UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE MONTH OF FEBRUARY 2025
COMMISSION FILE NUMBER 001-38976
Genmab A/S
(Exact name of Registrant as specified in its charter)
Carl Jacobsens Vej 30
2500 Valby
Denmark
+45 70 20 27 28
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ⌧ Form 40-F ◻
This report on Form 6-K shall be deemed to be incorporated by reference in Genmab A/S’s registration statements on Form S-8 (File No. 333-232693, 333-253519, 333-262970 and 333-277273) and to be a part thereof from the date on which this report is filed, to the extent not superseded by documents or reports subsequently filed or furnished.
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
GENMAB A/S |
|
|
|
|
|
BY: |
/s/ Anthony Pagano |
|
|
Name: Anthony Pagano |
|
|
Title: Executive Vice President & Chief Financial Officer |
DATE: February 12, 2025
EXHIBIT INDEX
Exhibit |
Description of Exhibit |
|
|
99.1 |
|
99.1 (a) |
|
101.INS |
Inline XBRL Instance Document |
101.SCH |
Inline XBRL Taxonomy Extension Schema Document |
101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF |
Inline XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB |
Inline XBRL Taxonomy Extension Labels Linkbase Document |
101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
Exhibit 99.1
GENMAB 2024 ANNUAL REPORT |
|
Table of Contents
MANAGEMENT’S REVIEW |
|
1 |
|
3 |
|
4 |
|
5 |
|
8 |
|
10 |
|
12 |
|
14 |
|
15 |
|
15 |
|
18 |
|
19 |
|
21 |
|
22 |
|
44 |
|
58 |
|
65 |
|
66 |
|
68 |
|
73 |
|
76 |
|
80 |
|
FINANCIAL STATEMENTS FOR THE GENMAB GROUP |
158 |
FINANCIAL STATEMENTS OF THE PARENT COMPANY |
224 |
DIRECTORS’ AND MANAGEMENT’S STATEMENT ON THE ANNUAL REPORT |
241 |
INDEPENDENT AUDITOR’S REPORTS |
242 |
OTHER INFORMATION |
251 |
FORWARD LOOKING STATEMENT |
251 |
CONTACT INFORMATION |
252 |
Our Reporting Suite
| ● | 2024 Corporate Governance Report |
| ● | 2024 Compensation Report |
Our Corporate Governance and Compensation Reports for 2024 can be found on our website Genmab.com.
*The Sustainability Statements are part of Management’s Review
Our 2030 Vision
By 2030, our KYSO (knock-your-socks-off) antibody medicines® are fundamentally transforming the lives of people with cancer and other serious diseases.
GENMAB 2024 ANNUAL REPORT |
|
Our Core Purpose, Supporting Our 2030 Vision
Our unstoppable team will improve the lives of patients through innovative and differentiated antibody therapeutics.
25 Years of Innovation
1999 – 2009
| ● | Genmab founded |
| ● | Nasdaq Copenhagen A/S (Nasdaq Copenhagen) Initial Public Offering (IPO) |
| ● | First partnership (F. Hoffmann-La Roche AG (Roche)) |
| ● | Ofatumumab program announced |
| ● | Daratumumab selected |
| ● | Arzerra®1 (ofatumumab) first approval |
2010 – 2020
| ● | DuoBody® technology platform announced |
| ● | Collaboration with Seagen Inc. (Seagen) |
| ● | DuoBody research and license agreement with Johnson & Johnson (J&J, legal entity Janssen Biotech, Inc.) |
| ● | Daratumumab agreement with J&J |
| ● | HexaBody® technology platform announced |
| ● | DARZALEX®2 (daratumumab) approval and launch |
| ● | BioNTech SE (BioNTech) partnership |
| ● | U.S. IPO under Nasdaq Global Select Market; dual listed as GMAB |
| ● | Japan Operations established under Genmab K.K. |
| ● | AbbVie Inc. (AbbVie) partnership |
| ● | DARZALEX FASPRO®2 (daratumumab and hyaluronidase fihj) approval and launch |
| ● | Kesimpta®3 (ofatumumab) approval and launch |
| ● | TEPEZZA®4 (teprotumumab) approval and launch |
2021 – 2024
| ● | Tivdak®5 (tisotumab vedotin-tftv) approval and launch |
| ● | DuoBody-based bispecifics RYBREVANT®2 (amivantamab), TECVAYLI®2 (teclistamab) and TALVEY®2 (talquetamab) approval and launch |
| ● | DuoBody-based bispecific EPKINLY®6 (epcoritamab-bysp)/TEPKINLY®6 (epcoritamab) approval and launch |
| ● | argenx SE (argenx) partnership |
| ● | ProfoundBio Inc. (ProfoundBio) acquisition, including rinatabart sesutecan (Rina-S™) |
| ● | Genmab assumes full ownership of acasunlimab |
| ● | Rina-S and acasunlimab move into Phase 3 development |
1. Developed and commercialized by GlaxoSmithKline (GSK); 2. Developed and commercialized by J&J; 3. Developed and commercialized by Novartis AG (Novartis); 4. Developed and commercialized by Amgen Inc. (Amgen); 5. Co-developed and commercialized with Pfizer Inc. (Pfizer); 6. Co-developed and commercialized with AbbVie
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 2/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Chair’s Statement
Dear Shareholder,
For 25 years, Genmab has pioneered antibody-based medicines to fundamentally transform the lives of people with cancer and other serious diseases. We take pride looking back at the great leaps we have made globally and within the foundations of Genmab; however, our focus remains steadfast on the future. Our progress has led us here: over 2,700 team members across five countries, eight antibody-based medicines having an impact on patients’ lives, and two wholly owned assets now in late-stage development.
GENMAB’S EVOLUTION
Founded in 1999, Genmab celebrated its 25th anniversary in 2024, and the year gave all of us at Genmab much to celebrate. Twenty-five years of scientific progress have had an impact on the lives of patients and have inspired us to continue on our path of becoming a fully integrated biotech. This year, Genmab successfully acquired ProfoundBio. In addition to gaining worldwide rights to three clinical candidates and novel antibody-drug conjugate (ADC) platforms, the acquisition is representative of our long-term growth potential. We welcomed talented new colleagues to our Research & Development (R&D) team, and accelerated the clinical development for Rina-S, a wholly owned asset now in Phase 3 clinical development.
Sustainability and social responsibility are fundamental to the way we work at Genmab. This Annual Report also marks the first year for Genmab incorporating our Environmental, Social and Governance (ESG) disclosures in compliance with the European Union’s (EU) Corporate Sustainability Reporting Directive (CSRD). Our first double materiality assessment (DMA) and its outcome supported the development of our first impacts, risks and opportunities that guided our future sustainability strategy. We remain committed to ensuring our actions benefit our stakeholders and society and that our Corporate Social Responsibility (CSR) practices are integrated as a core part of our business. In 2024, 800 Genmab employees completed 4,037 service hours to make a difference in the lives of patients and their families, our communities and the environment.
EXPERIENCED LEADERSHIP
Our future looks promising under our expanded leadership. In 2024, we had two additions to our Executive Committee: Rayne Waller as Chief Technical Operations Officer, and Brad Bailey as Chief Commercial Officer. Rayne Waller joined Genmab to further solidify and strengthen our technical operations and lead all the manufacturing and supply chain capabilities of our proprietary programs through preclinical, clinical and commercial stages. Brad Bailey, previously Genmab’s Senior Vice President and U.S. General Manager, expanded his role to lead the direction, planning, and execution of Genmab’s global commercial strategies as we expand beyond our two priority markets of the U.S. and Japan. These new additions strengthen our commitment to a bold future for our diverse and innovative mid- to late-stage clinical programs.
In 2024, our Board of Directors (Board) continued to provide governance, guidance, and dedicated leadership. Comprised of experts in their fields, the Board has supported organizational growth initiatives, driven global change, and contributed value across Genmab.
On behalf of the Board, I would like to thank Genmab’s dedicated team members, Chief Executive Officer Jan van de Winkel and the entire global leadership team for their inspiration and extraordinary leadership as well as our shareholders for your continued support.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 3/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Sincerely,
Deirdre P. Connelly
Board Chair
Letter from the CEO
Dear Shareholder,
As I reflect on 2024, I am proud to share a year of remarkable progress and strategic achievement at Genmab. This year has reinforced our commitment to transforming the lives of people with cancer and other serious diseases through groundbreaking antibody-based medicines. Our advances across research, development, and commercialization activities reflect the strength of our vision, our team, and our unwavering focus on delivering value for patients and stakeholders alike.
STRATEGIC GROWTH AND INNOVATION
This year, we achieved several key milestones that drive us closer to our 2030 Vision of being a fully integrated biotech innovation powerhouse. Central to this was our acquisition of ProfoundBio, completed in May, which significantly enhanced our long-term growth potential and brought assets such as Rina-S into our pipeline. Rina-S, a next-generation ADC with best-in-class potential, entered Phase 3 development this year.
We also assumed sole responsibility for the continued development and potential commercialization of acasunlimab, underscoring our commitment to building a robust pipeline of wholly owned, late-stage programs. These advancements are supported by a growing portfolio of proprietary technologies, including the novel ADC technology platforms we acquired with ProfoundBio, and our validated DuoBody platform, which underpins our success with innovative bispecific antibodies.
TRANSFORMING SCIENCE INTO MEDICINE
This year, we carefully evaluated our investments with a focus on portfolio prioritization, and we evaluated our clinical pipeline to ensure we are investing our resources in the best and most effective way possible. This strategic prioritization means we are very focused on maximizing the potential of turning science into medicine through our Phase 3 programs, EPKINLY, Rina-S and acasunlimab. After a thorough assessment, we also decided to terminate some early-stage clinical programs that did not meet our criteria for potential KYSO® antibody-based medicines. And we decided not to pursue a Phase 3 program for Tivdak in second line plus head and neck cancer.
This year we are pleased that our commercialized medicines reached significant achievements:
| ● | EPKINLY became the first and only subcutaneous (SC) bispecific antibody approved to treat both relapsed or refractory follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL) in the U.S. and Europe. Strong launches in Japan and other key markets have exceeded expectations. |
| ● | Tivdak, our tissue factor (TF)-directed ADC, received full U.S. Food and Drug Administration (U.S. FDA) approval based on data demonstrating significant overall survival benefits for patients with recurrent or metastatic cervical cancer. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 4/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
A STRONG FINANCIAL FOUNDATION TO ENABLE OUR EVOLUTION
Our financial performance this year has been a testament to the strength of our strategy. Recurring revenues grew significantly, driven by royalties from our collaborations and sales of EPKINLY and Tivdak, both of which delivered robust sales in 2024. This growth reinforces our financial position and enables continued investment based on our strategic prioritization efforts, which include our late-stage clinical programs and commercialization capabilities. This focused approach enables us to realize our vision and capitalize on significant growth opportunities ahead.
Genmab’s 25th anniversary also marked the beginning of a new era of opportunity as our company leverages the full potential of our late-stage clinical programs, the potential of the acquisition of ProfoundBio and continues to build on our existing cutting-edge antibody research and development to fulfill our mid- to long-term growth as a fully integrated biotech innovation powerhouse.
ACKNOWLEDGMENTS AND OUTLOOK
These accomplishments and our progress would not have been possible without the dedication of our exceptional team, the collaboration of our partners, and the trust of our shareholders. I want to express my deepest gratitude to all who have contributed to our success this year.
We are excited about the opportunities that lie ahead as we continue to evolve into a fully integrated biotech. With a strong foundation, an exceptional team, and a strong pipeline of innovative antibody medicines and investigational medicines, Genmab is well-positioned to deliver on our vision to have an impact on the lives of patients around the world.
Thank you for your continued confidence and support. Together, we will continue to drive forward and reach our inspirational 2030 vision.
Sincerely yours,
Jan van de Winkel, Ph.D.
President & CEO
2024 at a Glance
Operational
| ● | EPKINLY/TEPKINLY became the first and only SC bispecific antibody approved in both the U.S. and Europe to treat both relapsed or refractory FL and relapsed or refractory DLBCL after two or more lines of systemic therapy |
| ● | Acquisition of ProfoundBio, granting Genmab worldwide rights to multiple candidates in development (including Rina-S) plus ProfoundBio’s novel ADC technology platforms |
| ● | Genmab submitted a supplemental Japan New Drug Application (J-NDA) to the Ministry of Health, Labour and Welfare (MHLW) for SC EPKINLY for the treatment of relapsed or refractory FL after two or more lines of systemic therapy |
| ● | Tivdak received full U.S. FDA approval to treat recurrent or metastatic cervical cancer |
| ● | Genmab submitted a J-NDA to the MHLW for Tivdak for the treatment of advanced or recurrent cervical cancer |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 5/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| ● | Genmab assumed sole responsibility for the continued development and potential commercialization of acasunlimab |
| ● | Two Genmab wholly owned programs, Rina-S and acasunlimab, moved into Phase 3 development |
| ● | Multiple programs entered clinical-stage development including GEN1059 (BNT314, DuoBody-EpCAMx4-1BB), GEN1055 (BNT315, HexaBody-OX40), GEN1057 (DuoBody-FAPαxDR4) and GEN1286 (EGFRxcMET ADC) |
| ● | Additional regulatory approvals for J&J therapies DARZALEX FASPRO and RYBREVANT |
| ● | Approval of Amgen’s TEPEZZA in Japan for the treatment of active thyroid eye disease (TED) |
| ● | Continued development of Genmab’s broader organizational infrastructure with the addition of over 600 new colleagues |
Financial
| ● | DKK 99B |
o |
2024 year-end market cap |
| ● | DKK 21,526M |
o |
2024 revenue |
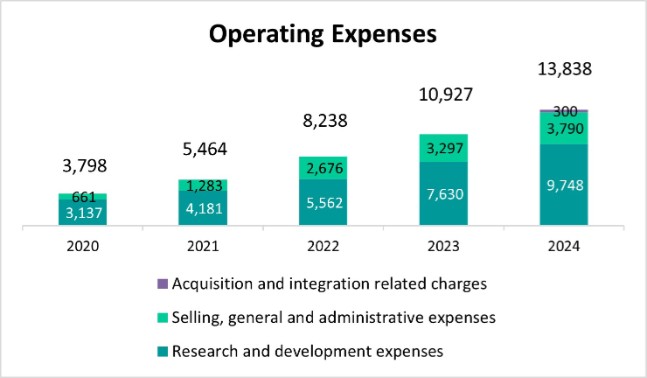
| ● | DKK 13,838M |
o |
2024 operating expenses, 72% invested in R&D |
| ● | Liquidity and Capital Resources |
| o | Marketable securities – DKK 11,243M |
| o | Cash and cash equivalents – DKK 9,858M |
| o | Shareholders’ equity – DKK 36,697M |
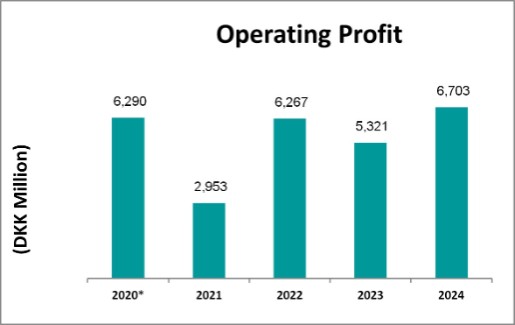
* 2020 Operating Profit impacted by one-time AbbVie upfront payment.
Sustainability
| ● | Completed first DMA in accordance with CSRD |
| ● | First year integrating our sustainability statements into Genmab’s Annual Report |
| ● | Environmental |
| o | Set first long-term emission reduction target for Scope 1 & 2 emissions |
| o | Achieved 77% renewable electricity across all sites |
| o | Established formalized processes governing supplier engagement on climate change |
| ● | Social |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 6/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| o | Exceeded life sciences industry benchmark for favorability and participation rate for Global Employee Engagement Survey |
| o | Zero workplace injuries |
| o | 100% of eligible team members with access to year-end performance process |
| o | Training available to all team members at varying degrees |
| ● | Governance |
| o | Code of Conduct applies to all Genmab team members |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 7/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Consolidated Key Figures
(DKK million) |
|
|
|
|
|
|
|
|
|
Income Statement |
2020 |
|
2021 |
|
2022 |
|
2023 |
|
2024 |
Revenue |
10,088 |
|
8,417 |
|
14,505 |
|
16,474 |
|
21,526 |
Cost of product sales |
- |
|
- |
|
- |
|
(226) |
|
(985) |
Research and development expenses |
(3,137) |
|
(4,181) |
|
(5,562) |
|
(7,630) |
|
(9,748) |
Selling, general and administrative expenses |
(661) |
|
(1,283) |
|
(2,676) |
|
(3,297) |
|
(3,790) |
Acquisition and integration related charges |
- |
|
- |
|
- |
|
- |
|
(300) |
Total costs and operating expenses |
(3,798) |
|
(5,464) |
|
(8,238) |
|
(11,153) |
|
(14,823) |
Operating profit |
6,290 |
|
2,953 |
|
6,267 |
|
5,321 |
|
6,703 |
Net financial items |
(409) |
|
965 |
|
678 |
|
316 |
|
2,461 |
Net profit |
4,740 |
|
2,957 |
|
5,452 |
|
4,352 |
|
7,844 |
|
|
|
|
|
|
|
|
|
|
Balance Sheet |
|
|
|
|
|
|
|
|
|
Total non-current assets |
2,352 |
|
1,891 |
|
1,901 |
|
2,150 |
|
17,957 |
Marketable securities |
8,819 |
|
10,381 |
|
12,431 |
|
13,268 |
|
11,243 |
Cash and cash equivalents |
7,260 |
|
8,957 |
|
9,893 |
|
14,867 |
|
9,858 |
Total assets |
21,105 |
|
24,538 |
|
30,119 |
|
35,289 |
|
45,811 |
Share capital |
66 |
|
66 |
|
66 |
|
66 |
|
66 |
Shareholders' equity |
19,083 |
|
22,107 |
|
27,282 |
|
31,610 |
|
36,697 |
|
|
|
|
|
|
|
|
|
|
Cash Flow Statement |
|
|
|
|
|
|
|
|
|
Cash flow from operating activities |
6,433 |
|
2,228 |
|
3,912 |
|
7,380 |
|
7,771 |
Cash flow from investing activities |
(2,351) |
|
(961) |
|
(2,761) |
|
(1,282) |
|
(9,907) |
Cash flow from financing activities |
71 |
|
(420) |
|
(789) |
|
(606) |
|
(3,919) |
Investments in intangible assets |
- |
|
- |
|
- |
|
(10) |
|
(117) |
Investments in tangible assets |
(307) |
|
(252) |
|
(317) |
|
(366) |
|
(187) |
|
|
|
|
|
|
|
|
|
|
Financial Ratios and Other Information |
|
|
|
|
|
|
|
|
|
Basic net profit per share |
72.72 |
|
45.22 |
|
83.38 |
|
66.64 |
|
122.21 |
Diluted net profit per share |
71.94 |
|
44.77 |
|
82.59 |
|
66.02 |
|
121.36 |
Year-end share market price |
2,463.00 |
|
2,630.00 |
|
2,941.00 |
|
2,155.00 |
|
1,492.50 |
Price / book value |
8.52 |
|
7.85 |
|
7.11 |
|
4.50 |
|
2.68 |
Shareholders' equity per share |
289.14 |
|
334.95 |
|
413.36 |
|
478.94 |
|
556.02 |
Equity ratio |
90% |
|
90% |
|
91% |
|
90% |
|
80% |
Shares outstanding |
65,545,748 |
|
65,718,456 |
|
65,961,573 |
|
66,074,535 |
|
66,187,186 |
Average number of employees (FTE)* |
656 |
|
1,022 |
|
1,460 |
|
2,011 |
|
2,535 |
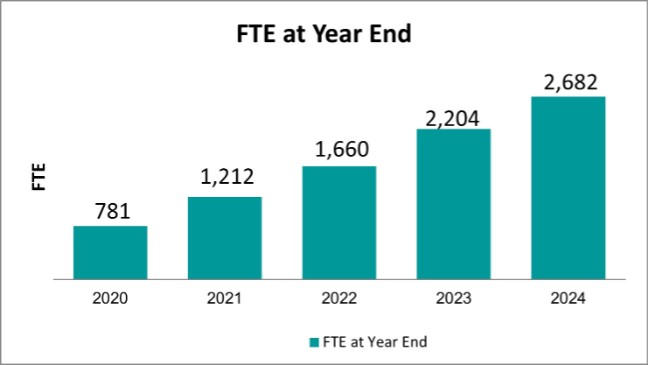
Number of employees (FTE) at year-end |
781 |
|
1,212 |
|
1,660 |
|
2,204 |
|
2,682 |
* Full-time equivalent (FTE) or team member

|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 8/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 9/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
2025 Outlook
2025 Outlook is presented in USD as management has determined it is appropriate to change the functional currency of Genmab A/S and the presentation currency to USD effective January 1, 2025. Refer to note 5.8 for additional details regarding the change in functional and presentation currency.
(USD millions) |
2024 Actual Result |
2025 Guidance |
2025 Guidance Mid-Point |
2024 Growth % |
2025 Growth %* |
Revenue |
3,124 |
3,340 - 3,660 |
3,500 |
31% |
12% |
Royalties |
2,518 |
2,785 - 3,015 |
2,900 |
27% |
15% |
Net product sales/Collaboration revenue** |
316 |
415 - 460 |
438 |
199% |
39% |
Milestones/Reimbursement revenue |
290 |
140 - 185 |
162 |
-2% |
-44% |
Gross profit |
2,981 |
3,120 - 3,420 |
3,270 |
26% |
10% |
Operating expenses |
(2,008) |
(2,055) - (2,225) |
(2,140) |
27% |
7% |
Operating profit |
973 |
895 - 1,365 |
1,130 |
26% |
16% |
*Mid-point of guidance range
**Net product sales and collaboration revenue consists of EPKINLY net product sales in the U.S. and Japan, and Tivdak (Genmab's share of gross profits).
Revenue
Genmab expects its 2025 revenue to be in the range of USD 3.3 – 3.7 billion, compared to USD 3.1 billion in 2024.
Genmab’s projected revenue growth for 2025 is driven by higher royalties, net product sales and collaboration revenue. Royalty growth relates mainly to DARZALEX and Kesimpta net sales growth. Net product sales and collaboration revenue growth is driven by strong performance for both EPKINLY and Tivdak. Net product sales and collaboration revenue consists of EPKINLY net product sales in the U.S. and Japan, and Tivdak (50% gross profit share).
Genmab’s projected revenue for 2025 primarily consists of DARZALEX royalties of approximately USD 2.2 billion at the midpoint. Such royalties are based on estimated DARZALEX 2025 net sales of USD 12.6 – 13.4 billion compared to actual net sales in 2024 of USD 11.7 billion. DARZALEX royalties are partly offset by Genmab’s share of J&J’s royalty payments to Halozyme Therapeutics, Inc. (Halozyme) in connection with SC net sales as well as royalty reduction in countries and territories where there is no Genmab patent coverage.
The remainder of Genmab’s revenue consists of royalties from Kesimpta, TEPEZZA, RYBREVANT, TECVAYLI, TALVEY and TEPKINLY, net product sales and collaboration revenue from EPKINLY and Tivdak, reimbursement revenue and milestones.
Operating Expenses
Genmab anticipates its 2025 operating expenses to be in the range of USD 2.1 – 2.2 billion, compared to USD 2.0 billion in 2024. The increase in operating expenses is primarily related to investments in late-stage programs and launch readiness in key markets.
Operating Profit
Genmab expects its 2025 operating profit to be in the range of USD 0.9 – 1.4 billion, compared to USD ~1.0 billion.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 10/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Outlook: Risks and Assumptions
In addition to factors already mentioned, the estimates above are subject to change due to numerous reasons, including but not limited to: the achievement of certain milestones associated with Genmab’s collaboration agreements; the timing and variation of development activities (including activities carried out by Genmab’s collaboration partners) and related income and costs; DARZALEX, DARZALEX FASPRO, Kesimpta, TEPEZZA, RYBREVANT, TECVAYLI, TALVEY and TEPKINLY net sales and royalties paid to Genmab; changing rates of inflation; and currency exchange rates (the 2025 guidance assumes a USD / DKK exchange rate of 7.2). The financial guidance assumes that no significant new agreements are entered into during 2025 that could materially affect the results.
The factors discussed above, as well as other factors that are currently unforeseeable, may result in further material adverse impacts on Genmab’s business and financial performance, including unfavorable impacts on the sales of Tivdak and EPKINLY/TEPKINLY, and on the net sales of DARZALEX, Kesimpta, TEPEZZA, RYBREVANT, TECVAYLI, and TALVEY by Genmab’s collaboration partners and on Genmab’s royalties, collaboration revenue and milestone revenue therefrom.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 11/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Our Strategy | |||
Business Strategy |
Priorities in 2024 |
Priorities for 2025 |
Related Risk |
|
Build a profitable and successful biotech ●
Maintain a flexible and capital-efficient model
●
Maximize relationships with partners
●
Retain ownership of select products
|
Invest in our people and culture ●
Further scale organization aligned with
differentiated antibody product portfolio growth and future launches Become a leading integrated biotech innovation powerhouse ●
Use solid financial base to grow and broaden antibody product and technology portfolio
|
Focus investments to optimize and enable growth strategy |
Please refer to the risks included in this Annual Report. |
|
Focus on core competence ●
Identify the best disease targets
●
Develop unique first-in-class or best-in-class antibodies
●
Develop next-generation technologies
|
Build world-class differentiated pipeline ●
Acasunlimab (GEN1046/BNT311, DuoBody-PD-L1x4-1BB)
o
Initiate Phase 3 study (2L NSCLC)
●
GEN1042 (DuoBody-CD40x4-1BB)1
o
Phase 2 data and determine next steps
●
Expand and advance proprietary
product portfolio |
Expand our pipeline through organic and inorganic opportunities |
Please refer to the risks included in this Annual Report. |
|
Turn science into medicine ●
Create differentiated antibody therapeutics with significant commercial potential
|
Bring our own medicines to patients & expand our markets ●
EPKINLY2
o
Initiate three Phase 3 trials
o
Expand label to include relapsed/refractory FL
●
Tivdak3
o
Initiate Phase 3 study in head and neck
●
Execute successful launches and growth in key markets
|
Advance mid-to-late-stage pipeline assets: epcoritamab, Rina-S, acasunlimab |
Please refer to the risks included in this Annual Report. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 12/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Sustainability Strategy |
Priorities in 2024 |
Priorities for 2025 |
Related Risk |
We are committed to embedding sustainability in our business operations with a focus on reducing our carbon footprint, upholding responsibility towards our people, patients and society while maintaining high standards of corporate governance |
●
Continue to grow our commitment to being a sustainable and responsible company
●
Ensure that policies and procedures are implemented in alignment with ESG-related reporting requirements, while continuing to monitor the regulatory landscape
●
Collaborate internally to integrate ESG into our strategic planning, business operations and risk management processes
●
Continue to develop and deliver treatments to improve lives of patients
●
Minimize our carbon footprint and map our Greenhouse Gas (GHG) emissions
●
Promote the Company’s efforts to attract, retain, motivate and recognize diverse, world-class talent
●
Invest in Diversity, Equity and Inclusion processes and efforts, which is critical to our future growth
|
General ●
Advance sustainability commitments by integrating action plans around material impacts, risks and opportunities into our business operations
●
Launch sustainability awareness training for all team members
Environmental ●
Focus on the continuous update and execution of action plans to achieve near and long-term emission reduction targets
Social ●
Continue to pursue science and innovation with the potential to improve patients’ lives through our medicines and facilitate access to these medicines
●
Continue efforts to promote employee well-being & vitality
●
Maintain training and skills development opportunities for all team members
●
Support our future business needs by attracting, retaining, developing, recognizing and motivating a diverse and talented team
Governance ●
Reasonably ensure compliance with regulatory reporting requirements in a transparent manner
●
Ensure strong governance by engaging key stakeholders, including the Board and its Committees, CSR & Sustainability Committee, senior leaders, employees and suppliers
|
Please refer to the risks included in this Annual Report. |
1. Co-development with BioNTech; 2. Co-development with AbbVie; 3. Co-development with Pfizer
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 13/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Who We Are
Our Core Values
In our quest to turn science into medicine, we use these guideposts to transform the future of cancer treatment:
| ● | Passion for innovation |
| ● | Determination — being the best at what we do |
| ● | Integrity — we do the right thing |
| ● | We work as one team and respect each other |
Genmab’s Growing Organization and Presence
| ● | Copenhagen, Denmark |
| o | Headquarters |
| o | Chemistry, Manufacturing and Controls (CMC) Operations |
| o | Development Operations |
| o | Quality Control (QC) Laboratory |
| o | Corporate Functions |
| ● | Utrecht, The Netherlands |
| o | Discovery and Antibody Research |
| o | Translational and Quantitative Sciences |
| o | Development Operations |
| o | Corporate Functions |
| ● | Princeton, U.S. |
| o | Translational and Quantitative Sciences |
| o | Development Operations |
| o | U.S. Market Operations |
| o | Corporate Functions |
| ● | Tokyo, Japan |
| o | Development Operations |
| o | Japan Market Operations |
| ● | Suzhou and Shanghai, China |
| o | Early-stage R&D |
| o | CMC Operations |
Our Key Accomplishments
Each of our achievements stands as evidence of our unyielding determination, including:
| ● | Two Genmab co-owned medicines on the market: Tivdak with Pfizer and EPKINLY/TEPKINLY with AbbVie |
| ● | Six additional medicines that were created by Genmab, or that leverage Genmab’s DuoBody technology, are being developed and marketed by global pharmaceutical and biotechnology companies |
| ● | Late-stage pipeline with high potential: EPKINLY, Rina-S and acasunlimab |
| ● | Suite of proprietary antibody technologies including bispecifics and ADC platform technologies fueling future innovations |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 14/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| ● | Robust clinical and preclinical pipeline fueling future growth |
| ● | Over 45 Investigational New Drugs (IND) filed by Genmab and/or partners, based on Genmab’s innovations and technology, since 1999 |
| ● | Industry-leading team with antibody know-how, and expertise in R&D and commercial fields |
| ● | Partnerships with industry leaders and innovators across the innovation ecosystem of pharma, biotech and academia |
| ● | Partnership with ChatGPT to launch “AI Everywhere,” providing ChatGPT access to more than 2,000 colleagues |
| ● | Solid financial foundation enabling our evolution to a fully integrated biotech |
| ● | Building and expanding our capabilities with more than 2,700 team members across our international locations and through the acquisition of ProfoundBio in 2024 |
Business Model
At Genmab, we have built a profitable and successful biotech that creates value for our stakeholders.
Our Strengths and Differentiators
| ● | World-class antibody biology knowledge and insight into disease targets |
| ● | Discovery and development engine with proprietary technologies that allow us to build a differentiated pipeline |
| ● | In-house expertise with a solid track record of building successful strategic partnerships |
| ● | Pipeline of potential best-in-class and/or first-in-class therapies |
| ● | Experienced, diverse leadership team |
Building a Fully Integrated Biotech Innovation Powerhouse
| ● | Team: deeply driven One Genmab team rooted in science and inspired by patients |
| ● | Translational and precision medicine, data science and artificial intelligence (AI): key to accelerating development and ensuring the right therapies get to the right patients |
| ● | Collaboration: reaches across the innovation ecosystem of pharma, biotech and academia, and drives our business forward |
| ● | Strong financials: growing recurring revenues and prioritization and focused investments |
Research: track record of success and investing for tomorrow
Development: scaling up capabilities to expand from early- to late-stage development
Technical Operations and Commercialization: the next step in our evolution
Enabling functions: supporting growth and managing risk
Value Chain
Genmab’s value chain is a comprehensive and integrated process that spans from early-stage R&D to the global commercialization of antibody-based therapies. Each step is designed to maximize innovation, ensure product quality, and optimize market reach, all while leveraging strategic partnerships to enhance efficiency and profitability.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 15/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Below is a breakdown of the key components as well as the key stakeholders relevant to each value chain component:
1. Research and Discovery
Target Identification: The first step in Genmab’s value chain involves identifying disease-related targets, particularly in oncology. This includes research into the biological mechanisms underlying diseases and identifying targets that can be addressed with antibody therapies.
Antibody Engineering: Genmab employs its proprietary technologies, such as DuoBody, HexaBody and its ADC platforms, to engineer and develop novel antibodies. These technologies allow for the creation of bispecific antibodies (capable of targeting two different antigens), enhanced antibody functionality and ADCs (antibodies with potent cytotoxic agents coupled to them).
2. Preclinical Development
Preclinical Development: Genmab conducts extensive preclinical testing to evaluate the efficacy, safety, and potential of these antibodies before advancing them to clinical trials.
3. Clinical Development
Clinical Trials: Genmab advances its antibody candidates through various phases of clinical trials (Phase 1, 2, and 3). This involves testing the candidates in human patients to assess safety, dosage, and efficacy.
Strategic Partnerships: During clinical development, Genmab may decide to partner with pharmaceutical or biotechnology companies to co-develop and co-fund the trials. These partnerships help mitigate risk and share the costs of development.
4. Manufacturing
Process Development: Genmab focuses on developing scalable and efficient manufacturing processes for its antibody products. Genmab does not currently own or operate large-scale manufacturing facilities but works closely with contract manufacturing organizations (CMOs) and partners to ensure high-quality production.
QC: Ensuring the consistency, safety, and quality of our products is a key part of the manufacturing process. Genmab implements strict quality control measures throughout production.
5. Registration and Launch
Regulatory Approval: Following successful clinical trials, Genmab seeks regulatory approval from authorities like the European Medicines Agency (EMA) (Europe), the U.S. FDA (U.S.) and MHLW (Japan), and other regulatory bodies. This step is crucial for bringing the investigational medicine to market.
6. Commercialization
Marketing and Sales: Once a product receives regulatory approval, Genmab, often through a variety of arrangements, markets and sells the approved medicine. This step includes establishing market strategies
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 16/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
including market access, engaging with payors, educating healthcare providers, and launching the product in various regions.
Distribution: Genmab collaborates with distribution networks, either through partners or on its own, to ensure the approved medicine reaches healthcare providers and patients in the approved jurisdictions.
Pharmacovigilance: After launch, Genmab monitors the product's performance in the market, collecting data on its safety and effectiveness in broader patient populations.
7. Partnerships and Alliances
Licensing: Genmab licenses its proprietary technologies (like the DuoBody and HexaBody platforms) to other biotech and pharmaceutical companies. This generates revenue through upfront payments, milestones, and royalties.
Business Development: Genmab’s Business Development team plays a crucial role in the value chain, particularly in bridging the gap between R&D and commercial success by identifying opportunities, helping form strategic alliances, and assisting in in-licensing and out-licensing business.
Co-Development: Genmab often enters into co-development agreements where the costs, risks, and profits are shared with partners. This collaboration is particularly important during clinical development and commercialization phases.
Strategic Alliances: Beyond traditional partnerships, Genmab engages in strategic alliances with research institutions and other biotech companies to access complementary technologies, expand its pipeline, and explore new therapeutic areas.
Refer to section SBM-2 in the sustainability statements for details of key stakeholders including a mapping to Genmab’s value chain.
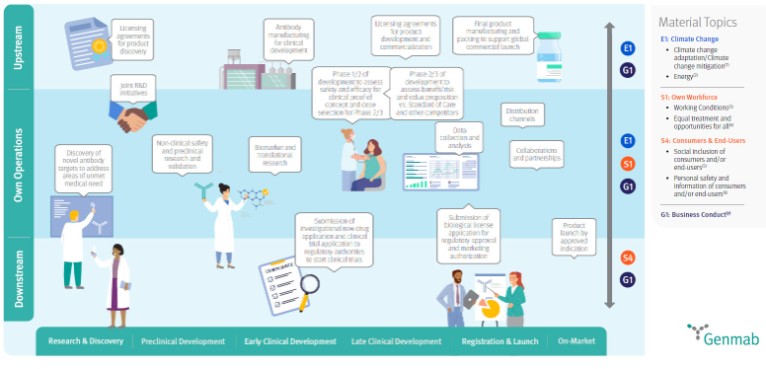
Refer to the sustainability statements within this Annual Report for material topics identified as part of Genmab’s DMA. The visual maps the material topics to Genmab’s value chain. Refer to the Who We Are section for Genmab’s presence.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 17/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Research and Development Capabilities
Inspired by Nature
At Genmab, we are inspired by nature and understand how antibodies work. We are deeply knowledgeable about antibody biology and our scientists harness this expertise to create and develop differentiated investigational antibody medicines. We utilize a sophisticated and highly automated process to efficiently generate, select, produce, and evaluate antibody-based products. Our teams have established a fully integrated R&D enterprise and streamlined process to coordinate the activities of antibody product discovery, preclinical testing, manufacturing, clinical trial design and execution, and regulatory submissions across Genmab’s international operations. We have expanded our scientific focus to use data science and artificial intelligence to aid in the discovery of new targets and biomarkers and bolster our in-depth precision medicine and translational laboratory capabilities. Through our expertise in antibody drug development, we pioneer technologies that allow us to create differentiated and potentially first-in-class or best-in-class investigational medicines with the potential to improve patients’ lives. Our antibody expertise has enabled us to create our cutting-edge technology platforms: DuoBody, HexaBody, DuoHexaBody® and HexElect®. With our acquisition of ProfoundBio we gained novel ADC technology platforms. Additional information about our technologies is available on Genmab’s website, www.genmab.com/antibody-science/antibody-technology-platforms.
Sustainable and State-of-the-Art Facilities
The Netherlands
Genmab’s presence in the Netherlands is composed of three buildings in the Utrecht area: The Genmab Research and Development Center (GRDC) and the Accelerator at the Utrecht Science Park and a Genmab office in nearby Zeist. Discovery and preclinical research is conducted at our GRDC and Accelerator facilities, which house state-of-the-art laboratories, including a new chemistry lab that opened at the GRDC in 2024. The GRDC was one of the first Building Research Establishment Environmental Assessment Method (BREEAM) Excellent laboratory buildings in the Netherlands. The Accelerator, a multi-tenant ultra-modern R&D facility, was opened in 2023, enabling our continued R&D growth trajectory. These three spaces are located in close proximity to other life science companies and a premier research university. They accommodate modern auditoriums, and innovative brainstorming and meeting rooms. They provide a bright, open, and collaborative atmosphere and enable the Genmab team to continue to innovate and find new ways to help patients.
Denmark
Denmark, with its rich history of scientific achievement and innovation, has been our home for Genmab's headquarters for 25 years. We are surrounded by a vibrant ecosystem of talent, with multiple biotech and pharma peers, academia and research centers, knowledge, and resources. Genmab opened our new headquarters in Valby, Denmark in 2023, a space designed specifically for Genmab. In addition, Genmab introduced our own Good Manufacturing Practice (GMP) QC laboratory in 2023. The new space insources certain business-critical processes and capabilities for our early clinical development. With our growing pipeline and commercial ambitions, we are taking control of processes, prioritization, people, and timing and taking another tremendous step toward becoming an end-to-end biotech innovation powerhouse.
United States
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 18/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Genmab opened our new U.S. facility in 2020. This space, modeled on the open and collaborative spirit of the R&D labs and offices in Utrecht and Zeist, includes both offices and laboratories. The U.S. precision medicine laboratories allow Genmab to expand our clinical and preclinical drug development expertise and are part of the strategic growth of the Company. As with our Utrecht facilities, our U.S. office and laboratories were designed and built with sustainability in mind and meet the requirements for Leadership in Energy and Environmental Design (LEED) Gold certification for sustainable design features.
Japan
Genmab’s Japan office is located in Roppongi, an international business district in the center of Tokyo. It offers an open and collaborative environment that fosters Genmab’s culture of innovation and teamwork.
China
As part of our acquisition of ProfoundBio, Genmab expanded our presence to our newest locations with state-of-the-art research and CMC capabilities in Suzhou, China.
As Genmab continues to grow our geographical footprint, we will endeavor to do so with minimal impact to the environment and with a focus on sustainable practices.
Bringing Our Own Innovative Medicines to Patients
We are creating lasting impact as we bring our medicines to more patients around the world.
We are making continuous progress in our ambition to become a fully-integrated, end-to-end biotech, which includes advancing the development of our fully owned medicines and delivering them to patients around the world.
Building on our legacy of innovation and our patient-first approach, we have successfully launched two therapies through partnerships. This year, we expanded the reach of these medicines to additional geographies and patient populations while also progressing the development of wholly-owned, late-stage investigational products.
Expanding the Potential of Bispecifics in Lymphoma
In June 2024, the U.S. FDA approved a second indication for EPKINLY (epcoritamab-bysp), specifically for the treatment of adults with relapsed or refractory FL after two or more lines of systemic therapy. With this approval, EPKINLY became the first and only T-cell engaging bispecific antibody administered subcutaneously approved in the U.S. to treat this patient population.
Subsequently in August 2024, epcoritamab, under the brand name TEPKINLY, became the first subcutaneous bispecific antibody approved as a monotherapy in the EU to treat relapsed or refractory FL after two or more lines of systemic therapy.
Additionally in September 2024, the U.S. FDA granted a second breakthrough therapy designation for EPKINLY in relapsed or refractory FL when administered in combination with rituximab and lenalidomide.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 19/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
In Japan, a supplemental J-NDA was submitted to the MHLW for EPKINLY for the treatment of patients with third line plus relapsed or refractory FL grade 1-3A.
Follicular lymphoma is the second most common form of non-Hodgkin’s lymphoma (NHL). It is considered incurable, and historically, patients may relapse or face a shortened duration of response with prior standards of care.
Since 2023, EPKINLY has received regulatory approvals in more than 50 countries. It was the first bispecific approved in the world with a dual indication in third line plus relapsed or refractory DLBCL and third line plus relapsed or refractory FL. In the U.S., it continues to be the only bispecific with this dual indication. In Japan, it remains the first and only T-cell engaging bispecific antibody administered subcutaneously approved for the treatment of adults with relapsed or refractory large B-cell lymphoma (LBCL) or grade 3B FL. EPKINLY/TEPKINLY is developed and commercialized in partnership with AbbVie.
Driving Progress in Cervical Cancer Care
Advanced cervical cancer remains a disease with high medical need. In the U.S., up to 16% of cervical cancer cases are diagnosed at the metastatic stage, and up to 61% of earlier-stage diagnoses progress to metastatic disease. Globally, cervical cancer is the fourth most common cause of cancer death in women.
In April 2024, the U.S. FDA approved the supplemental Biologics License Application (sBLA) granting approval for Tivdak (tisotumab vedotin-tftv), which we market in the U.S. in partnership with Pfizer. This U.S. FDA action converted the September 2021 accelerated approval of Tivdak to a full approval. Tivdak remains the first and only approved ADC for the treatment of patients with recurrent or metastatic cervical cancer in the U.S.
In Japan, the incidence and mortality rates for cervical cancer have been increasing, attributable in part to low vaccination rates for the human papillomavirus (HPV) (<5%) and regular checkups (40%). In April 2024, a J-NDA was submitted to the MHLW for Tivdak for the treatment of advanced or recurrent cervical cancer with disease progression after chemotherapy. Currently, patients whose disease progresses or recurs following initial treatment face limited options. If approved, Tivdak could provide renewed hope to the cervical cancer community.
Creating Community with CeMe™
The CeMe™ campaign in the U.S., created in partnership with Pfizer, builds community and a sense of belonging among those impacted by cervical cancer.
“After the treatment, I didn’t know what to do next,” said Anna Ogo. “That’s about when I found a community of women who had experienced cervical cancer like me, and they helped me tremendously. With their support, I began to find confidence in sharing my story.”
www.youtube.com/cemestories
Ensuring Rapid and Sustainable Access to Medicines
We are focused in our pursuit to turn innovative science into medicines that create value and deliver meaningful impact to patients and health systems.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 20/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Ultimately, we positively impact the lives of people with cancer when our science becomes medicine, our medicine creates value, and the value of our medicine is realized by patients who can benefit. Patient access and affordability are key components of this.
Our Approach to Value, Access, and Pricing
| ● | Value: The value of our medicines is driven by our innovative science. |
| ● | Access: Patient impact happens when our medicines reach the people who need them and help them live better. |
| ● | Pricing: The price of our medicines reflects the innovation behind our science, its impact on patients, and our commitment to bringing that science to patients. |
We aim to ensure that the price of our medicines allows patients, regardless of their socioeconomic or insurance status, to have timely access while considering the transformational potential of our science, its benefit to healthcare systems and society, and our ability to invest in the breakthrough science of the future.
At the same time, together with our partners, we work with local country regulatory and payer authorities in the U.S., Japan, and throughout Europe to facilitate registration and reimbursement to help enable patient access to our medicines around the world.
We recognize that true patient impact happens when our medicines reach the patients who need them. In the U.S., MyNavCare™ Patient Support by Genmab was created to offer support services to patients prescribed Genmab medicines to help them navigate each step of their unique treatment journey
MyNavCare
MyNavCare offers a range of resources and services, from financial assistance to ongoing support, to help patients access the Genmab medication they need.
Part of this can include ongoing support from Patient Engagement Liaisons – such as Ann Fodrey. During a hurricane in 2024, one of the patients Ann supports was impacted by the storm and the roof of his home was heavily damaged. Fodrey provided consistent support, checking in to make sure the patient, a retired military veteran, was able to safely get to his treatment and also connected him with a veteran’s organization that was able to install a temporary roof on his house.
“Every day we do small things like this. It doesn’t have to be pointing them to an organization that can provide the support they need. It could be connecting them with a peer-to-peer resource, so they have somebody that’s going through a similar experience – listening to them,” Fodrey said. “And that’s just as important.”
www.mynavcare.com
Antibody Discovery and Development
We are experts in antibody discovery and development. Our appreciation for, and understanding of, the power of the human immune system gives us a unique perspective on how to respond to the constant challenges of oncology drug development. We entered a new chapter in Genmab’s evolution with the commercialization and launch of our first medicine, Tivdak, co-owned with Pfizer, in 2021, and we successfully launched our second medicine, EPKINLY/TEPKINLY, in 2023 under our collaboration with
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 21/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
AbbVie. As part of our transformation to a fully integrated biotech, at the end of 2024, we also had two wholly owned programs in Phase 3 development, Rina-S and acasunlimab.
Products and Technologies
Pipeline
At the end of 2024, Genmab’s proprietary pipeline of investigational medicines, where we are responsible for at least 50% of development, consisted of twelve antibody products in clinical development. These include Genmab’s approved medicines, Tivdak, developed and commercialized together with Pfizer, and EPKINLY/TEPKINLY, developed and commercialized together with AbbVie. In addition to our own pipeline, there are multiple investigational medicines in development by global pharmaceutical and biotechnology companies, including six approved medicines powered by Genmab’s technology and innovations. Beyond the investigational medicines in clinical development, our pipeline includes multiple preclinical programs. An overview of the development status of our approved medicines and of each of our investigational medicines is provided in the following sections. Detailed descriptions of dosing and efficacy and safety data from certain clinical trials have been disclosed in company announcements and media releases published via the Nasdaq Copenhagen stock exchange and may also be found in Genmab’s filings with the U.S. Securities and Exchange Commission (SEC). Additional information is available on Genmab’s website, www.genmab.com. The information accessible through our website is not part of and is not incorporated by reference herein.
Genmab’s Proprietary1 Products
Approved Medicines
Approved Product |
Target |
Developed By |
Disease Indication(s)2 |
|
EPKINLY (epcoritamab-bysp, epcoritamab) TEPKINLY (epcoritamab) |
CD3xCD20 |
Co-development Genmab/AbbVie |
Approved in multiple territories including the U.S. and Europe for adult patients with relapsed or refractory DLBCL after two or more lines of systemic therapy and in Japan for adult patients with certain types of relapsed or refractory LBCL after two or more lines of systemic therapy |
|
|
|
Approved in multiple territories including the U.S. and Europe for adult patients with relapsed or refractory FL after two or more lines of systemic therapy |
|
Tivdak (tisotumab vedotin-tftv) |
TF |
Co-development Genmab/Pfizer |
Approved in the U.S. for adult patients with recurrent/metastatic cervical cancer with disease progression on or after chemotherapy |
1Approved and investigational medicines where Genmab has ≥50% ownership, in co-development with partners as indicated.
2Refer to local country prescribing information for precise indication and safety information.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 22/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Pipeline, Including Further Development for Approved Medicines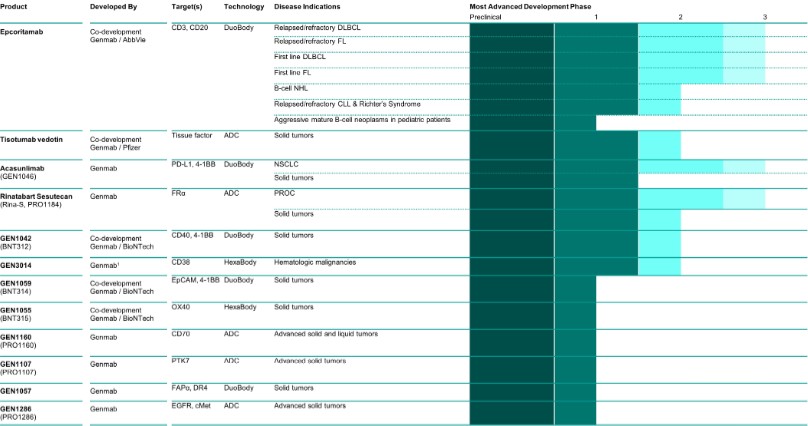
1Genmab is developing HexaBody-CD38 in an exclusive worldwide license and option agreement with J&J.
CLL = chronic lymphocytic leukemia
In 2024, Genmab discontinued the GEN1047 (DuoBody-CD3xB7H4), GEN3017 (DuoBody-CD3xCD30) and GEN1056 (with BioNTech, BNT322) programs following a strategic re-evaluation of Genmab’s portfolio. For similar reasons, Genmab and BioNTech took the decision to discontinue the clinical development of GEN1053 (HexaBody-CD27, BNT313) including the Phase 1/2 clinical trial (NCT05435339) in solid tumors.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 23/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Programs Incorporating Genmab’s Innovation and Technology1
Approved Medicines
Approved Product |
Discovered and/or Developed & Marketed By |
Disease Indication(s)2 |
|
DARZALEX (daratumumab)/DARZALEX FASPRO (daratumumab and hyaluronidase-fihj) |
J&J (Royalties to Genmab on net global sales) |
Multiple myeloma |
Light-chain (AL) Amyloidosis |
||
|
Kesimpta (ofatumumab) |
Novartis (Royalties to Genmab on net global sales) |
Relapsing multiple sclerosis (RMS) |
|
TEPEZZA (teprotumumab-trbw) |
Amgen (under sublicense from Roche, royalties to Genmab on net global sales) |
TED |
|
RYBREVANT (amivantamab/amivantamab-vmjw) |
J&J (Royalties to Genmab on net global sales) |
NSCLC |
|
TECVAYLI (teclistamab/teclistamab-cqyv) |
J&J (Royalties to Genmab on net global sales) |
Relapsed and refractory multiple myeloma |
|
TALVEY (talquetamab/talquetamab-tgvs) |
J&J (Royalties to Genmab on net global sales) |
Relapsed and refractory multiple myeloma |
1Approved and investigational medicines created by Genmab or created by collaboration partners leveraging Genmab’s DuoBody technology platform, under development, and where relevant, commercialized by a third party.
2See local prescribing information for precise indication and safety information.
Pipeline, Including Further Development for Approved Medicines, ≥Phase 2 Development
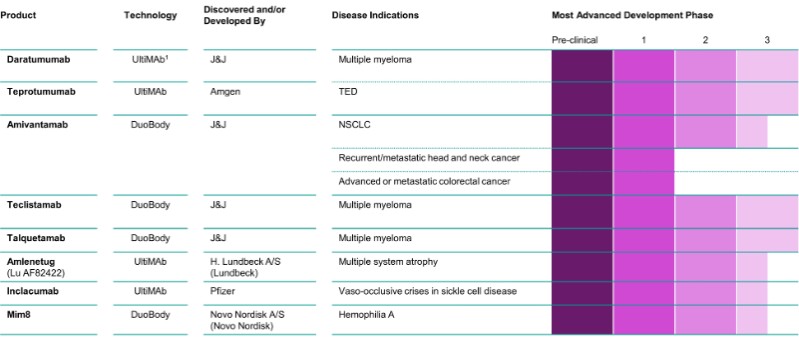
1UltiMab transgenic mouse technology licensed from Medarex, Inc. (Medarex), a wholly owned subsidiary of Bristol-Myers Squibb.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 24/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Genmab’s Proprietary Pipeline
Programs where Genmab has ≥50% ownership.
EPKINLY/TEPKINLY (epcoritamab)
First and only bispecific antibody approved in the U.S. and Europe to treat both relapsed or refractory FL and DLBCL after two or more lines of systemic therapy
| ● | SC bispecific antibody targeting CD3 and CD20 created using Genmab’s DuoBody technology platform |
| ● | Epcoritamab (approved as EPKINLY and TEPKINLY) has received regulatory approvals in multiple territories including in the U.S. and Europe for adult patients with relapsed or refractory DLBCL after two or more lines of systemic therapy, and in Japan for adult patients with certain types of relapsed or refractory LBCL after two or more lines of systemic therapy |
| ● | EPKINLY/TEPKINLY have also been approved in the U.S. and Europe for the treatment of adults with relapsed or refractory FL after two or more lines of systemic therapy. Regulatory submission for epcoritamab for this indication is currently under review in Japan |
| ● | Multiple clinical trials are ongoing across different settings and histologies, including five Phase 3 trials, with more trials in planning |
| ● | Co-developed and co-commercialized in collaboration with AbbVie |
Epcoritamab is a proprietary bispecific antibody created using Genmab’s DuoBody technology platform. Epcoritamab targets CD3, which is expressed on T-cells, and CD20, a clinically validated target on malignant B-cells. Genmab used technology licensed from Medarex to generate the CD20 antibody forming part of epcoritamab. Epcoritamab is marketed as EPKINLY in the U.S., Japan, and other regions, and as TEPKINLY in Europe and other regions. See local prescribing information for specific indications and safety information. In 2020, Genmab entered into a collaboration agreement with AbbVie to jointly develop and commercialize epcoritamab. The companies share commercialization responsibilities in the U.S. and Japan, with AbbVie responsible for further global commercialization. Genmab records sales in the U.S. and Japan and receives tiered royalties between 22% and 26% on remaining global sales outside of these territories, subject to certain royalty reductions. The companies have a broad clinical development program for epcoritamab including five ongoing Phase 3 trials and additional trials in planning. Please refer to Note 5.6 of the financial statements for further details regarding the epcoritamab collaboration with AbbVie. Please consult the U.S. Prescribing Information for EPKINLY and the European Summary of Product Characteristics for TEPKINLY for the labeled indication and safety information.
FOURTH QUARTER UPDATE
| ● | December: Multiple presentations at the 66th American Society of Hematology (ASH) Annual Meeting, including four oral presentations. Data from the EPCORE® CLL-1 trial of epcoritamab monotherapy in patients with relapsed or refractory CLL was selected for presentation during the prestigious ASH Annual Meeting Press Program. |
UPDATES FROM FIRST QUARTER TO THIRD QUARTER
| ● | September: The U.S. FDA granted a Breakthrough Therapy Designation (BTD) for epcoritamab in combination with rituximab and lenalidomide for the treatment of adult patients with relapsed or refractory FL after at least one line of systemic therapy. This is the second BTD granted for epcoritamab. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 25/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| ● | August: The European Commission (EC) granted conditional marketing authorization for TEPKINLY (epcoritamab) as a monotherapy for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy. The conditional marketing authorization was supported by data from the FL cohort of the EPCORE NHL-1 trial (NCT03625037). |
| ● | June: The U.S. FDA approved EPKINLY (epcoritamab-bysp) for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy. The approval was supported by data from the FL cohort of the EPCORE NHL-1 trial. |
| ● | June: The EMA’s Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending the granting of conditional marketing authorization of epcoritamab for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy. |
| ● | June: Multiple data presentations were featured at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting including two rapid oral presentations. These presentations included data from the pivotal and cycle 1 dose optimization FL cohorts of the EPCORE NHL-1 clinical trial, which was subsequently selected for presentation at the Best of ASCO conference. |
| ● | June: Multiple data presentations were featured at the 2024 European Hematology Association (EHA) Congress including three oral presentations. |
| ● | June: Data published in The Lancet Haematology, “Epcoritamab monotherapy in patients with relapsed or refractory follicular lymphoma (EPCORE NHL-1): a phase 2 cohort of a single-arm, multicentre study.” |
| ● | May: Epcoritamab monotherapy was added to the National Comprehensive Cancer Network® (NCCN®) Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for “B-cell Lymphomas” (Version 2.2024) for third-line and subsequent therapy for patients with FL as a Category 2A, preferred regimen. |
| ● | March: Genmab submitted a supplemental J-NDA to the MHLW in Japan for SC epcoritamab for the treatment of relapsed or refractory FL after two or more lines of systemic therapy. The application was supported by data from the FL cohort of the EPCORE NHL-1 trial and the EPCORE NHL-3 (NCT04542824) trial. |
| ● | February: The U.S. FDA granted Priority Review for the sBLA for epcoritamab-bysp for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy. The submission was supported by data from the FL cohort of the EPCORE NHL-1 trial. |
| ● | February: A new Phase 3 clinical trial was initiated, evaluating the safety and efficacy of SC epcoritamab in combination with rituximab and lenalidomide compared to chemoimmunotherapy in previously untreated FL (EPCORE FL-2, NCT06191744). |
| ● | February: Multiple data presentations, including oral presentations, at the 21st Annual Meeting of the Japanese Society of Medical Oncology (JSMO). |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 26/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
ONGOING CLINICAL TRIALS

R-CHOP = rituximab-cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone; ASCT = autologous stem cell transplant; R2 = rituximab, lenalidomide
About Diffuse Large B-cell Lymphoma
DLBCL is the most common type of B-cell NHL worldwide, accounting for approximately 25%-30% of all NHL cases.1,2 In the U.S. there are approximately 25,000 new cases of DLBCL diagnosed each year. 3 DLBCL can arise in lymph nodes as well as in organs outside of the lymphatic system, occurs more commonly in the elderly and is slightly more prevalent in men.4,5 DLBCL is a fast-growing type of NHL, a cancer that develops in the lymphatic system and affects B-cell lymphocytes, a type of white blood cell. For many people living with DLBCL, their cancer either relapses, which means it may return after treatment, or becomes refractory, meaning it does not respond to treatment. Although new therapies have become available, treatment management can remain a challenge.6,7
1. Lymphoma Research Foundation. Diffuse Large B-Cell Lymphoma. Accessed November 22, 2024. https://lymphoma.org/understanding-lymphoma/aboutlymphoma/nhl/dlbcl/
2. Padala, et al. Diffuse Large B-Cell Lymphoma. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. 2023 Apr 24.
3. Leukemia and Lymphoma Society. Diffuse Large B-Cell Lymphoma (DLBCL). Accessed November 22, 2024. https://www.lls.org/research/diffuse-large-b-cell-lymphoma-dlbcl
4. Sehn LH, Salles G. N Engl J Med. 2021;384:842-858.
5. Kanas G, Ge W, Quek RGW, et al. Leukemia & Lymphoma. 2022;63(1):54-63.
6. Sehn LH, Salles G. N Engl J Med. 2021;384:842-858.
7. Crump M, Neelapu SS, Farooq U, et al. Blood. 2017;130(16):1800-1808.
About Follicular Lymphoma
FL is typically an indolent (or slow-growing) form of NHL that arises from B-lymphocytes and is the second most common form of NHL accounting for 20-30% of all cases.1 About 15,000 people develop FL each year in the U.S.2 and it is considered incurable with current standard of care therapies.3 Patients often relapse and, with each relapse the remission and time to next treatment is shorter.4 Over time, transformation to DLBCL,
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 27/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
an aggressive form of NHL associated with poor survival outcomes, can occur in more than 25% of FL patients.5
1. Lymphoma Research Foundation official website. https://lymphoma.org/aboutlymphoma/nhl/fl/. Accessed November 22, 2024.
2. Leukemia & Lymphoma Society. https://www.lls.org/research/follicular-lymphoma-fl. Accessed November 22, 2024.
3. Ghione P, Palomba ML, Ghesquieres H, et al. Treatment patterns and outcomes in relapsed/refractory follicular lymphoma: results from the international SCHOLAR-5 study. Haematologica. 2023;108(3):822-832. doi: 10.3324/haematol.2022.281421.
4. Rivas-Delgado A, Magnano L, Moreno-Velázquez M, et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol. 2018;184(5):753-759. doi:10.1111/bjh.15708.
5. Al-Tourah AJ, Gill KK, Chhanabhai M, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin's lymphoma. J Clin Oncol. 2008 Nov 10;26(32):5165-9. doi: 10.1200/JCO.2008.16.0283. Epub 2008 Oct 6. PMID: 18838711.
Tivdak (tisotumab vedotin-tftv)
First and Only U.S. FDA Approved ADC for Recurrent or Metastatic Cervical Cancer
| ● | An ADC directed to TF, a protein highly prevalent on solid tumors, including cervical cancer, which is associated with poor prognosis |
| ● | Full approval granted by the U.S. FDA for tisotumab vedotin-tftv, marketed as Tivdak, for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy; Tivdak is the first ADC with demonstrated overall survival data to be granted full U.S. FDA approval in this patient population |
| ● | Regulatory submissions for tisotumab vedotin for the treatment of recurrent or metastatic cervical cancer are currently under review in both Japan and Europe |
| ● | Co-developed globally and co-promoted in the U.S. in collaboration with Pfizer |
Tisotumab vedotin is an ADC composed of Genmab’s human monoclonal antibody directed to TF and Pfizer’s ADC technology that utilizes a protease-cleavable linker that covalently attaches the microtubule-disrupting agent monomethyl auristatin E to the antibody. Genmab used technology licensed from Medarex to generate the TF antibody forming part of tisotumab vedotin. Tisotumab vedotin-tftv, marketed as Tivdak, is the first and only U.S. FDA approved ADC for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy. Tisotumab vedotin is being co-developed by Genmab and Pfizer. Under a joint commercialization agreement, Genmab is co-promoting Tivdak in the U.S. and will lead commercial operational activities in Japan once approved. Pfizer is leading commercial operational activities in the U.S. and will lead commercial operational activities in China once approved. In the U.S. market there will be a 50:50 profit split. Effective January 1, 2025, Genmab and Pfizer agreed to amend the License and Collaboration Agreement and the Joint Commercialization Agreement for Tivdak, assigning Genmab sole responsibility for the development and commercialization of Tivdak for second line plus recurrent or metastatic cervical cancer in Europe and all other regions globally, excluding the United States and the China region. Genmab will record sales for Europe, Japan and rest of world markets (excluding the United States and the China region), once commercialized, and will provide royalties to Pfizer on net sales in the low teens. The companies have joint decision-making power on the worldwide development and commercialization strategy for Tivdak. Please refer to Note 5.6 of the financial statements for further details regarding the tisotumab vedotin collaboration with Pfizer. Please consult the U.S. Prescribing Information for Tivdak for the labeled indication and safety information, including the boxed warning.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 28/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
FOURTH QUARTER UPDATE
| ● | December: The U.S. NCCN updated its Clinical Practice Guidelines in Oncology for Cervical Cancer with tisotumab vedotin-tftv changing from a category 2A to a category 1. Tisotumab vedotin-tftv plus pembrolizumab was also added as an option for PD-L1 positive tumors. |
| ● | October: Upon strategic evaluation we have decided to discontinue preparation for a Phase 3 study in second/third line squamous cell carcinoma of the head and neck. |
UPDATES FROM FIRST QUARTER TO THIRD QUARTER
| ● | July: Data from innovaTV 301 clinical trial (NCT04697628) published in New England Journal of Medicine, Tisotumab Vedotin as Second- or Third-Line Therapy for Recurrent Cervical Cancer. |
| ● | June: Two data presentations were featured at the 2024 ASCO Annual Meeting including a rapid oral presentation of data from the Phase 2 innovaTV 207 (NCT03485209) trial, evaluating tisotumab vedotin in pretreated patients with relapsed/metastatic head and neck squamous cell carcinoma. |
| ● | April: Genmab submitted a J-NDA to the MHLW in Japan for Tivdak for the treatment of adult patients with advanced or recurrent cervical cancer that has progressed on or after chemotherapy. |
| ● | April: The U.S. FDA granted full approval for Tivdak (tisotumab vedotin-tftv) for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy. This U.S. FDA action converted the September 2021 accelerated approval of Tivdak to a full approval. |
| ● | March: The U.S. NCCN updated their Clinical Practice Guidelines in Oncology for Vaginal Cancer to include tisotumab vedotin-tftv under “Other Recommended Regimens” as second-line or subsequent systemic therapy for patients with recurrent or metastatic squamous cell carcinoma/adenocarcinoma primary vaginal cancer. |
| ● | March: Multiple data presentations, including a late-breaking oral presentation, at the Society of Gynecologic Oncology (SGO) 2024 Annual Meeting. |
| ● | February: The EMA validated for review the marketing authorization application of tisotumab vedotin for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after systemic therapy. |
| ● | January: The U.S. FDA accepted the sBLA seeking to convert the accelerated approval of Tivdak to full approval for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after first-line therapy. |
About Cervical Cancer
Cervical cancer remains a disease with high unmet need despite advances in effective vaccination and screening practices to prevent and diagnose pre-/early-stage cancers for curative treatment. Recurrent and/or metastatic cervical cancer is a particularly devastating and mostly incurable disease; up to 15% of adults with cervical cancer present with metastatic disease at diagnosis1,2 and, for adults diagnosed at earlier stages who receive treatment, up to 61%3 will experience disease recurrence. It was estimated that in 2024, more than 13,820 new cases of invasive cervical cancer were diagnosed in the U.S. and 4,360 adults would die from the disease.1
1. National Cancer Institute. SEER Cancer Stat Facts: Cervical Cancer. 2023. https://seer.cancer.gov/statfacts/html/cervix.html. Accessed November 22, 2024.
2. McLachlan J, Boussios S, Okines A, et al. The impact of systemic therapy beyond first-line treatment for advanced cervical cancer. Clin Oncol (R Coll Radiol). 2017;29(3):153-60
3. Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol. 2016;214(1):22-30
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 29/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Acasunlimab (GEN1046)
Bispecific Next-generation Immunotherapy
| ● | Bispecific antibody targeting PD-L1 and 4-1BB, created using Genmab’s DuoBody technology platform |
| ● | A Phase 3 trial (NCT06635824, ABBIL1TY NSCLC-06) in NSCLC is recruiting |
| ● | In 2024 Genmab assumed sole responsibility for the continued development and potential commercialization of acasunlimab |
Acasunlimab (GEN1046, DuoBody-PD-L1x4-1BB) is a proprietary bispecific antibody, created using Genmab’s DuoBody technology platform. In August 2024, BioNTech opted not to participate in the further development of the acasunlimab program under the parties’ existing License and Collaboration Agreement for reasons related to BioNTech’s portfolio strategy. Genmab subsequently assumed sole responsibility for the continued development and potential commercialization of acasunlimab and the program will be subject to payment of certain milestones and a tiered single-digit royalty on net sales by Genmab to BioNTech. Please refer to Note 5.6 of the financial statements for further details regarding Genmab’s collaboration with BioNTech. Acasunlimab is designed to induce an antitumor immune response by simultaneous and complementary programmed death ligand 1 (PD-L1) blockade and conditional 4-1BB stimulation using an inert DuoBody format. A Phase 3 trial (ABBIL1TY NSCLC-06) of acasunlimab in combination with pembrolizumab compared to docetaxel in CPI-experienced, PD-L1 positive metastatic NSCLC is recruiting.
FOURTH QUARTER UPDATE
| ● | November: A Phase 3 open-label trial was initiated to evaluate the efficacy and safety of acasunlimab in combination with pembrolizumab versus docetaxel (standard of care) in patients with PD-L1-positive metastatic NSCLC who have been treated with PD-1/PD-L1 inhibitor and platinum-containing chemotherapy, administered either in combination or sequentially in the metastatic setting. |
| ● | November: Additional support for the acasunlimab every six weeks (Q6W) dosing schedule, along with preclinical data, was presented during poster sessions at the Society for Immunotherapy of Cancer (SITC) 2024 Conference. |
UPDATES FROM FIRST QUARTER TO THIRD QUARTER
| ● | September: Translational and pharmacokinetic/pharmacodynamic data supporting the Phase 3 dosing regimen for acasunlimab in combination with pembrolizumab in patients with relapsed/refractory metastatic NSCLC was presented as a poster at the 2024 World Conference on Lung Cancer (WCLC). |
| ● | August: As noted above, BioNTech opted not to participate in the further development of the acasunlimab program. Genmab subsequently assumed sole responsibility for the continued development and potential commercialization of acasunlimab. |
| ● | June: Data from the Phase 2 trial of acasunlimab as a single agent or in combination with pembrolizumab for the treatment of relapsed/refractory metastatic NSCLC after treatment with standard of care therapy with an immune checkpoint inhibitor was presented as a poster at the 2024 ASCO Annual Meeting. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 30/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Rinatabart Sesutecan (Rina-S, GEN1184)
Potential Best-in-class Folate Receptor Alpha (FRα)-targeted Type I Topoisomerase (TOPO1) ADC
| ● | FRα-targeted TOPO1 ADC being evaluated for potential treatment of FRα-expressing cancers |
| ● | Phase 3 clinical trial (NCT06619236) in Platinum-resistant Ovarian Cancer (PROC) is recruiting |
Rina-S is a novel FRα-targeted TOPO1 ADC being evaluated for the potential treatment of ovarian cancer and other FRα-expressing cancers. Dose escalation data suggests that Rina-S has robust single agent activity in various cancers across a broad range of FRα expression levels. In January 2024, Rina-S was granted Fast Track Designation by the U.S. FDA for the treatment of FRα-expressing high-grade serous or endometrioid PROC. A Phase 3 trial (NCT06619236) of Rina-S in second line plus PROC is recruiting.
FOURTH QUARTER UPDATE
| ● | December: A Phase 3 open-label trial was initiated to evaluate the efficacy and safety of Rina-S versus treatment of investigator’s choice chemotherapy in patients with PROC. |
UPDATE FROM FIRST QUARTER TO THIRD QUARTER
| ● | September: Data from the Phase 1/2 study of Rina-S were presented as a mini-oral presentation at the European Society of Medical Oncology Congress 2024 (ESMO). |
GEN1042 (BNT312)
Potential First-in-Class Bispecific Agonistic Antibody
| ● | Bispecific antibody targeting CD40 and 4-1BB, created using Genmab’s DuoBody technology platform |
| ● | Multiple clinical trials in solid tumors ongoing |
| ● | Co-developed in collaboration with BioNTech |
GEN1042 (DuoBody-CD40x4-1BB, BNT312) is a proprietary bispecific antibody, jointly owned by Genmab and BioNTech, created using Genmab’s DuoBody technology platform. It is being co-developed by Genmab and BioNTech under an agreement in which the companies share all costs and future potential profits for GEN1042 on a 50:50 basis. CD40 and 4-1BB were selected as targets to enhance activation of both dendritic cells and antigen-dependent T-cells. Three clinical trials of GEN1042 in solid tumors are ongoing. Please refer to Note 5.6 of the financial statements for further details regarding Genmab’s collaboration with BioNTech.
GEN3014
HexaBody-based Investigational Medicine with Potential in Hematological Malignancies
| ● | Antibody targeting CD38, created using Genmab’s HexaBody technology platform |
| ● | Phase 1/2 clinical trial (NCT04824794) in relapsed/refractory multiple myeloma and other hematological malignancies ongoing |
| ● | Developed in an exclusive worldwide license and option agreement with J&J |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 31/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
GEN3014 (HexaBody-CD38) is a human CD38 monoclonal antibody-based investigational medicine created using Genmab’s HexaBody technology platform. GEN3014 is a second generation CD38 targeting immunoglobulin G1 (IgG1) antibody with a hexamerization-enhancing modification. GEN3014 is designed to induce antitumor activity through highly potent complement-dependent cytotoxicity (CDC) and antitumor activity, which is enhanced compared to daratumumab as demonstrated in previously presented preclinical data and is effective at a wider range of target expression levels. In June 2019, Genmab entered into an exclusive worldwide license and option agreement with J&J to develop and commercialize GEN3014. A Phase 1/2 clinical trial in hematologic malignancies is ongoing and includes a cohort comparing GEN3014 to daratumumab in CD38 monoclonal antibody-naïve relapsed or refractory multiple myeloma patients.
FOURTH QUARTER UPDATE
| ● | December: Per the terms of the agreement between Genmab and J&J, Genmab submitted a data package to J&J comparing GEN3014 to daratumumab in CD38 monoclonal antibody-naïve relapsed or refractory multiple myeloma patients. This data will be used to inform J&J’s decision on whether to exercise its option to receive a worldwide license to develop, manufacture and commercialize GEN3014. |
GEN1059 (BNT314)
Bispecific Antibody with Potential in Solid Tumors
| ● | Bispecific antibody targeting epithelial cell adhesion molecule (EpCAM) and 4-1BB, created using Genmab’s DuoBody technology platform |
| ● | Phase 1/2 clinical trial (NCT06150183) in malignant solid tumors recruiting |
| ● | Co-developed in collaboration with BioNTech |
GEN1059 (DuoBody-EpCAMx4-1BB, BNT314), jointly owned by Genmab and BioNTech and created using Genmab’s DuoBody technology platform, is a bispecific antibody aimed at boosting antitumor immune responses through EpCAM-dependent 4-1BB agonistic activity. GEN1059 is being co-developed by Genmab and BioNTech under an agreement in which the companies share all costs and future potential profits for GEN1059 on a 50:50 basis. A Phase 1/2 clinical trial of GEN1059 in solid tumors is recruiting. Please refer to Note 5.6 of the financial statements for further details regarding Genmab’s collaboration with BioNTech.
UPDATE FROM FIRST QUARTER TO THIRD QUARTER
| ● | January: The first patient was treated in the first-in-human Phase 1/2 trial of GEN1059 in advanced or metastatic solid tumors. |
GEN1055 (BNT315)
HexaBody-based Antibody with Potential in Solid Tumors
| ● | Antibody targeting OX40, created using Genmab’s HexaBody technology platform |
| ● | Phase 1/2 clinical trial (NCT06391775) in malignant solid tumors recruiting |
| ● | Co-developed in collaboration with BioNTech |
GEN1055 (HexaBody-OX40, BNT315), jointly owned by Genmab and BioNTech and created using Genmab’s HexaBody technology platform, is an immune-modulating OX40 agonist antibody designed to promote
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 32/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
immunity by enhancing T-cell responses through FcγR-independent OX40 clustering on T cells. GEN1055 is being co-developed by Genmab and BioNTech under an agreement in which the companies
share all costs and future potential profits for GEN1055 on a 50:50 basis. A Phase 1/2 clinical trial of GEN1055 in solid tumors is recruiting. Please refer to Note 5.6 of the financial statements for further details regarding Genmab’s collaboration with BioNTech.
UPDATE FROM FIRST QUARTER TO THIRD QUARTER
| ● | June: The first patient was treated in the first-in-human Phase 1/2 trial of GEN1055 in malignant solid tumors. |
GEN1160
ADC with Potential in Both Solid Tumors and Hematological Malignancies
| ● | CD70-targeted ADC being evaluated in advanced solid and liquid tumors |
| ● | Phase 1/2 clinical trial (NCT05721222) in advanced solid and liquid tumors recruiting |
GEN1160 is a CD70-targeted ADC. CD70 is a protein expressed on both solid tumors and hematological malignancies. A Phase 1/2 clinical study of GEN1160 in advanced renal cell carcinoma, nasopharyngeal carcinoma and NHL is recruiting.
GEN1107
ADC with Potential in Solid Tumors
| ● | Protein tyrosine kinase 7 (PTK7)-targeted ADC being evaluated in advanced solid tumors |
| ● | Phase 1/2 clinical trial (NCT06171789) in advanced solid tumors recruiting |
GEN1107 is a PTK7-targeted ADC. PTK7 is a clinically validated ADC target with broad solid tumor expression, particularly in tumor-initiating cells. A Phase 1/2 clinical study of GEN1107 in advanced solid tumors is recruiting.
GEN1057
Bispecific antibody with potential in solid tumors
| ● | Bispecific antibody targeting fibroblast activation protein alpha (FAPα) and death receptor 4 (DR4), created using Genmab’s DuoBody technology platform |
| ● | Phase 1/2 clinical trial (NCT06573294) in malignant solid tumors recruiting |
GEN1057 (DuoBody-FAPαxDR4) is a bispecific antibody-based investigational medicine created using Genmab’s DuoBody technology platform. GEN1057 is designed for the conditional DR4 transactivation-mediated tumor cell killing by crosslinking FAPα expressed on cancer-associated fibroblasts with DR4 expressed on tumor cells. A Phase 1/2 clinical trial of GEN1057 in malignant solid tumors is recruiting.
UPDATE FROM FIRST QUARTER TO THIRD QUARTER
| ● | September: The first patient was dosed in the first-in-human Phase 1/2 clinical trial of GEN1057 in solid tumors. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 33/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
GEN1286
ADC with Potential in Solid Tumors
| ● | ADC that targets epidermal growth factor receptor (EGFR) and cellular-mesenchymal epithelial transition factor receptor tyrosine kinase (cMet) being evaluated in advanced solid tumors |
| ● | Phase 1/2 clinical trial (NCT06685068) in advanced solid tumors recruiting |
GEN1286 is an ADC targeting EGFR and cMet, two validated cancer targets. A Phase 1/2 clinical study of GEN1286 in advanced solid tumors is recruiting.
FOURTH QUARTER UPDATE
| ● | December: The first patient was dosed in the first-in-human Phase 1/2 clinical trial of GEN1286 in solid tumors. |
Preclinical Programs
| ● | Broad preclinical pipeline that includes both partnered products and in-house programs based on our proprietary technologies and/or antibodies |
| ● | Multiple new IND applications expected to be submitted over the coming years |
| ● | Genmab has entered multiple strategic collaborations to support the expansion of our innovative pipeline, including our acquisition of ProfoundBio in 2024 |
Our preclinical pipeline includes immune effector function enhanced antibodies developed with our HexaBody technology platform, bispecific antibodies created with our DuoBody technology platform and ADCs created with our ADC technology platforms. We are also collaborating with our partners to generate additional new antibody-based product concepts. A number of the preclinical programs are conducted in cooperation with our collaboration partners.
UPDATE FROM FIRST QUARTER TO THIRD QUARTER
| ● | September: Clinical Trial Application (CTA) submitted in Europe for GEN1078. |
Approved Medicines Incorporating Genmab’s Innovations and Technology
In addition to Genmab’s own pipeline of investigational medicines, our innovations and proprietary technology platforms are applied in the pipelines of global pharmaceutical and biotechnology companies. These companies are running clinical development programs with antibodies created by Genmab or created using Genmab’s proprietary DuoBody bispecific antibody technology platform.
The information in this section includes those therapies that have been approved by regulatory agencies in certain territories. Under the agreements for these medicines Genmab is entitled to certain potential milestones and royalties.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 34/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
DARZALEX (daratumumab)
Redefining the Treatment of Multiple Myeloma
| ● | First-in-class human CD38 monoclonal antibody |
| ● | Developed and commercialized by J&J under an exclusive worldwide license from Genmab |
| ● | Intravenous (IV) formulation approved in combination with other therapies and as monotherapy for certain multiple myeloma indications |
| ● | First and only SC CD38-directed antibody approved for the treatment of certain multiple myeloma indications, known as DARZALEX FASPRO in the U.S., and as DARZALEX SC in Europe |
| ● | SC daratumumab is the first and only approved therapy for AL amyloidosis in the U.S., Europe, and Japan |
| ● | 2024 net sales of DARZALEX by J&J were USD 11,670 million |
Daratumumab is a human monoclonal antibody that binds with high affinity to the CD38 molecule, which is highly expressed on the surface of multiple myeloma cells and is also expressed by AL amyloidosis plasma cells. Genmab used technology licensed from Medarex to generate the CD38 antibody. Daratumumab is being developed and commercialized by J&J under an exclusive worldwide license from Genmab. Under the terms of the agreement, Genmab receives royalties between 12% and 20% with J&J reducing such royalty payments for Genmab’s share of J&J’s royalty payments made to Halozyme; payments are further reduced in countries and territories where there are no relevant patents. Please refer to Note 5.6 of the financial statements for further details regarding the daratumumab collaboration with J&J. Daratumumab (marketed as DARZALEX for IV administration and as DARZALEX FASPRO in the U.S. and as DARZALEX SC in Europe for SC administration) is approved in a large number of territories for the treatment of adult patients with certain multiple myeloma indications and is the only approved therapy in the U.S., Europe and Japan for the treatment of adult patients with AL amyloidosis. Please consult the European Summary of Product Characteristics for DARZALEX and DARZALEX SC and the U.S. Prescribing Information for DARZALEX and DARZALEX FASPRO for the labeled indication and safety information.
Kesimpta (ofatumumab)
Approved in the Treatment of RMS
| ● | Human CD20 monoclonal antibody developed and commercialized by Novartis under a license agreement with Genmab |
| ● | Approved in territories including the U.S., EU and Japan for treatment of RMS in adults |
| ● | First B-cell therapy that can be self-administered by patients using the Sensoready® autoinjector pen |
Ofatumumab is a human monoclonal antibody that targets an epitope on the CD20 molecule encompassing parts of the small and large extracellular loops. Genmab used technology licensed from Medarex to generate the CD20 antibody. Ofatumumab, marketed as Kesimpta, is approved in territories including the U.S., Europe, and Japan for the treatment of certain adult patients with RMS. Kesimpta is the first B-cell therapy that can be self-administered by patients using the Sensoready autoinjector pen, once monthly after starting therapy. Ofatumumab is being developed and marketed worldwide by Novartis under a license agreement between Genmab and Novartis. Under the terms of the agreement, Genmab receives a 10% royalty on net sales of Kesimpta, and Genmab pays a low-single digit royalty to Medarex based on Kesimpta sales. Please refer to Note 5.6 of the financial statements for further details regarding the ofatumumab collaboration with Novartis.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 35/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Please consult the U.S. Prescribing Information and the European Summary of Product Characteristics for the labeled indication and safety information for Kesimpta.
TEPEZZA (teprotumumab-trbw)
First U.S. FDA Approved Medicine for the Treatment of TED
| ● | Developed and commercialized by Amgen for the treatment of TED |
| ● | First and only approved medicine for the treatment of TED in the U.S. and Japan |
| ● | Regulatory approval pending in Europe |
Teprotumumab, approved by the U.S. FDA and by Japan’s MHLW under the trade name TEPEZZA, is a human monoclonal antibody that targets the Insulin-like Growth Factor 1 Receptor (IGF-1R), a validated target. It is the first and only medicine approved in the U.S. and in Japan for the treatment of TED. Genmab used technology licensed from Medarex to generate the IGF-1R antibody. The antibody was created by Genmab under a collaboration with Roche. Development and commercialization of the product was subsequently conducted by Horizon Therapeutics plc (Horizon) under a sublicense from Roche. In October 2023, Amgen completed its acquisition of Horizon, including the rights to all commercialization and development of teprotumumab. Under the terms of Genmab’s agreement with Roche, Genmab receives a mid-single digit royalty on net sales (as defined) of TEPEZZA. Please refer to Note 5.6 of the financial statements for further details regarding the teprotumumab collaboration. Please consult the U.S. Prescribing Information for the labeled indication and safety information for TEPEZZA.
RYBREVANT (amivantamab)
First Regulatory Approvals for a DuoBody-based Medicine
| ● | Part of Genmab and J&J DuoBody research and license agreement |
| ● | First approved medicine created using Genmab’s proprietary DuoBody technology |
| ● | Under the agreement with J&J, Genmab is eligible to receive milestones and receives royalties on net sales of RYBREVANT |
In July 2012, and as amended in December 2013, Genmab entered into a collaboration with J&J to create and develop bispecific antibodies using Genmab’s DuoBody technology platform. One of these, J&J’s amivantamab, is a fully human bispecific antibody that targets EGFR and cMet, two validated cancer targets. The two antibody libraries used to produce amivantamab were both generated by Genmab. In collaboration with J&J, the antibody pair used to create amivantamab was co-discovered. Amivantamab, marketed as RYBREVANT, is approved in certain territories for the treatment of certain adult patients with NSCLC. J&J is responsible for the development and commercialization of amivantamab. Under the agreement with J&J, Genmab is eligible to receive milestones and receives royalties between 8% and 10% on net sales of RYBREVANT subject to a reduction of such royalty payments in countries and territories where there are no relevant patents, among other reductions. Genmab pays a royalty to Medarex based on RYBREVANT net sales. Please refer to Note 5.6 of the financial statements for further details regarding the DuoBody collaboration with J&J. Please consult the U.S. Prescribing Information and the European Summary of Product Characteristics for RYBREVANT for the labeled indication and safety information.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 36/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
TECVAYLI (teclistamab)
Bispecific Antibody Approved for the Treatment of Relapsed and Refractory Multiple Myeloma
| ● | Part of Genmab and J&J DuoBody research and license agreement |
| ● | Second approved medicine created using Genmab’s proprietary DuoBody technology |
| ● | Under the agreement with J&J, Genmab is eligible to receive milestones and receives royalties on net sales of TECVAYLI |
In July 2012, and as amended in December 2013, Genmab entered into a collaboration with J&J to create and develop bispecific antibodies using Genmab’s DuoBody technology platform. One of the products subsequently discovered and developed by J&J is teclistamab, a bispecific antibody that targets CD3, which is expressed on T-cells, and B-cell maturation antigen (BCMA), which is expressed in mature B lymphocytes. Teclistamab, marketed as TECVAYLI, is approved in certain territories for the treatment of certain adult patients with relapsed or refractory multiple myeloma. J&J is responsible for the development and commercialization of TECVAYLI. Under our agreement with J&J, Genmab is eligible to receive milestones and receives a mid-single digit royalty on net sales of TECVAYLI subject to a reduction of such royalty payments in countries and territories where there are no relevant patents, among other reductions. Please refer to Note 5.6 of the financial statements for further details regarding the DuoBody collaboration with J&J. Please consult the U.S. Prescribing Information and the European Summary of Product Characteristics for TECVAYLI for the labeled indication and safety information.
TALVEY (talquetamab)
Bispecific Antibody Approved for the Treatment of Relapsed and Refractory Multiple Myeloma
| ● | Part of Genmab and J&J DuoBody research and license agreement |
| ● | Fourth approved medicine created using Genmab’s proprietary DuoBody technology |
| ● | Under the agreement with J&J, Genmab is eligible to receive milestones and receives royalties on net sales of TALVEY |
In July 2012, and as amended in December 2013, Genmab entered into a collaboration with J&J to create and develop bispecific antibodies using Genmab’s DuoBody technology platform. One of the products subsequently discovered and developed by J&J is talquetamab, a bispecific antibody that targets CD3, which is expressed on T-cells, and G protein-coupled receptor, family C, group 5, member D (GPRC5D), an orphan receptor expressed in malignant plasma cells. Talquetamab, marketed as TALVEY, is approved in certain territories for the treatment of certain adult patients with relapsed or refractory multiple myeloma. J&J is responsible for the development and commercialization of TALVEY. Under our agreement with J&J, Genmab is eligible to receive milestones and receives a mid-single digit royalty on net sales of TALVEY subject to a reduction of such royalty payments in countries and territories where there are no relevant patents, among other reductions. Please refer to Note 5.6 of the financial statements for further details regarding the DuoBody collaboration with J&J. Please consult the U.S. Prescribing Information and the European Summary of Product Characteristics for TALVEY for the labeled indication and safety information.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 37/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Antibody Technologies
Antibodies are Y-shaped proteins that play a central role in immunity against bacteria and viruses (also known as pathogens). As we develop immunity, our bodies generate antibodies that bind to pathogen structures (known as antigens), which are specific to the pathogen. Once bound, the antibodies attract other parts of the immune system to eliminate the pathogen. In modern medicine, we have learned how to create and develop specific antibodies against antigens associated with diseased human cells for use in the treatment of diseases such as cancer and autoimmune disease. Genmab uses several types of technologies to create antibodies to treat disease and has developed proprietary antibody technologies including the DuoBody, HexaBody, DuoHexaBody and HexElect technology platforms. With our acquisition of ProfoundBio we also gained their novel ADC technology platforms. Information about these technologies can be found in the following sections and at www.genmab.com/antibody-science/antibody-technology-platforms.
We also use or license several other technologies to generate diverse libraries of high-quality, functional antibodies. In addition, we use or license technologies to increase the potency of some of our antibody therapeutics on a product-by-product basis.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 38/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Our Proprietary Technology Platform Suite
Platform |
|
Principle |
Applications |
DuoBody |
|
Bispecific antibodies |
Dual-targeting: · Recruitment (e.g., T cells) · Tumor heterogeneity |
ADC Technology |
|
Proprietary hydrophilic linker-drug platforms |
●
ADCs with more “antibody-like” PK
●
Pursue targets with clear opportunities for best- and/or first-in-class ADCs
|
HexaBody |
|
Target-mediated enhanced hexamerization |
Enhanced potency: · CDC · Target clustering, outside-in signaling, apoptosis |
DuoHexaBody |
|
Bispecific antibodies with target-mediated enhanced hexamerization |
Dual-targeting + enhanced potency: · CDC · Target clustering, outside-in signaling, apoptosis |
HexElect |
|
Two co-dependent antibodies with target-mediated enhanced hexamerization |
Dual-targeting + enhanced potency and selectivity: · Co-dependent unlocking of potency · New target space, previously inaccessible |
DuoBody Technology Platform
Innovative Technology for Bispecific Antibody Therapeutics
| ● | Bispecific antibody technology platform |
| ● | Potential in cancer, autoimmune, infectious, cardiovascular, central nervous system diseases, and hemophilia |
| ● | Commercial collaborations with AbbVie, J&J and BioNTech among others, plus multiple research collaborations |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 39/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| ● | Multiple regulatory approvals for medicines created using the DuoBody technology platform |
The DuoBody technology platform is Genmab’s innovative platform for the discovery and development of bispecific antibodies. Bispecific antibodies bind to two different epitopes (or “docking” sites) either on the same or on different targets (also known as dual-targeting). Dual-targeting may improve binding specificity and enhance therapeutic efficacy or bring two different cells together (for example, engaging a T cell to kill a tumor cell). Bispecific antibodies generated with the DuoBody technology platform can be used for the development of therapeutics for diseases such as cancer, autoimmune, infectious, cardiovascular, central nervous system diseases, and hemophilia. DuoBody molecules combine the benefits of bispecificity with the strengths of conventional antibodies, which allows DuoBody molecules to be administered and dosed the same way as other antibody therapeutics. Genmab’s DuoBody technology platform generates bispecific antibodies via a versatile and broadly applicable process that is easily performed at high throughput, standard bench, as well as at commercial manufacturing scale. Genmab uses the DuoBody technology platform to create its own bispecific antibody programs and the technology is also available for licensing. Genmab has numerous alliances for the DuoBody technology platform including commercial collaborations with AbbVie, J&J, Novo Nordisk and BioNTech.
Genmab’s proprietary DuoBody technology platform has been applied to a variety of bispecific antibody products in development, both in our own pipeline and in programs being developed by collaboration partners. The technology has been validated by the continued advancement of these investigational medicines through clinical development, including four approved medicines. Today four products created by use of the DuoBody technology platform have received regulatory approval.
|
The innovative DuoBody technology platform generates bispecific antibodies via a fast, versatile and broadly applicable process called controlled Fab-arm exchange. With only minimal protein engineering, the technology allows the binding arms of two distinct monoclonal antibodies to exchange, combining into one stable bispecific antibody, thereby retaining regular immunoglobulin structure and function. The DuoBody technology platform is also highly suitable for high throughput generation, screening and discovery of bispecific antibodies in final therapeutic format. |
DuoBody Collaborations
Advancing Our Pipeline
AbbVie
On June 10, 2020, Genmab entered into a broad oncology collaboration agreement with AbbVie to jointly develop and commercialize products including epcoritamab (DuoBody-CD3xCD20), and subsequently into a discovery research collaboration for up to four future differentiated antibody therapeutics for cancer. The companies will share commercial responsibilities for epcoritamab in the U.S. and Japan, with AbbVie responsible for further global commercialization. Genmab is the principal for net sales in the U.S. and Japan and receives tiered royalties on remaining global sales outside of these territories. For any product candidates developed as a result of the companies’ discovery research collaboration, Genmab and AbbVie will share responsibilities for global development and commercialization in the U.S. and Japan. Genmab retains the right to co-commercialize these products, along with AbbVie, outside of the U.S. and Japan.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 40/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Under the terms of the agreement, Genmab has the potential to receive regulatory and sales milestone payments, as well as tiered royalties between 22% and 26% on net sales for epcoritamab outside the U.S. and Japan. Except for these royalty-bearing sales, the parties will share in profit from the sale of epcoritamab on a 50:50 basis. If all four next-generation antibody product candidates developed as a result of the discovery research collaboration are successful, Genmab is eligible to receive up to USD 2.0 billion in option exercise and success-based milestones. Genmab and AbbVie split 50:50 the development costs related to epcoritamab, while Genmab will be responsible for 100% of the costs for the discovery research programs up to opt-in. Please refer to Note 5.6 of the financial statements for further details regarding the collaboration with AbbVie.
BioNTech
In May 2015, Genmab entered an agreement with BioNTech to jointly research, develop and commercialize bispecific antibody-based investigational medicines using Genmab’s DuoBody technology platform. Under the terms of the agreement, BioNTech will provide proprietary antibodies against key immunomodulatory targets, while Genmab provides proprietary antibodies and access to its DuoBody technology platform. Genmab paid an upfront fee of USD 10 million to BioNTech. If the companies jointly select any antibody-based product candidates for clinical development, development costs and product ownership will be shared equally going forward. If one of the companies does not wish to move an antibody product forward, the other company is entitled to continue developing it on predetermined licensing terms. The agreement also includes provisions which will allow the parties to opt out of joint development at key points. Genmab and BioNTech currently have two bispecific antibody products in clinical development, GEN1042 (BNT312, DuoBody-CD40x4-1BB) and GEN1059 (BNT314, DuoBody-EpCAMx4-1BB). In 2024, Genmab assumed sole responsibility for the continued development and potential commercialization of an additional bispecific antibody, acasunlimab (GEN1046, DuoBody-PD-L1x4-1BB) after BioNTech opted to not participate in further development of the program. Please refer to Note 5.6 of the financial statements for further details regarding Genmab’s collaboration with BioNTech.
Our Innovative Technology in Action
J&J
In July 2012, and as amended in December 2013, Genmab entered into a collaboration with J&J to create and develop bispecific antibodies using our DuoBody technology platform.
Three of the DuoBody-based investigational medicines created under this collaboration, RYBREVANT (amivantamab), TECVAYLI (teclistamab) and TALVEY (talquetamab) have received regulatory approval in various territories worldwide. Genmab is eligible to receive milestone payments and receives royalties on net sales of each commercialized DuoBody medicine. Please refer to Note 5.6 of the financial statements for further details regarding the DuoBody collaboration with J&J.
Novo Nordisk
In August 2015, Genmab entered an agreement to grant Novo Nordisk commercial licenses to use the DuoBody technology platform to create and develop bispecific antibody candidates for two therapeutic programs that would target a disease area outside of cancer therapeutics. After an initial period of exclusivity for both target combinations, Novo Nordisk extended exclusivity of the commercial license for one target combination in 2018, now in clinical development as Mim8. Under the exclusive license agreement, Genmab is entitled to potential milestones and will be entitled to mid-single digit royalties on sales of Mim8, should it receive regulatory approval.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 41/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
ADC Technology Platforms
Two Proprietary Hydrophilic Linker-drug Platforms with Clinical Validation
| ● | Combines both novel and validated components to create potentially best-in-class ADCs |
| ● | Acquired from ProfoundBio, basis of four products in the clinic, including Rina-S |
ADCs are antibodies with potent cytotoxic agents coupled to them. By using antibodies that recognize specific targets on tumor cells, these cytotoxic agents are preferentially delivered to the tumor cells. With Genmab’s acquisition of ProfoundBio we inherited proprietary hydrophilic antibody-drug linker technology that blends innovative and proven methods to design ADCs leading to potentially enhanced therapeutic outcomes. This technology leverages two decades of insights into ADC pharmacology and optimization. These novel, highly hydrophilic and stable cleavable linkers are designed to mask the hydrophobicity of payloads, leading to ADCs with more “antibody-like” pharmacokinetics. Initial focus has been on clinically proven targets where ADC viability has been established. Our goal is to pursue targets with clear opportunities for best- and/or first-in-class ADCs. We also have the potential to combine this technology with Genmab’s proprietary DuoBody technology to create bispecific ADCs. There are currently four wholly owned programs in the clinic based on this technology, Rina-S, GEN1286 (EGFR, cMet), GEN1107 (PTK7) and GEN1160 (CD70).
HexaBody Technology Platform
Creating Differentiated Therapeutics
| ● | Enhanced potency antibody technology platform |
| ● | Broadly applicable technology that builds on natural antibody biology |
| ● | HexaBody-based investigational medicines in clinical development; HexaBody-CD38 (GEN3014) and HexaBody-OX40 (GEN1055/BNT315) |
The HexaBody technology platform is a proprietary Genmab technology that is designed to increase the potency of antibodies. The HexaBody technology platform builds on natural biology and strengthens the natural killing ability of antibodies while retaining regular structure and specificity. The technology allows for the creation of potent therapeutics by inducing antibody hexamer formation (clusters of six antibodies) after binding to their target antigen on the cell surface. We have used the HexaBody technology platform to generate antibodies with enhanced complement-mediated killing, allowing antibodies with limited or absent killing capacity to be transformed into potent, cytotoxic antibodies. In addition to complement-mediated killing, the clustering of membrane receptors by the HexaBody technology platform can lead to subsequent outside-in signaling leading to cell death. The HexaBody technology platform creates opportunities to explore new antibody-based product candidates and repurpose drug candidates unsuccessful in previous clinical trials due to insufficient potency. The HexaBody technology platform is broadly applicable and can be combined with Genmab’s DuoBody technology platform (DuoHexaBody technology platform) as well as other antibody technologies. The technology has the potential to enhance antibody therapeutics for a broad range of applications including cancer and infectious diseases. Genmab is using the HexaBody technology platform for its own antibody programs and the technology is also available for licensing. Two HexaBody-based investigational medicines are currently in clinical development. Genmab entered into an exclusive worldwide license and option agreement with J&J to develop and commercialize GEN3014 (HexaBody-CD38), a next-generation CD38 monoclonal antibody-based investigational medicine. In 2022, Genmab and BioNTech expanded their global strategic collaboration to include co-development of monospecific antibody candidates
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 42/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
leveraging the HexaBody technology. Currently in the clinic under this collaboration is GEN1055 (BNT315, HexaBody-OX40).
DuoHexaBody Technology Platform
Combining Dual-Targeting and Enhanced Potency
| ● | Antibody technology that combines DuoBody and HexaBody technology platforms |
| ● | Creates bispecific antibodies with target-mediated enhanced potency |
The DuoHexaBody technology platform is a proprietary technology that combines the dual targeting of our DuoBody technology platform with the enhanced potency of our HexaBody technology platform, creating bispecific antibodies with target-mediated enhanced hexamerization.
HexElect Technology Platform
Enhancing Selectivity and Potency
| ● | Antibody technology platform inspired by the HexaBody technology platform |
| ● | Combines dual-targeting with enhanced selectivity and potency |
The HexElect antibody technology platform is Genmab’s newest proprietary antibody technology. This technology combines two HexaBody molecules designed to effectively and selectively hit only those cells that express both targets by making the activity of complexes of HexaBody molecules dependent on their binding to two different targets on the same cell. The HexElect technology platform maximizes efficacy while minimizing possible toxicity, potentially leading to more potent and safer investigational medicines.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 43/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
“We are increasingly prioritizing investment into programs that have a clear line of sight to generate meaningful revenue, especially our Phase 3 programs, EPKINLY, Rina-S and acasunlimab. In order to be well positioned at the end of this decade, these are the investments that we need to make today.”
Anthony Pagano, Executive Vice President and Chief Financial Officer
Financial Review - Group
The financial statements are prepared on a consolidated basis for Genmab A/S (parent company) and its subsidiaries. The Genmab financial statements are published in Danish Kroner (DKK). The Genmab consolidated Group is referenced herein as “Genmab” or the “Company.”
RESULT FOR THE YEAR
Guidance and Result for 2024 | ||
(DKK million) |
Latest Guidance |
Actual |
Revenue |
21,100 - 21,700 |
21,526 |
Royalties |
17,000 - 17,400 |
17,352 |
Net product sales/Collaboration revenue* |
2,000 - 2,200 |
2,176 |
Milestones/Reimbursement revenue |
2,100 - 2,100 |
1,996 |
Gross Profit** |
20,200 - 20,800 |
20,541 |
Operating expenses** |
(14,100) - (14,400) |
(13,838) |
Operating profit** |
5,800 - 6,700 |
6,703 |
*Net Product Sales and Collaboration Revenue consists of EPKINLY Net Product Sales in the U.S. and Japan and Tivdak (Genmab’s share of net profits) in the U.S.
**Operating Expenses Range excludes Cost of Product Sales Range, which is included in Gross Profit Range
Actual revenue, operating expenses and operating profit were in line with the latest guidance published on November 6, 2024.
REVENUE
Genmab’s revenue was DKK 21,526 million in 2024 compared to DKK 16,474 million in 2023. The increase of DKK 5,052 million, or 31%, was primarily driven by higher DARZALEX and Kesimpta royalties achieved under our collaborations with J&J and Novartis, respectively. Increased EPKINLY net product sales, driven by a strong product launch in 2023 with a full year of net sales in 2024, also contributed to increased revenue in 2024.
Genmab’s revenue was DKK 16,474 million in 2023 compared to DKK 14,505 million in 2022. The increase of DKK 1,969 million, or 14%, was primarily driven by higher DARZALEX and Kesimpta royalties achieved under our collaborations with Janssen and Novartis, respectively, partly offset by milestones achieved in 2022 under our collaboration with AbbVie. EPKINLY net product sales, driven by a strong product launch, also contributed to increased revenue in 2023.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 44/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
(DKK million) |
|
2024 |
2023 |
2022 |
|||
Royalties |
|
17,352 |
80% |
13,705 |
83% |
11,582 |
80% |
Reimbursement Revenue |
|
996 |
5% |
864 |
5% |
818 |
6% |
Milestone Revenue |
|
1,000 |
5% |
1,177 |
7% |
1,767 |
12% |
Collaboration Revenue |
|
433 |
2% |
307 |
2% |
332 |
2% |
Net Product Sales |
|
1,743 |
8% |
421 |
3% |
— |
— |
License Revenue |
|
2 |
0% |
— |
0% |
6 |
0% |
Total revenue |
|
21,526 |
100% |
16,474 |
100% |
14,505 |
100% |
Royalties
Royalty revenue amounted to DKK 17,352 million in 2024 compared to DKK 13,705 million in 2023. The increase of DKK 3,647 million, or 27%, was primarily driven by higher DARZALEX and Kesimpta royalties achieved under our daratumumab collaboration with J&J and ofatumumab collaboration with Novartis, respectively. The table below summarizes Genmab’s royalty revenue by product.
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
DARZALEX |
|
13,922 |
|
11,265 |
|
9,966 |
Kesimpta |
|
2,222 |
|
1,494 |
|
779 |
TEPEZZA |
|
737 |
|
704 |
|
796 |
Other |
|
471 |
|
242 |
|
41 |
Total royalties |
|
17,352 |
|
13,705 |
|
11,582 |
DARZALEX
J&J’s net sales of DARZALEX were USD 11,670 million in 2024 compared to USD 9,744 million in 2023 and USD 7,977 million in 2022. The increase from 2023 to 2024 of USD 1,926 million, or 20%, was driven by share gains in all regions. The increase from 2022 to 2023 of USD 1,767 million, or 22%, was also driven by share gains in all regions.
Royalty revenue on net sales of DARZALEX was DKK 13,922 million in 2024 compared to DKK 11,265 million in 2023 and DKK 9,966 million in 2022, an increase of DKK 2,657 million from 2023 to 2024, and DKK 1,299 million from 2022 to 2023.
The percentage increase in royalties of 24% from 2023 to 2024 is higher than the percentage increase in the underlying net sales of 20% primarily due to a higher effective royalty rate for 2024 and other positive foreign exchange rate impacts, partially offset by the increase in Genmab’s Halozyme royalty reductions in connection with the increase in SC product net sales and an increase in royalty reductions on net sales in countries and territories where there is no Genmab patent coverage as well as lower average exchange rate between the USD and DKK in 2024. Under our license agreement with Janssen for DARZALEX, for purposes of calculating royalties due to Genmab, DARZALEX net sales for non-U.S. dollar denominated currencies are translated to U.S. dollars at a specified annual Currency Hedge Rate. This contractual arrangement is the driver for the other foreign exchange impacts discussed above.
The percentage increase in royalties of 13% from 2022 to 2023 is lower than the percentage increase in the underlying net sales of 22% primarily due to a lower average exchange rate between the USD and DKK in 2023, other foreign exchange impacts, the increase in Genmab’s Halozyme royalty reductions in connection
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 45/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
with the increase in SC product net sales and an increase in royalty reductions on net sales in countries and territories where there is no Genmab patent coverage. Under our license agreement with Janssen for DARZALEX, for purposes of calculating royalties due to Genmab, net sales for non-U.S. denominated currencies are translated to U.S. dollars at a specific annual Currency Hedge Rate. This contractual agreement is the driver for the other foreign exchange rate impacts discussed above, which were significantly more favorable in 2022 compared to 2023.
Kesimpta
Novartis’ net sales of Kesimpta were USD 3,224 million in 2024 compared to USD 2,171 million in 2023 and USD 1,092 million in 2022. The increase of USD 1,053 million from 2023 to 2024, or 49%, was primarily driven by increased demand and strong access. The increase of USD 1,079 million from 2022 to 2023, or 99%, was primarily driven by increased demand, strong access, and a one-time positive revenue adjustment in Europe.
Royalty revenue on net sales of Kesimpta was DKK 2,222 million in 2024 compared to DKK 1,494 million in 2023, an increase of DKK 728 million, or 49%. Royalty revenue on net sales of Kesimpta was DKK 1,494 million in 2023 compared to DKK 779 million in 2022, an increase of DKK 715 million, or 92%.
TEPEZZA
Amgen’s net sales of TEPEZZA were USD 1,851 million in 2024 compared to USD 1,771 million in 2023 and USD 1,966 million in 2022. Royalty revenue on net sales of TEPEZZA was DKK 737 million in 2024 compared to DKK 704 million in 2023 and DKK 796 million in 2022, an increase of DKK 33 million, or 5% from 2023 to 2024 and a decrease of DKK 92 million, or 12% from 2022 to 2023.
Other royalties
Other royalties consist of royalties from net sales of RYBREVANT, TECVAYLI, TALVEY and TEPKINLY.
J&J was granted U.S. FDA approval for RYBREVANT during the second quarter of 2021, and Genmab subsequently started recognizing royalties on net sales of RYBREVANT. Royalties were not material for 2024, 2023 or 2022.
J&J was granted approval for TECVAYLI for the treatment of relapsed or refractory multiple myeloma during the third quarter of 2022 in Europe and in the fourth quarter of 2022 in the U.S. Royalties were not material for 2024, 2023 or 2022.
During the third quarter of 2023, J&J was granted approval in the U.S. and in Europe for TALVEY for the treatment of relapsed or refractory multiple myeloma. Royalties were not material for 2024 or 2023.
The EC granted conditional marketing authorization for TEPKINLY as a monotherapy for the treatment of adult patients with relapsed or refractory DLBCL after two or more lines of systemic therapy during the third quarter of 2023. Royalties from AbbVie, related to European net sales, were not material for 2024 or 2023.
Royalty revenue fluctuations from period to period are driven by the level of product net sales, foreign currency exchange rate movements and more specifically to DARZALEX, the contractual arrangement related to annual Currency Hedge Rate, Genmab’s share of J&J’s royalty payments to Halozyme in
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 46/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
connection with SC product net sales and royalty deductions on net sales in countries and territories where there is no patent protection.
Reimbursement Revenue
Reimbursement revenue, mainly comprised of the reimbursement of certain research and development costs related to the development work under Genmab’s collaboration agreements, amounted to DKK 996 million in 2024 compared to DKK 864 million in 2023 and DKK 818 million in 2022. The increase of DKK 132 million, or 15%, from 2023 to 2024 was primarily driven by higher activities under our collaboration agreements with BioNTech for DuoBody-CD40x4-1BB and acasunlimab, prior to Genmab assuming full ownership, as well as by higher activities under our collaboration agreement with Pfizer for Tivdak. The increase of DKK 46 million, or 6%, from 2022 to 2023 was primarily driven by higher activities under our collaboration agreements with BioNTech for DuoBody-CD40x4-1BB and acasunlimab.
Milestone Revenue
Milestone revenue was DKK 1,000 million in 2024 compared to DKK 1,177 million in 2023 and DKK 1,767 million in 2022, a decrease of DKK 177 million, or 15%, from 2023 to 2024, and a decrease of DKK 590 million, or 33%, from 2022 to 2023, primarily driven by the following:
2024 milestones:
| ● | Novartis milestone of DKK 582 million driven by worldwide net sales for Kesimpta, first exceeding DKK 17.4 billion in 2024, and |
| ● | AbbVie milestone of DKK 343 million (USD 50 million) due to the acceptance for filing of a Biologics License Application (BLA) by the U.S. FDA in the second indication of epcoritamab in the U.S. |
2023 milestones:
| ● | AbbVie milestone of DKK 348 million (USD 50 million) driven by the first commercial sale of EPKINLY in the U.S., |
| ● | AbbVie milestone of DKK 205 million (USD 30 million) due to the acceptance of the marketing authorization application (MAA) filing by the EMA of the type II variation for marketing authorization of TEPKINLY, |
| ● | AbbVie milestone of DKK 176 million (USD 25 million) due to the first commercial sale of TEPKINLY in Europe, and |
| ● | J&J milestone of DKK 169 million (USD 25 million) related to the BLA approval in the U.S. for talquetamab. |
2022 milestones:
| ● | AbbVie milestone of DKK 577 million (USD 80 million) driven by the acceptance of the BLA by the U.S. FDA for epcoritamab, |
| ● | AbbVie milestone of DKK 444 million (USD 60 million) triggered by the validation of the MAA by the EMA in the EU for epcoritamab, |
| ● | J&J milestones of DKK 189 million (USD 25 million) and DKK 112 million (USD 15 million) for the approval of TECVAYLI for the treatment of relapsed or refractory multiple myeloma in the U.S. and Europe, respectively, and |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 47/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| ● | AbbVie milestone of DKK 153 million (USD 20 million) driven by the initiation, or first patient dosed, of a pivotal trial (Phase 3) in the second indication for epcoritamab. |
Milestone revenue may fluctuate significantly from period to period due to both the timing of achievements and the varying amount of each individual milestone under our license and collaboration agreements.
Collaboration Revenue
Collaboration revenue, which reflects 50% of gross profit from net sales of Tivdak in the U.S. by Pfizer, was DKK 433 million in 2024 compared to DKK 307 million in 2023 and DKK 332 million in 2022. The increase of DKK 126 million, or 41%, from 2023 to 2024 was primarily driven by increased sales of Tivdak. The decrease of DKK 25 million from 2022 to 2023 was primarily driven by a one-off payment in 2022 from Pfizer of approximately DKK 112 million (USD 15 million) which reflects Genmab’s share (50%) of payments received by Pfizer in connection with the sublicense of its rights to develop and commercialize tisotumab vedotin in China to Zai Lab Hong Kong, partly offset by an increase in net sales of Tivdak in 2023.
Net Product Sales
Global net sales of EPKINLY/TEPKINLY were USD 281 million in 2024. Net product sales in the U.S. and Japan by Genmab were DKK 1,743 million in 2024 compared to DKK 421 million in 2023. EPKINLY was approved in the U.S. in May 2023 and in Japan in September 2023.
Net sales of TEPKINLY in territories where Genmab receives royalty revenue were USD 28 million in 2024, with immaterial net sales in 2023 due to regulatory approvals in such territories not occurring until late 2023.
As EPKINLY is Genmab’s first commercialized product for which Genmab is recording net product sales, there were no net product sales recognized during 2022.
Refer to Note 2.2 for further details about revenue.
COST OF PRODUCT SALES
Genmab recognized cost of product sales of DKK 985 million in 2024 compared to DKK 226 million in 2023. Cost of product sales related to EPKINLY sales is primarily comprised of profit-sharing amounts payable to AbbVie of DKK 831 million in 2024 compared to DKK 195 million in 2023, as well as product costs. There were no cost of product sales recognized during 2022 as EPKINLY was approved in the U.S. in May 2023 and in Japan in September 2023. Aside from these items, there are no other costs included within cost of product sales.
Refer to Notes 2.3, 3.5 and 5.6 for further details about cost of product sales.
RESEARCH AND DEVELOPMENT EXPENSES
Research and development expenses amounted to DKK 9,748 million in 2024 compared to DKK 7,630 million in 2023 and DKK 5,562 million in 2022. The increase from 2023 to 2024 of DKK 2,118 million, or 28%, was driven by the increased and accelerated advancement of epcoritamab under our collaboration with AbbVie, the addition of ProfoundBio related research and development expenses, primarily Rina-S, advancement of acasunlimab and DuoBody-CD40x4-1BB under our collaboration with BioNTech, further progression of pipeline products, and the increase in team members to support the continued expansion of our product portfolio. The increase from 2022 to 2023 of DKK 2,068 million, or 37% was driven by the increased and accelerated advancement of epcoritamab under our collaboration with AbbVie, advancement of acasunlimab
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 48/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
and DuoBody-CD40x4-1BB under our collaboration with BioNTech, further progression of pipeline products, and the increase in team members to support the continued expansion of our product portfolio.
Research and development costs accounted for 72% of total research and development expenses and selling, general and administration expenses in 2024 compared to 70% in 2023 and 68% in 2022.
The following table provides information regarding our research and development expenses for 2024 as compared to 2023 and 2022.
|
|
|
|
|
|
Percentage |
|
|
Percentage |
|
||
|
|
|
|
Change |
|
|
Change |
|
||||
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
|
2024/2023 |
|
|
2023/2022 |
|
Research(1) |
|
2,137 |
|
1,507 |
|
1,222 |
|
42 |
% |
|
23 |
% |
Development and contract manufacturing(2) |
|
3,555 |
|
2,324 |
|
1,556 |
|
53 |
% |
|
49 |
% |
Clinical(3) |
|
3,296 |
|
3,282 |
|
2,059 |
|
0 |
% |
|
59 |
% |
Upfront payments(4) |
|
— |
|
3 |
|
155 |
|
(100) |
% |
|
(98) |
% |
Other(5) |
|
760 |
|
514 |
|
570 |
|
48 |
% |
|
(10) |
% |
Total research and development expenses |
|
9,748 |
|
7,630 |
|
5,562 |
|
28 |
% |
|
37 |
% |
(1) Research expenses include, among other things, personnel, occupancy and laboratory expenses, technology access fees associated with identification of new monoclonal antibodies (mAbs), expenses associated with the development of new proprietary technologies and research activities associated with our product candidates, such as in vitro and in vivo studies, translational research, and IND enabling toxicology studies.
(2) Development and contract manufacturing expenses include personnel and occupancy expenses, external contract manufacturing costs for the scaleup and pre-approval manufacturing of drug product used in research and our clinical trials, costs for drug product supplied to our collaborators, costs related to preparation for the production of process validation batches to be used in potential future regulatory submissions, quality control and assurance activities, and storage and shipment of our product candidates.
(3) Clinical expenses include personnel, travel, occupancy costs, and external clinical trial costs including contract research organizations (CROs), investigator fees, clinical site fees, contractors and regulatory activities associated with conducting human clinical trials.
(4) Upfront payments include payments made to third parties upon entering into R&D license and collaboration agreements.
(5) Other research and development expenses primarily include share-based compensation, depreciation, amortization and impairment expenses.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 49/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The following table shows third-party costs incurred for research, contract manufacturing of our product candidates and clinical and regulatory services for 2024 as compared to 2023 and 2022. The table also presents unallocated costs and overhead consisting of third-party costs for our preclinical stage programs, personnel, facilities, and other indirect costs not directly charged to development programs.
|
|
|
|
|
|
Percentage |
|
|
Percentage |
|
||
|
|
|
|
Change |
|
|
Change |
|
||||
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
|
2024/2023 |
|
|
2023/2022 |
|
Epcoritamab |
|
2,854 |
|
1,323 |
|
801 |
|
116 |
% |
|
65 |
% |
Rina-S |
|
319 |
|
— |
|
— |
|
N/A |
|
|
N/A |
|
Tisotumab vedotin |
|
263 |
|
285 |
|
319 |
|
(8) |
% |
|
(11) |
% |
Acasunlimab |
|
707 |
|
553 |
|
369 |
|
28 |
% |
|
50 |
% |
DuoBody-CD40x4-1BB |
|
514 |
|
409 |
|
242 |
|
26 |
% |
|
69 |
% |
Other clinical stage programs |
|
503 |
|
743 |
|
393 |
|
(32) |
% |
|
89 |
% |
Total third-party costs for clinical stage programs |
|
5,160 |
|
3,313 |
|
2,124 |
|
56 |
% |
|
56 |
% |
Preclinical projects |
|
1,491 |
|
1,132 |
|
830 |
|
32 |
% |
|
36 |
% |
Upfront payments |
|
— |
|
3 |
|
155 |
|
(100) |
% |
|
(98) |
% |
Personnel, unallocated costs and overhead |
|
3,097 |
|
3,182 |
|
2,453 |
|
(3) |
% |
|
30 |
% |
Total research and development expenses |
|
9,748 |
|
7,630 |
|
5,562 |
|
28 |
% |
|
37 |
% |
Third-party costs for epcoritamab increased by DKK 1,531 million, or 116%, in 2024 as compared to 2023, primarily due to the advancement and acceleration of the epcoritamab program under Genmab’s collaboration with AbbVie. Third-party costs for epcoritamab increased by DKK 522 million, or 65%, in 2023 as compared to 2022, primarily due to the advancement and acceleration of the epcoritamab program under Genmab’s collaboration with AbbVie.
Third-party costs for Rina-S were DKK 319 million in 2024. Rina-S was acquired through the acquisition of ProfoundBio in the second quarter of 2024.
Third-party costs for tisotumab vedotin decreased by DKK 22 million, or 8%, in 2024 as compared to 2023, primarily due to the completion of certain clinical study activities in 2024. Third-party costs for tisotumab vedotin decreased by DKK 34 million, or 11%, in 2023 as compared to 2022, primarily due to the completion of certain clinical study activities in 2023.
Third-party costs for acasunlimab increased by DKK 154 million, or 28%, in 2024 as compared to 2023, primarily due to the continued advancement of the program, which Genmab obtained sole ownership during the third quarter of 2024. Third-party costs for acasunlimab increased by DKK 184 million, or 50%, in 2023 as compared to 2022, primarily due to the continued advancement and expansion of the program under Genmab’s prior collaboration with BioNTech on this project.
Third-party costs for DuoBody-CD40x4-1BB increased by DKK 105 million, or 26%, in 2024 as compared to 2023, primarily due to the continued advancement and expansion of the program under Genmab’s collaboration with BioNTech. Third-party costs for DuoBody-CD40x4-1BB increased by DKK 167 million, or
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 50/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
69%, in 2023 as compared to 2022, primarily due to the continued advancement and expansion of the program under Genmab’s collaboration with BioNTech.
Third-party costs for Genmab’s other clinical stage programs decreased by DKK 240 million, or 32%, in 2024 as compared to 2023, primarily related to advancements of DuoBody-CD3xB7H4 and DuoBody-CD3xCD30 in 2024. Third-party costs for Genmab’s other clinical stage programs increased by DKK 350 million, or 89%, in 2023 as compared to 2022, primarily related to advancements of DuoBody-CD3xB7H4 and DuoBody-CD3xCD30 in 2023.
Research and development expenses related to our preclinical projects increased by DKK 359 million, or 32%, in 2024 as compared to 2023, driven by the continued investment in new and existing preclinical programs. An IND was submitted for DuoBody-FAPαxDR4 and a CTA was submitted for GEN1078. Research and development expenses related to our preclinical projects increased by DKK 302 million, or 36%, in 2023 as compared to 2022, driven by the continued investment in new and existing preclinical programs.
Upfront payments were not material in either 2024 or 2023 driven by a decrease in the number of R&D license payments recorded as expense. Upfront payments decreased by DKK 152 million, or 98%, driven by a decrease in the number of R&D license payments in 2023 as compared to 2022.
Personnel, unallocated costs and overhead decreased by DKK 85 million, or 3%, in 2024 as compared to 2023, primarily due to travel costs, which were higher in 2023 due to the upcoming launch of EPKINLY in 2023. Our research and development FTEs increased from 1,541 at the end of 2023 to 1,886 at the end of 2024. Personnel, unallocated costs and overhead increased by DKK 729 million, or 30%, in 2023 as compared to 2022, primarily due to an increase in staffing levels and the expansion of our facilities to accommodate our growth. Our research and development FTEs increased from 1,193 at the end of 2022 to 1,541 at the end of 2023.
Refer to Note 2.3, 3.1, 3.2 and 5.5 for further details about staff costs, intangible assets, property and equipment and the acquisition of ProfoundBio.
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES
Selling, general and administrative expenses were DKK 3,790 million in 2024 compared to DKK 3,297 million in 2023 and DKK 2,676 million in 2022. The increase from 2023 to 2024 of DKK 493 million, or 15%, was driven by the continued expansion of Genmab’s commercialization capabilities through the increase in team members to support the continued launch of EPKINLY in the U.S. and Japan in 2023, and the investment in Genmab’s broader organizational capabilities. Selling, general and administration expense growth has been moderating during 2024 reflecting a focus on driving efficiency. We continue to increase team members and commercial support in a strategic manner. The increase from 2022 to 2023 of DKK 621 million, or 23%, was driven by the continued expansion of Genmab’s commercialization capabilities through the increase in team members to support the launch of EPKINLY in the U.S. and Japan in 2023, and the investment in Genmab’s broader organizational capabilities.
DKK 1,813 million, or 48% of selling, general and administrative expenses in 2024, was related to compensation of Genmab team members associated with selling, general and administrative activities, as compared to DKK 1,541 million, or 47% in 2023 and DKK 1,065 million, or 40% in 2022.
Refer to Note 2.3 and 3.2 for further details about staff costs and property and equipment.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 51/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Selling, general and administrative expenses accounted for 28% of total research and development expenses and selling, general and administration expenses in 2024 compared to 30% in 2023 and 32% in 2022.
ACQUISITION AND INTEGRATION RELATED CHARGES
Acquisition and integration related charges for the acquisition of ProfoundBio were DKK 300 million in 2024 compared to no acquisition and integration related charges for 2023 or 2022 as there were no acquisitions during these years.
Refer to Note 5.5 for further details about the acquisition of ProfoundBio.
OPERATING PROFIT
Operating profit was DKK 6,703 million in 2024 compared to DKK 5,321 million in 2023, an increase of DKK 1,382 million, or 26%. Operating profit was DKK 5,321 million in 2023 compared to DKK 6,267 million in 2022, a decrease of DKK 946 million, or 15%.
FINANCIAL INCOME AND EXPENSE
Financial income and expense was comprised of the following:
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
Financial income: |
|
|
|
|
|
|
Interest and other financial income |
|
995 |
|
982 |
|
324 |
Gain on marketable securities |
|
364 |
|
495 |
|
92 |
Gain on other investments |
|
146 |
|
72 |
|
58 |
Foreign exchange rate gain |
|
2,933 |
|
391 |
|
2,715 |
|
|
|
|
|
|
|
Total financial income |
|
4,438 |
|
1,940 |
|
3,189 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial expenses: |
|
|
|
|
|
|
Interest and other financial expenses |
|
(120) |
|
(70) |
|
(39) |
Loss on marketable securities |
|
(147) |
|
(176) |
|
(453) |
Loss on other investments |
|
(116) |
|
(98) |
|
(355) |
Foreign exchange rate loss |
|
(1,594) |
|
(1,280) |
|
(1,664) |
|
|
|
|
|
|
|
Total financial expenses |
|
(1,977) |
|
(1,624) |
|
(2,511) |
|
|
|
|
|
|
|
Net financial items |
|
2,461 |
|
316 |
|
678 |
Interest Income
Interest income was DKK 995 million in 2024 compared to DKK 982 million in 2023 and DKK 324 million in 2022. The increase of DKK 13 million, or 1% from 2023 to 2024, was primarily driven by the higher cash and cash equivalents and marketable securities in the first half of 2024 compared to 2023, almost entirely offset by lower cash and cash equivalents and marketable securities in the second half of 2024 compared to 2023 as a result of liquidating marketable securities and using cash to purchase ProfoundBio. The increase of 658 million, or 203% from 2022 to 2023 was primarily driven by higher effective interest rates in the U.S., Europe, and Denmark.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 52/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Foreign Exchange Rate Gains and Losses
Foreign exchange rate gain, net of DKK 1,339 million in 2024 compared to the foreign exchange rate loss, net of DKK 889 million in 2023 and foreign exchange rate gain, net of DKK 1,051 in 2022 were primarily driven by foreign exchange movements impacting Genmab’s USD denominated marketable securities and cash and cash equivalents; in particular, the USD/DKK foreign exchange rates were as follows for each period:
|
|
December 31, |
|
December 31, |
|
December 31, |
|
|
2024 |
|
2023 |
|
2022 |
USD/DKK Foreign Exchange Rates |
|
7.1429 |
|
6.7447 |
|
6.9722 |
% Increase/(Decrease) |
|
6% |
|
(3)% |
|
6% |
Marketable Securities Gains and Losses
GAIN ON MARKETABLE SECURITIES, NET WAS DKK 217 MILLION IN 2024 COMPARED TO GAIN ON MARKETABLE SECURITIES, NET OF DKK 319 MILLION IN 2023 AND LOSS ON MARKETABLE SECURITIES, NET OF DKK 361 MILLION IN 2022. THE DECREASE IN GAIN, NET OF DKK 102 MILLION, OR 32% FROM 2023 TO 2024 WAS PRIMARILY DRIVEN BY THE DECREASE IN MARKETABLE SECURITIES IN THE FIRST HALF OF 2024 TO FUND THE ACQUISITION OF PROFOUNDBIO AND SHARE REPURCHASE AS WELL AS CHANGING INTEREST RATE OUTLOOKS FOR THE U.S., PRIMARILY IN THE FOURTH QUARTER OF 2024. THE INCREASE IN GAIN, NET OF DKK 680 MILLION, OR 188% FROM 2022 TO 2023, WAS PRIMARILY DRIVEN BY INTEREST RATE OUTLOOKS FOR THE U.S. AND EUROPE.
Other Investments
Gains on other investments, net were DKK 30 million in 2024, losses on other investments, net were DKK 26 million in 2023 and DKK 297 million in 2022. The net gains and losses in 2024 and 2023 were primarily driven by changes in fair value of Genmab’s investments in certain strategic investment funds. The losses in 2022 were primarily driven by the change in fair value of Genmab’s investment in common shares of CureVac.
Refer to Notes 4.2 and 4.5 for further details regarding foreign currency risk and net financial items, respectively.
CORPORATE TAX
Corporate tax expense was DKK 1,320 million in 2024 compared to DKK 1,285 million in 2023 and DKK 1,493 million in 2022. Genmab’s estimated annual effective tax rate was 14.4% in 2024 compared to 22.8% in 2023 and 21.5% in 2022. The decrease from 2023 to 2024 in Genmab’s effective tax rate was primarily due to the integration of ProfoundBio which allowed for the deduction of previously unrecognized deferred tax assets in 2024. The increase from 2022 to 2023 in Genmab’s effective tax rate was mainly driven by the increase of unrecognized deferred tax assets.
We anticipate that our effective tax rate should be closer to the Danish statutory rate of 22% going forward.
Refer to Note 2.4 for additional information regarding the corporate tax, deferred tax assets and deferred tax liabilities including management’s significant judgements and estimates.
NET PROFIT
Net profit for 2024 was DKK 7,844 million compared to DKK 4,352 million in 2023 and DKK 5,452 million in 2022. The changes in net profit for the periods were driven by the items described above.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 53/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
LIQUIDITY AND CAPITAL RESOURCES
|
|
December 31, |
|
(DKK million) |
|
2024 |
2023 |
Marketable securities |
|
11,243 |
13,268 |
Cash and cash equivalents |
|
9,858 |
14,867 |
Shareholders' equity |
|
36,697 |
31,610 |
As of December 31, 2024, cash and cash equivalents and marketable securities denominated in USD represented 85% of Genmab’s total cash and cash equivalents and marketable securities compared to 90% as of December 31, 2023.
Marketable securities are invested in highly secure and liquid investments with short effective maturities. As of December 31, 2024, 71% of Genmab’s marketable securities were long-term A rated or higher, or short-term rated A-1 / P-1 by S&P, Moody’s or Fitch compared to 72% as of December 31, 2023.
As of December 31, 2024, DKK 9,858 million, as compared to DKK 14,867 million as of December 31, 2023, was held as cash and cash equivalents, and as of December 31, 2024, DKK 11,243 million, as compared to DKK 13,268 million as of December 31, 2023, was held as liquid investments in short-term government and other debt instruments.
Cash and cash equivalents included short-term marketable securities of DKK 82 million at the end of December 2024, compared to DKK 1,353 million at the end of December 2023. In accordance with Genmab’s accounting policy, securities purchased with a maturity of less than 90 days at the date of acquisition are classified as cash and cash equivalents.
Genmab requires cash to meet our operating expenses and capital expenditures. We have funded our cash requirements since inception, including through December 31, 2024, primarily with royalty and milestone payments from our partners, upfront payments, and equity financing. Genmab expects to continue to fund a significant portion of our development costs for proprietary product candidates as well as commercialization activities with cash received from royalties and milestone payments from partners, and net sales of Genmab products.
During the fourth quarter of 2024, Genmab entered into an unsecured three-year revolving credit facility (“Credit Facility”) of up to USD 300 million with a syndicate of lenders. Genmab intends to use the Credit Facility to finance working capital needs, and for general corporate purposes, of Genmab A/S and its subsidiaries. The Credit Facility includes options to increase the size of the facility up to USD 500 million as well as the ability to extend for an additional two years. The Credit Facility contains certain customary financial covenants. As of December 31, 2024, there were no outstanding amounts due on, nor any usage of, the Credit Facility and Genmab was in compliance with all financial covenants.
Genmab’s expenditures on current and future preclinical and clinical development programs are subject to numerous uncertainties in timing and cost to completion. In order to advance our product candidates toward commercialization, the product candidates are tested in numerous preclinical safety, toxicology and efficacy studies. Genmab then conducts clinical trials for those product candidates that take several years or more to complete. The length of time varies substantially based upon the type, complexity, novelty and intended use of a product candidate. The cost of clinical trials may vary significantly over the life of a project as a result of a variety of factors, including: the number of patients required in the clinical trials; the length of time required to
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 54/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
enroll trial participants; the number and location of sites included in the trials; the costs of producing supplies of the product candidates needed for clinical trials and regulatory submissions; the safety and efficacy profile of the product candidate; the use of CROs to assist with the management of the trials; and the costs and timing of, and the ability to secure, regulatory approvals.
Genmab’s expenses also fluctuate from period to period based on the degree of activities with collaborative partners, timing of manufacturing campaigns, numbers of patients enrolled in clinical trials and the outcome of each clinical trial event. As a result, Genmab is unable to determine with any degree of certainty the anticipated completion dates, duration and completion costs of research and development projects, or when and to what extent Genmab will receive cash inflows from the commercialization and sale of any product candidates. Genmab also cannot predict the actual amount or timing of future royalties and milestone payments, and these may differ from estimates.
Genmab expects to increase operating expenditures and make additional capital outlays over the next several years as Genmab supports preclinical development, manufacturing, clinical trial activities, product collaborations, commercialization activities and additional hiring of staff. As spending increases on research, development, business development and /or activities related to mergers and acquisitions, and commercialization activities related to product collaborations, Genmab may be required to make certain capital outlays against which Genmab expects to receive reimbursement to the extent the outlay exceeds Genmab’s share under the applicable collaboration agreement. Genmab expects that the time-lag between the expenditure by Genmab, and the reimbursement by a partner of its relevant share, may increase Genmab’s working capital needs. To the extent Genmab’s capital resources are insufficient to meet future capital requirements, Genmab will need to finance operating requirements and other cash needs through the use of the Credit Facility, public or private equity offerings, debt financings, or additional corporate collaboration and licensing arrangements.
Refer to Notes 4.1, 4.2 and 4.4 for additional information regarding our external source of liquidity, financial, risks and marketable securities, respectively.
CASH FLOWS
The following table provides information regarding Genmab’s cash flow for 2024, 2023 and 2022.
Cash Flow (DKK million) |
2024 |
2023 |
2022 |
Cash provided by operating activities |
7,771 |
7,380 |
3,912 |
Cash (used in) investing activities |
(9,907) |
(1,282) |
(2,761) |
Cash (used in) financing activities |
(3,919) |
(606) |
(789) |
Increase in cash and cash equivalents |
(6,055) |
5,492 |
362 |
|
|
|
|
Exchange rate adjustments |
1,046 |
(518) |
574 |
Net cash provided by operating activities is primarily related to our operating profit, changes in operating assets and liabilities, reversal of net financial items, and adjustments related to non-cash transactions. Cash provided by operating activities increased in 2024 compared to 2023 primarily driven by an increase in net profit before tax of DKK 3.5 billion, an increase in non-cash transactions of DKK 368 million, and a decrease in taxes paid of DKK 724 million in 2024 compared to 2023, partly offset by significant AbbVie milestones achieved during the fourth quarter of 2022 with related cash received during 2023 and an increase in DARZALEX royalty receivables in the fourth quarter of 2024 compared to the fourth quarter of 2023. Cash provided by operating activities increased in 2023 compared to 2022 primarily driven by significant AbbVie
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 55/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
milestones achieved during the fourth quarter of 2022 with related cash received during 2023, cash received for DARZALEX royalties in 2023, and higher corporate tax payments made in 2023 compared to 2022.
Net cash (used in) investing activities primarily reflects cash used in making acquisitions, differences between the proceeds received from the sale and maturity of our investments and amounts invested, and the cash paid for investments in tangible and intangible assets. The increase from 2023 to 2024 in net cash (used in) investing activities is primarily driven by the acquisition of ProfoundBio, partly offset by the sales and maturities of marketable securities exceeding purchases in 2024, compared to purchases exceeding sales and maturities in 2023. The decrease from 2022 to 2023 in net cash (used in) investing activities is primarily driven by purchases of marketable securities exceeding sales and maturities to a greater extent during 2022 compared to 2023.
Net cash (used in) financing activities is primarily related to the purchase of treasury shares, exercise of warrants, lease payments, and payment of withholding taxes on behalf of employees on net settled Restricted Stock Units (RSUs). The increase from 2023 to 2024 in net cash (used in) financing activities is primarily driven by cash payments for the purchase of treasury shares of DKK 3,879 million in 2024 compared to DKK 564 million in 2023. The decrease from 2022 to 2023 in net cash (used in) financing activities is primarily driven by cash payments for the purchase of treasury shares of DKK 564 million in 2023 compared to DKK 908 million in 2022.
Exchange rate adjustments represent foreign currency gains or losses on Genmab’s cash and cash equivalents, primarily driven by our cash and cash equivalents holdings denominated in USD. The USD/DKK foreign exchange rate increased 6% in 2024, decreased 3% in 2023 and increased 6% in 2022.
