2023FYfalse0000009326P3Y0.66660.6666http://fasb.org/us-gaap/2023#OtherComprehensiveIncomeLossCashFlowHedgeGainLossAfterReclassificationAndTaxParenthttp://fasb.org/us-gaap/2023#OtherComprehensiveIncomeLossCashFlowHedgeGainLossAfterReclassificationAndTaxParenthttp://fasb.org/us-gaap/2023#OtherComprehensiveIncomeLossCashFlowHedgeGainLossAfterReclassificationAndTaxParenthttp://fasb.org/us-gaap/2023#OtherComprehensiveIncomeDefinedBenefitPlansNetUnamortizedGainLossArisingDuringPeriodNetOfTaxhttp://fasb.org/us-gaap/2023#OtherComprehensiveIncomeDefinedBenefitPlansNetUnamortizedGainLossArisingDuringPeriodNetOfTaxhttp://fasb.org/us-gaap/2023#OtherComprehensiveIncomeDefinedBenefitPlansNetUnamortizedGainLossArisingDuringPeriodNetOfTax00000093262023-01-012023-12-3100000093262023-06-30iso4217:USD00000093262024-02-02xbrli:shares00000093262023-12-3100000093262022-12-31iso4217:USDxbrli:shares00000093262022-01-012022-12-3100000093262021-01-012021-12-3100000093262020-12-310000009326us-gaap:RetainedEarningsMember2020-12-310000009326us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-310000009326us-gaap:CommonStockMember2020-12-310000009326us-gaap:AdditionalPaidInCapitalMember2020-12-310000009326us-gaap:RetainedEarningsMember2021-01-012021-12-310000009326us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-01-012021-12-3100000093262021-12-310000009326us-gaap:CommonStockMember2021-01-012021-12-310000009326us-gaap:AdditionalPaidInCapitalMember2021-01-012021-12-310000009326us-gaap:RetainedEarningsMember2021-12-310000009326us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310000009326us-gaap:CommonStockMember2021-12-310000009326us-gaap:AdditionalPaidInCapitalMember2021-12-310000009326us-gaap:RetainedEarningsMember2022-01-012022-12-310000009326us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310000009326us-gaap:CommonStockMember2022-01-012022-12-310000009326us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310000009326us-gaap:RetainedEarningsMember2022-12-310000009326us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310000009326us-gaap:CommonStockMember2022-12-310000009326us-gaap:AdditionalPaidInCapitalMember2022-12-310000009326us-gaap:RetainedEarningsMember2023-01-012023-12-310000009326us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310000009326us-gaap:CommonStockMember2023-01-012023-12-310000009326us-gaap:AdditionalPaidInCapitalMember2023-01-012023-12-310000009326us-gaap:RetainedEarningsMember2023-12-310000009326us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310000009326us-gaap:CommonStockMember2023-12-310000009326us-gaap:AdditionalPaidInCapitalMember2023-12-310000009326us-gaap:BuildingMembersrt:MinimumMember2023-12-310000009326srt:MaximumMemberus-gaap:BuildingMember2023-12-310000009326us-gaap:EquipmentMembersrt:MinimumMember2023-12-310000009326srt:MaximumMemberus-gaap:EquipmentMember2023-12-310000009326bcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2022-01-012022-12-310000009326bcpc:CardinalAssociatesIncBergstromMember2022-01-012022-12-310000009326bcpc:CardinalAssociatesIncBergstromMember2023-01-012023-12-310000009326bcpc:HumanNutritionAndHealthMember2023-12-310000009326bcpc:HumanNutritionAndHealthMember2022-12-310000009326bcpc:AnimalNutritionAndHealthMember2023-12-310000009326bcpc:AnimalNutritionAndHealthMember2022-12-310000009326bcpc:SpecialtyProductsMember2023-12-310000009326bcpc:SpecialtyProductsMember2022-12-310000009326bcpc:IndustrialProductsMember2023-12-310000009326bcpc:IndustrialProductsMember2022-12-310000009326us-gaap:CustomerListsMembersrt:MinimumMember2023-12-310000009326us-gaap:CustomerListsMembersrt:MaximumMember2023-12-310000009326us-gaap:TrademarksAndTradeNamesMembersrt:MinimumMember2023-12-310000009326us-gaap:TrademarksAndTradeNamesMembersrt:MaximumMember2023-12-310000009326us-gaap:DevelopedTechnologyRightsMembersrt:MinimumMember2023-12-310000009326us-gaap:DevelopedTechnologyRightsMembersrt:MaximumMember2023-12-310000009326bcpc:RegulatoryRegistrationCostsMembersrt:MinimumMember2023-12-310000009326srt:MaximumMemberbcpc:RegulatoryRegistrationCostsMember2023-12-310000009326bcpc:PatentsTradeSecretsMembersrt:MinimumMember2023-12-310000009326bcpc:PatentsTradeSecretsMembersrt:MaximumMember2023-12-310000009326bcpc:OtherIntangibleAssetsExcludingRegulatoryRegistrationCostsAndPatentsTradeSecretsMembersrt:MinimumMember2023-12-310000009326bcpc:OtherIntangibleAssetsExcludingRegulatoryRegistrationCostsAndPatentsTradeSecretsMembersrt:MaximumMember2023-12-310000009326bcpc:CardinalAssociatesIncBergstromMember2022-08-302022-08-300000009326bcpc:CardinalAssociatesIncBergstromMemberbcpc:FormerShareholdersMember2022-08-302022-08-300000009326bcpc:FormerBankMemberbcpc:CardinalAssociatesIncBergstromMember2022-08-302022-08-300000009326bcpc:CardinalAssociatesIncBergstromMember2023-12-310000009326bcpc:CardinalAssociatesIncBergstromMember2023-07-012023-09-300000009326bcpc:CardinalAssociatesIncBergstromMember2022-08-300000009326bcpc:HumanNutritionAndHealthMember2022-08-302022-08-30xbrli:pure0000009326bcpc:AnimalNutritionAndHealthMember2022-08-302022-08-300000009326us-gaap:CustomerRelationshipsMemberbcpc:CardinalAssociatesIncBergstromMember2022-08-300000009326us-gaap:DevelopedTechnologyRightsMemberbcpc:CardinalAssociatesIncBergstromMember2022-08-300000009326bcpc:CardinalAssociatesIncBergstromMemberus-gaap:TrademarksMember2022-08-300000009326us-gaap:CustomerRelationshipsMemberbcpc:CardinalAssociatesIncBergstromMember2022-08-302022-08-300000009326bcpc:CorporateTrademarkMemberbcpc:CardinalAssociatesIncBergstromMember2022-08-302022-08-300000009326bcpc:ProductTrademarksMemberbcpc:CardinalAssociatesIncBergstromMember2022-08-302022-08-300000009326us-gaap:DevelopedTechnologyRightsMemberbcpc:CardinalAssociatesIncBergstromMember2022-08-302022-08-300000009326bcpc:CardinalAssociatesIncBergstromMember2021-01-012021-12-310000009326bcpc:CardinalAssociatesIncBergstromMember2023-10-012023-12-310000009326bcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2022-06-212022-06-21iso4217:NOK0000009326bcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMemberbcpc:FormerShareholdersMember2022-06-212022-06-210000009326bcpc:FormerShareholdersLendersAndCreditorsMemberbcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2022-06-212022-06-210000009326bcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2022-12-310000009326bcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2022-06-210000009326us-gaap:CustomerRelationshipsMemberbcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2022-06-210000009326us-gaap:DevelopedTechnologyRightsMemberbcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2022-06-210000009326bcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMemberus-gaap:TrademarksMember2022-06-210000009326us-gaap:CustomerRelationshipsMemberbcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2022-06-212022-06-210000009326bcpc:CorporateTrademarkMemberbcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2022-06-212022-06-210000009326bcpc:ProductTrademarksMemberbcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2022-06-212022-06-210000009326us-gaap:DevelopedTechnologyRightsMemberbcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2022-06-212022-06-210000009326bcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2023-01-012023-12-310000009326bcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2021-01-012021-12-310000009326bcpc:KechuBidCoASAndItsSubsidiaryCompaniesKappaMember2023-10-012023-12-310000009326us-gaap:CostOfSalesMember2023-01-012023-12-310000009326us-gaap:CostOfSalesMember2022-01-012022-12-310000009326us-gaap:CostOfSalesMember2021-01-012021-12-310000009326us-gaap:OperatingExpenseMember2023-01-012023-12-310000009326us-gaap:OperatingExpenseMember2022-01-012022-12-310000009326us-gaap:OperatingExpenseMember2021-01-012021-12-31bcpc:plan0000009326bcpc:OmnibusIncentivePlan2017Member2023-12-310000009326bcpc:OmnibusIncentivePlan2017Member2023-06-222023-06-220000009326bcpc:OmnibusIncentivePlan2017Member2023-01-012023-12-310000009326bcpc:OmnibusIncentivePlan2017Memberus-gaap:EmployeeStockOptionMember2023-12-310000009326us-gaap:ShareBasedPaymentArrangementNonemployeeMemberbcpc:OmnibusIncentivePlan2017Member2023-01-012023-12-310000009326us-gaap:PerformanceSharesMemberus-gaap:ShareBasedPaymentArrangementEmployeeMember2023-01-012023-12-310000009326us-gaap:EmployeeStockOptionMember2023-01-012023-12-310000009326us-gaap:EmployeeStockOptionMember2022-01-012022-12-310000009326us-gaap:EmployeeStockOptionMember2021-01-012021-12-310000009326us-gaap:PerformanceSharesMemberus-gaap:ShareBasedPaymentArrangementEmployeeMember2022-01-012022-12-310000009326us-gaap:PerformanceSharesMemberus-gaap:ShareBasedPaymentArrangementEmployeeMember2021-01-012021-12-310000009326srt:MinimumMemberus-gaap:EmployeeStockOptionMember2023-01-012023-12-310000009326srt:MaximumMemberus-gaap:EmployeeStockOptionMember2023-01-012023-12-310000009326us-gaap:ShareBasedPaymentArrangementEmployeeMemberus-gaap:RestrictedStockMember2023-01-012023-12-310000009326us-gaap:ShareBasedPaymentArrangementNonemployeeMemberus-gaap:RestrictedStockMember2023-01-012023-12-310000009326bcpc:ExercisePriceRange1Member2023-01-012023-12-310000009326bcpc:ExercisePriceRange1Member2023-12-310000009326bcpc:ExercisePriceRange2Member2023-01-012023-12-310000009326bcpc:ExercisePriceRange2Member2023-12-310000009326bcpc:ExercisePriceRange3Member2023-01-012023-12-310000009326bcpc:ExercisePriceRange3Member2023-12-310000009326us-gaap:RestrictedStockMember2022-12-310000009326us-gaap:RestrictedStockMember2021-12-310000009326us-gaap:RestrictedStockMember2020-12-310000009326us-gaap:RestrictedStockMember2023-01-012023-12-310000009326us-gaap:RestrictedStockMember2022-01-012022-12-310000009326us-gaap:RestrictedStockMember2021-01-012021-12-310000009326us-gaap:RestrictedStockMember2023-12-310000009326us-gaap:PerformanceSharesMember2022-12-310000009326us-gaap:PerformanceSharesMember2021-12-310000009326us-gaap:PerformanceSharesMember2020-12-310000009326us-gaap:PerformanceSharesMember2023-01-012023-12-310000009326us-gaap:PerformanceSharesMember2022-01-012022-12-310000009326us-gaap:PerformanceSharesMember2021-01-012021-12-310000009326us-gaap:PerformanceSharesMember2023-12-310000009326us-gaap:LandMember2023-12-310000009326us-gaap:LandMember2022-12-310000009326us-gaap:BuildingMember2023-12-310000009326us-gaap:BuildingMember2022-12-310000009326us-gaap:EquipmentMember2023-12-310000009326us-gaap:EquipmentMember2022-12-310000009326us-gaap:ConstructionInProgressMember2023-12-310000009326us-gaap:ConstructionInProgressMember2022-12-310000009326srt:NorthAmericaMember2023-12-310000009326srt:NorthAmericaMember2022-12-310000009326srt:EuropeMember2023-12-310000009326srt:EuropeMember2022-12-310000009326us-gaap:CustomerListsMember2023-12-310000009326us-gaap:CustomerListsMember2022-12-310000009326us-gaap:TrademarksAndTradeNamesMember2023-12-310000009326us-gaap:TrademarksAndTradeNamesMember2022-12-310000009326us-gaap:DevelopedTechnologyRightsMember2023-12-310000009326us-gaap:DevelopedTechnologyRightsMember2022-12-310000009326us-gaap:OtherIntangibleAssetsMembersrt:MinimumMember2023-12-310000009326srt:MaximumMemberus-gaap:OtherIntangibleAssetsMember2023-12-310000009326us-gaap:OtherIntangibleAssetsMember2023-12-310000009326us-gaap:OtherIntangibleAssetsMember2022-12-310000009326bcpc:StGabrielCCCompanyLLCMember2013-12-310000009326bcpc:EastmanChemicalCompanyMemberbcpc:StGabrielCCCompanyLLCMember2013-12-31bcpc:vote0000009326bcpc:StGabrielCCCompanyLLCMember2023-01-012023-12-310000009326bcpc:StGabrielCCCompanyLLCMember2022-01-012022-12-310000009326bcpc:StGabrielCCCompanyLLCMember2021-01-012021-12-310000009326bcpc:StGabrielCCCompanyLLCMember2023-12-310000009326bcpc:StGabrielCCCompanyLLCMember2022-12-310000009326us-gaap:RevolvingCreditFacilityMemberbcpc:A2018CreditAgreementMember2018-06-270000009326us-gaap:RevolvingCreditFacilityMemberbcpc:A2018CreditAgreementMember2022-04-012022-06-300000009326us-gaap:RevolvingCreditFacilityMemberbcpc:A2018CreditAgreementMember2022-07-270000009326us-gaap:RevolvingCreditFacilityMemberbcpc:A2018CreditAgreementMember2022-07-272022-07-270000009326us-gaap:RevolvingCreditFacilityMemberbcpc:A2018CreditAgreementMember2022-07-012022-09-300000009326us-gaap:RevolvingCreditFacilityMemberbcpc:A2018CreditAgreementMember2023-12-310000009326us-gaap:RevolvingCreditFacilityMemberbcpc:A2018CreditAgreementMember2022-12-310000009326us-gaap:RevolvingCreditFacilityMemberbcpc:A2018CreditAgreementMember2023-01-012023-12-310000009326bcpc:RevolvingCreditAgreementMember2023-12-310000009326srt:MinimumMemberbcpc:RevolvingCreditAgreementMember2023-01-012023-12-310000009326srt:MaximumMemberbcpc:RevolvingCreditAgreementMember2023-01-012023-12-310000009326bcpc:RevolvingCreditAgreementMember2023-01-012023-12-310000009326us-gaap:StateAndLocalJurisdictionMember2023-12-31bcpc:segment0000009326us-gaap:OperatingSegmentsMemberbcpc:HumanNutritionAndHealthMember2023-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:HumanNutritionAndHealthMember2022-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:AnimalNutritionAndHealthMember2023-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:AnimalNutritionAndHealthMember2022-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:SpecialtyProductsMember2023-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:SpecialtyProductsMember2022-12-310000009326us-gaap:CorporateNonSegmentMember2023-12-310000009326us-gaap:CorporateNonSegmentMember2022-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:HumanNutritionAndHealthMember2023-01-012023-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:HumanNutritionAndHealthMember2022-01-012022-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:HumanNutritionAndHealthMember2021-01-012021-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:AnimalNutritionAndHealthMember2023-01-012023-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:AnimalNutritionAndHealthMember2022-01-012022-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:AnimalNutritionAndHealthMember2021-01-012021-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:SpecialtyProductsMember2023-01-012023-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:SpecialtyProductsMember2022-01-012022-12-310000009326us-gaap:OperatingSegmentsMemberbcpc:SpecialtyProductsMember2021-01-012021-12-310000009326us-gaap:CorporateNonSegmentMember2023-01-012023-12-310000009326us-gaap:CorporateNonSegmentMember2022-01-012022-12-310000009326us-gaap:CorporateNonSegmentMember2021-01-012021-12-310000009326us-gaap:MaterialReconcilingItemsMember2023-01-012023-12-310000009326us-gaap:MaterialReconcilingItemsMember2022-01-012022-12-310000009326us-gaap:MaterialReconcilingItemsMember2021-01-012021-12-310000009326bcpc:ProductSalesMember2023-01-012023-12-310000009326bcpc:ProductSalesMember2022-01-012022-12-310000009326bcpc:ProductSalesMember2021-01-012021-12-310000009326us-gaap:RoyaltyMember2023-01-012023-12-310000009326us-gaap:RoyaltyMember2022-01-012022-12-310000009326us-gaap:RoyaltyMember2021-01-012021-12-310000009326country:US2023-01-012023-12-310000009326country:US2022-01-012022-12-310000009326country:US2021-01-012021-12-310000009326us-gaap:NonUsMember2023-01-012023-12-310000009326us-gaap:NonUsMember2022-01-012022-12-310000009326us-gaap:NonUsMember2021-01-012021-12-310000009326us-gaap:CurrencySwapMember2023-01-012023-12-310000009326us-gaap:CurrencySwapMember2022-01-012022-12-310000009326us-gaap:CurrencySwapMember2021-01-012021-12-310000009326us-gaap:AccumulatedTranslationAdjustmentMember2022-12-310000009326us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2022-12-310000009326us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-12-310000009326us-gaap:AccumulatedTranslationAdjustmentMember2023-01-012023-12-310000009326us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2023-01-012023-12-310000009326us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-01-012023-12-310000009326us-gaap:AccumulatedTranslationAdjustmentMember2023-12-310000009326us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2023-12-310000009326us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-12-3100000093262021-01-012021-01-010000009326us-gaap:DefinedBenefitPostretirementHealthCoverageMember2022-12-310000009326us-gaap:DefinedBenefitPostretirementHealthCoverageMember2021-12-310000009326us-gaap:DefinedBenefitPostretirementHealthCoverageMember2023-01-012023-12-310000009326us-gaap:DefinedBenefitPostretirementHealthCoverageMember2022-01-012022-12-310000009326us-gaap:DefinedBenefitPostretirementHealthCoverageMember2023-12-310000009326us-gaap:DefinedBenefitPostretirementHealthCoverageMember2021-01-012021-12-310000009326bcpc:CentralStatesSoutheastAndSouthwestAreasPensionFundMember2023-01-012023-12-310000009326bcpc:CentralStatesSoutheastAndSouthwestAreasPensionFundMember2022-01-012022-12-310000009326bcpc:CentralStatesSoutheastAndSouthwestAreasPensionFundMember2021-01-012021-12-310000009326bcpc:ChemogasDefinedPensionPlanMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310000009326bcpc:ChemogasDefinedPensionPlanMemberus-gaap:PensionPlansDefinedBenefitMember2021-12-310000009326bcpc:ChemogasDefinedPensionPlanMemberus-gaap:PensionPlansDefinedBenefitMember2023-01-012023-12-310000009326bcpc:ChemogasDefinedPensionPlanMemberus-gaap:PensionPlansDefinedBenefitMember2022-01-012022-12-310000009326bcpc:ChemogasDefinedPensionPlanMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310000009326us-gaap:PensionPlansDefinedBenefitMember2023-12-310000009326us-gaap:PensionPlansDefinedBenefitMember2022-12-310000009326bcpc:ChemogasDefinedPensionPlanMemberus-gaap:PensionPlansDefinedBenefitMember2021-01-012021-12-310000009326us-gaap:FairValueInputsLevel1Member2023-12-310000009326us-gaap:FairValueInputsLevel1Member2022-12-31bcpc:financial_instrument0000009326us-gaap:MoneyMarketFundsMember2023-12-310000009326us-gaap:MoneyMarketFundsMember2022-12-310000009326us-gaap:CurrencySwapMember2022-12-310000009326us-gaap:InterestRateSwapMember2022-12-310000009326us-gaap:CorporateJointVentureMemberbcpc:ServicesProvidedMember2023-01-012023-12-310000009326us-gaap:CorporateJointVentureMemberbcpc:ServicesProvidedMember2022-01-012022-12-310000009326us-gaap:CorporateJointVentureMemberbcpc:ServicesProvidedMember2021-01-012021-12-310000009326us-gaap:CorporateJointVentureMemberbcpc:RawMaterialsSoldMember2023-01-012023-12-310000009326us-gaap:CorporateJointVentureMemberbcpc:RawMaterialsSoldMember2022-01-012022-12-310000009326us-gaap:CorporateJointVentureMemberbcpc:RawMaterialsSoldMember2021-01-012021-12-310000009326us-gaap:CorporateJointVentureMember2023-01-012023-12-310000009326us-gaap:CorporateJointVentureMember2022-01-012022-12-310000009326us-gaap:CorporateJointVentureMember2021-01-012021-12-310000009326us-gaap:CorporateJointVentureMember2023-12-310000009326us-gaap:CorporateJointVentureMember2022-12-310000009326bcpc:NonContractualMoniesOwedMemberus-gaap:CorporateJointVentureMember2023-12-310000009326bcpc:NonContractualMoniesOwedMemberus-gaap:CorporateJointVentureMember2022-12-31bcpc:tranche0000009326bcpc:LesseeOperatingLeaseTrancheOneMembersrt:MinimumMember2023-12-310000009326bcpc:LesseeOperatingLeaseTrancheOneMembersrt:MaximumMember2023-12-310000009326srt:MinimumMemberbcpc:LesseeOperatingLeaseTrancheTwoMember2023-12-310000009326srt:MaximumMemberbcpc:LesseeOperatingLeaseTrancheTwoMember2023-12-310000009326bcpc:LesseeOperatingLeaseTrancheThreeMembersrt:MinimumMember2023-12-310000009326srt:MaximumMemberbcpc:LesseeOperatingLeaseTrancheThreeMember2023-12-310000009326bcpc:LesseeOperatingLeaseTrancheFourMember2023-12-310000009326bcpc:LesseeOperatingLeaseTrancheFourMembersrt:MinimumMember2023-12-310000009326srt:MaximumMemberbcpc:LesseeOperatingLeaseTrancheFourMember2023-12-310000009326us-gaap:InterestRateSwapMemberus-gaap:DesignatedAsHedgingInstrumentMemberbcpc:PayFixedInterestRateMember2019-05-280000009326us-gaap:InterestRateSwapMemberus-gaap:DesignatedAsHedgingInstrumentMember2019-05-280000009326us-gaap:InterestRateSwapMemberus-gaap:InterestExpenseMember2023-01-012023-12-310000009326us-gaap:InterestRateSwapMemberus-gaap:InterestExpenseMember2022-01-012022-12-310000009326us-gaap:InterestRateSwapMemberus-gaap:InterestExpenseMember2021-01-012021-12-310000009326us-gaap:DesignatedAsHedgingInstrumentMemberbcpc:PayFixedInterestRateMemberus-gaap:CurrencySwapMember2019-05-280000009326bcpc:ReceiveFixedInterestRateMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMember2019-05-280000009326us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMember2019-05-280000009326us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:InterestExpenseMember2023-01-012023-12-310000009326us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:InterestExpenseMember2022-01-012022-12-310000009326us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:InterestExpenseMember2021-01-012021-12-310000009326us-gaap:InterestRateSwapMember2023-01-012023-12-310000009326us-gaap:InterestRateSwapMember2022-01-012022-12-310000009326us-gaap:InterestRateSwapMember2021-01-012021-12-310000009326us-gaap:ForwardContractsMember2022-06-21bcpc:derivative0000009326us-gaap:ForwardContractsMember2023-01-012023-12-310000009326us-gaap:ForwardContractsMember2023-12-310000009326us-gaap:ForwardContractsMember2022-12-3100000093262023-01-012023-03-3100000093262023-04-012023-06-3000000093262023-07-012023-09-3000000093262023-10-012023-12-3100000093262022-01-012022-03-3100000093262022-04-012022-06-3000000093262022-07-012022-09-3000000093262022-10-012022-12-310000009326us-gaap:AllowanceForCreditLossMember2020-12-310000009326us-gaap:InventoryValuationReserveMember2020-12-310000009326us-gaap:AllowanceForCreditLossMember2021-01-012021-12-310000009326us-gaap:InventoryValuationReserveMember2021-01-012021-12-310000009326us-gaap:AllowanceForCreditLossMember2021-12-310000009326us-gaap:InventoryValuationReserveMember2021-12-310000009326us-gaap:AllowanceForCreditLossMember2022-01-012022-12-310000009326us-gaap:InventoryValuationReserveMember2022-01-012022-12-310000009326us-gaap:AllowanceForCreditLossMember2022-12-310000009326us-gaap:InventoryValuationReserveMember2022-12-310000009326us-gaap:AllowanceForCreditLossMember2023-01-012023-12-310000009326us-gaap:InventoryValuationReserveMember2023-01-012023-12-310000009326us-gaap:AllowanceForCreditLossMember2023-12-310000009326us-gaap:InventoryValuationReserveMember2023-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
|
|
|
|
|
| ☑ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR SECTION 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended December 31, 2023 |
|
OR |
| ☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from _____ to _____ |
Commission file number: 1-13648
_______________________________________________________________________________________________________________
Balchem Corporation
(Exact name of Registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
| Maryland |
|
13-2578432 |
| (State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification Number) |
|
|
|
|
|
|
|
|
|
5 Paragon Drive, Montvale, NJ 07645 |
| (Address of principal executive offices) (Zip Code) |
Registrant’s telephone number, including area code: (845) 326-5600 |
|
| Securities registered pursuant to Section 12(b) of the Act: |
|
|
|
| Title of each class |
Trading symbol |
Name of each exchange on which registered |
| Common Stock, par value $.06-2/3 per share |
BCPC |
The Nasdaq Stock Market LLC |
|
|
|
| Securities registered pursuant to Section 12(g) of the Act: None |
Indicate by check mark whether the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☑ No ☐
Indicate by check mark whether the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ☐ No ☑
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes ☑ No ☐
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
|
|
|
|
|
| (Check one): |
Large accelerated filer ☑ |
Accelerated filer ☐ |
|
| |
Non-accelerated filer ☐ |
Smaller reporting company ☐ |
Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☑
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant's executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☑
The aggregate market value of the common stock, par value $.06-2/3 per share (the “Common Stock”), issued and outstanding and held by non-affiliates of the Registrant, based upon the closing price for the Common Stock on the NASDAQ Stock Market LLC on June 30, 2023 was approximately $4,321,000,000. For purposes of this calculation, shares of the Registrant held by directors and officers of the Registrant and under the Registrant’s 401(k)/profit sharing plan have been excluded.
The number of shares outstanding of Common Stock was 32,266,941 as of February 2, 2024.
DOCUMENTS INCORPORATED BY REFERENCE
Selected portions of the Registrant’s proxy statement for its 2024 Annual Meeting of Shareholders (the “2024 Proxy Statement”) to be filed with the Securities and Exchange Commission pursuant to Regulation 14A within 120 days after Registrant’s fiscal year-end of December 31, 2023 are incorporated by reference in Part III of this Annual Report on Form 10-K to the extent stated therein.
Cautionary Statement Regarding Forward-Looking Statements
Certain statements in this Annual Report on Form 10-K, other than purely historical information, are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements are not statements of historical facts, but rather reflect our current expectations or beliefs concerning future events and results. We generally use the words “believe,” “expect,” “intend,” “plan,” “anticipate,” “likely,” “will,” “would,” “will be,” “will continue,” “will likely result,” “estimate,” “project,” “forecast,” “outlook,” “strategy,” “future,” “opportunity,” “may,” “should,” or the negative thereof or variations thereon or similar expressions generally intended to identify forward-looking statements. Such forward-looking statements, including those concerning our expectations, involve risks, uncertainties and other factors, some of which are beyond our control, which may cause our actual results, performance or achievements, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. The risks, uncertainties and factors that could cause our results to differ materially from our expectations and beliefs include, but are not limited to, those factors set forth in this Annual Report on Form 10-K under “Item 1A. - Risk Factors.” You should read that information in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Item 7 of this report and our Consolidated Financial Statements and related notes in Item 8 of this report. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
BALCHEM CORPORATION
ANNUAL REPORT ON FORM 10-K
PART I
Item 1. Business (All amounts in thousands, except share and per share data)
General
Balchem Corporation (“Balchem,” the “Company,” “we” or “us”), was incorporated in the State of Maryland in 1967. We develop, manufacture, distribute and market specialty performance ingredients and products for the nutritional, food, pharmaceutical, animal health, medical device sterilization, plant nutrition and industrial markets. Our three reportable segments are strategic businesses that offer products and services to different markets: Human Nutrition and Health, Animal Nutrition and Health, and Specialty Products. Sales and production of products outside of our reportable segments and other minor business activities are included in "Other and Unallocated".
We sell our products through our own sales force, independent distributors and sales agents. Financial information concerning our business, business segments and geographic information appears in Management’s Discussion and Analysis of Financial Condition and Results of Operations under Item 7 below and in the Notes to our Consolidated Financial Statements included under Item 8 below, which information is incorporated herein by reference.
Human Nutrition and Health
The Human Nutrition and Health ("HNH") segment provides human grade choline nutrients and mineral amino acid chelated products through this segment for nutrition and health applications. Choline is recognized to play a key role in the development and structural integrity of brain cell membranes in infants, processing dietary fat, reproductive development and neural functions, such as memory and muscle function. The Company's mineral amino acid chelates, specialized mineral salts, and mineral complexes are used as raw materials for inclusion in premier human nutrition products; proprietary technologies have been combined to create an organic molecule in a form the body can readily assimilate. Sales growth for human nutrition applications is reliant on differentiation from lower-cost competitive products through scientific data, intellectual property and customers' appreciation of brand value. Consequently, the Company makes investments in such activities for long-term value differentiation. This segment also manufactures specialty vitamin K2, which plays a crucial role in the human body for bone health, heart health and immunity, and methylsulfonylmethane ("MSM"), which is a widely used nutritional ingredient that helps provide benefits for joint health, sports nutrition, skin and beauty, and healthy aging. This segment also serves the food and beverage industry for beverage, bakery, dairy, confectionary, and savory manufacturers. The Company partners with its customers from ideation through commercialization to bring on-trend beverages, baked goods, confections, dairy and meat products to market. The Company has expertise in trends analysis and product development. With its strong manufacturing capabilities in customized spray dried and emulsified powders, extrusion and agglomeration, blended lipid systems, liquid flavor delivery systems, juice and dairy bases, chocolate systems, ice cream bases and variegates, the Company is a one-stop solutions provider for beverage and dairy product development needs. Additionally, this segment provides microencapsulation solutions to a variety of applications in food, pharmaceutical and nutritional ingredients to enhance performance of nutritional fortification, processing, mixing, and packaging applications and shelf-life. Major product applications are baked goods, refrigerated and frozen dough systems, processed meats, seasoning blends, confections, sports and protein bars, dietary plans, and nutritional supplements. The Company also creates cereal systems for ready-to-eat cereals, grain-based snacks, and cereal based ingredients.
Animal Nutrition and Health
The Company’s Animal Nutrition and Health ("ANH") segment provides nutritional products derived from its microencapsulation and chelation technologies in addition to the essential nutrient choline chloride. For ruminant animals, the Company’s microencapsulated products boost health and milk production by delivering nutrient supplements that are biologically available, providing required nutritional levels. The Company’s proprietary chelation technology provides enhanced nutrient absorption for various species of production and companion animals and is marketed for use in animal feed throughout the world. ANH also manufactures and supplies choline chloride, an essential nutrient for monogastric animal health, predominantly to the poultry, pet and swine industries. Choline, which is manufactured and sold in both dry and aqueous forms, plays a vital role in the metabolism of fat. In poultry, choline deficiency can result in reduced growth rates and perosis in young birds, while in swine production choline is a necessary and required component of gestating and lactating sow diets for both liver health and prevention of leg deformity. This segment also manufactures MSM, which is a widely used nutritional ingredient that provides benefits for pet health.
Sales of value-added encapsulated products are highly dependent on overall industry economics as well as the Company's ability to leverage the results of university and field research on the animal health and production benefits of our products. Management believes that success in the commodity-oriented choline chloride marketplace is highly dependent on the Company’s ability to maintain its strong reputation for excellent product quality and customer service. The Company continues to drive production efficiencies in order to maintain its competitive-cost position to effectively compete in a competitive global marketplace.
Specialty Products
The Company re-packages and distributes a number of performance gases and chemicals for various uses by its customers, notably ethylene oxide, propylene oxide, and ammonia. Ethylene oxide is sold as a sterilant gas, primarily for use in the health care industry. It is used to sterilize a wide range of medical devices because of its versatility and effectiveness in treating hard or soft surfaces, composites, metals, tubing and different types of plastics without negatively impacting the performance of the device being sterilized. Contract sterilizers and medical device manufacturers are principal customers for this product. Propylene oxide is marketed and sold as a fumigant to aid in the control of insects and microbiological spoilage, to reduce bacterial and mold contamination in certain shelled and processed nut meats, processed spices, cacao beans, cocoa powder, raisins, figs and prunes, and for various chemical synthesis applications, such as increasing paint durability and manufacturing specialty starches and textile coatings. Ammonia is used primarily as a refrigerant, for heat treatment of metals and various chemical synthesis applications, and is distributed in reusable and recyclable drum and cylinder packaging approved for use in the countries these products are shipped to.
The Company’s performance gases and chemicals are distributed worldwide in specially designed, reusable and recyclable drum and cylinder packaging, to assure compliance with safety, quality and environmental standards as outlined by the applicable regulatory agencies in the countries our products are shipped to. The Company’s inventory of these specially built drums and cylinders, along with its five filling facilities, represents a significant capital investment. The Company also sells single use canisters for use in sterilizing re-usable devices typically processed in autoclave units in hospitals.
The Company’s micronutrient agricultural nutrition business sells chelated minerals primarily to producers of high value crops. The Company has a unique and patented two-step approach to solving mineral deficiency in plants to optimize health, yield and shelf-life. First, the Company determines optimal mineral balance for plant health. The Company then has a foliar applied Metalosate® product range, utilizing patented amino acid chelate technology. Its products quickly and efficiently deliver mineral nutrients. As a result, the farmer/grower gets healthier crops that are more resistant to disease and pests, larger yields and healthier food for the consumer with extended shelf life for produce being shipped long distances.
Acquisitions
On August 30, 2022, the Company's wholly-owned subsidiary Albion Laboratories, Inc. ("Albion") entered into a Stock Purchase Agreement, and closed on such transaction with Cardinal Associates Inc. ("Cardinal"), a corporation organized under the laws of the State of Washington, pursuant to which Albion acquired Cardinal and its Bergstrom Nutrition business (collectively, "Bergstrom"). Bergstrom is a leading science-based manufacturer of MSM, based in Vancouver, Washington. Details related to the Bergstrom acquisition are disclosed in Note 2, Significant Acquisitions. The addition of OptiMSM®, Bergstrom Nutrition's MSM brand, to the Company's portfolio within the Human Nutrition and Health and Animal Nutrition and Health segments provides a synergistic scientific advantage in Balchem's key strategic therapeutic focus areas such as longevity and performance and is a strong fit with Balchem's specialty, science-backed mineral products.
