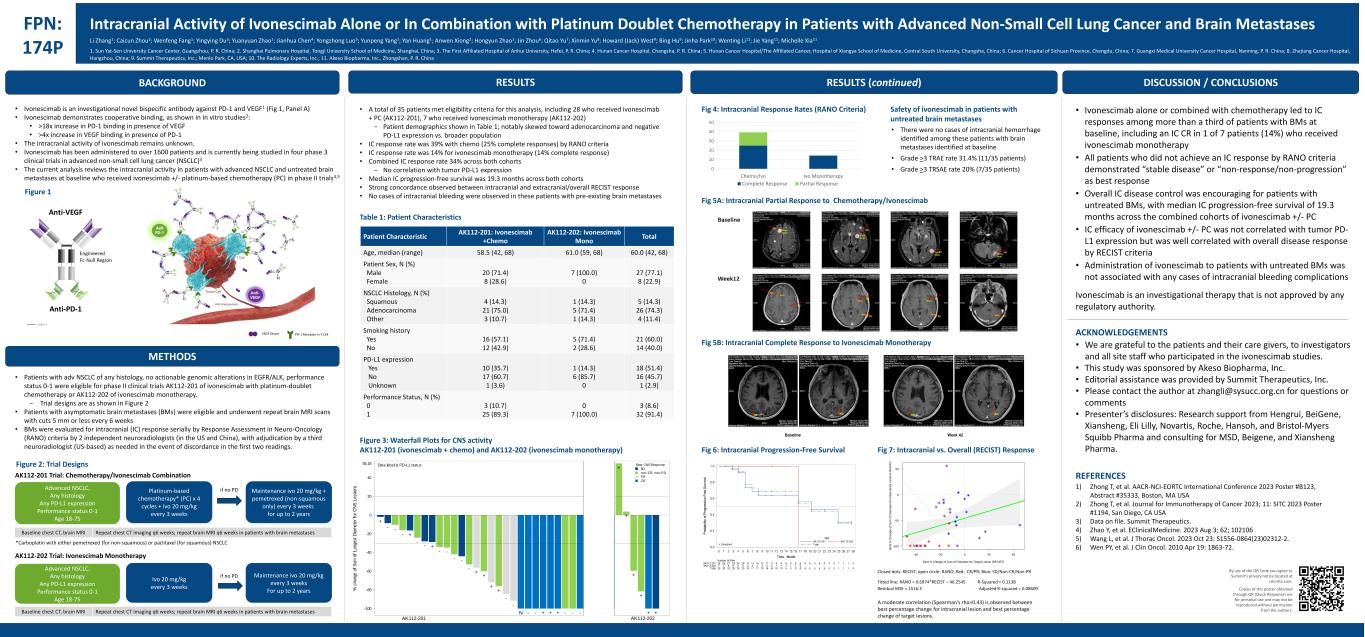
BACKGROUND FPN: 174P Intracranial Activity of Ivonescimab Alone or In Combination with Platinum Doublet Chemotherapy in Patients with Advanced Non-Small Cell Lung Cancer and Brain Metastases • Ivonescimab is an investigational novel bispecific antibody against PD-1 and VEGF1 (Fig 1, Panel A) • Ivonescimab demonstrates cooperative binding, as shown in in vitro studies2: • >18x increase in PD-1 binding in presence of VEGF • >4x increase in VEGF binding in presence of PD-1 • The intracranial activity of ivonescimab remains unknown. • Ivonescimab has been administered to over 1600 patients and is currently being studied in four phase 3 clinical trials in advanced non-small cell lung cancer (NSCLC)3 • The current analysis reviews the intracranial activity in patients with advanced NSCLC and untreated brain metastases at baseline who received ivonescimab +/- platinum-based chemotherapy (PC) in phase II trials4,5 METHODS • Patients with adv NSCLC of any histology, no actionable genomic alterations in EGFR/ALK, performance status 0-1 were eligible for phase II clinical trials AK112-201 of ivonescimab with platinum-doublet chemotherapy or AK112-202 of ivonescimab monotherapy. − Trial designs are as shown in Figure 2 • Patients with asymptomatic brain metastases (BMs) were eligible and underwent repeat brain MRI scans with cuts 5 mm or less every 6 weeks • BMs were evaluated for intracranial (IC) response serially by Response Assessment in Neuro-Oncology (RANO) criteria by 2 independent neuroradiologists (in the US and China), with adjudication by a third neuroradiologist (US-based) as needed in the event of discordance in the first two readings. • Ivonescimab alone or combined with chemotherapy led to IC responses among more than a third of patients with BMs at baseline, including an IC CR in 1 of 7 patients (14%) who received ivonescimab monotherapy • All patients who did not achieve an IC response by RANO criteria demonstrated “stable disease” or “non-response/non-progression” as best response • Overall IC disease control was encouraging for patients with untreated BMs, with median IC progression-free survival of 19.3 months across the combined cohorts of ivonescimab +/- PC • IC efficacy of ivonescimab +/- PC was not correlated with tumor PD- L1 expression but was well correlated with overall disease response by RECIST criteria • Administration of ivonescimab to patients with untreated BMs was not associated with any cases of intracranial bleeding complications Ivonescimab is an investigational therapy that is not approved by any regulatory authority. ACKNOWLEDGEMENTS • We are grateful to the patients and their care givers, to investigators and all site staff who