
May 2024 Unique biology Precision therapeutics Broad impact Exhibit 99.1

| Forward Looking Statements 2 This presentation contains forward-looking statements that are based on management's beliefs and assumptions and on information currently available to management. All statements other than statements of historical facts contained in this presentation are forward- looking statements. In some cases, you can identify forward-looking statements by terms such as “could,” “may,” “might,” “will,” “likely,” “anticipates,” “intends,” “plans,” “seeks,” “believes,” “estimates,” “expects,” “continues,” “projects” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements include, but are not limited to, statements concerning: the potential outcomes from our collaboration agreement with Lilly; the initiation, timing, progress and results of our research and development programs and pre-clinical studies and clinical trials, including with respect to our Phase 1 study of FHD-286 in combination with decitabine or cytarabine in relapsed and/or refractory AML patients and anticipated timing of release of clinical data, and the planned Phase 1 dose escalation study of FHD-909 with Loxo@Lilly; our ability to advance product candidates that we may develop and to successfully complete preclinical and clinical studies; our ability to leverage our initial programs to develop additional product candidates using our Gene Traffic Control Platform®; the impact of exogeneous factors, including macroeconomic and geopolitical circumstances, on our and our collaborators’ business operations, including our research and development programs and pre-clinical studies; developments related to our competitors and our industry; our ability to expand the target populations of our programs and the availability of patients for clinical testing; our ability to obtain regulatory approval for FHD- 286 and any future product candidates from the FDA and other regulatory authorities; our ability to identify and enter into future license agreements and collaborations; our ability to continue to rely on our CDMOs and CROs for our manufacturing and research needs; regulatory developments in the United States and foreign countries; our ability to attract and retain key scientific and management personnel; the scope of protection we are able to establish, maintain and enforce for intellectual property rights covering FHD-286, our future products and our Gene Traffic Control Platform; and our use of proceeds from capital-raising transactions, estimates of our expenses, capital requirements, and needs for additional financing. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Additional important factors to be considered in connection with forward-looking statements are described in the Company's filings with the Securities and Exchange Commission, including withing the section entitled "Risk Factors" in the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2023. Any forward-looking statements represent the Company’s views only as of the date of this presentation and should not be relied upon as representing its views as of any subsequent date. The Company explicitly disclaims any obligation to update any forward-looking statements. The Company’s business is subject to substantial risks and uncertainties.

| Developing First-In-Class Precision Medicines Targeting Major Unmet Needs in Cancer 3 Large Market Potential Chromatin biology is implicated in up to 50% of tumors, potentially impacting ~2.5 million patients Foghorn’s current pipeline potentially addresses more than 500,000 of these patients Broad pipeline across a range of targets and small molecule modalities Major Strategic Collaboration Strategic collaboration with Loxo@Lilly; $380 million upfront; 50/50 U.S. economic split on two lead programs Well- Funded $206.7 million in cash and equivalents (as of 3/31/2024) Provides runway into H1’26 Value Drivers Anticipate data from the Phase 1 study of FHD-286 in combination with decitabine in H2’24 BRM Selective Inhibitor (FHD-909), partnered with Loxo@Lilly, IND submitted to FDA, Phase 1 initiation anticipated in H2’24 Advancement of preclinical assets (BRM Selective Degrader, CBP, EP300, ARID1B) towards INDs Leader in Unique Area of Cancer Biology Foghorn is a leader in targeting chromatin biology, which has the potential to address underlying dependencies of many genetically defined cancers Platform with initial focus in oncology, therapeutic area expansion potential

| Unique Insights into Chromatin Biology to Prosecute Untapped Area for Novel Targets and Therapeutics 4 Novel Targets Guided by Genetic Dependencies Tailored Drugging Approaches Transcription Factor Mutations / Overexpression Chromatin Remodeling Complex Mutations / Overexpression Helicases & Other Chromatin Binding Proteins involved in gene expression / function Enzymatic Inhibitors Highly selective and allosteric small molecule inhibitors Transcription Factor Disruptors Disrupt interactions between chromatin remodeling complexes and transcription factors Potential druggable sites ATP ADP Chromatin Regulatory System Critical for Gene Expression Targeted Protein Degradation Molecular glue and bi-functional protein degraders Chromatin – compacted form of DNA inside the nucleus of the cell Chromatin Remodeling Complex – specialized multiprotein machineries that allow access to DNA Transcription Factor – proteins that help turn specific genes "on" or "off" by working in concert with the chromatin remodeling complex to bind to DNA

| Foghorn’s Validated Gene Traffic Control© Platform Enables an Integrated, Scalable, Efficient and Repeatable Paradigm 5 Deep mechanistic understanding of the chromatin regulatory system Biology first, small molecule modality agnostic Targeting Disease Selective Therapeutics What to Drug: Identify disease dependencies with novel targets How to Drug: Small molecules, degrader and delivery platform Biochemistry, biophysics and assays of large complexes and proteins Specialized Approach Where to Drug: Engineer selectivity via unique assays and protein capabilities Transcription Factor Disruptors Enzymatic Inhibitors Targeted Protein Degraders

