0001599901FALSE00015999012025-09-092025-09-090001599901dei:FormerAddressMember2025-09-092025-09-09
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________________________________
FORM 8-K
_________________________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): September 9, 2025
_________________________________________
AVIDITY BIOSCIENCES, INC.
(Exact name of registrant as specified in its charter)
_________________________________________
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Delaware |
|
001-39321 |
|
46-1336960 |
|
(State or other jurisdiction of
incorporation or organization)
|
|
(Commission
File Number)
|
|
(I.R.S. Employer
Identification No.)
|
3020 Callan Road
San Diego, California 92121 92121
(Address of principal executive offices) (Zip Code)
(858) 401-7900
(Registrant’s telephone number, include area code)
10578 Science Center Drive, Suite 125
San Diego, California 92121 92121
(Former Name or Former Address, if Changed Since Last Report)
_________________________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
|
|
|
|
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
|
|
|
|
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
|
|
|
|
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
|
|
|
|
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s)
|
|
Name of each exchange
on which registered
|
| Common Stock, par value $0.0001 per share |
|
RNA |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
|
|
|
|
|
|
| Item 5.02. |
Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers. |
On September 9, 2025, the board of directors of Avidity Biosciences, Inc. ("Avidity" or the "Company") approved an amendment to the Avidity Biosciences, Inc. 2022 Employment Inducement Incentive Award Plan to increase the authorized number of shares of the Company's common stock reserved for issuance thereunder from an aggregate of 4,500,000 shares to an aggregate of 8,000,000 shares. A copy of the full text of this amendment is attached as Exhibit 10.1 to this Current Report on Form 8-K and is incorporated herein by reference.
|
|
|
|
|
|
| Item 7.01. |
Regulation FD Disclosure. |
On September 10, 2025, the Company will host an investor and analyst event (the "Event") to discuss new data from participants treated continuously with delpacibart zotadirsen ("del-zota") for one year in the EXPLORE44® and EXPLORE44-OLE™ trials. The Event will begin at 8:00 a.m. Eastern Time and will be available via a live video webcast accessible under the "Events and Presentations" page in the "Investors" section of Avidity's corporate website, at https://www.aviditybiosciences.com. During the Event, the Company will present the corporate slide presentation attached as Exhibit 99.1 to this Current Report on Form 8-K, which is incorporated herein by reference.