BALANCE SHEET
As of December 31, 2024, total assets were DKK 45,811 million, compared to DKK 35,289 million as of December 31, 2023. As of December 31, 2024, assets are mainly comprised of intangible assets of DKK 12,343 million, primarily made up of intangible assets acquired in the ProfoundBio acquisition, marketable securities of DKK 11,243 million, current receivables of DKK 6,590 million, cash and cash equivalents of DKK 9,858 million and DKK 2,535 million of goodwill related to the acquisition of ProfoundBio. The current receivables consist primarily of amounts related to royalties from our collaboration agreements. The credit risk related to our receivables is not material based on no historical credit losses as well as the high-quality nature of Genmab’s collaboration partners and limited number of distributors with high credit standing.
Refer to Note 3.6 for additional information regarding receivables and Note 5.5 for additional details related to the acquisition of ProfoundBio.
As of December 31, 2024, total liabilities were DKK 9,114 million compared to DKK 3,679 million as of December 31, 2023. The increase in total liabilities of DKK 5,435 million, or 148%, was primarily driven by the DKK 2,359 million deferred tax liability related to the acquisition and integration activities for ProfoundBio, an increase of DKK 1,656 million in corporate taxes payable due to Genmab’s net profit before tax, an increase of DKK 1,170 million in accruals related to the expansion of our product pipeline, and an increase in lease liabilities of DKK 259 million driven by the commencement of a lease in the U.S. with respect to office and laboratory space.
Shareholders’ equity as of December 31, 2024 was DKK 36,697 million compared to DKK 31,610 million as of December 31, 2023. The increase of DKK 5,087 million, or 16%, was driven primarily by Genmab’s net profit for the period and share-based compensation expenses, partly offset by the purchase of treasury shares.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 56/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Genmab’s equity ratio was 80% as of December 31, 2024 compared to 90% as of December 31, 2023. The decrease was primarily attributable to assets acquired in the acquisition of ProfoundBio, net of cash paid, in addition to the share buy-back completed in June 2024 that offset Genmab’s net profit for the period.
LEGAL MATTERS – J&J BINDING ARBITRATIONS
In September 2020, Genmab commenced arbitration against J&J with respect to two different provisions of our license agreement for daratumumab, both relating to royalties payable to Genmab on net sales of daratumumab (marketed as DARZALEX for IV administration and as DARZALEX FASPRO in the U.S. and as DARZALEX SC in Europe for SC administration). In April 2022, the arbitral tribunal issued an award in that arbitration denying both of Genmab’s claims. Genmab did not seek review of the award. On June 9, 2022, Genmab commenced a second arbitration against J&J under the license agreement, in which Genmab sought additional compensation from J&J with respect to SC daratumumab based on Genmab’s position that the award in favor of J&J in the first arbitration was premised on that tribunal’s determination that IV daratumumab and SC daratumumab were separate “Licensed Products” as that term is defined in the license agreement. Genmab’s claim in that second arbitration was denied by the tribunal on April 21, 2023 on the ground that it should have been brought in the first arbitration, and the dismissal was affirmed by an appellate arbitrator on January 23, 2024.
In June 2024, Chugai Pharmaceutical Co., Ltd. filed a lawsuit in the Tokyo District Court, Japan against AbbVie’s and Genmab’s subsidiaries in Japan asserting that their activities with EPKINLY (epcoritamab) in Japan infringe two Japanese patents held by Chugai, JP6278598 and JP6773929. Chugai is claiming damages and injunctive relief.
Genmab and AbbVie believe that the two Japanese patents are invalid and not infringed and intend to vigorously defend against the lawsuit, and thus no provision has been recorded related to this matter.
Refer to Note 5.7 for further details about contingencies.
Financial Review – Parent
REVENUE
Genmab A/S’s revenue was DKK 22,167 million in 2024 compared to DKK 17,126 million in 2023. The increase of DKK 5,041 million, or 29%, was primarily driven by higher DARZALEX and Kesimpta royalties achieved under our collaborations with J&J and Novartis, respectively. Increased EPKINLY intercompany net product sales, driven by a strong product launch in 2023 with a full year of net sales in 2024, also contributed to increased revenue in 2024.
FINANCIAL INCOME AND EXPENSE
Genmab A/S’ financial income was DKK 17,404 million in 2024 compared to DKK 2,199 million in 2023. The increase of DKK 15,205 was primarily driven by the DKK 13.0 billion of dividend income Genmab A/S received related to the sale of ProfoundBio US intangible assets to Genmab A/S.
Genmab A/S’ financial expense was DKK 12,239 million in 2024 compared to DKK 1,871 million in 2023. The increase of DKK 10,368 was primarily driven by the DKK 10.4 billion impairment related to Genmab A/S’ investment in subsidiaries.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 57/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Refer to Note 14 in the parent company financial statements for further details related to the transfer of ProfoundBio US intangible assets to Genmab A/S.
Risk Management
Genmab has core facilities in five countries that perform research and development activities with clinical trials conducted around the globe. We also have commercial and sales organizations in the U.S. and Japan with manufacturing support activities in Europe. Through our activities, we are exposed to a variety of risks, some of which are inherent in our business and/or beyond our control including sustainability-related risks. These risks may have a significant impact on our business if not properly assessed and controlled. Maintaining a strong control environment, with adequate procedures for identification, prioritization and assessment of risks and adhering to operational policies designed to reduce such risks to an acceptable level, is essential for the continued evolution of Genmab. It is our policy to identify and reduce the risks derived from our operations and to establish insurance coverage and other enterprise risk reduction and resilience mechanisms to mitigate any residual risk, wherever considered practicable. The Audit and Finance Committee of the Board performs a yearly review of Genmab’s Enterprise Risk Program and relevant insurance coverage to ensure that they are appropriate for Genmab. For further information about the risks and uncertainties that Genmab faces, refer to the current Form 20-F filed with the SEC.
The use of data, as defined in the Danish Financial Statements Act, both personal and non-personal, is essential to fulfilling Genmab’s core purpose; and Genmab is committed to handling data with integrity and in an ethical and compliant manner considering the impact our actions may have on individuals and society.
Genmab has a policy for Data Ethics in compliance with Section 99d of the Danish Financial Statements Act in which Genmab adopted the Data Ethics principles of the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA).
These principles complement and strengthen already existing Genmab policies and procedures, and they focus on the following areas:
| 1. | Autonomy: Respect individuals’ privacy, protect their rights, and honor confidentiality |
| 2. | Transparency: Individuals should be able to understand how their personal data is used |
| 3. | Data Quality: The best quality data available should be used to make decisions |
| 4. | Fairness and Non-discrimination: Data acquisition should be inclusive, equitable, and seek to support the industry’s mission of responding to the needs of all patients |
| 5. | Ethics by Design: Controls to prevent harm and risks to individuals should be built into the design of data architecture and data processing |
| 6. | Responsible Data Sharing: Data sharing should be based on processes that actively and consistently consider, prioritize, and protect individual rights |
| 7. | Responsibility and Accountability: Data Ethics Principles should be operationalized through effective governance, clear standards, training, monitoring activities, and disciplinary sanctions |
Genmab will continue to focus on these principles, particularly in the areas of data privacy, DE&I, clinical trials, and the application of new technologies (e.g., Artificial Intelligence and Machine Learning), where policies, processes, and training materials will be aligned with the above-mentioned principles. The Genmab Data Ethics policy and its principles are anchored in the Genmab Code of Conduct as part of the overall Genmab Compliance program.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 58/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The following is a summary of Genmab’s key risk areas, including sustainability-related risks, and how we address and mitigate such risks.
Risk related to |
Risk areas |
Mitigation |
Risk trend |
|---|---|---|---|
Business and Products |
The identification and development of successful products is expensive and includes time-consuming clinical trials with uncertain outcomes and the risk of failure to obtain regulatory approval in one or more jurisdictions. |
Genmab has a disciplined approach to investment, focusing on areas with the potential to maximize success, including new technologies and formats, scaling up to expand from early- to late-stage development and commercialization. Genmab has established various committees to ensure optimal selection of disease targets and formats of our antibody candidates, and to monitor progress of preclinical and clinical development. We strive to have a well-balanced product pipeline, continuing to search for and identify new product candidates, and closely monitoring the market landscape. |
= |
|
Genmab is dependent on the identification and development of new proprietary technologies and access to new third-party technologies. This exposes us to safety issues as well as other failures and setbacks related to use of such new or existing technologies. |
Genmab strives to identify and develop new antibody-based products that harness new antibody technologies, such as the DuoBody, HexaBody, DuoHexaBody and HexElect technology platforms, ADC technology, and gain access to competitive and complementary new third-party technologies. We closely monitor our preclinical programs and clinical trials to mitigate any unforeseen safety issues or other failures, or setbacks associated with the use of these technology platforms. |
= |
|
Genmab faces ongoing uncertainty about the successful commercialization of product candidates. This is a result of factors including immense competition on the basis of cost and efficacy as well as rapid technological change, which may result in others discovering, developing or commercializing competing products before and/or more successfully than us. |
From early in the research phase and throughout development, commercial potential and product commercialization, associated risks are assessed to ensure that final products have the potential to be commercially viable. Genmab attempts to control commercial risks in part by regularly monitoring and evaluating current market conditions, competing products and new technologies, to potentially gain access to new technologies and products that may supplement our pipeline. Genmab also strives to ensure market exclusivity for its own technologies and products by seeking patent protection. Genmab engages with patients and caregivers to gather insights and improve patient outcomes. |
= |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 59/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Risk related to |
Risk areas |
Mitigation |
Risk trend |
|---|---|---|---|
|
Genmab’s near- and mid-term prospects are substantially dependent on continued clinical and commercial success of DARZALEX. The impact of DARZALEX patent expirations, typically followed by the introduction of competing generic, biosimilar or other products could have an adverse impact on Genmab’s future royalty revenue. DARZALEX is subject to intense competition in the multiple myeloma therapy market. |
Genmab focuses on its three-pronged strategy of focusing on our core competence, turning science into medicine and building a profitable and successful biotech to develop a broad pipeline of unique best-in-class or first-in-class antibody products with significant commercial potential. In addition, Genmab maintains a strong cash position, disciplined financial management, and a flexible and capital efficient business model to mitigate potential setbacks related to DARZALEX. To address the impact of DARZALEX loss of exclusivity, Genmab intends to mitigate this risk through its strong foundation in science and investments in launched medicines as well as its existing, and potential acquisition of new, late-stage assets. Genmab manages and maintains efficient operations through focused prioritization and increased productivity. Including DARZALEX there are eight commercialized medicines on the market that drive significant recurring revenue for the company. In 2020, two additional Genmab-created antibody products, Kesimpta and TEPEZZA, were approved by the U.S. FDA. In 2021, 2022, and 2023, respectively, Genmab’s bispecific DuoBody technology was the basis for the DuoBody-based medicines RYBREVANT, TECVAYLI and TALVEY, which were approved by the U.S. FDA and the EC. All of these provide Genmab with additional recurring royalty revenue. Tivdak, Genmab’s first medicine, in development with Pfizer, was approved by the U.S. FDA and product sales of Tivdak commenced in 2021. EPKINLY/TEPKINLY, Genmab’s second medicine, in development with AbbVie, was approved by the U.S. FDA, the Japan MHLW and the EC and product sales of EPKINLY/TEPKINLY commenced in 2023. In addition, we currently have two wholly owned programs, Rina-S and acasunlimab, which moved into Phase 3 development in 2024. |
> |
|
Genmab has exposure to product liability claims related to the use or misuse of our products and technologies. |
Product liability claims and/or litigation could materially affect our business and financial position, and Genmab therefore strives to maintain internal processes for the review, approval, and compliant use of promotion materials and also maintains appropriate product liability insurance for our clinical trials and our approved products and other coverage required under applicable laws. |
= |
|
Our core research and manufacturing activities are carried out at a limited number of locations. Any event resulting in Genmab’s or our vendors’/suppliers’ inability to operate these facilities could materially disrupt our business. |
Genmab employs oversight and quality risk management principles. In addition, Genmab follows current Good Laboratory Practices (cGLP) and current Good Manufacturing Practices (cGMP) and requires that our vendors operate with the same standards. Genmab’s quality assurance (QA) department ensures that high-quality standards are set and monitors adherence to these practices. |
= |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 60/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Risk related to |
Risk areas |
Mitigation |
Risk trend |
|---|---|---|---|
|
If we are unable to effectively manage Genmab’s fast-paced growth, or maintain our commercialization and other capabilities at adequate levels, and control operating costs within the scope of our overall business as well as properly integrate acquisitions, financial condition and net profits may be adversely affected. Any business disruption or failure to properly manage growth, maintain capabilities and transformation in a manner that reflects and supports our organizational strategies and priorities, while assuring ethical business practices, prudent risk management, and commercial compliance, could have a material adverse effect on our business, financial condition, results of operations and cash flows. |
We have experienced rapid growth over the last several years. We anticipate additional growth as our pipeline advances and we continue product commercialization activities. Such growth, including maintaining and enabling R&D, commercialization, and support functions, has placed significant demands on our management and infrastructure, including new operational and financial systems, as well as extending manufacturing and commercial outsource arrangements. Our success will depend in part upon our ability to manage and maintain operations and integrate acquisitions effectively through leadership, focused prioritization, increased productivity and talent management to maintain our values-based, collaborative culture. As we continue to grow and evolve, we must continuously improve our operational, commercial, compliance, financial and management practices, and controls. |
= |
|
Genmab is subject to government regulations on pricing/public reimbursement as well as other healthcare payer cost-containment initiatives; increased pressures by governmental and third-party payers to reduce healthcare costs. |
Genmab strives to develop differentiated antibody medicines that bring meaningful impact to patients and health systems and are well-positioned to secure reasonable price reimbursement by government healthcare programs and private health insurers. The impact our science has on patients today and in the future, particularly those with few treatment options, drives the value of our medicines. Genmab’s U.S. Government Affairs & Policy department interacts with U.S. federal and state policymakers to advance policies aimed at improving patients’ lives through access to quality healthcare and innovative science. Genmab’s U.S. Market Access department educates payers on the value of our products and works across the healthcare system to help ensure all appropriate patients gain access to our innovative medicines. |
> |
Strategic Collaborations |
Genmab is dependent on existing partnerships with major pharmaceutical or biotech companies to support our business and develop and extend the commercialization of our products. |
Our business may suffer if our collaboration partners do not devote sufficient resources to our programs and products, do not successfully maintain, defend and enforce their intellectual property rights or do not otherwise have the ability to successfully develop or commercialize our products, independently or in collaboration with others. Our business may also suffer if we are not able to continue our current collaborations or establish new collaborations. Genmab strives to be an attractive and respected collaboration partner, and to pursue a close and open dialogue with our collaboration partners to share ideas and align on best practices and decisions within clinical development and commercial operations to increase the likelihood that we reach our goals. |
= |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 61/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Risk related to |
Risk areas |
Mitigation |
Risk trend |
|---|---|---|---|
|
Genmab is primarily dependent on one contract manufacturing organization (CMO) and individual sites at the CMO to produce and supply our product candidates. Genmab is also dependent on clinical research organizations to conduct key aspects of our clinical trials, and on collaboration partners to conduct some of our clinical trials. CMOs may be subject to or affected by various U.S. legislation, executive orders, regulations, or investigations. |
Genmab oversees outsourcing and partnership relationships to ensure consistency with strategic objectives and service provider compliance with regulatory requirements, resources, and performance. This includes assessment of contingency plans, availability of alternative service providers and costs and resources required to switch service providers. We continually evaluate financial solvency and require our suppliers to abide by a code of conduct consistent with Genmab’s Code of Conduct. |
= |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 62/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Risk related to |
Risk areas |
Mitigation |
Risk trend |
|---|---|---|---|
Regulation, Legislation, and Compliance |
Genmab is subject to extensive legislative, regulatory and other requirements during preclinical and clinical development, commercialization, and post-marketing approval, including healthcare, marketing/labelling/promotion, fraud and abuse, competition/antitrust laws, and regulations, as well as transparency, privacy and data protection and other requirements.
Genmab is subject to strict disclosure obligations under applicable laws and regulations globally, including the EU Market Abuse Regulation and the U.S. Inflation Reduction Act (IRA). Being listed on the Nasdaq Global Select Market, we are subject to additional U.S. regulatory requirements, including U.S. securities laws and the U.S. Foreign Corrupt Practices Act, and may become more exposed to U.S. class actions. |
To ensure compliance with applicable healthcare laws and regulations, Genmab has established a compliance program, including a Code of Conduct that is regularly evaluated and sets high ethical standards on which all colleagues receive regular training. Genmab also maintains a Speak Up Policy and Hotline for reporting and response to potential misconduct. Our head of Global Compliance reports directly to the CEO. Genmab is committed to transparency of clinical trial research and has published our Clinical Trial Transparency Declaration. Genmab is also committed to ensuring equal access to Genmab clinical trials and that patients participating in our trials are representative of those living with the disease being studied. Genmab respects the privacy, protection, and appropriate use of data by ensuring compliance with all applicable privacy and data protection laws, regulations, and other standards. In support of this commitment, Genmab established its Global Data Privacy Office supported by a cross-functional team of privacy subject matter experts, including a Data Protection Officer, who collaborate in the development and maintenance of a forward-looking Global Data Privacy Program that seeks address shifts in both the internal and external environments, along with emerging challenges in the privacy and data protection regulatory landscape. The Program, through its policies, procedures, and centralized guidance for processing personal data, seeks to drive organizational accountability and empower Genmab colleagues, and our third party partners, to handle personal data consistent with our values of ethical behavior, integrity, fairness, inclusion, and transparency. To further support compliance with regulatory, legal, and other requirements applicable to our business and operations, including current Good Laboratory Practices (cGLP), current Good Clinical Practices (cGCP) and current Good Manufacturing Practices (cGMP), Genmab’s QA department is staying abreast of and adhering to regulatory and legislative changes relevant to quality standards. Genmab has also established relevant procedures and guidelines to ensure transparency with respect to providing timely, adequate, and correct information to the market and otherwise complying with applicable securities laws and other legal and regulatory requirements. Genmab has an Internal Audit function that reports to the Audit and Finance Committee of the Board and administratively reports to the CFO. |
> |
|
Legislation, regulations, industry codes and practices, and their application may change from time to time. |
To prevent unwarranted consequences of new and amended legislation, regulations, etc., Genmab strives to stay current with respect to all applicable legislation, regulations, industry codes and practices by means of its internal compliance function and related governance bodies as well as internal and external legal counsel. Also, internal procedures for review and refinement of contracts are ongoing to ensure contractual consistency and compliance with applicable legislation, regulation, and other standards. |
= |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 63/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Risk related to |
Risk areas |
Mitigation |
Risk trend |
|---|---|---|---|
Intellectual Property |
Genmab is dependent on protecting our own intellectual property rights to regain our investments and protect our competitive positions. We may become involved in lawsuits to protect or enforce our patents or other intellectual property which could result in costly litigation and unfavorable outcomes. Claims may be asserted against us that we infringe the intellectual property of third parties, which could result in costly litigation and unfavorable outcomes. |
Genmab files and prosecutes patent applications to optimally protect its products and technologies. To protect trade secrets and technologies, Genmab maintains strict confidentiality standards and agreements for employees and collaborating parties.
Genmab actively monitors third-party patent positions within our relevant fields to avoid violating any third-party patent rights. |
= |
Finances |
Genmab may need additional funding. |
Because Genmab’s future commercial potential and operating profits are hard to predict, Genmab’s policy is to maintain a strong capital base so as to maintain investor, creditor and market confidence, and a continuous advancement of Genmab’s product pipeline and business in general. Genmab also has access to revolving loan facility totaling USD 300 million, with options to increase size of the facility up to USD 500 million, which can be drawn down upon as another source of additional funding. |
= |
|
Genmab is exposed to different kinds of financial risks, including currency exposure and changes in interest rates as well as changes in Danish, U.S. or foreign tax laws or related compliance requirements. |
Genmab has established financial risk management guidelines to identify and analyze relevant risks, to set appropriate risk limits and controls, and to monitor the risks and adherence to limits. Please refer to Note 4.2 of the financial statements for additional information regarding financial risks. |
= |
Management and Workforce |
Genmab may have an inability to attract and retain suitably qualified team members as it continues to evolve. |
To attract and retain our highly skilled team, including the members of Genmab’s Executive Management, Genmab offers competitive remuneration packages, including share-based remuneration. Genmab strives to create a positive, safe, and energizing working environment. Genmab has strong core values that nourish high-integrity and ethical behavior, respectful and candid tone and a culture which prizes diversity, as well as trust and teamwork. Genmab has implemented strategies such as diversifying recruitment efforts, cross-training employees, fostering a culture of knowledge sharing, investing in talent development programs, and promoting a supportive work environment that values employee well-being and career growth. Please refer to Note 4.6 of the financial statements for additional information regarding share-based compensation. |
= |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 64/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Risk related to |
Risk areas |
Mitigation |
Risk trend |
|---|---|---|---|
Cybersecurity |
Genmab may be subject to malicious cyber attacks, and with the increased use of artificial intelligence within the biopharmaceutical industry, can lead to the theft or leakage of intellectual property, sensitive business data, or personal employee or patient data, with the result of significant business disruptions, negative impacts to patient or employee privacy, monetary loss or fines from authorities, or reputational damage. |
Genmab has implemented security controls and processes to enhance the identification of potential data/systems security issues and mitigate the risk of security breaches. Genmab makes use of the National Institute of Standards and Technology (NIST) Cybersecurity Framework and other security standards to define and implement such security controls. Due to the continually changing threat environment, regular assessments are executed to ensure that implemented security controls and processes follow the threat profile of the Company and effectively support Genmab’s ambitious business strategy. The risk of security breaches is regarded as enterprise risk and the Company’s threat profile, the security program and security incidents are presented and discussed in meetings of the Global Compliance and Risk Committee and the Audit and Finance Committee of the Board. Genmab’s Cybersecurity Program, in conjunction with Genmab’s Global Data Privacy Program, collaborate to manage and mitigate any cybersecurity and data privacy threats to the personal data processed in our systems and by our third party partners. |
= |
Environment |
Genmab could face transitional risks by its inability to manage the carbon footprint and energy mix from our business operations and physical risks from climate-related events that may impact our business operations or that of our third-party partners or suppliers. |
Genmab has oversight and manages its carbon footprint Scope 1 and 2 emissions from its business operations. Genmab is committed to tracking the Scope 3 emissions carbon footprint by partnering with suppliers. Genmab makes use of scenario analysis to evaluate risks and opportunities due to the rapid pace of world climate change. Genmab’s work with climate strategy, carbon reduction targets, climate-related financial risk, relevant prevention, and mitigation measures are presented to and reviewed by the Board biannually. Refer to the sustainability statements for details of Genmab’s targets in the future to mitigate risks. |
= |
Risk Level in Relation to Last Year: = Unchanged < Decreased > Increased
Enterprise Risk Management
As an international biotech company dedicated to improving the lives of cancer patients around the world, Genmab operates within a heavily regulated environment that exposes us to an ever-evolving set of risks, some of which are beyond our control. Genmab has core facilities in five countries, conduct activities in additional areas, and perform an array of essential innovation, research, development, manufacturing activities, commercial operations, and support functions, all of which pose risks to our operations and success. Specifically, these operations and activities expose us to risks that include but may not be limited to financial, research and development, regulatory, IT/data/technology, staffing, compliance, legal, and also environmental risks.
In order to assure that we are positioned to effectively identify and mitigate the potential impacts of these risks, Genmab has dedicated resources toward enabling its ERM framework under the Global Compliance & Risk function that reports directly to the CEO. In concert with a refreshed Code of Conduct, company policies and procedures, Genmab has chartered a Global Compliance and Risk Governance Committee (GCRC) co-chaired by the CEO and the head of Compliance & Risk. Genmab has also updated its risk model and
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 65/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
framework to include enhanced risk oversight, mitigation, governance, and reporting, all of which we believe positions us to better manage the risks associated with our business, now and into the future.
| ● | Board and Audit and Finance Committee: Board delegates ERM/Risk oversight to the Audit and Finance Committee but retains visibility of ERM progress. The Audit and Finance Committee is accountable to ensure management appropriately manages the risks to the business. |
| ● | Executive Management: Maintains ultimate ownership of and accountability for management of top risks, enabling proper linkage of risk management to strategic initiatives and business decisions. |
| ● | GCRC: Validates risk identification, prioritization, strategic and tactical ownership of risk mitigation plans and reporting. |
| ● | ERM Framework: Routinely gathers risks, evaluates with risk sponsors, prioritizes, and reports to the GCRC, Executive Management and Board, driving risk discussions, and supporting risk sponsors and management in facilitating ERM processes, risk-intelligent decision-making, and key risk capabilities. |
| ● | Risk Sponsors and Business Champions: Manage risks in the normal course of business, executing risk plans/mitigation activities, and monitoring and reporting key risk information. |
Corporate Governance
Genmab works diligently to improve its guidelines and policies for corporate governance, taking into account the recent trends in international and domestic requirements and recommendations. Genmab’s commitment to corporate governance is based on ethics and integrity and forms the basis of its effort to strengthen the confidence that existing and future shareholders, partners, employees, and other stakeholders have in Genmab. The role of shareholders and their interaction with Genmab is important. Genmab believes that open and transparent communication is necessary to maintain the confidence of Genmab’s shareholders and achieves this through company announcements, investor meetings and company presentations. Genmab is committed to providing reliable and transparent information about its business, financial results, development programs and scientific results in a clear and timely manner.
All Danish companies listed on the Nasdaq Copenhagen are required to disclose in their annual reports how they address the Recommendations for Corporate Governance issued by the Committee on Corporate Governance in December 2020 (the “Recommendations”), applying the “comply-or-explain” principle.
Genmab follows the Recommendations, except for one specific sub-area where Genmab’s corporate governance principles differ from the Recommendations:
| ● | The Recommendations provide that according to a company’s takeover contingency procedures, the Board abstains from countering any takeover bids by taking actions that seek to prevent the shareholders from deciding on the takeover bid, without the approval of the general meeting. Genmab does not have such a restriction in its takeover contingency procedures and retains the right in certain circumstances to reject takeover bids without consulting the shareholders. Genmab believes this provides the Board with the needed flexibility to best respond to takeover bids and to negotiate with bidders; retaining this flexibility helps the Board meet its objectives in protecting and creating value in the interest of the shareholders. Actions will be determined on a case-by-case basis |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 66/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| with due consideration of the interests of the shareholders and other stakeholders. |
Genmab publishes its statutory report on Corporate Governance for the financial year 2024 cf. Article 107b of the Danish Financial Statements Act (“Lovpligtig redegørelse for virksomhedsledelse jf. årsregnskabslovens § 107 b”) on the Company’s website, including a detailed description of the Board’s consideration in respect of all the Recommendations. The statutory report on Corporate Governance can be found on Genmab’s website https://ir.genmab.com/corporate-governance.
THE BOARD OF DIRECTORS
The Board is responsible for setting the overall strategy and goals for Genmab and monitoring its operations and results. Board duties include establishing policies for strategy, accounting, organization and finance and the appointment of Executive Management members. The Board also assesses Genmab’s capital and share structure and is responsible for approving share issues and the grant of warrants and RSUs.
The Board has established an annual process whereby the Board’s performance is assessed through self-evaluation to verify that the Board is capable of fulfilling its function and responsibilities. When performing these evaluations external assistance is obtained every year. The outcome of the Board‘s 2024 self-assessment was positive with only minor areas for improvement identified.
BOARD COMMITTEES
To support the Board in its duties, the Board has established and appointed a Compensation Committee, an Audit and Finance Committee, a Nominating and Corporate Governance Committee and a Scientific Committee. These committees are charged with reviewing issues pertaining to their respective fields that are due to be considered at Board meetings. Written charters specifying the tasks and responsibilities for each of the committees are available on Genmab’s website www.genmab.com.
For more details on the work, composition and evaluation of the Board and its committees, reference is made to the statutory report on Corporate Governance.
REMUNERATION POLICY
A Remuneration Policy applying to the compensation of members of the Board and the registered Executive Management of Genmab A/S has been prepared in accordance with Sections 139 and 139a of the Danish Companies Act and was most recently considered and adopted by the 2024 Annual General Meeting pursuant to the Danish Companies Act (in Danish “Selskabsloven”). The Remuneration Policy contains an exhaustive description of the remuneration components for members of the Board and the registered Executive Management and includes the reasons for choosing the individual components of the remuneration and a description of the criteria on which the balance between the individual components of the remuneration is based. The latest version can be downloaded from Genmab’s website https://ir.genmab.com/compensation.
COMPENSATION REPORT
In accordance with the Recommendations, Genmab has prepared a compensation report for the financial year 2024 that includes information on the total remuneration received by each member of the Board and the registered Executive Management of Genmab A/S for the last four years, including information on the most important content of retention and resignation arrangements and the correlation between the remuneration and company strategy and relevant related goals (the “Compensation Report”). The Compensation Report can be found on Genmab’s website https://ir.genmab.com/compensation.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 67/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
CHANGE OF CONTROL
The Danish Financial Statements Act (Section 107a) contains rules relating to listed companies with respect to certain disclosures that may be of interest to the stock market and potential takeover bidders, in particular in relation to disclosure of change of control provisions. In the event of a change of control, change of control clauses are included in some of our collaboration, development, and license agreements as well as in service agreements for certain employees.
Collaboration, Development and License Agreements
Genmab has entered into collaboration, development and license agreements with external parties, which may be subject to renegotiation in the case of a change of control event as specified in the individual agreements. However, any changes in the agreements are not expected to have significant impact on our financial position.
Service Agreements with Executive Management and Employees
The service agreements with each registered member of the Executive Management may be terminated by Genmab with no less than 12 months’ notice and by the registered member of the Executive Management with no less than six months’ notice. In the event of a change of control of Genmab, the termination notice due to the registered member of the Executive Management is extended to 24 months. In the event of termination by Genmab (unless for cause) or by a registered member of Executive Management as a result of a change of control of Genmab, Genmab is obliged to pay a registered member of Executive Management a compensation equal to his/her existing total salary (including benefits) for up to two years in addition to the notice period.
In addition, Genmab has entered into service agreements with a limited number of employees according to which Genmab may become obliged to compensate the employees in connection with a change of control of Genmab. If Genmab, as a result of a change of control, terminates the service agreement without cause or changes the working conditions to the detriment of the employee, the employee shall be entitled to terminate the employment relationship without further cause with one month’s notice in which case Genmab shall pay the employee a compensation equal to one-half, one or two times the employee’s existing annual salary (including benefits).
Change of control clauses related to our warrant and RSU programs are outlined in Note 4.6.
SHARE CAPITAL
Information on share capital is included in Note 4.7. Unless otherwise provided in the Danish Companies Act, the adoption of any resolution to amend Genmab A/S’ articles of association shall be subject to the affirmative vote of not less than two thirds of the votes cast, as well as of the voting share capital represented at the general meeting. Genmab A/S’ entire articles of association can be found on our website www.genmab.com.
Board of Directors
Refer to our website: Board of Directors | Genmab for the Board of Directors diversity and skills matrix.
Deirdre P. Connelly
Female, Hispanic/American, 64
Board Chair (Independent, elected by the General Meeting); Chair of the Nominating and Corporate Governance Committee, Member of the Audit and Finance Committee and the Compensation Committee
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 68/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
First elected 2017, current term expires 2025
Special Competencies and Qualifications
Deirdre P. Connelly has more than 30 years’ experience as a corporate leader and board member in publicly traded companies with global operations. She has comprehensive knowledge and experience with business conduct, business turnaround and product development and has successfully directed the launch of more than 20 new pharmaceutical drugs. As a former HR executive, Deirdre P. Connelly also has valuable insight in corporate culture transformation, talent development and managing large organizations. She furthermore has significant experience with the development of governance and other sustainability related responsibilities from various leadership roles and as a board member. Deirdre P. Connelly is former President of U.S. Operations of Eli Lilly and Company and former President, North America Pharmaceuticals for GlaxoSmithKline.
ESG Competencies: Social · Governance
Current Board Positions
Member: Lincoln Financial Corporation1, Macy’s Inc.2
1. Chair of Compensation Committee, Member of Audit Committee, Corporate Governance Committee and Executive Committee
2. Chair of Nominating and Corporate Governance Committee, Member of Compensation and Management Development Committee
Pernille Erenbjerg
Female, Danish, 57
Deputy Board Chair (Independent, elected by the General Meeting); Chair of the Audit and Finance Committee, Member of the Nominating and Corporate Governance Committee
First elected 2015, current term expires 2025
Special Competencies and Qualifications
Pernille Erenbjerg has broad executive management and business experience from the telecoms, media, and tech industries. She has extensive expertise with business conduct and in operation and strategic transformation of large and complex companies, including digital transformations and digitally based innovation, and has been responsible for major transformation processes in complex organizations including M&A. Pernille Erenbjerg furthermore has significant IT and cybersecurity expertise and sustainability related experience from various executive and non-executive positions. She has a Certified Public Accountant background (no longer practicing) and has a comprehensive all-around background within finance, including extensive exposure to public and private equity and debt investors. Pernille Erenbjerg is former CEO and President of TDC Group A/S. Pernille Erenbjerg is an audit committee financial expert based on her professional experience, including her background within accounting, her service in senior finance leadership at TDC Group A/S and as an audit committee chair or member at other public companies.
ESG Competencies: Environmental · Social · Governance
Current Board Positions
Chair: KK Wind Solutions
Member: RTL Group1, GlobalConnect
1. Chair of Audit Committee
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 69/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Anders Gersel Pedersen, M.D., Ph.D.
Male, Danish, 73
Board Member (Non-independent, elected by the General Meeting); Chair of the Compensation Committee and Member of the Scientific Committee and the Nominating and Corporate Governance Committee
First elected 2003, current term expires 2025
Special Competencies and Qualifications
Anders Gersel Pedersen has more than 30 years’ board and management experience in publicly traded, international pharmaceutical and biotech companies. He has significant knowledge and expertise in discovery and development of the product pipeline from preclinical activities to post-launch marketing studies as well as solid business experience. Anders Gersel Pedersen furthermore has extensive experience with the global pharmaceutical market and has built comprehensive knowledge and insight in governance, including business conduct, and the development of other sustainability related responsibilities from various leadership roles and as a board member. Anders Gersel Pedersen is former Executive Vice President of Research & Development of H. Lundbeck.
ESG Competencies: Environmental · Social · Governance
Current Board Positions
Chair: Aelis Farma S.A.S.
Deputy Chair: Bavarian Nordic A/S1
Member: Hansa Biopharma AB2, Bond 2 Development GP Limited
1. Member of Finance, Risk and Audit Committee, Member of Science, Technology & Investment Committee
2. Chair of Scientific Committee, Member of Remuneration Committee
Paolo Paoletti, M.D.
Male, Italian/American, 74
Board Member (Independent, elected by the General Meeting); Chair of the Scientific Committee and Member of the Compensation Committee
First elected 2015, current term expires 2025
Special Competencies and Qualifications
Paolo Paoletti has extensive experience in research, development and commercialization in the pharmaceutical industry, where he has been responsible for the development of several medicines approved globally and the related global commercial strategies. As an executive, he has led cross-functional teams on the development and registration of medicines and has been responsible for all compliance aspects for the R&D organization. Paolo Paoletti has successfully conducted submissions and approvals of new cancer drugs and new indications in the U.S., in Europe and in Japan. He furthermore has significant experience with governance, including business conduct, from various leadership roles and as a board member. Paolo Paoletti is former Vice President of Oncology Clinical Development with Eli Lilly and Company, former President of GSK Oncology with GlaxoSmithKline, and former CEO of GAMMADELTA Therapeutics.
ESG Competencies: Environmental · Social · Governance
Current Position, including Managerial Positions
Member of the Investment Committee for Apollo Therapeutics Limited
Scientific Advisor for 3B Future Health Fund
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 70/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Current Board Positions
None
Rolf Hoffmann
Male, German/Swiss, 65
Board Member (Independent, elected by the General Meeting); Member of the Audit and Finance Committee and the Scientific Committee
First elected 2017, current term expires 2025
Special Competencies and Qualifications
Rolf Hoffmann has more than 30 years’ experience in senior management and as a board member in the life science industry worldwide. He has significant expertise with business conduct and in creating and optimizing commercial opportunities in global markets and has managed companies across multiple continents with multibillion P&L and cross-functional accountability. Rolf Hoffmann furthermore has knowledge and experience with governance, compliance and ensuring organizational efficiency from various management positions as well as from being a board member. Rolf Hoffmann has held a variety of sales and marketing and executive management positions with Eli Lilly and Company, and is former Senior Vice President, International Commercial Operations and former Senior Vice President, U.S. Commercial Operations with Amgen.
ESG Competencies: Environmental · Social · Governance
Current Position, including Managerial Positions
Adjunct Professor of Strategy and Entrepreneurship at University of North Carolina Business School
Current Board Positions
Member: Semdor Pharma, Sun Pharmaceutical Industries Ltd.1
1. Member of Nomination and Remuneration Committee
Elizabeth A. O’Farrell
Female, American, 60
Board Member (Independent, elected by the General Meeting); Member of the Audit and Finance Committee and the Compensation Committee
First elected 2022, current term expires 2025
Special Competencies and Qualifications
Elizabeth O’Farrell has solid financial experience from her 25-year career in finance leadership roles and as a board member. During her career, she has led multiple strategy, planning and resource allocation processes in multiple roles and in cross-functional teams. Elizabeth O’Farrell has significant knowledge and expertise in business conduct and with driving paradigm changing contributions within finance and the enterprise through collaboration and influence. In addition to experience at Price Waterhouse and Whipple & Company Corporation, Elizabeth O’Farrell held various executive management positions at Eli Lilly and Company, including as former Chief Procurement Officer. Elizabeth O’Farrell is an audit committee financial expert based on her professional experience, including her service in senior finance leadership positions at Eli Lilly and as an audit committee chair or member at other public companies.
ESG Competencies: Social · Governance
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 71/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Current Board Positions
Chair: PDL BioPharma1
Member: LENSAR2, Geron Corporation3, Karius2
1. Chair of Compensation Committee, Member of Audit Committee, Member of Cost Committee
2. Chair of Audit Committee
3. Chair of Audit Committee, Member of Strategic Committee
Takahiro Hamatani
Male, Japanese, 50
Board Member (Non-independent, elected by the employees)
First elected 2022, current term expires 2025
Special Competencies and Qualifications
Takahiro Hamatani has over 20 years’ experience in the pharmaceutical industry in various roles including finance, sales, marketing, and corporate strategy. He has extensive expertise in strategic business planning and finance business partnering as well as experience in successful product launches, geographical expansions, and business development deals. Takahiro Hamatani has previously worked in International Operations at Takeda supporting commercial operations in North and South America and is a Certified Public Accountant in the US.
ESG Competencies: Social · Governance
Current Position, including Managerial Positions
Senior Director, Head of Finance Japan at Genmab
Martin Schultz
Male, Danish, 49
Board Member (Non-independent, elected by the employees)
First elected 2022, current term expires 2025
Special Competencies and Qualifications
Martin Schultz has broad experience within clinical project management with a substantial understanding and knowledge of research and development. He furthermore has specific expertise in project management, strategic sourcing, vendor collaboration, contract, and budget governance.
ESG Competencies: Social · Governance
Current Position, including Managerial Positions
Senior Director, Head of Development Business Partnership & Strategy at Genmab
Mijke Zachariasse, Ph.D.
Female, Dutch, 51
Board Member (Non-independent, elected by the employees)
First elected 2019, current term expires 2025
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 72/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Special Competencies and Qualifications
Mijke Zachariasse has broad experience in people and business management and expertise in building partnerships across sectors, research funding landscape, operational excellence and organizational strategy and change.
ESG Competencies: Environmental · Social · Governance
Current Position, including Managerial Positions
Vice President, Head of Antibody Research Materials at Genmab
Executive Management
As of December 31, 2024, there are nine members of Executive Management.
Jan G. J. van de Winkel, Ph.D.
Dutch, 63, Male
President & Chief Executive Officer
Special Competencies
Extensive antibody creation and development expertise, broad knowledge of the biotechnology industry and executive management skills.
ESG Competencies: Social · Governance
Anthony Pagano
American, 47, Male
Executive Vice President & Chief Financial Officer
Special Competencies
Significant knowledge and experience in the life sciences industry particularly as it relates to corporate finance, corporate development, strategic planning, general management, treasury, accounting, and corporate governance.
ESG Competencies: Social · Governance
Judith Klimovsky, M.D.
Argentinian (U.S. Citizen), 68, Female
Executive Vice President & Chief Development Officer
Special Competencies
Extensive expertise in oncology drug development from early clinical stages through to marketing approval, experience in clinical practice and leading large teams in pharmaceutical organizations.
ESG Competencies: Social · Governance
Current Board Positions
Member: Bio-Techne
Tahamtan Ahmadi, M.D., Ph.D.
Iranian-German (U.S. Citizen), 52, Male
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 73/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Executive Vice President & Chief Medical Officer, Head of Experimental Medicines
Special Competencies
Significant expertise in global regulatory and clinical drug development across entire spectrum from pre-IND to life cycle management; drug discovery and translational research.
ESG Competencies: Social · Governance
Birgitte Stephensen
Danish, 64, Female
Executive Vice President, Chief Legal Officer
Special Competencies
Intellectual property and legal expertise in the pharmaceutical and biotechnology fields.
ESG Competencies: Social · Governance
Christopher Cozic
American, 47, Male
Executive Vice President, Chief People Officer
Special Competencies
Expertise in strategic leadership, organization design, human resource management, policy development, employee relations, organizational development, and a heavy concentration in all aspects of corporate growth and expansion.
ESG Competencies: Social
Martine J. van Vugt, Ph.D.
Dutch, 54, Female
Executive Vice President, Corporate Strategy and Planning
Special Competencies
Extensive knowledge of and experience in Corporate Strategy, Corporate and Business Development, as well as Portfolio, Project, and Alliance Management.
ESG Competencies: Social · Governance
Rayne Waller
American, 57, Male
Executive Vice President & Chief Technical Operations Officer
Special Competencies
Expertise in all elements of technical operations from early-to-mid-stage product development through global manufacturing of both clinical and commercial products.
ESG Competencies: Social · Governance
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 74/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Brad Bailey
American, 57, Male
Executive Vice President & Chief Commercial Officer
Special Competencies
Extensive experience in strategic and operational commercial leadership roles across specialty biopharma, oncology, immunology, and other serious diseases.
ESG Competencies: Social
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 75/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Shareholders and Share Information
OWNERSHIP
Genmab is dual listed on the Nasdaq Copenhagen and the Nasdaq Global Select Market in the U.S. under the symbol GMAB. Our communication with the capital markets complies with the disclosure rules and regulations of these exchanges. As of December 31, 2024, the number of registered shareholders totaled 93,681 shareholders holding a total of 64,844,223 shares, which represented 98% of the total share capital of 66,187,186. The following table shows share data as of December 31, 2024.
Share Data |
|
|
|
|
|
Denmark |
|
U.S. |
|
|
|
|
|
|
Number of shares at December 31, 2024 |
66,187,186 |
|
6,280,456 |
(represented by 62,804,560 American Depository Shares (ADSs)) |
|
|
|
|
|
Listing |
Nasdaq Copenhagen |
|
Nasdaq Global Select Market, New York |
|
|
|
|
|
|
Ticker Symbol |
GMAB |
|
GMAB |
|
|
|
|
|
|
Index Membership |
OMX Nordic Large Cap Index |
|
Nasdaq Biotech Index |
|
The following shareholder is registered in Genmab’s register of shareholders as being the owner of a minimum of 5% of the voting rights or a minimum of 5% of the share capital (one share equals one vote) as of December 31, 2024:
| ● | BlackRock, Inc., 50 Hudson Yards, New York, New York 10001, United States of America (6.8%) |
Shareholders registered in the Company’s shareholder registry may sign up for electronic shareholder communications via Genmab’s investor portal. The investor portal can be accessed at Genmab’s website www.genmab.com/investors. Electronic shareholder communication enables Genmab to, among other things, quickly and efficiently call general meetings.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 76/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The charts included here illustrate the performance of the Genmab share during 2024, the performance of the Genmab share over the last five years, from 2020 through the end of 2024, and the geographical distribution of our shareholders. As of December 30, 2024, Genmab’s shares closed at DKK 1,492.50 and ADSs closed at USD 20.77.
Please refer to Note 4.7 of the financial statements for additional information regarding Genmab’s share capital including authorizations to issue shares and purchase its own shares.
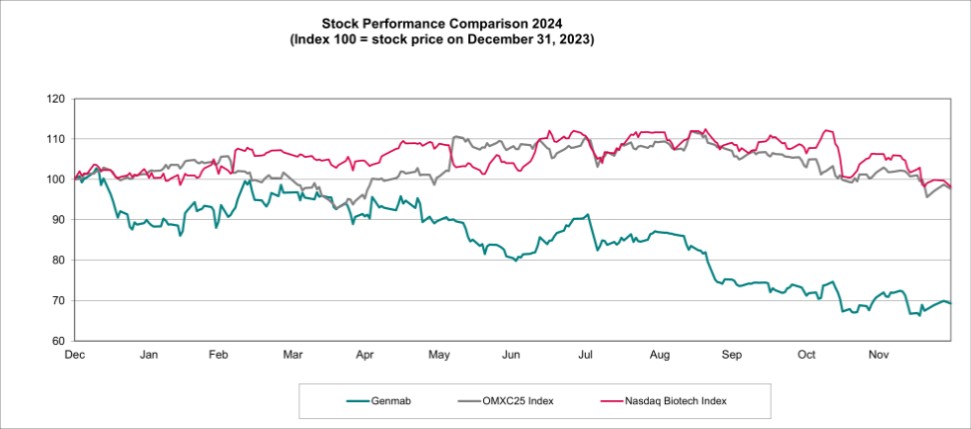
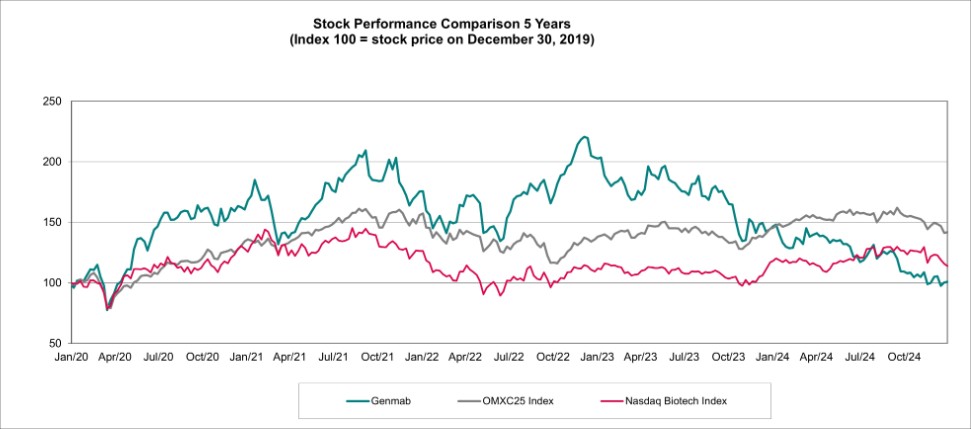
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 77/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
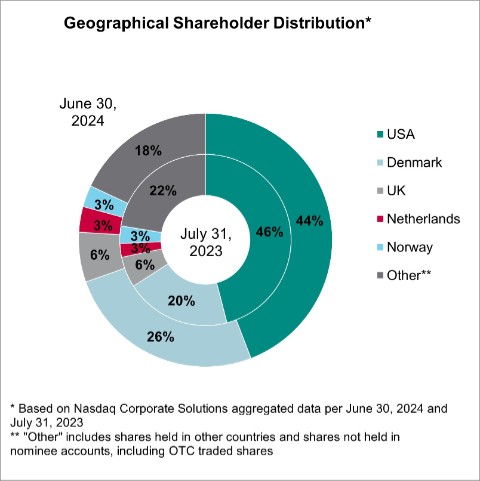
Genmab is a Foreign Private Issuer as defined in the SEC's rules and regulations. The determination of foreign private issuer status is made annually. We plan to make our next determination with respect to our foreign private issuer status on June 30, 2025.
AMERICAN DEPOSITARY RECEIPT (ADR) PROGRAM
Genmab has a sponsored Level 3 ADR program with Deutsche Bank Trust Company Americas. An ADS is a share certificate representing ownership of shares in a non-U.S. corporation. ADSs issued under Genmab’s ADR Program are quoted and traded in U.S. dollars on the Nasdaq Global Select Market in the United States. Ten Genmab ADSs correspond to one Genmab ordinary share. Genmab’s ADR ticker symbol is GMAB. For more information on Genmab’s ADR Program, visit https://ir.genmab.com/adr-program.
INVESTOR RELATIONS
Genmab’s Investor Relations department aims to ensure relevant, accurate and timely information is available to our investors and the financial community. We maintain an ongoing dialogue with sell-side equity analysts, as well as major institutional and retail shareholders. A list of the current analysts covering Genmab can be found at our website along with financial reports, company announcements, current presentations, fact sheets and other downloads.
Contact
For Media Relations:
Marisol Peron, Senior Vice President, Global Communications & Corporate Affairs
T: +1 609 524 0065; E: mmp@genmab.com
For Investor Relations:
Andrew Carlsen, Vice President, Head of Investor Relations
T: +45 33 77 95 58; E: acn@genmab.com
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 78/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Annual General Meeting
Genmab’s Annual General Meeting will be held on March 12, 2025 at 2:00 PM CEST. Further details will be included in the notice to convene the Annual General Meeting.
Financial Calendar for 2025 | |
Annual General Meeting 2025 |
Wednesday, March 12, 2025 |
Publication of the Interim Report for the first quarter 2025 |
Thursday, May 8, 2025 |
Publication of the Interim Report for the first half 2025 |
Thursday, August 7, 2025 |
Publication of the Interim Report for the first nine months 2025 |
Thursday, November 6, 2025 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 79/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Sustainability Statements
Table of Contents |
|
80 |
|
96 |
|
112 |
|
143 |
|
154 |
Sustainability Statements: General Information
Genmab recognizes that sustainability is fundamental to the way we work, and crucial for protecting the planet, fostering economic and social stability, and ensuring a viable future for all. As Genmab is committed to improving patients' lives through transformative medicines, Genmab understands the interconnectedness between human health and the health of our planet. By prioritizing sustainability initiatives, such as reducing carbon emissions, and fostering a diverse and inclusive workplace, Genmab ensures that its actions today contribute to a healthier and more equitable future for generations to come. Moreover, sustainability is integral to maintaining stakeholder trust and securing long-term success in an increasingly conscious global marketplace.
Section |
Disclosure Requirements Content |
Disclosure Requirements # |
Reference / Report |
1.1 Basis for preparation |
General basis for preparation of the sustainability statement |
BP-1 |
SUS |
|
Disclosures in relation to specific circumstances |
BP-2 |
SUS |
1.2 Governance |
The role of the administrative, management and supervisory bodies |
GOV-1 |
SUS, MR |
|
Information provided to, and sustainability matters addressed by the undertaking’s administrative, management and supervisory bodies |
GOV-2 |
SUS, MR |
|
Sustainability-related performance in incentive schemes |
GOV-3, E1, S1 |
SUS |
|
Statement on due diligence |
GOV-4 |
SUS |
|
Risk management and internal controls over sustainability reporting |
GOV-5 |
SUS, MR |
1.3 Strategy |
Strategy, business model and value chain |
SBM-1 |
SUS, MR, FS |
|
Interests and views of stakeholders |
SBM-2 |
SUS |
|
Material impacts, risks and opportunities and how they interact with our strategy and business model |
SBM-3 |
SUS |
1.4 Impact, risk and opportunity |
Process to identify and assess material impacts, risks and opportunities |
IRO-1 |
SUS |
|
Disclosure requirements in ESRS covered by the sustainability statement |
IRO-2 |
SUS |
|
|
|
|
SUS - Sustainability Statements --- MR - Management's Review --- FS - Financial Statements | |||
| 1.1 | Basis for presentation |
General basis for preparation of the sustainability statement (BP-1)
Frameworks
The Corporate Sustainability Reporting Directive (CSRD) directs public companies to report in line with the European Sustainability Reporting Standards (ESRS) starting on or after January 1, 2024. As a result, this 2024 annual report marks the first year in which Genmab is reporting in accordance with this directive and the underlying reporting standards. Genmab’s sustainability statements have been prepared in compliance with the ESRS as required by sections 99a of the Danish Financial Statements Act.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 80/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Consolidation
The sustainability statements have been prepared on a consolidated basis in line with our consolidated financial statements; therefore, the disclosures comprise the Genmab A/S (parent company) and its subsidiaries. The E1 disclosures in particular have been consolidated on the basis of both financial and operational control. The sustainability statements cover Genmab’s own operations and upstream and downstream value chains, where material, specifically regarding disclosures around impacts, risks and opportunities (IROs), policies, actions, targets and metrics. Genmab has not omitted any specific pieces of information corresponding to intellectual property, know-how or the results of innovation nor used the exemption from disclosure of impending developments or matters in the course of negotiation.
Disclosures in relation to specific circumstances (BP-2)
Disclosures Stemming from Other Regulation
Genmab’s sustainability statements also comply with sections 99d and 107(d) of the Danish Financial Statements Act. Refer to Appendix A for a full overview.
Accounting Policies
Genmab’s accounting policies have been applied, in all material respects, consistently in the financial year and for comparative figures.
Key Accounting Estimates and Judgements
Genmab uses estimates and judgements for the reporting of certain data points related to our Scope 3 emissions, which are detailed in the relevant accounting policies. Quantifying greenhouse gas (GHG) emissions inherently involves significant uncertainty due to the complexity of natural and anthropogenic systems. Measurement challenges arise from factors such as variability in emissions sources, accuracy of data and assumptions in emission factors. We regularly reassess our use of estimates and judgements based on experience, the development of sustainability reporting, and a number of other factors. Changes in estimates are recognized in the period in which the estimate in question is revised. In addition, we make judgements when we apply the accounting policies.
Refer to the quantitative data tables in the sustainability statements for further information on accounting policies, key estimates, judgements, and assumptions applied.
Incorporation by Reference
Genmab’s management report consists of a Management's Review section and these sustainability statements which reports disclosure requirements from the ESRS. Our sustainability statements are structured into four overall sections: ‘General Information,’ ‘Environmental,’ ‘Social,’ and ‘Governance.’ Genmab has chosen to incorporate some of the strategy and governance disclosures from the cross-cutting standard ESRS 2 in the Management’s Review as we believe this information is best read in close connection with our Financial Review and overview of our activities. Where incorporation by reference is used to refer outside of these sustainability statements, it is clearly defined.
Genmab considers forward-looking information including targets disclosed in the sustainability statements to be uncertain.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 81/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| 1.2 | Governance |
The role of the administrative, management and supervisory bodies (GOV-1), and
information provided to, and sustainability matters addressed by the undertaking’s administrative, management and supervisory bodies (GOV-2)
At Genmab, sustainability governance is ingrained in our overall corporate governance framework, reflecting our commitment to integrating sustainable practices into all aspects of our business. Genmab’s sustainability governance structure is robust, with clear roles and responsibilities defined at various levels of the organization.
At the highest level, the Board (which includes 0 executive members and 9 non-executive members) oversees sustainability strategy and performance, ensuring alignment with Genmab's long-term business objectives and stakeholder expectations. The Board receives updates on CSR and sustainability initiatives at least annually, has oversight of material impacts, risks, and opportunities (IROs) and targets identified by Genmab, and enables informed decision-making that incorporates environmental, social, and governance (ESG) considerations.
It is the Board’s aim to maintain an equitable gender representation in the Board. As of December 31, 2024, the six shareholder-elected board members are evenly split between 50% male (three persons) and 50% female (three persons) which constitutes equal gender representation in accordance with the guidelines from the Danish Business Authority. As of December 31, 2024, at the Board level, five or 56%, are independent, and four or 44%, are not independent. In accordance with the Danish corporate governance recommendations, we consider shareholder-elected board member Anders Gersel Pedersen non-independent solely by virtue of the length of his tenure on our Board, following his election to the Board in 2003. Also, the three employee-elected board members are non-independent.
Our Board’s Nominating and Corporate Governance Committee oversees our corporate governance, CSR, ESG and sustainability efforts and provides recommendations to the Board on corporate governance, CSR, ESG and sustainability matters. Additionally, the Board’s Audit and Finance Committee oversees our sustainability reporting requirements.
Within the management structure, Genmab has established a dedicated CSR & Sustainability Committee, which is co-chaired by our CEO and SVP of Global Communications and Corporate Affairs and is comprised of senior leaders from relevant functional areas, such as research and development, operations, compliance and risk, legal, human resources, and finance. This committee is responsible for setting strategic priorities, management of material IROs and targets, and monitoring progress towards sustainability goals. It also facilitates cross-functional collaboration to drive innovation and continuous improvement in sustainability performance.
Under the CSR & Sustainability Committee, Genmab has established a Corporate Sustainability Team comprised of Genmab employees responsible for driving the double materiality assessment process including the identification of material impacts, risks and opportunities, targets/goals, actions and metrics, and the collection and documentation of relevant data for sustainability reporting requirements. The Corporate Sustainability Team coordinates with internal stakeholders (other Genmab employees) responsible for integrating sustainability strategy into business operations. The Corporate Sustainability Team is responsible for monitoring and reporting on progress towards targets at least annually.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 82/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Sustainability reporting is approved annually by Executive Management (which includes 9 executive members and 0 non-executive members) and the Board. Executive Management and the Board have significant global expertise in the pharmaceutical, biotech and life sciences industries with specific expertise in Environmental, Social and Governance matters. Further, Genmab has access to experts and has utilized the expertise of outside consultants in preparing its DMA and with regards to environmental matters. Refer to the biographies in the “Board of Directors” and “Executive Management” sections in Management’s Review for an understanding of the relevant experience (including sustainability-related expertise).
Genmab maintains transparent communication channels with stakeholders, including investors and proxy advisors, employees, customers, suppliers, and the broader community, regarding its sustainability efforts. Genmab regularly discloses sustainability-related information through various channels, such as annual reports, investor presentations and engagement sessions. By fostering dialogue and incorporating feedback from stakeholders, Genmab ensures that its sustainability initiatives are responsive to evolving expectations and societal needs.
For Business Conduct matters, the leaders of our Global Compliance, Data Privacy, and Enterprise Risk Management Programs report directly to the CEO and the Board.
Overall, sustainability governance at Genmab is characterized by proactive leadership, robust oversight, and stakeholder engagement, reflecting our commitment to creating long-term value while contributing positively to society and the environment.
Refer to the “Corporate Governance” section in Management’s Review for additional information on governance structure.
Refer to section SBM-3 for a list of the material impacts, risks and opportunities addressed by management and those charged with governance during the reporting period.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 83/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
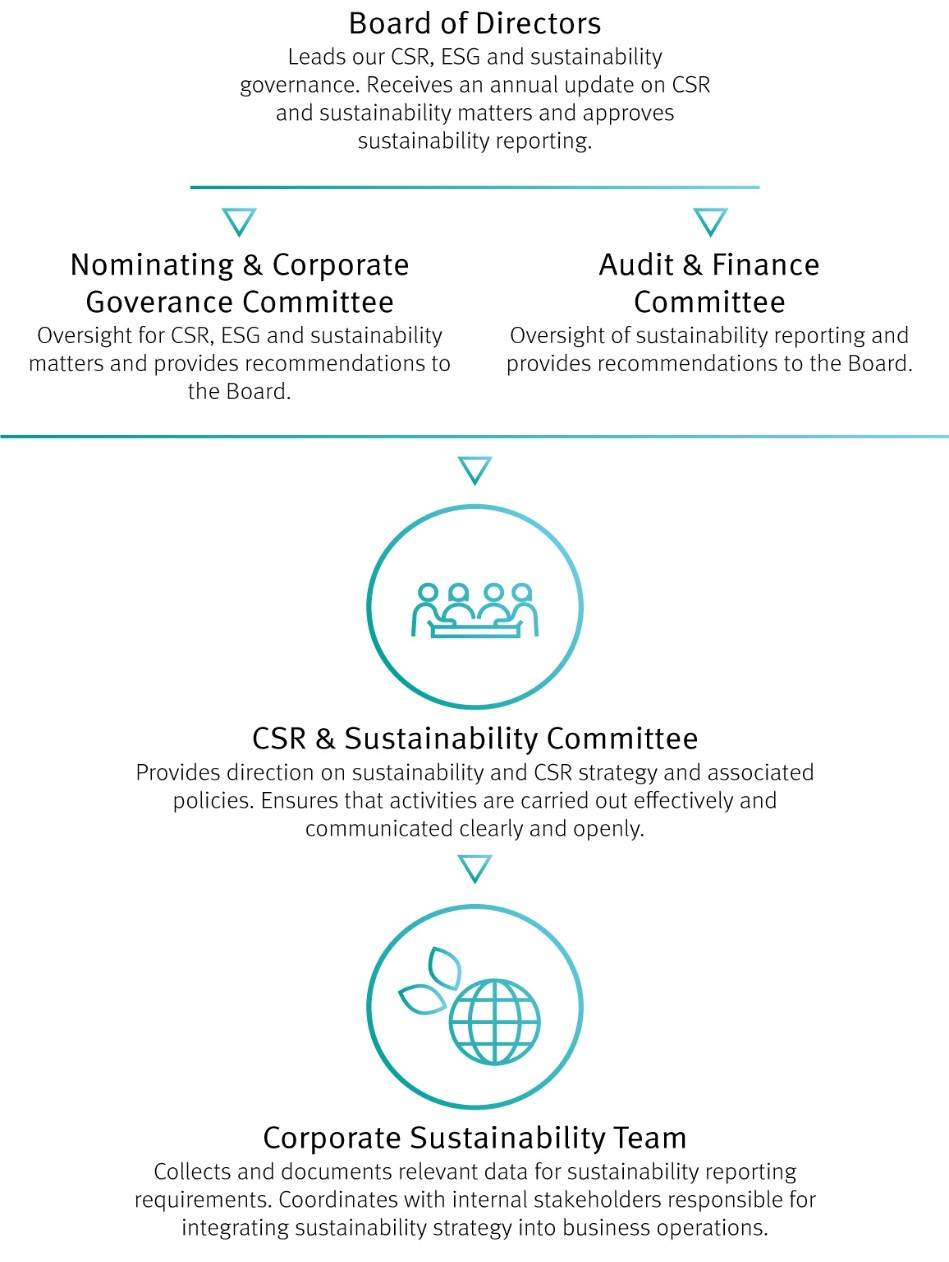
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 84/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Sustainability-related performance in incentive schemes (GOV-3)
Per Genmab’s Remuneration Policy, the variable compensation of the Executive Management is based on the achievement of specific Key Performance Indicators (KPIs) and performance goals that relate to the performance of each executive member and to Genmab A/S’ short and long-term business results. The KPIs and performance goals that the Board sets for the purposes of Genmab A/S’ incentive arrangements – both annual and share-based – are directly linked to the business strategy and our annual business plans. The KPIs/performance goals may be financial, operational, and/or strategic and organizational. The KPIs/performance goals are recommended by the Compensation Committee and approved by the Board.
Genmab grants restricted stock units (RSUs) to Executive Management which are performance-based and include sustainability-related performance goals, whereas RSUs granted to the Board are not performance-based and do not include sustainability-related performance goals. The key characteristics of the sustainability-related performance goals are tied to Climate, Diversity and Employee Well-Being targets with equal weighting against the overall goals of 10% for the 2024 and 2023 grants, and 15% for the 2022 grant. Share-based compensation granted is at a 4x target multiplier with maximum opportunity of 6x multiplier with no cap in 2024 and 2023, compared to a 2.4x target multiplier with 4x maximum with a grant date fair value cap of DKK 25 million in 2022. In 2025, Genmab plans to remove gender diversity balance targets from executive management performance criteria in future grants.
Refer to sections E1-4 and S1-5 for targets linked to Executive Management incentive compensation.
Total remuneration to registered Executive Management in 2024 was DKK 79.9 million. The proportion of total remuneration to registered Executive Management in 2024 linked to climate-related performance goals was DKK 1.8 million, or 2%. Total variable remuneration to registered Executive Management in 2024 was DKK 63.4 million and comprises annual cash bonus and share-based compensation expense. The proportion of variable remuneration to registered Executive Management in 2024 linked to all sustainability-related performance goals was DKK 5.7 million, or 9%.
Refer to Note 5.1 in the consolidated financial statements for details.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 85/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Statement on due diligence (GOV-4)
The following table provides a mapping of the core elements of due diligence, for impacts on people and the environment, to the relevant disclosures in Genmab’s sustainability statements:
Core elements of Due Diligence |
Sections in the Sustainability Statement |
Does the disclosure relate to people and/or the environment? |
a) Embedding due diligence in governance, strategy, and business model |
ESRS 2 GOV-1, GOV-2, GOV-3 ESRS 2 SBM-3: E1 |
People and Environment Environment |
b) Engaging with affected stakeholders in all key steps of the due diligence |
ESRS 2 GOV-1, GOV-2 ESRS 2 SBM-2 ESRS 2 IRO-1 ESRS 2 MDR-P: E1-2 |
People and Environment People and Environment People and Environment Environment |
c) Identifying and assessing adverse impacts |
ESRS 2 IRO-1: E1 |
Environment |
d) Taking actions to address those adverse impact |
ESRS 2 MDR-A: E1-3 |
Environment |
e) Tracking the effectiveness of these efforts and communicating |
ESRS 2 MDR-M: E1-4 ESRS 2 MDR-T: E1-4 |
Environment Environment |
Risk management and internal controls over sustainability reporting (GOV-5)
Genmab assesses potential risks related to sustainability mainly through our CSR & Sustainability Committee but also as part of Genmab’s Enterprise Risk Program led by our Head of Global Compliance and Risk who reports directly to our CEO, both of which are members of the CSR & Sustainability Committee. Clearly defined sustainability governance aids the overall risk management process. The Board has a supervisory duty and Executive Management has overall responsibility for Genmab’s risk management and internal controls in relation to sustainability reporting processes.
Genmab evaluates how sustainability-related risks could affect operations, reputation, and financial performance. Genmab has also implemented processes to ensure the accuracy and reliability of sustainability data collected from various internal stakeholders / departments, which help maintain transparency and traceability of reported information.
Genmab aligns its sustainability reporting with recognized frameworks to ensure consistency and comparability, and engages with stakeholders to gather feedback and ensure reporting meets their needs and expectations.
Internal and external audits are conducted of sustainability reporting processes to assess compliance with established controls and processes. Genmab has an Internal Audit function that reports to the Audit and Finance Committee of the Board and administratively reports to the CFO, and any findings would be communicated through this channel at least annually. The Corporate Sustainability Team integrates the findings of its risk assessment and internal controls regarding the sustainability reporting process into relevant internal functions and processes.
Genmab’s external auditor provides limited assurance on Genmab’s sustainability statements.
Genmab provides training to employees on sustainability issues, related risk management practices and reporting responsibilities to foster a culture of accountability. These components collectively help Genmab manage risks effectively and ensure the integrity of its sustainability reporting.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 86/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Refer to Risk Management section in Management’ Review for the followed risk assessment approach including the risk prioritization methodology, details of risks identified and mitigation strategies, and related controls.
| 1.3 | Strategy |
Strategy, business model and value chain (SBM-1)
A description of Genmab’s strategy including our response / priorities to the main challenges ahead, business model, value chain, products, and customers in relation to sustainability is provided in the following sections in Management’s Review:
| ● | Our Strategy |
| ● | Who We Are |
| ● | Business Model |
| ● | Value Chain |
| ● | Research and Development Capabilities |
| ● | Bringing Our Own Innovative Medicines to Patients |
| ● | Antibody Discovery and Development |
| ● | Products and Technologies |
Genmab’s sustainability-related goals have been broken out into relevant targets in the Environmental, Social and Governance sections of these sustainability statements.
Refer to section S1-6 for information on Genmab’s headcount by geographical areas.
Refer to Note 2.1 in the consolidated financial statements for disclosures related to Genmab’s revenue by type, collaboration partner and product, and Note 2.2 for Genmab’s revenue by geographical area. There are no additional significant ESRS sectors beyond those reflected in these disclosures. Genmab’s goals have been broken out into relevant targets in the Environmental, Social and Governance sections of the sustainability statements.
Interests and views of stakeholders (SBM-2)
As an international biotech company, Genmab engages continuously with a diverse range of stakeholders, all of whom have a vested interest in our business practices. This engagement is crucial to our success and reinforces our commitment to benefiting both our direct stakeholders and society as a whole. Through ongoing dialogue, we seek to understand their perspectives, concerns, and expectations. These interactions inform our sustainability efforts, due diligence processes and double materiality assessments, ensuring that our actions align with the needs and insights of those we serve.
The views and interests of affected key stakeholders regarding our sustainability-related impacts are regularly communicated to our CSR & Sustainability Committee through periodic meetings. For all key stakeholders listed in the table, Genmab listens to the views of stakeholders through engagement and takes these views into account, which has led to the outcomes disclosed.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 87/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Genmab's Key Stakeholders |
Description |
Value Chain Location |
How Engagement is Organized |
Purpose of Engagement |
Outcome from Engagement |
Academic and research institutions |
Partners contributing to early-stage research, technology development, and/or innovation. |
Upstream |
- Academic partnerships |
- Collaboration on research |
- Accelerated innovation |
Collaboration partners |
Companies that collaborate with Genmab in co-development, licensing, and/or commercialization. |
Upstream, |
- Joint team meetings |
- Innovation and development |
- Successful product development and commercialization |
Communities |
As part of Genmab’s ongoing commitment to Corporate Social Responsibility (CSR), we aim to contribute to and ensure the vibrancy and sustainability of the communities where we live and work. |
Upstream, |
- Community programs |
- Health education and awareness |
- Positive community impact |
Contract manufacturing organizations (CMOs) |
CMOs manage the production of Genmab’s antibody therapies, ensuring that they meet quality standards and are produced at scale. |
Upstream |
- Structured collaboration |
- Efficiency and flexibility to leverage the expertise and capacity of CMOs |
- Quality products |
Employees |
Internal stakeholders involved at every stage of our supply chain, from research to commercialization, ensuring that Genmab’s operations align with its strategic goals. Our talented teams are the cornerstone of our success and fundamental to achieving our 2030 Vision. |
Own Operations |
- Surveys and workplace assessments, including our Global Engagement Survey |
- Including employees' perceptions and experiences |
- Internal policy updates |
Healthcare providers |
Physicians, nurses, pharmacists and medical institutions that play a role in recruiting patients for clinical trials and prescribing / administering Genmab’s therapies. |
Upstream, |
- Clinical Trials |
- Understanding clinical needs |
- Refined clinical trials |
Investors |
Shareholders interested in the entire value chain, with a focus on how each stage contributes to Genmab's financial health and growth potential. Genmab has a diverse shareholder base with investors from across a spectrum of size and location. The support of Genmab’s investors is essential to the success of the Company. |
Upstream, |
- Earnings calls and reports |
- Transparency related strategy, financial performance, and research advancements |
- Increased investor confidence |
Patients / patient organizations |
The end-users of Genmab’s therapies or groups who represent them, whose outcomes and feedback are vital to develop and deliver better medicines. |
Upstream, |
- Standing Patient Advisory Council |
- Patient-centric approach |
- Insights on trial designs |
Payers |
Insurance companies, government healthcare programs, and other entities that reimburse or fund the cost of therapies, influencing pricing and market access. |
Downstream |
- Value assessment studies |
- Market access |
- Increased access |
Quality assurance teams |
Both internal teams (employees) and external partners ensure the consistency and safety of the products during manufacturing. |
Own Operations, Upstream |
- Cross-functional teams ensuring quality is integrated throughout product lifecycle |
- Regulatory compliance |
- High quality products |
Regulatory agencies |
Bodies like the EMA (Europe), the FDA (U.S.) and MHLW (Japan) are key stakeholders, as they approve trial designs, oversee progress, and ultimately decide on the approval of new therapies. |
Upstream, Downstream |
- Regular meetings |
- Regulatory compliance |
- Streamlined development process |
Scientists and research partners |
Internal teams (employees) and external partners are critical in the early stages of target identification and antibody engineering. These include our own antibody expert scientists as well as academic institutions, research organizations (including CROs) and technology providers. |
Own Operations, Upstream |
- Co-development of programs |
- Access to technology |
- Development of innovative / new antibody therapeutics and other treatment modalities |
Suppliers |
Providers of raw materials and other inputs necessary for the production of antibody therapies. |
Upstream |
- Supplier selection and qualification |
- Quality assurance |
- Consistent quality |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 88/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Material impacts, risks, and opportunities and how they interact with our strategy and business model (SBM-3)
The table describes Genmab’s material impacts, risks and opportunities resulting from our double materiality assessment (see section IRO-1), including a description of where in our business model, our own operations, and our value chain these material impacts, risks and opportunities are concentrated. We also describe whether our impacts are positive or negative. All impacts are actual impacts unless otherwise stated that they are potential impacts.
More information on how we respond to the effects of our impacts, risks and opportunities is included in the topical sections under ‘Environmental,’ ‘Social,’ and ‘Governance.’ There, we also expand on the linkage to our sustainability strategy and business model, expected time horizons and the nature of the business relationship.
There is no significant risk of a material adjustment within the next annual reporting period to the carrying amounts of assets and liabilities reported in the consolidated financial statements linked to material risks and opportunities identified in our sustainability reporting. Genmab has no material investment or disposal plans currently linked to our material IROs or planned sources of funding to implement our sustainability strategy.
We have one entity specific IRO relating to G1: Business Conduct, Cybersecurity. This is Genmab’s first year of sustainability reporting and therefore there are no changes since the previous reporting period.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 89/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Section |
Material Topic |
Material Impact |
Value Chain Location |
Time Horizon |
Material Risk |
Material Opportunity |
Environmental |
Climate Change |
Emissions from value chain (negative) |
Upstream |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Environment |
Partner with value chain to reduce Scope 3 emissions |
|
|
Emissions from own operations (negative) |
Own Operations |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Environment |
Renewable energy contracts (to mitigate climate change risks) |
|
Climate Change |
Energy consumption (negative) |
Own Operations |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Environment |
Increasing energy efficiency in the procurement of new locations as operations expand in the future and major renovations to existing locations. |
|
|
Generation of energy from onsite solar panels (positive) |
Own Operations |
Short, Medium, Long-Term |
|
|
Social |
Own Workforce |
Employee well-being and vitality (positive) |
Own Operations |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Management and Workforce |
Provide a voice to employees through our global engagement survey |
|
|
Safety in our facilities (positive) |
Own Operations |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Management and Workforce |
Safety training / ongoing education in our laboratories |
|
Own Workforce |
Career development through training and skill building (positive) |
Own Operations |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Management and Workforce |
Providing employees with opportunities to discuss their career at Genmab |
|
|
Diversity in the workplace (positive) |
Own Operations |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Management and Workforce |
|
|
Consumers and End-Users |
Access and integrity in clinical trials (positive) |
Downstream |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance |
|
|
|
Market access programs to allow for product availability for uninsured or underinsured (positive) |
Downstream |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Business and Products |
Assisting those having difficulty affording Genmab products prescribed to them |
|
Consumers and End-Users |
Patient engagement programs developed (positive) |
Downstream |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Business and Products |
|
|
|
Clinical trial transparency (positive) |
Downstream |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance |
|
Governance |
Business Conduct |
Healthy corporate culture aligned with core values and purpose (positive) |
Own Operations |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance |
Annual Code of Conduct training rollout as mandatory for all employees, with launch and completion rates monitored by Global Compliance team |
|
Business Conduct |
Cybersecurity and Global Data Privacy programs in place to protect the privacy of our business, our own workforce, patients and all individuals who entrust us with their information (positive) |
Upstream, Own Operations, Downstream |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance and Cybersecurity |
|
|
Business Conduct |
Protection of whistleblowers through anti-retaliation policies and procedures (positive) |
Upstream, Own Operations, Downstream |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance |
|
|
Business Conduct |
Animal welfare policy (positive) |
Own Operations |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance |
|
|
Business Conduct |
Strong management of suppliers, focused on compliance with supplier code of conduct (positive) |
Upstream |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Strategic Collaborations |
Continue to partner with suppliers on sustainability related commitments in the future |
|
Business Conduct |
Ethical business culture and business practices (positive) |
Own Operations |
Short, Medium, Long-Term |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance, Strategic Collaborations and Management and Workforce |
Annual Code of Conduct training rollout as mandatory for all employees, with launch and completion rates monitored by Global Compliance team |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 90/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Genmab has the resources in place to manage the effects of the IROs on our business across the Environmental, Social and Governance areas.
Refer to the Environmental section of the sustainability statements for information on Genmab’s resilience analysis.
| 1.4 | Impact, risk and opportunity management |
Process to identify and assess material impacts, risks and opportunities (IRO-1)
A cornerstone element of the CSRD and ESRS is the concept of double materiality for sustainability matters. The concept of double materiality means that a sustainability matter can have an inside-out impact (i.e., an impact that Genmab has or can have on people and/or the environment – this is called ‘impact materiality’) or an outside-in impact (i.e., a (actual/potential) financial impact (risk/opportunity) from a sustainability matter on Genmab – this is called ‘financial materiality’).
Genmab performed a double materiality assessment (DMA) for the first time as part of our 2024 sustainability reporting and we look forward to incorporating our sustainability strategy in our day-to-day business operations over the coming year(s).
From a governance perspective, the Corporate Sustainability Team drove the execution of the DMA. The approach for and outcomes of the double materiality were extensively discussed with and approved by Executive Management and our Board and its relevant Committees. Refer to GOV-1 and GOV-2 for further details on the governance structure with regards to sustainability.
Scope
The scope of the DMA entailed the parent company, Genmab A/S, and its subsidiaries on a consolidated basis as well as our upstream and downstream value chain.
Material topics
Genmab engaged a sustainability consultancy firm to assist in the performance of a DMA in accordance with the CSRD and the underlying ESRS. During this process, which involved numerous internal and external stakeholders, we determined seven (7) topics, sub-topics or sub-sub topics to be material for Genmab. These seven material topics link to four (4) of the standards (ESRS E1, S1, S4 and G1).
Non-material topics
Genmab screened site locations and assets across Denmark, the Netherlands, the U.S., Japan and China including business activities and considering all Application Requirement 16 topics, determined ESRS E2, E3, E4, E5, S1 (specific to other work-related rights), S2 and S3 to be not material from an impact or financial perspective. In our analysis, we considered our own operations and upstream and downstream value chain and were not aware of any material matters to disclose in our sustainability statements. We surveyed internal stakeholders and external stakeholders and performed desk research, held workshops and deep-dive sessions, and noted no issues triggering these topics to be material for Genmab. E4 followed the same approach as above and as such we did not perform a detailed assessment to identify related IROs.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 91/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
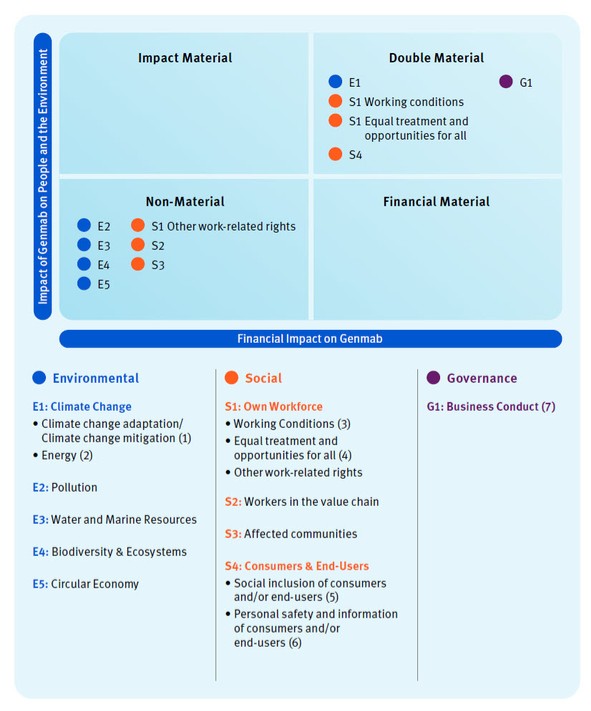
The process of how the DMA was performed, and description of how we arrived at these material topics is described below.
Value chain analysis and stakeholder selection
As an initial step, Genmab defined our value chain and key stakeholders, focusing both on our own operations, as well as on our value chain. We conducted desk research that included peer reviews on sustainability topics and research on the relevance of sustainability topics for the pharmaceutical sector. Based on the outcomes of this mapping exercise and desk research, we determined initial topics for the longlist used in the double materiality analysis.
We selected internal stakeholders from across our business operations to provide coverage across all ESRS topics, sub-topics, and sub-sub-topics. Given the global scope of our business, we included internal stakeholders from across all our locations.
We interviewed external stakeholders representing at least one across each of the following: patient associations, investors, collaboration partners, sector organizations and civil society organizations. The input
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 92/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
of these external stakeholders, combined with the expertise of the internal stakeholders, provided valuable insights.
Longlist and validation
Following the analysis as described above, a longlist of material topics was prepared. This longlist was based on the guidance provided by the ESRS (ESRS 1 – Application Requirement 16), further complemented by insights from the value chain and stakeholder analyses, other regulatory frameworks, and desktop peer review.
Based on this longlist, both impact materiality and financial materiality were assessed through surveys, interviews, and workshops. The combination of these three methods ensured coverage of the broad range of stakeholders and provided room to finetune the outcomes to reflect any (regional) nuances during the workshops.
The surveys provided input from a broad audience and led to initial scoring. Internal and external interviews conducted before the workshops and after the workshops provided expert insight and guidance to validate outcomes. Refer below for further detail on the execution of the impact materiality and financial materiality analyses.
Double Materiality
The ESRS prescribes companies to assess topics that are material to the business from an impact and/or financial perspective. The following paragraphs describe the methodology Genmab used to assess both impact and financial materiality.
Impact Materiality
ESRS 1, ‘General Requirements,’ highlights the indicators that companies should consider in assessing their impacts on sustainability matters. The assessment of impacts is aligned with other international standards, such as the United Nations Guiding Principles on Business and Human Rights (UNGP) and the Organization for Economic Co-Operation and Development (OECD) Guidelines. International standards prescribe that impacts should be rated based on their severity. The concept of severity is based on the factors scale, scope, and irremediable character of the impact. In case of positive impacts, scoring irremediability is unnecessary. For Genmab, all the mentioned individual factors were rated on a scale of 1 to 5, based on which an average score was calculated.
In case of potential impacts, participants also rated likelihood on a scale of 0 to 1. For actual impacts, the likelihood is automatically scored as 1 since the likelihood has already been proven. For completeness’ sake, we note that for potential negative impacts regarding human rights, severity took precedence over likelihood as also prescribed by the ESRS.
Other indicators that are prescribed by the ESRS for the assessment of impact materiality include: the topic’s location in the value chain (own operations, upstream or downstream), and whether the impact pertains to the undertaking in the short-, medium- or long-term scored as time horizons.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 93/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
To summarize, all longlisted sustainability matters were scored using the following indicators:
| (i) | the topic’s location in the value chain (own operations, upstream or downstream), |
| (ii) | whether it is an actual or potential impact, |
| (iii) | whether it is a positive or negative impact, |
| (iv) | time horizons, and |
| (v) | scale, scope and irremediability of the impacts. |
For a matter to be considered as impact material, we have used a relative threshold – i.e., we have used the average score for impact materiality based on the five-point scale and set the threshold a full point higher.
Financial Materiality
During the preparation of the longlist, as well as during the workshops, we considered impacts and dependencies with the risks and opportunities that may arise from these impacts and dependencies through extensively discussing the sustainability topic at hand and what impact it could have on Genmab and our business.
The scoring of financial materiality is largely similar to impact materiality; however, instead of focusing on impacts, financial materiality is based on risks and opportunities. Risks and opportunities are scored on the basis of magnitude (scale of 1 to 5), and likelihood (scale of 0 to 1). Participants also assess time horizons and location in the value chain.
For a matter to be considered as financial material, we initially used a similar relative threshold as for impact materiality: the average financial materiality score based on the five-point scale plus a full point. Our final results used a mix of qualitative and quantitative factors and our financial statement materiality level to conclude on material topics.
Validation
The survey outcomes were discussed with internal stakeholders during the workshops and deep-dive sessions. Where needed, changes and/or nuances were made. To further align and validate the outcomes of the DMA, these were presented to external stakeholders and internal interviews were conducted with subject matter experts.
All identified sustainability-related risks have been identified in prior year reporting under our ERM framework and continue to be updated and prioritized in line with other risks identified.
The final DMA for 2024 has been approved and adopted by the Board with the filing of this Annual Report.
Action
While conducting the DMA in 2024, we further integrated the various aspects of the DMA into our business. We focused on setting meaningful and appropriate targets for our material topics including defining the data gathering processes.
With our dedicated Corporate Sustainability Team, we intend to monitor our performance over the coming years, while remaining agile and current with our double materiality assessment (e.g., following acquisitions or other major business changes).
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 94/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Disclosure requirements in ESRS covered by the sustainability statement (IRO-2)
Following the outcome of our DMA, Genmab compiled a list of disclosure requirements including the page numbers and/or paragraphs where the related disclosures are located in the sustainability statements. This is presented as content indexes in the General, Environmental, Social and Governance sections of the sustainability statements. Genmab has determined the material information to be disclosed in relation to the impacts, risks and opportunities assessed to be material, utilizing a mix of qualitative and quantitative factors and our financial statement materiality level.
Genmab also included a table of datapoints derived from other EU legislation in the appendices to the sustainability statements.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 95/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
SUSTAINABILITY STATEMENTS: ENVIRONMENTAL
As a leading international biotech company, we recognize the responsibility we have to protect our planet and its natural resources, as well as the health and safety of our team members, business partners and society as a whole. By conducting our business in a safe and sustainable manner, we aim to reduce our environmental impact by refining our processes and incorporating best practices into our own operations and value chain, where applicable. To achieve this, our environmental strategy focuses on monitoring and evaluating targets for environmental activities, measuring our impact and communicating our progress.
|
|
Disclosure |
Section |
Disclosure Requirement Content |
Requirement # |
E1 Climate Change | ||
2.0 IROs |
Material Impacts, Risks and Opportunities and their interaction with strategy and business model |
SBM-3 |
2.1 E1 Climate Change Strategy |
Transition plan for climate change mitigation |
E1-1 |
2.2 E1 Climate Change |
Policies related to climate change mitigation and adaptation |
E1-2 |
IRO management |
Actions and resources in relation to climate change policies |
E1-3 |
2.3 E1 Climate Change |
Targets related to climate change mitigation and adaptation |
E1-4 |
Metrics and Targets |
Energy consumption and mix |
E1-5 |
|
Gross Scopes 1, 2, 3 and total GHG emissions |
E1-6 |
|
GHG removals and GHG mitigation projects financed through carbon credits |
E1-7* |
|
Internal carbon pricing |
E1-8* |
|
Anticipated financial effects from material physical and transition risks and potential climate-related opportunities |
E1-9** |
EU Taxonomy | ||
2.4 EU Taxonomy |
Disclosures pursuant to Article 8 of Regulation (EU) 2020/852 (Taxonomy Regulation) |
N/A |
* Disclosure requirements E1-7 and E1-8 are not applicable for Genmab.
** Genmab has adopted the phase-in for E1-9 and elected not to disclose for year 1 reporting.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 96/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
2.0 IROs
Material Impacts, Risks and Opportunities (IROs) and their interaction with strategy and business model (SBM-3)
Section |
Material Topic |
Material Impact |
Value Chain Location |
Material Risk |
Material Opportunity |
Environmental |
Climate Change |
Emissions from value chain (negative) |
Upstream |
Genmab could face transitional risks by its inability to manage the carbon footprint and energy mix from our business operations and physical risks from climate-related events that may impact our business operations or that of our third-party partners or suppliers. |
Partner with value chain to reduce Scope 3 emissions |
|
|
Emissions from own operations (negative) |
Own Operations |
|
Renewable energy contracts (to mitigate climate change risks) |
|
Climate Change |
Energy consumption (negative) |
Own Operations |
|
Increasing energy efficiency in the procurement of new locations as operations expand in the future and major renovations to existing locations. |
|
|
Generation of energy from onsite solar panels (positive) |
Own Operations |
|
|
All impacts, risks and opportunities have expected time horizons of short, medium and long-term.
Emissions from our value chain / Emissions from our own operations
Climate change adaptation and mitigation is material due to GHG emissions primarily in our value chain, but also in our own operations, which has a negative impact on the environment with transitional and physical risks associated. Genmab has adaptation and mitigation efforts aligned with the Paris Agreement for a future where warming is kept at 1.5°C. Genmab is committed to reducing GHG emissions to limit the global warming the planet is facing.
In line with our ambitions to reduce Scope 3 emissions through supplier engagement, Genmab has an opportunity to reduce GHG emissions in our upstream value chain by setting clear expectations and requirements, providing incentives and setting goals, which could lead to more efficient production processes and cost savings for both Genmab and its suppliers.
In terms of our own operations, Genmab plans to reduce emissions by sourcing electricity from renewable energy sources such as solar, wind, hydroelectric, or geothermal power. Genmab has an opportunity to reduce emissions by actively investing in renewable energy procurement through power purchase agreements (PPAs), green tariffs, or direct ownership of renewable energy assets which could mitigate climate change risks relating to carbon taxation. Genmab can decrease the carbon intensity of its electricity consumption by contracting with renewable energy providers or investing in onsite renewable energy projects. This not only helps in reducing emissions, but also supports the growth of renewable energy markets and contributes to broader sustainability goals.
Genmab has an opportunity to reduce GHG emissions and promote health in the context of climate change through various initiatives targeting employee behavior and operational practices. We aim to reduce energy consumption and GHG emissions by implementing sustainable practices in laboratories in line with MyGreenLab recommendations. Additionally, we encourage employees to minimize air travel to lower GHG emissions while ensuring business continuity, supported by guidelines that have been in place for several
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 97/253 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
years. Furthermore, establishing remote work policies and promoting alternative commuting methods, such as biking, carpooling, and public transit, can contribute to decreasing GHG emissions associated with employee commuting, fostering a healthier environment for all and could lead to cost savings.
Energy consumption
Genmab utilizes 61% renewable energy at our state-of-the-art buildings; therefore, there is a negative impact in terms of non-renewable energy usage with risk associated with our energy mix; however, there is room to achieve 100% renewable energy usage at all of our locations.
Genmab has an opportunity to increase energy efficiency in the procurement of new locations as operations expand in the future and major renovations to existing locations which could lead to financial savings on operational expenses.
Refer to E1-5 for energy mix and consumption table.
Generation of energy from onsite solar panels
Genmab also has a positive impact from generation of energy through solar panels at our headquarters in Denmark and certain R&D locations in the Netherlands.
Refer to E1-4 for climate change targets.
Genmab’s Resilience to Climate Change
Genmab's resilience analysis was conducted qualitatively in 2021, incorporating climate scenarios based on key reports from authoritative bodies such as the Intergovernmental Panel on Climate Change (IPCC) and the International Energy Agency (IEA). No material changes to the analysis were identified in 2024, 2023 or 2022. The analysis was conducted by assessing climate-related impacts across Genmab’s entire value chain, including supply chains, operations, energy consumption, and logistics.
This resilience analysis helps inform Genmab’s strategic planning, risk management, and financial planning processes, ensuring that climate-related risks and opportunities are integrated into the company’s Enterprise Risk Management (ERM) framework.
Genmab utilized two scenarios to explore potential transition and physical risks at a Paris Agreement aligned 1.5–2°C and a high emissions scenario at 4°C warming levels, considering both short-term (2030) and medium-to-long-term (2040/2050) time horizons in alignment with Genmab’s strategic planning horizons and its GHG emissions reduction targets.
| ● | The 1.5–2°C scenario assumes a transition to a low-carbon economy in line with global climate targets. This scenario evaluates risks and opportunities arising from regulatory actions such as carbon taxation, low-carbon technology adoption, and evolving consumer preferences toward sustainability. |
| ● | The 4°C scenario represents a business-as-usual pathway with high emissions and limited global mitigation efforts, leading to more severe physical risks such as extreme weather events, flooding, and disruptions to supply chains. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 98/253 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The key assumptions for the resilience analysis include the transition to a low-carbon economy, macroeconomic trends, energy consumption and mix, technology deployment and time horizons.
Based on the scenario analysis, Genmab identified several potential physical risks, transition risks and opportunities for both the 1.5–2°C and 4°C warming scenarios across short-term and medium/long-term time frames. The identified physical and transition risks and opportunities were evaluated based on likelihood, magnitude and duration to Genmab’s operations and taking into account Genmab’s physical geographical locations at the time of conducting the analysis. No aspects of Genmab’s business were identified as incompatible with a transition to a climate neutral economy.
| ● | Key physical risks to Genmab’s business activities and assets identified in the scenarios: Disruption of supply chain and operations from extreme weather events, increased cooling costs from more frequent and severe heat waves, increased costs of raw materials due to changes in weather patterns and extreme weather events, operations and supply chain disruption from coastal flooding and damage to physical assets and inventory. |
| ● | Key transition risks to Genmab’s business activities identified in the scenarios: Global carbon taxation and pricing impacting costs and financial returns, investor focus on climate performance limiting access to capital and investment, competition for talent in scientific and innovative fields becomes increasingly linked to climate performance, and cost of compliance with fragmented and drastic regulatory intervention. |
While the scenario analysis identified potential physical and transition risks, the risk related to the Environment was identified as the material risk to Genmab. Refer to the Risk Management section in Management’s Review for risks related to the Environment.
Genmab has committed to a science-aligned emissions reduction target aligned with the Paris Agreement, aimed at reducing its GHG emissions in line with the global goal to limit warming to 1.5°C. This target plays a critical role in mitigating both transition and physical risks by guiding risk mitigation, reducing exposure to physical risks and enhancing resilience to market shifts.
Uncertainties within the resilience analysis included climate projections under the scenarios and regulatory evolution over time.
Genmab’s resilience analysis, underpinned by qualitative scenario analysis and guided by a science-aligned emissions reduction target, highlights the company’s preparedness by adapting our strategy for both transition and physical climate risks in the medium and long term. The science-aligned emissions reduction target offers a clear pathway for mitigating these risks while also seizing opportunities associated with the transition to a low-carbon economy. Through its ongoing commitment to sustainability, Genmab is not only reducing its exposure to climate risks but also positioning itself for long-term business success in an increasingly climate-conscious world.
2.1 E1 Climate Change Strategy
Transition plan for climate change mitigation (E1-1)
Genmab approaches climate change mitigation through a comprehensive transition plan, addressing risks, setting targets, and engaging stakeholders including suppliers. We have assessed potential climate-related
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 99/253 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
risks to our own operations and our value chain – See the Risk Management section in Management’s Review for further details.
We have established science-aligned emissions reductions targets for reducing GHG emissions across our own operations and our value chain, aligning with the goals of the Paris Agreement to limit global warming to 1.5°C, and we have set targets for GHG emission reduction.
Genmab plans to reduce GHG emissions by:
| ◾ | engaging with suppliers and partners to ensure ongoing decarbonization of our value chain, |
| ◾ | sourcing electricity from renewable energy sources such as solar, wind, hydroelectric, or geothermal power, and |
| ◾ | encouraging and inspiring behavioral changes for our employees to reduce emissions from lab operations, business travel and employee commuting |
Genmab’s transition plan is embedded in and aligned with our overall business strategy including our financial planning. Refer to “Our Strategy” section in Management’s Review for details.
The transition plan is reviewed through our governance model and is approved by Executive Management and Board.
Refer to E1-4 for Genmab’s progress on the implementation of the transition plan.
2.2 E1 Climate Change IRO Management
Policies related to climate change mitigation and adaptation (E1-2)
Genmab published its Corporate Social Responsibility (CSR) Policy in 2023 which was adopted by the Board and includes a focus on environmental sustainability.
In 2024, Genmab published an overarching Commitment to the Environment and Sustainability to manage material environmental sustainability matters including material IROs covering climate change adaptation and mitigation, and energy, and matters not material to Genmab including water and circular economy. The Commitment to the Environment and Sustainability was adopted by the CSR & Sustainability Committee on December 11, 2024.
Genmab’s Commitment to the Environment and Sustainability applies to all employees and contractors and all activities within Genmab’s operations. It extends to all regions where Genmab operates, recognizing diverse environmental challenges and opportunities.
Genmab’s CSR & Sustainability Committee, composed of cross-functional senior leaders, and co-lead by our CEO and SVP Communications & Corporate Affairs, is responsible for the oversight of Genmab’s Commitment to the Environment & Sustainability and implementation.
Genmab has included the perspectives of internal and external key experts and stakeholders in determining material environmental topics and in formulating Genmab’s Commitment to the Environment and Sustainability.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 100/253 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Genmab's Commitment to the Environment and Sustainability is available for our key stakeholders including our employees on our website (Genmab.com).
Refer to the Commitment to the Environment and Sustainability on our website.
Actions and resources in relation to climate change policies (E1-3)
Engaged with suppliers to encourage them to set their own emissions targets
We engage in continuous dialogue on sustainability with our key suppliers so Genmab may meet our target as disclosed in E1-4.
In 2024, Genmab benchmarked the climate ambitions of our top suppliers to provide a clear baseline and identify actions and hotspots for improvement. We had in-person and virtual engagement with key suppliers identified as significant sources of emissions. Genmab established formalized processes governing supplier engagement on climate change by incorporating specific climate change topics in the supplier onboarding and annual contract review.
Continued investment in Renewable Energy Procurement
In 2024, Genmab renegotiated an electricity contract to ensure that our sites continue towards our ambition of sourcing 100% renewable electricity to help mitigate our Scope 2 emissions.
Refer to E1-5 and E1-6 for further details on energy usage and mix, and GHG emissions.
2.3 E1 Climate Metrics and Targets
Targets related to climate change mitigation and adaptation (E1-4)
Genmab plans to minimize its carbon footprint across our business to align with a future where warming is kept at 1.5°C in line with the Paris Agreement. Genmab has considered a diverse range of climate scenarios when setting the targets. Specific targets consistent with Genmab’s GHG inventory are summarized below and relate to the gross reduction of GHG emissions:
| (1) | Develop and execute on sustainable climate-related strategy by 20251 |
The development and execution of a sustainable climate-related strategy by 2025 is critical to ensuring that we align our operations with global climate targets. By disclosing Scope 1, 2, and 3 emissions in the 2023 annual reporting, Genmab has already laid the groundwork for transparency and accountability of our environmental impact. Building on this foundation, in 2024, Genmab achieved the development of a comprehensive climate strategy, with a clear commitment to climate targets aligned with the Paris Agreement. Executing this strategy will involve taking tangible actions toward GHG emissions reductions, with partial progress on Scope 1 and 2 reductions expected by the end of 2025. By executing these steps, Genmab plans to not only meet regulatory expectations
1 Executive Management received RSU grants in 2023 with performance linked to developing and executing on a sustainable climate-related strategy
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 101/253 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
but also contribute to global efforts to combat climate change toward a sustainable future for both our operations and the planet.
| (2) | Reduce Scope 1 and Scope 2 emissions by 42% through a reduction in Scope 2 emissions by 2030 from a 2024 base year2 |
Genmab intends to achieve a 42% reduction in Scope 1 and Scope 2 (market-based) GHG emissions by 2030 compared to a 2024 baseline year by sourcing renewable electricity at our facilities.
| (3) | Ensure 70% of suppliers by spend covering upstream purchases goods and services, capital goods and upstream transportation commit to have Science Based Targets (SBT) by 20303 |
Genmab intends to indirectly mitigate GHG emission impacts to the environment by 2030 through supplier engagement and responsible sourcing practices by ensuring at least 70% of our largest suppliers by spend (operating expenses), covering upstream purchased goods and services, capital goods and upstream transportation, commit to have science-based targets by 2030.
| (4) | Reduce Scope 1 and 2 emissions by 90% by 2050 from a 2024 base year |
Genmab intends to reduce Scope 1 and 2 (market-based) GHG emissions by 90% by 2050 from a 2024 base year to contribute to the obtainment of the ambitions laid out in the 2015 Paris Agreement by sourcing renewable electricity and switching to cleaner heating sources.
Refer to section GOV-3 for climate related targets related to Executive Management incentive compensation.
In 2022, Executive Management was granted RSUs linked to Genmab’s climate target to ensure 100% renewable electricity was achieved across all major operating sites, which at the time of grants included our headquarters in Denmark and sites in the Netherlands, the U.S. and Japan. As of December 31, 2024, all major operating sites under the RSU grant achieved 100% renewable electricity.
2 Executive Management received RSU grants in 2024 with performance linked to Scope 1 and Scope 2 emission reductions by 42% by 2030 from a 2021 base year. The grant occurred prior to our base year update to 2024 due to significant changes in our structure and corresponding emissions (ProfoundBio acquisition in May 2024) and achievement will be assessed prior to base year update.
3 Executive Management received RSU grants in 2024 with performance linked to supplier engagement ensuring two thirds of suppliers by spend committed to a Paris Agreement aligned climate target by 2030
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 102/253 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Energy consumption and mix (E1-5)
|
Energy consumption and mix |
|
2024 |
1 |
Total fossil energy consumption |
MWh |
4,616 |
|
Share of fossil sources in total energy consumption |
% |
38% |
|
|
|
|
2 |
Consumption from nuclear sources |
MWh |
92 |
|
Share of consumption from nuclear sources in total energy consumption |
% |
1% |
|
|
|
|
3 |
Fuel consumption for renewable sources, including biomass (also comprising industrial and municipal waste of biologic origin, biogas, renewable hydrogen, etc.) |
MWh |
- |
|
|
|
|
4 |
Consumption of purchased or acquired electricity, heat, steam, and cooling from renewable sources |
MWh |
7,414 |
|
|
|
|
5 |
The consumption of self-generated non-fuel renewable energy |
MWh |
77 |
|
|
|
|
6 |
Total renewable energy consumption1 |
MWh |
7,491 |
|
Share of renewable sources in total energy consumption |
% |
61% |
|
|
|
|
|
Total energy consumption2 |
MWh |
12,199 |
|
|
|
|
|
1 - Total renewable energy consumption (MWh) (calculated as the sum of lines 3 to 5) |
|
|
|
2 - Total energy consumption (MWh) (calculated as the sum of lines 1, 2 and 6) |
|
|
Accounting Policies
Total energy consumption is accounted for by combining both renewable and non-renewable energy sources used across our operations. We measure energy consumption in megawatt-hours (MWh), incorporating data from energy management systems and utility invoices to ensure accuracy. Renewable energy includes wind, solar, hydro and other sustainable sources which are supported by contractual agreements such as Energy Attribute Certificates (EACs), while non-renewable energy encompasses fossil fuels and grid electricity. This comprehensive approach allows us to monitor our overall energy usage effectively and conduct annual reviews of our consumption data to validate its completeness and reliability, ensuring compliance with relevant reporting standards and reinforcing our commitment to sustainability.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 103/253 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Gross Scopes 1, 2, 3 and total GHG emissions (E1-6)
Genmab calculates its Scope 1, 2 and 3 GHG emissions in accordance with the requirements of ESRS E1 Climate Change, considering the principles, requirements and guidance provided by the GHG Protocol.
|
|
Milestones and Target Years |
||||
|
Base Year |
2023 |
% Change |
2030 |
2050 |
Annual % Target / Base Year3 |
Scope 1 GHG emissions1 |
|
|
|
|
|
|
Gross Scope 1 GHG emissions (tCO2eq) |
534 |
317 |
68% |
534 |
54 |
0% |
|
|
|
|
|
|
|
Scope 2 GHG emissions |
|
|
|
|
|
|
Gross location-based Scope 2 GHG emissions (tCO2eq) |
2,705 |
1,200 |
125% |
- |
- |
- |
Gross market-based Scope 2 GHG emissions (tCO2eq) |
1,163 |
238 |
389% |
450 |
116 |
7% |
|
|
|
|
|
|
|
Total Scope 1 and market-based Scope 2 GHG emissions (tCO2eq) |
1,697 |
555 |
206% |
984 |
170 |
7% |
|
|
|
|
|
|
|
Significant Scope 3 GHG emissions2 |
|
|
|
|
|
|
Total Gross indirect (Scope 3) GHG emissions (tCO2eq) |
|
|
|
|
|
|
1 - Purchased Goods and services |
164,449 |
127,237 |
29% |
|
|
|
2 - Capital goods |
5,519 |
10,045 |
(45)% |
|
|
|
3 - Fuel and energy-related |
1,078 |
411 |
162% |
|
|
|
4 - Upstream transportation and distribution |
5,425 |
4,737 |
15% |
|
|
|
6 - Business travel |
10,559 |
7,675 |
38% |
|
|
|
7 - Employee commuting |
946 |
803 |
18% |
|
|
|
Total Scope 3 GHG emissions |
187,976 |
150,908 |
25% |
|
|
|
|
|
|
|
|
|
|
Total GHG emissions |
|
|
|
|
|
|
Total GHG emissions (location- based) (tCO2eq) |
191,215 |
152,425 |
25% |
|
|
|
Total GHG emissions (market- based) (tCO2eq) |
189,673 |
151,463 |
25% |
|
|
|
1 - Percentage of Scope 1 GHG emissions from regulated emission trading schemes not applicable to Genmab.
2 - Scope 3 GHG emissions categories excluded from the inventory include 5 – Waste generated in operations as it is included in category 1, 8 – Upstream leased assets, 9 – Downstream transportation and distribution, 10 – Processing of sold products, 11 – Use of products sold, 13 – Downstream leased assets and 14 – Franchises as they are all not applicable to Genmab, and 12 – End-of-life treatment of sold products and 15 – Investments as they are not material. Further, there are no emission reduction target percentages for Scope 3 GHG emissions. Refer to E1-4 for environmental targets.
3 – Annual % Target / Base Year represents the actual reduction target for 2030 (or 42%) over six years.
Accounting Policies
Scope 1 GHG Emissions
Scope 1 GHG emissions refer to the direct GHG emissions from sources that are financially or operationally (as required by ESRS 1) controlled by Genmab at our headquarters, office, laboratory and preclinical development space across all Genmab locations. Direct GHG emissions is comprised of the sum of greenhouse gases, which are converted to CO₂ equivalents (CO2eq). The GHG emissions arise from the combustion of fuel products and leakage of refrigerants. To calculate GHG emissions the newest version of DEFRA GHG Conversion factors (2024) has been used.
Scope 2 GHG Emissions
Scope 2 GHG emissions refer to the indirect GHG emissions resulting from the generation of purchased energy that is used by Genmab at our headquarters, office, laboratory and preclinical development space across all Genmab locations. Scope 2 GHG emissions occur at the facility where the energy is generated,
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 104/253 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
thus being classified as indirect GHG emissions. The GHG emissions are linked to the electricity and district heating consumption.
Scope 2 location-based GHG emissions are calculated by taking consumed energy multiplied with relevant location-based national emission factors provided by the International Energy Agency (IEA) (2023) and the Association of Issuing Bodies (AIB) (2023).
Scope 2 market-based GHG emissions are calculated by taking consumed energy multiplied with market-specific emission factors provided directly from the energy supplier. Renewable energy purchases and certificates are considered, when accounting for indirect GHG emissions, using the market-based approach.
Scope 3 GHG Emissions
Genmab has identified six categories, out of the 15 categories of Scope 3 GHG emissions defined by the GHG protocol, as relevant. The remaining nine categories are not separately reported on as they are either not applicable to Genmab or GHG emissions have been included in the other emission categories. Zero percent of Genmab’s Scope 3 GHG emissions are measured using primary data obtained from suppliers or other value chain partners. For spend-based calculations, all spend in foreign currencies has been converted into USD using the average exchange rate for the year.
Category 1 - Purchased goods and services
Purchased goods and services include GHG emissions related to all spend from external suppliers, except for investment (CapEx), travel, and transportation and distribution spend, which are included in other Scope 3 categories. Spend is converted into CO2eq emissions using the spend-based method by applying the Environmentally Extended Input-Output (EEIO) model with US EPA emission factors (2022) to estimate GHG emissions (CO2eq).
Category 2 - Capital goods
Capital goods include GHG emissions related to investments in tangible assets (CapEx). Spend is converted into CO2eq emissions using the spend-based method by applying the Environmentally Extended Input-Output (EEIO) model with US EPA emission factors (2022) to estimate GHG emissions (CO2eq).
Category 3 - Fuel and energy-related activities
Fuel and energy-related activities include all upstream Well-to-Tank (WTT) CO2eq emissions of purchased fuel and electricity and Transmission and Distribution (T&D) Loss of purchased electricity (beyond Scope 1 and 2 GHG emissions). Electricity and fuel consumption are multiplied by DEFRA's emission factors (2023 for fuel and 2021 for electricity) to estimate GHG emissions (CO2eq). The category primarily comprises upstream WTT and T&D emissions from electricity and WTT emissions from natural gas.
Category 4 - Upstream transportation and distribution
Upstream transportation and distribution include GHG emissions related to spend from external suppliers related to transportation and distribution of goods. Spend is converted into CO2eq emissions using the spend-based method by applying the Environmentally Extended Input-Output (EEIO) model with US EPA emission factors (2022) to estimate GHG emissions (CO2eq).
Category 6 – Business travel
Business travel includes GHG emissions related to spend from external suppliers related to flights, ground transportation, hotel stays and meals in connection with business travel. Spend is converted into CO2eq
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 105/253 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
emissions using the spend-based method by applying the Environmentally Extended Input-Output (EEIO) model with US EPA emission factors (2022) to estimate GHG emissions (CO2eq).
Category 7 – Employee Commuting
Employee commuting includes GHG emissions related to employees’ commuting between their homes and the Genmab sites. GHG emissions are estimated using the average data method and based on assumptions across our locations.
|
2024 |
GHG intensity per net revenue |
|
Total GHG emissions (location-based) per net revenue |
8.88 |
Total GHG emissions (market-based) per net revenue |
8.81 |
Refer to Note 2.1 in the consolidated financial statements for disclosures related to Genmab’s revenue.
2.4 EU Taxonomy
Disclosures pursuant to Article 8 of Regulation (EU) 2020/852
The EU Taxonomy is a classification system designed to provide a framework for identifying sustainable economic activities. It helps companies and investors distinguish between activities that contribute to environmental sustainability by establishing a common language for defining what constitutes "green" or sustainable business practices. The EU Taxonomy plays a role in supporting the transition towards a more sustainable economy.
In line with Article 8 of the EU Taxonomy and the Disclosure Delegated Act (EU) 2021/2178, Genmab is required to report on the sustainability profile of its activities, specifically focusing on the eligibility and alignment of its Turnover, Capital Expenditures (CapEx) and Operating Expenditures (OpEx).
Eligibility:
We screened our economic activities against those outlined in the Taxonomy, identifying eligible Turnover, CapEx and OpEx.
| ● | Turnover - We assessed turnover based on the net product sales of pharmaceutical products. We concluded that turnover from the sale of EPKINLY qualifies under the Manufacture of Medicinal Products (#1.2) activity, in line with the Taxonomy criteria for Pollution Prevention and Control (PPC). We did not include turnover from the sale of EPKINLY as eligible activities in 2023 as EPKINLY launched in the U.S. in May 2023 and Japan in September 2023. |
| ● | CapEx - Our assessment focused on investments that align with Taxonomy-eligible activities. We identified one eligible project under Renovation of Buildings (#7.2) in line with the Taxonomy criteria for Climate Change Mitigation (CCM). |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 106/253 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| ● | OpEx - We evaluated the eligibility of our OpEx by reviewing the eligible economic activities outlined in its Statement of Profit or Loss and examining the data available to us from our ERP system. Based on this evaluation, we did not identify eligible OpEx. |
Alignment:
We assessed whether any of our Taxonomy-eligible Turnover or CapEx for economic activities 1.2 and 7.2 could be considered Taxonomy-aligned; however, we were not able to obtain enough evidence to conclude alignment with the 'Substantial contribution' and 'Do No Significant Harm' (DNSH) criteria.
Accounting Policies
Turnover:
Total Turnover consists of total revenue as disclosed in note 2.1 in the consolidated financial statements. The Turnover KPI represents the ratio of net product sales from taxonomy-eligible or taxonomy-aligned economic activities to the total revenue in a fiscal year.
CapEx:
Total CapEx consists of additions to intangible assets, tangible assets and right-of-use assets during the fiscal year (refer to notes 3.1, 3.2 and 3.3, respectively) and considered before depreciation, amortization, and any re-measurements, including those resulting from revaluations and impairments, for the relevant financial year, excluding any fair value changes. Furthermore, total CapEx consists of any additions to tangible and intangible assets resulting from business combinations. The CapEx KPI represents the share of CapEx that is taxonomy-eligible or taxonomy-aligned divided by the total CapEx.
OpEx:
Total OpEx includes direct non-capitalized costs that relate to research and development, building renovation measures, short-term lease, maintenance and repair, and any other direct expenditures relating to the day-to-day servicing of assets of property, plant and equipment by the undertaking or third party to whom activities are outsourced that are necessary to ensure the continued and effective functioning of such assets. OpEx does not include amortization, depreciation, or impairments.
To avoid double counting related to the economic activities, Turnover, CapEx and OpEx are distinctly allocated to ensure that there is no overlap across these financial metrics.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 107/253 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Economic Activities — Turnover
TURNOVER |
Substantial Contribution Criteria |
DNSH criteria ('Does Not Significantly Harm') |
|
|
|
|
||||||||||||||
Economic Activities |
Codes |
Absolute Turnover |
Proportion of Turnover |
Climate Change Mitigation |
Climate Change Adaptation |
Water |
Pollution |
Circular Economy |
Biodiversity and ecosystems |
Climate Change Mitigation |
Climate Change Adaptation |
Water |
Pollution |
Circular Economy |
Biodiversity |
Minimum Safeguards |
Taxonomy Aligned Proportion of Total Turnover, 2024 |
Taxonomy Aligned Proportion of Turnover, 2023 |
Category (Enabling Activity) |
Category (Transitional Activity) |
|
|
DKK million |
% |
% |
% |
% |
% |
% |
% |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
% |
% |
E |
T |
A. TAXONOMY-ELIGIBLE ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
A.1. Environmentally sustainable activities (Taxonomy-aligned) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
None |
|
- |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
0% |
0% |
N/A |
N/A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Turnover of environmentally sustainable activities (Taxonomy-aligned) (A.1) |
- |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
0% |
0% |
N/A |
N/A |
|
A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) |
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Manufacture of medicinal products. |
PPC 1.2 |
1,743 |
8% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Turnover of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) |
1,743 |
8% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total (A.1+A.2) |
|
1,743 |
8% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B. TAXONOMY-NON-ELIGIBLE ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Turnover of Taxonomy-non-eligible activities |
19,783 |
92% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total (A+B) |
|
21,526 |
100% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 108/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Economic Activities — CapEX
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CAPEX |
Substantial Contribution Criteria |
DNSH criteria ('Does Not Significantly Harm') |
|
|
|
|
||||||||||||||
Economic Activities |
Codes |
Absolute CapEx |
Proportion of CapEx |
Climate Change Mitigation |
Climate Change Adaptation |
Water |
Pollution |
Circular Economy |
Biodiversity and ecosystems |
Climate Change Mitigation |
Climate Change Adaptation |
Water |
Pollution |
Circular Economy |
Biodiversity |
Minimum Safeguards |
Taxonomy Aligned Proportion of Total CapEx, 2024 |
Taxonomy Aligned Proportion of CapEx, 2023 |
Category (Enabling Activity) |
Category (Transitional Activity) |
|
|
DKK million |
% |
% |
% |
% |
% |
% |
% |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
% |
% |
E |
T |
A. TAXONOMY-ELIGIBLE ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
A.1. Environmentally sustainable activities (Taxonomy-aligned) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
None |
|
- |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
0% |
0% |
N/A |
N/A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CapEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) |
- |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
0% |
0% |
N/A |
N/A |
|
A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) |
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Renovation of existing buildings |
CCM 7.2 |
80 |
1% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CapEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) |
80 |
1% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total (A.1+A.2) |
|
80 |
1% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B. TAXONOMY-NON-ELIGIBLE ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CapEx of Taxonomy-non-eligible activities |
12,486 |
99% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total (A+B) |
|
12,566 |
100% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 109/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Economic Activities — OpEx
OPEX |
Substantial Contribution Criteria |
DNSH criteria ('Does Not Significantly Harm') |
|
|
|
|
||||||||||||||
Economic Activities |
Codes |
Absolute OpEx |
Proportion of OpEx |
Climate Change Mitigation |
Climate Change Adaptation |
Water |
Pollution |
Circular Economy |
Biodiversity and ecosystems |
Climate Change Mitigation |
Climate Change Adaptation |
Water |
Pollution |
Circular Economy |
Biodiversity |
Minimum Safeguards |
Taxonomy Aligned Proportion of Total OpEx, 2024 |
Taxonomy Aligned Proportion of OpEx, 2023 |
Category (Enabling Activity) |
Category (Transitional Activity) |
|
|
DKK million |
% |
% |
% |
% |
% |
% |
% |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
Y/N |
% |
% |
E |
T |
A. TAXONOMY-ELIGIBLE ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
A.1. Environmentally sustainable activities (Taxonomy-aligned) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
None |
|
- |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
0% |
0% |
N/A |
N/A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OpEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) |
- |
0% |
0% |
0% |
0% |
0% |
0% |
0% |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
0% |
0% |
N/A |
N/A |
|
A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) |
|
|
|
|
|
|
|
|
|
|
|
|||||||||
None |
|
- |
0% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OpEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) |
- |
0% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total (A.1+A.2) |
|
- |
0% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B. TAXONOMY-NON-ELIGIBLE ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OpEx of Taxonomy-non-eligible activities |
9,501 |
100% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total (A+B) |
|
9,501 |
100% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 110/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Annex XII
Row |
Nuclear energy related activities |
|
1 |
The undertaking carries out, funds or has exposures to research, development, demonstration and deployment of innovative electricity generation facilities that produce energy from nuclear processes with minimal waste from the fuel cycle. |
NO |
2 |
The undertaking carries out, funds or has exposures to construction and safe operation of new nuclear installations to produce electricity or process heat, including for the purposes of district heating or industrial processes such as hydrogen production, as well as their safety upgrades, using best available technologies. |
NO |
3 |
The undertaking carries out, funds or has exposures to safe operation of existing nuclear installations that produce electricity or process heat, including for the purposes of district heating or industrial processes such as hydrogen production from nuclear energy, as well as their safety upgrades. |
NO |
|
Fossil gas related activities |
|
4 |
The undertaking carries out, funds or has exposures to construction or operation of electricity generation facilities that produce electricity using fossil gaseous fuels. |
NO |
5 |
The undertaking carries out, funds or has exposures to construction, refurbishment, and operation of combined heat/cool and power generation facilities using fossil gaseous fuels. |
NO |
6 |
The undertaking carries out, funds or has exposures to construction, refurbishment and operation of heat generation facilities that produce heat/cool using fossil gaseous fuels. |
NO |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 111/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Sustainability Statements: Social
Genmab is dedicated to making a meaningful impact in the lives of patients and caregivers by developing innovative treatments to transform cancer care and address other serious diseases. We prioritize understanding the needs of patients and their families, ensuring that their experiences and insights guide our research, development and commercialization efforts.
Our workforce is our greatest asset; the dedication and innovation of our team members are essential to our success. The Genmab Commitment, our employee value proposition, grounds our culture and brings our vision, purpose, and core values to life. Team members, or full-time equivalents (FTEs) at Genmab are defined as all employees on our payroll, both full-time and part-time, as well as active and on-leave. All individuals have been included by reflecting the proportion of an FTE they represent based on their contractual agreement. Non-employees at Genmab include contingent workers and consultants provided by third party undertakings who are primarily engaged in employment activities.
We prioritize attracting and retaining qualified individuals who align with our core mission of improving patient lives. Teamwork and respect are central to our culture, fostering an inclusive, open, and supportive environment across our global locations. We believe that workplace diversity—encompassing social, educational, cultural, national, age, and gender differences—is crucial for our continued success. By recruiting employees with the right skills and competencies, we create interactive teams that drive our goals and ensure Genmab’s ongoing impact in healthcare, ultimately benefiting the patients and care partners we serve.
Own Workforce
Below are the list of Disclosure Requirements as it pertains to ESRS S1 - Own Workforce:
Section |
Disclosure requirement content |
Disclosure requirement # |
3.0 IROs |
Material Impacts, Risks and Opportunities and their interaction with strategy and business model |
SBM-3 |
3.1 Own Workforce IRO Management |
Policies related to own workforce |
S1-1 |
|
Processes for engaging with own workers and workers’ representatives about impacts |
S1-2 |
|
Processes to remediate negative impacts and channels for own workers to raise concerns |
S1-3 |
|
Taking action on material impacts on own workforce, and approaches to mitigating material risks and pursuing material opportunities related to own workforce, and effectiveness of those actions |
S1-4 |
3.2 Own Workforce Metrics and Targets |
Targets related to managing material negative impacts, advancing positive impacts, and managing material risks and opportunities |
S1-5 |
|
Characteristics of the Company’s employees |
S1-6 |
|
Characteristics of non-employee workers in the Company’s own workforce |
S1-7* |
|
Collective bargaining coverage and social dialogue |
S1-8 |
|
Diversity metrics |
S1-9 |
|
Adequate wages |
S1-10 |
|
Social protection |
S1-11 |
|
Persons with disabilities |
S1-12* |
|
Training and skills development metrics |
S1-13 |
|
Health and safety metrics |
S1-14 |
|
Work-life balance metrics |
S1-15* |
|
Compensation metrics (pay gap and total compensation) |
S1-16 |
|
Incidents, complaints and severe human rights impacts |
S1-17 |
*Genmab has adopted the phase-in for S1-7, S1-12 and S1-15 and elected not to disclose for year 1 reporting.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 112/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
3.0 IROs
The table describes Genmab’s material impacts, risks and opportunities related to our material Social topics on own workforce:
Section |
Material Topic |
Material Impact |
Value Chain Location |
Material Risk |
Material Opportunity |
Social |
Own Workforce |
Employee well-being and vitality (positive) |
Own Operations |
Genmab may have an inability to attract and retain suitably qualified team members |
Provide a voice to employees through our global engagement survey |
|
|
Safety in our facilities (positive) |
Own Operations |
|
Safety training / ongoing education in our laboratories |
|
Own Workforce |
Career development through training and skill building (positive) |
Own Operations |
|
Providing employees with opportunities to discuss their career at Genmab |
|
|
Diversity in the workplace (positive) |
Own Operations |
|
|
All impacts, risks and opportunities have expected time horizons of short, medium and long-term.
Employee well-being and vitality
Prioritizing employee well-being and vitality is not just a moral imperative but also a strategic investment in the success and sustainability of Genmab. It promotes a positive work environment, improves employee morale and engagement, enhances productivity, reduces turnover, and helps maintain compliance with legal requirements. Therefore, prioritizing the health and safety of our teams demonstrates our commitment to our most valuable asset—our people.
Promoting work-life balance is not just beneficial for employees, it also positively impacts Genmab by fostering a more productive, engaged, and satisfied workforce. It contributes to a positive organizational culture, supports recruitment and retention efforts, and ultimately enhances the overall performance and success of Genmab.
Genmab has a significant opportunity to enhance its workplace culture by providing a voice to our employees through ongoing initiatives aimed at improving employee engagement. By leveraging platforms such as our annual global engagement survey, Genmab can foster an environment where employees feel valued, motivated, and committed to both their work and the organization's goals. This focus on engagement is likely to lead to improved talent retention and overall employee well-being.
With both local and global surveys conducted annually or biennially, Genmab gathers valuable insights into employee satisfaction, including both physical and mental workplace conditions. The results of these surveys provide a foundation for actionable improvements, with the Human Resources team facilitating the process and Genmab leaders taking ownership of the outcomes. By actively addressing areas of concern highlighted in the surveys, Genmab can create a more supportive and responsive work environment, ultimately empowering employees to share their feedback, concerns, and ideas effectively.
Safety in our facilities
Safety in facilities at Genmab is essential not only for complying with legal requirements, but also for protecting employees, enhancing productivity, reducing costs, maintaining a positive reputation, and supporting overall business success and sustainability. It reflects Genmab's commitment to the well-being of its employees and its responsibility as a corporate citizen within the broader community.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 113/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
We have instituted mandatory safety training and ongoing education in all workplace areas, especially related to the proper handling of hazardous materials and chemicals in our labs to enhance productivity and reduce costs.
Career development through training and skill building
Employees with advanced skills contribute to higher productivity and efficiency, improving the overall performance of the organization. Organizations that invest in their employees’ development tend to have higher retention rates, as employees feel valued and are more likely to stay with Genmab. Companies known for their commitment to employee development attract top talent, as professionals seek employers who offer growth opportunities. A focus on continuous learning fosters a culture of growth and development, enhancing teamwork, collaboration, and overall morale.
Genmab provides employees with opportunities to discuss their future career at Genmab which should increase employee retention, provide for the ability to attract top talent and achieve higher productivity and efficiency. This is done formally through the year-end performance reviews, but also informally through ad hoc discussions with the manager, as needed. Employees are provided with several internal resources related to the performance process.
Diversity in the workplace
We strive to create, nurture, and maintain a global, inclusive culture where differences drive innovative solutions to meet the needs of patients, care partners, families, and employees. Our teams give Genmab its strength. We are committed to championing a corporate culture that accepts and promotes uniqueness and empowers each team member to bring their authentic self to work in a safe, open, and respectful environment. Our diversity, equity, and inclusion (“DE&I”) strategy aims to make Genmab an Extra[not]ordinary™ employer and inclusive workplace with DE&I efforts centered on gender, age and nationality. Genmab has team members representing over 75 nationalities.
Genmab also has several initiatives in place to connect employees across the company, including employee resource groups (ERGs), sharing individual and group experiences to create an inclusive and supporting work environment and to continuously improve employee engagement.
Refer to S1-5 for targets related to our Own Workforce and S1-9 for diversity metrics including gender and age.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 114/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
3.1 Own workforce IRO management
Policies related to own workforce (S1-1)
Genmab has a number of policies that address our material IROs for the topic of Working Conditions, and related to all of its own workforce, including the following:
| ● | Code of Conduct |
| ● | Global Speak Up Policy |
| ● | Human Rights Commitment |
| ● | Global Diversity, Equity & Inclusion Policy |
| ● | Corporate Social Responsibility (CSR) |
| ● | Global Lab Occupational Health and Safety Policy |
Genmab has other processes and systems in place including our Workplace Accident Prevention Management System and Employee Value Proposition.
Code of Conduct
The Code of Conduct policy aims to promote ethical and compliant conduct in all aspects of our business. Our Code of Conduct applies to everyone in our Company, at every level, including employees, managers, and board members. We expect our alliance partners and third parties, including agents, consultants, contingent workers, and suppliers, to act in a way that is consistent with our values and our Code of Conduct when conducting business on behalf of Genmab. As noted in the Code of Conduct, #11 - Genmab proactively seeks to foster a values-based performance culture and a safe and healthy workplace environment of mutual respect, dignity and inclusion, one that assures we can attract, develop and retain a highly talented, diverse workforce of engaged employees driven to deliver superior business outcomes. We are committed to equal opportunity and fair treatment of our employees. All forms of harassment and retaliation are unacceptable and counter to everything we stand for as a Company.
The Code of Conduct can be mapped to the following positive impacts:
| ● | Employee well-being and vitality |
| ● | Career development through training and skill building |
Genmab’s Head of Global Compliance is responsible for this policy and reports directly to the CEO, and both are members of the CSR and Sustainability Committee.
Refer to the Code of Conduct on our website.
Global Speak Up Policy
Enabling employees and external stakeholders to speak up when they observe potential misconduct or have concerns about matters relating to our business is vital to retaining our strong Genmab culture and to doing the right thing. The Genmab Global Speak Up Policy allows for confidential and (where allowed) anonymous reports that will be directed to Genmab Compliance for initial triage and handling. This approach renders the reporting process more accessible and easier to navigate. All reports made under this Policy will be received and treated sensitively and seriously, and will be dealt with promptly, fairly, and objectively. Any investigations commenced will be conducted in a timely manner and will be fair and independent from any persons to whom the report relates.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 115/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Importantly, our policy also extends protections not only to our regular employees, but also contingent staff, part-timers, temp staff, trainees, interns (both current and former) and others who choose to report their concerns.
All employees, in addition to the new hire onboarding training, are required to complete annual Code of Conduct training and attest to their commitment to adhere to our ethical standards. This training also reviews among other topics, the Global Speak Up Policy concepts.
The Global Speak Up Policy can be mapped to the positive impact of Employee well-being and vitality. Genmab’s Head of Global Compliance is responsible for this policy and reports directly to the CEO, and both are members of the CSR and Sustainability Committee.
Refer to the Global Speak Up Policy on our website.
Human Rights Commitment
We recognize and support human rights and are dedicated to conducting business in a way that respects the dignity of all people. Our Human Rights Commitment is guided by current human rights laws and the United Nations Guiding Principles on Business and Human Rights. Our Guiding Principles refer to the International Bill of Human Rights, which consists of the Universal Declaration of Human Rights and the two Covenants that implement it, as well as the International Labour Organization’s (ILO) Declaration on Fundamental Rights and Principles at Work and the core conventions that underpin it, and we are in alignment with these instruments. We are committed to respecting human rights, including labor rights in our own operations and complying with the laws of the countries in which we do business. As part of our commitment, we seek to identify, prevent, and address any potential and actual adverse human rights impacts that our business may contribute to or cause. Further, as disclosed in our Human Rights Commitment, privacy rights of our employees, patients, healthcare providers, customers and other stakeholders are protected. Additionally, per the Human Rights Commitment policy we ensure fair, non-discriminatory employment practices and the prohibition of any form of human trafficking, forced, indentured, slave or child labor. We ensure that the policy is publicly available to all affected stakeholders by publishing it on our website.
In 2024, through periodic checks and audits, we continued to provide assurance that our policies, procedures, and operations align with our Human Rights Commitment. Our Supplier Code of Conduct addresses human rights and labor relations to ensure suppliers understand our commitment to compliance with local human and labor laws and recognize the importance we place on human rights. The Supplier Code of Conduct includes provisions in line with the applicable ILO standards addressing the safety of all workers including those in precarious work, human trafficking and the use of forced labour or child labour. The Global Speak Up Policy and Hotline covers the measures provided to enable remedy for human rights impacts.
The Human Rights Commitment policy can be mapped to the positive impact of Employee well-being and vitality. Under the leadership of our Chief People Officer, our Human Resources function is responsible for ensuring our compliance with this Commitment.
Refer to the Human Rights Commitment on our website. Refer also to the Corporate Global Supplier Code of Conduct Policy on our website.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 116/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Global Diversity, Equity & Inclusion Policy
At Genmab, prioritizing DE&I means creating richer solutions, obtaining better results, and maximizing productivity, innovation, and creativity. Diversity allows for a variety of perspectives, and inclusion takes different ideas and perspectives into account, while equity ensures fair opportunities to succeed. We embrace each employee’s unique contribution to our culture by valuing differences including age, disabilities, gender, Genmab heritage, nationality, professional specialization race/ethnicity, problem-solving style, sexual orientation, and social class. Genmab’s Global Diversity, Equity & Inclusion Policy covers inclusion for groups that may be at particular risk of vulnerability including all employees, regardless of gender, race, ethnicity, religion, age, disability, and other characteristics.
By ensuring equal treatment and opportunity, Genmab has a positive impact on people in its workforce and value chain. Diversity in the workplace and harboring a safe workplace for all leads to innovation, creating the opportunity for Genmab to gather multiple perspectives into one place. Similarly, a lack of diversity can impact company performance negatively and hinder innovative solutions to support patients.
With regards to our people and measures against violence and harassment in the workplace, as noted in our Code of Conduct, all forms of harassment and retaliation are unacceptable and counter to everything we stand for as a Company.
The Global Diversity, Equity & Inclusion Policy (most recently adopted by the Board of Directors on June 12, 2024) can be mapped to the positive impact of Diversity in the workplace. The DE&I Council (made up of senior leaders at Genmab) is responsible for ensuring our compliance with this policy.
Refer to the Global Diversity, Equity & Inclusion Policy on our website.
Corporate Social Responsibility (CSR) Policy
As noted in the CSR Policy under No. #2, we care for our employees’ health, well-being, safety, and development and promote a collaborative culture that fosters passion for innovation, integrity, and respect. We believe that diversity, equity, and inclusion are fundamental to achieving our vision and are committed to championing a corporate culture that accepts and promotes uniqueness and empowers each team member to bring their authentic self to work in a safe, open, and respectful environment.
The CSR Policy is mapped to the positive impact of Career development through training and skill building. The Nominating and Corporate Governance Committee of Genmab A/S’ Board of Directors oversees all aspects of Genmab’s CSR efforts.
Refer to the Corporate Social Responsibility (CSR) Policy on our website.
Global Lab Occupational Health and Safety Policy
Genmab has an internal Global Lab Occupational Health and Safety Policy specific to our R&D labs across our locations. Formal committees responsible for monitoring and improving health and safety at each of our locations continued their work. Each committee reports to site operations and to the local management team to address and escalate any issues. Health and safety prevention workers continue to monitor and improve health and safety at our R&D labs. This policy is mapped to the positive impact of Safety in our facilities. Under the leadership of our Chief Medical Officer, our committees are responsible for ensuring our compliance with the policy.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 117/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Workplace Accident Prevention Management System
Genmab adheres to processes, assessments, inspections, and staff trainings and has reporting in place to prevent, monitor and manage any workplace accidents. We aim to create a workplace accident prevention policy over the coming years. Mandatory workplace assessments are conducted in compliance with local regulations. This strategy is mapped to the positive impact of Safety in our facilities. Under the leadership of our Chief People Officer, our Human Resources function is responsible for ensuring our compliance with this Commitment.
Employee Value Proposition
Genmab has developed the Genmab Commitment, which is our employee value proposition that is published internally and the essence of it is used across in our internal and external communications and social media platforms.
The Commitment is what grounds our culture in the day-to-day work and brings our vision, purpose, and core values to life.
Our Commitment is made up of four key ingredients that illustrate how “We are extra[not]ordinary™”:
| ● | Empowerment |
| ● | Care |
| ● | Authenticity |
| ● | Impact |
These principles capture the culture we aspire to — engaging our team members to go beyond business as usual and to be remarkable, unique, and authentic. We offer an extra[not] ordinary rewards and opportunities package that empowers team members to succeed and enhances their well-being.
Processes for engaging with own workers and workers’ representatives about impacts (S1-2)
Genmab promotes an environment that fosters individual empowerment that allows team members to achieve their maximum potential. Genmab encourages active dialogue directly between employees and management. Genmab drives initiatives that engage, develop, and inspire employees as a part of our overall Total Rewards strategy.
Genmab facilitates active dialogue between our team members and management on workplace issues and other topics of concern through HR business partners, Employee Representative Council and Works Council.
| ● | Danish Employee Representative Council presents topics of interest and concern to employees during the year (at least once a year) through meetings to enable us to gain insight into the perspectives of our workforce and to ensure we remain a preferred workplace. Team members in Denmark have furthermore exercised their right to elect representatives to the Board of Directors in accordance with Danish legislation, and three group employees were elected to the Board under a voluntary scheme allowing employes from other sites to be elected. This employee representation strengthens the involvement and decision-making process at Genmab. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 118/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| ● | Dutch Works Council is a statutory body with the legal right and obligation to monitor and work for the proper functioning of the Company in all its objectives. This advocacy group represents team members in the Netherlands to bring concerns from the workforce to management during the year (at least once a year). Under the Dutch Works Councils Act, our Council must consent on topics that directly affect employees’ everyday work and must be involved in, and consulted for, advice on major organizational changes and determine the impact on the local workforce. |
While the U.S., Japan and China do not have Work Councils, employee engagement occurs globally for all employees through surveys and workplace assessments including our annual global engagement survey, inclusion networks, personal development dialogues, employee-elected board positions, employment relations and occupational health and safety representation.
