UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2024
☐ |
TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ______________to________________
Commission File No. 001-37704
DARIOHEALTH CORP.
(Exact name of registrant as specified in its charter)
Delaware |
|
45-2973162 |
(State or other jurisdiction of |
|
(I.R.S. Employer |
|
|
|
322 W. 57th St. |
|
10019 |
(Address of principal executive offices) |
|
(Zip Code) |
(972)-4 770-6377
(Registrant’s telephone number, including area code)
Securities Registered pursuant to Section 12(b) of the Act
|
|
|||
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered: |
Common Stock, par value $0.0001 per share |
|
DRIO |
|
The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(b) of the Exchange Act: None
Securities registered pursuant to Section 12(g) of the Act: Common Stock, par value $0.0001 per share; Warrants to purchase Common Stock
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
|
|
|
Non-accelerated filer |
☑ |
Smaller reporting company |
☑ |
|
|
|
|
Emerging growth company |
☐ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
The aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the closing price as of the last business day of the registrant’s most recently completed second fiscal quarter is $31,031,352.
As of March 3, 2025, the registrant had outstanding 41,567,016 shares of common stock, $0.0001 par value per share.
Documents Incorporated By Reference: None.
TABLE OF CONTENTS
Item No. |
|
Description |
|
Page |
|
||||
3 |
||||
|
||||
|
||||
|
||||
5 |
||||
60 |
||||
85 |
||||
|
|
85 |
||
86 |
||||
86 |
||||
86 |
||||
|
||||
|
||||
|
||||
87 |
||||
90 |
||||
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
90 |
|||
102 |
||||
102 |
||||
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
102 |
|||
102 |
||||
103 |
||||
|
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
|
103 |
|
|
||||
|
||||
|
||||
104 |
||||
110 |
||||
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
117 |
|||
Certain Relationships and Related Transactions, and Director Independence |
118 |
|||
120 |
||||
|
||||
|
||||
|
|
|||
121 |
||||
125 |
||||
126 |
||||
2
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS AND SUMMARY RISK FACTORS
This Annual Report on Form 10-K, or the Annual Report, contains “forward-looking statements,” which includes information relating to future events, future financial performance, financial projections, strategies, expectations, competitive environment and regulation. Words such as “may,” “should,” “could,” “would,” “predicts,” “potential,” “continue,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” and similar expressions, as well as statements in future tense, identify forward-looking statements. Forward-looking statements should not be read as a guarantee of future performance or results and may not be accurate indications of when such performance or results will be achieved. Forward-looking statements are based on information we have when those statements are made or management’s good faith belief as of that time with respect to future events, and are subject to significant risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that could cause such differences include, but are not limited to:
| ● | our current and future capital requirements and our ability to satisfy our capital needs through financing transactions or otherwise; |
| ● | our ability to meet the requirements of our existing debt facility; |
| ● | our product launches and market penetration plans; |
| ● | the execution of agreements with various providers for our solution; |
| ● | our ability to maintain our relationships with key partners; |
| ● | our ability to complete required clinical trials of our product and obtain clearance or approval from the United States Food and Drug Administration (the “FDA”), or other regulatory agencies in different jurisdictions; |
| ● | our ability to maintain or protect the validity of our U.S. and other patents and other intellectual property; |
| ● | our ability to retain key executive members; |
| ● | our ability to internally develop new inventions and intellectual property; |
| ● | general market, political and economic conditions in the countries in which we operate, including those related to recent unrest and actual or potential armed conflict in Israel and other parts of the Middle East, such as the recent attack by Hamas and other terrorist organizations from the Gaza Strip and Israel’s war against them; |
| ● | interpretations of current laws and the passages of future laws; and |
| ● | acceptance of our business model by investors. |
The foregoing does not represent an exhaustive list of matters that may be covered by the forward-looking statements contained herein or risk factors that we are faced with that may cause our actual results to differ from those anticipated in our forward-looking statements. Please see “Risk Factors” for additional risks that could adversely impact our business and financial performance.
Moreover, new risks regularly emerge and it is not possible for our management to predict or articulate all the risks we face, nor can we assess the impact of all risks on our business or the extent to which any risk, or combination of risks, may cause actual results to differ from those contained in any forward-looking statements. All forward-looking statements included in this Annual Report are based on information available to us on the date of this Annual Report. Except to the extent required by applicable laws or rules, we undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements contained above and throughout this Annual Report.
3
When used in this Annual Report, the terms “Dario,” “DarioHealth,” “the Company,” “we,” “our,” and “us” refer to DarioHealth Corp., a Delaware corporation and our subsidiary LabStyle Innovation Ltd., an Israeli company, PsyInnovations Inc., a Delaware company, Twill, Inc., a Delaware company, Twill ISR Ltd, an Israeli company, and DarioHealth India Services Pvt. Ltd., an Indian company. “Dario” is registered as a trademark in the United States, Israel, China, Canada, Hong Kong, South Africa, Japan, Costa Rica, and Panama. “DarioHealth” is registered as a trademark in the United States and Israel.
Summary of Risk Factors
Our business is subject to a number of risks, including risks that may adversely affect our business, financial condition and results of operations. These risks are discussed more fully below and include, but are not limited to, risks related to:
Risks Related to Our Financial Position and Capital Requirements
| ● | Risks associated with our relatively new business; |
| ● | our future capital needs and their potential impact on our existing stockholders; |
| ● | our history of losses and stockholder’s inability to rely upon our historical operating performance; |
| ● | our revenues are concentrated with a major customer, and our revenues may decrease significantly if we were to lose our major customer; |
Risks Related to Our Business
| ● | the acceptance of our products in the market and our exposure to market trends; |
| ● | our risks of basing our business on the sale of our principal technology; |
| ● | our reliance on manufacturers and distributors; |
| ● | the impact of a failure of our digital marketing efforts; |
| ● | our reliance on the Apple App Store and Google’s Android platform; |
| ● | the risks associated with conducting business internationally; |
| ● | potential errors in our business processes and product offerings; |
| ● | our reliance on the performance of key members of our management team and our need to attract highly skilled personnel; |
| ● | the volatility of capital markets and other macroeconomic factors, including due to inflationary pressures, geopolitical tensions or the outbreak of hostilities or war; |
Risks Related to Product Development and Regulatory Approval
| ● | the expense and time required to obtain regulatory clearance of our products; |
| ● | our limited clinical studies and the susceptibility to varying interpretations of such studies; |
| ● | our ability to complete clinical trials; |
| ● | the failure to comply with the FDA’s Quality System Regulation or any applicable state equivalent; |
| ● | our reliance on third parties to conduct clinical trial work; |
| ● | the impact of legislation and federal, state and foreign laws on our business, including protecting the confidentiality of patient health information; |
| ● | the potential impact of product liability suits; |
Risks Related to Our Intellectual Property
| ● | the risks relating to obtaining or maintaining our intellectual property; |
| ● | potential litigation relating to the protection of our intellectual property; |
| ● | our limited foreign intellectual property rights; |
| ● | Our reliance on confidentiality agreements and the difficulty in enforcing such agreements; |
4
Risks Related to Our Industry
| ● | the intense competition we face in the markets we operate; |
| ● | our need to respond quickly to technological developments; |
| ● | the risks relating to obtaining or maintaining our intellectual property; |
| ● | the risks relating to third-party payors not providing for adequate coverage and reimbursement for our products; |
Risks Related to Our Operations in Israel
| ● | the risks relating to the political, economic and military instability that may exist in Israel; |
| ● | our principal executive offices and other significant operations are located in Israel, and, therefore, our results may be adversely affected by political, economic and military instability in Israel, including the multi front war Israel is currently facing; |
| ● | the potential for operations to be disrupted as a result of obligations of Israeli citizens to perform military service; |
| ● | the difficulty in enforcing judgements against us or certain of our executive officers and directors; |
Risks Related to the Ownership of Our Common Stock and Warrants
| ● | Nasdaq may delist our securities from trading on its exchange, which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions; |
| ● | the ability for our officers, directors and founding stockholders to exert influence over our affairs; |
| ● | the potential lack of liquidity, or volatility, of our common stock and warrants; |
| ● | the impact of analysts not publishing research or reports about us; |
| ● | the expense relating to our requirements as a U.S. public company; |
| ● | the potential failure to maintain effective internal controls over financial reporting; |
| ● | the existence of anti-takeover provisions in our charter documents and Delaware law; and |
| ● | that we do not intend to pay dividends on our common stock. |
PART I
Item 1. Business Overview
We are a leading global digital health company with a mission to power the behavior changes that drive better health. We are committed to transforming healthcare by delivering a comprehensive and highly engaging whole-person health platform, which enables us to create a future where healthy change is effortless and accessible to all.
At the core of our mission and vision is engagement. We believe that most existing digital health solutions in the market fail to deliver improved health outcomes because users are not engaged due to a lack relevance, personalization, consumerization, and longitudinal data and information. We, and our acquired companies, first commercialized our digital behavioral health products in the direct-to-consumer (“D2C”) marketplace, and we continue to use the D2C marketplace as a sandbox and laboratory to innovation. These consumers pay for these digital health products out of their own pockets and are therefore the most value driven among all healthcare consumers. These consumers demanded that we deliver highly engaging user experiences that deliver strong clinical health outcomes for which consumers will pay. The bottom line is that if users are not engaged in digital solutions over a long period of time, they cannot change their behavior and they cannot get healthier – we first deliver engagement followed by sustained behavior change that then leads to measurable health outcomes and improvement. We believe that our D2C marketplace roots and continued focus delivers better user experiences, longer sustained engagement, stronger clinical outcomes, at the most affordable prices, that then delivers the highest return on investment (“ROI”) in the industry.
Our whole-person health model includes the following five elements:
5
| 1. | Physical Health: Focuses on the prevention, and treatment of physical ailments; primarily cardiometabolic and musculoskeletal conditions. |
| 2. | Mental Health: Addresses emotional and psychological well-being, including stress management, as well as clinical anxiety, and depression across all levels of severity. |
| 3. | Social and Environmental Factors: Considers influences like socioeconomic status, community resources, housing, and education. |
| 4. | Individualized Care: Tailored user journey and care plans that respect personal goals, cultural values, and life circumstances. |
| 5. | Integration of Clinical Services: Combines different healthcare providers and systems to deliver seamless care for both physical and mental health needs. |
We have created our whole-person healthcare solution through both organic development and acquisitions of leading companies across several therapeutic areas. As a digital health consolidation leader, we have acquired companies that have spent over a decade and nearly $525 million, in combination with our own investment, to develop and deliver the most engaging whole-person health platform in the market to empower individuals to achieve their optimal health through data-driven, precision artificial intelligence (“AI”) personalized care solutions that integrate the management of physical and mental health needs.
Leveraging advanced analytics, data-driven AI precision and personalization, a deep understanding of consumer behavior, user-centric technology, and a holistic approach, we provide tailored interventions that meet the unique needs of each user to deliver the health industry’s highest levels of user activation and sustained engagement. Our digital self-care solutions ensure optimal levels of clinical outcomes with the highest levels of clinical efficacy by empowering users to overcome the psychological, social, and physical barriers to effective and sustainable behavior change.
With our whole-person digital health platform, we address a broad range of health needs, including chronic condition management (e.g., diabetes, hypertension, obesity, and musculoskeletal issues), behavioral health (e.g., stress, anxiety, and depression), and preventive care. By integrating digital therapeutics and well-being solutions with real-time data monitoring and access to professional care teams, we ensure an AI driven adaptive and continuous care experience that combines digital self-care, with virtual coaching, and virtual clinical care. As of 2024, our eligible user base spans millions of individuals worldwide, supported by partnerships with employers, health plans, pharmaceutical companies, and providers aiming to deliver instant access to the highest quality and most effective self-care and virtual human care that delivers the optimal level of clinical utilization to ensure the best value and outcomes to our users and customers.
Who We Serve
We serve four primary market segments that drive our business model. Our historic roots, as well as those of our largest acquisition, Twill, Inc. (“Twill”), began in the D2C market, which has forced us to create what we consider the industry’s most engaging and effective self-care whole-person digital health solutions; and we continue to operate in the D2C market in the U.S. by providing our chronic condition management and patient engagement solutions across many different health conditions. In addition, we use the D2C market as an innovation laboratory to develop and test new features and benefits to ensure that these innovations meet our high engagement and outcomes standards before deploying them in the business-to-business (“B2B”) market. From our D2C origins, Dario and Twill expanded into similar B2B market segments over the past seven years such that these market segments now represent three-fourths of our current revenues. These B2B market segments include medium-to-large employers, national and regional health plans, and global pharmaceutical companies.
Our medium-to-large employer market segment is focused on employers with over 1,000 employees and currently includes three of the five largest global technology companies and one of the two largest employers in the United States. In total, we had 53 employer customers in 2024. We seek to provide solutions to their employees that address the primary areas of health condition focus on by employers for meaningful costs savings that can deliver high and sustainable returns on investment, which can exceed 5:1. In this market segment, we go to market through a direct sales force, consultants, brokers, and channel partners that sell our solutions to their health plan and employer customers.
6
Our health plan customers include five of the nation’s largest organizations where we provide our solutions to their members both nationally and regionally. We have particularly specialized in providing its behavioral health offering to Medicare and Medicaid members, where we have established itself as the most engaging and effective solutions among these demographics. We are now leveraging this Medicare and Medicaid behavioral health specialty to expand access to its other chronic condition solutions to these populations. We go to market primarily through our own sales force and partners with large national health plans and other channel partners.
In 2018, Twill began to expand its offering to pharmaceutical companies, and in 2022 we entered into our first pharmaceutical company partnership. We now deliver these combined capabilities on our engagement platform to the pharmaceutical industry to provide three value propositions: 1) Top of the Funnel education and awareness to help companies find new patients for their treatments, 2) Mental and physical health support to improve medication adherence, persistence, and compliance, 3) Patient journey data analytics to better understand and target patients to get the right therapy to the right patient at the right time. We have provided our engagement platform services to a dozen global pharmaceutical companies across nearly as many medical conditions. We have a dedicated team with many years of experience selling into the pharmaceutical industry.
How We Generate Revenue
All of our customers have an interest in value-based health outcomes and care and have begun to work with us to align various types of outcomes with innovative revenue models. We differentiate ourselves versus our competitors by being willing to enter into risk-based agreements with performance guarantees that align the financial incentives with the employer, health plan, employee, plan member, and we deliver the best health outcomes at the most affordable price, thereby delivering the highest ROI. Across all market segments, we follow a digital health engagement platform subscription revenue model that is priced upon either a per user population basis (“PMPM”) or a per engaged user basis (“PEMPM”), and in some cases a customer may employee both pricing options together. These revenue models can also include milestone-based payments, as well as performance guarantees. Our digital health platform subscription model applies across our various product and therapeutic areas of focus.
We have the following product offerings with the engagement platform subscription revenue models:
Dario Connect (Well-being) – Our free D2C user engagement offering activates users, educates them, and encourages them to take the next best action on their patient journey. It is a digital front door to all other Dario products, as well as to our customer’s other digital offerings. For pharmaceutical companies, Dario Connect is used to create engaged communities around specific health conditions to increase awareness and education so that they will seek access to the most effective medication prescriptions. Previous claims-based analysis of script conversion completed by third parties demonstrated a 10:1 ROI for pharmaceutical companies who subscribed to this service. For pharmaceutical companies, their subscription fees are a function of the therapeutic area, the complexity of the patient journey, number of patients targeted, and platform configuration required to meet the needs of these patients. Among employers, this offering is used to increase access to our product benefits among non-employee dependents, and the payment for this service is included in the subscription for the primary product purchased for the various conditions addressed. For health plans, it provides support to patients across a broad array of medical conditions.
Dario Mind (Mental Health) – Our behavioral health offering decreases the symptoms of stress, anxiety, and depression by 25-30% in 8 weeks or less when used as directed. Many users will continue to use the product for many months and even years, with sustained engagement rates of close to 60% at 2 months, 40% at 12 months, and 30% at three years. Most of our customers see mental health condition prevalence among their employees at 20%. This is a digital first offering for empowered digital self-care that is then augmented with virtual coaching, and/or virtual clinical care from therapists and/or prescribers to address the needs of users across all levels of clinical severity. Customers can pay for digital self-care and virtual coaching on either a PMPM or PEMPM basis. When adding clinical care services, these can be paid for on a fee-for-service (“FFS”) basis, PMPM, or PEMPM basis, and is only offered through our B2B sales channel. We find prevalence of mental health conditions to be around 20% among employer and health plan populations, and more than 50% among some pharmaceutical company therapeutic areas of focus.
Dario Health (Diabetes) – Combining our proprietary glucometer as well as our Dario Health digital self-care application and virtual coaching, Dario Health for diabetes delivers the highest level of A1c reduction among the leading digital health companies focused on diabetes, of between 1.4 and 2.3 points, depending on the patients’ initial baseline.
7
This is offered through both our D2C and B2B sales channels. In the B2B sales channel, we see prevalence of this condition between 8-10% for most employers and health plans. Studies completed with a top 20 pharmaceutical company using claims data suggested total medical cost savings for patients using this solution reaching $5,000 per year. Customers can pay for digital self-care and virtual coaching on either a PMPM or PEMPM basis.
Dario Health (Hypertension) – We include our own proprietary blood pressure cuff with our Digital Health self-care application and virtual coaching. Clinical research has demonstrated that more than two-thirds of users with Hypertension stage 1 levels experience a 13mmHg reduction in systolic blood pressure when used as directed. As hypertension is one of the most prevalent conditions among adults in the United States, we find that among health plans and employers this solution is useful to 35% or more of their employees and members. This is offered through both our D2C and B2B sales channels. Customers can pay for digital self-care and virtual coaching on either a PMPM or PEMPM basis.
Dario Health (Weight management and GLP-1) – 30.7% of the U.S. population is overweight with about 42.4% being obese, meaning they have a body-mass index (“BMI”) of 30 or greater, creating the greatest level of prevalence among all of our product offerings. The recent introduction of GLP-1 medication has also increased the interest in this solution. We believe that we have demonstrated that our digital self-care solution, with our proprietary weight scale, Dario Health app and virtual coaching, delivers the industry’s highest level of digital self-care weight loss, at 10%, over 12 months. By educating users and helping them change their diet, exercise, and sleep behaviors, users can keep the weight off. We have modified our core offering to complement GLP-1 onboarding and offboarding, as well as GLP-1 prescription services. We offer this through B2B sales channels. Customers can pay for digital self-care and virtual coaching on either a PMPM or PEMPM basis. In 2024, we provided our GLP-1 offering to 20% of our employer customers, and we expect this to increase due to the strong demand for GLP-1s among patients and employers.
Dario Move (MSK) – Pain is one of the primary reasons for employees missing work and it is one of the key drivers of healthcare costs. Dario Move focuses on improving posture to alleviate pain with its proprietary medical device used with its Dario Move application and virtual coaching. We find that prevalence among employers is nearly 25%. This solution is only sold on a B2B and D2C basis, and is generally combined with our other offerings, and can be paid for on either a PMPM or PEMPM basis.
Bundled Full-suite or Partial-suite – While any one of our solutions can be subscribed to individually, increasingly employers and health plans are seeking vendors who are no longer point solution providers but who can consolidate multiple condition offerings into a single offering with one simple price. To accommodate these market demands, we now increasingly provides subscriptions for its full-suite or partial-suite on a PMPM or PEMPM basis, where additional clinical network services can be added on either a FFS basis or added to the PMPM or PEMPM subscription pricing. As one of the only whole-person digital health companies with a full suite of cardiometabolic, behavioral health, and musculoskeletal solutions, we believe that we have a competitive advantage with our bundled offering.
Why We Have Confidence in Our Digital Offerings
We have developed a comprehensive body of research, encompassing over 90 studies, that have demonstrated the transformative potential of our digital health solutions in advancing clinical care. We believe that these findings emphasize the platform’s ability to deliver long-term sustainable outcomes, including durable results over three years, while showcasing scalability with outcomes for more than 100,000 members.
We have established ourselves as a pioneer in the digital health sector, being the first to analyze multi-condition impacts by exploring relationships such as blood glucose and blood pressure, as well as blood glucose and weight monitoring. This work spans diverse clinical domains, including diabetes, hypertension, weight loss, pain, depression, and anxiety. Among diabetes users, significant improvements were recorded, i.e. those with a baseline HbA1c greater than 9, with a 2.3-point reduction in HbA1c compared to a 1.8-point reduction among non-Dario users.
Additionally, our members achieved substantial cost savings of $5,077 per person annually, driven by reductions in all-cause healthcare resource utilization (“HCRU”) and office visit costs.
8
These outcomes are underpinned by the demonstrated effectiveness of behavioral engagement, which serves as a critical driver of improved clinical results. Collectively, these achievements position us as a leader in digital health, delivering enhanced care and meaningful economic value.
The following provides a summary of many of our clinical research findings that support our claims to improving user clinical, health, and economic outcomes.
| • | 28% reduction in depression and anxiety symptoms |
| • | 2.0 reduction in A1c |
| • | 10% reduction in weight |
| • | 10% reduction in blood pressure |
| • | 10% improvement in medication adherence |
| • | 50% reduction in pain |
| • | $2,323 annual medical cost savings from behavioral interventions |
| • | $5,077 annual medical cost savings from cardiometabolic interventions |
Competitive Strengths
Our competitive advantage has emerged from our founding roots as a D2C company, that required us to develop the most engaging self-care digital behavioral programs and medical devices. Over more than a decade and over $500 million investment in technology, we have completed over 90 studies on the various whole-person health offerings to demonstrate their first-in-class clinical and health outcomes that results for a combination of high levels of sustained user engagement and highly effective clinically validated behavior change. We expanded from our initial diabetes focus to now include hypertension, obesity, weight management, musculoskeletal pain, stress, anxiety, and depression, that are all offered from the same digital health platform and can be bundled together or sold individually. By collecting billions of data points across all these health conditions over a decade, we have developed and commercialized it AI driven personalization that magnifies all of our competitive advantages to deliver more valuable results for users and customers. Claim-based analysis of our users has shown that we generate the highest ROI for its customers, whether they be employers, health plans, or pharmaceutical companies.
D2C Roots Ensured Highest Levels of Engagement
Unlike most of our competitors, we have always operated in the D2C market, and we continue to operate in both the D2C market as well as the B2B market, where the former drives rapid innovation cycles that require accelerated deployment of new features that can then be provided to our B2B customers. This ensures that our whole-person health platform continues to be highly intuitive and engaging so that it drives high user satisfaction and sustained engagement, ensuring better adherence to care plans that deliver the highest levels of quality outcomes and the industry’s leading ROI. Across all our offerings, we have had over 5 million users with billions of datapoints that have enabled the company to A/B test thousands of product features and functions and measure which deliver the highest levels of engagement.
Clinical Validation of The Highest Levels of Health Outcomes
Over more than a decade, we have completed over 90 randomized controlled trials (“RCTs”), real-world evidence studies (“RWE”), observational studies, interventional studies, and insurance-claims-based analysis. These have demonstrated our ability to generate best-in-class clinical and economic outcomes. Such studies provide the supporting evidence and confidence for us to provide at-risk offerings, performance guarantees, and value-based care.
9
Integrated Whole-Person Digital Health Platform
During the past five years, employers, health plans, healthcare providers, and pharmaceutical companies have expressed “point solution fatigue,” and have begun to prune the number of point solutions vendors and instead consolidate their digital health offering with vendors that provide a whole-person portfolio that covers all the major health conditions that drive their healthcare spending. Our whole-person digital health platform addresses these primary therapeutic areas of focus among employers, health plans, health systems, and pharmaceutical companies. These include all areas of cardiometabolic health (e.g., diabetes, hypertension, overweight/obesity), behavioral health (e.g., stress, anxiety, and depression), and musculoskeletal pain. In addition, we are partnering with clinical healthcare provides to provide virtual clinical care to its digital health offerings and virtual coaching. By integrating all these onto its whole-person digital health platform, it enables a hyper personalized, seamless, and individualized experience across all relevant health needs that ensure high levels of user engagement to deliver robust clinical outcomes that exceed those of nearly all competitors across all medical conditions.
AI and Data-Driven Personalization
Our proprietary AI and machine learning algorithms deliver highly personalized health insights and interventions, ensuring relevance and efficacy for each user. These were developed initially based upon our D2C experience with millions of users who pay out-of-pocket for our digital self-care solutions. By leveraging billions of data points with our D2C offering, we optimize our user recruitment, activation, and engagement algorithms for first-in-class engagement. Dario applies its AI, developed in the D2C market, to its B2B businesses to optimize the B2B businesses to enable Dario to outperform competitors in each market segment in engaging users and delivering valuable outcomes.
The Industries Highest ROIs
We have done claims-based analysis of our various offerings with its customers to measure the medical and economic outcomes that results from using its whole-person digital health offerings. These studies support our claims for providing the digital health industry’s strongest ROIs by decreasing medical costs, increasing medication adherence, and activating and engagement users in the healthcare system more effectively than alternatives. In addition, our offerings deliver impressive improvements in worker productivity by decreasing absenteeism, presenteeism, and healthy engagement in work. The key to these costs savings is that we collaborate with employers, payers, pharmaceutical companies, and providers to deliver comprehensive care solutions and improve accessibility. Across all these market segments, we power behavior change by providing digital first self-care, complemented with virtual coaching care, and then the optimal level of clinical care delivered through our clinical provider partners to accelerate access to care and improved whole-person health outcomes, at the lowest possible cost.
Our Growth Strategies
We have four core growth strategies that leverage all aspects of our whole-person digital health platform. After the acquisition of Twill in 2024 and integrating its commercial offerings and technological platform with ours, we are now prepared in 2025 to execute our growth strategies to both drive top line growth and to do so with greater operating efficiency that we believe will enable profitable growth.
Dario Health Comprehensive Cardiometabolic Health
Our core offering is our Dario Health cardiometabolic suite that provides FDA cleared devices and a digital behavioral health experience that enables users to decrease the symptoms of hypertension, diabetes, and obesity. Our primary market for this offering are medium-to-large sized employers that are seeking to control their chronic disease healthcare spending. We also work with large regional health plans to provide this offering to their members and self-insured employer customers. We provide virtual coaching to all of these users and provides access to virtual clinical services to these users as well. We find that among employees for most employers, the prevalence of diabetes is about 10%, while hypertension is 35%, and obesity and being overweight is between 35-75%.
10
Dario Connect is offered with Dario Health as a free digital experience that engages users across many different medical conditions. Users of Dario Connect participate in digital communities to learn more about their health condition, treatment options, learn from healthcare experts, including clinicians, and explore ways that they can improve their overall health. Dario Connect acts as a gateway and digital front door to get access to all of our whole-person digital offerings, as well as to get access to our customer products and services. Among employer customers, Dario Connect is how non-employee dependent learn about health care benefits they have access to and can access those benefits. Users can also get access to our virtual clinic partners and services through Dario Connect. Dario Connect is also the product that we configure for pharmaceutical companies to provide Top of Funnel awareness campaigns to help them find new patients.
Dario Mind Comprehensive Behavior Health
We have expanded our Dario Mind offering to include not only the industry leading self-care digital mental health application to decrease the symptoms of stress, anxiety, and depression, but also to provide access to virtual coaching, and virtual clinical services including virtual therapy and virtual clinical consultations, prescribing, and medication management. We are the only provider in the market with a comprehensive whole-person digital health offering supported by RCTs that demonstrate the clinical efficacy of its digital cognitive behavioral therapy (“CBT”) and mindfulness interventions. This offering has been deployed globally in 10 different languages among some of the world’s largest employers and health plans. We find that among most of our employer clients, over 20% will have a meaningful mental health challenge that require some level of treatment and support that Dario Mind can provide. In addition, we have several large pharmaceutical companies that provide Dario Mind to patients to address their comorbid stress, anxiety, and depression associated with their underlying medical condition. By addressing these symptoms, and providing imbedded psychoeducation about their condition, we have seen medication adherence rates increase by 10-20% over 6–12-month periods.
Bundled Full Suite Offering for Body and Mind
We offer the most comprehensive whole-person digital health offering in the market and can offer it as a bundled full-suite offering to employers and health plans where these customers can pay for the full bundle under any one of several revenue models such as PMPM, PEMPM, and milestone value-based. This full suite bundle includes Dario Mind, Dario Health, Dario Connect, and Dario Move. When pricing the full bundle, we price this based upon expected prevalence, engagement, outcomes for each component of the full bundle. By so doing, we believe that we can achieve meaningful economies of scale related cost savings that it can pass on to our customers and thereby deliver industry leading ROIs.
GLP-1 Opportunities with Employers and Pharmaceutical Companies
According to the Center for Disease Control (the “CDC”), 42.4% of Americans meet the BMI level of 30 for an obesity diagnosis. In the past few years, pharmaceutical companies like Eli Lilly and Novo Nordisk have launched expensive, blockbuster GLP-1 drugs for patient who have been diagnosed as obese that have demonstrated the ability to decrease BMI by 10-20%. Due to the success of these drugs in decreasing BMI, there are now nearly 100 drugs in the pipeline among these two pharmaceutical companies and their competitors that address many of the side effects that these drugs have with regards to tolerability, fatigue, and muscle loss. Research indicates that 26.2% of patients discontinue GLP-1s at 3 months, with an additional 30.8 doing so at 6 mots, and 36.5% at 12 months; with only 25% remaining on the medication after two years. Once a patient discontinues use of the GLP-1, the typical patient regained two-thirds of their weight within one year. For this reason, employers are eager to have their employees engaged in an effective digital behavioral health program while they are on a GLP-1 that will in crease their weight loss while on the medication and keep the weight off after discontinuation by helping the patient develop new, sustainable healthy habits. Presently, 20% of our employer customers now subscribe to its GLP-1 offering, and we see this as an important growth engine in the future. We have also found interest among pharmaceutical companies in providing our GLP-1 solution so support patients on their medication in developing healthy habits to improve outcomes.
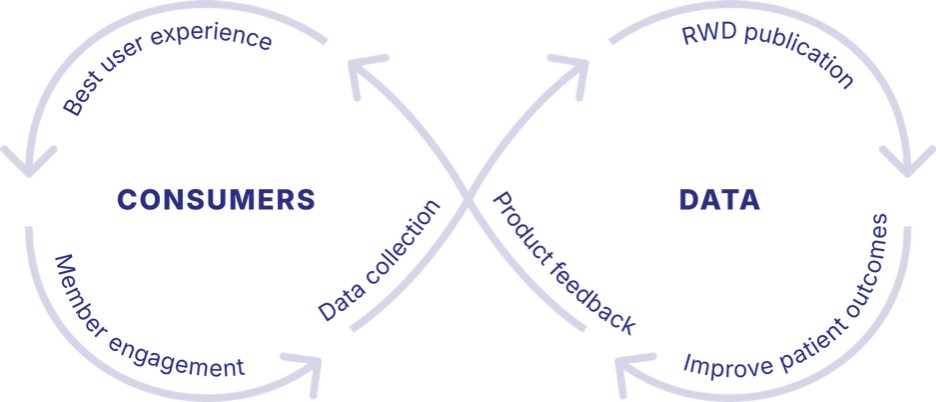
Dario Flywheel Integrates All These Growth Strategies
Our strategy is to integrate all these growth strategies into a united whole through its flywheel as diagramed below, which shows how its B2B and D2C strategies feed one another to create greater value as follows:
More B2B Clients: By getting more B2B customers it increases the number of users on the platform, which increases the data collected and used, increases the value to third-party and inhouse virtual clinical care networks, increases cross condition and cross client monetization, such as from behavior health to cardiometabolic health, which increases the average revenue per user, and then increases the D2C users and value.
11
More Users: User come from both our B2B and D2C market strategies, which drives our data driven innovation, and our value to virtual human care services.
More Value Per User: Data and associated AI, machine learning and analytics is what drives improved personalized user experience, which drives better outcomes, increased trust, more clients, and more users. In addition, our whole-person health model creates more value per user by providing access to different kinds of care and access to virtual clinical care.
Better Health Outcomes: As the users increase, the clients increase, the data increases with greater personalization, and expanded access to virtual human care, users achieve better health outcomes, that increase trust among both customers and users, which then increases the data, cross condition referrals.
Sales and Marketing
We employ a multi-channel sales and marketing approach designed to effectively reach diverse stakeholders.
Employer Sales
We employ a hybrid approach to the employer market due to its size and complexity. This means that it works with brokers and consultants as well as employing a direct sales force. Most medium-to-large employers go through a two-to-three-year request for proposal (“RFP”) cycle where they evaluate digital health vendors to select new ones based upon the value of their offering. Our value proposition with employers is to engage their employees at the highest levels, with effective, evidenced-based behavioral interventions that deliver meaningful clinical outcomes, and show compelling evidence of ROI at twice the industry average. We can contract directly with an employer or enable the employer to leverage their existing health plan benefits to pay for our services.
Health Plan Integration
We partner with payers to integrate our solutions into their benefit plan offerings. We can also provide our services on either a direct basis or through its claims-based billing. Health plans are a channel to employers as well as a channel to Medicare Advantage members. We have our own direct-to-health plan sales force but will also leverage consultants and brokers where appropriate. Marketing is focused on industry conferences, and highly targeted outreach.
12
Pharmaceutical Sales and Marketing
Many pharmaceutical companies work with consultants to identify how they can leverage digital health to complement and provide companion digital health services to support patients on their medications or to find new patients for their medications. We will often work with these consultants to design solutions with its engagement platform for their pharmaceutical clients. In addition, our pharma sales team will reach out to executives at pharmaceutical companies in the targeted areas we have identified to market and sell subscriptions to its engagement platform to these companies. Its primary areas of focus are oncology, rare disease, cardiometabolic health, dermatology, and mental health.
Dario AI Creating a Hyper-personalized Digital Health Future
We are successfully integrating AI to enhance patient care, streamline operations, and drive continuous innovation on its whole-person digital health platform. Our mission has been to empower individuals with personalized, data-driven health solutions while continuously improving clinical outcomes and healthcare efficiency. AI has been instrumental in harnessing the power of data to deliver precise, proactive, and personalized care.
We employ curated generative AI models trained on our decade of user journey data on millions of users and large content library to derive proprietary insights not otherwise attainable from publicly available data to drive significant competitive advantages in engagement, clinical and financial outcomes. We apply these insights to the Dario experience to create a sustainable competitive advantage.
Data and AI as a Strategic Asset
We, along with Twill (formerly a standalone entity before being acquired by us), has built an extensive repository of health data over a cumulative 25 years. This wealth of information serves as a critical asset, fueling AI-driven insights that enhance decision-making, predict health risks, and optimize patient engagement. Our proprietary datasets span multiple health conditions, providing a unique foundation for developing advanced AI models tailored to real-world patient needs.
25 Years of Cumulative Experience, With Billions of Proprietary Data Points
Our AI capabilities are built upon a rich foundation of proprietary data and research, including:
| • | Large available member datasets: Billions of data points from providing its products to over 5 million users over a cumulative 25 years. |
| • | Multi-condition focus: Addressing multiple therapeutic areas Cardiometabolic (diabetes, hypertension, weight management), Behavioral Health (stress, anxiety, depression), and musculoskeletal pain with over 75 million physical and behavioral health measurements collected through our medical devices and tens of billions of data points through its applications. |
| • | Uniquely matched datasets: Enriched with metadata such as biomedical and behavioral, as well as location, nutrition, and other timely and relevant health information for deeper insights. |
| • | Extensive AI research: Over 7 years of AI advancements, including the development of large language models, conversational AI chatbot, and advanced machine learning and deep learning algorithms, delivering over 15 million behavioral health interventions and 25 million digital behavioral health virtual coaching interactions. |
| • | Direct access to claims data: Utilized on an anonymized basis in-house to train proprietary models for optimized care delivery and outcomes. |
| • | Open architecture: Enabling integration with client-provided care modalities, seamlessly connecting them to Dario’s digital care pathways. |
13
Our AI strategy has three primary pillars:
| 1. | Operational Efficiency and Simplification: Optimizing workflows, reducing administrative burdens, and enhancing operational efficiency to drive better healthcare outcomes. |
| 2. | Whole-Person Patient Care: Expanding our product offerings to provide the most comprehensive and personalized care experience for every individual from digital self-care to virtual clinical care. |
| 3. | Curated generative AI: Leveraging a cumulative 25 years of proprietary data collection and content creation to develop a unique generative AI model tailored to personalized care in a highly regulated healthcare environment. Recent advancements in AI model training, particularly in reducing hardware costs, serve as a significant accelerator for Dario’s curated generative AI. These developments enable us to scale and refine our AI models more efficiently, further enhancing predictive accuracy and real-time personalization. |
| 1. | Operational Efficiency and Simplification |
Our AI has been instrumental in improving operational efficiency across multiple functions, enhancing productivity, automating routine tasks, and optimizing decision-making processes. Specific applications include:
| • | High-Quality Content Creation: AI-driven tools assist in generating engaging, evidence-based health content, improving efficiency and consistency. |
| • | Culturally Adapted Content Translation: Custom trained generative AI models based on our vast content library in more than 10 languages enable automatic translation of new content that maintains a consistent tone of voice and cultural sensitivities across all languages. |
| • | Software Development: AI accelerates software development by optimizing code generation, debugging, and deployment processes. |
| • | Member Services: Deploy AI-powered automation to optimize member engagement, enhance customer support, improve response times and member satisfaction. |
| • | Product Management: Enables data-driven product decisions, enhancing usability and personalization. |
| • | Human Resources (HR): Improve recruitment, employee engagement, and workforce planning, streamlining HR processes. |
| • | Finance: Optimize financial operations, forecasting, and risk management, reducing manual efforts and improving accuracy. |
| • | Internal Knowledge Retrieval: Retrieval-Augmented Generation (“RAG”) used to facilitate seamless access to organizational knowledge, enabling employees to quickly find relevant data, policies, and historical insights for improved decision-making and efficiency. |
Our AI development and adoption is enabling us to reduce operating expenses. Looking ahead, we expect ongoing AI advancements to drive an additional efficiency gains in the next few years, further reducing costs and improving user experience, customer service, innovation, and value.
| 2. | Curated Generative AI |
We are pioneering a proprietary generative AI model today, which delivers highly personalized care solutions in a tightly regulated healthcare environment. Our advancements in AI model training reduce hardware dependency and costs and further accelerate the scalability and performance of curated generative AI. RAG represents a key component of this model that enhances generative AI by integrating real-time, domain-specific proprietary data retrieval, which represents a proprietary advantage.
14
| • | Context-Aware Generative AI – Our RAG engine enhances AI-generated content by incorporating the latest medical insights, ensuring accuracy and relevance. |
| • | Unique and Certified Data - Unlike generic GenAI models relying on publicly available internet data, our generative AI is built upon a unique, healthcare-intelligent, and certified dataset. |
| • | Healthcare-Specific Intelligence – Our generative AI integrates domain-specific knowledge, ensuring high accuracy and contextual relevance for patient care applications. |
| • | Regulatory Compliance – We designed our RAG to adhere to strict healthcare regulations. As such, we supports safe, ethical, and privacy-compliant AI-driven health solutions. |
| • | Commitment to Ethical AI - We prioritize fairness, transparency, and ethical AI development in all our models. |
| • | Regulatory Adherence - Our AI systems comply with healthcare regulations, including Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) and the EU General Data Protection Regulation (“GDPR”), ensuring data security and patient privacy. |
| • | Bias Mitigation - Continuous refinement of AI models help minimize bias, improving accuracy and equitable healthcare delivery. |
| • | Patient Trust - AI systems are built with patient trust in mind, ensuring that AI-driven insights enhance rather than replace clinical decision-making. |
| 3. | Whole-Person Patient Care |
Our AI-powered solutions enhance patient engagement, provide predictive analytics, and deliver personalized treatment plans. AI applications in patient care include:
| • | Personalized Digital Therapeutics: AI-powered algorithms tailor interventions based on individual health data, improving adherence and engagement. |
| • | Predictive Analytics for Chronic Disease Management: Our predictive model for blood glucose levels demonstrates over 90% accuracy, which can support efforts to guide user behaviors to engage in timely interventions and proactive treatment in cases where early warning signs can ameliorate health deterioration. |
| • | Automated Coaching and Behavioral Insights: AI-driven behavioral coaching adapts to users in real-time, enhancing self-management and lifestyle modifications. |
| • | Clinical Decision Support: AI enhances provider decision-making by surfacing actionable insights based upon algorithms that are based upon vast data sources that enhance clinical judgments. |
| • | Recommendation Engine Optimization: AI-driven recommendation systems refine patient treatment strategies, enhance engagement and improve health outcomes. |
On Dario’s AI Roadmap
In 2025, we plan to widen and deepen its utilization of AI in the following four areas:
| • | GLP-1 optimization: Utilize AI-powered models to predict members’ level of adherence to GLP-1 prescription prior to prescribing and their level of success in maintaining outcomes post GLP-1 off-boarding. This capability will allow Dario to intelligently prioritize prescriptions over other weight loss and behavior change programs. |
| • | Advancing RAG-powered Generative AI: Refining AI-driven personalized healthcare by integrating real-time monitoring, improving predictive accuracy, and adapting models to personalized treatment plans. |
15
| Leveraging reinforcement learning enables continuous AI improvements based on patient feedback and outcomes. |
| • | Operational Efficiency and Simplification: We have several productivity initiatives in multiple areas such as Product Management, Member Services, and Human Resources. |
Competition
We operate in a highly competitive digital health market, characterized by rapid innovation and evolving consumer expectations. During the past five years, employers and health plans have expressed “point-solution fatigue” and have begun to decrease the number of their point-solution digital health vendors, and to consolidate their digital health vendors among those that provide multiple, integrated offerings. This has driven substantial industry consolidation and has enabled us to differentiate itself as a consolidation leader among competitors as it provides a whole-person health care solutions covering six different medical conditions.
We see two primary dimensions emerging: economies of scale and economies of scope. The first dimension is an increased focus on ROI driving by economies of scale. Recent surveys of employer’s priorities indicates that they want digital health solutions that have much lower costs that can be broadly deployed among their populations and deliver much higher ROIs than they have accepted in the past. A 2:1 ROI is no longer adequate to meet the needs of employers and health plans. For this reason, we have leveraged our AI investments, superior user engagement, highly effective user behavior change, and access to virtual digital care and services to deliver the highest ROIs in the industry that generally exceed 5:1.
The second dimension is driven by economies of scope that increases simplicity by decreasing the number of digital health vendor that deliver an increasingly broad scope of digital health offerings. This simplifies contracting, reporting, value creation, access to users, and the creation of value for users, employers, and health plans.
With these two emerging value creation dimensions that are driving competitive advantage, we see the following companies as its primary competitors:
Broad-based Digital Health Platforms
Companies such as Teladoc Health, Inc. and MDLive, an Evernorth company, offer a full array of integrated virtual care solutions for primary care, chronic and behavioral health. While these companies have both scale and scope, they have not yet developed an operating model that deliver their services profitably at scale.
Cardiometabolic Health Platforms
There are other more narrowly focused companies such as Omada Health, Inc., Vida Health, Inc., Virta Health Corp, and Welldoc, Inc. that focus on the B2B market. And other D2C oriented companies such as Ro, Inc., Hims & Hers, Inc., Weight Watchers International, Inc., and Noom, Inc., that compete with us in the cardiometabolic and GLP-1 space. Most of these companies lack the whole-personal digital health scope that we offer, and still deliver ROIs that are half of what customers expect and demand.
Behavioral Health Providers
Specialized mental health platforms like Spring Care, Inc., Lyra Health, Inc., Headspace, Inc., BetterHelp, Inc. (a division of Teladoc), Calm.com, Inc., and Talkspace, Inc., compete in the B2B behavioral health marketplace. All these companies focus narrowly on behavioral health with no plans to expand their scope to include additional chronic disease conditions.
Wearable Technology Firm
While we integrate with many wearable technology firm’s devices from companies such as Fitbit, Garmin, and Apple, these companies also provide some D2C offerings that could be considered as competitors to our D2C products. However, these companies do not truly compete in the B2B employer, health plan, and pharmaceutical marketplace with competitive comprehensive behavioral and cardiometabolic offerings.
16
As such, we do not see these companies are true competitors.
Traditional Healthcare Systems and Health Plans
While many health systems may have their own home-grown solutions for many of the conditions that we address, few really provide a commercially competitive offering beyond their own network. The same is generally true for most health plans, which primarily focus on vendors like us to provide chronic and behavioral health solutions to their members.
Given this competitive landscape, we differentiate ourself through our integrated whole-person health approach, AI and data-driven personalization, and commitment to user-centric design to deliver industry leading levels of sustained user engagement. Our focus on measurable outcomes and strong partnerships positions us as a leader in the digital health market that can deliver the economies of scale and scope that delivers the highest ROI and easiest delivery of its individual or bundled solutions.
Recent Developments
Aetna
In the first quarter of 2024, we launched the Aetna behavioral platform with approximately a dozen customers, whereby we began to generate revenue from that collaboration, and we expect Aetna to continue to add employers to the platform. A separate self-help program contracted with Aetna last year launched on the platform at the end of the first quarter and is also expected to grow throughout 2024.
Employer Contracts
In April 2024, we announced four new contracts to provide integrated chronic condition management solutions for two employers beginning in the second quarter of 2024.
In November 2024, we announced the signing of four contracts with self-insured employers that are expected to go live in the first quarter of 2025. The contracts span across the full suite of our platform, with no one opting for a single condition, showing evidence of the value of a multi-condition offering.
Presentation of New Studies
In March 2024, we announced two new clinical studies presented at the International Conference on Advanced Technologies and Treatments for Diabetes (ATTD) 2024, demonstrating a 6.38% reduction in weight for members with a baseline BMI of 30 and above and an overall reduction in blood glucose levels, with average blood glucose levels remaining below 140 mg/dL for one year.
In April 2024, we announced two new studies published in the leading peer-reviewed journal for digital health and medicine, Journal of Internet Medicine (JMIR), including a Randomized Controlled Trial (RCT) demonstrating the impact of a digital stress reduction program for teens.
In April 2024, we announced new research published in the leading peer-reviewed journal for digital health and medicine, JMIR, demonstrating a clinically significant reduction in blood glucose levels for members using Dario to manage weight alongside diabetes.
GLP-1 Updates
In June 2024, we announced a new contract with a national employer to provide its cardiometabolic solution with integrated support for GLP-1s to employees beginning in the third quarter of 2024.
In June 2024, we presented two studies presented at the 84th Annual American Diabetes Association (ADA) Scientific Sessions in Orlando. The first study provides an analysis of member data for those tracking a GLP-1 medication in Dario's cardiometabolic solution.
17
A retrospective analysis was conducted using data from members using Dario to manage Type 2 diabetes or prediabetes and taking either Metformin, a standard medication prescribed for diabetes, or a GLP-1. Both member groups were tracked for 12 months as they engaged with our digital chronic condition solution to measure blood glucose levels and track lifestyle activities such as diet and exercise. Members also utilized our Medication Cabinet feature to log Metformin or GLP-1 dataset reminders and report adherence. The data showed significant improvement in adopting healthy behaviors over a 12-month period across both groups as shown by increased tracking of healthy lifestyle behaviors as shown by logged meals and physical activity beginning in the first three months and sustained through the 12-month period.
Additional research presented by us at the ADA conference examined Dario's ability to help members realize the goal of diabetes remission for people living with Type 2 diabetes. The ADA considers remission to be achieve when an individual with Type 2 diabetes sustains normal blood glucose levels of less than 6.5% HbA1c for three months without the aid of a diabetic medication.1 This retrospective study analyzed the data of 7,240 individuals with Type 2 diabetes using our platform to help manage their condition without the help of insulin for at least six months. The results demonstrated a significant impact:
| ● | 31% of our members experienced blood glucose levels reflecting the goal of diabetes remission with average blood glucose readings of less than 140 mg/dL (A1c 6.5%) during a three-month period; |
| ● | 70% of our members achieved a blood glucose level of less than 140 mg/dL in their last month of usage maintained it for three-month period, indicating behavior change and improved long-term glycemic control; and |
| ● | Lifestyle activities as shown by logged meals and physical activity moderated the reduction in average blood glucose levels and high readings ratio. |
New Research Reveals How Physical Activity Mediates the Impact of Depression on Blood Glucose Levels in Individuals with Diabetes or Prediabetes
In September 2024, we announced the publication of a new study in the peer-reviewed journal Frontiers in Endocrinology. The study demonstrates the effectiveness of our cardiometabolic solution for members living with diabetes or prediabetes and depression.
Integration of Twill Behavioral Health Capabilities into Cardiometabolic Health Solution
In September 2024, we announced the integration of condition-specific communities and peer groups with personalized navigation capabilities into our cardiometabolic solution to improve outcomes-based engagement, marking a significant milestone in the integration of the Dario-Twill product offering.
Twill by Dario to Offer a Benefit for AARP Members
In October 2024, we announced a new AARP member benefit that provides members with proven digital behavioral health and well-being solutions from Twill by Dario. The new benefit is expected to launch in January of 2025 for AARP members.
Integration of Twill Capabilities Across Full Multi-Condition Platform
In October 2024, we announced the full integration of Twill's advanced behavioral health and navigation capabilities into our platform. The integration completed our effort to create one of the most comprehensive and effective end-to-end digital health solutions in the market. By unifying data across mental and physical health, we offer a more personalized and effective approach to care.
New Regional Health Plan, Expanding Reach in Medicaid Population
In October 2024, we announced a new contract with a regional health plan in the Medicaid space, which has launched with the full suite of cardiometabolic solutions. Starting with an initial pilot program, we will work closely with the health plan to bring its proven solutions to the plan's population, which includes nearly 10,000 Medicaid members.
18
Pharma Collaborations with Global Pharma Leader to Enhance User Engagement
In November 2024, we announced a new collaboration with a pharma company that will utilize Dario Connect (formerly Twill Care) among their patient populations to further their direct-to-consumer efforts using our refined engagement and navigation technologies. In an effort to strengthen and expand connections across patient populations that are candidates for a new drug to treat psoriasis, a top pharmaceutical company chose us to pilot a cutting-edge initiative aimed to help onboard patients that will engage with the drug via the platform. This collaboration is designed to collect critical insights on patient outcomes and drug effectiveness, with Dario Connect's innovative community-building capabilities playing a key role in enhancing patient engagement.
GLP-1 Solution with Prescribing Capabilities through Collaboration with MediOrbis LLC, Targeting Employers and Direct-to-Consumer Markets
In January 2025, we announced a collaboration with MediOrbis, a multi-specialty digital health provider, to add prescribing capabilities to our GLP-1 behavior change solution for a comprehensive medical weight loss program. This strategic addition creates a fully integrated solution for employers covering weight-loss medications and supports direct-to-consumer offerings, expanding our addressable market in one of the fastest-growing segments of digital health.
Sanofi
On November 26, 2024, we were notified of the termination of the Amended and Restated Exclusive Preferred Partner, Co-Promotion, Development and License Agreement with Sanofi U.S. Services Inc. (“Sanofi”), dated July 10, 2023, which was originally executed on February 28, 2022. On December 4, 2024, we transitioned our relationship with Sanofi and entered into a services agreement. Pursuant to the services agreement, we will configure our Twill Care platform for Sanofi and transition the relationship to a recuring revenue model.
Rula Health
In March 2025, we announced a strategic collaboration with Path CCM, Inc. d/b/a Rula Health ("Rula”), a leading provider of high-quality behavioral health services, to expand access to mental health support for employers and their workforce nationwide. Through this strategic collaboration, we will have exposure to Rula’s extensive network of over 15,000 providers. By leveraging Rula’s high-quality provider network with extensive coverage, we will offer an “easy button” solution for employers looking to implement behavioral health support seamlessly within their existing benefits structure.
19
Clinical Studies
Main Highlights
Our studies below demonstrate the clinical value of our legacy digital therapeutic devices and the ability of our solutions to deliver sustainable outcomes over time.
At the ADA 2018 session, Dario presented three real-world-data analysis studies, as detailed below.
Type 2 Diabetes Users of Dario Digital Diabetes Management System Experience a Shift from Greater than 180 mg/dL to Normal Glucose Levels with Sustainable Results
| ● | Reduction of 19.3% in high glucose readings within 12 months |
| ● | Increase of 11.3% in in-range readings within 12 months |
Methods: A retrospective data evaluation study was performed on the DarioTM cloud database. A population of all active Type 2 Diabetic (T2D) users that took measurements with DarioTM Blood Glucose Monitoring System (“BGMS”) on average of 20 measurements per month during 2017. The study assessed the ratio of all high blood glucose readings (180-400 mg/dL) and the ratio of all normal blood glucose readings (80-120 mg/dL) in their first month of use to their last month of use during 2017 as recorded in the database.
Results: For 17,156 T2D users activated during 2017 the average ratio of high events (180-400 mg/dL) was reduced by 19.3% (from 28.4% to 22.9% of the entire measurements). While at the same time, the ratio of normal range readings (80-120 mg/dL) was increased by 11.3% (from 25.6% to 28.5% of the entire measurements).
Updated Analysis combining 2017 and 2018 data totals 38,838 Type 2 Diabetes active users and 3,318,014 measurements show 14.3% decrease in high readings (180-400 mg/dL) and 9.2 % increase in In-range (80-120 mg/dL) readings.
A decrease in High Readings and Severe Hyperglycemic Events for People with T2D over the Full Year of 2017 in Users Monitoring with Dario Digital Diabetes Management System
| ● | Reduction of 20% of High events (180-400 mg/dL) in T2D sustained within 12 months |
| ● | Reduction of 58% of Hyper events (>400mg/dL) in T2D within 12 months |
Methods: A retrospective data evaluation study was performed on the DarioTM cloud database. A population of active T2D users that continuously measured their blood glucose using DarioTM BGMS during the full year of 2017 was evaluated. The study assessed the ratio of high (180-400 mg/dL) and hyperglycemic (>400mg/dL) blood glucose readings during full year of 2017 as recorded in the database. The average of high and hyperglycemic glucose readings were calculated in periods of 30-60, 60-90, 90-120, 120-150, 150-180, 180-210, 210-240, 240-270, 270-300, 300-330, 330-360 days and compared to first 30 days as a starting point of analysis.
Results: For 225 T2D active users the ratio of high events (180-400 mg/dL) was reduced gradually in 19.6% (from 23.4% to 18.8% of the entire measurements) from baseline compared to the 12th month of the year. Moreover, the ratio of severe hyperglycemia events (>400 mg/dL) was decreased in 57.8% (from 0.90% to 0.38% of the entire measurements) at the same period.
Continuous Reduction of Blood Glucose Average during One Year of Glucose Monitoring Using Dario Digital Monitoring System in a High-Risk Population
| ● | The study presented a reduction of 14% Blood Glucose average was observed in T2D within 12 months |
| ● | 76% of the population showed 24% improvement in Blood glucose average within 12 months |
20
Methods: An exploratory data analysis study reviewed a population of high-risk active type 2 Diabetic users with initial 30 days glucose average above 180 mg/dL during a full calendar year. The study assessed the average blood glucose readings along a year of usage. The average glucose readings was calculated per user in periods of 30 days intervals from 30-60 to 330-360 days and compared to the first 30 days as the starting point baseline of analysis.
Results: Overall of 238 highly engaged T2D users (more than one daily measurement in average) whose average blood glucose level was above 180mg/dL in the first 30 days of measurements (225±45 mg/dL) showed continuous reduction in glucose level average vs. baseline. Reduction in blood glucose average level was demonstrated gradually, in the succeeding 3, 6 and 12 months showing average decrease of 7%, 11% and 14% vs. baseline, respectively. Furthermore, 76% of the entire population (180 out of 238 users) improved their average blood glucose level over a year. Those 180 users (average blood glucose 228±46) showed an average decrease of 10%, 16% and 24% in their glucose average following 3, 6 and 12 months, respectively.
At the American Association of Diabetes Educators (AADE) 2018 Dario presented a study titled “Decrease in Estimated A1C for people in High-risk over a full year of users monitoring with a digital Diabetes management system.”
A reduction of 1.4% in estimated HbA1C in Type 2 Diabetes high risk users from baseline after one year of the Dario system use.
Methods: A retrospective data evaluation study was performed on the DarioTM cloud database. A population of high-risk (with baseline A1C > 7.5 percent), active users that continuously measured their blood glucose using DarioTM BGMS during a full year was evaluated. The study assessed estimated A1C values based on blood glucose readings during a full year as recorded in the database. The estimated A1C values were calculated in periods of 3, 6, 9 and 12 months and compared to first 30 days as a starting point of analysis.
Results: A group of 363 high-risk Dario BGMS users (A1C>7.5) with greater than two blood glucose measurements taken per day in the first 30 days and in the 12th month of the year was selected. Estimated A1C was improved by -0.7, -0.8 and -1 percent from baseline to 3, 6 and 9 months respectively, and remained -1 percent lower following 12 months of usage (8.65±0.96 vs.7.65±1.0). Moreover, subgroup analyses by diabetes type revealed substantial estimated A1C improvement among people with T2D showing improvement of -1 percent from baseline to 3, 6 months and 1.4 percent following 12 months (8.5 ± 0.91% vs. 7.14% ± 0.98%).
An additional study evaluated the potential improvement in glycemic variability in Type 2 diabetes over six months in patients monitoring with Dario Digital Diabetes Management System. Dario presented the study results at the Advance Technologies and Treatment for Diabetes (ATTD) conference in February 2019 in Berlin. We presented two additional studies outcomes at ADA 2019 conference.
Decrease in Glycemic Variability for T2D over Six Months in Patients Monitoring with Dario Digital Diabetes Management System
| ● | The study demonstrated a reduction of 14%-18% in measurements variability was observed in T2D within 6 months |
| ● | Hypo events (<70 mg/dL) remained <1 event on average |
Methods: A retrospective data evaluation study was performed on the DarioTM database. A population of T2D high-risk patients (blood glucose measurements average (GMavg) >180 mg/dL) measuring more than 20 times in the first 30 days (analysis baseline) was evaluated on days 60-90 (3 months) and 150-180 days (6 months). Standard deviation (SD) and GMavg were calculated and compared to the baseline.
Results: A group of 698 T2D high-risk DarioTM users was selected. GV was reduced by 10% and 14% from baseline through 3 and 6 months, respectively (SD of 55.7, 58.4 vs.65.0). GMavg was reduced by 8% and 12% from baseline through 3 and 6 months, respectively (201.1±25.57, 192.8±54.3 vs. 219.5±38.5) while patient’s hypoglycemic event (<70mg/dL) was in average, less than one (<1) during this period. Subgroup analyses (355 patients) revealed substantial Methods: A retrospective data evaluation study was performed on the DarioTM cloud database.
21
GV improvement among non-Insulin T2D patients. The GV was reduced by 14% and 18% from baseline through 3 and 6 months, respectively (SD of 52.8, 50.7 vs.61.7).
T2D Users of Dario Digital Diabetes Management System Experience an Increase of in-range Glucose Levels Linked to App Engagement
Relative Increase of 10 % In-range linked to App engagement
A population of active T2D users (>15 measurements per month on average) was evaluated. The study assessed the ratio of in-range blood glucose readings (70-140 mg/dL) as a function of App engagement level for 6 months as recorded in the database compared to first 30 days as a starting point of analysis.
Results: A population of 4917 T2D non-insulin users measuring more than 15 times per month on average during 6 months in a row was evaluated. The ratio of in-range (70-140 mg/dL) readings was increased following 3 months in correlation to the level of tagging meal reference/carbs/physical activity occurrences (4.0%, 9.1% and 11.9% for tagging 0-1, 1-2 and >2 times per day on average, respectively) and sustained for 6 months.
Reduction of Blood Glucose Average Less than 140mg/dL in People with Type2 Diabetes Using Dario Digital Diabetes Management System
30-40% of T2D Dario users experienced Reduction of Blood Glucose Average below 140 mg/dL
Methods: A retrospective data evaluation study was performed on the DarioTM cloud database. A population of active T2D users that continuously measured for 6 months was evaluated. The study assessed their BG avg and estimated A1C (eA1C) values based on blood glucose readings as recorded in the database. Values were calculated in periods of 3 and 6 months and compared to their first 30 days as a starting point analysis.
Results: A group of 1248 Dario BGMS T2D active users (1.98 measurements per day on average during 6 months in a row) with BG avg >140mg/dL (eA1C>6.5) was evaluated. All 1248 (100%) reduced their BG avg along 6 months on average.
A group of 31% (387) achieved BG avg of <140 mg/dL (eA1C<6.5) following 3 months showing 19% reduction on average from baseline (132.38±13.36 vs.162.79±25.41 mg/dL and eA1C 6.24±0.46 vs 7.3±0.88) and sustained their glycemic control during a 6 month period (131.57±13.86 mg/dL and eA1C 6.21±0.48).
Subgroup analyses of 568 non-insulin users revealed that 40% (226) achieved a BG avg <140 mg/dL following 3 months (131.95±13.21 vs.161.67±24.18 mg/dL and eA1C 6.22±0.46 vs 7.26±0.84) and sustained for 6 months period (131.03±13.70 mg/dL and eA1C 6.19±0.47). Along the 6 months period, hypo events (<50mg/dL) per user per month on average remained stable.
In August 2019 another study was presented at the AADE 2019 in Atlanta. The study evaluated the “Impact of Digital Intervention on In-range Glucose Levels in Users with Diabetes.” The study results showed 6% improvement in average blood glucose levels over 3 months intervention program for a group of 162 users. A 39% increase in the in-range (80-130 mg/dL; <180mg/dL post-meal) measurements was observed in a subgroup of 101 patients who started with average blood glucose levels of over 140mg/dL. In November 2019, another analysis was presented in Diabetes Technology conference “The Effect of Digital Intervention on Glycemic Control in Users with Diabetes” looking on total in-range measurements ratio 70-180 mg/dL showing increase of 19% following 3 months on the Dario Engage platform.
In February 2020, we presented an additional clinical study at the ATTD conference in Madrid, Spain. The presented data shows the Dario digital therapeutics platform successfully assists insulin dependent patients with diabetes in reducing hypoglycemic events.
22
Decrease in Hypoglycemia Events Over Two Years in Patients Monitoring with Dario’s Digital Diabetes Management System
Methods: A retrospective data analysis was performed on the Dario real-world database. Insulin dependent of users with type 1 or type 2 diabetes population was evaluated for two years of continuous system use. Average numbers of level 1 hypoglycemia (<70mg/dL) and level 2 hypoglycemia (<54 mg/dL) events were calculated monthly and compared to baseline (first month).
Results: For 1481 type 1 and type 2 insulin dependent users, average of level 1 hypoglycemia events and level 2 were reduced by 24% and by 17% after 6 months and by 50% and 57% after 2 years vs. baseline respectively. Users with type 1 diabetes (N=363) reduced level 1 hypoglycemia events by 50% and Level 2 by 55% after 2 years. Moreover, a 40% reduction in high blood glucose readings was observed as well after 2 years.
In June 2020, we presented two clinical studies at the ADA virtual conference. The presented data from these studies showed:
Estimated A1C Reduction in High-Risk Patients Over Two Years of Using a Digital Diabetes Management Platform
This study presented data indicated the potential for a digital diabetes management solution to effect and sustain glycemic control improvements and demonstrated long term reduction of blood glucose average (eA1c) and glycemic variability in type 2 diabetes over two years. The system assists users through a variety of mechanisms including behavior modification in diabetes self-management and in long-term routines for self-care.
Methods: A retrospective study of high-risk users (BG avg >180 mg/dL equivalent to e A1c 8.0) 2 with type 2 diabetes that measured their blood glucose using the Dario® platform database over two consecutive years was performed. The minimum engagement level for inclusion was at least two blood glucose measurements per day on average taken in Month 1 and Month 24. Actual blood glucose readings were taken by the Dario meter and loaded into the cloud database. These were evaluated for the blood glucose average (“BGavg”), estimated A1c (eA1c) values and glycemic variability (by Standard Deviation; SD) following 24 months compared to the first month (baseline).
Results: 368 high-risk, T2D active and engaged users for at least consecutive 2 years were identified and assessed for their risk-level and insulin usage. A group of 148 T2D, non-Insulin users that started with a blood glucose average (BG avg) >180 mg/dl (equivalent to eA1c>8.0) consistently reduced their BG avg by 18% on average and sustained these values (179±45 vs. 219±56 mg/dL) following 2 years on the Dario platform. Glycemic variability was reduced over two years by 20% on average (SD:45 vs. 56). Substantial reductions were observed for higher risk groups (insulin and non-insulin treated). The subset that started with average BG levels > 212 mg/dL (eA1c >9.0) and average BG levels >240 mg/dL (eA1c>10) reduced their average BG by 22.5% and 25.7% respectively on average over two years. The equivalent reductions in eA1c were 1.95% and 2.42%.
Users with type 2 diabetes using a digital platform experienced sustained improvement in blood glucose levels.
Methods: A retrospective data evaluation (Q1:2018-2019) was performed on the Dario® data base. A population of active users (18 measurements per month with the Dario® System on average) with T2D, non-Insulin treated was evaluated over a full year. High blood glucose readings (180-400 mg/dL, >250 mg/dL), fasting readings (<126 mg/dL) and post-meal readings (<180mg/dL) ratios were assessed in their first month of use until the 12th month.
Results: For 9,200 users with T2D, non-Insulin users, the average ratio of high glycemia events (180-400 mg/dL) from entire set of measurements was reduced by 26% (18.62% vs. 23.43%) while readings of >250mg/dL were reduced by 33% (4.65% vs. 6.93%) over a year. Fasting measurements analysis revealed an increase of 16% in ratios of readings <126 mg/dL per entire set of fasting measurements (40.59% vs. 34.92%) on average.
In August 2020, we presented an additional clinical study at the Virtual Association of Diabetes Care and Education Specialists (ADCES) conference. The presented observational study data demonstrated better glycemic and blood pressure control. Patients using an integrated chronic disease management digital platform have the potential to improve user activation which may assist to better manage their blood glucose and blood pressure levels and sustain behavioral change.
23
Impact of Digital Management on Clinical Outcome in Patients with Chronic Conditions: diabetes and hypertension.
The study presented Hypertension and Diabetes clinical outcomes.
Hypertension: Increase in normal level % measurements from 6% to 12% while hypertension stage 2 measurements decreased from 53% to 45%. 70% of the users (243 out of 345) improved their blood pressure levels by 8.4 mmHg Systolic and 6.2 mmHg Diastolic, on average.
Glucose levels: A reduction of 33% in high readings (>250 mg/dL) and 67% in severe events (>400 mg/dL) was observed over six months.
Methods: A retrospective data evaluation study was performed on the DarioTM cloud database. A population of active users that measured both blood pressure and blood glucose for at least 3 months was observed. Blood pressure and blood glucose levels were evaluated. First month measuring on Dario platform was used as study baseline. Clinical outcomes examined were blood pressure values, percentage of blood pressure categories, BGavg and high blood glucose readings (>250 mg/dL, >400 mg/dL) ratios.
Results: A group of 345 active users started at baseline with Hypertension stage 1, 2 or hypertensive crisis levels and measured following 3 months was evaluated.
| o | Normal levels increased from 6% to 12% and percentage of users with hypertension stage 2 decreased from 53% to 45% |
| o | 70% of the users (243 out of 345) improved their blood pressure levels in 8.4 mmHg Systolic and 6.2 mmHg on average (Systolic 134.2±12 vs.142.6±14; Diastolic 89.9 ±11 vs.83.7 ±8.7) |
| o | A group of 345 users measured with Dario their blood glucose in addition to blood pressure, 89% are type 2 and pre-diabetes - average age is 60.4. |
| o | For the group of 345 users a reduction of 33% (5.4% vs.8.0%) in high readings ratio (>250 mg/dL) and 67% (0.3%vs.0.9%) in severe events ratio (>400 mg/dL) was observed following six months on average. |
A subset of 114 users with diabetes in higher risk started with BG average >160 mg/dL improved their average blood glucose by 14% (207±47 vs.177±50 mg/dL) following six months compared to baseline.
In November 2020, we presented additional clinical study data at the Virtual Diabetes Technology Society (“DTS”) meeting.
The Effect of a Digital Therapeutic Platform on Glycemic Control in Adults above Age 65 with Type 2 Diabetes.
This study showed reduction of 13% blood glucose average in age group ≥65 (N=298) at six months sustained for 12 months, and reduction of 38.1% in high readings ratio (>250 mg/dL) in the ≥65 age group at six months and by 41.5% at 12 months.