| Foghorn Is Advancing a Pipeline of First-in-Class Precision Therapeutics with Potential for Broad Application in Oncology 6 Modality Program Phase 1 Enzyme Inhibitors Transcription Factor Disruptors Undisclosed Protein Degraders Commercial Rights FHD-286 (BRG1/BRM) FHD-909 (Selective BRM) 3 Discovery Programs Undisclosed Partnered Undisclosed Disease BRG1 mutant cancers (e.g., NSCLC, bladder, endometrial, colorectal) Relapsed/Refractory AML Selective BRM BRG1 mutant cancers (e.g., NSCLC, bladder, endometrial, colorectal) Selective ARID1B ARID1A mutant cancers (e.g., ovarian, endometrial, colorectal) Selective CBP EP300 mutant cancers (e.g., bladder, gastric, breast, NSCLC, colorectal) Selective EP300 EP300 dependent cancers (e.g., prostate, DLBCL), CBP mutant cancers (e.g., NSCLC, bladder) Undisclosed Undisclosed Undisclosed Pre-Clinical Phase 2 / 3Discovery

FHD-286: Dual BRM/BRG1 Inhibition Targeting BAF Dependency in Cancer FHD-609 is a Selective, Potent, Protein Degrader of the BRD9 component of the BAF complex

| Exploring BAF Dependency in Cancer with FHD-286 – Potent, Small Molecule Inhibitor Targeting BRM and BRG1 8 Clinical and pre-clinical data demonstrate broad- based differentiation across AML and multiple solid tumors BAF ATPase: BRG1/BRM FHD-286: • Allosteric modulation inhibiting the activity of both BRM and BRG1 • Oral, daily, potent small molecule inhibitor FHD-286 Differentiation Pre-clinical data support ability to overcome drug resistance (i.e., EGFR NSCLC, enzalutamide- resistant CRPC, PD-1 refractory) Overcoming Drug Resistance Clinical data demonstrate an increase of CD8+ T- cells and a reduction of T-regulatory cells Immune Modulation Current and Potential Future Opportunity Pre-clinical data support ability to address BAF mutated cancers (e.g., BRG1 mutant)Mutations

| First-in-Class Broad-Based Differentiation Agent With Significant Combination Potential in AML 9 Completed Phase I Monotherapy Safety and Efficacy Results Ongoing Phase I Combination Study Efficacy • Differentiation observed in heavily pre-treated patients, regardless of mutational status • Multiple patients with bone marrow and peripheral blast improvements and associated ANC recovery Safety • Adverse event profile consistent with late-line AML population • Most frequent ≥ grade 3 TRAEs: increased blood bilirubin, hypocalcemia, differentiation syndrome (DS), stomatitis, increased ALT • Adjudicated Differentiation Syndrome rate of 15% • Phase I dose escalation study evaluating oral daily dosing of FHD-286 with fixed dose decitabine or cytarabine • Standard 3+3 dose escalation design • Data anticipated in H2’2024

| Significant Opportunity for Novel, Effective Therapy in Patients with R/R AML 10 • More than half of patients will relapse post frontline treatment • Intensive chemotherapy has been standard of care for four decades with no meaningful improvement Most cases of AML are not curable 40% of AML cases have no actionable mutations • No meaningful developments for the broad AML patient population since the approval of Venetoclax • Recent development has focused predominantly on AML subsets harboring actionable mutations – FLT3, IDH1/2, and MLL** ̽ Source: Decision Resources Group 2025 Forecast: **Menin inhibitors not yet approved; R/R: relapsed/refractory; CRc: composite complete response • Post Ven/Aza, treatment options are limited – CRc rates 15-17% • Mortality remains high for this population, mOS ~3mo • Patients with actionable mutations who relapse post targeted therapy have high unmet need Initial FHD-286 Opportunity ~17,000 Drug Treatable R/R Patients* FHD-286 could provide a meaningful opportunity to improve outcomes in the R/R setting. We believe there is an additional opportunity in the newly diagnosed setting.

| Study Design for FHD-286 Phase 1 Multicenter Dose-Escalation in Combination with Decitabine in AML 11 Target Indication: • R/R AML Treatment Plan & Dose Escalation: • 3+3 escalation design • Oral FHD-286, QD, 28-day cycles • Standard decitabine dose schedule Key Objectives • Safety/Tolerability • Maximum Tolerated Dose (MTD) and/or Recommended Phase 2 Dose (RP2D) determinations Primary • Preliminary clinical activity • PK parameters of FHD-286 in combination with Decitabine in subjects on/off triazole antifungal agents classified as strong CYP3A4 inhibitors Secondary • PD effects of FHD-286 in combination with Decitabine • MRD Exploratory FHD-286: 1.5 mg QD + Decitabine FHD-286: 2.5 mg QD + Decitabine FHD-286: 5 mg QD + Decitabine FHD-286: 7.5 mg QD + Decitabine FHD-286: 2.5 mg QD + Decitabine FHD-286: 5 mg QD + Decitabine FHD-286: 7.5 mg QD + Decitabine Potential Next Steps: FHD-286 + Decitabine Expansion FHD-286 + Other Agent Escalation Subjects on a triazole antifungal agent classified as a strong CYP3A4 inhibitor Subjects NOT on a triazole antifungal agent classified as a strong CYP3A4 inhibitor Parallel Dose Escalations Potential additional escalations Potential additional escalations