The information contained in this Item 7.01, including in Exhibit 99.1 hereto and on Avidity’s corporate website, is being “furnished” and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), is not subject to the liabilities of that section and is not deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
On September 10, 2025, the Company announced positive new data from participants treated continuously with del-zota for one year in the EXPLORE44 and EXPLORE44-OLE trials. These data demonstrated reversal of disease progression and unprecedented improvement compared to baseline and natural history across multiple functional measures. Additional data from the EXPLORE44 program will be presented at upcoming scientific congresses.
Trial participants treated with del-zota demonstrated statistically significant increases of approximately 25 percent of normal in dystrophin production and restored total dystrophin up to 58 percent of normal. Creatine kinase (“CK”) levels reduced by greater than 80 percent compared to baseline and were sustained at near normal levels throughout the duration of evaluation with participants followed for up to 16 months. Additionally, 50 percent of participants had CK levels within the normal range at one year of treatment.
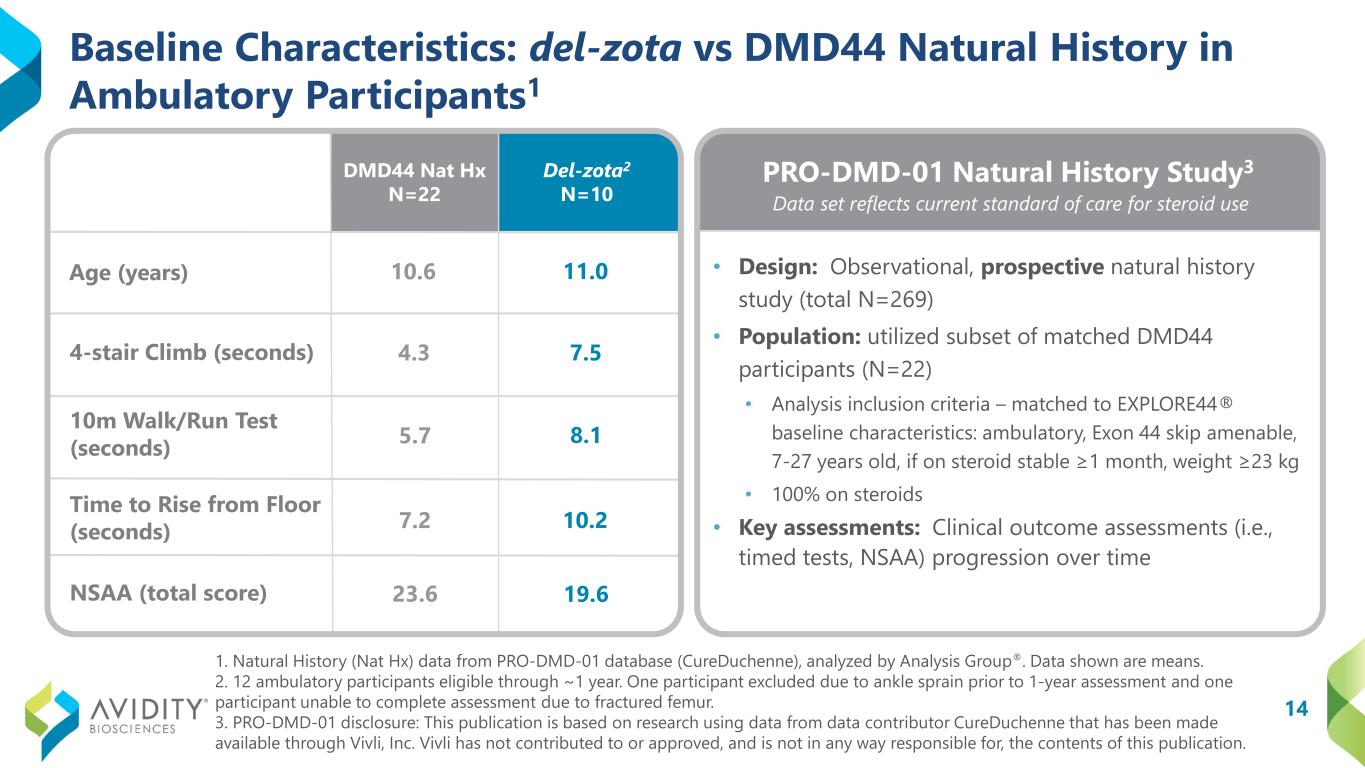
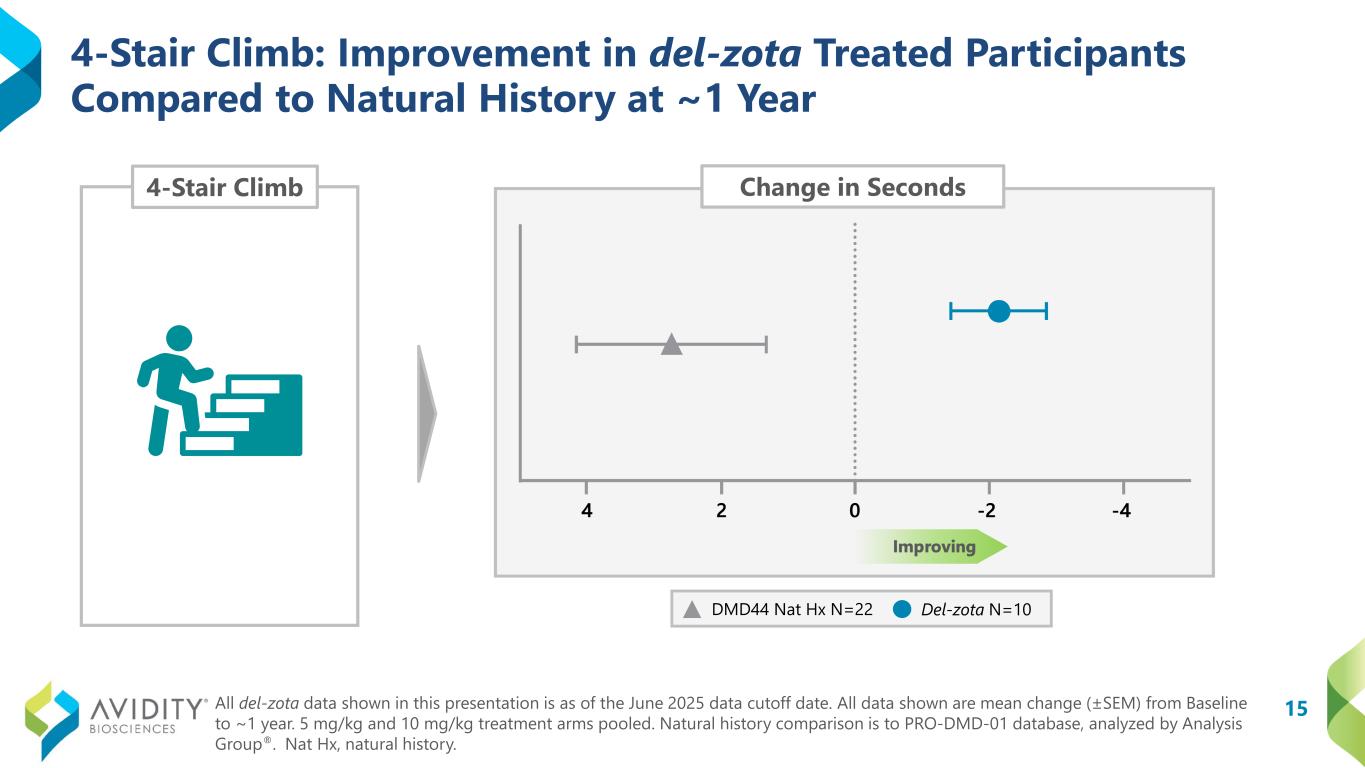
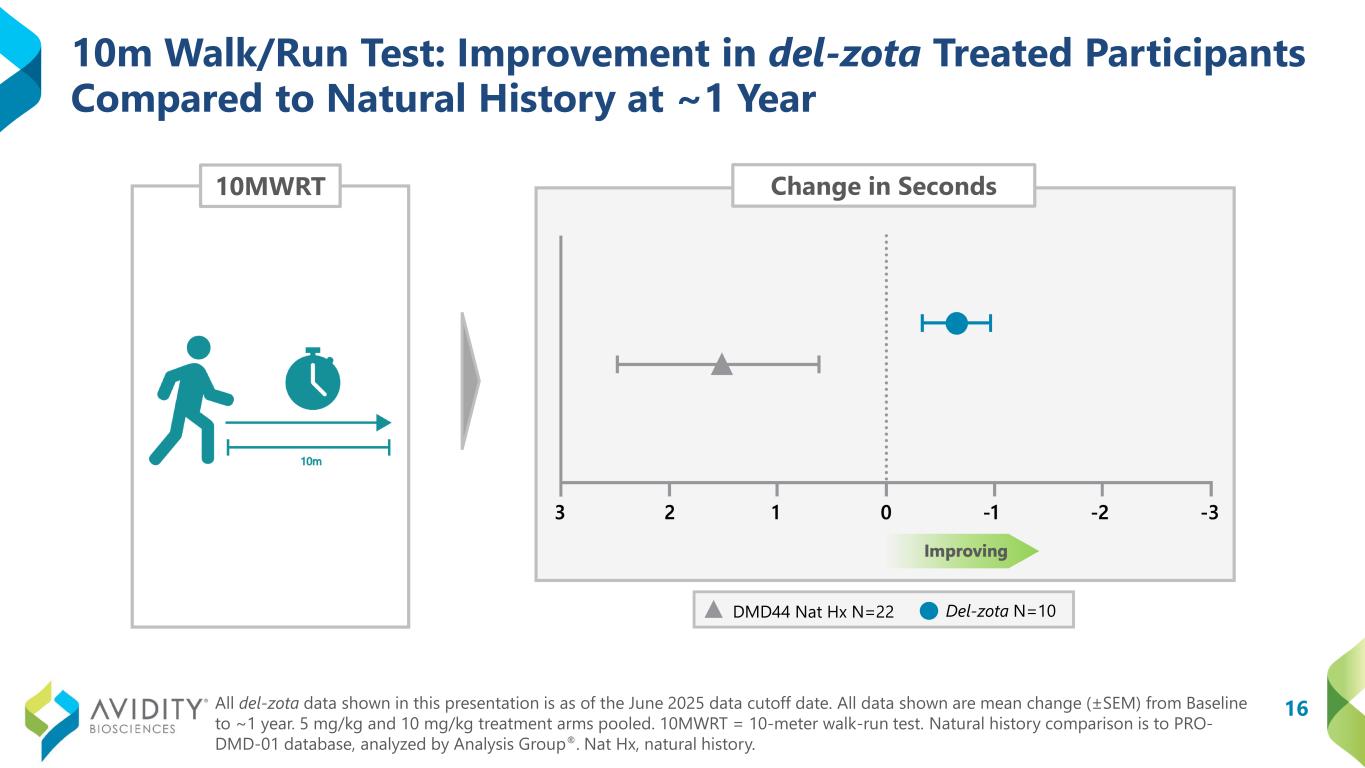
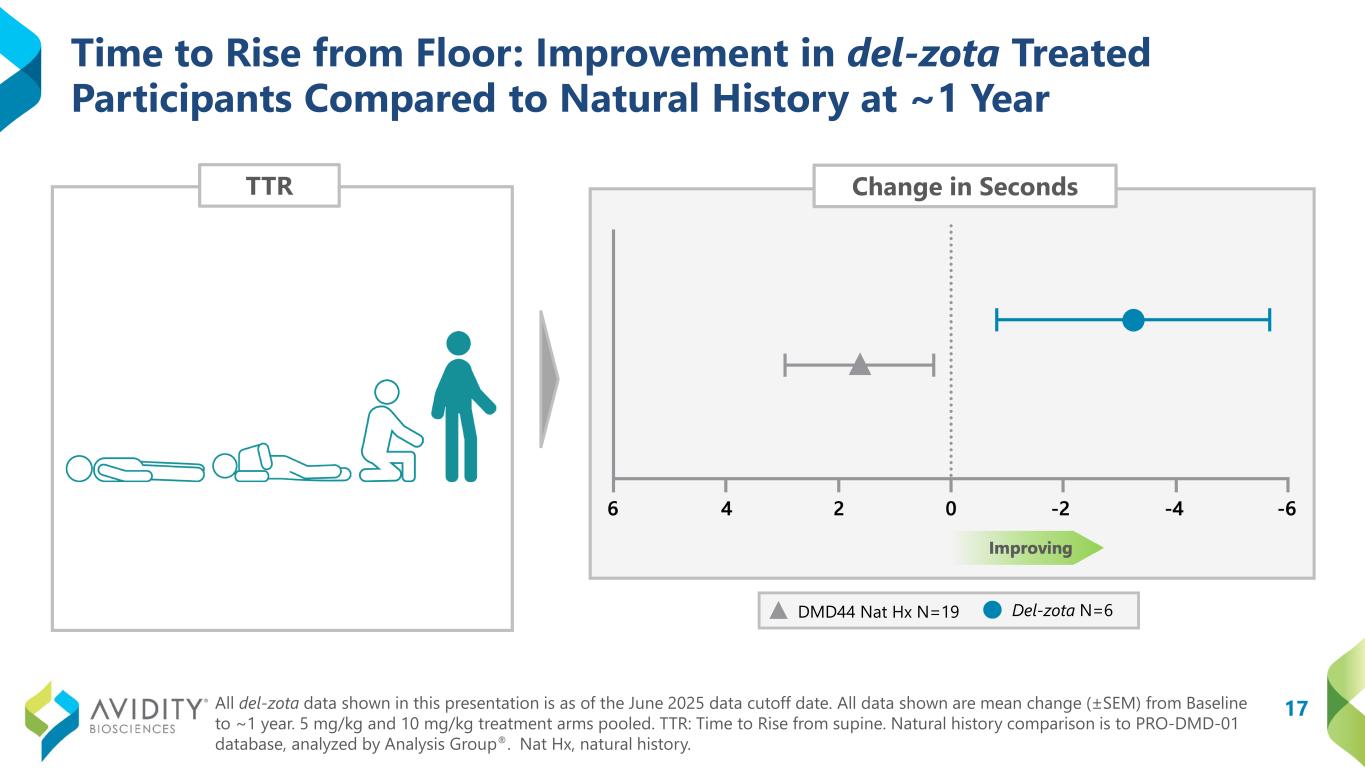
A total of 17 participants (12 ambulatory and 5 non-ambulatory) who began on the del-zota treated arm of EXPLORE44 and continued into the EXPLORE44-OLE have been followed for approximately one year. Given the study design, some participants received 5 mg/kg once every six weeks (“Q6W”) and some received 10 mg/kg once every eight weeks during EXPLORE44. All participants were transitioned to the 5 mg/kg (Q6W) dosing schedule during EXPLORE44-OLE. Not all participants could complete all assessments. Functional data from these pooled dosing cohorts for del-zota treated participants, compared to DMD44 natural history (PRO-DMD-01), demonstrated improvement:
•4-Stair Climb (“4SC”): Improved from baseline by 2.1 seconds. In contrast, the natural history group declined from baseline by 2.7 seconds (DMD44 Nat Hx N=22; del-zota N=10).