A Global Employee Engagement Survey is conducted on an annual basis. The results of the survey help us keep a pulse on Genmab’s areas of strength and opportunity. Focus groups are conducted to generate further insights on critical engagement issues. The organization shares results with all team members and encourages all people leaders to review feedback with their teams and develop actions to improve the overall employee experience. In addition, Executive Management reviews results as a group to analyze key findings, themes, reflect on areas of strengths and opportunities for employee engagement.
To inform teams on our business and our progress, Genmab hosts functional and/or regional town hall updates.
Under the leadership of our Chief People Officer, our Human Resources function is responsible for ensuring engagement with our own workers and workers’ representatives.
Refer to SBM-2 for summary table of key stakeholder engagement.
Processes to remediate negative impacts and channels for own workers to raise concerns (S1-3)
While we have not identified any material negative impacts regarding our team members, we do promote and encourage our people to speak up to report concerns, share feedback, address compliance issues, and express ethical dilemmas to promoting transparency and foster a culture of openness and accountability by raising awareness of potential risks or challenges that could affect the organization.
Employees can raise concerns through their immediate managers or a Human Resource colleague, who will track and monitor such concerns, and raise the issue as needed to the appropriate management to resolve it.
Employees can also raise concerns through our 24/7 full-service Speak Up (whistleblower) compliance hotline.
Speak Up Compliance Hotline
24/7 – Online or by Phone
GenmabSpeakUp.ethicspoint.com
Denmark: 80-83-01-69
Netherlands: 0800-020-1556
United States: 1-844-942-3289
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 119/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Japan: 0800-123-0136
China: 400 125 3055
Refer to the Global Speak Up Policy in S1-1 and on our website. Refer to G1-1 for details of Genmab’s anti-retaliation policies and procedures and how Genmab tracks and monitors issues raised.
Taking action on material impacts on own workforce, and approaches to mitigating material risks and pursuing material opportunities related to own workforce, and effectiveness of those actions (S1-4)
The following actions were taken around Genmab’s material impacts on our own workforce in 2024:
Employee Well-Being and Vitality
Genmab’s well-being pillars were implemented in 2024 which include:
| ● | Emotional well-being: thrive every day |
| o | Supporting resilience and mental health through manager training and tools |
| ● | Financial well-being: enjoy security and freedom |
| o | Promoting financial security with initiatives like Global Money Week |
| ● | Physical well-being: feel your best |
| o | Offering on-site and virtual fitness opportunities |
| ● | Social well-being: live connected |
| o | Facilitating volunteer opportunities to connect employees with the community |
Programs
Global Well-Being: In 2023, we created a strategic roadmap to help establish an effective Global Well-Being (GWB) Program to support our team members. Our roadmap was developed based on feedback from key stakeholders and the insights of well-being experts who use research-based best practices to design and implement a custom GWB program for our organization. The program that Genmab offered in 2024 and 2023 included the following workshops:
| ● | Care for you as the caregiver |
| ● | Leading with love – celebrating and affirming LGBTQ+ young people |
| ● | Creating financial well-being plan |
| ● | Design your work/life webinar |
| ● | International self-care day article posted on Everyday intranet |
Emotional and Mental Health: Genmab offered multiple programs, including self-care applications, in 2024 and 2023 and resources at each location to support emotional and mental health needs. A weeklong series on Well-being for employees that provided resources for managers to leverage in support of their employees’ mental health in 2024 and 2023 included the following workshops:
| ● | Resilience: protecting your mental health in stressful times |
| ● | How to maximize your day |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 120/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| ● | Stress busting: run from the bear |
| ● | The importance of unplugging |
| ● | Boundaries before burnout |
Volunteering
Genmab organizes events throughout the year to connect with each other and our communities. In 2024, 688 team members volunteered 2,952 hours on Global Volunteer Day compared to 571 team members and 2,668 hours in 2023.
Self-Care Applications
In 2023, we launched our own mental health application, GenCare, which is used by our team members globally. The app uses several learning approaches and preferences to support neurodiversity. GenCare evolved in 2024 with updated content and a focus on improved well-being.
Work-life Balance
Genmab continued to provide four additional days off and four meeting-free days throughout the year in both 2024 and 2023. Genmab recognizes the importance of time away from work and offers all employees paid time off, as well as leave of absence policies, to support our teams members through extended periods away from work on a paid and/or unpaid basis. Genmab offers family-related leave to all full-time employees across all our Genmab entities following statutory provisions as stated by the relevant local jurisdiction.
Total Rewards & Opportunities
As Genmab continues to grow, we’ve been enhancing systems to support transparency, understanding, and empowerment regarding employees’ Total Rewards & Opportunities which includes all of the tangible and intangible elements available to employees in return for helping Genmab fulfill our purpose and live our values.
In 2024, we introduced a new feature in our Human Resources Information System (HRIS) providing more information about employee salary and growth potential. Employees have the ability to view salary ranges and market ratios for comparable roles in the Biotech / Life Sciences industry. These ranges were created following consultation and alignment with leaders throughout Genmab to ensure that the market data leveraged was the most appropriate for the skills and responsibilities our employees possess. As a result, this exercise exemplifies our rigorous, data-driven processes to ensure our salaries are internally consistent as well as externally attractive – for both new hires and current employees.
Genmab also awards new hires with equity grants in the form of warrants and restricted stock units, allowing our team members the opportunity to become part owners in Genmab.
Lastly, Genmab offers a variety of market competitive benefits to our employees including physical, social, professional, emotional and financial well-being.
Refer to Note 4.6 in the consolidated financial statements for details related to grants of share-based instruments in 2024 and 2023.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 121/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Safety in Our Facilities
Our Safe & Sound and Sustainability Week recognizes the successes of workplace health and safety programs and provides information on how to keep our lab workers safe while bringing awareness to our sustainability footprint.
As part of the program for the weeks in 2024 and 2023, we highlighted important lab safety topics specifically for the laboratories in the Netherlands and the U.S. This included CPR certification to equip our team members with life-saving skills, eyewash demonstrations, management of expired/unused chemicals, fire extinguisher training and biosafety. We also celebrated our team members’ efforts in identifying and reporting potential hazards.
We have instituted mandatory safety training and ongoing education in all workplace areas, especially related to the proper handling of hazardous materials and chemicals in our labs. We are fully aware of the impact that chemicals can have on employee and patient health and safety. We are committed to ensuring that our chemical management practices comply with all relevant regulations and standards, and that we minimize the potential for harm to our workers, customers, and the environment. Risk assessments of chemicals used by Genmab in its operations indicate that many pose low or no hazard. However, we constantly monitor the use of chemicals with high risk factors, such as being corrosive, reactive, mutagenic or carcinogenic, and are constantly promoting investigation into their potential replacement or process enhancements. In alignment with our stringent safety protocols and adherence to legal requirements, our labs recorded no chemical-related incidents resulting in injuries requiring beyond basic first aid treatment or significant chemical emissions.
Career Development through Training and Skill Building
Global Employee Engagement Survey
As standard practice, Genmab reviews the survey results with the Executive Committee to reflect on employee feedback, current engagement strategy and understand future actions that could support the employee experience. It is also encouraged for people leaders to review survey results directly with their employees to discuss the feedback and establish any actions to improve the employee experience. Lastly, Genmab transparently displays survey results with the broader employee population by publishing them on the Company’s internal secured network.
In 2024 and 2023, Genmab conducted the survey with areas of focus including: diversity and inclusion, immediate manager, work environment, innovation, engagement, camaraderie & teamwork, thriving, communication effectiveness, work-life balance, career development, senior leadership, performance management and organization effectiveness. Team members scored Genmab on these areas of focus which helps us keep a pulse on areas of concern.
The engagement score, which measures favorability, is influenced by certain questions specifically designed to measure overall employee engagement on a five point rating scale. In 2024, we achieved a 79% engagement score and a 90% global participation rate compared to an 83% engagement score and an 88% global participation rate in 2023. Our results in 2024 and 2023 exceeded life sciences industry benchmarks of 78% engagement score and 80% participation rate.
Refer to S1-5 for targets related to the global employee engagement survey.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 122/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Learning & Development
In 2024, Genmab created multiple skills-based learning paths for team members focusing on specific skill development with focus areas including digital and AI, feedback, strategic planning, advance excel skills, leading different generations, informal leadership, and business communications.
In 2023, Genmab focused its efforts to continue building our learning culture, including curation and development of new virtual learning content through our GenSpire platform, as well as initiating custom, targeted learning programs. We continued to implement our cloud-based learning management system, together with an e-learning library of courses to help team members develop their skills while working remotely.
AI in learning
In 2024, Genmab focused on the usage of AI in learning to increase usability, course suggestions, and our collaboration with our learning providers.
Global Mentorship Program
In 2024, Genmab launched a Global Mentor Program, to ensure an inclusive and supporting environment. This new mentor program can impact the sense of belonging, as employees connect with other colleagues outside their area of expertise as well as connecting with leaders that can provide insights and feedback that is not linked to any performance plan. The feedback can be used to develop skills and traits important to that specific individual.
Diversity in the Workplace
Employee Satisfaction
We achieved an overall satisfaction score of 88% or greater on Diversity and Inclusion in the Global Employee Engagement Survey in 2024 and 2023.
DE&I Council
Our DE&I Council guides the alignment of our DE&I strategy with our overall business strategy. The Council includes representation of senior leaders, DE&I team, Internal Communication, and presidents of our ERGs, and meets to discuss cultural activities, employee feedback and future DE&I needs.
DE&I Trainings
Our DE&I team updates Genmab DE&I training each year to respond to the growth and diversity in Genmab as well as the geopolitical situation in the global environment. We conducted multiple trainings in 2024 and 2023. The trainings offered were a combination of culture Workshops, culture masterclasses and DE&I workshops with basic concepts and definitions.
Employee Resource Groups (ERGs)
Genmab currently has six (6) Employee Resource Groups (ERGs). ERGs are employee led, self-directed voluntary groups that enhance culture and aim to bring better business outcomes through leveraging diversity and inclusion by offering opportunities to network internally, attracting a diverse employee base, providing the inclusion of ideas and solutions, and creating opportunities for career development. ERGs are overseen by the DE&I team, under the Human Resource function. All ERGs are open to all employees of Genmab.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 123/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Talent Acquisition and Fair & Equal Hiring Practices
In 2024, we attended two (2) diversity career fairs. In 2023, our Talent Acquisition team, including 100% of our recruiters, received additional implicit bias training on eliminating bias in the hiring process. In partnership with the National Black MBA Association, Inc and Disability IN, in the U.S., as well as Women in Tech in Brussels, we continued outreach and relationship building in historically underserved communities to better understand their needs and identify ways to support local prosperity and the development of potential talent for our Company. We plan to continue to participate in three to four diversity career fairs annually, with the focus on expanding the talent pipeline and ensuring that diverse talent has access to apply for vacant positions.
Compliance with the Dutch Participation Act
We employ three individuals with disabilities who were trained, mentored, and coached on the job to support this law that aims to help everyone find work in the Netherlands, including people with disabilities.
Refer to S1-5 for targets linked to diversity.
Targets related to managing material negative impacts, advancing positive impacts, and managing material risks and opportunities (S1-5)
Targets related to Employee well-being and vitality
Genmab continues to promote an environment that fosters individual empowerment that allows team members to achieve their maximum potential, and drive initiatives that engage, develop and inspire employees as a part of our overall Total Rewards strategy. Specific target identified by the HR function related to promoting employee well-being and vitality is indicated below:
| ● | Meet or exceed the global benchmark for (1) employee engagement score and (2) participation rate for the Global Employee Engagement Survey in 2025 (annual target) 4 |
The target is measured annually after the administration of the Global Employee Engagement Survey. Genmab will compare the engagement score, which measures the favorability rate, and the participation rate for the Company's engagement in comparison to the life sciences industry average. Focusing on top-down governance, the survey results are reviewed with the Executive Committee. During the discussion, the team will reflect on any actions needed to maintain or improve overall employee engagement.
4 Executive Management received RSU grants in 2022, 2023 and 2024 with performance linked to sustaining at or better than the global benchmark for employee engagement.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 124/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Refer to S1-4 which shows how Genmab’s Global Employee Engagement Survey results in 2024 and 2023 outpaced life sciences industry benchmarks.
Targets related to Career development through training & skill building
The Genmab Learning & Development (L&D) team within the Global Talent Management strives to be data centric. The L&D team measures learning completion records for required learning, optional learning, and self-assigned learning. Aside from completion measures the team also measures the communication channels effectiveness. Specifically, L&D has set annual required career development, training & skill building targets as follows:
| ● | 100% of eligible employees are provided access to Genmab’s end of year performance process |
The Global Talent Management team at Genmab will ensure that all eligible employees are provided full access to the year-end performance process at Genmab, including providing internal resources and early engagement to the employees. This is set as an annual target for 2025.
| ● | 100% of employees are provided access to training programs that meet the development needs across various career stages and learnings styles |
The Global Talent Management team at Genmab will ensure that professional development skills trainings are offered to all employees. This is set as an annual target for 2025.
| ● | Launch sustainability awareness training by 2025 |
The Corporate Sustainability Team at Genmab plans to launch sustainability awareness training for team members in 2025.
Targets related to Diversity in the Workplace
Diversity targets are determined by the DE&I Council (made up of senior executives) and adopted by the Board of Directors. They are as follows:
| (1) | Maintain between 40% to 60% gender representation at a director level and above5 |
The Board of Directors has committed to maintaining an annual target of balanced gender representation, at a director level and above level positions by 2025. In order to provide equal employment and advancement opportunities to all individuals, employment decisions at Genmab are based on merit. The Company is committed to equal opportunity in the conduct of all our business activities. Genmab does not discriminate on the basis of race, color, creed, religion, gender, national
5 Executive Management received RSU grants in 2022, 2023 and 2024 with performance linked to gender diversity balance targets in director and above level roles. The range of payout varies based on gender splits (see GOV-3).
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 125/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
origin, age, marital status, disability, sexual orientation, gender identity or expression, status with regard to public assistance or any other classification protected by applicable law.
As of December 31, 2024, the gender split in director level and above was 52% female and 48% male as disclosed in the table in section S1-9.
| (2) | Target between 40% to 60% gender representation by 2025 in the Other Management Levels at Genmab A/S only in accordance with the guidelines from the Danish Business Authority |
As of December 31, 2024, the gender split in the Other Management Levels, as defined in the Danish Companies Act, was 33% female managers (six persons) and 67% male managers (12 persons) for Genmab A/S (Danish Parent only). As we do not currently have an equal share of men and women in the Other Management Levels at Genmab A/S as defined by the Danish Companies Act, the Board of Directors has committed to a target ratio of 40% to 60% for female and male splits in the Other Management Levels of Genmab A/S by 2025, or the target that comes closest to this target and which still constitutes an equal gender composition in accordance with the guidelines from the Danish Business Authority.
To pursue the fulfillment of the set target and to continue working towards and maintaining diversity and equal opportunities for employees at all management levels in the Genmab Group, we have implemented several initiatives related to, among other things, recruitment, employment terms and talent development. We also offer participation in internal network groups and focus on raising awareness of bias throughout the organization by conducting regular internal training.
Taking into account these initiatives and the existing composition of the Other Management Levels of Genmab A/S, the target is expected to be met by 2025.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 126/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Characteristics of the undertaking’s employees (S1-6)
|
|
December 31, |
|||||||
|
|
2024* |
|
2023 |
2022 |
||||
Employees (Headcount) |
|
Female |
|
Male |
|
Total |
|
Total |
Total |
Denmark |
|
326 |
|
228 |
|
554 |
|
495 |
410 |
Netherlands |
|
490 |
|
335 |
|
825 |
|
740 |
600 |
U.S. |
|
642 |
|
432 |
|
1,074 |
|
887 |
643 |
Japan |
|
55 |
|
131 |
|
186 |
|
140 |
58 |
China |
|
55 |
|
56 |
|
111 |
|
- |
- |
Total Headcount |
|
1,568 |
|
1,182 |
|
2,750 |
|
2,262 |
1,711 |
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|||||||
|
|
2024* |
|
2023 |
2022 |
||||
Employees (FTEs) |
|
Female |
|
Male |
|
Total |
|
Total |
Total |
Permanent |
|
1,498 |
|
1,136 |
|
2,634 |
|
2,159 |
1,627 |
Temporary |
|
28 |
|
20 |
|
48 |
|
45 |
33 |
Total FTEs |
|
1,526 |
|
1,156 |
|
2,682 |
|
2,204 |
1,660 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|||
FTEs (R&D vs. SG&A) |
|
|
|
|
|
2024 |
|
2023 |
2022 |
Research and development FTE |
|
1,886 |
|
1,541 |
1,193 |
||||
Selling, general and administrative FTE |
|
796 |
|
663 |
467 |
||||
|
|
|
|
|
|
|
|
|
|
Turnover |
|
|
|
|
|
2024 |
|
2023 |
2022 |
# of FTEs leaving Genmab |
|
|
|
|
|
190 |
|
157 |
119 |
Turnover Rate - Overall |
|
|
|
|
|
7% |
|
8% |
8% |
Turnover Rate - Voluntary |
|
|
|
|
|
6% |
|
5% |
6% |
* 2024 is a baseline year for female / male headcount and FTE reporting. Splits were not previously disclosed in prior year Annual Reports.
As of December 31, 2024, the total number of FTEs was 2,682 compared to 2,204 as of December 31, 2023. The increase was primarily driven by the expansion and acceleration of our pipeline, as well as the investment in the expansion of Genmab’s commercialization capabilities, including support for EPKINLY in
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 127/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
the U.S. and Japan post launch activities, and broader organizational capabilities and the acquisition of ProfoundBio.
Accounting Policies
Number of Employees
FTEs are defined as all employees on our payroll, both full-time and part-time, as well as active and on-leave. All individuals have been included by reflecting the proportion of an FTE they represent based on their contractual agreement.
Headcount are defined as all employees on our payroll, both full-time and part-time, as well as active and on-leave. All individuals have been included by reflecting a 1 equivalent per person.
Turnover Rate
Turnover rate is calculated by the overall number of FTEs leaving since the beginning of the year divided by the average FTE for the year.
Temporary
Includes interns, student workers, post Doctorate (post doc) and fixed term employees.
Refer to the Note 2.3 Staff Costs for cross reference to FTEs reported in Genmab’s financial statements.
Collective bargaining coverage and social dialogue (S1-8)
There are no employees covered by collective bargaining agreements at Genmab. There are workers councils in Denmark and the Netherlands. Refer to S1-2 for details of the work councils.
|
Collective Bargaining Coverage |
Social Dialogue |
Coverage Rate |
Employees - EEA |
Workplace representation (EEA only) |
0-19% |
|
|
20-39% |
|
|
40-59% |
|
|
60-79% |
|
|
80-100% |
|
Denmark, Netherlands |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 128/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Diversity metrics (S1-9)
Diversity metrics as of December 31:
|
2024 |
2023 |
2022 |
||||||
|
Female |
Male |
Total |
Female |
Male |
Total |
Female |
Male |
Total |
Board of Directors, Shareholder-Elected |
3 |
3 |
6 |
3 |
3 |
6 |
3 |
3 |
6 |
% of total |
50% |
50% |
100% |
50% |
50% |
100% |
50% |
50% |
100% |
|
|
|
|
|
|
|
|
|
|
Board of Directors, Including Employee-Elected |
4 |
5 |
9 |
4 |
5 |
9 |
4 |
5 |
9 |
% of total |
44% |
56% |
100% |
44% |
56% |
100% |
44% |
56% |
100% |
|
|
|
|
|
|
|
|
|
|
Executive Management |
3 |
6 |
9 |
3 |
5 |
8 |
2 |
5 |
7 |
% of total |
33% |
67% |
100% |
38% |
63% |
100% |
29% |
71% |
100% |
|
|
|
|
|
|
|
|
|
|
|
2024 |
2023 |
2022 |
||||||
|
Female |
Male |
Total |
Female |
Male |
Total |
Female |
Male |
Total |
Genmab Group |
1,525 |
1,157 |
2,682 |
1,270 |
934 |
2,204 |
964 |
696 |
1,660 |
% of total |
57% |
43% |
100% |
58% |
42% |
100% |
58% |
42% |
100% |
|
|
|
|
|
|
|
|
|
|
Director Level and Above |
547 |
507 |
1,054 |
449 |
414 |
863 |
348 |
332 |
680 |
% of total |
52% |
48% |
100% |
52% |
48% |
100% |
51% |
49% |
100% |
|
|
|
|
|
|
|
|
|
|
Below Director Level |
978 |
650 |
1,628 |
821 |
520 |
1,341 |
616 |
364 |
980 |
% of total |
60% |
40% |
100% |
61% |
39% |
100% |
63% |
37% |
100% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Age |
2024 |
2023 |
2022 |
|
|
|
|
|
|
< 30 |
11% |
13% |
14% |
|
|
|
|
|
|
30 - 50 |
63% |
63% |
62% |
|
|
|
|
|
|
> 50 |
26% |
24% |
24% |
|
|
|
|
|
|
|
100% |
100% |
100% |
|
|
|
|
|
|
Adequate wages (S1-10)
All of Genmab's employees receive adequate wages. We have a dedicated compensation & benefits team at Genmab that ensures that we are paying our people in line with local legal requirements and with peer and similar companies through benchmark analysis.
Social protection (S1-11)
All of Genmab's employees are covered by social protection, through public programs or through benefits offered by Genmab, against loss of income due to any of the following major life events including sickness, unemployment, employment injury and acquired disability, parental leave and retirement.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 129/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Training and skills development metrics (S1-13)
Genmab provides all employees training and skills development related activities within the context of continuous professional growth, to upgrade employees’ skills and facilitate continued employability.
|
2024 |
|
Training and skills development metrics |
Female |
Male |
% of Employees Who Have Completed Performance Reviews/Career Conversations |
100% |
100% |
% of Employees Who Have Not Completed Performance Reviews/Career Conversations |
0% |
0% |
Average Number of Training Hours |
10 |
8 |
Health and safety metrics (S1-14)
The health and safety of 100% of Genmab’s employees and non-employees are covered by legal requirements and recognized standards and guidelines. There were no fatalities in 2024 or 2023 as a result of work-related injuries and work-related ill health. In 2024, there were no work-related accidents. In 2023, we had one occupational incident that resulted in lost time at work in the U.S. We will continue our ongoing preventative health and safety activities to reinforce policies and procedures to all team members globally.
Remuneration metrics (pay gap and total remuneration) (S1-16)
The below table shows the percentage gap in pay between all our female and male employees and the ratio between the remuneration of our CEO and the median remuneration of our employees.
|
|
2024 |
Gender pay gap - Overall |
12% |
|
Gender pay gap - Excluding Executive Management |
5% |
|
CEO pay ratio |
64 |
|
Accounting Policies
Gender pay gap
Genmab calculates the gender pay gap for employees as the difference of average pay levels (gross hourly pay) between female and male employees, expressed as percentage of the average pay level of male employees. The calculation relies on a single variable (gender) and excludes other factors that would typically be included in a pay analysis, such as job level, amount of experience, performance rating, education level, typical market pay for a position, etc.
CEO pay ratio
Genmab calculates the CEO pay ratio for employees as the annual total remuneration ratio of the highest paid individual (our CEO) to the median annual total remuneration for all employees (excluding our CEO). Remuneration includes base salary, defined contribution plans, other benefits, annual cash bonus and share-based compensation. Refer to Note 5.1 in the consolidated financial statements for details of CEO pay for 2024.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 130/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Incidents, complaints and severe human rights impacts (S1-17)
During 2024, there were no work-related incidents of discrimination, including harassment, substantiated. No cases of severe human rights incidents (e.g., forced labor, human trafficking, or child labor) were identified during 2024.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 131/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Consumers and End-users
Genmab’s consumers include healthcare providers, pharmaceutical distributors, and end-users are its patients. Our consumers and/or end-users are dependent on accurate and accessible product-related information, such as manuals and product labels, to avoid potentially damaging use of a product. We also have a small group of pediatric consumers/end-users who are participating in a pediatric study, who are particularly vulnerable to health or privacy impacts or impacts from marketing and sales strategies due to the inherent nature of young age-group.
At Genmab, our work is anchored in our core purpose: to improve the lives of patients through innovative and differentiated antibody therapeutics. Driven by this purpose, we are transforming the way patients fight cancer while creating long-term value for all our stakeholders.
Below is the list of Disclosure Requirements as it pertains to ESRS S4 — Consumers and/or End-users:
Section |
Disclosure requirement content |
Disclosure requirement # |
4.0 IROs |
Material Impacts, Risks and Opportunities and their interaction with strategy and business model |
SBM-3 |
4.1 IRO Management |
Policies related to consumers and end-users |
S4-1 |
|
Processes for engaging with consumers and end-users about impacts |
S4-2 |
|
Processes to remediate negative impacts and channels for consumers and end-users to raise concerns |
S4-3 |
|
Taking action on material impacts on consumers and end-users, and approaches to managing material risks and pursuing material opportunities related to consumers and end-users, and effectiveness of those actions |
S4-4 |
4.2 Consumers & End-Users Metrics and Targets |
Targets related to managing material negative impacts, advancing positive impacts, and managing material risks and opportunities |
S4-5 |
4.0 IROs
The below table describes Genmab’s material impacts, risks and opportunities related to our material Social topics for Consumers and End-Users:
Section |
Material Topic |
Material Impact |
Value Chain Location |
Material Risk |
Material Opportunity |
Social |
Consumers and End-Users |
Access and integrity in clinical trials (positive) |
Downstream |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance |
|
|
|
Market access programs to allow for product availability for uninsured or underinsured (positive) |
Downstream |
Refer to the Risk Management section of the Annual Report for risks related to Business and Products |
Assisting those having difficulty affording Genmab products prescribed to them |
|
Consumers and End-Users |
Patient engagement programs developed (positive) |
Downstream |
Refer to the Risk Management section of the Annual Report for risks related to Business and Products |
|
|
|
Clinical trial transparency (positive) |
Downstream |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance |
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 132/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
All impacts, risks and opportunities have expected time horizons of short, medium and long-term.
Access and integrity in Clinical trials (positive)
We strive to enroll patients who will benefit from our clinical studies and Genmab is subject to extensive legislative, regulatory and other requirements which pose a risk to Genmab; however, Genmab is committed to ensuring equal access to Genmab clinical trials. We have DE&I initiatives to ensure we enroll diverse patients who represent the communities we serve.
Market access programs to allow for product availability for uninsured or underinsured (positive)
MyNavCare Patient Support is designed to provide comprehensive resources and support to help patients access EPKINLY (epcoritamab-bysp) throughout their treatment journey. Recognizing the challenges faced by uninsured or underinsured patients, Genmab has created a robust program through MyNavCare to provide financial assistance and free medicine for eligible patients struggling to afford their prescribed treatments. Without effective market access programs, Genmab could lose market share from competition.
Assisting those having difficulty affording Genmab products prescribed to them is an identified opportunity for Genmab and can lead to market growth in a competitive pharmaceutical landscape. Through MyNavCare, Genmab assists eligible patients with coverage for co-pays, co-insurance, and deductibles, helping to ensure that financial challenges do not become barriers to accessing life-changing therapies. This initiative reflects Genmab’s commitment to reducing disparities in healthcare access and empowering patients to focus on their treatment journey.
Patient engagement programs developed (positive)
Genmab is elevating the voices of patients and care partners by incorporating their perspectives into all aspects of our work — from early-stage R&D to clinical trials and commercialization. These insights are vital to our ability to innovate and to support people impacted by our medicines as they navigate the complex aspects of a serious illness. Without our patient engagement programs, there is risk of increased competition and decreased overall patient experience. Genmab engages with patients and caregivers to gather insights and improve patient outcomes.
The goal of these programs is to effectively and efficiently bring medicines to patients that incorporate the patient voice across the continuum of clinical development, including ensuring clinical protocols and informed consents have the patient insights required to attract and retain patients in the trials while meeting regulatory requirements.
Clinical trial transparency (positive)
As there is risk associated with transparency in our business, increased transparency in clinical trials is a key factor for advancing medical research and improving patient outcomes. As Genmab develops antibody therapeutics for cancer treatment and other serious diseases, increased transparency can have the following material impacts:
| ● | Help patients find trials that match their eligibility criteria and preferences, increasing the recruitment and retention rates of participants. |
| ● | Empower patients to make informed decisions about their health, enhancing their trust and satisfaction with the trial process and the Company. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 133/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| ● | Improve the evidence base for clinical care, allowing clinicians and scientists to access and evaluate the safety and efficacy of new treatments. |
| ● | Prevent duplication of unsuccessful trials, saving time and resources for the research community. |
| ● | Contribute to efficient design of clinical trials, enabling Genmab and the research community to learn and optimize new trial protocols and outcomes. |
Increased transparency in clinical trials benefits Genmab by facilitating development of innovative and effective therapies for patients with cancer or other serious diseases and ensuring their optimal use.
4.1 Consumers and End-Users IRO management
Policies related to Consumers and End-Users (S4-1)
Human Rights Commitment
Our Human Rights Commitment includes policies around our patient’s safety and privacy rights of our patients, healthcare providers and other customers.
Refer to section S1-1 for additional disclosures on our Human Rights Commitment and the Human Rights Commitment on our website.
Commitment to Quality
Our goal is to safeguard patient safety through state-of-the-art monitoring systems, stringent processes, and industry best practices. Our comprehensive safety program is designed to identify and mitigate potential risks associated with our products, and to ensure that our products are safe and effective for their intended use.
We work closely with regulatory agencies to ensure that our products meet all safety and efficacy standards. We also collaborate with healthcare providers and patient advocacy groups to ensure that patients have access to the information they need to make informed decisions about their treatment. Under the leadership of our Chief Development Officer, our functions are responsible for ensuring compliance with Commitment to Quality.
Refer to the Commitment to Quality on our website.
Clinical Trial Transparency
Genmab is committed to transparency of clinical trial research. We recognize the scientific and ethical value of sharing clinical trial information in a non-biased and timely manner to benefit diverse audiences. Transparency in clinical trials helps patients and healthcare providers to make well-informed decisions about patients’ health. Transparency is also about building and maintaining public trust in clinical research.
We are committed to sharing clinical trial information and results in a language understandable also for non-scientists, as part of our ongoing transparency efforts.
Our commitments apply to all Genmab-sponsored interventional clinical trials, Phase 1 and beyond, conducted worldwide. To the extent applicable, our commitments also apply to Genmab-sponsored non-interventional clinical studies and expanded access programs. The most senior level in Genmab that is accountable for the policy is the Chief Development Officer.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 134/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The Genmab Clinical Trial Transparency Declaration can be mapped to the positive impact of Clinical trial transparency and is publicly available via the website. The Declaration is also referenced in the onboarding program of new Development Operations employees.
Refer to the Clinical Trial Transparency Declaration on our website.
Code of Conduct
Within Genmab’s Code of Conduct, there is a section (#5) specifically on Clinical Trials, which is publicly available via Genmab’s website.
Our research and clinical trials are always guided by Our Purpose: to improve the lives of patients through innovative and differentiated antibody therapeutics. We are committed to conducting our research, development, and related data collection with scientific integrity and disclosing results in a timely manner.
Genmab’s Code of Conduct also describes our 20 ethical standards and covers responsible marketing practices. We at Genmab are committed to conducting all aspects of our business with integrity, and in an ethical, honest, and transparent manner. These standards and our associated corporate policies provide further guidance for our people with respect to this commitment. Genmab’s Head of Global Compliance is responsible for this policy and reports directly to the CEO, and both are members of the CSR and Sustainability Committee. The Code of Conduct can be mapped to our positive impact of Access and integrity in clinical trials.
Refer to the Code of Conduct on our website.
Commitment to Patients
Across Genmab, our talented and dedicated team works every day to improve the lives of patients through innovative and differentiated antibody therapeutics. Genmab’s Commitment to Patients describes how our work is guided by doing what’s best for patients. Through our Patient Advisory Council, patients contribute their insights ranging from how we design and conduct our clinical trials to how we may help to educate and support patients who have been prescribed our products. We are focused on understanding the many individuals who may support a patient throughout their disease experience and tailoring resources to the needs of these care partners to better facilitate their role in the care journey. Our goal is to conduct clinical research that reflects the real-world demographics of the diseases we study, and we strive to ensure our trials are patient centric and accessible for patients of all backgrounds to participate. We aim to ensure that all patients who are prescribed our medicines have timely access to them. We work with stakeholders across the healthcare system and have patient support services (PSS) in place to address patient access to our medications once prescribed. We aim to facilitate transparency in our work and interactions.
The Commitment to Patients can be mapped to the positive impacts of patient engagement and market access programs developed to allow for product availability for uninsured or underinsured. The Senior Vice President Communications and Corporate Affairs is responsible for the policy and reports directly to CEO.
Refer to the Commitment to Patients on our website.
Global Compliance Policy
Genmab has an internal Global Compliance Policy and playbook. The Global Compliance Policy covers interactions and engagements with healthcare professionals, healthcare organizations, patients, patient association groups and government officials (stakeholders). Genmab’s Head of Global Compliance is
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 135/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
responsible for this policy and reports directly to the CEO, and both are members of the CSR and Sustainability Committee.
Processes for engaging with consumers and end-users about impacts (S4-2)
Genmab’s engagement with consumers and end-users is a multi-faceted strategy that prioritizes patient safety, ethical marketing, and transparent communication.
Patient Engagement Programs
Genmab’s mission to improve the lives of patients with cancer and serious diseases is realized through its commitment to patient engagement programs. These efforts include clinical trials, advocacy initiatives, and also through tailored support programs like MyNavCare Patient Support™, which provides, with respect to Genmab products approved in the U.S., personalized assistance for patients and their care partners, as well as support for HCPs for the ultimate benefit of patients.
The MyNavCare Patient Support™ program offers comprehensive support, including insurance navigation, and financial support, designed to facilitate access to therapies for all eligible patients. The program supports, among others, those who are uninsured or underinsured, ensuring equitable access to treatment while alleviating the burdens associated with navigating complex healthcare systems. When someone has enrolled in the MyNavCare program, which is optional, they can call and engage with MyNavCare team members to discuss any questions or issues they have.
Genmab values patient input and actively involves patients in its decision-making processes through initiatives like the Patient Advisory Council. This council enables patients to share their experiences and insights, influencing how clinical trials are designed and how medicines are delivered. By listening to patient feedback, Genmab can better understand their needs and concerns, ultimately enhancing both the science behind its therapies and the support services offered, thereby contributing to the decision-making processes ranging from how we design and conduct our clinical trials to the way we deliver our medicines.
Engagement occurs on a regular basis, from early development through commercial. The Senior Vice President Communications and Corporate Affairs is responsible for ensuring this engagement and reports directly to CEO.
Clinical Trial Transparency
Transparency in clinical trials is another key focus for Genmab. Genmab engages on a regular basis with patient advocacy groups and healthcare professionals to ensure that the perspectives of consumers and end-users are integrated into trial design and execution. Genmab publishes its Clinical Trial Transparency Declaration on its website, keeping patients and other stakeholders informed about trial processes and expectations. The Development Operations team, organized under the Chief Development Officer, oversees this engagement, and ensures the effective planning, execution, and public disclosure of clinical trials.
Safety and Compliance
The Global Drug Safety team plays a crucial role in ensuring that Genmab’s products meet stringent safety and efficacy standards. They work closely with regulatory agencies to maintain compliance but do not engage directly with patients. This team's efforts are complemented by a comprehensive safety monitoring program designed to identify and mitigate potential risks associated with Genmab’s therapies. Trust in the safety and quality of these products is essential, as any breach of trust can adversely affect the Company’s reputation
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 136/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
and core business. Under the leadership of our Chief Development Officer, our internal functions are responsible for ensuring safety and compliance.
Ethical Marketing Practices
Genmab emphasizes responsible marketing practices in all interactions with healthcare providers and stakeholders. Genmab has established a Code of Conduct that sets high ethical standards for employees, reinforced through regular training. This ensures that all marketing and sales efforts align with local and national regulations, which may limit direct engagement with consumers and end-users. Ethical marketing practices are the responsibility of our Chief Commercial Officer.
Processes to remediate negative impacts and channels for consumers and end-users to raise concerns (S4-3)
While we have not identified any material negative impacts regarding our consumers and end-users, we do promote and encourage our consumers and end-users to speak up to report concerns, share feedback, address compliance issues, and enable remedy for human rights impacts. We believe our consumers and end-users trust the channels described in this section as we receive questions and requests from these channels.
Clinical Trials
Consumers and end-users can raise questions or concerns via Genmab’s publicly available mailbox, ClinicalTrial@genmab.com. This mailbox is provided along with all registered trials on disclosure platforms such as ClinicalTrials.gov and the EU CTIS public portal. At time of enrollment, the trial participants are provided an Informed Consent Form (ICF) to sign before joining a trial. The ICF contains additional information on channels to raise questions or concerns. The requests received are reviewed and assessed to ensure they are addressed by the appropriate Genmab function, while ensuring that personal information is handled securely and in compliance with privacy regulations.
MyNavCare
Patients enrolled in the MyNavCare program have access to dedicated team members who address their concerns, answer questions, and provide personalized assistance. The Patient Engagement Liaisons (PELs) serve as trusted points of contact, offering tailored support without providing medical advice or working under the direction of the prescribing healthcare providers. PELs are dedicated to helping patients and care partners by:
| ● | Providing information about the patient’s condition and what to expect while on treatment |
| ● | Connecting them with external organizations |
| ● | Offering resources tailored for the needs of both patients and care partners |
This optional program ensures that patients feel supported and informed, creating a compassionate framework that prioritizes patient empowerment while respecting the role of healthcare providers in treatment decisions.
The effectiveness of our work is tracked via regular meetings with our MyNavCare team members and through scheduled updates with the other cross functional teams leadership meetings. The Patient Services Steering Committee, made of senior U.S. Market leaders, meets every quarter to review the patient services program operational metrics and effectiveness.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 137/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Reporting a Side Effect or a Quality Concern
We take patient safety seriously. Reports of side effects and quality concerns enable us to ensure the safety of our medicines and the patients who take them. Genmab has a public number that patients may call. We state on all our resources the following:
You are encouraged to report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch or to Genmab US, Inc. at 1-855-4GENMAB (1-855-443-6622).
The company systematically monitors and evaluates adverse events and other safety-related information associated with its therapies post-approval. This ongoing surveillance helps identify and respond to potential safety concerns promptly.
Speak Up Hotline
If patients or caregivers have concerns, they can raise them through the Speak Up Hotline. Our 24/7 full-service Speak Up (whistleblower) compliance hotline enables the anonymous reporting of illegal, unethical, and/or non-compliant behavior and related concerns in connection with our organization. Our Compliance team regularly reviews these matters, and supports investigations as warranted, reporting to both management and the Audit & Finance Committee.
Refer to the Global Speak Up Policy and Hotline on our website, and S1-3 for further details.
Taking action on material impacts on consumers and end-users, and approaches to managing material risks and pursuing material opportunities related to consumers and end-users, and effectiveness of those actions (S4-4)
Genmab has safety measures in place for products, patients, and healthcare providers. Genmab believes that patient safety plays a critical role in our business operations. Genmab has a Comprehensive Safety Program that is designed to identify and mitigate potential risks associated with our products, and to ensure that our products are safe and effective for their intended use. We work closely with regulatory agencies to ensure that our products meet all safety and efficacy standards.
Genmab provided annual training programs on pharmacovigilance for global drug safety and pharmacovigilance (GDS&PV) staff, including regular updates and assessments to ensure continuous learning and compliance with the latest regulations. Also, Genmab developed and distributed educational materials on safety requirements to stakeholders and organized workshops to enhance their understanding and implementation of safety protocols. These actions are linked to the personal safety and information of consumers and end-users.
The following actions were taken around Genmab’s material impacts on our consumers and end-users for 2024:
Access and integrity in clinical trials
We have a Patient Advisory Council and a Study Coordinator Advisory Council who provide input to our clinical trials. In addition, our clinical trials are reviewed by institutional review boards, ethics committees, regulatory authorities, and data and safety monitoring boards.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 138/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
As of December 31, 2024, there was a total of 25 ongoing Genmab-sponsored clinical trials, all registered on ClinicalTrials.gov. Our clinical trials are taking place in 37 countries worldwide with 620 patients enrolled during the year.
Genmab complies with all applicable industry regulations, guidelines, and standards globally for drug development, such as cGLP, cGCP, cGMPs and good animal practice as defined by the Federation of European Laboratory Animal Science Associations (FELASA). We also monitor and comply with all relevant legislation and regulations, including guidelines issued by international regulatory authorities such as the European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA), the Pharmaceuticals and Medical Devices Agency (PMDA) and others. It is important to acknowledge our relationship with Japan PMDA, as it reflects our global strength. Our operations were periodically audited by relevant authorities.
Clinical trials generate the data necessary to evaluate the safety and efficacy of drugs, providing insight on how to use a therapy and which patients are most likely to benefit from treatment. Even with the advancements in understanding the incidence of different cancers between genders and racial or ethnic groups, inequities persist, resulting in underrepresentation in clinical trials.
Genmab's DE&I in Clinical Trials Project Team is responsible for defining and implementing a framework to deliver Genmab’s intentions to provide clinical trial treatment options to patients in a wider community and generate clinical trial data from currently underrepresented populations. The Project Team works to ensure all Genmab registration studies have a clear strategy and process for Diversity Plan success.
Genmab has been working to implement diversity in our pivotal clinical trials. In 2024, Genmab introduced a Diversity Action Plan (DAP) SOP and DAP template to be implemented for all pivotal trials. The inaugural Diversity Action Plan was submitted to the U.S. FDA in 2024. With the U.S. FDA final guidance expected in 2025 we will ensure compliance with the agency’s new rules and implement diversity in all applicable pivotal studies where appropriate.
Genmab systematically monitors and evaluates adverse events and other safety-related information associated with its therapies post-approval. This ongoing surveillance helps identify and respond to potential safety concerns promptly. Our cross functional team regularly reviews matters, and supports investigations as warranted. We have a mix of internal and external committees that regularly monitors clinical trial data to track and assess the effectiveness of these actions mentioned above.
Market Access Programs to Allow for Product Availability for Uninsured or Underinsured
All Market Access colleagues undergo comprehensive annual compliance training to ensure adherence to regulatory standards and ethical practices. Here is a list of some of the key compliance training modules they complete:
| ● | Data Privacy |
| ● | U.S. FDA guidelines |
| ● | The PhRMA code and Ad Promotion rules |
| ● | Compliance issues including Anti-bribery and Speak Up modules (including whistleblower training) |
| ● | Pharmacovigilance |
| ● | Sunshine Act (and various other transparency laws) |
| ● | Conflict of interest |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 139/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The MyNavCare team also trains annually on specific compliance related PSS polices and business rules.
These training modules ensure that colleagues in the Market Access group remain informed about the latest regulatory requirements and ethical standards, thereby safeguarding the Company's reputation and ensuring the continued availability of their products to patients. The tracking of the completion rates are monitored by the Global Compliance team.
Patient Engagement Programs Developed
Genmab is elevating the voices of patients and care partners by incorporating their perspectives into all aspects of our work from early-stage R&D to clinical trials and commercialization. These insights are vital to our ability to innovate and to support patients as they navigate the complex aspects of a serious illness. The effectiveness of these patient engagement programs are tracked via regular meetings with our patient advisory council and through scheduled updates with other internal teams. Our patient advocacy team facilitated multiple touchpoints with key stakeholders throughout the year to provide opportunities for mutual learning and insight sharing:
| ● | Our Patient Advisory Council, formed in 2023, which consisted of 16 members as of December 31, 2024 compared to 13 members as of December 31, 2023, met nine times in 2024 and four times in 2023. In 2024, topics discussed included feedback on patient materials, such as brand messaging and caregiver resources, the global and U.S. websites, and review of clinical trial materials, including informed consent forms, protocol, and lay summary. In 2023, topics discussed were around clinical trial recruitment and diversity in clinical trials, providing patient perspective on the cancer experience and how Genmab can better serve patients and care partners. Members of the Council represent people with a variety of tumor types, ages, geographies, and socioeconomic backgrounds. |
| ● | Our third annual Science Day in 2024 brought together 29 leaders from 24 organizations and six patient advisors for 1.5 days of scientific discussion and knowledge exchange. Our second annual Science Day in 2023 brought together 32 representatives from patient advocacy and professional groups, our Patient Advisory Council and Genmab team members to share information about our respective work to inform how we can best address the needs of patients and their care partners. Topics included AI, and the cancer journey for patients and care partners. We understand the need for broad stakeholder education to help break down barriers and reach underrepresented populations, along with the need to support the patient and care partner to address the psychosocial impacts of living with cancer, such as feelings of depression, anxiety, and fear. The actionable insights gained from Science Day are key to shaping our future patient engagement and education efforts. |
MyNavCare
We are bringing life-changing medicines and support services to patients through MyNavCare Patient Support. Since the 2023 launch of EPKINLY in the U.S, through 2024, we have supported patients directly through MyNavCare Patient Support, our robust patient support program, and continuously worked with stakeholders across the healthcare system to ensure rapid and sustainable access to appropriate patients.
In the U.S., MyNavCare supports each patient’s unique needs alongside the needs of care partners. We support:
| ● | Patients and care partners through case management, insurance navigation and financial assistance, among other services |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 140/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| ● | HCPs through navigating access, including reimbursement education, and billing and coverage information, among other resources with the overarching goal of supporting patients needs |
We also provide access support for eligible patients who are uninsured or underinsured through our Patient Assistance Program, which minimizes the burden of applying for assistance and quickly determines eligibility.
We aim to take great care and consideration to help ensure rapid and sustainable access for all appropriate patients who may benefit from our therapies as we look to bring our own medicines to additional markets in the future. We remain focused in our pursuit to turn innovative science into medicine that creates value and delivers meaningful impact to patients, their care partners and the HCPs who serve them.
Under the leadership of our Vice President, U.S. Market Access, our market access function is responsible for oversight of MyNavCare.
The effectiveness of our work is tracked via regular meetings with our MyNavCare team members and through scheduled updates with the other cross functional teams leadership meetings.
Clinical Trial Transparency
To ensure access to quality information for patients, consumers, and end-users, Genmab has taken the following action in 2024:
| ● | Offered trial descriptions in the ClinicalTrials.gov registration that are clear and understandable for non-scientists, benefiting potential trial participants. |
Tracking and assessment is done by reviewing requests received from consumers and end-users via Genmab’s publicly available mailbox, ClinicalTrial@genmab.com. The received requests are triaged to ensure they are addressed by the appropriate Genmab function.
Continuous Improvement
Genmab is committed to continuous improvement in patient engagement and safety monitoring. The Company regularly evaluates feedback from advocacy groups and utilizes social media to assess the effectiveness of its communications. By fostering a culture of open dialogue and ethical conduct, Genmab not only meets regulatory requirements but also builds trust with the patients and healthcare providers it serves.
Genmab is actively working to reduce the clinical trial burden for patients. Engaging our Patient Advisory Council to review protocols and integrating patient feedback into our trials, and developing health-literate materials are some of the ways in which we help to increase access to our clinical trials.
Our Clinical Trial Transparency Declaration acknowledges our strong commitment to the scientific and ethical aspects of increasing the transparency of clinical trial research. In alignment with the Declaration, we disclosed information and results from our clinical trials through publicly accessible study registries/databases such as ClinicalTrials.gov, and the European Union Clinical Trials Information System (EU CTIS), to ensure compliance with global and national laws in the evolving area of transparency.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 141/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
4.2 Consumers and End-Users Metrics and Targets
Targets related to managing material negative impacts, advancing positive impacts, and managing material risks and opportunities (S4-5)
As there are no identified negative impacts in this area, no targets were set, which is aligned with our strategy/priority for 2025.
Refer to Our Strategy in Management’s Review for details.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 142/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Sustainability Statements: Governance
Genmab’s oversight of sustainability is designed to ensure that our commitments are integrated as a core part of our business and aligned with international best practice. We are dedicated to complying with all laws, codes, and standards applicable to our business and operations, as well as ensuring transparency in our sustainability disclosures.
Business Conduct
Below are the list of disclosure requirements as it pertains to ESRS G1 – Business Conduct:
Section |
Disclosure requirement content |
Disclosure requirement # |
5.0 IROs |
Material Impacts, Risks and Opportunities and their interaction with strategy and business model |
SBM-3 |
5.1 Business Conduct IRO Management |
Business conduct policies and corporate culture |
G1-1 |
|
Management of relationships with suppliers |
G1-2 |
|
Prevention and detection of corruption and bribery |
G1-3 |
5.2 Business Conduct Metrics and Targets |
Incidents of corruption or bribery |
G1-4 |
|
Political influence and lobbying activities |
G1-5* |
|
Payment practices |
G1-6 |
* Disclosure requirement G1-5 is not material for Genmab.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 143/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
5.0 IROs
Material Impacts, Risks and Opportunities and their interaction with strategy and business model (SBM-3)
The table describes Genmab’s material impacts, risks and opportunities related to our material business conduct matters:
Section |
Material Topic |
Material Impact |
Value Chain Location |
Material Risk |
Material Opportunity |
Governance |
Business Conduct |
Healthy corporate culture aligned with core values and purpose (positive) |
Own Operations |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance |
Annual Code of Conduct training rollout as mandatory for all employees, with launch and completion rates monitored by Global Compliance team |
|
Business Conduct |
Cybersecurity and Global Data Privacy programs in place to protect the privacy of our business, our own workforce, patients and all individuals who entrust us with their information (positive) |
Upstream, Own Operations, Downstream |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance and Cybersecurity |
|
|
Business Conduct |
Protection of whistleblowers through anti-retaliation policies and procedures (positive) |
Upstream, Own Operations, Downstream |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance |
|
|
Business Conduct |
Animal welfare policy (positive) |
Own Operations |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance |
|
|
Business Conduct |
Strong management of suppliers, focused on compliance with supplier code of conduct (positive) |
Upstream |
Refer to the Risk Management section of the Annual Report for risks related to Strategic Collaborations |
Continue to partner with suppliers on sustainability related commitments in the future |
|
Business Conduct |
Ethical business culture and business practices (positive) |
Own Operations |
Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance, Strategic Collaborations and Management and Workforce |
Annual Code of Conduct training rollout as mandatory for all employees, with launch and completion rates monitored by Global Compliance team |
No actions were taken in 2024 across the business conduct section as no negative impacts were identified. Further, there were no instances of corruption and bribery (see G1-4) or late payments resulting in fines, penalties or litigation (see G1-6).
Other than the metrics disclosed in G1-4 and G1-6, no other metrics were determined to be relevant for reporting in 2024. One target across the business conduct section was identified related to suppliers (see G1-2) and is in collaboration with our environmental strategy / priorities for 2025 and the future. No other targets were identified due to the fact that there were no negative impacts identified.
All impacts, risks and opportunities have expected time horizons of short, medium and long-term.
Healthy corporate culture aligned with core values and purpose
In our quest to turn science into medicine, we use these guideposts to transform the future of cancer treatment:
| ● | Passion for innovation |
| ● | Determination - being the best at what we do |
| ● | Integrity - we do the right thing |
| ● | We work as one team and respect each other |
Without maintaining a healthy corporate culture, Genmab is exposed to compliance and other risks. Genmab has an opportunity to provide annual training to employees on key business conduct matters. Annual Code of Conduct training is mandatory for all employees, with launch and completion rates monitored by the Global
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 144/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Compliance team. The Code of Conduct training should decrease behaviors that go against our Code of Conduct that could lead to compliance or other issues.
Cybersecurity programs in place to protect the privacy of our business, our own workforce and patients
We focus on privacy and protection of personal data at Genmab, covering several data categories, such as the data of patients, employees, business partners, HCPs, and other stakeholders. Genmab acknowledges the risk related to cybersecurity breaches that could occur within our value chain. We have taken solid measures to protect personal data in compliance with the EU General Data Protection Regulation (GDPR) and other applicable personal data protection legislation and requirements. All our team members are educated in the GDPR.
The Global Information Security and Risk Management team reports to the Board on a quarterly basis. No security incidents with critical or material business impact have been reported in 2024.
Accounting policies
Genmab has a security incident management process and a separate security incident system which security incidents are processed. Incidents are entered through a number of channels including Genmab’s security monitoring partner, by employees through Genmab’s internal systems and by Genmab’s security operations team. Security incidents are analyzed and rated according to different priority categories ranging from priority 1 (critical) to priority 4 (low). We perform an analysis of the number of incidents with business impact utilizing the financial statement materiality threshold. Incidences with material impact are reported to Genmab’s Board on a quarterly basis.
Protection of whistleblowers through anti-retaliation policies and procedures
Genmab’s 24/7 full-service Speak Up (whistleblower) compliance hotline enables the anonymous reporting of illegal, unethical, and/or non-compliant behavior and related concerns in connection with our organization. Our Compliance team regularly reviews these matters which pose legal and regulatory risks to Genmab, and supports investigations as warranted, reporting to both management and the Audit & Finance Committee.
Animal welfare policy
Genmab understands the legal and regulatory risks associated with working with animals in our business, and our animal welfare policy represents Genmab’s commitment to sound practices that aim to replace, reduce, and refine the use of animals in Genmab’s research and development.
Strong management of suppliers, ensuring compliance with Genmab’s Global Supplier Code of Conduct
Supplier relationship management is a key business initiative that aims to build mutually beneficial relationships between the company and suppliers. Well-designed programs help companies to increase collaboration by identifying the right suppliers. Genmab understands the risk of being dependent on existing partnerships, and has mitigation efforts in place to address these risks.
Genmab has a real opportunity to partner with suppliers on Sustainability related commitments around the environment and our Supplier Code of Conduct which could lead to cost savings and stronger relationships in the future.
Refer to E1-4 for environmental targets linked to supplier engagement.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 145/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Ethical business culture and business practices
We are committed to operating all aspects of our business with the utmost integrity. We have an established global compliance program and incorporate compliance, ethics and transparency considerations into our business practices, policies, and procedures. We hold ourselves accountable to high ethical standards, promoting our Code of Conduct to employees and engaging with partners and suppliers committed to the same level of ethics in their operations. We are committed to conducting all aspects of our business in an ethical, honest, and transparent manner, and understand there are various regulatory, legislative and compliance risks.
All employees and contractors are required to complete annual training and attest to their commitment to adhere to our ethical standards. The Code of Conduct training provides an overview of our Ethical Standards, Company Values, and incorporates training vignettes that illustrate ethical approaches to common business practices. This training also reviews relevant anti-bribery and anti-corruption, regulatory, conflicts of interest, and Speak Up concepts. Compliance team members receive regular compliance training on key aspects of our compliance policies and procedures.
Refer to GOV-1 for details of the role and expertise of the administrative, management and supervisory bodies related to business conduct matters.
5.1 Business conduct IRO management
Business conduct policies and corporate culture (G1-1)
Our Code of Conduct and 20 ethical standards embody Genmab’s commitment to doing the right thing and ensure that the ways in which we work reflect the highest standards of integrity and compliance with applicable laws and regulations. We continue to mature our compliance, risk, and data privacy program foundations as we grow and evolve to further strengthen our culture of integrity, business continuity and corporate resilience. These steps help us assure a risk-based approach to our business, giving us the confidence to make the right decisions, drive value for patients and unite behind shared Company goals.
The leader of our Global Compliance and Enterprise Risk Management programs reports directly to the CEO and the Board.
Genmab’s policies on business conduct related matters linked to material IROs disclosed in section G1-1 include the following:
| ● | Code of Conduct |
| ● | Global Compliance Policy |
| ● | Global Speak Up Policy |
| ● | Anti-Fraud Policy |
| ● | Data Ethics Policy |
| ● | Cybersecurity Program |
| ● | Animal Welfare Policy |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 146/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Code of Conduct
Genmab’s Code of Conduct sets high ethical standards for all employees and the Board when conducting business on behalf of Genmab. The Code of Conduct encourages team members to conduct themselves in a manner reflecting our core values, determination, integrity, innovation, and teamwork when representing the Company. All employees are required to complete annual training and attest to their commitment to adhere to our ethical standards. The Code of Conduct training provides an overview of our Ethical Standards, Company Values, and incorporates training vignettes that illustrate ethical approaches to common business practices. This training also reviews relevant anti-bribery and anti-corruption, regulatory, conflicts of interest, and Speak Up concepts. Our head of Global Compliance is responsible for the Code of Conduct and reports directly to our CEO, and both are members of the CSR & Sustainability Committee. The Global Compliance team monitors completion of the annual Code of Conduct training and provides progress updates to function leaders and the Global Compliance and Risk Committee. The Code of Conduct can be mapped to the positive impact of a healthy corporate culture aligned with core values and purpose. Refer to the Code of Conduct on our website.
Global Compliance Policy
Our internal Global Compliance Policy, owned by our head of Global Compliance, outlines our standards on interactions and engagements with HCPs, healthcare organizations, patients, patient association groups and government officials consistent with applicable industry codes and standards. The policy aligns with the values and principles articulated in our Code of Conduct and is complemented by an associated Global Fair Market Value Policy and a Compliance Playbook tool to ensure stakeholder engagement is conducted in an ethical, compliant manner. As a result of the commercialization our first co-owned medicines including TIVDAK (2021) and EPKINLY (2023), we have expanded our compliance program to assure ethical market-based and customer-focused business practices. Genmab maintains a Global Compliance Program staffed by compliance professionals who monitor adherence to the policy.
Global Speak Up Policy
Genmab maintains a Speak Up (whistleblower) program featuring an independently operated hotline service available globally intended to provide anyone with information about potential misconduct related to Genmab or its business activities the opportunity to report the misconduct. Genmab's Speak Up program is intended to accommodate information from any group with information including all Genmab's current and former employees, directors, contractors, customers, suppliers, and other third parties wishing to report concerns.
All reports made through the Genmab Speak Up program are assessed and considered by the Genmab Global Compliance team in a prompt, fair, and compliant manner. Genmab has established procedures to protect whistleblowers and ensure they do not suffer retaliation for their report. On a quarterly basis, Genmab’s Audit and Finance Committee receives a summary of all reports made under the Speak Up program along with additional information about any material incidents raised. Summaries of all reports made to the Speak Up program are provided to Genmab’s Global Compliance and Risk Committee (GCRC) annually.
All Genmab employees and contractors are required to take Speak Up training, and completion metrics are monitored by the Compliance team. Speak Up program training is a mandatory component of employee onboarding and also an annual requirement.
Genmab has zero tolerance for any retaliation against anyone who raises concerns or participates in investigations. Retaliation includes any conduct or treatment that could discourage someone from speaking up. Genmab will protect the identity of people who participate in the Speak Up program as appropriate and
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 147/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
consistent with applicable law. Genmab utilizes a number of methods (determined by the scenario) to protect whistleblowers from retaliation or detriment including but not limited to discrimination, harassment, physical or psychological harm, isolation, impact to employee performance and/or compensation, damaging property, or varying employee's role or duties.
The Global Speak Up Policy can be mapped to the positive impact of protection of whistleblowers through anti-retaliation policies and procedures.
Refer to the Global Speak Up Policy on our website, and S1-3 for further details. Refer to G1-3 for Genmab’s procedures to investigate business conduct matters.
Anti-Fraud Policy
Genmab has an internal Anti-Fraud Policy that communicates anti-fraud principles and program elements, and management’s responsibility for detecting and responding to fraud and misconduct. This Policy applies to all employees, officers, directors of Genmab, and management regardless of legal entity or work location, and anyone supervising the performance of services for or on behalf of Genmab, including contractors, contingent workers, agents, suppliers, and consultants (collectively “Genmab Person”). Genmab’s Corporate Controller is responsible for the anti-fraud policy and reports to the CFO. Genmab monitors through Internal Audit assessing the potential for fraud risk when planning individual audits. On an annual basis, a fraud risk assessment is prepared with the participation of management. This annual fraud risk assessment is presented to the Audit & Finance Committee.
Data Ethics Policy
The use of data, both personal and non-personal, is essential to fulfilling our core purpose, and we are committed to handling data with integrity and in an ethical and compliant manner. Genmab has developed a global data privacy program supported by a cross-functional team of global data privacy subject matter experts and appointed an external Data Privacy Officer (DPO) dedicated to GDPR compliance and oversight.
Our Data Ethics Policy complies with Section 99d of the Danish Financial Statements Act, and we adopted the Data Ethics principles of the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA). As part of this commitment to ethical and responsible use of data and overall transparency, Genmab has made this Data Ethics Policy publicly available to all external stakeholders. Refer to the Data Ethics Policy on our website.
The policy and its principles are anchored in our Code of Conduct as part of our overall Compliance program, and has been communicated to our management so they can share and consider it with team members. Over the past year, we focused on further embedding these principles into our operations, particularly in the areas of data privacy, DE&I, clinical trials, and the application of new technologies such as AI and machine learning. This goal was supported by efforts to evolve our data privacy governance model into a forward-looking and comprehensive global data privacy program. Genmab, in 2025, will continue to optimize and enhance its approach to responsible and ethical use of data in its operations through continued transformation of and investment in its global data privacy program. There were no substantiated complaints concerning breaches of data privacy from individuals or data protection authorities in 2024 or 2023.
Cybersecurity Program
We maintain a comprehensive cybersecurity program based on the National Institute of Standards and Technology’s NIST 800 Special Publication Information Security standard (“NIST standard”) for managing
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 148/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
cybersecurity activities, including formulation of global objectives of the cybersecurity program and risk identification and mitigation activities.
Our Global Cybersecurity Program is under the leadership of the vice president and global head of cybersecurity and IT risk management who reports to the senior vice president, global head of IT & digital. The program is overseen by the Global Compliance and Risk Committee (co-chaired by our CEO and our global head of compliance and risk) and the Audit and Finance Committee.
The program includes activities and projects in all five functions of the NIST standard with the goal of further improving our security profile and adapting, where needed, to changes in the business strategy and threat environment of Genmab. Input for the program comes from the annual attack and penetration test, periodic threat landscape and security maturity assessments, as well as requirements of applicable cybersecurity regulations.
We have established a Cyber Response Task Force responsible for responding to potential cyber crisis situations that could have an impact on our Company, partners, or patients we serve. The Task Force provides assurance that our incident response and recovery capability is effective in increasing our ability to prevent, detect and respond to potential cyberattacks.
We regard the protection of the Company against cybersecurity attacks as a task for everyone in the organization. Therefore, employees are provided with information about cybersecurity risks and how to detect and report security incidents in online trainings and quarterly security events. The Cybersecurity Program can be mapped to the positive impact of cybersecurity and global data privacy programs in place to protect the privacy of our business, our own workforce, patients and all individuals who entrust us with their information.
Animal Welfare Policy
At Genmab, we use research animals for the purpose of addressing important scientific questions or to fulfill a regulatory requirement. Animals have intrinsic value and experiments on animals are only carried out when no appropriate alternative method is available.
Genmab has established an internal animal welfare policy and animal welfare management procedures for its locations in the U.S., Europe, Japan, and China that incorporate local laws and regulations and that reflect our high standards. We plan to harmonize these ways of working between our sites in 2025.
Our policies reflect our responsible and humane use of animals in research. We actively continue to drive the implementation of the 3Rs principles (Replacement, Reduction and Refinement) in all that we do. A dedicated animal welfare officer monitors animal welfare policies and practices, ensuring we continuously refine the care and use of the animals involved in our research.
In 2023, we implemented the use of low-stress handling techniques for mice, the use of optimized nesting material and started an ongoing effort to use minimally invasive techniques for the identification (marking) of rodents to improve the welfare of animals in our daily care.
In 2024, we launched a Global Animal Welfare Committee who owns the policies, to explore 3R opportunities, to advise on animal welfare matters and to work towards enhancing our transparency regarding the use of animals in research. In addition, we started to develop internal policies describing all the elements of working with animals.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 149/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Genmab provides continuing education to team members working with animals on ethical treatment of animals.
Genmab performs animal welfare audits at external contractors, when required, to ensure contractors maintain comparable high standards for animal use and care as we do internally. All animal studies performed, whether internally or externally, are Genmab’s moral and ethical responsibility and we strive to apply our high standards in all that we do.
Refer to G1-2 for details of the Global Procurement Policy and Global Supplier Code of Conduct.
Refer to G1-3 for details of the Anti-Corruption Anti-Bribery Policy which includes disclosure of functions within Genmab that are most at risk in respect of corruption and bribery.
Other Genmab policies disclosed on our website or internal Genmab policies include the Enterprise Risk Management and Resilience Policy, International Trade Controls Policy, and Tax Policy.
Management of relationships with suppliers (G1-2)
Genmab’s Global Procurement functions which include, R&D Contract Management, Lab Purchase Management, CMC Commercial Supply Chain and Global Procurement (collectively known as “Procurement”) are created to be a trusted value adding partner to both internal and external stakeholders across the entire Genmab value chain.
Global Procurement Policy
Genmab’s Global Procurement Policy is an important tool utilized by our team members to steer the procurement practices and increase financial cost control and transparency within Genmab. Furthermore, the Policy helps assess and mitigate risk, ensure selection and maintenance of high quality, regulatory compliant third parties, through a transparent, fair, and compliant process. Genmab’s CFO is responsible for the policy, which is available on Genmab’s intranet.
The Global Procurement Policy states that suppliers have to be on-boarded prior to engaging in a commercial relationship to ensure timely payment. The use of purchase orders, which is also mandated by the Procurement Policy, furthermore, facilitates approval of invoiced spend, ensuring that the agreed upon payment terms are being met. Suppliers that self-identify to Genmab as small and medium enterprises (SMEs), have 30 days net as a standard payment term unless contractually agreed otherwise in writing, which is clearly stated in the Global Procurement Policy.
The procurement process is set up to facilitate on-time payments, starting with proper on-boarding of the suppliers prior to engaging in a commercial relationship, followed by a mandatory use of Purchase Requisitions and Purchase Orders (with an exception list), ensuring that, when an invoice is received it will be paid in a timely manner.
Supplier Code of Conduct
Our Supplier Code of Conduct articulates expectations for all third parties conducting work on our behalf, minimizing risks to Genmab posed by our suppliers’ activities. The Supplier Code of Conduct addresses topics that include, but are not limited to, anti-bribery and anti-corruption, privacy, trade compliance, conflicts of interest, human and labor rights, diversity, compliance with environmental laws and regulations, supply chain and animal welfare, protecting information and intellectual property, protecting physical and digital
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 150/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
security and product compliance and quality. Our VP, Head of Global Procurement who reports to our CFO is responsible for the policy. In 2024 and 2023, all new Genmab suppliers have been required to attest to our Supplier Code of Conduct annually as part of the onboarding and contracting process.
Our Global Procurement function implemented a dedicated supplier vetting tool which serves as a single point of entry for all new suppliers. Information technology & digital, quality assurance, compliance and risk, and legal functions are involved when the risk score is elevated based on a fact-based approach. Our vetting process focuses on financial health, international sanctions, regulatory and reputational risks, and other key issues. Suppliers in sanctioned countries are subject to additional legal review before payments may be processed.
In 2023, we established a supplier diversity program in the U.S., working closely with entities such as the Veterans Administration to align on targets. Genmab defines a diverse supplier as a >51% women, minority, or veteran-owned small business. The Supplier Master Registry in SAP has been updated in 2024 with a specific field to allow Genmab to monitor supplier diversity for the U.S.
We have created our first-ever Company-wide supplier governance best practices guide to improve how we work together across lines of business, and how we partner with suppliers so all teams can successfully execute against their goals. In January 2024, contract managers, sourcing managers, procurement leads and alliance managers have been trained on how and when to apply these best practices in support of our lines of businesses’ priorities and goals. A Vendor Risk Manager is planned to be on-boarded in 2025 to ensure proper documentation and tracking of the initiative.
All the Genmab suppliers are vetted against unethical business practices, including adverse media, U.S. and EU Sanctions lists and the Global Corruption Index as well as a review of the financial health of the third party.
The Global Procurement Policy and Supplier Code of Conduct can be mapped to the positive impact of our strong management of suppliers, focused on compliance with supplier code of conduct, and targets set are the responsibility of our global procurement team.
Targets for supplier management:
| ● | Acceptance of Genmab’s Supplier Code of Conduct by 80% of suppliers by spend by 2025 |
The target specifically supports important governance topics covered in the Supplier Code of Conduct including areas like legal compliance, anti-corruption and bribery, labor practices, human rights, supply chain, animal welfare, protection of information and intellectual property / security. Genmab will monitor, review and report metrics related to this target in the next annual report.
Refer to E1-4 for details of Genmab’s engagement plans with suppliers on Scope 3 emission reductions targets.
Prevention and detection of corruption and bribery (G1-3)
Anti-Bribery and Anti-Corruption (ABAC) Policy
As a global company, we implement policies to mitigate risks related to bribery and corruption. Our Anti-Bribery and Anti-Corruption (ABAC) Policy educates team members on recognizing risks, emphasizes our
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 151/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
zero-tolerance stance, and outlines reporting mechanisms for suspected misconduct. All employees receive annual ABAC training, and we are enhancing due diligence and monitoring activities for better oversight. In 2023, we updated our Quarterly Financial Disclosure Questionnaire to improve management disclosures regarding potential bribery and corruption.
Management identifies the primary bribery and corruption risks as payments or gifts intended to secure preferential treatment for the company. Employees interacting with public officials or regulatory representatives face heightened risks, as do those dealing with healthcare providers and third parties on Genmab's behalf, especially due to limited oversight of third-party anti-corruption practices.
Reports of bribery or corruption are reviewed by the Global Compliance Program, which reports directly to the CEO. Annual summaries are provided to the Global Compliance and Risk Committee and the Board, detailing all reported incidents.
We consider all functions within the business to be potentially subject to corruption and bribery and as such, 100% of Genmab employees, contractors, and Board members must complete annual Code of Conduct training, which covers ABAC, regulatory issues, conflicts of interest, and reporting mechanisms. New hires also receive ABAC training. Our Compliance program maintains a SharePoint site for business conduct policies and uses the company intranet to communicate key concepts.
Every two years, business functions assess their vulnerability to corruption and report findings to the Compliance organization, which may implement further controls. Our Internal Audit function also conducts an annual fraud assessment. The Anti-Bribery and Anti-Corruption Policy can be mapped to the positive impact of our ethical business culture and business practices.
5.2 Business conduct metrics and targets
Incidents of corruption or bribery (G1-4)
Genmab defines bribery as acts designed to influence individuals to act dishonestly in the performance or discharge of their duty, and corruption as the misuse of office or power or influence for private gain. Genmab has a zero-tolerance policy for any acts of bribery or corruption by employees, contingent staff, management, officers, directors, or third-party agents or representatives. Genmab’s 24/7 Speak Up Compliance Hotline enables the anonymous reporting of behavior indicative of corruption or bribery. Our compliance team reviews these reports and supports investigations as warranted. A review of all hotline reported incidents indicates no submissions related to a Genmab employee, contingent staff, management, officer, director, or third-party agent or representative performing an act of bribery or corruption in 2024 or 2023, thus no incidents of corruption or bribery.
Payment practices (G1-6)
Standard payment terms are 45 days net.
For certain categories of suppliers there are other possible payment terms, for example 30 days net allowed for Small and Medium Enterprises (SMEs) and 7 days net for Grants/Sponsorships and Government organizations.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 152/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Genmab defines SMEs as enterprises which employ fewer than 10 persons or which have an annual turnover not exceeding EUR/USD 10 million.
There were no legal proceedings for late payments in 2024, including payments to SMEs.
Currently, Genmab does not have a process to track actual payment terms by main category of suppliers. However, the average time Genmab takes to pay an invoice for all suppliers with varying payment terms from the date when the contractual or statutory term of payment starts to be calculated, in number of days was 46 in 2024.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 153/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Sustainability Statements: Appendix A
Appendix A (Derived from ESRS 2 Appendix B)
Disclosure Requirement and related datapoint |
SFDR Reference |
Pillar 3 Reference |
Benchmark Regulation Reference |
EU Climate Law Reference |
Material/ Not Material |
Section, Paragraph or Page Reference |
ESRS 2 GOV-1 Board's gender diversity paragraph 21 (d) |
Indicator number 13 of Table #1 of Annex 1 |
|
Commission Delegated Regulation (EU) 2020/1816, Annex II |
|
Material |
GOV-1 Section |
ESRS 2 GOV-1 Percentage of board members who are independent paragraph 21 (e) |
|
|
Delegated Regulation (EU) 2020/1816, Annex II |
|
Material |
GOV-1 Section |
ESRS 2 GOV-4 Statement on due diligence paragraph 30 |
Indicator number 10 Table #3 of Annex 1 |
|
|
|
Material |
GOV-4 Section |
ESRS 2 SBM-1 Involvement in activities related to fossil fuel activities paragraph 40 (d) i |
Indicators number 4 Table #1 of Annex 1 |
Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 Table 1: Qualitative information on Environmental risk and Table 2: Qualitative information on Social risk |
Delegated Regulation (EU) 2020/1816, Annex II |
|
Not Material |
|
ESRS 2 SBM-1 Involvement in activities related to chemical production paragraph 40 (d) ii |
Indicator number 9 Table #2 of Annex 1 |
|
Delegated Regulation (EU) 2020/1816, Annex II |
|
Not Material |
|
ESRS 2 SBM-1 Involvement in activities related to controversial weapons paragraph 40 (d) iii |
Indicator number 14 Table #1 of Annex 1 |
|
Delegated Regulation (EU) 2020/1818, Article 12(1) Delegated Regulation (EU) 2020/1816, Annex II |
|
Not Material |
|
ESRS 2 SBM-1 Involvement in activities related to cultivation and production of tobacco paragraph 40 (d) iv |
|
|
Delegated Regulation (EU) 2020/1818, Article 12(1) Delegated Regulation (EU) 2020/1816, Annex II |
|
Not Material |
|
ESRS E1-1 Transition plan to reach climate neutrality by 2050 paragraph 14 |
|
|
|
Regulation (EU) 2021/1119, Article 2(1) |
Material |
ESRS E1-1 Section |
ESRS E1-1 Undertakings excluded from Paris-aligned Benchmarks paragraph 16 (g) |
|
Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 Template 1: Banking book- Climate Change transition risk: Credit quality of exposures by sector, emissions and residual maturity |
Delegated Regulation (EU) 2020/1818, Article12.1 (d) to (g), and Article 12.2 |
|
Not Material |
|
ESRS E1-4 GHG emission reduction targets paragraph 34 |
Indicator number 4 Table #2 of Annex 1 |
Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 Template 3: Banking book – Climate change transition risk: alignment metrics |
Delegated Regulation (EU) 2020/1818, Article 6 |
|
Material |
ESRS E1-4 Section |
ESRS E1-5 Energy consumption from fossil sources disaggregated by sources (only high climate impact sectors) paragraph 38 |
Indicator number 5 Table #1 and Indicator n. 5 Table #2 of Annex 1 |
|
|
|
Not Material |
|
ESRS E1-5 Energy consumption and mix paragraph 37 |
Indicator number 5 Table #1 of Annex 1 |
|
|
|
Material |
ESRS E1-5 Section |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 154/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
ESRS E1-5 Energy intensity associated with activities in high climate impact sectors paragraphs 40 to 43 |
Indicator number 6 Table #1 of Annex 1 |
|
|
|
Not Material |
|
ESRS E1-6 Gross Scope 1, 2, 3 and Total GHG emissions paragraph 44 |
Indicators number 1 and 2 Table #1 of Annex 1 |
Article 449a; Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 Template 1: Banking book – Climate change transition risk: Credit quality of exposures by sector, emissions and residual maturity |
Delegated Regulation (EU) 2020/1818, Article 5(1), 6 and 8(1) |
|
Material |
ESRS E1-6 Section |
ESRS E1-6 Gross GHG emissions intensity paragraphs 53 to 55 |
Indicators number 3 Table #1 of Annex 1 |
Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 Template 3: Banking book – Climate change transition risk: alignment metrics |
Delegated Regulation (EU) 2020/1818, Article 8(1) |
|
Material |
ESRS E1-6 Section |
ESRS E1-7 GHG removals and carbon credits paragraph 56 |
|
|
|
Regulation (EU) 2021/1119, Article 2(1) |
Not Material |
|
ESRS E1-9 Exposure of the benchmark portfolio to climate-related physical risks paragraph 66 |
|
|
Delegated Regulation (EU) 2020/1818, Annex II Delegated Regulation (EU) 2020/1816, Annex II |
|
Not Material |
|
ESRS E1-9 Disaggregation of monetary amounts by acute and chronic physical risk paragraph 66 (a) ESRS E1-9 Location of significant assets at material physical risk paragraph 66 (c). |
|
Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 paragraphs 46 and 47; Template 5: Banking book - Climate change physical risk: Exposures subject to physical risk. |
|
|
Not Material |
|
ESRS E1-9 Breakdown of the carrying value of its real estate assets by energy-efficiency classes paragraph 67 (c). |
|
Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 paragraph 34;Template 2:Banking book -Climate change transition risk: Loans collateralised by immovable property - Energy efficiency of the collateral |
|
|
Not Material |
|
ESRS E1-9 Degree of exposure of the portfolio to climate- related opportunities paragraph 69 |
|
|
Delegated Regulation (EU) 2020/1818, Annex II |
|
Not Material |
|
ESRS E2-4 Amount of each pollutant listed in Annex II of the E- PRTR Regulation (European Pollutant Release and Transfer Register) emitted to air, water and soil, paragraph 28 |
Indicator number 8 Table #1 of Annex 1 Indicator number 2 Table #2 of Annex 1 Indicator number 1 Table #2 of Annex 1 Indicator number 3 Table #2 of Annex 1 |
|
|
|
Not Material |
|
ESRS E3-1 Water and marine resources paragraph 9 |
Indicator number 7 Table #2 of Annex 1 |
|
|
|
Not Material |
|
ESRS E3-1 Dedicated policy paragraph 13 |
Indicator number 8 Table 2 of Annex 1 |
|
|
|
Not Material |
|
ESRS E3-1 Sustainable oceans and seas paragraph 14 |
Indicator number 12 Table #2 of Annex 1 |
|
|
|
Not Material |
|
ESRS E3-4 Total water recycled and reused paragraph 28 (c) |
Indicator number 6.2 Table #2 of Annex 1 |
|
|
|
Not Material |
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 155/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
ESRS E3-4 Total water consumption in m3 per net revenue on own operations paragraph 29 |
Indicator number 6.1 Table #2 of Annex 1 |
|
|
|
Not Material |
|
ESRS 2 - SBM 3 - E4 paragraph 16 (a) i |
Indicator number 7 Table #1 of Annex 1 |
|
|
|
Not Material |
|
ESRS 2 - SBM 3 - E4 paragraph 16 (b) |
Indicator number 10 Table #2 of Annex 1 |
|
|
|
Not Material |
|
ESRS 2 - SBM 3 - E4 paragraph 16 (c) |
Indicator number 14 Table #2 of Annex 1 |
|
|
|
Not Material |
|
ESRS E4-2 Sustainable land / agriculture practices or policies paragraph 24 (b) |
Indicator number 11 Table #2 of Annex 1 |
|
|
|
Not Material |
|
ESRS E4-2 Sustainable oceans / seas practices or policies paragraph 24 (c) |
Indicator number 12 Table #2 of Annex 1 |
|
|
|
Not Material |
|
ESRS E4-2 Policies to address deforestation paragraph 24 (d) |
Indicator number 15 Table #2 of Annex 1 |
|
|
|
Not Material |
|
ESRS E5-5 Non-recycled waste paragraph 37 (d) |
Indicator number 13 Table #2 of Annex 1 |
|
|
|
Not Material |
|
ESRS E5-5 Hazardous waste and radioactive waste paragraph 39 |
Indicator number 9 Table #1 of Annex 1 |
|
|
|
Not Material |
|
ESRS 2- SBM3 - S1 Risk of incidents of forced labour paragraph 14 (f) |
Indicator number 13 Table #3 of Annex I |
|
|
|
Not Material |
|
ESRS 2- SBM3 - S1 Risk of incidents of child labour paragraph 14 (g) |
Indicator number 12 Table #3 of Annex I |
|
|
|
Not Material |
|
ESRS S1-1 Human rights policy commitments paragraph 20 |
Indicator number 9 Table #3 and Indicator number 11 Table #1 of Annex I |
|
|
|
Material |
ESRS S1-1 Section |
ESRS S1-1 Due diligence policies on issues addressed by the fundamental International Labor Organisation Conventions 1 to 8, paragraph 21 |
|
|
Delegated Regulation (EU) 2020/1816, Annex II |
|
Material |
ESRS S1-1 Section |
ESRS S1-1 processes and measures for preventing trafficking in human beings paragraph 22 |
Indicator number 11 Table #3 of Annex I |
|
|
|
Material |
ESRS S1-1 Section |
ESRS S1-1 workplace accident prevention policy or management system paragraph 23 |
Indicator number 1 Table #3 of Annex I |
|
|
|
Material |
ESRS S1-1 Section |
ESRS S1-3 grievance/complaints handling mechanisms paragraph 32 (c) |
Indicator number 5 Table #3 of Annex I |
|
|
|
Material |
ESRS S1-3 Section |
ESRS S1-14 Number of fatalities and number and rate of work-related accidents paragraph 88 (b) and (c) |
Indicator number 2 Table #3 of Annex I |
|
Delegated Regulation (EU) 2020/1816, Annex II |
|
Material |
ESRS S1-14 Section |
ESRS S1-14 Number of days lost to injuries, accidents, fatalities or illness paragraph 88 (e) |
Indicator number 3 Table #3 of Annex I |
|
|
|
Material |
ESRS S1-14 Section |
ESRS S1-16 Unadjusted gender pay gap paragraph 97 (a) |
Indicator number 12 Table #1 of Annex I |
|
Delegated Regulation (EU) 2020/1816, Annex II |
|
Material |
ESRS S1-16 Section |
ESRS S1-16 Excessive CEO pay ratio paragraph 97 (b) |
Indicator number 8 Table #3 of Annex I |
|
|
|
Material |
ESRS S1-16 Section |
ESRS S1-17 Incidents of discrimination paragraph 103 (a) |
Indicator number 7 Table #3 of Annex I |
|
|
|
Material |
ESRS S1-17 Section |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 156/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
ESRS S1-17 Non-respect of UNGPs on Business and Human Rights and OECD Guidelines paragraph 104 (a) |
Indicator number 10 Table #1 and Indicator n. 14 Table #3 of Annex I |
|
Delegated Regulation (EU) 2020/1816, Annex II Delegated Regulation (EU) 2020/1818 Art 12 (1) |
|
Not Material |
|
ESRS 2- SBM3 – S2 Significant risk of child labour or forced labour in the value chain paragraph 11 (b) |
Indicators number 12 and n. 13 Table #3 of Annex I |
|
|
|
Not Material |
|
ESRS S2-1 Human rights policy commitments paragraph 17 |
Indicator number 9 Table #3 and Indicator n. 11 Table #1 of Annex 1 |
|
|
|
Not Material |
|
ESRS S2-1 Policies related to value chain workers paragraph 18 |
Indicator number 11 and n. 4 Table #3 of Annex 1 |
|
|
|
Not Material |
|
ESRS S2-1 Non- respect of UNGPs on Business and Human Rights principles and OECD guidelines paragraph 19 |
Indicator number 10 Table #1 of Annex 1 |
|
Delegated Regulation (EU) 2020/1816, Annex II Delegated Regulation (EU) 2020/1818, Art 12 (1) |
|
Not Material |
|
ESRS S2-1 Due diligence policies on issues addressed by the fundamental International Labor Organisation Conventions 1 to 8, paragraph 19 |
|
|
Delegated Regulation (EU) 2020/1816, Annex II |
|
Not Material |
|
ESRS S2-4 Human rights issues and incidents connected to its upstream and downstream value chain paragraph 36 |
Indicator number 14 Table #3 of Annex 1 |
|
|
|
Not Material |
|
ESRS S3-1 Human rights policy commitments paragraph 16 |
Indicator number 9 Table #3 of Annex 1 and Indicator number 11 Table #1 of Annex 1 |
|
|
|
Not Material |
|
ESRS S3-1 non-respect of UNGPs on Business and Human Rights, ILO principles or OECD guidelines paragraph 17 |
Indicator number 10 Table #1 Annex 1 |
|
Delegated Regulation (EU) 2020/1816, Annex II Delegated Regulation (EU) 2020/1818, Art 12 (1) |
|
Not Material |
|
ESRS S3-4 Human rights issues and incidents paragraph 36 |
Indicator number 14 Table #3 of Annex 1 |
|
|
|
Not Material |
|
ESRS S4-1 Policies related to consumers and end-users paragraph 16 |
Indicator number 9 Table #3 and Indicator number 11 Table #1 of Annex 1 |
|
|
|
Material |
ESRS S4-1 Section |
ESRS S4-1 Non-respect of UNGPs on Business and Human Rights and OECD guidelines paragraph 17 |
Indicator number 10 Table #1 of Annex 1 |
|
Delegated Regulation (EU) 2020/1816, Annex II Delegated Regulation (EU) 2020/1818, Art 12 (1) |
|
Not Material |
|
ESRS S4-4 Human rights issues and incidents paragraph 35 |
Indicator number 14 Table #3 of Annex 1 |
|
|
|
Not Material |
|
ESRS G1-1 United Nations Convention against Corruption paragraph 10 (b) |
Indicator number 15 Table #3 of Annex 1 |
|
|
|
Not Material |
|
ESRS G1-1 Protection of whistle- blowers paragraph 10 (d) |
Indicator number 6 Table #3 of Annex 1 |
|
|
|
Material |
ESRS G1-1 Section |
ESRS G1-4 Fines for violation of anti-corruption and anti-bribery laws paragraph 24 (a) |
Indicator number 17 Table #3 of Annex 1 |
|
Delegated Regulation (EU) 2020/1816, Annex II) |
|
Material |
ESRS G1-4 Section |
ESRS G1-4 Standards of anti- corruption and anti- bribery paragraph 24 (b) |
Indicator number 16 Table #3 of Annex 1 |
|
|
|
Not Material |
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 157/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Financial Statements for the Genmab Group
Introduction
The financial statements in the 2024 Annual Report are grouped into the following sections: Primary Statements; Basis of Presentation; Results for the Year; Operating Assets and Liabilities; Capital Structure, Financial Risk and Related Items; and Other Disclosures.
Each note to the financial statements includes information about the accounting policies applied and significant management judgements and estimates in addition to the financial numbers.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 158/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Table of Contents
160 |
|
160 |
|
161 |
|
162 |
|
163 |
|
|
|
164 |
|
164 |
|
168 |
|
169 |
|
171 |
|
|
|
172 |
|
172 |
|
174 |
|
176 |
|
177 |
|
179 |
|
|
|
181 |
|
181 |
|
184 |
|
186 |
|
187 |
|
188 |
|
189 |
|
190 |
|
191 |
|
|
|
SECTION 4 – CAPITAL STRUCTURE, FINANCIAL RISK AND RELATED ITEMS |
192 |
192 |
|
192 |
|
196 |
|
199 |
|
200 |
|
202 |
|
207 |
|
|
|
211 |
|
5.1 Remuneration of the Board of Directors and Executive Management |
211 |
212 |
|
213 |
|
5.4 Fees to Auditors Appointed at the Annual General Meeting |
213 |
213 |
|
218 |
|
222 |
|
222 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 159/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Primary Statements
Consolidated Statements of Comprehensive Income
INCOME STATEMENT
(DKK million) |
Note |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
2.1, 2.2 |
|
21,526 |
|
16,474 |
|
14,505 |
|
|
|
|
|
|
|
|
Cost of product sales |
2.3 |
|
(985) |
|
(226) |
|
- |
Research and development expenses |
2.3, 3.1, 3.2 |
|
(9,748) |
|
(7,630) |
|
(5,562) |
Selling, general and administrative expenses |
2.3, 3.2 |
|
(3,790) |
|
(3,297) |
|
(2,676) |
Acquisition and integration related charges |
5.5 |
|
(300) |
|
- |
|
- |
Total costs and operating expenses |
|
|
(14,823) |
|
(11,153) |
|
(8,238) |
|
|
|
|
|
|
|
|
Operating profit |
|
|
6,703 |
|
5,321 |
|
6,267 |
|
|
|
|
|
|
|
|
Financial income |
4.5 |
|
4,438 |
|
1,940 |
|
3,189 |
Financial expenses |
4.5 |
|
(1,977) |
|
(1,624) |
|
(2,511) |
|
|
|
|
|
|
|
|
Net profit before tax |
|
|
9,164 |
|
5,637 |
|
6,945 |
|
|
|
|
|
|
|
|
Corporate tax |
2.4 |
|
(1,320) |
|
(1,285) |
|
(1,493) |
|
|
|
|
|
|
|
|
Net profit |
|
|
7,844 |
|
4,352 |
|
5,452 |
|
|
|
|
|
|
|
|
Other comprehensive income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amounts which may be re-classified to the income statement: |
|
|
|
|
|
|
|
Exchange differences on translation of foreign operations |
|
|
430 |
|
(38) |
|
17 |
|
|
|
|
|
|
|
|
Total comprehensive income |
|
|
8,274 |
|
4,314 |
|
5,469 |
|
|
|
|
|
|
|
|
Basic net profit per share |
2.5 |
|
122.21 |
|
66.64 |
|
83.38 |
Diluted net profit per share |
2.5 |
|
121.36 |
|
66.02 |
|
82.59 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 160/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Consolidated Balance Sheets
|
|
|
December 31, |
||
(DKK million) |
Note |
|
2024 |
|
2023 |
|
|
|
|
|
|
ASSETS |
|
|
|
|
|
|
|
|
|
|
|
Goodwill |
3.1, 5.5 |
|
2,535 |
|
- |
Other intangible assets |
3.1, 5.5 |
|
12,343 |
|
101 |
Property and equipment |
2.2, 3.2 |
|
978 |
|
955 |
Right-of-use assets |
2.2, 3.3 |
|
913 |
|
686 |
Receivables |
2.2, 3.6 |
|
52 |
|
62 |
Deferred tax assets |
2.4 |
|
908 |
|
212 |
Other investments |
3.4 |
|
228 |
|
134 |
|
|
|
|
|
|
Total non-current assets |
|
|
17,957 |
|
2,150 |
|
|
|
|
|
|
|
|
|
|
|
|
Corporate tax receivable |
2.4 |
|
101 |
|
- |
Inventories |
3.5 |
|
62 |
|
57 |
Receivables |
3.6 |
|
6,590 |
|
4,947 |
Marketable securities |
4.2, 4.4 |
|
11,243 |
|
13,268 |
Cash and cash equivalents |
|
|
9,858 |
|
14,867 |
|
|
|
|
|
|
Total current assets |
|
|
27,854 |
|
33,139 |
|
|
|
|
|
|
Total assets |
|
|
45,811 |
|
35,289 |
|
|
|
|
|
|
SHAREHOLDERS' EQUITY AND LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
Share capital |
4.7 |
|
66 |
|
66 |
Share premium |
4.7 |
|
12,590 |
|
12,461 |
Other reserves |
|
|
490 |
|
60 |
Retained earnings |
|
|
23,551 |
|
19,023 |
|
|
|
|
|
|
Total shareholders' equity |
|
|
36,697 |
|
31,610 |
|
|
|
|
|
|
Lease liabilities |
3.3 |
|
937 |
|
680 |
Contract liabilities |
3.7 |
|
480 |
|
480 |
Deferred tax liabilities |
2.4 |
|
2,359 |
|
- |
Other payables |
3.8 |
|
30 |
|
35 |
|
|
|
|
|
|
Total non-current liabilities |
|
|
3,806 |
|
1,195 |
|
|
|
|
|
|
Corporate tax payable |
2.4 |
|
1,710 |
|
54 |
Lease liabilities |
3.3 |
|
92 |
|
90 |
Contract liabilities |
3.7 |
|
24 |
|
33 |
Other payables |
3.8 |
|
3,482 |
|
2,307 |
|
|
|
|
|
|
Total current liabilities |
|
|
5,308 |
|
2,484 |
|
|
|
|
|
|
Total liabilities |
|
|
9,114 |
|
3,679 |
|
|
|
|
|
|
Total shareholders' equity and liabilities |
|
|
45,811 |
|
35,289 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 161/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Consolidated Statements of Cash Flows
(DKK million) |
Note |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net profit before tax |
|
|
9,164 |
|
5,637 |
|
6,945 |
|
|
|
|
|
|
|
|
Financial income |
4.5 |
|
(4,438) |
|
(1,940) |
|
(3,189) |
Financial expenses |
4.5 |
|
1,977 |
|
1,624 |
|
2,511 |
Adjustment for non-cash transactions |
|
|
|
|
|
|
|
Share-based compensation expense |
2.3, 4.6 |
|
721 |
|
586 |
|
439 |
Depreciation |
3.2, 3.3 |
|
335 |
|
272 |
|
222 |
Amortization |
3.1 |
|
78 |
|
23 |
|
140 |
Impairment charges |
3.1 |
|
115 |
|
- |
|
- |
Change in operating assets and liabilities |
|
|
|
|
|
|
|
Receivables |
3.6 |
|
(1,590) |
|
797 |
|
(2,123) |
Inventories |
3.5 |
|
(5) |
|
(57) |
|
- |
Other payables |
3.8 |
|
857 |
|
622 |
|
283 |
|
|
|
|
|
|
|
|
Cash flows from operating activities before financial items |
|
|
7,214 |
|
7,564 |
|
5,228 |
|
|
|
|
|
|
|
|
Interest received |
|
|
935 |
|
908 |
|
283 |
Interest elements of lease payments |
3.3 |
|
(35) |
|
(24) |
|
(15) |
Interest paid |
|
|
- |
|
(1) |
|
(1) |
Corporate taxes paid |
|
|
(343) |
|
(1,067) |
|
(1,583) |
|
|
|
|
|
|
|
|
Net cash provided by operating activities |
|
|
7,771 |
|
7,380 |
|
3,912 |
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
Acquisition of business, net of cash acquired |
5.5 |
|
(12,246) |
|
- |
|
- |
Investment in intangible assets |
3.1 |
|
(117) |
|
(10) |
|
- |
Investment in tangible assets |
3.2 |
|
(187) |
|
(366) |
|
(317) |
Marketable securities bought |
4.3, 4.4 |
|
(8,581) |
|
(10,876) |
|
(9,659) |
Marketable securities sold |
4.3, 4.4 |
|
11,279 |
|
10,001 |
|
7,254 |
Other investments bought |
3.4 |
|
(55) |
|
(31) |
|
(39) |
|
|
|
|
|
|
|
|
Net cash (used in) investing activities |
|
|
(9,907) |
|
(1,282) |
|
(2,761) |
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
Warrants exercised |
4.6, 4.7 |
|
129 |
|
152 |
|
280 |
Principal elements of lease payments |
3.3 |
|
(60) |
|
(91) |
|
(73) |
Purchase of treasury shares |
4.7 |
|
(3,879) |
|
(564) |
|
(908) |
Payment of withholding taxes on behalf of employees on net settled RSUs |
|
|
(109) |
|
(103) |
|
(88) |
|
|
|
|
|
|
|
|
Net cash (used in) financing activities |
|
|
(3,919) |
|
(606) |
|
(789) |
|
|
|
|
|
|
|
|
Changes in cash and cash equivalents |
|
|
(6,055) |
|
5,492 |
|
362 |
Cash and cash equivalents at the beginning of the period |
|
|
14,867 |
|
9,893 |
|
8,957 |
Exchange rate adjustments |
|
|
1,046 |
|
(518) |
|
574 |
|
|
|
|
|
|
|
|
Cash and cash equivalents at the end of the period |
|
|
9,858 |
|
14,867 |
|
9,893 |
|
|
|
|
|
|
|
|
Cash and cash equivalents include: |
|
|
|
|
|
|
|
Bank deposits |
|
|
9,776 |
|
13,514 |
|
9,299 |
Short-term marketable securities |
|
|
82 |
|
1,353 |
|
594 |
|
|
|
|
|
|
|
|
Cash and cash equivalents at the end of the period |
|
|
9,858 |
|
14,867 |
|
9,893 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 162/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Consolidated Statements of Changes in Equity
(DKK million) |
|
Share capital |
|
Share premium |
|
Translation reserves |
|
Retained earnings |
|
Shareholders' equity |
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2021 |
|
66 |
|
12,029 |
|
81 |
|
9,931 |
|
22,107 |
|
|
|
|
|
|
|
|
|
|
|
Net profit |
|
- |
|
- |
|
- |
|
5,452 |
|
5,452 |
Other comprehensive income |
|
- |
|
- |
|
17 |
|
- |
|
17 |
Total comprehensive income |
|
- |
|
- |
|
17 |
|
5,452 |
|
5,469 |
|
|
|
|
|
|
|
|
|
|
|
Transactions with owners: |
|
|
|
|
|
|
|
|
|
|
Exercise of warrants |
|
- |
|
280 |
|
- |
|
- |
|
280 |
Purchase of treasury shares |
|
- |
|
- |
|
- |
|
(908) |
|
(908) |
Share-based compensation expenses |
|
- |
|
- |
|
- |
|
439 |
|
439 |
Withholding taxes on behalf of employees on net settled RSUs |
|
- |
|
- |
|
- |
|
(88) |
|
(88) |
Tax on items recognized directly in equity |
|
- |
|
- |
|
- |
|
(17) |
|
(17) |
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2022 |
|
66 |
|
12,309 |
|
98 |
|
14,809 |
|
27,282 |
|
|
|
|
|
|
|
|
|
|
|
Net profit |
|
- |
|
- |
|
- |
|
4,352 |
|
4,352 |
Other comprehensive income |
|
- |
|
- |
|
(38) |
|
- |
|
(38) |
Total comprehensive income |
|
- |
|
- |
|
(38) |
|
4,352 |
|
4,314 |
|
|
|
|
|
|
|
|
|
|
|
Transactions with owners: |
|
|
|
|
|
|
|
|
|
|
Exercise of warrants |
|
- |
|
152 |
|
- |
|
- |
|
152 |
Purchase of treasury shares |
|
- |
|
- |
|
- |
|
(564) |
|
(564) |
Share-based compensation expenses |
|
- |
|
- |
|
- |
|
586 |
|
586 |
Withholding taxes on behalf of employees on net settled RSUs |
|
- |
|
- |
|
- |
|
(103) |
|
(103) |
Tax on items recognized directly in equity |
|
- |
|
- |
|
- |
|
(57) |
|
(57) |
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2023 |
|
66 |
|
12,461 |
|
60 |
|
19,023 |
|
31,610 |
|
|
|
|
|
|
|
|
|
|
|
Net profit |
|
- |
|
- |
|
- |
|
7,844 |
|
7,844 |
Other comprehensive income |
|
- |
|
- |
|
430 |
|
- |
|
430 |
Total comprehensive income |
|
- |
|
- |
|
430 |
|
7,844 |
|
8,274 |
|
|
|
|
|
|
|
|
|
|
|
Transactions with owners: |
|
|
|
|
|
|
|
|
|
|
Exercise of warrants |
|
- |
|
129 |
|
- |
|
- |
|
129 |
Purchase of treasury shares |
|
- |
|
- |
|
- |
|
(3,879) |
|
(3,879) |
Share-based compensation expenses |
|
- |
|
- |
|
- |
|
721 |
|
721 |
Withholding taxes on behalf of employees on net settled RSUs |
|
- |
|
- |
|
- |
|
(109) |
|
(109) |
Tax on items recognized directly in equity |
|
- |
|
- |
|
- |
|
(49) |
|
(49) |
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2024 |
|
66 |
|
12,590 |
|
490 |
|
23,551 |
|
36,697 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 163/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Section 1 – Basis of Presentation
These consolidated financial statements include Genmab A/S (parent company) and subsidiaries over which the parent company has control. The Genmab consolidated Group is referenced herein as “Genmab” or the “Company.”
This section describes Genmab’s general accounting policies including management’s judgements and estimates under IFRS Accounting Standards as issued by the International Accounting Standards Board (IASB) and endorsed by the EU (IFRS Accounting Standards). The specific accounting policies are described in each note in conjunction with supplementary disclosures of the specific item with the aim to provide a more understandable description of each accounting area.
1.1 – Nature of the Business and Material Accounting Policies
Genmab A/S is a publicly traded, international biotechnology company that was founded in 1999 and specializes in the creation and development of differentiated antibody therapeutics for the treatment of cancer and other diseases. Genmab has six approved products commercialized by third parties, two approved products that are jointly commercialized with a collaboration partner, a broad clinical and preclinical product pipeline and proprietary next-generation antibody technologies.
The consolidated financial statements have been prepared in accordance with IFRS Accounting Standards as issued by the International Accounting Standards Board (IASB) and in accordance with IFRS as endorsed by the EU and further disclosure requirements for listed companies in Denmark. The consolidated financial statements were approved by the Board of Directors and authorized for issue on February 12, 2025. Except as outlined in Note 1.2, the consolidated financial statements have been prepared using the same accounting policies as 2023.
Please refer to the overview below to see in which note/section the detailed accounting policy is included.
Section 2 – Results for the Year |
3.4 Other Investments |
2.1 Revenue |
3.5 Inventories |
2.2 Information about Geographical Areas |
3.6 Receivables |
2.3 Staff Costs |
3.8 Other Payables |
2.4 Corporate and Deferred Tax |
Section 4 – Capital Structure, Financial Risk and Related Items |
2.5 Profit per Share |
4.3 Financial Assets and Liabilities |
Section 3 – Operating Assets and Liabilities |
4.4 Marketable Securities |
3.1 Intangible Assets and Goodwill |
4.5 Financial Income and Expenses |
3.2 Property and Equipment |
4.6 Share-Based Instruments |
3.3 Leases |
Section 5 – Other Disclosures |
|
5.5 Acquisition of Businesses |
|
|
Materiality
Genmab’s Annual Report is based on the concept of materiality and the Company focuses on information that is considered material and relevant to the users of the consolidated financial statements. The consolidated financial statements consist of a large number of transactions. These transactions are aggregated into classes according to their nature or function and presented in classes of similar items in the consolidated
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 164/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
financial statements as required by IFRS and the Danish Financial Statements Act. If items are individually immaterial, they are aggregated with other items of similar nature in the consolidated financial statements or in the notes.
Genmab provides these specific required disclosures unless the information is considered immaterial to the economic decision-making of the readers of the consolidated financial statements or not applicable.
Consolidated Financial Statements
The consolidated financial statements include Genmab A/S and subsidiaries over which the parent company has control. The parent controls a subsidiary when the parent is exposed to, or has rights to, variable returns from its involvement with the subsidiary and has the ability to affect those returns through its power to direct the activities of the subsidiary. Genmab A/S (parent company) holds investments either directly or indirectly in the following subsidiaries:
Name |
Domicile |
Ownership and votes 2024 |
Ownership and votes 2023 |
Genmab B.V. |
Utrecht, the Netherlands |
100% |
100% |
Genmab Holding B.V. |
Utrecht, the Netherlands |
100% |
100% |
Genmab US, Inc. |
New Jersey, USA |
100% |
100% |
Genmab K.K. |
Tokyo, Japan |
100% |
100% |
ProfoundBio, Inc. |
Delaware, USA |
100% |
N/A* |
ProfoundBio, US Co |
Delaware, USA |
100% |
N/A* |
Profound Limited |
Hong Kong, China |
100% |
N/A* |
ProfoundBio co., Ltd. |
Suzhou, China |
100% |
N/A* |
ProfoundBio Shanghai Branch, Co., Ltd. |
Shanghai, China |
100% |
N/A* |
Beijing Puyifang Biotechnology Co., Ltd. |
Beijing, China |
100% |
N/A* |
*These subsidiaries were added as a result of the acquisition of ProfoundBio during the second quarter of 2024.
Genmab’s consolidated financial statements have been prepared on the basis of the financial statements of the parent company and subsidiaries – prepared under Genmab’s accounting policies – by combining similar accounting items on a line-by-line basis. On consolidation, intercompany income and expenses, intercompany receivables and payables, and unrealized gains and losses on transactions between the consolidated companies are eliminated.
The recorded value of the equity interests in the consolidated subsidiaries is eliminated with the proportionate share of the subsidiaries’ equity. Subsidiaries are consolidated from the date when control is transferred to the Group.
Items included in the financial statements of Genmab's entities are measured using the currency of the primary economic environment in which the entity operates (functional currency).The income statements for
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 165/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
subsidiaries with a different functional currency than Genmab’s presentation currency are translated into Genmab’s presentation currency at average exchange rates, and the balance sheets are translated at the exchange rate in effect at the balance sheet date.
Exchange rate differences arising from the translation of foreign subsidiaries shareholders’ equity at the beginning of the year and exchange rate differences arising as a result of foreign subsidiaries’ income statements being translated at average exchange rates are recorded in translation reserves in shareholders’ equity.
Functional and Presentation Currency
The consolidated financial statements have been prepared in Danish Kroner (DKK), which is the functional and presentation currency of the parent company.
Foreign Currency
Transactions in foreign currencies are translated at the exchange rates in effect at the date of the transaction.
Exchange rate gains and losses arising between the transaction date and the settlement date are recognized in the Consolidated Statements of Comprehensive Income as financial income or expense.
Unsettled monetary assets and liabilities in foreign currencies are translated at the exchange rates in effect at the balance sheet date. Exchange rate gains and losses arising between the transaction date and the balance sheet date are recognized in the Consolidated Statements of Comprehensive Income as financial income or expense.
Classification of Costs and Operating Expenses in the Income Statement
Cost of Product Sales
Cost of product sales includes direct and indirect costs relating to the manufacturing of inventory mainly from third-party providers of manufacturing as well as costs related to internal resources and distribution and logistics. Inventory amounts written down as a result of excess or obsolescence are charged to cost of product sales. Also included in Cost of Product Sales are royalty payments on commercialized products. Aside from these items, there are no other costs included within cost of product sales.
Additionally, cost of product sales includes profit-sharing amounts owed to collaboration partners for the sale of commercial products when Genmab is determined to be the principal in sales to end customers. The only profit-sharing amounts owed to collaboration partners that are recorded as cost of product sales relate to sales of EPKINLY in the U.S. and Japan pursuant to the Collaboration Agreement with AbbVie.
Refer to Note 5.6 in the Annual Report for detailed information regarding Genmab’s Collaboration Agreement with AbbVie.
Research and Development Expenses
Research and development expenses primarily include salaries, benefits and other employee-related costs of Genmab’s research and development staff, license costs, manufacturing costs, preclinical costs, clinical trials, contractors and outside service fees, amortization and impairment of licenses and rights related to intangible assets, depreciation of property and equipment, and depreciation of right-of-use assets, to the extent that such costs are related to the Group’s research and development activities.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 166/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Refer to Note 3.1 for a more detailed description on the treatment of Genmab’s research and development expenses.
Selling, General and Administrative Expenses
Selling, general and administrative expenses relate to the management and administration of Genmab, including commercialization activities. This primarily includes salaries, benefits and other employee costs related to management and support functions including human resources, information technology and the finance departments. In addition, depreciation of property and equipment and depreciation of right-of-use assets, to the extent such expenses are related to administrative functions, are also included. Selling, general and administrative expenses are recognized in the Consolidated Statements of Comprehensive Income in the period to which they relate.
Acquisition and Integration Related Charges
Acquisition and integration related charges for the acquisition of ProfoundBio which occurred during the second quarter of 2024.
Refer to Note 5.5 for more information regarding Genmab’s Acquisition and Integration costs related to the acquisition of ProfoundBio.
Government Grants
Government grants are recognized at their fair value where there is reasonable assurance that the grant will be received and that Genmab will comply with all attaching conditions. When the grant relates to an expense item, it is recognized as a reduction of that expense on a systematic basis over the periods that the costs for which it is intended to compensate are incurred. Where the grant relates to an asset, the fair value is credited to a contract liability account and is released to the statement of comprehensive income as other operating income over the expected useful life of the relevant asset by equal annual installments.
Statements of Cash Flows
The cash flow statement is presented using the indirect method with basis in the net profit before tax.
Cash flows from operating activities are stated as the net profit before tax adjusted for financial income and expense, non-cash operating items including depreciation, amortization, impairment losses, share-based compensation expenses, and for changes in operating assets and liabilities, interest paid and received, interest elements of lease payments and corporate taxes paid or received. Operating assets and liabilities are mainly comprised of changes in receivables, inventories and other payables excluding the items included in cash and cash equivalents. Changes in non-current assets and liabilities are included in operating assets and liabilities, if related to the main revenue-producing activities of Genmab.
Cash flows from investing activities consist of acquisitions of businesses, net of cash acquired, purchases and sales of marketable securities and other investments, as well as purchases of intangible assets and property and equipment.
Cash flows from financing activities relate to the purchase of treasury shares, exercise of warrants, payments of withholding taxes on behalf of employees on net settled RSUs and payments of long-term loans including installments on lease liabilities.
Cash and cash equivalents are comprised of cash, bank deposits, and marketable securities with a maturity of less than 90 days on the date of acquisition.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 167/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The statements of cash flows cannot be derived solely from the consolidated financial statements.
Treasury Shares
The total amount paid to acquire treasury shares including directly attributable costs and the proceeds from the sale of treasury shares is recognized in retained earnings.
Collaborations, License Agreements and Collaborative Agreements
Collaborations and License Agreements
Genmab continues to pursue the establishment of research collaborations and licensing agreements. These arrangements often include upfront payments, expense reimbursements or payments to the collaboration partner, and milestone and royalty arrangements, contingent upon the occurrence of certain future events linked to the success of the asset in development.
In regard to Genmab’s license agreements with J&J, Novartis and Roche, each of these parties retain final decision-making authority over the relevant activities and as such no joint control exists.
Refer to Note 2.1 for additional information related to revenue from these parties.
Collaborative Agreements
Genmab has entered into a number of joint collaborative agreements. These agreements often include upfront payments, expense reimbursements or payments to the collaboration partner, and milestone and royalty arrangements, contingent upon the occurrence of certain future events linked to the success of the asset in development.
These agreements also provide Genmab with varying rights to develop, produce and market products together with its collaborative partners. Both parties in these arrangements share in the decision-making and therefore have joint control of the arrangement. In 2024, Genmab’s more significant collaboration agreements are with AbbVie (epcoritamab), Pfizer (tisotumab vedotin) and BioNTech.
Refer to Note 2.1 for additional information related to revenue from our joint collaborative agreements.
Refer to Note 5.6 for detailed information regarding Genmab’s significant Research Collaborations, License Agreements and Collaborative Agreements.
1.2 New Accounting Policies and Disclosures
NEW ACCOUNTING POLICIES AND DISCLOSURES FOR 2024
Genmab has, with effect from January 1, 2024, implemented the following standards and amendments:
| ● | Amendments to IFRS 16 Leases: Lease Liability in a Sale and Leaseback |
| ● | Amendments to IAS 1 Presentation of Financial Statements: Classification of Liabilities as Current or Non-current, Classification of Liabilities as Current or Non-current – Deferral of Effective Date, and Non-current Liabilities with Covenants, and |
| ● | Amendments to IAS 7 Statement of Cash Flows and IFRS 7 Financial Instruments: Disclosures: Supplier Finance Arrangements |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 168/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The implementation of these amendments did not have a material impact on the consolidated financial statements for the current or prior reporting periods and is not expected to have a significant impact in future reporting periods.
NEW ACCOUNTING POLICIES AND DISCLOSURES EFFECTIVE IN 2025 OR LATER
FURTHERMORE, AS IT RELATES TO NEW OR AMENDED ACCOUNTING STANDARDS AND INTERPRETATIONS (IFRSS) ISSUED BY THE IASB, MANAGEMENT DOES NOT ANTICIPATE ANY SIGNIFICANT IMPACT ON THE CONSOLIDATED FINANCIAL STATEMENTS IN THE PERIOD OF INITIAL APPLICATION FROM THE ADOPTION OF THESE NEW STANDARDS AND AMENDMENTS, APART FROM IFRS 18 ‘PRESENTATION AND DISCLOSURE IN FINANCIAL STATEMENTS’ WHICH REPLACES IAS 1 EFFECTIVE FROM 1 JANUARY 2027. THE NEW IFRS 18 IS EXPECTED TO CHANGE THE PRESENTATION OF THE FINANCIAL STATEMENTS, REQUIRING ITEMS OF INCOME AND EXPENSE TO BE CLASSIFIED INTO FIVE CATEGORIES: OPERATING, INVESTING, FINANCE, INCOME TAXES AND DISCONTINUED OPERATIONS ALONG WITH TWO NEW MANDATORY SUB-TOTALS, OPERATING PROFIT OR LOSS AND PROFIT OR LOSS BEFORE FINANCING AND INCOME TAXES. IFRS 18 WILL NOT IMPACT THE RECOGNITION OR MEASUREMENT OF ITEMS IN THE FINANCIAL STATEMENTS.
1.3 Management’s Judgements and Estimates under IFRS
In preparing financial statements under IFRS, certain provisions in the standards require management’s judgements, including various accounting estimates and assumptions. These judgements and estimates affect the application of accounting policies, as well as reported amounts within the consolidated financial statements and disclosures.
Determining the carrying amount of certain assets and liabilities requires judgements, estimates and assumptions concerning future events that are based on historical experience and other factors, which by their very nature are associated with uncertainty and unpredictability.
Accounting estimates are based on historical experience and various other factors relative to the circumstances in which they are applied. Estimates are generally made based on information available at the time.
Accounting judgements are made in the process of applying accounting policies. These judgements are typically made based on the guidance and information available at the time of application.
These estimates and judgements may prove incomplete or incorrect, and unexpected events or circumstances may arise. Genmab is also subject to risks and uncertainties which may lead actual results to differ from these estimates, both positively and negatively. Specific risks for Genmab are discussed in the relevant section of this Annual Report and in the notes to the consolidated financial statements.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 169/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The areas involving a high degree of judgement and estimation that are significant to the consolidated financial statements are summarized below. Refer to the identified notes for further information on the key accounting estimates and judgements utilized in the preparation of the consolidated financial statements.
Accounting policy |
Key accounting estimates and judgements |
Note reference |
Risk |
Revenue recognition |
Judgement in assessing whether a collaboration partner is a customer Estimation of partner net sales amounts in the calculation of royalties Estimation of variable consideration Judgement in assessing the nature of combined performance obligations within contracts |
Note 2.1 |
High |
Share-based compensation |
Judgement in selecting assumptions required for valuation of warrant grants Estimation in developing forfeiture rate RSUs/warrants and probability of achievement for PSUs |
Note 4.6 |
Moderate |
Current and deferred income taxes |
Judgement and estimation regarding valuation of deferred income taxes |
Note 2.4 |
Moderate |
Fair value and impairment assessment of other intangible assets and goodwill |
Estimation of the fair value of other intangible assets and assessment of impairment of other intangible assets Estimation regarding the valuation of goodwill and assessment of impairment of goodwill |
Notes 3.1 and 5.5 |
High |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 170/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
1.4 Revision of Prior Period Financial Statements
To facilitate comparison of information across periods, certain reclassifications and revisions have been made to prior period financial income and expense amounts to conform to the current period’s appropriate presentation.
Refer to Note 4.5 for additional information relating to financial income and expenses of the Group and Note 14 in the parent financial statements relating to financial income and expenses of the Parent.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 171/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Section 2 Results for the Year
This section includes disclosures related to revenue, information about geographical areas, staff costs, corporate and deferred tax, and profit per share.
2.1 – Revenue
(DKK million) |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
|
Revenue by type: |
|
|
|
|
|
|
|
Royalties |
|
|
17,352 |
|
13,705 |
|
11,582 |
Reimbursement revenue |
|
|
996 |
|
864 |
|
818 |
Milestone revenue |
|
|
1,000 |
|
1,177 |
|
1,767 |
Collaboration revenue |
|
|
433 |
|
307 |
|
332 |
Net product sales |
|
|
1,743 |
|
421 |
|
- |
License revenue |
|
|
2 |
|
- |
|
6 |
|
|
|
|
|
|
|
|
Total |
|
|
21,526 |
|
16,474 |
|
14,505 |
|
|
|
|
|
|
|
|
Revenue by collaboration partner: |
|
|
|
|
|
|
|
Janssen |
|
|
14,422 |
|
11,949 |
|
10,530 |
AbbVie |
|
|
394 |
|
732 |
|
1,174 |
Roche |
|
|
741 |
|
704 |
|
796 |
Novartis |
|
|
2,822 |
|
1,511 |
|
815 |
BioNTech |
|
|
869 |
|
784 |
|
708 |
Pfizer1 |
|
|
533 |
|
373 |
|
413 |
Other |
|
|
2 |
|
- |
|
69 |
|
|
|
|
|
|
|
|
Total2 |
|
|
19,783 |
|
16,053 |
|
14,505 |
|
|
|
|
|
|
|
|
Royalties by product: |
|
|
|
|
|
|
|
DARZALEX |
|
|
13,922 |
|
11,265 |
|
9,966 |
Kesimpta |
|
|
2,222 |
|
1,494 |
|
779 |
TEPEZZA |
|
|
737 |
|
704 |
|
796 |
Other3 |
|
|
471 |
|
242 |
|
41 |
|
|
|
|
|
|
|
|
Total |
|
|
17,352 |
|
13,705 |
|
11,582 |
1Pzifer acquired Seagen in December 2023
2 Excludes Genmab’s Net product sales
3Other consists of royalties from net sales of RYBREVANT, TECVAYLI, TALVEY and TEPKINLY
ACCOUNTING POLICIES
Genmab recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration that it expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that Genmab determines are within the scope of IFRS 15, Genmab
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 172/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. Genmab only applies the five-step model to contracts when it is probable that the Company will collect the consideration it is entitled to in exchange for the goods or services it transfers to the customer. At contract inception, once the contract is determined to be within the scope of IFRS 15, Genmab assesses the goods and services promised within each contract and identifies as a performance obligation each good or service that is distinct. Revenue is recognized in the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied.
Royalties: Certain of Genmab’s license and collaboration agreements include sales-based royalties based on the level of sales. The license has been deemed to be the predominant item to which the royalties relate under Genmab’s license and collaboration agreements. As a result, Genmab recognizes revenue when the related sales occur.
Reimbursement Revenue for R&D Services: Genmab’s research collaboration agreements include provisions for reimbursement or cost sharing for R&D services and payment for full time equivalents (“FTEs”) at contractual rates. R&D services are performed and satisfied over time given that the customer simultaneously receives and consumes the benefits provided by Genmab and revenue for research services is recognized over time rather than at a point in time.
Milestone Revenue: Certain of Genmab’s license and collaboration agreements include development, regulatory and commercial milestone payments based on the level of sales. At the inception of each arrangement that includes milestone payments, Genmab evaluates whether the achievement of milestones is considered highly probable and estimates the amount to be included in the transaction price using the most likely amount method. If it is highly probable that a significant revenue reversal would not occur, the associated milestone value is included in the transaction price. Milestone payments that are not within the control of Genmab or the license and collaboration partner, such as regulatory approvals, are not considered probable of being achieved until those approvals are received. The transaction price is then allocated to each performance obligation on a relative stand-alone selling price basis, for which Genmab recognizes revenue as or when the performance obligations under the contract are satisfied. At the end of each subsequent reporting period, Genmab re-evaluates the probability of achievement of such development milestones and commercial milestones and any related constraint, and if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue and earnings in the period of adjustment. Under all of Genmab’s existing license and collaboration agreements, milestone payments have been allocated to the license transfer performance obligation.
License Revenue for Intellectual Property: If the license to Genmab’s functional intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, Genmab recognizes revenues from non-refundable upfront fees allocated to the license at the point in time the license is transferred to the licensee and the licensee is able to use and benefit from the license. For licenses that are bundled with other promises, Genmab utilizes judgement to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, upfront fees. Under all of Genmab’s existing license and collaboration agreements the license to functional intellectual property has been determined to be distinct from other performance obligations identified in the agreement.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 173/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Collaboration Revenue: Collaboration revenue includes the result of profit sharing arrangements for the sale of commercial products by our collaboration partners. When Genmab’s collaboration partner is determined to be the principal in sales to end customers, Genmab’s share of profits for the sale of commercial products is included in collaboration revenue.
Net Product Sales: Revenue from the sale of goods is recognized when control is transferred to the customer and it is probable that Genmab will collect the consideration to which it is entitled for transferring the products. Control of the products is transferred at a single point in time which occurs upon delivery to the customer. The amount of sales to be recognized is based on the consideration Genmab expects to receive in exchange for its goods. When sales are recognized, an estimate for a variety of sales deductions is also recorded such as cash discounts, government rebates, chargebacks, wholesaler fees, other rebates and administrative fees, sales returns and allowances and other sales discounts. Sales deductions are estimated and recognized as a reduction of gross product sales to arrive at net product sales, by assessing the expected value of the sales deductions (variable consideration). Sales deductions are estimated and provided for at the time the related sales are recorded. Genmab’s estimates related to sales deductions require significant use of estimates as not all conditions are known at the time of sale. The estimates are based on analyses of existing contractual obligations, historical experience, drug product analogs and payer channel mix. Genmab considers the provisions established for sales deductions to be reasonable and appropriate based on currently available information; however, the actual amount of deductions may differ from the amounts estimated by management as more information becomes available. Estimates will be assessed each period and adjusted as required based on updated information and actual experience.
When Genmab is determined to be the principal in sales to end customers, all product sales are included in net product sales in the Consolidated Statements of Comprehensive Income. As of December 31, 2024, all net product sales relate to sales of EPKINLY in the U.S. and Japan pursuant to the Collaboration Agreement with AbbVie.
Refer to Note 5.6 for detailed information regarding Genmab’s significant Research Collaborations, License Agreements and Collaborative Agreements.
| ● | Refer to Note 1.3 for management’s judgements and estimates related to revenue recognition. |
2.2 – Information about Geographical Areas
Genmab is managed and operated as one business unit, which is reflected in the organizational structure and internal reporting. No separate lines of business or separate business entities have been identified with respect to any licensed products, marketed products, product candidates or geographical markets and no segment information is currently prepared for internal reporting.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 174/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Accordingly, it has been concluded that it is not relevant to include segment disclosures in the financial statements as Genmab’s business activities are not organized on the basis of differences in related product and geographical areas.
|
|
Revenue |
|
Non-current assets |
|
Revenue |
|
Non-current assets |
|
Revenue |
|
Non-current assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denmark |
|
19,783 |
|
12,710 |
|
16,053 |
|
496 |
|
14,505 |
|
211 |
Netherlands |
|
- |
|
767 |
|
- |
|
874 |
|
- |
|
793 |
United States |
|
902 |
|
3,196 |
|
380 |
|
378 |
|
- |
|
442 |
Japan |
|
841 |
|
100 |
|
41 |
|
56 |
|
- |
|
70 |
China |
|
- |
|
48 |
|
- |
|
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
21,526 |
|
16,821 |
|
16,474 |
|
1,804 |
|
14,505 |
|
1,516 |
Out of total non-current assets of DKK 16,821 million, DKK 12,340 million relates to intangible assets in Denmark and DKK 2,535 million relates to goodwill in the United States acquired as a part of the acquisition of ProfoundBio.
ACCOUNTING POLICIES
Geographical information is presented for Genmab’s revenue and non-current assets. Revenue is attributed to countries on the basis of the location of the legal entity holding the contract with the counterparty. Non-current assets comprise intangible assets, goodwill, property and equipment, right-of-use assets, and receivables.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 175/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
2.3 – Staff Costs
|
|
|
|
|
|
|
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
Wages and salaries |
|
3,168 |
|
2,631 |
|
1,913 |
Share-based compensation |
|
721 |
|
586 |
|
439 |
Defined contribution plans |
|
205 |
|
170 |
|
112 |
Other social security costs |
|
399 |
|
335 |
|
263 |
Government grants related to research and development expenses |
|
(149) |
|
(174) |
|
(144) |
|
|
|
|
|
|
|
Total |
|
4,344 |
|
3,548 |
|
2,583 |
|
|
|
|
|
|
|
Staff costs are included in the Consolidated Statements of Comprehensive Income as follows: |
|
|
|
|
|
|
Cost of product sales |
|
11 |
|
3 |
|
- |
Research and development expenses |
|
2,520 |
|
2,004 |
|
1,518 |
Selling, general and administrative expenses |
|
1,813 |
|
1,541 |
|
1,065 |
|
|
|
|
|
|
|
Total |
|
4,344 |
|
3,548 |
|
2,583 |
|
|
|
|
|
|
|
Average number of FTE |
|
2,535 |
|
2,011 |
|
1,460 |
Number of FTE at year-end |
|
2,682 |
|
2,204 |
|
1,660 |
Refer to Note 4.6 for additional information regarding share-based instruments and Note 5.1 for additional information regarding the remuneration of the Board and Executive Management.
ACCOUNTING POLICIES
STAFF COSTS
Wages and salaries, other social security costs, paid leave and bonuses, and other employee benefits are recognized in the financial year in which the employee performs the associated work.
Genmab’s pension plans are classified as defined contribution plans and, accordingly, no pension obligations are recognized in the balance sheet. Costs relating to defined contribution plans are included in the income statement in the period in which they are accrued, and outstanding contributions are included in other payables.
Termination benefits are recognized as an expense, when Genmab is committed demonstrably, without realistic possibility of withdrawal, to a formal detailed plan to terminate employment.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 176/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
2.4 – Corporate and Deferred Tax
TAXATION – INCOME STATEMENT & SHAREHOLDERS’ EQUITY
|
|
|
|
|
|
|
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
Current tax on profit |
|
1,799 |
|
1,301 |
|
1,478 |
Adjustment to deferred tax |
|
99 |
|
(59) |
|
107 |
Net Increase (decrease) of unrecognized deferred tax assets for the year |
|
(578) |
|
43 |
|
(92) |
|
|
|
|
|
|
|
Total tax for the period in the income statement |
|
1,320 |
|
1,285 |
|
1,493 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
Net profit before tax |
|
9,164 |
|
5,637 |
|
6,945 |
|
|
|
|
|
|
|
Tax at the Danish corporation tax rate of 22% for all periods |
|
2,016 |
|
1,240 |
|
1,528 |
|
|
|
|
|
|
|
Tax effect of: |
|
|
|
|
|
|
Net Increase (decrease) of unrecognized deferred tax assets for the year |
|
(579) |
|
43 |
|
(92) |
Net of non-taxable income over non-deductible expenses |
|
92 |
|
7 |
|
73 |
Other current and deferred tax adjustments |
|
(209) |
|
(5) |
|
(16) |
|
|
|
|
|
|
|
Total tax effect |
|
(696) |
|
45 |
|
(35) |
|
|
|
|
|
|
|
Total tax for the period in the income statement |
|
1,320 |
|
1,285 |
|
1,493 |
|
|
|
|
|
|
|
Total tax for the period in shareholders' equity |
|
49 |
|
57 |
|
(22) |
|
|
|
|
|
|
|
Effective Tax Rate |
|
14.4% |
|
22.8% |
|
21.5% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate tax consists of current tax and the adjustment of deferred taxes during the year. The corporate tax expense was DKK 1,320 million in 2024, DKK 1,285 million in 2023 and DKK 1,493 million in 2022. Tax benefits of DKK 49 million in 2024, DKK 57 million in 2023 and tax expenses of DKK 22 million in 2022, related to excess tax benefits for share-based compensation were recorded directly in shareholders’ equity.
As a result of the ProfoundBio integration activities, Genmab utilized approximately DKK 2.2 billion of previously unrecognized tax losses during 2024.
Genmab operates in multiple jurisdictions which have enacted new legislation to implement the global minimum top-up tax, which became effective on January 1, 2024. Under this legislation, the Company is liable to pay a top-up tax for the difference between its GloBE Effective Tax Rate per jurisdiction and the minimum rate of 15 percent. The rules have no impact on the tax position of Genmab in 2024.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 177/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
TAXATION – BALANCE SHEET
Significant components of the deferred tax (liabilities) assets are as follows:
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
Share-based instruments |
|
270 |
|
41 |
Deferred revenue |
|
120 |
|
113 |
Intangible assets |
|
(2,478) |
|
- |
Other temporary differences |
|
637 |
|
58 |
|
|
|
|
|
Total at December 31 |
|
(1,451) |
|
212 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Genmab recognizes deferred tax assets if it is probable that sufficient taxable income will be available in the future. Management has considered future taxable income and applied its judgement in assessing whether deferred tax assets should be recognized.
The difference between the deferred tax liability as of December 31, 2024 and the deferred tax liability acquired as part of the acquisition of ProfoundBio relates to the reestablishment of the deferred tax liability as a result of the transfer of intangible assets from ProfoundBio US to Genmab A/S during the fourth quarter of 2024.
As of December 31, 2024, Genmab had estimated gross unrecognized tax loss carryforwards in the Netherlands of DKK 0.7 billion to reduce future taxable income. As of December 31, 2023, Genmab had estimated gross unrecognized tax loss carryforwards in the U.S. and in the Netherlands of DKK 2.1 billion and DKK 0.5 billion, respectively. The tax losses in the Netherlands available as of December 31, 2024, can be carried forward indefinitely.
ACCOUNTING POLICIES
CORPORATE TAX
Corporate tax, which consists of current tax and deferred taxes for the year, is recognized in the income statement, except to the extent that the tax is attributable to items which directly relate to shareholders’ equity or other comprehensive income.
Current tax assets and liabilities for current and prior periods are measured at the amounts expected to be recovered from or paid to the tax authorities.
DEFERRED TAX
Deferred tax accounting requires recognition of deferred tax on all temporary differences between the carrying amount of assets and liabilities and the tax base of such assets and liabilities. This includes the tax value of certain tax losses carried forward.
Deferred tax is calculated in accordance with the tax regulations in the local countries and the tax rates expected to be in force at the time the deferred tax is utilized. Changes in deferred tax as a result of changes in tax rates are recognized in the income statement.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 178/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Deferred tax assets resulting from temporary differences, including the tax value of losses to be carried forward, are recognized only to the extent that it is probable that future taxable profit will be available against which the differences can be utilized.
Deferred tax liabilities are recognized for taxable temporary differences that arise when the carrying amount of an asset exceeds its tax basis or the carrying amount of a liability is less than its tax base.
MANAGEMENT’S JUDGEMENTS AND ESTIMATES
DEFERRED TAX
Genmab recognizes deferred tax assets if management assesses that these tax assets can be offset against positive taxable income within the foreseeable future. This judgement is made on an ongoing basis and is based on numerous factors, including actual results, budgets, and business plans for the coming years.
Realization of deferred tax assets is dependent upon a number of factors, including estimated future taxable earnings, the timing and amount of which are highly uncertain. A significant portion of Genmab’s future taxable income will be driven by future events that are highly susceptible to factors outside the control of Genmab including overall commercial growth , specific clinical outcomes, regulatory approvals, advancement of Genmab’s product pipeline and other matters. As such, changes in estimates of future taxable income could impact Genmab’s future taxable income in a positive or negative manner.
As a result of the ProfoundBio integration activities, Genmab, based on current business plans and estimates of future taxable income, recognized a significant portion of previously unrecognized deferred tax assets during 2024.
2.5 – Profit Per Share
(DKK million) |
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
Net profit |
7,844 |
|
4,352 |
|
5,452 |
|
|
|
|
|
|
(Shares) |
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares outstanding |
66,139,029 |
|
66,023,437 |
|
65,783,130 |
Weighted average number of treasury shares |
(1,952,382) |
|
(713,693) |
|
(395,829) |
|
|
|
|
|
|
Weighted average number of shares excl. treasury shares |
64,186,647 |
|
65,309,744 |
|
65,387,301 |
Adjustments for share-based instruments, dilution |
446,293 |
|
604,961 |
|
622,303 |
|
|
|
|
|
|
Weighted average number of shares, diluted |
64,632,940 |
|
65,914,705 |
|
66,009,604 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net profit per share |
122.21 |
|
66.64 |
|
83.38 |
Diluted net profit per share |
121.36 |
|
66.02 |
|
82.59 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 179/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
In the calculation of the diluted net profit per share for 2024, 788,967 potential ordinary shares related to share-based instruments have been excluded as they are anti-dilutive, compared to 248,649 and 68,728 for 2023 and 2022, respectively.
ACCOUNTING POLICIES
BASIC NET PROFIT PER SHARE
Basic net profit per share is calculated as the net profit for the period divided by the weighted average number of outstanding ordinary shares, excluding treasury shares.
DILUTED NET PROFIT PER SHARE
Diluted net profit per share is calculated as the net profit for the period divided by the weighted average number of outstanding ordinary shares, excluding treasury shares and adjusted for the dilutive effect of share equivalents.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 180/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Section 3 – Operating Assets and Liabilities
This section covers the operating assets and related liabilities that form the basis for Genmab’s activities. Deferred tax assets and liabilities are included in Note 2.4. Assets related to Genmab’s financing activities are shown in section 4.
3.1 – Other Intangible Assets and Goodwill
Intangible Assets
The increase in the gross carrying value of other intangible assets during 2024 was primarily due to the addition of DKK 10,577 million of in-process research and development (IPR&D) and DKK 1,243 million of a technology platform asset from the ProfoundBio acquisition. The technology platform asset is being amortized over its estimated useful life of 15 years. Refer to Note 5.5 for additional details.
(DKK million) |
|
Goodwill |
Licenses and Patents |
Technology Platform |
Acquired IPR&D |
Total Intangible Assets |
|
|
|
|
|
|
|
2024 |
|
|
|
|
|
|
Cost at the beginning of the year |
|
- |
901 |
- |
- |
901 |
Additions during the year |
|
2,436 |
163 |
1,243 |
10,577 |
14,419 |
Effect of exchange rate adjustment |
|
99 |
- |
44 |
369 |
512 |
Cost at the end of the year |
|
2,535 |
1,064 |
1,287 |
10,946 |
15,832 |
Amortization and impairment losses at the beginning of the year |
|
- |
800 |
- |
- |
800 |
Amortization for the year |
|
- |
25 |
53 |
- |
78 |
Impairment losses for the year |
|
- |
76 |
- |
- |
76 |
Amortization and impairment losses at the end of the year |
|
- |
901 |
53 |
- |
954 |
Carrying amount at the end of the year |
|
2,535 |
163 |
1,234 |
10,946 |
14,878 |
|
|
|
|
|
|
|
2023 |
|
|
|
|
|
|
Cost at the beginning of the year |
|
- |
891 |
- |
- |
891 |
Additions during the year |
|
- |
10 |
- |
- |
10 |
Cost at the end of the year |
|
- |
901 |
- |
- |
901 |
Amortization and impairment losses at the beginning of the year |
|
- |
745 |
- |
- |
745 |
Amortization for the year |
|
- |
55 |
- |
- |
55 |
Amortization and impairment losses at the end of the year |
|
- |
800 |
- |
- |
800 |
Carrying amount at the end of the year |
|
- |
101 |
- |
- |
101 |
Impairment losses for the year related to licenses and patents, which were not material, were recorded in Research and development expenses in the Consolidated Statements of Comprehensive Income.
Amortization expense was DKK 78 million, DKK 55 million, and DKK 108 million for 2024, 2023 and 2022, respectively, which was recorded in Research and development expenses in the Consolidated Statements of Comprehensive Income.
Goodwill
The carrying amount of goodwill was DKK 2,535 million as of December 31, 2024, due to the acquisition of ProfoundBio (refer to Note 5.5). No impairment of goodwill was recognized in 2024 as the annual impairment test showed that the estimated recoverable amount exceeded the carrying amount of the single cash-generating unit (CGU) to which all of Genmab’s goodwill was allocated. There was no goodwill balance as of December 31, 2023.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 181/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
ACCOUNTING POLICIES
RESEARCH AND DEVELOPMENT PROJECTS
Internal and subcontracted research costs are charged in full to research and development expenses in the Consolidated Statements of Comprehensive Income in the period in which they are incurred. Development costs are also expensed until regulatory approval is obtained or is probable. Genmab has no internally generated intangible assets from development, as the criteria for recognition of an intangible asset are not met.