In February 2021 we published in the first time in a peer-reviewed journal “Journal of Medical Internet research (JMIR) Diabetes”, the article:
“Role of Digital Engagement in Diabetes Care Beyond Measurement: Retrospective Cohort Study”
This study sheds light on the source of the association between user engagement with a diabetes tracking app and the clinical condition, highlighting the importance of within-person changes versus between-person differences. Our findings underscore the need for and provide a basis for a personalized approach to digital health.
24
Methods: This retrospective real-world analysis followed 998 people with type 2 diabetes who regularly tracked their blood glucose levels with the Dario digital therapeutics platform for chronic diseases. Subjects included “nontaggers” (users who rarely or never used app features to notice and track mealtime, food, exercise, mood, and location, n=585) and “taggers” (users who used these features, n=413) representing increased digital engagement. Within- and between-person variabilities in tagging behavior were disaggregated to reveal the association between tagging behavior and blood glucose levels. The associations between an individual’s tagging behavior in a given month and the monthly average blood glucose level in the following month were analyzed for quasicausal effects. A generalized mixed piecewise statistical framework was applied throughout.
Results: Analysis revealed significant improvement in the monthly average blood glucose level during the first 6 months (t=−10.01, P<.001), which was maintained during the following 6 months (t=−1.54, P=.12). Moreover, taggers demonstrated a significantly steeper improvement in the initial period relative to nontaggers (t=2.15, P=.03). Additional findings included a within-user quasicausal nonlinear link between tagging behavior and glucose control improvement with a 1-month lag. More specifically, increased tagging behavior in any given month resulted in a 43% improvement in glucose levels in the next month up to a person-specific average in tagging intensity (t=−11.02, P<.001). Above that within-person mean level of digital engagement, glucose levels remained stable but did not show additional improvement with increased tagging (t=0.82, P=.41). When assessed alongside within-person effects, between-person changes in tagging behavior were not associated with changes in monthly average glucose levels (t=1.30, P=.20).
In February 2021, we also presented two studies virtually in ATTD.
Impact of a Digital Intervention Engine on Diabetes Self-management
A digital diabetes platform has the potential to consistently interact with users, improve self-management and sustain among users who had not recently measured their blood glucose.
Methods: A retrospective study was performed on a population of 246 Dario active members who had not measured blood glucose for a 7-day period. 127 of these users were randomly assigned to a Test group and experienced a digital intervention flow, and the remaining 119 users were assigned to a Control group.
Results: Digital engagement levels were observed following 60 days in both groups. Differences between Test group and Control group were observed. In the Test group, the percent of users who measured blood glucose was significantly higher (P<0.001): 14% in first 30 days and 22% in 30-60 days; average number of measurements was 6% higher in the first 30 days and 17% in 30-60 days; number of interactions (e.g. logging fasting glucose) with the digital platform was 10% higher in first 30 days and 15% in 30-60 days. Difference in average days between measurements, defined as “recency” was 30% lower in the test group.
Impact of a Digital Therapeutic on Insulin Self-Management
The potential benefit of a digital diabetes management platform in the self-management required from insulin treated users, incorporating its use on a daily base, and sustaining behavioral change.
Methods: A retrospective study was performed on a population of 285 active Dario users (85% with type 2) under insulin therapy, that measured with Dario for at least three months and logged basal insulin usage. The group included 112 users whose starting average blood glucose >180 mg/dL. Among this group the average age was 50±20.8. The group also included 173 users whose starting average blood glucose was <180 mg/dL with average age 54±19.9. First month measuring on platform was used as study baseline.
Results: In the sub-group of 112 users the average amount of basal insulin increased by six units after three months (45 vs.39). Their fasting blood glucose was significantly reduced (9%) after three months (186±57 vs. 204±62) without change in hypoglycemia events ratio (<70 mg/dL) on average, and 15% of the users reduced their fasting average to <126 mg/dL. However, in the sub-group of 173 users, basal insulin usage and fasting glucose levels remained stable following three months.
25
In May 2021, a prospective pilot study was published in a peer reviewed journal “Journal of Diabetes Science and Technology”:
“Digital Therapeutics for Type 2 Diabetes: Incorporating Coaching Support and Validating Digital Monitoring”
The study suggests that a diabetes digital platform with real-time feedback and access to coaching improved diabetes outcome measures such as HbA1c with a reduction in GV. Importantly, we provide clinical validation for digital self-monitoring to deliver personalized care for patients with type 2 diabetes mellitus (“T2DM”). Future research should replicate our findings using a larger sample.
Methods: In this study (ClinicalTrials.gov: NCT04057248), 12 participants with baseline HbA1c >8.5% were provided with Dario digital therapeutic platform (connected blood glucose meter, test strips, mobile app and access to live certified diabetes care and education specialist (“CDCES”)). At both study enrollment and completion, participants completed blood testing and a satisfaction report. During 3-month intervention, participants tracked their blood glucose levels through the app and were routinely contacted by CDCES. Clinical outcomes and self reported data before and after intervention were compared.
Results:
| ● | Significant reduction in lab values such as HbA1C (2 points), Fasting Blood Glucose (18%) and BMI (10%) |
| ● | Statistically significant improvement in glucose variability (21%) |
| ● | Significant improvement in self-reported evaluation in weight and glucose control satisfaction |
| ● | Weekly engagement with CDCES predicted reduction of participants’ GV during the following week |
In June 2021, two studies were presented in 81th ADA:
Impact of Digital Intervention Tools on Engagement and Glycemic Outcomes
Product updates to digital platforms that guide on healthy eating and help users understand their glucose readings in context may assist users in improving the management of their diabetes.
Methods: A retrospective data evaluation study was performed on Dario TM members during the time before and after product modification. Digital engagement and clinical outcomes were measured on first to six months per each period to examine if habit formation was achieved.
Results: A group of total 9794 users who had enrolled in a membership for 6 months or longer was evaluated. The digital engagement was improved. The ratio of measurements logged with context (fasting, pre-meal, post-meal, bedtime) was increased significantly by 56% in the first month following product modification on average (51.3%. vs. 32.8%) (P<0.001). Differences in the level of digital engagement remained stable over a 6-month period. The average number of days between measurements, i.e. “recency” decreased by 21% on average (2.71 vs. 3.45). Average ratios of high readings (180-400 mg/dL) were reduced by 12% on average over six months.
Users with high-risk type 2 diabetes using a digital therapeutic platform experience a change in blood glucose levels
Digital diabetes platform has the potential to enhance self-care behaviors across socioeconomic statuses and among different language speakers.
Methods: A retrospective data evaluation study was performed on the DarioTM data base. A population (“high-risk users”) of all users with type 2 diabetes activated during 2017-2020 who took measurements with Dario in the first 2 months and who started with an average blood glucose above 180 mg/dL was evaluated. The ratios assessed were target range (70-180 mg/dL) and high blood glucose (>180 mg/dL) readings over a year. Socioeconomic status was matched by applying zip code data to census.gov data.
26
Results: For 11,101 users, the average ratio of target range readings (70-180 mg/dL) was significantly increased from 28.4% to 54.8% (P<0.001). Average high events ratio (>180 mg/dL) was significantly reduced from 71.3% to 44.4% over a full year usage (P<0.001). The change appeared in the earliest months and was maintained over a year. Average number of days between measurements, i.e., “recency” was 3.3 days. A subset of Spanish language app users (N=169) was also evaluated, and comparable trends were observed. Matching Census.gov data on study population showed that 20% of users resided in low-income zip codes, 70% in middle and 10% in upper income zip codes.
In August 2021, we presented additional clinical study data at the ADCES meeting.
Efficacy of a tailored digital intervention tool targeting patients with clustered recurrent high glucose readings
The potential benefit of implementing a real-time digital diabetes intervention journey to recognize episodes of high blood glucose measurement clusters and assist patients in improving self-management and clinical outcomes.
Methods: A retrospective data evaluation study was performed on a population of 3,609 users who experienced a cluster event of frequent high blood glucose levels above 250 mg/dL (>=4 times in 4 different days along 7 days) and measured with Dario at least one month before and after the event during 2021. A group of 1,084 users was assigned to a Test group who experienced a digital intervention flow with personalized messages via various channels. The remaining 2,525 users were assigned to a Control group. The clinical outcome examined was the monthly average of high blood glucose readings ratio calculated as the number of blood glucose measurements >250 mg/dL per total number of measurements in a month. This was measured during the event month and in the following month. T-test was used to compare the changes in high readings ratio in the Test group and Control group in the following month versus event month.
Results: A significant difference of 19% vs. control group (N=3,609), 18% for the group with type 2 (N=2307) and 42% for the group with type 2 non-insulin, in the reduction in average monthly ratio of high readings (above 250 mg/dL) per total blood glucose measurements in the following month. The results indicate personalized communications are effectively influencing positive lifestyle behavior change
A group of 454 users experienced the cluster event in a 6-month period before the digital journey was activated and after. A significant difference was observed after the digital journey versus before the digital journey in the following month’s change in high readings ratio (-8% vs. +5%; P-value <0.03)
In February 2022, another manuscript was published in “Journal of Medical Internet research (JMIR)”
“Blood Pressure Monitoring as a Digital Health Tool for Improving Diabetes Clinical Outcomes: Retrospective Real-world Study”
The results of this study shed light on the association between BG and BP levels and on the role of BP self-monitoring in diabetes management. Our findings also underscore the need and provide a basis for a comprehensive approach to understanding the mechanism of BP regulation associated with BG.
Methods: In this retrospective, real-world case-control study, we extracted the data of 269 people with T2D who tracked their BG levels using the Dario digital platform for a chronic condition. We analyzed the digital data of the users who, in addition to BG, monitored their BP using the same app (BP-monitoring [BPM] group, n=137) 6 months before and after starting their BP monitoring. Propensity score matching established a control group, no blood pressure monitoring (NBPM, n=132), matched on demographic and baseline clinical measures to the BPM group. A piecewise mixed model was used for analyzing the time trajectories of BG, BP, and their lagged association
Results: Analysis revealed a significant difference in BG time trajectories associated with BP monitoring in BPM and NBPM groups (t=–2.12, P=.03). The BPM group demonstrated BG reduction improvement in the monthly average BG levels during the first 6 months (t=–3.57, P<.001), while BG did not change for the NBPM group (t=0.39, P=.70). Both groups showed similarly stable BG time trajectories (B=0.98, t=1.16, P=.25) before starting the use of the BP-monitoring system. In addition, the BPM group showed a significant reduction in systolic (t=–6.42, P<.001) and diastolic (t=– 4.80, P<.001) BP during the first 6 months of BP monitoring.
27
Finally, BG levels were positively associated with systolic (B=0.24, t=2.77, P=.001) and diastolic (B=0.30, t=2.41, P=.02) BP.
In February 2022, we presented virtually in ATTD:
Impact of a digital therapeutic platform on weight loss and diabetes self-management
This observational study demonstrates the potential for digital platforms to durably improve diabetes and weight self-management among users with BMI of ≥30 kg/m2.
Methods: A retrospective study was performed on 715 Dario active members who started with a baseline BMI of ≥30 kg/m2 (51% male; 48% female; 80% with type 2 diabetes) and who recorded weight measurements for at least 12 months. Weight measurements and blood glucose readings were observed over 12 months.
Results: The total population of 715 users who participated in the study improved their weight level on average (p<0.05). Nearly two-thirds of the population improved their weight, with an average reduction of 7.4% (p<0.05) and an average reduction in BMI of 2.8 kg/m2. Over 30 percent achieved weight loss of 5% or greater over 12 months. A subset of 237 engaged users who started with BMI of ≥35 kg/m2 achieved weight loss of 5% over 12 months (p<0.05). The subgroup of 108 users that started at high-risk blood glucose levels (average blood glucose >180 mg/dL) reduced their weight by 4.9%, average blood glucose by 16.1% and high readings ratio by 38% over 12 months (p<0.05).
In June 2022, three retrospective data analysis studies were presented in 82th ADA:
Persons with high-risk diabetes, depression and stress using a Digital health platform experience improvement in glycemic management
The use of a multi-condition digital therapeutic platform may be associated with improved glucose management for persons with “high risk” glycemia who cope with depression and stress. The present study revealed that a digital multi-condition platform has the potential to enhance self-care behaviors among people with diabetes that suffer from stress and depression.
Methods: A retrospective data analysis on the DarioTM database of users who activated the mobile app during 2019-2021 and who self-reported stress and depression in the app questionnaire. Participants who took at least 5 measurements during their 1st and 12th months with Dario and who started with an average blood glucose >180 mg/dL were termed “high-risk”. A statistical analysis (T-test) was used to evaluate the differences in average blood glucose and high blood glucose (>180 mg/dL) readings ratio over a year.
Results: The high-risk group of 491 users significantly reduced their average blood glucose by 13% (204±60 vs. 234±55) (P<0.001). A subset of high-risk users with type 2 (N=379) was also evaluated and significantly reduced their average blood glucose by 14% (P<0.001) (201±66 vs. 233±53). Moreover, high glucose events ratio (>180 mg/dL) was significantly reduced from 72.6% to 55.8% over a full year of usage (P<0.001) (N=343).
Hypertension control among persons with diabetes using a self-management multicondition digital platform
A multi-condition digital therapeutic platform may promote behavioral modifications and result in sustainable improvements in both glycemic control and blood pressure levels. The study demonstrates an improvement in multiple chronic conditions (diabetes and hypertension) for people using one digital platform.
Methods: A retrospective data evaluation was performed on the Dario database. A population of active users who started with hypertension stage 1 (Systolic ≥130 mmHg or Diastolic ≥ 80mmHg) as their baseline since 2019 was identified. Blood glucose and blood pressure readings were assessed at first and sixth month of use. A subgroup of users who started at hypertension stage 2 was evaluated as well. A statistical analysis (T-test) was used to evaluate differences in Systolic and Diastolic pressures and average blood glucose.
28
Results:
For the 2554 users with diabetes and hypertension stage 1 and above, more than two thirds improved their systolic blood pressure by 13 mmHg (P<0.001; 144±14 to 131±13) and diastolic blood pressure by 8 mmHg (P<0.001; 91±12 to 83±10) over six months.
Additionally, a group of 38.7% (N=990) moved to a lower hypertensive stage (P<0.001) according to American Heart Association definitions.
The subset of 1367 users with stage 2 hypertension improved their systolic blood pressure from 150±12.4 to 141±15.2 mmHg on average and 43.9% (N=600) improved their blood pressure by more than 10 mmHg over six months (P<0.001).
The subgroup of 306 users who started at high-risk blood glucose levels significantly reduced their blood glucose average by 15% over 6 months (232.4±46 to 198±65 mg/dL) (P<0.001).
Blood Glucose Levels in High-Risk Type 2 Diabetes Users of a Digital Therapeutic Platform by Race/Ethnicity
Digital therapeutic platforms may promote behavior modification in high-risk patients with type 2 diabetes to create sustainable outcomes and allow the users to become more active participants in their chronic condition. The study revealed that the digital diabetes platform has the potential to enhance self-care behaviors across diverse populations.
Methods: A retrospective data study was performed on the Dario database. A group of Dario digital therapeutic users with type 2 diabetes that was active during 2019-2021 and took at least three blood glucose measurements in the first and 12th months was evaluated. The group started with average blood glucose above 180 mg/dL in the first month and reported Ethnicity in the app: White, Latino, Black, or Asian. The baseline was defined as the first month’s average blood glucose. A statistical analysis (Wilcoxon and Kruskal – Wallis tests) was used to evaluate the difference between groups in their average blood glucose levels over a year.
Results: A group of 1,000 users was analyzed, male 483 (48%) and female 517 (52%). Average blood glucose was significantly reduced in all users and per ethnic group over a year: All users by 14% (230±58 vs. 197±47) (p<0.001); White by 14% (229±58 vs. 197±47) ) (p<0.001); Latino by 15% (237±59 vs. 202±48) (p<0.001); Black by 15% (230±63 vs. 196±48) (p<0.001) and Asian by 15% (229±55 vs.195±43) (p<0.005).
No difference between the groups was found at 12th month(P=0.751).
In August 2022, we presented a retrospective data analysis study in the ADCES.
Digital therapeutic platforms improve blood glucose management across rural/nonrural groups.
The study supports the hypothesis that digital diabetes platforms have the potential to enhance self-care behaviors across challenging population from varied socioeconomic statuses in high-risk patients with T2DM.
Methods: A retrospective data study was performed on the Dario database. A group of T2DM “high-risk” users started with an average blood glucose of 180 mg/dL and above in the first month (baseline), was evaluated. The group of Dario users were active at 2019-2021 and took at least six blood glucose measurements in the first, 6th and 12th months. Members residency was defined as rural or nonrural based on whether their community was eligible to apply for Rural Health Grants by the Federal Office of Rural Health Policy (“FORHP”) (10). Nonparametric tests were used to evaluate the differences in average blood glucose levels over a year.
Results:
| ● | A group of 1333 users was analyzed with demographic characteristics as follows: Nonrural 1157 (87%) and Rural 176 (13%). |
29
| ● | The blood glucose average mg/dl was significantly reduced (Friedman tests) in all users and in each rural/nonrural group over a year: Nonrural reduced by 17% from T0 to T12 (228±59 vs. 190±47) (P<0.001); Rural reduced by 13% from T0 to T12 (224±60 vs. 196±51) (P<0.001). |
| ● | No significant difference between Rural/Nonrural groups was found at first, 6th and 12th months periods (Kruskal-Wallis, P=0.235/0.163/0.142 respectively). |
In August 2022, we published in the first-time two retrospective data analysis on behavioral health outcomes, Depression and Anxiety in the American Psychology Association (the “APA”).
Effectiveness of a Digital Behavioral Health Solution for Depression Symptoms
This study provides preliminary insights into the effectiveness of a digital chronic condition platform to facilitate symptom reduction in individuals screened for depression.
Methods: A retrospective data evaluation study was performed on the Dario database. The Patient Health Questionnaire-9 (“PHQ-9”) was utilized to screen for depression severity and track progress over time. The current sample is based on individuals who used the Dario Behavioral Health platform between 2019-2021, and completed at least two PHQ-9 assessments, one at baseline and the second between baseline and 12 weeks of platform utilization. Scores were calculated based on PHQ-9 scoring guidelines. Users were stratified based on severity as minimal-mild (score 0-9), mild-moderate and severe-moderate (10-19), or Severe (>=20).
Results: A group of 496 platform users (376 women, 108 men, 12 other) who completed two assessments of PHQ-9 was evaluated. The population included 269 users who started at minimal-mild severity and 227 who started at moderate or severe severity (175 moderate; 52 severe).The minimal-mild group mostly maintained at the same level of average PHQ-9 score post assessment. The moderate-severe group significantly improved their average PHQ-9 score (P<0.001).
A proportion of 72% of moderate-severe users showed improvement in their post PHQ-9 assessment and 38% of moderate-severe users reported scores in the minimal-mild range over the study period. Moreover, 44% of the moderate-severe population experienced a clinically significant score reduction (reduction of >5) in the full PHQ-9 over the study period. Out of 175 users who started at a moderate depression level, and 162 (93%) improved or maintained their level and out of 52 users who started at a severe depression level, 30 (58%) users reduced their level to moderate or minimal-mild.
Effectiveness of a Digital Behavioral Health Solution for Anxiety Symptoms
This study provides preliminary insights into the effectiveness of a digital chronic condition platform to facilitate symptom reduction in individuals screened for anxiety.
Methods: A retrospective data evaluation study was performed on the Dario database. The Generalized Anxiety Disorder Assessment (GAD-7) was utilized to screen for anxiety severity and track progress over time. The current sample is based on individuals who used the Dario Behavioral Health platform between 2019-2021, and completed at least two GAD-7 assessments, one at baseline and the second between baseline and 12 weeks of platform utilization. Scores were calculated based on GAD-7 scoring guidelines. Users were stratified based on severity as minimal-mild (score 0-9), moderate (10-14), or Severe (>=15).
Results:
| ● | The group of 523 platform users who completed two assessments of GAD-7 was evaluated; 297 users had baseline scores in the minimal-mild range and 226 were moderate or severe. The severe group significantly improved their average GAD-7 score (P<0.001; paired t-test). A proportion of 68% from the severe users improved their score, and 42% of the severe users reported scores in minimal-mild range over the study period (P<0.001). Moreover, 40% of the severe population experienced a clinically significant score reduction (reduction of >5) in GAD-7 over the study period. |
30
| ● | The minimal-mild group mostly maintained their levels and hence did not escalate to higher severity while using the care platform. Additionally, out of 100 users who started at a moderate anxiety level, 84 (84%) improved or maintained their level (P<0.001), and out of 126 users started at a severe anxiety level, 69 (55%) reduced their level to moderate or minimal-mild (P<0.001). |
In September 2022, a retrospective data analysis study was published in “International Association for the Study of Pain” large conference.
Pain level reduction mediated by perceived posture quality and training duration in patients using digital therapeutic biofeedback technology
The study sheds light on the nature of the linkage between posture biofeedback technology and pain reduction. Based on the findings of our mediation model constructed on a lagged association between training duration, perceived posture quality, and pain levels, we suggest that posture quality is a potential mechanism for posture training-related analgesia.
Methods: A retrospective real-world study examined 981 users who used the Dario posture trainer. Training duration, defined as the time the device is worn (hours), was recorded. This study utilized the Dario posture trainer, Upright in Dario Health, a wearable postural biofeedback device.
Results: Posture biofeedback training duration was significantly associated with pain levels (B=-0.0002, p<0.001). Also, the training duration predicted the following week’s posture quality (B=0.0004, p<0.001) and in turn posture quality predicted the following week’s pain. Finaly, posture quality mediated the effect of weekly training duration on the pain levels in two weeks.
In December 2022, the first manuscript was published in a peer-reviewed journal “Frontiers in Physiology” on retrospective data analysis on UpRight posture biofeedback platform.
The two-stage therapeutic effect of posture biofeedback training on back pain and the associated mechanism: A retrospective cohort study
The study findings provided a better understanding of the therapeutic dynamic during digital biofeedback intervention targeting pain, modeling the associated two-stage process. Moreover, the study sheds light on the biofeedback mechanism and may assist in developing a better therapeutic approach targeting perceived posture quality.
Methods: This retrospective real-world evidence study followed 981 users who used the UpRight posture biofeedback platform. Piecewise mixed models were used for modeling the two-stage trajectory of pain levels, perceived posture quality, and weekly training duration following an 8-week biofeedback training. Also, the mediation effect of perceived posture quality on the analgeic effect of training duration was tested using Monte Carlo simulations based on lagged effect mixed models.
Results: The analysis revealed significant pain level reduction of 50% (p <.0001) and posture quality improvement (p <.0001) during the first 4 weeks of the training, maintaining similar pain levels and perceived posture quality during the next 4 weeks. In addition, weekly training duration demonstrated an increase during the first 3 weeks (p <.001) and decreased during the next 5 weeks (p <.001). Moreover, training duration predicted following-week perceived posture quality (p <.001) and in turn perceived posture quality predicted following-week pain (p <.001) (p = 0.30). Finally, perceived posture quality mediated the effect of weekly training duration on the pain levels in 2 weeks (p <.0001).
In January 2023, a manuscript was published in a peer-reviewed journal “MDPI-Applied sciences” on retrospective data analysis glycemic management across Racial/Ethic groups:
31
Glycemic Management by a Digital Therapeutic Platform across Racial/Ethnic Groups: A Retrospective Cohort Study
Our findings demonstrate improvement in blood glucose levels in high-risk racial/ethnic minority populations with T2DM, showing that in this group of users who are motivated to use a digital device there appears to be no difference in the outcomes between racial/ethnic groups.
Methods: The retrospective real-world analysis followed a group of 1,000 people with Type 2 diabetes who used the Dario digital therapeutic platform over 12 months. Participants included in the study had a blood glucose average > 180 mg/dL (hyperglycemia, high-risk) in their first month. The differences between/within the groups’ average blood glucose level (Avg.bg) and glycemic variability were evaluated. Furthermore, three general linear models were constructed to predict the Avg.bg by the number of blood glucose measurements (Bgm) in Model 1 (with the moderator White persons ("WP")/people from racial and ethnic minority groups ("REM")) and by the frequency of measurements by months (F.m) within REM and WP in Model 2 and Model 3, respectively.
Results: The Avg.bg was significantly reduced in each group over a year with no differences between REM/WP users. Blood glucose measurements in Model 1 and frequency of measurements by months in Model 2 and Model 3 predicted the Avg.bg. Findings indicate a positive association between digital engagement and glycemia, with no differences between REM and WP participants.
In February 2023, a manuscript was published in a peer-reviewed journal “PAIN reports” on an analytical framework of retrospective data for personalized pain management using piecewise mixed-effects model trees:
Personalizing digital pain management with adapted machine learning approach
This analytical framework offers an opportunity for investigating the personalized efficacy of digital therapeutics for pain management, taking into account users' characteristics and boosting interpretability and can benefit from including more users' characteristics.
Methods: We demonstrated the implementation of the model with posture biofeedback training data of 3610 users collected during 8 weeks. The users reported their pain levels and posture quality. We developed personalized models for nonlinear time-related fluctuations of pain levels, posture quality, and weekly training duration using age, gender, and body mass index as potential moderating factors.
Results: Pain levels and posture quality demonstrated strong improvement during the first 3 weeks of the training, followed by a sustained pattern. The age of the users moderated the time fluctuations in pain levels, whereas age and gender interactively moderated the trajectories in the posture quality. Train duration increased during the first 3 weeks only for older users, whereas all the users decreased the training duration during the next 5 weeks.
In February 2023, we presented two additional clinical studies at the ATTD conference in Berlin, Germany:
Decrease in Hypoglycemia Events over Year in Older adults with Diabetes Monitoring with Digital Diabetes Management System
Older adults using a digital diabetes management platform have the potential to promote behavioral change and prevent hypoglycemia, demonstrating better glycemic outcome.
Methods: A retrospective data analysis was performed on the Dario database. A cohort of users aged 67 or older with Type 1 or Type 2 diabetes and using Dario over a year was evaluated. Average numbers of Hypoglycemia Level 1 (<70mg/dL) and Level 2 (<54 mg/dL) events as defined by the ADA were observed monthly and compared to baseline (first month).
Results: In the cohort of 2844 users, hypoglycemia level 1 events were reduced by 31% and 35% from baseline (0.54, 0.51 vs. 0.78) on average within 6 months and sustained over a year (p<0.05). Hypoglycemia level 2 events were reduced by 53% (0.08, 0.08 vs. 0.17) on average within 6 months and sustained over a year (<0.05). The ratio of hypoglycemia readings per total measurements significantly reduced as well. Subgroup analyses (1353 patients) of Dario users aged 67 or older with Type 1 or Type 2 using Insulin revealed a substantial reduction of severe hypoglycemia Level 2 of 42% (0.11 vs. 0.19) (p<0.05) and significant reduction in hypoglycemia events ratio as well over a year.
32
Impact of Digital Coaching on Diabetes
Self-management and Glycemic Outcomes for People with Type 2 Diabetes
Self-monitoring blood glucose and digital engagement have a mediating role in the effect of digital coaching on blood glucose levels. Coaching interactions have the potential to lead to behavioral change, which can positively impact the trajectory of a low-engaged user. By constantly learning from the user's data, the digital therapeutic platform can continually improve and adapt to the user's individual needs, leading to better outcomes. The study demonstrated that our platform is digital first where the human coaching intervention is provided to the right user at the right time and with the right intervention.
Methods: Retrospective data analysis was performed on a sample size of 712 users with type 2 diabetes with baseline average >180 mg/dL measured blood glucose over 12 months on the platform. 534 did not use a coaching service and 178 users interacted with a coach over a year.
Results: Monthly Average blood glucose level significantly reduced in both groups w and w/o coach interaction over a year (18% vs. 11%). Blood glucose measurements and digital engagement activities mediated the effect of coaching on average blood glucose levels. For individuals who are less inclined to measure their blood glucose, coaching can help establish regular monitoring habits and to understand the importance of monitoring their levels.
In June 2023, we presented three clinical studies at the 83th ADA conference:
Blood Glucose Reduction and Long-term Sustainability in High-risk Patients with Type 2 Diabetes Over Three Years Using a Digital Platform
The study showed that digital diabetes monitoring has the potential to enhance users’ awareness and affect and sustain glycemic control improvements over 3 years. Moreover, it is highly expected that engagement to app features in a chronic condition management may help users with type 2 diabetes consistently taking care of their lifestyle behaviors to maintain better clinical outcomes.
Methods: A retrospective data analysis was performed on users with T2D in high-risk (baseline BG avg>169 mg/dL in month1; equivalent to A1C 7.5) who activated between 2017-2020 and measured their BG using Dario platform over three consecutive years (at months 1,6,12,24 and 36). The outcomes assessed were average blood glucose (BG avg) and high BG readings ratio (>180 mg/dL). Digital engagement was assessed by additional parameters including measurement type (fasting/premeal/post meal/bedtime), carbohydrate intake, meal type and physical activity alongside glucose measurement.
Results: A group of 1,239 users significantly reduced their BG avg consistently over three years by 15.6% (179±55 vs. 212±42) (p<0.05) and high readings ratio by 39% (p<0.05). A subset of users who completed at least one engagement type following months 12, 24, and 36 (N=433) demonstrated significantly greater reductions versus the complementary group (N=806): BG avg by 18.8% (172±51 vs. 212±45) and high readings ratio by 45% (p<0.05).
Digital Platform Users Managing Three Chronic Conditions Diabetes, Hypertension and Overweight Experience Better Outcomes than those Who Manage One Condition Following Six Months
Monitoring several conditions on an integrated platform may have the potential to offer a greater means for a person with diabetes to effectively manage glycemia, engage with the treatment and improve outcomes. This study can illuminate the relative contribution of multi chronic conditions management driving behavioral change to successful diabetes outcomes.
Methods: A retrospective data analysis was performed on users who were active during 2019-2022 and measured blood glucose (BG) using Dario platform for at least six months. The test group included users who measured blood pressure (BP) and weight within 6 months from their first BG measurement; a matched control group of users who measured BG only in the first six months was generated. BG levels were assessed by average BG (BG avg) and BGMS engagement was assessed by counting BG measurements per month. High-risk users were defined as those with baseline BG avg>180mg/dL in Month 1.
33
Results: A total number of 17,108 users were included in the study: a test group of 2154 users measured BG, BP and Weight in the first six months on the platform and a control group of 14,954 users measured BG only in the first six months on the platform. The test group (N=2,154) demonstrated significantly greater (1.7-fold) engagement evaluated by higher number of BG measurements, than the control group (N=14,954) in Month 1 and Month 6 (p<0.01). A subgroup of the test group, 343 users at high-risk who measured 3 conditions BG, BP and weight was evaluated. A matched control group of 1,579 high-risk users was generated. The high-risk test group (N=343) demonstrated significantly greater reduction in BG avg versus the control subgroup (N=1579) measured BG only, after 6 months (17% vs.11%) (p<0.01). Moreover, the ratio of population that reduced their BG avg to lower than 180 mg/dL after 6 months (equivalent to A1C 8.0; HEDIS measure) was significantly higher in the test subgroup versus control subgroup in high-risk (by 30%; p<0.01).
Impact of a Digital Health Educational Feature on Engagement and Glycemic Outcomes
The present study demonstrates that by providing improved knowledge, increased motivation, and personalized learning digital health educational features can help users feel more empowered to manage their diabetes effectively.
Methods: A retrospective data evaluation study was performed on Dario TM members with type 2 (T2D) and prediabetes who experienced the educational feature. Engagement (blood glucose measurements and logging carbs to Dario mobile app) and glycemic outcomes were assessed three months pre-post experiencing the feature. Glycemic outcomes were assessed by average blood glucose (mg/dL) and glycemic variability (SD).
Results: A group of 994 people with type 2 and prediabetes who were active in the mobile app and measured their blood glucose three months before using the new feature and in the following three months after, was evaluated. The average number of blood glucose measurements increased by 34% (p<0.05) following the introduction of the new learning feature. A subgroup of 303 users with type 2 and prediabetes that reported depression in the app as a co-existing condition increased carbs logging event by 39%. In a subgroup of 234 high-risk users (baseline >180 mg/dL) the average blood glucose and glucose variability were significantly reduced by 13% and 11% on average, respectively (p<0.05).
In August 2023, we presented an additional clinical study on the benefits of engagement with a digital therapeutic for better clinical outcomes at the ADCES conference.
Users managing Diabetes with Large-scale Digital Therapeutics Platform Experience a Change in Blood Glucose and Engagement Over Two Years.
The present study demonstrates the benefits of engagement with a digital therapeutic platform for diabetes management in high-risk patients, demonstrating an improvement in glycemic outcomes and sustainment for a significant period.
Methods: A retrospective real-world data study was performed on the Dario database. The current sample is based on active users since 2019 with at least two months measurements for two years. Engagement was assessed by BG and weight measurements. Clinical outcomes assessed were average BG and high readings (>180mg/dL) ratios. Linear mixed effects models investigated changes in engagement and clinical outcomes.
Results: A population of 119,482 platform users was included, Age: 53 ± 15; Gender: 51% women. High-risk subgroup included 31,562 users with first month (baseline) average BG>180 mg/dL. Total users’ engagement increased significantly by 29% (14.3 to 18.5) over two years (p<0.001). High readings ratio (>180mg/dL) in high-risk subgroup decreased significantly by 38% (38.8% vs. 63.1%) over two years (p<0.001). The monthly average BG of the high-risk subgroup was reduced significantly by 16% (218.1mg/dL to 183.4mg/dL) over two years (p<0.001). A negative interaction effect was found with monthly engagements and the monthly average BG, as users with increased engagements (+1 SD) demonstrated stronger reductions in monthly average BG.
In September 2023, a manuscript was published in a peer-reviewed journal “Journal of Medical Internet research (JMIR) Diabetes” on the contribution of specific digital engagement tools to mental health conditions – Depression and Anxiety.
34
Specifying the Efficacy of Digital Therapeutic Tools for Depression and Anxiety: Retrospective, 2-Cohort, Real-World Analysis
This study demonstrated general improvement followed by a period of stability of depression and anxiety symptoms associated with cognitive behavioral therapy–based digital intervention. Interestingly, engagement with a coaching session but not a breathing exercise was associated with a reduction in depression symptoms. Moreover, breathing exercise but not engagement with a coaching session was associated with a reduction of anxiety symptoms. These findings emphasize the importance of using a personalized approach to behavioral health during digital health interventions.
Methods: Depression and general anxiety symptoms were evaluated in real-world data cohorts using the digital health platform for digital intervention and monitoring change. This retrospective real-world analysis of users on a mobile platform–based treatment followed two cohorts of people: (1) users who started with moderate levels of depression and completed at least 2 depression assessments (n=519) and (2) users who started with moderate levels of anxiety and completed at least 2 anxiety assessments (n=474). Levels of depression (Patient Health Questionnaire-9) and anxiety (Generalized Anxiety Disorder-7) were tracked throughout the first 16 weeks. A piecewise mixed-effects model was applied to model the trajectories of the Patient Health Questionnaire-9 and the Generalized Anxiety Disorder-7 mean scores in 2 segments (1-6 weeks and 7-16 weeks). Finally, simple slope analysis was used for the interpretation of the interactions probing the moderators: coaching sessions and breathing exercises in both depression and anxiety cohorts.
Results: Analysis revealed a significant decrease in depression symptoms (β=–.37, 95% CI –0.46 to 0.28; P≤.001) during the period of weeks 1-6 of app use, which was maintained during the period of 7-16 weeks. Coach interaction significantly moderated the reduction in depression symptoms during the period of weeks 1-6 (β=–.03, 95% CI –0.05 to –0.001; P=.02). A significant decrease in anxiety symptoms (β=–.41, 95% CI –0.50 to –0.33; P≤.001) was revealed during the period of 1-6 weeks, which was maintained during the period of 7-16 weeks. Breathing exercises significantly moderated the reduction in anxiety symptoms during the period of 1-6 weeks (β=–.07, 95% CI –0.14 to –0.01; P=.04).
In November 2023, we presented additional clinical study data at the virtual DTS meeting that investigated the association between monthly aggregated blood glucose measurements and walking activity (number of steps).
Non-linear Association between Blood Glucose Levels and Walking in an Integrated Digital Health Platform for Diabetes Management
This study sheds light on the importance of walking in diabetes management. Our findings highlight the potential of digital health platforms in promoting physical activity using a diabetes tracking app to improve clinical outcomes. By integrating the information that counts steps people with diabetes can access a single platform that helps them feel supported in their daily diabetes care and lifestyle management.
Methods:
In this retrospective real-world study, a cohort of 989 platform users with Type 2 diabetes and pre-diabetes, who regularly tracked their blood glucose levels for 12 months using the Dario digital platform was evaluated. The association between blood glucose levels and the number of steps was examined over time. A piecewise linear mixed effects model was applied to test the trajectories over time of monthly average blood glucose and monthly average number of steps a day in two time periods defined by previous research3 as well as to test non-linear association between them.
Results: The sample included 437 (44%) women, 546 (55%) men and 6 (0.6%) others. The average age was 62.5 (SD ±12.7) and the average BMI was 32.5 (SD ±6.9). The baseline average BG was 142.5 mg/dL (SD ±37.7). 808 (82%) users have type 2 diabetes and 181 (18%) have pre-diabetes. Analysis revealed that during the first 4 months there is a positive trend of monthly average steps (B=.02, P<.001) while there is a negative trend of blood glucose levels (B= -2.00, P<.001) in the same time frame. Significant improvement in monthly average blood glucose was observed in users with at least 400 steps a day (B= -2.26, P<.001), while for those with less than 400 steps a day there was no significant change.
Certain clinical studies were published by Sanofi on our data in 2023.
The first poster was published at ISPOR May 2023.
35
Comparison of All-Cause Healthcare Resource Utilization Rates between Patients with Type 2 Diabetes Who Use a Digital Diabetes Solution Versus Non-Users: A 12-Month Retrospective Cohort Study
In this retrospective cohort study, utilizing Dario Diabetes Solution (DDS) demonstrated a significantly greater reduction in all-cause HCRU and inpatient hospitalization rates during 12-month follow-up compared with non-users receiving usual care.
Methods: This retrospective cohort study (January 2017–April 2021) included adults (≥18 years) with T2DM receiving anti-diabetic medication(s) with ≥1 inpatient or ≥2 outpatient visits ≥30 days apart during baseline period. Baseline was 12 months before index date (first DDS registration [users] or first medical encounter in the quarter with medical claims [non-users]); follow-up was 12 months. User and non-user cohorts were matched 1:3 using exact and propensity score matching. Analysis included patients with access to care 12 months pre- and post-index date. Primary endpoint was all-cause HCRU (inpatient hospitalizations + ER visits) rates during follow-up. Data were analyzed using a generalized linear model with negative binomial distribution.
Results: Of 9779 patients, DDS users (n=2445) and non-users (n=7334) were matched; mean±SD age was 58.2±10.6 and 58.3±12.5 years, respectively. At 12 months, mean (95% CI) all-cause HCRU rate (inpatient hospitalization + ER visits) was 0.475 (0.438–0.516) and 0.524 (0.500–0.549) events/year for users and non-users, respectively. Users had 9.3% lower HCRU rate compared with non-users (incidence rate ratio [IRR], 0.907 [0.826–0.996]; P=0.04). Mean all-cause inpatient hospitalization rate was 0.166 (0.147–0.186) and 0.216 (0.203–0.230) events/year for users and non-users, respectively. Users had 23.5% lower inpatient hospitalization incident rate versus non-users (IRR, 0.765 [0.671–0.873]; P<0.0001); ER visit rates were similar in both cohorts (IRR, 1.01 [0.907–1.125]; P=0.86).
Three studies were presented by Sanofi at the 83th ADA conference:
Impact of Digital Diabetes Solution on Glycemic Control in Adults with Type 2 Diabetes Mellitus in the United States—A Retrospective Cohort Study
The study showed adults with uncontrolled T2DM using DDS had better outcomes at 6 months, with more significant HbA1c reductions than matched nonusers across various BL HbA1c levels, showing incremental improvements to usual care.
Methods: This retrospective cohort study included adults with T2DM with a baseline (BL) HbA1c ≥7% who used DDS (users) or received usual care (nonusers) between January 1, 2017, to October 31, 2021. BL period was 1 year before index date (first DDS registration [users] or first claim date in the quarter [nonusers]); follow-up period was 6 months. DDS user and nonuser cohorts were matched 1:3 using exact and propensity score matching. Primary endpoint was change in HbA1c from BL to 6 months, with subgroup analyses of patients (pts) with BL HbA1c >7.5%, >8%, >9%, and ≥1% drop from BL. Difference-in-difference results are reported using least squares (LS) means from linear models.
Results: The study included 568 DDS users and 1699 nonusers. For all 2267 pts, mean ± SD age was 57.5±11.3 years and HbA1c was 9.14±1.83% at BL. At 6 months, LS mean difference between groups was −0.23% (mean HbA1c change vs BL: users, −1.02% [95% CI, −1.15, −0.89]; nonusers, −0.79% [−0.87, −0.71]; P=0.004). HbA1c drop ≥1% from BL to 6 months was achieved by 47% of users vs 37% nonusers (difference: 10%; P<0.001). Subgroup analysis at all BL HbA1c showed users achieved more significant HbA1c reductions vs nonusers (P<0.05). For pts with BL HbA1c >9%, the healthcare effectiveness data and information set performance measure, mean difference between groups was -0.47% (users, -2.25% [-2.50, -1.99]; nonusers, -1.78% [-1.92, -1.63]; P=0.002).
Use of Digital Diabetes Solution Is Associated with Improved Glycemic Control without Increased Risk of Severe Hypoglycemia in Adults with Type 2 Diabetes Mellitus in the United States—Retrospective Cohort Study
In this retrospective study, a larger proportion of adults with uncontrolled T2DM (BL HbA1c ≥8%) achieved HbA1c <8% after using DDS vs nonusers, with no increased risk of severe hypoglycemia (SH).
Methods: This retrospective cohort analysis included adults with T2DM who used DDS (users) and nonusers from 1JAN2017 to 31OCT2021. BL period was 1 year before index date (users, first DDS registration; nonusers, first claim date in the quarter); follow up was 6 months.
36
DDS users and nonusers were sequentially matched 1:3 using exact and propensity score matching. Secondary endpoints included SH (event requiring medical intervention) rates for all patients (pts) and for pts with BL HbA1c ≥8% who achieved predefined target HbA1c <8%. SH incidence in users vs nonusers was examined.
Results: Overall cohort included 568 DDS users and 1699 nonusers: mean age, 57.5±11.3 years; mean BL HbA1c, 9.14±1.83%; oral antidiabetic drugs, 51%; insulin, 6%. BL SH was rare (users, 7/568; nonusers, 12/1699). There were 387 users and 1089 nonusers with BL HbA1c ≥8% (mean BL HbA1c, 10.0±1.7%). In this subgroup, HbA1c <8% was achieved by 9% more DDS users (174/387 [45%]) vs nonusers (393/1089 [36%]) at 6 months; P=0.002. At 6 months, overall SH rate was 38.8 (users) vs 10.6 (nonusers) events/1,000 pts per year (incidence rate ratio, 0.9; P=0.9). No observed increase in SH risk was associated with DDS in this population. SH was rare in pts who achieved HbA1c <8% (users, 1/174; nonusers, 3/393).
Effect of a Digital Diabetes Solution on All-Cause Health Care Resource Utilization Charges for Patients with Type 2 Diabetes—A Retrospective Cohort Study
In this retrospective cohort study, pts with T2DM who utilized DDS incurred significantly lower all-cause HCRU and OV charges vs nonusers.
Methods: This retrospective cohort study (pt selection window: 1JAN2017 - 31APR2021) included adults (≥18 years) with T2DM receiving antidiabetic medication(s) with ≥1 inpatient or ≥2 outpatient visits ≥30 days apart during the baseline (BL) period. BL was 1 year before index date (users, 1st DDS registration; nonusers, 1st claim date in the quarter); follow up was 1 year. User and nonuser cohorts were matched 1:3 using exact and propensity score matching. Study assessed HCRU rates and charges. A 2-part gamma distribution model was used to determine 1) likelihood (odds ratio, OR) of users vs nonusers to incur charges, then 2) total charges per patient per year (PPPY) including all-cause HCRU and office visit (OV) charges.
Results: Of 9779 pts, 2445 DDS users and 7334 nonusers were matched; mean age, 58.2±10.6 and 58.3±12.5 years, respectively. At 1 year, users were 9% less likely to incur all-cause HCRU charges vs nonusers (OR, 0.91; P=0.07). All-cause HCRU charges were 26% lower for users vs nonusers (P<0.0001; $12552 [adjusted] PPPY savings). Users were more likely to incur all-cause OV charges vs nonusers (P=0.04). However, users had 19% lower all-cause OV charges vs nonusers (P<0.0001; $1790 [adjusted] PPPY savings). The percentages of pts who incurred T2DM related HCRU charges were low (users, 3.1%; nonusers, 3.0%).
Three posters were published at AMCP-Nexus, in October 2023:
The impact of a digital health technology on healthcare quality measures and clinical outcomes in adults with type 2 diabetes mellitus
In this retrospective study, in adults with T2DM, a greater proportion of DDS users with BL A1c greater than or equal to 8% achieved A1c less than 8% and a smaller proportion with BL A1c greater than 9% remained greater than 9%, compared with a matched cohort of nonusers, without increasing the risk of severe hypoglycemia. DDS offers improved quality outcomes based on HEDIS A1c criteria.
Methods: Cohorts of 568 DDS users and 1,699 nonusers were compared. Inclusion criteria were as follows: adults with a diagnosis of T2DM, receiving at least 1 diabetes medication, A1c greater than or equal to 7.0%, and not using a continuous glucose monitor between January 1, 2017, and October 31, 2021. The primary endpoint was a change in A1c from baseline (BL) during a 180-day follow-up period, with subgroup analyses of people with BL A1c greater than 7.5%, greater than 8%, and greater than 9%. Exploratory analyses were conducted to evaluate whether DDS use could facilitate a lowering of BL A1c from greater than or equal to 8% to less than 8% and from greater than 9% to less than 9% in adults with T2DM. Secondary endpoints included severe hypoglycemia (event requiring medical intervention) rates for all included DDS users and nonusers and for those with BL A1c greater than or equal to 8% who achieved a predefined target A1c of less than 8%.
37
Results: Overall, DDS user and nonuser cohorts were well matched , including by payer type (70% commercial and 18% Medicare for both groups). Among 387 DDS users with BL A1c greater than or equal to 8%, 174 (45%) had A1c less than 8% during follow-up, compared with 393 (36%) of 1,089 nonusers (P=0.0021). In a subgroup analysis of people with BL A1c greater than 9%, among 237 DDS users, 86 (36%) had follow-up A1c greater than 9%, compared with 347 (49%) of 713 nonusers (P=0.0009). There was no increase in rates of severe hypoglycemia comparing groups (P>0.4).
Association between more frequent engagement with the Dario Diabetes Solution, a digital health technology, and a reduction in HbA1c in adults with type 2 diabetes mellitus
Higher DDS engagement was associated with a significantly greater reduction in A1c in adults with T2DM. The highest engagement was in the first 2 months of follow-up and correlated with the greatest reductions in HbA1c. Engagement decreased over time during follow-up. Further research is needed to assess additional interventions that may sustain engagement over time.
Methods: This analysis included DDS users receiving at least 1 diabetes medication, with A1c ≥7.0%, and not using a continuous glucose monitor between January 1, 2017, and October 31, 2021. Baseline (BL) was 1 year before the index date (first registration for DDS), with follow-up of 180 days from the index date. Engagement activities were collected via the DDS app. Engagement activity was measured in active days (ie, number of days when a user performed any engagement activity). Ten DDS engagement activities were evaluated, including measuring BG, tagging (timing of BG measurement or meal type), food logging, and sharing logbook. Associations between overall DDS engagement and change in A1c were analyzed over 180 days using a linear regression method, and associations were evaluated at 60- day intervals over those 180 days. Individual components of engagement were also analyzed.
Results: 568 DDS users were included. At BL, their mean age was 57.3 years (SD ±11.3) and mean A1c was 9.14±1.78%. Median engagement activity was 65 active days of 180. Each day with any DDS engagement activity was associated with a 0.01% change in A1c (P<0.0001). Users in the most engaged quartile had 5× greater reduction in A1c than the least engaged quartile. Individual engagement activities with significant associations with reduced A1c were BG measurement, tagging (meal type), and inputting insulin dose.
A retrospective cohort study comparing health care resource utilization, length of stay and 30-day readmissions in users and non-users of a digital diabetes health intervention for patients with type 2 diabetes mellitus.
In this real-world analysis, all-cause HCRU, inpatient, and 30-day readmission rates were significantly lower among DDS users vs nonusers (9%, 23%, and 36%, respectively), and LOS was significantly shorter (1.6 days).
Methods: In this retrospective cohort study, adults (aged >18 years) receiving therapy for type 2 diabetes who used DDS from January 1, 2017, to April 30, 2021, were identified; index date was defined as the date of first DDS registration. Anonymized DDS user data were linked to patient-level claims data within the Symphony Health Integrated Dataverse. The DDS cohort was matched 1:3 using exact and propensity score matching to a nonuser cohort from the Symphony Health Integrated Dataverse with medical claims for type 2 diabetes mellitus during the study period. For nonusers, the index date was the first medical claim date in the matched quarter. All patients were required to have 12 months’ post-index follow-up and to have at least 2 outpatient claims (>30 days apart) or at least 1 inpatient claim within this period. This analysis compared all-cause HCRU rates (inpatient + emergency department), LOS, and 30- day readmission rates in DDS users and nonusers. Negative binomial generalized linear models adjusting for baseline rates were used to generate incidence rates (per personyear) and incidence rate ratios (IRRs) and corresponding 95% Cis for all-cause HCRU and 30-day readmission rates. LOS was compared between groups using a two-sample t-test.
Results: DDS users (n=2,445) and nonusers (n=7,334) were well matched (mean+SD age: 58.2+10.6 vs 58.3+12.5 years; sex, 53.3% female for both). At follow-up, the all-cause HCRU rate was 0.47 (95% CI= 0.44-0.52) in DDS users and 0.52 (95% CI= 0.50-0.55) in nonusers (IRR=0.91; 95% CI=0.83-1.00; P=0.041). The mean all-cause inpatient event rate was 0.17 (95% CI= 0.15-0.19) in DDS users and 0.22 (95% CI= 0.20-0.23) in nonusers (IRR= 0.77; 95% CI= 0.67-0.87; P<0.0001); there was no significant difference in emergency department visits. DDS users with an inpatient event (users, n=327; nonusers, n=1,196) had a shorter LOS (7.2 vs 8.8 days; P=0.017) and lower 30-day readmission rate (IRR= 0.64; 95% CI= 0.45-0.92; P=0.014) vs nonusers.
38
Additional poster was presented at ISPOR EU, in November 2023.
The Impact of Patient Engagement with a Digital Diabetes Solution on All-Cause Healthcare Resource Utilization Rates and Charges
Overall engagement with DDS was associated with a reduction in all-cause HCRU and lower likelihood of incurring HCRU-related charges.
Methods: Patient-level claims data for adult DDS users (>18 years; registered 01-Jan-2017 to 30-Apr-2021) receiving therapy for T2DM were retrieved from Symphony Health Integrated Dataverse. All patients had >2 outpatient (>30 days apart) or >1 inpatient visit within 12 months prior to index (date of first DDS registration). This analysis reports all-cause HCRU (inpatient + emergency room visits) and odds of incurring HCRU-related charges >$0 based on user engagement with any of 10 DDS activity metrics (‘components’ of activity metrics: measuring BG, measuring blood pressure, measuring weight, tagging [BG timing and meal type], food logging, inputting insulin dose, recording physical activity, sharing logbooks, reading articles, coach interaction). Overall engagement was defined as number of days any component was used within 12 months post-index. All-cause HCRU rates and charges per 100 days of engagement were adjusted for baseline values with a negative binomial generalized linear model and logistic model, respectively; incidence rate ratios (IRR) for HCRU and odds ratios (OR) for charges >$0 were derived.
Results: We identified 2445 DDS users (mean+SD age, 58.2+10.6 years; 53.3% female). Overall engagement (use of any DDS component) was associated with a 10% reduction in all-cause HCRU (IRR: 0.90; p=0.0048). Overall DDS engagement was associated with 15% decreased odds of incurring all-cause HCRU-related charges >$0 (OR: 0.85; p=0.0004).
Another poster was presented at Diabetes Technology Society at Nov 2023, the abstract will be published in JDST early 2024. The title is: “Impact of a Digital Diabetes Solution on Medication Adherence in Adults in the United States with Type 2 Diabetes Mellitus”.
In March 2024, we presented two clinical studies at the ATTD conference in Florence, Italy:
Impact of a digital health intervention on awareness of vaccination against influenza among adults with diabetes
leveraging digital health technology to deliver knowledge and information can enhance influenza vaccination rates in diabetes population to reduce public health.
Methods: A real-world implementation study of a 6-month digital diabetes intervention was performed in U.S Dario users with diabetes. A group of 64,952 users were randomly sorted to: real-world group (RW) received messages designed in the digital platform; A RCT group received messages as designed in previous published RCT; Control group (C) received no intervention. Difference in self-reported vaccination rates and flu risk awareness levels were tested.
Results: A total of 8431 users (13% response rate) with diabetes typ1 or type 2 reported vaccination status. No compensation was offered during digital intervention. After a 6-month intervention, a numerical increase was observed in the real-world group (N=3068) versus the RCT (N=3505) and the Control group (N=1858) (71.9% vs. 71% and 70.5%, respectively) (p=0.521). Awareness to the risk in getting Flu in diabetes and the impact of the content materials were significantly higher (p<0.005) in the real-world group vs RCT (1.2-fold) and Control (1.35-fold).
Impact of digital therapeutic platform on blood glucose and weight levels in prediabetes population
This study demonstrates the potential of digital platforms to improve self-management and clinical outcomes among users with prediabetes, thus assisting in lowering the risk of developing type 2 diabetes.
Methods: A retrospective data analysis was performed on the prediabetes population using the platform in 2020-2023, with at least 2-months measuring BG or weight. Monthly Avg.BG, weight and digital activities were analyzed versus baseline (month 1). A linear mixed model tree was applied testing potential moderators.
39
Results: A group of 6853 users with prediabetes who managed their BG on the platform was included (Age 55.4±13; 53% females). In the subgroup with baseline ≥154 mg/dL (A1c 7.0) (N=819) a significant reduction of 15% in AvgBG was observed after 6 months and maintained along the year (198.9 to 168.2 mg/dL, p<0.001). In a subgroup with baseline ≥140 mg/dL (A1c 6.5) (N=1282) 66% have improved AvgBG and 40% reduced AvgBG <140mg/dL (p<0.001). A significant reduction of 6.3% in weight was observed in users with baseline BMI≥30 (N=1186, p<0.001). Meal tagging activity moderated the trajectory of weight improvement, monthly average >5 meal taggings had a statistically significant and stronger reduction in the average weight (-0.11, p<0.001) compared to ≤5 monthly average meal tagging (-0.08, p<0.001).
In February 2024 we published in a peer-reviewed journal “Journal of Medical Internet research (JMIR)”, the article:
The Impact of Digital Self-Monitoring of Weight on Improving Diabetes Clinical Outcomes: Quasi-Randomized Study
This study highlights the substantial benefits of self-monitoring of weight in managing BG levels in patients with diabetes, facilitated by a digital health platform, and advocates for the integration of digital self-monitoring tools in chronic disease management. We also provide initial evidence of testing the underlying mechanisms associated with BG management, underscoring the potential role of patient empowerment.
Methods: In this retrospective, real-world quasi-randomized study, 50% of the individuals who regularly used the weight monitoring (“WM”) feature were propensity score matched with 50% of the users who did not use the weight monitoring feature based on demographic and clinical characteristics. All the patients were diagnosed with T2D and tracked their BG levels. We analyzed monthly aggregated data 6 months before and after starting their weight monitoring. A piecewise mixed model was used for analyzing the time trajectories of BG and weight as well as exploring the disaggregation effect of between- and within-patient lagged effects of weight on BG.
Results: The WM group exhibited a significant reduction in BG levels post intervention (P<.001), whereas the non-monitoring group showed no significant changes (P=.59), and both groups showed no differences in BG pattern before the intervention (P=.59). Furthermore, the WM group achieved a meaningful decrease in BMI (P<.001). Finally, both within-patient (P<.001) and between-patient (P=.008) weight variability was positively associated with BG levels. However, 1-month lagged back BMI was not associated with BG levels (P=.36).
Three studies were presented at the 84th ADA conference in June 2024:
Digital platform users taking GLP-1 and managing Diabetes adapt lifestyle behavior change over twelve months
The study showed how lifestyle monitoring components in a metabolic digital disease management solution have the potential to drive behavior change and enhance clinical care among users taking GLP-1 medications. These lifestyle changes may be important in driving outcomes in users taking GLP-1 medications.
Methods: This study followed behavior change in users utilizing a new “medication cabinet” feature for medication logging and were identified as existing GLP-1 users, versus non-GLP-1 users. The purpose of the study was to investigate monthly activities over twelve months of using a digital platform in GLP-1 users versus non-GLP-1 Metformin users. A retrospective data analysis was performed on early users of “medication cabinet” with T2D and prediabetes who measured their blood glucose (BG) at months 1,3,6,9,12. The GLP-1 group included users who reported taking GLP-1 and non-GLP-1 group reported taking metformin. A piecewise mixed effects model was applied to follow monthly average BG and lifestyle digital activities (logging meal/reference, carbohydrate intake and calories burned).
Results: A cohort of 107 users was evaluated: GLP-1 (N=35) and non GLP-1 users (N=72). The groups were statistically equivalent in baseline average BG (150 vs. 152 mg/dL) and monthly average BG measurements (31.3 vs 30.5). GLP-1 users showed significant reduction in BG at months 1-5 (p=0.02) maintained throughout the year. GLP-1 and non-GLP-1 users both demonstrated a statistically significant increase in lifestyle activities during their first three months (p<0.001, p=0.02 respectively) maintained for a year. Moreover, mixed-effects model revealed a statistically significant increase in monthly average weight measurements over the year among GLP-1 users (p<0.001).
40
Deep sleep interaction with blood glucose levels in people using an integrated digital health platform for diabetes management
A digital health platform that aims to help users adjust deep sleep and manage blood glucose levels can be a powerful tool in promoting overall health and well-being. Sleep quality assessment connected with blood glucose monitoring on one single platform may provide actionable insights and help users understand the impact of sleep on their metabolic health.
Methods: A retrospective data analysis included people with T2D and prediabetes who used iPhone and integrated data into Dario digital health platform during Dec 2022-Dec 2023. Weekly aggregations of average BG and time of deep sleep were calculated. A mixed model analysis was used to determine whether the weekly avg BG can be predicted by weekly avg duration of deep sleep over time.
Results: A cohort of 417 users with type 2 and prediabetes was evaluated (Age 60.4±12.3; female 50.3%). A significant difference between weekly avg BG levels in different durations of weekly avg deep sleep was demonstrated by the model (B=-.00, CI 95% -0.00- -0.00, p=.029). Moreover, simple slope analysis showed that users with increased weekly avg deep sleep duration have significantly stronger reductions in avg BG (B=-.26, CI 95% -0.50- -0.03, p=.03) compared to users with low weekly avg deep sleep duration (B=-.13, CI 95% -0.37- 0.12, p=.31).
Sustained reduction in blood glucose levels reflecting diabetes remission in people with type 2 diabetes using a digital health platform
This study exhibits the potential role of digital health technology to change individuals' behaviors during management of T2D and support patients to achieve remission.
Methods: A retrospective data analysis was performed on users with T2D who have reported not using insulin and were active on Dario platform >6 months during 2018-2024, measured their BG in the first month and >3 measurements in the last month. Monthly Avg.BG and percentage of high readings (>180 mg/dL) versus baseline (first month) were evaluated Paired sample t-test was used for testing differences in AvgBG. A linear mixed-effects model was applied to evaluate the changes in AvgBG and high-readings percentage over time. Finally, a simple slope analysis was used for the interpretation of the interaction of AvgBG and high-readings percentage with lifestyle activities.
Results: A group of 7240 users was evaluated (Age 53.8 (SD ±24.6); 41% females). A ratio of 44% (3169/7240) reduced their last monthly AvgBG to <140 mg/dL (equivalent to estimated A1c of 6.5). A sustained AvgBG of <140 mg/dL for at least three consecutive months was observed in 70% (2223/3169) of the users reflecting diabetes remission. The AvgBG was reduced by 15% vs. baseline (P<.001) in this group.
In addition, users at high-risk with a baseline AvgBG ≥183 mg/dL demonstrated significant reductions in AvgBG and high-readings percentage in their last month moderated by the number of monthly lifestyle activities (logging carbs, meals and physical activities) as revealed by the model (B=-.00, P=.02 and B=-.00, P=.002 respectively).
In August 2024 we published in a peer-reviewed journal “Frontiers in Endocrinology” the article:
Walking away from depression: the mediating role of walking activity in depression impacting blood glucose levels of people with diabetes or prediabetes
This study shows how regular walking may reduce the negative impact of depression on BG levels in people with T2D. Our findings advocate for the integration of walking activity into treatment protocols as a cost-effective, accessible intervention strategy to improve glycemic management and depressive symptoms in this population.
Methods: A cohort of 989 users with T2D and prediabetes, who regularly tracked their steps levels and BG levels for 12 months using the Dario digital health platform was evaluated. The mediating role of the monthly average number of steps on the relationship between the self-reported depression status and lagged monthly average BG was assessed. Additionally, the association between monthly walking activity and monthly average BG was tested using a piecewise linear mixed effects model Results: Users with self-reported depression demonstrated increased BG levels compared to users without depression (B=8.00, P=.01).
41
The association between depression and monthly average number of steps was significant (B=-.27, P<.005) and monthly average number of steps significantly predicted the following months’ average BG (B=-.81, P=.001), adjusting for depression. The monthly average number of steps significantly mediated the effect of self-reported depression on the following month’s average BG (M=.22, P<.005). Further sensitivity analysis demonstrated model robustness over various periods. Finally, non-linear dynamics of walking activity over time was validated using unseen data showing a decrease in monthly average BG for users with over an average of 400 steps per day (B=-1.87, P<.01).
In March 2024 two studies were published in the behavioral health field.
An RCT study was published on Happify for Teens (Twill) we published in a peer-reviewed journal “Journal of Medical Internet research (JMIR)”, the article:
Effects of a Digital Mental Health Intervention on Perceived Stress and Rumination in Adolescents Aged 13 to 17 Years: Randomized Controlled Trial.
Happify for Teens was effective at reducing perceived stress, rumination, and loneliness among adolescents over 12 weeks when compared to a waitlist control group. Our data reveal the potential benefits of DMHIs for adolescents, which may present a more scalable, destigmatized, and cost-effective alternative to school-based programs.
Methods: This was a 12-week, 2-arm decentralized randomized controlled trial of adolescents aged 13 to 17 years who presented with elevated levels of perceived stress and brooding. Participants were randomly assigned to engage with a self-guided DMHI (Happify for Teens) or to a waitlist control. Participants assigned to the intervention group were given access to the program for 12 weeks. Happify for Teens consists of various evidence-based activities drawn from therapeutic modalities such as cognitive behavioral therapy, positive psychology, and mindfulness, which are then organized into several programs targeting specific areas of concern (eg, Stress Buster 101). Participants in the waitlist control received access to this product for 12 weeks upon completing the study. Participants in both groups completed measures of perceived stress, brooding, optimism, sleep disturbance, and loneliness at baseline, 4 weeks, 8 weeks, and 12 weeks. Changes in outcomes between the intervention and waitlist control groups were assessed using repeated-measures multilevel models.
Results: Of the 303 participants included in data analyses, 132 were assigned to the intervention and 171 to the waitlist. There were significantly greater improvements in the intervention condition for perceived stress (intervention: B=–1.50; 95% CI –1.82 to –1.19; P<.001 and control: B=–0.09; 95% CI –0.44 to 0.26; P=.61), brooding (intervention: B=–0.84; 95% CI –1.00 to –0.68; P<.001 and control: B=–0.30; 95% CI –0.47 to –0.12; P=.001), and loneliness (intervention: B=–0.96; 95% CI –1.2 to –0.73; P<.001 and control: B=–0.38; 95% CI: –0.64 to –0.12; P=.005) over the 12-week study period. Changes in optimism and sleep disturbance were not significantly different across groups (Ps≥.096).
A retrospective data analysis study published in a peer-reviewed journal “JMIR Formative Research” in the article:
Association of Digital Engagement with Relaxation Tools and Stress Level Reduction: Retrospective Cohort Study
This study sheds light on the association between stress level reduction and specific components of engagement in a digital health app, breathing exercises, and cognitive behavioral therapy–based video sessions. Our findings provide a basis for further investigation of current and moderating factors that contribute to the personalization of digital intervention. In addition, results may aid in developing a more comprehensive understanding of how digital intervention tools work for mental health and for whom they are most effective.
Methods: Stress levels were evaluated in a real-world data cohort using a behavioral health app for digital intervention and monitoring change. This retrospective real-world analysis of users on a mobile platform–based treatment followed users (N=490) who started with moderate and above levels of stress and completed at least 2 stress assessments. The levels of stress were tracked throughout the first 10 weeks. A piecewise mixed effects model was applied to model the trajectories of weekly stress mean scores in 2-time segments (1-6 weeks and 6-10 weeks). Next, a simple slope analysis was used for interpreting interactions probing the moderators: breathing exercises and video sessions.
42
Piecewise mixed-effects models were also used to model the trajectories of specific perceived stress item rates in the stress questionnaire in the 2 segments (1-6 weeks and 6-10 weeks) and whether they are moderated by the relaxation engagements. Simple slope analysis was also used here for the interpretation of the interactions.
Results: Analysis revealed a significant decrease in stress symptoms (β=–.25; 95% CI –0.32 to –0.17; P<.001) during the period of 1-6 weeks of app use that was maintained during the period of 6-10 weeks. Breathing exercises significantly moderated the reduction in stress symptoms during the period of 1-6 weeks (β=–.07; 95% CI –0.13 to –0.01; P=.03), while engagement in digital video sessions did not moderate stress scores. Engagement in digital video sessions, as well as breathing exercises, significantly moderated the reduction in perceived sense of burden and lack of productivity during weeks 1-6 and remained stable during weeks 6-10 on both items.
43
Government Regulation
The principal markets that we have initially targeted for Dario are the United States, Canada, the European Union, Australia, and New Zealand. The following is an overview of the regulatory regimes in these jurisdictions.
United States Regulation Generally
In the United States, medical devices are subject to extensive regulatory control at the federal level by the FDA under the Federal Food, Drug, and Cosmetic Act (“FDCA”) and its implementing regulations. Under Section 201(h) of the FDCA, a medical device is an article, which does not achieve its intended purpose through chemical action or metabolism in or on the body and, among other things, is intended (i) for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment or prevention of disease, in man or other animals or (ii) to affect the structure or any function of the body of man or other animals. The Dario Blood Glucose Monitoring System is classified as a medical device and subject to regulation by numerous agencies and legislative bodies, including the FDA and its foreign counterparts. FDA regulations govern, among other things, device design and development, nonclinical and clinical testing, manufacturing, packaging, labeling, storage, pre-market clearance or approval, establishment registration and device listing, advertising and promotion, sales and distribution, recalls and field actions, servicing and post-market surveillance. A number of U.S. states also impose licensing and compliance regimes on companies that manufacture or distribute prescription devices into or within the state.
The FDA classifies medical devices into one of three classes. Classification of a device is important because the class to which a device is assigned determines, among other things, the necessity and type of FDA review required prior to marketing the device. Unless an exemption applies, each medical device commercially distributed in the United States will require a clearance through the pre-market notification (or 510(k)) process, De Novo classification, or pre-market approval (“PMA”) from the FDA.
Class I devices are those for which reasonable assurance of safety and effectiveness can be maintained through adherence to general controls, which include compliance with the applicable portions of the FDA’s Quality System Regulation (“QSR”), as well as regulations requiring facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials. The Class I designation also applies to devices for which there is insufficient information to determine that general controls are sufficient to provide reasonable assurance of the safety and effectiveness of the device or to establish special controls to provide such assurance, but that are not life-supporting or life-sustaining or for a use which is of substantial importance in preventing impairment of human health, and that do not present a potential, unreasonable risk of illness or injury.