On June 21, 2022, the Company and its wholly-owned subsidiary, Balchem B.V., completed the acquisition of Kechu BidCo AS and its subsidiary companies, including Kappa Bioscience AS, a leading science-based manufacturer of specialty vitamin K2 for the human nutrition industry, headquartered in Oslo, Norway (all acquired companies collectively referred to as “Kappa”). Details related to the Kappa acquisition are disclosed in Note 2, Significant Acquisitions. The acquisition strengthens the Company's scientific and technical expertise, geographic reach, and marketplace leadership, which should ultimately lead to accelerated growth for the Company's portfolios within the Human Nutrition and Health segment.
Raw Materials
The raw materials utilized by us in the manufacture of our products are sourced from suppliers both domestically and internationally. Such raw materials include materials derived from petrochemicals, minerals, metals, agricultural commodities and other readily available commodities and are subject to price fluctuations due to market conditions. In 2023, supply reliability improved due to a weaker macroeconomic (demand) environment though we experienced some difficulties in procuring certain materials due to the challenging geopolitical environment impacting some supply lanes. In a year of mixed inflationary and deflationary trends across key categories we source, we were able to secure most necessary materials from our suppliers and continued to strive to ensure a sustainable supply chain to support our growing business operations.
Intellectual Property
We currently hold over 130 patents and over 400 trademarks in the United States and overseas. We also use know-how, trade secrets, formulae, and manufacturing techniques that assist in maintaining competitive positions of certain of our products. Formulae and know-how are of particular importance in the manufacture of a number of our proprietary products. We believe that our patents, in the aggregate, are advantageous to our business. However, we do not believe we are materially dependent on any particular patent or any particular group of patents. We believe that our sales and competitive position are dependent primarily upon the quality of our products, technical sales efforts and market conditions, rather than on patent protection.
Seasonality
While in general, the businesses of our segments are not seasonal to any material extent, the plant nutrition business within Specialty Products is a seasonal business with the vast majority of sales occurring in the first half of the year, based on the planting season in the northern hemisphere.
Backlog
At December 31, 2023, we had a total backlog of $42,957 (comprised of $32,418 for the HNH segment; $7,639 for the ANH segment; $2,678 for the Specialty Products segment, and $222 for other), as compared to a total backlog of $47,022 at December 31, 2022 (comprised of $31,550 for the HNH segment; $11,983 for the ANH segment; $2,980 for the Specialty Products segment and $509 for other). It has generally been our policy and practice to maintain an inventory of finished products and/or component materials for our segments to enable us to ship products within two months after receipt of a product order. All orders in the current backlog are expected to be filled in the 2024 fiscal year.
Competition
Our competitors include many large and small companies, some of which have greater financial, research and development, production and other resources than us. Competition in the supplement, food and beverage markets we serve are based primarily on product performance, customer support, quality, service and price. The development of new and improved products is important to our success. This competitive environment requires substantial investments in product and manufacturing process research and development. In addition, the winning and retention of customer acceptance of our food and nutrition products involve substantial expenditures for application testing, either internally or at customer/prospect sites, and sales efforts. Our competition in this market includes a variety of ingredient and nutritional supplement companies, many of which are privately-held. Therefore, it is difficult to assess the size of all of our segment competitors or where we rank in comparison to such privately-held competitors.
Competition in the animal feed and industrial markets we serve is based primarily on product performance, customer support, quality, service and price. The markets for our products are subject to competitive risks because these markets are highly price competitive. Our competition in this market includes a variety of animal nutrition and health ingredient companies, along with certain industrial companies, many of which are privately-held. Therefore, we are unable to assess the size of all of our competitors or where we rank in comparison to such privately-held competitors.
In the Specialty Products segment, competition within Performance Gases is based primarily on service, reliability, quality, and price. Our competitors in this market vary globally, many of which are regional privately-held companies. We also face competition from alternate technologies or substitute products. In our plant nutrition business, competition is based primarily on product performance, customer support, quality, and price. The development of new and improved products is also important to our ability to compete. Our competition in this market is primarily regional privately-held companies.
Research and Development
During the years ended December 31, 2023, 2022 and 2021, we incurred research and development expenses of approximately $15,049, $12,191, and $13,524, respectively, on Company-sponsored research and development for new products, improvements to existing products, and manufacturing processes. We have historically funded our research and development programs with funds available from current operations with the intent of recovering those costs from profits derived from future sales of products resulting from, or enhanced by, the research and development effort.
We prioritize our product development activities in an effort to allocate resources to those product candidates that, we believe, have the greatest commercial potential. Factors we consider in determining the products to pursue include projected markets and needs, status of our proprietary rights, technical feasibility, expected and known product attributes, and estimated costs to bring the product to market.
Capital Projects
We continue to invest in projects across all production facilities and capital expenditures were approximately $37,274, $49,086, and $36,142 for 2023, 2022 and 2021, respectively. In 2023, we invested $20,720 on projects expected to provide favorable returns on investment, including expanded capacity in key product lines in the HNH segment. In addition, we invested $6,900 for environmental, health, safety, and security upgrades to our facilities. In 2022, we invested $29,759 on projects expected to provide favorable returns on investment, including expanded capacity in key product lines in the HNH segment. In addition, we invested $6,020 for environmental, health, safety, and security upgrades to our facilities and $3,024 in automation projects that improved quality and efficiency of our operations. In 2021, we invested $20,544 on projects expected to provide favorable returns on investment, including expanded capacity in key product lines in the HNH segment. In addition, we invested $3,138 for environmental, health, safety, and security upgrades to our facilities, $2,330 in automation projects that improved quality and efficiency of our operations, and $2,222 in research and development projects. Capital expenditures are projected to range from $35,000 to $40,000 for 2024, including our continued efforts to invest in energy and water saving projects, while exploring additional renewable energy opportunities in support of the company's sustainability efforts.
Environmental and Regulatory Matters
The Federal Insecticide, Fungicide and Rodenticide Act (“FIFRA”), a health and safety statute, requires that certain products within our Specialty Products segment must be registered with the U.S. Environmental Protection Agency ("EPA") because they are considered pesticides. As part of the registration review process, the EPA assesses a wide variety of studies to determine the likelihood of risk to human health and the environment from exposure associated with use of the product. We hold EPA registrations permitting us to sell ethylene oxide as a medical device sterilant and spice fumigant and propylene oxide as a fumigant of nuts and spices.
In April 2008, the EPA issued a RED (“Re-registration Eligibility Decision”) for ethylene oxide which permitted the continued use of ethylene oxide “to sterilize medical or laboratory equipment, pharmaceuticals, and aseptic packaging, or to reduce microbial load on musical instruments, cosmetics, whole and ground spices and other seasoning materials and artifacts, archival material or library objects”. In 2013, the EPA initiated a new registration review of ethylene oxide, in line with and as part of the registration review scheduled for a large number of other pesticides. When the Final Work Plan was issued in March 2014, the EPA anticipated that this registration review process would take approximately seven years. In December 2016, the EPA issued its Integrated Risk Information System (“IRIS”) assessment of ethylene oxide (the "IRIS Assessment"), another aspect of the EPA’s safety review of ethylene oxide. In November 2020, the EPA issued a Draft Human Health Risk Assessment for Ethylene Oxide (“Draft HHRA”). In this Draft HHRA, the EPA presented multiple perspectives on risk extrapolation, including the IRIS Assessment. While acknowledging the necessity of maintaining the critical uses of ethylene oxide, based on the range of unit risk provided in this qualitative assessment, the EPA stated that there should be further mitigation measures implemented. In April 2023, the EPA released a Proposed Interim Decision and Draft Human Health Risk Assessment addendum which included certain proposed mitigation measures. We believe that the EPA intends to reregister ethylene oxide for the sterilization of medical or laboratory equipment, pharmaceuticals, aseptic packaging, and the reduction of microbes on spices/seasonings, with the proposed mitigation measures potentially impacting such users, including our customers. The product, when used as a sterilant for certain medical devices, has no known equally effective substitute. In October 2019, the U.S. Food and Drug Administration in a public statement said, "Although medical devices can be sterilized by several methods, ethylene oxide is the most common method of sterilization of medical devices in the U.S. and is a well-established and scientifically-proven method of preventing harmful microorganisms from reproducing and causing infections." Management believes the lack of availability of this product could not be reasonably tolerated by various medical device manufacturers or the health care industry due to the resultant infection potential.
Similarly, the EPA issued a RED for propylene oxide in August 2006. At that time, the EPA “determined that products containing the active ingredient propylene oxide ("PPO") are eligible for re-registration provided that…risk mitigation measures…are adopted.” In 2013, the EPA initiated a new registration review of propylene oxide, in line with and as part of the registration review scheduled for a large number of other pesticides. A Final Work Plan was issued in March 2014, and the EPA anticipated that this review process would take approximately seven years. In October 2020, the EPA issued both the Proposed Interim Decision and Draft Risk Assessment for propylene oxide.
In July 2021, the EPA issued the Interim Decision. Based on these documents, the use of propylene oxide to treat nuts and spices will continue to be permitted with minimal changes to the current approved usage. We submitted those changes and expect the EPA to review and approve them in the coming months during 2024.
Our facility in Verona, Missouri facility, while held by a prior owner, Syntex Agribusiness, Inc. (“Syntex”), was designated by the EPA as a Superfund site and placed on the National Priorities List in 1983 because of dioxin contamination on portions of the site. Remediation was conducted by Syntex under the oversight of the EPA and the Missouri Department of Natural Resources. We are indemnified by the sellers under our May 2001 asset purchase agreement covering our acquisition of the Verona, Missouri facility for potential liabilities associated with the Superfund site, and one of the sellers, in turn, has the benefit of certain contractual indemnification by Syntex in relation to the implementation of the above-described Superfund remedy. In June 2023, in response to a Special Notice Letter received from the EPA in 2022, BCP Ingredients, Inc. ("BCP"), the Company's subsidiary that operates the site, Syntex, EPA, and the State of Missouri entered into an Administrative Settlement Agreement and Order on Consent (“ASAOC”) for a focused remedial investigation/feasibility study ("RI/FS") under which (a) BCP will conduct a source investigation of potential source(s) of releases of 1,4-dioxane and chlorobenzene at a portion of the site and (b) BCP and Syntex will complete a RI/FS to determine a potential remedy, if any is required. Activities under the ASAOC are underway and are expected to continue for some period of time.
In connection with normal operations at our plant facilities, we are required to maintain environmental and other permits, including those relating to the use of ethylene oxide. From time to time, our manufacturing sites may be subject to inspections by the EPA and other agencies. To the extent any consent orders or other agreements are entered into as a result of findings from such inspections, the Company is committed to ensuring compliance with such orders or agreements. For a further discussion of our potential environmental liabilities, see Note 16, Commitments and Contingencies, to our Consolidated Financial Statements.
We believe we are in compliance in all material respects with applicable laws and regulations that have been enacted or adopted regulating the discharge of materials into the environment or otherwise relating to the protection of the environment. Such compliance includes the maintenance of required permits under air pollution regulations and compliance with requirements of the Occupational Safety and Health Administration. The cost of such compliance has not had a material effect upon the results of our operations or our financial condition.
We produce products which are required to be manufactured in conformity with current Good Manufacturing Practice (“cGMP”) regulations as interpreted and enforced by the FDA, through third party contract arrangement. Modifications, enhancements or changes in contracted manufacturing facilities or procedures relating to our pharmaceutical products are, in many circumstances, subject to FDA approval, which may be subject to a lengthy application process or which we may be unable to obtain. Any contracted manufacturing facilities that manufacture our pharmaceutical products are periodically subject to inspection by the FDA and other governmental agencies, and operations at these facilities could be interrupted or halted if the results of these inspections are unsatisfactory.
Human Capital
Our employees are our most valued asset and fundamental to our success. As of December 31, 2023, we employed approximately 1,302 full-time employees worldwide, with approximately 18% covered by collective bargaining agreements. We are seeing some modest improvement in most relevant labor markets and we believe that we have been successful in attracting skilled and experienced personnel in a competitive environment and that our human capital resources are adequate to perform all business functions. In addition, we continue to enhance technology to further optimize productivity and performance.
Health and Safety
Protecting the workplace environment and the health and safety of our employees, contractors, visitors, and neighbors is our top priority. Our recordable injury rate, which is defined as recordable injuries per 200,000 hours worked, was 1.39 and 1.17 in 2023 and 2022, respectively. The injuries were primarily the result of manual material handling and cultural/behavioral factors that influence outcome. We are adjusting our 2024 environmental, health, safety, and security management system to include an even greater emphasis on hazard identification/correction and cultural/behavioral aspects of personal safety. In addition, we continually upgrade our facilities to reduce health and safety risks and establish procedures with appropriate personnel protection for the safety of our employees.
Diversity and Inclusion
We recognize that our best performance is achieved when our teams are diverse, and accordingly, diversity and inclusion are important elements of Balchem's Human Resources strategy. We strive to promote inclusion through the implementation of inclusive leadership training across the Company and are committed to increasing representation of minorities throughout the organization. In 2023, our total workforce consisted of 74% male and 26% female among all employees and 47% male and 53% female when excluding supply chain and operations functions.
In 2022, our total workforce consisted of 75% male and 25% female among all employees and 50% male and 50% female when excluding supply chain and operations functions. With the support of our Board of Directors, we continue to explore additional diversity and inclusion initiatives.
Training and Well-Being Programs
We strive to develop employee skills and knowledge, which includes training for job-specific technical knowledge, regulatory requirements, and company policies, through our internal learning and development platform. The topics of trainings include the Company's Code of Conduct, anti-harassment and discrimination, foreign corrupt practices, antitrust, cyber security, and various other compliance subjects. Our sponsored employee continuing learning program offers a broad base of assistance for employees, including learning and development courses. We also deployed unconscious bias and inclusive leadership training to our management team. Employees have access to healthy lifestyle discounts through our Wellness Center, as well as debt, legal, and financial counseling. Leadership programs, peak performance training and multiple online services and courses enable our employees to choose their own learning paths and work towards achieving their goals for education, finances, and overall well-being.
Performance Review, Compensation and Benefits
Our annual performance review process is an important, objective-based dialogue to foster continuous growth and development by providing an opportunity to establish goals and deliver feedback relative to each employee's performance. Balchem's annual review process is closely aligned with a formal succession planning and talent review process designed to identify and develop the next generation of leaders.
We are dedicated to providing full-time employees with a competitive compensation package that includes medical, dental, vision, and prescription benefits in addition to a 401(k) matching program. Balchem also provides financial support for health and wellness programs such as online financial wellness content, sponsored weight loss programs and subsidized gym memberships. We also provide generous time off and leave benefits, which are important to help ensure employees can enjoy a healthy balance between work and family time.
For the years ended December 31, 2023 and 2022, our turnover rate was 11% and 15%, respectively, for salaried employees with an average length of service of over 9 years for both years. For the years ended December 31, 2023 and 2022, our turnover rate was 29% and 36%, respectively, for hourly employees with an average length of service of about 7 years for both years. We are continuing to improve employee retention with effective employment engagement efforts, a productive performance review process, and competitive compensation.
Sustainability
We operate as strong stewards of our shareholders, customers, suppliers, employees, and the communities in which we operate. We are working to make our workforce more inclusive, our business more sustainable, and our communities more engaged by maintaining strong environmental, social and governance practices.
In 2023, we published our 2022 Sustainability Report. This report provides detailed information regarding our Corporate Responsibility strategy, focus areas and governance structure. We are committed to reducing our greenhouse gas emissions by implementing new technologies, improving operational efficiencies, and expanding green energy usages. In addition, we are committed to reducing our global water use by reducing and recycling water usage and investing new technologies to improve water efficiency. For more information on our approach to sustainability management, refer to our 2022 Sustainability Report, which is available on our website at https://balchem.com/our-company/corporate-social-responsibility/sustainability. The information contained on, or that may be accessed through, the Company’s website is not incorporated by reference into, and is not part of, this Annual Report on Form 10-K.
In December 2023, Balchem was named on Newsweek's 2024 list of America's Most Responsible Companies and has earned a ranking amongst this prestigious list of companies for the fourth consecutive year. This prestigious list, compiled by Newsweek in partnership with Statista Inc., recognizes the most responsible companies in the U.S. across a variety of industries, and is based on their assessment of publicly available corporate responsibility data. We are pleased to be recognized by Newsweek and Statista for our leadership in corporate responsibility.
Available Information
Our headquarters is located at 5 Paragon Drive, Montvale, NJ 07645. Our telephone number is (845) 326-5600 and our Internet website address is www.balchem.com. We make available through our website, free of charge, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, and amendments to such reports, as soon as reasonably practicable after they have been electronically filed with the Securities and Exchange Commission (the "SEC").
Such reports are available via a link from the Investor Relations page on our website to a list of our reports on the SEC’s EDGAR website. The address of the SEC's website is www.sec.gov.
Item 1A. Risk Factors
We discuss our expectations regarding future performance, events and outcomes in this Form 10-K, quarterly and annual reports, press releases and other written and oral communications. All statements except for historical and present factual information are “forward-looking statements” and are based on financial data and business plans available only as of the time the statements are made, which may become outdated or incomplete. Forward-looking statements are inherently uncertain, and investors must recognize that events could significantly differ from our expectations. You should carefully consider the risk factors discussed below, together with all the other information included in this Form 10-K, in evaluating us and our ordinary shares. If any of the risks below actually occurs, our business, financial condition, results of operations and cash flows could be materially and adversely affected. Any such adverse effect may cause the trading price of our ordinary shares to decline, and as a result, you could lose all or part of your investment in us. Our business may also be adversely affected by risks and uncertainties not known to us or risks that we currently believe to be immaterial. We assume no obligation to update any forward-looking statements as a result of new information, future events or other factors.
Operational Risks
We face risks associated with our sales to customers and manufacturing operations outside the United States.
Our net sales consist of sales both within and outside the United States. In addition, we conduct a portion of our manufacturing outside the United States. The majority of our foreign sales occur through our foreign subsidiaries and the remainder of our foreign sales result from exports to foreign distributors, resellers and customers. Our foreign sales and operations are subject to a number of risks, including: longer accounts receivable collection periods; the impact of recessions and other economic conditions in economies outside the United States; export duties and quotas; imposition of, or changes in, tariffs, sanctions, trade restrictions, and trade relations including but not limited to those associated with the United States-Mexico-Canada Agreement ("USMCA") which replaced the North American Free Trade Agreement ("NAFTA"), other free trade agreements, and the exit of the United Kingdom from the European Union; unexpected changes in regulatory requirements; certification requirements; environmental regulations; reduced protection for intellectual property rights in some countries; potentially adverse tax consequences; political and economic instability; and preference for locally produced products. These factors could have a material adverse impact on our ability to increase or maintain our international sales.
Our sales and operations may be adversely affected by supply chain disruptions due to political unrest, terrorist acts, and national and international conflicts.
Our sales and operations are subject to a number of risks, including political and economic instability, which could have a material adverse impact on our ability to increase or maintain our international sales and operations. National and international conflicts such as war, border closures, civil disturbances and terrorist acts, including Russia's invasion of Ukraine and the ongoing conflict between Israel and Hamas, may increase the likelihood of already strained supply interruptions and further hinder our ability to access the materials and energy we need to manufacture our products. Additional supply chain disruptions will make it harder for us to find favorable pricing and reliable sources for the materials we need. As a result, such disruptions will put upward pressure on our costs and increase the risk that we may be unable to acquire the materials and services we need to continue to make certain products, in particular at our manufacturing facilities in Europe.
Our financial success depends in part on the reliability and sufficiency of our manufacturing facilities.
Our revenues depend on the effective operation of our manufacturing, packaging, and processing facilities. The operation of our facilities involves risks, including the breakdown, failure, or substandard performance of equipment, power outages, the improper installation or operation of equipment, explosions, fires, natural disasters, failure to achieve or maintain safety or quality standards, work stoppages, supply or logistical outages, and the need to comply with environmental and other directives of governmental agencies. The occurrence of material operational problems, including, but not limited to, the above events, could adversely affect our profitability during the period of such operational difficulties.
Our ability to successfully grow and expand our business depends on our ability to recruit and retain a highly qualified and diverse workforce.
Our ability to successfully grow and expand our business is dependent upon our ability to recruit and retain a workforce with the skills necessary to develop, manufacture and deliver the products and services desired by our customers. We need highly skilled and qualified personnel in multiple areas, including research and development, engineering, sales, manufacturing, information technology, cybersecurity, accounting, regulatory, and management. We must therefore continue to effectively recruit, retain and motivate highly qualified, skilled and diverse personnel to maintain our current business and support our projected growth. A shortage of these employees for various reasons, including intense competition for skilled employees, labor shortages, increased labor costs, candidates’ preference to work remotely, changes in laws and policies regarding immigration and work authorizations in jurisdictions where we have operations, or any government mandates that may result in workforce attrition and difficulty with recruiting, may jeopardize our ability to grow and expand our business.
We may, from time to time, experience problems in our labor relations.
A portion of our North American workforce is represented by a union under a single collective bargaining agreement. In Europe, employees at our Marano, Ticino, Italy facility and Bertinoro, Italy facility are covered by a national collective bargaining agreement, respectively. We believe that our present labor relations with all our union employees are satisfactory, however, our failure to renew these agreements on reasonable terms could result in labor disruptions and increased labor costs, which could adversely affect our financial performance. Similarly, if our relations with the union portion of our workforce do not remain positive, such employees could initiate a strike, work stoppage or slowdown in the future. In the event of such an action, we may not be able to adequately meet the needs of our customers using our remaining workforce and our operations and financial condition could be adversely affected. Additionally, other portions of our workforce could become subject to union campaigns.
The effects of global climate change or other unexpected events, including global health crises, may disrupt our operations and have a negative impact on our business.
The effects of global climate change, such as extreme weather conditions and natural disasters occurring more frequently or with more intense effects, or the occurrence of unexpected events including wildfires, tornadoes, hurricanes, earthquakes, floods, tsunamis and other severe hazards or global health crises, such as the outbreak of Ebola or the global COVID-19 pandemic, or other actual or threatened epidemic, pandemic, outbreak and spread of a communicable disease or virus, in the countries where we operate or sell products and provide services, could adversely affect our operations and financial performance. Extreme weather, natural disasters, power outages, global health crises or other unexpected events could disrupt our operations by impacting the availability and cost of materials needed for manufacturing, causing physical damage and partial or complete closure of our manufacturing sites or distribution centers, loss of human capital, temporary or long-term disruption in the manufacturing and supply of products and services and disruption in our ability to deliver products and services to customers. These events and disruptions could also adversely affect our customers’ and suppliers’ financial condition or ability to operate, resulting in reduced customer demand, delays in payments received or supply chain disruptions. Further, these events and disruptions could increase insurance and other operating costs, including impacting our decisions regarding construction of new facilities to select areas less prone to climate change risks and natural disasters, which could result in indirect financial risks passed through the supply chain or other price modifications to our products and services.
We may be subject to risks relating to our information technology and operational technology systems.
We rely extensively on information technology and operational technology systems, networks and services including hardware, software, firmware and technological applications and platforms (collectively, "IT Systems") to manage and operate our business from end-to-end, including ordering and managing materials from suppliers, design and development, manufacturing, marketing, selling and shipping to customers, invoicing and billing, managing our banking and cash liquidity systems, managing our enterprise resource planning and other accounting and financial systems and complying with regulatory, legal and tax requirements. We have invested and will continue to invest in improving our IT Systems. Some of these investments are significant and impact many important operational processes and procedures. There is no assurance that newly implemented IT Systems will improve our current systems, improve our operations or yield the expected returns on the investments. In addition, the implementation of new IT Systems may be more difficult, costly or time consuming than expected and cause disruptions in our operations and, if not properly implemented and maintained, negatively impact our business. If our IT Systems cease to function properly or if these systems do not provide the anticipated benefits, our ability to manage our operations could be impaired.
We currently rely on third-party service providers for many of the critical elements of our global information and operational technology infrastructure and their failure to provide effective support for such infrastructure could negatively impact our business and financial results.
We have outsourced many of the critical elements of our global information and operational technology infrastructure to third-party service providers in order to achieve efficiencies. If such service providers do not perform or do not perform effectively, we may not be able to achieve the expected efficiencies and may have to incur additional costs to address failures in providing service by the service providers.
Depending on the function involved, such non-performance, ineffective performance or failures of service may lead to business disruptions, processing inefficiencies or security breaches.
Disruptions or breaches of our information systems could adversely affect us.
Despite our implementation of cybersecurity measures which have focused on prevention (including a robust cybersecurity employee education program to train our employees on email and password security, recognizing phishing and related topics on a regular basis), mitigation, resilience and recovery, our network and products, including access solutions, may be vulnerable to cybersecurity attacks, computer viruses, malicious codes, malware, ransomware, phishing, social engineering, denial of service, hacking, break-ins and similar disruptions, including through use of new artificial intelligence tools or methods. Cybersecurity attacks and intrusion efforts are continuous and evolving, and in certain cases they have been successful at the most robust institutions. The scope and severity of risks that cyber threats present have increased dramatically and include, but are not limited to, malicious software, attempts to gain unauthorized access to data or premises, exploiting weaknesses related to vendors or other third parties that could be exploited to attack our systems, denials of service and other electronic security breaches that could lead to disruptions in systems, unauthorized release of confidential or otherwise protected information and corruption of data. Any such event could have a material adverse effect on our business, operating results and financial condition, as we face regulatory, reputational and litigation risks resulting from potential cyber incidents, as well as the potential of incurring significant remediation costs. Further, while we maintain insurance coverage that may, subject to policy terms and exclusions, cover certain aspects of our cyber risks, such insurance coverage may be insufficient to cover our losses or all types of claims that may arise in the continually evolving area of cyber risk.
We also face increasing and evolving disclosure obligations related to cybersecurity events. Despite rigorous processes, we may not adequately meet all our existing or future disclosure obligations and/or having our disclosures misinterpreted. Determining whether a cybersecurity incident is notifiable or reportable may not be straightforward and any such mandatory disclosures could lead to negative publicity, loss of customer confidence in the effectiveness of our security measures, diversion of management's attention and governmental investigations.
Our daily business operations also require us to collect and/or retain sensitive data such as intellectual property, proprietary business information and data related to customers, employees, suppliers and business partners within our networking infrastructure including data from individuals subject to the European Union's General Data Protection Regulation, that is subject to privacy and security laws, regulations and/or customer-imposed controls. Despite our efforts to protect such data, the loss or breach of such data due to various causes including material security breaches, catastrophic events, extreme weather, natural disasters, power outages, system failures, computer viruses, improper data handling, programming errors, unauthorized access and employee error or malfeasance could result in wide reaching negative impacts to our business, and as such, the ongoing maintenance and security of this information is pertinent to the success of our business operations and our strategic goals.
Our networking infrastructure and related assets may be subject to unauthorized access by hackers, employee error or malfeasance or other unforeseen activities. Such issues could result in the disruption of business processes, network degradation and system downtime, along with the potential that a third party will exploit our critical assets such as intellectual property, proprietary business information and data related to our customers, suppliers and business partners. To the extent that such disruptions occur and our business continuity plans do not effectively address these disruptions in a timely manner, they may cause delays in the manufacture or shipment of our products and the cancellation of customer orders and, as a result, our business, operating results and financial condition could be materially and adversely affected, resulting in a possible loss of business or brand reputation.
Business and Financial Risks
Increased competition could adversely affect our business and financial results.
We face competition in our markets from a number of large and small companies, some of which have greater financial, research and development, production and other resources than we do. Our competitive position is based principally on performance, quality, customer support, service, breadth of product line, manufacturing or packaging technology and the selling prices of our products. We may be unable to effectively compete on all these bases. Further, our competitors may improve the design and performance of their products and introduce new products with competitive price and performance characteristics. While we expect to do the same to maintain our current competitive position and market share, if we are unable to anticipate evolving trends in the market or the timing and scale of our competitors’ activities and initiatives, the demand for our products and services could be negatively impacted.
Global economic conditions may adversely affect our business, operating results and financial condition.
Unfavorable changes in economic conditions, including inflation, recession, changes in tariffs and trade relations amongst international trading partners, or other changes in economic conditions, may adversely impact the markets in which we operate.
These conditions may make it extremely difficult for our customers, our vendors and us to accurately forecast and plan future business activities, and they could cause U.S. and foreign businesses to slow spending on our products which would reduce our revenues and profitability. If inflation in costs such as raw materials, packaging, freight, labor and energy prices increase beyond our ability to control for them through measures such as implementing operating efficiencies, we may not be able to increase prices to sufficiently offset the effect of various costs increases without negatively impacting customer demand, thereby negatively impacting our margin performance and results of operations.
Furthermore, during challenging economic times our customers may face issues gaining timely access to sufficient credit, which could result in an impairment of their ability to make timely payments to us. If that were to occur, we may be required to increase our allowance for doubtful accounts and cash flow would be negatively impacted. We cannot predict the timing, depth or duration of any economic slowdown or subsequent economic recovery, worldwide, or in the markets in which we operate. Also, at any point in time we have funds in our cash accounts that are with third party financial institutions. These balances in the U.S. and other countries could exceed the Federal Deposit Insurance Corporation (“FDIC”) and other relevant insurance limits, respectively. While we monitor the cash balances in our accounts, these balances could be impacted if the underlying financial institutions fail or could be subject to other adverse conditions in the financial markets. Additionally, our future results of operations could be adversely affected by changes in the effective tax rate as a result of a change in the mix of earnings in jurisdictions with differing statutory tax rates, changes in tax laws, regulations and judicial rulings or changes in the interpretation thereof.
Raw material shortages or price increases could adversely affect our business and financial results.
The principal raw materials that we use in the manufacture of our products can be subject to price fluctuations due to market conditions and factors beyond our control, including the COVID-19 pandemic and inflationary pressures, both of which have impacted our business over the past several years and are likely to continue for some time. Such raw materials include materials derived from petrochemicals, minerals, metals, agricultural commodities and other commodities. While the selling prices of our products tend to increase or decrease over time with the cost of raw materials, these changes may not occur simultaneously or to the same degree. At times, including during periods of rapidly increasing raw material prices, we may be unable to pass increases in raw material costs through to our customers due to certain contractual obligations. Such increases in the price of raw materials, if not offset by product price increases, or substitute raw materials, would have an adverse impact on our profitability. We believe we have reliable sources of supply for our raw materials under normal market conditions. We cannot, however, predict the likelihood or impact of any future raw material shortages. Any shortages or unforeseen price increases could have a material adverse impact on our results of operations.
Our international operations subject us to currency translation risk and currency transaction risk which could cause our results to fluctuate from period to period.
The financial condition and results of operations of our foreign subsidiaries are reported in local currencies and then translated into U.S. dollars at the applicable currency exchange rate for inclusion in our consolidated financial statements. Exchange rates between these currencies in recent years have fluctuated and may do so in the future. Furthermore, we incur currency transaction risk whenever we enter into either a purchase or a sales transaction using a currency different than the functional currency. Given the volatility of exchange rates, we may not be able to effectively manage our currency transactions and/or translation risks. Volatility in currency exchange rates could impact our business and financial results.
Although we utilize risk management tools, such as derivative instruments, to mitigate market fluctuations in foreign currencies, any changes in strategy in regard to risk management tools can also affect revenue, expenses and results of operations and there can be no assurance that such measures will result in cost savings or that all market fluctuation exposure will be eliminated.
Our debt instruments are subject to interest rate risks and impose operating and financial restrictions which could have an adverse impact on our business and results of operations.
Our incurrence of indebtedness could have negative consequences to us, including limiting our ability to borrow additional monies for our working capital, capital expenditures, acquisitions, debt service requirements or other general corporate purposes; limiting our flexibility in planning for, or reacting to, changes in our operations, our business or the industries in which we compete; our leverage may place us at a competitive disadvantage by limiting our ability to invest in the business or in further research and development; making us more vulnerable to downturns in our business or the economy; and there would be a material adverse effect on our business and financial condition if we were unable to service our indebtedness or obtain additional financing, as needed.
Our ability to make payments on our indebtedness depends on our ability to generate cash in the future. If we do not generate sufficient cash flow to meet our debt service and working capital requirements, we may need to seek additional financing or sell assets. This may make it more difficult for us to obtain financing on terms that are acceptable to us, or at all. Without any such financing, we could be forced to sell assets to make up for any shortfall in our payment obligations under unfavorable circumstances.
Interest payable in accordance with our five-year senior secured revolving credit agreement (the "Credit Agreement") is based on a fluctuating rate. In light of potential fluctuations, including interest rate increases which may continue, we are exposed to risk resulting from adverse changes in interest rates.
Further, due to the cessation of the London Interbank Offered Rate (“LIBOR”), we have entered into financial transactions such as credit agreements that use the Secured Overnight Financing Rate (“SOFR”) as interest rate benchmarks. SOFR is calculated differently from LIBOR and has inherent differences which could give rise to uncertainties, including the limited historical data and volatility in the benchmark rates. The full effects of the transition to SOFR or other rates remain uncertain.
We may not be able to successfully consummate and manage acquisition, joint venture and divestiture activities which could have an impact on our results.
From time to time, we may acquire other businesses, enter into joint ventures and, based on an evaluation of our business portfolio, divest existing businesses. These acquisitions, joint ventures and divestitures may present financial, managerial and operational challenges, including diversion of management attention from existing businesses, difficulty with integrating or separating personnel and financial and other systems, increased expenses, difficulties in realizing synergies expected to result from acquisitions, potential loss of key employees, key contractual relationships or key customers of acquired companies or of us, difficulties in integrating financial reporting systems and implementing controls, procedures and policies, including disclosure controls and procedures and internal control over financial reporting, appropriate for public companies of our size at companies that, prior to the acquisition, had lacked such controls, procedures and policies, assumption of unknown liabilities and indemnities, and potential disputes with the buyers or sellers. In addition, we may be required to incur asset impairment charges (including charges related to tangible assets, goodwill and other intangible assets) in connection with acquired businesses which may reduce our profitability. If we are unable to consummate such transactions, or successfully integrate and grow acquisitions and achieve contemplated revenue synergies and cost savings, our financial results could be adversely affected. Additionally, joint ventures inherently involve a lesser degree of control over business operations, thereby potentially increasing the financial, legal, operational and/or compliance risks.
We may not be able to effectively manage and implement restructuring initiatives or other organizational changes.