participated in the ivonescimab studies. • This study was sponsored by Akeso Biopharma, Inc. • Editorial assistance was provided by Summit Therapeutics, Inc. • Please contact the author at zhangli@sysucc.org.cn for questions or comments • Presenter’s disclosures: Research support from Hengrui, BeiGene, Xiansheng, Eli Lilly, Novartis, Roche, Hansoh, and Bristol-Myers Squibb Pharma and consulting for MSD, Beigene, and Xiansheng Pharma. REFERENCES 1) Zhong T, et al. AACR-NCI-EORTC International Conference 2023 Poster #B123, Abstract #35333, Boston, MA USA 2) Zhong T, et al. Journal for Immunotherapy of Cancer 2023; 11: SITC 2023 Poster #1194, San Diego, CA USA 3) Data on file. Summit Therapeutics. 4) Zhao Y, et al. EClinicalMedicine. 2023 Aug 3; 62; 102106 5) Wang L, et al. J Thorac Oncol. 2023 Oct 23: S1556-0864(23)02312-2. 6) Wen PY, et al. J Clin Oncol. 2010 Apr 19: 1863-72. By use of the QR Code you agree to Summit’s privacy notice located at smmttx.com. Copies of this poster obtained through QR (Quick Response) are for personal use and may not be reproduced without permission from the authors. Figure 2: Trial Designs Platinum-based chemotherapy* (PC) x 4 cycles + ivo 20 mg/kg every 3 weeks Maintenance ivo 20 mg/kg + pemetrexed (non-squamous only) every 3 weeks for up to 2 years Advanced NSCLC, Any histology Any PD-L1 expression Performance status 0-1 Age 18-75 if no PD AK112-201 Trial: Chemotherapy/Ivonescimab Combination Repeat chest CT imaging q6 weeks; repeat brain MRI q6 weeks in patients with brain metastasesBaseline chest CT, brain MRI *Carboplatin with either pemetrexed (for non-squamous) or paclitaxel (for squamous) NSCLC Ivo 20 mg/kg every 3 weeks Maintenance ivo 20 mg/kg every 3 weeks For up to 2 years Advanced NSCLC, Any histology Any PD-L1 expression Performance status 0-1 Age 18-75 if no PD AK112-202 Trial: Ivonescimab Monotherapy DISCUSSION / CONCLUSIONS Table 1: Patient Characteristics • A total of 35 patients met eligibility criteria for this analysis, including 28 who received ivonescimab + PC (AK112-201), 7 who received ivonescimab monotherapy (AK112-202) − Patient demographics shown in Table 1; notably skewed toward adenocarcinoma and negative PD-L1 expression vs. broader population • IC response rate was 39% with chemo (25% complete responses) by RANO criteria • IC response rate was 14% for ivonescimab monotherapy (14% complete response) • Combined IC response rate 34% across both cohorts − No correlation with tumor PD-L1 expression • Median IC progression-free survival was 19.3 months across both cohorts • Strong concordance observed between intracranial and extracranial/overall RECIST response • No cases of intracranial bleeding were observed in these patients with pre-existing brain