Class II devices those for which general controls alone are insufficient to provide reasonable assurance of safety and effectiveness and there is sufficient information to establish “special controls.” These special controls can include performance standards, post-market surveillance requirements, patient registries and FDA guidance documents describing device-specific special controls. While most Class I devices are exempt from the pre-market notification requirement, most Class II devices require a pre-market notification prior to commercialization in the United States; however, the FDA has the authority to exempt Class II devices from the pre-market notification requirement under certain circumstances.
Class III devices are intended to be life sustaining or life supporting, devices that are implantable, devices that present a potential unreasonable risk of harm or are of substantial importance in preventing impairment of health, and devices that are not substantially equivalent to other lawfully marketed devices and for which safety and effectiveness cannot be assured solely by the general controls and special controls.
Pre-market Notification (510(k)) Clearance Process. Manufacturers of most Class II devices must submit premarket notifications to the FDA under Section 510(k) of the FDCA (21 U.S.C. § 360(k)) to obtain the necessary authorization to market or commercially distribute such devices. To obtain 510(k) clearance, manufacturers must submit to the FDA adequate information demonstrating that the proposed device is “substantially equivalent” to a “predicate device” that is already on the market. A predicate device is a legally marketed device that is not subject to PMA, meaning, (i) a device that was legally marketed prior to May 28, 1976, or pre amendments device, and for which a PMA is not required, (ii) a device that has been reclassified from Class III to Class II or I, or (iii) a device that was found substantially equivalent through the 510(k) process. The FDA typically issues a decision within 90 days of receipt of a 510(k)
44
submission but may stop the review clock for up to 180 days to request that the applicant respond to the agency’s requests for additional information about the proposed device. If the FDA agrees that the device is substantially equivalent to the predicate device identified by the applicant in a premarket notification submission, the agency will grant 510(k) clearance for the new device, permitting the applicant to commercialize the device. Premarket notifications are subject to user fees, unless a specific exemption applies.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could even require a premarket application approval. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If the FDA disagrees with the determination, the agency may retroactively require the manufacturer to seek 510(k) clearance or premarket application approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or premarket application approval is obtained.
De Novo Classification. This device regulatory pathway allows a manufacturer whose novel device is automatically classified into Class III to request that the FDA classify such device as Class I or Class II based on evidence that the device in fact presents low or moderate risk, instead of following the typical Class III device pathway requiring the submission and approval of a PMA application. If the manufacturer seeks reclassification into Class II, the classification request must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. The FDA typically issues a decision within 150 days of receipt on a De Novo classification request but, as with 510(k) submissions, may stop the review clock for up to 180 days to request that the applicant respond to the agency’s requests for additional information. If FDA grants the De Novo request, the device may be legally marketed in the United States. However, the FDA may reject the classification request if the agency identifies a suitable legally marketed predicate device that provides a reasonable basis for review of substantial equivalence or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and adequate special controls cannot be developed. De Novo classification requests are subject to user fees, unless a specific exemption applies.
Premarket Approval (PMA). The PMA process is more demanding than the 510(k) and De Novo classification processes. For a PMA, the manufacturer must demonstrate through extensive data, including data from nonclinical studies and one or more clinical trials, that the device is safe and effective for its proposed indication. The PMA application must also contain a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing, and proposed labeling. Following receipt of a PMA submission, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FDCA to complete its review and determine whether the proposed device can be approved for commercialization, although in practice, PMA reviews often take significantly longer, and it can take up to several years for the FDA to issue a final decision. Before approving a PMA, the FDA generally also performs an on-site inspection of manufacturing facilities for the product to ensure compliance with the QSR.
The FDA may refer any PMA submission, including applications for novel device candidates or device candidates that present difficult questions of safety or efficacy, to an advisory committee for review. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and, if so, under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it considers such recommendations when making final decisions on approval.
If the FDA’s evaluation of the PMA application and inspection of the manufacturing facility is favorable, the FDA may issue an approval order authorizing commercial marketing of the device, or an “approvable letter,” which usually contains a number of conditions that must be met in order to secure final approval of the PMA. When and if those conditions have been met to the satisfaction of the FDA, the agency will issue a PMA approval order, subject to the conditions of approval and the limitations established in the approval order. If the FDA’s evaluation of a PMA application or manufacturing facility is not favorable, the FDA will deny approval of the PMA or issue a “not approvable letter.” The FDA may also determine that additional studies are necessary, in which case the PMA approval may be delayed for several months or years while such additional studies are conducted, and data is submitted in an amendment to the PMA. The PMA process can be expensive, uncertain and lengthy, and each PMA submission is subject to a substantial user fee unless a specific exemption applies.
45
PMA approval may also be granted with post-approval requirements such as the need for additional patient follow-up or requirements to conduct additional clinical trials.
After approval of a premarket application, a new PMA application or PMA supplement may be required in the event of a modification to the labeling, manufacturing process, specifications, materials or design of the device. PMA supplements often require submission of the same type of information as an initial PMA application, except that the supplements are limited to information needed to support any changes from the device covered by the approved PMA application and may or may not require as extensive clinical data or the convening of an advisory committee. The PMA pathway is much more costly, lengthy and uncertain.
Breakthrough Devices. The Breakthrough Devices Program is a voluntary program intended to expedite the review, development, assessment and review of certain medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human diseases or conditions for which no approved or cleared treatment exists or that offer significant advantages over existing approved or cleared alternatives. Submissions for devices designated as Breakthrough Devices will receive priority review, meaning that the review of the submission is placed at the top of the appropriate review queue and receives additional review resources, as needed and available. Although Breakthrough Device designation or access to any other expedited program may expedite the development or clearance/authorization/approval process, it does not change the standards for clearance/authorization/approval. Designation for any expedited review procedure does not ensure that we will ultimately obtain regulatory clearance or approval for such a product. Our acquisition of Twill added a PDT that received a Breakthrough Device Designation.
In general, software that is intended for a medical purpose, whether it is included with a hardware device or is standalone software, is considered a medical device and subject to the same regulatory pathways described above, as long as it meets the definition of a “device” in Section 201(h) of the FDCA. However, the 21st Century Cures Act, which became law in December 2016, expressly excluded from the FDCA’s device definition some software functions, such as software to support healthcare facility administration, general wellness software, electronic health records and certain clinical decision support software. In September 2019, the FDA published a revised guidance, General Wellness: Policy for Low Risk Devices, describing its approach to general wellness products, including software, which states that the agency does not intend to examine the compliance of low risk general wellness products, as long as they are intended to (i) maintain or encourage general health or healthy activity and do not make any claims relating to specific diseases or conditions, or (ii) encourage a healthy lifestyle to help reduce the risk or impact of or help the user live well with certain chronic diseases or conditions where there is an established connection between a healthy lifestyle and the disease or condition. In addition, the FDA has published the Policy for Device Software Functions and Mobile Medical Applications guidance, which describes the agency’s approach to regulating software device functions, and in particular, the types of software that are the focus regulatory enforcement, under enforcement discretion, or not considered medical devices. Our Smart Diabetes Management Solution software and wireless blood pressure monitor software are considered medical devices under the FDCA and such software products have received the required marketing authorizations and are listed with FDA. We believe that our other current software products either are not intended for a medical purpose or meet the applicable criteria to be considered low risk products the FDA may exercise enforcement discretion, choosing not to enforce certain regulatory requirements.
Unlike regulatory exemptions from the 510(k) pre-market notification process, which still require compliance with general controls like registration and quality management systems, enforcement discretion means the FDA does not intend to enforce these controls for qualifying low-risk devices.
The FDA issued a Final Rule on February 2, 2024, describing amendments to harmonize the QSR with the 2016 edition of the International Organization for Standardization publication Medical Devices: Quality management systems—Requirements for regulatory purposes (ISO 13485:2016), which will become effective on February 2, 2026. The harmonization process is not expected to have a significant impact on the quality system compliance operations of device manufacturers because most requirements described in the QSR correspond to requirements set forth in ISO 13485:2016. However, device manufacturers will likely need to revise certain quality system procedures to ensure compliance with the harmonized regulations and any failure to make such revisions or adapt to the harmonized regulations, once they become effective, may result in observations of noncompliance during facility inspections by the FDA or comparable regulatory authorities.
46
European and Non-European Regulation Generally
Sales of medical devices outside the United States are subject to foreign regulatory requirements that vary widely from country to country. These laws and regulations range from simple product registration requirements in some countries to complex clearance and production controls in others. As a result, the processes and time periods required to obtain foreign marketing clearance may be longer or shorter than those necessary to obtain FDA clearance.
For example, the European Union (EU) has adopted specific directives and subsequently regulations regulating the design, manufacture, clinical investigations, labeling, conformity assessment, post-market surveillance and vigilance reporting for medical devices. The EU rules described below are generally applicable in the European Economic Area (EEA). Other countries, such as Switzerland, have entered into Mutual Recognition Agreements and allow the marketing of medical devices that meet EU requirements.
The EU presently requires that all medical products be certified to meet the EU’s safety and performance requirements for such products and bear a CE mark, an international symbol of adherence to quality assurance standards and demonstrated clinical effectiveness. Prior to May 26, 2021, all medical devices placed on the EU market had to meet the relevant essential requirements laid down in Council Directive 93/42/EEC, or the Medical Device Directive (MDD), and if applicable, the Council Directive 90/385/EEC, or the Active Implantable Medical Device Directive (AIMD), or for in vitro diagnostic devices, Council Directive 98/79/EC, or the In Vitro Diagnostic Directive (IVDD). Active Implantable Medical Devices (AIMD) are defined as medical devices that rely on a source of electrical energy or any source of power other than that generated by the body, which are totally or partially introduced, either surgically or medically, into the human body and intended to remain after the procedure.
On May 26, 2021, the Medical Devices Regulation, EU 2017/745, (MDR) became effective, repealing and replacing the MDD and the AIMDD. The MDR is directly applicable in all EU member states. The MDR changed several aspects of the regulatory framework for medical device marketing in Europe in order to increase regulatory oversight of all medical devices marketed in the EU (which, in turn, increased the costs, time and requirements to place innovative or high-risk medical devices on the European market). The MDR among other things (i) strengthens the rules on placing devices on the market and reinforces post-market surveillance; (ii) establishes explicit provisions on a manufacturer’s responsibilities for the follow-up of the quality, performance and safety; (iii) improves the traceability of medical devices through a unique identification number; and (iv) sets up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU.
An overarching requirement under the MDR is that any device must be designed and manufactured in such a way that it will not compromise the clinical condition or safety of patients, or the safety and health of users and others. In addition, the device must meet the performance specifications intended by the manufacturer and be designed, manufactured and packaged in a suitable manner. To that effect, the European Commission has adopted various standards applicable to medical devices. These include standards governing common requirements, such as sterilization and safety of medical electrical equipment and product standards for certain types of medical devices. There are also harmonized standards relating to design and manufacture. While not mandatory, compliance with harmonized standards is a way for manufacturers to demonstrate that products comply with relevant EU legislation.
To demonstrate compliance with the General Safety and Performance Requirements (GSPRs) set forth in the MDR, medical device manufacturers must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Conformity assessment procedures require an assessment of the technical documentation, including the device description, the design stages, the manufacturing process, available clinical evidence, literature data for the product, and post-market experience in respect of similar products already marketed. Except for low-risk medical devices (Class I non-sterile, non-measuring devices), where the manufacturer can self-declare the conformity of its products with the GSPRs (except for any parts which relate to sterility or metrology), a conformity assessment procedure requires the intervention of a Notified Body. Notified Bodies are independent organizations designated by EU member states to assess the conformity of devices before being placed on the market. A Notified Body typically audits and examines a product’s technical dossiers and the manufacturer’s quality management system (which must, in particular, comply with ISO 13485). If satisfied that the AIMD or other medical device conforms to the relevant GSPRs, the Notified Body issues a certificate of conformity, which the manufacturer uses as a basis for its own declaration of conformity.
47
The manufacturer may then apply the CE-Mark to the device, allowing the device to be legally marketed throughout the EU.
Notified Body certificates of conformity are valid for a fixed duration (which shall not exceed five years). Throughout the term of the certificate, the manufacturer will be subject to periodic surveillance audits to verify continued compliance with the applicable requirements. In particular, there will be a new audit by the Notified Body before it renews the relevant certificate(s).
Devices lawfully placed on the market pursuant to the MDD and the AIMDD prior to May 26, 2021 could initially continue to be made available on the market or put into service until May 26, 2025. Nevertheless, the European Parliament recently adopted legislation to extend this transitional period to give manufacturers more time to switch from the previously applicable provisions to the new certification requirements for medical devices as laid down by the MDR. For high risk, class III and class IIb implantable devices the transitional period is extended until December 31, 2027. For medium and low risk, class IIb devices and class IIa, Im, Is and Ir devices the transition period is extended until December 31, 2028.
In May 2022, the IVDD was replaced by the In Vitro Diagnostic Device Regulation, EU 2017/746, (IVDR) and given a 5-year transition period until its full implementation on May 26, 2022. Unlike the IVDD, the IVDR has binding legal force throughout every EU member state. The major goal of the IVDR was to standardize diagnostic procedures within the EU, increase reliability of diagnostic analysis and enhance patient safety. Under the IVDR, IVDs are subject to additional legal regulatory requirements. Among other things, the IVDR introduces a new risk-based classification system and requirements for conformity assessments. Under the IVDR and subsequent amendments, IVDs already certified by a Notified Body under the IVDD may remain on the market until May 26, 2025, and IVDs certified without the involvement of a Notified Body may be placed on, or remain in, the market for up to three additional years (until May 26, 2028) depending on the classification of the IVD. The manufacturers of such devices remaining on the market must comply with specific requirements in the IVDR, but ultimately, such products, as with all new IVDs, will have to undergo the IVDR’s conformity assessment procedures. In addition, the IVDR imposes additional requirements relating to post-market surveillance and submission of post-market performance follow-up reports.
In September 2013, we obtained ISO 13485 certification for our quality management system and CE Mark certification to market Dario, and in May 2015 Dario was cleared to fulfill the criteria according to EN ISO 15197:2013. The granting of the CE Mark allows Dario to be marketed and sold in 32 countries across Europe as well as in certain other countries worldwide. On November 21, 2014, MDSS, our European Authorized Representative, completed the registration of the Dario Blood Glucose Monitoring System with the German Authority as required by Article 10 of Directive 98/79/EC on in vitro diagnostic medical devices. We commenced an initial soft launch of the product in Europe in 2014, created initial demand for the product and established brand awareness and marketing techniques to reach our target market with a goal to continue expansion to new markets and territories.
We achieved regulatory clearance to market Dario in other countries that do not rely on the CE Mark. To date, the non-CE Mark jurisdictions which we have begun to market Dario include the United States, New Zealand, Canada, and Australia.
To the extent that we seek to market our product in other non-CE Mark countries in the future, we will be required to comply with the applicable regulatory requirements in each such country. Such regulatory requirements vary by country, and complying with such regulations may require substantial time and effort. As a result, no assurance can be given that we will be able to satisfy the regulatory requirements to sell our products in any such country. If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension of clinical trials, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, and criminal prosecution.
Clinical Trials
Clinical trials are almost always required to support PMA applications and are sometimes required to support 510(k) and De Novo classification submissions. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s good clinical practice (GCP), regulations, including the investigational device exemption (IDE) regulations that govern investigational device labeling, prohibit promotion of investigational devices, and specify recordkeeping, reporting and monitoring responsibilities of trial sponsors and investigators.
48
If the device presents a “significant risk,” as defined by the FDA, the agency requires the device sponsor to submit an IDE application to the FDA, which must become effective prior to commencing human clinical studies. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a patient. An IDE application must be supported by appropriate data, such as animal and laboratory test results, showing that the device has a safety profile appropriate for human testing and that the trial protocol is scientifically sound. The IDE will automatically become effective 30 days after receipt by the FDA, unless the FDA expressly approves or denies the application in writing or notifies the sponsor that the investigation is on hold and may not begin until the sponsor provides supplemental information about the investigation that satisfies the agency’s concerns. If the FDA determines that there are deficiencies or other concerns with an IDE that require modification of the trial, the FDA may permit a clinical trial to proceed under a conditional approval or the sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. In addition, the trial must be approved by, and conducted under the oversight of, an institutional review board, or IRB, for each clinical site. If the device presents a non-significant risk to the patient according to criteria established by FDA as part of the IDE regulations, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate authorization from the FDA, but must still comply with abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements.
As part of its clinical trial oversight responsibilities, an IRB must review and approve, among other things, the trial protocol and informed consent information to be provided to clinical trial subjects. An IRB must operate in compliance with FDA regulations. Information about certain clinical studies, including details of the protocol and eventually trial results, also must be submitted within specific timeframes to the National Institutes of Health (NIH), for public dissemination on the ClinicalTrials.gov data registry. Information related to the product, patient population, phase of investigation, trial sites and investigators and other aspects of the clinical trial is made public as part of the registration of the clinical trial. Sponsors are also obligated to disclose the results of their clinical studies after completion. Disclosure of the results of these studies can be delayed in some cases for up to two years after the date of completion of the trial. Failure to timely register a covered clinical study or to submit study results as provided for in the law can give rise to civil monetary penalties and also prevent the non-compliant party from receiving future grant funds from the federal government. The U.S. Department of Health and Human Services’ Final Rule and NIH’s corresponding policy on ClinicalTrials.gov registration and reporting requirements became effective in 2017, and the government has brought enforcement actions against non-compliant clinical trial sponsors.
Progress reports detailing the results of the clinical studies must be submitted at least annually to the FDA and more frequently if unanticipated serious adverse events, or SAEs, occur. The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the clinical protocol, GCP, or other IRB requirements or if the investigational product has been associated with unexpected serious harm to patients.
In the Consolidated Appropriations Act for 2023, Congress amended the FDCA to require the sponsor of any pivotal clinical trial that will be used to demonstrate the safety and effectiveness of a medical device marketing authorization submission to develop a diversity action plan for such trial, and if submission of an IDE application is required, to submit such diversity action plan to the FDA. The action plan must include the sponsor’s diversity goals for enrollment, as well as a rationale for the goals and a description of how the sponsor will meet them. The FDA may grant a waiver for some or all of the requirements for a diversity action plan. It is unknown at this time how the diversity action plan may affect device pivotal clinical trial planning and timing or what specific information FDA will expect in such plans, but if FDA objects to a sponsor’s diversity action plan and requires the sponsor to amend the plan or take other actions, it may delay trial initiation.
There is no assurance that a clinical study at any given site will progress as anticipated; the interim results of a study may not be satisfactory leading the sponsor or others to terminate the study, there may be an insufficient number of patients who qualify for the study or who agree to participate in the study or the investigator at the site may have priorities other than the study. Also, there can be no assurance that the clinical study will provide sufficient evidence to assure regulatory authorities that the product is safe, effective and performs as intended as a prerequisite for granting market clearance.
49
See “Clinical Trials” above for clinical trials performed to date.
Post-Clearance Matters
Even if the FDA or other non-US regulatory authorities approve or clear a device, they may limit its intended uses in such a way that manufacturing and distributing the device may not be commercially feasible. After clearance or approval to market is given, the FDA and foreign regulatory agencies, upon the occurrence of certain events, are authorized under various circumstances to withdraw the clearance or approval or require changes to a device, its manufacturing process or its labeling or additional proof that regulatory requirements have been met.
A manufacturer of a device approved through the premarket approval application process is not permitted to make changes to the device which affects its safety or effectiveness without first submitting a supplement application to its premarket approval application and obtaining FDA clearance for that supplement. In some instances, the FDA may require a clinical trial to support a supplement application. A manufacturer of a device cleared through a 510(k) submission or a 510(k)+ “de-novo” submission must submit another premarket notification if it intends to make a change or modification in the device that could significantly affect the safety or effectiveness of the device, such as a significant change or modification in design, material, chemical composition, energy source or manufacturing process. Any change in the intended uses of a premarket approval application device or a 510(k) device requires an approval supplement or cleared premarket notification. Exported devices are subject to the regulatory requirements of each country to which the device is exported, as well as certain FDA export requirements.
Ongoing Regulation by FDA
Even after a device receives clearance or approval and is placed on the market, numerous regulatory requirements apply. These include:
| ● | establishment registration and device listing; |
| ● | QSR requirements, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation, and other quality assurance procedures during all phases of the product life-cycle; |
| ● | labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or “off-label” uses, and other requirements related to promotional activities; |
| ● | medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; |
| ● | corrections, removal and recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; |
| ● | the FDA’s recall authority, whereby the agency can order device manufacturers to recall from the market a product that is in violation of governing laws and regulations; and |
| ● | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
The medical device reporting requirements also extend to healthcare facilities that use medical devices in providing care to patients, or “device user facilities,” which include hospitals, ambulatory surgical facilities, nursing homes, outpatient diagnostic facilities, or outpatient treatment facilities, but not physician offices. A device user facility must report any device-related death to both the FDA and the device manufacturer, or any device-related serious injury to the manufacturer (or, if the manufacturer is unknown, to the FDA) within 10 days of the event. Device user facilities are not required to report device malfunctions that would likely cause or contribute to death or serious injury if the malfunction were to recur but may voluntarily report such malfunctions through MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program.
50
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions: warning letters, fines, injunctions, civil or criminal penalties, recall or seizure of our current or future products, operating restrictions, partial suspension or total shutdown of production, refusing our request for 510(k) clearance or PMA approval of new products, rescinding previously granted 510(k) clearances or withdrawing previously granted PMA approvals.
We may be subject to announced and unannounced inspections by the FDA, and these inspections may include the manufacturing facilities of our subcontractors. If, as a result of these inspections, the FDA determines that our or our subcontractor’s equipment, facilities, laboratories or processes do not comply with applicable FDA regulations and conditions of product clearance, the FDA may seek civil, criminal or administrative sanctions and/or remedies against us, including the suspension of our manufacturing and selling operations.
Ongoing Regulation by International Regulators
International sales of medical devices are subject to foreign government regulations, which may vary substantially from country to country.
In order to maintain the right to affix the CE Mark to sell medical devices in the European Union, periodic surveillance audits of the company premises and, if needed, at major subcontractors’ premises must to be carried out by a Notified Body. In addition, all manufacturers placing medical devices into the market in the EU must comply with the EU medical device vigilance system. Under this system, serious incidents must be reported to the relevant authorities of the EU member states, and manufacturers are required to take Field Safety Corrective Actions (“FSCAs”), to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. A serious incident is defined as any malfunction or deterioration in the characteristics or performance of a device made available on the market, including use-error due to ergonomic features, as well as any inadequacy in the information supplied by the manufacturer and any undesirable side-effect that directly or indirectly led, might have led or might lead to death, temporary or permanent serious deterioration of health state, or a serious public health threat. An FSCA can include the withdrawal of the device from the market, or a recall thereof. FSCAs must be communicated by the manufacturer or its legal representative to the users of the device through Field Safety Notices.
The advertising and promotion of medical devices is subject to some general principles set forth by EU directives. Directive 2006/114/EC concerning misleading and comparative advertising and Directive 2005/29/EC on unfair commercial practices. While the aforementioned directives are not specific to the advertising of medical devices, the provisions of national law transposing them must also be complied with and contain general rules, for example requiring that advertisements are evidenced, balanced and not misleading. Specific requirements are defined at national level. EU member states laws related to the advertising and promotion of medical devices, which vary between jurisdictions, may limit or restrict the advertising and promotion of products to the general public and may impose limitations on promotional activities with healthcare professionals.
Many EU member states have adopted specific anti-gift statutes that further limit commercial practices for medical devices, in particular vis-à-vis healthcare professionals and organizations. Additionally, there has been a recent trend of increased regulation of payments and transfers of value provided to healthcare professionals or entities. In addition, many EU member states have adopted national “Sunshine Acts” which impose reporting and transparency requirements (often on an annual basis), similar to the requirements in the United States, on medical device manufacturers. Certain countries also mandate implementation of commercial compliance programs.
Failure to comply with applicable regulatory requirements can result in enforcement action by the applicable regulatory authorities, which may include any of the following sanctions: fines, injunctions, civil or criminal penalties, recall or seizure of our current or future products, operating restrictions, partial suspension or total shutdown of production, refusing our request for renewing marketing authorization of our products or for granting marketing authorization for new products.
51
Federal Trade Commission Regulatory Oversight
Our advertising for our products in the United States is subject to federal truth-in-advertising laws enforced by the Federal Trade Commission (“FTC”), as well as comparable state consumer protection laws. Under the Federal Trade Commission Act, or FTC Act, the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. The FTC has very broad enforcement authority, and failure to abide by the substantive requirements of the FTC Act and other consumer protection laws can result in administrative or judicial penalties, including civil penalties, injunctions affecting the manner in which we would be able to market services or products in the future, or criminal prosecution.
Federal Communications Commission Regulation
The Dario Blood Glucose Monitoring System includes a wireless radio frequency transmitter and receiver and, therefore, is subject to equipment authorization requirements in the United States. The Federal Communications Commission (“FCC”) requires advance clearance of all radio frequency devices before they can be imported into, sold or marketed in the United States. These clearances ensure that the proposed products comply with FCC radio frequency emission and power level standards and will not cause interference.
State Licensure Requirements
Several U.S. states require that Durable Medical Equipment (“DME”) providers be licensed in order to sell products to patients in that state. Certain of these states require that DME providers maintain an in-state location. Some states also require a device manufacturer or distributor to obtain a license in order to distribute prescription medical devices to customers in such states. If these rules are determined to be applicable to us and if we were found to be noncompliant, we could lose our licensure in that state, which could prohibit us from selling our current or future products to patients in that state.
Other U.S. Healthcare Laws and Regulations
We must comply with various U.S. federal and state laws, rules and regulations pertaining to healthcare fraud and abuse, including anti-kickback laws and physician self-referral laws, rules and regulations. Violations of the fraud and abuse laws are punishable by criminal and civil sanctions, including, in some instances, exclusion from participation in federal and state healthcare programs, including Medicare and Medicaid. These laws include the following:
| ● | The federal Anti-Kickback Statute (AKS) prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal health care program such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the AKS or specific intent to violate it to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the AKS constitutes a false or fraudulent claim for purposes of the federal False Claims Act (“FCA”) or federal civil money penalties statute; |
| ● | The federal civil and criminal false claims laws and civil monetary penalty laws, including the federal FCA, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, false or fraudulent claims for payment to, or approval by Medicare, Medicaid, or other federal healthcare programs, knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim or an obligation to pay or transmit money to the federal government, or knowingly concealing or knowingly and improperly avoiding or decreasing or concealing an obligation to pay money to the federal government. Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payers if they are deemed to “cause” the submission of false or fraudulent claims. The FCA also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery; |
52
| ● | The Civil Monetary Penalties Law, which prohibits, among other things, the offering or giving of remuneration, which includes, without limitation, any transfer of items or services for free or for less than fair market value (with limited exceptions), to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of items or services reimbursable by a federal or state governmental program; |
| ● | The HIPAA imposes criminal and civil liability for executing a scheme to defraud any health care benefit program or making false statements relating to health care matters; |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health (“HITECH”) Act, and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| ● | The federal transparency requirements under the Physician Payments Sunshine Act require manufacturers of FDA-approved drugs, devices, biologics and medical supplies covered by Medicare or Medicaid to report, on an annual basis, to the Center for Medicare and Medicaid Services (“CMS”) information related to payments and other transfers of value to physicians, certain advanced non-physician healthcare practitioners, and teaching hospitals or to entities or individuals at the request of, or designated on behalf of, such physicians, non-physician healthcare practitioners, and teaching hospitals as well as certain ownership and investment interests held by physicians and their immediate family members; and |
| ● | Analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by nongovernmental third-party payors, including private insurers. |
Certain states also have adopted marketing and/or transparency laws relevant to device manufacturers, some of which are broader in scope in comparison to applicable federal laws. Some state laws require medical device companies to comply with the relevant industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring device manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures. In addition, state and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Violation of any of the federal and state healthcare laws may result in penalties, including without limitation, civil, criminal and/or administrative penalties, damages, fines, disgorgement, exclusion from participation in government programs, such as Medicare and Medicaid, injunctions, private “qui tam” actions brought by individual whistleblowers in the name of the government, or refusal to enter into government contracts, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of operations. Our actual or perceived failure to comply with healthcare and data privacy laws could result in liability or reputational harm and could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our customer base and thereby decrease our future revenues.
U.S. and European Data Security and Data Privacy Laws
HIPAA’s administrative simplification provisions established comprehensive U.S. federal standards for the privacy and security of health information. In 2009, Congress enacted Subtitle D of the HITECH provisions of the American Recovery and Reinvestment Act of 2009, which expanded and strengthened HIPAA, created new targets for enforcement, imposed new penalties for noncompliance and established new breach notification requirements. HIPAA applies to health plans, healthcare clearing houses, and healthcare providers that conduct certain healthcare transactions electronically, which are referred to collectively as Covered Entities, as well as individuals or entities that perform services for Covered Entities involving the use, or disclosure of, individually identifiable health information or protected health information (PHI) under HIPAA. Such service providers are called "Business Associates." Under HIPAA, as amended by the HITECH Act, HHS has issued regulations to protect the privacy and security of PHI used or disclosed by Covered Entities and Business Associates. HIPAA also regulates and standardizes the codes, formats and identifiers used in certain healthcare transactions and standardization of identifiers for health plans and providers, for example insurance billing.
53
Any non-compliance with HIPAA and HITECH and related penalties, could adversely impact our business.
The HIPAA security standards require the adoption of administrative, physical, and technical safeguards and the adoption of written security policies and procedures to maintain the security of protected health information.
The HIPAA privacy regulations address the privacy of PHI by limiting the use and release of such information. They also set forth certain rights that an individual has with respect to his or her PHI maintained by a covered entity, including the right to access or amend certain records containing PHI, request an accounting of disclosures of PHI or to request restrictions on the use or disclosure of PHI. The HIPAA breach notification regulations impose certain reporting requirements on Covered Entities and their Business Associates in the event of a breach of PHI.
Significant civil and criminal fines and other penalties may be imposed for violating HIPAA directly, and in connection with acts or omissions of any agents, including a downstream Business associate, as determined according to the federal common law of agency. Civil penalties are adjusted for inflation on an annual basis and can exceed one million dollars per year for failure to comply with a HIPAA requirement. A single breach incident can violate multiple requirements. Additionally, a person who knowingly obtains or discloses PHI in violation of HIPAA may face a criminal penalties (including fines and imprisonment), which increase if the wrongful conduct involves false pretenses or the intent to sell, transfer or use PHI for commercial advantage, personal gain or malicious harm. Covered Entities are also subject to enforcement by state Attorneys General who were given authority to enforce HIPAA.
Additionally, while HIPAA does not create a private right of action allowing individuals to file suit against us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care cases in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI.
Even when HIPAA does not apply, according to the FTC, failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Individually identifiable health information is considered sensitive data that merits stronger safeguards. The FTC and states' Attorneys General have also brought enforcement actions and prosecuted some data breach cases as unfair and/or deceptive acts or practices under the FTC Act and comparable state laws.
The HIPAA privacy and security regulations establish a uniform federal "floor" and do not preempt state laws that are more stringent or provide individuals with greater rights with respect to the privacy or security of, and access to, their records containing PHI. These laws overlap with HIPAA and may differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. The State of California, for example, has implemented comprehensive laws and regulations. The California Confidentiality of Medical Information Act (“CMIA”), imposes restrictive requirements regulating the use and disclosure of health information and other personally identifiable information. The California Consumer Privacy Act of 2018 (the “CCPA”) went into effect January 1, 2020. The CCPA, among other things, creates new data privacy obligations for covered companies and provides new privacy rights to California residents, including the right to opt out of certain disclosures of their information. It also creates individual privacy rights for California consumers and increases the privacy and security obligations of entities handling certain personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches which has led to an increase data breach litigation. Although the law includes limited exceptions, including for PHI maintained by a covered entity or business associate under HIPAA and medical information maintained by healthcare providers under the CMIA, it may regulate or impact our processing of personal information depending on the context. Further, the California Privacy Rights Act (“CPRA”) went into effect January 1, 2023, amending the CCPA. The CPRA imposes additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data and expands the application of the CCPA to all human resources personal information of California-based employees. It also created a new regulatory entity, the California Privacy Protection Agency data protection agency, which is authorized to issue substantive regulations under the CPRA and is expected to result in increased privacy and information security enforcement.
54
Other states have implemented similar laws protecting identifiable health and personal information, and most such laws differ from each other in significant ways and may not be preempted by HIPAA, thus complicating compliance efforts.
In dealing with health information for the development of our technology or for commercial purposes, we will be indirectly affected by HIPAA and state-imposed health information privacy and security laws because these laws regulate the ability of our customers and research collaborators to share health information with us. Additionally, we must identify and comply with all applicable state laws for the protection of personal information with respect to employee information or other personal information that we collect.
In the European Union, increasingly stringent data protection and privacy rules that have and will continue to have substantial impact on the use of personal and patient data across the healthcare industry became stronger in May 2018. The GDPR applies across the European Union and includes, among other things, a requirement for prompt notice of data breaches to data subjects and supervisory authorities in certain circumstances and significant fines for non-compliance. The GDPR fine framework can be up to 20 million euros, or up to 4% of our total global turnover of the preceding fiscal year, whichever is higher. The GDPR sets out a number of requirements that must be complied with when handling the personal data of such European Union-based data subjects including: providing expanded disclosures about how their personal data will be used; higher standards for organizations to demonstrate that they have obtained valid consent or have another legal basis in place to justify their data processing activities; the obligation to appoint data protection officers in certain circumstances; new rights for individuals to be “forgotten” and rights to data portability, as well as enhanced current rights (e.g. access requests); the principal of accountability and demonstrating compliance through policies, procedures, training and audit; and the new mandatory data breach regime. In particular, medical or health data, genetic data and biometric data where the latter is used to uniquely identify an individual are all classified as “special category” data under the GDPR and are afforded greater protection and require additional compliance measures. Noncompliance could result in the imposition of fines, penalties, data lockup or orders to stop noncompliant activities.
We could also be subject to evolving European Union laws on data export, for transfers of data outside the European Union to themselves, group companies or third parties. The GDPR only permits exports of data outside the European Union to jurisdictions that ensure an adequate level of data protection. The United States has not been deemed to offer an adequate level of protection, so in order for us to transfer personal data from the EU to the United States, we must identify a legal basis for data transfer (e.g., the European Union Commission approved Standard Contractual Clauses). On July 16, 2020, the Court of Justice of the European Union or the CJEU, issued a landmark opinion in the case Maximilian Schrems vs. Facebook (Case C-311/18), called Schrems II. This decision (a) called into question commonly relied upon data transfer mechanisms as between the European Union member states and the United States (such as the Standard Contractual Clauses) and (b) invalidateds the EU-U.S. Privacy Shield on which many companies had relied as an acceptable mechanism for transferring such data from the EU to the United States. However, on July 10, 2023, the European Commission adopted an adequacy decision for a new mechanism for transferring data from the EU to the United States – the EU-U.S. Data Privacy Framework, which provides EU individuals with several new rights, including the right to obtain access to their data, or obtain correction or deletion of incorrect or unlawfully handled data. The adequacy decision followed the signing of an executive order introducing new binding safeguards to address the points raised in the Schrems II decision by the CJEU. The European Commission will continually review developments in the United States along with its adequacy decision. Adequacy decisions can be adapted or even withdrawn in the event of developments affecting the level of protection in the applicable jurisdiction. Future actions of EU data protection authorities are difficult to predict. Some customers or other service providers may respond to these evolving laws and regulations by asking us to make certain privacy or data-related contractual commitments that we are unable or unwilling to make. This could lead to the loss of current or prospective customers or other business relationships.
55
Intellectual Property
Patent applications
On May 8, 2011, certain of our founders filed a Patent Cooperation Treaty (“PCT”) Application No. PCT/IL2011/000369, titled “Fluids Testing Apparatus and Methods of Use.” This PCT claimed priority from two preceding U.S. provisional applications filed by our founders, with the earliest priority date being May 9, 2010. The PCT application was transferred to us by our founders on October 27, 2011.
This application covers the novel blood glucose measurement device, comprising the glucose meter; and an adaptor that connects the glucose meter to a smart-phone to receive power supply and data display, storage, and analysis. A PCT search report and written opinion on patentability that we received from World Intellectual Property Organization (“WIPO”) that included only two “Y” citations and one additional non-relevant reference. Corresponding national applications of our PCT were filed in the U.S., Europe, Japan, China, Australia and Israel.
On May 1, 2014, we announced the receipt of a U.S. Notice of Allowance for a key patent relating to how the Dario Blood Glucose Monitoring System draws power from and transmits data to a smartphone via the audio jack port. This patent was issued as U.S. Patent No. 8,797,180 in August 2014, and in August2015, we received U.S. patent (No. 9,125,549) that broadened our registered patent No. 8,797,180 to include testing of other bodily fluids through an audio jack connection. We believe these early patents represent critical intellectual property recognition and a significant initial validation of our intellectual property efforts. Further, a corresponding European patent was granted to us in May 2016, as European patent No. 2569622 for testing of fluids through an audio jack connection. An additional corresponding patent was granted in Israel in April 2016.
On November 11, 2017, U.S. patent No. 9,832,301 titled “Systems and methods for adjusting power levels on a monitoring device” was granted. This patent enhances the way the Dario Blood Glucose Monitoring System communicates with users’ smartphone devices. This family includes a corresponding pending application in China.
Additionally, we recently received U.S. patent No. 10,445,072 that enables optical communication between the Dario Blood Glucose Monitoring System and the end user’s smartphone devices.
Additional patent applications are in the process, of being discussed and developed, and we believe that we have a rich potential pipeline of future technologies that we intend to develop.
For example, we are further seeking to develop and protect new intellectual property around future generations of our hardware and software with the goal of achieving enhanced functionality, user interface, data usability, cyber protection, and artificial intelligence enhancement.
In early 2022, we acquired Physimax and acquired the following patent – US 10,709,374 B2 titled “System and Method for Assessment of Musculoskeletal Profile of a Target Individual.”
This patent was also submitted as EP application #19767795.8 on the 05/03/2019 and is currently pending.
Design patents and patent applications on the Dario Blood Glucose Monitoring System
To further protect our market distinction and branding for the Dario Blood Glucose Monitoring System, three U.S. Design Applications have been filed and granted covering the glucose meter, the cartridge, and connection dongle. At least some of these applications were granted and registered in the United States, as well as, Canada, Europe, and Hong Kong.
Trademark applications
We have also filed several families of trademark applications covering the “Dario” name (wordmark), the Dario name and logo (logo), the Dario logo alone (logo), the DARIO-LITE wordmark, the LABSTYLE INNOVATIONS wordmark, the DARIOHEALTH wordmark, and the DARIOHEALTH logo. In particular, the “Dario” wordmark is registered as a trademark in Australia, Canada, China, Costa Rica, United States, Israel, Hong Kong, South Africa, Japan, Costa Rica, Europe, Israel, Korea, Mexico, New Zealand, Panama and Russia.
56
The “DARIOHEALTH” wordmark is registered as a trademark in the United States, Canada, China and India.
Upright also added the following trademarks to our list: UPRIGHT, UPRIGHT GO – registered in the US, AU and EM, and UPRIGHT DASHBOARD, UPRIGHT DESKTOP, UPRIGHT GO 2, UPRIGHT POSTURE IS WITHING REACH – registered in the U.S.
Utility Models
We have been granted Utility Models for our core invention in Japan.
Other intangible assets
As the number of Dario users grows, an ever-growing amount of data is being collected from diabetic patients, including their blood sugar levels, meal compositions, routines, physical exercise (intensity and duration) as well as many other factors, and lately also blood pressure data, which are all useful for creating meaningful correlations between these factors and insulin use. We expect that this database will be highly valuable and may be capitalized in many ways. The accumulation of this type of know-how and related algorithms are protected as trade secrets using specialized confidentiality protocols.
Twill Intellectual Property
Granted Patents
On October 27, 2020, the United States Patent Office issued Patent No. US 10,813,584 B2 titled “ASSESSING ADHERENCE FIDELITY TO BEHAVIORAL INTERVENTIONS USING INTERACTIVITY AND NATURAL LANGUAGE PROCESSING”. The abstract contained in the patent award describes this patent as “A computer system apparatus and a method carried out by such apparatus for interacting with a user via a behavior intervention designed to cause an increase in emotional well-being of the user. The behavior intervention has a plurality of conditions to be satisfied. The process includes receiving input data from the user during the behavior intervention, performing, on at least a portion of the received input data having text, semantic analysis to identify terms that satisfy the plurality of conditions and assessing, based on an amount of completeness of satisfying the plurality of conditions, a level of adherence to the behavior intervention. When one or more of the plurality of conditions are determined not as satisfied, the process includes generating a prompt designed to elicit, from the user, a response specific to satisfying the missing conditions.”
There are also pending associated patent applications filed in the European Patent Office (Application 18835438.5), Canada (Application 3070229), and Hong Kong (Application 620200109481).
On February 7, 2023, the United States Patent Office issued Patent No. US 11,575,737 B2 titled “DYNAMIC INTERACTIVE NETWORK SYSTEM FOR PROVIDING ONLINE SERVICE AND SOCIAL COMMUNITY FOR ENGAGING, LEARNING, AND TRAINING SKILLS FOR MENTAL HEALTH”. The abstract contained in the patent award describes this patent as “A dynamic interactive network system provides an online service and social community for engaging, learning, and training skills for happiness. The system includes a processor and memory storing instructions which when executed by the processor configure the processor to provide the online service. The instructions further configure the processor to provide tracks including activities, provide an initial happiness level and a track to a user based on a self-assessment completed by the user upon signing up, monitor progress of the user based on self-assessments periodically completed by the user, modify the track based on the self-assessments, suggest followers to the user from the users whose profiles match the profile of the user in terms of demographics, psychographics, and rating of the users on the online service, and generate a happiness graph for the user that correlates the activities and the followers with their impact on happiness level of the user.”
On February 20, 2024, the United States Patent Office issued Patent No. US 11,909,811 B2 titled “DYNAMIC INTERACTIVE NETWORK SYSTEM FOR PROVIDING ONLINE SERVICE AND SOCIAL COMMUNITY FOR ENGAGING, LEARNING, AND TRAINING SKILLS FOR MENTAL HEALTH”. This patent is a continuation of Patent No. 11,575,737 with additional claims.
57
On August 10, 2023, the United States Patent Office issued Patent No. US 11,727,217 B2 titled “SYSTEMS AND METHODS FOR DYNAMIC USER INTERACTION FOR IMPROVING MENTAL HEALTH”. The abstract contained in the patent award describes this patent as “A computing system for interacting with a user comprises a processor and a memory storing executable software which, when executed by the processor, causes the processor to commence an interactive session with a user, receive input data from the user during the interactive session, analyze the received input data and output a response to the user to continue the interactive session with the user. The processor, prior to outputting the response, identifies one or more topics from the received input data, ascertains a tone of the received input data, generates a mirroring prompt based on the ascertained tone of the received input data, and output to the user the generated mirroring prompt. The processor outputs the mirroring prompt to the user during the interactive session to cause an increase in a level of engagement of the user with the interactive session”.
On October 10, 2023, the United States Patent Office issued Patent No. US 11,779,270 B2 titled “SYSTEMS AND METHODS FOR TRAINING ARTIFICIALLY-INTELLIGENT CLASSIFIER”. The abstract contained in the patent award describes the patent as “A computing system/method for enabling a user to improve, via training, a system designed to increase the emotional and/or physical well-being of persons or designed for other purposes. The system/method includes retrieving a user response from a dialogue database, the user response having already labeled thereto an assigned class having a highest confidence score, the confidence score indicating degree of confidence that context of the retrieved user response is of the assigned class, displaying, the assigned class, along with other classes each having a respective lower confidence score, and receiving an indication of validity of the assigned class. The system/method further includes retrieving of a pair of sequential user response and follow-up prompt from the database, displaying user-selectable ratings, each rating designating a respectively different quality to the follow-up prompt, receiving selection of a rating and a related comment, and associating the selection and the comment to the follow-up prompt.”
There are also pending associated patent applications filed in the European Patent Office (Application 19847959.4), Canada (Application 3109113).
Patent Applications
On December 8, 2021, patent application 17/546,020 was filed in the United States Patent office. Titled “CUSTOMIZABLE THERAPY SYSTEM AND PROCESS”, the abstract for this application describes as “The present invention is directed to a computing system and a process carried out by such system for providing a therapy session personalized to the circumstances of the user. The therapy session includes an audio component that is automatically generated by an algorithm that makes a voice seem to be that of an actual person. In a similar way, a video component to the therapy session may also be presented.”
On January 26, 2022, patent application 17/585,003 was filed in the United States Patent office. Titled “SENSOR TRACKING BASED PATIENT SOCIAL CONTENT SYSTEM”, the abstract for this application describes as “A computing system that includes a server, the system functions as a patient social content system (PSCS) which, among other functions, provides content to a patient/user. A device with a network connection to the computing system permits the patient to provide information to the computing system. In addition, one or more sensors are configured to detect changes in various physical parameters relevant to the patient. Electrical signals from the sensors are conveyed to the computing system so that the computing system is aware of changes in physical parameters of the patient. Based on the changed physical parameters of the patient, the computing system provides different content to the user that is relevant to the changes. Overall, processes carried out by the system establish and maintain a patient social content system that utilizes tracking data received from one or more sensors.”.
Associated applications have been filed in the WIPO (Application No.PCT/US 2022/046487 and EPO (Application No. 22881741.7)
58
Granted Trademarks
Country |
Mark |
Registration No. |
Registration Date |
USA |
Therapeutic Media |
7281217 |
01/16/2024 |
USA |
Duet |
7270430 |
01/09/2024 |
USA |
KOPA |
6531581 |
10/19/2021 |
USA |
|
6108363 |
07/21/2020 |
USA |
ANNA |
6024641 |
03/31/2020 |
USA |
|
5546023 |
08/21/2018 |
USA |
HAPPIFY HEALTH |
5565951 |
09/18/2018 |
Madrid Protocol |
HAPPIFY |
1216735 |
Renewal: 10/15/2023 |
USA |
HAPPIFY |
4475643 |
01/28/2014 |
Madrid Protocol |
SEQUENCE |
1697868 |
08/23/2022 |
USA |
SEQUENCE |
7231798 |
11/28/2023 |
Madrid Protocol |
ASPIRO |
1738488 (AU,UK) |
04/28/2023 |
USA |
|
7310387 |
02/20/2024 |
Employees
As of March 3, 2025, we had 196 full-time employees and 6 part-time employees. We have employment agreements with our four executive officers. See “Management – Employment Agreements.”
59
Item 1A. Risk Factors
Investing in our securities is highly speculative and involves a high degree of risk. You should carefully consider the following factors and other information in this Annual Report and our other SEC filings before making a decision to invest in our securities. Additional risks and uncertainties that we are unaware of may become important factors that affect us. If any of the following events occur, our business, financial conditions and operating results may be materially and adversely affected. In that event, the trading price of our common stock and warrants may decline, and you could lose all or part of your investment.
Risks Related to Our Financial Position and Capital Requirements
We were formed in August 2011 and are thus subject to the risks associated with new businesses.
We were formed in August 2011 as a new business and, commencing from 2015, we entered the commercialization stage of our technology. As such, this limited operating history may not be adequate to enable you to fully assess our ability to develop commercialize and achieve market acceptance of the Dario digital platform. We commenced a commercial launch of the free Dario Smart Diabetes Management application in the United Kingdom in late 2013 and commenced an initial soft launch of the full Dario Smart Diabetes Management Solution (including the app and the Dario Blood Glucose Monitoring System) in selected jurisdictions in March 2014 with the goal of collecting customer feedback to refine our longer-term roll-out strategy and continued to scale up launch during 2014 in the United Kingdom, the Netherlands and New Zealand, in 2015 in Australia, Israel and Canada and in 2016 in the United States. These efforts have not generated sufficient revenues, and we will need to generate additional revenues over the next years. Therefore, we are, and expect for the foreseeable future to be, subject to all the risks and uncertainties, inherent in a new business and the development and sale of new medical devices and related software applications. As a result, we may be unable to fully develop, obtain regulatory approval for, commercialize, manufacture, market, sell and derive material revenues in the timeframes we project, if at all, and our inability to do so would materially and adversely impact our viability as a company. In addition, we still must establish many functions necessary to operate a business, including finalizing our managerial and administrative structure, continuing product and technology development, assessing and commencing our marketing activities, implementing financial systems and controls and personnel recruitment.
Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in their initial revenue generating stages, particularly those in the medical device and mobile health fields. In particular, potential investors should consider that there is a significant risk that we will not be able to:
| ● | implement or execute our current business plan, or that our business plan is sound; |
| ● | maintain our management team and the Company’s board of directors (the “Board of Directors”); |
| ● | raise sufficient funds in the capital markets or otherwise to effectuate our business plan; |
| ● | determine that our technologies that we have developed are commercially viable; and/or |
| ● | attract, enter into or maintain contracts with, and retain customers. |
In the event that we do not successfully address these risks, our business, prospects, financial condition, and results of operations could be materially and adversely affected.
Given our limited revenue and lack of positive cash flow, we will need to raise additional capital, which may be unavailable to us or, even if consummated, may cause dilution or place significant restrictions on our ability to operate.
According to our management’s estimates, based on our current cash on hand and further based on our budget and the assumption that initial commercial sales will commence during our anticipated timeframes, we believe that we will have sufficient resources to continue our activities through 2025.
60
Since we might be unable to generate sufficient revenue or cash flow to fund our operations for the foreseeable future, we will need to seek additional equity or debt financing to provide the capital required to maintain or expand our operations. We may also need additional funding for developing products and services, increasing our sales and marketing capabilities, and promoting brand identity, as well as for working capital requirements and other operating and general corporate purposes. Moreover, the regulatory compliance arising out of being a publicly registered company has dramatically increased our costs.
We currently have a credit facility in place with Avenue Venture Opportunities Fund L.P. and Avenue Venture Opportunities Fund II, L.P., of which $30 million was made available in May 2023. However, there can be no assurance that we will be able to raise sufficient additional capital on acceptable terms, or at all. If such financing is not available on satisfactory terms, or is not available at all, we may be required to delay, scale back or eliminate the development of business opportunities and our operations and financial condition may be materially adversely affected. As of December 31, 2024, we have drawn down $30 million of the credit facility.
If we raise additional capital by issuing equity securities, the percentage ownership of our existing stockholders may be reduced, and accordingly these stockholders may experience substantial dilution. We may also issue equity securities that provide for rights, preferences and privileges senior to those of our common stock. Given our need for cash and that equity raising is the most common type of fundraising for companies like ours, the risk of dilution is particularly significant for stockholders of our company.
Debt financing, if obtained, may involve agreements that include liens on our assets, covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, could increase our expenses and require that our assets be provided as a security for such debt. Debt financing would also be required to be repaid regardless of our operating results.
If we raise additional funds through collaborations and licensing arrangements, we may be required to relinquish some rights to our technologies or candidate products, or to grant licenses on terms that are not favorable to us.
Funding from any source may be unavailable to us on acceptable terms, or at all. If we do not have sufficient capital to fund our operations and expenses, we may not be able to achieve or maintain competitiveness, which could lead to the failure of our business and the loss of your investment.
We have incurred significant losses since inception. As such, you cannot rely upon our historical operating performance to make an investment decision regarding our company.
Since our inception, we have engaged primarily in research and development activities and in 2015 entered the commercialization stage. We have financed our operations primarily through private placements, public offerings of common stock and certain credit facilities, and have incurred losses in each year since inception including net losses of $42,747,000 and $59,427,000 in 2024 and 2023, respectively. Our accumulated deficit at December 31, 2024 was approximately $390,343,000. We do not know whether or when we will become profitable. Our ability to generate revenue and achieve profitability depends upon our ability, alone or with others, to commercialize our digital health engagement platform. We may be unable to achieve this goal.
We may be subject to claims for rescission or damages in connection with certain sales of shares of our securities.
In March 2016, the Securities and Exchange Commission declared effective a registration statement that we filed to cover 66,667 shares 76,667 warrants to purchase common stock, 76,667 shares of common stock underlying such warrants, and underwriters’ warrants to purchase up to 7,172 shares of common stock. Sales of approximately 2,778 shares of common stock, approximately 12,778 shares of common stock underlying warrants and approximately 1,278 shares of common stock underlying underwriters’ warrants may not have been made in accordance with Section 5 of the Securities Act of 1933, as amended. Accordingly, the purchasers of those securities may have rescission rights or be entitled to damages. The amount of such liability, if any, is uncertain. In the event that we are required to make payments to investors as a result of these unregistered sales of securities, our liquidity could be negatively impacted.
61
Our revenues are concentrated with a major customer, and our revenues may decrease significantly if we were to lose our major customer.
Due to our limited operating history, we have a limited customer base and have depended on a major customer for a significant portion of our revenue. As of December 31, 2024, our major customer accounted for 41.6% of our accounts receivable balance. If the customer were to terminate the agreement, or if we fail to adequately perform under the agreement, and if we are unable to diversify our customer base, our revenue could decline, and our results of operations could be adversely affected.
Risks Related to Our Business
There is no assurance that our digital health engagement platform will succeed or be adopted by healthcare providers.
Our product offering consists of our digital health engagement platform, where we digitally engage with Dario users, assist them in monitoring their chronic illnesses and provide them with coaching, support, digital communications, and real-time alerts, trends and pattern analysis. We expect that our digital health engagement platform may be leveraged by our potential partners, such as clinics, health care service providers, employers, and payers for scalable monitoring of people with diabetes in a cost-effective manner, which we expect will open for us additional revenue streams. While we have begun to execute agreements with employers and health plans in the United States, we have not yet seen wide adoption of our platform. Therefore, the success of our digital health engagement platform will depend entirely on our potential partners’ adoption of the platform and we cannot assure you that our potential partners will do so, or, if adopted, that they will continue to use the platform continually and for an extended period of time. If we cannot encourage potential partners to utilize our digital health engagement platform we may not succeed in marketing the product to our potential partners, the failure of which may materially and adversely affect our business and operating results.
The success of our Dario product will depend on the acceptance of Dario in the healthcare market.
Dario has been CE marked since 2013, enabling us to commercialize in 32 countries across Europe as well as in certain other countries worldwide. It was also approved by the regulatory authorities in Australia, New Zealand, Canada, Israel and South Africa, and most recently in December 2015, we received FDA clearance. As a result, we have a limited history of commercializing Dario and commenced selling Dario in the United States in 2016. We have limited experience engaging in commercial activities and limited established relationships with physicians and hospitals as well as third-party suppliers on whom we depend for the manufacture of our product. We are faced with the risk that the marketplace will not be receptive to Dario over competing products and that we will be unable to compete effectively. Factors that could affect our ability to establish Dario or any potential future product include:
| ● | the development of products or devices which could result in a shift of customer preferences away from our device and services and significantly decrease revenue; |
| ● | the increased use of improved diabetes drugs that could encourage certain diabetics to test less often, resulting in less usage of a self-monitoring test device for certain types of diabetics; |
| ● | the challenges of developing (or acquiring externally-developed) technology solutions that are adequate and competitive in meeting the requirements of next-generation design challenges, including interoperability with various electronic health records; |
| ● | the significant number of current competitors in the BGMS market who have significantly greater brand recognition and more recognizable trademarks and who have established relationships with healthcare providers and payors; and |
| ● | intense competition to attract acquisition targets, which may make it more difficult for us to acquire companies or technologies at an acceptable price or at all. |
We cannot assure you that Dario or any future product will gain broad market acceptance. If the market for Dario or any future product fails to develop or develops more slowly than expected, or if any of the technology and standards supported by us do not achieve or sustain market acceptance, our business and operating results would be materially and adversely affected.
62
We cannot accurately predict the volume or timing of any future sales, making the timing of any revenues difficult to predict.
We may be faced with lengthy customer evaluation and approval processes associated with the adoption of our digital health engagement platform. Consequently, we may incur substantial expenses and devote significant management effort and expense in developing customer adoption of our platform which may not result in revenue generation. We must also obtain regulatory approvals of Dario in certain jurisdictions as well as approval for insurance reimbursement in order to initiate sales of Dario, each of which is subject to risk and potential delays, and neither of which may actually occur. As such, we cannot accurately predict the volume or timing of any future sales.
If Dario fails to satisfy current or future customer requirements, we may be required to make significant expenditures to redesign the product, and we may have insufficient resources to do so.
Dario is designed to address an evolving marketplace and must comply with current and evolving customer requirements in order to gain market acceptance. There is a risk that Dario will not meet anticipated customer requirements or desires. If we are required to redesign our products to address customer demands or otherwise modify our business model, we may incur significant unanticipated expenses and losses, and we may be left with insufficient resources to engage in such activities. If we are unable to redesign our products, develop new products or modify our business model to meet customer desires or any other customer requirements that may emerge, our operating results would be materially adversely affected, and our business might fail.
We expect to derive substantially all of our revenues from our principal technology, which leaves us subject to the risk of reliance on such technology.
We expect to derive substantially all of our revenues from sales of products derived from our principal technology, which is our digital health engagement platform. Our initial product utilizing this technology is Dario. As such, any factor adversely affecting sales of our digital health engagement platform, including the product release cycles, regulatory issues, market acceptance, product competition, performance and reliability, reputation, price competition and economic and market conditions, would likely harm our operating results. We may be unable to develop other products utilizing our technology, which would likely lead to the failure of our business.
We are dependent upon third-party manufacturers and suppliers making us vulnerable to supply shortages and problems and price fluctuations, which could harm our business.
We do not own or operate manufacturing facilities for clinical or commercial production of the Dario Blood Glucose Monitoring System, and we lack the resources and the capability to manufacture the Dario Blood Glucose Monitoring System on a commercial scale. Therefore, we rely on a limited number of suppliers who manufacture and assemble certain components of the Dario Blood Glucose Monitoring System. Our suppliers may encounter problems during manufacturing for a variety of reasons, including, for example, failure to follow specific protocols and procedures, failure to comply with applicable legal and regulatory requirements, equipment malfunction and environmental factors, failure to properly conduct their own business affairs, and infringement of third-party intellectual property rights, any of which could delay or impede their ability to meet our requirements. Our reliance on these third-party suppliers also subjects us to other risks that could harm our business, including:
| ● | we are not a major customer of many of our suppliers, and these suppliers may therefore give other customers’ needs higher priority than ours; |
| ● | third parties may threaten or enforce their intellectual property rights against our suppliers, which may cause disruptions or delays in shipment, or may force our suppliers to cease conducting business with us; |
| ● | we may not be able to obtain an adequate supply in a timely manner or on commercially reasonable terms; |
63
| ● | our suppliers, especially new suppliers, may make errors in manufacturing that could negatively affect the efficacy or safety of the Dario Blood Glucose Monitoring System or cause delays in shipment; |
| ● | we may have difficulty locating and qualifying alternative suppliers; |
| ● | switching components or suppliers may require product redesign and possibly submission to FDA, European Economic Area Notified Bodies, or other foreign regulatory bodies, which could significantly impede or delay our commercial activities; |
| ● | one or more of our sole- or single-source suppliers may be unwilling or unable to supply components of the Dario Blood Glucose Monitoring System; |
| ● | other customers may use fair or unfair negotiation tactics and/or pressures to impede our use of the supplier; |
| ● | the occurrence of a fire, natural disaster or other catastrophe impacting one or more of our suppliers may affect their ability to deliver products to us in a timely manner; and |
| ● | our suppliers may encounter financial or other business hardships unrelated to our demand, which could inhibit their ability to fulfill our orders and meet our requirements. |
We may not be able to quickly establish additional or alternative suppliers if necessary, in part because we may need to undertake additional activities to establish such suppliers as required by the regulatory approval process. Any interruption or delay in obtaining products from our third-party suppliers, or our inability to obtain products from qualified alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our customers and cause them to switch to competing products. Given our reliance on certain single-source suppliers, we are especially susceptible to supply shortages because we do not have alternate suppliers currently available.
We rely in part on a small group of third-party distributors to effectively distribute our products.
We depend in part on medical device distributors for the marketing and selling of our products in certain territories in which we have launched product sales. We depend on these distributors’ efforts to market our products, yet we are unable to control their efforts completely. These distributors typically sell a variety of other, non-competing products that may limit the resources they dedicate to selling Dario. In addition, we are unable to ensure that our distributors comply with all applicable laws regarding the sale of our products. If our distributors fail to effectively market and sell Dario, in full compliance with applicable laws, our operating results and business may suffer. Recruiting and retaining qualified third-party distributors and training them in our technology and product offering requires significant time and resources. To develop and expand our distribution, we must continue to scale and improve our processes and procedures that support our distributors. Further, if our relationship with a successful distributor terminates, we may be unable to replace that distributor without disruption to our business. If we fail to maintain positive relationships with our distributors, fail to develop new relationships with other distributors, including in new markets, fail to manage, train or incentivize existing distributors effectively, or fail to provide distributors with competitive products on attractive terms, or if these distributors are not successful in their sales efforts, our revenue may decrease and our operating results, reputation and business may be harmed.
Failure in our online and digital marketing efforts could significantly impact our ability to generate sales.
In several of our principal target markets, we utilize online and digital marketing in order to create awareness to Dario. Our management believes that using online advertisement through affiliate networks and a variety of other pay-for-performance methods will be superior for marketing and generating sales of Dario rather than utilizing traditional, expensive retail channels. However, there is a risk that our marketing strategy could fail. Because we plan to use non-traditional retail sales tools and to rely on healthcare providers to educate our customers about Dario, we cannot predict the level of success, if any, that we may achieve by marketing Dario via the internet. The failure of our online marketing efforts would significantly and negatively impact our ability to generate sales.
64
Our Dario application, which is a key to our business model, is available via Apple’s App Store and via Google’s Android platforms and maybe in the future via additional platforms. If we are unable to achieve or maintain a good relationship with each of Apple and Google or similar platforms, or if the Apple App Store or the Google Play Store or any other applicable platform were unavailable for any prolonged period of time, our business will suffer.
A key component of the Dario Solution is an iPhone or Android application which includes tools to help diabetic patients manage their disease. This application is compatible with Apple’s iOS and with Google’s Android platforms and may in the future become compatible via additional platforms. If we are unable to make our Dario Smart Diabetes Management application compatible with these platforms, or if there is any deterioration in our relationship with either Apple or Google or others after our application is available, our business would be materially harmed.
We are subject to each of Apple’s and Google’s standard terms and conditions for application developers, which govern the promotion, distribution, and operation of games and other applications on their respective storefronts. Each of Apple and Google has broad discretion to change its standard terms and conditions, including changes which could require us to pay to have our Dario application available for downloading. In addition, these standard terms and conditions can be vague and subject to changing interpretations by Apple or Google. We may not receive any advance warning of such changes. In addition, each of Apple and Google has the right to prohibit a developer from distributing its applications on its storefront if the developer violates its standard terms and conditions. In the event that either Apple or Google ever determines that we are in violation of its standard terms and conditions, including by a new interpretation, and prohibits us from distributing our Dario Management application on its storefront, it would materially harm our business.
Additionally, we will rely on the continued function of the Apple App Store and the Google Play Store as digital storefronts where our Dario application may be obtained. There have been occasions in the past when these digital storefronts were unavailable for short periods of time or where there have been issues with the in-app purchasing functionality within the storefront. In the event that either the Apple App Store or the Google Play Store is unavailable or if in-app purchasing functionality within the storefront is non-operational for a prolonged period of time, it would have a material adverse effect on the ability of our customers to secure the Dario Smart Diabetes Management application, which would materially harm our business.
We rely upon Software-as-a-Services, or SAAS, technologies from third parties to operate our business, and interruptions or performance problems with these technologies may adversely affect our business, financial condition and results of operations.
We rely on hosted SaaS applications from third parties in order to operate critical functions of our business, including platform delivery, enterprise resource planning, customer relationship management, billing, project management and accounting and financial reporting. If these services become unavailable due to extended outages, interruptions or because they are no longer available on commercially reasonable terms, our expenses could increase, our ability to manage finances could be interrupted and our processes for managing sales of our platform and products and supporting our customers could be impaired until equivalent services, if available, are identified, obtained and implemented, all of which could adversely affect our business, financial condition and results of operations.
The SaaS pricing model is evolving and our failure to manage its evolution and demand could lead to lower than expected revenue and profit.
We derive most of our revenue growth from subscription offerings and, specifically, SaaS offerings. This business model depends heavily on achieving economies of scale because the initial upfront investment is costly and the associated revenue is recognized on a ratable basis. If we fail to achieve appropriate economies of scale or if we fail to manage or anticipate the evolution and demand of the SaaS pricing model, then our business and operating results could be adversely affected.
Our results of operations may fluctuate significantly due to the timing of our recognition of SaaS revenues.
We may experience volatility in our reported revenues and operating results due to the differences in timing of revenue recognition between our SaaS offerings and our traditional on-premise software and hardware sales. SaaS revenues are generally recognized ratably over the life of the subscriptions. In contrast, revenue from our on-premise software and hardware sales is generally recognized in full at the time of delivery.
65
Accordingly, the SaaS delivery model creates risks related to the timing of revenue recognition not associated with our traditional on-premise software delivery model and hardware sales. A portion of our SaaS revenue results from the recognition of deferred revenue relating to subscription agreements entered into during prior reporting periods. A decline in new or renewed subscriptions in any period may not be immediately reflected in our reported financial results for that period, but may result in a decline in our revenue in future reporting periods. If any of our assumptions about revenue from our SaaS delivery model prove incorrect, our actual results may vary materially from those anticipated, estimated, or projected.
Any damage, failure or disruption of our SaaS network infrastructure or data centers could impair our ability to effectively provide our solution, harm our reputation and adversely affect our business.
Our SaaS network infrastructure is a critical part of our business operations. Our clients access our solution through standard web browsers, smart phones, tablets and other web-enabled devices and depend on us for fast and reliable access to our solution. We serve all of our clients from our data centers located in the United-States. Our SaaS network infrastructure and data centers are vulnerable to damage, failure and disruption.
In the future, we may experience issues with our computing and communications infrastructure, or data centers caused by the following factors:
•human error;
•telecommunications failures or outages from third-party providers;
• computer viruses or cyber-attacks;
•break-ins or other security breaches;
•acts of terrorism, sabotage, intentional acts of vandalism or other misconduct;
•tornadoes, fires, earthquakes, hurricanes, floods and other natural disasters;
•power loss; and
•other unforeseen interruptions or damages.
If our SaaS network infrastructure or our clients’ ability to access our solution is interrupted, client and employee data from recent transactions may be permanently lost, and we could be exposed to significant claims by clients, particularly if the access interruption is associated with problems in the timely delivery of funds payable to employees or tax authorities. Further, any adverse changes in service levels at our data centers resulting from damage to or failure of our data centers could result in disruptions in our services. Any significant instances of system downtime or performance problems at our data centers could negatively affect our reputation and ability to attract new clients, prevent us from gaining new or additional business from our current clients, or cause our current clients to terminate their use of our solution, any of which would adversely impact our revenues. In addition, if our network infrastructure and data centers fail to support increased capacity due to growth in our business, our clients may experience interruptions in the availability of our solution. Such interruptions may reduce our revenues, cause us to issue refunds to clients or adversely affect our retention of existing clients, any of which could have a negative impact on our business, operating results or financial condition.
Our products are subject to technological changes which may impact their use.
Our Dario Blood Glucose Monitoring System is currently designed to be plugged into the Lighting jack for Apple devices or the USB-C jack for other mobile devices. As a result, our products are subject to future technological changes to mobile devices that may occur in the future. If we are unable to modify our products to keep pace with such technological changes, it would have a material adverse effect the ability of our customers to use our products, which would materially harm our business.
66
As we conduct business internationally, we are susceptible to risks associated with international relationships.
Outside of the United States, we operate our business internationally, presently in Europe, Australia and Canada. The international operation of our business requires significant management attention, which could negatively affect our business if it diverts their attention from their other responsibilities. In the event that we are unable to manage the complications associated with international operations, our business prospects could be materially and adversely affected. In addition, as a result of the crisis in Ukraine, both the United States and the EU have implemented sanctions against certain Russian individuals and entities, as well with respect to Belarus, and may impact the economic and political stability in the EU. If the EU experiences economic and political instability as a result of these current tensions, our business, including revenue, profitability and cash flows, and operations could be adversely affected. In addition, doing business with foreign customers subjects us to additional risks that we do not generally face in the United States. These risks and uncertainties include:
| ● | management, communication and integration problems resulting from cultural differences and geographic dispersion; |
| ● | localization of products and services, including translation of foreign languages; |
| ● | delivery, logistics and storage costs; |
| ● | longer accounts receivable payment cycles and difficulties in collecting accounts receivable; |
| ● | difficulties supporting international operations; |
| ● | difficulties supporting customer services; |
| ● | changes in economic and political conditions; |
| ● | impact of trade protection measures; |
| ● | complying with import or export licensing requirements; |
| ● | exchange rate fluctuations; |
| ● | competition from companies with international operations, including large international competitors and entrenched local companies; |
| ● | potentially adverse tax consequences, including foreign tax systems and restrictions on the repatriation of earnings; |
| ● | maintaining and servicing computer hardware in distant locations; |
| ● | keeping current and complying with a wide variety of foreign laws and legal standards, including local labor laws; |
| ● | securing or maintaining protection for our intellectual property; and |
| ● | reduced or varied protection for intellectual property rights, including the ability to transfer such rights to third parties, in some countries. |
The occurrence of any or all of these risks could adversely affect our international business and, consequently, our results of operations and financial condition.