We may, from time to time, restructure or make other adjustments to our workforce and manufacturing footprint in response to market or product changes, performance issues, changes in strategy, acquisitions and/or other internal and external considerations. These restructuring activities and other organizational changes may result in increased restructuring costs, diversion of management’s time and attention from daily operations and temporarily reduced productivity. If we are unable to successfully manage and implement restructuring and other organizational changes, we may not achieve or sustain the expected growth or cost savings benefits of these activities or do so within the expected timeframe. These effects could recur in connection with future acquisitions and other organizational changes and our results of operations could be negatively affected.
Changes in our relationships with our vendors, changes in tax or trade policy, interruptions in our operations or supply chain or increased commodity or supply chain costs could adversely affect our results of operations.
We are dependent on our vendors, including common carriers, to supply raw materials to our manufacturing facilities. As we continue to add capabilities to quickly move the appropriate amount of inventory at optimal operational costs through our entire supply chain, operating our fulfillment network becomes more complex and challenging. If our fulfillment network does not operate properly, if a vendor fails to deliver on its commitments, or if common carriers have difficulty providing capacity to meet demands for their services, we could experience inventory shortages, delivery delays or increased delivery costs, which could lead to lost sales and decreased guest confidence, and adversely affect our results of operations.
A large portion of our raw materials are sourced, directly or indirectly, from outside the U.S. Any major changes in tax or trade policy, such as the imposition of additional tariffs or duties on imported products, between the U.S. and countries from which we source raw materials could require us to take certain actions, including for example raising prices on products we sell and seeking alternative sources of supply from vendors in other countries with whom we have less familiarity, which could adversely affect our reputation, sales, and our results of operations.
Political or financial instability, currency fluctuations, the outbreak of pandemics or other illnesses (such as the COVID-19 pandemic), labor unrest, transport capacity and costs, port security, weather conditions, natural disasters, or other events that could alter or suspend our operations, slow or disrupt port activities, or affect foreign trade are beyond our control and could materially disrupt our supply of raw materials, increase our costs, and/or adversely affect our results of operations. There have been periodic labor disputes impacting the U.S. ports that have caused us to make alternative arrangements to continue the flow of inventory, and if these types of disputes recur, worsen, or occur in other countries through which we source products, it may have a material impact on our costs or inventory supply.
Changes in the costs of procuring commodities used in our products or the costs related to our supply chain, could adversely affect our results of operations.
Adverse publicity or consumer concern regarding the safety or quality of food products containing our products, or health concerns, whether with our products, products in the same general class as our products or for food products containing our products, may result in the loss of sales. Also, consumer preferences for products containing our products may change.
We are dependent upon consumers’ perception of the safety, quality and possible dietary benefits of products containing our food ingredient products. As a result, substantial negative publicity concerning our products or other foods and beverages in which our products are used could lead to a loss of consumer confidence in those products, removal of those products from retailers’ shelves and reduced sales and prices of our products. Product quality issues, actual or perceived, or allegations of product contamination, even when false or unfounded, could hurt the image of our products or of brands of products containing our products, and cause consumers to choose other products. Further, any product recall, whether our own or by a third party, whether due to real or unfounded allegations, could impact demand on food products containing our products or even our products. Any of these events could have a material adverse effect on our business, results of operations and financial condition. Consumer preferences, as well as trends, within the food industries change often and our failure to anticipate, identify or react to changes in these preferences and trends could, among other things, lead to reduced demand and price reductions, and could have an adverse effect on our business, results of operations and financial condition. While we continue to diversify our product offerings, developing new products entails risks and we cannot be certain that demand for our products and products containing our products will continue at current levels or increase in the future.
Legal, Regulatory and Compliance Risks
Material adverse legal judgments, fines, penalties or settlements could adversely affect our business.
We may from time to time become involved in legal proceedings and disputes incidental to the operation of our business. Our business may be adversely affected by the outcome of these proceedings and other contingencies (including, without limitation, product liability, tort, environmental, intellectual property, antitrust, data protection, privacy, and labor and employment matters) that cannot be predicted with certainty. As required by GAAP, if applicable, we establish reserves based on our assessment of contingencies. Subsequent developments in legal proceedings and other contingencies may affect our assessment and estimates of the loss contingency recorded as a reserve, and we may be required to make additional material payments.
Our business exposes us to potential product liability claims and recalls, which could adversely impact our financial condition and performance.
Our development, manufacture and sales of food ingredient, pharmaceutical and nutritional supplement products involve an inherent risk of exposure to product liability claims, product recalls, product seizures and related adverse publicity. A product liability judgment against us could also result in substantial and unexpected expenditures, affect consumer confidence in our products, and divert management’s attention from other responsibilities. Although we maintain product liability insurance coverage in amounts we believe are customary within the industry, there can be no assurance that this level of coverage is adequate or that we will be able to continue to maintain our existing insurance or obtain comparable insurance at a reasonable cost, if at all. A product recall or a partially or completely uninsured judgment against us could have a material adverse effect on results of operations and financial condition.
Our brands are important assets of our businesses, and violation of our trademark rights by imitators could negatively impact revenues and brand reputation.
Our brands and trademarks enjoy a reputation for quality and value and are important to our success and competitive position. Unauthorized use of our trademarks may not only erode sales of our products but may also cause significant damage to our brand name and reputation, interfere with relationships with our customers and increase litigation costs. There can be no assurance that our on-going effort to protect our brand and trademark rights will prevent all violations.
Allegations that we have infringed the intellectual property rights of third parties could negatively affect us.
We may be subject to claims of infringement of intellectual property rights by third parties. In general, if it is determined that one or more of our technologies, products or services infringes the intellectual property rights owned by others, we may be required to cease marketing those products or services, to obtain licenses from the holders of the intellectual property at a material cost or to take other actions to avoid infringing such intellectual property rights. The litigation process is costly and subject to inherent uncertainties, and we may not prevail in litigation matters regardless of the merits of our position. Adverse intellectual property litigation or claims of infringement against us may become extremely disruptive if the plaintiffs succeed in blocking the trade of our products and services and may have a material adverse effect on our business.
We are subject to risks related to corporate social responsibility and reputational matters.
Our reputation and the reputation of our brands, including the perception held by our customers, end-users, business partners, investors, other key stakeholders and the communities in which we do business are influenced by various factors. There is an increased focus from our stakeholders on Environmental, Social and Governance (“ESG”) practices and disclosure – and if we fail, or are perceived to have failed, in any number of ESG matters, such as environmental stewardship, goals regarding our intended reduction of carbon emissions and water usage, inclusion and diversity, workplace conduct and support for local communities, or to effectively respond to changes in, or new, legal or regulatory requirements concerning climate change or other sustainability concerns, our reputation or the reputation of our brands may suffer. Such damage to our reputation and the reputation of our brands may negatively impact our business, financial condition and results of operations. Further, there are an increasing number of anti-ESG legislative initiatives that may conflict with other regulatory requirements or our stakeholders' expectations.
In addition, negative or inaccurate postings or comments on social media or networking websites about the Company or our brands could generate adverse publicity that could damage our reputation or the reputation of our brands. If we are unable to effectively manage real or perceived issues, including concerns about product quality, safety, corporate social responsibility or other matters, sentiments toward the Company or our products could be negatively impacted, and our financial results could suffer.
Our reputation, ability to do business and results of operations could be impaired by adverse publicity or improper conduct by any of our employees, agents or business partners.
We are subject to regulation under a variety of U.S. federal and state and non-U.S. laws, regulations and policies including laws related to anti-corruption, export and import compliance, anti-trust and money laundering due to our global operations. We cannot provide assurance that our internal controls will always protect us from the improper conduct of our employees, agents and business partners. Any improper conduct could damage our reputation and subject us to, among other things, civil and criminal penalties, material fines, equitable remedies (including profit disgorgement and injunctions on future conduct), securities litigation and a general loss of investor confidence.
Our operations are subject to regulatory risks and the loss of governmental permits and approvals would materially and adversely affect some of our businesses.
Our U.S. and non-U.S. operations are subject to a number of laws and regulations, including environmental, health and safety standards. We have incurred, and will be required to continue to incur, significant expenditures to comply with these laws and regulations. Changes to, or changes in interpretations of, current laws and regulations, including climate change legislation or other environmental mandates, could require us to increase our compliance expenditures, cause us to significantly alter or discontinue offering existing products and services or cause us to develop new products and services. Altering current products and services or developing new products and services to comply with changes in the applicable laws and regulations could require significant research and development investments, increase the cost of providing the products and services and adversely affect the demand for our products and services, including shifting demand to competitors in countries where laws and regulations may be less stringent.
In the event a regulatory authority concludes that we are not or have not at all times been in full compliance with these laws or regulations, we could be fined, criminally charged or otherwise sanctioned. Certain environmental laws assess liability on current or previous owners of real property or operators of manufacturing facilities for the costs of investigation, removal or remediation of hazardous substances or materials at such properties or at properties at which parties have disposed of hazardous substances. Liability for investigative, removal and remedial costs under certain U.S. federal and state laws and certain non-U.S. laws are retroactive, strict and joint and several. In addition to cleanup actions brought by governmental authorities, private parties could bring personal injury or other claims due to the presence of, or exposure to, hazardous substances. For more information, see "Item 1. Business – Environmental and Regulatory Matters" of this report.
While we have planned for future capital and operating expenditures to maintain compliance with environmental laws, our costs of compliance may exceed our estimates. We may also be subject to environmental claims for personal injury, liabilities arising from past, present or future releases of, or exposures to, hazardous substances, or cost recovery actions for remediation of facilities in the future based on our past, present or future business activities.
Further, pursuant to applicable environmental and safety laws and regulations, we are required to obtain and maintain certain governmental permits and approvals, including EPA registrations under FIFRA for some of our products. We maintain EPA FIFRA registrations for ethylene oxide as a medical device sterilant and spice fumigant and for propylene oxide as a fumigant of nuts and spices.
These products are progressing through a multi-year FIFRA re-registration review process. Recent draft documents indicate that the EPA intends to continue the registrations for both ethylene oxide and propylene oxide with certain additional mitigation measures. The EPA may re-examine the registrations in the future in accordance with the provisions of FIFRA. Any future determination by the EPA to discontinue permitted use of ethylene oxide or propylene oxide would have a material adverse effect on our business and financial results.
Commercial supply of pharmaceutical products that we may develop, subject to cGMP manufacturing regulations, would be performed by third-party cGMP manufacturers. Modifications, enhancements or changes in third-party manufacturing facilities or procedures of our pharmaceutical products are, in many circumstances, subject to FDA approval, which may be subject to a lengthy application process or which we may be unable to obtain. Any third-party cGMP manufacturers that we may use are periodically subject to inspection by the FDA and other governmental agencies, and operations at these facilities could be interrupted or halted if the results of these inspections are unsatisfactory. Failure to comply with the FDA or other governmental regulations can result in fines, unanticipated compliance expenditures, recall or seizure of products, total or partial suspension of production, enforcement actions, injunctions and criminal prosecution, which could have a material adverse effect on our business and financial results.
Permits and approvals may be subject to revocation, modification or denial under certain circumstances. Our operations or activities could result in administrative or private actions, revocation of required permits or licenses, or fines, penalties or damages, which could have an adverse effect on us. In addition, we cannot predict the extent to which any legislation or regulation may affect the market for our products or our cost of doing business.
Concerns about ethylene oxide emissions have resulted in certain state actions against certain of our customers that are currently impacting these customers’ ability to use the ethylene oxide process to sterilize medical devices, which may, in turn, affect sales to these customers.
There is increased focus on the use and emissions of ethylene oxide by the EPA and state environmental agencies. Certain of the Company’s customers who use ethylene oxide in the U.S. for the sterilization of medical devices have received ongoing state and local scrutiny for environmental concerns at their facilities. This scrutiny is associated with the IRIS Assessment described in “Item 1. Business – Environmental and Regulatory Matters” of this report, which deemed exposure to ethylene oxide as unsafe at levels far below those found in the environment. The EPA began using the IRIS Assessment in 2020 to regulate change to existing permissible emissions limits at facilities that produce or use ethylene oxide in non-sterilization processes, and subsequently proposed rules for ethylene oxide sterilization facilities as well. These rules have yet to be finalized. Additionally, some state and local regulators have drawn their own conclusions from the IRIS Assessment, which has resulted in certain state actions against our customers that continue to impact these customers’ ability to use the ethylene oxide process to sterilize medical devices. Due to these regulatory actions, many customers have taken or are expected to take some voluntary downtime to install new abatement equipment. The installation of the new abatement equipment is being done ahead of what is expected to be changes in the EPA regulations. The Company remains confident that the sterilization industry will be able to install abatement equipment to satisfy the new forthcoming EPA requirements. The Company is working with various stakeholders to ensure the EPA considers all available assessments to appropriately quantify ethylene oxide's risks. While the Company believes that EPA will, as it has in the past, ultimately regulate to lower emissions levels based on a combined consideration of the various assessments available and that industry will then adopt practices and procedures to ensure compliance with these new regulations, there is no assurance that this will be the case. Further, additional regulatory requirements associated with the use and emission of ethylene oxide may be imposed in the future, both within and outside of the U.S. Such increased regulation could require our customers and/or the Company to temporarily suspend operations to install additional fugitive emissions control technology, limit the use of ethylene oxide or take other actions which could impact our business, financial condition or results of operations.
Item 1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
Cybersecurity is a critical part of our enterprise risk management. The Board, through its Audit Committee, oversees enterprise risk management, including cybersecurity. To more effectively address cybersecurity threats, we have numerous security layers within our least privilege network approach which is managed by our Information Technology Department. Our cybersecurity programs align with numerous standards and continues to grow and develop as new technologies emerge. Further, we have regular user awareness testing and trainings in place which helps keep all end users and executive leadership up-to-date on the most current threats. The global head of Information Security, possessing credentials in both information technology (“IT”) and cybersecurity, provides regular updates to senior management. Additionally, they provide at least an annual update, or more frequently if necessary, to both the Audit Committee and the full Board regarding the current threat landscape at Balchem, cybersecurity technologies, mitigation strategies, industry trends and best practices that we follow, major cybersecurity incidents (if any), and other areas of importance. The global head of Information Security has responsibility over cybersecurity management globally and reports directly to the Chief Financial Officer. Additional activities to maintain and enhance information security are discussed below.
•Reliable, Scalable Systems and Infrastructure
Our information security systems, infrastructure, and processes are built on and follow the U.S. National Institute of Standards and Technology ("NIST") framework for information security, which is a set of guidelines, accepted standards, and best practices for mitigating organization cybersecurity risks published by NIST. We continue to make significant investments in industry-leading and advanced technologies as part of our strategy to strengthen our security posture, business continuity capabilities, and ability to protect and safeguard systems and stakeholder data. Our Information Security Program and systems are tested and assessed annually by an independent third party.
•Automation and Artificial Intelligence
We have implemented automated systems to proactively test attack vectors by emulating inside and outside threats resulting in the validation of our ability to detect and defend against a cyber attack. Artificial intelligence is used as part of early warning systems designed to detect, alert, and respond to potential cyber threats.
•Training
Recognizing that information security, stakeholder data, and privacy principles involve more than just systems and infrastructure, we provide semi-annual cybersecurity education and training to all users with access to IT systems, devices, or applications. Internal social engineering phishing campaigns are conducted regularly with the goal of building a culture of cybersecurity, as well as raising awareness and reinforcing best practices across the organization.
Third parties also play a role in our cybersecurity. We engage third-party services to conduct evaluations of our security controls, whether through penetration testing, independent audits or consulting on best practices to address new challenges. These evaluations include testing both the design and operational effectiveness of security controls.
We apply a risk-based approach to mitigate cybersecurity risks associated with our use of third-party service providers and cybersecurity considerations affect the selection and oversight of these third-party service providers. We perform due diligence on third parties that have access to our most critical systems, data or facilities that house such systems or data.
While we have experienced cybersecurity threats in the past in the normal course of business and expect to continue to experience such threats from time to time, to date, none have had a material adverse effect on our business, financial condition, results of operations or cash flows. Even with the extensive approach we take to cybersecurity, we may not be successful in preventing or mitigating a cybersecurity incident that could have a material adverse effect on us. See Item 1A. “Risk Factors - Operational Risks - Disruptions or breaches of our information systems could adversely affect us” for a discussion of cybersecurity risks.
In the event of a possible cybersecurity incident, we would immediately implement our crisis management plan, which includes the following steps:
(1) Internal reporting and review of the incident or development
(2) Gathering and assessing information
(3) Developing and implementing a communications strategy
(5) Debrief and recovery
(4) Monitoring and evaluating a response As part of the gathering and assessment of information in step 2, we will consider various factors to make a materiality determination of the incident, including business impact, potential costs, impacted data, scope of the incident, possible litigation or regulatory implications, and reputational damage.
Item 2. Properties
Our corporate headquarters is located in Montvale, New Jersey. Our operations are conducted at our owned and leased facilities throughout the U.S. and other foreign countries. These facilities house manufacturing and warehousing operations, as well as administrative offices. We have a total of 38 locations across the world and some of these manufacturing and warehousing locations serve multiple segments.
The following is a summary of our principal properties:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Segment |
|
Location |
|
Administrative |
|
Manufacturing |
|
Warehousing |
| Corporate |
|
5 U.S. cities |
|
5 |
|
— |
|
— |
| HNH |
|
17 U.S. cities and 6 foreign countries |
|
1 |
|
16 |
|
6 |
| ANH |
|
9 U.S. cities and 3 foreign countries |
|
— |
|
10 |
|
2 |
| Specialty Products |
|
6 U.S. cities and 6 foreign countries |
|
2 |
|
8 |
|
2 |
| Other |
|
2 U.S. cities and 1 foreign country |
|
— |
|
3 |
|
— |
We believe that our production facilities and related machinery and equipment are well maintained, suitable for their purpose, and adequate to support our businesses.
Item 3. Legal Proceedings
In the normal course of business, we are involved in a variety of lawsuits, claims and legal proceedings, from time to time, including commercial and contract disputes, labor and employment matters, product liability claims, environmental liabilities, trade regulation matters, intellectual property disputes and tax-related matters. Further, in connection with normal operations at our plant facilities, our manufacturing sites may, from time to time, be subject to inspections or inquiries by the EPA and other agencies. To the extent any consent orders or other agreements are entered into as a result of findings from such inspections or inquiries, the Company is committed to ensuring compliance with such orders or agreements.
Information with respect to certain legal proceedings is included in Note 16, Commitments and Contingencies, to our Consolidated Financial Statements for the year ended December 31, 2023 contained in this Annual Report on Form 10-K, and is incorporated herein by reference.
In our opinion, we do not expect pending legal matters to have a material adverse effect on our consolidated financial position, results of operations, liquidity or cash flows.
Item 4. Mine Safety Disclosures
Not applicable.
INFORMATION ABOUT OUR EXECUTIVE OFFICERS
The following is a list of executive officers of the Company as of February 16, 2024.
Theodore L. Harris, age 58, has served as our Chairman, President and Chief Executive Officer since 2017.
C. Martin Bengtsson, age 46, has served as our Executive Vice President and Chief Financial Officer since February 2019.
Hatsuki Miyata, age 48, has served as our Executive Vice President, General Counsel and Secretary since July 2022. Ms. Miyata previously served as Deputy General Counsel and Corporate Secretary at Allegion plc, a global manufacturing company in seamless access and security products, from October 2018 to July 2022.
Frederic Boned, age 46, has served as our Senior Vice President and General Manager, Human Nutrition and Health, since November 2022. Prior to that, he served as Regional Vice President, Health Nutrition and Care – North America from January 2022 to November 2022, and Vice President, Human Nutrition and Health – North America from September 2018 to January 2022, each at DSM, a Dutch multinational corporation in the fields of health and nutrition.
Jonathan H. Griffin, age 48, has served as our Senior Vice President and General Manager, Animal Nutrition and Health, since September 2022. Prior to that, he led that business segment as our Vice President and General Manager, Animal Nutrition and Health from 2016 to September 2022.
Martin L. Reid, age 57, has served as our Senior Vice President and Chief Supply Chain Officer since September 2022. Prior to that, he served as Vice President and Chief Supply Chain Officer from January 2021 to September 2022. Mr. Reid served as Chief Supply Chain Officer at Godiva Chocolate from May 2019 to December 2020, and as Vice President, Supply Chain – North America Manufacturing at The Estee Lauder Companies, Inc., a multinational cosmetics company, prior to that.
Michael R. Sestrick, Ph.D., age 60, has served as our Senior Vice President and Chief Technology Officer since September 2022. Prior to that he served as our Vice President and Chief Technology officer from April 2017 to September 2022.
M. Brent Tignor, age 46, has served as our Senior Vice President and Chief Human Resources Officer since September 2022. Prior to that, he led the Human Resources department as our Vice President and Chief Human Resources Officer from February 2022 to September 2022 and as our Vice President, Human Resources from 2016 to February 2022.
Job L. van Gunsteren, age 48, has served as our Senior Vice President and General Manager, Specialty Products, since September 2022. Prior to that, he served as our Vice President and General Manager, Special Products from August 2019 to September 2022 and as our Director for Animal Nutrition and Health – EMEA from 2013 to 2019.
William A. Backus, age 57, has served as our Vice President and Chief Accounting Officer since October 2017. He also served as interim Chief Financial Officer from October 2018 to February 2019.
All above-listed officers except for Ms. Miyata, Mr. Boned, and Mr. Reid have been employed by the Company for more than the past five years. No family relationship exists between any of the above-listed executive officers of the Company. All officers are elected to hold office for one year or until their successors are elected and qualified or their earlier death, resignation or removal from office by the Board of Directors of the Company.
PART II
Item 5. Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
The Common Stock is listed on the Nasdaq Stock Market LLC under the symbol “BCPC.”
On February 2, 2024, the closing price for the Common Stock on the Nasdaq Stock Market LLC was $143.14.
Record Holders
As of February 2, 2024, the approximate number of holders of record of Common Stock was 64. Such number does not include stockholders who hold their stock in street name.
Performance Graph
The graph below sets forth the cumulative total stockholder return on the Common Stock (referred to in the table as “BCPC”) for the five years ended December 31, 2023, the overall stock market return during such period for shares comprising the Russell 2000® Index (which we believe includes companies with market capitalization similar to that of us), and the overall stock market return during such period for shares comprising the Dow Jones U.S. Specialty Chemicals Index, in each case assuming a comparable initial investment of $100 on December 31, 2018 and the subsequent reinvestment of dividends. The Russell 2000® Index measures the performance of the shares of the 2000 smallest companies included in the Russell 3000® Index. In light of our industry segments, we do not believe that published industry-specific indices are necessarily representative of stocks comparable to us. Nevertheless, we consider the Dow Jones U.S. Specialty Chemicals Index to be potentially useful as a peer group index with respect to us. The performance of the Common Stock shown on the graph below is historical only and not necessarily indicative of future performance.
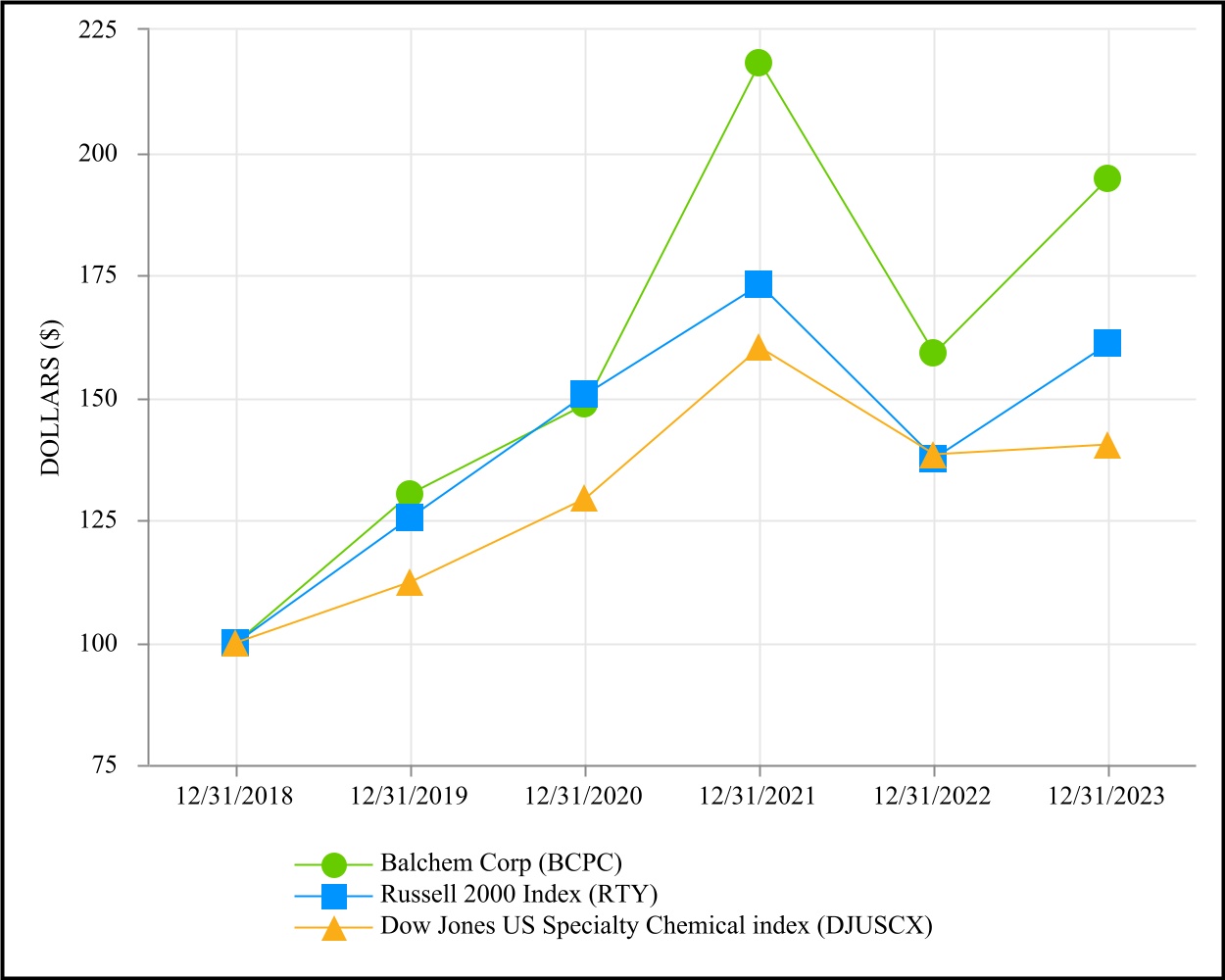
Issuer Purchase of Equity Securities
The following table summarizes the share repurchase activity for the year ended December 31, 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Number of Shares
Purchased (1)
|
|
Average Price Paid Per Share |
|
Total Number of Shares
Purchased as
Part of Publicly Announced
Programs(2)
|
|
Approximate Dollar Value of Shares that May Yet Be
Purchased Under the
Plans or Programs(2)(3)
|
| January 1-31, 2023 |
|
1,343 |
|
|
$ |
130.96 |
|
|
1,343 |
|
|
$ |
90,512,611 |
|
| February 1-28, 2023 |
|
26,766 |
|
|
$ |
137.24 |
|
|
26,766 |
|
|
$ |
91,178,224 |
|
| March 1-31, 2023 |
|
— |
|
|
$ |
— |
|
|
— |
|
|
$ |
91,178,224 |
|
| First Quarter |
|
28,109 |
|
|
|
|
28,109 |
|
|
|
|
|
|
|
|
|
|
|
|
| April 1-30, 2023 |
|
— |
|
|
$ |
— |
|
|
— |
|
|
$ |
91,178,224 |
|
| May 1-31, 2023 |
|
504 |
|
|
$ |
132.26 |
|
|
504 |
|
|
$ |
87,807,402 |
|
| June 1-30, 2023 |
|
63 |
|
|
$ |
134.81 |
|
|
63 |
|
|
$ |
89,488,765 |
|
| Second Quarter |
|
567 |
|
|
|
|
567 |
|
|
|
|
|
|
|
|
|
|
|
|
| July 1-31, 2023 |
|
482 |
|
|
$ |
128.54 |
|
|
482 |
|
|
$ |
85,264,695 |
|
| August 1-31, 2023 |
|
— |
|
|
$ |
— |
|
|
— |
|
|
$ |
85,264,695 |
|
| September 1-30, 2023 |
|
293 |
|
|
$ |
134.00 |
|
|
293 |
|
|
$ |
88,847,226 |
|
| Third Quarter |
|
775 |
|
|
|
|
775 |
|
|
|
|
|
|
|
|
|
|
|
|
| October 1-31, 2023 |
|
— |
|
|
$ |
— |
|
|
— |
|
|
$ |
88,847,226 |
|
| November 1-30, 2023 |
|
241 |
|
|
$ |
119.51 |
|
|
241 |
|
|
$ |
79,211,236 |
|
| December 1-31, 2023 |
|
2,866 |
|
|
$ |
144.94 |
|
|
2,866 |
|
|
$ |
95,651,484 |
|
| Fourth Quarter |
|
3,107 |
|
|
|
|
3,107 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
|
32,558 |
|
|
|
|
32,558 |
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) The Company repurchased (withheld) shares from employees solely in connection with the tax settlement of vested shares and/or exercised stock options under the Company's omnibus incentive plan. |
(2) Our Board of Directors has approved a stock repurchase program. The total authorization under this program is 3,763,038 shares. Since the inception of the program in June 1999, a total of 3,103,106 shares have been repurchased. Other than shares withheld for tax purposes, as described in footnote 1 above, no share repurchases were made under the Company's stock repurchase program during the year ended December 31, 2023. There is no expiration for this program. |
(3) Dollar amounts in this column equal the number of shares remaining available for repurchase under the stock repurchase program as of the last date of the applicable month multiplied by the monthly average price paid per share. |
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
(All amounts in thousands, except share and per share data)
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our Consolidated Financial Statements and the related notes included in this report. Refer to Part II, Item 7 in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 (filed with the SEC on February 24, 2023) for additional discussion of our financial condition and results of operations for the year ended December 31, 2021. In addition, discussion of year-to-year comparisons between 2022 and 2021 are not included in this Annual Report on Form 10-K, and can be found in "Management's Discussion and Analysis of Financial Condition and Results of Operations" in Part II, Item 7 of the Company's Annual Report on Form 10-K for the year ended December 31, 2022. Those statements in the following discussion that are not historical in nature should be considered to be forward-looking statements that are inherently uncertain. See “Cautionary Statement Regarding Forward-Looking Statements.”
Overview
We develop, manufacture, distribute and market specialty performance ingredients and products for the nutritional, food, pharmaceutical, animal health, medical device sterilization, plant nutrition and industrial markets. Our three reportable segments are strategic businesses that offer products and services to different markets: Human Nutrition and Health, Animal Nutrition and Health, and Specialty Products, as more fully described in Note 11, Segment Information, of the consolidated financial statements. Sales and production of products outside of our reportable segments and other minor business activities are included in "Other and Unallocated".
Segment Results
We sell products for all three segments through our own sales force, independent distributors, and sales agents.
The following tables summarize consolidated net sales by segment and business segment earnings from operations for the three years ended December 31, 2023, 2022 and 2021 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Business Segment Net Sales |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Human Nutrition and Health |
|
$ |
550,751 |
|
|
$ |
527,131 |
|
|
$ |
442,733 |
|
| Animal Nutrition and Health |
|
238,326 |
|
|
262,297 |
|
|
226,776 |
|
| Specialty Products |
|
125,965 |
|
|
131,438 |
|
|
117,020 |
|
Other and Unallocated (1) |
|
7,397 |
|
|
21,492 |
|
|
12,494 |
|
| Total |
|
$ |
922,439 |
|
|
$ |
942,358 |
|
|
$ |
799,023 |
|
|
|
|
|
|
|
|
| Business Segment Earnings From Operations |
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Human Nutrition and Health |
|
$ |
102,419 |
|
|
$ |
82,125 |
|
|
$ |
76,031 |
|
| Animal Nutrition and Health |
|
27,576 |
|
|
36,056 |
|
|
26,179 |
|
| Specialty Products |
|
34,579 |
|
|
32,789 |
|
|
30,020 |
|
Other and Unallocated (1) |
|
(5,381) |
|
|
(5,784) |
|
|
(4,728) |
|
| Total |
|
$ |
159,193 |
|
|
$ |
145,186 |
|
|
$ |
127,502 |
|
|
|
|
|
|
|
|
(1) Other and Unallocated consists of a few minor businesses which individually do not meet the quantitative thresholds for separate presentation and corporate expenses that have not been allocated to a segment. Unallocated corporate expenses consist of: (i) Transaction and integration costs, ERP implementation costs, and unallocated legal fees totaling $1,617, $3,581 and $1,264 for years ended December 31, 2023, 2022 and 2021, respectively, and (ii) Unallocated amortization expense of $312, $2,951, and $2,510 for years ended December 31, 2023, 2022, and 2021, respectively, related to an intangible asset in connection with a company-wide ERP system implementation. |
Acquisitions
On August 30, 2022, we completed the acquisition of Bergstrom, a leading science-based manufacturer of MSM, based in Vancouver, Washington, and on June 21, 2022, we completed the acquisition of Kappa, a leading science-based manufacturer of specialty vitamin K2 for the human nutrition industry, headquartered in Oslo, Norway. Details related to both acquisitions are disclosed in Note 2, Significant Acquisitions, and the "Acquisitions" section in Item 1. Business.
Results of Operations - Fiscal Year 2023 compared to Fiscal Year 2022
Summary of Consolidated Statements of Earnings
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
2023 |
|
2022 |
|
Increase
(Decrease) |
|
% Change |
| Net sales |
|
$ |
922,439 |
|
|
$ |
942,358 |
|
|
$ |
(19,919) |
|
|
(2.1) |
% |
| Gross margin |
|
302,056 |
|
|
280,451 |
|
|
21,605 |
|
|
7.7 |
% |
| Operating expenses |
|
142,863 |
|
|
135,265 |
|
|
7,598 |
|
|
5.6 |
% |
| Earnings from operations |
|
159,193 |
|
|
145,186 |
|
|
14,007 |
|
|
9.6 |
% |
| Interest and other expenses |
|
21,932 |
|
|
11,437 |
|
|
10,495 |
|
|
91.8 |
% |
| Income tax expense |
|
28,718 |
|
|
28,382 |
|
|
336 |
|
|
1.2 |
% |
| Net earnings |
|
$ |
108,543 |
|
|
$ |
105,367 |
|
|
$ |
3,176 |
|
|
3.0 |
% |
Management's discussion and analysis of the Consolidated Statements of Earnings is included below:
Net Sales
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase
(Decrease) |
|
|
| (in thousands) |
|
2023 |
|
2022 |
|
|
% Change |
| Human Nutrition and Health |
|
$ |
550,751 |
|
|
$ |
527,131 |
|
|
$ |
23,620 |
|
|
4.5 |
% |
| Animal Nutrition and Health |
|
238,326 |
|
|
262,297 |
|
|
(23,971) |
|
|
(9.1) |
% |
| Specialty Products |
|
125,965 |
|
|
131,438 |
|
|
(5,473) |
|
|
(4.2) |
% |
| Other |
|
7,397 |
|
|
21,492 |
|
|
(14,095) |
|
|
(65.6) |
% |
| Total |
|
$ |
922,439 |
|
|
$ |
942,358 |
|
|
$ |
(19,919) |
|
|
(2.1) |
% |
•The increase in net sales within the Human Nutrition and Health segment for 2023 compared to 2022 was primarily driven by the contribution from recent acquisitions, higher sales within the minerals and nutrients business, and a favorable impact related to changes in foreign currency rates, partially offset by lower sales within food and beverage markets. Total sales for this segment grew 4.5%, with average selling prices contributing 2.6%, volume and mix contributing 1.6%, and the change in foreign currency exchange rates contributing 0.3%.
