committee have been categorised as General Insiders. |
| Insiders |
General Insiders and Ad Hoc Insiders. |
| Insider Register |
IT platform comprising a list of General Insiders and Ad Hoc Insiders. |
| Investor Relations |
The investor relations department of Novo Nordisk. |
| Management |
The Board of Directors and Executive Management. |
| MAR |
Market Abuse Regulation as amended from time to time. |
7
|
|
|
|
|
|
|
Exhibit 11.1 2024 - Novo Nordisk |
|
|
|
|
|
|
| Term |
Definition |
| Market Manipulation |
Market Manipulation is defined in MAR Article 12 and may include, but is not limited to:
a.entering into a transaction, placing an order to trade in the Shares and the Bonds, respectively, of Novo Nordisk or any other behaviour which
i.gives or is likely to give, false or misleading signals as to the supply of, demand for, or price of Shares and the Bonds, respectively, of Novo Nordisk or
ii.secures, or is likely to secure, the price of one or several Shares and the Bonds, respectively, at an abnormal or artificial level unless carried out for legitimate reasons and conform with accepted market practice in accordance with MAR Article 13;
b.entering into a transaction, placing an order to trade in the Shares and the Bonds, respectively, of Novo Nordisk or any other activity or behaviour which affects or is likely to affect the price of Shares and the Bonds, respectively, of Novo Nordisk which employs a fictitious device or any other form of deception or contrivance;
c.disseminating information through the media, including the internet, or by any other means, which gives, or is likely to give, false or misleading signals as to the supply of, the demand for, or price of, Shares and the Bonds, respectively, of Novo Nordisk, or is likely to secure, the price of the Shares and the Bonds, respectively, of Novo Nordisk, at an abnormal or artificial level, including the dissemination of rumours, where the person who made the dissemination knew, or ought to have known, that the information was false or misleading; or
d.transmitting false or misleading information or providing false or misleading inputs in relation to a benchmark where the person who made the transmission or provided the input knew or ought to have known that it was false or misleading, or any other behaviours which manipulates the calculation of a benchmark.
The following behaviour shall, inter alia, be considered as market manipulation:
a.conduct by a person, or persons acting in collaboration, to secure a dominant position over the supply of or demand for Shares and the Bonds, respectively, of Novo Nordisk which has or I likely to have the effect of fixing, directly or indirectly, purchase or sale prices of the Shares and the Bonds, respectively, at an abnormal or artificial level or creating other unfair trading conditions for the transaction;
b.the buying or selling of Shares and the Bonds, respectively, of Novo Nordisk at the opening or close of the market which has or is likely to have the effect of misleading investors acting on the basis of the prices displayed, including the opening or closing price;
c.the placing of orders to a trading venue, including any cancellation or modification thereof, by any available means of trading, including by electronic means, such as algorithmic or high-frequency trading strategies, and which has one of the effects referred to above under a) or b) by; (i) disrupting or delaying the functioning of the trading system of the trading venue or being likely to do so, (ii) making it more difficult for other persons to identify genuine orders on the trading system of the trading venue or being likely to do so, including by entering orders which in the overloading or destabilisation of the order book, or (iii) creating or being likely to create a false or misleading signal about the supply of, or demand for or price of Shares and the Bonds, respectively, in particular by entering orders to initiate or exacerbate a trend; or
d.the taking advantage of occasional or regular access to the traditional or electronic media by voicing an opinion about the Shares and the Bonds, respectively, of Novo Nordisk while having previously taken positions on that Shares and the Bonds, respectively, and profiting subsequently from the impact of the opinions voiced on the price of the Shares and the Bonds, respectively, without having simultaneously disclosed the conflict of interest to the public in a proper and effective way.
|
8
|
|
|
|
|
|
|
Exhibit 11.1 2024 - Novo Nordisk |
|
|
|
|
|
|
| Term |
Definition |
| Material News |
News that constitute or may constitute inside information as defined in MAR.
MAR Article 7(1) defines inside information as:
information of a precise nature which has not been made public, relating, directly or indirectly, to one or more issuers or to one or more financial instruments, and which, if it were made public, would be likely to have a significant effect on the prices of those financial instruments or on the price of related derivative financial instruments.
Information of a “precise nature” shall mean information which indicates a set of circumstances which exists or may reasonably be expected to come into existence or an event which has occurred or may reasonably be expected to occur where it is specific enough to enable a conclusion to be drawn as to the possible effect of that set of circumstances or event on the prices of the financial instruments or related derivative financial instruments.
In the case of a protracted process that is intended to bring about, or that results in, particular circumstances or a particular event, those future circumstances or that future event, and also the intermediate steps of that process which are connected with bringing about or resulting in those future circumstances or that future event, may be deemed to be precise information.
An intermediate step in a protracted process shall be deemed to be Inside Information if, by itself, it satisfies the criteria of Inside Information.
Information which, if it were made public, would be likely to have a significant effect on the prices of financial instruments, derivative financial instruments etc. shall mean information which a reasonable investor would be likely to use as part of the basis of investment decisions.
Examples of information which may constitute Material News are set out in Schedule II. |
| Nasdaq Copenhagen |
Nasdaq Copenhagen A/S, Denmark. |
| Non-Insiders |
Employees not comprised by the Internal Rules on Insiders’ Trading in Shares, i.e. Employees who are not General Insiders or Ad Hoc Insiders. |
| Novo Nordisk |
Novo Nordisk A/S, company registration no. (CVR) 24 25 67 90, Novo Alle 1, 2880 Bagsværd, Denmark. |
| Novo Nordisk Group |
Novo Nordisk and its subsidiaries from time to time. |
| NYSE |
New York Stock Exchange, The United States. |
| Permanent Insiders |
Employees with access to all Material News at all times (as determined by the Disclosure Committee and listed by Corporate Legal). |
| Project Vice President |
A person responsible for important global project(s) with high business impact for Novo Nordisk. |
| Rules for Issuers |
Rules for issuers of shares on Nasdaq Copenhagen. |
| Safe Harbour Rules |
The rules issued pursuant to MAR’s Article 5. |
| Shares |
Listed as well as non-listed Novo Nordisk equity related securities including shares, ADRs, share related securities such as futures, options, warrants and convertible bonds and other debt instruments with similar economic effect, derivatives or other financial instruments linked there to. Listing includes listing on any stock exchange in the world. |
| Stock Exchanges |
Nasdaq Copenhagen, the New York Stock Exchange and Euronext Dublin. |
| Trading Window |
Period of 15 calendar days calculated from the day of the announcement of the annual results or publication of an interim report in which trading with Shares by Insiders may take place. |
|
|
|
|
9
|
|
|
|
|
|
|
Exhibit 11.1 2024 - Novo Nordisk |
EX-12.1
5
exhibit121certificationlfj.htm
EX-12.1
Document
Exhibit 12.1
CERTIFICATION ON THE EFFECTIVENESS OF DISCLOSURE CONTROLS AND PROCEDURES IN FORM 20-F FOR 2024
I, Lars Fruergaard Jørgensen, certify that:
1.I have reviewed this annual report on Form 20-F of Novo Nordisk A/S;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report;
4.The company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and
5.The company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting.
Date: February 5, 2025
|
|
|
|
|
|
| /s/ Lars Fruergaard Jørgensen |
|
Lars Fruergaard Jørgensen
President and chief executive officer |
|
EX-12.2
6
exhibit122certificationkmk.htm
EX-12.2
Document
Exhibit 12.2
CERTIFICATION ON THE EFFECTIVENESS OF DISCLOSURE CONTROLS AND PROCEDURES IN FORM 20-F FOR 2024
I, Karsten Munk Knudsen, certify that:
1.I have reviewed this annual report on Form 20-F of Novo Nordisk A/S;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report;
4.The company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and
5.The company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting.
Date: February 5, 2025
|
|
|
|
|
|
| /s/ Karsten Munk Knudsen |
|
Karsten Munk Knudsen
Executive vice president and
chief financial officer |
|
EX-13.1
7
exhibit131906certification.htm
EX-13.1
Document
Exhibit 13.1
CERTIFICATION OF LARS FRUERGAARD JØRGENSEN, CHIEF EXECUTIVE OFFICER OF NOVO NORDISK A/S, AND KARSTEN MUNK KNUDSEN, CHIEF FINANCIAL OFFICER OF NOVO NORDISK A/S, PURSUANT TO SECTION 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Novo Nordisk A/S (the "Company") on Form 20-F for the period ending December 31, 2024, as filed with the Securities and Exchange Commission on the date hereof (the "Report"), the undersigned hereby certify that to the best of our knowledge:
1.The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
2.The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: February 5, 2025
|
|
|
|
|
|
|
|
|
| /s/ Lars Fruergaard Jørgensen |
|
/s/ Karsten Munk Knudsen |
Lars Fruergaard Jørgensen
President and chief executive officer
|
|
Karsten Munk Knudsen
Executive vice president and
chief financial officer |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________________
FORM 6-K
________________________
REPORT OF FOREIGN PRIVATE ISSUER
Pursuant to rule 13a-16 or 15d-16
of the Securities Exchange Act of 1934
February 5, 2025
_________________________
NOVO NORDISK A/S
(Exact name of Registrant as specified in its charter)
_________________________
Novo Allé
DK-2880 Bagsværd
Denmark
(Adress of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F
Form 20-F x Form 40-F o
Indicate by check mark whether the registrant by furnishing the information contained in this Form is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes o No x
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g-32(b):82-_______

