UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended June 30, 2024
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 001-36410
Phibro Animal Health Corporation
(Exact name of registrant as specified in its charter)
Delaware |
13-1840497 |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
|
Glenpointe Centre East, 3rd Floor |
|
300 Frank W. Burr Boulevard, Suite 21, Teaneck, New Jersey |
07666-6712 |
(Address of Principal Executive Offices) |
(Zip Code) |
(Registrant’s telephone number, including area code) (201) 329-7300
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
Class A Common Stock, $0.0001 par value per share |
|
PAHC |
|
Nasdaq Stock Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files.) Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
Accelerated filer |
☒ |
Non-accelerated filer |
☐ |
Smaller reporting company |
☐ |
Emerging growth company |
☐ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ◻
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financing reporting under Section 404(b) of the Sarbanes Oxley Act (15 U.S.C 7262(b)) by the registered public accounting firm that prepared or issued its audit report Yes ☒ No ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of the registrant’s Class A common stock and Class B common stock held by non-affiliates of the registrant was $234,820,329 as of December 31, 2023, the last business day of the registrant’s most recently completed second fiscal quarter based on the closing price of the common stock on the Nasdaq Stock Market. The registrant has no non-voting common stock.
As of August 23, 2024, there were 20,337,574 shares of the registrant’s Class A common stock, par value $0.0001 per share, and 20,166,034 shares of the registrant’s Class B common stock, par value $0.0001 per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the registrant’s Proxy Statement for the 2024 Annual Meeting of Shareholders (hereinafter referred to as the “2024 Proxy Statement”) are incorporated herein by reference in Part III of this Annual Report on Form 10-K. Such proxy statement will be filed with the Securities and Exchange Commission within 120 days of the registrant’s fiscal year ended June 30, 2024.
PHIBRO ANIMAL HEALTH CORPORATION
TABLE OF CONTENTS
|
|
Page |
3 |
||
5 |
||
5 |
||
|
|
|
|
|
|
6 |
||
27 |
||
52 |
||
52 |
||
54 |
||
54 |
||
54 |
||
|
|
|
55 |
||
56 |
||
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
56 |
|
76 |
||
77 |
||
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
116 |
|
116 |
||
116 |
||
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
116 |
|
|
|
|
117 |
||
117 |
||
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
117 |
|
Certain Relationships and Related Transactions, and Director Independence |
117 |
|
117 |
||
|
|
|
118 |
||
122 |
||
|
123 |
|
2
Forward-Looking Statements and Risk Factors Summary
This Annual Report on Form 10-K contains forward-looking statements that are subject to risks and uncertainties. All statements other than statements of historical or current fact included in this report are forward-looking statements. Forward-looking statements discuss our current expectations and projections relating to our financial condition, results of operations, plans, objectives, future performance and business. You can identify forward-looking statements by the fact that they do not relate strictly to historical or current facts. These statements may include words such as “aim,” “anticipate,” “believe,” “estimate,” “expect,” “forecast,” “outlook,” “potential,” “project,” “projection,” “plan,” “intend,” “seek,” “may,” “could,” “would,” “will,” “should,” “can,” “can have,” “likely,” the negatives thereof and other words and terms of similar meaning in connection with any discussion of the timing or nature of future operating or financial performance or other events. For example, all statements we make relating to our estimated and projected earnings, revenues, costs, expenditures, cash flows, growth rates and financial results, our plans and objectives for future operations, growth or initiatives, strategies, or the expected outcome or impact of pending or threatened litigation are forward-looking statements. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. Examples of such risks and uncertainties include:
| ● | outbreaks of animal diseases could significantly reduce demand for our products or availability of raw materials; |
| ● | perceived adverse effects on human health linked to the consumption of food derived from animals that utilize our products could cause a decline in the sales of those products; |
| ● | restrictions on the use of antibacterials in food-producing animals may become more prevalent; |
| ● | the potential Food and Drug Administration (“FDA”) withdrawal of approval of our Mecadox® (carbadox) product; |
| ● | a material portion of our sales and gross profits are generated by antibacterials and other related products; |
| ● | competition in each of our markets from a number of large and small companies, some of which have greater financial, research and development (“R&D”), production and other resources than we have; |
| ● | our business may be negatively affected by weather conditions and the availability of natural resources; |
| ● | the negative effects of a pandemic, epidemic, or outbreak of an infectious disease in humans, such as COVID-19, on our business, financial results, manufacturing facilities and supply chain, as well as our customers, protein processors and markets; |
| ● | climate change could have a material adverse impact on our operations and our customers’ businesses; |
| ● | actions of regulatory bodies, including obtaining approvals related to the testing, manufacturing and marketing of certain of our products; |
| ● | the continuing trend toward consolidation of certain customer groups as well as the emergence of large buying groups; |
| ● | our ability to control costs and expenses; |
| ● | any unforeseen material loss or casualty; |
| ● | misuse or extra-label use of our products; |
| ● | exposure relating to rising costs and reduced customer income; |
| ● | heightened competition, including those from generics and those deriving from advances in veterinary medical practices and animal health technologies; |
| ● | unanticipated safety or efficacy concerns; |
| ● | our dependence on suppliers having current regulatory approvals; |
| ● | our raw materials are subject to price fluctuations and their availability can be limited; |
3
| ● | natural and man-made disasters, including but not limited to fire, snow and ice storms, flood, hail, hurricanes and earthquakes; |
| ● | business interruption from political and social instability, including crime, civil disturbance, terrorist activities, outbreaks of disease and pandemics and armed conflicts, such as the ongoing armed conflicts between Israel and Hamas (and potential broader military conflict in the region) and between Russia and Ukraine; |
| ● | terrorist attacks, particularly attacks on or within markets in which we operate, including the terrorist attack on Israel by Hamas militants and the ongoing related conflict; |
| ● | risks related to changes in tax rates and exposure; |
| ● | our ability to successfully implement our strategic initiatives; |
| ● | our reliance on the continued operation of our manufacturing facilities and application of our intellectual property; |
| ● | adverse U.S. and international economic market conditions, including currency fluctuations; |
| ● | failure of our product approval, R&D, acquisition and licensing efforts to generate new products; |
| ● | the risks of product liability claims, legal proceedings and general litigation expenses; |
| ● | the impact of current and future laws and regulatory changes, including risks related to the protection of our customers’ privacy and risks related to environmental, health and safety laws and regulations; |
| ● | modification of foreign trade policy may harm our food animal product customers; |
| ● | our ability to successfully integrate acquired businesses, including the medicated feed additive product portfolio, certain water-soluble products and related assets, which we have agreed to acquire from Zoetis Inc.; |
| ● | our dependence on our Israeli and Brazilian operations; |
| ● | impact of increased or decreased inventory levels at our direct customers or channel distributors; |
| ● | our substantial level of indebtedness and related debt-service obligations; |
| ● | restrictions imposed by covenants in our debt agreements; |
| ● | the risk of breaches of data security and cybersecurity attacks; |
| ● | the risk of work stoppages; and |
| ● | other factors as described in “Risk Factors” in Item 1A. of this Annual Report on Form 10-K. |
While we believe that our assumptions are reasonable, we caution that it is very difficult to predict the impact of known factors, and it is impossible for us to anticipate all factors that could affect our actual results. Important factors that could cause actual results to differ materially from our expectations, or cautionary statements, are disclosed under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” All forward-looking statements are expressly qualified in their entirety by these cautionary statements. You should evaluate all forward-looking statements made in this report in the context of these risks and uncertainties.
We caution you that the important factors referenced above may not contain all of the factors that are important to you. In addition, we cannot assure you that we will realize the results or developments we expect or anticipate or, even if substantially realized, that they will result in the consequences we anticipate or affect us or our operations in the way we expect. The forward-looking statements included in this report are made only as of the date hereof. We undertake no obligation to publicly update or revise any forward-looking statement as a result of new information, future events or otherwise, except as otherwise required by law. If we do update one or more forward-looking statements, no inference should be made that we will make additional updates with respect to those or other forward-looking statements.
4
Market, Ranking and Other Industry Data
Unless otherwise indicated, information contained in this report concerning our industry and the markets in which we operate, including our general expectations and market position, market opportunity and market share, is based on management estimates. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. We believe these estimates are reasonable as of the date of this report, or if an earlier date is specified, as of such earlier date. However, this information may prove to be inaccurate because of the method by which we obtained some of the data for our estimates or because this information is subject to change and cannot always be verified due to limits on the availability and reliability of independent sources, the voluntary nature of the data gathering process and other limitations and uncertainties inherent in any statistical survey of market shares. In addition, purchasing patterns and consumer preferences can and do change. As a result, you should be aware that market share, ranking and other similar data set forth in this report, and estimates and beliefs based on such data, may not be reliable.
Trademarks, Service Marks and Trade Names
The following trademarks and service marks used throughout this report belong to, are licensed to, or are otherwise used by us in our business: AB20®; Animate®; Aviax®; Aviax Plus®; Avi-Carb®; Banminth®; Bloat Guard®; Cellerate Yeast Solutions®; Carbigen®; Cerdimix®; Coxistac®; EASE®; Emulsigen®; Eskalin®; Gemstone®; Lactrol®; Magni-Phi®; MB-1®; Mecadox®; MicroLife® Prime; MJPRRS®; MVP adjuvants®; Neo-Terramycin®; Nicarb®; Nicarmix®; OmniGen®; Phi-Shield®; pHi-Tech®; Phivax®; Polygen®; Posistac®; Rejensa®; Rumatel®; Salmin Plus®; Stafac®; TAbic®; Tailor-Made®; Terramycin®; V.H.™; V-Max®; and Vistore®. In subsequent uses of the marks in this report, the symbols may be omitted.
5
PART I
Item 1. Business
Overview
Phibro Animal Health Corporation is a leading global diversified animal health and mineral nutrition company. We strive to be a trusted partner with livestock producers, farmers, veterinarians and consumers who raise and care for farm and companion animals by providing solutions to help them maintain and enhance the health of their animals. We market approximately 750 product lines in over 80 countries to approximately 4,200 customers. We develop, manufacture and market a broad range of products for food and companion animals including poultry, swine, beef and dairy cattle, aquaculture and dogs. Our products help prevent, control and treat diseases and support nutrition to help improve animal health and well-being. We sell animal health and mineral nutrition products either directly to integrated poultry, swine and cattle producers or through animal feed manufacturers, wholesalers, distributors and veterinarians.
Our products include:
| ● | Animal health products such as antibacterials, anticoccidials, nutritional specialty products, and vaccines and vaccine adjuvants that help improve the animal’s health and therefore improve performance, food safety and animal welfare. Our Animal Health segment also includes antibacterials and other processing aids used in the ethanol fermentation industry. |
| ● | Mineral nutrition products that fortify the animal’s diet and help maintain optimal health. |
We have focused our efforts in regions where the majority of livestock production is consolidated in large commercial farms. We believe we are well positioned to grow our sales with our established network of sales, marketing and distribution professionals in markets in North America, Latin America, Asia Pacific, Europe, Africa and the Middle East.
We are investing resources to further develop products for the companion animal sector. Our business is currently concentrated in the livestock sector.
In addition to animal health and mineral nutrition products, we manufacture and market specific ingredients for use in the personal care, industrial chemical and chemical catalyst industries. We sell performance products directly to customers in the aforementioned industries.
Our Class A common stock trades on the Nasdaq Stock Market (“Nasdaq”) under the trading symbol “PAHC.” Our Class B common stock is not listed or traded on any stock exchange. We are a Delaware corporation.
Unless otherwise indicated or the context requires otherwise, references in this report to “we,” “our,” “us,” the “Company,” “Phibro,” “PAHC” and similar expressions refer to Phibro Animal Health Corporation and its subsidiaries.
For discussion regarding the impact of the ongoing armed conflicts between Israel and Hamas and between Russia and Ukraine on our financial results, see Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Business Segments
We manage our business in three segments — Animal Health, Mineral Nutrition, and Performance Products — each with its own dedicated management and sales team, for enhanced focus and accountability. Net sales by segments, species and regions were:
|
|
Segments |
|
Change |
|
Percentage of total |
|
|||||||||||||||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023 / 2022 |
|
2024 |
|
2023 |
|
2022 |
|
|||||||||
|
|
|
|
|
($ in millions) |
|
|
|
|
|
|
|
||||||||||||||
Animal Health |
|
$ |
706 |
|
$ |
660 |
|
$ |
607 |
|
$ |
47 |
|
7 |
% |
$ |
53 |
|
9 |
% |
69 |
% |
67 |
% |
64 |
% |
Mineral Nutrition |
|
|
244 |
|
|
243 |
|
|
260 |
|
|
1 |
|
0 |
% |
|
(17) |
|
(6) |
% |
24 |
% |
25 |
% |
28 |
% |
Performance Products |
|
|
68 |
|
|
75 |
|
|
76 |
|
|
(8) |
|
(10) |
% |
|
(0) |
|
(0) |
% |
7 |
% |
8 |
% |
8 |
% |
Total |
|
$ |
1,018 |
|
$ |
978 |
|
$ |
942 |
|
$ |
40 |
|
4 |
% |
$ |
36 |
|
4 |
% |
|
|
|
|
|
|
6
|
|
Species |
|
Change |
|
Percentage of total |
|
|||||||||||||||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023 / 2022 |
|
2024 |
|
2023 |
|
2022 |
|
|||||||||
|
|
|
|
|
($ in millions) |
|
|
|
|
|
|
|
||||||||||||||
Poultry |
|
$ |
370 |
|
$ |
331 |
|
$ |
319 |
|
$ |
39 |
|
12 |
% |
$ |
12 |
|
4 |
% |
36 |
% |
34 |
% |
34 |
% |
Dairy |
|
|
161 |
|
|
190 |
|
|
186 |
|
|
(29) |
|
(15) |
% |
|
4 |
|
2 |
% |
16 |
% |
19 |
% |
20 |
% |
Cattle |
|
|
130 |
|
|
128 |
|
|
127 |
|
|
2 |
|
1 |
% |
|
1 |
|
1 |
% |
13 |
% |
13 |
% |
13 |
% |
Swine |
|
|
97 |
|
|
89 |
|
|
80 |
|
|
8 |
|
9 |
% |
|
9 |
|
11 |
% |
10 |
% |
9 |
% |
8 |
% |
Other (1) |
|
|
260 |
|
|
240 |
|
|
230 |
|
|
20 |
|
8 |
% |
|
10 |
|
4 |
% |
26 |
% |
25 |
% |
24 |
% |
Total |
|
$ |
1,018 |
|
$ |
978 |
|
$ |
942 |
|
$ |
40 |
|
4 |
% |
$ |
36 |
|
4 |
% |
|
|
|
|
|
|
|
|
Regions (2) |
|
Change |
|
Percentage of total |
|
|||||||||||||||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023 / 2022 |
|
2024 |
|
2023 |
|
2022 |
|
|||||||||
|
|
|
|
|
($ in millions) |
|
|
|
|
|
|
|
||||||||||||||
United States |
|
$ |
585 |
|
$ |
579 |
|
$ |
562 |
|
$ |
6 |
|
1 |
% |
$ |
17 |
|
3 |
% |
57 |
% |
59 |
% |
60 |
% |
Latin America and Canada |
|
|
248 |
|
|
220 |
|
|
191 |
|
|
28 |
|
13 |
% |
|
29 |
|
15 |
% |
24 |
% |
22 |
% |
20 |
% |
Europe, Middle East and Africa |
|
|
122 |
|
|
118 |
|
|
122 |
|
|
4 |
|
4 |
% |
|
(5) |
|
(4) |
% |
12 |
% |
12 |
% |
13 |
% |
Asia Pacific |
|
|
63 |
|
|
61 |
|
|
67 |
|
|
2 |
|
3 |
% |
|
(5) |
|
(8) |
% |
6 |
% |
6 |
% |
7 |
% |
Total |
|
$ |
1,018 |
|
$ |
978 |
|
$ |
942 |
|
$ |
40 |
|
4 |
% |
$ |
36 |
|
4 |
% |
|
|
|
|
|
|
| (1) | Other includes sales related to: Performance Products customers; the ethanol industry; aquaculture and other animal species; adjuvants for animal vaccine manufacturers; and Mineral Nutrition other customers. |
| (2) | Net sales by region are based on country of destination. |
Certain amounts and percentages may reflect rounding adjustments.
Adjusted EBITDA by segment was:
|
|
Adjusted EBITDA(1) |
|
Change |
|
Percentage of total(2) |
|
|||||||||||||||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023 / 2022 |
|
2024 |
|
2023 |
|
2022 |
|
|||||||||
|
|
|
|
|
($ in millions) |
|
|
|
|
|
|
|
||||||||||||||
Animal Health |
|
$ |
146 |
|
$ |
136 |
|
$ |
124 |
|
$ |
9 |
|
7 |
% |
$ |
12 |
|
10 |
% |
86 |
% |
84 |
% |
79 |
% |
Mineral Nutrition |
|
|
16 |
|
|
17 |
|
|
24 |
|
|
(1) |
|
(6) |
% |
|
(7) |
|
(28) |
% |
10 |
% |
11 |
% |
15 |
% |
Performance Products |
|
|
8 |
|
|
9 |
|
|
9 |
|
|
(2) |
|
(18) |
% |
|
1 |
|
7 |
% |
5 |
% |
6 |
% |
6 |
% |
Corporate |
|
|
(58) |
|
|
(50) |
|
|
(46) |
|
|
(8) |
|
17 |
% |
|
(4) |
|
10 |
% |
|
|
|
|
|
|
Total |
|
$ |
111 |
|
$ |
113 |
|
$ |
111 |
|
$ |
(2) |
|
(1) |
% |
$ |
2 |
|
2 |
% |
|
|
|
|
|
|
| (1) | See “Management’s Discussion and Analysis of Financial Condition and Results of Operations — General description of non-GAAP financial measures” for description of Adjusted EBITDA. |
| (2) | Before unallocated corporate costs. |
Certain amounts and percentages may reflect rounding adjustments.
Net identifiable assets by segment were:
|
|
Net Identifiable Assets |
|
Change |
|
Percentage of total |
|
|||||||||||||||||||
As of June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023 / 2022 |
|
2024 |
|
2023 |
|
2022 |
|
|||||||||
|
|
|
|
|
($ in millions) |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Animal Health |
|
$ |
684 |
|
$ |
699 |
|
$ |
655 |
|
$ |
(14) |
|
(2) |
% |
$ |
44 |
|
7 |
% |
70 |
% |
72 |
% |
70 |
% |
Mineral Nutrition |
|
|
67 |
|
|
76 |
|
|
87 |
|
|
(9) |
|
(12) |
% |
|
(12) |
|
(13) |
% |
7 |
% |
8 |
% |
9 |
% |
Performance Products |
|
|
51 |
|
|
50 |
|
|
39 |
|
|
1 |
|
2 |
% |
|
10 |
|
26 |
% |
5 |
% |
5 |
% |
4 |
% |
Corporate |
|
|
180 |
|
|
147 |
|
|
150 |
|
|
32 |
|
22 |
% |
|
(3) |
|
(2) |
% |
18 |
% |
15 |
% |
16 |
% |
Total |
|
$ |
982 |
|
$ |
971 |
|
$ |
932 |
|
$ |
11 |
|
1 |
% |
$ |
40 |
|
4 |
% |
|
|
|
|
|
|
7
Corporate assets include cash and cash equivalents, short-term investments, debt issuance costs, income tax related assets and certain other assets.
Certain amounts and percentages may reflect rounding adjustments.
Animal Health
Our Animal Health business develops, manufactures and markets about 280 product lines, including:
| ● | antibacterials, which inhibit the growth of pathogenic bacteria that cause infections in animals; anticoccidials, which inhibit the growth of coccidia (parasites) that damage the intestinal tract of animals; and related products (MFAs and other); |
| ● | nutritional specialty products, which support nutrition to help improve health and performance (nutritional specialties); and |
| ● | vaccines, which induce an increase in antibody levels against a specific virus or bacteria, thus preventing disease due to infection with wild strains of that virus or bacteria (vaccines). |
Our animal health products help our customers prevent, control and treat diseases and support nutrition to help improve health and well-being, enabling our customers to more efficiently produce high-quality, wholesome and affordable animal protein products for human consumption. We develop, manufacture and market a broad range of animal health products for food animals including poultry, swine, beef and dairy cattle and aquaculture. We provide technical and product support directly to our customers to ensure the optimal use of our products. The animal health industry and demand for many of our animal health products in a particular region are affected by changing disease pressures and by weather conditions, as usage of our products follows varying weather patterns and seasons. As a result, we may experience regional and seasonal fluctuations in our animal health segment.
We continue to build our companion animal business and pipeline. Our Rejensa® joint supplement for dogs continues to gain customer acceptance. Our companion animal development pipeline includes an early-stage atopic dermatitis compound, a potential treatment for mitral heart valve disease in dogs, a pain product and two oral care products.
Animal Health net sales by product group and regions were:
|
|
Product Groups |
|
Change |
|
Percentage of total |
|
|||||||||||||||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023 / 2022 |
|
2024 |
|
2023 |
|
2022 |
|
|||||||||
|
|
|
|
|
($ in millions) |
|
|
|
|
|
|
|
||||||||||||||
MFAs and other |
|
$ |
421 |
|
$ |
387 |
|
$ |
362 |
|
$ |
34 |
|
9 |
% |
$ |
26 |
|
7 |
% |
60 |
% |
59 |
% |
60 |
% |
Nutritional specialties |
|
|
165 |
|
|
173 |
|
|
157 |
|
|
(8) |
|
(5) |
% |
|
15 |
|
10 |
% |
23 |
% |
26 |
% |
26 |
% |
Vaccines |
|
|
121 |
|
|
100 |
|
|
88 |
|
|
21 |
|
21 |
% |
|
12 |
|
13 |
% |
17 |
% |
15 |
% |
15 |
% |
Animal Health |
|
$ |
706 |
|
$ |
660 |
|
$ |
607 |
|
$ |
47 |
|
7 |
% |
$ |
53 |
|
9 |
% |
|
|
|
|
|
|
|
|
Regions(1) |
|
Change |
|
Percentage of total |
|
|||||||||||||||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023 / 2022 |
|
2024 |
|
2023 |
|
2022 |
|
|||||||||
|
|
|
|
|
($ in millions) |
|
|
|
|
|
|
|
||||||||||||||
United States |
|
$ |
287 |
|
$ |
277 |
|
$ |
248 |
|
$ |
10 |
|
4 |
% |
$ |
29 |
|
12 |
% |
41 |
% |
42 |
% |
41 |
% |
Latin America and Canada |
|
|
238 |
|
|
207 |
|
|
175 |
|
|
31 |
|
15 |
% |
|
32 |
|
18 |
% |
34 |
% |
31 |
% |
29 |
% |
Europe, Middle East and Africa |
|
|
119 |
|
|
116 |
|
|
120 |
|
|
3 |
|
3 |
% |
|
(4) |
|
(3) |
% |
17 |
% |
18 |
% |
20 |
% |
Asia Pacific |
|
|
62 |
|
|
60 |
|
|
64 |
|
|
2 |
|
3 |
% |
|
(4) |
|
(6) |
% |
9 |
% |
9 |
% |
11 |
% |
Total |
|
$ |
706 |
|
$ |
660 |
|
$ |
607 |
|
$ |
46 |
|
7 |
% |
$ |
53 |
|
9 |
% |
|
|
|
|
|
|
| (1) | Net sales by region are based on country of destination. |
Certain amounts and percentages may reflect rounding adjustments.
8
MFAs and Other
Our MFAs and other products primarily consist of concentrated medicated products administered through animal feeds, commonly referred to as Medicated Feed Additives (“MFAs”). Our MFAs and other products primarily consist of the production and sale of antibacterials (including Stafac®, Terramycin®, Neo-Terramycin® and Mecadox®) and anticoccidials (including Nicarb®, Aviax®, Aviax Plus®, Coxistac® and amprolium). Antibacterials inhibit the growth of pathogenic bacteria that cause infections in animals, while anticoccidials inhibit growth of coccidia (parasites) that damage the intestinal tract of animals. The “MFAs and other products” product group also includes antibacterial products and other processing aids used in the ethanol fermentation industry.
Approximately 43% of our MFAs and other sales in fiscal year 2024 were to the poultry industry, with sales to swine, beef and dairy cattle and other customers accounting for the remainder. We market our MFAs and other products in all regions where we do business.
Nutritional Specialties
Nutritional specialty products enhance nutrition to help improve health and performance in areas such as immune system function and digestive health. Many of our proprietary nutritional specialty products have been developed through applied research in cooperation with private research companies or by leading universities with whom we collaborate and then further develop through commercial trials with customers. Our nutritional specialty products include the OmniGen® family of products, patented nutritional specialty products that have been shown in several studies to help maintain a cow’s healthy immune system; Animate®, an anionic nutritional specialty product that helps optimize the health and performance of the transition dairy cow; Magni-Phi®, a proprietary nutritional specialty product that has been shown to help improve intestinal health and immune response in poultry; MicroLife® Prime, a four-strain direct-fed microbial product for optimization of gut health, which leads to better pathogen control and improved performance in poultry; and, Cellerate Yeast Solutions®, a line of proprietary yeast culture products that are used to help improve digestive health, which may lead to improved animal health and performance.
We are also a developer, manufacturer and marketer of microbial products and bioproducts for a variety of applications serving animal health and nutrition, environmental, industrial and agricultural customers. We market our nutritional specialty products in all regions in which we operate.
Vaccines
Our vaccine products are primarily focused on preventing diseases in poultry, swine, beef and dairy cattle and aquaculture. We market our vaccine products to protect animals from either viral or bacterial disease challenges in all regions in which we operate.
We have developed and market approximately 50 product lines for the prevention of diseases in poultry, including vaccines to protect against Infectious Bursal Disease, Infectious Bronchitis, Newcastle Disease, Reovirus, Salmonella and Coryza.
We develop, manufacture and market autogenous vaccines against animal diseases for swine, poultry and beef and dairy cattle in the United States and Brazil. Our autogenous bacterial and viral vaccines enable us to produce custom vaccines for veterinarians that contain antigens specific to each farm, allowing Phibro to provide comprehensive and customized health management solutions to our customers. Our autogenous vaccine products include the Tailor-Made® and Phi-Shield® lines of vaccines and the MJPRRS® vaccine. We also develop, manufacture and market adjuvants to animal vaccine manufacturers globally.
We have developed TAbic®, an innovative and proprietary delivery platform for vaccines. TAbic is a patented technology for formulation and delivery of vaccine antigens in effervescent tablets, packaged in sealed aluminum blister packages. The technology replaces the glass bottles that are in common use today, and offers significant sustainability advantages including reduced storage requirements, customer handling and disposal. Several of our vaccine products are available in the patented TAbic format.
9
We also focus on innovation to produce new antigens or new presentations of antigens, and have developed new vaccines and related technologies, such as:
| ● | MB-1®, a live attenuated vaccine for Infectious Bursal disease, developed from the M.B. strain, adapted for in-ovo or subcutaneous injection at the hatchery; |
| ● | TAbic® IBVAR206, a live attenuated virus vaccine for Infectious Bronchitis developed from a unique genotype 2 variant strain; |
| ● | The inactivated subunit Infectious Bursal Disease Virus; |
| ● | Salmin Plus®, the first multi-variant inactivated vaccine containing Salmonella Enteritis, Salmonella Typhimurium and Salmonella Infantis; |
| ● | EASE® (Enhanced Antigen Surface Expression), a new bacterial growth procedure to improve the performance of our autogenous vaccines; and |
| ● | pHi-Tech®, a portable electronic vaccination device and software that ensures proper delivery of vaccines and provides health management information. |
We continue to invest in a vaccine production facility in Sligo, Ireland to manufacture poultry vaccines, with our first commercial sale of product from this facility having occurred in fiscal year 2022, and longer-term expectations to add swine and cattle vaccines production at this facility. Installation of additional machinery and equipment is planned while we continue to submit necessary registrations on a country-by-country basis in order to obtain regulatory approvals needed to sell these products to a broader geographic market.
We completed construction of a new vaccine production facility in Guarulhos, Brazil in fiscal year 2023 and are now marketing autogenous vaccines that combat disease in swine, poultry and aquaculture for the Brazilian market.
Mineral Nutrition
Our Mineral Nutrition business manufactures and markets approximately 380 formulations and concentrations of trace minerals such as zinc, manganese, copper, iron and other compounds, with a focus on customers in North America. Our customers use these products to fortify the daily feed requirements of their animals’ diets and maintain an optimal balance of trace elements in each animal. We manufacture and market a broad range of mineral nutrition products for food animals including poultry, swine and beef and dairy cattle. Volume growth in the mineral nutrition sector is primarily driven by livestock production and customer inventory levels, while pricing is largely based on costs of the underlying commodity metals. Demand for our mineral nutrition products can vary due to changes in customer buying patterns, seasonal variability and weather conditions in a particular region, which may cause animal feed consumption to fluctuate.
Performance Products
Our Performance Products business manufactures and markets specialty ingredients for use in the personal care, industrial chemical and chemical catalyst industries, predominantly in the United States.
Our Products
Animal Health
MFAs and Other
Our MFAs and other products primarily consist of the production and sale of antibacterials (Stafac®, Terramycin®, Neo-Terramycin® and Mecadox®) and anticoccidials (Nicarb®, Aviax®, Aviax Plus®, Coxistac® and amprolium). We sell our MFAs and other products in all regions where we do business.
10
Antibacterials and Anticoccidials
We manufacture and market a broad range of antibacterials and other medicated products to the global livestock industry. These products provide therapeutic benefits for the animals while helping to control pathogens that have a negative impact on animal health and productivity. The table below presents our core MFA products:
|
|
|
|
Market Entry of |
|
|
Product |
|
Active Ingredient |
|
Active Ingredient |
|
Description |
Terramycin® |
|
oxytetracycline |
|
1951 |
|
Antibacterial with multiple applications for a wide number of species |
Nicarb® |
|
nicarbazin |
|
1954 |
|
Anticoccidial for poultry |
Amprolium |
|
amprolium |
|
1960 |
|
Anticoccidial for poultry and cattle |
Bloat Guard® |
|
poloxalene |
|
1967 |
|
Anti-bloat treatment for cattle |
Banminth® |
|
pyrantel tartrate |
|
1972 |
|
Anthelmintic for livestock |
Mecadox® |
|
carbadox |
|
1972 |
|
Antibacterial for enteric pathogens in swine including salmonellosis and swine dysentery |
Stafac®/Eskalin®/V-Max® |
|
virginiamycin |
|
1975 |
|
Antibacterial used to prevent and control diseases in poultry, swine and cattle |
Coxistac® /Posistac® |
|
salinomycin |
|
1979 |
|
Anticoccidial for poultry, cattle and swine |
Rumatel® |
|
morantel tartrate |
|
1981 |
|
Anthelmintic for livestock |
Cerdimix® |
|
oxibendazole |
|
1982 |
|
Anthelmintic for livestock |
Aviax® |
|
semduramicin |
|
1995 |
|
Anticoccidial for poultry |
Neo-Terramycin® |
|
oxytetracycline + neomycin |
|
1999 |
|
Combination of two antibacterials with multiple applications for a wide number of species |
Aviax Plus®/Avi-Carb® |
|
semduramicin + nicarbazin |
|
2010 |
|
Anticoccidial for poultry |
Antibacterials are biological or chemical products used in the animal health industry to treat or to prevent bacterial diseases, thereby promoting animal health, resulting in more efficient livestock production. Several factors contribute to limit the efficiency, weight gain and feed conversions of livestock production, including stress, poor nutrition, environmental and management challenges and disease. Antibacterials help prevent, control and treat disease in livestock, which can also lead to improved overall health of the animals, improved rate of weight gain and more efficient feed conversion. Our antibacterial products include:
| ● | Virginiamycin. Virginiamycin is an antibacterial marketed under the brand names Stafac® to poultry, swine and cattle producers, Eskalin® to dairy cows and beef cattle producers and V-Max® for beef cattle producers. Virginiamycin is used primarily to prevent necrotic enteritis in chickens, treat and control swine dysentery and aid in the prevention or reduce the incidence of rumen acidosis and liver abscesses in cattle. Our experience in the development and production of virginiamycin has enabled us to develop significant intellectual property through trade secret know-how, which has helped protect against competition from generics. We are the sole worldwide manufacturer and marketer of virginiamycin. |
| ● | Carbadox. We market carbadox under the brand name Mecadox® for use in swine feeds to control swine salmonellosis and swine dysentery and, as a result, improve animal health and production efficiencies. Mecadox® is sold primarily in the United States to feed companies and large integrated swine producers. |
| ● | Oxytetracycline and Neomycin. Terramycin® utilizes the active ingredient oxytetracycline and Neo-Terramycin® combines the active ingredients neomycin and oxytetracycline to prevent, control and treat a wide range of diseases in chickens, turkeys, cattle, swine and aquaculture. We sell Terramycin and Neo-Terramycin products primarily to livestock and aquaculture producers, feed companies and distributors. |
Anticoccidials are produced through fermentation or chemical synthesis and are primarily used to prevent and control the disease coccidiosis in poultry and cattle, thereby promoting intestinal health, resulting in healthier animals. Coccidiosis is a disease of the digestive tract that has considerable health consequences to livestock and, as a result, is of great concern to livestock producers.
11
We sell our anticoccidials primarily to integrated poultry producers and feed companies and to international animal health companies. Our anticoccidial products include:
| ● | Nicarbazin. We produce and market nicarbazin, a broad-spectrum anticoccidial used for coccidiosis prevention in poultry. We market nicarbazin under the trademarks Nicarb® and Nicarmix® and as an active pharmaceutical ingredient. |
| ● | Amprolium. We produce and market amprolium primarily as an active pharmaceutical ingredient. |
| ● | Salinomycin and Semduramicin. We produce and market Coxistac®, Aviax®/Aviax Plus®/Avi-Carb® and Posistac®, which are in a class of compounds known as ionophores, to combat coccidiosis in poultry and increase feed efficiency in swine. |
Anthelmintics are used to treat infestations of parasitic intestinal worms. Our anthelmintic products, Rumatel®, Banminth® and Cerdimix® are marketed to control major intestinal parasites. Rumatel is indicated for cattle while Banminth and Cerdimix are used in swine.
Bloat Guard® is an anti-bloat treatment used in cattle to control bloat in animals grazing on legume or wheat-pasture.
Other includes products used in the ethanol fermentation industry, including antimicrobials, yeasts, process cleaning, corn oil recovery and other processing aids.
Nutritional Specialties
Our primary nutritional specialty products have been identified, developed and commercialized by our staff of nutritionists and veterinarians working with private research companies, leading universities and customers with whom we collaborate. For those of our nutritional specialty products that are not proprietary or exclusive to us, we typically maintain unique supply agreements or exclusive distributor status with the product developers giving us preferential access to trademarks, territories and research data.
Our nutritional specialty products include:
|
|
Market |
|
|
Product |
|
Entry |
|
Description |
AB20® |
|
1989 |
|
Natural flow agent that improves overall feed quality |
Animate® |
|
1999 |
|
Helps maintain proper blood calcium levels in dairy cows during critical transition period |
OmniGen® |
|
2004 |
|
Optimize immune function in dairy cows and improve productivity |
Magni-Phi® & Magni-Phi® Ultra |
|
2015 |
|
Proprietary blend that helps to improve intestinal health and immune response which may lead to improved absorption and utilization of nutrients for poultry |
Cellerate Yeast Solutions® |
|
2017 |
|
Proprietary yeast culture products for all classes of livestock to help improve digestive health |
MicroLife® Prime |
|
2019 |
|
4-way combination direct-fed microbial for optimization of gut health, which can lead to better pathogen control in poultry |
AB20® is a natural flow agent that, when added to feed, binds moisture to improve the overall feed quality. The product is one of the most thoroughly researched in the flow agent product category.
Animate® is a patented anionic mineral supplement that helps optimize the health and performance of the transition dairy cow and improves profitability for dairy producers.
OmniGen® is a proprietary nutritional specialty product line designed to help maintain a cow’s healthy immune system, improve their natural response to potential environmental stressors and health challenges, and improve productivity.
Magni-Phi® and Magni-Phi® Ultra are a proprietary blend of saponins, triterpenoids and polyphenols (classes of phytogenic feed additives or natural botanicals) that help improve intestinal health and immune response which may lead to improved absorption and utilization of nutrients for poultry.
12
Cellerate Yeast Solutions® is a line of proprietary yeast culture and yeast culture blends with yeast fractions and/or live cell yeast used in all classes of livestock and companion animals for improved digestive health. Improved digestive health may lead to improved animal health and performance.
MicroLife® Prime represents a proprietary combination of four strains of bacillus-based direct-fed microbials that have been shown to promote beneficial gut bacteria, which can help promote health, immunity and productivity in poultry, which leads to lower pathogen challenges in commercial poultry production. Phibro continues to work in the development of new bacillus-based products, which are being developed for multiple animal species.
We market nutritional specialty products to livestock producers with the support of key influencers, such as animal nutritionists and veterinarians.