metastases Patient Characteristic AK112-201: Ivonescimab +Chemo AK112-202: Ivonescimab Mono Total Age, median (range) 58.5 (42, 68) 61.0 (59, 68) 60.0 (42, 68) Patient Sex, N (%) Male Female 20 (71.4) 8 (28.6) 7 (100.0) 0 27 (77.1) 8 (22.9) NSCLC Histology, N (%) Squamous Adenocarcinoma Other 4 (14.3) 21 (75.0) 3 (10.7) 1 (14.3) 5 (71.4) 1 (14.3) 5 (14.3) 26 (74.3) 4 (11.4) Smoking history Yes No 16 (57.1) 12 (42.9) 5 (71.4) 2 (28.6) 21 (60.0) 14 (40.0) PD-L1 expression Yes No Unknown 10 (35.7) 17 (60.7) 1 (3.6) 1 (14.3) 6 (85.7) 0 18 (51.4) 16 (45.7) 1 (2.9) Performance Status, N (%) 0 1 3 (10.7) 25 (89.3) 0 7 (100.0) 3 (8.6) 32 (91.4) Figure 3: Waterfall Plots for CNS activity AK112-201 (ivonescimab + chemo) and AK112-202 (ivonescimab monotherapy) RESULTS Repeat chest CT imaging q6 weeks; repeat brain MRI q6 weeks in patients with brain metastasesBaseline chest CT, brain MRI RESULTS (continued) 0 10 20 30 40 50 Chemo/Ivo Ivo Monotherapy Complete Response Partial Response Safety of ivonescimab in patients with untreated brain metastases • There were no cases of intracranial hemorrhage identified among these patients with brain metastases identified at baseline • Grade >3 TRAE rate 31.4% (11/35 patients) • Grade >3 TRSAE rate 20% (7/35 patients) Fig 5A: Intracranial Partial Response to Chemotherapy/Ivonescimab Fig 5B: Intracranial Complete Response to Ivonescimab Monotherapy Fig 6: Intracranial Progression-Free Survival Fig 7: Intracranial vs. Overall (RECIST) Response VEGF Dimer PD-1 Receptor in T Cell Li Zhang1; Caicun Zhou2; Wenfeng Fang1; Yingying Du3; Yuanyuan Zhao1; Jianhua Chen4; Yongzhong Luo5; Yunpeng Yang1; Yan Huang1; Anwen Xiong2; Hongyun Zhao1; Jin Zhou6; Qitao Yu7; Xinmin Yu8; Howard (Jack) West9; Bing Hu9; Jinha Park10; Wenting Li11; Jie Yang11; Michelle Xia11 1. Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China; 2. Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China; 3. The First Affiliated Hospital of Anhui University, Hefei, P. R. China; 4. Hunan Cancer Hospital, Changsha, P. R. China; 5. Hunan Cancer Hospital/The Affiliated Cancer, Hospital of Xiangya School of Medicine, Central South University, Changsha, China; 6. Cancer Hospital of Sichuan Province, Chengdu, China; 7. Guangxi Medical University Cancer Hospital, Nanning, P. R. China; 8. Zhejiang Cancer Hospital, Hangzhou, China; 9. Summit Therapeutics, Inc.; Menlo Park, CA, USA; 10. The Radiology Experts, Inc.; 11. Akeso Biopharma, Inc., Zhongshan, P. R. China Figure 1 A moderate correlation (Spearman's rho=0.43) is observed between best percentage change for intracranial lesion and best percentage change of target lesions. + - - - + - - + - - - - - + + + + - - N - - + + + - - - + + + + + + -100 -80 -60 -40 -20 0 