•10-Meter Walk/Run Test (“10mWRT”): Improved from baseline by 0.7 seconds. In contrast, the natural history group declined from baseline by 1.5 seconds (DMD44 Nat Hx N=22; del-zota N=10).
•Time to Rise from Floor (“TTR”): Improved from baseline by 3.2 seconds. In contrast, the natural history group declined from baseline by 1.6 seconds (DMD Nat Hx N=19; del-zota N=6).
•North Star Ambulatory Assessment (“NSAA”): Remained stable. In contrast, the natural history group declined from baseline by 2.4 points (DMD44 Nat Hx N=20; del-zota N=10).
•Performance of Upper Limb (“PUL2”): Improved from baseline by 1.5 points. In contrast, the natural history group declined from baseline by 0.7 points. Similar PUL improvements were seen in both ambulatory and non-ambulatory participants (DMD44 Nat Hx N=27; del-zota N=17).
Safety was assessed in all participants in the EXPLORE44-OLE trial, and del-zota continued to demonstrate favorable long-term safety and tolerability as of the June 2025 data cutoff. Most treatment emergent adverse events (“TEAEs”) were mild or moderate with the most common TEAEs (occurring in greater than 3 participants) being upper respiratory tract symptoms, diarrhea, fall, backpain and headache.
One participant discontinued from EXPLORE44-OLE following an event of hypersensitivity.
Avidity remains on track to submit a Biologics License Application (“BLA”) to the U.S. Food and Drug Administration (“FDA”) at year end 2025. This will be Avidity’s first of three planned BLA submissions over a 12-month period. Avidity continues to prepare for a confirmatory study to support full global approval.
In the above studies, 4SC, 10mWRT, TTR and NSAA were only performed in ambulatory participants, and PUL was performed in ambulatory and non-ambulatory participants. PRO-DMD-01 was an observational, prospective natural history study (n=269) intended to study the progression of subjects with DMD. In collaboration with a third-party marketing research group engaged in the analysis of DMD progression based on natural history, a matched subset of DMD44 participants (n=22) was utilized to analyze progression on key ambulatory functional endpoints at 12 months. A different natural history comparator was used for the PUL 2.0 assessment based on a separate published natural history study (n=27). Natural history data may not be precisely matched to del-zota patient data or representative of the DMD44 patient population in other ways.
|
|
|
|
|
|
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits
|
|
|
|
|
|
|
|
|
|
Exhibit
Number
|
|
Description |
|
|
|
| 10.1 |
|
|
|
|
| 99.1 |
|
|
|
|
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AVIDITY BIOSCIENCES, INC. |
|
|
|
|
| Date: September 10, 2025 |
|
By: |
/s/ Michael F. MacLean |
|
|
|
Michael F. MacLean |
|
|
|
Chief Financial Officer |
EX-10.1
2
exhibit101-amendmenttoin.htm
EX-10.1
exhibit101-amendmenttoin

Exhibit 10.1 AMENDMENT TO THE AVIDITY BIOSCIENCES, INC. 2022 EMPLOYMENT INDUCEMENT INCENTIVE AWARD PLAN THIS AMENDMENT TO THE AVIDITY BIOSCIENCES, INC. 2022 EMPLOYMENT INDUCEMENT INCENTIVE AWARD PLAN (this “Amendment”), effective as of September 9, 2025 is made and adopted by Avidity Biosciences, Inc., a Delaware corporation (the “Company”). Capitalized terms used but not otherwise defined herein shall have the meanings ascribed to them in the Plan (as defined below). RECITALS WHEREAS, the Company maintains the Avidity Biosciences, Inc. 2022 Employment Inducement Incentive Award Plan (as amended from time to time, the “Plan”); WHEREAS, pursuant to Section 10.4 of the Plan, the Plan may be amended by the Administrator of the Plan at any time; WHEREAS, the Human Capital Management Committee of the Company’s Board of Directors (the “Board”) is the Administrator of the Plan; WHEREAS, pursuant to Section 3.1 of the Plan, the Board may re-vest administrative authority over the Plan to itself at any time; and WHEREAS, the Board has re-vested itself administrative authority over the Plan solely with respect to, and for purposes of, approving this Amendment and, pursuant to such authority, has adopted and approved this Amendment. NOW, THEREFORE, in consideration of the foregoing, the Company hereby amends the Plan as follows: 1. Section 11.28 of the Plan is hereby amended and restated in its entirety to read as follows: A. “11.28 ‘Overall Share Limit’ means 8,000,000 Shares.” 2. This Amendment shall be and is hereby incorporated in and forms a part of the Plan. 3. Except as expressly provided herein, all other terms and provisions of the Plan shall remain unchanged and in full force and effect.

IN WITNESS WHEREOF, I hereby certify that this Amendment was duly adopted by the Board of Directors of Avidity Biosciences, Inc. on September 9, 2025. Avidity Biosciences, Inc. By: /s/ John B. Moriarty, Jr., J.D. John B. Moriarty, Jr., J.D. Chief Legal Officer and Corporate Secretary Date: September 9, 2025
EX-99.1
3
sep_10x2025xdel-zotaxweb.htm
EX-99.1
sep_10x2025xdel-zotaxweb

1 September 10, 2025 NASDAQ: RNA | aviditybio.com Investor & Analyst Event Redefining the Treatment of Duchenne Muscular Dystrophy (DMD) Del-zota EXPLORE44-OLE Topline and Functional Data Exhibit 99.1

2 Forward-Looking Statements We caution the reader that this presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements other than statements of historical fact contained in this presentation are forward-looking statements. Forward-looking statements include, but are not limited to, statements regarding: our business strategy; the design, goals and conduct of our Phase 1/2 EXPLORE44 trial and EXPLORE44-OLE study; the characterization of data and results from our Phase 1/2 EXPLORE44 trial and EXPLORE44-OLE study and conclusions drawn therefrom; our plans to submit a BLA to the FDA and the timing thereof; research and development plans; plans and projected timelines for delpacibart zotadirsen (del-zota, formerly AOC 1044) and other product candidates; safety and tolerability and functional benefit of del-zota; key functional endpoints used in the study of del-zota and the data produced therefrom; the status and possibility of del-zota regarding accelerated approval; our ability to execute on plans for a potential commercial launch of del-zota and the timing thereof; our ability to successfully build commercial infrastructure and ensure access to del-zota; the potential of the AOC platform; the regulatory pathways available for del-zota and our other product candidates; the design, goals, statuses and details of EXPLORE44 and EXPLORE44-OLE; the significance of comparing results from the study of del-zota to the referenced natural history study; the status and potential of del-zota; dosage levels to be administered in our clinical trials of del-zota; enrollment status of EXPLORE44-OLE; the ability of del-zota to treat DMD44; timing and likelihood of success; product approvals; and plans and objectives of management for future operations. In some cases, the reader can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The inclusion of forward-looking statements should not be regarded as a representation by Avidity that any of our plans will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in our business and beyond our control, including, without limitation: the results of clinical trials are not necessarily predictive of future results; requests for data by the FDA or other regulatory authorities may result in significant additional expense and timing delays; data delivered to the regulators may not be satisfactory, or support a successful BLA submission, registration or accelerated approval, including, where applicable, as a result of our inability to establish that a novel biomarker may serve as a surrogate endpoint reasonably likely to predict clinical benefit; additional participant data related to del-zota that continues to become available may be inconsistent with the data produced as of the most recent date cutoffs, and further analysis of existing data and analysis of new data may lead to conclusions different from those established as of such date cutoffs; unexpected adverse side effects or inadequate efficacy of del-zota may delay or limit its development, regulatory approval and/or commercialization, or may result in clinical holds, recalls or product liability claims; our approach to the discovery and development of product candidates is unproven, and we do not know whether we will be able to develop any products of commercial value; potential delays in the commencement, enrollment and completion of clinical trials and data readouts therefrom; even if approved, we may not be able to execute any successful product launches; our dependence on third parties in connection with clinical testing and product manufacturing; regulatory developments in the United States and foreign countries, including acceptance of INDs and similar foreign regulatory submissions and our proposed design of future clinical trials; Fast Track and other designations by the FDA may not lead to a faster development or regulatory review or approval process; our ability to obtain and maintain intellectual property protection for our product candidates and proprietary technologies; we may exhaust our capital resources sooner than we expect and fail to raise additional needed funds; and other risks described under the heading “Risk Factors” in our Form 10-K for the year ended December 31, 2024, filed with the SEC on February 27, 2025, and in subsequent filings with the SEC. The reader is cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. All forward- looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry and disease indications. This data involves a number of assumptions and limitations, and the reader is cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities, nor shall there be any sale of securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.