67
We expect to be exposed to fluctuations in currency exchange rates, which could adversely affect our results of operations.
Because we expect to conduct a material portion of our business outside of the United States but report our financial results in U.S. Dollars, we face exposure to adverse movements in currency exchange rates. Our foreign operations will be exposed to foreign exchange rate fluctuations as the financial results are translated from the local currency into U.S. Dollars upon consolidation. Specifically, the U.S. Dollar cost of our operations in Israel is influenced by any movements in the currency exchange rate of the New Israeli Shekel (NIS). Such movements in the currency exchange rate may have a negative effect on our financial results. If the U.S. Dollar weakens against foreign currencies, the translation of these foreign currencies denominated transactions will result in increased revenue, operating expenses and net income. Similarly, if the U.S. Dollar strengthens against foreign currencies, the translation of these foreign currencies denominated transactions will result in decreased revenue, operating expenses and net income. As exchange rates vary, sales and other operating results, when translated, may differ materially from our or the capital market’s expectations.
Non-U.S. governments often impose strict price controls, which may adversely affect our future profitability.
We intend to seek approval to market Dario and any future product in both the U.S. and in non-U.S. jurisdictions. If we obtain approval in one or more non-U.S. jurisdictions, we will be subject to rules and regulations in those jurisdictions relating to our products. In some countries, particularly countries of the European Union, each of which has developed its own rules and regulations, pricing may be subject to governmental control under certain circumstances. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a medical device candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product to other available products. If reimbursement of our product candidates is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability.
Our Dario platform and associated business processes may contain undetected errors, which could limit our ability to provide our services and diminish the attractiveness of our service offerings.
The Dario platform may contain undetected errors, defects or bugs. As a result, our customers or end users may discover errors or defects in our products, software or the systems we design, or the products or systems incorporating our designs and intellectual property may not operate as expected. We may discover significant errors or defects in the future that we may not be able to fix. Our inability to fix any of those errors could limit our ability to provide our products, impair the reputation of our brand and diminish the attractiveness of our product offerings to our customers.
In addition, we may utilize third-party technology or components in our products, and we rely on those third parties to provide support services to us. Failure of those third parties to provide necessary support services could materially adversely impact our business.
Our future performance will depend on the continued engagement of key members of our management team.
Our future performance depends to a large extent on the continued services of members of our current management including, in particular, Erez Raphael, our Chief Executive Officer and a member of our Board of Directors and Zvi Ben David, our Chief Financial Officer, Treasurer and Secretary. In the event that we lose the continued services of such key personnel for any reason, this could have a material adverse effect on our business, operations, and prospects.
If we are not able to attract and retain highly skilled managerial, scientific and technical personnel, we may not be able to implement our business model successfully.
We believe that our management team must be able to act decisively to apply and adapt our business model in the rapidly changing markets in which we will compete. In addition, we will rely upon technical and scientific employees or third-party contractors to effectively establish, manage and grow our business. Consequently, we believe that our future viability will depend largely on our ability to attract and retain highly skilled managerial, sales, scientific and technical personnel. In order to do so, we may need to pay higher compensation or fees to our employees or consultants than we currently expect, and such higher compensation payments would have a negative effect on our operating results.
68
Competition for experienced, high-quality personnel is intense and we cannot assure that we will be able to recruit and retain such personnel. We may not be able to hire or retain the necessary personnel to implement our business strategy. Our failure to hire and retain such personnel could impair our ability to develop new products and manage our business effectively.
We may not generate the expected benefits of our acquisition of Twill.
Through our acquisitions of Twill, we expanded our product offering to include digital-first solutions with a mission to improve users mental and physical health. There can be no assurance that these acquisitions will provide the benefits we expect or that we will be able to develop the operations of Twill successfully. Any failure to do so could have a material adverse effect on our business, operating results and financial condition.
Risks Related to Product Development and Regulatory Approval
The regulatory clearance process which we must navigate is expensive, time-consuming, and uncertain and may prevent us from obtaining clearance for the commercialization of Dario or our any future product.
We are not permitted to market Dario in any jurisdiction until we receive marketing authorization from the applicable regulatory authority. To date, we have received regulatory authorization in Australia, Canada, Israel, Italy, the Netherlands, New Zealand, the United Kingdom, and the United States.
The research, design, testing, manufacturing, labeling, selling, marketing and distribution of medical devices are subject to extensive regulation by the FDA and non-U.S. regulatory authorities, which regulations differ from country to country. In particular, marketing authorization requirements vary between countries and can involve additional product testing and additional administrative review periods. The time required to obtain marketing authorization in other countries might differ from that required to obtain FDA clearance or other marketing authorization. Obtaining authorization for a device in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory authorization in one country may negatively impact the regulatory process in others. There can be no assurance that even after such time and expenditures, we will be able to obtain necessary regulatory approvals for clinical testing or for the manufacturing or marketing of any products. In addition, during the regulatory process, other companies may develop other technologies with the same intended use as our products. Significant delays in receiving, or the failure to receive, marketing authorization for our new products would have an adverse effect on our ability to expand our business.
We are also subject to numerous post-marketing regulatory requirements, which include quality management system regulations, labeling regulations and medical device reporting regulations. Specifically, the medical device reporting regulations require us to report to different regulatory agencies if our device causes or contributes to a death or serious injury, or malfunctions in a way that would likely cause or contribute to a death or serious injury. In addition, these regulatory requirements may change in the future in a way that adversely affects us, or various regulatory authorities may take other actions that could prevent or delay authorization of our products under development or impact our ability to gain authorization for modifications to our currently approved or cleared products in a timely manner. If we fail to comply with present or future regulatory requirements that are applicable to us, we may be subject to enforcement action by regulatory agencies, which may include, among others, any of the following sanctions:
| ● | untitled letters, warning letters, fines, injunctions, consent decrees, and civil penalties; |
| ● | customer notification, or orders for repair, replacement or refunds; |
| ● | voluntary or mandatory recall or seizure of our current or future products; |
| ● | imposing operating restrictions, suspension or shutdown of production; |
| ● | refusing our requests for marketing authorization of new products, new intended uses or modifications to Dario or future products; |
| ● | suspending or withdrawing marketing authorizations that have already been granted; and |
69
| ● | criminal prosecution. |
The occurrence of any of these events may have a material adverse effect on our business, financial condition and results of operations.
We have conducted limited clinical trials of Dario. Clinical and nonclinical data is susceptible to varying interpretations, which could delay, limit or prevent additional regulatory clearances.
To date, we have conducted limited clinical trials on Dario. There can be no assurance that we will successfully complete additional clinical trials necessary to receive additional regulatory approvals in certain jurisdictions. While studies conducted by us have produced results we believe to be encouraging and indicative of the potential efficacy of Dario, data already obtained, or in the future obtained, from nonclinical studies and clinical trials do not necessarily predict the results that will be obtained from later nonclinical studies or clinical trials. Moreover, nonclinical and clinical data are susceptible to varying interpretations, which could delay, limit or prevent additional regulatory approvals. A number of companies in the medical device and pharmaceutical industries have suffered significant setbacks in advanced clinical trials, even after promising results in earlier studies. If we fail to adequately demonstrate the safety and effectiveness of a product candidate under development, it could delay or prevent regulatory authorization of the device, resulting in delays to commercialization, and could materially harm our business. Even though we have received CE mark and FDA clearance of Dario, there can be no assurance that we will be able to receive authorization for other potential applications of our principal technology, or that we will receive regulatory authorizations from other targeted regions or countries.
We may be unable to complete required clinical trials, or we may experience significant delays in completing such clinical trials, which could significantly delay our targeted product launch timeframe and impair our viability and business plan.
The completion of any future clinical trials for Dario or other trials that we may be required to undertake in the future could be delayed, suspended or terminated for several reasons, including:
| ● | delay or failure in reaching agreement with regulatory authorities on a trial design that we are able to execute; |
| ● | delay or failure in obtaining authorization to commence a trial, including approval from the appropriate IRB to conduct testing of a product candidate on human subjects, or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a clinical trial; |
| ● | delay in reaching, or failure to reach, agreement on acceptable terms with prospective contract research organizations and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| ● | failure or inability to conduct the clinical trial in accordance with regulatory requirements; |
| ● | sites participating in the trial may drop out of the trial, which may require us to engage new sites for an expansion of the number of sites that are permitted to be involved in the trial; |
| ● | failure to initiate or delay of or inability to complete a clinical trial as a result of a clinical hold imposed by a regulatory authority due to observed safety findings or other reasons; |
| ● | delays that we may experience in patient enrollment or completion of certain trials; |
| ● | patients may not enroll in, remain in or complete, the clinical trial at the rates we expect; and |
| ● | clinical investigators may not perform our clinical trial on our anticipated schedule or consistent with the clinical trial protocol and good clinical practices. |
In addition, the U.S. Congress recently amended the FDCA to require sponsors of any pivotal study to support marketing authorization of a medical device to design and submit a diversity action plan for such clinical trial. The action plan must describe appropriate diversity goals for enrollment, as well as a rationale for the goals and a description of how the sponsor will meet them.
70
For any future pivotal studies involving our device products or product candidates, we must submit a diversity action plan to the FDA by the time a pivotal study protocol is submitted to the agency for review, as applicable, unless we are able to obtain a waiver for some or all of the requirements for a diversity action plan. It is unknown at this time how the diversity action plan may affect the planning and timing of any future pivotal study for our products or product candidates or what specific information FDA will expect in such plan. However, initiation of such studies may be delayed if the FDA objects to a proposed diversity action plans for any future pivotal study of our product candidates, and we may experience difficulties recruiting a diverse population of patients in attempting to fulfill the requirements of any approved diversity action plan.
If our ongoing or future clinical trials are delayed it will take us longer to further commercialize Dario and generate additional revenues. Moreover, our development costs will increase if we have material delays in our clinical trial or if we need to perform more or larger clinical trials than planned. We may be faced with similar risks in connection with future trials we conduct. See “Business - Clinical Trials” for a description of our clinical trials performed to date.
If we or our manufacturers fail to comply with the FDA’s Quality System Regulation or any applicable state equivalent, our operations could be interrupted, and our operating results could suffer.
We, our manufacturers and suppliers must, unless specifically exempt by regulation, follow the FDA’s QSR, as well as similar regulations of foreign jurisdictions regarding the manufacturing process. In addition, we and certain of our manufacturers and suppliers are subject to inspection by regulatory authorities to assess regulatory compliance from time to time and may not be able to demonstrate adequate compliance with applicable regulations. If we, our affiliates, our manufacturers or suppliers are found to be in significant non-compliance or fail to take satisfactory corrective action in response to adverse inspectional findings, the FDA or other applicable regulatory authority could take enforcement actions against us and our manufacturers which could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. Accordingly, our operating results could suffer.
We are subject to the risk of reliance on third parties to conduct our clinical trial work.
We depend on independent clinical investigators to conduct our clinical trials. Contract research organizations may also assist us in the collection and analysis of data. These investigators and contract research organizations will not be our employees and we will not be able to control, other than by contract, the number of resources, including the time that they devote to products that we develop. Nevertheless, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the applicable protocol and legal, regulatory and scientific standards, and our reliance on the investigators or contract research organizations does not relieve us of our regulatory responsibilities. If the independent investigators or contract research organizations fail to devote sufficient resources to our clinical trials, or if their performance is substandard, it will delay the approval or clearance and commercialization of any products that we develop. Further, the FDA and other regulatory bodies around the world require that we comply with GCP standards for conducting, recording and reporting clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity, and confidentiality of trial subjects are protected. If our independent clinical investigators and contract research organizations fail to comply with GCP, the results of our clinical trials could be called into question and the clinical development of our product candidates could be delayed. Failure of clinical investigators or contract research organizations to meet their obligations to us or comply with federal regulations could adversely affect the clinical development of our product candidates and harm our business. Moreover, we intend to conduct several clinical trials in order to support our marketing efforts and business development purposes. Such clinical trials will be conducted by third parties as well. Failure of such clinical trials to meet their primary endpoints could adversely affect our marketing efforts.
If any of our relationships with the investigators or contract research organizations conducting our ongoing or future trials terminate, we may not be able to enter into arrangements with alternative third parties on commercially reasonable terms, or at all. Entering into arrangements with alternative contract research organizations, trial investigators or other third parties involves additional cost and requires management focus and time, in addition to requiring a transition period when a new contract research organization, trial investigator or other third party begins work. If third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain are compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, any clinical trials such third parties are associated with may be extended, delayed or terminated, and we may not be able to obtain marketing authorization for or successfully commercialize our product candidates.
71
Because we have relied on third parties to conduct our clinical trials, our internal capacity to perform these functions is limited. Outsourcing these functions involves risk that third parties may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. In addition, the use of third-party service providers requires us to disclose our proprietary information to these parties, which could increase the risk that this information will be misappropriated. To the extent we are unable to identify and successfully manage the performance of third-party service providers in the future, our business may be adversely affected. Though we carefully manage our relationships with our contract research organizations and investigators, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
Recent initiatives by the FDA to enhance and modernize various regulatory pathways for device products and its overall approach to safety and innovation in the medical technology industry creates the possibility of changing product development costs, requirements, and other factors and additional uncertainty for our future products and business.
Regulatory requirements may change in the future in a way that adversely affects us. Any change in the laws or regulations that govern the clearance and approval processes or the post-market compliance requirements relating to our current and future products could make it more difficult and costly to obtain clearance or approval for new products, or to produce, market and distribute existing products. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing authorization that we otherwise may have obtained, and we may not achieve or sustain profitability, which would adversely affect our business, prospects, financial condition and results of operations.
In recent years, the U.S. government, including the FDA and other government agencies, have been focusing on the cybersecurity risks associated with certain medical devices and encouraging device manufacturers to take a more proactive approach to assessing the cybersecurity risks of their devices both during development and on a periodic basis after the devices are in commercial distribution. For example, in December 2022, the Congress enacted the Consolidated Appropriations Act for 2023, an omnibus appropriations bill, which included amendments to the FDCA under the Food and Drug Omnibus Reform Act of 2022 (“FDORA”). In addition to the requirement that sponsors of pivotal trials submit diversity action plans for pivotal trials (see “Government Regulation—Clinical Trials”), FDORA included new requirements for cyber devices, defined as any medical device that is or includes software that is validated, installed, or authorized by the manufacturer; can connect to the internet; and may be vulnerable to cybersecurity threats. Under the FDORA amendments to the FDCA, any application for marketing authorization of the cyber device must include a software bill of materials and a cybersecurity plan describing the methods by which the manufacturer will monitor, identify and address cybersecurity vulnerabilities. Any failure by a cyber device manufacturer to comply with applicable cybersecurity requirements is considered a violation of the FDCA and will subject the manufacturer to enforcement actions and possibly legal sanctions. Further regulatory efforts by the FDA or other federal or state regulatory authorities could lead to new, onerous cybersecurity requirements in the future as well as additional product liability or other litigation risks if any of our products is considered to be susceptible to third-party tampering.
In addition, Congress passed the 21st Century Cures Act in December 2016, which made multiple changes to the FDA’s rules for medical devices as well as for clinical trials, and the Medical Device User Fee reauthorization package in September 2022, which affects medical device regulation both pre- and post-approval and could have certain impacts on our business. In recent years, the FDA has also considered a series of efforts to modernize and streamline the 510(k) notification and regulatory review process and monitoring post-market safety. For example, as of October 2023, all 510(k) applications (unless specifically exempted) must be submitted to the FDA electronically using the electronic submission template and resource, or eSTAR, and the Center for Devices and Radiological Health (“CDRH”) Portal. Further changes in the FDA 510(k) process could make clearance more difficult to obtain, increase delay, add uncertainty and have other significant adverse effects on our ability to obtain and maintain clearance for our products.
Furthermore, the FDA issued a Final Rule on February 2, 2024 describing amendments to harmonize the QSR with ISO 13485:2016, which will become effective on February 2, 2026. The harmonization process is not expected to have a significant impact on the quality system compliance operations of device manufacturers because most requirements described in the QSR correspond to requirements set forth in ISO 13485:2016.
72
However, device manufacturers will likely need to revise certain quality system procedures to ensure compliance with the harmonized regulations and any failure by us or our third-party manufacturers to make such revisions or adapt to the harmonized regulations, once they become effective, may result in observations of noncompliance during facility inspections by the FDA or comparable regulatory authorities.
Broad-based domestic and international government initiatives to reduce spending, particularly those related to healthcare costs, may reduce reimbursement rates for medical procedures, or make it more difficult for customers to purchase our products and services, all of which could adversely affect our business.
Healthcare reforms, changes in healthcare policies and changes to third-party coverage and reimbursements, including legislation enacted reforming the U.S. healthcare system and both domestic and foreign healthcare cost containment legislation, and any future changes to such legislation, may affect demand for our products and services and may have a material adverse effect on our financial condition and results of operations. Reforms implemented under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, (the “ACA”) in the United States, as well as state-level healthcare reform proposals, could reduce medical procedure volumes and impact the demand for medical device products or the prices at which we can sell products. The impact of healthcare reform legislation, and practices including price regulation, competitive pricing, comparative effectiveness of therapies, technology assessments, and managed care arrangements are uncertain. There can be no assurance that current levels of reimbursement will not be decreased in the future, or that future legislation, regulation, or reimbursement policies of third parties will not adversely affect the demand for our products and services or our ability to sell products and provide services on a profitable basis. The adoption of significant changes to the healthcare system in the United States, the EEA or other jurisdictions in which we may market our products and services, could limit the prices we are able to charge for our products and services or the amounts of reimbursement available for our products and services, could limit the acceptance and availability of our products and services, reduce medical procedure volumes and increase operational and other costs.
Legislative and regulatory changes under the ACA remain possible, but it is unknown what form any such changes or any law would take, and how or whether it may affect the medical device industry as a whole or our business in the future. In addition to the ACA, there have been and will likely continue to be other federal and state changes that affect the provision of healthcare goods and services in the United States. While we are unable to predict what changes may ultimately be enacted, to the extent that future changes affect how our products and services are paid for and reimbursed by government and private payers, our business could be adversely impacted. Moreover, complying with any new legislation or reversing changes implemented under the ACA could be time-intensive and expensive, resulting in a material adverse effect on the business.
In addition, there has been heightened governmental scrutiny, including increasing legislative and enforcement interest, in recent years over the manner in which manufacturers set prices for their marketed healthcare products, which has resulted in several Congressional inquiries and proposed and enacted legislation designed, among other things, to bring more transparency to healthcare product pricing, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for healthcare products. Individual states in the United States have also become increasingly active in implementing regulations designed to control healthcare product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures and, in some cases, mechanisms to encourage importation of healthcare products from other countries. Additionally, third-party payors and governmental authorities have become increasingly interested in reference pricing systems and publication of discounts and list prices.
We are subject to federal, state and foreign laws prohibiting “kickbacks” and false or fraudulent claims, and other fraud and abuse laws, transparency laws, and other healthcare laws and regulations, which, if violated, could subject us to substantial penalties. Additionally, any challenge to or investigation into our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business.
Our relationships with customers and third-party payors are subject to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain our sales, marketing and other promotional activities by limiting the kinds of financial arrangements, including sales programs and certain customer and product support programs, we may have with hospitals, physicians or other purchasers of medical devices.
73
Other federal and state laws generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, or are for items or services that were not provided as claimed. These laws include, among others, the federal Anti-Kickback Statute, the federal civil False Claims Act, other federal healthcare false statement and fraud statutes, the Open Payments program under the Physician Payments Sunshine Act, the Civil Monetary Penalties Law, and analogous fraud and abuse and transparency laws in most states, as described in “Government Regulation—Other U.S. Healthcare Laws and Regulations.” Although the federal laws generally apply only to products or services for which payment may be made by a government healthcare program, state laws often apply regardless of whether federal funds may be involved.
While we believe and strive to ensure that our business arrangements with third parties and other activities and programs comply with all applicable laws, these laws are complex, and our activities may be found not to be compliant with one or more of these laws, which may result in significant civil, criminal and/or administrative penalties, fines, damages and exclusion from participation in government healthcare programs. Even an unsuccessful challenge or investigation into our practices could cause adverse publicity, and be costly to respond to, and thus could have a material adverse effect on our business, financial condition and results of operations. Our compliance with Medicare and Medicaid regulations may be reviewed by federal or state agencies, including the Office of Inspector General for the U.S. Department of Health and Human Services (HHS-OIG), CMS, and the Department of Justice, or may be subject to whistleblower lawsuits under federal and state false claims laws.
Product liability suits, whether or not meritorious, could be brought against us due to an alleged defective product or for the misuse of Dario or our potential future products. These suits could result in expensive and time-consuming litigation, payment of substantial damages, and an increase in our insurance rates.
If Dario or any of our future products are defectively designed or manufactured, contain defective components, or are misused, or if someone claims any of the foregoing, whether or not meritorious, we may become subject to substantial and costly litigation. Misusing our device or failing to adhere to the operating guidelines or the device producing inaccurate meter readings could cause significant harm to patients, including death. In addition, if our operating guidelines are found to be inadequate, we may be subject to liability. Product liability claims could divert management’s attention from our core business, be expensive to defend and result in sizable damage awards against us. While we maintain product liability insurance, we may not have sufficient insurance coverage for all future claims. Any product liability claims brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing continuing coverage, could harm our reputation in the industry and could reduce revenue. Product liability claims in excess of our insurance coverage would be paid out of cash reserves harming our financial condition and adversely affecting our results of operations.
If we are found to have violated laws protecting the confidentiality of patient health information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business.
Part of our business plan includes the storage and potential monetization of medical data of users of Dario. There are a number of federal and state laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services promulgated patient privacy rules under the HIPAA. These privacy rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their own health information and limiting most use and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose. We may face difficulties in holding such information in compliance with applicable law. If we are found to be in violation of the privacy rules under HIPAA, we could be subject to civil or criminal penalties, which could increase our liabilities, harm our reputation and have a material adverse effect on our business, financial condition and results of operations.
In addition to data protection laws passed by the U.S. federal government, many U.S. states and foreign countries have implemented their own data protection laws, some of which may apply simultaneously and conflict with U.S. federal law. Many of these laws create consumer rights including the right to know what personal information is collected, the right to know whether the data is sold or disclosed and to whom, the right to request that a company delete personal information collected, the right to opt-out of the sale of personal information and the right to non-discrimination in terms of price or service when a consumer exercises a privacy right.
74
If we fail to comply with these regulations, we could be subject to civil sanctions, including fines and penalties for noncompliance.
In particular, data protection, privacy, and other laws and regulations adopted in jurisdictions outside of the United States can be more restrictive than corresponding U.S. laws and regulations. Data localization laws in some countries generally mandate that certain types of data collected in a particular country be stored and/or processed within that country. We could be subject to audits in Europe and around the world, particularly in the areas of consumer and data protection, as we continue to grow and expand our operations. Legislators and regulators may make legal and regulatory changes, or interpret and apply existing laws, in ways that make our products less useful to customers, require us to incur substantial costs, expose us to unanticipated civil or criminal liability, or cause us to change our business practices. These changes or increased costs could negatively impact our business and results of operations in material ways. For example, the GDPR imposes requirements in the European Economic Area relating to, among other things, consent to process personal data of individuals, the information provided to individuals regarding the processing of their personal data, the security and confidentiality of personal data, notifications in the event of data breaches and use of third-party processors. The GDPR also imposes restrictions on the transfer of personal data from the European Economic Area to third countries like the United States, although the European Commission recently adopted an adequacy decision for the EU-U.S. Data Privacy Framework. If we fail to comply with these standards, we could be subject to criminal penalties and civil sanctions, including fines and penalties and amounts could be significant.
Our employees, independent contractors, consultants, manufacturers and suppliers may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, consultants, manufacturers and suppliers may engage in fraudulent or illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: (i) the laws of the FDA and other similar foreign regulatory bodies, including those laws requiring the reporting of true, complete and accurate information to such regulators; (ii) manufacturing standards; (iii) healthcare fraud and abuse laws in the United States and similar foreign fraudulent misconduct laws; or (iv) laws that require the true, complete and accurate reporting of financial information or data. These laws may impact, among other things, future sales, marketing and education programs. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commissions, certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials.
Although we have a code of business conduct and ethics, it is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent these activities may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant fines or other sanctions, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, disgorgement, individual imprisonment, additional integrity reporting and oversight obligations, possible exclusion from participation in Medicare, Medicaid and other government healthcare programs, contractual damages, reputational harm, diminished profits and future earnings and curtailment of operations, any of which could adversely affect our ability to operate our business and our results of operations. Whether or not we are successful in defending against any such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these claims or investigations, which could have a material adverse effect on our business, financial condition and results of operations.
75
Risks Related to Our Intellectual Property
The failure to obtain or maintain patents, licensing agreements and other intellectual property could materially impact our ability to compete effectively.
In order for our business to be viable and to compete effectively, we need to develop and maintain, and we will heavily rely on, our proprietary position with respect to our technologies and intellectual property. We filed a PCT application for a “Fluids Testing Apparatus and Methods of Use” in May 2011 which incorporates two U.S. provisional applications submitted in the preceding year. The PCT covers the specific processes related to blood glucose level measurement as well as more general methods of rapid tests of body fluids and has subsequently been converted into several national phase patent applications. We have also filed patent applications for other aspects of the Dario Blood Glucose Monitoring Solution. We have also obtained numerous Web domains.
However, to date, we have only been issued four patents (three of which were issued in the United States) relating to how the Dario Blood Glucose Monitoring System draws power from and transmits data to a smartphone via the audio jack port. None of our other patents have been granted by a patent office. In addition, there are significant risks associated with our actual or proposed intellectual property. The risks and uncertainties that we face with respect to our pending patent and other proprietary rights principally include the following:
| ● | pending patent applications we have filed or will file may not result in issued patents or may take longer than we expect to result in issued patents; |
| ● | we may be subject to interference proceedings; |
| ● | we may be subject to opposition proceedings in foreign countries; |
| ● | any patents that are issued to us may not provide meaningful protection; |
| ● | we may not be able to develop additional proprietary technologies that are patentable; |
| ● | other companies may challenge patents licensed or issued to us; |
| ● | other companies may have independently developed and/or patented (or may in the future independently develop and patent) similar or alternative technologies, or duplicate our technologies; |
| ● | other companies may design their technologies around technologies we have licensed or developed; and |
| ● | enforcement of patents is complex, uncertain and very expensive. |
We cannot be certain that patents will be issued as a result of any of our pending or future applications, or that any of our patents, once issued, will provide us with adequate protection from competing products. For example, issued patents may be circumvented or challenged, declared invalid or unenforceable, or narrowed in scope. In addition, since the publication of discoveries in scientific or patent literature often lags behind actual discoveries, we cannot be certain that we were the first to make our inventions or to file patent applications covering those inventions.
It is also possible that others may have or may obtain issued patents that could prevent us from commercializing our products or require us to obtain licenses requiring the payment of significant fees or royalties in order to enable us to conduct our business. As to those patents that we have licensed, our rights depend on maintaining our obligations to the licensor under the applicable license agreement, and we may be unable to do so.
Costly litigation may be necessary to protect our intellectual property rights and we may be subject to claims alleging the violation of the intellectual property rights of others.
We may face significant expense and liability as a result of litigation or other proceedings relating to patents and intellectual property rights of others. In the event that another party has also filed a patent application or been issued a patent relating to an invention or technology claimed by us in pending applications, we may be required to participate in an interference proceeding declared by the United States Patent and Trademark Office to determine priority of invention, which could result in substantial uncertainties and costs for us, even if the eventual outcome was favorable to us.
76
We, or our licensors, also could be required to participate in interference proceedings involving issued patents and pending applications of another entity. An adverse outcome in an interference proceeding could require us to cease using the technology, substantially modify it or to license rights from prevailing third parties.
The cost to us of any patent litigation or other proceeding relating to our licensed patents or patent applications, even if resolved in our favor, could be substantial, especially given our early stage of development. Our ability to enforce our patent protection could be limited by our financial resources and may be subject to lengthy delays. A third party may claim that we are using inventions claimed by their patents and may go to court to stop us from engaging in our normal operations and activities, such as research, development and the sale of any future products. Such lawsuits are expensive and would consume significant time and other resources. There is a risk that a court will decide that we are infringing the third party’s patents and will order us to stop the activities claimed by the patents. In addition, there is a risk that a court will order us to pay the other party damages for having infringed their patents.
Moreover, there is no guarantee that any prevailing patent owner would offer us a license so that we could continue to engage in activities claimed by the patent, or that such a license if made available to us, could be acquired on commercially acceptable terms. In addition, third parties may, in the future, assert other intellectual property infringement claims against us with respect to our services, technologies or other matters.
We have limited foreign intellectual property rights and may not be able to protect our intellectual property rights throughout the world.
We have limited intellectual property rights outside the United States. Filing, prosecuting and defending patents on devices in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property to the same extent as laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patents to develop their own products and further, may export otherwise infringing products to territories where we have patents, but enforcement is not as strong as that in the United States.
Many companies have encountered significant problems in protecting and defending intellectual property in foreign jurisdictions. The legal systems of certain countries, particularly China and certain other developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property, particularly those relating to medical devices and biopharmaceutical products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. To date, we have not sought to enforce any issued patents in these foreign jurisdictions. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. The requirements for patentability may differ in certain countries, particularly developing countries. Certain countries in Europe and developing countries, including China and India, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In those countries, we and our licensors may have limited remedies if patents are infringed or if we or our licensors are compelled to grant a license to a third party, which could materially diminish the value of those patents. This could limit our potential revenue opportunities. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
We rely on confidentiality agreements that could be breached and may be difficult to enforce, which could result in third parties using our intellectual property to compete against us.
Although we believe that we take reasonable steps to protect our intellectual property, including the use of agreements relating to the non-disclosure of confidential information to third parties, as well as agreements that purport to require the disclosure and assignment to us of the rights to the ideas, developments, discoveries and inventions of our employees and consultants while we employ them, the agreements can be difficult and costly to enforce.
77
Although we seek to enter into these types of agreements with our contractors, consultants, advisors and research collaborators, to the extent that employees and consultants utilize or independently develop intellectual property in connection with any of our projects, disputes may arise as to the intellectual property rights associated with our technology. If a dispute arises, a court may determine that the right belongs to a third party. In addition, enforcement of our rights can be costly and unpredictable. We also rely on trade secrets and proprietary know-how that we seek to protect in part by confidentiality agreements with our employees, contractors, consultants, advisors or others. Despite the protective measures we employ, we still face the risk that:
| ● | these agreements may be breached; |
| ● | these agreements may not provide adequate remedies for the applicable type of breach; |
| ● | our proprietary know-how will otherwise become known; or |
| ● | our competitors will independently develop similar technology or proprietary information. |
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an interest in our patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. In addition, the Israeli Supreme Court ruled in 2012 that an employee who receives a patent or contributes to an invention during his employment may be allowed to seek compensation for such contributions from his or her employer, even if the employee’s contract of employment specifically states otherwise and the employee has transferred all intellectual property rights to the employer. The Israeli Supreme Court ruled that the fact that a contract revokes an employee’s right for royalties and compensation, does not rule out the right of the employee to claim their right for royalties. As a result, it is unclear whether and, if so, to what extent our employees may be able to claim compensation with respect to our future revenue. We may receive less revenue from future products if any of our employees successfully claim for compensation for their work in developing our intellectual property, which in turn could impact our future profitability.
Risks Related to Our Industry
We face intense competition in the digital support solution and the self-monitoring of blood glucose market, and as a result we may be unable to effectively compete in our industry.
In recent years, a number of digitally supported solutions have emerged to manage diabetes and other chronic conditions. Competitors are developing new technologies rapidly and, in some cases, are also expanding to manage other chronic conditions. With our first product, Dario, we compete directly and primarily with large pharmaceutical and medical device companies such as Abbott Laboratories, Asensia (formerly Bayer Diabetes Care), Johnson & Johnson LifeScan, Roche Diagnostics and Sanofi. The first four of these companies has a combined majority market share of the BGMS business and strong research and development capacity for next-generation products. Their dominant market position since the late 1990s, and significant control over the market could significantly limit our ability to introduce Dario or effectively market and generate sales of the product. We will also compete with numerous second-tier and third-tier competitors.
In addition, we only recently transformed our business to primarily focus on the sale of our digital support solution, which joins a crowded field of competitors such as Amazon, Apple and Google. Our competitors vary by intervention (devices, applications, coaching and analytics), by channel (health plan, pharma, provider, employer) and by condition (including, for example, diabetes, MSK, blood hypertension, and others).
78
Certain of our competitors offer this integrated approach in varying degrees, including, among others, Teladoc Health, Inc. and MDLive, an Evernorth company, offer a full array of integrated virtual care solutions for primary care, chronic and behavioral health. While these companies have both scale and scope, they have not yet developed an operating model that deliver their services profitably at scale. There are other more narrowly focused companies such as Omada Health, Inc., Vida Health, Inc., Virta Health Corp, and Welldoc, Inc. that focus on the B2B market. And other D2C oriented companies such as Ro, Inc., Hims & Hers, Inc., Weight Watchers International, Inc., and Noom, Inc., that compete with us in the cardiometabolic and GLP-1 space. Specialized mental health platforms like Spring Care, Inc., Lyra Health, Inc., Headspace, Inc., BetterHelp, Inc. (a division of Teladoc), Calm.com, Inc., and Talkspace, Inc., compete in the B2B behavioral health marketplace.
Competition in the digitally supported solutions market and BGMS market is extremely intense, which can lead to, among other things, price reductions, longer selling cycles, lower product margins, loss of market share and additional working capital requirements. To succeed, we must, among other critical matters, gain consumer acceptance for Dario and potential future devices incorporating our principal technology and offer better strategic concepts, technical solutions, prices and response time, or a combination of these factors, than those of other competitors. If our competitors offer significant discounts on certain products, we may need to lower our prices or offer other favorable terms in order to compete successfully. Moreover, any broad-based changes to our prices and pricing policies could make it difficult to generate revenues or cause our revenues, if established, to decline. Some of our competitors may bundle certain software products offering competing applications for diabetes management at low prices for promotional purposes or as a long-term pricing strategy. These practices could significantly reduce demand for Dario or potential future products or constrain prices we can charge. Moreover, if our competitors develop and commercialize products that are more effective or desirable than Dario or the other products that we may develop, we may not convince our customers to use our products. Any such changes would likely reduce our commercial opportunity and revenue potential and could materially adversely impact our operating results.
If we fail to respond quickly to technological developments our products may become uncompetitive and obsolete.
The BGMS market and other markets in which we plan to compete experience rapid technological developments, changes in industry standards, changes in customer requirements and frequent new product introductions and improvements. If we are unable to respond quickly to these developments, we may lose competitive position, and Dario or any other device or technology may become uncompetitive or obsolete, causing revenues and operating results to suffer. In order to compete, we must develop or acquire new devices and improve our existing device on a schedule that keeps pace with technological developments and the requirements for products addressing a broad spectrum and designers and designer expertise in our industries. We must also be able to support a range of changing customer preferences. For instance, as non-invasive technologies become more readily available in the market, we may be required to adopt our platform to accommodate the use of non-invasive or continuous blood glucose sensors. We cannot guarantee that we will be successful in any manner in these efforts.
If third-party payors do not provide adequate coverage and reimbursement for the use of our products and services, our revenue will be negatively impacted.
In the United States and other jurisdictions such as Germany and England, we expect that our products and services should generally be available for full or partial patient reimbursement by third-party payers. Our success in marketing our services depend and will depend in large part on whether U.S. and international government health administrative authorities, private health insurers and other organizations adequately cover and reimburse customers for the cost of our products and services.
In the United States, we expect to derive nearly all our sales from sales directly to consumers as well as retail pharmacy and DME distributors who typically bill various third-party payors, including Medicare, Medicaid, private commercial insurance companies, health maintenance organizations, health plans and other healthcare-related organizations, to cover all or a portion of the costs and fees associated with our products and services and bill patients for any applicable deductibles or co-payments. Access to adequate coverage and reimbursement for CMS procedures using our products and services (and our other products and services in development) by third-party payors is essential to the acceptance of our products by our customers.
79
Third-party payors, whether foreign or domestic, or governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. In addition, in the United States, no uniform policy of coverage and reimbursement for medical device products and services exists among third-party payors. Therefore, coverage and reimbursement for medical device products and services can differ significantly from payor to payor. In addition, payors continually review new technologies for possible coverage and can, without notice, deny coverage for these new products and procedures. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained, or maintained if obtained.
Reimbursement systems in international markets vary significantly by country and by region within some countries, and reimbursement approvals must be obtained on a country-by-country basis. In many international markets, a product must be approved for reimbursement before it can be approved for sale in that country. Further, many international markets have government-managed healthcare systems that control reimbursement for new devices and procedures. In most markets, there are private insurance systems as well as government-managed systems. If sufficient coverage and reimbursement are not available for our current or future products, in either the United States or internationally, the demand for our products and our revenues will be adversely affected.
Risks Related to Our Operations in Israel
Our principal executive offices and other significant operations are located in Israel, and, therefore, our results may be adversely affected by political, economic and military instability in Israel, including a multi front war against Israel.
Our executive offices and corporate headquarters are located in Israel. In addition, most of our executive officers are residents of Israel, although the majority of our employees are located outside of Israel. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its Arab neighbors. On October 7, 2023, Hamas terrorists infiltrated Israel’s southern border from the Gaza Strip and conducted a series of attacks on civilian and military targets. Hamas also launched extensive rocket attacks on Israeli population and industrial centers located along Israel’s border with the Gaza Strip and in other areas within the State of Israel. Following the attack, Israel’s security cabinet declared war against Hamas and the Israeli military began to call-up reservists for active duty. At the same time, and because of the war declaration against Hamas, the clash between Israel and Hezbollah in Lebanon had escalated to an armed conflict, which included daily attacks on Israel.
Furthermore, certain of our employees may be obligated to perform annual reserve duty in the Israel Defense Forces and are subject to being called up for active military duty at any time. Many Israeli citizens who have served in the army are required to perform reserve duty until they reach the age of 40 or older, depending upon the nature of their military service. Our operations could be disrupted by such call-ups, which may include the call-up of members of our management. Such disruption could materially adversely affect our business, financial condition and results of operations. None of our executive officers have been called up for active military duty.
Our commercial insurance does not cover losses that may occur as a result of events associated with war and terrorism. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained or that it will sufficiently cover our potential damages.
Investors may have difficulties enforcing a U.S. judgment, including judgments based upon the civil liability provisions of the U.S. federal securities laws, against us, or our executive officers and directors or asserting U.S. securities laws claims in Israel.
Certain of our directors and officers are not residents of the United States and whose assets may be located outside the United States. Service of process upon us or our non-U.S. resident directors and officers and enforcement of judgments obtained in the United States against us or our non-U.S. our directors and executive officers may be difficult to obtain within the United States. We have been informed by our legal counsel in Israel that it may be difficult to assert claims under U.S. securities laws in original actions instituted in Israel or obtain a judgment based on the civil liability provisions of U.S. federal securities laws. Israeli courts may refuse to hear a claim based on a violation of U.S. securities laws against us or our officers and directors because Israel may not be the most appropriate forum to bring such a claim.
80
In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel addressing the matters described above. Israeli courts might not enforce judgments rendered outside Israel, which may make it difficult to collect on judgments rendered against us or our officers and directors.
Moreover, among other reasons, including but not limited to, fraud or absence of due process, or the existence of a judgment which is at variance with another judgment that was given in the same matter if a suit in the same matter between the same parties was pending before a court or tribunal in Israel, an Israeli court will not enforce a foreign judgment if it was given in a state whose laws do not provide for the enforcement of judgments of Israeli courts (subject to exceptional cases) or if its enforcement is likely to prejudice the sovereignty or security of the State of Israel.
Risks Related to the Ownership of Our Common Stock
Nasdaq may delist our securities from trading on its exchange, which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions.
On September 16, 2024, we received a written notice from the Nasdaq Stock Market LLC (“Nasdaq”) indicating that we are not in compliance with Nasdaq Listing Rule 5550(a)(2), as our closing bid price for our common stock was below $1.00 per share for the last 30 consecutive business days. Pursuant to Nasdaq Listing Rule 5810(c)(3)(A), we have been granted a 180-calendar day compliance period, or until March 17, 2025, to regain compliance with the minimum bid price requirement. During the compliance period, our common stock will continue to be listed and traded on the Nasdaq Stock Market. To regain compliance, the closing bid price of our common stock must meet or exceed $1.00 per share for at least 10 consecutive business days during the 180-calendar day compliance period.
If we are not in compliance by March 17, 2025, we may be afforded a second 180-calendar day compliance period. To qualify for this additional time, we will be required to meet the continued listing requirement for market value of publicly held shares and all other initial listing standards for Nasdaq with the exception of the minimum bid price requirement, and will need to provide written notice of our intention to cure the deficiency during the second compliance period. If we do not regain compliance within the allotted compliance period(s), including any extensions that may be granted by Nasdaq, Nasdaq will provide notice that our common stock will be subject to delisting.
We intend to monitor the closing bid price of our common stock between now and March 17, 2025, and will consider available options to resolve our noncompliance with the minimum bid price requirement as may be necessary. There can be no assurance that we will be able to regain compliance with the minimum bid price requirement or that we will otherwise be in compliance with other Nasdaq listing criteria.
Our officers and directors may exert significant influence over our affairs, including the outcome of matters requiring stockholder approval.
As of the date of this Annual Report, our officers and directors collectively have a beneficial ownership interest of approximately 14.4% of our Company. As a result, such individuals will have the ability, acting together, to control the election of our directors and the outcome of corporate actions requiring stockholder approval, such as: (i) a merger or a sale of our company, (ii) a sale of all or substantially all of our assets, and (iii) amendments to our certificate of incorporation and bylaws. This concentration of voting power and control could have a significant effect in delaying, deferring or preventing an action that might otherwise be beneficial to our other stockholders and be disadvantageous to our stockholders with interests different from those individuals. Certain of these individuals also have significant control over our business, policies and affairs as officers or directors of our company. Therefore, you should not invest in reliance on your ability to have any control over our company.
81
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they change their recommendations regarding our common stock adversely, the price of our common stock and trading volume could decline.
The trading market for our common stock may be influenced by the research and reports that securities or industry analysts may publish about us, our business, our market or our competitors. If any of the analysts who may cover us change their recommendation regarding our common stock adversely, or provide more favorable relative recommendations about our competitors, the price of our common stock would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the price of our common stock or trading volume to decline.
The market price of our common stock has been extremely volatile and may continue to be volatile due to numerous circumstances beyond our control.
The market price of our common stock has fluctuated, and may continue to fluctuate, widely, due to many factors, some of which may be beyond our control. These factors include, without limitation:
| ● | “short squeezes”; |
| ● | comments by securities analysts or other third parties, including blogs, articles, message boards and social and other media; |
| ● | large stockholders exiting their position in our securities or an increase or decrease in the short interest in our securities; |
| ● | actual or anticipated fluctuations in our financial and operating results; |
| ● | changes in foreign currency exchange rates; |
| ● | the commencement, enrollment or results of our planned or future clinical trials of our product candidates or those of our competitors; |
| ● | the success of competitive drugs or therapies; |
| ● | regulatory or legal developments in the United States and other countries; |
| ● | the success of competitive products or technologies; |
| ● | developments or disputes concerning patent applications, issued patents or other proprietary rights; |
| ● | the recruitment or departure of key personnel; |
| ● | the level of expenses related to our product candidates or clinical development programs; |
| ● | litigation matters, including amounts which may or may not be recoverable pursuant to our officer and director insurance policies, regulatory actions affecting the Company and the outcome thereof; |
| ● | the results of our efforts to discover, develop, acquire or in-license additional product candidates; |
| ● | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
| ● | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
82
| ● | significant lawsuits, including patent or stockholder litigation; |
| ● | variations in our financial results or those of companies that are perceived to be similar to us; |
| ● | market conditions in our market sector; |
| ● | general economic, political, and market conditions and overall fluctuations in the financial markets in the United States and abroad; and |
| ● | investors’ general perception of us and our business. |
Stock markets in general and our stock price in particular have recently experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies and our company. For example, the closing sale prices of our Common Stock from January 1, 2024 through December 31, 2024, ranged from a high of $2.55 per share (on February 15, 2024) to a low of $0.65 per share (on December 18, 2024). During that time, we have not experienced any material changes in our financial condition or results of operations that would explain such price volatility or trading volume; however, we have sold equity which was dilutive to existing stockholders. These broad market fluctuations may adversely affect the trading price of our securities. Additionally, these and other external factors have caused and may continue to cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent our stockholders from readily selling their shares of our common stock and may otherwise negatively affect the liquidity of our common stock.
In addition, if the stock price of our common stock continues to trade at its current level, it may imply as a negative indicator of the valuation of our intangible assets and our goodwill, which could result in an impairment for these assets.
Shares eligible for future sale may adversely affect the market for our common stock and warrants.
From time to time, certain of our stockholders may be eligible to sell all or some of their shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, promulgated under the Securities Act, subject to certain limitations. Specifically, we have 49,585 shares of various classes of preferred stock that are convertible into 39,142,798 shares of common stock, and which are also entitled to receive dividends of up to 4,072,297 shares of common stock. In general, pursuant to Rule 144, after satisfying a six month holding period: (i) affiliated stockholder (or stockholders whose shares are aggregated) may, under certain circumstances, sell within any three month period a number of securities which does not exceed the greater of 1% of the then outstanding shares of common stock or the average weekly trading volume of the class during the four calendar weeks prior to such sale and (ii) non-affiliated stockholders may sell without such limitations, provided we are current in our public reporting obligations. Rule 144 also permits the sale of securities by non-affiliates that have satisfied a one year holding period without any limitation or restriction. Any substantial sale of our common stock pursuant to Rule 144 or pursuant to any resale report may have a material adverse effect on the market price of our securities.
Our compliance with complicated U.S. regulations concerning corporate governance and public disclosure is expensive. Moreover, our ability to comply with all applicable laws, rules and regulations is uncertain given our management’s relative inexperience with operating U.S. public companies.
As a publicly reporting company, we are faced with expensive and complicated and evolving disclosure, governance and compliance laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act and the Dodd-Frank Act, and, to the extent we complete our anticipated public offering, the rules of the Nasdaq Stock Market. New or changing laws, regulations and standards are subject to varying interpretations in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies, which could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. As a result, our efforts to comply with evolving laws, regulations and standards of a U.S. public company are likely to continue to result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities.
83
Moreover, our executive officers have little experience in operating a U.S. public company, which makes our ability to comply with applicable laws, rules and regulations uncertain. Our failure to company with all laws, rules and regulations applicable to U.S. public companies could subject us or our management to regulatory scrutiny or sanction, which could harm our reputation and stock price.
If we fail to maintain effective internal control over financial reporting, the price of our common stock may be adversely affected.
Our internal control over financial reporting may have weaknesses and conditions that could require correction or remediation, the disclosure of which may have an adverse impact on the price of our common stock. We are required to establish and maintain appropriate internal control over financial reporting. Failure to establish those controls, or any failure of those controls once established, could adversely affect our public disclosures regarding our business, prospects, financial condition or results of operations. In addition, management’s assessment of internal control over financial reporting may identify weaknesses and conditions that need to be addressed in our internal control over financial reporting or other matters that may raise concerns for investors. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting or disclosure of management’s assessment of our internal control over financial reporting may have an adverse impact on the price of our common stock.
Anti-takeover provisions in our charter documents and Delaware law could discourage, delay or prevent a change in control of our company and may affect the trading price of our common stock and warrants.
We are a Delaware corporation and the anti-takeover provisions of the Delaware General Corporation Law may discourage, delay or prevent a change in control by prohibiting us from engaging in a business combination with an interested stockholder for a period of three years after the person becomes an interested stockholder, even if a change in control would be beneficial to our existing stockholders. In addition, our certificate of incorporation and bylaws may discourage, delay or prevent a change in our management or control over us that stockholders may consider favorable. Our certificate of incorporation and bylaws:
| ● | authorize the issuance of “blank check” preferred stock that could be issued by our Board of Directors to thwart a takeover attempt; |
| ● | provide that vacancies on our Board of Directors, including newly created directorships, may be filled only by a majority vote of directors then in office; |
| ● | provide that special meetings of stockholders may only be called by our Chairman, Chief Executive Officer and/or President or other executive officer, our Board of Directors or a super-majority (66 2/3%) of our stockholders; |
| ● | place restrictive requirements (including advance notification of stockholder nominations and proposals) on how special meetings of stockholders may be called by our stockholders; |
| ● | do not provide stockholders with the ability to cumulate their votes; and |
| ● | provide that our Board of Directors or a super-majority of our stockholders (66 2/3%) may amend our bylaws. |
84
We are a smaller reporting company and the reduced reporting requirements applicable to smaller reporting companies may make our common stock less attractive to investors.
We are a smaller reporting company (“SRC”) and a non-accelerated filer, which allows us to take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not SRCs or non-accelerated filers, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, reduced disclosure obligations regarding executive compensation in our Annual Report and our periodic reports and proxy statements and providing only two years of audited financial statements in our Annual Report and our periodic reports. We will remain an SRC until (a) the aggregate market value of our outstanding common stock held by non-affiliates as of the last business day our most recently completed second fiscal quarter exceeds $250 million or (b) (1) we have over $100 million in annual revenues and (2) the aggregate market value of our outstanding common stock held by non-affiliates as of the last business day our most recently completed second fiscal quarter exceeds $700 million. We cannot predict whether investors will find our common stock less attractive if we rely on certain or all of these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile and may decline.
We do not currently intend to pay dividends on our common stock in the foreseeable future, and consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We have never declared or paid cash dividends on our common stock and do not anticipate paying any cash dividends to holders of our common stock in the foreseeable future. Consequently, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investments. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
Item 1B. Unresolved Staff Comments
Not applicable.
Item 1C. Cybersecurity
We maintain a comprehensive process for identifying, assessing, and managing material risks from cybersecurity threats as part of our broader risk management system and processes. We obtain input, as appropriate, for our cybersecurity risk management program on the security industry and threat trends from external experts and internal threat intelligence team. A team of dedicated privacy, safety, and security professionals oversees cybersecurity risk management and mitigation, incident prevention, detection, and remediation. Leadership of this team includes professionals with deep cybersecurity expertise, including our Chief Information Security Officer. Our executive leadership team, along with input from the above team, are responsible for our overall enterprise risk management system and processes and regularly consider cybersecurity risks in the context of other material risks to the company.
As part of our cybersecurity risk management system, our incident management team tracks and logs privacy and security incidents across the Company, our vendors, and other third-party service providers to remediate and resolve any such incidents. Significant incidents are reviewed regularly by a cross-functional working group to determine whether further escalation is appropriate. Any incident assessed as potentially being or potentially becoming material is immediately escalated for further assessment, and then reported to designated members of our senior management. We consult with outside counsel as appropriate, including on materiality analysis and disclosure matters, and our senior management makes the final materiality determinations and disclosure and other compliance decisions.
The Audit Committee has oversight responsibility for risks and incidents relating to cybersecurity threats, including compliance with disclosure requirements, cooperation with law enforcement, and related effects on financial and other risks, and it reports any findings and recommendations, as appropriate, to the full Board for consideration. Senior management regularly discusses cyber risks and trends and, should they arise, any material incidents with the Audit Committee.
85
Our business strategy, results of operations and financial condition have not been materially affected by risks from cybersecurity threats, including as a result of previously identified cybersecurity incidents, but we cannot provide assurance that they will not be materially affected in the future by such risks or any future material incidents. For more information on our cybersecurity related risks, see Item 1A Risk Factors of this Annual Report on Form 10-K.
Item 2. Properties
We do not own any real property. Currently, we maintain offices at 5 Tarshish St., Caesarea Industrial Park, 3088900, Israel. On June 6, 2023, we signed a lease agreement for these facilities for a period of 5 years commencing upon the completion of adjustments of the office space. We moved into these offices during August 2023. The rental agreement will be extended automatically for an additional 60 months following expiration of the initial term. The monthly rent and management services under this lease are approximately $21,800.
Item 3. Legal Proceedings
We are currently not a party to any pending legal proceeding, nor is our property the subject of a pending legal proceeding, that we believe is not ordinary routine litigation incidental to our business or otherwise material to the financial condition of our business.
Item 4. Mine Safety Disclosures
Not applicable.
86
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock is quoted on the Nasdaq Capital Market under the symbol “DRIO”.
Record Holders
As of March 3, 2025, we had 328 stockholders of record of our common stock.
Dividends
We have never paid any cash dividends on our common stock. We anticipate that we will retain funds and future earnings to support operations and to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends in the foreseeable future. Any future determination to pay dividends will be at the discretion of our Board of Directors and will depend on our financial condition, results of operations, capital requirements and other factors that our Board of Directors deems relevant. In addition, the terms of any future debt or credit financings may preclude us from paying dividends.
Securities Authorized for Issuance Under Equity Compensation Plans as of December 31, 2024:
The following table provides information as of December 31, 2024, with respect to options outstanding under the Company’s Amended and Restated 2012 Equity Incentive Plan (the “2012 Equity Incentive Plan”), the Company’s 2020 Equity Incentive Plan (the “2020 Equity Incentive Plan”), and the Company’s other equity compensation arrangements.
|
|
|
|
Number of securities |
|
|
|
|
|
|
|
|
|
to be issued upon |
|
Weighted-average |
|
|
|
|
|
|
|
exercise of |
|
exercise price of |
|
Number of securities |
|
|
|
|
|
outstanding options, |
|
outstanding options, |
|
remaining available |
|
Plan category |
|
Forfeited shares (6) |
|
warrants and rights |
|
warrants and rights |
|
for future issuance |
|
Equity compensation plans approved by security holders |
|
334,522 |
|
5,881,675 |
|
$ |
3.24 |
|
7,583,836 |
Equity compensation plans not approved by security holders (1) |
|
|
|
90,000 |
|
$ |
8.41 |
|
— |
Equity compensation plans not approved by security holders (2) |
|
|
|
50,000 |
|
$ |
5.75 |
|
— |
Equity compensation plans not approved by security holders (3) |
|
|
|
20,000 |
|
$ |
18.62 |
|
— |
Equity compensation plans not approved by security holders (4) |
|
|
|
1,931,074 |
|
$ |
2.55 |
|
— |
Equity compensation plans not approved by security holders (5) |
|
|
|
2,250,000 |
|
$ |
1.35 |
|
— |
Total |
|
334,522 |
|
10,222,749 |
|
|
|
|
7,583,836 |
In March 2013, our Board adopted a non-employee director’s remuneration policy.
| (1) | In January 2020, our Board approved the grant of non-plan options as a material inducement for employment, in accordance with Nasdaq Listing Rule 5635(c)(4), to our newly hired President and General Manager for North America. The options have an exercise price of $8.41 per share. 90,000 options are time based and vest over a three-year period. One third vests after one year and the balance vests over eight quarterly installments after the first anniversary; these options have a cashless exercise feature and a six-year term. An additional 90,000 options are performance based, and vest over a three-year period. One third vest after one year and the balance vest over eight quarterly installments after the first anniversary; these options have a cashless exercise feature and a six- |
87
| year term. 22,500 options will commence vesting every calendar year for the next four years, commencing in 2021, and only if certain performance milestones were met in the immediately preceding year. 22,500 of these options have expired on each of January 1, 2021, January 1, 2022, January 1, 2023 and January 1, 2024 as the performance milestones were not met. |
| (2) | In March 2020, our Board approved the grant of certain non-plan options as a material inducement for employment, in accordance with Nasdaq Listing Rule 5635(c)(4), to our newly hired Chief Medical Officer. The options have an exercise price of $5.75 per share, and vest over a three-year period with one third vesting after one year and the balance vesting over eight quarterly installments after the first anniversary; these options have a cashless exercise feature and a six-year term. |
| (3) | In July 2021, our Board approved the grant of certain non-plan options as a material inducement for employment, in accordance with Nasdaq Listing Rule 5635(c)(4), to our newly hired Special Vice President of Market Access. The options have an exercise price of $18.62 per share, and vest over a three-year period with one third vesting after one year and the balance vesting over eight quarterly installments after the first anniversary; these options have a cashless exercise feature and a ten-year term. The option expired on February 4, 2025 following the departure of the employee. |
| (4) | In February 2024, our Board approved the grant of certain non-plan options as a material inducement for employment, in accordance with Nasdaq Listing Rule 5635(c)(4), to the employees of Twill, Inc. The options have an exercise price of $2.55 per share, the options are time based and vest over a two-year period in eight equal amounts. These options have a cashless exercise feature and a ten-year term. |
| (5) | In June 2024, our Board approved the grant of certain non-plan options as a material inducement for employment, in accordance with Nasdaq Listing Rule 5635(c)(4), to our newly hired Chief Commercial Officer. The options have an exercise price of $1.35 per share, 500,000 options are time based and vest over a three-year period. One third vests after one year and the balance vests over eight quarterly installments after the first anniversary; these options have a cashless exercise feature and a ten-year term. An additional 1,750,000 options are performance based, and vest upon achieving personal objective during the years 2025 to 2028. |
| (6) | 334,522 restricted shares of common stock issued to certain of our employees were forfeited, as they were not vested upon certain employee departures. |
On January 23, 2012, our Board of Directors and a majority of the holders of our then outstanding shares of our common stock adopted our 2012 Equity Incentive Plan (which includes both U.S. and Israeli sub-plans). On January 23, 2012, an Israeli sub-plan was adopted under our 2012 Equity Incentive Plan, which sets forth the terms for the grant of stock awards to Israeli employees or Israeli non-employees. The sub-plan was adopted in accordance with the amended sections 102 and 3(i) of Israel’s Income Tax Ordinance. The sub-plan is part of the 2012 Equity Incentive Plan and subject to the same terms and conditions. On September 26, 2016 and November 30, 2016, respectively, our Board of Directors and stockholders approved an amendment to the 2012 Equity Incentive Plan increasing the number of shares of common stock available under the plan to 1,873,000 as well as amended the 2012 Equity Incentive Plan to permit grants of shares of common stock. On February 2, 2017 and March 9, 2017, respectively, our Board of Directors and stockholders approved an amendment to the 2012 Equity Incentive Plan increasing the number of shares of common stock available under the plan to 2,373,000. On October 9, 2017 and December 4, 2017, respectively, our Board of Directors and stockholders approved an amendment to the 2012 Equity Incentive Plan increasing the number of shares of common stock available under the plan to 3,873,000. On March 26, 2018 and May 18, 2018, respectively, our Board of Directors and stockholders approved an amendment to the 2012 Equity Incentive Plan increasing the number of shares of common stock available under the plan to 5,373,000. On October 7, 2018 and November 29, 2018, respectively, our Board of Directors and stockholders approved an amendment to the 2012 Equity Incentive Plan increasing the number of shares of common stock available under the plan to 7,873,000. On September 3, 2019 and November 6, 2019, respectively, our Board of Directors and stockholders approved an amendment to the 2012 Equity Incentive Plan increasing the number of shares of common stock available under the plan to 618,650 on a post reverse stock split basis. On December 26, 2019 and February 5, 2020, respectively, our Board of Directors and stockholders approved an amendment to the 2012 Equity Incentive Plan increasing the number of shares of common stock available under the plan to 1,968,650. The 2012 Equity Incentive Plan expired on January 23, 2022. On September 2, 2020 and October 14, 2020, respectively, our Board of Directors and stockholders approved and adopted the Company’s 2020 Equity Incentive Plan (the “2020 Equity Incentive Plan”), reserving for issuance a pool of 900,000 shares of the Company’s common stock under the plan.
88
On January 1, 2021, the number of shares of common stock available under the plan increased to 1,828,890 according to the terms thereof. On June 7, 2021, the number of shares of common stock available under the plan increased to 2,528,890 according to the terms thereof. On January 1, 2022, the number of shares of common stock available under the plan increased to 3,868,514 according to the terms thereof. On January 1, 2023, the number of shares of common stock available under the plan increased to 5,862,860 according to the terms thereof. On January 1, 2024, the number of shares of common stock available under the plan increased to 8,356,624 according to the terms thereof. On June 25, 2024, the number of shares of common stock available under the plan increased to 11,356,624. On January 1, 2025, the numbers of shares of common stock available under the plan increased to 17,897,652. As of March 3, 2025, there were 7,583,836 shares of Common Stock reserved for issuance thereunder. The Company’s officers and directors are among the persons eligible to receive awards under the 2020 Equity Incentive Plan in accordance with the terms and conditions thereunder.
The purpose of our 2020 Equity Incentive Plan is to attract and retain directors, officers, consultants, advisors and employees whose services are considered valuable, to encourage a sense of proprietorship and to stimulate an active interest of such persons in our development and financial achievements The 2020 Equity Incentive Plan will be administered by the Compensation Committee of our Board of Directors or by the full board, which may determine, among other things, the (a) terms and conditions of any option or stock purchase right granted, including the exercise price and the vesting schedule, (b) persons who are to receive options and stock purchase rights and (c) the number of shares to be subject to each option and stock purchase right. The 2020 Equity Incentive Plan will each provide for the grant of (i) ”incentive” options (qualified under section 422 of the Internal Revenue Code of 1986, as amended) to employees of our company and (ii) non-qualified options to directors and consultants of our company. In addition, our Board of Directors has authorized the appointment of IBI Capital Compensation and Trusts (2004) Ltd. to act as a trustee for grants of options under the Israeli sub-plan to Israeli residents.
In connection with the administration of our 2020 Equity Incentive Plan, our Compensation Committee will:
| ● | determine which employees and other persons will be granted awards under our 2020 Equity Incentive Plan; |
| ● | grant the awards to those selected to participate; |
| ● | determine the exercise price for options; and |
| ● | prescribe any limitations, restrictions and conditions upon any awards, including the vesting conditions of awards. |
Our Compensation Committee will: (i) interpret our 2020 Equity Incentive Plan; and (ii) make all other determinations and take all other action that may be necessary or advisable to implement and administer our 2020 Equity Incentive Plan.
The 2020 Equity Incentive Plan provides that in the event of a change of control event, the Compensation Committee or our Board of Directors shall have the discretion to determine whether and to what extent to accelerate the vesting, exercise or payment of an award.
In addition, our Board of Directors may amend our 2020 Equity Incentive Plan at any time. However, without stockholder approval, our 2020 Equity Incentive Plan may not be amended in a manner that would:
| ● | increase the number of shares that may be issued under such Equity Incentive Plan; |
| ● | materially modify the requirements for eligibility for participation in such Equity Incentive Plan; |
| ● | materially increase the benefits to participants provided by such Equity Incentive Plan; or |
| ● | otherwise disqualify such Equity Incentive Plan for coverage under Rule 16b-3 promulgated under the Exchange Act. |
89
Awards previously granted under our 2020 Equity Incentive Plan may not be impaired or affected by any amendment of such without the consent of the affected grantees.
Option Exercises
To date, no options have been exercised by our directors or officers.
Unregistered Sales of Equity Securities and Use of Proceeds
During the fourth quarter of 2024, we issued an aggregate 418,550 shares of our common stock to certain of our service providers as compensation to them for services rendered.
We claimed exemption from registration under the Securities Act of 1933, as amended, or the Securities Act, for the foregoing transactions under Section 4(a)(2) of the Securities Act.
Prior Awards to Management
On November 4, 2024, our Compensation Committee approved amendments to certain previously issued awards of restricted Common Stock in the aggregate amount of 68,750 and 250,000, respectively, granted to Mr. Erez Raphael, our Chief Executive Officer, and Mr. Zvi Ben David, our Chief Financial Officer, in the aggregate amount of 23,750 and 125,000, respectively, on May 18, 2022 and March 6, 2024 (collectively, the “Prior Awards”) to permit an immediate acceleration of the unvested portion of the Prior Awards in the event of change in control of the Company
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation
Readers are advised to review the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes and other financial information included elsewhere in this Annual Report. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. See “Cautionary Note Regarding Forward-Looking Statements”. You should review the “Risk Factors” section of this Annual Report for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a leading global DTx company revolutionizing the way people manage their health across the chronic condition spectrum to live a better and healthier life. Our mission is to transform how affected individuals manage their health and chronic conditions by empowering our customers to easily manage their conditions and take steps to improve their overall health. Most chronic conditions are driven by personal behaviors and the actions that are or are not taken. We believe that changing these behaviors can dramatically improve our customers’ overall health and substantially reduce unnecessary health spending. However, behavioral change and habit formation are difficult, especially in managing chronic disease and related conditions. Our digital therapeutics endeavor to produce lasting behavior changes in our customers by applying a novel combination of AI-driven dynamic personalization and behavioral science at scale. This allows us to engage and support our customers, and offer them a complete virtual care solution, ideally resulting in improved health outcomes and reduced total cost of care.
Our principal operating subsidiary, LabStyle Innovation Ltd., is an Israeli company (“LabStyle”) with its headquarters in Caesarea, Israel. We were formed on August 11, 2011, as a Delaware corporation with the name LabStyle Innovations Corp. On July 28, 2016, we changed our name to DarioHealth Corp. We began our sales in the direct-to-consumer space, solving first for what we deemed the most difficult problems: how to engage users and support behavior change to improve clinical outcomes in diabetes. Our most developed AI tools leverage the direct-to-consumer experience from over 150,000 members to drive superior engagement and outcomes.
90
In early 2020, we broadened our solutions to include other medical conditions in addition to diabetes, and to serve business customers who seek to improve the health of their stakeholders. We also subsequently acquired Upright, PsyInnovations, Physimax Technology, and most recently Twill, to further our platform. Presently, we have deployed solutions for diabetes, hypertension, pre-diabetes, MSK and behavioral health, which conditions will also be powered by our AI-driven behavior change platform. We are currently delivering our solutions to providers, employers, health plans and pharmaceutical companies. We continue to achieve key benchmarks as we rapidly scale our B2B2C model, including more than 100 total signed contracts as of today. We believe we have a unique and defensible position in the market thanks to our unique solution origin in consumer markets.
On January 26, 2021, Dario, Labstyle, Upright Technologies Ltd., an Israeli limited company, Vertex C (C.I.) Fund L.P. (in its capacity as the representative of the Selling Shareholders), and all holders of Upright’s outstanding securities (the “Selling Shareholders”), entered into a share purchase agreement (the “Upright Agreement”) pursuant to which Dario, through Labstyle, acquired all of the outstanding securities of Upright. The agreement was consummated on February 1, 2021, and Upright now operates as our wholly owned subsidiary. As part of the acquisition, we issued the Selling Shareholders 1,687,612 shares of our common stock and agreed to assume options to purchase up to 100,193 shares of our common stock, subject to certain escrow and indemnity provisions contained in the Upright Agreement (in the aggregate, the “Consideration Shares”). In addition, the shares issued are subject to the terms of a lock-up agreement, pursuant to which the Selling Shareholders (subject to certain exceptions) have agreed to restrict their ability to transfer their shares as follows: (i) shares representing 20% of their respective Consideration Shares will be restricted from transfer for a period of one hundred and eighty (180) days from the date of the closing of the acquisition, (ii) shares representing 30% of their respective Consideration Shares will be restricted from transfer for a period of two hundred and seventy (270) days from the closing date, (iii) shares representing 30% of their respective Consideration Shares will be restricted from transfer for a period of three hundred and sixty (360) days from the closing date and (iv) shares representing 20% of their respective Consideration Shares will be restricted from transfer for a period of four hundred and fifty (450) days from the closing date.