Vaccines
We develop, manufacture and market fully licensed and autogenous vaccines for poultry, swine, beef and dairy cattle and aquaculture globally. We also develop, manufacture and market vaccination devices. We produce vaccines that protect animals from either viral or bacterial disease challenges. Our vaccine products include:
|
|
Market |
|
|
Product |
|
Entry |
|
Description |
V.H.TM |
|
1974 |
|
Live vaccine for the prevention of Newcastle Disease in poultry |
Tailor-Made® Vaccines |
|
1982 |
|
Autogenous vaccines against either bacterial or viral diseases in poultry, swine and beef and dairy cattle in the U.S. |
MVP adjuvants® |
|
1982 |
|
Components of veterinary vaccines that enhance the immune response to a vaccine |
TAbic® M.B. |
|
2004 |
|
Live vaccine for the prevention of Infectious Bursal Disease in poultry |
MJPRRS® |
|
2007 |
|
Autogenous vaccine for the prevention of porcine reproductive and respiratory syndrome (“PRRS”) in swine |
TAbic® IB VAR |
|
2009 |
|
Live vaccine for the prevention of Infectious Bronchitis variant 1 strain 233A in poultry |
TAbic® IBVAR206 |
|
2010 |
|
Live vaccine for the prevention of Infectious Bronchitis variant 206 in poultry |
MB-1® |
|
2017 |
|
Live vaccine for the prevention of Infections Bursal Disease in the hatchery in poultry |
pHi-Tech® |
|
2019 |
|
Portable electronic vaccination device and software that ensures proper delivery of vaccines and provides health management information |
Phivax® SLE |
|
2019 |
|
A live attenuated Salmonella Enteritidis vaccine for the control of Salmonella infection in poultry |
Phi-Shield® Vaccines |
|
2023 |
|
Autogenous vaccines against either bacterial or viral diseases in poultry, swine and aquaculture in Brazil |
The V.H. strain of Newcastle Disease vaccine is a pathogenic strain and is effective when applied by aerosol, coarse spray, drinking water or eye-drops. It has been used successfully under various management and climate conditions in many breeds of poultry.
Tailor-Made® vaccines are autogenous vaccines against either bacterial or viral diseases which contain antigens specific to each farm. We manufacture and sell these vaccines to U.S. veterinarians for use primarily in swine, poultry and beef and dairy cattle.
MVP adjuvants® are integral components used in veterinary vaccines which enhance the immune response to a vaccine. Our adjuvants include Emulsigen®, Emulsigen® D, Emulsigen® P, Carbigen® and Polygen®.
The M.B. strain of Gumboro vaccine is an intermediate virulence live vaccine strain used for the prevention of Infectious Bursal Disease in poultry. The intermediate strain was developed to provide protection against the new field epidemic virus, which is more virulent than those previously encountered.
13
MJPRRS®, an autogenous vaccine for swine, is administered to pregnant sows to protect their offspring from PRRS. This vaccine includes multiple PRRS isolates representing different virus strains of PRRS.
TAbic® IB VAR and TAbic® IBVAR206 vaccines are intermediate virulence live vaccine strains used for the prevention of infectious bronchitis in poultry. Both vaccines have become significant tools in the increasing fight against infectious bronchitis in regions throughout the world.
MB-1® is a live attenuated vaccine for Infectious Bursal disease in poultry, developed from the M.B. strain, adapted for in-ovo or subcutaneous injection in the hatchery.
pHi-Tech® is a portable electronic vaccination device and software that ensures proper delivery of vaccines and provides health management information.
Phivax® SLE is a vaccine used as an aid in the reduction of Salmonella Enteritidis colonization in layers and breeder broiler chickens.
Phi-Shield® vaccines are autogenous vaccines against either bacterial or viral diseases which contain antigens specific to each farm. We manufacture and sell these vaccines to Brazilian producers for use primarily in swine, poultry and aquaculture.
We focus on innovation to produce new antigens or new presentations of antigens, and have developed new vaccines, such as the inactivated subunit Infectious Bursal Disease Virus and Egg Drop Syndrome vaccines, being sold as monovalent vaccines or in combinations with other antigens.
Mineral Nutrition
Our mineral nutrition products principally include inorganic and organic compounds of copper, zinc, cobalt, iron, selenium, manganese, magnesium and iodine.
Our mineral nutrition products also include GemStone®, our exclusive line of chelated organic trace minerals, including zinc, manganese, copper and iron glycine chelates. Our formulas feature high metal content to ensure greater mineral presence and preserve critical ration space. Each product is also highly chelated for superior bioavailability to maximize mineral absorption and minimize environmental impact. These organic trace minerals are available in a highly concentrated, easy-flowing granule.
Our mineral nutrition products also include the Vistore® portfolio of products, our chloride mineral option of value-driven trace mineral offerings. Our formulas feature high metal content to ensure optimal mineral presence and preserve critical ration space. High bioavailability also promotes maximized absorption for enhanced results and minimized waste.
Our major mineral nutrition customers are U.S. regional and national feed companies, distributors, co-ops, pre-mixers, integrated swine, beef and poultry producers and pet food manufacturers. The majority of our customers have nutrition staffs who determine their specific formulas for custom trace mineral premixes. Trace mineral costs and our selling prices fluctuate with commodity markets, and therefore, these products are price sensitive. Their sale requires a focused effort on cost and inventory management, quality control, customer service, pricing and logistics execution to be profitable.
Performance Products
Our Performance Products business manufactures and markets specialty ingredients for use in the personal care, industrial chemical and chemical catalyst industries. We operate the business through our PhibroChem (a division of PAHC), Ferro Metal and Chemical Corporation Limited and Phibro-Tech, Inc. (“Phibro-Tech”) business units.
Sales and Marketing
Our sales organization includes sales, marketing and technical support employees. In markets where we do not have a direct commercial presence, we generally contract with distributors that provide logistics and sales and marketing support for our products. Together, our Animal Health and Mineral Nutrition businesses have a sales, marketing and technical support organization of more than 400 employees and approximately 200 distributors who market our portfolio of approximately 670 product lines to livestock producers, veterinarians, nutritionists, animal feed companies and distributors in over 80 countries.
14
In markets where we have a direct commercial presence, we sell our animal health and mineral nutrition products through our local sales offices, either directly to integrated poultry, swine and beef and dairy cattle producers or through commercial animal feed manufacturers, wholesalers and distributors. Our sales representatives visit our customers, including livestock producers, veterinarians, nutritionists, animal feed companies and distributors, to inform, promote and sell our products and services. In direct service markets, our technical operations specialists provide scientific consulting focused on disease management and herd management, and training and education on diverse topics, including responsible product use.
We sell our Performance Products through our local sales offices to the personal care, industrial chemical and chemical catalyst industries. We market these products predominately in the United States.
Customers
We have approximately 4,200 customers, of which approximately 3,900 customers are served by our Animal Health and Mineral Nutrition businesses. We consider a diverse set of livestock producers, including poultry and swine operations and beef and dairy cattle farmers, to be the primary customers of our livestock products. We sell our animal health and mineral nutrition products directly to livestock and aquaculture producers and to distributors that typically re-sell the products to livestock producers. We sell our companion animal product using a distributor calling on veterinary clinics. We do not consider the business to be dependent on a single customer or a few customers, and we believe the loss of any one customer would not have a material adverse effect on our results.
We typically sell pursuant to purchase orders from customers and generally do not enter into long-term delivery contracts.
Product Registrations, Patents and Trademarks
We own certain product registrations, patents, trade names and trademarks, and use know-how, trade secrets, formulae and manufacturing techniques, which assist in maintaining the competitive positions of certain of our products. We believe that technology is an important component of our competitive position, and it provides us with low-cost positions enabling us to produce high quality products. Patents protect some of our technology, but a significant portion of our competitive advantage is based on know-how built up over many years of commercial operation, which is protected as trade secrets. We own, or have exclusive rights to use under license, approximately 300 patents or pending applications in more than 40 countries but we believe that no single patent is of material importance to our business and, accordingly, that the expiration or termination thereof would not materially affect our business.
We market our animal health products under hundreds of governmental product registrations approving many of our products with respect to animal drug safety and efficacy. The use of many of our medicated products is regulated by authorities that are specific to each country, e.g., the FDA in the United States, Health Canada in Canada and European Food Safety Authority (“EFSA”) and the European Medicines Agency (“EMA”) in Europe. Medicated product registrations and requirements are country- and product-specific for each country in which they are sold. We continuously monitor, maintain and update the appropriate registration files pertaining to such regulations and approvals. In certain countries where we work with a third-party distributor, local regulatory requirements may require registration in the name of such distributor. As of June 30, 2024, we had approximately 1,050 Animal Health product registrations globally, including approximately 390 MFA registrations, 320 vaccine registrations (including autogenous vaccines) and 340 registrations for nutritional specialty products. Our MFA global registrations included approximately 90 registrations for virginiamycin.
Additionally, many of our vaccine products are based on proprietary master seeds, proprietary adjuvant formulations or patented virus grouping technology. We actively seek to protect our proprietary information, including our trade secrets and proprietary know-how, by seeking to require our employees, consultants, advisors and partners to enter into confidentiality agreements and other arrangements upon the commencement of their employment or engagement.
We seek to file and maintain trademark registrations around the world based on commercial activities in most regions where we have, or desire to have, a business presence for a particular product or service. We currently maintain, or have rights to use under license, approximately 3,400 trademark registrations or pending applications globally, identifying goods and services related to our business.
15
Our technology, brands and other intellectual property are important elements of our business. We rely on patent, trademark, copyright and trade secret laws, as well as non-disclosure agreements, to protect our intellectual property rights. Our policy is to vigorously protect, enforce and defend our rights to our intellectual property, as appropriate.
Compliance with Government Regulation
Many of our animal health and mineral nutrition products require licensing by a governmental agency before marketing. To maintain compliance with these regulatory requirements, we have established processes, systems and dedicated resources with end-to-end involvement from product concept to launch and maintenance in the market. Our regulatory function seeks to engage in dialogue with various global agencies regarding their policies that relate to animal health products. For products that are currently subject to formal licensing by government agencies, our business relies on the ongoing approval and/or periodic re-approval of those licenses. Failure to maintain and, where applicable, renew those licenses for any reason including, but not limited to, changing regulations, more stringent technical, legal or regulatory requirements, or failure of the company or its agents to make timely, complete or accurate submissions, could result in suspension or loss of the company’s rights to market its products in one or more countries.
United States
In the United States, governmental oversight of animal nutrition and health products is conducted primarily by the FDA and/or the United States Department of Agriculture (“USDA”). The United States Environmental Protection Agency (the “EPA”) has jurisdiction over certain products applied topically to animals or to premises to control external parasites and shares regulatory jurisdiction of ethanol manufactured in biofuel manufacturing facilities with the FDA.
The FDA regulates foods intended for human consumption and, through the Center for Veterinary Medicine (“CVM”), regulates the manufacture and distribution of animal drugs marketed in the U.S. including those administered to animals from which human foods are derived. All manufacturers of animal health pharmaceuticals marketed in the United States, must show their products to be safe, effective and produced by a consistent method of manufacture as defined under the Federal Food, Drug, and Cosmetic Act. To protect the food and drug supply, the FDA develops technical standards for human and animal drug safety, effectiveness, labeling and Good Manufacturing Practice. The CVM evaluates data necessary to support approvals of veterinary drugs. Drug sponsors are required to file reports of certain product quality defects and adverse events in accordance with agency requirements.
FDA approval of Type A Medicated Feed Articles and drugs is based on satisfactory demonstration of safety, efficacy, manufacturing quality standards and appropriate labeling. Efficacy requirements are based on the desired label claim and encompass all species for which label indication is desired. Safety requirements include target animal safety and, in the case of food animals, human food safety (“HFS”). HFS reviews include drug residue levels and the safety of those residue levels. In addition to the safety and efficacy requirements for animal drugs used in food-producing animals, environmental safety must be demonstrated. Depending on the compound, the environmental studies may be quite extensive and expensive. In many instances, the regulatory hurdles for a drug that will be used in food-producing animals are at least as stringent as, if not more so than, those required for a drug used in humans. In addition, certain safety requirements relating to antimicrobial resistance must be met for antimicrobial products.
The CVM Office of New Animal Drug Evaluation is responsible for reviewing information submitted by drug sponsors who wish to obtain approval to manufacture and sell animal drugs. A new animal drug is deemed unsafe unless there is an approved New Animal Drug Application (“NADA”). Virtually all animal drugs are “new animal drugs” within the meaning of the Federal Food, Drug, and Cosmetic Act. An approved Abbreviated New Animal Drug Application (“ANADA”) is a generic equivalent of an NADA previously approved by the FDA. Both are regulated by the FDA. The drug development process for human therapeutics is generally more involved than that for animal drugs. However, because human food safety and environmental safety are issues for food-producing animals, the animal drug approval process for food-producing animals typically takes longer than for companion animals.
The FDA may deny an NADA or ANADA if applicable regulatory criteria are not satisfied, require additional testing or information, or require post-marketing testing and surveillance to monitor the safety or efficacy of a product. There can be no assurances that FDA approval of any NADA or ANADA will be granted on a timely basis, or at all. Moreover, if regulatory approval of a product is granted, such approval may entail limitations on the indicated uses for which it may be marketed.
16
Finally, product approvals may be withdrawn if compliance with regulatory standards is not maintained or if problems occur following initial marketing. Among the conditions for NADA or ANADA approval is the requirement that the prospective manufacturer’s quality control and manufacturing procedures conform to FDA’s current Good Manufacturing Practice (“cGMP”) regulations. A manufacturing facility is periodically inspected by the FDA for determination of compliance with cGMP after an initial pre-approval inspection. Certain subsequent manufacturing changes must be approved by the FDA prior to implementation. In complying with standards set forth in these regulations, manufacturers must continue to expend time, monies and effort in the area of production and quality control to ensure compliance. The process of seeking FDA approvals can be costly, time consuming and subject to unanticipated and significant delays. There can be no assurance that such approvals will be granted on a timely basis, or at all. Any delay in obtaining or any failure to obtain FDA or foreign government approvals, or the suspension or revocation of such approvals, would adversely affect our ability to introduce and market our products and to generate revenue.
The issue of the potential for increased bacterial resistance to certain antibiotics used in certain food-producing animals is the subject of discussions on a worldwide basis and, in certain instances, has led to government restrictions on the use of antibiotics in these food-producing animals. The sale of antibiotics is a material portion of our business. Legislative bills are introduced in the United States Congress from time to time that, if adopted, could have an adverse effect on our business. One of these initiatives is a proposed bill called the Preservation of Antibiotics for Medical Treatment Act, which has been introduced in almost every Congress since the mid 2000’s. To date, such bills have not had sufficient support to become law. Should statutory, regulatory or other developments result in restrictions on the sale of our products, it could have a material adverse impact on our financial position, results of operations and cash flows.
The USDA regulates the U.S. veterinary vaccines through the Center for Veterinary Biologics (“CVB”), which implements the Virus-Serum-Toxin Act to assure that pure, safe, potent and effective veterinary biologics are available for diagnosis, prevention and treatment of animal diseases. The CVB monitors and inspects vaccine products and the manufacturing facilities.
The EPA has established and monitors the Renewable Fuel Standard program, for which some of our biofuel manufacturing facilities must comply. Compliance includes generating and tracking renewable identification numbers documentation over transfer, blending and exporting, and quarterly reporting.
Virginiamycin. In November 2004, the CVM released a draft for comment of its risk assessment of streptogramin resistance for treatment of certain infections in humans attributable to the use of streptogramins in animals (the “risk assessment”). The risk assessment was initiated after approval of a human drug called Synercid® (quinupristin/dalfopristin) for treating vancomycin-resistant Enterococcus faecium (“VREf”), which led to increased attention regarding the use of streptogramins in animals. Synercid and virginiamycin (the active ingredient in our Stafac product) are both members of the streptogramin class of antimicrobial drugs. The risk assessment was unable to produce any firm conclusions as to whether, and, if so, how much, the use of virginiamycin in food animals contributes to the occurrence of streptogramin-resistant infections in humans via a foodborne pathway.
In classifying streptogramins in 2003 as a “medically important antimicrobial” (“MIA”) on the CVM’s Guidance for Industry (“GFI”) 152 list, a guidance document for evaluating the microbial safety of antimicrobial new animal drugs on food for human consumption, the FDA’s stated concern was the potential impact on use of Synercid for treating VREf in humans. In 2010, the U.S. label for Synercid was changed and the VREf indication was removed. The FDA determined that data submitted by the sponsor of Synercid failed to verify clinical benefit of the product for the treatment of VREf infections in humans. We requested that the FDA remove the streptogramin class of antimicrobials from GFI 152 to reflect that they are not “medically important” for human therapy, however, the FDA declined our request. The FDA has issued a draft of GFI 152 and the streptogram class of antimicrobials was still included as medically important. Phibro submitted comments again to the open docket recommending that streptogramins be listed as not medically important, particularly in light of the withdrawal of Synercid from the U.S. market by the sponsor. There is no certainty surrounding the outcome of the current review of the GFI 152 list and actions that may be taken by the FDA.
MIAs. Effective January 2017, the CVM’s revised Veterinary Feed Directive (“VFD”) regulations, which included changes to the control and use of antimicrobial products for use in animal feed, require that affected antimicrobial products may only be used if authorized by a veterinarian in accordance with the regulations. Prior to implementation of the revised VFD regulations, many approved antimicrobial products could be obtained and used without formal veterinary authorization.
17
In January 2017, the FDA and industry, including us, completed the process of label changes for MIA products to remove production claims and to limit the use of MIAs to those uses that are considered necessary for assuring animal health, namely for the prevention, control and/or treatment of disease, and that MIA use in food-producing animals should include veterinary oversight or consultation. The label changes were the result of recommendations from the CVM, as described in GFI 213 (“New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food-Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI 209”) and GFI 209 (“The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals”).
Carbadox. In April 2016, the FDA began initial steps to withdraw approval of carbadox (the active ingredient in our Mecadox product) via a regulatory process known as a Notice of Opportunity for Hearing (“NOOH”), due to concerns that certain residues from the product may persist in animal tissues for longer than previously determined. In the years following, Phibro has continued an ongoing process of responding collaboratively and transparently to the FDA’s Center for Veterinary Medicine (“CVM”) inquiries and has provided extensive and meticulous research and data that confirmed the safety of carbadox. In July 2020, the FDA announced it would not proceed to a hearing on the scientific concerns raised in the 2016 NOOH, consistent with the normal regulatory procedure, but instead announced that it was withdrawing the 2016 NOOH and issuing a proposed order to review the regulatory method for carbadox. Phibro reiterated the safety of carbadox and the appropriateness of the regulatory method and offered to work with the CVM to generate additional data to support the existing regulatory method or select a suitable alternative regulatory method.
In March 2022, the FDA held a Part 15 virtual public hearing seeking data and information related to the safety of carbadox in which Phibro participated and again detailed the research and data that confirm the safety of carbadox. In November 2023, the FDA issued a final order to revoke the approved method for detecting carbadox residues. The FDA also provided notice in the Federal Register proposing to withdraw approval of all NADAs providing for use of carbadox in medicated swine feed and announcing an opportunity for Phibro to request a hearing on this proposal. This second action is based on CVM’s determination that there is no approved regulatory method to detect carbadox residues in the edible tissues of the treated swine. Phibro is continuing to defend swine producers’ ability to use Mecadox. We have requested a full evidentiary hearing on the merits before an administrative law judge. In January 2024, Phibro filed a lawsuit in the D.C. Federal District Court asking the court to invalidate the order which revoked the regulatory method for carbadox. Should we be unable to successfully defend the safety of the product, the loss of carbadox sales will have an adverse effect on our financial condition and results of operations. Sales of Mecadox (carbadox) for the year ended June 30, 2024 were approximately $22 million. As of the date of this Annual Report on Form 10-K, Mecadox continues to be available for use by swine producers.
Manufacturing. The FDA routinely carries out audits related to cGMP standards for manufacturing facilities that make veterinary drug products and active pharmaceutical ingredients approved for sale in the U.S. The FDA inspectors may make observations during these inspections, which may require corrective action in order for the manufacturing facility to remain in compliance with cGMP standards. Failure to take such corrective actions could result in the manufacturing facility being ineligible to receive future FDA approvals. In very serious cases of noncompliance with cGMP standards, the FDA may issue a warning letter which could result in products produced in such manufacturing facilities to be ineligible for sale in the U.S. Although it is our objective to remain in full conformance with U.S. cGMP standards, we have in the past received adverse observations and may in the future receive adverse observations or warning letters. Failure to comply with cGMP standards could have a material impact on our business and financial results.
European Union
European Union (“E.U.”) legislation requires that veterinary medicinal products must have a marketing authorization before they are placed on the market in the European Union. A veterinary medicinal product must meet certain quality, safety, efficacy and environmental criteria to receive a marketing authorization. The European Medicines Agency (and its main veterinary scientific committee, the Committee for Medicinal Products for Veterinary Use) and the national authorities in the various E.U. Member States, are responsible for administering this regime.
A separate E.U. regime applies to feed additives. It provides for a re-registration process for existing additives and this process is ongoing. For certain types of additives, the authorizations are not generic in nature (so that they can be relied upon by any operator) but are limited to the company that obtained the marketing authorization. They are known as Brand Specific Approvals (“BSA”).
18
The system is similar to the U.S. system, where, for certain types of additives, regulatory approval is for the formulated product or “brand.”
The EFSA is responsible for the E.U. risk assessment regarding food and feed safety. Operating under the European Commission, in close collaboration with national authorities and in open consultation with its stakeholders, the EFSA provides independent scientific advice and communication on existing and emerging risks. The EFSA may issue advice regarding the process of adopting or revising European legislation on food or feed safety, deciding whether to approve regulated substances such as pesticides and food additives, or developing new regulatory frameworks and policies, for instance, in the field of nutrition. The EFSA aims to provide appropriate, consistent, accurate and timely communications on food safety issues to all stakeholders and the public at large, based on the Authority’s risk assessments and scientific expertise. The containment of antimicrobial resistance is one of the key areas of concern for the EFSA, EMA, the European Commission and its Directorates, the European Parliament and European Member State Governments.
A number of manufacturers, including us, submitted dossiers in order to re-register various anticoccidials for the purpose of obtaining regulatory approval from the European Commission. The BSA for our nicarbazin product was published in October 2010. Our reauthorization submission was made on time and is pending. We sell nicarbazin under our own BSA and as an active ingredient for another marketer’s product that has obtained a BSA and is sold in the European Union. Similarly, a BSA for our semduramicin product, Aviax®, was published in 2006 and our reauthorization submission was made on time and is pending. We have submitted a dossier for reauthorization in accordance with the requirements of the EFSA and responded to requests for additional information from the EFSA by submitting additional data for each product. The current BSAs remain valid while the EFSA reviews the additional data we have submitted. There can be no guarantee that these submissions will be reviewed favorably or in a timely manner. Failure to gain reauthorization in a timely manner could have an adverse financial impact on our business.
The Delegating and Implementing Acts under E.U. Regulation 2019/6 includes provisions that could require animals or animal origin products imported into the E.U. from other countries to be produced under the same conditions as are required in the E.U. This may preclude the use of veterinary products not approved in the E.U. or require animal health products to be used in the manner approved in the E.U. If such restrictions are implemented, they could result in a reduction or elimination of the use of our products, especially our antibacterial products, in countries that export animals or animal origin products to the E.U. and other countries that align their regulations with E.U. regulations.
Brazil
The Ministry of Agriculture, Livestock Production and Supply (“MAPA”) is the regulatory body in Brazil responsible for the regulation and control of pharmaceuticals, biologicals and medicinal feed additives for animal use. MAPA’s regulatory activities are conducted through the Secretary of Agricultural Defense and its Livestock Products Inspection Department. These activities include the inspection and licensing of both manufacturing and commercial establishments for veterinary products, as well as the submission, review and approval of pharmaceuticals, biologicals and medicinal feed additives.
Other Countries
We are subject to regulatory requirements governing investigation, clinical trials and marketing approval for animal drugs in many other countries in which our products are sold. The regulatory approval process includes similar risks to those associated with the FDA and European Commission approvals set forth above.
Global Policy and Guidance
Country-specific regulatory laws have provisions that include requirements for certain labeling, safety, efficacy and manufacturers’ quality procedures (to assure the consistency of the products), as well as company records and reports. With the exception of Australia, Canada, Japan and New Zealand, most other countries’ regulatory agencies will generally refer to the FDA, USDA, European Union and other international animal health entities, including the World Organization for Animal Health, Codex Alimentarius Commission, the recognized international standard-setting body for food (“Codex”), before establishing their own standards and regulations for veterinary pharmaceuticals and vaccines.
19
The Joint FAO/WHO Expert Committee on Food Additives is an international expert scientific committee that is administered jointly by the Food and Agriculture Organization of the United Nations and the World Health Organization. It provides risk assessments and safety evaluations of residues of veterinary drugs in animal products as well as exposure and residue definition and maximum residue limit proposals for veterinary drugs in traded food commodities. These internationally published references may also be used by national authorities when setting domestic standards. We work with the national authorities to establish acceptable safe levels of residual product in food-producing animals after treatment. This in turn enables the calculation of appropriate withdrawal times for our products prior to an animal entering the food chain.
In July 2014, the Codex adopted risk management advice language for a number of compounds including carbadox. The advice language states “authorities should prevent residues of carbadox in food. This can be accomplished by not using carbadox in food producing animals.” The advice language is to provide advice only and is not binding on individual national authorities, and almost all national authorities already have long-established regulatory standards for carbadox, including prohibiting the use of carbadox in swine production within their territory, prohibiting the importation of pork from swine that are fed carbadox, or permitting the importation of pork from swine that are fed carbadox provided there is no detection of carbadox residues in the meat. The advice language may be considered by national authorities in making future risk management determinations. To the extent additional national authorities elect to follow the advice and prohibit the use of carbadox in food-producing animals and/or the importation of pork from swine that are fed carbadox, such decisions could have an adverse effect on our sales of carbadox in those countries or in countries that produce meat for export to those countries.
Advertising and Promotion Review
Promotion of animal health products is controlled by regulations in many countries. These rules generally restrict advertising and promotion to those approved claims and uses that have been reviewed and endorsed by the applicable agency. We conduct a review of promotion material for compliance with the local and regional requirements in the markets where we sell animal health products.
Food Safety Inspection Service/Generally Recognized As Safe
The FDA is authorized to determine the safety of substances (including “generally recognized as safe” or “GRAS” substances, and food and feed additives), as well as prescribing safe conditions of use. The FDA, which has the responsibility for determining the safety of substances, together with the Food Safety and Inspection Service, the food safety branch within the USDA, maintain the authority in the United States to determine that new substances and new uses of previously approved substances are suitable for use in meat, milk and poultry products.
Competition
We are engaged in highly competitive industries and, with respect to all our major products, face competition from a substantial and continually evolving number of global and regional competitors. Some competitors have greater financial, R&D, manufacturing and other resources than we have. Our competitive position is based principally on our product registrations, customer service and support, breadth of product line, product quality, manufacturing technology, facility locations and product prices. We face competition in every market in which we participate. Many of our products face competition from products that may be used as an alternative or substitute.
There has been, and there may continue to be, consolidation in the animal health market, which could strengthen our competitors. Our competitors can be expected to continue to improve the design and performance of their products and to introduce new products with competitive price and performance characteristics. There can be no assurance that we will have sufficient resources to maintain our current competitive position, however, we believe the following strengths create sustainable competitive advantages that will enable us to continue our growth as a leader in our industry.
Products Aligned with Need for Increased Protein Production
Increased scarcity of natural resources is increasing the need for efficient production of food animals such as poultry, swine and cattle. Our animal health products, including our MFAs, vaccines and nutritional specialty products, help prevent and manage disease outbreaks and enhance nutrition to help support natural defenses against diseases. These products are often critical to our customers’ efficient production of healthy animals. Our leading MFAs product franchise, Stafac®/V-Max®/Eskalin®, is approved in over 30 countries for use in poultry, swine and beef and dairy cattle and is regarded as one of the leading MFA products for production animals.
20
Our nicarbazin and amprolium MFAs are globally recognized anticoccidials. Our nutritional specialty product offerings such as OmniGen-AF and Animate are used increasingly in the global dairy industry, and Magni-Phi® and MicroLife® Prime are rapidly becoming important products for poultry producers. Our vaccine products are effective against critical diseases in poultry, swine and beef and dairy cattle.
Global Presence with Existing Infrastructure in Key High-Growth Markets
We have an established direct presence in many important emerging markets, and we believe we are a leader in many of the emerging markets in which we operate. Our existing operations and established sales, marketing and distribution network in over 80 countries provide us with opportunities to take advantage of global growth opportunities. Outside of the United States, our global footprint reaches to key high growth regions (countries where the livestock production growth rate is expected to be higher than the average growth rate) including Brazil and other countries in South America, China, India and Southeast Asia, Mexico, Turkey, Australia, Canada, Poland and other Eastern European countries and South Africa and other countries in Africa. Our operations in countries outside of the United States contributed approximately 59% of our Animal Health segment revenues for the year ended June 30, 2024.
Leading Positions in High Growth Sub-sectors of the Animal Health Market
We are a global leader in the development, manufacture and commercialization of MFAs and nutritional specialty products for the animal health market. We believe we are well positioned in the fastest growing food animal species segments of the animal health market with significant presence in poultry and swine. We believe our sales of MFA products were third largest in the animal health market.
Diversified and Complementary Product Portfolio with Strong Brand Name Recognition
We market products across the three largest livestock species (poultry, swine, and beef and dairy cattle) and aquaculture and in the major product categories (MFAs, vaccines and nutritional specialty products). We believe our diversity of species and product categories enhances our sales mix and lowers our sales concentration risk. The complementary nature of our Animal Health and Mineral Nutrition portfolio provides us with unique cross-selling opportunities that can be used to gain access to new customers or deepen our relationships with existing customers. We believe we have strong brand name recognition for the Phibro name and for many of our animal health and mineral nutrition products, and we believe Phibro vaccines are recognized as an industry standard in efficacy against highly virulent disease challenges. Our diverse portfolio of products also allows us to address the distinct growing conditions of livestock in different regions.
Experienced Sales Force and Technical Support Staff with Strong, Consultative Customer Relationships
Within our Animal Health and Mineral Nutrition segments, utilizing both our sales, marketing and technical support organization of approximately 400 employees and a broad distribution network, we market our portfolio of more than 670 product lines to livestock producers and veterinarians in over 80 countries. We interact with customers at both their corporate and operating level, which we believe allows us to develop an in-depth understanding of their needs. Our technical support and research personnel are also important contributors to our overall sales effort. We have a total of approximately 180 technical, field service and quality control/quality assurance personnel throughout the world. These professionals interface directly with our key customers to provide practical solutions to derive optimum benefits from our products.
21
Experienced, Committed Employees and Management Team
We have a diverse and highly skilled team of animal health professionals, including technical and field service personnel located in key countries throughout the world. These individuals have extensive field experience and are vital to helping us maintain and grow our business. Many of our field team have more than 20 years of experience in the animal health industry and many have been with us for more than 10 years.
We have a strong management team with a proven track record of success at both the corporate and operating levels. The executive management team has diverse backgrounds and on average more than 30 years of experience in the animal health or related industry.
Human Capital
As of June 30, 2024, we had approximately 1,940 employees in 53 locations spanning 32 countries. Certain of our Brazilian employees are covered by multi-employer regional industry-specific unions. Certain of our Israeli employees are covered by site-specific collective bargaining agreements. Certain employees globally are covered by individual employment agreements.
We strive to nurture a strong culture that empowers team members and provides opportunities for growth and development. The Denison Organization Culture survey was administered to all employees globally in 2017 and 2021, with an abbreviated survey on key points conducted in 2023, and will continue to be used as a key metric to measure our ongoing organizational health initiative focused on building employee capability, leadership development, employee onboarding and sales force effectiveness.
At Phibro we view the strength of our team as a critical component of our success. The following principles, which guide our decisions and actions, provide an overview of how we approach management of human capital resources.
Our Most Valuable Asset – The Company and its Employees
We recognize that our employees provide the competitive edge needed to compete successfully in world markets. We adhere to human resources policies and practices that meet the needs of the business and the individual, so that we can attract and retain the highest caliber employees. Talent development is a strategic priority at Phibro, and we offer opportunities for growth at all levels of the company. Our goal is to ensure we have the right colleagues with the right skills in the right roles and with the appropriate support to build leadership capabilities and drive organizational results. As business priorities evolve and we seek to innovate, we work to nurture and develop current talent to best serve future needs. We take a programmatic and focused approach to developing our people.
Achievement of business objectives and the fulfillment of individual career aspirations are reinforced by our competitive compensation and benefit programs, comprehensive training and development programs, health and safety programs that promote and safeguard employees’ well-being, and work environments that are conducive to the successful application of skills and knowledge. In addition to traditional professional development, we offer a robust, cloud-based online training curriculum from one of the leading providers of development material for learning-focused organizations.
Employee safety is paramount. We have implemented our Road to Zero initiative, which utilizes teaming concepts to elevate employee involvement in project-based improvement activities. Participation drives a strong culture of safety and quality. Road to Zero provides a formal system for engagement, shared responsibility, leadership opportunities, meaningful contributions and accountability. We have and will continue to take the necessary daily precautions as recommended by local government authorities to keep our employees safe.
Strength Through Diversity & Inclusion
We create a positive and supportive work environment for our employees. Our approach enables opportunity for inclusion and encourages diverse perspectives and thinking to maximize the achievement of innovative and successful outcomes. We aim to protect employees from being discriminated against because of gender, sexual orientation, age, marital status, race, religion, political beliefs, ethnic background, country of origin, language or non-job-related disabilities, and we follow these same principles when recruiting new talent to our organization.
22
Respecting Employees
Phibro employees are our greatest strength and most valuable asset. When we equip team members to apply their skills, talent and passions to contribute and make a positive impact, everyone succeeds. When we thrive as individuals and teams, the Company thrives. We promote from within wherever possible, safeguard the confidentiality of employee records and keep employees informed of issues affecting them.
Cultivating One Leader at a Time
Our proprietary Leadership Model is a framework that guides how our people plan and act to advance company priorities. We strive for each executive/manager/employee to be consistently challenged to:
| ● | See what needs to be done (strategy, vision, growth); |
| ● | Get it done (execution); and |
| ● | Get it done the right way (how you do it). |
Recognizing that leadership may be exhibited differently by an individual contributor versus a first-line manager versus an upper-level manager, all Phibro employees are consistently expected to demonstrate leadership behaviors.
Manufacturing
The Animal Health business segment manufactures many products internally and supplements that production with contract manufacturing organizations (“CMOs”) as necessary.
We manufacture active pharmaceutical ingredients for certain of our antibacterial and anticoccidial products in Guarulhos, Brazil and Braganca Paulista, Brazil. We manufacture active pharmaceutical ingredients for certain of our anticoccidial and antimicrobial products in Neot Hovav, Israel. We produce vaccines in Beit Shemesh, Israel, Sligo, Ireland, Omaha, Nebraska, and Guarulhos, Brazil. We produce adjuvants in Omaha, Nebraska. We produce pharmaceuticals, disinfectants and other animal health products in Petach Tikva, Israel. We produce certain of our nutritional specialty products in Quincy and Chillicothe, Illinois and Sarasota, Florida. We produce certain of our mineral nutrition products in Quincy, Illinois and Omaha, Nebraska.
We supplement internal manufacturing and production capabilities with CMOs. We purchase certain active pharmaceutical ingredients for other medicated products from CMOs in China, India and other locations. We then formulate the final dosage form in our facilities and in contract facilities located in Argentina, Australia, Brazil, Canada, China, Israel, Malaysia, Mexico, South Africa and the United States.
We purchase certain raw materials necessary for the commercial production of our products from a variety of third-party suppliers. Such raw materials are generally available from multiple sources, are purchased worldwide and are normally available in quantities adequate to meet the needs of the Company’s business.
We believe that our existing facilities, as supplemented by CMOs, are adequate for our current requirements and for our operations in the foreseeable future.
Research and Development
Most of our manufacturing facilities have chemists and technicians on staff involved in product development, quality assurance, quality control and providing technical services to customers. Research, development and technical service efforts are conducted by our veterinarians (DVMs) and nutritionists at various facilities.
We operate Animal Health R&D and product testing at several of our domestic and international facilities. We also engage various independent contract research organizations to undertake research and development activities.
These facilities provide R&D services relating to: fermentation development and micro-biological strain improvement; vaccine development; chemical synthesis and formulation development; nutritional specialty product development; and ethanol-related products.
23
Environmental, Health and Safety
Our operations and properties are subject to Environmental Laws (as defined below) and regulations. We have incurred, and will continue to incur, expenses to attain and maintain compliance with Environmental Laws. While we believe that our operations are currently in material compliance with Environmental Laws, we have, from time to time, received notices of violation from governmental authorities, and have been involved in civil or criminal action for such violations. Additionally, at various sites, our subsidiaries are engaged in continuing investigation, remediation and/or monitoring to address contamination associated with historical operations. We maintain accruals for costs and liabilities associated with Environmental Laws, which we currently believe are adequate. In many instances, it is difficult to predict the ultimate costs under Environmental Laws and the time period during which such costs are likely to be incurred.
Governmental authorities have the power to enforce compliance with their regulations. Violators of Environmental Laws may be subject to civil, criminal and administrative penalties, injunctions or both. Failure to comply with Environmental Laws may result in the temporary or permanent suspension of operations and/or permits, limitations on production, or increased operating costs. In addition, private plaintiffs may initiate lawsuits for personal injury, property damage, diminution in property value or other relief as a result of our operations. Environmental Laws, and the interpretation or enforcement thereof, are subject to change and may become more stringent in the future, potentially resulting in substantial future costs or capital or operating expenses. We devote considerable resources to complying with Environmental Laws and managing environmental liabilities. We have developed programs to identify requirements under and maintain compliance with Environmental Laws; however, we cannot predict with certainty the impact of increased and more stringent regulation on our operations, future capital expenditure requirements, or the cost of compliance. Based upon our experience to date, we believe that the future cost of compliance with existing Environmental Laws, and liabilities for known environmental claims pursuant to such Environmental Laws, will not have a material adverse effect on our financial position, results of operations, cash flows or liquidity.