3 OUR VISION To profoundly improve people’s lives by revolutionizing the delivery of RNA therapeutics Yannick, living with DMD

4Redefining the Treatment of DMD Today’s Presenters Sarah Boyce President & CEO Steve Hughes, MD Chief Medical Officer
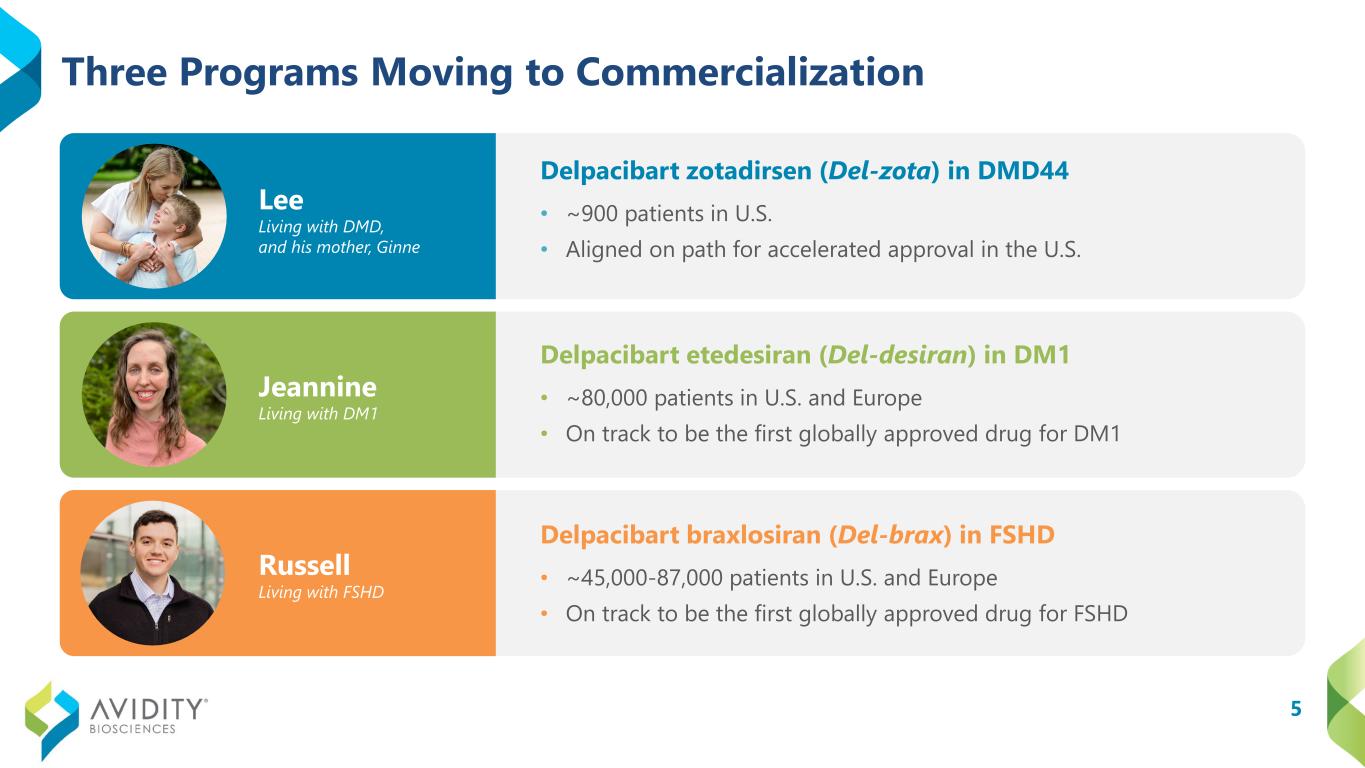
5 Three Programs Moving to Commercialization Lee Living with DMD, and his mother, Ginne Jeannine Living with DM1 Russell Living with FSHD Delpacibart etedesiran (Del-desiran) in DM1 • ~80,000 patients in U.S. and Europe • On track to be the first globally approved drug for DM1 Delpacibart braxlosiran (Del-brax) in FSHD • ~45,000-87,000 patients in U.S. and Europe • On track to be the first globally approved drug for FSHD Delpacibart zotadirsen (Del-zota) in DMD44 • ~900 patients in U.S. • Aligned on path for accelerated approval in the U.S.
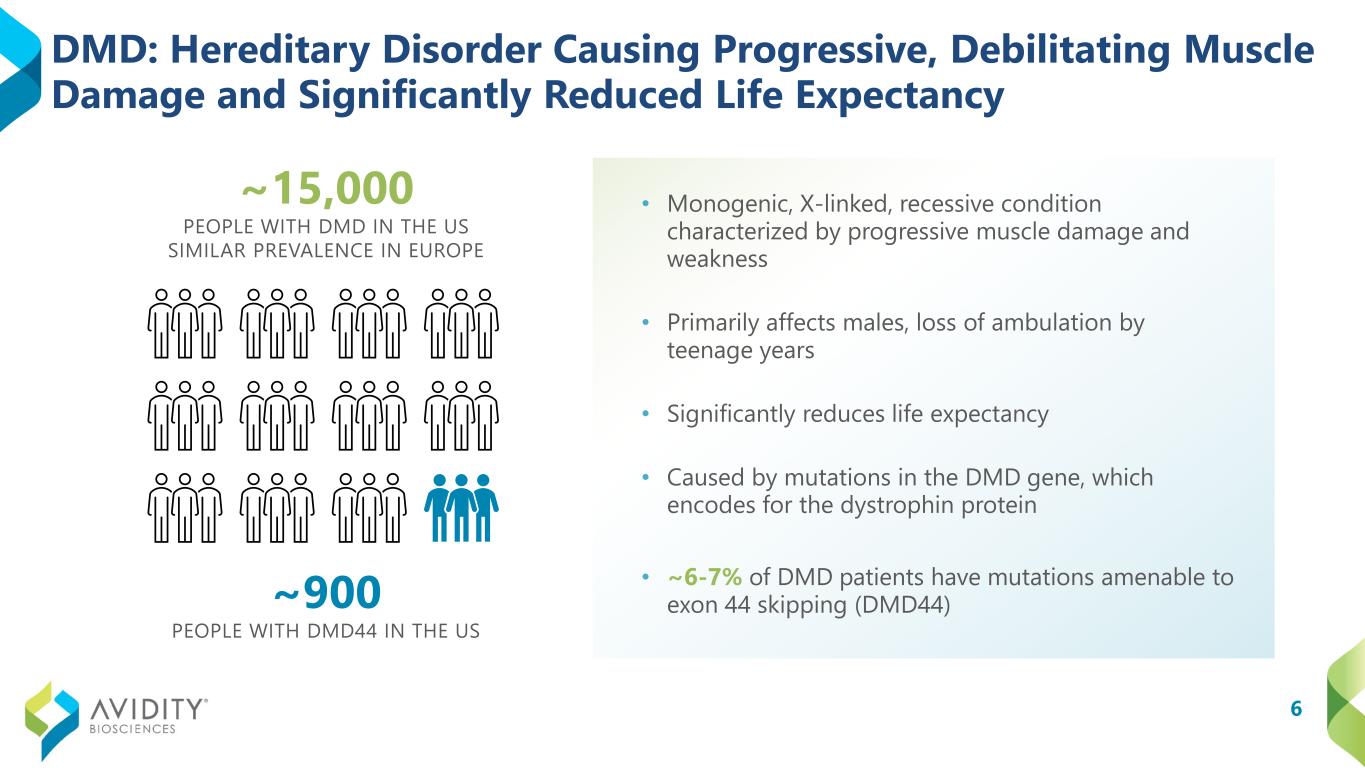
6 DMD: Hereditary Disorder Causing Progressive, Debilitating Muscle Damage and Significantly Reduced Life Expectancy • Monogenic, X-linked, recessive condition characterized by progressive muscle damage and weakness • Primarily affects males, loss of ambulation by teenage years • Significantly reduces life expectancy • Caused by mutations in the DMD gene, which encodes for the dystrophin protein • ~6-7% of DMD patients have mutations amenable to exon 44 skipping (DMD44) ~15,000 PEOPLE WITH DMD IN THE US SIMILAR PREVALENCE IN EUROPE ~900 PEOPLE WITH DMD44 IN THE US
7 Today’s Update Unprecedented functional improvement on all key measures demonstrated at one year across EXPLORE44® & EXPLORE44-OLE Compelling biomarker data of muscle health including rapid, significant and sustained creatine kinase (CK) reductions Continued favorable safety and tolerability with most TEAEs mild or moderate On track for BLA submission by year end 2025 and launch in 2026 delpacibart zotadirsen abbreviation: del-zota mAb PMO mAb: monoclonal antibody PMO: phosphorodiamidate morpholino oligomer