20 40 55.18 % c ha ng e of S um o f L on ge st D ia m et er f or C N S L es si on s CR PR non-CR, non-PD SD Best CNS ResponseData label is PD-L1 status AK112-201 AK112-202 + - - - + - - + - - - - - + + + + - - N - - + + + - - - + + + + + + -100 -80 -60 -40 -20 0 20 40 55.18 % c ha ng e of S um o f L on ge st D ia m et er f or C N S L es si on s CR PR non-CR, non-PD SD Best CNS ResponseData label is PD-L1 status AK112-201 AK112-202 Closed dots: RECIST; open circle: RANO; Red: CR/PR; Blue: SD/Non-CR/Non-PR Best % Change of Sum of Diameter for Target Lesion (RECIST) B e s t % C h a n g e o f S u m o f D ia m e te r fo r In tr a c ra n ia l L e s io n s ( R A N O ) -40 -20 0 20 40 -100 -50 0 50 Fig 4: Intracranial Response Rates (RANO Criteria) Fitted line: RANO = 0.6974*RECIST – 46.2545 R-Squared = 0.1138 Residual MSE = 1516.3 Adjusted R-squared = 0.08609
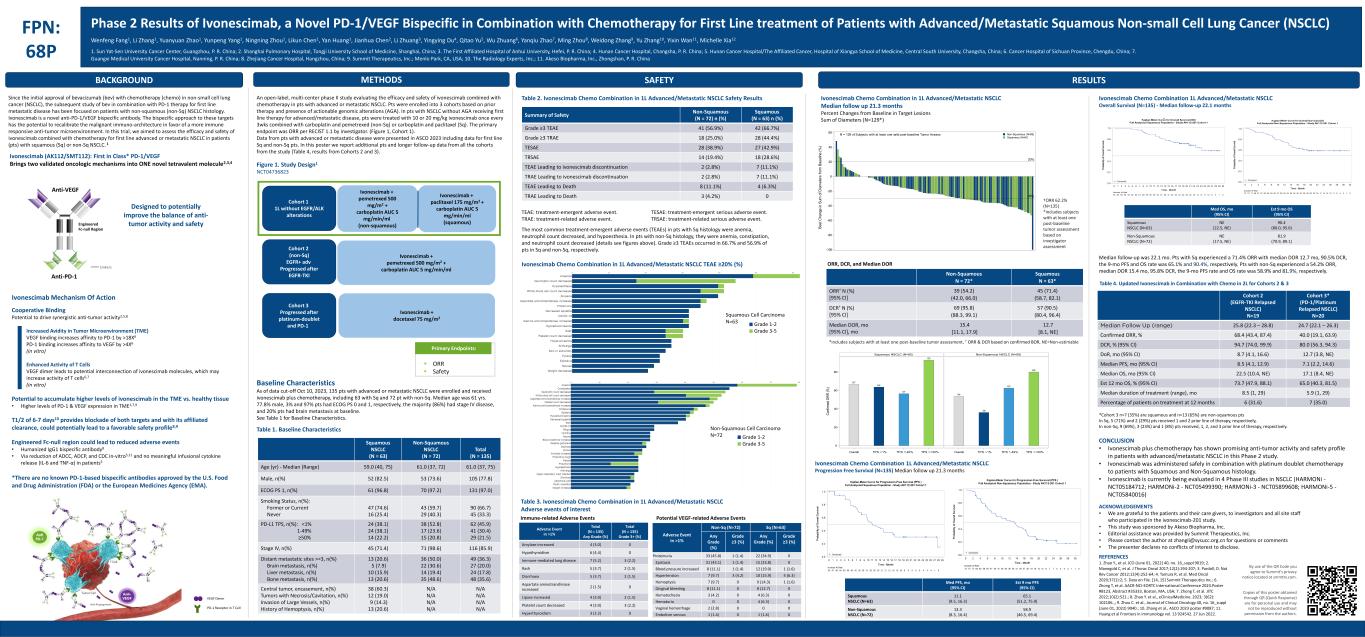
BACKGROUND FPN: 68P Phase 2 Results of Ivonescimab, a Novel PD-1/VEGF Bispecific in Combination with Chemotherapy for First Line treatment of Patients with Advanced/Metastatic Squamous Non-small Cell Lung Cancer (NSCLC) By use of the QR Code you agree to Summit’s privacy notice located at smmttx.com. Copies of this poster obtained through QR (Quick Response) are for personal use and may not be reproduced without permission from the authors. RESULTSMETHODS SAFETY Wenfeng Fang1, Li Zhang1, Yuanyuan Zhao1, Yunpeng Yang1, Ningning Zhou1, Likun Chen1, Yan Huang1, Jianhua Chen2, Li Zhuang3, Yingying Du4, Qitao Yu5, Wu Zhuang6, Yanqiu Zhao7, Ming Zhou8, Weidong Zhang9, Yu Zhang10, Yixin Wan11, Michelle Xia12 1. Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China; 2. Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China; 3. The First Affiliated Hospital of Anhui University, Hefei, P. R. China; 4. Hunan Cancer Hospital, Changsha, P. R. China; 5. Hunan Cancer Hospital/The Affiliated Cancer, Hospital of Xiangya School of Medicine, Central South University, Changsha, China; 6. Cancer Hospital of Sichuan Province, Chengdu, China; 7. Guangxi Medical University Cancer Hospital, Nanning, P. R. China; 8. Zhejiang Cancer Hospital, Hangzhou, China; 9. Summit Therapeutics, Inc.; Menlo Park, CA, USA; 10. The Radiology Experts, Inc.; 11. Akeso Biopharma, Inc., Zhongshan, P. R. China Table 1. Baseline Characteristics Table 2. Ivonescimab Chemo Combination in 1L Advanced/Metastatic NSCLC Safety Results Squamous NSCLC (N = 63) Non-Squamous NSCLC (N = 72) Total (N = 135) Age (yr) - Median (Range) 59.0 (40, 75) 61.0 (37, 72) 61.0 (37, 75) Male, n(%) 52 (82.5) 53 (73.6) 105 (77.8) ECOG PS 1, n(%) 61 (96.8) 70 (97.2) 131 (97.0) Smoking Status, n(%): Former or Current Never 47 (74.6) 16 (25.4) 43 (59.7) 29 (40.3) 90 (66.7) 45 (33.3) PD-L1 