•The decrease in net sales within the Animal Nutrition and Health segment for 2023 compared to 2022 was primarily driven by lower sales in both the monogastric and ruminant species markets, partially offset by incremental sales related to the Bergstrom acquisition, and a favorable impact related to changes in foreign currency exchange rates. Total sales for this segment decreased by 9.1%, with volume and mix contributing -6.3%, average selling prices contributing -3.5%, and the change in foreign currency exchange rates contributing 0.7%.
•The decrease in Specialty Products segment sales for 2023 compared to 2022 was primarily due to lower sales in both the plant nutrition and performance gases businesses, partially offset by a favorable impact related to changes in foreign currency exchange rates. Total sales for this segment decreased by 4.2%, with volume and mix contributing -9.4%, the change in foreign currency exchange rates contributing 0.7%, and average selling prices contributing 4.5%.
•Sales relating to Other decreased from the prior year primarily due to lower demand.
•Sales may fluctuate in future periods based on macroeconomic conditions, competitive dynamics, changes in customer preferences, and our ability to successfully introduce new products to the market.
Gross Margin
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
2023 |
|
2022 |
|
Increase
(Decrease) |
|
% Change |
| Gross margin |
|
$ |
302,056 |
|
|
$ |
280,451 |
|
|
$ |
21,605 |
|
|
7.7 |
% |
| % of net sales |
|
32.7 |
% |
|
29.8 |
% |
|
|
|
|
Gross margin dollars increased for 2023 compared to 2022 due to a decrease in cost of goods sold of $41,524. The 6.3% decrease in cost of goods sold was mainly driven by lower sales and certain lower manufacturing input costs.
Operating Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
2023 |
|
2022 |
|
Increase
(Decrease) |
|
% Change |
| Operating expenses |
|
$ |
142,863 |
|
|
$ |
135,265 |
|
|
$ |
7,598 |
|
|
5.6 |
% |
| % of net sales |
|
15.5 |
% |
|
14.4 |
% |
|
|
|
|
The increase in operating expenses was primarily due to restructuring-related impairment and asset disposal charges of $7,764, incremental operating expenses related to the Kappa and Bergstrom acquisitions of $7,699, and higher compensation-related expenses of $2,323, partially offset by favorable adjustments to transaction costs of $10,828.
Earnings From Operations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
2023 |
|
2022 |
|
Increase
(Decrease) |
|
% Change |
| Human Nutrition and Health |
|
$ |
102,419 |
|
|
$ |
82,125 |
|
|
$ |
20,294 |
|
|
24.7 |
% |
| Animal Nutrition and Health |
|
27,576 |
|
|
36,056 |
|
|
(8,480) |
|
|
(23.5) |
% |
| Specialty Products |
|
34,579 |
|
|
32,789 |
|
|
1,790 |
|
|
5.5 |
% |
| Other and unallocated |
|
(5,381) |
|
|
(5,784) |
|
|
403 |
|
|
7.0 |
% |
| Earnings from operations |
|
$ |
159,193 |
|
|
$ |
145,186 |
|
|
$ |
14,007 |
|
|
9.6 |
% |
|
|
|
|
|
|
|
|
|
| % of net sales (operating margin) |
|
17.3 |
% |
|
15.4 |
% |
|
|
|
|
•Human Nutrition & Health segment earnings from operations increased $20,294 and the gross margin contribution was $30,144. This was partially offset by an increase in operating expenses of $9,850, primarily due to the incremental operating expenses related to the Kappa and Bergstrom acquisitions of $7,502, restructuring-related impairment and asset disposal charges of $6,031, and an increase in amortization of $2,435, partially offset by favorable adjustments to transaction costs of $7,855.
•Animal Nutrition & Health segment earnings from operations decreased $8,480. Gross margin decreased $7,547 primarily due to aforementioned lower sales.
•Specialty Products segment earnings from operations increased $1,790, which was primarily driven by a 410 basis point increase in gross margin as a percent of sales. The increase in gross margin was due to higher average selling prices and decreases in certain manufacturing input costs. The increase was partially offset by an increase in operating expenses of $897, primarily driven by higher compensation-related expenses of $1,586.
•The increase in Other and unallocated was primarily driven by decreases of unallocated corporate expenses, partially offset by the aforementioned lower sales.
Other Expenses (Income)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
2023 |
|
2022 |
|
Increase
(Decrease) |
|
% Change |
| Interest expense, net |
|
$ |
22,613 |
|
|
$ |
10,268 |
|
|
$ |
12,345 |
|
|
120.2 |
% |
| Other, net |
|
(681) |
|
|
1,169 |
|
|
(1,850) |
|
|
(158.3) |
% |
|
|
$ |
21,932 |
|
|
$ |
11,437 |
|
|
$ |
10,495 |
|
|
91.8 |
% |
Interest expense for 2023 and 2022 was primarily related to outstanding borrowings under the 2022 Credit Agreement. The increase in interest expense is due to the additional borrowings in connection with the acquisitions and higher interest rates.
Income Tax Expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
2023 |
|
2022 |
|
Increase
(Decrease) |
|
% Change |
| Income tax expense |
|
$ |
28,718 |
|
|
$ |
28,382 |
|
|
$ |
336 |
|
|
1.2 |
% |
| Effective tax rate |
|
20.9 |
% |
|
21.2 |
% |
|
|
|
|
The decrease in the effective tax rate was primarily due to an increase in certain tax credits.
Liquidity and Capital Resources
(All amounts in thousands, except share and per share data)
Contractual Obligations
Our short-term purchase obligations primarily include contractual arrangements in the form of purchase orders with suppliers. As of December 31, 2023, such purchase obligations were $72,958. For debt obligations, see Note 8, Revolving Loan, and for operating and finance lease obligations, see Note 19, Leases.
We know of no current or pending demands on, or commitments for, our liquid assets that will materially affect our liquidity.
There were no material changes during the year ended December 31, 2023 outside the ordinary course of business in the specified contractual obligations set forth in our Annual Report on Form 10-K for the year ended December 31, 2022 other than the reduction of the contingent consideration liabilities to $100.
We expect our operations to continue generating sufficient cash flow to fund working capital requirements and necessary capital investments. We are actively pursuing additional acquisition candidates. We could seek additional bank loans or access to financial markets to fund such acquisitions, our operations, working capital, necessary capital investments or other cash requirements should we deem it necessary to do so.
Cash
Cash and cash equivalents decreased to $64,447 at December 31, 2023 from $66,560 at December 31, 2022. At December 31, 2023, we had $53,152 of cash and cash equivalents held by our foreign subsidiaries. We presently intend to permanently reinvest these funds in foreign operations by continuing to make additional plant related investments, and potentially invest in partnerships or acquisitions; therefore, we do not currently expect to repatriate these funds in order to fund U.S. operations or obligations. However, if these funds are needed for U.S. operations, we could be required to pay additional withholding taxes to repatriate these funds. Due to prevailing economic conditions of increased interest rates and subsequent borrowing costs, we remitted approximately $18,000 from our Belgium subsidiary to pay down U.S. debt, resulting in income tax expense of $20. The remittance was used to pay down U.S. debt. Working capital was $165,751 at December 31, 2023 as compared to $195,761 at December 31, 2022, a decrease of $30,010. Significant cash payments during the year included net payments on the revolving loan of $131,000, capital expenditures and intangible assets acquired of $37,892, and the payment of the 2022 declared dividend in 2023 of $22,872.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
2023 |
|
2022 |
|
Increase
(Decrease) |
|
% Change |
| Cash flows provided by operating activities |
|
$ |
183,761 |
|
|
$ |
138,536 |
|
|
$ |
45,225 |
|
|
32.6 |
% |
| Cash flows used in investing activities |
|
(34,813) |
|
|
(416,014) |
|
|
381,201 |
|
|
91.6 |
% |
| Cash flows (used in) provided by financing activities |
|
(153,321) |
|
|
246,679 |
|
|
(400,000) |
|
|
(162.2) |
% |
Operating Activities
The increase in cash flows from operating activities was primarily driven by the impact from changes in working capital.
Investing Activities
We continue to invest in corporate projects, improvements across all production facilities, and intangible assets. Total investments in property, plant and equipment and intangible assets were $37,892 and $49,945 for the years ended December 31, 2023 and 2022, respectively. Capital expenditures are projected to be approximately $35,000 to $40,000 for 2024. As mentioned above, we expect that our operations will continue to generate sufficient cash flow to fund the commitments for capital expenditures. These capital expenditures are part of our continuous efforts to support our growing businesses.
In 2022, we completed the acquisitions of Kappa and Bergstrom. Cash paid for these acquisitions, net of cash acquired, amounted to $1,252 and $365,780, for years ended December 31, 2023 and 2022, respectively.
Financing Activities
In 2023, we borrowed $18,000 to fund the payment of the 2022 dividend and made total loan payments of $149,000, resulting in $240,431 available under the 2022 Credit Agreement (see Note 8, Revolving Loan) as of December 31, 2023.
We have an approved stock repurchase program. The total authorization under this program is 3,763,038 shares. Since the inception of the program in June 1999, a total of 3,103,106 shares have been repurchased. We intend to acquire shares from time to time at prevailing market prices if and to the extent we deem it is advisable to do so based on our assessment of corporate cash flow, market conditions and other factors. Open market repurchases of common stock could be made pursuant to a trading plan established pursuant to Rule 10b5-1 under the Securities Exchange Act of 1934, as amended, which would permit common stock to be repurchased at a time that we might otherwise be precluded from doing so under insider trading laws or self-imposed trading restrictions. We also purchase (withhold) shares from employees in connection with the tax settlement of vested shares and/or exercised stock options under the Company's omnibus incentive plan. Share repurchases are funded with existing cash on hand.
Proceeds from stock options exercised were $5,242 and $3,212 for the years ended December 31, 2023 and 2022, respectively. Dividend payments were $22,872 and $20,713 during 2023 and 2022, respectively.
Other Matters Impacting Liquidity
We have a liability of $4,650 for uncertain tax positions, including the related interest and penalties, recorded in accordance with ASC 740-10, for which we are unable to reasonably estimate the timing of settlement, if any.
We currently provide postretirement benefits in the form of two retirement medical plans, as discussed in Note 15, Employee Benefit Plans. The liability recorded in other long-term liabilities on the consolidated balance sheets as of December 31, 2023 and December 31, 2022 was $1,395 and $1,465, respectively, and the plans are not funded. Historical cash payments made under these plans have typically been less than $200 per year. We do not anticipate any changes to the payments made in the current year for the plans.
Balchem NV ("Chemogas") has an unfunded defined benefit plan. The plan provides for the payment of a lump sum at retirement or payments in case of death of the covered employees. The amount recorded for these obligations on our balance sheet as of December 31, 2023 and December 31, 2022 was $420 and $393, respectively, and was included in other long-term obligations.
We provide an unfunded, nonqualified deferred compensation plan maintained for the benefit of a select group of management or highly compensated employees. Assets of the plan are held in a rabbi trust, which are included in "Other non-current assets" on the consolidated balance sheet. They are subject to additional risk of loss in the event of bankruptcy or insolvency of the Company. The deferred compensation liability as of December 31, 2023 and December 31, 2022 was $10,188 and $8,543, respectively, and is included in "Other long-term obligations" on the consolidated balance sheets. The related rabbi trust assets were $10,188 and $8,547 as of December 31, 2023 and December 31, 2022, respectively, and were included in "Other non-current assets" on the consolidated balance sheets.
Related Party Transactions
We were engaged in related party transactions with St. Gabriel CC Company, LLC for the years ended December 31, 2023 and December 31, 2022. Refer to Note 18, Related Party Transactions.
Critical Accounting Estimates
Critical accounting estimates are those estimates made in accordance with generally accepted accounting principles that involve a significant level of estimation uncertainty and have had or are reasonably likely to have a material impact on our financial condition or results of operations. Our management is required to make these critical accounting estimates and assumptions during the preparation of consolidated financial statements in accordance with accounting principles generally accepted in the United States of America. These estimates and assumptions impact the reported amount of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the consolidated financial statements. Estimates and assumptions are reviewed periodically, and the effects of revisions are reflected in the consolidated financial statements in the period they are determined to be necessary. Actual results could differ from those estimates.
Our critical accounting estimates are those that require application of management's most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and that may change in subsequent periods. Management considers the following to be critical accounting estimates.
Goodwill and Intangible Assets
The valuation methods and assumptions used in valuing goodwill and identified intangibles and assessing the impairment of goodwill and identified intangibles involves a significant level of estimation uncertainty. In addition, the assumptions used in determining the useful life of an intangible asset involves a significant level of estimation uncertainty. Refer to the Goodwill and Acquired Intangible Assets section in Note 1, Business Description and Summary of Significant Accounting Policies, for details related to the valuation and impairment process of both goodwill and intangible assets. Changes in market conditions, laws and regulations, and key assumptions made in future quantitative assessments, including expected cash flows, competitive factors and discount rates, could result in the recognition of an impairment charge, and in turn could have a material impact on our financial condition or results of operations in subsequent periods.
Contingent Consideration Liabilities
In connection with recent acquisitions (see Note 2, Significant Acquisitions), the sellers of each of the acquired entities had an opportunity to receive an additional payment if certain financial performance targets and other metrics were met, thereby requiring us to record contingent consideration liabilities on our balance sheet. The valuation methods and assumptions used in assessing the contingent consideration liabilities involve a significant level of estimation uncertainty, however, as of December 31, 2023, the earn-out periods concluded and the Company recorded a contingent consideration liability of $100.
Income Taxes
The valuation methods and assumptions used in calculating income taxes, deferred tax assets and liabilities, and valuation allowances involve a significant level of estimation uncertainty. Refer to the Income Taxes in Note 1, Business Description and Summary of Significant Accounting Policies, for details. Changes in the assumptions such as our forecast of future market growth, forecasted earnings, future taxable income, and prudent and feasible tax planning strategies could result in income taxes adjustments, and in turn could have a material impact on our financial condition or results of operations in subsequent periods.
Significant Accounting Policies and Recent Accounting Pronouncements
See Note 1, Business Description and Summary of Significant Accounting Policies, in Notes to Consolidated Financial Statements regarding significant accounting policies and recent accounting pronouncements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Our cash and cash equivalents are held primarily in checking accounts, certificates of deposit, and money market investment funds. In 2019, we entered into an interest rate swap and cross-currency swap for hedging purposes. These derivatives settled on their maturity date of June 27, 2023. Refer to details noted below (see Note 20, Derivative Instruments and Hedging Activities). Additionally, as of December 31, 2023, our borrowings were under a revolving loan bearing interest at a fluctuating rate as defined by the 2022 Credit Agreement plus an applicable rate (see Note 8, Revolving Loan). The applicable rate is based upon our consolidated net leverage ratio, as defined in the 2022 Credit Agreement. A 100 basis point increase or decrease in interest rates, applied to our borrowings at December 31, 2023, would result in an increase or decrease in annual interest expense and a corresponding reduction or increase in cash flow of approximately $3,096. We are exposed to commodity price risks, including prices of our primary raw materials. Our objective is to seek a reduction in the potential negative earnings impact of raw material pricing arising in our business activities. We manage these financial exposures, where possible, through pricing and operational means. Our practices may change as economic conditions change.
Interest Rate Risk
We have exposure to market risk for changes in interest rates, including the interest rate relating to the 2022 Credit Agreement. In the second quarter of 2019, we began to manage our interest rate exposure through the use of derivative instruments. These derivatives were utilized for risk management purposes, and were not used for trading or speculative purposes. We hedged a portion of our floating interest rate exposure using an interest rate swap (see Note 20, Derivative Instruments and Hedging Activities). This derivative settled on its maturity date of June 27, 2023.
Foreign Currency Exchange Risk
The financial condition and results of operations of our foreign subsidiaries are reported in local currencies and then translated into U.S. dollars at the applicable currency exchange rate for inclusion in our consolidated financial statements. Therefore, we are exposed to foreign currency exchange risk related to these currencies. In 2019, we entered into a cross-currency swap, with a notional amount of $108,569, which we designated as a hedge of our net investment in Chemogas (see Note 20, Derivative Instruments and Hedging Activities). This derivative settled on its maturity date of June 27, 2023.
Item 8. Financial Statements and Supplementary Data
|
|
|
|
|
|
|
|
|
| Index to Financial Statements and Supplementary Data: |
|
Page Numbers |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Balchem Corporation
Opinions on the Financial Statements and Internal Control Over Financial Reporting
We have audited the accompanying consolidated balance sheets of Balchem Corporation and its subsidiaries (the Company) as of December 31, 2023 and 2022, and the related consolidated statements of earnings, comprehensive income, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2023, and the related notes and schedule listed at Item 8 (collectively, the financial statements). We also have audited the Company’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013.
Basis for Opinions
The Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's financial statements and an opinion on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the financial statements included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control Over Financial Reporting
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Valuation of Reporting Units for Goodwill Impairment Testing
As described in Notes 1 and 6 to the financial statements, the Company’s goodwill balance was $779 million as of December 31, 2023. The Company performed an annual goodwill impairment test as of October 1, 2023 using a quantitative evaluation for each of its reporting units. The Company determines the fair value of its reporting units using the income approach, based on a discounted cash flow valuation model. To test for goodwill impairment, the Company compares the fair value of each reporting unit to its carrying value. When determining the fair value of each reporting unit, management makes significant estimates and assumptions related to a number of factors. The Company considers the impact of factors that are specific to each of the reporting units such as industry and economic changes as well as projected sales and expense growth rates based upon annual budgets and longer-range strategic plans, which are highly sensitive to changes in domestic and foreign economic conditions, and the selection of appropriate discount rates.
Given the significant estimates and assumptions management makes to determine the fair value of the reporting units and the sensitivity of the operations to changes in U.S. and foreign economic conditions, we identified management’s assumptions related to the sales and expense growth rates, the discount rates, and the terminal value calculation utilized in the valuation of the reporting units within the Company’s goodwill impairment tests as a critical audit matter. Auditing the reasonableness of management’s estimates and assumptions required a high degree of auditor judgment and an increased extent of effort, including the need to involve our fair value specialists.
Our audit procedures related to sales and expense growth rates, discount rates, and the terminal value calculation utilized in the valuation of the Company’s reporting units included the following, among others:
•We obtained an understanding of the relevant controls related to the valuation of the Company’s reporting units and tested such controls for design and operating effectiveness, including management review controls related to sales and expense growth rates and the selection of appropriate discount rates.
•We evaluated the reasonableness of management’s forecasts of sales and expense growth rates by comparing the forecasts to (1) the historical results, (2) internal communications to management and the Board of Directors, and (3) external communications made by management to analysts and investors, as applicable.
•We evaluated changes in the regulatory environment using industry reports containing analysis of the Company’s markets and assessed whether these changes were reflected in management’s forecasts of sales and expense growth rates.
•With the assistance of our fair value specialists, we evaluated the reasonableness of the discount rates and tested the relevance and reliability of source information underlying the determination of the discount rates, tested the mathematical accuracy of the calculation, and developed a range of independent estimates and compared those to the discount rates selected by management.
•With the assistance of our fair value specialists, we evaluated the reasonableness and tested the mathematical accuracy of the terminal value calculations.
/s/ RSM US LLP
We have served as the Company's auditor since 2004.
|
|
|
| New York, New York |
| February 16, 2024 |
BALCHEM CORPORATION
Consolidated Balance Sheets
December 31, 2023 and 2022
(Dollars in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
| Current assets: |
|
|
|
| Cash and cash equivalents |
$ |
64,447 |
|
|
$ |
66,560 |
|
|
Accounts receivable, net of allowance for doubtful accounts of $908 and $1,226 at
December 31, 2023 and 2022, respectively
|
125,284 |
|
|
131,578 |
|
| Inventories, net |
109,521 |
|
|
119,668 |
|
| Prepaid expenses |
7,798 |
|
|
4,903 |
|
| Derivative assets |
— |
|
|
5,993 |
|
| Other current assets |
7,192 |
|
|
7,101 |
|
| Total current assets |
314,242 |
|
|
335,803 |
|
| Property, plant and equipment, net |
276,039 |
|
|
271,355 |
|
| Goodwill |
778,907 |
|
|
769,509 |
|
| Intangible assets with finite lives, net |
191,212 |
|
|
213,295 |
|
| Right of use assets - operating leases |
17,763 |
|
|
17,094 |
|
| Right of use assets - finance lease |
2,101 |
|
|
2,338 |
|
| Other non-current assets |
16,947 |
|
|
15,118 |
|
| Total assets |
$ |
1,597,211 |
|
|
$ |
1,624,512 |
|
|
|
|
|
| Liabilities and Stockholders’ Equity |
|
|
|
| Current liabilities: |
|
|
|
| Trade accounts payable |
$ |
55,503 |
|
|
$ |
57,322 |
|
| Accrued expenses |
40,855 |
|
|
36,745 |
|
| Accrued compensation and other benefits |
17,228 |
|
|
16,544 |
|
| Dividends payable |
25,717 |
|
|
23,129 |
|
| Income tax payable |
4,967 |
|
|
2,280 |
|
| Operating lease liabilities - current |
3,949 |
|
|
3,796 |
|
| Finance lease liabilities - current |
272 |
|
|
226 |
|
| Total current liabilities |
148,491 |
|
|
140,042 |
|
| Revolving loan |
309,569 |
|
|
440,569 |
|
| Deferred income taxes |
52,046 |
|
|
62,784 |
|
| Operating lease liabilities - non-current |
14,601 |
|
|
13,806 |
|
| Finance lease liabilities - non-current |
1,943 |
|
|
2,213 |
|
| Other long-term obligations |
16,577 |
|
|
26,814 |
|
| Total liabilities |
543,227 |
|
|
686,228 |
|
| Commitments and contingencies (Note 16) |
|
|
|
| Stockholders’ equity: |
|
|
|
Preferred stock, $25 par value. Authorized 2,000,000 shares; none issued and outstanding |
— |
|
|
— |
|
Common stock, $.0667 par value. Authorized 120,000,000 shares; 32,254,728 shares issued and outstanding at December 31, 2023 and 32,152,787 shares issued and outstanding at December 31, 2022, respectively |
2,152 |
|
|
2,145 |
|
| Additional paid-in capital |
145,653 |
|
|
128,806 |
|
| Retained earnings |
897,488 |
|
|
814,487 |
|
| Accumulated other comprehensive income (loss) |
8,691 |
|
|
(7,154) |
|
| Total stockholders’ equity |
1,053,984 |
|
|
938,284 |
|
| Total liabilities and stockholders’ equity |
$ |
1,597,211 |
|
|
$ |
1,624,512 |
|
See accompanying notes to consolidated financial statements.
BALCHEM CORPORATION
Consolidated Statements of Earnings
Years Ended December 31, 2023, 2022 and 2021
(In thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
| Net sales |
$ |
922,439 |
|
|
$ |
942,358 |
|
|
$ |
799,023 |
|
|
|
|
|
|
|
| Cost of sales |
620,383 |
|
|
661,907 |
|
|
555,849 |
|
|
|
|
|
|
|
| Gross margin |
302,056 |
|
|
280,451 |
|
|
243,174 |
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
| Selling expenses |
74,397 |
|
|
67,409 |
|
|
60,413 |
|
| Research and development expenses |
15,049 |
|
|
12,191 |
|
|
13,524 |
|
| General and administrative expenses |
53,417 |
|
|
55,665 |
|
|
41,735 |
|
|
142,863 |
|
|
135,265 |
|
|
115,672 |
|
|
|
|
|
|
|
| Earnings from operations |
159,193 |
|
|
145,186 |
|
|
127,502 |
|
|
|
|
|
|
|
| Other expenses: |
|
|
|
|
|
|
|
|
|
|
|
| Interest expense, net |
22,613 |
|
|
10,268 |
|
|
2,456 |
|
| Other (income) expense, net |
(681) |
|
|
1,169 |
|
|
(187) |
|
|
21,932 |
|
|
11,437 |
|
|
2,269 |
|
|
|
|
|
|
|
| Earnings before income tax expense |
137,261 |
|
|
133,749 |
|
|
125,233 |
|
|
|
|
|
|
|
| Income tax expense |
28,718 |
|
|
28,382 |
|
|
29,129 |
|
|
|
|
|
|
|
| Net earnings |
$ |
108,543 |
|
|
$ |
105,367 |
|
|
$ |
96,104 |
|
|
|
|
|
|
|
| Basic net earnings per common share |
$ |
3.38 |
|
|
$ |
3.29 |
|
|
$ |
2.98 |
|
|
|
|
|
|
|
| Diluted net earnings per common share |
$ |
3.35 |
|
|
$ |
3.25 |
|
|
$ |
2.94 |
|
See accompanying notes to consolidated financial statements.
BALCHEM CORPORATION
Consolidated Statements of Comprehensive Income
Years Ended December 31, 2023, 2022 and 2021
(In thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
|
|
|
|
|
| Net earnings |
|
$ |
108,543 |
|
|
$ |
105,367 |
|
|
$ |
96,104 |
|
|
|
|
|
|
|
|
| Other comprehensive income (loss), net of tax: |
|
|
|
|
|
|
| Net foreign currency translation adjustment |
|
16,809 |
|
|
(4,799) |
|
|
(11,255) |
|
Unrealized (loss) gain on cash flow hedge, net of taxes of $341, $868, and $654 at December 31, 2023, 2022, and 2021, respectively |
|
(1,065) |
|
|
2,696 |
|
|
2,053 |
|
Net change in postretirement benefit plan, net of taxes of $39, $24, and $13 at December 31, 2023, 2022 and 2021, respectively |
|
101 |
|
|
(58) |
|
|
36 |
|
| Other comprehensive income (loss), net of tax |
|
15,845 |
|
|
(2,161) |
|
|
(9,166) |
|
|
|
|
|
|
|
|
| Comprehensive income |
|
$ |
124,388 |
|
|
$ |
103,206 |
|
|
$ |
86,938 |
|
See accompanying notes to consolidated financial statements.
BALCHEM CORPORATION
Consolidated Statements of Stockholders’ Equity
Years Ended December 31, 2023, 2022 and 2021
(Dollars in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
Stockholders'
Equity |
|
Retained
Earnings |
|
Accumulated
Other
Comprehensive
Income (Loss) |
|
Common Stock |
|
Additional
Paid-in
Capital |
| Shares |
|
Amount |
| Balance - December 31, 2020 |
$ |
828,233 |
|
|
$ |
656,740 |
|
|
$ |
4,173 |
|
|
32,372,621 |
|
|
$ |
2,160 |
|
|
$ |
165,160 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net earnings |
96,104 |
|
|
96,104 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
| Other comprehensive loss |
(9,166) |
|
|
— |
|
|
(9,166) |
|
|
— |
|
|
— |
|
|
— |
|
Dividends ($.64 per share) |
(20,706) |
|
|
(20,706) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
| Repurchases of common stock |
(35,239) |
|
|
— |
|
|
— |
|
|
(249,848) |
|
|
(17) |
|
|
(35,222) |
|
| Shares and options issued under stock plans |
17,789 |
|
|
— |
|
|
— |
|
|
164,377 |
|
|
11 |
|
|
17,778 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance - December 31, 2021 |
877,015 |
|
|
732,138 |
|
|
(4,993) |
|
|
32,287,150 |
|
|
2,154 |
|
|
147,716 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net earnings |
105,367 |
|
|
105,367 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
| Other comprehensive loss |
(2,161) |
|
|
— |
|
|
(2,161) |
|
|
— |
|
|
— |
|
|
— |
|
Dividends ($.71 per share) |
(23,018) |
|
|
(23,018) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
| Repurchases of common stock |
(35,423) |
|
|
— |
|
|
— |
|
|
(252,304) |
|
|
(16) |
|
|
(35,407) |
|
| Shares and options issued under stock plans |
16,504 |
|
|
— |
|
|
— |
|
|
117,941 |
|
|
7 |
|
|
16,497 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance - December 31, 2022 |
938,284 |
|
|
814,487 |
|
|
(7,154) |
|
|
32,152,787 |
|
|
2,145 |
|
|
128,806 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net earnings |
108,543 |
|
|
108,543 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
| Other comprehensive income |
15,845 |
|
|
— |
|
|
15,845 |
|
|
— |
|
|
— |
|
|
— |
|
Dividends ($.79 per share) |
(25,542) |
|
|
(25,542) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
| Repurchases of common stock, including excise tax * |
(4,514) |
|
|
— |
|
|
— |
|
|
(32,558) |
|
|
(2) |
|
|
(4,512) |
|
| Shares and options issued under stock plans |
21,368 |
|
|
— |
|
|
— |
|
|
134,499 |
|
|
9 |
|
|
21,359 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance - December 31, 2023 |
$ |
1,053,984 |
|
|
$ |
897,488 |
|
|
$ |
8,691 |
|
|
32,254,728 |
|
|
$ |
2,152 |
|
|
$ |
145,653 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| * On August 16, 2022, the U.S. government enacted the Inflation Reduction Act (the "IRA") into law. The IRA imposes a 1% excise tax on share repurchases, which is effective for repurchases completed after December 31, 2022. The excise tax is recorded within equity as part of the repurchase of the common stock. |
See accompanying notes to consolidated financial statements.
BALCHEM CORPORATION
Consolidated Statements of Cash Flows
Years Ended December 31, 2023, 2022 and 2021
(In thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
2023 |
|
2022 |
|
2021 |
| Cash flows from operating activities: |
|
|
|
|
|
| Net earnings |
$ |
108,543 |
|
|
$ |
105,367 |
|
|
$ |
96,104 |
|
|
|
|
|
|
|
| Adjustments to reconcile net earnings to net cash provided by operating activities: |
|
|
|
|
|
| Depreciation and amortization |
54,935 |
|
|
51,848 |
|
|
48,879 |
|
| Stock compensation expense |
16,052 |
|
|
13,224 |
|
|
10,802 |
|
| Deferred income taxes |
(10,814) |
|
|
(8,362) |
|
|
(5,944) |
|
| Provision for doubtful accounts |
37 |
|
|
401 |
|
|
180 |
|
| Unrealized (gain) loss on foreign currency transactions and deferred compensation |
(733) |
|
|
914 |
|
|
(384) |
|
| Asset impairment charge and (gain) loss on disposal of assets |
7,031 |
|
|
366 |
|
|
(53) |
|
| Change in fair value of contingent consideration liability |
(11,300) |
|
|
— |
|
|
— |
|
| Changes in assets and liabilities, net of acquired balances |
|
|
|
|
|
| Accounts receivable |
6,969 |
|
|
(3,618) |
|
|
(20,700) |
|
| Inventories |
10,530 |
|
|
(7,804) |
|
|
(21,023) |
|
| Prepaid expenses and other current assets |
(3,540) |
|
|
1,870 |
|
|
(881) |
|
| Accounts payable and accrued expenses |
3,552 |
|
|
(15,543) |
|
|
47,067 |
|
| Income taxes |
2,194 |
|
|
296 |
|
|
4,787 |
|
| Other |
305 |
|
|
(423) |
|
|
1,680 |
|
| Net cash provided by operating activities |
183,761 |
|
|
138,536 |
|
|
160,514 |
|
|
|
|
|
|
|
| Cash flows from investing activities: |
|
|
|
|
|
| Cash paid for acquisitions, net of cash acquired |
(1,252) |
|
|
(365,780) |
|
|
— |
|
| Capital expenditures and intangible assets acquired |
(37,892) |
|
|
(49,945) |
|
|
(37,363) |
|
| Proceeds from sale of assets |
1,881 |
|
|
206 |
|
|
318 |
|
| Proceeds from settlement of net investment hedge |
2,740 |
|
|
— |
|
|
— |
|
| Proceeds from insurance |
— |
|
|
— |
|
|
1,831 |
|
| Investment in affiliates |
(290) |
|
|
(495) |
|
|
(86) |
|
| Net cash used in investing activities |
(34,813) |
|
|
(416,014) |
|
|
(35,300) |
|
|
|
|
|
|
|
| Cash flows from financing activities: |
|
|
|
|
|
| Proceeds from revolving loan |
18,000 |
|
|
435,000 |
|
|
5,000 |
|
| Principal payments on revolving debt |
(149,000) |
|
|
(103,000) |
|
|
(60,000) |
|
| Principal payment on acquired debt |
— |
|
|
(30,988) |
|
|
— |
|
| Cash paid for financing costs |
— |
|
|
(1,232) |
|
|
— |
|
| Principal payments on finance lease |
(222) |
|
|
(177) |
|
|
(159) |
|
| Proceeds from stock options exercised |
5,242 |
|
|
3,212 |
|
|
6,943 |
|
| Dividends paid |
(22,872) |
|
|
(20,713) |
|
|
(18,723) |
|
| Repurchases of common stock |
(4,469) |
|
|
(35,423) |
|
|
(35,239) |
|
| Net cash (used in) provided by financing activities |
(153,321) |
|
|
246,679 |
|
|
(102,178) |
|
|
|
|
|
|
|
| Effect of exchange rate changes on cash |
2,260 |
|
|
(5,880) |
|
|
(4,368) |
|
|
|
|
|
|
|
| (Decrease) increase in cash and cash equivalents |
(2,113) |
|
|
(36,679) |
|
|
18,668 |
|
|
|
|
|
|
|
| Cash and cash equivalents beginning of period |
66,560 |
|
|
103,239 |
|
|
84,571 |
|
| Cash and cash equivalents end of period |
$ |
64,447 |
|
|
$ |
66,560 |
|
|
$ |
103,239 |
|
Supplemental Cash Flow Information - see Note 13
See accompanying notes to consolidated financial statements.
BALCHEM CORPORATION
Notes to Consolidated Financial Statements
(All amounts in thousands, except share and per share data)
NOTE 1 - BUSINESS DESCRIPTION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business Description
Balchem Corporation (“Balchem” or the “Company”), including, unless the context otherwise requires, its wholly-owned subsidiaries, incorporated in the State of Maryland in 1967, is engaged in the development, manufacture and marketing of specialty performance ingredients and products for the food, nutritional, feed, pharmaceutical, agricultural, and medical sterilization industries.