8 Agenda/Outline Revolutionizing the Delivery of RNA Sarah Boyce, President & CEO Del-zota: Redefining the Treatment of DMD Steve Hughes, M.D., CMO Closing Remarks Sarah Boyce, President & CEO Q&A Session Avidity Management Moderator: Kat Lange, CBO
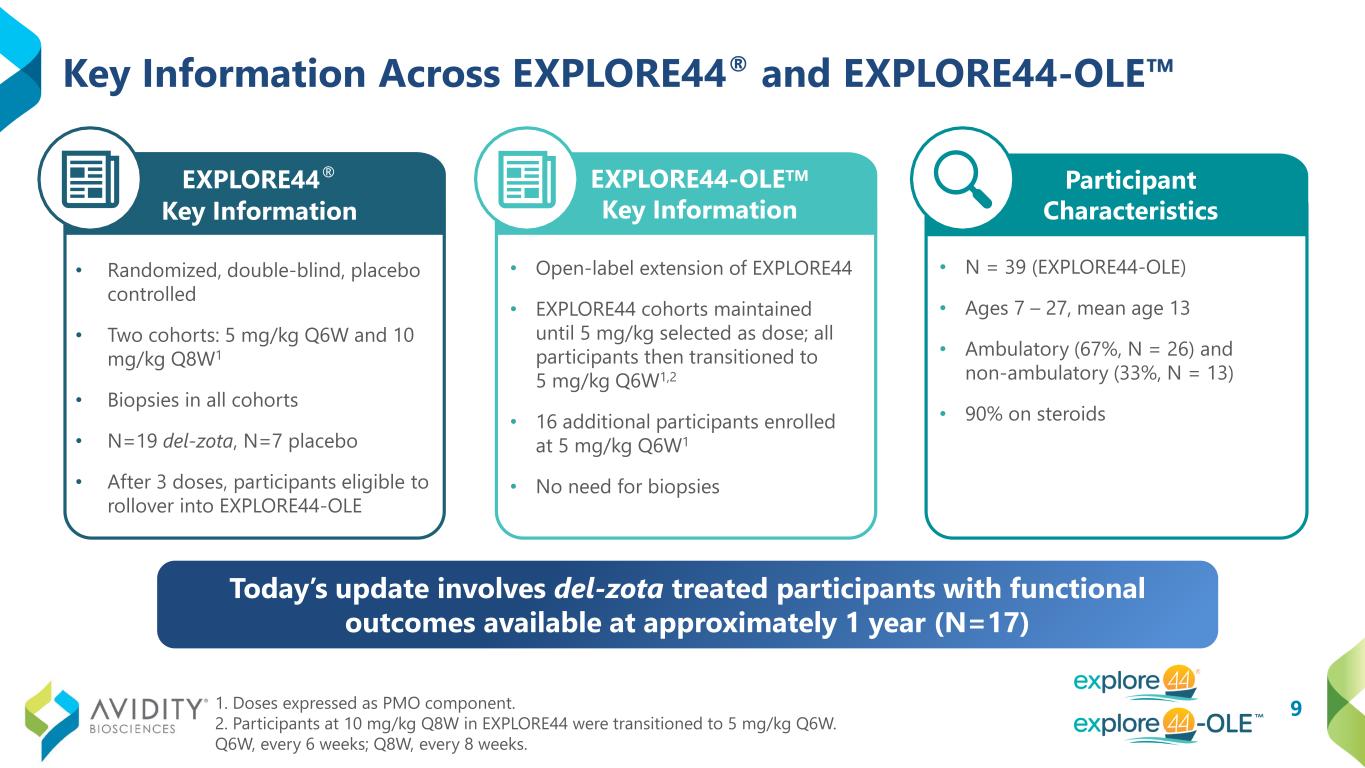
9 Key Information Across EXPLORE44® and EXPLORE44-OLE 1. Doses expressed as PMO component. 2. Participants at 10 mg/kg Q8W in EXPLORE44 were transitioned to 5 mg/kg Q6W. Q6W, every 6 weeks; Q8W, every 8 weeks. EXPLORE44® Key Information • Randomized, double-blind, placebo controlled • Two cohorts: 5 mg/kg Q6W and 10 mg/kg Q8W1 • Biopsies in all cohorts • N=19 del-zota, N=7 placebo • After 3 doses, participants eligible to rollover into EXPLORE44-OLE • Open-label extension of EXPLORE44 • EXPLORE44 cohorts maintained until 5 mg/kg selected as dose; all participants then transitioned to 5 mg/kg Q6W1,2 • 16 additional participants enrolled at 5 mg/kg Q6W1 • No need for biopsies EXPLORE44-OLETM Key Information Key Participant Characteristics (N = 39) Ages 7 – 27, mean age 13 Ambulatory (67%) and non- ambulatory (33%) 90% on steroids • N = 39 (EXPLORE44-OLE) • Ages 7 – 27, mean age 13 • Ambulatory (67%, N = 26) and non-ambulatory (33%, N = 13) • 90% on steroids Participant Characteristics Today’s update involves del-zota treated participants with functional outcomes available at approximately 1 year (N=17)
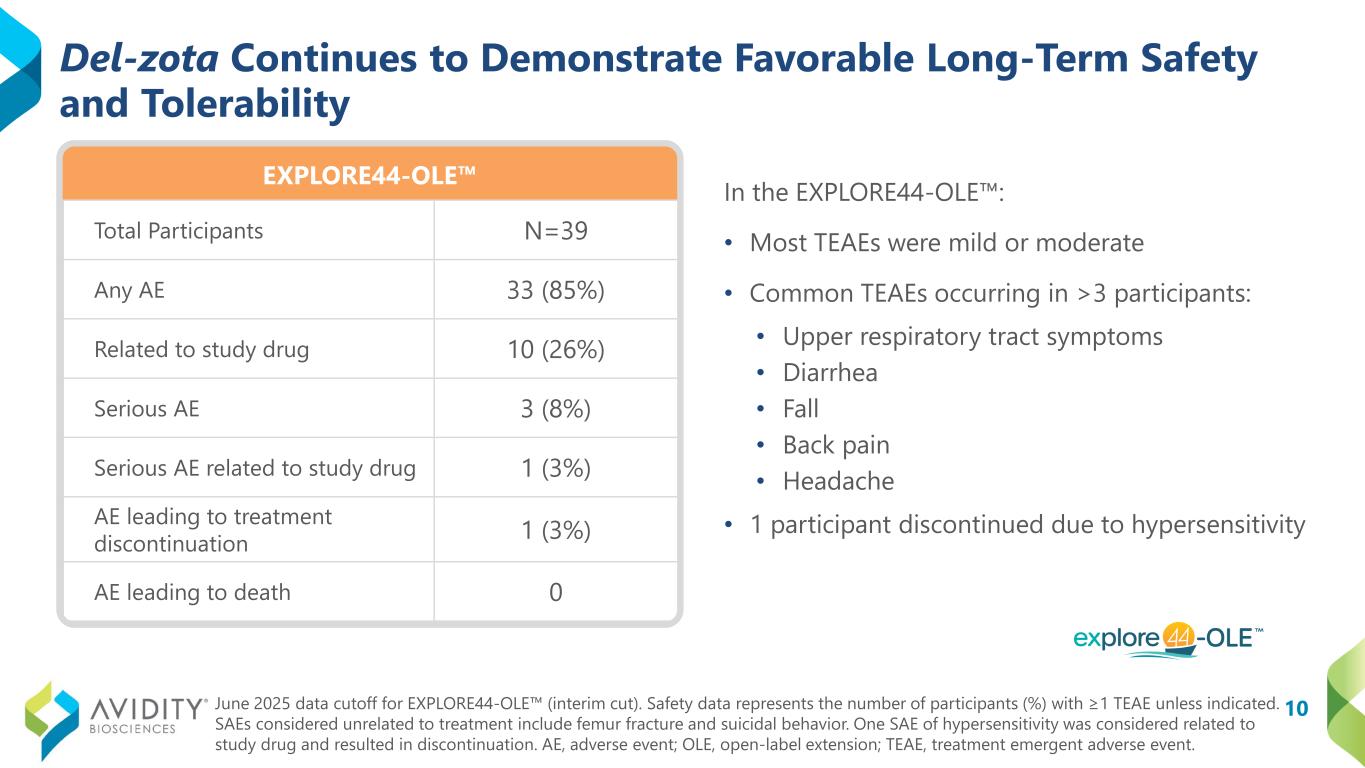
10 EXPLORE44-OLE Del-zota Continues to Demonstrate Favorable Long-Term Safety and Tolerability Total Participants N=39 Any AE 33 (85%) Related to study drug 10 (26%) Serious AE 3 (8%) Serious AE related to study drug 1 (3%) AE leading to treatment discontinuation 1 (3%) AE leading to death 0 In the EXPLORE44-OLE : • Most TEAEs were mild or moderate • Common TEAEs occurring in >3 participants: • Upper respiratory tract symptoms • Diarrhea • Fall • Back pain • Headache • 1 participant discontinued due to hypersensitivity June 2025 data cutoff for EXPLORE44-OLE (interim cut). Safety data represents the number of participants (%) with ≥1 TEAE unless indicated. SAEs considered unrelated to treatment include femur fracture and suicidal behavior. One SAE of hypersensitivity was considered related to study drug and resulted in discontinuation. AE, adverse event; OLE, open-label extension; TEAE, treatment emergent adverse event.