TPS, n(%): <1% 1-49% ≥50% 24 (38.1) 24 (38.1) 14 (22.2) 38 (52.8) 17 (23.6) 15 (20.8) 62 (45.9) 41 (30.4) 29 (21.5) Stage IV, n(%) 45 (71.4) 71 (98.6) 116 (85.9) Distant metastatic sites >=3, n(%) Brain metastasis, n(%) Liver metastasis, n(%) Bone metastasis, n(%) 13 (20.6) 5 (7.9) 10 (15.9) 13 (20.6) 36 (50.0) 22 (30.6) 14 (19.4) 35 (48.6) 49 (36.3) 27 (20.0) 24 (17.8) 48 (35.6) Central tumor, encasement, n(%) Tumors with Necrosis/Cavitation, n(%) Invasion of Large Vessels, n(%) History of Hemoptysis, n(%) 38 (60.3) 12 (19.0) 9 (14.3) 13 (20.6) N/A N/A N/A N/A N/A N/A N/A N/A Summary of Safety Non-Squamous (N = 72) n (%) Squamous (N = 63) n (%) Grade ≥3 TEAE 41 (56.9%) 42 (66.7%) Grade ≥3 TRAE 18 (25.0%) 28 (44.4%) TESAE 28 (38.9%) 27 (42.9%) TRSAE 14 (19.4%) 18 (28.6%) TEAE Leading to ivonescimab discontinuation 2 (2.8%) 7 (11.1%) TRAE Leading to ivonescimab discontinuation 2 (2.8%) 7 (11.1%) TEAE Leading to Death 8 (11.1%) 4 (6.3%) TRAE Leading to Death 3 (4.2%) 0 TEAE: treatment-emergent adverse event. TRAE: treatment-related adverse event. TESAE: treatment-emergent serious adverse event. TRSAE: treatment-related serious adverse event. Adverse Event in >1% Total (N = 135) Any Grade (%) Total (N = 135) Grade 3+ (%) Amylase increased 4 (3.0) 0 Hypothyroidism 6 (4.4) 0 Immune-mediated lung disease 7 (5.2) 3 (2.2) Rash 5 (3.7) 2 (1.5) Diarrhoea 5 (3.7) 2 (1.5) Aspartate aminotransferase increased 2 (1.5) 0 Lipase increased 4 (3.0) 2 (1.5) Platelet count decreased 4 (3.0) 3 (2.2) Hyperthyroidism 3 (2.2) 0 Adverse Event in >1% Non-Sq (N=72) Sq (N=63) Any Grade (%) Grade ≥3 (%) Any Grade (%) Grade ≥3 (%) Proteinuria 33 (45.8) 1 (1.4) 22 (34.9) 0 Epistaxis 31 (43.1) 1 (1.4) 15 (23.8) 0 Blood pressure increased 8 (11.1) 1 (1.4) 12 (19.0) 1 (1.6) Hypertension 7 (9.7) 3 (4.2) 10 (15.9) 4 (6.3) Hemoptysis 7 (9.7) 0 9 (14.3) 1 (1.6) Gingival bleeding 8 (11.1) 0 8 (12.7) 0 Hematochezia 3 (4.2) 0 4 (6.3) 0 Hematuria 0 0 4 (6.3) 0 Vaginal hemorrhage 2 (2.8) 0 0 0 Embolism venous 1 (1.4) 0 1 (1.6) 0 Immune-related Adverse Events Potential VEGF-related Adverse Events -30% 20% -100 -80 -60 -40 -20 0 20 40 60 B e s t C h a n g e in S u m o f D ia m e te rs f ro m B a s e lin e ( % ) Squamous (N=60) Non-Squamous (N=69)N = 129 of Subjects with at least one valid post-baseline Tumor Assessment Non-Squamous N = 72* Squamous N = 63* ORR† N (%) [95% CI] 39 (54.2) (42.0, 66.0) 45 (71.4) (58.7, 82.1) DCR† N (%) [95% CI] 69 (95.8) (88.3, 99.1) 57 (90.5) (80.4, 96.4) Median DOR, mo [95% CI], mo 15.4 [11.1, 17.9] 12.7 [8.1, NE] Since the initial approval of bevacizumab (bev) with chemotherapy (chemo) in non-small cell lung cancer (NSCLC), the subsequent study of bev in combination with PD-1 therapy for first line metastatic