|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review |
|
Sustainability statement |
|
Financial statements
and additional information |
|
|
4
5
7
8
9
10
11
12
17
26
32
38
41
42
45
|
Introducing Novo Nordisk
Letter from the Chair and the CEO
Key figures
Purpose and strategy
Value creation
Strategic Aspirations 2025 progress
Strategic Aspirations
Purpose and sustainability
Innovation and therapeutic focus
Commercial execution
Financials
Risks
Management
Board of Directors
Executive Management
|
|
47
47
49
50
51
52
54
54
60
64
65
67
69
71
71
80
88
90
90
95
|
General information
ESG Performance
Basis for preparation of the
Sustainability statement
Sustainability governance
Interests and views of stakeholders
Double materiality assessment
Environment
Climate change
Resource use and circular economy
Pollution
Water
Biodiversity and ecosystems
EU Taxonomy
Social
Patient protection and quality of life
Own workforce
Workers in the value chain
Governance
Business conduct
Appendix
|
|
|
101
102
103
104
105
106
138
138
139
141
143
144
145
146
|
Consolidated financial statements
Income statement and Statement
of comprehensive income
Cash flow statement
Balance sheet
Equity statement
Notes to the Consolidated
financial statements
Statements and auditor’s reports
Statement by the Board of Directors
and Executive Management
Left blank intentionally
Left blank intentionally
Additional information
More information
Product overview
Financial statements of the parent company
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A new chapter in our integrated reporting |
|
The Annual Report 2024 marks a significant step in the evolution of Novo Nordisk’s integrated reporting. This year,
our Sustainability statement is for the first time prepared according to the EU Corporate Sustainability Reporting
Directive (CSRD) requirements. |
|
|
|
|
|
We have been committed to integrated reporting since 2004, when we
first started evaluating our performance based on social, environmental
and financial impact. This commitment was further strengthened in 2019
with the adoption of our Strategic Aspirations 2025, which cover our
financial and sustainability ambitions.
This year, in line with the CSRD, we have conducted a double materiality assessment to identify the sustainability matters that are most important
to Novo Nordisk, considering both societal and financial implications.
The essential topics identified include patient protection and quality
of life, climate change, resource use and circular economy, and own workforce – reflecting our aspirations of progress towards zero environmental impact, being respected for adding value to society
and being a sustainable employer.
The outcomes of this assessment have provided us with key metrics to
track our performance across our material sustainability topics. You can
read more about our progress towards achieving our sustainability
ambitions in the Annual review on page 12, while detailed breakdowns of
our performance can be found in the Sustainability statement on page 46.
Together, these sections make up this year’s Management report.
Moreover, our commitment to sustainability is reflected in our incentive programmes, which incorporate our Strategic Aspirations 2025 into
both individual and corporate performance targets. This highlights our dedication to driving sustainable growth and creating long-term value
for all stakeholders. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Introducing Novo Nordisk / Letter from the Chair and the CEO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Building a healthier tomorrow |
|
|
|
|
|
|
|
|
2024 was a year of significant growth for Novo Nordisk, characterised by continued innovation, capacity expansions and strong
commercial execution. As we reflect on our progress, we also recognise the magnitude of the challenges that lie ahead. |
Chair of the Board
of Directors,
Helge Lund (left) and
President and CEO,
Lars Fruergaard
Jørgensen (right). |
|
|
|
The global prevalence of serious chronic diseases is growing by the day, impacting millions
of lives and placing a heavy burden on overstretched healthcare systems. This has created unprecedented demand for our life-changing GLP-1-based medicines. Over the past four
years, we have more than quadrupled the number of people reached with these treatments
and increased our volume market share in the GLP-1 segment to 63%. In 2024, we served
more than 45.2 million people living with serious chronic diseases, while our global sales
and operating profit both grew by 26% at constant exchange rates.
As we strive to keep pace with the growing demand for our medicines, our production
capacity has been stretched. In response, we have continued to invest heavily in scaling up
our manufacturing capabilities with capital expenditure and acquisitions amounting to more than DKK 129 billion in 2024. The acquisition of three fill-finish sites formerly run by contract
and development manufacturer Catalent Inc., along with significant expansions of our existing production facilities in Denmark, France, Brazil, China and the US, are testament to our commitment to improving supply stability.
In order to meet increasing demand and ensure a stable supply of our medicines, we are also taking steps to consolidate our product portfolio by gradually phasing out some of our older insulin products. This will create much-needed space in our global manufacturing network as
we seek to reach millions more people with our medicines over the next decade. At the same time, we strive not to leave existing patients without alternative treatment options, either from Novo Nordisk or other companies, and we remain committed to working closely with local health authorities and the medical community to enable access to affordable care.
Our belief that health is a fundamental human right drives our extensive partnership
programmes and access initiatives. In times of geopolitical instability, safeguarding access to care for those in conflict zones and underserved areas is paramount. Our partnerships with humanitarian organisations such as the Danish Red Cross play a crucial role in this effort, demonstrating our dedication to making a difference where it is needed most. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Introducing Novo Nordisk / Letter from the Chair and the CEO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Moreover, we are increasing our investment in preventive health measures through initiatives
like Cities for Better Health – a pioneering urban health partnership now active in 51 cities worldwide – and our collaboration with UNICEF to prevent childhood obesity. These efforts
aim to address the root causes of serious chronic diseases, thereby reducing the global health
burden and fostering a healthier future. Our Transformational Prevention Unit complements
our partnership-driven approach, looking to develop scalable, science-based solutions that can predict and pre-empt obesity and its consequences.
The same scientific rigour is being applied across our R&D activities, which are driving
transformative change across multiple therapy areas. Rooted in our deep understanding of
proteins and peptides and fuelled by research partnerships, AI-driven drug discovery and the acquisition of new technology platforms, we are striving to accelerate the discovery of new
targets and optimise our clinical trials to the benefit of people living with serious chronic diseases.
Innovation remains our core contribution to society and the driving force behind our continued growth. The past year has seen us add to the growing body of clinical evidence supporting the broad cardiometabolic and societal benefits of semaglutide – the molecule at the heart of our flagship GLP-1-based medicines Ozempic®, Wegovy® and Rybelsus® – and we are confident that our pipeline has the potential to add even more value.
In obesity, we completed the first phase 3 trial of CagriSema, currently in development for the treatment of obesity or overweight and type 2 diabetes. After 68 weeks, if all people adhered to treatment, CagriSema demonstrated a statistically significant weight loss of 22.7% vs 2.3% with placebo alone. This is among the highest weight reductions yet seen in a phase 3a programme for a GLP-1 combination therapy. We intend to further explore the weight loss potential of CagriSema in an additional study.
Earlier in our obesity pipeline, topline results from a phase 1b/2a trial of subcutaneous
amycretin have demonstrated the weight lowering potential of the unimolecular GLP-1 and amylin receptor agonist, supporting previous data seen with the oral formulation. When evaluating the effects of treatment if all people adhered to treatment, those receiving a 20 mg dose of amycretin experienced an estimated average weight loss of 22.0% over 36 weeks compared to 2% weight gain with placebo.
In diabetes, the first launches of Awiqli® – the world’s first once-weekly basal insulin – exemplify our enduring commitment to innovation in this space more than 100 years after we first started producing insulin. Moreover, our dedication to addressing unmet needs within rare disease is exemplified by the pending regulatory submission of Mim8 for the treatment of haemophilia A.
The growth of our business has inevitably led to an increase in our environmental footprint, and we are stepping up efforts to mitigate this impact. We have introduced comprehensive, updated roadmaps targeting reductions in our emissions, plastic footprint and impact on nature and
|
|
biodiversity. Achieving these ambitions will be no small feat given the increasing global demand for
our medicines, but we are rising to the challenge. Our roadmaps include measures to decouple our environmental impact from our continued growth by incorporating the use of low-carbon materials across our value chain, supporting our suppliers through a transition to renewable energy and facilitating a switch from disposable to reusable injection devices for our medicines wherever possible.
Our operating environment is also becoming more complex, shaped by geopolitical tensions,
global conflicts and technological advancements. Our unique ownership structure, underpinned
by the Novo Nordisk Foundation as controlling shareholder, provides us with the stability we need
to navigate these uncertainties. This model supports our sustainable growth by allowing us to take
a long-term view on our investments and strategies; crucial in a volatile world where short-term market pressures can often lead to reactive decision-making.
We are similarly mindful of the importance of sustainably scaling our organisation. We are now
77,349 colleagues worldwide – an increase of 20% compared to 2023 that reflects our commitment
to scaling up in the face of growing demand. Our focus is on ensuring new hires receive the
support and resources they need to fully integrate into our global workforce and connect with the Novo Nordisk Way – the core guiding principles that underpin everything we do. This approach
also safeguards our focus on diversity and inclusion, fostering an environment where every
employee feels valued and included.
As we look forward to 2025 and beyond, we are optimistic about the opportunities that lie ahead
as we strive to serve millions more people with serious chronic diseases. However, we are also mindful of the challenges inherent to our growth and the need to balance short-term costs with
long-term societal value.
Our purpose remains clear: driving change to defeat serious chronic diseases. By staying true
to our purpose and values, we are confident in our ability to navigate the complexities of the
ever-evolving global healthcare landscape and to continue making a meaningful difference in
the lives of millions of people worldwide.
We would like to extend our gratitude to all Novo Nordisk colleagues worldwide for their hard
work and dedication at a time of unprecedented demand for our life-changing medicines, and
to our shareholders for their continued support of our company.
Helge Lund Lars Fruergaard Jørgensen
Chair of the Board of Directors President and CEO
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Introducing Novo Nordisk / Key figures |
|
Key figures
Novo Nordisk is a leading global healthcare company, founded in 1923 and headquartered in Denmark.
|
|
|
|
|
|
|
|
|
|
45.2
million people living with
diabetes and obesity reached
|
|
5
countries with
R&D facilities
|
13
countries with
production facilities
|
80
countries with
affiliates
|
|
77,349
employees worldwide
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
2020 |
2021 |
2022 |
2023 |
|
2024 |
2023-24 |
| Financial performance |
|
|
|
|
|
|
Change |
| Net sales |
126,946 |
140,800 |
176,954 |
232,261 |
|
290,403 |
25 |
% |
| Sales growth as reported |
4.0 |
% |
10.9 |
% |
25.7 |
% |
31.3 |
% |
|
25.0 |
% |
|
Sales growth in constant exchange rates1 |
6.7 |
% |
13.8 |
% |
16.4 |
% |
35.6 |
% |
|
25.7 |
% |
|
| Operating profit |
54,126 |
58,644 |
74,809 |
102,574 |
|
128,339 |
25 |
% |
| Operating profit growth as reported |
3.1 |
% |
8.3 |
% |
27.6 |
% |
37.1 |
% |
|
25.1 |
% |
|
Operating profit growth in constant exchange rates1 |
6.8 |
% |
12.7 |
% |
14.6 |
% |
43.7 |
% |
|
26.2 |
% |
|
| Depreciation, amortisation and impairment losses |
5,753 |
6,025 |
7,362 |
9,413 |
|
19,107 |
103 |
% |
EBITDA1,2 |
59,879 |
64,669 |
82,171 |
111,987 |
|
147,446 |
32 |
% |
| EBITDA growth as reported |
3.0 |
% |
8.0 |
% |
27.1 |
% |
36.3 |
% |
|
31.7 |
% |
|
| EBITDA growth in constant exchange rates |
6.7 |
% |
12.0 |
% |
14.9 |
% |
42.4 |
% |
|
32.7 |
% |
|
| Net financials |
(996) |
436 |
(5,747) |
2,100 |
|
(1,148) |
|
| Profit before income taxes |
53,130 |
59,080 |
69,062 |
104,674 |
|
127,191 |
22 |
% |
Effective tax rate3 |
20.7 |
% |
19.2 |
% |
19.6 |
% |
20.1 |
% |
|
20.6 |
% |
|
| Net profit |
42,138 |
47,757 |
55,525 |
83,683 |
|
100,988 |
21 |
% |
Purchase of property, plant and equipment3 |
5,825 |
6,335 |
12,146 |
25,806 |
|
47,164 |
83 |
% |
Purchase of intangible assets3 |
16,256 |
1,050 |
2,607 |
13,090 |
|
4,145 |
(68 |
%) |
| Cash used for acquisition of businesses |
— |
18,283 |
7,075 |
— |
|
82,163 |
|
Free cash flow1 |
28,565 |
29,319 |
57,362 |
68,326 |
|
(14,707) |
|
| Total assets |
144,922 |
194,508 |
241,257 |
314,486 |
|
465,795 |
48 |
% |
| Equity |
63,325 |
70,746 |
83,486 |
106,561 |
|
143,486 |
35 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
2020 |
2021 |
2022 |
2023 |
|
2024 |
2023-24 |
| Financial ratios |
|
|
|
|
|
|
Change |
Gross margin3 |
83.5 |
% |
83.2 |
% |
83.9 |
% |
84.6 |
% |
|
84.7 |
% |
|
| Sales and distribution costs in percentage of sales |
25.9 |
% |
26.3 |
% |
26.1 |
% |
24.4 |
% |
|
21.4 |
% |
|
| Research and development costs in percentage of sales |
12.2 |
% |
12.6 |
% |
13.6 |
% |
14.0 |
% |
|
16.6 |
% |
|
Operating margin3 |
42.6 |
% |
41.7 |
% |
42.3 |
% |
44.2 |
% |
|
44.2 |
% |
|
Net profit margin3 |
33.2 |
% |
33.9 |
% |
31.4 |
% |
36.0 |
% |
|
34.8 |
% |
|
Cash to earnings1 |
67.8 |
% |
61.4 |
% |
103.3 |
% |
81.6 |
% |
|
(14.6 |
%) |
|
Return on invested capital1 |
82.8 |
% |
69.0 |
% |
73.6 |
% |
88.5 |
% |
|
63.9 |
% |
|
| Share performance and capital allocation |
|
|
|
|
|
|
|
Basic earnings per share/ADR in DKK3 |
9.03 |
10.40 |
12.26 |
18.67 |
|
22.67 |
21 |
% |
Diluted earnings per share/ADR in DKK3 |
9.01 |
10.37 |
12.22 |
18.62 |
|
22.63 |
22 |
% |
| Total number of shares (million), end of year |
4,700 |
4,620 |
4,560 |
4,510 |
|
4,465 |
(1 |
%) |
Dividend per share in DKK4 |
4.55 |
5.20 |
6.20 |
9.40 |
|
11.40 |
21 |
% |
Total dividend (DKK million)4 |
21,066 |
23,711 |
27,950 |
41,987 |
|
50,683 |
21 |
% |
Dividend payout ratio3 |
50.0 |
% |
49.6 |
% |
50.3 |
% |
50.2 |
% |
|
50.2 |
% |
|
| Share repurchases (DKK million) |
16,855 |
19,447 |
24,086 |
29,924 |
|
20,181 |
(33 |
%) |
| Closing share price (DKK) |
214 |
368 |
469 |
698 |
|
624 |
(11 |
%) |
1. See Non-IFRS financial measures. 2. EBITDA is defined as ’net profit’, adjusted for 'income taxes', 'financial items', 'depreciation and amortisation' and 'impairment losses and reversals'. 3. See Financial definitions and ratios. 4. Total dividend for the year including interim dividend of DKK 3.50 per share, corresponding to DKK 15,583 million, which was paid in August 2024. The remaining DKK 7.90 per share, corresponding to DKK 35,100 million, will be paid subject to approval at the Annual General Meeting in March 2025.
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Introducing Novo Nordisk / Purpose and strategy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Purpose and strategy |
At Novo Nordisk, our purpose is clear: driving change to defeat serious chronic diseases. Through our
life-changing innovations, we are building a healthier future for generations to come. |
|
|
|
|
|
|
|
|
We are dedicated to reinforcing our leadership in diabetes and obesity, securing a leading position in rare diseases and establishing ourselves as a key player in cardiovascular disease. Additionally,
we are actively building our presence in the treatment of metabolic dysfunction-associated steatohepatitis, chronic kidney disease and Alzheimer’s disease.
We create value on multiple fronts. Through the Novo Nordisk Way, we ensure our employees thrive in a supportive and innovative environment. We operate as a responsible business, striving to address environmental and social impacts, to create value for society and fulfil our financial commitments to shareholders, ensuring sustainable growth and success.
Our value chain is similarly comprehensive, encompassing every stage from the initial concept of a new treatment to its final delivery to people living with serious chronic diseases. This includes our own operations in R&D and manufacturing, as well as collaborations with suppliers to source materials and distribute our treatments effectively.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Introducing Novo Nordisk / Value creation |
|
Value creation
We focus on creating lasting value for society and our business with a strong commitment to financial, environmental
and social responsibility. Following the Novo Nordisk Way, we are dedicated to delivering long-term value for people living
with serious chronic diseases, our employees, partners, shareholders and society at large.
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Introducing Novo Nordisk / Strategic Aspirations 2025 progress |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Strategic Aspirations 2025 progress |
|
|
|
|
Strategic Aspirations 2025 |
|
Progress |
|
|
Purpose and
sustainability |
|
Progress towards zero environmental impact |
|
•Overall CO2e emissions (scope 1, 2 and full scope 3) increased by 23% compared to 2023 |
|
|
|
Being respected for adding value to society |
|
•Medical treatment provided to 43.0 million people living with diabetes and 2.2 million people living with obesity
•Reached more than 64,000 children in the Changing Diabetes® in Children programme
|
|
|
|
|
Being recognised as a sustainable employer |
|
•Share of women in senior leadership positions has increased by 0.7 percentage point to 42% compared to 2023 |
|
|
|
|
Sustainable supply chain |
|
•Acquisition of Catalent by Novo Holdings and the related acquisition by Novo Nordisk of three manufacturing sites from Novo Holdings completed |
|
|
Innovation and
therapeutic focus |
|
Further raise the innovation-bar for Diabetes treatment |
|
•Awiqli® approved in the EU, Japan and China
•Complete Response Letter received for insulin icodec in the US
•Successful completion of phase 3a programme with IcoSema
•US approval and positive EU opinion for an update of the Ozempic® label based on the FLOW kidney trial
•Submission of the SOUL cardiovascular outcomes trial and STRIDE functional outcomes trial in the US and EU
|
|
|
|
Develop a leading portfolio of superior treatment solutions for Obesity |
|
•Phase 2 trial initiated with once-weekly GIP/GLP-1 dual agonist
•Phase 2a trial with monlunabant completed
•CagriSema demonstrated superior weight loss in the REDEFINE 1 trial
•Phase 3b trials, STEP UP and STEP UP T2D, with semaglutide 7.2 mg successfully completed
•Phase 1b/2a trial with injectable amycretin successfully completed
•Phase 1 trial with a tri-agonist (Triple) initiated
|
|
|
|
|
Strengthen and progress the Rare disease pipeline |
|
•Phase 3a trial, FRONTIER 2, with Mim8 successfully completed in people with haemophilia A
•Successful completion of the phase 2 part (interim) of the etavopivat HIBISCUS phase 2/3 trial
•Alhemo® (Concizumab) approved in the US and EU for the treatment of haemophilia A and B with inhibitors
•Alhemo® submitted in the EU for the treatment of haemophilia A and B without inhibitors
|
|
|
|
|
Establish presence in Cardiovascular & Emerging Therapy Areas focusing
on CVD, MASH and CKD |
|
•Agreement to acquire Cardior Pharmaceuticals and lead asset CDR132L in phase 2 development for treatment of heart failure
•Phase 3 development initiated with ziltivekimab in HFpEF and AMI
•Phase 3 trial CLARION-CKD trial stopped as ocedurenone failed to meet primary endpoint
•Successful completion of part I of phase 3 trial ESSENCE with semaglutide 2.4 mg in MASH
|
|
|
Commercial
execution |
|
Strengthen Diabetes leadership – aim at global value market share of more than 1/3 |
|
•Diabetes value market share remained unchanged at 33.7% (MAT) |
|
|
|
More than DKK 25 billion in Obesity sales by 2025 |
|
•Obesity care sales increased by 57% (CER) to DKK 65.1 billion |
|
|
|
|
Secure a sustained growth outlook for Rare disease |
|
•Rare disease sales increased by 9% (CER) to DKK 18.6 billion |
|
|
Financials |
|
Deliver solid sales and operating profit growth |
|
•Sales growth of 26% (CER)
•Operating profit growth of 26% (CER), negatively impacted by impairment losses related to intangible assets
|
|
|
|
|
Drive operational efficiencies across the value chain to enable investments in future growth assets |
|
•Operational leverage reflecting sales growth, when excluding impairment losses |
|
|
|
|
Deliver free cash flow to enable attractive capital allocation to shareholders |
|
•Free cash flow of DKK (14.7) billion, negatively impacted by the Catalent transaction
•DKK 64.3 billion returned to shareholders
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Introducing Novo Nordisk / Purpose and sustainability |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PURPOSE AND SUSTAINABILITY
Driving change in human
and planetary health
|
|
As the global prevalence of serious chronic diseases continues to increase, overburdened healthcare systems face growing pressure to deliver cost-effective, quality care, while millions of people lack access to essential treatments. In 2024, we reached more than 45.2 million people with our life-changing medicines – an increase of 3.6 million compared to 2023. As our business grows, so does our social responsibility to support vulnerable populations, and this year we were able to reach 8.4 million vulnerable people living with diabetes – a slight decrease compared to 2023. With the aim
of addressing growing health inequities, we are broadening our access and affordability initiatives, including programmes like Changing Diabetes® in Children. Since its inception in 2009, this programme has provided care and support to more than 64,000 young people – keeping us on
track to achieve our ambition of reaching a total of 100,000 children by 2030.
Prevention is similarly critical to reducing the global health burden, and we are investing more in preventive health measures than ever before. Our GLP-1-based medicines hold the potential to deliver substantial long-term healthcare savings by improving patient outcomes and reducing the need for more intensive treatments. Meanwhile, the 2024 expansion of our pioneering urban health initiative, Cities for Better Health, showcases our growing ambition to drive change outside the clinic. Building upon a decade of insights, this expanded partnership programme now includes a Childhood Obesity Prevention Initiative (COPI) aiming to deliver measurable, community-driven interventions that promote healthy eating and physical activity among children living in underprivileged urban communities. Initially launching in six cities across five continents, COPI complements our existing work with UNICEF to prevent this escalating problem.
We also prioritise environmental sustainability – including nature and biodiversity – across our value
chain and have a clear focus on decoupling our environmental impact from our growth as we progress towards our net zero 2045 emissions target. This will be a significant challenge with emissions continuing to rise as our business expands to keep pace with demand, but we are determined to step up to the task.
To this end, we have updated roadmaps targeting reductions in our emissions, plastic footprint and
impact on nature and biodiversity, each laying out a clear path towards creating a more sustainable business. Key focus areas include supporting our suppliers through a transition to renewable energy, switching to reusable injection devices for our medicines wherever possible and exploring the
use of low-impact glucose alternatives in our production processes.
Despite the scale of the challenges ahead, our commitment to improving human and planetary
health remains unwavering. We are determined to do more with less – reaching more vulnerable people with our life-saving medicines and doing more to curb the rising prevalence of serious
chronic diseases, all while minimising our environmental impact.
|
|
|
In an increasingly complex and unpredictable world, the intersection
of climate change, health inequity and the rising prevalence of serious chronic diseases presents an unprecedented risk to both human and planetary health. Recognising the magnitude of these challenges,
we are aiming to expand the reach and societal impact of our life-changing medicines and preventive health initiatives while striving to reduce our CO2e emissions, plastic footprint and impact on nature.
|
|
|
|
Strategic Aspirations 2025
|
|
|
|
|
|
Progress towards zero
environmental impact
Being respected for adding
value to society
Being recognised as
a sustainable employer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Introducing Novo Nordisk / Purpose and sustainability |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Driving change in chronic
disease prevention |
|
|
Tackling growing
health disparities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
We are taking determined action to prevent serious chronic diseases, focusing on improving urban health for vulnerable communities and preventing childhood overweight and obesity. These efforts are complemented by our Transformational Prevention Unit, which aims to develop scalable and accessible science-based solutions that can predict and pre-empt obesity and its consequences.
Our pioneering urban health programme, Cities for Better Health (CBH), sits at the forefront of our prevention efforts. Now with a broadened scope that aligns with our expansion into new therapy areas, this public-private partnership drives action to prevent serious chronic diseases across a global network of 51 large cities.
The Childhood Obesity Prevention Initiative (COPI) is the
latest initiative to come out of CBH. Taking aim at childhood overweight and obesity, it seeks to deliver measurable, community-driven interventions promoting healthy eating
and physical activity among children living in underprivileged urban communities.
Guided by a global evidence-based framework, these measures will target children aged between six and 13, aiming to positively affect diet and physical activity, improve health-related quality of life and promote healthy weight. The initiative complements our ongoing collaboration with UNICEF to tackle childhood obesity, where we are focusing on building healthy environments that enable and empower children to eat well and be active.
|
|
|
|
|
Millions currently lack access to diabetes care due to high costs or unavailability, often with devastating consequences.
In 2024, we reached 8.4 million vulnerable people with diabetes,
a 5% decrease from last year, mainly due to reduced tender sales of human insulins. Despite this, our commitment to addressing health inequity remains unwavering. We are intensifying efforts to make care more affordable for vulnerable populations, improve supply chains and build capacity for diagnosis and disease management.
Key initiatives include Changing Diabetes® in Children (CDiC), which has reached over 64,000 children with type 1 diabetes
in low- and middle-income countries since 2009. Support can include free life-saving medicine, blood glucose monitoring equipment and medical supplies for young people under 25.
In the past year, the programme integrated new digital elements
to support access to care in vulnerable settings. This includes the ‘Diabetes Besties’ video series, which helps bridge the gap in patient education for children living with diabetes.
Other initiatives include Partnering for Change, a collaboration with the Danish Red Cross to address health issues in humanitarian crises, and iCARE, an integrated business model aimed at breaking down barriers to diabetes care in Middle Africa and Indonesia. iCARE provides affordable insulin, trains healthcare providers and empowers people with diabetes to improve their health and quality of life.
1. The 2023 figure has been restated. Read more on page 75.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Purpose and sustainability |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Decoupling environmental
impact from our growth |
|
|
Reducing our
plastic footprint |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Our commitment to delivering life-changing medicines to
millions of people worldwide compels us to responsibly manage our use of water, energy and resources.
We have made significant progress in reducing our scope 1 and
2 emissions since 2019. However, our scope 3 emissions, which comprise about 96% of our total emissions, continue to rise as
we grow to meet increasing demand for our medicines. To achieve net zero emissions by 2045, we have a roadmap to reduce scope 3 emissions by 33% by 2033, using 2024 as the baseline. This
target – which covers nearly 70% of our scope 3 emissions in accordance with Science Based Targets initiative (SBTi) provisions – is aligned with climate science and has been submitted to the SBTi for validation.
Key decarbonisation measures include switching to low-carbon materials and feedstock across our production network, shifting our distribution model to low-emissions transportation and supporting our suppliers in transitioning to renewable energy. To date, more than 1,800 suppliers have already committed to make the switch. At the same time, we acknowledge that these measures will not be enough to meet our target, and will therefore investigate additional levers – including new technologies – to close this gap.
Additionally, we have sharpened our focus on the impact of our operations on nature and biodiversity, setting an ambition to halt nature loss across our value chain by 2033 and achieving nature-positive status by 2045.
|
|
|
|
|
Around the world, millions of people with serious chronic diseases depend on medical devices. Once used, many of
these devices end up in landfills or are incinerated, wasting tonnes of valuable materials that could be recycled. As the number of people who rely on our medicines increases, so
does our obligation to help address the related environmental issues – including plastic waste.
To this end, we are targeting a 30% reduction in the amount of plastic used per patient by 2033, underpinned by the adoption of a reduce, change and avoid approach across our diabetes and obesity portfolio. We aim to achieve this by transitioning from disposable to reuseable devices and by developing new medicines designed to be administered less frequently.
In addition, we are scaling up our ReMed™ device take-back scheme to avoid plastic waste ending up in landfills. ReMed™
is built on the success of our local take-back pilot programmes, enabling pen users to return their used devices to give the plastic a new life. Four years on, and more than four million returned pens since the launch of the first pilot, the scheme is now active in seven key markets – including Denmark, where
we collaborate with other healthcare companies to offer a unique industry-wide solution. The same collaborative model will be piloted in the UK in 2025.
|
|
“We are targeting a 30% reduction in the amount
of plastic used per patient
by 2033”
|
|
|
1. The 2023 figure has been restated; read more about this and our emissions targets on page 57. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Purpose and sustainability |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainably scaling
our organisation |
The extraordinary surge in demand for our life-changing medicines in recent years has led to
a substantial increase in the number of new hires as we expand our workforce to keep pace.
Last year alone, we added 13,030 employees across our global organisation, which now comprises 77,349 colleagues worldwide.
“Our focus is on sustainably scaling our organisation; ensuring it is run efficiently, our priorities remain clear and our resources are used optimally”
Our focus is on sustainably scaling our organisation; ensuring it is run efficiently, our priorities remain clear and our resources are used optimally. This approach helps safeguard the
wellbeing of our expanding workforce and bolsters our reputation as a highly engaged and supportive place to work. Last year, we recorded an overall engagement score of 85% in our annual company survey, which saw a record 90% of all employees participate.
To support the integration of our new colleagues, we aim to equip all new hires with the support and resources they need to onboard and connect with our strong company culture
and purpose, which remain essential to our success.
By dedicating additional time and resources to this integration process, we also help to foster
an environment that values diverse perspectives and ensures every employee feels included.
Moreover, it is crucial that we maintain a sustainable work-life balance for all our employees.
As our business grows, we are carefully monitoring workplace stress levels, targeting a 10% annual reduction in the number of employees reporting symptoms of stress. Although we did
not meet this target in 2024, when overall stress levels remained unchanged year-on-year at 13.8%, we will continue to implement new measures to address symptoms of stress at the
earliest opportunity.
The foundation of our commitment to supporting the wellbeing and development of our employees is the Novo Nordisk Way; a set of guiding principles constituting the core of our identity and operations. It bridges our company’s past, present and future, steering our
strategy, decisions and behaviours. By familiarising new employees with the 10 Essentials that direct the decisions and actions of every Novo Nordisk colleague, we uphold our dedication to
the company’s core values of openness, accountability and respect. We employ a distinctive, systematic approach known as facilitation – value audits – to ensure that all employees adhere
to these Essentials.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Purpose and sustainability |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate governance
|
|
|
|
|
|
|
|
|
|
Governance structure
The shareholders of Novo Nordisk exercise their rights at the Annual General Meeting, which
is the supreme governing body of the company. The general meeting, inter alia, adopts the company’s Articles of Association, approves the Annual Report and elects the Board of Directors.
Any shareholder has the right to raise questions at general meetings. Resolutions can generally be passed by a simple majority. However, resolutions to amend the Articles of Association require two-thirds of the votes cast and capital represented, unless other adoption requirements are imposed by the Danish Companies Act.
Novo Nordisk has a two-tier management structure consisting of the Board of Directors and Executive Management. The governance structure and rules of Novo Nordisk are further described in our Articles of Association and our Corporate Governance Report, both available
at: www.novonordisk.com/about/corporate-governance.html.
Foundation ownership
Novo Holdings A/S, a Danish company wholly owned by the Novo Nordisk Foundation, holds
the majority of votes at Novo Nordisk A/S’ general meetings. The combination of foundation ownership and stock listing enables Novo Nordisk to embark on long-term sustainable strategies while maintaining short-term transparency on performance. Our foundation ownership supports the overarching imperative to be both commercially successful and responsive to the wider needs of society.
The Novo Nordisk Foundation has two objectives: to provide a stable basis for the commercial and research activities of Novo Nordisk, Novonesis and additional companies in Novo Holdings’ investment portfolio; and to support scientific, humanitarian and social causes. Please refer to the section on value creation on page 9. For more information about the ownership structure of Novo Nordisk, see page 36.
Corporate governance reporting
Novo Nordisk reports in accordance with the Danish Corporate Governance Recommendations, which are implemented by Nasdaq Copenhagen in the Nordic Main Market Rulebook for Issuer
of Shares, as well as the Corporate Governance Standards of the New York Stock Exchange applicable to foreign private issuers.
|
|
Novo Nordisk complies with the Danish Corporate Governance Recommendations as we account for which recommendations we comply with or deviate from and explain our chosen approach.
You can find further information about our corporate governance practices and statement on our approach to each of the Danish Corporate Governance Recommendations as well as the Corporate Governance Standards of the New York Stock Exchange in our Corporate Governance Report, available at: www.novonordisk.com/about/corporate-governance.html.
Remuneration
Executive remuneration is linked to financial performance as well as non-financial performance
(e.g. innovation and sustainability). Novo Nordisk has prepared a separate Remuneration Report describing the remuneration awarded or due during 2024 to the Board of Directors and Executive Management members registered with the Danish Business Authority. The Remuneration Report
is submitted to the Annual General Meeting for an advisory vote. The Remuneration Policy and the Remuneration Report are available at: www.novonordisk.com/about/corporate-governance.html.
Disclosure regarding change of control provisions
It is disclosed that Novo Nordisk does not have any material contracts that take effect, alter or terminate upon a change of control of Novo Nordisk following implementation of a takeover bid.
In the event of termination – whether by Novo Nordisk or by the individual – due to a merger, acquisition or takeover of Novo Nordisk, members of Executive Management registered with the Danish Business Authority are, in addition to the notice period, entitled to a severance payment of 24 months’ base salary plus pension contribution.
Ethics and compliance
In Novo Nordisk, we have an ethics and compliance programme which comprises of a code of conduct (OneCode), requirements (The Ethics Navigator), processes and awareness and capability building as stipulated in the seven elements of an effective compliance programme. Data privacy is a key component in our ethical principles, ensuring guardrails are in place to manage and mitigate risks, thus safeguarding our patients and society at large. We have also adopted a set of principles for data and artificial intelligence (AI) ethics to support ethical decision-making. We have initiated building our AI Ethics & Compliance framework, incorporating elements such as principles, requirements and risk assessments, as well as building AI literacy training and capabilities.
You can read more about these principles, in accordance with the Danish Financial Statements
Act Section 99d, at: www.novonordisk.com/data-privacy-and-user-rights/data-ethics.html.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Innovation and therapeutic focus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INNOVATION AND THERAPEUTIC FOCUS
Empowering patients
with life-changing
innovations
|
|
Our evolution from a diabetes-centric company to an organisation with a broader focus on
metabolic and cardiovascular health requires even sharper prioritisation across our portfolio.
To do this, we have established the role, purpose and ambition level for each therapy area based
on future opportunities, while at the same time assessing our competitive strengths and the
capabilities required to unlock these opportunities.
The result is a clear set of priorities that guide our R&D and external business development
activities across therapy areas. These include significant investments in novel technological
platforms as well as strategic collaborations and acquisitions that expand our research horizons
and ensure we remain at the forefront of therapeutic innovation.
Our primary strategic focus remains on strengthening our leadership position in diabetes and
obesity. The latter is an increasingly critical area of unmet medical need, impacting more than one
billion people worldwide. Our robust pipeline underscores our ambition to develop transformative treatment solutions. Notable advancements include the phase 3 development of CagriSema, an innovative once-weekly combination of an amylin analogue (cagrilintide) and GLP-1 receptor agonist (semaglutide), and successfully completing the phase 1b/2a trial with subcutaneous amycretin,
a unimolecular long-acting GLP-1 and amylin receptor agonist.
Driven by a strong focus on strategic partnerships and external innovation, our modality portfolio
has expanded significantly in recent years, and now incorporates diverse approaches including
proteins and peptides, small interfering ribonucleic acid (siRNA), small molecules, cell therapy
and gene editing. This diversification enables us to leverage multiple modalities for target biology, enhancing our ability to address complex diseases. Ongoing projects include collaborations with biotech firms including Heartseed (cell therapy) and Ventus Therapeutics (small molecules) to
identify novel drug candidates for the treatment of heart failure and atherosclerotic cardiovascular disease, while the acquisition of the megaTAL technology platform from longstanding partner
2seventy bio has enhanced our in-house gene editing capabilities in haemophilia.
Artificial intelligence (AI) and human data also play a pivotal role in our R&D activities. By leveraging real-world evidence and diverse data cohorts, we are able to enhance our early discovery processes, while our AI-driven data mining and analyses help us mitigate risks involved in translating findings
from animal models to humans. This approach accelerates the discovery of new targets and
increases the likelihood of clinical success. Our R&D hub in the greater Boston area, a world-leading
life sciences cluster, exemplifies this forward-thinking approach, working with local partners to
leverage the power of machine learning, big data and AI to enhance our R&D capabilities.
|
|
|
As healthcare innovation accelerates at an unprecedented rate,
Novo Nordisk is driving transformative change across multiple therapy
areas, with a particular focus on meeting unmet patient needs in
diabetes, obesity, cardiovascular diseases and rare blood disorders.
Through strategic investments in AI-driven drug discovery, clinical trial optimisation and new technological platforms, our ambition is to set new standards for innovation that deliver tangible, lasting improvements to
the lives of the people we serve. |
|
|
Strategic Aspirations 2025
|
|
|
|
|
Further raise the innovation-bar for Diabetes treatment
Develop a leading portfolio of superior treatment
solutions for Obesity
Strengthen and progress the Rare disease pipeline
Establish presence in Cardiovascular & Emerging
Therapy Areas focusing on CVD, MASH and CKD1
|
|
|
1. Cardiovascular disease, metabolic dysfunction-associated steatohepatitis and chronic kidney disease. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Innovation and therapeutic focus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Developing breakthrough
innovations in obesity |
|
Obesity is a public health crisis impacting more than one billion people worldwide. Meeting unmet needs in obesity is a critical focus area for Novo Nordisk, and our aim is to build a differentiated portfolio of superior treatment solutions that go beyond weight loss to deliver meaningful improvements in overall metabolic and cardiovascular health and physical function. Over the past year, we have strengthened our leadership position in this dynamic and rapidly-growing space. At the forefront of these advancements are two promising investigational therapies: CagriSema and amycretin.
CagriSema, currently in phase 3 development for the treatment of obesity or overweight and type 2 diabetes, aims to combine the proven efficacy of semaglutide with the potential complementary benefits of cagrilintide, a novel amylin analogue. Topline results from the pivotal REDEFINE 1 phase 3a trial demonstrated 22.7% weight loss vs 2.3% with placebo alone after 68 weeks if all people adhered to treatment – among the highest reductions yet seen in a phase 3a programme for a GLP-1 combination therapy. CagriSema appeared to have a safe and well-tolerated profile in the study. The most common adverse events were gastrointestinal, and the vast majority were mild to moderate and diminished over time, consistent with the GLP-1 receptor agonist class. With the insights obtained from the REDEFINE 1 trial, we plan to further explore the weight loss potential of CagriSema in an additional study.
Amycretin, a novel unimolecular GLP-1 and amylin receptor agonist, aims to combine the physiological effects of these two biologies, enhancing glucose-dependent insulin secretion, inhibiting glucagon release, slowing gastric emptying and promoting satiety. Findings from a phase 1b/2a study of subcutaneous amycretin demonstrated a safety profile consistent with incretin-based therapies. The most common adverse events were gastrointestinal and the vast majority were mild to moderate in severity. When evaluating the effects of treatment if all people adhered to treatment, amycretin demonstrated an estimated body weight loss of 9.7% on
1.25 mg (20 weeks), 16.2% on 5 mg (28 weeks) and 22.0% on 20 mg (36 weeks). People treated with placebo experienced an estimated 1.9%, 2.3% and 2.0% body weight gain, respectively. These results support the weight lowering potential of amycretin previously seen with the once-daily oral formulation, which demonstrated 13.1% weight loss after 12 weeks in a phase 1 study.
In addition to these developments, we successfully completed two phase 3b obesity trials with semaglutide 7.2 mg. When evaluating the effects of treatment if all people adhered to treatment over 72 weeks, semaglutide 7.2 mg demonstrated 20.7% weight loss vs 2.4% with placebo in people with obesity in the STEP UP study, and 14.1% weight loss vs 3.6% with placebo in people with obesity and type 2 diabetes in the STEP UP T2D study.
We are also continuing to unpack the full data sets from our landmark SELECT trial programme, which include samples from approximately 11,000 people collected over a five-year period. Enhanced by AI and digital capabilities, these data can help us identify new targets and biomarkers for future projects and predict disease progression and treatment response.
|
|
| Patricio Argüelles lives with obesity in Mexico. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Innovation and therapeutic focus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pioneering transformational
treatments for diabetes |
|
The discovery of insulin more than 100 years ago transformed diabetes from a death sentence into a manageable disease.
Today, we are still driving change in diabetes by improving quality of life through innovative new treatments and delivery devices. The past year has been no exception, characterised by advancements in our diabetes pipeline that demonstrate our commitment to raising the bar for innovation in this ever-evolving therapy area.
CagriSema is a once-weekly combination of an amylin analogue (cagrilintide) and a GLP-1 receptor agonist (semaglutide). It is currently in phase 3 development for the treatment of
type 2 diabetes in the REIMAGINE programme to assess its effects on blood glucose
regulation, body weight and broader metabolic health parameters. A separate phase 3 programme – REDEFINE – is also investigating CagriSema for the treatment of obesity.
We are also making progress in the development of a once-weekly GIP/GLP-1 receptor dual agonist, aiming to leverage the combined benefits of two powerful incretin hormones. By activating both GIP (gastric inhibitory polypeptide) and GLP-1 receptors, this investigational therapy aims to enhance blood sugar control, increase insulin secretion, reduce glucagon levels and promote weight loss.
In type 1 diabetes, our early-stage pipeline has similarly transformative potential. Key projects include Pumpsulin, which aims to deliver a novel fast-acting insulin optimised for use in
future insulin pump-based fully closed-loop CSII (Continuous Subcutaneous Insulin Infusion) systems, and our work on developing a glucose-sensitive insulin. Currently in phase 1 clinical development, this cutting-edge therapy is designed to automatically respond to the body’s glucose levels, offering a more dynamic and physiological approach to insulin treatment.
“The therapy aims to preserve the body’s natural ability to produce insulin, potentially preventing or delaying the onset of type 1 diabetes”
Another notable example is our DNA immunotherapy project. Targeted at individuals recently diagnosed and at risk of developing type 1 diabetes, this investigational therapy aims to transform the management of the disease by addressing the root cause of the immune system’s harmful response. Administered through regular injections, it seeks to ‘retrain’ the immune system to stop attacking insulin-producing cells in the pancreas. By doing so, the therapy aims to preserve the body’s natural ability to produce insulin, potentially preventing
or delaying the onset of type 1 diabetes.
|
|
Novo Nordisk employee Jacob Sten Petersen and his daughter Vita at the Breakthrough T1D Walk in the US. Vita was diagnosed with type 1 diabetes at age three. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Innovation and therapeutic focus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cardiovascular disease,
the world’s biggest killer |
|
Cardiovascular diseases (CVD) are the leading cause of death globally, taking an estimated 17.9 million lives each year. The prevalence of CVD is on the rise, driven by factors such as ageing populations, lifestyle changes and increasing rates of obesity and diabetes. This growing burden underscores the urgent need for innovative treatments to manage and mitigate the impact of cardiovascular (CV) conditions.
Although CVD is a crowded, highly competitive therapy area, significant unmet needs persist.
Our GLP-1-based therapies Ozempic®, Wegovy® and Rybelsus® have all demonstrated a reduction in risk of major adverse CV events in separate cardiovascular outcomes trials, adding
to the growing body of evidence supporting the robust cardiometabolic profile of semaglutide. Beyond our portfolio of GLP-1-based medicines, we are also developing a pipeline of CV assets targeting specific, underserved areas where we can leverage our expertise in metabolic
diseases. Central to these efforts is ziltivekimab, our lead CV candidate currently in phase 3 development across multiple CV indications.
Acquired as part of a business development deal to bring Boston-based biotech firm Corvidia Therapeutics in-house back in 2020, ziltivekimab is an investigational monoclonal antibody designed to target interleukin-6 (IL-6), a protein in the inflammation pathway linked to the development of different CV conditions. By targeting IL-6, ziltivekimab is under investigation to reduce inflammation and potentially improve outcomes across a spectrum of CV conditions – including atherosclerotic cardiovascular disease, heart failure with preserved ejection fraction, and acute myocardial infarction.
“Phase 2 data demonstrated that ziltivekimab significantly lowers inflammation biomarkers linked to atherosclerosis in individuals with advanced chronic kidney disease”
Phase 2 data demonstrated that ziltivekimab significantly lowers inflammation biomarkers
linked to atherosclerosis in individuals with advanced chronic kidney disease. With phase 3 trials now in progress, our goal is to establish the first-in-class therapy as a foundational treatment
for high-risk cardiovascular patients, aiming to improve cardiovascular outcomes by targeting systemic inflammation.
With the potential to improve outcomes across several indications, ziltivekimab exemplifies our commitment to strengthening our position in the CVD space.
|
|
| Greg Patterson lives with cardiovascular disease in the US. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Innovation and therapeutic focus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Emerging therapies
for MASH |
|
|
Pioneering AI in
research and development |
|
|
|
|
|
|
|
|
|
|
|
|
Semaglutide has already proven its effectiveness in enhancing glycaemic control, promoting weight loss and reducing cardiovascular risk. Now, it has demonstrated potential as a treatment for metabolic dysfunction-associated steatohepatitis (MASH), a progressive liver disease that affects more than
250 million people worldwide.
MASH is characterised by liver inflammation and damage
due to fat accumulation. If left untreated, this condition can progress to cirrhosis and liver failure, posing a significant health risk. Yet with only one pharmacological treatment approved specifically for MASH, there is significant unmet
need in the space for effective therapeutic options.
According to the headline results from part one of the
ESSENCE trial, semaglutide 2.4 mg demonstrated a statistically significant and superior improvement in liver fibrosis with
no worsening in steatohepatitis – as well as resolution of steatohepatitis with no worsening of liver fibrosis at 72 weeks. This initial phase of the study included 800 people with MASH and moderate to advanced liver fibrosis.
Part two of the trial, designed to evaluate the long-term impact of semaglutide 2.4 mg on liver-related clinical events, is set to continue until 2029. Meanwhile, Novo Nordisk plans to file for regulatory approval in the US and EU in the first half of 2025.
|
|
“Semaglutide 2.4 mg demonstrated a statistically significant and superior improvement in liver fibrosis with no worsening in steatohepatitis”
|
|
|
We are revolutionising our R&D efforts through artificial intelligence (AI), particularly in drug discovery, molecular design and clinical trial optimisation.
In drug discovery, AI is playing a pivotal role in identifying
new compounds. By combining AI with high-throughput experimentation, we have assessed one billion virtual molecules
via computer modelling and screened approximately 2,500 compounds in the lab. This led to the discovery of a highly
selective amylin compound that closely mimics the natural hormone, requiring 50-75% fewer design rounds.
Molecular design has also advanced through AI. By leveraging predictive pharmacology and knowledge mining, we are able
to accelerate the design cycles of new molecules, expediting development and enhancing the precision of targeted therapies.
AI is also optimising our clinical trials by identifying subpopulations, improving trial design and site selection and forecasting outcomes. For example, harmonising data from around 1,600 clinical trials, including SELECT and STEP, has provided best-in-class cardiometabolic data, leading to improved disease insights,
patient stratification and drug target identification.
We are also enhancing our AI capabilities through strategic partnerships. Our recently expanded collaboration with Valo
Health is a prime example of our approach, seeking to accelerate the development of up to 20 novel drug programmes within the cardiometabolic space by leveraging cutting-edge AI technology and extensive human datasets.
|
|
“By combining AI
with high-throughput
experimentation, we have assessed one billion virtual molecules via computer
modelling and screened
approximately 2,500
compounds in the lab”
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Innovation and therapeutic focus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pioneering new treatments for rare blood disorders |
|
Novo Nordisk has a long-standing legacy of pioneering advancements in the treatment of rare
blood disorders, and our pipeline is primed to extend this tradition. In haemophilia A, our investigational treatment Mim8 represents an optimised therapeutic approach that could redefine the standard of care for patients worldwide, while a novel oral Factor VIIIa mimetic could be on
the horizon with Inno8.
Traditional treatments for haemophilia A often require intravenous infusions and cumbersome administration procedures, posing a significant burden for patients. Mim8 offers a promising alternative, administered subcutaneously in a weekly, bi-weekly or monthly dose. It mimics the function of missing clotting Factor VIII (FVIII) by bridging Factor IXa and Factor X to restore the
body’s ability to form blood clots. Mim8 is currently pending submission for regulatory review.
Inno8 holds the potential to become the first-ever oral treatment for haemophilia A. Inno8 is a
small antibody fragment that – like Mim8 – mimics FVIIIa function, but the size of the molecule
is small enough to enable oral absorption. The Inno8 development programme is focused on a fast-to-market approach with overlapping clinical trials, seeking to provide a convenient and efficacious alternative to regular infusions.
We have also partnered with a pioneering biotech firm, 2seventy bio, to develop a groundbreaking gene editing treatment for haemophilia A. This collaboration – which was initiated in 2019, extended in 2022 and resulted in the acquisition of the megaTAL technology platform in 2024
– aims to correct the clotting factor deficiency in patients, potentially eliminating the need for regular treatments.
Our efforts extend beyond haemophilia to haemoglobinopathies, a group of inherited genetic blood disorders affecting the structure or production of the haemoglobin molecule. Here, we are building a research portfolio to address the underlying disease pathophysiology. We are utilising our innovative technology platforms to restore red blood cell health and reduce inflammation
and organ damage. Etavopivat, an investigational oral once-daily therapeutic developed to improve anaemia and red blood cell health in people with sickle cell disease (SCD), is at the forefront of our efforts in this area.
Etavopivat was acquired as part of the deal that brought Forma Therapeutics in-house back in 2022, and is currently in a phase 3 clinical trial in adolescents and adults with SCD, and a phase 2 trial for people with transfusion-dependent SCD and thalassemia, another hereditary haemoglobinopathy disorder. Results from the phase 2 part of the HIBISCUS trial programme were presented at the Annual Meeting of the American Society of Hematology in 2024, and indicate that etavopivat has the potential to improve haemoglobin levels and reduce the incidence of vaso-occlusive crises compared to placebo – severe pain caused when blood vessels are blocked and deprive tissues of oxygen – in people with SCD.
|
|
| Ebrar Oruc lives with haemophilia A in Turkey. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Innovation and therapeutic focus |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Pipeline overview |
|
|
|
|
| Project |
Indication |
Description |
Phase |
IcoSema
NN1535
|
T2D1 |
A combination of GLP-12 receptor agonist semaglutide and insulin icodec intended for once-weekly subcutaneous treatment. |
|
Icodec
NN1436
|
T1D3
and T2D
|
A long-acting basal insulin analogue intended for once-weekly
subcutaneous treatment. |
|
CagriSema
NN9388
|
T2D |
A combination of amylin analogue
cagrilintide and GLP-1 receptor
agonist semaglutide intended for
once-weekly subcutaneous treatment. |
|
|
OW GIP4/GLP-1
NN9541
|
T2D |
A dual GLP-1/GIP receptor agonist intended for once-weekly subcutaneous treatment. |
|
GELA
NN9506 |
T2D |
A collaboration with GE Healthcare,
using ultrasound for once-monthly
treatment. |
|
Amycretin
NN9490
|
T2D |
A unimolecular long-acting GLP-1 and amylin receptor agonist intended for once-daily oral treatment and once-weekly subcutaneous treatment. |
|
Pumpsulin
NN1471 |
T1D |
A novel insulin analogue for use in closed loop pump systems. |
|
DNA
immunotherapy
NN9041 |
T1D |
A novel plasmid encoding
pre-proinsulin, TGF5-beta1, IL6-10 and IL-2 intended for subcutaneous treatment.
|
|
OW Oral
Semaglutide
NN9904
|
T2D |
An oral version of the GLP-1 receptor agonist intended for once-weekly treatment. |
|
|
GSI7
NN1644
|
T1D |
An injectable glucose sensitive insulin intended for once daily treatment. |
|
|
|
|
|
1. T2D: Type 2 diabetes. 2. GLP-1: Glucagon-like peptide-1. 3. T1D: Type 1 diabetes. 4. GIP: Gastric inhibitory polypeptide. 5. TGF: Transforming growth factor. 6. IL: Interleukin. 7. GSI: Glucose-sensitive insulin. 8. CB-1: Cannabinoid receptor-1.
9. PKR: Pyruvate kinase-R. 10. RNAi: Ribonucleic acid interference. 11. CKD: Chronic kidney disease. 12. ASCVD: Atherosclerotic cardiovascular disease. 13. AMI: Acute miocardial infarction. 14. HFpEF: Heart failure with preserved ejection fraction. 15. CVD: Cardiovascular disease. 16. ANGPTL3: Angiopoietin-like 3. 17. MASH: Metabolic dysfunction-associated steatohepatitis. 18. FGF21: Fibroblast growth factor 21. 19. RNA: Ribonucleic acid. 20. LXRa: Liver X receptor alpha.
21. siRNA: Small interfering RNA. 22. MARC1: Mitochondrial amidoxime-reducing component 1. 23. NLRP3i: NOD-like receptor protein 3 inhibitor. 24. PD-L1: Programmed death ligand 1.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Project |
Indication |
Description |
Phase |
|
Oral Semaglutide
NN9932
|
Obesity |
A long-acting GLP-1 receptor agonist, 25 mg and 50 mg, intended for
once-daily oral treatment. |
|
|
Semaglutide
7.2 mg
NN9536
|
Obesity |
A long-acting GLP-1 receptor agonist, 7.2 mg, intended for once-weekly subcutaneous treatment. |
|
|
CagriSema
NN9838
|
Obesity |
A combination of amylin analogue cagrilintide and GLP-1 receptor
agonist semaglutide intended for
once-weekly subcutaneous treatment. |
|
|
GELA
NN9505 |
Obesity |
A collaboration with GE Healthcare,
using ultrasound for once-monthly
treatment. |
|
|
Monlunabant
NN9440
|
Obesity |
CB-18 receptor inverse agonist
intended for once-daily oral
treatment.
|
|
|
Cagrilintide
NN9833
|
Obesity |
An amylin analogue intended for
once-weekly subcutaneous treatment. |
|
|
Amycretin
NN9487
|
Obesity |
A unimolecular long-acting GLP-1 and amylin receptor agonist intended for once-daily oral treatment and once-weekly subcutaneous treatment. |
|
|
INV-347
NN9441
|
Obesity |
CB-1 receptor inverse agonist
intended for once-daily oral
treatment. |
|
|
OW GIP/GLP-1
NN9542 |
Obesity |
A dual GLP-1/GIP receptor agonist intended for once-weekly subcutaneous treatment. |
|
|
Triple
NN9662
|
Obesity |
Tri-agonist. |
|
|
Amylin 355
NN9638
|
Obesity |
Amylin analogue developed for
once-weekly subcutaneous treatment. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Project |
Indication |
Description |
Phase |
Mim8
NN7769 |
Haemophilia
A w/wo inhibitors |
A next generation FVIIIa mimetic
bispecific antibody intended for subcutaneous prophylaxis for
haemophilia A. |
|
| Etavopivat NN7535 |
Sickle cell disease |
A second-generation small molecule
PKR9-activator intended for once-daily
oral treatment. |
|
| Etavopivat NN7536 |
Thalassemia |
A second-generation small molecule
PKR-activator intended for once-daily
oral treatment. |
|
NDec
NN7533
|
Sickle cell disease |
An oral combination of decitabine and tetrahydrouridine. The project is
developed in collaboration with
EpiDestiny. |
|
|
TMPRSS2
RNAi10
|
Hereditary haemo-
chromatosis |
Small interfering RNA intended for
once every 1 to 3 months
subcutaneous treatment. |
|
Inno8
NN7441
|
Haemophilia
A w/wo
inhibitors
|
An antibody intended for oral administration. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Project |
Indication |
Description |
Phase |
| Ziltivekimab NN6018 |
CKD11
ASCVD12
AMI13
HFpEF14
|
A once-monthly monoclonal antibody intended for inhibition of IL-6 activity. |
|
| Coramitug NN6019 |
CVD15 |
An anti-amyloid immunotherapy
intended for intravenous treatment. |
|
CM4HF
NN9003 |
CVD |
An investigational cell therapy
intended for restoring heart function
in people with chronic heart failure. |
|
Anti-ANGPTL3
mAb NN6491
|
CVD |
An ANGPTL316 neutralising sweeping antibody intended for once-monthly subcutaneous treatment. |
|
| Semaglutide NN6535 |
Alzheimer’s |
A long-acting GLP-1 receptor agonist intended for once-daily oral or
once-weekly subcutaneous treatment. |
|
| Semaglutide NN9931 |
MASH17 |
A long-acting GLP-1 receptor
agonist intended for once-weekly subcutaneous treatment. |
|
| CagriSema NN9588 |
MASH |
A combination of amylin analogue cagrilintide and GLP-1 analogue semaglutide intended for once-weekly subcutaneous treatment. |
|
Zalfermin
NN9500
|
MASH |
A long-acting FGF2118 analogue
intended for once-weekly
subcutaneous treatment.
|
|
CDR132L
NN6706
|
Heart
failure |
An RNA19-based oligonucleotide
inhibitor developed for once-monthly intravenous treatment.
|
|
|
LXRa20
NN6582
|
MASH |
A siRNA21 targeting LXRa intended for once-monthly subcutaneous treatment. |
|
|
MARC122
NN6581
|
MASH |
A siRNA molecule targeting MARC1 intended for once-monthly
subcutaneous treatment. |
|
SC4PD
NN9001
|
Parkinson’s |
Cryopreserved cell therapy developed
for disease modifying treatment. |
|
DCR-XDH
NN4004
|
Gout |
An RNA-based oligonucleotide
intended for subcutaneous treatment. |
|
|
Ventus NLRP3i23
NN6022
|
CVD |
Small molecule NLRP3 inhibitor
intended for once-daily oral treatment. |
|
CNP HF
NN6537 |
Heart failure |
C-type natriuretic peptide intended for once-weekly subcutaneous treatment. |
|
|
PD-L124
NN4003
|
Oncology |
A PD-L1 GalXC™-derived lipid
conjugate intended for once-monthly subcutaneous treatment. |
|
STAT3
NN4002 |
Oncology |
A GalXC™-derived lipid conjugate one-time subcutaneous treatment. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Innovation and therapeutic focus |
|
Research and development progress
Regulatory events
•Awiqli®, once-weekly insulin icodec, was approved by the EMA (European Medicines Agency) and PMDA (Pharmaceuticals and Medical Devices Agency) for the treatment of T2D and T1D and by the CDE (Center for
Drug Evaluation) for the treatment of T2D.
• Icodec received a complete response letter from the FDA (Food and Drug Administration).
• Rybelsus® (oral semaglutide) received approval by the
EMA and the FDA for three formulation changes of
tablets (1.5 mg, 4 mg, 9 mg).
• Ozempic® label expansion was approved by the EMA to reflect the reduction in kidney disease related events in people with T2D based on FLOW results.
• FLOW results were submitted for Ozempic® (semaglutide injection, 1 mg) to FDA, PMDA and CDE for the treatment
of chronic kidney disease in patients with T2D.
• IcoSema was submitted to the EMA and CDE for initial marketing authorisation for the treatment of T2D.
• Zegalogue® (dasiglucagon) was approved by the EMA for treating severe hypoglycaemia (low blood glucose levels)
in adults and children from 6 years.
• DuraTouch® device has received the CE (Conformitée Européenne) mark in all countries of the European union.
Clinical progress
•Phase 3a trial programme, COMBINE, investigating
once-weekly IcoSema in people with T2D was completed.
• Phase 3a trials, REIMAGINE 1 and 3, investigating CagriSema as monotherapy and as add-on to insulin in people with T2D respectively were initiated.
• Phase 3b trials of the REIMAGINE programme
comparing CagriSema (2.4/2.4 mg) vs tirzepatide (15 mg) and comparing CagriSema (1/1 mg) vs tirzepatide (5 mg)
in patients with T2D were initiated.
• Phase 3b trials, ONWARDS 8 and 10, investigating icodec
in people with T2D were initiated.
• Phase 3b trial, ONWARDS 9, investigating icodec in
insulin-naive people with T2D was completed.
• Phase 3b trial, COMBINE 4, investigating IcoSema vs glargine in a post OAD (oral anti-diabetic) population
was initiated.
• Phase 3b CVOT (cardiovascular outcomes trial), SOUL, investigating Rybelsus®, oral semaglutide 14 mg, on cardiovascular outcomes in people with T2D and established cardiovascular disease and/or chronic kidney disease was completed.
• Phase 3b trial, STRIDE, investigating semaglutide subcutaneous 1.0 mg in people living with T2D and peripheral arterial disease was completed.
• Phase 3b outcomes trial, FLOW, investigating
semaglutide sc. 1.0 mg in people living with T2D and chronic kidney disease was completed.
• Phase 2 programme investigating OW GIP/GLP-1 in
people living with chronic kidney disease and a dose finding study in people living with diabetes were initiated.
• Phase 2 trial, a dose finding trial, investigating subcutaneous amycretin in people living with diabetes
was initiated.
• Phase 2 trial investigating CagriSema in people living
with T2D and chronic kidney disease was initiated.
• Phase 1/2, a first in human dose and multiple dose trial, investigating DNA immunotherapy in development for
T1D was completed.
• Phase 1 trial investigating GSI in people living with T1D
was initiated.
• Once-monthly GIP/GLP-1 developed for glycaemic
control in people with T2D was terminated.
Regulatory events
•Wegovy® was approved by the FDA to reduce the risk of
major cardiovascular events (MACE) and EMA adopted a positive opinion to reflect risk reduction of major cardiovascular events in people with overweight or
obesity and established cardiovascular disease in the
label based on SELECT CVOT results.
• Wegovy® was approved by the CDE for weight
management in people living with overweight or obesity.
• Wegovy® label expansion was approved by the EMA to
reflect the reduction in symptoms and improved physical limitations and exercise function in people with
obesity-related heart failure with preserved ejection
fraction (HFpEF) based on STEP-HFpEF results.
• Wegovy® label expansion was approved by the EMA to
reflect the reduction of pain and improved physical
function related to knee osteoarthritis in people living
with obesity based on the results of the STEP 9 trial.
Clinical progress
•Phase 3a trial, REDEFINE 1, investigating efficacy and
safety of cagrilintide (2.4 mg) in combination with
semaglutide (2.4 mg) in people with overweight or
obesity was completed. The extension study of
REDEFINE 1 is ongoing.
• Phase 3b trial, REDEFINE 9, investigating CagriSema
(1.7 mg/1.7 mg) and CagriSema (1.0 mg/1.0 mg) effects
on weight reduction in people with overweight or
obesity was initiated.
• Phase 3b trial, OASIS 4, investigating oral semaglutide
(25 mg) weight loss in people living with overweight with weight-related comorbidities or obesity was completed.
• Phase 3b trials, STEP UP and STEP UP T2D, investigating semaglutide (7.2 mg) on weight loss were completed.
• Phase 2a trial investigating monlunabant (INV-202) in
patients with obesity and metabolic syndrome has been completed.
• Phase 1 first in human dose trial investigating amylin
355 in people with overweight or obesity was initiated.
• Phase 1 trial studying the safety and tolerability of oral amycretin was completed.
• Phase 1b trial, a dose-finding study investigating oral amycretin was initiated.
• Phase 1b/2a trial, investigating subcutaneous amycretin
was completed.
• Phase 1 Triple first in human trial was initiated.
Regulatory events
•Esperoct® was approved by the CDE for treatment and prevention or reduction of number of bleeding episodes in people with haemophilia A.
• Alhemo® (concizumab) was approved by the EMA and FDA for the treatment of haemophilia A and B disease with inhibitors and by the PMDA for the treatment of haemophilia A and B disease with and without inhibitors.
Clinical progress
•Phase 3a trials, FRONTIER 2 and 5, investigating
once-weekly to once-monthly subcutaneous Mim8 in people aged 12 or older with haemophilia A were completed.
• Phase 3a trial, HIBISCUS 2, investigating etavopivat in adolescents and adults living with sickle cell disease was initiated.
• Phase 2 trial investigating etavopivat on cerebral haemodynamic response in children with sickle cell disease was initiated.
• Phase 2 trials investigating etavopivat for the treatment of myelodysplastic syndromes (MDS) have been closed. The MDS programme was terminated.
• Phase 1 first in human trial investigating Inno8 was initiated.
• Phase 1 trial investigating TMPRSS6 RNAi in people living with hereditary haemochromatosis was initiated.
Clinical progress
•Phase 3a trial, ESSENCE, investigating semaglutide subcutaneous 2.4 mg efficacy and safety in people with MASH completed its primary interim data readout, the trial is continuing to investigate the effect on outcomes in people with MASH.
• Phase 3a trial, CLARION-CKD, investigating ocedureone in patients with uncontrolled hypertension and advanced chronic kidney disease was terminated. The ocedureone programme was terminated.
• Phase 3a CVOT, ARTEMIS, investigating the effect of ziltivekimab on outcomes in people with acute myocardial infarction was initiated.
• Phase 3a trial, ATHENA, investigating the effect of ziltivekimab on functional outcomes in HFpEF patients was initiated (SPA).
• Novo Nordisk acquired Cardior with lead asset CDR132L.
• Phase 1 trial investigating VAP-1i, a GLP-1/GIP receptor agonist for people living with MASH was terminated.
• Phase 1 first in human trial investigating Ventus NRLP31 was completed.
• Phase 1 trial investigating DCR-XHD in people living with refractory gout was initiated.
• Phase 1 first in human and single ascending dose trial investigating CNP HF was initiated.
• Phase 1 trial, investigating safety and tolerability of
PD-L1 in adult oncology patients with solid tumours refractory to standard therapy was initiated.
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Innovation and therapeutic focus |
|
Patent status for products with marketing authorisation
The patent expiry dates for products with marketing authorisation1 are shown in the tables on the right.
The dates provided are for expiry in the US, China, Japan and Europe of patents on the active ingredient, unless otherwise indicated, and include actual and estimated extensions of patent term, when applicable. For several products, in addition to the active ingredient patent, Novo Nordisk holds other patents on manufacturing processes, formulations or uses that may be relevant for exclusivity beyond the expiration of the active ingredient patent. Furthermore, regulatory data protection and/or orphan exclusivity may apply.
|
|
|
|
1. This overview does not include products whose sales represent less than 0.5% of Novo Nordisk’s total sales. 2. Patent status varies from country to country. The figures in the table are based on Germany.
3. For Ozempic® in Canada, regulatory data protection applies until 2026. 4. Modern insulins are NovoRapid® (NovoLog®), NovoMix® 30 (NovoLog® Mix 70/30) and Levemir®. 5. Formulation patent; active ingredient patent has expired.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Product |
US |
China |
Japan |
Europe2 |
Ozempic®3 |
2032 |
2026 |
2031 |
2031 |
Human insulin and Modern insulins4 |
Expired |
Expired |
Expired |
Expired |
Rybelsus® |
2032 |
2026 |
2031 |
2031 |
Tresiba® |
2029 |
Expired |
2027 |
2028 |
Victoza® |
Expired |
Expired |
Expired |
Expired |
Ryzodeg® |
2029 |
Expired |
Expired |
2028 |
Xultophy® |
2029 |
Expired |
Expired |
2028 |
Fiasp® |
20305 |
20305 |
20305 |
20305 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Product |
US |
China |
Japan |
Europe2 |
Wegovy® |
2032 |
2026 |
2031 |
2031 |
Saxenda® |
Expired |
Expired |
Expired |
Expired |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Product |
US |
China |
Japan |
Europe2 |
NovoSeven® |
Expired |
Expired |
Expired |
Expired |
Norditropin® (SimpleXx®) |
Expired |
Expired |
Expired |
Expired |
Esperoct® |
2032 |
2029 |
2034 |
2034 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Commercial execution |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COMMERCIAL EXECUTION
Safeguarding supply and improving access across expanding markets
|
|
Balancing the growing needs of our patients with effective management of our resources
is key to how we operate. As global demand increases we have refined our portfolio
strategy to maximise the reach and impact of our treatments. This includes efforts to
optimise our diabetes portfolio by gradually phasing out some of our older insulin products
to free up manufacturing capacity and resources across our supply chain. By doing so, we
can dedicate more space in our manufacturing network to innovative, scalable solutions
– and ultimately expand the reach of our life-changing innovations to millions more
patients over the next decade.
At the same time, we are striving to provide those who are impacted by the changes to
our portfolio with access to alternative treatment options, either from Novo Nordisk or
other companies. We are working closely with local health authorities and the medical
community in affected markets to develop new access initiatives for at-risk individuals.
Furthermore, our extensive range of partnership programmes – including iCARE and
our Access to Insulin Commitment – continue to provide access to affordable care for
vulnerable populations living in low- and middle-income countries.
We are also increasing our production capacity through site expansions and acquisitions.
A significant milestone in 2024 was the acquisition of three fill-finish sites previously
run by the global contract manufacturing and development organisation Catalent Inc.
This move will enable us to expand our manufacturing capacity and provide future
optionality and flexibility for our existing supply network, while complementing our
significant ongoing internal supply chain expansions.
The unprecedented scale of our capital expenditure, which includes record investments
in the expansion of existing production sites, underscores our commitment to meeting
the growing demand for our medicines. In 2024, work continued on major expansions of
our production sites in Denmark, France, Brazil, China and the US – investments that will
ultimately enable us to reach millions more people worldwide with our innovations.
Ensuring uninterrupted access to treatment options for people already using Novo Nordisk
medicines also remains a top priority. By adopting clear prioritisation principles, we are
focusing on the responsible and equitable launch and distribution of new and existing
products across geographies and patient groups. This includes allocating a proportion of
Wegovy® volumes in every new launch market for people with a high medical need and
low socioeconomic status.
|
|
|
Amid escalating diabetes and obesity crises, Novo Nordisk is
experiencing unprecedented global demand for our life-changing medicines. With mounting evidence of the broad systemic impact
and societal value of our GLP-1-based treatments, we have developed innovative commercial strategies to safeguard patient access and strengthen supply chain resilience worldwide. |
|
|
Strategic Aspirations 2025
|
|
|
|
|
|
Strengthen Diabetes leadership
– aim at global value market
share of more than 1/3
More than DKK 25 billion
in Obesity sales by 2025
Secure a sustained growth
outlook for Rare disease
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Commercial execution |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ozempic® sales uptake further strengthens our leadership in diabetes care |
|
Demand for Novo Nordisk’s GLP-1-based medicines, particularly Ozempic®, continued to soar throughout 2024, reflecting the growing global prevalence of diabetes.
Administered as a once-weekly injection for the treatment of type 2 diabetes, Ozempic® maintains
its position as the world’s biggest-selling diabetes medicine, backed by its proven efficacy in controlling blood sugar and reducing body weight, as well as a growing body of evidence demonstrating broader cardiometabolic benefits. Over the past year alone, the clinical profile of Ozempic® has been further boosted by data demonstrating a reduction in the risk of kidney disease progression in people with type 2 diabetes and chronic kidney disease, as well as functional improvements in people with type 2 diabetes and symptomatic peripheral artery disease vs placebo.
“Demand has been fuelled by a broader acceptance and understanding of
the importance of GLP-1-based therapies”
Now available in more than 70 markets, Ozempic® sales have been central to the continued growth in sales of our diabetes products. Our strategic aspiration to secure a value market share of at least one-third by 2025 has already been achieved, and the continued uptake of Ozempic® across launch markets has enabled us to maintain a value market share of 33.7% in 2024. This demand has been fuelled by a broader acceptance and understanding of the importance of GLP-1-based therapies among healthcare professionals, patients and payers as a cornerstone of effective diabetes care
and management.
Novo Nordisk is not the only healthcare company investing in the growth and development of
the GLP-1 segment, and competition has increased significantly over the past year. Nevertheless,
we remain the market leader in the diabetes GLP-1 space with a value share of 55.1%, a slight increase compared to 2023 when our value share stood at 54.8%.
Despite the sales penetration of Ozempic®, high demand has also posed challenges, necessitating strategic decisions to prioritise distribution to regions and patient groups with the most pressing
needs. We have also continued to invest heavily in expanding production capacity, seeking to stabilise supply and ensure that Ozempic® remains accessible to the growing number of patients who have already initiated treatment.
Through our industry-leading portfolio, relentless focus on innovation and robust pipeline of
next-generation treatments, we remain well-positioned to maintain and enhance our leadership position in diabetes care.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Commercial execution |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Awiqli® approval underscores our continuing commitment to insulin |
|
Our company is built upon a century-long legacy of innovation in diabetes care, and we are
still pushing boundaries as we search for new breakthroughs in this ever-evolving space.
These efforts are exemplified by the launch of Awiqli® – the world’s first once-weekly basal
insulin – in China, Germany and Canada.
“Awiqli® represents a critical and innovative addition to our diabetes portfolio
and a key milestone for patients seeking to reduce some of the challenges of diabetes management”
Awiqli® represents a critical and innovative addition to our diabetes portfolio and a key
milestone for patients seeking to reduce some of the challenges of diabetes management –
particularly the burden of multiple injections. Its approval in the EU was based on phase 3a
clinical trial results demonstrating superior blood sugar reduction and superior time in range
(time spent within the recommended blood sugar range), compared with daily basal insulin in
people living with type 2 diabetes not previously treated with insulin. Trial data also showed low
rates of clinically significant or severe hypoglycaemia – less than one event per patient-year of
exposure – with no statistically significant difference compared to daily basal insulin in insulin
naïve people living with type 2 diabetes.
However, the therapy’s journey to market in the US has been more challenging, with the US
Food and Drug Administration (FDA) issuing a Complete Response Letter (CRL) in July 2024.
This followed a meeting of the FDA Endocrinologic and Metabolic Drugs Advisory Committee
in May 2024, where a panel of independent scientific experts discussed the benefit-risk of
once-weekly insulin icodec in type 1 diabetes. The panel determined that the data available
were not sufficient to conclude on a positive benefit-risk in type 1 diabetes.
In the CRL, the FDA requests more information relating to the manufacturing process and
the type 1 diabetes indication before the review of the application can be completed. The CRL
did not mention the use of once-weekly insulin icodec in type 2 diabetes. Novo Nordisk is
evaluating the content of the CRL and will work closely with the FDA to fulfil the requests.
Despite this setback in the US, the rollout of Awiqli® in other major markets underscores our
continuing commitment to insulin innovation more than 100 years after our founders first
commercialised production of this life-saving medicine.
|
|
|
|
|
|
|
Kyle Sam lives with type 2 diabetes
and is part of the
DUDES Club, a brotherhood to support men’s health and wellbeing in British Columbia, Canada. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Commercial execution |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wegovy® maintains
market-leading position in
increasingly dynamic sector
|
|
The past year has been transformative for the burgeoning obesity market, marked by
increasing competition and soaring demand for GLP-1 receptor agonists and other
incretin-based therapies. Wegovy®, our flagship obesity therapy, has been at the forefront
of this competitive landscape, maintaining its market-leading position despite new entrants
to the segment.
Following its initial launch in the US, Wegovy® is now available in more than 15 markets
worldwide. As the obesity market continues to grow worldwide, so does the demand for
Wegovy®. This is driven by the rising global prevalence of obesity – which has more than
tripled over the past 50 years – and a broader shift in the perception of treatment. Once
considered a lifestyle issue, obesity is now widely recognised as a serious chronic disease
that requires medical intervention.
We have responded by investing heavily in scaling up our production capacity and carefully prioritising launches and distribution. Our expanding global production network is operating
around the clock to ensure a stable and consistent supply of Wegovy® and a proportion of
Wegovy® volumes is being allocated in every launch market for people with a high medical
need and low socioeconomic status.
The continued success of Wegovy® is underpinned by its clinical profile as the world’s
first weight management therapy also approved to reduce the risk of major adverse
cardiovascular events – a key differentiator in an increasingly competitive segment. This
has enabled us to capture much of the growth to date in a rapidly-expanding and dynamic
market, helping us to build on our position of strength and reputation as first-movers in
the space following the success of our first-generation GLP-1-based therapy, Saxenda®.
Despite advancements in treatment and growing acceptance of obesity as a serious chronic
disease, significant unmet needs remain. Many people living with obesity still lack access to
effective therapies, and there is a clear need for continued innovation to develop treatments
that can deliver greater efficacy and additional benefits. Moreover, there is a need for more
holistic, preventive approaches that can address the multifaceted nature of obesity – including behavioural, psychological and environmental factors.
Novo Nordisk is dedicated to addressing these unmet needs through a steadfast commitment
to innovation. Our obesity pipeline includes numerous promising candidates aiming to further reduce the burden of obesity and related conditions on patients and healthcare systems alike.
By leveraging our expertise in GLP-1-based therapies and exploring new therapeutic avenues,
we are well-placed to continue leading the way in obesity treatment.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Commercial execution |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wegovy® label expansions underscore
broader cardiometabolic benefits
|
|
Gearing up for new launches
in our rare disease portfolio |
|
The robust clinical profile of our market-leading weight loss therapy, Wegovy®, has been further validated after regulatory bodies approved label expansions acknowledging its efficacy
in mitigating cardiovascular risks. These updates highlight the extensive cardiometabolic benefits of our market-leading GLP-1-based therapy, extending beyond weight reduction.
The new indications are based on robust clinical evidence
from key trial programmes SELECT and STEP HFpEF. Data
from SELECT demonstrated that Wegovy® reduced the risk
of major adverse cardiovascular events in people with overweight or obesity and established cardiovascular disease, on top of cardiovascular standard of care treatments vs placebo. Findings from the STEP HFpEF trials, meanwhile, showed that Wegovy® reduced symptoms of heart failure and physical limitations in people with obesity and heart failure
with preserved ejection fraction (HFpEF) vs placebo.
These data add to a growing body of evidence showcasing semaglutide’s potential to address critical unmet needs in cardiovascular health.
By broadening the approved uses of Wegovy® and helping
to differentiate the treatment in an increasingly competitive market, we are aspiring to both strengthen our leadership position in obesity and enhance our impact on broader
public health.
|
|
“These data add to a growing body of evidence showcasing semaglutide’s potential to address critical unmet needs in cardiovascular health” |
|
|
2024 has been a year of significant progress in our rare disease portfolio, building on our strong legacy of innovation in rare blood and endocrine disorders. With the pending submission of Mim8, first launches of Alhemo® and continued rollout of Sogroya®, we are building on a return to growth for our Rare Disease franchise following a positive year in which overall sales increased 9% at constant exchange rates (CER).
Sales of our rare endocrine disorder products increased by 31% at CER over the course of the year, mainly driven by the rollout of Sogroya® – the world’s first once-weekly treatment for both children and adults with growth hormone deficiency. Sogroya® is now available in seven countries, including the US.
In rare blood disorders, phase 3 clinical trials have demonstrated the transformative potential of Mim8 in reducing bleeds. The investigational therapy is designed
to mimic the activity of Factor VIIIa, the clotting protein missing or defective in people with haemophilia A.
Alhemo® addresses significant unmet needs in haemophilia
A and B with inhibitors – the latter being an area with very limited treatment options. Administered once-daily by subcutaneous injection, the therapy offers routine treatment
to prevent bleeding in a prefilled pen device. Alhemo® is now approved in several markets worldwide, including the US and EU.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
“Alhemo® addresses significant unmet needs in haemophilia A and B with inhibitors – the latter being an area with very limited treatment options” |
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Commercial execution |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unprecedented investment
in production lays foundation for continued growth |
|
Over the past four years, we have more than quadrupled the global reach of our GLP-1-based medicines, increasing our volume market share in the GLP-1 segment to 63% over the same period. With demand still growing, we continued to expand our production network throughout 2024, making significant investments in capital expenditure (CapEx) and acquisitions totalling more than DKK 129 billion.
Through the targeted acquisitions of brownfield sites, the strategic expansion of existing facilities, the establishment of new sites and the upscaling of our global production workforce, we are equipping ourselves to support the launch of multiple next-generation therapies and meet the needs of millions more people worldwide.
The scope of these investments is measured not only in financial terms, but also by the increased
volume of active pharmaceutical ingredients (API) and the number of devices we can produce.
By investing in state-of-the-art, multi-product facilities designed to accommodate current and future products, we are laying a foundation for sustainable long-term growth.
“We are investing more than DKK 80 billion into expanding our API production capacity, including the construction of a new 170,000 square metre, multi-product API facility in Kalundborg, Denmark”
The ongoing expansions of our production sites across the globe exemplify our approach to scaling up. We are investing more than DKK 80 billion into expanding our API production capacity, including the construction of a new 170,000 square metre, multi-product API facility in Kalundborg, Denmark. Alongside additional expansions of sites in Denmark, France, Brazil, China and the US, these efforts will significantly enhance our ability to meet future demand across our portfolio.
Our acquisition of three new fill-finish sites at the turn of the year complements the ongoing expansion of our internal supply chain, enabling us to expand our manufacturing capacity and provide future optionality and flexibility for our existing supply network. The three former Catalent sites were acquired as part of a transaction that saw Novo Holdings – the holding and investment company responsible for managing the wealth and assets of the Novo Nordisk Foundation – acquire the US-based contract manufacturing and development organisation. Approximately 3,200 highly skilled Catalent employees became part of Novo Nordisk under the terms of the agreement, which will ensure that existing obligations towards other customers currently being served by the three sites will be honoured.
Removing bottlenecks in our existing supply chain also remains a top priority as we seek to keep pace with demand. The consolidation of our insulin portfolio will free up vital capacity and resources for production of our next-generation innovations, while our transition towards reusable devices and once-weekly rather than once-daily formulations continues.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Financials |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial performance
Sales increased by 25% measured in Danish kroner and by 26% at CER to DKK 290,403 million
in 2024. Novo Nordisk's 2024 sales and operating profit performance measured at CER were within the ranges provided in November 2024. The effective tax rate, capital expenditure as
well as depreciation, amortisation and impairment losses were all in line with the guidance.
The free cash flow in 2024 was realised at DKK -14.7 billion, mainly impacted by the USD 11.7 billion acquisition price related to the three Catalent manufacturing sites.
Geographic sales development
Sales in North America Operations increased by 30% in both Danish kroner and at CER.
Sales in International Operations increased by 17% measured in Danish kroner and by 19% at CER. Sales in EMEA increased by 19% in both Danish kroner and at CER. Sales in Region China increased by 11% measured in Danish kroner and by 13% at CER. Sales in Rest of World
increased by 19% measured in Danish kroner and by 23% at CER.
Sales development across therapeutic areas
Sales in Diabetes care increased by 19% measured in Danish kroner and by 20% at CER. Sales of Obesity care products, Wegovy® and Saxenda®, increased by 56% measured in Danish kroner and by 57% at CER. Sales of Rare disease products increased by 9% in both Danish kroner and at CER.
In the following sections, unless otherwise noted, market data are based on moving annual total (MAT) from November 2023 and November 2024 provided by the independent data provider IQVIA.
|
|
|
FINANCIALS
2024 performance
and 2025 outlook
|
|
|
|
|
|
|
|
Strategic Aspirations 2025 |
|
|
|
|
|
Deliver solid sales and operating
profit growth
Drive operational efficiencies across
the value chain to enable investments
in future growth assets
Deliver free cash flow to enable attractive
capital allocation to shareholders
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Financials |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diabetes care
Sales in Diabetes care increased by 19% measured in Danish kroner and by 20% at CER to DKK 206,618 million driven by growth of GLP-1-based products and insulins. Novo Nordisk's global diabetes value market share remains unchanged over the last 12 months at 33.7%.
The market share was driven by market share gains in North America Operations, offset by a market share decline in International Operations.
GLP-1-based therapy for type 2 diabetes
Sales of GLP-1-based products for type 2 diabetes (Rybelsus®, Ozempic® and Victoza®) increased by 21% measured in Danish kroner and by 22% at CER to DKK 149,125 million. The estimated global GLP-1 share of total diabetes prescriptions has increased to 6.7% compared with 6.0% 12 months ago. Novo Nordisk is the global market leader in the GLP-1 segment with a 55.1% value market share.
Ozempic® sales increased by 26% in both Danish kroner and at CER to DKK 120,342 million. Sales growth was driven by both North America Operations and International Operations. Sales growth has resulted in periodic supply constraints and related drug shortage notifications across geographies.
Rybelsus® sales increased by 24% measured in Danish kroner and by 26% at CER to DKK 23,301 million. Sales growth was driven by EMEA and Rest of World.
Victoza® sales decreased by 37% measured in Danish kroner and by 36% at CER to DKK 5,482 million. The decline was driven by the GLP-1 diabetes market moving towards once-weekly treatments in both North America Operations and International Operations.
Insulin sales
Sales of insulin increased by 15% measured in Danish kroner and by 17% at CER to DKK 55,373 million.
Obesity care
Sales of Obesity care products, Wegovy® and Saxenda®, increased by 56% measured in Danish kroner and by 57% at CER to DKK 65,146 million. Sales growth was driven by both North America Operations and International Operations. The volume growth of the global branded obesity market was 119%. Novo Nordisk is the global market leader with a volume market share of 70.4%.
Rare disease
Rare disease sales increased by 9% in both Danish kroner and at CER to DKK 18,639 million.
|
|
Rare endocrine disorders
Sales of Rare endocrine disorder products increased by 30% measured in Danish kroner and by 31% at CER to DKK 4,993 million. Novo Nordisk is working on gradually re-establishing supply of rare endocrine disorder products following a reduction of manufacturing output. Sogroya® has been launched in six countries, and the initial feedback from patients and physicians is encouraging.
Rare blood disorders
Sales of Rare blood disorder products increased by 3% in both Danish kroner and at CER to DKK 12,138 million mainly driven by increased haemophilia B sales.
Development in costs and operating profit
The cost of goods sold increased by 24% measured in Danish kroner and by 25% at CER to DKK 44,522 million, resulting in a gross margin of 84.7% measured in Danish kroner, compared with 84.6% 2023. The increase in gross margin mainly reflects a positive product mix driven by increased sales of GLP-1-based treatments and a positive price impact due to gross-to-net sales adjustments in the US. This is partially countered by costs related to ongoing capacity expansions.
Sales and distribution costs increased by 9% measured in Danish kroner and by 10% at CER to DKK 62,101 million. The increase in costs is driven by both North America Operations and International Operations. In North America Operations, the cost increase is mainly driven by promotional activities related to Wegovy®. In International Operations, the increase is mainly related to Obesity care market development activities and Wegovy® launch activities as well as promotional activities for GLP-1 diabetes products. The increase in sales and distribution costs is negatively impacted by adjustments to legal provisions in 2023. Sales and distribution costs amounted to 21.4% as a percentage of sales.
|
|
|
|
|
|
|
|
|
|
Research and development costs increased by 48% in both Danish kroner and at CER to DKK 48,062 million compared to 2023, mainly reflecting increased late-stage clinical trial activity, increased early research activities as well as impairment losses related to intangible assets. Research and development costs amounted to 16.6% as a percentage of sales. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Financials |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Administration costs increased by 9% in both Danish kroner and at CER to DKK 5,276 million.
Other operating income and expenses (net) showed a loss of DKK 2,103 million compared to an income of DKK 119 million in 2023. The loss is mainly reflecting impairments related to a partnership agreement of a company previously acquired by Novo Nordisk and transaction costs related to the Catalent transaction.
Operating profit increased by 25% measured in Danish kroner and by 26% at CER to DKK 128,339 million, reflecting the sales growth and impairments related to intangible assets. EBITDA increased by 32% measured in Danish kroner and by 33% at CER.
Financial items (net) and tax
Financial items (net) showed a net loss of DKK 1,148 million compared with a net gain of DKK 2,100 million in 2023. This primarily reflects losses on non-hedged currencies.
In line with Novo Nordisk’s treasury policy, the most significant foreign exchange risks for Novo Nordisk have been hedged, primarily through foreign exchange forward contracts. The foreign exchange result was a net loss of DKK 1,023 million compared with a net gain of DKK 1,652 million in 2023.
As per the end of December 2024, a negative market value of financial contracts of approximately DKK 5.8 billion has been deferred for recognition in 2025.
The effective tax rate was 20.6% in 2024 compared with an effective tax rate of 20.1% in 2023.
Net profit increased by 21% to DKK 100,988 million and diluted earnings per share increased by 22% to DKK 22.63. Net profit and diluted earnings per share are impacted by impairments related to intangible assets.
|
|
2025 outlook
Sales growth is expected to be 16% to 24% at CER. Given the current exchange rates versus the Danish krone, sales growth reported in DKK is expected to be 3 percentage points higher than at CER.
The guidance reflects expectations for sales growth in both North America Operations and International Operations, mainly driven by volume growth of GLP-1-based treatments for Obesity and Diabetes care. Intensifying competition and continued pricing pressure within Diabetes and Obesity care is included in the guidance.
Following higher-than-expected volume growth in recent years, including GLP-1-based products such as Ozempic® and Wegovy®, combined with the expectation of continued volume growth and capacity limitations at some manufacturing sites, the outlook also reflects expected continued periodic supply constraints and related drug shortage notifications across a number of products and geographies. Novo Nordisk is investing in internal and external capacity to increase supply both short and long-term.
Operating profit growth is expected to be 19% to 27% at CER. Given the current exchange rates versus the Danish krone, growth reported in DKK is expected to be 5 percentage points higher than at CER. The expectation for operating profit growth primarily reflects the sales growth outlook and continued investments in future and current growth drivers within Research, Development, Commercial and Manufacturing. Within R&D, investments are related to the continued expansion and progression of the early and late-stage pipeline. Commercial investments are mainly related to Obesity care market development and activities as well as investments within GLP-1 diabetes care. Within Manufacturing, investments are mainly related to ongoing scaling of capacity efforts, and a negative mid-single-digit operating profit growth impact related to the acquisition of the three Catalent manufacturing sites is also included in the guidance.
Novo Nordisk expects financial items (net) for 2025 to amount to a loss of around DKK 9 billion. This is driven by expected losses on hedged currencies, primarily the US dollar due to the increased USD/DKK rate, and increased interest expenses related to funding of the Catalent transaction, as the acquisition is mainly debt-financed.
The effective tax rate for 2025 is expected to be in the range of 21-23%. The increase compared to 2024 is mainly driven by country and therapy sales mix.
Capital expenditure is expected to be around 65 billion DKK in 2025, reflecting expansion of the global supply chain. The investments will create additional capacity across the supply chain, including manufacturing of active pharmaceutical ingredients (API), additional aseptic production and finished production processes as well as packaging capacity. In the coming years, the capital expenditure to sales ratio is still expected to be low double-digit.
Depreciation, amortisation and impairment losses are expected to be around DKK 17 billion, and include depreciations and amortisations related to the Catalent transaction.
|
|
|
|
|
|
|
|
|
|
|
|
1. Expectations for 2025.
|
Cash flow and capital allocation
Free cash flow in 2024 was realised at DKK -14.7 billion compared to DKK 68.3 billion in 2023. The lower free cash flow in 2024 is mainly impacted by the USD 11.7 billion acquisition price related to the three Catalent manufacturing sites. Free cash flow is also impacted by increasing capital expenditure, partially countered by net cash generated from operating activities.
Capital expenditure for property, plant and equipment was DKK 47.2 billion compared with DKK 25.8 billion in 2023, primarily reflecting investments in additional capacity for active pharmaceutical ingredient (API) production and fill-finish capacity for both current and future injectable and oral products. Capital expenditure related to intangible assets was DKK 4.1 billion in 2024 compared with DKK 13.1 billion in 2023 reflecting business development activities.
Income under the 340B Program has been partially recognised.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Financials |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The free cash flow is expected to be DKK 75-85 billion reflecting the sales growth, a favourable impact from rebates in the US, countered by increased investments in capital expenditure.
All of the above expectations are based on assumptions that the global or regional macroeconomic and political environment will not significantly change business conditions for Novo Nordisk during 2025, including energy and supply chain disruptions, the potential implications from major healthcare reforms and legislative changes, taxation changes, including changes in tariffs and duties, as well as outcome of legal cases including litigations related to the 340B Drug Pricing Program in the US, and that the currency exchange rates, especially the US dollar, will remain at the current level versus the Danish krone. The guidance is also based on assumptions in relation to the estimation of gross-to-net developments in the US gross sales. Finally, the guidance does not include the financial implications of any new significant business development transactions and significant impairments of intangible assets during 2025.
Novo Nordisk has hedged expected net cash flows in a number of invoicing currencies, and, all other things being equal, movements in key invoicing currencies will impact Novo Nordisk’s operating profit as outlined in note 4.4 on Financial risks.
|
|
Forward-looking statements
Novo Nordisk’s statutory Annual Report 2024, Form 20-F, any quarterly financial reports, and written information released, shown, or oral statements made, to the public in the future by
or on behalf of Novo Nordisk, may contain certain forward-looking statements relating to the operating, financial and sustainability performance and results of Novo Nordisk and/or the industry in which it operates. Forward-looking statements can be identified by the fact that they do not relate to historical or current facts and include guidance. Words such as ‘believe’, ‘expect’, ‘may’, ‘will’, ‘plan’, ‘strategy’, ‘transition plan’, ‘prospect’, ‘foresee’, ‘estimate’, ‘project’, ‘anticipate’, ‘can’, ‘intend’, ‘target’ and other words and terms of similar meaning in connection with any discussion of future operating, financial or sustainability performance identify forward-looking statements. Examples of such forward-looking statements include, but are not limited to:
•Statements of targets, future guidance, (transition) plans, objectives or goals for future operations, including those related to operating, financial and sustainability matters,
Novo Nordisk’s products, product research, product development, product introductions
and product approvals as well as cooperation in relation thereto;
•Statements containing projections of or targets for revenues, costs, income (or loss), earnings per share, capital expenditures, dividends, capital structure, net financials and other financial measures;
•Statements regarding future economic performance, future actions and outcome of contingencies, such as legal proceedings; and
•Statements regarding the assumptions underlying or relating to such statements.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expectations are as reported, if not otherwise stated |
Expectations
5 February 2025 |
|
These statements are based on current plans, estimates, opinions, views and projections. Although Novo Nordisk believes that the expectation reflected in such forward-looking statements are reasonable, there can be no assurance that such expectation will prove to be correct. By their very nature, forward-looking statements involve risks, uncertainties and assumptions, both general and specific, and actual results may differ materially from those contemplated, expressed or implied by any forward-looking statement.
Factors that may affect future results include, but are not limited to, global as well as local political, economic and environmental conditions, such as interest rate and currency exchange rate fluctuations or climate change, delay or failure of projects related to research and/or development, unplanned loss of patents, interruptions of supplies and production, including
as a result of interruptions or delays affecting supply chains on which Novo Nordisk relies, shortages of supplies, including energy supplies, product recalls, unexpected contract breaches or terminations, government-mandated or market-driven price decreases for Novo Nordisk’s products, introduction of competing products, reliance on information technology including the risk of cybersecurity breaches, Novo Nordisk’s ability to successfully market current and new products, exposure to product liability and legal proceedings and investigations, changes in
|
|
|
Sales growth |
|
|
|
|
at CER |
16% to 24% |
|
|
|
as reported |
Around 3 percentage points higher than at CER |
|
|
|
Operating profit growth |
|
|
|
|
at CER |
19% and 27% |
|
|
|
as reported |
Around 5 percentage points higher than at CER |
|
|
|
Financial items (net) |
Loss of around 9 bDKK |
|
|
|
Effective tax rate |
21% to 23% |
|
|
|
Capital expenditure (PP&E) |
Around 65 bDKK |
|
|
|
Depreciation, amortisation and impairment losses |
Around DKK 17 billion |
|
|
|
Free cash flow (excluding impact from business development) |
Between 75 and 85 bDKK |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Financials |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
governmental laws and related interpretation thereof, including on reimbursement,
intellectual property protection and regulatory controls on testing, approval, manufacturing
and marketing, and taxation changes, including changes in tariffs and duties, perceived or
actual failure to adhere to ethical marketing practices, investments in and divestitures of
domestic and foreign companies, unexpected growth in costs and expenses, strikes and other labour market disputes, failure to recruit and retain the right employees, failure to maintain a culture of compliance, epidemics, pandemics or other public health crises, effects of domestic
or international crises, civil unrest, war or other conflict and factors related to the foregoing matters and other factors not specifically identified herein.
For an overview of some, but not all, of the risks that could adversely affect Novo Nordisk’s
results or the accuracy of forward-looking statements in this Annual Report 2024, reference is made to the overview of risk factors in ‘Risks’ of this Annual Report 2024.
|
|
None of Novo Nordisk or its subsidiaries or any such person’s officers, or employees accept any responsibility for the future accuracy of the opinions and forward-looking statements expressed
in the Annual Report 2024, Form 20-F, any quarterly financial reports, and written information released, shown, or oral statements made, to the public in the future by or on behalf of Novo Nordisk or the actual occurrence of the forecasted developments.
Unless required by law, Novo Nordisk has no duty and undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events, or otherwise.
Shares and capital structure
Through open and proactive communication, Novo Nordisk aims to provide the basis for fair and efficient pricing of our shares.
Share capital and ownership
Novo Nordisk’s share capital of DKK 446.5 million is divided into A and B share capital. The A and
B shares are calculated in units of DKK 0.10, amounting to 4.5 billion shares. The A share capital, consisting of 1,075 million shares, has a nominal value of DKK 107,487,200 and the B share capital, consisting of 3,390 million shares, has a nominal value of DKK 339,012,800. Each A share carries
100 votes and each B share carries 10 votes. Novo Nordisk’s B shares are listed on Nasdaq Copenhagen and on the New York Stock Exchange (NYSE) as American Depository Receipts (ADRs).
The general meeting has authorised the Board of Directors to distribute extraordinary dividends,
issue new shares in accordance with the Articles of Association and repurchase shares in
accordance with authorisations granted.
|
|
|
|
|
|
Ownership structure2
2. Treasury shares
are included;
however, voting
rights of treasury
shares cannot be exercised.
3. Novo Holdings
A/S’s registered
address is Tuborg Havnevej 19,
DK-2900 Hellerup, Denmark.
|
|
|
|
|
The company’s A shares are not listed and are held by Novo Holdings A/S3, a Danish public limited liability company wholly owned by the Novo Nordisk Foundation. According to the Articles of Association of the Foundation, the A shares cannot be divested. Special rights attached to A shares include pre-emptive subscription rights in the event of an increase in the A share capital and pre-emptive purchase rights in the event of a sale of A shares, while B shares take priority for liquidation proceedings. A shares take priority for dividends below 0.5%, and B shares take priority for dividends between 0.5 and 5%. However, in practice, A and B shares receive the same amount of dividend per share. |
|
4. Split of
shareholders
is denoted
according to the
location of legal
deposit-owners. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Financials |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of 31 December 2024, Novo Holdings A/S held a B share capital of nominally DKK 17,756,050. Together with the A shares, Novo Holdings A/S’s total ownership amounted to nominally DKK 125,243,250. Novo Holdings A/S ownership is reflected in the ‘Ownership structure’ chart.
There is no complete record of all shareholders; however, based on available sources of information, as of 31 December 2024 it is estimated that shares were geographically distributed
as shown in the ‘Geographical split of shareholders’ chart. As of 31 December 2024, the free float of listed B shares was 94.06% (of which approximately 13.82% are listed as ADRs), excluding
Novo Holdings A/S and Novo Nordisk’s holding of shares. As of 31 December 2024, Novo
Holdings A/S and Novo Nordisk’s holding of B shares equalled 201,220,032 shares and had a nominal value of DKK 20,122,003. For details about the share capital, see note 4.3 to the Consolidated financial statements.
Capital structure
Novo Nordisk’s Board of Directors and Executive Management consider that the current capital and share structure of Novo Nordisk serve the interests of the shareholders and the company
well. Novo Nordisk’s capital structure strategy offers a balance between long-term shareholder value creation and competitive shareholder return in the short term.
In 2024, Novo Nordisk issued Eurobonds totaling EUR 4.65 billion. The total outstanding Eurobonds as of the end of 2024 amounted to 6.8 billion.
Dividend policy
The company’s dividend policy applies a pharmaceutical industry benchmark to ensure a competitive payout ratio for dividend payments, may be complemented by share repurchase programmes. The final dividend for 2023 paid in March 2024 was equal to DKK 6.40 per A and B share of DKK 0.10 as well as for ADRs. The total dividend for 2023 was DKK 9.40 per A and B
share of DKK 0.10, corresponding to a payout ratio of 50.2%. The 2023 pharma peer group
average was 53.0%.
In August 2024, an interim dividend was paid equalling DKK 3.50 per A and B share of DKK 0.10 as well as for ADRs. For 2024, the Board of Directors will propose a final dividend of DKK 7.90 to be paid in March 2025, equivalent to a total dividend for 2024 of DKK 11.40 and a payout ratio of 50.2%. The company expects to distribute an interim dividend in August 2025. Further information regarding this interim dividend will be announced in connection with the financial report for the first six months of 2025. Dividends are paid from distributable reserves. Novo Nordisk does not pay a dividend on its holding of treasury shares.
|
|
Share repurchase programme for 2024/2025
During the twelve-month period beginning 6 February 2024, Novo Nordisk repurchased
shares worth DKK 20 billion. The share repurchase programme has primarily been
conducted in accordance with the safe harbour rules in the EU Market Abuse Regulation
(MAR). Novo Nordisk’s capital allocation priorities focus on internal growth investments, including supply chain expansions, dividends as well as external growth opportunities,
including acquiring the three Catalent manufacturing sites. Consequently, Novo Nordisk
is not expecting to initiate a share buyback programme in 2025.
Share price development
Since end of December 2023 until end of December 2024, Novo Nordisk’s share price
decreased from DKK 698 to DKK 624, a decrease of 10.6%. The total market value of Novo Nordisk’s B shares, excluding treasury shares and Novo Holdings A/S shares, was DKK 1,990,516,353,626 as of 30 December 2024.
|
|
|
|
|
|
|
|
|
|
|
5. OMXC25 and pharmaceutical
industry
development
have been
rebased to Novo
Nordisk share
price in January
2024.
6. AstraZeneca,
Bristol-Meyers,
Eli Lilly,
GlaxoSmithKline, Lundbeck, Merck, Novartis, Pfizer,
Roche and Sanofi. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Risk management |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Risk management
Rigorous and systematic risk management is essential. The current risk landscape is impacted by elevated geopolitical uncertainties and market dynamics in the segments in which we operate.
We apply a dual-lensed approach to risk management. This means that we aim to identify and mitigate both operational risks that pose a threat to our short- to medium-term plans, and strategic risks that could reduce
our ability to realise our corporate strategy over the long term.
Addressing risks in our strategic planning
Scenario and risk-thinking exercises are part of our strategic planning. These exercises involve analysing
market trends and considering the effects of socioeconomic, environmental, geopolitical and political
changes that could pose risks to, or create opportunities for, our business. Annually, Executive Management
and the Board of Directors review and discuss a strategic risk profile. Further, strategic risks and the
conclusions from our double materiality assessment in the Sustainability statement are compared to better
integrate sustainability risks in our risk outlooks and strategic direction. The main strategic risks are:
Innovation and competition
Novo Nordisk faces a concentration risk with multiple brands being dependent on the semaglutide
compound as the active pharmaceutical ingredient. To remain competitive in the long term and thereby
mitigate the innovation risk, we invest in internal and external pipeline opportunities as well as effectively
attracting talent to continue providing patients with innovative treatments.
Production capacity and supply chain risks
Demand fluctuations, resource shortages, geopolitical instability, trade disputes and local manufacturing requirements are all factors that can pressure global supply chains. Furthermore, expanding production
capacity is complex and associated with a long lead times. Therefore, planning and management of our
production capacity and supply chain are key to mitigate this risk.
Access and affordability
Access to affordable care is a global issue as healthcare systems struggle to provide quality care at a
sustainable cost, while the burden of chronic diseases keeps rising. Ensuring access and affordability is a
risk and responsibility Novo Nordisk shares with all stakeholders involved in healthcare. We continue to
scale our capacity to meet patient demand and thereby broaden access to medicines and to
meet our social responsibilities.
Healthcare reform
Some governments are adopting changes to their pharmaceutical frameworks that introduce further
complexity in healthcare systems and uncertainties in the regulatory environment. This may lead to additional
price pressure, potentially impacting our profitability. We continuously educate healthcare providers about
the value and benefits of our products as well as engage in a dialogue with policymakers and stakeholders, communicating potential consequences of healthcare reform to the innovative life science environment.
|
|
Digital disruption
New digital technologies could provide an opportunity to deliver more value to our stakeholders and help patients live a life with fewer limitations. We recognise that there is also a risk of digital disruption leading to increased competition through accelerated and enhanced drug discovery and development. To ensure we remain competitive, we continuously innovate and integrate these technologies into our processes.
Environmental impact
Novo Nordisk's current expansion efforts, including scaling of production capacity, significantly increases our current and projected greenhouse gas emissions. We are addressing this challenge through our Circular for Zero strategy. This includes an increased focus on our global emissions, also encompassing scope 3 emissions, as well as assessing, monitoring and mitigating environmental risks across the value chain.
Geopolitical uncertainty
Ongoing conflicts, geopolitical tensions and social unrest represent a volatile landscape which has led to governments introducing trade restrictions and tariffs. If escalating tensions persist, there is a risk that
further tariffs may be imposed. Most notably, the new US administration considers to impose a range of trade actions on all articles imported into the US. We navigate this elevated degree of geopolitical uncertainty by monitoring geopolitical developments, actively engaging in policy making and diversifying our supply chain.
Ethics and compliance
Our commitment to ethics and compliance remains at the forefront of all our operations. Any inability to uphold our ethical standards could lead to reputational implications, with potential effects on market access and pricing negotiations. Our values, encapsulated in the Novo Nordisk Way, guide every decision we make. OneCode, our code of conduct, further empowers us to conduct our operations responsibly. These guidelines help us to maintain integrity, thereby enabling us to fulfil our purpose effectively.
Operational risk management process
In the short- to medium-term, we are exposed to risks throughout our value chain. Some risks are inherent in the pharmaceutical industry, such as delays or failures of potential late-stage medicines in the R&D pipeline. Other risks, such as geopolitical instability, supply disruptions and competition, are common amongst manufacturing companies with global production. We will not compromise on product quality, patient safety and business ethics: these are front and centre of our enterprise-wide risk management set-up. We assess risks with regard to their corresponding likelihood of financial loss or reputational damage.
Executive Management, the Board of Directors and the Audit Committee review a risk grid of our biggest operational risks every three months. This grid is based on insights from management teams across the organisation and includes all types of risks that could cause significant disruptions to the business over a three-year horizon, including potential environmental, social and governance risks. An overview of our key operational risks, along with detailed descriptions, is provided on the next page. For more information, see our Corporate Governance Report available at: www.novonordisk.com/about/corporate-governance.html.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Key operational risks |
|
Key operational risks
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Risk area |
Description |
Impact |
Mitigating actions |
|
|
Research and
clinical pipeline
risks1
|
Findings in clinical activities, regulatory processes or misjudging of commercial potential, leading to delays or failure of products in the pipeline. |
•Patients would not be provided with innovative treatment options.
• Could have an adverse impact on sales, profits and market position.
|
•Pre-clinical and clinical activities to demonstrate safety and
efficacy.
• Consultations with regulators to review pre-clinical and
clinical findings and obtain guidance on development path.
|
|
Product supply, quality and
safety risks
|
Higher-than-expected demand or disruption of product supply due to, e.g. geopolitical instability or quality issues, may compromise product availability, ultimately impacting patients and representing a lost commercial opportunity. In addition, there could be risks related to safety and product liability. |
•Product shortages could have potential implications for patients.
• Could jeopardise reputation and license to operate if regulatory compliance is not ensured.
• Compromised patient safety and exposure to product liability legal proceedings.
• Could diminish trust in Novo Nordisk, impacting our reputation.
• Could have an adverse impact on sales, profits and market position.
|
•Significantly expanding global production with multiple
facilities and safety stock to reduce supply risk.
• Planning and management of supply chain.
• Regular quality audits of internal units and suppliers to
document Good Manufacturing Practice (GMP) compliance.
• Identification and correction of root causes when issues are identified. If necessary, products are recalled.
|
|
Commercialisation
risks1
|
Competitive pressures, as well as market dynamics and geopolitical, macroeconomic or healthcare crises (e.g. pandemics) leading to reduced payer ability and willingness to pay. |
•Market dynamics could impact price levels and patient access.
• Could have an adverse impact on sales, profits and market position.
|
•Innovation of novel products, clinical trial data and real-world evidence demonstrate added value of new products.
• Payer negotiations to ensure improved patient access.
• Increased and new access and affordability initiatives.
|
|
IT security
risks
|
Disruption to IT systems, such as
cyber-attacks or infrastructure failure, resulting in business disruption or breach of data confidentiality. |
•Could limit our ability to produce and safeguard product quality.
• Could compromise patients’ or other individuals’ privacy.
• Could limit our ability to maintain operations or limit future business opportunities
if proprietary information is lost.
• Could have an adverse impact on sales, profits and market position.
|
•Proactive company-wide information security awareness initiatives.
• Continuity plans for non-availability of IT systems.
• Company-wide internal audit of IT security controls.
• Detection and protection mechanisms in IT systems and
business processes.
|
|
Financial
risks
|
Exchange rate fluctuations (mainly in USD, CNY, JPY, CAD and BRL), geopolitical risks (e.g. tariffs), disputes with tax authorities and changes to tax legislation and interpretation. |
•Could lead to tax adjustments, fines and higher-than-expected tax level.
• Could have an adverse impact on sales and profits.
• Geopolitical actions could lead to an increase in corporate taxes and duties.
|
•Hedging for selected currencies.
• Integrated treasury management.
• Applicable taxes paid in jurisdictions where business activity generates profits and multi-year Advance Pricing Agreements
with tax authorities.
|
|
Legal, patents
and compliance
risks1
|
Breach of legislation, industry codes or company policies. Competitors asserting patents against Novo Nordisk or challenging patents critical for protection of commercial product and pipeline candidates. |
•Potential exposure to investigations, criminal and civil sanctions and other penalties.
• Could compromise our reputation and the rights and integrity of individuals
involved.
• Could lead to unexpected loss of exclusivity for, or injunctions against, existing and pipeline products.
• Could have an adverse impact on sales, profits and market position.
|
•Code of Conduct integrated in our business.
• Compliance Hotline in place.
• Legal review of key activities and internal audit of compliance
with business ethics standards.
• Internal controls to minimise vulnerability to patent
infringement and invalidity actions.
|
|
1. Risk is also captured as part of the double materiality assessment conclusions in the Sustainability statement; read more on page 52. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Strategic Aspirations / Financials |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Management / Board of Directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Board of Directors |
|
|
|
|
Helge Lund
Chair
|
|
|
Henrik Poulsen
Vice chair
|
|
|
Elisabeth Dahl Christensen
|
|
|
Laurence
Debroux
|
|
|
Andreas
Fibig
|
|
|
Sylvie
Grégoire
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Norwegian. Born October 1962. Male. Member since 20171. Term 2025.
Chair of the People and Governance Committee and the Chair Committee.
Positions and management duties
Chair of the board of directors and chair
of the people, culture and governance committee of BP p.l.c. Chair of the board
of directors of Inkerman AS. Chair of the board of directors of Stiftelsen Værekraft. Member of the board of directors and member of the remuneration committee
of Belron SA. Member of the board of
directors of P/F Tjaldur. Member of the board of trustees of the International
Crisis Group. Operating advisor to
Clayton Dubilier & Rice.
Competences
Global corporate leadership;
healthcare and pharma industry;
finance and accounting; business development, M&A and external innovation sourcing; human capital management; environmental, social
and governance (ESG).
|
|
Danish. Born September 1967. Male. Member since 2021. Term 2025. Chair
of the Remuneration Committee and member of the Audit Committee and
the Chair Committee.
Positions and management duties
Chair of the supervisory board, chair
of the people & culture committee and member of the remuneration committee of Carlsberg A/S. Chair of the board of directors and chair of the nomination
& remuneration committee of Faerch
A/S. Member of the board of directors
of Novo Holdings A/S. Member of the supervisory board of Bertelsmann
SE & Co. KGaA. Senior advisor to
A.P. Møller Holding A/S.
Competences
Global corporate leadership;
finance and accounting; business development, M&A and external innovation sourcing; human capital management; environmental, social
and governance (ESG).
|
|
Danish. Born November 1965. Female. Member since 2022. Term 2026.
Employee representative. Member
of the Remuneration Committee.
Positions and management duties
Full-time union representative at
Novo Nordisk A/S.
Competences
Not mapped for employee
representatives.
|
|
French. Born July 1969. Female.
Member since 2019. Term 2025. Chair
of the Audit Committee and member
of the Remuneration Committee.
Positions and management duties
Member of the board of directors, chair
of the audit committee and member of
the ESG committee of Exor N.V. Member
of the supervisory board and chair of the audit committee of Randstad N.V. Member of the board of directors of Institut Mérieux, HEC Paris Business School and Kite Insights Limited (the Climate School).
Competences
Global corporate leadership;
healthcare and pharma industry;
finance and accounting; business development, M&A and external innovation sourcing; human capital management; environmental, social
and governance (ESG).
|
|
German. Born February 1962. Male. Member since 2018. Term 2025.
Member of the Research & Development Committee.
Positions and management duties
Member of the board of directors of Indigo Agriculture Inc., Evodiabio ApS and ExlService Holdings, Inc. Honorary director of the German American Chamber of Commerce.
Competences
Global corporate leadership; healthcare and pharma industry; technology, data and digital; finance and accounting; business development, M&A and external innovation sourcing; human capital management; environmental, social and governance (ESG).
|
|
Canadian and American. Born
November 1961. Female. Member
since 2015. Term 2025. Member of
the Audit Committee, the Research
& Development Committee and the
People and Governance Committee.
Positions and management duties
Co-founder and member of the board of directors of CervoMed, Inc. Chair
of the board of directors of Abivax SA. Member of the board of directors of
F2G Ltd. Advisor to the Soffinova Telethon Fund.
Competences
Global corporate leadership; healthcare and pharma industry; medicine and science; finance and accounting; business development, M&A and external innovation sourcing; human capital management.
|
|
|
1. Helge Lund was also a member of the Board of Directors for one one-year term from 2014-2015. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Management / Board of Directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Board of Directors (continued) |
|
|
|
|
Liselotte Hyveled
|
|
|
Mette Bøjer Jensen
|
|
|
Kasim
Kutay
|
|
|
Christina
Law
|
|
|
Martin
Mackay
|
|
|
Thomas Rantzau
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Danish. Born January 1966. Female. Member since 20222. Term 2026. Employee representative. Member
of the Research & Development Committee.
Positions and management duties
Chief patient officer and principal vice president of Patient Voice Strategy & Alliances, Novo Nordisk A/S. Member
of the board of directors of TriSalus
Life Sciences.
Competences
Not mapped for employee
representatives.
|
|
Danish. Born December 1975. Female. Member since 2018. Term 2026. Employee representative. Member
of the Audit Committee.
Positions and management duties
Wash & Sterilisation specialist in
Product Supply, Novo Nordisk A/S.
Competences
Not mapped for employee
representatives.
|
|
British. Born May 1965. Male. Member since 2017. Term 2025. Member of the People and Governance Committee and the Research & Development Committee.
Positions and management duties
CEO of Novo Holdings A/S. Member
of the board of directors and member
of the nomination and remuneration committee of Novonesis A/S.
Competences
Global corporate leadership; healthcare and pharma industry; finance and accounting; business development,
M&A and external innovation sourcing; human capital management.
|
|
Chinese. Born January 1967. Female. Member since 2022. Term 2025. Member of the Audit Committee.
Positions and management duties
Group CEO of Raintree Group of Companies. Member of the board of directors of Raintree Group Limited, Raintree Investment Pte Ltd. and Air Liquide S.A. Member of the board of directors of La Fondation des Champions. Member of the board of directors and chair of the development committee of National Gallery Singapore.
Competences
Global corporate leadership; technology, data and digital; business development, M&A and external innovation sourcing; human capital management.
|
|
American and British. Born April 1956. Male. Member since 2018. Term 2025. Chair of the Research & Development Committee and member of the Remuneration Committee.
Positions and management duties
Co-founder and non-executive chair of the board of directors of Rallybio LLC. Member of the board of directors and member of the science and technology committee and the responsible animal use committee of Charles River Laboratories International, Inc. Member of the board of directors and member
of the compensation committee and research and development committee of SpringWorks Therapeutics, Inc. Scientific advisor at Pivotal BioVenture Partners. Member of the external advisory board of Boston Children’s Hospital.
Competences
Global corporate leadership; healthcare and pharma industry; medicine and science; technology, data and digital; business development, M&A and external innovation sourcing; human capital management.
|
|
Danish. Born March 1972. Male.
Member since 2018. Term 2026. Employee representative. Member of
the People and Governance Committee.
Positions and management duties
Lead auditor, Internal Audits,
Novo Nordisk A/S.
Competences
Not mapped for employee
representatives.
|
|
|
2. Liselotte Hyveled was also an employee-elected member of the Board of Directors for one four-year term from 2014-2018. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Management / Board of Directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Independence and meeting attendance overview |
|
|
|
|
Meeting attendance in 20243 |
|
|
Name |
Independence4 |
Board of
Directors |
Chair
Committee |
Audit
Committee10
|
People and Governance Committee |
Remuneration
Committee |
R&D
Committee |
|
|
Helge Lund |
Independent |
9/9 |
8/8 |
|
3/3 |
|
|
|
|
Henrik Poulsen |
Not independent5, 6, 7, 8 |
9/9 |
8/8 |
5/5 |
|
6/6 |
|
|
|
Elisabeth Dahl Christensen |
Not independent9 |
9/9 |
|
|
|
5/6 |
|
|
|
Laurence Debroux |
Independent6, 7, 8 |
9/9 |
|
5/5 |
|
6/6 |
|
|
|
Andreas Fibig |
Independent |
8/9 |
|
|
|
|
5/6 |
|
|
Sylvie Grégoire |
Independent6 |
9/9 |
|
5/5 |
3/3 |
|
6/6 |
|
|
Liselotte Hyveled |
Not independent9 |
8/9 |
|
|
|
|
6/6 |
|
|
Mette Bøjer Jensen |
Not independent6, 9 |
9/9 |
|
5/5 |
|
|
|
|
|
Kasim Kutay |
Not independent5 |
8/911 |
|
|
3/3 |
|
5/6 |
|
|
Christina Law |
Independent6 |
9/9 |
|
5/5 |
|
|
|
|
|
Martin Mackay |
Independent |
9/9 |
|
|
|
6/6 |
6/6 |
|
|
Thomas Rantzau |
Not independent9 |
9/9 |
|
|
3/3 |
|
|
|
|
3. Number of meetings attended by each Board member out of the total number of meetings within the member’s term. 4. In accordance with recommendation 3.2.1 of the Danish Corporate Governance Recommendations. 5. Member of the board of directors or executive management of Novo Holdings A/S. 6. Pursuant to the US Securities Exchange Act, Laurence Debroux, Sylvie Grégoire and Christina Law qualify as independent Audit Committee members, while Mette Bøjer Jensen and Henrik Poulsen rely on an exemption from the independence requirements. 7. Laurence Debroux and Henrik Poulsen possess the qualifications within accounting and auditing required under part 8 of the Danish Act on Approved Auditors and Audit Firms. 8. Designated as financial experts as defined by the US Securities and Exchange Commission (SEC). 9. Elected by employees of Novo Nordisk. 10. Collectively, the members have relevant industry expertise. 11. Kasim Kutay was recused from an extraordinary meeting of the Board of Directors due to a conflict of interest. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual review / Management / Executive Management |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Executive Management |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lars Fruergaard Jørgensen1 |
|
|
Maziar Mike Doustdar
|
|
|
Ludovic
Helfgott
|
|
|
Karsten Munk Knudsen1 |
|
|
Martin Holst Lange
|
|
|
President and Chief Executive Officer (CEO).
Born November 1966. Male.
Other positions and management duties
President of the European Federation of Pharmaceutical Industries and Associations
(EFPIA). Member of the board of directors at Danmarks Nationalbank (the Danish central bank).
|
|
Executive Vice President. International
Operations. Born August 1970. Male.
Other positions and management duties
Member of the board of directors and the personnel and remuneration committee of
Orion Corporation.
|
|
Executive Vice President. Rare Disease.
Born July 1974. Male.
Other positions and management duties
President of the Novo Nordisk Haemophilia Foundation Council.
|
|
Executive Vice President. Chief Financial
Officer (CFO). Born December 1971. Male.
Other positions and management duties
Member of the board of directors and chair of
the audit committee of Hempel A/S. Member of
the board of directors, chair of the audit & ESG committee of 3Shape Holding A/S.
|
|
Executive Vice President. Development.
Born October 1970. Male.
Other positions and management duties
Member of the board of directors of Pharmacosmos A/S.
|
|
|
|
David
Moore |
|
|
Tania
Sabroe |
|
|
Marcus
Schindler |
|
|
Camilla
Sylvest |
|
|
Henrik
Wulff |
|
|
Executive Vice President. US Operations & Business Development. Born January 1974. Male.
Other positions and management duties
Member of the board of directors of
Novasenta Inc.
|
|
Executive Vice President. Global People & Organisation. Born July 1977. Female.
Other positions and management duties
No other management positions.
|
|
Executive Vice President. Research & Early Development and Chief Scientific Officer (CSO).
Born September 1966. Male.
Other positions and management duties
Adjunct Professor of Pharmacology at
the University of Gothenburg.
|
|
Executive Vice President. Commercial Strategy & Corporate Affairs. Born November 1972. Female.
Other positions and management duties
Member of the board of directors of Danish Crown A/S and Argenx SE.
|
|
Executive Vice President. Product Supply,
Quality & IT. Born November 1970. Male.
Other positions and management duties
Member of the board of directors of
Grundfos Holding A/S.
|
|
|
1. Lars Fruergaard Jørgensen and Karsten Munk Knudsen are registered as executives with the Danish Business Authority. The other members of Executive Management are not registered as executives with the Danish Business Authority. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / General information / 1.1 ESG performance |
|
1. General information
1.1 ESG performance
Novo Nordisk strives to conduct its activities in a financially, environmentally, and socially responsible way. The year 2024 marks an important milestone for Novo Nordisk’s sustainability reporting. This is our first Sustainability statement prepared in accordance with the EU Corporate Sustainability Reporting Directive (CSRD). Through our disclosures, we endeavour to ensure transparency across all sustainability matters deemed material to Novo Nordisk, including the ways in which we impact people and society as a pharmaceutical company, both positively and negatively.
The Sustainability statement covers our essential sustainability topics, aligned with our strategic aspirations as highlighted in the Annual review, which are
the sustainability topics found to be material from both an impact and financial perspective. The statement also covers other important sustainability topics, deemed material due to our commitment to being transparent regarding our impacts. The Sustainability statement includes information on relevant processes, policies, actions, performance metrics and targets in accordance
with the requirements of the European Sustainability Reporting Standards (ESRS) for each sustainability topic.
In this first CSRD implementation year, we have focused on ensuring a concise Sustainability statement, in line with our financial reporting. In addition, we
have strived to translate the sector-agnostic CSRD requirements into relevant information to Novo Nordisk. In particular, we have ensured that the standard for consumers and end-users reflects our reality of serving patients. We remain committed to continuing to improve the standardisation and transparency of sustainability information, while also doing our part to ensure our sustainability reporting is relevant and clear to all stakeholders. In parallel to implementing the new reporting requirements, we have accelerated on our strategic ambitions to further progress on sustainability in 2024.
To help serve people living with serious chronic diseases, we reached a total number of 45.2 million people with Diabetes and Obesity care treatment in 2024
(41.6 million in 2023). The growth in demand has resulted in further investments
to expand capacity to be able to serve many more patients in the future.
"To help serve people living with serious chronic diseases, we reached a total number of 45.2 million people with Diabetes and Obesity care treatment in 2024."
We acknowledge that, as we continue to reach more patients, our social responsibility to improve access and affordability for vulnerable populations also grows. The number of vulnerable patients reached with our Diabetes products was 8.4 million, a decrease of 5% compared to 2023 due to reduced reach with human insulin tender sales. We remain committed to our broader access and affordability efforts to help address global health inequities while adhering to high quality standards. In regards to our target on reaching more children through our Changing Diabetes® programme, we have reached 64,743 children since its start in 2009, which is in line with our target of reaching 100,000 children by 2030.
Our prevention initiatives continue to be core to our social responsibility, and we broadened their scope with the relaunch of Cities for Better Health and our new Childhood Obesity Prevention Initiative, focusing on childhood overweight and obesity in underprivileged urban communities.
In 2024, we strengthened our Circular for Zero ambition by setting a target, aligned with climate science, of a 33% reduction of our absolute scope 3 emissions by 20331 compared to a 2024 baseline. In addition, we have a scope 1 and 2 emissions target of zero CO2e by 2030 and overall net zero emissions by 2045. Due to our rapid growth, in 2024 scope 1 and 2 emissions increased by 9%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Strategic aspiration |
ESG metric |
Unit |
Target |
Base year |
Target year |
Being respected for adding value to society |
Children reached via Changing Diabetes® in Children |
Number |
100,000 |
2009 |
2030 |
|
Progress towards zero
environmental impact
|
Scope 1 and 2 (market-based) GHG emissions |
1,000 tonnes CO2e |
0 |
– |
2030 |
Scope 3 GHG emissions |
% |
(33%) |
2024 |
2033 |
Total GHG emissions (net zero) |
1,000 tonnes CO2e |
0 |
– |
2045 |
Plastic footprint per patient |
% |
(30%) |
2024 |
2033 |
Being respected as a sustainable employer |
Gender in senior leadership positions |
% men:women |
min. 45% |
– |
2025 |
1. The target covers nearly 70% of our scope 3 emissions in accordance with SBTi provisions.
Read more on page 57.
and scope 3 emissions by 24%, underlining the importance of our updated decarbonisation roadmap to decouple emissions from future growth.
In 2024, we also set a global target to reduce our plastic footprint per
patient by 30% by 2033. This will be achieved through circular product
design, innovating treatment methods and efforts to convert to reusable devices. Furthermore, we developed a new nature roadmap with the overarching ambition of halting the loss of nature in our value chain by
2033 and becoming nature positive by 2045.
To remain respected as a sustainable employer, we maintain a focus on diversity and inclusion and continue to work towards a balanced gender representation across all managerial levels globally. In 2024, we reached 42% female representation in senior leadership positions, and we are aiming for a minimum of 45% women and 45% men in senior leadership positions by the end of 2025.
To improve safety, physical and mental wellbeing of our workforce, we monitor
short-term targets to reduce the number of accidents, as well as number of employees experiencing physical pain and symptoms of stress. In 2024, our year-on-year reduction targets were not met, due to different factors including the scaling of our organisation. A number of actions have been initiated in 2024 as we remain committed to protecting the health, safety and wellbeing of our workforce.
The table below shows selected targets related to our strategic priorities, with key performance metrics disclosed on the following page. A complete list of all metrics is placed under relevant topical chapters.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / General information / 1.1 ESG performance |
|
Key ESG performance metrics for the year ended 31 December
As a result of our double materiality assessment (see section 1.5 'Double materiality assessment' on page 52), we identified four essential sustainability topics with both financial and impact materiality which are closely aligned with our strategic sustainability aspirations described in the Annual review: patient protection and quality of life, climate change, resource
use and circular economy, and own workforce. Other important sustainability topics have been identified, which are only impact material. Listed below are selected performance metrics for both essential and important sustainability topics.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Essential sustainability topics |
Unit |
Table |
|
2024 |
|
2023 |
2022 |
|
Important sustainability topics |
Unit |
Table |
|
2024 |
|
2023 |
2022 |
 Patient protection and quality of life |
|
|
|
|
|
|
|
|
 Business conduct |
|
|
|
|
|
|
|
Patients reached with Diabetes and Obesity care products |
Number in millions |
3.1.1 |
|
45.2 |
|
41.6 |
36.9 |
|
Substantiated cases reported within accounting issues, fraud |
Number |
4.1.4 |
|
242 |
|
221 |
227 |
Vulnerable patients reached with Diabetes care products2 |
Number in millions |
3.1.1 |
|
8.4 |
|
8.8 |
- |
|
and business ethics matters via the Compliance Hotline6 |
|
|
Children reached through the Changing Diabetes® in Children programme (cumulative) |
Number |
3.1.2 |
|
64,743 |
|
52,249 |
41,033 |
|
Animals purchased for research |
Number |
4.1.8 |
|
49,284 |
|
56,508 |
79,750 |
|
|
|
 Water |
|
|
|
|
|
|
|
Product recalls |
Number |
3.1.4 |
|
3 |
|
|
2 |
3 |
|
Total water consumption |
1,000 m3 |
2.4.1 |
|
630 |
|
- |
- |
| Failed inspections |
Number |
3.1.4 |
|
0 |
|
0 |
0 |
|
 Pollution |
|
|
|
|
|
|
|
 Climate change |
|
|
|
|
|
|
|
|
Total amount of substances of very high concern that leave facilities |
Tonnes |
2.3.1 |
|
1 |
|
- |
- |
Scope 1 GHG emissions |
1,000 tonnes CO2e |
2.1.1 |
|
85 |
|
78 |
76 |
|
Total amount of substances of concern that leave facilities |
Tonnes |
2.3.1 |
|
10 |
|
- |
- |
Scope 2 GHG emissions (market-based) |
1,000 tonnes CO2e |
2.1.1 |
|
16 |
|
15 |
16 |
|
|
Scope 3 GHG emissions3 |
1,000 tonnes CO2e |
2.1.1 |
|
2,160 |
|
1,743 |
- |
|
 Resource use and circular economy |
|
|
|
|
|
|
|
|
Plastic footprint (absolute) |
Tonnes |
2.2.2 |
|
15,654 |
|
- |
- |
|
Plastic footprint per patient |
kg/patient |
2.2.2 |
|
0.35 |
|
- |
- |
|
 Own workforce |
|
|
|
|
|
|
|
|
Employees (headcount)4 |
Number |
3.2.3 |
|
74,156 |
|
64,319 |
55,185 |
|
Gender in senior leadership positions |
% men:women |
3.2.7 |
|
58:42 |
|
59:41 |
61:39 |
|
Rate of recordable work-related accidents for own workforce5 |
Accidents per million hours worked |
3.2.6 |
|
1.2 |
|
1.3 |
1.3 |
|
Employees reporting symptoms of stress |
% |
3.2.6 |
|
13.8 |
|
13.8 |
13.8 |
|
Employees reporting symptoms of work-related physical pain |
% |
3.2.6 |
|
6.8 |
|
7.1 |
7.8 |
|
|
2. 2023 figure has been restated. For more information, please see section 3.1 'Patient protection and quality of life' on page 75.
3. 2023 figure has been restated. For more information, please see section 2.1 'Climate change' on page 58.
4. Total headcount of 77,349 cf. note 2.4 in the Consolidated financial statements. The variance of 3,193 employees is due to Catalent employees not being included.
5. 2023 and 2022 figures have been restated. For more information, please see section 3.2 'Own workforce' on page 84.
6. 2023 and 2022 figures have been restated. For more information, please see section 4.1 'Business conduct' on page 92.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / General information / 1.2 Basis for preparation of the Sustainability statement |
|
1.2 Basis for preparation of the
Sustainability statement
General reporting standards and principles
Our Sustainability statement has been prepared in accordance with the ESRS
as required by the Danish Financial Statement Act. Information derived from other EU legislations is listed in section 5, table 1.
Certain disclosures have been prepared taking other sustainability reporting standards and guidelines into account, such as the Greenhouse Gas (GHG) Protocol, Science Based Targets initiative (SBTi), Science Based Targets
Network (SBTN), Taskforce on Climate-related Financial Disclosures (TCFD),
the Global Reporting Initiative Standards and the Danish Financial Statements Act’s sections 99d and 107d (see pages 16 and 85-87). The International Sustainability Standards Board (ISSB) recently issued IFRS S1 and IFRS S2, making them effective, but voluntary, for annual reporting beginning on
or after 1 January 2024. While we are not required to follow these standards
and have not adopted the rules, we took them into account during the preparation of the Sustainability statement.
The time horizons considered for the preparation of the Sustainability statement are in line with those advised by the CSRD, and specifically up to one year as short-term, from one to five years as medium-term and more than five years
for long-term.
We have not opted to omit information corresponding to intellectual property, know-how, results of innovation, impending developments or matters in the course of negotiation, but in this first year of preparation of the Sustainability statement we opted to use the phase-in provisions listed in ESRS 1 Appendix C applicable to Novo Nordisk. Similarly, all voluntary disclosures that we consider required for a fair representation have been included. Table 2 in section 5 includes an index of all the ESRS requirements we comply with.
Scope of consolidation
The organisational boundaries applied to Novo Nordisk’s consolidated Environmental, Social, and Governance (ESG) reporting align with those of
the Consolidated financial statements. For disclosures on GHG emissions and pollution, we considered operational control when determining the consolidation scope. For policies, actions and targets related to 'Own workforce', NNE is excluded, as the subsidiary has its own processes in place.
On 18 December 2024, Novo Nordisk acquired three fill-finish sites located in
the US, Belgium and Italy from Novo Holdings, upon completion of its acquisition
of Catalent Inc., a global contract development and manufacturing organisation.
The impact of the acquisition has been deemed immaterial for sustainability reporting and is not included in our Sustainability statement, including the additional headcount, explaining the difference with Annual review and Consolidated financial statements.
The Sustainability statement addresses the material impacts, risks and opportunities (IROs) of both our own operations and our upstream and downstream value chain. For a visualisation of our value chain, please refer
to page 9. The extent to which our policies, actions and targets include our
value chain depends on our double materiality assessment. We have applied transitional provisions relating to some value chain information; please refer
to the topical sections for additional information.
Sources of estimation and outcome uncertainty
The use of estimates for performance metrics, including when upstream
and downstream value chain data is included, is described in the individual accounting policies. Overall, metrics related to our own operations have a higher amount of primary data, while value chain metrics are often estimated and therefore have a higher level of measurement uncertainty. All assumptions and potential uncertainties are documented in the accounting policies. Forward-looking information, such as targets, are uncertain in nature and we refer to the section 'Forward-looking statements' on page 35 for further details.
Changes in preparation, presentation or due to specific circumstances
Restatements of historical data due to reporting errors in previous periods,
and/or changes to accounting policies, are only performed if the materiality threshold defined in our restatement guidelines is exceeded. Management provides the specific disclosures required by CSRD unless the information
is not applicable or is considered immaterial to the decision-making of the primary users of the Sustainability statement. Restatements are primarily due
to improvements in calculation methodology or new scientific evidence as we continuously work to improve the accuracy of our sustainability reporting. In 2024, the organisational scope of some metrics was expanded to include all entities, but this has not resulted in any restatements.
In 2024, the following metrics have been restated due to improved calculation methodology: the comparative figures for total scope 3 GHG emissions and its
categories 1 (Purchased goods and services), 2 (Capital goods), 4 (Upstream transportation and distribution) and 6 (Business travel), vulnerable patients
reached with Diabetes care products, rate of recordable work-related accidents
for own workforce, and number of substantiated cases reported within accounting issues, fraud and business ethics matters via the Compliance Hotline. For more information, see sections 2.1 'Climate change', 3.1 'Patient protection and quality
of life', 3.2 'Own workforce' and 4.1 'Business conduct'.
Comparative figures
Comparative figures are provided for metrics that have been disclosed in one or more prior periods, where their definition and scope were aligned with the ESRS requirements or required only minor adjustments. In accordance with the ESRS transitional provision, no comparative figures are disclosed for new metrics introduced in 2024.
Incorporation by reference
An overview of all incorporation by references used within the Sustainability statement is listed in section 5, table 3.
Statement on sustainability due diligence
Novo Nordisk performs due diligence activities relating to people and the environment. The table below outlines the specific processes and their location in the Sustainability statement.
|
|
|
|
|
|
|
|
|
|
Core elements of environmental
and social due diligence
|
Pages |
| a) |
Embedding due diligence in governance, strategy and business model |
50-55, 60, 64, 65, 67,
68, 71, 80, 88, 90
|
| b) |
Engaging with affected stakeholders in
all key steps of the due diligence
|
50-52, 60, 66, 68, 72, 80,
86, 88, 90, 91, 94, 95
|
| c) |
Identifying and assessing adverse impacts |
52-55, 60, 64, 65, 67, 71, 80, 88, 90 |
| d) |
Taking actions to address those
adverse impacts
|
55, 56, 60-61, 64, 66, 68, 72-74, 76-79, 81-82, 84, 86, 89, 90-92, 94 |
| e) |
Tracking effectiveness of these efforts
and communicating
|
55-59, 62-68, 74,
75, 82-87, 91-93
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / General information / 1.3 Sustainability governance |
|
1.3 Sustainability governance
The role of the Board of Directors and Executive Management
Sustainability matters are addressed in relevant governance bodies across
the organisation to ensure integration into Novo Nordisk's strategy and
core business.
Our sustainability governance is anchored with the Board of Directors, which
has the overall responsibility of providing oversight and advice on sustainability matters, including the strategic direction and ambition level as set by Executive Management. In addition, the Audit Committee assists the Board with oversight of financial and sustainability reporting while the Remuneration Committee
and the People and Governance Committee assist the Board in ensuring integration of sustainability into our executive remuneration and board competency assessment, respectively. The oversight and relevant reporting lines are outlined in the visualisation on the right, including approval of our sustainability reporting of material impacts, risks and opportunities, target performance and variable remuneration components. The mandates outlined are addressed in relevant governance documents pertaining to each of the governance bodies.
Strategy and targets are set by Executive Management, who is responsible for material sustainability matters in the day-to-day management of Novo Nordisk. In March 2024, the Board of Directors and Executive Management attended an educational session on sustainability trends and key topics, which included a discussion of the double materiality assessment and a deep-dive on nature
and biodiversity, to increase their expertise with regards to specific impacts, risks and opportunities.
Operational decisions are delegated to management level, with the Sustainable Business Execution Steering Group – comprising senior representatives from
across the business – providing guidance on implementation of our environmental, social and governance-related disclosure initiatives. In addition to the dedicated sustainability steering groups, sustainability is also integrated into existing governance structures, such as our product governance, where we aim to
integrate social and environmental considerations across product lifecycles.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / General information / 1.4 Interests and views of stakeholders |
|
We actively seek external input through our Sustainability Advisory Council,
an independent body offering perspectives that challenge us to improve
our sustainability efforts continuously. See more about the council and its members at: www.novonordisk.com/sustainable-business/esg-portal.html.
Reward structures at Novo Nordisk are designed to support our strategy and motivate our executives to deliver sustainable growth and successful outcomes
in relation to our strategic aspirations. Both the short- and long-term incentive programmes include sustainability-related metrics, such as our target on GHG emissions, aligning with Novo Nordisk's sustainability-related objectives.
For further details on sustainability governance and remuneration of the
Board of Directors and Executive Management, we refer to the incorporation
by reference table 3 in section 5, on page 96.
Risk management and internal controls over sustainability reporting
At Novo Nordisk, risks and controls over sustainability reporting are assessed annually. We assess risks associated with incomplete or inconsistent sustainability reporting, including risks related to the accuracy of data and manual errors when consolidating data from different systems. We use a centralised, online repository to document our financial and sustainability-related risks and controls and focus on the highest risks. With regards to the double materiality assessment, we performed controls on the process for identifying material impacts, risks and opportunities and underlying documentation.
Novo Nordisk’s lines of business and responsible data owners assess the
risks associated with sustainability data and implement appropriate controls.
A headquarter function maintains an overall risk assessment of sustainability reporting and determines the level of internal controls required for each process, depending on the materiality of the risks. Group Internal Audit conducts independent audits to assess the design and operating effectiveness
of the risk and control processes.
Executive Management is responsible for the overall internal control framework. The Disclosure Committee – a management committee established by Executive Management – reviews changes to sustainability reporting in the Company Announcement on a quarterly basis. The Audit Committee oversees financial and sustainability reporting and is informed about actions and progress on essential sustainability metrics and targets on a quarterly basis.
1.4 Interests and views of stakeholders
Novo Nordisk strives to understand and reflect the interests and views of patients (gathered via engagement with patient organisations), employees,
and other key stakeholders across the value chain, through our standard due diligence processes, as we deliver on our sustainability priorities and our double materiality assessment. We ensure that the interests and views are taken into account in the strategic direction of Novo Nordisk by informing Executive
|
|
|
|
|
|
|
|
|
Stakeholder group |
Purpose and engagement channels |
Examples of how outcomes are taken into account |
| Patient organisations, healthcare professionals and healthcare organisations |
We ensure a patient-centred business approach to improve prevention, detection, treatment and access to quality care for people living with serious chronic diseases, through research collaboration and trials, conferences, and scientific and medical communications.
|
•Development of new treatments and product improvements.
•Efforts to strengthen the resilience of healthcare systems, for example through prevention efforts.
|
| Employees |
We strive to improve health, safety and wellbeing of our employees. Talent attraction and retention, driving innovation and providing employees with equitable opportunities to realise their potential, are essential to our strategy. We enable this via our annual employee survey, Evolve, individual career development and training, workers’ councils, ongoing social dialogue, and investing in onboarding of new employees.
|
•Novo Nordisk Way facilitations to live up to our cultural commitments.
•Fostering a culture of safety with attention to increase employee health and total wellbeing.
•Improving employee benefits, for example parental leave.
|
| Suppliers and third-party representatives |
Our Responsible Sourcing Programme, human rights due diligence and established contracting and engagement processes drive our supplier and third-party engagement. We leverage this when purchasing goods and services to manufacture or distribute pharmaceutical products and partnering with third-party representatives, for example on filling or assembling final products or performing clinical trials.
|
•Renewable electricity commitments in our supply chain.
•Informed supplier and third-party representative selection.
|
| Public officials and regulators |
To advocate for improvement of public health and meet current and future regulatory requirements we organise and sponsor events, engage with industry associations, drive bilateral dialogues with local, national and international agencies and authorities. |
•Enable new innovation.
•Improve existing healthcare to the benefit of patients.
|
| Partners and peers |
We seek perspectives from partners and peers to advance on many of our commitments, especially when it comes to our access and prevention efforts. Example of such collaborations include our Sustainability Advisory Council, Cities for Better Health, and other industry partnerships. |
•Advance our environmental commitments, for example through Sustainable Marine Fuels (SMF) and lower carbon plastics.
•Prevent childhood overweight and obesity through UNICEF partnership and the Childhood Obesity Prevention Initiative with partners such as city governments and academic institutions.
•Provide input to sustainability strategies and targets.
|
| Investors |
We strive to provide timely, accurate and transparent information to our investors through engagements such as Capital Markets Day, the annual general meeting, ESG raters and rankers, and recurring engagement in response to investor queries.
|
•Strengthened sustainability performance, reporting and communication efforts. |
Management and the Board of Directors on an ongoing basis as per our sustainability governance.
We continuously measure the extent to which we live up to societies’ expectations through our company reputation score. Novo Nordisk has maintained its 'Excellent' reputation in 2024, achieving a global reputation
score of 81.6 (on a 100-scale) across 20 tracked markets and five stakeholder groups, in line with the performance of 2023.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / General information / 1.5 Double materiality assessment |
|
1.5 Double materiality assessment
Processes to identify and assess material impacts, risks and opportunities
In 2024, we completed a double materiality assessment in accordance with
the requirements of ESRS 1 to determine material sustainability topics for
the entire Novo Nordisk Group. The assessment considered both the impacts
of our business on society and the environment (impact materiality) and
how sustainability topics affect the Group in the form of business risks
and opportunities (financial materiality). In addition, we considered the implementation guidance provided by EFRAG1 (EFRAG IG 1), including how
to set qualitative and quantitative thresholds, and previous materiality
analyses. Results will be reviewed annually.
The double materiality assessment was initiated combining the list of sub-sub-topics as per ESRS 1 with additional entity-specific sustainability matters, in consideration of Novo Nordisk’s industry, using screening tools and existing voluntary standards. Furthermore, an outside-in perspective was considered,
by consulting our stakeholders and actors in the value chain. All identified
topics have followed a four-phase process: 1) input from internal subject
matter experts; 2) engagement with external stakeholders; 3) calibration by internal leaders including discussion by senior management; and 4) Audit Committee review and approval.
Subject matter experts in our workforce provided initial input by scoring relevant topics related to their areas of expertise, through a survey covering
the full scope of the Novo Nordisk Group. The scores were accompanied by qualitative rationales that included considerations of specific geographies, processes, due diligence findings and actors across the value chain to identify material IROs.
By using an average scoring approach, impacts were assessed based on their scale, scope, irremediability (in the case of negative impacts) and likelihood;
and risks and opportunities on their magnitude, likelihood and type of financial effect. Likelihood was assessed only for potential IROs.
The methodology to determine impact materiality was largely aligned with EFRAG's implementation guideline, assessing scale, scope and irremediability against qualitative criteria. For scale, we assessed the size of the impact to the environment and people; for scope its reach or geographical span and, where
1. European Financial Reporting Advisory Group, a private association providing technical advice
to the European Commission on both financial and sustainability reporting standards.
applicable, irremediability scores considered how difficult it would be to
remedy our negative impacts.
The methodology to determine financial materiality was largely aligned
with Novo Nordisk’s enterprise risk management framework, including the quantitative and qualitative scales and the different types of the financial
effects: classified as monetary, reputational, ethical or quality-related. The main differences to the enterprise risk management framework include longer time horizons and the risks being assessed before mitigating actions in accordance with ESRS 1. We will continue to assess how sustainability is considered in our overall risk profile, to strengthen our integrated risk management process.
External stakeholder groups were involved through surveys of: a selection of international patient organisations (serving as proxy for our patients’ interests across different therapy areas), a selection of our top 20 investors by ownership size; and a selection of Tier 1 suppliers across different sourcing categories. Affected communities were not directly consulted. Other consultations are specified in relevant sections of the Sustainability statement. Additionally,
we used a data analysis tool to reflect the perspectives of wider society, using sources such as peers’ public reports, regulatory guidance and news.
The preliminary results were aggregated, discussed and calibrated at workshops with sustainability leaders from various functions in the Group
and by senior management. This was to include a top-down perspective,
prevent subjective bias and ensure consistency across the sustainability topics.
IRO conclusions were determined against our pre-defined thresholds. For impact materiality, all sustainability topics classified as critical, significant and important and considered current or likely/very likely to occur, were deemed material (see double materiality assessment illustration on the next page). For risks and opportunities, a sustainability topic was considered financially material with scores categorised as critical and significant if either current or likely/very likely to occur. The thresholds for impact materiality were set lower than those for financial materiality, reflecting our commitment to transparency of our impacts on and contributions to society and the planet. The results of the
double materiality assessment and material IROs were reviewed and approved
by the Audit Committee.
We have mapped the material IROs to the applicable ESRS data points and further assessed their materiality, to determine whether they were relevant
for our business model and/or for the decision-making needs of the users
of the Sustainability statement. This analysis determined the material sustainability information disclosed in this Sustainability statement.
Interaction with strategy and business model
The results of our double materiality assessment reflect Novo Nordisk's sustainability strategy and business model. Our positive, social impacts associated with providing access to life-saving medicines without compromising safety or quality, help to improve quality of life and healthcare systems for people around
the world. For an overview of our products, see section 'Innovation and therapeutic focus' on pages 17-25. To remain a relevant and attractive workplace, we depend on people's wellbeing, within and outside our operations, to contribute to continued innovation to society. The results also underscore our negative environmental impact when manufacturing medicine, including associated carbon emissions and plastic footprint. Coupled with our dependence on nature-based resources, our environmental strategy strives to limit any such negative impacts. We acknowledge the impacts of our business conduct, and of upholding the highest ethical standards in order to continue to be respected for our societal contributions..
To assess the resilience of Novo Nordisk's strategy and business model, sustainability is included in our annual strategy review. Executive Management
and the Board of Directors meet annually to discuss strategic risks and opportunities within and beyond the next five years to ensure that we continue
to meet society’s needs. Discussions include sustainability impacts such as how we reach more vulnerable patients while striving to minimise environmental impacts.
Resilience discussions are based on input and recommendations from across the organisation with a focus on the topics that are most likely to impact our long-term strategy and forward-looking trend analyses, resulting in adjustments to the strategy if necessary. Sustainability is therefore integrated in relevant functional strategies and sustainability strategies are discussed on an ongoing basis with Executive Management. Descriptions of topic-specific resilience analysis are included where relevant in the topical sections. For more on our strategy that relates to sustainability matters, see section 'Purpose and sustainability' on pages 12-16.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / General information / 1.5 Double materiality assessment |
|
Outcomes of the double materiality assessment
The illustration to the right provides an overview of the material IROs associated with each material sustainability topic and where the IROs are in our business model across time horizons.
Climate change, resource use and circular economy, patient protection and quality of life, and own workforce are all essential sustainability topics to Novo Nordisk, reflecting our focus of managing the associated impacts, risks and opportunities through our strategic priorities. Furthermore, water, pollution, biodiversity, workers in the value chain and business conduct are all important sustainability topics where Novo Nordisk has impacts, but is not materially affected by related risks or opportunities. A detailed description of the material IROs is given in the topical sections of this Sustainability statement.
We focus on setting strategic targets for our essential topics as these are considered our top sustainability priorities. For other important sustainability topics we measure progress against relevant metrics or project KPIs as specified for relevant actions in each topical section.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DMA topics |
Category |
Resources |
|
R&D |
|
Manufacturing |
|
Distribution |
|
Patients |
Time horizon |
Page |
|
Climate change |
|
CO2e emissions across our operations and value chain contribute to climate change |
|
54 |
|
|
|
Potential reputational risks associated with rising CO2e emissions |
|
54 |
|
|
|
Potential weather-related hazards impacting safety at our sites and in our value chain |
|
54 |
|
Resource use and circular economy |
|
Resource use and waste associated with manufacturing and products |
|
60 |
|
|
|
Potential reputational risks associated with resource consumption |
|
60 |
|
Pollution |
|
Chemicals affecting human health or ecosystems |
|
64 |
|
Water |
|
Availability and deterioration of water resources |
|
|
|
65 |
|
Biodiversity and ecosystems |
|
Reliance on natural resources and ecosystem services |
|
|
|
67 |
|
|
|
Reliance on vulnerable species in research |
|
|
|
|
|
|
|
67 |
|
Patient protection and quality of life |
|
|
|
|
|
|
|
Improving quality of life through medicine |
|
71 |
|
Innovation2 |
|
|
|
Potential new discoveries to serve patient needs |
|
71 |
|
Prevention2 |
|
|
|
|
|
|
|
Reducing and preventing serious chronic diseases |
|
71 |
|
|
|
|
|
Health equity in clinical trials |
|
|
|
Health equity for vulnerable patients |
|
71 |
|
|
|
|
|
Safe clinical trials |
|
Product quality and safety |
|
71 |
|
Falsified medicines2 |
|
|
|
|
|
|
|
Protection against falsified medicines |
|
71 |
|
|
|
|
|
Protecting clinical
trial information
|
|
|
|
Protecting patient information |
|
71 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Potential reputational and regulatory risks |
|
|
|
|
|
71 |
|
Own workforce |
|
|
|
Employee benefits and flexible working conditions |
|
|
|
|
|
80 |
|
|
|
|
|
Potential human rights incidents |
|
|
|
|
|
80 |
|
|
|
|
|
Healthy and safe work environment |
|
|
|
|
|
80 |
|
|
|
|
|
Equal opportunities fostering innovation |
|
|
|
|
|
80 |
|
|
|
|
|
Attracting talent to enable continued innovation |
|
|
|
|
|
80 |
|
Workers in the value chain |
|
Protecting working conditions and human rights |
|
|
|
|
|
Protecting working conditions and human rights |
|
88 |
|
|
|
|
|
|
|
|
|
|
|
Business conduct |
|
|
|
Ethical working culture through Novo Nordisk Way |
|
|
|
|
|
90 |
|
|
|
Interacting with all stakeholders in accordance with our business ethics standards |
|
90 |
|
|
|
|
|
|
|
|
|
Promoting public health |
|
90 |
|
Bioethics2 |
|
|
|
Upholding high bioethical standards |
|
|
|
|
|
90 |
|
|
|
Reliance on animals in research |
|
|
|
|
|
|
|
90 |
 Positive impact  Negative impact  Opportunity  Risk |
|
|
|
|
|
|
2. Entity-specific topics.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.1 Climate change |
|
2. Environment
2.1 Climate change
As a global company with sourcing, manufacturing, and distribution to reach patients across the world, Novo Nordisk has impacts on climate change and the environment. We address these impacts through the execution of our Circular for Zero strategy, including decarbonising our own operations and working
with our suppliers to reduce GHG emissions across our value chain.
Material impacts, risks and opportunities (IROs)
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
|
CO2e emissions across our operations and value
chain contribute to climate change
|
|
•Upstream
•Own operations
•Downstream
|
Potential reputational risks associated with rising CO2e emissions |
|
•Upstream
•Own operations
•Downstream
|
The majority (96%) of Novo Nordisk’s GHG emissions originate in our upstream
and downstream value chain, from sourcing and services to distributing
our products (scope 3). GHG emissions from our own operations (scopes 1 and 2) have a relatively lower impact (4%). Our emissions have a negative impact on global warming, while material transition risks of our climate change mitigation efforts include potential reputational risks associated with an increase in GHG emissions.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Potential weather-related hazards impacting safety at our sites and in our value chain |
|
•Upstream
•Own operations
•Downstream
|
Weather-related hazards could potentially have negative impacts on the protection of employees or workers in the value chain, as well as on patients' health and wellbeing if access to treatments is disrupted. The worst impacts from climate-related weather hazards will likely materialise in the medium- and

