Document
Exhibit 99.1
Inspire Medical Systems, Inc. Publishes Inspire V Data at
ISSS/AAO-HNS Meetings
MINNEAPOLIS, MN – October 10, 2025 – Inspire Medical Systems, Inc. (NYSE: INSP) (Inspire), a medical technology company focused on the development and commercialization of innovative, minimally invasive solutions for patients with obstructive sleep apnea (OSA), today published Inspire V clinical outcomes data from its Singapore clinical study as well as the Company’s limited market release in the United States including single site experience at two leading centers. These data will be presented at the American Otolaryngology-Head and Neck Surgery (AAO-HNS) and International Surgical Sleep Society (ISSS) meetings being conducted in Indianapolis between October 9 and October 14.
“We are incredibly excited to publish first-time clinical evidence on the Inspire V system. The design of the Inspire V system captures experience built over 25 years with Inspire therapy including over 100,000 patients implanted, and we are proud to provide the initial data from this advanced platform,” stated Tim Herbert, Chairman and Chief Executive Officer of Inspire Medical Systems. “Our initial experience raises the bar for what a successful therapy needs to achieve for both safety and efficacy. The data show that the Inspire V system provides very high and consistent patient adherence of over six hours per night which a patient’s physician can monitor with our SleepSync™ patient management platform.”
“The data presented at the ISSS/AAO-HNS meetings in Indianapolis this weekend demonstrate the benefits that Inspire therapy brings to the many patients struggling with OSA,” stated Paul Hoff, M.D., M.S., Inspire Vice President, Senior Medical Director. “The strong safety, superior respiratory sensing, and increased adherence, combined with a 20% reduction in surgical times and features designed to enhance patient comfort, demonstrate a truly significant advancement with the Inspire V system.”
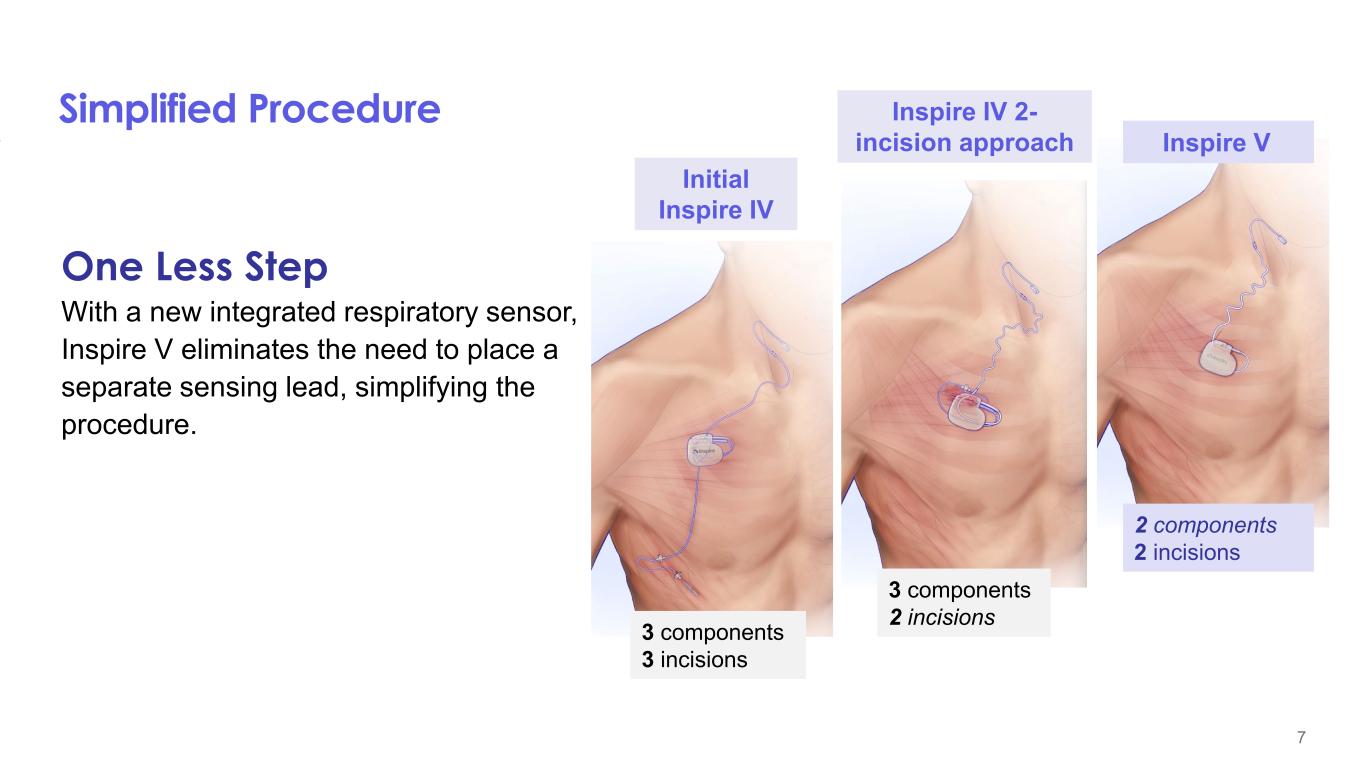
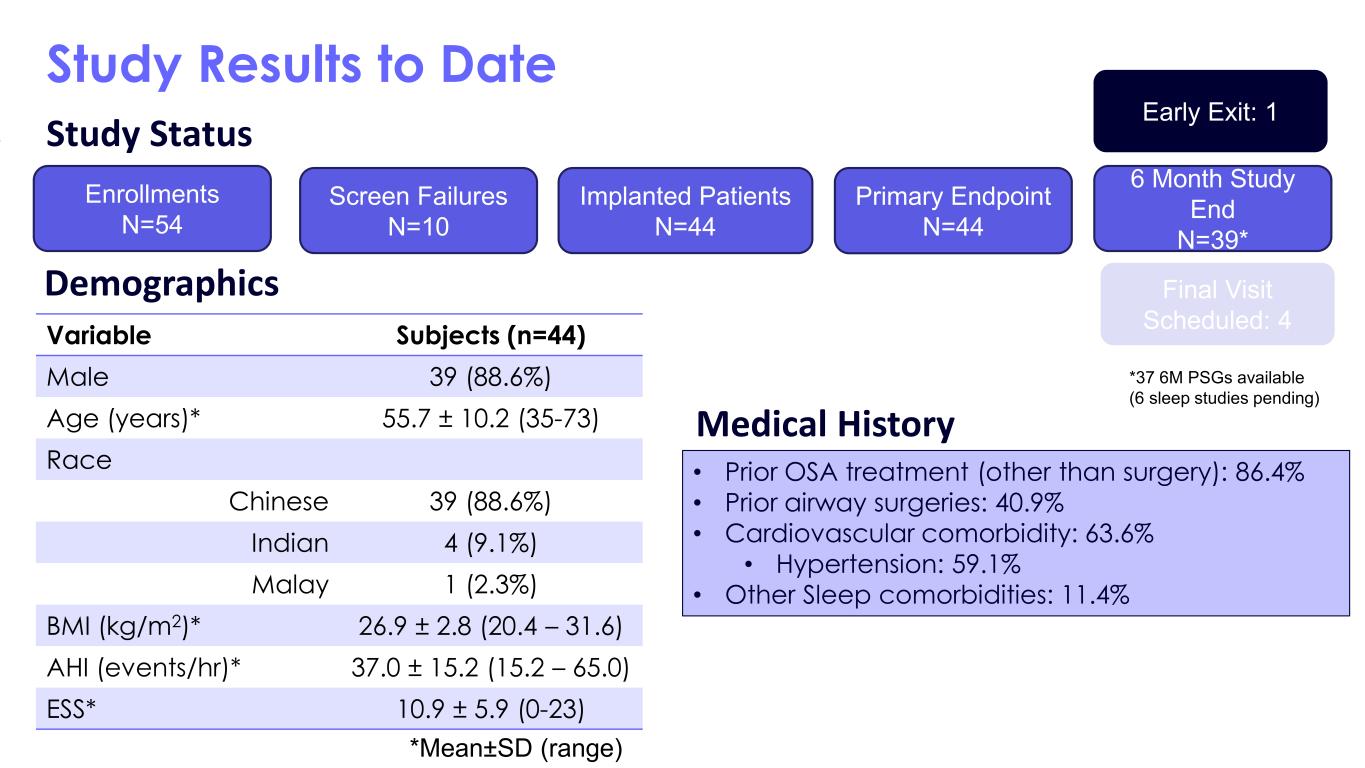
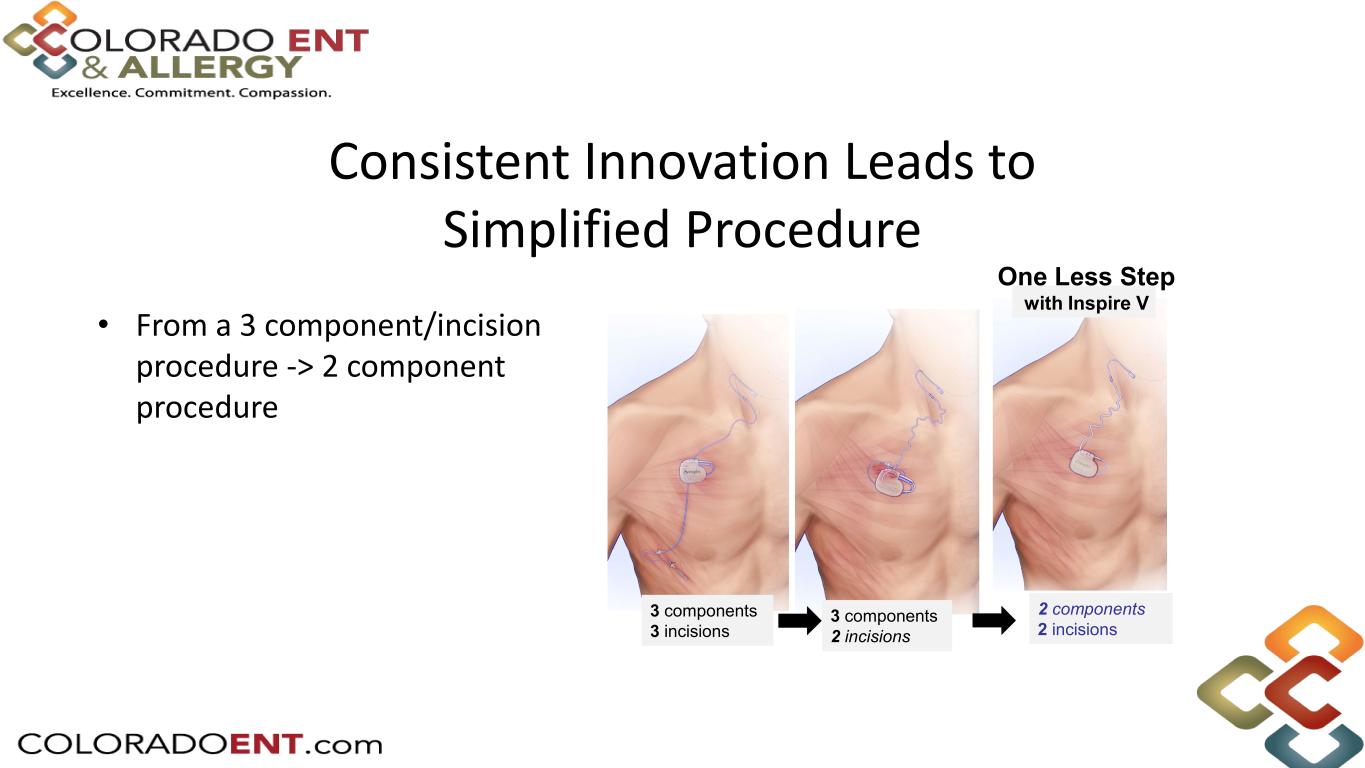
The Inspire V system trial conducted in Singapore at two centers was the initial experience with the new device. The study included 44 patients who received the Inspire V system and are being followed for six months post implant. The surgeons demonstrated a 20% reduction in surgical times as compared to their Inspire IV case times and 100% of the procedures were completed successfully. A key feature of the Inspire V system is that the respiratory sensing is internal to the neurostimulator, eliminating the need for the pressure sensing lead required with prior generations. The Inspire design provides for closed-loop stimulation to optimize therapy outcomes, and the primary endpoint of this Inspire V study was to measure the inspiratory phase overlap percentage (IPOP), which measures how well inspiration is covered by stimulation. The results showed the Inspire V device to be superior to the Inspire IV device (Inspire V IPOP of 87.1% vs 79.4% for Inspire IV with a non-inferiority margin of 4.6%). Important secondary measures included patient adherence to therapy of all 44 patients averaged 5.5±1.7 hours/night at a mean of 246 days post-implant, as well as the median reduction in the Apnea Hypopnea Index (AHI), as measured by overnight polysomnography (PSG), to be 34.4 events/hour at baseline to 8.3 events/hour at month six for the 37 patients who have completed this clinical visit to date. The safety profile was equally strong with only two patients requiring post-operative antibiotics for wound care that resolved within two weeks following the procedure. Once the remaining patients complete their six-month visit, these data will be submitted for publication.