We, along with TWILL Merger Sub, Inc. (“Merger Sub”), Twill and Bilal Khan, solely in his capacity as the representatives of Twill’s stockholders and other equity holders, entered into an Agreement and Plan of Merger (the “Merger Agreement”), dated February 15, 2024 (the “Closing Date”). Pursuant to the provisions of the Merger Agreement, on the Closing Date, (i) Merger Sub was merged with and into Twill (the “Merger”), the separate corporate existence of Merger Sub ceased and Twill continued as the surviving company and a wholly owned subsidiary of the Company, (ii) we paid to Twill’s debt holders and equity holders aggregate consideration (“Merger Consideration”) of (A) $10.0 million in cash, (B) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 10,000,400 shares (the “Warrant Shares”) of our common stock issuable to a trust (the “Trust”) formed for the benefit of certain equity and debt holders of Twill, issuable in 4 equal tranches, (C) stock options to purchase up to 2,963,459 shares of common stock issued to employees of Twill as an inducement to their employment with us, issued outside of our equity compensation plans, pursuant to Nasdaq Rule 5635(c)(4), with an exercise price of $2.55 per share, and (D) a combination of warrants and restricted stock units (“RSUs”) to acquire up to 1,766,508 shares of common stock issued to certain outgoing board members, consultants and outgoing officers of Twill (all of such RSUs and warrants being subject to the approval of the Company’s stockholders, pursuant to Nasdaq Rule 5635), and (iii) the parties to the Merger Agreement consummated the transactions contemplated thereby. The Merger Agreement contains various customary representations, warranties and covenants. As a result of the Merger, Twill will operate as our wholly owned subsidiary.
The Pre-Funded Warrants are subject to a non-waivable 19.99% ownership blocker and the issuance of any shares of common stock underlying such warrants that are in excess of such amount shall be subject to the approval of our stockholders. In addition, the Company, the Trust and WhiteHawk Capital Partner LP (the “Beneficiary”), have executed a Lock Up/Leak Out Agreement (the “Leak Out Agreement”), pursuant to which until such time as the Trust receives $10,600,000 in aggregate net proceeds (the “Leak Out Period”), (i) the Trust shall only be allowed to sell such Warrant Shares at a rate of up to 10% of the average daily trading volume of the common stock in a manner which will not negatively affect the share price, (ii) all such sales shall be conducted pursuant to Rule 144 and (iii) that the Beneficiary shall not cause the Trust to engage in any short selling of such Warrant Shares during the Leak-Out Period. The Company has agreed to seek stockholder approval within 135 days following the closing of the Merger to permit the full exercise of the Pre-Funded Warrants (the “Warrant Vote”). In addition, we entered into voting agreements with certain existing stockholders to vote in favor of the Warrant Vote. We have agreed to call a stockholder meeting each fiscal quarter thereafter to the extent the Warrant Vote is not approved by the Company’s stockholders.
91
Pursuant to the terms of the Merger Agreement, we also agreed to appoint a new member to our board of directors, nominated by Twill equity holders and subject to such nominee being acceptable to us, within 90 days following the closing of the Merger. Such appointment right shall continue until the earlier of 540 days following the closing of the Merger, or the date which the Trust exercises its third tranche of Pre-Funded Warrants.
In addition, we executed certain consulting agreements (the “Consulting Agreements”) with Ofer Leidner and Bilal Khan, each former officers of Twill. Pursuant to the terms of the Consulting Agreements, we agreed to retain the services of Messrs. Leidner and Khan for a period of at least 14 months and 6 months respectively, in exchange for monthly consulting fees of $35,416 and $35,417, respectively. In addition, the Company agreed to issue to Mr. Leidner warrants to purchase up to 1,032,946 shares of common stock, of which 717,946 are subject to time vesting and 315,000 are subject to certain performance-based metrics, and to issue to Mr. Khan 350,000 fully vested RSUs which shall be vest subject to stockholder approval.
Readers are cautioned that, according to our management’s estimates, based on our budget and the initial launch of our commercial sales, we believe that we will have sufficient resources to continue our activity through 2025 without raising additional capital. This includes an amount of anticipated inflows from sales of Dario through direct sales in the United States and through distribution partners. If we are unable to scale up our commercial launch of Dario or meet our commercial sales targets (or if we are unable to ramp up revenues), and if we are unable to obtain additional capital resources in the near term, we may be unable to continue activities, absent material alterations in our business plans, and our business might fail.
Critical Accounting Policies
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). Our fiscal year ends December 31.
This Management’s Discussion and Analysis of Financial Condition and Results of Operations discuss our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these consolidated financial statements requires making estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, as well as the reported revenues and expenses for the reporting periods. On an ongoing basis, we evaluate such estimates and judgments. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ (perhaps significantly) from these estimates under different assumptions or conditions.
While all the accounting policies impact the consolidated financial statements, certain policies may be viewed to be critical. Our management believes that the accounting policies which involve more significant judgments and estimates used in the preparation of our consolidated financial statements, include revenue recognition, inventories, liability related to certain warrants, and accounting for production lines and its related useful life and impairment.
Revenue Recognition
Revenue is recognized under the five-step methodology in accordance with Accounting Standards Codification (“ASC”) 606, “Revenue from contracts with customers,” (“ASC 606”), which requires us to identify the contract with the customer, identify the performance obligations in the contract, determine the transaction price, allocate the transaction price to the performance obligations identified, and recognize revenue when (or as) each performance obligation is satisfied.
We derive our revenue principally from:
Consumers revenue
We consider customer and distributers purchase orders to be the contracts with a customer. For each contract, we consider the promise to transfer tangible products and/or services, each of which are distinct, to be the identified performance obligations. In determining the transaction price, we evaluate whether the price is subject to rebates and adjustments to determine the net consideration to which we expect to receive.
92
As our standard payment terms are less than one year, the contracts have no significant financing component. We allocate the transaction price to each distinct performance obligation based on their relative standalone selling price. Revenue from tangible products is recognized when control of the product is transferred to the customer (i.e., when our performance obligation is satisfied), which typically occurs at shipment. The revenues from fixed-price services are recognized ratably over the contract period and the costs associated with these contracts are recognized as incurred.
Commercial revenue - B2B2C
We provide mobile and web-based digital therapeutics health management programs to employers and health plans for their employees or covered individuals including live clinical coaching, content, automated journeys, hardware, and life-style coaching, currently supporting diabetes, prediabetes and obesity, hypertension, behavioral health (BH) and musculoskeletal health (MSK). At contract inception, we assess the type of services being provided and assess the performance obligations in the contract. Revenue is recognized either on a per engaged member per month (PEMPM) or a per employee per month (PEPM) basis. Our contracts consist of a fixed price that is based on the monthly number of members and clinical programs consumed by each member. The price is determined during contract negotiations with customers.
Certain of our contracts include client performance guarantees and a portion of the fees in those contracts are subject to performance-based metrics such as clinical outcomes or minimum member utilization rate. We include in the transaction price some or all of an amount of variable consideration only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. Refund to a customer that results from performance levels that were not met by the end of the measurement period are adjusted to the transaction price, and therefore estimated at the outset of the arrangement.
In the 2024 fiscal year, our B2B2C channel included 36 new employers and health plan clients, which brought our total client base to 83.
Commercial revenue - Strategic partnerships
We have also entered into contracts with a preferred partner and a health plan provider in which we provide data license, development and implementation services.
Inventories
Inventory write-down is measured as the difference between the cost of the inventory and net realized value based upon assumptions about future demand, and is charged to the cost of sales. At the point of the loss recognition, a new, lower-cost basis for that inventory is established, and subsequent changes in facts and circumstances do not result in the restoration or increase in that newly established cost basis.
If there were to be a sudden and significant decrease in demand for our products or if there were a higher incidence of inventory obsolescence because of rapidly changing technology and customer requirements, we could be required to increase our inventory write-downs and our gross margin could be adversely affected. Inventory and supply chain management remain areas of focus as we balance the need to maintain supply chain flexibility, to help ensure competitive lead times with the risk of inventory obsolescence.
During the year ended December 31, 2024, total inventory write-downs expenses amounted to $301.
Production Lines
Capitalization of Costs. We capitalize direct incremental costs of third-party manufacturers related to the equipment in our production lines. We cease construction cost capitalization relating to our production lines once they are ready for its intended use and held available for occupancy. All renovations and betterments that extend the economic useful lives of assets and/or improve the performance of the production lines are capitalized.
93
Useful Lives of Assets. We are required to make subjective assessments as to the useful lives of our production lines for purposes of determining the amount of depreciation to record on an annual basis with respect to our construction of the production lines. These assessments have a direct impact on our net income (loss). Production lines are usually depreciated on a straight-line basis over a period of up to seven years, except any renovations and betterments which are depreciated over the remaining life of the production lines.
Impairment of production lines. We are required to review our production lines for impairment in accordance with ASC 360, “Property, Plant and Equipment,” whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the assets. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets.
Business combination and asset acquisitions. We apply the provisions of ASC 805, “Business Combination” and allocates the fair value of purchase consideration to the tangible assets acquired, liabilities assumed, and intangible assets acquired based on their estimated fair values. The excess of the fair value of purchase consideration over the fair values of these identifiable assets and liabilities is recorded as goodwill. When determining the fair values of assets acquired and liabilities assumed, management makes significant estimates and assumptions, especially with respect to intangible assets.
Acquisition-related expenses are recognized separately from the business combination and are expensed as incurred.
We account for a transaction as an asset acquisition when substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or group of similar identifiable assets, or otherwise does not meet the definition of a business. Asset acquisition-related direct costs are capitalized as part of the asset or assets acquired
Results of Operations
Comparison of the Year Ended December 31, 2024 to Year Ended December 31, 2023 (dollar amounts in thousands)
Revenues
Revenues for the year ended December 31, 2024, amounted to $27,040 compared to $20,352 during the year ended December 31, 2023. The increase in revenues for the year ended December 31, 2024, compared to the year ended December 31, 2023, resulted from an increase in our revenues from our commercial channel. The revenues also include the consolidation of Twill’s revenues, as a result of its acquisition during the first quarter of 2024. The pro forma revenues for the year ended December 31, 2024, if the closing of the acquisition of Twill would have taken place on the first day of the year would have amounted to $29,003.
Revenues generated during the year ended December 31, 2024, were derived from the sale of services to our commercial customers and consumers located mainly in the United States.
Cost of Revenues
During the years ended December 31, 2024 and 2023, we recorded costs related to revenues in the amount of $13,773 and $14,368, respectively. The decrease in cost of revenues was mainly due to a reduction in payroll related expenses included in the cost of revenues and stock-based compensation during the period, partially offset by an increase in amortization of technology related to the acquisition of Twill, and hosting costs.
Cost of revenues consist mainly of cost of device production, employees’ salaries and related overhead costs, stock-based compensation, depreciation of production lines and related cost of equipment used in production, amortization of technologies, hosting costs, shipping and handling costs and inventory write-downs.
Gross Profit
Gross profit for the year ended December 31, 2024, amounted to $13,267 (49.1% of revenues) compared to $5,984 (29.4% of revenues) for the year ended December 31, 2023
94
. The increase in gross profit as a percentage of revenue for the year ended December 31, 2024, compared to the year ended December 31, 2023, resulted mainly from the increase in the revenues from the commercial channel, mainly related to the acquisition of Twill. Gross profit for the year ended December 31, 2024, excluding amortization of acquired technology, depreciation and stock-based compensation was $18,366 (67.9% of revenues) compared to $10,801 (53.1% of revenues) during the year ended December 31, 2023.
Research and Development Expenses
Our research and development expenses increased by $3,931 to $24,179 for the year ended December 31, 2024, compared to $20,248 for the year ended December 31, 2023. This increase was mainly a result of higher payroll related expenses, software licenses and an increase in subcontractor and consulting expenses due to the consolidation of Twill during the year ended December 31, 2024, partially offset by stock-based compensation and bonus payments. Our research and development expenses, excluding stock-based compensation and depreciation, for the year ended December 31, 2024, were $20,645 compared to $16,367 for the year ended December 31, 2023, an increase of $4,278. This increase was mainly a result of higher payroll related expenses, software licenses, and an increase in subcontractor and consulting expenses due to the consolidation of Twill.
Research and development expenses consist mainly of employees’ salaries and related overhead costs involved in research and development activities, expenses related to: (i) our solutions including our Dario Smart Diabetes Management Solution, Dario Move solution and our digital behavioral health solution, (ii) labor, stock-based compensation contractors and engineering expenses, (iii) depreciation and maintenance fees related to equipment and software tools used in research and development, (iv) clinical trials performed in the United States to satisfy the FDA approval requirements and (v) facilities expenses associated with and allocated to research and development activities.
Sales and Marketing
Our sales and marketing expenses increased by $2,565 to $26,350 for the year ended December 31, 2024, compared to $23,785 for the year ended December 31, 2023. This increase was mainly a result of higher payroll related expenses, amortization of customer relationship, software licenses and an increase in subcontractor and consulting expenses due to the consolidation of Twill, partially offset by a decrease in bonus payments and stock-based compensation expenses during the year ended December 31, 2024. Our sales and marketing expenses, excluding stock-based compensation, depreciation and amortization, for the year ended December 31, 2024, were $20,277 compared to $17,146 for the year ended December 31, 2023, an increase of $3,131. This increase was mainly due to an increase in payroll expenses, subcontractor and consulting services and software licenses resulting from the consolidation of Twill during the year ended December 31, 2024.
Sales and marketing expenses consist mainly of employees’ salaries and related overhead costs, stock-based compensation, online marketing campaigns of our service offering, trade show expenses and marketing consultants, marketing expenses and subcontractors.
General and Administrative Expenses
Our general and administrative expenses increased by $2,342 to $20,482 for the year ended December 31, 2024, compared to $18,140 for the year ended December 31, 2023. The increase was mainly due to higher payroll related expenses and acquisition costs related to the consolidation of Twill during the year ended December 31, 2024, partially offset by a decrease in stock-based compensation. Our general and administrative expenses, excluding stock-based compensation, acquisition costs and depreciation, for the year ended December 31, 2024, were $11,236 compared to $8,663 for the year ended December 31, 2023, an increase of $2,573. This increase was mainly due to higher payroll related expenses and acquisition costs related to the consolidation of Twill.
Our general and administrative expenses consist mainly of employees’ salaries and related overhead costs, stock-based compensation, directors’ fees, legal and accounting fees, patent registration, expenses related to investor relations, as well as our office rent and related expenses.
95
Finance income (expenses), net
Our finance income, net, increased by $16,319 to $13,145 for the year ended December 31, 2024, compared to $3,174 financing expenses for the year ended December 31, 2023. The increase in the finance income, net for the year ended December 31, 2024, compared to the year ended December 31, 2023, was due to income from revaluation of the pre-funded warrants issued as part of the consideration for the acquisition of Twill, as these warrants are classified as a liability under U.S. GAAP.
Financial expenses, net primarily consist of credit facility interest expense, interest income from cash balances, revaluation of warrants, revaluation of short-term investments, bank charges, lease liability and foreign currency translation differences.
Income tax
Income from tax was $1,852 for the year ended December 31, 2024, representing an increase of $1,916 as compared to $64 of income tax expenses for the year ended December 31, 2023. The increase in our income from tax was due to a change in the valuation allowance for deferred tax liability that resulted from the acquisition of Twill.
Net loss
Net loss for the year ended December 31, 2024 was $42,747. Net loss for the year ended December 31, 2023, was $59,427. The decrease from 2023 was mainly due to the increase in our financial income.
Net operating loss carryforwards
As of December 31, 2024, we, WayForward and Twill had a U.S. federal net operating loss carryforward of approximately $192,404, of which $27,270 were generated from tax years 2011-2017 and can be carried forward and offset against taxable income, which expires during the years 2031 to 2037.
On December 22, 2017, the U.S. Tax Cuts and Jobs Act of 2017 (the “TCJA”) modified the rules regarding utilization of net operating loss and net operating losses generated subsequent to the TCJA can only be used to offset 80% of taxable income with an indefinite carryforward period for unused carryforwards (i.e., they should not expire). The remaining net operating losses carryforwards of approximately $165,134 were generated during 2018 - 2024, and are not subject to the annual limitation described above.
Our Israeli subsidiary, Labstyle, accumulated net operating losses for Israeli income tax purposes as of December 31, 2024, in the amount of approximately $221,030. The net operating losses may be carried forward and offset against taxable income in the future for an indefinite period.
In accordance with U.S. GAAP, it is required that a deferred tax asset be reduced by a valuation allowance if, based on the weight of available evidence it is more likely than not (a likelihood of more than 50 percent) that some portion or all of the deferred tax assets will not be realized. The valuation allowance should be sufficient to reduce the deferred tax asset to the amount which is more likely than not to be realized. As a result, we recorded a valuation allowance with respect to our deferred tax asset. Under Sections 382 and 383 of the Internal Revenue Code, if an ownership change occurs with respect to a “loss corporation” (as defined in the Internal Revenue Code), there are annual limitations on the amount of the net operating loss and other deductions which are available to us.
The factors described above resulted in net loss attributable to common stockholders of $40,982 and $63,511 for the year ended December 31, 2024 and 2023, respectively.
Non-GAAP Financial Measures
To supplement our consolidated financial statements presented in accordance with U.S. GAAP within this Annual Report on Form 10-K, management provides certain non-GAAP financial measures (“NGFM”) of the Company’s financial results, including such amounts captioned: “net loss before interest, taxes, depreciation, and amortization” or “EBITDA,” and “Non-GAAP Adjusted Loss,” as presented herein below.
96
Importantly, we note the NGFM measures captioned “EBITDA” and “Non-GAAP Adjusted Loss” are not recognized terms under U.S. GAAP, and as such, they are not a substitute for, considered superior to, considered separately from, nor as an alternative to, U.S. GAAP and /or the most directly comparable U.S. GAAP financial measures.
Such NGFM are presented with the intent of providing greater transparency of information used by us in our financial performance analysis and operational decision-making. Additionally, we believe these NGFM provide meaningful information to assist investors, shareholders, and other readers of our consolidated financial statements, in making comparisons to our historical financial results, and analyzing the underlying financial results of our operations. The NGFM are provided to enhance readers’ overall understanding of our current financial results and to provide further information to enhance the comparability of results between the current year period and the prior year period.
We believe the NGFM provide useful information by isolating certain expenses, gains, and losses, which are not necessarily indicative of our operating financial results and business outlook. In this regard, the presentation of the NGFM herein below, is to help the reader of our consolidated financial statements to understand the effects of the non-cash impact on our (U.S. GAAP) audited statement of operations of the revaluation of the warrants and the expense related to stock-based compensation, each as discussed herein above.
A reconciliation to the most directly comparable U.S. GAAP measure to NGFM, as discussed above, is as follows:
|
|
Year Ended December 31, |
|||||||
|
|
(in thousands) |
|||||||
|
|
2024 |
|
2023 |
|
$ Change |
|||
Net Loss Reconciliation |
|
|
|
|
|
|
|
|
|
Net loss - as reported |
|
$ |
(42,747) |
|
$ |
(59,427) |
|
$ |
16,680 |
|
|
|
|
|
|
|
|
|
|
Adjustments |
|
|
|
|
|
|
|
|
|
Depreciation and impairment expense |
|
|
1,327 |
|
|
473 |
|
|
854 |
Amortization of acquired technology, brand and customer relationship |
|
|
6,100 |
|
|
4,512 |
|
|
1,588 |
Other financial (income) expenses, net |
|
|
(13,145) |
|
|
3,174 |
|
|
(16,319) |
Income tax |
|
|
(1,852) |
|
|
64 |
|
|
(1,916) |
|
|
|
|
|
|
|
|
|
|
EBITDA |
|
|
(50,317) |
|
|
(51,204) |
|
|
887 |
|
|
|
|
|
|
|
|
|
|
Acquisition costs |
|
|
729 |
|
|
128 |
|
|
601 |
Stock-based compensation expenses |
|
|
15,796 |
|
|
19,701 |
|
|
(3,905) |
|
|
|
|
|
|
|
|
|
|
Non-GAAP adjusted loss |
|
$ |
(33,792) |
|
$ |
(31,375) |
|
$ |
(2,417) |
Liquidity and Capital Resources (amounts in thousands except for share and share amounts)
We have incurred net losses since its inception. As of December 31, 2024, we had incurred recurring losses and negative cash flows since inception and has an accumulated deficit of $390,343 as of December 31, 2024. For the year ended December 31, 2024, we used approximately $38,562 of cash in operations. We expect to incur future net losses and our transition to profitability is dependent upon, among other things, the successful development and commercialization of our products and the achievement of a level of revenues adequate to support the cost structure. Until we achieve profitability or generate positive cash flows, we will continue to be dependent on raising additional funds. We intend to fund our future operations through cash on hand, additional private and/or public offerings of debt or equity securities or a combination of the foregoing. There are no assurances, however, that we will be able to obtain an adequate level of financial resources that are required for the long-term development and commercialization of our product offerings.
As of December 31, 2024, we had approximately $27,764 in cash and cash equivalents compared to $36,797 at December 31, 2023.
97
We have experienced cumulative losses of $390,343 from inception (August 11, 2011) through December 31, 2024 and have a stockholders’ equity of $72,019 at December 31, 2024. In addition, we have not completed our efforts to establish a stable recurring source of revenues sufficient to cover our operating costs and expect to continue to generate losses for the foreseeable future.
Since inception, we have financed our operations primarily through private placements and public offerings of our common stock and warrants to purchase shares of our common stock, receiving aggregate net proceeds totaling $282,925 and a credit facility of $25,564 as of December 31, 2024.
On January 26, 2021, we entered into securities purchase agreements with institutional accredited investors relating to an offering with respect to the sale of an aggregate of 3,278,688 shares of the Company’s common stock at a purchase price of $21.35 per share, for aggregate gross proceeds of $70,000. The closing of the offering was consummated on February 1, 2021. The purchase price per share represents the “Minimum Price” of the Company’s Common Stock pursuant to Nasdaq Rule 5635(d) as of the date of execution of each respective securities purchase agreement. We and the investors participating in the offering also executed a registration rights agreement pursuant to which we agreed to file a registration statement covering the resale of the shares within sixty (60) days following the final closing of the offering.
On October 22, 2021, we entered into a Sales Agreement (“Sales Agreement”) with Cowen and Company, LLC (“Cowen”), pursuant to which we may issue and sell shares of our common stock having an aggregate offering price of up to $50,000 from time to time through Cowen. Upon entering into the Sales Agreement, we filed a new shelf registration statement on Form S-3, which was declared effective by the SEC on November 12, 2021. During the year ended December 31, 2023, we sold 408,043 shares of our common stock under the Sales Agreement for aggregate net proceeds of approximately $1,614, The Sales Agreement has since expired.
On February 28, 2022, we entered into securities purchase agreements with institutional accredited investors relating to a registered direct offering with respect to the sale of an aggregate of 4,674,454 shares of our common stock and pre-funded warrants to purchase an aggregate of 667,559 shares of our common stock, at a purchase price of $7.49 per share. The aggregate gross proceeds were approximately $40,000.
On June 9, 2022, we entered into a Credit Agreement (the “Credit Agreement”), with OrbiMed Royalty and Credit Opportunities III, LP (“Orbimed”), as the lender for a five-year senior secured credit facility in an aggregate principal amount of up to $50 million (the “Loan Facility”), of which $25 million was made available on the closing date and up to $25 million was to be made available on or prior to June 30, 2023, subject to certain revenue requirements.
On May 1, 2023, we entered into a Loan and Security Agreement, and Supplement thereto (the “LSA”), with our subsidiary, PsyInnovations, collectively as the borrowers (the “Borrowers”) and Avenue Venture Opportunities Fund II, L.P. and Avenue Venture Opportunities Fund, L.P., collectively as the lenders (the “Avenue Lenders”). The LSA provides for a four-year secured credit facility in an aggregate principal amount of up to $40,000 (the “Loan Facility”), of which $30,000 was made available on the closing date (the “Initial Tranche”) and up to $10,000 (the “Discretionary Tranche”) may be made available on the later of July 1, 2023, or the date the Lender approves the issuance of the Discretionary Tranche. On May 1, 2023, the Borrowers closed on the Initial Tranche, less certain fees and expenses payable to or on behalf of the Avenue Lenders. As a result of the execution of the LSA and the funding of the Initial Tranche, we satisfied our prior Credit Agreement we previously executed with OrbiMed, on June 9, 2022, and terminated the Credit Agreement with Orbimed.
All obligations under the LSA are guaranteed by our wholly owned subsidiary, Labstyle. All obligations under the LSA, and the guarantees of those obligations, are secured by substantially all of our, PsyInnovations’ and the guarantor's assets. Subject to certain milestones set forth in the LSA, the Borrowers shall make monthly payments to the Avenue Lenders of the interest at the then effective rate. If the Borrowers fail to meet the milestones set forth in the LSA, the Borrowers shall make monthly principal installments in advance in an amount sufficient to fully amortize the Loan. The Borrowers shall repay amounts outstanding under the Loan Facility in full immediately upon an acceleration as a result of an event of default as set forth in the LSA.
During the term of the Loan Facility, interest payable in cash by the Borrowers shall accrue on any outstanding balance due under the Loan Facility at a rate per annum equal to the higher of (x) the sum of four one-half percent (4.50%)
98
plus the prime rate as published in the Wall Street Journal and (y) twelve and one-half percent (12.50%). During an event of default, any outstanding amount under the Loan Facility will bear interest at a rate of 5.00% in excess of the otherwise applicable rate of interest. The Borrowers will pay certain fees with respect to the Loan Facility, including an upfront commitment fee, an administration fee and a prepayment premium, as well as certain other fees and expenses of the Avenue Lenders.
On February 15, 2024, we entered into the First Amendment to Loan and Security Agreement and Supplement (the “Avenue Amendment”) with the Avenue Lenders. Pursuant to the Avenue Amendment, the parties agreed to include the Merger Sub and Twill as parties to our existing loan facility with the Avenue Lenders. In addition, the Avenue Amendment provides (i) that we will seek stockholder approval to reprice the warrants issued to the lenders on May 1, 2023 to permit an amendment to the exercise price of such warrants to the “minimum price” as defined by Nasdaq rules as of the closing of the Twill Agreement and (ii) permit the Avenue Lenders, subject to Nasdaq rules, to convert up to two million of the principal amount of its loan to us at a conversion price of $4.001 per share.
On December 16, 2024, we entered into the Third Amendment to Loan and Security Agreement and Supplement (the “Third Avenue Amendment”) with Avenue Lenders. Pursuant to the Third Avenue Amendment, the parties agreed to (i) amend the potential interest only period under the loan facility such that the existing interest only period ending on April 30, 2024 was extended by a period of six months provided that we net certain proceeds from an equity financing on or before March 31, 2025 in the aggregate; (ii) an additional sixth month interest only extension period was added, which is conditioned on our achieving a multi-million dollar net revenue milestone, with cash burn not to exceed a certain multi-million dollar level, for the trailing six month period ending September 30, 2025; (iii) the interest only period may not exceed a total of 36 months from the closing of the loan as of May 1, 2023; and (iv) the maturity date of the loan will be extended from May 1, 2027 to November 1, 2027, provided that we meet the foregoing amended milestones.
In addition, the Third Avenue Amendment provides (i) that we will seek stockholder approval to reprice the warrants issued to the Avenue Lenders on May 1, 2023 to permit an amendment to the exercise price of such warrants to the “minimum price” as defined by Nasdaq rules as of the closing of the Avenue Amendment (or $0.7208 per share) and (ii) permit the Avenue Lenders, subject to Nasdaq rules, to convert up to two million of the principal amount of its loan to us at a conversion price of $0.8650 per share. As of December 31, 2024, we have not yet obtained stockholder approval for the warrant repricing.
In consideration for the Third Avenue Amendment, we agreed to pay a certain amendment fee at closing, and the exit payment due under the loan was increased by a certain amount, in addition to accrued interest and then outstanding principal.
On May 1, 2023, we entered into securities purchase agreements (each, a “Series B Purchase Agreement”) with accredited investors relating to an offering and the sale of an aggregate of 6,200 shares of newly designated Series B Preferred Stock (the “Series B Preferred Stock”), an aggregate of 7,946 shares of Series B-1 Preferred Stock (the “Series B-1 Preferred Stock”), and an aggregate of 150 shares of Series B-2 Preferred Stock (the “Series B-2 Preferred Stock”) at a purchase price of $1,000 for each share of preferred stock. Certain of our executive officers and directors purchased shares of Series B-2 Preferred Stock in the Offering. On May 5, 2023, we entered into purchase agreements (the “Series B-3 Purchase Agreement” and together with the Series B Purchase Agreement, the “Purchase Agreement”) with accredited investors, relating to the Offering, to an offering and the sale of an aggregate of 1,106 shares of newly designated Series B-3 Preferred Stock (the “Series B-3 Preferred Stock”), at a purchase price of $1,000 for each share of preferred stock. As a result of the sale of the preferred stock, the aggregate gross proceeds to us from the Offering are approximately $15.4 million. The closing of the Series B Preferred Stock, Series B-1 Preferred Stock and Series B-2 Preferred Stock occurred on May 4, 2023, and the closing of the Series B-3 Preferred Stock occurred on May 9, 2023.
On May 1, 2023, we executed an agreement (the “Preferred Agreement”) with existing holders of our Series A-1 Convertible Preferred Stock (the “Series A-1 Preferred Stock”). Pursuant to the Preferred Agreement, we agreed to issue such holders of Series A-1 Preferred Stock up to an aggregate of an additional 382,050 shares of common stock, in addition to the 1,273,499 shares of common stock issuable upon conversion of the Series A-1 Preferred Stock, in consideration for such holders agreeing not to convert their shares of Series A-1 Preferred Stock.
99
Such shares of common stock are issuable on the following dates, assuming the Series A-1 Preferred Stock has not yet been converted: (i) up to an aggregate of 63,675 shares of Common Stock before July 1, 2023, if not converted for at least one quarter, (ii) up to an aggregate of 127,350 shares of Common Stock before October 1, 2023, if not converted for at least two quarters, (iii) up to an aggregate of 191,026 shares of Common Stock before January 1, 2024, if not converted for at least three quarters, (iv) up to an aggregate of 254,700 shares of Common Stock before April 1, 2024, if not converted for at least four quarters, and (v) up to an aggregate of 382,050 shares of Common Stock before July 1, 2024, if not converted for at least five quarters. The holders of Series A-1 Preferred Stock will not be entitled to receive any such shares if the issuance of such shares will exceed a non-waivable 19.99% ownership blocker.
On February 15, 2024, we entered into securities purchase agreements (each, a “Series C Purchase Agreement”) with accredited investors relating to an offering (the “Series C Offering”) and the sale of an aggregate of (i) 17,307 shares of newly designated Series C Preferred Stock (the “Series C Preferred Stock”), and (ii) 4,000 shares of Series C-1 Preferred Stock (the “Series C-1 Preferred Stock”), at a purchase price of $1,000 for each share of preferred stock. In addition, on February 16, 2024, we entered into Series C Purchase Agreements with accredited investors relating to the Offering and the sale of an aggregate of 1,115 shares of Series C-2 Preferred Stock (the “Series C-2 Preferred Stock” and together with the Series C Preferred Stock and the Series C-1 Preferred Stock, the “Series C Preferred Stock”), at a purchase price of $1,000 for each share of preferred stock. As a result of the sale of the preferred stock, the aggregate gross proceeds to us from the Series C Offering were approximately $22,422. The closing of the Series C Preferred Stock, Series C-1 Preferred Stock and Series C-2 Preferred Stock occurred on February 21, 2024.
On December 16, 2024, we entered into securities purchase agreements with accredited investors relating to an offering and the sale of an aggregate of (i) 7,055 shares of newly designated Series D Preferred Stock, and (ii) 11,750 shares of Series D-1 Preferred Stock, at a purchase price of $1,000 for each share of preferred stock. As a result of the sale of the preferred stock, the aggregate gross proceeds to us from the offering were approximately $18,805. The closing of the offering occurred on December 18, 2024.
On January 7, 2025, we entered into securities purchase agreements with accredited investors relating to an offering and the sale of an aggregate of (i) 4,950 shares of newly designated Series D-2 Preferred Stock, and (ii) 1,850 shares of Series D-3 Preferred Stock, at a purchase price of $1,000 for each share of preferred stock. As a result of the sale of the preferred stock, the aggregate gross proceeds to us from the offering were approximately $6,800. The closing of the offering occurred on January 14, 2025.
Readers are advised that available resources may be consumed more rapidly than currently anticipated, resulting in the need for additional funding sooner than expected. Should this occur, we will need to seek additional capital earlier than anticipated in order to fund (1) further development and, if needed (2) expenses which will be required in order to expand manufacturing of our products, (3) sales and marketing efforts and (4) general working capital. Such funding may be unavailable to us on acceptable terms, or at all. Our failure to obtain such funding when needed could create a negative impact on our stock price or could potentially lead to the failure of our company. This would particularly be the case if we are unable to commercially distribute our products and services in the jurisdictions and in the timeframes we expect. We believe that we have sufficient cash to fund our operations for at least the next twelve months.
100
Cash Flows (dollar amounts in thousands)
The following tables sets forth selected cash flow information for the periods indicated:
|
|
December 31, |
||
|
|
2024 |
|
2023 |
|
|
$ |
|
$ |
Cash used in operating activities: |
|
(38,562) |
|
(30,379) |
Cash used in investing activities: |
|
(8,934) |
|
(547) |
Cash provided by financing activities: |
|
38,531 |
|
18,253 |
|
|
(8,965) |
|
(12,673) |
Net cash used in operating activities
Net cash used in operating activities was $38,562 for the year ended December 31, 2024, compared to $30,379 used in operations for the year ended December 31, 2023. Cash used in operations increased mainly due to the increase in our operating expenses and an increase in our working capital.
Net cash used in investing activities
Net cash used for investing activities was $8,934 for the year ended December 31, 2024, compared to cash used in investing activities of $547 for the year ended December 31, 2023. The increase is mainly due to the acquisition of Twill in the year ended December 31, 2024, compared to the year ended December 31, 2023.
Net cash provided by financing activities
Net cash provided by financing activities was $38,531 for the year ended December 31, 2024, compared to $18,253 for the year ended December 31, 2023. During the year ended December 31, 2024, we raised net proceeds in an amount of approximately $38,531 through our February and December 2024 private placements.
Contractual Obligations
Set forth below is a summary of our current obligations as of December 31, 2024, to make future payments due by the period indicated below, excluding payables and accruals. We expect to be able to meet our obligations in the ordinary course. Operating lease obligations are for motor vehicle and real property leases which we use in our business. Purchasing obligations consists of outstanding purchase orders for materials and services from our vendors.
|
|
Payments due by period |
||||||||||
|
|
(In U.S. dollars thousands) |
||||||||||
Contractual Obligations |
|
Total |
|
Less than 1 year |
|
1-3 years |
|
Over 4 years |
||||
Operating Lease Obligations |
|
$ |
1,461 |
|
$ |
525 |
|
$ |
936 |
|
$ |
— |
Purchasing Obligations |
|
|
3,423 |
|
|
3,423 |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total contractual cash obligations |
|
$ |
4,884 |
|
$ |
3,948 |
|
$ |
936 |
|
$ |
— |
Contingencies
We account for our contingent liabilities in accordance with ASC 450 “Contingencies”. A provision is recorded when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated.
With respect to legal matters, provisions are reviewed and adjusted to reflect the impact of negotiations, estimated settlements, legal rulings, advice of legal counsel and other information and events pertaining to a particular matter. Currently, we are not a party to any ligation that we believe could have a material adverse effect on our business, financial position, results of operations or cash flows.
101
Recently Issued and Adopted Accounting Pronouncements
In November 2023, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) 2023-07, Segment Reporting (Topic 280), Improvements to Reportable Segment Disclosures, which expands annual and interim disclosure requirements for reportable segments, primarily through enhanced disclosures about significant segment expenses. In addition, it provides new segment disclosure requirements for entities with a single reportable segment. The guidance will be effective for us for annual periods beginning January 1, 2024, and for interim periods beginning January 1, 2025. Early adoption is permitted. We adopted the fiscal year standard for the period beginning January 1, 2024 on a retrospective basis which resulted in updated segment disclosures. For more information on the updated segment disclosures, refer to Note 18 of our consolidated financial statements.
Recently issued accounting pronouncements, not yet adopted:
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740), Improvements to Income Tax Disclosures, which requires disaggregated information about the effective tax rate reconciliation as well as information on income taxes paid. The guidance will be effective for us for annual periods beginning January 1, 2025, with early adoption permitted. We are currently evaluating the impact on its financial statement disclosures.
In November 2024, the FASB issued ASU 2024-03, Income Statement-Reporting Comprehensive Income-Expense Disaggregation Disclosures (Subtopic 220-40) - Disaggregation of Income Statement Expenses. The ASU requires, among other items, additional disaggregated disclosures in the notes to financial statements for certain categories of expenses that are included on the Statements of Operations. ASU 2024-03 is effective for fiscal years beginning after December 15, 2026, and for interim periods within fiscal years beginning after December 15, 2027, with early adoption permitted. We are currently evaluating the effect of adopting the ASU on our disclosures.
Item 7A. Quantitative and Qualitative Disclosure About Market Risk
Not applicable.
Item 8. Financial Statements and Supplementary Data
Our consolidated financial statements and notes thereto and the report of Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global, our independent registered public accounting firm, are set forth on pages F-2 through F-54 of this Annual Report.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, we carried out an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act. Based on that evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded that, at December 31, 2024, such disclosure controls and procedures were effective.
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, as appropriate, to allow timely decisions regarding required disclosure.
102
Limitations on the Effectiveness of Internal Controls
Readers are cautioned that our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will necessarily prevent all fraud and material error. An internal control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our control have been detected. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any control design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2024, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control Over Financial Reporting
As required by the SEC rules and regulations, our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our consolidated financial statements for external reporting purposes in accordance with U.S. GAAP. Our internal control over financial reporting includes those policies and procedures that:
| (1) | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of our company; |
| (2) | provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with accounting principles generally accepted in the United States of America, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| (3) | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the consolidated financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect errors or misstatements in our consolidated financial statements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree or compliance with the policies or procedures may deteriorate. Management assessed the effectiveness of our internal control over financial reporting at December 31, 2024. In making these assessments, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework). Based on our assessments and those criteria, management determined that we maintained effective internal control over financial reporting at December 31, 2024.
Item 9B. Other Information
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
103
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The following sets forth information regarding our executive officers and the members of our Board of Directors as of the date of this Annual Report. All directors hold office for one-year terms until the election and qualification of their successors. Officers are appointed by our Board of Directors and serve at the discretion of our Board of Directors, subject to applicable employment agreements.
Name |
|
Age |
|
Position(s) |
Erez Raphael |
|
51 |
|
Chief Executive Officer and Director |
Zvi Ben David |
|
64 |
|
Chief Financial Officer, Treasurer and Secretary |
Steven Nelson |
|
52 |
|
Chief Commercial Officer |
Yoav Shaked |
|
53 |
|
Chairman of the Board of Directors |
Dennis Matheis |
|
64 |
|
Director |
Hila Karah |
|
56 |
|
Director |
Dennis M. McGrath |
|
68 |
|
Director |
Lawrence Leisure |
|
74 |
|
Director |
Adam Stern |
|
60 |
|
Director |
Erez Raphael has served as our Chief Executive Officer since August 9, 2013 and as a director of our company since December 2013. Mr. Raphael served as Chairman of the Board of Directors from November 2014 to July 2018, and as a director from November 2014 to the present. He previously and until October 2012 served as our Vice President of Research and Development. Mr. Raphael has over 17 years of industry experience, having been responsible in his career for product delivery, technology and business development. Prior to joining us, from 2010 to 2012, Mr. Raphael served as Head of Business Operations for Nokia Siemens Networks, where he was responsible for establishing and implementing a new portfolio business unit directed towards marketing and sales of complimentary products. Prior to that, from 1998 to 2010, he held increasingly senior positions at Amdocs Limited (Nasdaq:DOX) where he was ultimately responsible for advising the Chief Technology Officer and implementing matters of overall business strategy. Mr. Raphael holds a B.A. in economics and business management from Haifa University. We believe Mr. Raphael is qualified to serve on our Board of Directors because of his extensive experience with technology companies and in sales and marketing.
Zvi Ben David has served as our Chief Financial Officer, Treasurer and Secretary since January 7, 2015. Mr. Ben David has over 25 years of experience in corporate and international financial management, including at both publicly-listed and private companies. Since 2012, he has acted as an independent entrepreneur with, and investor in, various medical device ventures. From 2005 to 2012, Mr. Ben David served as the Chief Financial Officer of UltraShape Medical Ltd., a developer, manufacturer and marketer of innovative non-invasive technologies for fat cell destruction and body sculpting. While with UltraShape, he helped lead the company through $35 million in private financing, followed by the company’s merger with a Tel Aviv Stock Exchange company and ultimately the company’s sale to Syneron Medical Ltd. From 2000 to 2005, he served as Vice President and Chief Financial Officer of Given Imaging Ltd., where he was part of the management team that led that company’s 2001 initial public offering and 2004 follow-on offering, and served as a director of that company from its establishment in 1998 to 2000. From 1995 to June 2000, Mr. Ben David served as Vice President and Chief Financial Officer of RDC Rafael Development Corporation, one of Given Imaging Ltd.’s principal shareholders. From 1994 to 1995, Mr. Ben David served as manager of the finance division of Electrochemical Industries (Frutarom) Ltd., an Israeli company traded on the Tel-Aviv Stock Exchange and the American Stock Exchange, and from 1989 to 1993, Mr. Ben David served as the manager of that company’s economy and control department. From 1984 to 1988, Mr. Ben David worked at Avigosh & Kerbs, an accounting firm in Haifa, Israel. Mr. Ben David is a certified public accountant in Israel and holds a B.A. in economics and accounting from Haifa University.
Steven C. Nelson has served as our Chief Commercial Officer since June 5, 2024. From October 2018 to September 2023, Mr. Nelson served as President and Chief Executive Officer of Contigo Health, a Premier Inc. subsidiary, where he previously served as Chief Operating Officer from 2017 to 2018, Vice President of Strategy, Planning, & Innovation from 2015 to 2016 and Chief of Staff in 2016. From 2007 to 2014, he served as Senior Vice President of Strategy, Product, & Marketing at Highmark Inc., a healthcare company. Prior to Highmark, from 2012 to 2014, he served as the Senior Vice President of Executive Oversight at Allegheny Health Network, a healthcare company.
104
Earlier in his career, Mr. Nelson was a Senior Vice President for General Nutrition Centers, General Manager of Brand Marketing & Promotions for MET-Rx &Worldwide Sports Nutrition, Vice President of International Marketing for 141 Communicator, and Director of Marketing & Omnicom Integration for GMR Marketing. Mr. Nelson holds a Bachelor of Science in Education from the University of Pittsburgh at Johnstown and a Master of Arts in Business Administration from Ohio University.
Yoav Shaked has served as the Chairman of our Board of Directors since July 5, 2018. Since 2011, Mr. Shaked has served as a partner at Sequoia Capital, a leading global venture capital firm. In 2005, he co-founded Medpoint Ltd., a private medical device distribution company offering a wide range of medical products. Previously, he founded and served as Chief Executive Officer of Y-Med Inc. from May 2004 through November 2009, until its sale to C.R. Bard, Inc. After the sale of Y-Med Inc., Mr. Shaked served as the director of research at ThermopeutiX, a developer of innovative products for strokes and peripheral artery disease. Mr. Shaked currently serves on the board of directors of several biotechnology companies, including Endospan, Vibrant Gastro, B-Lite (G&G Biotechnology) and Orasis Pharmaceuticals, the latter of which he serves as Chairman of the Board. Mr. Shaked has a B.A. in biology from The Hebrew University of Jerusalem. We believe that Mr. Shaked is qualified to serve as Chairman of the Board because of his extensive experience both in biotechnology companies and in the venture capital realm.
Dennis Matheis has been a director of our company since July 2, 2020. Mr. Matheis spent nearly 30 years in various senior leadership roles in health insurance and healthcare. Since September 2022 he serves as the President and Chief Executive Officer of Sentara Healthcare, Inc. Prior to that, he served for 5 years as the President of Optima Health, Inc. and spent 13 years in leadership roles at Anthem, Inc., serving as President of Central Region and Exchanges encompassing six states and representing $12 billion in annual revenue. Mr. Matheis also served in senior leadership roles at Anthem Blue Cross and Blue Shield of Missouri, CIGNA Healthcare and Humana Health Plan, as well as Advocate Health Care in Chicago. Mr. Matheis has a B.S. in Accounting from the University of Kentucky and practiced as a Certified Public Accountant before entering the healthcare industry. We believe that Mr. Matheis is qualified to serve on our Board of Directors because of his experience in the healthcare business.
Hila Karah has been a director of our company since November 23, 2014. Ms. Karah is an independent business consultant and an investor in several high-tech, biotech and internet companies. From 2006 to 2013, she served as a partner and Chief Investment Officer of Eurotrust Ltd., a family office. From 2002 to 2005, she served as a research analyst at Perceptive Life Sciences Ltd., a New York-based hedge fund. Prior to that, Ms. Karah served as research analyst at Oracle Partners Ltd., a health care-focused hedge fund. Ms. Karah has served as a director in several private and public companies including Intec Pharma, since 2009 and Cyren Ltd since 2008. Ms. Karah holds a B.A. in Molecular and Cell Biology from the University of California, Berkeley, and studied at the University of California, Berkeley-University of California, San Francisco Joint Medical Program. We believe Ms. Karah is qualified to serve on our Board of Directors because of her experience as an investor in and advisor to high-tech, biotech and internet companies.
Dennis M. McGrath has been a director of our company since November 12, 2013. Mr. McGrath is a seasoned medical device industry executive with extensive public company leadership experience possessing a broad range of skills in corporate finance, business development, corporate strategy, operations, and administration. After an 18 year career at PhotoMedex, Inc. (Nasdaq: PHMD), he recently joined PAVmed, Inc (Nasdaq: PAVM, PAVMW) as its Executive Vice President and Chief Financial Officer. Previously, from 2000 to 2017 Mr. McGrath served in several senior level positions of PhotoMedex, Inc. (Nasdaq: PHMD), a global manufacturer and distributor of medical device equipment and services, including from 2011 to 2017 as director, President, and Chief Financial Officer. Prior to PhotoMedex’s reverse merger with Radiancy, Inc. in December 2011, he also served as Chief Executive Officer from 2009 to 2011 and served as Vice President of Finance and Chief Financial Officer from 2000 to 2009. He received honors as a P.A.C.T. (Philadelphia Alliance for Capital and Technology) finalist for the 2011 Investment Deal of the Year, award winner for the SmartCEO Magazine 2012 CEO of the Year for Turnaround Company, and finalist for the Ernst & Young 2013 Entrepreneur of the Year. He has extensive experience in mergers and acquisitions, both domestically and internationally, and particularly involving public company acquisitions, including Surgical Laser Technologies, Inc, (formerly, Nasdaq: SLTI), ProCyte Corporation (formerly, Nasdaq: PRCY), LCA Vision, Inc. (formerly, Nasdaq: LCAV) and Think New Ideas, Inc. (formerly, Nasdaq: THNK). Prior to PhotoMedex, he served in several senior level positions of AnswerThink Consulting Group, Inc.
105
(then, Nasdaq: ANSR, now, The Hackett Group, Nasdaq: HCKT), a business consulting and technology integration company, including from 1999 to 2000 as Chief Operating Officer of the Internet Practice, the largest division of AnswerThink Consulting Group, Inc., while concurrently during the merger of the companies, serving as the acting Chief Financial Officer of Think New Ideas, Inc. (then, Nasdaq: THNK, now, Nasdaq: HCKT), an interactive marketing services and business solutions company. Mr. McGrath also served from 1996 until 1999 as Chief Financial Officer, Executive Vice President and director of TriSpan, Inc., an internet commerce solutions and technology consulting company, which was acquired by AnswerThink Consulting Group, Inc. in 1999. During his tenure at Arthur Andersen & Co., where he began his career, he became a Certified Public Accountant in 1981 and he holds a B.S., maxima cum laude, in accounting from LaSalle University. In addition to serving as the audit and compensation committee chair and director of several biopharmaceutical and medical device companies, including our Company, Citius Pharmaceuticals, Inc. (Nasdaq: CTXR), Citius Oncology, Inc (Nasdaq: CTOR), and LIV Process, Inc. formerly BioVector, Inc. Previously from 2014 to 2024 Mr. McGrath served as a director and audit chair of Cagent Vascular, Inc, and from 2007 to 2009 served as a director of Embrella Cardiovascular, Inc. (sold to Edwards Lifesciences Corporation, NYSE: EW). He also serves on the Board of Trustees for Manor College and the Board of Visitors for Taylor University. We believe Mr. McGrath is qualified to serve on our Board of Directors because of his accounting expertise and his experiences serving as an officer and director of public and private companies.
Lawrence Leisure has been a director of our company since February 25, 2025. Mr. Leisure has extensive business experience consulting and advising in the senior living, provider services, value based care and technology enabled services sectors. Since 2014, Mr. Leisure has served as the Co-Founder and Co-Managing Partner of Chicago Pacific Founders, a private equity firm focused on senior living, provider services, value based care, and technology enabled services. Mr. Leisure is serving on the Board of Directors, and the compensation committee, of P3 Health Partners Inc. (NASDAQ: PIII), a publicly traded health management company. Mr. Leisure has also served on the boards of directors of several private companies backed by Chicago Pacific Founders as well as several venture capital backed companies. Since 2009, he has served as Co-Founder and Chairman of Healthspottr, a strategic networking and consulting firm focused on health innovation and collaboration in the healthcare industry and he also served as the Co-Founder of the Employer Health Innovation Roundtable since 2015. Prior to Chicago Pacific Partners, Mr. Leisure held senior management roles at Accenture, PricewaterhouseCoopers, Towers Perrin, Kaiser Foundation Health Plans and UnitedHealth Group. Mr. Leisure was also employed by the venture capital firm, Kleiner Perkins, as an Operating Partner within the Life Sciences & Digital Health Practice. Mr. Leisure is Chairman of the UCSF Rosenman Institute, member of the UCLA Anderson School of Management Board of Advisors, and a Senior Advisor at the Mussalem Center for BIODESIGN at Stanford University. Mr. Leisure holds a B.A. from Stanford University and an MBA from the University of California-Los Angeles Anderson School of Management. We believe Mr. Leisure is qualified to serve on our Board of Directors because of his accounting expertise and his experiences serving as an officer and director of public and private companies.
Adam Stern has been a director of our company since March 1, 2020. Mr. Stern, has been the head Private Equity Banking at Aegis Capital Corp. and CEO of SternAegis Ventures since 2012 and was a member of our board of directors between October 2011 and May 2014. Prior to Aegis, from 1997 to November 2012, he was with Spencer Trask Ventures, Inc., most recently as a Senior Managing Director, where he managed the structured finance group focusing primarily on the technology and life science sectors. Mr. Stern held increasingly responsible positions from 1989 to 1997 with Josephthal & Co., Inc., members of the New York Stock Exchange, where he served as Senior Vice President and Managing Director of Private Equity Marketing. He has been a FINRA licensed securities broker since 1987 and a General Securities Principal since 1991. Mr. Stern is a director of Aerami Therapeutics Holdings (formerly Dance Biopharm, Inc.), Matinas BioPharma Holdings, Inc. Adgero Biopharmaceuticals Holdings and Hydrofarm Holdings Group, Inc. Mr. Stern is a former director of InVivo Therapeutics Holdings Corp. (OTCQB: NVIV), Organovo Holdings, Inc. (NYSE MKT: ONVO) and PROLOR Biotech Ltd., which was sold to Opko Health, Inc. (NYSE: OPK) for approximately $600 million in 2013. Mr. Stern holds a Bachelor of Arts degree with honors from The University of South Florida in Tampa. We believe Mr. Stern is qualified to serve on our Board of Directors because of his experience in the capital markets, his experiences serving as a director of public and private companies and his experience with life sciences companies.
Scientific Advisory Board
We have established a Scientific Advisory Board (SAB), whose members will be available to us to advise on our scientific and business plans and operational strategies. Below is the biography of our current SAB member.
Dr. David Horwitz – Advisory Board Member, is presently a Senior Consultant with Numerof & Associates and also President of DLH Biomedical Consulting. He previously served as the Global Chief Medical Officer of the Johnson
106
and Johnson Diabetes Institute. Prior to this, he was Vice President, Worldwide Clinical Affairs & Evidence-Based Medicine at LifeScan, Inc., a Johnson & Johnson company. During his time at LifeScan, Dr. Horwitz had, at various times, been responsible for Clinical Research, Medical Affairs, Regulatory Affairs, and Advocacy & Professional Affairs. Dr. Horwitz has previously held faculty positions in the medical schools at the University of Chicago and the University of Illinois, where he was a clinical professor of medicine. He is a Board-Certified internist and endocrinologist, and a Fellow of the American College of Physicians. He has published over 100 articles in scientific and clinical journals, primarily in the areas of diabetes and metabolism. He has completed a term as an industry representative on the Clinical Chemistry and Toxicology advisory panel of the U.S. Food and Drug Administration. He is presently serving as a volunteer physician for a charity-supported clinic.
Dr. Marilyn Ritholz –Advisory Board Member, is a Senior Psychologist at the Joslin Diabetes Center and treats both adults and adolescents with diabetes. In addition, she is on the faculty at Beth Israel Deaconess Medical Center (BIDMC) and is an Assistant Professor of Psychology at Harvard Medical School. Dr. Ritholz is an experienced qualitative researcher. In collaboration with colleagues, she has explored qualitative aspects of healthcare regarding the patient-provider relationship, provider communication about diabetes complications, and psychosocial factors associated with diabetes technology, including continuous glucose monitoring. She has published more than 20 qualitative articles on these topics.
Arnaud Robert – Advisory Board Member, is an accomplished international executive with 25 years of experience in creating new strategies, transforming companies, and driving business outcomes by leveraging digital, AI, and technology. His journey through the realms of Disney, Nike, Viking Cruises, Shaw Communications, and most recently at Sanofi as the EVP & Chief Digital Officer, has been marked by thoughtful leadership and deep digital transformations across business, operations, technology, people, and culture. He also launched several best-in-class experiences, including the Nike Apple watch running app and Disney Movies Anywhere (precursor to Disney+), both used by millions of consumers and widely profiled in the media. Currently, Arnaud is managing director of an advisory firm that helps CEOs, Private Equity Partners, and a large consultancy achieve their strategic objectives through M&A, operational excellence, and digital / AI / GenAI solutions. Arnaud was voted Business Transformation Top 150 (2022), Top 100 Global CDOs (2022 & 2023), and is a former member of the World Economic Forum Media Council. He holds a PhD in Computer Science from the Swiss Institute of Technology and has filed 50 patents.
Board Composition
Our business is managed under the direction of our Board of Directors. Our Board of Directors currently consists of seven members.
Pursuant to the terms of the placement agency agreement between us and Aegis Capital Corp., dated October 22, 2019, we granted Aegis the right to nominate an individual to the Board of Directors for a period of three years, which resulted in the appointment of Mr. Stern to serve on our Board of Directors.
There are no arrangements between our directors and any other person pursuant to which our directors were nominated or elected for their positions.
Board Committees
Our Board of Directors has three standing committees: An Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee.
Audit Committee
Our Audit Committee is comprised of Messrs. Shaked, McGrath and Matheis, each of whom is an independent director. Mr. McGrath is the Chairman of the Audit Committee. Mr. McGrath is an “audit committee financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K.
Our Audit Committee oversees our corporate accounting, financial reporting practices and the audits of financial statements. For this purpose, the Audit Committee has a charter (which is reviewed annually) and performs several functions.
107
The Audit Committee charter is available on our website at dariohealth.investorroom.com under the “For Investors / Corporate Governance” section. The Audit Committee:
| ● | evaluates the independence and performance of, and assesses the qualifications of, our independent auditor and engage such independent auditor; |
| ● | approves the plan and fees for the annual audit, quarterly reviews, tax and other audit-related services and approve in advance any non-audit service to be provided by our independent auditor; |
| ● | monitors the independence of our independent auditor and the rotation of partners of the independent auditor on our engagement team as required by law; |
| ● | reviews the financial statements to be included in our Annual Report on Form 10-K and Quarterly Reports on Form 10-Q and reviews with management and our independent auditor the results of the annual audit and reviews of our quarterly financial statements; and |
| ● | oversees all aspects our systems of internal accounting control and corporate governance functions on behalf of the Board of Directors. |
Compensation Committee
Our Compensation Committee is comprised of Messrs. Shaked, McGrath and Ms. Karah. Mr. McGrath is the Chairman of the Compensation Committee.
The Compensation Committee reviews or recommends the compensation arrangements for our management and employees and also assists our Board of Directors in reviewing and approving matters such as company benefit and insurance plans, including monitoring the performance thereof. The Compensation Committee has a charter (which is reviewed annually) and performs several functions. The Compensation Committee charter is available on our website at dariohealth.investorroom.com under the “For Investors / Corporate Governance” section.
The Compensation Committee has the authority to directly engage, at our expense, any compensation to consultants, or other advisers as it deems necessary to carry out its responsibilities in determining the amount and form of employee, executive and director compensation.
Nominating and Corporate Governance Committee
Our Nominating and Corporate Governance Committee is currently comprised of Messers. Matheis and Shaked. Mr. Matheis is the Chairman of the Nominating and Corporate Governance Committee.
The Nominating and Corporate Governance Committee is charged with the responsibility of reviewing our corporate governance policies and with proposing potential director nominees to the Board of Directors for consideration. This committee also has the authority to oversee the hiring of potential executive positions in our company. The Nominating and Corporate Governance Committee operates under a written charter, which will be reviewed and evaluated at least annually.
Director Independence
Our Board of Directors has reviewed the materiality of any relationship that each of our directors has with us, either directly or indirectly. Based on this review, our Board of Directors has determined that, Messrs. Shaked, Matheis and McGrath, and Ms. Karah are “independent directors” as defined in the Nasdaq Listing Rules and Rule 10A-3 promulgated under the Exchange Act.
Code of Ethics
On March 5, 2013, our Board of Directors adopted a Code of Business Conduct and Ethics and Insider Trading Policy which applies to all insiders including our principal executive officer, principal financial officer, and principal accounting officer.
108
Our Code of Business Conduct and Ethics is available on our website at www.mydario.com under the Investors/Governance section. The information on our website is not incorporated by reference into this Report. We intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding amendment to, or waiver from, a provision of our Code of Ethics by posting such information on the website address specified above.
Insider Trading Policy
We have adopted an insider trading policy, governing the purchase, sale and other transactions in our securities that applies to our directors, executive officers, employees, and other covered persons, including immediate family members and entities controlled by any of the foregoing persons, as well as by the Company itself.
The insider trading policy prohibits, among other things, insider trading and certain speculative transactions in our securities (including short sales, buying put and selling call options and other hedging or derivative transactions in our securities) and establishes a regular blackout period schedule during which directors, executive officers, employees, and other covered persons may not trade in the Company’s securities, as well as certain pre-clearance procedures that directors and executive officers must observe prior to effecting any transaction in our securities.
We believe that the insider trading policy is reasonably designed to promote compliance with insider trading laws, rules and regulations, and listing standards applicable to us. A copy of the insider trading policy is filed as Exhibit 19.1 to this Form 10-K.
Limitation of Directors Liability and Indemnification
The Delaware General Corporation Law authorizes corporations to limit or eliminate, subject to certain conditions, the personal liability of directors to corporations and their stockholders for monetary damages for breach of their fiduciary duties. Our certificate of incorporation limits the liability of our directors to the fullest extent permitted by Delaware law.
We have director and officer liability insurance to cover liabilities our directors and officers may incur in connection with their services to us, including matters arising under the Securities Act. Our certificate of incorporation and bylaws also provide that we will indemnify our directors and officers who, by reason of the fact that he or she is one of our officers or directors, is involved in a legal proceeding of any nature.
We have entered into indemnification agreements with our directors and officers pursuant to which we agreed to indemnify each director and officer for any liability he or she may incur by reason of the fact that he or she serves as our director or officer, to the maximum extent permitted by law.
There is no pending litigation or proceeding involving any of our directors, officers, employees or agents in which indemnification will be required or permitted. We are not aware of any threatened litigation or proceeding that may result in a claim for such indemnification.
109
Item 11. Executive Compensation
The following table summarizes compensation of our named executive officers, as of December 31, 2024 and 2023.
Summary Compensation Table
|
|
|
|
|
|
|
|
|
|
|
|
|
Option |
|
Non-equity |
|
Non-qualified |
|
All Other |
|
|
|
||
Name and |
|
|
|
|
|
|
|
|
|
|
|
|
Awards |
|
incentive plan |
|
incentive plan |
|
Compensation |
|
Total |
|||
Principal Position |
|
Year |
|
Salary ($)* |
|
Bonus ($) |
|
Stock Awards |
|
($)** |
|
compensation |
|
compensation |
|
($) |
|
($) |
||||||
Erez Raphael |
|
2024 |
|
$ |
448,293 |
(1) |
$ |
— |
(2) |
$ |
1,344,000 |
(3) |
$ |
— |
|
— |
|
— |
|
$ |
169,791 |
(4) |
$ |
1,962,084 |
(Chief Executive Officer) |
|
2023 |
|
$ |
446,307 |
(1) |
$ |
101,939 |
(2) |
$ |
— |
(3) |
$ |
— |
|
— |
|
— |
|
$ |
173,941 |
(4) |
$ |
722,187 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Zvi Ben David |
|
2024 |
|
$ |
327,311 |
(5) |
|
— |
(6) |
$ |
672,000 |
(7) |
$ |
— |
|
— |
|
— |
|
$ |
87,144 |
(8) |
$ |
1,086,455 |
(Chief Financial Officer) |
|
2023 |
|
$ |
243,419 |
(5) |
|
31,479 |
(6) |
$ |
— |
(7) |
$ |
— |
|
— |
|
— |
|
$ |
67,686 |
(8) |
$ |
342,584 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Richard Anderson |
|
2024 |
|
$ |
345,000 |
(9) |
|
— |
(10) |
$ |
— |
(11) |
$ |
962,250 |
(12) |
— |
|
— |
|
$ |
59,074 |
(12) |
$ |
1,366,324 |
(Former President) |
|
2023 |
|
$ |
400,000 |
(9) |
|
80,000 |
(10) |
$ |
— |
(11) |
$ |
— |
(12) |
— |
|
— |
|
$ |
56,648 |
(12) |
$ |
536,648 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Steven Nelson |
|
2024 |
|
$ |
212,308 |
(13) |
$ |
— |
|
$ |
— |
|
$ |
532,500 |
(14) |
— |
|
— |
|
$ |
44,564 |
(15) |
$ |
789,372 |
(Chief Commercial Officer) (20) |
|
2023 |
|
$ |
— |
(13) |
$ |
— |
|
$ |
— |
|
$ |
— |
(14) |
— |
|
— |
|
$ |
— |
(15) |
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tomer Ben Kiki |
|
2024 |
|
$ |
384,041 |
(16) |
$ |
— |
|
$ |
— |
|
$ |
1,573,421 |
(17) |
— |
|
— |
|
$ |
38,690 |
(18) |
$ |
1,996,152 |
(Former Chief Operations Officer) (20) |
|
2023 |
|
$ |
— |
(16) |
$ |
— |
|
$ |
— |
|
$ |
— |
(17) |
— |
|
— |
|
$ |
— |
(18) |
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
Certain compensation paid by the company is denominated in New Israeli Shekel (or the NIS). Such compensation is calculated for purposes of this table based on the annual average currency exchange for such period. |
** |
Amount shown does not reflect dollar amount actually received. Instead, this amount reflects the aggregate grant date fair value of each stock option granted in the fiscal years ended December 31, 2024, and December 31, 2023, computed in accordance with the provisions of ASC 718 “Compensation-Stock Compensation” (“ASC 718”). Assumptions used in accordance with ASC 718 are included in Note 16 to our consolidated financial statements included in this Annual Report. |
| (1) | In accordance with his second amendment to the employment agreement with our company effective August 11, 2013, Mr. Raphael was initially entitled to a monthly salary of NIS 44,000. Since August 2013, the Compensation Committee has approved several increases to Mr. Raphael’s monthly salary, most recently on April 1, 2021, when his monthly salary was increased to NIS 137,466 (approximately $37,078 per month). |
| (2) | In March 2023, Mr. Raphael was paid a bonus of $101,939 for his performance during 2022. |
| (3) | On March 6, 2024, Mr. Raphael was granted 800,000 restricted shares of our common stock under our 2020 Equity Incentive Plan. |
| (4) | In addition to his salary, Mr. Raphael is entitled to receive a leased automobile and mobile phone during his employment as well as reimbursements for expenses accrued. These benefits, as well as other social benefits under Israeli law, are included as part of his “All Other Compensation.” |
| (5) | In accordance with his employment agreement with our company effective January 8, 2015, Mr. Ben David was initially entitled to a monthly salary and additional compensation (excluding social benefits under applicable Israeli law) of NIS 31,200 (approximately $8,415) for providing eighty percent of his working time to our company. Beginning on March 1, 2015, Mr. Ben David began working for us on a full-time basis pursuant to the terms of his employment agreement at which point Mr. Ben David’s salary was increased to NIS 39,000 (approximately $10,519 per month. Since March 2015, the Compensation Committee has approved several increases to Mr. Ben David’s salary, most recently on March 1, 2024, when his monthly salary was updated to NIS 105,000 (approximately $28,471) |
| (6) | In March 2023, Mr. Ben David was paid a bonus of $31,479 for his performance during 2022. |
110
| (7) | On March 6, 2024, Mr. Ben David was granted 400,000 restricted shares of our common stock under our 2020 Equity Incentive Plan. |
| (8) | In addition to his salary, Mr. Ben David is entitled to receive a mobile phone during his employment as well as reimbursements for expenses accrued. These benefits, as well as other social benefits under Israeli law, are included as part of his “All Other Compensation.” |
| (9) | In accordance with his employment agreement, effective in January 2020, Mr. Anderson was entitled to a monthly salary of $27,916.67. As of April 2022, Mr. Anderson was entitled to a monthly salary of $33,333.33. |
| (10) | In April 2023, Mr. Anderson was paid a bonus of $80,000 for his performance during 2022. |
| (11) | On March 6, 2024, Mr. Anderson was granted 750,000 options to purchase shares of our common stock under our 2020 Equity Incentive Plan, at an exercise price of $1.68 per share. |
| (12) | In addition to his salary, Mr. Anderson was entitled to participate in any and other benefit plans and programs that we may offer to our employees from time to time according to the terms of such plans and our practices and policies as well as reimbursements for expenses accrued. These benefits are included as part of his “All Other Compensation.” |
| (13) | In accordance with his employment agreement, effective in June 2024, Mr. Nelson was entitled to a monthly salary of $33,333.33. |
| (14) | On June 5, 2024, Mr. Nelson was granted 500,000 options to purchase shares of our common stock as an inducement grant pursuant to Nasdaq Listing Rule 5635 (c)(4), at an exercise price of $1.35 per share. |
| (15) | In addition to his salary, Mr. Nelson is entitled to participate in any and other benefit plans and programs that we may offer to our employees from time to time according to the terms of such plans and the Company’s practices and policies as well as reimbursements for expenses accrued. These benefits are included as part of his “All Other Compensation.” |
| (16) | In accordance with his employment agreement, effective in February 2024, Mr. Ben-Kiki was entitled to a monthly salary of $35,417. |
| (17) | On February 15, 2024, Mr. Ben-Kiki was granted 717,946 options to purchase shares of our common stock as an inducement grant pursuant to Nasdaq Listing Rule 5635 (c)(4), at an exercise price of $2.55 per share. On November 4, 2024, Mr. Ben-Kiki was granted a Warrant to purchase 319,653 shares of our common stock at an exercise price of $0.9475 per share. |
| (18) | In addition to his salary, Mr. Ben-Kiki was entitled to participate in any and other benefit plans and programs that we may offer to our employees from time to time according to the terms of such plans and our practices and policies as well as reimbursements for expenses accrued. These benefits are included as part of his “All Other Compensation.” |
All compensation awarded to our executive officers was independently reviewed by our Compensation Committee.
Employment and Related Agreements
Except as set forth below, we currently have no other written employment agreements with any of our officers and directors. The following is a description of our current executive employment agreements:
Erez Raphael, Chief Executive Officer and a Member of the Board of Directors – On August 30, 2013, LabStyle Innovation Ltd., our Israeli subsidiary, entered into an amendment to a Personal Employment Agreement with Mr. Raphael in connection with his August 2013 appointment as our President and Chief Executive Officer. Pursuant to the terms of his employment agreement as amended, Mr. Raphael is entitled to a monthly salary of NIS 137,466 (approximately $37,275) and a target bonus equal to 75% of his annual base salary.