Genmab acquires licenses and rights primarily to gain access to targets and technologies identified by third parties. Payments to third parties under collaboration and license agreements are assessed to determine whether such payments should be expensed as incurred as research and development expenses or capitalized as an intangible asset. Licenses and rights that meet the criteria for capitalization as intangible assets are measured at cost less accumulated amortization and any impairment losses. Milestone payments related to capitalized licenses and rights are accounted for as an increase in the cost to acquire licenses and rights.
For acquired research and development projects, and intellectual property rights, including acquisition in a business combination, the likelihood of obtaining future commercial sales is reflected in the cost of the asset, and thus the probability recognition criteria is always considered to be satisfied. As the cost of acquired research and development projects can often be measured reliably, these projects fulfil the capitalization criteria as intangible assets on acquisition. Development costs incurred subsequent to acquisition are treated consistently with internal project development costs.
GOODWILL
Goodwill represents the excess of purchase price over the fair value of net identifiable assets acquired and liabilities assumed in a business combination accounted for by the acquisition method of accounting. Goodwill is allocated to each of the group’s CGU (or groups of CGUs) expected to benefit from the synergies of the combination. Genmab consists of one single CGU which represents its single operating segment.
RECOGNITION AND MEASUREMENT
Intangible assets are initially measured at cost and are subsequently measured at cost less any accumulated amortization and any impairment loss. Goodwill is not amortized but is subject to impairment testing.
For intellectual property rights acquired for research and development projects, upfront fees and acquisition costs are capitalized as the historical cost. Subsequent milestone payments payable on achievement of a contingent event will be capitalized when the contingent event being achieved is probable. Intangible assets acquired in a business combination are recognized at fair value at the acquisition date.
Amortization
Intangible assets with definite useful lives are amortized based on the straight-line method over their estimated useful lives. This corresponds to the legal duration or the economic useful life depending on which is shorter. The amortization of intellectual property rights, including IPR&D, commences after regulatory approval has been obtained or when assets are put in use.
Impairment
Goodwill and intangible assets not yet available for use (IPR&D) are tested for impairment when indicators of impairment exist. However, they are tested at least annually, irrespective of whether there is any indication that they may be impaired.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 182/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
If circumstances or changes in Genmab’s operations indicate that the carrying amount of Goodwill, IPR&D or definite-lived intangible assets may not be recoverable, management performs an impairment test of the asset for impairment.
Amortization, impairment losses, and gains or losses on the disposal of other intangible assets related to licenses and rights are recognized in Consolidated Statements of Comprehensive Income as research and development expenses.
MANAGEMENT’S JUDGMENTS AND ESTIMATES
Impairment Assessment of Goodwill and Other Intangible Assets
CGUs to which goodwill has been allocated are tested for impairment at least annually, or more frequently when there is an indication that the unit may be impaired by assessing qualitative factors or performing a quantitative analysis. Goodwill is monitored for impairment at the operating segment level, which is the lowest level CGU to which goodwill is allocated and monitored by Management. Goodwill impairment tests are based on management’s estimate of recoverable amount determined as the greater of the fair value less cost to sell, or its value in use. Value in use is calculated based on a multiple applied on steady earnings from operations before tax generated from the CGU. If the carrying amount of goodwill exceeds the recoverable amount, any impairment is measured as the difference between the recoverable amount and the carrying amount. Any impairment is first allocated to reduce the carrying amount of goodwill and any exceeding amount is allocated pro-rata to Genmab’s other assets in the CGU in the scope of IAS 36 but not less than their recoverable amount. An impairment loss is recognized in the Consolidated Statements of Comprehensive Income when the impairment is identified. Impairments of goodwill are prohibited from future reversals.
As permitted under IAS 36 (Impairment of Assets), use of a short-cut quantitative impairment test may be relied on if conservative assumptions are utilized which would result in an underestimation of the recoverable amount. By applying a multiple of four on the previous twelve consecutive months operating profit before tax the estimated recoverable amount is higher than the carrying amount of the CGU. It is management’s assessment that a multiple of four is a conservative assumption compared to market observations. The operating profit before tax for the previous twelve consecutive months is based on recurring earnings and is considered a conservative measure of earnings for the next four years compared to the budget and forecast prepared by the Group. Thus, management has concluded that there is no impairment on goodwill. As Genmab has a single CGU with a quoted market price of the entire Group (level 1 observable input), a high-level comparison between Genmab’s market capitalization and the recoverable amount was also performed to reaffirm the reasonableness of the short-cut approach to estimate recoverable amount. The carrying amount of the single CGU includes Genmab’s net assets, less cash and marketable securities as returns on such balances are not included in operating profit before tax and therefore are not included in the recoverable amount either.
The basis for the review of IPR&D impairment is also the recoverable amount. If the carrying amount of an intangible asset is greater than the recoverable amount, the intangible asset is written down to the recoverable amount. An impairment loss is recognized in the Consolidated Statements of Comprehensive Income when the impairment is identified. Impairments on intangible assets are reviewed at each reporting date for possible reversal.
Factors considered material that could trigger an impairment test include the following:
• Development of a competing drug
• Realized sales trending below predicted sales
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 183/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
• Inconsistent or unfavorable clinical readouts
• Changes in the legal framework covering patents, rights, and licenses
• Advances in medicine and/or technology that affect the medical treatments
• Adverse impact on reputation and/or brand names
3.2 – PROPERTY AND EQUIPMENT
(DKK million) |
|
|
Leasehold improvements |
|
Equipment, furniture and fixtures |
|
Assets under construction |
|
Total property and equipment |
|
|
|
|
|
|
|
|
|
|
2024 |
|
|
|
|
|
|
|
|
|
Cost at January 1 |
|
|
684 |
|
908 |
|
39 |
|
1,631 |
Additions for the year |
|
|
5 |
|
81 |
|
116 |
|
202 |
Acquisitions through business combinations |
|
|
11 |
|
41 |
|
- |
|
52 |
Transfers between the classes |
|
|
10 |
|
38 |
|
(48) |
|
- |
Disposals for the year |
|
|
(5) |
|
(86) |
|
(4) |
|
(95) |
Exchange rate adjustment |
|
|
16 |
|
9 |
|
- |
|
25 |
|
|
|
|
|
|
|
|
|
|
Cost at December 31 |
|
|
721 |
|
991 |
|
103 |
|
1,815 |
|
|
|
|
|
|
|
|
|
|
Accumulated depreciation and impairment at January 1 |
|
|
(194) |
|
(482) |
|
- |
|
(676) |
Depreciation for the year |
|
|
(75) |
|
(158) |
|
- |
|
(233) |
Exchange rate adjustment |
|
|
(7) |
|
(4) |
|
- |
|
(11) |
Accumulated depreciation on disposals |
|
|
5 |
|
78 |
|
- |
|
83 |
Accumulated depreciation and impairment at December 31 |
|
|
(271) |
|
(566) |
|
- |
|
(837) |
|
|
|
|
|
|
|
|
|
. |
Carrying amount at December 31 |
|
|
450 |
|
425 |
|
103 |
|
978 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Leasehold improvements |
|
Equipment, furniture and fixtures |
|
Assets under construction |
|
Total property and equipment |
(DKK million) |
|
|
|
|
|
|
|
|
|
2023 |
|
|
|
|
|
|
|
|
|
Cost at January 1 |
|
|
412 |
|
649 |
|
233 |
|
1,294 |
Additions for the year |
|
|
6 |
|
129 |
|
222 |
|
357 |
Transfers between the classes |
|
|
276 |
|
134 |
|
(410) |
|
- |
Disposals for the year |
|
|
- |
|
- |
|
(6) |
|
(6) |
Exchange rate adjustment |
|
|
(10) |
|
(4) |
|
- |
|
(14) |
|
|
|
|
|
|
|
|
|
|
Cost at December 31 |
|
|
684 |
|
908 |
|
39 |
|
1,631 |
|
|
|
|
|
|
|
|
|
|
Accumulated depreciation and impairment at January 1 |
|
|
(132) |
|
(363) |
|
- |
|
(495) |
Depreciation for the year |
|
|
(64) |
|
(121) |
|
- |
|
(185) |
Exchange rate adjustment |
|
|
2 |
|
2 |
|
- |
|
4 |
Accumulated depreciation and impairment at December 31 |
|
|
(194) |
|
(482) |
|
- |
|
(676) |
|
|
|
|
|
|
|
|
|
. |
Carrying amount at December 31 |
|
|
490 |
|
426 |
|
39 |
|
955 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2024 |
|
2023 |
|
2022 |
(DKK million) |
|
|
|
|
|
|
|
|
|
Depreciation and impairment included in the income statement as follows: |
|
|
|
|
|
|
|
|
|
Research and development expenses |
|
|
|
|
197 |
|
140 |
|
108 |
Selling, general and administrative expenses |
|
|
|
|
36 |
|
45 |
|
38 |
|
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
233 |
|
185 |
|
146 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 184/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Capital expenditures in 2024 were primarily related to the expansion Genmab’s facilities in the United States and Japan. Capital expenditures in 2023 were primarily related to the expansion of our facilities in the Netherlands and our new headquarters in Denmark.
ACCOUNTING POLICIES
Property and equipment is comprised of leasehold improvements, assets under construction, and equipment, furniture, and fixtures, which are measured at cost less accumulated depreciation and any impairment losses.
The cost is comprised of the acquisition price and direct costs related to the acquisition until the asset is ready for use. Costs include direct costs and costs to subcontractors.
DEPRECIATION
Depreciation is calculated on a straight-line basis to allocate the cost of the assets, net of any residual value, over the estimated useful lives, which are as follows:
Equipment, furniture, and fixtures |
3-5 years |
Leasehold improvements |
15 years or the lease term, if shorter |
Depreciation commences when the asset is available for use, including when it is in the location and condition necessary for it to be capable of operating in the manner intended by management. The useful lives and residual values are reviewed and adjusted if appropriate on a yearly basis. Assets under construction are not depreciated.
IMPAIRMENT
If circumstances or changes in Genmab’s operations indicate that the carrying amount of property and equipment may not be recoverable, management performs an impairment test of the asset.
The basis for the performance of an impairment test is the recoverable amount of the asset, determined as the greater of the fair value less cost to sell or its value in use. Value in use is calculated as the net present value of future cash inflow expected to be generated from the asset.
If the carrying amount of an asset is greater than the recoverable amount, the asset is written down to the recoverable amount. An impairment loss is recognized in the Consolidated Statements of Comprehensive Income when the impairment is identified.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 185/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
3.3 - Leases
Genmab has entered into lease agreements with respect to office and laboratory space, vehicles, and IT equipment. The expense, lease liability, and right-of-use assets balances related to vehicles and IT equipment are immaterial. The leases are non-cancellable over various periods through 2038.
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
Right-of-use assets |
|
|
|
|
|
|
Balance at January 1 |
|
686 |
|
523 |
|
354 |
Additions to right-of-use assets1 |
|
329 |
|
250 |
|
243 |
Depreciation charge for the year |
|
(102) |
|
(87) |
|
(74) |
|
|
|
|
|
|
|
Balance at December 31 |
|
913 |
|
686 |
|
523 |
|
|
|
|
|
|
|
Lease liabilities |
|
|
|
|
|
|
Current |
|
92 |
|
90 |
|
74 |
Non-current |
|
937 |
|
680 |
|
523 |
|
|
|
|
|
|
|
Total at December 31 |
|
1,029 |
|
770 |
|
597 |
|
|
|
|
|
|
|
(1) Additions to right-of-use assets also includes modifications to existing leases and adjustments to the provisions for contractual restoration obligations related to leases of Genmab offices. | ||||||
|
|
|
|
|
|
|
Cash outflow for lease payments |
|
96 |
|
115 |
|
88 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Variable lease payments, short-term lease expense, lease interest expense, low-value assets, and sublease income are immaterial.
Future minimum payments under leases are as follows:
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
Payment due |
|
|
|
|
|
|
Less than 1 year |
|
127 |
|
106 |
|
89 |
1 to 3 years |
|
284 |
|
199 |
|
167 |
More than 3 years but less than 5 years |
|
281 |
|
183 |
|
136 |
More than 5 years |
|
553 |
|
412 |
|
271 |
|
|
|
|
|
|
|
Total at December 31 |
|
1,245 |
|
900 |
|
663 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 186/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
ACCOUNTING POLICIES
All leases are recognized in the Consolidated Balance Sheets as a right-of-use (ROU) asset with a corresponding lease liability, except for short-term leases in which the term is 12 months or less, or low-value leases.
ROU assets represent Genmab’s right to use an underlying asset for the lease term and lease liabilities represent Genmab’s obligation to make lease payments arising from the lease. The ROU asset is depreciated over the shorter of the asset’s useful life or the lease term on a straight-line basis. In the Consolidated Statements of Comprehensive Income, depreciation of the ROU asset is recognized over the lease term in operating expenses and interest expenses related to the lease liability are classified in financial items.
Genmab determines if an arrangement is a lease at inception. Genmab leases various properties, vehicles, and IT equipment. Rental contracts are typically made for fixed periods. Lease terms are negotiated on an individual basis and contain a wide range of terms and conditions.
Assets and liabilities arising from a lease are initially measured on a present value basis. Lease liabilities include the net present value of fixed payments, less any lease incentives receivable. As Genmab’s leases generally do not provide an implicit interest rate, Genmab uses an incremental borrowing rate based on the information available at the commencement date of the lease in determining the present value of lease payments. Lease terms utilized by Genmab may include options to extend or terminate the lease when it is reasonably certain that Genmab will exercise that option. In determining the lease term, management considers all facts and circumstances that create an economic incentive to exercise an extension option, or not exercise a termination option. Extension options (or periods after termination options) are only included in the lease term if the lease is reasonably certain to be extended.
ROU assets are measured at cost and include the amount of the initial measurement of the lease liability, any lease payments made at or before the commencement date less any lease incentives received, any initial direct costs, and restoration costs.
Payments associated with short-term leases and leases of low-value assets are recognized on a straight-line basis as an expense in the Consolidated Statements of Comprehensive Income.
3.4 - Other Investments
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
|
|
|
Publicly traded equity securities |
|
38 |
|
47 |
Fund investments |
|
176 |
|
87 |
Privately held equity securities |
|
14 |
|
- |
|
|
|
|
|
Total at December 31 |
|
228 |
|
134 |
Other investments includes strategic investments in publicly traded common stock of companies, including common stock of companies with whom Genmab has entered into collaboration arrangements, investments in certain investment funds, as well as investments in shares of privately held companies.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 187/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
ACCOUNTING POLICIES
Other investments are measured on initial recognition at fair value, and subsequently at fair value. Changes in fair value are recognized in the Consolidated Statements of Comprehensive Income within financial income or expense.
Other investments primarily consist of investments in certain strategic investment funds. Genmab’s share of the fair value of these fund investments is determined based on the valuation of the underlying investments included in the fund. Investments in publicly traded equity securities included in these strategic investment funds are valued based at the most recent sale price or official closing price reported on the exchange or over-the-counter market on which they trade, while investments in non-publicly traded equity securities are based on other factors, including but not limited to, type of the security, the size of the holding, the initial cost of the security, the price and extent of public trading in similar securities of the comparable companies, an analysis of the company's or issuer's financial statements and with respect to debt securities, the maturity and creditworthiness. As such, these fund investments have been characterized as Level 3 investments as fair values are based on significant unobservable inputs.
3.5 – Inventories
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
Raw materials |
|
4 |
|
14 |
Work in progress |
|
- |
|
- |
Finished goods |
|
79 |
|
59 |
Total inventories (gross) at December 31 |
|
83 |
|
73 |
|
|
|
|
|
Allowances at year end |
|
(21) |
|
(16) |
|
|
|
|
|
Total inventories (net) at December 31 |
|
62 |
|
57 |
In 2024 and 2023, allowances related to write downs of excess and obsolete inventories were immaterial and recognized as expense within cost of product sales in the Consolidated Statements of Comprehensive Income.
Inventory write down in 2023 pertaining to pre-launch inventories of EPKINLY was also immaterial. The write down was recorded as research and development expense in Genmab’s Consolidated Statements of Comprehensive Income and was subsequently reversed upon receiving U.S. FDA approval during the second quarter of 2023.
ACCOUNTING POLICIES
Inventories are measured at the lower of cost and net realizable value with costs determined on a first-in, first-out basis. Costs comprise direct and indirect costs relating to the manufacture of inventory mainly from third-party providers of manufacturing as well as costs related to internal resources and distribution and logistics. Genmab assesses the recoverability of capitalized inventories during each reporting period and will write down excess or obsolete inventories to their net realizable value in the period in which the impairment is identified. Write downs of inventory are included within Cost of product sales in the Consolidated Statements of Comprehensive Income.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 188/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Included in inventories are materials with the intended purpose of being made available for sale. If the materials are later used in the production of clinical products, the materials are charged to research and development expense when shipped to the clinical packaging site. Materials ordered exclusively to be used in Genmab’s research and development process (e.g., early research/clinical trials) are immediately expensed to research and development based on the relevant shipping terms (FOB destination/shipping point).
Inventory manufactured prior to regulatory approval of a product (prelaunch inventory) is written down to its net realizable value (that is the probable amount expected to be realized from its sale or use at the time of production). The amount of this write down is recognized in the Consolidated Statements of Comprehensive Income as research and development expenses. Once there is a high probability of regulatory approval being obtained for the product, inventory costs begin to be capitalized. Additionally, the write-down is reversed, up to no more than the original cost. The reversal of the write-down is recognized as a reduction to research and development expenses in the Consolidated Statements of Comprehensive Income.
3.6 – Receivables
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
Receivables related to collaboration agreements |
|
5,434 |
|
4,148 |
Prepayments |
|
256 |
|
241 |
Trade receivables related to product sales |
|
466 |
|
184 |
Interest receivables |
|
133 |
|
150 |
Other receivables |
|
353 |
|
286 |
Total at December 31 |
|
6,642 |
|
5,009 |
|
|
|
|
|
Non-current receivables |
|
52 |
|
62 |
Current receivables |
|
6,590 |
|
4,947 |
|
|
|
|
|
Total at December 31 |
|
6,642 |
|
5,009 |
During 2024 and 2023, there were no losses related to receivables and the credit risk on receivables is considered to be limited. The provision for expected credit losses was zero given that there have been no credit losses over the last three years and the limited credit risk due to high-quality nature with high credit ratings (top tier life science companies and major distributors) of Genmab’s customers are not likely to result in future default risk.
The receivables are mainly comprised of royalties, trade receivables, milestones and amounts due under collaboration agreements and are non-interest bearing receivables which are due less than one year from the balance sheet date.
Refer to Note 4.2 for additional information about interest receivables and related credit risk.
ACCOUNTING POLICIES
Initially, trade receivables are designated as financial assets measured at transaction price and other receivables are measured at fair value. Subsequently receivables are measured in the balance sheet at amortized cost, which generally corresponds to nominal value less expected credit losses.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 189/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Accounts receivable arising from product sales consists of amounts due from customers, net of customer allowances for chargebacks, cash and other discounts and estimated credit losses. Genmab’s contracts with customers have initial payment terms that range from 30 to 180 days.
Genmab utilizes a simplified approach to measuring expected credit losses and uses a lifetime expected loss allowance for all receivables. To measure the expected credit losses, receivables have been grouped based on credit risk characteristics and the days past due.
Prepayments include expenditures related to a future financial period. Prepayments are measured at nominal value.
3.7 – Contract Liabilities
Genmab has recognized the following liabilities related to the AbbVie collaboration agreement.
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
Contract liabilities at January 1 |
|
513 |
|
513 |
Payment received |
|
- |
|
- |
Revenue recognized during the year |
|
(9) |
|
- |
|
|
|
|
|
Total at December 31 |
|
504 |
|
513 |
|
|
|
|
|
Non-current contract liabilities |
|
480 |
|
480 |
Current contract liabilities |
|
24 |
|
33 |
|
|
|
|
|
Total at December 31 |
|
504 |
|
513 |
Contract liabilities were recognized in connection with the AbbVie collaboration agreement. An upfront payment of USD 750 million (DKK 4,911 million) was received in July 2020 of which DKK 4,398 million was recognized as license revenue during 2020.
The revenue deferred at the initiation of the AbbVie agreement in June 2020 related to four product concepts to be identified and subject to a research agreement to be negotiated between Genmab and AbbVie.
During the first quarter of 2022, Genmab and AbbVie entered into the aforementioned research agreement that governs the research and development activities in regard to the product concepts.
As part of the continued evaluation of contract liabilities related to the AbbVie collaboration agreement, Genmab’s classification of contract liabilities reflects the current estimate of co-development activities as of December 31, 2024. Contract liabilities have been recognized as reimbursement revenue during the second half of 2024.
Refer to Note 5.6 for additional information related to the AbbVie collaboration.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 190/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
3.8 – Other Payables
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
|
|
|
Liabilities related to collaboration agreements |
|
275 |
|
145 |
Staff cost liabilities |
|
720 |
|
637 |
Accounts payable |
|
644 |
|
330 |
Other liabilities |
|
1,873 |
|
1,230 |
|
|
|
|
|
Total at December 31 |
|
3,512 |
|
2,342 |
|
|
|
|
|
Non-current other payables |
|
30 |
|
35 |
Current other payables |
|
3,482 |
|
2,307 |
|
|
|
|
|
Total at December 31 |
|
3,512 |
|
2,342 |
ACCOUNTING POLICIES
Other payables, excluding provisions, are initially measured at fair value and subsequently measured in the balance sheet at amortized cost.
The current other payables are comprised of liabilities that are due less than one year from the balance sheet date and are in general not interest bearing and settled on an ongoing basis during the next financial year.
Non-current payables are measured at the present value of the expenditures expected to be required to settle the obligation using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the obligation. The increase in the liability due to passage of time is recognized as interest expense.
ACCOUNTS PAYABLE
Accounts payable are measured in the Consolidated Balance Sheets at amortized cost.
OTHER LIABILITIES
Other liabilities primarily include accrued expenses related to our research and development project costs and are measured in the Consolidated Balance Sheets at amortized cost.
Refer to Note 2.3 for accounting policies related to staff costs.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 191/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Section 4 – Capital Structure, Financial Risk and Related Items
This section includes disclosures related to how Genmab manages its capital structure, cash position and related risks and items. Genmab is primarily financed through partnership collaborations.
4.1 – Capital Management
Genmab’s goal is to maintain a strong capital base so as to maintain investor, creditor and market confidence, and to have adequate liquidity to support the continuous advancement of Genmab’s product pipeline and business in general. To achieve this goal Genmab invests in different liquidity tiers. To meet operational goals, Genmab invests in cash and cash equivalents (marketable securities). To ensure sufficient reserves, Genmab invests in short-term securities with an average duration of about six months, which serves as back-up liquidity for the operating tier. For strategic purposes, Genmab has short term investments to support the Company’s growth over the longer term. Most of Genmab’s cash and marketable securities are in USD due to having a larger USD expenditure base than DKK, which provides better matching of investment balances with actual expenditures. Genmab is primarily financed through revenues under various collaboration agreements and had, as of December 31, 2024, cash, and cash equivalents of DKK 9,858 million and marketable securities of DKK 11,243 million compared to DKK 14,867 million and DKK 13,268 million, respectively, as of December 31, 2023. Genmab’s cash and cash equivalents and marketable securities support the advancement of our product pipeline and operations.
The adequacy of our available funds will depend on many factors, including the level of DARZALEX and other royalty streams, progress in our research and development programs, the magnitude of those programs, our commitments to existing and new clinical collaborators, our ability to establish commercial and licensing arrangements, our capital expenditures, market developments, and any future acquisitions. Accordingly, Genmab may require additional funds and may attempt to raise additional funds through equity or debt financings, collaborative agreements with partners, or from other sources.
During the fourth quarter of 2024, Genmab entered into an unsecured three-year revolving credit facility (“Credit Facility”) of up to USD 300 million with a syndicate of lenders. Genmab intends to use the Credit Facility to finance working capital needs, and for general corporate purposes, of Genmab A/S and its subsidiaries. The Credit Facility includes options to increase the size of the facility up to USD 500 million as well as the ability to extend for an additional two years. The Credit Facility contains certain customary financial covenants. As of December 31, 2024, there were no outstanding amounts due on, nor any usage of, the Credit Facility and Genmab was in compliance with all financial covenants.
The Board monitors the share and capital structure to ensure that Genmab’s capital resources support its strategic goals.
Neither Genmab A/S nor any of its subsidiaries are subject to externally imposed capital requirements.
4.2 – Financial Risk
The financial risks of Genmab are managed centrally.
The overall risk management guidelines have been approved by the Board of Directors and include the Group’s investment policy related to our marketable securities. The Group’s risk management guidelines are
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 192/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
established to identify and analyze the risks faced by the Genmab Group, to set the appropriate risk limits and controls and to monitor the risks and adherence to limits. It is Genmab’s policy not to actively speculate in financial risks. The Group’s financial risk management is directed solely towards monitoring and reducing financial risks which are directly related to Genmab’s operations.
The primary objective of Genmab’s investment activities is to preserve capital and ensure liquidity with a secondary objective of maximizing the return derived from security investments without significantly increasing risk. Therefore, our investment policy includes among other items, guidelines and ranges for which investments (which are primarily shorter-term in nature) are considered to be eligible investments for Genmab and which investment parameters are to be applied, including maturity limitations and credit ratings. In addition, the policy includes specific diversification criteria and investment limits to minimize the risk of loss resulting from over-concentration of assets in a specific class, issuer, currency, country, or economic sector.
Genmab’s marketable securities are administered by external investment managers. The investment guidelines and managers are reviewed regularly to reflect changes in market conditions, Genmab’s activities and financial position. Genmab’s investment policy allows investments in debt rated BBB- or greater by S&P or Fitch and in debt rated Baa3 or greater by Moody’s. The policy also includes additional allowable investment types such as corporate debt, commercial paper, certificates of deposit, and certain types of AAA rated asset-backed securities.
In addition to the capital management and financing risk mentioned in Note 4.1, Genmab has identified the following key financial risk areas, which are mainly related to our marketable securities portfolio:
· credit risk; |
· foreign currency risk; and |
· interest rate risk |
All of Genmab’s marketable securities are traded in established markets. Given the current market conditions, all future cash inflows, including re-investments of proceeds from the disposal of marketable securities, are invested in highly liquid, investment grade securities. Refer to Note 4.4 for additional information regarding marketable securities.
CREDIT RISK
Genmab is exposed to credit risk and losses on marketable securities, bank deposits and receivables. The maximum credit exposure related to Genmab’s cash and cash equivalents and marketable securities was DKK 21,101 million as of December 31, 2024, compared to DKK 28,135 million as of December 31, 2023. The maximum credit exposure to Genmab’s receivables was DKK 6,642 million as of December 31, 2024 compared to DKK 5,009 million as of December 31, 2023.
Marketable Securities
To manage and reduce credit risks on our securities, Genmab’s policy is to ensure only securities from investment grade issuers are eligible for our portfolios. No issuer of marketable securities can be accepted if the issuer, at the time of purchase, does not have the credit quality equal to or better than the rating shown in the table below from at least one of the rating agencies. If an issuer is rated by more than one of the rating agencies listed below, the credit assessment is made against the lowest rating available for the issuer.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 193/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Category |
S&P |
Moody’s |
Fitch |
Short-term |
A-2 |
P-2 |
F-2 |
Long-term |
BBB- |
Baa3 |
BBB- |
Genmab’s current portfolio is spread over a number of different securities with a focus on liquidity and security. As of December 31, 2024, 71% of Genmab’s marketable securities were long-term A rated or higher, or short-term A-1 / P-1 rated by S&P, Moody’s or Fitch compared to 72% as of December 31, 2023. The total value of marketable securities amounted to DKK 11,243 million at the end of 2024 compared to DKK 13,268 million at the end of 2023.
Cash and Cash Equivalents
To reduce the credit risk on our bank deposits, Genmab’s policy is only to invest its cash deposits with highly rated financial institutions. Currently, these financial institutions have a short-term Fitch and S&P rating of at least F-1 and A-1, respectively. In addition, Genmab maintains bank deposits at a level necessary to support the short-term funding requirements of Genmab. The total value of bank deposits including AAA rated money market funds and short-term marketable securities classified as cash equivalents amounted to DKK 9,858 million as of December 31, 2024 compared to DKK 14,867 million at the end of 2023. The decrease was primarily the result of cash used to acquire ProfoundBio in the second half of 2024.
Receivables
The credit risk related to our receivables is not significant based on the high-quality nature of Genmab’s collaboration partners. As disclosed in Note 2.2, J&J, Novartis, Roche, AbbVie and BioNTech are Genmab’s primary partners in which receivables are established for royalties, milestone revenue and reimbursement revenue. These are long-standing relationships and Genmab does not have a history of writing off receivables from collaboration partners.
FOREIGN CURRENCY RISK
Genmab’s presentation currency is the DKK; however, Genmab’s revenues and expenses are in a number of different currencies. Consequently, there is a substantial risk of exchange rate fluctuations having an impact on Genmab’s cash flows, profit (loss) and/or financial position in DKK.
The majority of Genmab’s revenue is generated in USD. Exchange rate changes to the USD will result in changes to the translated value of future net profit before tax and cash flows. Genmab’s revenue in USD was 79% of total revenue in 2024 as compared to 86% in 2023 and 89% in 2022.
Under our license agreement with J&J for DARZALEX, for purposes of calculating royalties due to Genmab, DARZALEX net sales for non-U.S. dollar denominated currencies are translated to U.S. dollars at a specified annual Currency Hedge Rate. Movements in foreign exchanges against the annual Currency Hedge Rate will result in changes to royalties due to Genmab impacting net profit before tax and cash flows.
There is also exposure that exchange rate fluctuations may impact equity as part of the currency translation adjustments required to convert the investments in foreign subsidiaries from their respective functional currencies to the presentation currency during consolidation, however any such fluctuations would be immaterial. The foreign subsidiaries are not significantly affected by currency risks as both revenues and expenses are primarily settled in the foreign subsidiaries’ functional currencies.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 194/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Foreign currency risk is primarily concentrated at the Genmab A/S level as transactions with subsidiaries are primarily in the functional currency of the subsidiary. To manage and reduce this foreign currency risk, Genmab maintains a large portion of its investment portfolio in marketable securities in USD (approximately 76%) as well as a portion of our investment portfolio in DKK, EUR, and GBP denominated securities as a natural partial hedge of Genmab A/S’ liability exposures in these currencies.
Assets and Liabilities in Foreign Currency
Genmab’s marketable securities denominated in USD, DKK, EUR, and GBP as a percentage of total marketable securities were as follows:
|
|
|
|
|
|
Percent |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
USD |
|
76 |
% |
81 |
% |
DKK |
|
15 |
% |
12 |
% |
EUR |
|
8 |
% |
6 |
% |
GBP |
|
1 |
% |
1 |
% |
Total at December 31 |
|
100 |
% |
100 |
% |
Genmab’s USD currency exposure is mainly related to cash and cash equivalents, marketable securities, and receivables related to our collaborations with J&J, AbbVie, and Roche. Significant changes in the exchange rate of USD to DKK could cause net profit before tax to change materially as gains and losses are recognized in the Consolidated Statements of Comprehensive Income. Based on the amount of assets and liabilities denominated in USD as of December 31, 2024 and 2023, a 10% increase/decrease in the USD to DKK exchange rate is estimated to impact Genmab’s net profit before tax by approximately DKK 1.9 billion and DKK 2.7 billion, respectively. The analysis assumes that all other variables, in particular interest rates, remain constant. The movements in the income statement and equity arise from monetary items (cash and cash equivalents, marketable securities, receivables, and liabilities) where the functional currency of the entity differs from the currency that the monetary items are denominated in.
Genmab’s EUR exposure is mainly related to our marketable securities, receivables under our collaboration with BioNTech, and other costs denominated in EUR. Since the introduction of the EUR in 1999, Denmark has committed to maintaining a central rate of 7.46 DKK to the EUR. This rate may fluctuate within a +/- 2.25% band. Should Denmark’s policy toward the EUR change, the DKK values of our EUR denominated assets and costs could be materially different compared to what is calculated and reported under the existing Danish policy toward the DKK/EUR. As of December 31, 2024 and 2023, Genmab’s EUR exposure is not material.
Genmab’s GBP currency exposure is mainly related to contracts and marketable securities denominated in GBP. As of December 31, 2024 and 2023, Genmab’s GBP exposure is not material.
INTEREST RATE RISK
Genmab’s exposure to interest rate risk is primarily related to marketable securities, as Genmab currently does not have significant interest-bearing debts.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 195/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Marketable Securities
The securities in which the Group has invested bear interest rate risk, as a change in market-derived interest rates may cause fluctuations in the fair value of the investments. In accordance with the objective of the investment activities, the portfolio of securities is monitored on a total return basis.
To control and minimize the interest rate risk, Genmab maintains an investment portfolio in a variety of securities with a relatively short effective duration with both fixed and variable interest rates.
A sensitivity analysis was performed on Genmab’s marketable securities, and based on exposures in 2023 and 2024, a hypothetical +/- 1% interest rate change would not have resulted in a material change in the fair values of these financial instruments. Due to the short-term nature of the current investments and to the extent that Genmab is able to hold the investments to maturity, the current exposure to changes in fair value due to interest rate changes is considered to be insignificant compared to the fair value of the portfolio.
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
Year of Maturity |
|
|
|
|
2024 |
|
- |
|
6,742 |
2025 |
|
5,000 |
|
3,717 |
2026 |
|
3,209 |
|
2,175 |
2027 |
|
2,314 |
|
232 |
2028 |
|
329 |
|
143 |
2029+ |
|
391 |
|
259 |
Total at December 31 |
|
11,243 |
|
13,268 |
4.3 —Financial Assets and Liabilities
CATEGORIES OF FINANCIAL ASSETS AND LIABILITIES
|
|
December 31, |
|
(DKK million) |
Note |
2024 |
2023 |
Financial assets measured at fair value through profit or loss |
|
|
|
Marketable securities |
4.4 |
11,243 |
13,268 |
Other investments |
3.4 |
228 |
134 |
Financial assets measured at amortized cost |
|
|
|
Receivables excluding prepayments |
3.6 |
6,386 |
4,768 |
Cash and cash equivalents |
|
9,858 |
14,867 |
Financial liabilities measured at amortized cost |
|
|
|
Lease liabilities |
3.3 |
(1,029) |
(770) |
Other payables excluding provisions |
3.8 |
(3,482) |
(2,316) |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 196/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
FAIR VALUE MEASUREMENT
|
|
December 31, |
|||||||
|
|
2024 |
2023 |
||||||
(DKK million) |
Note |
Level 1 |
Level 2 |
Level 3 |
Total |
Level 1 |
Level 2 |
Level 3 |
Total |
Assets Measured at Fair Value |
|
|
|
|
|
|
|
|
|
Marketable securities |
4.4 |
11,243 |
- |
- |
11,243 |
13,268 |
- |
- |
13,268 |
Other investments |
3.4 |
38 |
14 |
176 |
228 |
47 |
- |
87 |
134 |
Marketable Securities
Substantially all fair market values are determined by reference to external sources using unadjusted quoted prices in established markets for our marketable securities (Level 1).
Other Investments
Other investments primarily consist of investments in certain strategic investment funds. Genmab’s share of the fair value of these fund investments is determined based on the valuation of the underlying investments included in the fund. Investments in publicly traded equity securities included in these strategic investment funds are valued based at the most recent sale price or official closing price reported on the exchange or over-the-counter market on which they trade, while investments in non-publicly traded equity securities are based on other factors, including but not limited to, type of the security, the size of the holding, the initial cost of the security, the price and extent of public trading in similar securities of the comparable companies, an analysis of the company's or issuer's financial statements and with respect to debt securities, the maturity and creditworthiness. As such, these fund investments have been characterized as Level 3 investments as fair values are not entirely based on observable market data.
There were no transfers into or out of Level 3 during 2024 or 2023. Acquisitions (capital calls), fair value changes and foreign currency changes on Level 3 investments in 2024 and 2023 were as follows:
(DKK million) |
Other Investments |
Fair value at December 31, 2022 |
66 |
Acquisitions |
30 |
Fair value changes |
(9) |
Fair value at December 31, 2023 |
87 |
Acquisitions |
42 |
Fair value changes |
43 |
Foreign currency changes |
4 |
Fair value at December 31, 2024 |
176 |
ACCOUNTING POLICIES
CLASSIFICATION OF CATEGORIES OF FINANCIAL ASSETS AND LIABILITIES
Genmab classifies its financial assets held into the following measurement categories:
| ● | those to be measured subsequently at fair value (either through other comprehensive income, or through profit or loss), and |
| ● | those to be measured at amortized cost. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 197/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The classification depends on the business model for managing the financial assets and the contractual terms of the cash flows.
For assets measured at fair value, gains and losses will either be recorded in profit or loss or other comprehensive income.
Genmab reclassifies debt investments only when its business model for managing those assets changes.
Further details about the accounting policy for each of the categories are outlined in the respective notes.
FAIR VALUE MEASUREMENT
Genmab measures financial instruments, such as marketable securities, at fair value at each balance sheet date. Management assessed that the fair value of financial assets and liabilities measured at amortized cost such as bank deposits, receivables and other payables approximate their carrying amounts largely due to the short-term maturities of these instruments.
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value measurement is based on the presumption that the transaction to sell the asset or transfer the liability takes place either:
| ● | In the principal market for the asset or liability, or |
| ● | In the absence of a principal market, in the most advantageous market for the asset or liability. |
The principal or the most advantageous market must be accessible by Genmab.
The fair value of an asset or a liability is measured using the assumptions that market participants would use when pricing the asset or liability, assuming that market participants act in their economic best interest.
Genmab uses valuation techniques that are appropriate in the circumstances and for which sufficient data are available to measure fair value, maximizing the use of relevant observable inputs and minimizing the use of unobservable inputs.
For financial instruments that are measured in the balance sheet at fair value, IFRS 13 requires disclosure of fair value measurements by level of the following fair value measurement hierarchy:
· Level 1 - Quoted prices (unadjusted) in active markets for identical assets or liabilities |
· Level 2 - Inputs other than quoted prices included within level 1 that are observable for the asset or liability, either directly (that is, as prices) or indirectly (that is, derived from prices) |
· Level 3 - Inputs for the asset or liability that are not based on observable market data (that is, unobservable inputs). |
For assets and liabilities that are recognized in the financial statements at fair value on a recurring basis, Genmab determines whether transfers have occurred between levels in the hierarchy by re-assessing categorization (based on the lowest level input that is significant to the fair value measurement as a whole) at the end of each reporting period. Any transfers between the different levels are carried out at the end of the reporting period.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 198/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
4.4 – Marketable Securities
|
|
Market value |
Share |
|
Market value |
Share |
(DKK million) |
|
2024 |
% |
|
2023 |
% |
|
|
|
|
|
|
|
USD portfolio |
|
|
|
|
|
|
Corporate bonds |
|
5,082 |
45% |
|
6,039 |
46% |
US government bonds and treasury bills |
|
2,533 |
22% |
|
3,247 |
24% |
Commercial paper |
|
191 |
2% |
|
451 |
3% |
Other |
|
816 |
7% |
|
1,003 |
8% |
Total USD portfolio |
|
8,622 |
76% |
|
10,740 |
81% |
|
|
|
|
|
|
|
DKK portfolio |
|
|
|
|
|
|
Kingdom of Denmark bonds and treasury bills |
|
429 |
4% |
|
419 |
3% |
Danish mortgage-backed securities |
|
1,217 |
11% |
|
1,170 |
9% |
Total DKK portfolio |
|
1,646 |
15% |
|
1,589 |
12% |
|
|
|
|
|
|
|
EUR portfolio |
|
|
|
|
|
|
European government bonds and treasury bills |
|
886 |
8% |
|
858 |
6% |
|
|
|
|
|
|
|
GBP portfolio |
|
|
|
|
|
|
UK government bonds and treasury bills |
|
89 |
1% |
|
81 |
1% |
|
|
|
|
|
|
|
Total portfolio at December 31 |
|
11,243 |
100% |
|
13,268 |
100% |
|
|
|
|
|
|
|
Marketable securities at December 31 |
|
11,243 |
|
|
13,268 |
|
Refer to Note 4.2 for additional information regarding the risks related to our marketable securities.
ACCOUNTING POLICIES
Marketable securities are debt instruments that consist of investments in securities with a maturity of 90 days or greater at the time of acquisition. Measurement of marketable securities depends on the business model for managing the asset and the cash flow characteristics of the asset. Genmab assesses its debt instruments to determine classification based on the following measurement categories:
| ● | Amortized cost: Assets that are held for collection of contractual cash flows, where those cash flows represent solely payments of principal and interest, are measured at amortized cost. Interest income from these financial assets is included in finance income using the effective interest rate method. Any gain or loss arising on derecognition is recognized directly in profit or loss and presented in other financial income or expenses, together with foreign exchange gains and losses. Impairment losses, when material, are presented as a separate line item in the Consolidated Statements of Comprehensive Income. |
| ● | Fair value through other comprehensive income (FVOCI): Assets that are held to achieve an objective by both collecting contractual cash flows as well as selling financial assets and where those cash flows represent solely payments of principal and interest, are measured at FVOCI. Changes in fair value on a debt investment that is subsequently measured at FVOCI are recognized in other comprehensive income. Impairment gains and losses, interest income and foreign exchange gains and losses are recognized in the Consolidated Statements of Comprehensive Income and presented within financial income or expenses in the period in which they arise. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 199/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
| ● | Fair value through profit and loss (FVPL): Assets that do not meet the criteria for amortized cost or FVOCI are measured at FVPL. A gain or loss on a debt investment that is subsequently measured at FVPL is recognized in the Consolidated Statements of Comprehensive Income and presented net within financial income or expenses in the period in which it arises. |
Genmab’s portfolio is managed and evaluated on a fair value basis in accordance with its stated investment guidelines and the information provided internally to management. This business model does not meet the criteria for amortized cost or FVOCI and as a result marketable securities are measured at FVPL. This classification is consistent with the prior year’s classification.
Genmab invests its cash in deposits with major financial institutions, in AAA rated money market funds, Danish mortgage bonds, investment grade rated corporate debt, commercial paper, certificates of deposit, certain types of AAA rated asset backed securities, U.S. Agency bonds, and notes issued by the Danish, European and U.S. governments. The securities can be purchased and sold using established markets.
Transactions are recognized at the trade date.
4.5 – Financial Income and Expenses
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
Financial income: |
|
|
|
|
|
|
Interest and other financial income |
|
995 |
|
982 |
|
324 |
Gain on marketable securities |
|
364 |
|
495 |
|
92 |
Gain on other investments |
|
146 |
|
72 |
|
58 |
Foreign exchange rate gain |
|
2,933 |
|
391 |
|
2,715 |
|
|
|
|
|
|
|
Total financial income |
|
4,438 |
|
1,940 |
|
3,189 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial expenses: |
|
|
|
|
|
|
Interest and other financial expenses |
|
(120) |
|
(70) |
|
(39) |
Loss on marketable securities |
|
(147) |
|
(176) |
|
(453) |
Loss on other investments |
|
(116) |
|
(98) |
|
(355) |
Foreign exchange rate loss |
|
(1,594) |
|
(1,280) |
|
(1,664) |
|
|
|
|
|
|
|
Total financial expenses |
|
(1,977) |
|
(1,624) |
|
(2,511) |
|
|
|
|
|
|
|
Net financial items |
|
2,461 |
|
316 |
|
678 |
|
|
|
|
|
|
|
INTEREST INCOME
Interest income was DKK 995 million in 2024 compared to DKK 982 million in 2023 and DKK 324 million in 2022. The increase of DKK 13 million, or 1% from 2023 to 2024, was primarily driven by the higher cash and cash equivalents and marketable securities in the first half of 2024 compared to 2023, almost entirely offset by lower cash and cash equivalents and marketable securities in the second half of 2024 compared to 2023 as a result of liquidating marketable securities and using cash to purchase ProfoundBio. The increase of 658 million, or 203% from 2022 to 2023 was primarily driven by higher effective interest rates in the U.S., Europe, and Denmark.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 200/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
FOREIGN EXCHANGE RATE GAINS AND LOSSES
Foreign exchange rate gains, net of DKK 1,339 million in 2024 compared to foreign exchange rate loss, net of DKK 889 million in 2023 was primarily driven by foreign exchange movements impacting Genmab’s USD denominated marketable securities and cash and cash equivalents. Foreign exchange rate loss, net of DKK 889 million in 2023 compared to foreign exchange rate gain, net of DKK 1,051 million in 2022 was primarily driven by foreign exchange movements impacting Genmab’s USD denominated marketable securities and cash and cash equivalents; in particular, the USD/DKK foreign exchange rates were as follows for each period:
|
|
December 31, |
|
December 31, |
|
December 31, |
|
|
2024 |
|
2023 |
|
2022 |
USD/DKK Foreign Exchange Rates |
|
7.1429 |
|
6.7447 |
|
6.9722 |
% Increase/(Decrease) |
|
6% |
|
(3)% |
|
6% |
Refer to Note 4.2 for additional information on foreign currency risk.
MARKETABLE SECURITIES GAINS AND LOSSES
Gain on marketable securities, net was DKK 217 million in 2024 compared to gain on marketable securities, net of DKK 319 million in 2023 and loss on marketable securities, net of DKK 361 million in 2022. The decrease in gain of DKK 102 million, or 32% from 2023 to 2024 was primarily driven by the decrease in marketable securities in the first half of 2024 to fund the acquisition of ProfoundBio and share repurchase as well as changing interest rate outlooks for the U.S., primarily in the fourth quarter of 2024. The increase in gain of DKK 680 million, or 188% from 2022 to 2023, was primarily driven by interest rate outlooks for the U.S. and Europe.
OTHER INVESTMENTS
Gains on other investments, net were DKK 30 million in 2024, losses on other investments, net were DKK 26 million in 2023 and DKK 297 million in 2022. The net gains and losses in 2024 and 2023 were primarily driven by changes in fair value of Genmab’s investments in certain strategic investment funds. The losses in 2022 were primarily driven by the change in fair value of Genmab’s investment in common shares of CureVac.
ACCOUNTING POLICIES
Financial income and expenses include interest as well as foreign exchange rate adjustments and gains and losses on marketable securities (designated as FVPL) and realized gains and losses and write-downs of other securities and equity interests.
Interest income is shown separately from gains and losses on marketable securities and other securities and equity interests.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 201/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
4.6 – Share-Based Instruments
Restricted Stock Unit Program
Genmab A/S has established an RSU program (equity-settled share-based payment transactions) as an incentive for Genmab’s employees, members of the Executive Management, and members of the Board of Directors. RSUs granted to Executive Management are performance-based (PSUs).
RSUs are granted by the Board of Directors. RSU grants to members of the Board of Directors and members of the registered Executive Management are subject to the Remuneration Policy adopted at the Annual General Meeting.
See the table below for a summary of key terms of Genmab’s RSU programs:
RSUs Granted in Periods |
||
Key Terms |
December 2019 - February 2021 |
From February 2021 |
Grants |
RSUs are granted at no cost to employees. Number of shares granted is determined based on closing share price on the grant date. |
|
Vesting (Settlement) |
Cliff vesting – RSUs become fully vested on the first banking day of the month following a period of three years from the grant date. The three year cliff vesting also applies to PSUs, while also subject to the degree of fulfilment of the applicable performance criteria. After RSUs vest, the holder receives one share in Genmab A/S for each RSU granted. In jurisdictions in which Genmab as an employer is required to withhold tax and settle with the tax authority on behalf of the employee, Genmab withholds the number of RSUs that are equal to the monetary value of the employee’s tax obligation from the total number of RSUs that otherwise would have been issued to the employee upon vesting (“net settlement”). Genmab A/S may at its sole discretion in extraordinary circumstances choose to make a cash settlement instead of delivering shares. |
|
Leaver |
Leavers – Forfeit all unvested RSUs except when due to retirement, death, serious sickness, or serious injury, in which case granted but not yet vested RSUs shall remain outstanding and will be settled in accordance with their terms. Notwithstanding the above, the December 2020 RSU grant to members of the Board was made subject to pro-rata vesting upon termination of board services. |
Good-Leavers1- May maintain a pro-rata portion of unvested RSUs. Voluntary leavers forfeit unvested RSUs. |
1 – “Good-Leaver” – Dismissal without cause or termination of employment due to Genmab’s material breach of the RSU or Warrant holder’s employment terms, or if the participant is a member of the Board, if the membership of the Board ceases for any other reason than as a result of the participant’s death.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 202/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
2 - “Bad-leaver” - Dismissed for cause or during the employment probationary period.
The RSU program contains anti-dilution provisions if changes occur in Genmab’s share capital prior to the vesting date and provisions to accelerate vesting of RSUs in the event of change of control as defined in the RSU program.
RSU Activity in 2024, 2023 and 2022
|
|
Number of RSUs held by the Board of Directors |
|
Number of RSUs held by the Executive Management |
|
Number of RSUs held by employees |
|
Number of RSUs held by former members of the Executive Management, Board of Directors and employees |
|
Total RSUs |
Weighted Average Fair Value - RSUs Granted - DKK |
Total Fair Value of RSUs Granted - DKK million |
Outstanding at January 1, 2022 |
|
10,965 |
|
89,043 |
|
293,031 |
|
12,952 |
|
405,991 |
|
|
Granted* |
|
4,295 |
|
40,453 |
|
221,000 |
|
6,383 |
|
272,131 |
2,250.18 |
612 |
Settled |
|
(3,420) |
|
(17,165) |
|
(67,945) |
|
(12,847) |
|
(101,377) |
|
|
Transferred |
|
(2,368) |
|
- |
|
(13,749) |
|
16,117 |
|
- |
|
|
Forfeited |
|
(653) |
|
- |
|
(9,195) |
|
(18,759) |
|
(28,607) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2022 |
|
8,819 |
|
112,331 |
|
423,142 |
|
3,846 |
|
548,138 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at January 1, 2023 |
|
8,819 |
|
112,331 |
|
423,142 |
|
3,846 |
|
548,138 |
|
|
Granted* |
|
3,361 |
|
75,854 |
|
208,353 |
|
11,643 |
|
299,211 |
2,619.35 |
784 |
Settled |
|
(1,880) |
|
(35,773) |
|
(54,871) |
|
(9,805) |
|
(102,329) |
|
|
Transferred |
|
- |
|
12,918 |
|
(55,103) |
|
42,185 |
|
- |
|
|
Forfeited |
|
- |
|
(4,357) |
|
(35) |
|
(37,984) |
|
(42,376) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2023 |
|
10,300 |
|
160,973 |
|
521,486 |
|
9,885 |
|
702,644 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at January 1, 2024 |
|
10,300 |
|
160,973 |
|
521,486 |
|
9,885 |
|
702,644 |
|
|
Granted* |
|
7,097 |
|
121,063 |
|
344,068 |
|
14,484 |
|
486,712 |
1,977.87 |
963 |
Settled |
|
(3,367) |
|
(35,320) |
|
(112,663) |
|
(12,465) |
|
(163,815) |
|
|
Transferred |
|
- |
|
(19,924) |
|
(37,348) |
|
57,272 |
|
- |
|
|
Forfeited |
|
- |
|
(11,667) |
|
(71) |
|
(38,178) |
|
(49,916) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2024 |
|
14,030 |
|
215,125 |
|
715,472 |
|
30,998 |
|
975,625 |
|
|
*RSUs held by the Board of Directors include RSUs granted to employee-elected Board Members as employees of Genmab A/S or its subsidiaries.
Refer to Note 5.1 for additional information regarding compensation of the Executive Management and the Board of Directors.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 203/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Warrant Program
Genmab A/S has established a warrant program (equity-settled share-based payment transactions) as an incentive for all the Genmab Group’s employees.
Warrants are granted by the Board of Directors in accordance with authorizations given to it by Genmab A/S’ shareholders.
Following Genmab’s Annual General Meeting on March 29, 2023, members of the registered Executive Management and members of the Board may only be granted RSUs.
See the table below for a summary of key terms of Genmab’s warrant programs:
Warrants Granted in Periods |
|||
Key Terms |
April 2012 - March 2017 |
March 2017 - February 2021 |
From February 2021 |
Grants |
Warrants are granted at no cost to employees. Granted at an exercise price equal to the closing share price on the grant date. |
||
Vesting (Exercisable) |
Annually over 4-year period (25% per year) |
Cliff vesting over 3-year period (100% after 3 years) |
|
Leaver |
Leavers - Forfeit all unvested warrants; however, will be able to exercise pro-rata portion of warrants on a regular schedule in instances where the employment relationship is terminated by Genmab without cause. |
Good-Leavers - Maintain a pro-rata portion of unvested warrants. Bad-Leavers - Forfeit all unvested warrants. Death - Forfeit all unvested warrants. Voluntary leavers forfeit all unvested warrants. |
|
Lapse |
7th anniversary of grant date |
||
The warrant program contains anti-dilution provisions if changes occur in Genmab’s share capital prior to the warrants being exercised and provisions to accelerate vesting of warrants in the event of change of control or certain other extraordinary transactions as defined in the warrant program.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 204/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Warrant Activity in 2024, 2023 and 2022
|
|
Number of warrants held by the Board of Directors |
|
Number of warrants held by the Executive Management |
|
Number of warrants held by employees |
|
Number of warrants held by former members of the Executive Management, Board of Directors and employees |
|
Total warrants |
|
Weighted average exercise price - DKK |
Weighted average share price at exercise date - DKK |
Outstanding Warrants - % of Share Capital |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at January 1, 2022 |
|
10,658 |
|
159,634 |
|
739,000 |
|
59,159 |
|
968,451 |
|
1,501.49 |
|
|
Granted* |
|
1,541 |
|
- |
|
250,005 |
|
7,412 |
|
258,958 |
|
2,244.22 |
|
|
Exercised |
|
(1,558) |
|
(29,836) |
|
(176,948) |
|
(34,775) |
|
(243,117) |
|
1,154.95 |
2,815.33 |
|
Expired |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
|
Forfeited |
|
- |
|
- |
|
(13,670) |
|
(32,654) |
|
(46,324) |
|
2,029.00 |
|
|
Transfers |
|
(8,721) |
|
- |
|
(25,373) |
|
34,094 |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2022 |
|
1,920 |
|
129,798 |
|
773,014 |
|
33,236 |
|
937,968 |
|
1,770.31 |
|
1% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at year end |
|
617 |
|
118,571 |
|
282,296 |
|
32,695 |
|
434,179 |
|
1,265.68 |
|
|
Exercisable warrants in the money at year end |
|
617 |
|
118,571 |
|
282,296 |
|
32,695 |
|
434,179 |
|
1,265.68 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at January 1, 2023 |
|
1,920 |
|
129,798 |
|
773,014 |
|
33,236 |
|
937,968 |
|
1,770.31 |
|
|
Granted* |
|
403 |
|
- |
|
198,001 |
|
10,973 |
|
209,377 |
|
2,632.02 |
|
|
Exercised |
|
- |
|
(11,900) |
|
(74,672) |
|
(26,390) |
|
(112,962) |
|
1,341.40 |
2,657.76 |
|
Expired |
|
- |
|
- |
|
(1,200) |
|
(117) |
|
(1,317) |
|
1,225.18 |
|
|
Forfeited |
|
- |
|
- |
|
(32) |
|
(43,143) |
|
(43,175) |
|
2,274.50 |
|
|
Transfers |
|
- |
|
21,295 |
|
(103,396) |
|
82,101 |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2023 |
|
2,323 |
|
139,193 |
|
791,715 |
|
56,660 |
|
989,891 |
|
1,980.25 |
|
1% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at year end |
|
875 |
|
123,345 |
|
246,635 |
|
45,686 |
|
416,541 |
|
1,416.25 |
|
|
Exercisable warrants in the money at year end |
|
617 |
|
123,345 |
|
192,945 |
|
43,632 |
|
360,539 |
|
1,272.37 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at January 1, 2024 |
|
2,323 |
|
139,193 |
|
791,715 |
|
56,660 |
|
989,891 |
|
1,980.25 |
|
|
Granted* |
|
694 |
|
- |
|
354,255 |
|
14,898 |
|
369,847 |
|
1,974.71 |
|
|
Exercised |
|
- |
|
(63,811) |
|
(31,721) |
|
(17,119) |
|
(112,651) |
|
1,143.29 |
1,877.19 |
|
Expired |
|
- |
|
- |
|
(155) |
|
(132) |
|
(287) |
|
1,032.00 |
|
|
Forfeited |
|
- |
|
- |
|
(73) |
|
(39,564) |
|
(39,637) |
|
2,300.10 |
|
|
Transfers |
|
- |
|
555 |
|
(53,903) |
|
53,348 |
|
- |
|
- |
|
|
|
|
|
|
. |
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2024 |
|
3,017 |
|
75,937 |
|
1,060,118 |
|
68,091 |
|
1,207,163 |
|
2,046.38 |
|
2% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at year end |
|
1,226 |
|
63,405 |
|
321,099 |
|
60,686 |
|
446,416 |
|
1,759.86 |
|
|
Exercisable warrants in the money at year end |
|
- |
|
46,166 |
|
77,669 |
|
25,477 |
|
149,312 |
|
1,131.68 |
|
|
*Warrants held by the Board include warrants granted to employee-elected Board Members as employees of Genmab A/S or its subsidiaries.
Refer to Note 5.1 for additional information regarding compensation of the Executive Management and the Board of Directors.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 205/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Weighted Average Outstanding Warrants at December 31, 2024
As of December 31, 2024, the range of exercise prices for outstanding warrants was DKK 962 to DKK 3,172 with a weighted average remaining contractual life of 4.24 years. As of December 31, 2023, the range of exercise prices for outstanding warrants was DKK 962 to DKK 3,172 with a weighted average remaining contractual life of 4.11 years.
ACCOUNTING POLICIES
SHARE-BASED COMPENSATION EXPENSES
Share-based compensation expense is recognized in the Consolidated Statements of Comprehensive Income based on the estimated fair value of the awards at grant date. Subsequently, the fair value is not remeasured. The expense recognized reflects an estimate of the number of awards expected to vest after taking into consideration an estimate of award forfeitures based on historical experience and is recognized on a straight-line basis over the requisite service period, which is the vesting period. Genmab reassesses its estimate of the number of shares expected to vest periodically.
Management expectations related to the achievement of performance goals associated with performance-based RSU grants are assessed periodically, and that assessment is used to determine whether such grants are expected to vest or if any revision to the current estimate is required. Genmab recognizes the impact of the revised estimate of the number of awards expected to vest, if any, as an adjustment to the income statement over the remaining vesting period. If performance-based milestones related to performance-based RSU grants are not met or not expected to be met, any share-based compensation expense recognized to date associated with grants that are not expected to vest will be reversed.
Share-based compensation expenses represent calculated values of warrants, RSUs and performance-based RSUs granted and do not represent actual cash expenditures. A corresponding amount is recognized in shareholders’ equity as the warrant, RSU and performance-based RSU programs are designated as equity-settled share-based payment transactions.
The fair value of each RSU and performance-based RSU granted during the year are calculated using the closing share price on the grant date. Below is a description on how the fair value of warrants is measured and the estimates involved.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 206/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
MANAGEMENT’S JUDGEMENTS AND ESTIMATES
SHARE-BASED COMPENSATION EXPENSES
The fair value of each warrant granted during the year is calculated using the Black-Scholes pricing model. This pricing model requires the input of subjective assumptions such as:
The expected stock price volatility, which is based upon the historical volatility of Genmab’s stock price; |
The risk-free interest rate, which is determined as the interest rate on Danish government bonds (bullet issues) with an average maturity of five years; |
The expected life of warrants, which is based on vesting terms, expected rate of exercise and life terms in the current warrant program. |
These assumptions can vary over time and can change the fair value of future warrants granted.
Valuation Assumptions for Warrants Granted in 2024, 2023 and 2022
The fair value of each warrant granted during the year is calculated using the Black-Scholes pricing model with the following assumptions:
Weighted average |
2024 |
2023 |
2022 |
Fair value per warrant on grant date |
639.67 |
924.10 |
664.08 |
Share price |
1,974.71 |
2,632.02 |
2,244.22 |
Exercise price |
1,974.71 |
2,632.02 |
2,244.22 |
Expected dividend yield |
0% |
0% |
0% |
Expected stock price volatility |
32.3% |
35.3% |
33.5% |
Risk-free interest rate |
2.26% |
2.48% |
0.15% |
Expected life of warrants |
5 years |
5 years |
5 years |
|
|
|
|
Total Fair Value of Amounts Granted |
2024 |
2023 |
2022 |
Total fair value of warrants granted |
DKK 237 million |
DKK 193 million |
DKK 172 million |
4.7 – Share Capital
SHARE CAPITAL
The share capital comprises the nominal amount of Genmab A/S ordinary shares, each at a nominal value of DKK 1. All shares are fully paid.
As of December 31, 2024, the share capital of Genmab A/S comprised 66,187,186 shares of DKK 1 each with one vote. There are no restrictions related to the transferability of the shares. All shares are regarded as negotiable instruments and do not confer any special rights upon the holder, and no shareholder shall be under an obligation to allow his/her shares to be redeemed.
Genmab’s Board is authorized to increase the share capital by subscription of new shares, issue warrants to subscribe for shares and raise loans against bonds as well as other financial instruments of Genmab A/S as set out in articles 4A-5B of Genmab A/S’ articles of association. Further, Genmab’s share capital is in compliance with the capital requirements of the Danish Companies Act and the rules of Nasdaq Copenhagen.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 207/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
See table below for warrants issued and reissued and warrants available for reissue under active authorizations as of December 31, 2024:
|
March 13, 2024 |
April 13, 2021 |
March 29, 2019 |
|
Authorization |
Authorization |
Authorization |
Warrants issued |
- |
585,692 |
500,000 |
Warrants reissued |
- |
41,143 |
81,684 |
Warrants available for issue |
750,000 |
164,308 |
- |
Warrants available for reissue |
- |
4,550 |
- |
SHARE PREMIUM
The share premium reserve is comprised of the amount received, attributable to shareholders’ equity, in excess of the nominal amount of the shares issued at the parent company’s offerings, reduced by any external expenses directly attributable to the offerings. The share premium reserve can be distributed.
CHANGES IN SHARE CAPITAL DURING 2022 TO 2024
The share capital of DKK 66 million at December 31, 2024, is divided into 66,187,186 shares at a nominal value of DKK 1 each.
|
Number of shares |
|
Share capital |
|
Share Price Ranges1 |
|
|
|
(DKK million) |
|
|
|
|
|
|
|
|
December 31, 2021 |
65,718,456 |
|
65.7 |
|
|
|
|
|
|
|
|
Exercise of warrants |
243,117 |
|
0.3 |
|
DKK 466.20 to DKK 1,615.00 |
|
|
|
|
|
|
December 31, 2022 |
65,961,573 |
|
66.0 |
|
|
|
|
|
|
|
|
Exercise of warrants |
112,962 |
|
0.1 |
|
DKK 815.50 to DKK 1,948.00 |
|
|
|
|
|
|
December 31, 2023 |
66,074,535 |
|
66.1 |
|
|
|
|
|
|
|
|
Exercise of warrants |
112,651 |
|
0.1 |
|
DKK 962.00 to DKK 1,615.00 |
|
|
|
|
|
|
December 31, 2024 |
66,187,186 |
|
66.2 |
|
|
1 - New shares were subscribed at share prices in connection with the exercise of warrants under Genmab’s warrant program.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 208/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
TREASURY SHARES
|
Number of shares |
|
Share capital |
|
Proportion of share capital |
|
Cost |
|
|
|
(DKK million) |
|
% |
|
(DKK million) |
|
|
|
|
|
|
|
|
Shareholding at December 31, 2021 |
288,325 |
|
0.3 |
|
0.4 |
|
550 |
|
|
|
|
|
|
|
|
Purchase of treasury shares |
370,000 |
|
0.4 |
|
0.6 |
|
908 |
Shares used for funding RSU program |
(68,377) |
|
(0.1) |
|
(0.1) |
|
(80) |
|
|
|
|
|
|
|
|
Shareholding at December 31, 2022 |
589,948 |
|
0.6 |
|
0.9 |
|
1,378 |
|
|
|
|
|
|
|
|
Purchase of treasury shares |
220,000 |
|
0.2 |
|
0.3 |
|
564 |
Shares used for funding RSU program |
(65,778) |
|
(0.1) |
|
(0.1) |
|
(126) |
|
|
|
|
|
|
|
|
Shareholding at December 31, 2023 |
744,170 |
|
0.7 |
|
1.1 |
|
1,816 |
|
|
|
|
|
|
|
|
Purchase of treasury shares |
2,011,853 |
|
2.0 |
|
3.0 |
|
3,879 |
Shares used for funding RSU program |
(109,016) |
|
(0.1) |
|
(0.1) |
|
(246) |
|
|
|
|
|
|
|
|
Shareholding at December 31, 2024 |
2,647,007 |
|
2.6 |
|
4.0 |
|
5,449 |
SHARE REPURCHASES
As of December 31, 2024, Genmab’s 2021 and 2023 authorizations have shares available for
repurchase, whereas Genmab’s 2019 authorization has expired. In addition, at Genmab’s Annual General
Meeting on March 13, 2024, a new authorization to acquire treasury shares up to a nominal amount of
DKK 3,500,000 was granted.
|
|
2024 |
|
2023 |
|
2021 |
Number of shares authorized for repurchase1 |
|
3,500,000 |
|
500,000 |
|
500,000 |
Actual shares repurchased under authorization |
|
1,821,853 |
|
— |
|
450,000 |
Shares available for repurchase as of December 31, 2024 |
|
1,678,147 |
|
500,000 |
|
50,000 |
|
|
|
|
|
|
|
1 Nominal value of DKK 3,500,000 for 2024, and DKK 500,000 for 2023 and 2021 Authorizations |
|
|
|
|||
As announced on February 14, 2024, and March 15, 2024, Genmab initiated two share buy-back programs. The purpose of the share buy-back program announced on February 14, 2024, was to honor Genmab’s commitments under the RSU program. The share buy-back program announced on March 15, 2024, was in support of Genmab’s capital allocation strategy. During 2024, Genmab acquired 2,011,853 of its own shares, representing approximately 3.0% of share capital as of December 31, 2023. The total amount paid to acquire the shares, including directly attributable costs, was DKK 3,879 million and was recognized as a deduction to shareholders’ equity. During 2023, Genmab acquired 220,000 of its own shares, representing approximately 0.3% of share capital as of December 31, 2022. The total amount paid to acquire the shares, including directly attributable costs, was DKK 564 million and was recognized as a deduction to shareholders’ equity.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 209/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
These shares are classified as treasury shares and are presented within retained earnings on the Consolidated Balance Sheets as of December 31, 2024.
As of December 31, 2024, 2,647,007 treasury shares were held by Genmab.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 210/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Section 5 – Other Disclosures
This section is comprised of various statutory disclosures or notes that are of secondary importance for the understanding of Genmab’s financials.
5.1 – Remuneration of the Board of Directors and Executive Management
The total remuneration of the Board and Executive Management is as follows:
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
Wages and salaries |
|
101 |
|
71 |
|
55 |
Share-based compensation expenses |
|
157 |
|
100 |
|
70 |
Defined contribution plans |
|
4 |
|
3 |
|
2 |
|
|
|
|
|
|
|
Total |
|
262 |
|
174 |
|
127 |
The remuneration packages for the Board and Executive Management are described in further detail in Genmab’s 2024 Compensation Report. The remuneration packages are denominated in DKK, EUR, or USD. The Compensation Committee of the Board performs an annual review of the remuneration packages. All incentive and variable remuneration is considered and adopted at the Company’s Annual General Meeting.
Share-based compensation is included in the Consolidated Statements of Comprehensive Income and reported in the table above. Share-based compensation expense represents the estimated fair value of the awards at grant date and does not represent actual cash compensation received by the Board Members or Executive Management. Refer to Note 4.6 for additional information regarding Genmab’s share-based compensation programs and accounting policies.
REMUNERATION TO THE BOARD OF DIRECTORS
|
Base Board Fee |
|
Committee |
|
Share-Based Compensation Expenses |
|
Total |
|
||||||||
(DKK million) |
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deirdre P. Connelly |
1.2 |
1.2 |
1.2 |
|
0.5 |
0.5 |
0.5 |
|
1.6 |
1.1 |
0.9 |
|
3.3 |
2.8 |
2.6 |
|
Pernille Erenbjerg |
0.9 |
0.9 |
0.9 |
|
0.4 |
0.4 |
0.4 |
|
1.3 |
0.8 |
0.7 |
|
2.6 |
2.1 |
2.0 |
|
Anders Gersel Pedersen |
0.6 |
0.6 |
0.6 |
|
0.5 |
0.5 |
0.4 |
|
1.0 |
0.6 |
0.5 |
|
2.1 |
1.7 |
1.5 |
|
Paolo Paoletti |
0.6 |
0.6 |
0.6 |
|
0.3 |
0.3 |
0.3 |
|
1.0 |
0.6 |
0.5 |
|
1.9 |
1.5 |
1.4 |
|
Rolf Hoffmann |
0.6 |
0.6 |
0.6 |
|
0.4 |
0.3 |
0.3 |
|
1.0 |
0.6 |
0.5 |
|
2.0 |
1.5 |
1.4 |
|
Elizabeth O’Farrell1 |
0.6 |
0.6 |
0.5 |
|
0.4 |
0.3 |
0.2 |
|
1.6 |
1.0 |
0.6 |
|
2.6 |
1.9 |
1.3 |
|
Mijke Zachariasse2 |
0.6 |
0.6 |
0.6 |
|
- |
- |
- |
|
1.0 |
0.5 |
0.4 |
|
1.6 |
1.1 |
1.0 |
|
Martin Schultz2 |
0.6 |
0.6 |
0.5 |
|
- |
- |
- |
|
0.8 |
0.2 |
- |
|
1.4 |
0.8 |
0.5 |
|
Takahiro Hamatani2 |
0.6 |
0.6 |
0.5 |
|
- |
- |
- |
|
0.8 |
0.2 |
- |
|
1.4 |
0.8 |
0.5 |
|
Peter Storm Kristensen3 |
- |
- |
0.1 |
|
- |
- |
- |
|
- |
- |
0.1 |
|
- |
- |
0.2 |
|
Rima Bawarshi Nassar3 |
- |
- |
0.1 |
|
- |
- |
- |
|
- |
- |
0.1 |
|
- |
- |
0.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
6.3 |
6.3 |
6.2 |
|
2.5 |
2.3 |
2.1 |
|
10.1 |
5.6 |
4.3 |
|
18.9 |
14.2 |
12.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 – Elizabeth O’Farrell was newly elected to the Board at the Annual General Meeting in March 2022.
2 – Employee elected board members were elected at the Annual General Meeting in March 2022.
3 – Peter Storm Kristensen and Rima Bawarshi Nassar stepped down from the Board as employee elected board members at the Annual General Meeting in March 2022.
Refer to the section “Board of Directors” in Management’s Review for additional information regarding the Board.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 211/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
REMUNERATION TO THE EXECUTIVE MANAGEMENT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Base Salary |
|
Defined Contribution Plans |
|
Other Benefits |
|
Annual Cash Bonus |
|
Share-Based Compensation Expenses |
|
Total |
||||||||||||
(DKK million) |
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
|
2024 |
2023 |
2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Jan van de Winkel |
9.7 |
9.2 |
8.6 |
|
1.7 |
1.3 |
1.3 |
|
0.2 |
0.3 |
0.3 |
|
8.8 |
9.2 |
8.6 |
|
34.4 |
24.3 |
22.9 |
|
54.8 |
44.3 |
41.7 |
Anthony Pagano |
4.7 |
4.4 |
4.3 |
|
0.1 |
0.1 |
0.1 |
|
- |
- |
- |
|
2.7 |
2.6 |
2.6 |
|
16.5 |
12.5 |
9.5 |
|
24.0 |
19.6 |
16.5 |
Anthony Mancini3 |
3.0 |
4.9 |
4.7 |
|
0.1 |
0.1 |
0.1 |
|
16.5 |
- |
- |
|
3.0 |
2.9 |
2.8 |
|
28.9 |
13.9 |
11.4 |
|
51.5 |
21.8 |
19.0 |
Judith Klimovsky |
5.3 |
5.0 |
4.9 |
|
0.1 |
0.1 |
0.1 |
|
0.1 |
- |
- |
|
3.0 |
3.0 |
2.9 |
|
19.4 |
13.6 |
14.1 |
|
27.9 |
21.7 |
22.0 |
Tahamtan Ahmadi |
5.0 |
4.7 |
4.6 |
|
0.1 |
0.1 |
0.1 |
|
- |
- |
- |
|
2.8 |
2.9 |
2.8 |
|
17.9 |
12.1 |
7.7 |
|
25.8 |
19.8 |
15.2 |
Birgitte Stephensen1 |
2.9 |
2.6 |
- |
|
0.3 |
0.3 |
- |
|
- |
- |
- |
|
1.7 |
1.5 |
- |
|
8.3 |
5.7 |
- |
|
13.2 |
10.1 |
- |
Christopher Cozic1 |
3.4 |
3.3 |
- |
|
0.1 |
0.1 |
- |
|
- |
- |
- |
|
2.0 |
2.0 |
- |
|
11.1 |
7.8 |
- |
|
16.6 |
13.2 |
- |
Martine van Vugt2 |
2.9 |
2.5 |
- |
|
0.7 |
0.6 |
- |
|
0.1 |
0.1 |
- |
|
1.6 |
1.6 |
- |
|
5.7 |
4.1 |
- |
|
11.0 |
8.9 |
- |
Brad Bailey4 |
3.9 |
- |
- |
|
0.1 |
- |
- |
|
0.6 |
- |
- |
|
2.4 |
- |
- |
|
4.1 |
- |
- |
|
11.1 |
- |
- |
Rayne Waller4 |
1.7 |
- |
- |
|
0.1 |
- |
- |
|
3.8 |
- |
- |
|
0.9 |
- |
- |
|
0.8 |
- |
- |
|
7.3 |
- |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
42.5 |
36.6 |
27.1 |
|
3.4 |
2.7 |
1.7 |
|
21.3 |
0.4 |
0.3 |
|
28.9 |
25.7 |
19.7 |
|
147.1 |
94.0 |
65.6 |
|
243.2 |
159.4 |
114.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 – Birgitte Stephensen and Christopher Cozic were appointed Chief Legal Officer and Chief People Officer, respectively, and members of the Executive Management in March 2022.
2 – Martine van Vugt was appointed Chief Strategy Officer and member of the Executive Management in March 2023.
3 – Anthony Mancini stepped down as Executive Vice President and Chief Operating Officer in September 2024.
4 – Brad Bailey and Rayne Waller were appointed Executive Vice President and Chief Commercial Officer, and Executive Vice President and Chief Technical Operations Officer, respectively, and members of the Executive Management in August 2024.
Jan van de Winkel, President and Chief Executive Officer, and Anthony Pagano, Executive Vice President and Chief Financial Officer, are formally registered as executive managers with the Danish Business Authority.
Refer to the section “Executive Management” in Management’s Review for additional information regarding the Executive Management.
Severance Payments
In the event Genmab terminates the service agreements with any member of the Executive Management team without cause, Genmab is obliged to pay his/her existing salary for one or two years after the end of the one-year notice period. However, in the event of termination by Genmab (unless for cause) or by any member of Executive Management as a result of a change of control of Genmab, Genmab is obliged to pay compensation equal to his/her existing total salary (including benefits) for up to two years in addition to the notice period. The total value of remuneration relating to the notice period for new members of Executive Management cannot exceed two years of remuneration, including all components of the remuneration. In case of the termination of the service agreements of the Executive Management without cause, the total impact on Genmab’s financial position is estimated to be approximately DKK 120 million as of December 31, 2024 (2023: DKK 103 million, 2022: DKK 82 million).
5.2 – Related Party Disclosures
Genmab’s related parties are its Board, Executive Management, and close members of the family of these persons.
Genmab has not granted any loans, guarantees or other commitments to or on behalf of any of the members of the Board or members of the Executive Management.
Other than the remuneration and other transactions relating to the Board and the Executive Management described in Note 5.1, there were no material related party transactions during 2024, 2023 or 2022.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 212/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
5.3 – Commitments
PURCHASE OBLIGATIONS
Genmab has entered into a number of agreements related to research and development activities that contain various obligations. These contractual obligations amounted to approximately DKK 2,879 million as of December 31, 2024 (2023: approximately DKK 3,212 million).
Genmab also has certain contingent commitments under license and collaboration agreements that may become due in the future. As of December 31, 2024, these contingent commitments amounted to approximately DKK 15,433 million (USD 2,161 million) in potential future development, regulatory and commercial milestone payments to third parties under license and collaboration agreements for our preclinical and clinical stage development programs as compared to approximately DKK 15,393 million (USD 2,282 million) as of December 31, 2023. These milestone payments generally become due and payable only upon the achievement of certain development, clinical, regulatory or commercial milestones. The events triggering such payments or obligations have not yet occurred.
In addition to the above obligations, Genmab enters into a variety of agreements and financial commitments in the normal course of business. The terms generally allow Genmab the option to cancel, reschedule and adjust our requirements based on our business needs prior to the delivery of goods or performance of services. It is not possible to predict the maximum potential amount of future payments under these agreements due to the conditional nature of our obligations and the unique facts and circumstances involved in each particular agreement.
5.4 – Fees to Auditors Appointed at the Annual General Meeting
(DKK million) |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
Audit fees |
|
10.6 |
|
6.1 |
|
5.8 |
Audit-related fees |
|
2.3 |
|
3.4 |
|
2.0 |
All other fees |
|
- |
|
0.1 |
|
- |
|
|
|
|
|
|
|
Total |
|
12.9 |
|
9.6 |
|
7.8 |
Genmab changed auditors from PricewaterhouseCoopers Statsautoriseret Revisionspartnerselskab (PwC) to Deloitte Statsautoriseret Revisionspartnerselskab (Deloitte) as Genmab’s new statutory auditor and independent registered public accounting firm for the fiscal year beginning January 1, 2024, replacing PwC. As such, fees in the table above reflect those incurred by Deloitte in 2024 and by PwC in 2023 and 2022
Fees for other services than statutory audit of the financial statements provided by Deloitte amounted to DKK 2.3 million in 2024 (DKK 3.4 million and DKK 2.0 million in 2023 and 2022, respectively provided by PwC). These services primarily include agreed-upon procedures, other assurance assessments and reports, and accounting advice.
5.5 – Acquisition of Businesses
On May 21, 2024 (Acquisition Date), Genmab completed the previously announced acquisition of all of the outstanding shares of ProfoundBio, resulting in ProfoundBio becoming a wholly owned subsidiary of Genmab.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 213/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The acquisition of ProfoundBio gave Genmab worldwide rights to three candidates in clinical development, including ProfoundBio’s lead drug candidate, rinatabart sesutecan (Rina-S). In addition, Genmab acquired ProfoundBio’s novel ADC technology platforms. Rina-S is a clinical-stage, FRαtargeted, TOPO1 ADC, which was in Phase 2 of a Phase 1/2 clinical trial at the time of the acquisition, for the treatment of ovarian cancer and other FRα-expressing solid tumors. Based on the data from the ongoing Phase 1/2 clinical trial Genmab intends to broaden the development plans for Rina-S within ovarian cancer and other FRαexpressing solid tumors. In January 2024, the U.S. FDA granted Fast Track designation to Rina-S for the treatment of patients with FRα-expressing high-grade serous or endometrioid platinum-resistant ovarian cancer.
In addition to payment of USD 1.72 billion (DKK 11.80 billion) for all of the outstanding shares of ProfoundBio, Genmab also made a USD 199 million (DKK 1,368 million) payment to holders of outstanding ProfoundBio equity awards for settlement of such vested and non-vested awards. Of the USD 199 million (DKK 1,368 million) payment, USD 187 million (DKK 1,289 million) related to the portion of awards where the vesting period was completed prior to the Acquisition Date. This portion of the payment was therefore determined to be attributable to the pre-combination period and included in purchase consideration. The remaining USD 11 million (DKK 79 million) payment related to the portion of awards with future vesting conditions, and therefore is attributable to post-combination services. The amount attributable to the post-combination service does not form part of the consideration and was therefore instead recognized as Acquisition and integration related charges in Genmab’s Consolidated Statements of Comprehensive Income.
The acquisition has been accounted for using the acquisition method of accounting which requires that assets acquired and liabilities assumed be recognized at their fair values as of the Acquisition Date and consolidated into Genmab’s Consolidated Balance Sheets. The results of operations for ProfoundBio have been included in Genmab’s consolidated financial statements from the Acquisition Date. A fair value measurement has been performed and the purchase price has been allocated to intangible assets, associated deferred tax liabilities, other assets and liabilities, as well as goodwill being the excess value of the purchase price over the fair value of assets acquired and liabilities assumed (the purchase price allocation). Adjustments may be applied to the purchase price allocation for a period of up to 12 months from the Acquisition Date. During the fourth quarter of 2024, the Company recorded a measurement period adjustment impacting non-current deferred tax liabilities and goodwill that was not material. The total consideration for the acquisition of ProfoundBio is summarized as follows:
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 214/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
|
Total Consideration |
|
|
Amounts in USD millions |
Amounts in DKK millions |
Cash paid for outstanding shares |
1,718 |
11,798 |
Cash for equity compensation attributable to pre-combination service |
187 |
1,289 |
Total consideration |
1,905 |
13,087 |
Cash acquired |
(122) |
(841) |
Cash used for acquisition of business |
1,783 |
12,246 |
The purchase price allocation resulted in the following amounts being allocated to the assets acquired and liabilities assumed at the Acquisition Date based upon their respective fair values summarized below:
|
Amounts Recognized as of the Acquisition Date |
|
|
Amounts in USD millions |
Amounts in DKK millions |
Cash and cash equivalents |
122 |
841 |
Other current assets* |
4 |
29 |
Property and equipment |
6 |
41 |
IPR&D |
1,540 |
10,577 |
Technology platform intangible asset |
181 |
1,243 |
Other non-current assets** |
3 |
21 |
Deferred tax liability |
(292) |
(2,010) |
Other current liabilities*** |
(13) |
(91) |
Total identifiable net assets |
1,551 |
10,651 |
Goodwill |
354 |
2,436 |
Total consideration |
1,905 |
13,087 |
*Includes receivables and other investments
**Includes other investments and right-of-use assets
***Includes other payables, contract liabilities, lease and other liabilities
The carrying values of other current assets, property and equipment, other non-current assets and other current liabilities were determined to approximate their fair values.
The fair value assigned to acquired IPR&D, which was calculated using the multi-period excess earnings method of the income approach, was based on the present value of expected after-tax cash flows attributable to Rina-S, which was in Phase 1/2 testing. The present value of expected after-tax cash flows obtainable from Rina-S and assigned to IPR&D was determined by estimating the after-tax costs to complete development of Rina-S into a commercially viable product, estimating future revenue and ongoing expenses to produce, support and sell Rina-S, on an after-tax basis, and discounting the resulting net cash flows to present value. The revenue and costs projections used were reduced based on the probability that compounds at similar stages of development will become commercially viable products. The rate utilized to discount the net cash flows to their present value reflects the risk associated with the future earnings attributable to the intangible asset. Acquired IPR&D will be accounted for as an intangible asset not yet available for use until regulatory approval in a major market is received or development is discontinued.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 215/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
The fair value of the technology platform intangible asset was calculated using the relief from royalty method of the income approach. This method includes assigning value based on the economic savings from owning, rather than in-licensing, the technology platform intangible asset supported by observable market data for peer companies, then discounting the resulting probability adjusted net post-tax cash flows using a discount rate commensurate with the risk associated with the future income or cost savings attributable to the intangible asset.
The significant assumptions used to estimate the value of the acquired intangible assets include discount rates and certain assumptions that form the basis of future cash flows (such as probabilities of technical and regulatory success, revenue growth rates, operating margins, and royalty rates).
The excess of purchase price over the fair value amounts assigned to identifiable assets acquired and liabilities assumed represents the goodwill amount resulting from the acquisition. The goodwill recorded as part of the acquisition is attributable to the intangible assets that do not qualify for separate recognition at the time of the acquisition, assembled workforce and deferred tax consequences of the IPR&D and technology platform intangible asset recorded for financial statement purposes. Genmab does not expect any portion of this goodwill to be deductible for tax purposes. The goodwill attributable to the acquisition has been recorded as a non-current asset in Genmab’s Consolidated Balance Sheets and is not amortized, but is subject to review for impairment annually. Refer to Note 3.1 for further details related to the accounting for goodwill.
From the Acquisition Date through December 31, 2024, Genmab’s Consolidated Statements of Comprehensive Income include no revenue and the following expenses associated with the acquisition and operations of ProfoundBio (in DKK millions):
Consolidated Statements of Comprehensive Income (DKK million): |
Acquisition Date through December 31, 2024 |
Research and development expenses |
403 |
Selling, general and administrative expenses |
27 |
Acquisition and integration related charges* |
187 |
Total |
617 |
*Acquisition related charges incurred from the Acquisition Date through December 31, 2024, are comprised of payments to holders of outstanding ProfoundBio equity awards related to post-combination services (DKK 79 million). The remaining expenses are integration related charges incurred from the Acquisition Date through December 31, 2024, which are comprised of professional fees incurred to assist with the integration of ProfoundBio into Genmab’s operations post-acquisition. Additionally, prior to the Acquisition Date, Genmab recorded DKK 113 million in Acquisition and integration related charges in Genmab’s Consolidated Statements of Comprehensive Income related to professional due diligence procedures in connection with the acquisition of ProfoundBio. The DKK 113 million of Acquisition and integration related charges incurred prior to the Acquisition Date and the DKK 187 million of Acquisition and integration charges incurred from the Acquisition Date through December 31, 2024 total DKK 300 million through the fourth quarter of 2024.
The following table provides Genmab’s consolidated revenue and net profit for 2024 as if the acquisition of ProfoundBio had occurred on January 1, 2024 (in DKK millions):
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 216/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
(DKK million) |
Twelve Month Period Ended December 31, 2024 |
Revenue |
21,526 |
Net Profit |
7,622 |
The unaudited pro forma information does not necessarily reflect the actual results of operations of the combined entities that would have been achieved, nor are they necessarily indicative of future results of operations. The unaudited pro forma information reflects certain adjustments that were directly attributable to the acquisition of ProfoundBio, including additional amortization adjustments for the fair value of the technology platform intangible asset acquired.
As of December 31, 2024, Cash and cash equivalents in Genmab’s Consolidated Balance Sheets includes USD 30 million (DKK 214 million) of restricted cash balances for funds held in escrow related to the acquisition of ProfoundBio.
ACCOUNTING POLICIES
BUSINESS COMBINATIONS
The acquisition method of accounting is used to account for all acquisitions where the target company meets the definition of a business in accordance with IFRS 3 (Business Combinations). The purchase price for a business is comprised of the fair value of the assets transferred and liabilities owned to the former owners, including option holders, of the acquired business and the fair value of any asset or liability resulting from a contingent consideration arrangement. Any amount of the purchase price which effectively comprises a settlement of a pre-existing relationship is not part of the exchange for the acquiree and is therefore not included in the consideration for the purpose of applying the acquisition method. Settlements of pre-existing relationships are accounted for as separate transactions in accordance with the relevant IFRS standards.
Identifiable assets and liabilities and contingent liabilities assumed are measured at fair value on the date of acquisition by applying relevant valuation methods. Goodwill is recognized as the excess of purchase price over the fair value of net identifiable assets acquired and liabilities assumed. Acquisition-related charges are expensed as incurred and included within Acquisition and integration related charges in the Consolidated Statements of Comprehensive Income.
MANAGEMENT’S JUDGMENTS AND ESTIMATES – OTHER INTANGIBLE ASSETS AND GOODWILL
Fair Value and Impairment Assessment of Other Intangible Assets and Goodwill
The application of the acquisition method involves the use of significant estimates because the identifiable net assets of the acquiree are recognized at their fair values for which observable market prices are typically not available. This is particularly relevant for intangible assets which require use of valuation techniques typically based on estimates of present value of future uncertain cash flows. The significant assumptions used to estimate the value of the acquired intangible assets include discount rates and certain assumptions that form the basis of future cash flows (such as probabilities of technical and regulatory success, revenue growth rates, operating margins, and royalty rates).
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 217/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
5.6 – Collaborations and Licenses
Collaborations
Genmab enters into collaborations with biotechnology and pharmaceutical companies to advance the development and commercialization of Genmab’s product candidates and to supplement its internal pipeline. Genmab seeks collaborations that will allow Genmab to retain significant future participation in product sales through either profit-sharing or royalties paid on net sales. Below is an overview of certain of Genmab’s collaborations that have had, or are expected in the near term to have, a significant impact on financial results.
J&J (Daratumumab/DARZALEX)
In 2012, Genmab entered into a global license, development and commercialization agreement with J&J for daratumumab (marketed for the treatment of certain multiple myeloma indications as DARZALEX for IV administration and as DARZALEX FASPRO in the U.S. and DARZALEX SC in Europe for SC administration). Under this agreement, J&J is fully responsible for developing and commercializing daratumumab, and all costs associated therewith. Genmab receives tiered royalty payments between 12% and 20% based on J&J’s annual net product sales with J&J reducing such royalty payments for Genmab’s share of J&J’s royalty payments made to Halozyme. In addition, the royalties payable by J&J are limited in time and subject to reduction on a country-by-country basis for customary reduction events, including for lack of Genmab patent coverage or upon patent expiration or invalidation in the relevant country and upon the first commercial sale of a biosimilar product in the relevant country (for as long as the biosimilar product remains for sale in that country). Pursuant to the terms of the agreement, J&J’s obligation to pay royalties to us will expire on a country-by-country basis on the later of the date that is 13 years after the first commercial sale of daratumumab in such country or upon the expiration or invalidation of the last-to-expire relevant Genmab patent covering daratumumab in such country. The first U.S., European and Japanese sales of daratumumab occurred in 2015, 2016 and 2017, respectively. We have issued patents and pending patent applications covering daratumumab in numerous jurisdictions, including patents issued in the U.S., Europe and Japan. J&J owns a separate patent portfolio related to the subcutaneous formulation of daratumumab used in DARZALEX FASPRO/DARZALEX SC, but a binding arbitration determined that we are not entitled to royalties based on these separate patents.
Our issued U.S., European and Japanese patents covering daratumumab, after giving effect to issued U.S., European and Japanese patent term extensions and supplementary protection certificates, expire in 2029, 2031 and begin to expire in 2030, respectively. Assuming constant underlying sales of DARZALEX, we expect that our royalties from sales of DARZALEX will begin to decline materially in 2029 following expiration of our U.S. patent rights on daratumumab. Genmab is also eligible to receive certain additional payments in connection with development, regulatory and sales milestones.
In September 2020, Genmab commenced arbitration against J&J with respect to two different provisions of our license agreement for daratumumab, both relating to royalties payable to Genmab on net sales of daratumumab (marketed as DARZALEX for IV administration and as DARZALEX FASPRO in the U.S. and as DARZALEX SC in Europe for SC administration). In April 2022, the arbitral tribunal issued an award in that arbitration denying both of Genmab’s claims. Genmab did not seek review of the award.
On June 9, 2022, Genmab announced the commencement of a second arbitration under the daratumumab license agreement with Janssen with claims for milestone payments for daratumumab SC of USD 405 million and a separate 13-year royalty term for daratumumab SC on a country-by-country basis, from the date of the first commercial sale of daratumumab SC in each such country. This second arbitration followed from the
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 218/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
award in the prior arbitration, where the tribunal ruled in favor of Janssen on the question as to whether Genmab is required to share in Janssen’s royalty payments to Halozyme for its technology used in the daratumumab SC product. The tribunal based its ruling on the finding that DARZALEX FASPRO constitutes a new licensed product under the license agreement.
On April 21, 2023, the arbitral tribunal dismissed Genmab’s claims regarding the second arbitration, on the basis that these claims should have been brought in the first arbitration. One arbitrator dissented Genmab filed a request for review of the award, which was denied on January 23, 2024. As a result, the dismissal of Genmab’s claims in the second arbitration is now final.
Novartis (Ofatumumab/Kesimpta)
Genmab and GlaxoSmithKline (GSK) entered a co-development and collaboration agreement for ofatumumab in 2006. The full rights to ofatumumab were transferred from GSK to Novartis in 2015. Novartis is now fully responsible for the development and commercialization of ofatumumab in all potential indications, including autoimmune diseases. Genmab is entitled to a 10% royalty payment on net sales for non-cancer treatments. Genmab pays a royalty to Medarex based on Kesimpta net sales. Novartis’s obligation to pay royalties to Genmab under this agreement expire on a country-by-country basis only in the event Novartis is no longer selling such product in a given country. The royalties are on a country-by-country basis subject to reduction in case of significant competition by competing products (as defined in the agreement) or a joint committee determination that a license of intellectual property owned by a third-party is necessary for commercialization.
Roche (Teprotumumab/TEPEZZA)
In May 2001, Genmab entered a research collaboration with Roche to develop human antibodies to disease targets identified by Roche. In 2002, this alliance was expanded. Under the agreement, Genmab will receive milestones as well as royalty payments on successful products.
Teprotumumab was initially developed in collaboration between Genmab and Roche, and later investigated under license from Roche by River Vision Development Corporation and subsequently Horizon Therapeutics for ophthalmic use. The product was approved under the brand name TEPEZZA in 2020 by the U.S. FDA for the treatment of TED and in 2024 by Japan's MHLW for the treatment of active or high clinical activity score (CAS) TED. In October 2023, Amgen completed its acquisition of Horizon Therapeutics, including all rights to the development and commercialization of teprotumumab. Under the terms of Genmab’s agreement with Roche, Genmab receives a mid-single digit royalty on net sales of TEPEZZA, on a country-by-country basis, for 10 years following the first commercial sale in such country.
Pfizer (Tisotumab vedotin/Tivdak)
In September 2010, Genmab and Pfizer entered into an ADC collaboration, and a commercial license and collaboration agreement was executed in October 2011. In October 2020, Genmab and Pfizer entered into a Joint Commercialization Agreement where Genmab would co-promote tisotumab vedotin, marketed as Tivdak, in the U.S., and lead commercial operational activities and record sales in Japan, while Pfizer would lead operational commercial activities in the U.S., Europe and China with a 50:50 profit split in those markets. In all other markets, if any, Pfizer would be responsible for commercializing tisotumab vedotin and Genmab would receive royalties based on a percentage of aggregate net sales ranging from the mid-teens to the mid-twenties. Effective January 1, 2025, Genmab and Pfizer agreed to amend the License and Collaboration Agreement and the Joint Commercialization Agreement for Tivdak, assigning Genmab sole responsibility for the development and commercialization of Tivdak for second line plus recurrent or metastatic cervical cancer in Europe and all other regions globally, excluding the United States and the China region. With this
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 219/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
amendment, Genmab will continue to co-promote Tivdak with Pfizer in the U.S. and will record sales for Europe, Japan and rest of world markets (excluding the United States and China regions), once commercialized, and will provide royalties to Pfizer on net sales in the low teens. Pfizer will continue to lead commercialization activities in China, when approved. The companies will continue the practice of joint decision-making on the worldwide development and commercialization strategy for tisotumab vedotin.
AbbVie (Epcoritamab/EPKINLY/TEPKINLY)
On June 10, 2020, Genmab entered into a broad oncology collaboration agreement with AbbVie to jointly develop and commercialize products including epcoritamab, and subsequently into a discovery research collaboration for up to four future differentiated antibody therapeutics for cancer. The companies will share commercial responsibilities for epcoritamab in the U.S. and Japan, with AbbVie responsible for further global commercialization. Genmab is the principal for net sales in the U.S. and Japan and receives tiered royalties between 22% and 26% on remaining net sales outside of these territories, subject to certain royalty reductions. For any product candidates developed as a result of the companies’ discovery research collaboration, Genmab and AbbVie will share responsibilities for global development and commercialization in the U.S. and Japan. Genmab retains the right to co-commercialize these products, along with AbbVie, outside of the U.S. and Japan.
Under the terms of the agreement, Genmab received a USD 750 million (DKK 4,911 million) upfront payment in June 2020 and was initially entitled to receive an aggregate of up to USD 3.15 billion in additional development, regulatory and sales milestone payments for all programs. Included in these potential milestones were up to USD 1.15 billion in payments related to clinical development and commercial success across the three bispecific antibody programs originally included in the agreement.
As a result of two programs being stopped, Genmab is instead contractually entitled to receive an aggregate of up to USD 2.55 billion in additional development, regulatory and sales milestone payments for all programs including an aggregate of up to USD 550 million in payments related to clinical development and commercial success for the one remaining bispecific antibody program, epcoritamab, included in the original agreement. In addition, and also included in these potential milestones, if all four next-generation antibody product candidates developed as a result of the discovery research collaboration are successful, Genmab is eligible to receive up to USD 2.0 billion in option exercise and success-based milestones.
In May 2023, epcoritamab received initial approval from the U.S. FDA and is marketed under the tradename EPKINLY. In September 2023, epcoritamab received initial approval from the EC and the Japan MHLW and is marketed under the tradenames TEPKINLY and EPKINLY, respectively. Genmab is entitled to tiered royalties between 22% and 26% on net sales for epcoritamab outside the U.S. and Japan. Except for these royalty-bearing sales, Genmab will share with AbbVie profits from the sale of licensed products on a 50:50 basis. Genmab and AbbVie split 50:50 the development costs related to epcoritamab, while Genmab will be responsible for 100% of the costs of the discovery research programs up to opt-in.
The total transaction price of USD 750 million (DKK 4,911 million) was allocated to the four performance obligations based on the best estimate of relative stand-alone selling prices. The allocation of the transaction price to the performance obligations is summarized below:
• Delivery of licenses for the three programs: USD 672 million (DKK 4,398 million)
• Co-development activities for the product concepts: USD 78 million (DKK 513 million)
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 220/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
For the license grants, Genmab based the stand-alone selling price on a discounted cash flow approach and considered several factors including, but not limited to, discount rate, development timeline, regulatory risks, estimated market demand and future revenue potential. For co-development activities related to up to four product concepts, a cost-plus margin approach was utilized.
The performance obligations related to the delivery of licenses were completed at a point in time (June 2020) and Genmab recognized USD 672 million (DKK 4,398 million) as license fee revenue in June 2020. After delivery of the licenses, Genmab shares further development and commercial costs equally with AbbVie. AbbVie is not assessed as a customer but as a collaboration partner, and as such this part of the collaboration is not in scope of IFRS 15.
Refer to Note 3.7 for information pertaining to the remaining performance obligation related to co-development activities for the product concepts.
BioNTech
In May 2015, Genmab entered into an agreement with BioNTech to jointly research, develop and commercialize bispecific antibody products using Genmab’s DuoBody technology platform. Under the terms of the agreement, BioNTech will provide proprietary antibodies against key immunomodulatory targets, while Genmab provides proprietary antibodies and access to its DuoBody technology platform. Genmab paid an upfront fee of USD 10 million to BioNTech and an additional fee as certain BioNTech assets were selected for further development. If the companies jointly select any product candidates for clinical development, development costs and product ownership will be shared equally going forward. If one of the companies does not wish to move a product candidate forward, the other company is entitled to continue developing the product on predetermined licensing terms. The agreement also includes provisions which will allow the parties to opt out of joint development at key points. During July 2022, Genmab and BioNTech expanded this collaboration to include the joint research, development and commercialization of monospecific antibody candidates using Genmab’s HexaBody technology platform.
Genmab and BioNTech have three investigational medicines currently in clinical development: DuoBody-CD40x4-1BB (GEN1042/BNT312), HexaBody-OX40 (GEN1055/BNT315) and DuoBody-EpCAMx4-1BB (GEN1059/BNT314). In August 2024, BioNTech opted not to participate in the further development of the acasunlimab (GEN1046) program under the parties’ existing License and Collaboration Agreement for reasons related to BioNTech’s portfolio strategy. Genmab assumed sole responsibility for the continued development and potential commercialization of acasunlimab and the program will be subject to payment of certain milestones and a tiered single-digit royalty on net sales by Genmab to BioNTech.
J&J (DuoBody)
In July 2012, and as amended in December 2013, Genmab entered into a collaboration with J&J to create and develop bispecific antibodies using our DuoBody technology platform.
As of December 31, 2024, three DuoBody-based products created under this collaboration were in active clinical development and had been approved by regulatory authorities: RYBREVANT, TECVAYLI and TALVEY. Under our agreement with J&J, Genmab is eligible to receive milestones and receives royalties between 8% and 10% on net sales of RYBREVANT, a mid-single digit royalty on net sales of TECVAYLI, and a mid-single digit royalty on net sales of TALVEY, all of which are subject to a reduction of such royalty payment in countries and territories where there are no relevant patents (as defined in the agreement), among other reductions. Pursuant to the terms of the DuoBody agreement, J&J’s obligation to pay these royalties will expire on a country-by-country and licensed product-by-licensed product basis on the later of the
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 221/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
date that is 10 years after the first sale of each licensed product in such country or upon the expiration of the last-to-expire relevant patent (as defined in the agreement) covering the licensed product in such country. Genmab pays a royalty to Medarex based on RYBREVANT net sales.
5.7 – Contingencies
Legal Contingency
In 2024, Chugai filed a lawsuit in the Tokyo District Court, Japan against AbbVie’s and Genmab’s subsidiaries in Japan asserting that their activities with EPKINLY (epcoritamab) in Japan infringe two Japanese patents held by Chugai, JP6278598 and JP6773929. Chugai is claiming damages and injunctive relief.
Genmab and AbbVie believe that the two Japanese patents are invalid and not infringed and intend to vigorously defend against the lawsuit, and thus no provision has been recognized related to this matter.
Financial Guarantees
As of December 31, 2024 and December 31, 2023, Genmab has financial bank guarantees of DKK 16 million issued as security for lease obligations under certain lease agreements. The likelihood of a claim under the guarantees has been assessed to be remote due to Genmab’ strong financial position and history of fulfilling lease payments. Accordingly, no provision has been recognized related to this matter.
5.8 – Subsequent Events
Management has determined it is appropriate to change the functional currency of the Genmab A/S legal entity from DKK to USD effective January 1, 2025. This determination was made based on the growing number and significance of the underlying USD transactions, triggered by the commercialization of EPKINLY. Effective for the first quarter of 2025, the consolidated financial statements will also be presented in USD, which will be both the functional and presentation currency of the parent company.
No other events have occurred subsequent to the balance sheet date that could significantly affect the financial statements as of December 31, 2024.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 222/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Table of Contents
224 |
|
224 |
|
225 |
|
226 |
|
227 |
|
|
|
NOTES |
|
228 |
|
229 |
|
230 |
|
231 |
|
232 |
|
233 |
|
234 |
|
235 |
|
235 |
|
235 |
|
236 |
|
236 |
|
237 |
|
237 |
|
15 Remuneration of the Board of Directors and Executive Management |
238 |
238 |
|
239 |
|
239 |
|
240 |
|
|
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 223/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Financial Statements of the Parent Company
Income Statements
(DKK million) |
|
Note |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
|
|
Revenue |
|
2 |
|
22,167 |
|
17,126 |
|
|
|
|
|
|
|
|
|
Cost of product sales |
|
|
|
(502) |
|
(86) |
|
Research and development expenses |
|
3, 5, 6 |
|
(10,358) |
|
(8,826) |
|
Selling, general and administrative expenses |
|
3, 6 |
|
(1,949) |
|
(2,521) |
|
Integration related charges |
|
|
|
(30) |
|
- |
|
Total costs and operating expenses |
|
|
|
(12,839) |
|
(11,433) |
|
|
|
|
|
|
|
|
|
Operating profit |
|
|
|
9,328 |
|
5,693 |
|
|
|
|
|
|
|
|
|
Financial income |
|
14, 17 |
|
17,404 |
|
2,199 |
|
Financial expenses |
|
14, 17 |
|
(12,239) |
|
(1,871) |
|
|
|
|
|
|
|
|
|
Net profit before tax |
|
|
|
14,493 |
|
6,021 |
|
|
|
|
|
|
|
|
|
Corporate tax |
|
4 |
|
(2,626) |
|
(1,277) |
|
|
|
|
|
|
|
|
|
Net profit |
|
|
|
11,867 |
|
4,744 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 224/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Balance Sheets
|
|
|
December 31, |
|
December 31, |
|
(DKK million) |
Note |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
|
ASSETS |
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets |
5 |
|
13,369 |
|
378 |
|
Property and equipment |
6 |
|
109 |
|
129 |
|
Right-of-use assets |
7 |
|
239 |
|
232 |
|
Investments in subsidiaries |
17 |
|
6,114 |
|
3,308 |
|
Receivables |
10 |
|
22 |
|
49 |
|
Deferred tax assets |
4 |
|
- |
|
198 |
|
Other investments |
8 |
|
179 |
|
87 |
|
|
|
|
|
|
|
|
Total non-current assets |
|
|
20,032 |
|
4,381 |
|
|
|
|
|
|
|
|
Corporate tax receivable |
4 |
|
101 |
|
- |
|
Inventories |
9 |
|
10 |
|
31 |
|
Receivables |
10 |
|
5,726 |
|
4,528 |
|
Receivables from subsidiaries |
10 |
|
964 |
|
650 |
|
Marketable securities |
13 |
|
11,243 |
|
13,268 |
|
Cash and cash equivalents |
|
|
8,993 |
|
14,467 |
|
|
|
|
|
|
|
|
Total current assets |
|
|
27,037 |
|
32,944 |
|
|
|
|
|
|
|
|
Total assets |
|
|
47,069 |
|
37,325 |
|
|
|
|
|
|
|
|
SHAREHOLDERS' EQUITY AND LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
Share capital |
|
|
66 |
|
66 |
|
Share premium |
|
|
12,590 |
|
12,461 |
|
Retained earnings |
|
|
28,940 |
|
20,347 |
|
|
|
|
|
|
|
|
Total shareholders' equity |
|
|
41,596 |
|
32,874 |
|
|
|
|
|
|
|
|
Lease liabilities |
7 |
|
235 |
|
227 |
|
Deferred revenue |
11 |
|
480 |
|
480 |
|
Deferred tax liabilities |
4 |
|
2,359 |
|
- |
|
Other payables |
12 |
|
20 |
|
20 |
|
|
|
|
|
|
|
|
Total non-current liabilities |
|
|
3,094 |
|
727 |
|
|
|
|
|
|
|
|
Corporate tax payable |
4 |
|
- |
|
45 |
|
Payable to subsidiaries |
12 |
|
887 |
|
2,525 |
|
Lease liabilities |
7 |
|
16 |
|
19 |
|
Deferred revenue |
11 |
|
24 |
|
33 |
|
Other payables |
12 |
|
1,452 |
|
1,102 |
|
|
|
|
|
|
|
|
Total current liabilities |
|
|
2,379 |
|
3,724 |
|
|
|
|
|
|
|
|
Total liabilities |
|
|
5,473 |
|
4,451 |
|
|
|
|
|
|
|
|
Total shareholders' equity and liabilities |
|
|
47,069 |
|
37,325 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 225/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Statements of Cash Flows
(DKK million) |
Note |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net profit before tax |
|
|
14,493 |
|
6,021 |
|
|
|
|
|
|
|
|
Financial Income |
14 |
|
(17,404) |
|
(2,199) |
|
Financial Expenses |
14 |
|
12,239 |
|
1,871 |
|
Adjustment for non-cash transactions |
|
|
|
|
|
|
Share-based compensation expense |
|
|
88 |
|
84 |
|
Depreciation |
|
|
40 |
|
32 |
|
Amortization |
|
|
36 |
|
29 |
|
Impairment charges |
|
|
282 |
|
- |
|
Change in operating assets and liabilities |
|
|
|
|
|
|
Receivables |
|
|
(1,173) |
|
1,062 |
|
Inventories |
|
|
22 |
|
(31) |
|
Other Payables |
|
|
352 |
|
207 |
|
|
|
|
|
|
|
|
Cash provided by operating activities before financial items |
|
|
8,975 |
|
7,076 |
|
|
|
|
|
|
|
|
Interest received |
|
|
905 |
|
888 |
|
Interest elements of lease payments |
7 |
|
(10) |
|
(9) |
|
Interest paid |
|
|
- |
|
(1) |
|
Corporate taxes (paid)/received |
|
|
(317) |
|
(1,056) |
|
|
|
|
|
|
|
|
Net cash provided by operating activities |
|
|
9,553 |
|
6,898 |
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
Transactions with subsidiaries |
|
|
(14,198) |
|
868 |
|
Investment in intangible assets |
5 |
|
(198) |
|
(82) |
|
Investment in tangible assets |
6 |
|
(6) |
|
(117) |
|
Marketable securities bought |
|
|
(8,581) |
|
(10,876) |
|
Marketable securities sold |
|
|
11,279 |
|
10,001 |
|
Other investments bought |
|
|
(42) |
|
(30) |
|
|
|
|
|
|
|
|
Net cash (used in) investing activities |
|
|
(11,746) |
|
(236) |
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
Warrants exercised |
|
|
129 |
|
152 |
|
Principal elements of lease payments |
7 |
|
(13) |
|
(15) |
|
Purchase of treasury shares |
|
|
(3,879) |
|
(564) |
|
Payment of withholding taxes on behalf of employees on net settled RSUs |
|
|
(109) |
|
(103) |
|
|
|
|
|
|
|
|
Net cash (used in) financing activities |
|
|
(3,872) |
|
(530) |
|
|
|
|
|
|
|
|
Changes in cash and cash equivalents |
|
|
(6,065) |
|
6,132 |
|
Cash and cash equivalents at the beginning of the period |
|
|
14,467 |
|
8,830 |
|
Exchange rate adjustments |
|
|
591 |
|
(495) |
|
|
|
|
|
|
|
|
Cash and cash equivalents at the end of the period |
|
|
8,993 |
|
14,467 |
|
|
|
|
|
|
|
|
Cash and cash equivalents include: |
|
|
|
|
|
|
Bank deposits |
|
|
8,911 |
|
13,114 |
|
Short-term marketable securities |
|
|
82 |
|
1,353 |
|
Cash and cash equivalents at the end of the period |
|
|
8,993 |
|
14,467 |
|
|
|
|
|
|
|
|
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 226/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
|
|
|
|
|
|
|
Statements of Changes in Equity
(DKK million) |
|
Share capital |
|
Share premium |
|
Retained earnings |
|
Shareholders' equity |
|
|
|
|
|
|
|
|
|
Balance at December 31, 2022 |
|
66 |
|
12,309 |
|
15,741 |
|
28,116 |
|
|
|
|
|
|
|
|
|
Net profit |
|
- |
|
- |
|
4,744 |
|
4,744 |
Exercise of warrants |
|
- |
|
152 |
|
- |
|
152 |
Purchase of treasury shares |
|
- |
|
- |
|
(564) |
|
(564) |
Share-based compensation expenses |
|
- |
|
- |
|
586 |
|
586 |
Withholding taxes on behalf of employees on net settled RSUs |
|
- |
|
- |
|
(103) |
|
(103) |
Tax on items recognized directly in equity |
|
- |
|
- |
|
(57) |
|
(57) |
|
|
|
|
|
|
|
|
|
Balance at December 31, 2023 |
|
66 |
|
12,461 |
|
20,347 |
|
32,874 |
|
|
|
|
|
|
|
|
|
Net profit |
|
- |
|
- |
|
11,867 |
|
11,867 |
Exercise of warrants |
|
- |
|
129 |
|
- |
|
129 |
Purchase of treasury shares |
|
- |
|
- |
|
(3,879) |
|
(3,879) |
Share-based compensation expenses |
|
- |
|
- |
|
721 |
|
721 |
Withholding taxes on behalf of employees on net settled RSUs |
|
- |
|
- |
|
(109) |
|
(109) |
Tax on items recognized directly in equity |
|
- |
|
- |
|
(7) |
|
(7) |
|
|
|
|
|
|
|
|
|
Balance at December 31, 2024 |
|
66 |
|
12,590 |
|
28,940 |
|
41,596 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DISTRIBUTION OF THE YEAR’S PROFIT
The Board proposes that the parent company’s 2024 net profit of DKK 11,867 million (2023: net profit of DKK 4,744 million) be carried forward to next year by transfer to retained earnings.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 227/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
1 – Accounting Policies
The financial statements of the parent company have been prepared in accordance with the IFRS Accounting Standards as issued by the International Accounting Standards Board (IASB) and in accordance with IFRS as endorsed by the EU and further disclosure requirements for listed companies in Denmark.
A number of new or amended standards became applicable for the current reporting period. Genmab A/S did not have to change its accounting policies as a result of the adoption of these standards.
Refer to Note 1.2 in the consolidated financial statements for a description of new accounting policies and disclosures of the Group.
Refer to Note 1.3 in the consolidated financial statements for a description of management’s judgements and estimates under IFRS.
Refer to Note 1.4 in the consolidated financial statements for additional information regarding the immaterial reclassifications and revisions of the Group financial statements.
Supplementary Accounting Policies for the Parent Company
Investments in Subsidiaries
The cost method is used for measuring the investments in subsidiaries. Under the cost method, investments in subsidiaries are measured at historical cost. Equity interests in foreign currencies are translated to the reporting currency by use of historical exchange rates prevailing at the time of investment.
Additions to the carrying value of investment in subsidiaries include capital contributions made by the parent and share-based payment transactions related to employees of the respective subsidiaries based on where the employee has rendered service.
Distributions from the investment are recognized as income when declared, if any. If the distribution exceeds the current period income or if circumstances or changes in Genmab’s operations indicate that the carrying amount of the subsidiary may not be recoverable, the carrying amount is tested for impairment. Where the recoverable amount of the investments is lower than cost, the investments are written down to this lower value.
Refer to Note 1.1 in the consolidated financial statements for a description of the accounting policies of the Group.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 228/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
2 – Revenue
|
|
|
|
|
|
|
(DKK million) |
|
|
|
2024 |
|
2023 |
|
|
|
|
|
|
|
Revenue by type: |
|
|
|
|
|
|
Royalties |
|
|
|
17,352 |
|
13,705 |
Reimbursement revenue - External |
|
|
|
996 |
|
864 |
Reimbursement revenue - Intercompany |
|
|
|
1,140 |
|
937 |
Milestone revenue |
|
|
|
1,000 |
|
1,177 |
Collaboration revenue |
|
|
|
433 |
|
307 |
License revenue |
|
|
|
2 |
|
- |
Net product sales - Intercompany |
|
|
|
1,244 |
|
136 |
|
|
|
|
|
|
|
Total |
|
|
|
22,167 |
|
17,126 |
|
|
|
|
|
|
|
Revenue by collaboration partner: |
|
|
|
|
|
|
Janssen |
|
|
|
14,422 |
|
11,949 |
AbbVie |
|
|
|
394 |
|
732 |
Roche |
|
|
|
741 |
|
704 |
Novartis |
|
|
|
2,822 |
|
1,511 |
BioNTech |
|
|
|
869 |
|
784 |
Pfizer1 |
|
|
|
533 |
|
373 |
Other |
|
|
|
2 |
|
- |
|
|
|
|
|
|
|
Total2 |
|
|
|
19,783 |
|
16,053 |
|
|
|
|
|
|
|
Royalties by product: |
|
|
|
|
|
|
DARZALEX |
|
|
|
13,922 |
|
11,265 |
Kesimpta |
|
|
|
2,222 |
|
1,494 |
TEPEZZA |
|
|
|
737 |
|
704 |
Other3 |
|
|
|
471 |
|
242 |
|
|
|
|
|
|
|
Total |
|
|
|
17,352 |
|
13,705 |
1Pzifer acquired Seagen in December 2023
2 Excludes Genmab’s intercompany revenue
3Other consist of royalties from net sales of RYBREVANT, TECVAYLI, TALVEY and TEPKINLY
Refer to Note 2.1 in the consolidated financial statements for additional information regarding revenue of the Group.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 229/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
3 – Staff Costs
|
|
|
|
|
|
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
Wages and salaries |
|
566 |
|
500 |
|
Share-based compensation |
|
88 |
|
84 |
|
Defined contribution plans |
|
48 |
|
39 |
|
Other social security costs |
|
2 |
|
9 |
|
|
|
|
|
|
|
Total |
|
704 |
|
632 |
|
|
|
|
|
|
|
Staff costs are included in the income statement as follows: |
|
|
|
|
|
Research and development expenses |
|
539 |
|
501 |
|
Selling, general and administrative expenses |
|
165 |
|
131 |
|
|
|
|
|
|
|
Total |
|
704 |
|
632 |
|
|
|
|
|
|
|
Average number of FTE |
|
492 |
|
440 |
|
Number of FTE at year-end |
|
519 |
|
465 |
|
Refer to Note 2.3 in the consolidated financial statements for additional information regarding staff costs of the Group.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 230/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
4 – Corporate and Deferred Tax
TAXATION – INCOME STATEMENT & SHAREHOLDERS’ EQUITY
(DKK million) |
2024 |
|
2023 |
|
|
|
|
|
|
Current tax: |
|
|
|
|
Current tax on profit |
82 |
|
1,288 |
|
Deferred taxes |
2,544 |
|
(11) |
|
|
|
|
|
|
Total tax for the period in the income statement |
2,626 |
|
1,277 |
|
|
|
|
|
|
A reconciliation of Genmab's effective tax rate relative to the Danish statutory tax rate is as follows: |
|
|
|
|
(DKK million) |
2024 |
|
2023 |
|
|
|
|
|
|
Net profit before tax |
14,493 |
|
6,021 |
|
|
|
|
|
|
Tax at the Danish statutory corporation tax rate of 22% for all periods |
3,188 |
|
1,325 |
|
|
|
|
|
|
Tax effect of: |
|
|
|
|
Net of non-taxable income over non-deductible expenses |
(659) |
|
(52) |
|
Other current and deferred taxes adjustments |
97 |
|
4 |
|
Total tax effect |
(562) |
|
(48) |
|
|
|
|
|
|
Total tax for the period in the income statement |
2,626 |
|
1,277 |
|
Total tax for the period in shareholders' equity |
7 |
|
57 |
|
Effective Tax Rate |
18.1% |
|
21.2% |
|
TAXATION – BALANCE SHEET
Significant components of the deferred tax (liabilities) assets are as follows:
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
Share-based instruments |
|
39 |
|
37 |
Deferred revenue |
|
120 |
|
113 |
Intangible Assets |
|
(2,874) |
|
- |
Other temporary differences |
|
356 |
|
48 |
|
|
|
|
|
Total deferred tax (liabilities) assets |
|
(2,359) |
|
198 |
Refer to Note 2.4 in the consolidated financial statements for additional information regarding corporate and deferred tax of the Group.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 231/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
5 – Intangible Assets
(DKK million) |
|
Licenses and Patents |
Technology Platform |
Acquired IPR&D |
Total Intangible Assets |
|
|
|
|
|
|
|
|
2024 |
|
|
|
|
|
|
Cost at the beginning of the year |
|
1,093 |
- |
- |
1,093 |
|
Additions during the year |
|
244 |
1,237 |
11,789 |
13,270 |
|
Cost at the end of the year |
|
1,337 |
1,237 |
11,789 |
14,363 |
|
Amortization and impairment losses at the beginning of the year |
|
715 |
- |
- |
715 |
|
Amortization for the year |
|
33 |
3 |
- |
36 |
|
Impairment losses for the year |
|
243 |
- |
- |
243 |
|
Amortization and impairment losses at the end of the year |
|
991 |
3 |
- |
994 |
|
Carrying amount at the end of the year |
|
346 |
1,234 |
11,789 |
13,369 |
|
|
|
|
|
|
|
|
2023 |
|
|
|
|
|
|
Cost at the beginning of the year |
|
1,011 |
- |
- |
1,011 |
|
Additions during the year |
|
82 |
- |
- |
82 |
|
Cost at the end of the year |
|
1,093 |
- |
- |
1,093 |
|
Amortization and impairment losses at the beginning of the year |
|
654 |
- |
- |
654 |
|
Amortization for the year |
|
61 |
- |
- |
61 |
|
Amortization and impairment losses at the end of the year |
|
715 |
- |
- |
715 |
|
Carrying amount at the end of the year |
|
378 |
- |
- |
378 |
|
Parent Company intangible assets include IPR&D, a technology platform asset and licenses and rights primarily to gain access to targets and technologies identified by third parties as well as subsidiaries.
Refer to Note 3.1 in the consolidated financial statements for additional information regarding intangible assets of the Group. Refer to Note 17 in the parent financial statements for additional information regarding the intangible assets and goodwill acquired through the ProfoundBio acquisition.
Intangible Assets
The increase in the gross carrying value of intangible assets during 2024 was primarily due to the addition of approximately DKK 11,789 million of IPR&D and DKK 1,237 million of a technology platform asset from the ProfoundBio acquisition. The technology platform asset is being amortized over its estimated useful life of 15 years. These intellectual property rights were transferred from ProfoundBio US to Genmab A/S during the fourth quarter of 2024.
Impairment expenses related to licenses and patents were DKK 243 million in 2024 and were not material in 2023. Impairment expenses were recorded in Research and development expenses in the Parent Company Income Statements.
Amortization expense was DKK 36 million and DKK 61 million for 2024 and 2023, respectively, which was recorded in Research and development expenses in the Income Statements of the Parent Company.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 232/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
6 – Property and Equipment
(DKK million) |
|
Leasehold improvements |
|
Equipment, furniture and fixtures |
|
Assets under construction |
|
Total property and equipment |
|
|
|
|
|
|
|
|
|
2024 |
|
|
|
|
|
|
|
|
Cost at January 1 |
|
78 |
|
82 |
|
- |
|
160 |
Additions for the year |
|
- |
|
2 |
|
2 |
|
4 |
Transfers between the classes |
|
- |
|
1 |
|
- |
|
1 |
Disposals for the year |
|
(4) |
|
(9) |
|
(2) |
|
(15) |
|
|
|
|
|
|
|
|
|
Cost at December 31 |
|
74 |
|
76 |
|
- |
|
150 |
|
|
|
|
|
|
|
|
|
Accumulated depreciation and impairment at January 1 |
|
(7) |
|
(24) |
|
- |
|
(31) |
Depreciation for the year |
|
(4) |
|
(19) |
|
- |
|
(23) |
Disposals for the year |
|
4 |
|
9 |
|
- |
|
13 |
|
|
|
|
|
|
|
|
|
Accumulated depreciation and impairment at December 31 |
|
(7) |
|
(34) |
|
- |
|
(41) |
|
|
|
|
|
|
|
|
|
Carrying amount at December 31 |
|
67 |
|
42 |
|
- |
|
109 |
|
|
|
|
|
|
|
|
|
(DKK million) |
|
Leasehold improvements |
|
Equipment, furniture and fixtures |
|
Assets under construction |
|
Total property and equipment |
|
|
|
|
|
|
|
|
|
2023 |
|
|
|
|
|
|
|
|
Cost at January 1 |
|
4 |
|
24 |
|
17 |
|
45 |
Additions for the year |
|
5 |
|
10 |
|
100 |
|
115 |
Transfers between the classes |
|
69 |
|
48 |
|
(117) |
|
- |
Disposals for the year |
|
- |
|
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
Cost at December 31 |
|
78 |
|
82 |
|
- |
|
160 |
|
|
|
|
|
|
|
|
|
Accumulated depreciation and impairment at January 1 |
|
(4) |
|
(15) |
|
- |
|
(19) |
Depreciation for the year |
|
(3) |
|
(9) |
|
- |
|
(12) |
Disposals for the year |
|
- |
|
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
Accumulated depreciation and impairment at December 31 |
|
(7) |
|
(24) |
|
- |
|
(31) |
|
|
|
|
|
|
|
|
|
Carrying amount at December 31 |
|
71 |
|
58 |
|
- |
|
129 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(DKK million) |
|
|
|
|
|
2024 |
|
2023 |
|
|
|
|
|
|
|
|
|
Depreciation and impairment included in the income statement as follows: |
|
|
|
|
|
|
|
|
Research and development expenses |
|
|
|
|
|
18 |
|
6 |
Selling, general and administrative expenses |
|
|
|
|
|
5 |
|
6 |
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
|
23 |
|
12 |
Refer to Note 3.2 in the consolidated financial statements for additional information regarding property and equipment of the Group.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 233/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
7 – Leases
The parent company has entered into lease agreements with respect to office and laboratory space.
The leases are non-cancellable over various periods through 2038.
|
|
|
|
|
|
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
Right-of-use assets |
|
|
|
|
|
Balance at January 1 |
|
232 |
|
9 |
|
Additions to right-of-use assets1 |
|
24 |
|
242 |
|
Depreciation charge for the year |
|
(17) |
|
(19) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31 |
|
239 |
|
232 |
|
|
|
|
|
|
|
Lease liabilities |
|
|
|
|
|
Current |
|
16 |
|
19 |
|
Non-current |
|
235 |
|
227 |
|
|
|
|
|
|
|
Total at December 31 |
|
251 |
|
246 |
|
(1) Additions to right-of-use assets also includes modifications to existing leases and adjustments to the provisions for contractual restoration obligations related to leases of Genmab offices. |
|
|
|
|
|
|
|
|
|
|
|
Cash outflow for lease payments |
|
23 |
|
24 |
|
|
|
|
|
|
|
Variable lease payments, lease interest expense, and low-value assets are immaterial.
Future minimum payments under leases are as follows:
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
Payment due |
|
|
|
|
|
Less than 1 year |
|
27 |
|
23 |
|
1 to 3 years |
|
53 |
|
45 |
|
More than 3 years but less than 5 years |
|
54 |
|
45 |
|
More than 5 years |
|
186 |
|
202 |
|
|
|
|
|
|
|
Total at December 31 |
|
320 |
|
315 |
|
Refer to Note 3.3 in the consolidated financial statements for additional information regarding leases of the Group.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 234/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
8 – Other Investments
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
Fund Investments |
|
165 |
|
87 |
Privately held equity securities |
|
14 |
|
- |
Total at December 31 |
|
179 |
|
87 |
Refer to Note 3.4 to the consolidated financial statements for additional information on other investments of the Group.
9 – Inventories
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
Raw materials |
|
4 |
|
14 |
Work in progress |
|
- |
|
- |
Finished goods |
|
6 |
|
19 |
Total inventories (gross) at December 31 |
|
10 |
|
33 |
|
|
|
|
|
Allowances at year end |
|
- |
|
(2) |
|
|
|
|
|
Total inventories (net) at December 31 |
|
10 |
|
31 |
Refer to Note 3.5 in the consolidated financial statements for additional information regarding inventories of the Group.
10 – Receivables
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
Receivables related to collaboration agreements |
|
5,434 |
|
4,148 |
Prepayments |
|
107 |
|
121 |
Receivables from subsidiaries |
|
964 |
|
650 |
Interest receivables |
|
135 |
|
149 |
Other receivables |
|
72 |
|
159 |
|
|
|
|
|
Total at December 31 |
|
6,712 |
|
5,227 |
|
|
|
|
|
Non-current receivables |
|
22 |
|
49 |
Current receivables |
|
6,690 |
|
5,178 |
|
|
|
|
|
Total at December 31 |
|
6,712 |
|
5,227 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 235/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Refer to Note 3.6 in the consolidated financial statements for additional information regarding receivables of the Group.
11 – Contract Liabilities
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
Contract liabilities at January 1 |
|
513 |
|
513 |
Customer payment received |
|
- |
|
- |
Revenue recognized during the year |
|
(9) |
|
- |
|
|
|
|
|
Total at December 31 |
|
504 |
|
513 |
|
|
|
|
|
Non-current contract liabilities |
|
480 |
|
480 |
Current contract liabilities |
|
24 |
|
33 |
|
|
|
|
|
Total at December 31 |
|
504 |
|
513 |
Refer to Note 3.7 in the consolidated financial statements for additional information regarding contract liabilities of the Group.
12 – Other Payables
(DKK million) |
|
|
2024 |
|
2023 |
|
|
|
|
|
|
Liabilities related to collaboration agreements |
|
|
126 |
|
47 |
Staff cost liabilities |
|
|
91 |
|
106 |
Accounts payable |
|
|
187 |
|
107 |
Payable to subsidiaries |
|
|
887 |
|
2,525 |
Other liabilities |
|
|
1,068 |
|
862 |
|
|
|
|
|
|
Total at December 31 |
|
|
2,359 |
|
3,647 |
|
|
|
|
|
|
Non-current other payables |
|
|
20 |
|
20 |
Current other payables |
|
|
2,339 |
|
3,627 |
|
|
|
|
|
|
Total at December 31 |
|
|
2,359 |
|
3,647 |
Refer to Note 3.8 in the consolidated financial statements for additional information regarding other payables of the Group.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 236/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
13 – Marketable Securities
Refer to Note 4.4 in the consolidated financial statements for additional information on marketable securities of the Group.
14 – Financial Income and Expenses
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
Financial income: |
|
|
|
|
|
Dividend income from subsidiaries |
|
13,026 |
|
- |
|
Interest and other financial income |
|
964 |
|
962 |
|
Interest from subsidiaries |
|
6 |
|
1 |
|
Gain on marketable securities |
|
364 |
|
495 |
|
Gain on other investments |
|
121 |
|
6 |
|
Foreign exchange rate gain |
|
2,923 |
|
735 |
|
|
|
|
|
|
|
Total financial income |
|
17,404 |
|
2,199 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial expenses: |
|
|
|
|
|
Impairment of investment in subsidiaries |
|
(10,402) |
|
- |
|
Interest and other financial expenses |
|
(95) |
|
(55) |
|
Interest to subsidiaries |
|
(7) |
|
(10) |
|
Loss on marketable securities |
|
(147) |
|
(175) |
|
Loss on other investments |
|
(7) |
|
(14) |
|
Foreign exchange rate loss |
|
(1,581) |
|
(1,617) |
|
|
|
|
|
|
|
Total financial expenses |
|
(12,239) |
|
(1,871) |
|
|
|
|
|
|
|
Net financial items |
|
5,165 |
|
328 |
|
|
|
|
|
|
|
During the fourth quarter of 2024, ProfoundBio US (an indirect subsidiary of Genmab A/S) sold its intangible assets to Genmab A/S. Following this transaction, Genmab A/S ultimately received dividend income. The dividend income received of DKK 13.0 billion was recognized as Financial Income in the financial statements of the parent company.
As a result of the above, due to the significant deterioration in the value of Genmab A/S’ indirect investment in ProfoundBio US, Genmab A/S ultimately recorded a DKK 10.4 billion loss on impairment of its investment in subsidiaries. The difference between the dividend income received by Genmab A/S in this transaction and the loss on impairment of investment in subsidiaries relates to goodwill retained at the subsidiary level. The DKK 10.4 billion impairment loss was recognized as Financial Expense in the financial statements of the parent company.
Refer to Note 5.5 in the consolidated financial statements for additional information regarding the acquisition of ProfoundBio and Note 17 in the parent company financial statements for additional information related to investment in subsidiaries.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 237/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
15 – Remuneration of the Board of Directors and Executive Management
Remuneration of the Board for the parent, excluding employee elected board members not directly employed by the parent, is the same as the Group.
Remuneration of Executive Management not directly employed by the parent company is between 10% and 20% of their total compensation, as defined in their individual service agreement and as reported in Note 5.1 in the consolidated financial statements
Refer to Note 5.1 in the consolidated financial statements for additional information regarding the remuneration of the Board of Directors and Executive Management.
16 – Related Party Disclosures
GENMAB A/S’ RELATED PARTIES ARE THE PARENT COMPANY’S SUBSIDIARIES, BOARD, EXECUTIVE MANAGEMENT, AND CLOSE MEMBERS OF THE FAMILY OF THESE PERSONS.
TRANSACTIONS WITH SUBSIDIARIES
Genmab B.V., Genmab Holding B.V., Genmab US, Inc., Genmab K.K., ProfoundBio, Inc., ProfoundBio, US Co., Profound Limited, ProfoundBio co., Ltd., ProfoundBio Shanghai Branch, Co., Ltd., and Bejing Puyifang Biotechnology Co., Ltd. are 100% (directly or indirectly) owned subsidiaries of Genmab A/S and are included in the consolidated financial statements. During 2024, various intercompany transactions and services between the aforementioned companies took place in the field of product sales, research and development, selling, general and administration, finance and management. All intercompany transactions have been eliminated in the consolidated financial statements of the Genmab Group.
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
Transactions with subsidiaries: |
|
|
|
|
|
Income statement: |
|
|
|
|
|
Net product sales |
|
1,244 |
|
136 |
|
Reimbursement revenue |
|
1,140 |
|
937 |
|
Cost of product sales |
|
(28) |
|
(62) |
|
Service fee costs |
|
(5,752) |
|
(5,326) |
|
Milestone costs |
|
(545) |
|
(893) |
|
Impairment investment in subsidiaries |
|
(10,402) |
|
- |
|
Dividend income |
|
13,026 |
|
- |
|
Financial income |
|
6 |
|
1 |
|
Financial expense |
|
(7) |
|
(10) |
|
|
|
|
|
|
|
Balance sheet: |
|
|
|
|
|
Intangible assets |
|
1,029 |
|
291 |
|
Current receivables |
|
964 |
|
650 |
|
Current payables |
|
(887) |
|
(2,525) |
|
Genmab A/S has placed at each subsidiary’s disposal a credit facility (denominated in local currency) that the subsidiary may use to draw from in order to secure the necessary funding of its activities.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 238/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Refer to Note 5.2 to the consolidated financial statements for additional information regarding transactions with related parties of the Group.
17 – Investments in Subsidiaries
(DKK million) |
2024 |
|
2023 |
|
|
|
|
Cost at January 1 |
5,237 |
|
4,735 |
Additions |
13,208 |
|
502 |
Cost at December 31 |
18,445 |
|
5,237 |
|
|
|
|
Impairment at January 1 |
(1,929) |
|
(1,929) |
Impairment for the year |
(10,402) |
|
- |
|
|
|
|
Impairment at December 31 |
(12,331) |
|
(1,929) |
|
|
|
|
Carrying amount at December 31 |
6,114 |
|
3,308 |
Additions primarily related to the DKK 12.5 billion capital contribution to Genmab US, for the acquisition of ProfoundBio.
A DKK 10.4 billion impairment loss was recorded related to Genmab A/S’ indirect investment in ProfoundBio US.
Refer to Note 1.1 in the consolidated financial statements for a listing of subsidiaries owned by Genmab A/S, Note 5.5 in the consolidated financial statements of the group for additional information regarding the acquisition of ProfoundBio and Note 14 in the parent company financial statements for further details related to the transfer of ProfoundBio US intangible assets to Genmab A/S.
18 – Commitments and Contingencies
PURCHASE OBLIGATIONS
Genmab A/S has entered into a number of agreements related to research and development activities that contain various obligations. These contractual obligations amounted to approximately DKK 2,867 million as of December 31, 2024 (2023: approximately DKK 3,145 million).
Genmab A/S also has certain contingent commitments under our license and collaboration agreements that may become due in the future. As of December 31, 2024, these contingent commitments amounted to approximately DKK 12,304 million (USD 1,723 million) in potential future development, regulatory and commercial milestone payments to third parties under license and collaboration agreements for our preclinical and clinical stage development programs as compared to approximately DKK 9,991 million (USD 1,481 million) as of December 31, 2023. These milestone payments generally become due and payable only upon the achievement of certain development, clinical, regulatory or commercial milestones. The events triggering such payments or obligations have not yet occurred.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 239/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
In addition to the above obligations, Genmab A/S enters into a variety of agreements and financial commitments in the normal course of business. The terms generally allow us the option to cancel, reschedule and adjust our requirements based on our business needs prior to the delivery of goods or performance of services. It is not possible to predict the maximum potential amount of future payments under these agreements due to the conditional nature of our obligations and the unique facts and circumstances involved in each particular agreement.
FINANCIAL GUARANTEES
As of December 31, 2024 and December 31, 2023, Genmab A/S has financial bank guarantees of DKK 16 million issued as security for lease obligations under certain lease agreements. The likelihood of a claim under the guarantees has been assessed to be remote due to Genmab A/S’s strong financial position and history of fulfilling lease payments. Accordingly, no provision has been recognized related to this matter.