Environmental, Health and Safety Regulations
The following summarizes the principal Environmental Laws affecting our business.
Waste Management. Our operations are subject to statutes and regulations addressing the contamination by, and management of, hazardous substances and solid and hazardous wastes. In the United States, the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended (“CERCLA”), also known as the “Superfund” law, and comparable state laws, generally impose strict joint and several liability for costs of investigation and remediation and related liabilities, on defined classes of “potentially responsible parties” (“PRPs”). PRPs can be required to bear all of such costs regardless of fault, the legality of the original disposal or ownership of the disposal site. We have been, and may become, subject to liability under CERCLA for cleanup costs or investigation or clean up obligations or related third-party claims in connection with releases of hazardous substances at or from our current or former sites or offsite waste disposal facilities used by us, including those caused by predecessors or relating to divested properties or operations.
We must also comply with the Resource Conservation and Recovery Act of 1976, as amended (“RCRA”), and comparable state laws regulating the treatment, storage, disposal, remediation and transportation of solid and hazardous wastes. These laws impose management requirements on generators and transporters of such wastes and on the owners and operators of treatment, storage and disposal facilities. As current or historic recyclers of chemical waste, certain of our subsidiaries have been, and are likely to be, the focus of extensive compliance reviews by environmental regulatory authorities under RCRA. Our subsidiary Phibro-Tech currently has a RCRA operating permit for its Santa Fe Springs, California facility, for which a renewal application is under review and a draft permit has been issued for public review and comment. Phibro-Tech initially submitted an application for renewal of its permit for the Santa Fe Springs facility in 1996. We are unable to predict when the State of California will make a final permitting decision. Until the State of California issues its final decision on the renewal application, the facility is continuing to operate under the exiting permit. Phibro-Tech has updated its permit application on several occasions, and Department of Toxic Substances Control has approved a number of permit modifications to the existing permit. In addition, because we or our subsidiaries have closed several facilities that had been the subject of RCRA permits, we or our subsidiaries have been and will be required to investigate and remediate certain environmental contamination conditions at these closed plant sites within the requirements of RCRA corrective action programs.
24
Federal Water Pollution Control Act, as amended. We must comply with regulations related to the discharge of pollutants to the waters of the United States without governmental authorization, including those pursuant to the Federal Water Pollution Control Act.
Chemical Product Registration Requirements. We must comply with regulations related to the testing, manufacturing, labeling, registration and safety analysis of our products in order to distribute many of our products, including, for example, in the United States, the federal Toxic Substances Control Act and Federal Insecticide, Fungicide and Rodenticide Act, and in the European Union, the Regulation on Registration, Evaluation, Authorization and Restriction of Chemical Substances (“REACH”).
Air Emissions. Our operations are subject to the U.S. Clean Air Act (the “CAA”) and comparable U.S. state and foreign statutes and regulations, which regulate emissions of various air pollutants and contaminants. Certain of the CAA’s regulatory programs are the subject of ongoing review and/or are subject to ongoing litigation, such as the rules establishing new Maximum Achievable Control Technology for industrial boilers; significant expenditures may be required to meet current and emerging air quality standards. Regulatory agencies can also impose administrative, civil and criminal penalties for non-compliance with air permits or other air quality regulations. States may choose to set more stringent air emissions rules than those in the CAA. State, national and international authorities have also issued requirements focusing on greenhouse gas reductions. In the United States, the EPA has promulgated federal greenhouse gas regulations under the CAA affecting certain sources. In addition, a number of state, local and regional greenhouse gas initiatives are also being developed or are already in place. In Israel and Brazil, implementation of the Kyoto Protocol requirements regarding greenhouse gas emission reductions consists of energy efficiency regulations, carbon dioxide emissions allowances trading and renewable energy requirements.
Capital Expenditures
We have incurred and expect to continue to incur costs to maintain compliance with environmental, health and safety laws and regulations. Our capital expenditures relating to environmental, health and safety regulations were $2.6 million for the fiscal year ended June 30, 2024. See “Business — Environmental, Health and Safety Regulations” for further descriptions. Our environmental capital expenditure plans cover, among other things, the currently expected costs associated with known permit requirements relating to facility improvements.
Contamination and Hazardous Substance Risks
Investigation, Remediation and Monitoring Activities. Certain of PAHC’s subsidiaries that are currently or were historically engaged in recycling and other activities involving hazardous materials have been required to perform site investigations at their active, closed and former facilities and neighboring properties. Contamination of soil, groundwater and other environmental media has been identified or is suspected at several of these locations, including Santa Fe Springs, California; Powder Springs, Georgia; Union, Illinois; Sewaren, New Jersey; Sumter, South Carolina; and Joliet, Illinois, and regulatory authorities have required, and will continue to require, further investigation, corrective action and monitoring over future years. These subsidiaries also have been, and in the future may be, required to undertake additional capital improvements as part of these actions. In addition, RCRA and other applicable statutes and regulations require these subsidiaries to develop closure and post-closure plans for their facilities and in the event of a facility closure, obtain a permit that sets forth a closure plan for investigation, remediation and monitoring and requires post-closure monitoring and maintenance for up to 30 years. We believe we are in material compliance with these requirements and maintain adequate reserves to complete remediation and monitoring obligations at these locations.
In connection with past acquisitions and divestitures, we have undertaken certain indemnification obligations that require us, or may in the future require us, to conduct or finance environmental cleanups at sites we no longer own or operate. Under the terms of the sale of the former facility in Joliet, Illinois, Phibro-Tech remains responsible for any required investigation and remediation of the site attributable to conditions at the site at the time of the February 2011 sale date, and we believe we have sufficient reserves to cover the cost of the remediation.
Participating Responsible Parties (“PRPs”) at Omega Chemical Superfund Site. The EPA oversees remediation of contaminated groundwater that has migrated from the Omega Chemical Corporation Superfund Site (“Omega Chemical Site”), which is upgradient of Phibro-Tech’s Santa Fe Springs, California facility. The EPA entered into a settlement agreement and court-approved consent decree (the “Consent Decree”) with a group of companies, including Phibro-Tech and certain other subsidiaries of PAHC due to groundwater contamination from Phibro-Tech’s Santa Fe Springs facility that has allegedly commingled with contaminated groundwater from the Omega Chemical Site.
25
In February 2023, Phibro-Tech signed a definitive settlement agreement that provided for a “cash-out” settlement, with contribution protection, for Phibro-Tech and its affiliates releasing Phibro-Tech and its affiliates from liability for contamination of the groundwater plume affected by the Omega Chemical Site (with certain exceptions). The settlement agreement does not constitute an admission of liability on the part of Phibro-Tech or its affiliates. As part of the settlement, Phibro-Tech also resolved all claims for indemnification and contribution between Phibro-Tech and the successor to the prior owner of the Phibro-Tech site. The EPA, the Department of Justice and the district court have approved the definitive settlement agreement and the Consent Decree. The district court’s order can be appealed within 60 days, which period will expire on September 10, 2024. As of June 30, 2024, Phibro-Tech and one of its affiliates have made settlement payments totaling $5 million, which represents all cash payments required by the definitive settlement agreement.
Potential Claims. In addition to cleanup obligations, we could also be held liable for all consequences arising out of human exposure to hazardous substances or other environmental damage, which liability may not be covered by insurance.
Environmental Accruals and Financial Assurance. We have established environmental accruals to cover known remediation and monitoring costs at certain of our current and former facilities. Our accruals for environmental liabilities are recorded by calculating our best estimate of probable and reasonably estimable future costs using current information that is available at the time of the accrual. Our accruals for environmental liabilities totaled $4.3 million and $8.5 million as of June 30, 2024 and 2023, respectively.
In certain instances, regulatory authorities have required us to provide financial assurance for estimated costs of remediation, corrective action, monitoring and closure and post-closure plans. Our subsidiaries, in most instances, have chosen to provide the required financial assurance by means of surety bonds or letters of credit issued pursuant to our revolving credit facility. As of June 30, 2024, surety bonds and letters of credit provided $15.5 million of financial assurance.
Workplace Health and Safety
We are committed to manufacturing safe products and achieving a safe workplace. Our Environmental Health and Safety (“EHS”) Global Director, along with regional and site-based EHS professionals, manage environmental, health and safety matters throughout the Company. The site managers are responsible for implementing the established EHS controls. To protect employees, we have established health and safety policies, programs and processes at all our manufacturing sites. An external EHS audit is performed at each of our sites as needed based on the conditions at the respective sites.
Proposed Acquisition
In April 2024, we entered into a Purchase and Sale Agreement (the “Purchase Agreement”) with Zoetis Inc. (“Zoetis”) to acquire Zoetis’s medicated feed additive (MFA) product portfolio, certain water- soluble products and related assets (the “Proposed Acquisition”). The purchase price is $350 million, subject to certain adjustments set forth in the Purchase Agreement, payable in cash at closing. The product portfolio to be acquired, which generated approximately $400 million in revenue in 2023, is comprised of more than 37 product lines that are sold in approximately 80 countries. Also included in the Proposed Acquisition are six manufacturing sites, comprised of four in the U.S., one in Italy and one in China. We anticipate that the Proposed Acquisition will be completed between October and December 2024. Completion of the Proposed Acquisition is subject to satisfaction of customary closing conditions, including clearances by applicable regulatory authorities.
Where You Can Find More Information
We are subject to the information and periodic and current reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and, in accordance therewith, will file periodic and current reports, proxy statements and other information with the Securities and Exchange Commission (“SEC”). Such periodic and current reports, proxy statements and other information will be available to the public on the SEC’s website at www.sec.gov and through our website at www.pahc.com.
26
None of the information accessible on or through our website is incorporated into this Annual Report on Form 10-K.
Item 1A. Risk Factors
Risk Factors Summary
For a summary of risk factors, see our “Forward-Looking Statements and Risk Factors Summary” on page 3.
Risk Factors
You should carefully consider all of the information set forth in this Annual Report on Form 10-K, including the following risk factors, before deciding to invest in our Class A common stock. If any of the following risks occurs, our business, financial condition, results of operation or cash flows could be materially adversely affected. In any such case, the trading price of our Class A common stock could decline, and you could lose all or part of your investment. The risks below are not the only ones the Company faces. Additional risks not currently known to the Company or that the Company presently deems immaterial may also impair its business operations. This Annual Report on Form 10-K also contains forward-looking statements that involve risks and uncertainties. The Company’s results could materially differ from those anticipated in these forward-looking statements as a result of certain factors, including the risks it faces described below and elsewhere. See also “Forward-Looking Statements and Risk Factors Summary.”
Risk Factors Relating to Our Business
Outbreaks of animal diseases could significantly reduce demand for our products.
Sales of our food animal products could be materially adversely affected by the outbreak of disease carried by food animals, which could lead to the widespread death or precautionary destruction of food animals as well as the reduced consumption and demand for animal protein. The demand for our products could be significantly affected by outbreaks of animal diseases, and such occurrences may have a material adverse impact on the sale of our products and our financial condition and results of operations. The outbreaks of disease are beyond our control and could significantly affect demand for our products and consumer perceptions of certain meat products. An outbreak of disease could result in governmental restrictions on the import and export of chicken, pork, beef or other products to or from our customers. This could also create adverse publicity that may have a material adverse effect on our ability to sell our products successfully and on our financial condition and results of operations. In addition, outbreaks of disease carried by animals may reduce regional or global sales of particular animal-derived food products or result in reduced exports of such products, either due to heightened export restrictions or import prohibitions, which may reduce demand for our products due to reduced herd or flock sizes.
In recent years, outbreaks of African Swine Fever, primarily in China, have reduced animal populations and have reduced consumer demand for pork in the affected markets. In the past decade, there has been substantial publicity regarding H1N1, known as North American (or Swine) Influenza and, H5N1, known as Highly Pathogenic Avian Influenza, in the human population, birds and, most recently, dairy cattle. According to the WHO, in 2022, 67 countries in five continents reported H5N1 high pathogenicity avian influenza outbreaks in poultry and wild birds to the World Organization for Animal Health, with more than 131 million domestic poultry lost due to death or culling in affected farms and villages. In 2023, another 14 countries reported outbreaks, mainly in the Americas, as the disease continued to spread. There have also been concerns relating to E. coli in beef and Salmonella in poultry and other food poisoning micro-organisms in meats and other foods. Consumers may associate human health fears with animal diseases, food, food production or food animals whether or not it is scientifically valid, which may have an adverse impact on the demand for animal protein. Occurrences of this type could significantly affect demand for animal protein, which in turn could affect the demand for our products in a manner that has a significant adverse effect on our financial condition and results of operations. Also, the outbreak of any highly contagious disease near our main production sites could require us to immediately halt production of our products at such sites or force us to incur substantial expenses in procuring raw materials or products elsewhere. Outbreaks of an exotic or highly contagious disease in a country where we produce our products may result in other countries halting importation of our products for fear that our product may be contaminated with the exotic organism.
27
Perceived adverse effects on human health linked to the consumption of food derived from animals that utilize our products could cause a decline in the sales of those products.
Our business depends heavily on a healthy and growing livestock industry. Some in the public perceive risks to human health related to the consumption of food derived from animals that utilize certain of our products, including certain of our MFA products. In particular, there is increased focus in the United States, the E.U., China and other countries on the use of antimicrobials in the livestock industry. In the United States, this focus is primarily on the use of medically important antimicrobials, which include classes that are prescribed in animal and human health and are listed in the Appendix of the FDA-CVM Guidance for Industry (GFI) 152. As defined by the FDA, medically important antimicrobials (“MIAs”) include classes that are prescribed in animal and human health and are listed in the Appendix of GFI 152. Our products that contain virginiamycin, oxytetracycline or neomycin are classified by the FDA as medically important antimicrobials and are included in the GFI 152 list. The FDA announced its intention to further review the GFI 152 list and to review labeling directions of products on the GFI 152 list, which may lead to increased restrictions on the use of these products. In addition to the United States, the World Health Organization (WHO), the E.U., Australia and Canada have promulgated rating lists for antimicrobials that are used in veterinary medicine and that include certain of our products. The classification of our products as MIAs or similar listings may lead to a decline in the demand for and production of food products derived from animals that utilize our products and, in turn, demand for our products. Rules or regulations adopted by any territory that restrict the use of our products, especially our antibacterial products, which require animals or animal origin products imported into that territory to be produced under the same conditions as are required within the territory could result in a reduction or elimination of the use of our products in countries that export animals or animal origin products to such territories. Livestock producers may experience decreased demand for their products or reputational harm as a result of evolving consumer views of nutrition and health-related concerns, animal rights and other concerns. Any reputational harm to the livestock industry may also extend to companies in related industries, including us. In addition, campaigns by interest groups, activists and others with respect to perceived risks associated with the use of our products in animals, including position statements by livestock producers and their customers based on non-use of certain medicated products in livestock production, whether or not scientifically supported, could affect public perceptions and reduce the use of our products. Those adverse consumer views related to the use of one or more of our products in animals could have a material adverse effect on our financial condition and results of operations.
Restrictions on the use of antibacterials in food-producing animals may become more prevalent.
The issue of the potential transfer of antibacterial resistance from bacteria from food-producing animals to human bacterial pathogens, and the causality and impact of that transfer, are the subject of global scientific and regulatory discussion. Antibacterials refer to molecules that can be used to treat or prevent bacterial infections and are a sub-categorization of the products that make up our medicated feed additives portfolios. In some countries, this issue has led to government restrictions on the use of specific antibacterials in some food-producing animals, regardless of the route of administration (in feed, water, intra-mammary, topical, injectable or other route of administration). These restrictions include prohibitions on use of antibacterials for non-therapeutic uses, preventative use, duration of use and requiring veterinary oversight to use products. These restrictions are more prevalent in countries where animal protein is plentiful and governments are willing to take action even when there is scientific uncertainty.
Effective January 1, 2017, we voluntarily removed non-therapeutic claims from several of our antibacterial products sold in the United States, in order to align with the FDA’s GFI 209 and GFI 213. The FDA objective, as described in GFI 209 and GFI 213, was to eliminate the production (non-therapeutic) uses of medically important antimicrobials administered in feed or water to food producing animals while providing for the continued use of medically important antimicrobials in food-producing animals for treatment, control and prevention of disease (“therapeutic” use) under the supervision of a veterinarian. The FDA indicated that it took this action to help preserve the efficacy of medically important antimicrobials to treat infections in humans.
Our global sales of antibacterials, anticoccidials and other products, including our Mecadox product, were $421 million, $387 million and $362 million for the years ended June 30, 2024, 2023 and 2022, respectively. We cannot predict whether concerns regarding the use of antibacterials will result in additional restrictions, expanded regulations or consumer preferences to discontinue or reduce use of antibacterials in food-producing animals, which could materially adversely affect our operating results and financial condition.
28
If the FDA withdraws approval of our Mecadox (carbadox) product, the loss of sales of such product could have a material adverse effect on our business, financial condition and results of operations.
Our Mecadox (carbadox) product has been approved for use in food animals in the United States for over 50 years. Certain regulatory bodies have raised concerns about the possible presence of certain residues of our carbadox product in meat from animals that consume the product. The product was banned for use in the European Union in 1998 and has been banned in several other countries outside the United States.
In April 2016, the FDA began initial steps to withdraw approval of carbadox via a regulatory process known as a Notice of Opportunity for Hearing (“NOOH”), due to concerns that certain residues from the product may persist in animal tissues for longer than previously determined. In the years following, Phibro has continued an ongoing process of responding collaboratively and transparently to the FDA’s Center for Veterinary Medicine (“CVM”) inquiries and has provided extensive and meticulous research and data that confirmed the safety of carbadox. In July 2020, the FDA announced it would not proceed to a hearing on the scientific concerns raised in the 2016 NOOH, consistent with the normal regulatory procedure, but instead announced that it was withdrawing the 2016 NOOH and issuing a proposed order to review the regulatory method for carbadox. Phibro reiterated the safety of carbadox and the appropriateness of the regulatory method and offered to work with the CVM to generate additional data to support the existing regulatory method or select a suitable alternative regulatory method.
In March 2022, the FDA held a Part 15 virtual public hearing seeking data and information related to the safety of carbadox in which Phibro participated and again detailed the research and data that confirm the safety of carbadox. In November 2023, the FDA issued a final order to revoke the approved method for detecting carbadox residues. The FDA also provided notice in the Federal Register proposing to withdraw approval of all NADAs providing for use of carbadox in medicated swine feed and announcing an opportunity for Phibro to request a hearing on this proposal. This second action is based on CVM’s determination that there is no approved regulatory method to detect carbadox residues in the edible tissues of the treated swine. Phibro is continuing to defend swine producers’ ability to use Mecadox. We have requested a full evidentiary hearing on the merits before an administrative law judge. In January 2024, Phibro filed a lawsuit in the D.C. Federal District Court asking the court to invalidate the order which revoked the regulatory method for carbadox. Should we be unable to successfully defend the safety of the product, the loss of carbadox sales will have an adverse effect on our financial condition and results of operations. Sales of Mecadox (carbadox) for the year ended June 30, 2024 were approximately $22 million.
See also “ — We are subject to product registration and authorization regulations in many of the jurisdictions in which we operate and/or distribute our products, including the United States and member states of the European Union”; “Business —Compliance with Government Regulation — United States — Carbadox”; and “Business — Compliance with Government Regulation — Global Policy and Guidance.”
A material portion of our sales are generated by antibacterials and other related products.
Our medicated products business is comprised of a relatively small number of compounds and accounted for approximately 40% of net sales for each of the years ended June 30, 2024 and 2023. The significant loss of antibacterial or other related product sales for any reason, including product bans or restrictions, public perception, competition or any of the other risks related to such products as described in this Annual Report on Form 10-K, could have a material adverse effect on our business.
Our business may be negatively affected by weather conditions and the availability of natural resources.
The animal health industry and demand for many of our animal health products in a particular region are affected by changing disease pressures and by weather conditions, as usage of our products follows varying weather patterns and weather-related pressures from pests and diseases. As a result, we may experience regional and seasonal fluctuations in our results of operations.
In addition, livestock producers depend on the availability of natural resources, including abundant rainfall to sustain large supplies of drinking water, grasslands and grain production. Their animals’ health and their ability to operate could be adversely affected if they experience a shortage of fresh water due to human population growth or floods, droughts or other weather conditions. In the event of adverse weather conditions or a shortage of fresh water, livestock producers may purchase less of our products.
29
Adverse weather conditions, including excessive cold or heat, natural disasters, floods, droughts and other events, could negatively impact our livestock customers by impairing the health or growth of their animals or the production or availability of feed. Such events can also interfere with our customers’ operations due to power outages, fuel shortages, damage to their farms or facilities or disruption of transportation channels. In addition, droughts can lead to reduced availability of grazing pastures, forcing cattle producers to cull their herds. Fewer heads of cattle could result in reduced demand for our products. Further heat waves may cause stress in animals and lead to increased vulnerability to disease, reduced fertility rates and reduced milk production. Adverse weather conditions and natural disasters may also have a material impact on the aquaculture business. Changes in water temperatures could affect the timing of reproduction and growth of various fish species, as well as trigger the outbreak of certain water borne diseases.
Adverse weather events and natural disasters may also interfere with and negatively impact operations at our manufacturing sites, research and development facilities and offices, which could have a material adverse effect on our financial condition and results of operations, especially if the impact of an event or disaster is frequent or prolonged.
A pandemic, epidemic, or outbreak of an infectious disease in humans, such as COVID-19, may materially and adversely affect our business and our financial results.
Our business is exposed to risks associated with public health crises, including epidemics and pandemics such as the novel coronavirus and its variants (COVID-19). The COVID-19 pandemic adversely affected workforces, customers, suppliers, consumer sentiment, economies and financial markets and led to an economic downturn in many countries in which we operate. Disruptions due to a resurgence of COVID-19 or other similar health epidemics could negatively impact our manufacturing facilities, and our logistics and supply chain operations, as well as those of our customers, third-party manufacturers, suppliers and end users of our products who raise animals or who process meat, milk, eggs and seafood for human consumption and may result in a period of economic and business disruption and could have a material adverse impact on our business and financial results.
The COVID-19 pandemic and similar outbreaks could lead to decreased demand for protein, which may lead to end users of our products reducing their herd or flock sizes. In addition, demand for protein could be reduced because consumers may associate human health fears related to COVID-19 or other outbreaks with animal diseases, food, food production or food animals, whether or not it is scientifically valid. Reductions in demand for animal protein resulting from these factors could in turn affect the demand for our products in a manner that has a significant adverse effect on our financial condition and results of operations.
The impact of a pandemic or similar public health crises is uncertain and subject to change and could also exacerbate the other risks discussed in this “Risk Factors” section. We cannot predict with certainty the full scope and severity of any potential disruptions to our business, operating results, cash flows and/or financial condition, but we expect that the resulting adverse impact on our business and financial results could be material.
Climate change could have a material adverse impact on our operations and our customers’ businesses.
Our operations, and the activities of our customers, could be disrupted by climate change. The physical impact of climate change may prompt shifts in regulations or consumer preferences which in turn could have negative consequences for our and our customers’ businesses. Climate change may negatively impact our customers’ operations, particularly those in the livestock industry, through climate-related impacts such as increased air and water temperatures, rising water levels and increased incidence of disease in livestock. Potential physical risks from climate change may include altered distribution and intensity of rainfall, prolonged droughts or flooding, increased frequency of wildfires and other natural disasters, rising sea levels and a rising heat index, any of which could cause negative impacts to our and our customers’ businesses. If such events affect our customers’ businesses, they may purchase fewer of our products, and our revenues may be negatively impacted. Climate driven changes could have a material adverse effect on our financial condition and results of operations.
There has been a broad range of proposed and promulgated state, national and international regulations aimed at reducing the effects of climate change. Such regulations could result in additional costs to maintain compliance and additional income or other taxes. Climate change regulations continue to evolve, and it is not possible to accurately estimate potential future compliance costs.
30
The testing, manufacturing and marketing of certain of our products are subject to extensive regulation by numerous government authorities in the United States and other countries, including, but not limited to, the FDA.
Among other requirements, FDA approval of antibacterials and other medicated products, including the manufacturing processes and facilities used to produce such products, is required before such products may be marketed in the United States. Further, cross-clearance approvals are generally required for such products to be used in combination in animal feed. Similarly, marketing approval by a foreign governmental authority is typically required before such products may be marketed in a particular foreign country. In addition to approval of the product and its labeling, regulatory authorities typically require approval and periodic inspection of the manufacturing facilities. In order to obtain FDA approval of a new animal health product, we must, among other things, demonstrate to the satisfaction of the FDA that the product is safe and effective for its intended uses and that we are capable of manufacturing the product with procedures that conform to FDA’s current cGMP regulations, which must be followed at all times.
Audits related to cGMP standards are typically carried out by the FDA on a two-year cycle. We are routinely subject to these inspections and respond to the FDA to address any concerns they may make in their inspectional observations (Form 483). Although it is our objective to remain in full conformance with U.S. cGMP standards, there can be no assurance that future inspections will not raise adverse inspectional observations. Failure to comply with cGMP standards could have a material impact on our business and financial results.
The process of seeking FDA approvals can be costly, time-consuming, and subject to unanticipated and significant delays. There can be no assurance that such approvals will be granted to us on a timely basis, or at all. Any delay in obtaining or any failure to obtain FDA or foreign government approvals or the suspension or revocation of such approvals would adversely affect our ability to introduce and market medicated feed additive products and to generate product revenue. For more information on FDA and foreign government approvals and cGMP issues, see “Business — Compliance with Government Regulation.”
We may experience declines in the sales volume and prices of our products as the result of the continuing trend toward consolidation of certain customer and distributor groups as well as the emergence of large buying groups.
We make a majority of our sales to integrated poultry, swine and beef and dairy cattle operations and to a number of regional and national feed companies, distributors, co-ops and blenders. Food animal producers, particularly, swine and poultry producers, and our distributors have seen recent consolidation in their industries. Significant consolidation of our customers and distributors may result in these groups gaining additional purchasing leverage and consequently increasing the product pricing pressures facing our business. Additionally, the emergence of large buying groups potentially could enable such groups to attempt to extract price discounts on our products. Moreover, if, as a result of increased leverage, customers require us to reduce our pricing such that our gross margins are diminished, we could decide not to sell our products to a particular customer, which could result in a decrease in our revenues. Consolidation among our customer base may also lead to reduced demand for our products and replacement of our products by the combined entity with those of our competitors. The result of these developments could have a material adverse effect on our business, financial condition and results of operations.
Our business is subject to risk based on customer exposure to rising costs and reduced customer income.
Livestock producers may experience increased feed, fuel, transportation and other key costs or may experience decreased animal protein prices or sales, inflationary pressures as a result of interest rate increases or otherwise and including as a result of the uncertainties and potential economic downturn relating to a resurgence of the COVID-19 pandemic or similar public health crises, or relating to armed conflicts, including the ongoing conflicts between Israel and Hamas and between Russia and Ukraine. International sanctions, trade disputes and tariffs could reduce demand for our customers’ products. These trends could cause deterioration in the financial condition of our livestock producer customers, potentially inhibiting their ability to purchase our products or pay us for products delivered. Our livestock producer customers may offset rising costs by reducing spending on our products, including by switching to lower-cost alternatives to our products.
Generic products may be viewed as more cost-effective than certain of our products.
We face competition from products produced by other companies, including generic alternatives to certain of our products. We depend primarily on trade secrets to provide us with competitive advantages for many of our products. The protection afforded is limited by the availability of new competitive products or generic versions of existing products that can successfully compete with our products.
31
As a result, we may face competition from new competitive products or lower-priced generic alternatives to many of our products. Generic competitors are becoming more aggressive in terms of pricing, and generic products are an increasing percentage of overall animal health sales in certain regions. If animal health customers increase their use of new or existing generic products, our financial condition and results of operations could be materially adversely affected.
Advances in veterinary medical practices and animal health technologies could negatively affect the market for our products.
The market for our products could be impacted negatively by the introduction and/or broad market acceptance of newly developed or alternative products that address the diseases and conditions for which we sell products, including “green” or “holistic” health products or specially bred disease-resistant animals. In addition, technological breakthroughs by others may obviate our technology and reduce or eliminate the market for our products. Introduction or acceptance of such products or technologies could materially adversely affect our business, financial condition and results of operations.
The misuse or extra-label use of our products may harm our reputation or result in financial or other damages.
Our products have been approved for use under specific circumstances for, among other things, the prevention, control and/or treatment of certain diseases and conditions in specific species, in some cases subject to certain dosage levels or minimum withdrawal periods prior to the slaughter date. There may be increased risk of product liability if livestock producers or others attempt any extra-label use of our products, including the use of our products in species for which they have not been approved, or at dosage levels or periods prior to withdrawal that have not been approved. If we are deemed by a governmental or regulatory agency to have engaged in the promotion of any of our products for extra-label use, such agency could request that we modify our training or promotional materials and practices and we could be subject to significant fines and penalties. The imposition of such fines and penalties could also affect our reputation and position within the industry. Even if we were not responsible for having promoted the extra-label use, concerns could arise about the safety of the resulting meat in the human food supply. Any of these events could materially adversely affect our financial condition and results of operations.
The public perception of the safety, quality and efficacy of certain of our animal health products may harm our reputation.
The public perception of the safety, quality and efficacy of certain of our animal health products, whether or not these concerns are scientifically or clinically supported, may lead to product recalls, withdrawals, suspensions or declining sales as well as product liability and other claims.
Regulatory actions based on these types of safety, quality or efficacy concerns could impact all, or a significant portion of a product’s sales and could, depending on the circumstances, materially adversely affect our results of operations.
In addition, we depend on positive perceptions of the safety, quality and efficacy of our products, and animal health products generally, by our customers, veterinarians and end-users, and such concerns may harm our reputation. In some countries, these perceptions may be exacerbated by the existence of counterfeit versions of our products, which, depending on the legal and law enforcement recourse available in the jurisdiction where the counterfeiting occurs, may be difficult to police or stop. These concerns and the related harm to our reputation could materially adversely affect our financial condition and results of operations, regardless of whether such reports are accurate.
We are dependent on suppliers having current regulatory approvals, and the failure of those suppliers to maintain these approvals or other challenges in replacing any of those suppliers could affect our supply of materials or affect the distribution or sale of our products.
Suppliers and third-party contract manufacturers for our animal health and mineral nutrition products or the active pharmaceutical ingredients or other materials we use in our products, like us, are subject to extensive regulatory compliance. If any one of these third parties discontinues its supply to us because of changes in the regulatory environment to which such third parties are subject, significant regulatory violations or for any other reason, or an adverse event occurs at one of their facilities, the interruption in the supply of these materials could decrease sales of our affected products.
32
In this event, we may seek to enter into agreements with third parties to purchase active ingredients, raw materials or products or to lease or purchase new manufacturing facilities. We may be unable to find a third party willing or able to provide the necessary products or facilities suitable for manufacturing pharmaceuticals on terms acceptable to us or the cost of those pharmaceuticals may be prohibitive. If we have to obtain substitute materials or products, additional regulatory approvals will likely be required, as approvals are typically specific to a single product produced by a specified manufacturer in a specified facility and there can be no assurances that such regulatory approvals will be obtained. As such, the use of new facilities also requires regulatory approvals. While we take measures where economically feasible and available to secure back-up suppliers, the continued receipt of active ingredients or products from a sole source supplier could create challenges if a sole source was interrupted. We may not be able to provide adequate and timely product to eliminate any threat of interruption of supply of our products to customers and these problems may materially adversely impact our business.
The raw materials used by us and our third-party contract manufacturers in the manufacture of our products can be subject to price fluctuations and their availability can be limited.
While the selling prices of our products tend to increase or decrease over time with the cost of raw materials, such changes may not occur simultaneously or to the same degree. The costs of certain of our significant raw materials are subject to considerable volatility, and we generally do not engage in activities to hedge the costs of our raw materials and our third-party contract manufacturers may demand price increases related to increases in the costs of raw materials. In addition, we may be subject to new or increased tariffs on imported raw materials with limited ability to pass those increased costs through to our customers. Although no single raw material accounted for more than 5% of our cost of goods sold for the year ended June 30, 2024, volatility in raw material costs can result in significant fluctuations in our cost of goods sold of the affected products. The costs of raw materials used by our Mineral Nutrition business are particularly subject to fluctuations in global commodities markets and cost changes in the underlying commodities markets typically lead directly to a corresponding change in our revenues. Although we attempt to adjust the prices of our products to reflect significant changes in raw material costs, we may not be able to pass any increases in raw material costs through to our customers in the form of price increases. Significant increases in the costs of raw materials, if not offset by product price increases, could have a material adverse effect on our financial condition and results of operations. The supply of certain of our raw materials is dependent on third party suppliers. There is no guarantee that supply shortages or disruptions of such raw materials will not occur and the likelihood of such supply shortages and disruptions has been, and may continue to be, increased due to global supply chain disruptions, including those caused by the COVID-19 pandemic or similar health crises and the ongoing conflicts between Israel and Hamas and between Russia and Ukraine. In addition, if any one of these third parties discontinues its supply to us, or an adverse event occurs at one of their facilities, the interruption in the supply of these materials could decrease sales of our affected products. In the event that we cannot procure necessary major raw materials from other suppliers, the occurrence of any of these may have an adverse impact on our business.
Our revenues are dependent on the continued operation of our various manufacturing facilities.
Although presently all our manufacturing facilities are considered to be in good condition, the operation of our manufacturing facilities involves many risks which could cause product interruptions, including the breakdown, failure or substandard performance of equipment, construction delays, mislabeling, shortages of materials, labor problems, power outages, political and social instability, the improper installation or operation of equipment, natural disasters, terrorist activities, armed conflicts, the outbreak of any highly contagious diseases, such as COVID-19 in humans or African Swine Fever in swine, near our production sites and the need to comply with environmental and other directives of governmental agencies. In addition, regulatory authorities such as the FDA typically require approval and periodic inspection of the manufacturing facilities to confirm compliance with applicable regulatory requirements, and those requirements may be enforced by various means, including seizures and injunctions. Certain of our product lines are manufactured at a single facility, and certain of our product lines are manufactured at a single facility with limited capacity at a second facility, and production would not be easily transferable to another site. The occurrence of material operational problems, including but not limited to the above events, may adversely affect our financial condition and results of operations.
Our manufacturing network may be unable to meet the demand for our products or we may have excess capacity if demand for our products changes. The unpredictability of a product’s regulatory or commercial success or failure, the lead time necessary to construct highly technical and complex manufacturing sites and shifting customer demand (including as a result of market conditions or entry of branded or generic competition) increase the potential for capacity imbalances.
33
In addition, construction of manufacturing sites is expensive, and our ability to recover costs will depend on the market acceptance and success of the products produced at the new sites, which is uncertain.
We could be subject to changes in our tax rates, the adoption of new U.S. or foreign tax legislation or exposure to additional tax liabilities.
We are subject to income taxes in the U.S. and numerous foreign jurisdictions. Changes in the relevant tax laws, regulations and interpretations could adversely affect our future effective tax rates. Modifications to key elements of the U.S. or international tax framework could have a material adverse effect on our consolidated financial statements.
Our consolidated effective tax rate is subject to potential risks that various taxing authorities may challenge the pricing of our cross-border arrangements and subject us to additional tax, adversely affecting our expected consolidated effective tax rate and our tax liability. If our effective tax rates were to increase, particularly in the U.S. or other material foreign jurisdictions, our business, financial condition and results of operations could be materially adversely affected. In addition, our tax returns and other tax filings and positions are subject to review by the Internal Revenue Service (the “IRS”) and other tax authorities and governmental bodies. We regularly assess the likelihood of an adverse outcome resulting from these examinations to determine the adequacy of our provision for taxes. There can be no assurance as to the outcome of these examinations or the effects on our consolidated financial statements.
A significant portion of our operations are conducted in foreign jurisdictions and are subject to the economic, political, legal and business environments of the countries in which we do business.
Our international operations could be limited or disrupted by any of the following:
| ● | volatility in the international financial markets; |
| ● | compliance with governmental controls; |
| ● | difficulties enforcing contractual and intellectual property rights; |
| ● | compliance with a wide variety of laws and regulations, such as the U.S. Foreign Corrupt Practices Act (“FCPA”) and similar non-U.S. laws and regulations; |
| ● | compliance with foreign labor laws; |
| ● | compliance with Environmental Laws; |
| ● | burdens to comply with multiple and potentially conflicting foreign laws and regulations, including those relating to EHS requirements; |
| ● | changes in laws, regulations, government controls or enforcement practices with respect to our business and the businesses of our customers; |
| ● | political and social instability, including crime, civil disturbance, terrorist activities, outbreaks of disease and pandemics and armed conflicts, such as the ongoing conflicts between Israel and Hamas (and potential broader military conflict in the region) and between Russia and Ukraine; |
| ● | trade restrictions, export controls and sanctions laws and restrictions on direct investments by foreign entities, including restrictions administered by the Office of Foreign Assets Control of the U.S. Department of the Treasury; |
| ● | government limitations on foreign ownership; |
| ● | government takeover or nationalization of businesses; |
| ● | changes in tax laws and tariffs; |
| ● | changes in the economic, business, competitive and regulatory environment, including changes in the value of foreign currencies relative to the U.S. dollar or high inflation; |
34
| ● | imposition of anti-dumping and countervailing duties or other trade-related sanctions; |
| ● | costs and difficulties and compliance risks in staffing, managing and monitoring international operations; |
| ● | corruption risk inherent in business arrangements and regulatory contacts with foreign government entities; |
| ● | longer payment cycles and increased exposure to counterparty risk; and |
| ● | additional limitations on transferring personal information between countries or other restrictions on the processing of personal information. |
The multinational nature of our business subjects us to potential risks that various taxing authorities may challenge the pricing of our cross-border arrangements and subject us to additional tax, adversely impacting our effective tax rate and our tax liability.