111
On July 25, 2017, we, through our Israeli subsidiary, LabStyle Innovation Ltd., executed an Amended and Restated Employment Agreement with Mr. Raphael. Pursuant to the agreement, Mr. Raphael kept his monthly salary and shall be eligible for an annual bonus equal to up to 60% of his annual base salary. Mr. Raphael’s employment agreement expires on December 31, 2020. In the event Mr. Raphael’s employment agreement is terminated by us at will, by Mr. Raphael for good reason as provided thereby, or in conjunction with a change of control, Mr. Raphael shall be entitled to receive 24 months base salary and severance payment pursuant to applicable Israeli severance law, provided, however, that in the event such termination occurs during the final year of the term, or within the last 6 months of a renewal period of the term, Mr. Raphael shall be entitled to receive 12 months base salary and severance payment pursuant to applicable Israeli severance law. In the event the employment agreement is terminated by us for cause, Mr. Raphael will only be entitled to a severance pay under applicable Israeli severance law. Mr. Raphael’s employment agreement also includes a one-year non-competition and non-solicitation provision, certain confidentiality covenants and assignment of any of his company-related inventions. Under the terms of the agreement, Mr. Raphael is entitled to certain expense reimbursements and other standard benefits, including vacation, sick leave, contributions to a manager’s insurance policy and study fund and car and mobile phone allowances. On February 12, 2020, we extended the term of Mr. Raphael’s employment to expire on December 31, 2022.
Zvi Ben David, Chief Financial Officer, Treasurer and Secretary – On January 8, 2015, LabStyle Innovation Ltd., our Israeli subsidiary, entered into a Personal Employment Agreement with Mr. Ben David. Pursuant to the terms of his employment agreement, as amended, Mr. Ben David is entitled to a monthly salary of NIS 105,000 (approximately $28,471) and a target bonus equal to 40% of his base salary.
Mr. Ben David's employment agreement may be terminated by either party at will upon 90 days prior written notice or terminated by us for cause, as defined under the employment agreement. In the event the employment agreement is terminated by us at will, Mr. Ben David shall be entitled to receive 90 days of severance plus any required severance payment pursuant to applicable Israeli severance law. In the event the employment agreement is terminated by us for cause, Mr. Ben David will only be entitled to a severance pay under applicable Israeli severance law. The employment agreement also includes a twelve-month non-competition and non-solicitation provision, certain confidentiality covenants and assignment of any of his company-related inventions to the company. Under the terms of the employment agreement, Mr. Ben David is entitled to certain expense reimbursements and other standard benefits, including vacation, sick leave, contributions to a manager’s insurance policy and study fund and mobile phone allowances.
Steven Nelson, Chief Commercial Officer – On June 5, 2024, we appointed Mr. Nelson as our Chief Commercial Officer. In connection with Mr. Nelson’s appointment, we agreed to pay Mr. Nelson an annual base salary of $400,000. Mr. Nelson shall also be subject to a six-month non-competition and one-year non-solicitation provision, certain confidentiality covenants and assignment of any of his company-related inventions. Mr. Nelson will also be entitled to certain expense reimbursements and other standard benefits, including vacation and sick leave. In addition, Mr. Nelson will be entitled to receive an annual incentive bonus of up to $400,000, subject to certain milestones and performance targets. In conjunction with his appointment as Chief Commercial Officer, we agreed to issue Mr. Nelson a stock option to purchase up to 500,000 shares of common stock, with such shares vesting over three years, with one third of such shares vesting on June 1, 2025, and the remaining shares vesting in equal quarterly amounts. In addition, we agreed to issue Mr. Nelson a stock option to purchase 400,000 shares of common stock, of which 150,000 shares commence vest upon our achieving 92% of a targeted revenue amount for the year ended December 31, 2024, and the remaining balance of 250,000 shares if the annual revenue is 100% or more of a targeted amount, provided, however that such options shall be canceled if the annual revenue does not reach at least 92% of the targeted amount. We also agreed to issue Mr. Nelson a stock option to purchase 450,000 shares of common stock, for each of the years ending December 31, 2025, December 31, 2026 and December 2027, of which 150,000 shares commence vesting if the Company’s annual revenue is at least 92% of a targeted amount, 150,000 shares commence vesting if the Company’s annual revenue is 100% or more of the targeted amount and an additional 150,000 shares will commence vesting upon reaching certain annual goals as determined by the Company’s Board of Directors for such fiscal year, provided, however that such options shall be canceled if the annual revenue does not reach at least 92% of the targeted amount. Each of the performance options vests over a three-year period commencing on the first day of the following fiscal year to which such option relates, at the rate of 8.33% per quarter, at the last day of the relevant fiscal quarter. All options were granted as an inducement material to Mr. Nelson becoming an employee of the Company, in accordance with Nasdaq Listing Rule 5635(c)(4).
112
Richard Anderson, Former President and General Manager of North America – On January 7, 2020, we appointed Mr. Anderson as our President and General Manager of North America. In connection with Mr. Anderson’s appointment, we agreed to pay Mr. Anderson an annual base salary of $335,000. Mr. Anderson was also entitled to certain expense reimbursements and other standard benefits, including vacation and sick leave. On April 1, 2022 Mr. Anderson’s base salary was increased to $400,000. In addition, Mr. Anderson was entitled to receive an annual incentive bonus of up to $250,000, subject to certain milestones and performance targets. In addition, and in conjunction with his appointment as President and General Manager of North America, we agreed to issue Mr. Anderson a stock option to purchase up to 90,000 shares of common stock at an exercise price of $8.41 per share, subject to vesting. Mr. Anderson was also issued a stock option to purchase up to 90,000 shares of common stock at an exercise price of $8.41 per share, subject to vesting and the achievement of certain business revenue targets. In that regard, Mr. Anderson’s option will vest as follows: (i) 22,500 shares shall vest following fiscal year 2020 if our business-to-business revenues reach or exceed $6 million in the aggregate, or a pro-rated amount equal to the percentage achievement of such target, assuming the Company’s GAAP revenues in 2020 will reach at least $11 million in the aggregate; (ii) 22,500 shares shall vest following fiscal year 2021 if our business-to-business revenues reach or exceed $15 million in the aggregate, or a pro-rated amount equal to the percentage achievement of such target, assuming the Company’s GAAP revenues in 2021 will reach at least $19.5 million in the aggregate; (iii) 22,500 shares shall vest following fiscal year 2022 if our business-to-business revenues reach or exceed $40 million in the aggregate, or a pro-rated amount equal to the percentage achievement of such target, assuming the Company’s GAAP revenues in 2022 will reach at least $38 million in the aggregate; and (iv) 22,500 shares shall vest following fiscal year 2023 if our business-to-business revenues reach or exceed $80 million in the aggregate, or a pro-rated amount equal to the percentage achievement of such target, assuming the Company’s GAAP revenues in 2023 will reach at least $62 million in the aggregate. The performance options for 2020, 2021, 2022 and 2023 did not vest and have expired.
On May 30, 2024, we and Mr. Anderson, mutually agreed that Mr. Anderson would cease serving in his role as President of the Company and would continue as a consultant and member of Advisory Board.
Tomer Ben-Kiki, Former Chief Operating Officer. Mr. Ben-Kiki acted as the Company’s Chief Operating Officer between February 15, 2024 and August 7, 2024. Under Mr. Ben-Kiki’s employment agreement he was entitled to earn an annual salary of $212,000 for his work in the United States, and 65,000 NIS per month for his work in Israel. Mr. Ben-Kiki was also entitled to a bonus of up to 20% of his base salary, subject to certain performance objectives as defined by the Board of Directors. In addition, he was entitled to receive a stock option to purchase up to 1,017,947 shares of Common Stock, at an exercise price of $2.55 per share, which were granted as an inducement material to Mr. Ben-Kiki becoming an employee of the company, in accordance with Nasdaq Listing Rule 5635(c)(4).. On August 7, 2024, we and Tomer Ben-Kiki, mutually agreed that Mr. Ben-Kiki would be relieved from his role as Chief Operating Officer and re-assigned to serve in a non-executive management role as our Chief Technology Officer. In connection with his departure from his role, we made a consulting agreement with Mr. Ben-Kiki.
113
Outstanding Equity Awards at December 31, 2024
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
|
|
|
|
|
incentive |
|
|
|
|
|
|
|
|
|
|
|
plan awards: |
|
|
|
|
|
|
|
Number of |
|
Number of |
|
Number of |
|
|
|
|
|
|
|
securities |
|
securities |
|
securities |
|
|
|
|
|
|
|
underlying |
|
underlying |
|
underlying |
|
|
|
|
|
|
|
unexercised |
|
unexercised |
|
unexercised |
|
Option |
|
Option |
|
|
|
options (#) |
|
options (#) |
|
unearned |
|
exercise |
|
expiration |
|
Name |
|
exercisable |
|
unexercisable |
|
options (#) |
|
price ($) |
|
date |
|
Zvi Ben David |
|
27,827 |
|
— |
|
— |
|
$ |
7.74 |
|
February 12, 2026 |
(Chief Financial Officer, Secretary and Treasurer) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Richard Anderson |
|
90,000 |
|
|
|
|
|
$ |
8.410 |
|
January 30, 2026 |
(President and General Manager of North America) |
|
91,652 |
|
|
|
|
|
$ |
17.890 |
|
January 19, 2031 |
|
|
112,500 |
|
22,500 |
(1) |
|
|
$ |
7.190 |
|
May 18, 2032 |
|
|
515,625 |
|
234,375 |
(1) |
|
|
$ |
1.680 |
|
March 6, 2034 |
|
|
|
|
|
|
|
|
|
|
|
|
Steven Nelson |
|
|
|
500,000 |
(2) |
— |
|
$ |
1.35 |
|
June 5, 2034 |
(Chief Commercial Officer) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tomer Ben-Kiki |
|
398,293 |
|
319,654 |
(3) |
|
|
$ |
2.55 |
|
February 15, 2034 |
(Former Chief Operating Officer) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Option Shares |
|
1,235,897 |
|
1,076,529 |
|
|
|
|
|
|
|
| (1) | Vests in 12 equal quarterly installments over a three-year period. |
| (2) | One third vests after one year followed by 8 equal quarterly amounts over the following two years. |
| (3) | Unvested options were cancelled. |
We do not have any formal policy that requires us to grant, or avoid granting, equity-based compensation at certain times. We do not grant equity awards in anticipation of the release of material nonpublic information that is likely to result in changes to the price of our common stock, and do not time the public release of such information based on award grant dates. The timing of any equity grants to executive officers or directors in connection with new hires, promotions, or other non-routine grants is tied to the event giving rise to the award (such as an executive officer’s commencement of employment or promotion effective date).
During the year ended December 31, 2024, there were no equity grants made to our executive officers during any period beginning four business days before the filing of a periodic report or current report disclosing material non-public information and ending one business day after the filing or furnishing of such report with the Securities and Exchange Commission that were likely to result in changes to the price of our common stock, and do not time the public release of such information based on award grant dates.
Non-Employee Director Remuneration Policy
In March 2013, our Board of Directors adopted the following non-employee director remuneration policy:
Cash Awards
Our non-employee directors (currently Messrs. Shaked, Matheis, McGrath, Kaplan, Stern and Ms. Karah) will receive the following cash payments for each fiscal year: (i) $50,000 per year, to be paid quarterly in arrears and (ii)
114
$20,000 for Board committee service, to be paid quarterly in arrears. Commencing the second quarter of 2024 Messrs Shaked, Mathies, Kaplan, Stern and Ms. Karah have waived their cash compensation.
Stock and Option Awards
On March 6, 2024, the Compensation Committee of our Board of Directors approved the following issuances, each was done under our 2020 Equity Incentive Plan: (i) 90,000 restricted shares of our common stock to Mr. Shaked; (ii) 80,000 restricted shares of our common stock to Mr. McGrath; and (iii) 70,000 restricted shares of our common stock to each of Ms. Karah, Mr. Matheis, Mr. Stern and Mr. Kaplan.
On February 24, 2025, our Compensation Committee of our Board of Directors approved to accelerate the vesting of 35,000 restricted shares of our common stock as well as approved the grant of 30,000 restricted shares of Common Stock to Mr. Kaplan.
Compensation Committee Review
The Compensation Committee shall, if it deems necessary or prudent in its discretion, reevaluate and approve in January of each such year (or in any event prior to the first board meeting of such fiscal year) the cash and equity awards (amount and manner or method of payment) to be made to non-employee directors for such fiscal year. In making this determination, the Compensation Committee shall utilize such market standard metrics as it deems appropriate, including, without limitation, an analysis of cash compensation paid to independent directors of our peer group.
The Compensation Committee shall also have the power and discretion to determine in the future whether non-employee directors should receive annual or other grants of options to purchase shares of common stock or other equity incentive awards in such amounts and pursuant to such policies as the Compensation Committee may determine utilizing such market standard metrics as it deems appropriate, including, without limitation, an analysis of equity awards granted to independent directors of our peer group.
Participation of Employee Directors; New Directors
Unless separately and specifically approved by the Compensation Committee in its discretion, no employee director of our company shall be entitled to receive any remuneration for service as a director (other than expense reimbursement as per prevailing policy).
New directors joining our Board of Directors shall be entitled to a pro-rated portion (based on months to be served in the fiscal year in which they join) of cash and stock option or other equity incentive awards (if applicable) for the applicable fiscal year at the time they join the board.
Prior Awards to Management
On November 4, 2024, our Compensation Committee approved amendments to certain previously issued awards of restricted shares of common stock in the aggregate amount of 68,750 and 250,000 shares of common stock, respectively, granted to Mr. Erez Raphael, our Chief Executive Officer, and Mr. Zvi Ben David, our Chief Financial Officer, in the aggregate amount of 23,750 and 125,000 shares of common stock, respectively, on May 18, 2022 and March 6, 2024 (collectively, the “Prior Awards”) to permit an immediate acceleration of the unvested portion of the Prior Awards in the event of change in control of the Company.
115
Summary Director Compensation Table
The following table summarizes the annual compensation paid to our non-employee directors for the fiscal year ended December 31, 2024:
|
|
|
|
Fees Paid |
|
|
|
|
|
|
|
|
|
|
Non- |
|
|
|
|
|
|
||
|
|
|
|
or |
|
|
|
|
|
|
|
Non-equity |
|
qualified |
|
|
|
|
|
|
|||
Name and |
|
|
|
Earned in |
|
|
|
|
Option |
|
incentive |
|
deferred |
|
All other |
|
|
|
|||||
Principal |
|
|
|
Cash |
|
Stock |
|
Awards |
|
plan |
|
compensation |
|
compensation |
|
|
|
||||||
Position |
|
Year |
|
($) |
|
Awards |
|
($)* |
|
compensation |
|
earnings |
|
($) |
|
Total ($) |
|||||||
Dennis McGrath |
|
2024 |
|
$ |
70,000 |
|
$ |
134,400 |
(1) |
$ |
— |
(2) |
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
204,400 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jon Kaplan (13) |
|
2024 |
|
$ |
12,500 |
|
$ |
117,600 |
(3) |
$ |
— |
(4) |
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
130,100 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dennis Matheis |
|
2024 |
|
$ |
17,500 |
|
$ |
117,600 |
(5) |
$ |
— |
(6) |
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
135,100 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hila Karah |
|
2024 |
|
$ |
17,500 |
|
$ |
117,600 |
(7) |
$ |
— |
(8) |
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
135,100 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yoav Shaked |
|
2024 |
|
$ |
17,500 |
|
$ |
151,200 |
(9) |
$ |
— |
(10) |
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
168,700 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adam Stern |
|
2024 |
|
$ |
12,500 |
|
$ |
117,600 |
(11) |
$ |
— |
(12) |
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
130,100 |
* |
Amount shown does not reflect dollar amount actually received. Instead, this amount reflects the aggregate grant date fair value of each stock option granted in the fiscal year ended December 31, 2024, computed in accordance with the provisions of ASC 718. Assumptions used in accordance with ASC 718 are included in Note 9 to our consolidated financial statements included in this Annual Report. |
| (1) | 154,744 stock awards are outstanding as of December 31, 2024. |
| (2) | No option awards are outstanding as of December 31, 2024. |
| (3) | 70,000 stock awards are outstanding as of December 31, 2024. |
| (4) | No option awards are outstanding as of December 31, 2024. |
| (5) | 102,620 stock awards are outstanding as of December 31, 2024. |
| (6) | 55,000 option awards are outstanding as of December 31, 2024. |
| (7) | 218,751 stock awards are outstanding as of December 31, 2024. |
| (8) | No option awards are outstanding as of December 31, 2024. |
| (9) | 253,896 stock awards are outstanding as of December 31, 2024. |
| (10) | No option awards are outstanding as of December 31, 2024. |
| (11) | 178,341 stock awards are outstanding as of December 31, 2024. |
| (12) | No option awards are outstanding as of December 31, 2024. |
| (13) | Mr. Kaplan resigned from the Board of Directors on February 23, 2025. |
116
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth information regarding the beneficial ownership of our common stock as of March 3, 2025 by:
each person known by us to be the beneficial owner of more than 5% of our outstanding shares of common stock;
| ● | each of our named executive officers and directors; and |
| ● | all our executive officers and directors as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. Except as otherwise indicated, each person or entity named in the table has sole voting and investment power with respect to all shares of our capital shown as beneficially owned, subject to applicable community property laws.
In computing the number and percentage of shares beneficially owned by a person, shares that may be acquired by such person within 60 days of the date of this Annual Report are counted as outstanding, while these shares are not counted as outstanding for computing the percentage ownership of any other person. Unless otherwise indicated, the address of each person listed below is c/o DarioHealth Corp., 322 W. 57th St. #33B, New York, New York 10019.
|
|
|
|
Percent of |
|
|
|
Shares of |
|
Common |
|
|
|
Common |
|
Stock |
|
|
|
Beneficially |
|
Beneficially |
|
Name of Beneficial Owner |
|
Stock Owned |
|
Owned (1) |
|
Officers and Directors |
|
|
|
|
|
Erez Raphael (2) |
|
1,813,593 |
|
4.4 |
% |
Zvi Ben David (3) |
|
692,732 |
|
1.7 |
% |
Steven C. Nelson (4) |
|
55,000 |
|
* |
% |
Richard Anderson (5) |
|
976,779 |
|
2.3 |
% |
Tomer Ben Kiki (6) |
|
504,846 |
|
1.2 |
|
Dennis M. McGrath (7) |
|
116,411 |
|
* |
% |
Lawrence Leisure (8) |
|
287,500 |
|
* |
% |
Hila Karah (9) |
|
199,499 |
|
* |
% |
Yoav Shaked (10) |
|
250,032 |
|
* |
% |
Adam Stern (11) |
|
913,065 |
|
2.2 |
% |
Dennis Mathies (12) |
|
206,493 |
|
* |
% |
All Executive Officers and Directors as a group (11 persons) ** |
|
6,015,950 |
|
14.4 |
% |
|
|
|
|
|
|
5% Stockholders |
|
|
|
|
|
Nantahala Capital Management, LLC. (13) |
|
4,235,023 |
|
9.9 |
% |
Solid Financial, LLC. (14) |
|
2,139,642 |
|
5.1 |
% |
Tasso Partners, LLC. (15) |
|
3,394,043 |
|
8.1 |
% |
* |
less than 1%. |
| (1) | Percentage ownership is based on 41,567,016 shares of our common stock outstanding as of March 3, 2025 and, for each person or entity listed above, warrants or options to purchase shares of our common stock which exercisable within 60 days of such date. |
| (2) | Includes 1,315,242 vested restricted shares. Also includes 37,876 shares of our common stock, held by Dicilyon Consulting and Investment Ltd. Erez Raphael is the natural person with voting and dispositive power over our securities held by Dicilyon Consulting and Investment Ltd. The address of Dicilyon Consulting and Investment Ltd. is 10 Nataf St., Ramat Hasharon 4704063, Israel. |
117
| (3) | Includes 27,827 vested options to purchase common stock and 523,312 vested restricted shares. Includes 1,786 shares owned by his spouse, for which Mr. Ben David disclaims beneficial ownership except to the extent of his pecuniary interest therein. |
| (4) | Excludes 500,000 options which are not vested. |
| (5) | Includes 867,902 vested options to purchase common stock and 93,695 vested restricted shares. Excludes 198,750 options which are not vested. |
| (6) | Includes 398,294 vested options to purchase common stock and 106,552 vested warrants to purchase common stock. Excludes 213,101 warrants which are not vested. |
| (7) | Includes 116,411 vested restricted shares. |
| (8) | Includes 162,500 vested restricted shares and warrants to purchase 125,000 shares of common stock. |
| (9) | Includes 150,356 vested restricted shares. |
| (10) | Includes 143,350 vested restricted shares. Includes 1,667 shares owned by his spouse, for which Mr. Shaked disclaims beneficial ownership except to the extent of his pecuniary interest therein. |
| (11) | Includes 133,017 vested restricted shares. Includes 250 C preferred shares exercisable into 124,000 shares of common stock. Includes warrants exercisable into 507,648 shares of common stock, subject to a contractual beneficial ownership limitation of 4.99%. |
| (12) | Includes 52,084 vested options to purchase common stock and 70,120 vested restricted shares. Excludes 2,916 options which have not been vested. |
| (13) | Based solely on information contained in Form 13G/A filed with the SEC on February 14, 2025, and data provided by the holder. Includes 277,546 pre-funded warrants to purchase common stock issued in May 2019 and preferred shares convertible into 933,451 shares of common stock, subject to a contractual beneficial ownership limitation of 9.99% and excludes preferred shares convertible into 3,803,747 shares of common stock, 386,129 pre-funded warrants issued on July 31, 2020, and 331,814 pre-funded warrants issued on February 28, 2022. |
| (14) | Based solely on information contained in Form 13G/A filed with the SEC on February 12, 2025. The securities are held in discretionary accounts managed by Solid Financial LLC, where the firm has the power to vote and dispose of such securities on behalf of its clients. |
| (15) | Based solely on information contained in Form 13G filed with the SEC on December 13 2024, and data provided by the holder. Includes (i) 1,394,721 shares of Common Stock, (ii) shares of Preferred C Stock convertible into 1,222,772 shares of Common Stock, and (iii) shares of Preferred C-2 Stock convertible into 23,364 shares of Common Stock, 385,000 warrants to purchase common stock which are beneficially owned by Dana Carrera is the trustee of the GCL Family Trust, and included pursuant to Rule 13d-3(d)(1)(i) of the Securities Exchange Act of 1934, as amended. |
Item 13. Certain Relationships and Related Party Transactions
Executive Officers and Directors
We have entered into employment and consulting agreements and granted stock awards to our executive officers and directors as more fully described in “Executive Compensation” above.
Executive Officers and Directors
We have entered into employment agreements and granted stock awards to our executive officers as more fully described in “Executive Compensation” above.
118
Statement of Policy
All transactions (if any) between us and our officers, directors or five percent stockholders, and respective affiliates will be on terms no less favorable than could be obtained from unaffiliated third parties and will be approved by a majority of our independent directors who do not have an interest in the transactions and who had access, at our expense, to our legal counsel or independent legal counsel.
On December 28, 2023, we entered into a placement agency agreement (the “Placement Agency Agreement”) with Aegis Capital Corp. (“Aegis”), as amended on January 31, 2024, with respect to the offering of the Series C Preferred Stock. Pursuant to the terms of the Placement Agency Agreement, in connection with each closing of the offering, we agreed to pay Aegis an aggregate cash fee representing 10% of aggregate proceeds raised in the offering (and fees representing 5% and 1.5% for certain company introduced investors), non-accountable expense allowance representing 3% of aggregate proceeds raised in the offering (and fees representing 1.5% and none for certain Company introduced investors). In addition, we will issue to Aegis or its designees warrants (the “Placement Agent Warrant”) to purchase shares of common stock representing 14.5% of the equivalent shares of Common Stock issuable upon initial conversion of the Series C Preferred Stock at an exercise price equal to the consolidated bid price of the common stock as of the date of such closing. The Placement Agent Warrant provides for a cashless exercise feature and are exercisable for a period of five years from the date of closing. We also granted the Placement Agent the right of first refusal, for a twelve (12) month period after the final closing of the offering, to serve as the Company’s lead or co-placement agent for any proposed private placement of our securities (equity or debt) that is proposed to be consummated to investors in the United States with the assistance of a registered broker dealer.
Adam Stern, a member of our Board of Directors, has an interest, and will receive fees due to Aegis.
On April 3, 2020, we entered into a financial advisory agreement with Aegis Capital Corp., pursuant to which we agreed to pay Aegis Capital Corp. (“Aegis”) certain a fee of up to 3% of any proceeds from sales derived by us through commercial transactions entered into with parties introduced by Aegis. In addition, on April 3, 2020, we entered into a Sales Fee Agreement with Aegis, pursuant to which we agreed to pay Aegis a fee of up 4.5% of consideration we may receive in a business development transaction (including, any joint-venture, partnership, strategic collaboration or investment, licensing transaction, co-promotion or distribution agreement or other profit or revenue sharing, or similar business arrangement) from parties introduced by Aegis. To date, we have not paid Aegis any fees as a result of these agreements. Adam Stern, a member of our Board, has an interest, and will receive fees due to, Aegis.
On September 3, 2021, we entered into to a consulting agreement, as amended (the “Consulting Agreement”), with NearWater Growth LCC (“NearWater”). As remuneration for such services, we agreed to pay NearWater a monthly cash retainer upon the successful completion of an equity financing resulting in gross proceeds in excess of $25 million and we further agreed to issue NearWater shares of our common stock as equity compensation if certain milestones are met such as NearWater introducing entities to us for acquisition or partnership purposes, provided certain revenue milestones are met. Further, the Consulting Agreement provides for the payment to NearWater of 150,000 shares of our common stock which shall vest quarterly over a four year period. To date, NearWater has received approximately 258,000 shares of our common stock and common stock purchase warrants to purchase up to 125,000 shares of common stock. On February 24, 2025, we and NearWater entered into a Second Amendment to the Consulting Agreement pursuant to which we agreed to pay NearWater a monthly retainer of $5,000 beginning on September 1, 2024, increasing to $10,000 per month on January 1, 2025.
Lawrence Leisure, a member of our Board of Directors is a Member of NearWater. Mr. Leisure has in interest in, and will receive, the compensation due to NearWater.
To the best of our knowledge, other than as set forth above, there were no material transactions, or series of similar transactions, or any currently proposed transactions, or series of similar transactions, to which we were or are to be a party, in which the amount involved exceeds the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years, and in which any director or executive officer, or any security holder who is known by us to own of record or beneficially more than 5% of any class of our common stock, or any member of the immediate family of any of the foregoing persons, has an interest (other than compensation to our officers and directors in the ordinary course of business).
119
Item 14. Principal Accounting Fees and Services
The following table sets forth fees billed to us by Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global, our independent registered public accounting firm, during the fiscal years ended December 31, 2024 and December 31, 2023 for: (i) services rendered for the audit of our annual financial statements and the review of our quarterly financial statements; (ii) services by our independent registered public accounting firms that are reasonably related to the performance of the audit or review of our financial statements and that are not reported as audit fees; (iii) services rendered in connection with tax compliance, tax advice and tax planning; and (iv) all other fees for services rendered.
|
|
December 31, 2024 |
|
December 31, 2023 |
||
Audit Fees |
|
$ |
487,573 |
|
$ |
303,296 |
Tax Fees (1) |
|
$ |
82,535 |
|
$ |
16,686 |
All Other Fees (2) |
|
$ |
273,150 |
|
$ |
102,250 |
Total |
|
$ |
843,258 |
|
$ |
422,232 |
| (1) | Consists of fees relating to our tax compliance and tax planning. |
| (2) | Consists of fees relating to our private placements. |
Audit Committee Policies
The Audit Committee of our Board of Directors is solely responsible for the approval in advance of all audit and permitted non-audit services to be provided by the independent auditors (including the fees and other terms thereof), subject to the de minimus exceptions for non-audit services provided by Section 10A(i)(1)(B) of the Exchange Act, which services are subsequently approved by the Board of Directors prior to the completion of the audit. None of the fees listed above are for services rendered pursuant to such de minimus exceptions.
120
121
3.17 |
|
|||
4.1 |
|
|||
4.2 |
|
|||
4.3* |
|
|||
4.4 |
|
|||
4.5 |
|
|||
4.6 |
|
|||
4.7 |
|
|||
4.8 |
|
|||
4.9 |
|
|||
4.10 |
|
|||
4.11* |
|
|||
4.12* |
|
|||
10.1+ |
|
|||
10.2+ |
|
|||
10.3+ |
|
|||
10.4+ |
|
|||
10.5+ |
|
|||
10.6+ |
|
|||
10.7+ |
|
|||
10.8+ |
|
|||
122
10.9+ |
|
|||
10.10+ |
|
|||
10.11 |
|
|||
10.12+ |
||||
10.13˄ |
|
|||
10.14 |
|
|||
10.15+ |
|
|||
10.16 |
|
|||
10.17 |
|
|||
10.18˄ |
|
|||
10.19 |
|
|||
10.20 |
|
|||
10.21˄ |
|
|||
10.22 |
|
|||
10.23 |
|
Redemption Agreement by and between DarioHealth Corp. and Richard Allan Anderson dated June 9, 2022. |
||
10.24 |
|
|||
10.25 |
|
|||
123
10.26 |
|
|||
10.27 |
|
|||
10.28 |
|
|||
10.29 |
|
|||
10.30 |
|
|||
10.31 |
|
|||
10.32 |
|
|||
10.33 |
|
|||
10.34+ |
|
|||
10.35+ |
|
|||
10.36 |
|
|||
10.37 |
|
|||
10.38 |
|
|||
10.39 |
|
|||
19.1* |
|
|||
21.1* |
|
|||
23.1* |
|
|||
31.1* |
|
|||
31.2* |
|
|||
32.1** |
|
Certification of the Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. 1350. |
||
97.1 |
|
|||
124
101* |
|
The following financial statements from the Company’s annual report on Form 10-K for the year ended December 31, 2024, formatted in Inline XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Comprehensive Loss, (iii) Statements of Changes in Stockholders’ Equity, (iv) Consolidated Statements of Cash Flows and (v) the Notes to Consolidated Financial Statements, tagged as blocks of text and in detail. |
||
104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
||
+ |
Management contract or compensatory plan or arrangement |
* |
Filed herewith |
** |
Furnished herewith |
˄ |
Certain identified information in the exhibit has been excluded from the exhibit because it is both (i) not material and (ii) would likely cause competitive harm to DarioHealth Corp. if publicly disclosed |
Item 16. Form 10-K Summary.
None.
125
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: March 10, 2025 |
DARIOHEALTH CORP. |
||
|
|
||
|
By: |
/s/ Erez Raphael |
|
|
|
Name: |
Erez Raphael |
|
|
Title: |
Chief Executive Officer |
|
|
||
|
By: |
/s/ Zvi Ben David |
|
|
|
Name: |
Zvi Ben David |
|
|
Title: |
Chief Financial Officer, Secretary and Treasurer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Person |
|
Capacity |
|
Date |
|
|
|
|
|
|
|
/s/ Erez Raphael |
|
Chief Executive Officer and |
|
March 10, 2025 |
|
Erez Raphael |
|
Director (Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
/s/ Zvi Ben David |
|
Chief Financial Officer, Secretary and |
|
March 10, 2025 |
|
Zvi Ben David |
|
Treasurer (Principal Financial and Accounting Officer) |
|
|
|
|
|
|
|
|
|
/s/ Yoav Shaked |
|
Chairman of the Board |
|
March 10, 2025 |
|
Yoav Shaked |
|
|
|
|
|
|
|
|
|
|
|
/s/ Dennis Matheis |
|
Director |
|
March 10, 2025 |
|
Dennis Matheis |
|
|
|
|
|
|
|
|
|
|
|
/s/ Hila Karah |
|
Director |
|
March 10, 2025 |
|
Hila Karah |
|
|
|
|
|
|
|
|
|
|
|
/s/ Dennis M. McGrath |
|
Director |
|
March 10, 2025 |
|
Dennis M. McGrath |
|
|
|
|
|
|
|
|
|
|
|
/s/ Lawrence Leisure |
|
Director |
|
March 10, 2025 |
|
Lawrence Leisure |
|
|
|
|
|
|
|
|
|
|
|
/s/ Adam Stern |
|
Director |
|
March 10, 2025 |
|
Adam Stern |
|
|
|
|
|
|
|
|
|
|
|
126
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2024
INDEX
|
Page |
|
Report of Independent Registered Public Accounting Firm (PCAOB - Firm ID: 1281) |
|
F-2 – F-5 |
|
||
|
F-6 – F-7 |
|
|
||
|
F-8 |
|
|
||
|
F-9 |
|
|
||
|
F-10 |
|
|
||
|
F-11 – F-54 |
- - - - - - - - - - - - - - -
|
Kost Forer Gabbay & Kasierer 144 Menachem Begin Road, Building A, Tel-Aviv 6492102, Israel |
Tel: +972-3-6232525 Fax: +972-3-5622555 ey.com |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of
DARIOHEALTH CORP.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of DarioHealth Corp. and its subsidiaries (the Company) as of December 31, 2024 and 2023, the related consolidated statements of comprehensive loss, changes in stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2024, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2024, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
F - 2
|
Kost Forer Gabbay & Kasierer 144 Menachem Begin Road, Building A, Tel-Aviv 6492102, Israel |
Tel: +972-3-6232525 Fax: +972-3-5622555 ey.com |
Revenue Recognition |
|
Description of the Matter |
As described in Note 2 and Note 6 to the consolidated financial statements, a significant portion of the Company's revenue is derived from agreements with enterprise business market groups to provide a mobile and web-based digital therapeutics health management programs, data license and related development and implementation services. The Company contracts with customers often include promises to transfer multiple promises to provide goods and services, which are accounted for separately if they are distinct performance obligations. In such contracts, the transaction price is then allocated to the distinct performance obligations on a relative standalone selling price basis and revenue is recognized when control of the distinct performance obligation is transferred. The accounting for contracts with multiple promises requires the company to exercise significant judgment in determining revenue recognition for these contracts and includes: (a) identification and determination of whether products and services are considered distinct performance obligations that should be accounted for separately based on the terms and conditions of the relevant agreements, (b) determination of stand-alone selling prices for each distinct performance obligation that are not sold separately. (c) the pattern of transferring control (i.e., timing of when revenue is recognized) for each distinct performance obligation. . Given these factors, the related audit effort in evaluating management’s judgments in determining revenue recognition for these customer contracts was extensive and required a high degree of auditor judgment.. |
|
|
How We Addressed the Matter in Our Audit |
For a sample of customers, we: (1) obtained and read contract source documents, including master agreements, and other documents that were part of the agreement and evaluating management's identification of the contract and the distinct performance obligations based on the terms of the arrangements and the company's accounting policies, (2) tested management’s identification of significant terms for completeness, including the identification and determination of distinct performance obligations, (3) evaluating the methodology and reasonableness of management’s assumptions used for the estimate of stand-alone selling prices on a sample basis for products and services that are not sold separately (4) tested management’s calculations of revenue and the associated timing of revenue recognition. In addition, we have also evaluated the Company’s disclosures in relation to this matter. |
F - 3
|
Kost Forer Gabbay & Kasierer 144 Menachem Begin Road, Building A, Tel-Aviv 6492102, Israel |
Tel: +972-3-6232525 Fax: +972-3-5622555 ey.com |
Going concern assessment |
|
Description of the Matter |
As discussed in Note 1 to the consolidated financial statements, the Company has incurred operating losses and negative cash-flow from operations since inception. The Company’s operations are dependent on its ability to raise additional funds. This dependency will continue until the Company will be able to completely finance its operations by generating revenue from its products and services. Management has concluded that, based on its current projections and plans, the Company will be able to satisfy its liquidity requirements for at least one year from the date these financial statements were issued. We identified the assessment of liquidity and the Company’s ability to continue as a going concern as a critical audit matter due to the subjective judgments required of management to conclude the Company would have sufficient liquidity to sustain itself for at least a year beyond the date of the issuance of the consolidated financial statements. This in turn led to a high degree of auditor subjectivity and judgment to evaluate the audit evidence supporting the liquidity conclusions. |
|
|
How We Addressed the Matter in Our Audit |
In addressing the matter, our audit procedures included, among others, assessing the reasonableness of forecasted revenue, operating expenses and sources of cash used in management’s assessment to determine whether the company has sufficient liquidity to fund operations for at least one year from the consolidated financial statement issuance date. This testing included inquiries with management, comparison of prior period forecasts to actual results and consideration of positive and negative evidence impacting management’s forecasts and liquidity. We also performed sensitivity analyses to assess the impact of changes in the key assumptions included in management's liquidity forecast models. In addition, we assessed the adequacy of the company’s going concern disclosures included in note 1 to the consolidated financial statements. |
|
|
Business combinations |
|
Description of the Matter |
As described in Note 4 to the consolidated financial statements, On February 15, 2024, the Company acquired 100% of the equity shares of Twill Inc. (Twill"). The fair value of the consideration was estimated to be 34,456 $Twill Acquisition was accounted for as a business combination in accordance with ASC 805 “Business Combinations”. Auditing the Company's accounting for the Twill Acquisition was complex due to the significant estimation uncertainty in determining the fair values of identified intangible assets, which consisted of technology of $ 5.6 million and customer relationships of $13.7 million. The significant estimation uncertainty in determining the fair values of identified intangible assets was primarily due to the sensitivity of the respective fair values to underlying assumptions about the future performance of the acquired business. The significant assumptions used to estimate the fair value of the technology and customer relationships intangible assets included discount rates and certain assumptions that form the basis of the forecasted results, such as revenue growth rates, profitability margins and estimated costs. These significant assumptions are forward-looking and could be affected by future economic and market conditions. |
F - 4
|
Kost Forer Gabbay & Kasierer 144 Menachem Begin Road, Building A, Tel-Aviv 6492102, Israel |
Tel: +972-3-6232525 Fax: +972-3-5622555 ey.com |
How We Addressed the Matter in Our Audit |
To test the estimated fair value of the developed technology and customer relationships intangible assets, we performed audit procedures that included, among others, evaluating the Company's selection of the valuation methodology, evaluating the methods and significant assumptions used by the Company, and evaluating the completeness and accuracy of the underlying data supporting the significant assumptions and estimates. For example, we compared the significant assumptions to current industry, market and economic trends and to the Company's budgets and forecasts, and Twill’s historical operating results. We involved our valuation specialists to assist with our evaluation of the methodology used by the Company and significant assumptions included in the fair value estimates. Our valuation specialists’ procedures included, among others, developing a range of independent estimates for the discount rates used in the valuation models and comparing those to the discount rates selected by management. |
/s/ KOST FORER GABBAY & KASIERER |
|
A Member of Ernst & Young Global |
|
We have served as the Company’s auditor since 2012.
Tel-Aviv, Israel
March 10, 2025
F - 5
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
U.S. dollars in thousands
|
|
December 31, |
|
December 31, |
||
|
|
2024 |
|
2023 |
||
ASSETS |
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT ASSETS: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
27,764 |
|
$ |
36,797 |
Short-term bank deposits |
|
|
697 |
|
|
- |
Short-term restricted bank deposits |
|
|
175 |
|
|
292 |
Trade receivables, net |
|
|
4,804 |
|
|
3,155 |
Inventories |
|
|
4,753 |
|
|
5,062 |
Other accounts receivable and prepaid expenses |
|
|
2,336 |
|
|
2,024 |
|
|
|
|
|
|
|
Total current assets |
|
|
40,529 |
|
|
47,330 |
|
|
|
|
|
|
|
NON-CURRENT ASSETS: |
|
|
|
|
|
|
Deposits |
|
|
79 |
|
|
6 |
Operating lease right of use assets |
|
|
1,065 |
|
|
967 |
Long-term assets |
|
|
313 |
|
|
143 |
Property and equipment, net |
|
|
709 |
|
|
899 |
Intangible assets, net |
|
|
18,762 |
|
|
5,404 |
Goodwill |
|
|
57,427 |
|
|
41,640 |
|
|
|
|
|
|
|
Total non-current assets |
|
|
78,355 |
|
|
49,059 |
|
|
|
|
|
|
|
Total assets |
|
$ |
118,884 |
|
$ |
96,389 |
The accompanying notes are an integral part of the consolidated financial statements.
F-6
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
U.S. dollars in thousands (except stock and stock data)
|
|
December 31, |
|
December 31, |
||
|
|
2024 |
|
2023 |
||
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES: |
|
|
|
|
|
|
Trade payables |
|
$ |
3,045 |
|
$ |
1,131 |
Deferred revenues |
|
|
1,583 |
|
|
997 |
Operating lease liabilities |
|
|
504 |
|
|
111 |
Other accounts payable and accrued expenses |
|
|
6,052 |
|
|
6,300 |
Current maturity of long-term loan |
|
|
5,451 |
|
|
3,954 |
|
|
|
|
|
|
|
Total current liabilities |
|
|
16,635 |
|
|
12,493 |
|
|
|
|
|
|
|
NON-CURRENT LIABILITIES |
|
|
|
|
|
|
Operating lease liabilities |
|
|
765 |
|
|
885 |
Long-term loan |
|
|
23,472 |
|
|
24,591 |
Warrant liability |
|
|
5,968 |
|
|
240 |
Other long-term liabilities |
|
|
25 |
|
|
36 |
|
|
|
|
|
|
|
Total non-current liabilities |
|
|
30,230 |
|
|
25,752 |
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
Common stock of $0.0001 par value - authorized: 160,000,000 shares; issued and outstanding: 38,388,431 and 27,191,849 shares on December 31, 2024 and December 31, 2023, respectively |
|
|
4 |
|
|
3 |
Preferred stock of $0.0001 par value - authorized: 5,000,000 shares; issued and outstanding: 49,585 and 18,959 shares on December 31, 2024 and December 31, 2023, respectively |
|
|
*) - |
|
|
*) - |
Additional paid-in capital |
|
|
462,358 |
|
|
407,502 |
Accumulated deficit |
|
|
(390,343) |
|
|
(349,361) |
|
|
|
|
|
|
|
Total stockholders’ equity |
|
|
72,019 |
|
|
58,144 |
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity |
|
$ |
118,884 |
|
$ |
96,389 |
The accompanying notes are an integral part of the consolidated financial statements.
*) Represents an amount lower than $1.
F-7
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
U.S. dollars in thousands (except stock and stock data)
|
|
Year ended |
||||
|
|
December 31, |
||||
|
|
2024 |
|
2023 |
||
Revenues: |
|
|
|
|
|
|
Services |
|
$ |
20,197 |
|
$ |
13,084 |
Consumer hardware |
|
|
6,843 |
|
|
7,268 |
Total revenues |
|
|
27,040 |
|
|
20,352 |
|
|
|
|
|
|
|
Cost of revenues: |
|
|
|
|
|
|
Services |
|
|
3,606 |
|
|
4,679 |
Consumer hardware |
|
|
5,139 |
|
|
5,303 |
Amortization of acquired intangible assets |
|
|
5,028 |
|
|
4,386 |
Total cost of revenues |
|
|
13,773 |
|
|
14,368 |
|
|
|
|
|
|
|
Gross profit |
|
|
13,267 |
|
|
5,984 |
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
Research and development |
|
$ |
24,179 |
|
$ |
20,248 |
Sales and marketing |
|
|
26,350 |
|
|
23,785 |
General and administrative |
|
|
20,482 |
|
|
18,140 |
|
|
|
|
|
|
|
Total operating expenses |
|
|
71,011 |
|
|
62,173 |
|
|
|
|
|
|
|
Operating loss |
|
|
57,744 |
|
|
56,189 |
|
|
|
|
|
|
|
Total financial expenses (income), net |
|
|
(13,145) |
|
|
3,174 |
|
|
|
|
|
|
|
Loss before taxes |
|
|
44,599 |
|
|
59,363 |
|
|
|
|
|
|
|
Income Tax |
|
|
(1,852) |
|
|
64 |
|
|
|
|
|
|
|
Net loss |
|
$ |
42,747 |
|
$ |
59,427 |
|
|
|
|
|
|
|
Deemed dividend (contribution) |
|
$ |
(1,765) |
|
$ |
4,084 |
|
|
|
|
|
|
|
Net loss attributable to common shareholders |
|
$ |
40,982 |
|
$ |
63,511 |
|
|
|
|
|
|
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share of common stock |
|
$ |
0.61 |
|
$ |
1.93 |
Weighted average number of common stock used in computing basic and diluted net loss per share |
|
|
49,039,410 |
|
|
28,371,979 |
The accompanying notes are an integral part of the consolidated financial statements.
F-8
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
U.S. dollars in thousands (except stock and stock data)
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
Total |
||
|
|
Common Stock |
|
Preferred Stock |
|
paid-in |
|
Accumulated |
|
stockholders’ |
|||||||||
|
|
Number |
|
Amount |
|
Number |
|
Amount |
|
capital |
|
deficit |
|
equity |
|||||
Balance as of January 1, 2023 |
|
25,724,470 |
|
$ |
3 |
|
3,567 |
|
$ |
*) - |
|
$ |
365,846 |
|
$ |
(285,850) |
|
$ |
79,999 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of options |
|
4,800 |
|
|
*) - |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
*) - |
Exercise of warrants |
|
86,983 |
|
|
*) - |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
Extinguishment of preferred stock in connection with preferred stock modification |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
984 |
|
|
(984) |
|
|
— |
Deemed dividend related to issuance of preferred stock |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
3,100 |
|
|
(3,100) |
|
|
— |
Conversion of preferred stock to common stock |
|
3,582 |
|
|
*) - |
|
(10) |
|
|
— |
|
|
— |
|
|
— |
|
|
*) - |
Issuance of warrants to service providers |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
3,516 |
|
|
— |
|
|
3,516 |
Stock-based compensation |
|
887,334 |
|
|
*) - |
|
— |
|
|
— |
|
|
16,185 |
|
|
— |
|
|
16,185 |
Issuance of Common Stock, net of issuance cost |
|
408,043 |
|
|
*) - |
|
15,402 |
|
|
— |
|
|
16,482 |
|
|
— |
|
|
16,482 |
Common stock related to earnout consideration |
|
76,637 |
|
|
*) - |
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
*) - |
Issuance of warrants related to loan agreement, net of issuance cost |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
1,389 |
|
|
— |
|
|
1,389 |
Net loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(59,427) |
|
|
(59,427) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2023 |
|
27,191,849 |
|
$ |
3 |
|
18,959 |
|
$ |
*) - |
|
$ |
407,502 |
|
$ |
(349,361) |
|
$ |
58,144 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of options |
|
10,714 |
|
|
*) - |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
*) - |
Exercise of pre-funded warrant to common stock |
|
3,187,101 |
|
|
*) - |
|
— |
|
|
— |
|
|
2,225 |
|
|
— |
|
|
2,225 |
Extinguishment of preferred stock in connection with preferred stock modification |
|
— |
|
|
— |
|
— |
|
|
|
|
|
(12,194) |
|
|
12,194 |
|
|
— |
Deemed dividend related to issuance of preferred stock |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
10,429 |
|
|
(10,429) |
|
|
— |
Conversion of preferred stock to common stock |
|
4,807,489 |
|
|
*) - |
|
(10,601) |
|
|
*) - |
|
|
— |
|
|
— |
|
|
*) - |
Issuance of warrants to service providers |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
2,704 |
|
|
— |
|
|
2,704 |
Stock-based compensation |
|
3,191,278 |
|
|
*) - |
|
— |
|
|
— |
|
|
13,092 |
|
|
— |
|
|
13,092 |
Issuance of preferred stock, net of issuance cost |
|
— |
|
|
1 |
|
41,227 |
|
|
*) - |
|
|
38,530 |
|
|
— |
|
|
38,531 |
Modification of warrants |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
70 |
|
|
— |
|
|
70 |
Net loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(42,747) |
|
|
(42,747) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2024 |
|
38,388,431 |
|
$ |
4 |
|
49,585 |
|
$ |
*) - |
|
$ |
462,358 |
|
$ |
(390,343) |
|
$ |
72,019 |
*) Represents an amount lower than $1.
The accompanying notes are an integral part of the consolidated financial statements.
F-9
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENT OF CASH FLOWS
U.S. dollars in thousands
|
|
Year ended |
||||
|
|
December 31, |
||||
|
|
2024 |
|
2023 |
||
Cash flows from operating activities: |
|
|
|
|
|
|
Net loss |
|
$ |
(42,747) |
|
$ |
(59,427) |
Adjustments required to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
Stock-based compensation |
|
|
15,796 |
|
|
19,701 |
Depreciation and impairment |
|
|
1,327 |
|
|
473 |
Change in operating lease right of use assets |
|
|
907 |
|
|
239 |
Amortization of acquired intangible assets |
|
|
6,100 |
|
|
4,512 |
Decrease in trade receivables, net |
|
|
1,680 |
|
|
3,261 |
Increase in other accounts receivable, prepaid expense and long-term assets |
|
|
(80) |
|
|
(426) |
Decrease in inventories |
|
|
308 |
|
|
2,894 |
Decrease in trade payables |
|
|
(496) |
|
|
(1,191) |
Decrease in other accounts payable and accrued expenses |
|
|
(3,483) |
|
|
(256) |
Decrease in deferred revenues |
|
|
(156) |
|
|
(323) |
Change in operating lease liabilities |
|
|
(1,150) |
|
|
(124) |
Change in fair value of warrant liability |
|
|
(16,504) |
|
|
(670) |
Non-Cash financial expenses |
|
|
516 |
|
|
1,198 |
Other |
|
|
(580) |
|
|
(240) |
|
|
|
|
|
|
|
Net cash used in operating activities |
|
|
(38,562) |
|
|
(30,379) |
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
Purchase of property and equipment |
|
|
(138) |
|
|
(584) |
Purchase of short-term investments |
|
|
— |
|
|
(4,996) |
Proceeds from redemption of short-term investments |
|
|
— |
|
|
5,033 |
Payments for business acquisitions, net of cash acquired |
|
|
(8,796) |
|
|
— |
|
|
|
|
|
|
|
Net cash used in investing activities |
|
|
(8,934) |
|
|
(547) |
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
Proceeds from issuance of common stock, net of issuance costs |
|
|
— |
|
|
1,614 |
Proceeds from issuance of preferred stock, net of issuance costs |
|
|
38,531 |
|
|
14,868 |
Proceeds from borrowings on credit agreement |
|
|
— |
|
|
29,604 |
Repayment of long-term loan |
|
|
— |
|
|
(27,833) |
|
|
|
|
|
|
|
Net cash provided by financing activities |
|
|
38,531 |
|
|
18,253 |
|
|
|
|
|
|
|
Decrease in cash, cash equivalents and restricted cash and cash equivalents |
|
|
(8,965) |
|
|
(12,673) |
Effect of exchange rate differences on cash, cash equivalents and restricted cash and cash equivalents |
|
|
(68) |
|
|
— |
Cash, cash equivalents and restricted cash and cash equivalents at beginning of period |
|
|
36,797 |
|
|
49,470 |
Cash, cash equivalents and restricted cash and cash equivalents at end of period |
|
$ |
27,764 |
|
$ |
36,797 |
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
Cash paid during the period for interest on long-term loan |
|
$ |
3,927 |
|
$ |
4,031 |
Non-cash activities: |
|
|
|
|
|
|
Right-of-use assets obtained in exchange for lease liabilities |
|
$ |
428 |
|
$ |
136 |
Exercise of pre-funded warrant to common stock upon acquisition |
|
$ |
2,225 |
|
$ |
- |
F-10
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 1:- GENERAL
| a. | DarioHealth Corp. (the “Company” or “DarioHealth”) was incorporated in the State of Delaware and commenced operations on August 11, 2011. |
DarioHealth is a global digital therapeutics (DTx) company delivering personalized evidence-based interventions that are driven by precision data analytics, software, and personalized coaching, DarioHealth has developed an approach with the intent to empower individuals to adjust their lifestyle in holistic way.
DarioHealth’s cross-functional team operates at the intersection of life sciences, behavioral science, and software technology to deliver seamlessly integrated and highly engaging digital therapeutics interventions. Our platform and suite of solutions deliver personalized and dynamic interventions driven by data analytics and one-on-one coaching for diabetes, hypertension, weight management, musculoskeletal pain, and behavioral health.
DarioHealth’s digital therapeutic platform has been designed with a ‘user-first’ strategy, focusing on the user’s needs first and foremost, and user experience and satisfaction. User satisfaction is constantly measured and drives, all company processes, including our technology design.
The Company has one reporting unit and one operating segment.
| b. | The Company has a wholly owned subsidiary, LabStyle Innovation Ltd. (“LabStyle”), which was incorporated and commenced operations on September 14, 2011, in Israel. Its principal business activity is to hold the Company’s intellectual property and to perform research and development, manufacturing, marketing, and other business activities. |
| c. | On February 15, 2024 (the “Closing Date”), the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Twill, Inc. (“Twill”), (see note 4). Pursuant to the provisions of the Merger Agreement, on the Closing Date, TWILL Merger Sub, Inc. (“Merger Sub”) was merged with and into Twill, the separate corporate existence of Merger Sub ceased and Twill continued as the surviving company and a wholly owned subsidiary of the Company. Twill is a clinical grade technology company working to shorten the distance between need and care by configuring personalized digital therapeutics and care solutions at scale for the modern healthcare cloud. Twill’s Intelligent Healing Platform(tm): integrates artificial intelligence (AI) with empathy, making healing more personal, precise, and connected for the entire care journey. Twill deploys a full spectrum of science-backed care solutions-including digital therapeutics, coaching, community, and well-being products for pharma, health plans, enterprises, and individuals everywhere. |
| d. | The Company has incurred net losses since its inception. As of December 31, 2024, The Company has incurred recurring losses and negative cash flows since inception and has an accumulated deficit of $390,343 as of December 31, 2024. For the year ended December 31, 2024, the Company used approximately $38,562 of cash in operations. Management believes the Company has sufficient funds to support its operation for at least a period of twelve months from the date of the issuance of these consolidated financial statements. The Company expects to incur future net losses and our transition to profitability is dependent upon, among other things, the successful development and commercialization of the Company’s products and the achievement of a level of revenues adequate to support the cost structure. Until the Company achieves profitability or generates positive cash flows, it will continue to be dependent on raising additional funds to fund its operations. The Company intends to fund its future operations through cash on hand, additional private and/or public offerings of debt or equity securities or a combination of the foregoing. There are no assurances, however, that the Company will be able to obtain an adequate level of financial resources that are required for the long-term development and commercialization of its product offerings. |
| e. | The Company’s common stock (the “Common Stock”) is listed on the Nasdaq Capital Market under the symbol “DRIO”. |
F-11
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES
The consolidated financial statements are prepared according to United States generally accepted accounting principles (“U.S. GAAP”).
a. |
Use of estimates: |
The preparation of the consolidated financial statements and related disclosures in conformity with U.S. GAAP requires the Company’s management to make judgments, assumptions and estimates that affect the amounts reported in its consolidated financial statements and accompanying notes. Management bases its estimates on historical experience and on various other assumptions it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates, and such differences may be material.
These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements including other accounts receivable and prepaid expenses and other accounts payable and accrued expenses, and the reported amounts of revenue, cost of revenues and operational expenses during the reporting period. Actual results could differ from those estimates.
b. |
Financial statements in U.S. dollars (“$,” “dollar” or “dollars”): |
The functional currency of the Company and its subsidiaries is the U.S dollar.
The Company’s revenues and financing activities are incurred in U.S. dollars. Although a portion of the Company’s expenses is denominated in New Israeli Shekels (“NIS”), a substantial portion of its expenses is denominated in dollars. Accordingly, the Company’s management believes that the currency of the primary economic environment in which the Company and its subsidiaries operate is the dollar; thus, the dollar is the functional currency of the Company. Transactions and balances denominated in dollars are presented at their original amounts. Monetary accounts denominated in currencies other than the dollar are re-measured into dollars in accordance with Accounting Standards Codification (“ASC”) 830, “Foreign Currency Matters”. All transaction gains and losses of the re-measurement of monetary balance sheet items are reflected in the consolidated statements of comprehensive loss as financial income or expenses, as appropriate.
c. |
Principles of consolidation: |
The consolidated financial statements include the accounts of the Company and its subsidiaries. Intercompany accounts and transactions have been eliminated upon consolidation.
d. |
Segment information: |
Operating segments are defined as components of an enterprise about which separate financial information is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and assessing performance. The Company identified its Chief Executive Officer as the Chief Operating Decision Maker (CODM). The Company’s Chief Executive Officer reviews the financial information presented on consolidated basis for purposes of allocating resources and evaluating its financial performance.
e. |
Cash and cash equivalents: |
The Company considers all highly liquid investments, which are readily convertible to cash with a maturity of three months or less at the date of acquisition, to be cash equivalents.
F-12
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
f. |
Short-term restricted bank deposits: |
Short-term restricted bank deposits are restricted deposits with maturities of up to one year and are pledged in favor of the bank as a security for the bank guarantees issued to the landlords of the Company’s offices and credit card payments. The short-term restricted bank deposits are denominated in NIS and USD and bear interest at an average rate of 4.56% and 5.2% as of December 31, 2024 and 2023, respectively. The short-term restricted bank deposits are presented at their cost, including accrued interest.
As of December 31, 2024, and 2023, the Company had a short-term restricted bank deposit which are used as collateral for rent in the amount of $175 and $229, respectively.
As of December 31, 2024, and 2023, the Company had short-term restricted bank deposits which were used as collateral for credit payments in amounts of $0 and $63, respectively.
The following table provides a reconciliation of the cash balances reported on the balance sheets and the cash, cash equivalents and short-term restricted bank deposits balances reported in the statements of cash flows:
|
|
December 31, |
|
December 31, |
|
||
|
|
2024 |
|
2023 |
|
||
Cash, and cash equivalents as reported on the balance sheets |
|
$ |
27,764 |
|
$ |
36,797 |
|
|
|
|
|
|
|
|
|
Cash, restricted cash, cash equivalents, and restricted cash and cash equivalents as reported in the statements of cash flows |
|
$ |
27,764 |
|
$ |
36,797 |
|
g. |
Trade receivables, net: |
The Company records trade receivables when an enforceable right to payment exists, net of allowance for credit losses. The Company’s expected credit loss allowance methodology for trade receivables is based upon its assessment of various factors, including historical experience, the age of the trade receivable balances, credit quality of its customers, current economic conditions, reasonable and supportable forecasts of future economic conditions, and other factors that may affect its ability to collect from customers. The estimated credit loss allowance is recorded as general and administrative expenses on the Company's Consolidated Statements of Comprehensive Loss. As of December 31, 2024, and 2023, the credit loss allowance was $169 and $163, respectively.
h. |
Inventories: |
Inventories are stated at the lower of cost or net realized value. Cost is determined on a first in first out (“FIFO”) basis. Inventory write-downs are provided to cover technological obsolescence, excess inventories and discontinued products. Inventory write-downs represent the difference between the cost of the inventory and net realizable value. Inventory write-downs are charged to the cost of revenues when a new lower cost basis is established. Subsequent changes in facts and circumstances do not result in the restoration or increase in that newly established cost basis.
Work-in-process is immaterial, given the typically short manufacturing cycle, and therefore is disclosed in conjunction with raw materials.
Total write-downs during the years ended December 31, 2024, and 2023 amounted to $301 and $121, respectively.
F-13
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
i. |
Property and equipment: |
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets at the following annual rates:
|
|
% |
Computers, and peripheral equipment |
|
15-33 |
Office furniture and equipment |
|
6-15 |
Production lines |
|
14-20 |
Leasehold improvements |
|
Over the shorter of the lease term or |
j. |
Impairment of long-lived assets: |
The Company's long-lived assets are reviewed for impairment in accordance with ASC 360, “Property, Plant and Equipment,” whenever events or changes in circumstances indicate that the carrying amount of an asset (or asset group) may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset (or asset group) to the future undiscounted cash flows expected to be generated by the assets. If such assets (or asset group) are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets (or asset group). For the years ended December 31, 2024, and 2023, the Company recorded an impairment loss of $860 and $0, respectively.
k. |
Revenue recognition |
The Company recognizes revenue in accordance with ASC 606, when (or as) it satisfies performance obligations by transferring promised hardware or services to its customers in an amount that reflects the consideration the Company expects to receive. The Company applies the following five steps: (1) identify the contract with a customer, (2) identify the performance obligations in the contract, (3) determine the transaction price, (4) allocate the transaction price to the performance obligations in the contract, and (5) recognize revenue when a performance obligation is satisfied.
If the contract contains a single performance obligation, the entire transaction price is allocated to the single performance obligation. For contracts that contain multiple performance obligations, the Company allocates the transaction price to each performance obligation based on the relative standalone selling price (“SSP”) for each performance obligation. The Company uses judgment in determining the SSP for its performance obligations. To determine SSP, the Company maximizes the use of observable standalone sales and observable data, where available. In instances where performance obligations do not have observable standalone sales, the Company may use alternative methods to estimate the standalone selling price, such as cost plus margin approach.
F-14
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
The Company’s payment terms are generally 45 days or less. In instances where the timing of revenue recognition differs from the timing of invoicing, the Company determines its contracts generally do not include a significant financing component since the Company's selling prices are not subject to billing terms nor is the Company’s purpose to receive financing from its customers or to provide customers with financing. In addition, the Company elected to apply the practical expedient not to adjust the promised amount of consideration for the effects of a significant financing component if the Company expects, at the inception of a contract, that the period between when the Company will transfer a promised good or service to a customer and when the customer will pay for that good or service will be one year or less. Revenue is recognized net of any taxes collected from customers which are subsequently remitted to governmental entities. The Company elected to account for shipping and handling activities as fulfillment activities. Shipping and handling activities are classified as part of cost of revenues.
Consumers revenue
The Company considers customer and distributor purchase orders to be contracts with customers. For each contract, the Company considers the promise to transfer tangible hardware and/or services, each of which are distinct, and accounted for as separate performance obligations. In determining the transaction price, the Company evaluates whether the price is subject to rebates and adjustments to determine the net consideration to which the Company expects to receive. Revenue from tangible hardware is recognized when control of the hardware is transferred to the customer (i.e., when the Company’s performance obligation is satisfied), which typically occurs at shipment. The revenues from fixed-price services are recognized ratably over the contract period as the services have a consistent continuous pattern of transfer to a customer during the contract period.
Commercial revenue – B2B2C
The Company provides a mobile and web-based digital therapeutics health management programs to employers and health plans for their employees or covered individuals. Such programs include live clinical coaching, content, automated journeys, hardware, and lifestyle coaching, currently supporting diabetes, prediabetes and obesity, hypertension, behavioral health (BH) and musculoskeletal health (MSK). At contract inception, the Company assesses the type of services being provided and assesses the performance obligations in the contract. These solutions integrate access to the Company’s web-based platform, and clinical and data services to provide an overall health management solution. The promises to transfer these goods and services are not separately identifiable and are considered a single continuous service comprised of a series of distinct services recognized over time that are substantially the same and have the same pattern of transfer (i.e., distinct days of service). Revenues related to the Company's newly acquired Twill platform are recognized over time, since the customer simultaneously receives and consumes the benefits provided by the Company’s performance. Revenues related to health management programs and to the Twill platform are recognized using a time-elapsed measure of progress, since those services have a consistent continuous pattern of transfer to the customer.
To the extent the transaction price includes variable consideration, revenue is recognized using the variable consideration allocation exception, or, if the allocation exception is not met, the Company recognizes revenue ratably based on estimates of the variable consideration to the extent it is probable that a significant reversal of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. When the variable consideration allocation exception is met, the Company recognizes revenue each month using either on a per engaged member per month (PEMPM) or a per employee per month (PEPM) basis.
Since the acquisition of Twill (note 4), the Company also provides professional services and advertisement serving services related to the Twill platform. Revenues related to professional services are recognized over time, since the customer simultaneously receives and consumes the benefits provided by the Company’s performance.
F-15
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
The Company generally recognizes revenues for professional services using an input method, based on labor hours consumed, which the Company believes best depicts the transfer of the services to the customer. Revenues related to advertisement serving services are recognized when impressions are delivered. The Company recognizes revenue from the display of advertisements in the contracted period in which the impressions are delivered. Impressions are considered delivered when an advertisement is displayed.
Certain of the Company’s contracts include client performance guarantees and a portion of the fees in those contracts are subject to performance-based metrics such as clinical outcomes or minimum member utilization rates. The Company includes in the transaction price some or all of an amount of variable consideration only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. Refunds to a customer that result from performance levels that were not met by the end of the measurement period are adjusted to the transaction price, and therefore estimated at the outset of the arrangement.
The Company follows the guidance provided in ASC 606 for determining whether it is a principal (i.e., report revenues on a gross basis) or an agent (i.e., report revenues on a net basis) in arrangements with customers that involve another party that contributes to providing specified services to a customer, based on whether the Company controls the specified good or service.
Commercial revenue - Strategic partnerships
The Company has also entered into contracts (Note 6) with a preferred partner and a health plan provider in which the Company provides data license, development and implementation services.
l. |
Cost of revenues: |
Cost of revenues is comprised of the cost of production, data center costs, shipping and handling inventory, hosting services, personnel and related overhead costs, depreciation of production line and related equipment costs, amortization of costs to fulfill a contract and inventory write-downs.
F-16
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
m. |
Concentrations of credit risk: |
Financial instruments that potentially subject the Company to credit risk primarily consist of cash and cash equivalents, short-term deposits, restricted deposits, and trade receivables. For cash and cash equivalents, the Company is exposed to credit risks in the event of default by the financial institutions to the extent that amounts recorded on the accompanying consolidated balance sheets exceed federally insured limits. The Company places its cash and cash equivalents and short-term deposits with financial institutions with high-quality credit ratings and has not experienced any losses in such accounts.
For trade receivables, the Company is exposed to credit risk in the event of non-payment by customers to the extent of the amounts recorded on the accompanying consolidated balance sheets.
|
|
|
Balance at |
|
|
|
|
|
|
|
|
Balance at |
|
|
|
beginning of period |
|
|
Additions |
|
|
Deduction |
|
|
end of period |
Year ended December 31, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
Allowance for credit losses |
|
$ |
163 |
|
$ |
268 |
|
$ |
(262) |
|
$ |
169 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
Allowance for credit losses |
|
$ |
23 |
|
$ |
140 |
|
$ |
— |
|
$ |
163 |
The Company has no off-balance-sheet concentration of credit risk.
Customer A: As of December 31, 2024, and as December 31, 2023, the Company's major customer accounted for 41.6% and 0%, respectively of the Company's accounts receivable balance.
For the year ended December 31, 2024 and December 31, 2023, the Company's major customer accounted for 24.6% and 0%, respectively, of the Company's revenue in the period.
Customer B: As of December 31, 2024, and as December 31, 2023, the Company's major customer accounted for 0% and 66.5%, respectively of the Company's accounts receivable balance.
For the year ended December 31, 2024, and December 31, 2023, the Company's major customer accounted for 0% and 29.9%, respectively, of the Company's revenue in the period.
n. |
Income taxes: |
The Company accounts for income taxes in accordance with ASC 740, “Income Taxes” (“ASC 740”). This guidance prescribes the use of the liability method whereby deferred tax asset and liability account balances are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance, if necessary, to reduce deferred tax assets to amounts that are more likely than not to be realized.
ASC 740 contains a two-step approach to recognizing and measuring a liability for uncertain tax positions. The first step is to evaluate the tax position taken or expected to be taken in a tax return by determining if the weight of available evidence indicates that it is more likely than not that, on an evaluation of the technical merits, the tax position will be sustained on audit, including resolution of any related appeals or litigation processes. The second step is to measure the tax benefit as the largest amount that is more than 50% likely to be realized upon ultimate settlement. As of December 31, 2024, and 2023, no liability for unrecognized tax benefits was recorded.
F-17
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
o. |
Research and development costs: |
Research and development costs are charged to the consolidated statements of comprehensive loss, as incurred.
p. |
Accounting for stock-based compensation: |
The Company accounts for stock-based compensation in accordance with ASC 718, “Compensation - Stock Compensation” (“ASC 718”), which requires companies to estimate the fair value of equity-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in the Company’s consolidated statement of comprehensive loss.