| FHD-286 Demonstrated Differentiation Across a Broad Range of Genetic Backgrounds in Phase 1 Trial 12 Dose Level Mutations Cytogenetics Risk Starting CD11b% Max CD11b% CD11b+ Fold Change Starting CD34% Min CD34% CD34+ % Decrease 10mg N/A Adverse 7 62 9.2x 94 27 (71%) 7.5mg CBFB (locus at 16q22) 2 94 59.4x 70 2 (97%) 7.5mg KMT2A rearrangement Adverse 3 58 21.4x 85 9 (90%) 7.5mg RUNX1, KRAS, ASXL1, JAK2, TET2, EZH2, ETNK1 Adverse 5 73 15x 95 18 (81%) 7.5mg N/A Adverse 8 52 6.3x 94 33 (65%) 7.5mg ASXL1, TP53, U2AF1 Adverse 19 63 3.3x 92 51 (45%) 5mg RUNX1, NRAS, KRAS, SF3B1, ASXL2, CSF3R, GATA2 Adverse 3 74 29x 94 19 (80%) 5mg RUNX1, NRAS, ASLX1 Adverse 4 97 22.8x 98 7 (93%) 5mg N/A Adverse 6 79 13x 93 11 (88%) 5mg TET2, WT1 GATA2 PLCG2 ARHGEF28, BRD4, CDK12, DDX41, KMT20, PARP1, ZRSR2 3 24 8.1x 86 62 (27%) 5mg N/A Adverse 4 28 6.5x 93 66 (29%) 5mg DNMT3a, TET2 21 88 4.1x 30 4 (88%) 2.5mg NRAS, WT1 Adverse 3 13 4.8x 93 89 (4%) CD11b (marker of differentiation) increases CD34 (leukemic stem cell marker) decreases

| Bone Marrow Aspirate: Clear Evidence of Differentiation Bone Marrow Blast Reduction from 40% to 6% Signs of Differentiation in Heavily Pre-Treated, Secondary AML Patient with Abnormal Karyotype in Phase 1 Trial 13 Patient Background: • 47-year-old male, secondary AML • Abnormal karyotype: Del (7Q), Inv (3), Der (7;12), -8, ADD(1) Prior AML Treatment: • Progressive disease: 4 lines prior treatment and 2 bone marrow transplants Prior non-AML treatment: • MDS with inv(3) and der(7;12) and ASXL1 mut. Received AZA x 4. Initiation of FHD-286 at 10 MG Dose: • Bone marrow blast from 40% to 6% with clear evidence of differentiation with persistence of cytogenetics abnormalities. ANC recovery.

| Absolute Neutrophil Count (ANC) Peripheral Blast Count Clinical Benefit in Heavily Pre-Treated Patient in Phase 1 Trial 14 100% 0% 50% 10 5 10/13/21 10/24/21 11/4/21 11/14/21 11/25/21 12/6/21 0 Initiation of FHD 286 1st SCT 2nd SCT Patient Background: • 25-year-old male, treatment-related AML • KMT2A rearrangement Prior AML Treatment: • Progressive disease with CNS Leukemia: 7 lines prior treatment and 2 bone marrow transplants Prior non-AML treatment: • Ewing’s sarcoma: Treated with Chemo/RT/Surgery (VCR, doxo, cyclophos, ifos, etoposide) Initiation of FHD-286 at 10 MG Dose: • Drop in peripheral blast, 97% to 5% • Bone marrow reduction from 89% to 48%, with ANC recovery 5/23/19 11/22/19 5/22/20 5/23/2111/21/20 11/22/21 Initiation of FHD 286

| FHD-286 + decitabine (MLL-AFP + FLT3 TKD Luc/GFP PDX) Pre-Clinical Data Demonstrated Combination Potential with Multiple Agents in AML 15 FHD-286 + BET inhibitor (mtNPM1 + FLT3 ITD Luc/GFP AML PDX) FHD-286 + menin inhibitor (mtNPM1 + FLT3 ITD Luc/GFP AML PDX) FHD-286 + cytarabine (MV4, 11 FLT3 ITD CDX) SNDX-5613: Menin inhibitor ✱ ✱ ✱ ✱ ✱

Selective BRM Modulators For BRG1 Mutated Cancers FHD-286 is a Potent, Selective, Allosteric, Small Molecule Inhibitor of the BRG1 and BRM subunits of the BAF complex

| BRM Selective Inhibitor FHD-909 IND Submitted in Q2’24, BRM Selective Degrader Continues Late-Stage Pre-Clinical Development 17 BRM Selective Inhibitor (FHD-909) BRM Selective Degrader Biology Exploit the synthetic lethal relationship between BRM (SMARCA2) and mutated BRG1 (SMARCA4) Stage IND submitted in Q2’24 Advancing in parallel through late pre- clinical development Opportunity BRG1 mutated cancer including ~10% of NSCLC and up to 5% of all solid tumors Loxo@Lilly Partnership 50/50 global R&D cost share | 50/50 U.S. economics | tiered ex-U.S. royalties starting in the low double-digit range and escalating into the twenties