Positive impact

Negative impact

Opportunity

Risk
long-term and are currently not considered financially material to Novo Nordisk. However, we continuously assess physical climate risks and the extent to which they could impact our operations and value chain, as described in this section.
Based on our climate adaptation efforts and decarbonisation initiatives throughout our value chain, we assess that our strategy is resilient in relation to climate change. This assessment was made based on the processes described below, including a high-level climate scenario analysis conducted in 2024.
Processes to identify impacts, risks and opportunities
To identify our climate impacts, we track our GHG emissions by monitoring our direct emissions and energy consumption across sites, as well as emissions in our upstream and downstream value chain through supplier data, activity data and financial data. We screen our activities to assess actual and potential climate impacts in line with our corporate strategy and decarbonisation roadmap.
Climate-related risks are identified and assessed as part of Novo Nordisk’s
risk management process and systems (see section ‘Risk management’ on page 39 with regards to the main strategic risk 'environmental impact'). Short- and medium-term climate risks are assessed across business areas, while long-term risks are assessed as part of our company-wide strategic risk identification
process. When identifying material climate risks, the same time horizons have been applied as for the double materiality assessment, with climate-related
risks expected to materialise in the long-term (beyond 5 years). We use natural hazard screening for all relevant physical hazards at our production sites and in our supply chain, excluding warehouses, on an annual basis and as part of our sourcing due diligence process. Transition risks are assessed qualitatively in accordance with the double materiality assessment methodology.
In 2024, we conducted a forward-looking, high-level assessment of physical
and transition climate-related risk, considering a 4°C and a 1.5°C scenario to capture how ‘business as usual’ and rapid decarbonisation pathways would each impact our business. For physical risks, the analysis was performed under high emission Representative Concentration Pathway (RCP) 8.5 and a low emissions RCP 2.6. pathway across 2030- and 2050-time horizons, focusing on selected
raw materials and on acute and chronic hazards. This was done using an external meteorological database, as well as asset information and geospatial coordinates for production sites. Transition risks were modelled using an Integrated Assessment Model (IAM) scenario analysis tool to account for sector- and region-specific macroeconomic shifts and considering energy supply, raw material pricing, labour cost, and revenue changes. No aspects of our business were identified as incompatible with a transition to a climate-neutral economy.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.1 Climate change |
|
Mitigation, adaptation and energy
Policies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Policy |
Environmental policy |
|
Purpose |
Guides action across material environmental topics |
|
Scope |
All of Novo Nordisk's global activities |
|
Most senior level accountable |
Executive Management |
|
Availability |
Externally available:
Our environmental policy
|
|
Applicability across Sustainability statement |
•Climate change, page 54
•Resource use and circular economy, page 60
•Pollution, page 64
•Water, page 65
•Biodiversity and ecosystems, page 67
|
|
|
|
|
Novo Nordisk’s environmental policy states our commitment to ensuring treatment for people living with serious chronic diseases with the lowest
possible impact on the environment, by reducing our GHG emissions in line
with the Science Based Targets initiative (SBTi). As such, the policy sets out a
key objective of mitigating our climate impact through energy efficiency measures, deployment of renewable energy, and other decarbonisation levers
as detailed further in this section. The policy also states our commitment to protecting the business through evaluating climate-related risks and taking measures to adapt to climate change.
Implementation of the environmental policy is ensured by management teams across the business and by on-site environmental partners at all production facilities globally, who safeguard compliance and continuous improvements. Our production facilities are ISO 14001 certified for environmental management to ensure continuous improvements and mitigate, control and prevent negative environmental impacts.
Actions and transition plan for climate change mitigation
To mitigate our impacts on climate change, Novo Nordisk is committed to reaching net zero emissions across scope 1, scope 2 (market-based) and scope 3 GHG emissions by 2045 in alignment with the Corporate Net-Zero Standard from the SBTi. In addition, we have targets of zero scope 1 and scope 2 (market-based) CO2e emissions by 2030, and a new target of 33% absolute reduction of scope
3 CO2e emissions by 2033 compared to our base-year of 20241. The targets have been approved by our Board of Directors and Executive Management. Our new scope 3 target is not 1.5℃ aligned, but is consistent with SBTi’s well-below 2.0℃ pathway. We will start executing the transition plan in 2025 to achieve our scope 3 target by 2033. However, many of our scope 3 decarbonisation levers will not materialise until at least 2030. With the projected growth and the delayed effect of our decarbonisation levers, we anticipate an overall growth in GHG emissions until 2030 compared to the baseline.
We have made progress in reducing our environmental impact since the launch
of our Circular for Zero strategy in 2019, including converting to renewable electricity in our own operations and among suppliers, and curbing the climate impact from transportation. In 2024, we updated our scope 3 decarbonisation roadmap, expanding the scope of our target from operations and transportation to cover our operations and value chain globally. The roadmap is aligned with Novo Nordisk's overall business strategy, taking into account our projected growth. Our growth increases the challenges of decarbonising and thus underscores the importance of decoupling our ability to serve more patients
from our climate impact, to lower GHG emissions. The expected reductions2, including anticipated growth in emissions, are currently estimated at 2,200 thousand tonnes of CO2e across scope 3 by 2033. This includes initiatives
already identified, estimated at 1,500 thousand tonnes CO2e, and therefore a
gap towards our target of an estimated 700 thousand tonnes of CO2. We will address these remaining emissions as we progress with implementing the roadmap and assess the scalability and effectiveness of identified initiatives,
while investigating additional levers such as potential new technological advancements. Furthermore, we estimate 131 thousand tonnes of CO2e of expected reductions across scope 1 and 2 by 2030. The expected GHG emission reduction pathway is illustrated on page 57.
1. The target covers nearly 70% of our scope 3 emissions in accordance with SBTi provisions.
Read more on page 57.
2. Expected reductions is defined as the overall quantitative contribution to achieve the GHG emission reduction targets from all decarbonisation levers, including expected growth projections.
Emission reduction efforts in our own operations (scope 1 and 2) are focused
on switching to renewable energy sources and increased energy efficiency. Within our upstream and downstream value chain (scope 3), which generates 96% of our total GHG emissions, key levers include converting to lower carbon materials, requiring that our tier 1 suppliers convert to renewable energy, and lowering the emissions from our distribution, as detailed in the table on the
next page. Beyond 2033, Novo Nordisk expects to use carbon removals to neutralise residual CO2e emissions of up to 10% of the baseline emissions towards our net zero 2045 target, in line with SBTi requirements and guidance from the Intergovernmental Panel on Climate Change (IPCC). We are exploring opportunities for removing and storing GHG involving both nature- and technology-based solutions. Our potential locked-in GHG emissions are limited but relevant in relation to our partially fossil-fuel-powered production sites, which we are working to convert to electric and renewable energy.
We strive to expand our production capacity in a sustainable way, and in
2024 we aligned a proportion of the buildings in two of our capacity expansion projects to the EU Taxonomy, resulting in 6% aligned CapEx in 2024 (3% including Catalent), thus contributing to climate change mitigation3. See
section 2.6 'EU Taxonomy' on page 69 for more information on our approach and ambitions to align future capacity expansion projects where feasible.
We continuously invest in integrating our Circular for Zero priorities across our business as part of how we operate, from working with suppliers and designing
our manufacturing processes to reducing the end-of-life impact of our products. Further, we will commit additional resources to accelerate the implementation of our updated environmental roadmaps developed in 2024 across climate, plastic and nature. In addition, extended value is created through grants to health, sustainability and the life science ecosystem, via our unique ownership structure, by the Novo Nordisk Foundation, our majority shareholder through Novo Holdings A/S. We also expect to update our incentive programmes, described in our Remuneration report as referenced in Table 3 on page 96, to reflect our new decarbonisation roadmap.
3. Novo Nordisk is not excluded from the EU Paris-aligned Benchmarks.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.1 Climate change |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key action to address climate change |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we track effectiveness |
|
|
Energy efficiency
and optimisation
(scope 1 and 2)
|
Sites and processes are optimised through our energy savings programme, rethinking the design of our site infrastructure to ensure a resource-efficient energy supply, for example through the construction of a district cooling ring at site Kalundborg, Denmark, to be completed in 2026. Energy efficiency and optimisation across sites is an ongoing action until at least 2030. |
Own operations globally |
Yes |
•Progressed with the construction of district cooling ring at site Kalundborg, Denmark,
with expected energy savings of over 20,000 MWh/year after completion in 2026.
•In 2024, energy savings initiatives across sites resulted in total energy reduction of
13,740 MWh.
|
|
|
Switching to renewable electricity (scope 2) |
Reach 100% renewable electricity for Novo Nordisk’s own production sites and affiliates, continuously striving for best practice solutions. Action is ongoing until at least 2030. |
Own operations globally |
Yes |
•Maintained 100% of Novo Nordisk’s electricity consumption procured from renewable
energy sources at all production sites.
•Continued the transition towards renewable electricity at our affiliates,
reaching 99% coverage.
|
|
|
Reducing emissions
from heat and steam (scope 1 and 2)
|
To reach zero CO2e emissions from production, we are converting steam and heat in our production processes towards renewable energy sources by electrifying processes and covering natural gas consumption by biogas certificates. Action is ongoing until at least 2030. |
Own operations globally |
Yes |
•Progressed plans to electrify heat and steam production where possible and to cover the natural gas consumption of sites in the US with biogas (renewable natural gas) certificates.
•Energy consumption from renewable resources accounted for 54% of total energy consumption (excluding steam and heat derived from biomass).
|
|
|
Remaining scope 1 and
2 emissions reductions
|
Main reductions from remaining scope 1 and 2 GHG emissions will come from lowering emissions from refrigerants, back-up systems and transitioning fossil-based vehicles in own operations to battery electric or plug-in hybrid vehicles. Action is ongoing until at least 2030. |
Own operations globally |
Yes |
•Continued the transition away from fossil-based vehicles with market-specific guidance
based on local infrastructure conditions.
|
|
|
Reducing emissions from high impact sourcing categories (scope 3, category
1 and 2)
|
Reducing emissions from high impact sourcing categories through three focus areas:
1) converting to lower carbon raw materials and feedstocks for our device and drug manufacturing, as well as lower carbon construction materials; 2) process optimisations
to lower material use; 3) renewable energy for tier 1 suppliers. Action to be implemented
from 2025-2033.
|
Supply chain globally |
Yes |
•To date, more than 1,800 suppliers have committed to transitioning to renewable power.
•In 2024, initiatives were identified together with suppliers and relevant business units across Novo Nordisk, which are expected to account for the majority of scope 3 emission reductions.
•Effectiveness is assessed on a quarterly basis through tracking estimated GHG emissions.
|
|
|
Reducing emissions from product distribution (scope 3, category 4 and 9) |
Lower distribution emissions by air, sea, and road: 1) by air, converting additional upstream
air freight to sea freight, while securing Sustainable Aviation Fuel (SAF) via long-term off-take agreements; 2) by sea, securing Sustainable Marine Fuel (SMF) in upstream distribution; 3) by road, through low-carbon road freight solutions upstream and downstream. Action to be completed by 2033.
|
Upstream and downstream distribution globally |
Yes |
•Initiatives identified together with suppliers and relevant business units across Novo Nordisk.
•Effectiveness is assessed on a quarterly basis through tracking estimated GHG emissions.
|
|
|
Climate adaptation |
To address physical climate risks faced by some of our sites in the medium and long term, all medium- and high-risk sites are covered by mitigation plans, including procedures in cases of
a temporary production shutdown and safety rooms for employees, as well as leak detection,
drainage, and protection against storm surge. The natural hazard exposure of our 400 most
critical suppliers is reviewed as part of our comprehensive supply chain risk assessment.
|
Own production sites and suppliers |
No |
•Conducted an updated assessment based on new tools and data, including several new suppliers and acquisition sites, in addition to own sites.
•Effectiveness of actions are tracked through an annual NatCat report, distributed to own sites to ensure cross-site learning and best practice.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.1 Climate change |
|
Performance
GHG emissions targets
We set GHG emission reduction targets across scope 1 and 2 (market-based)
and scope 3. The base year is 2024, which is considered representative of
Novo Nordisk's business activities. Our scope 3 target covers nearly 70% of our scope 3 emissions, in accordance with the SBTi’s provision of postponing up to one third of base year GHG emissions associated with the highest estimation uncertainty and lowest abatement potential. Hence, categories 3, 5, 7, 12, and
a part of category 1 and 2 are not included in the scope of the 2033 target5.
The target is derived using a sectoral decarbonisation pathway and has been submitted for validation by SBTi. Our 2045 net zero target covers all emissions across scope 1, 2 and 3.
5. Categories 1 - Purchased goods and services, 2 - Capital goods, 3 - Fuel- and energy-related activities, 5 - Waste generated in operations, 7 - Employee commuting, 12 End-of-life treatment of sold products.
In setting the scope 3 target, we estimated projected emissions based on the most ambitious growth forecasts aligned with expected production volumes and expansion projects between 2024 and 2033. To identify and assess the feasibility of decarbonisation levers, we engaged with selected suppliers and with business units across Novo Nordisk. Achieving our targets depends in
part on the availability of lower carbon technologies and materials that meet
our quality standards as part of the general transition to a lower carbon economy. We will monitor the effectiveness of our targets starting in 2025.
Progress on GHG emissions
In 2024, during the process of developing a new scope 3 target, we have updated our methodology to provide a more reliable data foundation for
our target baseline. The current methodology has been reviewed and
adjusted across the most material scope 3 categories as follows:
•Purchased goods and services (category 1) updates include a combination
of transferring contract manufacturing categories from spend- to activity-based accounting, adjustment of emission factors and volume conversions.
•Capital goods (category 2) updates include adjusting existing spend-based factors, using supplier-specific information when available, and developing new spend-based factors from bills of material.
•Emission factors of Upstream transportation and distribution (category 4),
and Business travel (category 6) have been updated.
The review indicated that categories 1 and 2 were previously overstated due
to the inherent uncertainty of spend-based emission factors. We have therefore restated scope 3 GHG emissions for 2023. Our efforts to enhance the accuracy of our scope 3 inventory will continue with a further focus on the less material categories. The inherent uncertainties in scope 3 calculation methodologies, together with ongoing scientific advancements, mean that the risk of future restatements will continue to be present for this metric. In 2024, approximately 12% of scope 3 emissions were calculated using primary data.
In 2024, Novo Nordisk’s gross scope 1 and 2 (market-based) emissions grew
by 9% and 7% respectively due to the increase in natural gas consumption in
our US production and at our newly acquired site in Ireland, Athlone; and the increased consumption of fossil-based steam in China. Scope 3 emissions increased by 24% due to substantial investments in production capacity and increase in supply chain activities to serve more patients. Combined categories
1 (Purchased goods and services) and 2 (Capital goods) account for almost 80%
of total scope 3 emissions. These categories are part of our efforts to increase supply to reach more patients and responsible for the majority of the increase
in scope 3 emissions in 2024. Our efforts to decarbonise categories 4 (Upstream distribution) and 6 (Business travel) have been challenged by the growth of
the company. The procurement of sustainable aviation fuel (SAF) and sustainable marine fuels (SMF) have helped to curb the growth.
In 2024, we disclosed for the first time our biogenic emissions, which amounted to 110,000 tonnes CO2e. Biogenic emissions, sometimes referred to as out-of-scope emissions, originate primarily from the procurement of biogas and bio-based steam, and the combustion of bio-based fuels (wood).
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.1 Climate change |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.1.1 Scope 1, 2 and 3 GHG emissions6 |
Unit |
2024 |
2023 |
2022 |
% change |
| Scope 1 GHG emissions |
1,000 tCO2e |
85 |
78 |
76 |
9% |
Percentage of scope 1 GHG emissions from regulated emission trading schemes |
% |
0.3 |
– |
– |
– |
Biogenic emissions (Out-of-scope emissions) scope 1 |
1,000 tCO2 |
37 |
– |
– |
– |
Scope 2 GHG emissions – location-based |
1,000 tCO2e |
174 |
– |
– |
– |
Scope 2 GHG emissions – market-based |
1,000 tCO2e |
16 |
15 |
16 |
7% |
Biogenic emissions (Out-of-scope emissions) scope 2 |
1,000 tCO2 |
73 |
– |
– |
– |
Scope 1 and 2 (market-based) GHG emissions |
1,000 tCO2e |
101 |
93 |
92 |
9% |
| Scope 3 GHG emissions |
1,000 tCO2e |
2,160 |
|
1,743 |
|
– |
24% |
• Category 1: Purchased goods and services7 |
1,000 tCO2e |
1,215 |
|
1,018 |
|
– |
19% |
• Category 2: Capital goods7 |
1,000 tCO2e |
465 |
|
303 |
– |
53% |
• Category 3: Fuel- and energy-related activities |
1,000 tCO2e |
74 |
56 |
– |
32% |
• Category 4: Upstream transportation and distribution7 |
1,000 tCO2e |
101 |
108 |
– |
(6%) |
• Category 5: Waste generated in operations |
1,000 tCO2e |
6 |
6 |
– |
0% |
• Category 6: Business travel7 |
1,000 tCO2e |
188 |
154 |
– |
22% |
• Category 7: Employee commuting |
1,000 tCO2e |
52 |
43 |
– |
21% |
• Category 9: Downstream transportation and distribution |
1,000 tCO2e |
57 |
52 |
– |
10% |
• Category 12: End-of-life treatment of sold products |
1,000 tCO2e |
2 |
3 |
– |
(33%) |
Percentage of scope 3 GHG emissions calculated using primary data |
% |
12.3 |
– |
– |
- |
Total GHG emissions – location-based |
1,000 tCO2e |
2,419 |
|
– |
– |
- |
GHG emission intensity, location-based (total GHG emissions per net revenue8) |
tCO2e/mDKK |
8.3 |
– |
– |
- |
Total GHG emissions – market-based |
1,000 tCO2e |
2,261 |
|
1,836 |
|
– |
23% |
GHG emission intensity, market-based (total GHG emissions per net revenue8) |
tCO2e/mDKK |
7.8 |
– |
– |
- |
6. Operational control = financial control approach. 7. 2023 figures have been restated for categories 1, 2, 4 and 6 from 2,067; 1,315; 113 and 83 thousand tonnes CO2e, respectively, as disclosed in the Annual Report 2023. 8. Please see note 2.1 'Net sales and rebates' on page 107 in the Consolidated financial statements. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.1.2 Scope 1, 2 and 3 GHG emissions targets |
Unit |
20249 |
2030 |
2033 |
2045 |
Average annual reduction |
Target type |
Target |
| Scope 1 and 2 (market-based) GHG emissions |
1,000 tCO2e |
101 |
0 |
0 |
0 |
17% |
Absolute |
(100%) |
Scope 3 GHG emissions10 |
1,000 tCO2e |
1,493 |
|
– |
1,000 |
|
011 |
4% |
Absolute |
(33%) |
9. Base year. 10. The target covers nearly 70% of our scope 3 emissions in accordance with SBTi provisions. Read more on page 57. 11. Novo Nordisk estimates residual emissions after the decarbonisation levers to be 150 thousand tonnes CO2e. |
Progress on energy consumption
In 2024, energy consumption from own operations increased by 33%.
More than half of this increase is due to the inclusion of car fuel in the
metric, that was previously not included. The remaining increase is due to higher consumption of steam, electricity and natural gas. In line with our
major decarbonisation levers, we continued working with energy-saving
and optimisation projects during 2024, as detailed in the table of key actions
on page 56.
Total energy consumption from contractual renewable sources – primarily renewable electricity and biogas – accounted for 54% of total energy consumption. The remaining 46% of energy consumption originated from
fossil and other sources, of which fossil sources accounted for approximately 474 thousand MWh and non-contractual biomass for further 173 thousand MWh. We adopt a conservative approach when differentiating between renewable and non-renewable sources for electricity, steam, and heat. Only energy specified as renewable in the supplier's contractual agreement is classified as renewable energy. Due to this conservative approach, steam
and heat derived from biomass are not included under renewable sources.
In 2024, almost all of Novo Nordisk’s electricity consumption was procured from renewable electricity. Since 2020, Novo Nordisk has transitioned to sourcing renewable electricity for all production sites with a range of solutions, primarily Power Purchase Agreements (PPA), Renewable Electricity Certificates (REC), and Guarantees of Origin (GO). All our production sites now source renewable electricity, as do the majority of our offices and laboratories.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.1.3 Energy consumption and mix |
Unit |
2024 |
2023 |
2022 |
Total energy consumption related to own operations12 |
MWh |
1,400,228 |
|
1,051,111 |
1,021,389 |
Percentage of fossil sources in total energy consumption |
% |
46% |
– |
– |
Percentage of renewable sources in total energy consumption |
% |
54% |
– |
– |
Energy intensity (total energy consumption per net revenue13) |
MWh/mDKK |
4.82 |
– |
– |
|
12. Equals to total energy consumption from activities in high climate impact sectors.
13. Please see note 2.1 'Net sales and rebates' on page 107 in the Consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.1 Climate change |
|
ACCOUNTING POLICIES
Scope 1 GHG emissions
The reporting of scope 1 and 2 CO2e emissions follows the ESRS and GHG Protocol Guidance. Includes CO2e emissions from fuels, as well as fugitive emissions of purchased refrigerants. Production sites report all leaks of more than 1 kg refrigerant from cooling systems with a filling of more than 1 kg. Emission factors for the respective energy types are the most recent available from third parties, such as the US Environmental Protection Agency and the UK Government GHG Conversion Factors for Company Reporting. GHG removals, carbon credits and avoided emissions are not included. N2O and CH4 emissions from the consumption of biofuels are included in scope 1 and 2, while bio-based CO₂ emission are assumed to be zero and are not included but disclosed separately under biogenic emissions. Biogenic emissions refer to out of scope emissions of CO2 from the combustion of biomass- based primary fuels (scope 1) and biomass-derived electricity, steam and district heating (scope 2). Biogenic
emissions from our fermentation process are not included due to high calculation uncertainty but we will investigate potential estimation methodology going forward.
Percentage of scope 1 GHG emissions from regulated emission trading schemes
The share of emissions controlled and managed within the framework of the
EU ETS scheme of the total scope 1. Other national and non-EU ETS are not assessed applicable to Novo Nordisk.
Scope 2 emissions
Indirect GHG emissions from electricity, heat and steam, purchased and consumed by Novo Nordisk. Location-based emissions are based on national
grid average emission factors for defined locations. Market-based scope 2 emissions refer to indirect GHG emissions associated with purchased electricity, heat and steam through procurement of contractual instruments such as Energy Attribute Certificates, Power Purchase Agreements and Guarantees of Origin from sources such as wind, hydro, solar and biomass. For sites without such contractual agreements and for other scope 2 energy types in the absence of supplier specific emission factors and / or residual mix emission factors, the national average emission factor has been applied.
Scope 3 emissions
Indirect GHG emissions that originate from our value chain. Novo Nordisk
has identified nine categories of scope 3 emissions out of the fifteen defined
by the GHG Protocol as significant. The remaining six categories are not reported on separately, as they are either not applicable to Novo Nordisk or have been included in the other emission categories. Accounting policies are detailed only for the two most material categories of scope 3 – category 1 and 2. Our calculation methods for remaining categories 3, 4, 5, 6, 7, 9 and 12 are in line with the GHG Protocol and include the supplier-specific method, distance-based approach, average-activity method, average spend-based method and other hybrid method. In general, major sources of emission factors include DEFRA, EXIOBASE, GaBi and other industry databases and standards.
Category 1: Purchased goods and services
Emissions related to all spend from external suppliers, except for investment spend and travel categories, which are included in other scope 3 categories. Purchased goods and services mainly comprise raw materials for products, marketing, packaging materials and consumables for laboratory and IT office equipment. Direct spend is converted into CO2e emissions using the average data method. Material weights are matched with CO2e factors depending on data availability. A spend-based factor is applied for direct spend data where
no weight can be obtained. Indirect spend is converted into CO2e using a
spend-based method.
Category 2: Capital goods
Emissions related to all indirect investment spend from external suppliers, mainly production utilities and equipment. Indirect spend is converted into
CO2e emissions via the average spend-based method.
Percentage of GHG scope 3 calculated using primary data
Scope 3 emissions where primary data from suppliers or other value chain partners have been utilised in the calculation. This includes all of category 3, parts of category 4 emissions from inter-site distribution and the distribution
of finished products (provided directly by an external supplier managing the transportation and distribution processes), business flights (part of category
6) and two materials of category 1.
Total energy consumption from fossil sources under Novo Nordisk control
Primary energy consumption from coal, crude oil, petroleum products, and natural gas, as well as consumption of externally purchased secondary non-renewable energy such as electricity, heat, steam and cooling; and non-contractual biomass energy. Energy consumption is based on meter readings and/or invoices. In line with the ESRS requirements, we have enlarged the scope of the total energy consumption metric to include all entities under operational control, including fuel consumption in leased vehicles.
Total energy consumption from renewable sources
Wood, biogas and externally purchased electricity from renewable sources,
such as wind, solar, hydropower, biomass or biogas, as defined in the contractual agreements. Due to the conservative accounting approach requirement, steam and heat derived from biomass are not included under renewable sources. Consumption is based on meter readings and/or invoices and complemented with data on renewable energy certificates for each site. The previously reported metric ‘Share of renewable power for production sites’ was adjusted in scope to fully reflect the ESRS requirement.
Energy intensity/GHG intensity
Total energy consumption/total GHG emissions per net revenue. For energy intensity this corresponds to energy intensity from activities in high climate impact sectors. It is assumed that all activities of the Novo Nordisk Group are
in a high climate impact sector (NACE code C21). Net revenue refers to total
net sales generated by Novo Nordisk.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.2 Resource use and circular economy |
|
2.2 Resource use and circular economy
At the core of our Circular for Zero strategy is our commitment to decoupling resource use and waste from our ability to serve patients. The urgency of innovation in this regard is heightened by the stringent regulatory standards
for the pharmaceutical industry, for example restriction of the use of recycled materials in our medicines and devices. Similarly, while many materials in
our products are theoretically recyclable individually, the lack of established recycling infrastructure for pharmaceutical products makes this difficult to
be achieved in practice. We also recognise the challenges related to the
inflow of materials and resources into our construction projects within
the upstream value chain.
Material impacts, risks and opportunities (IROs)
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Resource use and waste associated with manufacturing and products |
|
•Upstream
•Own operations
•Downstream
|
Potential reputational risks related to resource consumption |
|
•Upstream
•Own operations
•Downstream
|
When manufacturing our products, we use materials and rely on natural resources, while also generating waste through our production processes
or during our products’ end-of-life cycle; all of which contribute to negative environmental impacts. Plastic components and plastic raw materials are among our top purchasing categories by weight, together with biological materials such as agricultural commodities and packaging materials, as well
as various technical materials used in our manufacturing processes. The impact of sourcing these raw materials has increased in recent years due to our high growth rate, a trend we are working to address by decoupling environmental impact from growth as part of our Circular for Zero strategy. As our devices are core to our business, reputational risks related to our resource consumption
are also considered material.

Positive impact

Negative impact

Opportunity

Risk
Processes to identify impacts, risks and opportunities
To assess impacts, risks and opportunities related to our resource inflows
and outflows, including waste, we consulted suppliers, investors and patient organisations. We continuously assess impacts and risks through environmental assessments, annually at each production site, and as part of all product development processes. The primary business areas in Novo Nordisk associated with resource use and circular economy are production, supply and sourcing units; however, our efforts to achieve our Circular for Zero ambitions affect all
of Novo Nordisk and are core to our operations across the business.
Resource inflow, resource outflow and waste
Policies
Circularity is the foundation of Novo Nordisk’s Circular for Zero strategy
and is anchored in our environmental policy, which states our commitment
to designing out waste and pollution and keeping materials in loops, thus addressing our material impacts and risks related to resource use and circular economy. Our environmental policy states our commitment to promoting low impact products and processes, when possible, for example by finding ways to use waste from one process as a resource in another process, including in our downstream value chain through our ReMedTM programme.
We also strive to source reused, recycled, and renewable biological materials, while always considering patient safety and the stringent regulatory requirements applicable to the pharmaceutical sector.
As stated in our environmental policy, Novo Nordisk works systematically
with third-party-validated life cycle assessments to better understand and reduce the environmental impact of our products. We prioritise the avoidance and reduction of waste over waste treatment where possible. As such, our environmental policy and associated actions related to products and production processes address all levels of the waste hierarchy, from prevention and reuse to recycling, energy recovery and disposal. For information on our reuse of chemicals and treatment of chemical waste and water, see sections
2.3 'Pollution' and 2.4 'Water' on pages 64 and 65, respectively.
Actions
Novo Nordisk continuously takes action to reduce resource use, increase circularity, and minimise waste across product design and development, manufacturing, and product end-of-life. To further strengthen these efforts,
in 2024 we set new targets for reducing our plastic footprint per patient in addition to our target addressing landfill waste, as detailed further under Performance in this section. The key actions to reach these targets and our wider Circular for Zero objectives are listed in the table on the next page.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.2 Resource use and circular economy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key action to address resource use and circular economy |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we track effectiveness |
|
|
Lower-carbon plastics |
Industrial partnership to buy e-methanol to produce a lower-carbon alternative to one of
Novo Nordisk's top two plastic types. Production is expected to start in 2025 and continue
on an ongoing basis, pending completion of plant construction.
|
Global, injection devices |
No |
•Partnership launched in 2024.
•Tracking to be established in connection with receipt of first batch of materials.
|
|
|
ReMedTM: recycling
of used pens
|
Take-back scheme to recycle injection pens launched in 2020 in Denmark, and subsequently
in six other markets. These seven markets represent approximately 20% of injection pens
currently supplied in the market. An industry-wide scheme was launched in Denmark in 2023
with three other pharmaceutical companies as part of our ongoing efforts to address the
lack of recycling infrastructures in our industry. Collected pens are recycled by an external
recycling partner.
|
Denmark, Brazil, France, Italy, UK, Japan, Germany |
No |
•ReMedTM initiatives launched in Italy, Japan and Germany.
•Return rate of 32% achieved for all injection devices in Danish industry scheme. Recycling
rate for returned pens increased from 50% in early 2024 to 70% by end of 2024.
•Effectiveness is tracked through monitoring return rates across markets in scope. We work continuously on making patients, society and partners aware of the scheme to drive up return rates and increase recycled volumes.
|
|
|
Converting to reusable devices |
Converting from single and multi-use devices to reusable devices, to lower the lifetime environmental impact per product, including developing a cost-efficient reusable
pen with a competitive environmental profile across our injection pen portfolio as part of
our recurring efforts to increase circularity and reduce our plastic footprint per patient.
|
Global |
Yes |
•Plastic footprint of 0.35 kg/patient in 2024.
•Development of new reusable device, planned for launch in 2026.
•Future plans include delivering the majority of daily insulins in reusable devices.
•Strengthened implementation of circular design framework through organisational
anchoring and tools.
•Awiqli® entered the market for the first time.
|
|
|
Circular design guidelines |
Guidelines tailored to our devices and packaging and applied to every design process,
which consider 1) design for expected lifetime; 2) design for sustainable materials; 3) no unnecessary waste in production; and 4) recyclability after use. This is a recurring action.
|
Global |
|
Innovating treatment methods |
Optimising our material use through Awiqli®, the world’s first once-weekly basal insulin, as
part of our efforts to reduce our plastic footprint towards 2033. Going from daily to weekly injection reduces the plastic footprint of the treatment by approximately two thirds (compared to once-daily treatment).
|
Canada, Germany,
and China
|
|
Making production processes more circular |
Circular production processes, including internal reuse of ethanol at our two
largest API production sites, as a recurring action. At site Kalundborg, Denmark, most
remaining ethanol waste together with yeast slurry is turned into energy and fertiliser for local farmers as part of the Kalundborg Symbiosis.
|
API production in Denmark and US |
No |
•Resource use is monitored at site level to track our levels of circularity and resource efficiency across production processes, thus enabling the ongoing identification of improvement areas. For example, the reuse of ethanol reduces use of new ethanol by almost 90%.
|
|
|
Eliminating
landfill waste
|
Divert waste from landfill to either incineration, other recovery operations or recycling by
2030 to avoid harmful environmental impacts from landfill waste.
|
Production sites globally |
Yes |
•Waste to landfill reduced at site Clayton, US, most of it via waste-to-energy, leading to a significant reduction of landfill waste. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.2 Resource use and circular economy |
|
Performance
Targets on plastic footprint per patient and zero landfill
To track the effectiveness of our actions, we have set a global target to reduce our plastic footprint per patient from Diabetes and Obesity products by 30%
by 2033, compared to our baseline of 0.35 kg/patient in 2024. This target addresses both resource inflows and outflows, including the minimisation of primary raw materials, thus preventing plastic waste via efforts to convert to reusable devices, circular product design, and innovating treatment methods. The scope of the target includes plastic in devices and primary packaging for Diabetes and Obesity products, and internal experts across areas were involved in setting it.
To address our waste impact, we also have a global target of zero landfill from production sites by 2030, thus seeking to redirect waste to incineration or recycling. In 2024, the total volume of production waste directed to landfill was 94 tonnes, hereby we achieved a 92% reduction compared to our baseline (2019).
Progress on resource inflows
In 2024, the overall total weight of products and technical and biological materials used for the manufacture of our medicines amounted to 226 thousand tonnes, with approximately two thirds being technical materials
and one third biological components. We do not currently procure certified sustainably sourced biological materials, leading us to report their share as 0%. In 2024, the total weight of reused or recycled materials was 3 tonnes, primarily from the sourcing of gowning, and recycled pallet shippers and shipper boxes.
In addition to plastic components, the main products and materials sourced
that relate to resource inflows include biological materials, such as agricultural commodities (for example glucose) and printed packaging, as well as technical materials, such as acids and bases, and solvents. While we do not directly purchase critical raw materials and rare earths, some purchased items include critical raw materials such as magnesium, manganese, and phosphorus. Resource outflows leaving Novo Nordisk’s facilities include medicines, injection devices, packaging materials, and waste.
Progress on resource outflows
The durability of our prefilled devices is determined by the shelf-life of the medicine and the number of doses it contains. Durability is estimated between 24 and 36 months and approximately 7 uses, depending on the medical substance. Reusable devices have a durability of 60 months, which is at the higher end of the industry range.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.2.1 Resource inflows |
Unit |
2024 |
2023 |
2022 |
| Overall total weight of products and technical and biological materials used during the reporting period |
1,000 tonnes |
226 |
– |
– |
| Percentage of biological materials (and biofuels used for non-energy purposes) that are sustainably sourced |
% |
0 |
– |
– |
| Absolute weight of secondary reused or recycled components, secondary intermediary products and secondary materials used to manufacture the undertaking’s products and services (including packaging) |
1,000 tonnes |
3 |
– |
– |
| Percentage of secondary reused or recycled components, secondary intermediary products and secondary materials |
% |
1 |
|
– |
– |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.2.2 Resource outflows |
Unit |
Novo Nordisk
2024 |
Industry
2024 |
Novo Nordisk
2023
|
Industry
2023 |
Novo Nordisk
2022
|
Industry
2022 |
Expected durability of unopened prefilled devices |
Months |
24-36 |
12-36 |
– |
– |
– |
– |
• Prefilled devices for single use |
Number of uses |
1 |
1 |
– |
– |
– |
– |
• Prefilled devices for multiple use |
Number of uses |
7 |
7 |
– |
– |
– |
– |
Expected durability of reusable devices |
Months |
60 |
12-72 |
– |
– |
– |
– |
Recyclable content in products |
% |
0 |
– |
– |
– |
– |
– |
Recyclable content in products packaging |
% |
28 |
– |
– |
– |
– |
– |
Plastic footprint (absolute) |
Tonnes |
15,654 |
N/A |
|
|
|
|
Plastic footprint per patient |
kg/patient |
0.35 |
N/A |
|
|
|
|
|
|
|
|
|
|
|
|
| Target |
|
2024 |
2033 |
|
|
|
|
Plastic footprint per patient |
% reduction from 2024 |
N/A |
(30%) |
|
|
|
|
While many components of our devices can be recycled individually, there
is no established recycling infrastructure for pharmaceutical waste in many
of our markets, and hence we conservatively assume zero recyclable content
in products.
A conservative estimate of recyclable content in our products' packaging is
28%, reflecting the lowest share of recyclable content according to our product lifecycle assessments. The full range is 28-88%, with the difference being due
to varying packaging compositions across our core products and three key
geographies (Europe, US and Japan). Data on total packaging weights by geography have not been available for 2024, but it is a priority to establish the data foundation for reporting recyclable packaging across all key products as weighted average in 2025.
Progress on resource outflows – waste
Novo Nordisk’s largest waste streams in terms of volume are the generation
of organic residue (for example yeast slurry), water waste (waste fraction with
a high water content treated as waste) and ethanol waste. The majority of organic residues is diverted from disposal through recycling and other recovery operations. Total waste increased by 21% in 2024, mainly due to increased production volumes (19% increase in waste generated in production) and the inclusion of non-production entities in the total waste metric (2%). 15% of the total waste was directed to disposal and therefore non-recycled, while 85% was recycled, recovered or prepared for reuse. Hazardous waste accounted for 23% of the total, with equal split between further recycling/recovery operations and waste directed to disposal through incineration.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.2 Resource use and circular economy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.2.3 Resource outflow – Waste |
Unit |
2024 |
2023 |
2022 |
| Total waste generated |
Tonnes |
229,690 |
189,091 |
213,505 |
Non-recycled waste |
Tonnes |
34,132 |
– |
– |
Percentage of non-recycled waste |
% |
15% |
– |
– |
Total amount of radioactive waste |
kg |
871 |
– |
– |
Waste to landfill |
Tonnes |
120 |
638 |
906 |
Progress on target |
|
2024 |
2030 |
|
Waste to landfill (production) |
Tonnes |
94 |
0 |
|
1. 20kg Isotope 125-I solid, 15kg Isotope 125-I Liquid, 15kg Isotope 3-H, solid, and 37kg Isotope 3-H, solid. |
ACCOUNTING POLICIES
Overall total weight of technical and biological products and materials
Total amount of materials used in our operations. Technical materials cannot
be processed by the biological cycle, while biological materials can. Total weight includes all raw materials, associated process materials and semi-manufactured goods or parts sourced into production. Approximately 3% of the total cannot be categorised into biological or technical material but is still included in the total. No material was included in both categories to avoid double-counting.
Percentage of biological materials that are sustainably sourced
Proportion of biologically sourced materials deemed sustainable, according to our internally approved list of eco-labels (including those from the Forest Stewardship Council (FSC), Programme for the Endorsement of Forest Certification (PEFC), and Rainforest Alliance), in the total materials used.
Absolute weight of secondary reused or recycled components
Total weight of previously used or recycled materials used in the production process, such as gowning, recycled pallet shippers and shipper boxes. Internal
re-use and multiple re-use within Novo Nordisk are not included in the metric, for example the reuse of ethanol and pallets.
Percentage of secondary reused or recycled components
The weight of secondary reused and recycled materials, components and products divided by the total weight of all materials used.
Recyclable content in products and packaging
Share of recyclable materials used. Novo Nordisk's definition of recyclable content reflects practical recyclability in line with the Ellen McArthur Foundation’s definition and the EU Packaging and Packaging Waste Regulation. For recyclable content in product packaging, data on total packaging weights by geography have not been available and the metric shows the shows the lower end of the range of recyclable content across markets and not the weighted average.
The expected durability of products
For reusable devices technical durability of the device. For prefilled devices, expected number of uses and anticipated shelf-life for unopened medicines, with shelf-life referring to the expected ability of the products to maintain their stability, efficacy, and quality over a specified period under recommended storage conditions. The metric is a minimum to maximum range per product category for both Novo Nordisk products placed on the market and the industry average available from market research, industry reports, regulatory guidelines, supply chain data and external certifications. Novo Nordisk’s product range is included in
the industry average. Number of uses for multi-use prefilled devices is based on the most sold device-medicine combination and the daily dose defined by the WHO.
Plastic footprint
Absolute plastic footprint is defined as the total amount of plastic placed on the market by Novo Nordisk in connection with Diabetes and Obesity products, including plastic from Novo Nordisk devices (pens and needles) and primary packaging (cartridges, vials, blister packs and tablet bottles). The metric does not capture additional plastic used in the process. Plastic footprint per patient refers to the absolute volume divided by patients reached.
Total waste generated by Novo Nordisk
Waste collected by a certified waste management company and waste intended for collection. It is measured by weight receipts or other data from the waste management company, including all waste fractions and disposal methods. December data was estimated. Waste data for offices and affiliates outside Denmark are extrapolated based on headcount data available for their Danish counterparts. All waste subcategories are split between hazardous and non-hazardous waste according to the EU’s Waste Framework Directive. Radioactive waste is reported separately and not included in the total.
Hazardous and non-hazardous waste diverted from disposal due to preparation for reuse, recycling or other recovery operations
All waste directed for reuse without any further processing and waste directed for recycling or any other recovery operation, excluding energy recovery by incineration. We estimate the preparation for reuse to be negligible.
Hazardous and non-hazardous waste directed to disposal by incineration,
landfill and other disposal operations
All waste directed to disposal by incineration, both with and without energy recovery, and by landfill at designated landfill sites.
Percentage of non-recycled waste
Share of all waste directed to disposal out of total waste.
Total amount of radioactive waste
Total amount of waste materials that contain radioactive substances generated at Novo Nordisk and transferred to a radioactive waste management facility. We handle radioactive waste in compliance with applicable regulations.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.3 Pollution |
|
2.3 Pollution
Novo Nordisk relies on both biological processes and chemicals to produce medicines, devices and packaging materials. As a pharmaceutical company, chemical substances have particular relevance to our business, and we comply with and report in accordance with all relevant regulations to prevent and control all types of pollution.
Material impacts, risks and opportunities (IROs)
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Chemicals affecting human health or ecosystems |
|
•Upstream
•Own operations
•Downstream
|
While chemicals are an essential component in the production of medicines, some Substances of Concern (SCs) and Substances of Very High Concern (SVHCs)1 can potentially have a negative impact on health or the environment. Material impacts related to SCs and SVHCs concern both substances procured or used in production, and substances that leave our facilities in products or as emissions (see illustration on the right hand side).
Processes to identify impacts, risks and opportunities
To identify pollution-related impacts, Novo Nordisk conducts annual environmental assessments at all production sites, covering air emissions, waste, noise, water withdrawal and water discharge, soil and ground water. We screen for harmful chemicals, including those covered by relevant regulations such as REACH during the product development process, and we use the EU Commission’s definition of Most Harmful Substances when defining chemicals in scope.

Positive impact

Negative impact

Opportunity

Risk
1. SVHCs are chemicals that have serious irreversible effects on human health or the environment, in accordance with REACH. SCs are additional chemicals that have harmful effects.
Substances of concern and very high concern
Policies
Novo Nordisk’s environmental policy states our commitment to handling chemicals safely and striving to avoid the use of harmful chemicals, when developing or designing new products and processes. Our screening of
harmful chemicals applies to both in-house and outsourced product development processes. For treatments already on the market, we strive to minimise the use of these chemicals and substitute them where possible.
All of Novo Nordisk’s production facilities are ISO 14001 certified for environmental management and thus mitigate, control, and prevent negative environmental impacts, including pollution-related incidents and emergency situations. We reuse chemicals where possible and ensure the best possible handling of hazardous waste and emissions. We also ensure compliance with the terms of the environmental permit for each production site. In case of breaches of regulatory terms, we register an environmental non-conformity, investigate, and implement corrective actions.
Actions
We work with several innovation projects in both our own and outsourced production processes to reduce the environmental impacts of our products
on an ongoing basis, as described in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key action to address chemical pollution |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we
track effectiveness
|
|
|
Reducing chemicals
in production and medicines
|
Recurring action to minimise the use of specific chemicals in production processes through substitution or purification for reuse, and to
optimise efficacy of medicines with lower
chemical use.
|
In-house and outsourced production processes |
No |
•The use of relevant chemicals is tracked through environmental assessments at production sites and during product design and development processes to monitor effectiveness.
|
|
Performance
We continuously track our use of chemicals through various internal KPIs and environmental assessments to ensure that we mitigate negative impacts
on the environment or human health.
A total of 2,304 tonnes of SCs and SVHCs were procured in 2024. Most of their use is linked to solvents used in the production of our API. We are seeking to reduce the use of these chemicals through substitution initiatives or purification for reuse, as described in the key action below. During the production process, almost all of SCs and SVHCs are collected as waste and handled safely in accordance with our policies and any relevant regulations.

The remaining substances, leaving Novo Nordisk in the form of emissions or as part of products, were estimated to be 11 tonnes of SCs and SVHCs during 2024. SCs leaving our facilities as part of our products make up 5 tonnes, and originate primarily from one substance used as an excipient in extremely low concentrations to preserve our medicines. The remaining SCs and SVHCs leave our facilities as emissions to air and water. In 2024, this amounted to approximately 5 tonnes of SCs and 1 tonne of SVHCs, primarily linked to the production of API. The reported volumes of SCs and SVHCs leaving our facilities are within regulatory limits.
In addition to the disclosures above, we also report in accordance with the European Pollutant Release and Transfer Register.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.4 Water |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2.3.1 Substances of Concern and Substances of Very High Concern2 |
|
Substances of concern |
|
Substances of very high concern |
| Unit |
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
| Substances procured |
Tonnes |
445 |
– |
– |
|
1,859 |
– |
– |
| • Human health hazard |
Tonnes |
355 |
– |
– |
|
N/A |
– |
– |
| • Environmental hazard |
Tonnes |
111 |
– |
– |
|
N/A |
– |
– |
| • Physical hazard |
Tonnes |
0 |
– |
– |
|
N/A |
– |
– |
| Substances leaving facilities as emissions, as products, or as part of products |
Tonnes |
10 |
– |
– |
|
1 |
– |
– |
| Substances leaving facilities as emissions |
Tonnes |
5 |
– |
– |
|
1 |
– |
– |
| • Human health hazard |
Tonnes |
5 |
– |
– |
|
N/A |
– |
– |
| • Environmental hazard |
Tonnes |
0 |
– |
– |
|
N/A |
– |
– |
| • Physical hazard |
Tonnes |
0 |
– |
– |
|
N/A |
– |
– |
| Substances leaving facilities as products, or part of products |
Tonnes |
5 |
– |
– |
|
0.003 |
– |
– |
| • Human health hazard |
Tonnes |
5 |
– |
– |
|
N/A |
– |
– |
| • Environmental hazard |
Tonnes |
0 |
– |
– |
|
N/A |
– |
– |
| • Physical hazard |
Tonnes |
0 |
– |
– |
|
N/A |
– |
– |
2. If a material belongs to more than one main hazard class, the weight of the substance is reported in both hazard classes. Consequently, the sum of the sub-categories exceeds the total. |
ACCOUNTING POLICIES
The figures reported in Table 2.3.1 are manually calculated from the available data and are subject to significant uncertainty. The weight of substances is calculated according to their concentration in the material. If information on concentration is not available, the assumption is that 100% of the material consists of substance(s)
in scope. Consequently, certain metrics might be overestimated. The scoping of
the materials included in the calculations may not be exhaustive.
Main hazard classes (human health, environmental and physical)
Defined by chemical subject matter experts, based on the specific hazards they present and includes following hazard class codes: Human health hazard (hazard class code H3xx or EUH3xx), environmental hazard (hazard class code H4xx or EUH4xx or EUH059) and physical hazard (hazard class code H2xx or EUH2xx).
If a material belongs to more than one main hazard class (human health and environmental hazard) the weight of the substance is reported in both hazard classes. Consequently, the sum of the sub-categories exceeds the total. Main
hazard classes are not applicable to SVHCs.
Total weight of substances of concern/ substances of very high concern
Comprise the total weight of substances procured into production, categorised into main hazard classes. Data sources include receipts of materials and purchase orders mapped against a chemical database indicating hazard class.
Amount of substances of concern and substances of very high concern
that leave facilities as emissions
Total weight of SCs and SVHCs that leave production sites as emissions to air
or water, split into main hazard classes. The estimated volumes of substances are based on available data for our API production and estimated for Chemistry, Manufacturing and Control (CMC) processes. Laboratories were deemed immaterial and are not in scope. Novo Nordisk Pharmatech A/S will be
included from 2025.
Amount of substances of concern that leave facilities as products or part of products
Total weight of SCs and SVHCs that leave Novo Nordisk as products or part of products split into main hazard classes. Products or parts of products are defined as either excipients or devices. Data sources include production data (with final product quantities), bills of materials and purchase orders mapped against a chemical database indicating hazard class.
2.4 Water
Water is an essential natural resource in the manufacturing of Novo Nordisk’s pharmaceutical products and a key input to many commodities in our supply chain. Hence, water management is key to both our own operations and to our value chain, where we as part of our new nature roadmap, further described on page 68, are expanding actions and engaging suppliers to mitigate our negative impacts on water resources.
Material impacts, risks and opportunities (IROs)
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Availability and deterioration of water resources |
|
•Upstream
•Own operations
•Downstream
|
Negative impacts on water availability occur when we source water for production purposes, especially in water-stressed areas, and at the end-stage
of production, when water is discharged to water treatment plants, which can impact water quality. Resilience is assessed as part of our nature and biodiversity efforts and is further described in the transition plan in section 2.5 'Biodiversity and ecosystems' on page 68.
Processes to identify impacts, risks and opportunities
To assess water-related impacts, risks, and opportunities, we conduct screenings
of our production sites for areas of water stress and risk using the tool Aqueduct
4.0 from the World Resource Institute (WRI). As part of our engagement in water stewardship, in 2024 we conducted stakeholder engagements with water management authorities and other industrial water users at our sites in Montes Claros, Brazil, and Hillerød, Denmark, and at our largest API production sites: Clayton, US, and Kalundborg, Denmark.

Positive impact

Negative impact

Opportunity

Risk
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.4 Water |
|
Water withdrawal, consumption and discharge
Policies
Novo Nordisk’s environmental policy addresses water management and sets out our ambition to design less water-intensive processes by reusing and recycling water. We treat production-related water discharge onsite and/or offsite and avoid water pollution by discharging water in accordance with local regulations, and in case of any breaches, we take corrective actions.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key action to
address water |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we track effectiveness |
|
|
Water withdrawal savings programme |
Efficiency projects at production sites in scope, including optimisation of water use, creating systems for single and multiple reuse and recycling. We systematically map opportunities for water savings to create detailed savings plans, and the programme is planned to run until 2033. |
Production sites with high water withdrawals and/or high water-risk/stress area |
No |
•To track the effectiveness, sites in scope forecast expected water withdrawals and implement water savings initiatives. Water savings are registered per initiative via an internal dashboard.
•Total water savings in 2024 amounted to 105,600 m3, of which 51,000 m3 was in areas of high water-stress and/or water risk.
|
|
|
Engaging priority suppliers |
Engaging priority suppliers on their water impacts through a capability-building programme. Planned for 2025-2033 |
Key suppliers to be identified |
No |
•Action to be initiated in 2025, when processes for tracking effectiveness will be established. |
|
|
Taking actions outside
of sites
|
Enhancing water quantity and quality outside of
our sites, including replenishing water. Planned
for 2025-2033.
|
Upstream value chain |
No |
•Action to be initiated in 2025, when processes for tracking effectiveness will be established. |
|
|
Saving water through the establishment of district cooling in Kalundborg |
Establishment of district cooling at our largest production site in Kalundborg, Denmark, which accounts for half of our total water withdrawals, including phasing out the use of water from Lake Tissø. Industrial collaboration with the Kalundborg Symbiosis, Kalundborg Utility, and Novonesis. Expected completion of construction during 2026. |
Production site in Kalundborg, Denmark |
No |
•Progressed with the construction process, which is expected to be finalised during 2026, with surrounding factories connected from 2026 onwards.
•Expected annual water withdrawal savings upon completion estimated at 400,000 m3.
|
|
|
Doubling wastewater treatment capacity in Kalundborg |
Expansion of on-site wastewater treatment
operated by Novonesis, doubling the industrial wastewater and biomass treatment capacity.
Energy is recovered in the treatment process as
part of the Kalundborg Symbiosis. To be completed in 2026.
|
Production site in Kalundborg, Denmark |
No |
•Project launched and construction has started. |
|
In addition, our environmental policy also addresses our special focus on
water withdrawal in areas of high water-stress or risk, both at our own sites
and with suppliers of key commodities, for example through our water withdrawal savings programme and planned efforts to engage priority suppliers. The policy also addresses our support of water stewardship principles and collective actions in water basins that are under pressure, for example in our current efforts to establish district cooling in Kalundborg and planned efforts to enhance water quantity and quality as detailed in the key actions below.
Actions
We implemented and planned several actions in 2024 to mitigate our impacts on water, as part of our Circular for Zero strategy and our new nature roadmap, described further on page 68. Key actions to mitigate negative impacts on water are listed in the table on this page.
Performance
We have not set external targets on water, but systematically track water withdrawals and water savings as part of our water withdrawal savings programme, as described in the table of key actions. In 2024, we experienced an increase in water withdrawal of 26% compared to 2023 due to our growth. Most of the increase is due to increased API production, as well as water used in expansion and construction projects. In 2024, the organisational scope of water withdrawal expanded to also include water withdrawals for offices and research facilities outside of Denmark, which increased the total water withdrawal by further 3%.
Most of water drawn into the boundaries of Novo Nordisk is used for cooling and the fermentation process, and is subsequently discharged, making the actual water consumption relatively low. The largest share of water consumed comes from evaporation, water waste (sludge) and water in our products. We estimate that, in 2024, over 400 thousand m3 of water were reused or recycled at our production facilities.
Production sites in China (Tianjin), US (Clayton and Durham), Iran (Tehran) and Algeria (Blida) are located in areas with high or extremely high water-stress
and/or water risk. These sites account for approximately 23% of the total water withdrawal, with the biggest withdrawal occurring in the US and China.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2.4.1 Water consumption |
Unit |
2024 |
2023 |
2022 |
| Total water consumption |
1,000 m3 |
630 |
– |
– |
• Water withdrawal1 |
1,000 m3 |
5,213 |
|
4,150 |
3,918 |
• Water discharge |
1,000 m3 |
4,583 |
|
– |
– |
| Total water consumption in areas at water risk, including areas of high water stress |
1,000 m3 |
191 |
– |
– |
• Water withdrawal |
1,000 m3 |
1,217 |
|
– |
– |
| Total water recycled and reused |
1,000 m3 |
416 |
– |
– |
| Water intensity ratio |
m3/mDKK |
2.17 |
– |
– |
1. Water withdrawal was previously reported as 'Water consumption'. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.5 Biodiversity and ecosystems |
|
ACCOUNTING POLICIES
Water withdrawal
Includes all types of water such as drinking water, industrial water, steam, rain water and water from remediation wells and rainwater. Data is based on meter readings and invoices. Data for offices and affiliates outside Denmark are extrapolated based on data available for their Danish counterparts (approximately 97% of the total is based on primary data).
Water discharge
Includes discharge of process- and sanitary water and discharge from storm water to outside Novo Nordisk’s boundaries, and water discharge used for irrigation. For offices where discharge data are not available, it has been assumed that water discharge equals water withdrawal.
Water consumption
Water drawn into Novo Nordisk’s boundaries but not discharged, calculated
as the balance between total water withdrawal and total water discharge.
Total water consumed in areas at water risk, including areas of high-water stress
Total water consumed by sites located in areas at water risk, including areas of high water stress. The identification of production sites in scope is performed using the Aqueduct 4.0 Water Risk Atlas tool provided by the World Resource Institute using two indicators: baseline water stress and overall water risk. 'High' means high or extremely high ratings in Aqueduct 4.0. The data reported are based on primary data.
Total water recycled and reused
Total quantity of water and water discharge (treated or untreated) that has
been used more than once at the production sites before being discharged.
The volume is estimated based on key indicators for specific water treatment equipment and technologies available at the sites. This includes steam condensate returned to steam generator, reverse osmosis water treatment,
and water discharge from water treatment for irrigation. The metric is estimated with a conservative approach.
Water intensity ratio
Total water consumption (as outlined above) per DKK million in net revenue, defined as total net sales generated by Novo Nordisk.
2.5 Biodiversity and ecosystems
We rely on natural resources in our production of pharmaceutical products, primarily agricultural (glucose), forestry (paper), fossil-based (plastic) commodities, and water. In 2024, we developed a new nature roadmap
to accelerate our efforts to address these impacts.
Material impacts, risks and opportunities (IROs)
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Reliance on natural resources and ecosystem services |
|
•Upstream
•Own operations
|
Our resource use in production processes leads to negative impacts on nature. Such impacts can also occur due to land-use change when establishing new production sites impacting local ecosystems and contributing to the loss of natural habitats. Along our value chain, the sourcing of raw materials that require fresh-water or land-use change also have impacts on nature, especially agricultural production or deforestation for wood-based materials, where pollution, for example from pesticides used for agricultural purposes, can exacerbate negative impacts.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Reliance on vulnerable species in research |
|
•Upstream
•Own operations
|
Novo Nordisk's material impact on vulnerable species includes our reliance on horseshoe crab blood for endotoxin testing, which is required to ensure the safety of our medicines. Extraction of horseshoe crab blood can harm survival rates of the animals even though they are released back into their natural habitats. We no longer use products from endangered horseshoe crab species, and are working to phase out the use of lysate from vulnerable horseshoe crab species.