Principles of Consolidation
The consolidated financial statements include the financial statements of the Company and its subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation. Certain reclassifications have been made to prior period amounts to conform with the current period's presentation.
Revenue Recognition
Revenue for each of the Company’s business segments is recognized when control of the promised goods is transferred to our customers, in an amount that reflects the consideration we expect to realize in exchange for those goods. The Company reports amounts billed to customers related to shipping and handling as revenue and includes costs incurred for shipping and handling in cost of sales. Amounts received for unshipped merchandise are not recognized as revenue but rather they are recorded as customer deposits and are included in current liabilities. In instances of shipments made on consignment, revenue is recognized when control is transferred to the customer.
In accordance with Accounting Standards Codification ("ASC") 606, Revenue from Contracts with Customers, revenue-generating contracts are assessed to identify distinct performance obligations, allocating transaction prices to those performance obligations, and criteria for satisfaction of a performance obligation. The standard allows for recognition of revenue only when we have satisfied a performance obligation through transferring control of the promised good or service to a customer. Control, in this instance, may mean the ability to prevent other entities from directing the use of, and receiving benefit from, a good or service. The standard indicates that an entity must determine at contract inception whether it will transfer control of a promised good or service over time or satisfy the performance obligation at a point in time through analysis of the following criteria: (i) the entity has a present right to payment, (ii) the customer has legal title, (iii) the customer has physical possession, (iv) the customer has the significant risks and rewards of ownership and (v) the customer has accepted the asset. The Company assesses collectability based primarily on the customer’s payment history and on the creditworthiness of the customer.
Cash and Cash Equivalents
The Company considers all highly liquid investments with a maturity of three months or less to be cash equivalents. The Company has funds in its cash accounts that are with third party financial institutions, primarily in certificates of deposit and money market funds. The Company's balances of cash and cash equivalents in the U.S. and other countries exceed the insurance limits of the Federal Deposit Insurance Corporation (“FDIC”) and other relevant insurance limits in other countries.
Accounts Receivable
Credit terms are granted in the normal course of business to the Company’s customers and on-going credit evaluations are performed on the Company’s customers. In June 2016, the FASB issued Accounting Standards Update ("ASU") No. 2016-13, "Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments", which requires that credit losses be reported based on expected losses instead of the incurred loss model. Based on this ASU, customers' credit limits are adjusted based upon their reasonably expected credit worthiness which is determined through review of their payment history, their current credit information, and any foreseeable future events. Collections and payments from customers are continuously monitored and allowances for doubtful accounts for estimated losses resulting from the inability of the Company’s customers to make required payments are maintained. Estimated losses are based on historical experience, any specific customer collection issues identified, and any reasonably expected future adverse events. If the financial condition of our customers were to deteriorate resulting in an impairment of their ability to make payments, additional allowances and related bad debt expense may be required.
Inventories
Inventories are valued at the lower of cost (first in, first out) or net realizable value and have been reduced by an allowance for excess or obsolete inventories. Cost elements include material, labor and manufacturing overhead.
Property, Plant and Equipment and Depreciation
Property, plant and equipment are stated at cost.
Depreciation of plant and equipment is calculated using the straight-line method over the estimated useful lives of the assets as follows:
|
|
|
|
|
|
| Buildings |
15-25 years |
| Equipment |
2-28 years |
Expenditures for repairs and maintenance are charged to expense. Alterations and major overhauls that extend the lives or increase the capacity of plant assets are capitalized. When assets are retired or otherwise disposed of, the cost of the assets and the related accumulated depreciation are removed from the accounts and any resultant gain or loss is included in earnings from operations.
Business Concentrations
Financial instruments that subject the Company to credit risk consist primarily of accounts receivable and money market investments. Investments are managed within established guidelines to mitigate risks. Accounts receivable subject the Company to credit risk partially due to the concentration of amounts due from customers. The Company extends credit to its customers based upon an evaluation of the customers’ financial condition and credit histories. In 2023, 2022 and 2021, no customer accounted for more than 10% of total net sales or accounts receivable.
Post-employment Benefits
We provide life insurance, health care benefits, and defined benefit pension plan payments for certain eligible retirees and health care benefits for certain retirees’ eligible survivors. The costs and obligations related to these benefits reflect our assumptions as to health care cost trends and key economic conditions including discount rates, expected rate of return on plan assets, and expected salary increases. The cost of providing plan benefits also depends on demographic assumptions including retirements, mortality, turnover, and plan participation. If actual experience differs from these assumptions, the cost of providing these benefits could increase or decrease.
In accordance with ASC 715, “Compensation-Retirement Benefits,” we are required to recognize the overfunded or underfunded status of a defined benefit post retirement plan (other than a multiemployer plan) as an asset or liability in our statement of financial position, and to recognize changes in that funded status in the year in which the changes occur through comprehensive income.
Goodwill and Acquired Intangible Assets
Goodwill represents the excess of purchase price over the fair value of net assets acquired in accordance with ASC 805, "Business Combinations". Goodwill and intangible assets acquired in a business combination that have indefinite useful lives are not amortized but are instead assessed for impairment annually and more frequently if events and circumstances indicate that the assets might be impaired, in accordance with the provisions of ASC 350, "Intangibles-Goodwill and Other". The Company performed its annual test as of October 1. ASC 350 also requires that intangible assets with estimable useful lives be amortized over their respective estimated useful lives to their estimated residual values, and reviewed for impairment if events and circumstances indicate that the assets might be impaired.
In January 2017, the FASB issued ASU No. 2017-04, “Simplifying the Test for Goodwill Impairment” (“ASU 2017-04”), which addresses changes to the testing for goodwill impairment by eliminating Step 2 of the process. In accordance with this update, a goodwill impairment test will be performed by comparing the fair value of a reporting unit with its carrying amount. An impairment charge should be recognized for the amount by which the carrying amount exceeds the reporting unit’s fair value.
As of October 1, 2023 and 2022, the Company opted to bypass the qualitative assessment and proceeded directly to performing the quantitative goodwill impairment test. The Company assessed the fair values of its reporting units by utilizing the income approach, based on a discounted cash flow valuation model as the basis for its conclusions. The Company's estimates of future cash flows included significant management assumptions such as revenue growth rates, operating margins, discount rates, estimated terminal values and future economic and market conditions. The Company's assessment concluded that the fair values of the reporting units exceeded their carrying amounts, including goodwill. Accordingly, the goodwill of the reporting units was not considered impaired as of October 1, 2023 and 2022. The Company may resume performing the qualitative assessment in subsequent periods.
The Company had goodwill in the amount of $778,907 and $769,509 as of December 31, 2023 and 2022, respectively, subject to the provisions of ASC 350, “Intangibles-Goodwill and Other.”
|
|
|
|
|
|
|
|
|
| Goodwill at December 31, 2021 |
|
$ |
523,949 |
|
| Goodwill as a result of the Kappa acquisition |
|
216,295 |
|
| Goodwill as a result of the Bergstrom acquisition |
|
31,209 |
|
| Impact due to change in foreign exchange rates |
|
(1,944) |
|
| Goodwill at December 31, 2022 |
|
769,509 |
|
| Goodwill as a result of the Bergstrom acquisition |
|
341 |
|
| Impact due to change in foreign exchange rates |
|
9,057 |
|
| Goodwill at December 31, 2023 |
|
$ |
778,907 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
December 31, 2023 |
|
December 31, 2022 |
| HNH |
|
$ |
673,207 |
|
|
$ |
665,804 |
|
| ANH |
|
24,469 |
|
|
24,218 |
|
| Specialty Products |
|
81,175 |
|
|
79,429 |
|
| Other and Unallocated |
|
56 |
|
|
58 |
|
| Total |
|
$ |
778,907 |
|
|
$ |
769,509 |
|
The following intangible assets with finite lives are stated at cost and are amortized either on an accelerated basis or on a straight-line basis over the following estimated useful lives:
|
|
|
|
|
|
|
|
|
| |
|
Amortization Period
(in years) |
| Customer relationships and lists |
|
10 - 20 |
| Trademarks and trade names |
|
2 - 17 |
| Developed technology |
|
5 - 12 |
| Regulatory registration costs |
|
5 - 10 |
| Patents and trade secrets |
|
15 - 17 |
| Other |
|
2 - 18 |
Intangible assets with finite lives are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset, which is generally based on discounted cash flows. The useful life of an intangible asset is based on our assumptions regarding expected use of the asset; the relationship of the intangible asset to another asset or group of assets; any legal, regulatory or contractual provisions that may limit the useful life of the asset or that enable renewal or extension of the asset’s legal or contractual life without substantial cost; the effects of obsolescence, demand, competition and other economic factors; and the level of maintenance expenditures required to obtain the expected future cash flows from the asset and their related impact on the asset’s useful life. If events or circumstances indicate that the life of an intangible asset has changed, it could result in higher future amortization charges or recognition of an impairment loss. For the year ended December 31, 2023, there were no triggering events which required intangible asset impairment reviews.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are measured using enacted tax rates in effect for the fiscal year in which those temporary differences are expected to be recovered or settled. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. In evaluating our ability to recover our deferred tax assets, in full or in part, we consider all available positive and negative evidence, including our past operating results, our forecast of future market growth, forecasted earnings, future taxable income, and prudent and feasible tax planning strategies. The assumptions utilized in determining future taxable income require judgment and are consistent with the plans and estimates we are using to manage the underlying businesses.
We recognize uncertain income tax positions taken on income tax returns at the largest amount that is more likely than not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a fifty percent likelihood of being sustained.
Our policy for recording interest and penalties associated with uncertain tax positions is to record such items as a component of our income tax provision.
Use of Estimates
Management is required to make certain estimates and assumptions during the preparation of consolidated financial statements in accordance with accounting principles generally accepted in the United States of America. These estimates and assumptions impact the reported amount of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the consolidated financial statements and revenues and expenses during the reporting period. Estimates and assumptions are reviewed periodically, and the effects of revisions are reflected in the consolidated financial statements in the period they are determined to be necessary. Actual results could differ from those estimates.
Fair Value of Financial Instruments
The Company has a number of financial instruments, none of which are held for trading purposes. The estimated fair value amounts have been determined by the Company using available market information and appropriate valuation methodologies. Considerable judgment is required in interpreting market data to develop the estimates of fair value, and, accordingly, the estimates are not necessarily indicative of the amounts that the Company could realize in a current market exchange. The carrying value of debt approximates fair value as the interest rate is based on market and the Company’s consolidated leverage ratio. The Company’s financial instruments also include cash equivalents, accounts receivable, accounts payable and accrued liabilities, and are carried at cost which approximates fair value due to the short-term maturity of these instruments.
In addition, non-current assets includes rabbi trust funds related to the Company's deferred compensation plan. The money market and rabbi trust funds are valued using level one inputs, as defined by ASC 820, "Fair Value Measurement."
The Company also had derivative financial instruments, consisting of a cross-currency swap and an interest rate swap, which were included in derivative assets and derivative liabilities, in the consolidated balance sheets (see Note 20, Derivative Instruments and Hedging Activities). The fair values of these derivative instruments were determined based on Level 2 inputs, using significant inputs that were observable either directly or indirectly, including interest rate curves and implied volatilities. These derivatives were settled on their maturity date on June 27, 2023 and there were no other derivatives outstanding as of December 31, 2023.
Cost of Sales
Cost of sales are primarily comprised of raw materials and supplies consumed in the manufacture of product, as well as manufacturing labor, maintenance labor, depreciation expense, and direct overhead expense necessary to convert purchased materials and supplies into finished product. Cost of sales also includes inbound freight costs, outbound freight costs for shipping products to customers, warehousing costs, quality control and obsolescence expense.
Selling, General and Administrative Expenses
Selling expenses consist primarily of compensation and benefit costs, amortization of customer relationships and lists, trade promotions, advertising, commissions and other marketing costs. General and administrative expenses consist primarily of payroll and benefit costs, occupancy and operating costs of corporate offices, depreciation and amortization expense on non-manufacturing assets, information systems costs and other miscellaneous administrative costs.
Research and Development
Research and development costs are associated directly with the Company's efforts to develop, design, and enhance its products, services, technologies, or processes. Such costs are expensed as incurred.
Net Earnings Per Common Share
Basic net earnings per common share is calculated by dividing net earnings by the weighted average number of common shares outstanding during the period. Diluted net earnings per common share is calculated in a manner consistent with basic net earnings per common share except that the weighted average number of common shares outstanding also includes the dilutive effect of stock options outstanding, unvested restricted stock, and unvested performance shares (using the treasury stock method).
Stock-based Compensation
The Company has stock-based employee compensation plans, which are described more fully in Note 3, Stockholders' Equity. The Company accounts for stock-based compensation in accordance with ASC 718, “Compensation-Stock Compensation,” which requires all share-based payments, including grants of stock options, to be recognized in the statement of earnings as an operating expense, based on their fair values. The Company estimates the fair value of each option award on the date of grant using either the Black-Scholes model or the Binomial model, whichever is deemed to be most appropriate. Estimates of and assumptions about forfeiture rates, terms, volatility, interest rates and dividend yields are used to calculate stock-based compensation. A significant change to these estimates could materially affect the Company’s operating results.
Impairment of Long-lived Assets
Long-lived assets, such as property, plant, and equipment, and purchased intangibles subject to amortization, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset group to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset, which is generally based on discounted cash flows.
Derivative Instruments and Hedging Activities
The Company is exposed to market fluctuations in interest rates as well as variability in foreign exchange rates. In May 2019, the Company entered into an interest rate swap with JP Morgan Chase, N.A. (the "Swap Counterparty") and a cross-currency swap with JP Morgan Chase, N.A. (the "Bank Counterparty"). The Company's primary objective for holding derivative financial instruments was to manage interest rate risk and foreign currency risk. The Company does not enter into derivative financial instruments for trading or speculative purposes.
The derivative instruments were with the above single counterparty and were subject to a contractual agreement that provided for the net settlement of all contracts through a single payment in a single currency in the event of default on or termination of any one contract. As such, the derivative instruments were categorized as a master netting arrangement and presented as a net derivative asset or derivative liability on the consolidated balance sheet as of December 31, 2022. The Company settled its derivative instruments on their maturity date of June 27, 2023 and had no other derivatives outstanding as of December 31, 2023.
On a quarterly basis through their maturity, we assessed the effectiveness of the hedging relationships for the interest rate swap and cross-currency swap by reviewing the critical terms indicated in the applicable agreement. The hedging relationships were determined to be highly effective. As such, the net change in fair values of the interest rate swap, that qualified as a cash flow hedge, was recorded in accumulated other comprehensive income/(loss) and subsequently reclassified into interest expense as interest payments were made on our debt. For the cross-currency swap, the amounts that have not yet been recognized in earnings remain in the cumulative translation adjustment section of accumulated other comprehensive income until the hedged net investment is sold or liquidated in accordance with paragraphs 815-35-35-5A, "Derivatives and Hedging - Net Investment Hedges", and 830-30-40-1 through 40-1A, "Foreign Currency Matters - Derecognition". Refer to Note 20, Derivative Instruments and Hedging Activities, for detailed information about our derivative financial instruments.
Recently Issued Accounting Pronouncements
In December 2023, the FASB issued ASU 2023-09, "Income Taxes (Topic 740) - Improvements to Income Tax Disclosures." The new guidance is intended to enhance the transparency and decision usefulness of income tax disclosures by requiring disaggregated information about a reporting entity's effective tax rate reconciliation and information on income taxes paid. The amendment is effective for fiscal years beginning after December 15, 2024, with early adoption permitted. The amendment in this Update should be applied on a prospective basis, with retrospective application permitted. The Company is in the process of evaluating the impact that the adoption of ASU 2023-09 will have to the financial statements and related disclosures.
In November 2023, the FASB issued ASU 2023-07, "Segment Reporting (Topic 280) - Improvements to Reportable Segment Disclosures." The ASU expands reportable segment disclosure requirements by requiring disclosures of significant reportable segment expenses that are regularly provided to the Chief Operating Decision Maker (“CODM”) and included within each reported measure of a segment's profit or loss. The ASU also requires disclosure of the title and position of the individual identified as the CODM and an explanation of how the CODM uses the reported measures of a segment's profit or loss in assessing segment performance and deciding how to allocate resources. Additionally, ASU 2023-07 requires all segment profit or loss and assets disclosures to be provided on an annual and interim basis. ASU 2023-07 is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning December 15, 2024. Early adoption is permitted and the amendments must be applied retrospectively to all prior periods presented. The adoption of this guidance will not affect the Company's consolidated results of operations, financial position or cash flows. The Company is currently evaluating the effect the guidance will have on its disclosures.
In August 2023, the FASB issued ASU 2023-05, "Business Combinations - Joint Venture Formations (Subtopic 805-60): Recognition and Initial Measurement." The new guidance applies to the formation of a joint venture and requires a joint venture to initially measure all contributions received upon its formation at fair value. The guidance is intended to reduce diversity in practice and is applicable to joint venture entities with a formation date on or after January 1, 2025 on a prospective basis. While ASU 2023-05 is not currently applicable to Balchem, the Company will apply this guidance in future reporting periods after the guidance is effective to any future arrangements meeting the definition of a joint venture.
In March 2020, the FASB issued ASU 2020-04, "Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting", and in December 2022 subsequently issued ASU 2022-06, “Reference Rate Reform (Topic 848): Deferral of the Sunset Date of Topic 848.” These ASU’s provide temporary optional guidance to ease the potential burden in accounting for reference rate reform. The Standards Updates provide optional expedients and exceptions for applying accounting principles generally accepted in the United States to contract modifications and hedging relationships that reference LIBOR or another reference rate that are expected to be discontinued. The Standards Updates were effective upon issuance and can generally be applied through December 31, 2024. Due to the discontinuation of LIBOR and under the relief provided by Topic 848, during the third quarter of 2022, the Company modified its interest rate swap and replaced LIBOR with 1-month CME Term SOFR. The modification of the agreement did not have a significant impact on the Company's consolidated financial statements and disclosures. The interest rate swap matured on June 27, 2023.
NOTE 2 – SIGNIFICANT ACQUISITIONS
Cardinal Associates Inc. ("Bergstrom")
On August 30, 2022, the Company's wholly-owned subsidiary Albion Laboratories, Inc. ("Albion") entered into a Stock Purchase Agreement, and closed on such transaction with Cardinal Associates Inc. ("Cardinal"), a corporation organized under the laws of the State of Washington, pursuant to which Albion acquired Cardinal and its Bergstrom Nutrition business (collectively, "Bergstrom"). Bergstrom Nutrition is a leading science-based manufacturer of MSM, based in Vancouver, Washington. MSM is a widely used nutritional ingredient with strong scientific evidence supporting its benefits for joint health, sports nutrition, skin and beauty, healthy aging, and pet health. The addition of OptiMSM®, Bergstrom Nutrition's MSM brand, to the Company's portfolio within the Human Nutrition and Health and Animal Nutrition and Health segments provides a synergistic scientific advantage in Balchem's key strategic therapeutic focus areas such as longevity and performance and is a strong fit with Balchem's specialty, science-backed mineral products.
The Company made payments of $72,143 for the acquisition, amounting to $71,937 to the former shareholders or on behalf of the former shareholders and $206 to pay off Bergstrom's bank debt. Net of cash acquired of $773, total payments made to the former shareholders or on behalf of the former shareholders of Bergstrom were $71,164. The acquisition was primarily financed through the 2022 Credit Agreement (see Note 8, Revolving Loan). In connection with this transaction, the former shareholders of Bergstrom had an opportunity to receive an additional payment in 2024 if certain financial performance targets and other metrics were met. As of December 31, 2023, the earn-out periods concluded and the Company recorded a contingent consideration liability of $100 which was included in "Accrued expenses" on the consolidated balance sheets. The Company also made an additional post-closing payment of $910 in the third quarter of 2023 that was negotiated as a deduction of the cash consideration at closing. As a result, total payments related to the transaction are expected to be $72,243, comprised of the upfront cash consideration of $70,892, a working capital adjustment of $341, an additional post-closing payment of $910, and the fair value of the earn-out payment of $100.
The goodwill of $31,550 that arose on the acquisition date consists largely of expected synergies, including the combined entities' experience and technical problem-solving capabilities, and acquired workforce. 80% of the goodwill is assigned to the Human Nutrition and Health business segment and 20% of the goodwill is assigned to the Animal Nutrition and Health business segment. For tax purposes, a joint election under 338(h)(10) was made to treat the stock acquisition as a deemed asset acquisition, therefore generating tax amortizable goodwill.
The following table summarizes the fair values of the assets acquired and liabilities assumed:
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
773 |
|
| Accounts receivable |
|
4,699 |
|
| Inventories |
|
3,972 |
|
| Property, plant and equipment |
|
2,243 |
|
| Right of use assets |
|
866 |
|
| Customer relationships |
|
29,900 |
|
| Developed technology |
|
4,600 |
|
| Trademarks |
|
2,300 |
|
| Other assets |
|
197 |
|
| Accounts payable |
|
(699) |
|
| Bank debt |
|
(206) |
|
| Lease liabilities |
|
(871) |
|
| Other liabilities |
|
(462) |
|
| Goodwill |
|
31,550 |
|
| Total consideration on acquisition date and working capital adjustment |
|
78,862 |
|
| Net decrease to contingent consideration liability and other post-closing payments |
|
(6,825) |
|
| Total expected consideration |
|
72,037 |
|
| To pay off bank debt |
|
206 |
|
| Total expected payments |
|
$ |
72,243 |
|
The fair value of tangible and intangible assets acquired and liabilities assumed is based on management’s estimates and assumptions. In preparing our fair value estimates of the intangible assets and certain tangible assets acquired, management, among other things, consulted an independent advisor. Valuation methods utilized include net realizable value for inventory, multi-period excess earnings method for customer relationships, the relief from royalty method for other intangible assets, and a scenario-based approach for the contingent consideration.
Customer relationships are amortized over a 15-year period utilizing a percentage of excess earnings over economic life method. The corporate trademark and product trademarks are amortized over 2 years and 10 years, respectively, and developed technology is amortized over 12 years, utilizing the straight-line method as the consumption pattern of the related economic benefits cannot be reliably determined.
Transaction and integration costs related to the Bergstrom acquisition are included in general and administrative expenses and were $(10,614) and $4,604 for the years ended December 31, 2023 and 2022, respectively. There were no such amounts related to this acquisition for the year ended December 31, 2021. These amounts included favorable adjustments to transaction costs of $11,300 for the year ended December 31, 2023 and an unfavorable adjustment to transaction costs of $3,565 for the year ended December 31, 2022.
Kechu BidCo AS and Its Subsidiary Companies ("Kappa")
On June 21, 2022, Balchem Corporation and its wholly-owned subsidiary, Balchem B.V., completed the acquisition of Kechu BidCo AS and its subsidiary companies, including Kappa Bioscience AS, a leading science-based manufacturer of specialty vitamin K2 for the human nutrition industry, headquartered in Oslo, Norway (all acquired companies collectively referred to as “Kappa”). Kappa manufactures specialty vitamin K2, which plays a crucial role in the human body for bone health, heart health and immunity. Primarily, vitamin K2 supports the transport and distribution of calcium in the body. Vitamin K2 is important at all life stages, from pregnancy and early life to healthy aging. The acquisition strengthens the Company's scientific and technical expertise, geographic reach, and marketplace leadership, which should ultimately lead to accelerated growth for the Company's portfolios within the Human Nutrition and Health segment.
The Company made payments of approximately kr3,305,653 ("kr" indicates the Norwegian krone), amounting to approximately kr3,001,981 to the former shareholders and approximately kr303,672 to Kappa's lenders to pay off all Kappa bank debt. Net of cash acquired of kr63,064, total payments to the former shareholders were kr2,938,917. Net of gains on foreign currency forward contracts of $512 (see Note 20, Derivative Instruments and Hedging Activities), these payments translated to approximately $333,112, amounting to approximately $302,464 paid to the former shareholders and approximately $30,648 to Kappa's lenders. Net of cash acquired of $6,365, total payments made to the former shareholders of Kappa were approximately $296,099. The acquisition was primarily financed through the 2018 Credit Agreement (see Note 8, Revolving Loan). In connection with this transaction, the former shareholders of Kappa had an opportunity to receive an additional payment in 2024 if certain financial performance targets and other metrics were met. There was no contingent consideration liability recorded as of December 31, 2023.
The goodwill of $216,383 that arose on the acquisition date consists largely of expected synergies, including the combined entities' experience and technical problem-solving capabilities, and acquired workforce. The goodwill is assigned to the Human Nutrition and Health business segment and is not deductible for income tax purposes.
The following table summarizes the fair values of the assets acquired and liabilities assumed. The transactions were completed in Norwegian kroner ("NOK") and the amounts were translated to U.S. dollars ("USD") using the foreign currency exchange rate as of June 21, 2022.
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
$ |
6,365 |
|
| Accounts receivable |
|
8,036 |
|
| Inventories |
|
17,600 |
|
| Property, plant and equipment |
|
9,854 |
|
| Right of use assets |
|
3,349 |
|
| Customer relationships |
|
88,813 |
|
| Developed technology |
|
15,643 |
|
| Trademarks |
|
5,046 |
|
| Other assets |
|
2,399 |
|
| Accounts payable |
|
(3,301) |
|
| Bank debt |
|
(30,648) |
|
| Lease liabilities |
|
(3,349) |
|
| Other liabilities |
|
(4,461) |
|
| Deferred income taxes, net |
|
(24,716) |
|
| Goodwill |
|
216,383 |
|
| Total consideration on acquisition date |
|
307,013 |
|
| Decrease to contingent consideration liability |
|
(4,037) |
|
| Net gain on foreign currency exchange forward contracts |
|
(512) |
|
| Total expected consideration |
|
302,464 |
|
| Kappa bank debt paid on acquisition date |
|
30,648 |
|
| Total expected payments |
|
$ |
333,112 |
|
The fair value of tangible and intangible assets acquired and liabilities assumed is based on management’s estimates and assumptions. In preparing our fair value estimates of the intangible assets and certain tangible assets acquired, management, among other things, consulted an independent advisor. Valuation methods utilized include net realizable value for inventory, multi-period excess earnings method for customer relationships, the relief from royalty method for other intangible assets, and a scenario-based approach for the contingent consideration.
Customer relationships are amortized over a 15-year period utilizing a percentage of excess earnings over economic life method. The corporate trademark and product trademarks are amortized over 2 years and 10 years, respectively, and developed technology is amortized over 12 years, utilizing the straight-line method as the consumption pattern of the related economic benefits cannot be reliably determined.
Transaction and integration costs related to the Kappa acquisition are included in general and administrative expenses and were $533 and $(2,306) for the years ended December 31, 2023 and 2022, respectively. There were no such amounts related to this acquisition for the year ended December 31, 2021. The amount included a favorable adjustment to transaction costs of $4,037 for the year ended December 31, 2022.
The following selected unaudited pro forma information presents the consolidated results of operations as if the business combinations in 2022 had occurred as of January 1, 2021.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Twelve Months ended December 31, |
|
|
Net Sales |
|
Net Earnings |
Kappa & Bergstrom actual results included in the Company's consolidated income statement in 2023 |
|
$ |
59,532 |
|
|
$ |
5,487 |
|
|
|
|
|
|
Kappa & Bergstrom actual results included in the Company's consolidated income statement in 2022 |
|
$ |
22,158 |
|
|
$ |
(5,359) |
|
|
|
|
|
|
2023 Supplemental pro forma combined financial |
|
$ |
922,439 |
|
|
$ |
116,317 |
|
|
|
|
|
|
2022 Supplemental pro forma combined financial |
|
$ |
982,021 |
|
|
$ |
110,181 |
|
|
|
|
|
|
2021 Supplemental pro forma combined financial |
|
$ |
859,252 |
|
|
$ |
90,672 |
|
The above selected unaudited pro forma information includes the following acquisition-related adjustments: (1) additional amortization of intangible assets and depreciation of fixed assets; (2) adjustments related to the fair value of the acquired inventory, (3) adjustments to interest expense on borrowings at rates in effect during the related period, factoring in estimated payments based on free cash flow, and (4) other one-time adjustments.
The pro forma information presented does not purport to be indicative of the results that actually would have been attained if these acquisitions had occurred at the beginning of the periods presented and is not intended to be a projection of future results.
NOTE 3 - STOCKHOLDERS’ EQUITY
Stock-Based Compensation
All share-based payments, including grants of stock options, are recognized in the statements of earnings as operating expenses, based on their fair values.
The Company has made an estimate of expected forfeitures, based on its historical experience, and is recognizing compensation cost only for those stock-based compensation awards expected to vest.
The Company’s results for the years ended December 31, 2023, 2022 and 2021 reflected the following compensation cost and such compensation cost had the following effects on net earnings:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Increase/(Decrease) for the
Year Ended December 31, |
| |
|
2023 |
|
2022 |
|
2021 |
| Cost of sales |
|
$ |
1,900 |
|
|
$ |
1,302 |
|
|
$ |
845 |
|
| Operating expenses |
|
14,152 |
|
|
11,922 |
|
|
9,957 |
|
| Net earnings |
|
(12,375) |
|
|
(10,214) |
|
|
(8,370) |
|
On December 31, 2023, the Company had one share-based compensation plan under which awards may be granted, which is described below.
In June 2017, the Company’s shareholders approved the Balchem Corporation 2017 Omnibus Incentive Plan (“2017 Plan”) for officers, employees and directors of the Company and its subsidiaries. The 2017 Plan replaced the 1999 Stock Plan and amendments and restatements thereto (collectively to be referred to as the “1999 Plan"), which expired in April 2018. No further awards will be made under the 1999 Plan, and the shares that remained available for grant under the 1999 Plan will only be used to settle outstanding awards granted under the 1999 Plan and will not become available under the 2017 Plan. On June 22, 2023, the Company’s shareholders approved an amendment and restatement of the 2017 Plan (the “Amended 2017 Plan”). The Amended 2017 Plan is administered by the Compensation Committee of the Board of Directors of the Company. The Amended 2017 Plan provides as follows: (i) for a termination date of June 22, 2033; (ii) the authorization of 2,400,000 shares for future grants (which represents an increase of 800,000 shares from the amount approved under the 2017 Plan); (iii) for the making of grants of stock options, stock appreciation rights, restricted stock awards, restricted stock units, and other stock-based awards, as well as for the making of cash performance awards; (iv) except as provided by the Compensation Committee or in an employment agreement as in effect on the effective date of the Amended 2017 Plan, no automatic acceleration of outstanding awards upon the occurrence of a change in control of the Company; (v) certain annual limits on the number of shares and amount of cash that may be granted; (vi) for dividends or dividend equivalents otherwise payable on an unvested award to accrue and be paid only at such time as the vesting conditions applicable to the underlying award have been satisfied; (vii) for incentive compensation recovery if the Company is required to prepare an accounting restatement of its financial statements, in accordance with any compensation recovery policy adopted by the Company, applicable law, government regulations or national securities exchange requirements, or in the discretion of the Compensation Committee in the event of a restatement due to the Company’s material noncompliance with any financial reporting requirements under the securities laws; and (viii) for compliance with the requirements of Section 409A of the Internal Revenue Code of 1986, as amended (the “Internal Revenue Code” or the “Code”). No option will be exercisable for longer than ten years after the date of grant.
The shares to be issued upon exercise of the outstanding options have been approved, reserved and are adequate to cover all exercises. As of December 31, 2023, the Amended 2017 Plan had 1,034,260 shares available for future awards.
The Company has Restricted Stock Grant Agreements with the Company's non–employee directors and certain employees. Under the Restricted Stock Grant Agreements, certain shares of the Common Stock have been granted, ranging from 70 shares to 54,000 shares, to its non-employee directors and certain employees, subject to time-based vesting requirements.
The Company also has performance share (“PS”) awards, which provide the recipients the right to receive a certain number of shares of the Common Stock in the future, subject to an (1) EBITDA performance hurdle, where vesting is dependent upon the Company achieving a certain EBITDA percentage growth over the performance period, and (2) relative total shareholder return (“TSR”) market condition where vesting is dependent upon the Company’s TSR performance over the performance period (typically three years) relative to a comparator group consisting of the Russell 2000 index constituents.
The fair value of each option award issued under the Company’s stock plans is estimated on the date of grant using either the Black-Scholes model or the Binomial model, whichever is deemed to be most appropriate. For the years ended December 31, 2023, 2022, and 2021, the fair value of each option grant uses the assumptions noted in the following table. Expected volatilities are based on historical volatility of the Company’s stock. The expected term of the options is based on the Company’s historical experience of employees’ exercise behavior. Dividend yields are based on the Company’s historical dividend yields. Risk-free interest rates are based on the implied yields currently available on U.S. Treasury zero coupon issues with a remaining term equal to the expected life.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
| Weighted Average Assumptions: |
|
2023 |
|
2022 |
|
2021 |
| Expected Volatility |
|
28.1 |
% |
|
30.3 |
% |
|
32.9 |
% |
| Expected Term (in years) |
|
4.8 |
|
7.3 |
|
4.9 |
| Risk-Free Interest Rate |
|
3.9 |
% |
|
2.8 |
% |
|
0.5 |
% |
| Dividend Yield |
|
0.5 |
% |
|
0.5 |
% |
|
0.5 |
% |
The value of the restricted shares is based on the fair value of the award at the date of grant.
Performance Share expense is measured based on the fair value at the date of grant utilizing a Black-Scholes methodology to produce a Monte-Carlo simulation model which allows for the incorporation of the performance hurdles that must be met before the Performance Share vests. The assumptions used in the fair value determination were risk free interest rates of 4.2%, 1.8%, and 0.2%; dividend yields of 0.5%, 0.5%, and 0.6%; volatilities of 32%, 32%, and 33%; and initial TSR’s of 4.2%, -15.7%, and 11.7% in each case for the years ended December 31, 2023, 2022, and 2021, respectively. Expense is based on the estimated number of shares expected to vest, assuming the requisite service period is rendered and the probable outcome of the performance condition is achieved. The estimate is revised if subsequent information indicates that the actual number of shares likely to vest differs from previous estimates. Expense is ultimately adjusted based on the actual achievement of service and performance targets. The Performance Shares will cliff vest 100% at the end of the third year following the grant in accordance with the performance metrics set forth.
Compensation expense for stock options and stock awards is recognized on a straight-line basis over the vesting period, generally three to five years for stock options, three years for employee restricted stock awards, three years for employee performance share awards, and three years for non-employee director restricted stock awards.