The Company recognizes compensation expenses for the value of its awards granted based on the straight-line method over the requisite service period of each of the awards, net of estimated forfeitures. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
The Company estimates the fair value of stock options granted using the Black-Scholes-Merton option-pricing model. The option-pricing model requires a number of assumptions, of which the most significant are the expected stock price volatility and the expected option term. Expected volatility was calculated based upon historical volatility of the Company. The expected option term represents the period that the Company’s stock options are expected to be outstanding and is determined based on the simplified method until sufficient historical exercise data will support using expected life assumptions. The risk-free interest rate is based on the yield from U.S. treasury bonds with an equivalent term. The Company has historically not paid dividends and has no foreseeable plans to pay dividends.
q. |
Fair value of financial instruments: |
The Company applies ASC 820, “Fair Value Measurements and Disclosures” (“ASC 820”). Under this standard, fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (i.e., the “exit price”) in an orderly transaction between market participants at the measurement date.
In determining fair value, the Company uses various valuation approaches. ASC 820 establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent from the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances.
The hierarchy is broken down into three levels based on the inputs as follows:
Level 1 - |
Valuations based on quoted prices in active markets for identical assets that the Company has the ability to access. Valuation adjustments and block discounts are not applied to Level 1 instruments. Since valuations are based on quoted prices that are readily and regularly available in an active market, valuation of these products does not entail a significant degree of judgment. |
Level 2 - |
Valuations based on one or more quoted prices in markets that are not active or for which all significant inputs are observable, either directly or indirectly. |
F-18
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
Level 3 - |
Valuations based on inputs that are unobservable and significant to the overall fair value measurement. |
The availability of observable inputs can vary from instrument to instrument and is affected by a wide variety of factors, including, for example, the type of investment, the liquidity of markets and other characteristics particular to the transaction. To the extent that valuation is based on models or inputs that are unobservable in the market, the determination of fair value requires more judgment, and the fair value are categorized as Level 3.
The carrying amounts of cash and cash equivalents, short-term restricted bank deposits, trade receivables, other accounts receivable and prepaid expenses, trade payables and other accounts payable and accrued expenses approximate their fair value due to the short-term maturity of such instruments. The Company's Loan Facility, and warrants liability were measured at fair value using Level 3 unobservable inputs (see note 8).
r. |
Warrants: |
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance. The assessment considers whether the warrants are freestanding financial instruments, meet the definition of a liability under ASC 480, and meet all of the requirements for equity classification, including whether the warrants are indexed to the Company’s own common stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent reporting period end date while the warrants are outstanding. Warrants that meet all the criteria for equity classification, are required to be recorded as a component of additional paid-in capital. Warrants that do not meet all the criteria for equity classification, are required to be recorded as liabilities at their initial fair value on the date of issuance and remeasured to fair value at each balance sheet date thereafter. The liability-classified warrants are recorded under non-current liabilities. Changes in the estimated fair value of the warrants are recognized in “Financial expenses, net” in the consolidated statements of comprehensive loss.
s. |
Basic and diluted net loss per share: |
The Company computes net loss per share using the two-class method required for participating securities. The two-class method requires income available to common stockholders for the period to be allocated between shares of Common Stock and participating securities based upon their respective rights to receive dividends as if all income for the period had been distributed. The Company's Convertible Preferred shares would be entitled to dividends that would be distributed to the holders of Common Stock, based on the conversion ratio, assuming conversion of all Convertible Preferred shares into shares of Common Stock.
The Company’s basic net loss per share is calculated by dividing net loss attributable to common and preferred stockholders by the weighted-average number of shares, without consideration of potentially dilutive securities. The diluted net loss per share is calculated by giving effect to all potentially dilutive securities outstanding for the period using the treasury share method or the if-converted method based on the nature of such securities. Diluted net loss per share is the same as basic net loss per share in periods when the effects of potentially dilutive shares of Common Stock are anti-dilutive.
F-19
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
t. |
Severance pay: |
Since inception date, all Ltd. employees who are entitled to receive severance pay in accordance with the applicable law in Israel, have been included under section 14 of the Israeli Severance Compensation Law (“Section 14”). Under this section, they are entitled only to monthly deposits, at a rate of 8.33% of their monthly salary, made by the employer on their behalf with insurance companies. Payments in accordance with Section 14 release Ltd. from any future severance payments in respect of those employees. Payments under Section 14 are not recorded as an asset in the Company’s balance sheet.
Severance pay expense for the year ended December 31, 2024 and 2023 amounted to $935 and $947, respectively.
The Company has a 401(k) defined contribution plan covering certain employees in the U.S. All eligible employees may elect to contribute up to $23 per year (for certain employees over 50 years of age the maximum contribution is $30.5 per year), of their annual compensation to the plan through salary deferrals, subject to Internal Revenue Service limits.
u. |
Legal and other contingencies: |
The Company accounts for its contingent liabilities in accordance with ASC 450 “Contingencies”. A provision is recorded when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated. With respect to legal matters, provisions are reviewed and adjusted to reflect the impact of negotiations, estimated settlements, legal rulings, advice of legal counsel and other information and events pertaining to a particular matter. As of December 31, 2024, and 2023, the Company is not a party to any litigation that could have a material adverse effect on the Company’s business, financial position, results of operations or cash flows. Legal costs incurred in connection with loss contingencies are expensed as incurred.
v. |
Leases: |
Lessee accounting:
The Company determines if an arrangement is a lease and the classification of that lease at inception based on: (1) whether the contract involves the use of a distinct identified asset, (2) whether the Company obtains the right to substantially all the economic benefits from the use of the asset throughout the period, and (3) whether the Company has a right to direct the use of the asset. The Company elected not to recognize a lease liability or right-of-use (“ROU”) asset for leases with a term of twelve months or less. The Company also elected the practical expedient not to separate lease and non-lease components for its leases.
ROU assets represent the right to use an underlying asset for the lease term and lease liabilities represent the obligation to make lease payments arising from the lease. ROU assets are initially measured at amounts, which represents the discounted present value of the lease payments over the lease, plus any initial direct costs incurred. The ROU assets are reviewed for impairment. The lease liability is initially measured at lease commencement date based on the discounted present value of the lease payments over the lease term. The implicit rate within the operating leases is generally not determinable; therefore, the Company uses the Incremental Borrowing Rate (“IBR”) based on the information available at commencement date in determining the present value of lease payments. The Company’s IBR is estimated to approximate the interest rate on similar terms and payments and in economic environments where the leased asset is located.
F-20
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
Certain leases include options to extend or terminate the lease. An option to extend the lease is considered in connection with determining the ROU asset and lease liability when it is reasonably certain that the Company will exercise that option. An option to terminate is considered unless it is reasonably certain that the Company will not exercise the option. See also Note 9.
w. |
Business combination and asset acquisitions: |
The Company applies the provisions of ASC 805, “Business Combination” and allocates the fair value of purchase consideration to the tangible assets acquired, liabilities assumed, and intangible assets acquired based on their estimated fair values. The excess of the fair value of purchase consideration over the fair values of these identifiable assets and liabilities is recorded as goodwill. When determining the fair values of assets acquired and liabilities assumed, management makes significant estimates and assumptions, especially with respect to intangible assets.
Acquisition-related expenses are recognized separately from the business combination and are expensed as incurred.
The Company accounts for a transaction as an asset acquisition when substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or group of similar identifiable assets, or otherwise does not meet the definition of a business. Asset acquisition-related direct costs are capitalized as part of the asset or assets acquired.
x. |
Intangible Assets: |
Acquired identifiable finite-lived intangible assets are amortized on a straight-line basis over the estimated useful lives of the assets. The basis of amortization approximates the pattern in which the assets are utilized, over their estimated useful lives. The Company routinely reviews the remaining estimated useful lives of finite-lived intangible assets. In case the Company reduces the estimated useful life for any asset, the remaining unamortized balance is amortized over the revised estimated useful life (see Note 11).
y. |
Goodwill: |
Goodwill represents the excess of the purchase price in a business combination over the fair value of the net tangible and intangible assets acquired. Under ASC 350, "Intangible - Goodwill and Other" ("ASC 350"), goodwill is not amortized, but rather is subject to an annual impairment test.
ASC 350 allows an entity to first assess qualitative factors to determine whether it is necessary to perform the quantitative goodwill impairment test. If the qualitative assessment does not result in a more likely than not indication of impairment, no further impairment testing is required. If the Company elects not to use this option, or if the Company determines that it is more likely than not that the fair value of a reporting unit is less than its carrying value, then the Company prepares a quantitative analysis to determine whether the carrying value of a reporting unit exceeds its estimated fair value. If the carrying value of a reporting unit would exceed its estimated fair value, the Company would have recognized an impairment of goodwill for the amount of this excess.
For the years ended December 31, 2024 and 2023, no impairment of goodwill has been recorded.
F-21
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
z. |
Recently Adopted Accounting Pronouncements |
(i) |
In November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2023-07, Segment Reporting (Topic 280), Improvements to Reportable Segment Disclosures requiring public entities to disclose information about their reportable segments’ significant expenses and other segment items on an interim and annual basis. Public entities with a single reportable segment are required to apply the disclosure requirements in ASU 2023-07, as well as all existing segment disclosures and reconciliation requirements in ASC 280 on an interim and annual basis. ASU 2023-07 is effective for fiscal years beginning after December 15, 2023, and interim periods for the fiscal years beginning after December 15, 2024. The Company adopted the ASU 2023-07 during the year ended December 31, 2024, on a retrospective basis which resulted in updated segment disclosures. See Note 18 in the accompanying notes to the consolidated financial statements for further detail. |
aa. |
Recently issued accounting pronouncements, not yet adopted: |
(i) |
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740) - Improvements to Income Tax Disclosures. The ASU requires that an entity disclose specific categories in the effective tax rate reconciliation as well as provide additional information for reconciling items that meet a quantitative threshold. Further, the ASU requires certain disclosures of state versus federal income tax expense and taxes paid. The amendments in this ASU are required to be adopted for fiscal years beginning after December 15, 2024. Early adoption is permitted, and the amendments should be applied on a prospective basis. The Company is currently evaluating the effect of adopting the ASU on its disclosures. |
(ii) |
In November 2024, the FASB issued ASU 2024-03, Income Statement-Reporting Comprehensive Income-Expense Disaggregation Disclosures (Subtopic 220-40) - Disaggregation of Income Statement Expenses. The ASU requires, among other items, additional disaggregated disclosures in the notes to financial statements for certain categories of expenses that are included on the Statements of Operations. ASU 2024-03 is effective for fiscal years beginning after December 15, 2026, and for interim periods within fiscal years beginning after December 15, 2027, with early adoption permitted, and may be applied either prospectively or retrospectively. The Company is currently evaluating the effect of adopting the ASU on its disclosures. |
NOTE 3:- OTHER ACCOUNTS RECEIVABLE AND PREPAID EXPENSES
|
|
December 31, |
||||
|
|
2024 |
|
2023 |
||
|
|
|
|
|
|
|
Prepaid expenses |
|
$ |
1,788 |
|
$ |
1,536 |
Costs to fulfill a contract |
|
|
259 |
|
|
238 |
Government authorities |
|
|
289 |
|
|
250 |
|
|
|
|
|
|
|
|
|
$ |
2,336 |
|
$ |
2,024 |
F-22
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 4:- ACQUISITIONS
Acquisition of Twill
On February 15, 2024, the Company completed the merger and the associated acquisition of all issued and outstanding shares of Twill for an aggregate consideration of (A) $10.0 million in cash, and (B) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 10,000,400 shares of the Company’s Common Stock, per value $0.0001 per share (the "common stock"). In addition, the Company issued stock options to purchase up to 2,963,459 shares of Common Stock and a combination of warrants and restricted stock units (“RSUs”) to acquire up to 1,766,508 shares of Common Stock to certain employees and officers of Twill. The Company accounted for the stock options, warrants and RSUs separately from the acquisition. The Company incurred acquisition-related costs in a total amount of $857, which were included in general and administrative expenses in the Consolidated Statements of Comprehensive loss.
The acquisition of Twill advances the Company’s strategy to evolve from a point solution to a comprehensive multi condition platform. With the integration of Twill, the Company believes it can achieve multiple advantages as well as synergies in multiple fronts like its product offering, commercial channels, improved clients and member experience.
Preliminary purchase price allocation:
Pursuant to ASC 805, the total purchase price was allocated to Twill’s net tangible and intangible assets based on their estimated fair values as set forth below. The excess of the purchase price over the net tangible and identifiable intangible assets was recorded as goodwill. A portion of the acquisition price was recorded as goodwill due to the synergies with Twill and is not expected to be deductible for tax purposes.
The allocation of the purchase price to the assets acquired and liabilities assumed based on management’s estimate of fair values at the date of acquisition as follows:
F-23
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 4 – ACQUISITIONS (Cont.)
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
531 |
Short-term restricted bank deposits |
|
|
673 |
Trade receivables |
|
|
3,329 |
Other accounts receivable and prepaid expenses |
|
|
475 |
Property and equipment, net |
|
|
580 |
Operating lease right of use assets |
|
|
995 |
Acquisition-related intangibles |
|
|
19,435 |
Other assets |
|
|
23 |
Total assets acquired |
|
|
26,041 |
Trade payables |
|
|
2,411 |
Other accounts payable and accrued expenses |
|
|
1,223 |
Deferred revenues |
|
|
742 |
Operating lease liabilities |
|
|
995 |
Deferred tax liability |
|
|
2,001 |
Liabilities assumed |
|
|
7,372 |
Fair value of net assets acquired |
|
|
18,669 |
Goodwill |
|
|
15,787 |
Total purchase consideration |
|
$ |
34,456 |
Following are details of the purchase consideration allocated to acquired intangible assets:
|
|
|
|
Amortization |
||
|
|
Fair value |
|
period (Years) |
||
Technology (1) |
|
$ |
5,644 |
|
|
7.9 |
Customer relationship healthcare (2) |
|
|
13,791 |
|
|
11.9 |
Total identified intangible assets acquired |
|
$ |
19,435 |
|
|
|
| (1) | The technology has been calculated through the Income Approach, in particular the Relief from Royalty method. |
| (2) | The fair value of Twill’s customer relationships has been calculated using the Multi-Period excess earnings method (“MPEEM method”). |
|
|
Amount |
|
Number of shares of common stock issuable upon the exercise of the consideration warrants. |
|
|
10,000,400 |
Value of each warrant issued |
|
$ |
2.446 |
Total consideration warrant shares |
|
|
24,456 |
|
|
|
|
Cash consideration |
|
|
10,000 |
Total purchase price |
|
$ |
34,456 |
F-24
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 4 – ACQUISITIONS (Cont.)
The consolidated statement of comprehensive loss includes the following revenue and net loss attributable to Twill in 2024:
|
|
Year ended |
|
|
|
December 31, 2024 |
|
Revenues |
|
$ |
12,931 |
Net loss |
|
$ |
5,645 |
The Company recognized $2,001 of a deferred tax liability which relates to the purchase price allocation fair value adjustments, other than goodwill. The Company is planning to file a consolidated tax return in the U.S. together with Twill and to utilize the benefit of the Company's loss carryforwards against the future taxable income of Twill and consequently decreased its valuation allowance in an amount equal to the deferred tax liability recognized in the business combination.
Supplemental Unaudited Pro Forma Information
The following table sets forth a summary of the unaudited pro forma results of the Company as if the acquisition of Twill, which closed in February 2024, had taken place had Twill been acquired as of January 1, 2023.
|
|
Year ended |
||||
|
|
December 31, |
||||
|
|
2024 |
|
2023 |
||
Total revenues |
|
$ |
29,003 |
|
$ |
38,542 |
Net loss |
|
$ |
55,985 |
|
$ |
69,711 |
|
|
|
|
|
|
|
The unaudited pro forma financial information presented is for informational purposes only and is not necessarily indicative of the results of operations that would have been achieved if the Twill acquisition was completed at the beginning of 2023 and are not indicative of the future operating results of the combined company. The pro forma results include adjustments related primarily to purchase accounting, and amortization of acquisition-related intangible assets. The pro forma results also include income from revaluation of the pre-funded warrants issued as part of the consideration for the acquisition of Twill, as these warrants are classified as a liability under U.S. GAAP.
NOTE 5:- INVENTORIES
Inventory consists of the following:
|
|
December 31, |
|
December 31, |
||
|
|
2024 |
|
2023 |
||
Raw materials |
|
$ |
563 |
|
$ |
1,015 |
Finished products |
|
|
4,190 |
|
|
4,047 |
|
|
|
|
|
|
|
|
|
$ |
4,753 |
|
$ |
5,062 |
F-25
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 6:- REVENUE
The Company is operating a multi-condition healthcare business, empowering individuals to manage their chronic conditions and take steps to improve their overall health. The Company generates revenue directly from individuals through a la carte offering and membership plans. The Company also contracts with enterprise business market groups to provide digital therapeutics solutions for individuals to receive access to services through the Company’s commercial arrangements.
Agreement with Preferred Partner
On February 28, 2022, the Company entered into an exclusive preferred partner, co-promotion, development and license agreement for a term of five (5) years (the “Exclusive Agreement”). Pursuant to the Exclusive Agreement, the Company will provide a license to access and use certain Company data. The contract included fixed and variable amounts, which were fully constrained. In addition, the Company may provide development services for new products of the other party.
During fiscal year 2022 and 2023, three development plans were approved and accounted for as separate contracts. The Company's measured the progress of the development services performance obligations using an input method, based on labor hours consumed as the Company believes that this method best depicts the transfer of services to the customer. In the years ended December 31, 2022, and 2023, the Company recognized revenues in the amounts of $5,506 and $4,592, respectively. During fiscal 2024, the Company provided a price concession to the preferred partner in the amount of $1,088 to resolve an outstanding dispute. As a result, the Company has recorded the price concession amount as a reduction in revenue.
On December 26, 2024, the agreement with the preferred partner was terminated. Concurrently, the Company entered into a new agreement (the "New Agreement") to provide its SaaS services. As part of these arrangements, the preferred partner shall pay $1,580. The Company accounted for the termination and the creation of a new contract as a contract modification. Since the additional services were not priced at SSP, the contract modification was accounted for prospectively. Under the new contract, the Company will provide the preferred customer with SaaS and professional services and ad-serving services. During the year ended December 31, 2024, the Company recognized revenues related to the modified contract in the amount of $292.
Agreement with National Health Plan
On October 1, 2021, the Company entered into a Master Service Agreement (the “MSA”) and a statement of work (“SOW”, and such SOW, the “October SOW”) with a national health plan (“Health Plan”). Pursuant to the October SOW, the Company will provide the Health Plan access to the Company’s web and app-based platform for behavioral health. The Company has concluded that the contract contained a single performance obligation – to provide access to the Company’s platform. The consideration in the contract was based entirely on customer usage.
On August 2022, the Company entered into an additional SOW (the “August SOW”) with the Health Plan according to which the Company will provide implementation services and shall develop additional features to be included in the platform.
The Company concluded that the August SOW should be accounted for as a separate contract. The Company has concluded that the August SOW contained two performance obligations as follows:
| (i) | Digital Behavioral Health Navigation Platform Implementation. This performance obligation includes configuration and implementation of the platform. |
| (ii) | Enhancements to the Digital Behavioral Health Navigation Platform. This performance obligation includes adding additional features and capabilities to the platform. |
F-26
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 6: - REVENUES (Cont.)
The August SOW includes a fixed consideration in the amount of $2,650. The Company allocated the consideration between the two performance obligations based on standalone selling prices. The Company determined the standalone selling prices based on the expected cost plus a margin approach.
On February 21, 2023, and July 1, 2024, the Company entered into change orders with the Health Plan according to which the Company will provide additional implementation services and shall develop additional features to be included in the platform. The change orders include a fixed consideration in the amount of $90 and $85, respectively.
For the year ended December 31, 2023, the Company recognized revenues of $962. The August SOW was completed during the second quarter of 2023.
Revenue Source:
The following table represent the Company total revenues for the year ended December 31, 2024 and 2023 disaggregated by revenue source:
|
|
Year ended |
||||
|
|
December 31, |
||||
|
|
2024 |
|
2023 |
||
Commercial - Business-to-Business-to-Consumer (“B2B2C”) |
|
$ |
20,023 |
|
$ |
5,005 |
Commercial - Strategic partnerships |
|
|
(514) |
|
|
7,054 |
Consumers |
|
|
7,531 |
|
|
8,293 |
|
|
|
|
|
|
|
|
|
$ |
27,040 |
|
$ |
20,352 |
Deferred Revenue
The Company recognizes contract liabilities, or deferred revenues, when it receives advance payments from customers prior to the satisfaction of the Company's performance obligations. The balance of deferred revenues approximates the aggregate amount of the transaction price allocated to the unsatisfied performance obligations at the end of the reporting period. The Company expects to recognize approximately $1,525 over the next 12 months and the remainder thereafter.
The Company elected to not disclose information about remaining performance obligations for which the variable consideration is allocated to a wholly unsatisfied promise to transfer a distinct good or service that is subject to the variable consideration allocation exception.
The following table presents the significant changes in the deferred revenue balance during the year ended December 31, 2024:
Balance, beginning of the period |
|
$ |
997 |
Additions through acquisition of Twill |
|
|
742 |
New performance obligations |
|
|
5,191 |
Reclassification to revenue as a result of satisfying performance obligations |
|
|
(5,347) |
|
|
|
|
Balance, end of the period |
|
$ |
1,583 |
Costs to fulfill a contract
F-27
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
The Company defers costs incurred to fulfill contracts that: (1) relate directly to the contract; (2) are expected to generate resources that will be used to satisfy the Company’s performance obligations under the contract; and (3) are
NOTE 6:- REVENUE (Cont.)
expected to be recovered through revenue generated under the contract. Contract fulfillment costs are expensed as the Company satisfies its performance obligations and recorded into cost of revenue.
Costs to fulfill a contract are recorded in other accounts receivable and prepaid expenses and long-term assets.
Costs to fulfill a contract consist of (1) deferred consumer hardware costs incurred in connection with the delivery of services that are deferred, and (2) deferred costs incurred, related to future performance obligations which are capitalized.
Costs to fulfill a contract as of December 31, 2024, and December 2023, consisted of the following:
|
|
December 31, |
|
December 31, |
||
|
|
2024 |
|
2023 |
||
|
|
Unaudited |
|
|
|
|
Costs to fulfill a contract, current |
|
$ |
259 |
|
$ |
238 |
Costs to fulfill a contract, noncurrent |
|
|
65 |
|
|
59 |
|
|
|
|
|
|
|
Total costs to fulfill a contract |
|
$ |
324 |
|
$ |
297 |
Costs to fulfill a contract were as follows:
|
|
|
Costs to |
|
|
|
fulfill a contract |
|
|
|
|
Beginning balance as of December 31, 2023 |
|
$ |
297 |
Additions |
|
|
391 |
Cost of revenue recognized |
|
|
(364) |
|
|
|
|
Ending balance as of December 31, 2024 |
|
|
324 |
F-28
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 7:- DEBT
Loan Facility
On May 1, 2023, the Company refinanced its existing $25,000 credit facility with a new $30,000 credit facility in the Loan and Security Agreement, and Supplement thereto (the “LSA” or the “Avenue Loan Facility”) by and between the Company and its subsidiary PsyInnovations Inc., collectively as the borrowers (the “Borrowers”) and Avenue Venture Opportunities Fund II, L.P. and Avenue Venture Opportunities Fund, L.P., collectively as the lenders (the “Avenue Lenders”). The LSA provides for a four-year secured credit facility in an aggregate principal amount of up to $40,000, of which $30,000 was made available on the closing date (the “Initial Tranche”) and up to $10,000 (the “Discretionary Tranche”) may be made available on the later of July 1, 2023, or the date the Avenue Lenders approve the issuance of the Discretionary Tranche. On May 1, 2023, the Borrowers closed on the Initial Tranche, less certain fees and expenses payable to or on behalf of the Avenue Lenders.
During the term of the Avenue Loan Facility, interest payable in cash by the Borrowers shall accrue on any outstanding balance due under the Avenue Loan Facility at a rate per annum equal to the higher of (x) the sum of four one-half percent (4.50%) plus the prime rate as published in the Wall Street Journal and (y) twelve and one-half percent (12.50%). During an event of default, any outstanding amount under the Avenue Loan Facility will bear interest at a rate of 5.00% in excess of the otherwise applicable rate of interest and the outstanding balance shall be due and payable. As part of the LSA, the Company issued a warrant (the “Warrant”) to purchase up to 584,882 shares of the Company’s Common Stock, at an exercise price of $3.334 per share, which has a term of five years from the issuance date.
On February 15, 2024, the Company and the Borrowers entered into the First Amendment to Loan and Security Agreement and Supplement (the “Avenue Amendment”) with the Avenue Lenders. Pursuant to the Avenue Amendment, the parties agreed to include the Merger Sub and Twill as parties to the Company’s existing loan facility with the lenders. In addition, the Avenue Amendment permit the lenders, subject to Nasdaq rules, to convert up to $2,000 of the principal amount of its loan to the Company at a fixed conversion price of $4.001 per share.
On June 25, 2024, the Company stockholders approved the Avenue Amendment and repriced the Warrant to purchase up to 584,882 shares of Common Stock issued to the lenders on May 1, 2023 at an exercise price $3.334 per share, to permit an amendment to the exercise price of such Warrants to $2.02 which is the “minimum price” as defined by Nasdaq rules as of the closing of the Twill Agreement and (ii) permit the lenders, subject to Nasdaq rules, to convert up to $2,000 of the principal amount of the outstanding Avenue Loan Facility into Borrower’s unrestricted shares of the Company’s Common Stock at a conversion price of $4.001.
Pursuant to the LSA and the Avenue Amendment, the Company is obligated to maintain at least $5,000 of unrestricted cash in deposit accounts located in the United States.
The Company concluded that Avenue Loan Facility and the Warrant are freestanding financial instruments since these instruments are legally detachable and separately exercisable. The Company has concluded that the Warrant meets all the conditions to be classified as equity pursuant to ASC 480 and ASC 815-40. In addition, the Company elected to account for the Avenue Loan Facility under the fair value option in accordance with ASC 825, “Financial Instruments.” Under the fair value option, changes in fair value are recorded in earnings except for fair value adjustments related to instrument specific credit risk, which are recorded as other comprehensive income or loss. As such, the proceeds were first allocated to the Avenue Loan Facility at fair value in the amount of $28,215 and the remaining amount of $1,389 was allocated to the Warrant.
F-29
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 7: - DEBT (Cont.)
The Company remeasurement expenses related to the Avenue Loan for the year ended December 31, 2024 were $378, compared to $42 of remeasurement income for the year ended December 31, 2023, which were included as part of the financial (income) expenses in the Company's statements comprehensive loss. During the years ended December 31, 2024, and December 31, 2023, the Company did not recognize any instrument specific credit risk fair value adjustment.
In connection with the debt modification, the Company also modified the Warrant by reducing the exercise price of the Warrant from $3.334 to $2.02. The Company recognized the incremental fair value resulting from the modification through earnings in the amount of $70.
On December 16, 2024, the Company, entered into the Third Amendment to Loan and Security Agreement and Supplement (the “Third Avenue Amendment”) with the Avenue Lenders. Pursuant to the Third Avenue Amendment, the parties agreed to (i) amend the potential interest only period under the loan facility such that the existing interest only period ending on May 30, 2025 was extended by a period of six months provided the Company achieving certain net proceeds from an equity financing on or before March 31, 2025 in the aggregate; (ii) an additional sixth month interest only extension period was added, which is conditioned on the Company achieving a certain net revenue milestone, with cash burn not to exceed a certain level, for the trailing six month period ending September 30, 2025; (iii) the interest only period may not exceed a total of 36 months from the closing of the loan as of May 1, 2023; and (iv) the maturity date of the loan will be extended from May 1, 2027 to November 1, 2027, provided that the Company meets the foregoing amended milestones. In January 2025, the Company met the equity financing milestone.
In addition, the Avenue Amendment provides (i) that the Company will seek stockholder approval to reprice the Warrant issued to the Avenue Lenders on May 1, 2023 to permit an amendment to the exercise price of such Warrant to the “minimum price” as defined by Nasdaq rules as of the closing of the Avenue Amendment (or $0.7208 per share) and (ii) permit the Avenue Lenders, subject to Nasdaq rules, to convert up to $2,000 of the principal amount of its loan to the Company’s common stock at a conversion price of $0.8650 per share. As of December 31, 2024, the Company has not yet obtained stockholder approval for these items.
Orbimed Warrants
On June 9, 2022 (the closing date of the Orbimed Loan, which was repaid in May 2023), the Company agreed to issue Orbimed a warrant (the “Orbimed Warrant”) to purchase up to 226,586 shares of the Company’s Common Stock, at an exercise price of $6.62 per share, which shall have a term of 7 years from the issuance date. The Orbimed Warrant contains customary share adjustment provisions, as well as weighted average price protection in certain circumstances but in no event will the exercise price of the Warrant be adjusted to a price less than $4.00 per share. Following the issuance and sale of the Company’s Series C Preferred Stock in February 2024, and as a result of a certain price protection provision in the Orbimed Warrant, the exercise price of the Orbimed Warrant was adjusted to a price per share of $4.00.
The Company has concluded that the Orbimed Warrant is not indexed to the Company's own stock and should be recorded as a liability measured at fair value with changes in fair value recognized in earnings. The Company remeasurement income related to the Orbimed Warrant for the year ended December 31, 2024, were $168, compared to $670 for the year ended December 31, 2023.
Pre-funded warrants
On February 15, 2024, as part of the acquisition of Twill (See note 4) the Company issued Pre-Funded Warrants to purchase up to 10,000,400 shares of Company Common Stock, issuable to a trust (the “Trust”) formed for the benefit of certain equity and debt holders of Twill.
F-30
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 7: - DEBT (Cont.)
The Company has classified the Pre-Funded Warrants as liability pursuant to ASC 815-40 since the Pre-Funded Warrants do not meet all the equity classification conditions. Accordingly, the Company measured the Pre-Funded Warrants at their fair value. The warrants liability is subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in our statement of comprehensive loss.
In November 2024, a total of 2,500,100 Pre-Funded Warrants were exercised into 2,499,828 shares of Common Stock. During the year ended December 31, 2024, the Company recognized $16,336 respectively, of remeasurement income related to the Pre-Funded Warrants. The estimated fair value of the Pre-Funded Warrants liabilities is determined using Level 1 inputs based on quoted prices in active markets.
NOTE 8:- FAIR VALUE MEASUREMENTS
Under U.S. GAAP, fair value is defined as the amount that would be received for selling an asset or paid to transfer a liability in an orderly transaction between market participants and requires that assets and liabilities carried at fair value are classified and disclosed in the following three categories:
Level 1 - |
Valuations based on quoted prices in active markets for identical assets that the Company has the ability to access. Valuation adjustments and block discounts are not applied to Level 1 instruments. Since valuations are based on quoted prices that are readily and regularly available in an active market, valuation of these products does not entail a significant degree of judgment. |
Level 2 - |
Valuations based on one or more quoted prices in markets that are not active or for which all significant inputs are observable, either directly or indirectly. |
Level 3 - |
Valuations based on inputs that are unobservable and significant to the overall fair value measurement. |
The availability of observable inputs can vary from instrument to instrument and is affected by a wide variety of factors, including, for example, the type of investment, the liquidity of markets and other characteristics particular to the transaction. To the extent that valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment, and the investments are categorized as Level 3.
The carrying amounts of cash and cash equivalents, short-term restricted bank deposits, trade receivables, other accounts receivable and prepaid expenses, trade payables and other accounts payable and accrued expenses approximate their fair value due to the short-term maturity of such instruments. The Company’s Orbimed loan facility was measured at fair value using Level 3 unobservable inputs until the payoff date of May 1, 2023. The Orbimed Warrant liability was measured at fair value using Level 3 unobservable inputs. In addition, the Avenue Loan Facility is also measured at fair value using level 3 inputs.
F-31
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 8:- FAIR VALUE MEASUREMENTS (Cont.)
The following tables present information about the Company’s financial liabilities measured at fair value on a recurring basis and indicate the level of the fair value hierarchy used to determine such fair values:
|
|
December 31, 2024 |
|||||||||||
|
|
Fair Value |
|
|
Level 1 |
|
Level 2 |
|
Level 3 |
||||
|
|
|
|
|
(in thousands) |
|
|
||||||
Financial assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. treasury notes |
|
$ |
7,305 |
|
|
$ |
— |
|
$ |
7,305 |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total financial assets |
|
$ |
7,305 |
|
|
$ |
— |
|
$ |
7,305 |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term loan |
|
|
28,923 |
|
|
|
— |
|
|
— |
|
|
28,923 |
Orbimed Warrant liability |
|
|
72 |
|
|
|
— |
|
|
— |
|
|
72 |
Pre-funded warrant liability |
|
|
5,896 |
|
|
|
— |
|
|
5,896 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total financial liabilities |
|
$ |
34,891 |
|
|
$ |
— |
|
$ |
5,896 |
|
$ |
28,995 |
|
|
December 31, 2023 |
|||||||||||
|
|
Fair Value |
|
|
Level 1 |
|
Level 2 |
|
Level 3 |
||||
|
|
|
|
|
(in thousands) |
|
|
||||||
Financial liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term loan |
|
|
28,545 |
|
|
|
— |
|
|
— |
|
|
28,545 |
Orbimed Warrant liability |
|
$ |
240 |
|
|
|
— |
|
|
— |
|
|
240 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total financial liabilities |
|
$ |
28,785 |
|
|
$ |
— |
|
$ |
— |
|
$ |
28,785 |
Loan Facilities
The fair value of the Avenue Loan Facility was determined based on significant inputs not observable in the market, which represents a Level 3 measurement within the fair value hierarchy. The Avenue Loan Facility fair value estimate incorporates comparisons to instruments with similar covenants, collateral, and risk profiles and was obtained using a discounted cash flow technique. On the date of Avenue Loan Facility origination, or May 1, 2023, the discount rate was arrived at by calibrating the loan amount of $30 million with the fair value of the warrants of $1,413 and the loan terms interest rate equal to the greater of (i) the sum of four and one-half percent (4.50%) plus the Prime Rate, and (ii) twelve and one-half percent (12.50%). During an event of default, any outstanding amount under the Avenue Loan Facility will bear interest at a rate of 5.00% in excess of the otherwise applicable rate of interest. The fair value of the Avenue Loan Facility, as of December 31, 2024, was estimated using a discount rate of 19% which reflects the internal rate of return of the Avenue Loan Facility at closing, as of May 1, 2023. For the years ended December 31, 2024 and December 31, 2023, the change in the fair value of the loan was recorded in earnings since the Company concluded that the change in fair value was not related to instrument-specific credit risk.
Orbimed Warrant Liability
The fair value of the Orbimed Warrant liability was determined based on significant inputs not observable in the market, which represents a Level 3 measurement within the fair value hierarchy. The fair value of the Orbimed Warrant liability is estimated by the Company based on the Monte-Carlo simulation valuation technique, in order to predict the probability of different outcomes that rely on repeated random variables.
F-32
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 8:- FAIR VALUE MEASUREMENTS (Cont.)
The following inputs were used to estimate the fair value of the Orbimed Warrant liability:
|
|
|
December 31, |
|
|
December 31, |
||
|
|
|
2024 |
|
|
2023 |
||
Stock price |
|
$ |
0.79 |
|
|
$ |
1.72 |
|
Exercise price |
|
|
4.00 |
|
|
|
5.79 |
|
Expected term (in years) |
|
|
4.44 |
|
|
|
5.44 |
|
Volatility |
|
|
91.2% |
|
|
|
96.8% |
|
Dividend rate |
|
|
— |
|
|
|
— |
|
Risk-free interest rate |
|
|
4.53% |
|
|
|
3.88% |
|
The following tables present the summary of the changes in the fair value of our financial instruments:
|
|
|
Long-Term Loan |
|
|
Orbimed Warrant Liability |
|
|
Pre-funded Warrant Liability |
Balance as of January 1, 2023 |
|
$ |
26,928 |
|
$ |
910 |
|
$ |
— |
Issuance |
|
|
28,587 |
|
|
— |
|
|
— |
Principal repayments on long-term loan |
|
|
(27,833) |
|
|
— |
|
|
— |
Change in fair value |
|
|
863 |
|
|
(670) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2023 |
|
$ |
28,545 |
|
$ |
240 |
|
$ |
— |
|
|
|
Long-Term Loan |
|
|
Orbimed Warrant Liability |
|
|
Pre-funded Warrant Liability |
Balance as of January 1, 2024 |
|
$ |
28,545 |
|
$ |
240 |
|
$ |
— |
Issuance |
|
|
— |
|
|
— |
|
|
24,457 |
Exercise |
|
|
— |
|
|
— |
|
|
(2,225) |
Change in fair value |
|
|
378 |
|
|
(168) |
|
|
(16,336) |
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2024 |
|
$ |
28,923 |
|
$ |
72 |
|
$ |
5,896 |
F-33
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 9:- LEASES
The Company has entered into various non-cancelable operating lease agreements for certain of its offices and car leases. The Company's leases have original lease periods expiring between 2024 and 2028. Many leases include one or more options to renew. The Company does not assume renewals in determination of the lease term unless the renewals are deemed to be reasonably certain at lease commencement. The Company's lease agreements do not contain any material residual value guarantees or material restrictive covenants, the Company elected to not recognize a lease liability and a ROU asset for lease with a term of twelve months or less.
The components of lease costs, lease term and discount rate are as follows:
|
|
Year Ended |
|
|
|
|
December 31, |
|
|
|
|
2024 |
|
|
Lease cost |
|
|
|
|
Operating lease cost |
|
$ |
1,050 |
|
Sublease rent income |
|
|
(371) |
|
Short term lease cost |
|
|
133 |
|
Variable lease cost |
|
|
13 |
|
Total lease cost |
|
$ |
825 |
|
|
|
|
|
|
Weighted Average Remaining Lease Term |
|
|
|
|
Operating leases |
|
|
3.14 |
|
|
|
|
|
|
Weighted Average Discount Rate |
|
|
|
|
Operating leases |
|
|
9.35 |
% |
The following is a schedule, by years, of maturities of lease liabilities as of December 31, 2024:
|
|
Operating |
|
|
|
Leases |
|
|
|
|
|
2025 |
|
$ |
525 |
2026 |
|
|
376 |
2027 |
|
|
277 |
2028 |
|
|
283 |
2029 |
|
|
— |
Total undiscounted cash flows |
|
|
1,461 |
Less imputed interest |
|
|
(192) |
Present value of lease liabilities |
|
$ |
1,269 |
Supplemental cash flow information related to leases are as follows:
|
|
Year ended |
|
|
|
December 31, |
|
|
|
2024 |
|
Cash paid for amounts included in the measurement of lease liabilities: |
|
|
|
Operating cash flows from operating leases |
|
$ |
1,289 |
Lease liabilities arising from obtaining right-of-use assets: |
|
|
|
Operating leases |
|
$ |
428 |
F-34
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 10:- PROPERTY AND EQUIPMENT, NET
Composition of assets, grouped by major classification, is as follows:
|
|
December 31, |
||||
|
|
2024 |
|
2023 |
||
Cost: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Computers and peripheral equipment |
|
$ |
1,122 |
|
$ |
937 |
Office furniture and equipment |
|
|
132 |
|
|
137 |
Production lines |
|
|
1,030 |
|
|
988 |
Leasehold improvement |
|
|
336 |
|
|
326 |
|
|
|
|
|
|
|
|
|
|
2,620 |
|
|
2,388 |
Accumulated depreciation: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Computers and peripheral equipment |
|
|
835 |
|
|
542 |
Office furniture and equipment |
|
|
48 |
|
|
43 |
Production lines |
|
|
930 |
|
|
874 |
Leasehold improvement |
|
|
98 |
|
|
30 |
|
|
|
|
|
|
|
|
|
|
1,911 |
|
|
1,489 |
|
|
|
|
|
|
|
Property and equipment, net |
|
$ |
709 |
|
$ |
899 |
Depreciation expenses for the year ended December 31, 2024, and 2023 amounted to $466 and $392, respectively.
F-35
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 11:- OTHER INTANGIBLE ASSETS, NET
a. Finite-lived other intangible assets: |
|
|
|
|
|
|
|
|
|
|
December 31, |
|
December 31, |
|
Weighted Average |
||
|
|
2024 |
|
2023 |
|
Remaining Life |
||
|
|
|
|
|
|
As of December 31, 2024 |
||
Original amounts: |
|
|
|
|
|
|
|
|
Technology |
|
$ |
22,580 |
|
$ |
16,936 |
|
5.9 |
Brand |
|
|
376 |
|
|
376 |
|
0.0 |
Customer Relationship Healthcare |
|
|
13,791 |
|
|
— |
|
11.0 |
Domains |
|
|
23 |
|
|
— |
|
|
|
|
|
36,770 |
|
|
17,312 |
|
|
Accumulated amortization: |
|
|
|
|
|
|
|
|
Technology |
|
|
16,611 |
|
|
11,586 |
|
|
Brand |
|
|
376 |
|
|
322 |
|
|
Customer Relationship Healthcare |
|
|
1,018 |
|
|
— |
|
|
Domains |
|
|
3 |
|
|
— |
|
|
|
|
|
18,008 |
|
|
11,908 |
|
|
Other intangible assets, net |
|
$ |
18,762 |
|
$ |
5,404 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
b. Amortization expenses for the years ended December 31, 2024 and December 31, 2023 amounted to $6,100 and $4,512, respectively. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
c. Estimated amortization expense: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, |
|
|
|
|
|
|
|
|
2025 |
|
|
2,827 |
|
|
|
|
|
2026 |
|
|
1,875 |
|
|
|
|
|
2027 |
|
|
1,875 |
|
|
|
|
|
2028 |
|
|
1,880 |
|
|
|
|
|
2029 |
|
|
1,877 |
|
|
|
|
|
Thereafter |
|
|
8,428 |
|
|
|
|
|
|
|
$ |
18,762 |
|
|
|
|
|
F-36
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 12:-GOODWILL
The following table set forth the changes in the carrying amount of the Company’s goodwill during the year ended December 31, 2024 and the year ended December 31, 2023 (in thousands):
|
|
December 31, |
|
|
|
2024 |
|
|
|
|
|
As of December 31, 2022 |
|
$ |
41,640 |
Additions |
|
|
— |
As of December 31, 2023 |
|
|
41,640 |
Additions |
|
|
15,787 |
As of December 31, 2024 |
|
$ |
57,427 |
|
|
|
|
NOTE 13:- OTHER ACCOUNTS PAYABLE AND ACCRUED EXPENSES
|
|
December 31, |
||||
|
|
2024 |
|
2023 |
||
Employees and payroll accruals |
|
$ |
4,112 |
|
$ |
4,073 |
Accrued expenses |
|
|
1,940 |
|
|
2,227 |
|
|
|
|
|
|
|
|
|
$ |
6,052 |
|
$ |
6,300 |
NOTE 14:- COMMITMENTS AND CONTINGENT LIABILITIES
From time to time, the Company is involved in claims and legal proceedings. The Company reviews the status of each matter and assesses its potential financial exposure. If the potential loss from any claim or legal proceeding is considered probable and the amount can be reasonably estimated, the Company accrues a liability for the estimated loss.
Royalties
The Company has a liability to pay future royalties to the Israeli Innovation Authority (the “IIA”) for participation in programs sponsored by the Israeli government for the support of research and development activities. The Company is obligated to pay royalties to the IIA, amounting to 3% of the sales of the products and other related revenues (based on the U.S. dollar) generated from such projects, up to 100% of the grants received. Royalty payment obligations also bear interest at the LIBOR rate. The obligation to pay these royalties is contingent on actual sales of the products and in the absence of such sales, no payment is required.
In connection with specific research and development activities, Physimax Technology (“Physimax”), prior to its acquisition by the Company, received $1,012 of participation payments from the IIA. The Company’s total commitment for royalties payable with respect to future sales, based on IIA participations received, net of royalties accrued or paid, totaled $932 as of December 31, 2024.
During the year ended December 31,2024 and the year ended December 31, 2023 the company recorded IIA royalties related to the acquisition of Physimax Technology in amount of $2 and $1, respectively.
F-37
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 15:- TAXES ON INCOME
The Company and its subsidiaries are separately taxed under the domestic tax laws of the country of incorporation of each entity.
Tax Reform
On December 22, 2017, the U.S. Tax Cuts and Jobs Act of 2017 (the “TCJA”) was signed into law. The TCJA makes broad and complex changes to the Internal Revenue Code of 1986 (the “Code”) that may impact the Company’s provision for income taxes. The changes include, but are not limited to:
| ● | Decreasing the corporate income tax rate from 35% to 21% effective for tax years beginning after December 31, 2017 (“Rate Reduction”); |
| ● | The Deemed Repatriation Transition Tax; and |
| ● | Taxation of Global Intangible Low-Taxed Income (“GILTI”) earned by foreign subsidiaries beginning after December 31, 2017. The GILTI tax imposes a tax on foreign income in excess of a deemed return on tangible assets of foreign corporations. |
Net Operating Losses- Before the TCJA, taxable losses generated in the U.S. were able to be carried back for two years or carried forward for 20 years to offset prior/future year taxable income. TCJA changes the rule, and allows losses generated after 2017 (i.e. starting in 2018) to be carried forward indefinitely, but only to offset 80% of future year income. Carryback losses are no longer allowed
In response to the COVID-19 pandemic, the U.S. passed the Coronavirus Aid, Relief, and Economic Security Act (CARES) in March 2020. The CARES Act changed the treatment of net operating losses (“NOLS”) generated in tax years 2018, 2019 and 2020. Losses generated in these years are able to be carried backward for 5 years, and carried forward indefinitely, without the 80% limitation.
Tax rates applicable to Labstyle:
The Corporate tax rate in Israel in 2024 and 2023 was 23%.
Net operating loss carryforward:
Labstyle has accumulated net operating losses for Israeli income tax purposes as of December 31, 2024, in the amount of approximately $221,030. The net operating losses may be carried forward and offset against taxable income in the future for an indefinite period.
As of December 31, 2024, the Company, WayForward and Twill had a U.S. federal net operating loss carryforward of approximately $42,429, $6,305 and $143,670 of which $7,120, $371 and $19,779 respectively, were generated from tax years 2011-2017 and can be carried forward and offset against taxable income and that expires during the years 2031 to 2037. Under Sections 382 and 383 of the IRC, utilization of the U.S. loss carryforward may be subject to substantial annual limitation due to the “change in ownership” provisions of the Code and similar state provisions. The annual limitations may result in the expiration of losses before utilization. A "change in ownership" has occurred during 2024 when Twill was acquired by Dario, resulting in a limitation of $2,316 per year for the years 2025-2028.
The remaining NOLs of the Company, WayForward and Twill are approximately $35,309, $5,934 and $123,891 were generated in years 2018-2024, and are subject to the TCJA, which modified the rules regarding utilization of NOLs. NOLs generated after December 31, 2017, can only be used to offset 80% of taxable income with an indefinite carryforward period for unused carryforwards (i.e., they should not expire). Utilization of the federal and state net operating losses and credits may be subject to a substantial annual limitation due to an additional ownership change.
F-38
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 15:- TAXES ON INCOME (Cont.)
The annual limitation may result in the expiration of net operating losses and credits before utilization and in the event, the Company has a change of ownership, utilization of the carryforwards could be restricted.
As discussed above, under the CARES Act, the losses from 2018-2024 are excluded from the limitation and can be carried forward indefinitely to offset 100% of future net income.
Deferred income taxes:
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets are as follows:
|
|
December 31, |
||||
|
|
2024 |
|
2023 |
||
Deferred tax assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net operating loss and capital losses carry forward |
|
$ |
63,016 |
|
$ |
51,533 |
Research and development expenses |
|
|
3,163 |
|
|
3,815 |
Accrued employees costs |
|
|
432 |
|
|
370 |
Credit losses |
|
|
36 |
|
|
— |
Deferred revenue |
|
|
41 |
|
|
— |
Debt issuance costs |
|
|
139 |
|
|
— |
Stock-based compensation |
|
|
3,441 |
|
|
4,328 |
R&D Capitalization under section 174 |
|
|
10,245 |
|
|
— |
Depreciation |
|
|
72 |
|
|
— |
Loan |
|
|
226 |
|
|
306 |
Intangible Assets |
|
|
240 |
|
|
152 |
Lease |
|
|
312 |
|
|
229 |
|
|
|
|
|
|
|
Deferred tax assets: |
|
|
81,363 |
|
|
60,733 |
Less: Valuation allowance |
|
|
(77,189) |
|
|
(58,303) |
|
|
|
|
|
|
|
Deferred tax assets |
|
|
4,174 |
|
|
2,430 |
|
|
|
|
|
|
|
Deferred tax liability: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible Assets |
|
|
(3,909) |
|
|
(2,208) |
ROU assets |
|
|
(265) |
|
|
(222) |
|
|
|
|
|
|
|
Deferred tax liability |
|
|
(4,174) |
|
|
(2,430) |
|
|
|
|
|
|
|
Net deferred tax asset |
|
$ |
— |
|
$ |
— |
The deferred tax balances included in the consolidated financial statements as of December 31, 2024, are calculated according to the tax rates that were in effect as of the reporting date and do not take into account the potential effects of the changes in the tax rate.
F-39
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands
NOTE 15:- TAXES ON INCOME (Cont.)
The net change in the total valuation allowance for the year ended December 31, 2024, was an increase of $18,886 and is mainly relates to increase in deferred taxes on net operating loss for which a full valuation allowance was recorded. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets depends on the generation of future taxable income during the periods in which those temporary differences and tax loss carryforward are deductible. Management considers the projected taxable income and tax-planning strategies in making this assessment. In consideration of the Company’s accumulated losses and the uncertainty of its ability to utilize its deferred tax assets in the future, management currently believes that it is more likely than not that the Company will not realize all of its deferred tax assets and accordingly recorded a valuation allowance to offset the deferred tax assets.
a. |
Loss before taxes on income consists of the following: |
|
|
Year ended |
||||
|
|
December 31, |
||||
|
|
2024 |
|
2023 |
||
Domestic |
|
$ |
17,863 |
|
$ |
23,477 |
Foreign |
|
|
26,736 |
|
|
35,886 |
|
|
|
|
|
|
|
|
|
$ |
44,599 |
|
$ |
59,363 |
b. |
The main reconciling item between the statutory tax rate of the Company and the effective tax rate is the recognition of valuation allowance in respect of deferred taxes relating to accumulated net operating losses carried forward due to the uncertainty of the realization of such deferred taxes. |
Income tax expense (benefit) was as follows:
|
|
|
Year ended |
|||
|
|
|
December 31, |
|||
Current: |
|
2024 |
|
2023 |
||
Domestic |
|
$ |
— |
|
$ |
— |
Foreign |
|
|
149 |
|
|
64 |
Total current income tax expense |
|
|
149 |
|
|
64 |
Deferred: |
|
|
|
|
|
|
Domestic |
|
|
(2,001) |
|
|
— |
Foreign |
|
|
— |
|
|
— |
Total deferred income tax expense (benefit) |
|
|
(2,001) |
|
|
— |
Income tax |
|
$ |
(1,852) |
|
$ |
64 |
F-40
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 16:- STOCKHOLDERS’ EQUITY
| a. | The holders of Common Stock have the right to one vote for each share of Common Stock held of record by such holder with respect to all matters on which holders of Common Stock are entitled to vote, to receive dividends as they may be declared at the discretion of the Company’s Board of Directors and to participate in the balance of the Company’s assets remaining after liquidation, dissolution or winding up, ratably in proportion to the number of shares of Common Stock held by them after giving effect to any rights of holders of preferred stock. Except for contractual rights of certain investors, the holders of Common Stock have no pre-emptive or similar rights and are not subject to redemption rights and carry no subscription or conversion rights. |
| b. | In January and March 2024, the Compensation Committee (the “Compensation Committee”) of the Board of Directors of the Company approved the grant of 1,946,500 restricted shares, subject to time vesting, to directors, officers and employees of the Company and approved the grant of 1,100,400 options to purchase Common Stock, and 320,000 performance-based options to purchase Common Stock to officers, employees, and consultants of the Company, at exercise prices between $1.68 and $2.14 per share. The time vesting restricted shares of Common Stock and stock options vest over various periods between two to three years commencing on the respective grant dates. The options have a ten-year term. The restricted shares of Common Stock and the options were issued under the Company’s 2020 Equity incentive Plan, as amended (the “2020 Plan”). |
| c. | In April 2024, the Compensation Committee approved the grant of 260,500 restricted shares of Common Stock subject to time vesting to employees of the Company and approved the grant of options to purchase up to 564,900 shares of Common Stock, to employees and consultants of the Company, at exercise prices between $1.38 and $1.48 per share. The time vesting restricted shares of Common Stock and stock options vest over various periods between two to three years commencing on the respective grant dates. The options have a ten-year term. The restricted shares of Common Stock and the options were issued under the 2020 Plan. |
| d. | In May 2024, the Compensation Committee approved the grant of a non-qualified stock option to purchase up to 2,250,000 shares of Common Stock as an inducement grant to the new Chief Commercial Officer. Out of the 2,250,000 shares of Common Stock, 500,000 shares will vest over three years, with one third of such shares vesting on June 1, 2025, and the remaining shares vesting in equal quarterly amounts during the following two years. 1,750,000 of the options are performance-based and will commence vesting upon reaching certain performance goals during the fiscal years between 2024 and 2027. Each performance option will vest over a three-year period commencing on the first day of the following fiscal year to which such option relates, in eight equal quarterly instalments. |
| e. | In August 2024, the Compensation Committee approved the grant of 590,000 restricted shares of Common Stock subject to time vesting to employees of the Company and approved the grant of 1,692,250 options to purchase Common Stock, and 300,000 performance-based options to purchase Common Stock to officers, employees, and consultants of the Company, at exercise prices between $0.93 to $1.05 per share. The time vesting restricted shares of Common Stock and stock options vest over three years commencing on the respective grant dates. The options have a ten-year term. The restricted shares of Common Stock and the options were issued under the Company’s 2020 Plan. |
| f. | In November 2024, the Compensation Committee approved the grant of 10,000 restricted shares of Common Stock subject to time vesting to employees of the Company and approved the grant of options to purchase up to 367,500 shares of Common Stock, to employees and consultants of the Company, at an exercise price of $0.9475 per share. The time vesting restricted shares of Common Stock and stock options vest over various periods between one to three years commencing on the respective grant dates. The options have a ten-year term. The restricted shares of Common Stock and the options were issued under the 2020 Plan. |
F-41
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 16:- STOCKHOLDERS’ EQUITY (Cont.)
| g. | In December 2024, the Compensation Committee approved the grant of 300,000 restricted shares of Common Stock subject to time vesting to an employee of the Company and approved the grant of options to purchase up to 363,500 shares of Common Stock, to employees of the Company, at an exercise price of $0.80 per share. The time vesting restricted shares of Common Stock and stock options vest over three years commencing on the respective grant dates. The options have a ten-year term. The restricted shares of Common Stock and the options were issued under the 2020 Plan. |
| h. | In August 2024, the Compensation Committee approved the grant of 418,550 restricted shares of Common Stock to certain service providers. During the year ended December 31, 2024, the Company recorded compensation expense in the amount of $354 related to the issuance of these shares of Common Stock. |
| i. | On November 4, 2024, the Compensation Committee approved amendments to certain previously issued awards of restricted Common Stock in the aggregate amount of 68,750 and 250,000, respectively, granted to Mr. Erez Raphael, our Chief Executive Officer, and Mr. Zvi Ben David, our Chief Financial Officer, in the aggregate amount of 23,750 and 125,000, respectively, on May 18, 2022 and March 6, 2024 (collectively, the “Prior Awards”) to permit an immediate acceleration of the unvested portion of the Prior Awards in the event of change in control of the Company. |
| j. | In December 2024, the Compensation Committee approved amendments to certain previously issued awards of restricted Common Stock in the aggregate amount of 176,001, granted to certain employees, to permit an immediate acceleration of the unvested portion of the Prior Awards. 152,000 of the accelerated shares will be subject to a lockup until December 31, 2025. During the year ended December 31, 2024, the Company recorded stock-based compensation expenses related to the acceleration of the unvested portion of the Prior Awards at the amount of $75. |
| k. | In April 2024, the Compensation Committee approved the grant of warrants to purchase up to 1,471,250 shares of Common Stock, with exercise prices between $1.43 to $2.00 per share, to certain consultants. The warrants are exercisable into shares of Common Stock on or before December 31, 2026. In addition, the Compensation Committee approved a reduction in the exercise price of warrants to purchase up to 700,000 shares of Common Stock issued to certain consultants in the past at exercise prices between $5.20 to $6.45 per share, to an exercise price of $1.60 per share. During the year ended December 31, 2024, the Company recorded stock-based compensation expenses related to those repriced warrants at the amount of $332. |
In August 2024, the Compensation Committee approved the grant of warrants to purchase up to 600,000 shares of Common Stock subject to time vesting of one to two years, with exercise prices of $0.93 per share to certain consultants. Out of the warrants to purchase 600,000 shares of Common Stock, 100,000 shares are exercisable into Common Stock on or before December 31, 2027, and 500,000 shares are exercisable into Common Stock on or before August 31, 2029. In addition, the Compensation Committee approved the grant performance-based warrants to purchase up to 800,000 shares of Common Stock, with exercise prices of $0.93 per share to a certain consultant. The warrants are exercisable into Common Stock on or before August 31, 2029. Management's assumption is that it is not probable that the condition will be met. During the year ended December 31, 2024, the Company recorded stock-based compensation expenses related to the warrants at the amount of $67.
| l. | In November 2024, the Compensation Committee approved the grant of warrants to purchase up to 469,653 shares of Common Stock, with an exercise price of $0.9475 per share to a certain consultant. The warrants are exercisable into Common Stock on or before the five years anniversary of the grant date. |
During the year ended December 31, 2024, the Company recorded stock-based compensation expenses related to the warrants at the amount of $46.
F-42
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 16:- STOCKHOLDERS’ EQUITY (Cont.)
| m. | In December 2024, the Compensation Committee approved a reduction in the exercise price of warrants to purchase up to 881,250 shares of Common Stock issued to certain consultants in the past at exercise prices between $1.60 to $12.00 per share, to an exercise price of $0.70 per share. During the year ended December 31, 2024, the Company recorded stock-based compensation expenses related to those repriced warrants at the amount of $164. |
| n. | In January 2023, the Compensation Committee approved the grant of warrants to purchase up to 280,000 shares of common stock, with an exercise price of $5.20, per share to certain consultants. The warrants are exercisable into common stock on or before December 31, 2026. During the year ended December 31, 2023, the Company recorded compensation expenses for certain consultants in the amount of $650. |
| o. | In January 2023, the Compensation Committee approved a reduction in the exercise price of warrants to purchase up to 350,000 shares of common stock issued to certain consultants in the past at exercise prices between $7.50 to $30.00 per share, to an exercise price of $5.20 per share, subject to the performance of additional services. The Company has accounted for the change as a modification and recorded the increase in fair value as compensation expense for those certain consultants in the amount of $960. |
| p. | On July 25, 2023, the Compensation Committee approved the grant of warrants to purchase up to 40,000 shares of common stock, with an exercise price of $3.46, per share to a certain consultant, the stock options vests over a three-year period. The warrants are exercisable into common stock on or before December 31, 2026. During the year ended December 31, 2023, the Company recorded compensation expenses for this certain consultant in the amount of $29. |
| q. | On February 15, 2024, the Company executed a consulting agreement with a former officer of Twill. Pursuant to the terms of the consulting agreement, the Company agreed to issue to the former officer of Twill 350,000 fully vested RSUs. |
On June 25, 2024, the stockholders of the Company approved the issuance of certain restricted shares of Common Stock and warrants of the Company, under certain consulting agreements with former officers and directors of Twill. Pursuant to the terms of the consulting agreements, the Company agreed to issue to those former officers of Twill warrants to purchase up to 1,032,946 shares of the Company’s Common Stock, of which 717,946 are subject to time vesting and 315,000 are subject to certain performance-based metrics, with an exercise price of $2.55 and 383,562 fully vested RSUs.
During the year ended December 31, 2024, the Company recorded share-based compensation expenses related to these service providers in the amount of $3,196. For the year ended December 31, 2024, no expenses were recorded related to the performance-based warrants.
| r. | On January 30, and September 27, 2024, out of the pre-funded warrants that were issued in July 2020, 400,017 and 287,303 were exercised on a cashless basis into 400,000 and 287,273 shares of Common Stock respectively. As of December 31, 2024, the Company’s total outstanding prefunded warrants were exercisable into 995,499 shares of Common Stock. |
On July 11, 2023, out of the pre-funded warrants that were issued in July 2020 and February 2022, 86,985 were exercised on a cashless basis into 86,983 shares of Common Stock.
| s. | In April 2023, the Company issued 76,637 shares of common stock to settle an earn-out payment owed in connection with the acquisition of PsyInnovations, Inc. (dba wayForward). |
| t. | In February 2024, the Company issued 17,307, 4,000 and 1,115 Series C, C-1 and C-2 preferred shares, respectively, at a purchase price of $1,000 per preferred share. The Series C and C-1 Preferred Stock are |
F-43
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 16:- STOCKHOLDERS’ EQUITY (Cont.)
convertible into Common Stock at $2.02 per Common Stock. The Series C-2 Preferred Stock is convertible into Common Stock at $2.14 per Common Stock. As a result of the sale of the preferred stock, the aggregate gross proceeds to the Company from the Offering were approximately $22,422.
In addition, the holders of preferred stock will also be entitled to dividends payable as follows: (i) a number of shares of Common Stock equal to seven and a half five percent (7.5%) of the number of shares of Common Stock issuable upon conversion of the preferred stock then held by such holder for each full quarter anniversary of holding for a total of four (4) quarters from the Closing Date, and (ii) a number of shares of Common Stock equal to fifteen percent (15%) of the number of shares of Common Stock issuable upon conversion of the preferred stock then held by such holder on the fifth full quarter from the Closing Date.
In May, September and November 2024, a total of 100, 25 and 20 shares of certain Series C Convertible Preferred Stock were converted into 49,505, 14,234 and 12,129 shares of Common Stock, respectively.
During the year ended December 31, 2024, the Company accounted for the dividend shares of Common Stock upon the dividend shares earned by Series C, C-1 and C-2 Preferred Stock as a deemed dividend in a total amount of $6,542.
| u. | In December 2024, the Company issued 7,055 and 11,750 Series D and D-1 preferred shares, respectively, at a purchase price of $1,000 per preferred share. The Series D and D-1 Preferred Stock are convertible into Common Stock at $0.73 per Common Stock. As a result of the sale of the preferred stock, the aggregate gross proceeds to the Company from the Offering were approximately $18,805. |
The conversion of the preferred stock is subject to stockholder approval, the preferred stock will automatically convert into shares of Common Stock, subject to certain beneficial ownership limitations, including a non-waivable 19.99% ownership blocker, on the 12-month anniversary of the issuance date. The holders of preferred stock will also be entitled to dividends equal to a number of shares of Common Stock equal to ten percent (10%) of the number of shares of Common Stock issuable upon conversion of the preferred stock then held by such holder for each full quarter anniversary of holding for a total of four (4) quarters from the Closing Date, all issuable upon conversion of the preferred stock.
In addition, the Company and certain purchasers in the Offering that are holders of the Company's Series B and C Preferred Stock executed lock up agreements (the “Lock Up Agreement”), pursuant to which the Company agreed to issue, subject to stockholder approval, up to forty percent (40%) of the shares of Common Stock conversion shares of the preferred stock held by such purchaser, including dividend shares of Common Stock due upon conversion of these shares into shares of Common Stock, over the course of twelve (12) months (the “Additional Shares”). Each holder shall be entitled to receive 10% of the Additional Shares for each three (3) month period each holder agrees not to transfer or otherwise sell (subject to certain limitations) the shares of Common Stock issuable upon conversion of the Series B Preferred Stock and Series C Preferred Stock and the dividend shares of Common Stock due upon conversion.
During the year ended December 31, 2024, the Company accounted for the dividend shares of Common Stock upon the dividend shares earned by Series D and D-1 Preferred Stock as a deemed dividend in a total amount of $318.
| v. | On May 1, 2023, the Company entered into securities purchase agreements with accredited investors relating to an offering and the sale of an aggregate of 6,200 shares of newly designated Series B Preferred Stock (the “Series B Preferred Stock”), an aggregate of 7,946 shares of Series B-1 Preferred Stock (the “Series B-1 Preferred Stock”), and an aggregate of 150 shares of Series B-2 Preferred Stock (the “Series B-2 Preferred Stock”) at a purchase price of $1,000 for each share of preferred stock. Certain of our executive officers and directors |
F-44
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 16:- STOCKHOLDERS’ EQUITY (Cont.)
purchased shares of Series B-2 Preferred Stock in the offering. On May 5, 2023, the Company entered into purchase agreements with accredited investors, relating to the offering of 1,106 shares of newly designated Series B-3 Preferred Stock (the “Series B-3 Preferred Stock”), at a purchase price of $1,000 for each share of preferred stock. The initial conversion price for the Series B, B-1, B-2, and B-3 Preferred Stock was $3.334, $3.334, $3.370 and $3.392, respectively, subject to adjustment in the event of stock splits, stock dividends, and similar transactions. As a result of the sale of the preferred stock, the aggregate gross proceeds to the Company from the offerings were approximately $15,402 ($14,868 net of issuance expenses).
The preferred stock will automatically convert into shares of common stock, on the 15-month anniversary of the issuance date. The holders of preferred stock will also be entitled dividends payable as follows: (i) a number of shares of common stock equal to five percent (5.0%) of the number of shares of common stock issuable upon conversion of the preferred stock then held by such holder for each full quarter anniversary of holding for a total of four (4) quarters from the closing date, and (ii) a number of shares of common stock equal to ten percent (10%) of the number of shares of common stock issuable upon conversion of the preferred stock then held by such holder on the fifth full quarter from the closing. The Series B-2 Preferred Stock dividend is subject to receipt of the approval of the Company’s shareholders.
| w. | On June 25, 2024, following the approval of the necessary preferred holders, the Company modified the terms of the Series B and B-1 preferred stock to (i) extend the mandatory conversion period from fifteen (15) to eighteen |
(18) months from the original issue date and (ii) increase the percentage of dividends the holders of the preferred stock will be entitled to receive by including a dividend of ten percent (10%) for the fifth full quarter from the closing date and a dividend of twenty five percent (25%) for the sixth quarter from the closing date.
On September 11, 2024, following the approval of the necessary preferred holders, the Company modified the terms of the Series B-3 preferred stock to (i) extend the mandatory conversion period from fifteen (15) to eighteen (18) months from the original issue date and (ii) increase the percentage of dividends the holders of the preferred stock will be entitled to receive by including a dividend of ten percent (10%) for the fifth full quarter from the closing date and a dividend of twenty five percent (25%) for the sixth quarter from the closing date.
The Company concluded that the Series B, B-1 and B-3 preferred shares modification should be accounted for as an extinguishment transaction. As such, the Company recorded the modified preferred shares at their fair value and derecognized the carrying amount of the previous unmodified shares, resulting in a deemed contribution of $12,194.
In November, on the 18-month anniversary of the Series B, B-1 and B-3 Effective Date, 5,950 Series B, 3,000 B-1 and 406 B-3 Preferred Stock converted into 2,766,201, 1,394,721 and 185,526 shares of Common Stock.
During the year ended December 31, 2024, the Company accounted for the dividend shares of common stock upon the deferred conversion of the Series B, B-1, B-2, and B-3 Convertible Preferred Stock as a deemed dividend in a total amount of $2,953.
| x. | In April and June 2024, a total of 700 and 250 of certain Series B Convertible Preferred Stock were converted into 237,323 and 89,982 shares of Common Stock, respectively, including the issuance of dividend shares. In addition, during the year ended December 31, 2024, the Company recorded deemed dividend in the amount of $616 with respect to the Series B convertible Preferred Stock. |
| y. | In August 2024, 150 Series B-2 Preferred Stock automatically converted into 57,868 shares of Common Stock after the completion of the fifteen (15)-month anniversary of the Series B-2 Preferred Stock. The conversion included accumulative dividends payable upon conversion of Series B-2 Preferred Stock. |
F-45
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 16:- STOCKHOLDERS’ EQUITY (Cont.)
| z. | Through November 2022, December 2022 and January 2023, 6,355 Series A Preferred Stock automatically converted into 2,133,904 shares of Common Stock after the completion of the 36-month anniversary of each the Series A Preferred Stock. The conversion included accumulative dividends payable available upon conversion of each Series A Preferred Stock. |
| aa. | As of December 31, 2024, there were 3,557 shares of Series A-1 Preferred stock issued and outstanding. The outstanding Series A-1 Preferred stock is convertible into approximately 1,655,548 shares of Common Stock, including the issuance of dividend shares. |
As of December 31, 2024, there were 4,946 shares of Series B-1 Preferred stock issued and outstanding. The outstanding Series B-1 Preferred stock is convertible into approximately 2,299,432 shares of Common Stock, including the issuance of dividend shares.