Positive impact

Negative impact

Opportunity

Risk
Processes to identify impacts, risks and opportunities
To assess Novo Nordisk's impacts on biodiversity and ecosystems, we undertook an assessment aligned with the Science Based Targets Network (SBTN) methodology, covering our direct operations and upstream value chain and
using primary activity data and lifecycle assessment (LCA) databases, along with
data on the state of nature. Furthermore, we screened our value chain for high-impact commodities that cause an increased pressure on nature.
Dependencies on nature were assessed across our value chain using the ENCORE (Exploring Natural Capital Opportunities, Risks and Exposure) tool
to evaluate relevant sub-industries, based on the areas most likely to disrupt Novo Nordisk in terms of raw material inputs, production processes, and testing. Physical and transition risks were assessed separately using the
World Wildlife Fund Biodiversity Risk Filter and through a qualitative scenario analysis. Systemic risks were not considered, and consultations with affected communities were not conducted. As we have not yet been able to develop
a methodology to assess whether our operational sites are having negative impacts on biodiversity sensitive areas, we do not currently have mitigation measures in place.
Biodiversity loss, conditions of and dependencies
on ecosystems and state of species
Policies
Novo Nordisk's environmental policy is aligned with our nature roadmap and
it covers the key drivers of biodiversity loss such as water- and land-use,
over-exploitation, pollution, and climate change, to mitigate material nature-
related impacts. Its scope covers all our owned, leased, or managed operational sites, including those near biodiversity sensitive areas.
The policy sets out our commitment to protecting and restoring nature and biodiversity, as well as our ambition to work with suppliers to create solutions with less impact on water-, forest-, and land-use, for example, through regenerative agriculture. We will also work with partners on restoration activities beyond our value chain. As such, the policy relates to both our impacts and dependencies on nature, as described in this section. The policy does not address social consequences of biodiversity and ecosystems-related impacts.
In addition to the provisions in our environmental policy, Novo Nordisk adheres to all local regulations related to nature and biodiversity in the establishment of new sites and in our production sourcing and processes.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.5 Biodiversity and ecosystems |
|
Actions and transition plan for nature
Novo Nordisk's nature roadmap, formalised in 2024, has an overarching aspiration to halt the loss of nature in our value chain by 2033, and become nature positive by 2045. This will be achieved by reducing our impact on
land, water, and biodiversity, while driving positive impacts through restoration and transformative actions. In pursuing our ambition, we seek to mitigate the
risks nature loss poses to our business and drive action for nature in line
with global policy frameworks, including the Kunming-Montreal Biodiversity Framework and the EU Biodiversity Strategy for 2030. The nature roadmap has been approved by Executive Management and Board of Directors. The key actions to address our impacts and deliver on our nature roadmap are outlined in the table to the right, including actions incorporating nature-based solutions, such
as restoration projects. Local and indigenous knowledge will be incorporated into the development of restoration projects where appropriate, and we do not use biodiversity offsets as part of our nature roadmap.
To inform the development of our nature roadmap in 2024, we conducted a
high-level resilience analysis of the exposure of our current business model
to ecosystem-related risks. The analysis considered one scenario where we
meet our nature roadmap ambitions and one in which we do not. The results indicated that implementation of the roadmap could decrease Novo Nordisk’s exposure to nature-related risks related to raw material shortage and emerging deforestation regulation and highlighted the need for continued focus on water management. In conducting the analysis, it was assumed that high nature degradation would continue along the current trajectory towards 2030 and 2050. The scope included the upstream value chain of strategically significant raw materials in selected geographies, water withdrawal at key sites in our own operations, and chemicals in water discharge in the downstream value chain. External stakeholders were not involved in the analysis.
Performance
As part of our nature roadmap, we are continuously working to understand and measure our impacts and dependencies, and will continue this effort as we begin implementing the roadmap in 2025, also establishing processes to track the effectiveness of our actions.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key action to
address biodiversity and ecosystems
|
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we
track effectiveness
|
|
|
Reduce impact on water |
Reduced impact on water through water
withdrawal savings at production sites in scope, together with other actions as detailed in section
2.4 'Water' on page 66.
|
Own operations and key suppliers |
No |
•Water withdrawals and water savings are tracked.
•For more details, see section 2.4 'Water'.
•Process to track effectiveness of supplier engagement to be established during roadmap implementation in 2025.
|
|
|
Reduce impact on
land by addressing deforestation, soil degradation and pollution in
supply chain
|
Avoid degradation of land in our supply chain
by ensuring a deforestation free paper and cardboard supply chain, and strive for all glucose
to be sourced from regenerative agriculture.
|
Supply chain |
No |
•Novo Nordisk’s nature roadmap was endorsed in 2024 and will be implemented from 2025 onwards, during which processes to track effectiveness will be established. |
|
|
Reduce impact on biodiversity |
Restore biodiversity at key sites, ensuring
positive impacts by 2033. Avoid impacts on endangered species.
|
Own production sites |
|
|
Restoration projects |
Initiate restoration projects near key sites
by 2033. Develop a global restoration plan linked
to our value chain by 2026 to become nature
positive by 2045.
|
Own value chain and beyond |
|
|
Transformation |
Through transformative approaches, optimise and replace glucose in API production to bring our glucose land footprint close to zero by 2045. |
Own operations |
|
|
Minimise and phase out the use of biological products from vulnerable and endangered species |
Minimise and phase out the use of horseshoe crab materials, Tachypleus amebocyte lysate, TAL, and Limulus amebocyte lysate, LAL. The use of lysate from the endangered Chinese horseshoe crab, TAL, has been phased out, and the remaining phase-out of LAL from the vulnerable American horseshoe crab is tentatively expected between 2025 and 2035, with the majority of testing expected to be phased out by 2027. The complete discontinuation depends on regulatory approvals of alternative testing methods.
|
Own operations |
No |
•Phase-out of TAL has been completed as of 2023.
•Use of LAL in our research areas was discontinued in 2024, and we continue working to phase out use for remaining testing of samples. The recent acceptance by the European Pharmacopoeia and the USP Microbiology Expert Committee of the use of recombinant reagents for relevant testing marked an important milestone towards this goal.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.6 EU Taxonomy |
|
2.6 EU Taxonomy
The EU Taxonomy provides a classification system that defines which economic activities are environmentally sustainable, while meeting standards for human
rights, anti-corruption, fair competition and taxation. Novo Nordisk welcomes the introduction of comparable sustainability definitions and is working to incorporate relevant EU Taxonomy criteria into our operations to support
our sustainability ambitions.
The Taxonomy-related disclosure process can be broken down into three
major steps.
•The first step is a screening of potentially eligible economic activities (screening in accordance with the technical annexes of the Climate Delegated Act: Annex 1 on Climate change mitigation and Annex 2 on Climate change adaptation and a screening of the Environmental Delegated Act: Annex I on Sustainable use and protection of water and marine resources, Annex II on Transition to a circular economy, Annex III on Pollution prevention and control and Annex IV on Protection and restoration of biodiversity and ecosystems), which results in an already shortened list.
•In the next step, each of the economic activities are assessed against how Novo Nordisk performs the activity, considering a financial and strategic materiality. This is followed by the detailed assessment of the alignment of identified economic activities, comprising substantial contribution, 'Do No Significant Harm' (DNSH) and minimum safeguards.
•Finally, the KPIs required for Taxonomy reporting are extracted (see Novo Nordisk adjusted tabular overview on the next page and the detailed mandatory reporting templates in section 5, tables 4 a-c on pages 97-99).
Taxonomy-eligibility
Based on our annual review process and materiality considerations, the following economic activities defined in the EU Taxonomy have been identified as relevant to Novo Nordisk:
•P1.2: Manufacture of medicinal products (environmental objective Pollution prevention and control) - relevant for the Turnover, CapEx and OpEx KPIs (eligibility).
•CCM7.1: Construction of new buildings (environmental objective
Climate change mitigation) - relevant for CapEx (eligibility and alignment).
•CCM7.2: Renovation of existing buildings (environmental objective
Climate change mitigation) - relevant for CapEx (eligibility).
Taxonomy-alignment – Substantial contribution and 'Do No Significant Harm' (DNSH)
Novo Nordisk has assessed the technical screening criteria for eligible economic activities deemed material and has conducted an internal analysis of the feasibility of Taxonomy-alignment. This leads to the following result for 2024:
•7.1 Construction of new buildings: An evaluation of the Climate Delegated Act Annex 1 on climate change mitigation has been conducted, leading to the conclusion that alignment of new building construction can be claimed following a positive gap analysis. A proportion of two major ongoing construction projects in Hillerød and Kalundborg, Denmark, have met the requirements of Taxonomy- alignment. These projects demonstrate compliance with a substantial number of the technical screening criteria for 2024. Certain criteria are yet to be fulfilled because the relevant construction phase has not been reached. We assume fulfilment of these on the basis of pre-calculations in the design phases, robust processes, and controls throughout the entire construction process. We thereby claim alignment based on the expectations that our construction continues to follow our plans. As part of Novo Nordisk's commitment to expanding production capacity in a sustainable way, future building projects will incorporate alignment criteria when feasible in the upcoming years.
•7.2 Renovation of existing buildings: This economic activity represents the second phase in implementing Taxonomy-alignment within our construction and real estate activities. No alignment is reported for this activity in 2024.
•1.2 Manufacture of medicinal products: We are also conducting an initial assessment of alignment criteria focused on our Danish manufacturing sites for Ozempic® and Wegovy® for economic activity 1.2. We currently meet many of the criteria, such as using readily biodegradable ingredients in our medicinal products and managing emissions of pollutants to air, water, and soil. However, we have encountered challenges in obtaining evidence for, or addressing the practical aspects of, meeting some of the criteria, which will require further consideration in alignment with our Circular for Zero strategy.
Taxonomy-alignment – Minimum safeguards
Operating as a responsible business is a core value for Novo Nordisk, including meeting the minimum safeguards defined in the EU Taxonomy through the following practices:
•Commitment to respecting human rights across our value chain. Our human rights due diligence process is aligned with the UN Guiding Principles on Business and Human Rights and the OECD Guidelines for Multinational Enterprises (see section 3.3 'Workers in the value chain' on page 89).
•Strict prohibition of bribery and corruption, and adherence to relevant
laws and industry codes. Our robust anti-corruption programme comprises internal audits, training for employees and business partners as well as due diligence around third-party representatives (see section 4.1 'Business conduct' on page 91).
•Managing our tax affairs responsibly, while complying with both the letter
and spirit of the law. We are committed to tax transparency and comply with applicable tax regulations.
•Valuing fair competition and complying with laws governing relationships
with suppliers, customers, and competitors. Employee awareness of competition laws is promoted, and all business practices are aligned with these regulations.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Environment / 2.6 EU Taxonomy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Novo Nordisk adjusted EU Taxonomy overview1
(see section 5, tables 4 a-c for the mandatory reporting templates)
|
Turnover |
|
CapEx1 |
|
OpEx |
|
|
2024 |
|
2024 |
|
2024 |
| Environmental objective |
Economic activity |
(mDKK) |
(%) |
|
(mDKK) |
(%) |
|
(mDKK) |
(%) |
| Total Turnover, CapEx, OpEx |
|
290,403 |
100 |
|
57,720 |
100 |
|
39,933 |
100 |
Taxonomy-non-eligible activities |
|
0 |
0 |
|
18,698 |
32 |
|
38,014 |
95 |
| Climate change mitigation |
7.1 Construction of new buildings |
0 |
0 |
|
13,050 |
23 |
|
0 |
0 |
|
7.2 Renovation of existing buildings |
0 |
0 |
|
2,336 |
4 |
|
0 |
0 |
| Pollution prevention and control |
1.2 Manufacture of medicinal products |
290,403 |
100 |
|
20,142 |
35 |
|
1,919 |
5 |
Eligible not aligned |
|
290,403 |
100 |
|
35,528 |
62 |
|
1,919 |
5 |
Eligible and aligned |
7.1 Construction of new buildings (in Hillerød and
Kalundborg, Denmark)
|
0 |
0 |
|
3,494 |
6 |
|
0 |
0 |
1. Excluding impact of Catalent acquisition. With the inclusion of Catalent, total CapEx would have been DKK 123,972 million, eligible not aligned CapEx 29% and eligible and aligned CapEx 3%. |
Contextual information about the KPIs
In 2024, no additional economic activities were added to the reporting scope and no changes were made to the data collection process. As a result of our Taxonomy screening process:
•Turnover in 2024 was 100% Taxonomy-eligible, but 0% Taxonomy-aligned.
•CapEx in 2024 was 68% Taxonomy-eligible (32% including Catalent), hereof
6% eligible and aligned (3% including Catalent) under economic activity 7.1 'Construction of new buildings'.
•OpEx in 2024 was 5% Taxonomy-eligible, but 0% Taxonomy-aligned.
We consider all Novo Nordisk’s turnover Taxonomy-eligible under economic activity 1.2. Taxonomy-eligible CapEx includes only CapEx directly associated with the manufacturing process or related to construction or renovation of buildings; intangible assets are therefore excluded. This is the main reason
for reported Taxonomy-eligibility under economic activity 1.2 being less than 100%. Eligible CapEx mainly relates to the expansion of production capacity
and additions to property, plant and equipment, as per note 3.2 'Property,
plant and equipment' on page 115 in the Consolidated financial statements.
Eligible OpEx includes R&D directly linked to the manufacturing processes.
As a result, only R&D costs from Chemistry, Manufacturing and Control Development & Scaling (CMC) are counted as Taxonomy-eligible OpEx.
Taxonomy reporting templates in accordance with Article 8 of Commission Delegated Regulation (EU) 2021/2178, as amended by the Taxonomy Environmental Delegated Act (Commission Delegated Regulation (EU) 2023/2486) can be found in section 5, tables 4 a-c.
We do not carry out any activities relating to the generation of nuclear energy and fossil gas as per delegated regulation 2022/1214 and hence consider these economic activities not applicable. Novo Nordisk purchases fossil gas for own production processes (see section 2.1 'Climate change' for further details).
Moreover, the EU Taxonomy Regulation and the related Delegated Acts contain formulations and terms that are still subject to considerable interpretation uncertainty and for which clarifications have not yet been published in every case. Due to the inherent risk that undefined legal terms can be interpreted differently, the legal compliance of the interpretation is subject to uncertainty (we refer to the section 'Forward-looking statements' on page 35 for further details).
ACCOUNTING POLICIES
The Taxonomy KPIs include all fully consolidated companies of the Novo Nordisk Group. The CapEx resulting from the acquisition of Catalent in December 2024 (see section 1.2 ‘Basis for preparation of the Sustainability statement’ on page 49) is recognised accordingly in the Taxonomy tables in section 5 in order to reconcile this with the financial reporting.
Total Turnover
Total revenue from sale of goods, as defined under IFRS Accounting Standards (see note 2.1 'Net sales and rebates' on page 107 in the Consolidated financial statements). The turnover KPI is defined as Taxonomy-eligible turnover divided by total turnover.
Capital expenditures (CapEx)
Additions to fixed assets (including finance leases) and intangible assets. Additions resulting from business combinations are also included. Goodwill is not included in CapEx as it is not defined as an intangible asset in accordance with IAS 38. The CapEx KPI is defined as Taxonomy-eligible CapEx divided by total CapEx (see notes 3.1 'Intangible assets' on page 113 and 3.2 'Property, plant and equipment' on page 115 in the Consolidated financial statements).
Operating expenses (OpEx)
Direct non-capitalised costs that relate to R&D (see note 2.3 'Research and development costs' on page 110 in the Consolidated financial statements), building renovation, short-term leases, maintenance and repairs, and any
other direct expenditures relating to the day-to-day servicing of property,
plant and equipment. OpEx excludes amortisations and impairments. The
OpEx KPI is defined as Taxonomy-eligible OpEx divided by total OpEx.
None of our activities contribute to multiple environmental objectives, and so
no disaggregation of KPIs is required. For the allocation of Turnover, CapEx
and OpEx we have identified the relevant income, purchases and measures,
and we have identified the primary related economic activities in the Climate Delegated Act. In this way, we ensure that no activity is double-counted. We are adjusting the R&D cost for amortisations. This is in order not to double count these costs, as the amortisation would have been part of CapEx in prior years. Moreover, there are no updates or restatements performed in 2024 for the information reported in 2023.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social information / 3.1 Patient protection and quality of life |
|
3. Social
3.1 Patient protection and quality of life1
Novo Nordisk's purpose is to drive change to defeat serious chronic diseases, and our efforts to make our innovative medicines accessible to patients throughout the world are associated with material impacts, risks and opportunities.
Material impacts, risks and opportunities (IROs)
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Improving quality of life through medicines |
|
•Downstream
|
Potential new discoveries to serve patient needs |
|
•Own operations
•Downstream
|
From discovery and clinical trials through to the production and sale of our innovative products, Novo Nordisk has material positive impacts on the lives of patients. With our investments to become a broader cardiometabolic-focused company, our efforts to further raise the innovation bar to tackle global health challenges creates potential new opportunities to help patients.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Reducing and preventing serious chronic diseases |
|
•Downstream
|
To live up to our purpose, our social sustainability roadmap includes prevention efforts to help reduce the global health burden, with potential positive impacts when improving urban health for vulnerable communities. We especially focus on children to bend the obesity and diabetes curves, with the aim of having long-term health impacts and improving the resiliency of healthcare systems.

Positive impact

Negative impact

Opportunity

Risk
1. Includes ESRS topics related to S4: ‘Consumers and end-users’ and entity-specific topics such as prevention of serious chronic diseases and falsified medicines.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
|
Health equity for vulnerable patients and in
clinical trials
|
|
•Own operations
•Downstream
|
Potential reputational risks related to access efforts |
|
•Own operations
|
As part of our social sustainability roadmap, Novo Nordisk has a positive impact when we help tackle growing health disparities, by strengthening capacity and increasing access to affordable care globally. We focus our efforts on vulnerable patients and children with serious chronic diseases, especially in low- and middle-income countries. In addition, we support accessibility through our clinical trials efforts. Global access and affordability challenges persist, which is considered a material reputational risk to Novo Nordisk. Due to different healthcare systems, medicines to which patients have access and the price they are charged vary significantly. We continue to collaborate with relevant stakeholders to ensure widespread, affordable access.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Safe clinical trials and product quality and safety |
|
•Own operations
•Downstream
|
Protection against falsified medicines |
|
•Downstream
|
Ensuring safe clinical trials and the efficacy, safety and optimal use of our
products is fundamental to everything we do. Patient safety is therefore a top priority, in order to mitigate any adverse health impacts and risks related to
our products or clinical trials. In addition, we fight against falsified medicines related to our products in the market, in order to keep patients safe against
any serious adverse health effects that may be caused by using illegally manufactured products.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Protecting clinical trial and patient information |
|
•Own operations
•Downstream
|
We strive to protect clinical trial and patient information, ensuring patient privacy, responsible product communication and correct labelling of our medicines or devices, to mitigate any adverse health-related consequences for our patients.
We consider insights from patients and patient organisations to be vital for the continued improvement of products, treatment and care, and adhere to applicable laws and human rights governing these interactions to limit any negative impacts.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Potential reputational and regulatory risks |
|
•Own operations
|
Any failure to protect patients is not only a material negative impact, but also a risk to Novo Nordisk's business and reputation. We therefore do not compromise on product quality or patient safety.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social information / 3.1 Patient protection and quality of life |
|
General process for patient engagement
The exchange of information and insights with patients and patient organisations is vital for Novo Nordisk’s continued improvement of research, products, treatments, and care. We support patient empowerment, guided by our Patient Voice Strategy, and collaborate with patient organisations to improve prevention, treatment, and access to quality care for people living with serious chronic diseases. Our Patient Voice Strategy aims to directly benefit our trial participants and patients, while
also informing our corporate and therapy area strategies. We are currently implementing our Patient Voice Strategy across all product development projects.
Patient engagement takes place either directly with individual patients or
with their caregivers, healthcare professionals, experts and relevant patient organisations as their legitimate representatives, in compliance with relevant laws and regulation. Engagement occurs before, during and after the launch
of our products, with the frequency of engagement dependent on the stage
of development and the type of treatment or product. Relevant patient populations are selected based on the insights needed and various channels
are used for our patients to express thoughts and concerns, for example through patient advisory boards, workshops, and surveys.
The engagement process is owned by our two chief patient officers, who have overall responsibility for ensuring that the needs and perspectives of patients and care partners are incorporated into our decision-making processes.
Channels to raise patient safety concerns
We routinely monitor the safety and quality of all our products by reviewing safety data from clinical trials, reported side effects and quality complaints.
By monitoring the quality and safety of our products, we can take timely and appropriate actions to safeguard patient safety and fulfil our reporting obligations to health authorities under relevant legislation.
Patients can use our publicly available portals for any issues in relation to Novo Nordisk products, including product complaints or if they wish to report a side effect or falsified products. In addition, all affiliates have call centres operated in the local language where patients and healthcare professionals can get help in relation to a Novo Nordisk product.
The channels are tracked by our Customer Complaints Department and Global Safety Department, which records, investigates and responds to customer complaints globally concerning the quality, labelling, durability, reliability, effectiveness, safety, performance or malfunction of Novo Nordisk’s products and reports these to health authorities in accordance with applicable legislation. Depending on the nature of the information received, there are strict timelines for when to escalate the matters for further investigation. All personal
information related to the reporting of a product complaint or side effect is processed in accordance with data protection legislation.
When necessary, we recall products affected by a safety or quality issue, update labels for marketed products, or communicate directly with affected patients, healthcare professionals or health authorities, informing them about product safety risks.
Innovation
Policies
Novo Nordisk's purpose is to bring innovative medicines to help the millions
of people worldwide living with serious chronic diseases. Our decades-long commitment to the development of GLP-1-based medications is not only reshaping diabetes and obesity management but also opening potential new avenues of treatment for cardiovascular, kidney, liver, and Alzheimer's diseases. Our innovations help improve quality of life for patients and at the same time, reduce the cost of hospitalisations for healthcare systems.
To ensure that we impact society positively through our innovations, patients are at the centre of everything we do. Many of our policies therefore encompass our patient-related efforts. Our OneCode policy outlines the key requirements with respect to how we act across all our policies and standard operating procedures, including how we provide medicines in a responsible way, engage with all patients and adhere to high quality standards to advance the quality of care. Every year, all employees must confirm they have read and understood OneCode. We respect the human rights of patients, in accordance with our Human Rights Commitment further described in section 3.3 ‘Workers in the value chain’ on page 88.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key action to address sustainability-related innovation |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and
how we track effectiveness
|
|
|
Integration of sustainability in
product development
|
Ongoing integration of sustainability in product development and product-related governance. The framework assesses the social and environmental profile across the product's lifecycle to support development decisions and identify areas of improvement across product portfolio. |
40+ products, including R&D pipeline and marketed products |
No |
•Piloted in five products across five different product development stages and five different therapy areas to test the robustness and applicability of the framework.
•Rolled out the framework for +80% of the products in scope and will continue the implementation in 2025.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Policy |
|
OneCode |
|
Purpose |
|
Guide on how to act as a company and as individuals |
|
Scope |
|
Everyone employed by or working on behalf of
Novo Nordisk
|
|
Most senior level accountable |
|
Executive Management |
|
Availability |
|
Externally available: OneCode |
|
Applicability across Sustainability statement |
|
•Patient protection and quality of life, page 71
•Own workforce, page 80
•Business conduct, page 90
|
|
|
Supporting policy documentation |
|
•Position papers (Access to diabetes care and medicine pricing, clinical trials ethics, falsified medicines)
•Principles (Data and Al ethics, processing of personal data)
•Standard operating procedure
|
|
|
|
|
|
Actions
For an overview of opportunities to accelerate healthcare innovation across Obesity, Diabetes, Rare Diseases and Cardiovascular & Emerging Therapy Areas, see section 'Innovation and therapeutic focus' on page 17. The action table provides an overview of other, sustainability-linked innovation efforts. We will continuously assess relevant sustainability performance indicators to include in future disclosures.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.1 Patient protection and quality of life |
|
Prevention of serious chronic diseases
Policies
Novo Nordisk invests in primary prevention of serious chronic diseases, with
a focus on early prevention in childhood and targeting vulnerable populations such as socially disadvantaged communities. As a gateway to other chronic diseases, we have a specific focus on prevention of obesity.
While we do not have a formal policy related to prevention, we
have integrated our prevention activities into our therapy area strategies,
as part of our social responsibility.
Actions
To implement our social responsibility within primary prevention, we take a
multi-level approach through partnerships, working in urban environments
to address issues such as nutrition, education and physical activity. We will continuously assess relevant sustainability performance indicators to include
in future disclosures.
Sustainability-related costs are integrated in our ongoing business. In addition we set aside further dedicated funding across our social sustainability roadmaps related to both prevention and access to deliver on our global health equity ambitions (see also next section). Combined, we have further committed DKK 1.3 billion in investments in 2024 and 2025, excluding donations and other contributions that we report on in table 3.1.3. We will continuously assess needed investments as part of our financial planning processes. Extended value is also created through grants to health, sustainability and the life science ecosystem, via our unique ownership structure, by the Novo Nordisk Foundation, our majority shareholder through Novo Holdings A/S.
Social inclusion of patients
ACCESS TO MEDICINES
Policies
Health inequity is a global challenge, with overburdened healthcare systems
facing growing pressure to deliver quality care while managing costs, disproportionately affecting people in resource-poor settings. To help drive positive impacts for our patients, Novo Nordisk has a commitment to help improve patient access and affordability. Our publicly available position papers further detail how we work with our social responsibility.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key actions to address prevention |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we
track effectiveness
|
|
|
Partnership with UNICEF |
Partnership with UNICEF to prevent childhood overweight and obesity by building healthy environments that enable and empower children to eat well and be active. Partnership runs until 2026. |
Primary focus is on Brazil, Colombia, Mexico,
and Indonesia
|
No |
•Roll-out of programmatic activities by UNICEF,
for example strengthening nutrition education in schools and promoting use of food labelling.
•UNICEF evaluates effectiveness against KPIs
covering both indirect and direct impacts of the programmatic activities and report these in a publicly available report.
|
|
|
Cities for Better Health |
Global network of prevention partnerships at city level, addressing three core challenges to drive better health in cities: healthy food, physical activity and sustainable financing models to ensure ongoing funding. |
51 cities across the world |
No |
•In 2024, a new childhood obesity prevention initiative, The Childhood Obesity Prevention Initiative (COPI), was launched in six cities across Brazil, Canada, France, Japan, South Africa and Spain to accelerate the prevention of childhood obesity in disadvantaged urban communities.
•In addition, 15 affiliates received technical and financial support to start local prevention initiatives.
•A monitoring and evaluation framework is used to assess improved health-related outcomes.
|
|
|
Transformational Prevention Unit (TPU) |
Develop scientific and scalable commercial solutions that predict and pre-empt obesity and its consequences for those at greatest risk. Established in 2023, the TPU is committed to building multi-sector partnerships with the ambition to support overall prevention efforts with substantial societal value, including socially disadvantaged groups. |
Individuals globally with higher risk of obesity |
No |
•Combining scientific insights with clinical, as well as public health data, with the aim to develop tailored and targeted interventions that meet specific individual and societal needs.
•The tailored approach aims to enable earlier and more accessible prediction of health and disease, improving patient outcomes and minimising unnecessary treatments and reducing healthcare costs.
|
|
Our position on access to diabetes care advocates for equal rights and accessibility to healthcare for all, as stated in the UN Universal Declaration of Human Rights. The position outlines our commitment to overcoming the barriers to effective diabetes care in low- and middle-income countries, including limited healthcare capacity and unreliable supply of medicine and equipment.
Our position on medicine pricing outlines how pricing should reflect the medicine's value to patients, society and the healthcare system. This includes multiple factors such as the medical need the product meets for clinicians and patients and how the clinical profile improves the patients' short- and long-term health outcomes and quality of life. Other factors include contracting, pricing and reimbursement system of a given country. Each country has its own healthcare system, which can provide patients with different medicines at different costs. We acknowledge global affordability challenges, including in
high income countries, and collaborate with policymakers and health authorities to help develop solutions to ensure affordable access for all patients.
Actions
In support of our position statements, several action plans are underway,
as outlined in the table, in order to overcome barriers to effective care for vulnerable patients, including collaborating with external partners to improve access and affordability, enhancing supply chains and improve healthcare capacity. In the US, Novo Nordisk provides rebates and sales discounts to insurance companies and other payers to secure coverage for commercially and government-insured patients. For vulnerable patient populations, we offer low-cost or no-cost programmes. Unless otherwise indicated, actions are considered recurring. Across all actions, patients reached is considered a key performance indicator to ensure progress and effectiveness of our efforts.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.1 Patient protection and quality of life |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key actions to address access to medicines |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we track effectiveness |
|
|
Ringfenced volumes Wegovy® |
A proportion of Wegovy® volumes in each launch market is ringfenced for access pathways such as public reimbursement, public institution purchase or other patient access and support programmes. The focus is to improve health equity and provide affordable care. |
People with high medical need and low socioeconomic status |
No |
•Seven countries have agreements in place for access pathways such as public reimbursement, individual reimbursement or private insurance: UK, Japan, Switzerland, Qatar, Iceland, Norway, and Canada. |
|
|
Access to Insulin Commitment |
A ceiling price of USD 3 per vial in low- and middle-income countries around the world and
USD 2 per vial for organisations providing relief in humanitarian settings.
|
77 low- and middle-income countries |
No |
•The patients reached through the Commitment are a part of the overall metric on vulnerable patients with diabetes reached through Novo Nordisk Diabetes care products in 2024. |
|
|
Changing Diabetes®
in Children
|
Public-private partnership founded in 2009 to provide diabetes care to children and youth with type 1 diabetes living in low and middle-income countries. This includes free life-saving medicine, blood glucose monitoring equipment and medical supplies for young people under the age of 25. |
30 countries across Africa, Middle East, Asia and South America |
Yes |
•An accumulate 64,743 children and youth have been reached through Changing Diabetes®
in Children.
•Patient education and healthcare capacity-building supported across more than 500 clinics.
|
|
|
iCARE business model |
Improve access to diabetes care to vulnerable populations. Implementation is integrated in affiliates' business strategies and targets through four main building blocks of health equity focused diabetes management: capacity, affordability, reach, and empowerment. |
49 countries in Sub-Saharan Africa, and Indonesia |
No |
•Expansion of iCARE business model to Indonesia.
•Served 433 thousand people with diabetes with insulin and trained 3,778 healthcare professionals through capacity building programmes through partnerships.
•We assess effectiveness together with our partners.
|
|
|
Human Thermal Solution (HITS) |
New flexible storage options for two Novo Nordisk human insulin products: Actrapid® and Insulatard®, making Novo Nordisk the first insulin manufacturer to introduce flexible storage options for people with diabetes in settings where refrigeration is a challenge. |
All countries where Actrapid® and Insulatard® are launched |
No |
•38 countries have received approvals of label update.
•Expanding to all countries where Actrapid® and Insulatard® are launched depends on country approvals.
|
|
|
Access Innovation Incubator |
Identification of new and innovative solutions to support people with diabetes. Solutions include a global partnership with MedtronicLABS to scale a digital patient pathway for diabetes management in three African countries. Our Senselet partnership in Ethiopia strengthens supply chain capacity through higher education and on-the-job training. |
Ghana, Kenya, Rwanda, Ethiopia |
No |
•To date, the MedtronicLABS partnership has supported the enrolment of approximately 22,380 patients across 27 reference centres in three African countries.
•To date, Senselet has supported more than 1,000 front-line workers and 900 academics to receive training in healthcare supply chain management.
•From 2025 onwards, these initiatives will be transitioned and integrated into the iCARE business model to ensure alignment with local and regional strategies and activities.
|
|
|
Collaboration with World Diabetes Foundation |
Donations to the independent and non-profit foundation, World Diabetes Foundation (WDF), to improve diabetes care by strengthening national health systems as well as primary prevention. |
Low and middle-income countries |
No |
•In 2024, donations to World Diabetes Foundation (WDF) reached DKK 120 million.
|
|
|
Partnering for Change programme |
Public-private knowledge-partnership between the International Committee of the Red Cross, London School of Hygiene & Tropical Medicine and the Danish Red Cross to address the growing need for chronic disease treatment for people in humanitarian crisis areas. |
Lebanon and Iraq |
No |
•11 peer-reviewed research publications, informing humanitarian efforts.
•Support handbook with guidance to patients in times of crises where continuity in care is disrupted.
•The partnership is ending in 2024 but the Red Cross is staying on the ground despite the escalating crises in Lebanon. New commitment is in development.
|
|
|
Affordability programmes in the US |
Creating comprehensive, affordable patient access by focusing our efforts on key levers:
•Ensure affordable access to Novo Nordisk products to address challenges within the complex US healthcare system.
•Increase product access among low-income population and/or individuals with disabilities through Medicaid.
•Continue to offer programmes to maintain insulin affordability.
•Support vulnerable patient populations with free products across Diabetes and Rare Disease portfolios through Novo Nordisk’s patient assistance programmes.
|
United States |
No |
•In 2024, 80% of US patients with insurance coverage for Ozempic® or Wegovy® paid USD 25 or less for each prescription, and almost 90% of US patients paid USD 50 or less.
•Substantially increased access to Wegovy® for Medicaid eligible patients with lower incomes and/or disabilities, which now accounts for 10% of Novo Nordisk’s US Wegovy® sales.
•Upheld 5+ year commitment to provide diverse insulin affordability support options, including MyInsulinRx™ programme, unbranded biologic and human insulin treatment options.
•Continued commitment of long-standing patient assistance programme to support eligible patients.
•Visit novocare.com for more information on our affordability programmes.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.1 Patient protection and quality of life |
|
Performance
Performance on patients reached
To track our impact worldwide we monitor the number of patients reached
with Novo Nordisk's Diabetes and Obesity care products. The total number of patients treated with our Diabetes products increased 6% from 40.5 million
in 2023 to 43 million in 2024. The development was primarily driven by the increase in Diabetes GLP-1-based products. We also increased the number of patients reached with Obesity treatments from 1.1 million in 2023 to 2.2 million in 2024. The 100% increase was primarily driven by the launch of Wegovy® in +10 further countries in 2024. In addition to acquiring production sites in 2024, we are currently expanding and expect to see the effect of this on the number of patients reached in the future.
Moreover, in 2024 we applied a new methodology to the number of vulnerable patients reached with Novo Nordisk's Diabetes care products. Due to different methodologies applied, vulnerable patients reached with Diabetes care products are not fully to be considered a portion of overall patients reached.
In 2024, the number of vulnerable patients treated with our Diabetes care products decreased 5% from 8.8 million in 2023 to 8.4 million in 2024. This decrease was driven by fewer vulnerable patients reached through human insulin tender sales and access and affordability initiatives.
Performance on children reached through Changing Diabetes® in Children
To track progress on our programme Changing Diabetes® in Children (CDiC),
we have a target to reach 100,000 children and young people living with type 1 diabetes by 2030, starting from the inception of the programme in 2009. By the end of 2024, a total number of 64,743 children were reached. The countries in scope that contribute to reaching the target are the 30 partner countries of CDiC. The target was set by using international estimations, from the International Diabetes Federation (IDF), of number of children living with type 1 diabetes in low and middle-income countries. As part of the partnership agreements with local implementing partners, project milestones are set with the aim to improve diabetes care. The progress to reach the target is monitored quarterly and through annual reports received from local implementing partners.
Performance on donations
We have slightly increased our donations and other contributions to the World Diabetes Foundation in accordance with the donation agreement. With regards to the Novo Nordisk Haemophilia Foundation, we have increased our donation from DKK 19 million in 2023 to DKK 26 million in 2024 to support ongoing projects.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1.1 Patients reached with Novo Nordisk's products |
Unit |
2024 |
2023 |
2022 |
|
Patients reached with Novo Nordisk's Diabetes and Obesity
care products
|
Number in millions |
45.2 |
41.6 |
36.9 |
| Patients reached with Novo Nordisk's Diabetes care products |
Number in millions |
43.0 |
40.5 |
36.3 |
| Patients reached with Novo Nordisk's Obesity care products |
Number in millions |
2.2 |
1.1 |
0.6 |
Vulnerable patients reached with Diabetes care products1 |
Number in millions |
8.4 |
8.8 |
– |
1. 2023 figure for Vulnerable patients reached with Diabetes care products has been restated from
6.7 millions.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1.2 Changing Diabetes® in children |
Unit |
2024 |
2023 |
2022 |
Children reached through the Changing Diabetes® in children programme |
Number |
64,743 |
52,249 |
41,033 |
2030 target |
Number |
100,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1.3. Donations and other contributions |
Unit |
2024 |
2023 |
2022 |
| Total donations and other contributions |
mDKK |
146 |
138 |
126 |
| World Diabetes Foundation (WDF) |
mDKK |
120 |
119 |
93 |
| Novo Nordisk Haemophilia Foundation (NNHF) |
mDKK |
26 |
19 |
33 |
ACCOUNTING POLICIES
Patients reached with Novo Nordisk's Diabetes and Obesity care products
Estimated by dividing Novo Nordisk's annual sales, samples and donations volume by the annual usage dose per patient for each product class, as defined by the WHO (for Diabetes) or in accordance with the dose strength of the product (for Obesity). Devices are excluded. Methodology has been changed compared to previous years as samples and donations have been added in 2024. The impact on the comparative figures is deemed immaterial and hence no restatement has been made.
Vulnerable patients reached with Novo Nordisk's Diabetes care products
Defined as a patient who received Novo Nordisk Diabetes care products either through products sold under local affordability thresholds, based on World Bank data and local healthcare expenditures, or public tenders in low-, lower middle- or upper middle- income countries (LMICs) as defined by the World Bank, or through specific diabetes access and affordability programmes or humanitarian donations. Vulnerable patients are estimated by using two methods: firstly, reach of one vulnerable patient is defined as sales volume in LMICs corresponding to an annual drug usage dose per patient as defined by WHO through public tender sales, products sold under affordability thresholds, or humanitarian donations and for vulnerable patients reached in the US through products supplied in select programmes. Secondly, for US access and affordability programmes, reach of one vulnerable patients is defined at the time of enrolment based on patient programme reports.
Children reached through the Changing Diabetes® in Children programme
Estimated as the total accumulated number of children and youth enrolled since the initiation of the partnership in 2009. Children participating for multiple years are only included once in the year of enrolment. Children and youth are defined as 0-25 years old and living in poverty as defined by the World Bank.
Donations and other contributions
The monetary donations from Novo Nordisk to the World Diabetes Foundation (WDF) and the Novo Nordisk Haemophilia Foundation (NNHF) are recognised when the donation or contribution is paid out.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.1 Patient protection and quality of life |
|
ACCESS TO CLINICAL TRIALS
Policies
In accordance with our OneCode policy, we believe health equity is central to
the development of new treatments, as everyone should have access to medical products regardless of demographics, underlying diseases or social factors.
We focus our efforts on promoting diversity, equity and inclusion (DE&I) in clinical trial conduct to ensure that scientific data are representative of the patient population and have internal procedures in place to support such efforts. We acknowledge that accomplishing this demands tailored solutions specific to each trial programme.
Actions
To further advance our health equity efforts in clinical trial conduct, we focus our efforts on implementing DE&I considerations and decentralised trial elements
(DCT) as outlined in the table. Unless otherwise indicated, actions are considered recurring.
Global efforts to enhance DE&I in clinical trials are led by an expert function in our Global Clinical operations area. Local efforts focusing on US activities are driven by our North American Organisation. However, DE&I efforts impact all functions across the value chain involved in the design, planning and execution of clinical trials. We will continuously assess relevant sustainability performance indicators to include in future disclosures.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key action to address access to clinical trials |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we
track effectiveness
|
|
|
Promotion of DE&I in clinical trials |
Framework for integrating DE&I in clinical trial planning and execution to ensure that clinical trial participants are representative of the patient population.
|
Global trials on a fit-for-purpose basis |
No |
•Current focus has primarily been on US population.
•Global actions include launch of a training programme in order to upskill relevant staff.
•We participate in a public-private partnership (IHI READI) that aims to improve representation and inclusion in clinical research.
•Overall effectiveness is assessed on an ongoing basis and we continuously assess how to optimise internal processes, including technology and data capture.
|
|
|
Integration of decentralised trials
(DCT) elements
|
Integrating DCT elements to help improve access
to clinical trials, a more diverse pool of participants and a higher retention rate, for example by
allowing assessments to be conducted at patients’ preferred location.
|
Global trials on a fit-for-purpose basis |
No |
•In 2024, over two thirds of our active phase 2-3 clinical trials included one or more DCT elements.
•We actively engage with regulatory bodies, clinical research sites and patient advocacy groups to address the various barriers for implementing DCT elements in clinical studies.
|
|
Patient safety
PRODUCT QUALITY AND SAFETY
Policies
Every day, people rely on the quality and safety of our products. Various systems and standard operating procedures help us to safeguard this, including Novo Nordisk’s quality management system, which ensures we adhere to the highest quality standards and mitigate negative impacts and risks related to the safety of patients, for our authorised medical products and devices.
Furthermore, our global pharmacovigilance system collates all safety information to monitor the safety of our products and devices and ensure that we meet all regulatory requirements to protect the safety of patients and clinical trial participants. The pharmacovigilance system has three key processes:
1) safety data collection, 2) data analysis and evaluation, and 3) routine reporting to health authorities and communication with relevant parties.
The Global Head of Safety is responsible for the global pharmacovigilance system, ensuring that all relevant safety data reported to Novo Nordisk on investigational,
authorised, and that marketed pharmaceutical products and medical devices are recorded, evaluated, and collated for surveillance and reporting.
CLINICAL TRIAL SAFETY
Policies
All Novo Nordisk’s clinical trials and clinical research activities are governed
by national laws and international conventions2 as described in our publicly available position on clinical trials ethics and are integrated into our standard operating procedures to ensure safe global clinical research activities.
Special consideration is given to vulnerable patient populations, including children and the elderly. If clinical research involves vulnerable patients, it is always evaluated whether the study should have an external Data Safety Monitoring Board to ensure independent safety review of the study. To ensure that our medicinal products and formulations are safe and effective for a paediatric population, we develop paediatric plans as required by the
European Medicines Agency, the UK Medicines & Healthcare Products Regulatory Agency, and the FDA and Good Clinical Practice Guidelines. We conduct paediatric clinical trials with minimal disruption and interference with the children’s and their families’ daily lives.
2. Including The Declaration of Helsinki, the International Conference on Harmonisation Guideline for Good Clinical Practice, Good Pharmacoepidemiology Practices, the Nuremberg Code, the UN Guiding Principles on Business and Human Rights, the Belmont Report and UNESCO’s Universal Declaration on Bioethics and Human Rights.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.1 Patient protection and quality of life |
|
Our internal, multidisciplinary Paediatric Expert Group offers guidance to aid such principles. Additionally, processes for seeking informed consent from a minor or their legally authorised representative must take place in accordance with local regulations and Novo Nordisk’s instructions.
Actions
The safety and quality of our products and clinical trials are prerequisites to Novo Nordisk's operating model. We routinely monitor the safety and quality by adhering to all relevant procedures and regulations.
Patient safety is managed through our quality management and pharmacovigilance systems with the involvement of various internal functions. The pharmacovigilance system is owned and operated by the Global Patient Safety Department. There is close cooperation and alignment with the regions and affiliates, each responsible for managing matters related to local pharmacovigilance. Our quality management system is operated by the Global Quality organisation with the system applicable throughout the product development process, from R&D activities to production sites and across affiliates.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key actions to address quality and safety |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we
track effectiveness
|
|
|
Mitigating risks related to product safety and quality signals |
Monitoring and using safety information from patients to take timely and appropriate action to improve product quality and safety. Outcomes are monitored in our risk management system and a
risk management plan is prepared in accordance with regulatory requirements.
|
All Novo Nordisk products with marketing authorisation
and products in Novo Nordisk sponsored
clinical trials
|
No |
•A safety committee with members from all relevant functional areas is established prior to any clinical investigation of a new pharmaceutical product or medical device, providing assessments of safety data throughout the product or device’s life cycle.
•Number of product recalls and failed inspections are reported to track effectiveness. In 2024, we tracked three product recalls and 0 failed inspections.
|
|
|
Mitigating safety risks
in clinical trials
|
Detailed protocol for each clinical research activity based on scientific methodology and ethical considerations, to be approved by an Independent Ethics Committee, Institutional Review Board or other appropriate bodies, as well as by regulatory authorities prior to study start. |
All Novo Nordisk sponsored clinical trials |
No |
•Relevant safety information is continuously assessed during Novo Nordisk-sponsored clinical research activities and appropriate actions taken if risks outweigh potential benefits. In the event of any clinical research-related injury, participants are compensated according to domestic laws. |
|
Performance
To manage impacts and risks related to product safety and quality signals, Novo Nordisk tracks the number of product recalls. In 2024, we had three product recalls, due to cracked cartridges of insulin products in South Africa, underfilled vials in the Czech Republic and incorrect labelling of products in clinical trials. None of the recalls had serious adverse health consequences.
Furthermore, we actively monitor inspections to ensure compliance with health inspection requirements. In 2024, 180 inspections were conducted, and we did not fail any inspections. At year-end, 144 inspections were passed and 36 were in-progress, as final inspection reports had not yet been received, or the final authority’s acceptance was pending. Follow-up on in-progress inspections will continue in 2025.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1.4. Product recalls and
failed inspections
|
Unit |
2024 |
2023 |
2022 |
| Product recalls |
Number |
3 |
2 |
3 |
| Failed inspections |
Number |
0 |
0 |
0 |
ACCOUNTING POLICIES
Failed inspections
Inspections where FDA warning letters or European Medicines Agency non-compliance letters related to Good Medical Practice inspections are received, Good Medical Practice/ISO certificates for strategic sites are lost, pre-approval inspections result in a complete response letter, study conclusions are changed due to Good Clinical Practice/Good Laboratory Practice inspection issues, or marketing or import authorisations are withdrawn due to inspection issues. Strategic sites are defined as the manufacturing sites in Brazil, China, Denmark, France, and the US. Acquired companies' inspections are defined as inspections run by the acquired company. Inspections at acquired companies run by Novo Nordisk are reported as Novo Nordisk inspections.
Product recalls
Number of times Novo Nordisk has instituted a recall of a product from the market due to patient safety reasons, including recalls in connection with clinical trials. A recall may affect multiple countries.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.1 Patient protection and quality of life |
|
FALSIFIED MEDICINES
Policies
Falsified medicines are a global problem that pose severe risk to public health
and patient safety worldwide. These illegally manufactured products may contain the wrong or incorrectly dosed active pharmaceutical ingredients or harmful substances leading to serious adverse effects. Falsified products are at an all-time high, driven by the surge in demand for weight-loss drugs and injectable products. Novo Nordisk helps investigate suspected cases of pharmaceutical crime and takes a proactive approach to managing negative impacts and risks to patients.
The handling of suspected falsified Novo Nordisk products is outlined in our OneCode policy and covered in our quality management system and standard operating procedures, supporting the monitoring, signal detection and reporting
to health authorities of alleged occurrences. Further details can be found in our publicly available position on falsified medicines.
Novo Nordisk detects falsified medicines through reported product complaints from patients, healthcare professionals and authorities, as well as through field- and online surveillance by investigative firms. Specialised security services are used to conduct investigations, test purchases and for decommissioning. With the support of local investigation firms, we also perform market searches to help health authorities locate and seize falsified products. We collaborate with authorities in over 20 countries, including Europol and Interpol, to support and facilitate the detection of falsified medicines.
Global responsibility for product protection lies with the Head of Global Security who leads our efforts to protect key products and patents.
Actions
To further prevent falsified or mislabelled medicines entering the pharmaceutical distribution system, Novo Nordisk pursues targeted actions as outlined in the table. Unless otherwise indicated, actions are considered recurring.
Resources for our product protection programme lie with Global Security, however, the programme is implemented globally at regional and affiliate-level, with more extensive efforts being conducted in high-risk markets. To ensure the continued effectiveness of our actions, Novo Nordisk is a member of the Pharmaceutical Security Institute, which fights falsified medicines worldwide. We will continuously assess relevant sustainability performance indicators to include in future disclosures.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key actions to address falsified medicines |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we
track effectiveness
|
|
|
Product protection
end-to-end solutions
|
Applying relevant security features based on risk assessment and regulatory requirements, including reinforcing supply chain integrity through security specifications in distribution and warehouse contracts. |
Global operations and downstream value chain |
No |
•In 2024, we continued to review the security of our supply chain including agile testing solutions and rapid testing devices to support swift identification of falsified medicines.
•We continuously review our approach to the protective features of products, from overt to covert solutions.
|
|
|
Awareness campaigns and training |
Awareness campaigns and onboarding programme to prevent patients from buying medicines outside legitimate channels, including more information for healthcare professionals. |
Global operations and downstream value chain |
No |
•Rolled out a dedicated, onboarding programme to over 1,600 employees in relevant functions.
•Launch of awareness programme for law enforcement agencies enabling an increase in seizures of falsified medicines.
•Launch of other external awareness campaigns for patients via social media and our website.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.1 Patient protection and quality of life |
|
Information-related impacts for patients and
clinical trial participants
Policies
Data protection is integrated in our global ethics and compliance framework,
as outlined in our OneCode policy and relevant standard operating procedures. Strict data protection applies to all personal data related to patients and clinical trials and is implemented in accordance with all applicable data protection requirements. We outline how we work with data protection in our publicly available data ethics standards and personal data processing principles. Before initiating clinical trials, we ensure that participants are well informed about their privacy rights including the legitimate disclosure of data.
We disseminate scientific knowledge obtained through clinical trials for the benefit of society. Our commitment to transparency of clinical research activities is outlined in our clinical disclosure and reporting instructions, which are aligned with legal requirements and ethical principles3 and owned by the Head of Regulatory, Quality and Clinical Reporting. Through our clinical reporting and transparency efforts we ensure that results from Novo Nordisk sponsored clinical research activities are disclosed in public registers. To make research more accessible, we aim to publish Plain Language Summaries (PLS) for our phase 3 primary data publications to translate complex scientific information in an easy-to-understand format in accordance with our standard operating procedures.
Following successful completion of a clinical trial programme, we work in close collaboration with global health authorities to ensure informative and accurate product labels to guide patients’ use and outcomes of treatment. Processes for safeguarding labelling quality in the markets in which Novo Nordisk operates are outlined in standard operating procedures, which are the responsibility of Global Regulatory Affairs.
We communicate with healthcare professionals about our products to encourage informed use, so they can make the best treatment choices for
the benefit of their patient’s health. We have strict guidelines in place to only promote our products for uses that have been approved by the appropriate regulatory authority in a manner that is truthful, accurate, non-misleading, balanced and consistent with the approved product label. Off-label promotion
is prohibited as outlined in our OneCode policy.
3. Including, but not limited to, World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects.
Actions
The management of information-related impacts and risks is a prerequisite
to our operating model. We therefore routinely take action to prevent and
mitigate any information-related risks and impacts for patients and clinical
trial participants while maintaining strict adherence to all relevant regulations
and standards.
The management of information-related impacts involves various functions. Our Global Ethics and Compliance Office oversees the global data privacy agenda, together with expert functions throughout the organisation. Ensuring informative and accurate labels is a cross-functional undertaking anchored in our Global Regulatory Affairs Department, working closely with our affiliates, regions and functions engaged in global development programmes. The Clinical Reporting Department ensures transparency of clinical research activities together with colleagues across our global development programme. We will continuously assess relevant sustainability performance indicators to include in future disclosures.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key actions to address information-related impacts |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we
track effectiveness
|
|
|
Improving transparency of our Patient Information and Informed Consent
forms (PIIC)
|
Update of PIIC forms to enhance general transparency with respect to i) engaging in Novo Nordisk sponsored clinical trials and ii) how privacy rights of patients are protected. Project expected to be completed by May 2025. |
Intended for use in global clinical trials. Will be adaptable to
local deviations
|
No |
•We continuously assess improvement areas when it comes to privacy rights of patients. |
|
|
Communicating and raising awareness of informed use of our products |
All promotional materials for our respective
products undergo robust legal, medical and regulatory review processes. We continuously strengthen our guidance and communication to ensure healthcare professionals are equipped
with appropriate information about our products and the underlying clinical data to make the best decisions for patients.
|
Global
operations related to product communication
|
No |
•Training healthcare professionals on approved indications of our products and key messages around responsible use.
•Alignment with authorities to support proactive communication to emphasis the indication of
our products.
•Field forces and commercial functions in all markets have been provided with clear guidance on how only to engage in conversations for approved labels of our products.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.2 Own workforce |
|
3.2 Own workforce
As a pharmaceutical company, we depend on talented people and innovative ideas. Our workforce spans employees working at our production sites and in laboratories to sales representatives and administrative employees. In light of our current expansion, our workforce has grown substantially to help meet the surge in demand of our medicines. We have in 2024 implemented a sustainable growth strategy, moderating the pace of recruitments in order to ensure a good workplace for all employees.
Material impacts, risks and opportunities (IROs)
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Employee benefits and flexible working conditions |
|
•Own operations
|
Potential human rights incidents |
|
•Own operations
|
Novo Nordisk upholds good working conditions globally for all employees hired
on an employment contract, offering benefits and flexibility to promote employee wellbeing. Novo Nordisk has been voted a top workplace across several countries and regions as part of the Best Places to work programme, highlighting our positive impact when it comes to offering an attractive, global workplace. We do not tolerate any potential human rights violations and will manage any harm according to our procedures. Examples of human rights incidents related to own workforce in Novo Nordisk include potential incidents related to safe and healthy working conditions, right to privacy and data protection as well as employee’s rights. No specific parts
of our operations have been found to be at specific risk of forced or child labour, and most of our employees work in low-risk countries, according to the Global Rights Index.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Healthy and safe work environment |
|
•Own operations
|
When we research, produce and manufacture pharmaceutical products, we aim to operate to the highest health and safety standards, which includes ensuring that employees feel physically and mentally safe regardless of whether they work in a physically demanding or sedentary working environment. We recognise that health and safety incidents can lead to negative impacts for
those affected.