In addition, international transactions may involve increased financial and legal risks due to differing legal systems and customs, as well as restrictions and sanctions that may be imposed on one or more persons and/or jurisdictions in which we operate, including those arising from the ongoing armed conflicts between Israel and Hamas (and potential broader military conflict in the region) and between Russia and Ukraine. Compliance with these requirements may prohibit the import or export of certain products and technologies or may require us to obtain a license before importing or exporting certain products or technology. A failure to comply with any of these laws, regulations or requirements could result in civil or criminal legal proceedings, monetary or non-monetary penalties, or both, disruptions to our business, limitations on our ability to import and export products and services, and damage to our reputation. In addition, variations in the pricing of our products in different jurisdictions may result in the unauthorized importation of our products between jurisdictions. While the impact of these factors is difficult to predict, any of them could materially adversely affect our financial condition and results of operations. Changes in any of these laws, regulations or requirements, or the political environment in a particular country, may affect our ability to engage in business transactions in certain markets, including investment, procurement and repatriation of earnings.
We are subject to product registration and authorization regulations in many of the jurisdictions in which we operate and/or distribute our products, including the United States and member states of the European Union.
We are subject to regulations related to testing, manufacturing, labeling, registration and safety analysis in order to lawfully distribute many of our products, including for example, in the United States, the Federal Toxic Substances Control Act and the Federal Insecticide, Fungicide, and Rodenticide Act, and in the European Union, the Regulation on REACH. We are also subject to similar requirements in many of the other jurisdictions in which we operate and/or distribute our products. In some cases, such registrations are subject to periodic review by relevant authorities. Such regulations may lead to governmental restrictions or cancellations of, or refusal to issue, certain registrations or authorizations, or cause us or our customers to make product substitutions in the future. Such regulations may also lead to increased third party scrutiny and personal injury or product liability claims. Compliance with these regulations can be difficult, costly and time consuming and liabilities or costs relating to such regulations could have a material adverse effect on our business, financial condition and results of operations.
We have significant assets located outside the United States and a significant portion of our sales and earnings is attributable to operations conducted abroad that may be adversely affected by foreign currency exchange rate fluctuations and other inherent risks.
As of June 30, 2024, we had manufacturing and direct sales operations in 24 countries and sold our products in over 80 countries. Our operations outside the United States accounted for 59% and 58% of our consolidated assets as of June 30, 2024 and 2023, respectively, and 43% and 41% of our consolidated net sales for the years ended June 30, 2024 and 2023, respectively. Our foreign operations are subject to currency exchange fluctuations and restrictions, political instability in some countries, and uncertainty of, and governmental control over, commercial rights.
Changes in the relative values of currencies take place from time to time and could in the future adversely affect our results of operations as well as our ability to meet interest and principal obligations on our indebtedness. To the extent that the U.S. dollar fluctuates relative to the applicable foreign currency, our results are favorably or unfavorably affected. We may from time to time manage this exposure by entering into foreign currency contracts. Such contracts generally are entered into with respect to anticipated costs denominated in foreign currencies for which timing of the payment can be reasonably estimated.
35
No assurances can be given that such hedging activities will not result in, or will be successful in preventing, losses that could have an adverse effect on our financial condition or results of operations. There are times when we do not hedge against foreign currency fluctuations and therefore are subject to the risks associated with fluctuations in currency exchange rates.
In addition, international manufacturing, sales and raw materials sourcing are subject to other inherent risks, including possible nationalization or expropriation, labor unrest, political instability, price and exchange controls, limitation on foreign participation in local enterprises, health-care regulation, export duties and quotas, domestic and international customs and tariffs, compliance with export controls and sanctions laws, the Foreign Corrupt Practices Act and other laws and regulations governing international trade, unexpected changes in regulatory environments, difficulty in obtaining distribution and support, and potentially adverse tax consequences. Although such risks have not had a material adverse effect on us in the past, these factors could have a material adverse impact on our ability to increase or maintain our international sales or on our results of operations in the future.
We have manufacturing facilities located in Israel and a portion of our net sales and earnings is attributable to products produced and operations conducted in Israel.
Our Israeli manufacturing facilities and local operations accounted for 28% and 27% of our consolidated assets, as of June 30, 2024 and 2023, and 21% and 19% of our consolidated net sales for the years ended June 30, 2024 and 2023, respectively. We maintain manufacturing facilities in Israel, which manufacture:
| ● | anticoccidials and antimicrobials, most of which are exported; |
| ● | vaccines, a substantial portion of which are exported; and |
| ● | animal health pharmaceuticals, nutritional specialty products and trace minerals for the domestic animal industry. |
A substantial portion of this production is exported from Israel to major world markets. Accordingly, our Israeli operations are dependent on foreign markets and the ability to reach those markets. Hostilities between Israel and its neighbors, including the ongoing conflict between Israel and Hamas (and potential broader military conflict in the region), may hinder Israel’s international trade. This, in turn, could have a material adverse effect on our business, financial condition and results of operations. See “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations – Factors affecting our performance – Armed conflicts – Israel and Hamas.”
Certain countries, companies and organizations continue to participate in a boycott of Israeli firms and other companies doing business in Israel or with Israeli companies. We do not believe that the boycott has had a material adverse effect on us, but we cannot provide assurance that restrictive laws, policies or practices directed toward Israel or Israeli businesses will not have an adverse impact on our operations or expansion of our business. Our business, financial condition and results of operations in Israel may be adversely affected by factors outside of our control, such as currency fluctuations, energy shortages and other political, social and economic developments in or affecting Israel, including as a result of the impact of any resurgence of the COVID-19 pandemic or the ongoing conflict between Israel and Hamas.
We have manufacturing facilities located in Brazil and a portion of our sales and earnings is attributable to products produced and operations conducted in Brazil.
Our Brazilian manufacturing facilities and local operations accounted for 14% of our consolidated assets, as of June 30, 2024 and 2023, and 16% of our consolidated net sales for the years ended June 30, 2024 and 2023. We maintain manufacturing facilities in Brazil, which manufacture virginiamycin, semduramicin, salinomycin and nicarbazin. Our Brazilian facilities also produce Stafac, Aviax, Aviax Plus, Coxistac, Nicarb, Kamoran®, and Terramycin granular formulations. A substantial portion of the production is exported from Brazil to major world markets. Accordingly, our Brazilian operations are dependent on foreign markets and the ability to reach those markets.
Our business, financial condition and results of operations in Brazil may be adversely affected by factors outside of our control, such as currency fluctuations, energy shortages and other political, social and economic developments in or affecting Brazil, including as a result of the impact of a resurgence of the COVID-19 pandemic or similar public health crises in Brazil.
36
Certain of our employees are covered by collective bargaining or other labor agreements.
As of June 30, 2024, approximately 300 of our Israeli employees and 600 of our Brazilian employees were covered by collective bargaining agreements. We believe we have satisfactory relations with our employees. There can be no assurance that we will not experience a work stoppage or strike at our manufacturing facilities. A prolonged work stoppage or strike at any of our manufacturing facilities could have a material adverse effect on our business, financial condition and results of operations.
The loss of key personnel may disrupt our business and adversely affect our financial results.
Our operations and future success are dependent on the continued efforts of our senior executive officers and other key personnel. Although we have entered into employment agreements with certain executives, we may not be able to retain all of our senior executive officers and key employees. These senior executive officers and other key employees may be hired by our competitors, some of which have considerably more financial resources than we do. The loss of the services of any of our senior executive officers or other key personnel, or the inability to hire and retain qualified employees, could have a material adverse effect on our business, financial condition and results of operations.
Our R&D relies on evaluations in animals, which may become subject to bans or additional regulations.
As a company that produces animal health medicines and vaccines, evaluation of our existing and new products in animals is required in order to be able to register our products. Animal testing in certain industries has been the subject of controversy and adverse publicity. Some organizations and individuals have attempted to ban animal testing or encourage the adoption of additional regulations applicable to animal testing. To the extent that the activities of such organizations and individuals are successful, our R&D, and by extension our financial condition and results of operations, could be materially adversely affected. In addition, negative publicity about us or our industry could harm our reputation.
Our operations, properties and subsidiaries are subject to a wide variety of complex and stringent federal, state, local and foreign environmental laws and regulations.
We are subject to environmental, health and safety laws and regulations, including those governing pollution; protection of the environment; the use, management and release of hazardous materials, substances and wastes; air emissions; greenhouse gas emissions; water use, supply and discharges; the investigation and remediation of contamination; the manufacture, distribution and sale of regulated materials, including pesticides; the importing, exporting and transportation of products; and the health and safety of our employees and the public (collectively, “Environmental Laws”). See “Business — Environmental, Health and Safety.”
Pursuant to Environmental Laws, certain of our subsidiaries are required to obtain and maintain numerous governmental permits, licenses, registrations, authorizations and approvals, including “RCRA Part B” hazardous waste permits, to conduct various aspects of their operations (collectively “Environmental Permits”), any of which may be subject to suspension, revocation, modification, termination or denial under certain circumstances or which may not be renewed upon their expiration for various reasons, including noncompliance. See “Business — Environmental, Health and Safety.” These Environmental Permits can be difficult, costly and time consuming to obtain and may contain conditions that limit our operations. Additionally, any failure to obtain and maintain such Environmental Permits could restrict or otherwise prohibit certain aspects of our operations, which could have a material adverse effect on our business, financial condition and results of operations.
We have expended, and may be required to expend in the future, substantial funds for compliance with Environmental Laws. As recyclers of hazardous metal-containing chemical wastes, certain of our subsidiaries have been, and are likely to be, the focus of extensive compliance reviews by environmental regulatory authorities under Environmental Laws, including those relating to the generation, transportation, treatment, storage and disposal of solid and hazardous wastes under the RCRA. In the past, some of our subsidiaries have paid fines and entered into consent orders to address alleged environmental violations. See “Business — Environmental, Health and Safety.” We cannot assure you that our operations or activities or those of certain of our subsidiaries, including with respect to compliance with Environmental Laws, will not result in civil or criminal enforcement actions or private actions, regulatory or judicial orders enjoining or curtailing operations or requiring corrective measures, installation of pollution control equipment or remedial measures or costs, revocation of required Environmental Permits, or fines, penalties or damages, which could have a material adverse effect on our business, financial condition and results of operations.
37
In addition, we cannot predict the extent to which Environmental Laws, and the interpretation or enforcement thereof, may change or become more stringent in the future, each of which may affect the market for our products or give rise to additional capital expenditures, compliance costs or liabilities that could be material.
Our operations or products may impact the environment or cause or contribute to contamination or exposure to hazardous substances.
Given the nature of our current and former operations, particularly at our chemical manufacturing sites, we have incurred, are currently incurring and may in the future incur liabilities under CERCLA, or under other federal, state, local and foreign Environmental Laws related to releases of or contamination by hazardous substances, with respect to our current or former sites, adjacent or nearby third-party sites, or offsite disposal locations. See “Business — Environmental, Health and Safety.” Certain Environmental Laws, including CERCLA, can impose strict, joint, several and retroactive liability for the cost of investigation and cleanup of contaminated sites on owners and operators of such sites, as well as on persons who dispose of or arrange for disposal of hazardous substances at such sites. Accordingly, we could incur liability, whether as a result of government enforcement or private claims, for known or unknown liabilities at, or caused by migration from or hazardous waste transported from, any of our current or former facilities or properties, including those owned or operated by predecessors or third parties. See “Business — Environmental, Health and Safety.” Such liability could have a material adverse effect on our business, financial condition and results of operations.
The nature of our current and former operations also exposes us to the risk of claims under Environmental Laws. We could be subject to claims by environmental regulatory authorities, individuals and other third parties seeking damages for alleged personal injury, property damage and damages to natural resources resulting from hazardous substance contamination or human exposure caused by our operations, facilities or products, and there can be no assurance that material costs and liabilities will not be incurred in connection with any such claims. Our insurance may not be sufficient to cover any of these exposures, product, injury or damage claims.
Furthermore, regulatory agencies are showing increasing concern over the impact of animal health products and livestock operations on the environment. This increased regulatory scrutiny may necessitate that additional time and resources be spent to address these concerns for both new and existing products and could affect product sales and materially adversely affect our business, financial condition or results of operations.
We cannot assure you that our liabilities arising from past or future releases of, or exposure to, hazardous substances will not materially adversely affect our business, financial condition or results of operations.
We have been and may continue to be subject to claims of injury from direct exposure to certain of our products that constitute or contain hazardous substances and from indirect exposure when such substances are incorporated into other companies’ products.
Because certain of our products constitute or contain hazardous substances, and because the production of certain chemicals involves the use, handling, processing, storage and transportation of hazardous substances, from time to time we are subject to claims of injury from direct exposure to such substances and from indirect exposure when such substances are incorporated into other companies’ products. There can be no assurance that as a result of past or future operations, there will not be additional claims of injury by employees or members of the public due to exposure, or alleged exposure, to such substances. We are also party to a number of claims and lawsuits arising out of the normal course of business, including product liability claims and allegations of violations of governmental regulations, and face present and future claims with respect to workplace exposure, workers’ compensation and other matters. In most cases, such claims are covered by insurance and, where applicable, workers’ compensation insurance, subject to policy limits and exclusions; however, our insurance coverage, to the extent available, may not be adequate to protect us from all liabilities that we might incur in connection with the manufacture, sale and use of our products. Insurance is expensive and, in the future, may not be available on acceptable terms, if at all. A successful claim or series of claims brought against us in excess of our insurance coverage could have a materially adverse effect on our business, financial condition and results of operations. In addition, any claims, even if not ultimately successful, could adversely affect the marketplace’s acceptance of our products.
38
We are subject to risks from litigation that may materially impact our operations.
We face an inherent business risk of exposure to various types of claims and lawsuits. We are involved in various legal proceedings that arise in the ordinary course of our business. Although it is not possible to predict with certainty the outcome of every pending claim or lawsuit or the range of probable loss, we believe these pending lawsuits and claims will not individually or in the aggregate have a material adverse impact on our results of operations. However, we could, in the future, be subject to various lawsuits, including intellectual property, product liability, personal injury, product warranty, environmental or antitrust claims, among others, and incur judgments or enter into settlements of lawsuits and claims that could have a material adverse effect on our results of operations in any particular period.
We are subject to risks that may not be covered by our insurance policies.
In addition to pollution and other environmental risks, we are subject to risks inherent in the animal health, mineral nutrition and performance products industries, such as explosions, fires, spills or releases. Any significant interruption of operations at our principal facilities could have a material adverse effect on us. We maintain general liability insurance, pollution legal liability insurance and property and business interruption insurance with coverage limits that we believe are adequate. Because of the nature of industry hazards, it is possible that liabilities for pollution and other damages arising from a major occurrence may not be covered by our insurance policies or could exceed insurance coverages or policy limits or that such insurance may not be available at reasonable rates in the future. Any such liabilities, which could arise due to injury or loss of life, severe damage to and destruction of property and equipment, pollution or other environmental damage or suspension of operations, could have a material adverse effect on our business.
We may fail to consummate the proposed acquisition of certain Zoetis products and assets, may not consummate the proposed acquisition on expected terms, or may not achieve the anticipated benefits.
In April 2024, we entered into a definitive agreement with Zoetis to acquire Zoetis’s MFA product portfolio, certain water-soluble products and related assets. It is currently anticipated that the Proposed Acquisition will be completed between October and December 2024. Completion of the Proposed Acquisition is subject to customary closing conditions, including clearances by applicable regulatory authorities. Until all such closing conditions are satisfied or waived, the possible timing and likelihood of completion of the Proposed Acquisition are uncertain, and, accordingly, there can be no assurance that the Proposed Acquisition will be completed on the expected terms, on the anticipated schedule or at all. Any delay in consummation of the Proposed Acquisition may result in greater transaction costs and professional fees.
Our efforts to complete the Proposed Acquisition could cause substantial disruptions in our business. A substantial amount of our management’s attention is being directed towards the completion of the Proposed Acquisition and such distraction could affect our management’s ability to service our existing business, pursue other business opportunities or could otherwise adversely affect our business.
If consummated, the success of the Proposed Acquisition will depend, in significant part, on our ability to successfully integrate the acquired business, establish and maintain good relationships with new and existing customers, suppliers, and other business partners, grow the revenue of the consolidated company and realize the anticipated strategic benefits and synergies. The combination of businesses is a complex, costly and time-consuming process. As a result, we expect to devote significant management attention and resources prior to closing to prepare for integration, and we expect to devote significant management attention and resources post-closing to integrate the business practices and operations. The integration process may disrupt the businesses and, if implemented ineffectively, would impair the realization of the full expected benefits. The growth and the anticipated benefits of the Proposed Acquisition may not be realized fully or at all, or may take longer to realize than we expect. Actual operating, strategic and revenue opportunities, if achieved at all, may be less significant than we expect or may take longer to achieve than anticipated. If we are not able to achieve these objectives and realize the anticipated benefits and synergies expected from the Proposed Acquisition within a reasonable time, our business, financial condition and operating results may be adversely affected.
Adverse U.S. and international economic and market conditions may adversely affect our product sales and business.
Current U.S. and international economic and market conditions are uncertain. The COVID-19 pandemic adversely affected international economic conditions and financial markets and led to economic downturns in many countries in which we operate. Our revenues and operating results may be affected by uncertain or changing economic and market conditions, including as a result of a resurgence of the COVID-19 pandemic or other similar public health crises, and other challenges faced in the credit markets and financial services industry.
39
Economic, business, political and financial disruptions from the ongoing armed conflicts between Israel and Hamas (and potential broader military conflict in the region) and between Russia and Ukraine and the imposition of sanctions and business disruptions as well as inflation, could also have a material adverse effect on our operating results, financial condition, and liquidity. Certain of our customers and suppliers could be affected directly by an economic downturn and could face credit issues or cash flow problems that could give rise to payment delays, increased credit risk, bankruptcies and other financial hardships that could decrease the demand for our products or hinder our ability to collect amounts due from customers. Customers may seek lower price alternatives to our products if they are negatively impacted by poor economic conditions. Furthermore, our exposure to credit and collectability risk and cybersecurity risk is higher in certain international markets and as a result of the crisis resulting from the ongoing armed conflicts between Israel and Hamas and between Russia and Ukraine, our ability to mitigate such risks may be limited. While we have procedures to monitor and limit exposure to credit and collectability risk and we have defensive measures in place to prevent and mitigate cyberattacks, there can be no assurance that such procedures and measures will effectively limit such risks and avoid losses.
If domestic and global economic and market conditions remain uncertain or persist or deteriorate further, we may experience material impacts on our business, financial condition and results of operations. Adverse economic conditions impacting our customers, including, among others, increased taxation, higher unemployment, lower customer confidence in the economy, higher customer debt levels, lower availability of customer credit, higher interest rates and hardships relating to declines in the stock markets, could cause purchases of meat products to decline, resulting in a decrease in purchases of our products, which could adversely affect our financial condition and results of operation. Adverse economic and market conditions could also negatively impact our business by negatively affecting the parties with whom we do business, including among others, our customers, our manufacturers and our suppliers.
We may not be able to realize the expected benefits of our investments in emerging markets.
We have been taking steps to take advantage of the rise in global demand for animal protein in emerging markets, including by expanding our manufacturing presence, sales, marketing and distribution in these markets. Failure to continue to maintain and expand our business in emerging markets could also materially adversely affect our operating results and financial condition.
Some countries within emerging markets may be especially vulnerable to periods of local, regional or global economic, political or social instability or crisis. For example, our sales in certain emerging markets have suffered from extended periods of disruption due to natural disasters. Furthermore, we have also experienced lower than expected sales in certain emerging markets due to local, regional and global restrictions on banking and commercial activities in those countries. For all these and other reasons, sales within emerging markets carry significant risks.
Modification of foreign trade policy may harm our food animal product customers.
Changes in laws, agreements and policies governing foreign trade in the territories and countries where our customers do business could negatively impact such customers’ businesses and adversely affect our results of operations. A number of our customers, particularly U.S.-based food animal producers have benefited from free trade agreements, including, in the past, the North American Free Trade Agreement (“NAFTA”). The U.S., Canada and Mexico reached an agreement to replace NAFTA with the United States-Mexico-Canada Agreement. Any other changes to international trade agreements or policies could harm our customers, and as a result, negatively impact our financial condition and results of operations. Additionally, in response to new U.S. tariffs affecting foreign imports, some foreign governments, including China, have instituted or are considering instituting tariffs on certain U.S. goods. While the scope and duration of these and any future tariffs remain uncertain, tariffs imposed by the U.S. or foreign governments on our customers’ products, or on our products or the active pharmaceutical ingredients or other components thereof, could negatively impact our financial condition and results of operations.
40
Our product approval, R&D, acquisition and licensing efforts may fail to generate new products and product lifecycle developments.
Our future success depends on both our existing product portfolio, including our ability to obtain cross-clearances enabling the use of our medicated products in conjunction with other products, approval for use of our products with new species, approval for new claims for our products, approval of our products in new markets and our pipeline of new products, including new products that we may develop through joint ventures and products that we are able to obtain through license or acquisition. The majority of our R&D programs focus on product lifecycle development, which is defined as R&D programs that leverage existing animal health products by adding new species or claims, achieving approvals in new markets or creating new combinations and reformulations. We commit substantial effort, funds and other resources to expanding our product approvals and R&D, both through our own dedicated resources and through collaborations with third parties.
We may be unable to determine with accuracy when or whether any of our expanded product approvals for our existing product portfolio or any of our products now under development will be approved or launched, or we may be unable to obtain expanded product approvals or develop, license or otherwise acquire product candidates or products. In addition, we cannot predict whether any products, once launched, will be commercially successful or will achieve sales and revenues that are consistent with our expectations. The animal health industry is subject to regional and local trends and regulations and, as a result, products that are successful in some of our markets may not achieve similar success when introduced into new markets. Furthermore, the timing and cost of our R&D may increase, and our R&D may become less predictable. For example, changes in regulations applicable to our industry may make it more time-consuming and/or costly to research, test and develop products.
Products in the animal health industry are sometimes derived from molecules and compounds discovered or developed as part of human health research. We may enter into collaboration or licensing arrangements with third parties to provide us with access to compounds and other technology for purposes of our business. Such agreements are typically complex and require time to negotiate and implement. If we enter into these arrangements, we may not be able to maintain these relationships or establish new ones in the future on acceptable terms or at all. In addition, any collaboration that we enter into may not be successful, and the success may depend on the efforts and actions of our collaborators, which we may not be able to control. If we are unable to access human health-generated molecules and compounds to conduct R&D on cost-effective terms, our ability to develop new products could be limited.
The actual or purported intellectual property rights of third parties may negatively affect our business.
A third party may sue us, our distributors or licensors, or otherwise make a claim, alleging infringement or other violation of the third-party’s patents, trademarks, trade dress, copyrights, trade secrets, domain names or other intellectual property rights. If we do not prevail in this type of litigation, we may be required to:
| ● | pay monetary damages; |
| ● | obtain a license in order to continue manufacturing or marketing the affected products, which may not be available on commercially reasonable terms, or at all; or |
| ● | stop activities, including any commercial activities, relating to the affected products, which could include a recall of the affected products and/or a cessation of sales in the future. |
The costs of defending an intellectual property claim could be substantial and could materially adversely affect our operating results and financial condition, even if we successfully defend such claims. We may also incur costs in connection with an obligation to indemnify a distributor, licensor or other third party. Moreover, even if we believe that we do not infringe a validly existing third-party patent, we may choose to license such patent, which would result in associated costs and obligations.
The intellectual property positions of animal health medicines and vaccines businesses frequently involve complex legal and factual questions, and an issued patent does not guarantee us the right to practice the patented technology or develop, manufacture or commercialize the patented product. We cannot be certain that a competitor or other third party does not have or will not obtain rights to intellectual property that may prevent us from manufacturing, developing or marketing certain of our products, regardless of whether we believe such intellectual property rights are valid and enforceable or we believe we would be otherwise able to develop a more commercially successful product, which may harm our financial condition and results of operations.
41
If our intellectual property rights are challenged or circumvented, competitors may be able to take advantage of our R&D efforts. We are also dependent upon trade secrets, which in some cases may be difficult to protect.
Our long-term success largely depends on our ability to market technologically competitive products. We rely and expect to continue to rely on a combination of intellectual property, including patent, trademark, trade dress, copyright, trade secret and domain name protection laws, as well as confidentiality and license agreements with our employees and others, to protect our intellectual property and proprietary rights. If we fail to obtain and maintain adequate intellectual property protection, we may not be able to prevent third parties from using our proprietary technologies or from marketing products that are very similar or identical to ours. Our currently pending or future patent applications may not result in issued patents, or be approved on a timely basis, or at all. Similarly, any term extensions that we seek may not be approved on a timely basis, if at all. In addition, our issued patents may not contain claims sufficiently broad to protect us against third parties with similar technologies or products or provide us with any competitive advantage, including exclusivity in a particular product area. The scope of our patent claims also may vary between countries, as individual countries have their own patent laws. For example, some countries only permit the issuance of patents covering a novel chemical compound itself, and its first use, and thus further methods of use for the same compound, may not be patentable. We may be subject to challenges by third parties regarding our intellectual property, including claims regarding validity, enforceability, scope and effective term. The validity, enforceability, scope and effective term of patents can be highly uncertain and often involve complex legal and factual questions and proceedings. Our ability to enforce our patents also depends on the laws of individual countries and each country’s practice with respect to enforcement of intellectual property rights. In addition, if we are unable to maintain our existing license agreements or other agreements pursuant to which third parties grant us rights to intellectual property, including because such agreements expire or are terminated, our financial condition and results of operations could be materially adversely affected.
Patent law changes in the United States and other countries may also weaken our ability to enforce our patent rights or make such enforcement financially unattractive. Any such changes could result in increased costs to protect our intellectual property or limit our ability to obtain and maintain patent protection for our products in these jurisdictions.
Additionally, certain foreign governments have indicated that compulsory licenses to patents may be granted in the case of national emergencies, which could diminish or eliminate sales and profits from those regions and materially adversely affect our operating results and financial condition.
Likewise, in the United States and other countries, we currently hold issued trademark registrations and have trademark applications pending, any of which may be the subject of a governmental or third-party objection, which could prevent the maintenance or issuance of the same and thus create the potential need to rebrand or relabel a product. As our products mature, our reliance on our trademarks to differentiate us from our competitors increases and as a result, if we are unable to prevent third parties from adopting, registering or using trademarks and trade dress that infringe, dilute or otherwise violate our trademark rights, our business could be materially adversely affected.
Our competitive position is also dependent upon unpatented trade secrets, which in some cases may be difficult to protect. Others may independently develop substantially equivalent proprietary information and techniques or may otherwise gain access to our trade secrets and trade secrets may be disclosed or we may not be able to protect our rights to unpatented trade secrets.
Many of our vaccine products and other products are based on or incorporate proprietary information, including proprietary master seeds and proprietary or patented adjuvant formulations. We actively seek to protect our proprietary information, including our trade secrets and proprietary know-how, by requiring our employees, consultants, other advisors and other third parties to execute confidentiality agreements upon the commencement of their employment, engagement or other relationship. Despite these efforts and precautions, we may be unable to prevent a third party from copying or otherwise obtaining and using our trade secrets or our other intellectual property without authorization and legal remedies may not adequately compensate us for the damages caused by such unauthorized use. Further, others may independently and lawfully develop substantially similar or identical products that circumvent our intellectual property by means of alternative designs or processes or otherwise.
42
The misappropriation and infringement of our intellectual property, particularly in foreign countries where the laws may not protect our proprietary rights as fully as in the United States, may occur even when we take steps to prevent it. In the future, we may be party to patent lawsuits and other intellectual property rights claims that are expensive and time consuming, and if resolved adversely, could have a significant impact on our business and financial condition. In the future, we may not be able to enforce intellectual property that relates to our products for various reasons, including licensor restrictions and other restrictions imposed by third parties, and the costs of doing so may outweigh the value of doing so, and this could have a material adverse impact on our business and financial condition.
We are subject to the U.S. Foreign Corrupt Practices Act and other anti-corruption laws or trade control laws, as well as other laws governing our international operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties, other remedial measures and legal expenses, which could adversely affect our business, financial condition and results of operations.
Our operations are subject to anti-corruption laws, including the FCPA and other anti-corruption laws that apply in countries where we do business. The FCPA, UK Bribery Act and other laws generally prohibit us and our employees and intermediaries from bribing, being bribed or making other prohibited payments to government officials or other persons to obtain or retain business or gain some other business advantage. We operate in a number of jurisdictions that pose a high risk of potential FCPA violations, and we participate in relationships with third parties whose actions could potentially subject us to liability under the FCPA or local anti-corruption laws. In addition, we cannot predict the nature, scope or effect of future regulatory requirements to which our international operations might be subject or the manner in which existing laws might be administered or interpreted.
We are also subject to other laws and regulations governing our international operations, including regulations administered by the U.S. Department of Commerce’s Bureau of Industry and Security, the U.S. Department of Treasury’s Office of Foreign Asset Control and various non-U.S. government entities, including applicable export control regulations, economic sanctions on countries and persons, customs requirements, currency exchange regulations and transfer pricing regulations (collectively, the “Trade Control laws”).
There is no assurance that we will be completely effective in ensuring our compliance with all applicable anticorruption laws, including the FCPA or other legal requirements, including Trade Control laws. If we are not in compliance with the FCPA and other anti-corruption laws or Trade Control laws, we may be subject to criminal and civil penalties, disgorgement and other sanctions and remedial measures, and legal expenses, which could have an adverse impact on our business, financial condition, results of operations and liquidity. Likewise, any investigation of any potential violations of the FCPA other anti-corruption laws or Trade Control laws by U.S. or foreign authorities could also have an adverse impact on our reputation, business, financial condition and results of operations.
Increased regulation or decreased governmental financial support for the raising, processing or consumption of food animals could reduce demand for our animal health products.
Companies in the animal health industry are subject to extensive and increasingly stringent regulations. If livestock producers are adversely affected by new regulations or changes to existing regulations, they may reduce herd sizes or become less profitable and, as a result, they may reduce their use of our products, which may materially adversely affect our operating results and financial condition. Furthermore, adverse regulations related, directly or indirectly, to the use of one or more of our products may injure livestock producers’ market position. More stringent regulation of the livestock industry or our products could have a material adverse effect on our operating results and financial condition. Also, many industrial producers, including livestock producers, benefit from governmental subsidies, and if such subsidies were to be reduced or eliminated, these companies may become less profitable and, as a result, may reduce their use of our products.
Increased or decreased inventory levels at our channel distributors can lead to fluctuations in our revenues and variations in payment terms extended to our distributors can impact our cash flows.
In addition to selling our products directly to customers, we also sell to distributors who, in turn, sell our products to third parties. Inventory levels at our distributors may increase or decrease as a result of various factors, including end customer demand, new customer contracts, the influence of competition, political and socio-economic climate, contractual obligations related to minimum inventory levels, changing perceptions, including those of alternative products, our ability to renew distribution contracts with expected terms, our ability to implement commercial strategies, regulatory restrictions, armed conflicts, unexpected customer behavior, proactive measures taken by us in response to shifting market dynamics and procedures and environmental factors beyond our control, including weather conditions or an outbreak of infectious disease such as COVID-19 or diseases carried by farm animals such as African Swine fever.
43
These increases and decreases can lead to variations in our quarterly and annual revenues.
In addition, we have policies that govern the payment terms that we extend to our customers. From time to time, our distributors have requested exceptions to the payment term policies that we extend to them for various reasons, including consolidation amongst our distributors, changes in the buying patterns of end customers, as well as the perception of our distributors regarding the need to maintain certain inventory levels to avoid supply disruptions. Extensions of anticipated customer payment terms can impact our cash flows, liquidity and results of operations.
We have substantial debt and interest payment requirements that may restrict our future operations and impair our ability to meet our obligations under our indebtedness. Restrictions imposed by our outstanding indebtedness, including the restrictions contained in our 2024 Credit Facilities, may limit our ability to operate our business and to finance our future operations or capital needs or to engage in other business activities.
As of June 30, 2024, we had outstanding indebtedness (reflecting the principal amounts) of $256.9 million under our 2021 Term A loan (as defined below), $45.0 million under our 2023 Incremental Term loan (as defined below), $176.0 million of outstanding borrowings under our revolving credit facility, $11.3 million under our 2022 Term Loan (as defined below) and $2.3 million of outstanding letters of credit. In July 2024, to refinance our existing indebtedness and to fund the pending Proposed Acquisition, we entered into the 2024 Credit Facilities (as defined below). Subject to restrictions in our 2024 Credit Facilities, we may incur significant additional indebtedness. If we and our subsidiaries incur significant additional indebtedness, the related risks that we face could intensify.
Our substantial debt may have important consequences. For instance, it could:
| ● | make it more difficult for us to satisfy our financial obligations, including those relating to the 2024 Credit Facilities; |
| ● | require us to dedicate a substantial portion of any cash flow from operations to the payment of interest and principal due under our debt, which will reduce funds available for other business purposes, including capital expenditures and acquisitions; |
| ● | increase our vulnerability to general adverse economic and industry conditions; |
| ● | limit our flexibility in planning for or reacting to changes in our business and the industry in which we operate; |
| ● | place us at a competitive disadvantage compared with some of our competitors that may have less debt and better access to capital resources; and |
| ● | limit our ability to obtain additional financing required to fund working capital and capital expenditures and for other general corporate purposes. |
Our ability to satisfy our obligations and to reduce our total debt depends on our future operating performance and on economic, financial, competitive and other factors, many of which are beyond our control. Our business may not generate sufficient cash flow, and future financings may not be available to provide sufficient net proceeds, to meet these obligations or to successfully execute our business strategy.
The terms of the 2024 Credit Facilities contain certain covenants that limit our ability and that of our subsidiaries to create liens, merge or consolidate, dispose of assets, incur indebtedness and guarantees, repurchase or redeem capital stock and indebtedness, make certain investments or acquisitions, enter into certain transactions with affiliates or change the nature of our business. As a result of these covenants and restrictions, we will be limited in how we conduct our business, and we may be unable to raise additional debt or equity financing to compete effectively or to take advantage of new business opportunities. The terms of any future indebtedness we may incur could include more restrictive covenants. We may not be able to maintain compliance with the covenants in any of our debt instruments in the future and, if we fail to do so, we may not be able to obtain waivers from the lenders and/or amend the covenants.
44
We may not be able to generate sufficient cash to service all of our indebtedness and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or refinance our debt obligations depends on our financial condition and operating performance, which are subject to prevailing economic and competitive conditions and to certain financial, business, legislative, regulatory and other factors beyond our control, including the impact of any public health crises, such as the COVID-19 pandemic, the ongoing armed conflicts between Israel and Hamas and between Russia and Ukraine, and the related economic downturn in the debt markets. In connection with the pending Proposed Acquisition and corresponding refinancing of our existing indebtedness through the 2024 Credit Facilities, we expect our debt interest payments to increase substantially. We may be unable to maintain a level of cash flows from operating activities sufficient to permit us to pay the principal and interest on our indebtedness.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we could face substantial liquidity problems and could be forced to reduce or delay investments and capital expenditures, or to dispose of material assets or operations, alter our dividend policy, seek additional debt or equity capital or restructure or refinance our indebtedness. We may not be able to effect any such alternative measures on commercially reasonable terms or at all and, even if successful, those alternative actions may not allow us to meet our scheduled debt service obligations. The instruments that govern our indebtedness may restrict our ability to dispose of assets and may restrict the use of proceeds from those dispositions and may also restrict our ability to raise debt or equity capital to be used to repay other indebtedness when it becomes due. We may not be able to consummate those dispositions or to obtain proceeds in an amount sufficient to meet any debt service obligations when due.
In addition, we conduct our operations through our subsidiaries. Accordingly, repayment of our indebtedness will depend on the generation of cash flow by our subsidiaries, including our international subsidiaries, and their ability to make such cash available to us, by dividend, debt repayment or otherwise. Our subsidiaries may not have any obligation to pay amounts due on our indebtedness or to make funds available for that purpose. Our subsidiaries may not be able to, or may not be permitted to, make distributions to enable us to make payments in respect of our indebtedness. Each subsidiary is a distinct legal entity, and under certain circumstances, legal, tax and contractual restrictions may limit our ability to obtain cash from our subsidiaries or may subject any transfer of cash from our subsidiaries to substantial tax liabilities. In the event that we do not receive distributions from our subsidiaries, we may be unable to make required principal and interest payments on our indebtedness.