In the United States, the Company conducted a limited market release evaluation of the Inspire V system at 10 leading centers with 101 patients. All device procedures were completed successfully with no serious adverse events, and all patients continue to use their Inspire therapy. At the 60-day check, all 101 patients were using their therapy with an average stimulation amplitude of 1.7 volts and an average usage of 6.8 hours/night.
At the subsequent sleep study, data on the first 34 patients demonstrated a median reduction in AHI from 30 to 4.5 events/hours, noting that these are therapeutic values. With these 34 patients, the average amplitude remained steady at 1.7 volts and therapy adherence remained consistent at 6.7 hours/night.
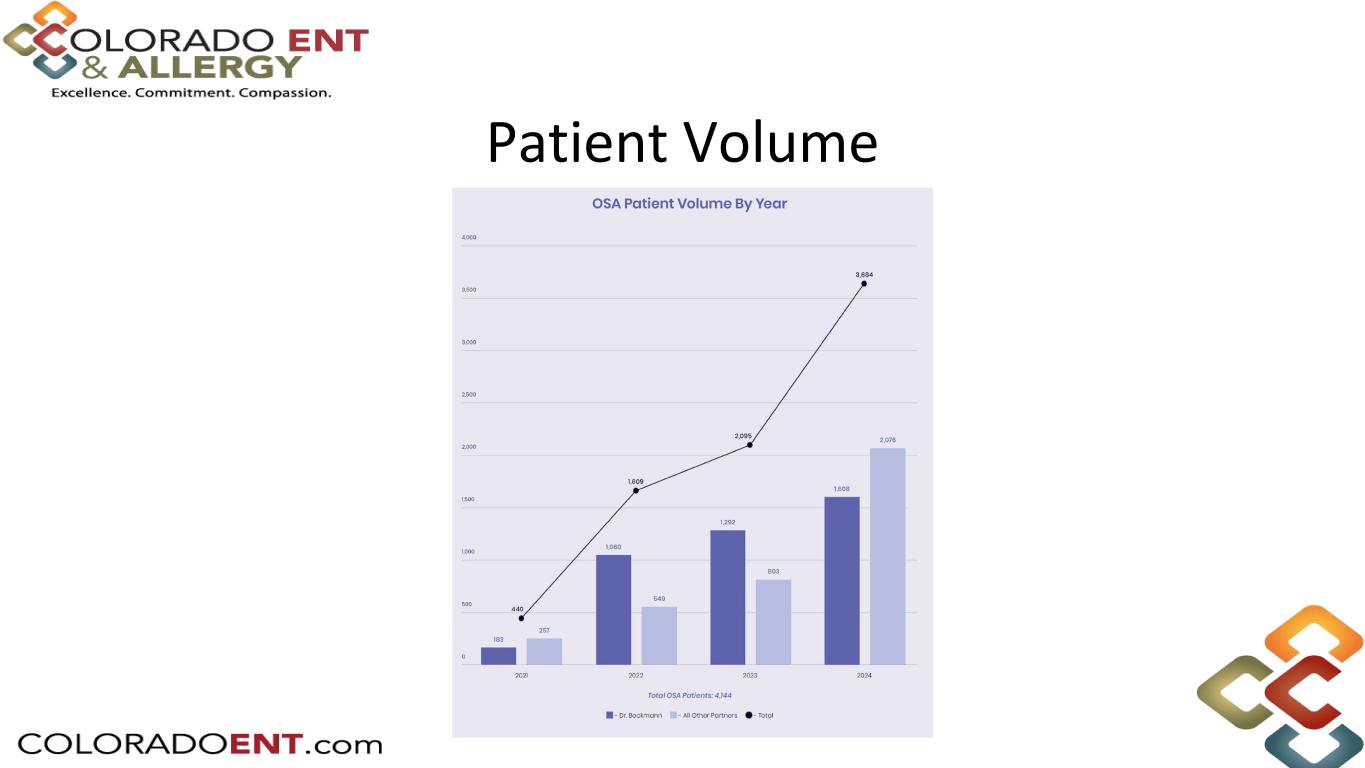
The limited market release also provided single-site results from two of the centers. Dr. Phil Huyett from the Massachusetts Eye and Ear Infirmary in Boston reported that for his first nine patients, all procedures were completed successfully with an average surgical implant time of 34.5 minutes for a standard Inspire placement, and slightly longer for revised techniques. Dr. Nic Beckmann at Colorado ENT and Allergy in Colorado Springs reported an increase in implant volume with Inspire V due to the shorter procedure times averaging 12 implants per surgery day compared to an average of 9 cases per surgery day with Inspire IV. Dr. Beckmann also discussed the current reimbursement levels as well as the proposed 2026 Medicare reimbursement for the Inspire V CPT code.
Sessions of Interest
Friday, October 10 7:00 a.m. – 8:00 a.m. ET: Next Generation Hypoglossal Nerve Stimulation for the Treatment of Obstructive Sleep Apnea: Results of the Inspire V Study (Ryan Soose, M.D., Tom Kaffenberger, M.D., Nic Beckmann, D.O., F.A.A.O.A., Phil Huyett, M.D.)
Sunday, October 12 2:30 p.m. – 3:30 p.m. ET: Next Generation Hypoglossal Nerve Stimulation for the Treatment of Obstructive Sleep Apnea: Results of the Inspire V Study (Nic Beckmann, D.O., F.A.A.O.A., Maria Suurna, M.D., Mark Aloia, Ph.D., Michael Coleman)
About Inspire Medical Systems
Inspire is a medical technology company focused on the development and commercialization of innovative, minimally invasive solutions for patients with obstructive sleep apnea. Inspire’s proprietary Inspire therapy is the first and only FDA, EU MDR, and PDMA-approved neurostimulation technology that provides a safe and effective treatment for moderate to severe obstructive sleep apnea.