Refer to Note 5.3 in the consolidated financial statements for additional information regarding commitments of the Group and Note 5.7 in the consolidated financial statements for additional information regarding contingencies of the Group.
19 – Fees to Auditors Appointed at the Annual General Meeting
(DKK million) |
|
2024 |
|
2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Audit fees |
|
10.4 |
|
6.1 |
|
Audit-related fees |
|
2.3 |
|
3.4 |
|
|
|
|
|
|
|
Total |
|
12.7 |
|
9.5 |
|
Fees for other services than statutory audit of the financial statements provided by Deloitte Statsautoriseret Revisionspartnerselskab amounted to DKK 2.3 million in 2024 (DKK 3.4 million in 2023 provided by PricewaterhouseCoopers Statsautoriseret Revisionspartnerselskab. These services primarily include agreed-upon procedures, other assurance assessments and reports, and accounting advice.
Refer to Note 5.4 in the consolidated financial statements for additional information regarding fees to auditors of the Group.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 240/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Directors’ and Management’s Statement on the Annual Report
The Board of Directors and the Executive Management have today considered and approved the Annual Report of Genmab A/S for the financial year January 1 to December 31, 2024.
The Annual Report has been prepared in accordance with IFRS Accounting Standards as issued by the International Accounting Standards Board (IASB) and in accordance with IFRS as endorsed by the EU and further disclosure requirements for listed companies in Denmark.
In our opinion, the Consolidated Financial Statements and the Parent Company Financial Statements give a true and fair view of the Group’s and the Parent Company’s financial position at December 31, 2024 as well as of the results of their operations and the Group's cash flows for the financial year January 1 to December 31, 2024.
In our opinion, the Management Review is prepared in accordance with relevant laws and regulations and contains a fair review of the development of the Group's and the Parent Company’s business and financial matters, the results for the year and of the Parent Company’s financial position and the financial position as a whole of the entities included in the Consolidated Financial Statements, together with a description of the principal risks and uncertainties that the Group and the Parent Company face.
The Sustainability Statements are prepared in accordance with the European Sustainability Reporting Standards (ESRS) as required by the Danish Financial Statements Act as well as article 8 in the EU Taxonomy regulation.
In our opinion, the Annual Report of Genmab A/S for the financial year January 1 to December 31, 2024, with the file name Genmab-2024-12-31-0-en.zip is prepared, in all material respects, in compliance with the ESEF Regulation.
We recommend that the Annual Report be adopted at the Annual General Meeting.
Copenhagen, February 12, 2025
EXECUTIVE MANAGEMENT
|
|
Jan van de Winkel |
Anthony Pagano |
(President & CEO) |
(Executive Vice President & CFO) |
BOARD OF DIRECTORS
|
|
|
Deirdre P. Connelly |
Pernille Erenbjerg |
Anders Gersel Pedersen |
(Chair) |
(Deputy Chair) |
|
|
|
|
Rolf Hoffmann |
Paolo Paoletti |
Elizabeth O’Farrell |
|
|
|
Mijke Zachariasse |
Takahiro Hamatani |
Martin Schultz |
(Employee elected) |
(Employee elected) |
(Employee elected) |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 241/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Independent Auditor’s Reports
Report on the consolidated financial statements and the parent financial statements
To the shareholders of Genmab A/S
Opinion
We have audited the consolidated financial statements and the parent financial statements of Genmab A/S for the financial year January 1, 2024 - December 31, 2024, which comprise balance sheet, statements of cash flow, statement of changes in equity and notes, including material accounting policy information for the Group as well as the Parent, statement of comprehensive income of the Group and income statement of the Parent. The consolidated financial statements and the parent financial statements are prepared in accordance with IFRS Accounting Standards as endorsed by the EU and additional disclosure requirements for listed entities in Denmark.
In our opinion, the consolidated financial statements and the parent financial statements give a true and fair view of the Group’s and the Parent’s financial position at December 31, 2024, and of the results of its operations and cash flows for the financial year January 1, 2024 - December 31, 2024 in accordance with IFRS Accounting Standards as endorsed by the EU and additional disclosure requirements for listed entities in Denmark.
Our opinion is consistent with our Long Form Audit Report issued to the Audit & Finance Committee and the Board of Directors.
Basis for opinion
We conducted our audit in accordance with International Standards on Auditing (ISAs) and the additional requirements applicable in Denmark. Our responsibilities under those standards and requirements are further described in the "Auditor’s responsibilities for the audit of the consolidated financial statements and the parent financial statements" section of this auditor’s report. We are independent of the Group in accordance with the International Ethics Standards Board for Accountants’ International Code of Ethics for Professional Accountants (IESBA Code) and the additional ethical requirements applicable in Denmark, and we have fulfilled our other ethical responsibilities in accordance with these requirements and the IESBA Code. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion.
To the best of our knowledge and belief, we have not provided any prohibited non-audit services as referred to in Article 5(1) of Regulation (EU) No 537/2014.
We were appointed auditors of Genmab A/S for the first time on March 13, 2024, for the financial year 2024.
Key audit matters
Key audit matters are those matters that, in our professional judgement, were of most significance in our audit of the consolidated financial statements and the parent financial statements for the financial year January 1, 2024 - December 31, 2024. These matters were addressed in the context of our audit of the consolidated financial statements and the parent financial statements as a whole, and in forming our opinion thereon, and we do not provide a separate opinion on these matters.
Key audit matter |
How our audit addressed the key audit matter |
Valuation of Acquired IPR&D Assets in the ProfoundBio, Inc. Acquisition |
We tested the effectiveness of controls over the valuation of the Acquired IPR&D assets, including the Company’s controls over the significant estimates |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 242/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
|
Refer to Notes 3.1 and 5.5 to the financial statements. The Company completed the acquisition of ProfoundBio, Inc. (“ProfoundBio”) for USD 1.72 billion (DKK 11.8 billion) on May 21, 2024. The Company accounted for the acquisition as a business combination and, accordingly, has performed procedures to identify all assets and liabilities and allocated the purchase price to the assets acquired and liabilities assumed based on their respective estimated fair values as of the date of acquisition. Intangible assets acquired primarily included the in-process research and development intangible assets (“Acquired IPR&D assets”). The Company estimated the fair value of the Acquired IPR&D assets using an income approach. The fair value determination of the Acquired IPR&D assets required the Company to make significant estimates and assumptions related to the forecasted future cash flows, such as probabilities of technical and regulatory success, and the determination of the discount rates. We identified the valuation of Acquired IPR&D assets for the ProfoundBio acquisition as a key audit matter because of the high level of complexity and management judgement involved in determining the above outlined significant estimates and assumptions used by the Company to determine the fair value of these assets. This required a high degree of auditor judgement and an increased extent of effort when performing audit procedures to evaluate the reasonableness of management’s estimates and assumptions. |
and assumptions related to the forecasted future cash flows, such as probabilities of technical and regulatory success, and the determination of the discount rates. We assessed the reasonableness of the Company’s probabilities of technical and regulatory success used in determination of the fair value of the Acquired IPR&D assets by comparing to internal and external market studies and certain peer companies/products in the industry. We assessed the reasonableness of the Company’s forecasts of future cash flows used in determination of the fair value of the Acquired IPR&D assets by comparing the forecasts to historical results of operations, certain peer companies within comparable industries, and internal and external market studies. With the assistance of our valuation specialists, we evaluated the reasonableness of the discount rates by testing the source information and inputs underlying the determination of the discount rates, including in relation to publicly available information for comparable companies and testing the mathematical accuracy of the calculation. |
|
Revenue recognition of royalty revenue Refer to Note 2.1 to the financial statements. The Company recognized royalty revenue, where revenue is recognized based on net sales by collaboration partners. The Company uses net sales provided by its collaboration partners as an input to their calculation of the amount of royalty revenue to recognize in each period. The preliminary net sales data provided by the collaboration partner may |
We tested the effectiveness of controls relating to the evaluation for reasonableness of the estimated net sales used in the determination of royalty revenue recognition. We tested the overall reasonableness of the estimated net sales reported by the collaboration partners by assessing the historical accuracy of the estimates. We obtained external confirmations from selected collaboration partners on the estimated and actual net sales amounts reported. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 243/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
|
change once final net sales data is available. We identified the revenue recognition of royalty contracts as a key audit matter because of the significant estimation uncertainty related to the net sales data provided by collaboration partners. Specifically, the collaboration partner’s estimate of net sales could change based on the final net sales impacting the royalty revenue recognized in each period. This required a high degree of auditor judgement and an increased extent of effort when performing audit procedures to evaluate the reasonableness of management’s estimates of the net sales. |
Statement on Management’s Review
Management is responsible for the Management Review.
Our opinion on the consolidated financial statements and the parent financial statements does not cover the Management Review, and we do not as part of the audit express any form of assurance conclusion thereon.
In connection with our audit of the consolidated financial statements and the parent financial statements, our responsibility is to read the Management Review and, in doing so, consider whether the Management Review is materially inconsistent with the consolidated financial statements and the parent financial statements or our knowledge obtained in the audit or otherwise appears to be materially misstated.
Moreover, it is our responsibility to consider whether the Management Review provides the information required by the Danish Financial Statements Act. This does not include the requirements in paragraph 99a related to the Sustainability Statement covered by the separate auditor’s limited assurance report hereon.
Based on the work we have performed, in our view, the Management Review is in accordance with the consolidated financial statements and the parent financial statements and has been prepared in accordance with the requirements of the Danish Financial Statements Act except for the requirements in paragraph 99a related to the Sustainability Statement, see above. We did not identify any material misstatement of the Management Review.
Management's responsibilities for the consolidated financial statements and the parent financial statements
Management is responsible for the preparation of consolidated financial statements and parent financial
statements that give a true and fair view in accordance with IFRS Accounting Standards as endorsed by the EU and additional requirements of the Danish Financial Statements Act, and for such internal control as Management determines is necessary to enable the preparation of consolidated financial statements and parent financial statements that are free from material misstatement, whether due to fraud or error.
In preparing the consolidated financial statements and the parent financial statements, Management is responsible for assessing the Group’s and the Parent’s ability to continue as a going concern, for disclosing, as applicable, matters related to going concern, and for using the going concern basis of accounting in preparing the consolidated financial statements and the parent financial statements unless Management either intends to liquidate the Group or the Entity or to cease operations, or has no realistic alternative but to do so.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 244/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Auditor's responsibilities for the audit of the consolidated financial statements and the parent financial statements
Our objectives are to obtain reasonable assurance about whether the consolidated financial statements and the parent financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance, but is not a guarantee that an audit conducted in accordance with ISAs and the additional requirements applicable in Denmark will always detect a material misstatement when it exists. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of these consolidated financial statements and these parent financial statements.
As part of an audit conducted in accordance with ISAs and the additional requirements applicable in Denmark, we exercise professional judgement and maintain professional scepticism throughout the audit. We also:
| ● | Identify and assess the risks of material misstatement of the consolidated financial statements and the parent financial statements, whether due to fraud or error, design and perform audit procedures responsive to those risks, and obtain audit evidence that is sufficient and appropriate to provide a basis for our opinion. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. |
| ● | Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Group’s and the Parent’s internal control. |
| ● | Evaluate the appropriateness of accounting policies used and the reasonableness of accounting estimates and related disclosures made by Management. |
| ● | Conclude on the appropriateness of Management’s use of the going concern basis of accounting in preparing the consolidated financial statements and the parent financial statements, and, based on the audit evidence obtained, whether a material uncertainty exists related to events or conditions that may cast significant doubt on the Group's and the Parent’s ability to continue as a going concern. If we conclude that a material uncertainty exists, we are required to draw attention in our auditor’s report to the related disclosures in the consolidated financial statements and the parent financial statements or, if such disclosures are inadequate, to modify our opinion. Our conclusions are based on the audit evidence obtained up to the date of our auditor’s report. However, future events or conditions may cause the Group and the Entity to cease to continue as a going concern. |
| ● | Evaluate the overall presentation, structure and content of the consolidated financial statements and the parent financial statements, including the disclosures in the notes, and whether the consolidated financial statements and the parent financial statements represent the underlying transactions and events in a manner that gives a true and fair view. |
| ● | Plan and perform the group audit to obtain sufficient and appropriate audit evidence regarding the financial information of the entities or business units within the group as a basis for forming an opinion on the consolidated financial statements and the parent financial statements. We are responsible for the direction, supervision and review of the audit work performed for purposes of the group audit. We remain solely responsible for our audit opinion. |
We communicate with those charged with governance regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control that we identify during our audit.
We also provide those charged with governance with a statement that we have complied with relevant ethical requirements regarding independence, and to communicate with them all relationships and other matters that may
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 245/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
reasonably be thought to bear on our independence, and, where applicable, safeguards put in place and measures taken to eliminate threats.
From the matters communicated with those charged with governance, we determine those matters that were of most significance in the audit of the consolidated financial statements and the parent financial statements of the current period and are therefore the key audit matters. We describe these matters in our auditor’s report unless law or regulation precludes public disclosure about the matter or when, in extremely rare circumstances, we determine that a matter should not be communicated in our report because the adverse consequences of doing so would reasonably be expected to outweigh the public interest benefits of such communication.
Report on compliance with the ESEF Regulation
As part of our audit of the consolidated financial statements and the parent financial statements of Genmab A/S we performed procedures to express an opinion on whether the annual report for the financial year January 1, 2024 - December 31, 2024, with the file name Genmab-2024-12-31-0-en.zip, is prepared, in all material respects, in compliance with the Commission Delegated Regulation (EU) 2019/815 on the European Single Electronic Format (ESEF Regulation), which includes requirements related to the preparation of the annual report in XHTML format and iXBRL tagging of the consolidated financial statements including notes.
Management is responsible for preparing an annual report that complies with the ESEF Regulation. This responsibility includes:
| ● | The preparing of the annual report in XHTML format; |
| ● | The selection and application of appropriate iXBRL tags, including extensions to the ESEF taxonomy and the anchoring thereof to elements in the taxonomy, for financial information required to be tagged using judgement where necessary; |
| ● | Ensuring consistency between iXBRL tagged data and the consolidated financial statements presented in human readable format; and |
| ● | For such internal control as Management determines necessary to enable the preparation of an annual report that is compliant with the ESEF Regulation. |
Our responsibility is to obtain reasonable assurance on whether the annual report is prepared, in all material respects, in compliance with the ESEF Regulation based on the evidence we have obtained, and to issue a report that includes our opinion. The nature, timing and extent of procedures selected depend on the auditor’s judgement, including the assessment of the risks of material departures from the requirements set out in the ESEF Regulation, whether due to fraud or error. The procedures include:
| ● | Testing whether the annual report is prepared in XHTML format; |
| ● | Obtaining an understanding of the company’s iXBRL tagging process and of internal control over the tagging process; |
| ● | Evaluating the completeness of the iXBRL tagging of the consolidated financial statements including notes; |
| ● | Evaluating the appropriateness of the company’s use of iXBRL elements selected from the ESEF taxonomy and the creation of extension elements where no suitable element in the ESEF taxonomy has been identified; |
| ● | Evaluating the use of anchoring of extension elements to elements in the ESEF taxonomy; and |
| ● | Reconciling the iXBRL tagged data with the audited consolidated and parent financial statements. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 246/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
In our opinion, the annual report of Genmab A/S for the financial year January 1, 2024 - December 31, 2024, with the file name Genmab-2024-12-31-0-en.zip, is prepared, in all material respects, in compliance with the ESEF Regulation.
Copenhagen, February 12, 2025
Deloitte
Statsautoriseret Revisionspartnerselskab
CVR no 33 96 35 56
|
|
Sumit Sudan |
Niels Skannerup Vendelbo |
State Authorised Public Accountant |
State Authorised Public Accountant |
mne33716 |
mne34532 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 247/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Independent auditor’s limited assurance report on Sustainability Statements
To the stakeholders of Genmab A/S
Limited assurance conclusion
We have conducted a limited assurance engagement on the Sustainability Statements of Genmab A/S (the “Group”) included in the Management’s Review (the “Sustainability Statements”), for the financial year January 1 – December 31, 2024.
Based on the procedures we have performed and the evidence we have obtained, nothing has come to our attention that causes us to believe that the Sustainability Statements is not prepared, in all material respects, in accordance with the Danish Financial Statements Act section 99a, including:
| ● | compliance with the European Sustainability Reporting Standards (ESRS), including that the process carried out by the management to identify the information reported in the Sustainability Statements (the “Process”) is in accordance with the description set out in 1.4 Impact, risk, and opportunity management; and |
| ● | compliance of the disclosures in subsection 2.4 EU Taxonomy within the environmental section of the Sustainability Statements with Article 8 of EU Regulation 2020/852 (the “Taxonomy Regulation”). |
Basis for conclusion
We conducted our limited assurance engagement in accordance with ISAE 3000 (Revised), Assurance engagements other than audits or reviews of historical financial information, and additional requirements applicable in Denmark.
The procedures in a limited assurance engagement vary in nature and timing from, and are less in extent than for, a reasonable assurance engagement. Consequently, the level of assurance obtained in a limited assurance engagement is substantially lower than the assurance that would have been obtained had a reasonable assurance engagement been performed.
We believe that the evidence we have obtained is sufficient and appropriate to provide a basis for our conclusion. Our responsibilities under this standard are further described in the “Auditor’s responsibilities for the assurance engagement” section of our report.
Our independence and quality management
We are independent of the Group in accordance with the International Ethics Standards Board for Accountants’ International Code of Ethics for Professional Accountants (IESBA Code) and the additional ethical requirements applicable in Denmark. We have also fulfilled our other ethical responsibilities in accordance with these requirements and the IESBA Code.
Deloitte Statsautoriseret Revisionspartnerselskab applies International Standard on Quality Management 1, ISQM1, which requires the firm to design, implement and operate a system of quality management including policies or procedures regarding compliance with ethical requirements, professional standards and applicable legal and regulatory requirements.
Other matter
The comparative information included in the Sustainability Statements of the Group was not subject to an assurance engagement. Our conclusion is not modified in respect of this matter.
Inherent limitations in preparing the Sustainability Statements
In reporting forward-looking information in accordance with ESRS, management is required to prepare the forward-looking information on the basis of disclosed assumptions about events that may occur in the future and possible future actions by the Group. Actual outcomes are likely to be different since anticipated events frequently do not occur as expected.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 248/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Management’s responsibilities for the Sustainability Statements
Management is responsible for designing and implementing a process to identify the information reported in the Sustainability Statements in accordance with the ESRS and for disclosing this Process in 1.4 Impact, risk, and opportunity management of the Sustainability Statements. This responsibility includes:
| ● | understanding the context in which the Group’s activities and business relationships take place and developing an understanding of its affected stakeholders; |
| ● | the identification of the actual and potential impacts (both negative and positive) related to sustainability matters, as well as risks and opportunities that affect, or could reasonably be expected to affect, the Group’s financial position, financial performance, cash flows, access to finance or cost of capital over the short-, medium-, or long-term; |
| ● | the assessment of the materiality of the identified impacts, risks and opportunities related to sustainability matters by selecting and applying appropriate thresholds; and |
| ● | making assumptions that are reasonable in the circumstances. |
Management is further responsible for the preparation of the Sustainability Statements, in accordance with the Danish Financial Statements Act section 99a, including:
| ● | compliance with the ESRS; |
| ● | preparing the disclosures in subsection 2.4 EU Taxonomy within the environmental section of the Sustainability Statements, in compliance with Article 8 of the Taxonomy Regulation; |
| ● | designing, implementing and maintaining such internal control that management determines is necessary to enable the preparation of the Sustainability Statements that is free from material misstatement, whether due to fraud or error; and |
| ● | the selection and application of appropriate sustainability reporting methods and making assumptions and estimates that are reasonable in the circumstances. |
Auditor’s responsibilities for the assurance engagement
Our objectives are to plan and perform the assurance engagement to obtain limited assurance about whether the Sustainability Statements is free from material misstatement, whether due to fraud or error, and to issue a limited assurance report that includes our conclusion. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence decisions of users taken on the basis of the Sustainability Statements as a whole.
As part of a limited assurance engagement in accordance with ISAE 3000 (Revised) we exercise professional judgement and maintain professional scepticism throughout the engagement.
Our responsibilities in respect of the Process include:
| ● | Obtaining an understanding of the Process but not for the purpose of providing a conclusion on the effectiveness of the Process, including the outcome of the Process; |
| ● | Considering whether the information identified addresses the applicable disclosure requirements of the ESRS, and |
| ● | Designing and performing procedures to evaluate whether the Process is consistent with the Group’s description of its Process, as disclosed in 1.4 Impact, risk, and opportunity management. |
Our other responsibilities in respect of the Sustainability Statements include:
| ● | Identifying disclosures where material misstatements are likely to arise, whether due to fraud or error; and |
| ● | Designing and performing procedures responsive to disclosures in the Sustainability Statements where material misstatements are likely to arise. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 249/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Summary of the work performed
A limited assurance engagement involves performing procedures to obtain evidence about the Sustainability Statements.
The nature, timing and extent of procedures selected depend on professional judgement, including the identification of disclosures where material misstatements are likely to arise, whether due to fraud or error, in the Sustainability Statements.
In conducting our limited assurance engagement, with respect to the Process, we:
| ● | Obtained an understanding of the Process by performing inquiries to understand the sources of the information used by management; and reviewing the Group’s internal documentation of its Process; and |
| ● | Evaluated whether the evidence obtained from our procedures about the Process implemented by the Group’s was consistent with the description of the Process set out in 1.4 Impact, risk, and opportunity management. |
In conducting our limited assurance engagement, with respect to the Sustainability Statements, we:
| ● | Obtained an understanding of the Group’s reporting processes relevant to the preparation of its Sustainability Statements including the consolidation processes by obtaining an understanding of the Group’s control environment, processes and information systems relevant to the preparation of the Sustainability Statements but not evaluating the design of particular control activities, obtaining evidence about their implementation or testing their operating effectiveness; |
| ● | Evaluated whether material information identified by the Process is included in the Sustainability Statements; |
| ● | Evaluated whether the structure and the presentation of the Sustainability Statements are in accordance with the ESRS; |
| ● | Performed inquiries of relevant personnel and analytical procedures on selected information in the Sustainability Statements; |
| ● | Performed substantive assurance procedures on selected information in the Sustainability Statements; |
| ● | Evaluated methods, assumptions and data for developing material estimates and forward-looking information and how these methods were applied; |
| ● | Obtained an understanding of the process to identify taxonomy-eligible and taxonomy-aligned economic activities and the corresponding disclosures in the Sustainability Statements. |
Copenhagen, February 12, 2025
Deloitte
Statsautoriseret Revisionspartnerselskab
CVR no 33 96 35 56
|
|
Sumit Sudan |
Niels Skannerup Vendelbo |
State Authorised Public Accountant |
State Authorised Public Accountant |
mne33716 |
mne34532 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 250/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
Other Information
Forward Looking Statement
This Annual Report contains forward looking statements. The words “believe,” “expect,” “anticipate,” “intend” and “plan” and similar expressions identify forward looking statements. Actual results or performance may differ materially from any future results or performance expressed or implied by such statements. The important factors that could cause our actual results or performance to differ materially include, among others, risks associated with product discovery and development, uncertainties related to the outcome and conduct of clinical trials including unforeseen safety issues, uncertainties related to product manufacturing, our inability to manage growth, the competitive environment in relation to our business area and markets, our inability to attract and retain suitably qualified personnel, the unenforceability or lack of protection of our patents and proprietary rights, our relationships with affiliated entities, changes and developments in technology which may render our products obsolete, and other factors. Additional factors that could cause our actual results or performance to differ materially could also include and are not limited to the risk and uncertainties related to regulatory action, reimbursement, market adoption by physicians or lack of market acceptance of our products, the risk that the Company or our collaborators may be delayed or unsuccessful in planned clinical trial initiations, enrollment and planned regulatory submissions and approvals in the U.S. and other countries. For a further discussion of these risks, please refer to the section “Risk Management” in this Annual Report and the risk factors included in Genmab’s 2024 Annual Report on Form 20-F and other filings with the U.S. Securities and Exchange Commission (SEC). Genmab does not undertake any obligation to update or revise forward looking statements in this Annual Report nor to confirm such statements to reflect subsequent events or circumstances after the date made or in relation to actual results, unless required by law.
Genmab A/S and/or its subsidiaries own the following trademarks: Genmab®; the Y-shaped Genmab logo®; Genmab in combination with the Y-shaped Genmab logo®; HuMax®; DuoBody®; DuoBody in combination with the DuoBody logo®; HexaBody®; HexaBody in combination with the HexaBody logo®; DuoHexaBody®, HexElect®; KYSO® and MyNavCare™. ProfoundBio™ and Rina-S™ are trademarks of ProfoundBio, US Co. and ProfoundBio (Suzhou) Co., Ltd. Tivdak® and CeMe™ are trademarks of Seagen Inc.; Arzerra® is a trademark of Novartis Pharma AG. Kesimpta® and Sensoready® are trademarks of Novartis AG or its affiliates; DARZALEX®, DARZALEX FASPRO®, RYBREVANT®, TECVAYLI® and TALVEY® are trademarks of Johnson & Johnson; EPCORE®, EPKINLY®, TEPKINLY® and their designs are trademarks of AbbVie Biotechnology Ltd.; TEPEZZA® is a trademark of Horizon Therapeutics Ireland DAC. ©2025, Genmab A/S. All rights reserved.
Photograph credits:
Andrei Jackamets
Stijn Doors
Tuala Hjarnø
Rob Walbers
3FX, Inc.
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 251/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
GENMAB 2024 ANNUAL REPORT |
|
About Genmab A/S
Genmab is an international biotechnology company with a core purpose of guiding its unstoppable team to strive toward improving the lives of patients with innovative and differentiated antibody therapeutics. For more than 25 years, its passionate, innovative and collaborative team has invented next-generation antibody technology platforms and leveraged translational, quantitative and data sciences, resulting in a proprietary pipeline including bispecific T-cell engagers, antibody-drug conjugates, next-generation immune checkpoint modulators and effector function-enhanced antibodies. By 2030, Genmab’s vision is to transform the lives of people with cancer and other serious diseases with knock-your-socks-off (KYSO) antibody medicines®.
Established in 1999, Genmab is headquartered in Copenhagen, Denmark, with international presence across North America, Europe, and Asia Pacific. For more information, please visit Genmab.com and follow us on LinkedIn and X.
|
Addresses GENMAB A/S Carl Jacobsens Vej 30 2500 Valby Denmark T. +45 70 20 27 28 GENMAB US, INC. 777 Scudders Mill Road Plainsboro, NJ 08536 USA GENMAB QC LAB Baltorpvej 154 2750 Ballerup Denmark T. +45 70 20 27 28 |
GENMAB B.V. & GENMAB HOLDING B.V. Uppsalalaan 15 3584 CT Utrecht The Netherlands T. +31 30 2 123 123 GENMAB K.K. 35F Midtown Tower 9-7-1 Akasaka, Minato-ku Tokyo 107-6235 T. +81 3 5403 6330 PROFOUNDBIO CO. LTD. * Suite 101 & 102 Building 1 Phase 3A of BioBAY No. 1 Xinze Road Suzhou Industrial Park Suzhou, China 215021 T. +86 512 6799 1868 *ProfoundBio was acquired by Genmab in May 2024 www.genmab.com LEI Code 529900MTJPDPE4MHJ122 |
|
|
|
Genmab A/S |
Tel: +45 7020 2728 |
Company Announcement no. 03 |
Carl Jacobsens Vej 30 |
|
Page 252/252 |
2500 Valby, Denmark |
www.genmab.com |
CVR no. 2102 3884 |
|
2024 Annual Report CVR No. 21 02 38 84 Genmab A/S Carl Jacobsens Vej 30 2500 Valby Denmark Leading Antibody Science for Better Futures |
|
Management’s Review 4 Our 2030 Vision 5 Chair’s Statement 7 Letter from the CEO 9 2024 at a Glance 10 Consolidated Key Figures 11 2025 Outlook 12 Our Strategy 13 Who We Are 14 Business Model 15 Value Chain 17 Research and Development Capabilities 19 Bringing Our Own Innovative Medicines to Patients 21 Antibody Discovery and Development 22 Products and Technologies 51 Financial Review 62 Risk Management 71 Enterprise Risk Management 72 Corporate Governance 74 Board of Directors 77 Executive Management 79 Shareholders and Share Information Sustainability Statements* 82 General Information 94 Environmental 106 Social 124 Governance 131 Appendix A Financial Statements 139 Financial Statements for the Genmab Group 183 Financial Statements of the Parent Company 197 Directors’ and Management’s Statement on the Annual Report 198 Independent Auditor’s Reports Other Information 204 Forward Looking Statement 205 Contact Information Table of Contents Our Reporting Suite 2024 Corporate Governance Report 2024 Compensation Report Our Corporate Governance and Compensation Reports for 2024 can be found on our website Genmab.com. *The Sustainability Statements are part of Management’s Review Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 2 |
|
01 Management’s Review In this section 4 Our 2030 Vision 5 Chair’s Statement 7 Letter from the CEO 9 2024 at a Glance 10 Consolidated Key Figures 11 2025 Outlook 12 Our Strategy 13 Who We Are 14 Business Model 15 Value Chain 17 Research and Development Capabilities 19 Bringing Our Own Innovative Medicines to Patients 21 Antibody Discovery and Development 22 Products and Technologies 51 Financial Review 62 Risk Management 71 Enterprise Risk Management 72 Corporate Governance 74 Board of Directors 77 Executive Management 79 Shareholders and Share Information Sustainability Statement 82 General Information 94 Environmental 106 Social 124 Governance 131 Appendix A Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 3 |
|
Our Core Purpose, Supporting Our 2030 Vision Our unstoppable team will improve the lives of patients through innovative and differentiated antibody therapeutics. ® Our 2030 Vision By 2030, our KYSO (knock-your-socks-off) antibody medicines® are fundamentally transforming the lives of people with cancer and other serious diseases. • Genmab founded • Nasdaq Copenhagen A/S (Nasdaq Copenhagen) Initial Public Offering (IPO) • First partnership (F. Hoffmann-La Roche AG (Roche)) • Ofatumumab program announced • Daratumumab selected • Arzerra®1 (ofatumumab) first approval • Tivdak®5 (tisotumab vedotin-tftv) approval and launch • DuoBody-based bispecifics RYBREVANT®2 (amivantamab), TECVAYLI®2 (teclistamab) and TALVEY®2 (talquetamab) approval and launch • DuoBody-based bispecific EPKINLY®6 (epcoritamab-bysp)/ TEPKINLY®6 (epcoritamab) approval and launch • argenx SE (argenx) partnership • ProfoundBio Inc. (ProfoundBio) acquisition, including rinatabart sesutecan (Rina-S™) • Genmab assumes full ownership of acasunlimab • Rina-S and acasunlimab move into Phase 3 development • DuoBody® technology platform announced • Collaboration with Seagen Inc. (Seagen) • DuoBody research and license agreement with Johnson & Johnson (J&J, legal entity Janssen Biotech, Inc.) • Daratumumab agreement with J&J • HexaBody® technology platform announced • DARZALEX®2 (daratumumab) approval and launch • BioNTech SE (BioNTech) partnership • U.S. IPO under Nasdaq Global Select Market; dual listed as GMAB • Japan Operations established under Genmab K.K. • AbbVie Inc. (AbbVie) partnership • DARZALEX FASPRO®2 (daratumumab and hyaluronidase fihj) approval and launch • Kesimpta®3 (ofatumumab) approval and launch • TEPEZZA®4 (teprotumumab) approval and launch 1999–2009 2010–2020 2021–2024 25 Years of Innovation 1. Developed and commercialized by GlaxoSmithKline (GSK); 2. Developed and commercialized by J&J; 3. Developed and commercialized by Novartis AG (Novartis); 4. Developed and commercialized by Amgen Inc. (Amgen); 5. Co-developed and commercialized with Pfizer Inc. (Pfizer); 6. Co-developed and commercialized with AbbVie Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 4 |
|
Dear Shareholder, For 25 years, Genmab has pioneered antibody-based medicines to fundamentally transform the lives of people with cancer and other serious diseases. We take pride looking back at the great leaps we have made globally and within the foundations of Genmab; however, our focus remains steadfast on the future. Our progress has led us here: over 2,700 team members across five countries, eight antibody-based medicines having an impact on patients’ lives, and two wholly owned assets now in late-stage development. Genmab’s Evolution Founded in 1999, Genmab celebrated its 25th anniversary in 2024, and the year gave all of us at Genmab much to celebrate. Twenty-five years of scientific progress have had an impact on the lives of patients and have inspired us to continue on our path of becoming a fully integrated biotech. This year, Genmab successfully acquired ProfoundBio. In addition to gaining worldwide rights to three clinical candidates and novel antibody-drug conjugate (ADC) platforms, the acquisition is representative of our long-term growth potential. We welcomed talented new colleagues to our Research & Development (R&D) team, and accelerated the clinical development for Rina-S, a wholly owned asset now in Phase 3 clinical development. Sustainability and social responsibility are fundamental to the way we work at Genmab. This Annual Report also marks the first year for Genmab incorporating our Environmental, Social and Governance (ESG) disclosures in compliance with the European Union’s (EU) Corporate Sustainability Reporting Directive (CSRD). Our first double materiality assessment (DMA) and its outcome supported the development of our first impacts, risks and opportunities that guided our future sustainability strategy. We remain committed to ensuring our actions benefit our stakeholders and society and that our Corporate Social Responsibility (CSR) practices are integrated as a core part of our business. In 2024, 800 Genmab employees completed 4,037 service hours to make a difference in the lives of patients and their families, our communities and the environment. Experienced Leadership Our future looks promising under our expanded leadership. In 2024, we had two additions to our Executive Committee: Rayne Waller as Chief Technical Operations Officer, and Brad Bailey as Chief Commercial Officer. Rayne Waller joined Genmab to further solidify and strengthen our technical operations Deirdre P. Connelly Board Chair Chair’s Statement Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 5 |
|
and lead all the manufacturing and supply chain capabilities of our proprietary programs through preclinical, clinical and commercial stages. Brad Bailey, previously Genmab’s Senior Vice President and U.S. General Manager, expanded his role to lead the direction, planning, and execution of Genmab’s global commercial strategies as we expand beyond our two priority markets of the U.S. and Japan. These new additions strengthen our commitment to a bold future for our diverse and innovative mid- to late-stage clinical programs. In 2024, our Board of Directors (Board) continued to provide governance, guidance, and dedicated leadership. Comprised of experts in their fields, the Board has supported organizational growth initiatives, driven global change, and contributed value across Genmab. On behalf of the Board, I would like to thank Genmab’s dedicated team members, Chief Executive Officer Jan van de Winkel and the entire global leadership team for their inspiration and extraordinary leadership as well as our shareholders for your continued support. Sincerely, Deirdre P. Connelly Board Chair Chair’s Statement Genmab 2024 Annual Report Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 6 |
|
Jan van de Winkel, Ph.D. President & Chief Executive Officer Dear Shareholder, As I reflect on 2024, I am proud to share a year of remarkable progress and strategic achievement at Genmab. This year has reinforced our commitment to transforming the lives of people with cancer and other serious diseases through groundbreaking antibody-based medicines. Our advances across research, development, and commercialization activities reflect the strength of our vision, our team, and our unwavering focus on delivering value for patients and stakeholders alike. Strategic Growth and Innovation This year, we achieved several key milestones that drive us closer to our 2030 Vision of being a fully integrated biotech innovation powerhouse. Central to this was our acquisition of ProfoundBio, completed in May, which significantly enhanced our long-term growth potential and brought assets such as Rina-S into our pipeline. Rina-S, a next-generation ADC with best-in-class potential, entered Phase 3 development this year. We also assumed sole responsibility for the continued development and potential commercialization of acasunlimab, underscoring our commitment to building a robust pipeline of wholly owned, late-stage programs. These advancements are supported by a growing portfolio of proprietary technologies, including the novel ADC technology platforms we acquired with ProfoundBio, and our validated DuoBody platform, which underpins our success with innovative bispecific antibodies. Transforming Science into Medicine This year, we carefully evaluated our investments with a focus on portfolio prioritization, and we evaluated our clinical pipeline to ensure we are investing our resources in the best and most effective way possible. This strategic prioritization means we are very focused on maximizing the potential of turning science into medicine through our Phase 3 programs, EPKINLY, Rina-S and acasunlimab. After a thorough assessment, we also decided to terminate some early-stage clinical programs that did not meet our criteria for potential KYSO ® antibody-based medicines. And we decided not to pursue a Phase 3 program for Tivdak in second line plus head and neck cancer. This year we are pleased that our commercialized medicines reached significant achievements: • EPKINLY became the first and only subcutaneous (SC) bispecific antibody approved to treat both relapsed or refractory Letter from the CEO Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 7 |
|
Letter from the CEO follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL) in the U.S. and Europe. Strong launches in Japan and other key markets have exceeded expectations. • Tivdak, our tissue factor (TF)-directed ADC, received full U.S. Food and Drug Administration (U.S. FDA) approval based on data demonstrating significant overall survival benefits for patients with recurrent or metastatic cervical cancer. A Strong Financial Foundation to Enable Our Evolution Our financial performance this year has been a testament to the strength of our strategy. Recurring revenues grew significantly, driven by royalties from our collaborations and sales of EPKINLY and Tivdak, both of which delivered robust sales in 2024. This growth reinforces our financial position and enables continued investment based on our strategic prioritization efforts, which include our late-stage clinical programs and commercialization capabilities. This focused approach enables us to realize our vision and capitalize on significant growth opportunities ahead. Genmab’s 25th anniversary also marked the beginning of a new era of opportunity as our company leverages the full potential of our late-stage clinical programs, the potential of the acquisition of ProfoundBio and continues to build on our existing cutting-edge antibody research and development to fulfill our mid-to long-term growth as a fully integrated biotech innovation powerhouse. Acknowledgments and Outlook These accomplishments and our progress would not have been possible without the dedication of our exceptional team, the collaboration of our partners, and the trust of our shareholders. I want to express my deepest gratitude to all who have contributed to our success this year. We are excited about the opportunities that lie ahead as we continue to evolve into a fully integrated biotech. With a strong foundation, an exceptional team, and a strong pipeline of innovative antibody medicines and investigational medicines, Genmab is well-positioned to deliver on our vision to have an impact on the lives of patients around the world. Thank you for your continued confidence and support. Together, we will continue to drive forward and reach our inspirational 2030 vision. Sincerely yours, Jan van de Winkel, Ph.D. President & CEO Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 8 |
|
Liquidity and Capital Resources DKK 11,243M Marketable securities DKK 9,858M Cash and cash equivalents DKK 36,697M Shareholders’ equity Financial DKK 99B 2024 year-end market cap DKK 21,526M 2024 revenue DKK 13,538M 2024 operating expenses, 72% invested in R&D 2024 at a Glance Operational • EPKINLY/TEPKINLY became the first and only SC bispecific antibody approved in both the U.S. and Europe to treat both relapsed or refractory FL and relapsed or refractory DLBCL after two or more lines of systemic therapy • Acquisition of ProfoundBio, granting Genmab worldwide rights to multiple candidates in development (including Rina-S) plus ProfoundBio’s novel ADC technology platforms • Genmab submitted a supplemental Japan New Drug Application (J-NDA) to the Ministry of Health, Labour and Welfare (MHLW) for SC EPKINLY for the treatment of relapsed or refractory FL after two or more lines of systemic therapy • Tivdak received full U.S. FDA approval to treat recurrent or metastatic cervical cancer • Genmab submitted a J-NDA to the MHLW for Tivdak for the treatment of advanced or recurrent cervical cancer • Genmab assumed sole responsibility for the continued development and potential commercialization of acasunlimab • Two Genmab wholly owned programs, Rina-S and acasunlimab, moved into Phase 3 development • Multiple programs entered clinical-stage development including GEN1059 (BNT314, DuoBody-EpCAMx4-1BB), GEN1055 (BNT315, HexaBody-OX40), GEN1057 (DuoBody-FAPαxDR4) and GEN1286 (EGFRxcMET ADC) • Additional regulatory approvals for J&J therapies DARZALEX FASPRO and RYBREVANT • Approval of Amgen’s TEPEZZA in Japan for the treatment of active thyroid eye disease (TED) • Continued development of Genmab’s broader organizational infrastructure with the addition of over 600 new colleagues Operating Profit* (DKK million) *2020 Operating Profit impacted by one-time AbbVie upfront payment. Sustainability • Completed first DMA in accordance with CSRD • First year integrating our sustainability statements into Genmab’s Annual Report 2020* 2021 2022 2023 2024 2,953 6,267 6,703 5,321 6,290 Environmental • Set first long-term emission reduction target for Scope 1 & 2 emissions • Achieved 77% renewable electricity across all sites • Established formalized processes governing supplier engagement on climate change Social • Exceeded life sciences industry benchmark for favorability and participation rate for Global Employee Engagement Survey • Zero workplace injuries • 100% of eligible team members with access to year-end performance process • Training available to all team members at varying degrees Governance • Code of Conduct applies to all Genmab team members Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 9 |
|
(DKK million) 2020 2021 2022 2023 2024 Income Statement Revenue 10,088 8,417 14,505 16,474 21,526 Cost of product sales – – – (226) (985) Research and development expenses (3,137) (4,181) (5,562) (7,630) (9,748) Selling, general and administrative expenses (661) (1,283) (2,676) (3,297) (3,790) Acquisition and integration related charges – – – – (300) Total costs and operating expenses (3,798) (5,464) (8,238) (11,153) (14,823) Operating profit 6,290 2,953 6,267 5,321 6,703 Net financial items (409) 965 678 316 2,461 Net profit 4,740 2,957 5,452 4,352 7,844 Balance Sheet Total non-current assets 2,352 1,891 1,901 2,150 17,957 Marketable securities 8,819 10,381 12,431 13,268 11,243 Cash and cash equivalents 7,260 8,957 9,893 14,867 9,858 Total assets 21,105 24,538 30,119 35,289 45,811 Share capital 66 66 66 66 66 Shareholders’ equity 19,083 22,107 27,282 31,610 36,697 Cash Flow Statement Cash flow from operating activities 6,433 2,228 3,912 7,380 7,771 Cash flow from investing activities (2,351) (961) (2,761) (1,282) (9,907) Cash flow from financing activities 71 (420) (789) (606) (3,919) Investments in intangible assets – – – (10) (117) Investments in tangible assets (307) (252) (317) (366) (187) Financial Ratios and Other Information Basic net profit per share 72.72 45.22 83.38 66.64 122.21 Diluted net profit per share 71.94 44.77 82.59 66.02 121.36 Year-end share market price 2,463.00 2,630.00 2,941.00 2,155.00 1,492.50 Price/book value 8.52 7.85 7.11 4.50 2.68 Shareholders’ equity per share 289.14 334.95 413.36 478.94 556.02 Equity ratio 90% 90% 91% 90% 80% Shares outstanding 65,545,748 65,718,456 65,961,573 66,074,535 66,187,186 Average number of employees (FTE)* 656 1,022 1,460 2,011 2,535 Number of employees (FTE) at year-end 781 1,212 1,660 2,204 2,682 *Full-time equivalent (FTE) or team member. Consolidated Key Figures 2020 2021 2022 2023 2024 8,417 14,505 21,526 16,474 10,088 Revenue* (DKK million) 2020 2021 2022 2023 2024 1,212 1,660 2,682 2,204 781 FTE at Year End FTE 2020 2021 2022 2023 2024 4,181 5,562 9,748 3,137 1,283 2,676 3,790 300 661 5,464 8,238 13,838 7,630 3,297 10,927 3,798 Research and development expenses Selling, general and administrative expenses Acquisition and integration related charges Operating Expenses (DKK million) Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 10 |
|
Revenue Genmab expects its 2025 revenue to be in the range of USD 3.3–3.7 billion, compared to USD 3.1 billion in 2024. Genmab’s projected revenue growth for 2025 is driven by higher royalties, net product sales and collaboration revenue. Royalty growth relates mainly to DARZALEX and Kesimpta net sales growth. Net product sales and collaboration revenue growth is driven by strong performance for both EPKINLY and Tivdak. Net product sales and collaboration revenue consists of EPKINLY net product sales in the U.S. and Japan, and Tivdak (50% gross profit share). Genmab’s projected revenue for 2025 primarily consists of DARZALEX royalties of approximately USD 2.2 billion at the midpoint. Such royalties are based on estimated DARZALEX 2025 net sales of USD 12.6–13.4 billion compared to actual net sales in 2024 of USD 11.7 billion. DARZALEX royalties are partly offset by Genmab’s share of J&J’s royalty payments to Halozyme Therapeutics, Inc. (Halozyme) in connection with SC net sales as well as royalty reduction in countries and territories where there is no Genmab patent coverage. The remainder of Genmab’s revenue consists of royalties from Kesimpta, TEPEZZA, RYBREVANT, TECVAYLI, TALVEY and TEPKINLY, net product sales and collaboration revenue from EPKINLY and Tivdak, reimbursement revenue and milestones. 2025 Outlook Operating Expenses Genmab anticipates its 2025 operating expenses to be in the range of USD 2.1–2.2 billion, compared to USD 2.0 billion in 2024. The increase in operating expenses is primarily related to investments in late-stage programs and launch readiness in key markets. Operating Profit Genmab expects its 2025 operating profit to be in the range of USD 0.9–1.4 billion, compared to USD ~1.0 billion. Outlook: Risks and Assumptions In addition to factors already mentioned, the estimates above are subject to change due to numerous reasons, including but not limited to: the achievement of certain milestones associated with Genmab’s collaboration agreements; the timing and variation of development activities (including activities carried out by Genmab’s collaboration partners) and related income and costs; DARZALEX, DARZALEX FASPRO, Kesimpta, TEPEZZA, RYBREVANT, TECVAYLI, TALVEY and TEPKINLY net sales and royalties paid to Genmab; changing rates of inflation; and currency exchange rates (the 2025 guidance assumes a USD/DKK exchange rate of 7.2). The financial guidance assumes that no significant new agreements are entered into during 2025 that could materially affect the results. The factors discussed above, as well as other factors that are currently unforeseeable, may result in further material adverse impacts on Genmab’s business and financial performance, including unfavorable impacts on the sales of Tivdak and EPKINLY/TEPKINLY, and on the net sales of DARZALEX, Kesimpta, TEPEZZA, RYBREVANT, TECVAYLI, and TALVEY by Genmab’s collaboration partners and on Genmab’s royalties, collaboration revenue and milestone revenue therefrom. 2025 Outlook is presented in USD as management has determined it is appropriate to change the functional currency of Genmab A/S and the presentation currency to USD effective January 1, 2025. Refer to Note 5.8 for additional details regarding the change in functional and presentation currency. (USD millions) 2024 Actual Result 2025 Guidance 2025 Guidance Mid-Point 2024 Growth % 2025 Growth %* Revenue 3,124 3,340–3,660 3,500 31% 12% Royalties 2,518 2,785–3,015 2,900 27% 15% Net product sales/ Collaboration revenue** 316 415–460 438 199% 39% Milestones/Reimbursement revenue 290 140–185 162 -2% -44% Gross profit 2,981 3,120–3,420 3,270 26% 10% Operating expenses (2,008) (2,055)–(2,225) (2,140) 27% 7% Operating profit 973 895–1,365 1,130 26% 16% *Mid-point of guidance range **Net product sales and collaboration revenue consists of EPKINLY net product sales in the U.S. and Japan, and Tivdak (Genmab’s share of gross profits). Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 11 |
|
Our Strategy Business Strategy Priorities in 2024 Priorities for 2025 Related Risk Build a profitable and successful biotech ― Maintain a flexible and capital-efficient model ― Maximize relationships with partners ― Retain ownership of select products Invest in our people and culture ― Further scale organization aligned with differentiated antibody product portfolio growth and future launches Become a leading integrated biotech innovation powerhouse ― Use solid financial base to grow and broaden antibody product and technology portfolio Focus investments to optimize and enable growth strategy Please refer to the risks included in this Annual Report. Focus on core competence ― Identify the best disease targets ― Develop unique first-in-class or best-in-class antibodies ― Develop next-generation technologies Build world-class differentiated pipeline ― Acasunlimab (GEN1046/BNT311, DuoBody-PD-L1x4-1BB) ― Initiate Phase 3 study (2L NSCLC) ― GEN1042 (DuoBody-CD40x4-1BB)1 ― Phase 2 data and determine next steps ― Expand and advance proprietary product portfolio Expand our pipeline through organic and inorganic opportunities Please refer to the risks included in this Annual Report. Turn science into medicine ― Create differentiated antibody therapeutics with significant commercial potential Bring our own medicines to patients & expand our markets ― EPKINLY2 ― Initiate three Phase 3 trials ― Expand label to include relapsed/refractory FL ― Tivdak3 ― Initiate Phase 3 study in head and neck ― Execute successful launches and growth in key markets Advance mid-to-late-stage pipeline assets: epcoritamab, Rina-S, acasunlimab Please refer to the risks included in this Annual Report. Sustainability Strategy Priorities in 2024 Priorities for 2025 Related Risk We are committed to embedding sustainability in our business operations with a focus on reducing our carbon footprint, upholding responsibility towards our people, patients and society while maintaining high standards of corporate governance ― Continue to grow our commitment to being a sustainable and responsible company ― Ensure that policies and procedures are implemented in alignment with ESG-related reporting requirements, while continuing to monitor the regulatory landscape ― Collaborate internally to integrate ESG into our strategic planning, business operations and risk management processes ― Continue to develop and deliver treatments to improve lives of patients ― Minimize our carbon footprint and map our Greenhouse Gas (GHG) emissions ― Promote the Company’s efforts to attract, retain, motivate and recognize diverse, world-class talent ― Invest in Diversity, Equity and Inclusion processes and efforts, which is critical to our future growth General ― Advance sustainability commitments by integrating action plans around material impacts, risks and opportunities into our business operations ― Launch sustainability awareness training for all team members Environmental ― Focus on the continuous update and execution of action plans to achieve near and long-term emission reduction targets Social ― Continue to pursue science and innovation with the potential to improve patients’ lives through our medicines and facilitate access to these medicines ― Continue efforts to promote employee well-being & vitality ― Maintain training and skills development opportunities for all team members ― Support our future business needs by attracting, retaining, developing, recognizing and motivating a diverse and talented team Governance ― Reasonably ensure compliance with regulatory reporting requirements in a transparent manner ― Ensure strong governance by engaging key stakeholders, including the Board and its Committees, CSR & Sustainability Committee, senior leaders, employees and suppliers Please refer to the risks included in this Annual Report. 1. Co-development with BioNTech; 2. Co-development with AbbVie; 3. Co-development with Pfizer Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 12 |
|
Our Core Values In our quest to turn science into medicine, we use these guideposts to transform the future of cancer treatment: • Passion for innovation • Determination—being the best at what we do • Integrity—we do the right thing • We work as one team and respect each other Our Key Accomplishments Each of our achievements stands as evidence of our unyielding determination, including: • Two Genmab co-owned medicines on the market: Tivdak with Pfizer and EPKINLY/ TEPKINLY with AbbVie • Six additional medicines that were created by Genmab, or that leverage Genmab’s DuoBody technology, are being developed and marketed by global pharmaceutical and biotechnology companies • Late-stage pipeline with high potential: EPKINLY, Rina-S and acasunlimab • Suite of proprietary antibody technologies including bispecifics and ADC platform technologies fueling future innovations • Robust clinical and preclinical pipeline fueling future growth • Over 45 Investigational New Drugs (IND) filed by Genmab and/or partners, based on Genmab’s innovations and technology, since 1999 • Industry-leading team with antibody know-how, and expertise in R&D and commercial fields • Partnerships with industry leaders and innovators across the innovation ecosystem of pharma, biotech and academia • Partnership with ChatGPT to launch “AI Everywhere,” providing ChatGPT access to more than 2,000 colleagues • Solid financial foundation enabling our evolution to a fully integrated biotech • Building and expanding our capabilities with more than 2,700 team members across our international locations and through the acquisition of ProfoundBio in 2024 Genmab’s Growing Organization and Presence Princeton, U.S. – Translational and Quantitative Sciences – Development Operations – U.S. Market Operations – Corporate Functions Utrecht, The Netherlands – Discovery and Antibody Research – Translational and Quantitative Sciences – Development Operations – Corporate Functions Copenhagen, Denmark – Headquarters – Chemistry, Manufacturing and Controls (CMC) Operations – Development Operations – Quality Control (QC) Laboratory – Corporate Functions Tokyo, Japan – Development Operations – Japan Market Operations Who We Are Suzhou and Shanghai, China – Early-stage R&D – CMC Operations Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 13 |
|
Building a Fully Integrated Biotech Innovation Powerhouse Team Deeply driven One Genmab team rooted in science and inspired by patients Strong financials Growing recurring revenues and prioritization and focused investments Collaboration Reaches across the innovation ecosystem of pharma, biotech and academia, and drives our business forward Translational and precision medicine, data science and artificial intelligence (AI): Key to accelerating development and ensuring the right therapies get to the right patients Research Track record of success and investing for tomorrow Development Scaling up capabilities to expand from early- to late-stage development Technical Operations and Commercialization The next step in our evolution Enabling functions: Supporting growth and managing risk Business Model At Genmab, we have built a profitable and successful biotech that creates value for our stakeholders. Our Strengths and Differentiators World-class antibody biology knowledge and insight into disease targets Discovery and development engine with proprietary technologies that allow us to build a differentiated pipeline In-house expertise with a solid track record of building successful strategic partnerships Pipeline of potential best-in-class and/or first-in-class therapies Experienced, diverse leadership team Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 14 |
|
1. Research and Discovery Target Identification: The first step in Genmab’s value chain involves identifying disease-related targets, particularly in oncology. This includes research into the biological mechanisms underlying diseases and identifying targets that can be addressed with antibody therapies. Antibody Engineering: Genmab employs its proprietary technologies, such as DuoBody, HexaBody and its ADC platforms, to engineer and develop novel antibodies. These tech-nologies allow for the creation of bispecific antibodies (capable of targeting two different antigens), enhanced antibody functionality and ADCs (antibodies with potent cytotoxic agents coupled to them). 2. Preclinical Development Preclinical Development: Genmab conducts extensive preclinical testing to evaluate the efficacy, safety, and potential of these antibodies before advancing them to clinical trials. 3. Clinical Development Clinical Trials: Genmab advances its antibody candidates through various phases of clinical trials (Phase 1, 2, and 3). This involves testing the candidates in human patients to assess safety, dosage, and efficacy. Strategic Partnerships: During clinical devel-opment, Genmab may decide to partner with pharmaceutical or biotechnology companies to co-develop and co-fund the trials. These part-nerships help mitigate risk and share the costs of development. 4. Manufacturing Process Development: Genmab focuses on developing scalable and efficient manufacturing processes for its antibody products. Genmab does not currently own or operate large-scale manufacturing facilities but works closely with contract manufacturing organizations (CMOs) and partners to ensure high-quality production. QC: Ensuring the consistency, safety, and quality of our products is a key part of the manufac-turing process. Genmab implements strict quality control measures throughout production. 5. Registration and Launch Regulatory Approval: Following successful clinical trials, Genmab seeks regulatory approval from authorities like the European Medicines Agency (EMA) (Europe), the U.S. FDA (U.S.) and MHLW (Japan), and other regulatory bodies. This step is crucial for bringing the investigational medicine to market. 6. Commercialization Marketing and Sales: Once a product receives regulatory approval, Genmab, often through a variety of arrangements, markets and sells the approved medicine. This step includes establishing market strategies including market access, engaging with payors, educating healthcare providers, and launching the product in various regions. Distribution: Genmab collaborates with distribution networks, either through partners or on its own, to ensure the approved medicine reaches healthcare providers and patients in the approved jurisdictions. Pharmacovigilance: After launch, Genmab monitors the product’s performance in the market, collecting data on its safety and effectiveness in broader patient populations. 7. Partnerships and Alliances Licensing: Genmab licenses its proprietary technologies (like the DuoBody and HexaBody platforms) to other biotech and pharmaceutical companies. This generates revenue through upfront payments, milestones, and royalties. Business Development: Genmab’s Business Development team plays a crucial role in the value chain, particularly in bridging the gap between R&D and commercial success by identifying opportunities, helping form strategic alliances, and assisting in in-licensing and out-licensing business. Co-Development: Genmab often enters into co-development agreements where the costs, risks, and profits are shared with partners. This collaboration is particularly important during clinical development and commercialization phases. Strategic Alliances: Beyond traditional partnerships, Genmab engages in strategic alliances with research institutions and other biotech companies to access complementary technologies, expand its pipeline, and explore new therapeutic areas. Refer to section SBM-2 in the sustainability statements for details of key stakeholders including a mapping to Genmab’s value chain. Genmab’s value chain is a comprehensive and integrated process that spans from early-stage R&D to the global commercialization of anti-body-based therapies. Each step is designed to maximize innovation, ensure product quality, and optimize market reach, all while leveraging strategic partnerships to enhance efficiency and profitability. Below is a breakdown of the key components as well as the key stakeholders relevant to each value chain component: Value Chain Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 15 |
|
Refer to the sustainability statements within this Annual Report for material topics identified as part of Genmab’s DMA. The visual maps the material topics to Genmab’s value chain. Refer to the Who We Are section for Genmab’s presence. Value Chain E1: Climate Change • Climate change adaptation/Climate change mitigation(1) • Energy(2) S1: Own Workforce • Working Conditions(3) • Equal treatment and opportunities for all(4) S4: Consumers & End-Users • Social inclusion of consumers and/or end-users(5) • Personal safety and information of consumers and/or end-users(6) G1: Business Conduct(7) Material Topics E1 S1 G1 E1 S4 G1 G1 COMPLIANCE Research & Discovery Preclinical Development Early Clinical Development Late Clinical Development Registration & Launch Discovery of novel antibody targets to address areas of unmet medical need Licensing agreements for product discovery Non-clinical safety and preclinical research and validation Antibody manufacturing for clinical development Licensing agreements for product development and commercialization On-Market Final product manufacturing and packing to support global commercial launch Data collection and analysis Submission of biological license application for regulatory approval and marketing authorization Product launch by approved indication Collaborations and partnerships Distribution channels Submission of investigational new drug application and clinical trial application to regulatory authorities to start clinical trials Joint R&D initiatives Biomarker and translational research Phase 1/2 of development to assess safety and e‘cacy for clinical proof of concept and dose selection for Phase 2/3 Phase 2/3 of development to assess bene’t/risk and value proposition vs. Standard of Care and other competitors Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 16 |
|
Inspired by Nature At Genmab, we are inspired by nature and understand how antibodies work. We are deeply knowledgeable about antibody biology and our scientists harness this expertise to create and develop differentiated inves-tigational antibody medicines. We utilize a sophisticated and highly automated process to efficiently generate, select, produce, and evaluate antibody-based products. Our teams have established a fully integrated R&D enter-prise and streamlined process to coordinate the activities of antibody product discovery, preclinical testing, manufacturing, clinical trial design and execution, and regulatory submissions across Genmab’s international operations. We have expanded our scientific focus to use data science and artificial intel-ligence to aid in the discovery of new targets and biomarkers and bolster our in-depth precision medicine and translational labo-ratory capabilities. Through our expertise in antibody drug development, we pioneer tech-nologies that allow us to create differentiated and potentially first-in-class or best-in-class investigational medicines with the potential to improve patients’ lives. Our antibody expertise has enabled us to create our cutting-edge technology platforms: DuoBody, HexaBody, DuoHexaBody® and HexElect®. With our acquisition of ProfoundBio we gained novel ADC technology platforms. Additional information about our technol-ogies is available on Genmab’s website, www.genmab.com/antibody-science/ antibody-technology-platforms. Research and Development Capabilities Genmab 2024 Annual Report Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 17 |
|
Sustainable and State-of-the-Art Facilities The Netherlands Genmab’s presence in the Netherlands is composed of three buildings in the Utrecht area: The Genmab Research and Development Center (GRDC) and the Accelerator at the Utrecht Science Park and a Genmab office in nearby Zeist. Discovery and preclinical research is conducted at our GRDC and Accelerator facilities, which house state-of-the-art laboratories, including a new chemistry lab that opened at the GRDC in 2024. The GRDC was one of the first Building Research Establishment Environmental Assessment Method (BREEAM) Excellent laboratory buildings in the Netherlands. The Accelerator, a multi-tenant ultra-modern R&D facility, was opened in 2023, enabling our continued R&D growth trajectory. These three spaces are located in close proximity to other life science companies and a premier research university. They accommodate modern auditoriums, and innovative brainstorming and meeting rooms. They provide a bright, open, and collaborative atmosphere and enable the Genmab team to continue to innovate and find new ways to help patients. Denmark Denmark, with its rich history of scientific achievement and innovation, has been our home for Genmab’s headquarters for 25 years. We are surrounded by a vibrant ecosystem of talent, with multiple biotech and pharma peers, academia and research centers, knowledge, and resources. Genmab opened our new headquarters in Valby, Denmark in 2023, a space designed specifically for Genmab. In addition, Genmab introduced our own Good Manufacturing Practice (GMP) QC laboratory in 2023. The new space insources certain business-critical processes and capabilities for our early clinical development. With our growing pipeline and commercial ambitions, we are taking control of processes, prioritization, people, and timing and taking another tremendous step toward becoming an end-to-end biotech innovation powerhouse. United States Genmab opened our new U.S. facility in 2020. This space, modeled on the open and collaborative spirit of the R&D labs and offices in Utrecht and Zeist, includes both offices and laboratories. The U.S. precision medicine laboratories allow Genmab to expand our clinical and preclinical drug development expertise and are part of the strategic growth of the Company. As with our Utrecht facilities, our U.S. office and laboratories were designed and built with sustainability in mind and meet the requirements for Leadership in Energy and Environmental Design (LEED) Gold certification for sustainable design features. Japan Genmab’s Japan office is located in Roppongi, an international business district in the center of Tokyo. It offers an open and collaborative environment that fosters Genmab’s culture of innovation and teamwork. China As part of our acquisition of ProfoundBio, Genmab expanded our presence to our newest locations with state-of-the-art research and CMC capabilities in Suzhou, China. As Genmab continues to grow our geographical footprint, we will endeavor to do so with minimal impact to the environment and with a focus on sustainable practices. Research and Development Capabilities Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 18 |
|
Bringing Our Own Innovative Medicines to Patients We are making continuous progress in our ambition to become a fully-integrated, end-to-end biotech, which includes advancing the development of our fully owned medicines and delivering them to patients around the world. Building on our legacy of innovation and our patient-first approach, we have successfully launched two therapies through partnerships. This year, we expanded the reach of these medicines to additional geographies and patient populations while also progressing the development of wholly-owned, late-stage investigational products. Expanding the Potential of Bispecifics in Lymphoma In June 2024, the U.S. FDA approved a second indication for EPKINLY (epcoritamab-bysp), specifically for the treatment of adults with relapsed or refractory FL after two or more lines of systemic therapy. With this approval, EPKINLY became the first and only T-cell engaging bispecific antibody administered subcutaneously approved in the U.S. to treat this patient population. Subsequently in August 2024, epcoritamab, under the brand name TEPKINLY, became the first subcutaneous bispecific antibody approved as a monotherapy in the EU to treat relapsed or refractory FL after two or more lines of systemic therapy. Additionally in September 2024, the U.S. FDA granted a second breakthrough therapy designa-tion for EPKINLY in relapsed or refractory FL when administered in combination with rituximab and lenalidomide. In Japan, a supplemental J-NDA was submitted to the MHLW for EPKINLY for the treatment of patients with third line plus relapsed or refractory FL grade 1-3A. Follicular lymphoma is the second most common form of non-Hodgkin’s lymphoma (NHL). It is considered incurable, and historically, patients may relapse or face a shortened duration of response with prior standards of care. Since 2023, EPKINLY has received regulatory approvals in more than 50 countries. It was the first bispecific approved in the world with a dual indication in third line plus relapsed or refractory DLBCL and third line plus relapsed or refractory FL. In the U.S., it continues to be the only bispecific with this dual indication. In Japan, it remains the first and only T-cell engaging bispecific antibody administered subcutaneously approved for the treatment of adults with relapsed or refractory large B-cell lymphoma (LBCL) or grade 3B FL. EPKINLY/TEPKINLY is developed and commercialized in partnership with AbbVie. We are creating lasting impact as we bring our medicines to more patients around the world. Creating Community with CeMe™ The CeMe™ campaign in the U.S., created in partnership with Pfizer, builds community and a sense of belonging among those impacted by cervical cancer. “After the treatment, I didn’t know what to do next,” said Anna Ogo. “That’s about when I found a community of women who had experienced cervical cancer like me, and they helped me tremendously. With their support, I began to find confidence in sharing my story.” www.youtube.com/cemestories Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 19 |
|
Bringing Our Own Innovative Medicines to Patients Driving Progress in Cervical Cancer Care Advanced cervical cancer remains a disease with high medical need. In the U.S., up to 16% of cervical cancer cases are diagnosed at the metastatic stage, and up to 61% of earlier-stage diagnoses progress to metastatic disease. Globally, cervical cancer is the fourth most common cause of cancer death in women. In April 2024, the U.S. FDA approved the supplemental Biologics License Application (sBLA) granting approval for Tivdak (tisotumab vedotin-tftv), which we market in the U.S. in partnership with Pfizer. This U.S. FDA action converted the September 2021 accelerated approval of Tivdak to a full approval. Tivdak remains the first and only approved ADC for the treatment of patients with recurrent or metastatic cervical cancer in the U.S. In Japan, the incidence and mortality rates for cervical cancer have been increasing, attributable in part to low vaccination rates for the human papillomavirus (HPV) (<5%) and regular checkups (40%). In April 2024, a J-NDA was submitted to the MHLW for Tivdak for the treatment of advanced or recurrent cervical cancer with disease progression after chemotherapy. Currently, patients whose disease progresses or recurs following initial treatment face limited options. If approved, Tivdak could provide renewed hope to the cervical cancer community. Ensuring Rapid and Sustainable Access to Medicines We are focused in our pursuit to turn innovative science into medicines that create value and deliver meaningful impact to patients and health systems. Ultimately, we positively impact the lives of people with cancer when our science becomes medicine, our medicine creates value, and the value of our medicine is realized by patients who can benefit. Patient access and affordability are key components of this. We aim to ensure that the price of our medicines allows patients, regardless of their socioeconomic or insurance status, to have timely access while considering the transformational potential of our science, its benefit to healthcare systems and society, and our ability to invest in the breakthrough science of the future. At the same time, together with our partners, we work with local country regulatory and payer authorities in the U.S., Japan, and throughout Europe to facilitate registration and reimbursement to help enable patient access to our medicines around the world. Our Approach to Value, Access, and Pricing • Value: The value of our medicines is driven by our innovative science. • Access: Patient impact happens when our medicines reach the people who need them and help them live better. • Pricing: The price of our medicines reflects the innovation behind our science, its impact on patients, and our commitment to bringing that science to patients. We recognize that true patient impact happens when our medicines reach the patients who need them. In the U.S., MyNavCare™ Patient Support by Genmab was created to offer support services to patients prescribed Genmab medicines to help them navigate each step of their unique treatment journey. MyNavCare offers a range of resources and services, from financial assistance to ongoing support, to help patients access the Genmab medication they need. Part of this can include ongoing support from Patient Engagement Liaisons—such as Ann Fodrey. During a hurricane in 2024, one of the patients Ann supports was impacted by the storm and the roof of his home was heavily damaged. Fodrey provided consistent support, checking in to make sure the patient, a retired military veteran, was able to safely get to his treatment and also connected him with a veteran’s organization that was able to install a temporary roof on his house. “Every day we do small things like this. It doesn’t have to be pointing them to an organization that can provide the support they need. It could be connecting them with a peer-to-peer resource, so they have somebody that’s going through a similar experience—listening to them,” Fodrey said. “And that’s just as important.” www.mynavcare.com Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 20 |
|
Antibody Discovery and Development T1/2 Antibody-Drug Conjugate/ Immunocytokine Designer Polyclonals/ Bispecifics Half-Life Extended/Inert/ Fc-Enhanced/Isotypes We are experts in antibody discovery and development. Our appreciation for, and understanding of, the power of the human immune system gives us a unique perspective on how to respond to the constant challenges of oncology drug development. We entered a new chapter in Genmab’s evolution with the commercialization and launch of our first medicine, Tivdak, co-owned with Pfizer, in 2021, and we successfully launched our second medicine, EPKINLY/TEPKINLY, in 2023 under our collaboration with AbbVie. As part of our transformation to a fully integrated biotech, at the end of 2024, we also had two wholly owned programs in Phase 3 development, Rina-S and acasunlimab. ® Target Antibody Format Indication Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 21 |
|
Products and Technologies Pipeline At the end of 2024, Genmab’s proprietary pipeline of investigational medicines, where we are responsible for at least 50% of development, consisted of twelve antibody products in clinical development. These include Genmab’s approved medicines, Tivdak, developed and commercialized together with Pfizer, and EPKINLY/TEPKINLY, developed and commercialized together with AbbVie. In addition to our own pipeline, there are multiple investigational medicines in development by global pharmaceutical and biotechnology companies, including six approved medicines powered by Genmab’s technology and innovations. Beyond the investigational medicines in clinical development, our pipeline includes multiple preclinical programs. An overview of the development status of our approved medicines and of each of our investigational medicines is provided in the following sections. Detailed descriptions of dosing and efficacy and safety data from certain clinical trials have been disclosed in company announcements and media releases published via the Nasdaq Copenhagen stock exchange and may also be found in Genmab’s filings with the U.S. Securities and Exchange Commission (SEC). Additional information is available on Genmab’s website, www.genmab.com. The information accessible through our website is not part of and is not incorporated by reference herein. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 22 |
|
Products and Technologies Genmab’s Proprietary1 Products Approved Medicines Approved Product Target Developed By Disease Indication(s)2 EPKINLY (epcoritamab-bysp, epcoritamab) TEPKINLY (epcoritamab) CD3xCD20 Co-development Genmab/AbbVie Approved in multiple territories including the U.S. and Europe for adult patients with relapsed or refractory DLBCL after two or more lines of systemic therapy and in Japan for adult patients with certain types of relapsed or refractory LBCL after two or more lines of systemic therapy Approved in multiple territories including the U.S. and Europe for adult patients with relapsed or refractory FL after two or more lines of systemic therapy Tivdak (tisotumab vedotin-tftv) TF Co-development Genmab/Pfizer Approved in the U.S. for adult patients with recurrent/metastatic cervical cancer with disease progression on or after chemotherapy 1. Approved and investigational medicines where Genmab has ≥50% ownership, in co-development with partners as indicated. 2. Refer to local country prescribing information for precise indication and safety information. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 23 |
|
Pipeline, Including Further Development for Approved Medicines Product Developed By Target(s) Technology Disease Indications Most Advanced Development Phase Preclinical 1 2 3 Epcoritamab Co-development Genmab/AbbVie CD3, CD20 DuoBody Relapsed/refractory DLBCL Relapsed/refractory FL First line DLBCL First line FL B-cell NHL Relapsed/refractory CLL & Richter’s Syndrome Aggressive mature B-cell neoplasms in pediatric patients Tisotumab vedotin Co-development Genmab/Pfizer Tissue factor ADC Solid tumors Acasunlimab (GEN1046) Genmab PD-L1, 4-1BB DuoBody NSCLC Solid tumors Rinatabart Sesutecan (Rina-S, PRO1184) Genmab FRα ADC PROC Solid tumors GEN1042 (BNT312) Co-development Genmab/BioNTech CD40, 4-1BB DuoBody Solid tumors GEN3014 Genmab1 CD38 HexaBody Hematologic malignancies GEN1059 (BNT314) Co-development Genmab/BioNTech EpCAM, 4-1BB DuoBody Solid tumors GEN1055 (BNT315) Co-development Genmab/BioNTech OX40 HexaBody Solid tumors GEN1160 (PRO1160) Genmab CD70 ADC Advanced solid and liquid tumors GEN1107 (PRO1107) Genmab PTK7 ADC Advanced solid tumors GEN1057 Genmab FAPα, DR4 DuoBody Solid tumors GEN1286 (PRO1286) Genmab EGFR, cMet ADC Advanced solid tumors 1. Genmab is developing HexaBody-CD38 in an exclusive worldwide license and option agreement with J&J. CLL = chronic lymphocytic leukemia In 2024, Genmab discontinued the GEN1047 (DuoBody-CD3xB7H4), GEN3017 (DuoBody-CD3xCD30) and GEN1056 (with BioNTech, BNT322) programs following a strategic re-evaluation of Genmab’s portfolio. For similar reasons, Genmab and BioNTech took the decision to discontinue the clinical development of GEN1053 (HexaBody-CD27, BNT313) including the Phase 1/2 clinical trial (NCT05435339) in solid tumors. Products and Technologies Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 24 |
|
Programs Incorporating Genmab’s Innovation and Technology1 Approved Medicines Approved Product Discovered and/or Developed & Marketed By Disease Indication(s)2 DARZALEX (daratumumab)/DARZALEX FASPRO (daratumumab and hyaluronidase-fihj) J&J (Royalties to Genmab on net global sales) Multiple myeloma Light-chain (AL) Amyloidosis Kesimpta (ofatumumab) Novartis (Royalties to Genmab on net global sales) Relapsing multiple sclerosis (RMS) TEPEZZA (teprotumumab-trbw) Amgen (under sublicense from Roche, royalties to Genmab on net global sales) TED RYBREVANT (amivantamab/amivantamab-vmjw) J&J (Royalties to Genmab on net global sales) NSCLC TECVAYLI (teclistamab/teclistamab-cqyv) J&J (Royalties to Genmab on net global sales) Relapsed and refractory multiple myeloma TALVEY (talquetamab/talquetamab-tgvs) J&J (Royalties to Genmab on net global sales) Relapsed and refractory multiple myeloma 1. Approved and investigational medicines created by Genmab or created by collaboration partners leveraging Genmab’s DuoBody technology platform, under development, and where relevant, commercialized by a third party. 2. See local prescribing information for precise indication and safety information. Pipeline, Including Further Development for Approved Medicines, ≥Phase 2 Development Product Technology Discovered and/or Developed By Disease Indications Most Advanced Development Phase Pre-clinical 1 2 3 Daratumumab UltiMAb1 J&J Multiple myeloma Teprotumumab UltiMAb Amgen TED Amivantamab DuoBody J&J NSCLC Recurrent/metastatic head and neck cancer Advanced or metastatic colorectal cancer Teclistamab DuoBody J&J Multiple myeloma Talquetamab DuoBody J&J Multiple myeloma Amlenetug (Lu AF82422) UltiMAb H. Lundbeck A/S (Lundbeck) Multiple system atrophy Inclacumab UltiMAb Pfizer Vaso-occlusive crises in sickle cell disease Mim8 DuoBody Novo Nordisk A/S (Novo Nordisk) Hemophilia A 1. UltiMab transgenic mouse technology licensed from Medarex, Inc. (Medarex), a wholly owned subsidiary of Bristol-Myers Squibb. Products and Technologies Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 25 |
|
Genmab’s Proprietary Pipeline Programs where Genmab has ≥50% ownership. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 26 |
|
Genmab’s Proprietary Pipeline EPKINLY/TEPKINLY (epcoritamab) First and only bispecific antibody approved in the U.S. and Europe to treat both relapsed or refractory FL and DLBCL after two or more lines of systemic therapy • SC bispecific antibody targeting CD3 and CD20 created using Genmab’s DuoBody technology platform • Epcoritamab (approved as EPKINLY and TEPKINLY) has received regulatory approvals in multiple territories including in the U.S. and Europe for adult patients with relapsed or refractory DLBCL after two or more lines of systemic therapy, and in Japan for adult patients with certain types of relapsed or refractory LBCL after two or more lines of systemic therapy • EPKINLY/TEPKINLY have also been approved in the U.S. and Europe for the treatment of adults with relapsed or refractory FL after two or more lines of systemic therapy. Regulatory submission for epcoritamab for this indication is currently under review in Japan • Multiple clinical trials are ongoing across different settings and histologies, including five Phase 3 trials, with more trials in planning • Co-developed and co-commercialized in collaboration with AbbVie Epcoritamab is a proprietary bispecific antibody created using Genmab’s DuoBody technology platform. Epcoritamab targets CD3, which is expressed on T-cells, and CD20, a clinically validated target on malignant B-cells. Genmab used technology licensed from Medarex to generate the CD20 antibody forming part of epcoritamab. Epcoritamab is marketed as EPKINLY in the U.S., Japan, and other regions, and as TEPKINLY in Europe and other regions. See local prescribing information for specific indications and safety information. In 2020, Genmab entered into a collaboration agreement with AbbVie to jointly develop and commer-cialize epcoritamab. The companies share commercialization responsibilities in the U.S. and Japan, with AbbVie responsible for further global commercialization. Genmab records sales in the U.S. and Japan and receives tiered royalties between 22% and 26% on remaining global sales outside of these territories, subject to certain royalty reductions. The companies have a broad clinical development program for epcoritamab including five ongoing Phase 3 trials and additional trials in planning. Please refer to Note 5.6 of the financial statements for further details regarding the epcoritamab collaboration with AbbVie. Please consult the U.S. Prescribing Information for EPKINLY and the European Summary of Product Characteristics for TEPKINLY for the labeled indication and safety information. Fourth Quarter Update • December: Multiple presentations at the 66th American Society of Hematology (ASH) Annual Meeting, including four oral presentations. Data from the EPCORE® CLL-1 trial of epcoritamab monotherapy in patients with relapsed or refractory CLL was selected for presentation during the prestigious ASH Annual Meeting Press Program. Updates from First Quarter to Third Quarter • September: The U.S. FDA granted a Breakthrough Therapy Designation (BTD) for epcoritamab in combination with rituximab and lenalidomide for the treatment of adult patients with relapsed or refractory FL after at least one line of systemic therapy. This is the second BTD granted for epcoritamab. • August: The European Commission (EC) granted conditional marketing authorization for TEPKINLY (epcoritamab) as a monotherapy for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy. The conditional marketing authorization was supported by data from the FL cohort of the EPCORE NHL-1 trial (NCT03625037). Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 27 |
|
• June: The U.S. FDA approved EPKINLY (epcoritamab-bysp) for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy. The approval was supported by data from the FL cohort of the EPCORE NHL-1 trial. • June: The EMA’s Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending the granting of conditional marketing authorization of epcoritamab for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy. • June: Multiple data presentations were featured at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting including two rapid oral presentations. These presentations included data from the pivotal and cycle 1 dose optimization FL cohorts of the EPCORE NHL-1 clinical trial, which was subsequently selected for presentation at the Best of ASCO conference. • June: Multiple data presentations were featured at the 2024 European Hematology Association (EHA) Congress including three oral presentations. • June: Data published in The Lancet Haematology, “Epcoritamab monotherapy in patients with relapsed or refractory follicular lymphoma (EPCORE NHL-1): a phase 2 cohort of a single-arm, multicentre study.” • May: Epcoritamab monotherapy was added to the National Comprehensive Cancer Network® (NCCN®) Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for “B-cell Lymphomas” (Version 2.2024) for third-line and subsequent therapy for patients with FL as a Category 2A, preferred regimen. • March: Genmab submitted a supplemental J-NDA to the MHLW in Japan for SC epcoritamab for the treatment of relapsed or refractory FL after two or more lines of systemic therapy. The application was supported by data from the FL cohort of the EPCORE NHL-1 trial and the EPCORE NHL-3 (NCT04542824) trial. • February: The U.S. FDA granted Priority Review for the sBLA for epcoritamab-bysp for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy. The submission was supported by data from the FL cohort of the EPCORE NHL-1 trial. • February: A new Phase 3 clinical trial was initiated, evaluating the safety and efficacy of SC epcoritamab in combination with rituximab and lenalidomide compared to chemoimmunotherapy in previously untreated FL (EPCORE FL-2, NCT06191744). • February: Multiple data presentations, including oral presentations, at the 21st Annual Meeting of the Japanese Society of Medical Oncology (JSMO). Genmab’s Proprietary Pipeline About Diffuse Large B-cell Lymphoma DLBCL is the most common type of B-cell NHL worldwide, accounting for approximately 25%–30% of all NHL cases.1,2 In the U.S. there are approximately 25,000 new cases of DLBCL diagnosed each year.3 DLBCL can arise in lymph nodes as well as in organs outside of the lymphatic system, occurs more commonly in the elderly and is slightly more prevalent in men.4,5 DLBCL is a fast-growing type of NHL, a cancer that develops in the lymphatic system and affects B-cell lymphocytes, a type of white blood cell. For many people living with DLBCL, their cancer either relapses, which means it may return after treatment, or becomes refractory, meaning it does not respond to treatment. Although new therapies have become available, treatment management can remain a challenge.6,7 1. Lymphoma Research Foundation. Diffuse Large B-Cell Lymphoma. Accessed November 22, 2024. https://lymphoma.org/understanding-lymphoma/aboutlymphoma/nhl/dlbcl/ 2. Padala, et al. Diffuse Large B-Cell Lymphoma. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. 2023 Apr 24. 3. Leukemia and Lymphoma Society. Diffuse Large B-Cell Lymphoma (DLBCL). Accessed November 22, 2024. https://www.lls.org/research/diffuse-large-b-cell-lymphoma-dlbcl 4. Sehn LH, Salles G. N Engl J Med. 2021;384:842-858. 5. Kanas G, Ge W, Quek RGW, et al. Leukemia & Lymphoma. 2022;63(1):54-63. 6. Sehn LH, Salles G. N Engl J Med. 2021;384:842-858. 7. Crump M, Neelapu SS, Farooq U, et al. Blood. 2017;130(16):1800-1808. DLBCL accounts for ~25%–30% of all NHL cases worldwide ~25,000 new cases diagnosed in the U.S. each year Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 28 |
|
Genmab’s Proprietary Pipeline Ongoing Clinical Trials B-NHL Type Stage Development Phase Pre-clinical 1 2 3 DLBCL Relapsed/Refractory EPCORE DLBCL-1 Front-line + R-CHOP EPCORE DLBCL-2 Relapsed/Refractory + lenalidomide, ASCT ineligible EPCORE DLBCL-4 Front-line +/- lenalidomide EPCORE DLBCL-3 FL Relapsed/Refractory (Combo) EPCORE FL-1 Front-line +R2 EPCORE FL-2 DLBCL & FL Outpatient EPCORE NHL-6 B-NHL Relapsed/Progressive/Refractory EPCORE NHL-1 Relapsed/Progressive/Refractory (Japan) EPCORE NHL-3 Relapsed/Refractory Pediatric EPCORE Peds-1 Previously Untreated/Relapsed/Refractory (Combo) EPCORE NHL-2 Previously Untreated/Relapsed/Refractory (China) EPCORE NHL-4 Previously Untreated/Relapsed/Refractory (Combo) EPCORE NHL-5 CLL/Richter’s Syndrome Relapsed/Refractory EPCORE CLL-1 R-CHOP = rituximab-cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone; ASCT = autologous stem cell transplant; R2 = rituximab, lenalidomide About Follicular Lymphoma FL is typically an indolent (or slow-growing) form of NHL that arises from B-lymphocytes and is the second most common form of NHL accounting for 20–30% of all cases.1 About 15,000 people develop FL each year in the U.S.2 and it is considered incurable with current standard of care therapies.3 Patients often relapse and, with each relapse the remission and time to next treatment is shorter.4 Over time, transformation to DLBCL, an aggressive form of NHL associated with poor survival outcomes, can occur in more than 25% of FL patients.5 1. Lymphoma Research Foundation official website. https://lymphoma.org/aboutlymphoma/nhl/fl/. Accessed November 22, 2024. 2. Leukemia & Lymphoma Society. https://www.lls. org/research/follicular-lymphoma-fl. Accessed November 22, 2024. 3. Ghione P, Palomba ML, Ghesquieres H, et al. Treatment patterns and outcomes in relapsed/ refractory follicular lymphoma: results from the international SCHOLAR-5 study. Haematologica. 2023;108(3):822-832. doi: 10.3324/ haematol.2022.281421. 4. Rivas-Delgado A, Magnano L, Moreno-Velázquez M, et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol. 2018;184(5):753-759. doi:10.1111/bjh.15708. 5. Al-Tourah AJ, Gill KK, Chhanabhai M, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol. 2008 Nov 10;26(32):5165-9. doi: 10.1200/JCO.2008.16.0283. Epub 2008 Oct 6. PMID: 18838711. FL accounts for 20%–30% of all NHL cases ~15,000 people develop FL each year in the U.S. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 29 |
|
Genmab’s Proprietary Pipeline Tivdak (tisotumab vedotin-tftv) First and Only U.S. FDA Approved ADC for Recurrent or Metastatic Cervical Cancer • An ADC directed to TF, a protein highly prevalent on solid tumors, including cervical cancer, which is associated with poor prognosis • Full approval granted by the U.S. FDA for tisotumab vedotin-tftv, marketed as Tivdak, for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy; Tivdak is the first ADC with demonstrated overall survival data to be granted full U.S. FDA approval in this patient population • Regulatory submissions for tisotumab vedotin for the treatment of recurrent or metastatic cervical cancer are currently under review in both Japan and Europe • Co-developed globally and co-promoted in the U.S. in collaboration with Pfizer Tisotumab vedotin is an ADC composed of Genmab’s human monoclonal antibody directed to TF and Pfizer’s ADC technology that utilizes a protease-cleavable linker that covalently attaches the microtubule-disrupting agent monomethyl auristatin E to the antibody. Genmab used technology licensed from Medarex to generate the TF antibody forming part of tisotumab vedotin. Tisotumab vedotin-tftv, marketed as Tivdak, is the first and only U.S. FDA approved ADC for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy. Tisotumab vedotin is being co-developed by Genmab and Pfizer. Under a joint commercialization agreement, Genmab is co-promoting Tivdak in the U.S. and will lead commercial operational activi-ties in Japan once approved. Pfizer is leading commercial operational activities in the U.S. and will lead commercial operational activities in China once approved. In the U.S. market there will be a 50:50 profit split. Effective January 1, 2025, Genmab and Pfizer agreed to amend the License and Collaboration Agreement and the Joint Commercialization Agreement for Tivdak, assigning Genmab sole responsibility for the development and commercialization of Tivdak for second line plus recurrent or meta-static cervical cancer in Europe and all other regions globally, excluding the United States and the China region. Genmab will record sales for Europe, Japan and rest of world markets (excluding the United States and the China region), once commercialized, and will provide royalties to Pfizer on net sales in the low teens. The companies have joint decision-making power on the worldwide development and commercialization strategy for Tivdak. Please refer to Note 5.6 of the financial statements for further details regarding the tisotumab vedotin collaboration with Pfizer. Please consult the U.S. Prescribing Information for Tivdak for the labeled indication and safety information, including the boxed warning. Fourth Quarter Update • December: The U.S. NCCN updated its Clinical Practice Guidelines in Oncology for Cervical Cancer with tisotumab vedotin-tftv changing from a category 2A to a category 1. Tisotumab vedotin-tftv plus pembrolizumab was also added as an option for PD-L1 positive tumors. • October: Upon strategic evaluation we have decided to discontinue preparation for a Phase 3 study in second/third line squamous cell carcinoma of the head and neck. Updates from First Quarter to Third Quarter • July: Data from innovaTV 301 clinical trial (NCT04697628) published in New England Journal of Medicine, Tisotumab Vedotin as Second- or Third-Line Therapy for Recurrent Cervical Cancer. • June: Two data presentations were featured at the 2024 ASCO Annual Meeting including a rapid oral presentation of data from the Phase 2 innovaTV 207 (NCT03485209) trial, evaluating tisotumab vedotin in pretreated patients with relapsed/metastatic head and neck squamous cell carcinoma. • April: Genmab submitted a J-NDA to the MHLW in Japan for Tivdak for the treatment of adult patients with advanced or recurrent Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 30 |
|
cervical cancer that has progressed on or after chemotherapy. • April: The U.S. FDA granted full approval for Tivdak (tisotumab vedotin-tftv) for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy. This U.S. FDA action converted the September 2021 accelerated approval of Tivdak to a full approval. • March: The U.S. NCCN updated their Clinical Practice Guidelines in Oncology for Vaginal Cancer to include tisotumab vedotin-tftv under “Other Recommended Regimens” as second-line or subsequent systemic therapy for patients with recurrent or metastatic squamous cell carcinoma/adenocarcinoma primary vaginal cancer. • March: Multiple data presentations, including a late-breaking oral presentation, at the Society of Gynecologic Oncology (SGO) 2024 Annual Meeting. • February: The EMA validated for review the marketing authorization application of tisotumab vedotin for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after systemic therapy. • January: The U.S. FDA accepted the sBLA seeking to convert the accelerated approval of Tivdak to full approval for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after first-line therapy. Genmab’s Proprietary Pipeline About Cervical Cancer Cervical cancer remains a disease with high unmet need despite advances in effective vaccination and screening practices to prevent and diagnose pre-/early-stage cancers for curative treatment. Recurrent and/or metastatic cervical cancer is a particularly devastating and mostly incurable disease; up to 15% of adults with cervical cancer present with metastatic disease at diagnosis1,2 and, for adults diagnosed at earlier stages who receive treatment, up to 61%3 will experience disease recurrence. It was estimated that in 2024, more than 13,820 new cases of invasive cervical cancer were diagnosed in the U.S. and 4,360 adults would die from the disease.1 1. National Cancer Institute. SEER Cancer Stat Facts: Cervical Cancer. 2023. https://seer.cancer.gov/ statfacts/html/cervix.html. Accessed November 22, 2024. 2. McLachlan J, Boussios S, Okines A, et al. The impact of systemic therapy beyond first-line treatment for advanced cervical cancer. Clin Oncol (R Coll Radiol). 2017;29(3):153-60 3. Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol. 2016;214(1):22-30 In 2024 >13,820 new cases of invasive cervical cancer were diagnosed in the U.S. 4,360 adults would die from the disease Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 31 |
|
Genmab’s Proprietary Pipeline Acasunlimab (GEN1046) Bispecific Next-generation Immunotherapy • Bispecific antibody targeting PD-L1 and 4-1BB, created using Genmab’s DuoBody technology platform • A Phase 3 trial (NCT06635824, ABBIL1TY NSCLC-06) in NSCLC is recruiting • In 2024 Genmab assumed sole responsibility for the continued development and potential commercialization of acasunlimab Acasunlimab (GEN1046, DuoBody-PD-L1x4-1BB) is a proprietary bispecific antibody, created using Genmab’s DuoBody technology platform. In August 2024, BioNTech opted not to participate in the further development of the acasunlimab program under the parties’ existing License and Collaboration Agreement for reasons related to BioNTech’s portfolio strategy. Genmab subsequently assumed sole responsibility for the continued development and potential commercialization of acasunlimab and the program will be subject to payment of certain milestones and a tiered single-digit royalty on net sales by Genmab to BioNTech. Please refer to Note 5.6 of the financial statements for further details regarding Genmab’s collaboration with BioNTech. Acasunlimab is designed to induce an antitumor immune response by simultaneous and complementary programmed death ligand 1 (PD-L1) blockade and conditional 4-1BB stimulation using an inert DuoBody format. A Phase 3 trial (ABBIL1TY NSCLC-06) of acasunlimab in combination with pembrolizumab compared to docetaxel in CPI-experienced, PD-L1 positive metastatic NSCLC is recruiting. Fourth Quarter Update • November: A Phase 3 open-label trial was initiated to evaluate the efficacy and safety of acasunlimab in combination with pembrolizumab versus docetaxel (standard of care) in patients with PD-L1-positive metastatic NSCLC who have been treated with PD-1/PD-L1 inhibitor and platinum-containing chemotherapy, administered either in combination or sequentially in the metastatic setting. • November: Additional support for the acasunlimab every six weeks (Q6W) dosing schedule, along with preclinical data, was presented during poster sessions at the Society for Immunotherapy of Cancer (SITC) 2024 Conference. Updates from First Quarter to Third Quarter • September: Translational and pharmacokinetic/pharmacodynamic data supporting the Phase 3 dosing regimen for acasunlimab in combination with pembrolizumab in patients with relapsed/ refractory metastatic NSCLC was presented as a poster at the 2024 World Conference on Lung Cancer (WCLC). • August: As noted above, BioNTech opted not to participate in the further development of the acasunlimab program. Genmab subsequently assumed sole responsibility for the continued development and potential commercialization of acasunlimab. • June: Data from the Phase 2 trial of acasunlimab as a single agent or in combination with pembrolizumab for the treatment of relapsed/refractory metastatic NSCLC after treatment with standard of care therapy with an immune checkpoint inhibitor was presented as a poster at the 2024 ASCO Annual Meeting. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 32 |
|
Genmab’s Proprietary Pipeline GEN1042 (BNT312) Potential First-in-Class Bispecific Agonistic Antibody • Bispecific antibody targeting CD40 and 4-1BB, created using Genmab’s DuoBody technology platform • Multiple clinical trials in solid tumors ongoing • Co-developed in collaboration with BioNTech GEN1042 (DuoBody-CD40x4-1BB, BNT312) is a proprietary bispecific antibody, jointly owned by Genmab and BioNTech, created using Genmab’s DuoBody technology platform. It is being co-developed by Genmab and BioNTech under an agreement in which the companies share all costs and future potential profits for GEN1042 on a 50:50 basis. CD40 and 4-1BB were selected as targets to enhance activation of both dendritic cells and antigen-dependent T-cells. Three clinical trials of GEN1042 in solid tumors are ongoing. Please refer to Note 5.6 of the financial statements for further details regarding Genmab’s collaboration with BioNTech. Rinatabart Sesutecan (Rina-S, GEN1184) Potential Best-in-class Folate Receptor Alpha (FRα)-targeted Type I Topoisomerase (TOPO1) ADC • FRα-targeted TOPO1 ADC being evaluated for potential treatment of FRα-expressing cancers • Phase 3 clinical trial (NCT06619236) in Platinum-resistant Ovarian Cancer (PROC) is recruiting Rina-S is a novel FRα-targeted TOPO1 ADC being evaluated for the potential treatment of ovarian cancer and other FRα-expressing cancers. Dose escalation data suggests that Rina-S has robust single agent activity in various cancers across a broad range of FRα expression levels. In January 2024, Rina-S was granted Fast Track Designation by the U.S. FDA for the treatment of FRα-expressing high-grade serous or endometrioid PROC. A Phase 3 trial (NCT06619236) of Rina-S in second line plus PROC is recruiting. Fourth Quarter Update • December: A Phase 3 open-label trial was initiated to evaluate the efficacy and safety of Rina-S versus treatment of investigator’s choice chemotherapy in patients with PROC. Update from First Quarter to Third Quarter • September: Data from the Phase 1/2 study of Rina-S were presented as a mini-oral presentation at the European Society of Medical Oncology Congress 2024 (ESMO). Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 33 |
|
Genmab’s Proprietary Pipeline GEN3014 HexaBody-based Investigational Medicine with Potential in Hematological Malignancies • Antibody targeting CD38, created using Genmab’s HexaBody technology platform • Phase 1/2 clinical trial (NCT04824794) in relapsed/ refractory multiple myeloma and other hematological malignancies ongoing • Developed in an exclusive worldwide license and option agreement with J&J GEN3014 (HexaBody-CD38) is a human CD38 monoclonal antibody-based investigational medicine created using Genmab’s HexaBody technology platform. GEN3014 is a second generation CD38 targeting immunoglobulin G1 (IgG1) antibody with a hexamerization-enhancing modification. GEN3014 is designed to induce antitumor activity through highly potent complement-dependent cytotoxicity (CDC) and antitumor activity, which is enhanced compared to daratumumab as demonstrated in previously presented preclinical data and is effective at a wider range of target expression levels. In June 2019, Genmab entered into an exclusive worldwide license and option agreement with J&J to develop and commercialize GEN3014. A Phase 1/2 clinical trial in hematologic malignancies is ongoing and includes a cohort comparing GEN3014 to daratumumab in CD38 monoclonal antibody-naïve relapsed or refractory multiple myeloma patients. Fourth Quarter Update • December: Per the terms of the agreement between Genmab and J&J, Genmab submitted a data package to J&J comparing GEN3014 to daratumumab in CD38 monoclonal antibody-naïve relapsed or refractory multiple myeloma patients. This data will be used to inform J&J’s decision on whether to exercise its option to receive a worldwide license to develop, manufacture and commercialize GEN3014. GEN1059 (BNT314) Bispecific Antibody with Potential in Solid Tumors • Bispecific antibody targeting epithelial cell adhesion molecule (EpCAM) and 4-1BB, created using Genmab’s DuoBody technology platform • Phase 1/2 clinical trial (NCT06150183) in malignant solid tumors recruiting • Co-developed in collaboration with BioNTech GEN1059 (DuoBody-EpCAMx4-1BB, BNT314), jointly owned by Genmab and BioNTech and created using Genmab’s DuoBody technology platform, is a bispecific antibody aimed at boosting antitumor immune responses through EpCAM-dependent 4-1BB agonistic activity. GEN1059 is being co-developed by Genmab and BioNTech under an agreement in which the companies share all costs and future potential profits for GEN1059 on a 50:50 basis. A Phase 1/2 clinical trial of GEN1059 in solid tumors is recruiting. Please refer to Note 5.6 of the financial statements for further details regarding Genmab’s collaboration with BioNTech. Update from First Quarter to Third Quarter • January: The first patient was treated in the first-in-human Phase 1/2 trial of GEN1059 in advanced or metastatic solid tumors. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 34 |
|
Genmab’s Proprietary Pipeline GEN1055 (BNT315) HexaBody-based Antibody with Potential in Solid Tumors • Antibody targeting OX40, created using Genmab’s HexaBody technology platform • Phase 1/2 clinical trial (NCT06391775) in malignant solid tumors recruiting • Co-developed in collaboration with BioNTech GEN1055 (HexaBody-OX40, BNT315), jointly owned by Genmab and BioNTech and created using Genmab’s HexaBody technology platform, is an immune-modulating OX40 agonist antibody designed to promote immunity by enhancing T-cell responses through FcγR-independent OX40 clustering on T cells. GEN1055 is being co-developed by Genmab and BioNTech under an agreement in which the companies share all costs and future potential profits for GEN1055 on a 50:50 basis. A Phase 1/2 clinical trial of GEN1055 in solid tumors is recruiting. Please refer to Note 5.6 of the financial statements for further details regarding Genmab’s collaboration with BioNTech. Update from First Quarter to Third Quarter • June: The first patient was treated in the first-in-human Phase 1/2 trial of GEN1055 in malignant solid tumors. GEN1160 ADC with Potential in Both Solid Tumors and Hematological Malignancies • CD70-targeted ADC being evaluated in advanced solid and liquid tumors • Phase 1/2 clinical trial (NCT05721222) in advanced solid and liquid tumors recruiting GEN1160 is a CD70-targeted ADC. CD70 is a protein expressed on both solid tumors and hematological malignancies. A Phase 1/2 clinical study of GEN1160 in advanced renal cell carcinoma, nasopharyngeal carcinoma and NHL is recruiting. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 35 |
|
Genmab’s Proprietary Pipeline GEN1107 ADC with Potential in Solid Tumors • Protein tyrosine kinase 7 (PTK7)- targeted ADC being evaluated in advanced solid tumors • Phase 1/2 clinical trial (NCT06171789) in advanced solid tumors recruiting GEN1107 is a PTK7-targeted ADC. PTK7 is a clinically validated ADC target with broad solid tumor expression, particularly in tumor-initiating cells. A Phase 1/2 clinical study of GEN1107 in advanced solid tumors is recruiting. GEN1057 Bispecific antibody with potential in solid tumors • Bispecific antibody targeting fibroblast activation protein alpha (FAPα) and death receptor 4 (DR4), created using Genmab’s DuoBody technology platform • Phase 1/2 clinical trial (NCT06573294) in malignant solid tumors recruiting GEN1057 (DuoBody-FAPαxDR4) is a bispecific antibody-based investigational medicine created using Genmab’s DuoBody technology platform. GEN1057 is designed for the conditional DR4 transactivation-mediated tumor cell killing by crosslinking FAPα expressed on cancer-associated fibroblasts with DR4 expressed on tumor cells. A Phase 1/2 clinical trial of GEN1057 in malignant solid tumors is recruiting. Update from First Quarter to Third Quarter • September: The first patient was dosed in the first-in-human Phase 1/2 clinical trial of GEN1057 in solid tumors. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 36 |
|
Genmab’s Proprietary Pipeline GEN1286 ADC with Potential in Solid Tumors • ADC that targets epidermal growth factor receptor (EGFR) and cellular-mesenchymal epithelial transition factor receptor tyrosine kinase (cMet) being evaluated in advanced solid tumors • Phase 1/2 clinical trial (NCT06685068) in advanced solid tumors recruiting GEN1286 is an ADC targeting EGFR and cMet, two validated cancer targets. A Phase 1/2 clinical study of GEN1286 in advanced solid tumors is recruiting. Fourth Quarter Update • December: The first patient was dosed in the first-in-human Phase 1/2 clinical trial of GEN1286 in solid tumors. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 37 |
|
Preclinical Programs • Broad preclinical pipeline that includes both partnered products and in-house programs based on our proprietary technologies and/or antibodies • Multiple new IND applications expected to be submitted over the coming years • Genmab has entered multiple strategic collaborations to support the expansion of our innovative pipeline, including our acquisition of ProfoundBio in 2024 Our preclinical pipeline includes immune effector function enhanced antibodies developed with our HexaBody technology platform, bispecific antibodies created with our DuoBody technology platform and ADCs created with our ADC technology platforms. We are also collaborating with our partners to generate additional new antibody-based product concepts. A number of the preclinical programs are conducted in cooperation with our collaboration partners. Update from First Quarter to Third Quarter • September: Clinical Trial Application (CTA) submitted in Europe for GEN1078. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 38 |
|
Approved Medicines Incorporating Genmab’s Innovations and Technology In addition to Genmab’s own pipeline of investigational medicines, our innovations and proprietary technology platforms are applied in the pipelines of global pharmaceutical and biotechnology companies. These companies are running clinical development programs with antibodies created by Genmab or created using Genmab’s proprietary DuoBody bispecific antibody technology platform. The information in this section includes those therapies that have been approved by regulatory agencies in certain territories. Under the agreements for these medicines Genmab is entitled to certain potential milestones and royalties. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 39 |
|
Redefining the Treatment of Multiple Myeloma • First-in-class human CD38 monoclonal antibody • Developed and commercialized by J&J under an exclusive worldwide license from Genmab • Intravenous (IV) formulation approved in combination with other therapies and as monotherapy for certain multiple myeloma indications • First and only SC CD38-directed antibody approved for the treatment of certain multiple myeloma indications, known as DARZALEX FASPRO in the U.S., and as DARZALEX SC in Europe • SC daratumumab is the first and only approved therapy for AL amyloidosis in the U.S., Europe, and Japan • 2024 net sales of DARZALEX by J&J were USD 11,670 million Daratumumab is a human monoclonal antibody that binds with high affinity to the CD38 molecule, which is highly expressed on the surface of multiple myeloma cells and is also expressed by AL amyloidosis plasma cells. Genmab used technology licensed from Medarex to generate the CD38 antibody. Daratumumab is being developed and commer-cialized by J&J under an exclusive worldwide license from Genmab. Under the terms of the agreement, Genmab receives royalties between 12% and 20% with J&J reducing such royalty payments for Genmab’s share of J&J’s royalty payments made to Halozyme; payments are further reduced in countries and territories where there are no relevant patents. Please refer to Note 5.6 of the financial statements for further details regarding the daratumumab collaboration with J&J. Daratumumab (marketed as DARZALEX for IV administration and as DARZALEX FASPRO in the U.S. and as DARZALEX SC in Europe for SC administration) is approved in a large number of territories for the treatment of adult patients with certain multiple myeloma indications and is the only approved therapy in the U.S., Europe and Japan for the treatment of adult patients with AL amyloidosis. Please consult the European Summary of Product Characteristics for DARZALEX and DARZALEX SC and the U.S. Prescribing Information for DARZALEX and DARZALEX FASPRO for the labeled indication and safety information. Approved Medicines Incorporating Genmab’s Innovations and Technology Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 40 |
|
Approved Medicines Incorporating Genmab’s Innovations and Technology Approved in the Treatment of RMS • Human CD20 monoclonal antibody developed and commercialized by Novartis under a license agreement with Genmab • Approved in territories including the U.S., EU and Japan for treatment of RMS in adults • First B-cell therapy that can be self-administered by patients using the Sensoready® autoinjector pen Ofatumumab is a human monoclonal antibody that targets an epitope on the CD20 molecule encompassing parts of the small and large extracellular loops. Genmab used technology licensed from Medarex to generate the CD20 antibody. Ofatumumab, marketed as Kesimpta, is approved in territories including the U.S., Europe, and Japan for the treatment of certain adult patients with RMS. Kesimpta is the first B-cell therapy that can be self-administered by patients using the Sensoready autoinjector pen, once monthly after starting therapy. Ofatumumab is being developed and marketed worldwide by Novartis under a license agreement between Genmab and Novartis. Under the terms of the agreement, Genmab receives a 10% royalty on net sales of Kesimpta, and Genmab pays a low-single digit royalty to Medarex based on Kesimpta sales. Please refer to Note 5.6 of the financial statements for further details regarding the ofatumumab collaboration with Novartis. Please consult the U.S. Prescribing Information and the European Summary of Product Characteristics for the labeled indication and safety information for Kesimpta. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 41 |
|
Approved Medicines Incorporating Genmab’s Innovations and Technology First U.S. FDA Approved Medicine for the Treatment of TED • Developed and commercialized by Amgen for the treatment of TED • First and only approved medicine for the treatment of TED in the U.S. and Japan • Regulatory approval pending in Europe Teprotumumab, approved by the U.S. FDA and by Japan’s MHLW under the trade name TEPEZZA, is a human monoclonal antibody that targets the Insulin-like Growth Factor 1 Receptor (IGF-1R), a validated target. It is the first and only medicine approved in the U.S. and in Japan for the treatment of TED. Genmab used technology licensed from Medarex to generate the IGF-1R antibody. The antibody was created by Genmab under a collaboration with Roche. Development and commercialization of the product was subsequently conducted by Horizon Therapeutics plc (Horizon) under a sublicense from Roche. In October 2023, Amgen completed its acquisition of Horizon, including the rights to all commercialization and development of teprotumumab. Under the terms of Genmab’s agreement with Roche, Genmab receives a mid-single digit royalty on net sales (as defined) of TEPEZZA. Please refer to Note 5.6 of the financial statements for further details regarding the teprotumumab collaboration. Please consult the U.S. Prescribing Information for the labeled indication and safety information for TEPEZZA. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 42 |
|
First Regulatory Approvals for a DuoBody-based Medicine • Part of Genmab and J&J DuoBody research and license agreement • First approved medicine created using Genmab’s proprietary DuoBody technology • Under the agreement with J&J, Genmab is eligible to receive milestones and receives royalties on net sales of RYBREVANT In July 2012, and as amended in December 2013, Genmab entered into a collaboration with J&J to create and develop bispecific antibodies using Genmab’s DuoBody technology platform. One of these, J&J’s amivantamab, is a fully human bispecific antibody that targets EGFR and cMet, two validated cancer targets. The two antibody libraries used to produce amivantamab were both generated by Genmab. In collaboration with J&J, the antibody pair used to create amivantamab was co-discovered. Amivantamab, marketed as RYBREVANT, is approved in certain territories for the treatment of certain adult patients with NSCLC. J&J is responsible for the development and commercialization of amivantamab. Under the agreement with J&J, Genmab is eligible to receive milestones and receives royalties between 8% and 10% on net sales of RYBREVANT subject to a reduction of such royalty payments in countries and territories where there are no relevant patents, among other reductions. Genmab pays a royalty to Medarex based on RYBREVANT net sales. Please refer to Note 5.6 of the financial statements for further details regarding the DuoBody collaboration with J&J. Please consult the U.S. Prescribing Information and the European Summary of Product Characteristics for RYBREVANT for the labeled indication and safety information. Approved Medicines Incorporating Genmab’s Innovations and Technology Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 43 |
|
Approved Medicines Incorporating Genmab’s Innovations and Technology Bispecific Antibody Approved for the Treatment of Relapsed and Refractory Multiple Myeloma • Part of Genmab and J&J DuoBody research and license agreement • Second approved medicine created using Genmab’s proprietary DuoBody technology • Under the agreement with J&J, Genmab is eligible to receive milestones and receives royalties on net sales of TECVAYLI In July 2012, and as amended in December 2013, Genmab entered into a collaboration with J&J to create and develop bispecific antibodies using Genmab’s DuoBody technology platform. One of the products subsequently discovered and developed by J&J is teclistamab, a bispecific antibody that targets CD3, which is expressed on T-cells, and B-cell maturation antigen (BCMA), which is expressed in mature B lymphocytes. Teclistamab, marketed as TECVAYLI, is approved in certain territories for the treatment of certain adult patients with relapsed or refractory multiple myeloma. J&J is responsible for the development and commercialization of TECVAYLI. Under our agreement with J&J, Genmab is eligible to receive milestones and receives a mid-single digit royalty on net sales of TECVAYLI subject to a reduction of such royalty payments in countries and territories where there are no relevant patents, among other reductions. Please refer to Note 5.6 of the financial statements for further details regarding the DuoBody collaboration with J&J. Please consult the U.S. Prescribing Information and the European Summary of Product Characteristics for TECVAYLI for the labeled indication and safety information. (teclistamab) TECVAYLI TM Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 44 |
|
Approved Medicines Incorporating Genmab’s Innovations and Technology Bispecific Antibody Approved for the Treatment of Relapsed and Refractory Multiple Myeloma • Part of Genmab and J&J DuoBody research and license agreement • Fourth approved medicine created using Genmab’s proprietary DuoBody technology • Under the agreement with J&J, Genmab is eligible to receive milestones and receives royalties on net sales of TALVEY In July 2012, and as amended in December 2013, Genmab entered into a collaboration with J&J to create and develop bispecific antibodies using Genmab’s DuoBody technology platform. One of the products subsequently discovered and developed by J&J is talquetamab, a bispecific antibody that targets CD3, which is expressed on T-cells, and G protein-coupled receptor, family C, group 5, member D (GPRC5D), an orphan receptor expressed in malignant plasma cells. Talquetamab, marketed as TALVEY, is approved in certain territories for the treatment of certain adult patients with relapsed or refractory multiple myeloma. J&J is responsible for the development and commercialization of TALVEY. Under our agreement with J&J, Genmab is eligible to receive milestones and receives a mid-single digit royalty on net sales of TALVEY subject to a reduction of such royalty payments in countries and territories where there are no relevant patents, among other reductions. Please refer to Note 5.6 of the financial statements for further details regarding the DuoBody collaboration with J&J. Please consult the U.S. Prescribing Information and the European Summary of Product Characteristics for TALVEY for the labeled indication and safety information. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 45 |
|
Antibodies are Y-shaped proteins that play a central role in immunity against bacteria and viruses (also known as pathogens). As we develop immunity, our bodies generate antibodies that bind to pathogen structures (known as antigens), which are specific to the pathogen. Once bound, the antibodies attract other parts of the immune system to eliminate the pathogen. In modern medicine, we have learned how to create and develop specific antibodies against antigens associated with diseased human cells for use in the treatment of diseases such as cancer and autoimmune disease. Genmab uses several types of technologies to create antibodies to treat disease and has developed proprietary antibody technologies including the DuoBody, HexaBody, DuoHexaBody and HexElect technology platforms. With our acquisition of ProfoundBio we also gained their novel ADC technology platforms. Information about these technologies can be found in the following sections and at www.genmab.com/antibody-science/ antibody-technology-platforms. We also use or license several other technologies to generate diverse libraries of high-quality, functional antibodies. In addition, we use or license technologies to increase the potency of some of our antibody therapeutics on a product-by-product basis. Antibody Technologies Our Proprietary Technology Platform Suite Platform Principle Applications DuoBody Bispecific antibodies Dual-targeting: • Recruitment (e.g., T cells) • Tumor heterogeneity ADC Technology Proprietary hydrophilic linker-drug platforms • ADCs with more “antibody-like” PK • Pursue targets with clear opportunities for best-and/or first-in-class ADCs HexaBody Target-mediated enhanced hexamerization Enhanced potency: • CDC • Target clustering, outside-in signaling, apoptosis DuoHexaBody Bispecific antibodies with target-mediated enhanced hexamerization Dual-targeting + enhanced potency: • CDC • Target clustering, outside-in signaling, apoptosis HexElect + + + Two co-dependent antibodies with target-mediated enhanced hexamerization Dual-targeting + enhanced potency and selectivity: • Co-dependent unlocking of potency • New target space, previously inaccessible Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 46 |
|
Innovative Technology for Bispecific Antibody Therapeutics • Bispecific antibody technology platform • Potential in cancer, autoimmune, infectious, cardiovascular, central nervous system diseases, and hemophilia • Commercial collaborations with AbbVie, J&J and BioNTech among others, plus multiple research collaborations • Multiple regulatory approvals for medicines created using the DuoBody technology platform The DuoBody technology platform is Genmab’s innovative platform for the discovery and development of bispecific antibodies. Bispecific antibodies bind to two different epitopes (or “docking” sites) either on the same or on different targets (also known as dual-targeting). Dual-targeting may improve binding specificity and enhance therapeutic efficacy or bring two different cells together (for example, engaging a T cell to kill a tumor cell). Bispecific antibodies generated with the DuoBody technology platform can be used for the development of therapeutics for diseases such as cancer, autoimmune, infectious, cardiovascular, central nervous system diseases, and hemophilia. DuoBody molecules combine the benefits of bispecificity with the strengths of conventional antibodies, which allows DuoBody molecules to be administered and dosed the same way as other antibody therapeutics. Genmab’s DuoBody technology platform generates bispecific antibodies via a versatile and broadly applicable process that is easily performed at high throughput, standard bench, as well as at commercial manufacturing scale. Genmab uses the DuoBody technology platform to create its own bispecific antibody programs and the technology is also available for licensing. Genmab has numerous alliances for the DuoBody technology platform including commercial collaborations with AbbVie, J&J, Novo Nordisk and BioNTech. Genmab’s proprietary DuoBody technology platform has been applied to a variety of bispecific antibody products in development, both in our own pipeline and in programs being developed by collaboration partners. The technology has been validated by the continued advancement of these investiga-tional medicines through clinical development, including four approved medicines. Today four products created by use of the DuoBody technology platform have received regula-tory approval. Antibody Technologies The innovative DuoBody technology platform generates bispecific antibodies via a fast, versatile and broadly applicable process called controlled Fab-arm exchange. With only minimal protein engineering, the technology allows the binding arms of two distinct monoclonal antibodies to exchange, combining into one stable bispecific antibody, thereby retaining regular immunoglobulin structure and function. The DuoBody technology platform is also highly suitable for high throughput generation, screening and discovery of bispecific antibodies in final therapeutic format. DuoBody Technology Platform Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 47 |
|
Advancing Our Pipeline AbbVie On June 10, 2020, Genmab entered into a broad oncology collaboration agreement with AbbVie to jointly develop and commercialize products including epcoritamab (DuoBody-CD3xCD20), and subsequently into a discovery research collaboration for up to four future differentiated antibody therapeutics for cancer. The companies will share commercial responsibilities for epcoritamab in the U.S. and Japan, with AbbVie responsible for further global commercialization. Genmab is the principal for net sales in the U.S. and Japan and receives tiered royalties on remaining global sales outside of these territories. For any product candidates developed as a result of the companies’ discovery research collaboration, Genmab and AbbVie will share responsibilities for global development and commercialization in the U.S. and Japan. Genmab retains the right to co-commercialize these products, along with AbbVie, outside of the U.S. and Japan. Under the terms of the agreement, Genmab has the potential to receive regulatory and sales milestone payments, as well as tiered royalties between 22% and 26% on net sales for epcoritamab outside the U.S. and Japan. Except for these royalty-bearing sales, the parties will share in profit from the sale of epcoritamab on a 50:50 basis. If all four next-generation antibody product candidates developed as a result of the discovery research collaboration are successful, Genmab is eligible to receive up to USD 2.0 billion in option exercise and success-based milestones. Genmab and AbbVie split 50:50 the development costs related to epcoritamab, while Genmab will be responsible for 100% of the costs for the discovery research programs up to opt-in. Please refer to Note 5.6 of the financial statements for further details regarding the collaboration with AbbVie. BioNTech In May 2015, Genmab entered an agreement with BioNTech to jointly research, develop and commercialize bispecific antibody-based investigational medicines using Genmab’s DuoBody technology platform. Under the terms of the agreement, BioNTech will provide proprietary antibodies against key immunomodulatory targets, while Genmab provides proprietary antibodies and access to its DuoBody technology platform. Genmab paid an upfront fee of USD 10 million to BioNTech. If the companies jointly select any antibody-based product candidates for clinical development, development costs and product ownership will be shared equally going forward. If one of the companies does not wish to move an antibody product forward, the other company is entitled to continue developing it on predetermined licensing terms. The agreement also includes provisions which will allow the parties to opt out of joint development at key points. Genmab and BioNTech currently have two bispecific antibody products in clinical development, GEN1042 (BNT312, DuoBody-CD40x4-1BB) and GEN1059 (BNT314, DuoBody-EpCAMx4-1BB). In 2024, Genmab assumed sole responsibility for the continued development and potential commercialization of an additional bispecific antibody, acasunlimab (GEN1046, DuoBody-PD-L1x4-1BB) after BioNTech opted to not participate in further development of the program. Please refer to Note 5.6 of the financial statements for further details regarding Genmab’s collaboration with BioNTech. Our Innovative Technology in Action J&J In July 2012, and as amended in December 2013, Genmab entered into a collaboration with J&J to create and develop bispecific antibodies using our DuoBody technology platform. Three of the DuoBody-based investigational medicines created under this collaboration, RYBREVANT (amivantamab), TECVAYLI (teclistamab) and TALVEY (talquetamab) have received regulatory approval in various territories worldwide. Genmab is eligible to receive milestone payments and receives royalties on net sales of each commercialized DuoBody medicine. Please refer to Note 5.6 of the financial statements for further details regarding the DuoBody collaboration with J&J. Novo Nordisk In August 2015, Genmab entered an agreement to grant Novo Nordisk commercial licenses to use the DuoBody technology platform to create and develop bispecific antibody candidates for two therapeutic programs that would target a disease area outside of cancer therapeutics. After an initial period of exclusivity for both target combinations, Novo Nordisk extended exclusivity of the commercial license for one target combination in 2018, now in clinical development as Mim8. Under the exclusive license agreement, Genmab is entitled to potential milestones and will be entitled to mid-single digit royalties on sales of Mim8, should it receive regulatory approval. Antibody Technologies DuoBody Collaborations Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 48 |
|
ADC Technology Platforms Two Proprietary Hydrophilic Linker-drug Platforms with Clinical Validation • Combines both novel and validated components to create potentially best-in-class ADCs • Acquired from ProfoundBio, basis of four products in the clinic, including Rina-S ADCs are antibodies with potent cytotoxic agents coupled to them. By using antibodies that recognize specific targets on tumor cells, these cytotoxic agents are preferentially delivered to the tumor cells. With Genmab’s acquisition of ProfoundBio we inherited proprietary hydrophilic antibody-drug linker technology that blends innovative and proven methods to design ADCs leading to potentially enhanced therapeutic outcomes. This technology leverages two decades of insights into ADC pharmacology and optimization. These novel, highly hydrophilic and stable cleavable linkers are designed to mask the hydrophobicity of payloads, leading to ADCs with more “antibody-like” pharmacokinetics. Initial focus has been on clinically proven targets where ADC viability has been established. Our goal is to pursue targets with clear opportunities for best- and/or first-in-class ADCs. We also have the potential to combine this technology with Genmab’s proprietary DuoBody technology to create bispecific ADCs. There are currently four wholly owned programs in the clinic based on this technology, Rina-S, GEN1286 (EGFR, cMet), GEN1107 (PTK7) and GEN1160 (CD70). HexaBody Technology Platform Creating Differentiated Therapeutics • Enhanced potency antibody technology platform • Broadly applicable technology that builds on natural antibody biology • HexaBody-based investigational medicines in clinical development; HexaBody-CD38 (GEN3014) and HexaBody-OX40 (GEN1055/ BNT315) The HexaBody technology platform is a proprietary Genmab technology that is designed to increase the potency of antibodies. The HexaBody technology platform builds on natural biology and strengthens the natural killing ability of antibodies while retaining regular structure and specificity. The technology allows for the creation of potent therapeutics by inducing antibody hexamer formation (clusters of six antibodies) after binding to their target antigen on the cell surface. We have used the HexaBody technology platform to generate antibodies with enhanced complement-mediated killing, allowing antibodies with limited or absent killing capacity to be transformed into potent, cytotoxic antibodies. In addition to complement-mediated killing, the clustering of membrane receptors by the HexaBody technology platform can lead to subsequent outside-in signaling leading to cell death. The HexaBody technology platform creates opportunities to explore new antibody-based product candidates and repurpose drug candidates unsuccessful in previous clinical trials due to insufficient potency. The HexaBody technology platform is broadly applicable and can be combined with Genmab’s DuoBody technology platform (DuoHexaBody technology platform) as well as other antibody technologies. The technology has the potential to enhance antibody therapeutics for a broad range of applications including cancer and infectious diseases. Genmab is using the HexaBody technology platform for its own antibody programs and the technology is also available for licensing. Two HexaBody-based investigational medicines are currently in clinical development. Genmab entered into an exclusive worldwide license and option agreement with J&J to develop and commercialize GEN3014 (HexaBody-CD38), a next-generation CD38 monoclonal antibody-based investigational medicine. In 2022, Genmab and BioNTech expanded their global strategic collaboration to include co-development of monospecific antibody candidates leveraging the HexaBody technology. Currently in the clinic under this collaboration is GEN1055 (BNT315, HexaBody-OX40). Antibody Technologies Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 49 |
|
Antibody Technologies DuoHexaBody Technology Platform Combining Dual-Targeting and Enhanced Potency • Antibody technology that combines DuoBody and HexaBody technology platforms • Creates bispecific antibodies with target-mediated enhanced potency The DuoHexaBody technology platform is a proprietary technology that combines the dual targeting of our DuoBody technology platform with the enhanced potency of our HexaBody technology platform, creating bispecific antibodies with target-mediated enhanced hexamerization. HexElect Technology Platform Enhancing Selectivity and Potency • Antibody technology platform inspired by the HexaBody technology platform • Combines dual-targeting with enhanced selectivity and potency The HexElect antibody technology platform is Genmab’s newest proprietary antibody technology. This technology combines two HexaBody molecules designed to effectively and selectively hit only those cells that express both targets by making the activity of complexes of HexaBody molecules dependent on their binding to two different targets on the same cell. The HexElect technology platform maximizes efficacy while minimizing possible toxicity, potentially leading to more potent and safer investigational medicines. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 50 |
|
Financial Review “We are increasingly prioritizing investment into programs that have a clear line of sight to generate meaningful revenue, especially our Phase 3 programs, EPKINLY, Rina-S and acasunlimab. In order to be well positioned at the end of this decade, these are the investments that we need to make today.” Anthony Pagano Executive Vice President and Chief Financial Officer Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 51 |
|
Result for the Year Guidance and Result for 2024 Guidance and Result for 2024 (DKK million) Latest Guidance Actual Revenue 21,100–21,700 21,526 Royalties 17,000–17,400 17,352 Net product sales/Collaboration revenue* 2,000–2,200 2,176 Milestones/Reimbursement revenue 2,100–2,100 1,996 Gross Profit** 20,200–20,800 20,541 Operating expenses** (14,100)–(14,400) (13,838) Operating profit** 5,800–6,700 6,703 *Net Product Sales and Collaboration Revenue consists of EPKINLY Net Product Sales in the U.S. and Japan and Tivdak (Genmab’s share of net profits) in the U.S. **Operating Expenses Range excludes Cost of Product Sales Range, which is included in Gross Profit Range Actual revenue, operating expenses and operating profit were in line with the latest guidance published on November 6, 2024. Financial Review— Group The financial statements are prepared on a consolidated basis for Genmab A/S (parent company) and its subsidiaries. The Genmab financial statements are published in Danish Kroner (DKK). The Genmab consolidated Group is referenced herein as “Genmab” or the “Company.” Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 52 |
|
Revenue Genmab’s revenue was DKK 21,526 million in 2024 compared to DKK 16,474 million in 2023. The increase of DKK 5,052 million, or 31%, was primarily driven by higher DARZALEX and Kesimpta royalties achieved under our collaborations with J&J and Novartis, respectively. Increased EPKINLY net product sales, driven by a strong product launch in 2023 with a full year of net sales in 2024, also contributed to increased revenue in 2024. Genmab’s revenue was DKK 16,474 million in 2023 compared to DKK 14,505 million in 2022. The increase of DKK 1,969 million, or 14%, was primarily driven by higher DARZALEX and Kesimpta royalties achieved under our collaborations with Janssen and Novartis, respectively, partly offset by milestones achieved in 2022 under our collaboration with AbbVie. EPKINLY net product sales, driven by a strong product launch, also contributed to increased revenue in 2023. (DKK million) 2024 2023 2022 Royalties 17,352 80% 13,705 83% 11,582 80% Reimbursement Revenue 996 5% 864 5% 818 6% Milestone Revenue 1,000 5% 1,177 7% 1,767 12% Collaboration Revenue 433 2% 307 2% 332 2% Net Product Sales 1,743 8% 421 3% – – License Revenue 2 0% – – 6 0% Total revenue 21,526 100% 16,474 100% 14,505 100% Royalties Royalty revenue amounted to DKK 17,352 million in 2024 compared to DKK 13,705 million in 2023. The increase of DKK 3,647 million, or 27%, was primarily driven by higher DARZALEX and Kesimpta royalties achieved under our daratumumab collaboration with J&J and ofatumumab collaboration with Novartis, respectively. The table below summarizes Genmab’s royalty revenue by product. (DKK million) 2024 2023 2022 DARZALEX 13,922 11,265 9,966 Kesimpta 2,222 1,494 779 TEPEZZA 737 704 796 Other 471 242 41 Total royalties 17,352 13,705 11,582 DARZALEX J&J’s net sales of DARZALEX were USD 11,670 million in 2024 compared to USD 9,744 million in 2023 and USD 7,977 million in 2022. The increase from 2023 to 2024 of USD 1,926 million, or 20%, was driven by share gains in all regions. The increase from 2022 to 2023 of USD 1,767 million, or 22%, was also driven by share gains in all regions. Royalty revenue on net sales of DARZALEX was DKK 13,922 million in 2024 compared to DKK 11,265 million in 2023 and DKK 9,966 million in 2022, an increase of DKK 2,657 million from 2023 to 2024, and DKK 1,299 million from 2022 to 2023. The percentage increase in royalties of 24% from 2023 to 2024 is higher than the percentage increase in the underlying net sales of 20% primarily due to a higher effective royalty rate for 2024 and other positive foreign exchange rate impacts, partially offset by the increase in Genmab’s Halozyme royalty reductions in connection with the increase in SC product net sales and an increase in royalty reductions on net sales in countries and territories where there is no Genmab patent coverage as well as lower average exchange rate between the USD and DKK in 2024. Under our license agreement with Janssen for DARZALEX, for purposes of calcu-lating royalties due to Genmab, DARZALEX net sales for non-U.S. dollar denominated currencies are translated to U.S. dollars at a specified annual Currency Hedge Rate. This contractual arrange-ment is the driver for the other foreign exchange impacts discussed above. The percentage increase in royalties of 13% from 2022 to 2023 is lower than the percentage increase in the underlying net sales of 22% primarily due to a lower average exchange rate between the USD and DKK in 2023, other foreign exchange impacts, the increase in Genmab’s Halozyme royalty reductions in connection with the increase in SC product net sales and an increase in royalty reductions on net sales in countries and territories where there is no Genmab patent coverage. Under our license agreement with Janssen for DARZALEX, for purposes of calculating royalties due to Genmab, net sales for non-U.S. denominated currencies are translated to U.S. dollars at a specific annual Currency Hedge Rate. This contractual agreement is the driver for the other foreign exchange rate impacts discussed above, which were signifi-cantly more favorable in 2022 compared to 2023. Kesimpta Novartis’ net sales of Kesimpta were USD 3,224 million in 2024 compared to USD 2,171 million in 2023 and USD 1,092 million in 2022. The Financial Review—Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 53 |
|
increase of USD 1,053 million from 2023 to 2024, or 49%, was primarily driven by increased demand and strong access. The increase of USD 1,079 million from 2022 to 2023, or 99%, was primarily driven by increased demand, strong access, and a one-time positive revenue adjustment in Europe. Royalty revenue on net sales of Kesimpta was DKK 2,222 million in 2024 compared to DKK 1,494 million in 2023, an increase of DKK 728 million, or 49%. Royalty revenue on net sales of Kesimpta was DKK 1,494 million in 2023 compared to DKK 779 million in 2022, an increase of DKK 715 million, or 92%. TEPEZZA Amgen’s net sales of TEPEZZA were USD 1,851 million in 2024 compared to USD 1,771 million in 2023 and USD 1,966 million in 2022. Royalty revenue on net sales of TEPEZZA was DKK 737 million in 2024 compared to DKK 704 million in 2023 and DKK 796 million in 2022, an increase of DKK 33 million, or 5% from 2023 to 2024 and a decrease of DKK 92 million, or 12% from 2022 to 2023. Other Royalties Other royalties consist of royalties from net sales of RYBREVANT, TECVAYLI, TALVEY and TEPKINLY. J&J was granted U.S. FDA approval for RYBREVANT during the second quarter of 2021, and Genmab subsequently started recognizing royalties on net sales of RYBREVANT. Royalties were not material for 2024, 2023 or 2022. J&J was granted approval for TECVAYLI for the treatment of relapsed or refractory multiple myeloma during the third quarter of 2022 in Europe and in the fourth quarter of 2022 in the U.S. Royalties were not material for 2024, 2023 or 2022. During the third quarter of 2023, J&J was granted approval in the U.S. and in Europe for TALVEY for the treatment of relapsed or refractory multiple myeloma. Royalties were not material for 2024 or 2023. The EC granted conditional marketing autho-rization for TEPKINLY as a monotherapy for the treatment of adult patients with relapsed or refractory DLBCL after two or more lines of systemic therapy during the third quarter of 2023. Royalties from AbbVie, related to European net sales, were not material for 2024 or 2023. Royalty revenue fluctuations from period to period are driven by the level of product net sales, foreign currency exchange rate movements and more specifically to DARZALEX, the contractual arrangement related to annual Currency Hedge Rate, Genmab’s share of J&J’s royalty payments to Halozyme in connection with SC product net sales and royalty deductions on net sales in countries and territories where there is no patent protection. Reimbursement Revenue Reimbursement revenue, mainly comprised of the reimbursement of certain research and development costs related to the development work under Genmab’s collaboration agreements, amounted to DKK 996 million in 2024 compared to DKK 864 million in 2023 and DKK 818 million in 2022. The increase of DKK 132 million, or 15%, from 2023 to 2024 was primarily driven by higher activities under our collaboration agreements with BioNTech for DuoBody-CD40x4-1BB and acasunlimab, prior to Genmab assuming full ownership, as well as by higher activities under our collaboration agreement with Pfizer for Tivdak. The increase of DKK 46 million, or 6%, from 2022 to 2023 was primarily driven by higher activities under our collaboration agreements with BioNTech for DuoBody-CD40x4-1BB and acasunlimab. Milestone Revenue Milestone revenue was DKK 1,000 million in 2024 compared to DKK 1,177 million in 2023 and DKK 1,767 million in 2022, a decrease of DKK 177 million, or 15%, from 2023 to 2024, and a decrease of DKK 590 million, or 33%, from 2022 to 2023, primarily driven by the following: 2024 milestones: • Novartis milestone of DKK 582 million driven by worldwide net sales for Kesimpta, first exceeding DKK 17.4 billion in 2024, and • AbbVie milestone of DKK 343 million (USD 50 million) due to the acceptance for filing of a Biologics License Application (BLA) by the U.S. FDA in the second indication of epcoritamab in the U.S. 2023 milestones: • AbbVie milestone of DKK 348 million (USD 50 million) driven by the first commercial sale of EPKINLY in the U.S., • AbbVie milestone of DKK 205 million (USD 30 million) due to the acceptance of the marketing authorization application (MAA) filing by the EMA of the type II variation for marketing authorization of TEPKINLY, • AbbVie milestone of DKK 176 million (USD 25 million) due to the first commercial sale of TEPKINLY in Europe, and • J&J milestone of DKK 169 million (USD 25 million) related to the BLA approval in the U.S. for talquetamab. 2022 milestones: • AbbVie milestone of DKK 577 million (USD 80 million) driven by the acceptance of the BLA by the U.S. FDA for epcoritamab, • AbbVie milestone of DKK 444 million (USD 60 million) triggered by the validation of the MAA by the EMA in the EU for epcoritamab, • J&J milestones of DKK 189 million (USD 25 million) and DKK 112 million (USD 15 million) for the approval of TECVAYLI for the treatment of relapsed or refractory multiple myeloma in the U.S. and Europe, respectively, and • AbbVie milestone of DKK 153 million (USD 20 million) driven by the initiation, or first patient dosed, of a pivotal trial (Phase 3) in the second indication for epcoritamab. Milestone revenue may fluctuate significantly from period to period due to both the timing of achievements and the varying amount of each individual milestone under our license and collaboration agreements. Collaboration Revenue Collaboration revenue, which reflects 50% of gross profit from net sales of Tivdak in the U.S. by Pfizer, was DKK 433 million in 2024 compared to DKK 307 million in 2023 and DKK 332 million in 2022. The increase of DKK 126 million, or 41%, from 2023 to 2024 was primarily driven by increased sales of Tivdak. The decrease of DKK 25 million from 2022 to 2023 was primarily driven by a one-off payment in 2022 from Pfizer of approximately DKK 112 million (USD 15 million) which reflects Financial Review—Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 54 |
|
Genmab’s share (50%) of payments received by Pfizer in connection with the sublicense of its rights to develop and commercialize tisotumab vedotin in China to Zai Lab Hong Kong, partly offset by an increase in net sales of Tivdak in 2023. Net Product Sales Global net sales of EPKINLY/TEPKINLY were USD 281 million in 2024. Net product sales in the U.S. and Japan by Genmab were DKK 1,743 million in 2024 compared to DKK 421 million in 2023. EPKINLY was approved in the U.S. in May 2023 and in Japan in September 2023. Net sales of TEPKINLY in territories where Genmab receives royalty revenue were USD 28 million in 2024, with immaterial net sales in 2023 due to regulatory approvals in such territories not occurring until late 2023. As EPKINLY is Genmab’s first commercialized product for which Genmab is recording net product sales, there were no net product sales recognized during 2022. Refer to Note 2.2 for further details about revenue. Cost of Product Sales Genmab recognized cost of product sales of DKK 985 million in 2024 compared to DKK 226 million in 2023. Cost of product sales related to EPKINLY sales is primarily comprised of profit-sharing amounts payable to AbbVie of DKK 831 million in 2024 compared to DKK 195 million in 2023, as well as product costs. There were no cost of product sales recog-nized during 2022 as EPKINLY was approved in the U.S. in May 2023 and in Japan in September 2023. Aside from these items, there are no other costs included within cost of product sales. Refer to Notes 2.3, 3.5 and 5.6 for further details about cost of product sales. Research and Development Expenses Research and development expenses amounted to DKK 9,748 million in 2024 compared to DKK 7,630 million in 2023 and DKK 5,562 million in 2022. The increase from 2023 to 2024 of DKK 2,118 million, or 28%, was driven by the increased and accelerated advancement of epcoritamab under our collaboration with AbbVie, the addition of ProfoundBio related research and development expenses, primarily Rina-S, advancement of acasunlimab and DuoBody-CD40x4-1BB under our collaboration with BioNTech, further progression of pipeline products, and the increase in team members to support the continued expansion of our product portfolio. The increase from 2022 to 2023 of DKK 2,068 million, or 37% was driven by the increased and accelerated advancement of epcoritamab under our collaboration with AbbVie, advancement of acasunlimab and DuoBody-CD40x4-1BB under our collaboration with BioNTech, further progression of pipeline products, and the increase in team members to support the continued expansion of our product portfolio. Research and development costs accounted for 72% of total research and development expenses and selling, general and administration expenses in 2024 compared to 70% in 2023 and 68% in 2022. The following table provides information regarding our research and development expenses for 2024 as compared to 2023 and 2022. (DKK million) 2024 2023 2022 Percentage Change 2024/2023 Percentage Change 2023/2022 Research1 2,137 1,507 1,222 42% 23% Development and contract manufacturing2 3,555 2,324 1,556 53% 49% Clinical3 3,296 3,282 2,059 0% 59% Upfront payments4 – 3 155 (100)% (98)% Other5 760 514 570 48% (10)% Total research and development expenses 9,748 7,630 5,562 28% 37% 1. Research expenses include, among other things, personnel, occupancy and laboratory expenses, technology access fees associated with identification of new monoclonal antibodies (mAbs), expenses associated with the development of new proprietary technologies and research activities associated with our product candidates, such as in vitro and in vivo studies, translational research, and IND enabling toxicology studies. 2. Development and contract manufacturing expenses include personnel and occupancy expenses, external contract manufacturing costs for the scaleup and pre-approval manufacturing of drug product used in research and our clinical trials, costs for drug product supplied to our collaborators, costs related to preparation for the production of process validation batches to be used in potential future regulatory submissions, quality control and assurance activities, and storage and shipment of our product candidates. 3. Clinical expenses include personnel, travel, occupancy costs, and external clinical trial costs including contract research organizations (CROs), investigator fees, clinical site fees, contractors and regulatory activities associated with conducting human clinical trials. 4. Upfront payments include payments made to third parties upon entering into R&D license and collaboration agreements. 5. Other research and development expenses primarily include share-based compensation, depreciation, amortization and impairment expenses. Financial Review—Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 55 |
|
The following table shows third-party costs incurred for research, contract manufacturing of our product candidates and clinical and regulatory services for 2024 as compared to 2023 and 2022. The table also presents unallocated costs and overhead consisting of third-party costs for our preclinical stage programs, personnel, facilities, and other indirect costs not directly charged to development programs. (DKK million) 2024 2023 2022 Percentage Change 2024/2023 Percentage Change 2023/2022 Epcoritamab 2,854 1,323 801 116% 65% Rina-S 319 – – N/A N/A Tisotumab vedotin 263 285 319 (8)% (11)% Acasunlimab 707 553 369 28% 50% DuoBody-CD40x4-1BB 514 409 242 26% 69% Other clinical stage programs 503 743 393 (32)% 89% Total third-party costs for clinical stage programs 5,160 3,313 2,124 56% 56% Preclinical projects 1,491 1,132 830 32% 36% Upfront payments – 3 155 (100)% (98)% Personnel, unallocated costs and overhead 3,097 3,182 2,453 (3)% 30% Total research and development expenses 9,748 7,630 5,562 28% 37% Third-party costs for epcoritamab increased by DKK 1,531 million, or 116%, in 2024 as compared to 2023, primarily due to the advancement and acceleration of the epcoritamab program under Genmab’s collaboration with AbbVie. Third-party costs for epcoritamab increased by DKK 522 million, or 65%, in 2023 as compared to 2022, primarily due to the advancement and acceleration of the epcoritamab program under Genmab’s collaboration with AbbVie. Third-party costs for Rina-S were DKK 319 million in 2024. Rina-S was acquired through the acquisition of ProfoundBio in the second quarter of 2024. Third-party costs for tisotumab vedotin decreased by DKK 22 million, or 8%, in 2024 as compared to 2023, primarily due to the completion of certain clinical study activities in 2024. Third-party costs for tisotumab vedotin decreased by DKK 34 million, or 11%, in 2023 as compared to 2022, primarily due to the comple-tion of certain clinical study activities in 2023. Third-party costs for acasunlimab increased by DKK 154 million, or 28%, in 2024 as compared to 2023, primarily due to the continued advancement of the program, which Genmab obtained sole ownership during the third quarter of 2024. Third-party costs for acasunlimab increased by DKK 184 million, or 50%, in 2023 as compared to 2022, primarily due to the continued advancement and expansion of the program under Genmab’s prior collaboration with BioNTech on this project. Third-party costs for DuoBody-CD40x4-1BB increased by DKK 105 million, or 26%, in 2024 as compared to 2023, primarily due to the continued advancement and expansion of the program under Genmab’s collaboration with BioNTech. Third-party costs for DuoBody-CD40x4-1BB increased by DKK 167 million, or 69%, in 2023 as compared to 2022, primarily due to the continued advancement and expansion of the program under Genmab’s collaboration with BioNTech. Third-party costs for Genmab’s other clinical stage programs decreased by DKK 240 million, or 32%, in 2024 as compared to 2023, primarily related to advancements of DuoBody-CD3xB7H4 and DuoBody-CD3xCD30 in 2024. Third-party costs for Genmab’s other clinical stage programs increased by DKK 350 million, or 89%, in 2023 as compared to 2022, primarily related to advancements of DuoBody-CD3xB7H4 and DuoBody-CD3xCD30 in 2023. Research and development expenses related to our preclinical projects increased by DKK 359 million, or 32%, in 2024 as compared to 2023, driven by the continued investment in new and existing preclinical programs. An IND was submitted for DuoBody-FAPαxDR4 and a CTA was submitted for GEN1078. Research and development expenses related to our preclinical projects increased by DKK 302 million, or 36%, in 2023 as compared to 2022, driven by the continued investment in new and existing preclinical programs. Upfront payments were not material in either 2024 or 2023 driven by a decrease in the number of R&D license payments recorded as expense. Upfront payments decreased by DKK 152 million, or 98%, driven by a decrease in the number of R&D license payments in 2023 as compared to 2022. Personnel, unallocated costs and overhead decreased by DKK 85 million, or 3%, in 2024 as compared to 2023, primarily due to travel costs, which were higher in 2023 due to the upcoming launch of EPKINLY in 2023. Our research and development FTEs increased from 1,541 at the end of 2023 to 1,886 at the end of 2024. Personnel, unallocated costs and overhead increased by DKK 729 million, or 30%, in 2023 as compared to 2022, primarily due to an increase in staffing levels and the expansion of our facilities to accommodate our growth. Our research and development FTEs increased from 1,193 at the end of 2022 to 1,541 at the end of 2023. Refer to Note 2.3, 3.1, 3.2 and 5.5 for further details about staff costs, intangible assets, property and equipment and the acquisition of ProfoundBio. Financial Review—Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 56 |