| BRM Selective Inhibition is a Promising Strategy to Exploit the Synthetic Lethal Relationship Between BRM and Mutated BRG1 18 No BRG1 Mutations BRG1 Mutation BRG1 Mutation and BRMi Cell Survival Cell Death Healthy Cells Cancer Cells BRG1 BRM BAF Complex BRM BAF Complex Mutant BRG1 BAF Complex BRMiBRG1 Precision medicine targeting synthetic lethal relationships is a proven clinical approach now used in multiple cancers (e.g., PARP inhibitors)

| BRG1 is Mutated in Up to 10% of NSCLC; Up to 5% of Solid Tumors 19 BRG1 mutated across a broad range of tumors Accounts for ~5% of solid tumors AACR GENIE via cBioPortal

| BRG1 mutated in up to 10% of NSCLC tumors, minimal overlap with other mutations2Overall Survival for SMARCA4wt vs SMARCA4mut1 Patients with NSCLC Harboring BRG1 Mutations Have Significantly Worse Clinical Outcomes and Define a High Unmet Need Patient Population 20 BRG1 10% KRAS 29% EGFR 26% ALK 7% RET 4% MET 7% 1. Alessi JV, et al., 2021; 2. TCGA via cBioPortal

| BRG1 WT BRG1 MUT 0.0001 0.001 0.01 0.1 1 A b so lu te IC 50 ( μ M ) NCI-H2122 CALU6 NCI-H2172 NCI-H441 NCI-H2170 NCI-H460 NCI-H1703 NCI-H358 NCI-H2009 NCI-H1944 NCI-H1299 A549 RERFLCAI NCI-H1693 NCI-H1568 NCI-H2023 NCI-H838 BRG1 (WT) BRG1 (MUT) FHD-909 Demonstrated Approximately 30-fold Selectivity Across 17 BRG1 (SMARCA4) Mutant and Wild-Type Cell Lines 21 Spread in potency for wild type versus mutant cell lines indicates 33-fold selectivity observed Selectivity = 33x Median IC50 (µM) 0.0932BRG1 WT 0.0028BRG1 MUT

| FHD-909 Monotherapy Demonstrated In Vivo Activity in H2126 BRG1 Mutant NSCLC Model; Well Tolerated 22 H2126 Reduction in Tumor Volume H2126 Body Weight Genetic Background: BRG1 W764R, TP53 E62*, STK11-/-, CDKN2A-/-, KEAP1 R272C NOTE: All doses were well tolerated. Dosing holidays were applied at the high dose, as appropriate T u m o r vo lu m e (m m 3 ) 0 7 14 21 28 80 90 100 110 120 Days of Dosing (Dose Start Day1)

| RERF-LC-AI Model FHD-909 Monotherapy Demonstrated 96% TGI in A549 and Tumor Stasis in RERF-LC-AI Mutant NSCLC Models 23 A549 Model NOTE: All doses were well tolerated. Dosing holidays were applied at the high dose, as appropriate BRG1 Q729fs / H736Y, KRAS G12S, STK11-/-, CDKN2A-/-, KEAP1 G333C BRG1 mut p.E1496*, TP53 p.Q104*, NF1 p.E1699* Genetic Background Genetic Background