disease has been focused on patients with non-squamous (non-Sq) NSCLC histology. Ivonescimab is a novel anti-PD-1/VEGF bispecific antibody. The bispecific approach to these targets has the potential to recalibrate the malignant immuno-architecture in favor of a more immune responsive anti-tumor microenvironment. In this trial, we aimed to assess the efficacy and safety of ivonescimab combined with chemotherapy for first line advanced or metastatic NSCLC in patients (pts) with squamous (Sq) or non-Sq NSCLC. 1 Ivonescimab (AK112/SMT112): First in Class* PD-1/VEGF Brings two validated oncologic mechanisms into ONE novel tetravalent molecule2,3,4 Designed to potentially improve the balance of anti- tumor activity and safety Ivonescimab Mechanism Of Action Cooperative Binding Potential to drive synergistic anti-tumor activity2,5,8 Increased Avidity in Tumor Microenvironment (TME) VEGF binding increases affinity to PD-1 by >18X6 PD-1 binding increases affinity to VEGF by >4X6 (in vitro) Enhanced Activity of T Cells VEGF dimer leads to potential interconnection of ivonescimab molecules, which may increase activity of T cells6,7 (in vitro) Potential to accumulate higher levels of ivonescimab in the TME vs. healthy tissue • Higher levels of PD-1 & VEGF expression in TME1,7,9 T1/2 of 6-7 days10 provides blockade of both targets and with its affiliated clearance, could potentially lead to a favorable safety profile8,9 Engineered Fc-null region could lead to reduced adverse events • Humanized IgG1 bispecific antibody8 • Via reduction of ADCC, ADCP, and CDC in-vitro5,11 and no meaningful infusional cytokine release (IL-6 and TNF-α) in patients5 *There are no known PD-1-based bispecific antibodies approved by the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). An open-label, multi-center phase II study evaluating the efficacy and safety of ivonescimab combined with chemotherapy in pts with advanced or metastatic NSCLC. Pts were enrolled into 3 cohorts based on prior therapy and presence of actionable genomic alterations (AGA). In pts with NSCLC without AGA receiving first line therapy for advanced/metastatic disease, pts were treated with 10 or 20 mg/kg ivonescimab once every 3wks combined with carboplatin and pemetrexed (non-Sq) or carboplatin and paclitaxel (Sq). The primary endpoint was ORR per RECIST 1.1 by investigator. (Figure 1, Cohort 1). Data from pts with advanced or metastatic disease were presented in ASCO 2023 including data for first line Sq and non-Sq pts. In this poster we report additional pts and longer follow-up data from all the cohorts from the study (Table 4, results from Cohorts 2 and 3). Baseline Characteristics As of data cut-off Oct 10, 2023, 135 pts with advanced or metastatic