Positive impact

Negative impact

Opportunity

Risk
In 2024, we witnessed serious incidents in connection with the expansion of our production capacity which have resulted in heightened levels of safety measures being implemented across the organisation.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Equal opportunities fostering innovation |
|
•Own operations
|
To sustain an innovative work culture, Novo Nordisk has positive impacts on
our employees by continuously strengthening our efforts in diversity, equity, and inclusion to ensure that every employee can contribute, feel a sense of belonging and has equitable career opportunities. Furthermore, by offering comprehensive training and development opportunities for all, we support our employees to keep learning and growing.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Attracting talent to enable continued innovation |
|
•Own operations
|
We recognise that, as an innovation company, we are exposed to potential
risks because our business depends on attracting and retaining talent. We
are especially dependent on research and development to sustain continued innovation. The deliberate slowdown in recruitment does not affect our commitment to ensure that we can attract the right skills, experience and qualifications across our global operations.
General processes for workforce engagement
We engage with our own workforce both directly and indirectly through
multiple processes to inform our decisions. The main way we obtain direct feedback from our employees is through our yearly employee survey Evolve. The survey ensures that we continue to monitor and improve Novo Nordisk
as a workplace and all teams work actively with the results every year.
Furthermore, we engage with workers’ representatives. In Denmark, employees are represented by local unions and associations, and in European affiliates, workers’ representatives are elected by the employees. In some of our international affiliates, engagement will take place directly with employees.
The frequency of engagement varies across our operations; in Denmark, we
have scheduled dialogue meetings between management and workers’ representatives at least every quarter. Dialogue with the European Works
Council (EWC) secretariat takes place on an ongoing basis, and includes an
annual meeting with all EWC representatives. Negotiations on topics such as salary and collective bargaining agreements depend on the agreed time frame. These dialogues support us in assessing the general effectiveness of our employee-related efforts and is implemented by local People & Organisation teams and managers. In Denmark, worker's representatives are also represented at the Board of Directors, further enhancing dialogue and representation.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.2 Own workforce |
|
General process for remediation
There are multiple ways in which employees can raise workplace-related grievances and concerns, including through the local or global People & Organisation or Legal function, Business Ethics Compliance Office, Group Internal Audit, Novo Nordisk Way facilitations, the annual Evolve survey, onboarding surveys and the Ombudsman function.
Individual cases concerning unfair treatment of a particular employee
will usually be handled by the Ombudsman function. We will not tolerate discrimination or retaliation against persons who file a report or participate
in an investigation in good faith.
Employees can always report any concerns anonymously through the publicly available Novo Nordisk Compliance Hotline. The Compliance Hotline is further described in section 4.1 ‘Business conduct’, under 'Compliance Hotline and protection of whistleblowers', on page 91, including our anti-retaliation policy.
We place importance on the provision of effective remedy wherever employees’ rights have been found to have been negatively impacted.
Working conditions and other work-related rights
Policies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Policy |
|
Labour Code of Conduct |
|
Purpose |
|
Minimum labour standards for our employees |
|
Scope |
|
All Novo Nordisk employees |
|
|
Most senior level accountable |
|
Executive Management |
|
|
Availability |
|
Externally available:
Novo Nordisk Labour Code of Conduct
|
|
|
Applicability across Sustainability statement |
|
•Own Workforce, p. 80 |
|
|
Supporting policy documentation |
|
•Anti-harassment Framework |
|
|
|
|
|
Novo Nordisk’s Labour Code of Conduct1 details globally adopted minimum labour standards for our employees, to safeguard employees' rights and promote favourable working conditions to remain an attractive workplace. As detailed in
our policy, we operate in accordance with all applicable laws and regulations.
All employees are required to receive secure employment and adequate income in a standard working week to meet their basic needs, along with discretionary income. This is achieved by maintaining employee salaries and benefits above
the living wage of a given country, the statutory minimum wage given by law, prevailing industry benchmarks, or the wage negotiated in collective agreements, whichever is the highest. Periodic assessments and adjustments are made to account for changes in the cost of living and economic conditions.
We track permissible working hours to ensure adequate work-life balance. To empower employees, we offer various flexible working solutions. In line with
local business requirements, employees can apply for options such as a career break, a compressed working week or reduced working hours according to personal needs. Pay and benefits are adjusted accordingly. Employees are covered by social protection through public programmes or benefits offered
by Novo Nordisk.
Novo Nordisk respects our employees’ right to associate freely and to join or refrain from joining labour unions and workers’ councils without fear of discrimination or retaliation. Where the right of freedom of association and collective bargaining is restricted or prohibited under law, we do not hinder employees from developing alternative mechanisms to express their grievances and protect their rights regarding working conditions. To encourage social dialogue, Novo Nordisk also engages with workers' representatives, for example, through Novo Nordisk’s European Works Council (EWC).
We protect equal treatment and opportunities for all employees, including a working environment free from discrimination and harassment. Equality means free from discrimination due to grounds of gender, family status, race and ethnic origin (including colour), national or social origin, religious beliefs, political orientation, sexual orientation and identity, marital status, age, disability or
other categories protected by national, state or local laws. Our internal Anti-harassment Framework sets out the global minimum standards for a fair process when handling any cases of harassment at Novo Nordisk and is implemented by local People & Organisation and Ethics & Compliance units. Employees working in North America are safeguarded from harassment and discrimination through a local process and framework.
We process employee data as part of conducting our business. Our OneCode policy and Ethics & Compliance programme are the basis for our global privacy and data ethics compliance across the value chain. These set the minimum
global standards for how we handle and protect personal data, together with applicable laws and regulations.
The Labour Code of Conduct also outlines expected minimum requirements regarding Novo Nordisk employees’ human rights at work, in line with our Human Rights Commitment. Our policy commits to prohibition, prevention,
and mitigation of forced, bonded or debt labour, slavery, servitude, human trafficking and child labour. For information on our human rights policy, we refer to section 3.3 ‘Workers in the value chain’ on page 88.
Actions
Recurring actions related to working conditions are outlined in the table.
Some of our actions supporting our positive working conditions are described
in other sections, for example, strengthening of parental leave as described in
the section 'Equal treatment and opportunities for all' on page 86. Overall, the effectiveness of our actions is assessed through continuous engagement with our employees and all leaders are expected to tend to the wellbeing of their employees.
Resources allocated to managing impacts and risks related to own working conditions are handled by our Global People and Compliance units as well as local People & Organisation teams depending on affiliate size.
1. The Labour Code of Conduct is aligned with the UN Guiding Principles on Business and Human Rights, the International Bill of Human Rights, the International Labour Organization’s Declaration on Fundamental Principles and Rights at Work and the UN Global Compact Ten Principles.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.2 Own workforce |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key actions to address working conditions in own workforce |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we track effectiveness |
|
|
Mitigating risks related
to own workforce or potential human rights breaches
|
Risks are assessed and addressed through mitigating actions on an ongoing basis, in accordance with the enterprise risk management framework. Any remedy is applicable in accordance with, local, legal requirements. |
Global operations |
No |
•Risk mitigation efforts are carried out continuously, and includes support to relevant affiliates on compliance with for example local legislation.
•With respect to human rights efforts, we are in the process of upskilling our workforce to strengthen our due diligence for our own operations.
|
|
|
Due diligence assessment of Labour Code of Conduct |
In 2024, we have initiated a due diligence assessment of our Labour Code of Conduct to evaluate the effectiveness of its global implementation since its launch in 2019 to ensure we protect our working condition standards. To be completed in 2025. |
Global operations |
No |
•The finalisation of the assessment in 2025 will provide insights for potential future action plans.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Permanent employees (headcount) |
|
Temporary employees (headcount) |
|
Non-guaranteed hours employees (headcount) |
|
Total |
3.2.2 Characteristics of Novo Nordisk's employees2 |
Unit |
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
Men |
Number |
34,720 |
|
– |
– |
|
2,696 |
– |
– |
|
0 |
– |
– |
|
37,416 |
– |
– |
Women |
Number |
33,920 |
|
– |
– |
|
2,791 |
– |
– |
|
0 |
– |
– |
|
36,711 |
– |
– |
Other/not reported |
Number |
29 |
– |
– |
|
0 |
– |
– |
|
0 |
– |
– |
|
29 |
– |
– |
2. Total headcount of 77,349 as per note 2.4 'Employee cost' on page 110 in the Consolidated financial statements. The variance of 3,193 employees is due to Catalent employees not being included. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2.3 Employees and employee turnover3 |
Unit |
2024 |
2023 |
2022 |
Total number of employees (FTEs) – excluding Catalent |
FTEs |
73,109 |
63,370 |
54,393 |
Total number of employees (headcount) – including Catalent |
Number |
77,349 |
64,319 |
55,185 |
Total number of employees (headcount) – excluding Catalent |
Number |
74,156 |
64,319 |
55,185 |
• Denmark |
Number |
34,185 |
28,692 |
22,916 |
• EMEA (Europe, the Middle East and Africa), excluding Denmark |
Number |
9,928 |
8,808 |
7,954 |
• North America (US, Canada) |
Number |
9,279 |
8,315 |
7,250 |
• Region China (Mainland China, Hong Kong, Taiwan) |
Number |
6,977 |
6,485 |
6,148 |
• Rest of World (all other countries) |
Number |
13,787 |
12,019 |
10,917 |
Number of leavers |
Number |
3,574 |
– |
– |
Employee turnover |
% |
5.5 |
5.5 |
8.2 |
3. Total headcount of 77,349 as per note 2.4 'Employee cost' on page 110 in the Consolidated financial statements. The variance of 3,193 employees is due to Catalent employees not being included. |
|
Performance
To measure our employee's engagement, we track our yearly employee survey (Evolve) index score. The result of the 2024 employee survey is broadly in line with the one from 2023, with a slight decrease of 1 percentage point of favourable answers. Novo Nordisk continues to score in the top quartile when benchmarked against external organisations regarding having a purpose-driven workplace.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2.1 Enterprise Evolve score |
Unit |
2024 |
2023 |
2022 |
Enterprise Evolve score |
% |
85 |
86 |
85 |
In 2024, we continued to expand our business while focusing on being an attractive workplace. We increased our workforce by 9,837 employees since 2023, ending the year with 74,156 employees. The most significant increase was at our production sites, particularly within manufacturing and quality, as well as professionals within Digital & IT. Aligned with our sustainable growth objectives, we continue to safeguard the wellbeing of our employees. Our focus resulted in
an employee turnover of 5.5%, consistent with 2023.
Currently, Novo Nordisk's HR systems allow employees to select the gender with which they most identify. We are committed to increasing awareness of this self-identification option for future disclosures to disclose on other/not reported.
To support our policy of employees having the right to form or join associations
and to bargain collectively, we began reporting on the number and percentage
of collective bargaining coverage for Denmark in 2024. There are five collective bargaining agreements currently in effect, covering 32% of the workforce in Denmark. All employees covered by collective bargaining agreements in Denmark are also covered by workers' representatives, resulting in a 32% representation.
With respect to adequate wages, we provide employees with pay that is above
the living wage in the given country.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2.4 Collective bargaining agreements and workers' representatives coverage |
Unit |
2024 |
2023 |
2022 |
Number of collective bargaining agreements – Denmark |
Number |
5 |
– |
– |
Percentage of employees covered by collective bargaining agreements and workers' representatives – Denmark |
% |
32 |
– |
– |
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.2 Own workforce |
|
In 2024, Novo Nordisk had 167 substantiated people-related cases, which is based on people who have filed a complaint or concern of different levels of severity and which have been confirmed. Hereof, 139 cases related to harassment, including discrimination. None were deemed as severe cases of human rights incidents. Various activities took place during the year to ensure awareness of speak-up channels and completeness of data. In 2024, the architecture of our grievance mechanisms has been improved and the internal governance has been strengthened, to support registration of incidents reported and investigated locally. We will continue to promote our speak-up culture and anti-harassment framework as part of our company-wide campaigns. We acknowledge that there may be cases which are not being reported to our Compliance Hotline.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2.5 Incidents, complaints and severe human rights impacts |
Unit |
2024 |
2023 |
2022 |
Substantiated people-related cases |
Number |
167 |
– |
– |
• Hereof substantiated cases of harassment, including discrimination |
Number |
139 |
– |
– |
• Hereof substantiated cases of severe human rights incidents |
Number |
0 |
– |
– |
• Hereof breaches of the UNGPs |
Number |
0 |
– |
– |
• Hereof number of complaints filed against Novo Nordisk to National Contact Points for OECD Multinational Enterprises |
Number |
0 |
– |
– |
Amount of material fines, penalties and compensation related to the above mentioned incidents |
mDKK |
0 |
– |
– |
ACCOUNTING POLICIES
Enterprise Evolve score
Measures the average percentage of favourable answers to the 18 engagement
items shared in Novo Nordisk’s annual employee survey. Favourable answers are defined as ‘Agree’ and ‘Strongly agree’ to positively framed questions. The survey is administered by an external vendor.
Employees (headcount)
Measured as the headcount of all employees at year-end, excluding externals, employees on unpaid leave, interns, Bachelor’s and Master’s thesis employees and substitutes. Employee data is based on registrations in Novo Nordisk’s HR
systems. Employees are attributed to geographical regions according to their primary workplace.
Number of leavers
The number of employees, excluding temporary employees, who left the
Novo Nordisk Group during the year.
Employee turnover
Measured as the number of leavers during the financial year, divided by the average number of employees, excluding temporary employees. Employees working for Group companies that have been sold are not counted as having left the Group.
Collective bargaining agreements and worker's representatives
Comprises the absolute number of the different types of collective bargaining agreements based on specific employee sub-groups (administrative, technicians, operators, skilled workers, etc.) in Denmark. Percentage of employees covered by collective bargaining agreements and workers' representatives are calculated as headcount covered in Denmark at year-
end divided by total headcount in Denmark at year-end.
Substantiated people-related cases
Cases that, through a formal process, have been reported to or filed with the Compliance Hotline and have been substantiated or partially substantiated based on an investigation during the year. Cases are within the overarching categories of the global anti-harassment framework, the Novo Nordisk Way and Ombudsman, as well as other potential human rights breaches for internal employees, consultants and other externally hired individual workers.
Substantiated cases of harassment, including discrimination
Cases that have been closed as substantiated or partially substantiated based on an investigation under the Novo Nordisk Way and the global anti-harassment framework for our own workforce.
Severe human rights incidents
Any substantiated case of severe adverse human rights impacts (child labour or forced labour) reported via Novo Nordisk’s Compliance Hotline for our own workforce, that has been closed during the year based on an investigation.
Breaches of the UNGPs
Incidents presenting a breach to the United Nations Guiding Principles on Business and Human Rights.
Complaints filed with OECD Multinational Enterprise Contact Points
Cases filed against a Novo Nordisk legal entity (parent or affiliate) under the OECD’s database of specific instances.
Amount of material fines, penalties and compensation related to the above-mentioned incidents
Damages resulting from violations of social or human rights laws, including discrimination and severe human rights incidents, where a Novo Nordisk legal entity (parent or affiliate) has been found in violation by a court of law and been condemned to pay material fines, penalties or compensation.
Health and safety
Policies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Policy |
|
Health and Safety |
|
Purpose |
|
Ensure safety, mental and physical wellbeing |
|
Scope |
|
Applies across all operations, including contractors |
|
Most senior level accountable |
|
Executive Management |
|
|
Availability |
|
Externally available: Health and Safety |
|
|
Applicability across Sustainability statement |
|
•Own workforce, page 80 |
|
|
Supporting policy documentation |
|
•Local health and safety instructions |
|
|
|
|
|
Novo Nordisk's Health and Safety policy focuses on a holistic approach and encompasses safety, physical wellbeing, mental wellbeing and health promotion, with the overall goal of prevention and continuous health improvements. The objectives of the policy are to ensure high standards
in our operations, promote a healthy lifestyle, make employees and leaders accountable for workplace safety, ensure that the working environment is not compromised for economic or productivity reasons, and fulfil all necessary
legal requirements.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.2 Own workforce |
|
Our policy is implemented through our health and safety management system, which applies to Novo Nordisk employees globally. The system includes specific health and safety requirements, for instance regarding risk assessments, emergency procedures and preparedness. The procedures are supported by dedicated training of all managers and health and safety employees as well as
basic training of employees globally. All our production facilities are certified by international standards for health and safety (ISO 45001) and are regularly audited internally and externally.
Actions
Our health and safety actions are implemented in a partnering approach with all business areas across the Group. Internal functions responsible for the actions include a global Health and Safety unit, which sets the direction and collaborates across business areas dedicated specifically to health and safety. Furthermore, the health and safety management system includes requirements for the involvement of employees across the group.
Performance
In 2024, Novo Nordisk had 173 accidents, primarily related to production expansion. Several actions were immediately taken across Novo Nordisk, including to ensure that work permits are in place for all non-routine high-hazard work such as hot work and working at height. Moreover, emergency procedures have been reviewed and emergency response teams have been established where not already in place. From 2025, Novo Nordisk will introduce a new, global safety target to sustain and enhance focus on driving prevention of potential and serious accidents.
To measure progress against our local and global health initiatives, we set targets applicable across our global operations to reduce and prevent accidents from occurring. Our safety target has the aim of 10% annual improvement in rate of recordable work-related accidents (also commonly referred to as Lost Time Accident Frequency (LTAF) or Lost Time Injury Frequency (LTIF)).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key actions to address health and safety |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we
track effectiveness
|
|
|
Local health and safety action plans |
A bottom-up management review is conducted
on an annual basis to assess the effectiveness of
the health and safety management system. Each business area has committed to a local health and safety plan addressing all strategic focus areas and relevant risks associated.
Any remedy is provided based on local, legal requirements and global support options are
made available for affected employees.
|
Global operations |
Yes |
•Safety: Performance against health and safety metrics include 173 recordable work-related accidents. Several actions were taken to ensure
that safety is systematically addressed.
•Mental wellbeing: 13.8% of employees reported symptoms of stress. Areas with a high level of
stress symptoms have been offered support from
an organisational psychologist, focusing on organisational aspects, psycho-social factors
and leadership.
•Physical wellbeing: 6.8% reported symptoms of work-related physical pain. Targeted efforts in areas with a high level of work-related pain has been piloted supporting local business areas to address root causes systematically. Further, interventions in Denmark and competency building on work-related pain at global production sites have been conducted.
•NovoHealth: The employee health promotion programme focuses on physical activity, healthy eating, individual mental wellbeing, nicotine cessation, weight management and health checks.
|
|
|
Specific actions related to health and safety incidents |
In response to expansion-related health and safety risks, further measures have been implemented in 2024 to prevent fire-related accidents in the future, including mapping of high-risk activities, establishing work permit offices, and review of emergency response plans. |
Global operations,
with a focus
on capacity expansion projects
|
No |
•New safety KPI was developed, applicable from 2025, expanding the scope of reported accidents and investigations to further prevent incidents across 10 high-risk hazards, including activities related to working at heights and hot work.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2.6 Health and safety (own employees) |
Unit |
2024 |
2023 |
2022 |
Year-on-year
reduction target
|
| Workforce covered by health and safety management system (headcount) |
% |
100 |
– |
– |
|
Recordable work-related accidents |
Number |
173 |
153 |
128 |
|
Rate of recordable work-related accidents4 |
Accidents per million hours worked |
1.2 |
1.3 |
1.3 |
10% |
Fatalities as result of work-related injuries |
Number |
0 |
1 |
2 |
|
Employees reporting symptoms of stress |
% |
13.8 |
13.8 |
13.8 |
10% |
Employees reporting symptoms of work-related physical pain |
% |
6.8 |
7.1 |
7.8 |
5% |
4. Rate of recordable work-related accidents was previously reported as Frequency of occupational accidents. Figures for 2022 and 2023 have been restated from 1.5 accidents per million hours worked in both years. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.2 Own workforce |
|
The calculation method for the rate of recordable work-related accidents was changed as of January 2024 to follow the international standard definition, harmonising the FTE equivalent working hours to 2,000 hours a year compared to the previous 1,600 hours, and using an average FTE number instead of year-end figures.
The rate of recordable work-related accidents decreased by 8% in 2024, which does not meet the 10% annual improvement target. In 2024, it was reduced to 1.2 accidents per million hours worked compared to 1.3% in 2023. The decrease is primarily caused by an increased safety focus from top management, based
on awareness of the elevated risk level connected to production expansion.
This focus has been communicated down through line of business and has
been supported by clearer safety requirements and safety behaviour initiatives.
In 2024, Novo Nordisk had 173 accidents with reported absence compared to 153 in 2023, which is in line with the increase in the number of employees. Furthermore, Novo Nordisk had zero work-related fatalities in 2024 compared
to 1 in 2023.
To improve mental and physical well-being, we have set targets to reduce the number of employees reporting symptoms of stress by 10% year-on-year and
to reduce employees reporting symptoms of work-related physical pain by 5% year-on-year. In 2024, 13.8% of employees responded that they had experienced symptoms of stress, which is in line with the 2023 result, hence the target of 10% annual improvement has not been met. The reasons are many and vary across the organisation. The overarching cause is the growth of the company, leading to both onboarding-related burdens and organisational changes which are known risk factors to mental wellbeing. In Production and Operations, the demand for supply adds significantly to the strain. In many other areas, the increased complexity arising from the expansion of our portfolio is a contributing factor to stress. In 2024, specific training and follow-up guidance with focus on mental well-being was offered for relevant teams. Furthermore, a global mental well-being delivery model targeting areas with a high stress level has been implemented to both reduce and prevent stress. We will continue to implement new initiatives throughout 2025 to further improve performance.
Reported symptoms of work-related physical pain decreased by 4% from 7.1%
in 2023 to 6.8% in 2024. Hence, the target of 5% year-on-year improvement has not been met. The delivery model for physical wellbeing was only initiated in 2024 and the full results of this remain to be seen.
Across all targets, the health and safety management system ensures that we involve our own workforce, including health and safety representatives, in target setting, performance tracking and when identifying lessons learned. Insights from the annual employee engagement survey, Evolve, also informs our target setting. Monitoring occurs on an ongoing basis and is reported annually.
ACCOUNTING POLICIES
Workforce covered by health and safety management system (headcount)
The percentage of employees in Novo Nordisk's own workforce who are covered by our health and safety management system based on legal requirements and/or recognised standards or guidelines is defined as the number of employees covered by health and safety management systems (headcount) divided by all employees (headcount).
Recordable work-related accidents
Total number of work-related injuries causing at least one day of absence in addition to the day of the accident.
Fatalities as a result of work-related injuries
Work-related accidents resulting in the death of an employee. All employees (headcount), permanent, temporary, and non-guaranteed hours, have been included in this metric.
Rate of recordable work-related accidents
Rate of recordable work-related accidents for our own workforce, measured in accidents per million hours worked, also referred to as the lost-time accident frequency (LTAF). Contractors, visitors, employees on unpaid leave, interns, and Bachelor's and Master's thesis students are not included. The number of hours worked is based on 2,000 working hours annually per full-time equivalent and the monthly records of number of employees converted into full-time equivalents.
Percentages of employees reporting symptoms of stress/work-related physical pain
Reported via the annual employee survey Evolve. In the survey, stress is defined as a situation where the employee feels tense, restless, nervous or troubled, or unable to sleep at night due to thoughts about their problems. Regarding symptoms of physical pain, the survey asks if an employee's work generally causes them physical pain. The two relative targets of improving mental and physical wellbeing are measured as the percentage of employees responding 'Quite much' or 'Very much' for mental wellbeing or 'Unfavourable' to the statement related to physical pain.
Equal treatment and opportunities for all
Policies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Policy |
|
Diversity and Inclusion |
|
Purpose |
|
Guides our actions to promote equal opportunities |
|
Scope |
|
All Novo Nordisk employees |
|
|
Most senior level accountable |
|
Executive Management |
|
|
Availability |
|
Externally available: Diversity and inclusion policy |
|
|
Applicability across Sustainability statement |
|
•Own workforce, page 80 |
|
|
|
|
|
By offering a diverse and inclusive workplace with opportunities to continuously learn and grow, Novo Nordisk can help foster the best conditions for employees
and sustain continued innovation.
Our diversity and inclusion (D&I) policy defines diversity as the mix of employees, perspectives, and backgrounds we have in our business, and
inclusion as creating a culture where all employees feel valued and have a sense of belonging. We recognise that diversity is any dimension that differentiates our people and enables diverse thinking, for example gender, ethnicity, race, nationality, disability and sexual orientation.
To foster equal treatment, our D&I policy focuses on mitigating bias, creating
an inclusive workplace, and having leaders serve as role models. Novo Nordisk actively seeks input from employees, senior leadership and peers to ensure that efforts reflect the needs and aspirations of our workforce and aligns with societal values and expectations.
Equal opportunities in Novo Nordisk also means creating a strong learning culture, embedded in a set of core beliefs that focus on how we ensure a shared and deliberate approach to personal and professional growth. While we do not have a specific training policy, we are guided by internal standard operating procedures for compliance driven and job specific training.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.2 Own workforce |
|
The procedures serve as process guides for identifying, providing, evaluating and documenting relevant training for each employee to comply with the healthcare-regulated requirements related to their job. Compliance-driven training varies depending on the need of the business unit.
To maximise the potential of ongoing dialogue between employees and managers, we focus on individual development plans and dedicated talks to discuss growth, where employees engage with their managers regarding,
for example, training opportunities and career aspirations.
Actions
To foster equal opportunities, the outlined action plans support the implementation of our policy commitments. Unless otherwise indicated, actions are considered recurring. In consideration of the local context and societal norms we ask all areas to determine local aspirations and action plans applicable to their geography.
Internal functions involved in execution of our actions include our Global
D&I team, which sets the strategic direction and targets for Novo Nordisk.
D&I professionals and champions align aspirations and action plans with the global direction.
Our balanced gender representation targets are aspirational goals and Novo Nordisk is dedicated to providing equal employment opportunities for all, regardless of gender. We have a merit-based recruitment strategy and endeavour to hire the most qualified person for the job based on their
skills, experience and qualifications across our global operations.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key actions to address equal treatment and opportunities |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we
track effectiveness
|
|
|
Mitigate bias through equal pay reviews |
Ensure that individuals with similar roles and responsibilities are compensated equitably, regardless of background, gender, or ethnicity. Equal pay reviews are conducted on a quarterly
basis with corrective actions for confirmed equal
pay risk cases.
|
Global operations, excluding US and Canada following own processes |
No |
•In 2024, out of the around 62 thousand positions covered in the pay review, we identified 0.13% – compared to 0.6% in 2023 – with an equal pay gap and we are taking corrective actions.
•The equal pay review goes beyond gender and considers various parameters to identify gaps using employee’s job level, job family, tenure, country.
|
|
|
Inclusive workplace through balanced gender representation |
Striving for balanced gender representation across managerial levels, through for example ensuring a diverse slate of candidates, diverse recruitment panel and pipeline of diverse talents. |
Global operations |
Yes |
•Men account for 54% in leadership positions and 58% in senior leadership positions.
•Women account for 46% in leadership positions
and 42% in senior leadership positions.
|
|
|
Inclusive workplace through flexible working policies |
Improved minimum global standards for paid maternity leave and paid parental leave for
non-birthing parents as well as paid leave for employees to handle serious health conditions of their dependents. Changes are applicable from January 2025.
|
Global operations |
No |
•Introduction of a global minimum standard of parental leave within the first year of becoming a parent extended from 8 to 14 weeks for all non-birthing parents globally.
•Introduction of a global minimum standard of 2 weeks of paid leave annually for employees who need time to handle a serious health condition
of dependents.
|
|
|
Roll-out of training offerings |
Employee training based on target group, qualifications and job requirements to inspire
positive leadership habits and empower potential at all levels. Training offerings cover both compliance-related training but also development options through global talent and development programmes, virtual and face-to-face skill courses, and online learning content.
|
Global operations |
No |
•In 2024, new compliance-related training was established regarding product quality, safety and efficacy, impacting around 6,000 managers.
•Approximately 4,000 out of nearly 9,000 leaders engaged in our development programmes, and about 2,000 employees completed global strategic capability development programmes.
•More than 40,000 times employees and leaders have completed a learning item online to help develop specific skills.
•Progress of training programmes are monitored through voluntary surveys following course completion.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.2 Own workforce |
|
Performance
In 2021, we set a global target to achieve balanced gender representation across all managerial levels, and a minimum of 45% women and 45% men
in senior leadership roles by the end of 2025. The target is applicable across
our operations. The target was set based on various benchmarks, including pharmaceutical peers, other global Danish companies, industry-leaders and research, and was developed in collaboration with leaders and People & Organisation representatives and approved by the Board of Directors. We
are transparent regarding tracking performance against this target.
In 2024, 46% of all leadership positions were filled by women, the same as
in 2023. Within senior leadership, 42% of positions were filled by women at
the end of 2024, in line with 41% at the end of 2023.
As of 31 December 2024, the Board of Directors had equal gender representation, consisting of six female and six male members. Moreover,
when excluding employee representatives, the shareholder-elected Board members comprise of three female and five male members. According to the Danish Companies Act, this is regarded as having equal gender representation, and Novo Nordisk is therefore not legally required to set a gender target. Since diversity remains important for the Board, it has maintained a voluntary 2026 target of having at least three shareholder-elected Board members who are women and three who are men. Diversity in the broadest sense remains a
focus area for the Board of Directors, including Board member searches.
Novo Nordisk is reporting on the gender pay gap for the first time. In 2024,
the aggregated gender pay gap is 3% in favour of women. We continue to work actively with equal pay, for example through our equal pay reviews as described in the action section, according to which 0.13% of positions where identified to have an equal pay gap, when taking into account various parameters beyond gender.
To ensure that we provide equal treatment and opportunities for all, we track the age distribution among our employees, which largely remains unchanged year over year.
Across all of our D&I efforts, we monitor our global Inclusion Index, which is part of our annual employee engagement survey, Evolve. It indicates how our employees rate the state of inclusion at Novo Nordisk, and it resulted in 82%
of our employees rating the inclusion statements favourably in 2024.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Men |
|
Women |
3.2.7 Diversity metrics – Management levels |
Unit |
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
Number of employees (headcount) at senior leadership – CEO, EVP, SVP |
Number |
38 |
– |
– |
|
22 |
– |
– |
Percentage of employees (headcount) at senior leadership – CEO, EVP, SVP |
% |
63 |
64 |
71 |
|
37 |
36 |
29 |
Number of employees (headcount) at senior leadership – CVP, VP |
Number |
466 |
– |
– |
|
339 |
– |
– |
Percentage of employees (headcount) at senior leadership – CVP, VP |
% |
58 |
59 |
60 |
|
42 |
41 |
40 |
Number of employees (headcount) at other leadership levels – Director, manager, team leader |
Number |
4,726 |
– |
– |
|
4,171 |
– |
– |
Percentage of employees (headcount) at other leadership levels – Director, manager, team leader |
% |
53 |
54 |
55 |
|
47 |
46 |
45 |
Gender in leadership positions (overall) |
% |
54 |
54 |
56 |
|
46 |
46 |
44 |
Gender in senior leadership positions (CEO, EVP, SVP, CVP and VP) |
% |
58 |
59 |
61 |
|
42 |
41 |
39 |
| Target: minimum 45% men and 45% women |
|
|
|
|
|
|
|
|
Gender on the Board of Directors |
% |
50 |
50 |
54 |
|
50 |
50 |
46 |
Gender on the Board of Directors without employee representatives |
% |
62 |
62 |
67 |
|
38 |
38 |
33 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2.8 Remuneration metrics |
Unit |
2024 |
2023 |
2022 |
| Gender pay gap |
% |
(3)5 |
– |
– |
| Annual total remuneration ratio |
Ratio |
63 |
– |
– |
5. Negative gender pay gap shows a pay gap in favour of women. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2.9 Employees by age group |
Unit |
2024 |
2023 |
2022 |
| Under 30 years old |
Headcount |
11,538 |
– |
– |
|
% |
16 |
17 |
15 |
| Between 30 and 50 years old |
Headcount |
48,429 |
– |
– |
|
% |
65 |
64 |
65 |
| Over 50 years old |
Headcount |
14,189 |
– |
– |
|
% |
19 |
19 |
20 |
ACCOUNTING POLICIES
Gender in leadership and senior leadership positions
Reported as the percentage split by gender in leadership and senior leadership positions. Senior leadership positions are defined as employees in the global
job levels chief executive officer (CEO), executive vice president (EVP), senior
vice president (SVP), corporate vice president (CVP) and vice president (VP). These are the top management positions in the Novo Nordisk Group. Other leadership levels are defined as employees in the global job levels of director, manager and team leader. Leadership positions overall are defined as directors, managers, team leaders and senior leadership positions. Diversity on the Board of Directors is reported as the percentage split by gender among all members, including employee elected members.
Gender pay gap
Calculated as the difference between the average annualised salary for men and women divided by the average annualised salary for men, and expressed as the percentage of the average annualised salary for men. All employees at all job levels and in all countries have been included in this metric. Calculations were performed for the full consolidation, regardless of job level and country.
Annual total remuneration ratio
Calculated as the ratio between the annual retribution of the highest paid individual and the annual total remuneration for all employees.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.3 Workers in the value chain |
|
3.3 Workers in the value chain
As a global company, Novo Nordisk depends on a large value chain of more
than 60,000 suppliers providing goods or services to enable our business.
We therefore impact workers in our global supply chain both directly and indirectly, for example when sourcing materials used to manufacture our
medical products, when hiring external contractors for our expansion projects,
or through logistic partners when distributing our products. While our suppliers are concentrated primarily in Denmark, the US, and China, we aim to ensure
all of our partners across our global value chain meet and uphold the expected minimum requirements for human rights, social, health and safety and environment.
Material impacts, risks and opportunities (IROs)
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Protecting working conditions and human rights |
|
•Upstream
•Downstream
|
Given the size of our value chain, we acknowledge negative impacts can occur where we fail to identify or follow-up on cases where suppliers do not meet our standards. Based on supplier audit findings, negative impacts relate primarily
to individual incidents, such as worker protection issues or working hours not meeting our standards. We acknowledge that human rights violations can occur across our value chain, and we will continuously review our due diligence and risk assessments to identify potential and actual human rights violations that we may cause or contribute to. Currently, we have not identified any geographies or commodities in our value chain that are exposed to significant risks of human rights violations. We also have not identified any specific group of value chain workers that is particularly vulnerable to negative impacts.
Process for engagement and remediation
We engage directly and indirectly with supplier representatives through our ongoing engagements across sourcing units as well as through supplier audits. Our various procurement functions are responsible for ensuring engagement is conducted in accordance with Novo Nordisk’s Responsible Sourcing Programme.

Positive impact

Negative impact

Opportunity

Risk
Our policy specifies that suppliers must implement procedures that allow all employees to raise and address workplace grievances anonymously without
fear of reprisal or retaliation. Furthermore, all value chain workers can raise any concerns, including grievances related to human rights through our Compliance Hotline. The Compliance Hotline is further described in the section ‘Business conduct’ on page 91. We do not have other formalised processes for engaging directly with value chain workers.
Working conditions and equal treatment and
opportunities for all
Policies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Policy |
|
Responsible Sourcing Standards |
|
Purpose |
|
Expected minimum requirements for our partners |
|
Scope |
|
All global suppliers providing goods or services to Novo Nordisk |
|
|
Most senior level accountable |
|
Senior Vice President of Global Solutions |
|
|
Availability |
|
Externally available:
Responsible Sourcing Standards
|
|
|
Applicability across Sustainability statement |
|
•Workers in the value chain, page 88
•Business conduct, page 90
|
|
|
|
|
|
The purpose of Novo Nordisk’s Responsible Sourcing Standards is to safeguard human rights in the workplace, protect labour and social rights, establish safe, secure and healthy working conditions, and minimise negative environmental impacts. The policy is built on internationally recognised legislations and standards such as the Corporate Sustainability Due Diligence Directive, the
UN Guiding Principles on Business and Human Rights, the OECD Guidelines
for Multinational Enterprises on Responsible Business Conduct and the International Labour Organisation.
The minimum requirements outlined in our policy cover all global suppliers and include, but are not limited to, manufacturers, contractors, agencies, distributors, transportation carriers and technology partners. In 2024, we have updated our Responsible Sourcing Standards by strengthening existing policy principles as
well as covering additional social and human rights requirements aligned with the Corporate Sustainability Due Diligence Directive (CSDDD). Going forward, the policy is a requirement in all new contracts and will be rolled out in a phased approach across our global value chain.
To promote good working conditions for value chain workers, our policy includes principles for appropriate working hours, adequate wages, secure employment, and ensuring that workers are paid on time and in full, according to applicable wage laws, including minimum wages, over-time and mandated benefits. Labour rights should be promoted, as set forth in applicable laws, enabling workers to associate freely, join or not join labour unions, seek representation, and join workers’ councils in support of social dialogue.
Suppliers are also required to protect workers from exposure to workplace hazards through regular review of health and safety policies, regulations and processes, provide necessary worker protection and safety equipment, implement emergency plans and train workers accordingly. With Novo Nordisk undertaking several global capacity expansion projects we have implemented global minimum construction safety standards. Safe working conditions also refer to physical and mental health including a workplace free from harassment.
To monitor the implementation of the Responsible Sourcing Standards, we conduct selected audits of our strategic suppliers each year, during which corrective action plans can be devised in event of breach of the policy. We follow up to ensure resolution of issues. If a supplier fails to comply with the corrective action plan, Novo Nordisk reserves the right to terminate the contract with the supplier, depending on the extent of the breach.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Social / 3.3 Workers in the value chain |
|
Other work-related rights
Policies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Policy |
|
Human Rights Commitment |
|
Purpose |
|
Guiding all behaviour with respect to human rights |
|
|
Scope |
|
All individuals who can be impacted by Novo Nordisk's activities and business relationships |
|
|
Most senior level accountable
|
|
Chief compliance officer |
|
|
Availability |
|
Externally available:
Novo Nordisk Human Rights Commitment
|
|
|
Applicability across Sustainability statement |
|
•Patient protection and quality of life, page 71
•Own workforce, page 80
•Workers in the value chain, page 88
•Business conduct, page 90
|
|
|
|
|
|
Our Human Rights Commitment defines adequate human rights protection
and refers to all internationally recognised human rights instruments, including the International Bill of Human Rights, the International Labour Organisation Declaration on Fundamental Principles and Rights at Work, and the Convention on the Rights of the Child.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key actions to
address workers in
the value chain
|
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we
track effectiveness
|
|
|
Strengthened the Responsible Sourcing Standard |
Update of policy from October 2024, making it mandatory for all new contracts going forward.
To initiate the process we focus on larger contracts and strategic suppliers. Policy requirements are aligned with external legal firm and CSDDD.
|
Global suppliers |
No |
•Since October, we have started to work in a phased approach to ensure that all our suppliers globally adopt Responsible Sourcing Standards in new or renegotiated contracts. We aim to complete our efforts by 2027. Effectiveness and progress will be assessed continuously.
|
|
To live up to the commitment, we pay particular attention to the rights of,
as well as the challenges faced by vulnerable groups. In addition, we strictly prohibit the use of any form of forced labour or human trafficking, and expect our suppliers to take the necessary steps to prevent this from happening in
their own business or supply chain. Suppliers are also expected to ensure the protection, security, and lawful use of personal data of workers in the value chain, and to ensure, at a minimum, compliance with all applicable privacy
and data protection laws.
The process for monitoring the implementation of the Human Rights Commitment within the value chain and our own operations include identifying and assessing both materialised and emerging human rights risks within business relationships. Assessment is in accordance with our enterprise risk management framework. For suppliers in scope for a responsible sourcing audit, audits may be extended if indicators from a pre-audit survey show heightened human rights risks.
Actions
The Responsible Sourcing Programme is being implemented across our
global procurement teams responsible for the various sourcing categories.
This is to ensure the identification and mitigation of negative impacts and risks by engaging with suppliers, conducting supplier audits, developing corrective action plans and monitoring progress. Global actions have been initiated as outlined in the table. Individual incidents found in 2024 as part of our responsible sourcing audits is further described in the section 4.1 'Business conduct' with regards to management of relationships with suppliers on page 92, for which remediation of findings is still ongoing. We will continuously assess relevant sustainability performance indicators to include in future disclosures.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Governance / 4.1 Business conduct |
|
4. Governance
4.1 Business conduct
Novo Nordisk takes a proactive approach to ensuring uniform and ethical business conduct across markets to increase trust in our company and
maintain and improve relationships with our key stakeholders. Furthermore,
we endeavour to further strengthen the trust of our investors, business partners, employees, and the public through open and transparent
corporate communication.
Material impacts, risks and opportunities (IROs)
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Ethical working culture through Novo Nordisk Way |
|
•Own operations |
At the core of our efforts to uphold high standards of business ethics is the Novo Nordisk Way. This is a set of corporate culture commitments which help guide our employees’ ethical behaviour and interaction with our value chain partners. We conduct facilitations to ensure that all employees live up to our cultural commitments. This is to safeguard our employees, and reinforce that we live up to our cultural commitments also outside of Novo Nordisk. We continue to assess if we live by our commitments, including through our reputational score.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Interacting with all stakeholders in accordance with our business ethics standards |
|
•Upstream
•Own operations
•Downstream
|
While we follow all relevant local and international laws, principles, standards and codes when it comes to business conduct, negative impacts can occur if
we fail to uphold our business ethics standards in our interactions, especially
in geographies that rank low in Transparency International’s Corruption Perceptions Index (CPI). The identification of material IROs in relation to