Our inability to generate sufficient cash flows to satisfy our debt obligations, or to refinance our indebtedness on commercially reasonable terms or at all, may materially adversely affect our operating results, financial condition and liquidity and our ability to satisfy our obligations under our indebtedness or pay dividends on our common stock.
We are subject to change of control provisions.
We are a party to certain contractual arrangements that are subject to change of control provisions. In this context, “change of control” is generally defined as including (a) any person or group, other than Mr. Jack C. Bendheim and his family and affiliates (the current holders of approximately 90.9% of the combined voting power of all classes of our outstanding common stock), becoming the beneficial owner of more than 50% of the total voting power of our stock, and (b) a change in any twelve month period in the majority of the members of the Board that is not approved by Mr. Bendheim and/or his family and affiliates or by the majority of directors in office at the start of such period.
Mr. Bendheim and his family and affiliates may choose to dispose of part or all of their stakes in us and/or may cease to exercise the current level of control they have over the appointment and removal of members of our Board. Any such changes may trigger a “change of control” event that could result in us being forced to repay the 2024 Credit Facilities or lead to the termination of a significant contract to which we are a party. If any such event occurs, this may negatively affect our financial condition and operating results. In addition, we may not have sufficient funds to finance repayment of any of such indebtedness upon any such “change in control.”
We depend on sophisticated information technology and infrastructure.
We rely on various information systems to manage our operations, and we increasingly depend on third parties and applications on virtualized, or “cloud,” infrastructure to operate and support our information technology systems. These third parties include large established vendors as well as small, privately owned companies.
45
Failure by these providers to adequately service our operations or a change in control or insolvency of these providers could have an adverse effect on our business, which in turn may materially adversely affect our business, financial condition or results of operations.
We may be required to write down goodwill or identifiable intangible assets.
Under generally accepted accounting principles in the United States (“GAAP”), if we determine goodwill or identifiable intangible assets are impaired, we will be required to write down these assets and record a non-cash impairment charge. As of June 30, 2024, we had goodwill of $54.6 million and identifiable intangible assets, less accumulated amortization, of $45.0 million. Identifiable intangible assets consist primarily of developed technology rights and patents and customer relationships.
Determining whether an impairment exists and the amount of the potential impairment involves quantitative data and qualitative criteria that are based on estimates and assumptions requiring significant management judgment. Future events or new information may change management’s valuation of goodwill or an intangible asset in a short amount of time. The timing and amount of impairment charges recorded in our consolidated statements of operations and write-downs recorded in our consolidated balance sheets could vary if management’s conclusions change. Any impairment of goodwill or identifiable intangible assets could have a material adverse effect on our financial condition and results of operations.
We may be unable to adequately protect our customers’ privacy or we may fail to comply with privacy laws.
The protection of customer, employee and company data is critical and the regulatory environment surrounding information security, storage, use, processing, disclosure and privacy is demanding, with the frequent imposition of new and changing requirements. In addition, our customers expect that we will adequately protect their personal information. Any actual or perceived significant breakdown, intrusion, interruption, cyber-attack or corruption of customer, employee or company data or our failure to comply with federal, state, local and foreign privacy laws could damage our reputation and result in lost sales, fines and lawsuits. Despite our considerable efforts and technology to secure our computer network, security could be compromised, confidential information could be misappropriated or system disruptions could occur. Any actual or perceived access, disclosure or other loss of information or any significant breakdown, intrusion, interruption, cyber-attack or corruption of customer, employee or company data or our failure to comply with federal, state, local and foreign privacy laws or contractual obligations with customers, vendors, payment processors and other third parties, could result in legal claims or proceedings, liability under laws or contracts that protect the privacy of personal information, regulatory penalties, disruption of our operations and damage to our reputation, all of which could materially adversely affect our business, revenue and competitive position.
We may be subject to information technology system failures, network disruptions and breaches in data security.
We are increasingly dependent upon information technology systems and infrastructure to conduct critical operations and generally operate our business, which includes using information technology systems to process, transmit and store electronic information in our day-to-day operations, including customer, employee and company data. The changes to “in-office” expectations resulting from the COVID-19 pandemic have resulted in a substantial portion of our employees working remotely and have increased our dependence on tools that facilitate employees working from home and gaining remote access to our information technology systems. As a result, any disruption to our information technology systems, our industrial machinery, software used in our manufacturing facilities, firmware or software embedded in our equipment or machinery, including from cyber incidents, could have a material adverse effect on our business. The increased use of these tools could also make our information technology systems more vulnerable to breaches of data security and cybersecurity attacks. The size and complexity of our computer systems make them potentially vulnerable to breakdown, malicious intrusion and random attack. We also store certain information with third parties. Our information systems and those of our third-party vendors are subjected to computer viruses or other malicious codes, unauthorized access attempts, and cyber, phishing or ransomware attacks and also are vulnerable to an increasing threat of continually evolving cybersecurity risks and external hazards. Disruption, degradation, or manipulation of these systems and infrastructure through intentional or accidental means could impact key business processes. Cyber-attacks against the Company’s systems and infrastructure could result in exposure of confidential information, the modification of critical data and/or the failure of critical operations. Likewise, improper or inadvertent employee behavior, including data privacy breaches by employees and others with permitted access to our systems may pose a risk that sensitive data may be exposed to unauthorized persons or to the public. Any such breach could compromise our networks, and the information stored therein could be accessed, publicly disclosed, lost or stolen.
46
Such attacks could result in our intellectual property and other confidential information being lost or stolen, disruption of our operations and other negative consequences, such as increased costs for security measures or remediation costs, and diversion of management attention. Although the aggregate impact on the Company’s operations and financial condition has not been material to date, the Company has been the target of events of this nature and expects them to continue as cyber-attacks are becoming more sophisticated and frequent, and the techniques used in such attacks change rapidly. The Company monitors its data, information technology and personnel usage of Company systems to reduce these risks and continues to do so on an ongoing basis for any current or potential threats.
If any of our operational technologies, software or hardware or other control systems are compromised, fail or have other significant shortcomings, it could disrupt our business, require us to incur substantial additional expenses or result in potential liability or reputational damage. While we have invested in protection of data and information technology, there can be no assurance that our efforts will prevent such breakdowns, cybersecurity attacks or breaches in our systems that could cause reputational damage, business disruption and legal and regulatory costs; could result in third-party claims; could result in compromise or misappropriation of our intellectual property, trade secrets and sensitive information; and could otherwise adversely affect our business and financial results.
Implementing new business lines or offering new products and services may subject us to additional risks.
From time to time, we may implement new business lines or offer new products and services within existing lines of business. There may be substantial risks and uncertainties associated with these efforts. We may invest significant time and resources in developing, marketing, or acquiring new lines of business and/or offering new products and services. Initial timetables for the introduction and development or acquisition of new lines of business and/or the offering of new products or services may not be achieved, and price and profitability targets may prove to be unachievable. Our lack of experience or knowledge, as well as external factors, such as compliance with regulations, competitive alternatives and shifting market preferences, may also impact the success of an acquisition or the implementation of a new line of business or a new product or service. New business lines or new products and services within existing lines of business could affect the sales and profitability of existing lines of business or products and services. Failure to successfully manage these risks in the implementation or acquisition of new lines of business or the offering of new products or services could have a material adverse effect on our reputation, business, results of operations, and financial condition.
Risks Related to Ownership of Our Class A Common Stock
Our multiple class structure and the concentration of our voting power with certain of our stockholders will limit your ability to influence corporate matters, and conflicts of interest between certain of our stockholders and us or other investors could arise in the future.
As of August 23, 2024, BFI Co., LLC (“BFI”) beneficially owns 59,480 shares of our Class A common stock and 20,166,034 shares of our Class B common stock, which together represent approximately 90.9% of the combined voting power of all classes of our outstanding common stock. As of August 23, 2024, our other stockholders collectively own interests representing approximately 9.1% of the combined voting power of all classes of our outstanding common stock. Because of our multiple class structure and the concentration of voting power with BFI, BFI will continue to be able to control all matters submitted to our stockholders for approval for so long as BFI holds common stock representing greater than 50% of the combined voting power of all classes of our outstanding common stock. BFI will therefore have significant influence over management and affairs and control the approval of all matters requiring stockholder approval, including the election of directors and significant corporate transactions, such as a merger or other sale of the Company or its assets, for the foreseeable future.
We are classified as a “controlled company” and, as a result, we qualify for, and intend to rely on, exemptions from certain corporate governance requirements. You will not have the same protections afforded to stockholders of companies that are subject to such requirements.
BFI controls a majority of the combined voting power of all classes of our outstanding common stock. As a result, we are a “controlled company” within the meaning of the Nasdaq corporate governance standards. Under Nasdaq rules, a company of which more than 50% of the voting power is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance requirements, including:
47
| ● | the requirement that a majority of the Board consists of independent directors; |
| ● | the requirement that we have a nominating and corporate governance committee and that it is composed entirely of independent directors; and |
| ● | the requirement for an annual performance evaluation of the nominating and corporate governance and compensation committees. |
We utilize and intend to continue to utilize these exemptions. As a result, while we currently have a majority of independent directors:
| ● | we may not have a majority of independent directors in the future; |
| ● | we will not have a nominating and corporate governance committee; and |
| ● | we will not be required to have an annual performance evaluation of the compensation committee. |
Accordingly, you will not have the same protections afforded to stockholders of companies that are subject to all of the Nasdaq corporate governance requirements.
Our stock price may be volatile or may decline regardless of our operating performance.
The market price of our Class A common stock may fluctuate significantly in response to a number of factors, many of which we cannot control, including those described under “— Risk Factors Relating to Our Business” and the following:
| ● | changes in financial estimates by any securities analysts who follow our Class A common stock, our failure to meet these estimates or failure of those analysts to initiate or maintain coverage of our Class A common stock; |
| ● | downgrades by any securities analysts who follow our Class A common stock; |
| ● | future sales of our Class A common stock by our officers, directors and significant stockholders; |
| ● | market conditions or trends in our industry or the economy as a whole and, in particular, in the animal health industry; |
| ● | investors’ perceptions of our prospects; |
| ● | announcements by us or our competitors of significant contracts, acquisitions, joint ventures or capital commitments; and |
| ● | changes in key personnel. |
In addition, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. The COVID-19 pandemic and the ongoing armed conflicts between Israel and Hamas and between Russia and Ukraine have contributed to significant volatility in stock and financial markets in the United States and globally. In the past, stockholders have instituted securities class action litigation following periods of market volatility. If we were involved in securities litigation, we could incur substantial costs, and our resources and the attention of management could be diverted from our business.
Our majority stockholder has the ability to control significant corporate activities and our majority stockholder’s interests may not coincide with yours.
As of August 23, 2024, approximately 90.9% of the combined voting power of all classes of our outstanding common stock is held by BFI. As a result of its ownership, so long as it holds a majority of the combined voting power of all classes of our outstanding common stock, BFI will have the ability to control the outcome of matters submitted to a vote of stockholders and, through our Board of Directors, the ability to control decision-making with respect to our business direction and policies. Matters over which BFI, directly or indirectly, exercises control include:
| ● | the election of our Board of Directors and the appointment and removal of our officers; |
48
| ● | mergers and other business combination transactions, including proposed transactions that would result in our stockholders receiving a premium price for their shares; |
| ● | other acquisitions or dispositions of businesses or assets; |
| ● | incurrence of indebtedness and the issuance of equity securities; |
| ● | repurchase of stock and payment of dividends; and |
| ● | the issuance of shares to management under our equity incentive plans. |
Even if BFI’s ownership of our shares falls below a majority of the combined voting power of all classes of our outstanding common stock, it may continue to be able to influence or effectively control our decisions.
Future sales of our Class A common stock, or the perception in the public markets that these sales may occur, may depress our stock price.
Sales of substantial amounts of our Class A common stock in the public market, or the perception that these sales could occur, could adversely affect the price of our Class A common stock and could impair our ability to raise capital through the sale of additional shares. In addition, subject to certain restrictions on converting Class B common stock into Class A common stock, all of our outstanding shares of Class B common stock may be converted into Class A common stock and sold in the public market by existing stockholders. As of August 23, 2024, we had 20,337,574 shares of Class A common stock and 20,166,034 shares of Class B common stock outstanding.
BFI, which holds all of our outstanding Class B common stock, has the right to require us to register the sales of its shares under the Securities Act under the terms of an agreement between us and the holders of these securities. In the future, we may also issue our securities in connection with investments or acquisitions. The amount of shares of our Class A common stock issued in connection with an investment or acquisition could constitute a material portion of our then-outstanding shares of our Class A common stock.
Anti-takeover provisions in our charter documents and Delaware law might discourage or delay acquisition attempts for us that you might consider favorable.
Our certificate of incorporation and bylaws contain provisions that may make the acquisition of the Company more difficult without the approval of our Board of Directors. These provisions:
| ● | authorize the issuance of undesignated preferred stock, the terms of which may be established and the shares of which may be issued without stockholder approval, and which may include super voting, special approval, dividend, or other rights or preferences superior to the rights of the holders of Class A common stock; |
| ● | prohibit, at any time after BFI and its affiliates cease to hold at least 50% of the combined voting power of all classes of our outstanding common stock, stockholder action by written consent, without the express prior consent of the Board of Directors; |
| ● | provide that the Board of Directors is expressly authorized to make, alter or repeal our amended and restated bylaws; |
| ● | establish advance notice requirements for nominations for elections to our Board of Directors or for proposing matters that can be acted upon by stockholders at stockholder meetings; and |
| ● | establish a classified Board of Directors, as a result of which our Board of Directors will be divided into three classes, with each class serving for staggered three-year terms, which prevents stockholders from electing an entirely new Board of Directors at an annual meeting; and require, at any time after BFI and its affiliates cease to hold at least 50% of the combined voting power of all classes of our outstanding common stock, the approval of holders of at least three quarters of the combined voting power of all classes of our outstanding common stock for stockholders to amend the amended and restated bylaws or amended and restated certificate of incorporation. |
These anti-takeover provisions and other provisions under Delaware law could discourage, delay or prevent a transaction involving a change in control of the Company, even if doing so would benefit our stockholders. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing and to cause us to take other corporate actions you desire.
49
Our certificate of incorporation designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our certificate of incorporation provides that, subject to limited exceptions, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders, (iii) any action asserting a claim against us arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or our by-laws or (iv) any other action asserting a claim against us that is governed by the internal affairs doctrine. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and to have consented to the provisions of our certificate of incorporation described above. This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and employees. Alternatively, if a court were to find these provisions of our restated certificate of incorporation inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business, financial condition and results of operations.
Provisions of our certificate of incorporation could have the effect of preventing us from having the benefit of certain business opportunities that we would otherwise be entitled to pursue.
Our certificate of incorporation provides that BFI and its affiliates are not required to offer corporate opportunities of which they become aware to us and could, therefore, offer such opportunities instead to other companies including affiliates of BFI. In the event that BFI obtains business opportunities from which we might otherwise benefit but chooses not to present such opportunities to us, these provisions of our certificate of incorporation could have the effect of preventing us from pursuing transactions or relationships that would otherwise be in the best interests of our stockholders.
We may not pay cash dividends in the future and, as a result, you may not receive any return on investment unless you are able to sell your Class A common stock for a price greater than your initial investment.
We have a paid a quarterly dividend since September 2014 on our Class A and Class B common stock and our Board of Directors has declared a cash dividend of $0.12 per share on our Class A common stock and Class B common stock that is payable September 25, 2024. Any determination to pay dividends in the future will be at the discretion of our Board of Directors and will depend upon results of operations, financial condition, contractual restrictions and our ability to obtain funds from our subsidiaries to meet our obligations. Our 2024 Credit Facilities permit us to pay distributions to stockholders out of available cash subject to certain annual limitations and so long as no default or event of default under the 2024 Credit Facilities shall have occurred and be continuing at the time such distribution is declared. Realization of a gain on your investment will depend on the appreciation of the price of our Class A common stock.
General Risk Factors
We face competition in each of our markets from a number of large and small companies, some of which have greater financial, R&D, production and other resources than we have.
Many of our products face competition from alternative or substitute products. We are engaged in highly competitive industries and, with respect to all of our major products, face competition from a substantial number of global and regional competitors. Several new start-up companies also compete in the animal health industry. We believe many of our competitors are conducting R&D activities in areas served by our products and in areas in which we are developing products. Some competitors have greater financial, R&D, production and other resources than we have. Some of our principal competitors include Boehringer Ingelheim International GmbH, Ceva Santé Animale, Elanco Animal Health Incorporated, Huvepharma Inc., Merck & Co., Inc. (Merck Animal Health and MSD Animal Health), Southeastern Minerals, Inc. and Zoetis. To the extent these companies or new entrants offer comparable animal health, mineral nutrition or performance products at lower prices, our business could be adversely affected.
50
New entrants could substantially reduce our market share or render our products obsolete. Furthermore, many of our competitors have relationships with key distributors and, because of their size, have the ability to offer attractive pricing incentives, which may negatively impact or hinder our relationships with these distributors.
In certain countries, because of our size and product mix, we may not be able to capitalize on changes in competition and pricing as fully as our competitors. In recent years, there have been new generic medicated products introduced to the livestock industry, particularly in the United States.
There has been and likely will continue to be consolidation in the animal health market, which could strengthen our competitors. Our competitors can be expected to continue to improve the formulation and performance of their products and to introduce new products with competitive price and performance characteristics. There can be no assurance that we will have sufficient resources to maintain our current competitive position or market share. We also face competitive pressures arising from, among other things, more favorable safety and efficacy product profiles, limited demand growth or a significant number of additional competitive products being introduced into a particular market, price reductions by competitors, the ability of competitors to capitalize on their economies of scale and the ability of competitors to produce or otherwise procure animal health products at lower costs than us. To the extent that any of our competitors are more successful with respect to any key competitive factor, or we are forced to reduce, or are unable to raise, the price of any of our products in order to remain competitive, our business, financial condition and results of operations could be materially adversely affected.
Failure to comply with requirements to design, implement and maintain effective internal controls could have a material adverse effect on our business and stock price.
As a public company, we have significant requirements for enhanced financial reporting and internal controls. The process of designing and implementing effective internal controls is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company. If we are unable to establish or maintain appropriate internal financial reporting controls and procedures, it could cause us to fail to meet our reporting obligations on a timely basis, result in material misstatements in our consolidated financial statements and harm our operating results. In addition, we are required, pursuant to Section 404, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. This assessment includes disclosure of any material weaknesses identified by our management in our internal control over financial reporting and a statement that our auditors have issued an attestation report on the effectiveness of our internal controls. Testing and maintaining internal controls may divert our management’s attention from other matters that are important to our business. We may not be able to conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with Section 404 or our independent registered public accounting firm may not issue an unqualified opinion. We cannot provide any assurance we will not identify material weaknesses in the future. If we suffer deficiencies or material weaknesses in our internal controls, we may be unable to report financial information in a timely and accurate manner and it could result in a material misstatement of our annual or interim financial statements that would not be prevented or detected on a timely basis, which could cause investors to lose confidence in our financial reporting, negatively affect the trading price of our common stock and could cause a default under the agreements governing our indebtedness. If either we are unable to conclude that we have effective internal control over financial reporting or our independent registered public accounting firm is unable to provide us with an unqualified opinion, investors could lose confidence in our reported financial information, which could have a material adverse effect on the trading price of our stock.
As a public company, we are subject to financial and other reporting and corporate governance requirements that may be difficult for us to satisfy and may divert management’s attention from our business.
As a public company, we are required to file annual and quarterly reports and other information pursuant to the Exchange Act with the SEC. We are required to ensure that we have the ability to prepare consolidated financial statements that comply with SEC reporting requirements on a timely basis. We are also subject to other reporting and corporate governance requirements, including the applicable stock exchange listing standards and certain provisions of the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act and the regulations promulgated thereunder, which impose significant compliance obligations upon us.
51
As a public company, we are required to commit significant resources and management time and attention to these requirements, which cause us to incur significant costs and which may place a strain on our systems and resources. As a result, our management’s attention might be diverted from other business concerns. Compliance with these requirements place significant demands on our legal, accounting and finance staff and on our accounting, financial and information systems and increase our legal and accounting compliance costs as well as our compensation expense as we have been or may be required to hire additional accounting, tax, finance and legal staff with the requisite technical knowledge.
We may not be able to expand through acquisitions or successfully integrate the products, services and personnel of acquired businesses.
From time to time, we may make selective acquisitions to expand our range of products and services and to expand the geographic scope of our business, such as the Proposed Acquisition. However, we may be unable to identify suitable targets in the future, and competition for acquisitions may make it difficult for us to consummate acquisitions on acceptable terms or at all. We may not be able to locate any complementary products that meet our requirements or that are available to us on acceptable terms or we may not have sufficient capital resources to consummate a proposed acquisition. In addition, assuming we identify suitable products or partners, the process of effectively entering into these arrangements involves risks that our management’s attention may be diverted from other business concerns. Further, if we succeed in identifying and consummating appropriate acquisitions on acceptable terms, we may not be able to successfully integrate the products, services and personnel of any acquired businesses, including the Proposed Acquisition, on a basis consistent with our current business practice. In particular, we may face greater than expected costs, time and effort involved in completing and integrating such acquisitions and potential disruption of our ongoing business. Furthermore, we may realize fewer, if any, synergies than envisaged. Our ability to manage acquired businesses may also be limited if we enter into joint ventures or do not acquire full ownership or a controlling stake in the acquired business. In addition, continued growth through acquisitions may significantly strain our existing management and operational resources. As a result, we may need to recruit additional personnel, particularly at the level below senior management, and we may not be able to recruit qualified management and other key personnel to manage our growth. Moreover, certain transactions could adversely impact earnings as we incur development and other expenses related to the transactions and we could incur debt to complete these transactions. Debt instruments could contain contractual commitments and covenants that could adversely affect our cash flow and our ability to operate our business, financial condition and results of operations. See also “— Risk Factors Relating to Our Business — We may fail to consummate the proposed acquisition of certain Zoetis products and assets, may not consummate the proposed acquisition on expected terms, or may not achieve the anticipated benefits.”
We may not successfully implement our business strategies or achieve expected gross margin improvements.
We are pursuing and may continue to pursue strategic initiatives that management considers critical to our long-term success, including, but not limited to, increasing sales in emerging markets, base revenue growth through new product development and value-added product lifecycle development; improving operational efficiency through manufacturing efficiency improvement and other programs; and expanding our complementary products and services. There are significant risks involved with the execution of these types of initiatives, including significant business, economic and competitive uncertainties, many of which are outside of our control. Accordingly, we cannot predict whether we will succeed in implementing these strategic initiatives. It could take several years to realize the anticipated benefits from these initiatives, if any benefits are achieved at all. We may be unable to achieve expected gross margin improvements on our products or technologies. Additionally, our business strategy may change from time to time, which could delay our ability to implement initiatives that we believe are important to our business.
Item 1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
Risk Management and Strategy
As a leading global diversified animal health and mineral nutrition company, we are increasingly reliant on information technology systems and infrastructure to conduct critical operations and generally operate our business, which includes using information technology systems to process, transmit and store electronic information, including customer, employee and company data.
52
The use of information technology systems makes us vulnerable to breaches of data security and cybersecurity attacks. For information on the potential risks related to cybersecurity, see “Item 1A. Risk Factors — Risk Factors Relating to Our Business — We may be subject to information technology system failures, network disruptions and breaches in data security.”
Although the aggregate impact on our operations and financial condition has not been material to date, the Company has been the target of cybersecurity attacks and expects them to continue as such attacks are becoming more sophisticated and frequent, and the techniques used in such attacks change rapidly. Any such future incidents could have a material impact on our business.
Our information security team, headed by our Director of IT Cyber Security and Compliance, who reports to our Chief Information Officer (“CIO”), monitors our technology systems to prevent, detect, mitigate and remediate any cybersecurity incidents. Our enterprise-wide cybersecurity program is aligned with the U.S. National Institute of Standards Technology Cybersecurity Framework and the Israel National Cyber Directorate, and we are members of the New Jersey Cybersecurity and Communications Integration Cell. This collaboration enables us to increase our cybersecurity knowledge, threat awareness, and awareness of cyber incidents within the industry. It also helps us take proactive measures to prevent incidents by defining attack group identifiers and implementing learning and improvement processes.
We use tools and techniques to continually assess, monitor, manage, and mitigate security risks to our technology systems. Our processes extend to third-party service providers, who have access to data on our systems. We engage a third party to perform an independent assessment of our cybersecurity penetration test and risk assessment program at least once every two years.
Our preventive and protected stratagem include the following:
| ● | Security Operations Center (SOC) and Managed Detection and Response (MDR) services to ensure continuous monitoring and response to potential threats. |
| ● | A cyber training and awareness process based on an annual work plan, which includes monthly training, bi-weekly simulations, an interactive magazine sent once a month to all users and a cyber incident simulation for the infrastructure team. The contents are based on relevant market trends. |
In cybersecurity or privacy incidents, the probable frequency and magnitude of loss are evaluated by the triage team. The incidents are categorized as either high- or low-level severity and communicated to the CIO and Chief Executive Officer and the Senior Vice President, General Counsel and Corporate Secretary (“Legal Counsel”), at which time a determination is made on any additional communication requirements. Our Board of Directors is notified of high-level severity incidents. Our approach to incidents generally includes a process of investigation, learning, and subsequent improvement. In addition, we believe cyber insurance is a key mechanism for supporting and managing critical cyber incidents. Aligning the policy involves a thorough review of our protection layers and processes.
Governance
Our information technology systems are managed by our CIO. He has over 45 years of experience in digital systems and technology in the animal health, bio-tech pharmaceutical, pharmaceutical and oil and gas industries. He has held multiple leadership roles driving business value for investments in digital solutions. He also spent six years as a leader within internal auditing, providing experience and wisdom in business-driven risk management.
The CIO provides periodic reports to the Board of Directors and the executive management team, including our Legal Counsel. These reports include updates on our cybersecurity risks and threats, assessments of our information security program, and any changes in the threat landscape. Our information technology systems are regularly evaluated by internal and external consultants with the results of the review reported to the executive management team and the Board of Directors.
53
Item 2. Properties
The following table lists our material properties:
|
|
|
|
Owned/ |
|
Approx. sq. |
|
|
Business Segment(s) |
|
Location |
|
Leased |
|
Footage |
|
Purpose(s) |
Animal Health |
|
Buenos Aires, Argentina |
|
Owned |
|
43,000 |
|
Manufacturing and Administrative |
Animal Health |
|
Braganca Paulista, Brazil |
|
Owned |
|
50,000 |
|
Manufacturing and Administrative |
Animal Health |
|
Guarulhos, Brazil |
|
Owned |
|
1,294,000 |
|
Manufacturing, Sales, Premixing, |
Animal Health |
|
Heliópolis, Brazil |
|
Owned |
|
15,000 |
|
Manufacturing and Administrative |
Animal Health |
|
Sligo, Ireland |
|
Owned |
|
45,000 |
|
Manufacturing |
Animal Health |
|
Beit Shemesh, Israel |
|
Owned/ land lease |
|
79,000 |
|
Manufacturing and Research |
Animal Health |
|
Neot Hovav, Israel |
|
Owned/land lease |
|
140,000 |
|
Manufacturing and Research |
Animal Health |
|
Petach Tikva, Israel |
|
Owned |
|
60,000 |
|
Manufacturing |
Animal Health |
|
Sarasota, Florida |
|
Leased |
|
93,000 |
|
Manufacturing, Sales, Research and Administrative |
Animal Health |
|
Chillicothe, Illinois |
|
Owned |
|
19,000 |
|
Manufacturing |
Animal Health |
|
Mendon, Illinois |
|
Owned |
|
64,000 |
|
Research |
Animal Health |
|
St. Paul, Minnesota |
|
Leased |
|
5,000 |
|
Research |
Animal Health |
|
Omaha, Nebraska |
|
Owned |
|
59,000 |
|
Manufacturing, Sales and Research |
Animal Health |
|
State College, Pennsylvania |
|
Owned |
|
13,000 |
|
Research |
Animal Health and Mineral Nutrition |
|
Quincy, Illinois |
|
Owned |
|
306,000 |
|
Manufacturing, Sales, Research and Administrative |
Mineral Nutrition |
|
Omaha, Nebraska |
|
Owned |
|
84,000 |
|
Manufacturing |
Performance Products |
|
Santa Fe Springs, California |
|
Owned |
|
108,000 |
|
Manufacturing |
Corporate |
|
Teaneck, New Jersey |
|
Leased |
|
50,000 |
|
Corporate and Administrative |
Item 3. Legal Proceedings
We are from time to time subject to claims and litigation arising in the ordinary course of business. These claims and litigation may include, among other things, allegations of violation of United States and foreign competition law, labor laws, consumer protection laws, data protection laws and Environmental Laws and regulations, as well as claims or litigation relating to product liability, intellectual property, securities, breach of contract and tort. We operate in multiple jurisdictions and, as a result, a claim in one jurisdiction may lead to claims or regulatory penalties in other jurisdictions.
We do not believe that the ultimate resolution of existing claims and litigation will have a material adverse effect on our financial position, results of operations, liquidity or capital resources. However, one or more unfavorable outcomes in any claim or litigation against us could have a material adverse effect for the period in which they are resolved. In addition, regardless of their merits or their ultimate outcomes, such matters are costly, divert management’s attention and may materially adversely affect our reputation, even if resolved in our favor.
See “Notes to Consolidated Financial Statements — Commitments and Contingencies” in Part II. Item 8 on this Annual Report on Form 10-K, which is incorporated herein by reference, for further information on our legal proceedings. For an additional discussion of certain risks associated with legal proceedings see “Risk Factors” above.
Item 4. Mine Safety Disclosures
Not applicable.
54
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information for Common Stock
Our Class A common stock is traded on Nasdaq under the trading symbol “PAHC.” Our Class B common stock is not listed or traded on any stock exchange. At June 30, 2024, there were 20,337,574 shares of Class A common stock outstanding.
During the fiscal year ended June 30, 2024, we did not sell any unregistered securities nor did we purchase any of our equity securities.
Holders of Record
As of August 23, 2024, there were 20,337,574 shares of our Class A common stock outstanding, which were held by one stockholder of record, not including beneficial owners of shares registered in nominee or street name. As of August 23, 2024, there were 20,166,034 shares of our Class B common stock outstanding, which were held by one stockholder of record. Each share of Class B common stock is convertible at any time at the option of the holder into one share of Class A common stock. Information about 5% beneficial owners of our common stock is incorporated by reference from the discussion in our 2024 Proxy Statement under the heading Security Ownership of Certain Beneficial Owners and Management.
Dividend Policy
We intend to pay regular quarterly dividends to holders of our Class A and Class B common stock out of assets legally available for this purpose. Any future determination to pay dividends is subject to review and approval by our Board of Directors and will depend upon our results of operations, financial condition, capital requirements, our ability to obtain funds from our subsidiaries and other factors that our Board of Directors deem relevant. Additionally, the terms of our current and any future agreements governing our indebtedness could limit our ability to pay dividends or make other distributions.
Stock Performance Graph
This performance graph is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference in any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act.
The following graph shows a comparison from June 30, 2019 through June 30, 2024 of the cumulative stockholder return of our Class A common stock, the S&P 500 Index, the Nasdaq Composite Index, the Russell 2000 Index and the S&P Pharmaceuticals Index. The graph assumes that $100 was invested in our Class A common stock and each of the aforementioned indexes at the market close on June 30, 2019, and assumes dividends, if any, are reinvested. The stock price performance shown on the graph is not necessarily indicative of future stock price performance, and we do not make any projections of future stockholder returns.
55
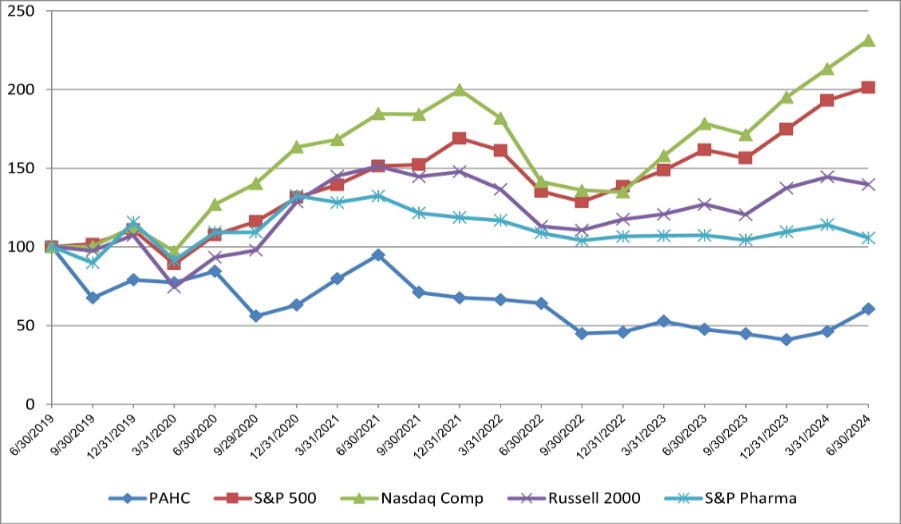
Item 6. (Reserved)
Not applicable
Item 7.Management’s Discussion and Analysis of Financial Condition and Results of Operations
Introduction
Our management’s discussion and analysis of financial condition and results of operations (“MD&A”) is provided to assist readers in understanding our performance, as reflected in the results of our operations, our financial condition and our cash flows. The following discussion summarizes the significant factors affecting our consolidated operating results, financial condition, liquidity and cash flows as of and for the periods presented. This MD&A should be read in conjunction with our consolidated financial statements and related notes thereto included under the section entitled “Financial Statements and Supplementary Data.” Our future results could differ materially from our historical performance as a result of various factors such as those discussed in “Risk Factors” and “Forward-Looking Statements and Risk Factors Summary.”
Overview of our business
Phibro Animal Health Corporation is a leading global diversified animal health and mineral nutrition company. We develop, manufacture and market a broad range of products for food and companion animals including poultry, swine, beef and dairy cattle, aquaculture and dogs. Our products help prevent, control and treat diseases, and support nutrition to help improve animal health and well-being. In addition to animal health and mineral nutrition products, we manufacture and market specific ingredients for use in the personal care, industrial chemical and chemical catalyst industries. We market approximately 750 product lines in over 80 countries to approximately 4,200 customers.
56
Proposed Acquisition
In April 2024, we entered into the Purchase Agreement with Zoetis to acquire Zoetis’s medicated feed additive product portfolio, certain water-soluble products and related assets. The purchase price is $350 million, subject to certain adjustments set forth in the Purchase Agreement, payable in cash at closing. The product portfolio to be acquired, which generated approximately $400 million in revenue in 2023, is comprised of more than 37 product lines that are sold in approximately 80 countries. Also included in the Proposed Acquisition are six manufacturing sites, comprised of four in the U.S., one in Italy and one in China. We anticipate that the Proposed Acquisition will be completed between October and December 2024. Completion of the Proposed Acquisition is subject to satisfaction of customary closing conditions, including clearances by applicable regulatory authorities.
2024 Credit Agreement
In July 2024, we entered into a Credit Agreement (the “2024 Credit Agreement”) with a group of lenders. Initial borrowings were used to refinance all our outstanding debt, to pay fees and expenses of the transaction, and for ongoing working capital requirements and general corporate purposes. Borrowings under the Delayed Draw Term A-1 and A-2 Loans will be used to finance the purchase price of the Proposed Acquisition upon completion of the acquisition. See “Notes to Consolidated Financial Statements — Subsequent Event — 2024 Credit Agreement” for additional information.
Factors affecting our performance
Armed conflicts
Israel and Hamas
On October 7, 2023, Hamas militants crossed into Israel from Gaza in a large-scale, surprise terrorist attack. Hamas terrorists invaded Israel, first firing rockets into the country and then carrying out attacks inflicting mass casualties with hundreds more taken hostage. In order to provide immediate assistance to the victims of the attacks and their families, we and our employees provided monetary donations that were distributed to charities that offered relief services, welfare, equipment, food and other necessities.
We have three manufacturing sites in Israel. A manufacturing plant in Neot Hovav that produces active pharmaceutical ingredients for certain of our anticoccidial and antimicrobial products, a facility in Beit Shemesh that produces vaccines and a plant in Petah Tikvah that manufactures premix products and nutritional products. In addition, we have an office location near Tel Aviv in Airport City. As of June 30, 2024, we had approximately 500 employees located in Israel. While we initially had some disruption to our operations at the onset of the Israel-Hamas conflict, at the current time, we have confidence in our ability to meet our supply commitment to customers and maintain sufficient inventory to continue regional support. A significant escalation of the tensions in Israel occurred on April 13, 2024, when Iran launched more than 300 drones and missiles against Israel, but we had no material disruption to our business. While the situation surrounding the ongoing conflict remains fluid, our operations in Israel have navigated numerous challenging situations over the years.
The prolonged continuation or escalation of this conflict may trigger bans, economic and other sanctions, as well as broader military conflict, which could include neighboring nations. The potential impact of the current conflict, or escalation thereof, on our business is unclear but may include, without limitation, the possible disruption of our operations, particularly at our facilities in Israel, supply chain and logistics disruptions, personnel and raw material shortages, and other consequences, including as a result of the actions of, or disruption of the operations of, certain regulatory and governmental authorities and of certain of our suppliers, collaborative partners, licensees, manufacturing sites, distributors and customers. Our Israeli manufacturing facilities and local operations account for 28% of our consolidated assets as of June 30, 2024, and 21% of our consolidated net sales, for the year ended June 30, 2024.