A summary of stock option plan activity for 2023, 2022, and 2021 for all plans is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
|
|
# of
Shares
(000s) |
|
Weighted Average
Exercise Price |
|
# of
Shares
(000s) |
|
Weighted Average
Exercise Price |
|
# of
Shares
(000s) |
|
Weighted Average
Exercise Price |
| Outstanding at beginning of year |
|
1,045 |
|
|
$ |
99.82 |
|
|
867 |
|
|
$ |
88.19 |
|
|
858 |
|
|
$ |
80.58 |
|
| Granted |
|
109 |
|
|
138.09 |
|
|
239 |
|
|
139.04 |
|
|
129 |
|
|
119.12 |
|
| Exercised |
|
(64) |
|
|
81.98 |
|
|
(44) |
|
|
73.58 |
|
|
(109) |
|
|
63.42 |
|
| Forfeited |
|
(11) |
|
|
131.79 |
|
|
(17) |
|
|
124.89 |
|
|
(10) |
|
|
106.93 |
|
| Cancelled |
|
(1) |
|
|
138.07 |
|
|
— |
|
|
— |
|
|
(1) |
|
|
74.57 |
|
| Outstanding at end of year |
|
1,078 |
|
|
$ |
104.38 |
|
|
1,045 |
|
|
$ |
99.82 |
|
|
867 |
|
|
$ |
88.19 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Exercisable at end of year |
|
720 |
|
|
$ |
88.49 |
|
|
654 |
|
|
$ |
81.95 |
|
|
538 |
|
|
$ |
75.51 |
|
The aggregate intrinsic value for outstanding stock options was $47,889, $27,221 and $69,711 at December 31, 2023, 2022 and 2021, respectively, with a weighted average remaining contractual term of 5.7 years at December 31, 2023. Exercisable stock options at December 31, 2023 had an aggregate intrinsic value of $43,364 with a weighted average remaining contractual term of 4.4 years.
Other information pertaining to option activity during the years ended December 31, 2023, 2022 and 2021 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Years Ended December 31, |
| |
|
2023 |
|
2022 |
|
2021 |
| Weighted-average fair value of options granted |
|
$ |
40.91 |
|
|
$ |
44.77 |
|
|
$ |
33.11 |
|
| Total intrinsic value of stock options exercised ($000s) |
|
$ |
3,241 |
|
|
$ |
2,713 |
|
|
$ |
7,866 |
|
Additional information related to stock options outstanding under all plans at December 31, 2023 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
Options Outstanding |
|
Options Exercisable |
Range of Exercise
Prices |
|
Shares
Outstanding
(000s) |
|
Weighted
Average
Remaining
Contractual
Term |
|
Weighted
Average
Exercise
Price |
|
Number
Exercisable
(000s) |
|
Weighted
Average
Exercise
Price |
$54.87 - $85.33 |
|
387 |
|
|
3.5 |
|
$ |
72.41 |
|
|
387 |
|
|
$ |
72.41 |
|
$85.40 - $118.60 |
|
250 |
|
|
4.9 |
|
101.84 |
|
|
248 |
|
|
101.71 |
|
$118.96 - $150.85 |
|
441 |
|
|
8.1 |
|
133.93 |
|
|
85 |
|
|
123.47 |
|
| |
|
1,078 |
|
|
5.7 |
|
$ |
104.38 |
|
|
720 |
|
|
$ |
88.49 |
|
Non-vested restricted stock activity for the years ended December 31, 2023, 2022 and 2021 is summarized below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| |
|
Shares (000s) |
|
Weighted
Average Grant
Date Fair
Value |
|
Shares (000s) |
|
Weighted
Average Grant
Date Fair
Value |
|
Shares (000s) |
|
Weighted
Average Grant
Date Fair
Value |
| Non-vested balance at beginning of year |
|
122 |
|
|
$ |
124.42 |
|
|
166 |
|
|
$ |
99.7 |
|
|
159 |
|
|
$ |
90.71 |
|
| Granted |
|
40 |
|
|
137.20 |
|
|
46 |
|
|
137.17 |
|
|
42 |
|
|
123.58 |
|
| Vested |
|
(42) |
|
|
112.30 |
|
|
(82) |
|
|
82.15 |
|
|
(24) |
|
|
85.83 |
|
| Forfeited |
|
(4) |
|
|
128.06 |
|
|
(8) |
|
|
118.07 |
|
|
(11) |
|
|
90.49 |
|
| Non-vested balance at end of year |
|
116 |
|
|
$ |
133.06 |
|
|
122 |
|
|
$ |
124.42 |
|
|
166 |
|
|
$ |
99.7 |
|
Non-vested performance share activity for the years ended December 31, 2023, 2022 and 2021 is summarized below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| |
|
Shares (000s) |
|
Weighted
Average Grant
Date Fair
Value |
|
Shares (000s) |
|
Weighted
Average Grant
Date Fair
Value |
|
Shares (000s) |
|
Weighted
Average Grant
Date Fair
Value |
| Non-vested balance at beginning of year |
|
70 |
|
|
$ |
127.69 |
|
|
69 |
|
|
$ |
110.72 |
|
|
71 |
|
|
$ |
91.99 |
|
| Granted |
|
42 |
|
|
139.66 |
|
|
39 |
|
|
114.22 |
|
|
36 |
|
|
108.74 |
|
| Vested |
|
(36) |
|
|
98.84 |
|
|
(35) |
|
|
53.17 |
|
|
(24) |
|
|
70.64 |
|
| Forfeited |
|
— |
|
|
— |
|
|
(3) |
|
|
84.09 |
|
|
(14) |
|
|
81.03 |
|
| Non-vested balance at end of year |
|
76 |
|
|
$ |
135.25 |
|
|
70 |
|
|
$ |
127.69 |
|
|
69 |
|
|
$ |
110.72 |
|
As of December 31, 2023, 2022 and 2021, there was $18,817, $20,791 and $13,980, respectively, of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the plans. As of December 31, 2023, the unrecognized compensation cost is expected to be recognized over a weighted-average period of approximately 1.6 years. We estimate that share-based compensation expense for the year ended December 31, 2024 will be approximately $14,800.
Repurchase of Common Stock
The Company's Board of Directors has approved a stock repurchase program. The total authorization under this program is 3,763,038 shares. Since the inception of the program in June 1999, a total of 3,103,106 shares have been purchased. The Company intends to acquire shares from time to time at prevailing market prices if and to the extent it deems it is advisable to do so based on its assessment of corporate cash flow, market conditions and other factors. Open market repurchases of common stock could be made pursuant to trading plan established pursuant to Rule 10b5-1 under the Securities Exchange Act of 1934, as amended, which would permit common stock to be repurchased at a time that the Company might otherwise be precluded from doing so under insider trading laws or self-imposed trading restrictions. The Company also repurchases (withholds) shares from employees in connection with the tax settlement of vested shares and/or exercised stock options under the Company's omnibus incentive plan. Such repurchases of shares from employees are funded with existing cash on hand. During 2023, 2022, and 2021, the Company purchased 32,558, 252,304, and 249,848 shares, respectively, from open market purchases and from employees on a net-settlement basis to provide cash to employees to cover the associated employee payroll taxes. These shares were purchased at an average cost of $137.29, $140.40, and $141.04 per share, respectively.
NOTE 4 - INVENTORIES
Inventories, net of reserves at December 31, 2023 and 2022 consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Raw materials |
|
$ |
39,517 |
|
|
$ |
44,477 |
|
| Work in progress |
|
3,960 |
|
|
3,143 |
|
| Finished goods |
|
66,044 |
|
|
72,048 |
|
| Total inventories |
|
$ |
109,521 |
|
|
$ |
119,668 |
|
On a regular basis, the Company evaluates its inventory balances for excess quantities and obsolescence by analyzing demand, inventory on hand, sales levels and other information. Based on these evaluations, inventory balances are reserved, if necessary. The reserve for inventory was $2,463 and $2,640 at December 31, 2023 and 2022, respectively.
NOTE 5 - PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment at December 31, 2023 and 2022 are summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Land |
|
$ |
11,787 |
|
|
$ |
11,415 |
|
| Building |
|
104,363 |
|
|
90,644 |
|
| Equipment |
|
312,704 |
|
|
278,851 |
|
| Construction in progress |
|
59,981 |
|
|
79,928 |
|
| |
|
488,835 |
|
|
460,838 |
|
| Less: Accumulated depreciation |
|
212,796 |
|
|
189,483 |
|
| Property, plant and equipment, net |
|
$ |
276,039 |
|
|
$ |
271,355 |
|
Geographic Area Data - Long-Lived Assets (excluding intangible assets):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| United States |
|
$ |
203,692 |
|
|
$ |
211,588 |
|
| Foreign Countries |
|
72,347 |
|
|
59,767 |
|
| Total |
|
$ |
276,039 |
|
|
$ |
271,355 |
|
Depreciation expense was $26,373, $24,033 and $23,295 for the years ended December 31, 2023, 2022 and 2021, respectively.
In accordance with Topic 360, the Company reviews long-lived assets for impairment on an annual basis and also whenever events indicate that the carrying amount of the assets may not be fully recoverable. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset, which is generally based on discounted cash flows. Included in “General and administrative expenses” were restructuring-related impairment and asset disposal charges of $7,764 related to building, equipment, and construction in progress mainly in the Human Nutrition and Health and the Animal Nutrition and Health segments, for the year ended December 31, 2023. Such expenses for the year ended December 31, 2022 were not material.
NOTE 6 - INTANGIBLE ASSETS
The Company had goodwill in the amount of $778,907 and $769,509 as of December 31, 2023 and 2022, respectively, subject to the provisions of ASC 350, “Intangibles-Goodwill and Other.” The increase in goodwill is primarily due to foreign currency translation adjustments.
As of December 31, 2023 and 2022, the Company had identifiable intangible assets as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
| |
|
Amortization
Period
(In years) |
|
Gross
Carrying
Amount |
|
Accumulated
Amortization |
|
Gross
Carrying
Amount |
|
Accumulated
Amortization |
| Customer relationships and lists |
|
10-20 |
|
$ |
362,032 |
|
|
$ |
209,651 |
|
|
$ |
357,131 |
|
|
$ |
190,576 |
|
| Trademarks and trade names |
|
2-17 |
|
50,286 |
|
|
37,773 |
|
|
50,058 |
|
|
33,416 |
|
| Developed technology |
|
5-12 |
|
41,184 |
|
|
17,516 |
|
|
40,473 |
|
|
16,171 |
|
| Other |
|
2-18 |
|
25,733 |
|
|
23,083 |
|
|
25,041 |
|
|
19,245 |
|
| |
|
|
|
$ |
479,235 |
|
|
$ |
288,023 |
|
|
$ |
472,703 |
|
|
$ |
259,408 |
|
Amortization of identifiable intangible assets was $28,035, $27,271 and $25,092 for 2023, 2022 and 2021, respectively. Assuming no change in the gross carrying value of identifiable intangible assets, the estimated amortization expense is approximately $18,971 in 2024, $15,509 in 2025, $15,308 in 2026, $14,784 in 2027, and $14,387 in 2028. At December 31, 2023 and 2022, there were no identifiable intangible assets with indefinite useful lives as defined by ASC 350, “Intangibles-Goodwill and Other.” Identifiable intangible assets are reflected in the Company’s consolidated balance sheets under Intangible assets with finite lives, net. There were no changes to the useful lives of intangible assets subject to amortization in 2023 and 2022.
The Federal Insecticide, Fungicide and Rodenticide Act, (“FIFRA”), a health and safety statute, requires that certain products within our specialty products segment must be registered with the U.S. Environmental Protection Agency (the "EPA") because they are considered pesticides. Costs of such registrations are included as other in the table above.
NOTE 7 – EQUITY-METHOD INVESTMENT
In 2013, the Company and Eastman Chemical Company formed a joint venture (66.66% / 33.34% ownership), St. Gabriel CC Company, LLC, to design, develop, and construct an expansion of the Company’s St. Gabriel aqueous choline chloride plant. The Company contributed the St. Gabriel plant, at cost, and all continued expansion and improvements are funded by the owners. The joint venture became operational as of July 1, 2016. St. Gabriel CC Company, LLC is a Variable Interest Entity ("VIE") because the total equity at risk is not sufficient to permit the joint venture to finance its own activities without additional subordinated financial support. Additionally, voting rights (2 votes each) are not proportionate to the owners’ obligation to absorb expected losses or receive the expected residual returns of the joint venture. The Company will receive up to 2/3 of the production offtake capacity and absorbs operating expenses approximately proportional to the actual percentage of offtake. The joint venture is accounted for under the equity method of accounting since the Company is not the primary beneficiary as the Company does not have the power to direct the activities of the joint venture that most significantly impact its economic performance. The Company recognized a loss of $509, $559, and $557 for the years ended December 31, 2023, 2022, and 2021, respectively, relating to its portion of the joint venture’s expenses in other expense. The Company made capital contributions to the investment totaling $290, $355, and $85 for the years ended December 31, 2023, 2022, and 2021 respectively. The carrying value of the joint venture at December 31, 2023 and 2022 was $4,076 and $4,295, respectively, and is recorded in "Other non-current assets" on the consolidated balance sheets.
NOTE 8 – REVOLVING LOAN
On June 27, 2018, the Company and a bank syndicate entered into a credit agreement (the "2018 Credit Agreement"), which provided for revolving loans up to $500,000, due on June 27, 2023. During the second quarter of 2022, the Company borrowed $345,000 under the 2018 Credit Agreement to fund the Kappa acquisition (see Note 2, Significant Acquisitions). On July 27, 2022, the Company entered into an Amended and Restated Credit Agreement (the "2022 Credit Agreement") with certain lenders in the form of a senior secured revolving credit facility, due on July 27, 2027. The 2022 Credit Agreement allows for up to $550,000 of borrowing. The loans may be used for working capital, letters of credit, and other corporate purposes and may be drawn upon at the Company’s discretion. The Company used initial proceeds from the 2022 Credit Agreement to repay the outstanding balance of $433,569 due in June 2023 under the 2018 Credit Agreement. During the third quarter of 2022, the Company borrowed another $70,000 to fund the Bergstrom acquisition (see Note 2, Significant Acquisitions). As of December 31, 2023 and 2022, the total balance outstanding on the 2022 Credit Agreement amounted to $309,569 and $440,569, respectively. There are no installment payments required on the revolving loans; they may be voluntarily prepaid in whole or in part without premium or penalty, and all outstanding amounts are due on the maturity date.
Amounts outstanding under the 2022 Credit Agreement are subject to an interest rate equal to a fluctuating rate as defined by the 2022 Credit Agreement plus an applicable rate. The applicable rate is based upon the Company’s consolidated net leverage ratio, as defined in the 2022 Credit Agreement, and the interest rate was 6.580% at December 31, 2023. The Company is also required to pay a commitment fee on the unused portion of the revolving loan, which is based on the Company’s consolidated net leverage ratio as defined in the 2022 Credit Agreement and ranges from 0.150% to 0.225% (0.175% at December 31, 2023). The unused portion of the revolving loan amounted to $240,431 at December 31, 2023. The Company is also required to pay, as applicable, letter of credit fees, administrative agent fees, and other fees to the arrangers and lenders.
Costs associated with the issuance of the revolving loans are capitalized and amortized on a straight-line basis over the term of the 2022 Credit Agreement. Capitalized costs net of accumulated amortization totaled $1,030 and $1,317 at December 31, 2023 and 2022, respectively, and are included in "Other non-current assets" on the consolidated balance sheets. Amortization expense pertaining to these costs totaled $287, $335, and $282 for the years ended December 31, 2023, 2022, and 2021, respectively, and are included in "Interest expense" in the accompanying consolidated statements of earnings.
The 2022 Credit Agreement contains quarterly covenants requiring the consolidated leverage ratio to be less than a certain maximum ratio and the consolidated interest coverage ratio to exceed a certain minimum ratio. At December 31, 2023, the Company was in compliance with these covenants. Indebtedness under the Company’s loan agreements is secured by assets of the Company.
NOTE 9 - NET EARNINGS PER COMMON SHARE
The following presents a reconciliation of the net earnings and shares used in calculating basic and diluted net earnings per common share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
|
2023 |
|
2022 |
|
2021 |
| Net Earnings - Basic and Diluted |
|
$ |
108,543 |
|
|
$ |
105,367 |
|
|
$ |
96,104 |
|
|
|
|
|
|
|
|
| Share (000s) |
|
|
|
|
|
|
| Weighted Average Common Shares - Basic |
|
32,108 |
|
|
32,019 |
|
|
32,215 |
|
| Effect of Dilutive Securities – Stock Options, Restricted Stock, and Performance Shares |
|
340 |
|
|
374 |
|
|
457 |
|
| Weighted Average Common Shares - Diluted |
|
32,448 |
|
|
32,393 |
|
|
32,672 |
|
|
|
|
|
|
|
|
| Net Earnings Per Share - Basic |
|
$ |
3.38 |
|
|
$ |
3.29 |
|
|
$ |
2.98 |
|
| Net Earnings Per Share - Diluted |
|
$ |
3.35 |
|
|
$ |
3.25 |
|
|
$ |
2.94 |
|
The number of anti-dilutive shares were 354,619, 371,513, and 155,294 for 2023, 2022, and 2021. Anti-dilutive shares could potentially dilute basic earnings per share in future periods and therefore, were not included in diluted earnings per share.
NOTE 10 - INCOME TAXES
The Company’s effective tax rate for 2023, 2022 and 2021 was 20.9%, 21.2%, and 23.3%, respectively. The decrease from 2022 to 2023 is primarily due to an increase in certain tax credits.
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company regularly reviews its deferred tax assets for recoverability and would establish a valuation allowance if it believed that such assets may not be recovered, taking into consideration historical operating results, expectations of future earnings, changes in its operations and the expected timing of the reversals of existing temporary differences.
The Company considers the undistributed earnings of certain non-U.S. subsidiaries to be indefinitely reinvested outside of the United States on the basis of estimates that future domestic cash generation will be sufficient to meet future domestic cash needs. However, due to prevailing economic conditions of increased interest rates and subsequent borrowing costs, the Company remitted approximately $18,000 from its Belgium subsidiary and has incurred an income tax expense of approximately $20. The remittance was used to pay down U.S. debt. The Company had unremitted foreign earnings of approximately $109,000 and $94,000 for the years ended December 31, 2023 and 2022, respectively. The determination of the unrecognized deferred tax liability on those undistributed earnings is not practicable due to its legal entity structure and the complexity of U.S. and local country tax laws. If the Company decides to change its assertion on its remaining undistributed foreign earnings, it will need to recognize the income tax effects in the period it changes its assertion.
Income tax expense consists of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Current: |
|
|
|
|
|
|
| Federal |
|
$ |
27,306 |
|
|
$ |
26,423 |
|
|
$ |
25,019 |
|
| Foreign |
|
7,634 |
|
|
7,103 |
|
|
7,553 |
|
| State |
|
4,403 |
|
|
3,964 |
|
|
3,664 |
|
| Deferred: |
|
|
|
|
|
|
| Federal |
|
(7,737) |
|
|
(7,532) |
|
|
(3,709) |
|
| Foreign |
|
(2,285) |
|
|
(215) |
|
|
(3,038) |
|
| State |
|
(603) |
|
|
(1,361) |
|
|
(360) |
|
| Total income tax provision |
|
$ |
28,718 |
|
|
$ |
28,382 |
|
|
$ |
29,129 |
|
The provision for income taxes differs from the amount computed by applying the Federal statutory rate of 21% for 2023, 2022, and 2021 to earnings before income tax expense due to the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Income tax at Federal statutory rate |
|
$ |
28,825 |
|
|
$ |
28,087 |
|
|
$ |
26,299 |
|
| State income taxes, net of Federal income taxes |
|
2,513 |
|
|
1,862 |
|
|
2,406 |
|
| Stock Options |
|
(1,004) |
|
|
(676) |
|
|
(924) |
|
| Foreign-derived intangible income (FDII) |
|
(1,752) |
|
|
(1,778) |
|
|
(1,540) |
|
| Foreign rate differential |
|
946 |
|
|
2,066 |
|
|
1,188 |
|
| Other |
|
(810) |
|
|
(1,179) |
|
|
1,700 |
|
| Total income tax provision |
|
$ |
28,718 |
|
|
$ |
28,382 |
|
|
$ |
29,129 |
|
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities at December 31, 2023 and 2022 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Deferred tax assets: |
|
|
|
|
| Inventories |
|
$ |
1,049 |
|
|
$ |
1,038 |
|
| Restricted stock and stock options |
|
5,565 |
|
|
3,932 |
|
| Lease liabilities |
|
4,812 |
|
|
5,439 |
|
| Research and development |
|
12,653 |
|
|
4,134 |
|
| Other |
|
3,874 |
|
|
3,717 |
|
| Total deferred tax assets |
|
27,953 |
|
|
18,260 |
|
| Deferred tax liabilities: |
|
|
|
|
| Amortization |
|
$ |
(42,351) |
|
|
$ |
(46,688) |
|
| Depreciation |
|
(28,937) |
|
|
(25,097) |
|
| Prepaid expenses |
|
(421) |
|
|
(462) |
|
| Foreign currency and interest rate swaps |
|
(647) |
|
|
(1,456) |
|
| Right of use assets |
|
(4,574) |
|
|
(5,324) |
|
| Other |
|
(3,047) |
|
|
(1,995) |
|
| Total deferred tax liabilities |
|
(79,977) |
|
|
(81,022) |
|
|
|
|
|
|
| Valuation allowance |
|
(22) |
|
|
(22) |
|
|
|
|
|
|
| Net deferred tax liability |
|
$ |
(52,046) |
|
|
$ |
(62,784) |
|
As of December 31, 2023, the Company has state income tax net operating loss (NOL) carryforwards of $348. The state NOL carryforwards will expire between 2026 and 2035. The Company believes that the benefit from the state NOL carryforwards will not be realized, therefore, a valuation allowance has been established in the amount of $22.
Provisions of ASC 740-10 clarify whether or not to recognize assets or liabilities for tax positions taken that may be challenged by a tax authority. A reconciliation of the beginning and ending amount of unrecognized tax benefits, which is included in other long-term obligations on the Company’s consolidated balance sheets, is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Balance at beginning of period |
|
$ |
5,815 |
|
|
$ |
5,881 |
|
|
$ |
5,335 |
|
| Increases for tax positions of prior years |
|
1,353 |
|
|
2,194 |
|
|
806 |
|
| Decreases for tax positions of prior years |
|
(2,518) |
|
|
(2,260) |
|
|
(260) |
|
| Balance at end of period |
|
$ |
4,650 |
|
|
$ |
5,815 |
|
|
$ |
5,881 |
|
All of Balchem's unrecognized tax benefits, if recognized in future periods, would impact the Company's effective tax rate in such future periods.
The Company recognizes both interest and penalties as part of the income tax provision. During the years ended December 31, 2023 and 2022, these amounts were reduced by $322 and $371, respectively. During the year ended December 31, 2021, this amounted to $262. As of December 31, 2023 and 2022, accrued interest and penalties were $1,413 and $1,735, respectively.
Balchem files income tax returns in the U.S. and in various states and foreign countries. In the major jurisdictions where the Company operates, it is generally no longer subject to income tax examinations by tax authorities for years before 2019 and management does not anticipate any material change in the total amount of unrecognized tax benefits to occur within the next twelve months.
The European Union (“EU”) member states formally adopted the EU’s Pillar Two Directive, which was established by the Organization for Economic Co-operation and Development. Pillar Two generally provides for a 15 percent minimum effective tax rate for the jurisdictions where multinational enterprises operate. While the Company does not anticipate that this will have a material impact on its tax provision or effective tax rate, the Company continues to monitor evolving tax legislation in the jurisdictions in which it operates.
NOTE 11 - SEGMENT INFORMATION
Balchem Corporation reports three reportable segments: Human Nutrition and Health, Animal Nutrition and Health, and Specialty Products. Sales and production of products outside of our reportable segments and other minor business activities are included in "Other and Unallocated."
Human Nutrition and Health
The Human Nutrition and Health ("HNH") segment provides human grade choline nutrients and mineral amino acid chelated products through this segment for nutrition and health applications. Choline is recognized to play a key role in the development and structural integrity of brain cell membranes in infants, processing dietary fat, reproductive development and neural functions, such as memory and muscle function. The Company's mineral amino acid chelates, specialized mineral salts, and mineral complexes are used as raw materials for inclusion in premier human nutrition products; proprietary technologies have been combined to create an organic molecule in a form the body can readily assimilate. Sales growth for human nutrition applications is reliant on differentiation from lower-cost competitive products through scientific data, intellectual property and customers' appreciation of brand value. Consequently, the Company makes investments in such activities for long-term value differentiation. This segment also manufactures specialty vitamin K2, which plays a crucial role in the human body for bone health, heart health and immunity, and methylsulfonylmethane ("MSM"), which is a widely used nutritional ingredient that helps provide benefits for joint health, sports nutrition, skin and beauty, and healthy aging. This segment also serves the food and beverage industry for beverage, bakery, dairy, confectionary, and savory manufacturers. The Company partners with its customers from ideation through commercialization to bring on-trend beverages, baked goods, confections, dairy and meat products to market. The Company has expertise in trends analysis and product development. With its strong manufacturing capabilities in customized spray dried and emulsified powders, extrusion and agglomeration, blended lipid systems, liquid flavor delivery systems, juice and dairy bases, chocolate systems, ice cream bases and variegates, the Company is a one-stop solutions provider for beverage and dairy product development needs. Additionally, this segment provides microencapsulation solutions to a variety of applications in food, pharmaceutical and nutritional ingredients to enhance performance of nutritional fortification, processing, mixing, and packaging applications and shelf-life. Major product applications are baked goods, refrigerated and frozen dough systems, processed meats, seasoning blends, confections, sports and protein bars, dietary plans, and nutritional supplements. The Company also creates cereal systems for ready-to-eat cereals, grain-based snacks, and cereal based ingredients.
Animal Nutrition and Health
The Company’s Animal Nutrition and Health ("ANH") segment provides nutritional products derived from its microencapsulation and chelation technologies in addition to the essential nutrient choline chloride. For ruminant animals, the Company’s microencapsulated products boost health and milk production by delivering nutrient supplements that are biologically available, providing required nutritional levels. The Company’s proprietary chelation technology provides enhanced nutrient absorption for various species of production and companion animals and is marketed for use in animal feed throughout the world. ANH also manufactures and supplies choline chloride, an essential nutrient for monogastric animal health, predominantly to the poultry, pet and swine industries. Choline, which is manufactured and sold in both dry and aqueous forms, plays a vital role in the metabolism of fat. In poultry, choline deficiency can result in reduced growth rates and perosis in young birds, while in swine production choline is a necessary and required component of gestating and lactating sow diets for both liver health and prevention of leg deformity. This segment also manufactures MSM, which is a widely used nutritional ingredient that provides benefits for pet health.
Sales of value-added encapsulated products are highly dependent on overall industry economics as well as the Company's ability to leverage the results of university and field research on the animal health and production benefits of our products. Management believes that success in the commodity-oriented choline chloride marketplace is highly dependent on the Company’s ability to maintain its strong reputation for excellent product quality and customer service. The Company continues to drive production efficiencies in order to maintain its competitive-cost position to effectively compete in a competitive global marketplace.
Specialty Products
The Company re-packages and distributes a number of performance gases and chemicals for various uses by its customers, notably ethylene oxide, propylene oxide, and ammonia. Ethylene oxide is sold as a sterilant gas, primarily for use in the health care industry.
It is used to sterilize a wide range of medical devices because of its versatility and effectiveness in treating hard or soft surfaces, composites, metals, tubing and different types of plastics without negatively impacting the performance of the device being sterilized. Contract sterilizers and medical device manufacturers are principal customers for this product. Propylene oxide is marketed and sold as a fumigant to aid in the control of insects and microbiological spoilage, to reduce bacterial and mold contamination in certain shelled and processed nut meats, processed spices, cacao beans, cocoa powder, raisins, figs and prunes, and for various chemical synthesis applications, such as increasing paint durability and manufacturing specialty starches and textile coatings. Ammonia is used primarily as a refrigerant, for heat treatment of metals and various chemical synthesis applications, and is distributed in reusable and recyclable drum and cylinder packaging approved for use in the countries these products are shipped to.
The Company’s performance gases and chemicals are distributed worldwide in specially designed, reusable and recyclable drum and cylinder packaging, to assure compliance with safety, quality and environmental standards as outlined by the applicable regulatory agencies in the countries our products are shipped to. The Company’s inventory of these specially built drums and cylinders, along with its five filling facilities, represents a significant capital investment. The Company also sells single use canisters for use in sterilizing re-usable devices typically processed in autoclave units in hospitals.
The Company’s micronutrient agricultural nutrition business sells chelated minerals primarily to producers of high value crops. The Company has a unique and patented two-step approach to solving mineral deficiency in plants to optimize health, yield and shelf-life. First, the Company determines optimal mineral balance for plant health. The Company then has a foliar applied Metalosate® product range, utilizing patented amino acid chelate technology. Its products quickly and efficiently deliver mineral nutrients. As a result, the farmer/grower gets healthier crops that are more resistant to disease and pests, larger yields and healthier food for the consumer with extended shelf life for produce being shipped long distances.
The segment information is summarized as follows:
Business Segment Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Human Nutrition and Health |
|
$ |
1,180,527 |
|
|
$ |
1,170,238 |
|
| Animal Nutrition and Health |
|
166,994 |
|
|
175,972 |
|
| Specialty Products |
|
168,307 |
|
|
177,187 |
|
Other and Unallocated (1) |
|
81,383 |
|
|
101,115 |
|
| Total |
|
$ |
1,597,211 |
|
|
$ |
1,624,512 |
|
Business Segment Net Sales
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Human Nutrition and Health |
|
$ |
550,751 |
|
|
$ |
527,131 |
|
|
$ |
442,733 |
|
| Animal Nutrition and Health |
|
238,326 |
|
|
262,297 |
|
|
226,776 |
|
| Specialty Products |
|
125,965 |
|
|
131,438 |
|
|
117,020 |
|
Other and Unallocated (2) |
|
7,397 |
|
|
21,492 |
|
|
12,494 |
|
| Total |
|
$ |
922,439 |
|
|
$ |
942,358 |
|
|
$ |
799,023 |
|
Business Segment Earnings Before Income Taxes
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Human Nutrition and Health |
|
$ |
102,419 |
|
|
$ |
82,125 |
|
|
$ |
76,031 |
|
| Animal Nutrition and Health |
|
27,576 |
|
|
36,056 |
|
|
26,179 |
|
| Specialty Products |
|
34,579 |
|
|
32,789 |
|
|
30,020 |
|
Other and Unallocated (2) |
|
(5,381) |
|
|
(5,784) |
|
|
(4,728) |
|
| Interest and other expense |
|
(21,932) |
|
|
(11,437) |
|
|
(2,269) |
|
| Total |
|
$ |
137,261 |
|
|
$ |
133,749 |
|
|
$ |
125,233 |
|
Depreciation/Amortization
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Human Nutrition and Health |
|
$ |
38,568 |
|
|
$ |
33,728 |
|
|
$ |
30,012 |
|
| Animal Nutrition and Health |
|
7,876 |
|
|
6,685 |
|
|
7,414 |
|
| Specialty Products |
|
7,278 |
|
|
7,507 |
|
|
8,332 |
|
Other and Unallocated (2) |
|
1,213 |
|
|
3,928 |
|
|
3,121 |
|
| Total |
|
$ |
54,935 |
|
|
$ |
51,848 |
|
|
$ |
48,879 |
|
|
|
|
|
|
|
|
Capital Expenditures
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Human Nutrition and Health |
|
$ |
26,415 |
|
|
$ |
33,668 |
|
|
$ |
23,714 |
|
| Animal Nutrition and Health |
|
6,993 |
|
|
10,809 |
|
|
8,100 |
|
| Specialty Products |
|
3,535 |
|
|
4,004 |
|
|
3,804 |
|
Other and Unallocated (2) |
|
331 |
|
|
605 |
|
|
524 |
|
| Total |
|
$ |
37,274 |
|
|
$ |
49,086 |
|
|
$ |
36,142 |
|
|
|
|
(1) Other and Unallocated assets consist of certain cash, capitalized loan issuance costs, other assets, investments, and income taxes, which the Company does not allocate to its individual business segments. It also includes assets associated with a few minor businesses which individually do not meet the quantitative thresholds for separate presentation. |
(2) Other and Unallocated consists of a few minor businesses which individually do not meet the quantitative thresholds for separate presentation and corporate expenses that have not been allocated to a segment. Unallocated corporate expenses consist of: (i) Transaction and integration costs, ERP implementation costs, and unallocated legal fees totaling $1,617, $3,581 and $1,264 for years ended December 31, 2023, 2022 and 2021, respectively, and (ii) Unallocated amortization expense of $312, $2,951, and $2,510 for years ended December 31, 2023, 2022, and 2021, respectively, related to an intangible asset in connection with a company-wide ERP system implementation. |
NOTE 12 - REVENUE
Revenue Recognition
Revenues are recognized when control of the promised goods is transferred to customers, in an amount that reflects the consideration we expect to realize in exchange for those goods.
The following table presents revenues disaggregated by revenue source. Sales and usage-based taxes are excluded from revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Product Sales Revenue |
|
$ |
919,951 |
|
|
$ |
939,166 |
|
|
$ |
794,518 |
|
| Royalty Revenue |
|
2,488 |
|
|
3,192 |
|
|
4,505 |
|
| Total Revenue |
|
$ |
922,439 |
|
|
$ |
942,358 |
|
|
$ |
799,023 |
|
The following table presents revenues disaggregated by geography, based on customers' delivery addresses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| United States |
|
$ |
689,601 |
|
|
$ |
682,238 |
|
|
$ |
584,661 |
|
| Foreign Countries |
|
232,838 |
|
|
260,120 |
|
|
214,362 |
|
| Total |
|
$ |
922,439 |
|
|
$ |
942,358 |
|
|
$ |
799,023 |
|
Product Sales Revenues
The Company’s primary operation is the manufacturing and sale of health and wellness ingredient products, in which the Company receives an order from a customer and fulfills that order. The Company’s product sales are considered point-in-time revenue.
Royalty Revenues
Royalty revenue consists of agreements with customers to use the Company’s intellectual property in exchange for a sales-based royalty. Royalties are considered over time revenue and are recorded in the HNH segment.
Contract Liabilities
The Company records contract liabilities when cash payments are received or due in advance of performance, including amounts which are refundable.
The Company’s payment terms vary by the type and location of customers and the products offered. The term between invoicing and when payment is due is not significant. For certain products or services and customer types, the Company requires payment before the products are delivered to the customer.
Practical Expedients and Exemptions
The Company generally expenses sales commissions when incurred because the amortization period would have been one year or less. These costs are recorded within selling and marketing expenses.