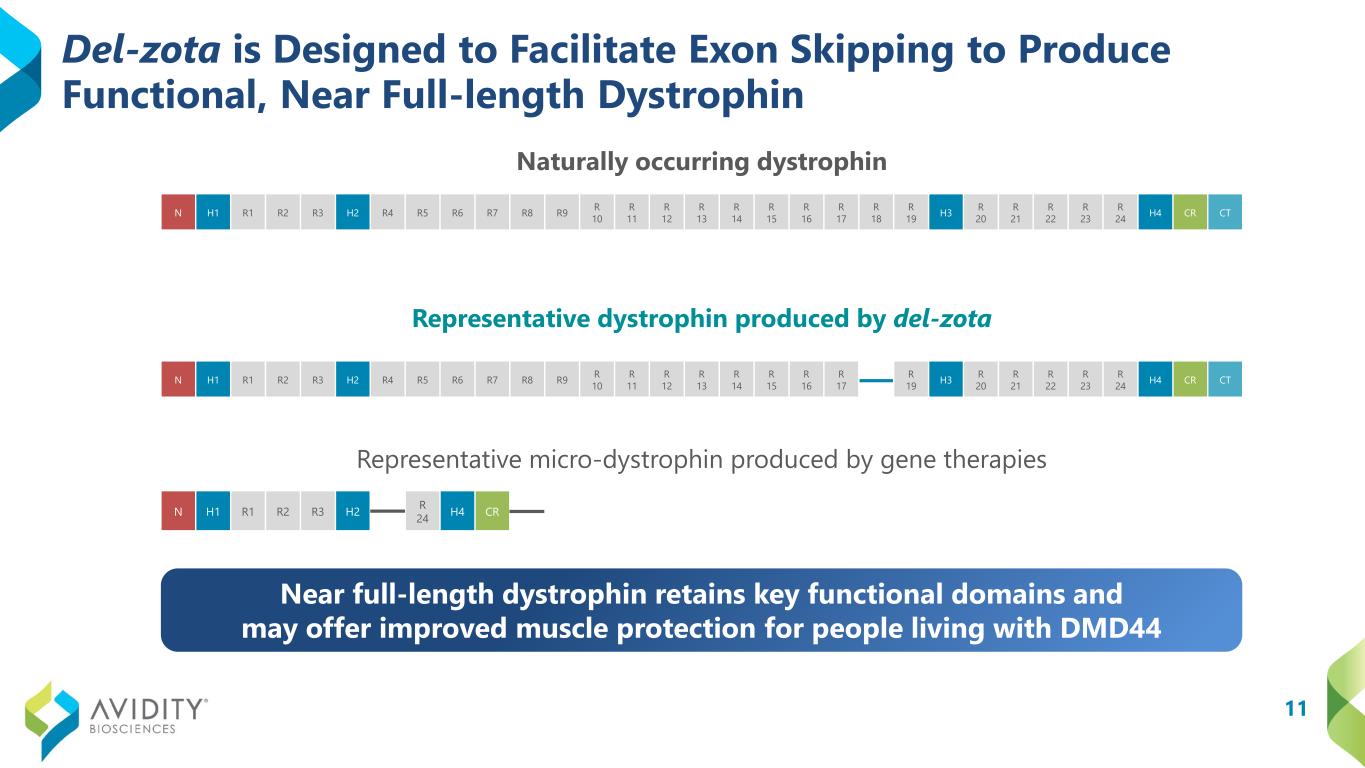
11 Del-zota is Designed to Facilitate Exon Skipping to Produce Functional, Near Full-length Dystrophin Naturally occurring dystrophin Representative dystrophin produced by del-zota Representative micro-dystrophin produced by gene therapies N H1 R1 R2 R3 H2 R 24 H4 CR N H1 R1 R2 R3 H2 R4 R5 R6 R7 R8 R9 R 10 R 11 R 12 R 13 R 14 R 15 R 16 R 17 R 18 R 19 H3 R 20 R 21 R 22 R 23 R 24 H4 CR CT N H1 R1 R2 R3 H2 R4 R5 R6 R7 R8 R9 R 10 R 11 R 12 R 13 R 14 R 15 R 16 R 17 R 19 H3 R 20 R 21 R 22 R 23 R 24 H4 CR CT Near full-length dystrophin retains key functional domains and may offer improved muscle protection for people living with DMD44
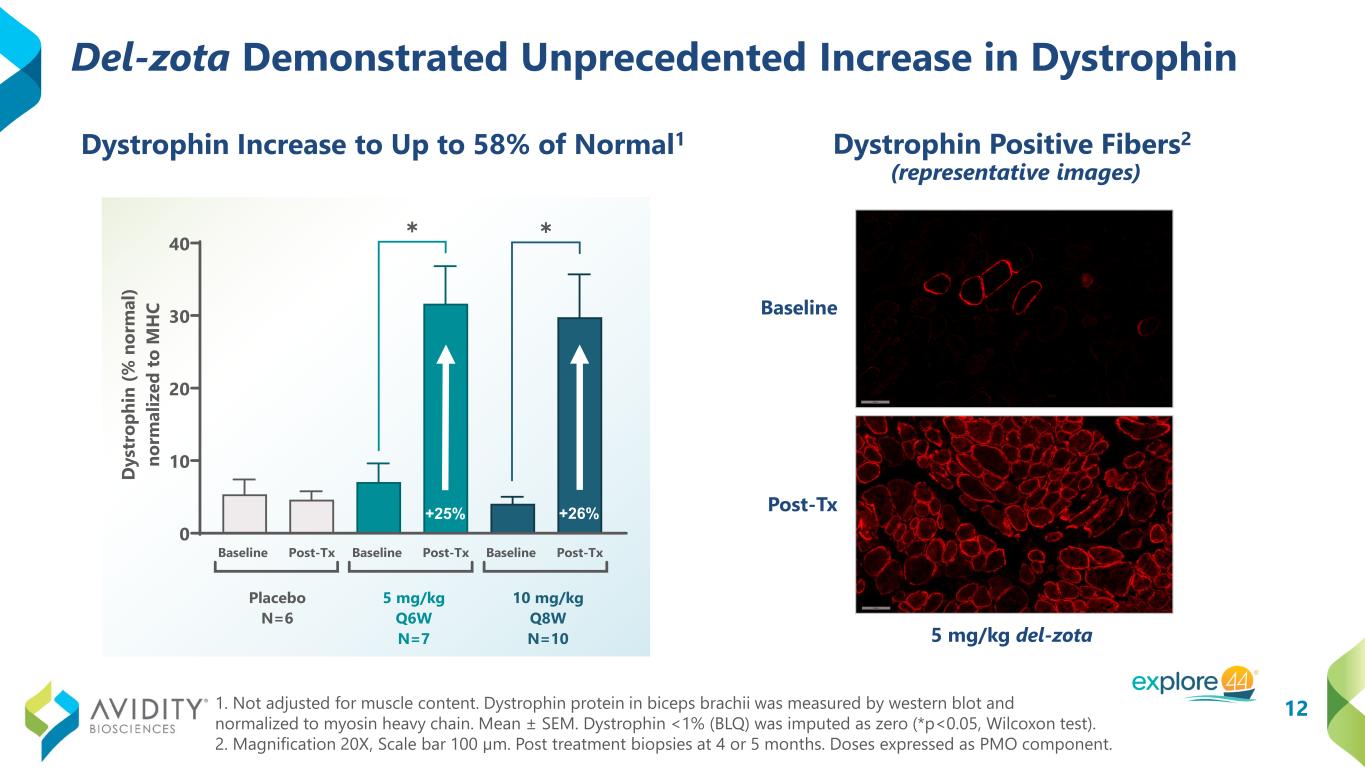
12 Del-zota Demonstrated Unprecedented Increase in Dystrophin 1. Not adjusted for muscle content. Dystrophin protein in biceps brachii was measured by western blot and normalized to myosin heavy chain. Mean ± SEM. Dystrophin <1% (BLQ) was imputed as zero (*p<0.05, Wilcoxon test). 2. Magnification 20X, Scale bar 100 µm. Post treatment biopsies at 4 or 5 months. Doses expressed as PMO component. Baseline Post-Tx Baseline Post-Tx Baseline Post-Tx 0 10 20 30 40 D ys tr op hi n (% n or m al ) no rm al iz ed to M H C ✱ Placebo N=6 5 mg/kg Q6W N=7 10 mg/kg Q8W N=10 ✱ +25% +26% Baseline Post-Tx 5 mg/kg del-zota Dystrophin Increase to Up to 58% of Normal1 Dystrophin Positive Fibers2 (representative images)
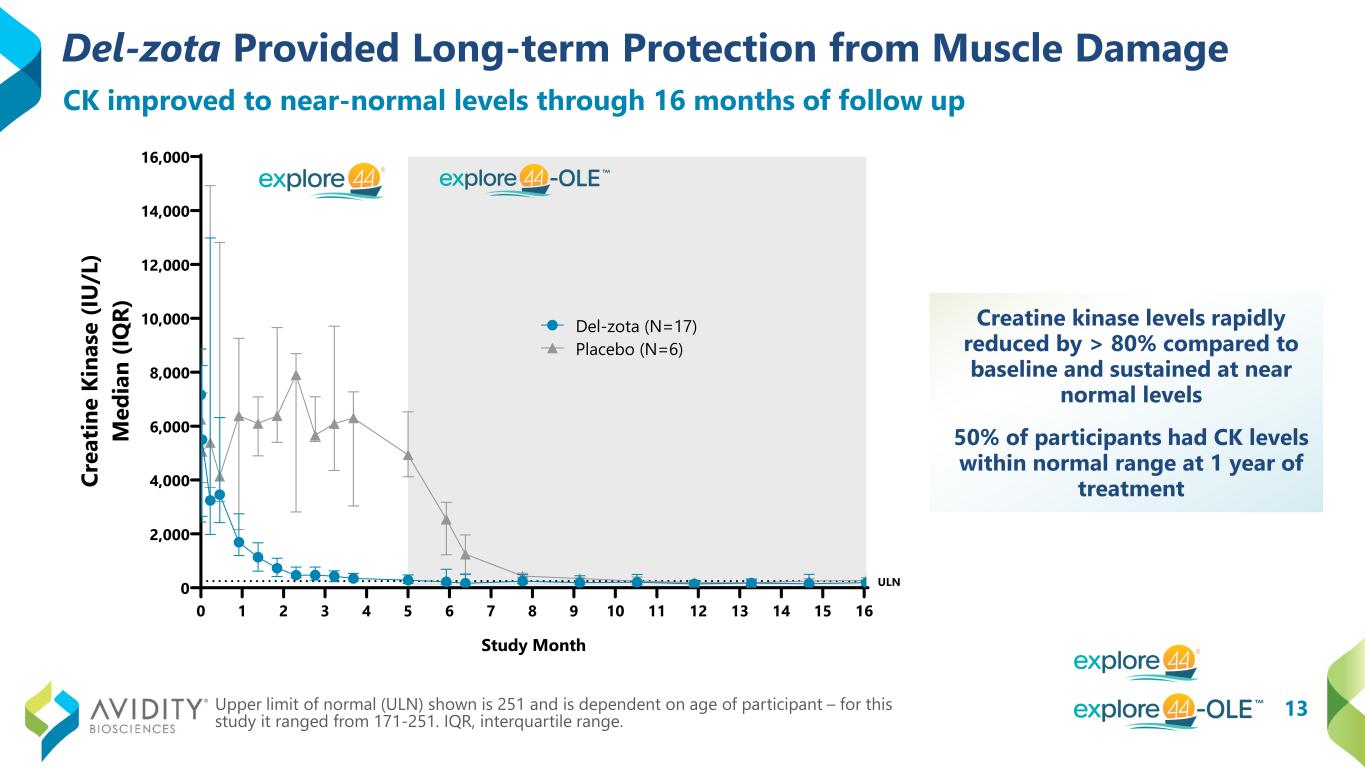
13 Del-zota Provided Long-term Protection from Muscle Damage Creatine kinase levels rapidly reduced by > 80% compared to baseline and sustained at near normal levels 50% of participants had CK levels within normal range at 1 year of treatment Upper limit of normal (ULN) shown is 251 and is dependent on age of participant – for this study it ranged from 171-251. IQR, interquartile range. CK improved to near-normal levels through 16 months of follow up 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 0 2,000 4,000 6,000 8,000 10,000 12,000 14,000 16,000 Study Month Cr ea tin e Ki na se (I U /L ) M ed ia n (IQ R) Del-zota (N=17) Placebo (N=6) ULN