Positive impact

Negative impact

Opportunity

Risk
business conduct matters was undertaken at a global level, but with consideration of countries ranked low in the CPI. We ensure that interactions
with our stakeholders are compliant with our business ethics standards, including with healthcare professionals, public officials and third party representatives. Despite having strong compliance procedures in place,
we recognise that any violations can have negative impacts on society or stakeholders in our value chain.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Promoting public health |
|
•Downstream |
In our interactions with public healthcare systems and their stakeholders, we promote public policy and societal positions through our public affairs efforts on public health and serious chronic diseases, thus helping to protect the wellbeing of patients, the healthcare system and wider society.
|
|
|
|
|
|
|
|
|
Identified IRO |
Category |
Value chain |
Upholding high bioethical standards |
|
•Own operations |
Novo Nordisk faces several complex bioethical issues during the discovery, development and production of pharmaceutical products. We set high bioethical standards to ensure good business conduct during the innovation phase. These are essential for protecting and preventing harm to society while simultaneously promoting trust when advancing public health and scientific knowledge. We do not compromise the protection of ethical considerations in the pursuit of new scientific breakthroughs.
|
|
|
|
|
|
|
|
|
| Identified IRO |
Category |
Value chain |
Reliance on animals in research |
|
•Upstream
•Own operations
|
The use of living animals is still crucial in research for new medicines. Novo Nordisk's use of animals for research has negative impacts for the animals. While the strictest procedures are in place to ensure high ethical and welfare standards, it is still expected that the quality of life of animals is affected.
Corporate culture
Policies
The Novo Nordisk Way, through its 10 Essentials (see page 15), describes the values and behaviours that guide everything we do, rooted in the principles
and vision of our founders.
Our OneCode policy supports us in living up to the Novo Nordisk Way, guiding everyone employed by, or working on behalf of, Novo Nordisk, on how to act as a company and as individuals, including what constitutes a healthy workspace and our speak-up culture. For more on our OneCode policy, see section 3.1 'Patient protection and quality of life' on page 72.
Performance
A team of facilitators evaluates the adherence to the Novo Nordisk Way of selected units on rotation every year. In 2024, a total of 51 units were facilitated, compared to 42 in 2023. We will continue to increase the number of facilitations to match Novo Nordisk's growth. The units facilitated in 2024 represent 29,021 employees across Novo Nordisk's operations, of which approximately 3,000 employees were interviewed, as well as 600 employees collaborating closely with the units in scope of facilitation. All units were assessed to be working in accordance with the Novo Nordisk Way and no immediate actions were required. Across all units facilitated, Novo Nordisk’s rapid growth, organisational changes, and efforts to increase product supply are the main factors driving improvement opportunities.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.1.1 Facilitations of the
Novo Nordisk Way |
Unit |
2024 |
2023 |
2022 |
| Facilitations of the Novo Nordisk Way |
Number |
51 |
42 |
36 |
ACCOUNTING POLICIES
Facilitations of the Novo Nordisk Way
A facilitation is an internal process for assessing adherence to the Novo Nordisk Way. The number of facilitations is measured as the number of facilitations completed. The assessments are based on a review of documentation and feedback from stakeholders, followed by an on-site visit during which randomly selected employees and management are interviewed. Identified gaps and improvement opportunities related to the Novo Nordisk Way are presented to, and discussed with, Executive Management. The facilitators and Executive Management agree
on an action plan to address any gaps and improvement opportunities.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Governance / 4.1 Business conduct |
|
Anti-corruption and anti-bribery
Policies
Rooted in the Novo Nordisk Way, our OneCode policy also reflects our company-wide commitment to doing business ethically and with integrity, to protect Novo Nordisk and our business partners from engaging in any form of corruption
and bribery.
To ensure our employees understand the implications of the OneCode policy, we conduct annual ethics and compliance training and tests in the form of mandatory e-learning for all employees and monitor the completion rate, while following up with employees to ensure completion of the training. Additionally, Group Internal Audit performs business ethics reviews to ensure compliance with our business ethics standards.
In Novo Nordisk we have not yet defined functions-at-risk, however, ethics
and compliance training is mandatory for all employees globally, including
for employee-elected members of the Board of Directors. As they are not Novo Nordisk employees, the shareholder-elected members of the Board of Directors receive annual training in our OneCode policy.
As a part of our OneCode policy, we focus on complying with all local and international anti-corruption regulations that may apply to our business, such
as the US Foreign Corrupt Practices Act, the UK Bribery Act and the UN Guiding Principles on Business and Human Rights.
Performance
We continue to have almost full coverage of our global mandatory ethics and compliance training. The remaining 1% is mainly due to employees being on leave. Additional targeted measures, such as the annual Ethics Days, help raise awareness, and we will continue to assess such initiatives in the future to further strengthen performance.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4.1.2 Prevention and detection of corruption and bribery |
Unit |
2024 |
2023 |
2022 |
Employees trained in ethics and compliance |
% |
99 |
99 |
99 |
As in 2023, we continue to report zero convictions for breaches of anti-corruption and anti-bribery laws. The amount of fines for violation of anti-corruption and anti-bribery laws, an additional metric introduced in 2024, also amounted to zero.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4.1.3 Incidents of corruption or bribery |
Unit |
2024 |
2023 |
2022 |
Convictions for violation of anti-corruption and anti-bribery laws |
Number |
0 |
0 |
0 |
ACCOUNTING POLICIES
Employees trained in ethics and compliance
The mandatory ethics and compliance training for employees working at Novo Nordisk comprises globally applicable e-learning. The percentage of employees trained is calculated as the number of employees that have completed the training divided by the total number of employees at year-end.
Number of convictions for violation of anti-corruption and anti-bribery laws
Anti-corruption and anti-bribery instances where any reported undertaking
has been found in violation by a court of law.
Compliance Hotline and protection of whistleblowers
Policies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Policy |
|
Anti-retaliation policy |
|
Purpose |
|
Protection of any persons who report or participate in an investigation in good faith |
|
Scope |
|
Any user of the Compliance Hotline, whether employees or external stakeholders |
|
Most senior level accountable |
|
Chief Compliance Officer |
|
|
Availability |
|
Externally available: Compliance Hotline |
|
|
Applicability across Sustainability statement |
|
•Patient protection and quality of life, page 71
•Own workforce, page 80
•Workers in the value chain, page 88
|
|
|
|
|
|
Our employees are encouraged to speak up about ethical or compliance concerns and thereby contribute to an ethical culture at Novo Nordisk.
All employees and external stakeholders can report concerns of misconduct in
a secure and confidential manner, with the option of anonymity, through the Compliance Hotline or in person. All employees are informed about our hotline
as part of their annual ethics and compliance training. We continually assess the effectiveness of the Compliance Hotline, including an assessment every two years, conducted by a third party to ensure trust of the channel and processes.
The concerns reported can be related to business ethics misconduct, accounting issues, fraud, bribery and corruption, quality misconduct, breaches of antitrust laws, environmental legislation and data privacy, departures from the Novo Nordisk Way, and misconduct such as espionage, sabotage, information security violations or other serious offences.
For each report in scope of the investigational process, an internal lead investigator will be appointed working objectively and independently, under confidentiality obligations, and trained to safeguard investigative information. We reply to all complaints within a few days and confirm the closure of the investigation when finalised. Results and significant ongoing investigations
are reported on a quarterly basis to the Audit Committee and Executive Management, including updates on severe cases, general trends, and corrective actions, such as sanctions. Novo Nordisk has established a global Disciplinary Sanction Guideline to ensure the best possible alignment of disciplinary sanctions across the organisation. The guideline is based on two severity factors: intent and frequency. The processes and sanctions in this guideline do not overwrite local employment laws and any relevant collective agreements, which shall be followed at all times.
We have zero tolerance for discrimination or retaliation against whistleblowers. Anyone who retaliates against an employee reporting misconduct will be subject
to disciplinary action, up to and including termination in accordance with our
policy. Novo Nordisk’s measures to protect whistleblowers are in line with the EU Whistleblowing Directive (EU Directive 2019/1937), with which we must comply, and we ensure adherence to local regulations during investigations outside Europe.
Performance
In 2024, 242 cases reported via the Compliance Hotline relating to accounting issues, fraud and business ethics matters were substantiated. The 9% increase
in number of substantiated cases compared to 2023 is driven by the business growth, including increased number of employees. The numbers for 2022 and 2023 have been restated, as cases involving Novo Nordisk Way violations have been moved to table 3.2.5 'Incidents, complaints and severe human rights impacts', where we report on substantiated people-related cases to avoid
'double-counting'.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Governance / 4.1 Business conduct |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.1.4 Substantiated cases reported within accounting issues, fraud and business ethics matters |
Unit |
2024 |
2023 |
2022 |
Substantiated cases reported within accounting issues, fraud and business ethics matters via the Compliance Hotline1 |
Number |
242 |
221 |
227 |
| 1. Substantiated cases reported within accounting issues, fraud and business ethics matters was previously reported as Number of substantiated cases reported via the Compliance Hotline. For 2023 and 2022, 314 and 288 cases have been reported, respectively, considering the previous definition. |
ACCOUNTING POLICIES
Substantiated cases reported within accounting issues, fraud and business
ethics matters via the Compliance Hotline
Number of cases reported to the Compliance Hotline, where reported allegations of suspected misconduct have been substantiated or partially substantiated. When a case has been substantiated or partially substantiated, corrective actions are initiated.
Management of relationships with suppliers
Policies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Policy |
|
Global procurement policy |
|
Purpose |
|
Ensure good conduct in how we source goods and services, select suppliers and negotiate agreements |
|
Scope |
|
All sourced goods and services, excluding those used for manufacturing of Novo Nordisk products |
|
|
Most senior level accountable |
|
Corporate vice president of Corporate
Procurement |
|
|
Availability |
|
Externally available: Procurement in Novo Nordisk |
|
|
Applicability across Sustainability statement |
|
•Business conduct, p. 90 |
|
|
Supporting policy documentation |
|
•Internal standard operating procedure on procurement for manufacturing |
|
|
|
|
|
We are dependent on our suppliers and aim to ensure good conduct in how we source goods and services, select suppliers and negotiate agreements, including fair and transparent payment practices. Our procurement policy defines the
guidelines for contracting with us, including qualification and tendering to issuing invoices and using a spend management platform. The policy applies to our largest sourcing group, indirect spend, but does not apply to goods and services used in the manufacturing of Novo Nordisk's products. In those circumstances, an internal standard operating procedure supplements the policy.
We continue to conduct regular supplier audits, including re-visits, to ensure high quality with the suppliers with which we engage. The risk-based minimum frequencies for quality audits are governed through our procurement for manufacturing setup and the frequencies are based on usage (categories for goods and services). For responsible sourcing audits, we are considering risk parameters such as country of operation and spend.
We make procurement decisions that are financially, environmentally and socially responsible as outlined in our Responsible Sourcing Standards in section 3.3 ‘Workers in the value chain’ on page 88. We use e-sourcing and e-auction solutions to provide faster tendering and ensure a fair and transparent process during negotiations. We work with preferred suppliers to help us build better relationships. Becoming a preferred supplier is dependent on many factors, including openness to continuous improvement and innovation, and delivering quality products in a timely manner.
Our standard payment terms are 60 days, but other terms may be agreed as part of contract negotiations. We are committed to preventing late payments to suppliers, particularly when these are small enterprises. During the COVID-19 pandemic, to mitigate the impacts of the crisis, we implemented a payment guideline, that ensured payment to small suppliers as soon as possible upon receipt and approval of invoices (which may have been earlier than the terms specified in invoices or contracts). This guideline was then made permanent
and continues to apply today.
Performance
In 2024, new metrics on payment practices were implemented for the first time.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4.1.5 Payment practices |
Unit |
2024 |
2023 |
2022 |
Average number of days to pay invoice |
Days |
42 |
– |
– |
• Small suppliers |
Days |
24 |
– |
– |
• Large suppliers |
Days |
49 |
– |
– |
Percentage of payments aligned with standard payment terms |
% |
83 |
– |
– |
• Small suppliers |
% |
77 |
– |
– |
• Large suppliers |
% |
84 |
– |
– |
Outstanding legal proceedings for late payments |
Number |
0 |
– |
– |
We continued to increase the number of supplier audits to 429 in 2024 from 382 in 2023, reflecting the increased activity level in Novo Nordisk. Three critical findings were issued during 2024. Two of these were related to responsible sourcing, concerning management of contract labour, for which the remediation is still ongoing. The last finding was related to quality audits, concerning protection against cross contamination, and agreements regarding actions to address it have been made with the affected supplier.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4.1.6 Supplier audits |
Unit |
2024 |
2023 |
2022 |
| Total supplier audits |
Number |
429 |
382 |
294 |
ACCOUNTING POLICIES
Average number of days to pay invoice
Average number of days it takes Novo Nordisk to settle an invoice from the invoice date (when contractual or statutory term of payment starts to be calculated) until the invoice has been cleared.
Percentage of payments aligned with standard payment terms
Includes all transactions where the invoice cycle time is equal to or less than
the specified payment terms, divided by the total number of transactions. Small suppliers (with less than DKK 1 million in spend over the last twelve months)
are measured based on 30-day payment terms, whereas other suppliers are assessed using payment terms from the invoice document recorded in our internal systems.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Governance / 4.1 Business conduct |
|
Number of outstanding legal proceedings for late payments
Number of all outstanding legal proceedings (litigation or arbitration)
for late payment.
Supplier audits
Total number of supplier audits, concluded by Novo Nordisk's Corporate Quality & Inspections function, consisting of the number of responsible sourcing audits
and quality audits conducted at suppliers, selected using various risk parameters. Audits for responsible sourcing are conducted according to Novo Nordisk's Responsible Sourcing Standard to ensure compliance. In addition, suppliers of goods and services used in the manufacture of Novo Nordisk pharmaceuticals are subject to extensive quality audits in accordance with different quality standards, including third-party audits.
Political influence and lobbying activities
Policies
Novo Nordisk actively engages with various stakeholders, including public officials, to advocate for important issues affecting patients, our business, our partners and the communities in which we operate globally. Our OneCode policy sets out the objectives of having patients’ interests as our first priority, acting with professionalism and integrity and adhering to local regulation on public engagement. It also outlines our zero-tolerance of giving or offering anything of value to a politician, public official or decision-maker to seek undue influence. This is essential for us and guides our interactions.
Our advocacy is grounded in realising the potential that innovation in our industry can bring to patients, healthcare systems and society, while ensuring transparency and adherence to business ethics in our interactions. We strive
to achieve this by advocating for industry-level initiatives and regulation that promote the following:
•Evidence-based chronic disease prevention, public health and improvement
of care for people living with serious chronic diseases.
•Innovation and provision of optimal conditions for making new discoveries
to benefit patients.
•Improvements of resilience of healthcare systems.
•A more environmentally sustainable way of operating in the pharma industry.
We are a member of various industry and trade associations representing the pharmaceutical industry, to bring about consensus on broad policy issues that affect the patients we serve and our business. Our membership of these organisations is evaluated on an ongoing basis, considering their expertise in policy, advocacy and ability to drive the agenda on issues important to us.
To ensure transparency around our activities, we are registered in the EU Transparency Register under ID 29570313329-11. No members of our Board
of Directors have held a comparable position in public administration in the
two years preceding their appointment.
Actions
Through our engagement with various stakeholders, such as industry and trade associations, we have taken actions for the implementation of our objectives, with the key objectives listed in following the table. Unless otherwise indicated, actions are considered recurring.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key actions to address advocacy |
Description and year of completion |
Scope of action |
Target in place |
Overall progress in 2024 and how we track effectiveness |
|
|
Presidency of the European Federation
of Pharmaceutical Industries and Associations (EFPIA)
|
Novo Nordisk’s President and CEO Lars Fruergaard Jørgensen is President of EFPIA 2023-2025, focusing on the review of the EU General Pharmaceutical Legislation, advocating for innovation and providing optimal conditions for making new discoveries accessible to patients. |
Patients in Europe |
No |
•Our CEO's presidency of EFPIA supported the collaboration with policy makers, to establish industrial policies aimed at fostering an ecosystem that encourages innovation and prioritises life sciences as a strategic industry. |
|
|
Obesity advocacy |
Advocacy through EFPIA Obesity Policy Platform to improve healthcare solutions for people living with obesity, recognise obesity as a relapsing chronic disease and increase knowledge of its financial cost. Recurring collaboration with EFPIA Health Systems Working Group, to address some of the major challenges facing health system resilience. |
Patients in Europe |
No |
•In 2024, Novo Nordisk joined the newly established Obesity Policy Platform.
•The Health Systems Working Group has made progress on improving efficiencies between health system resources and fostering collaboration on creating more sustainable health systems.
|
|
|
Diabetes advocacy |
Advocacy through the European Diabetes Forum
for policy change that enables healthcare systems
to better manage diabetes care.
|
Patients in Europe |
No |
•Campaigned, together with the European cardiovascular community, for cardiovascular disease and diabetes within European
policy priorities.
|
|
Performance
In 2024, a new metric on trade association membership fees was introduced.
A zero-tolerance policy applies at Novo Nordisk with regards to in-kind political contributions.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.1.7 Trade association membership fees and in-kind political contributions |
Unit |
2024 |
2023 |
2022 |
Trade association membership fees |
mDKK |
177 |
– |
– |
| In-kind political contributions made |
mDKK |
0 |
– |
– |
ACCOUNTING POLICIES
Trade association membership fees
The total monetary value of trade association membership fees during the financial year reported in DKK millions. Data is collected at country level for Brazil, Canada, China, Denmark, France, Germany, India, Italy, Japan, the United Kingdom and the US, where Novo Nordisk focuses its public affairs activities.
In-kind political contributions
In-kind contributions can include advertising, use of facilities, design and printing, donation of equipment, provision of board membership, employment or consultancy work for elected politicians or candidates for office.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Governance / 4.1 Business conduct |
|
Bioethics
Policies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Policy |
|
Bioethics policy |
|
Purpose |
|
Guide bioethical behaviour in our research and development |
|
Scope |
|
Applies globally |
|
Most senior level accountable |
|
Executive management |
|
Availability |
|
Externally available: Bioethics |
|
|
Applicability across Sustainability statement |
|
•Business conduct, page 90
•Patient protection and quality of life, page 71
|
|
|
Supporting policy documentation |
|
•Position papers (for each bioethical focus area)
•Standard operating procedures
|
|
|
|
|
|
Bioethics refers to all ethical issues related to the use of life science technologies for the discovery, development and production of pharmaceutical products. Novo Nordisk’s bioethics policy sets out general operational guidelines for research and development and informs day-to-day business decisions.
Our policy states our commitment to high global ethical standards in research involving people, animals, human materials and gene technology. We extend
these requirements to our external partners, contract research organisations
and suppliers, and monitor their performance. We act in accordance with relevant international conventions and standards, and actively promote bioethical awareness at Novo Nordisk. We implement the policy objectives by integrating these in standard operating procedures, processes and decision-making, and we have cross-functional governance that addresses emerging bioethical dilemmas. On an annual basis we define strategic focus areas, and report to the leadership teams of Research and Early Development, and Development, on the bioethical strategy execution, highlighting potential bioethical issues.
Our policy commitments are further detailed in position statements for specific bioethical focus areas, including clinical trial and human biosample ethics, animal ethics, cell and gene therapy ethics and gene technology ethics, which are all publicly available on our website.
Animal welfare
Policies
It is not currently possible to examine the complex interactions in a living organism using only methods that do not involve animals, such as in silico methods, cell cultures and tissues. Therefore, research involving living
animals remains crucial in the discovery, development and production of
new pharmaceutical products, to ensure that our products meet high quality and safety standards throughout their life cycle.
Novo Nordisk’s bioethics policy, with reference to animal ethics, sets out our high ethical and welfare standards and applies to all animals purchased for research undertaken by us either in-house or by external contractors. The policy includes animal ethics principles that we uphold, namely: Replace, Reduce and Refine (3Rs) research when using animals, defines practices related to housing, husbandry, care and transportation of animals, and their health control, and thereby ensures that every precaution is taken to reduce suffering and distress.
Following the principles of the 3Rs, we continually strive to reduce the number of animals used and to replace animal testing with in vitro methods. We approach the use of non-human primates with care and consideration, only using them when absolutely necessary for efficacy and safety prior to testing
in humans, for example when testing potential new therapies where homology to the human genome is essential.
We have various channels for the expression of any concerns, such as via the attending veterinarian, the local Animal Unit Manager or the Ethical Review Council, an internal group established to ensure the ethical review of all experiments on living and sentient animals performed at, or on behalf of, Novo Nordisk.
Novo Nordisk’s animal rights principles as set out in our bioethics policy comply with the Council of Europe’s Convention for the Protection of Vertebrate Animals
used for Experimental and Other Scientific Purposes, forming the basis for Directive 2010/63/EU, which is focused on the protection of animals used for scientific purposes. Novo Nordisk is also a signatory to the Marseille Declaration, establishing, together with our pharmaceutical peers, the worldwide implementation of high standards for animals housed and used internally and externally by the industry for scientific purposes. We continue to engage in discussion on animal ethics and welfare issues, gaining insights from stakeholder dialogues collaborations with animal welfare organisations2, regulators, researchers, students and journalists.
Performance
The number of animals purchased for research in 2024 decreased by 13% compared to 2023. 96% of the animals were rodents. The decrease is due to
our continuous efforts to reduce the number of animals used in research. It
also reflects the nature and maturity of the research projects, where species qualification determines the number needed for testing in non-human primates (decreased by 55% from 2023). The significant decrease in the number of fish since 2022, to none in 2024, is due to specific research projects using fish larvae that have been discontinued.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4.1.8 Animals purchased for research |
Unit |
2024 |
2023 |
2022 |
| Mice, rats and other rodents |
Number |
47,478 |
54,410 |
63,760 |
| Pigs |
Number |
615 |
608 |
427 |
| Rabbits |
Number |
689 |
289 |
606 |
| Dogs |
Number |
126 |
356 |
146 |
| Non-human primates |
Number |
366 |
807 |
700 |
| Fish |
Number |
0 |
36 |
14,098 |
| Other vertebrates |
Number |
10 |
2 |
13 |
| Total animals purchased |
Number |
49,284 |
56,508 |
79,750 |
ACCOUNTING POLICIES
Animals purchased for research
Number of animals purchased for all research undertaken by Novo Nordisk, either in-house or by external contractors. It is based on internal registration
of purchased animals and yearly reports from external contractors.
2. These include the Danish Animal Welfare Society, the UK’s Royal Society for the Prevention of Cruelty to Animals, the Danish Association of the Pharmaceutical Industry and the Universities Federation for Animal Welfare.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Appendix |
|
Tables in accordance with ESRS 2 General Disclosures and the EU Taxonomy Regulation:
Table 1 – Other legislation
The table below includes all of the data points that derive from other EU legislation as listed in ESRS 2 appendix B, indicating were the data points can be found in our report and which data points are assessed as not applicable to Novo Nordisk.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Disclosure requirement |
Data point |
SFDR reference |
Pillar 3 reference |
Benchmark regulation reference |
EU Climate Law reference |
Section |
Page |
| ESRS 2 GOV-1 |
21 (d) |
x |
|
x |
|
Sustainability statement |
87 |
|
| ESRS 2 GOV-1 |
21 (e) |
|
|
x |
|
Sustainability statement |
42-44 |
| ESRS 2 GOV-4 |
30 |
x |
|
|
|
Sustainability statement |
49 |
| ESRS 2 SBM-1 |
40 (d) i |
x |
x |
x |
|
Not applicable to NN |
- |
| ESRS 2 SBM-1 |
40 (d) ii |
x |
|
x |
|
Not applicable to NN |
- |
| ESRS 2 SBM-1 |
40 (d) iii |
x |
|
x |
|
Not applicable to NN |
- |
| ESRS 2 SBM-1 |
40 (d) iv |
|
|
x |
|
Not applicable to NN |
- |
| ESRS E1-1 |
14 |
|
|
|
x |
Sustainability statement |
55 |
| ESRS E1-1 |
16 (g) |
|
x |
x |
|
Sustainability statement |
55 |
| ESRS E1-4 |
34 |
x |
x |
x |
|
Sustainability statement |
55, 57, 58 |
| ESRS E1-5 |
38 |
x |
|
|
|
Sustainability statement |
58, 59 |
| ESRS E1-5 |
37 |
x |
|
|
|
Sustainability statement |
58, 59 |
| ESRS E1-5 |
40-43 |
x |
|
|
|
Sustainability statement |
58 |
| ESRS E1-6 |
44 |
x |
x |
x |
|
Sustainability statement |
58 |
| ESRS E1-6 |
53-55 |
x |
x |
x |
|
Sustainability statement |
58 |
| ESRS E1-7 |
56 |
|
|
|
x |
Not applicable to NN |
- |
| ESRS E1-9 |
66 |
|
|
x |
|
Not applicable to NN |
- |
| ESRS E1-9 |
66 (a); 66 (c) |
|
x |
|
|
Not applicable to NN |
- |
| ESRS E1-9 |
67 (c) |
|
x |
|
|
Not applicable to NN |
- |
| ESRS E1-9 |
69 |
|
|
x |
|
Not applicable to NN |
- |
| ESRS E2-4 |
28 |
x |
|
|
|
Not applicable to NN |
- |
| ESRS E3-1 |
9 |
x |
|
|
|
Sustainability statement |
66 |
| ESRS E3-1 |
13 |
x |
|
|
|
Not applicable to NN |
- |
| ESRS E3-1 |
14 |
x |
|
|
|
Not applicable to NN |
- |
| ESRS E3-4 |
28 (C) |
x |
|
|
|
Sustainability statement |
66 |
| ESRS E3-4 |
29 |
x |
|
|
|
Sustainability statement |
66 |
ESRS 2- IRO 1 - E4 |
16 (a) i |
x |
|
|
|
Not applicable to NN |
- |
ESRS 2- IRO 1 - E4 |
16 (b) |
x |
|
|
|
Not applicable to NN |
- |
ESRS 2- IRO 1 - E4 |
16 (c) |
x |
|
|
|
Sustainability statement |
67 |
| ESRS E4-2 |
24 (b) |
x |
|
|
|
Sustainability statement |
67 |
| ESRS E4-2 |
24 (c) |
x |
|
|
|
Not applicable to NN |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Disclosure requirement |
Data point |
SFDR reference |
Pillar 3 reference |
Benchmark regulation reference |
EU Climate Law reference |
Section |
Page |
| ESRS E4-2 |
24 (d) |
x |
|
|
|
Sustainability statement |
67 |
| ESRS E5-5 |
37 (d) |
x |
|
|
|
Sustainability statement |
62, 63 |
| ESRS E5-5 |
39 |
x |
|
|
|
Sustainability statement |
62, 63 |
ESRS 2- SBM 3 - S1 |
14 (f) |
x |
|
|
|
Sustainability statement |
80 |
ESRS 2- SBM 3 - S1 |
14 (g) |
x |
|
|
|
Sustainability statement |
80 |
| ESRS S1-1 |
20 |
x |
|
|
|
Sustainability statement |
80, 81 |
| ESRS S1-1 |
21 |
|
|
x |
|
Sustainability statement |
81 |
| ESRS S1-1 |
22 |
x |
|
|
|
Sustainability statement |
81 |
| ESRS S1-1 |
23 |
x |
|
|
|
Sustainability statement |
83, 84 |
| ESRS S1-3 |
32 (c) |
x |
|
|
|
Sustainability statement |
81 |
| ESRS S1-14 |
88 (b), 88 (c) |
x |
|
x |
|
Sustainability statement |
84, 85 |
| ESRS S1-14 |
88 (e) |
x |
|
|
|
Not applicable to NN |
- |
| ESRS S1-16 |
97 (a) |
x |
|
x |
|
Sustainability statement |
87 |
| ESRS S1-16 |
97 (b) |
x |
|
|
|
Sustainability statement |
87 |
| ESRS S1-17 |
103 (a) |
x |
|
|
|
Sustainability statement |
83 |
| ESRS S1-17 |
104 (a) |
x |
|
x |
|
Sustainability statement |
83 |
ESRS 2- SBM 3 - S2 |
11 (b) |
x |
|
|
|
Sustainability statement |
88 |
| ESRS S2-1 |
17 |
x |
|
|
|
Sustainability statement |
88, 89 |
| ESRS S2-1 |
18 |
x |
|
|
|
Sustainability statement |
88 |
| ESRS S2-1 |
19 |
x |
|
x |
|
Sustainability statement |
88 |
| ESRS S2-1 |
19 |
|
|
x |
|
Sustainability statement |
88 |
| ESRS S2-4 |
36 |
x |
|
|
|
Not applicable to NN |
- |
| ESRS S3-1 |
16 |
x |
|
|
|
Not applicable to NN |
- |
| ESRS S3-1 |
17 |
x |
|
x |
|
Not applicable to NN |
- |
| ESRS S3-4 |
36 |
x |
|
|
|
Not applicable to NN |
- |
| ESRS S4-1 |
16 |
x |
|
|
|
Sustainability statement |
72 |
| ESRS S4-1 |
17 |
x |
|
x |
|
Sustainability statement |
72 |
| ESRS S4-4 |
35 |
x |
|
|
|
Not applicable to NN |
- |
| ESRS G1-1 |
10 (b) |
x |
|
|
|
Not applicable to NN |
- |
| ESRS G1-1 |
10 (d) |
x |
|
|
|
Not applicable to NN |
- |
| ESRS G1-4 |
24 (a) |
x |
|
x |
|
Sustainability statement |
91 |
| ESRS G1-4 |
24 (b) |
x |
|
|
|
Sustainability statement |
91 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Appendix |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 2 – Disclosure requirements in ESRS covered by the Sustainability statement |
|
Table 3 – List of incorporations by reference |
| ESRS 2 – General disclosures |
|
ESRS E2 – Pollution |
|
ESRS E5 – Resource use and
circular economy |
|
ESRS S2 – Workers in the
value chain |
|
ESRS disclosure requirement |
|
Incorporation by reference |
| Disclosure requirement |
Page |
|
Disclosure requirement |
Page |
|
|
|
ESRS 2 GOV-1 (21 a-e, 23 a, b); G1 GOV-1 (5 a, b): Roles and responsibilities of Board of Directors and Executive Management |
|
See Annual review, subheading ‘Competences' on pages 42 and 43 and section 'Independence and meeting attendance overview' on page 44 (and
as additional reference within the Sustainability statement: see table 3.2.7 'Diversity metrics – Management levels' on page 87).
|
| BP-1: Basis for preparation |
49 |
|
ESRS 2 IRO-1: Processes |
52, 53, 64 |
|
Disclosure requirement |
Page |
|
Disclosure requirement |
Page |
|
|
| BP-2: Specific circumstances |
49 |
|
E2-1: Policies |
64 |
|
ESRS 2 IRO-1: Processes |
52, 53, 60 |
|
ESRS 2 SBM 2: Stakeholders |
51 |
|
|
| GOV-1: Governance roles |
50, 51 |
|
E2-2: Actions |
64 |
|
E5-1: Policies |
60 |
|
ESRS 2 SBM 3: Strategy |
88 |
|
ESRS 2 GOV-2 (26 b): Overseeing sustainability matters |
|
See Corporate governance report, page 4, sub-section ‘Strategy’ and Annual review: page 39, sub-sections ‘Access and affordability’, ‘Environmental impact’ and ‘Ethics and compliance’. |
| GOV-2: Governance |
50, 51 |
|
E2-3: Targets |
64 |
|
E5-2: Actions |
60, 61 |
|
S2-1: Policies |
88, 89 |
|
|
| GOV-3: Incentives schemes |
50-52, 55 |
|
E2-4: Pollution |
N/A |
|
E5-3: Targets |
61, 62 |
|
S2-2: Processes |
88 |
|
|
| GOV-4: Due diligence |
49 |
|
E2-5: Substances |
64, 65 |
|
E5-4: Resource inflows |
62, 63 |
|
S2-3: Remediate impacts |
88 |
|
ESRS 2 GOV-2 (26 a): Sustainability
matters discussed
|
|
See Corporate governance report, page 4, sub-section ‘Strategy’. |
| GOV-5: Risk management |
51 |
|
E2-6: Financial effects |
N/A |
|
E5-5: Resource outflows |
62, 63 |
|
S2-4: Actions |
89 |
|
|
| SBM-1: Value chain |
49, 52 |
|
|
|
|
E5-6: Financial effects |
N/A |
|
S2-5: Targets |
89 |
|
ESRS 2 GOV-3 (29 a-e): Incentive schemes dependent on sustainability-related targets and performance metrics |
|
See Remuneration report, pages 13-16, 3.5 ‘Short-term incentive programme 2024’ and pages 16-19, 3.6-3.8 ‘Long-term incentive programmes 2022, 2023 and 2024 – programme design’; page 5, table 1, rows: Short-term cash-based incentive programme and Long-term share-based incentive programme for the Board of Directors; and page 9, table 7, rows: Short-term incentive programme (STIP) and Long-term incentive programme (LTIP) for
Executive Management.
|
| SBM-2: Stakeholders |
51 |
|
ESRS E3 – Water and
marine resources |
|
|
|
|
|
|
|
|
| SBM-3: Strategy |
52 |
|
|
ESRS S1 – Own workforce |
|
ESRS S4 – Patient protection and
quality of life |
|
|
| IRO-1: Processes |
52, 53 |
|
Disclosure requirement |
Page |
|
Disclosure requirement |
Page |
|
|
| IRO-2: ESRS DR's covered |
95, 96 |
|
ESRS 2 IRO-1: Processes |
52, 53, 65 |
|
ESRS 2 SBM 2: Stakeholders |
51 |
|
Disclosure requirement |
Page |
|
|
|
|
|
E3-1: Policies |
66 |
|
ESRS 2 SBM 3: Strategy |
80 |
|
ESRS 2 SBM 2: Stakeholders |
51 |
|
ESRS E1, 13 (related to ESRS 2 GOV-3): Portion of total expensed remuneration to registered executives dependent on performance against climate related targets; ESRS 2 GOV-3 (29 d): Portion of total expensed variable remuneration to registered executives dependent on performance against ESG related targets |
|
See Remuneration report, page 20, table 25. |
| ESRS E1 – Climate change |
|
E3-2: Actions |
66 |
|
S1-1: Policies |
81, 83, 85 |
|
ESRS 2 SBM 3: Strategy |
71 |
|
|
| Disclosure requirement |
Page |
|
E3-3: Targets |
66 |
|
S1-2: Processes |
80, 81 |
|
S4-1: Policies |
72, 73, 76-79 |
|
|
| ESRS 2 GOV-3: Governance |
51, 96 |
|
E3-4: Water consumption |
66, 67 |
|
S1-3: Remediate impacts |
81 |
|
S4-2: Processes |
72 |
|
|
| E1-1: Transition plan |
57 |
|
E3-5: Financial effects |
N/A |
|
S1-4: Actions |
81, 84, 86 |
|
S4-3: Remediate impacts |
72 |
|
|
| ESRS 2 SBM-3: Strategy |
55 |
|
|
|
|
S1-5: Targets |
82, 84, 86 |
|
S4-4: Actions |
72-74, 76-79 |
|
|
| ESRS 2 IRO-1: Processes |
52-54 |
|
ESRS E4 – Biodiversity
and ecosystems |
|
S1-6: Own employees |
82-87 |
|
S4-5: Targets |
72-79 |
|
ESRS 2 SBM-1 (42 a-c): Business model
and value chain
|
|
See Annual review, page 9, illustration of the stages from
resources to patients.
|
| E1-2: Policies |
55 |
|
|
S1-7: Non-employees |
N/A |
|
|
|
|
|
| E1-3: Actions |
55, 56 |
|
Disclosure requirement |
Page |
|
S1-8: Bargaining coverage |
82, 83 |
|
ESRS G1 – Business conduct |
|
ESRS 2 BP-2 (12): Forward-looking information |
|
See Annual review, page 35, section ‘Financials’, sub-chapter 'Forward-looking statements' for information on forward-looking information such as targets. |
| E1-4: Targets |
56, 57 |
|
E4-1: Transition plan |
68 |
|
S1-9: Diversity |
87 |
|
Disclosure requirement |
Page |
|
|
| E1-5: Energy consumption |
58, 59 |
|
ESRS 2 SBM-3: Strategy |
67 |
|
S1-10: Adequate wages |
82 |
|
ESRS 2 GOV-1: Governance |
90 |
|
ESRS 2 SBM-1 (40 a, e-g): Sustainability-related goals, significant products,
value chain
|
|
See Annual review, section 'Purpose and sustainability', pages 12-16, for
more on our strategy that relate to sustainability matters (and as additional reference within the Sustainability statement: See page 82, table 3.2.3 'Employees and employee turnover').
|
| E1-6: Scopes 1, 2, and 3 |
57, 58 |
|
ESRS 2 - IRO 1: Processes |
52, 53, 67 |
|
S1-11: Social protection |
N/A |
|
ESRS 2 IRO-1: Processes |
90 |
|
|
| E1-7: GHG removals |
57 |
|
E4-2: Policies |
67 |
|
S1-12: Disabilities |
N/A |
|
G1-1: Corporate culture |
90 |
|
|
| E1-8: Internal carbon pricing |
N/A |
|
E4-3: Actions |
68 |
|
S1-13:Training |
N/A |
|
G1-2: Suppliers |
92, 93 |
|
ESRS E1-1 (disclosure requirement related to ESRS 2 IRO-1 20 b, c): Process to identify and assess climate-related risks |
|
See Annual review, section ‘Risk management’ on page 39 with regards to
the main strategic risk 'environmental impact'.
|
| E1-9: Financial effects |
N/A |
|
E4-4: Targets |
68 |
|
S1-14: Health and safety |
83-85 |
|
G1-3: Prevention |
91 |
|
|
|
|
|
E4-5: Impacts |
67, 68 |
|
S1-15: Work-life balance |
81, 86 |
|
G1-4: Incidents |
91, 92 |
|
|
|
|
|
E4-6: Financial effects |
N/A |
|
S1-16: Compensation |
87 |
|
G1-5: Political influence |
93 |
|
ESRS S4-4 MDR-A (33b): Overview of what action is planned or underway to pursue material opportunities for the undertaking in relation to consumers and/or end-users |
|
See Annual review, section 'Innovation and therapeutic focus', page 17-25,
for an overview of opportunities to accelerate healthcare innovation
across Obesity, Diabetes, Rare Diseases and Cardiovascular & Emerging Therapy Areas.
|
|
|
|
|
|
|
S1-17: Complaints |
83 |
|
G1-6: Payment practices |
92, 93 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1. In addition, a detailed description of the material IROs is given in the topical sections of this Sustainability statement. |
|
|
|
|
ESRS 2 MDR-P (65a): The Novo Nordisk
Way Essentials
|
|
See Annual review, page 15, visualisation ‘The Novo Nordisk Way Essentials’. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Appendix |
|
Tables 4a – Proportion of Turnover from products or services associated with Taxonomy-aligned economic activities – disclosure covering year 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial year 2024 |
2024 |
Substantial contribution criteria |
DNSH criteria (“Does Not Significantly Harm”) |
|
|
|
|
| Economic activities (1) |
Code
(2)
|
Turnover
(3)
|
Proportion of turnover, 2024
(4)
|
Climate change mitigation (5) |
Climate change adaptation (6) |
Water
(7)
|
Pollution
(8)
|
Circular economy (9) |
Biodiversity (10) |
Climate change mitigation (11) |
Climate change adaptation (12) |
Water
(13)
|
Pollution (14) |
Circular economy (15) |
Biodiversity (16) |
Minimum safeguards (17) |
Proportion of Taxonomy-aligned (A.1.) or -eligible (A.2.) turnover, 2023
(18)
|
Category enabling activity
(19)
|
Category transitional activity
(20)
|
|
|
mDkk |
% |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
% |
E |
T |
| A. TAXONOMY-ELIGIBLE ACTIVITIES |
| A.1. Environmentally sustainable activities (Taxonomy-aligned) |
Turnover of environmentally sustainable activities
(Taxonomy-aligned) (A.1) |
|
0 |
0% |
0% |
|
|
|
|
|
|
|
|
|
|
|
|
0% |
|
| Of which enabling |
|
0 |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
|
|
|
|
|
|
|
0% |
|
|
| Of which transitional |
|
0 |
0% |
0% |
|
|
|
|
|
|
|
|
0% |
|
|
| A.2. Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) |
|
EL; N/EL |
EL; N/EL |
EL; N/EL |
EL; N/EL |
EL; N/EL |
EL; N/EL |
|
| Manufacture of medicinal products |
PPC 1.2 |
290,403 |
100% |
N/EL |
N/EL |
N/EL |
EL |
N/EL |
N/EL |
|
|
|
|
|
|
|
100% |
|
|
| Turnover of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2.) |
|
290,403 |
100% |
0% |
0% |
0% |
100% |
0% |
0% |
|
|
|
|
|
|
|
100% |
|
|
| Turnover of Taxonomy-eligible activities (A.1. + A.2.) |
|
290,403 |
100% |
0% |
0% |
0% |
100% |
0% |
0% |
|
|
|
|
|
|
|
100% |
|
|
| B. TAXONOMY-NON-ELIGIBLE ACTIVITIES |
|
| Turnover of Taxonomy-non-eligible activities (B) |
|
0 |
0% |
| TOTAL |
|
290,403 |
100% |
Y – Yes, Taxonomy-eligible and Taxonomy-aligned activity with the relevant environmental objective N – No, Taxonomy-eligible but not Taxonomy-aligned activity with the relevant environmental objective N/EL – Not eligible, Taxonomy-non-eligible activity for the relevant environmental objective |
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Appendix |
|
Tables 4b – Proportion of CapEx from products or services associated with Taxonomy-aligned economic activities – disclosure covering year 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial year 2024 |
2024 |
Substantial contribution criteria |
DNSH criteria ("Does Not Significantly Harm") |
|
| Economic activities (1) |
Code
(2)
|
CapEx
(3)
|
Proportion of CapEx, 2024
(4)
|
Climate change mitigation (5) |
Climate change adaptation (6) |
Water
(7)
|
Pollution
(8)
|
Circular economy (9) |
Biodiversity (10) |
Climate change mitigation (11) |
Climate change adaptation (12) |
Water
(13)
|
Pollution (14) |
Circular economy (15) |
Biodiversity (16) |
Minimum safeguards (17) |
Proportion of Taxonomy-aligned (A.1.) or eligible (A.2.)
CapEx,
2023
(18)
|
Category enabling activity
(19)
|
Category transitional activity
(20)
|
|
|
mDkk |
% |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
% |
E |
T |
| A. TAXONOMY-ELIGIBLE ACTIVITIES |
| A.1. Environmentally sustainable activities (Taxonomy-aligned) |
| Construction on new buildings |
CCM 7.1 |
3,494 |
|
3% |
Y |
N/EL |
N/EL |
N/EL |
N/EL |
N/EL |
Y |
Y |
Y |
Y |
Y |
Y |
Y |
0% |
|
|
CapEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) |
|
3,494 |
3% |
3% |
|
|
|
|
|
|
|
|
|
|
|
|
0% |
|
| Of which enabling |
|
0 |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
|
|
|
|
|
|
|
0% |
|
|
| Of which transitional |
|
0 |
0% |
0% |
|
|
|
|
|
|
|
|
0% |
|
|
| A.2. Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) |
|
EL; N/EL |
EL; N/EL |
EL; N/EL |
EL; N/EL |
EL; N/EL |
EL; N/EL |
|
| Construction on new buildings |
CCM 7.1 |
13,050 |
|
11% |
EL |
N/EL |
N/EL |
N/EL |
N/EL |
N/EL |
|
|
|
|
|
|
|
14% |
|
|
| Renovation of buildings |
CCM 7.2 |
2,336 |
|
2% |
EL |
N/EL |
N/EL |
N/EL |
N/EL |
N/EL |
|
|
|
|
|
|
|
5% |
|
|
| Manufacture of medicinal products |
PPC 1.2 |
20,142 |
|
16% |
N/EL |
N/EL |
N/EL |
EL |
N/EL |
N/EL |
|
|
|
|
|
|
|
41% |
|
|
| CapEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2.) |
|
35,528 |
|
29% |
13% |
0% |
0% |
16% |
0% |
0% |
|
|
|
|
|
|
|
60% |
|
|
| CapEx of Taxonomy-eligible activities (A.1. + A.2.) |
|
39,022 |
|
32% |
16% |
0% |
0% |
16% |
0% |
0% |
|
|
|
|
|
|
|
60 |
% |
|
|
| B. TAXONOMY-NON-ELIGIBLE ACTIVITIES |
|
| CapEx of Taxonomy-non-eligible activities (B) |
|
84,950 |
|
68% |
| TOTAL |
|
123,972 |
|
100% |
| Y – Yes, Taxonomy-eligible and Taxonomy-aligned activity with the relevant environmental objective N – No, Taxonomy-eligible but not Taxonomy-aligned activity with the relevant environmental objective N/EL – Not eligible, Taxonomy-non-eligible activity for the relevant environmental objective |
|
|
|
|
|
|
|
|
|
|
|
|
|
Sustainability statement / Appendix |
|
Tables 4c – Proportion of OpEx from products or services associated with Taxonomy-aligned economic activities – disclosure covering year 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial year 2024 |
2024 |
Substantial contribution criteria |
DNSH criteria (“Does Not Significantly Harm”) |
|
| Economic activities (1) |
Code
(2)
|
OpEx
(3)
|
Proportion of OpEx, 2024
(4)
|
Climate change mitigation (5) |
Climate change adaptation (6) |
Water
(7)
|
Pollution
(8)
|
Circular economy (9) |
Biodiversity (10) |
Climate change mitigation (11) |
Climate change adaptation (12) |
Water
(13)
|
Pollution (14) |
Circular economy (15) |
Biodiversity (16) |
Minimum safeguards (17) |
Proportion of Taxonomy-aligned (A.1.) or -eligible (A.2.)
OpEx,
2023
(18)
|
Category enabling activity
(19)
|
Category transitional activity
(20)
|
|
|
mDkk |
% |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y; N; N/EL |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
% |
E |
T |
| A. TAXONOMY-ELIGIBLE ACTIVITIES |
| A.1. Environmentally sustainable activities (Taxonomy-aligned) |
OpEx of environmentally sustainable activities
(Taxonomy-aligned) (A.1) |
|
0 |
0% |
0% |
|
|
|
|
|
|
|
|
|
|
|
|
0% |
|
| Of which enabling |
|
0 |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
|
|
|
|
|
|
|
0% |
|
|
| Of which transitional |
|
0 |
0% |
0% |
|
|
|
|
|
|
|
|
0% |
|
|
| A.2. Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) |
|
EL; N/EL |
EL; N/EL |
EL; N/EL |
EL; N/EL |
EL; N/EL |
EL; N/EL |
|
| Manufacture of medicinal products |
P 1.2 |
1,919 |
|
5% |
N/EL |
N/EL |
N/EL |
EL |
N/EL |
N/EL |
|
5% |
|
| OpEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2.) |
|
1,919 |
|
5% |
0% |
0% |
0% |
5% |
0% |
0% |
5% |
| OpEx of Taxonomy-eligible activities (A.1. + A.2.) |
|
1,919 |
|
5% |
0% |
0% |
0% |
5% |
0% |
0% |
5% |
| B. TAXONOMY-NON-ELIGIBLE ACTIVITIES |
|
| OpEx of Taxonomy-non-eligible activities (B) |
|
38,014 |
|
95% |
| TOTAL |
|
39,933 |
|
100% |
Y – Yes, Taxonomy-eligible and Taxonomy-aligned activity with the relevant environmental objective N – No, Taxonomy-eligible but not Taxonomy-aligned activity with the relevant environmental objective N/EL – Not eligible, Taxonomy-non-eligible activity for the relevant environmental objective |
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements |
|
Consolidated financial statements
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Income statement and Statement of comprehensive income |
102 |
|
|
| Cash flow statement |
103 |
|
|
| Balance sheet |
104 |
|
|
| Equity statement |
105 |
|
|
| Notes to the Consolidated financial statements |
106 |
|
|
| Section 1 |
106 |
|
|
| Basis of preparation |
106 |
|
|
|
Material accounting policies and key accounting estimates and judgements |
106 |
|
|
|
Changes in accounting policies and disclosures |
106 |
|
|
| Section 2 |
107 |
|
|
| Results for the year |
107 |
|
|
| 2.1 |
Net sales and rebates |
107 |
|
|
| 2.2 |
Segment information |
108 |
|
|
| 2.3 |
Research and development costs |
110 |
|
|
| 2.4 |
Employee costs |
110 |
|
|
| 2.5 |
Other operating income and expenses |
111 |
|
|
| 2.6 |
Income taxes and deferred income taxes |
111 |
|
|
| Section 3 |
113 |
|
|
| Operating assets and liabilities |
113 |
|
|
| 3.1 |
Intangible assets |
113 |
|
|
| 3.2 |
Property, plant and equipment |
115 |
|
|
| 3.3 |
Inventories |
116 |
|
|
| 3.4 |
Trade receivables |
116 |
|
|
| 3.5 |
Provisions and contingent liabilities |
117 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Section 4 |
119 |
|
Capital structure and financial items |
119 |
|
4.1 |
Earnings per share |
119 |
|
4.2 |
Distribution to shareholders |
119 |
|
4.3 |
Share capital, Treasury shares and Other reserves |
119 |
|
4.4 |
Financial risks |
120 |
|
4.5 |
Derivative financial instruments |
122 |
|
4.6 |
Borrowings |
123 |
|
4.7 |
Cash flow statement specifications |
125 |
|
4.8 |
Financial assets and liabilities |
126 |
|
4.9 |
Financial income and expenses |
127 |
|
Section 5 |
128 |
|
Other disclosures |
128 |
|
5.1 |
Share-based payment schemes |
128 |
|
5.2 |
Commitments |
130 |
|
5.3 |
Acquisition of businesses |
130 |
|
5.4 |
Related party transactions |
131 |
|
5.5 |
Fees to statutory auditors |
132 |
|
5.6 |
General accounting policies |
132 |
|
5.7 |
Companies in the Novo Nordisk Group |
133 |
|
Part of the Annual review (not audited) |
134 |
|
Financial definitions and ratios |
134 |
|
Non-IFRS financial measures |
135 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Income statement and Statement of comprehensive income |
|
Income statement and Statement of comprehensive income
for the year ended 31 December
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Note |
|
2024 |
|
2023 |
2022 |
| Income statement |
|
|
|
|
|
|
| Net sales |
2.1, 2.2 |
|
290,403 |
|
232,261 |
176,954 |
| Cost of goods sold |
2.2 |
|
(44,522) |
|
(35,765) |
(28,448) |
| Gross profit |
|
|
245,881 |
|
196,496 |
148,506 |
| Sales and distribution costs |
2.2 |
|
(62,101) |
|
(56,743) |
(46,217) |
| Research and development costs |
2.2, 2.3 |
|
(48,062) |
|
(32,443) |
(24,047) |
| Administrative costs |
2.2 |
|
(5,276) |
|
(4,855) |
(4,467) |
| Other operating income and expenses |
2.2, 2.5 |
|
(2,103) |
|
119 |
1,034 |
| Operating profit |
|
|
128,339 |
|
102,574 |
74,809 |
| Financial income |
4.9 |
|
6,198 |
|
2,945 |
239 |
| Financial expenses |
4.9 |
|
(7,346) |
|
(845) |
(5,986) |
| Profit before income taxes |
|
|
127,191 |
|
104,674 |
69,062 |
| Income taxes |
2.6 |
|
(26,203) |
|
(20,991) |
(13,537) |
| Net profit |
|
|
100,988 |
|
83,683 |
55,525 |
|
|
|
|
|
|
|
| Earnings per share |
|
|
|
|
|
|
| Basic earnings per share (DKK) |
4.1 |
|
22.67 |
|
18.67 |
12.26 |
| Diluted earnings per share (DKK) |
4.1 |
|
22.63 |
|
18.62 |
12.22 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Note |
|
2024 |
|
2023 |
2022 |
| Statement of comprehensive income |
|
|
|
|
|
|
| Net profit |
|
|
100,988 |
|
83,683 |
55,525 |
|
|
|
|
|
|
|
| Other comprehensive income: |
|
|
|
|
|
|
| Remeasurements of retirement benefit obligations |
|
|
(119) |
|
13 |
615 |
|
|
|
|
|
|
|
| Items that will not be reclassified subsequently to the income statement |
|
|
(119) |
|
13 |
615 |
|
|
|
|
|
|
|
| Exchange rate adjustments of investments in subsidiaries |
4.3 |
|
3,096 |
|
(1,404) |
2,289 |
| Cash flow hedges: |
|
|
|
|
|
|
| Realisation of previously deferred (gains)/losses |
4.3, 4.5 |
|
(1,612) |
|
(1,026) |
1,740 |
| Deferred gains/(losses) related to acquisition of businesses |
4.3, 4.5 |
|
1,154 |
|
— |
— |
| Deferred gains/(losses) on hedges open at year-end |
4.3, 4.5 |
|
(5,763) |
|
1,612 |
1,026 |
| Tax and other items |
4.3 |
|
1,343 |
|
(355) |
(892) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Items that will be reclassified subsequently to the income statement |
|
|
(1,782) |
|
(1,173) |
4,163 |
|
|
|
|
|
|
|
| Other comprehensive income |
|
|
(1,901) |
|
(1,160) |
4,778 |
| Total comprehensive income |
|
|
99,087 |
|
82,523 |
60,303 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Cash flow statement |
|
Cash flow statement
for the year ended 31 December
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Note |
|
2024 |
|
2023 |
2022 |
| Cash flow statement |
|
|
|
|
|
|
| Net profit |
|
|
100,988 |
|
83,683 |
55,525 |
| Adjustment of non-cash items: |
|
|
|
|
|
|
| Income taxes in the income statement |
2.6 |
|
26,203 |
|
20,991 |
13,537 |
| Depreciation, amortisation and impairment losses |
3.1, 3.2 |
|
19,107 |
|
9,413 |
7,362 |
| Other non-cash items |
4.7 |
|
15,029 |
|
33,517 |
22,509 |
| Changes in working capital |
4.7 |
|
(11,995) |
|
(13,380) |
(5,535) |
| Interest received |
|
|
1,884 |
|
1,072 |
276 |
| Interest paid |
|
|
(612) |
|
(491) |
(272) |
| Income taxes paid |
2.6 |
|
(29,636) |
|
(25,897) |
(14,515) |
| Net cash flows from operating activities |
|
|
120,968 |
|
108,908 |
78,887 |
| Purchase of intangible assets |
3.1 |
|
(4,145) |
|
(13,090) |
(2,607) |
|
|
|
|
|
|
|
| Purchase of property, plant and equipment |
3.2 |
|
(47,164) |
|
(25,806) |
(12,146) |
| Cash used for acquisition of businesses |
5.3 |
|
(82,163) |
|
— |
(7,075) |
| Proceeds from other financial assets |
|
|
— |
|
33 |
— |
| Purchase of other financial assets |
|
|
(786) |
|
(271) |
(169) |
| Purchase of marketable securities |
|
|
(19,028) |
|
(13,018) |
(9,566) |
| Sale of marketable securities |
|
|
24,391 |
|
8,260 |
6,645 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net cash flows from investing activities |
|
|
(128,895) |
|
(43,892) |
(24,918) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Note |
|
2024 |
|
2023 |
2022 |
| Purchase of treasury shares |
4.2 |
|
(20,181) |
|
(29,924) |
(24,086) |
| Dividends paid |
4.2 |
|
(44,140) |
|
(31,767) |
(25,303) |
| Proceeds from borrowings |
4.6 |
|
79,391 |
|
— |
11,215 |
| Repayment of borrowings |
4.6 |
|
(6,335) |
|
(1,467) |
(13,623) |
| Net cash flows from financing activities |
|
|
8,735 |
|
(63,158) |
(51,797) |
|
|
|
|
|
|
|
| Net cash generated from activities |
|
|
808 |
|
1,858 |
2,172 |
| Cash and cash equivalents at the beginning of the year |
|
|
14,392 |
|
12,653 |
10,719 |
|
|
|
|
|
|
|
| Exchange gains/(losses) on cash and cash equivalents |
|
|
455 |
|
(119) |
(238) |
| Cash and cash equivalents at the end of the year |
|
|
15,655 |
|
14,392 |
12,653 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Balance sheet |
|
Balance sheet
at 31 December
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Note |
|
2024 |
|
2023 |
| Assets |
|
|
|
|
|
| Intangible assets |
3.1 |
|
111,090 |
|
60,406 |
| Property, plant and equipment |
3.2 |
|
162,488 |
|
90,961 |
| Investments in associated companies |
|
|
400 |
|
410 |
| Deferred income tax assets |
2.6 |
|
24,627 |
|
20,380 |
| Other receivables and prepayments |
4.8 |
|
4,016 |
|
1,430 |
| Other financial assets |
4.8 |
|
2,277 |
|
1,253 |
| Total non-current assets |
|
|
304,898 |
|
174,840 |
|
|
|
|
|
|
| Inventories |
3.3 |
|
40,849 |
|
31,811 |
| Trade receivables |
3.4 |
|
71,949 |
|
64,770 |
| Tax receivables |
|
|
2,853 |
|
2,423 |
| Other receivables and prepayments |
4.8 |
|
12,612 |
|
8,068 |
| Marketable securities |
4.4 |
|
10,653 |
|
15,838 |
| Derivative financial instruments |
4.5 |
|
6,326 |
|
2,344 |
| Cash at bank |
4.4 |
|
15,655 |
|
14,392 |
| Total current assets |
|
|
160,897 |
|
139,646 |
|
|
|
|
|
|
| Total assets |
|
|
465,795 |
|
314,486 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Note |
|
2024 |
|
|
2023 |
| Equity and liabilities |
|
|
|
|
|
|
| Share capital |
4.3 |
|
446 |
|
|
451 |
| Treasury shares |
4.3 |
|
(2) |
|
|
(5) |
| Retained earnings |
|
|
144,448 |
|
|
104,839 |
| Other reserves |
4.3 |
|
(1,406) |
|
|
1,276 |
| Total equity |
|
|
143,486 |
|
|
106,561 |
|
|
|
|
|
|
|
| Borrowings |
4.6 |
|
89,674 |
|
|
20,528 |
| Deferred income tax liabilities |
2.6 |
|
5,426 |
|
|
10,162 |
| Retirement benefit obligations |
|
|
903 |
|
|
742 |
| Other liabilities |
4.8 |
|
23 |
|
|
189 |
| Provisions |
3.5 |
|
8,755 |
|
|
6,649 |
| Total non-current liabilities |
|
|
104,781 |
|
|
38,270 |
|
|
|
|
|
|
|
| Borrowings |
4.6 |
|
13,113 |
|
|
6,478 |
| Trade payables |
|
|
28,846 |
|
|
25,606 |
| Tax payables |
|
|
9,716 |
|
|
7,116 |
| Other liabilities |
4.8 |
|
37,993 |
|
|
28,705 |
| Derivative financial instruments |
4.5 |
|
7,531 |
|
|
1,272 |
| Provisions |
3.5 |
|
120,329 |
|
|
100,478 |
| Total current liabilities |
|
|
217,528 |
|
|
169,655 |
| Total liabilities |
|
|
322,309 |
|
|
207,925 |
|
|
|
|
|
|
|
| Total equity and liabilities |
|
|
465,795 |
|
|
314,486 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Equity statement |
|
Equity statement
at 31 December
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2024 |
|
2023 |
|
2022 |
| DKK million |
Share capital |
Treasury shares |
Retained earnings |
Other reserves |
|
Total |
|
Share capital |
Treasury shares |
Retained earnings |
Other reserves |
|
Total |
|
Share capital |
Treasury shares |
Retained earnings |
Other reserves |
|
Total |
| Balance at the beginning of the year |
451 |
(5) |
104,839 |
1,276 |
|
106,561 |
|
456 |
(6) |
80,587 |
2,449 |
|
83,486 |
|
462 |
(6) |
72,004 |
(1,714) |
|
70,746 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net profit |
|
|
100,988 |
|
|
100,988 |
|
|
|
83,683 |
|
|
83,683 |
|
|
|
55,525 |
|
|
55,525 |
| Other comprehensive income |
|
|
(119) |
(1,782) |
|
(1,901) |
|
|
|
13 |
(1,173) |
|
(1,160) |
|
|
|
615 |
4,163 |
|
4,778 |
| Total comprehensive income |
|
|
100,869 |
(1,782) |
|
99,087 |
|
|
|
83,696 |
(1,173) |
|
82,523 |
|
|
|
56,140 |
4,163 |
|
60,303 |
Transfer of cash flow hedge reserve to intangible assets (note 4.3) |
|
|
|
(900) |
|
(900) |
|
|
|
|
— |
|
— |
|
|
|
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Transactions with owners: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dividends (note 4.2) |
|
|
(44,140) |
|
|
(44,140) |
|
|
|
(31,767) |
|
|
(31,767) |
|
|
|
(25,303) |
|
|
(25,303) |
Share-based payments (note 5.1) |
|
|
2,289 |
|
|
2,289 |
|
|
|
2,149 |
|
|
2,149 |
|
|
|
1,539 |
|
|
1,539 |
Purchase of treasury shares (note 4.3) |
|
(2) |
(20,179) |
|
|
(20,181) |
|
|
(4) |
(29,920) |
|
|
(29,924) |
|
|
(6) |
(24,080) |
|
|
(24,086) |
Reduction of the B share capital (note 4.3) |
(5) |
5 |
|
|
|
— |
|
(5) |
5 |
|
|
|
— |
|
(6) |
6 |
|
|
|
— |
| Tax related to transactions with owners |
|
|
770 |
|
|
770 |
|
|
|
94 |
|
|
94 |
|
|
|
287 |
|
|
287 |
| Balance at the end of the year |
446 |
(2) |
144,448 |
(1,406) |
|
143,486 |
|
451 |
(5) |
104,839 |
1,276 |
|
106,561 |
|
456 |
(6) |
80,587 |
2,449 |
|
83,486 |
Refer to note 4.3 for details of movements in Other reserves.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 1.1 Material accounting policies and key accounting estimates and judgements |
|
Notes to the Consolidated financial statements
Section 1
Basis of preparation
1.1 Material accounting policies and key accounting estimates and judgements
The Consolidated financial statements included in this Annual Report have been prepared in accordance with IFRS® Accounting Standards as issued by the International Accounting Standards Board (IASB) and in accordance with IFRS Accounting Standards as endorsed by the EU and further requirements in the Danish Financial Statements Act.
Measurement basis
The Consolidated financial statements have been prepared on the historical cost basis except for derivative financial instruments, equity investments, marketable securities and trade receivables in a factoring portfolio, which are measured at fair value.
Material accounting policies
Apart from the general accounting policies, which are described in note 5.6, Novo Nordisk’s accounting policies are described in each of the individual notes to the Consolidated financial statements. The accounting policies have been applied consistently in the preparation of the Consolidated financial statements for all the years presented.
Key accounting estimates and judgements
The use of reasonable estimates and judgements is an essential part of the preparation of the Consolidated financial statements. Given the uncertainties inherent in Novo Nordisk’s business activities, Management must make certain estimates regarding valuation and make judgements on the reported amounts of assets, liabilities, net sales, expenses and related disclosures.
The key accounting estimates identified are those that have a significant risk of resulting in a material adjustment to the carrying amount of assets and liabilities in the following reporting period. An example being the estimation of US sales deductions and provisions for sales rebates.
When determining estimates and assumptions, Management has assessed the qualitative and quantitative impact of climate-related matters. It is Management’s assessment that the effect of climate-related matters does not significantly impact estimates and assumptions.
Management bases its estimates on historical experience and various other assumptions that are held to be reasonable under the circumstances. The estimates and underlying assumptions are reviewed on an ongoing basis. If necessary, changes are recognised in the period in which the estimate is revised. Management considers the key accounting estimates to be reasonable and appropriate based on currently available information. The actual amounts may differ from the amounts estimated as more detailed information becomes available.
In addition, Management may make certain judgements in the process of applying
the entity’s accounting policies, for example the Judgement of whether intangible assets acquired in a business combination are separately identifiable.
Management regards those listed below as the key accounting estimates and judgements applied in the preparation of the Consolidated financial statements.
Refer to the specific notes for further information on the key accounting estimates
and judgements as well as assumptions applied.
Applying materiality
The Consolidated financial statements are a result of processing large numbers
of transactions and aggregating those transactions into classes according to their
nature or function. The transactions are presented in classes of similar items in
the Consolidated financial statements. If a line item is not individually material, it
is aggregated with other items of a similar nature in the Consolidated financial statements or in the notes.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Key accounting estimates and judgements |
Risk |
Note(s) |
| Estimate of US sales deductions and provisions for sales rebates |
High |
2.1, 3.5 |
| Estimate in determining fair values of assets acquired in a business combination and in impairment reviews of intangible assets |
High |
3.1, 5.3 |
| Judgement of whether intangible assets acquired in a business combination are separately identifiable |
High |
5.3 |
|
|
|
| Estimate regarding deferred income tax assets and provision for uncertain tax positions |
Medium |
2.6 |
| Estimate of ongoing legal disputes, litigation and investigations |
Medium |
3.5 |
|
|
|
Management provides the specific disclosures required by IFRS Accounting Standards unless the information is not applicable or is considered immaterial to the decision-making of the primary users of these financial statements.
1.2 Changes in accounting policies and disclosures
Management has assessed that new or amended IFRS Accounting Standards and interpretations issued by the IASB and endorsed by the EU effective on or after 1 January 2024 has not had a significant effect on the Consolidated financial statements.
Furthermore, new or amended IFRS Accounting Standards and interpretations issued by the IASB that have not yet become effective are generally not adopted until they become effective and endorsed by the EU. Management does not anticipate any significant impact on the Consolidated financial statements in the period of initial application from the adoption of these new standards and amendments, apart from IFRS 18 ‘Presentation and Disclosure in Financial Statements’ which replaces IAS 1 effective from 1 January 2027. The new IFRS 18 is expected to change the presentation of the Income statement and to differentiate between earnings from operating activities, investment activities and financing activities. IFRS 18 will also add additional disclosures but will not change any accounting policies on recognition and measurement, hence it will not change reported net results.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 2.1 Net sales and rebates |
|
Section 2
Results for the year
2.1 Net sales and rebates
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Gross-to-net sales reconciliation |
| DKK million |
|
2024 |
|
2023 |
2022 |
| Gross sales |
|
680,563 |
|
608,645 |
455,692 |
| US Managed Care and Medicare |
|
(238,946) |
|
(223,191) |
(161,123) |
| US wholesaler charge-backs |
|
(64,437) |
|
(74,435) |
(56,443) |
| US Medicaid rebates |
|
(32,919) |
|
(31,821) |
(24,667) |
| Other US discounts and sales returns |
|
(30,737) |
|
(28,481) |
(18,300) |
| US rebates, discounts and sales returns |
|
(367,039) |
|
(357,928) |
(260,533) |
| Non-US rebates, discounts and sales returns |
|
(23,121) |
|
(18,456) |
(18,205) |
| Total gross-to-net sales adjustments |
|
(390,160) |
|
(376,384) |
(278,738) |
| Net sales |
|
290,403 |
|
232,261 |
176,954 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Provisions for sales rebates |
| DKK million |
|
2024 |
|
2023 |
2022 |
| At the beginning of the year |
|
99,878 |
|
69,499 |
50,822 |
| Additional provisions, including increases to existing provisions |
|
318,812 |
|
285,266 |
206,354 |
| Amount paid during the year |
|
(299,334) |
|
(250,316) |
(189,580) |
| Adjustments regarding prior years, including unused amounts reversed during the year |
|
(6,452) |
|
(2,364) |
(1,141) |
| Effect of exchange rate adjustment |
|
5,612 |
|
(2,207) |
3,044 |
| At the end of the year |
|
118,516 |
|
99,878 |
69,499 |
Sales discounts and sales rebates are predominantly issued in the US. As such, total US rebates, discounts and sales returns amounts to DKK (367,039) million, corresponding to 69% of gross sales in the US (74% in 2023 and 75% in 2022).
Provisions for sales rebates include US Managed Care, Medicare, Medicaid, 340B
Drug Pricing Program and other US rebate types, as well as rebates in a number
of European countries and Canada.
Pricing mechanisms in the US market
In the US, sales rebates are paid in connection with public healthcare insurance programmes, including Medicare and Medicaid, as well as rebates to pharmacy benefit managers (PBMs) and managed healthcare plans. Key customers in the US include private payers, PBMs and government payers. PBMs and managed healthcare plans play a role in negotiating price concessions with drug manufacturers for both the commercial and government channels, and determine which drugs are covered
on their formularies (or 'preferred drug lists').
US Managed Care and Medicare
For Managed Care and Medicare, rebates are offered to a number of PBMs and managed healthcare plans. These rebate programmes allow the customer to receive a rebate after attaining certain performance parameters relating to formulary status or pre-established market share thresholds. Rebate provisions are estimated according to the specific terms in each agreement, historical experience, anticipated channel mix, growth rates and market share information. Novo Nordisk adjusts the provision periodically to reflect actual sales performance. Managed Care and Medicare rebates are generally settled around 100 days from the transaction date.
US wholesaler charge-backs
Wholesaler charge-backs relate to contractual arrangements between Novo Nordisk and indirect customers in the US whereby products are sold at contract prices lower than the list price originally charged to wholesalers. Chargeback provisions are estimated using a combination of factors such as historical experience, current wholesaler inventory levels, contract terms and the value of claims received but not yet processed. Wholesaler charge-backs are generally settled within 30 days after receipt of claim.
In January 2021, Novo Nordisk changed its policy in the US related to the 340B Drug Pricing Program, whereby Novo Nordisk no longer provides 340B statutory discounts to certain pharmacies that contract with covered entities participating in the 340B Drug Pricing Program. Novo Nordisk has recognised revenue related to the 340B Drug Pricing Program to the extent that it is highly probable that its inclusion will not result in a significant revenue reversal in the future. Management’s assessment considers interpretations of applicable laws, legal and administrative rulings, as well as attrition and experience from historical claims. During 2024, additional provisions for 340B statutory discounts of net USD 0.8 billion were recognised. As of 31 December 2024, provisions for sales rebates comprise a provision for 340B statutory discounts of
USD 4.6 billion.
Refer to note 3.5 for a more elaborate description of the ongoing litigation related to the 340B Drug Pricing Program.
US Medicaid rebates
Medicaid is a government insurance programme. Medicaid rebates have been estimated using a combination of historical experience, product and population growth, price changes and the impact of contracting strategies. The calculation
also involves interpretation of relevant regulations that are subject to changes in interpretative guidance from government authorities. Novo Nordisk adjusts the provision periodically to reflect actual sales performance. Medicaid rebates
are generally settled around 150 days from the transaction date.
Other US and non-US discounts and sales returns
Other discounts are provided to distributors, wholesalers, hospitals, pharmacies, etc. Further, discounts are provided to patients through different programmes.They are usually linked to sales volume or provided as cash discounts. Discounts are calculated based on historical data and recorded as a reduction in gross sales at the time the related sales are recorded. Sales returns relate to damaged or expired products.
Other net sales disclosures
In 2024, Novo Nordisk had 3 major wholesalers distributing products in the US, representing 23%, 17% and 17% respectively of global net sales (22%, 17% and 15% in 2023 and 19%, 14% and 13% in 2022). Sales to these 3 wholesalers are within both Diabetes and Obesity care and Rare disease.
Net sales to be recognised from existing customer contracts containing fixed or minimum sales volumes, with an original term greater than 12 months, are expected to be DKK 3,753 million within 12 months (DKK 3,166 million in 2023) and DKK 5,822 million thereafter (DKK 443 million).
KEY ACCOUNTING ESTIMATES OF SALES DEDUCTIONS AND
PROVISIONS FOR SALES REBATES
Sales deductions are estimated and provided for at the time the related sales
are recorded. These estimates of unsettled rebate, discount and product return obligations is considered a key accounting estimate as not all conditions are known
at the time of sale, for example total sales volume to a given customer. The estimates are based on analyses of existing contractual obligations and historical experience. Provisions are calculated on the basis of a percentage of sales for each product
as defined by the contracts with the various customer groups. Provisions for
sales rebates are adjusted to actual amounts as rebates, discounts and returns
are processed.
Revenue related to the 340B Drug Pricing Program can only be recognised to the extent that it is highly probable that a significant reversal of the recognised revenue will not occur.
Novo Nordisk considers the provisions established for sales rebates to be reasonable and appropriate based on the information currently available. However, the actual amount of rebates and discounts may differ from the amounts estimated by Management as more detailed information becomes available.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 2.2 Segment information |
|
ACCOUNTING POLICIES
Revenue from sale of goods is recognised when Novo Nordisk has transferred
control of products sold to the buyer and it is probable that Novo Nordisk will collect the consideration to which it is entitled for transferring the products. Control of the products is transferred at a single point in time, typically on delivery. The amount of sales to be recognised is based on the consideration Novo Nordisk expects to receive in exchange for its goods. When sales are recognised, Novo Nordisk also records estimates for a variety of sales deductions; including product returns as well as rebates and discounts to government agencies, wholesalers, health insurance companies, managed healthcare organisations and retail customers. Sales deductions are recognised as a reduction of gross sales to arrive at net sales, by assessing the expected value of the sales deductions (variable consideration). Where contracts contain customer acceptance criteria, Novo Nordisk recognises sales when the acceptance criteria are satisfied.
In some markets, Novo Nordisk sells products on a sale-or-return basis. Where there
is historical experience or a reasonably accurate estimate of future returns, estimated product returns are recorded as a reduction in sales. Where shipments of new products are made on a sale-or-return basis, without sufficient historical experience for estimating sales returns, revenue is recorded based on estimated demand and acceptance rates for well-established products with similar market characteristics. If similar market characteristics do not exist, revenue is recorded when there is evidence of consumption or when the right of return has expired.
Unsettled rebates are recognised as provisions when the timing or amount is uncertain (note 3.5).
Where absolute amounts are known, the rebates are recognised as other liabilities. Wholesaler charge-backs that are absolute are netted against trade receivable balances.
The impact of foreign currency hedging in the income statement is recognised as
part of financial items. Refer to notes 4.4, 4.5 and 4.9 for more details on hedging.
2.2 Segment information
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating segments – Key figures |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diabetes and Obesity care |
|
Rare disease |
|
Total |
| DKK million |
2024 |
|
2023 |
2022 |
|
2024 |
|
2023 |
2022 |
|
2024 |
|
2023 |
2022 |
| Net sales |
271,764 |
|
215,098 |
156,412 |
|
18,639 |
|
17,163 |
20,542 |
|
290,403 |
|
232,261 |
176,954 |
| Cost of goods sold |
(37,760) |
|
(30,483) |
(23,405) |
|
(6,762) |
|
(5,282) |
(5,043) |
|
(44,522) |
|
(35,765) |
(28,448) |
| Sales and distribution costs |
(57,840) |
|
(52,477) |
(42,392) |
|
(4,261) |
|
(4,266) |
(3,825) |
|
(62,101) |
|
(56,743) |
(46,217) |
| Research and development costs |
(41,490) |
|
(28,073) |
(20,157) |
|
(6,572) |
|
(4,370) |
(3,890) |
|
(48,062) |
|
(32,443) |
(24,047) |
| Administrative costs |
(4,881) |
|
(4,435) |
(3,955) |
|
(395) |
|
(420) |
(512) |
|
(5,276) |
|
(4,855) |
(4,467) |
| Other operating income and expenses |
(2,074) |
|
(7) |
892 |
|
(29) |
|
126 |
142 |
|
(2,103) |
|
119 |
1,034 |
| Segment operating profit |
127,719 |
|
99,623 |
67,395 |
|
620 |
|
2,951 |
7,414 |
|
128,339 |
|
102,574 |
74,809 |
| Operating margin |
47.0 |
% |
|
46.3 |
% |
43.1 |
% |
|
3.3 |
% |
|
17.2 |
% |
36.1 |
% |
|
44.2 |
% |
|
44.2 |
% |
42.3 |
% |
| Depreciation and amortisation expenses |
(7,104) |
|
(6,042) |
(5,421) |
|
(1,441) |
|
(1,247) |
(1,132) |
|
(8,545) |
|
(7,289) |
(6,553) |
| Impairment losses and reversals |
(9,262) |
|
(2,153) |
(280) |
|
(1,300) |
|
29 |
(529) |
|
(10,562) |
|
(2,124) |
(809) |
| Total depreciation, amortisation, impairment losses and reversals |
|
(16,366) |
|
(8,195) |
(5,701) |
|
(2,741) |
|
(1,218) |
(1,661) |
|
(19,107) |
|
(9,413) |
(7,362) |
Operating segments
Novo Nordisk operates in two segments based on therapies: Diabetes and Obesity care and Rare disease, representing the entirety of the Group's operations. The activities of the segments include research, development, manufacturing and marketing of products within the following areas:
•Diabetes and Obesity care: diabetes, obesity, cardiovascular and emerging therapy areas
•Rare disease: rare blood disorders, rare endocrine disorders and hormone replacement therapy.
Segment performance is evaluated on the basis of operating profit, consistent with the Consolidated financial statements. Financial income and expenses and income taxes are managed at Group level and are not allocated to segments. There are no sales or other transactions between the segments. Costs have generally been split between segments according to a specific allocation. Certain corporate overhead costs are allocated between segments based on overall allocation keys. Other operating income and expenses have been allocated to the two segments based on the same principle.
ACCOUNTING POLICIES
Operating segments are reported in a manner consistent with the internal reporting provided to Executive Management and the Board of Directors. We consider Executive Management to be the operating decision-making body.
Geographical areas
In 2024, Novo Nordisk operated in two main commercial units:
•International Operations
•EMEA: Europe, the Middle East and Africa.
•Region China: Mainland China, Hong Kong and Taiwan.
•Rest of World: All other countries except for North America.
•North America Operations (the US and Canada).
In 2024, the US contributed 10% or more of total net sales. In 2023, the US also contributed 10% or more of total net sales. The country of domicile is Denmark, which is part of EMEA. Denmark is immaterial to Novo Nordisk's activities in terms of sales as 99.2% of total net sales are realised outside Denmark (99.2 % in 2023). Sales are attributed to geographical areas according to the location of the customer.
Total property, plant and equipment and intangible assets amounts to DKK 273,578 million (DKK 151,367 million in 2023), of which DKK 177,471 million is located in Denmark (DKK 82,274 million in 2023) and DKK 57,141 million is located in the US (DKK 46,609 million in 2023).
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 2.2 Segment information |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net sales – Segments and geographical areas |
|
|
|
|
|
Total International Operations |
Total North America Operations |
Total Novo Nordisk
net sales |
|
|
|
|
|
Total IO |
EMEA |
Region China |
Rest of World |
Total NAO |
US |
|
|
|
|
| DKK million |
2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
|
|
|
|
| Diabetes and Obesity care segment: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ozempic® |
29,055 |
26,378 |
17,369 |
16,001 |
14,327 |
10,417 |
5,762 |
4,821 |
2,196 |
7,292 |
7,230 |
4,756 |
91,287 |
69,340 |
42,381 |
84,201 |
63,010 |
38,750 |
120,342 |
95,718 |
59,750 |
|
|
|
|
Rybelsus® |
12,231 |
7,389 |
3,155 |
7,136 |
4,232 |
1,714 |
511 |
131 |
63 |
4,584 |
3,026 |
1,378 |
11,070 |
11,361 |
8,144 |
10,795 |
11,060 |
8,011 |
23,301 |
18,750 |
11,299 |
|
|
|
|
Victoza® |
3,686 |
4,850 |
5,672 |
1,422 |
2,166 |
2,724 |
975 |
1,256 |
1,478 |
1,289 |
1,428 |
1,470 |
1,796 |
3,814 |
6,650 |
1,699 |
3,613 |
6,406 |
5,482 |
8,664 |
12,322 |
|
|
|
|
| Total GLP-1 |
44,972 |
38,617 |
26,196 |
24,559 |
20,725 |
14,855 |
7,248 |
6,208 |
3,737 |
13,165 |
11,684 |
7,604 |
104,153 |
84,515 |
57,175 |
96,695 |
77,683 |
53,167 |
149,125 |
123,132 |
83,371 |
|
|
|
|
| Long-acting insulin |
12,884 |
11,339 |
11,403 |
7,686 |
7,103 |
7,157 |
2,696 |
1,649 |
1,636 |
2,502 |
2,587 |
2,610 |
6,211 |
3,566 |
5,338 |
5,538 |
2,931 |
4,685 |
19,095 |
14,905 |
16,741 |
|
|
|
|
•of which Awigli® |
15 |
— |
— |
9 |
— |
— |
6 |
— |
— |
— |
— |
— |
4 |
— |
— |
— |
— |
— |
19 |
— |
— |
|
|
|
|
•of which Tresiba® |
6,494 |
5,864 |
6,092 |
3,876 |
3,435 |
3,485 |
978 |
848 |
1,050 |
1,640 |
1,581 |
1,557 |
3,411 |
1,888 |
3,261 |
2,806 |
1,333 |
2,723 |
9,905 |
7,752 |
9,353 |
|
|
|
|
•of which Xultophy® |
4,217 |
2,887 |
2,400 |
2,185 |
1,831 |
1,716 |
1,414 |
409 |
45 |
618 |
647 |
639 |
286 |
332 |
409 |
281 |
325 |
399 |
4,503 |
3,219 |
2,809 |
|
|
|
|
•of which Levemir® |
2,158 |
2,588 |
2,911 |
1,616 |
1,837 |
1,956 |
298 |
392 |
541 |
244 |
359 |
414 |
2,510 |
1,346 |
1,668 |
2,451 |
1,273 |
1,563 |
4,668 |
3,934 |
4,579 |
|
|
|
|
| Premix insulin |
10,143 |
9,342 |
10,023 |
2,637 |
2,570 |
2,622 |
4,784 |
4,441 |
4,912 |
2,722 |
2,331 |
2,489 |
646 |
232 |
539 |
632 |
216 |
517 |
10,789 |
9,574 |
10,562 |
|
|
|
|
•of which Ryzodeg® |
4,929 |
3,730 |
2,889 |
701 |
587 |
495 |
2,782 |
1,965 |
1,218 |
1,446 |
1,178 |
1,176 |
— |
— |
— |
— |
— |
— |
4,929 |
3,730 |
2,889 |
|
|
|
|
•of which NovoMix® |
5,214 |
5,612 |
7,134 |
1,936 |
1,983 |
2,127 |
2,002 |
2,476 |
3,694 |
1,276 |
1,153 |
1,313 |
646 |
232 |
539 |
632 |
216 |
517 |
5,860 |
5,844 |
7,673 |
|
|
|
|
| Fast-acting insulin |
10,563 |
10,415 |
10,826 |
6,934 |
6,695 |
6,456 |
1,474 |
1,545 |
1,942 |
2,155 |
2,175 |
2,428 |
7,959 |
5,534 |
6,637 |
7,773 |
5,265 |
6,247 |
18,522 |
15,949 |
17,463 |
|
|
|
|
•of which Fiasp® |
1,609 |
1,512 |
1,354 |
1,289 |
1,266 |
1,138 |
— |
— |
— |
320 |
246 |
216 |
260 |
661 |
649 |
213 |
618 |
606 |
1,869 |
2,173 |
2,003 |
|
|
|
|
•of which NovoRapid® |
8,954 |
8,903 |
9,472 |
5,645 |
5,429 |
5,318 |
1,474 |
1,545 |
1,942 |
1,835 |
1,929 |
2,212 |
7,699 |
4,873 |
5,988 |
7,560 |
4,647 |
5,641 |
16,653 |
13,776 |
15,460 |
|
|
|
|
| Human insulin |
5,388 |
6,134 |
6,508 |
1,762 |
1,919 |
1,983 |
806 |
1,213 |
1,812 |
2,820 |
3,002 |
2,713 |
1,579 |
1,460 |
1,678 |
1,535 |
1,406 |
1,605 |
6,967 |
7,594 |
8,186 |
|
|
|
|
| Total insulin |
38,978 |
37,230 |
38,760 |
19,019 |
18,287 |
18,218 |
9,760 |
8,848 |
10,302 |
10,199 |
10,095 |
10,240 |
16,395 |
10,792 |
14,192 |
15,478 |
9,818 |
13,054 |
55,373 |
48,022 |
52,952 |
|
|
|
|
| Other Diabetes care |
1,856 |
1,987 |
2,428 |
688 |
661 |
717 |
782 |
892 |
1,181 |
386 |
434 |
530 |
264 |
325 |
797 |
213 |
267 |
660 |
2,120 |
2,312 |
3,225 |
|
|
|
|
| Total Diabetes care |
85,806 |
77,834 |
67,384 |
44,266 |
39,673 |
33,790 |
17,790 |
15,948 |
15,220 |
23,750 |
22,213 |
18,374 |
120,812 |
95,632 |
72,164 |
112,386 |
87,768 |
66,881 |
206,618 |
173,466 |
139,548 |
|
|
|
|
Wegovy® |
11,425 |
1,913 |
54 |
7,513 |
1,913 |
54 |
196 |
— |
— |
3,716 |
— |
— |
46,781 |
29,430 |
6,134 |
45,770 |
29,430 |
6,134 |
58,206 |
31,343 |
6,188 |
|
|
|
|
Saxenda® |
5,563 |
6,402 |
5,832 |
2,920 |
3,780 |
3,561 |
102 |
146 |
133 |
2,541 |
2,476 |
2,138 |
1,377 |
3,887 |
4,844 |
777 |
3,306 |
4,368 |
6,940 |
10,289 |
10,676 |
|
|
|
|
| Total Obesity care |
16,988 |
8,315 |
5,886 |
10,433 |
5,693 |
3,615 |
298 |
146 |
133 |
6,257 |
2,476 |
2,138 |
48,158 |
33,317 |
10,978 |
46,547 |
32,736 |
10,502 |
65,146 |
41,632 |
16,864 |
|
|
|
|
| Diabetes and Obesity care total |
102,794 |
86,149 |
73,270 |
54,699 |
45,366 |
37,405 |
18,088 |
16,094 |
15,353 |
30,007 |
24,689 |
20,512 |
168,970 |
128,949 |
83,142 |
158,933 |
120,504 |
77,383 |
271,764 |
215,098 |
156,412 |
|
|
|
|
Rare disease segment: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Rare blood disorders |
6,442 |
6,432 |
6,671 |
3,924 |
4,021 |
3,795 |
363 |
372 |
604 |
2,155 |
2,039 |
2,272 |
5,696 |
5,344 |
5,035 |
5,387 |
5,070 |
4,710 |
12,138 |
11,776 |
11,706 |
|
|
|
|
•of which Haemophilia A |
1,906 |
1,939 |
1,769 |
1,231 |
1,271 |
1,137 |
236 |
223 |
81 |
439 |
445 |
551 |
548 |
483 |
569 |
537 |
468 |
543 |
2,454 |
2,422 |
2,338 |
|
|
|
|
•of which Haemophilia B |
649 |
584 |
479 |
436 |
377 |
294 |
17 |
13 |
13 |
196 |
194 |
172 |
657 |
477 |
280 |
486 |
336 |
152 |
1,306 |
1,061 |
759 |
|
|
|
|
•of which NovoSeven® |
3,735 |
3,789 |
4,335 |
2,168 |
2,285 |
2,311 |
110 |
136 |
510 |
1,457 |
1,368 |
1,514 |
4,248 |
4,169 |
3,973 |
4,135 |
4,065 |
3,811 |
7,983 |
7,958 |
8,308 |
|
|
|
|
| Rare endocrine disorders |
2,032 |
2,045 |
4,904 |
1,038 |
699 |
2,232 |
41 |
216 |
246 |
953 |
1,130 |
2,426 |
2,961 |
1,791 |
2,234 |
2,922 |
1,757 |
2,205 |
4,993 |
3,836 |
7,138 |
|
|
|
|
| Other Rare disease |
963 |
1,006 |
1,002 |
741 |
781 |
804 |
9 |
5 |
6 |
213 |
220 |
192 |
545 |
545 |
696 |
160 |
203 |
358 |
1,508 |
1,551 |
1,698 |
|
|
|
|
| Rare disease total |
9,437 |
9,483 |
12,577 |
5,703 |
5,501 |
6,831 |
413 |
593 |
856 |
3,321 |
3,389 |
4,890 |
9,202 |
7,680 |
7,965 |
8,469 |
7,030 |
7,273 |
18,639 |
17,163 |
20,542 |
|
|
|
|
| Total sales by geographical area |
112,231 |
95,632 |
85,847 |
60,402 |
50,867 |
44,236 |
18,501 |
16,687 |
16,209 |
33,328 |
28,078 |
25,402 |
178,172 |
136,629 |
91,107 |
167,402 |
127,534 |
84,656 |
290,403 |
232,261 |
176,954 |
|
|
|
|
| Total sales growth as reported |
17.4 |
% |
11.4 |
% |
16.7 |
% |
18.7 |
% |
15.0 |
% |
17.3 |
% |
10.9 |
% |
2.9 |
% |
1.2 |
% |
18.7 |
% |
10.5 |
% |
28.2 |
% |
30.4 |
% |
50.0 |
% |
35.4 |
% |
31.3 |
% |
50.6 |
% |
34.4 |
% |
25.0 |
% |
31.3 |
% |
25.7 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 2.3 Research and development costs |
|
2.3 Research and development costs
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
2022 |
Employee costs (note 2.4) |
|
15,923 |
|
12,429 |
9,952 |
Amortisation, intangible assets (note 3.1) |
|
931 |
|
649 |
604 |
Impairment losses and reversals, intangible assets (note 3.1) |
|
7,912 |
|
1,108 |
760 |
Depreciation, property, plant and equipment (note 3.2) |
|
1,120 |
|
1,053 |
898 |
Impairment losses, property, plant and equipment (note 3.2) |
|
78 |
|
260 |
24 |
| Clinical trial cost |
|
12,232 |
|
9,468 |
6,313 |
| Other research and development costs |
|
9,866 |
|
7,476 |
5,496 |
| Total research and development costs |
|
48,062 |
|
32,443 |
24,047 |
| As percentage of net sales |
|
16.6% |
|
14.0% |
13.6% |
Novo Nordisk's research and development is mainly focused on:
•Insulins, GLP-1s and other therapeutic compounds for diabetes treatment
•GLP-1s, combinations and new modes of action for Obesity care
•Blood-clotting factors and new modes of action for treatment of haemophilia
and other rare blood disorders
•Novel targets within cardiovascular disease focusing on ASCVD and Heart failure
•Human growth hormone and new modes of action for treatment of growth disorders and other rare endocrine disorders
•New indications with existing assets within MASH, Alzheimer’s disease and
chronic kidney disease
•Research technology platforms including cell therapy and RNAi for treatment of MASH, cardiovascular disease, chronic kidney disease and Parkinson's disease, among others
The research activities mainly utilise biotechnological methods based on advanced protein chemistry and protein engineering. These methods have played a key role in the development of the production technology used to manufacture insulin, GLP-1, recombinant blood-clotting factors and human growth hormone. Research activities further utilise digital scientific methodologies and other technology platforms including stem cells, gene therapy, small molecules and RNAi therapies.
Research and development activities are mainly carried out by Novo Nordisk's research and development centres in Denmark, the US, the UK and China. Clinical trials are carried out all over the world. Novo Nordisk also enters into partnerships
and licence agreements.
Other research and development costs mainly comprise external consulting
fees, IT services, facilities, consumables and other operational costs.
ACCOUNTING POLICIES
Novo Nordisk expenses all research costs. Due to significant regulatory uncertainties and other uncertainties inherent in the development of new products, internal and subcontracted development costs are also expensed as they are incurred, in line with industry practice. This means that they do not qualify for capitalisation as intangible assets until marketing approval by a regulatory authority is obtained or considered highly probable. Costs for post-approval activities that are required by authorities
as a condition for obtaining regulatory approval are recognised as research and development costs.
Research and development costs primarily comprise employee costs as well as
internal and external costs related to execution of studies, including manufacturing costs and facility costs of the research centres. The costs also comprise amortisation, depreciation and impairment losses related to intellectual property rights and property, plant and equipment used in the research and development activities.
Amortisations of intellectual property rights related to marketed products are recognised in cost of goods sold. Royalty expenses paid to partners after regulatory approval are also expensed as cost of goods sold.
Contractual research and development obligations to be paid in the future are disclosed separately as commitments in note 5.2.
2.4 Employee costs
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
2024 |
|
2023 |
2022 |
| Wages and salaries |
52,311 |
|
42,867 |
34,575 |
Share-based payment costs (note 5.1) |
2,289 |
|
2,149 |
1,539 |
| Pensions – defined contribution plans |
4,235 |
|
3,267 |
2,472 |
| Pensions – defined benefit plans |
156 |
|
126 |
185 |
| Other social security contributions |
3,505 |
|
3,039 |
2,713 |
| Other employee costs |
4,929 |
|
4,066 |
3,105 |
| Total employee costs for the year |
67,425 |
|
55,514 |
44,589 |
| Employee costs capitalised as intangible assets and property, plant and equipment |
(3,540) |
|
(2,337) |
(1,451) |
Change in employee costs capitalised
as inventories |
(470) |
|
(409) |
(70) |
Total employee costs
in the income statement |
63,415 |
|
52,768 |
43,068 |
| Included in the income statement: |
|
|
|
|
| Cost of goods sold |
20,074 |
|
15,490 |
11,766 |
| Sales and distribution costs |
22,920 |
|
20,810 |
17,700 |
| Research and development costs |
15,923 |
|
12,429 |
9,952 |
| Administrative costs |
4,265 |
|
3,962 |
3,517 |
| Other operating income and expenses |
233 |
|
77 |
133 |
Total employee costs in the
income statement |
63,415 |
|
52,768 |
43,068 |
Number of employees
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number |
|
2024 |
|
2023 |
2022 |
| Average number of full-time employees |
|
69,480 |
|
59,552 |
51,046 |
| Year-end number of full-time employees |
|
76,302 |
|
63,370 |
54,393 |
| Year-end employees (total) |
|
77,349 |
|
64,319 |
55,185 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 2.5 Other operating income and expenses |
|
ACCOUNTING POLICIES
Wages, salaries, social security contributions, annual leave and sick leave, bonuses
and non-monetary benefits are recognised in the year in which the associated services are rendered by employees of Novo Nordisk. Where Novo Nordisk provides long-term employee benefits, the costs are accrued to match the rendering of the services by the employees concerned.
2.5 Other operating income and expenses
ACCOUNTING POLICIES
Other operating income and expenses, include mainly licence income and amortisations and impairment losses, which are of a secondary nature in relation
to the main activities of Novo Nordisk.
Operating profit from wholly owned subsidiaries, not related to Novo Nordisk's
main activities, as well as operating profit from non-core manufacturing contracts,
are recognised as other operating income and expenses.
Other operating income and expenses, also includes transaction costs in
connection with acquisition of businesses. Refer to note 5.3 for details on the acquisition of businesses.
2.6 Income taxes and deferred income taxes
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Income taxes expensed |
| DKK million |
2024 |
|
2023 |
2022 |
| Current tax on profit for the year |
32,082 |
|
25,918 |
17,829 |
| Deferred tax on profit for the year |
(5,484) |
|
(4,464) |
(3,806) |
| Tax on profit for the year |
26,598 |
|
21,454 |
14,023 |
Current tax adjustments recognised
for prior years |
172 |
|
(916) |
339 |
Deferred tax adjustments recognised
for prior years |
(567) |
|
453 |
(825) |
| Income taxes in the income statement |
26,203 |
|
20,991 |
13,537 |
Tax on other comprehensive income
for the year, (income)/expense |
(1,343) |
|
359 |
889 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Computation of effective tax rate |
| DKK million |
|
|
2024 |
|
2023 |
2022 |
| Statutory corporate income tax rate in Denmark |
|
|
22.0 |
% |
|
22.0 |
% |
22.0 |
% |
| Deviation in foreign subsidiaries' tax rates compared to the Danish tax rate (net) |
|
|
(0.5%) |
|
(0.9%) |
(1.1%) |
| Non-taxable income less non-tax-deductible expenses (net) |
|
|
(0.7%) |
|
(0.7%) |
(0.5%) |
| Other adjustments (net) |
|
|
(0.2%) |
|
(0.3%) |
(0.8%) |
| Effective tax rate |
|
|
20.6 |
% |
|
20.1 |
% |
19.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Income taxes paid |
| DKK million |
|
2024 |
|
2023 |
2022 |
Income taxes paid in Denmark for
current year |
|
21,810 |
|
16,899 |
7,481 |
Income taxes paid outside Denmark
for current year |
|
7,826 |
|
8,998 |
7,034 |
|
|
|
|
|
|
| Income taxes paid |
|
29,636 |
|
25,897 |
14,515 |
The deviation in foreign subsidiaries' tax rates from the Danish tax rate is mainly driven by Swiss and US business activities. Other adjustments consist of tax related
to prior years.
From 1 January 2024 Novo Nordisk is subject to Global Minimum Tax (OECD BEPS Pillar 2 rules). The rules did not have a material impact on the tax position of Novo Nordisk in 2024.
KEY ACCOUNTING ESTIMATES REGARDING DEFERRED INCOME TAX ASSETS AND PROVISIONS FOR UNCERTAIN TAX POSITIONS
Management has considered future taxable income and has estimated the amount
of deferred income tax assets that should be recognised. The estimate is based on an assessment of whether sufficient taxable income will be available in the future, against which the temporary differences and unused tax losses can be utilised. The total tax value of unrecognised tax loss carry-forwards amounts to DKK 602 million in 2024 (DKK 360 million in 2023).
In the course of conducting business globally, tax and transfer pricing disputes with tax authorities may occur. Management has estimated the expected outcome of the disputes by using the ‘most likely outcome’ method to determine the provisions for uncertain tax positions. Management considers the provisions made to be adequate. However, the actual obligation may deviate and depends on the result of litigation
and settlements with the relevant tax authorities.
ACCOUNTING POLICIES
The tax expense for the period comprises current and deferred tax. It also includes adjustments to previous years and changes in provisions for uncertain tax positions. Tax is recognised in the income statement except to the extent that it relates to items recognised in equity or other comprehensive income. Provisions for ongoing tax disputes are included as part of deferred tax assets, tax receivables and tax payables.
Deferred income taxes arise from temporary differences between the accounting
and tax values of the individual consolidated companies and from realisable tax loss carry-forwards. Deferred tax liabilities are not recognised if they arise from the initial recognition of goodwill. Deferred income tax is also not accounted for if it arises
from initial recognition of an asset or liability in a transaction other than a business combination that, at the time of the transaction, affects neither accounting nor taxable profit or loss and does not give rise to equal taxable and deductible temporary differences. The tax value of tax loss carry-forwards is included in deferred tax
assets to the extent that these are expected to be utilised in future taxable income. The deferred income taxes are measured according to current tax rules and at the
tax rates assumed in the year in which the assets are expected to be utilised.
In general, the Danish tax rules related to dividends from group companies provide exemption from tax for most repatriated profits. In some countries withholding tax will be applied to dividends paid to Denmark. A provision for withholding tax is only recognised if a concrete distribution of dividends is planned. The unrecognised potential withholding tax amounts to DKK 1,228 million (DKK 1,026 million in 2023).
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 2.6 Income taxes and deferred income taxes |
|
The value of future tax deductions in relation to share programmes is recognised
as a deferred tax asset until the shares are paid out to the employees. Any estimated excess tax deduction compared to the costs realised in the income statement is charged to equity.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Development in deferred income tax assets and liabilities |
Property,
plant and
equipment |
Intangible
assets |
Inventories |
Liabilities |
Other |
Offset
within
countries |
|
Total |
|
| DKK million |
|
|
| 2024 |
|
|
|
|
|
|
|
|
|
| Net deferred tax asset/(liability) at the beginning of the year |
(2,561) |
(10,241) |
1,717 |
14,427 |
6,876 |
— |
|
10,218 |
|
| Income/(charge) to the income statement |
(207) |
427 |
2,142 |
3,485 |
204 |
— |
|
6,051 |
|
| Income/(charge) to other comprehensive income |
— |
(254) |
(71) |
17 |
1,622 |
— |
|
1,314 |
|
| Income/(charge) to equity |
— |
254 |
— |
— |
(314) |
— |
|
(60) |
|
| Additions from acquisitions |
(2,723) |
3,693 |
— |
25 |
102 |
— |
|
1,097 |
|
| Effect of exchange rate adjustment |
(116) |
(145) |
2 |
773 |
67 |
— |
|
581 |
|
| Net deferred tax asset/(liability) at the end of the year |
(5,607) |
(6,266) |
3,790 |
18,727 |
8,557 |
— |
|
19,201 |
|
| Classified as follows: |
|
|
|
|
|
|
|
|
|
| Deferred tax asset at the end of the year |
497 |
225 |
3,847 |
18,989 |
13,112 |
(12,043) |
|
24,627 |
|
| Deferred tax liability at the end of the year |
(6,104) |
(6,491) |
(57) |
(262) |
(4,555) |
12,043 |
|
(5,426) |
|
| 2023 |
|
|
|
|
|
|
|
|
|
| Net deferred tax asset/(liability) at the beginning of the year |
(2,402) |
(8,279) |
2,595 |
11,007 |
3,922 |
— |
|
6,843 |
|
| Income/(charge) to the income statement |
(213) |
(2,106) |
(645) |
3,973 |
3,002 |
— |
|
4,011 |
|
| Income/(charge) to other comprehensive income |
— |
— |
(224) |
(6) |
(129) |
— |
|
(359) |
|
| Income/(charge) to equity |
— |
— |
— |
— |
(120) |
— |
|
(120) |
|
| Additions from acquisitions |
— |
— |
— |
— |
62 |
— |
|
62 |
|
| Effect of exchange rate adjustment |
54 |
144 |
(9) |
(547) |
139 |
— |
|
(219) |
|
| Net deferred tax asset/(liability) at the end of the year |
(2,561) |
(10,241) |
1,717 |
14,427 |
6,876 |
— |
|
10,218 |
|
| Classified as follows: |
|
|
|
|
|
|
|
|
|
| Deferred tax asset at the end of the year |
433 |
245 |
1,820 |
14,792 |
6,986 |
(3,896) |
|
20,380 |
|
| Deferred tax liability at the end of the year |
(2,994) |
(10,486) |
(103) |
(365) |
(110) |
3,896 |
|
(10,162) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 3.1 Intangible assets |
|
Section 3
Operating assets and liabilities
3.1 Intangible assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Amortisation |
|
| DKK million |
|
2024 |
|
2023 |
2022 |
| Cost of goods sold |
|
1,400 |
|
|
982 |
846 |
| Sales and distribution costs |
|
— |
|
|
9 |
34 |
| Research and development costs |
|
931 |
|
|
649 |
604 |
| Administrative costs |
|
14 |
|
|
41 |
19 |
| Other operating income and expenses |
|
167 |
|
|
153 |
96 |
| Total amortisation |
|
2,512 |
|
|
1,834 |
1,599 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Impairment losses and reversals |
|
| DKK million |
|
2024 |
|
2023 |
2022 |
| Research and development costs |
|
7,912 |
|
|
1,108 |
760 |
| Other operating income and expenses |
|
1,601 |
|
|
306 |
— |
| Total impairment losses and reversals |
|
9,513 |
|
|
1,414 |
760 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
Goodwill |
Intellectual property rights and know-how |
Software and other intangibles |
|
|
Total intangible assets |
|
| 2024 |
|
|
|
|
|
|
|
|
| Cost at the beginning of the year |
|
4,464 |
|
60,745 |
|
5,584 |
|
|
|
70,793 |
|
|
Additions from acquisition of businesses (note 5.3) |
|
15,323 |
|
41,154 |
|
311 |
|
|
|
56,788 |
|
|
| Additions during the year |
|
— |
|
4,165 |
|
710 |
|
|
|
4,875 |
|
|
| Disposals during the year |
|
— |
|
(213) |
(70) |
|
|
(283) |
|
|
|
|
|
|
|
|
|
|
| Effect of exchange rate adjustment |
|
277 |
|
858 |
|
89 |
|
|
|
1,224 |
|
|
| Cost at the end of the year |
|
20,064 |
|
106,709 |
|
6,624 |
|
|
|
133,397 |
|
|
| Amortisation and impairment losses at the beginning of the year |
|
— |
|
8,225 |
|
2,162 |
|
|
|
10,387 |
|
|
| Amortisation for the year |
|
— |
|
2,257 |
|
255 |
|
|
|
2,512 |
|
|
| Impairment losses for the year |
|
— |
|
9,441 |
|
72 |
|
|
|
9,513 |
|
|
|
|
|
|
|
|
|
|
|
| Amortisation and impairment losses reversed on disposals during the year |
|
— |
|
(213) |
(70) |
|
|
(283) |
|
| Effect of exchange rate adjustment |
|
— |
|
163 |
|
15 |
|
|
|
178 |
|
|
| Amortisation and impairment losses at the end of the year |
|
— |
|
19,873 |
|
2,434 |
|
|
|
22,307 |
|
|
| Carrying amount at the end of the year |
|
20,064 |
|
86,836 |
|
4,190 |
|
|
|
111,090 |
|
|
| 2023 |
|
|
|
|
|
|
|
|
| Cost at the beginning of the year |
|
4,615 |
|
49,731 |
|
5,281 |
|
|
|
59,627 |
|
|
|
|
|
|
|
|
|
|
|
| Additions during the year |
|
— |
|
12,567 |
|
500 |
|
|
|
13,067 |
|
|
| Disposals during the year |
|
— |
|
(1,629) |
(158) |
|
|
(1,787) |
|
| Effect of exchange rate adjustment |
|
(151) |
76 |
|
(39) |
|
|
(114) |
|
| Cost at the end of the year |
|
4,464 |
|
60,745 |
|
5,584 |
|
|
|
70,793 |
|
|
| Amortisation and impairment losses at the beginning of the year |
|
— |
|
6,737 |
|
1,951 |
|
|
|
8,688 |
|
|
| Amortisation for the year |
|
— |
|
1,621 |
|
213 |
|
|
|
1,834 |
|
|
| Impairment losses for the year |
|
— |
|
1,776 |
|
20 |
|
|
|
1,796 |
|
|
| Impairment losses reversed during the year |
|
— |
|
(382) |
— |
|
|
|
(382) |
|
| Amortisation and impairment losses reversed on disposals during the year |
|
— |
|
(1,629) |
(16) |
|
|
(1,645) |
|
| Effect of exchange rate adjustment |
|
— |
|
102 |
|
(6) |
|
|
96 |
|
|
| Amortisation and impairment losses at the end of the year |
|
— |
|
8,225 |
|
2,162 |
|
|
|
10,387 |
|
|
| Carrying amount at the end of the year |
|
4,464 |
|
52,520 |
|
3,422 |
|
|
|
60,406 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 3.1 Intangible assets |
|
Material intangible assets
Intellectual property rights and know-how with a carrying value of DKK 86,836 million (DKK 52,520 million in 2023), comprise intellectual property and licenses related mainly to marketed products, know-how attributable to manufacturing, products and technologies in development as well as technologies used in the research and development phase.
Know-how with a carrying value of DKK 40,944 million (DKK nil in 2023), and a remaining useful life of 10 years, is recognised in the acquisition of three fill-finish sites in 2024 and is primarily attributable to the documented processes and systems for efficient and large-scale production of GLP-1 products as well as know-how to expand capacity in an efficient way. Intellectual property and licenses related to marketed products include Rybelsus® with a carrying value of DKK 5,453 million (DKK 6,018 million in 2023) and a remaining useful life of 10 years (11 years in 2023). Technologies used in the research and development phase include a RNAi technology platform with a carrying value of DKK 9,530 million (DKK 9,480 million in 2023), with
a remaining estimated useful life of 20 years (21 years in 2023).
Intellectual property rights and know-how as well as Software and other intangibles contain assets not yet available for use amounting to DKK 23,893 million (DKK 29,548 million in 2023).
Impairment losses on intellectual property rights
Impairment losses on intellectual property rights amounted to DKK 9,441 million (DKK 1,776 million in 2023). The single-largest impairment loss recognised in 2024 amounted to DKK 5,650 million arising from the impairment of ocedurenone. The impairment loss is linked to the termination of a phase 3 trial with ocedurenone which failed to meet its primary endpoints, hence the recoverable amount was estimated to nil. The impairment loss is recognised in research and development costs in the segment Diabetes and Obesity.
Impairment review of goodwill
Goodwill is allocated to the segments Diabetes and Obesity care by DKK 19,592 million (DKK 4,018 million in 2023) and to Rare Disease by DKK 472 million (DKK 446 million in 2023). The annual impairment review showed that the recoverable amount in the forecast period significantly exceeds the carrying amount of the cash-generating
units to which goodwill was allocated.
Goodwill is monitored for impairment at the operating segment level, which is the lowest level CGU to which consolidated goodwill is allocated and monitored by Management. CGUs are therefore defined as Novo Nordisk's operating segments, Diabetes and Obesity care and Rare disease. The recoverable amount is estimated based on fair value, with fair value being estimated at net present value using an income-approach. The applied post-tax discount rates are 7.0% (Pre-tax discount rate of 8.3%). Cash flow projections are based on budgets approved by Management. The forecast period comprises 9 years.
The key estimations relate to volume of market share, growth rates, pricing, development of new markets and the success rate for introducing new products and treatments. Assumptions are affected by external factors such as market and generic competition, and price regulation.
Key assumptions reflect past experience adjusted for market specific risks or expected changes. Fair value is determined using largely unobservable inputs.
KEY ACCOUNTING ESTIMATES IN DETERMINING FAIR VALUES OF INTANGIBLE ASSETS IN IMPAIRMENT REVIEWS
Impairment tests are based on Management’s projections and anticipated net present value of estimated future cash flows.
Goodwill and intangible assets not yet available for use are tested for impairment at least annually or when indicators of impairment are identified. Goodwill is allocated to operating segments based on expected future cash flow from products utilising the synergies and know-how acquired.
Impairment tests are based on Management’s projections and anticipated net present value of estimated future cash flows from marketable products. The discount rate used is based on the Group WACC, adjusted where appropriate, to reflect the risk of the specific asset tested. Fair value is determined using largely unobservable inputs. Accordingly, the valuation technique and inputs used to measure fair value are classified as level 3 in the fair value hierarchy.
Assets that are subject to amortisation are reviewed for impairment whenever
events or changes in circumstances indicate that the carrying amount may not be recoverable. Factors considered material that could trigger an impairment test include the following:
•Development of a competing drug
•Realised sales trending below predicted sales
•Changes or anticipated changes in participation rates or reimbursement policies
•Inconsistent or unfavourable clinical readouts
•Changes in the legal framework covering patents, rights and licences
•Advances in medicine and/or technology that affect the medical treatments
•Adverse impact on reputation and/or brand names
•Changes in the economic lives of similar assets
•Relationship to other intangible assets or property, plant and equipment
An impairment loss is recognised when the carrying amount of intangible assets exceeds the recoverable amount. Impairments on intangible assets, other than goodwill, are reviewed at each reporting date for possible reversal.
ACCOUNTING POLICIES
Research and development projects
Internal and subcontracted research costs are fully charged to the consolidated income statement in the period in which they are incurred. Consistent with industry practice, development costs are also expensed until regulatory approval is obtained
or is probable; refer to note 2.3.
Payments to third parties under collaboration and licence agreements are assessed for the substance of their nature. Payments which represent subcontracted research and development work are expensed as the services are received. Payments which represent transfer of rights of intellectual property are capitalised.
For acquired research and development projects, and intellectual property rights,
the likelihood of obtaining future commercial sales is reflected in the cost of the asset, and thus the probability recognition criteria is always considered to be satisfied. As the cost of acquired research and development projects can often be measured reliably, these projects fulfil the capitalisation criteria as intangible assets on acquisition. Subsequent milestone payments payable on achievement of a contingent event (e.g. commencement of phase 3 trials) are accrued and capitalised into the cost of the intangible asset when the achievement of the event is probable. Development costs incurred subsequent to acquisition are treated consistently with internal project development costs.
Recognition and measurement
Intangible assets acquired separately are initially measured at cost and are subsequently measured at cost less any accumulated amortisation and any impairment loss. Identifiable intangible assets acquired in a business combination
are initially measured at fair value.
Amortisation of intellectual property rights is based on the straight-line method over the estimated useful life. This corresponds to the legal duration or the economic useful life depending on which is shorter, and not exceeding 25 years in either case. The amortisation of intellectual property rights commences after regulatory approval
has been obtained or when assets are put in use.
Amortisation of know-how, which arises from business combinations, is based on
the straight-line method over the estimated useful life of 10 years corresponding
to the period in which economic benefits are expected to be realised.
Amortisation of software is based on the straight-line method over the estimated useful life of 3-15 years. The amortisation commences when the asset is in the
location and condition necessary for it to be capable of operating in the manner intended by Management.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 3.2 Property, plant and equipment |
|
3.2 Property, plant and equipment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
Land and buildings |
Plant and machinery |
Other equipment |
Assets
under
construction |
|
Property, plant and equipment |
| 2024 |
|
|
|
|
|
|
|
| Cost at the beginning of the year |
|
48,990 |
|
40,951 |
|
8,979 |
|
39,663 |
|
|
138,583 |
|
|
|
|
|
|
|
|
|
| Additions from acquisition of businesses (note 5.3) |
|
6,709 |
|
18,460 |
|
278 |
|
— |
|
|
25,447 |
|
| Additions during the year |
|
3,789 |
|
872 |
|
874 |
|
46,650 |
|
|
52,185 |
|
| Disposals during the year |
|
(632) |
(1,305) |
(547) |
(524) |
|
(3,008) |
| Transfer and reclassifications |
|
2,342 |
|
3,602 |
|
509 |
|
(6,453) |
|
— |
|
| Effect of exchange rate adjustment |
|
943 |
|
618 |
|
74 |
|
42 |
|
|
1,677 |
|
| Cost at the end of the year |
|
62,141 |
|
63,198 |
|
10,167 |
|
79,378 |
|
|
214,884 |
|
| Depreciation and impairment losses at the beginning of the year |
|
18,325 |
|
23,834 |
|
5,463 |
|
— |
|
|
47,622 |
|
| Depreciation for the year |
|
2,786 |
|
2,099 |
|
1,148 |
|
— |
|
|
6,033 |
|
| Impairment losses for the year |
|
43 |
|
474 |
|
8 |
|
524 |
|
|
1,049 |
|
| Depreciation and impairment losses reversed on disposals during the year |
|
(563) |
(918) |
(538) |
(524) |
|
(2,543) |
| Effect of exchange rate adjustment |
|
113 |
|
69 |
|
53 |
|
— |
|
|
235 |
|
| Depreciation and impairment losses at the end of the year |
|
20,704 |
|
25,558 |
|
6,134 |
|
— |
|
|
52,396 |
|
| Carrying amount at the end of the year |
|
41,437 |
|
37,640 |
|
4,033 |
|
79,378 |
|
|
162,488 |
|
| 2023 |
|
|
|
|
|
|
|
| Cost at the beginning of the year |
|
43,403 |
|
37,548 |
|
8,114 |
|
22,361 |
|
|
111,426 |
|
| Additions during the year |
|
2,681 |
|
47 |
|
873 |
|
27,830 |
|
|
31,431 |
|
| Disposals during the year |
|
(690) |
(952) |
(624) |
(562) |
|
(2,828) |
| Transfer and reclassifications |
|
4,246 |
|
4,679 |
|
731 |
|
(9,656) |
|
|
— |
|
| Effect of exchange rate adjustment |
|
(650) |
(371) |
(115) |
(310) |
|
(1,446) |
| Cost at the end of the year |
|
48,990 |
|
40,951 |
|
8,979 |
|
39,663 |
|
|
138,583 |
|
| Depreciation and impairment losses at the beginning of the year |
|
16,781 |
|
22,935 |
|
5,039 |
|
— |
|
|
44,755 |
|
| Depreciation for the year |
|
2,450 |
|
1,919 |
|
1,086 |
|
— |
|
|
5,455 |
|
| Impairment losses for the year |
|
6 |
|
118 |
|
24 |
|
562 |
|
|
710 |
|
| Depreciation and impairment losses reversed on disposals during the year |
|
(664) |
(942) |
(597) |
(562) |
|
(2,765) |
| Effect of exchange rate adjustment |
|
(248) |
(196) |
(89) |
— |
|
|
(533) |
| Depreciation and impairment losses at the end of the year |
|
18,325 |
|
23,834 |
|
5,463 |
|
— |
|
|
47,622 |
|
| Carrying amount at the end of the year |
|
30,665 |
|
17,117 |
|
3,516 |
|
39,663 |
|
|
90,961 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Depreciation |
| DKK million |
|
2024 |
|
2023 |
2022 |
| Cost of goods sold |
|
3,799 |
|
3,522 |
3,205 |
| Sales and distribution costs |
|
487 |
|
500 |
423 |
| Research and development costs |
|
1,120 |
|
1,053 |
898 |
| Administrative costs |
|
554 |
|
354 |
408 |
| Other operating income and expenses |
|
73 |
|
26 |
20 |
| Total depreciation |
|
6,033 |
|
5,455 |
4,954 |
| Of which related to leased assets |
|
1,500 |
|
1,251 |
1,052 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Impairment losses |
|
| DKK million |
|
2024 |
|
2023 |
2022 |
| Cost of goods sold |
|
962 |
|
446 |
24 |
| Sales and distribution costs |
|
9 |
|
4 |
1 |
| Research and development costs |
|
78 |
|
260 |
24 |
|
|
|
|
|
|
|
|
|
|
|
|
| Total impairment losses |
|
1,049 |
|
710 |
49 |
| Of which related to leased assets |
|
9 |
|
— |
— |
Novo Nordisk mainly leases office buildings, warehouses, laboratories and vehicles. The right-of-use asset is presented in property, plant and equipment and the lease liability in borrowings.
|
|
|
|
|
|
|
|
|
|
|
|
| Leased property, plant and equipment |
| DKK million |
2024 |
|
2023 |
| Land and buildings |
6,067 |
|
5,157 |
| Other equipment |
775 |
|
768 |
| Total |
6,842 |
|
5,925 |
The total cash outflow for leases amounted to DKK 2,211 million (DKK 2,022 million in 2023 and DKK 1,438 million in 2022). Refer to note 4.6 for a maturity analysis of
lease payments and 5.2 for commitments not recognised in the balance sheet Property, plant and equipment is measured at historical cost less accumulated depreciations and any impairment loss.
related to leases.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 3.3 Inventories |
|
ACCOUNTING POLICIES
The cost of self-constructed assets includes costs directly attributable to the construction of the assets. Any subsequent cost is included in the asset’s carrying amount or recognised as a separate asset only when it is probable that future economic benefits associated with the item will flow to Novo Nordisk, and the cost of the item can be measured reliably. Depreciation is based on the straight-line method over the estimated useful life of the assets (buildings: 10-50 years, plant and machinery: 5-25 years and other equipment: 3-10 years. Land is not depreciated). Climate-related matters, including the commitment to reach net zero emissions, were considered when estimating the useful lives of property, plant
and equipment.
Depreciation commences when the asset is available for use, i.e. when it is in the location and condition necessary for it to be capable of operating in the manner intended by Management. The asset's residual value and useful life is reviewed and adjusted, if appropriate, at the end of each reporting period. If an asset’s carrying amount is higher than its estimated recoverable amount, it is written down to the recoverable amount. Plant and equipment with no alternative use developed as part
of a research and development project are expensed. However, plant and equipment with an alternative use or used for general research and development purposes are capitalised and depreciated over the estimated useful life as research and development costs.
For contracts which are, or contain, a lease, the Group recognises a right-of-use asset and a lease liability. The right-of-use asset is initially measured at cost, being the initial amount of the lease liability. The right-of-use asset is subsequently depreciated using the straight-line method over the lease term.
The lease term comprises the non-cancellable period of a lease, together with periods covered by extension options if these are reasonably certain to be exercised.
3.3 Inventories
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
| Raw materials |
|
13,369 |
|
9,500 |
| Work in progress |
|
22,335 |
|
17,601 |
| Finished goods |
|
8,873 |
|
7,224 |
| Total inventories (gross) |
|
44,577 |
|
34,325 |
| Write-downs at year-end |
|
(3,728) |
|
(2,514) |
| Total inventories (net) |
|
40,849 |
|
31,811 |
Indirect production costs included in work in
progress and finished goods |
|
15,082 |
|
13,101 |
| Share of total inventories (net) |
|
37 |
% |
|
41 |
% |
| Movements in inventory write-downs: |
|
|
|
|
| Write-downs at the beginning of the year |
|
2,514 |
|
1,715 |
| Write-downs during the year |
|
2,660 |
|
1,808 |
| Utilisation of write-downs |
|
(1,401) |
|
(718) |
| Reversal of write-downs |
|
(45) |
|
(291) |
| Write-downs at the end of the year |
|
3,728 |
|
2,514 |
All write-downs in both 2024 and 2023 relate to fully impaired inventory.
ACCOUNTING POLICIES
Inventories are stated at cost or net realisable value, whichever is lower. Cost is determined using the first-in, first-out method. Cost comprises direct production
costs such as raw materials, consumables and labour. Production costs for work in progress and finished goods include indirect production costs such as employee costs,
depreciation, maintenance, etc. If the expected sales price less completion costs to execute sales (net realisable value) is lower than the carrying amount, a write-down is recognised for the amount by which the carrying amount exceeds its net realisable value.
Inventory manufactured prior to regulatory approval (prelaunch inventory) is capitalised but immediately written down, until there is a high probability of regulatory approval for the product. The cost is recognised in the income statement as research and development costs. Once there is a high probability of regulatory approval being obtained, the write-down is reversed, up to no more than the original cost.
3.4 Trade receivables
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Gross carrying amount |
Loss allowance |
|
Net carrying amount |
|
| 2024 |
|
|
|
|
|
| Not yet due |
71,245 |
(1,049) |
|
70,196 |
|
| 1-90 days |
1,452 |
(230) |
|
1,222 |
|
| 91-180 days |
415 |
(110) |
|
305 |
|
| 181-270 days |
328 |
(102) |
|
226 |
|
| 271-360 days |
341 |
(341) |
|
— |
|
| More than 360 days past due |
295 |
(295) |
|
— |
|
| Trade receivables |
74,076 |
(2,127) |
|
71,949 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2023 |
|
|
|
|
|
| Not yet due |
64,327 |
(1,095) |
|
63,232 |
|
| 1-90 days |
1,557 |
(160) |
|
1,397 |
|
| 91-180 days |
211 |
(100) |
|
111 |
|
| 181-270 days |
111 |
(81) |
|
30 |
|
| 271-360 days |
90 |
(90) |
|
— |
|
| More than 360 days past due |
268 |
(268) |
|
— |
|
| Trade receivables |
66,564 |
(1,794) |
|
64,770 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allowance for doubtful trade receivables |
| DKK million |
2024 |
|
2023 |
| Carrying amount at the beginning of the year |
1,794 |
|
1,520 |
| Reversal of allowance on realised losses |
(70) |
|
(39) |
| Net movement recognised in income statement |
445 |
|
413 |
| Effect of exchange rate adjustment |
(42) |
|
(100) |
| Allowance at the end of the year |
2,127 |
|
1,794 |
Novo Nordisk’s customer base is comprised of government agencies, wholesalers, retail pharmacies and other customers. Novo Nordisk closely monitors the current economic conditions of countries impacted by currency fluctuations, high inflation
and an unstable political climate. These indicators, as well as payment history, are taken into account in the valuation of trade receivables. No loss allowance has been recognised on trade receivables in factoring portfolios in 2024 and 2023.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 3.5 Provisions and contingent liabilities |
|
Refer to note 4.4 for more information on credit exposures.
ACCOUNTING POLICIES
Trade receivables are initially recognised at transaction price and subsequently measured at amortised cost using the effective interest method, less allowance
for doubtful trade receivables.
Before being sold, trade receivables in factoring portfolios are measured at fair value with changes recognised in other comprehensive income. The allowance for doubtful receivables is deducted from the carrying amount of trade receivables in sales and distribution costs.
Management measures allowance for doubtful trade receivables based on the simplified approach to provide for expected credit losses, which requires the use
of the lifetime expected loss provision for all trade receivables. The allowance is an estimate based on shared credit risk characteristics and the days past due. Generally, invoices are due for payment within 90 days from shipment of goods. Loss allowance is calculated using an ageing factor, geographical risk and specific customer knowledge. The allowance is based on a provision matrix on days past due and a forward looking element relating mainly to incorporation of S&P Ratings country risk ratings and an individual assessment. Refer to note 4.4 for a general description of credit risk.
3.5 Provisions and contingent liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Provisions
for sales
rebates1
|
|
Provisions
for legal
disputes
|
|
Provisions
for product
returns
|
Other
provisions2
|
|
2024
Total |
|
2023
Total |
| At the beginning of the year |
99,878 |
|
3,786 |
|
1,532 |
1,931 |
|
107,127 |
|
74,877 |
| Additional provisions, including increases to existing provisions |
318,812 |
|
202 |
|
2,148 |
798 |
|
321,960 |
|
288,801 |
Additional provisions from acquisition of businesses (note 5.3) |
— |
|
— |
|
— |
1,084 |
|
1,084 |
|
— |
| Amount used during the year |
(299,334) |
|
— |
|
(693) |
(191) |
|
(300,218) |
|
(251,246) |
| Adjustments regarding prior years, including unused amounts reversed during the year |
(6,452) |
|
(31) |
|
80 |
(320) |
|
(6,723) |
|
(3,023) |
| Effect of exchange rate adjustment |
5,612 |
|
222 |
|
27 |
(7) |
|
5,854 |
|
(2,282) |
| At the end of the year |
118,516 |
|
4,179 |
|
3,094 |
3,295 |
|
129,084 |
|
107,127 |
Non-current liabilities3 |
548 |
|
4,154 |
|
908 |
3,145 |
|
8,755 |
|
6,649 |
| Current liabilities |
117,968 |
|
25 |
|
2,186 |
150 |
|
120,329 |
|
100,478 |
1. Provisions for sales rebates are related to US Managed Care, Medicare, Medicaid, 340B Drug Pricing Program and other types of US rebates, as well as rebates in a number of European countries and Canada. 2. Other provisions consist of various types of provisions, including contingent payments arising from business combinations and obligations in relation to employee benefits such as jubilee benefits. 3. For non-current liabilities, provisions for sales rebates are expected to be settled after one year, provisions for product returns will be utilised in 2025 and 2026. In the case of provisions for legal disputes, the timing of settlement cannot be determined. |
Contingent liabilities
Novo Nordisk is currently involved in pending litigations, claims and investigations arising out of the normal conduct of its business. While provisions that Management deems to be reasonable and appropriate have been made for probable losses, there are inherent uncertainties connected with these estimates.
Since January 2021, Novo Nordisk has made a number of changes to its policy in
the US related to facilitating delivery of its discounted medicines to commercial pharmacies that contract with covered entities participating in the 340B Drug Pricing Program. On 30 January 2023, the US Court of Appeals for the Third Circuit issued a ruling holding that Novo Nordisk’s drug distribution policy was consistent with the 340B statute. On 21 May 2024, the US Court of Appeals for the DC Circuit issued a ruling in a related case involving other pharmaceutical manufacturers that similarly held that their drug distribution policies were consistent with the 340B statute. However, an appeal in another related case is still pending before the US Court of Appeals for the Seventh Circuit, and as such these cases may be subject to further discretionary appellate review before the US Supreme Court. Depending on the outcome of the pending Seventh Circuit ruling and any subsequent appeals in these matters, there may be a material impact on Novo Nordisk’s financial position, net sales, operating profit and cash flow.
Pending litigation against Novo Nordisk
Mosaic Health Inc. and Central Virginia Health Services, Inc. (both 340B covered entities) filed a putative class action lawsuit in Federal Court in New York against Novo Nordisk, Eli Lilly and Company, Sanofi and AstraZeneca alleging a conspiracy among the manufacturers to artificially fix prices of diabetes medications through changes to their policies relating to the distribution of 340B drugs. The lawsuit was subsequently dismissed by the Court on 2 September 2022. The plaintiffs appealed the dismissal of the complaint to the United States Court of Appeals for the Second Circuit. That appeal is currently pending. Novo Nordisk does not expect this matter to have a material impact on Novo Nordisk’s financial position, operating profit or cash flow.
Novo Nordisk is currently defending numerous lawsuits, including putative class actions, relating to the pricing of diabetes medicines in the US. The first lawsuit was filed in 2017 and in August 2023 a multi-district litigation was created in the United States District court for the District of New Jersey. Nearly all pending matters also name Eli Lilly and Company and Sanofi as defendants, while certain matters also
name Pharmacy Benefit Managers (PBMs) and related entities. Plaintiffs generally allege that the manufacturers and PBMs colluded to artificially inflate list prices paid by consumers for diabetes products, while offering reduced prices to PBMs through rebates used to secure formulary access. Novo Nordisk does not expect these matters to have a material impact on Novo Nordisk’s financial position, operating profit or
cash flow.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 3.5 Provisions and contingent liabilities |
|
In 2016, Novo Nordisk received a Civil Investigative Demand ("CID") from the US Department of Justice ("DOJ") relating to potential off-label marketing of NovoSeven® (including high dose and for prophylactic use) and interactions with physicians and patients. The DOJ investigation was likely prompted by a lawsuit filed in 2015 by a former Novo Nordisk employee (the “Relator”), who alleged Novo Nordisk caused the submission of false claims to Medicare, Medicaid, Federal Employees Health Benefits Program and private insurers in California. In September 2022, DOJ ceased its investigation and declined to intervene in the lawsuit. The Relator and the Washington State Attorney General have proceeded with the lawsuit, which was transferred to the United States District Court for the Western District of Washington in May 2023. Novo Nordisk does not expect this matter to have a material impact on Novo Nordisk’s financial position, operating profit or cash flow.
Novo Nordisk, along with Eli Lilly, are defendants in numerous product liability lawsuits (mainly in in the US) related to the use of GLP-1-based medicines. Plaintiffs have alleged that the use of these treatments, including Victoza®, Ozempic®,
Wegovy® and Rybelsus®, have caused various gastrointestinal and other injuries.
The US lawsuits have been consolidated in a multi-district litigation in the United States District Court for the Eastern District of Pennsylvania. Novo Nordisk does not expect these matters to have a material impact on Novo Nordisk’s financial position, operating profit or cash flow.
On 13 September 2024, five former employees filed a putative class action against Novo Nordisk Inc. ("NNI"), the NNI Board of Directors, and the NNI Retirement Committee alleging claims for breach of fiduciary duty in connection with the management of the NNI Retirement Plan. The complaint alleges that, from September 2018 to the present, certain conduct violated the Employee Retirement Income Security Act of 1974. Novo Nordisk does not expect this matter to have a material impact on Novo Nordisk’s financial position, operating profit or cash flow.
On 24 January 2025, a class-action lawsuit was filed against Novo Nordisk A/S,
Chief Executive Officer Lars Fruergaard Jorgensen and Executive Vice President, Development Martin Holst Lange in the United States District Court for the District