NSCLC were enrolled and received ivonescimab plus chemotherapy, including 63 with Sq and 72 pt with non-Sq. Median age was 61 yrs. 77.8% male, 3% and 97% pts had ECOG PS 0 and 1, respectively, the majority (86%) had stage IV disease, and 20% pts had brain metastasis at baseline. See Table 1 for Baseline Characteristics. The most common treatment-emergent adverse events (TEAEs) in pts with Sq histology were anemia, neutrophil count decreased, and hypoesthesia. In pts with non-Sq histology, they were anemia, constipation, and neutrophil count decreased (details see figures above). Grade ≥3 TEAEs occurred in 66.7% and 56.9% of pts in Sq and non-Sq, respectively. Ivonescimab Chemo Combination in 1L Advanced/Metastatic NSCLC TEAE ≥20% (%) Table 3. Ivonescimab Chemo Combination in 1L Advanced/Metastatic NSCLC Adverse events of interest Ivonescimab Chemo Combination in 1L Advanced/Metastatic NSCLC Median follow up 21.3 months Percent Changes from Baseline in Target Lesions Sum of Diameters (N=129*) †ORR 62.2% (N=135) *includes subjects with at least one post-baseline tumor assessment based on investigator assessment ORR, DCR, and Median DOR *includes subjects with at least one post-baseline tumor assessment, † ORR & DCR based on confirmed BOR, NE=Non-estimable Ivonescimab Chemo Combination 1L Advanced/Metastatic NSCLC Progression Free Survival (N=135) Median follow up 21.3 months Ivonescimab Chemo Combination 1L Advanced/Metastatic NSCLC Overall Survival (N=135) - Median follow-up 22.1 months Median follow-up was 22.1 mo. Pts with Sq experienced a 71.4% ORR with median DOR 12.7 mo, 90.5% DCR, the 9-mo PFS and OS rate was 65.1% and 90.4%, respectively. Pts with non-Sq experienced a 54.2% ORR, median DOR 15.4 mo, 95.8% DCR, the 9-mo PFS rate and OS rate was 58.9% and 81.9%, respectively. Table 4. Updated Ivonescimab in Combination with Chemo in 2L for Cohorts 2 & 3 Med PFS, mo (95% CI) Est 9 mo PFS (95% CI) Squamous NSCLC (N=63) 11.1 (9.5, 16.3) 65.1 (51.2, 75.9) Non-Squamous NSCLC (N=72) 13.3 (8.3, 16.4) 58.9 (46.5, 69.4) *Cohort 3 n=7 (35%) are squamous and n=13 (65%) are non-squamous pts In Sq, 5 (71%) and 2 (29%) pts received 1 and 2 prior line of therapy, respectively, In non-Sq, 9 (69%), 3 (23%) and 1 (8%) pts received, 1, 2, and 3 prior line of therapy, respectively. Cohort 2 (EGFR-TKI Relapsed NSCLC) N=19 Cohort 3* (PD-1/Platinum Relapsed NSCLC) N=20 Median Follow Up (range) 25.8 (22.3 – 28.8) 24.7 (22.1 – 26.3) Confirmed ORR, % 68.4 (43.4, 87.4) 40.0 (19.1, 63.9) DCR, % (95% CI) 94.7 (74.0, 99.9) 80.0 (56.3, 94.3) DoR, mo (95% CI) 8.7 (4.1, 16.6) 12.7 (3.8, NE) Median PFS, mo (95% CI) 8.5 (4.1, 12.9) 7.1 (2.2, 14.6) Median OS, mo (95% CI) 22.5 (10.4, NE) 17.1 (8.4, NE) Est 12 mo OS, % (95% CI) 73.7 (47.9, 