14 1. Natural History (Nat Hx) data from PRO-DMD-01 database (CureDuchenne), analyzed by Analysis Group®. Data shown are means. 2. 12 ambulatory participants eligible through ~1 year. One participant excluded due to ankle sprain prior to 1-year assessment and one participant unable to complete assessment due to fractured femur. 3. PRO-DMD-01 disclosure: This publication is based on research using data from data contributor CureDuchenne that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication. Baseline Characteristics: del-zota vs DMD44 Natural History in Ambulatory Participants1 10.6Age (years) 11.0 23.6NSAA (total score) 19.6 7.2 Time to Rise from Floor (seconds) 10.2 5.710m Walk/Run Test (seconds) 8.1 4.34-stair Climb (seconds) 7.5 DMD44 Nat Hx N=22 • Design: Observational, prospective natural history study (total N=269) • Population: utilized subset of matched DMD44 participants (N=22) • Analysis inclusion criteria – matched to EXPLORE44® baseline characteristics: ambulatory, Exon 44 skip amenable, 7-27 years old, if on steroid stable ≥1 month, weight ≥23 kg • 100% on steroids • Key assessments: Clinical outcome assessments (i.e., timed tests, NSAA) progression over time Data set reflects current standard of care for steroid use PRO-DMD-01 Natural History Study3Del-zota2 N=10
15 4-Stair Climb: Improvement in del-zota Treated Participants Compared to Natural History at ~1 Year 4-Stair Climb DMD44 Nat Hx N=22 Del-zota N=10 Change in Seconds All del-zota data shown in this presentation is as of the June 2025 data cutoff date. All data shown are mean change (±SEM) from Baseline to ~1 year. 5 mg/kg and 10 mg/kg treatment arms pooled. Natural history comparison is to PRO-DMD-01 database, analyzed by Analysis Group®. Nat Hx, natural history.