| FHD-909 Monotherapy Demonstrated Regression in H1793 BRG1 Mutant NSCLC Models 24 H1793 Model • FHD 909 delivered across range of BRG1 mut xenograft models • Results ranging from impressive TGI to regression as monotherapy • All doses across all four models were well tolerated NOTE: All doses were well tolerated. Dosing holidays were applied at the high dose, as appropriate 0 7 14 21 28 100 200 300 400 500 Days of Dosing (Dose Start Day1) Vehicle Control FHD-909, 20mg/kg, BID, PO FHD-909, 40mg/kg, BID, PO FHD-909, 60mg/kg, BID, PO BRG1 E514*, TP53 R209* R273H, ARID1A C884*Genetic Background:
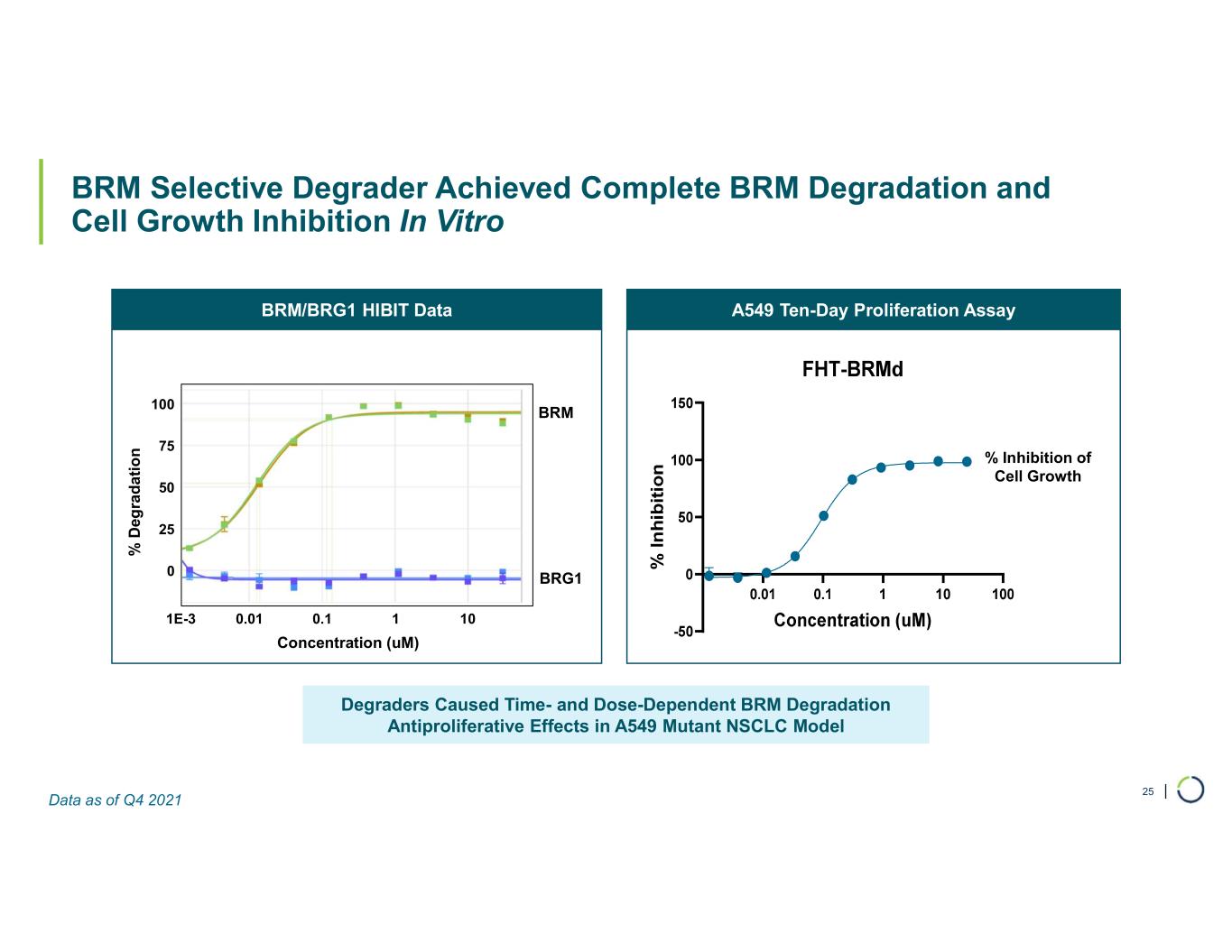
| BRM Selective Degrader Achieved Complete BRM Degradation and Cell Growth Inhibition In Vitro 25 Degraders Caused Time- and Dose-Dependent BRM Degradation Antiproliferative Effects in A549 Mutant NSCLC Model A549 Ten-Day Proliferation AssayBRM/BRG1 HIBIT Data BRM BRG1 % D eg ra d a ti o n Concentration (uM) % Inhibition of Cell Growth 0 25 50 75 100 1E-3 0.01 0.1 1 10 Data as of Q4 2021

Selective CBP Protein Degrader For EP300 Mutated Cancers

| Summary: Selective CBP Protein Degrader for EP300 Mutated Cancers 27* Per year incidence in the U.S., EU5, Japan Source: Clarivate RDG Mature Markets Data • CREB binding protein (CBP) • Targeted protein degrader Target / Approach • EP300 mutated cancers (e.g., subsets of bladder, colorectal, breast, gastric and lung cancers) Initial Indication • EP300 mutated cancers Mutation / Aberration • Pre-clinicalStage • Over 100,000 New Patients Impacted / Year* 10% 10% 8% 7% 6% 6% 5% 5% 3% 0% 5% 10% 15% Melanoma NSCLC Bladder Cancer Endometrial Gastric Breast Pancreatic Cancer Colorectal Cancer Commercial Opportunity SCCHN % of Patients with EP300 Mutation

| Colorectal RKO (EP300 Null) Model Selective CBP Degradation Resulted in Significant Tumor Growth Inhibition in Colorectal & Bladder in EP300 Null Models 28 Bladder 639V (EP300 Null) Model B o d y W ei g h t C h a n g e (% ) T u m o r V o lu m e ( m m ³ + S E M ) T u m o r V o lu m e ( m m ³ + S E M ) B o d y W ei g h t C h a n g e ( % )