|
Selling, General and Administrative Expenses Selling, general and administrative expenses were DKK 3,790 million in 2024 compared to DKK 3,297 million in 2023 and DKK 2,676 million in 2022. The increase from 2023 to 2024 of DKK 493 million, or 15%, was driven by the continued expansion of Genmab’s commercialization capabilities through the increase in team members to support the continued launch of EPKINLY in the U.S. and Japan in 2023, and the investment in Genmab’s broader organizational capabilities. Selling, general and administration expense growth has been moderating during 2024 reflecting a focus on driving efficiency. We continue to increase team members and commercial support in a strategic manner. The increase from 2022 to 2023 of DKK 621 million, or 23%, was driven by the continued expansion of Genmab’s commercialization capabilities through the increase in team members to support the launch of EPKINLY in the U.S. and Japan in 2023, and the investment in Genmab’s broader organizational capabilities. DKK 1,813 million, or 48% of selling, general and administrative expenses in 2024, was related to compensation of Genmab team members associated with selling, general and administrative activities, as compared to DKK 1,541 million, or 47% in 2023 and DKK 1,065 million, or 40% in 2022. Refer to Note 2.3 and 3.2 for further details about staff costs and property and equipment. Selling, general and administrative expenses accounted for 28% of total research and development expenses and selling, general and administration expenses in 2024 compared to 30% in 2023 and 32% in 2022. Acquisition and Integration Related Charges Acquisition and integration related charges for the acquisition of ProfoundBio were DKK 300 million in 2024 compared to no acquisition and integration related charges for 2023 or 2022 as there were no acquisitions during these years. Refer to Note 5.5 for further details about the acquisition of ProfoundBio. Operating Profit Operating profit was DKK 6,703 million in 2024 compared to DKK 5,321 million in 2023, an increase of DKK 1,382 million, or 26%. Operating profit was DKK 5,321 million in 2023 compared to DKK 6,267 million in 2022, a decrease of DKK 946 million, or 15%. Financial Income and Expense Financial income and expense was comprised of the following: (DKK million) 2024 2023 2022 Financial income: Interest and other financial income 995 982 324 Gain on marketable securities 364 495 92 Gain on other investments 146 72 58 Foreign exchange rate gain 2,933 391 2,715 Total financial income 4,438 1,940 3,189 Financial expenses: Interest and other financial expenses (120) (70) (39) Loss on marketable securities (147) (176) (453) Loss on other investments (116) (98) (355) Foreign exchange rate loss (1,594) (1,280) (1,664) Total financial expenses (1,977) (1,624) (2,511) Net financial items 2,461 316 678 Interest Income Interest income was DKK 995 million in 2024 compared to DKK 982 million in 2023 and DKK 324 million in 2022. The increase of DKK 13 million, or 1% from 2023 to 2024, was primarily driven by the higher cash and cash equivalents and marketable securities in the first half of 2024 compared to 2023, almost entirely offset by lower cash and cash equivalents and marketable securities in the second half of 2024 compared to 2023 as a result of liquidating marketable securities and using cash to purchase ProfoundBio. The increase of 658 million, or 203% from 2022 to 2023 was primarily driven by higher effective interest rates in the U.S., Europe, and Denmark. Financial Review—Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 57 |
|
Foreign Exchange Rate Gains and Losses Foreign exchange rate gain, net of DKK 1,339 million in 2024 compared to the foreign exchange rate loss, net of DKK 889 million in 2023 and foreign exchange rate gain, net of DKK 1,051 in 2022 were primarily driven by foreign exchange movements impacting Genmab’s USD denominated marketable securities and cash and cash equivalents; in particular, the USD/DKK foreign exchange rates were as follows for each period: December 31, 2024 December 31, 2023 December 31, 2022 USD/DKK Foreign Exchange Rates 7.1429 6.7447 6.9722 % Increase/(Decrease) 6% (3)% 6% Marketable Securities Gains and Losses Gain on marketable securities, net was DKK 217 million in 2024 compared to gain on marketable securities, net of DKK 319 million in 2023 and loss on marketable securities, net of DKK 361 million in 2022. The decrease in gain, net of DKK 102 million, or 32% from 2023 to 2024 was primarily driven by the decrease in marketable securities in the first half of 2024 to fund the acquisition of ProfoundBio and share repurchase as well as changing interest rate outlooks for the U.S., primarily in the fourth quarter of 2024. The increase in gain, net of DKK 680 million, or 188% from 2022 to 2023, was primarily driven by interest rate outlooks for the U.S. and Europe. Other Investments Gains on other investments, net were DKK 30 million in 2024, losses on other investments, net were DKK 26 million in 2023 and DKK 297 million in 2022. The net gains and losses in 2024 and 2023 were primarily driven by changes in fair value of Genmab’s investments in certain strategic investment funds. The losses in 2022 were primarily driven by the change in fair value of Genmab’s investment in common shares of CureVac. Refer to Notes 4.2 and 4.5 for further details regarding foreign currency risk and net financial items, respectively. Corporate Tax Corporate tax expense was DKK 1,320 million in 2024 compared to DKK 1,285 million in 2023 and DKK 1,493 million in 2022. Genmab’s estimated annual effective tax rate was 14.4% in 2024 compared to 22.8% in 2023 and 21.5% in 2022. The decrease from 2023 to 2024 in Genmab’s effective tax rate was primarily due to the inte-gration of ProfoundBio which allowed for the deduction of previously unrecognized deferred tax assets in 2024. The increase from 2022 to 2023 in Genmab’s effective tax rate was mainly driven by the increase of unrecognized deferred tax assets. We anticipate that our effective tax rate should be closer to the Danish statutory rate of 22% going forward. Refer to Note 2.4 for additional information regarding the corporate tax, deferred tax assets and deferred tax liabilities including manage-ment’s significant judgements and estimates. Net Profit Net profit for 2024 was DKK 7,844 million compared to DKK 4,352 million in 2023 and DKK 5,452 million in 2022. The changes in net profit for the periods were driven by the items described above. Liquidity and Capital Resources December 31, (DKK million) 2024 2023 Marketable securities 11,243 13,268 Cash and cash equivalents 9,858 14,867 Shareholders’ equity 36,697 31,610 As of December 31, 2024, cash and cash equivalents and marketable securities denominated in USD represented 85% of Genmab’s total cash and cash equivalents and marketable securities compared to 90% as of December 31, 2023. Marketable securities are invested in highly secure and liquid investments with short effective maturities. As of December 31, 2024, 71% of Genmab’s marketable securities were long-term A rated or higher, or short-term rated A-1/P-1 by S&P, Moody’s or Fitch compared to 72% as of December 31, 2023. As of December 31, 2024, DKK 9,858 million, as compared to DKK 14,867 million as of December 31, 2023, was held as cash and cash equivalents, and as of December 31, 2024, DKK 11,243 million, as compared to DKK 13,268 million as of December 31, 2023, was held as liquid investments in short-term government and other debt instruments. Cash and cash equivalents included short-term marketable securities of DKK 82 million at the end of December 2024, compared to DKK 1,353 million at the end of December 2023. In accordance with Genmab’s accounting policy, securities purchased with a maturity of less than 90 days at the date of acquisition are classified as cash and cash equivalents. Genmab requires cash to meet our operating expenses and capital expenditures. We have funded our cash requirements since inception, including through December 31, 2024, primarily with royalty and milestone payments from our partners, upfront payments, and equity financing. Genmab expects to continue to fund a significant portion of our development costs for proprietary product candidates as well as commercialization activities with cash received from royalties and milestone payments from partners, and net sales of Genmab products. During the fourth quarter of 2024, Genmab entered into an unsecured three-year revolving credit facility (“Credit Facility”) of up to USD 300 million with a syndicate of lenders. Genmab intends to use the Credit Facility to finance working capital needs, and for general corporate purposes, of Genmab A/S and its subsidiaries. The Credit Facility includes options to increase the size of the facility up to USD 500 million as well as the ability to extend Financial Review—Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 58 |
|
for an additional two years. The Credit Facility contains certain customary financial covenants. As of December 31, 2024, there were no outstanding amounts due on, nor any usage of, the Credit Facility and Genmab was in compliance with all financial covenants. Genmab’s expenditures on current and future preclinical and clinical development programs are subject to numerous uncertainties in timing and cost to completion. In order to advance our product candidates toward commercialization, the product candidates are tested in numerous preclinical safety, toxicology and efficacy studies. Genmab then conducts clinical trials for those product candidates that take several years or more to complete. The length of time varies substantially based upon the type, complexity, novelty and intended use of a product candidate. The cost of clinical trials may vary significantly over the life of a project as a result of a variety of factors, including: the number of patients required in the clinical trials; the length of time required to enroll trial participants; the number and location of sites included in the trials; the costs of producing supplies of the product candidates needed for clinical trials and regulatory submissions; the safety and efficacy profile of the product candidate; the use of CROs to assist with the management of the trials; and the costs and timing of, and the ability to secure, regulatory approvals. Genmab’s expenses also fluctuate from period to period based on the degree of activities with collaborative partners, timing of manufacturing campaigns, numbers of patients enrolled in clinical trials and the outcome of each clinical trial event. As a result, Genmab is unable to determine with any degree of certainty the anticipated completion dates, duration and completion costs of research and development projects, or when and to what extent Genmab will receive cash inflows from the commercialization and sale of any product candidates. Genmab also cannot predict the actual amount or timing of future royalties and milestone payments, and these may differ from estimates. Genmab expects to increase operating expenditures and make additional capital outlays over the next several years as Genmab supports preclinical development, manufacturing, clinical trial activities, product collaborations, commercialization activities and additional hiring of staff. As spending increases on research, development, business development and /or activities related to mergers and acquisitions, and commercialization activities related to product collaborations, Genmab may be required to make certain capital outlays against which Genmab expects to receive reimbursement to the extent the outlay exceeds Genmab’s share under the applicable collaboration agreement. Genmab expects that the time-lag between the expenditure by Genmab, and the reimbursement by a partner of its relevant share, may increase Genmab’s working capital needs. To the extent Genmab’s capital resources are insufficient to meet future capital requirements, Genmab will need to finance operating requirements and other cash needs through the use of the Credit Facility, public or private equity offerings, debt financings, or additional corporate collaboration and licensing arrangements. Refer to Notes 4.1, 4.2 and 4.4 for additional information regarding our external source of liquidity, financial, risks and marketable securities, respectively. Cash Flows The following table provides information regarding Genmab’s cash flow for 2024, 2023 and 2022. Cash Flow (DKK million) 2024 2023 2022 Cash provided by operating activities 7,771 7,380 3,912 Cash (used in) investing activities (9,907) (1,282) (2,761) Cash (used in) financing activities (3,919) (606) (789) Increase in cash and cash equivalents (6,055) 5,492 362 Exchange rate adjustments 1,046 (518) 574 Net cash provided by operating activities is primarily related to our operating profit, changes in operating assets and liabilities, reversal of net financial items, and adjustments related to non-cash transactions. Cash provided by operating activities increased in 2024 compared to 2023 primarily driven by an increase in net profit before tax of DKK 3.5 billion, an increase in non-cash transactions of DKK 368 million, and a decrease in taxes paid of DKK 724 million in 2024 compared to 2023, partly offset by signif-icant AbbVie milestones achieved during the fourth quarter of 2022 with related cash received during 2023 and an increase in DARZALEX royalty receivables in the fourth quarter of 2024 compared to the fourth quarter of 2023. Cash provided by operating activities increased in 2023 compared to 2022 primarily driven by significant AbbVie milestones achieved during the fourth quarter of 2022 with related cash received during 2023, cash received for DARZALEX royalties in 2023, and higher corporate tax payments made in 2023 compared to 2022. Net cash (used in) investing activities primarily reflects cash used in making acquisitions, differences between the proceeds received from the sale and maturity of our investments and amounts invested, and the cash paid for investments in tangible and intangible assets. The increase from 2023 to 2024 in net cash (used in) investing activities is primarily driven by the acquisition of ProfoundBio, partly offset by the sales and maturities of marketable securities exceeding purchases in 2024, compared to purchases exceeding sales and maturities in 2023. The decrease from 2022 to 2023 in net cash (used in) investing activities is primarily driven by purchases of marketable securities exceeding sales and maturities to a greater extent during 2022 compared to 2023. Net cash (used in) financing activities is primarily related to the purchase of treasury shares, exercise of warrants, lease payments, and payment of withholding taxes on behalf of employees on net settled Restricted Stock Units (RSUs). The increase from 2023 to 2024 in net cash (used in) financing activities is primarily driven by cash payments for the purchase of treasury shares of DKK 3,879 million in 2024 compared to DKK 564 million in 2023. The decrease from 2022 to 2023 in net cash (used in) financing activities is primarily driven by cash payments for the purchase of treasury shares of DKK 564 million in 2023 compared to DKK 908 million in 2022. Financial Review—Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 59 |
|
Exchange rate adjustments represent foreign currency gains or losses on Genmab’s cash and cash equivalents, primarily driven by our cash and cash equivalents holdings denominated in USD. The USD/DKK foreign exchange rate increased 6% in 2024, decreased 3% in 2023 and increased 6% in 2022. Balance Sheet As of December 31, 2024, total assets were DKK 45,811 million, compared to DKK 35,289 million as of December 31, 2023. As of December 31, 2024, assets are mainly comprised of intangible assets of DKK 12,343 million, primarily made up of intangible assets acquired in the ProfoundBio acquisition, marketable securities of DKK 11,243 million, current receivables of DKK 6,590 million, cash and cash equivalents of DKK 9,858 million and DKK 2,535 million of goodwill related to the acquisition of ProfoundBio. The current receivables consist primarily of amounts related to royalties from our collaboration agreements. The credit risk related to our receivables is not material based on no historical credit losses as well as the high-quality nature of Genmab’s collaboration partners and limited number of distributors with high credit standing. Refer to Note 3.6 for additional information regarding receivables and Note 5.5 for additional details related to the acquisition of ProfoundBio. As of December 31, 2024, total liabilities were DKK 9,114 million compared to DKK 3,679 million as of December 31, 2023. The increase in total liabilities of DKK 5,435 million, or 148%, was primarily driven by the DKK 2,359 million deferred tax liability related to the acquisition and integration activities for ProfoundBio, an increase of DKK 1,656 million in corporate taxes payable due to Genmab’s net profit before tax, an increase of DKK 1,170 million in accruals related to the expansion of our product pipeline, and an increase in lease liabilities of DKK 259 million driven by the commencement of a lease in the U.S. with respect to office and laboratory space. Shareholders’ equity as of December 31, 2024 was DKK 36,697 million compared to DKK 31,610 million as of December 31, 2023. The increase of DKK 5,087 million, or 16%, was driven primarily by Genmab’s net profit for the period and share-based compensation expenses, partly offset by the purchase of treasury shares. Genmab’s equity ratio was 80% as of December 31, 2024 compared to 90% as of December 31, 2023. The decrease was primarily attributable to assets acquired in the acquisition of ProfoundBio, net of cash paid, in addition to the share buy-back completed in June 2024 that offset Genmab’s net profit for the period. Financial Review—Group Legal Matters—J&J Binding Arbitrations In September 2020, Genmab commenced arbitration against J&J with respect to two different provisions of our license agreement for daratumumab, both relating to royalties payable to Genmab on net sales of daratumumab (marketed as DARZALEX for IV administration and as DARZALEX FASPRO in the U.S. and as DARZALEX SC in Europe for SC administration). In April 2022, the arbitral tribunal issued an award in that arbitration denying both of Genmab’s claims. Genmab did not seek review of the award. On June 9, 2022, Genmab commenced a second arbitration against J&J under the license agreement, in which Genmab sought additional compensation from J&J with respect to SC daratumumab based on Genmab’s position that the award in favor of J&J in the first arbitration was premised on that tribunal’s determination that IV daratumumab and SC daratumumab were separate “Licensed Products” as that term is defined in the license agreement. Genmab’s claim in that second arbitration was denied by the tribunal on April 21, 2023 on the ground that it should have been brought in the first arbitration, and the dismissal was affirmed by an appellate arbitrator on January 23, 2024. In June 2024, Chugai Pharmaceutical Co., Ltd. filed a lawsuit in the Tokyo District Court, Japan against AbbVie’s and Genmab’s subsidiaries in Japan asserting that their activities with EPKINLY (epcoritamab) in Japan infringe two Japanese patents held by Chugai, JP6278598 and JP6773929. Chugai is claiming damages and injunctive relief. Genmab and AbbVie believe that the two Japanese patents are invalid and not infringed and intend to vigorously defend against the lawsuit, and thus no provision has been recorded related to this matter. Refer to Note 5.7 for further details about contingencies. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 60 |
|
Financial Review— Parent Revenue Genmab A/S’s revenue was DKK 22,167 million in 2024 compared to DKK 17,126 million in 2023. The increase of DKK 5,041 million, or 29%, was primarily driven by higher DARZALEX and Kesimpta royalties achieved under our collab-orations with J&J and Novartis, respectively. Increased EPKINLY intercompany net product sales, driven by a strong product launch in 2023 with a full year of net sales in 2024, also contrib-uted to increased revenue in 2024. Financial Income and Expense Genmab A/S’ financial income was DKK 17,404 million in 2024 compared to DKK 2,199 million in 2023. The increase of DKK 15,205 was primarily driven by the DKK 13.0 billion of dividend income Genmab A/S received related to the sale of ProfoundBio US intangible assets to Genmab A/S. Genmab A/S’ financial expense was DKK 12,239 million in 2024 compared to DKK 1,871 million in 2023. The increase of DKK 10,368 was primarily driven by the DKK 10.4 billion impairment related to Genmab A/S’ investment in subsidiaries. Refer to Note 14 in the parent company financial statements for further details related to the transfer of ProfoundBio US intangible assets to Genmab A/S. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 61 |
|
Risk Management 5. Ethics by Design Controls to prevent harm and risks to individuals should be built into the design of data architecture and data processing 6. Responsible Data Sharing Data sharing should be based on processes that actively and consistently consider, prioritize, and protect individual rights 7. Responsibility and Accountability Data Ethics Principles should be operationalized through effective governance, clear standards, training, monitoring activities, and disciplinary sanctions Genmab has core facilities in five countries that perform research and development activities with clinical trials conducted around the globe. We also have commercial and sales organizations in the U.S. and Japan with manufacturing support activities in Europe. Through our activities, we are exposed to a variety of risks, some of which are inherent in our business and/or beyond our control including sustainability-related risks. These risks may have a significant impact on our business if not properly assessed and controlled. Maintaining a strong control environment, with adequate procedures for identification, prioritization and assessment of risks and adhering to operational policies designed to reduce such risks to an acceptable level, is essential for the continued evolution of Genmab. It is our policy to identify and reduce the risks derived from our operations and to establish insurance coverage and other enterprise risk reduction and resilience mechanisms to mitigate any residual risk, wherever considered practicable. The Audit and Finance Committee of the Board performs a yearly review of Genmab’s Enterprise Risk Program and relevant insurance coverage to ensure that they are appropriate for Genmab. For further information about the risks and uncertainties that Genmab faces, refer to the current Form 20-F filed with the SEC. The use of data, as defined in the Danish Financial Statements Act, both personal and non-personal, is essential to fulfilling Genmab’s core purpose; and Genmab is committed to handling data with integrity and in an ethical and compliant manner considering the impact our actions may have on individuals and society. Genmab has a policy for Data Ethics in compliance with Section 99d of the Danish Financial Statements Act in which Genmab adopted the Data Ethics principles of the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA). These principles complement and strengthen already existing Genmab policies and procedures, and they focus on the following areas: Genmab will continue to focus on these principles, particularly in the areas of data privacy, DE&I, clinical trials, and the application of new technologies (e.g., Artificial Intelligence and Machine Learning), where policies, processes, and training materials will be aligned with the above-mentioned principles. The Genmab Data Ethics policy and its principles are anchored in the Genmab Code of Conduct as part of the overall Genmab Compliance program. 1. Autonomy Respect individuals’ privacy, protect their rights, and honor confidentiality 2. Transparency Individuals should be able to understand how their personal data is used 3. Data Quality The best quality data available should be used to make decisions 4. Fairness and Non-discrimination Data acquisition should be inclusive, equitable, and seek to support the industry’s mission of responding to the needs of all patients Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 62 |
|
Risk Management Risk related to Risk areas Mitigation Risk trend Business and Products The identification and development of successful products is expensive and includes time-consuming clinical trials with uncertain outcomes and the risk of failure to obtain regulatory approval in one or more jurisdictions. Genmab has a disciplined approach to investment, focusing on areas with the potential to maximize success, including new technologies and formats, scaling up to expand from early- to late-stage development and commercialization. Genmab has established various committees to ensure optimal selection of disease targets and formats of our antibody candidates, and to monitor progress of preclinical and clinical development. We strive to have a well-balanced product pipeline, continuing to search for and identify new product candidates, and closely monitoring the market landscape. Genmab is dependent on the identification and development of new proprietary technologies and access to new third-party technologies. This exposes us to safety issues as well as other failures and setbacks related to use of such new or existing technologies. Genmab strives to identify and develop new antibody-based products that harness new antibody technologies, such as the DuoBody, HexaBody, DuoHexaBody and HexElect technology platforms, ADC technology, and gain access to competitive and complementary new third-party technologies. We closely monitor our preclinical programs and clinical trials to mitigate any unforeseen safety issues or other failures, or setbacks associated with the use of these technology platforms. Genmab faces ongoing uncertainty about the successful commercialization of product candidates. This is a result of factors including immense competition on the basis of cost and efficacy as well as rapid technological change, which may result in others discovering, developing or commercializing competing products before and/or more successfully than us. From early in the research phase and throughout development, commercial potential and product commercialization, associated risks are assessed to ensure that final products have the potential to be commercially viable. Genmab attempts to control commercial risks in part by regularly monitoring and evaluating current market conditions, competing products and new technologies, to potentially gain access to new technologies and products that may supplement our pipeline. Genmab also strives to ensure market exclusivity for its own technologies and products by seeking patent protection. Genmab engages with patients and caregivers to gather insights and improve patient outcomes. The following is a summary of Genmab’s key risk areas, including sustainability-related risks, and how we address and mitigate such risks. Risk Level in Relation to Last Year: Unchanged Decreased Increased Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 63 |
|
Risk Management Risk related to Risk areas Mitigation Risk trend Business and Products (continued) Genmab’s near- and mid-term prospects are substantially dependent on continued clinical and commercial success of DARZALEX. The impact of DARZALEX patent expirations, typically followed by the introduction of competing generic, biosimilar or other products could have an adverse impact on Genmab’s future royalty revenue. DARZALEX is subject to intense competition in the multiple myeloma therapy market. Genmab focuses on its three-pronged strategy of focusing on our core competence, turning science into medicine and building a profitable and successful biotech to develop a broad pipeline of unique best-in-class or first-in-class antibody products with significant commercial potential. In addition, Genmab maintains a strong cash position, disciplined financial management, and a flexible and capital efficient business model to mitigate potential setbacks related to DARZALEX. To address the impact of DARZALEX loss of exclusivity, Genmab intends to mitigate this risk through its strong foundation in science and investments in launched medicines as well as its existing, and potential acquisition of new, late-stage assets. Genmab manages and maintains efficient operations through focused prioritization and increased productivity. Including DARZALEX there are eight commercialized medicines on the market that drive significant recurring revenue for the company. In 2020, two additional Genmab-created antibody products, Kesimpta and TEPEZZA, were approved by the U.S. FDA. In 2021, 2022, and 2023, respectively, Genmab’s bispecific DuoBody technology was the basis for the DuoBody-based medicines RYBREVANT, TECVAYLI and TALVEY, which were approved by the U.S. FDA and the EC. All of these provide Genmab with additional recurring royalty revenue. Tivdak, Genmab’s first medicine, in development with Pfizer, was approved by the U.S. FDA and product sales of Tivdak commenced in 2021. EPKINLY/TEPKINLY, Genmab’s second medicine, in development with AbbVie, was approved by the U.S. FDA, the Japan MHLW and the EC and product sales of EPKINLY/TEPKINLY commenced in 2023. In addition, we currently have two wholly owned programs, Rina-S and acasunlimab, which moved into Phase 3 development in 2024. Genmab has exposure to product liability claims related to the use or misuse of our products and technologies. Product liability claims and/or litigation could materially affect our business and financial position, and Genmab therefore strives to maintain internal processes for the review, approval, and compliant use of promotion materials and also maintains appropriate product liability insurance for our clinical trials and our approved products and other coverage required under applicable laws. Risk Level in Relation to Last Year: Unchanged Decreased Increased Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 64 |
|
Risk Management Risk related to Risk areas Mitigation Risk trend Business and Products (continued) Our core research and manufacturing activities are carried out at a limited number of locations. Any event resulting in Genmab’s or our vendors’/suppliers’ inability to operate these facilities could materially disrupt our business. Genmab employs oversight and quality risk management principles. In addition, Genmab follows current Good Laboratory Practices (cGLP) and current Good Manufacturing Practices (cGMP) and requires that our vendors operate with the same standards. Genmab’s quality assurance (QA) department ensures that high-quality standards are set and monitors adherence to these practices. If we are unable to effectively manage Genmab’s fast-paced growth, or maintain our commercialization and other capabilities at adequate levels, and control operating costs within the scope of our overall business as well as properly integrate acquisitions, financial condition and net profits may be adversely affected. Any business disruption or failure to properly manage growth, maintain capabilities and transformation in a manner that reflects and supports our organizational strategies and priorities, while assuring ethical business practices, prudent risk management, and commercial compliance, could have a material adverse effect on our business, financial condition, results of operations and cash flows. We have experienced rapid growth over the last several years. We anticipate additional growth as our pipeline advances and we continue product commercialization activities. Such growth, including maintaining and enabling R&D, commercialization, and support functions, has placed significant demands on our management and infrastructure, including new operational and financial systems, as well as extending manufacturing and commercial outsource arrangements. Our success will depend in part upon our ability to manage and maintain operations and integrate acquisitions effectively through leadership, focused prioritization, increased productivity and talent management to maintain our values-based, collaborative culture. As we continue to grow and evolve, we must continuously improve our operational, commercial, compliance, financial and management practices, and controls. Genmab is subject to government regulations on pricing/public reimbursement as well as other healthcare payer cost-containment initiatives; increased pressures by governmental and third-party payers to reduce healthcare costs. Genmab strives to develop differentiated antibody medicines that bring meaningful impact to patients and health systems and are well-positioned to secure reasonable price reimbursement by government healthcare programs and private health insurers. The impact our science has on patients today and in the future, particularly those with few treatment options, drives the value of our medicines. Genmab’s U.S. Government Affairs & Policy department interacts with U.S. federal and state policymakers to advance policies aimed at improving patients’ lives through access to quality healthcare and innovative science. Genmab’s U.S. Market Access department educates payers on the value of our products and works across the healthcare system to help ensure all appropriate patients gain access to our innovative medicines. Risk Level in Relation to Last Year: Unchanged Decreased Increased Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 65 |
|
Risk Management Risk related to Risk areas Mitigation Risk trend Strategic Collaborations Genmab is dependent on existing partnerships with major pharmaceutical or biotech companies to support our business and develop and extend the commercialization of our products. Our business may suffer if our collaboration partners do not devote sufficient resources to our programs and products, do not successfully maintain, defend and enforce their intellectual property rights or do not otherwise have the ability to successfully develop or commercialize our products, independently or in collaboration with others. Our business may also suffer if we are not able to continue our current collaborations or establish new collaborations. Genmab strives to be an attractive and respected collaboration partner, and to pursue a close and open dialogue with our collaboration partners to share ideas and align on best practices and decisions within clinical development and commercial operations to increase the likelihood that we reach our goals. Genmab is primarily dependent on one contract manufacturing organization (CMO) and individual sites at the CMO to produce and supply our product candidates. Genmab is also dependent on clinical research organizations to conduct key aspects of our clinical trials, and on collaboration partners to conduct some of our clinical trials. CMOs may be subject to or affected by various U.S. legislation, executive orders, regulations, or investigations. Genmab oversees outsourcing and partnership relationships to ensure consistency with strategic objectives and service provider compliance with regulatory requirements, resources, and performance. This includes assessment of contingency plans, availability of alternative service providers and costs and resources required to switch service providers. We continually evaluate financial solvency and require our suppliers to abide by a code of conduct consistent with Genmab’s Code of Conduct. Risk Level in Relation to Last Year: Unchanged Decreased Increased Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 66 |
|
Risk Management Risk related to Risk areas Mitigation Risk trend Regulation, Legislation, and Compliance Genmab is subject to extensive legislative, regulatory and other requirements during preclinical and clinical development, commercialization, and post-marketing approval, including healthcare, marketing/ labelling/promotion, fraud and abuse, competition/antitrust laws, and regulations, as well as transparency, privacy and data protection and other requirements. Genmab is subject to strict disclosure obligations under applicable laws and regulations globally, including the EU Market Abuse Regulation and the U.S. Inflation Reduction Act (IRA). Being listed on the Nasdaq Global Select Market, we are subject to additional U.S. regulatory requirements, including U.S. securities laws and the U.S. Foreign Corrupt Practices Act, and may become more exposed to U.S. class actions. To ensure compliance with applicable healthcare laws and regulations, Genmab has established a compliance program, including a Code of Conduct that is regularly evaluated and sets high ethical standards on which all colleagues receive regular training. Genmab also maintains a Speak Up Policy and Hotline for reporting and response to potential misconduct. Our head of Global Compliance reports directly to the CEO. Genmab is committed to transparency of clinical trial research and has published our Clinical Trial Transparency Declaration. Genmab is also committed to ensuring equal access to Genmab clinical trials and that patients participating in our trials are representative of those living with the disease being studied. Genmab respects the privacy, protection, and appropriate use of data by ensuring compliance with all applicable privacy and data protection laws, regulations, and other standards. In support of this commitment, Genmab established its Global Data Privacy Office supported by a cross-functional team of privacy subject matter experts, including a Data Protection Officer, who collaborate in the development and maintenance of a forward-looking Global Data Privacy Program that seeks address shifts in both the internal and external environments, along with emerging challenges in the privacy and data protection regulatory landscape. The Program, through its policies, procedures, and centralized guidance for processing personal data, seeks to drive organizational accountability and empower Genmab colleagues, and our third party partners, to handle personal data consistent with our values of ethical behavior, integrity, fairness, inclusion, and transparency. To further support compliance with regulatory, legal, and other requirements applicable to our business and operations, including current Good Laboratory Practices (cGLP), current Good Clinical Practices (cGCP) and current Good Manufacturing Practices (cGMP), Genmab’s QA department is staying abreast of and adhering to regulatory and legislative changes relevant to quality standards. Risk Level in Relation to Last Year: Unchanged Decreased Increased Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 67 |
|
Risk Management Risk related to Risk areas Mitigation Risk trend Regulation, Legislation, and Compliance (continued) (continued) Genmab has also established relevant procedures and guidelines to ensure transparency with respect to providing timely, adequate, and correct information to the market and otherwise complying with applicable securities laws and other legal and regulatory requirements. Genmab has an Internal Audit function that reports to the Audit and Finance Committee of the Board and administratively reports to the CFO. Legislation, regulations, industry codes and practices, and their application may change from time to time. To prevent unwarranted consequences of new and amended legislation, regulations, etc., Genmab strives to stay current with respect to all applicable legislation, regulations, industry codes and practices by means of its internal compliance function and related governance bodies as well as internal and external legal counsel. Also, internal procedures for review and refinement of contracts are ongoing to ensure contractual consistency and compliance with applicable legislation, regulation, and other standards. Intellectual Property Genmab is dependent on protecting our own intellectual property rights to regain our investments and protect our competitive positions. We may become involved in lawsuits to protect or enforce our patents or other intellectual property which could result in costly litigation and unfavorable outcomes. Claims may be asserted against us that we infringe the intellectual property of third parties, which could result in costly litigation and unfavorable outcomes. Genmab files and prosecutes patent applications to optimally protect its products and technologies. To protect trade secrets and technologies, Genmab maintains strict confidentiality standards and agreements for employees and collaborating parties. Genmab actively monitors third-party patent positions within our relevant fields to avoid violating any third-party patent rights. Finances Genmab may need additional funding. Because Genmab’s future commercial potential and operating profits are hard to predict, Genmab’s policy is to maintain a strong capital base so as to maintain investor, creditor and market confidence, and a continuous advancement of Genmab’s product pipeline and business in general. Genmab also has access to revolving loan facility totaling USD 300 million, with options to increase size of the facility up to USD 500 million, which can be drawn down upon as another source of additional funding. Risk Level in Relation to Last Year: Unchanged Decreased Increased Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 68 |
|
Risk Management Risk related to Risk areas Mitigation Risk trend Finances (continued) Genmab is exposed to different kinds of financial risks, including currency exposure and changes in interest rates as well as changes in Danish, U.S. or foreign tax laws or related compliance requirements. Genmab has established financial risk management guidelines to identify and analyze relevant risks, to set appropriate risk limits and controls, and to monitor the risks and adherence to limits. Please refer to Note 4.2 of the financial statements for additional information regarding financial risks. Management and Workforce Genmab may have an inability to attract and retain suitably qualified team members as it continues to evolve. To attract and retain our highly skilled team, including the members of Genmab’s Executive Management, Genmab offers competitive remuneration packages, including share-based remuneration. Genmab strives to create a positive, safe, and energizing working environment. Genmab has strong core values that nourish high-integrity and ethical behavior, respectful and candid tone and a culture which prizes diversity, as well as trust and teamwork. Genmab has implemented strategies such as diversifying recruitment efforts, cross-training employees, fostering a culture of knowledge sharing, investing in talent development programs, and promoting a supportive work environment that values employee well-being and career growth. Please refer to Note 4.6 of the financial statements for additional information regarding share-based compensation. Cybersecurity Genmab may be subject to malicious cyber attacks, and with the increased use of artificial intelligence within the biopharmaceutical industry, can lead to the theft or leakage of intellectual property, sensitive business data, or personal employee or patient data, with the result of significant business disruptions, negative impacts to patient or employee privacy, monetary loss or fines from authorities, or reputational damage. Genmab has implemented security controls and processes to enhance the identification of potential data/systems security issues and mitigate the risk of security breaches. Genmab makes use of the National Institute of Standards and Technology (NIST) Cybersecurity Framework and other security standards to define and implement such security controls. Due to the continually changing threat environment, regular assessments are executed to ensure that implemented security controls and processes follow the threat profile of the Company and effectively support Genmab’s ambitious business strategy. The risk of security breaches is regarded as enterprise risk and the Company’s threat profile, the security program and security incidents are presented and discussed in meetings of the Global Compliance and Risk Committee and the Audit and Finance Committee of the Board. Genmab’s Cybersecurity Program, in conjunction with Genmab’s Global Data Privacy Program, collaborate to manage and mitigate any cybersecurity and data privacy threats to the personal data processed in our systems and by our third party partners. Risk Level in Relation to Last Year: Unchanged Decreased Increased Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 69 |
|
Risk Management Risk related to Risk areas Mitigation Risk trend Environment Genmab could face transitional risks by its inability to manage the carbon footprint and energy mix from our business operations and physical risks from climate-related events that may impact our business operations or that of our third-party partners or suppliers. Genmab has oversight and manages its carbon footprint Scope 1 and 2 emissions from its business operations. Genmab is committed to tracking the Scope 3 emissions carbon footprint by partnering with suppliers. Genmab makes use of scenario analysis to evaluate risks and opportunities due to the rapid pace of world climate change. Genmab’s work with climate strategy, carbon reduction targets, climate-related financial risk, relevant prevention, and mitigation measures are presented to and reviewed by the Board biannually. Refer to the sustainability statements for details of Genmab’s targets in the future to mitigate risks. Risk Level in Relation to Last Year: Unchanged Decreased Increased Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 70 |
|
As an international biotech company dedicated to improving the lives of cancer patients around the world, Genmab operates within a heavily regulated environment that exposes us to an ever-evolving set of risks, some of which are beyond our control. Genmab has core facilities in five countries, conduct activities in additional areas, and perform an array of essential innovation, research, development, manufacturing activities, commercial operations, and support functions, all of which pose risks to our operations and success. Specifically, these operations and activities expose us to risks that include but may not be limited to financial, research and development, regulatory, IT/data/technology, staffing, compliance, legal, and also environmental risks. In order to assure that we are positioned to effectively identify and mitigate the potential impacts of these risks, Genmab has dedicated resources toward enabling its ERM framework under the Global Compliance & Risk function that reports directly to the CEO. In concert with a refreshed Code of Conduct, company policies and procedures, Genmab has chartered a Global Compliance and Risk Governance Committee (GCRC) co-chaired by the CEO and the head of Compliance & Risk. Genmab has also updated its risk model and framework to include enhanced risk oversight, mitigation, governance, and reporting, all of which we believe positions us to better manage the risks associated with our business, now and into the future. Enterprise Risk Management Effective ERM starts with strong governance Board and Audit and Finance Committee Board delegates ERM/Risk oversight to the Audit and Finance Committee but retains visibility of ERM progress. The Audit and Finance Committee is accountable to ensure management appropriately manages the risks to the business. Executive Management Maintains ultimate ownership of and accountability for management of top risks, enabling proper linkage of risk management to strategic initiatives and business decisions. GCRC Validates risk identification, prioritization, strategic and tactical ownership of risk mitigation plans and reporting. ERM Framework Routinely gathers risks, evaluates with risk sponsors, prioritizes, and reports to the GCRC, Executive Management and Board, driving risk discussions, and supporting risk sponsors and management in facilitating ERM processes, risk-intelligent decision-making, and key risk capabilities. Risk Sponsors and Business Champions Manage risks in the normal course of business, executing risk plans/mitigation activities, and monitoring and reporting key risk information. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 71 |
|
Corporate Governance Genmab works diligently to improve its guidelines and policies for corporate governance, taking into account the recent trends in international and domestic requirements and recommendations. Genmab’s commitment to corporate governance is based on ethics and integrity and forms the basis of its effort to strengthen the confidence that existing and future shareholders, partners, employees, and other stakeholders have in Genmab. The role of shareholders and their interaction with Genmab is important. Genmab believes that open and transparent communication is necessary to maintain the confidence of Genmab’s shareholders and achieves this through company announcements, investor meetings and company presentations. Genmab is committed to providing reliable and transparent information about its business, financial results, development programs and scientific results in a clear and timely manner. All Danish companies listed on the Nasdaq Copenhagen are required to disclose in their annual reports how they address the Recommendations for Corporate Governance issued by the Committee on Corporate Governance in December 2020 (the “Recommendations”), applying the “comply-or-explain” principle. Genmab follows the Recommendations, except for one specific sub-area where Genmab’s corporate governance principles differ from the Recommendations: • The Recommendations provide that according to a company’s takeover contingency procedures, the Board abstains from countering any takeover bids by taking actions that seek to prevent the shareholders from deciding on the takeover bid, without the approval of the general meeting. Genmab does not have such a restriction in its takeover contingency procedures and retains the right in certain circumstances to reject takeover bids without consulting the shareholders. Genmab believes this provides the Board with the needed flexibility to best respond to takeover bids and to negotiate with bidders; retaining this flexibility helps the Board meet its objectives in protecting and creating value in the interest of the shareholders. Actions will be determined on a case-by-case basis with due consideration of the interests of the shareholders and other stakeholders. Genmab publishes its statutory report on Corporate Governance for the financial year 2024 cf. Article 107b of the Danish Financial Statements Act (“Lovpligtig redegørelse for virksomhedsledelse jf. årsregnskabslovens § 107 b”) on the Company’s website, including a detailed description of the Board’s consideration in respect of all the Recommendations. The statutory report on Corporate Governance can be found on Genmab’s website https://ir.genmab. com/corporate-governance. The Board of Directors The Board is responsible for setting the overall strategy and goals for Genmab and monitoring its operations and results. Board duties include establishing policies for strategy, accounting, organization and finance and the appointment of Executive Management members. The Board also assesses Genmab’s capital and share structure and is responsible for approving share issues and the grant of warrants and RSUs. The Board has established an annual process whereby the Board’s performance is assessed through self-evaluation to verify that the Board is capable of fulfilling its function and responsibilities. When performing these evaluations external assistance is obtained every year. The outcome of the Board‘s 2024 self-assessment was positive with only minor areas for improvement identified. Board Committees To support the Board in its duties, the Board has established and appointed a Compensation Committee, an Audit and Finance Committee, a Nominating and Corporate Governance Committee and a Scientific Committee. These committees are charged with reviewing issues pertaining to their respective fields that are due to be considered at Board meetings. Written charters specifying the tasks and responsibilities for each of the committees are available on Genmab’s website www.genmab.com. For more details on the work, composition and evaluation of the Board and its committees, reference is made to the statutory report on Corporate Governance. Remuneration Policy A Remuneration Policy applying to the compensation of members of the Board and the registered Executive Management of Genmab A/S has been prepared in accordance with Sections 139 and 139a of the Danish Companies Act and was most recently considered and adopted by the 2024 Annual General Meeting pursuant to the Danish Companies Act (in Danish “Selskabsloven”). The Remuneration Policy contains an exhaustive description of the remuneration components for members of the Board and the registered Executive Management and includes the reasons for choosing the individual components of the remuneration and a description of the criteria on which the balance between the individual components of the remuneration is based. The latest version can be downloaded from Genmab’s website https:// ir.genmab.com/compensation. Compensation Report In accordance with the Recommendations, Genmab has prepared a compensation report for the financial year 2024 that includes information on the total remuneration received by each member of the Board and the registered Executive Management of Genmab A/S for the last four years, including information on the most important content of retention and resignation arrangements and the correlation between the remuneration and company strategy and relevant related goals (the “Compensation Report”). The Compensation Report can be found on Genmab’s website https://ir.genmab.com/compensation. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 72 |
|
Change of Control The Danish Financial Statements Act (Section 107a) contains rules relating to listed companies with respect to certain disclosures that may be of interest to the stock market and potential takeover bidders, in particular in relation to disclosure of change of control provisions. In the event of a change of control, change of control clauses are included in some of our collaboration, development, and license agreements as well as in service agreements for certain employees. Collaboration, Development and License Agreements Genmab has entered into collaboration, development and license agreements with external parties, which may be subject to renegotiation in the case of a change of control event as specified in the individual agreements. However, any changes in the agreements are not expected to have significant impact on our financial position. Service Agreements with Executive Management and Employees The service agreements with each registered member of the Executive Management may be terminated by Genmab with no less than 12 months’ notice and by the registered member of the Executive Management with no less than six months’ notice. In the event of a change of control of Genmab, the termination notice due to the registered member of the Executive Management is extended to 24 months. In the event of termination by Genmab (unless for cause) or by a registered member of Executive Management as a result of a change of control of Genmab, Genmab is obliged to pay a registered member of Executive Management a compensation equal to his/her existing total salary (including benefits) for up to two years in addition to the notice period. In addition, Genmab has entered into service agreements with a limited number of employees according to which Genmab may become obliged to compensate the employees in connection with a change of control of Genmab. If Genmab, as a result of a change of control, terminates the service agreement without cause or changes the working conditions to the detriment of the employee, the employee shall be entitled to terminate the employment relationship without further cause with one month’s notice in which case Genmab shall pay the employee a compensation equal to one-half, one or two times the employee’s existing annual salary (including benefits). Change of control clauses related to our warrant and RSU programs are outlined in Note 4.6. Share capital Information on share capital is included in Note 4.7. Unless otherwise provided in the Danish Companies Act, the adoption of any resolution to amend Genmab A/S’ articles of association shall be subject to the affirmative vote of not less than two thirds of the votes cast, as well as of the voting share capital represented at the general meeting. Genmab A/S’ entire articles of association can be found on our website www.genmab.com. Corporate Governance Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 73 |
|
Deirdre P. Connelly Female, Hispanic/American, 64 Board Chair (Independent, elected by the General Meeting); Chair of the Nominating and Corporate Governance Committee, Member of the Audit and Finance Committee and the Compensation Committee First elected 2017, current term expires 2025 Special Competencies and Qualifications Deirdre P. Connelly has more than 30 years’ experience as a corporate leader and board member in publicly traded companies with global operations. She has comprehensive knowledge and experience with business conduct, business turnaround and product development and has successfully directed the launch of more than 20 new pharmaceutical drugs. As a former HR executive, Deirdre P. Connelly also has valuable insight in corporate culture transformation, talent development and managing large organizations. She furthermore has significant experience with the development of governance and other sustainability related responsibilities from various leadership roles and as a board member. Deirdre P. Connelly is former President of U.S. Operations of Eli Lilly and Company and former President, North America Pharmaceuticals for GlaxoSmithKline. ESG Competencies: Social • Governance Current Board Positions • Member: Lincoln Financial Corporation1 , Macy’s Inc.2 1. Chair of Compensation Committee, Member of Audit Committee, Corporate Governance Committee and Executive Committee 2. Chair of Nominating and Corporate Governance Committee, Member of Compensation and Management Development Committee Pernille Erenbjerg Female, Danish, 57 Deputy Board Chair (Independent, elected by the General Meeting); Chair of the Audit and Finance Committee, Member of the Nominating and Corporate Governance Committee First elected 2015, current term expires 2025 Special Competencies and Qualifications Pernille Erenbjerg has broad executive management and business experience from the telecoms, media, and tech industries. She has extensive expertise with business conduct and in operation and strategic transformation of large and complex companies, including digital transformations and digitally based innovation, and has been responsible for major transformation processes in complex organizations including M&A. Pernille Erenbjerg furthermore has significant IT and cybersecurity expertise and sustainability related experience from various executive and non-executive positions. She has a Certified Public Accountant background (no longer practicing) and has a comprehensive all-around background within finance, including extensive exposure to public and private equity and debt investors. Pernille Erenbjerg is former CEO and President of TDC Group A/S. Pernille Erenbjerg is an audit committee financial expert based on her professional experience, including her background within accounting, her service in senior finance leadership at TDC Group A/S and as an audit committee chair or member at other public companies. ESG Competencies: Environmental • Social • Governance Current Board Positions • Chair: KK Wind Solutions • Member: RTL Group1 , GlobalConnect 1. Chair of Audit Committee Anders Gersel Pedersen, M.D., Ph.D. Male, Danish, 73 Board Member (Non-independent, elected by the General Meeting); Chair of the Compensation Committee and Member of the Scientific Committee and the Nominating and Corporate Governance Committee First elected 2003, current term expires 2025 Special Competencies and Qualifications Anders Gersel Pedersen has more than 30 years’ board and management experience in publicly traded, international pharmaceutical and biotech companies. He has significant knowledge and expertise in discovery and development of the product pipeline from preclinical activities to post-launch marketing studies as well as solid business experience. Anders Gersel Pedersen furthermore has extensive experience with the global pharmaceutical market and has built comprehensive knowledge and insight in governance, including business conduct, and the development of other sustainability related responsibilities from various leadership roles and as a board member. Anders Gersel Pedersen is former Executive Vice President of Research & Development of H. Lundbeck. ESG Competencies: Environmental • Social • Governance Current Board Positions • Chair: Aelis Farma S.A.S. • Deputy Chair: Bavarian Nordic A/S1 • Member: Hansa Biopharma AB2, Bond 2 Development GP Limited 1. Member of Finance, Risk and Audit Committee, Member of Science, Technology & Investment Committee 2. Chair of Scientific Committee, Member of Remuneration Committee Board of Directors Refer to our website: Board of Directors | Genmab for the Board of Directors diversity and skills matrix. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 74 |
|
Paolo Paoletti, M.D. Male, Italian/American, 74 Board Member (Independent, elected by the General Meeting); Chair of the Scientific Committee and Member of the Compensation Committee First elected 2015, current term expires 2025 Special Competencies and Qualifications Paolo Paoletti has extensive experience in research, development and commercialization in the pharmaceutical industry, where he has been responsible for the development of several medicines approved globally and the related global commercial strategies. As an executive, he has led cross-functional teams on the development and registration of medicines and has been responsible for all compliance aspects for the R&D organization. Paolo Paoletti has successfully conducted submissions and approvals of new cancer drugs and new indications in the U.S., in Europe and in Japan. He furthermore has significant experience with governance, including business conduct, from various leadership roles and as a board member. Paolo Paoletti is former Vice President of Oncology Clinical Development with Eli Lilly and Company, former President of GSK Oncology with GlaxoSmithKline, and former CEO of GAMMADELTA Therapeutics. ESG Competencies: Environmental • Social • Governance Current Position, including Managerial Positions • Member of the Investment Committee for Apollo Therapeutics Limited • Scientific Advisor for 3B Future Health Fund Current Board Positions • None Rolf Hoffmann Male, German/Swiss, 65 Board Member (Independent, elected by the General Meeting); Member of the Audit and Finance Committee and the Scientific Committee First elected 2017, current term expires 2025 Special Competencies and Qualifications Rolf Hoffmann has more than 30 years’ experience in senior management and as a board member in the life science industry worldwide. He has significant expertise with business conduct and in creating and optimizing commercial opportunities in global markets and has managed companies across multiple continents with multibillion P&L and cross-functional accountability. Rolf Hoffmann furthermore has knowledge and experience with governance, compliance and ensuring organizational efficiency from various management positions as well as from being a board member. Rolf Hoffmann has held a variety of sales and marketing and executive management positions with Eli Lilly and Company, and is former Senior Vice President, International Commercial Operations and former Senior Vice President, U.S. Commercial Operations with Amgen. ESG Competencies: Environmental • Social • Governance Current Position, including Managerial Positions • Adjunct Professor of Strategy and Entrepreneurship at University of North Carolina Business School Current Board Positions • Member: Semdor Pharma, Sun Pharmaceutical Industries Ltd.1 1. Member of Nomination and Remuneration Committee Elizabeth A. O’Farrell Female, American, 60 Board Member (Independent, elected by the General Meeting); Member of the Audit and Finance Committee and the Compensation Committee First elected 2022, current term expires 2025 Special Competencies and Qualifications Elizabeth O’Farrell has solid financial experience from her 25-year career in finance leadership roles and as a board member. During her career, she has led multiple strategy, planning and resource allocation processes in multiple roles and in cross-functional teams. Elizabeth O’Farrell has significant knowledge and expertise in business conduct and with driving paradigm changing contributions within finance and the enterprise through collaboration and influence. In addition to experience at Price Waterhouse and Whipple & Company Corporation, Elizabeth O’Farrell held various executive management positions at Eli Lilly and Company, including as former Chief Procurement Officer. Elizabeth O’Farrell is an audit committee financial expert based on her professional experience, including her service in senior finance leadership positions at Eli Lilly and as an audit committee chair or member at other public companies. ESG Competencies: Social • Governance Current Board Positions • Chair: PDL BioPharma1 • Member: LENSAR2, Geron Corporation3 , Karius2 1. Chair of Compensation Committee, Member of Audit Committee, Member of Cost Committee 2. Chair of Audit Committee 3. Chair of Audit Committee, Member of Strategic Committee Board of Directors Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 75 |
|
Takahiro Hamatani Male, Japanese, 50 Board Member (Non-independent, elected by the employees) First elected 2022, current term expires 2025 Special Competencies and Qualifications Takahiro Hamatani has over 20 years’ experience in the pharmaceutical industry in various roles including finance, sales, marketing, and corporate strategy. He has extensive expertise in strategic business planning and finance business partnering as well as experience in successful product launches, geographical expansions, and business development deals. Takahiro Hamatani has previously worked in International Operations at Takeda supporting commercial operations in North and South America and is a Certified Public Accountant in the US. ESG Competencies: Social • Governance Current Position, including Managerial Positions • Senior Director, Head of Finance Japan at Genmab Martin Schultz Male, Danish, 49 Board Member (Non-independent, elected by the employees) First elected 2022, current term expires 2025 Special Competencies and Qualifications Martin Schultz has broad experience within clinical project management with a substantial understanding and knowledge of research and development. He furthermore has specific expertise in project management, strategic sourcing, vendor collaboration, contract, and budget governance. ESG Competencies: Social • Governance Current Position, including Managerial Positions • Senior Director, Head of Development Business Partnership & Strategy at Genmab Mijke Zachariasse, Ph.D. Female, Dutch, 51 Board Member (Non-independent, elected by the employees) First elected 2019, current term expires 2025 Special Competencies and Qualifications Mijke Zachariasse has broad experience in people and business management and expertise in building partnerships across sectors, research funding landscape, operational excellence and organizational strategy and change. ESG Competencies: Environmental • Social • Governance Current Position, including Managerial Positions • Vice President, Head of Antibody Research Materials at Genmab Board of Directors Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 76 |
|
Jan G. J. van de Winkel, Ph.D. Dutch, 63, Male President & Chief Executive Officer Special Competencies Extensive antibody creation and development expertise, broad knowledge of the biotechnology industry and executive management skills. ESG Competencies: Social • Governance Anthony Pagano American, 47, Male Executive Vice President & Chief Financial Officer Special Competencies Significant knowledge and experience in the life sciences industry particularly as it relates to corporate finance, corporate development, strategic planning, general management, treasury, accounting, and corporate governance. ESG Competencies: Social • Governance Judith Klimovsky, M.D. Argentinian (U.S. Citizen), 68, Female Executive Vice President & Chief Development Officer Special Competencies Extensive expertise in oncology drug development from early clinical stages through to marketing approval, experience in clinical practice and leading large teams in pharmaceutical organizations. ESG Competencies: Social • Governance Current Board Positions • Member: Bio-Techne Tahamtan Ahmadi, M.D., Ph.D. Iranian-German (U.S. Citizen), 52, Male Executive Vice President & Chief Medical Officer, Head of Experimental Medicines Special Competencies Significant expertise in global regulatory and clinical drug development across entire spectrum from pre-IND to life cycle management; drug discovery and translational research. ESG Competencies: Social • Governance Birgitte Stephensen Danish, 64, Female Executive Vice President, Chief Legal Officer Special Competencies Intellectual property and legal expertise in the pharmaceutical and biotechnology fields. ESG Competencies: Social • Governance Executive Management As of December 31, 2024, there are nine members of Executive Management. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 77 |
|
Christopher Cozic American, 47, Male Executive Vice President, Chief People Officer Special Competencies Expertise in strategic leadership, organization design, human resource management, policy development, employee relations, organizational development, and a heavy concentration in all aspects of corporate growth and expansion. ESG Competencies: Social Martine J. van Vugt, Ph.D. Dutch, 54, Female Executive Vice President, Corporate Strategy and Planning Special Competencies Extensive knowledge of and experience in Corporate Strategy, Corporate and Business Development, as well as Portfolio, Project, and Alliance Management. ESG Competencies: Social • Governance Rayne Waller American, 57, Male Executive Vice President & Chief Technical Operations Officer Special Competencies Expertise in all elements of technical operations from early-to-mid-stage product development through global manufacturing of both clinical and commercial products. ESG Competencies: Social • Governance Brad Bailey American, 57, Male Executive Vice President & Chief Commercial Officer Special Competencies Extensive experience in strategic and operational commercial leadership roles across specialty biopharma, oncology, immunology, and other serious diseases. ESG Competencies: Social Executive Management Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 78 |
|
Ownership Genmab is dual listed on the Nasdaq Copenhagen and the Nasdaq Global Select Market in the U.S. under the symbol GMAB. Our communication with the capital markets complies with the disclosure rules and regulations of these exchanges. As of December 31, 2024, the number of registered shareholders totaled 93,681 shareholders holding a total of 64,844,223 shares, which represented 98% of the total share capital of 66,187,186. The following shareholder is registered in Genmab’s register of shareholders as being the owner of a minimum of 5% of the voting rights or a minimum of 5% of the share capital (one share equals one vote) as of December 31, 2024: • BlackRock, Inc., 50 Hudson Yards, New York, New York 10001, United States of America (6.8%) Shareholders registered in the Company’s shareholder registry may sign up for electronic shareholder communications via Genmab’s investor portal. The investor portal can be accessed at Genmab’s website www.genmab.com/investors. Electronic shareholder communication enables Genmab to, among other things, quickly and efficiently call general meetings. The charts included here illustrate the performance of the Genmab share during 2024, the performance of the Genmab share over the last five years, from 2020 through the end of 2024, and the geographical distribution of our shareholders. As of December 30, 2024, Genmab’s shares closed at DKK 1,492.50 and ADSs closed at USD 20.77. Shareholders and Share Information The following table shows share data as of December 31, 2024. Share Data Denmark U.S. Number of shares at December 31, 2024 66,187,186 6,280,456 (represented by 62,804,560 American Depository Shares (ADSs)) Listing Nasdaq Copenhagen Nasdaq Global Select Market, New York Ticker Symbol GMAB GMAB Index Membership OMX Nordic Large Cap Index OMX Copenhagen Benchmark Index OMX Copenhagen 25 Index (OMXC25) Nasdaq Biotech Index Stock Performance Comparison 2024 (Index 100 = stock price on December 31, 2023) Stock Performance Comparison 5 Years (Index 100 = stock price on December 31, 2019) Geographical Shareholder Distribution* * Based on Nasdaq Corporate Solutions aggregated data per June 30, 2024 and July 31, 2023 ** “Other” includes shares held in other countries and shares not held in nominee accounts, including OTC traded shares 60 70 80 90 100 110 120 Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Genmab OMXC25 Index Nasdaq Biotech Index 50 100 150 200 250 Oct 2024 Jul 2024 Jan 2024 Apr 2024 Jul 2023 Oct 2023 Jan 2023 Apr 2023 Jul 2022 Oct 2022 Jan 2022 Apr 2022 Jul 2020 Oct 2020 Jan 2020 Apr 2020 Jul 2021 Oct 2021 Jan 2021 Apr 2021 Genmab OMXC25 Index Nasdaq Biotech Index 22% 18% 3% 3% July 31, 2023 June 30, 2024 3% 3% 6% 6% 20% 26% 46% 44% USA Denmark UK Netherlands Norway Other** Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 79 |
|
Shareholders and Share Information Please refer to Note 4.7 of the financial statements for additional information regarding Genmab’s share capital including authorizations to issue shares and purchase its own shares. Genmab is a Foreign Private Issuer as defined in the SEC’s rules and regulations. The determination of foreign private issuer status is made annually. We plan to make our next determination with respect to our foreign private issuer status on June 30, 2025. American Depositary Receipt (ADR) Program Genmab has a sponsored Level 3 ADR program with Deutsche Bank Trust Company Americas. An ADS is a share certificate representing ownership of shares in a non-U.S. corporation. ADSs issued under Genmab’s ADR Program are quoted and traded in U.S. dollars on the Nasdaq Global Select Market in the United States. Ten Genmab ADSs correspond to one Genmab ordinary share. Genmab’s ADR ticker symbol is GMAB. For more information on Genmab’s ADR Program, visit https://ir.genmab.com/adr-program. Investor Relations Genmab’s Investor Relations department aims to ensure relevant, accurate and timely information is available to our investors and the financial community. We maintain an ongoing dialogue with sell-side equity analysts, as well as major institutional and retail shareholders. A list of the current analysts covering Genmab can be found at our website along with financial reports, company announcements, current presentations, fact sheets and other downloads. Contact For Media Relations: Marisol Peron Senior Vice President Global Communications & Corporate Affairs T: +1 609 524 0065; E: mmp@genmab.com For Investor Relations: Andrew Carlsen Vice President Head of Investor Relations T: +45 33 77 95 58; E: acn@genmab.com Annual General Meeting Genmab’s Annual General Meeting will be held on March 12, 2025 at 2:00 PM CEST. Further details will be included in the notice to convene the Annual General Meeting. Financial Calendar for 2025 Annual General Meeting 2025 Wednesday, March 12, 2025 Publication of the Interim Report for the first quarter 2025 Thursday, May 8, 2025 Publication of the Interim Report for the first half 2025 Thursday, August 7, 2025 Publication of the Interim Report for the first nine months 2025 Thursday, November 6, 2025 Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 80 |
|
Management’s Review: Sustainability Statements In this section 82 General Information 94 Environmental 106 Social 124 Governance 131 Appendix A Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 81 |
|
Sustainability Statements Sustainability Statements General Information Genmab recognizes that sustainability is fundamental to the way we work, and crucial for protecting the planet, fostering economic and social stability, and ensuring a viable future for all. As Genmab is committed to improving patients’ lives through transformative medicines, Genmab understands the interconnectedness between human health and the health of our planet. By prioritizing sustainability initiatives, such as reducing carbon emissions, and fostering a diverse and inclusive workplace, Genmab ensures that its actions today contribute to a healthier and more equitable future for generations to come. Moreover, sustainability is integral to maintaining stakeholder trust and securing long-term success in an increasingly conscious global marketplace. Section Disclosure Requirements Content Disclosure Requirements # Reference/Report 1.1 Basis for preparation General basis for preparation of the sustainability statement BP-1 SUS Disclosures in relation to specific circumstances BP-2 SUS 1.2 Governance The role of the administrative, management and supervisory bodies GOV-1 SUS, MR Information provided to, and sustainability matters addressed by the undertaking’s administrative, management and supervisory bodies GOV-2 SUS, MR Sustainability-related performance in incentive schemes GOV-3, E1, S1 SUS Statement on due diligence GOV-4 SUS Risk management and internal controls over sustainability reporting GOV-5 SUS, MR 1.3 Strategy Strategy, business model and value chain SBM-1 SUS, MR, FS Interests and views of stakeholders SBM-2 SUS Material impacts, risks and opportunities and how they interact with our strategy and business model SBM-3 SUS 1.4 Impact, risk and opportunity management Process to identify and assess material impacts, risks and opportunities IRO-1 SUS Disclosure requirements in ESRS covered by the sustainability statement IRO-2 SUS SUS—Sustainability Statements MR—Management’s Review FS—Financial Statements Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 82 |
|
Sustainability Statements 1.1 Basis for presentation General basis for preparation of the sustainability statement (BP-1) Frameworks The Corporate Sustainability Reporting Directive (CSRD) directs public companies to report in line with the European Sustainability Reporting Standards (ESRS) starting on or after January 1, 2024. As a result, this 2024 annual report marks the first year in which Genmab is reporting in accordance with this directive and the underlying reporting standards. Genmab’s sustainability statements have been prepared in compliance with the ESRS as required by sections 99a of the Danish Financial Statements Act. Consolidation The sustainability statements have been prepared on a consolidated basis in line with our consolidated financial statements; therefore, the disclosures comprise the Genmab A/S (parent company) and its subsidiaries. The E1 disclosures in particular have been consolidated on the basis of both financial and operational control. The sustainability statements cover Genmab’s own operations and upstream and downstream value chains, where material, specifically regarding disclosures around impacts, risks and opportuni-ties (IROs), policies, actions, targets and metrics. Genmab has not omitted any specific pieces of information corresponding to intellectual property, know-how or the results of innova-tion nor used the exemption from disclosure of impending developments or matters in the course of negotiation. Disclosures in relation to specific circumstances (BP-2) Disclosures Stemming from Other Regulation Genmab’s sustainability statements also comply with sections 99d and 107(d) of the Danish Financial Statements Act. Refer to Appendix A for a full overview. Accounting Policies Genmab’s accounting policies have been applied, in all material respects, consistently in the financial year and for comparative figures. Key Accounting Estimates and Judgements Genmab uses estimates and judgements for the reporting of certain data points related to our Scope 3 emissions, which are detailed in the relevant accounting policies. Quantifying greenhouse gas (GHG) emissions inherently involves significant uncertainty due to the complexity of natural and anthropogenic systems. Measurement challenges arise from factors such as variability in emissions sources, accuracy of data and assumptions in emission factors. We regularly reassess our use of estimates and judgements based on experience, the development of sustainability reporting, and a number of other factors. Changes in estimates are recognized in the period in which the estimate in question is revised. In addition, we make judgements when we apply the accounting policies. Refer to the quantitative data tables in the sustainability statements for further informa-tion on accounting policies, key estimates, judgements, and assumptions applied. Incorporation by Reference Genmab’s management report consists of a Management’s Review section and these sustain-ability statements which reports disclosure requirements from the ESRS. Our sustainability statements are structured into four overall sections: ‘General Information,’ ‘Environmental,’ ‘Social,’ and ‘Governance.’ Genmab has chosen to incorporate some of the strategy and gover-nance disclosures from the cross-cutting standard ESRS 2 in the Management’s Review as we believe this information is best read in close connection with our Financial Review and overview of our activities. Where incorporation by reference is used to refer outside of these sustainability statements, it is clearly defined. Genmab considers forward-looking information including targets disclosed in the sustainability statements to be uncertain. 1.2 Governance The role of the administrative, management and supervisory bodies (GOV-1), and information provided to, and sustainability matters addressed by the undertaking’s administrative, management and supervisory bodies (GOV-2) At Genmab, sustainability governance is ingrained in our overall corporate governance framework, reflecting our commitment to inte-grating sustainable practices into all aspects of our business. Genmab’s sustainability gover-nance structure is robust, with clear roles and responsibilities defined at various levels of the organization. At the highest level, the Board (which includes 0 executive members and 9 non-executive members) oversees sustainability strategy and performance, ensuring alignment with Genmab’s long-term business objectives and stakeholder expectations. The Board receives updates on CSR and sustainability initiatives at least annually, has oversight of material impacts, risks, and oppor-tunities (IROs) and targets identified by Genmab, and enables informed decision-making that incor-porates environmental, social, and governance (ESG) considerations. It is the Board’s aim to maintain an equitable gender representation in the Board. As of December 31, 2024, the six shareholder-elected board members are evenly split between 50% male (three persons) and 50% female (three persons) which constitutes equal gender representation in accordance with the guidelines from the Danish Business Authority. As of December 31, 2024, at the Board level, five or 56%, are independent, and four or 44%, are not independent. In accordance with the Danish corporate governance recommendations, we consider shareholder-elected board member Anders Gersel Pedersen non-independent solely by virtue of the length of his tenure on our Board, following his election to the Board in 2003. Also, the three employee-elected board members are non-independent. Our Board’s Nominating and Corporate Governance Committee oversees our corporate governance, CSR, ESG and sustainability efforts and provides recommendations to the Board on corporate governance, CSR, ESG and sustain-ability matters. Additionally, the Board’s Audit and Finance Committee oversees our sustain-ability reporting requirements. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 83 |
|
Sustainability Statements Within the management structure, Genmab has established a dedicated CSR & Sustainability Committee, which is co-chaired by our CEO and SVP of Global Communications and Corporate Affairs and is comprised of senior leaders from relevant functional areas, such as research and development, operations, compliance and risk, legal, human resources, and finance. This committee is responsible for setting strategic priorities, management of material IROs and targets, and monitoring progress towards sustain - ability goals. It also facilitates cross-functional collaboration to drive innovation and continuous improvement in sustainability performance. Under the CSR & Sustainability Committee, Genmab has established a Corporate Sustainability Team comprised of Genmab employees respon - sible for driving the double materiality assessment process including the identification of material impacts, risks and opportunities, targets/goals, actions and metrics, and the collection and documentation of relevant data for sustain - ability reporting requirements. The Corporate Sustainability Team coordinates with internal stakeholders (other Genmab employees) respon - sible for integrating sustainability strategy into business operations. The Corporate Sustainability Team is responsible for monitoring and reporting on progress towards targets at least annually. Sustainability reporting is approved annually by Executive Management (which includes 9 executive members and 0 non-executive members) and the Board. Executive Management and the Board have significant global expertise in the pharmaceutical, biotech and life sciences industries with specific expertise in Environmental, Social and Governance matters. Further, Genmab has access to experts and has utilized the expertise of outside consultants in preparing its DMA and with regards to envi - ronmental matters. Refer to the biographies in the “Board of Directors” and “Executive Management” sections in Management’s Review for an understanding of the relevant experience (including sustainability-related expertise). Genmab maintains transparent communication channels with stakeholders, including investors and proxy advisors, employees, customers, suppliers, and the broader community, regarding its sustainability efforts. Genmab regularly discloses sustainability-related information through various channels, such as annual reports, investor presentations and engagement sessions. By fostering dialogue and incorporating feedback from stakeholders, Genmab ensures that its sustainability initiatives are responsive to evolving expectations and societal needs. For Business Conduct matters, the leaders of our Global Compliance, Data Privacy, and Enterprise Risk Management Programs report directly to the CEO and the Board. Overall, sustainability governance at Genmab is characterized by proactive leadership, robust oversight, and stakeholder engagement, reflecting our commitment to creating long-term value while contributing positively to society and the environment. Refer to the “Corporate Governance” section in Management’s Review for additional informa - tion on governance structure. Refer to section SBM-3 for a list of the material impacts, risks and opportunities addressed by management and those charged with gover - nance during the reporting period. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 84 |
|
Sustainability Statements Sustainability-related performance in incentive schemes (GOV-3) Per Genmab’s Remuneration Policy, the variable compensation of the Executive Management is based on the achievement of specific Key Performance Indicators (KPIs) and performance goals that relate to the performance of each executive member and to Genmab A/S’ short and long-term business results. The KPIs and perfor-mance goals that the Board sets for the purposes of Genmab A/S’ incentive arrangements—both annual and share-based—are directly linked to the business strategy and our annual business plans. The KPIs/performance goals may be financial, operational, and/or strategic and organizational. The KPIs/performance goals are recommended by the Compensation Committee and approved by the Board. Genmab grants restricted stock units (RSUs) to Executive Management which are performance-based and include sustainability-related performance goals, whereas RSUs granted to the Board are not performance-based and do not include sustainability-related performance goals. The key characteristics of the sustainability-related performance goals are tied to Climate, Diversity and Employee Well-Being targets with equal weighting against the overall goals of 10% for the 2024 and 2023 grants, and 15% for the 2022 grant. Share-based compensation granted is at a 4x target multiplier with maximum oppor-tunity of 6x multiplier with no cap in 2024 and 2023, compared to a 2.4x target multiplier with 4x maximum with a grant date fair value cap of DKK 25 million in 2022. In 2025, Genmab plans to remove gender diversity balance targets from executive management performance criteria in future grants. Refer to sections E1-4 and S1-5 for targets linked to Executive Management incentive compensation. Total remuneration to registered Executive Management in 2024 was DKK 79.9 million. The proportion of total remuneration to regis-tered Executive Management in 2024 linked to climate-related performance goals was DKK 1.8 million, or 2%. Total variable remunera-tion to registered Executive Management in 2024 was DKK 63.4 million and comprises annual cash bonus and share-based compensation expense. The proportion of variable remuneration to regis-tered Executive Management in 2024 linked to all sustainability-related performance goals was DKK 5.7 million, or 9%. Refer to Note 5.1 in the consolidated financial statements for details. Statement on due diligence (GOV-4) The following table provides a mapping of the core elements of due diligence, for impacts on people and the environment, to the relevant disclosures in Genmab’s sustainability statements: Core elements of Due Diligence Sections in the Sustainability Statement Does the disclosure relate to people and/or the environment? a) Embedding due diligence in governance, strategy, and business model ESRS 2 GOV-1, GOV-2, GOV-3 ESRS 2 SBM-3: E1 People and Environment Environment b) Engaging with affected stakeholders in all key steps of the due diligence ESRS 2 GOV-1, GOV-2 ESRS 2 SBM-2 ESRS 2 IRO-1 ESRS 2 MDR-P: E1-2 People and Environment People and Environment People and Environment Environment c) Identifying and assessing adverse impacts ESRS 2 IRO-1: E1 Environment d) Taking actions to address those adverse impact ESRS 2 MDR-A: E1-3 Environment e) Tracking the effectiveness of these efforts and communicating ESRS 2 MDR-M: E1-4 ESRS 2 MDR-T: E1-4 Environment Environment Risk management and internal controls over sustainability reporting (GOV-5) Genmab assesses potential risks related to sustainability mainly through our CSR & Sustainability Committee but also as part of Genmab’s Enterprise Risk Program led by our Head of Global Compliance and Risk who reports directly to our CEO, both of which are members of the CSR & Sustainability Committee. Clearly defined sustainability governance aids the overall risk management process. The Board has a supervisory duty and Executive Management has overall responsibility for Genmab’s risk management and internal controls in relation to sustainability reporting processes. Genmab evaluates how sustainability-related risks could affect operations, reputation, and financial performance. Genmab has also imple-mented processes to ensure the accuracy and reliability of sustainability data collected from various internal stakeholders/departments, which help maintain transparency and trace-ability of reported information. Genmab aligns its sustainability reporting with recognized frameworks to ensure consis-tency and comparability, and engages with Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 85 |
|
Sustainability Statements stakeholders to gather feedback and ensure reporting meets their needs and expectations. Internal and external audits are conducted of sustainability reporting processes to assess compliance with established controls and processes. Genmab has an Internal Audit function that reports to the Audit and Finance Committee of the Board and administratively reports to the CFO, and any findings would be communicated through this channel at least annually. The Corporate Sustainability Team integrates the findings of its risk assessment and internal controls regarding the sustainability reporting process into relevant internal functions and processes. Genmab’s external auditor provides limited assurance on Genmab’s sustainability statements. Genmab provides training to employees on sustainability issues, related risk management practices and reporting responsibilities to foster a culture of accountability. These compo-nents collectively help Genmab manage risks effectively and ensure the integrity of its sustain-ability reporting. Refer to Risk Management section in Management’s Review for the followed risk assessment approach including the risk prioritization methodology, details of risks identified and mitigation strategies, and related controls. 1.3 Strategy Strategy, business model and value chain (SBM-1) A description of Genmab’s strategy including our response/priorities to the main challenges ahead, business model, value chain, products, and customers in relation to sustainability is provided in the following sections in Management’s Review: • Our Strategy • Who We Are • Business Model • Value Chain • Research and Development Capabilities • Bringing Our Own Innovative Medicines to Patients • Antibody Discovery and Development • Products and Technologies Genmab’s sustainability-related goals have been broken out into relevant targets in the Environmental, Social and Governance sections of these sustainability statements. Refer to section S1-6 for information on Genmab’s headcount by geographical areas. Refer to Note 2.1 in the consolidated financial statements for disclosures related to Genmab’s revenue by type, collaboration partner and product, and Note 2.2 for Genmab’s revenue by geographical area. There are no additional significant ESRS sectors beyond those reflected in these disclosures. Genmab’s goals have been broken out into relevant targets in the Environmental, Social and Governance sections of the sustainability statements. Interests and views of stakeholders (SBM-2) As an international biotech company, Genmab engages continuously with a diverse range of stakeholders, all of whom have a vested interest in our business practices. This engage-ment is crucial to our success and reinforces our commitment to benefiting both our direct stakeholders and society as a whole. Through ongoing dialogue, we seek to understand their perspectives, concerns, and expectations. These interactions inform our sustainability efforts, due diligence processes and double materiality assessments, ensuring that our actions align with the needs and insights of those we serve. The views and interests of affected key stake-holders regarding our sustainability-related impacts are regularly communicated to our CSR & Sustainability Committee through periodic meetings. For all key stakeholders listed in the table, Genmab listens to the views of stakeholders through engagement and takes these views into account, which has led to the outcomes disclosed. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 86 |
|
Sustainability Statements Genmab’s Key Stakeholders Description Value Chain Location How Engagement is Organized Purpose of Engagement Outcome from Engagement Academic and research institutions Partners contributing to early-stage research, technology development, and/or innovation. Upstream ― Academic partnerships ― Scientific conferences and workshops ― Sponsorship of academic research ― Collaborative research programs ― Collaboration on research ― Access to innovation ― Training and knowledge sharing ― Accelerated innovation ― Enhanced scientific knowledge ― Strengthened reputation ― Talent development Collaboration partners Companies that collaborate with Genmab in co-development, licensing, and/or commercialization. Upstream, Downstream ― Joint team meetings ― Steering committees ― Project reviews ― Innovation and development ― Strategic alignment ― Successful product development and commercialization ― Shared knowledge and expertise ― Strengthened relationships Communities As part of Genmab’s ongoing commitment to Corporate Social Responsibility (CSR), we aim to contribute to and ensure the vibrancy and sustainability of the communities where we live and work. Upstream, Own Operations, Downstream ― Community programs ― Partnerships with nonprofits ― Employee involvement ― Health education and awareness ― Social responsibility ― Building trust ― Positive community impact ― Increased employee satisfaction and morale ― Stronger relationships and rapport with the community Contract manufacturing organizations (CMOs) CMOs manage the production of Genmab’s antibody therapies, ensuring that they meet quality standards and are produced at scale. Upstream ― Structured collaboration ― Regular communication ― Quality assurance processes ― Efficiency and flexibility to leverage the expertise and capacity of CMOs ― Focus on core competencies ― Quality products ― Timely production ― Building long-term relationships with CMOs fosters innovation Employees Internal stakeholders involved at every stage of our supply chain, from research to commercialization, ensuring that Genmab’s operations align with its strategic goals. Our talented teams are the cornerstone of our success and fundamental to achieving our 2030 Vision. Own Operations ― Surveys and workplace assessments, including our Global Engagement Survey ― Inclusion networks ― Employee-elected board members ― Personal development dialogues and trainings ― Employment relations and occupational health and safety representation ― Dutch Works Council ― Danish Employee Representative Council ― Including employees’ perceptions and experiences ― Contributing to a sustainable workplace and working life ― Social dialogue with workers councils ― Internal policy updates ― Improvement and action plans ― Communications from management ― Global initiatives and campaigns ― Employee engagement scores Healthcare providers Physicians, nurses, pharmacists and medical institutions that play a role in recruiting patients for clinical trials and prescribing/administering Genmab’s therapies. Upstream, Downstream ― Clinical Trials ― Advisory Boards ― Educational Initiatives ― Feedback Mechanisms ― Understanding clinical needs ― Enhancing clinical research ― Improving patient outcomes ― Building relationships ― Refined clinical trials ― Increased adoption of therapies ― Enhanced safety and efficacy ― Stronger provider networks Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 87 |
|
Sustainability Statements Genmab’s Key Stakeholders Description Value Chain Location How Engagement is Organized Purpose of Engagement Outcome from Engagement Investors Shareholders interested in the entire value chain, with a focus on how each stage contributes to Genmab’s financial health and growth potential. Genmab has a diverse shareholder base with investors from across a spectrum of size and location. The support of Genmab’s investors is essential to the success of the Company. Upstream, Downstream ― Earnings calls and reports ― Investor conferences ― One-on-one meetings with investors and proxy advisors ― Roadshows ― Transparency related strategy, financial performance, and research advancements ― Trust building ― Feedback gathering to understand investor perspectives and concerns ― Increased investor confidence ― Enhanced market understanding ― Diverse shareholder base Patients/patient organizations The end-users of Genmab’s therapies or groups who represent them, whose outcomes and feedback are vital to develop and deliver better medicines. Upstream, Downstream ― Standing Patient Advisory Council ― Survey and focus groups ― Support patient organizations ― Clinical trial participation ― Annual science day ― Patient-centric approach ― Feedback mechanisms ― Education and awareness ― Evaluate patient information of Genmab medicines and clinical trials ― Insights on trial designs ― Enhanced communication ― Informed decision making ― Safer and more effective products Payers Insurance companies, government healthcare programs, and other entities that reimburse or fund the cost of therapies, influencing pricing and market access. Downstream ― Value assessment studies ― Direct meetings and presentations ― Participation in advisory boards ― Collaboration with health economists ― Market access ― Value demonstration ― Refine pricing strategies, improve product offerings, and align with market needs. ― Increased access ― Stronger relationships ― Enhanced value proposition Quality assurance teams Both internal teams (employees) and external partners ensure the consistency and safety of the products during manufacturing. Own Operations, Upstream ― Cross-functional teams ensuring quality is integrated throughout product lifecycle ― Regular audits and reviews ― Standard operating procedures (SOPs) ― Trainings ― Regulatory compliance ― Product integrity ― Identifying continuous improvement within processes ― High quality products ― Reduced risk of non-compliance ― Enhanced reputation Regulatory agencies Bodies like the EMA (Europe), the FDA (U.S.) and MHLW (Japan) are key stakeholders, as they approve trial designs, oversee progress, and ultimately decide on the approval of new therapies. Upstream, Downstream ― Regular meetings ― Submissions and reports ― Advisory committees ― Guidance consultation ― Regulatory compliance ― Gaining insights and recommendations ― Risk mitigation ― Approval pathway clarity ― Streamlined development process ― Regulatory approvals ― Enhanced safety and efficacy data ― Stronger regulatory relationships Scientists and research partners Internal teams (employees) and external partners are critical in the early stages of target identification and antibody engineering. These include our own antibody expert scientists as well as academic institutions, research organizations (including CROs) and technology providers. Own Operations, Upstream ― Co-development of programs ― Licensing of our technology platforms ― Involvement in clinical trials including partnering with clinical research organizations (CROs) ― Indirectly through work with industry groups ― Access to technology ― Accelerated drug development ― Networking and community building ― Development of innovative/new antibody therapeutics and other treatment modalities ― Publications and patents ― Enhanced research capabilities ― Increased visibility and reputation Suppliers Providers of raw materials and other inputs necessary for the production of antibody therapies. Upstream ― Supplier selection and qualification ― Collaboration and communication ― Contracts and agreements ― Quality assurance ― Supply chain reliability ― Innovation and improvement ― Drive actions toward sustainability related targets/goals ― Consistent quality ― Improved efficiency ― Strong relationships Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 88 |
|
Sustainability Statements Material impacts, risks, and opportunities and how they interact with our strategy and business model (SBM-3) The table describes Genmab’s material impacts, risks and opportunities resulting from our double materiality assessment (see section IRO-1), including a description of where in our business model, our own operations, and our value chain these material impacts, risks and opportunities are concentrated. We also describe whether our impacts are positive or negative. All impacts are actual impacts unless otherwise stated that they are potential impacts. More information on how we respond to the effects of our impacts, risks and opportuni-ties is included in the topical sections under ‘Environmental,’ ‘Social,’ and ‘Governance.’ There, we also expand on the linkage to our sustainability strategy and business model, expected time horizons and the nature of the business relationship. There is no significant risk of a material adjust-ment within the next annual reporting period to the carrying amounts of assets and liabilities reported in the consolidated financial statements linked to material risks and opportunities iden-tified in our sustainability reporting. Genmab has no material investment or disposal plans currently linked to our material IROs or planned sources of funding to implement our sustain-ability strategy. We have one entity specific IRO relating to G1: Business Conduct, Cybersecurity. This is Genmab’s first year of sustainability reporting and therefore there are no changes since the previous reporting period. Section Material Topic Material Impact Value Chain Location Time Horizon Material Risk Material Opportunity Environmental Climate Change ― Adaptation and Mitigation Emissions from value chain (negative) Upstream Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Environment Partner with value chain to reduce Scope 3 emissions Emissions from own operations (negative) Own Operations Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Environment Renewable energy contracts (to mitigate climate change risks) Behavioral changes of employees (labs, travel patterns) Climate Change ― Energy Energy consumption (negative) Own Operations Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Environment Increasing energy efficiency in the procurement of new locations as operations expand in the future and major renovations to existing locations. Generation of energy from onsite solar panels (positive) Own Operations Short, Medium, Long-Term Social Own Workforce ― Working Conditions Employee well-being and vitality (positive) Own Operations Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Management and Workforce Provide a voice to employees through our global engagement survey Safety in our facilities (positive) Own Operations Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Management and Workforce Safety training/ongoing education in our laboratories Own Workforce ― Equal Treatment and Opportunities for All Career development through training and skill building (positive) Own Operations Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Management and Workforce Providing employees with opportunities to discuss their career at Genmab Diversity in the workplace (positive) Own Operations Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Management and Workforce Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 89 |
|
Sustainability Statements Section Material Topic Material Impact Value Chain Location Time Horizon Material Risk Material Opportunity Social (continued) Consumers and End-Users ― Social inclusion of consumers and/or end-users Access and integrity in clinical trials (positive) Downstream Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance Market access programs to allow for product availability for uninsured or underinsured (positive) Downstream Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Business and Products Assisting those having difficulty affording Genmab products prescribed to them Consumers and End-Users ― Personal safety and information of consumers and/or end users Patient engagement programs developed (positive) Downstream Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Business and Products Clinical trial transparency (positive) Downstream Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance Governance Business Conduct ― Corporate Culture Healthy corporate culture aligned with core values and purpose (positive) Own Operations Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance Annual Code of Conduct training rollout as mandatory for all employees, with launch and completion rates monitored by Global Compliance team Business Conduct ― Privacy Cybersecurity and Global Data Privacy programs in place to protect the privacy of our business, our own workforce, patients and all individuals who entrust us with their information (positive) Upstream, Own Operations, Downstream Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance and Cybersecurity Business Conduct ― Protection of whistle-blowers Protection of whistleblowers through anti-retaliation policies and procedures (positive) Upstream, Own Operations, Downstream Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance Business Conduct ― Animal Welfare Animal welfare policy (positive) Own Operations Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance Business Conduct ― Management of relationships with suppliers (including payment practices) Strong management of suppliers, focused on compliance with supplier code of conduct (positive) Upstream Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Strategic Collaborations Continue to partner with suppliers on sustainability related commitments in the future Business Conduct ― Corruption and bribery Ethical business culture and business practices (positive) Own Operations Short, Medium, Long-Term Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance, Strategic Collaborations and Management and Workforce Annual Code of Conduct training rollout as mandatory for all employees, with launch and completion rates monitored by Global Compliance team Genmab has the resources in place to manage the effects of the IROs on our business across the Environmental, Social and Governance areas. Refer to the Environmental section of the sustainability statements for information on Genmab’s resilience analysis. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 90 |
|
Sustainability Statements 1.4 Impact, risk and opportunity management Process to identify and assess material impacts, risks and opportunities (IRO-1) A cornerstone element of the CSRD and ESRS is the concept of double materiality for sustain-ability matters. The concept of double materiality means that a sustainability matter can have an inside-out impact (i.e., an impact that Genmab has or can have on people and/or the environ-ment—this is called ‘impact materiality’) or an outside-in impact (i.e., a (actual/potential) financial impact (risk/opportunity) from a sustainability matter on Genmab—this is called ‘financial materiality’). Genmab performed a double materiality assess-ment (DMA) for the first time as part of our 2024 sustainability reporting and we look forward to incorporating our sustainability strategy in our day-to-day business operations over the coming year(s). From a governance perspective, the Corporate Sustainability Team drove the execution of the DMA. The approach for and outcomes of the double materiality were extensively discussed with and approved by Executive Management and our Board and its relevant Committees. Refer to GOV-1 and GOV-2 for further details on the governance structure with regards to sustainability. Scope The scope of the DMA entailed the parent company, Genmab A/S, and its subsidiaries on a consolidated basis as well as our upstream and downstream value chain. Material topics Genmab engaged a sustainability consultancy firm to assist in the performance of a DMA in accordance with the CSRD and the underlying ESRS. During this process, which involved numerous internal and external stakeholders, we determined seven (7) topics, sub-topics or sub-sub topics to be material for Genmab. These seven material topics link to four (4) of the standards (ESRS E1, S1, S4 and G1). Non-material topics Genmab screened site locations and assets across Denmark, the Netherlands, the U.S., Japan and China including business activities and considering all Application Requirement 16 topics, determined ESRS E2, E3, E4, E5, S1 (specific to other work-related rights), S2 and S3 to be not material from an impact or financial perspective. In our analysis, we considered our own operations and upstream and down-stream value chain and were not aware of any material matters to disclose in our sustainability statements. We surveyed internal stakeholders and external stakeholders and performed desk research, held workshops and deep-dive sessions, and noted no issues triggering these topics to be material for Genmab. E4 followed the same approach as above and as such we did not perform a detailed assessment to identify related IROs. Impact of Genmab on People and the Environment Double Material E1 G1 S1 Working conditions S1 Equal treatment and opportunities for all S4 Environmental E1: Climate Change • Climate change adaptation/ Climate change mitigation (1) • Energy (2) E2: Pollution E3: Water and Marine Resources E4: Biodiversity & Ecosystems E5: Circular Economy Financial Impact on Genmab Non-Material E2 S1 Other work-related rights E3 S2 E4 S3 E5 Impact Material Financial Material Social S1: Own Workforce • Working Conditions (3) • Equal treatment and opportunities for all (4) • Other work-related rights S2: Workers in the value chain S3: Affected communities S4: Consumers & End-Users • Social inclusion of consumers and/or end-users (5) • Personal safety and information of consumers and/or end-users (6) Governance G1: Business Conduct (7) Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 91 |
|
Sustainability Statements The process of how the DMA was performed, and description of how we arrived at these material topics is described below. Value chain analysis and stakeholder selection As an initial step, Genmab defined our value chain and key stakeholders, focusing both on our own operations, as well as on our value chain. We conducted desk research that included peer reviews on sustainability topics and research on the relevance of sustainability topics for the pharmaceutical sector. Based on the outcomes of this mapping exercise and desk research, we determined initial topics for the longlist used in the double materiality analysis. We selected internal stakeholders from across our business operations to provide coverage across all ESRS topics, sub-topics, and sub-sub-topics. Given the global scope of our business, we included internal stakeholders from across all our locations. We interviewed external stakeholders repre-senting at least one across each of the following: patient associations, investors, collaboration partners, sector organizations and civil society organizations. The input of these external stake-holders, combined with the expertise of the internal stakeholders, provided valuable insights. Longlist and validation Following the analysis as described above, a longlist of material topics was prepared. This longlist was based on the guidance provided by the ESRS (ESRS 1—Application Requirement 16), further complemented by insights from the value chain and stakeholder analyses, other regulatory frameworks, and desktop peer review. Based on this longlist, both impact materiality and financial materiality were assessed through surveys, interviews, and workshops. The combi-nation of these three methods ensured coverage of the broad range of stakeholders and provided room to finetune the outcomes to reflect any (regional) nuances during the workshops. The surveys provided input from a broad audience and led to initial scoring. Internal and external interviews conducted before the workshops and after the workshops provided expert insight and guidance to validate outcomes. Refer below for further detail on the execution of the impact mate-riality and financial materiality analyses. Double Materiality The ESRS prescribes companies to assess topics that are material to the business from an impact and/or financial perspective. The following para-graphs describe the methodology Genmab used to assess both impact and financial materiality. Impact Materiality ESRS 1, ‘General Requirements,’ highlights the indicators that companies should consider in assessing their impacts on sustainability matters. The assessment of impacts is aligned with other international standards, such as the United Nations Guiding Principles on Business and Human Rights (UNGP) and the Organization for Economic Co-Operation and Development (OECD) Guidelines. International standards prescribe that impacts should be rated based on their severity. The concept of severity is based on the factors scale, scope, and irremediable character of the impact. In case of positive impacts, scoring irremediability is unnecessary. For Genmab, all the mentioned individual factors were rated on a scale of 1 to 5, based on which an average score was calculated. In case of potential impacts, participants also rated likelihood on a scale of 0 to 1. For actual impacts, the likelihood is automatically scored as 1 since the likelihood has already been proven. For completeness’ sake, we note that for potential negative impacts regarding human rights, severity took precedence over likelihood as also prescribed by the ESRS. Other indicators that are prescribed by the ESRS for the assessment of impact materiality include: the topic’s location in the value chain (own opera-tions, upstream or downstream), and whether the impact pertains to the undertaking in the short-, medium- or long-term scored as time horizons. To summarize, all longlisted sustainability matters were scored using the following indicators: (i) the topic’s location in the value chain (own operations, upstream or downstream), (ii) whether it is an actual or potential impact, (iii) whether it is a positive or negative impact, (iv) time horizons, and (v) scale, scope and irremediability of the impacts. For a matter to be considered as impact material, we have used a relative threshold—i.e., we have used the average score for impact materi-ality based on the five-point scale and set the threshold a full point higher. Financial Materiality During the preparation of the longlist, as well as during the workshops, we considered impacts and dependencies with the risks and opportu-nities that may arise from these impacts and dependencies through extensively discussing the sustainability topic at hand and what impact it could have on Genmab and our business. The scoring of financial materiality is largely similar to impact materiality; however, instead of focusing on impacts, financial materiality is based on risks and opportunities. Risks and opportunities are scored on the basis of magnitude (scale of 1 to 5), and likelihood (scale of 0 to 1). Participants also assess time horizons and location in the value chain. For a matter to be considered as financial material, we initially used a similar relative threshold as for impact materiality: the average financial materiality score based on the five-point scale plus a full point. Our final results used a mix of qualitative and quantitative factors and our financial statement materiality level to conclude on material topics. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 92 |
|
Sustainability Statements Validation The survey outcomes were discussed with internal stakeholders during the workshops and deep-dive sessions. Where needed, changes and/or nuances were made. To further align and validate the outcomes of the DMA, these were presented to external stakeholders and internal interviews were conducted with subject matter experts. All identified sustainability-related risks have been identified in prior year reporting under our ERM framework and continue to be updated and prioritized in line with other risks identified. The final DMA for 2024 has been approved and adopted by the Board with the filing of this Annual Report. Action While conducting the DMA in 2024, we further integrated the various aspects of the DMA into our business. We focused on setting meaningful and appropriate targets for our material topics including defining the data gathering processes. With our dedicated Corporate Sustainability Team, we intend to monitor our performance over the coming years, while remaining agile and current with our double materiality assess-ment (e.g., following acquisitions or other major business changes). Disclosure requirements in ESRS covered by the sustainability statement (IRO-2) Following the outcome of our DMA, Genmab compiled a list of disclosure requirements including the page numbers and/or paragraphs where the related disclosures are located in the sustainability statements. This is presented as content indexes in the General, Environmental, Social and Governance sections of the sustain-ability statements. Genmab has determined the material information to be disclosed in relation to the impacts, risks and opportunities assessed to be material, utilizing a mix of qualitative and quantitative factors and our financial statement materiality level. Genmab also included a table of datapoints derived from other EU legislation in the appen-dices to the sustainability statements. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 93 |
|
Sustainability Statements Environmental As a leading international biotech company, we recognize the responsibility we have to protect our planet and its natural resources, as well as the health and safety of our team members, business partners and society as a whole. By conducting our business in a safe and sustainable manner, we aim to reduce our environmental impact by refining our processes and incorporating best practices into our own operations and value chain, where applicable. To achieve this, our environmental strategy focuses on monitoring and evaluating targets for environmental activities, measuring our impact and communicating our progress. Section Disclosure Requirement Content Disclosure Requirement # E1 Climate Change 2.0 IROs Material Impacts, Risks and Opportunities and their interaction with strategy and business model SBM-3 2.1 E1 Climate Change Strategy Transition plan for climate change mitigation E1-1 2.2 E1 Climate Change IRO management Policies related to climate change mitigation and adaptation E1-2 Actions and resources in relation to climate change policies E1-3 2.3 E1 Climate Change Metrics and Targets Targets related to climate change mitigation and adaptation E1-4 Energy consumption and mix E1-5 Gross Scopes 1, 2, 3 and total GHG emissions E1-6 GHG removals and GHG mitigation projects financed through carbon credits E1-7* Internal carbon pricing E1-8* Anticipated financial effects from material physical and transition risks and potential climate-related opportunities E1-9** EU Taxonomy 2.4 EU Taxonomy Disclosures pursuant to Article 8 of Regulation (EU) 2020/852 (Taxonomy Regulation) N/A *Disclosure requirements E1-7 and E1-8 are not applicable for Genmab. **Genmab has adopted the phase-in for E1-9 and elected not to disclose for year 1 reporting. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 94 |
|
Sustainability Statements 2.0 IROs Material Impacts, Risks and Opportunities (IROs) and their interaction with strategy and business model (SBM-3) Section Material Topic Material Impact Value Chain Location Material Risk Material Opportunity Environmental Climate Change ― Adaptation and Mitigation Emissions from value chain (negative) Upstream Genmab could face transitional risks by its inability to manage the carbon footprint and energy mix from our business operations and physical risks from climate-related events that may impact our business operations or that of our third-party partners or suppliers. Partner with value chain to reduce Scope 3 emissions Emissions from own operations (negative) Own Operations Refer to the Risk Management section of the Annual Report under Environment for details Renewable energy contracts (to mitigate climate change risks) Behavioral changes of employees (labs, travel patterns) Climate Change ― Energy Energy consumption (negative) Own Operations Increasing energy efficiency in the procurement of new locations as operations expand in the future and major renovations to existing locations. Generation of energy from onsite solar panels (positive) Own Operations All impacts, risks and opportunities have expected time horizons of short, medium and long-term. Emissions from our value chain/ Emissions from our own operations Climate change adaptation and mitigation is material due to GHG emissions primarily in our value chain, but also in our own operations, which has a negative impact on the environment with transitional and physical risks associated. Genmab has adaptation and mitigation efforts aligned with the Paris Agreement for a future where warming is kept at 1.5°C. Genmab is committed to reducing GHG emissions to limit the global warming the planet is facing. In line with our ambitions to reduce Scope 3 emissions through supplier engagement, Genmab has an opportunity to reduce GHG emissions in our upstream value chain by setting clear expec-tations and requirements, providing incentives and setting goals, which could lead to more efficient production processes and cost savings for both Genmab and its suppliers. In terms of our own operations, Genmab plans to reduce emissions by sourcing electricity from renewable energy sources such as solar, wind, hydroelectric, or geothermal power. Genmab has an opportunity to reduce emissions by actively investing in renewable energy procurement through power purchase agreements (PPAs), green tariffs, or direct ownership of renewable energy assets which could mitigate climate change risks relating to carbon taxation. Genmab can decrease the carbon intensity of its electricity consumption by contracting with renewable energy providers or investing in onsite renewable energy projects. This not only helps in reducing emissions, but also supports the growth of renewable energy markets and contributes to broader sustainability goals. Genmab has an opportunity to reduce GHG emissions and promote health in the context of climate change through various initiatives targeting employee behavior and operational practices. We aim to reduce energy consumption and GHG emissions by implementing sustainable practices in laboratories in line with MyGreenLab recommendations. Additionally, we encourage employees to minimize air travel to lower GHG emissions while ensuring business continuity, supported by guidelines that have been in place for several years. Furthermore, establishing remote work policies and promoting alternative commuting methods, such as biking, carpooling, and public transit, can contribute to decreasing GHG emissions associated with employee commuting, fostering a healthier environment for all and could lead to cost savings. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 95 |
|
Sustainability Statements Energy consumption Genmab utilizes 61% renewable energy at our state-of-the-art buildings; therefore, there is a negative impact in terms of non-renewable energy usage with risk associated with our energy mix; however, there is room to achieve 100% renewable energy usage at all of our locations. Genmab has an opportunity to increase energy efficiency in the procurement of new locations as operations expand in the future and major renovations to existing locations which could lead to financial savings on operational expenses. Refer to E1-5 for energy mix and consumption table. Generation of energy from onsite solar panels Genmab also has a positive impact from generation of energy through solar panels at our headquarters in Denmark and certain R&D locations in the Netherlands. Refer to E1-4 for climate change targets. Genmab’s Resilience to Climate Change Genmab’s resilience analysis was conducted qualitatively in 2021, incorporating climate scenarios based on key reports from authorita-tive bodies such as the Intergovernmental Panel on Climate Change (IPCC) and the International Energy Agency (IEA). No material changes to the analysis were identified in 2024, 2023 or 2022. The analysis was conducted by assessing climate-related impacts across Genmab’s entire value chain, including supply chains, operations, energy consumption, and logistics. This resilience analysis helps inform Genmab’s strategic planning, risk management, and financial planning processes, ensuring that climate-related risks and opportunities are integrated into the company’s Enterprise Risk Management (ERM) framework. Genmab utilized two scenarios to explore potential transition and physical risks at a Paris Agreement aligned 1.5–2°C and a high emissions scenario at 4°C warming levels, considering both short-term (2030) and medium-to-long-term (2040/2050) time horizons in alignment with Genmab’s strategic planning horizons and its GHG emissions reduction targets. • The 1.5–2°C scenario assumes a transition to a low-carbon economy in line with global climate targets. This scenario evaluates risks and opportunities arising from regulatory actions such as carbon taxation, low-carbon technology adoption, and evolving consumer preferences toward sustainability. • The 4°C scenario represents a business-as-usual pathway with high emissions and limited global mitigation efforts, leading to more severe physical risks such as extreme weather events, flooding, and disruptions to supply chains. The key assumptions for the resilience analysis include the transition to a low-carbon economy, macroeconomic trends, energy consumption and mix, technology deployment and time horizons. Based on the scenario analysis, Genmab iden-tified several potential physical risks, transition risks and opportunities for both the 1.5–2°C and 4°C warming scenarios across short-term and medium/long-term time frames. The identified physical and transition risks and opportunities were evaluated based on likelihood, magnitude and duration to Genmab’s operations and taking into account Genmab’s physical geographical locations at the time of conducting the analysis. No aspects of Genmab’s business were identified as incompatible with a transition to a climate neutral economy. • Key physical risks to Genmab’s business activities and assets identified in the scenarios: Disruption of supply chain and operations from extreme weather events, increased cooling costs from more frequent and severe heat waves, increased costs of raw materials due to changes in weather patterns and extreme weather events, operations and supply chain disruption from coastal flooding and damage to physical assets and inventory. • Key transition risks to Genmab’s business activities identified in the scenarios: Global carbon taxation and pricing impacting costs and financial returns, investor focus on climate performance limiting access to capital and investment, competition for talent in scientific and innovative fields becomes increasingly linked to climate performance, and cost of compliance with fragmented and drastic regulatory intervention. While the scenario analysis identified potential physical and transition risks, the risk related to the Environment was identified as the material risk to Genmab. Refer to the Risk Management section in Management’s Review for risks related to the Environment. Genmab has committed to a science-aligned emissions reduction target aligned with the Paris Agreement, aimed at reducing its GHG emissions in line with the global goal to limit warming to 1.5°C. This target plays a critical role in mitigating both transition and physical risks Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 96 |
|
Sustainability Statements by guiding risk mitigation, reducing exposure to physical risks and enhancing resilience to market shifts. Uncertainties within the resilience analysis included climate projections under the scenarios and regulatory evolution over time. Genmab’s resilience analysis, underpinned by qualitative scenario analysis and guided by a science-aligned emissions reduction target, high-lights the company’s preparedness by adapting our strategy for both transition and physical climate risks in the medium and long term. The science-aligned emissions reduction target offers a clear pathway for mitigating these risks while also seizing opportunities associated with the transition to a low-carbon economy. Through its ongoing commitment to sustainability, Genmab is not only reducing its exposure to climate risks but also positioning itself for long-term business success in an increasingly climate-conscious world. 2.1 E1 Climate Change Strategy Transition plan for climate change mitigation (E1-1) Genmab approaches climate change mitiga-tion through a comprehensive transition plan, addressing risks, setting targets, and engaging stakeholders including suppliers. We have assessed potential climate-related risks to our own operations and our value chain—See the Risk Management section in Management’s Review for further details. We have established science-aligned emissions reductions targets for reducing GHG emissions across our own operations and our value chain, aligning with the goals of the Paris Agreement to limit global warming to 1.5°C, and we have set targets for GHG emission reduction. Genmab plans to reduce GHG emissions by: • engaging with suppliers and partners to ensure ongoing decarbonization of our value chain, • sourcing electricity from renewable energy sources such as solar, wind, hydroelectric, or geothermal power, and • encouraging and inspiring behavioral changes for our employees to reduce emissions from lab operations, business travel and employee commuting Genmab’s transition plan is embedded in and aligned with our overall business strategy including our financial planning. Refer to “Our Strategy” section in Management’s Review for details. The transition plan is reviewed through our governance model and is approved by Executive Management and Board. Refer E1-4 for Genmab’s progress on the imple-mentation of the transition plan. 2.2 E1 Climate Change IRO Management Policies related to climate change mitigation and adaptation (E1-2) Genmab published its Corporate Social Responsibility (CSR) Policy in 2023 which was adopted by the Board and includes a focus on environmental sustainability. In 2024, Genmab published an overarching Commitment to the Environment and Sustainability to manage material environ-mental sustainability matters including material IROs covering climate change adaptation and mitigation, and energy, and matters not material to Genmab including water and circular economy. The Commitment to the Environment and Sustainability was adopted by the CSR & Sustainability Committee on December 11, 2024. Genmab’s Commitment to the Environment and Sustainability applies to all employees and contractors and all activities within Genmab’s operations. It extends to all regions where Genmab operates, recognizing diverse environ-mental challenges and opportunities. Genmab’s CSR & Sustainability Committee, composed of cross-functional senior leaders, and co-lead by our CEO and SVP Communications & Corporate Affairs, is responsible for the oversight of Genmab’s Commitment to the Environment & Sustainability and implementation. Genmab has included the perspectives of internal and external key experts and stakeholders in determining material environmental topics and in formulating Genmab’s Commitment to the Environment and Sustainability. Genmab’s Commitment to the Environment and Sustainability is available for our key stake-holders including our employees on our website (Genmab.com). Refer to the Commitment to the Environment and Sustainability on our website. Actions and resources in relation to climate change policies (E1-3) Engaged with suppliers to encourage them to set their own emissions targets We engage in continuous dialogue on sustain-ability with our key suppliers so Genmab may meet our target as disclosed in E1-4. In 2024, Genmab benchmarked the climate ambitions of our top suppliers to provide a clear baseline and identify actions and hotspots for improvement. We had in-person and virtual engagement with key suppliers identified as significant sources of emissions. Genmab estab-lished formalized processes governing supplier engagement on climate change by incorporating specific climate change topics in the supplier onboarding and annual contract review. Continued investment in Renewable Energy Procurement In 2024, Genmab renegotiated an electricity contract to ensure that our sites continue towards our ambition of sourcing 100% renewable elec-tricity to help mitigate our Scope 2 emissions. Refer to E1-5 and E1-6 for further details on energy usage and mix, and GHG emissions. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 97 |
|
Sustainability Statements 2.3 E1 Climate Metrics and Targets Targets related to climate change mitigation and adaptation (E1-4) Genmab plans to minimize its carbon footprint across our business to align with a future where warming is kept at 1.5°C in line with the Paris Agreement. Genmab has considered a diverse range of climate scenarios when setting the targets. Specific targets consistent with Genmab’s GHG inventory are summarized below and relate to the gross reduction of GHG emissions: (1) Develop and execute on sustainable climate-related strategy by 20251 The development and execution of a sustain-able climate-related strategy by 2025 is critical to ensuring that we align our operations with global climate targets. By disclosing Scope 1, 2, and 3 emissions in the 2023 annual report-ing, Genmab has already laid the groundwork for transparency and accountability of our envi-ronmental impact. Building on this foundation, in 2024, Genmab achieved the development of a comprehensive climate strategy, with a clear commitment to climate targets aligned with the Paris Agreement. Executing this strategy will involve taking tangible actions toward GHG emissions reductions, with partial progress on Scope 1 and 2 reductions expected by the end of 2025. By executing these steps, Genmab plans to not only meet regulatory expecta-tions but also contribute to global efforts to combat climate change toward a sustainable future for both our operations and the planet. 1. Executive Management received RSU grants in 2023 with performance linked to developing and executing on a sustainable climate-related strategy. 2. Executive Management received RSU grants in 2024 with performance linked to Scope 1 and Scope 2 emission reductions by 42% by 2030 from a 2021 base year. The grant occurred prior to our base year update to 2024 due to significant changes in our structure and corresponding emissions (ProfoundBio acquisition in May 2024) and achievement will be assessed prior to base year update. 3. Executive Management received RSU grants in 2024 with performance linked to supplier engagement ensuring two thirds of suppliers by spend committed to a Paris Agreement aligned climate target by 2030. (2) Reduce Scope 1 and Scope 2 emissions by 42% through a reduction in Scope 2 emissions by 2030 from a 2024 base year2 Genmab intends to achieve a 42% reduction in Scope 1 and Scope 2 (market-based) GHG emissions by 2030 compared to a 2024 baseline year by sourcing renewable electricity at our facilities. (3) Ensure 70% of suppliers by spend covering upstream purchases goods and services, capital goods and upstream transportation commit to have Science Based Targets (SBT) by 20303 Genmab intends to indirectly mitigate GHG emission impacts to the environment by 2030 through supplier engagement and responsible sourcing practices by ensuring at least 70% of our largest suppliers by spend (operating expenses), covering upstream purchased goods and services, capital goods and upstream transportation, commit to have science-based targets by 2030. (4) Reduce Scope 1 and 2 emissions by 90% by 2050 from a 2024 base year Genmab intends to reduce Scope 1 and 2 (market-based) GHG emissions by 90% by 2050 from a 2024 base year to contribute to the obtainment of the ambitions laid out in the 2015 Paris Agreement by sourcing renewable electricity and switching to cleaner heating sources. Refer to section GOV-3 for climate related targets related to Executive Management incentive compensation. In 2022, Executive Management was granted RSUs linked to Genmab’s climate target to ensure 100% renewable electricity was achieved across all major operating sites, which at the time of grants included our headquarters in Denmark and sites in the Netherlands, the U.S. and Japan. As of December 31, 2024, all major operating sites under the RSU grant achieved 100% renewable electricity. Energy consumption and mix (E1-5) Energy consumption and mix 2024 1 Total fossil energy consumption MWh 4,616 Share of fossil sources in total energy consumption % 38% 2 Consumption from nuclear sources MWh 92 Share of consumption from nuclear sources in total energy consumption % 1% 3 Fuel consumption for renewable sources, including biomass (also comprising industrial and municipal waste of biologic origin, biogas, renewable hydrogen, etc.) MWh – 4 Consumption of purchased or acquired electricity, heat, steam, and cooling from renewable sources MWh 7,414 5 The consumption of self-generated non-fuel renewable energy MWh 77 6 Total renewable energy consumption1 MWh 7,491 Share of renewable sources in total energy consumption % 61% Total energy consumption2 MWh 12,199 1. Total renewable energy consumption (MWh) (calculated as the sum of lines 3 to 5) 2. Total energy consumption (MWh) (calculated as the sum of lines 1, 2 and 6) Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 98 |
|
Sustainability Statements Accounting Policies Total energy consumption is accounted for by combining both renewable and non-renewable energy sources used across our operations. We measure energy consumption in megawatt-hours (MWh), incorporating data from energy manage-ment systems and utility invoices to ensure accuracy. Renewable energy includes wind, solar, hydro and other sustainable sources which are supported by contractual agreements such as Energy Attribute Certificates (EACs), while non-renewable energy encompasses fossil fuels and grid electricity. This comprehensive approach allows us to monitor our overall energy usage effectively and conduct annual reviews of our consumption data to validate its completeness and reliability, ensuring compliance with relevant reporting standards and reinforcing our commit-ment to sustainability. Gross Scopes 1, 2, 3 and total GHG emissions (E1-6) Genmab calculates its Scope 1, 2 and 3 GHG emissions in accordance with the requirements of ESRS E1 Climate Change, considering the principles, require-ments and guidance provided by the GHG Protocol. Base Year 2024 Milestones and Target Years 2023 % Change 2030 2050 Annual % Target/ Base Year3 Scope 1 GHG emissions1 Gross Scope 1 GHG emissions (tCO2eq) 534 317 68% 534 54 0% Scope 2 GHG emissions Gross location-based Scope 2 GHG emissions (tCO2eq) 2,705 1,200 125% – – – Gross market-based Scope 2 GHG emissions (tCO2eq) 1,163 238 389% 450 116 7% Total Scope 1 and market-based Scope 2 GHG emissions (tCO2eq) 1,697 555 206% 984 170 7% Significant Scope 3 GHG emissions2 Total Gross indirect (Scope 3) GHG emissions (tCO2eq) 1–Purchased Goods and services 164,449 127,237 29% 2–Capital goods 5,519 10,045 (45)% 3–Fuel and energy-related Activities (not included in Scope 1 or Scope 2) 1,078 411 162% 4–Upstream transportation and distribution 5,425 4,737 15% 6–Business travel 10,559 7,675 38% 7–Employee commuting 946 803 18% Total Scope 3 GHG emissions 187,976 150,908 25% Total GHG emissions Total GHG emissions (location-based) (tCO2eq) 191,215 152,425 25% Total GHG emissions (market-based) (tCO2eq) 189,673 151,463 25% 1. Percentage of Scope 1 GHG emissions from regulated emission trading schemes not applicable to Genmab. 2. Scope 3 GHG emissions categories excluded from the inventory include 5 – Waste generated in operations as it is included in category 1, 8 – Upstream leased assets, 9 – Downstream transportation and distribution, 10 – Processing of sold products, 11 – Use of products sold, 13 – Downstream leased assets and 14 – Franchises as they are all not applicable to Genmab, and 12 – End-of-life treatment of sold products and 15 – Investments as they are not material. Further, there are no emission reduction target percentages for Scope 3 GHG emissions. Refer to E1-4 for environmental targets. 3. Annual % Target/Base Year represents the actual reduction target for 2030 (or 42%) over six years. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 99 |
|
Sustainability Statements Accounting Policies Scope 1 GHG Emissions Scope 1 GHG emissions refer to the direct GHG emissions from sources that are financially or operationally (as required by ESRS 1) controlled by Genmab at our headquarters, office, laboratory and preclinical development space across all Genmab locations. Direct GHG emissions is comprised of the sum of greenhouse gases, which are converted to CO2 equivalents (CO2eq). The GHG emissions arise from the combustion of fuel products and leakage of refrigerants. To calculate GHG emissions the newest version of DEFRA GHG Conversion factors (2024) has been used. Scope 2 GHG Emissions Scope 2 GHG emissions refer to the indirect GHG emissions resulting from the generation of purchased energy that is used by Genmab at our headquarters, office, laboratory and preclinical development space across all Genmab locations. Scope 2 GHG emissions occur at the facility where the energy is generated, thus being classified as indirect GHG emissions. The GHG emissions are linked to the electricity and district heating consumption. Scope 2 location-based GHG emissions are calcu-lated by taking consumed energy multiplied with relevant location-based national emission factors provided by the International Energy Agency (IEA) (2023) and the Association of Issuing Bodies (AIB) (2023). Scope 2 market-based emissions are calculated by taking consumed energy multiplied with market-specific emission factors provided directly from the energy supplier. Renewable energy purchases and certificates are considered, when accounting for indirect GHG emissions, using the market-based approach. Scope 3 GHG Emissions Genmab has identified six categories, out of the 15 categories of Scope 3 GHG emissions defined by the GHG protocol, as relevant. The remaining nine categories are not separately reported on as they are either not applicable to Genmab or GHG emissions have been included in the other emission categories. Zero percent of Genmab’s Scope 3 GHG emissions are measured using primary data obtained from suppliers or other value chain partners. For spend-based calcula-tions, all spend in foreign currencies has been converted into USD using the average exchange rate for the year. Category 1—Purchased goods and services Purchased goods and services include GHG emissions related to all spend from external suppliers, except for investment (CapEx), travel, and transportation and distribution spend, which are included in other Scope 3 catego-ries. Spend is converted into CO2eq emissions using the spend-based method by applying the Environmentally Extended Input-Output (EEIO) model with US EPA emission factors (2022) to estimate GHG emissions (CO2eq). Category 2—Capital goods Capital goods include GHG emissions related to investments in tangible assets (CapEx). Spend is converted into CO2eq emissions using the spend-based method by applying the Environmentally Extended Input-Output (EEIO) model with US EPA emission factors (2022) to estimate GHG emissions (CO2eq). Category 3—Fuel and energy-related activities Fuel and energy-related activities include all upstream Well-to-Tank (WTT) CO2eq emissions of purchased fuel and electricity and Transmission and Distribution (T&D) Loss of purchased electricity (beyond Scope 1 and 2 GHG emissions). Electricity and fuel consumption are multiplied by DEFRA’s emission factors (2023 for fuel and 2021 for electricity) to estimate GHG emissions (CO2eq). The category primarily comprises upstream WTT and T&D emissions from electricity and WTT emissions from natural gas. Category 4—Upstream transportation and distribution Upstream transportation and distribution include GHG emissions related to spend from external suppliers related to transportation and distribution of goods. Spend is converted into CO2eq emissions using the spend-based method by applying the Environmentally Extended Input-Output (EEIO) model with US EPA emission factors (2022) to estimate GHG emissions (CO2eq). Category 6—Business travel Business travel includes GHG emissions related to spend from external suppliers related to flights, ground transportation, hotel stays and meals in connection with business travel. Spend is converted into CO2eq emissions using the spend-based method by applying the Environmentally Extended Input-Output (EEIO) model with US EPA emission factors (2022) to estimate GHG emissions (CO2eq). Category 7—Employee Commuting Employee commuting includes GHG emissions related to employees’ commuting between their homes and the Genmab sites. GHG emissions are estimated using the average data method and based on assumptions across our locations. 2024 GHG intensity per net revenue Total GHG emissions (location-based) per net revenue (tCO2eq/DKK million ) 8.88 Total GHG emissions (market-based) per net revenue (tCO2eq/DKK million ) 8.81 Refer to Note 2.1 in the consolidated financial statements for disclosures related to Genmab’s revenue. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 100 |
|
Sustainability Statements 2.4 EU Taxonomy Disclosures pursuant to Article 8 of Regulation (EU) 2020/852 The EU Taxonomy is a classification system designed to provide a framework for identi-fying sustainable economic activities. It helps companies and investors distinguish between activities that contribute to environmental sustainability by establishing a common language for defining what constitutes “green” or sustainable business practices. The EU Taxonomy plays a role in supporting the transition towards a more sustainable economy. In line with Article 8 of the EU Taxonomy and the Disclosure Delegated Act (EU) 2021/2178, Genmab is required to report on the sustainability profile of its activities, specifically focusing on the eligibility and alignment of its Turnover, Capital Expenditures (CapEx) and Operating Expenditures (OpEx). Eligibility: We screened our economic activities against those outlined in the Taxonomy, identifying eligible Turnover, CapEx and OpEx. • Turnover—We assessed turnover based on the net product sales of pharmaceutical products. We concluded that turnover from the sale of EPKINLY qualifies under the Manufacture of Medicinal Products (#1.2) activity, in line with the Taxonomy criteria for Pollution Prevention and Control (PPC). We did not include turnover from the sale of EPKINLY as eligible activities in 2023 as EPKINLY launched in the U.S. in May 2023 and Japan in September 2023. • CapEx—Our assessment focused on investments that align with Taxonomy-eligible activities. We identified one eligible project under Renovation of Buildings (#7.2) in line with the Taxonomy criteria for Climate Change Mitigation (CCM). • OpEx—We evaluated the eligibility of our OpEx by reviewing the eligible economic activities outlined in its Statement of Profit or Loss and examining the data available to us from our ERP system. Based on this evaluation, we did not identify eligible OpEx. Alignment: We assessed whether any of our Taxonomy-eligible Turnover or CapEx for economic activities 1.2 and 7.2 could be considered Taxonomy-aligned; however, we were not able to obtain enough evidence to conclude alignment with the ‘Substantial contribution’ and ‘Do No Significant Harm’ (DNSH) criteria. Accounting Policies Turnover: Total Turnover consists of total revenue as disclosed in Note 2.1 in the consolidated financial statements. The Turnover KPI represents the ratio of net product sales from taxonomy-eligible or taxonomy-aligned economic activities to the total revenue in a fiscal year. CapEx: Total CapEx consists of additions to intangible assets, tangible assets and right-of-use assets during the fiscal year (refer to Notes 3.1, 3.2 and 3.3, respectively) and considered before depre-ciation, amortization, and any re-measurements, including those resulting from revaluations and impairments, for the relevant financial year, excluding any fair value changes. Furthermore, total CapEx consists of any additions to tangible and intangible assets resulting from business combinations. The CapEx KPI represents the share of CapEx that is taxonomy-eligible or taxonomy-aligned divided by the total CapEx. OpEx: Total OpEx includes direct non-capitalized costs that relate to research and development, building renovation measures, short-term lease, maintenance and repair, and any other direct expenditures relating to the day-to-day servicing of assets of property, plant and equipment by the undertaking or third party to whom activities are outsourced that are necessary to ensure the continued and effective functioning of such assets. OpEx does not include amortization, depreciation, or impairments. To avoid double counting related to the economic activities, Turnover, CapEx and OpEx are distinctly allocated to ensure that there is no overlap across these financial metrics. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 101 |
|
Sustainability Statements Economic Activities—Turnover TURNOVER Codes Absolute Turnover Proportion of Turnover Substantial Contribution Criteria DNSH criteria (‘Does Not Significantly Harm’) Taxonomy Aligned Proportion of Total Turnover, 2024 Taxonomy Aligned Proportion of Turnover, 2023 Category (Enabling Activity) Category (Transitional Activity) Economic Activities Climate Change Mitigation Climate Change Adaptation Water Pollution Circular Economy Biodiversity and ecosystems Climate Change Mitigation Climate Change Adaptation Water Pollution Circular Economy Biodiversity Minimum Safeguards DKK million % % % % % % % Y/N Y/N Y/N Y/N Y/N Y/N Y/N % % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) None – 0% 0% 0% 0% 0% 0% 0% N/A N/A N/A N/A N/A N/A N/A 0% 0% N/A N/A Turnover of environmentally sustainable activities (Taxonomy-aligned) (A.1) – 0% 0% 0% 0% 0% 0% 0% N/A N/A N/A N/A N/A N/A N/A 0% 0% N/A N/A A.2. Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) Manufacture of medicinal products. PPC 1.2 1,743 8% Turnover of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) 1,743 8% Total (A.1+A.2) 1,743 8% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES Turnover of Taxonomy-non-eligible activities 19,783 92% Total (A+B) 21,526 100% Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 102 |
|
Sustainability Statements Economic Activities—CapEX CAPEX Codes Absolute CapEx Proportion of CapEx Substantial Contribution Criteria DNSH criteria (‘Does Not Significantly Harm’) Taxonomy Aligned Proportion of Total CapEx, 2024 Taxonomy Aligned Proportion of CapEx, 2023 Category (Enabling Activity) Category (Transitional Activity) Economic Activities Climate Change Mitigation Climate Change Adaptation Water Pollution Circular Economy Biodiversity and ecosystems Climate Change Mitigation Climate Change Adaptation Water Pollution Circular Economy Biodiversity Minimum Safeguards DKK million % % % % % % % Y/N Y/N Y/N Y/N Y/N Y/N Y/N % % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) None – 0% 0% 0% 0% 0% 0% 0% N/A N/A N/A N/A N/A N/A N/A 0% 0% N/A N/A CapEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) – 0% 0% 0% 0% 0% 0% 0% N/A N/A N/A N/A N/A N/A N/A 0% 0% N/A N/A A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) Renovation of existing buildings CCM 7.2 80 1% CapEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) 80 1% Total (A.1+A.2) 80 1% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES CapEx of Taxonomy-non-eligible activities 12,486 99% Total (A+B) 12,566 100% Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 103 |
|
Sustainability Statements Economic Activities—OpEx OpEx Codes Absolute OpEx Proportion of OpEx Substantial Contribution Criteria DNSH Criteria (Does Not Significantly Harm) Taxonomy Aligned Proportion of Total OpEx, 2024 Taxonomy Aligned Proportion of OpEx, 2023 Category (Enabling Activity) Category (Transitional Activity) Economic Activities Climate Change Mitigation Climate Change Adaptation Water Pollution Circular Economy Biodiversity and ecosystems Climate Change Mitigation Climate Change Adaptation Water Pollution Circular Economy Biodiversity Minimum Safeguards DKK million % % % % % % % Y/N Y/N Y/N Y/N Y/N Y/N Y/N % % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) None – 0% 0% 0% 0% 0% 0% 0% N/A N/A N/A N/A N/A N/A N/A 0% 0% N/A N/A OpEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) – 0% 0% 0% 0% 0% 0% 0% N/A N/A N/A N/A N/A N/A N/A 0% 0% N/A N/A A.2 Taxonomy-Eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) None – 0% OpEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) – 0% Total (A.1+A.2) – 0% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES OpEx of Taxonomy-non-eligible activities 9,501 100% Total (A+B) 9,501 100% Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 104 |
|
Sustainability Statements Annex XII Row Nuclear energy related activities 1 The undertaking carries out, funds or has exposures to research, development, demonstration and deployment of innovative electricity generation facilities that produce energy from nuclear processes with minimal waste from the fuel cycle. NO 2 The undertaking carries out, funds or has exposures to construction and safe operation of new nuclear installations to produce electricity or process heat, including for the purposes of district heating or industrial processes such as hydrogen production, as well as their safety upgrades, using best available technologies. NO 3 The undertaking carries out, funds or has exposures to safe operation of existing nuclear installations that produce electricity or process heat, including for the purposes of district heating or industrial processes such as hydrogen production from nuclear energy, as well as their safety upgrades. NO Fossil gas related activities 4 The undertaking carries out, funds or has exposures to construction or operation of electricity generation facilities that produce electricity using fossil gaseous fuels. NO 5 The undertaking carries out, funds or has exposures to construction, refurbishment, and operation of combined heat/cool and power generation facilities using fossil gaseous fuels. NO 6 The undertaking carries out, funds or has exposures to construction, refurbishment and operation of heat generation facilities that produce heat/ cool using fossil gaseous fuels. NO Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 105 |
|
Sustainability Statements Social—Own Workforce Genmab is dedicated to making a meaningful impact in the lives of patients and caregivers by developing innovative treatments to transform cancer care and address other serious diseases. We prioritize understanding the needs of patients and their families, ensuring that their experiences and insights guide our research, development and commercialization efforts. Our workforce is our greatest asset; the dedication and innovation of our team members are essential to our success. The Genmab Commitment, our employee value proposition, grounds our culture and brings our vision, purpose, and core values to life. Team members, or full-time equivalents (FTEs) at Genmab are defined as all employees on our payroll, both full-time and part-time, as well as active and on-leave. All individuals have been included by reflecting the proportion of an FTE they represent based on their contractual agreement. Non-employees at Genmab include contingent workers and consultants provided by third party undertakings who are primarily engaged in employment activities. We prioritize attracting and retaining qualified individuals who align with our core mission of improving patient lives. Teamwork and respect are central to our culture, fostering an inclusive, open, and supportive environment across our global locations. We believe that workplace diversity— encompassing social, educational, cultural, national, age, and gender differences— is crucial for our continued success. By recruiting employees with the right skills and competencies, we create interactive teams that drive our goals and ensure Genmab’s ongoing impact in healthcare, ultimately benefiting the patients and care partners we serve. Below are the list of Disclosure Requirements as it pertains to ESRS S1—Own Workforce: Section Disclosure requirement content Disclosure requirement # 3.0 IROs Material Impacts, Risks and Opportunities and their interaction with strategy and business model SBM-3 3.1 Own Workforce IRO Management Policies related to own workforce S1-1 Processes for engaging with own workers and workers’ representatives about impacts S1-2 Processes to remediate negative impacts and channels for own workers to raise concerns S1-3 Taking action on material impacts on own workforce, and approaches to mitigating material risks and pursuing material opportunities related to own workforce, and effectiveness of those actions S1-4 3.2 Own Workforce Metrics and Targets Targets related to managing material negative impacts, advancing positive impacts, and managing material risks and opportunities S1-5 Characteristics of the Company’s employees S1-6 Characteristics of non-employee workers in the Company’s own workforce S1-7* Collective bargaining coverage and social dialogue S1-8 Diversity metrics S1-9 Adequate wages S1-10 Social protection S1-11 Persons with disabilities S1-12* Training and skills development metrics S1-13 Health and safety metrics S1-14 Work-life balance metrics S1-15* Compensation metrics (pay gap and total compensation) S1-16 Incidents, complaints and severe human rights impacts S1-17 *Genmab has adopted the phase-in for S1-7, S1-12 and S1-15 and elected not to disclose for year 1 reporting. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 106 |
|
Sustainability Statements 3.0 IROs The table describes Genmab’s material impacts, risks and opportunities related to our material Social topics on own workforce: Section Material Topic Material Impact Value Chain Location Material Risk Material Opportunity Social Own Workforce ― Working Conditions Employee well-being and vitality (positive) Own Operations Genmab may have an inability to attract and retain suitably qualified team members Provide a voice to employees through our global engagement survey Safety in our facilities (positive) Own Operations Refer to the Risk Management section of the Annual Report under Management and Workforce for details Safety training/ongoing education in our laboratories Own Workforce ― Equal Treatment and Opportunities for All Career development through training and skill building (positive) Own Operations Providing employees with opportunities to discuss their career at Genmab Diversity in the workplace (positive) Own Operations All impacts, risks and opportunities have expected time horizons of short, medium and long-term. Employee well-being and vitality Prioritizing employee well-being and vitality is not just a moral imperative but also a strategic investment in the success and sustainability of Genmab. It promotes a positive work envi-ronment, improves employee morale and engagement, enhances productivity, reduces turnover, and helps maintain compliance with legal requirements. Therefore, prioritizing the health and safety of our teams demon-strates our commitment to our most valuable asset—our people. Promoting work-life balance is not just beneficial for employees, it also positively impacts Genmab by fostering a more productive, engaged, and satisfied workforce. It contributes to a positive organizational culture, supports recruitment and retention efforts, and ultimately enhances the overall performance and success of Genmab. Genmab has a significant opportunity to enhance its workplace culture by providing a voice to our employees through ongoing initiatives aimed at improving employee engagement. By leveraging platforms such as our annual global engage-ment survey, Genmab can foster an environment where employees feel valued, motivated, and committed to both their work and the organiza-tion’s goals. This focus on engagement is likely to lead to improved talent retention and overall employee well-being. With both local and global surveys conducted annually or biennially, Genmab gathers valuable insights into employee satisfaction, including both physical and mental workplace conditions. The results of these surveys provide a foun-dation for actionable improvements, with the Human Resources team facilitating the process and Genmab leaders taking ownership of the outcomes. By actively addressing areas of concern highlighted in the surveys, Genmab can create a more supportive and responsive work environment, ultimately empowering employees to share their feedback, concerns, and ideas effectively. Safety in our facilities Safety in facilities at Genmab is essential not only for complying with legal requirements, but also for protecting employees, enhancing productivity, reducing costs, maintaining a positive reputation, and supporting overall business success and sustainability. It reflects Genmab’s commitment to the well-being of its employees and its responsibility as a corporate citizen within the broader community. We have instituted mandatory safety training and ongoing education in all workplace areas, especially related to the proper handling of hazardous materials and chemicals in our labs to enhance productivity and reduce costs. Career development through training and skill building Employees with advanced skills contribute to higher productivity and efficiency, improving the overall performance of the organization. Organizations that invest in their employees’ development tend to have higher retention rates, as employees feel valued and are more likely to stay with Genmab. Companies known for their commitment to employee development attract top talent, as professionals seek employers who offer growth opportunities. A focus on continuous learning fosters a culture of growth and develop-ment, enhancing teamwork, collaboration, and overall morale. Genmab provides employees with opportunities to discuss their future career at Genmab which should increase employee retention, provide for the ability to attract top talent and achieve higher productivity and efficiency. This is done formally Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 107 |
|