16 10m Walk/Run Test: Improvement in del-zota Treated Participants Compared to Natural History at ~1 Year 10MWRT DMD44 Nat Hx N=22 Del-zota N=10 Change in Seconds All del-zota data shown in this presentation is as of the June 2025 data cutoff date. All data shown are mean change (±SEM) from Baseline to ~1 year. 5 mg/kg and 10 mg/kg treatment arms pooled. 10MWRT = 10-meter walk-run test. Natural history comparison is to PRO- DMD-01 database, analyzed by Analysis Group®. Nat Hx, natural history.
17 Time to Rise from Floor: Improvement in del-zota Treated Participants Compared to Natural History at ~1 Year TTR DMD44 Nat Hx N=19 Del-zota N=6 Change in Seconds All del-zota data shown in this presentation is as of the June 2025 data cutoff date. All data shown are mean change (±SEM) from Baseline to ~1 year. 5 mg/kg and 10 mg/kg treatment arms pooled. TTR: Time to Rise from supine. Natural history comparison is to PRO-DMD-01 database, analyzed by Analysis Group®. Nat Hx, natural history.
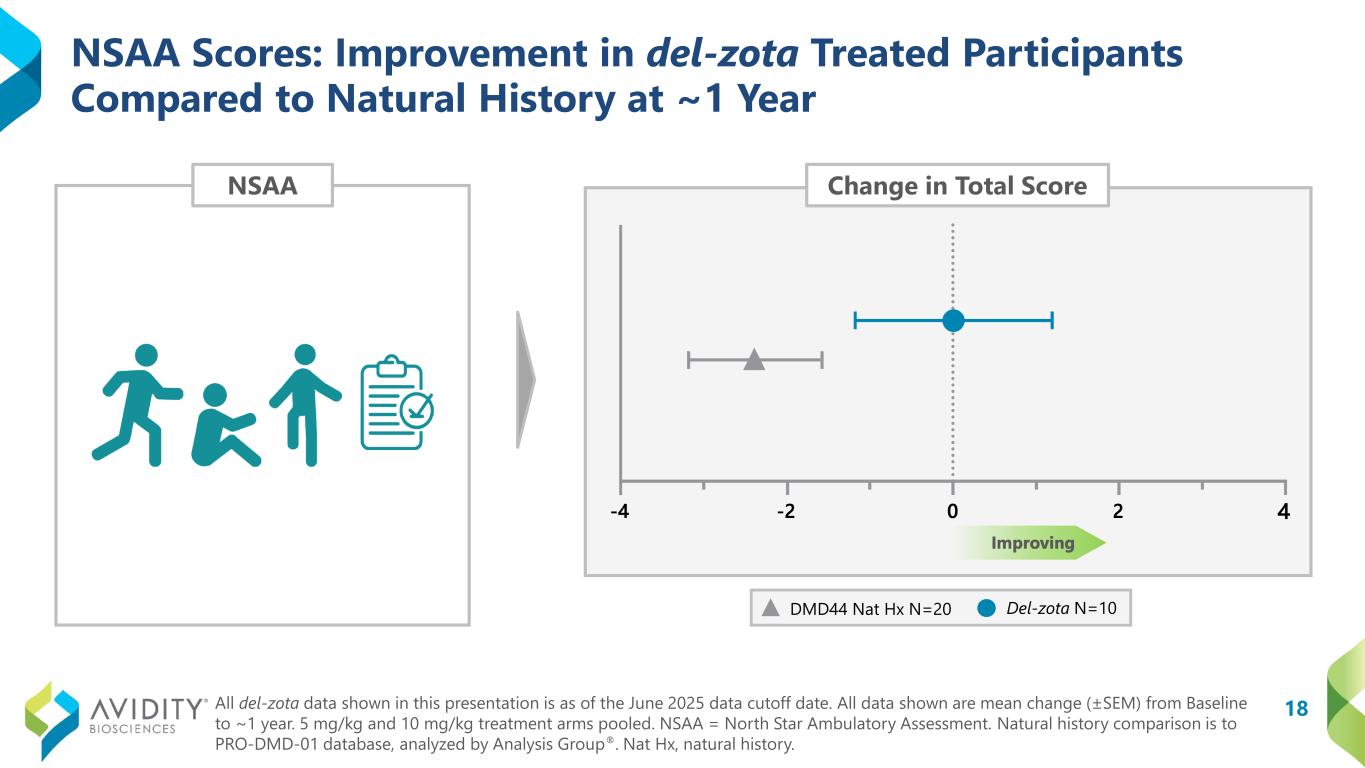
18 NSAA Scores: Improvement in del-zota Treated Participants Compared to Natural History at ~1 Year NSAA DMD44 Nat Hx N=20 Del-zota N=10 Change in Total Score All del-zota data shown in this presentation is as of the June 2025 data cutoff date. All data shown are mean change (±SEM) from Baseline to ~1 year. 5 mg/kg and 10 mg/kg treatment arms pooled. NSAA = North Star Ambulatory Assessment. Natural history comparison is to PRO-DMD-01 database, analyzed by Analysis Group®. Nat Hx, natural history.

19 Performance of Upper Limb (PUL): Improvement in del-zota Treated Participants Compared to Natural History at ~1 Year All del-zota data shown in this presentation is as of the June 2025 data cutoff date. All data shown are mean change (±SEM) from Baseline to ~1 year. 5 mg/kg and 10 mg/kg treatment arms pooled. PUL = Performance of Upper Limb 2.0. 1. Natural history comparison is to Brogna et al, Children (Basel), 2023. N=27 participants at 1 year. Nat Hx, natural history. PUL DMD44 Nat Hx N=271 Del-zota N=17 Change in Total Score

20 Del-zota Has Shown Improvement Across Key Functional Endpoints at ~1 Year of Treatment • Statistically-significant dystrophin increase delivering sustained improvements in CK and muscle protection • Consistent, clinically meaningful improvements across functional endpoints at ~1 year • Favorable long-term safety and tolerability profile in EXPLORE44® and EXPLORE44-OLE Improving Del-zota data as of the June 2025 data cutoff date and are mean change (±SEM) from Baseline to ~1 year. 5 and 10 mg/kg arms pooled. For 4SC, 10MWRT and TTR, N=19-22 for DMD44 Nat Hx (PRO-DMD-01 database, Analysis Group®) and 6-10 for del-zota. PUL: N=27 for Nat Hx, 17 for del-zota; Nat Hx (Brogna et al, 2023). Nat Hx, natural history. DMD44 Nat Hx Del-zota 10MWRT (sec) 4 Stair Climb (sec) Time to Rise (sec) PUL (total score)

21 Agenda/Outline Revolutionizing the Delivery of RNA Sarah Boyce, President & CEO Del-zota: Redefining the Treatment of DMD Steve Hughes, M.D., CMO Closing Remarks Sarah Boyce, President & CEO Q&A Session Avidity Management Moderator: Kat Lange, CBO

22 Revolutionizing RNA Delivery Key Participant Characteristics (N = 39) Ages 7 – 27, mean age 13 Ambulatory (67%) and non- ambulatory (33%) 90% on steroids REDEFINING DMD UNMATCHED COMMITMENT ACCELERATED PATHWAY • Unprecedented functional improvements and reversal of disease progression vs natural history • Compelling biomarker data of muscle health • Favorable long-term safety and tolerability • Commercial readiness to ensure access • Deep commitment to patient community • Finalizing Phase 3 confirmatory study design • On track to submit BLA by year end • Functional data further reinforce potential accelerated approval • Preparing global development pathway

23Redefining the Treatment of DMD Sarah Boyce President & CEO Steve Hughes, MD Chief Medical Officer W. Michael Flanagan, PhD Chief Scientific Officer Q&A Kat Lange Chief Business Officer

24 OUR VISION To profoundly improve people’s lives by revolutionizing the delivery of RNA therapeutics Nathan, living with DMD, and his mother, Misty

