| Selective CBP Degradation Resulted in Tumor Regression in Gastric EP300 Null Models 29 Gastric AGS (EP300 Null) Model B o d y w e ig h t C h a n g e ( % ) 20 30 40 50 60 0 500 1,000 1,500 Days After Tumor Inoculation T u m o r V o lu m e ( m m ³ + S E M ) Vehicle (BID) FHT-CBPd - 9, 10mg/kg (QD) FHT-CBPd - 9, 3mg/kg (QD) FHT-CBPd - 9, 1mg/kg (QD) FHT-CBPd - 9, 0.3mg/kg (QD) FHT-CBPd - 9, 0.1mg/kg (QD)
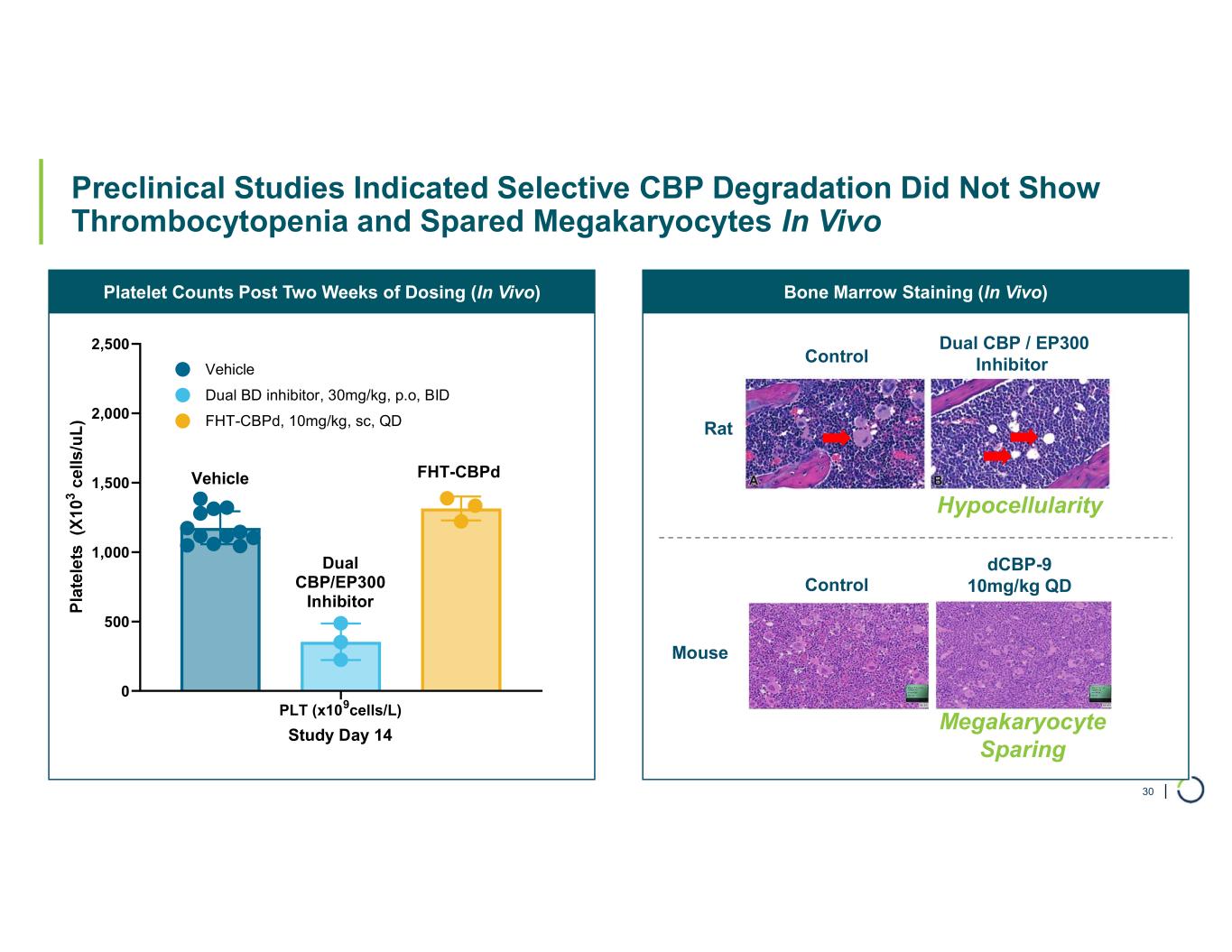
| Bone Marrow Staining (In Vivo)Platelet Counts Post Two Weeks of Dosing (In Vivo) Preclinical Studies Indicated Selective CBP Degradation Did Not Show Thrombocytopenia and Spared Megakaryocytes In Vivo 30 PLT (x109cells/L) 0 500 1,000 1,500 2,000 2,500 Study Day 14 Vehicle FHT-CBPd, 10mg/kg, sc, QD Dual BD inhibitor, 30mg/kg, p.o, BID FHT-CBPd Dual CBP/EP300 Inhibitor Vehicle Dual CBP / EP300 InhibitorControl Rat Control dCBP-9 10mg/kg QD Mouse Hypocellularity Megakaryocyte Sparing

| Preclinical Studied Indicated Long-Acting Injectable Formulations of CBP Degrader Could Enable Once Every 2 Weeks, or Less Frequent, Dosing 31 dCBP-9 PK/PD (60mg/kg, sc) dCBP-9 PK/PD (150mg/kg, sc) dCBP-9 PK/PD (150mg/kg, im) C B P L ev el s / % V e h ic le P la sm a P K (n g /m L )

Selective EP300 Protein Degrader For CBP Mutated and EP300 Dependent Cancers

| Summary: Selective EP300 Protein Degrader for CBP Mutant & EP300 Dependent Cancers 33 * Per year incidence in the U.S., EU5, Japan Source: Clarivate RDG Mature Markets Data • E1A binding protein p300 (EP300) • Targeted protein degrader Target / Approach • AR+ Prostate • DLBCL • Bladder, melanoma, others Initial Indications • EP300 dependent cancers • CBP mutant cancers Mutation / Aberration • Pre-clinicalStage • Over 100,000 New Patients Impacted / Year* Commercial Opportunity EP300 Dependent Cancers • Solid Tumors • AR+ mCRPC • HR+ breast • Hematologic malignancies • DLBCL • Multiple Myeloma CBP Mutant Cancers 10% 10% 9% 8% 8% 8% 6% 0% 5% 10% 15% Melanoma Bladder NSCLC Endometrial Colorectal Gastric Breast % of Patients with CBP Mutation