Russia and Ukraine
In response to the armed conflict between Russia and Ukraine that began in February 2022, we and our employees have provided support to Ukraine in the form of monetary donations, free products and humanitarian services. Our limited intent for the Russian market is to continue to provide medicines and vaccines, and related regulatory and technical support, to help existing customers combat disease challenges in the production of food animals on their farms.
57
We have no production or direct distribution operations and no planned investments in Russia.
Since the conflict began, the United States and other North Atlantic Treaty Organization (“NATO”) member states, as well as non-member states, announced targeted economic sanctions on Russia, including certain Russian citizens and enterprises. The continuation or escalation of the conflict may trigger additional economic and other sanctions, as well as broader military conflict. The potential impacts of any resulting bans, sanctions, boycotts or broader military conflicts on our business are uncertain. The potential impacts could include supply chain and logistics disruptions, macroeconomic impacts resulting from the exclusion of Russian financial institutions from the global banking system, volatility in foreign exchange rates and interest rates, inflationary pressures on raw materials and energy as well as heightened cybersecurity threats. Our sales to Russia and Ukraine for the 12 months ended June 30, 2024 represented approximately 1% of consolidated net sales.
We cannot know if the conflict could escalate and result in broader economic and security concerns that could adversely affect our business, financial condition, or results of operations.
Industry growth
We believe global population growth, the growth of the global middle class and the productivity improvements needed due to limitations of arable land and water supplies have supported and will continue to support growth of the animal health industry.
Regulatory developments
Our business depends heavily on a healthy and growing livestock industry. Some in the public perceive risks to human health related to the consumption of food derived from animals that utilize certain of our products, including certain of our MFA products. In particular, there is increased focus, in the United States and other countries, on the use of medically important antimicrobials. As defined by the FDA, medically important antimicrobials (“MIAs”) include classes that are prescribed in animal and human health and are listed in the Appendix of the FDA-CVM Guidance for Industry (GFI) 152. Our products that contain virginiamycin, oxytetracycline or neomycin are classified by the FDA as medically important antimicrobials. In addition to the United States, the World Health Organization (WHO), the E.U., Australia and Canada have promulgated rating lists for antimicrobials that are used in veterinary medicine and that include certain of our products.
The classification of our products as MIAs or similar listings may lead to a decline in the demand for and production of food products derived from animals that utilize our products and, in turn, demand for our products. Livestock producers may experience decreased demand for their products or reputational harm as a result of evolving consumer views of nutrition and health-related concerns, animal rights and other concerns. Any reputational harm to the livestock industry may also extend to companies in related industries, including us. In addition, campaigns by interest groups, activists and others with respect to perceived risks associated with the use of our products in animals, including position statements by livestock producers and their customers based on non-use of certain medicated products in livestock production, whether or not scientifically-supported, could affect public perceptions and reduce the use of our products. Those adverse consumer views related to the use of one or more of our products in animals could have a material adverse effect on our financial condition and results of operations.
In April 2016, the FDA began initial steps to withdraw approval of carbadox (the active ingredient in our Mecadox product) via a regulatory process known as a NOOH, due to concerns that certain residues from the product may persist in animal tissues for longer than previously determined. In the years following, Phibro has continued an ongoing process of responding collaboratively and transparently to the CVM inquiries and has provided extensive and meticulous research and data that confirmed the safety of carbadox. In July 2020, the FDA announced it would not proceed to a hearing on the scientific concerns raised in the 2016 NOOH, consistent with the normal regulatory procedure, but instead announced that it was withdrawing the 2016 NOOH and issuing a proposed order to review the regulatory method for carbadox. Phibro reiterated the safety of carbadox and the appropriateness of the regulatory method and offered to work with the CVM to generate additional data to support the existing regulatory method or select a suitable alternative regulatory method.
In March 2022, the FDA held a Part 15 virtual public hearing seeking data and information related to the safety of carbadox in which Phibro participated and again detailed the research and data that confirm the safety of carbadox. In November 2023, the FDA issued a final order to revoke the approved method for detecting carbadox residues.
58
The FDA also provided notice in the Federal Register proposing to withdraw approval of all NADAs providing for use of carbadox in medicated swine feed and announcing an opportunity for Phibro to request a hearing on this proposal. This second action is based on CVM’s determination that there is no approved regulatory method to detect carbadox residues in the edible tissues of the treated swine. Phibro is continuing to defend swine producers’ ability to use Mecadox. We have requested a full evidentiary hearing on the merits before an administrative law judge. In January 2024, Phibro filed a lawsuit in the D.C. Federal District Court asking the court to invalidate the order which revoked the regulatory method for carbadox. Should we be unable to successfully defend the safety of the product, the loss of carbadox sales will have an adverse effect on our financial condition and results of operations. Sales of Mecadox (carbadox) for the year ended June 30, 2024 were approximately $22 million. As of the date of this Annual Report on Form 10-K, Mecadox continues to be available for use by swine producers.
See also “Business — Compliance with Government Regulation — United States — Carbadox”; and “Business — Compliance with Government Regulation — Global Policy and Guidance.”
Our global sales of antibacterials, anticoccidials and other products were $421 million, $387 million and $362 million for the years ended June 30, 2024, 2023 and 2022, respectively.
Competition
The animal health industry is highly competitive. We believe many of our competitors are conducting R&D activities in areas served by our products and in areas in which we are developing products. Our competitors include stand-alone animal health businesses and the animal health businesses of large pharmaceutical companies. In addition to competition from established participants, there could be new entrants to the animal health medicines and vaccines industry in the future. Principal methods of competition vary depending on the region, species, product category or individual products, including reliability, reputation, quality, price, service and promotion to veterinary professionals and livestock producers.
Foreign exchange
We conduct operations in many areas of the world, involving transactions denominated in a variety of currencies. For the year ended June 30, 2024, we generated approximately 40% of our revenues from operations outside the United States. Although a portion of our revenues are denominated in various currencies, the selling prices of the majority of our sales outside the United States are referenced in U.S. dollars, and as a result, our revenues have not been significantly affected by currency movements. We are subject to currency risk to the extent that our costs are denominated in currencies other than those in which we earn revenues. We manufacture some of our major products in Brazil and Israel and production costs are largely denominated in local currencies, while the selling prices of the products are largely set in U.S. dollars. As such, we are exposed to changes in cost of goods sold resulting from currency movements and may not be able to adjust our selling prices to offset such movements. In addition, we incur selling and administrative expenses in various currencies and are exposed to changes in such expenses resulting from currency movements. For the year ended June 30, 2024, our expenses were not significantly affected by currency movements. Because we have transactions denominated in various currencies, changes in currency exchange rates have had, and will continue to have, an impact on our results of operations.
Climate
Adverse weather events and natural disasters may interfere with and negatively impact operations at our manufacturing sites, research and development facilities and offices, which could have a material adverse effect on our financial condition and results of operations, especially if the impact of an event or disaster is frequent or prolonged.
Our operations, and the activities of our customers, could be disrupted by climate change. The physical changes caused by climate change may prompt changes in regulations or consumer preferences which in turn could have negative consequences for our and our customers’ businesses. Climate change may negatively impact our customers’ operations, particularly those in the livestock industry, through climate-related impacts such as increased air and water temperatures, rising water levels and increased incidence of disease in livestock. Potential physical risks from climate change may include altered distribution and intensity of rainfall, prolonged droughts or flooding, increased frequency of wildfires and other natural disasters, rising sea levels and a rising heat index, any of which could cause negative impacts to our and our customers’ businesses.
59
If such events affect our customers’ businesses, they may purchase fewer of our products, and our revenues may be negatively impacted. Climate driven changes could have a material adverse effect on our financial condition and results of operations.
There has been a broad range of proposed and promulgated state, national and international regulations aimed at reducing the effects of climate change. Such regulations could result in additional costs to maintain compliance and additional income or other taxes. Climate change regulations continue to evolve, and it is not possible to accurately estimate potential future compliance costs.
Product development initiatives
Our future success depends on our existing product portfolio, including additional approvals for new claims for our products, for use of our products in new markets, for use of our products with new species and for cross-clearances enabling the use of our medicated products in conjunction with other products. Our future success also depends on our pipeline of new products, including new products that we may develop through joint ventures and products that we are able to obtain through license or acquisition. The majority of our R&D programs focus on product lifecycle development, which is defined as R&D programs that leverage existing animal health products by adding new species or claims, achieving approvals in new markets or creating new combinations and reformulations. We commit substantial effort, funds and other resources to expanding our product approvals and R&D, both through our own dedicated resources and through collaborations with third parties. We also commit significant resources to development of new vaccine technologies.
Our current strategic initiatives include several projects. We continue to invest in a vaccine production facility in Sligo, Ireland to manufacture poultry vaccines, with our first commercial sale of product realized in 2022, and longer-term expectations to add swine and cattle vaccines. We are developing microbial products and bioproducts for a variety of applications serving animal health and nutrition, environmental, industrial and agricultural customers. We began operations at a new vaccine production facility in Guarulhos, Brazil in fiscal year 2023 that manufactures and markets autogenous vaccines against animal diseases for swine, poultry and aquaculture. We continue to build our companion animal business and pipeline. Our Rejensa joint supplement for dogs continues to gain customer acceptance. Our companion animal development pipeline includes an early-stage atopic dermatitis compound, a potential treatment for mitral heart valve disease in dogs, a pain product and two oral care products.
60
Analysis of the consolidated statements of operations
Summary Results of Operations
|
|
|
|
|
|
|
|
|
|
|
Change |
|
|||||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023/ 2022 |
|
||||||||||
|
|
(in thousands, except per share amounts and percentages) |
|||||||||||||||||||
Net sales |
|
$ |
1,017,679 |
|
$ |
977,889 |
|
$ |
942,261 |
|
$ |
39,790 |
|
|
4 |
% |
$ |
35,628 |
|
4 |
% |
Gross profit |
|
|
313,092 |
|
|
298,237 |
|
|
285,400 |
|
|
14,855 |
|
|
5 |
% |
|
12,837 |
|
4 |
% |
Selling, general and administrative expenses |
|
|
259,777 |
|
|
226,390 |
|
|
206,414 |
|
|
33,387 |
|
|
15 |
% |
|
19,976 |
|
10 |
% |
Operating income |
|
|
53,315 |
|
|
71,847 |
|
|
78,986 |
|
|
(18,532) |
|
|
(26) |
% |
|
(7,139) |
|
(9) |
% |
Interest expense, net |
|
|
18,536 |
|
|
15,321 |
|
|
11,875 |
|
|
3,215 |
|
|
21 |
% |
|
3,446 |
|
29 |
% |
Foreign currency (gains) losses, net |
|
|
23,863 |
|
|
2,455 |
|
|
(5,216) |
|
|
21,408 |
|
|
|
* |
|
7,671 |
|
|
* |
Income before income taxes |
|
|
10,916 |
|
|
54,071 |
|
|
72,327 |
|
|
(43,155) |
|
|
(80) |
% |
|
(18,256) |
|
(25) |
% |
Provision for income taxes |
|
|
8,500 |
|
|
21,465 |
|
|
23,152 |
|
|
(12,965) |
|
|
(60) |
% |
|
(1,687) |
|
(7) |
% |
Net income |
|
$ |
2,416 |
|
$ |
32,606 |
|
$ |
49,175 |
|
$ |
(30,190) |
|
|
(93) |
% |
$ |
(16,569) |
|
(34) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
0.06 |
|
$ |
0.81 |
|
$ |
1.21 |
|
$ |
(0.75) |
|
|
|
|
$ |
(0.40) |
|
|
|
Diluted |
|
$ |
0.06 |
|
$ |
0.81 |
|
$ |
1.21 |
|
$ |
(0.75) |
|
|
|
|
$ |
(0.41) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares outstanding |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
40,504 |
|
|
40,504 |
|
|
40,504 |
|
|
|
|
|
|
|
|
|
|
|
|
Diluted |
|
|
40,523 |
|
|
40,504 |
|
|
40,504 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ratio to net sales |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
30.8 |
% |
|
30.5 |
% |
|
30.3 |
% |
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative expenses |
|
|
25.5 |
% |
|
23.2 |
% |
|
21.9 |
% |
|
|
|
|
|
|
|
|
|
|
|
Operating income |
|
|
5.2 |
% |
|
7.3 |
% |
|
8.4 |
% |
|
|
|
|
|
|
|
|
|
|
|
Income before income taxes |
|
|
1.1 |
% |
|
5.5 |
% |
|
7.7 |
% |
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
|
0.2 |
% |
|
3.3 |
% |
|
5.2 |
% |
|
|
|
|
|
|
|
|
|
|
|
Effective tax rate |
|
|
77.9 |
% |
|
39.7 |
% |
|
32.0 |
% |
|
|
|
|
|
|
|
|
|
|
|
Certain amounts and percentages may reflect rounding adjustments.
* |
Calculation not meaningful |
Changes in net sales from period to period primarily result from changes in volumes and average selling prices. Although a portion of our net sales is denominated in various currencies, the selling prices of the majority of our sales outside the United States are referenced in U.S. dollars, and as a result, currency movements have not significantly affected our revenues.
Our effective income tax rate has varied from period to period and from the federal statutory rate, due to the mix of taxable profits in various jurisdictions; changes in tax rates from period to period, including changes in income tax legislation in the United States and various international jurisdictions; and the effects of changes in uncertain tax positions and valuation allowances. Our future effective income tax rate will vary due to the relative amounts of taxable income in various jurisdictions, future changes in tax rates and legislation and other factors. We expect to repatriate approximately $80.0 million of international earnings, which will be subject to applicable non-U.S. withholding and related taxes, net of reductions in U.S. income taxes. We intend to continue to reinvest indefinitely all other undistributed earnings of our foreign subsidiaries where we could be subject to applicable non-U.S. withholding and related taxes if amounts are repatriated to the U.S. See “Notes to Consolidated Financial Statements — Income Taxes” for additional information.
61
Net sales, Adjusted EBITDA and reconciliation of GAAP net income to Adjusted EBITDA
We report Net sales and Adjusted EBITDA by segment to understand the operating performance of each segment. This enables us to monitor changes in net sales, costs and other actionable operating metrics at the segment level. See “— General description of non-GAAP financial measures” for descriptions of EBITDA and Adjusted EBITDA.
Segment net sales and Adjusted EBITDA:
|
|
|
|
|
|
|
|
|
|
|
Change |
|
||||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023 / 2022 |
|
|||||||||
Net sales |
|
(in thousands, except percentages) |
|
|||||||||||||||||
MFAs and other |
|
$ |
420,959 |
|
$ |
387,349 |
|
$ |
361,538 |
|
$ |
33,610 |
|
9 |
% |
$ |
25,811 |
|
7 |
% |
Nutritional specialties |
|
|
164,671 |
|
|
172,504 |
|
|
157,196 |
|
|
(7,833) |
|
(5) |
% |
|
15,308 |
|
10 |
% |
Vaccines |
|
|
120,852 |
|
|
99,998 |
|
|
88,321 |
|
|
20,854 |
|
21 |
% |
|
11,677 |
|
13 |
% |
Animal Health |
|
|
706,482 |
|
|
659,851 |
|
|
607,055 |
|
|
46,631 |
|
7 |
% |
|
52,796 |
|
9 |
% |
Mineral Nutrition |
|
|
243,663 |
|
|
242,656 |
|
|
259,512 |
|
|
1,007 |
|
0 |
% |
|
(16,856) |
|
(6) |
% |
Performance Products |
|
|
67,534 |
|
|
75,382 |
|
|
75,694 |
|
|
(7,848) |
|
(10) |
% |
|
(312) |
|
(0) |
% |
Total |
|
$ |
1,017,679 |
|
$ |
977,889 |
|
$ |
942,261 |
|
$ |
39,790 |
|
4 |
% |
$ |
35,628 |
|
4 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjusted EBITDA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Animal Health |
|
$ |
145,606 |
|
$ |
136,139 |
|
$ |
124,106 |
|
$ |
9,467 |
|
7 |
% |
$ |
12,033 |
|
10 |
% |
Mineral Nutrition |
|
|
16,449 |
|
|
17,417 |
|
|
24,038 |
|
|
(968) |
|
(6) |
% |
|
(6,621) |
|
(28) |
% |
Performance Products |
|
|
7,662 |
|
|
9,346 |
|
|
8,706 |
|
|
(1,684) |
|
(18) |
% |
|
640 |
|
7 |
% |
Corporate |
|
|
(58,480) |
|
|
(50,149) |
|
|
(45,767) |
|
|
(8,331) |
|
17 |
% |
|
(4,382) |
|
10 |
% |
Total |
|
$ |
111,237 |
|
$ |
112,753 |
|
$ |
111,083 |
|
$ |
(1,516) |
|
(1) |
% |
$ |
1,670 |
|
2 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjusted EBITDA as a percentage of segment net sales |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Animal Health |
|
|
20.6 |
% |
|
20.6 |
% |
|
20.4 |
% |
|
|
|
|
|
|
|
|
|
|
Mineral Nutrition |
|
|
6.8 |
% |
|
7.2 |
% |
|
9.3 |
% |
|
|
|
|
|
|
|
|
|
|
Performance Products |
|
|
11.3 |
% |
|
12.4 |
% |
|
11.5 |
% |
|
|
|
|
|
|
|
|
|
|
Corporate(1) |
|
|
(5.7) |
% |
|
(5.1) |
% |
|
(4.9) |
% |
|
|
|
|
|
|
|
|
|
|
Total(1) |
|
|
10.9 |
% |
|
11.5 |
% |
|
11.8 |
% |
|
|
|
|
|
|
|
|
|
|
| (1) | Reflects ratio to total net sales. |
Certain amounts and percentages may reflect rounding adjustments.
* |
Calculation not meaningful |
62
A reconciliation of net income, as reported under GAAP, to Adjusted EBITDA:
|
|
|
|
|
|
|
|
|
|
|
Change |
|
||||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024/ 2023 |
|
2023/ 2022 |
|
|||||||||
|
|
(in thousands, except percentages) |
|
|||||||||||||||||
Net income |
|
$ |
2,416 |
|
$ |
32,606 |
|
$ |
49,175 |
|
$ |
(30,190) |
|
(93) |
% |
$ |
(16,569) |
|
(34) |
% |
Interest expense, net |
|
|
18,536 |
|
|
15,321 |
|
|
11,875 |
|
|
3,215 |
|
21 |
% |
|
3,446 |
|
29 |
% |
Provision for income taxes |
|
|
8,500 |
|
|
21,465 |
|
|
23,152 |
|
|
(12,965) |
|
(60) |
% |
|
(1,687) |
|
(7) |
% |
Depreciation and amortization |
|
|
36,178 |
|
|
34,012 |
|
|
32,705 |
|
|
2,166 |
|
6 |
% |
|
1,307 |
|
4 |
% |
EBITDA |
|
|
65,630 |
|
|
103,404 |
|
|
116,907 |
|
|
(37,774) |
|
(37) |
% |
|
(13,503) |
|
(12) |
% |
Acquisition-related cost of goods sold |
|
|
521 |
|
|
— |
|
|
316 |
|
|
521 |
|
* |
|
|
(316) |
|
* |
|
Acquisition-related transaction costs |
|
|
6,405 |
|
|
— |
|
|
279 |
|
|
6,405 |
|
* |
|
|
(279) |
|
* |
|
Pension settlement cost |
|
|
10,674 |
|
|
— |
|
|
— |
|
|
10,674 |
|
* |
|
|
— |
|
* |
|
Brazil employment taxes |
|
|
4,202 |
|
|
— |
|
|
— |
|
|
4,202 |
|
* |
|
|
— |
|
* |
|
Stock-based compensation |
|
|
475 |
|
|
— |
|
|
— |
|
|
475 |
|
* |
|
|
— |
|
* |
|
Phibro Forward income growth initiatives implementation costs (1) |
|
|
366 |
|
|
— |
|
|
— |
|
|
366 |
|
* |
|
|
— |
|
* |
|
Insurance proceeds |
|
|
(899) |
|
|
— |
|
|
— |
|
|
(899) |
|
* |
|
|
— |
|
* |
|
Environmental remediation costs |
|
|
— |
|
|
6,894 |
|
|
— |
|
|
(6,894) |
|
* |
|
|
6,894 |
|
* |
|
Gain on sale of investment |
|
|
— |
|
|
— |
|
|
(1,203) |
|
|
— |
|
* |
|
|
1,203 |
|
* |
|
Foreign currency (gains) losses, net |
|
|
23,863 |
|
|
2,455 |
|
|
(5,216) |
|
|
21,408 |
|
* |
|
|
7,671 |
|
* |
|
Adjusted EBITDA |
|
$ |
111,237 |
|
$ |
112,753 |
|
$ |
111,083 |
|
$ |
(1,516) |
|
(1) |
% |
$ |
1,670 |
|
2 |
% |
| (1) | Phibro Forward is a company-wide initiative focused on unlocking additional areas of revenue growth and cost savings. |
Certain amounts and percentages may reflect rounding adjustments.
* |
Calculation not meaningful |
Comparison of the years ended June 30, 2024 and 2023
Net sales
Net sales of $1,017.7 million for the year ended June 30, 2024 increased $39.8 million, or 4%, as compared to the year ended June 30, 2023. Animal Health and Mineral Nutrition sales increased $46.6 million and $1.0 million, respectively. Performance Products sales decreased $7.8 million.
Animal Health
Net sales of $706.5 million for the year ended June 30, 2024 increased $46.6 million, or 7%. Net sales of MFAs and other increased $33.6 million, or 9%, due to increased volumes in all regions and higher demand for processing aids used in the ethanol fermentation industry. Net sales of nutritional specialty products decreased $7.8 million, or 5%, due to decreased demand for dairy and microbial products, partially offset by increased sales of poultry products. Net sales of vaccines increased $20.9 million, or 21%, primarily due to poultry product introductions in Latin America, plus an increase in domestic demand.
Mineral Nutrition
Net sales of $243.7 million for the year ended June 30, 2024 increased $1.0 million, or less than 1%, primarily due to increase in demand for trace minerals.
Performance Products
Net sales of $67.5 million for the year ended June 30, 2024 decreased $7.8 million, or 10%, driven by decreased demand for personal care product ingredients and industrial chemicals.
63
Gross profit
Gross profit of $313.1 million for the year ended June 30, 2024 increased $14.9 million, or 5%, as compared to the year ended June 30, 2023. Gross margin increased 30 basis points to 30.8% of net sales for the year ended June 30, 2024 as compared to 30.5% for the year ended June 30, 2023.
Animal Health gross profit increased $17.5 million due to higher product demand. Mineral Nutrition gross profit decreased $0.3 million. Performance Products gross profit decreased $1.8 million, due to lower demand and unfavorable product mix. Acquisition-related cost of goods sold reduced gross profit by $0.5 million.
Selling, general and administrative expenses
SG&A expenses of $259.8 million for the year ended June 30, 2024 increased $33.4 million, or 15%, as compared to the year ended June 30, 2023. SG&A for the year ended June 30, 2024 included a $10.7 million pension settlement charge, $6.4 million for acquisition-related costs, $4.2 million cost related to Brazil employment taxes, $0.5 million in stock-based compensation and $0.4 million related to consultant fees associated with Phibro Forward income growth initiatives, slightly offset by a gain from insurance proceeds of $0.9 million. SG&A for the year ended June 30, 2023, included $6.9 million of environmental remediation costs mostly related to the definitive settlement agreement related to the Omega Chemical Site. Excluding these items, SG&A increased $19.1 million, or 9%.
Animal Health SG&A increased $10.5 million, primarily due to an increase in employee-related costs and new product launches in Brazil. Mineral Nutrition SG&A increased $0.4 million due to an increase in employee-related costs. Performance Products SG&A decreased $0.1 million. Corporate expenses increased $8.3 million due to higher incentive-related employee costs and strategic investments.
Interest expense, net
Interest expense, net of $18.5 million for the year ended June 30, 2024 increased by $3.2 million, or 21%, as compared to the year ended June 30, 2023, as a result of higher variable interest rates and increased debt levels, partially offset by higher returns on short-term investments.
Foreign currency (gains) losses, net
Foreign currency losses, net for the year ended June 30, 2024 were $23.9 million, as compared to net losses of $2.5 million for the year ended June 30, 2023. Current period losses were driven by fluctuations in certain currencies relative to the U.S. dollar, including a major devaluation in the Argentine peso and the weakening of the Brazilian real.
Provision for income taxes
The provision for income taxes was $8.5 million and $21.5 million for the years ended June 30, 2024 and 2023, respectively. The effective income tax rate was 77.9% and 39.7% for the years ended June 30, 2024 and 2023, respectively. The effective income tax rate for the year ended June 30, 2024 was unfavorably affected by the proportionally greater effect of certain items such as Global Intangible Low-Tax Income (“GILTI”) taxes when compared with reduced pre-tax income. The provision for income taxes for the year ended June 30, 2024 included (i) a $2.8 million expense for applicable non-U.S. withholding and related taxes, net of reductions in U.S. incomes taxes, related to an planned repatriation of approximately $80.0 million of international earnings, (ii) a $1.2 million benefit related to the determination of whether a foreign tax is eligible for a U.S. foreign tax credit related to our fiscal year 2023, based on Internal Revenue Service (“IRS”) guidance provided subsequent to June 30, 2023, (iii) a $1.2 million benefit related to the release of certain valuation allowances on non-U.S. companies and (iv) a $1.6 million expense from changes in uncertain tax positions related to prior years and certain other items. The provision for income taxes for the year ended June 30, 2023, included a net cost of $1.5 million for certain one-time items including (i) GILTI income taxes that were modified by IRS guidance issued subsequent to June 30, 2023, (ii) the net cost of withholding taxes related to dividends received from an international affiliate and (iii) the net benefit related to certain unrecognized tax benefits from adjustments to and the lapse of statute of limitations of prior years. The effective income tax rate without these items would have been 59.2% and 36.9% for the years ended June 30, 2024 and 2023, respectively.
We record the GILTI-related aspects of comprehensive U.S. income tax legislation as a period expense. The provision for income taxes for the years ended June 30, 2024 and 2023, included $2.0 million and $1.8 million, respectively, of federal tax expense from the effects of GILTI.
64
Our effective income tax rate included 18.3% and 3.3% related to GILTI income tax expense for the years ended June 30, 2024 and 2023, respectively.
Net income
Net income of $2.4 million for the year ended June 30, 2024 decreased $30.2 million, as compared to net income of $32.6 million for the year ended June 30, 2023. Operating income decreased $18.5 million driven by higher SG&A, partially offset by higher gross profit. SG&A expenses increased by $33.4 million, which included pension settlement costs of $10.7 million, acquisition-related costs of $6.4 million, Brazil employment taxes of $4.2 million, stock-based compensation of $0.5 million and consultant fees related to Phibro Forward income growth initiatives of $0.4 million, slightly offset by a gain from insurance proceeds of $0.9 million. Changes in interest expense, net and foreign currency losses resulted in a $3.2 million and $21.4 million reduction in income before income taxes, respectively. This was partially offset by a $13.0 million decrease in income tax expense.
Adjusted EBITDA
Adjusted EBITDA of $111.2 million for the year ended June 30, 2024 decreased $1.5 million, or 1%, as compared to the year ended June 30, 2023. Animal Health Adjusted EBITDA increased $9.5 million, driven by higher sales and increased gross profit, partially offset by an increase in SG&A. Mineral Nutrition Adjusted EBITDA decreased $1.0 million, due to decreased gross profit and an increase in SGA. Performance Products Adjusted EBITDA decreased by $1.7 million due to lower sales and decreased gross profit. Corporate expenses increased $8.3 million due to higher incentive-related employee costs and strategic investments.
Comparison of the years ended June 30, 2023 and 2022
For a comparison of our results of operations for the years ended June 30, 2023 and 2022, and an analysis of our financial condition, liquidity and capital resources for the year ended June 30, 2023, see “Part II. Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” of our Annual Report on Form 10-K for the fiscal year ended June 30, 2023, filed with the SEC on August 30, 2023.
Adjusted net income and adjusted diluted earnings per share
We report adjusted net income to portray the results of our operations prior to considering certain income statement elements. See “—General description of non-GAAP financial measures” for more information.
A reconciliation of net income, as reported under GAAP, to adjusted net income is as follows:
|
|
|
|
|
|
|
|
|
|
|
Change |
|
||||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024/ 2023 |
|
2023/ 2022 |
|
|||||||||
|
|
|
(in thousands, except per share amounts and percentages) |
|
||||||||||||||||
Reconciliation of GAAP Net Income to Adjusted Net Income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
$ |
2,416 |
|
$ |
32,606 |
|
$ |
49,175 |
|
$ |
(30,190) |
|
(93) |
% |
$ |
(16,569) |
|
(34) |
% |
Adjustments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition-related items, net of income tax (1) |
|
|
13,063 |
|
|
6,959 |
|
|
7,113 |
|
|
6,104 |
|
* |
|
|
(154) |
|
* |
|
Certain items, net of income tax (1) |
|
|
11,420 |
|
|
4,929 |
|
|
(927) |
|
|
6,491 |
|
* |
|
|
5,856 |
|
* |
|
Foreign currency (gains) losses, net of income tax (1) |
|
|
19,429 |
|
|
2,936 |
|
|
(2,979) |
|
|
16,493 |
|
* |
|
|
5,915 |
|
* |
|
Certain income tax items (1) |
|
|
2,035 |
|
|
1,533 |
|
|
772 |
|
|
502 |
|
* |
|
|
761 |
|
* |
|
Total adjustments, net of income tax |
|
|
45,947 |
|
|
16,357 |
|
|
3,979 |
|
|
29,590 |
|
* |
|
|
12,378 |
|
* |
|
Adjusted net income |
|
$ |
48,363 |
|
$ |
48,963 |
|
$ |
53,154 |
|
$ |
(600) |
|
(1) |
% |
$ |
(4,191) |
|
(8) |
% |
| (1) | See table titled “Items Excluded from Adjusted Net Income” below for further details. |
65
A reconciliation of reported diluted earnings per share (EPS), as reported under GAAP, to non-GAAP adjusted diluted EPS is:
|
|
|
|
|
|
|
|
|
|
|
Change |
|
||||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024/ 2023 |
|
2023/ 2022 |
|
|||||||||
|
|
|
(in thousands, except per share amounts and percentages) |
|
||||||||||||||||
Reconciliation of GAAP diluted EPS to Adjusted diluted EPS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GAAP EPS, diluted |
|
$ |
0.06 |
|
$ |
0.81 |
|
$ |
1.21 |
|
$ |
(0.75) |
|
(93) |
% |
$ |
(0.40) |
|
(33) |
% |
Adjustments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition-related items, net of income tax |
|
|
0.32 |
|
|
0.17 |
|
|
0.17 |
|
|
0.15 |
|
* |
|
|
0.00 |
|
* |
|
Certain items, net of income tax |
|
|
0.28 |
|
|
0.12 |
|
|
(0.02) |
|
|
0.16 |
|
* |
|
|
0.14 |
|
* |
|
Foreign currency (gains) losses, net of income tax |
|
|
0.48 |
|
|
0.07 |
|
|
(0.07) |
|
|
0.41 |
|
* |
|
|
0.14 |
|
* |
|
Certain income tax items |
|
|
0.05 |
|
|
0.04 |
|
|
0.02 |
|
|
0.01 |
|
* |
|
|
0.02 |
|
* |
|
Adjustments EPS, diluted |
|
|
1.13 |
|
|
0.40 |
|
|
0.10 |
|
|
0.73 |
|
* |
|
|
0.30 |
|
* |
|
Adjusted EPS, diluted |
|
$ |
1.19 |
|
$ |
1.21 |
|
$ |
1.31 |
|
$ |
(0.02) |
|
(2) |
% |
$ |
(0.10) |
|
(8) |
% |
Items excluded from adjusted net income consisted of:
|
|
|
|||||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
|
|
|
(in thousands) |
||||||
Items Excluded from Adjusted Net Income |
|
|
|
|
|
|
|
|
|
Acquisition-related items |
|
|
|
|
|
|
|
|
|
Acquisition-related intangible amortization in cost of goods sold |
|
$ |
6,675 |
|
$ |
6,651 |
|
$ |
5,943 |
Acquisition-related cost of goods sold |
|
|
521 |
|
|
— |
|
|
316 |
Acquisition-related intangible amortization in SG&A |
|
|
2,986 |
|
|
3,045 |
|
|
2,981 |
Acquisition-related transaction costs in SG&A |
|
|
6,405 |
|
|
— |
|
|
279 |
Acquisition-related items - income taxes |
|
|
(3,524) |
|
|
(2,737) |
|
|
(2,406) |
Total acquisition-related items, net of income taxes |
|
|
13,063 |
|
|
6,959 |
|
|
7,113 |
|
|
|
|
|
|
|
|
|
|
Certain items (in SG&A) |
|
|
|
|
|
|
|
|
|
Pension settlement cost |
|
|
10,674 |
|
|
— |
|
|
— |
Brazil employment taxes |
|
|
4,202 |
|
|
— |
|
|
— |
Stock-based compensation |
|
|
475 |
|
|
— |
|
|
— |
Phibro Forward income growth initiatives implementation costs |
|
|
366 |
|
|
— |
|
|
— |
Insurance proceeds |
|
|
(899) |
|
|
— |
|
|
— |
Environmental remediation costs |
|
|
— |
|
|
6,894 |
|
|
— |
Gain on sale of investment |
|
|
— |
|
|
— |
|
|
(1,203) |
Certain items - income taxes |
|
|
(3,398) |
|
|
(1,965) |
|
|
276 |
Total certain items, net of income taxes |
|
|
11,420 |
|
|
4,929 |
|
|
(927) |
|
|
|
|
|
|
|
|
|
|
Foreign currency (gains) losses, net |
|
|
|
|
|
|
|
|
|
Foreign currency (gains) losses, net |
|
|
23,863 |
|
|
2,455 |
|
|
(5,216) |
Foreign currency (gains) losses, net - income taxes |
|
|
(4,434) |
|
|
481 |
|
|
2,237 |
Total foreign currency (gains) losses, net, net of income taxes |
|
|
19,429 |
|
|
2,936 |
|
|
(2,979) |
|
|
|
|
|
|
|
|
|
|
Certain income tax items |
|
|
|
|
|
|
|
|
|
Non-U.S. withholding and related taxes, net, on planned repatriation |
|
|
2,828 |
|
|
— |
|
|
— |
Foreign tax credit regulations |
|
|
(1,223) |
|
|
1,223 |
|
|
— |
Change in valuation allowance |
|
|
(1,204) |
|
|
— |
|
|
(1,001) |
Changes in uncertain tax positions and certain other items |
|
|
1,634 |
|
|
310 |
|
|
1,773 |
Total certain income tax items |
|
|
2,035 |
|
|
1,533 |
|
|
772 |
|
|
|
|
|
|
|
|
|
|
Total adjustments, net of income taxes |
|
$ |
45,947 |
|
$ |
16,357 |
|
$ |
3,979 |
66
Analysis of financial condition, liquidity and capital resources
Net increase (decrease) in cash and cash equivalents was:
|
|
|
|
|
|
|
|
|
|
|
Change |
||||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024/ 2023 |
|
2023/ 2022 |
|||||
|
|
(in thousands) |
|||||||||||||
Cash provided (used) by: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
87,594 |
|
$ |
13,310 |
|
$ |
31,649 |
|
$ |
74,284 |
|
$ |
(18,339) |
Investing activities |
|
|
(48,194) |
|
|
(74,018) |
|
|
(22,582) |
|
|
25,824 |
|
|
(51,436) |
Financing activities |
|
|
(6,768) |
|
|
26,987 |
|
|
16,343 |
|
|
(33,755) |
|
|
10,644 |
Effect of exchange-rate changes on cash and cash equivalents |
|
|
(3,300) |
|
|
754 |
|
|
(1,374) |
|
|
(4,054) |
|
|
2,128 |
Net increase (decrease) in cash and cash equivalents |
|
$ |
29,332 |
|
$ |
(32,967) |
|
$ |
24,036 |
|
$ |
62,299 |
|
$ |
(57,003) |
Operating activities
Operating activities provided $87.6 million of net cash for the year ended June 30, 2024. Cash provided by net income, adjusted for non-cash items, including depreciation and amortization, was $54.5 million. Cash provided in the ordinary course of business from changes in operating assets and liabilities and other items was $33.1 million. Accounts receivable used $8.7 million of cash due to sales growth, partially offset by an improvement in days sales outstanding. Inventory provided $2.6 million of cash due to a decrease in quantities on hand, offset by increased raw materials and production costs. Other current assets provided $11.0 million due to timing of tax payments in international regions. Accounts payable provided $12.0 million of cash due to timing of purchases and payments. Accrued expenses and other liabilities provided cash of $12.2 million, primarily due to increased employee-related liabilities and accrued transaction costs.
Operating activities provided $13.3 million of net cash for the year ended June 30, 2023. Net cash provided by operating activities was driven by business performance, resulting in cash provided by net income and non-cash items, including depreciation and amortization, of $54.4 million, offset by cash used in the ordinary course of business for changes in operating assets and liabilities of $41.1 million. Cash provided by accounts receivable was $5.3 million as a result of a favorable reduction in days sales outstanding. Cash used for inventory was $11.2 million due to increased raw material and production costs, product mix, lower than anticipated demand in our Mineral Nutrition products and a projected increase in future demand of other products in our portfolio. Other current assets used $7.4 million due to the timing of insurance payments. Accounts payable used $22.8 million due to timing of purchases and payments. Accrued expenses and other liabilities used $5.7 million, primarily due to changes in employee-related and lease liabilities.