For additional information about Inspire, please visit www.inspiresleep.com.
About AAO-HNS
The American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) is one of the world’s largest organizations representing specialists who treat the ears, nose, throat, and related structures of the head and neck. Otolaryngologist–head and neck surgeons diagnose and treat medical disorders that are among the most common affecting patients of all ages in the United States and around the world. Those medical conditions include chronic ear disease, hearing and balance disorders, hearing loss, sinusitis, snoring and sleep apnea, allergies, swallowing disorders, nosebleeds, hoarseness, dizziness, and tumors of the head and neck as well as aesthetic and reconstructive surgery and intricate micro-surgical procedures of the head and neck. The Academy has approximately 13,000 members.
About ISSS
The International Surgical Sleep Society (ISSS) is dedicated to improving equitable care for all patients with sleep disordered breathing through collaboration, research, education, advocacy and innovation and has over 600 global members.
Forward Looking Statements
This press release may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts are forward-looking statements, including, those relating to the strength and potential benefit of the Inspire V system clinical data. Forward-looking statements involve inherent risks and uncertainties, and important factors could cause actual results to differ materially from those anticipated, including the factors identified under “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations“ in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024, as updated in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2025, and as such factors may be updated from time to time in our other filings with the SEC, which are accessible on the SEC’s website at www.sec.gov and the Investors page of our website at www.inspiresleep.com. Forward-looking statements speak only as of the date they are made, and we undertake no obligation to update them in light of new information or future events.
Investor and Media Contact
Ezgi Yagci
Vice President, Investor Relations
ezgiyagci@inspiresleep.com
617-549-2443