| EP300 Degradation Resulted in Significant Tumor Growth Inhibition in AR+ VCAP Prostate and KARPAS422 DLBCL Models 34 Prostate AR+ VCAP Model DLBCL KARPAS422 (CBP Null) Model 5 10 15 20 25 30 35 40 45 50 0 500 1,000 1,500 2,000 Days After Tumor Inoculation T u m o r V o lu m e ( m m ³ + S E M ) Vehicle Control Enzalutamide, 30mg/kg, QD FHT-EP300d, 50mg/kg, BID 10 20 30 40 50 -20 -15 -10 -5 0 5 10 15 20 Days After Tumor Inoculation B o d y W ei g h t C h a n g e ( % ) B o d y W ei g h t C h a n g e ( % ) T u m o r V o lu m e ( m m ³ + S E M )

| Selective EP300 Degradation Does Not Show Thrombocytopenia In Vivo 35 Platelet Counts Post Two Weeks of Dosing (In-Vivo) PLT (x109cells/L) 0 500 1,000 1,500 2,000 2,500 Study Day 14 Vehicle FHT-EP300d, 50mg/kg, sc, BID Dual BD inhibitor, 30mg/kg, p.o, BID FHT-EP300d Dual CBP/EP300 Inhibitor Vehicle

Selective ARID1B Protein Degrader For ARID1A Mutated Cancers

| ARID1B is a Major Synthetic Lethal Target Implicated in Up To 5% of All Solid Tumors 37* Per year incidence in the U.S., EU5, Japan Source: Clarivate RDG Mature Markets Data • ARID1B • Targeted protein degrader Target / Approach • ARID1A mutated cancersInitial Indication • ARID1A mutations (e.g., ovarian, endometrial, colorectal, bladder and other cancers) Mutation / Aberration • Pre-clinicalStage • > 175,000 New Patients Impacted / Year* ARID1A ARID1B ~5% of all solid tumors harbor ARID1A mutations Uterine Bladder Stomach Cholangiocarcinoma Liver Esophageal Ovarian Colorectal Melanoma 0% 10% 20% 30% 40% Commercial Opportunity % of Patients with ARID1A Mutation

| Compound Screening and Structure-Based Optimization Yields Selective ARID1B Binders 38 • Mapped and purified several potential ligandable regions of ARID, which were then screened against various compound libraries • Characterized binding using multiple biochemical and biophysical techniques: e.g., DSF, ASMS, NMR, and SPR • Determined X-ray crystal structure of ARID ligandable domains with specific binders • Leveraged these structures to drive binding affinities and expand binding chemotypes Identification of Selective ARID1B Binders X-Ray Crystal Structures Detail Selective ARID1B Binding

| Structure-Based Optimization Drives Improved ARID1B Binding Affinity from 100 uM to less than 200 nM 39 1.4 Å co-xtal structure 2.0 Å soak structure 1.7 Å soak structure ARIDb-2 ARID1B Kd: 15 uM ARIDb-1 ARID1B Kd: 100 uM ARIDb-3 ARID1B Kd: 0.5 uM ARIDb-9 ARID1B Kd: 0.2 uM 1.9 Å co-xtal structure Gen 1: Screening Hit Gen 2: Early Optimization Gen 3: Sub-uM Affinity

Transcription Factors A Novel Approach FHD-609 is a Selective, Potent, Protein Degrader of the BRD9 component of the BAF complex