Investing activities
Investing activities used $48.2 million of net cash for the year ended June 30, 2024. Capital expenditures were $41.2 million, related primarily to continued investments in expanded production capacity and productivity improvements. Net purchases and maturities of short-term investments used $4.0 million in cash. We acquired a business for $3.3 million, net of cash acquired. Other investing activities provided $0.3 million of cash.
Investing activities used $74.0 million of net cash for the year ended June 30, 2023. Capital expenditures were $51.8 million, related primarily to continued investments in expanded production capacity and productivity improvements, and the $15.0 million purchase of additional land and building at an operating facility. Net purchases and maturities of short-term investments used $23.0 million in cash. Other investing activities provided $0.8 million of cash.
Financing activities
Financing activities used $6.8 million of net cash for the year ended June 30, 2024. Net borrowings on our 2021 Revolver (as defined below) provided $35.0 million in cash. We paid $22.3 million in scheduled long-term debt maturities. We paid $19.4 million in dividends to holders of our Class A common stock and Class B common stock.
Financing activities provided $27.0 million of net cash for the year ended June 30, 2023. Proceeds from the 2023 Incremental Term Loan and 2022 Term Loan provided $62.0 million, with debt issuance costs of $1.5 million. We paid $15.3 million in scheduled debt maturities. Net payments on our revolving credit facility reduced the outstanding balance by $4.0 million.
67
Proceeds from insurance premium financing, net of cash payments, provided cash of $5.2 million. We paid $19.4 million in dividends to holders of our Class A common stock and Class B common stock.
Liquidity and capital resources
We believe our cash on hand, our operating cash flows and our financing arrangements, including the availability of borrowings under the Revolving Credit Commitments (as defined below), will be sufficient to support our ongoing cash needs. We have considered the current and potential future effects of the macroeconomic market conditions in the financial markets. At this time, we expect adequate liquidity for at least the next twelve months.
Our future debt levels and liquidity are expected to be affected by the Proposed Acquisition and our intent to finance the transaction with new debt in the amount of $350.0 million. See “Notes to Consolidated Financial Statements — Proposed Acquisition.”
Aggregate maturities of long-term debt under the 2024 Credit Agreement, assuming completion of the Proposed Acquisition, are:
For the Years Ending June 30 |
|
|
Annual Maturities |
|
|
Interest Payments |
2025 |
|
$ |
11,875 |
|
$ |
46,400 |
2026 |
|
|
16,250 |
|
|
65,479 |
2027 |
|
|
25,025 |
|
|
63,892 |
2028 |
|
|
25,025 |
|
|
61,933 |
2029 |
|
|
25,025 |
|
|
59,973 |
Total |
|
$ |
103,200 |
|
$ |
297,677 |
For purposes of estimating future interest payments until maturity, we assume (i) long-term debt decreases in accordance with the scheduled amortization, (ii) the Revolving Credit Commitments remains constant at $201,000, (iii) the March 2020 interest rate swap agreement remains in place through its June 2025 maturity, (iv) future floating interest rates are the same as the rates at June 30, 2024, and (v) applicable rates are based on the 2024 Credit Facilities and the expected Net Leverage Ratio, assuming completion of the Proposed Acquisition.
We can provide no assurance that our liquidity and capital resources will be adequate for future funding requirements. We believe we will be able to comply with the terms of the covenants under the 2024 Credit Facilities based on our operating plan. In the event of adverse operating results and/or violation of covenants under the facilities, there can be no assurance we would be able to obtain waivers or amendments. Other risks to our meeting future funding requirements include global economic conditions and macroeconomic, business and financial disruptions that could arise, including ongoing conflicts between Israel and Hamas and between Russia and Ukraine. There can be no assurance that a challenging economic environment or an economic downturn would not affect our liquidity or our ability to obtain future financing or fund operations or investment opportunities. In addition, our debt covenants may restrict our ability to invest. Certain relevant measures of our liquidity and capital resources are as follows:
|
|
|
|
|
|
|
|
|
|
|
Change |
||||
As of June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023 / 2022 |
|||||
|
|
(in thousands, except ratios) |
|||||||||||||
Cash and cash equivalents and short-term investments |
|
$ |
114,613 |
|
$ |
81,281 |
|
$ |
91,248 |
|
$ |
33,332 |
|
$ |
(9,967) |
Working capital |
|
|
312,031 |
|
|
350,737 |
|
|
299,152 |
|
|
(38,706) |
|
|
51,585 |
Ratio of current assets to current liabilities |
|
|
2.79:1 |
|
|
3.28:1 |
|
|
2.70:1 |
|
|
|
|
|
|
We define working capital as total current assets (excluding cash and cash equivalents and short-term investments) less total current liabilities (excluding current portion of long-term debt). We calculate the ratio of current assets to current liabilities based on this definition.
At June 30, 2024, we had $176.0 million in outstanding borrowings under the 2021 Revolver. We had outstanding letters of credit and other commitments of $2.3 million, leaving $131.7 million available for borrowings and letters of credit, subject to restrictions in our 2021 Credit Facilities.
We currently intend to pay quarterly dividends on our Class A and Class B common stock, subject to approval by the Board of Directors. Our Board of Directors has declared a cash dividend of $0.12 per share on Class A common stock and Class B common stock, payable on September 25, 2024.
68
Our future ability to pay dividends will depend upon our results of operations, financial condition, capital requirements, our ability to obtain funds from our subsidiaries and other factors that our Board of Directors deems relevant. Additionally, the terms of our current and any future agreements governing our indebtedness could limit our ability to pay dividends or make other distributions.
We do not expect to contribute to the domestic pension plan during 2025.
At June 30, 2024, our cash and cash equivalents and short-term investments included $111.9 million held by our international subsidiaries. There are no restrictions on cash distributions to PAHC from our international subsidiaries.
Contractual obligations
Our contractual obligations include maturities under the 2021 Credit Facilities and the 2022 Term Loan, including future interest accruals, operating lease commitments, and certain purchase obligations. See “Notes to Consolidated Financial Statements — Debt, Leases, and Commitment and Contingencies.”
Analysis of the consolidated balance sheets
|
|
|
|
|
|
|
|
|
|
|
Change |
||||
As of June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023 / 2022 |
|||||
|
|
(in thousands) |
|||||||||||||
Accounts receivable - trade |
|
$ |
169,452 |
|
$ |
163,479 |
|
$ |
166,537 |
|
$ |
5,973 |
|
$ |
(3,058) |
DSO |
|
|
56 |
|
|
58 |
|
|
59 |
|
|
|
|
|
|
Payment terms outside the U.S. are typically longer than in the United States. We regularly monitor our accounts receivable for collectability, particularly in countries where economic conditions remain uncertain. We believe that our reserve for credit losses is appropriate. Our assessment is based on such factors as past due history, historical and expected collection patterns, the financial condition of our customers, the robust nature of our credit and collection practices and the economic environment. We calculate DSO based on a 360-day year and compare accounts receivable with sales for the quarter ending at the balance sheet date.
|
|
|
|
|
|
|
|
|
|
|
Change |
||||
As of June 30 |
|
2024 |
|
2023 |
|
2022 |
|
2024 / 2023 |
|
2023 / 2022 |
|||||
|
|
(in thousands) |
|||||||||||||
Inventories |
|
$ |
265,911 |
|
$ |
277,570 |
|
$ |
259,158 |
|
$ |
(11,659) |
|
$ |
18,412 |
Inventory decreased by $11.7 million in 2024, primarily due to an effort to manage inventory levels and the effect of currency fluctuations, partially offset by increased raw material and production costs.
Off-balance sheet arrangements
We currently do not use off-balance sheet arrangements for the purpose of credit enhancement, hedging transactions, investment or other financial purposes.
In the ordinary course of business, we may indemnify our counterparties against certain liabilities that may arise. These indemnifications typically pertain to environmental matters. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we would be required to reimburse the loss. These indemnifications generally are subject to certain restrictions and limitations.
69
Selected Quarterly Financial Data (Unaudited)
To facilitate quarterly comparisons, the following unaudited information presents the quarterly results of operations, including segment data, for the years ended June 30, 2024 and 2023. This quarterly financial data was prepared on the same basis as, and should be read in conjunction with, the audited consolidated financial statements and related notes included herein.
|
|
Quarters |
|
Year |
|||||||||||
|
|
September 30, |
|
December 31, |
|
March 31, |
|
June 30, |
|
June 30, |
|||||
For the Periods Ended |
|
2023 |
|
2023 |
|
2024 |
|
2024 |
|
2024 |
|||||
|
|
(in thousands) |
|||||||||||||
Net sales |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MFAs and other |
|
$ |
94,104 |
|
$ |
101,941 |
|
$ |
108,216 |
|
$ |
116,698 |
|
$ |
420,959 |
Nutritional Specialties |
|
|
40,210 |
|
|
41,436 |
|
|
40,194 |
|
|
42,831 |
|
|
164,671 |
Vaccines |
|
|
26,216 |
|
|
29,727 |
|
|
32,923 |
|
|
31,986 |
|
|
120,852 |
Animal Health |
|
$ |
160,530 |
|
$ |
173,104 |
|
$ |
181,333 |
|
$ |
191,515 |
|
$ |
706,482 |
Mineral Nutrition |
|
|
56,026 |
|
|
61,347 |
|
|
64,228 |
|
|
62,062 |
|
|
243,663 |
Performance Products |
|
|
14,793 |
|
|
15,492 |
|
|
17,662 |
|
|
19,587 |
|
|
67,534 |
Total net sales |
|
|
231,349 |
|
|
249,943 |
|
|
263,223 |
|
|
273,164 |
|
|
1,017,679 |
Cost of goods sold |
|
|
163,623 |
|
|
171,327 |
|
|
183,623 |
|
|
186,014 |
|
|
704,587 |
Gross profit |
|
|
67,726 |
|
|
78,616 |
|
|
79,600 |
|
|
87,150 |
|
|
313,092 |
Selling, general and administrative expenses |
|
|
68,452 |
|
|
62,915 |
|
|
59,676 |
|
|
68,734 |
|
|
259,777 |
Operating income (loss) |
|
|
(726) |
|
|
15,701 |
|
|
19,924 |
|
|
18,416 |
|
|
53,315 |
Interest expense, net |
|
|
4,564 |
|
|
4,659 |
|
|
4,575 |
|
|
4,738 |
|
|
18,536 |
Foreign currency (gains) losses, net |
|
|
6,689 |
|
|
7,477 |
|
|
2,427 |
|
|
7,270 |
|
|
23,863 |
Income (expense) before income taxes |
|
|
(11,979) |
|
|
3,565 |
|
|
12,922 |
|
|
6,408 |
|
|
10,916 |
Provision (benefit) for income taxes |
|
|
(3,964) |
|
|
2,291 |
|
|
4,517 |
|
|
5,656 |
|
|
8,500 |
Net income (loss) |
|
$ |
(8,015) |
|
$ |
1,274 |
|
$ |
8,405 |
|
$ |
752 |
|
$ |
2,416 |
Net income (loss) per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
basic |
|
$ |
(0.20) |
|
$ |
0.03 |
|
$ |
0.21 |
|
$ |
0.02 |
|
$ |
0.06 |
diluted |
|
$ |
(0.20) |
|
$ |
0.03 |
|
$ |
0.21 |
|
$ |
0.02 |
|
$ |
0.06 |
Adjusted EBITDA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Animal Health |
|
$ |
28,494 |
|
$ |
39,299 |
|
$ |
36,524 |
|
$ |
41,289 |
|
$ |
145,606 |
Mineral Nutrition |
|
|
2,881 |
|
|
3,507 |
|
|
4,665 |
|
|
5,396 |
|
|
16,449 |
Performance Products |
|
|
1,409 |
|
|
817 |
|
|
2,371 |
|
|
3,065 |
|
|
7,662 |
Corporate |
|
|
(14,133) |
|
|
(14,171) |
|
|
(13,856) |
|
|
(16,320) |
|
|
(58,480) |
Adjusted EBITDA |
|
$ |
18,651 |
|
$ |
29,452 |
|
$ |
29,704 |
|
$ |
33,430 |
|
$ |
111,237 |
Reconciliation of net income (loss) to Adjusted EBITDA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
(8,015) |
|
$ |
1,274 |
|
$ |
8,405 |
|
$ |
752 |
|
$ |
2,416 |
Interest expense, net |
|
|
4,564 |
|
|
4,659 |
|
|
4,575 |
|
|
4,738 |
|
|
18,536 |
Provision (benefit) for income taxes |
|
|
(3,964) |
|
|
2,291 |
|
|
4,517 |
|
|
5,656 |
|
|
8,500 |
Depreciation and amortization |
|
|
8,871 |
|
|
8,910 |
|
|
9,196 |
|
|
9,201 |
|
|
36,178 |
EBITDA |
|
|
1,456 |
|
|
17,134 |
|
|
26,693 |
|
|
20,347 |
|
|
65,630 |
Acquisition-related cost of goods sold |
|
|
— |
|
|
310 |
|
|
211 |
|
|
— |
|
|
521 |
Acquisition-related transaction costs |
|
|
— |
|
|
— |
|
|
512 |
|
|
5,893 |
|
|
6,405 |
Pension settlement cost |
|
|
10,425 |
|
|
249 |
|
|
— |
|
|
— |
|
|
10,674 |
Brazil employment taxes |
|
|
— |
|
|
4,202 |
|
|
— |
|
|
— |
|
|
4,202 |
Stock-based compensation |
|
|
81 |
|
|
80 |
|
|
135 |
|
|
179 |
|
|
475 |
Phibro Forward income growth initiatives implementation costs |
|
|
— |
|
|
— |
|
|
— |
|
|
366 |
|
|
366 |
Insurance proceeds |
|
|
— |
|
|
— |
|
|
(274) |
|
|
(625) |
|
|
(899) |
Foreign currency (gains) losses, net |
|
|
6,689 |
|
|
7,477 |
|
|
2,427 |
|
|
7,270 |
|
|
23,863 |
Adjusted EBITDA |
|
$ |
18,651 |
|
$ |
29,452 |
|
$ |
29,704 |
|
$ |
33,430 |
|
$ |
111,237 |
70
|
|
Quarters |
|
Year |
|||||||||||
|
|
September 30, |
|
December 31, |
|
March 31, |
|
June 30, |
|
June 30, |
|||||
For the Periods Ended |
|
2022 |
|
2022 |
|
2023 |
|
2023 |
|
2023 |
|||||
|
|
(in thousands) |
|||||||||||||
Net sales |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MFAs and other |
|
$ |
92,790 |
|
$ |
97,179 |
|
$ |
93,217 |
|
$ |
104,163 |
|
$ |
387,349 |
Nutritional Specialties |
|
|
39,054 |
|
|
43,856 |
|
|
45,016 |
|
|
44,578 |
|
|
172,504 |
Vaccines |
|
|
23,015 |
|
|
22,768 |
|
|
26,201 |
|
|
28,014 |
|
|
99,998 |
Animal Health |
|
$ |
154,859 |
|
$ |
163,803 |
|
$ |
164,434 |
|
$ |
176,755 |
|
$ |
659,851 |
Mineral Nutrition |
|
|
59,646 |
|
|
61,644 |
|
|
62,922 |
|
|
58,444 |
|
|
242,656 |
Performance Products |
|
|
18,016 |
|
|
19,199 |
|
|
18,317 |
|
|
19,850 |
|
|
75,382 |
Total net sales |
|
|
232,521 |
|
|
244,646 |
|
|
245,673 |
|
|
255,049 |
|
|
977,889 |
Cost of goods sold |
|
|
163,875 |
|
|
167,261 |
|
|
170,133 |
|
|
178,383 |
|
|
679,652 |
Gross profit |
|
|
68,646 |
|
|
77,385 |
|
|
75,540 |
|
|
76,666 |
|
|
298,237 |
Selling, general and administrative expenses |
|
|
54,962 |
|
|
61,541 |
|
|
56,987 |
|
|
52,900 |
|
|
226,390 |
Operating income |
|
|
13,684 |
|
|
15,844 |
|
|
18,553 |
|
|
23,766 |
|
|
71,847 |
Interest expense, net |
|
|
3,067 |
|
|
3,884 |
|
|
3,871 |
|
|
4,499 |
|
|
15,321 |
Foreign currency (gains) losses, net |
|
|
5,200 |
|
|
(149) |
|
|
(422) |
|
|
(2,174) |
|
|
2,455 |
Income before income taxes |
|
|
5,417 |
|
|
12,109 |
|
|
15,104 |
|
|
21,441 |
|
|
54,071 |
Provision for income taxes |
|
|
1,561 |
|
|
4,899 |
|
|
5,062 |
|
|
9,943 |
|
|
21,465 |
Net income |
|
$ |
3,856 |
|
$ |
7,210 |
|
$ |
10,042 |
|
$ |
11,498 |
|
$ |
32,606 |
Net income per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
basic |
|
$ |
0.10 |
|
$ |
0.18 |
|
$ |
0.25 |
|
$ |
0.28 |
|
$ |
0.81 |
diluted |
|
$ |
0.10 |
|
$ |
0.18 |
|
$ |
0.25 |
|
$ |
0.28 |
|
$ |
0.81 |
Adjusted EBITDA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Animal Health |
|
$ |
26,964 |
|
$ |
37,059 |
|
$ |
34,217 |
|
$ |
37,899 |
|
$ |
136,139 |
Mineral Nutrition |
|
|
5,297 |
|
|
4,399 |
|
|
3,859 |
|
|
3,862 |
|
|
17,417 |
Performance Products |
|
|
2,364 |
|
|
2,292 |
|
|
2,413 |
|
|
2,277 |
|
|
9,346 |
Corporate |
|
|
(12,491) |
|
|
(12,838) |
|
|
(13,122) |
|
|
(11,698) |
|
|
(50,149) |
Adjusted EBITDA |
|
$ |
22,134 |
|
$ |
30,912 |
|
$ |
27,367 |
|
$ |
32,340 |
|
$ |
112,753 |
Reconciliation of net income to Adjusted EBITDA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
$ |
3,856 |
|
$ |
7,210 |
|
$ |
10,042 |
|
$ |
11,498 |
|
$ |
32,606 |
Interest expense, net |
|
|
3,067 |
|
|
3,884 |
|
|
3,871 |
|
|
4,499 |
|
|
15,321 |
Provision for income taxes |
|
|
1,561 |
|
|
4,899 |
|
|
5,062 |
|
|
9,943 |
|
|
21,465 |
Depreciation and amortization |
|
|
8,450 |
|
|
8,499 |
|
|
8,489 |
|
|
8,574 |
|
|
34,012 |
EBITDA |
|
|
16,934 |
|
|
24,492 |
|
|
27,464 |
|
|
34,514 |
|
|
103,404 |
Environmental remediation costs |
|
|
— |
|
|
6,569 |
|
|
325 |
|
|
— |
|
|
6,894 |
Foreign currency (gains) losses, net |
|
|
5,200 |
|
|
(149) |
|
|
(422) |
|
|
(2,174) |
|
|
2,455 |
Adjusted EBITDA |
|
$ |
22,134 |
|
$ |
30,912 |
|
$ |
27,367 |
|
$ |
32,340 |
|
$ |
112,753 |
General description of non-GAAP financial measures
Adjusted EBITDA
Adjusted EBITDA is an alternative view of performance used by management as our primary operating measure, and we believe that investors’ understanding of our performance is enhanced by disclosing this performance measure. We report Adjusted EBITDA to reflect the results of our operations prior to considering certain income statement elements and to make financial and operating decisions. We calculate EBITDA as net income plus (i) interest expense, net, (ii) provision for income taxes or less benefit for income taxes and (iii) depreciation and amortization. We calculate Adjusted EBITDA as EBITDA plus (a) other expense or less other income, as separately reported on our consolidated statements of operations, including foreign currency (gains) losses, net and (b) certain items that we consider to be unusual, non-operational or non-recurring. The Adjusted EBITDA measure is not, and should not be viewed as, a substitute for GAAP reported net income and should not be considered as a measure of liquidity.
71
The Adjusted EBITDA measure is an important internal measurement for us. We measure our overall performance on this basis in conjunction with other performance metrics. The following are examples of how our Adjusted EBITDA measure is utilized:
| ● | senior management receives a monthly analysis of our operating results that is prepared on an Adjusted EBITDA basis; |
| ● | our annual budgets are prepared on an Adjusted EBITDA basis; and |
| ● | other goal-setting and performance measurements are prepared on an Adjusted EBITDA basis. |
Despite the importance of this measure to management in goal setting and performance measurement, Adjusted EBITDA is a non-GAAP financial measure that has no standardized meaning prescribed by GAAP and, therefore, has limits in its usefulness to investors. Because of its non-standardized definition, Adjusted EBITDA, unlike GAAP net income, may not be comparable to the calculation of similar measures of other companies. Adjusted EBITDA is presented to permit investors to more fully understand how management assesses performance.
We also recognize that, as an internal measure of performance, the Adjusted EBITDA measure has limitations, and we do not restrict our performance management process solely to this metric. A limitation of the Adjusted EBITDA measure is that it provides a view of our operations without including all events during a period, such as the depreciation of property, plant and equipment or amortization of acquired intangibles, and does not provide a comparable view of our performance to other companies.
Adjusted net income and adjusted diluted earnings per share
Adjusted net income and adjusted diluted earnings per share represent alternative views of performance and we believe investors’ understanding of our performance is enhanced by disclosing these performance measures. We report adjusted net income and adjusted diluted earnings per share to portray the results of our operations prior to considering certain income statement elements. We calculate adjusted net income as net income plus (i) acquisition-related intangible amortization and other acquisition-related items, (ii) certain items we consider to be unusual, non-operational or non-recurring, (iii) stock-based compensation, (iv) foreign currency (gains) losses, as separately reported on our consolidated statements of operations, and (v) the income tax effect of pre-tax income adjustments and certain income tax items. Adjusted diluted earnings per share is calculated by dividing adjusted net income by the diluted weighted average number of shares. The adjusted net income and adjusted diluted earnings per share measures are not, and should not be viewed as, a substitute for GAAP reported net income.
Adjusted net income and adjusted diluted earnings per share are non-GAAP financial measures that have no standardized meaning prescribed by GAAP and, therefore, have limits in their usefulness to investors. Because of its non-standardized definition, adjusted net income and adjusted diluted earnings per share, unlike GAAP net income, may not be comparable to the calculation of similar measures of other companies. Adjusted net income and adjusted diluted earnings per share are presented to permit investors to more fully understand how management assesses performance.
Certain significant items
Adjusted EBITDA, adjusted net income and adjusted diluted earnings per share are calculated prior to considering acquisition-related items and certain other items, as detailed in the table titled “Items Excluded from Adjusted Net Income” above. We evaluate such other items on an individual basis. Such evaluation considers both the quantitative and the qualitative aspect of their unusual, non-operational or non-recurring nature. Unusual, in this context, may represent items that are not part of our ongoing business; items that, either because of their nature or size, we would not expect to occur as part of our normal business on a regular basis. We consider business restructuring costs related to productivity and cost saving initiatives to be unusual items that we do not expect to occur as part of our normal business on a regular basis. We consider foreign currency gains and losses to be non-operational because they arise principally from intercompany transactions and are largely non-cash in nature.
New accounting standards
For discussion of new accounting standards, see “Notes to Consolidated Financial Statements — Summary of Significant Accounting Policies and New Accounting Standards.”
72
Critical accounting policies
Critical accounting policies are those that require application of management’s most difficult, subjective and/or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Not all accounting policies require management to make difficult, subjective or complex judgments or estimates. In presenting our consolidated financial statements in accordance with GAAP, we are required to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses. Actual results that differ from our estimates and assumptions could have an unfavorable effect on our financial position and results of operations.
The following is a summary of accounting policies that we consider critical to the consolidated financial statements.
Revenue Recognition
We recognize revenue from product sales when control of the products has transferred to the customer, typically when title and risk of loss transfer to the customer. Certain of our businesses have terms where control of the underlying product transfers to the customer on shipment, while others have terms where control transfers to the customer on delivery.
Revenue reflects the total consideration to which we expect to be entitled, in exchange for delivery of products or services, net of variable consideration. Variable consideration includes customer programs and incentive offerings, including pricing arrangements, rebates and other volume-based incentives. We record reductions to revenue for estimated variable consideration at the time we record the sale. Our estimates for variable consideration reflect the amount by which we expect variable consideration to affect the revenue recognized. Such estimates are based on contractual terms and historical experience and are adjusted to reflect future expectations as new information becomes available. Historically, we have not had significant adjustments to our estimates of customer incentives. Sales returns and product recalls have been insignificant and infrequent due to the nature of the products we sell.
Business Combinations
Our consolidated financial statements reflect the operations of an acquired business beginning as of the date of acquisition. Assets acquired and liabilities assumed are recorded at their fair values at the date of acquisition; goodwill is recorded for any excess of the purchase price over the fair values of the net assets acquired. Significant judgment may be required to determine the fair values of certain tangible and intangible assets and in assigning their respective useful lives. Significant judgment also may be required to determine the fair values of contingent consideration, if any. We typically utilize third-party valuation specialists to assist us in determining fair values of significant tangible and intangible assets and contingent consideration. The fair values are based on available historical information and on future expectations and assumptions deemed reasonable by management but are inherently uncertain. We typically use an income method to measure the fair value of intangible assets, based on forecasts of the expected future cash flows attributable to the respective assets. Significant estimates and assumptions inherent in the valuations reflect consideration of other marketplace participants and include the amount and timing of future cash flows, specifically the expected revenue growth rate applied to the cash flows. Unanticipated market or macroeconomic events and circumstances could affect the accuracy or validity of the estimates and assumptions. Determining the useful life of an intangible asset also requires judgment. Our estimates of the useful lives of intangible assets primarily are based on a number of factors including the competitive environment, underlying product life cycles, operating plans and the macroeconomic environment of the countries in which the products are sold. Intangible assets are amortized over their estimated lives.
Long-Lived Assets and Goodwill
We periodically review our long-lived and amortizable intangible assets for impairment and assess whether significant events or changes in business circumstances indicate that the carrying value of the assets may not be recoverable. Such circumstances may include a significant decrease in the market price of an asset, a significant adverse change in the manner in which the asset is being used or in its physical condition or a history of operating or cash flow losses associated with the use of an asset. We recognize an impairment loss when the carrying amount of an asset exceeds the anticipated future undiscounted cash flows expected to result from the use of the asset and its eventual disposition. The amount of the impairment loss is the excess of the asset’s carrying value over its fair value. In addition, we periodically reassess the estimated remaining useful lives of our long-lived and amortizable intangible assets.
73
Changes to estimated useful lives would affect the amount of depreciation and amortization recorded in the consolidated statements of operations.
Goodwill represents the excess of the purchase price over the fair value of the identifiable net assets acquired in a business combination. We assess goodwill for impairment annually during the fourth quarter, or more frequently if impairment indicators exist. Impairment exists when the carrying amount of goodwill exceeds its implied fair value. We may elect to assess our goodwill for impairment using a qualitative or a quantitative approach, to determine whether it is more likely than not that the fair value of goodwill is greater than its carrying value.
Income Taxes
The provision for income taxes includes U.S. federal, state and foreign income taxes and foreign withholding taxes. Our annual effective income tax rate is determined based on our income, statutory tax rates and tax planning opportunities available in the various jurisdictions in which we operate and the tax impacts of items treated differently for tax purposes than for financial reporting purposes. Tax law requires certain items be included in the tax return at different times than the items are reflected in the financial statements. Some of these differences are permanent, such as expenses that are not deductible in our tax return, and some differences are temporary, reversing over time, such as depreciation expense. These temporary differences give rise to deferred tax assets and liabilities. Deferred tax assets generally represent the tax effect of items that can be used as a tax deduction or credit in future years for which we have already recorded the tax benefit in our income statement. Deferred tax liabilities generally represent the tax effect of items recorded as tax expense in our income statement for which payment has been deferred, the tax effect of expenditures for which a deduction has already been taken in our tax return but has not yet been recognized in our income statement or the tax effect of assets recorded at fair value in business combinations for which there was no corresponding tax basis adjustment.
The recognition and measurement of a tax position is based on management’s best judgment given the facts, circumstances and information available at the reporting date. Inherent in determining our annual effective income tax rate are judgments regarding business plans, planning opportunities and expectations about future outcomes. Realization of certain deferred tax assets, including research and development costs capitalized for income tax purposes and net operating loss carryforwards, is dependent upon generating sufficient future taxable income in the appropriate jurisdiction prior to the expiration of the amortization or carryforward periods. We establish valuation allowances for deferred tax assets when the amount of expected future taxable income is not likely to support the use of the deduction or credit.
We may take tax positions that management believes are supportable but are potentially subject to successful challenge by the applicable taxing authority in the jurisdictions where we operate. We evaluate our tax positions and establish liabilities in accordance with the applicable accounting guidance on uncertainty in income taxes. We review these tax uncertainties in light of changing facts and circumstances, such as the progress of tax audits, and adjust them accordingly.
We account for income tax contingencies using a benefit recognition model. If our initial assessment does not result in the recognition of a tax benefit, we regularly monitor our position and subsequently recognize the tax benefit if: (i) there are changes in tax law or there is new information that sufficiently raise the likelihood of prevailing on the technical merits of the position to “more likely than not”; (ii) the statute of limitations expires; or (iii) there is a completion of an audit resulting in a favorable settlement of that tax year with the appropriate agency. We regularly re-evaluate our tax positions based on the results of audits of federal, state and foreign income tax filings, statute of limitations expirations, and changes in tax law or receipt of new information that would either increase or decrease the technical merits of a position relative to the “more-likely-than-not” standard.
Our assessments concerning uncertain tax positions are based on estimates and assumptions that have been deemed reasonable by management, but our estimates of unrecognized tax benefits and potential tax benefits may not be representative of actual outcomes, and variation from such estimates could materially affect our financial statements in the period of settlement or when the statutes of limitations expire. Finalizing audits with the relevant taxing authorities can include formal administrative and legal proceedings, and, as a result, it is difficult to estimate the timing and range of possible changes related to our uncertain tax positions, and such changes could be significant.
74
Because there are a number of estimates and assumptions inherent in calculating the various components of our income tax provision, certain future events such as changes in tax legislation, geographic mix of earnings, completion of tax audits or earnings repatriation plans could have an impact on those estimates and our effective income tax rate.
We expect to repatriate approximately $80.0 million of international earnings, which will be subject to applicable non-U.S. withholding and related taxes, net of reductions in U.S. income taxes. We intend to continue to reinvest indefinitely all other undistributed earnings of our foreign subsidiaries where we could be subject to applicable non-U.S. withholding and related taxes if amounts are repatriated to the U.S.
For more information regarding our significant accounting policies, estimates and assumptions, see “Notes to Consolidated Financial Statements — Summary of Significant Accounting Policies and New Accounting Standards.”
Contingencies
Legal matters
We are subject to numerous contingencies arising in the ordinary course of business, such as product liability and other product-related litigation, commercial litigation, environmental claims and proceedings and government investigations. Certain of these contingencies could result in losses, including damages, fines and/or civil penalties, and/or criminal charges, which could be substantial. We believe that we have strong defenses in these types of matters, but litigation is inherently unpredictable and excessive verdicts do occur. We do not believe that any of these matters will have a material adverse effect on our financial position. However, we could incur judgments, enter into settlements or revise our expectations regarding the outcome of certain matters, and such developments could have a material adverse effect on our results of operations or cash flows in the period in which the amounts are paid and/or accrued.
We have accrued for losses that are both probable and reasonably estimable. Substantially all of these contingencies are subject to significant uncertainties and, therefore, determining the likelihood of a loss and/or the measurement of any loss can be complex. Consequently, we are unable to estimate the range of reasonably possible loss in excess of amounts accrued. Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but the assessment process relies heavily on estimates and assumptions that may prove to be incomplete or inaccurate, and unanticipated events and circumstances may occur that might cause us to change those estimates and assumptions.
Environmental
Our operations and properties are subject to Environmental Laws and regulations. As such, the nature of our current and former operations exposes us to the risk of claims with respect to such matters, including fines, penalties and remediation obligations that may be imposed by regulatory authorities. Under certain circumstances, we might be required to curtail operations until a particular problem is remedied. Known costs and expenses under Environmental Laws incidental to ongoing operations, including the cost of litigation proceedings relating to environmental matters, are generally included within operating results. Potential costs and expenses may also be incurred in connection with the repair or upgrade of facilities to meet existing or new requirements under Environmental Laws or to investigate or remediate potential or actual contamination and from time to time we establish reserves for such contemplated investigation and remediation costs. In many instances, the ultimate costs under Environmental Laws and the time period during which such costs are likely to be incurred are difficult to predict.
While we believe that our operations are currently in material compliance with Environmental Laws, we have, from time to time, received notices of violation from governmental authorities, and have been involved in civil or criminal action for such violations. Additionally, at various sites, our subsidiaries are engaged in continuing investigation, remediation and/or monitoring efforts to address contamination associated with historic operations of the sites. We devote considerable resources to complying with Environmental Laws and managing environmental liabilities. We have developed programs to identify requirements under, and maintain compliance with Environmental Laws; however, we cannot predict with certainty the impact of increased and more stringent regulation on our operations, future capital expenditure requirements, or the cost of compliance.
The nature of our current and former operations exposes us to the risk of claims with respect to environmental matters and we cannot assure we will not incur material costs and liabilities in connection with such claims. Based upon our experience to date, we believe that the future cost of compliance with existing Environmental Laws, and liabilities for known environmental claims pursuant to such Environmental Laws, will not have a material adverse effect on our financial position, results of operations, cash flows or liquidity.
75
For additional details, see “Notes to Consolidated Financial Statements — Commitments and Contingencies.”
For additional details, see “Business — Environmental, Health and Safety.”
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Foreign exchange risk
Portions of our net sales and costs are exposed to changes in foreign exchange rates. Our products are sold in more than 80 countries and, as a result, our revenues are influenced by changes in foreign exchange rates. Because we operate in multiple foreign currencies, changes in those currencies relative to the U.S. dollar could affect our revenue and expenses, and consequently, net income. Exchange rate fluctuations may also have an effect beyond our reported financial results and directly affect operations. These fluctuations may affect the ability to buy and sell our goods and services in markets affected by significant exchange rate variances.
Our primary foreign currency exposures are to the Brazilian and Israeli currencies. From time to time, we manage foreign exchange risk through the use of foreign currency derivative contracts. We use these contracts to mitigate the potential earnings effects from exposure to foreign currencies.
We analyzed our foreign currency derivative contracts at June 30, 2024 to determine their sensitivity to exchange rate changes. The analysis indicates that if the U.S. dollar were to appreciate or depreciate by 10%, the fair value of these contracts would decrease by $0.3 million or increase by $0.2 million. For additional details, see “Notes to Consolidated Financial Statements — Derivatives.”
Interest rate risk
Our 2021 Credit Facilities and 2022 Term Loan carry floating interest rates based on the Secured Overnight Financing Rate (“SOFR”) or the Prime Rate. Therefore, our profitability and cash flows are exposed to interest rate fluctuations. Our interest rates also include variable applicable rates in addition to the SOFR portion of our interest obligation. The applicable rates for SOFR borrowings vary from 1.50% to 2.75%, based on the First Lien Net Leverage Ratio. We are a party to an interest rate swap agreement on $300,000 of notional principal that effectively converts the floating portion of our interest obligation on that amount of debt to a fixed rate of 0.61% through June 2025.
Based on our outstanding debt balances and the applicable rates in effect as of June 30, 2024, and considering the interest rate swap agreement, a 100-basis point increase in SOFR would increase annual interest expense and decrease cash flows by $1.9 million. For additional details, see “Notes to Consolidated Financial Statements — Debt” and “Notes to Consolidated Financial Statements — Derivatives.”
76
Item 8.Financial Statements and Supplementary Data
PHIBRO ANIMAL HEALTH CORPORATION
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
77
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Phibro Animal Health Corporation
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Phibro Animal Health Corporation and its subsidiaries (the "Company") as of June 30, 2024 and 2023, and the related consolidated statements of operations, of comprehensive income (loss), of changes in stockholders’ equity and of cash flows for each of the three years in the period ended June 30, 2024, including the related notes (collectively referred to as the "consolidated financial statements"). We also have audited the Company's internal control over financial reporting as of June 30, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of June 30, 2024 and 2023, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2024 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of June 30, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
78
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Revenue Recognition – Net Sales
As described in Notes 2 and 4 to the consolidated financial statements, the Company’s total net sales were $1,017.7 million for the year ended June 30, 2024. The Company recognizes revenue from product sales when obligations under the terms of a contract with the customer are satisfied; generally, this occurs with the transfer of control of the goods to the customers. Certain of the Company’s businesses have terms where control of the products transfers to the customer on shipment, while others have terms where control transfers to the customer on delivery. Revenue reflects the total consideration to which management expects to be entitled, in exchange for delivery of products or services, net of variable consideration, if any.
The principal consideration for our determination that performing procedures relating to revenue recognition is a critical audit matter is the high degree of auditor effort in performing procedures related to the Company’s revenue recognition.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the revenue recognition process, including controls over the recording of net sales at the transaction price once control has passed to the customer. These procedures also included, among others, (i) testing revenue transactions by evaluating the settlement of invoices and credit memos, (ii) tracing transactions not settled to a detailed listing of accounts receivable, (iii) testing credit memos issued during the year, (iv) confirming a sample of outstanding customer invoice balances at year end and, for confirmations not returned, obtaining and inspecting source documents, including purchase orders, invoices, sales contracts, proof of delivery, and subsequent cash receipts, where applicable, and (v) testing the completeness and accuracy of data provided by management.