Sustainability Statements through the year-end performance reviews, but also informally through ad hoc discussions with the manager, as needed. Employees are provided with several internal resources related to the performance process. Diversity in the workplace We strive to create, nurture, and maintain a global, inclusive culture where differences drive innovative solutions to meet the needs of patients, care partners, families, and employees. Our teams give Genmab its strength. We are committed to championing a corporate culture that accepts and promotes uniqueness and empowers each team member to bring their authentic self to work in a safe, open, and respectful environment. Our diversity, equity, and inclusion (“DE&I”) strategy aims to make Genmab an Extra[not]ordinary™ employer and inclusive workplace with DE&I efforts centered on gender, age and nationality. Genmab has team members representing over 75 nationalities. Genmab also has several initiatives in place to connect employees across the company, including employee resource groups (ERGs), sharing individual and group experiences to create an inclusive and supporting work environment and to continuously improve employee engagement. Refer to S1-5 for targets related to our Own Workforce and S1-9 for diversity metrics including gender and age. 3.1 Own workforce IRO management Policies related to own workforce (S1-1) Genmab has a number of policies that address our material IROs for the topic of Working Conditions, and related to all of its own workforce, including the following: • Code of Conduct • Global Speak Up Policy • Human Rights Commitment • Global Diversity, Equity & Inclusion Policy • Corporate Social Responsibility (CSR) • Global Lab Occupational Health and Safety Policy Genmab has other processes and systems in place including our Workplace Accident Prevention Management System and Employee Value Proposition. Code of Conduct The Code of Conduct policy aims to promote ethical and compliant conduct in all aspects of our business. Our Code of Conduct applies to everyone in our Company, at every level, including employees, managers, and board members. We expect our alliance partners and third parties, including agents, consultants, contingent workers, and suppliers, to act in a way that is consistent with our values and our Code of Conduct when conducting business on behalf of Genmab. As noted in the Code of Conduct, #11—Genmab proactively seeks to foster a values-based performance culture and a safe and healthy workplace environment of mutual respect, dignity and inclusion, one that assures we can attract, develop and retain a highly talented, diverse workforce of engaged employees driven to deliver superior business outcomes. We are committed to equal opportunity and fair treatment of our employees. All forms of harassment and retalia-tion are unacceptable and counter to everything we stand for as a Company. The Code of Conduct can be mapped to the following positive impacts: • Employee well-being and vitality • Career development through training and skill building Genmab’s Head of Global Compliance is respon-sible for this policy and reports directly to the CEO, and both are members of the CSR and Sustainability Committee. Refer to the Code of Conduct on our website. Global Speak Up Policy Enabling employees and external stakeholders to speak up when they observe potential miscon-duct or have concerns about matters relating to our business is vital to retaining our strong Genmab culture and to doing the right thing. The Genmab Global Speak Up Policy allows for confi-dential and (where allowed) anonymous reports that will be directed to Genmab Compliance for initial triage and handling. This approach renders the reporting process more accessible and easier to navigate. All reports made under this Policy will be received and treated sensitively and seriously, and will be dealt with promptly, fairly, and objectively. Any investigations commenced will be conducted in a timely manner and will be fair and independent from any persons to whom the report relates. Importantly, our policy also extends protections not only to our regular employees, but also contingent staff, part-timers, temp staff, trainees, interns (both current and former) and others who choose to report their concerns. All employees, in addition to the new hire onboarding training, are required to complete annual Code of Conduct training and attest to their commitment to adhere to our ethical standards. This training also reviews among other topics, the Global Speak Up Policy concepts. The Global Speak Up Policy can be mapped to the positive impact of Employee well-being and vitality. Genmab’s Head of Global Compliance is responsible for this policy and reports directly to the CEO, and both are members of the CSR and Sustainability Committee. Refer to the Global Speak Up Policy on our website. Human Rights Commitment We recognize and support human rights and are dedicated to conducting business in a way that respects the dignity of all people. Our Human Rights Commitment is guided by current human rights laws and the United Nations Guiding Principles on Business and Human Rights. Our Guiding Principles refer to the International Bill of Human Rights, which consists of the Universal Declaration of Human Rights and the two Covenants that implement it, as well as the International Labour Organization’s (ILO) Declaration on Fundamental Rights and Principles at Work and the core conventions that underpin it, and we are in alignment with these instruments. We are committed to respecting human rights, including labor rights in our own operations and complying with the laws of the Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 108 |
|
Sustainability Statements countries in which we do business. As part of our commitment, we seek to identify, prevent, and address any potential and actual adverse human rights impacts that our business may contribute to or cause. Further, as disclosed in our Human Rights Commitment, privacy rights of our employees, patients, healthcare providers, customers and other stakeholders are protected. Additionally, per the Human Rights Commitment policy we ensure fair, non-discriminatory employment practices and the prohibition of any form of human trafficking, forced, indentured, slave or child labor. We ensure that the policy is publicly available to all affected stakeholders by publishing it on our website. In 2024, through periodic checks and audits, we continued to provide assurance that our policies, procedures, and operations align with our Human Rights Commitment. Our Supplier Code of Conduct addresses human rights and labor relations to ensure suppliers understand our commitment to compliance with local human and labor laws and recognize the importance we place on human rights. The Supplier Code of Conduct includes provisions in line with the applicable ILO standards addressing the safety of all workers including those in precarious work, human trafficking and the use of forced labour or child labour. The Global Speak Up Policy and Hotline covers the measures provided to enable remedy for human rights impacts. The Human Rights Commitment policy can be mapped to the positive impact of Employee well-being and vitality. Under the leadership of our Chief People Officer, our Human Resources function is responsible for ensuring our compli-ance with this Commitment. Refer to the Human Rights Commitment on our website. Refer also to the Corporate Global Supplier Code of Conduct Policy on our website. Global Diversity, Equity & Inclusion Policy At Genmab, prioritizing DE&I means creating richer solutions, obtaining better results, and maximizing productivity, innovation, and creativity. Diversity allows for a variety of perspectives, and inclusion takes different ideas and perspectives into account, while equity ensures fair opportunities to succeed. We embrace each employee’s unique contribution to our culture by valuing differences including age, disabilities, gender, Genmab heritage, nation-ality, professional specialization race/ethnicity, problem-solving style, sexual orientation, and social class. Genmab’s Global Diversity, Equity & Inclusion Policy covers inclusion for groups that may be at particular risk of vulnerability including all employees, regardless of gender, race, ethnicity, religion, age, disability, and other characteristics. By ensuring equal treatment and opportunity, Genmab has a positive impact on people in its workforce and value chain. Diversity in the workplace and harboring a safe workplace for all leads to innovation, creating the opportunity for Genmab to gather multiple perspectives into one place. Similarly, a lack of diversity can impact company performance negatively and hinder innovative solutions to support patients. With regards to our people and measures against violence and harassment in the workplace, as noted in our Code of Conduct, all forms of harassment and retaliation are unacceptable and counter to everything we stand for as a Company. The Global Diversity, Equity & Inclusion Policy (most recently adopted by the Board of Directors on June 12, 2024) can be mapped to the positive impact of Diversity in the workplace. The DE&I Council (made up of senior leaders at Genmab) is responsible for ensuring our compliance with this policy. Refer to the Global Diversity, Equity & Inclusion Policy on our website. Corporate Social Responsibility (CSR) Policy As noted in the CSR Policy under No. #2, we care for our employees’ health, well-being, safety, and development and promote a collaborative culture that fosters passion for innovation, integrity, and respect. We believe that diversity, equity, and inclusion are fundamental to achieving our vision and are committed to championing a corporate culture that accepts and promotes uniqueness and empowers each team member to bring their authentic self to work in a safe, open, and respectful environment. The CSR Policy is mapped to the positive impact of Career development through training and skill building. The Nominating and Corporate Governance Committee of Genmab A/S’ Board of Directors oversees all aspects of Genmab’s CSR efforts. Refer to the Corporate Social Responsibility (CSR) Policy on our website. Global Lab Occupational Health and Safety Policy Genmab has an internal Global Lab Occupational Health and Safety Policy specific to our R&D labs across our locations. Formal committees respon-sible for monitoring and improving health and safety at each of our locations continued their work. Each committee reports to site operations and to the local management team to address and escalate any issues. Health and safety prevention workers continue to monitor and improve health and safety at our R&D labs. This policy is mapped to the positive impact of Safety in our facilities. Under the leadership of our Chief Medical Officer, our committees are responsible for ensuring our compliance with the policy. Workplace Accident Prevention Management System Genmab adheres to processes, assessments, inspections, and staff trainings and has reporting in place to prevent, monitor and manage any workplace accidents. We aim to create a workplace accident prevention policy over the coming years. Mandatory workplace assessments are conducted in compliance with local regula-tions. This strategy is mapped to the positive impact of Safety in our facilities. Under the lead-ership of our Chief People Officer, our Human Resources function is responsible for ensuring our compliance with this Commitment. Employee Value Proposition Genmab has developed the Genmab Commitment, which is our employee value proposition that is published internally and the essence of it is used across in our internal and external communica-tions and social media platforms. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 109 |
|
Sustainability Statements The Commitment is what grounds our culture in the day-to-day work and brings our vision, purpose, and core values to life. Our Commitment is made up of four key ingre-dients that illustrate how “We are extra[not] ordinary™”: • Empowerment • Care • Authenticity • Impact These principles capture the culture we aspire to—engaging our team members to go beyond business as usual and to be remarkable, unique, and authentic. We offer an extra[not] ordinary rewards and opportunities package that empowers team members to succeed and enhances their well-being. Processes for engaging with own workers and workers’ representatives about impacts (S1-2) Genmab promotes an environment that fosters individual empowerment that allows team members to achieve their maximum potential. Genmab encourages active dialogue directly between employees and management. Genmab drives initiatives that engage, develop, and inspire employees as a part of our overall Total Rewards strategy. Genmab facilitates active dialogue between our team members and management on workplace issues and other topics of concern through HR business partners, Employee Representative Council and Works Council. • Danish Employee Representative Council presents topics of interest and concern to employees during the year (at least once a year) through meetings to enable us to gain insight into the perspectives of our workforce and to ensure we remain a preferred workplace. Team members in Denmark have furthermore exercised their right to elect representatives to the Board of Directors in accordance with Danish legislation, and three group employees were elected to the Board under a voluntary scheme allowing employees from other sites to be elected. This employee representation strengthens the involvement and decision-making process at Genmab. • Dutch Works Council is a statutory body with the legal right and obligation to monitor and work for the proper functioning of the Company in all its objectives. This advocacy group represents team members in the Netherlands to bring concerns from the workforce to management during the year (at least once a year). Under the Dutch Works Councils Act, our Council must consent on topics that directly affect employees’ everyday work and must be involved in, and consulted for, advice on major organizational changes and determine the impact on the local workforce. While the U.S., Japan and China do not have Work Councils, employee engagement occurs globally for all employees through surveys and workplace assessments including our annual global engage-ment survey, inclusion networks, personal development dialogues, employee-elected board positions, employment relations and occupa-tional health and safety representation. A Global Employee Engagement Survey is conducted on an annual basis. The results of the survey help us keep a pulse on Genmab’s areas of strength and opportunity. Focus groups are conducted to generate further insights on critical engagement issues. The organization shares results with all team members and encourages all people leaders to review feedback with their teams and develop actions to improve the overall employee experience. In addition, Executive Management reviews results as a group to analyze key findings, themes, reflect on areas of strengths and opportunities for employee engagement. To inform teams on our business and our progress, Genmab hosts functional and/or regional town hall updates. Under the leadership of our Chief People Officer, our Human Resources function is responsible for ensuring engagement with our own workers and workers’ representatives. Refer to SBM-2 for summary table of key stakeholder engagement. Processes to remediate negative impacts and channels for own workers to raise concerns (S1-3) While we have not identified any material negative impacts regarding our team members, we do promote and encourage our people to speak up to report concerns, share feedback, address compliance issues, and express ethical dilemmas to promoting transparency and foster a culture of openness and accountability by raising awareness of potential risks or challenges that could affect the organization. Employees can raise concerns through their immediate managers or a Human Resource colleague, who will track and monitor such concerns, and raise the issue as needed to the appropriate management to resolve it. Employees can also raise concerns through our 24/7 full-service Speak Up (whistleblower) compliance hotline. Speak Up Compliance Hotline 24/7—Online or by Phone GenmabSpeakUp.ethicspoint.com Denmark: 80-83-01-69 Netherlands: 0800-020-1556 United States: 1-844-942-3289 Japan: 0800-123-0136 China: 400 125 3055 Refer to the Global Speak Up Policy in S1-1 and on our website. Refer to G1-1 for details of Genmab’s anti-retaliation policies and procedures and how Genmab tracks and monitors issues raised. Taking action on material impacts on own workforce, and approaches to mitigating material risks and pursuing material opportunities related to own workforce, and effectiveness of those actions (S1-4) The following actions were taken around Genmab’s material impacts on our own workforce in 2024: Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 110 |
|
Sustainability Statements Employee Well-Being and Vitality Genmab’s well-being pillars were implemented in 2024 which include: • Emotional well-being: thrive every day Supporting resilience and mental health through manager training and tools • Financial well-being: enjoy security and freedom Promoting financial security with initiatives like Global Money Week • Physical well-being: feel your best Offering on-site and virtual fitness opportunities • Social well-being: live connected Facilitating volunteer opportunities to connect employees with the community Programs Global Well-Being: In 2023, we created a strategic roadmap to help establish an effective Global Well-Being (GWB) Program to support our team members. Our roadmap was developed based on feedback from key stakeholders and the insights of well-being experts who use research-based best practices to design and implement a custom GWB program for our organi-zation. The program that Genmab offered in 2024 and 2023 included the following workshops: • Care for you as the caregiver • Leading with love—celebrating and affirming LGBTQ+ young people • Creating financial well-being plan • Design your work/life webinar • International self-care day article posted on Everyday intranet Emotional and Mental Health: Genmab offered multiple programs, including self-care applica-tions, in 2024 and 2023 and resources at each location to support emotional and mental health needs. A weeklong series on Well-being for employees that provided resources for managers to leverage in support of their employees’ mental health in 2024 and 2023 included the following workshops: • Resilience: protecting your mental health in stressful times • How to maximize your day • Stress busting: run from the bear • The importance of unplugging • Boundaries before burnout Volunteering Genmab organizes events throughout the year to connect with each other and our communities. In 2024, 688 team members volunteered 2,952 hours on Global Volunteer Day compared to 571 team members and 2,668 hours in 2023. Self-Care Applications In 2023, we launched our own mental health application, GenCare, which is used by our team members globally. The app uses several learning approaches and preferences to support neurodi-versity. GenCare evolved in 2024 with updated content and a focus on improved well-being. Work-life Balance Genmab continued to provide four additional days off and four meeting-free days throughout the year in both 2024 and 2023. Genmab recognizes the importance of time away from work and offers all employees paid time off, as well as leave of absence policies, to support our teams members through extended periods away from work on a paid and/or unpaid basis. Genmab offers family-related leave to all full-time employees across all our Genmab entities following statutory provisions as stated by the relevant local jurisdiction. Total Rewards & Opportunities As Genmab continues to grow, we’ve been enhancing systems to support transparency, understanding, and empowerment regarding employees’ Total Rewards & Opportunities which includes all of the tangible and intangible elements available to employees in return for helping Genmab fulfill our purpose and live our values. In 2024, we introduced a new feature in our Human Resources Information System (HRIS) providing more information about employee salary and growth potential. Employees have the ability to view salary ranges and market ratios for comparable roles in the Biotech/Life Sciences industry. These ranges were created following consultation and alignment with leaders throughout Genmab to ensure that the market data leveraged was the most appropriate for the skills and responsibilities our employees possess. As a result, this exercise exemplifies our rigorous, data-driven processes to ensure our salaries are internally consistent as well as externally attractive—for both new hires and current employees. Genmab also awards new hires with equity grants in the form of warrants and restricted stock units, allowing our team members the opportunity to become part owners in Genmab. Lastly, Genmab offers a variety of market competitive benefits to our employees including physical, social, professional, emotional and financial well-being. Refer to Note 4.6 in the consolidated financial statements for details related to grants of share-based instruments in 2024 and 2023. Safety in Our Facilities Our Safe & Sound and Sustainability Week recog-nizes the successes of workplace health and safety programs and provides information on how to keep our lab workers safe while bringing awareness to our sustainability footprint. As part of the program for the weeks in 2024 and 2023, we highlighted important lab safety topics specifically for the laboratories in the Netherlands and the U.S. This included CPR certification to equip our team members with life-saving skills, eyewash demonstrations, management of expired/unused chemicals, fire extinguisher training and biosafety. We also cele-brated our team members’ efforts in identifying and reporting potential hazards. We have instituted mandatory safety training and ongoing education in all workplace areas, especially related to the proper handling of hazardous materials and chemicals in our labs. We are fully aware of the impact that chemicals can have on employee and patient health and safety. We are committed to ensuring that our chemical management practices comply with all relevant regulations and standards, and that we minimize the potential for harm to our workers, customers, and the environment. Risk assessments of chemicals used by Genmab in its operations indicate that many pose low or no hazard. However, we constantly monitor the use Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 111 |
|
Sustainability Statements of chemicals with high risk factors, such as being corrosive, reactive, mutagenic or carcinogenic, and are constantly promoting investigation into their potential replacement or process enhance-ments. In alignment with our stringent safety protocols and adherence to legal requirements, our labs recorded no chemical-related incidents resulting in injuries requiring beyond basic first aid treatment or significant chemical emissions. Career Development through Training and Skill Building Global Employee Engagement Survey As standard practice, Genmab reviews the survey results with the Executive Committee to reflect on employee feedback, current engagement strategy and understand future actions that could support the employee experience. It is also encouraged for people leaders to review survey results directly with their employees to discuss the feedback and establish any actions to improve the employee experience. Lastly, Genmab trans-parently displays survey results with the broader employee population by publishing them on the Company’s internal secured network. In 2024 and 2023, Genmab conducted the survey with areas of focus including: diversity and inclusion, immediate manager, work environ-ment, innovation, engagement, camaraderie & teamwork, thriving, communication effectiveness, work-life balance, career development, senior leadership, performance management and orga-nization effectiveness. Team members scored Genmab on these areas of focus which helps us keep a pulse on areas of concern. The engagement score, which measures favor-ability, is influenced by certain questions specifically designed to measure overall employee engagement on a five point rating scale. In 2024, we achieved a 79% engage-ment score and a 90% global participation rate compared to an 83% engagement score and an 88% global participation rate in 2023. Our results in 2024 and 2023 exceeded life sciences industry benchmarks of 78% engagement score and 80% participation rate. Refer to S1-5 for targets related to the global employee engagement survey. Learning & Development In 2024, Genmab created multiple skills-based learning paths for team members focusing on specific skill development with focus areas including digital and AI, feedback, strategic planning, advance excel skills, leading different generations, informal leadership, and business communications. In 2023, Genmab focused its efforts to continue building our learning culture, including curation and development of new virtual learning content through our GenSpire platform, as well as initi-ating custom, targeted learning programs. We continued to implement our cloud-based learning management system, together with an e-learning library of courses to help team members develop their skills while working remotely. AI in learning In 2024, Genmab focused on the usage of AI in learning to increase usability, course suggestions, and our collaboration with our learning providers. Global Mentorship Program In 2024, Genmab launched a Global Mentor Program, to ensure an inclusive and supporting environment. This new mentor program can impact the sense of belonging, as employees connect with other colleagues outside their area of expertise as well as connecting with leaders that can provide insights and feedback that is not linked to any performance plan. The feedback can be used to develop skills and traits important to that specific individual. Diversity in the Workplace Employee Satisfaction We achieved an overall satisfaction score of 88% or greater on Diversity and Inclusion in the Global Employee Engagement Survey in 2024 and 2023. DE&I Council Our DE&I Council guides the alignment of our DE&I strategy with our overall business strategy. The Council includes representation of senior leaders, DE&I team, Internal Communication, and presidents of our ERGs, and meets to discuss cultural activities, employee feedback and future DE&I needs. DE&I Trainings Our DE&I team updates Genmab DE&I training each year to respond to the growth and diversity in Genmab as well as the geopolitical situation in the global environment. We conducted multiple trainings in 2024 and 2023. The trainings offered were a combination of culture Workshops, culture masterclasses and DE&I workshops with basic concepts and definitions. Employee Resource Groups (ERGs) Genmab currently has six (6) Employee Resource Groups (ERGs). ERGs are employee led, self-directed voluntary groups that enhance culture and aim to bring better business outcomes through leveraging diversity and inclusion by offering opportunities to network internally, attracting a diverse employee base, providing the inclusion of ideas and solutions, and creating opportunities for career development. ERGs are overseen by the DE&I team, under the Human Resource function. All ERGs are open to all employees of Genmab. Talent Acquisition and Fair & Equal Hiring Practices In 2024, we attended two (2) diversity career fairs. In 2023, our Talent Acquisition team, including 100% of our recruiters, received additional implicit bias training on eliminating bias in the hiring process. In partnership with the National Black MBA Association, Inc and Disability IN, in the U.S., as well as Women in Tech in Brussels, we continued outreach and relationship building in historically underserved communities to better understand their needs and identify ways to support local prosperity and the development of potential talent for our Company. We plan to continue to participate in three to four diversity career fairs annually, with the focus on expanding the talent pipeline and ensuring that diverse talent has access to apply for vacant positions. Compliance with the Dutch Participation Act We employ three individuals with disabilities who were trained, mentored, and coached on the job to support this law that aims to help everyone find work in the Netherlands, including people with disabilities. Refer to S1-5 for targets linked to diversity. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 112 |
|
Sustainability Statements Targets related to managing material negative impacts, advancing positive impacts, and managing material risks and opportunities (S1-5) Targets related to Employee well-being and vitality Genmab continues to promote an environment that fosters individual empowerment that allows team members to achieve their maximum potential, and drive initiatives that engage, develop and inspire employees as a part of our overall Total Rewards strategy. Specific target identified by the HR function related to promoting employee well-being and vitality is indicated below: • Meet or exceed the global benchmark for (1) employee engagement score and (2) participation rate for the Global Employee Engagement Survey in 2025 (annual target)1 The target is measured annually after the administration of the Global Employee Engagement Survey. Genmab will compare the engagement score, which measures the favorability rate, and the participation rate for the Company’s engagement in comparison to the life sciences industry average. Focusing on top-down governance, the survey results are reviewed with the Executive Committee. During the discussion, the team will reflect on any actions needed to maintain or improve overall employee engagement. Refer to S1-4 which shows how Genmab’s Global Employee Engagement Survey results in 2024 and 2023 outpaced life sciences industry benchmarks. 1. Executive Management received RSU grants in 2022, 2023 and 2024 with performance linked to sustaining at or better than the global benchmark for employee engagement. 2. Executive Management received RSU grants in 2022, 2023 and 2024 with performance linked to gender diversity balance targets in director and above level roles. The range of payout varies based on gender splits (see GOV-3). Targets related to Career development through training & skill building The Genmab Learning & Development (L&D) team within the Global Talent Management strives to be data centric. The L&D team measures learning completion records for required learning, optional learning, and self-assigned learning. Aside from completion measures the team also measures the communication channels effectiveness. Specifically, L&D has set annual required career development, training & skill building targets as follows: • 100% of eligible employees are provided access to Genmab’s end of year performance process The Global Talent Management team at Genmab will ensure that all eligible employees are provided full access to the year-end performance process at Genmab, including providing internal resources and early engagement to the employees. This is set as an annual target for 2025. • 100% of employees are provided access to training programs that meet the development needs across various career stages and learnings styles The Global Talent Management team at Genmab will ensure that professional development skills trainings are offered to all employees. This is set as an annual target for 2025. • Launch sustainability awareness training by 2025 The Corporate Sustainability Team at Genmab plans to launch sustainability awareness training for team members in 2025. Targets related to Diversity in the Workplace Diversity targets are determined by the DE&I Council (made up of senior executives) and adopted by the Board of Directors. They are as follows: (1) Maintain between 40% to 60% gender representation at a director level and above2 The Board of Directors has committed to maintaining an annual target of balanced gender representation, at a director level and above level positions by 2025. In order to provide equal employment and advancement opportunities to all individuals, employment decisions at Genmab are based on merit. The Company is committed to equal opportunity in the conduct of all our business activities. Genmab does not discriminate on the basis of race, color, creed, religion, gender, national origin, age, marital status, disability, sexual orientation, gender identity or expression, status with regard to public assistance or any other classification protected by appli-cable law. As of December 31, 2024, the gender split in director level and above was 52% female and 48% male as disclosed in the table in section S1-9. (2) Target between 40% to 60% gender repre-sentation by 2025 in the Other Management Levels at Genmab A/S only in accordance with the guidelines from the Danish Business Authority As of December 31, 2024, the gender split in the Other Management Levels, as defined in the Danish Companies Act, was 33% female managers (six persons) and 67% male managers (12 persons) for Genmab A/S (Danish Parent only). As we do not currently have an equal share of men and women in the Other Management Levels at Genmab A/S as defined by the Danish Companies Act, the Board of Directors has committed to a target ratio of 40% to 60% for female and male splits in the Other Management Levels of Genmab A/S by 2025, or the target that comes closest to this target and which still constitutes an equal gender composition in accordance with the guidelines from the Danish Business Authority. To pursue the fulfillment of the set target and to continue working towards and main-taining diversity and equal opportunities for employees at all management levels in the Genmab Group, we have implemented several initiatives related to, among other things, recruitment, employment terms and talent development. We also offer participation in internal network groups and focus on raising awareness of bias throughout the organiza-tion by conducting regular internal training. Taking into account these initiatives and the existing composition of the Other Management Levels of Genmab A/S, the target is expected to be met by 2025. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 113 |
|
Sustainability Statements Characteristics of the undertaking’s employees (S1-6) December 31, 2024* 2023 2022 Female Male Total Total Total Employees (Headcount) Denmark 326 228 554 495 410 Netherlands 490 335 825 740 600 U.S. 642 432 1,074 887 643 Japan 55 131 186 140 58 China 55 56 111 – – Total Headcount 1,568 1,182 2,750 2,262 1,711 December 31, 2024* 2023 2022 Female Male Total Total Total Employees (FTEs) Female Male Total Total Total Permanent 1,498 1,136 2,634 2,159 1,627 Temporary 28 20 48 45 33 Total FTEs 1,526 1,156 2,682 2,204 1,660 December 31, 2024 2023 2022 FTEs (R&D vs. SG&A) Research and development FTE 1,886 1,541 1,193 Selling, general and administrative FTE 796 663 467 2024 2023 2022 Turnover # of FTEs leaving Genmab 190 157 119 Turnover Rate—Overall 7% 8% 8% Turnover Rate—Voluntary 6% 5% 6% *2024 is a baseline year for female/male headcount and FTE reporting. Splits were not previously disclosed in prior year Annual Reports. As of December 31, 2024, the total number of FTEs was 2,682 compared to 2,204 as of December 31, 2023. The increase was primarily driven by the expansion and acceleration of our pipeline, as well as the investment in the expansion of Genmab’s commercialization capabilities, including support for EPKINLY in the U.S. and Japan post launch activities, and broader organizational capabilities and the acquisition of ProfoundBio. Accounting Policies Number of Employees FTEs are defined as all employees on our payroll, both full-time and part-time, as well as active and on-leave. All individuals have been included by reflecting the proportion of an FTE they represent based on their contractual agreement. Headcount are defined as all employees on our payroll, both full-time and part-time, as well as active and on-leave. All individuals have been included by reflecting a 1 equivalent per person. Turnover Rate Turnover rate is calculated by the overall number of FTEs leaving since the beginning of the year divided by the average FTE for the year. Temporary Includes interns, student workers, post Doctorate (post doc) and fixed term employees. Refer to the Note 2.3 Staff Costs for cross reference to FTEs reported in Genmab’s financial statements. Collective bargaining coverage and social dialogue (S1-8) There are no employees covered by collective bargaining agreements at Genmab. There are workers councils in Denmark and the Netherlands. Refer to S1-2 for details of the work councils. Coverage Rate Collective Bargaining Coverage Employees—EEA Social Dialogue Workplace representation (EEA only) 0–19% 20–39% 40–59% 60–79% 80–100% Denmark, Netherlands Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 114 |
|
Sustainability Statements Diversity metrics (S1-9) Diversity metrics as of December 31: 2024 2023 2022 Female Male Total Female Male Total Female Male Total Board of Directors, Shareholder-Elected 3 3 6 3 3 6 3 3 6 % of total 50% 50% 100% 50% 50% 100% 50% 50% 100% Board of Directors, Including Employee-Elected 4 5 9 4 5 9 4 5 9 % of total 44% 56% 100% 44% 56% 100% 44% 56% 100% Executive Management 3 6 9 3 5 8 2 5 7 % of total 33% 67% 100% 38% 63% 100% 29% 71% 100% 2024 2023 2022 Female Male Total Female Male Total Female Male Total Genmab Group 1,525 1,157 2,682 1,270 934 2,204 964 696 1,660 % of total 57% 43% 100% 58% 42% 100% 58% 42% 100% Director Level and Above 547 507 1,054 449 414 863 348 332 680 % of total 52% 48% 100% 52% 48% 100% 51% 49% 100% Below Director Level 978 650 1,628 821 520 1,341 616 364 980 % of total 60% 40% 100% 61% 39% 100% 63% 37% 100% 2024 2023 2022 Age < 30 11% 13% 14% 30–50 63% 63% 62% > 50 26% 24% 24% 100% 100% 100% Adequate wages (S1-10) All of Genmab’s employees receive adequate wages. We have a dedicated compensation & benefits team at Genmab that ensures that we are paying our people in line with local legal require-ments and with peer and similar companies through benchmark analysis. Social protection (S1-11) All of Genmab’s employees are covered by social protection, through public programs or through benefits offered by Genmab, against loss of income due to any of the following major life events including sickness, unemployment, employment injury and acquired disability, parental leave and retirement. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 115 |
|
Sustainability Statements Training and skills development metrics (S1-13) Genmab provides all employees training and skills development related activities within the context of continuous professional growth, to upgrade employees’ skills and facilitate continued employability. 2024 Female Male Training and skills development metrics % of Employees Who Have Completed Performance Reviews/Career Conversations 100% 100% % of Employees Who Have Not Completed Performance Reviews/Career Conversations 0% 0% Average Number of Training Hours 10 8 Health and safety metrics (S1-14) The health and safety of 100% of Genmab’s employees and non-employees are covered by legal requirements and recognized standards and guidelines. There were no fatalities in 2024 or 2023 as a result of work-related injuries and work-related ill health. In 2024, there were no work-related accidents. In 2023, we had one occupational incident that resulted in lost time at work in the U.S. We will continue our ongoing preventative health and safety activities to reinforce policies and procedures to all team members globally. Remuneration metrics (pay gap and total remuneration) (S1-16) The below table shows the percentage gap in pay between all our female and male employees and the ratio between the remuneration of our CEO and the median remuneration of our employees. 2024 Gender pay gap—Overall 12% Gender pay gap—Excluding Executive Management 5% CEO pay ratio 64 Accounting Policies Gender pay gap Genmab calculates the gender pay gap for employees as the difference of average pay levels (gross hourly pay) between female and male employees, expressed as percentage of the average pay level of male employees. The calculation relies on a single variable (gender) and excludes other factors that would typically be included in a pay analysis, such as job level, amount of experience, performance rating, education level, typical market pay for a position, etc. CEO pay ratio Genmab calculates the CEO pay ratio for employees as the annual total remuneration ratio of the highest paid individual (our CEO) to the median annual total remuneration for all employees (excluding our CEO). Remuneration includes base salary, defined contribution plans, other benefits, annual cash bonus and share-based compensation. Refer to Note 5.1 in the consolidated financial statements for details of CEO pay for 2024. Incidents, complaints and severe human rights impacts (S1-17) During 2024, there were no work-related incidents of discrimination, including harassment, substantiated. No cases of severe human rights incidents (e.g., forced labor, human trafficking, or child labor) were identified during 2024. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 116 |
|
Sustainability Statements Below is the list of Disclosure Requirements as it pertains to ESRS S4—Consumers and/or End-users: Section Disclosure requirement content Disclosure requirement # 4.0 IROs Material Impacts, Risks and Opportunities and their interaction with strategy and business model SBM-3 4.1 IRO Management Policies related to consumers and end-users S4-1 Processes for engaging with consumers and end-users about impacts S4-2 Processes to remediate negative impacts and channels for consumers and end-users to raise concerns S4-3 Taking action on material impacts on consumers and end-users, and approaches to managing material risks and pursuing material opportunities related to consumers and end-users, and effectiveness of those actions S4-4 4.2 Consumers & End-Users Metrics and Targets Targets related to managing material negative impacts, advancing positive impacts, and managing material risks and opportunities S4-5 Social— Consumers and End-users Genmab’s consumers include healthcare providers, pharmaceu-tical distributors, and end-users are its patients. Our consumers and/or end-users are dependent on accurate and accessible product-related infor-mation, such as manuals and product labels, to avoid potentially damaging use of a product. We also have a small group of pediatric consumers/ end-users who are participating in a pediatric study, who are particu-larly vulnerable to health or privacy impacts or impacts from marketing and sales strategies due to the inherent nature of young age-group. At Genmab, our work is anchored in our core purpose: to improve the lives of patients through innovative and differentiated antibody therapeutics. Driven by this purpose, we are transforming the way patients fight cancer while creating long-term value for all our stakeholders. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 117 |
|
Sustainability Statements 4.0 IROs The below table describes Genmab’s material impacts, risks and opportunities related to our material Social topics for Consumers and End-Users: Section Material Topic Material Impact Value Chain Location Material Risk Material Opportunity Social Consumers and End-Users ― Social inclusion of consumers and/or end-users Access and integrity in clinical trials (positive) Downstream Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance Market access programs to allow for product availability for uninsured or underinsured (positive) Downstream Refer to the Risk Management section of the Annual Report for risks related to Business and Products Assisting those having difficulty affording Genmab products prescribed to them Consumers and End-Users ― Personal safety and information of consumers and/ or end users Patient engagement programs developed (positive) Downstream Refer to the Risk Management section of the Annual Report for risks related to Business and Products Clinical trial transparency (positive) Downstream Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance All impacts, risks and opportunities have expected time horizons of short, medium and long-term. Access and integrity in Clinical trials (positive) We strive to enroll patients who will benefit from our clinical studies and Genmab is subject to extensive legislative, regulatory and other requirements which pose a risk to Genmab; however, Genmab is committed to ensuring equal access to Genmab clinical trials. We have DE&I initiatives to ensure we enroll diverse patients who represent the communities we serve. Market access programs to allow for product availability for uninsured or underinsured (positive) MyNavCare Patient Support is designed to provide comprehensive resources and support to help patients access EPKINLY (epcoritam-ab-bysp) throughout their treatment journey. Recognizing the challenges faced by uninsured or underinsured patients, Genmab has created a robust program through MyNavCare to provide financial assistance and free medicine for eligible patients struggling to afford their prescribed treatments. Without effective market access programs, Genmab could lose market share from competition. Assisting those having difficulty affording Genmab products prescribed to them is an iden-tified opportunity for Genmab and can lead to market growth in a competitive pharmaceutical landscape. Through MyNavCare, Genmab assists eligible patients with coverage for co-pays, co-insurance, and deductibles, helping to ensure that financial challenges do not become barriers to accessing life-changing therapies. This initia-tive reflects Genmab’s commitment to reducing disparities in healthcare access and empowering patients to focus on their treatment journey. Patient engagement programs developed (positive) Genmab is elevating the voices of patients and care partners by incorporating their perspectives into all aspects of our work—from early-stage R&D to clinical trials and commercialization. These insights are vital to our ability to innovate and to support people impacted by our medicines as they navigate the complex aspects of a serious illness. Without our patient engagement programs, there is risk of increased competi-tion and decreased overall patient experience. Genmab engages with patients and caregivers to gather insights and improve patient outcomes. The goal of these programs is to effectively and efficiently bring medicines to patients that incor-porate the patient voice across the continuum of clinical development, including ensuring clinical protocols and informed consents have the patient insights required to attract and retain patients in the trials while meeting regulatory requirements. Clinical trial transparency (positive) As there is risk associated with transparency in our business, increased transparency in clinical trials is a key factor for advancing medical research and improving patient outcomes. As Genmab develops antibody therapeutics for cancer treatment and other serious diseases, increased transparency can have the following material impacts: • Help patients find trials that match their eligibility criteria and preferences, increasing the recruitment and retention rates of participants. • Empower patients to make informed decisions about their health, enhancing their trust and satisfaction with the trial process and the Company. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 118 |
|
Sustainability Statements • Improve the evidence base for clinical care, allowing clinicians and scientists to access and evaluate the safety and efficacy of new treatments. • Prevent duplication of unsuccessful trials, saving time and resources for the research community. • Contribute to efficient design of clinical trials, enabling Genmab and the research community to learn and optimize new trial protocols and outcomes. Increased transparency in clinical trials benefits Genmab by facilitating development of innovative and effective therapies for patients with cancer or other serious diseases and ensuring their optimal use. 4.1 Consumers and End-Users IRO management Policies related to Consumers and End-Users (S4-1) Human Rights Commitment Our Human Rights Commitment includes policies around our patient’s safety and privacy rights of our patients, healthcare providers and other customers. Refer to section S1-1 for additional disclosures on our Human Rights Commitment and the Human Rights Commitment on our website. Commitment to Quality Our goal is to safeguard patient safety through state-of-the-art monitoring systems, stringent processes, and industry best practices. Our comprehensive safety program is designed to identify and mitigate potential risks asso-ciated with our products, and to ensure that our products are safe and effective for their intended use. We work closely with regulatory agencies to ensure that our products meet all safety and efficacy standards. We also collaborate with healthcare providers and patient advocacy groups to ensure that patients have access to the information they need to make informed decisions about their treatment. Under the lead-ership of our Chief Development Officer, our functions are responsible for ensuring compli-ance with Commitment to Quality. Refer to the Commitment to Quality on our website. Clinical Trial Transparency Genmab is committed to transparency of clinical trial research. We recognize the scientific and ethical value of sharing clinical trial information in a non-biased and timely manner to benefit diverse audiences. Transparency in clinical trials helps patients and healthcare providers to make well-informed decisions about patients’ health. Transparency is also about building and main-taining public trust in clinical research. We are committed to sharing clinical trial information and results in a language understandable also for non-scientists, as part of our ongoing transpar-ency efforts. Our commitments apply to all Genmab-sponsored interventional clinical trials, Phase 1 and beyond, conducted worldwide. To the extent applicable, our commitments also apply to Genmab-sponsored non-interventional clinical studies and expanded access programs. The most senior level in Genmab that is accountable for the policy is the Chief Development Officer. The Genmab Clinical Trial Transparency Declaration can be mapped to the positive impact of Clinical trial transparency and is publicly available via the website. The Declaration is also referenced in the onboarding program of new Development Operations employees. Refer to the Clinical Trial Transparency Declaration on our website. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 119 |
|
Sustainability Statements Code of Conduct Within Genmab’s Code of Conduct, there is a section (#5) specifically on Clinical Trials, which is publicly available via Genmab’s website. Our research and clinical trials are always guided by Our Purpose: to improve the lives of patients through innovative and differentiated antibody therapeutics. We are committed to conducting our research, development, and related data collec-tion with scientific integrity and disclosing results in a timely manner. Genmab’s Code of Conduct also describes our 20 ethical standards and covers respon-sible marketing practices. We at Genmab are committed to conducting all aspects of our business with integrity, and in an ethical, honest, and transparent manner. These standards and our associated corporate policies provide further guidance for our people with respect to this commitment. Genmab’s Head of Global Compliance is responsible for this policy and reports directly to the CEO, and both are members of the CSR and Sustainability Committee. The Code of Conduct can be mapped to our positive impact of Access and integrity in clinical trials. Refer to the Code of Conduct on our website. Commitment to Patients Across Genmab, our talented and dedicated team works every day to improve the lives of patients through innovative and differentiated antibody therapeutics. Genmab’s Commitment to Patients describes how our work is guided by doing what’s best for patients. Through our Patient Advisory Council, patients contribute their insights ranging from how we design and conduct our clinical trials to how we may help to educate and support patients who have been prescribed our products. We are focused on understanding the many indi-viduals who may support a patient throughout their disease experience and tailoring resources to the needs of these care partners to better facilitate their role in the care journey. Our goal is to conduct clinical research that reflects the real-world demographics of the diseases we study, and we strive to ensure our trials are patient centric and accessible for patients of all back-grounds to participate. We aim to ensure that all patients who are prescribed our medicines have timely access to them. We work with stakeholders across the healthcare system and have patient support services (PSS) in place to address patient access to our medications once prescribed. We aim to facilitate transparency in our work and interactions. The Commitment to Patients can be mapped to the positive impacts of patient engagement and market access programs developed to allow for product availability for uninsured or underin-sured. The Senior Vice President Communications and Corporate Affairs is responsible for the policy and reports directly to CEO. Refer to the Commitment to Patients on our website. Global Compliance Policy Genmab has an internal Global Compliance Policy and playbook. The Global Compliance Policy covers interactions and engagements with healthcare professionals, healthcare organizations, patients, patient association groups and government officials (stakeholders). Genmab’s Head of Global Compliance is respon-sible for this policy and reports directly to the CEO, and both are members of the CSR and Sustainability Committee. Processes for engaging with consumers and end-users about impacts (S4-2) Genmab’s engagement with consumers and end-users is a multi-faceted strategy that prior-itizes patient safety, ethical marketing, and transparent communication. Patient Engagement Programs Genmab’s mission to improve the lives of patients with cancer and serious diseases is realized through its commitment to patient engagement programs. These efforts include clinical trials, advocacy initiatives, and also through tailored support programs like MyNavCare Patient Support™, which provides, with respect to Genmab products approved in the U.S., person-alized assistance for patients and their care partners, as well as support for HCPs for the ultimate benefit of patients. The MyNavCare Patient Support™ program offers comprehensive support, including insurance navigation, and financial support, designed to facilitate access to therapies for all eligible patients. The program supports, among others, those who are uninsured or underinsured, ensuring equitable access to treatment while alle-viating the burdens associated with navigating complex healthcare systems. When someone has enrolled in the MyNavCare program, which is optional, they can call and engage with MyNavCare team members to discuss any questions or issues they have. Genmab values patient input and actively involves patients in its decision-making processes through initiatives like the Patient Advisory Council. This council enables patients to share their experiences and insights, influ-encing how clinical trials are designed and how medicines are delivered. By listening to patient feedback, Genmab can better understand their needs and concerns, ultimately enhancing both the science behind its therapies and the support services offered, thereby contributing to the decision-making processes ranging from how we design and conduct our clinical trials to the way we deliver our medicines. Engagement occurs on a regular basis, from early development through commercial. The Senior Vice President Communications and Corporate Affairs is responsible for ensuring this engage-ment and reports directly to CEO. Clinical Trial Transparency Transparency in clinical trials is another key focus for Genmab. Genmab engages on a regular basis with patient advocacy groups and healthcare professionals to ensure that the perspectives of consumers and end-users are integrated into trial design and execution. Genmab publishes its Clinical Trial Transparency Declaration on its website, keeping patients and other stakeholders informed about trial processes and expectations. The Development Operations team, organized under the Chief Development Officer, oversees this engagement, and ensures the effective planning, execution, and public disclosure of clinical trials. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 120 |
|
Sustainability Statements Safety and Compliance The Global Drug Safety team plays a crucial role in ensuring that Genmab’s products meet stringent safety and efficacy standards. They work closely with regulatory agencies to maintain compliance but do not engage directly with patients. This team’s efforts are complemented by a comprehensive safety monitoring program designed to identify and mitigate potential risks associated with Genmab’s therapies. Trust in the safety and quality of these products is essential, as any breach of trust can adversely affect the Company’s reputation and core business. Under the leadership of our Chief Development Officer, our internal functions are responsible for ensuring safety and compliance. Ethical Marketing Practices Genmab emphasizes responsible marketing practices in all interactions with healthcare providers and stakeholders. Genmab has estab-lished a Code of Conduct that sets high ethical standards for employees, reinforced through regular training. This ensures that all marketing and sales efforts align with local and national regulations, which may limit direct engagement with consumers and end-users. Ethical marketing practices are the responsibility of our Chief Commercial Officer. Processes to remediate negative impacts and channels for consumers and end-users to raise concerns (S4-3) While we have not identified any material negative impacts regarding our consumers and end-users, we do promote and encourage our consumers and end-users to speak up to report concerns, share feedback, address compli-ance issues, and enable remedy for human rights impacts. We believe our consumers and end-users trust the channels described in this section as we receive questions and requests from these channels. Clinical Trials Consumers and end-users can raise questions or concerns via Genmab’s publicly available mailbox, ClinicalTrial@genmab.com. This mailbox is provided along with all registered trials on disclosure platforms such as ClinicalTrials. gov and the EU CTIS public portal. At time of enrollment, the trial participants are provided an Informed Consent Form (ICF) to sign before joining a trial. The ICF contains additional information on channels to raise questions or concerns. The requests received are reviewed and assessed to ensure they are addressed by the appropriate Genmab function, while ensuring that personal information is handled securely and in compliance with privacy regulations. MyNavCare Patients enrolled in the MyNavCare program have access to dedicated team members who address their concerns, answer questions, and provide personalized assistance. The Patient Engagement Liaisons (PELs) serve as trusted points of contact, offering tailored support without providing medical advice or working under the direction of the prescribing healthcare providers. PELs are dedicated to helping patients and care partners by: • Providing information about the patient’s condition and what to expect while on treatment • Connecting them with external organizations • Offering resources tailored for the needs of both patients and care partners This optional program ensures that patients feel supported and informed, creating a compas-sionate framework that prioritizes patient empowerment while respecting the role of health-care providers in treatment decisions. The effectiveness of our work is tracked via regular meetings with our MyNavCare team members and through scheduled updates with the other cross functional teams leadership meetings. The Patient Services Steering Committee, made of senior U.S. Market leaders, meets every quarter to review the patient services program operational metrics and effectiveness. Reporting a Side Effect or a Quality Concern We take patient safety seriously. Reports of side effects and quality concerns enable us to ensure the safety of our medicines and the patients who take them. Genmab has a public number that patients may call. We state on all our resources the following: You are encouraged to report side effects to the FDA at (800) FDA-1088 or www.fda.gov/ medwatch or to Genmab US, Inc. at 1-855- 4GENMAB (18554436622). The company systematically monitors and evaluates adverse events and other safety-related information associated with its therapies post-approval. This ongoing surveillance helps identify and respond to potential safety concerns promptly. Speak Up Hotline If patients or caregivers have concerns, they can raise them through the Speak Up Hotline. Our 24/7 full-service Speak Up (whistle-blower) compliance hotline enables the anonymous reporting of illegal, unethical, and/ or non-compliant behavior and related concerns in connection with our organization. Our Compliance team regularly reviews these matters, and supports investigations as warranted, reporting to both management and the Audit & Finance Committee. Refer to the Global Speak Up Policy and Hotline on our website, and S1-3 for further details. Taking action on material impacts on consumers and end-users, and approaches to managing material risks and pursuing material opportunities related to consumers and end-users, and effectiveness of those actions (S4-4) Genmab has safety measures in place for products, patients, and healthcare providers. Genmab believes that patient safety plays a critical role in our business operations. Genmab has a Comprehensive Safety Program that is designed to identify and mitigate potential risks associated with our products, and to ensure that our products are safe and effective for their intended use. We work closely with regulatory agencies to ensure that our products meet all safety and efficacy standards. Genmab provided annual training programs on pharmacovigilance for global drug safety and pharmacovigilance (GDS&PV) staff, including regular updates and assessments to ensure continuous learning and compliance with the latest regulations. Also, Genmab developed and distributed educational materials on safety requirements to stakeholders and organized workshops to enhance their understanding and implementation of safety protocols. These Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 121 |
|
Sustainability Statements actions are linked to the personal safety and information of consumers and end-users. The following actions were taken around Genmab’s material impacts on our consumers and end-users for 2024: Access and integrity in clinical trials We have a Patient Advisory Council and a Study Coordinator Advisory Council who provide input to our clinical trials. In addition, our clinical trials are reviewed by institutional review boards, ethics committees, regulatory authorities, and data and safety monitoring boards. As of December 31, 2024, there was a total of 25 ongoing Genmab-sponsored clinical trials, all registered on ClinicalTrials.gov. Our clinical trials are taking place in 37 countries worldwide with 620 patients enrolled during the year. Genmab complies with all applicable industry regulations, guidelines, and standards globally for drug development, such as cGLP, cGCP, cGMPs and good animal practice as defined by the Federation of European Laboratory Animal Science Associations (FELASA). We also monitor and comply with all relevant legislation and regulations, including guidelines issued by international regulatory authorities such as the European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA), the Pharmaceuticals and Medical Devices Agency (PMDA) and others. It is important to acknowl-edge our relationship with Japan PMDA, as it reflects our global strength. Our operations were periodically audited by relevant authorities. Clinical trials generate the data necessary to evaluate the safety and efficacy of drugs, providing insight on how to use a therapy and which patients are most likely to benefit from treatment. Even with the advancements in understanding the incidence of different cancers between genders and racial or ethnic groups, inequities persist, resulting in underrepresenta-tion in clinical trials. Genmab’s DE&I in Clinical Trials Project Team is responsible for defining and implementing a framework to deliver Genmab’s intentions to provide clinical trial treatment options to patients in a wider community and generate clinical trial data from currently underrepresented popu-lations. The Project Team works to ensure all Genmab registration studies have a clear strategy and process for Diversity Plan success. Genmab has been working to implement diversity in our pivotal clinical trials. In 2024, Genmab introduced a Diversity Action Plan (DAP) SOP and DAP template to be implemented for all pivotal trials. The inaugural Diversity Action Plan was submitted to the U.S. FDA in 2024. With the U.S. FDA final guidance expected in 2025 we will ensure compliance with the agency’s new rules and implement diversity in all applicable pivotal studies where appropriate. Genmab systematically monitors and evaluates adverse events and other safety-related informa-tion associated with its therapies post-approval. This ongoing surveillance helps identify and respond to potential safety concerns promptly. Our cross functional team regularly reviews matters, and supports investigations as warranted. We have a mix of internal and external committees that regularly monitors clinical trial data to track and assess the effectiveness of these actions mentioned above. Market Access Programs to Allow for Product Availability for Uninsured or Underinsured All Market Access colleagues undergo compre-hensive annual compliance training to ensure adherence to regulatory standards and ethical practices. Here is a list of some of the key compli-ance training modules they complete: • Data Privacy • U.S. FDA guidelines • The PhRMA code and Ad Promotion rules • Compliance issues including Anti-bribery and Speak Up modules (including whistleblower training) • Pharmacovigilance • Sunshine Act (and various other transparency laws) • Conflict of interest The MyNavCare team also trains annually on specific compliance related PSS polices and business rules. These training modules ensure that colleagues in the Market Access group remain informed about the latest regulatory requirements and ethical standards, thereby safeguarding the Company’s reputation and ensuring the continued availability of their products to patients. The tracking of the completion rates are monitored by the Global Compliance team. Patient Engagement Programs Developed Genmab is elevating the voices of patients and care partners by incorporating their perspectives into all aspects of our work from early-stage R&D to clinical trials and commercialization. These insights are vital to our ability to innovate and to support patients as they navigate the complex aspects of a serious illness. The effec-tiveness of these patient engagement programs are tracked via regular meetings with our patient advisory council and through scheduled updates with other internal teams. Our patient advocacy team facilitated multiple touchpoints with key stakeholders throughout the year to provide opportunities for mutual learning and insight sharing: • Our Patient Advisory Council, formed in 2023, which consisted of 16 members as of December 31, 2024 compared to 13 members as of December 31, 2023, met nine times in 2024 and four times in 2023. In 2024, topics discussed included feedback on patient materials, such as brand messaging and caregiver resources, the global and U.S. websites, and review of clinical trial materials, including informed consent forms, protocol, and lay summary. In 2023, topics discussed were around clinical trial recruitment and diversity in clinical trials, providing patient perspective on the cancer experience and how Genmab can better serve patients and care partners. Members of the Council represent people with a variety of tumor types, ages, geographies, and socioeconomic backgrounds. • Our third annual Science Day in 2024 brought together 29 leaders from 24 organizations and six patient advisors for 1.5 days of scientific discussion and knowledge exchange. Our second annual Science Day in 2023 brought together 32 representatives from patient advocacy and professional groups, our Patient Advisory Council and Genmab team members to share information about our respective work to inform how we can best address the needs of patients and their care partners. Topics included AI, and the Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 122 |
|
Sustainability Statements cancer journey for patients and care partners. We understand the need for broad stakeholder education to help break down barriers and reach underrepresented populations, along with the need to support the patient and care partner to address the psychosocial impacts of living with cancer, such as feelings of depression, anxiety, and fear. The actionable insights gained from Science Day are key to shaping our future patient engagement and education efforts. MyNavCare We are bringing life-changing medicines and support services to patients through MyNavCare Patient Support. Since the 2023 launch of EPKINLY in the U.S., through 2024, we have supported patients directly through MyNavCare Patient Support, our robust patient support program, and continuously worked with stakeholders across the healthcare system to ensure rapid and sustainable access to appro-priate patients. In the U.S., MyNavCare supports each patient’s unique needs alongside the needs of care partners. We support: • Patients and care partners through case management, insurance navigation and financial assistance, among other services • HCPs through navigating access, including reimbursement education, and billing and coverage information, among other resources with the overarching goal of supporting patients needs We also provide access support for eligible patients who are uninsured or underinsured through our Patient Assistance Program, which minimizes the burden of applying for assistance and quickly determines eligibility. We aim to take great care and consideration to help ensure rapid and sustainable access for all appropriate patients who may benefit from our therapies as we look to bring our own medicines to additional markets in the future. We remain focused in our pursuit to turn innovative science into medicine that creates value and delivers meaningful impact to patients, their care partners and the HCPs who serve them. Under the leadership of our Vice President, U.S. Market Access, our market access function is responsible for oversight of MyNavCare. The effectiveness of our work is tracked via regular meetings with our MyNavCare team members and through scheduled updates with the other cross functional teams leader-ship meetings. Clinical Trial Transparency To ensure access to quality information for patients, consumers, and end-users, Genmab has taken the following action in 2024: • Offered trial descriptions in the ClinicalTrials.gov registration that are clear and understandable for non-scientists, benefiting potential trial participants. Tracking and assessment is done by reviewing requests received from consumers and end-users via Genmab’s publicly available mailbox, ClinicalTrial@genmab.com. The received requests are triaged to ensure they are addressed by the appropriate Genmab function. Continuous Improvement • Genmab is committed to continuous improvement in patient engagement and safety monitoring. The Company regularly evaluates feedback from advocacy groups and utilizes social media to assess the effectiveness of its communications. By fostering a culture of open dialogue and ethical conduct, Genmab not only meets regulatory requirements but also builds trust with the patients and healthcare providers it serves. • Genmab is actively working to reduce the clinical trial burden for patients. Engaging our Patient Advisory Council to review protocols and integrating patient feedback into our trials, and developing health-literate materials are some of the ways in which we help to increase access to our clinical trials. • Our Clinical Trial Transparency Declaration acknowledges our strong commitment to the scientific and ethical aspects of increasing the transparency of clinical trial research. In alignment with the Declaration, we disclosed information and results from our clinical trials through publicly accessible study registries/ databases such as ClinicalTrials.gov, and the European Union Clinical Trials Information System (EU CTIS), to ensure compliance with global and national laws in the evolving area of transparency. 4.2 Consumers and End-Users Metrics and Targets Targets related to managing material negative impacts, advancing positive impacts, and managing material risks and opportunities (S4-5) As there are no identified negative impacts in this area, no targets were set, which is aligned with our strategy/priority for 2025. Refer to Our Strategy in Management’s Review for details. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 123 |
|
Sustainability Statements Business Conduct Below are the list of disclosure requirements as it pertains to ESRS G1—Business Conduct: Section Disclosure requirement content Disclosure requirement # 5.0 IROs Material Impacts, Risks and Opportunities and their interaction with strategy and business model SBM-3 5.1 Business Conduct IRO Management Business conduct policies and corporate culture G1-1 Management of relationships with suppliers G1-2 Prevention and detection of corruption and bribery G1-3 5.2 Business Conduct Metrics and Targets Incidents of corruption or bribery G1-4 Political influence and lobbying activities G1-5* Payment practices G1-6 *Disclosure requirement G1-5 is not material for Genmab. Governance Genmab’s oversight of sustainability is designed to ensure that our commitments are integrated as a core part of our business and aligned with international best practice. We are dedicated to complying with all laws, codes, and standards applicable to our business and operations, as well as ensuring transparency in our sustainability disclosures. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 124 |
|
Sustainability Statements 5.0 IROs Material Impacts, Risks and Opportunities and their interaction with strategy and business model (SBM-3) The table describes Genmab’s material impacts, risks and opportunities related to our material business conduct matters: Section Material Topic Material Impact Value Chain Location Material Risk Material Opportunity Governance Business Conduct ― Corporate Culture Healthy corporate culture aligned with core values and purpose (positive) Own Operations Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance Annual Code of Conduct training rollout as mandatory for all employees, with launch and completion rates monitored by Global Compliance team Business Conduct ― Privacy Cybersecurity and Global Data Privacy programs in place to protect the privacy of our business, our own workforce, patients and all individuals who entrust us with their information (positive) Upstream, Own Operations, Downstream Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance and Cybersecurity Business Conduct ― Protection of whistle-blowers Protection of whistleblowers through anti-retaliation policies and procedures (positive) Upstream, Own Operations, Downstream Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance Business Conduct ― Animal Welfare Animal welfare policy (positive) Own Operations Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance Business Conduct ― Management of relationships with suppliers (including payment practices) Strong management of suppliers, focused on compliance with supplier code of conduct (positive) Upstream Refer to the Risk Management section of the Annual Report for risks related to Strategic Collaborations Continue to partner with suppliers on sustainability related commitments in the future Business Conduct ― Corruption and bribery Ethical business culture and business practices (positive) Own Operations Refer to the Risk Management section of the Annual Report for risks related to Regulation, Legislation and Compliance, Strategic Collaborations and Management and Workforce Annual Code of Conduct training rollout as mandatory for all employees, with launch and completion rates monitored by Global Compliance team No actions were taken in 2024 across the business conduct section as no negative impacts were identified. Further, there were no instances of corruption and bribery (see G1-4) or late payments resulting in fines, penalties or litigation (see G1-6). Other than the metrics disclosed in G1-4 and G1-6, no other metrics were determined to be relevant for reporting in 2024. One target across the business conduct section was identified related to suppliers (see G1-2) and is in collaboration with our environmental strategy/priorities for 2025 and the future. No other targets were identified due to the fact that there were no negative impacts identified. All impacts, risks and opportunities have expected time horizons of short, medium and long-term. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 125 |
|
Sustainability Statements Healthy corporate culture aligned with core values and purpose In our quest to turn science into medicine, we use these guideposts to transform the future of cancer treatment: • Passion for innovation • Determination—being the best at what we do • Integrity—we do the right thing • We work as one team and respect each other Without maintaining a healthy corporate culture, Genmab is exposed to compliance and other risks. Genmab has an opportunity to provide annual training to employees on key business conduct matters. Annual Code of Conduct training is mandatory for all employees, with launch and completion rates monitored by the Global Compliance team. The Code of Conduct training should decrease behaviors that go against our Code of Conduct that could lead to compliance or other issues. Cybersecurity programs in place to protect the privacy of our business, our own workforce and patients We focus on privacy and protection of personal data at Genmab, covering several data catego-ries, such as the data of patients, employees, business partners, HCPs, and other stakeholders. Genmab acknowledges the risk related to cyber-security breaches that could occur within our value chain. We have taken solid measures to protect personal data in compliance with the EU General Data Protection Regulation (GDPR) and other applicable personal data protection legisla-tion and requirements. All our team members are educated in the GDPR. The Global Information Security and Risk Management team reports to the Board on a quarterly basis. No security incidents with critical or material business impact have been reported in 2024. Accounting policies Genmab has a security incident management process and a separate security incident system which security incidents are processed. Incidents are entered through a number of channels including Genmab’s security monitoring partner, by employees through Genmab’s internal systems and by Genmab’s security operations team. Security incidents are analyzed and rated according to different priority categories ranging from priority 1 (critical) to priority 4 (low). We perform an analysis of the number of incidents with business impact utilizing the financial statement materiality threshold. Incidences with material impact are reported to Genmab’s Board on a quarterly basis. Protection of whistleblowers through anti-retaliation policies and procedures Genmab’s 24/7 full-service Speak Up (whis-tleblower) compliance hotline enables the anonymous reporting of illegal, unethical, and/ or non-compliant behavior and related concerns in connection with our organization. Our Compliance team regularly reviews these matters which pose legal and regulatory risks to Genmab, and supports investigations as warranted, reporting to both management and the Audit & Finance Committee. Animal welfare policy Genmab understands the legal and regulatory risks associated with working with animals in our business, and our animal welfare policy represents Genmab’s commitment to sound practices that aim to replace, reduce, and refine the use of animals in Genmab’s research and development. Strong management of suppliers, ensuring compliance with Genmab’s Global Supplier Code of Conduct Supplier relationship management is a key business initiative that aims to build mutually beneficial relationships between the company and suppliers. Well-designed programs help companies to increase collaboration by identi-fying the right suppliers. Genmab understands the risk of being dependent on existing part-nerships, and has mitigation efforts in place to address these risks. Genmab has a real opportunity to partner with suppliers on Sustainability related commitments around the environment and our Supplier Code of Conduct which could lead to cost savings and stronger relationships in the future. Refer to E1-4 for environmental targets linked to supplier engagement. Ethical business culture and business practices We are committed to operating all aspects of our business with the utmost integrity. We have an established global compliance program and incorporate compliance, ethics and transpar-ency considerations into our business practices, policies, and procedures. We hold ourselves accountable to high ethical standards, promoting our Code of Conduct to employees and engaging with partners and suppliers committed to the same level of ethics in their operations. We are committed to conducting all aspects of our business in an ethical, honest, and transparent manner, and understand there are various regula-tory, legislative and compliance risks. All employees and contractors are required to complete annual training and attest to their commitment to adhere to our ethical standards. The Code of Conduct training provides an overview of our Ethical Standards, Company Values, and incorporates training vignettes that illustrate ethical approaches to common business practices. This training also reviews relevant anti-bribery and anti-corruption, regulatory, conflicts of interest, and Speak Up concepts. Compliance team members receive regular compliance training on key aspects of our compliance policies and procedures. Refer to GOV-1 for details of the role and expertise of the administrative, management and supervisory bodies related to business conduct matters. 5.1 Business conduct IRO management Business conduct policies and corporate culture (G1-1) Our Code of Conduct and 20 ethical standards embody Genmab’s commitment to doing the right thing and ensure that the ways in which we work reflect the highest standards of integrity and compliance with applicable laws and regulations. We continue to mature our compliance, risk, and data privacy program foundations as we grow and evolve to further strengthen our culture of Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 126 |
|
Sustainability Statements integrity, business continuity and corporate resil-ience. These steps help us assure a risk-based approach to our business, giving us the confi-dence to make the right decisions, drive value for patients and unite behind shared Company goals. The leader of our Global Compliance and Enterprise Risk Management programs reports directly to the CEO and the Board. Genmab’s policies on business conduct related matters linked to material IROs disclosed in section G1-1 include the following: • Code of Conduct • Global Compliance Policy • Global Speak Up Policy • Anti-Fraud Policy • Data Ethics Policy • Cybersecurity Program • Animal Welfare Policy Code of Conduct Genmab’s Code of Conduct sets high ethical standards for all employees and the Board when conducting business on behalf of Genmab. The Code of Conduct encourages team members to conduct themselves in a manner reflecting our core values, determination, integrity, inno-vation, and teamwork when representing the Company. All employees are required to complete annual training and attest to their commitment to adhere to our ethical standards. The Code of Conduct training provides an overview of our Ethical Standards, Company Values, and incor-porates training vignettes that illustrate ethical approaches to common business practices. This training also reviews relevant anti-bribery and anti-corruption, regulatory, conflicts of interest, and Speak Up concepts. Our head of Global Compliance is responsible for the Code of Conduct and reports directly to our CEO, and both are members of the CSR & Sustainability Committee. The Global Compliance team monitors completion of the annual Code of Conduct training and provides progress updates to function leaders and the Global Compliance and Risk Committee. The Code of Conduct can be mapped to the positive impact of a healthy corporate culture aligned with core values and purpose. Refer to the Code of Conduct on our website. Global Compliance Policy Our internal Global Compliance Policy, owned by our head of Global Compliance, outlines our standards on interactions and engagements with HCPs, healthcare organizations, patients, patient association groups and government officials consistent with applicable industry codes and standards. The policy aligns with the values and principles articulated in our Code of Conduct and is complemented by an associated Global Fair Market Value Policy and a Compliance Playbook tool to ensure stakeholder engagement is conducted in an ethical, compliant manner. As a result of the commercialization our first co-owned medicines including TIVDAK (2021) and EPKINLY (2023), we have expanded our compliance program to assure ethical market-based and customer-focused business practices. Genmab maintains a Global Compliance Program staffed by compliance professionals who monitor adherence to the policy. Global Speak Up Policy Genmab maintains a Speak Up (whistleblower) program featuring an independently operated hotline service available globally intended to provide anyone with information about potential misconduct related to Genmab or its business activities the opportunity to report the miscon-duct. Genmab’s Speak Up program is intended to accommodate information from any group with information including all Genmab’s current and former employees, directors, contractors, customers, suppliers, and other third parties wishing to report concerns. All reports made through the Genmab Speak Up program are assessed and considered by the Genmab Global Compliance team in a prompt, fair, and compliant manner. Genmab has estab-lished procedures to protect whistleblowers and ensure they do not suffer retaliation for their report. On a quarterly basis, Genmab’s Audit and Finance Committee receives a summary of all reports made under the Speak Up program along with additional information about any material incidents raised. Summaries of all reports made to the Speak Up program are provided to Genmab’s Global Compliance and Risk Committee (GCRC) annually. All Genmab employees and contractors are required to take Speak Up training, and comple-tion metrics are monitored by the Compliance team. Speak Up program training is a mandatory component of employee onboarding and also an annual requirement. Genmab has zero tolerance for any retaliation against anyone who raises concerns or partic-ipates in investigations. Retaliation includes any conduct or treatment that could discourage someone from speaking up. Genmab will protect the identity of people who participate in the Speak Up program as appropriate and consistent with applicable law. Genmab utilizes a number of methods (determined by the scenario) to protect whistleblowers from retaliation or detriment including but not limited to discrimination, harassment, physical or psychological harm, isolation, impact to employee performance and/ or compensation, damaging property, or varying employee’s role or duties. The Global Speak Up Policy can be mapped to the positive impact of protection of whistleblowers through anti-retaliation policies and procedures. Refer to the Global Speak Up Policy on our website, and S1-3 for further details. Refer to G1-3 for Genmab’s procedures to investigate business conduct matters. Anti-Fraud Policy Genmab has an internal Anti-Fraud Policy that communicates anti-fraud principles and program elements, and management’s responsibility for detecting and responding to fraud and miscon-duct. This Policy applies to all employees, officers, directors of Genmab, and management regardless of legal entity or work location, and anyone supervising the performance of services for or on behalf of Genmab, including contractors, contingent workers, agents, suppliers, and consul-tants (collectively “Genmab Person”). Genmab’s Corporate Controller is responsible for the anti-fraud policy and reports to the CFO. Genmab monitors through Internal Audit assessing the potential for fraud risk when planning indi-vidual audits. On an annual basis, a fraud risk assessment is prepared with the participation of management. This annual fraud risk assessment is presented to the Audit & Finance Committee. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 127 |
|
Sustainability Statements Data Ethics Policy The use of data, both personal and non-personal, is essential to fulfilling our core purpose, and we are committed to handling data with integrity and in an ethical and compliant manner. Genmab has developed a global data privacy program supported by a cross-functional team of global data privacy subject matter experts and appointed an external Data Privacy Officer (DPO) dedicated to GDPR compliance and oversight. Our Data Ethics Policy complies with Section 99d of the Danish Financial Statements Act, and we adopted the Data Ethics principles of the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA). As part of this commitment to ethical and responsible use of data and overall transparency, Genmab has made this Data Ethics Policy publicly available to all external stakeholders. Refer to the Data Ethics Policy on our website. The policy and its principles are anchored in our Code of Conduct as part of our overall Compliance program, and has been communicated to our management so they can share and consider it with team members. Over the past year, we focused on further embedding these principles into our operations, particularly in the areas of data privacy, DE&I, clinical trials, and the applica-tion of new technologies such as AI and machine learning. This goal was supported by efforts to evolve our data privacy governance model into a forward-looking and comprehensive global data privacy program. Genmab, in 2025, will continue to optimize and enhance its approach to respon-sible and ethical use of data in its operations through continued transformation of and invest-ment in its global data privacy program. There were no substantiated complaints concerning breaches of data privacy from individuals or data protection authorities in 2024 or 2023. Cybersecurity Program We maintain a comprehensive cybersecurity program based on the National Institute of Standards and Technology’s NIST 800 Special Publication Information Security standard (“NIST standard”) for managing cybersecurity activities, including formulation of global objectives of the cybersecurity program and risk identification and mitigation activities. Our Global Cybersecurity Program is under the leadership of the vice president and global head of cybersecurity and IT risk management who reports to the senior vice president, global head of IT & digital. The program is overseen by the Global Compliance and Risk Committee (co-chaired by our CEO and our global head of compliance and risk) and the Audit and Finance Committee. The program includes activities and projects in all five functions of the NIST standard with the goal of further improving our security profile and adapting, where needed, to changes in the business strategy and threat environment of Genmab. Input for the program comes from the annual attack and penetration test, periodic threat landscape and security maturity assess-ments, as well as requirements of applicable cybersecurity regulations. We have established a Cyber Response Task Force responsible for responding to potential cyber crisis situations that could have an impact on our Company, partners, or patients we serve. The Task Force provides assurance that our incident response and recovery capability is effective in increasing our ability to prevent, detect and respond to potential cyberattacks. We regard the protection of the Company against cybersecurity attacks as a task for everyone in the organization. Therefore, employees are provided with information about cybersecurity risks and how to detect and report security incidents in online trainings and quarterly security events. The Cybersecurity Program can be mapped to the positive impact of cybersecu-rity and global data privacy programs in place to protect the privacy of our business, our own workforce, patients and all individuals who entrust us with their information. Animal Welfare Policy At Genmab, we use research animals for the purpose of addressing important scientific questions or to fulfill a regulatory requirement. Animals have intrinsic value and experiments on animals are only carried out when no appropriate alternative method is available. Genmab has established an internal animal welfare policy and animal welfare management procedures for its locations in the U.S., Europe, Japan, and China that incorporate local laws and regulations and that reflect our high standards. We plan to harmonize these ways of working between our sites in 2025. Our policies reflect our responsible and humane use of animals in research. We actively continue to drive the implementation of the 3Rs principles (Replacement, Reduction and Refinement) in all that we do. A dedicated animal welfare officer monitors animal welfare policies and practices, ensuring we continuously refine the care and use of the animals involved in our research. In 2023, we implemented the use of low-stress handling techniques for mice, the use of optimized nesting material and started an ongoing effort to use minimally invasive techniques for the identifi-cation (marking) of rodents to improve the welfare of animals in our daily care. In 2024, we launched a Global Animal Welfare Committee who owns the policies, to explore 3R opportunities, to advise on animal welfare matters and to work towards enhancing our trans-parency regarding the use of animals in research. In addition, we started to develop internal policies describing all the elements of working with animals. Genmab provides continuing education to team members working with animals on ethical treatment of animals. Genmab performs animal welfare audits at external contractors, when required, to ensure contractors maintain comparable high standards for animal use and care as we do internally. All animal studies performed, whether internally or externally, are Genmab’s moral and ethical responsibility and we strive to apply our high standards in all that we do. Refer to G1-2 for details of the Global Procurement Policy and Global Supplier Code of Conduct. Refer to G1-3 for details of the Anti-Corruption Anti-Bribery Policy which includes disclosure of functions within Genmab that are most at risk in respect of corruption and bribery. Other Genmab policies disclosed on our website or internal Genmab policies include the Enterprise Risk Management and Resilience Policy, International Trade Controls Policy, and Tax Policy. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 128 |
|
Sustainability Statements Management of relationships with suppliers (G1-2) Genmab’s Global Procurement functions which include, R&D Contract Management, Lab Purchase Management, CMC Commercial Supply Chain and Global Procurement (collectively known as “Procurement”) are created to be a trusted value adding partner to both internal and external stakeholders across the entire Genmab value chain. Global Procurement Policy Genmab’s Global Procurement Policy is an import-ant tool utilized by our team members to steer the procurement practices and increase financial cost control and transparency within Genmab. Furthermore, the Policy helps assess and miti-gate risk, ensure selection and maintenance of high quality, regulatory compliant third parties, through a transparent, fair, and compliant pro-cess. Genmab’s CFO is responsible for the policy, which is available on Genmab’s intranet. The Global Procurement Policy states that suppliers have to be on-boarded prior to engaging in a commercial relationship to ensure timely payment. The use of purchase orders, which is also mandated by the Procurement Policy, furthermore, facilitates approval of invoiced spend, ensuring that the agreed upon payment terms are being met. Suppliers that self-identify to Genmab as small and medium enterprises (SMEs), have 30 days net as a standard payment term unless contractually agreed otherwise in writing, which is clearly stated in the Global Procurement Policy. The procurement process is set up to facili-tate on-time payments, starting with proper on-boarding of the suppliers prior to engaging in a commercial relationship, followed by a mandatory use of Purchase Requisitions and Purchase Orders (with an exception list), ensuring that, when an invoice is received it will be paid in a timely manner. Supplier Code of Conduct Our Supplier Code of Conduct articulates expec-tations for all third parties conducting work on our behalf, minimizing risks to Genmab posed by our suppliers’ activities. The Supplier Code of Conduct addresses topics that include, but are not limited to, anti-bribery and anti-corruption, privacy, trade compliance, conflicts of interest, human and labor rights, diversity, compliance with environmental laws and regulations, supply chain and animal welfare, protecting information and intellectual property, protecting physical and digital security and product compliance and quality. Our VP, Head of Global Procurement who reports to our CFO is responsible for the policy. In 2024 and 2023, all new Genmab suppliers have been required to attest to our Supplier Code of Conduct annually as part of the onboarding and contracting process. Our Global Procurement function implemented a dedicated supplier vetting tool which serves as a single point of entry for all new suppliers. Information technology & digital, quality assurance, compliance and risk, and legal functions are involved when the risk score is elevated based on a fact-based approach. Our vetting process focuses on financial health, inter-national sanctions, regulatory and reputational risks, and other key issues. Suppliers in sanctioned countries are subject to additional legal review before payments may be processed. In 2023, we established a supplier diversity program in the U.S., working closely with entities such as the Veterans Administration to align on targets. Genmab defines a diverse supplier as a >51% women, minority, or veteran-owned small business. The Supplier Master Registry in SAP has been updated in 2024 with a specific field to allow Genmab to monitor supplier diversity for the U.S. We have created our first-ever Company-wide supplier governance best practices guide to improve how we work together across lines of business, and how we partner with suppliers so all teams can successfully execute against their goals. In January 2024, contract managers, sourcing managers, procurement leads and alliance managers have been trained on how and when to apply these best practices in support of our lines of businesses’ priorities and goals. A Vendor Risk Manager is planned to be on-boarded in 2025 to ensure proper documen-tation and tracking of the initiative. All the Genmab suppliers are vetted against unethical business practices, including adverse media, U.S. and EU Sanctions lists and the Global Corruption Index as well as a review of the financial health of the third party. The Global Procurement Policy and Supplier Code of Conduct can be mapped to the positive impact of our strong management of suppliers, focused on compliance with supplier code of conduct, and targets set are the responsibility of our global procurement team. Targets for supplier management: • Acceptance of Genmab’s Supplier Code of Conduct by 80% of suppliers by spend by 2025 The target specifically supports important gover-nance topics covered in the Supplier Code of Conduct including areas like legal compliance, anti-corruption and bribery, labor practices, human rights, supply chain, animal welfare, protection of information and intellectual property/security. Genmab will monitor, review and report metrics related to this target in the next annual report. Refer to E1-4 for details of Genmab’s engage-ment plans with suppliers on Scope 3 emission reductions targets. Prevention and detection of corruption and bribery (G1-3) Anti-Bribery and Anti-Corruption (ABAC) Policy As a global company, we implement policies to mitigate risks related to bribery and corruption. Our Anti-Bribery and Anti-Corruption (ABAC) Policy educates team members on recognizing risks, emphasizes our zero-tolerance stance, and outlines reporting mechanisms for suspected misconduct. All employees receive annual ABAC training, and we are enhancing due diligence and monitoring activities for better oversight. In 2023, we updated our Quarterly Financial Disclosure Questionnaire to improve management disclo-sures regarding potential bribery and corruption. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 129 |
|
Sustainability Statements Management identifies the primary bribery and corruption risks as payments or gifts intended to secure preferential treatment for the company. Employees interacting with public officials or regulatory representatives face heightened risks, as do those dealing with healthcare providers and third parties on Genmab’s behalf, especially due to limited oversight of third-party anti-corruption practices. Reports of bribery or corruption are reviewed by the Global Compliance Program, which reports directly to the CEO. Annual summaries are provided to the Global Compliance and Risk Committee and the Board, detailing all reported incidents. We consider all functions within the business to be potentially subject to corruption and bribery and as such, 100% of Genmab employees, contractors, and Board members must complete annual Code of Conduct training, which covers ABAC, regulatory issues, conflicts of interest, and reporting mechanisms. New hires also receive ABAC training. Our Compliance program maintains a SharePoint site for business conduct policies and uses the company intranet to communicate key concepts. Every two years, business functions assess their vulnerability to corruption and report findings to the Compliance organization, which may implement further controls. Our Internal Audit function also conducts an annual fraud assess-ment. The Anti-Bribery and Anti-Corruption Policy can be mapped to the positive impact of our ethical business culture and business practices. 5.2 Business conduct metrics and targets Incidents of corruption or bribery (G1-4) Genmab defines bribery as acts designed to influence individuals to act dishonestly in the performance or discharge of their duty, and corruption as the misuse of office or power or influence for private gain. Genmab has a zero-tolerance policy for any acts of bribery or corruption by employees, contingent staff, management, officers, directors, or third-party agents or representatives. Genmab’s 24/7 Speak Up Compliance Hotline enables the anonymous reporting of behavior indicative of corruption or bribery. Our compliance team reviews these reports and supports investigations as warranted. A review of all hotline reported incidents indicates no submissions related to a Genmab employee, contingent staff, management, officer, director, or third-party agent or representative performing an act of bribery or corruption in 2024 or 2023, thus no incidents of corruption or bribery. Payment practices (G1-6) Standard payment terms are 45 days net. For certain categories of suppliers there are other possible payment terms, for example 30 days net allowed for Small and Medium Enterprises (SMEs) and 7 days net for Grants/Sponsorships and Government organizations. Genmab defines SMEs as enterprises which employ fewer than 10 persons or which have an annual turnover not exceeding EUR/USD 10 million. There were no legal proceedings for late payments in 2024, including payments to SMEs. Currently, Genmab does not have a process to track actual payment terms by main category of suppliers. However, the average time Genmab takes to pay an invoice for all suppliers with varying payment terms from the date when the contractual or statutory term of payment starts to be calculated, in number of days was 46 in 2024. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 130 |
|
Disclosure Requirement and related datapoint SFDR Reference Pillar 3 Reference Benchmark Regulation Reference EU Climate Law Reference Material/Not Material Section, Paragraph or Page Reference ESRS 2 GOV-1 Board’s gender diversity paragraph 21 (d) Indicator number 13 of Table #1 of Annex 1 Commission Delegated Regulation (EU) 2020/1816, Annex II Material GOV-1 Section ESRS 2 GOV-1 Percentage of board members who are independent paragraph 21 (e) Delegated Regulation (EU) 2020/1816, Annex II Material GOV-1 Section ESRS 2 GOV-4 Statement on due diligence paragraph 30 Indicator number 10 Table #3 of Annex 1 Material GOV-4 Section ESRS 2 SBM-1 Involvement in activities related to fossil fuel activities paragraph 40 (d) i Indicators number 4 Table #1 of Annex 1 Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 Table 1: Qualitative information on Environmental risk and Table 2: Qualitative information on Social risk Delegated Regulation (EU) 2020/1816, Annex II Not Material ESRS 2 SBM-1 Involvement in activities related to chemical production paragraph 40 (d) ii Indicator number 9 Table #2 of Annex 1 Delegated Regulation (EU) 2020/1816, Annex II Not Material ESRS 2 SBM-1 Involvement in activities related to controversial weapons paragraph 40 (d) iii Indicator number 14 Table #1 of Annex 1 Delegated Regulation (EU) 2020/1818, Article 12(1) Delegated Regulation (EU) 2020/1816, Annex II Not Material ESRS 2 SBM-1 Involvement in activities related to cultivation and production of tobacco paragraph 40 (d) iv Delegated Regulation (EU) 2020/1818, Article 12(1) Delegated Regulation (EU) 2020/1816, Annex II Not Material ESRS E1-1 Transition plan to reach climate neutrality by 2050 paragraph 14 Regulation (EU) 2021/1119, Article 2(1) Material ESRS E1-1 Section Appendix A (Derived from ESRS 2 Appendix B) Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 131 |
|
Disclosure Requirement and related datapoint SFDR Reference Pillar 3 Reference Benchmark Regulation Reference EU Climate Law Reference Material/Not Material Section, Paragraph or Page Reference ESRS E1-1 Undertakings excluded from Paris-aligned Benchmarks paragraph 16 (g) Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 Template 1: Banking book – Climate Change transition risk: Credit quality of exposures by sector, emissions and residual maturity Delegated Regulation (EU) 2020/1818, Article12.1 (d) to (g), and Article 12.2 Not Material ESRS E1-4 GHG emission reduction targets paragraph 34 Indicator number 4 Table #2 of Annex 1 Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 Template 3: Banking book – Climate change transition risk: alignment metrics Delegated Regulation (EU) 2020/1818, Article 6 Material ESRS E1-4 Section ESRS E1-5 Energy consumption from fossil sources disaggregated by sources (only high climate impact sectors) paragraph 38 Indicator number 5 Table #1 and Indicator n. 5 Table #2 of Annex 1 Not Material ESRS E1-5 Energy consumption and mix paragraph 37 Indicator number 5 Table #1 of Annex 1 Material ESRS E1-5 Section ESRS E1-5 Energy intensity associated with activities in high climate impact sectors paragraphs 40 to 43 Indicator number 6 Table #1 of Annex 1 Not Material ESRS E1-6 Gross Scope 1, 2, 3 and Total GHG emissions paragraph 44 Indicators number 1 and 2 Table #1 of Annex 1 Article 449a; Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 Template 1: Banking book – Climate change transition risk: Credit quality of exposures by sector, emissions and residual maturity Delegated Regulation (EU) 2020/1818, Article 5(1), 6 and 8(1) Material ESRS E1-6 Section Appendix A (Derived from ESRS 2 Appendix B) Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 132 |
|
Disclosure Requirement and related datapoint SFDR Reference Pillar 3 Reference Benchmark Regulation Reference EU Climate Law Reference Material/Not Material Section, Paragraph or Page Reference ESRS E1-6 Gross GHG emissions intensity paragraphs 53 to 55 Indicators number 3 Table #1 of Annex 1 Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 Template 3: Banking book – Climate change transition risk: alignment metrics Delegated Regulation (EU) 2020/1818, Article 8(1) Material ESRS E1-6 Section ESRS E1-7 GHG removals and carbon credits paragraph 56 Regulation (EU) 2021/1119, Article 2(1) Not Material ESRS E1-9 Exposure of the benchmark portfolio to climate-related physical risks paragraph 66 Delegated Regulation (EU) 2020/1818, Annex II Delegated Regulation (EU) 2020/1816, Annex II Not Material ESRS E1-9 Disaggregation of monetary amounts by acute and chronic physical risk paragraph 66 (a) ESRS E1-9 Location of significant assets at material physical risk paragraph 66 (c). Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 paragraphs 46 and 47; Template 5: Banking book—Climate change physical risk: Exposures subject to physical risk. Not Material ESRS E1-9 Breakdown of the carrying value of its real estate assets by energy-efficiency classes paragraph 67 (c). Article 449a Regulation (EU) No 575/2013; Commission Implementing Regulation (EU) 2022/2453 paragraph 34;Template 2:Banking book -Climate change transition risk: Loans collateralised by immovable property— Energy efficiency of the collateral Not Material ESRS E1-9 Degree of exposure of the portfolio to climate-related opportunities paragraph 69 Delegated Regulation (EU) 2020/1818, Annex II Not Material Appendix A (Derived from ESRS 2 Appendix B) Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 133 |
|
Disclosure Requirement and related datapoint SFDR Reference Pillar 3 Reference Benchmark Regulation Reference EU Climate Law Reference Material/Not Material Section, Paragraph or Page Reference ESRS E2-4 Amount of each pollutant listed in Annex II of the E- PRTR Regulation (European Pollutant Release and Transfer Register) emitted to air, water and soil, paragraph 28 Indicator number 8 Table #1 of Annex 1 Indicator number 2 Table #2 of Annex 1 Indicator number 1 Table #2 of Annex 1 Indicator number 3 Table #2 of Annex 1 Not Material ESRS E3-1 Water and marine resources paragraph 9 Indicator number 7 Table #2 of Annex 1 Not Material ESRS E3-1 Dedicated policy paragraph 13 Indicator number 8 Table 2 of Annex 1 Not Material ESRS E3-1 Sustainable oceans and seas paragraph 14 Indicator number 12 Table #2 of Annex 1 Not Material ESRS E3-4 Total water recycled and reused paragraph 28 (c) Indicator number 6.2 Table #2 of Annex 1 Not Material ESRS E3-4 Total water consumption in m3 per net revenue on own operations paragraph 29 Indicator number 6.1 Table #2 of Annex 1 Not Material ESRS 2—SBM 3—E4 paragraph 16 (a) i Indicator number 7 Table #1 of Annex 1 Not Material ESRS 2—SBM 3—E4 paragraph 16 (b) Indicator number 10 Table #2 of Annex 1 Not Material ESRS 2—SBM 3—E4 paragraph 16 (c) Indicator number 14 Table #2 of Annex 1 Not Material ESRS E4-2 Sustainable land/agriculture practices or policies paragraph 24 (b) Indicator number 11 Table #2 of Annex 1 Not Material ESRS E4-2 Sustainable oceans/seas practices or policies paragraph 24 (c) Indicator number 12 Table #2 of Annex 1 Not Material ESRS E4-2 Policies to address deforestation paragraph 24 (d) Indicator number 15 Table #2 of Annex 1 Not Material ESRS E5-5 Non-recycled waste paragraph 37 (d) Indicator number 13 Table #2 of Annex 1 Not Material ESRS E5-5 Hazardous waste and radioactive waste paragraph 39 Indicator number 9 Table #1 of Annex 1 Not Material Appendix A (Derived from ESRS 2 Appendix B) Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 134 |
|
Disclosure Requirement and related datapoint SFDR Reference Pillar 3 Reference Benchmark Regulation Reference EU Climate Law Reference Material/Not Material Section, Paragraph or Page Reference ESRS 2- SBM3—S1 Risk of incidents of forced labour paragraph 14 (f) Indicator number 13 Table #3 of Annex I Not Material ESRS 2- SBM3—S1 Risk of incidents of child labour paragraph 14 (g) Indicator number 12 Table #3 of Annex I Not Material ESRS S1-1 Human rights policy commitments paragraph 20 Indicator number 9 Table #3 and Indicator number 11 Table #1 of Annex I Material ESRS S1-1 Section ESRS S1-1 Due diligence policies on issues addressed by the fundamental International Labor Organisation Conventions 1 to 8, paragraph 21 Delegated Regulation (EU) 2020/1816, Annex II Material ESRS S1-1 Section ESRS S1-1 processes and measures for preventing trafficking in human beings paragraph 22 Indicator number 11 Table #3 of Annex I Material ESRS S1-1 Section ESRS S1-1 workplace accident prevention policy or management system paragraph 23 Indicator number 1 Table #3 of Annex I Material ESRS S1-1 Section ESRS S1-3 grievance/complaints handling mechanisms paragraph 32 (c) Indicator number 5 Table #3 of Annex I Material ESRS S1-3 Section ESRS S1-14 Number of fatalities and number and rate of work-related accidents paragraph 88 (b) and (c) Indicator number 2 Table #3 of Annex I Delegated Regulation (EU) 2020/1816, Annex II Material ESRS S1-14 Section ESRS S1-14 Number of days lost to injuries, accidents, fatalities or illness paragraph 88 (e) Indicator number 3 Table #3 of Annex I Material ESRS S1-14 Section ESRS S1-16 Unadjusted gender pay gap paragraph 97 (a) Indicator number 12 Table #1 of Annex I Delegated Regulation (EU) 2020/1816, Annex II Material ESRS S1-16 Section ESRS S1-16 Excessive CEO pay ratio paragraph 97 (b) Indicator number 8 Table #3 of Annex I Material ESRS S1-16 Section ESRS S1-17 Incidents of discrimination paragraph 103 (a) Indicator number 7 Table #3 of Annex I Material ESRS S1-17 Section Appendix A (Derived from ESRS 2 Appendix B) Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 135 |
|
Disclosure Requirement and related datapoint SFDR Reference Pillar 3 Reference Benchmark Regulation Reference EU Climate Law Reference Material/Not Material Section, Paragraph or Page Reference ESRS S1-17 Non-respect of UNGPs on Business and Human Rights and OECD Guidelines paragraph 104 (a) Indicator number 10 Table #1 and Indicator n. 14 Table #3 of Annex I Delegated Regulation (EU) 2020/1816, Annex II Delegated Regulation (EU) 2020/1818 Art 12 (1) Not Material ESRS 2- SBM3 – S2 Significant risk of child labour or forced labour in the value chain paragraph 11 (b) Indicators number 12 and n. 13 Table #3 of Annex I Not Material ESRS S2-1 Human rights policy commitments paragraph 17 Indicator number 9 Table #3 and Indicator n. 11 Table #1 of Annex 1 Not Material ESRS S2-1 Policies related to value chain workers paragraph 18 Indicator number 11 and n. 4 Table #3 of Annex 1 Not Material ESRS S2-1 Non-respect of UNGPs on Business and Human Rights principles and OECD guidelines paragraph 19 Indicator number 10 Table #1 of Annex 1 Delegated Regulation (EU) 2020/1816, Annex II Delegated Regulation (EU) 2020/1818, Art 12 (1) Not Material ESRS S2-1 Due diligence policies on issues addressed by the fundamental International Labor Organisation Conventions 1 to 8, paragraph 19 Delegated Regulation (EU) 2020/1816, Annex II Not Material ESRS S2-4 Human rights issues and incidents connected to its upstream and downstream value chain paragraph 36 Indicator number 14 Table #3 of Annex 1 Not Material ESRS S3-1 Human rights policy commitments paragraph 16 Indicator number 9 Table #3 of Annex 1 and Indicator number 11 Table #1 of Annex 1 Not Material ESRS S3-1 non-respect of UNGPs on Business and Human Rights, ILO principles or OECD guidelines paragraph 17 Indicator number 10 Table #1 Annex 1 Delegated Regulation (EU) 2020/1816, Annex II Delegated Regulation (EU) 2020/1818, Art 12 (1) Not Material ESRS S3-4 Human rights issues and incidents paragraph 36 Indicator number 14 Table #3 of Annex 1 Not Material Appendix A (Derived from ESRS 2 Appendix B) Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 136 |
|
Disclosure Requirement and related datapoint SFDR Reference Pillar 3 Reference Benchmark Regulation Reference EU Climate Law Reference Material/Not Material Section, Paragraph or Page Reference ESRS S4-1 Policies related to consumers and end-users paragraph 16 Indicator number 9 Table #3 and Indicator number 11 Table #1 of Annex 1 Material ESRS S4-1 Section ESRS S4-1 Non-respect of UNGPs on Business and Human Rights and OECD guidelines paragraph 17 Indicator number 10 Table #1 of Annex 1 Delegated Regulation (EU) 2020/1816, Annex II Delegated Regulation (EU) 2020/1818, Art 12 (1) Not Material ESRS S4-4 Human rights issues and incidents paragraph 35 Indicator number 14 Table #3 of Annex 1 Not Material ESRS G1-1 United Nations Convention against Corruption paragraph 10 (b) Indicator number 15 Table #3 of Annex 1 Not Material ESRS G1-1 Protection of whistle-blowers paragraph 10 (d) Indicator number 6 Table #3 of Annex 1 Material ESRS G1-1 Section ESRS G1-4 Fines for violation of anti-corruption and anti-bribery laws paragraph 24 (a) Indicator number 17 Table #3 of Annex 1 Delegated Regulation (EU) 2020/1816, Annex II) Material ESRS G1-4 Section ESRS G1-4 Standards of anti-corruption and anti-bribery paragraph 24 (b) Indicator number 16 Table #3 of Annex 1 Not Material Appendix A (Derived from ESRS 2 Appendix B) Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 137 |
|
02 Financial Statements In this section 139 Financial Statements for the Genmab Group 183 Financial Statements of the Parent Company 197 Directors’ and Management’s Statement on the Annual Report 198 Independent Auditor’s Reports Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 138 |
|
Financial Statements for the Genmab Group Introduction The financial statements in the 2024 Annual Report are grouped into the following sections: Primary Statements; Basis of Presentation; Results for the Year; Operating Assets and Liabilities; Capital Structure, Financial Risk and Related Items; and Other Disclosures. Each note to the financial statements includes information about the accounting policies applied and significant management judgements and estimates in addition to the financial numbers. Table of Contents Primary Statements 140 Consolidated Statements of Comprehensive Income 141 Consolidated Balance Sheets 142 Consolidated Statements of Cash Flows 143 Consolidated Statements of Changes in Equity Section 1 Basis of Presentation 144 1.1 Nature of the Business and Material Accounting Policies 147 1.2 New Accounting Policies and Disclosures 147 1.3 Management’s Judgements and Estimates under IFRS 148 1.4 Revision of Prior Period Financial Statements Section 2 Results for the Year 149 2.1 Revenue 151 2.2 Information about Geographical Areas 151 2.3 Staff Costs 152 2.4 Corporate and Deferred Tax 153 2.5 Profit Per Share Section 3 Operating Assets and Liabilities 154 3.1 Other Intangible Assets and Goodwill 156 3.2 Property and Equipment 158 3.3 Leases 158 3.4 Other Investments 159 3.5 Inventories 159 3.6 Receivables 160 3.7 Contract Liabilities 160 3.8 Other Payables Section 4 Capital Structure, Financial Risk and Related Items 161 4.1 Capital Management 161 4.2 Financial Risk 163 4.3 Financial Assets and Liabilities 165 4.4 Marketable Securities 166 4.5 Financial Income and Expenses 167 4.6 Share-Based Instruments 172 4.7 Share Capital Section 5 Other Disclosures 174 5.1 Remuneration of the Board of Directors and Executive Management 177 5.2 Related Party Disclosures 177 5.3 Commitments 177 5.4 Fees to Auditors Appointed at the Annual General Meeting 178 5.5 Acquisition of Businesses 180 5.6 Collaborations and Licenses 182 5.7 Contingencies 182 5.8 Subsequent Events Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 139 |
|
Primary Statements Consolidated Statements of Comprehensive Income Income Statement (DKK million) Note 2024 2023 2022 Revenue 2.1, 2.2 21,526 16,474 14,505 Cost of product sales 2.3 (985) (226) – Research and development expenses 2.3, 3.1, 3.2 (9,748) (7,630) (5,562) Selling, general and administrative expenses 2.3, 3.2 (3,790) (3,297) (2,676) Acquisition and integration related charges 5.5 (300) – – Total costs and operating expenses (14,823) (11,153) (8,238) Operating profit 6,703 5,321 6,267 Financial income 4.5 4,438 1,940 3,189 Financial expenses 4.5 (1,977) (1,624) (2,511) Net profit before tax 9,164 5,637 6,945 Corporate tax 2.4 (1,320) (1,285) (1,493) Net profit 7,844 4,352 5,452 Other comprehensive income: Amounts which may be re-classified to the income statement: Exchange differences on translation of foreign operations 430 (38) 17 Total comprehensive income 8,274 4,314 5,469 Basic net profit per share 2.5 122.21 66.64 83.38 Diluted net profit per share 2.5 121.36 66.02 82.59 Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 140 |
|
Primary Statements Consolidated Balance Sheets (DKK million) Note December 31, 2024 December 31, 2023 Assets Goodwill 3.1, 5.5 2,535 – Other intangible assets 3.1, 5.5 12,343 101 Property and equipment 2.2, 3.2 978 955 Right-of-use assets 2.2, 3.3 913 686 Receivables 2.2, 3.6 52 62 Deferred tax assets 2.4 908 212 Other investments 3.4 228 134 Total non-current assets 17,957 2,150 Corporate tax receivable 2.4 101 – Inventories 3.5 62 57 Receivables 3.6 6,590 4,947 Marketable securities 4.2, 4.4 11,243 13,268 Cash and cash equivalents 9,858 14,867 Total current assets 27,854 33,139 Total assets 45,811 35,289 Shareholders’ Equity and Liabilities Share capital 4.7 66 66 Share premium 4.7 12,590 12,461 Other reserves 490 60 Retained earnings 23,551 19,023 Total shareholders’ equity 36,697 31,610 Lease liabilities 3.3 937 680 Contract liabilities 3.7 480 480 Deferred tax liabilities 2.4 2,359 – Other payables 3.8 30 35 Total non-current liabilities 3,806 1,195 Corporate tax payable 2.4 1,710 54 Lease liabilities 3.3 92 90 Contract liabilities 3.7 24 33 Other payables 3.8 3,482 2,307 Total current liabilities 5,308 2,484 Total liabilities 9,114 3,679 Total shareholders’ equity and liabilities 45,811 35,289 Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 141 |
|
Primary Statements Consolidated Statements of Cash Flows (DKK million) Note 2024 2023 2022 Cash flows from operating activities: Net profit before tax 9,164 5,637 6,945 Financial income 4.5 (4,438) (1,940) (3,189) Financial expenses 4.5 1,977 1,624 2,511 Adjustment for non-cash transactions Share-based compensation expense 2.3, 4.6 721 586 439 Depreciation 3.2, 3.3 335 272 222 Amortization 3.1 78 23 140 Impairment charges 3.1 115 – – Change in operating assets and liabilities Receivables 3.6 (1,590) 797 (2,123) Inventories 3.5 (5) (57) – Other payables 3.8 857 622 283 Cash flows from operating activities before financial items 7,214 7,564 5,228 Interest received 935 908 283 Interest elements of lease payments 3.3 (35) (24) (15) Interest paid – (1) (1) Corporate taxes paid (343) (1,067) (1,583) Net cash provided by operating activities 7,771 7,380 3,912 Cash flows from investing activities: Acquisition of business, net of cash acquired 5.5 (12,246) – – Investment in intangible assets 3.1 (117) (10) – Investment in tangible assets 3.2 (187) (366) (317) Marketable securities bought 4.3, 4.4 (8,581) (10,876) (9,659) Marketable securities sold 4.3, 4.4 11,279 10,001 7,254 Other investments bought 3.4 (55) (31) (39) Net cash (used in) investing activities (9,907) (1,282) (2,761) Cash flows from financing activities: Warrants exercised 4.6, 4.7 129 152 280 Principal elements of lease payments 3.3 (60) (91) (73) Purchase of treasury shares 4.7 (3,879) (564) (908) Payment of withholding taxes on behalf of employees on net settled RSUs (109) (103) (88) Net cash (used in) financing activities (3,919) (606) (789) Changes in cash and cash equivalents (6,055) 5,492 362 Cash and cash equivalents at the beginning of the period 14,867 9,893 8,957 Exchange rate adjustments 1,046 (518) 574 Cash and cash equivalents at the end of the period 9,858 14,867 9,893 Cash and cash equivalents include: Bank deposits 9,776 13,514 9,299 Short-term marketable securities 82 1,353 594 Cash and cash equivalents at the end of the period 9,858 14,867 9,893 Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 142 |
|
Primary Statements Consolidated Statements of Changes in Equity (DKK million) Share capital Share premium Translation reserves Retained earnings Shareholders’ equity Balance at December 31, 2021 66 12,029 81 9,931 22,107 Net profit – – – 5,452 5,452 Other comprehensive income – – 17 – 17 Total comprehensive income – – 17 5,452 5,469 Transactions with owners: Exercise of warrants – 280 – – 280 Purchase of treasury shares – – – (908) (908) Share-based compensation expenses – – – 439 439 Withholding taxes on behalf of employees on net settled RSUs – – – (88) (88) Tax on items recognized directly in equity – – – (17) (17) Balance at December 31, 2022 66 12,309 98 14,809 27,282 Net profit – – – 4,352 4,352 Other comprehensive income – – (38) – (38) Total comprehensive income – – (38) 4,352 4,314 Transactions with owners: Exercise of warrants – 152 – – 152 Purchase of treasury shares – – – (564) (564) Share-based compensation expenses – – – 586 586 Withholding taxes on behalf of employees on net settled RSUs – – – (103) (103) Tax on items recognized directly in equity – – – (57) (57) Balance at December 31, 2023 66 12,461 60 19,023 31,610 Net profit – – – 7,844 7,844 Other comprehensive income – – 430 - 430 Total comprehensive income – – 430 7,844 8,274 Transactions with owners: Exercise of warrants – 129 – – 129 Purchase of treasury shares – – – (3,879) (3,879) Share-based compensation expenses – – – 721 721 Withholding taxes on behalf of employees on net settled RSUs – – – (109) (109) Tax on items recognized directly in equity – – – (49) (49) Balance at December 31, 2024 66 12,590 490 23,551 36,697 Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 143 |
|
Section 1 Basis of Presentation These consolidated financial statements include Genmab A/S (parent company) and subsidiaries over which the parent company has control. The Genmab consolidated Group is referenced herein as “Genmab” or the “Company.” This section describes Genmab’s general accounting policies including management’s judgements and estimates under IFRS Accounting Standards as issued by the International Accounting Standards Board (IASB) and endorsed by the EU (IFRS Accounting Standards). The specific accounting policies are described in each note in conjunction with supplementary disclo-sures of the specific item with the aim to provide a more understandable description of each accounting area. 1.1 Nature of the Business and Material Accounting Policies Genmab A/S is a publicly traded, international biotechnology company that was founded in 1999 and specializes in the creation and devel-opment of differentiated antibody therapeutics for the treatment of cancer and other diseases. Genmab has six approved products commer-cialized by third parties, two approved products that are jointly commercialized with a collabo-ration partner, a broad clinical and preclinical product pipeline and proprietary next-generation antibody technologies. The consolidated financial statements have been prepared in accordance with IFRS Accounting Standards as issued by the International Accounting Standards Board (IASB) and in accordance with IFRS as endorsed by the EU and further disclosure requirements for listed companies in Denmark. The consolidated financial statements were approved by the Board of Directors and authorized for issue on February 12, 2025. Except as outlined in Note 1.2, the consolidated financial statements have been prepared using the same accounting policies as 2023. Please refer to the overview below to see in which note/section the detailed accounting policy is included. Section 2 Results for the Year 2.1 Revenue 2.2 Information about Geographical Areas 2.3 Staff Costs 2.4 Corporate and Deferred Tax 2.5 Profit per Share Section 3 Operating Assets and Liabilities 3.1 Intangible Assets and Goodwill 3.2 Property and Equipment 3.3 Leases 3.4 Other Investments 3.5 Inventories 3.6 Receivables 3.8 Other Payables Section 4 Capital Structure, Financial Risk and Related Items 4.3 Financial Assets and Liabilities 4.4 Marketable Securities 4.5 Financial Income and Expenses 4.6 Share-Based Instruments Section 5 Other Disclosures 5.5 Acquisition of Businesses Materiality Genmab’s Annual Report is based on the concept of materiality and the Company focuses on information that is considered material and relevant to the users of the consolidated financial statements. The consolidated financial statements consist of a large number of trans-actions. These transactions are aggregated into classes according to their nature or function and presented in classes of similar items in the consolidated financial statements as required by IFRS and the Danish Financial Statements Act. If items are individually immaterial, they are aggre-gated with other items of similar nature in the consolidated financial statements or in the notes. Genmab provides these specific required disclo-sures unless the information is considered immaterial to the economic decision-making of the readers of the consolidated financial state-ments or not applicable. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 144 |
|
Consolidated Financial Statements The consolidated financial statements include Genmab A/S and subsidiaries over which the parent company has control. The parent controls a subsidiary when the parent is exposed to, or has rights to, variable returns from its involvement with the subsidiary and has the ability to affect those returns through its power to direct the activities of the subsidiary. Genmab A/S (parent company) holds invest-ments either directly or indirectly in the following subsidiaries: Name Domicile Ownership and votes 2024 Ownership and votes 2023 Genmab B.V. Utrecht, the Netherlands 100% 100% Genmab Holding B.V. Utrecht, the Netherlands 100% 100% Genmab US, Inc. New Jersey, USA 100% 100% Genmab K.K. Tokyo, Japan 100% 100% ProfoundBio, Inc. Delaware, USA 100% N/A* ProfoundBio, US Co Delaware, USA 100% N/A* Profound Limited Hong Kong, China 100% N/A* ProfoundBio co., Ltd. Suzhou, China 100% N/A* ProfoundBio Shanghai Branch, Co., Ltd. Shanghai, China 100% N/A* Beijing Puyifang Biotechnology Co., Ltd. Beijing, China 100% N/A* *These subsidiaries were added as a result of the acquisition of ProfoundBio during the second quarter of 2024. Genmab’s consolidated financial statements have been prepared on the basis of the financial statements of the parent company and subsid-iaries—prepared under Genmab’s accounting policies—by combining similar accounting items on a line-by-line basis. On consolidation, inter-company income and expenses, intercompany receivables and payables, and unrealized gains and losses on transactions between the consoli-dated companies are eliminated. The recorded value of the equity interests in the consolidated subsidiaries is eliminated with the proportionate share of the subsidiaries’ equity. Subsidiaries are consolidated from the date when control is transferred to the Group. Items included in the financial statements of Genmab’s entities are measured using the currency of the primary economic environment in which the entity operates (functional currency). The income statements for subsidiaries with a different functional currency than Genmab’s presentation currency are translated into Genmab’s presentation currency at average exchange rates, and the balance sheets are trans-lated at the exchange rate in effect at the balance sheet date. Exchange rate differences arising from the trans-lation of foreign subsidiaries shareholders’ equity at the beginning of the year and exchange rate differences arising as a result of foreign subsid-iaries’ income statements being translated at average exchange rates are recorded in transla-tion reserves in shareholders’ equity. Functional and Presentation Currency The consolidated financial statements have been prepared in Danish Kroner (DKK), which is the functional and presentation currency of the parent company. Foreign Currency Transactions in foreign currencies are translated at the exchange rates in effect at the date of the transaction. Exchange rate gains and losses arising between the transaction date and the settlement date are recognized in the Consolidated Statements of Comprehensive Income as financial income or expense. Unsettled monetary assets and liabilities in foreign currencies are translated at the exchange rates in effect at the balance sheet date. Exchange rate gains and losses arising between the transaction date and the balance sheet date are recognized in the Consolidated Statements of Comprehensive Income as financial income or expense. Classification of Costs and Operating Expenses in the Income Statement Cost of Product Sales Cost of product sales includes direct and indirect costs relating to the manufacturing of inventory mainly from third-party providers of manufacturing as well as costs related to internal resources and distribution and logistics. Inventory amounts written down as a result of excess or obsolescence are charged to cost of product sales. Also included in Cost of Product Sales are royalty payments on commercialized products. Aside from these items, there are no other costs included within cost of product sales. Additionally, cost of product sales includes profit-sharing amounts owed to collaboration partners for the sale of commercial products when Genmab is determined to be the principal in sales to end customers. The only profit-sharing amounts owed to collaboration partners that are recorded as cost of product sales relate to sales of EPKINLY in the U.S. and Japan pursuant to the Collaboration Agreement with AbbVie. Refer to Note 5.6 in the Annual Report for detailed information regarding Genmab’s Collaboration Agreement with AbbVie. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 145 |
|
Research and Development Expenses Research and development expenses primarily include salaries, benefits and other employee-related costs of Genmab’s research and development staff, license costs, manufacturing costs, preclinical costs, clinical trials, contrac-tors and outside service fees, amortization and impairment of licenses and rights related to intangible assets, depreciation of property and equipment, and depreciation of right-of-use assets, to the extent that such costs are related to the Group’s research and development activities. Refer to Note 3.1 for a more detailed description on the treatment of Genmab’s research and development expenses. Selling, General and Administrative Expenses Selling, general and administrative expenses relate to the management and administration of Genmab, including commercialization activities. This primarily includes salaries, benefits and other employee costs related to management and support functions including human resources, information technology and the finance depart-ments. In addition, depreciation of property and equipment and depreciation of right-of-use assets, to the extent such expenses are related to administrative functions, are also included. Selling, general and administrative expenses are recognized in the Consolidated Statements of Comprehensive Income in the period to which they relate. Acquisition and Integration Related Charges Acquisition and integration related charges for the acquisition of ProfoundBio which occurred during the second quarter of 2024. Refer to Note 5.5 for more information regarding Genmab’s Acquisition and Integration costs related to the acquisition of ProfoundBio. Government Grants Government grants are recognized at their fair value where there is reasonable assurance that the grant will be received and that Genmab will comply with all attaching conditions. When the grant relates to an expense item, it is recognized as a reduction of that expense on a systematic basis over the periods that the costs for which it is intended to compensate are incurred. Where the grant relates to an asset, the fair value is credited to a contract liability account and is released to the statement of comprehensive income as other operating income over the expected useful life of the relevant asset by equal annual installments. Statements of Cash Flows The cash flow statement is presented using the indirect method with basis in the net profit before tax. Cash flows from operating activities are stated as the net profit before tax adjusted for financial income and expense, non-cash operating items including depreciation, amortization, impairment losses, share-based compensation expenses, and for changes in operating assets and liabili-ties, interest paid and received, interest elements of lease payments and corporate taxes paid or received. Operating assets and liabilities are mainly comprised of changes in receivables, inventories and other payables excluding the items included in cash and cash equivalents. Changes in non-current assets and liabilities are included in operating assets and liabilities, if related to the main revenue-producing activities of Genmab. Cash flows from investing activities consist of acquisitions of businesses, net of cash acquired, purchases and sales of marketable securities and other investments, as well as purchases of intan-gible assets and property and equipment. Cash flows from financing activities relate to the purchase of treasury shares, exercise of warrants, payments of withholding taxes on behalf of employees on net settled RSUs and payments of long-term loans including installments on lease liabilities. Cash and cash equivalents are comprised of cash, bank deposits, and marketable securities with a maturity of less than 90 days on the date of acquisition. The statements of cash flows cannot be derived solely from the consolidated financial statements. Treasury Shares The total amount paid to acquire treasury shares including directly attributable costs and the proceeds from the sale of treasury shares is recognized in retained earnings. Collaborations, License Agreements and Collaborative Agreements Collaborations and License Agreements Genmab continues to pursue the establishment of research collaborations and licensing agree-ments. These arrangements often include upfront payments, expense reimbursements or payments to the collaboration partner, and milestone and royalty arrangements, contingent upon the occurrence of certain future events linked to the success of the asset in development. In regard to Genmab’s license agreements with J&J, Novartis and Roche, each of these parties retain final decision-making authority over the relevant activities and as such no joint control exists. Refer to Note 2.1 for additional information related to revenue from these parties. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 146 |
|
Collaborative Agreements Genmab has entered into a number of joint collaborative agreements. These agreements often include upfront payments, expense reim-bursements or payments to the collaboration partner, and milestone and royalty arrangements, contingent upon the occurrence of certain future events linked to the success of the asset in development. These agreements also provide Genmab with varying rights to develop, produce and market products together with its collaborative partners. Both parties in these arrangements share in the decision-making and therefore have joint control of the arrangement. In 2024, Genmab’s more significant collaboration agreements are with AbbVie (epcoritamab), Pfizer (tisotumab vedotin) and BioNTech. Refer to Note 2.1 for additional information related to revenue from our joint collabora-tive agreements. Refer to Note 5.6 for detailed information regarding Genmab’s significant Research Collaborations, License Agreements and Collaborative Agreements. 1.2 New Accounting Policies and Disclosures New Accounting Policies and Disclosures for 2024 Genmab has, with effect from January 1, 2024, implemented the following standards and amendments: • Amendments to IFRS 16 Leases: Lease Liability in a Sale and Leaseback • Amendments to IAS 1 Presentation of Financial Statements: Classification of Liabilities as Current or Non-current, Classification of Liabilities as Current or Non-current—Deferral of Effective Date, and Non-current Liabilities with Covenants, and • Amendments to IAS 7 Statement of Cash Flows and IFRS 7 Financial Instruments: Disclosures: Supplier Finance Arrangements The implementation of these amendments did not have a material impact on the consolidated financial statements for the current or prior reporting periods and is not expected to have a significant impact in future reporting periods. New Accounting Policies and Disclosures Effective in 2025 or Later Furthermore, as it relates to new or amended accounting standards and interpretations (IFRSs) issued by the IASB, management does not anticipate any significant impact on the Consolidated Financial Statements in the period of initial application from the adoption of these new standards and amend-ments, apart from IFRS 18 ‘Presentation and Disclosure in Financial Statements’ which replaces IAS 1 effective from 1 January 2027. The new IFRS 18 is expected to change the presentation of the financial statements, requiring items of income and expense to be classified into five categories: operating, investing, finance, income taxes and discon-tinued operations along with two new mandatory sub-totals, operating profit or loss and profit or loss before financing and income taxes. IFRS 18 will not impact the recognition or measurement of items in the financial statements. 1.3 Management’s Judgements and Estimates under IFRS In preparing financial statements under IFRS, certain provisions in the standards require management’s judgements, including various accounting estimates and assumptions. These judgements and estimates affect the applica-tion of accounting policies, as well as reported amounts within the consolidated financial state-ments and disclosures. Determining the carrying amount of certain assets and liabilities requires judgements, estimates and assumptions concerning future events that are based on historical experience and other factors, which by their very nature are associated with uncertainty and unpredictability. Accounting estimates are based on historical experience and various other factors relative to the circumstances in which they are applied. Estimates are generally made based on informa-tion available at the time. Accounting judgements are made in the process of applying accounting policies. These judgements are typically made based on the guidance and information available at the time of application. These estimates and judgements may prove incomplete or incorrect, and unexpected events or circumstances may arise. Genmab is also subject to risks and uncertainties which may lead actual results to differ from these estimates, both positively and negatively. Specific risks for Genmab are discussed in the relevant section of this Annual Report and in the notes to the consol-idated financial statements. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 147 |
|
The areas involving a high degree of judgement and estimation that are significant to the consoli-dated financial statements are summarized below. Refer to the identified notes for further information on the key accounting estimates and judgements utilized in the preparation of the consolidated financial statements. Accounting policy Key accounting estimates and judgements Note reference Risk Revenue recognition Judgement in assessing whether a collaboration partner is a customer Estimation of partner net sales amounts in the calculation of royalties Estimation of variable consideration Judgement in assessing the nature of combined performance obligations within contracts Note 2.1 High Share-based compensation Judgement in selecting assumptions required for valuation of warrant grants Estimation in developing forfeiture rate RSUs/warrants and probability of achievement for PSUs Note 4.6 Moderate Current and deferred income taxes Judgement and estimation regarding valuation of deferred income taxes Note 2.4 Moderate Fair value and impairment assessment of other intangible assets and goodwill Estimation of the fair value of other intangible assets and assessment of impairment of other intangible assets Estimation regarding the valuation of goodwill and assessment of impairment of goodwill Notes 3.1 and 5.5 High 1.4 Revision of Prior Period Financial Statements To facilitate comparison of information across periods, certain reclassifications and revisions have been made to prior period financial income and expense amounts to conform to the current period’s appropriate presentation. Refer to Note 4.5 for additional information relating to financial income and expenses of the Group and Note 14 in the parent financial statements relating to financial income and expenses of the Parent. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 148 |
|
Section 2 Results for the Year This section includes disclosures related to revenue, information about geographical areas, staff costs, corporate and deferred tax, and profit per share. 2.1 Revenue (DKK million) 2024 2023 2022 Revenue by type: Royalties 17,352 13,705 11,582 Reimbursement revenue 996 864 818 Milestone revenue 1,000 1,177 1,767 Collaboration revenue 433 307 332 Net product sales 1,743 421 – License revenue 2 – 6 Total 21,526 16,474 14,505 Revenue by collaboration partner: Janssen 14,422 11,949 10,530 AbbVie 394 732 1,174 Roche 741 704 796 Novartis 2,822 1,511 815 BioNTech 869 784 708 Pfizer1 533 373 413 Other 2 – 69 Total2 19,783 16,053 14,505 Royalties by product: DARZALEX 13,922 11,265 9,966 Kesimpta 2,222 1,494 779 TEPEZZA 737 704 796 Other3 471 242 41 Total 17,352 13,705 11,582 1. Pzifer acquired Seagen in December 2023 2. Excludes Genmab’s Net product sales 3.Other consists of royalties from net sales of RYBREVANT, TECVAYLI, TALVEY and TEPKINLY Accounting Policies Genmab recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration that it expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that Genmab determines are within the scope of IFRS 15, Genmab performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. Genmab only applies the five-step model to contracts when it is probable that the Company will collect the consideration it is entitled to in exchange for the goods or services it transfers to the customer. At contract inception, once the contract is determined to be within the scope of IFRS 15, Genmab assesses the goods and services promised within each contract and identifies as a performance obligation each good or service that is distinct. Revenue is recognized in the amount of the transaction price that is allocated to the respective performance obli-gation when (or as) the performance obligation is satisfied. Royalties: Certain of Genmab’s license and collaboration agreements include sales-based royalties based on the level of sales. The license has been deemed to be the predominant item to which the royalties relate under Genmab’s license and collaboration agreements. As a result, Genmab recognizes revenue when the related sales occur. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 149 |
|
Reimbursement Revenue for R&D Services: Genmab’s research collaboration agreements include provisions for reimbursement or cost sharing for R&D services and payment for full time equivalents (“FTEs”) at contractual rates. R&D services are performed and satisfied over time given that the customer simultaneously receives and consumes the benefits provided by Genmab and revenue for research services is recognized over time rather than at a point in time. Milestone Revenue: Certain of Genmab’s license and collaboration agreements include devel-opment, regulatory and commercial milestone payments based on the level of sales. At the inception of each arrangement that includes milestone payments, Genmab evaluates whether the achievement of milestones is considered highly probable and estimates the amount to be included in the transaction price using the most likely amount method. If it is highly probable that a significant revenue reversal would not occur, the associated milestone value is included in the transaction price. Milestone payments that are not within the control of Genmab or the license and collaboration partner, such as regulatory approvals, are not considered probable of being achieved until those approvals are received. The transaction price is then allocated to each performance obligation on a relative stand-alone selling price basis, for which Genmab recognizes revenue as or when the performance obligations under the contract are satisfied. At the end of each subsequent reporting period, Genmab re-evaluates the probability of achievement of such development milestones and commercial milestones and any related constraint, and if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue and earnings in the period of adjustment. Under all of Genmab’s existing license and collaboration agreements, milestone payments have been allocated to the license transfer performance obligation. License Revenue for Intellectual Property: If the license to Genmab’s functional intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, Genmab recognizes revenues from non-refundable upfront fees allocated to the license at the point in time the license is trans-ferred to the licensee and the licensee is able to use and benefit from the license. For licenses that are bundled with other promises, Genmab utilizes judgement to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, upfront fees. Under all of Genmab’s existing license and collaboration agreements the license to functional intellectual property has been determined to be distinct from other perfor-mance obligations identified in the agreement. Collaboration Revenue: Collaboration revenue includes the result of profit sharing arrangements for the sale of commercial products by our collab-oration partners. When Genmab’s collaboration partner is determined to be the principal in sales to end customers, Genmab’s share of profits for the sale of commercial products is included in collaboration revenue. Net Product Sales: Revenue from the sale of goods is recognized when control is transferred to the customer and it is probable that Genmab will collect the consideration to which it is entitled for transferring the products. Control of the products is transferred at a single point in time which occurs upon delivery to the customer. The amount of sales to be recognized is based on the consideration Genmab expects to receive in exchange for its goods. When sales are recognized, an estimate for a variety of sales deductions is also recorded such as cash discounts, government rebates, chargebacks, wholesaler fees, other rebates and administrative fees, sales returns and allowances and other sales discounts. Sales deductions are estimated and recognized as a reduction of gross product sales to arrive at net product sales, by assessing the expected value of the sales deductions (variable consideration). Sales deductions are estimated and provided for at the time the related sales are recorded. Genmab’s estimates related to sales deductions require significant use of estimates as not all conditions are known at the time of sale. The estimates are based on analyses of existing contractual obligations, historical experience, drug product analogs and payer channel mix. Genmab considers the provisions established for sales deductions to be reason-able and appropriate based on currently available information; however, the actual amount of deductions may differ from the amounts estimated by management as more information becomes available. Estimates will be assessed each period and adjusted as required based on updated information and actual experience. When Genmab is determined to be the principal in sales to end customers, all product sales are included in net product sales in the Consolidated Statements of Comprehensive Income. As of December 31, 2024, all net product sales relate to sales of EPKINLY in the U.S. and Japan pursuant to the Collaboration Agreement with AbbVie. Refer to Note 5.6 for detailed information regarding Genmab’s significant Research Collaborations, License Agreements and Collaborative Agreements. Refer to Note 1.3 for management’s judgements and estimates related to revenue recognition. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 150 |
|
2.2 Information about Geographical Areas Genmab is managed and operated as one business unit, which is reflected in the organizational structure and internal reporting. No separate lines of business or separate business entities have been identified with respect to any licensed products, marketed products, product candidates or geograph-ical markets and no segment information is currently prepared for internal reporting. Accordingly, it has been concluded that it is not relevant to include segment disclosures in the financial statements as Genmab’s business activities are not organized on the basis of differences in related product and geographical areas. Revenue Non-current assets Revenue Non-current assets Revenue Non-current assets (DKK million) 2024 2023 2022 Denmark 19,783 12,710 16,053 496 14,505 211 Netherlands – 767 – 874 – 793 United States 902 3,196 380 378 – 442 Japan 841 100 41 56 – 70 China – 48 – – – – Total 21,526 16,821 16,474 1,804 14,505 1,516 Out of total non-current assets of DKK 16,821 million, DKK 12,340 million relates to intangible assets in Denmark and DKK 2,535 million relates to goodwill in the United States acquired as a part of the acquisition of ProfoundBio. Accounting Policies Geographical information is presented for Genmab’s revenue and non-current assets. Revenue is attributed to countries on the basis of the location of the legal entity holding the contract with the counterparty. Non-current assets comprise intangible assets, goodwill, property and equipment, right-of-use assets, and receivables. 2.3 Staff Costs (DKK million) 2024 2023 2022 Wages and salaries 3,168 2,631 1,913 Share-based compensation 721 586 439 Defined contribution plans 205 170 112 Other social security costs 399 335 263 Government grants related to research and development expenses (149) (174) (144) Total 4,344 3,548 2,583 Staff costs are included in the Consolidated Statements of Comprehensive Income as follows: Cost of product sales 11 3 – Research and development expenses 2,520 2,004 1,518 Selling, general and administrative expenses 1,813 1,541 1,065 Total 4,344 3,548 2,583 Average number of FTE 2,535 2,011 1,460 Number of FTE at year-end 2,682 2,204 1,660 Refer to Note 4.6 for additional informa-tion regarding share-based instruments and Note 5.1 for additional information regarding the remuneration of the Board and Executive Management. Accounting Policies Staff Costs Wages and salaries, other social security costs, paid leave and bonuses, and other employee benefits are recognized in the financial year in which the employee performs the associ-ated work. Genmab’s pension plans are classified as defined contribution plans and, accordingly, no pension obligations are recognized in the balance sheet. Costs relating to defined contribution plans are included in the income statement in the period in which they are accrued, and outstanding contri-butions are included in other payables. Termination benefits are recognized as an expense, when Genmab is committed demonstrably, without realistic possibility of withdrawal, to a formal detailed plan to terminate employment. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 151 |
|
2.4 Corporate and Deferred Tax Taxation—Income Statement & Shareholders’ Equity (DKK million) 2024 2023 2022 Current tax on profit 1,799 1,301 1,478 Adjustment to deferred tax 99 (59) 107 Net Increase (decrease) of unrecognized deferred tax assets for the year (578) 43 (92) Total tax for the period in the income statement 1,320 1,285 1,493 (DKK million) 2024 2023 2022 Net profit before tax 9,164 5,637 6,945 Tax at the Danish corporation tax rate of 22% for all periods 2,016 1,240 1,528 Tax effect of: Net Increase (decrease) of unrecognized deferred tax assets for the year (579) 43 (92) Net of non-taxable income over non-deductible expenses 92 7 73 Other current and deferred tax adjustments (209) (5) (16) Total tax effect (696) 45 (35) Total tax for the period in the income statement 1,320 1,285 1,493 Total tax for the period in shareholders’ equity 49 57 (22) Effective Tax Rate 14.4% 22.8% 21.5% Corporate tax consists of current tax and the adjustment of deferred taxes during the year. The corporate tax expense was DKK 1,320 million in 2024, DKK 1,285 million in 2023 and DKK 1,493 million in 2022. Tax benefits of DKK 49 million in 2024, DKK 57 million in 2023 and tax expenses of DKK 22 million in 2022, related to excess tax benefits for share-based compensation were recorded directly in share-holders’ equity. As a result of the ProfoundBio integration activ-ities, Genmab utilized approximately DKK 2.2 billion of previously unrecognized tax losses during 2024. Genmab operates in multiple jurisdictions which have enacted new legislation to implement the global minimum top-up tax, which became effective on January 1, 2024. Under this legisla-tion, the Company is liable to pay a top-up tax for the difference between its GloBE Effective Tax Rate per jurisdiction and the minimum rate of 15 percent. The rules have no impact on the tax position of Genmab in 2024. Taxation—Balance Sheet Significant components of the deferred tax (liabilities) assets are as follows: (DKK million) 2024 2023 Share-based instruments 270 41 Deferred revenue 120 113 Intangible assets (2,478) – Other temporary differences 637 58 Total at December 31 (1,451) 212 Genmab recognizes deferred tax assets if it is probable that sufficient taxable income will be available in the future. Management has considered future taxable income and applied its judgement in assessing whether deferred tax assets should be recognized. The difference between the deferred tax liability as of December 31, 2024 and the deferred tax liability acquired as part of the acquisition of ProfoundBio relates to the reestablishment of the deferred tax liability as a result of the transfer of intangible assets from ProfoundBio US to Genmab A/S during the fourth quarter of 2024. As of December 31, 2024, Genmab had estimated gross unrecognized tax loss carryforwards in the Netherlands of DKK 0.7 billion to reduce future taxable income. As of December 31, 2023, Genmab had estimated gross unrecognized tax loss carryforwards in the U.S. and in the Netherlands of DKK 2.1 billion and DKK 0.5 billion, respectively. The tax losses in the Netherlands available as of December 31, 2024, can be carried forward indefinitely. Accounting Policies Corporate Tax Corporate tax, which consists of current tax and deferred taxes for the year, is recognized in the income statement, except to the extent that the tax is attributable to items which directly relate to shareholders’ equity or other comprehen-sive income. Current tax assets and liabilities for current and prior periods are measured at the amounts expected to be recovered from or paid to the tax authorities. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 152 |
|
Deferred Tax Deferred tax accounting requires recognition of deferred tax on all temporary differences between the carrying amount of assets and liabilities and the tax base of such assets and liabilities. This includes the tax value of certain tax losses carried forward. Deferred tax is calculated in accordance with the tax regulations in the local countries and the tax rates expected to be in force at the time the deferred tax is utilized. Changes in deferred tax as a result of changes in tax rates are recognized in the income statement. Deferred tax assets resulting from temporary differences, including the tax value of losses to be carried forward, are recognized only to the extent that it is probable that future taxable profit will be available against which the differences can be utilized. Deferred tax liabilities are recognized for taxable temporary differences that arise when the carrying amount of an asset exceeds its tax basis or the carrying amount of a liability is less than its tax base. Management’s Judgements and Estimates Deferred Tax Genmab recognizes deferred tax assets if management assesses that these tax assets can be offset against positive taxable income within the foreseeable future. This judgement is made on an ongoing basis and is based on numerous factors, including actual results, budgets, and business plans for the coming years. Realization of deferred tax assets is dependent upon a number of factors, including estimated future taxable earnings, the timing and amount of which are highly uncertain. A significant portion of Genmab’s future taxable income will be driven by future events that are highly susceptible to factors outside the control of Genmab including overall commercial growth , specific clinical outcomes, regulatory approvals, advancement of Genmab’s product pipeline and other matters. As such, changes in estimates of future taxable income could impact Genmab’s future taxable income in a positive or negative manner. As a result of the ProfoundBio integration activities, Genmab, based on current business plans and estimates of future taxable income, recognized a significant portion of previously unrecognized deferred tax assets during 2024. 2.5 Profit Per Share (DKK million) 2024 2023 2022 Net profit 7,844 4,352 5,452 (Shares) Weighted average number of shares outstanding 66,139,029 66,023,437 65,783,130 Weighted average number of treasury shares (1,952,382) (713,693) (395,829) Weighted average number of shares excl. treasury shares 64,186,647 65,309,744 65,387,301 Adjustments for share-based instruments, dilution 446,293 604,961 622,303 Weighted average number of shares, diluted 64,632,940 65,914,705 66,009,604 Basic net profit per share 122.21 66.64 83.38 Diluted net profit per share 121.36 66.02 82.59 In the calculation of the diluted net profit per share for 2024, 788,967 potential ordinary shares related to share-based instruments have been excluded as they are anti-dilutive, compared to 248,649 and 68,728 for 2023 and 2022, respectively. Accounting Policies Basic Net Profit per Share Basic net profit per share is calculated as the net profit for the period divided by the weighted average number of outstanding ordinary shares, excluding treasury shares. Diluted Net Profit per Share Diluted net profit per share is calculated as the net profit for the period divided by the weighted average number of outstanding ordinary shares, excluding treasury shares and adjusted for the dilutive effect of share equivalents. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 153 |
|
Section 3 Operating Assets and Liabilities This section covers the operating assets and related liabilities that form the basis for Genmab’s activities. Deferred tax assets and liabilities are included in Note 2.4. Assets related to Genmab’s financing activities are shown in section 4. 3.1 Other Intangible Assets and Goodwill Intangible Assets The increase in the gross carrying value of other intangible assets during 2024 was primarily due to the addition of DKK 10,577 million of in-process research and development (IPR&D) and DKK 1,243 million of a technology platform asset from the ProfoundBio acquisition. The technology platform asset is being amortized over its estimated useful life of 15 years. Refer to Note 5.5 for additional details. (DKK million) Goodwill Licenses and Patents Technology Platform Acquired IPR&D Total Intangible Assets 2024 Cost at the beginning of the year – 901 – – 901 Additions during the year 2,436 163 1,243 10,577 14,419 Effect of exchange rate adjustment 99 – 44 369 512 Cost at the end of the year 2,535 1,064 1,287 10,946 15,832 Amortization and impairment losses at the beginning of the year – 800 – – 800 Amortization for the year – 25 53 – 78 Impairment losses for the year – 76 – – 76 Amortization and impairment losses at the end of the year – 901 53 – 954 Carrying amount at the end of the year 2,535 163 1,234 10,946 14,878 2023 Cost at the beginning of the year – 891 – – 891 Additions during the year – 10 – – 10 Cost at the end of the year – 901 – – 901 Amortization and impairment losses at the beginning of the year – 745 – – 745 Amortization for the year – 55 – – 55 Amortization and impairment losses at the end of the year – 800 – – 800 Carrying amount at the end of the year – 101 – – 101 Impairment losses for the year related to licenses and patents, which were not material, were recorded in Research and development expenses in the Consolidated Statements of Comprehensive Income. Amortization expense was DKK 78 million, DKK 55 million, and DKK 108 million for 2024, 2023 and 2022, respectively, which was recorded in Research and development expenses in the Consolidated Statements of Comprehensive Income. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 154 |
|