of New Jersey by a proposed class of purchasers of Novo Nordisk American Depository Receipts (ADRs) between 2 November 2022 and 19 December 2024. The lawsuit relates to REDEFINE-1 and alleges that the company failed to disclose or otherwise misled investors as to the nature of the dosages provided to patients in the study and that the company misleadingly exhibited confidence in its expected 25% average weight loss outcome. Novo Nordisk does not expect the litigation to have a material impact on Novo Nordisk’s financial position, operating profit or cash flow.
Other provisions and contingent liabilities
In February 2023, a class action lawsuit was filed by the City of Warwick Retirement System (“City of Warwick”) against Catalent, Inc. (“Catalent”) and co-defendants in the United States District Court for the District of New Jersey. The lawsuit alleges that the defendants artificially inflated Catalent’s revenue and made misleading statements and omissions concerning Catalent’s quality control issues; compliance with the US
Generally Accepted Accounting Principles; and the general demand for non-vaccine products. In December 2024, Novo Nordisk acquired three Catalent fill-finish sites from Novo Holding A/S, including a portion of any potential financial liability associated with the City of Warwick lawsuit. Novo Nordisk does not expect these matters to have a material impact on Novo Nordisk’s financial position, operating profit or cash flow.
In addition to the above, Novo Nordisk is engaged in certain litigation proceedings and various ongoing audits and investigations. In the opinion of Management, neither settlement nor continuation of such proceedings, nor such pending audits and investigations, are expected to have a material effect on Novo Nordisk’s financial position, operating profit or cash flow.
KEY ACCOUNTING ESTIMATES REGARDING ONGOING LEGAL DISPUTES, LITIGATION AND INVESTIGATIONS
Provisions for legal disputes consist of various types of provisions linked to ongoing legal disputes. Management makes estimates regarding provisions and contingencies, including the probability of pending and potential future litigation outcomes. These are by nature dependent on inherently uncertain future events. When determining likely outcomes of litigation, etc., Management considers the input of external counsel on each case, as well as known outcomes in case law. Although Management believes that the total provisions for legal proceedings are adequate based on currently available information, there can be no assurance that there will not be any changes in facts or matters, or that any future lawsuits, claims, proceedings or investigations will not be material.
ACCOUNTING POLICIES
Provisions for sales rebates and discounts granted to government agencies, wholesalers, retail pharmacies, Managed Care and other customers are recorded
at the time the related revenues are recorded or when the incentives are offered. Provisions are calculated based on Management's interpretation of applicable
laws and regulations, historical experience and the specific terms in the individual agreements. Unsettled rebates are recognised as provisions when the timing
or amount is uncertain. Where absolute amounts are known, the rebates are recognised as other liabilities. Refer to note 2.1 for further information on sales rebates and provisions.
Provisions for legal disputes are recognised where a legal or constructive obligation has been incurred as a result of past events and it is probable that there will be an outflow of resources that can be reliably estimated. In this case, Novo Nordisk arrives at an estimate based on an evaluation of the most likely outcome. Disputes for which no reliable estimate can be made are disclosed as contingent liabilities.
Provisions are measured at the present value of the anticipated expenditure for settlement. This is calculated using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the obligation.
Novo Nordisk issues credit notes for expired goods as a part of normal business. Where there is historical experience or a reasonably accurate estimate of expected future returns can otherwise be made, a provision for estimated product returns is recorded. The provision is measured at net sales value.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 4.1 Earnings per share |
|
Section 4
Capital structure and financial items
4.1 Earnings per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2024 |
|
2023 |
2022 |
| Net profit |
DKK million |
|
100,988 |
|
83,683 |
55,525 |
Average number of shares outstanding1 |
in million shares |
|
4,453.9 |
|
4,482.8 |
4,530.6 |
Dilutive effect of restricted
stock units |
in million shares |
|
9.1 |
|
12.0 |
14.0 |
Average number of shares outstanding, including
dilutive effect |
in million shares |
|
4,463.0 |
|
4,494.8 |
4,544.6 |
| Basic earnings per share |
DKK |
|
22.67 |
|
18.67 |
12.26 |
| Diluted earnings per share |
DKK |
|
22.63 |
|
18.62 |
12.22 |
| 1. Excluding treasury shares. |
The trading unit of the Novo Nordisk B shares listed on NASDAQ Copenhagen was changed from DKK 0.20 to DKK 0.10 as of 13 September 2023. The ADRs listed on
the New York Stock Exchange (NYSE) were similarly split as of 20 September 2023. Comparative figures have been restated to reflect the change in trading unit from DKK 0.20 to DKK 0.10.
ACCOUNTING POLICIES
Earnings per share is presented as both basic and diluted earnings per share. Basic earnings per share is calculated as net profit divided by the monthly average number of shares outstanding. Diluted earnings per share is calculated as net profit divided by the sum of monthly average number of shares outstanding, including the dilutive effect of the outstanding share pool. Refer to 'Financial definitions and ratios' for a description of calculation of the dilutive effect.
4.2 Distribution to shareholders
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
2024 |
|
2023 |
2022 |
| Interim dividend for the year |
15,583 |
|
13,430 |
9,613 |
| Dividend for prior year |
28,557 |
|
18,337 |
15,690 |
| Dividend payout in the year |
44,140 |
|
31,767 |
25,303 |
| Share repurchases for the year |
20,181 |
|
29,924 |
24,086 |
| Total distribution for the year |
64,321 |
|
61,691 |
49,389 |
Novo Nordisk's dividend pay-outs in the year was complemented by share repurchase programmes. Novo Nordisk's guiding principle is that any excess capital after the funding of organic growth opportunities and potential acquisitions should be returned to investors. No dividend is declared on treasury shares.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
2024 |
|
2023 |
2022 |
Interim dividend1 |
15,583 |
|
13,430 |
9,613 |
Final dividend2 |
35,100 |
|
28,557 |
18,337 |
| Total dividend |
50,683 |
|
41,987 |
27,950 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK per share |
2024 |
|
2023 |
2022 |
Interim dividend1 |
3.50 |
|
3.00 |
2.12 |
Final dividend2 |
7.90 |
|
6.40 |
4.08 |
| Total dividend |
11.40 |
|
9.40 |
6.20 |
1. Interim dividend was declared and paid in August 2024. 2. Final dividend for 2024 is expected to be distributed pending approval at the Annual General Meeting in March 2025. Final dividend for 2023 was declared and paid in March 2024. |
4.3 Share capital, Treasury shares and Other reserves
Development in number of shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of shares (million) |
A shares |
B shares |
Total issued shares |
|
Treasury shares |
Out-standing shares |
|
| Shares beginning of 2023 |
1,075 |
3,485 |
4,560 |
|
(60) |
4,500 |
|
| Shares cancelled in 2023 |
— |
(50) |
(50) |
|
50 |
— |
|
| Released allocated shares to employees |
— |
— |
— |
|
9 |
9 |
|
| Shares purchased in 2023 |
— |
— |
— |
|
(51) |
(51) |
|
Number of shares end
of 2023 |
1,075 |
3,435 |
4,510 |
|
(52) |
4,458 |
|
| Shares cancelled in 2024 |
— |
(45) |
(45) |
|
45 |
— |
|
| Released allocated shares to employees |
— |
— |
— |
|
8 |
8 |
|
| Shares purchased in 2024 |
— |
— |
— |
|
(25) |
(25) |
|
Number of shares end of 2024 |
1,075 |
3,390 |
4,465 |
|
(24) |
4,441 |
|
The A share capital and number of A shares of DKK 0.10 was unchanged in 2024, 2023 and 2022. In 2024, the B share capital decreased by DKK 4.5 million (equal to cancellation of 45 million shares of DKK 0.10). The corresponding decrease in 2023 was DKK 5 million (equal to cancellation of 50 million shares of DKK 0.10) and decrease in 2022 of DKK 6 million (equal to cancellation of 60 million shares of DKK 0.10).
Each A share of DKK 0.10 per share carries 100 votes and each B share of DKK 0.10 per share carries 10 votes.
At the end of 2024, the holding of treasury shares amounted to 0.5% of the total outstanding shares (1.1% of the outstanding shares in 2023). Treasury shares are primarily acquired to reduce the company's share capital. In addition, a limited part is used to finance Novo Nordisk's long-term share-based incentive programme and restricted stock units to employees. Treasury shares are deducted from the share capital on cancellation at their nominal value of DKK 0.10 per share. Differences between this amount and the amount paid to acquire or received for disposing of treasury shares are deducted directly in retained earnings.
The purchase of treasury shares during the year relates to the remaining part of the 2023 share repurchase programme, totalling DKK 1.6 billion, and the DKK 20 billion Novo Nordisk B share repurchase programme for 2024, of which DKK 1.4 billion was outstanding at year-end. The programme ended on 3 February 2025.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 4.4 Financial risks |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Specification of Other reserves |
| DKK million |
Exchange rate adjustments |
Cash flow hedges1 |
Tax and other items |
|
Total |
|
|
|
|
|
|
Reserve at 1 January 2022 |
(904) |
(1,740) |
930 |
|
(1,714) |
Other comprehensive
income, net |
2,289 |
2,766 |
(892) |
|
4,163 |
|
|
|
|
|
|
Reserve at 31 December 2022 |
1,385 |
1,026 |
38 |
|
2,449 |
|
|
|
|
|
|
| Other comprehensive income, net |
(1,404) |
586 |
(355) |
|
(1,173) |
Reserve at 31 December 2023 |
(19) |
1,612 |
(317) |
|
1,276 |
|
|
|
|
|
|
Other comprehensive
income, net |
3,096 |
(6,221) |
1,343 |
|
(1,782) |
|
|
|
|
|
|
|
Transferred to
intangible assets2
|
— |
(1,154) |
254 |
|
(900) |
Reserve at 31 December 2024 |
3,077 |
(5,763) |
1,280 |
|
(1,406) |
1. Refer to note 4.5 for information on cash flow hedges. 2. A gain from cash flow hedges related to acquisition of businesses of DKK 1,154 million is transferred directly from the cash flow hedge reserve on an after-tax basis to the initial cost of net assets acquired leading to a net hedging effect of DKK 900 million. Refer to note 5.3 for information of acquisition of businesses. |
According to Danish corporate law, reserves available for distribution as dividends
are based on the financial statements of the parent company, Novo Nordisk A/S. Dividends are declared and paid from distributable reserves. As of 31 December
2024, distributable reserves total DKK 121,931 million (DKK 78,779 million in 2023), corresponding to the parent company's retained earnings and Reserve for cash flow hedges and exchange rate adjustments.
4.4 Financial risks
Management has assessed the following key financial risks:
|
|
|
|
|
|
| Type |
Financial risk |
| Foreign exchange risk |
High |
| Credit risk |
Low |
| Interest rate risk |
Low |
| Liquidity risk |
Low |
Novo Nordisk has centralised management of the Group's financial risks. The overall objectives and policies for the company's financial risk management are outlined in the internal Treasury Policy, which is approved by the Board of Directors. The Treasury Policy consists of the Foreign Exchange Policy, the Investment Policy, the Financing Policy and the Policy regarding Credit Risk on Financial Counterparts, and includes a description of permitted use of financial instruments and risk limits.
Novo Nordisk only hedges commercial exposures and consequently does not enter into derivative transactions for trading or speculative purposes. Novo Nordisk uses a fully integrated treasury management system to manage all financial positions, and
all positions are marked-to-market.
Foreign exchange risk
Foreign exchange risk is the most important financial risk for Novo Nordisk and can have a significant impact on the income statement, statement of comprehensive income, balance sheet and cash flow statement. The majority of Novo Nordisk's foreign exchange exposures are in USD, EUR, CNY, CAD, JPY and BRL. The foreign exchange risk is most significant in USD. The exchange rate risk exposure in EUR is regarded as low because of Denmark's fixed exchange rate policy towards EUR. The overall objective of foreign exchange risk management is to reduce the short-term negative impact of exchange rate fluctuations on earnings and cash flow, thereby contributing to the predictability of the financial results. In selected currencies, Novo Nordisk hedges assets and liabilities as well as future expected cash flows up to a maximum of 24 months, including selected business development activities (acquisition of businesses).
Hedge accounting is applied to match the impact of the hedged item and the hedging instrument in the consolidated income statement. The currency hedging strategy balances risk reduction and cost of hedging by use of foreign exchange forwards and foreign exchange options matching the due dates of the hedged items. Expected cash flows are continually assessed using historical inflows, budgets and monthly sales forecasts. Hedge effectiveness is assessed on a regular basis.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Exchange rates applied for selected currencies |
|
|
|
USD |
CNY |
CAD |
JPY |
BRL |
|
| Average exchange rate applied (DKK per 100) |
| 2024 |
689 |
96 |
503 |
4.56 |
129 |
|
| 2023 |
689 |
97 |
511 |
4.91 |
138 |
|
| 2022 |
708 |
105 |
543 |
5.40 |
137 |
|
| Year-end exchange rate applied (DKK per 100) |
| 2024 |
714 |
98 |
496 |
4.53 |
115 |
|
| 2023 |
674 |
95 |
509 |
4.77 |
139 |
|
| 2022 |
697 |
101 |
515 |
5.29 |
132 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sensitivity of an immediate 5% decrease in currency rates on
31 December vs DKK1
|
| DKK million |
2024 |
|
2023 |
| Sensitivity of all currencies |
|
|
|
| Income statement |
(323) |
|
(117) |
| Other comprehensive income |
8,012 |
|
6,058 |
| Total |
7,689 |
|
5,941 |
| Hereof sensitivity of USD |
|
|
|
| Income statement |
148 |
|
70 |
| Other comprehensive income |
7,178 |
|
5,082 |
| Total |
7,326 |
|
5,152 |
1. An immediate 5% increase would have the opposite impact of the above. |
The foreign exchange sensitivity analysis comprises effects from the Group's financial instruments, including cash, trade receivables and trade payables, current loans, current and non-current financial investments, lease liabilities and foreign exchange forwards. Anticipated currency transactions, investments in foreign subsidiaries and non-current assets are not included. The main impact is driven by forward contracts used for hedging activities.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 4.4 Financial risks |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial contracts coverage at year end |
| Months |
USD |
CNY2 |
CAD |
JPY |
BRL |
| 2024 |
12 |
12 |
0 |
12 |
0 |
| 2023 |
12 |
12 |
9 |
12 |
0 |
| 2. Chinese yuan traded offshore (CNH) is used to hedge Novo Nordisk's CNY currency exposure. |
The table above shows hedge coverage horizon existing at year-end to cover the expected future cash flow for the disclosed number of months. The hedging of CAD has been phased out during 2024. Average hedge rate for USD cash flow hedges is 676 at the end of 2024 (676 at the end of 2023).
Credit risk
Credit risk arises from the possibility that transactional counterparties may default
on their obligations towards the Group.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Credit exposure for cash at bank, marketable securities and
derivative financial instruments (fair value) |
| DKK million |
|
Cash at
bank |
Marketable securities |
Derivative financial instruments |
Total |
|
| 2024 |
|
|
|
|
|
|
|
| AAA range |
|
— |
10,653 |
— |
|
10,653 |
|
| AA range |
|
6,582 |
— |
1,773 |
|
8,355 |
|
| A range |
|
8,278 |
— |
4,553 |
|
12,831 |
|
| BBB range |
|
172 |
— |
— |
|
172 |
|
Not rated or below
BBB range |
|
623 |
— |
— |
|
623 |
|
| Total |
|
15,655 |
10,653 |
6,326 |
|
32,634 |
|
| 2023 |
|
|
|
|
|
|
|
| AAA range |
|
— |
15,838 |
— |
|
15,838 |
|
| AA range |
|
6,451 |
— |
912 |
|
7,363 |
|
| A range |
|
7,292 |
— |
1,432 |
|
8,724 |
|
| BBB range |
|
17 |
— |
— |
|
17 |
|
Not rated or below
BBB range |
|
632 |
— |
— |
|
632 |
|
| Total |
|
14,392 |
15,838 |
2,344 |
|
32,574 |
|
Credit risk exposure to financial counterparties
Novo Nordisk considers its maximum credit exposure to financial counterparties
to be DKK 32,634 million (DKK 32,574 million in 2023).
To manage credit risk regarding financial counterparties, Novo Nordisk only enters into derivative financial contracts and money market deposits with financial counterparties possessing a satisfactory long-term credit rating from at least two
of the three selected rating agencies: Standard and Poor's, Moody's and Fitch. Furthermore, maximum credit lines defined for each counterparty diversify the
overall counterparty risk. The credit risk on marketable securities is low, as investments are made in highly liquid bonds with AAA credit ratings.
Credit risk exposure to non-financial counterparties
Novo Nordisk considers its maximum credit exposure to trade receivables, other receivables (less prepayments and VAT receivables) and other financial assets to be DKK 77,572 million (DKK 67,209 million in 2023). Refer to note 4.8 for details of the Group's total financial assets.
Outside the US, Novo Nordisk has no significant concentration of credit risk related
to trade receivables or other receivables and prepayments, because the exposure in general is spread over a large number of counterparties and customers. In the US, the three major wholesalers account for a large proportion of total net sales, see note 2.1. However, US wholesaler credit ratings are monitored, and part of the trade receivables are sold on full non-recourse terms; see below for details.
Novo Nordisk closely monitors the current economic conditions of countries impacted by currency fluctuations, high inflation and an unstable political climate. These indicators, as well as payment history are taken into account in the valuation of
trade receivables.
Trade receivable programmes
At year-end, the Group had derecognised receivables without recourse having
due dates after 31 December 2024 amounting to:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
2022 |
| US |
|
3,214 |
|
5,059 |
1,394 |
| Japan |
|
1,834 |
|
2,050 |
2,273 |
Novo Nordisk's subsidiaries in the US and Japan employ trade receivable programmes in which trade receivables are sold on full non-recourse terms to optimise working capital.
Interest rate risk
Novo Nordisk's exposure to interest rate risk is deemed low, primarily attributable
to the capital structure. The company's interest-bearing liabilities comprise a mix of fixed rate Eurobonds and variable rate instruments. The risk associated with variable interest-bearing liabilities is offset to some extent by variable interest-bearing assets. These assets consist of cash, cash equivalents, and marketable securities with a low portfolio duration. Taking into account these balancing factors, the overall interest rate risk is assessed to be low.
Liquidity risk
Novo Nordisk´s liquidity risk is considered to be low. The availability of the required liquidity is ensured through a combination of cash pools for cash centralisation, highly liquid investment portfolios and both uncommitted and committed credit facilities. In combination these factors mitigate short-term liquidity risk. Furthermore, the Board of Directors has decided not to initiate a new share repurchase program in 2025.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial reserves |
| DKK million |
|
2024 |
|
2023 |
2022 |
| Cash at bank |
|
15,655 |
|
14,392 |
12,653 |
| Marketable securities |
|
10,653 |
|
15,838 |
10,921 |
Undrawn committed credit facility3 |
|
22,380 |
|
11,552 |
11,527 |
| Undrawn bridge facility |
|
6,341 |
|
— |
— |
| Borrowings |
|
(11,775) |
|
(5,431) |
(480) |
| Financial reserves |
|
43,254 |
|
36,351 |
34,621 |
|
3. The undrawn committed credit facility comprises a facility of EUR 3,000 million in 2024
(EUR 1,550 million in 2023 and 2022) committed by a portfolio of international banks. The facility matures in 2029.
|
Financial reserves comprise of sources of liquidity, as shown in the table above, less borrowings that are contractually obliged to be repaid within 12 months. Borrowings, which reduces the financial reserves, consist of current borrowings (DKK 13,113 million) excluding leasing (DKK 1,338 million).
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 4.5 Derivative financial instruments |
|
4.5 Derivative financial instruments
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2024 |
|
2023 |
| DKK million |
Average rate |
|
Contract
amount
at year-end |
Positive
fair value
at year-end |
Negative
fair value
at year-end |
|
Average rate |
|
Contract
amount
at year-end |
Positive
fair value
at year-end |
Negative
fair value
at year-end |
| Forward contracts USD |
676 |
|
137,781 |
13 |
5,704 |
|
676 |
|
104,022 |
1,600 |
193 |
Forward contracts CNH and JPY1 |
|
|
16,910 |
109 |
181 |
|
|
|
20,246 |
295 |
90 |
|
|
|
|
|
|
|
|
|
|
|
|
| Forward contracts, cash flow hedges |
|
|
154,691 |
122 |
5,885 |
|
|
|
124,268 |
1,895 |
283 |
| Forward contracts USD |
683 |
|
75,864 |
6,135 |
1,577 |
|
675 |
|
65,870 |
330 |
946 |
| Forward contracts EUR, CNH, JPY and others |
|
|
17,451 |
69 |
69 |
|
|
|
28,520 |
119 |
43 |
| Forward contracts, fair value hedges |
|
|
93,315 |
6,204 |
1,646 |
|
|
|
94,390 |
449 |
989 |
| Total derivative financial instruments |
|
|
248,006 |
6,326 |
7,531 |
|
|
|
218,658 |
2,344 |
1,272 |
| Recognised in the income statement |
|
|
|
6,204 |
1,646 |
|
|
|
|
449 |
989 |
| Recognised in other comprehensive income |
|
|
|
122 |
5,885 |
|
|
|
|
1,895 |
283 |
1. For 2023 the relevant currencies are CNH, CAD and JPY. |
Deferred losses of DKK 5,763 million from cash flow hedges open at 31 December 2024 were recorded in Other Comprehensive Income along with deferred gains from cash flow hedges related to acquisition of businesses of DKK 1,154 million which was, upon maturity, transferred directly from the cash flow hedge reserve to the initial cost of net assets acquired on an after-tax basis.
Forward contracts are expected to impact the income statement within the next 12 months through financial income or expenses.
There is no ineffectiveness recognised at 31 December 2024.
ACCOUNTING POLICIES
On initiation of the contract, Novo Nordisk designates each derivative financial contract that qualifies for hedge accounting as one of:
•hedges of the fair value of a recognised asset or liability (fair value hedge)
•hedges of a forecast financial transaction (cash flow hedge).
All contracts are initially recognised at fair value and subsequently remeasured
at fair value at the end of the reporting period.
Fair value hedges
Value adjustments of fair value hedges are recognised in the income statement along with any value adjustments of the hedged asset or liability that are attributable to the hedged risk.
Cash flow hedges
Value adjustments of the effective part of cash flow hedges are recognised in other comprehensive income. The cumulative value adjustment of these contracts is transferred from other comprehensive income to the income statement when the hedged transaction is recognised in the income statement. For cash flow hedges of foreign currency risk on highly probable non-financial asset purchases, the cumulative
value adjustments are transferred directly from the cash flow hedge reserve to the initial cost of the asset when recognised.
Discontinuance of cash flow hedging
When a hedging instrument expires or is sold, or when a hedge no longer meets the criteria for hedge accounting, any cumulative gain or loss existing in equity at that time remains in equity and is transferred when the forecasted transaction is ultimately recognised in the income statement. When a forecasted transaction is no longer expected to occur, the cumulative gain
or loss that was reported in equity is immediately transferred to the income statement under financial income or financial expenses.
For additional disclosures on accounting policies for financial instruments refer to note 4.8.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 4.6 Borrowings |
|
4.6 Borrowings
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Reconciliation of liabilities arising from financing activities |
Non-cash movements |
|
| DKK million |
Beginning of the year |
Re-payments |
Proceeds |
Additions1 |
Disposals |
Exchange rates |
Other |
End of the year |
2024 |
|
|
|
|
|
|
|
|
|
| Lease liabilities |
|
5,726 |
(1,417) |
— |
2,383 |
(3) |
71 |
6 |
6,766 |
| Eurobonds |
|
20,824 |
(4,849) |
34,513 |
— |
— |
12 |
28 |
50,528 |
| Loans |
|
— |
— |
39,494 |
201 |
— |
6 |
— |
39,701 |
| Commercial papers |
|
— |
— |
5,344 |
— |
— |
(1) |
— |
5,343 |
| Bank overdrafts |
|
456 |
(69) |
40 |
— |
— |
22 |
— |
449 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total borrowings |
|
27,006 |
(6,335) |
79,391 |
2,584 |
(3) |
110 |
34 |
102,787 |
2023 |
|
|
|
|
|
|
|
|
|
| Lease liabilities |
|
4,529 |
(1,448) |
— |
2,809 |
(4) |
(170) |
10 |
5,726 |
| Eurobonds |
|
20,775 |
— |
— |
— |
— |
46 |
3 |
20,824 |
|
|
|
|
|
|
|
|
|
|
| Bank overdrafts |
|
480 |
(19) |
— |
— |
— |
(4) |
(1) |
456 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total borrowings |
|
25,784 |
(1,467) |
— |
2,809 |
(4) |
(128) |
12 |
27,006 |
| 1. Non-cash additions include additions from acquisitions of businesses. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Issuance of Eurobonds |
Nominal value in millions |
| Interest |
Issue date |
Maturity |
EUR |
DKK |
0.750% Fixed |
Mar 2022 |
Mar 2025 |
500 |
3,730 |
3.375% Fixed |
May 2024 |
May 2026 |
1,300 |
9,698 |
1.125% Fixed |
Mar 2022 |
Sep 2027 |
500 |
3,730 |
0.125% Fixed |
Jun 2021 |
Jun 2028 |
650 |
4,849 |
3.125% Fixed |
May 2024 |
Jan 2029 |
1,000 |
7,460 |
1.375% Fixed |
Mar 2022 |
Mar 2030 |
500 |
3,730 |
3.250% Fixed |
May 2024 |
Jan 2031 |
1,000 |
7,460 |
3.375% Fixed |
May 2024 |
May 2034 |
1,350 |
10,071 |
Eurobonds
Four tranches of Eurobonds with an aggregate nominal amount of EUR 4.65 billion, corresponding to DKK 34.7 billion, were issued under the Novo Nordisk’s European Medium Term Note (EMTN) programme in 2024. Net proceeds of the issuances contributed to the financing of the acquisition of three fill-finish sites from Novo Holdings A/S in connection with a transaction where Novo Holdings A/S acquired Catalent, Inc. (note 5.3). No bonds were issued in 2023.
The fair value of Eurobonds approximates the carrying value.
Loans
Loans comprise mainly of unsecured bank loans, intended as temporary funding of the acquisition of three fill-finish sites from Novo Holdings A/S, which carries a variable interest rate. The fair value of the loans approximates their carrying value.
A portion of loans arises from a sale and repurchase agreement of marketable securities (REPO). On 31 December 2024, the carrying amount of the assets transferred was DKK 2,200 million, and the carrying value of associated liabilities amounted to DKK 2,200 million. The repurchase is fixed, and Novo Nordisk has therefore retained full exposure from fair value changes of the marketable securities. Therefore, the transaction is treated as a collateralised lending arrangement. Where substantially all the risks and rewards of ownership are retained in financial assets that have been transferred, the assets are not derecognised and the proceeds obtained are recognised as a financial liability.
Commercial papers
Commercial papers comprise of short-term, unsecured promissory notes, intended as temporary funding of the acquisition of three fill-finish sites from Novo Holdings A/S, which carries a fixed interest rate. The fair value of the commercial papers approximates their carrying value.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 4.6 Borrowings |
|
ACCOUNTING POLICIES
Issued bonds, loans, commercial papers and bank overdrafts are initially recognised
at the fair value of the proceeds received less transaction costs. In subsequent periods these are measured at amortised cost using the effective interest method. The difference between the proceeds received and the nominal value is recognised in financial income or financial expenses over the term of the loan. For fair value determination refer to note 4.8.
Lease liabilities are related to right-of-use assets primarily premises and company cars and include the present value of future lease payments during the lease term. Lease liabilities are initially measured at the present value of the lease payments outstanding at the commencement date, discounted using the incremental borrowing rate. Lease liabilities are measured using the effective interest method. Lease liabilities are subsequently remeasured to reflect changes in future lease payments, e.g. changes
in lease terms.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Contractual undiscounted cash flows |
2024 |
| DKK million |
Leases |
Eurobonds |
Loans |
Commercial papers |
Bank overdrafts |
Total |
2024 |
|
|
|
|
|
|
| Within 1 year |
1,510 |
4,842 |
3,279 |
5,356 |
449 |
15,436 |
| 1-3 years |
2,327 |
11,829 |
37,945 |
— |
— |
52,101 |
| 3-5 years |
1,558 |
17,700 |
28 |
— |
— |
19,286 |
| More than 5 years |
2,056 |
23,403 |
— |
— |
— |
25,459 |
| Total |
7,451 |
57,774 |
41,252 |
5,356 |
449 |
112,282 |
| Carrying amount end of the year |
6,766 |
50,528 |
39,701 |
5,343 |
449 |
102,787 |
| Non-current borrowings |
5,428 |
46,799 |
37,447 |
— |
— |
89,674 |
| Current borrowings |
1,338 |
3,729 |
2,254 |
5,343 |
449 |
13,113 |
2023 |
|
|
|
|
|
|
| Within 1 year |
1,318 |
4,975 |
— |
— |
456 |
6,749 |
| 1-3 years |
1,902 |
3,948 |
— |
— |
— |
5,850 |
| 3-5 years |
1,253 |
8,695 |
— |
— |
— |
9,948 |
| More than 5 years |
1,612 |
3,819 |
— |
— |
— |
5,431 |
| Total |
6,085 |
21,437 |
— |
— |
456 |
27,978 |
| Carrying amount end of the year |
5,726 |
20,824 |
— |
— |
456 |
27,006 |
| Non-current borrowings |
4,552 |
15,976 |
— |
— |
— |
20,528 |
| Current borrowings |
1,174 |
4,848 |
— |
— |
456 |
6,478 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 4.7 Cash flow statement specifications |
|
4.7 Cash flow statement specifications
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other non-cash items |
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
2022 |
|
|
|
|
|
|
Interest income and interest expenses, net (note 4.9) |
|
(198) |
|
(527) |
139 |
|
Capital gain/(loss) on investments, net
(note 4.9)
|
|
19 |
|
106 |
124 |
Result of associated companies (note 4.9) |
|
17 |
|
(81) |
189 |
|
|
|
|
|
|
|
|
|
|
|
|
Share-based payment costs (note 5.1) |
|
2,289 |
|
2,149 |
1,539 |
| Increase/(decrease) in provisions and retirement benefit obligations |
|
22,118 |
|
32,230 |
18,465 |
| Exchange rate effects on provisions and retirement benefit obligations |
|
(5,846) |
|
2,277 |
(3,238) |
| Adjustment for remeasurements of retirement benefit obligations |
|
(119) |
|
13 |
615 |
Adjustment of provisions and retirement benefit obligations related to acquisition
of businesses |
|
(1,088) |
|
— |
— |
Unrealised gain/(loss) on fair value hedge through profit or loss (note 4.9) |
|
(5,098) |
|
(662) |
2,448 |
|
|
|
|
|
|
| Other |
|
2,935 |
|
(1,988) |
2,228 |
| Total other non-cash items |
|
15,029 |
|
33,517 |
22,509 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Change in working capital |
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
2022 |
| Inventories |
|
(9,038) |
|
(7,423) |
(4,767) |
| Trade receivables |
|
(7,179) |
|
(14,210) |
(9,917) |
| Other receivables and prepayments |
|
(4,544) |
|
(2,063) |
(968) |
| Trade payables |
|
3,240 |
|
10,019 |
6,717 |
| Other liabilities |
|
9,288 |
|
5,099 |
4,006 |
Adjustment for payables related to
non-current assets |
|
(3,520) |
|
(2,432) |
(1,567) |
Adjustment related to acquisition
of businesses |
|
1,134 |
|
— |
(143) |
|
Other non-current receivables
and prepayments1
|
|
(2,586) |
|
(1,224) |
61 |
Other non-current liabilities1 |
|
(166) |
|
89 |
(260) |
| Change in working capital including exchange rate adjustments |
|
(13,371) |
|
(12,145) |
(6,838) |
| Exchange rate adjustments |
|
1,376 |
|
(1,235) |
1,303 |
| Cash flow change in working capital |
|
(11,995) |
|
(13,380) |
(5,535) |
| 1. Other non-current receivables and prepayments and Other non-current liabilities relating to 2023 and 2022 have been reclassified from Total other non-cash items to Cash flow changes in operating assets, net. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 4.8 Financial assets and liabilities |
|
4.8 Financial assets and liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
|
2023 |
| Financial assets by category |
|
|
|
|
|
| Other financial assets |
|
1,530 |
|
|
571 |
| Marketable securities |
|
10,653 |
|
|
15,838 |
Financial assets at fair value through
the income statement |
|
12,183 |
|
|
16,409 |
Derivative financial instruments (note 4.5) |
|
6,326 |
|
|
2,344 |
| Derivatives used as hedging instruments (assets) |
|
6,326 |
|
|
2,344 |
| Other financial assets |
|
747 |
|
|
682 |
| Trade receivables |
|
25,996 |
|
|
31,729 |
Other receivables and prepayments
(current and non-current) |
|
16,628 |
|
|
9,498 |
•less prepayments and VAT receivables |
|
(13,282) |
|
|
(8,312) |
Cash at bank (note 4.4) |
|
15,655 |
|
|
14,392 |
| Financial assets at amortised cost |
|
45,744 |
|
|
47,989 |
| Trade receivables eligible for factoring |
|
45,953 |
|
|
33,041 |
| Financial assets at fair value through other comprehensive income |
|
45,953 |
|
|
33,041 |
Total financial assets at the end
of the year by category |
|
110,206 |
|
|
99,783 |
|
|
|
|
|
|
| Financial liabilities by category |
|
|
|
|
|
Derivative financial instruments (note 4.5) |
|
7,531 |
|
|
1,272 |
Derivatives used as hedging
instruments (liability) |
|
7,531 |
|
|
1,272 |
Borrowings (non-current) (note 4.6)1 |
|
89,674 |
|
|
20,528 |
Borrowings (current) (note 4.6)1 |
|
13,113 |
|
|
6,478 |
| Trade payables |
|
28,846 |
|
|
25,606 |
| Other liabilities (non-current) |
|
23 |
|
|
189 |
| Other liabilities (current) |
|
37,993 |
|
|
28,705 |
•less VAT and duties payable |
|
(960) |
|
|
(600) |
Financial liabilities measured at
amortised cost |
|
168,689 |
|
|
80,906 |
Total financial liabilities at the end
of the year by category |
|
176,220 |
|
|
82,178 |
1. Refer to note 4.6 for a maturity analysis for non-current and current borrowings. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Fair value measurement hierarchy |
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
| Active market data (level 1) |
|
10,833 |
|
16,052 |
| Directly or indirectly observable market data (level 2) |
|
6,326 |
|
2,344 |
| Not based on observable market data (level 3) |
|
47,303 |
|
33,398 |
| Total financial assets at fair value |
|
64,462 |
|
51,794 |
| Active market data (level 1) |
|
— |
|
— |
| Directly or indirectly observable market data (level 2) |
|
7,531 |
|
1,272 |
| Not based on observable market data (level 3) |
|
— |
|
— |
| Total financial liabilities at fair value |
|
7,531 |
|
1,272 |
Financial assets and liabilities measured at fair value can be categorised using the fair value measurement hierarchy above. There were no transfers between the 'Active market data' and 'Directly or indirectly observable market data' categories during 2024 or 2023. The fair value of issued Eurobonds, which is disclosed in note 4.6, are based on 'Active market data'. There are no significant intangible assets or items of property, plant and equipment measured at fair value.
Cash at bank at 31 December 2024 includes DKK 867 million that is restricted (DKK 857 million in 2023). The restricted cash balance relates to subsidiaries in which availability of currency for remittance of funds is temporarily scarce.
ACCOUNTING POLICIES
Depending on purpose, Novo Nordisk classifies financial instruments into the following categories:
•Financial assets at fair value through the income statement
•Derivatives used as hedging instruments
•Financial assets at amortised cost
•Financial assets at fair value through other comprehensive income
•Financial liabilities at amortised cost
Recognition and measurement
Financial assets measured at fair value through the income statement consist of other financial assets, which comprise of equity investments, and marketable securities. These financial instruments are initially recognised at fair value. Net gains and losses arising from changes in the fair value of equity instruments and marketable securities are recognised in the income statement as financial income or expenses.
For a description of accounting policies on derivative financial instruments used as hedging instruments, refer to note 4.5.
Financial assets at amortised cost are cash at bank and non-derivative financial assets solely with payments of principal and interest. Novo Nordisk normally 'holds-to-collect' the financial assets to attain the contractual cash flows. If collection is expected within one year (or in the normal operating cycle of the business, if longer), they are classified as current assets. If not, they are presented as non-current assets. These are initially measured at fair value less transaction costs, except for trade receivables that are initially measured at the transaction price. Subsequently, they are measured at amortised cost using the effective interest method less impairment. For a description of accounting policies on trade receivables, refer to note 3.4.
Financial assets at fair value through other comprehensive income are trade receivables that are held to collect or to sell in factoring agreements.
Financial liabilities at amortised cost consist of borrowings (issued Eurobonds, bank overdrafts and lease liabilities), trade payables and other liabilities (primarily accruals for promotional and distribution activities, accrued employee-related costs and accrued payables related to assets under construction). These are initially recognised at the fair value less transaction costs. Subsequently, they are measured at amortised cost using the effective interest method. For initial recognition of lease liabilities refer to note 4.6.
Fair value measurement
If an active market exists, the fair value of a financial instrument is based on the
most recently observed market price at the end of the reporting period. If a financial instrument is quoted in a market that is not active, Novo Nordisk bases its valuation on the most recent transaction price. Adjustment is made for subsequent changes
in market conditions, for instance by including transactions in similar financial instruments assumed to be motivated by normal business considerations. The fair values of quoted investments are based on current bid prices at the end of the reporting period.
Financial assets for which no active market exists are carried at fair value based on a valuation methodology. The fair value of such financial instruments are determined
on the basis of quoted market prices of financial instruments traded in active markets. The fair value of standard and simple financial instruments, such as foreign exchange forward contracts, interest rate swaps, currency swaps and unlisted bonds, is measured according to generally accepted valuation techniques. Market-based input is used to measure the fair value.
The fair value of trade receivables held to collect or sell in factoring agreements is calculated based on the net invoice amount (invoice amount less charge-backs) less the fee payable to the factoring entity. The factoring fee is insignificant due to the short period between the time of sale to the factoring entity and the invoice due date and the rate applicable. Inputs into the estimate of US wholesaler charge-backs are described in note 2.1.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 4.9 Financial income and expenses |
|
4.9 Financial income and expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
2022 |
| Financial income |
|
|
|
|
|
Interest income1 |
|
1,838 |
|
1,069 |
239 |
| Foreign exchange gain (net) |
|
— |
|
308 |
— |
Financial gain from forward
contracts (net) |
|
4,358 |
|
1,344 |
— |
|
|
|
|
|
|
| Capital gain on marketable securities |
|
2 |
|
143 |
— |
| Result of associated companies |
|
— |
|
81 |
— |
| Total financial income |
|
6,198 |
|
2,945 |
239 |
| Financial expenses |
|
|
|
|
|
| Interest expenses on debts and borrowings |
|
1,640 |
|
542 |
378 |
| Foreign exchange loss (net) |
|
5,381 |
|
— |
2,885 |
Financial loss from forward
contracts (net) |
|
— |
|
— |
1,766 |
| Capital loss on investments |
|
19 |
|
106 |
124 |
| Capital loss on marketable securities |
|
— |
|
— |
463 |
| Result of associated companies |
|
17 |
|
— |
189 |
| Other financial expenses |
|
289 |
|
197 |
181 |
| Total financial expenses |
|
7,346 |
|
845 |
5,986 |
1. Interest income include DKK 399 million from marketable securities at fair value through the income statement (2023: DKK 370 million; 2022: DKK 78 million) while the remaining interest income is derived from financial assets at amortised cost. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Financial impact from forward contracts, specified |
| DKK million |
|
2024 |
|
2023 |
2022 |
| Income/(loss) transferred from other comprehensive income |
|
1,612 |
|
1,026 |
(1,740) |
| Realised fair value adjustment of transferred contracts |
|
(2,903) |
|
214 |
(3,772) |
Unrealised fair value adjustments of forward contracts2 |
|
4,558 |
|
(540) |
(1,202) |
| Realised foreign exchange gain/(loss) on forward contracts |
|
1,091 |
|
644 |
4,948 |
| Financial income/(expense) from forward contracts |
|
4,358 |
|
1,344 |
(1,766) |
| 2. Refer to note 4.5 for information on open fair value hedge contracts at 31 December. |
ACCOUNTING POLICIES
Management has chosen to classify the result of hedging activities as part of financial items in the income statement, except for foreign currency-risk cash flow hedges on highly probable non-financial asset purchases where the cumulative value adjustments are transferred directly from the cash flow hedge reserve to the initial cost of the asset when recognised.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 5.1 Share-based payment schemes |
|
Section 5
Other disclosures
5.1 Share-based payment schemes
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based payment expensed in the income statement |
|
| DKK million |
2024 |
|
2023 |
2022 |
|
| Restricted stock units to employees |
380 |
|
365 |
265 |
|
| Long-term share-based incentive programme (Management Board) |
314 |
|
304 |
250 |
|
| Long-term share-based incentive programme (Management group below Management Board) |
1,403 |
|
1,271 |
819 |
|
| Restricted stock units to individual employees |
192 |
|
209 |
205 |
|
| Share-based payment expensed in the income statement |
2,289 |
|
2,149 |
1,539 |
|
|
Restricted stock units to employees
In connection with Novo Nordisk's 100 year anniversary and in appreciation of the efforts of employees during recent years, as of 1 February 2023, all eligible employees in the company were offered 74 restricted stock units. Each restricted stock unit gives the holder the right to receive one Novo Nordisk B share free of charge in August 2026, subject to continued employment. The cost of the DKK 1,331 million programme is amortised over the vesting period.
Long-term share-based incentive programme
Management Board
The LTIPs commenced in 2022, 2023 and 2024 have a three-year performance
period, subject to continued employment, and a subsequent two-year holding
period. Targets are set at the beginning of the performance period and include determination of threshold, on-target level of performance and level of performance to achieve maximum allocation of shares. The maximum share allocation at grant cannot exceed 30 months' base salary for the CEO, 24 months' base salary for executive vice presidents and up to 15.6 months' base salary for senior vice presidents. Hence the LTIP is capped at a number of shares at the time of grant. For 2024 onward, the Board sets both financial and non-financial targets for a three-year period which are linked to three-year average growth in sales, operating profit and non-financial performance. All targets are aligned to Novo Nordisk's Strategic Aspirations 2025: Purpose & Sustainability, Innovation & Therapeutic Focus, Commercial Execution and Financials. Target achievement is assessed by the Board.
The grant date of the 2024-programme was 31 January 2024, and the share price
used for the determining the grant date fair value of the award (DKK 767) was the average share price for Novo Nordisk B shares on Nasdaq Copenhagen in the period 31 January 2024 to 13 February 2024, adjusted for the expected dividend. Based on
the split of participants at the grant date, 50% of the shares is allocated to members
of Executive Management and 50% to other members of the Management Board.
All restricted stock units and shares allocated to Management are settled by transfers of treasury shares at the time of vesting.
Management group below the Management Board
The Management group below the Management Board has a share-based
incentive programme with similar performance criteria as Management Board.
For 2024 onward, the Board sets both financial and non-financial targets for a
three-year period.
On 31 December 2024, a total of 13.3 million shares (18.9 million in 2023 and 21.4 million in 2022) were outstanding including all ongoing programmes.
ACCOUNTING POLICIES
Novo Nordisk operates equity-settled, share-based compensation plans.
The fair value of the employee services received in exchange for the grant of
shares is recognised as an expense and allocated over the vesting period.
The total amount to be expensed over the performance and vesting period is determined by reference to the fair value of the shares granted, excluding the
impact of any non-market vesting conditions. The fair value is fixed at the grant
date, and adjusted for expected dividends during the vesting period. Non-market vesting conditions are included in assumptions about the number of shares that
are expected to vest. At the end of each reporting period, Novo Nordisk revises its estimates of the number of shares expected to vest. Novo Nordisk recognises the impact of the revision of the original estimates, if any, in the income statement and in a corresponding adjustment to equity (change in proceeds) over the remaining vesting period. Adjustments relating to previous years are included in the income statement
in the year of adjustment.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 5.1 Share-based payment schemes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| General terms and conditions of 2022-2024 programmes |
|
|
|
|
|
|
|
Employees' 100 year anniversary programme |
|
|
Management Board |
|
Management group below
Management Board |
|
Individual employees |
| Year of launch |
2023 |
|
|
2024 |
|
2023 |
2022 |
|
2024 |
|
2023 |
2022 |
|
2024 |
|
2023 |
2022 |
|
Preliminary number of shares to be allocated1 (million) |
3.0 |
|
|
|
0.3 |
|
|
0.6 |
|
0.7 |
|
|
1.5 |
|
|
3.1 |
|
3.3 |
|
|
0.2 |
|
|
0.3 |
|
0.8 |
|
|
| Fair value per restricted stock unit at grant date (DKK) |
446 |
|
|
|
767 |
|
|
456 |
|
320 |
|
|
767 |
|
|
456 |
|
320 |
|
|
794 |
|
|
544 |
|
371 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Performance and vesting period |
2023 to 2026 |
|
|
2024 to 2026 |
|
2023 to 2025 |
2022 to 2024 |
|
2024 to 2026 |
|
2023 to 2025 |
2022 to 2024 |
|
2024 to 2027 |
|
2023 to 2026 |
2022 to 2025 |
|
| Allocation date |
Aug 2026 |
|
|
Feb 2027 |
|
Feb 2026 |
Feb 2025 |
|
Feb 2027 |
|
Feb 2026 |
Feb 2025 |
|
2027 |
|
2026 |
2025 |
|
| Amortisation period |
3.5 years |
|
|
3 years |
|
3 years |
3 years |
|
3 years |
|
3 years |
3 years |
|
3 years |
|
3 years |
3 years |
|
1. The number of shares to be allocated under the LTIPs to Management Board and management group below Management Board, respectively, may potentially be reduced or increased depending on whether Novo Nordisk's performance during the 3-year performance period is higher or lower compared to targets determined by the Board. The maximum number is capped. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 5.2 Commitments |
|
5.2 Commitments
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contractual obligations not recognised in the balance sheet |
| DKK million (undiscounted) |
Current |
Non-current |
|
Total |
|
| 2024 |
|
|
|
|
|
Leases1 |
288 |
|
3,893 |
|
|
4,181 |
|
| Research and development obligations |
12,101 |
|
23,215 |
|
|
35,316 |
|
Research and development – potential milestone payments2 |
2,076 |
|
32,507 |
|
|
34,583 |
|
Commercial product launch – potential milestone payments2 |
384 |
|
16,543 |
|
|
16,927 |
|
| Purchase obligations relating to investments in property, plant and equipment |
8,305 |
|
3,354 |
|
|
11,659 |
|
| Purchase obligations relating to contract manufacturers |
8,925 |
|
62,136 |
|
|
71,061 |
|
| Other purchase obligations |
10,531 |
|
8,463 |
|
|
18,994 |
|
Total obligations not recognised
in the balance sheet |
42,610 |
|
150,111 |
|
|
192,721 |
|
| 2023 |
|
|
|
|
|
Leases1 |
144 |
|
2,053 |
|
|
2,197 |
|
|
| Research and development obligations |
8,678 |
|
13,235 |
|
|
21,913 |
|
|
Research and development – potential milestone payments2 |
1,234 |
|
27,311 |
|
|
28,545 |
|
|
Commercial product launch – potential milestone payments2 |
— |
|
12,952 |
|
|
12,952 |
|
|
| Purchase obligations relating to investments in property, plant and equipment |
4,222 |
|
1,693 |
|
|
5,915 |
|
|
| Purchase obligations relating to contract manufacturers |
6,315 |
|
26,792 |
|
|
33,107 |
|
| Other purchase obligations |
7,151 |
|
5,888 |
|
|
13,039 |
|
|
Total obligations not recognised
in the balance sheet |
27,744 |
|
89,924 |
|
|
117,668 |
|
|
1. Predominantly relates to estimated variable property taxes, leases committed but not yet commenced and low value leases. 2. Potential milestone payments are associated with uncertainty because they are linked to successful achievements in research activities.
Contractual obligations
Research and development obligations include commitments relating to clinical trials, contingent payments related to achieving development milestones. Such amounts entail uncertainties in relation to the period in which payments are due because a proportion of the obligations are dependent on milestone achievements. Exercise fees and subsequent milestone payments under in-licensing option agreements are excluded, as Novo Nordisk is not contractually obligated to make such payments. Commercial product launch milestones include contingent payments solely related to achievement of a commercial product launch following regulatory approval. The increase in research and development obligation is driven by the general increase in business activities.
Commercial milestones, royalties and other payments based on a percentage of sales generated from sale of goods following marketing approval are excluded from the contractual commitments analysis because of their contingent nature, related to future sales.
Purchase obligations related to investments in property, plant and equipment primarily relates to production capacity expansion projects. Novo Nordisk expects
to fund these commitments with existing cash and cash flow from operations.
Purchase obligations related to contract manufacturers relate to commitments entered to secure future manufacturing capacity.
Other purchase obligations mainly consist of commitments related to promotional and media activities, professional and consulting activities and strategic sourcing contracts.
The contractual obligations not recognised in the balance sheet represent contractual payments and are not discounted and are not risk-adjusted.
Other guarantees
Other guarantees amount to DKK 2,380 million (DKK 1,878 million in 2023) and primarily relate to performance guarantees issued by Novo Nordisk.
5.3 Acquisition of businesses
|
|
|
|
|
|
|
|
|
|
|
|
| Fair value recognised at date of acquisition |
|
|
2024 |
| DKK million |
Fill-finish sites (Catalent) |
Other acquisitions |
Total |
|
|
|
|
| Know-how |
41,102 |
— |
41,102 |
| Intellectual property rights and other intangible assets |
311 |
52 |
363 |
| Property, plant and equipment |
24,839 |
608 |
25,447 |
|
|
|
|
|
|
|
|
|
|
|
|
| Deferred tax assets (liabilities), net |
992 |
(7) |
985 |
| Provisions |
(1,084) |
— |
(1,084) |
| Other net assets |
1,290 |
(2) |
1,288 |
| Net identifiable assets acquired |
67,450 |
651 |
68,101 |
| Goodwill |
15,293 |
30 |
15,323 |
|
|
|
|
|
|
|
|
|
|
|
|
| Purchase price |
82,743 |
681 |
83,424 |
| Settlement of pre-existing relationships |
(597) |
— |
(597) |
| Cash consideration transferred |
82,146 |
681 |
82,827 |
| Cash acquired |
(664) |
— |
(664) |
| Cash used for acquisition of businesses; net of cash acquired |
81,482 |
681 |
82,163 |
|
|
|
|
|
|
|
|
Business combinations in 2024
Three fill-finish sites (Catalent)
On 18 December 2024, Novo Nordisk acquired three fill-finish sites from Novo Holdings A/S in connection with a transaction where Novo Holdings A/S acquired Catalent, Inc. (“Catalent”), a global contract development and manufacturing organisation.
The three fill-finish sites are specialised in the sterile filling of drugs and located in Bloomington (Indiana, US), Anagni (Italy) and Brussels (Belgium) and employ around 3,500 people.
Novo Nordisk and Novo Holdings are related parties. Novo Nordisk’s Board of Directors has approved the acquisition, finding it to be in the best interest of Novo Nordisk and its shareholders.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 5.4 Related party transactions |
|
Strategic rationale
The acquisition of the fill-finish sites is aligned with Novo Nordisk’s strategy of reaching more people living with diabetes and obesity with current and future treatments. It is expected to enable an expansion of the manufacturing capacity
and provide future optionality and flexibility for Novo Nordisk’s existing supply network. The acquisition is expected to gradually increase Novo Nordisk's filling
and finish capacity.
Details of the acquisition
The total cash consideration transferred was USD 11,723 million (DKK 82,146 million including hedging effects).
The purchase price allocation for the acquisition is considered provisional since the transaction was closed only on 18 December 2024, leaving limited time to identify
and determine fair value of assets acquired and liabilities assumed.
Know-how is primarily comprised of the documented processes and systems for efficient and large-scale production of GLP-1 products as well as know-how to expand capacity in an efficient way. The fair value of both property, plant and equipment and know-how incorporate a significant value of accelerated access to capacity as a reflection of the current shortage of fill-finish capacity and high demand for GLP-1 products in the market.
Goodwill primarily reflects the value of a highly-skilled assembled workforce in place
at the three fill-finish sites and expected synergies from Novo Nordisk’s existing know-how and production capabilities. Goodwill is fully allocated to the Diabetes and Obesity care segment.
Acquisition related costs of DKK 978 million are included in other operating income and expenses and a gain on pre-existing relationships of DKK 597 million is included
in cost of goods sold.
Had the business combination taken place on 1 January 2024, Net profit would have likely included additional net costs of around DKK 9 billion reflecting significant integration costs, amortisation of fair value adjustments made in purchase price allocation and interest expenses from planned borrowings incurred to finance the transaction. Net sales would have remained largely unchanged as revenues from existing manufacturing and development contracts are included in Other operating income and expenses as these are not part of the main revenue-generating activities of Novo Nordisk.
Other acquisitions
Other acquisitions of businesses in 2024 comprise the acquisition of a production
site in Ireland for a total purchase price of DKK 681 million.
KEY ACCOUNTING ESTIMATES IN DETERMINING THE FAIR VALUE OF
ASSETS ACQUIRED IN A BUSINESS COMBINATION AND JUDGEMENT OF
WHETHER INTANGIBLE ASSETS ACQUIRED IN A BUSINESS COMBINATION
ARE SEPARATELY IDENTIFIABLE
Management makes judgements when determining whether intangible assets,
such as know-how related to large-scale production of GLP-1 products as well as
know-how to expand capacity in an efficient way, are separately identifiable. This involves assessing if the know-how meets the separability criterion, which means it can be separated from the acquiree and sold, transferred, licensed, rented, or exchanged independently.
The application of the acquisition method of accounting involves the use of significant estimates because the identifiable net assets of the acquiree are recognised at their fair value for which observable market prices are typically not available. This is particularly relevant for assets which require use of valuation techniques typically based on estimates of present value of future uncertain cash flows.
The fair value is based on assumptions made by market participants, which in this business combination is assessed to be a company with similar needs and capacity
to acquire assets of the same nature and size as those of the acquired business.
The valuation of know-how identified in the acquisition is based on the multi-period excess earnings method, which is used to value unique assets that generate earnings. The economic benefit of the know-how is comprised by net cash flows attributable to the asset which also includes the benefit of accelerated access to production capacity compared to a greenfield construction scenario without the know-how required for commercial production at scale. The net present value of future estimated cash flows is based on projections of sales volumes and prices, valuation period and royalty rates.
The valuation of property, plant and equipment identified in the acquisition of the three fill-finish sites is mainly based on the depreciated replacement cost method in combination with the present value of accelerated access to production facilities. The depreciated replacement cost method reflects adjustments for physical deterioration as well as functional and economic obsolescence. Land has been valued using the market approach based on comparable transactions.
ACCOUNTING POLICIES
The acquisition method of accounting is used to account for all business combinations.
The purchase price for a business comprises the fair values of the assets transferred, liabilities incurred to the former owners including warrant holders of the acquired business and the fair value of any asset or liability resulting from a contingent consideration arrangement. Any amount of the purchase price which effectively comprises a settlement of a pre-existing relationship is not part of the exchange for the acquiree and is therefore not included in the consideration for the purpose of applying the acquisition method. Settlements of pre-existing relationships are
accounted for as separate transactions in accordance with the relevant IFRS Accounting Standards.
Identifiable assets and liabilities and contingent liabilities assumed are measured
at fair value at the date of acquisition by applying relevant valuation methods. Acquisition-related costs are expensed as incurred. Goodwill is recognised at the excess of purchase price over the fair value of net identifiable assets acquired and liabilities assumed.
5.4 Related party transactions
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Material transactions with related parties |
| DKK million |
2024 |
|
2023 |
2022 |
| Novo Holdings A/S |
|
|
|
|
| Purchase of Novo Nordisk B shares |
10,164 |
|
8,775 |
6,984 |
| Acquisition of fill-finish sites (note 5.3) |
82,146 |
|
— |
— |
| Dividend payment to Novo Holdings A/S |
12,502 |
|
9,028 |
7,207 |
| Services provided by Novo Nordisk |
(33) |
|
(17) |
(24) |
|
|
|
|
|
Novonesis Group |
|
|
|
|
| Services provided by Novo Nordisk |
(48) |
|
(48) |
(78) |
| Services provided by Novonesis |
117 |
|
112 |
92 |
| Altasciences Group |
|
|
|
|
|
|
|
|
|
| Services provided by Altasciences |
146 |
|
229 |
70 |
| Other subsidiaries of Novo Holding A/S |
|
|
|
|
| Services provided to Novo Nordisk |
93 |
|
— |
— |
| NNIT Group |
|
|
|
|
| Services provided by NNIT |
257 |
|
436 |
660 |
Novo Nordisk A/S is controlled by Novo Holdings A/S (incorporated in Denmark), which owns 28.1% of the share capital in Novo Nordisk A/S, representing 77.3% of the total number of votes. The remaining shares are widely held. The ultimate parent of the Group is the Novo Nordisk Foundation (incorporated in Denmark). Both entities are considered related parties.
Novonesis Group, Altasciences Company Inc., and other subsidiaries of Novo Holdings A/S are considered related parties to Novo Nordisk A/S. As an associated company of Novo Nordisk A/S, NNIT Group is also considered related party.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 5.5 Fees to statutory auditors |
|
In 2024, Novo Nordisk A/S acquired 12.6 million B shares, worth DKK 10,164 million, from Novo Holdings A/S as part of the DKK 20,000 million share repurchase programme. The transaction price for each transaction was calculated as the average market price in the open window period following the announcements of the financial results for the first and third quarters in 2024.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Remuneration to Executive Management and Board of Directors |
| DKK million |
|
2024 |
|
2023 |
2022 |
| Salary and short-term incentive |
|
180 |
|
173 |
141 |
| Pension |
|
18 |
|
17 |
13 |
Benefits1 |
|
55 |
|
19 |
9 |
Long-term incentive2 |
|
112 |
|
121 |
97 |
|
|
|
|
|
|
Executive Management in total3 |
|
365 |
|
330 |
260 |
Fees to Board of Directors4 |
|
23 |
|
22 |
20 |
| Total |
|
388 |
|
352 |
280 |
|
1. In 2024, an amount of DKK 45.4 million relates to recruitment arrangements as well as
a conditional amount payable at the end of employment. 2. Refer to note 5.1 for further
information on share-based payment schemes. 3. Total remuneration for persons registered as members of Executive Management with the Danish Business Authority amounts to DKK 88 million (DKK 195 million in 2023 and DKK 175 million in 2022). 4. All members of the Board of Directors
are registered with the Danish Business Authority.
|
There were no transactions with the Board of Directors or Executive Management besides remuneration.
There were no material unsettled balances with related parties at the end of the year.
5.5 Fees to statutory auditors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
2022 |
Statutory audit1 |
|
35 |
|
30 |
38 |
| Audit-related services |
|
5 |
|
3 |
2 |
| Tax advisory services |
|
9 |
|
8 |
3 |
| Other services |
|
13 |
|
18 |
12 |
| Total fees to statutory auditors |
|
62 |
|
59 |
55 |
1. Statutory audit fees in 2024 include DKK 5 million of additional fees mainly related to business acquisitions. Statutory audit fees in 2022 include DKK 9 million of additional fee related to 2021. |
Fees for services other than statutory audit of the financial statements amount to DKK 27 million (DKK 29 million in 2023 and DKK 17 million in 2022).
In 2024, Deloitte Statsautoriseret Revisionspartnerselskab provided other services than statutory audit in the amount of DKK 6 million (DKK 18 million in 2023 and
DKK 12 million in 2022) which relate to tax services relating to acquisitions, tax compliance, financial due diligence, management consulting, educational training and other assurance assessments and opinions.
5.6 General accounting policies
Principles of consolidation
The Consolidated financial statements incorporate the financial statements of the parent company Novo Nordisk A/S and entities controlled by Novo Nordisk A/S. Control exists when Novo Nordisk has effective power over the entity and has the right to variable returns from the entity. The results of subsidiaries acquired or disposed of during the year are included in the consolidated income statement from the effective date of acquisition and up to the effective date of disposal.
Functional and presentation currency
Items included in the financial statements of Novo Nordisk's entities are measured using the currency of the primary economic environment in which the entity operates (functional currency). The Consolidated financial statements are presented in Danish kroner (DKK), which is also the functional and presentation currency of the parent company.
Translation of transactions and balances
Foreign currency transactions are translated into the functional currency using the prevailing exchange rates at the transaction dates. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation at year-end exchange rates of monetary assets and liabilities are recognised in the income statement. Foreign currency differences arising from the translation of effective qualifying cash flow hedges are recognised in other comprehensive income.
Translation of Group companies
Financial statements of foreign subsidiaries are translated into DKK at the exchange rates prevailing at the end of the reporting period for balance sheet items, and at average exchange rates for income statement items. All effects of exchange rate adjustments are recognised in other comprehensive income.
Cash flow statement
The Cash flow statement is presented in accordance with the indirect method commencing with net profit for the year.
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated financial statements / Notes to the Consolidated financial statement / 5.7 Companies in the Novo Nordisk Group |
|
5.7 Companies in the Novo Nordisk Group
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Activity: |
• |
Sales and marketing |
• |
Production |
|
• |
Research and development |
• |
Services/investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Company and country |
|
Activity |
| Parent company |
|
|
|
|
|
| Novo Nordisk A/S, Denmark |
|
• |
• |
• |
• |
|
|
|
|
|
|
|
| Subsidiaries by geographical area |
|
|
|
|
|
|
|
|
|
|
|
|
| Company and country |
Percentage of shares owned |
Activity |
| North America Operations |
| Inversago Pharma Inc., Canada |
100 |
|
|
• |
|
| Novo Nordisk Canada Inc., Canada |
100 |
• |
|
|
|
| Novo Nordisk North America Operations A/S, Denmark |
100 |
|
|
|
• |
| Novo Nordisk Inc., US |
100 |
• |
|
|
|
| Novo Nordisk Pharmaceutical Industries LP, US |
100 |
|
• |
|
|
| Novo Nordisk Pharmatech US, Inc., US |
100 |
• |
|
|
|
| Novo Nordisk Pharma, Inc., US |
100 |
• |
|
|
|
| NN Corporate Development US, Inc., US |
100 |
|
|
• |
|
| NN Research & Development US, Inc., US |
100 |
|
|
• |
|
| Novo Nordisk US Bio Production, Inc., US |
100 |
|
• |
|
|
| Novo Nordisk US Holdings Inc., US |
100 |
|
|
|
• |
| Dicerna Pharmaceuticals, Inc., US |
100 |
|
|
• |
|
| Emisphere Technologies, Inc., US |
100 |
|
|
|
• |
| Forma Therapeutics, Inc., US |
100 |
|
|
• |
|
| Catalent Indiana LLC, US |
100 |
|
• |
|
|
|
|
|
|
|
|
|
| Region International Operations |
| Novo Nordisk Pharmaceuticals A/S, Denmark |
100 |
|
|
|
• |
| Novo Nordisk Pharma Operations A/S, Denmark |
100 |
• |
|
|
• |
| Novo Nordisk Region AAMEO and LATAM A/S, Denmark |
100 |
|
|
|
• |
| Novo Nordisk Region Europe A/S, Denmark |
100 |
|
|
|
• |
| Novo Nordisk Region Japan & Korea A/S, Denmark |
100 |
|
|
|
• |
|
|
|
|
|
|
|
| Region EMEA |
| Aldaph SpA, Algeria |
100 |
• |
• |
|
|
| Novo Nordisk Pharma GmbH, Austria |
100 |
• |
|
|
|
| S.A. Novo Nordisk Pharma N.V., Belgium |
100 |
• |
|
|
|
| Catalent Belgium S.A, Belgium |
100 |
|
• |
|
|
| Novo Nordisk Pharma d.o.o., Bosnia and Herzegovina |
100 |
• |
|
|
|
| Novo Nordisk Pharma EAD, Bulgaria |
100 |
• |
|
|
|
| Novo Nordisk Hrvatska d.o.o., Croatia |
100 |
• |
|
|
|
| Novo Nordisk s.r.o., Czech Republic |
100 |
• |
|
|
|
| Novo Nordisk Production Czech s.r.o, Czech Republic |
100 |
|
• |
|
|
| Novo Nordisk Denmark A/S, Denmark |
100 |
• |
|
|
|
| Novo Nordisk Pharmatech A/S, Denmark |
100 |
• |
• |
|
|
| Novo Nordisk Egypt LLC, Egypt |
100 |
• |
|
|
|
| Novo Nordisk Egypt Pharmaceuticals Ltd., Egypt |
100 |
• |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Company and country |
Percentage of shares owned |
Activity |
| Novo Nordisk Estonia OÜ, Estonia |
100 |
• |
|
|
|
| Novo Nordisk Farma OY, Finland |
100 |
• |
|
|
|
| Biocorp Production S.A., France |
100 |
|
• |
• |
|
| Novo Nordisk, France |
100 |
• |
|
|
|
| Novo Nordisk Production SAS, France |
100 |
|
• |
|
|
| Novo Nordisk Pharma GmbH, Germany |
100 |
• |
|
|
|
| Cardior Pharmaceuticals GmbH, Germany |
100 |
|
|
• |
|
| Novo Nordisk Hellas Epe., Greece |
100 |
• |
|
|
|
| Novo Nordisk Hungária Kft., Hungary |
100 |
• |
|
|
|
| Novo Nordisk Limited, Ireland |
100 |
• |
|
|
|
| Novo Nordisk Production Ireland Ltd., Ireland |
100 |
|
• |
|
|
| Novo Nordisk Ltd, Israel |
100 |
• |
|
|
|
| Novo Nordisk S.P.A., Italy |
100 |
• |
|
|
|
| Catalent Anagni S.R.L, Italy |
100 |
|
• |
|
|
| Novo Nordisk Kazakhstan LLP, Kazakhstan |
100 |
• |
|
|
|
| Novo Nordisk Kenya Ltd., Kenya |
100 |
• |
|
|
|
| Novo Nordisk Latvia SIA, Latvia |
100 |
• |
|
|
|
| Novo Nordisk Pharma SARL, Lebanon |
100 |
• |
|
|
|
| UAB Novo Nordisk Pharma, Lithuania |
100 |
• |
|
|
|
| Novo Nordisk Farma dooel, North Macedonia |
100 |
• |
|
|
|
| Novo Nordisk Pharma SAS, Morocco |
100 |
• |
|
|
|
| Novo Nordisk B.V., Netherlands |
100 |
• |
|
|
|
| Novo Nordisk Finance (Netherlands) B.V., Netherlands |
100 |
|
|
|
• |
| Novo Nordisk Pharma Limited, Nigeria |
100 |
• |
|
|
|
| Novo Nordisk Norway AS, Norway |
100 |
• |
|
|
|
| Novo Nordisk Pharmaceutical Services Sp. z.o.o., Poland |
100 |
• |
|
|
|
| Novo Nordisk Pharma Sp.z.o.o., Poland |
100 |
• |
|
|
|
| Novo Nordisk Portugal, Lda., Portugal |
100 |
• |
|
|
|
| Novo Nordisk Farma S.R.L., Romania |
100 |
• |
|
|
|
| Novo Nordisk Limited Liability Company, Russia |
100 |
• |
• |
|
|
| Novo Nordisk Production Support LLC, Russia |
100 |
|
|
|
• |
| Novo Nordisk Saudi for Trading, Saudi Arabia |
100 |
• |
|
|
|
| Novo Nordisk Pharma d.o.o. Belgrade (Serbia), Serbia |
100 |
• |
|
|
|
| Novo Nordisk Slovakia s.r.o., Slovakia |
100 |
• |
|
|
|
| Novo Nordisk, d.o.o., Slovenia |
100 |
• |
|
|
|
| Novo Nordisk (Pty) Limited, South Africa |
100 |
• |
|
|
|
| Novo Nordisk Pharma S.A., Spain |
100 |
• |
|
|
|
| Novo Nordisk Scandinavia AB, Sweden |
100 |
• |
|
|
|
| Novo Nordisk Health Care AG, Switzerland |
100 |
• |
|
• |
• |
| Novo Nordisk Pharma AG, Switzerland |
100 |
• |
|
|
|
| Novo Nordisk Tunisie SARL, Tunisia |
100 |
• |
|
|
|
| Novo Nordisk Saglik Ürünleri Tic. Ltd. Sti., Turkey |
100 |
• |
|
|
|
| Novo Nordisk Ukraine, LLC, Ukraine |
100 |
• |
|
|
|
| Novo Nordisk Pharma Gulf FZE, United Arab Emirates |
100 |
• |
|
|
|
| Novo Nordisk Limited, UK |
100 |
• |
|
|
|
| Novo Nordisk Research Centre Oxford Limited, UK |
100 |
|
|
• |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Company and country |
Percentage of shares owned |
Activity |
| Region China |
| Novo Nordisk (China) Pharmaceuticals Co. Ltd., China |
100 |
• |
• |
|
|
| Novo Nordisk (Shanghai) Pharma Trading Co., Ltd., China |
100 |
• |
|
|
|
| Novo Nordisk Region China A/S, Denmark |
100 |
|
|
|
• |
| Novo Nordisk Hong Kong Limited, Hong Kong |
100 |
• |
|
|
|
| Novo Nordisk Pharma (Taiwan) Ltd., Taiwan |
100 |
• |
|
|
|
| Beijing Novo Nordisk Pharmaceuticals Science & Technology Co., Ltd., China |
100 |
|
|
• |
|
|
|
|
|
|
|
|
| Region Rest of World |
| Novo Nordisk Pharma Argentina S.A., Argentina |
100 |
• |
|
|
|
| Novo Nordisk Pharmaceuticals Pty. Ltd., Australia |
100 |
• |
|
|
|
| Novo Nordisk Pharma (Private) Limited, Bangladesh |
100 |
• |
|
|
|
| Novo Nordisk Produção Farmacêutica do Brasil Ltda., Brazil |
100 |
|
• |
|
|
| Novo Nordisk Farmacêutica do Brasil Ltda., Brazil |
100 |
• |
|
|
|
| Novo Nordisk Farmacéutica Limitada, Chile |
100 |
• |
|
|
|
| Novo Nordisk Colombia SAS, Colombia |
100 |
• |
|
|
|
| Novo Nordisk India Private Limited, India |
100 |
• |
|
|
|
| Novo Nordisk Service Centre (India) Pvt. Ltd., India |
100 |
|
|
|
• |
| PT. Novo Nordisk Indonesia, Indonesia |
100 |
• |
|
|
|
| Novo Nordisk Pars Co. (PJS), Iran |
100 |
• |
• |
|
|
| Novo Nordisk Pharma Ltd., Japan |
100 |
• |
• |
|
|
| Novo Nordisk Pharma (Malaysia) Sdn Bhd, Malaysia |
100 |
• |
|
|
|
| Novo Nordisk Pharma Operations Sdn Bhd, Malaysia |
100 |
• |
|
|
|
| Novo Nordisk Mexico S.A. de C.V., Mexico |
100 |
• |
|
|
|
| Novo Nordisk Service Centre Mexico, Sociedad Anonim, Mexico |
100 |
|
|
|
• |
| Novo Nordisk Pharmaceuticals Ltd., New Zealand |
100 |
• |
|
|
|
| Novo Nordisk Pharma (Private) Limited, Pakistan |
100 |
• |
|
|
|
| Novo Nordisk Panama S.A., Panama |
100 |
• |
|
|
|
| Novo Nordisk Peru S.A.C., Peru |
100 |
• |
|
|
|
| Novo Nordisk Pharmaceuticals (Philippines) Inc., Philippines |
100 |
• |
|
|
|
| Novo Nordisk Pharma (Singapore) Pte Ltd., Singapore |
100 |
• |
|
|
|
| Novo Nordisk Pharma Korea Ltd., South Korea |
100 |
• |
|
|
|
| Novo Nordisk Lanka (PVT) Ltd, Sri Lanka |
100 |
• |
|
|
|
| Novo Nordisk Pharma (Thailand) Ltd., Thailand |
100 |
• |
|
|
|
| Novo Nordisk Vietnam Ltd., Vietnam |
100 |
• |
|
|
|
|
|
|
|
|
|
|
| Other subsidiaries and associated companies |
|
|
|
|
|
| NNE A/S, Denmark |
100 |
|
|
|
• |
| NNIT A/S, Denmark |
18 |
|
|
|
|
• |
| CS Solar Fund XIV, LLC, US |
99 |
|
|
|
|
• |
Companies without significant activities are not included in the list.
NNE A/S subsidiaries are not included in the list.
|
|
|
|
|
|
|
|
|
|
|
|
|
Part of the Annual review – not audited |
|
Financial definitions and ratios
(part of the Annual review – not audited)
Financial ratios have been calculated in accordance with the guidelines from the Danish Society of Financial Analysts, and supplemented by certain key ratios for
Novo Nordisk. Financial ratios are described below and in the section 'Non-IFRS financial measures'.
FINANCIAL DEFINITIONS
ADR
An American Depository Receipt (ADR) represents ownership of shares in a non-US company and trades in US financial markets.
EBITDA
EBITDA is defined as ’net profit’, adjusted for 'income taxes', 'financial items', 'depreciation and amortisation' and 'impairment losses and reversals'.
Number of shares outstanding
The total number of shares, excluding the holding of treasury shares.
Shares
The share capital of Novo Nordisk comprises of A-shares and B-shares, with B-shares listed on Nasdaq Copenhagen in trading units of nominal value DKK 0.10 and ADRs, that equals B-shares of nominal value DKK 0.10, being listed on New York Stock Exchange (NYSE). Key ratios per share, including number of outstanding shares, are aligned with trading units of nominal value DKK 0.10.
Working capital
Working capital is the net of operating assets and operating liabilities.
FINANCIAL RATIOS
Basic earnings per share (EPS)
Net profit divided by the average number of shares outstanding.
Diluted earnings per share
Net profit divided by average number of shares outstanding, including the dilutive effect of the outstanding restricted stock units.
Dividend payout ratio
Total dividends for the year as a percentage of net profit. Total dividends for the year comprise of interim dividend paid during the year and proposed ordinary dividend for the year.
Effective tax rate
Income taxes as a percentage of profit before income taxes.
Gross margin
Gross profit as a percentage of net sales.
Operating margin
Operating profit as a percentage of net sales.
Net profit margin
Net profit as a percentage of net sales.
|
|
|
|
|
|
|
|
|
|
|
|
|
Part of the Annual review – not audited |
|
Non-IFRS financial measures
(part of the Annual review – not audited)
In the Annual review, Novo Nordisk discloses certain financial measures of the Group’s financial performance, financial position and cash flows that reflect adjustments to the most directly comparable measures calculated and presented in accordance with IFRS Accounting Standards. These non-IFRS financial measures may not be defined and calculated by other companies in the same manner, and may therefore not be comparable.
The non-IFRS financial measures presented in the Annual review are:
•Net sales and operating profit in constant exchange rates (CER)
•'Net profit’, adjusted for 'income taxes', 'financial items', 'depreciation and amortisation' and 'impairment losses and reversals' (EBITDA) and EBITDA at constant exchange rates
•Return on invested capital (ROIC)
•Free cash flow
•Cash to earnings
IFRS refers to an IFRS financial measure.
Net sales and operating profit growth in constant exchange rates
'Growth in constant exchange rates' means that the effect of changes in exchange rates is excluded. It is defined as sales/operating profit for the period measured at the average exchange rates for the same period of the prior year, compared with net sales/operating profit for the same period of the prior year. Price adjustments within hyperinflation countries as defined in IAS 29 'Financial reporting in hyperinflation economies' are excluded from the calculation to avoid growth in constant exchange rates being artificially inflated. Growth in constant exchange rates is considered to be relevant information for investors in order to understand the underlying development in sales and operating profit by adjusting for the impact of currency fluctuations.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net sales in constant exchange rates |
| DKK million |
|
2024 |
|
2023 |
2022 |
Net sales IFRS |
|
290,403 |
|
232,261 |
176,954 |
| Effect of exchange rate |
|
1,575 |
|
7,658 |
(13,024) |
| Net sales in constant exchange rates |
291,978 |
|
239,919 |
163,930 |
| Net sales previous year |
|
232,261 |
|
176,954 |
140,800 |
| % increase/(decrease) in reported currencies |
|
25.0 |
% |
|
31.3 |
% |
25.7 |
% |
| % increase/(decrease) in constant exchange rates |
|
25.7 |
% |
|
35.6 |
% |
16.4 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating profit in constant exchange rates |
| DKK million |
|
2024 |
|
2023 |
2022 |
Operating profit IFRS |
|
128,339 |
|
102,574 |
74,809 |
| Effect of exchange rate |
|
1,096 |
|
4,898 |
(7,578) |
Operating profit in constant
exchange rates |
|
129,435 |
|
107,472 |
67,231 |
| Operating profit previous year |
|
102,574 |
|
74,809 |
58,644 |
| % increase/(decrease) in reported currencies |
|
25.1 |
% |
|
37.1 |
% |
27.6 |
% |
| % increase/(decrease) in constant exchange rates |
|
26.2 |
% |
|
43.7 |
% |
14.6 |
% |
EBITDA and EBITDA at constant exchange rates
Novo Nordisk defines EBITDA as ’net profit’ adjusted for 'income taxes', 'financial items', 'depreciation and amortisation' and 'impairment losses and reversals'. Management believes EBITDA is a useful measure as it helps analyse operating
results from core business operations without including the effects of capital structure, tax rates, depreciation, amortisation and impairment losses and reversals.
"EBITDA at CER" means that the effect of changes in exchange rates is excluded by measuring EBITDA (as defined above) at the average exchange rates for the same period prior year. EBITDA at CER is considered to be useful information for investors
in order to understand the underlying development by adjusting for the impact of currency fluctuations.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| EBITDA and EBITDA at constant exchange rates |
| DKK million |
|
2024 |
|
2023 |
2022 |
Net profit IFRS |
|
100,988 |
|
83,683 |
55,525 |
Income taxes IFRS |
|
26,203 |
|
20,991 |
13,537 |
Financial income IFRS |
|
(6,198) |
|
(2,945) |
(239) |
Financial expenses IFRS |
|
7,346 |
|
845 |
5,986 |
Operating profit (EBIT) IFRS |
|
128,339 |
|
102,574 |
74,809 |
| Depreciation and amortisations |
|
8,545 |
|
7,289 |
6,553 |
| Impairment losses and reversals |
|
10,562 |
|
2,124 |
809 |
| EBITDA |
|
147,446 |
|
111,987 |
82,171 |
| Effect of exchange rate |
|
1,146 |
|
5,043 |
(7,841) |
| EBITDA in constant exchange rates |
|
148,592 |
|
117,030 |
74,330 |
| EBITDA previous year |
|
111,987 |
|
82,171 |
64,669 |
| % increase/(decrease) in reported currencies |
|
31.7 |
% |
|
36.3 |
% |
27.1 |
% |
| % increase/(decrease) in constant exchange rates |
|
32.7 |
% |
|
42.4 |
% |
14.9 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
Part of the Annual review – not audited |
|
Return on invested capital (ROIC)
ROIC is defined as 'operating profit after tax' (using the effective tax rate) as a percentage of average inventories, receivables, property, plant and equipment, intangible assets and deferred tax assets, less non-interest-bearing liabilities including provisions and deferred tax liabilities (where the average is the sum of the above assets and liabilities at the beginning of the year and at year-end divided by two).
Management believes ROIC is a useful measure in providing investors and Management with information regarding the Group's performance. The calculation
of this financial target is a widely accepted measure of earnings efficiency in relation to total capital employed.
The following tables show the reconciliation of ROIC with operating profit/equity in
%, the most directly comparable IFRS financial measure:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating profit/equity in % |
| DKK million |
|
2024 |
|
2023 |
2022 |
Operating profit IFRS |
|
128,339 |
|
102,574 |
74,809 |
/ Equity IFRS |
|
143,486 |
|
106,561 |
83,486 |
| Operating profit/equity in % |
|
89.4 |
% |
|
96.3 |
% |
89.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ROIC |
| DKK million |
|
2024 |
|
2023 |
2022 |
| Operating profit after tax |
|
101,901 |
|
81,957 |
60,146 |
| / Average net operating assets |
|
159,548 |
|
92,566 |
81,744 |
| ROIC in % |
|
63.9 |
% |
|
88.5 |
% |
73.6 |
% |
ROIC numerator
Reconciliation of operating profit to operating profit after tax
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
2022 |
Operating profit IFRS |
|
128,339 |
|
102,574 |
74,809 |
| Tax on operating profit (using effective tax rate) |
|
(26,438) |
|
(20,617) |
(14,663) |
| Operating profit after tax |
|
101,901 |
|
81,957 |
60,146 |
ROIC denominator
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
2022 |
| Intangible assets |
|
111,090 |
|
60,406 |
50,939 |
| Property, plant and equipment |
|
162,488 |
|
90,961 |
66,671 |
| Deferred income tax assets |
|
24,627 |
|
20,380 |
13,904 |
| Other receivables and prepayments (non-current) |
|
4,016 |
|
1,430 |
206 |
| Inventories |
|
40,849 |
|
31,811 |
24,388 |
| Trade receivables |
|
71,949 |
|
64,770 |
50,560 |
| Tax receivables |
|
2,853 |
|
2,423 |
940 |
| Other receivables and prepayments (current) |
|
12,612 |
|
8,068 |
6,005 |
| Deferred income tax liabilities |
|
(5,426) |
|
(10,162) |
(7,061) |
| Retirement benefit obligations |
|
(903) |
|
(742) |
(762) |
| Other liabilities (non-current) |
|
(23) |
|
(189) |
(100) |
| Provisions (non-current) |
|
(8,755) |
|
(6,649) |
(4,590) |
| Trade payables |
|
(28,846) |
|
(25,606) |
(15,587) |
| Tax payables |
|
(9,716) |
|
(7,116) |
(7,091) |
| Other liabilities (current) |
|
(37,993) |
|
(28,705) |
(23,606) |
| Provisions (current) |
|
(120,329) |
|
(100,478) |
(70,287) |
| Net operating assets |
|
218,493 |
|
100,602 |
84,529 |
| Average net operating assets |
|
159,548 |
|
92,566 |
81,744 |
Reconciliation of net operating assets to equity IFRS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
2022 |
Equity IFRS |
|
143,486 |
|
106,561 |
83,486 |
| Investment in associated companies |
|
(400) |
|
(410) |
(327) |
| Other financial assets |
|
(2,277) |
|
(1,253) |
(1,016) |
| Marketable securities |
|
(10,653) |
|
(15,838) |
(10,921) |
| Derivative financial instruments |
|
(6,326) |
|
(2,344) |
(2,727) |
| Cash at bank |
|
(15,655) |
|
(14,392) |
(12,653) |
| Borrowings – non-current |
|
89,674 |
|
20,528 |
24,318 |
| Borrowings – current |
|
13,113 |
|
6,478 |
1,466 |
| Derivative financial instruments |
|
7,531 |
|
1,272 |
2,903 |
| Net operating assets |
|
218,493 |
|
100,602 |
84,529 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Part of the Annual review – not audited |
|
Free cash flow
Free cash flow is a measure of the amount of cash generated in the period which is available for the Board to allocate between Novo Nordisk's capital providers, through measures such as dividends, share repurchases and repayment of debt (excluding lease liability repayments) or for retaining within the business to fund future growth.
The following table shows a reconciliation of free cash flow with net cash generated from operating activities, the most directly comparable IFRS financial measure:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Free cash flow |
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
2022 |
Net cash generated from operating activities IFRS |
|
120,968 |
|
108,908 |
78,887 |
Net cash used in investing activities IFRS |
|
(128,895) |
|
(43,892) |
(24,918) |
Net purchase/(net sale) of marketable securities IFRS |
|
(5,363) |
|
4,758 |
2,921 |
Addition on marketable securities through acquisition of business IFRS |
|
— |
|
— |
1,470 |
Repayment on lease liabilities IFRS |
|
(1,417) |
|
(1,448) |
(998) |
| Free cash flow |
|
(14,707) |
|
68,326 |
57,362 |
Cash to earnings
Cash to earnings is defined as 'free cash flow as a percentage of net profit'.
Management believes that cash to earnings is an important performance metric because it measures the Group’s ability to turn earnings into cash. Since Management wants this measure to capture the ability of the Group’s operations to generate cash, free cash flow is used as the numerator instead of net cash flow.
The following table shows the reconciliation of cash to earnings to cash flow
from operating activities/net profit in %, the most directly comparable IFRS
financial measure:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash flow from operating activities/net profit in % |
|
| DKK million |
|
2024 |
|
2023 |
2022 |
Net cash generated from operating activities IFRS |
|
120,968 |
|
108,908 |
78,887 |
/ Net profit IFRS |
|
100,988 |
|
83,683 |
55,525 |
Cash flow from operating
activities/net profit in % |
|
119.8 |
% |
|
130.1 |
% |
142.1 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash to earnings |
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
2022 |
| Free cash flow |
|
(14,707) |
|
68,326 |
57,362 |
/ Net profit IFRS |
|
100,988 |
|
83,683 |
55,525 |
| Cash to earnings |
|
(14.6%) |
|
81.6 |
% |
103.3 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
Statements and Auditor´s Report / Statement by the Board of Directors and Executive Management |
|
Statement by the Board of Directors and Executive Management
The Board of Directors and Executive Management have today considered and approved the Annual Report of Novo Nordisk A/S
for the financial year 1 January 2024 – 31 December 2024.
The Consolidated financial statements are prepared in accordance
with IFRS Accounting Standards as adopted by the EU and disclosure requirements for listed companies in Denmark. The parent financial statements are presented in accordance with the Danish Financial Statements Act. Furthermore, the Annual Report is prepared in accordance with disclosure requirements for listed companies.
In our opinion, the Consolidated financial statements and the parent financial statements give a true and fair view of the Group’s and the Parent's financial position at 31 December 2024 as well as of the
results of their operations and the Group's cash flows for the
financial year 1 January 2024 – 31 December 2024.
In our opinion, the Management report is prepared in accordance with relevant laws and regulations and contains a fair review of the development of the Group's and the Parent’s business and financial matters, the results for the year and of the Parent’s financial position and the financial position as a whole of the entities included in the Consolidated financial statements, together with a description of the principal risks and uncertainties that the Group and the Parent face.
The Sustainability statement is prepared in accordance with the European Sustainability Reporting Standards (ESRS) as required by
the Danish Financial Statements Act, as well as article 8 in the
EU Taxonomy regulation.
Furthermore, in our opinion, the Annual Report of Novo Nordisk A/S
for the financial year 1 January 2024 – 31 December 2024, with the file name NOVO-2024-12-31-0-en.zip, is prepared, in all material respects, in accordance with the ESEF Regulation.
We recommend the Annual Report for adoption at the Annual
General Meeting.
Bagsværd, 5 February 2025
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Registered Executive Management |
Board of Directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
Lars Fruergaard Jørgensen
President and CEO |
|
Karsten Munk Knudsen
CFO |
|
Helge Lund
Chair |
|
Henrik Poulsen
Vice Chair |
|
Elisabeth Dahl Christensen |
|
Laurence Debroux |
|
|
|
|
Andreas Fibig |
|
Sylvie Grégoire |
|
Liselotte Hyveled |
|
Mette Bøjer Jensen |
|
|
|
|
Kasim Kutay |
|
Christina Law |
|
Martin Mackay |
|
Thomas Rantzau |
This page is intentionally left blank
This page is intentionally left blank
This page is intentionally left blank
This page is intentionally left blank
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial statements and additional information |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial statements and additional information / Additional information / More information |
|
More information
Novo Nordisk provides additional disclosure to satisfy legal requirements and stakeholder interests. Supplementary reports can be downloaded at: www.novonordisk.com/annualreport, while additional information can be found at: www.novonordisk.com.
Annual Report
This Annual Report is Novo Nordisk’s full statutory Annual Report pursuant to Section 149(1) of
the Danish Financial Statements Act. The statutory Annual Report will be presented and adopted
at the Annual General Meeting on 27 March 2025 and will subsequently be submitted to and
be available at the Danish Business Authority. The Consolidated financial statements included in
this Annual Report have been prepared in accordance with IFRS Accounting Standards (IFRS) as
issued by the International Accounting Standards Board (IASB) and in accordance with IFRS
Accounting Standards endorsed by the EU and further requirements in the Danish Financial
Statements Act.
The Sustainability statement included in this Annual Report has been prepared in accordance
with the European Sustainability Reporting Standards (ESRS) as required by the Danish Financial
Statement Act, as well as article 8 in the EU Taxonomy regulation.
Form 20-F
The Form 20-F is filed using a standardised reporting form so that investors can evaluate the company alongside US domestic equities. It is an annual reporting requirement by the US Securities and
Exchange Commission (SEC) for foreign private issuers with equity shares listed on exchanges in the
United States.
Corporate Governance Report
The Corporate Governance Report discloses Novo Nordisk’s compliance with corporate governance to
meet the requirements of the Danish Financial Statements Act.
Remuneration Report
The Remuneration Report describes the remuneration awarded or due during 2024 to members of the Board and Executive Management registered with the Danish Business Authority in accordance with
section 139b of the Danish Companies Act. The Remuneration Report is submitted to the Annual
General Meeting for an advisory vote.
Disclaimer
The patients, employees and relatives portrayed in this Annual Report and ancillary reports
have participated of their own accord and solely to express their own personal opinions on
topics referred to, which do not necessarily reflect the views and opinions of Novo Nordisk.
Use of the pictures as illustrations is in no way intended to associate the patients, employees
or relatives with the promotion of any Novo Nordisk products.
Credits
Design and production: Kontrapunkt.
Illustrations: Kontrapunkt.
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial statements and additional information / Additional information / Product overview |
|
|
|
|
|
|
|
|
|
|
|
|
|
Product overview1 |
|
|
|
|
|
|
|
|
Once-weekly insulin
•Awiqli®, insulin icodec
New generation insulin and combinations
•Tresiba®, insulin degludec
•Ryzodeg®, insulin degludec/insulin aspart
•Fiasp®, fast-acting insulin aspart
•Xultophy®2, insulin degludec/liraglutide
Modern insulin
•Levemir®, insulin detemir
•NovoRapid®3, insulin aspart
•NovoMix® 30, biphasic insulin aspart
•NovoMix® 50, biphasic insulin aspart
Human insulin
•Insulatard® isophane (NPH) insulin
•Actrapid®, regular human insulin
•Mixtard® 30, biphasic human insulin
•Mixtard® 50, biphasic human insulin
Glucagon-like peptide-1
•Victoza®, liraglutide
•Ozempic®, semaglutide
•Rybelsus®, oral semaglutide
Pre-filled delivery systems
•FlexTouch®, U100, U200
•FlexPen®
•InnoLet®
•Ozempic®, FlexTouch®
|
Durable delivery systems
•NovoPen® 6
•NovoPen® 5
•NovoPen® 4
•NovoPen Echo® Plus
•NovoPen Echo®
Other delivery systems
•PumpCart®, NovoRapid® and Fiasp® cartridge
to be used in pump
•Penfill® cartridge
•Mallya®
Oral antidiabetic agents
•NovoNorm®, repaglinide
Glucagon
•GlucaGen®, glucagon (vial and Hypokit®)
•Zegalogue®, dasiglucagon
Needles
•NovoFine® Plus
•NovoFine®
•NovoTwist®
•NovoFine® AutoCover®
|
Glucagon-like peptide-1
•Saxenda®, liraglutide 3.0 mg
•Wegovy®, semaglutide 2.4 mg
Obesity delivery systems
•Saxenda®, FlexTouch®
•Wegovy®, Single Dose Device and FlexTouch®
|
Rare blood disorders
•NovoSeven®, eptacog alfa (recombinant activated factor VII)
•NovoEight®4, turoctocog alfa (recombinant factor VIII)
•Esperoct®, turoctocog alfa pegol, N8-GP (recombinant factor VIII)
•Alhemo®, concizumab (anti-TFPI monoclonal antibody)
•Refixia®5, nonacog beta pegol, N9-GP (recombinant factor IX)
•NovoThirteen®6, catridecacog (recombinant factor XIII)
Rare haemato-renal disorders
•Rivfloza™, nedosiran (small interfering RNA)
Rare endocrine disorders
•Norditropin®, somatropin (rDNA origin)
•Sogroya®, somapacitan (rDNA origin)
Pre-filled human growth hormone delivery systems
•FlexPro®
•NordiFlex®
Other delivery systems
•PenMate®, automatic needle inserter for FlexPro®
Hormone replacement therapies
•Vagifem®7, estradiol hemihydrate
•Activelle®, estradiol/norethisterone acetate
•Kliogest®, estradiol/norethisterone acetate
•Novofem®, estradiol/norethisterone acetate
•Trisequens®, estradiol/norethisterone acetate
•Estrofem®, estradiol
|
|
1. Products listed may not be available or approved in all markets. 2. In the US approved under the brand name Xultophy® 100/3.6. 3. In the US called NovoLog®. 4. In the US written Novoeight®.
5. In the US approved under the name of REBINYN®. 6. In the US approved under the name tretten®. 7. In the UK also called gina®.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial statements and additional information / Financial statements of the parent company 2024 |
|
Financial statements of the parent company 2024
The following pages comprise the financial statements of the parent company, the legal entity Novo Nordisk A/S. Apart from ownership of the subsidiaries in the
Novo Nordisk Group, activities of the parent company mainly comprises sales, research and development, production, corporate activities and support functions.
Income statement
For the year ended 31 December
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Note |
|
2024 |
|
2023 |
| Net sales |
2 |
|
261,712 |
|
198,078 |
| Cost of goods sold |
3 |
|
(48,930) |
|
(38,433) |
| Gross profit |
|
|
212,782 |
|
159,645 |
| Sales and distribution costs |
3 |
|
(48,921) |
|
(42,291) |
| Research and development costs |
3 |
|
(40,296) |
|
(28,731) |
| Administrative costs |
3 |
|
(1,905) |
|
(2,002) |
| Other operating income and expenses |
|
|
692 |
|
1,315 |
| Operating profit |
|
|
122,352 |
|
87,936 |
| Profit in subsidiaries, net of tax |
8 |
|
8,578 |
|
15,973 |
| Financial income |
4 |
|
6,230 |
|
3,636 |
| Financial expenses |
4 |
|
(12,568) |
|
(4,581) |
| Profit before income taxes |
|
|
124,592 |
|
102,964 |
| Income taxes |
|
|
(22,908) |
|
(19,557) |
| Net profit |
|
|
101,684 |
|
83,407 |
Balance sheet
At 31 December
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Note |
|
2024 |
|
2023 |
| Assets |
|
|
|
|
|
| Intangible assets |
6 |
|
93,202 |
|
28,755 |
| Property, plant and equipment |
7 |
|
86,376 |
|
53,822 |
| Financial assets |
8 |
|
116,186 |
|
87,543 |
|
|
|
|
|
|
| Other receivables and prepayments |
9 |
|
3,429 |
|
1,238 |
| Total non-current assets |
|
|
299,193 |
|
171,358 |
| Raw materials |
|
|
11,075 |
|
8,415 |
| Work in progress |
|
|
20,439 |
|
16,211 |
| Finished goods |
|
|
5,038 |
|
4,311 |
| Inventories |
|
|
36,552 |
|
28,937 |
| Trade receivables |
|
|
3,289 |
|
2,348 |
| Amounts owed by affiliated companies |
|
|
47,106 |
|
30,398 |
| Tax receivables |
|
|
7 |
|
8 |
| Other receivables and prepayments |
9 |
|
6,402 |
|
5,494 |
| Receivables |
|
|
56,804 |
|
38,248 |
| Marketable securities |
|
|
10,653 |
|
15,838 |
| Derivative financial instruments |
11 |
|
6,326 |
|
2,344 |
| Cash at bank |
|
|
11,750 |
|
10,623 |
| Total current assets |
|
|
122,085 |
|
95,990 |
| Total assets |
|
|
421,278 |
|
267,348 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Note |
|
2024 |
|
2023 |
| Equity and liabilities |
|
|
|
|
|
| Share capital |
10 |
|
446 |
|
451 |
| Net revaluation reserve |
|
|
18,952 |
|
24,696 |
| Development costs reserve |
|
|
1,994 |
|
1,756 |
| Reserve for cash flow hedges and exchange rate adjustments |
|
|
(4,243) |
|
1,594 |
| Retained earnings |
|
|
126,174 |
|
77,185 |
| Total equity |
|
|
143,323 |
|
105,682 |
| Borrowings |
12 |
|
85,368 |
|
16,855 |
| Deferred income tax liabilities |
5 |
|
4,886 |
|
6,282 |
| Other provisions |
13 |
|
1,576 |
|
1,280 |
| Total non-current liabilities |
|
|
91,830 |
|
24,417 |
| Borrowings |
12 |
|
11,557 |
|
5,072 |
| Derivative financial instruments |
11 |
|
7,531 |
|
1,272 |
| Trade payables |
|
|
9,099 |
|
6,778 |
| Amounts owed to affiliated companies |
|
|
137,678 |
|
108,865 |
| Tax payables |
|
|
3,883 |
|
3,046 |
| Other liabilities |
|
|
16,377 |
|
12,216 |
| Total current liabilities |
|
|
186,125 |
|
137,249 |
| Total liabilities |
|
|
277,955 |
|
161,666 |
| Total equity and liabilities |
|
|
421,278 |
|
267,348 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial statements and additional information / Financial statements of the parent company 2024 |
|
Equity statement
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Share
capital |
Net
revaluation
reserve |
Development costs reserve |
Reserve for cash flow hedges and exchange rate adjustments |
Retained
earnings |
|
2024 |
|
2023 |
| Balance at the beginning of the year |
451 |
24,696 |
1,756 |
1,594 |
77,185 |
|
105,682 |
|
82,901 |
|
|
|
|
|
|
|
|
|
|
| Appropriated from net profit |
|
|
|
|
59,946 |
|
59,946 |
|
33,116 |
| Appropriated from net profit to net revaluation reserve |
|
(8,945) |
|
|
|
|
(8,945) |
|
8,304 |
| Exchange rate adjustments of investments in subsidiaries |
|
3,201 |
|
(135) |
|
|
3,066 |
|
(1,393) |
|
|
|
|
|
|
|
|
|
|
| Realisation of previously deferred (gains)/losses on cash flow hedges |
|
|
|
(1,547) |
|
|
(1,547) |
|
(998) |
| Deferred gains/(losses) on cash flow hedges incurred during the period |
|
|
|
(5,763) |
|
|
(5,763) |
|
1,547 |
| Tax related to cash flow hedges |
|
|
|
1,608 |
|
|
1,608 |
|
(121) |
|
|
|
|
|
|
|
|
|
|
| Development costs |
|
|
238 |
|
(238) |
|
— |
|
— |
| Other adjustments |
|
|
|
|
155 |
|
155 |
|
(496) |
|
|
|
|
|
|
|
|
|
|
| Transactions with owners: |
|
|
|
|
|
|
|
|
|
| Total dividend for the year |
|
|
|
|
50,683 |
|
50,683 |
|
41,987 |
| Interim dividends paid during the year |
|
|
|
|
(15,583) |
|
(15,583) |
|
(13,430) |
| Dividends paid for prior year |
|
|
|
|
(28,557) |
|
(28,557) |
|
(18,337) |
|
|
|
|
|
|
|
|
|
|
| Reduction of the B share capital |
(5) |
|
|
|
5 |
|
— |
|
— |
| Purchase of treasury shares |
|
|
|
|
(20,181) |
|
(20,181) |
|
(29,924) |
|
|
|
|
|
|
|
|
|
|
| Share-based payments (note 3) |
|
|
|
|
626 |
|
626 |
|
562 |
| Share-based payments in subsidiaries |
|
|
|
|
1,663 |
|
1,663 |
|
1,587 |
| Tax related to share-based payments |
|
|
|
|
470 |
|
470 |
|
377 |
| Balance at the end of the year |
446 |
18,952 |
1,994 |
(4,243) |
126,174 |
|
143,323 |
|
105,682 |
|
|
|
|
|
|
|
|
|
|
| Proposed appropriation of net profit: |
|
|
|
|
|
|
|
|
|
| Interim dividend for the year |
|
|
|
|
|
|
15,583 |
|
13,430 |
| Final dividend for the year |
|
|
|
|
|
|
35,100 |
|
28,557 |
| Appropriated to net revaluation reserve |
|
|
|
|
|
|
(8,945) |
|
8,304 |
| Transferred to retained earnings |
|
|
|
|
|
|
59,946 |
|
33,116 |
| Distribution of net profit |
|
|
|
|
|
|
101,684 |
|
83,407 |
Refer to note 4.3 in the Consolidated financial statements for details on the number of shares, treasury shares and total number of A and B shares in Novo Nordisk A/S. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial statements and additional information / Financial statements of the parent company 2024 |
|
Notes
1 Accounting policies
The financial statements of the parent company have been prepared in accordance with the Danish Financial Statements Act (Class D) and other accounting regulations for companies listed on Nasdaq Copenhagen.
The accounting policies for the financial statements of the parent company are unchanged from the previous financial year, except for the addition of access to capacity under intangible assets. The accounting policies are the same as for the Consolidated financial statements with the adjustments described below. For a description of the accounting policies of the Group, refer to the Consolidated
financial statements.
No separate statement of cash flows has been prepared for the parent company;
refer to the statement of cash flows for the Group.
Supplementary accounting policies for the parent company
In the Parent Financial Statements the acquisition of three fill-finish sites from Novo Holdings A/S is accounted for as acquisition of shares in subsidiaries and intangible assets (access to capacity).
Intangible assets
Access to capacity asset is amortised over 10 years.
Financial assets
In the financial statements of the parent company, investments in subsidiaries and associated companies are recorded under the equity method, using the respective share of the net asset values in subsidiaries and associated companies. The equity method is used as a measurement method rather than a consolidation method.
The net profit of subsidiaries and associated companies less unrealised intra-group profits and amortisation of goodwill is recorded in the income statement of the parent company. To the extent that net profit exceeds declared dividends from such companies, the net revaluation of investments in subsidiaries and associated companies is transferred to net revaluation reserve under equity according to the equity method.
Goodwill recognised in subsidiaries is amortised over 10-23 years, which reflects
the useful life of the underlying assets and activities generating the goodwill.
Amounts owed by affiliates, where settlement is neither planned nor likely within
the foreseeable future, are treated as part of net-investments in subsidiaries, with exchange rate adjustments recognised directly in equity through reserve for cash
flow hedges and exchange rate adjustments.
Tax
For Danish tax purposes, the parent company is assessed jointly with its Danish subsidiaries. The Danish jointly taxed companies are included in a Danish on-account tax payment scheme for Danish corporate income tax. All current taxes under the scheme are recorded in the individual companies. Novo Nordisk A/S and its jointly taxed subsidiaries are included in the joint taxation of the parent company,
Novo Holdings A/S.
2 Sales
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
| Sales by segment |
|
|
|
|
| Diabetes and Obesity care |
|
261,556 |
|
|
197,969 |
|
| Rare disease |
|
156 |
|
|
109 |
|
| Total sales |
|
261,712 |
|
|
198,078 |
|
| Sales by geographical segment |
|
|
|
|
| North America Operations |
|
165,689 |
|
|
124,860 |
|
| International Operations: |
|
|
|
|
| EMEA |
|
47,810 |
|
|
40,038 |
|
| Region China |
|
23,356 |
|
|
12,800 |
|
| Rest of World |
|
24,857 |
|
|
20,380 |
|
| Total sales |
|
261,712 |
|
|
198,078 |
|
Sales are attributed to a geographical segment based on location of the customer.
For definitions of segments, refer to note 2.2 in the Consolidated financial statements.
3 Employee costs
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
| Wages and salaries |
|
25,252 |
|
|
19,525 |
|
| Share-based payment costs |
|
626 |
|
|
562 |
|
| Pensions |
|
2,211 |
|
|
1,709 |
|
| Other social security contributions |
|
417 |
|
|
301 |
|
| Other employee costs |
|
1,371 |
|
|
1,039 |
|
| Total employee costs |
|
29,877 |
|
|
23,136 |
|
| Average number of full-time employees |
|
29,288 |
|
|
23,754 |
|
| Year-end number of full-time employees |
|
31,096 |
|
|
26,111 |
|
For information regarding remuneration to the Board of Directors and Executive Management, refer to note 5.4 in the Consolidated financial statements.
4 Financial income and financial expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
| Interest income relating to subsidiaries |
|
227 |
|
|
487 |
|
| Interest income relating to external counterparties |
|
1,589 |
|
|
936 |
|
|
|
|
|
|
| Foreign exchange gain (net) |
|
— |
|
|
772 |
|
| Financial gain from forward contracts (net) |
|
4,355 |
|
|
1,263 |
|
| Capital gain from marketable securities (net) |
|
2 |
|
|
144 |
|
| Other financial income |
|
57 |
|
|
34 |
|
| Total financial income |
|
6,230 |
|
|
3,636 |
|
| Interest expenses relating to subsidiaries |
|
6,763 |
|
|
4,225 |
|
| Interest expense relating to external counterparties |
|
529 |
|
|
148 |
|
| Result of associated company |
|
4 |
|
|
38 |
|
| Foreign exchange loss (net) |
|
5,076 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
| Other financial expenses |
|
196 |
|
|
170 |
|
| Total financial expenses |
|
12,568 |
|
|
4,581 |
|
5 Deferred income tax assets/(liabilities)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
Net deferred tax asset/(liability) at the beginning
of the year |
|
(6,282) |
|
(2,967) |
| Income/(charge) to the income statement |
|
(349) |
|
(2,797) |
| Additions from acquisitions |
|
254 |
|
|
— |
|
| Income/(charge) to equity |
|
1,491 |
|
|
(518) |
|
Net deferred tax asset/(liability)
at the end of the year |
|
(4,886) |
|
(6,282) |
|
The Danish corporate tax rate is 22% in 2024 (22% in 2023), which is used for the calculation of the deferred tax liability.
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial statements and additional information / Financial statements of the parent company 2024 |
|
6 Intangible assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
Intellectual property rights and similar rights |
Software and other intangibles |
|
2024 |
|
2023 |
| Cost at the beginning of the year |
|
31,514 |
|
4,143 |
|
|
35,657 |
|
|
23,820 |
|
| Additions during the year |
|
72,095 |
|
597 |
|
|
72,692 |
|
|
11,837 |
|
| Disposals during the year |
|
(949) |
(70) |
|
(1,019) |
|
— |
|
| Cost at the end of the year |
|
102,660 |
|
4,670 |
|
|
107,330 |
|
|
35,657 |
|
| Amortisation at the beginning of the year |
|
5,011 |
|
1,891 |
|
|
6,902 |
|
|
4,371 |
|
| Amortisation during the year |
|
1,178 |
|
221 |
|
|
1,399 |
|
|
1,011 |
|
| Impairment losses for the year |
|
5,985 |
|
71 |
|
|
6,056 |
|
|
1,520 |
|
| Amortisation and impairment losses reversed on disposals during the year |
|
(159) |
(70) |
|
(229) |
|
— |
|
| Amortisation at the end of the year |
|
12,015 |
|
2,113 |
|
|
14,128 |
|
|
6,902 |
|
| Carrying amount at the end of the year |
|
90,645 |
|
2,557 |
|
|
93,202 |
|
|
28,755 |
|
|
Intangible assets primarily relate to intellectual property rights, access to capacity asset amounting to DKK 57,496 million (acquired in 2024), internally developed software and costs related to major IT projects. Intangible assets which are not yet available for use amount to DKK 17,610 million (DKK 19,993 million in 2023).
For further information on impairments, refer to note 3.1 in the Consolidated financial statements.
|
7 Property, plant and equipment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Land and
buildings |
Plant and
machinery |
Other
equipment |
Assets under construction |
|
2024 |
|
2023 |
| Cost at the beginning of the year |
24,890 |
|
25,554 |
|
4,882 |
|
31,025 |
|
|
86,351 |
|
|
65,692 |
|
| Additions during the year |
1,127 |
|
337 |
|
171 |
|
34,184 |
|
|
35,819 |
|
|
22,420 |
|
| Disposals during the year |
(135) |
(375) |
(180) |
(244) |
|
(934) |
|
(1,761) |
| Transfer from/(to) other items |
1,286 |
|
2,388 |
|
261 |
|
(3,935) |
|
— |
|
|
— |
|
| Cost at the end of the year |
27,168 |
|
27,904 |
|
5,134 |
|
61,030 |
|
|
121,236 |
|
|
86,351 |
|
| Depreciation and impairment losses at the beginning of the year |
12,149 |
|
17,310 |
|
3,070 |
|
— |
|
|
32,529 |
|
|
31,145 |
|
| Depreciation for the year |
1,297 |
|
1,248 |
|
393 |
|
— |
|
|
2,938 |
|
|
2,748 |
|
| Impairment losses for the year |
22 |
|
50 |
|
6 |
|
244 |
|
|
322 |
|
|
409 |
|
| Depreciation reversed on disposals during the year |
(130) |
(378) |
(177) |
(244) |
|
(929) |
|
(1,773) |
| Depreciation and impairment losses at the end of the year |
13,338 |
|
18,230 |
|
3,292 |
|
— |
|
|
34,860 |
|
|
32,529 |
|
| Carrying amount at the end of the year |
13,830 |
|
9,674 |
|
1,842 |
|
61,030 |
|
|
86,376 |
|
|
53,822 |
|
| Of which related to leased property, plant and equipment |
1,377 |
|
— |
|
84 |
|
— |
|
|
1,461 |
|
|
1,083 |
|
| Leased property, plant and equipment primarily relates to lease of office buildings, warehouses, laboratories and vehicles. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial statements and additional information / Financial statements of the parent company 2024 |
|
8 Financial assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
Investments
in subsidiaries |
Amounts owed by affiliated companies |
Investment in
associated
company |
Other
securities and investments |
2024 |
|
2023 |
| Cost at the beginning of the year |
59,801 |
|
2,447 |
|
105 |
|
818 |
|
|
63,171 |
|
|
60,497 |
|
| Investments during the year |
33,977 |
|
868 |
|
|
145 |
|
|
34,990 |
|
|
6,094 |
|
| Divestments and repayments during the year |
— |
|
(476) |
|
— |
|
|
(476) |
|
(3,420) |
| Cost at the end of the year |
93,778 |
|
2,839 |
|
105 |
|
963 |
|
|
97,685 |
|
|
63,171 |
|
| Value adjustments at the beginning of the year |
41,271 |
|
21 |
|
52 |
|
(345) |
|
40,999 |
|
|
34,521 |
|
| Profit/(loss) before tax |
20,823 |
|
|
|
|
|
20,823 |
|
|
18,112 |
|
| Share of result after tax in associated company |
|
|
(4) |
|
|
(4) |
|
(38) |
| Income taxes on profit for the year |
(3,377) |
|
|
|
|
(3,377) |
|
(1,332) |
| Market value adjustment |
|
|
|
(34) |
|
(34) |
|
(6) |
| Dividends received |
(21,762) |
|
|
|
|
(21,762) |
|
(9,127) |
| Divestments during the year |
— |
|
|
|
— |
|
|
— |
|
|
54 |
|
| Effect of exchange rate adjustment charged to the income statement |
|
24 |
|
|
18 |
|
|
42 |
|
|
(367) |
| Effect of exchange rate adjustment charged to equity |
2,920 |
|
(135) |
|
|
|
2,785 |
|
|
(2,285) |
| Other adjustments |
4,243 |
|
|
|
|
|
4,243 |
|
|
1,467 |
|
|
|
|
|
|
|
|
|
|
| Value adjustments at the end of the year |
44,118 |
|
(90) |
48 |
|
(361) |
|
|
43,715 |
|
|
40,999 |
|
| Unrealised internal profit at the beginning of the year |
(16,627) |
|
|
|
|
(16,627) |
|
(16,712) |
| Unrealised internal profit movements in the year |
(8,868) |
|
|
|
|
(8,868) |
|
(807) |
| Effect of exchange rate adjustment charged to equity |
281 |
|
|
|
|
|
281 |
|
|
892 |
|
|
|
|
|
|
|
|
|
|
| Unrealised internal profit at the end of the year |
(25,214) |
— |
|
— |
|
— |
|
|
(25,214) |
|
(16,627) |
| Carrying amount at the end of the year |
112,682 |
|
2,749 |
|
153 |
|
602 |
|
|
116,186 |
|
|
87,543 |
|
For a list of companies in the Novo Nordisk Group, refer to note 5.7 in the Consolidated financial statements. |
9 Other receivables and prepayments
Other receivables and prepayments includes prepayments of DKK 7,571 million
(DKK 5,375 million in 2023), primarily related to prepaid contract manufacturing
and R&D activities
10 Share capital
For information on share capital, refer to note 4.3 in the Consolidated financial statements.
11 Derivatives
For information on derivative financial instruments, refer to note 4.5 in the Consolidated financial statements. All derivatives in the group are entered into
with Novo Nordisk A/S as the counterpart.
12 Borrowings
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
| Within 1 year |
|
11,557 |
|
|
5,072 |
|
| 1-5 years |
|
63,815 |
|
|
12,889 |
|
| More than 5 years |
|
21,553 |
|
|
3,966 |
|
| Total borrowings |
|
96,925 |
|
|
21,927 |
|
Borrowings mainly consist of debt to fund the acquisition of shares in subsidiaries and intangible assets (access to capacity), and loans from Novo Nordisk Finance (Netherlands) B.V. related to issuance of Eurobonds. For further information on borrowings, refer to note 4.6 in the Consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial statements and additional information / Financial statements of the parent company 2024 |
|
13 Other provisions
Provisions for pending litigations are recognised as other provisions. For information on pending litigations, refer to note 3.5 in the Consolidated financial statements. Furthermore, as part of normal business Novo Nordisk issues credit notes for
expired goods. Consequently, a provision for future returns is made, based on historical product return statistics.
14 Related party transactions
For information on transactions with related parties, refer to note 5.4 in the Consolidated financial statements.
The parent company’s share of services provided by NNIT Group amounts to
DKK 189 million (DKK 327 million in 2023). The parent company’s share of services provided to Novonesis Group amounts to DKK 38 million (DKK 38 million in 2023).
Novo Nordisk A/S is included in the Consolidated financial statements of the
Novo Nordisk Foundation.
15 Fee to statutory auditors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
Statutory audit1 |
|
14 |
|
|
9 |
|
| Audit-related services |
|
3 |
|
|
2 |
|
| Tax advisory services |
|
4 |
|
|
4 |
|
| Other services |
|
13 |
|
|
15 |
|
| Total fee to statutory auditors |
|
34 |
|
|
30 |
|
1. 2024 statutory audit fee includes DKK 5 million of additional fees mainly related to business acquisitions |
16 Commitments and contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DKK million |
|
2024 |
|
2023 |
| Commitments |
|
|
|
|
Leases1 |
|
2,657 |
|
|
804 |
|
| Research and development obligations |
|
31,511 |
|
|
18,448 |
|
Research and development - potential milestones2 |
|
33,614 |
|
|
25,218 |
|
Commercial product launch - potential milestones2 |
|
15,749 |
|
|
11,780 |
|
| Purchase obligations relating to investments in property plant and equipment |
|
4,956 |
|
|
1,072 |
|
| Purchase obligation relating to contract manufacturers |
|
71,061 |
|
|
33,107 |
|
| Other purchase obligations |
|
5,027 |
|
|
2,742 |
|
Guarantees given for subsidiaries3 |
|
68,081 |
|
|
35,608 |
|
| Other guarantees |
|
1,003 |
|
|
993 |
|
1. Lease commitments predominantly relate to lease agreements executed but not commenced
and estimated variable property taxes and low value assets.
2. Potential milestone payments are associated with uncertainty as they are linked to successful achievements in research activities; refer to note 5.2 in the Consolidated financial statements.
3. Guarantees given for subsidiaries mainly relate to guarantees towards Novo Nordisk Finance (Netherlands) B.V. related to issuance of Eurobonds. |
Novo Nordisk A/S and its Danish subsidiaries are jointly taxed with the Danish companies in Novo Holdings A/S. The joint taxation also covers withholding taxes
in the form of dividend tax, royalty tax and interest tax. The Danish companies are jointly and severally liable for the joint taxation. Any subsequent adjustments to income taxes and withholding taxes may lead to a larger liability. The tax for the individual companies is allocated in full on the basis of the expected taxable income.
For information on Purchase obligation related to contract manufacturers, refer to note 5.2 in the Consolidated financial statements. For information on pending litigation and other contingencies, refer to notes 3.5 and 5.2 in the Consolidated financial statements.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf of the undersigned, thereunto duly authorized.
Date: February 5, 2025
Novo Nordisk A/S
Lars Fruergaard Jørgensen
Chief Executive Officer
EX-15.2
9
exhibit152consentofindepen.htm
EX-15.2
Document
Exhibit 15.2
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference in Registration Statement Nos. 333-221244, 333-222923, and 333-263809 on Form S-8 of our report dated February 5, 2025 relating to the financial statements of Novo Nordisk A/S and the effectiveness of Novo Nordisk A/S’s internal control over financial reporting appearing in this Annual Report on Form 20-F for the year ended December 31, 2024.
/s/ Deloitte Statsautoriseret Revisionspartnerselskab
Copenhagen, Denmark
February 5, 2025
EX-97
10
exhibit97compensationrecov.htm
EX-97
Document
Exhibit 97
Appendix – Dodd Frank Recoupment Policy
NOVO NORDISK A/S
Dodd Frank Recoupment Policy
1. Purpose.
The Board of Directors (“Board”) of Novo Nordisk A/S (the “Company”), based upon the recommendation of its Remuneration Committee (the “Committee”), has adopted this Dodd Frank Recoupment Policy (this “Policy”) applicable to the Novo Nordisk Group in order to implement a mandatory clawback policy in the event of a Restatement in compliance with the Applicable Rules. Any capitalized terms used, but not immediately defined, in this Policy have the meanings set forth in Section 9.
2. Administration.
This Policy shall be administered by the Committee, which shall make all determinations with respect to this Policy in its sole discretion; provided that this Policy shall be interpreted in a manner consistent with the requirements of the Applicable Rules. Notwithstanding the foregoing, subject to the Applicable Rules, the Board may assume any or all powers and authority of the Committee with respect to this Policy, in which case references to the Committee shall be deemed to include the Board, as applicable.
3. Recovery on a Restatement.
In the event that the Company is required to prepare a Restatement, the Company (potentially through one or more of its subsidiaries) shall reasonably promptly recover from an Executive Officer the amount of any erroneously awarded Incentive-Based Compensation that is Received by such Executive Officer during the Recovery Period. The amount of erroneously Received Incentive-Based Compensation will be the excess of the Incentive-Based Compensation Received by the Executive Officer (whether in cash or shares) based on the erroneous data in the original financial statements over the Incentive-Based Compensation (whether in cash or in shares) that would have been Received by the Executive Officer had such Incentive-Based Compensation been based on the restated results, without respect to any tax liabilities incurred or paid by the Executive Officer.
Without limiting the foregoing, for Incentive-Based Compensation based on the Company’s stock price or total shareholder return, where the amount of erroneously awarded compensation is not subject to mathematical recalculation directly from the information in the Restatement, (i) the amount shall be based on the Company’s reasonable estimate of the effect of the Restatement on the stock price or total shareholder return upon which the Incentive-Based Compensation was Received and (ii) the Company shall maintain documentation of the determination of that reasonable estimate and provide such estimate to NYSE.
4. Coverage and Application.
This Policy covers all persons who are Executive Officers at any time during the Recovery Period for which Incentive-Based Compensation is Received. Incentive-Based Compensation shall not be recovered under this Policy to the extent Received by any person before the date the person served as an Executive Officer. Subsequent changes in an Executive Officer’s employment status, including retirement or termination of employment, do not affect the Company’s or a subsidiary’s right to recover Incentive-Based Compensation pursuant to this Policy.
This Policy shall apply to Incentive-Based Compensation that is Received by any Executive Officer on or after the Effective Date and that results from attainment of a Financial Reporting Measure based on or derived from financial information for any fiscal period ending on or after the Effective Date.
5. Exceptions to Policy.
No recovery of Incentive-Based Compensation shall be required if any of the following conditions are met and the Committee determines that, on such basis, recovery would be impracticable:
a.the direct expense paid to a third party to assist in enforcing this Policy would exceed the amount to be recovered; provided that prior to making a determination that it would be impracticable to recover any Incentive-Based Compensation based on the expense of enforcement, the Company or an applicable subsidiary shall (i) have made a reasonable attempt to recover the Incentive-Based Compensation, (ii) have documented such reasonable attempts to recover, and (iii) provide the documentation to NYSE;
b.recovery would violate Danish law or such other jurisdiction(s) as may govern the actual Incentive Based Compensation where that law was adopted prior to 28 November 2022; or
c.recovery would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees, to fail to meet the requirements of Section 401(a)(13) or Section 411(a) of the Internal Revenue Code of 1986, as amended (the “Code”), and U.S. Treasury regulations promulgated thereunder.
1
|
|
|
|
|
|
|
Exhibit 97 2024 - Novo Nordisk |
6. Methods of Recovery.
In the event of a Clawback Event, subject to applicable law, the Committee may take any such actions as it deems necessary or appropriate, including, without limitation:
a.the reduction or cancellation of any Incentive-Based Compensation in the form of vested or unvested equity or equity-based awards that have not been distributed or otherwise settled prior to the date of determination;
b.the recovery of any Incentive-Based Compensation that was previously paid to the Executive Officer;
c.the recovery of any gain realized on the vesting, exercise, settlement, sale, transfer, or other disposition of any Incentive-Based Compensation in the form of equity or equity-based awards;
d.the offset, withholding, or elimination of any amount that could be paid or awarded to the Executive Officer after the date of determination;
e.the recoupment of any amount in respect of Incentive-Based Compensation contributed to a plan that takes into account Incentive-Based Compensation (excluding certain tax-qualified plans, but including long-term disability, life insurance, supplemental executive retirement plans and deferred compensation plans, in each case to the extent permitted by applicable law, including Section 409A of the Code) and any earnings accrued to date on any such amount; and
f.the taking any other remedial and recovery action permitted by law, as determined by the Committee.
In addition, the Committee may authorize other actions vis-à-vis an Executive Officer as the Company (or any of its subsidiaries) may be entitled to take and the Committee deems appropriate.
7. Other Employees
The Committee may decide that this Policy is applicable to incentives received by Other Employees of the Company and its subsidiaries. If so, this Policy would become an integral part of a specific incentive scheme for such Other Employees. The Committee may decide when enforcing the policy to deviate from the Policy to the advantage of the Other Employees, including rescinding claw back and/or refraining from making public disclosures in relation to Other Employees unless required by law.
8. Miscellaneous.
a. Effective Date. This Policy shall be effective as of 1 December 2023 (“Effective Date”).
b. Public Disclosure. The Company shall make all required disclosures and filings with the Regulators with respect to this Policy in accordance with the requirements of the Applicable Rules, and any other requirements applicable to the Company, including any disclosures required in connection with SEC filings.
c. No Indemnification. The Company or any subsidiary shall not indemnify any current or former Executive Officer against the loss of erroneously awarded compensation and shall not pay or reimburse any Executive Officer for premiums incurred or paid for any insurance policy to fund such Executive Officer’s potential recovery obligations.
d. No Substitution of Rights; Non-Exhaustive Rights. Any right of recoupment under this Policy is in addition to, and not in lieu of, any other remedies or rights of recoupment that may be available to the Company or any subsidiary pursuant to (i) any equity or equity-based incentive compensation plan or any successor plan thereto, or any other incentive plan of the Company or any of its subsidiaries or affiliates or (ii) the terms of any similar policy or provision in any employment agreement, compensation agreement or arrangement, or similar agreement and any other legal remedies available to the Company or any subsidiary. In addition to recovery of compensation as provided for in this Policy, the Company or any subsidiary may take any and all other actions as it deems necessary, appropriate and in the Company’s or a subsidiary’s best interest in connection with a Clawback Event and nothing in this Policy limits the Company’s or a subsidiary’s rights to take any such or other appropriate actions.
e. Amendment; Termination; Sunset. The Board, based upon the recommendation of the Committee, may amend this Policy at any time for any reason, subject to any limitations under the Applicable Rules. Unless otherwise required by applicable law, this Policy shall no longer be effective from and after the date that the Company no longer has a class of securities publicly listed on a U.S. national securities exchange or is otherwise not subject to the Applicable Rules.
9. Defined Terms.
For the purposes of this Policy, the following terms shall have the meanings set forth below:
a.“Applicable Rules” means Section 10D of the Exchange Act and Rule 10D-1 promulgated thereunder, Section 303A.14 of the Listed Company Manual of the New York Stock Ex-change LLC, and any other national stock exchange rules that the Company is or may become subject to.
b.“Clawback Event” means a required recoupment of Incentive-Based Compensation in the event of a Restatement under the Applicable Rules.
c.“Exchange Act” means the Securities Exchange Act of 1934, as amended.
d.“Executive Officer” means any officer who is required to be covered by this Policy pursuant to Section 10D of the Exchange Act.
2
|
|
|
|
|
|
|
Exhibit 97 2024 - Novo Nordisk |
e.“Financial Reporting Measures” means (i) measures that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and any measures that are derived wholly or in part from such measures,
(ii) the Company’s stock price, and (iii) total shareholder return in respect of the Company. A “Financial Reporting
Measure” need not be presented within the financial statements or included in a filing with the SEC.
f.“Incentive-Based Compensation” means any compensation that is granted, earned, or vested, based wholly or in part upon the attainment of a Financial Reporting Measure. Incentive-Based Compensation does not include, among other forms of compensation, equity awards that vest exclusively upon completion of a specified employment period, without any performance condition, and bonus awards that are discretionary or based on subjective goals or goals unrelated to Financial Reporting Measures.
g.“Novo Nordisk Group” means Novo Nordisk A/S and its subsidiaries.
h.“NYSE” means the New York Stock Exchange.
i.“Other Employees” mean any other employees of the Company and its subsidiaries identified by the Committee from time to time.
j.“Received” means Incentive-Based Compensation is deemed “Received” for the purposes of this Policy in the Company’s fiscal period during which the Financial Reporting Measure applicable to the Incentive-Based Compensation award is attained, even if the payment or grant of the Incentive-Based Compensation occurs after the end of that period.
k.“Recovery Period” means the three completed fiscal years immediately preceding the date on which the Company is required to prepare a Restatement, which date is the earlier of (i) the date the Board, a committee of the Board, or the officer or officers of the Company authorized to take such action if Board action is not required, concludes, or reasonably should have concluded, that the Company is required to prepare a Restatement or (ii) a date that a court, regulator, or other legally authorized body directs the Company to prepare a Restatement.
l.“Regulators” means, as applicable, the SEC and the NYSE.
m.“Restatement” means that the Company is required under U.S. federal securities laws to prepare an accounting restatement due to a material noncompliance of the Company with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements (i) that is material to the previously issued financial statements, or (ii) that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period.
n.“SEC” means the U.S. Securities and Exchange Commission.
This Dodd Frank Recoupment Policy was adopted by the Board of Directors on November 1, 2023.
3
|
|
|
|
|
|
|
Exhibit 97 2024 - Novo Nordisk |











































































































































