The Company does not disclose the value of unsatisfied performance obligations for (i) contracts with an original expected length of one year or less and (ii) contracts for which the Company recognizes revenue at the amount to which it has the right to invoice for products shipped.
NOTE 13 - SUPPLEMENTAL CASH FLOW INFORMATION
Cash paid during the year for:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Income taxes |
|
$ |
35,725 |
|
|
$ |
33,016 |
|
|
$ |
25,355 |
|
| Interest |
|
$ |
25,933 |
|
|
$ |
11,879 |
|
|
$ |
4,547 |
|
Non-cash financing and investing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2023 |
|
2022 |
|
2021 |
| Dividends payable |
|
$ |
25,717 |
|
|
$ |
23,129 |
|
|
$ |
20,886 |
|
| Contingent consideration liability |
|
$ |
— |
|
|
$ |
11,872 |
|
|
$ |
— |
|
NOTE 14 – ACCUMULATED OTHER COMPREHENSIVE INCOME
The changes in accumulated other comprehensive income (loss) were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Years Ended December 31, |
| |
|
2023 |
|
2022 |
|
2021 |
| Net foreign currency translation adjustment |
|
$ |
16,809 |
|
|
$ |
(4,799) |
|
|
$ |
(11,255) |
|
|
|
|
|
|
|
|
| Net change of cash flow hedge (see Note 20 for further information) |
|
|
|
|
|
|
| Unrealized (loss) gain on cash flow hedge |
|
(1,406) |
|
|
3,564 |
|
|
2,707 |
|
| Tax |
|
341 |
|
|
(868) |
|
|
(654) |
|
| Net of tax |
|
(1,065) |
|
|
2,696 |
|
|
2,053 |
|
|
|
|
|
|
|
|
| Net change in postretirement benefit plan (see Note 15 for further information) |
|
|
|
|
|
|
| Prior service loss (gain) arising during the period |
|
132 |
|
|
(41) |
|
|
(4) |
|
| Amortization of prior service gain |
|
— |
|
|
9 |
|
|
74 |
|
| Amortization of loss (gain) |
|
8 |
|
|
(2) |
|
|
(21) |
|
| Total before tax |
|
140 |
|
|
(34) |
|
|
49 |
|
| Tax |
|
(39) |
|
|
(24) |
|
|
(13) |
|
| Net of tax |
|
101 |
|
|
(58) |
|
|
36 |
|
|
|
|
|
|
|
|
| Total other comprehensive income/(loss) |
|
$ |
15,845 |
|
|
$ |
(2,161) |
|
|
$ |
(9,166) |
|
Included in "Net foreign currency translation adjustment" was loss of $1,455 related to a net investment hedge, which was net of tax benefit of $471 for the year ended December 31, 2023, and gains of $3,851, and $4,766, related to a net investment hedge, net of tax expenses of $1,236, and $1,527, for the years ended December 31, 2022 and 2021, respectively. See Note 20, Derivative Instruments and Hedging Activities.
Accumulated other comprehensive loss at December 31, 2023 and 2022 consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Foreign currency
translation
adjustment |
|
Cash flow hedge |
|
Postretirement benefit plan |
|
Total |
| Balance December 31, 2022 |
|
$ |
(8,401) |
|
|
$ |
1,065 |
|
|
$ |
182 |
|
|
$ |
(7,154) |
|
| Other comprehensive income (loss) |
|
16,809 |
|
|
(1,065) |
|
|
101 |
|
|
15,845 |
|
| Balance December 31, 2023 |
|
$ |
8,408 |
|
|
$ |
— |
|
|
$ |
283 |
|
|
$ |
8,691 |
|
NOTE 15 - EMPLOYEE BENEFIT PLANS
Defined Contribution Plans
The Company sponsors one 401(k) savings plan for eligible employees, which allows participants to make pretax or after tax contributions and the Company matches certain percentages of those contributions. The plan also has a discretionary profit sharing portion and matches 401(k) contributions with shares of the Company’s Common Stock. All amounts contributed to the plan are deposited into a trust fund administered by independent trustees. On June 21, 2022, the Company completed the acquisition of Kappa, which sponsors one defined contribution plan for its employees. In addition, on August 30, 2022, the Company completed the acquisition of Bergstrom, which sponsored one defined contribution plan for its employees. The Bergstrom plan merged into the Company sponsored 401(k) savings plan on January 1, 2023. The Company provided for matching 401(k) savings plan contributions of $4,381, $4,363, and $4,142 in 2023, 2022 and 2021, respectively. Profit sharing contributions in 2023, 2022, and 2021 were not material.
Postretirement Medical Plans
The Company provides postretirement benefits in the form of two unfunded postretirement medical plans; one that is under a collective bargaining agreement and covers eligible retired employees of the Verona, Missouri facility and a plan for executive officers of the Company who meet eligibility requirements as set forth in the Company's Officer Retiree Program. The Company uses a December 31 measurement date for its postretirement medical plans. In accordance with ASC 715, “Compensation—Retirement Benefits,” the Company is required to recognize the over funded or underfunded status of a defined benefit post retirement plan (other than a multiemployer plan) as an asset or liability in its statement of financial position, and to recognize changes in that funded status in the year in which the changes occur through comprehensive income.
The actuarial recorded liabilities for such unfunded postretirement benefits are as follows:
Change in benefit obligation:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Benefit obligation at beginning of year |
|
$ |
1,465 |
|
|
$ |
1,293 |
|
| Service cost with interest to end of year |
|
108 |
|
|
79 |
|
| Interest cost |
|
62 |
|
|
26 |
|
| Participant contributions |
|
23 |
|
|
27 |
|
| Benefits paid |
|
(30) |
|
|
(69) |
|
| Actuarial (gain) loss |
|
(233) |
|
|
109 |
|
| Benefit obligation at end of year |
|
$ |
1,395 |
|
|
$ |
1,465 |
|
Change in plan assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Fair value of plan assets at beginning of year |
|
$ |
— |
|
|
$ |
— |
|
| Employer contributions |
|
7 |
|
|
42 |
|
| Participant contributions |
|
23 |
|
|
27 |
|
| Benefits paid |
|
(30) |
|
|
(69) |
|
| Fair value of plan assets at end of year |
|
$ |
— |
|
|
$ |
— |
|
Amounts recognized in consolidated balance sheet:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Accumulated postretirement benefit obligation |
|
$ |
(1,395) |
|
|
$ |
(1,465) |
|
| Fair value of plan assets |
|
— |
|
|
— |
|
| Funded status |
|
(1,395) |
|
|
(1,465) |
|
| Unrecognized prior service cost |
|
9 |
|
|
74 |
|
| Unrecognized net loss (gain) |
|
(2) |
|
|
(24) |
|
| Net amount recognized in consolidated balance sheet (after ASC 715) (included in "Other long-term obligations") |
|
$ |
(1,395) |
|
|
$ |
(1,465) |
|
| Accrued postretirement benefit cost (included in "Other long-term obligations") |
|
N/A |
|
N/A |
Components of net periodic benefit cost:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Service cost with interest to end of year |
|
$ |
108 |
|
|
$ |
79 |
|
|
$ |
87 |
|
| Interest cost |
|
62 |
|
|
26 |
|
|
23 |
|
| Amortization of prior service cost |
|
— |
|
|
9 |
|
|
74 |
|
| Amortization of loss (gain) |
|
8 |
|
|
(2) |
|
|
(24) |
|
| Total net periodic benefit cost |
|
$ |
178 |
|
|
$ |
112 |
|
|
$ |
160 |
|
Estimated future employer contributions and benefit payments are as follows:
|
|
|
|
|
|
|
|
|
| Year |
|
|
| 2024 |
|
$ |
131 |
|
| 2025 |
|
132 |
|
| 2026 |
|
99 |
|
| 2027 |
|
101 |
|
| 2028 |
|
85 |
|
| Years 2029-2033 |
|
615 |
|
Assumptions to determine benefit obligations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Discount rate |
|
4.15 |
% |
|
4.40 |
% |
Assumptions to determine net cost:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Discount rate |
|
4.40 |
% |
|
2.10 |
% |
|
1.75 |
% |
Defined Benefit Pension Plans
The Company contributes to one multi-employer defined benefit plan under the terms of a collective-bargaining agreement covering its union-represented employees of the Verona, Missouri facility. The risks of participation in this multiemployer plan are different from single-employer plans in the following aspects: (a) assets contributed to the multiemployer plan by one employer may be used to provide benefits to employees of other participating employers, (b) if a participating employer stops contributing to the plan, the unfunded obligations of the plan may be borne by the remaining participating employers, and (c) if the Company was to stop participating in its multiemployer plan, the Company would be required to pay that plan an amount based on the underfunded status of the plan, referred to as the withdrawal liability.
The Company’s participation in this plan for the annual period ended December 31, 2023 is outlined in the table below. The “EIN/Pension Plan Number” column provides the Employee Identification Number (EIN). The zone status is based on information that the Company received from the plan and is certified by the plan’s actuary. Among other factors, plans in the red zone or critical and declining zone are generally less than 65 percent funded, plans in the yellow zone are less than 80 percent funded, and plans in the green zone are at least 80 percent funded. The “FIP/RP Status Pending/Implemented” column indicates plans for which a financial improvement plan (FIP) or a rehabilitation plan (RP) is either pending or has been implemented. The last column lists the expiration date of the collective-bargaining agreement to which the plan is subject. Finally, the period-to-period comparability of the contributions for 2023 and 2022 was affected by a 4.0% increase in the 2023 contribution rate. There have been no other significant changes that affect the comparability of 2023 and 2022 contributions. The Company does not represent more than 5% of the contributions to this pension fund.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pension
Fund |
|
EIN/Pension
Plan
Number |
|
Pension Plan Protection Act Zone Status |
|
FIP/RP Status
Pending/ Implemented |
|
Contributions of Balchem Corporation |
|
Surcharge
Imposed |
|
Expiration Date of Collective-
Bargaining
Agreement |
|
2023 |
|
2022 |
|
|
2023 |
|
2022 |
|
2021 |
|
Central States,
Southeast and
Southwest Areas
Pension Fund |
|
36-6044243 |
|
Critical & Declining as of 1/1/23 |
|
Critical & Declining as of 1/1/22 |
|
Implemented |
|
$1,020 |
|
$939 |
|
$816 |
|
No |
|
7/12/2025 |
The Company provides an unfunded defined benefit pension plan for employees working in Belgium. The plan provides for the payment of a lump sum at retirement or payments in case of death of the covered employees.
The actuarial recorded liabilities for such unfunded defined benefit pension plan are as follows:
Change in benefit obligation:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Benefit obligation at beginning of year |
|
$ |
1,589 |
|
|
$ |
1,859 |
|
| Service cost with interest to end of year |
|
65 |
|
|
44 |
|
| Interest cost |
|
65 |
|
|
17 |
|
| Participant contributions |
|
— |
|
|
27 |
|
| Benefits paid |
|
(188) |
|
|
(60) |
|
| Actuarial loss (gain) |
|
80 |
|
|
(194) |
|
| Exchange rate changes |
|
49 |
|
|
(104) |
|
| Benefit obligation at end of year |
|
$ |
1,660 |
|
|
$ |
1,589 |
|
Change in plan assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Fair value of plan assets at beginning of year |
|
$ |
1,196 |
|
|
$ |
1,175 |
|
| Actual return on plan assets |
|
56 |
|
|
26 |
|
| Employer contributions |
|
138 |
|
|
94 |
|
| Participant contributions |
|
— |
|
|
27 |
|
| Benefits paid |
|
(188) |
|
|
(60) |
|
| Exchange rate changes |
|
38 |
|
|
(66) |
|
| Fair value of plan assets at end of year |
|
$ |
1,240 |
|
|
$ |
1,196 |
|
Amounts recognized in consolidated balance sheet:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Benefit obligation |
|
$ |
(1,660) |
|
|
$ |
(1,589) |
|
| Fair value of plan assets |
|
1,240 |
|
|
1,196 |
|
| Funded status |
|
(420) |
|
|
(393) |
|
| Unrecognized prior service cost |
|
N/A |
|
N/A |
| Unrecognized net (gain)/loss |
|
N/A |
|
N/A |
| Net amount recognized in consolidated balance sheet (after ASC 715) (included in other long-term obligations) |
|
$ |
(420) |
|
|
$ |
(393) |
|
| Accrued postretirement benefit cost (included in other long-term obligations) |
|
N/A |
|
N/A |
Components of net periodic benefit cost:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Service cost with interest to end of year |
|
$ |
65 |
|
|
$ |
44 |
|
|
$ |
67 |
|
| Interest cost |
|
65 |
|
|
17 |
|
|
14 |
|
| Expected return on plan assets |
|
(42) |
|
|
(37) |
|
|
(34) |
|
| Amortization of net loss |
|
— |
|
|
— |
|
|
3 |
|
| Total net periodic benefit cost |
|
$ |
88 |
|
|
$ |
24 |
|
|
$ |
50 |
|
Estimated future benefit payments are as follows:
|
|
|
|
|
|
|
|
|
| Year |
|
|
| 2024 |
|
$ |
1 |
|
| 2025 |
|
52 |
|
| 2026 |
|
1 |
|
| 2027 |
|
1 |
|
| 2028 |
|
1 |
|
| Years 2029-2033 |
|
1,096 |
|
Assumptions to determine benefit obligations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| Discount rate |
|
3.45 |
% |
|
4.00 |
% |
Assumptions to determine net cost:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
|
2021 |
| Discount rate |
|
4.00 |
% |
|
1.00 |
% |
|
0.75 |
% |
| Expected return on assets |
|
3.25 |
% |
|
3.25 |
% |
|
3.25 |
% |
Deferred Compensation Plan
The Company maintains an unfunded, non-qualified deferred compensation plan for the benefit of a select group of management or highly compensated employees. Assets of the plan are held in a rabbi trust, which are subject to additional risk of loss in the event of bankruptcy or insolvency of the Company. The deferred compensation liability as of December 31, 2023 and 2022 was $10,188 and $8,543, respectively, and was included in "Other long-term obligations" on the Company's balance sheet. The related rabbi trust assets were $10,188 and $8,547 as of December 31, 2023 and 2022, respectively, and were included in "Other non-current assets" on the Company's consolidated balance sheets.
NOTE 16 - COMMITMENTS AND CONTINGENCIES
The Company is obligated to make rental payments under non-cancelable operating and finance leases. Aggregate future minimum rental payments required under these leases at December 31, 2023 are disclosed in Note 19, Leases.
The Company’s Verona, Missouri facility, while held by a prior owner, Syntex Agribusiness, Inc. (“Syntex”), was designated by the U.S. Environmental Protection Agency (the "EPA") as a Superfund site and placed on the National Priorities List in 1983 because of dioxin contamination on portions of the site. Remediation was conducted by Syntex under the oversight of the EPA and the Missouri Department of Natural Resources. The Company is indemnified by the sellers under its May 2001 asset purchase agreement covering its acquisition of the Verona, Missouri facility for potential liabilities associated with the Superfund site. One of the sellers, in turn, has the benefit of certain contractual indemnification by Syntex in relation to the implementation of the above-described Superfund remedy. In June 2023, in response to a Special Notice Letter received from the EPA in 2022, BCP Ingredients, Inc. ("BCP"), the Company's subsidiary that operates the site, Syntex, EPA, and the State of Missouri entered into an Administrative Settlement Agreement and Order on Consent (“ASAOC”) for a focused remedial investigation/feasibility study ("RI/FS") under which (a) BCP will conduct a source investigation of potential source(s) of releases of 1,4-dioxane and chlorobenzene at a portion of the site and (b) BCP and Syntex will complete a RI/FS to determine a potential remedy, if any is required. Activities under the ASAOC are underway and are expected to continue for some period of time.
Separately, in June 2022, the EPA conducted an inspection of BCP’s Verona, Missouri facility (“2022 EPA Inspection”) which was followed by BCP entering into an Administrative Order for Compliance on Consent (“AOC”) with the EPA in relation to its risk management program at the Verona facility. Further, in January 2023, BCP entered into an Amended AOC with the EPA whereby the parties agreed to the extension of certain timelines.
BCP timely completed all requirements under the Amended AOC. In November 2023, BCP received a notice from the Environment and Natural Resources Division of the U.S Department of Justice (“DOJ”) primarily related to the 2022 EPA Inspection, which extended the opportunity to discuss alleged violations of Sections 112(r)(7) of the Clean Air Act and regulations in 40 C.F.R. Part 68, commonly known as the Risk Management Plan Rule (“RMP Rule”). BCP intends to participate in such discussions in 2024. In connection with the 2022 EPA Inspection, the Company believes that a loss contingency in this matter is probable and reasonably estimable and has recorded a loss contingency in an amount that is not material to its financial performance or operations.
In addition to the above, from time to time, the Company is a party to various legal proceedings, litigation, claims and assessments. While it is not possible to predict the ultimate disposition of each of these matters, management believes that the ultimate outcome of such matters will not have a material effect on the Company's consolidated financial position, results of operations, liquidity or cash flows.
NOTE 17 – FAIR VALUE OF FINANCIAL INSTRUMENTS
The Company has a number of financial instruments, none of which are held for trading purposes. The Company estimates that the fair value of all financial instruments at December 31, 2023 and 2022 does not differ materially from the aggregate carrying values of its financial instruments recorded in the accompanying consolidated balance sheets. The estimated fair value amounts have been determined by the Company using available market information and appropriate valuation methodologies. Considerable judgment is necessarily required in interpreting market data to develop the estimates of fair value, and, accordingly, the estimates are not necessarily indicative of the amounts that the Company could realize in a current market exchange. The carrying value of debt approximates fair value as the interest rate is based on market and the Company’s consolidated leverage ratio. The Company’s financial instruments also include cash equivalents, accounts receivable, accounts payable, and accrued liabilities, which are carried at cost and approximate fair value due to the short-term maturity of these instruments. Cash and cash equivalents at December 31, 2023 and 2022 included $959 and $934 in money market funds and other interest-bearing deposit accounts, respectively.
Non-current assets at December 31, 2023 and 2022 included $10,188 and $8,547, respectively, of rabbi trust funds related to the Company's deferred compensation plan. The money market and rabbi trust funds are valued using level one inputs, as defined by ASC 820, “Fair Value Measurement.”
The contingent consideration liabilities included on the balance sheet at of December 31, 2023 and 2022 amount to $100 and $11,400, respectively, and were valued using level three inputs, as defined by ASC 820, "Fair Value Measurement".
The Company also had derivative financial instruments, consisting of a cross-currency swap and an interest rate swap, which were included in "Derivative assets" in the Company's consolidated balance sheets. The fair values of these derivative instruments were determined based on Level 2 inputs, using significant inputs that are observable either directly or indirectly, including interest rate curves and implied volatilities. The Company settled its cross-currency swap and interest rate swap on June 27, 2023 and had no other derivatives outstanding as of December 31, 2023. The derivative assets related to the cross-currency swap and the interest rate swap were $4,587 and $1,406 at December 31, 2022, respectively.
NOTE 18 – RELATED PARTY TRANSACTIONS
The Company provides services under a contractual agreement to St. Gabriel CC Company, LLC. These services include accounting, information technology, quality control, and purchasing services, as well as operation of the St. Gabriel CC Company, LLC plant. The Company also sells raw materials to St. Gabriel CC Company, LLC. These raw materials are used in the production of finished goods that are, in turn, sold by Saint Gabriel CC Company, LLC to the Company for resale to unrelated parties. As such, the sale of these raw materials to St. Gabriel CC Company, LLC in this scenario lacks economic substance and therefore the Company does not include them in net sales within the consolidated statements of earnings.
Payments for the services the Company provided amounted to $4,363, $4,213, and $3,637, respectively, for the years ended December 31, 2023, 2022, and 2021. The raw materials purchased and subsequently sold amounted to $34,219, $39,853, and $27,915, respectively, for the years ended December 31, 2023, 2022, and 2021. These services and raw materials are primarily recorded in cost of goods sold, net of the finished goods received from St. Gabriel CC Company, LLC of $28,099, $29,062, and $22,043, respectively, for the years ended December 31, 2023, 2022, and 2021. At December 31, 2023 and 2022, the Company had receivables of $8,314 and $8,820, respectively, recorded in accounts receivable from St. Gabriel CC Company, LLC for services rendered and raw materials sold.
At December 31, 2023 and 2022, the Company had payables of $6,050 and $5,224, respectively, recorded in accounts payable for finished goods received from St. Gabriel CC Company, LLC. In addition, the Company had payables in the amount of $329 and $296, respectively, related to non-contractual monies owed to St. Gabriel CC Company, LLC, recorded in accounts payable as of December 31, 2023 and 2022.
NOTE 19 – LEASES
The Company has both real estate leases and equipment leases. The main types of equipment leases include forklifts, trailers, printers and copiers, railcars, and trucks. Leases are categorized as both operating leases and finance leases. As a result of electing the practical expedient within ASU 2016-02, variable lease payments are combined and recognized on the balance sheet in the event that those charges and any related increases are explicitly stated in the lease. Such payments include common area maintenance charges, property taxes, and insurance charges and are recorded in the right of use asset and corresponding liability when the payments are stated in the lease with (a) fixed or in-substance fixed amounts, or (b) a variable payment based on an index or rate. Due to the acquisitive nature of the Company and the potential for synergies upon integration of acquired entities, the Company determined that the reasonably certain criterion could not be met for any renewal periods beginning two years from December 31, 2023. In addition, the Company has historically not been exercising purchase options under the equipment leases as it does not make economic sense to buy the equipment. Instead, the Company has historically replaced the equipment with new leases. Therefore, the Company determined that the reasonably certain criterion could not be met as it relates to purchase options. The Company has no residual value guarantees in lease transactions.
The Company did not identify any embedded leases. As indicated above, the Company elected the practical expedient to combine lease and non-lease components and recognizes the combined amount on the consolidated balance sheet. Management determined that since the Company has a centralized treasury function, the parent company would either fund or guarantee a subsidiary's loan for borrowing over a similar term. As such, the Company's management determined it is appropriate to utilize a corporate based borrowing rate for all locations. The Company developed four tranches of leases based on lease terms and these tranches reflect the composition of the current lease portfolio. The Company's borrowing history shows that interest rates of a term loan or a line of credit depend on the duration of the loan rather than the nature of the assets purchased by those funds. Based on this understanding, the Company elected to use a portfolio approach to discount rates, applying corporate rates to the tranches of leases based on lease terms. Based on the Company's risk rating, the company applied the following discount rates for new leases entered into during 2023: (1) 1-2 years, 5.45%-6.72% (2) 3-4 years, 6.04%-7.31% (3) 5-9 years, 6.38%-7.65% and (4) 10+ years, 7.10%-8.37%.
Right of use assets and lease liabilities at December 31, 2023 and 2022 are summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Right of use assets |
|
2023 |
|
2022 |
| Operating leases |
|
$ |
17,763 |
|
|
$ |
17,094 |
|
| Finance lease |
|
2,101 |
|
|
2,338 |
|
| Total |
|
$ |
19,864 |
|
|
$ |
19,432 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lease liabilities - current |
|
2023 |
|
2022 |
| Operating leases |
|
$ |
3,949 |
|
|
$ |
3,796 |
|
| Finance lease |
|
272 |
|
|
226 |
|
| Total |
|
$ |
4,221 |
|
|
$ |
4,022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Lease liabilities - non-current |
|
2023 |
|
2022 |
| Operating leases |
|
$ |
14,601 |
|
|
$ |
13,806 |
|
| Finance lease |
|
1,943 |
|
|
2,213 |
|
| Total |
|
$ |
16,544 |
|
|
$ |
16,019 |
|
For the years ended December 31, 2023, 2022, and 2021, the Company's total lease costs were as follows, which included both amounts recognized in profits or losses during the period and amounts capitalized on the balance sheet, and the cash flows arising from lease transactions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
|
2023 |
|
2022 |
|
2021 |
| Lease Cost |
|
|
|
|
|
|
| Operating lease cost |
|
$ |
5,307 |
|
|
$ |
4,478 |
|
|
$ |
3,143 |
|
|
|
|
|
|
|
|
| Finance Lease cost |
|
|
|
|
|
|
| Amortization of ROU asset |
|
242 |
|
|
210 |
|
|
210 |
|
| Interest on lease liabilities |
|
115 |
|
|
125 |
|
|
129 |
|
| Total finance lease |
|
357 |
|
|
335 |
|
|
339 |
|
|
|
|
|
|
|
|
| Total lease cost |
|
$ |
5,664 |
|
|
$ |
4,813 |
|
|
$ |
3,482 |
|
|
|
|
|
|
|
|
| Cash paid for amounts included in the measurement of lease liabilities |
|
|
|
|
|
|
| Operating cash flows from operating leases |
|
$ |
4,757 |
|
|
$ |
4,269 |
|
|
$ |
3,097 |
|
| Operating cash flows from finance leases |
|
115 |
|
|
125 |
|
|
129 |
|
| Financing cash flows from finance leases |
|
222 |
|
|
177 |
|
|
159 |
|
|
|
$ |
5,094 |
|
|
$ |
4,571 |
|
|
$ |
3,385 |
|
|
|
|
|
|
|
|
| ROU assets obtained in exchange for new operating lease liabilities, net of ROU asset disposals |
|
$ |
6,365 |
|
|
$ |
11,488 |
|
|
$ |
3,804 |
|
|
|
|
|
|
|
|
| Weighted-average remaining lease term - operating leases |
|
9.33 years |
|
5.63 years |
|
4.21 years |
| Weighted-average remaining lease term - finance leases |
|
9.07 years |
|
9.95 years |
|
11.41 years |
|
|
|
|
|
|
|
| Weighted-average discount rate - operating leases |
|
7.4 |
% |
|
2.7 |
% |
|
3.5 |
% |
| Weighted-average discount rate - finance leases |
|
5.0 |
% |
|
5.0 |
% |
|
5.1 |
% |
Rent expense charged to operations under operating lease agreements for 2023, 2022, and 2021 aggregated approximately $5,307, $4,478, and $3,143, respectively.
Aggregate future minimum rental payments required under non-cancelable operating and finance leases at December 31, 2023 are as follows:
|
|
|
|
|
|
|
|
|
| Year |
|
|
| 2024 |
|
$ |
5,407 |
|
| 2025 |
|
4,219 |
|
| 2026 |
|
3,638 |
|
| 2027 |
|
2,736 |
|
| 2028 |
|
2,219 |
|
| Thereafter |
|
7,717 |
|
| Total minimum lease payments |
|
$ |
25,936 |
|
NOTE 20 – DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
On May 28, 2019, the Company entered into a pay-fixed (2.05%), receive-floating interest rate swap with a notional amount of $108,569 and a maturity date of June 27, 2023, which was designated as cash flow hedge. The net interest income related to the interest rate swap contract were $1,518 and $400 for the years ended December 31, 2023 and 2022, respectively. The net interest expense related to the interest rate swap contract was $2,144 for the year ended December 31, 2021. The net interest income and expense were recorded in the consolidated statements of earnings under "Interest expense, net."
On May 28, 2019, the Company also entered into a pay-fixed (0.00%), receive-fixed (2.05%) cross-currency swap to manage foreign exchange risk related to the Company's net investment in Chemogas, which was designated as net investment hedge. The derivative has a notional amount of $108,569, an effective date of May 28, 2019, and a maturity date of June 27, 2023. The interest income related to the cross-currency swap contract was $1,119, $2,250, and $2,257 for the years ended December 31, 2023, 2022, and 2021, respectively. The interest income was recorded in the consolidated statements of earnings under "Interest expense, net."
The Company settled its derivative instruments on their maturity date of June 27, 2023 and had no other derivatives outstanding as of December 31, 2023. The proceeds from the settlement of the cross-currency swap in the amount of $2,740 were classified as investing activities in the Consolidated Statements of Cash Flows.
As of December 31, 2022, the fair value of the derivative instruments is presented as follows in the Company's consolidated balance sheets:
|
|
|
|
|
|
|
|
|
| Derivative assets |
|
December 31, 2022 |
| Interest rate swap |
|
$ |
1,406 |
|
| Cross-currency swap |
|
4,587 |
|
| Derivative assets |
|
$ |
5,993 |
|
Gains and losses on our hedging instruments were recognized in accumulated other comprehensive income (loss) and categorized as follows for the years ended December 31, 2023, 2022, and 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Location within Statements of Comprehensive Income |
|
Year ended December 31, |
|
|
|
2023 |
|
2022 |
|
2021 |
| Cash flow hedge (interest rate swap), net of tax |
|
Unrealized (loss) gain on cash flow hedge, net |
|
$ |
(1,065) |
|
|
$ |
2,696 |
|
|
$ |
2,053 |
|
| Net investment hedge (cross-currency swap), net of tax |
|
Net foreign currency translation adjustment |
|
(1,455) |
|
|
3,851 |
|
|
4,766 |
|
|
|
|
|
$ |
(2,520) |
|
|
$ |
6,547 |
|
|
$ |
6,819 |
|
In connection with the Kappa acquisition (see Note 2, Significant Acquisitions), the Company entered into four short-term foreign currency exchange forward contracts to manage fluctuations in foreign currency exchange rates. The Company did not designate these contracts as hedged transactions under the applicable sections of ASC Topic 815, "Derivatives and Hedging". For the year ended December 31, 2022, the net gains on these forward contracts of $512 were recorded in other income or loss in the consolidated statements of earnings. As of December 31, 2023, the Company did not maintain any open foreign currency exchange forward contracts as all four contracts expired during 2022.
NOTE 21 - QUARTERLY FINANCIAL INFORMATION (UNAUDITED)
(In thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
2023 |
|
2022 |
| |
|
First
Quarter |
|
Second
Quarter |
|
Third
Quarter |
|
Fourth
Quarter |
|
First
Quarter |
|
Second
Quarter |
|
Third
Quarter |
|
Fourth
Quarter |
| Net sales |
|
$ |
232,540 |
|
|
$ |
231,252 |
|
|
$ |
229,948 |
|
|
$ |
228,699 |
|
|
$ |
228,867 |
|
|
$ |
236,693 |
|
|
$ |
244,267 |
|
|
$ |
232,531 |
|
| Gross margin |
|
73,170 |
|
|
77,349 |
|
|
76,544 |
|
|
74,993 |
|
|
71,506 |
|
|
71,876 |
|
|
68,430 |
|
|
68,639 |
|
| Earnings before income taxes |
|
29,119 |
|
|
38,400 |
|
|
36,475 |
|
|
33,267 |
|
|
37,630 |
|
|
39,258 |
|
|
31,085 |
|
|
25,776 |
|
| Net earnings |
|
22,710 |
|
|
30,110 |
|
|
29,075 |
|
|
26,648 |
|
|
28,930 |
|
|
29,782 |
|
|
25,249 |
|
|
21,406 |
|
| Basic net earnings per common share |
|
$ |
0.71 |
|
|
$ |
0.94 |
|
|
$ |
0.91 |
|
|
$ |
0.83 |
|
|
$ |
0.90 |
|
|
$ |
0.93 |
|
|
$ |
0.79 |
|
|
$ |
0.67 |
|
| Diluted net earnings per common share |
|
$ |
0.70 |
|
|
$ |
0.93 |
|
|
$ |
0.90 |
|
|
$ |
0.82 |
|
|
$ |
0.89 |
|
|
$ |
0.92 |
|
|
$ |
0.78 |
|
|
$ |
0.66 |
|
BALCHEM CORPORATION
Valuation and Qualifying Accounts
Years Ended December 31, 2023, 2022 and 2021
(In thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allowance
for Doubtful Accounts |
|
Inventory
Reserve |
| Balance - December 31, 2020 |
|
$ |
2,092 |
|
|
$ |
2,782 |
|
| Additions charged to costs and expenses |
|
180 |
|
|
7,312 |
|
Adjustments/deductions (a) |
|
(1,344) |
|
|
(8,669) |
|
|
|
|
|
|
| Balance - December 31, 2021 |
|
928 |
|
|
1,425 |
|
| Additions charged to costs and expenses |
|
401 |
|
|
6,786 |
|
Adjustments/deductions (a) |
|
(103) |
|
|
(5,571) |
|
|
|
|
|
|
| Balance - December 31, 2022 |
|
1,226 |
|
|
2,640 |
|
| Additions charged to costs and expenses |
|
37 |
|
|
2,450 |
|
Adjustments/deductions (a) |
|
(355) |
|
|
(2,627) |
|
|
|
|
|
|
| Balance - December 31, 2023 |
|
$ |
908 |
|
|
$ |
2,463 |
|
|
|
|
|
|
(a) Represents write-offs and other adjustments |
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
We maintain “disclosure controls and procedures,” as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (the “Exchange Act”), that are designed to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, management recognized that disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the disclosure controls and procedures are met. Additionally, in designing disclosure controls and procedures, our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible disclosure controls and procedures. The design of any disclosure controls and procedures also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2023. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of such date, our disclosure controls and procedures were effective.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting is a process designed under the supervision of our principal executive and principal financial officers to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external reporting purposes in accordance with U.S. generally accepted accounting principles.
Our internal control over financial reporting includes policies and procedures that pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect transactions and dispositions of assets; provide reasonable assurances that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of our management and our directors; and provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. In addition, projections of any evaluation of controls effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
As of December 31, 2023, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in the 2013 Internal Control-Integrated Framework (New Framework) to conduct an assessment of the effectiveness of our internal control over financial reporting. Based on this assessment, management has determined that our internal control over financial reporting was effective as of December 31, 2023.
Attestation Report of Registered Public Accounting Firm
The independent registered public accounting firm of RSM US LLP has issued an attestation report on our internal control over financial reporting, which is included herein.
Changes in Internal Control Over Financial Reporting
There has been no significant change in our internal control over financial reporting that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. As of December 31, 2023, management's assessment of and conclusion of the effectiveness of our internal controls over financial reporting of both Kappa and Bergstrom have been completed.
Therefore, management's assessment of and conclusion of the effectiveness of our internal control over financial reporting also includes the internal controls over financial reporting of Kappa and Bergstrom.
Item 9B. Other Information
No directors or officers adopted, modified or terminated a Rule 10b5-1 trading arrangement during the fiscal quarter ended December 31, 2023.
PART III
Item 10. Directors, Executive Officers of the Registrant, and Corporate Governance.
The information regarding our executive officers is included in Part I of this report under the heading “Information about our Executive Officers.”
The other information required by this item is incorporated by reference to the information contained under the headings “Proposal 1. Election of Directors”, “Delinquent Section 16(a) Reports,” and “Corporate Governance” in our Proxy Statement for the 2024 Annual Meeting of Shareholders which will be filed no later than 120 days after December 31, 2023 (the “2024 Proxy Statement”).
Item 11. Executive Compensation.
The information required by this item is incorporated by reference to the information contained under the headings “Executive Compensation,” “Compensation Committee Report,” and “Compensation Committee Interlocks and Insider Participation” in our 2024 Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item is incorporated by reference to the information contained under the headings “Security Ownership of Certain Beneficial Owners and of Management” and Equity Compensation Plan Information” in our 2024 Proxy Statement.