As of December 31, 2024, there were 22,277 shares of Series C, C-1 and C-2 Preferred stock issued and outstanding. The outstanding Series C, C-1 and C-2 Preferred stock is convertible into approximately 13,497,265 shares of Common Stock, including the issuance of dividend shares.
As of December 31, 2024, there were 18,805 shares of Series D, and D-1 Preferred stock issued and outstanding. The outstanding Series D, and D-1 Preferred stock is convertible into approximately 25,762,850 shares of Common Stock, including the issuance of dividend shares.
| bb. | During the year ended December 31, 2024, options were exercised into 10,714 shares of Common Stock. |
| cc. | Stock-based compensation: |
On January 23, 2012, the Company’s Amended and Restated 2012 Equity Incentive Plan (the “2012 Plan”) was adopted by the Board of Directors of the Company and approved by a majority of the Company’s stockholders, under which options to purchase shares of the Company’s Common Stock have been reserved. Under the 2012 Plan, options to purchase shares of Common Stock may be granted to employees and non-employees of the Company or any affiliate, each option granted can be exercised to one share of Common Stock. The 2012 Plan has expired.
On October 14, 2020, the Company’s stockholders approved the 2020 Plan. Under the 2020 Plan, options to purchase shares of Common Stock may be granted to employees and non-employees of the Company or any affiliate, each option granted can be exercised to one share of Common Stock.
In January 2023, pursuant to the terms of the 2020 Plan as approved by the Company’s stockholders, the Company increased the number of shares authorized for issuance under the 2020 Plan by 1,994,346 shares, from 3,868,514 to 5,862,860.
In January 2024, pursuant to the terms of the 2020 Plan as approved by the Company’s stockholders, the Company increased the number of shares authorized for issuance under the 2020 Plan by 2,493,764 shares, from 5,862,860 to 8,356,624.
In June 2024, pursuant to the terms of the 2020 Plan as approved by the Company’s stockholders, the Company increased the number of shares authorized for issuance under the 2020 Plan by 3,000,000 shares, from 8,356,624 to 11,356,624.
F-46
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 16:- STOCKHOLDERS’ EQUITY (Cont.)
The table below summarizes the outstanding warrants as of December 31, 2024:
|
|
Warrants |
|
|
|
|
|
|
outstanding as of |
|
Exercise |
|
|
|
|
December 31, 2024 |
|
price $ |
|
Expiration date |
Agent warrants July 31 2020 |
|
150,070 |
|
7.47 |
|
July 31, 2025 |
Agent warrants July 31 2020 |
|
2,393 |
|
7.94 |
|
July 31, 2025 |
Consultants |
|
25,000 |
|
13.88 |
|
September 26, 2025 |
Consultants |
|
500,000 |
|
1.08 |
|
December 16, 2025 |
Consultants |
|
50,000 |
|
1.60 |
|
December 31, 2025 |
Consultants |
|
8,000 |
|
13.60 |
|
December 31, 2025 |
Consultants |
|
285,000 |
|
1.60 |
|
December 31, 2026 |
Consultants |
|
145,000 |
|
0.70 |
|
December 31, 2026 |
Consultants |
|
155,000 |
|
0.70 |
|
December 31, 2026 |
Consultants |
|
40,000 |
|
3.46 |
|
December 31, 2026 |
Consultants |
|
130,000 |
|
1.60 |
|
December 31, 2026 |
Consultants |
|
56,250 |
|
2.00 |
|
December 31, 2026 |
Consultants |
|
500,000 |
|
1.60 |
|
December 31, 2026 |
Consultants |
|
30,000 |
|
2.00 |
|
December 31, 2026 |
Consultants |
|
300,000 |
|
1.43 |
|
December 31, 2026 |
Consultants |
|
100,000 |
|
1.60 |
|
December 31, 2026 |
Consultants |
|
100,000 |
|
0.93 |
|
August 24, 2027 |
Consultants |
|
60,000 |
|
0.70 |
|
December 31, 2027 |
Consultants |
|
87,500 |
|
0.70 |
|
December 31, 2027 |
Consultants |
|
45,000 |
|
0.70 |
|
December 31, 2027 |
Consultants |
|
25,000 |
|
0.70 |
|
December 31, 2027 |
Consultants |
|
125,000 |
|
0.70 |
|
December 31, 2027 |
Consultants |
|
125,000 |
|
0.70 |
|
December 31, 2027 |
Consultants |
|
80,000 |
|
0.70 |
|
December 31, 2027 |
Consultants |
|
10,000 |
|
0.70 |
|
December 31, 2027 |
Consultants |
|
10,000 |
|
0.70 |
|
December 31, 2027 |
Lender of loan facility |
|
292,441 |
|
2.02 |
|
May 1, 2028 |
Lender of loan facility |
|
292,441 |
|
2.02 |
|
May 1, 2028 |
Agent warrants C Feb. 21, 2024 |
|
1,068,670 |
|
2.02 |
|
February 21, 2029 |
Agent warrants C-2 Feb. 21, 2024 |
|
70,818 |
|
2.14 |
|
February 21, 2029 |
Lender of loan facility |
|
226,586 |
|
4.00 |
|
June 9, 2029 |
Consultants |
|
13,750 |
|
0.70 |
|
August 1, 2029 |
Consultants |
|
500,000 |
|
0.93 |
|
August 25, 2029 |
Consultants |
|
850,000 |
|
0.93 |
|
August 25, 2029 |
Consultants |
|
319,653 |
|
0.95 |
|
November 4, 2029 |
Consultants |
|
150,000 |
|
0.95 |
|
November 4, 2029 |
Consultants |
|
717,946 |
|
2.55 |
|
February 15, 2034 |
Consultants |
|
315,000 |
|
2.55 |
|
February 15, 2034 |
|
|
7,961,518 |
|
|
|
|
F-47
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 16:- STOCKHOLDERS’ EQUITY (Cont.)
In January 2023 and April 2023, the Board of Directors approved the acceleration of the unvested portion of 42,500 restricted shares of Common Stock to a certain employee of the Company. The share acceleration was part of a separation agreement with the employees. The Company has accounted for the acceleration as a type-3 modification and recorded compensation expenses in the amount of $153.
During the year ended December 31, 2023, the Company’s Compensation Committee approved the grant of 927,100 restricted shares of Common Stock to employees and consultants of which 537,100 are under the Company’s 2020 Equity Incentive Plan, as amended (“2020 Plan”). Out of the restricted shares granted, 235,000 restricted shares of Common Stock will vest immediately, 30,000 restricted shares will vest over a period of six months, and the remaining 662,100 restricted shares of Common Stock will vest over a period between two to four years commencing on the respective grant dates. The Compensation Committee also approved the grant of options to purchase up to 833,900 shares of Common Stock for employees and consultants of the Company, at exercise prices between $3.69 and $4.48 per share. Stock options to purchase 528,900 shares of common stock vest over a three-year period commencing on the respective grant dates, and options to purchase 305,000 shares of common stock are performance-based. The options have a ten-year term and were issued under the 2020 Plan.
Transactions related to the grant of options to employees, directors and non-employees under the above plans and non-plan options during the year ended December 31, 2024 were as follows:
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
Weighted |
|
average |
|
|
|
|
|
|
average |
|
remaining |
|
Aggregate |
|
|
|
|
exercise |
|
contractual |
|
Intrinsic |
|
|
Number of |
|
price |
|
life |
|
value |
|
|
options |
|
$ |
|
Years |
|
$ |
Options outstanding at beginning of period |
|
2,550,829 |
|
9.27 |
|
7.02 |
|
36 |
Options granted |
|
9,922,009 |
|
1.65 |
|
— |
|
— |
Options exercised |
|
(8,693) |
|
— |
|
— |
|
— |
Options expired |
|
(525,725) |
|
11.94 |
|
— |
|
— |
Options forfeited |
|
(1,715,671) |
|
3.13 |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
Options outstanding at end of period |
|
10,222,749 |
|
2.78 |
|
8.55 |
|
9 |
|
|
|
|
|
|
|
|
|
Options vested and expected to vest at end of period |
|
8,324,872 |
|
2.78 |
|
8.48 |
|
9 |
|
|
|
|
|
|
|
|
|
Exercisable at end of period |
|
3,378,508 |
|
5.39 |
|
7.11 |
|
9 |
Weighted average grant date fair value of options granted during the year ended December 31, 2024, and 2023 is $0.76 and $2.14, respectively.
The aggregate intrinsic value in the table above represents the total intrinsic value (the difference between the Company’s closing stock price on the last day of fiscal 2023 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their options on December 31, 2024. This amount is impacted by the changes in the fair market value of the Common Stock. The average intrinsic value of the options exercised in 2024 and 2023 were $1.20 and $3.26, respectively.
F-48
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 16:- STOCKHOLDERS’ EQUITY (Cont.)
Transactions related to the grant of restricted shares of Common Stock to employees, directors, and non-employees under the above plans during the year ended December 31, 2024 were as follows:
|
|
|
|
|
|
|
|
Number of |
|
|
|
|
|
|
|
|
Restricted shares |
|
|
|
|
|
|
|
|
|
Restricted shares outstanding at beginning of year |
|
|
|
|
|
|
|
2,635,926 |
Restricted shares granted |
|
|
|
|
|
|
|
3,107,000 |
Restricted shares forfeited |
|
|
|
|
|
|
|
(334,522) |
|
|
|
|
|
|
|
|
|
Restricted shares outstanding at end of period |
|
|
|
|
|
|
|
5,408,404 |
The Company estimates the fair value of stock options granted using the Black-Scholes option-pricing model.
The fair value of the restricted shares vested during 2024 amounts to $1,644.
The assumptions used are determined as follows:
Volatility. The expected volatility was derived from the historical volatilities of the Company’s stock price over a period equivalent to the expected term of the stock option grants.
Expected Term. The expected term represents the period that the stock-based awards are expected to be outstanding. When establishing the expected term assumption, the Company utilizes the simplified method.
Risk-Free Interest Rate. The risk-free interest rate is based on U.S. Treasury zero-coupon issues with terms similar to the expected term on the options.
Dividend Yield. The Company has never declared or paid any cash dividends and does not plan to pay cash dividends in the foreseeable future, and therefore, it used an expected dividend yield of zero.
The following table presents the assumptions used to estimate the fair values of the options granted to employees, non-employees and directors in the period presented:
|
|
Year ended |
|
||
|
|
December 31, |
|
||
|
|
2024 |
|
2023 |
|
Volatility |
|
94.75-97.97 |
% |
90.90-92.62 |
% |
Risk-free interest rate |
|
3.67-4.72 |
% |
3.45-4.13 |
% |
Dividend yield |
|
0 |
% |
0 |
% |
Expected life (years) |
|
5.00-5.87 |
|
5.81-5.88 |
|
As of December 31, 2024, the total unrecognized estimated compensation cost related to non-vested stock options and restricted shares granted prior to that date was $8,367, which is expected to be recognized over a weighted average period of approximately 1.10 years.
F-49
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 16:- STOCKHOLDERS’ EQUITY (Cont.)
The total compensation cost related to all the Company’s equity-based awards, recognized during year ended December 31, 2024 and 2023 were comprised as follows:
|
|
Year ended |
||||
|
|
December 31, |
||||
|
|
2024 |
|
2023 |
||
|
|
Unaudited |
||||
Cost of revenues |
|
$ |
13 |
|
$ |
327 |
Research and development |
|
|
3,296 |
|
|
3,803 |
Sales and marketing |
|
|
4,890 |
|
|
6,468 |
General and administrative |
|
|
7,597 |
|
|
9,103 |
|
|
|
|
|
|
|
Total stock-based compensation expenses |
|
$ |
15,796 |
|
$ |
19,701 |
NOTE 17:- SELECTED STATEMENTS OF OPERATIONS DATA
Financial expenses (income), net:
|
|
Year ended |
||||
|
|
December 31, |
||||
|
|
2024 |
|
2023 |
||
|
|
Unaudited |
||||
Bank charges |
|
$ |
96 |
|
$ |
112 |
Foreign currency adjustments expenses, net |
|
|
4 |
|
|
210 |
Interest income |
|
|
(1,116) |
|
|
(1,868) |
Revaluation of short-term investments |
|
|
— |
|
|
(37) |
Remeasurement of long-term loan |
|
|
4,305 |
|
|
4,894 |
Remeasurement of warrant liability |
|
|
(16,504) |
|
|
(670) |
Modification of warrants |
|
|
70 |
|
|
— |
Debt issuance cost |
|
|
— |
|
|
533 |
|
|
|
|
|
|
|
Total Financial expenses (income), net |
|
$ |
(13,145) |
|
$ |
3,174 |
F-50
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 18:- SEGMENT REPORTING
The Company identifies operating segments in accordance with ASC Topic 280, “Segment Reporting,” as components of an entity for which discrete financial information is available and is regularly reviewed by the chief operating decision maker, or decision-making group, in making decisions regarding resource allocation and evaluating financial performance.
The Company operates as one operating segment. Operating segments are defined as components of an enterprise for which separate financial information is regularly evaluated by the CODM, which is the Company’s chief executive officer, in deciding how to allocate resources and assess performance. The Company’s CODM evaluates the Company’s financial information and resources and assesses the performance of these resources on a consolidated basis. There is no expense or asset information that are supplemental to those disclosed in these consolidated financial statements, that are regularly provided to the CODM. The allocation of resources and assessment of performance of the operating segment is based on consolidated net loss as shown in our consolidated statement of comprehensive loss. The CODM considers net loss in the annual forecasting process and reviews actual results when making decisions about allocating resources. Since the Company operates as one operating segment, financial segment information, including profit or loss and asset information, can be found in the consolidated financial statements.
Geographic Information
•As of December 31, 2024, the majority of the Company’s long live assets are located in Israel.
•As of December 31, 2024, the majority of the Company's revenue is generated in the U.S.
F-51
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 19: - BASIC AND DILUTED NET LOSS PER COMMON STOCK
The Company computes net loss per share of common and preferred stock using the two-class method. Basic and diluted net earnings or loss per share is computed using the weighted-average number of shares outstanding during the period. This calculation includes the total weighted average number of the Common Stock, which includes prefunded warrants.
The total number of potential common shares related to the outstanding options, warrant and preferred shares excluded from the calculations of diluted net loss per share due to their anti-dilutive effect was 35,670,853 for the year ended December 31, 2024, and for the year ended December 31, 2023, was 12,188,189.
The following table sets forth the computation of the Company’s basic net earnings (loss) per common and preferred stock:
|
|
Year ended |
||||||||||||||||||||||||||||||||||
|
|
December 31, |
||||||||||||||||||||||||||||||||||
|
|
2024 |
||||||||||||||||||||||||||||||||||
|
|
|
Common |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock |
|
Preferred A-1 |
|
Preferred B |
|
Preferred B-1 |
|
Preferred B-2 |
|
Preferred B-3 |
|
Preferred C |
|
Preferred C-1 |
|
Preferred C-2 |
|
Preferred D |
|
Preferred D* |
|
Preferred D-1 |
||||||||||||
Basic earnings (loss) per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allocation of undistributed loss |
|
$ |
(30,088,977) |
|
$ |
(956,504) |
|
$ |
(1,145,280) |
|
$ |
(1,710,847) |
|
$ |
(18,996) |
|
$ |
(117,692) |
|
$ |
(4,933,826) |
|
$ |
(1,146,033) |
|
$ |
(301,423) |
|
$ |
(161,819) |
|
$ |
(49,399) |
|
$ |
(351,781) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of shares used in per share computation |
|
|
49,039,410 |
|
|
3,557 |
|
|
5,152 |
|
|
7,486 |
|
|
89 |
|
|
550 |
|
|
14,817 |
|
|
3,441 |
|
|
959 |
|
|
193 |
|
|
59 |
|
|
418 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic earnings (loss) per share amounts: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Distributed earnings - deemed dividends (contribution) |
|
|
— |
|
|
173.23 |
|
|
(744.18) |
|
|
(689.74) |
|
|
278.13 |
|
|
(487.79) |
|
|
341.29 |
|
|
341.36 |
|
|
323.19 |
|
|
306.32 |
|
|
543.04 |
|
|
543.04 |
Undistributed loss - allocated |
|
|
(0.61) |
|
|
(268.91) |
|
|
(222.29) |
|
|
(228.55) |
|
|
(213.01) |
|
|
(213.81) |
|
|
(332.98) |
|
|
(333.04) |
|
|
(314.24) |
|
|
(840.59) |
|
|
(840.59) |
|
|
(840.59) |
Basic earnings (loss) per share |
|
$ |
(0.61) |
|
$ |
(95.67) |
|
$ |
(966.47) |
|
$ |
(918.29) |
|
$ |
65.12 |
|
$ |
(701.61) |
|
$ |
8.31 |
|
$ |
8.32 |
|
$ |
8.95 |
|
$ |
(534.27) |
|
$ |
(297.55) |
|
$ |
(297.55) |
* Series D Preferred Stock which are subject to the lockup agreements (see Note 16).
F-52
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 19: - BASIC AND DILUTED NET LOSS PER COMMON STOCK (Cont.)
|
|
Year ended |
||||||||||||||||
|
|
December 31, |
||||||||||||||||
|
|
2023 |
||||||||||||||||
|
|
Common Stock |
|
Preferred A-1 |
|
Preferred B |
|
Preferred B-1 |
|
Preferred B-2 |
|
Preferred B-3 |
||||||
Basic earnings (loss) per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allocation of undistributed loss |
|
$ |
(54,860,245) |
|
$ |
(2,528,086) |
|
$ |
(2,476,171) |
|
$ |
(3,173,493) |
|
$ |
(59,308) |
|
$ |
(413,441) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of shares used in per share computation |
|
|
28,371,979 |
|
|
3,557 |
|
|
4,094 |
|
|
5,247 |
|
|
99 |
|
|
697 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic earnings (loss) per share amounts: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Distributed earnings - deemed dividends (contribution) |
|
|
— |
|
|
450.47 |
|
|
244.63 |
|
|
244.63 |
|
|
242.18 |
|
|
248.52 |
Undistributed loss - allocated |
|
|
(1.93) |
|
|
(710.74) |
|
|
(604.87) |
|
|
(604.87) |
|
|
(598.83) |
|
|
(593.23) |
Basic earnings (loss) per share |
|
$ |
(1.93) |
|
$ |
(260.26) |
|
$ |
(360.24) |
|
$ |
(360.24) |
|
$ |
(356.64) |
|
$ |
(344.71) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-53
DARIOHEALTH CORP. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except stock and stock data)
NOTE 20:- SUBSEQUENT EVENTS
| a. | On January 7, 2025, the Company entered into securities purchase agreements (each, a “Series D Purchase Agreement”) with accredited investors relating to an offering (the “Series D Offering”) and the sale of an aggregate of (i) 4,950 shares of newly designated Series D-2 Preferred Stock (the “Series D-2 Preferred Stock”), and (ii) 1,850 shares of Series D-3 Preferred Stock (the “Series D-3 Preferred Stock”), at a purchase price of $1,000 for each share of preferred stock. As a result of the sale of the preferred stock, the aggregate gross proceeds to the Company from the Series D Offering are approximately $6,800. The closing of the Series D-2 Preferred Stock, and Series D-3 Preferred Stock occurred on January 14, 2025. |
The conversion of the preferred stock is subject to stockholder approval. In addition, the preferred stock will automatically convert into shares of Common Stock, subject to certain beneficial ownership limitations, on the 12-month anniversary of the issuance date. The holders of preferred stock will also be entitled to dividends equal to a number of shares of Common Stock equal to ten percent (10%) of the number of shares of Common Stock issuable upon conversion of the preferred stock then held by such holder for each full quarter anniversary of holding for a total of four quarters from the Closing Date, all issuable upon conversion of the preferred stock.
| b. | In January 2025, pursuant to the terms of the 2020 Plan as approved by the Company’s stockholders, the Company increased the number of shares authorized for issuance under the 2020 Plan by 6,541,028 shares, from 11,356,624 to 17,897,652. |
| c. | In February 2025, the Compensation Committee approved the grant of 575,000 restricted shares of Common Stock to certain service providers and approved the grant of warrants to purchase up to 1,050,000 shares of Common Stock, with exercise prices of $0.64 per share, to certain consultants. The warrants are exercisable into shares of Common Stock on or before February 10, 2028. |
| d. | In February 2025, a total of 2,500,100 Pre-Funded Warrants were exercised into 2,499,698 shares of Common Stock. |
| e. | In February 2025, the Compensation Committee approved the grant of 30,000 restricted shares of Common Stock to a director of the Company and an amendment to certain previously issued awards of restricted Common Stock in the aggregate amount of 35,000, granted to the director, to permit an immediate acceleration of the unvested portion of the Prior Awards. |
- - - - - - - - - - - - - - -
F-54
Exhibit 4.3
DESCRIPTION OF THE REGISTRANT’S SECURITIES REGISTERED PURSUANT TO SECTION 12 OF THE SECURITIES EXCHANGE ACT OF 1934
The following description of the securities of DarioHealth Corp. (the “Company”) is a summary only and pertains to the Company’s common stock and preferred stock, which are the Company’s only securities registered under Section 12 of the Securities Exchange Act of 1934, as amended. This summary is not complete and is subject to and qualified by the applicable provisions of the Delaware General Corporation Law as well as provisions of the Company’s Certificate of Incorporation, as amended (the “Charter”), which is filed as exhibits to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2019 and is incorporated by reference herein, and By-laws, as amended (the “By-laws”), which is filed as exhibits to the Company’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2021 and is incorporated by reference herein.
Common Stock
Pursuant to the Company’s Charter, the Company is authorized to issue up to one hundred sixty million (160,000,000) shares of common stock, par value $0.0001 per share (the “Common Stock”).
The Common Stock is traded on The Nasdaq Capital Market under the symbol “DRIO.”
The holders of shares of Common Stock vote together as one class on all matters as to which holders of Common Stock are entitled to vote. Except as otherwise required by applicable law, all voting rights, subject to the preferential rights of any outstanding preferred stock, are vested in and exercised by the holders of Common Stock with each share of Common Stock being entitled to one vote, including in all elections of directors. The Company does not have a classified board of directors (the “Board”).
The holders of Common Stock are entitled to receive ratably such dividends, if any, as may be declared from time to time by the Board out of legally available funds therefore. The Company has not declared any dividends on its Common Stock and does not anticipate paying any dividends on its Common Stock in the foreseeable future.
In the event of the Company’s liquidation, dissolution or winding up, holders of the Common Stock are entitled to share ratably in all assets remaining after payment of liabilities. The Common Stock has no cumulative voting rights and no preemptive or other rights to subscribe for shares of the Company.
There are no redemption or sinking fund provisions applicable to the Common Stock. All shares of Common Stock currently outstanding are fully paid and non-assessable.
The Company is permitted to issue, and has from time to time, issued warrants and options to purchase shares of the Common Stock, as well as restricted stock units.
Preferred Stock
Pursuant to the Company’s Charter, the Company is authorized to issue, up to five million (5,000,000) shares of preferred stock, par value $0.0001 per share (the “Preferred Stock”).
There can be one or more series of Preferred Stock. The Company can establish from time to time the number of shares to be included in each such series, as well as to fix the designation and any preferences, conversion and other rights and limitations of such series. These rights and limitations may include voting powers, limitations as to dividends, and qualifications and terms and conditions of redemption of the shares of each such series.
To date, the Company has 3,557 shares of Series A-1 Convertible Preferred Stock (convertible into 878,273 shares of Common Stock), with a conversion price of $4.05 per share (the “Series A-1 Preferred Stock”), 4,946 shares of Series B-1 Convertible Preferred Stock (convertible into 1,483,503 shares of Common Stock), with a conversion price of $3.334 per share (the “Series B-1 Preferred Stock”), 17,037 shares of Series C Convertible Preferred Stock (convertible into 8,450,352 shares of Common Stock), with a conversion price of $2.02 per share (the “Series C Preferred Stock”), 4,000 shares of Series C-1 Convertible Preferred Stock (convertible into 1,984,000 shares of Common Stock), with a conversion price of $2.02 per share (the “Series C-1 Preferred Stock”), 1,115 shares of Series C-2 Convertible Preferred Stock (convertible into 521,820 shares of Common Stock), with a conversion price of $2.14 per share (the “Series C-2 Preferred Stock”), 7,055 shares of Series D Preferred Stock (convertible into 9,665,350 shares of Common Stock), with a conversion price of $0.73 per share (the “Series D Preferred Stock”), 11,750 shares of Series D-1 Preferred Stock (convertible into 16,097,500 shares of Common Stock), with a conversion price of $0.73 per share (the “Series D-1 Preferred Stock”), 4,950 shares of Series D-2 Preferred Stock (convertible into 5,964,750 shares of Common Stock), with a conversion price of $0.83 per share (the “Series D-2 Preferred Stock”) and 1,850 shares of Series D-3 Preferred Stock (convertible into 2,229,250 shares of Common Stock), with a conversion price of $0.83 per share (the “Series D-3 Preferred Stock”).
Each share Preferred Stock, except for the Series A-1 Preferred Stock, Series B-1 Preferred Stock, and Series C-1 Preferred Stock, the Series D-1 Preferred Stock, and the Series D-3 Preferred Stock is entitled to one vote per share on an as-converted basis. The Series A-1 Preferred Stock, Series B-1 Preferred Stock, Series C-1 Preferred Stock, Series D-1 Preferred Stock, and Series D-3 Preferred Stock do not possess any voting rights. In addition, the Series D Preferred Stock and Series D-2 Preferred Stock will only be entitled to voting rights after the receipt of stockholder approval.
In addition, the Preferred Stock is also entitled to the receipt of the following dividends: (i) the holders of Series C Preferred Stock, Series C-1 Preferred Stock and Series C-2 Preferred Stock are entitled to dividends equal to (A) a number of shares of Common Stock equal to seven and a half five percent (7.5%) of the number of shares of Common Stock issuable upon conversion of the Preferred Stock then held by such holder for each full quarter anniversary of holding for a total of four (4) quarters from the Closing Date, and (B) a number of shares of Common Stock equal to fifteen percent (15%) of the number of shares of Common Stock issuable upon conversion of the Preferred Stock then held by such holder on the fifth full quarter from the Closing Date; and (ii) the holders of Series D Preferred Stock, Series D-1 Preferred Stock, Series D-2 Preferred Stock and Series D-3 Preferred Stock are entitled to dividends equal to a number of shares of Common Stock equal to ten percent (10%) of the number of shares of Common Stock issuable upon conversion of such shares of Preferred Stock then held by such holder for each full quarter anniversary of holding for a total of four (4) quarters from the Closing Date, all issuable upon conversion of the Preferred Stock.
Anti-Takeover Effects of the Company’s Charter and By-Laws
In addition to provisions under Delaware law, the Company’s Charter and By-Laws contain provisions that could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder might consider favorable. In particular, the Charter and/or By-Laws, as applicable, among other things:
· |
provide the Board with the exclusive authority to call special meetings of the stockholders; |
· |
provide the Board with the ability to alter the By-Laws without stockholder approval; |
· |
provide the Board with the exclusive authority to fix the number of directors constituting the whole Board; and |
· |
provide that vacancies on the Board may be filled by a majority of directors then in office, although less than a quorum. |
Such provisions may have the effect of discouraging a third-party from acquiring the Company, even if doing so would be beneficial to the Company’s stockholders. These provisions are intended to enhance the likelihood of continuity and stability in the composition of the Board and in its policies, and to discourage some types of transactions that may involve an actual or threatened change in control of the Company. These provisions are designed to reduce the Company’s vulnerability to an unsolicited acquisition proposal and to discourage some tactics that may be used in proxy fights. The Company believes that the benefits of increased protection of its potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure the Company outweigh the disadvantages of discouraging such proposals because, among other things, negotiation of such proposals could result in an improvement of their terms.
However, these provisions could have the effect of discouraging others from making tender offers for shares of the Company’s Common Stock and, as a consequence, they also may inhibit fluctuations in the market price of the shares of the Company’s Common Stock that could result from actual or rumored takeover attempts. These provisions also may have the effect of preventing changes in the Company’s management.
Exhibit 4.11
AMENDMENT NO. 2 TO WARRANT TO PURCHASE
SHARES OF STOCK OF
DARIOHEALTH CORP.
THIS AMENDMENT NO. 2 TO WARRANT TO PURCHASE SHARES OF STOCK OF DARIOHEALTH CORP. is made as of February [__], 2024, by and between AVENUE VENTURE OPPORTUNITIES FUND II, L.P., (“Holder”) and DARIOHEALTH CORP., a Delaware corporation (the “Company”).
WHEREAS, Holder is the holder of that certain Warrant to Purchase Shares of Stock of the Company dated as of May 1, 2023, and as amended on June 14, 2023, issued by the Company to Holder (the “Warrant”);
WHEREAS, the parties now desire to amend the Warrant;
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows:
“Applicable Number” means 292,441.
“Stock Purchase Price” means $[__] (the Nasdaq “minimum price” as of the day prior to the Merger Closing Date).
2.Warrant Exercisability. The Warrant is amended to reflect that, subject to Sections 4.3 and 4.8, the Warrant may be exercised following receipt of Stockholder Approval, at any time or from time to time up to and including 5:00 p.m. (Pacific time) on May 1, 2028 (the “Expiration Date”), upon surrender to Company at its principal office at 18 W. 18th Street, 5th Floor, New York, NY 10011 (or at such other location as Company may advise Holder in writing) of this Warrant properly endorsed with the Form of Subscription attached hereto as Exhibit “A” (the “Form of Subscription”) duly completed and signed and upon payment in cash or by check of the aggregate Stock Purchase Price for the number of shares for which this Warrant is being exercised determined in accordance with the provisions hereof. The Stock Purchase Price and the number of shares purchasable hereunder are subject to further adjustment as provided in Section 4 of this Warrant. For purposes of the Warrant, “Stockholder Approval” means such approval as may be required by the applicable rules and regulations of the Nasdaq Capital Market (or any successor entity) from the stockholders of the Company with respect to issuance of the Warrant and the shares of Common Stock issuable upon the exercise thereof.
3.No Other Amendments.Except as amended hereby, the Warrant shall remain in full force and effect as originally written.
3.Governing Law. This Amendment No. 2 shall be governed by and construed in accordance with the laws of the State of Delaware, without regard to its conflict of laws provisions.
[Remainder of page left blank intentionally]
[Signature page follows]
IN WITNESS WHEREOF, the parties have executed this Amendment No. 2 to Warrant to Purchase Stock as of the date first above written.
DARIOHEALTH CORP. |
|
|
|
|
|
|
|
|
By: |
|
|
Name: |
Zvi Ben-David |
|
Title: |
Chief Financial Officer |
|
|
|
|
|
|
|
AVENUE VENTURE OPPORTUNITIES FUND II, LP | ||
| ||
By: |
Avenue Venture Opportunities Partners II, LLC |
|
Its: |
General Partner |
|
|
|
|
|
|
|
By: |
|
|
Name: |
Sonia Gardner |
|
Title: |
Member |
|
2
Exhibit 4.12
AMENDMENT NO. 3 TO WARRANT TO PURCHASE
SHARES OF STOCK OF
DARIOHEALTH CORP.
THIS AMENDMENT NO. 3 TO WARRANT TO PURCHASE SHARES OF STOCK OF DARIOHEALTH CORP. is made as of December 17, 2024, by and between AVENUE VENTURE OPPORTUNITIES FUND, L.P., (“Holder”) and DARIOHEALTH CORP., a Delaware corporation (the “Company”).
WHEREAS, Holder is the holder of that certain Warrant to Purchase Shares of Stock of DarioHealth Corp. dated as of May 1, 2023 issued by the Company to Holder (as amended by that certain Amendment No. 1 to Warrant effective as of June 14, 2023, and by that certain Amendment No. 2 to Warrant effective as of February 15, 2024, the “Warrant”);
WHEREAS, the parties now desire to amend the Warrant;
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows:
1.Amendment of Warrant. The defined term “Stock Purchase Price” is hereby amended and restated as follows:
“Stock Purchase Price” means $0.7208, subject to stockholder approval as required by Nasdaq rules.
2.No Other Amendments.Except as amended hereby, the Warrant shall remain in full force and effect as originally written.
3.Governing Law. This Amendment No. 3 shall be governed by and construed in accordance with the laws of the State of Delaware, without regard to its conflict of laws provisions.
[Remainder of page left blank intentionally]
[Signature page follows]
IN WITNESS WHEREOF, the parties have executed this Amendment No. 3 to Warrant to Purchase Stock as of the date first above written.
DARIOHEALTH CORP. |
|
|
|
|
|
|
|
|
By: |
|
|
Name: |
Zvi Ben-David |
|
Title: |
Chief Financial Officer |
|
|
|
|
|
|
|
AVENUE VENTURE OPPORTUNITIES FUND, LP | ||
| ||
By: |
Avenue Venture Opportunities Partners, LLC |
|
Its: |
General Partner |
|
|
|
|
|
|
|
By: |
|
|
Name: |
Sonia Gardner |
|
Title: |
Member |
|
2
Exhibit 10.23
REDEMPTION AGREEMENT
This Redemption Agreement, effective as of June 9, 2022 (this “Agreement”), is entered into by and between Richard Allan Anderson (“Executive”) and DarioHealth Corp. (“Dario”).
WHEREAS, pursuant to that certain Restricted Stock Award Agreements, dated as of January 19, 2021 and July 18, 2021, respectively (collectively, the “Restricted Stock Agreements”) issued pursuant to Dario’s 2020 Equity Incentive Plan, Dario granted to Executive 91,652 and 20,000 shares of restricted stock of Dario, respectively (collectively, the “Restricted Stock”), 33,885 of which have vested during the 2022 fiscal year.
WHEREAS, under the Restricted Stock Agreements, Executive agreed to pay to Dario, or make arrangements satisfactory to Dario’s Compensation Committee regarding the payment of, any federal, state, social security, Medicare and local taxes of any kind required by law to be withheld or paid with respect to the Restricted Stock (the “Withholding Tax Obligation”).
WHEREAS, as a result of the vesting of the Restricted Stock, Executive recognized approximately $321,500 in compensation income for United States federal, state, social security, Medicare and local tax purposes, which income is subject to approximately $170,275 in Withholding Tax Obligation.
WHEREAS, the Executive and Dario desire that Dario redeem sufficient shares of Restricted Stock from Executive for an aggregate redemption price equal to the Withholding Tax Obligation in satisfaction of the same on the terms and conditions set forth herein, and Dario’s Compensation Committee has approved such arrangement.
NOW, THEREFORE, in consideration of the mutual covenants contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto, intending to be legally bound, hereby agree as follows:
1.Redemption of Shares. Upon the terms and subject to the conditions of this Agreement, Dario hereby redeems from Executive 17,957 shares of Restricted Stock for an aggregate redemption price equal to the Withholding Tax Obligation. For avoidance of doubt, the redemption price shall be paid by Dario by deeming the Withholding Tax Obligation to be fully satisfied.
2.Representations and Warranties. Each of the parties hereby represents and warrants, severally as to himself or itself and not jointly, as follows:
(a)Executive has full power and authority to execute and deliver this Agreement and the other agreements and instruments contemplated hereby, to consummate the transactions contemplated hereby and to perform his obligations hereunder. This Agreement has been duly executed and delivered by Executive and constitutes the legal, valid and binding obligation of Executive, enforceable against him in accordance with its terms. Executive owns beneficially and of record the Restricted Stock and has good and valid title to the Restricted Stock, free and clear of all liens, encumbrances and adverse claims. The execution and delivery of this Agreement and each other agreement or instrument contemplated hereby by Executive, the performance by Executive of his obligations hereunder and the consummation by Executive of the transactions contemplated hereunder do not and will not (with or without notice or passage of time, or both), violate, conflict with, or result in a breach of, any of the terms or provisions of, or constitute a default under, or give rise to a right of termination, acceleration, violation or loss of rights under, or result in the creation or imposition of any liens, encumbrances or other adverse claims upon, any of the Restricted Stock under (i) any contract, agreement, note, bond, debenture or other instrument to which Executive is a party or by which he is bound, or (ii) applicable law. Executive acknowledges that Dario has not made any representation or warranty regarding the value of the Restricted Stock.
Executive (i) is a sophisticated individual familiar with transactions similar to those contemplated by this Agreement, (ii) has adequate information concerning the business and financial condition of Dario to make an informed decision regarding the redemption of the Restricted Stock, (iii) has voluntarily agreed to the redemption of the Restricted Stock, and has had an opportunity to consult with his legal, tax and financial advisors concerning this Agreement and its subject matter and (iv) has independently and without reliance upon Dario, and based on such information and the advice of such advisors as Executive has deemed appropriate, made its own analysis and decision to enter into this Agreement.
(b)Dario has full power and authority to execute and deliver this Agreement and the other agreements and instruments contemplated hereby, to consummate the transactions contemplated hereby and to perform its obligations hereunder. This Agreement has been duly executed and delivered Dario and constitutes the legal, valid and binding obligation of Dario, enforceable against Dario in accordance with its terms.
3.Further Assurances. Each party hereto, without additional consideration, shall cooperate, shall take such further action and shall execute and deliver such further documents as may be reasonably requested by the other party hereto in order to carry out the provisions and purposes of this Agreement.
4.Counterparts. This Agreement may be signed in counterparts with the same effect as if the signature on each counterpart were upon the same instrument. In the event that any signature is delivered by facsimile transmission or by e-mail delivery of a “.pdf” format data file, such signature shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such facsimile or “.pdf” signature page were an original thereof.
5.Headings. The headings of Articles and Sections in this Agreement are provided for convenience only and will not affect its construction or interpretation.
6.Waiver. Neither any failure nor any delay by any party in exercising any right, power or privilege under this Agreement or any of the documents referred to in this Agreement will operate as a waiver of such right, power or privilege, and no single or partial exercise of any such right, power or privilege will preclude any other or further exercise of such right, power or privilege.
7.Severability. The invalidity or unenforceability of any provisions of this Agreement pursuant to any applicable law shall not affect the validity of the remaining provisions hereof, but this Agreement shall be construed as if not containing the provision held invalid or unenforceable in the jurisdiction in which so held, and the remaining provisions of this Agreement shall remain in full force and effect. If the Agreement may not be effectively construed as if not containing the provision held invalid or unenforceable, then the provision contained herein that is held invalid or unenforceable shall be reformed so that it meets such requirements as to make it valid or enforceable.
8.Governing Law. This Agreement shall be governed by and construed in accordance with Section 18 of the 2020 Equity Incentive Plan.
[signature page follows]
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the day and year first above written.
|
By: |
/s/ Richard Allan Anderson |
|
Name: |
Richard Allan Anderson |
|
|
|
|
|
|
|
DARIOHEALTH CORP. |
|
|
|
|
|
|
|
|
By: |
/s/ Zvi Ben-David |
|
Name: |
Chief Financial Officer |
Exhibit 21.1
Subsidiaries of the Registrant
Labstyle Innovation Ltd., an Israeli company
PsyInnovations Inc., a Delaware company
DarioHealth India Services Pvt. Ltd., an Indian company
Twill, Inc., a Delaware company
Twill ISR Ltd., an Israeli company We consent to the incorporation by reference in the following Registration Statements:
Exhibit 23.1
Consent of Independent Registered Public Accounting Firm
(1) |
Registration Statement (Form S-3 Nos. 333-278865, 333-273019, 333-269092, and 333-265992) of DarioHealth Corp., and |
(2) |
Registration Statement (Form S-8 Nos. 333-284346, 333-280706, 333-277215, 333-276617, 333-269147, 333-262056, 333-256897, 333-251968, 333-249474) pertaining to 2020 Equity incentive Plan of DarioHealth Corp.; |
of our report dated March 10, 2025, with respect to the consolidated financial statements of DarioHealth Corp. included in this Annual Report (Form 10-K) for the year ended December 31, 2024.
Tel-Aviv, Israel |
|
/s/ KOST FORER GABBAY & KASIERER |
March 10, 2025 |
|
A Member of Ernst & Young Global |
Exhibit 31.1
CERTIFICATION
OF PRINCIPAL EXECUTIVE OFFICER
PURSUANT TO RULE 13A-14(A) AND 15D-14(A)
UNDER THE SECURITIES EXCHANGE ACT OF 1934
I, Erez Raphael, certify that:
1. I have reviewed this Annual Report on Form 10-K of DarioHealth Corp. (the “Registrant”);
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13-a13a-15(f) and 15d-15(f)) for the registrant and have:
5. The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
Date: March 10, 2025 |
/s/ Erez Raphael |
|
Erez Raphael |
|
Chief Executive Officer |
Exhibit 31.2
CERTIFICATION
OF PRINCIPAL FINANCIAL OFFICER
PURSUANT TO RULE 13A-14(A) AND 15D-14(A)
UNDER THE SECURITIES EXCHANGE ACT OF 1934
I, Zvi Ben David, certify that:
1. I have reviewed this Annual Report on Form 10-K of DarioHealth Corp. (the “Registrant”);
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13-a13a-15(f) and 15d-15(f)) for the registrant and have:
5. The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
Date: March 10, 2025 |
/s/ Zvi Ben David |
|
Zvi Ben David |
|
Chief Financial Officer, Secretary and Treasurer |
|
(Principal Financial Officer) |
Exhibit 32.1
CERTIFICATION
OF PRINCIPAL EXECUTIVE OFFICER AND
PRINCIPAL FINANCIAL OFFICER
PURSUANT TO 18 U. S. C. SECTION 1350,
In connection with the Annual Report of DarioHealth Corp. (the “Company”) on Form 10-K for the period ended December 31, 2024 (the “Report”), I, Erez Raphael, Chief Executive Officer of the Company, and I, Zvi Ben David, Chief Financial Officer of the Company, hereby certify pursuant to 18 U.S.C. Section 1350, that to my knowledge:
1. The Report fully complies with the requirements of Section 13(a) or 15(d), as applicable, of the Securities Exchange Act of 1934; and
2. The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: March 10, 2025 |
/s/ Erez Raphael |
|
Erez Raphael |
|
Chief Executive Officer |
|
(Principal Executive Officer) |
|
|
Date: March 10,2025 |
/s/ Zvi Ben David |
|
Zvi Ben David |
|
Chief Financial Officer, Secretary and Treasurer |
|
(Principal Financial Officer) |
Exhibit 19.1

INSIDER TRADING COMPLIANCE MANUAL
DarioHealth Corp.
Adopted March 5, 2013, Updated February 19, 2025
In order to take an active role in the prevention of insider trading violations by its officers, directors, employees, consultants, attorneys, advisors and other related persons, the Board of Directors (the “Board”) of DarioHealth Corp., a Delaware corporation (the “Company”) has adopted the policies and procedures described in this Insider Trading Compliance Manual.
I.Adoption of Insider Trading Policy.
Effective as of the date written above, the Company has adopted the Insider Trading Policy attached hereto as Exhibit A (the “Policy”), which prohibits trading based on material, nonpublic information regarding the Company (“Inside Information”). The Policy covers all officers and directors of the Company, all other employees of the Company and its subsidiaries, all secretaries and assistants supporting such directors and consultants or contractors to the Company or its subsidiaries who have or may have access to Inside Information and members of the immediate family or household of any such person. The Policy (and/or a summary thereof) is to be delivered to all new directors, employees, consultants and related individuals who are within the categories of covered persons upon the commencement of their relationships with the Company, and is to be circulated to all covered personnel at least annually.
In addition, the Company itself must comply with securities laws applicable to its own securities trading activities, and must not engage in any transaction involving a purchase or sale of its securities, including any offer to purchase or offer to sell or other disposition of its securities, when it is in possession of Material Nonpublic Information, as defined in the Policy, concerning the Company, other than in compliance with applicable law, subject to the policies and procedures adopted by the Company and the exceptions listed in the Policy to the extent applicable.
II.Designation of Certain Persons.
A.Section 16 Individuals. All directors and executive officers of the Company are subject to the reporting and liability provisions of Section 16 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and the rules and regulations promulgated thereunder (“Section 16 Individuals”).
B.Other Persons Subject to Policy. In addition, certain employees, consultants, and advisors of the Company as described in Section I above have, or are likely to have, from time to time access to Inside Information and together with the Section 16 Individuals, are subject to the Policy, including the pre-clearance requirement described in Section IV. A. below.
III.Appointment of Insider Trading Compliance Officer.
Page 1 of 13

By adoption of this Policy, the Board has appointed Zvi Ben-David as the Company’s insider trading compliance officer (the “Compliance Officer”). The Board shall have the power to designate other persons to act as the Compliance Officer.
IV.Duties of Compliance Officer.
The Compliance Officer has been designated by the Board to handle any and all matters relating to the Company’s insider trading compliance program. Certain of those duties may be delegated to outside counsel with special expertise in securities issues and relevant law. The duties of the Compliance Officer shall include the following:
A.Pre-clearing all transactions involving the Company’s securities by all officers and directors of the Company, the Company’s controller, the heads of sales and operations, the investor relations department of the Company and other employees of the Company who have routine access to Inside Information as determined by the Compliance Officer, who were notified that these additional restrictions apply to them, as well as members of the immediate family or household of such individuals, in order to determine compliance with the Policy, insider trading laws, Section 16 of the Exchange Act and Rule 144 promulgated under the Securities Act of 1933, as amended. Attached hereto as Exhibit B is a Pre-Clearance Checklist to assist the Compliance Officer’s performance of this duty.
B.Assisting in the preparation and filing of Section 16 reports (Forms 3, 4 and 5) for all Section 16 Individuals.
C.Serving as the designated recipient at the Company of copies of reports filed with the Securities and Exchange Commission by Section 16 Individuals under Section 16 of the Exchange Act.
D.Performing periodic reviews of available materials, which may include Forms 3, 4 and 5, Form 144, officers and director’s questionnaires, and reports received from the Company’s stock transfer agent, to determine trading activity by officers, directors and others who have, or may have, access to Inside Information.
E.Circulating the Policy (and/or a summary thereof) to all covered employees, including Section 16 Individuals, on an annual basis, and providing the Policy and other appropriate materials to new officers, directors and others who have, or may have, access to Inside Information.
F.Assisting the Board in implementation of the Policy.
G.Coordinating with Company legal counsel regarding all securities compliance matters.
H.Retaining copies of all appropriate securities reports, and maintaining records of his activities as Compliance Officer.
Page 2 of 13

ACKNOWLEDGEMENT
I hereby acknowledge that I have received a copy of DarioHealth Corp.’s Insider Trading Policy (the “Insider Trading Policy”). Further, I certify that I have reviewed the Insider Trading Policy, understand the policies and procedures contained therein and agree to be bound by and adhere to Insider Trading Policy, the policies and procedures contained herein, and any related policies and procedures of the Company.
Dated: |
|
|
|
|
|
Signature |
|
|
|
Name: |
|
Page 3 of 13

Exhibit A
DarioHealth Corp.
INSIDER TRADING POLICY
and Guidelines with Respect to Certain Transactions in Company Securities
APPLICABILITY OF POLICY
This Policy applies to all transactions in the securities of DarioHealth Corp. (the “Company”) securities, including common stock, options and warrants to purchase common stock and any other securities the Company may issue from time to time, such as preferred stock and convertible debentures, as well as to derivative securities relating to the Company’s stock, whether or not issued by the Company, such as exchange-traded options. It applies to all officers and directors of the Company, all other employees of the Company and its subsidiaries, all secretaries and assistants supporting such directors, officers and employees, and consultants or contractors to the Company or its subsidiaries who have or may have access to Material Nonpublic Information (as defined below) regarding the Company (collectively, “Company Affiliated Persons”). Company Affiliated Persons and members of the immediate family or household of any such persons are sometimes referred to in this Policy as “Insiders.” This Policy also applies to any trust or other estate in which an Insider has a substantial beneficial interest or as to which he or she serves as trustee or in a similar fiduciary capacity, and to any trust, corporation, partnership or other entity which the Insider controls, including venture capital partnerships. This Policy also applies to any person who receives Material Nonpublic Information from any Insider.
Any person who possesses Material Nonpublic Information regarding the Company is an Insider for so long as such information is not publicly known.
In addition, the Company itself must comply with securities laws applicable to its own securities trading activities, and must not engage in any transaction involving a purchase or sale of its securities, including any offer to purchase or offer to sell or other disposition of its securities, when it is in possession of Material Nonpublic Information, concerning the Company, other than in compliance with applicable law, subject to the policies and procedures adopted by the Company and the exceptions listed in this Policy to the extent applicable.
The Company has adopted this Policy in order to ensure compliance with securities laws and to avoid even the appearance of improper conduct by anyone associated with the Company. Failure to comply with these procedures could result in a serious violation of the securities laws by you and/or the Company and can result in both civil penalties and criminal fines and imprisonment. The appearance of insider trading can cause a substantial loss of confidence in the Company and its shares on the part of the public and the securities markets. This could result in an adverse impact on the Company and its shareholders. Accordingly, avoiding the appearance of engaging in share transactions on the basis of Material Nonpublic Information can be as important as avoiding a transaction actually based on such information.
DEFINITION OF MATERIAL NONPUBLIC INFORMATION
It is not possible to define all categories of material information. However, information should be regarded as: (i) “material” if there is a reasonable likelihood that it would be considered important to an investor in making an investment decision regarding the purchase or sale of the Company’s securities and (ii)
Page 4 of 13

“nonpublic” if such information not been previously disclosed to the general public and is otherwise not available to the general public (all such information for purposes of this Policy is considered “Material Nonpublic Information”). If there are any questions about whether a particular item of information is Material Nonpublic Information, do not act on it. Check first with the insider trading compliance officer, (the “Compliance Officer”).
While it may be difficult to determine whether particular information is material, there are various categories of information that are particularly sensitive and, as a general rule, should always be considered material. In addition, material information may be positive or negative. Information may be material even if it relates to future, speculative or contingent events and even if it is significant only when considered in combination with publicly available information. Nonpublic information can be material even with respect to companies that do not have publicly traded stock, such as those with outstanding bonds or bank loans. Examples of such information may include:
❍ |
Financial results; |
❍ |
Projections of future earnings or losses; |
❍ |
Information regarding regulatory review of Company products; |
❍ |
Sales and marketing information; |
❍ |
Major contract awards, cancellations or write-offs; |
❍ |
Joint ventures/commercial partnerships with third parties; |
❍ |
Intellectual property and other proprietary/scientific information; |
❍ |
News of a pending or proposed merger or acquisition; |
❍ |
News of the disposition of material assets; |
❍ |
Impending bankruptcy or financial liquidity problems; |
❍ |
Expansion or curtailment of operations or the gain or loss of a substantial customer or supplier; |
❍ |
New product announcements of a significant nature; |
❍ |
Significant pricing changes; |
❍ |
Events regarding the Company’s securities (e.g., stock splits, repurchases or changes in dividend policy); |
❍ |
New equity or debt offerings, significant borrowings, or other material financial transactions; |
❍ |
Significant litigation exposure due to actual or threatened litigation; |
❍ |
Changes in control of the Company or major changes in senior management; |
❍ |
Capital investment plans; |
❍ |
Changes in dividend policy; |
❍ |
Changes in auditors or auditor notification that the Company may no longer rely on an audit report; and |
❍ |
Significant actions by regulatory bodies. |
CERTAIN EXCEPTIONS
For purposes of this Policy, the Company considers that the exercise of stock options for cash under the Company’s stock option plan (but not the sale of any shares underlying such stock options) is exempt from this Policy, since the other party to the transaction is the Company itself and the price does not vary with the market but is fixed by the terms of the option agreement or the plan.
Bona fide gifts of securities are not deemed to be transactions for the purposes of this Policy. Whether a gift is truly bona fide will depend on the circumstances surrounding each gift. The more unrelated the donee is to the donor, the more likely the gift would be considered “bona fide” and not a “transaction”.
Page 5 of 13

For example, gifts to charities, religious institutions and service organizations would likely not be “transactions”. On the other hand, gifts to dependent children followed by a sale of the “gift” securities in close proximity to the time of the gift may imply some economic benefit to the donor and, therefore, make the gift non bona fide.
STATEMENT OF POLICY
General Policy
It is the policy of the Company to prohibit the unauthorized disclosure of any nonpublic information acquired in the workplace and the misuse of Material Nonpublic Information in securities trading.
Specific Policies
1.Trading on Material Nonpublic Information. With certain exceptions, no Insider shall engage in any transaction involving a purchase or sale of the Company’s securities, including any offer to purchase or offer to sell or gift or other disposition of the Company’s securities, during any period commencing with the date that he or she possesses Material Nonpublic Information concerning the Company, and ending at the close of business on the second Trading Day following the date of public disclosure of that information, or at such time as such nonpublic information is no longer material. However, see Section 2 under “Permitted Trading Period” below for a full discussion of trading pursuant to a pre-established plan or by delegation.
As used herein, the term “Trading Day” shall mean a day on which national stock exchanges in the United States are open for trading.
2.Tipping. No Insider shall disclose (“tip”) Material Nonpublic Information to any other person (including family members) where such information may be used by such person to his or her profit by trading in the securities of companies to which such information relates, nor shall such Insider or related person make recommendations or express opinions on the basis of Material Nonpublic Information as to trading in the Company’s securities.
Regulation FD (Fair Disclosure) is an issuer disclosure rule implemented by the SEC that addresses selective disclosure of Material Nonpublic Information. The regulation provides that when the Company, or person acting on its behalf, discloses material nonpublic information to certain enumerated persons (in general, securities market professionals and holders of the Company’s securities who may well trade on the basis of the information), it must make public disclosure of that information. The timing of the required public disclosure depends on whether the selective disclosure was intentional or unintentional; for an intentional selective disclosure, the Company must make public disclosures simultaneously; for a non-intentional disclosure the Company must make public disclosure promptly. Under the regulation, the required public disclosure may be made by filing or furnishing a Form 8-K, or by another method or combination of methods that is reasonably designed to effect broad, non-exclusionary distribution of the information to the public.
It is the policy of the Company that all communications with the press be handled only through our Chief Executive Officer or our investor/public relations firm. Please refer all press, analyst or similar requests for information to the Company’s Chief Executive Officer and do not respond to any inquiries without prior authorization from the Company’s Chief Executive Officer. If the Chief Executive Officer is unavailable, the Chief Executive Officer will designate another Company officer to fill this role.
Page 6 of 13

3.Confidentiality of Nonpublic Information. Nonpublic information relating to the Company is the property of the Company and the unauthorized disclosure of such information (including, without limitation, via email or by posting on Internet message boards or blogs) is strictly forbidden. Each Company officer and employee has executed a confidentiality agreement in favor of the Company which must be complied with. The Company’s Code of Ethics also governs this.
4.Duty to Report Inappropriate and Irregular Conduct. All officers and employees, and particularly managers and/or supervisors, have a responsibility for maintaining integrity within the Company. Consistent with the Company’s Code of Ethics, any violation of this Policy must be reported to the Company’s executive officers or to the Company’s Audit Committee.
Page 7 of 13

POTENTIAL CRIMINAL AND CIVIL LIABILITY
AND/OR DISCIPLINARY ACTION
1.Liability for Insider Trading. Insiders may be subject to penalties of up to $5,000,000 and up to twenty (20) years in jail for engaging in transactions in the Company’s securities at a time when they possess Material Nonpublic Information regarding the Company. In addition, the SEC has the authority to seek a civil monetary penalty of up to three times the amount of profit gained or loss avoided by illegal insider trading. “Profit gained” or “loss avoided” generally means the difference between the purchase or sale price of the Company’s stock and its value as measured by the trading price of the stock a reasonable period after public dissemination of the nonpublic information.
2.Liability for Tipping. Insiders may also be liable for improper transactions by any person (commonly referred to as a “tippee”) to whom they have disclosed Material Nonpublic Information regarding the Company or to whom they have made recommendations or expressed opinions on the basis of such information as to trading in the Company’s securities. The SEC has imposed large penalties even when the disclosing person did not profit from the trading. The SEC, the stock exchanges and the Financial Industry Regulatory Authority, Inc. (FINRA) use sophisticated electronic surveillance techniques to monitor and uncover insider trading.
3.Possible Disciplinary Actions. Individuals subject to this Policy who violate this Policy shall also be subject to disciplinary action by the Company, which may include suspension, forfeiture of perquisites, ineligibility for future participation in the Company’s equity incentive plans and/or termination of employment or association with the Company.
PERMITTED TRADING PERIOD
1.Black-Out Period and Trading Window. To ensure compliance with this Policy and applicable federal and state securities laws, the Company requires that all Insiders refrain from conducting any transactions involving the purchase or sale of the Company’s securities, other than during the period in any fiscal quarter commencing at the close of business on the second Trading Day following the date of public disclosure of the financial results for the prior fiscal quarter or year and ending on the twenty-fifth day of the third month of the fiscal quarter (the “Trading Window”). If such public disclosure occurs on a Trading Day before the markets close, then such date of disclosure shall be considered the first Trading Day following such public disclosure.
The safest period for trading in the Company’s securities, assuming the absence of Material Nonpublic Information, is generally the first ten (10) Trading Days of the Trading Window. It is the Company’s policy that the period when the Trading Window is “closed” is a particularly sensitive periods of time for transactions in the Company’s securities from the perspective of compliance with applicable securities laws. This is because officers, directors and certain other employees will, as any quarter progresses, are increasingly likely to possess Material Nonpublic Information about the expected financial results for the quarter. The purpose of the Trading Window is to avoid any unlawful or improper transactions or even the appearance of any such transactions.
It should be noted that even during the Trading Window any person possessing Material Nonpublic Information concerning the Company shall not engage in any transactions in the Company’s securities until such information has been known publicly for at least two Trading Days. The Company has adopted the policy of delaying trading for “at least two Trading Days” because the securities laws require that the public be informed effectively of previously undisclosed material information before Insiders trade in the Company’s stock.
Page 8 of 13

Public disclosure may occur through a widely disseminated press release or through filings, such as Forms 10-Q and 8-K, with the SEC. Furthermore, in order for the public to be effectively informed, the public must be given time to evaluate the information disclosed by the Company. Although the amount of time necessary for the public to evaluate the information may vary depending on the complexity of the information, generally two (2) Trading Days is a sufficient period of time.
From time to time, the Company may also require that directors, officers, selected employees and others suspend trading because of developments known to the Company and not yet disclosed to the public. In such event, such persons may not engage in any transaction involving the purchase or sale of the Company’s securities during such period and may not disclose to others the fact of such suspension of trading.
Although the Company may from time to time require during a Trading Window that directors, officers, selected employees and others suspend trading because of developments known to the Company and not yet disclosed to the public, each person is individually responsible at all times for compliance with the prohibitions against insider trading. Trading in the Company’s securities during the Trading Window should not be considered a “safe harbor,” and all directors, officers and other persons should use good judgment at all times.
Notwithstanding these general rules, Insiders may trade outside of the Trading Window provided that such trades are made pursuant to a pre-established plan (a so called “10b5-1 plan”) or by delegation; these alternatives are discussed in the next section.
2.Trading According to a Pre-established Plan or by Delegation. Trading which is not “on the basis of” material non-public information may not give rise to insider trading liability. The SEC has adopted Rule 10b5-1 under which insider trading liability can be avoided if Insiders follow very specific procedures. In general, such procedures involve trading according to pre-established instructions (a “Pre-established Trade”).
Pre-established Trades must:
(a)Be documented by a contract, written plan, or formal instruction which provides that the trade take place in the future. For example, an Insider can contract to sell his or her shares on a specific date, or simply delegate such decisions to an investment manager, 401(k) plan administrator or similar third party. This documentation must be provided to the Company’s Compliance Officer;
(b)Include in its documentation the specific amount, price and timing of the trade, or the formula for determining the amount, price and timing. For example, the Insider can buy or sell shares in a specific amount and on a specific date each month, or according to a pre-established percentage (of the Insider’s salary, for example) each time that the share price falls or rises to pre-established levels. In the case where trading decisions have been delegated, the specific amount, price and timing need not be provided;
(c)Be implemented at a time when the Insider does not possess material non-public information. As a practical matter, this means that the Insider may set up Pre-established Trades, or delegate trading discretion, only during a “Trading Window” (discussed in Section 1, above); and,
(d)Remain beyond the scope of the Insider’s influence after implementation.
Page 9 of 13

In general, the Insider must allow the Pre-established Trade to be executed without changes to the accompanying instructions, and the Insider cannot later execute a hedge transaction that modifies the effect of the Pre-established Trade. An Insider wishing to change the amount, price or timing of a Pre-established Trade, or terminate a Pre-established Trade, can do so only during a “Trading Window” (discussed in Section 1, above). If the Insider has delegated decision-making authority to a third party, the Insider cannot subsequently influence the third party in any way and such third party must not possess material non-public information at the time of any of the trades.
Prior to implementing a pre-established plan for trading, all officers and directors must receive the approval for such plan from the Compliance Officer.
3.Pre-Clearance of Trades. Even during a Trading Window, all officers and directors of the Company, the Company’s controller, the investor relations department of the Company and other employees of the Company who have routine access to Material Nonpublic Information as determined by the Compliance Officer, who were notified that these additional restrictions apply to them (collectively, “Access Insiders”), as well as members of the immediate family or household of such Access Insiders, must comply with the Company’s “pre-clearance” process prior to trading in the Company’s securities, implementing a pre-established plan for trading, or delegating decision-making authority over the Insider’s trades. To do so, each Access Insider must contact the Compliance Officer prior to initiating any of these actions. The Company may also find it necessary, from time to time, to require compliance with the pre-clearance process from certain employees, consultants and contractors, other than and in addition to Access Insiders.
4.Individual Responsibility. As Insiders, every person subject to this Policy has the individual responsibility to comply with this Policy against insider trading, regardless of whether the Company has established a Trading Window applicable to that Insider or any other Insiders of the Company. Each individual, and not necessarily the Company, is responsible for his or her own actions and will be individually responsible for the consequences of their actions. Therefore, appropriate judgment, diligence and caution should be exercised in connection with any trade in the Company’s securities. An Insider may, from time to time, have to forego a proposed transaction in the Company’s securities even if he or she planned to make the transaction before learning of the Material Nonpublic Information and even though the Insider believes he or she may suffer an economic loss or forego anticipated profit by waiting.
5.Exceptions to the Policy. Any exceptions to this Policy may only be made by advance written approval of each of: (i) the Company’s President, (ii) the Compliance Officer and (iii) the Chairman of the Audit Committee of the Company’s Board of Directors. Any such exceptions shall be immediately reported to the remaining members of the Board of Directors.
APPLICABILITY OF POLICY TO INSIDE INFORMATION
REGARDING OTHER COMPANIES
This Policy and the guidelines described herein also apply to Material Nonpublic Information relating to other companies, including the Company’s partners, customers, vendors or suppliers (“Business Partners”), when that information is obtained in the course of employment with, or other services performed on behalf of the Company. Civil and criminal penalties, as well as termination of employment, may result from trading on inside information regarding the Company’s Business Partners. All employees should treat Material Nonpublic Information about the Company’s Business Partners with the same care as is required with respect to information relating directly to the Company.
Page 10 of 13

RULE 144 AND SECTION 16 MATTERS FOR DIRECTORS AND OFFICERS
Directors and principal officers of the Company must also comply with Rule 144, or another applicable exemption from registration if selling any securities. The practical effect of Rule 144 is that directors and officers who sell the Company’s securities may be required to comply with a number of requirements including holding period, volume limitation, manner of sale and SEC filing requirements. The Company may provide separate memoranda and other appropriate materials to its directors and officers regarding compliance with Rule 144. In addition, purchases and sales (or sales and purchases) of Company common stock occurring within any six-month period in which profit is realized result in illegal “short-swing profits,” as further described below. The prohibition against short-swing profits is found in Section 16 of the Exchange Act. Section 16 contains a prohibition against profitable “insider trading” in a company’s securities within any six-month period regardless of the presence or absence of Material Nonpublic Information that may affect the market price of those securities. Each executive officer, director and 10% shareholder of the Company is subject to the prohibition against short-swing profits under Section 16. Such persons are required to file Forms 3, 4 and 5 reports reporting his or her initial ownership of the Company’s common stock and any subsequent changes in such ownership. The Sarbanes-Oxley Act of 2002 requires officers and directors who must report transactions on Form 4 to do so by the end of the second business day following the transaction date. Profit realized, for the purposes of Section 16, is calculated generally to provide maximum recovery by the Company. The measure of damages is the profit computed from any purchase and sale or any sale and purchase within the short-swing (i.e., six-month) period, without regard to any setoffs for losses, any first-in or first-out rules, or the identity of the shares of common stock. This approach sometimes has been called the “lowest price in, highest price out” rule. The Company may provide separate memoranda and other appropriate materials to its directors and officers regarding compliance with Section 16.
SPECIFIC REQUIREMENTS
1.Speculative Trading. No Insider may engage in transactions of a speculative nature at any time. All Insiders are prohibited from short selling the Company’s securities or engaging in transactions involving the Company’s based derivative securities. A short sale, for these purposes, means any transaction whereby one may benefit from a decline in the price of the Company’s securities. “Derivative Securities” are options, warrants, stock appreciation rights or similar rights whose value is derived from the value of an equity security, such as the Company’s common stock. This prohibition includes, but is not limited to, trading in the Company’s put and call option contracts, transacting in straddles, hedging or monetization transaction with respect to the Company’s securities, and the like. In addition, no Insider shall engage in a transaction with respect to securities of the Company if he or she owns the security, but does not deliver it against such sale (a “short sale against the box”) within twenty days thereafter, or does not within five days after such sale deposit it in the mails or other usual channels of transportation. The above does not derogate from Insiders’ right to hold and exercise options or other derivative securities granted under the Company’s employee share option or equity incentive plans as long as such exercise is not prohibited by this Policy.
2.Post-Termination Transactions. If an Insider is aware of Material Nonpublic Information at the time such Insider’s association with the Company is terminated, whether by the Insider or the Company, the Insider may not trade in Company securities until such information is no longer material or until two Trading Days after such information has become public. In addition, if the Company is not in a Trading Window at the time such association with the Company is terminated, the Insider may not trade in Company securities until two Trading Days after the next announcement of quarterly earnings or of the material, non-public information.
Page 11 of 13

3.Ad hoc Restrictions. The Compliance Officer has the authority to impose restrictions on trading in the Company’s securities by appropriate individuals at any time. In such event, the Compliance Officer will notify the affected individuals, either personally, by email or by voicemail, to inform them of the restrictions.
4.Open Orders. Any Insider who has placed a limit order or open instruction to buy or sell the Company’s securities shall bear responsibility for canceling such instructions immediately upon becoming in possession of Material Nonpublic Information.
INQUIRIES
Please direct your questions as to any of the matters discussed in this Policy to the Compliance Officer.
Page 12 of 13

Exhibit B
DARIOHEALTH CORP.
INSIDER TRADING COMPLIANCE PROGRAM - PRE-CLEARANCE CHECKLIST
Individual Proposing to Trade: |
|
|
|
|
|
Number of Shares covered by Proposed Trade: |
|
|
|
|
|
Date: |
|
|
|
Trading Window. Confirm that the trade will be made during the Company’s “trading window.” |
|
Section 16 Compliance. Confirm, if the individual is subject to Section 16, that the proposed trade will not give rise to any potential liability under Section 16 as a result of matched past (or intended future) transactions. Also, ensure that a Form 4 has been or will be completed and will be timely filed. |
|
Prohibited Trades. Confirm, if the individual is subject to Section 16, that the proposed transaction is not a “short sale,” put, call or other prohibited or strongly discouraged transaction. |
|
Rule 144 Compliance. Confirm that: |
|
Current public information requirement has been met; |
|
Shares are not restricted or, if restricted, the one year holding period has been met; |
|
Volume limitations are not exceeded (confirm that the individual is not part of an aggregated group); |
|
The manner of sale requirements have been met; and |
|
The Notice of Form 144 Sale has been completed and filed. |
|
Rule 10b-5 Concerns. Confirm that (i) the individual has been reminded that trading is prohibited when in possession of any material information regarding the Company that has not been adequately disclosed to the public, and (ii) the Insider Trading Compliance Officer has discussed with the individual any information known to the individual or the Insider Trading Compliance Officer which might be considered material, so that the individual has made an informed judgment as to the presence of inside information. |
Signature of Insider Trading Compliance Officer |
|
Page 13 of 13