Goodwill The carrying amount of goodwill was DKK 2,535 million as of December 31, 2024, due to the acquisition of ProfoundBio (refer to Note 5.5). No impairment of goodwill was recognized in 2024 as the annual impairment test showed that the estimated recoverable amount exceeded the carrying amount of the single cash-generating unit (CGU) to which all of Genmab’s goodwill was allocated. There was no goodwill balance as of December 31, 2023. Accounting Policies Research and Development Projects Internal and subcontracted research costs are charged in full to research and development expenses in the Consolidated Statements of Comprehensive Income in the period in which they are incurred. Development costs are also expensed until regulatory approval is obtained or is probable. Genmab has no internally generated intangible assets from development, as the criteria for recognition of an intangible asset are not met. Genmab acquires licenses and rights primarily to gain access to targets and technologies iden-tified by third parties. Payments to third parties under collaboration and license agreements are assessed to determine whether such payments should be expensed as incurred as research and development expenses or capitalized as an intangible asset. Licenses and rights that meet the criteria for capitalization as intangible assets are measured at cost less accumulated amor-tization and any impairment losses. Milestone payments related to capitalized licenses and rights are accounted for as an increase in the cost to acquire licenses and rights. For acquired research and development projects, and intellectual property rights, including acquisition in a business combination, the likelihood of obtaining future commercial sales is reflected in the cost of the asset, and thus the probability recognition criteria is always considered to be satisfied. As the cost of acquired research and development projects can often be measured reliably, these projects fulfil the capitalization criteria as intangible assets on acquisition. Development costs incurred subsequent to acquisition are treated consistently with internal project development costs. Goodwill Goodwill represents the excess of purchase price over the fair value of net identifiable assets acquired and liabilities assumed in a business combination accounted for by the acquisition method of accounting. Goodwill is allocated to each of the group’s CGU (or groups of CGUs) expected to benefit from the synergies of the combination. Genmab consists of one single CGU which represents its single operating segment. Recognition and Measurement Intangible assets are initially measured at cost and are subsequently measured at cost less any accumulated amortization and any impairment loss. Goodwill is not amortized but is subject to impairment testing. For intellectual property rights acquired for research and development projects, upfront fees and acquisition costs are capitalized as the historical cost. Subsequent milestone payments payable on achievement of a contingent event will be capitalized when the contingent event being achieved is probable. Intangible assets acquired in a business combination are recognized at fair value at the acquisition date. Amortization Intangible assets with definite useful lives are amortized based on the straight-line method over their estimated useful lives. This corresponds to the legal duration or the economic useful life depending on which is shorter. The amortization of intellectual property rights, including IPR&D, commences after regulatory approval has been obtained or when assets are put in use. Impairment Goodwill and intangible assets not yet available for use (IPR&D) are tested for impairment when indicators of impairment exist. However, they are tested at least annually, irrespective of whether there is any indication that they may be impaired. If circumstances or changes in Genmab’s oper-ations indicate that the carrying amount of Goodwill, IPR&D or definite-lived intangible assets may not be recoverable, management performs an impairment test of the asset for impairment. Amortization, impairment losses, and gains or losses on the disposal of other intangible assets related to licenses and rights are recognized in Consolidated Statements of Comprehensive Income as research and development expenses. Management’s Judgments and Estimates Impairment Assessment of Goodwill and Other Intangible Assets CGUs to which goodwill has been allocated are tested for impairment at least annually, or more frequently when there is an indication that the unit may be impaired by assessing qualitative factors or performing a quantitative analysis. Goodwill is monitored for impairment at the operating segment level, which is the lowest level CGU to which goodwill is allocated and monitored by Management. Goodwill impairment tests are based on management’s estimate of recoverable amount determined as the greater of the fair value less cost to sell, or its value in use. Value in use is calculated based on a multiple applied on steady earnings from oper-ations before tax generated from the CGU. If the carrying amount of goodwill exceeds the recov-erable amount, any impairment is measured as the difference between the recoverable amount and the carrying amount. Any impairment is first allocated to reduce the carrying amount of goodwill and any exceeding amount is allocated pro-rata to Genmab’s other assets in the CGU in the scope of IAS 36 but not less than their recoverable amount. An impairment loss is recognized in the Consolidated Statements of Comprehensive Income when the impairment is identified. Impairments of goodwill are prohibited from future reversals. As permitted under IAS 36 (Impairment of Assets), use of a short-cut quantitative impair-ment test may be relied on if conservative assumptions are utilized which would result in an underestimation of the recoverable amount. By applying a multiple of four on the previous twelve consecutive months operating profit before tax the estimated recoverable amount is higher than the carrying amount of the CGU. It is management’s assessment that a multiple of four is a conservative assumption compared to market observations. The operating profit before tax for the previous twelve consecutive months is based on recurring earnings and is considered a conser-vative measure of earnings for the next four years compared to the budget and forecast prepared by the Group. Thus, management has concluded that there is no impairment on goodwill. As Genmab has a single CGU with a quoted market price of Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 155 |
|
the entire Group (level 1 observable input), a high-level comparison between Genmab’s market capitalization and the recoverable amount was also performed to reaffirm the reasonableness of the short-cut approach to estimate recover-able amount. The carrying amount of the single CGU includes Genmab’s net assets, less cash and marketable securities as returns on such balances are not included in operating profit before tax and therefore are not included in the recoverable amount either. The basis for the review of IPR&D impairment is also the recoverable amount. If the carrying amount of an intangible asset is greater than the recoverable amount, the intangible asset is written down to the recoverable amount. An impairment loss is recognized in the Consolidated Statements of Comprehensive Income when the impairment is identified. Impairments on intan-gible assets are reviewed at each reporting date for possible reversal. Factors considered material that could trigger an impairment test include the following: • Development of a competing drug • Realized sales trending below predicted sales • Inconsistent or unfavorable clinical readouts • Changes in the legal framework covering patents, rights, and licenses • Advances in medicine and/or technology that affect the medical treatments • Adverse impact on reputation and/or brand names 3.2 Property and Equipment (DKK million) Leasehold improvements Equipment, furniture and fixtures Assets under construction Total property and equipment 2024 Cost at January 1 684 908 39 1,631 Additions for the year 5 81 116 202 Acquisitions through business combinations 11 41 – 52 Transfers between the classes 10 38 (48) – Disposals for the year (5) (86) (4) (95) Exchange rate adjustment 16 9 – 25 Cost at December 31 721 991 103 1,815 Accumulated depreciation and impairment at January 1 (194) (482) – (676) Depreciation for the year (75) (158) – (233) Exchange rate adjustment (7) (4) – (11) Accumulated depreciation on disposals 5 78 – 83 Accumulated depreciation and impairment at December 31 (271) (566) – (837) Carrying amount at December 31 450 425 103 978 Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 156 |
|
(DKK million) Leasehold improvements Equipment, furniture and fixtures Assets under construction Total property and equipment 2023 Cost at January 1 412 649 233 1,294 Additions for the year 6 129 222 357 Transfers between the classes 276 134 (410) – Disposals for the year – – (6) (6) Exchange rate adjustment (10) (4) – (14) Cost at December 31 684 908 39 1,631 Accumulated depreciation and impairment at January 1 (132) (363) – (495) Depreciation for the year (64) (121) – (185) Exchange rate adjustment 2 2 – 4 Accumulated depreciation and impairment at December 31 (194) (482) – (676) Carrying amount at December 31 490 426 39 955 (DKK million) 2024 2023 2022 Depreciation and impairment included in the income statement as follows: Research and development expenses 197 140 108 Selling, general and administrative expenses 36 45 38 Total 233 185 146 Capital expenditures in 2024 were primarily related to the expansion Genmab’s facilities in the United States and Japan. Capital expenditures in 2023 were primarily related to the expansion of our facilities in the Netherlands and our new headquarters in Denmark. Accounting Policies Property and equipment is comprised of leasehold improvements, assets under construc-tion, and equipment, furniture, and fixtures, which are measured at cost less accumulated depreciation and any impairment losses. The cost is comprised of the acquisition price and direct costs related to the acquisition until the asset is ready for use. Costs include direct costs and costs to subcontractors. Depreciation Depreciation is calculated on a straight-line basis to allocate the cost of the assets, net of any residual value, over the estimated useful lives, which are as follows: Equipment, furniture, and fixtures 3–5 years Leasehold improvements 15 years or the lease term, if shorter Depreciation commences when the asset is available for use, including when it is in the location and condition necessary for it to be capable of operating in the manner intended by management. The useful lives and residual values are reviewed and adjusted if appropriate on a yearly basis. Assets under construction are not depreciated. Impairment If circumstances or changes in Genmab’s oper-ations indicate that the carrying amount of property and equipment may not be recoverable, management performs an impairment test of the asset. The basis for the performance of an impairment test is the recoverable amount of the asset, deter-mined as the greater of the fair value less cost to sell or its value in use. Value in use is calculated as the net present value of future cash inflow expected to be generated from the asset. If the carrying amount of an asset is greater than the recoverable amount, the asset is written down to the recoverable amount. An impairment loss is recognized in the Consolidated Statements of Comprehensive Income when the impairment is identified. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 157 |
|
3.3 Leases Genmab has entered into lease agreements with respect to office and laboratory space, vehicles, and IT equipment. The expense, lease liability, and right-of-use assets balances related to vehicles and IT equipment are immaterial. The leases are non-cancellable over various periods through 2038. (DKK million) 2024 2023 2022 Right-of-use assets Balance at January 1 686 523 354 Additions to right-of-use assets1 329 250 243 Depreciation charge for the year (102) (87) (74) Balance at December 31 913 686 523 Lease liabilities Current 92 90 74 Non-current 937 680 523 Total at December 31 1,029 770 597 Cash outflow for lease payments 96 115 88 1. Additions to right-of-use assets also includes modifications to existing leases and adjustments to the provisions for contractual restoration obligations related to leases of Genmab offices. Variable lease payments, short-term lease expense, lease interest expense, low-value assets, and sublease income are immaterial. Future minimum payments under leases are as follows: (DKK million) 2024 2023 2022 Payment due Less than 1 year 127 106 89 1 to 3 years 284 199 167 More than 3 years but less than 5 years 281 183 136 More than 5 years 553 412 271 Total at December 31 1,245 900 663 Accounting Policies All leases are recognized in the Consolidated Balance Sheets as a right-of-use (ROU) asset with a corresponding lease liability, except for short-term leases in which the term is 12 months or less, or low-value leases. ROU assets represent Genmab’s right to use an underlying asset for the lease term and lease liabilities represent Genmab’s obligation to make lease payments arising from the lease. The ROU asset is depreciated over the shorter of the asset’s useful life or the lease term on a straight-line basis. In the Consolidated Statements of Comprehensive Income, depreciation of the ROU asset is recognized over the lease term in operating expenses and interest expenses related to the lease liability are classified in financial items. Genmab determines if an arrangement is a lease at inception. Genmab leases various properties, vehicles, and IT equipment. Rental contracts are typically made for fixed periods. Lease terms are negotiated on an individual basis and contain a wide range of terms and conditions. Assets and liabilities arising from a lease are initially measured on a present value basis. Lease liabilities include the net present value of fixed payments, less any lease incentives receivable. As Genmab’s leases generally do not provide an implicit interest rate, Genmab uses an incremental borrowing rate based on the information available at the commencement date of the lease in determining the present value of lease payments. Lease terms utilized by Genmab may include options to extend or terminate the lease when it is reasonably certain that Genmab will exercise that option. In determining the lease term, management considers all facts and circumstances that create an economic incentive to exercise an extension option, or not exercise a termination option. Extension options (or periods after termination options) are only included in the lease term if the lease is reasonably certain to be extended. ROU assets are measured at cost and include the amount of the initial measurement of the lease liability, any lease payments made at or before the commencement date less any lease incentives received, any initial direct costs, and restoration costs. Payments associated with short-term leases and leases of low-value assets are recognized on a straight-line basis as an expense in the Consolidated Statements of Comprehensive Income. 3.4 Other Investments (DKK million) 2024 2023 Publicly traded equity securities 38 47 Fund investments 176 87 Privately held equity securities 14 – Total at December 31 228 134 Other investments includes strategic investments in publicly traded common stock of companies, including common stock of companies with whom Genmab has entered into collaboration arrange-ments, investments in certain investment funds, as well as investments in shares of privately held companies. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 158 |
|
Accounting Policies Other investments are measured on initial recognition at fair value, and subsequently at fair value. Changes in fair value are recognized in the Consolidated Statements of Comprehensive Income within financial income or expense. Other investments primarily consist of investments in certain strategic investment funds. Genmab’s share of the fair value of these fund investments is determined based on the valuation of the underlying investments included in the fund. Investments in publicly traded equity securities included in these strategic investment funds are valued based at the most recent sale price or official closing price reported on the exchange or over-the-counter market on which they trade, while investments in non-publicly traded equity securities are based on other factors, including but not limited to, type of the security, the size of the holding, the initial cost of the security, the price and extent of public trading in similar securities of the comparable companies, an analysis of the company’s or issuer’s financial statements and with respect to debt securities, the maturity and creditworthiness. As such, these fund investments have been characterized as Level 3 investments as fair values are based on significant unobservable inputs. 3.5 Inventories (DKK million) 2024 2023 Raw materials 4 14 Work in progress – – Finished goods 79 59 Total inventories (gross) at December 31 83 73 Allowances at year end (21) (16) Total inventories (net) at December 31 62 57 In 2024 and 2023, allowances related to write downs of excess and obsolete inventories were immaterial and recognized as expense within cost of product sales in the Consolidated Statements of Comprehensive Income. Inventory write down in 2023 pertaining to pre-launch inventories of EPKINLY was also immaterial. The write down was recorded as research and development expense in Genmab’s Consolidated Statements of Comprehensive Income and was subsequently reversed upon receiving U.S. FDA approval during the second quarter of 2023. Accounting Policies Inventories are measured at the lower of cost and net realizable value with costs determined on a first-in, first-out basis. Costs comprise direct and indirect costs relating to the manu-facture of inventory mainly from third-party providers of manufacturing as well as costs related to internal resources and distribution and logistics. Genmab assesses the recoverability of capitalized inventories during each reporting period and will write down excess or obsolete inventories to their net realizable value in the period in which the impairment is identified. Write downs of inventory are included within Cost of product sales in the Consolidated Statements of Comprehensive Income. Included in inventories are materials with the intended purpose of being made available for sale. If the materials are later used in the production of clinical products, the materials are charged to research and development expense when shipped to the clinical packaging site. Materials ordered exclusively to be used in Genmab’s research and development process (e.g., early research/clinical trials) are immedi-ately expensed to research and development based on the relevant shipping terms (FOB desti-nation/shipping point). Inventory manufactured prior to regulatory approval of a product (prelaunch inventory) is written down to its net realizable value (that is the probable amount expected to be realized from its sale or use at the time of production). The amount of this write down is recognized in the Consolidated Statements of Comprehensive Income as research and development expenses. Once there is a high probability of regulatory approval being obtained for the product, inventory costs begin to be capitalized. Additionally, the write-down is reversed, up to no more than the original cost. The reversal of the write-down is recognized as a reduction to research and development expenses in the Consolidated Statements of Comprehensive Income. 3.6 Receivables (DKK million) 2024 2023 Receivables related to collaboration agreements 5,434 4,148 Prepayments 256 241 Trade receivables related to product sales 466 184 Interest receivables 133 150 Other receivables 353 286 Total at December 31 6,642 5,009 Non-current receivables 52 62 Current receivables 6,590 4,947 Total at December 31 6,642 5,009 During 2024 and 2023, there were no losses related to receivables and the credit risk on receivables is considered to be limited. The provision for expected credit losses was zero given that there have been no credit losses over the last three years and the limited credit risk due to high-quality nature with high credit ratings (top tier life science companies and major distrib-utors) of Genmab’s customers are not likely to result in future default risk. The receivables are mainly comprised of royalties, trade receivables, milestones and amounts due under collaboration agreements and are non-interest bearing receivables which are due less than one year from the balance sheet date. Refer to Note 4.2 for additional informa-tion about interest receivables and related credit risk. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 159 |
|
Accounting Policies Initially, trade receivables are designated as financial assets measured at transaction price and other receivables are measured at fair value. Subsequently receivables are measured in the balance sheet at amortized cost, which generally corresponds to nominal value less expected credit losses. Accounts receivable arising from product sales consists of amounts due from customers, net of customer allowances for chargebacks, cash and other discounts and estimated credit losses. Genmab’s contracts with customers have initial payment terms that range from 30 to 180 days. Genmab utilizes a simplified approach to measuring expected credit losses and uses a lifetime expected loss allowance for all receiv-ables. To measure the expected credit losses, receivables have been grouped based on credit risk characteristics and the days past due. Prepayments include expenditures related to a future financial period. Prepayments are measured at nominal value. 3.7 Contract Liabilities Genmab has recognized the following liabilities related to the AbbVie collaboration agreement. (DKK million) 2024 2023 Contract liabilities at January 1 513 513 Payment received – – Revenue recognized during the year (9) – Total at December 31 504 513 Non-current contract liabilities 480 480 Current contract liabilities 24 33 Total at December 31 504 513 Contract liabilities were recognized in connec-tion with the AbbVie collaboration agreement. An upfront payment of USD 750 million (DKK 4,911 million) was received in July 2020 of which DKK 4,398 million was recognized as license revenue during 2020. The revenue deferred at the initiation of the AbbVie agreement in June 2020 related to four product concepts to be identified and subject to a research agreement to be negotiated between Genmab and AbbVie. During the first quarter of 2022, Genmab and AbbVie entered into the aforementioned research agreement that governs the research and development activities in regard to the product concepts. As part of the continued evaluation of contract liabilities related to the AbbVie collaboration agreement, Genmab’s classification of contract liabilities reflects the current estimate of co-development activities as of December 31, 2024. Contract liabilities have been recognized as reimbursement revenue during the second half of 2024. Refer to Note 5.6 for additional information related to the AbbVie collaboration. 3.8 Other Payables (DKK million) 2024 2023 Liabilities related to collaboration agreements 275 145 Staff cost liabilities 720 637 Accounts payable 644 330 Other liabilities 1,873 1,230 Total at December 31 3,512 2,342 Non-current other payables 30 35 Current other payables 3,482 2,307 Total at December 31 3,512 2,342 Accounting Policies Other payables, excluding provisions, are initially measured at fair value and subsequently measured in the balance sheet at amortized cost. The current other payables are comprised of liabilities that are due less than one year from the balance sheet date and are in general not interest bearing and settled on an ongoing basis during the next financial year. Non-current payables are measured at the present value of the expenditures expected to be required to settle the obligation using a pre-tax discount rate that reflects current market assess-ments of the time value of money and the risks specific to the obligation. The increase in the liability due to passage of time is recognized as interest expense. Accounts Payable Accounts payable are measured in the Consolidated Balance Sheets at amortized cost. Other Liabilities Other liabilities primarily include accrued expenses related to our research and devel-opment project costs and are measured in the Consolidated Balance Sheets at amortized cost. Refer to Note 2.3 for accounting policies related to staff costs. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 160 |
|
Section 4 Capital Structure, Financial Risk and Related Items This section includes disclosures related to how Genmab manages its capital structure, cash position and related risks and items. Genmab is primarily financed through partnership collaborations. 4.1 Capital Management Genmab’s goal is to maintain a strong capital base so as to maintain investor, creditor and market confidence, and to have adequate liquidity to support the continuous advancement of Genmab’s product pipeline and business in general. To achieve this goal Genmab invests in different liquidity tiers. To meet operational goals, Genmab invests in cash and cash equivalents (marketable securities). To ensure sufficient reserves, Genmab invests in short-term securities with an average duration of about six months, which serves as back-up liquidity for the operating tier. For strategic purposes, Genmab has short term investments to support the Company’s growth over the longer term. Most of Genmab’s cash and marketable securities are in USD due to having a larger USD expenditure base than DKK, which provides better matching of investment balances with actual expenditures. Genmab is primarily financed through revenues under various collaboration agreements and had, as of December 31, 2024, cash, and cash equivalents of DKK 9,858 million and marketable securities of DKK 11,243 million compared to DKK 14,867 million and DKK 13,268 million, respectively, as of December 31, 2023. Genmab’s cash and cash equivalents and marketable securities support the advancement of our product pipeline and operations. The adequacy of our available funds will depend on many factors, including the level of DARZALEX and other royalty streams, progress in our research and development programs, the magnitude of those programs, our commitments to existing and new clinical collaborators, our ability to establish commercial and licensing arrangements, our capital expenditures, market developments, and any future acquisitions. Accordingly, Genmab may require additional funds and may attempt to raise additional funds through equity or debt financings, collaborative agreements with partners, or from other sources. During the fourth quarter of 2024, Genmab entered into an unsecured three-year revolving credit facility (“Credit Facility”) of up to USD 300 million with a syndicate of lenders. Genmab intends to use the Credit Facility to finance working capital needs, and for general corporate purposes, of Genmab A/S and its subsidiaries. The Credit Facility includes options to increase the size of the facility up to USD 500 million as well as the ability to extend for an addi-tional two years. The Credit Facility contains certain customary financial covenants. As of December 31, 2024, there were no outstanding amounts due on, nor any usage of, the Credit Facility and Genmab was in compliance with all financial covenants. The Board monitors the share and capital structure to ensure that Genmab’s capital resources support its strategic goals. Neither Genmab A/S nor any of its subsid-iaries are subject to externally imposed capital requirements. 4.2 Financial Risk The financial risks of Genmab are managed centrally. The overall risk management guidelines have been approved by the Board of Directors and include the Group’s investment policy related to our marketable securities. The Group’s risk management guidelines are established to identify and analyze the risks faced by the Genmab Group, to set the appropriate risk limits and controls and to monitor the risks and adherence to limits. It is Genmab’s policy not to actively speculate in financial risks. The Group’s financial risk management is directed solely towards monitoring and reducing financial risks which are directly related to Genmab’s operations. The primary objective of Genmab’s invest-ment activities is to preserve capital and ensure liquidity with a secondary objective of maximizing the return derived from security investments without significantly increasing risk. Therefore, our investment policy includes among other items, guidelines and ranges for which investments (which are primarily shorter-term in nature) are considered to be eligible investments for Genmab and which investment parameters are to be applied, including maturity limitations and credit ratings. In addition, the policy includes specific diversification criteria and investment limits to minimize the risk of loss resulting from over-concentration of assets in a specific class, issuer, currency, country, or economic sector. Genmab’s marketable securities are administered by external investment managers. The investment guidelines and managers are reviewed regularly to reflect changes in market conditions, Genmab’s activities and financial position. Genmab’s investment policy allows investments in debt rated BBB- or greater by S&P or Fitch and in debt rated Baa3 or greater by Moody’s. The policy also includes additional allowable investment types such as corporate debt, commercial paper, certif-icates of deposit, and certain types of AAA rated asset-backed securities. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 161 |
|
In addition to the capital management and financing risk mentioned in Note 4.1, Genmab has identified the following key financial risk areas, which are mainly related to our marketable securities portfolio: • credit risk; • foreign currency risk; and • interest rate risk All of Genmab’s marketable securities are traded in established markets. Given the current market conditions, all future cash inflows, including re-investments of proceeds from the disposal of marketable securities, are invested in highly liquid, investment grade securities. Refer to Note 4.4 for additional information regarding marketable securities. Credit Risk Genmab is exposed to credit risk and losses on marketable securities, bank deposits and receiv-ables. The maximum credit exposure related to Genmab’s cash and cash equivalents and marketable securities was DKK 21,101 million as of December 31, 2024, compared to DKK 28,135 million as of December 31, 2023. The maximum credit exposure to Genmab’s receiv-ables was DKK 6,642 million as of December 31, 2024 compared to DKK 5,009 million as of December 31, 2023. Marketable Securities To manage and reduce credit risks on our securi-ties, Genmab’s policy is to ensure only securities from investment grade issuers are eligible for our portfolios. No issuer of marketable securities can be accepted if the issuer, at the time of purchase, does not have the credit quality equal to or better than the rating shown in the table below from at least one of the rating agencies. If an issuer is rated by more than one of the rating agencies listed below, the credit assessment is made against the lowest rating available for the issuer. Category S&P Moody’s Fitch Short-term A-2 P-2 F-2 Long-term BBB- Baa3 BBB-Genmab’s current portfolio is spread over a number of different securities with a focus on liquidity and security. As of December 31, 2024, 71% of Genmab’s marketable securities were long-term A rated or higher, or short-term A-1/P-1 rated by S&P, Moody’s or Fitch compared to 72% as of December 31, 2023. The total value of marketable securities amounted to DKK 11,243 million at the end of 2024 compared to DKK 13,268 million at the end of 2023. Cash and Cash Equivalents To reduce the credit risk on our bank deposits, Genmab’s policy is only to invest its cash deposits with highly rated financial institutions. Currently, these financial institutions have a short-term Fitch and S&P rating of at least F-1 and A-1, respectively. In addition, Genmab maintains bank deposits at a level necessary to support the short-term funding requirements of Genmab. The total value of bank deposits including AAA rated money market funds and short-term marketable securities classified as cash equivalents amounted to DKK 9,858 million as of December 31, 2024 compared to DKK 14,867 million at the end of 2023. The decrease was primarily the result of cash used to acquire ProfoundBio in the second half of 2024. Receivables The credit risk related to our receivables is not significant based on the high-quality nature of Genmab’s collaboration partners. As disclosed in Note 2.2, J&J, Novartis, Roche, AbbVie and BioNTech are Genmab’s primary partners in which receivables are established for royalties, milestone revenue and reimbursement revenue. These are long-standing relationships and Genmab does not have a history of writing off receivables from collaboration partners. Foreign Currency Risk Genmab’s presentation currency is the DKK; however, Genmab’s revenues and expenses are in a number of different currencies. Consequently, there is a substantial risk of exchange rate fluctu-ations having an impact on Genmab’s cash flows, profit (loss) and/or financial position in DKK. The majority of Genmab’s revenue is generated in USD. Exchange rate changes to the USD will result in changes to the translated value of future net profit before tax and cash flows. Genmab’s revenue in USD was 79% of total revenue in 2024 as compared to 86% in 2023 and 89% in 2022. Under our license agreement with J&J for DARZALEX, for purposes of calculating royalties due to Genmab, DARZALEX net sales for non-U.S. dollar denominated currencies are translated to U.S. dollars at a specified annual Currency Hedge Rate. Movements in foreign exchanges against the annual Currency Hedge Rate will result in changes to royalties due to Genmab impacting net profit before tax and cash flows. There is also exposure that exchange rate fluctu-ations may impact equity as part of the currency translation adjustments required to convert the investments in foreign subsidiaries from their respective functional currencies to the presen-tation currency during consolidation, however any such fluctuations would be immaterial. The foreign subsidiaries are not significantly affected by currency risks as both revenues and expenses are primarily settled in the foreign subsidiaries’ functional currencies. Foreign currency risk is primarily concentrated at the Genmab A/S level as transactions with subsidiaries are primarily in the functional currency of the subsidiary. To manage and reduce this foreign currency risk, Genmab maintains a large portion of its investment portfolio in marketable securities in USD (approximately 76%) as well as a portion of our investment portfolio in DKK, EUR, and GBP denominated securities as a natural partial hedge of Genmab A/S’ liability exposures in these currencies. Assets and Liabilities in Foreign Currency Genmab’s marketable securities denominated in USD, DKK, EUR, and GBP as a percentage of total marketable securities were as follows: Percent 2024 2023 USD 76% 81% DKK 15% 12% EUR 8% 6% GBP 1% 1% Total at December 31 100% 100% Genmab’s USD currency exposure is mainly related to cash and cash equivalents, marketable securities, and receivables related to our collab-orations with J&J, AbbVie, and Roche. Significant changes in the exchange rate of USD to DKK could cause net profit before tax to change materially as gains and losses are recognized in Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 162 |
|
the Consolidated Statements of Comprehensive Income. Based on the amount of assets and liabilities denominated in USD as of December 31, 2024 and 2023, a 10% increase/decrease in the USD to DKK exchange rate is estimated to impact Genmab’s net profit before tax by approximately DKK 1.9 billion and DKK 2.7 billion, respectively. The analysis assumes that all other variables, in particular interest rates, remain constant. The movements in the income statement and equity arise from monetary items (cash and cash equiv-alents, marketable securities, receivables, and liabilities) where the functional currency of the entity differs from the currency that the monetary items are denominated in. Genmab’s EUR exposure is mainly related to our marketable securities, receivables under our collaboration with BioNTech, and other costs denominated in EUR. Since the introduction of the EUR in 1999, Denmark has committed to maintaining a central rate of 7.46 DKK to the EUR. This rate may fluctuate within a +/- 2.25% band. Should Denmark’s policy toward the EUR change, the DKK values of our EUR denominated assets and costs could be materially different compared to what is calculated and reported under the existing Danish policy toward the DKK/EUR. As of December 31, 2024 and 2023, Genmab’s EUR exposure is not material. Genmab’s GBP currency exposure is mainly related to contracts and marketable securities denomi-nated in GBP. As of December 31, 2024 and 2023, Genmab’s GBP exposure is not material. Interest Rate Risk Genmab’s exposure to interest rate risk is primarily related to marketable securities, as Genmab currently does not have significant interest-bearing debts. Marketable Securities The securities in which the Group has invested bear interest rate risk, as a change in market-derived interest rates may cause fluctuations in the fair value of the investments. In accordance with the objective of the investment activities, the portfolio of securities is monitored on a total return basis. To control and minimize the interest rate risk, Genmab maintains an investment portfolio in a variety of securities with a relatively short effective duration with both fixed and variable interest rates. A sensitivity analysis was performed on Genmab’s marketable securities, and based on exposures in 2023 and 2024, a hypothetical +/- 1% interest rate change would not have resulted in a material change in the fair values of these financial instruments. Due to the short-term nature of the current investments and to the extent that Genmab is able to hold the investments to maturity, the current exposure to changes in fair value due to interest rate changes is considered to be insignificant compared to the fair value of the portfolio. (DKK million) 2024 2023 Year of Maturity 2024 – 6,742 2025 5,000 3,717 2026 3,209 2,175 2027 2,314 232 2028 329 143 2029+ 391 259 Total at December 31 11,243 13,268 4.3 Financial Assets and Liabilities Categories of Financial Assets and Liabilities December 31, (DKK million) Note 2024 2023 Financial assets measured at fair value through profit or loss Marketable securities 4.4 11,243 13,268 Other investments 3.4 228 134 Financial assets measured at amortized cost Receivables excluding prepayments 3.6 6,386 4,768 Cash and cash equivalents 9,858 14,867 Financial liabilities measured at amortized cost Lease liabilities 3.3 (1,029) (770) Other payables excluding provisions 3.8 (3,482) (2,316) Fair Value Measurement December 31, 2024 2023 (DKK million) Note Level 1 Level 2 Level 3 Total Level 1 Level 2 Level 3 Total Assets Measured at Fair Value Marketable securities 4.4 11,243 – – 11,243 13,268 – – 13,268 Other investments 3.4 38 14 176 228 47 – 87 134 Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 163 |
|
Marketable Securities Substantially all fair market values are deter-mined by reference to external sources using unadjusted quoted prices in established markets for our marketable securities (Level 1). Other Investments Other investments primarily consist of invest-ments in certain strategic investment funds. Genmab’s share of the fair value of these fund investments is determined based on the valuation of the underlying investments included in the fund. Investments in publicly traded equity securities included in these strategic investment funds are valued based at the most recent sale price or official closing price reported on the exchange or over-the-counter market on which they trade, while investments in non-publicly traded equity securities are based on other factors, including but not limited to, type of the security, the size of the holding, the initial cost of the security, the price and extent of public trading in similar securities of the comparable companies, an analysis of the company’s or issuer’s financial statements and with respect to debt securities, the maturity and creditwor-thiness. As such, these fund investments have been characterized as Level 3 investments as fair values are not entirely based on observable market data. There were no transfers into or out of Level 3 during 2024 or 2023. Acquisitions (capital calls), fair value changes and foreign currency changes on Level 3 investments in 2024 and 2023 were as follows: (DKK million) Other Investments Fair value at December 31, 2022 66 Acquisitions 30 Fair value changes (9) Fair value at December 31, 2023 87 Acquisitions 42 Fair value changes 43 Foreign currency changes 4 Fair value at December 31, 2024 176 Accounting Policies Classification Of Categories Of Financial Assets And Liabilities Genmab classifies its financial assets held into the following measurement categories: • those to be measured subsequently at fair value (either through other comprehensive income, or through profit or loss), and • those to be measured at amortized cost. The classification depends on the business model for managing the financial assets and the contractual terms of the cash flows. For assets measured at fair value, gains and losses will either be recorded in profit or loss or other comprehensive income. Genmab reclassifies debt investments only when its business model for managing those assets changes. Further details about the accounting policy for each of the categories are outlined in the respec-tive notes. Fair Value Measurement Genmab measures financial instruments, such as marketable securities, at fair value at each balance sheet date. Management assessed that the fair value of financial assets and liabil-ities measured at amortized cost such as bank deposits, receivables and other payables approx-imate their carrying amounts largely due to the short-term maturities of these instruments. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market partic-ipants at the measurement date. The fair value measurement is based on the presumption that the transaction to sell the asset or transfer the liability takes place either: • In the principal market for the asset or liability, or • In the absence of a principal market, in the most advantageous market for the asset or liability. The principal or the most advantageous market must be accessible by Genmab. The fair value of an asset or a liability is measured using the assumptions that market participants would use when pricing the asset or liability, assuming that market participants act in their economic best interest. Genmab uses valuation techniques that are appropriate in the circumstances and for which sufficient data are available to measure fair value, maximizing the use of relevant observable inputs and minimizing the use of unobservable inputs. For financial instruments that are measured in the balance sheet at fair value, IFRS 13 requires disclosure of fair value measurements by level of the following fair value measurement hierarchy: • Level 1—Quoted prices (unadjusted) in active markets for identical assets or liabilities • Level 2—Inputs other than quoted prices included within level 1 that are observable for the asset or liability, either directly (that is, as prices) or indirectly (that is, derived from prices) • Level 3—Inputs for the asset or liability that are not based on observable market data (that is, unobservable inputs). For assets and liabilities that are recognized in the financial statements at fair value on a recurring basis, Genmab determines whether transfers have occurred between levels in the hierarchy by re-assessing categorization (based on the lowest level input that is significant to the fair value measurement as a whole) at the end of each reporting period. Any transfers between the different levels are carried out at the end of the reporting period. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 164 |
|
4.4 Marketable Securities (DKK million) Market value 2024 Share % Market value 2023 Share % USD portfolio Corporate bonds 5,082 45% 6,039 46% US government bonds and treasury bills 2,533 22% 3,247 24% Commercial paper 191 2% 451 3% Other 816 7% 1,003 8% Total USD portfolio 8,622 76% 10,740 81% DKK portfolio Kingdom of Denmark bonds and treasury bills 429 4% 419 3% Danish mortgage-backed securities 1,217 11% 1,170 9% Total DKK portfolio 1,646 15% 1,589 12% EUR portfolio European government bonds and treasury bills 886 8% 858 6% GBP portfolio UK government bonds and treasury bills 89 1% 81 1% Total portfolio at December 31 11,243 100% 13,268 100% Marketable securities at December 31 11,243 13,268 Refer to Note 4.2 for additional information regarding the risks related to our marketable securities. Accounting Policies Marketable securities are debt instruments that consist of investments in securities with a maturity of 90 days or greater at the time of acquisition. Measurement of marketable securi-ties depends on the business model for managing the asset and the cash flow characteristics of the asset. Genmab assesses its debt instruments to determine classification based on the following measurement categories: • Amortized cost: Assets that are held for collection of contractual cash flows, where those cash flows represent solely payments of principal and interest, are measured at amortized cost. Interest income from these financial assets is included in finance income using the effective interest rate method. Any gain or loss arising on derecognition is recognized directly in profit or loss and presented in other financial income or expenses, together with foreign exchange gains and losses. Impairment losses, when material, are presented as a separate line item in the Consolidated Statements of Comprehensive Income. • Fair value through other comprehensive income (FVOCI): Assets that are held to achieve an objective by both collecting contractual cash flows as well as selling financial assets and where those cash flows represent solely payments of principal and interest, are measured at FVOCI. Changes in fair value on a debt investment that is subsequently measured at FVOCI are recognized in other comprehensive income. Impairment gains and losses, interest income and foreign exchange gains and losses are recognized in the Consolidated Statements of Comprehensive Income and presented within financial income or expenses in the period in which they arise. • Fair value through profit and loss (FVPL): Assets that do not meet the criteria for amortized cost or FVOCI are measured at FVPL. A gain or loss on a debt investment that is subsequently measured at FVPL is recognized in the Consolidated Statements of Comprehensive Income and presented net within financial income or expenses in the period in which it arises. Genmab’s portfolio is managed and evaluated on a fair value basis in accordance with its stated investment guidelines and the information provided internally to management. This business model does not meet the criteria for amortized cost or FVOCI and as a result marketable securi-ties are measured at FVPL. This classification is consistent with the prior year’s classification. Genmab invests its cash in deposits with major financial institutions, in AAA rated money market funds, Danish mortgage bonds, investment grade rated corporate debt, commercial paper, certifi-cates of deposit, certain types of AAA rated asset backed securities, U.S. Agency bonds, and notes issued by the Danish, European and U.S. govern-ments. The securities can be purchased and sold using established markets. Transactions are recognized at the trade date. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 165 |
|
4.5 Financial Income and Expenses (DKK million) 2024 2023 2022 Financial income: Interest and other financial income 995 982 324 Gain on marketable securities 364 495 92 Gain on other investments 146 72 58 Foreign exchange rate gain 2,933 391 2,715 Total financial income 4,438 1,940 3,189 Financial expenses: Interest and other financial expenses (120) (70) (39) Loss on marketable securities (147) (176) (453) Loss on other investments (116) (98) (355) Foreign exchange rate loss (1,594) (1,280) (1,664) Total financial expenses (1,977) (1,624) (2,511) Net financial items 2,461 316 678 Interest Income Interest income was DKK 995 million in 2024 compared to DKK 982 million in 2023 and DKK 324 million in 2022. The increase of DKK 13 million, or 1% from 2023 to 2024, was primarily driven by the higher cash and cash equivalents and marketable securities in the first half of 2024 compared to 2023, almost entirely offset by lower cash and cash equivalents and marketable securities in the second half of 2024 compared to 2023 as a result of liquidating marketable securities and using cash to purchase ProfoundBio. The increase of 658 million, or 203% from 2022 to 2023 was primarily driven by higher effective interest rates in the U.S., Europe, and Denmark. Foreign Exchange Rate Gains and Losses Foreign exchange rate gains, net of DKK 1,339 million in 2024 compared to foreign exchange rate loss, net of DKK 889 million in 2023 was primarily driven by foreign exchange movements impacting Genmab’s USD denominated marketable securities and cash and cash equivalents. Foreign exchange rate loss, net of DKK 889 million in 2023 compared to foreign exchange rate gain, net of DKK 1,051 million in 2022 was primarily driven by foreign exchange movements impacting Genmab’s USD denominated marketable securities and cash and cash equivalents; in particular, the USD/DKK foreign exchange rates were as follows for each period: December 31, 2024 December 31, 2023 December 31, 2022 USD/DKK Foreign Exchange Rates 7.1429 6.7447 6.9722 % Increase/(Decrease) 6% (3)% 6% Refer to Note 4.2 for additional information on foreign currency risk. Marketable Securities Gains and Losses Gain on marketable securities, net was DKK 217 million in 2024 compared to gain on marketable securities, net of DKK 319 million in 2023 and loss on marketable securities, net of DKK 361 million in 2022. The decrease in gain of DKK 102 million, or 32% from 2023 to 2024 was primarily driven by the decrease in marketable securities in the first half of 2024 to fund the acquisition of ProfoundBio and share repurchase as well as changing interest rate outlooks for the U.S., primarily in the fourth quarter of 2024. The increase in gain of DKK 680 million, or 188% from 2022 to 2023, was primarily driven by interest rate outlooks for the U.S. and Europe. Other Investments Gains on other investments, net were DKK 30 million in 2024, losses on other investments, net were DKK 26 million in 2023 and DKK 297 million in 2022. The net gains and losses in 2024 and 2023 were primarily driven by changes in fair value of Genmab’s investments in certain strategic investment funds. The losses in 2022 were primarily driven by the change in fair value of Genmab’s investment in common shares of CureVac. Accounting Policies Financial income and expenses include interest as well as foreign exchange rate adjustments and gains and losses on marketable securities (designated as FVPL) and realized gains and losses and write-downs of other securities and equity interests. Interest income is shown separately from gains and losses on marketable securities and other securities and equity interests. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 166 |
|
4.6 Share-Based Instruments Restricted Stock Unit Program Genmab A/S has established an RSU program (equity-settled share-based payment transac-tions) as an incentive for Genmab’s employees, members of the Executive Management, and members of the Board of Directors. RSUs granted to Executive Management are performance-based (PSUs). RSUs are granted by the Board of Directors. RSU grants to members of the Board of Directors and members of the registered Executive Management are subject to the Remuneration Policy adopted at the Annual General Meeting. See the table below for a summary of key terms of Genmab’s RSU programs: RSUs Granted in Periods Key Terms December 2019–February 2021 From February 2021 Grants RSUs are granted at no cost to employees. Number of shares granted is determined based on closing share price on the grant date. Vesting (Settlement) Cliff vesting—RSUs become fully vested on the first banking day of the month following a period of three years from the grant date. The three year cliff vesting also applies to PSUs, while also subject to the degree of fulfilment of the applicable performance criteria. After RSUs vest, the holder receives one share in Genmab A/S for each RSU granted. In jurisdictions in which Genmab as an employer is required to withhold tax and settle with the tax authority on behalf of the employee, Genmab withholds the number of RSUs that are equal to the monetary value of the employee’s tax obligation from the total number of RSUs that otherwise would have been issued to the employee upon vesting (“net settlement”). Genmab A/S may at its sole discretion in extraordinary circumstances choose to make a cash settlement instead of delivering shares. Leaver Leavers—Forfeit all unvested RSUs except when due to retirement, death, serious sickness, or serious injury, in which case granted but not yet vested RSUs shall remain outstanding and will be settled in accordance with their terms. Notwithstanding the above, the December 2020 RSU grant to members of the Board was made subject to pro-rata vesting upon termination of board services. Employees and Executive Management—RSUs remain outstanding and vest accordingly when the employment relationship is terminated by Genmab without cause. Good-Leavers1—May maintain a pro-rata portion of unvested RSUs. Bad-Leavers2—Forfeit all unvested RSUs. Death—Forfeit all unvested RSUs. Voluntary leavers forfeit unvested RSUs. 1. “Good-Leaver”—Dismissal without cause or termination of employment due to Genmab’s material breach of the RSU or Warrant holder’s employment terms, or if the participant is a member of the Board, if the membership of the Board ceases for any other reason than as a result of the participant’s death. 2. “Bad-leaver”—Dismissed for cause or during the employment probationary period. The RSU program contains anti-dilution provisions if changes occur in Genmab’s share capital prior to the vesting date and provisions to accelerate vesting of RSUs in the event of change of control as defined in the RSU program. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 167 |
|
RSU Activity in 2024, 2023 and 2022 Number of RSUs held by the Board of Directors Number of RSUs held by the Executive Management Number of RSUs held by employees Number of RSUs held by former members of the Executive Management, Board of Directors and employees Total RSUs Weighted Average Fair Value—RSUs Granted—DKK Total Fair Value of RSUs Granted— DKK million Outstanding at January 1, 2022 10,965 89,043 293,031 12,952 405,991 Granted* 4,295 40,453 221,000 6,383 272,131 2,250.18 612 Settled (3,420) (17,165) (67,945) (12,847) (101,377) Transferred (2,368) – (13,749) 16,117 – Forfeited (653) – (9,195) (18,759) (28,607) Outstanding at December 31, 2022 8,819 112,331 423,142 3,846 548,138 Outstanding at January 1, 2023 8,819 112,331 423,142 3,846 548,138 Granted* 3,361 75,854 208,353 11,643 299,211 2,619.35 784 Settled (1,880) (35,773) (54,871) (9,805) (102,329) Transferred – 12,918 (55,103) 42,185 – Forfeited – (4,357) (35) (37,984) (42,376) Outstanding at December 31, 2023 10,300 160,973 521,486 9,885 702,644 Outstanding at January 1, 2024 10,300 160,973 521,486 9,885 702,644 Granted* 7,097 121,063 344,068 14,484 486,712 1,977.87 963 Settled (3,367) (35,320) (112,663) (12,465) (163,815) Transferred – (19,924) (37,348) 57,272 – Forfeited – (11,667) (71) (38,178) (49,916) Outstanding at December 31, 2024 14,030 215,125 715,472 30,998 975,625 *RSUs held by the Board of Directors include RSUs granted to employee-elected Board Members as employees of Genmab A/S or its subsidiaries. Refer to Note 5.1 for additional information regarding compensation of the Executive Management and the Board of Directors. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 168 |
|
Warrant Program Genmab A/S has established a warrant program (equity-settled share-based payment trans-actions) as an incentive for all the Genmab Group’s employees. Warrants are granted by the Board of Directors in accordance with authorizations given to it by Genmab A/S’ shareholders. Following Genmab’s Annual General Meeting on March 29, 2023, members of the registered Executive Management and members of the Board may only be granted RSUs. See the table below for a summary of key terms of Genmab’s warrant programs: Warrants Granted in Periods Key Terms April 2012–March 2017 March 2017–February 2021 From February 2021 Grants Warrants are granted at no cost to employees. Granted at an exercise price equal to the closing share price on the grant date. Vesting (Exercisable) Annually over 4-year period (25% per year) Cliff vesting over 3-year period (100% after 3 years) Leaver Leavers—Forfeit all unvested warrants; however, will be able to exercise pro-rata portion of warrants on a regular schedule in instances where the employment relationship is terminated by Genmab without cause. Good-Leavers—Maintain a pro-rata portion of unvested warrants. Bad-Leavers—Forfeit all unvested warrants. Death—Forfeit all unvested warrants. Voluntary leavers forfeit all unvested warrants. Lapse 7th anniversary of grant date The warrant program contains anti-dilution provisions if changes occur in Genmab’s share capital prior to the warrants being exercised and provisions to accelerate vesting of warrants in the event of change of control or certain other extraordinary transactions as defined in the warrant program. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 169 |
|
Warrant Activity in 2024, 2023 and 2022 Number of warrants held by the Board of Directors Number of warrants held by the Executive Management Number of warrants held by employees Number of warrants held by former members of the Executive Management, Board of Directors and employees Total warrants Weighted average exercise price—DKK Weighted average share price at exercise date—DKK Outstanding Warrants— % of Share Capital Outstanding at January 1, 2022 10,658 159,634 739,000 59,159 968,451 1,501.49 Granted* 1,541 – 250,005 7,412 258,958 2,244.22 Exercised (1,558) (29,836) (176,948) (34,775) (243,117) 1,154.95 2,815.33 Expired – – – – – – Forfeited – – (13,670) (32,654) (46,324) 2,029.00 Transfers (8,721) – (25,373) 34,094 – – Outstanding at December 31, 2022 1,920 129,798 773,014 33,236 937,968 1,770.31 1% Exercisable at year end 617 118,571 282,296 32,695 434,179 1,265.68 Exercisable warrants in the money at year end 617 118,571 282,296 32,695 434,179 1,265.68 Outstanding at January 1, 2023 1,920 129,798 773,014 33,236 937,968 1,770.31 Granted* 403 – 198,001 10,973 209,377 2,632.02 Exercised – (11,900) (74,672) (26,390) (112,962) 1,341.40 2,657.76 Expired – – (1,200) (117) (1,317) 1,225.18 Forfeited – – (32) (43,143) (43,175) 2,274.50 Transfers – 21,295 (103,396) 82,101 – – Outstanding at December 31, 2023 2,323 139,193 791,715 56,660 989,891 1,980.25 1% Exercisable at year end 875 123,345 246,635 45,686 416,541 1,416.25 Exercisable warrants in the money at year end 617 123,345 192,945 43,632 360,539 1,272.37 Outstanding at January 1, 2024 2,323 139,193 791,715 56,660 989,891 1,980.25 Granted* 694 – 354,255 14,898 369,847 1,974.71 Exercised – (63,811) (31,721) (17,119) (112,651) 1,143.29 1,877.19 Expired – – (155) (132) (287) 1,032.00 Forfeited – – (73) (39,564) (39,637) 2,300.10 Transfers – 555 (53,903) 53,348 – – Outstanding at December 31, 2024 3,017 75,937 1,060,118 68,091 1,207,163 2,046.38 2% Exercisable at year end 1,226 63,405 321,099 60,686 446,416 1,759.86 Exercisable warrants in the money at year end – 46,166 77,669 25,477 149,312 1,131.68 *Warrants held by the Board include warrants granted to employee-elected Board Members as employees of Genmab A/S or its subsidiaries. Refer to Note 5.1 for additional information regarding compensation of the Executive Management and the Board of Directors. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 170 |
|
Weighted Average Outstanding Warrants at December 31, 2024 As of December 31, 2024, the range of exercise prices for outstanding warrants was DKK 962 to DKK 3,172 with a weighted average remaining contractual life of 4.24 years. As of December 31, 2023, the range of exercise prices for outstanding warrants was DKK 962 to DKK 3,172 with a weighted average remaining contractual life of 4.11 years. Accounting Policies Share-Based Compensation Expenses Share-based compensation expense is recognized in the Consolidated Statements of Comprehensive Income based on the estimated fair value of the awards at grant date. Subsequently, the fair value is not remeasured. The expense recognized reflects an estimate of the number of awards expected to vest after taking into consideration an estimate of award forfeitures based on historical experience and is recognized on a straight-line basis over the requisite service period, which is the vesting period. Genmab reassesses its estimate of the number of shares expected to vest periodically. Management expectations related to the achieve-ment of performance goals associated with performance-based RSU grants are assessed periodically, and that assessment is used to determine whether such grants are expected to vest or if any revision to the current estimate is required. Genmab recognizes the impact of the revised estimate of the number of awards expected to vest, if any, as an adjustment to the income statement over the remaining vesting period. If performance-based milestones related to performance-based RSU grants are not met or not expected to be met, any share-based compensation expense recognized to date asso-ciated with grants that are not expected to vest will be reversed. Share-based compensation expenses represent calculated values of warrants, RSUs and performance-based RSUs granted and do not represent actual cash expenditures. A corre-sponding amount is recognized in shareholders’ equity as the warrant, RSU and performance-based RSU programs are designated as equity-settled share-based payment transactions. The fair value of each RSU and performance-based RSU granted during the year are calculated using the closing share price on the grant date. Below is a description on how the fair value of warrants is measured and the estimates involved. Management’s Judgements and Estimates Share-Based Compensation Expenses The fair value of each warrant granted during the year is calculated using the Black-Scholes pricing model. This pricing model requires the input of subjective assumptions such as: • The expected stock price volatility, which is based upon the historical volatility of Genmab’s stock price; • The risk-free interest rate, which is determined as the interest rate on Danish government bonds (bullet issues) with an average maturity of five years; • The expected life of warrants, which is based on vesting terms, expected rate of exercise and life terms in the current warrant program. These assumptions can vary over time and can change the fair value of future warrants granted. Valuation Assumptions for Warrants Granted in 2024, 2023 and 2022 The fair value of each warrant granted during the year is calculated using the Black-Scholes pricing model with the following assumptions: 2024 2023 2022 Weighted average Fair value per warrant on grant date 639.67 924.10 664.08 Share price 1,974.71 2,632.02 2,244.22 Exercise price 1,974.71 2,632.02 2,244.22 Expected dividend yield 0% 0% 0% Expected stock price volatility 32.3% 35.3% 33.5% Risk-free interest rate 2.26% 2.48% 0.15% Expected life of warrants 5 years 5 years 5 years Total Fair Value of Amounts Granted Total fair value of warrants granted DKK 237 million DKK 193 million DKK 172 million Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 171 |
|
4.7 Share Capital Share Capital The share capital comprises the nominal amount of Genmab A/S ordinary shares, each at a nominal value of DKK 1. All shares are fully paid. As of December 31, 2024, the share capital of Genmab A/S comprised 66,187,186 shares of DKK 1 each with one vote. There are no restric-tions related to the transferability of the shares. All shares are regarded as negotiable instruments and do not confer any special rights upon the holder, and no shareholder shall be under an obli-gation to allow his/her shares to be redeemed. Genmab’s Board is authorized to increase the share capital by subscription of new shares, issue warrants to subscribe for shares and raise loans against bonds as well as other financial instru-ments of Genmab A/S as set out in articles 4A-5B of Genmab A/S’ articles of association. Further, Genmab’s share capital is in compliance with the capital requirements of the Danish Companies Act and the rules of Nasdaq Copenhagen. See table below for warrants issued and reissued and warrants available for reissue under active authorizations as of December 31, 2024: March 13, 2024 Authorization April 13, 2021 Authorization March 29, 2019 Authorization Warrants issued – 585,692 500,000 Warrants reissued – 41,143 81,684 Warrants available for issue 750,000 164,308 – Warrants available for reissue – 4,550 – Share Premium The share premium reserve is comprised of the amount received, attributable to shareholders’ equity, in excess of the nominal amount of the shares issued at the parent company’s offerings, reduced by any external expenses directly attributable to the offerings. The share premium reserve can be distributed. Changes in Share Capital During 2022 to 2024 The share capital of DKK 66 million at December 31, 2024, is divided into 66,187,186 shares at a nominal value of DKK 1 each. Number of shares Share capital (DKK million) Share Price Ranges1 December 31, 2021 65,718,456 65.7 Exercise of warrants 243,117 0.3 DKK 466.20 to DKK 1,615.00 December 31, 2022 65,961,573 66.0 Exercise of warrants 112,962 0.1 DKK 815.50 to DKK 1,948.00 December 31, 2023 66,074,535 66.1 Exercise of warrants 112,651 0.1 DKK 962.00 to DKK 1,615.00 December 31, 2024 66,187,186 66.2 1. New shares were subscribed at share prices in connection with the exercise of warrants under Genmab’s warrant program. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 172 |
|
Treasury Shares Number of shares Share capital (DKK million) Proportion of share capital % Cost (DKK million) Shareholding at December 31, 2021 288,325 0.3 0.4 550 Purchase of treasury shares 370,000 0.4 0.6 908 Shares used for funding RSU program (68,377) (0.1) (0.1) (80) Shareholding at December 31, 2022 589,948 0.6 0.9 1,378 Purchase of treasury shares 220,000 0.2 0.3 564 Shares used for funding RSU program (65,778) (0.1) (0.1) (126) Shareholding at December 31, 2023 744,170 0.7 1.1 1,816 Purchase of treasury shares 2,011,853 2.0 3.0 3,879 Shares used for funding RSU program (109,016) (0.1) (0.1) (246) Shareholding at December 31, 2024 2,647,007 2.6 4.0 5,449 Share Repurchases As of December 31, 2024, Genmab’s 2021 and 2023 authorizations have shares available for repur-chase, whereas Genmab’s 2019 authorization has expired. In addition, at Genmab’s Annual General Meeting on March 13, 2024, a new authorization to acquire treasury shares up to a nominal amount of DKK 3,500,000 was granted. 2024 Authorization 2023 Authorization 2021 Authorization Number of shares authorized for repurchase1 3,500,000 500,000 500,000 Actual shares repurchased under authorization 1,821,853 – 450,000 Shares available for repurchase as of December 31, 2024 1,678,147 500,000 50,000 1.Nominal value of DKK 3,500,000 for 2024, and DKK 500,000 for 2023 and 2021 Authorizations Financial Statements for the Genmab Group As announced on February 14, 2024, and March 15, 2024, Genmab initiated two share buy-back programs. The purpose of the share buy-back program announced on February 14, 2024, was to honor Genmab’s commitments under the RSU program. The share buy-back program announced on March 15, 2024, was in support of Genmab’s capital allocation strategy. During 2024, Genmab acquired 2,011,853 of its own shares, representing approximately 3.0% of share capital as of December 31, 2023. The total amount paid to acquire the shares, including directly attributable costs, was DKK 3,879 million and was recognized as a deduction to shareholders’ equity. During 2023, Genmab acquired 220,000 of its own shares, repre-senting approximately 0.3% of share capital as of December 31, 2022. The total amount paid to acquire the shares, including directly attributable costs, was DKK 564 million and was recog-nized as a deduction to shareholders’ equity. These shares are classified as treasury shares and are presented within retained earnings on the Consolidated Balance Sheets as of December 31, 2024. As of December 31, 2024, 2,647,007 treasury shares were held by Genmab. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 173 |
|
Section 5 Other Disclosures This section is comprised of various statutory disclosures or notes that are of secondary importance for the understanding of Genmab’s financials. 5.1 Remuneration of the Board of Directors and Executive Management The total remuneration of the Board and Executive Management is as follows: (DKK million) 2024 2023 2022 Wages and salaries 101 71 55 Share-based compensation expenses 157 100 70 Defined contribution plans 4 3 2 Total 262 174 127 The remuneration packages for the Board and Executive Management are described in further detail in Genmab’s 2024 Compensation Report. The remuneration packages are denominated in DKK, EUR, or USD. The Compensation Committee of the Board performs an annual review of the remuneration packages. All incentive and variable remuneration is considered and adopted at the Company’s Annual General Meeting. Share-based compensation is included in the Consolidated Statements of Comprehensive Income and reported in the table above. Share-based compensation expense represents the estimated fair value of the awards at grant date and does not represent actual cash compen-sation received by the Board Members or Executive Management. Refer to Note 4.6 for additional information regarding Genmab’s share-based compensation programs and accounting policies. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 174 |
|
Remuneration to the Board of Directors Base Board Fee Committee Fees Share-Based Compensation Expenses Total (DKK million) 2024 2023 2022 2024 2023 2022 2024 2023 2022 2024 2023 2022 Deirdre P. Connelly 1.2 1.2 1.2 0.5 0.5 0.5 1.6 1.1 0.9 3.3 2.8 2.6 Pernille Erenbjerg 0.9 0.9 0.9 0.4 0.4 0.4 1.3 0.8 0.7 2.6 2.1 2.0 Anders Gersel Pedersen 0.6 0.6 0.6 0.5 0.5 0.4 1.0 0.6 0.5 2.1 1.7 1.5 Paolo Paoletti 0.6 0.6 0.6 0.3 0.3 0.3 1.0 0.6 0.5 1.9 1.5 1.4 Rolf Hoffmann 0.6 0.6 0.6 0.4 0.3 0.3 1.0 0.6 0.5 2.0 1.5 1.4 Elizabeth O’Farrell1 0.6 0.6 0.5 0.4 0.3 0.2 1.6 1.0 0.6 2.6 1.9 1.3 Mijke Zachariasse2 0.6 0.6 0.6 – – – 1.0 0.5 0.4 1.6 1.1 1.0 Martin Schultz2 0.6 0.6 0.5 – – – 0.8 0.2 – 1.4 0.8 0.5 Takahiro Hamatani2 0.6 0.6 0.5 – – – 0.8 0.2 – 1.4 0.8 0.5 Peter Storm Kristensen3 – – 0.1 – – – – – 0.1 – – 0.2 Rima Bawarshi Nassar3 – – 0.1 – – – – – 0.1 – – 0.2 Total 6.3 6.3 6.2 2.5 2.3 2.1 10.1 5.6 4.3 18.9 14.2 12.6 1. Elizabeth O’Farrell was newly elected to the Board at the Annual General Meeting in March 2022. 2. Employee elected board members were elected at the Annual General Meeting in March 2022. 3. Peter Storm Kristensen and Rima Bawarshi Nassar stepped down from the Board as employee elected board members at the Annual General Meeting in March 2022. Refer to the section “Board of Directors” in Management’s Review for additional information regarding the Board. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 175 |
|
Remuneration to the Executive Management Base Salary Defined Contribution Plans Other Benefits Annual Cash Bonus Share-Based Compensation Expenses Total (DKK million) 2024 2023 2022 2024 2023 2022 2024 2023 2022 2024 2023 2022 2024 2023 2022 2024 2023 2022 Jan van de Winkel 9.7 9.2 8.6 1.7 1.3 1.3 0.2 0.3 0.3 8.8 9.2 8.6 34.4 24.3 22.9 54.8 44.3 41.7 Anthony Pagano 4.7 4.4 4.3 0.1 0.1 0.1 – – – 2.7 2.6 2.6 16.5 12.5 9.5 24.0 19.6 16.5 Anthony Mancini3 3.0 4.9 4.7 0.1 0.1 0.1 16.5 – – 3.0 2.9 2.8 28.9 13.9 11.4 51.5 21.8 19.0 Judith Klimovsky 5.3 5.0 4.9 0.1 0.1 0.1 0.1 – – 3.0 3.0 2.9 19.4 13.6 14.1 27.9 21.7 22.0 Tahamtan Ahmadi 5.0 4.7 4.6 0.1 0.1 0.1 – – – 2.8 2.9 2.8 17.9 12.1 7.7 25.8 19.8 15.2 Birgitte Stephensen1 2.9 2.6 – 0.3 0.3 – – – – 1.7 1.5 – 8.3 5.7 – 13.2 10.1 – Christopher Cozic1 3.4 3.3 – 0.1 0.1 – – – – 2.0 2.0 – 11.1 7.8 – 16.6 13.2 – Martine van Vugt2 2.9 2.5 – 0.7 0.6 – 0.1 0.1 – 1.6 1.6 – 5.7 4.1 – 11.0 8.9 – Brad Bailey4 3.9 – – 0.1 – – 0.6 – – 2.4 – – 4.1 – – 11.1 – – Rayne Waller4 1.7 – – 0.1 – – 3.8 – – 0.9 – – 0.8 – – 7.3 – – Total 42.5 36.6 27.1 3.4 2.7 1.7 21.3 0.4 0.3 28.9 25.7 19.7 147.1 94.0 65.6 243.2 159.4 114.4 1. Birgitte Stephensen and Christopher Cozic were appointed Chief Legal Officer and Chief People Officer, respectively, and members of the Executive Management in March 2022. 2. Martine van Vugt was appointed Chief Strategy Officer and member of the Executive Management in March 2023. 3. Anthony Mancini stepped down as Executive Vice President and Chief Operating Officer in September 2024. 4. Brad Bailey and Rayne Waller were appointed Executive Vice President and Chief Commercial Officer, and Executive Vice President and Chief Technical Operations Officer, respectively, and members of the Executive Management in August 2024. Jan van de Winkel, President and Chief Executive Officer, and Anthony Pagano, Executive Vice President and Chief Financial Officer, are formally registered as executive managers with the Danish Business Authority. Refer to the section “Executive Management” in Management’s Review for additional informa-tion regarding the Executive Management. Severance Payments In the event Genmab terminates the service agreements with any member of the Executive Management team without cause, Genmab is obliged to pay his/her existing salary for one or two years after the end of the one-year notice period. However, in the event of termination by Genmab (unless for cause) or by any member of Executive Management as a result of a change of control of Genmab, Genmab is obliged to pay compensation equal to his/her existing total salary (including benefits) for up to two years in addition to the notice period. The total value of remuneration relating to the notice period for new members of Executive Management cannot exceed two years of remuneration, including all components of the remuneration. In case of the termination of the service agreements of the Executive Management without cause, the total impact on Genmab’s financial position is estimated to be approximately DKK 120 million as of December 31, 2024 (2023: DKK 103 million, 2022: DKK 82 million). Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 176 |
|
5.2 Related Party Disclosures Genmab’s related parties are its Board, Executive Management, and close members of the family of these persons. Genmab has not granted any loans, guarantees or other commitments to or on behalf of any of the members of the Board or members of the Executive Management. Other than the remuneration and other trans-actions relating to the Board and the Executive Management described in Note 5.1, there were no material related party transactions during 2024, 2023 or 2022. 5.3 Commitments Purchase Obligations Genmab has entered into a number of agree-ments related to research and development activities that contain various obligations. These contractual obligations amounted to approxi-mately DKK 2,879 million as of December 31, 2024 (2023: approximately DKK 3,212 million). Genmab also has certain contingent commit-ments under license and collaboration agreements that may become due in the future. As of December 31, 2024, these contingent commitments amounted to approximately DKK 15,433 million (USD 2,161 million) in potential future development, regulatory and commercial milestone payments to third parties under license and collaboration agreements for our preclinical and clinical stage development programs as compared to approximately DKK 15,393 million (USD 2,282 million) as of December 31, 2023. These milestone payments generally become due and payable only upon the achievement of certain development, clinical, regulatory or commercial milestones. The events trig-gering such payments or obligations have not yet occurred. In addition to the above obligations, Genmab enters into a variety of agreements and financial commitments in the normal course of business. The terms generally allow Genmab the option to cancel, reschedule and adjust our require-ments based on our business needs prior to the delivery of goods or performance of services. It is not possible to predict the maximum potential amount of future payments under these agree-ments due to the conditional nature of our obligations and the unique facts and circum-stances involved in each particular agreement. 5.4 Fees to Auditors Appointed at the Annual General Meeting (DKK million) 2024 2023 2022 Audit fees 10.6 6.1 5.8 Audit-related fees 2.3 3.4 2.0 All other fees – 0.1 – Total 12.9 9.6 7.8 Genmab changed auditors from PricewaterhouseCoopers Statsautoriseret Revisionspartnerselskab (PwC) to Deloitte Statsautoriseret Revisionspartnerselskab (Deloitte) as Genmab’s new statutory auditor and independent registered public accounting firm for the fiscal year beginning January 1, 2024, replacing PwC. As such, fees in the table above reflect those incurred by Deloitte in 2024 and by PwC in 2023 and 2022 Fees for other services than statutory audit of the financial statements provided by Deloitte amounted to DKK 2.3 million in 2024 (DKK 3.4 million and DKK 2.0 million in 2023 and 2022, respectively provided by PwC). These services primarily include agreed-upon proce-dures, other assurance assessments and reports, and accounting advice. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 177 |
|
5.5 Acquisition of Businesses On May 21, 2024 (Acquisition Date), Genmab completed the previously announced acquisition of all of the outstanding shares of ProfoundBio, resulting in ProfoundBio becoming a wholly owned subsidiary of Genmab. The acquisition of ProfoundBio gave Genmab worldwide rights to three candidates in clinical development, including ProfoundBio’s lead drug candidate, rinatabart sesutecan (Rina-S). In addition, Genmab acquired ProfoundBio’s novel ADC technology platforms. Rina-S is a clinical-stage, FRαtargeted, TOPO1 ADC, which was in Phase 2 of a Phase 1/2 clinical trial at the time of the acquisition, for the treatment of ovarian cancer and other FRα-expressing solid tumors. Based on the data from the ongoing Phase 1/2 clinical trial Genmab intends to broaden the development plans for Rina-S within ovarian cancer and other FRαexpressing solid tumors. In January 2024, the U.S. FDA granted Fast Track designation to Rina-S for the treatment of patients with FRα-expressing high-grade serous or endometrioid platinum-resistant ovarian cancer. In addition to payment of USD 1.72 billion (DKK 11.80 billion) for all of the outstanding shares of ProfoundBio, Genmab also made a USD 199 million (DKK 1,368 million) payment to holders of outstanding ProfoundBio equity awards for settlement of such vested and non-vested awards. Of the USD 199 million (DKK 1,368 million) payment, USD 187 million (DKK 1,289 million) related to the portion of awards where the vesting period was completed prior to the Acquisition Date. This portion of the payment was therefore determined to be attributable to the pre-combination period and included in purchase consideration. The remaining USD 11 million (DKK 79 million) payment related to the portion of awards with future vesting conditions, and therefore is attributable to post-combination services. The amount attributable to the post-combination service does not form part of the consideration and was therefore instead recognized as Acquisition and integration related charges in Genmab’s Consolidated Statements of Comprehensive Income. The acquisition has been accounted for using the acquisition method of accounting which requires that assets acquired and liabilities assumed be recognized at their fair values as of the Acquisition Date and consolidated into Genmab’s Consolidated Balance Sheets. The results of operations for ProfoundBio have been included in Genmab’s consolidated financial statements from the Acquisition Date. A fair value measurement has been performed and the purchase price has been allocated to intangible assets, associated deferred tax liabilities, other assets and liabili-ties, as well as goodwill being the excess value of the purchase price over the fair value of assets acquired and liabilities assumed (the purchase price allocation). Adjustments may be applied to the purchase price allocation for a period of up to 12 months from the Acquisition Date. During the fourth quarter of 2024, the Company recorded a measurement period adjustment impacting non-current deferred tax liabilities and goodwill that was not material. The total consideration for the acquisition of ProfoundBio is summarized as follows: Total Consideration Amounts in USD millions Amounts in DKK millions Cash paid for outstanding shares 1,718 11,798 Cash for equity compensation attributable to pre-combination service 187 1,289 Total consideration 1,905 13,087 Cash acquired (122) (841) Cash used for acquisition of business 1,783 12,246 The purchase price allocation resulted in the following amounts being allocated to the assets acquired and liabilities assumed at the Acquisition Date based upon their respective fair values summa-rized below: Amounts Recognized as of the Acquisition Date Amounts in USD millions Amounts in DKK millions Cash and cash equivalents 122 841 Other current assets* 4 29 Property and equipment 6 41 IPR&D 1,540 10,577 Technology platform intangible asset 181 1,243 Other non-current assets** 3 21 Deferred tax liability (292) (2,010) Other current liabilities*** (13) (91) Total identifiable net assets 1,551 10,651 Goodwill 354 2,436 Total consideration 1,905 13,087 *Includes receivables and other investments **Includes other investments and right-of-use assets ***Includes other payables, contract liabilities, lease and other liabilities Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 178 |
|
The carrying values of other current assets, property and equipment, other non-current assets and other current liabilities were deter-mined to approximate their fair values. The fair value assigned to acquired IPR&D, which was calculated using the multi-period excess earnings method of the income approach, was based on the present value of expected after-tax cash flows attributable to Rina-S, which was in Phase 1/2 testing. The present value of expected after-tax cash flows obtainable from Rina-S and assigned to IPR&D was determined by estimating the after-tax costs to complete development of Rina-S into a commercially viable product, esti-mating future revenue and ongoing expenses to produce, support and sell Rina-S, on an after-tax basis, and discounting the resulting net cash flows to present value. The revenue and costs projections used were reduced based on the probability that compounds at similar stages of development will become commercially viable products. The rate utilized to discount the net cash flows to their present value reflects the risk associated with the future earnings attrib-utable to the intangible asset. Acquired IPR&D will be accounted for as an intangible asset not yet available for use until regulatory approval in a major market is received or development is discontinued. The fair value of the technology platform intan-gible asset was calculated using the relief from royalty method of the income approach. This method includes assigning value based on the economic savings from owning, rather than in-licensing, the technology platform intangible asset supported by observable market data for peer companies, then discounting the resulting probability adjusted net post-tax cash flows using a discount rate commensurate with the risk associated with the future income or cost savings attributable to the intangible asset. The significant assumptions used to estimate the value of the acquired intangible assets include discount rates and certain assumptions that form the basis of future cash flows (such as probabilities of technical and regulatory success, revenue growth rates, operating margins, and royalty rates). The excess of purchase price over the fair value amounts assigned to identifiable assets acquired and liabilities assumed represents the goodwill amount resulting from the acquisition. The goodwill recorded as part of the acquisition is attributable to the intangible assets that do not qualify for separate recognition at the time of the acquisition, assembled workforce and deferred tax consequences of the IPR&D and technology platform intangible asset recorded for financial statement purposes. Genmab does not expect any portion of this goodwill to be deductible for tax purposes. The goodwill attributable to the acquisition has been recorded as a non-current asset in Genmab’s Consolidated Balance Sheets and is not amortized, but is subject to review for impairment annually. Refer to Note 3.1 for further details related to the accounting for goodwill. From the Acquisition Date through December 31, 2024, Genmab’s Consolidated Statements of Comprehensive Income include no revenue and the following expenses associated with the acquisition and operations of ProfoundBio (in DKK millions): Acquisition Date through December 31, 2024 Consolidated Statements of Comprehensive Income (DKK million): Research and development expenses 403 Selling, general and administrative expenses 27 Acquisition and integration related charges* 187 Total 617 *Acquisition related charges incurred from the Acquisition Date through December 31, 2024, are comprised of payments to holders of outstanding ProfoundBio equity awards related to post-combination services (DKK 79 million). The remaining expenses are integration related charges incurred from the Acquisition Date through December 31, 2024, which are comprised of professional fees incurred to assist with the integration of ProfoundBio into Genmab’s operations post-acqui-sition. Additionally, prior to the Acquisition Date, Genmab recorded DKK 113 million in Acquisition and integration related charges in Genmab’s Consolidated Statements of Comprehensive Income related to professional due diligence procedures in connection with the acquisition of ProfoundBio. The DKK 113 million of Acquisition and integration related charges incurred prior to the Acquisition Date and the DKK 187 million of Acquisition and integration charges incurred from the Acquisition Date through December 31, 2024 total DKK 300 million through the fourth quarter of 2024. The following table provides Genmab’s consol-idated revenue and net profit for 2024 as if the acquisition of ProfoundBio had occurred on January 1, 2024 (in DKK millions): (DKK million) Twelve Month Period Ended December 31, 2024 Revenue 21,526 Net Profit 7,622 The unaudited pro forma information does not necessarily reflect the actual results of opera-tions of the combined entities that would have been achieved, nor are they necessarily indicative of future results of operations. The unaudited pro forma information reflects certain adjustments that were directly attributable to the acquisition of ProfoundBio, including additional amortization adjustments for the fair value of the technology platform intangible asset acquired. As of December 31, 2024, Cash and cash equiv-alents in Genmab’s Consolidated Balance Sheets includes USD 30 million (DKK 214 million) of restricted cash balances for funds held in escrow related to the acquisition of ProfoundBio. Accounting Policies Business Combinations The acquisition method of accounting is used to account for all acquisitions where the target company meets the definition of a business in accordance with IFRS 3 (Business Combinations). The purchase price for a business is comprised of the fair value of the assets transferred and liabilities owned to the former owners, including option holders, of the acquired business and the fair value of any asset or liability resulting from Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 179 |
|
a contingent consideration arrangement. Any amount of the purchase price which effectively comprises a settlement of a pre-existing relation-ship is not part of the exchange for the acquiree and is therefore not included in the consider-ation for the purpose of applying the acquisition method. Settlements of pre-existing relationships are accounted for as separate transactions in accordance with the relevant IFRS standards. Identifiable assets and liabilities and contin-gent liabilities assumed are measured at fair value on the date of acquisition by applying relevant valuation methods. Goodwill is recog-nized as the excess of purchase price over the fair value of net identifiable assets acquired and liabilities assumed. Acquisition-related charges are expensed as incurred and included within Acquisition and integration related charges in the Consolidated Statements of Comprehensive Income. Management’s Judgements and Estimates—Other Intangible Assets and Goodwill Fair Value and Impairment Assessment of Other Intangible Assets and Goodwill The application of the acquisition method involves the use of significant estimates because the identifiable net assets of the acquiree are recognized at their fair values for which observ-able market prices are typically not available. This is particularly relevant for intangible assets which require use of valuation techniques typically based on estimates of present value of future uncertain cash flows. The significant assump-tions used to estimate the value of the acquired intangible assets include discount rates and certain assumptions that form the basis of future cash flows (such as probabilities of technical and regulatory success, revenue growth rates, operating margins, and royalty rates). 5.6 Collaborations and Licenses Collaborations Genmab enters into collaborations with biotechnology and pharmaceutical companies to advance the development and commercial-ization of Genmab’s product candidates and to supplement its internal pipeline. Genmab seeks collaborations that will allow Genmab to retain significant future participation in product sales through either profit-sharing or royalties paid on net sales. Below is an overview of certain of Genmab’s collaborations that have had, or are expected in the near term to have, a significant impact on financial results. J&J (Daratumumab/DARZALEX) In 2012, Genmab entered into a global license, development and commercialization agreement with J&J for daratumumab (marketed for the treatment of certain multiple myeloma indica-tions as DARZALEX for IV administration and as DARZALEX FASPRO in the U.S. and DARZALEX SC in Europe for SC administration). Under this agreement, J&J is fully responsible for devel-oping and commercializing daratumumab, and all costs associated therewith. Genmab receives tiered royalty payments between 12% and 20% based on J&J’s annual net product sales with J&J reducing such royalty payments for Genmab’s share of J&J’s royalty payments made to Halozyme. In addition, the royalties payable by J&J are limited in time and subject to reduction on a country-by-country basis for customary reduction events, including for lack of Genmab patent coverage or upon patent expiration or invalidation in the relevant country and upon the first commercial sale of a biosimilar product in the relevant country (for as long as the biosim-ilar product remains for sale in that country). Pursuant to the terms of the agreement, J&J’s obligation to pay royalties to us will expire on a country-by-country basis on the later of the date that is 13 years after the first commercial sale of daratumumab in such country or upon the expiration or invalidation of the last-to-expire relevant Genmab patent covering daratumumab in such country. The first U.S., European and Japanese sales of daratumumab occurred in 2015, 2016 and 2017, respectively. We have issued patents and pending patent applications covering daratumumab in numerous jurisdictions, including patents issued in the U.S., Europe and Japan. J&J owns a separate patent portfolio related to the subcutaneous formulation of dara-tumumab used in DARZALEX FASPRO/DARZALEX SC, but a binding arbitration determined that we are not entitled to royalties based on these separate patents. Our issued U.S., European and Japanese patents covering daratumumab, after giving effect to issued U.S., European and Japanese patent term extensions and supplementary protection certificates, expire in 2029, 2031 and begin to expire in 2030, respectively. Assuming constant underlying sales of DARZALEX, we expect that our royalties from sales of DARZALEX will begin to decline materially in 2029 following expiration of our U.S. patent rights on daratumumab. Genmab is also eligible to receive certain additional payments in connection with development, regu-latory and sales milestones. In September 2020, Genmab commenced arbi-tration against J&J with respect to two different provisions of our license agreement for dara-tumumab, both relating to royalties payable to Genmab on net sales of daratumumab (marketed as DARZALEX for IV administration and as DARZALEX FASPRO in the U.S. and as DARZALEX SC in Europe for SC administration). In April 2022, the arbitral tribunal issued an award in that arbi-tration denying both of Genmab’s claims. Genmab did not seek review of the award. On June 9, 2022, Genmab announced the commencement of a second arbitration under the daratumumab license agreement with Janssen with claims for milestone payments for daratu-mumab SC of USD 405 million and a separate 13-year royalty term for daratumumab SC on a country-by-country basis, from the date of the first commercial sale of daratumumab SC in each such country. This second arbitration followed from the award in the prior arbitration, where the tribunal ruled in favor of Janssen on the question as to whether Genmab is required to share in Janssen’s royalty payments to Halozyme for its technology used in the daratumumab SC product. The tribunal based its ruling on the finding that DARZALEX FASPRO constitutes a new licensed product under the license agreement. On April 21, 2023, the arbitral tribunal dismissed Genmab’s claims regarding the second arbi-tration, on the basis that these claims should have been brought in the first arbitration. One arbitrator dissented. Genmab filed a request for review of the award, which was denied on January 23, 2024. As a result, the dismissal of Genmab’s claims in the second arbitration is now final. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 180 |
|
Novartis (Ofatumumab/Kesimpta) Genmab and GlaxoSmithKline (GSK) entered a co-development and collaboration agreement for ofatumumab in 2006. The full rights to ofatu-mumab were transferred from GSK to Novartis in 2015. Novartis is now fully responsible for the development and commercialization of ofatu-mumab in all potential indications, including autoimmune diseases. Genmab is entitled to a 10% royalty payment on net sales for non-cancer treatments. Genmab pays a royalty to Medarex based on Kesimpta net sales. Novartis’s obli-gation to pay royalties to Genmab under this agreement expire on a country-by-country basis only in the event Novartis is no longer selling such product in a given country. The royalties are on a country-by-country basis subject to reduction in case of significant competition by competing products (as defined in the agreement) or a joint committee determination that a license of intellectual property owned by a third-party is necessary for commercialization. Roche (Teprotumumab/TEPEZZA) In May 2001, Genmab entered a research collaboration with Roche to develop human anti-bodies to disease targets identified by Roche. In 2002, this alliance was expanded. Under the agreement, Genmab will receive milestones as well as royalty payments on successful products. Teprotumumab was initially developed in collaboration between Genmab and Roche, and later investigated under license from Roche by River Vision Development Corporation subsequently and Horizon Therapeutics for ophthalmic use. The product was approved under the brand name TEPEZZA in 2020 by the U.S. FDA for the treatment of TED and in 2024 by Japan’s MHLW for the treatment of active or high clinical activity score (CAS) TED. In October 2023, Amgen completed its acquisition of Horizon Therapeutics, including all rights to the development and commercialization of teprotumumab. Under the terms of Genmab’s agreement with Roche, Genmab receives a mid-single digit royalty on net sales of TEPEZZA, on a country-by-country basis, for 10 years following the first commercial sale in such country. Pfizer (Tisotumab vedotin/Tivdak) In September 2010, Genmab and Pfizer entered into an ADC collaboration, and a commer-cial license and collaboration agreement was executed in October 2011. In October 2020, Genmab and Pfizer entered into a Joint Commercialization Agreement where Genmab would co-promote tisotumab vedotin, marketed as Tivdak, in the U.S., and lead commercial operational activities and record sales in Japan, while Pfizer would lead operational commercial activities in the U.S., Europe and China with a 50:50 profit split in those markets. In all other markets, if any, Pfizer would be responsible for commercializing tisotumab vedotin and Genmab would receive royalties based on a percentage of aggregate net sales ranging from the mid-teens to the mid-twenties. Effective January 1, 2025, Genmab and Pfizer agreed to amend the License and Collaboration Agreement and the Joint Commercialization Agreement for Tivdak, assigning Genmab sole responsibility for the development and commercialization of Tivdak for second line plus recurrent or metastatic cervical cancer in Europe and all other regions globally, excluding the United States and the China region. With this amendment, Genmab will continue to co-promote Tivdak with Pfizer in the U.S. and will record sales for Europe, Japan and rest of world markets (excluding the United States and China regions), once commercialized, and will provide royalties to Pfizer on net sales in the low teens. Pfizer will continue to lead commercial-ization activities in China, when approved. The companies will continue the practice of joint deci-sion-making on the worldwide development and commercialization strategy for tisotumab vedotin. AbbVie (Epcoritamab/ EPKINLY/TEPKINLY) On June 10, 2020, Genmab entered into a broad oncology collaboration agreement with AbbVie to jointly develop and commercialize products including epcoritamab, and subsequently into a discovery research collaboration for up to four future differentiated antibody therapeutics for cancer. The companies will share commercial responsibilities for epcoritamab in the U.S. and Japan, with AbbVie responsible for further global commercialization. Genmab is the principal for net sales in the U.S. and Japan and receives tiered royalties between 22% and 26% on remaining net sales outside of these territories, subject to certain royalty reductions. For any product candidates developed as a result of the companies’ discovery research collaboration, Genmab and AbbVie will share responsibilities for global development and commercialization in the U.S. and Japan. Genmab retains the right to co-commercialize these products, along with AbbVie, outside of the U.S. and Japan. Under the terms of the agreement, Genmab received a USD 750 million (DKK 4,911 million) upfront payment in June 2020 and was initially entitled to receive an aggregate of up to USD 3.15 billion in additional development, regulatory and sales milestone payments for all programs. Included in these potential milestones were up to USD 1.15 billion in payments related to clinical development and commercial success across the three bispecific antibody programs originally included in the agreement. As a result of two programs being stopped, Genmab is instead contractually entitled to receive an aggregate of up to USD 2.55 billion in additional development, regulatory and sales milestone payments for all programs including an aggregate of up to USD 550 million in payments related to clinical development and commer-cial success for the one remaining bispecific antibody program, epcoritamab, included in the original agreement. In addition, and also included in these potential milestones, if all four next-generation antibody product candidates developed as a result of the discovery research collaboration are successful, Genmab is eligible to receive up to USD 2.0 billion in option exercise and success-based milestones. In May 2023, epcoritamab received initial approval from the U.S. FDA and is marketed under the tradename EPKINLY. In September 2023, epcoritamab received initial approval from the EC and the Japan MHLW and is marketed under the tradenames TEPKINLY and EPKINLY, respec-tively. Genmab is entitled to tiered royalties between 22% and 26% on net sales for epcor-itamab outside the U.S. and Japan. Except for these royalty-bearing sales, Genmab will share with AbbVie profits from the sale of licensed products on a 50:50 basis. Genmab and AbbVie split 50:50 the development costs related to epcoritamab, while Genmab will be responsible for 100% of the costs of the discovery research programs up to opt-in. The total transaction price of USD 750 million (DKK 4,911 million) was allocated to the four performance obligations based on the best estimate of relative stand-alone selling prices. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 181 |
|
The allocation of the transaction price to the performance obligations is summarized below: • Delivery of licenses for the three programs: USD 672 million (DKK 4,398 million) • Co-development activities for the product concepts: USD 78 million (DKK 513 million) For the license grants, Genmab based the stand-alone selling price on a discounted cash flow approach and considered several factors including, but not limited to, discount rate, devel-opment timeline, regulatory risks, estimated market demand and future revenue potential. For co-development activities related to up to four product concepts, a cost-plus margin approach was utilized. The performance obligations related to the delivery of licenses were completed at a point in time (June 2020) and Genmab recognized USD 672 million (DKK 4,398 million) as license fee revenue in June 2020. After delivery of the licenses, Genmab shares further development and commercial costs equally with AbbVie. AbbVie is not assessed as a customer but as a collaboration partner, and as such this part of the collaboration is not in scope of IFRS 15. Refer to Note 3.7 for information pertaining to the remaining performance obligation related to co-development activities for the product concepts. BioNTech In May 2015, Genmab entered into an agreement with BioNTech to jointly research, develop and commercialize bispecific antibody products using Genmab’s DuoBody technology platform. Under the terms of the agreement, BioNTech will provide proprietary antibodies against key immunomodu-latory targets, while Genmab provides proprietary antibodies and access to its DuoBody technology platform. Genmab paid an upfront fee of USD 10 million to BioNTech and an additional fee as certain BioNTech assets were selected for further development. If the companies jointly select any product candidates for clinical development, development costs and product ownership will be shared equally going forward. If one of the companies does not wish to move a product candidate forward, the other company is entitled to continue developing the product on prede-termined licensing terms. The agreement also includes provisions which will allow the parties to opt out of joint development at key points. During July 2022, Genmab and BioNTech expanded this collaboration to include the joint research, devel-opment and commercialization of monospecific antibody candidates using Genmab’s HexaBody technology platform. Genmab and BioNTech have three investigational medicines currently in clinical development: DuoBody-CD40x4-1BB (GEN1042/BNT312), HexaBody-OX40 (GEN1055/BNT315) and DuoBody-EpCAMx4-1BB (GEN1059/BNT314). In August 2024, BioNTech opted not to participate in the further development of the acasunlimab (GEN1046) program under the parties’ existing License and Collaboration Agreement for reasons related to BioNTech’s portfolio strategy. Genmab assumed sole responsibility for the continued development and potential commercialization of acasunlimab and the program will be subject to payment of certain milestones and a tiered single-digit royalty on net sales by Genmab to BioNTech. J&J (DuoBody) In July 2012, and as amended in December 2013, Genmab entered into a collaboration with J&J to create and develop bispecific antibodies using our DuoBody technology platform. As of December 31, 2024, three DuoBody-based products created under this collaboration were in active clinical development and had been approved by regulatory authorities: RYBREVANT, TECVAYLI and TALVEY. Under our agreement with J&J, Genmab is eligible to receive milestones and receives royalties between 8% and 10% on net sales of RYBREVANT, a mid-single digit royalty on net sales of TECVAYLI, and a mid-single digit royalty on net sales of TALVEY, all of which are subject to a reduction of such royalty payment in countries and territories where there are no relevant patents (as defined in the agreement), among other reductions. Pursuant to the terms of the DuoBody agreement, J&J’s obligation to pay these royalties will expire on a country-by-country and licensed product-by-licensed product basis on the later of the date that is 10 years after the first sale of each licensed product in such country or upon the expiration of the last-to-expire relevant patent (as defined in the agreement) covering the licensed product in such country. Genmab pays a royalty to Medarex based on RYBREVANT net sales. 5.7 Contingencies Legal Contingency In 2024, Chugai filed a lawsuit in the Tokyo District Court, Japan against AbbVie’s and Genmab’s subsidiaries in Japan asserting that their activities with EPKINLY (epcoritamab) in Japan infringe two Japanese patents held by Chugai, JP6278598 and JP6773929. Chugai is claiming damages and injunctive relief. Genmab and AbbVie believe that the two Japanese patents are invalid and not infringed and intend to vigorously defend against the lawsuit, and thus no provision has been recognized related to this matter. Financial Guarantees As of December 31, 2024 and December 31, 2023, Genmab has financial bank guarantees of DKK 16 million issued as security for lease obligations under certain lease agreements. The likelihood of a claim under the guarantees has been assessed to be remote due to Genmab’ strong financial position and history of fulfilling lease payments. Accordingly, no provision has been recognized related to this matter. 5.8 Subsequent Events Management has determined it is appropriate to change the functional currency of the Genmab A/S legal entity from DKK to USD effective January 1, 2025. This determination was made based on the growing number and significance of the underlying USD transactions, triggered by the commercialization of EPKINLY. Effective for the first quarter of 2025, the consolidated financial statements will also be presented in USD, which will be both the functional and presentation currency of the parent company. No other events have occurred subsequent to the balance sheet date that could signifi-cantly affect the financial statements as of December 31, 2024. Financial Statements for the Genmab Group Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 182 |
|
Financial Statements of the Parent Company Table of Contents Financial Statements of the Parent Company 184 Income Statements 185 Balance Sheets 186 Statements of Cash Flows 187 Statements of Changes in Equity Notes to the Financial Statements of the Parent Company 188 1 Accounting Policies 189 2 Revenue 189 3 Staff Costs 189 4 Corporate and Deferred Tax 190 5 Intangible Assets 191 6 Property and Equipment 192 7 Leases 192 8 Other Investments 192 9 Inventories 193 10 Receivables 193 11 Contract Liabilities 193 12 Other Payables 193 13 Marketable Securities 194 14 Financial Income and Expenses 194 15 Remuneration of the Board of Directors and Executive Management 195 16 Related Party Disclosures 195 17 Investments in Subsidiaries 196 18 Commitments and Contingencies 196 19 Fees to Auditors Appointed at the Annual General Meeting Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 183 |
|
Financial Statements of the Parent Company Income Statements (DKK million) Note 2024 2023 Revenue 2 22,167 17,126 Cost of product sales (502) (86) Research and development expenses 3, 5, 6 (10,358) (8,826) Selling, general and administrative expenses 3, 6 (1,949) (2,521) Integration related charges (30) – Total costs and operating expenses (12,839) (11,433) Operating profit 9,328 5,693 Financial income 14, 17 17,404 2,199 Financial expenses 14, 17 (12,239) (1,871) Net profit before tax 14,493 6,021 Corporate tax 4 (2,626) (1,277) Net profit 11,867 4,744 Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 184 |
|
Financial Statements of the Parent Company Balance Sheets (DKK million) Note December 31, 2024 December 31, 2023 Assets Intangible assets 5 13,369 378 Property and equipment 6 109 129 Right-of-use assets 7 239 232 Investments in subsidiaries 17 6,114 3,308 Receivables 10 22 49 Deferred tax assets 4 – 198 Other investments 8 179 87 Total non-current assets 20,032 4,381 Corporate tax receivable 4 101 – Inventories 9 10 31 Receivables 10 5,726 4,528 Receivables from subsidiaries 10 964 650 Marketable securities 13 11,243 13,268 Cash and cash equivalents 8,993 14,467 Total current assets 27,037 32,944 Total assets 47,069 37,325 Shareholders’ Equity and Liabilities Share capital 66 66 Share premium 12,590 12,461 Retained earnings 28,940 20,347 Total shareholders’ equity 41,596 32,874 Lease liabilities 7 235 227 Deferred revenue 11 480 480 Deferred tax liabilities 4 2,359 – Other payables 12 20 20 Total non-current liabilities 3,094 727 Corporate tax payable 4 – 45 Payable to subsidiaries 12 887 2,525 Lease liabilities 7 16 19 Deferred revenue 11 24 33 Other payables 12 1,452 1,102 Total current liabilities 2,379 3,724 Total liabilities 5,473 4,451 Total shareholders’ equity and liabilities 47,069 37,325 Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 185 |
|
Financial Statements of the Parent Company Statements of Cash Flows (DKK million) Note 2024 2023 Cash flows from operating activities: Net profit before tax 14,493 6,021 Financial Income 14 (17,404) (2,199) Financial Expenses 14 12,239 1,871 Adjustment for non-cash transactions Share-based compensation expense 88 84 Depreciation 40 32 Amortization 36 29 Impairment charges 282 – Change in operating assets and liabilities Receivables (1,173) 1,062 Inventories 22 (31) Other Payables 352 207 Cash provided by operating activities before financial items 8,975 7,076 Interest received 905 888 Interest elements of lease payments 7 (10) (9) Interest paid – (1) Corporate taxes (paid)/received (317) (1,056) Net cash provided by operating activities 9,553 6,898 Cash flows from investing activities: Transactions with subsidiaries (14,198) 868 Investment in intangible assets 5 (198) (82) Investment in tangible assets 6 (6) (117) Marketable securities bought (8,581) (10,876) Marketable securities sold 11,279 10,001 Other investments bought (42) (30) Net cash (used in) investing activities (11,746) (236) Cash flows from financing activities: Warrants exercised 129 152 Principal elements of lease payments 7 (13) (15) Purchase of treasury shares (3,879) (564) Payment of withholding taxes on behalf of employees on net settled RSUs (109) (103) Net cash (used in) financing activities (3,872) (530) Changes in cash and cash equivalents (6,065) 6,132 Cash and cash equivalents at the beginning of the period 14,467 8,830 Exchange rate adjustments 591 (495) Cash and cash equivalents at the end of the period 8,993 14,467 Cash and cash equivalents include: Bank deposits 8,911 13,114 Short-term marketable securities 82 1,353 Cash and cash equivalents at the end of the period 8,993 14,467 Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 186 |
|
Financial Statements of the Parent Company Statements of Changes in Equity (DKK million) Share capital Share premium Retained earnings Shareholders’ equity Balance at December 31, 2022 66 12,309 15,741 28,116 Net profit – – 4,744 4,744 Exercise of warrants – 152 – 152 Purchase of treasury shares – – (564) (564) Share-based compensation expenses – – 586 586 Withholding taxes on behalf of employees on net settled RSUs – – (103) (103) Tax on items recognized directly in equity – – (57) (57) Balance at December 31, 2023 66 12,461 20,347 32,874 Net profit – – 11,867 11,867 Exercise of warrants – 129 – 129 Purchase of treasury shares – – (3,879) (3,879) Share-based compensation expenses – – 721 721 Withholding taxes on behalf of employees on net settled RSUs – – (109) (109) Tax on items recognized directly in equity – – (7) (7) Balance at December 31, 2024 66 12,590 28,940 41,596 Distribution of the Year’s Profit The Board proposes that the parent company’s 2024 net profit of DKK 11,867 million (2023: net profit of DKK 4,744 million) be carried forward to next year by transfer to retained earnings. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 187 |
|
Notes to the Financial Statements of the Parent Company 1 Accounting Policies The financial statements of the parent company have been prepared in accordance with the IFRS Accounting Standards as issued by the International Accounting Standards Board (IASB) and in accordance with IFRS as endorsed by the EU and further disclosure requirements for listed companies in Denmark. A number of new or amended standards became applicable for the current reporting period. Genmab A/S did not have to change its accounting policies as a result of the adoption of these standards. Refer to Note 1.2 in the consolidated financial statements for a description of new accounting policies and disclosures of the Group. Refer to Note 1.3 in the consolidated financial statements for a description of management’s judgements and estimates under IFRS. Refer to Note 1.4 in the consolidated financial statements for additional information regarding the immaterial reclassifications and revisions of the Group financial statements. Supplementary Accounting Policies for the Parent Company Investments in Subsidiaries The cost method is used for measuring the investments in subsidiaries. Under the cost method, investments in subsidiaries are measured at historical cost. Equity interests in foreign currencies are translated to the reporting currency by use of historical exchange rates prevailing at the time of investment. Additions to the carrying value of investment in subsidiaries include capital contributions made by the parent and share-based payment trans-actions related to employees of the respective subsidiaries based on where the employee has rendered service. Distributions from the investment are recog-nized as income when declared, if any. If the distribution exceeds the current period income or if circumstances or changes in Genmab’s oper-ations indicate that the carrying amount of the subsidiary may not be recoverable, the carrying amount is tested for impairment. Where the recoverable amount of the investments is lower than cost, the investments are written down to this lower value. Refer to Note 1.1 in the consolidated financial statements for a description of the accounting policies of the Group. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 188 |
|
2 Revenue (DKK million) 2024 2023 Revenue by type: Royalties 17,352 13,705 Reimbursement revenue—External 996 864 Reimbursement revenue—Intercompany 1,140 937 Milestone revenue 1,000 1,177 Collaboration revenue 433 307 License revenue 2 – Net product sales—Intercompany 1,244 136 Total 22,167 17,126 Revenue by collaboration partner: Janssen 14,422 11,949 AbbVie 394 732 Roche 741 704 Novartis 2,822 1,511 BioNTech 869 784 Pfizer1 533 373 Other 2 – Total2 19,783 16,053 Royalties by product: DARZALEX 13,922 11,265 Kesimpta 2,222 1,494 TEPEZZA 737 704 Other3 471 242 Total 17,352 13,705 1. Pzifer acquired Seagen in December 2023 2. Excludes Genmab’s intercompany revenue 3.Other consist of royalties from net sales of RYBREVANT, TECVAYLI, TALVEY and TEPKINLY Refer to Note 2.1 in the consolidated financial statements for additional information regarding revenue of the Group. 3 Staff Costs (DKK million) 2024 2023 Wages and salaries 566 500 Share-based compensation 88 84 Defined contribution plans 48 39 Other social security costs 2 9 Total 704 632 Staff costs are included in the income statement as follows: Research and development expenses 539 501 Selling, general and administrative expenses 165 131 Total 704 632 Average number of FTE 492 440 Number of FTE at year-end 519 465 Refer to Note 2.3 in the consolidated financial statements for additional information regarding staff costs of the Group. 4 Corporate and Deferred Tax Taxation—Income Statement & Shareholders’ Equity (DKK million) 2024 2023 Current tax: Current tax on profit 82 1,288 Deferred taxes 2,544 (11) Total tax for the period in the income statement 2,626 1,277 Notes to the Financial Statements of the Parent Company Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 189 |
|
A reconciliation of Genmab’s effective tax rate relative to the Danish statutory tax rate is as follows: (DKK million) 2024 2023 Net profit before tax 14,493 6,021 Tax at the Danish statutory corporation tax rate of 22% for all periods 3,188 1,325 Tax effect of: Net of non-taxable income over non-deductible expenses (659) (52) Other current and deferred taxes adjustments 97 4 Total tax effect (562) (48) Total tax for the period in the income statement 2,626 1,277 Total tax for the period in shareholders’ equity 7 57 Effective Tax Rate 18.1% 21.2% Taxation—Balance Sheet Significant components of the deferred tax (liabilities) assets are as follows: (DKK million) 2024 2023 Share-based instruments 39 37 Deferred revenue 120 113 Intangible Assets (2,874) Other temporary differences 356 48 Total deferred tax (liabilities) assets (2,359) 198 Refer to Note 2.4 in the consolidated financial statements for additional information regarding corporate and deferred tax of the Group. 5 Intangible Assets (DKK million) Licenses and Patents Technology Platform Acquired IPR&D Total Intangible Assets 2024 Cost at the beginning of the year 1,093 – – 1,093 Additions during the year 244 1,237 11,789 13,270 Cost at the end of the year 1,337 1,237 11,789 14,363 Amortization and impairment losses at the beginning of the year 715 – – 715 Amortization for the year 33 3 – 36 Impairment losses for the year 243 – – 243 Amortization and impairment losses at the end of the year 991 3 – 994 Carrying amount at the end of the year 346 1,234 11,789 13,369 2023 Cost at the beginning of the year 1,011 – – 1,011 Additions during the year 82 – – 82 Cost at the end of the year 1,093 – – 1,093 Amortization and impairment losses at the beginning of the year 654 – – 654 Amortization for the year 61 – – 61 Amortization and impairment losses at the end of the year 715 – – 715 Carrying amount at the end of the year 378 – – 378 Parent Company intangible assets include IPR&D, a technology platform asset and licenses and rights primarily to gain access to targets and technologies identified by third parties as well as subsidiaries. Refer to Note 3.1 in the consolidated financial statements for additional information regarding intangible assets of the Group. Refer to Note 17 in the parent financial statements for additional information regarding the intangible assets and goodwill acquired through the ProfoundBio acquisition. Notes to the Financial Statements of the Parent Company Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 190 |
|
Intangible Assets The increase in the gross carrying value of intan-gible assets during 2024 was primarily due to the addition of approximately DKK 11,789 million of IPR&D and DKK 1,237 million of a technology platform asset from the ProfoundBio acquisition. The technology platform asset is being amortized over its estimated useful life of 15 years. These intellectual property rights were transferred from ProfoundBio US to Genmab A/S during the fourth quarter of 2024. Impairment expenses related to licenses and patents were DKK 243 million in 2024 and were not material in 2023. Impairment expenses were recorded in Research and development expenses in the Parent Company Income Statements. Amortization expense was DKK 36 million and DKK 61 million for 2024 and 2023, respectively, which was recorded in Research and develop-ment expenses in the Income Statements of the Parent Company. 6 Property and Equipment (DKK million) Leasehold improvements Equipment, furniture and fixtures Assets under construction Total property and equipment 2024 Cost at January 1 78 82 – 160 Additions for the year – 2 2 4 Transfers between the classes – 1 – 1 Disposals for the year (4) (9) (2) (15) Cost at December 31 74 76 – 150 Accumulated depreciation and impairment at January 1 (7) (24) – (31) Depreciation for the year (4) (19) – (23) Disposals for the year 4 9 – 13 Accumulated depreciation and impairment at December 31 (7) (34) – (41) Carrying amount at December 31 67 42 – 109 2023 Cost at January 1 4 24 17 45 Additions for the year 5 10 100 115 Transfers between the classes 69 48 (117) – Disposals for the year – – – – Cost at December 31 78 82 – 160 Accumulated depreciation and impairment at January 1 (4) (15) – (19) Depreciation for the year (3) (9) – (12) Disposals for the year – – – – Accumulated depreciation and impairment at December 31 (7) (24) – (31) Carrying amount at December 31 71 58 – 129 (DKK million) 2024 2023 Depreciation and impairment included in the income statement as follows: Research and development expenses 18 6 Selling, general and administrative expenses 5 6 Total 23 12 Refer to Note 3.2 in the consolidated financial statements for additional information regarding property and equipment of the Group. Notes to the Financial Statements of the Parent Company Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 191 |
|
7 Leases The parent company has entered into lease agreements with respect to office and laboratory space. The leases are non-cancellable over various periods through 2038. (DKK million) 2024 2023 Right-of-use assets Balance at January 1 232 9 Additions to right-of-use assets1 24 242 Depreciation charge for the year (17) (19) Balance at December 31 239 232 Lease liabilities Current 16 19 Non-current 235 227 Total at December 31 251 246 Cash outflow for lease payments 23 24 1. Additions to right-of-use assets also includes modifications to existing leases and adjustments to the provisions for contractual restoration obligations related to leases of Genmab offices. Variable lease payments, lease interest expense, and low-value assets are immaterial. Future minimum payments under leases are as follows: (DKK million) 2024 2023 Payment due Less than 1 year 27 23 1 to 3 years 53 45 More than 3 years but less than 5 years 54 45 More than 5 years 186 202 Total at December 31 320 315 Refer to Note 3.3 in the consolidated financial statements for additional information regarding leases of the Group. 8 Other Investments (DKK million) 2024 2023 Fund Investments 165 87 Privately held equity securities 14 – Total at December 31 179 87 Refer to Note 3.4 to the consolidated financial statements for additional information on other investments of the Group. 9 Inventories (DKK million) 2024 2023 Raw materials 4 14 Work in progress – – Finished goods 6 19 Total inventories (gross) at December 31 10 33 Allowances at year end – (2) Total inventories (net) at December 31 10 31 Refer to Note 3.5 in the consolidated financial statements for additional information regarding inventories of the Group. Notes to the Financial Statements of the Parent Company Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 192 |
|
10 Receivables (DKK million) 2024 2023 Receivables related to collaboration agreements 5,434 4,148 Prepayments 107 121 Receivables from subsidiaries 964 650 Interest receivables 135 149 Other receivables 72 159 Total at December 31 6,712 5,227 Non-current receivables 22 49 Current receivables 6,690 5,178 Total at December 31 6,712 5,227 Refer to Note 3.6 in the consolidated financial statements for additional information regarding receivables of the Group. 11 Contract Liabilities (DKK million) 2024 2023 Contract liabilities at January 1 513 513 Customer payment received – – Revenue recognized during the year (9) – Total at December 31 504 513 Non-current contract liabilities 480 480 Current contract liabilities 24 33 Total at December 31 504 513 Refer to Note 3.7 in the consolidated financial statements for additional information regarding contract liabilities of the Group. 12 Other Payables (DKK million) 2024 2023 Liabilities related to collaboration agreements 126 47 Staff cost liabilities 91 106 Accounts payable 187 107 Payable to subsidiaries 887 2,525 Other liabilities 1,068 862 Total at December 31 2,359 3,647 Non-current other payables 20 20 Current other payables 2,339 3,627 Total at December 31 2,359 3,647 Refer to Note 3.8 in the consolidated financial statements for additional information regarding other payables of the Group. 13 Marketable Securities Refer to Note 4.4 in the consolidated financial statements for additional information on marketable securities of the Group. Notes to the Financial Statements of the Parent Company Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 193 |
|
14 Financial Income and Expenses (DKK million) 2024 2023 Financial income: Dividend income from subsidiaries 13,026 – Interest and other financial income 964 962 Interest from subsidiaries 6 1 Gain on marketable securities 364 495 Gain on other investments 121 6 Foreign exchange rate gain 2,923 735 Total financial income 17,404 2,199 Financial expenses: Impairment of investment in subsidiaries (10,402) – Interest and other financial expenses (95) (55) Interest to subsidiaries (7) (10) Loss on marketable securities (147) (175) Loss on other investments (7) (14) Foreign exchange rate loss (1,581) (1,617) Total financial expenses (12,239) (1,871) Net financial items 5,165 328 During the fourth quarter of 2024, ProfoundBio US (an indirect subsidiary of Genmab A/S) sold its intangible assets to Genmab A/S. Following this transaction, Genmab A/S ultimately received dividend income. The dividend income received of DKK 13.0 billion was recognized as Financial Income in the financial statements of the parent company. As a result of the above, due to the significant deterioration in the value of Genmab A/S’ indirect investment in ProfoundBio US, Genmab A/S ultimately recorded a DKK 10.4 billion loss on impairment of its investment in subsidiaries. The difference between the dividend income received by Genmab A/S in this transaction and the loss on impairment of investment in subsidiaries relates to goodwill retained at the subsidiary level. The DKK 10.4 billion impairment loss was recognized as Financial Expense in the financial statements of the parent company. Refer to Note 5.5 in the consolidated financial statements for additional information regarding the acquisition of ProfoundBio and Note 17 in the parent company financial statements for additional information related to investment in subsidiaries. 15 Remuneration of the Board of Directors and Executive Management Remuneration of the Board for the parent, excluding employee elected board members not directly employed by the parent, is the same as the Group. Remuneration of Executive Management not directly employed by the parent company is between 10% and 20% of their total compensation, as defined in their individual service agreement and as reported in Note 5.1 in the consolidated financial statements. Refer to Note 5.1 in the consolidated financial statements for additional information regarding the remuneration of the Board of Directors and Executive Management. Notes to the Financial Statements of the Parent Company Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 194 |
|
16 Related Party Disclosures Genmab A/S’ related parties are the parent company’s subsidiaries, Board, Executive Management, and close members of the family of these persons. Transactions With Subsidiaries Genmab B.V., Genmab Holding B.V., Genmab US, Inc., Genmab K.K., ProfoundBio, Inc., ProfoundBio, US Co., Profound Limited, ProfoundBio co., Ltd., ProfoundBio Shanghai Branch, Co., Ltd., and Bejing Puyifang Biotechnology Co., Ltd. are 100% (directly or indirectly) owned subsidiaries of Genmab A/S and are included in the consolidated financial statements. During 2024, various intercompany transactions and services between the aforementioned companies took place in the field of product sales, research and development, selling, general and administration, finance and management. All intercompany transactions have been eliminated in the consolidated financial statements of the Genmab Group. (DKK million) 2024 2023 Transactions with subsidiaries: Income statement: Net product sales 1,244 136 Reimbursement revenue 1,140 937 Cost of product sales (28) (62) Service fee costs (5,752) (5,326) Milestone costs (545) (893) Impairment investment in subsidiaries (10,402) – Dividend income 13,026 – Financial income 6 1 Financial expense (7) (10) Balance sheet: Intangible assets 1,029 291 Current receivables 964 650 Current payables (887) (2,525) Genmab A/S has placed at each subsidiary’s disposal a credit facility (denominated in local currency) that the subsidiary may use to draw from in order to secure the necessary funding of its activities. Refer to Note 5.2 to the consolidated financial statements for additional information regarding transactions with related parties of the Group. 17 Investments in Subsidiaries (DKK million) 2024 2023 Cost at January 1 5,237 4,735 Additions 13,208 502 Cost at December 31 18,445 5,237 Impairment at January 1 (1,929) (1,929) Impairment for the year (10,402) – Impairment at December 31 (12,331) (1,929) Carrying amount at December 31 6,114 3,308 Additions primarily related to the DKK 12.5 billion capital contribution to Genmab US, for the acquisi-tion of ProfoundBio. A DKK 10.4 billion impairment loss was recorded related to Genmab A/S’ indirect investment in ProfoundBio US. Refer to Note 1.1 in the consolidated financial statements for a listing of subsidiaries owned by Genmab A/S, Note 5.5 in the consolidated financial statements of the group for additional infor-mation regarding the acquisition of ProfoundBio and Note 14 in the parent company financial statements for further details related to the transfer of ProfoundBio US intangible assets to Genmab A/S. Notes to the Financial Statements of the Parent Company Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 195 |
|
Notes to the Financial Statements of the Parent Company 18 Commitments and Contingencies Purchase Obligations Genmab A/S has entered into a number of agreements related to research and development activities that contain various obligations. These contractual obligations amounted to approximately DKK 2,867 million as of December 31, 2024 (2023: approximately DKK 3,145 million). Genmab A/S also has certain contingent commitments under our license and collaboration agree-ments that may become due in the future. As of December 31, 2024, these contingent commitments amounted to approximately DKK 12,304 million (USD 1,723 million) in potential future development, regulatory and commercial milestone payments to third parties under license and collaboration agree-ments for our preclinical and clinical stage development programs as compared to approximately DKK 9,991 million (USD 1,481 million) as of December 31, 2023. These milestone payments generally become due and payable only upon the achievement of certain development, clinical, regulatory or commercial milestones. The events triggering such payments or obligations have not yet occurred. In addition to the above obligations, Genmab A/S enters into a variety of agreements and financial commitments in the normal course of business. The terms generally allow us the option to cancel, reschedule and adjust our requirements based on our business needs prior to the delivery of goods or performance of services. It is not possible to predict the maximum potential amount of future payments under these agreements due to the conditional nature of our obligations and the unique facts and circumstances involved in each particular agreement. Financial Guarantees As of December 31, 2024 and December 31, 2023, Genmab A/S has financial bank guarantees of DKK 16 million issued as security for lease obligations under certain lease agreements. The likelihood of a claim under the guarantees has been assessed to be remote due to Genmab A/S’s strong financial position and history of fulfilling lease payments. Accordingly, no provision has been recognized related to this matter. Refer to Note 5.3 in the consolidated financial statements for additional information regarding commitments of the Group and Note 5.7 in the consolidated financial statements for additional information regarding contingencies of the Group. 19 Fees to Auditors Appointed at the Annual General Meeting (DKK million) 2024 2023 Audit fees 10.4 6.1 Audit-related fees 2.3 3.4 Total 12.7 9.5 Fees for other services than statutory audit of the financial statements provided by Deloitte Statsautoriseret Revisionspartnerselskab amounted to DKK 2.3 million in 2024 (DKK 3.4 million in 2023 provided by PricewaterhouseCoopers Statsautoriseret Revisionspartnerselskab. These services primarily include agreed-upon procedures, other assurance assessments and reports, and accounting advice. Refer to Note 5.4 in the consolidated financial statements for additional information regarding fees to auditors of the Group. Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 196 |
|
The Board of Directors and the Executive Management have today considered and approved the Annual Report of Genmab A/S for the financial year January 1 to December 31, 2024. The Annual Report has been prepared in accordance with IFRS Accounting Standards as issued by the International Accounting Standards Board (IASB) and in accordance with IFRS as endorsed by the EU and further disclosure requirements for listed companies in Denmark. In our opinion, the Consolidated Financial Statements and the Parent Company Financial Statements give a true and fair view of the Group’s and the Parent Company’s financial position at December 31, 2024 as well as of the results of their operations and the Group’s cash flows for the financial year January 1 to December 31, 2024. In our opinion, the Management Review is prepared in accordance with relevant laws and regulations and contains a fair review of the development of the Group’s and the Parent Company’s business and financial matters, the results for the year and of the Parent Company’s financial position and the financial position as a whole of the entities included in the Consolidated Financial Statements, together with a description of the principal risks and uncertainties that the Group and the Parent Company face. The Sustainability Statements are prepared in accordance with the European Sustainability Reporting Standards (ESRS) as required by the Danish Financial Statements Act as well as article 8 in the EU Taxonomy regulation. In our opinion, the Annual Report of Genmab A/S for the financial year January 1 to December 31, 2024, with the file name Genmab-2024-12-31-0-en.zip is prepared, in all material respects, in compliance with the ESEF Regulation. We recommend that the Annual Report be adopted at the Annual General Meeting. Copenhagen, February 12, 2025 Directors’ and Management’s Statement on the Annual Report Executive Management Jan van de Winkel (President & CEO) Anthony Pagano (Executive Vice President & CFO) Board Of Directors Deirdre P. Connelly (Chair) Pernille Erenbjerg (Deputy Chair) Anders Gersel Pedersen Rolf Hoffmann Paolo Paoletti Elizabeth O’Farrell Mijke Zachariasse (Employee elected) Takahiro Hamatani (Employee elected) Martin Schultz (Employee elected) Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 197 |
|
Report on the consolidated financial statements and the parent financial statements To the shareholders of Genmab A/S Opinion We have audited the consolidated financial statements and the parent financial state-ments of Genmab A/S for the financial year January 1, 2024–December 31, 2024, which comprise balance sheet, statements of cash flow, statement of changes in equity and notes, including material accounting policy information for the Group as well as the Parent, statement of comprehensive income of the Group and income statement of the Parent. The consol-idated financial statements and the parent financial statements are prepared in accordance with IFRS Accounting Standards as endorsed by the EU and additional disclosure requirements for listed entities in Denmark. In our opinion, the consolidated financial state-ments and the parent financial statements give a true and fair view of the Group’s and the Parent’s financial position at December 31, 2024, and of the results of its operations and cash flows for the financial year January 1, 2024–December 31, 2024 in accordance with IFRS Accounting Standards as endorsed by the EU and additional disclosure requirements for listed entities in Denmark. Our opinion is consistent with our Long Form Audit Report issued to the Audit & Finance Committee and the Board of Directors. Basis for opinion We conducted our audit in accordance with International Standards on Auditing (ISAs) and the additional requirements applicable in Denmark. Our responsibilities under those standards and requirements are further described in the “Auditor’s responsibilities for the audit of the consolidated financial statements and the parent financial state-ments” section of this auditor’s report. We are independent of the Group in accordance with the International Ethics Standards Board for Accountants’ International Code of Ethics for Professional Accountants (IESBA Code) and the additional ethical requirements applicable in Denmark, and we have fulfilled our other ethical responsibilities in accordance with these requirements and the IESBA Code. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion. To the best of our knowledge and belief, we have not provided any prohibited non-audit services as referred to in Article 5(1) of Regulation (EU) No 537/2014. We were appointed auditors of Genmab A/S for the first time on March 13, 2024, for the financial year 2024. Key audit matters Key audit matters are those matters that, in our professional judgement, were of most significance in our audit of the consolidated financial statements and the parent financial statements for the financial year January 1, 2024–December 31, 2024. These matters were addressed in the context of our audit of the consolidated financial statements and the parent financial statements as a whole, and in forming our opinion thereon, and we do not provide a separate opinion on these matters. Key audit matter How our audit addressed the key audit matter Valuation of Acquired IPR&D Assets in the ProfoundBio, Inc. Acquisition Refer to Notes 3.1 and 5.5 to the financial statements. The Company completed the acquisition of ProfoundBio, Inc. (“ProfoundBio”) for USD 1.72 billion (DKK 11.8 billion) on May 21, 2024. The Company accounted for the acquisition as a business combination and, accordingly, has performed procedures to identify all assets and liabilities and allocated the purchase price to the assets acquired and liabilities assumed based on their respective estimated fair values as of the date of acquisition. Intangible assets acquired primarily included the in-process research and development intangible assets (“Acquired IPR&D assets”). The Company estimated the fair value of the Acquired IPR&D assets using an income approach. The fair value determination of the Acquired IPR&D assets required the Company to make significant estimates and assumptions related to the forecasted future cash flows, such as probabilities of technical and regulatory success, and the determination of the discount rates. We identified the valuation of Acquired IPR&D assets for the ProfoundBio acquisition as a key audit matter because of the high level of complexity and management judgement involved in determining the above outlined significant estimates and assumptions used by the Company to determine the fair value of these assets. This required a high degree of auditor judgement and an increased extent of effort when performing audit procedures to evaluate the reasonableness of management’s estimates and assumptions. We tested the effectiveness of controls over the valuation of the Acquired IPR&D assets, including the Company’s controls over the significant estimates and assumptions related to the forecasted future cash flows, such as probabilities of technical and regulatory success, and the determination of the discount rates. We assessed the reasonableness of the Company’s probabilities of technical and regulatory success used in determination of the fair value of the Acquired IPR&D assets by comparing to internal and external market studies and certain peer companies/ products in the industry. We assessed the reasonableness of the Company’s forecasts of future cash flows used in determination of the fair value of the Acquired IPR&D assets by comparing the forecasts to historical results of operations, certain peer companies within comparable industries, and internal and external market studies. With the assistance of our valuation specialists, we evaluated the reasonableness of the discount rates by testing the source information and inputs underlying the determination of the discount rates, including in relation to publicly available information for comparable companies and testing the mathematical accuracy of the calculation. Independent Auditor’s Reports Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 198 |
|
Key audit matter How our audit addressed the key audit matter Revenue recognition of royalty revenue Refer to Note 2.1 to the financial statements. The Company recognized royalty revenue, where revenue is recognized based on net sales by collaboration partners. The Company uses net sales provided by its collaboration partners as an input to their calculation of the amount of royalty revenue to recognize in each period. The preliminary net sales data provided by the collaboration partner may change once final net sales data is available. We identified the revenue recognition of royalty contracts as a key audit matter because of the significant estimation uncertainty related to the net sales data provided by collaboration partners. Specifically, the collaboration partner’s estimate of net sales could change based on the final net sales impacting the royalty revenue recognized in each period. This required a high degree of auditor judgement and an increased extent of effort when performing audit procedures to evaluate the reasonableness of management’s estimates of the net sales. We tested the effectiveness of controls relating to the evaluation for reasonableness of the estimated net sales used in the determination of royalty revenue recognition. We tested the overall reasonableness of the estimated net sales reported by the collaboration partners by assessing the historical accuracy of the estimates. We obtained external confirmations from selected collaboration partners on the estimated and actual net sales amounts reported. Statement on Management’s Review Management is responsible for the Management Review. Our opinion on the consolidated financial state-ments and the parent financial statements does not cover the Management Review, and we do not as part of the audit express any form of assurance conclusion thereon. In connection with our audit of the consolidated financial statements and the parent financial statements, our responsibility is to read the Management Review and, in doing so, consider whether the Management Review is materially inconsistent with the consolidated financial state-ments and the parent financial statements or our knowledge obtained in the audit or otherwise appears to be materially misstated. Moreover, it is our responsibility to consider whether the Management Review provides the information required by the Danish Financial Statements Act. This does not include the requirements in paragraph 99a related to the Sustainability Statement covered by the separate auditor’s limited assurance report hereon. Based on the work we have performed, in our view, the Management Review is in accordance with the consolidated financial statements and the parent financial statements and has been prepared in accordance with the requirements of the Danish Financial Statements Act except for the requirements in paragraph 99a related to the Sustainability Statement, see above. We did not identify any material misstatement of the Management Review. Management’s responsibilities for the consolidated financial statements and the parent financial statements Management is responsible for the preparation of consolidated financial statements and parent financial statements that give a true and fair view in accordance with IFRS Accounting Standards as endorsed by the EU and additional requirements of the Danish Financial Statements Act, and for such internal control as Management determines is necessary to enable the preparation of consol-idated financial statements and parent financial statements that are free from material misstate-ment, whether due to fraud or error. In preparing the consolidated financial state-ments and the parent financial statements, Management is responsible for assessing the Group’s and the Parent’s ability to continue as a going concern, for disclosing, as applicable, matters related to going concern, and for using the going concern basis of accounting in preparing the consolidated financial statements and the parent financial statements unless Management either intends to liquidate the Group or the Entity or to cease operations, or has no realistic alternative but to do so. Independent Auditor’s Reports Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 199 |
|
Auditor’s responsibilities for the audit of the consolidated financial statements and the parent financial statements Our objectives are to obtain reasonable assurance about whether the consolidated financial statements and the parent financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance, but is not a guarantee that an audit conducted in accordance with ISAs and the additional requirements applicable in Denmark will always detect a material misstatement when it exists. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of these consoli-dated financial statements and these parent financial statements. As part of an audit conducted in accordance with ISAs and the additional requirements applicable in Denmark, we exercise professional judgement and maintain professional scepticism throughout the audit. We also: • Identify and assess the risks of material misstatement of the consolidated financial statements and the parent financial statements, whether due to fraud or error, design and perform audit procedures responsive to those risks, and obtain audit evidence that is sufficient and appropriate to provide a basis for our opinion. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. • Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Group’s and the Parent’s internal control. • Evaluate the appropriateness of accounting policies used and the reasonableness of accounting estimates and related disclosures made by Management. • Conclude on the appropriateness of Management’s use of the going concern basis of accounting in preparing the consolidated financial statements and the parent financial statements, and, based on the audit evidence obtained, whether a material uncertainty exists related to events or conditions that may cast significant doubt on the Group’s and the Parent’s ability to continue as a going concern. If we conclude that a material uncertainty exists, we are required to draw attention in our auditor’s report to the related disclosures in the consolidated financial statements and the parent financial statements or, if such disclosures are inadequate, to modify our opinion. Our conclusions are based on the audit evidence obtained up to the date of our auditor’s report. However, future events or conditions may cause the Group and the Entity to cease to continue as a going concern. • Evaluate the overall presentation, structure and content of the consolidated financial statements and the parent financial statements, including the disclosures in the notes, and whether the consolidated financial statements and the parent financial statements represent the underlying transactions and events in a manner that gives a true and fair view. • Plan and perform the group audit to obtain sufficient and appropriate audit evidence regarding the financial information of the entities or business units within the group as a basis for forming an opinion on the consolidated financial statements and the parent financial statements. We are responsible for the direction, supervision and review of the audit work performed for purposes of the group audit. We remain solely responsible for our audit opinion. We communicate with those charged with governance regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control that we identify during our audit. We also provide those charged with governance with a statement that we have complied with relevant ethical requirements regarding inde-pendence, and to communicate with them all relationships and other matters that may reason-ably be thought to bear on our independence, and, where applicable, safeguards put in place and measures taken to eliminate threats. From the matters communicated with those charged with governance, we determine those matters that were of most significance in the audit of the consolidated financial statements and the parent financial statements of the current period and are therefore the key audit matters. We describe these matters in our auditor’s report unless law or regulation precludes public disclo-sure about the matter or when, in extremely rare circumstances, we determine that a matter should not be communicated in our report because the adverse consequences of doing so would reasonably be expected to outweigh the public interest benefits of such communication. Independent Auditor’s Reports Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 200 |
|
Report on compliance with the ESEF Regulation As part of our audit of the consolidated financial statements and the parent financial statements of Genmab A/S we performed procedures to express an opinion on whether the annual report for the financial year January 1, 2024–December 31, 2024, with the file name Genmab-2024-12-31- 0-en.zip, is prepared, in all material respects, in compliance with the Commission Delegated Regulation (EU) 2019/815 on the European Single Electronic Format (ESEF Regulation), which includes requirements related to the preparation of the annual report in XHTML format and iXBRL tagging of the consolidated financial statements including notes. Management is responsible for preparing an annual report that complies with the ESEF Regulation. This responsibility includes: • The preparing of the annual report in XHTML format; • The selection and application of appropriate iXBRL tags, including extensions to the ESEF taxonomy and the anchoring thereof to elements in the taxonomy, for financial information required to be tagged using judgement where necessary; • Ensuring consistency between iXBRL tagged data and the consolidated financial statements presented in human readable format; and • For such internal control as Management determines necessary to enable the preparation of an annual report that is compliant with the ESEF Regulation. Our responsibility is to obtain reasonable assurance on whether the annual report is prepared, in all material respects, in compliance with the ESEF Regulation based on the evidence we have obtained, and to issue a report that includes our opinion. The nature, timing and extent of procedures selected depend on the auditor’s judgement, including the assessment of the risks of material departures from the require-ments set out in the ESEF Regulation, whether due to fraud or error. The procedures include: • Testing whether the annual report is prepared in XHTML format; • Obtaining an understanding of the company’s iXBRL tagging process and of internal control over the tagging process; • Evaluating the completeness of the iXBRL tagging of the consolidated financial statements including notes; • Evaluating the appropriateness of the company’s use of iXBRL elements selected from the ESEF taxonomy and the creation of extension elements where no suitable element in the ESEF taxonomy has been identified; • Evaluating the use of anchoring of extension elements to elements in the ESEF taxonomy; and • Reconciling the iXBRL tagged data with the audited consolidated and parent financial statements. In our opinion, the annual report of Genmab A/S for the financial year January 1, 2024– December 31, 2024, with the file name Genmab-2024-12-31-0-en.zip, is prepared, in all material respects, in compliance with the ESEF Regulation. Copenhagen, February 12, 2025 Deloitte Statsautoriseret Revisionspartnerselskab CVR no 33 96 35 56 Sumit Sudan State Authorised Public A c countant mne33716 Niels Skannerup Vendelbo State Authorised Public A c countant mne34532 Independent Auditor’s Reports Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 201 |
|
Independent auditor’s limited assurance report on Sustainability Statements To the stakeholders of Genmab A/S Limited assurance conclusion We have conducted a limited assurance engage-ment on the Sustainability Statements of Genmab A/S (the “Group”) included in the Management’s Review (the “Sustainability Statements”), for the financial year January 1–December 31, 2024. Based on the procedures we have performed and the evidence we have obtained, nothing has come to our attention that causes us to believe that the Sustainability Statements is not prepared, in all material respects, in accordance with the Danish Financial Statements Act section 99a, including: • compliance with the European Sustainability Reporting Standards (ESRS), including that the process carried out by the management to identify the information reported in the Sustainability Statements (the “Process”) is in accordance with the description set out in 1.4 Impact, risk, and opportunity management; and • compliance of the disclosures in subsection 2.4 EU Taxonomy within the environmental section of the Sustainability Statements with Article 8 of EU Regulation 2020/852 (the “Taxonomy Regulation”). Basis for conclusion We conducted our limited assurance engage-ment in accordance with ISAE 3000 (Revised), Assurance engagements other than audits or reviews of historical financial information, and additional requirements applicable in Denmark. The procedures in a limited assurance engage-ment vary in nature and timing from, and are less in extent than for, a reasonable assurance engagement. Consequently, the level of assurance obtained in a limited assurance engagement is substantially lower than the assurance that would have been obtained had a reasonable assurance engagement been performed. We believe that the evidence we have obtained is sufficient and appropriate to provide a basis for our conclusion. Our responsibilities under this standard are further described in the “Auditor’s responsibilities for the assurance engagement” section of our report. Our independence and quality management We are independent of the Group in accordance with the International Ethics Standards Board for Accountants’ International Code of Ethics for Professional Accountants (IESBA Code) and the additional ethical requirements applicable in Denmark. We have also fulfilled our other ethical responsibilities in accordance with these require-ments and the IESBA Code. Deloitte Statsautoriseret Revisionspartnerselskab applies International Standard on Quality Management 1, ISQM1, which requires the firm to design, implement and operate a system of quality management including policies or procedures regarding compliance with ethical requirements, professional standards and appli-cable legal and regulatory requirements. Other matter The comparative information included in the Sustainability Statements of the Group was not subject to an assurance engagement. Our conclu-sion is not modified in respect of this matter. Inherent limitations in preparing the Sustainability Statements In reporting forward-looking information in accordance with ESRS, management is required to prepare the forward-looking information on the basis of disclosed assumptions about events that may occur in the future and possible future actions by the Group. Actual outcomes are likely to be different since anticipated events frequently do not occur as expected. Management’s responsibilities for the Sustainability Statements Management is responsible for designing and implementing a process to identify the informa-tion reported in the Sustainability Statements in accordance with the ESRS and for disclosing this Process in 1.4 Impact, risk, and opportunity management of the Sustainability Statements. This responsibility includes: • understanding the context in which the Group’s activities and business relationships take place and developing an understanding of its affected stakeholders; • the identification of the actual and potential impacts (both negative and positive) related to sustainability matters, as well as risks and opportunities that affect, or could reasonably be expected to affect, the Group’s financial position, financial performance, cash flows, access to finance or cost of capital over the short-, medium-, or long-term; • the assessment of the materiality of the identified impacts, risks and opportunities related to sustainability matters by selecting and applying appropriate thresholds; and • making assumptions that are reasonable in the circumstances. Management is further responsible for the preparation of the Sustainability Statements, in accordance with the Danish Financial Statements Act section 99a, including: • compliance with the ESRS; • preparing the disclosures in subsection 2.4 EU Taxonomy within the environmental section of the Sustainability Statements, in compliance with Article 8 of the Taxonomy Regulation; • designing, implementing and maintaining such internal control that management determines is necessary to enable the preparation of the Sustainability Statements that is free from material misstatement, whether due to fraud or error; and • the selection and application of appropriate sustainability reporting methods and making assumptions and estimates that are reasonable in the circumstances. Independent Auditor’s Reports Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 202 |
|
Independent Auditor’s Reports Auditor’s responsibilities for the assurance engagement Our objectives are to plan and perform the assurance engagement to obtain limited assurance about whether the Sustainability Statements is free from material misstatement, whether due to fraud or error, and to issue a limited assurance report that includes our conclu-sion. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence decisions of users taken on the basis of the Sustainability Statements as a whole. As part of a limited assurance engagement in accordance with ISAE 3000 (Revised) we exercise professional judgement and maintain profes-sional scepticism throughout the engagement. Our responsibilities in respect of the Process include: • Obtaining an understanding of the Process but not for the purpose of providing a conclusion on the effectiveness of the Process, including the outcome of the Process; • Considering whether the information identified addresses the applicable disclosure requirements of the ESRS, and • Designing and performing procedures to evaluate whether the Process is consistent with the Group’s description of its Process, as disclosed in 1.4 Impact, risk, and opportunity management. Our other responsibilities in respect of the Sustainability Statements include: • Identifying disclosures where material misstatements are likely to arise, whether due to fraud or error; and • Designing and performing procedures responsive to disclosures in the Sustainability Statements where material misstatements are likely to arise. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. Summary of the work performed A limited assurance engagement involves performing procedures to obtain evidence about the Sustainability Statements. The nature, timing and extent of procedures selected depend on professional judgement, including the identification of disclosures where material misstatements are likely to arise, whether due to fraud or error, in the Sustainability Statements. In conducting our limited assurance engagement, with respect to the Process, we: • Obtained an understanding of the Process by performing inquiries to understand the sources of the information used by management; and reviewing the Group’s internal documentation of its Process; and • Evaluated whether the evidence obtained from our procedures about the Process implemented by the Group’s was consistent with the description of the Process set out in 1.4 Impact, risk, and opportunity management. In conducting our limited assurance engagement, with respect to the Sustainability Statements, we: • Obtained an understanding of the Group’s reporting processes relevant to the preparation of its Sustainability Statements including the consolidation processes by obtaining an understanding of the Group’s control environment, processes and information systems relevant to the preparation of the Sustainability Statements but not evaluating the design of particular control activities, obtaining evidence about their implementation or testing their operating effectiveness; • Evaluated whether material information identified by the Process is included in the Sustainability Statements; • Evaluated whether the structure and the presentation of the Sustainability Statements are in accordance with the ESRS; • Performed inquiries of relevant personnel and analytical procedures on selected information in the Sustainability Statements; • Performed substantive assurance procedures on selected information in the Sustainability Statements; • Evaluated methods, assumptions and data for developing material estimates and forward-looking information and how these methods were applied; • Obtained an understanding of the process to identify taxonomy-eligible and taxonomy-aligned economic activities and the corresponding disclosures in the Sustainability Statements. Copenhagen, February 12, 2025 Deloitte Statsautoriseret Revisionspartnerselskab CVR no 33 96 35 56 Sumit Sudan State Authorised Public Accountant mne33716 Niels Skannerup Vendelbo State Authorised Public Accountant mne34532 Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 203 |
|
This Annual Report contains forward looking statements. The words “believe,” “expect,” “anticipate,” “intend” and “plan” and similar expressions identify forward looking state-ments. Actual results or performance may differ materially from any future results or performance expressed or implied by such statements. The important factors that could cause our actual results or performance to differ materially include, among others, risks associated with product discovery and development, uncertainties related to the outcome and conduct of clinical trials including unforeseen safety issues, uncer-tainties related to product manufacturing, our inability to manage growth, the competitive environment in relation to our business area and markets, our inability to attract and retain suitably qualified personnel, the unenforce-ability or lack of protection of our patents and proprietary rights, our relationships with affiliated entities, changes and developments in technology which may render our products obsolete, and other factors. Additional factors that could cause our actual results or perfor-mance to differ materially could also include and are not limited to the risk and uncertainties related to regulatory action, reimbursement, market adoption by physicians or lack of market acceptance of our products, the risk that the Company or our collaborators may be delayed or unsuccessful in planned clinical trial initiations, enrollment and planned regu-latory submissions and approvals in the U.S. and other countries. For a further discussion of these risks, please refer to the section “Risk Management” in this Annual Report and the risk factors included in Genmab’s 2024 Annual Report on Form 20-F and other filings with the U.S. Securities and Exchange Commission (SEC). Genmab does not undertake any obli-gation to update or revise forward looking statements in this Annual Report nor to confirm such statements to reflect subsequent events or circumstances after the date made or in relation to actual results, unless required by law. Genmab A/S and/or its subsidiaries own the following trademarks: Genmab®; the Y-shaped Genmab logo®; Genmab in combination with the Y-shaped Genmab logo®; HuMax®; DuoBody®; DuoBody in combination with the DuoBody logo®; HexaBody®; HexaBody in combination with the HexaBody logo®; DuoHexaBody®, HexElect®; KYSO® and MyNavCare™. ProfoundBio™ and Rina-S™ are trademarks of ProfoundBio, US Co. and ProfoundBio (Suzhou) Co., Ltd. Tivdak® and CeMe™ are trademarks of Seagen Inc.; Arzerra® is a trademark of Novartis Pharma AG. Kesimpta® and Sensoready® are trademarks of Novartis AG or its affiliates; DARZALEX®, DARZALEX FASPRO®, RYBREVANT®, TECVAYLI® and TALVEY® are trademarks of Johnson & Johnson; EPCORE®, EPKINLY®, TEPKINLY® and their designs are trademarks of AbbVie Biotechnology Ltd.; TEPEZZA® is a trademark of Horizon Therapeutics Ireland DAC. ©2025, Genmab A/S. All rights reserved. Photograph credits: Andrei Jackamets Stijn Doors Tuala Hjarnø Rob Walbers 3FX, Inc. About Genmab A/S Genmab is an international biotechnology company with a core purpose of guiding its unstoppable team to strive toward improving the lives of patients with innovative and differentiated antibody therapeutics. For more than 25 years, its passionate, innovative and collaborative team has invented next-generation antibody technology platforms and leveraged translational, quantitative and data sciences, resulting in a proprietary pipeline including bispecific T-cell engagers, antibody-drug conjugates, next-generation immune checkpoint modulators and effector function-enhanced antibodies. By 2030, Genmab’s vision is to transform the lives of people with cancer and other serious diseases with knock-your-socks-off (KYSO) antibody medicines®. Established in 1999, Genmab is headquartered in Copenhagen, Denmark, with international presence across North America, Europe, and Asia Pacific. For more information, please visit Genmab.com and follow us on LinkedIn and X. Forward Looking Statement 03 Other Information Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 204 |
|
Genmab A/S Carl Jacobsens Vej 30 2500 Valby Denmark T. +45 70 20 27 28 Genmab US, Inc. 777 Scudders Mill Road Plainsboro, NJ 08536 USA T. +1 609 430 2481 Genmab QC Lab Baltorpvej 154 2750 Ballerup Denmark T. +45 70 20 27 28 Genmab B.V. & Genmab Holding B.V. Uppsalalaan 15 3584 CT Utrecht The Netherlands T. +31 30 2 123 123 Genmab K.K. 35F Midtown Tower 9-7-1 Akasaka, Minato-ku Tokyo 107-6235 Japan T. +81 3 5403 6330 ProfoundBio Co. Ltd. * Suite 101 & 102 Building 1 Phase 3A of BioBAY No. 1 Xinze Road Suzhou Industrial Park Suzhou, China 215021 T. +86 512 6799 1868 *ProfoundBio was acquired by Genmab in May 2024 LEI Code 529900MTJPDPE4MHJ122 www.genmab.com Contact Information Genmab 2024 Annual Report Table of Contents Management’s Review Financial Statements Other Information 205 |