| Foghorn’s Novel Approach to Drugging Transcription Factors Enabled by Its Protein Production and Discovery Capabilities 41 • Highly involved in gene expression • Implicated in range of cancers and other diseases • Featureless surface: no druggable binding pocket • Tight interactions with DNA: undruggable affinities • Druggable binding pockets • Druggable affinities Transcription Factors are Compelling Drug Targets… …But Historically Difficult to Target… Foghorn Has a New Approach Focusing on Interaction with BAF HISTORICAL FOCUS POTENTIAL DRUGGABLE SITES FOGHORN’S FOCUS
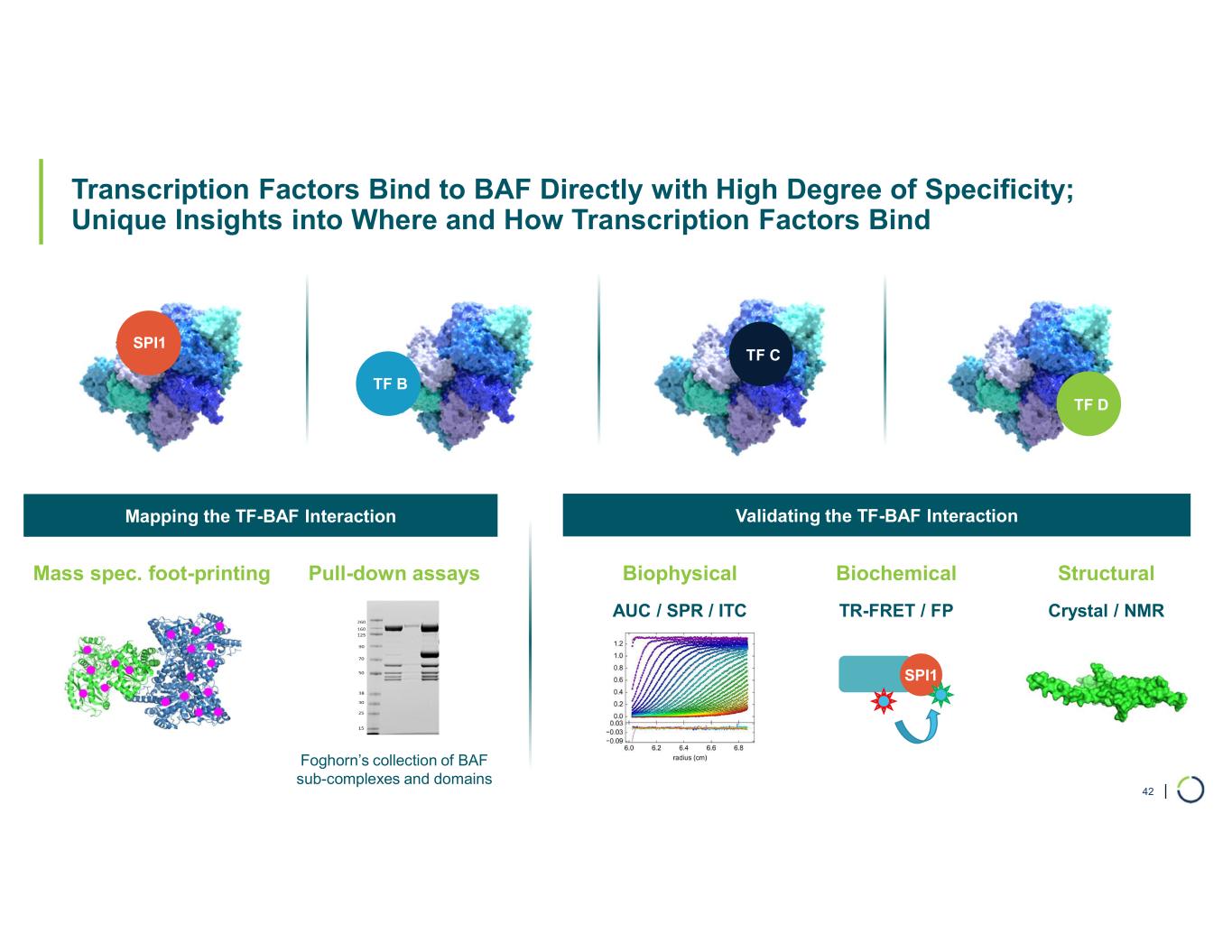
| Transcription Factors Bind to BAF Directly with High Degree of Specificity; Unique Insights into Where and How Transcription Factors Bind 42 Mass spec. foot-printing Pull-down assays Foghorn’s collection of BAF sub-complexes and domains Biophysical Biochemical Structural AUC / SPR / ITC TR-FRET / FP Crystal / NMR SPI1 Mapping the TF-BAF Interaction Validating the TF-BAF Interaction SPI1 TF B TF C TF D

| Foghorn Is Advancing a Pipeline of First-in-Class Precision Therapeutics with Potential for Broad Application in Oncology 43 Modality Program Phase 1 Enzyme Inhibitors Transcription Factor Disruptors Undisclosed Protein Degraders Commercial Rights FHD-286 (BRG1/BRM) FHD-909 (Selective BRM) 3 Discovery Programs Undisclosed Partnered Undisclosed Disease BRG1 mutant cancers (e.g., NSCLC, bladder, endometrial, colorectal) Relapsed/Refractory AML Selective BRM BRG1 mutant cancers (e.g., NSCLC, bladder, endometrial, colorectal) Selective ARID1B ARID1A mutant cancers (e.g., ovarian, endometrial, colorectal) Selective CBP EP300 mutant cancers (e.g., bladder, gastric, breast, NSCLC, colorectal) Selective EP300 EP300 dependent cancers (e.g., prostate, DLBCL), CBP mutant cancers (e.g., NSCLC, bladder) Undisclosed Undisclosed Undisclosed Pre-Clinical Phase 2 / 3Discovery

| Developing First-In-Class Precision Medicines Targeting Major Unmet Needs in Cancer 44 Large Market Potential Chromatin biology is implicated in up to 50% of tumors, potentially impacting ~2.5 million patients Foghorn’s current pipeline potentially addresses more than 500,000 of these patients Broad pipeline across a range of targets and small molecule modalities Major Strategic Collaboration Strategic collaboration with Loxo@Lilly; $380 million upfront; 50/50 U.S. economic split on two lead programs Well- Funded $206.7 million in cash and equivalents (as of 3/31/2024) Provides runway into H1’26 Value Drivers Anticipate data from the Phase 1 study of FHD-286 in combination with decitabine in H2’24 BRM Selective Inhibitor (FHD-909), partnered with Loxo@Lilly, IND submitted to FDA, Phase 1 initiation anticipated in H2’24 Advancement of preclinical assets (BRM Selective Degrader, CBP, EP300, ARID1B) towards INDs Leader in Unique Area of Cancer Biology Foghorn is a leader in targeting chromatin biology, which has the potential to address underlying dependencies of many genetically defined cancers Platform with initial focus in oncology, therapeutic area expansion potential











