Item 13. Certain Relationships and Related Transactions and Director Independence.
The information required by this item is incorporated by reference to the information contained under the headings “Related Party Transactions” and “Director Independence” in our 2024 Proxy Statement.
Item 14. Principal Accountant Fees and Services.
The information required by this item is incorporated by reference to the information contained under the heading “Information Relating to Proposal 2. Ratification of Appointment of Independent Registered Public Accounting Firm” of our 2024 Proxy Statement.
PART IV
Item 15. Exhibits and Financial Statement Schedules.
The following documents are filed as part of this Form 10-K:
|
|
|
|
|
|
|
|
|
|
|
|
| 1. |
|
Financial Statements |
Page Number |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2. |
|
Financial Statement Schedules |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 3. |
|
Exhibits |
|
|
|
|
|
| 2.1 |
|
|
|
|
|
|
| 3.1 |
|
|
| |
|
|
|
| 3.2 |
|
|
| |
|
|
|
| 3.3 |
|
|
| |
|
|
|
| 3.4 |
|
|
|
|
|
|
| 4.1 |
|
|
|
|
|
|
| 10.1 |
|
|
|
|
|
|
| 10.2 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.3 |
|
|
|
|
|
|
| 10.4 |
|
|
| |
|
|
|
| 10.5 |
|
|
|
|
|
|
| 10.6 |
|
|
|
|
|
|
| 10.7 |
|
|
|
|
|
|
| 10.8 |
|
|
|
|
|
|
| 10.9 |
|
|
|
|
|
|
| 10.10 |
|
|
|
|
|
|
| 10.11 |
|
|
|
|
|
|
| 10.12 |
|
|
|
|
|
|
| 10.13 |
|
|
|
|
|
|
| 10.14 |
|
|
|
|
|
|
| 21.1 |
|
|
|
|
|
|
| 23.1 |
|
|
|
|
|
|
| 31.1 |
|
|
|
|
|
|
| 31.2 |
|
|
|
|
|
|
| 32.1 |
|
|
|
|
|
|
| 32.2 |
|
|
|
|
|
|
| 97.1 |
|
|
|
|
|
|
| 101.INS |
|
XBRL Instance Document |
|
|
|
|
|
| 101.SCH |
|
XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
| 101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
| 101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 101.LAB |
|
XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
| 101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
|
| 104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
|
|
|
|
|
| * Each of the Exhibits noted by an asterisk is a management compensatory plan or arrangement. |
|
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
| Date: February 16, 2024 |
BALCHEM CORPORATION |
| |
By:/s/ Theodore L. Harris |
| |
Theodore L. Harris, Chairman, President and |
| |
and Chief Executive Officer |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
|
|
|
|
|
| /s/ Theodore L. Harris |
|
| Theodore L. Harris, Chairman, President and |
| Chief Executive Officer |
| Date: February 16, 2024 |
|
|
|
| /s/ C. Martin Bengtsson |
|
| C. Martin Bengtsson, Executive Vice President and |
| Chief Financial Officer |
| Date: February 16, 2024 |
|
|
|
| /s/ William A. Backus |
|
| William A. Backus, Vice President and |
| Chief Accounting Officer |
| Date: February 16, 2024 |
|
|
|
| /s/ David B. Fischer |
|
| David B. Fischer, Director |
| Date: February 16, 2024 |
|
|
|
| /s/ Kathleen B. Fish |
|
| Kathleen B. Fish, Director |
| Date: February 16, 2024 |
|
|
|
| /s/ Daniel E. Knutson |
|
| Daniel E. Knutson, Director |
| Date: February 16, 2024 |
|
|
|
| /s/ Joyce J. Lee |
|
| Joyce J. Lee, Director |
| Date: February 16, 2024 |
|
|
|
| /s/ Olivier Rigaud |
|
| Olivier Rigaud, Director |
| Date: February 16, 2024 |
|
|
|
| /s/ Monica Vicente |
|
| Monica Vicente, Director |
| Date: February 16, 2024 |
|
|
|
| /s/ Matthew D. Wineinger |
|
| Matthew D. Wineinger, Director |
| Date: February 16, 2024 |
|
EX-4.1
2
axex41descriptionofsecurit.htm
EX-4.1
Document
Exhibit 4.1
DESCRIPTION OF THE REGISTRANT’S SECURITIES
REGISTERED PURSUANT TO SECTION 12 OF THE
SECURITIES EXCHANGE ACT OF 1934
As of the date of the Annual Report on Form 10-K of which this exhibit is a part, Balchem Corporation had one class of securities registered under Section 12 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”): its common stock, par value $0.06-2/3 per share (“common stock”). References herein to “we,” “us,” “our” and the “Company” refer to Balchem Corporation and not to any of its subsidiaries.
The following description of our common stock is a summary and does not purport to be complete. It is subject to and qualified in its entirety by reference to the applicable provisions of the Maryland General Corporation law (the “MGCL”), the Company’s Composite Articles of Incorporation (our “charter”), and its Amended and Restated Bylaws (our “bylaws”). Each of our charter and our bylaws is incorporated by reference as an exhibit to the Annual Report on Form 10-K of which this Exhibit is a part. We encourage you to read our charter, bylaws, our Corporate Governance Guidelines (which are available on our website), and the applicable provisions of the MGCL for additional information.
General
Under our charter, we may issue up to 122,000,000 shares of stock, comprised of 120,000,000 shares of common stock and 2,000,000 shares of preferred stock, par value $25.00 per share (“preferred stock”).
Our charter authorizes our board of directors (our “Board”) to issue shares of stock of the Company or securities convertible into shares of its stock. As allowed by the MGCL, our charter authorizes our Board, without stockholder approval, to issue preferred stock, in one or more series, each series to be with such preferences, conversion and other rights, voting powers, restrictions, limitations as to dividends, qualifications and terms and conditions of redemption as our Board shall determine, with the foregoing potentially having the effect of delaying, deferring or preventing a change in control of the Company. We believe that the power to issue additional shares of our stock and to classify unissued shares of our preferred stock and to issue the classified shares provides us with increased flexibility in structuring possible future financings and acquisitions and in meeting other needs which might arise, but our issuance of additional shares of stock could dilute voting and other stockholder rights. See “Certain Provisions of Our Charter and Bylaws and of Maryland Law” below.
Common Stock
All of the outstanding shares of our common stock are duly authorized, fully paid and nonassessable. Our common stockholders are entitled to receive dividends when authorized by our Board and declared by us out of assets legally available for the payment of dividends. They are also: (i) entitled to share ratably in our assets legally available for distribution to our stockholders in the event of our liquidation, dissolution or winding up, after payment of, or adequate provision for, all of our known debts and liabilities, and (ii) generally are not liable for our debts or obligations. These rights may be subject to the preferential rights of any other class or series of our stock, including any preferred stock. All shares of common stock have equal dividend and liquidation rights.
Each outstanding share of common stock is entitled to one vote on all matters submitted to a vote of the stockholders and, except for the rights that may be held by the holders of shares of any subsequently issued class or series of preferred stock, the holders of our common stock possess exclusive voting power. There is no cumulative voting in the election of our directors. A majority of votes cast at a meeting of stockholders duly called and at which a quorum is present is sufficient to elect a director, other than in the case of a “contested election,” in which case a plurality of the votes cast is sufficient to elect a director. A “contested election” is any election of directors with respect to which (i) the Company receives notice that a stockholder has nominated an individual for election as a director in compliance with our bylaws and (ii) such nomination has not been withdrawn by the stockholder prior to the date when the Company first mails notice of the meeting to its stockholders and, as a result, the number of nominees for director is greater than the number of directors to be elected at the meeting. The Company’s Corporate Governance Guidelines set forth the procedures that are applicable if an incumbent director in an uncontested election receives a greater number of “withhold” votes for election than “for” votes. For all other matters, unless a different number is required by law, our charter or our bylaws, a majority of votes cast at a meeting of stockholders duly called and at which a quorum is present is sufficient to approve or authorize such other matter.
Our stockholders have no exchange, sinking fund or redemption rights, and have no preemptive rights to subscribe for any future issuance of our securities. Because our stockholders do not have preemptive rights, we may issue additional shares of stock that may reduce their proportionate voting and financial interest in the Company. Rights to receive dividends on our common stock may be limited by the terms of any future classified and issued shares of our preferred stock.
A Maryland corporation generally may not dissolve, amend its charter, merge, convert, consolidate, sell all or substantially all of its assets or engage in a statutory share exchange unless declared advisable by its board of directors and approved by the affirmative vote of stockholders holding at least two-thirds of the shares entitled to vote on the matter.
Transfer Agent and Registrar
The transfer agent and registrar for shares of our common stock is Broadridge Corporate Issuer Solutions, Inc.
Certain Provisions of Our Charter and Bylaws and of Maryland Law
Our Board of Directors
Our Board is divided into three classes, with the term of each class of directors expiring in a different successive year. Directors of each class are elected to serve until the third annual meeting following their election and until their successors are duly elected and qualify. As a general matter, a classified board can render a change in control of the Company or removal of incumbent management more difficult. We believe that the classification of our Board helps to assure the continuity and stability of our strategies and policies as determined by our Board.
Board vacancies may be filled by a majority of the remaining members of our Board even if such majority is less than a quorum. A director elected by our Board to fill a vacancy shall be elected to serve until the next annual meeting of stockholders and until his or her successor is duly elected and qualifies.
Under our bylaws, directors may be removed from office by the affirmative vote of a majority of all of the votes of stockholders entitled to be cast for the election of directors and any resulting vacancy for the unexpired term of the removed director shall be filled by action of the stockholders. In addition, the MGCL provides that, for so long as our Board is classified, a director may only be removed for cause.
Business Combinations
Under the MGCL, “business combinations” (as defined therein) between the Company and an “interested stockholder” or any affiliate thereof are prohibited for five years after the most recent date on which the interested stockholder became an interested stockholder. An interested stockholder is defined as:
•any person that beneficially owns, directly or indirectly, 10% or more of the voting power of the corporation’s outstanding voting stock; or
•an affiliate or associate of the corporation and was the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then outstanding stock of the corporation at any time within the two-year period immediately prior to the date in question.
Our Board may approve in advance the transaction by which such person otherwise would have become an interested stockholder, in which case, that person will not be an interested stockholder.
After the five-year prohibition, any business combination between us and an interested stockholder or any affiliate thereof generally must be recommended by our Board and approved by the affirmative vote of at least:
•80% of the votes entitled to be cast by holders of outstanding shares of voting stock; and
•two-thirds of the votes entitled to be cast by holders of voting stock other than shares held by the interested stockholder with whom or with whose affiliate the business combination is to be effected or shares held by any affiliate or associate of the interested stockholder.
These super-majority vote requirements do not apply if, among other conditions, our stockholders receive a minimum price, as defined under the MGCL, for their shares in the form of cash or other consideration in the same form as previously paid by the interested stockholder for its shares. The statute permits various exemptions from its provisions, including, without limitation, business combinations that are exempted by our Board prior to the time that an interested stockholder becomes an interested stockholder.
Control Share Acquisitions
The MGCL provides that a holder of “control shares” of a Maryland corporation acquired in a “control share acquisition” has no voting rights with respect to such shares except to the extent approved by the affirmative vote of two-thirds of all the votes entitled to be cast on the matter, excluding “interested shares,” as defined in MGCL section 3-701(g). A “control share acquisition” means the acquisition of ownership of, or the power to direct the exercise of voting power with respect to, control shares, subject to certain exceptions.
Control shares are voting shares of stock that, if aggregated with all other shares of stock owned by the acquiror or in respect of which the acquiror is entitled to exercise or direct the exercise of voting power, except solely by virtue of a revocable proxy, would entitle the acquiror to exercise voting power in electing directors within one of the ranges of voting power specified in the MGCL section 3-701(d)(i) – (iii). Control shares do not include shares the acquiror is entitled to vote as a result of having previously obtained stockholder approval or shares acquired directly from the corporation.
A person who has made or proposes to make a control share acquisition may compel our Board to call a special meeting of stockholders to consider the voting rights of the shares within 50 days of demand. This right is subject to the satisfaction of certain conditions, including an undertaking to pay the expenses of the meeting and delivering an “acquiring person statement” as described in the MGCL. If no request for a meeting is made, the Company may present the question at any stockholders’ meeting.
If voting rights are not approved at the meeting or if the acquiring person does not deliver an “acquiring person statement,” then we may redeem for “fair value” any or all of the control shares, except those for which voting rights have previously been approved. Fair value is determined, without regard to the absence of voting rights for the control shares, as of the meeting date of the non-approval of the voting rights or, if no meeting is held, as of the date of the last control share acquisition by the acquiror. If voting rights for control shares are approved at a stockholders’ meeting and the acquiror becomes entitled to vote a majority of the shares entitled to vote, all other stockholders may exercise appraisal rights. The fair value of the shares as determined for purposes of appraisal rights may not be less than the highest price per share paid by the acquiror in the control share acquisition.
The control share acquisition statute does not apply to shares acquired in a merger, consolidation, or statutory share exchange if we are a party to the transaction or to acquisitions approved or exempted by the charter or our bylaws.
Our bylaws contain a provision exempting any and all acquisitions by any person of our shares of stock from the Control Share Acquisition Act. This bylaw provision may be amended or eliminated at any time in the future.
Subtitle 8
Title 3, Subtitle 8, of the Corporations and Associations Article of the Annotated Code of Maryland (“Subtitle 8”) permits a Maryland corporation, such as the Company, with at least three directors who are not officers or employees of the corporation or affiliates of, or nominated by, a person seeking to acquire control of the corporation and a class of stock registered under the Exchange Act, to elect to be subject to any or all of the following provisions, by provision in its charter or bylaws or a resolution of its board of directors and notwithstanding any contrary provision in the charter or bylaws and without any action by stockholders:
•a classified board;
•a two-thirds vote requirement for removing a director;
•a requirement that the number of directors be fixed only by the board of directors;
•a requirement that a vacancy on the board be filled only by the remaining directors and for the remainder of the full term of the class of directors in which the vacancy occurred; or
•a majority requirement for the calling by stockholders of a special meeting of stockholders.
We have not elected to be subject to any provisions of Subtitle 8. However, through a provision in our bylaws unrelated to Subtitle 8, we have had a classified board for more than 25 years. In the future, our Board may elect, without stockholder approval, to be subject to one or more of the provisions of Subtitle 8.
Meetings of Stockholders
Under our bylaws, annual meetings of stockholders must be held each year at a date, time and place determined by our Board. Special meetings of stockholders may be called by the chair of our Board, our chief executive officer, our president and our Board. Additionally, subject to the provisions of our bylaws, a special meeting of stockholders to act on any matter that may properly be considered at a meeting of stockholders must be called by our secretary upon the written request of stockholders entitled to cast at least 25% of all of the votes entitled to be cast on the matter at such meeting. Only matters set forth in the notice of a special meeting of stockholders may be considered and acted upon at such a meeting.
Advance Notice of Director Nominations and New Business
Our bylaws provide that, with respect to an annual meeting of stockholders, nominations of individuals for election to our Board and the proposal of business to be considered by stockholders may be made only:
•pursuant to our notice of the meeting (or any supplement thereto) by or at the direction of our Board;
•otherwise properly brought before the meeting by or at the direction of our Board; or
•by a stockholder of record of the Company at the time such notice of meeting is delivered, who is entitled to vote at the meeting, and who complies with the advance notice procedures of our bylaws.
With respect to special meetings of stockholders, only the business specified in the notice of the meeting may be brought before the meeting. Nominations of individuals for election to our Board at a special meeting at which directors are to be elected may only be made:
•by or at the direction of our Board; or
•provided that our Board has determined that directors will be elected at the meeting, by a stockholder of record at the time of giving notice, who is entitled to vote at the meeting and upon such election, and who complies with the advance notice procedures of our bylaws.
The purpose of requiring stockholders to give advance notice of nominations and other proposals is to afford our Board and our stockholders the opportunity to consider the qualifications of the proposed nominees or the advisability of the other proposals and, to the extent considered necessary by our Board, to inform stockholders and make recommendations regarding the nominations or other proposals.
Limitation of Liability and Indemnification of Directors and Officers
The MGCL permits a Maryland corporation to include in its charter a provision limiting the liability of its directors and officers to the corporation and its stockholders for money damages except for liability resulting from (i) actual receipt of an improper benefit or profit in money, property or services or (ii) active and deliberate dishonesty that is established by a final judgment and that is material to the cause of action. Our charter contains such a provision that eliminates directors’ and officers’ liability to the maximum extent permitted by Maryland law.
Our bylaws obligate us, to the maximum extent permitted by Maryland law, to indemnify any present or former director or officer or any individual who, while a director or officer of the Company and at the request of the Company, serves or has served another corporation, real estate investment trust, limited liability company, partnership, joint venture, trust, employee benefit plan or other enterprise as a director, officer, partner, trustee, member or manager, is made or threatened to be made a party to, or witness in, a proceeding by reason of his or her service in that capacity, and to pay or reimburse his or her reasonable expenses in advance of final disposition of a proceeding to the maximum extent permitted by law.
EX-10.10
6
axex1010xofficerretireepro.htm
EX-10.10
Document
Exhibit 10.10
Balchem Corporation Officer Retiree Program
Balchem Corporation (the “Company”) sets forth the below Officer Retiree Program (the “Program”) with the intent to help attract and retain top talent at the executive level. For purposes of this Program, the term "Retirement" shall mean voluntary termination of employment at a time when the sum of the Officer’s age and years of service is at least 70, provided that the Officer has at least ten years of service and has served at least three consecutive years as an Officer. Further, “Officer” shall mean only those individuals approved by the Board of Directors to be either an Executive Officer and/or Section 16 Officer of the Company.
In order to be eligible for this program, the Officer must, in addition to meeting all eligibility requirements, cooperate in good faith to ensure a smooth transition in relation to his/her anticipated retirement, including providing no less than one (1) year’s prior notice to the Company (unless otherwise agreed to by the Company).
a)Officer Retiree Medical Program
1.Retirement — an Officer is entitled to receive medical benefits (see coverage below) if he or she meets the definition of Retirement, as described above and has executed an appropriate non-compete agreement (subject to local laws and regulations) with the Company.
2.Included dependents — the spouse and any eligible dependents, who, at the time of retiree’s departure, are eligible to be covered under the retiree’s medical plan, are eligible for coverage.
3.Coverage — eligible persons are covered from the date of retiree’s departure, at the same coverage available to an active employee for the retiree’s and spouse’s life, or until Medicare is available. Eligible dependents are covered until the dependent ceases to be eligible under the then current Company medical plan. In addition, the maximum years of coverage for any person eligible for coverage under this program is ten years.
4.Premiums — The Company charges the retiree (or his or her spouse) that portion of the coverage premiums that the Company would have paid if the retiree were an active employee.
b)Stock Options
Notwithstanding anything to the contrary in the 2017 Omnibus Incentive Plan (as amended from time to time) or in the Officer’s applicable stock option agreements, if an Officer ceases to be employed by the Company or its subsidiary companies, as may be applicable, by reason of Retirement, meets the definition of Retirement, as described above and has executed and is in compliance with their non-compete agreement with the Company, options granted shall continue to vest and become exercisable in accordance with their original vesting schedule. Except as otherwise provided, any unexercised portion of the option may be exercised prior to the later of (i) two years after optionee's termination of employment or (ii) two years after the vesting date of the option, but in any case not beyond the specified expiration date of the option.
c)Restricted Stock – Performance and Time Based
Notwithstanding anything to the contrary in the 2017 Omnibus Incentive Plan (as amended from time to time) or in the Officer’s applicable restricted stock agreements, if an Officer ceases to be employed by the Company or its subsidiary companies, as may be applicable, by reason of Retirement, meets the definition of Retirement, as described above and has executed and is in compliance with their non-compete agreement with the Company, all Restricted Stock grants shall continue to vest in accordance with their original vesting schedule.
d)Performance Shares
Notwithstanding anything to the contrary in the 2017 Omnibus Incentive Plan (as amended from time to time) or in the Officer’s applicable performance share agreements, if an Officer ceases to be employed by the Company or its subsidiary companies, as may be applicable, by reason of Retirement, meets the definition of Retirement, as described above and has executed and is in compliance with their non-compete agreement with the Company, all Performance Share grants shall continue to vest in accordance with their original vesting schedule.
Last updated: February 7, 2024
EX-21.1
7
bcpc202310k-ex211.htm
EX-21.1
Document
Exhibit 21.1
LIST OF SUBSIDIARIES
|
|
|
|
|
|
| Subsidiaries of the Registrant |
Jurisdiction of Organization |
| |
|
| Aberco, Inc. |
Maryland |
| |
|
| Albion Laboratories, Inc. |
Nevada |
| |
|
| Balchem BV |
Netherlands |
|
|
| Balchem Italia Srl |
Italy |
| |
|
| Balchem Ltd. |
Canada |
| |
|
| Balchem NV |
Belgium |
| |
|
| Balchem Pty Ltd. |
Australia |
| |
|
| Balchem Sdn Bhd |
Malaysia |
| |
|
| BCP Ingredients, Inc. |
Delaware |
|
|
| Kappa Bioscience AS |
Norway |
|
|
| Kappa Bioscience Europe GmbH |
Germany |
|
|
| Kappa Solutions AS |
Norway |
|
|
| Kechu BidCo AS |
Norway |
|
|
| SensoryEffects, Inc. |
Delaware |
|
|
| SensoryEffects Cereal Systems, Inc. |
Delaware |
|
|
| Stereo Gas Philippines Inc. |
Philippines |
EX-23.1
8
bcpc202310k-ex231.htm
EX-23.1
Document
Exhibit 23.1
Consent of Independent Registered Public Accounting Firm
We consent to the incorporation by reference in Registration Statements (Nos. 333-272998, 333-219722 and 333-155655) on Form S-8 of Balchem Corporation of our report dated February 16, 2024, relating to the consolidated financial statements, the financial statement schedules and the effectiveness of internal control over financial reporting of Balchem Corporation and its subsidiaries, appearing in this Annual Report on Form 10-K of Balchem Corporation for the year ended December 31, 2023.
/s/ RSM US LLP
|
|
|
| New York, New York |
|
| February 16, 2024 |
EX-31.1
9
bcpc202310k-ex311.htm
EX-31.1
Document
Exhibit 31.1
CERTIFICATIONS
I, Theodore L. Harris, certify that:
1.I have reviewed this annual report on Form 10-K of Balchem Corporation;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
| Date: February 16, 2024 |
/s/ Theodore L. Harris |
| |
Theodore L. Harris |
| |
Chairman, President and
Chief Executive Officer |
| |
(Principal Executive Officer) |
EX-31.2
10
bcpc202310k-ex312.htm
EX-31.2
Document
Exhibit 31.2
CERTIFICATIONS
I, C. Martin Bengtsson, certify that:
1.I have reviewed this annual report on Form 10-K of Balchem Corporation;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
| Date: February 16, 2024 |
/s/ C. Martin Bengtsson |
| |
C. Martin Bengtsson |
| |
Executive Vice President and
Chief Financial Officer |
| |
(Principal Financial Officer) |
EX-32.1
11
bcpc202310k-ex321.htm
EX-32.1
Document
Exhibit 32.1
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Balchem Corporation (the “Company”) on Form 10-K for the period ended December 31, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Theodore L. Harris, President, and Chief Executive Officer of the Company, certify, pursuant to 18 U.S.C. section 1350, as adopted pursuant to section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge:
(1)The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2)The information contained in the Report fairly presents, in all material respects, the financial condition and result of operations of the Company.
|
|
|
|
|
|
| |
/s/ Theodore L. Harris |
| |
Theodore L. Harris |
| |
Chairman, President and |
| |
Chief Executive Officer |
| |
(Principal Executive Officer) |
| |
February 16, 2024 |
This certification accompanies the above-described Report on Form 10-K pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 and shall not, except to the extent required by such Act, be deemed filed by the Company for purposes of Section 18 of the Securities Exchange Act of 1934, as amended.
EX-32.2
12
bcpc202310k-ex322.htm
EX-32.2
Document
Exhibit 32.2
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Balchem Corporation (the "Company") on Form 10-K for the period ended December 31, 2023 as filed with the Securities and Exchange Commission on the date hereof (the "Report"), I, C. Martin Bengtsson, Chief Financial Officer and Treasurer of the Company, certify, pursuant to 18 U.S.C. section 1350, as adopted pursuant to section 906 of the Sarbanes-Oxley Act of 2002, that to my knowledge:
(1)The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2)The information contained in the Report fairly presents, in all material respects, the financial condition and result of operations of the Company.
|
|
|
|
|
|
| |
/s/ C. Martin Bengtsson |
| |
C. Martin Bengtsson |
| |
Executive Vice President and
Chief Financial Officer |
| |
(Principal Financial Officer) |
| |
February 16, 2024 |
This certification accompanies the above-described Report on Form 10-K pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 and shall not, except to the extent required by such Act, be deemed filed by the Company for purposes of Section 18 of the Securities Exchange Act of 1934, as amended.
EX-97.1
13
axex971xbalchemclawbackpol.htm
EX-97.1
Document
Exhibit 97.1
BALCHEM CORPORATION
INCENTIVE-BASED COMPENSATION RECOVERY POLICY
PURPOSE
The Board of Directors (the “Board”) of Balchem Corporation (the “Company”) believes that it is in the best interests of the Company and its shareholders to create and maintain a culture that emphasizes integrity and accountability and that reinforces the Company’s pay-for-performance compensation philosophy. The Board has therefore adopted this clawback policy which provides for the recoupment of certain executive compensation in the event that the Company is required to prepare an accounting restatement due to material noncompliance with any financial reporting requirement under the federal securities laws (the “Policy”). This Policy is designed to comply with Section 10D of the Securities Exchange Act of 1934 (the “Exchange Act”), the rules promulgated thereunder, and the listing standards of the Nasdaq Stock Market LLC and shall be construed and interpreted in accordance with such intent.
ADMINISTRATION
This Policy shall be administered by the Compensation Committee of the Board (the “Compensation Committee”). Any determinations made by the Compensation Committee shall be final and binding on all affected individuals.
COVERED EXECUTIVES
This Policy applies to the Company’s current and former executive officers as determined by the Board in accordance with Section 10D of the Exchange Act, the rules promulgated thereunder, and the listing standards of the Nasdaq Stock Market LLC (“Covered Executives”).
RECOUPMENT; ACCOUNTING RESTATEMENT
In the event the Company is required to prepare an accounting restatement of its financial statements due to the Company’s material noncompliance with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period (each an “Accounting Restatement”), the Compensation Committee will require reimbursement or forfeiture of the Overpayment (as defined below) received by any Covered Executive during the three completed fiscal years immediately preceding the date on which the Company is required to prepare an Accounting Restatement and any transition period (that results from a change in the Company’s fiscal year) within or immediately following those three completed fiscal years.
INCENTIVE-BASED COMPENSATION
For purposes of this Policy, “Incentive-Based Compensation” means any compensation that is granted, earned, or vested based wholly or in part upon the attainment of a Financial Reporting Measure (as defined below), including, but not limited to: (i) non-equity incentive plan awards that are earned solely or in part by satisfying a financial reporting measure performance goal; (ii) bonuses paid from a bonus pool, where the size of the pool is determined solely or in part by satisfying a financial reporting measure performance goal; (iii) other cash awards based on satisfaction of a financial reporting measure performance goal; (iv) restricted stock, restricted stock units, stock options, stock appreciation rights, and performance share units that are granted or vest solely or in part on satisfying a financial reporting measure performance goal; and (v) proceeds from the sale of shares acquired through an incentive plan that were granted or vested solely or in part on satisfying a financial reporting measure performance goal. Compensation that would not be considered Incentive-Based Compensation include, but is not limited to: (a) salaries; (b) bonuses paid solely on satisfying subjective standards, such as demonstrating leadership, and/or completion of a specified employment period; (c) non-equity incentive plan awards earned solely on satisfying strategic or operational measures; (d) wholly time-based equity awards; and (e) discretionary bonuses or other compensation that is not paid from a bonus pool that is determined by satisfying a financial reporting measure performance goal.
A “Financial Reporting Measure” is: (i) any measure that is determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, or any measure derived wholly or in part from such measure, such as revenues, EBITDA, or net income and (ii) stock price and total shareholder return. Financial reporting measures include, but are not limited to: revenues; net income; operating income; profitability of one or more reportable segments; financial ratios (e.g., accounts receivable turnover and inventory turnover rates); net assets or net asset value per share (e.g., for registered investment companies and business development companies that are subject to the rule); earnings before interest, taxes, depreciation and amortization; funds from operations and adjusted funds from operations; liquidity measures (e.g., working capital, operating cash flow); return measures (e.g., return on invested capital, return on assets); earnings measures (e.g., earnings per share); sales per square foot or same store sales, where sales is subject to an accounting restatement; revenue per user, or average revenue per user, where revenue is subject to an accounting restatement; cost per employee, where cost is subject to an accounting restatement; any of such financial reporting measures relative to a peer group, where the issuer’s financial reporting measure is subject to an accounting restatement; and tax basis income.
OVERPAYMENT: AMOUNT SUBJECT TO RECOVERY
The amount of erroneously awarded compensation to be recovered will be the amount of Incentive-Based Compensation received that exceeds the amount of Incentive-Based Compensation that otherwise would have been received had it been determined based on the restated amounts, and must be computed without regard to any taxes paid (the “Overpayment”). Incentive-Based Compensation is deemed received in the issuer’s fiscal period during which the financial reporting measure specified in the Incentive-Based Compensation award is attained, even if the payment or grant of the Incentive-Based Compensation occurs after the end of that period.
For Incentive-Based Compensation based on stock price or total shareholder return, where the amount of erroneously awarded compensation is not subject to mathematical recalculation directly from the information in the Accounting Restatement, the amount must be based on a reasonable estimate of the effect of the Accounting Restatement on the stock price or total shareholder return upon which the Incentive-Based Compensation was received; and the Company must maintain documentation of the determination of that reasonable estimate and provide such documentation to the Nasdaq Stock Market LLC.
METHOD OF RECOUPMENT
The Compensation Committee will determine, in its sole discretion, the method for recouping Overpayment hereunder which may include, without limitation:
•requiring reimbursement of cash Incentive-Based Compensation previously paid;
•seeking recovery of any gain realized on the vesting, exercise, settlement, sale, transfer, or other disposition of any equity-based awards;
•offsetting the recouped amount from any compensation otherwise owed by the Company to the Covered Executive;
•cancelling outstanding vested or unvested equity awards; and/or
•taking any other remedial and recovery action permitted by law, as determined by the Compensation Committee.
LIMITATION ON RECOVERY; NO ADDITIONAL PAYMENTS
The right to recovery will be limited to Overpayments paid or distributed during the three years prior to the date on which the Company is required to prepare an Accounting Restatement and any transition period (that results from a change in the Company’s fiscal year) within or immediately following those three completed fiscal years. In no event shall the Company be required to award Covered Executives an additional payment if the restated or accurate financial results would have resulted in a higher Incentive-Based Compensation payment.
NO INDEMNIFICATION
The Company is prohibited from indemnifying any Covered Executives against the loss of any incorrectly awarded Incentive-Based Compensation. Further, the Company is prohibited from paying or reimbursing a Covered Executive for purchasing insurance to cover any such loss.
INTERPRETATION
The Compensation Committee is authorized to interpret and construe this Policy and to make all determinations necessary, appropriate, or advisable for the administration of this Policy. It is intended that this Policy be interpreted in a manner that is consistent with the requirements of Section 10D of the Exchange Act and the applicable rules or standards adopted by the Securities and Exchange Commission or the listing standards of the Nasdaq Stock Market LLC.
EFFECTIVE DATE
This Policy shall be effective as of the date it is adopted by the Board (the “Effective Date”) and shall apply to Incentive-Based Compensation received on or after October 2, 2023 (including Incentive-Based Compensation granted pursuant to arrangements existing prior to the Effective Date).
AMENDMENT; TERMINATION
The Board may amend this Policy from time to time in its discretion and shall amend this Policy as it deems necessary to reflect final rules or additional standards adopted by the Nasdaq Stock Market LLC. The Board may terminate this Policy at any time.
OTHER RECOUPMENT RIGHTS
The Board intends that this Policy will be applied to the fullest extent of the law. The Compensation Committee may require that any employment agreement, equity award agreement, or similar agreement entered into on or after the Effective Date shall, as a condition to the grant of any benefit thereunder, require a Covered Executive to agree to abide by the terms of this Policy. To the extent that the application of this Policy would provide for recovery of Incentive-Based Compensation that the Company recovers pursuant to Section 304 of the Sarbanes-Oxley Act or other recovery obligations, the amount the relevant Covered Executive has already reimbursed the Company will be credited to the required recovery under this Policy. Any right of recoupment under this Policy is in addition to, and not in lieu of, any other remedies or rights of recoupment that may be available to the Company pursuant to the terms of any similar policy in any compensatory or incentive plan, employment agreement, equity award agreement, or similar plan or agreement and any other legal remedies available to the Company.
IMPRACTICABILITY
The Compensation Committee shall recover any Overpayment in accordance with this Policy except to the extent that the Compensation Committee determines such recovery would be impracticable because:
(A) The direct expense paid to a third party to assist in enforcing the Policy would exceed the amount to be recovered. Before concluding that it would be impractical to recover any amount of erroneously awarded compensation based on expense of enforcement, the Company must make a reasonable attempt to recover such erroneously awarded compensation, document such reasonable attempts to recover and provide that documentation to the Nasdaq Stock Market LLC;
(B) Recovery would violate home country law where that law was adopted prior to November 28, 2022. Before concluding that it would be impractical to recover any amount of erroneously awarded compensation based on violation of home country law, the Company must obtain an opinion of home country counsel, acceptable to the Nasdaq Stock Market LLC, that recovery would result in such a violation, and much provide such opinion to the Nasdaq Stock Market LLC; or
(C) Recovery would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of the Company, to fail to meet the requirements of Section 401(a)(13) or Section 411(a) of the Code.
INDEMNIFICATION OF BOARD AND COMPENSATION COMMMITTEE
Any members of the Compensation Committee, and any other members of the Board who assist in the administration of this Policy, shall not be personally liable for any action, determination or interpretation made with respect to this Policy and shall be fully indemnified by the Company to the fullest extent under applicable law and Company policy with respect to any such action, determination or interpretation. The foregoing sentence shall not limit any other rights to indemnification of the members of the Board or Compensation Committee under applicable law or Company policy.
SUCCESSORS
This Policy shall be binding and enforceable against all Covered Executives and their beneficiaries, heirs, executors, administrators or other legal representatives.
Adopted by the Board on September 13, 2023