88.1) 65.0 (40.3, 81.5) Median duration of treatment (range), mo 8.5 (1, 29) 5.9 (1, 29) Percentage of patients on treatment at 12 months 6 (31.6) 7 (35.0) Med OS, mo (95% CI) Est 9 mo OS (95% CI) Squamous NSCLC (N=63) NE (22.5, NE) 90.4 (80.0, 95.6) Non-Squamous NSCLC (N=72) NE (17.5, NE) 81.9 (70.9, 89.1) CONCLUSION • Ivonescimab plus chemotherapy has shown promising anti-tumor activity and safety profile in patients with advanced/metastatic NSCLC in this Phase 2 study. • Ivonescimab was administered safely in combination with platinum doublet chemotherapy to patients with Squamous and Non-Squamous histology. • Ivonescimab is currently being evaluated in 4 Phase III studies in NSCLC (HARMONi - NCT05184712; HARMONi-2 - NCT05499390; HARMONi-3 - NCT05899608; HARMONi-5 - NCT05840016) VEGF Dimer PD-1 Receptor in T Cell ACKNOWLEDGEMENTS • We are grateful to the patients and their care givers, to investigators and all site staff who participated in the ivonescimab-201 study. • This study was sponsored by Akeso Biopharma, Inc. • Editorial assistance was provided by Summit Therapeutics, Inc. • Please contact the author at zhangli@sysucc.org.cn for questions or comments • The presenter declares no conflicts of interest to disclose. REFERENCES 1. Zhao Y., et al. JCO (June 01, 2022) 40, no. 16_suppl 9019; 2. Manegold C, et al. J Thorac Oncol 2017;12(2):194-207; 3. Pardoll, D. Nat Rev Cancer 2012;12(4):252-64; 4. Tamura R, et al. Med Oncol 2020;37(1):2; 5. Data on File. [14, 15] Summit Therapeutics Inc.; 6. Zhong T, et al. AACR-NCI-EORTC International Conference 2023.Poster #B123, Abstract #35333, Boston, MA, USA; 7. Zhong T, et al. JITC 2022;10(2):521.; 8. Zhao Y. et al., eClinicalMedicine. 2023; 3(62): 102106.,; 9. Zhou C. et al., Journal of Clinical Oncology 40, no. 16_suppl (June 01, 2022) 9040.; 10. Zhang et al., ASCO 2023 poster #9087; 11. Huang et al Frontiers in immunology vol. 13 924542. 27 Jun 2022. Anti-VEGF Engineered Fc-null Region Anti-PD-1 Linkers Ivonescimab + paclitaxel 175 mg/m2 + carboplatin AUC 5 mg/min/ml (squamous) Ivonescimab + pemetrexed 500 mg/m2 + carboplatin AUC 5 mg/min/ml (non-squamous) Cohort 1 1L without EGFR/ALK alterations Cohort 2 (non-Sq) EGFR+ adv Progressed after EGFR-TKI Cohort 3 Progressed after platinum-doublet and PD-1 Ivonescimab + pemetrexed 500 mg/m2 + carboplatin AUC 5 mg/min/ml Ivonescimab + docetaxel 75 mg/m2 ▪ ORR ▪ Safety Primary Endpoints: Figure 1. Study Design1 NCT04736823 Grade 1-2 Grade 3-5 Squamous Cell Carcinoma N=63 Non-Squamous Cell Carcinoma N=72 Grade 1-2 Grade 3-5 54 36 63 80 67 64 57 93 Overall TPS <1% TPS 1-49% TPS >=50%Overall TPS <1% TPS 1-49% TPS >=50% 0 20 40 60 80 C on fir m ed O R R ( % ) Non-Squamous NSCLC (N=69)Squamous NSCLC (N=60)