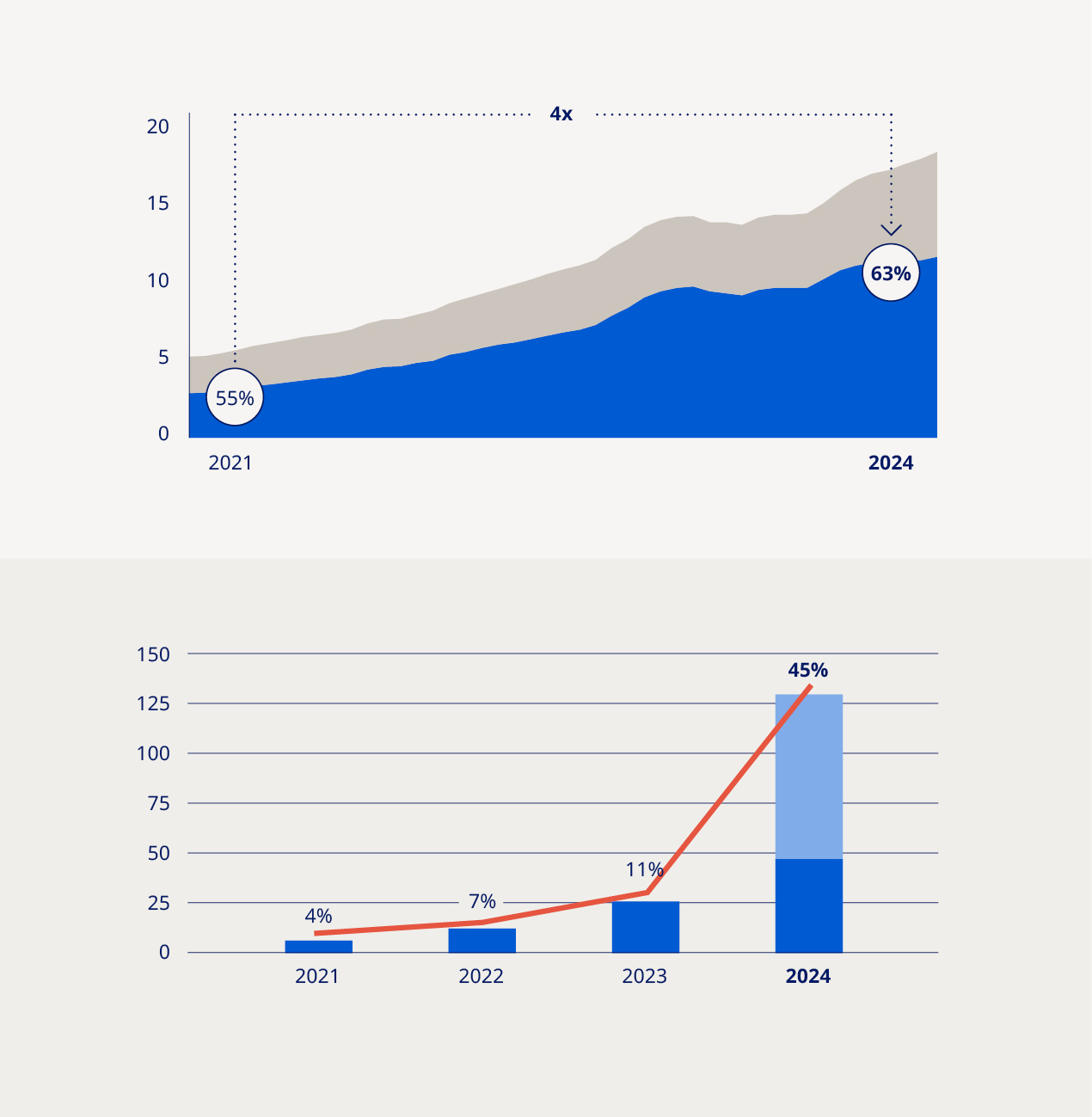










































































 Patient protection and quality of life
Patient protection and quality of life Business conduct
Business conduct Water
Water Pollution
Pollution Climate change
Climate change
 Resource use and circular economy
Resource use and circular economy Own workforce
Own workforce














































































 Positive impact
Positive impact  Negative impact
Negative impact  Opportunity
Opportunity  Risk
Risk





 Positive impact
Positive impact  Negative impact
Negative impact  Opportunity
Opportunity  Risk
Risk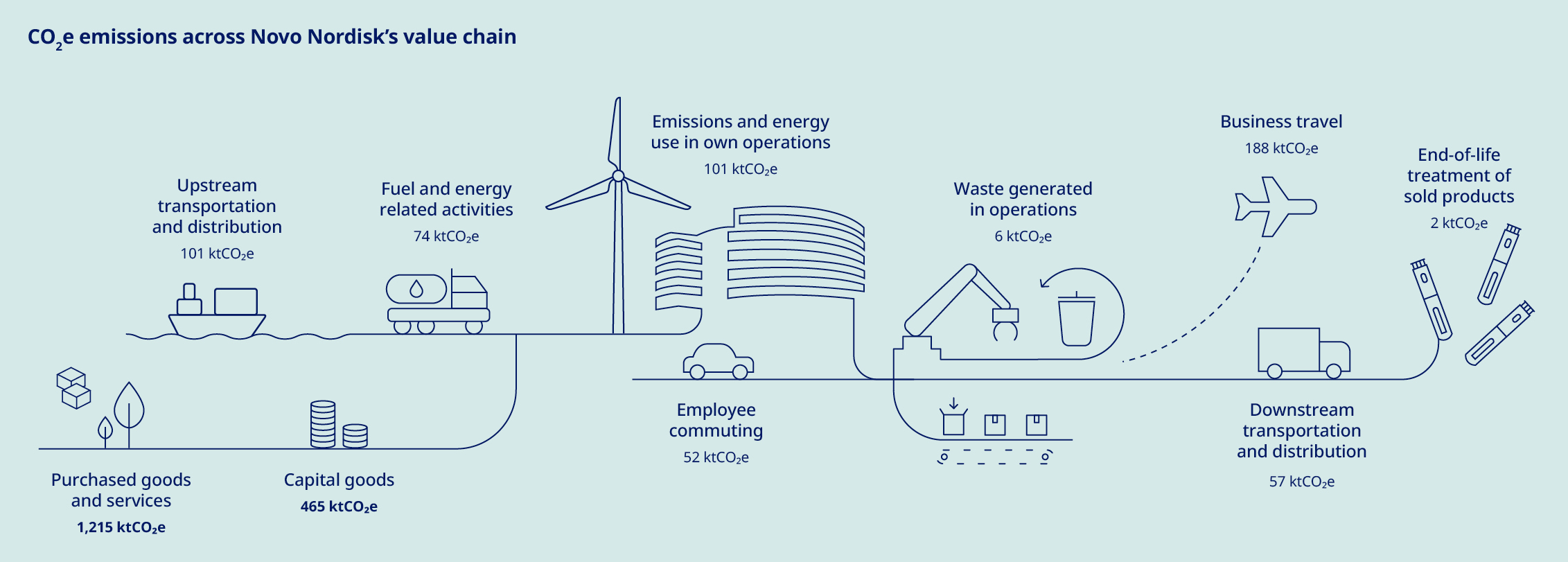
















 Positive impact
Positive impact  Negative impact
Negative impact  Opportunity
Opportunity  Risk
Risk










 Positive impact
Positive impact  Negative impact
Negative impact  Opportunity
Opportunity  Risk
Risk



 Positive impact
Positive impact  Negative impact
Negative impact  Opportunity
Opportunity  Risk
Risk





 Positive impact
Positive impact  Negative impact
Negative impact  Opportunity
Opportunity  Risk
Risk










 Positive impact
Positive impact  Negative impact
Negative impact  Opportunity
Opportunity  Risk
Risk



























 Positive impact
Positive impact  Negative impact
Negative impact  Opportunity
Opportunity  Risk
Risk



















 Positive impact
Positive impact  Negative impact
Negative impact  Opportunity
Opportunity  Risk
Risk





 Positive impact
Positive impact  Negative impact
Negative impact  Opportunity
Opportunity  Risk
Risk






















































































