/s/ PricewaterhouseCoopers LLP
Florham Park, New Jersey
August 28, 2024
We have served as the Company’s auditor since 1998.
79
PHIBRO ANIMAL HEALTH CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
|
|
(in thousands, except per share amounts) |
|||||||
Net sales |
|
$ |
1,017,679 |
|
$ |
977,889 |
|
$ |
942,261 |
Cost of goods sold |
|
|
704,587 |
|
|
679,652 |
|
|
656,861 |
Gross profit |
|
|
313,092 |
|
|
298,237 |
|
|
285,400 |
Selling, general and administrative expenses |
|
|
259,777 |
|
|
226,390 |
|
|
206,414 |
Operating income |
|
|
53,315 |
|
|
71,847 |
|
|
78,986 |
Interest expense, net |
|
|
18,536 |
|
|
15,321 |
|
|
11,875 |
Foreign currency (gains) losses, net |
|
|
23,863 |
|
|
2,455 |
|
|
(5,216) |
Income before income taxes |
|
|
10,916 |
|
|
54,071 |
|
|
72,327 |
Provision for income taxes |
|
|
8,500 |
|
|
21,465 |
|
|
23,152 |
Net income |
|
$ |
2,416 |
|
$ |
32,606 |
|
$ |
49,175 |
|
|
|
|
|
|
|
|
|
|
Net income per share |
|
|
|
|
|
|
|
|
|
basic |
|
$ |
0.06 |
|
$ |
0.81 |
|
$ |
1.21 |
diluted |
|
$ |
0.06 |
|
$ |
0.81 |
|
$ |
1.21 |
Weighted average common shares outstanding |
|
|
|
|
|
|
|
|
|
basic |
|
|
40,504 |
|
|
40,504 |
|
|
40,504 |
diluted |
|
|
40,523 |
|
|
40,504 |
|
|
40,504 |
The accompanying notes are an integral part of these consolidated financial statements
80
PHIBRO ANIMAL HEALTH CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
|
|
(in thousands) |
|||||||
Net income |
|
$ |
2,416 |
|
$ |
32,606 |
|
$ |
49,175 |
Change in fair value of derivative instruments |
|
|
(11,485) |
|
|
3,698 |
|
|
21,681 |
Foreign currency translation adjustment |
|
|
(8,942) |
|
|
3,972 |
|
|
(18,939) |
Pension settlement recognition |
|
|
10,674 |
|
|
— |
|
|
— |
Unrecognized net pension gains (losses) |
|
|
310 |
|
|
212 |
|
|
(4,235) |
(Provision) benefit for income taxes |
|
|
126 |
|
|
(979) |
|
|
(4,327) |
Other comprehensive income (loss) |
|
|
(9,317) |
|
|
6,903 |
|
|
(5,820) |
Comprehensive income (loss) |
|
$ |
(6,901) |
|
$ |
39,509 |
|
$ |
43,355 |
The accompanying notes are an integral part of these consolidated financial statements
81
PHIBRO ANIMAL HEALTH CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
As of June 30 |
|
2024 |
|
2023 |
||
|
|
(in thousands, except share and per share amounts) |
||||
ASSETS |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
70,613 |
|
$ |
41,281 |
Short-term investments |
|
|
44,000 |
|
|
40,000 |
Accounts receivable, net |
|
|
169,452 |
|
|
163,479 |
Inventories, net |
|
|
265,911 |
|
|
277,570 |
Other current assets |
|
|
51,021 |
|
|
63,393 |
Total current assets |
|
|
600,997 |
|
|
585,723 |
Property, plant and equipment, net |
|
|
203,300 |
|
|
195,568 |
Intangibles, net |
|
|
45,033 |
|
|
54,987 |
Goodwill |
|
|
54,557 |
|
|
53,274 |
Other assets |
|
|
78,297 |
|
|
81,845 |
Total assets |
|
$ |
982,184 |
|
$ |
971,397 |
LIABILITIES AND STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
Current portion of long-term debt |
|
$ |
29,795 |
|
$ |
22,295 |
Accounts payable |
|
|
85,567 |
|
|
73,853 |
Accrued expenses and other current liabilities |
|
|
88,786 |
|
|
79,852 |
Total current liabilities |
|
|
204,148 |
|
|
176,000 |
Revolving credit facility |
|
|
176,000 |
|
|
141,000 |
Long-term debt |
|
|
282,289 |
|
|
311,541 |
Other liabilities |
|
|
63,106 |
|
|
60,347 |
Total liabilities |
|
|
725,543 |
|
|
688,888 |
Commitments and contingencies (Note 13) |
|
|
|
|
|
|
Common stock, par value $0.0001 per share; 300,000,000 Class A shares authorized, 20,337,574 shares issued and outstanding at June 30, 2024, and June 30, 2023; 30,000,000 Class B shares authorized, 20,166,034 shares issued and outstanding at June 30, 2024, and June 30, 2023 |
|
|
4 |
|
|
4 |
Preferred stock, par value $0.0001 per share; 16,000,000 shares authorized, no shares issued and outstanding |
|
|
— |
|
|
— |
Paid-in capital |
|
|
136,278 |
|
|
135,803 |
Retained earnings |
|
|
243,886 |
|
|
260,912 |
Accumulated other comprehensive loss |
|
|
(123,527) |
|
|
(114,210) |
Total stockholders’ equity |
|
|
256,641 |
|
|
282,509 |
Total liabilities and stockholders’ equity |
|
$ |
982,184 |
|
$ |
971,397 |
The accompanying notes are an integral part of these consolidated financial statements
82
PHIBRO ANIMAL HEALTH CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
|
|
(in thousands) |
|||||||
OPERATING ACTIVITIES |
|
|
|
|
|
|
|
|
|
Net income |
|
$ |
2,416 |
|
$ |
32,606 |
|
$ |
49,175 |
Adjustments to reconcile net income to |
|
|
|
|
|
|
|
|
|
net cash provided by operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
36,178 |
|
|
34,012 |
|
|
32,705 |
Amortization of debt issuance costs |
|
|
1,040 |
|
|
727 |
|
|
590 |
Deferred income taxes |
|
|
(12,042) |
|
|
(2,838) |
|
|
(806) |
Foreign currency (gains) losses, net |
|
|
13,114 |
|
|
(10,398) |
|
|
(4,808) |
Acquisition-related items |
|
|
521 |
|
|
— |
|
|
316 |
Pension settlement cost |
|
|
10,674 |
|
|
— |
|
|
— |
Brazil employment taxes |
|
|
4,202 |
|
|
— |
|
|
— |
Stock-based compensation |
|
|
475 |
|
|
— |
|
|
— |
Gain on sale of investment |
|
|
— |
|
|
— |
|
|
(1,203) |
Other |
|
|
(2,098) |
|
|
334 |
|
|
319 |
Changes in operating assets and liabilities, net of business acquisition: |
|
|
|
|
|
|
|
|
|
Accounts receivable, net |
|
|
(8,678) |
|
|
5,335 |
|
|
(23,625) |
Inventories, net |
|
|
2,641 |
|
|
(11,222) |
|
|
(46,999) |
Other current assets |
|
|
11,040 |
|
|
(7,419) |
|
|
(1,804) |
Other assets |
|
|
3,922 |
|
|
750 |
|
|
(1,721) |
Accounts payable |
|
|
12,000 |
|
|
(22,830) |
|
|
26,358 |
Accrued expenses and other liabilities |
|
|
12,189 |
|
|
(5,747) |
|
|
3,152 |
Net cash provided by operating activities |
|
|
87,594 |
|
|
13,310 |
|
|
31,649 |
INVESTING ACTIVITIES |
|
|
|
|
|
|
|
|
|
Purchases of short-term investments |
|
|
(65,523) |
|
|
(40,000) |
|
|
(64,100) |
Maturities of short-term investments |
|
|
61,523 |
|
|
17,000 |
|
|
90,100 |
Capital expenditures |
|
|
(41,238) |
|
|
(51,794) |
|
|
(37,044) |
Business acquisition, net of cash acquired |
|
|
(3,282) |
|
|
— |
|
|
(13,511) |
Sale of investment |
|
|
— |
|
|
— |
|
|
1,353 |
Other, net |
|
|
326 |
|
|
776 |
|
|
620 |
Net cash used by investing activities |
|
|
(48,194) |
|
|
(74,018) |
|
|
(22,582) |
FINANCING ACTIVITIES |
|
|
|
|
|
|
|
|
|
Revolving credit facility borrowings |
|
|
276,000 |
|
|
264,000 |
|
|
297,000 |
Revolving credit facility repayments |
|
|
(241,000) |
|
|
(268,000) |
|
|
(247,000) |
Proceeds from long-term debt |
|
|
— |
|
|
62,000 |
|
|
— |
Payments of long-term debt |
|
|
(22,295) |
|
|
(15,315) |
|
|
(9,375) |
Debt issuance costs |
|
|
— |
|
|
(1,473) |
|
|
— |
Proceeds from insurance premium financing and other short-term debt |
|
|
8,593 |
|
|
6,356 |
|
|
— |
Payments of insurance premium financing and other short-term debt |
|
|
(8,624) |
|
|
(1,139) |
|
|
— |
Payment of contingent consideration |
|
|
— |
|
|
— |
|
|
(4,840) |
Dividends paid |
|
|
(19,442) |
|
|
(19,442) |
|
|
(19,442) |
Net cash (used) provided by financing activities |
|
|
(6,768) |
|
|
26,987 |
|
|
16,343 |
Effect of exchange rate changes on cash |
|
|
(3,300) |
|
|
754 |
|
|
(1,374) |
Net increase (decrease) in cash and cash equivalents |
|
|
29,332 |
|
|
(32,967) |
|
|
24,036 |
Cash and cash equivalents at beginning of period |
|
|
41,281 |
|
|
74,248 |
|
|
50,212 |
Cash and cash equivalents at end of period |
|
$ |
70,613 |
|
$ |
41,281 |
|
$ |
74,248 |
|
|
|
|
|
|
|
|
|
|
Supplemental cash flow information |
|
|
|
|
|
|
|
|
|
Interest paid, net |
|
$ |
17,253 |
|
$ |
14,575 |
|
$ |
11,159 |
Income taxes paid, net |
|
|
15,430 |
|
|
20,410 |
|
|
17,854 |
Non-cash investing and financing activities |
|
|
|
|
|
|
|
|
|
Property, plant and equipment |
|
|
2,367 |
|
|
2,764 |
|
|
2,953 |
The accompanying notes are an integral part of these consolidated financial statements
83
PHIBRO ANIMAL HEALTH CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
Shares of |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other |
|
|
|
|
|
|
Common |
|
Common |
|
Preferred |
|
Paid-in |
|
Retained |
|
Comprehensive |
|
|
|
|||||
|
|
Stock |
|
Stock |
|
Stock |
|
Capital |
|
Earnings |
|
Income (Loss) |
|
Total |
||||||
As of June 30, 2021 |
|
40,503,608 |
|
$ |
4 |
|
$ |
— |
|
$ |
135,803 |
|
$ |
218,015 |
|
$ |
(115,293) |
|
$ |
238,529 |
Comprehensive income (loss) |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
49,175 |
|
|
(5,820) |
|
|
43,355 |
Dividends declared ($0.48 per share) |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(19,442) |
|
|
— |
|
|
(19,442) |
As of June 30, 2022 |
|
40,503,608 |
|
$ |
4 |
|
$ |
— |
|
$ |
135,803 |
|
$ |
247,748 |
|
$ |
(121,113) |
|
$ |
262,442 |
Comprehensive income |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
32,606 |
|
|
6,903 |
|
|
39,509 |
Dividends declared ($0.48 per share) |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(19,442) |
|
|
— |
|
|
(19,442) |
As of June 30, 2023 |
|
40,503,608 |
|
$ |
4 |
|
$ |
— |
|
$ |
135,803 |
|
$ |
260,912 |
|
$ |
(114,210) |
|
$ |
282,509 |
Comprehensive income (loss) |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
2,416 |
|
|
(9,317) |
|
|
(6,901) |
Dividends declared ($0.48 per share) |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(19,442) |
|
|
— |
|
|
(19,442) |
Stock-based compensation expense |
|
— |
|
|
— |
|
|
— |
|
|
475 |
|
|
— |
|
|
— |
|
|
475 |
As of June 30, 2024 |
|
40,503,608 |
|
$ |
4 |
|
$ |
— |
|
$ |
136,278 |
|
$ |
243,886 |
|
$ |
(123,527) |
|
$ |
256,641 |
The accompanying notes are an integral part of these consolidated financial statements
84
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except per share amounts)
1. Description of Business
Phibro Animal Health Corporation (“Phibro” or “PAHC”) and its subsidiaries (together, the “Company”) is a diversified global developer, manufacturer and marketer of a broad range of animal health and mineral nutrition products for food and companion animals including poultry, swine, beef and dairy cattle, aquaculture and dogs. The Company is also a manufacturer and marketer of performance products for use in the personal care, industrial chemical and chemical catalyst industries. Unless otherwise indicated or the context requires otherwise, references in this report to “we,” “our,” “us,” and similar expressions refer to Phibro and its subsidiaries.
2. Summary of Significant Accounting Policies and New Accounting Standards
Principles of Consolidation and Basis of Presentation
The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) and include the accounts of Phibro and its consolidated subsidiaries. Intercompany balances and transactions have been eliminated from the consolidated financial statements. The decision whether to consolidate an entity requires consideration of majority voting interests, as well as effective control over the entity.
We present our financial statements on the basis of our fiscal year ending June 30. All references to years in these consolidated financial statements refer to the fiscal year ending or ended on June 30 of that year.
Risks and Uncertainties
The issue of the potential for increased bacterial resistance to certain antibiotics used in certain food-producing animals is the subject of discussions on a worldwide basis and, in certain instances, has led to government restrictions on or banning of the use of antibiotics in food-producing animals. The sale of antibiotics and antibacterials is a material portion of our business. Should product bans or restrictions, public perception, competition or other developments result in restrictions on the sale of such products, it could have a material adverse effect on our financial position, results of operations and cash flows.
An outbreak of disease carried by food animals, which could lead to the widespread death or precautionary destruction of food animals as well as reduced consumption and demand for animal protein, could adversely affect demand for our products. Such occurrences could have a material adverse effect on our financial condition, results of operations and cash flows.
The testing, manufacturing, and marketing of certain of our products are subject to extensive regulation by numerous government authorities in the United States and other countries.
We have significant assets in Israel, Brazil and other locations outside of the United States and a significant portion of our sales and earnings are attributable to operations conducted abroad. Our assets, results of operations and future prospects are subject to currency exchange fluctuations and restrictions, energy shortages, other economic developments, political or social instability in some countries, and uncertainty of, and governmental control over, commercial rights, which could result in a material adverse effect on our financial position, results of operations and cash flows.
We are subject to environmental laws and regulations governing the use, storage, handling, generation, treatment, emission, release, discharge and disposal of certain materials and wastes, the remediation of contaminated soil and groundwater, the manufacture, sale and use of regulated materials, including pesticides, and the health and safety of employees. As such, the nature of our current and former operations and those of our subsidiaries expose Phibro and our subsidiaries to the risk of claims with respect to such matters.
Our business could be impacted by economic sanctions, bans, boycotts, or broader military conflicts, including the ongoing armed conflicts between Israel and Hamas and between Russia and Ukraine. Other potential impacts include supply chain and logistics disruptions, macroeconomic impacts from the exclusion of Russian financial institutions from the global banking system, volatility in foreign exchange rates and interest rates, inflationary pressures on raw materials and energy, as well as heightened cybersecurity threats.
85
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Pandemics and similar outbreaks could directly or indirectly impact our business, results of operations and financial condition. A pandemic or any other similar health crisis could have economic impacts on customers, suppliers and markets. A pandemic could affect our future revenues, expenses, reserves and allowances, manufacturing operations and employee-related costs.
Use of Estimates
The Company’s consolidated financial statements have been prepared in accordance with GAAP. Preparation of these financial statements requires management to make certain estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures. Actual results could differ from these estimates. Estimates are used when accounting for the valuation of intangible assets, depreciation and amortization periods of long-lived and intangible assets, recoverability of long-lived and intangible assets and goodwill, realizability of deferred income tax assets, sales discounts, rebates, allowances and incentives, contingencies, employee compensation and actuarial assumptions related to our pension plans. We regularly evaluate our estimates and assumptions using historical experience and other factors. Our estimates are based on complex judgments, probabilities and assumptions that we believe to be reasonable.
Revenue Recognition
We recognize revenue from product sales when obligations under the terms of a contract with the customer are satisfied; generally, this occurs with the transfer of control of the goods to the customers. Certain of our businesses have terms where control of the underlying product transfers to the customer on shipment, while others have terms where control transfers to the customer on delivery.
Revenue reflects the total consideration to which we expect to be entitled in exchange for delivery of products or services, net of variable consideration. Variable consideration includes customer programs and incentive offerings, including pricing arrangements, rebates and other volume-based incentives. We record reductions to revenue for estimated variable consideration at the time we record the sale. Our estimates for variable consideration reflect the amount by which we expect variable consideration to affect the revenue recognized. Such estimates are generally based on contractual terms and historical experience and are adjusted to reflect future expectations as new information becomes available. Historically, we have not had significant adjustments to our estimates of variable compensation. Sales returns and product recalls have been insignificant and infrequent due to the nature of the products we sell.
Net sales include shipping and handling fees billed to customers. The associated costs are considered fulfillment activities and are included in cost of goods sold in the consolidated statements of operations when the related revenue is recognized. Net sales exclude value-added and other taxes based on sales.
Cash and Cash Equivalents
Cash equivalents include highly liquid investments with maturities of three months or less when purchased. Cash and cash equivalents held at financial institutions may at times exceed insured amounts. We believe we mitigate such risk by investing in or through major financial institutions.
Short-term Investments
Short-term investments include highly liquid investments with maturities greater than three months and less than one year at the time of purchase. We classify these investments as held to maturity and we record the related interest income as earned. We determine the appropriate balance sheet classification at the time of purchase and at each balance sheet date. Investments held at financial institutions may at times exceed insured amounts. We believe we mitigate such risk by investing in or through major financial institutions.
Accounts Receivable and Reserve for Credit Losses
Trade accounts receivable are recorded at the invoiced amount and do not bear interest. We grant credit terms in the normal course of business and generally do not require collateral or other security to support credit sales. Our ten largest customers represented, in aggregate, approximately 13% and 16% of accounts receivable at June 30, 2024 and 2023, respectively.
86
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The reserve for credit losses is our best estimate of the credit losses in existing accounts receivable. We monitor the financial performance, historical and expected collection patterns, and creditworthiness of our customers so that we can properly assess and respond to changes in their credit profile. We also monitor domestic and international economic conditions for the potential future effect on our customers. Past due balances are reviewed individually for collectability. Account balances are charged against the reserve when we determine it is probable the receivable will not be recovered.
Inventories
Inventories are valued at the lower of cost or net realizable value. Cost is determined principally under weighted average and standard cost methods, which approximate first-in, first-out (FIFO) cost. Obsolete and unsalable inventories, if any, are reflected at estimated net realizable value. Inventory costs include materials, direct labor and manufacturing overhead.
Property, Plant and Equipment
Property, plant and equipment are stated at cost.
Depreciation is charged to results of operations using the straight-line method based upon the assets’ estimated useful lives, ranging from two to thirty years for buildings and improvements, and three to ten years for machinery and equipment. We capitalize costs that extend the useful life or productive capacity of an asset. Repair and maintenance costs are expensed as incurred. In the case of disposals, the assets and related accumulated depreciation are removed from the accounts, and the net amounts, less proceeds from disposal, are included in the consolidated statements of operations.
Leases
We determine at the inception of an arrangement whether the arrangement contains a lease. If an arrangement contains a lease, we assess the lease term when the underlying asset is available for use (“lease commencement”). Individual lease terms reflect the non-cancellable period of the lease, reasonably certain renewal periods and consideration of termination options. We determine the lease classification as either operating or financing at lease commencement, which governs the pattern of expense recognition and presentation in our consolidated financial statements. Our current lease portfolio only includes operating leases.
We recognize a right-of-use (“ROU”) asset and a corresponding lease liability at lease commencement for leases with terms exceeding twelve months. Short-term leases with terms of twelve months or less are not recognized on the consolidated balance sheet and lease payments are recognized on a straight-line basis over the term.
The values of the ROU assets and lease liabilities are calculated based on the present value of the fixed payment obligations over the lease term, using our incremental borrowing rate (“IBR”), determined at lease commencement. The IBR reflects the rate of interest we would expect to pay on a secured basis to borrow an amount equal to the lease payments under similar terms. The IBR incorporates the term and economic environment of the respective lease arrangements.
We have elected to account for lease and non-lease components together as a single lease component and include fixed payment obligations related to such non-lease components in the measurement of ROU assets and lease liabilities. Fixed lease payments are recognized on a straight-line basis over the lease term. Variable lease payments can include index-based lease payments, real estate taxes, maintenance costs, utilization charges and other non-lease services paid to lessors and are not determinable at lease commencement. Variable lease payments are not included in the measurement of ROU assets and lease liabilities and are recognized in the period incurred.
Capitalized Software Costs
We capitalize costs to obtain, develop and implement software for internal use. Amounts paid to third parties and costs of internal employees who are directly associated with the software project are also capitalized, depending on the stage of development.
87
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
We expense software costs that do not meet the capitalization criteria. Capitalized software costs are included in property, plant and equipment on the consolidated balance sheets and are amortized on a straight-line basis over three to seven years.
Debt Issuance Costs
Costs and original issue discounts or premiums related to issuance or modification of our debt are deferred on the consolidated balance sheet and amortized over the lives of the respective debt instruments. Amortization of debt issuance costs is included in interest expense in the consolidated statements of operations.
Business Combinations
Our consolidated financial statements reflect the operations of an acquired business beginning as of the date of acquisition. Assets acquired and liabilities assumed are recorded at their fair values at the date of acquisition; goodwill is recorded for any excess of the purchase price over the fair values of the net assets acquired.
Significant judgment may be required to determine the fair values of certain tangible and intangible assets and in assigning their respective useful lives. Significant judgment also may be required to determine the fair values of contingent consideration, if any. We typically utilize third-party valuation specialists to assist us in determining fair values of significant tangible and intangible assets and contingent consideration. The fair values are based on available historical information and on future expectations and assumptions deemed reasonable by management, but are inherently uncertain. We typically use an income method to measure the fair value of intangible assets, based on forecasts of the expected future cash flows attributable to the respective assets. Significant estimates and assumptions inherent in the valuations reflect consideration of other marketplace participants and include the amount and timing of future cash flows, specifically the expected revenue growth rate applied to the cash flows. Unanticipated market or macroeconomic events and circumstances could affect the accuracy or validity of the estimates and assumptions. Determining the useful life of an intangible asset also requires judgment. Our estimates of the useful lives of intangible assets primarily are based on a number of factors including the competitive environment, underlying product life cycles, operating plans and the macroeconomic environment of the countries in which the products are sold. Intangible assets are amortized over their estimated lives. Intangible assets associated with acquired in-process research and development activities (“IPR&D”) are not amortized until a product is available for sale and regulatory approval is obtained.
Long-Lived Assets and Goodwill
We periodically review our long-lived and amortizable intangible assets for impairment and assess whether significant events or changes in business circumstances indicate that the carrying value of the assets may not be recoverable. Such circumstances may include a significant decrease in the market price of an asset, a significant adverse change in the manner in which the asset is being used or in its physical condition or a history of operating or cash flow losses associated with the use of an asset. We recognize an impairment loss when the carrying amount of an asset exceeds the anticipated future undiscounted cash flows expected to result from the use of the asset and its eventual disposition. The amount of the impairment loss is the excess of the asset’s carrying value over its fair value. In addition, we periodically reassess the estimated remaining useful lives of our long-lived and amortizable intangible assets. Changes to estimated useful lives would affect the amount of depreciation and amortization recorded in the consolidated statements of operations.
Goodwill represents the excess of the purchase price over the fair value of the identifiable net assets acquired in a business combination. We assess goodwill for impairment annually during our fourth quarter, or more frequently if impairment indicators exist. Impairment exists when the carrying amount of goodwill exceeds its implied fair value. We may elect to assess our goodwill for impairment using a qualitative or a quantitative approach, to determine whether it is more likely than not that the fair value of goodwill is greater than its carrying value. During the three months ended June 30, 2024, we tested goodwill using the quantitative approach and determined goodwill was not impaired. We have not recorded any goodwill impairment charges in the periods included in the consolidated financial statements.
88
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Foreign Currency Translation
We generally use local currency as the functional currency to measure the financial position and results of operations of each of our international subsidiaries. We translate assets and liabilities of these operations at the exchange rates in effect at the balance sheet date. We translate income statement accounts at the average rates of exchange prevailing during the period. Translation adjustments that arise from the use of differing exchange rates from period to period are included as a component of accumulated other comprehensive income (loss) in stockholders’ equity. Certain of our Israeli operations have designated the U.S. dollar as their functional currency. Gains and losses arising from re-measurement of local currency accounts into U.S. dollars are included in determining net income.
Comprehensive Income
Comprehensive income consists of net income and the changes in: (i) the fair value of derivative instruments that qualify for hedge accounting; (ii) foreign currency translation adjustments; (iii) pension settlement recognition and unrecognized net pension gains (losses); and (iv) the related (provision) benefit for income taxes.
Derivative Financial Instruments
We record all derivative financial instruments on the consolidated balance sheets at fair value. Changes in the fair value of derivatives are recorded in results of operations or other comprehensive income (loss), depending on whether a derivative is designated and effective as part of a hedge transaction and, if so, the type of hedge transaction. Gains and losses on derivative instruments designated and effective as part of a hedge transaction are included in the results of operations in the periods in which operations are affected by the underlying hedged item.
From time to time, we use certain derivative instruments to mitigate the risk associated with certain economic factors, such as exchange rates and interest rates, which may potentially affect our future cash flows. As of June 30, 2024, we used (i) foreign currency option contracts to mitigate certain exposures related to changes in foreign currency exchange rates on forecasted inventory purchases, and (ii) an interest rate swap on $300,000 of notional principal to manage future cash flow exposure resulting from variable interest rates on that amount of debt. To qualify a derivative as a hedge, we document the nature and relationships between hedging instruments and hedged items, the prospective effectiveness of the hedging instrument as well as the ultimate effectiveness, the risk-management objectives, the strategies for undertaking the various hedge transactions and the methods of assessing hedge effectiveness. We do not engage in trading or other speculative uses of financial instruments.
Environmental Liabilities
Expenditures for ongoing compliance with environmental regulations are expensed or capitalized as appropriate. We capitalize expenditures made to extend the useful life or productive capacity of an asset, including expenditures that prevent future environmental contamination. Other expenditures are expensed as incurred and are recorded in selling, general and administrative expenses in the consolidated statements of operations. We record the expense and related liability in the period an environmental assessment indicates remedial efforts are probable and the costs can be reasonably estimated. Estimates of the liability are based upon currently available facts, existing technology and presently enacted laws and regulations taking into consideration the likely effects of inflation and other societal and economic factors. All available evidence is considered, including prior experience in remediation of contaminated sites, other companies’ experiences and data released by the U.S. Environmental Protection Agency and other organizations. The estimated liabilities are not discounted. We record anticipated recoveries under existing insurance contracts if probable.
Income Taxes
The provision for income taxes includes U.S. federal, state, and foreign income taxes and foreign withholding taxes. Our annual effective income tax rate is determined based on our income, statutory tax rates and tax planning opportunities available in the various jurisdictions in which we operate and the tax effects of items treated differently for tax purposes than for financial reporting purposes. Tax law requires certain items be included in the tax return at different times than the items are reflected in the financial statements. Some of these differences are permanent, such as expenses that are not deductible in our tax return, and some differences are temporary, reversing over time, such as depreciation expense.
89
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
These temporary differences give rise to deferred tax assets and liabilities. Deferred tax assets generally represent the tax effect of items that can be used as a tax deduction or credit in future years for which we have already recorded the tax benefit in our income statement. Deferred tax liabilities generally represent the tax effect of items recorded as tax expense in our income statement for which payment has been deferred, the tax effect of expenditures for which a deduction has already been taken in our tax return but has not yet been recognized in our income statement, and the tax effect of assets recorded at fair value in business combinations for which there was no corresponding tax basis adjustment.
The recognition and measurement of a tax position is based on management’s best judgment given the facts, circumstances and information available at the reporting date. Inherent in determining our annual effective income tax rate are judgments regarding business plans, planning opportunities and expectations about future outcomes. Realization of certain deferred tax assets, including research and development costs capitalized for income tax purposes and net operating loss carryforwards, is dependent upon generating sufficient future taxable income in the appropriate jurisdiction prior to the expiration of the amortization or carryforward periods. We establish valuation allowances for deferred tax assets when the amount of expected future taxable income is not likely to support the use of the deduction or credit.
We may take tax positions that management believes are supportable but are potentially subject to successful challenge by the applicable taxing authority in the jurisdictions where we operate. We evaluate our tax positions and establish liabilities in accordance with the applicable accounting guidance on uncertainty in income taxes. We review these tax uncertainties in light of changing facts and circumstances, such as the progress of tax audits, and adjust them accordingly.
Because there are a number of estimates and assumptions inherent in calculating the various components of our income tax provision, future events such as changes in tax legislation, the geographic mix of earnings, completion of tax audits or earnings repatriation plans could have an effect on those estimates and our effective income tax rate.
Advertising
Advertising and marketing costs are expensed as incurred and are reflected in selling, general and administrative expenses.
Research and Development Expenditures
Research and development expenditures are expensed as incurred and are recorded in selling, general and administrative expenses in the consolidated statements of operations. Most of our manufacturing facilities have scientists and technicians on staff involved in product development, quality assurance and providing technical services to customers. Research, development and technical service efforts are conducted at various facilities. Our animal health research and development activities relate to: companion animal product development, fermentation development and microbiological strain improvement; vaccine development; chemical synthesis and formulation development; nutritional specialties development; and ethanol-related products.
Stock-Based Compensation
We recognize expense for stock-based compensation to employees, including grants of restricted stock units, over the requisite service period based on the grant date fair value of the awards. We determine the fair value of performance-based restricted stock units using the Monte Carlo simulation models. The models use historical and current market data to estimate the fair value. The models incorporate various assumptions such as the risk-free interest rate, expected volatility, expected dividend yield and expected life of the awards.
Net Income per Share and Weighted Average Shares
Basic net income per share is calculated by dividing net income by the weighted average number of common shares outstanding during the reporting period.
Diluted net income per share is calculated by dividing net income by the weighted average number of common shares outstanding during the reporting period after giving effect to dilutive common shares equivalents, resulting from the assumed vesting of restricted stock units, unless the effect would be antidilutive.
90
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Common share equivalents were included in the calculation of diluted net income per share for the periods included in the consolidated financial statements, when applicable.
|
|
|
|
|
|
|
|
|
|
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Net income |
|
$ |
2,416 |
|
$ |
32,606 |
|
$ |
49,175 |
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares – basic |
|
|
40,504 |
|
|
40,504 |
|
|
40,504 |
Dilutive effect of restricted stock units |
|
|
19 |
|
|
— |
|
|
— |
Weighted average number of shares - diluted |
|
|
40,523 |
|
|
40,504 |
|
|
40,504 |
|
|
|
|
|
|
|
|
|
|
Net income per share |
|
|
|
|
|
|
|
|
|
basic |
|
$ |
0.06 |
|
$ |
0.81 |
|
$ |
1.21 |
diluted |
|
$ |
0.06 |
|
$ |
0.81 |
|
$ |
1.21 |
New Accounting Standards
Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, requires the disclosure of significant segment expenses that are included in segment profit or loss and how the segment measures are used for decision-making. The ASU will be effective for Phibro’s fiscal year ending June 30, 2025, including retrospective disclosure for all prior periods presented, and interim periods subsequent to June 30, 2025. We are evaluating the impact to our segment disclosures.
ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, enhances income tax disclosures primarily related to the rate reconciliation and income taxes paid information. The ASU outlines specific categories to be provided in the rate reconciliation and requires additional information for those reconciling items that meet a quantitative threshold. The ASU requires disaggregated disclosure of federal, state and foreign income taxes paid, including disaggregation by individual jurisdictions in which income taxes paid (net of refunds received) is equal to or greater than five percent of total income taxes paid (net of refunds received). The ASU also requires disaggregated disclosure of federal, state and foreign income (loss) from continuing operations before income taxes. The enhanced disclosures will be applied on a prospective basis and are required for Phibro’s fiscal year ending June 30, 2026. We are evaluating the impact of the additional income tax-related disclosures.
3. Proposed Acquisition
In April 2024, we entered into a Purchase and Sale Agreement (the “Purchase Agreement”) with Zoetis Inc. (“Zoetis”) to acquire Zoetis’s medicated feed additive (MFA) product portfolio, certain water-soluble products and related assets (the “Proposed Acquisition”). The purchase price is $350,000, subject to certain adjustments set forth in the Purchase Agreement, payable in cash at closing. Completion of the Proposed Acquisition is subject to satisfaction of customary closing conditions, including clearances by applicable regulatory authorities.
4. Statements of Operations—Additional Information
Disaggregated revenue and customer payment terms
We develop, manufacture and market a broad range of products for food and companion animals including poultry, swine, beef and dairy cattle, aquaculture and dogs. The products help prevent, control and treat diseases and enhance nutrition to help improve animal health and well-being. We sell animal health and mineral nutrition products directly to integrated poultry, cattle and swine customers and through commercial animal feed manufacturers, distributors and veterinarians The animal health industry and demand for many of the animal health products in a particular region are affected by changing disease pressures and by weather conditions, as product usage follows varying weather patterns and seasons.
91
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Our operations are primarily focused on regions where the majority of livestock production is consolidated in large commercial farms.
We have a diversified portfolio of products that are classified within our three reportable business segments — Animal Health, Mineral Nutrition and Performance Products. Each segment has its own dedicated management and sales team.
Animal Health
The Animal Health business develops, manufactures and markets products in three main categories:
| ● | MFAs and other: MFAs and other products primarily consist of concentrated medicated products administered through animal feeds, commonly referred to as Medicated Feed Additives (“MFAs”). Specific product classifications include antibacterials, which inhibit the growth of pathogenic bacteria that cause infections in animals; anticoccidials, which inhibit the growth of coccidia (parasites) that damage the intestinal tract of animals; and other related products. The MFAs and other category also includes antibacterials and other processing aids used in the ethanol fermentation industry. |
| ● | Nutritional specialties: Nutritional specialty products enhance nutrition to help improve health and performance in areas such as immune system function and digestive health. We are also a developer, manufacturer and marketer of microbial products and bioproducts for a variety of applications serving animal health and nutrition, environmental, industrial and agricultural customers. |
| ● | Vaccines: Vaccine products are primarily focused on preventing diseases in poultry, swine, beef and dairy cattle and aquaculture. They protect animals from either viral or bacterial disease challenges. We develop, manufacture and market conventionally licensed and autogenous vaccine products, as well as adjuvants for animal vaccine manufacturers. We have developed and market an innovative and proprietary delivery platform for vaccines. |
Mineral Nutrition
The Mineral Nutrition business is comprised of formulations and concentrations of trace minerals such as zinc, manganese, copper, iron and other compounds, with a focus on customers in North America. Our customers use these products to fortify the daily feed requirements of their livestock’s diets and maintain an optimal balance of trace elements in each animal. We manufacture and market a broad range of mineral nutrition products for food animals including poultry, swine, and beef and dairy cattle.
Performance Products
The Performance Products business manufactures and markets specialty ingredients for use in the personal care, industrial chemical and chemical catalyst industries.
The following tables present our revenues disaggregated by major product category and geographic region:
Net Sales by Product Type
|
|
|
|
|
|
|
|||
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Animal Health |
|
|
|
|
|
|
|
|
|
MFAs and other |
|
$ |
420,959 |
|
$ |
387,349 |
|
$ |
361,538 |
Nutritional specialties |
|
|
164,671 |
|
|
172,504 |
|
|
157,196 |
Vaccines |
|
|
120,852 |
|
|
99,998 |
|
|
88,321 |
Total Animal Health |
|
$ |
706,482 |
|
$ |
659,851 |
|
$ |
607,055 |
Mineral Nutrition |
|
|
243,663 |
|
|
242,656 |
|
|
259,512 |
Performance Products |
|
|
67,534 |
|
|
75,382 |
|
|
75,694 |
Total |
|
$ |
1,017,679 |
|
$ |
977,889 |
|
$ |
942,261 |
92
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Net Sales by Region
|
|
|
|
|
|
|
|
|
|
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
United States |
|
$ |
584,763 |
|
$ |
578,773 |
|
$ |
561,803 |
Latin America and Canada |
|
|
247,705 |
|
|
219,846 |
|
|
191,047 |
Europe, Middle East and Africa |
|
|
121,977 |
|
|
117,815 |
|
|
122,480 |
Asia Pacific |
|
|
63,234 |
|
|
61,455 |
|
|
66,931 |
Total |
|
$ |
1,017,679 |
|
$ |
977,889 |
|
$ |
942,261 |
Net sales by region are based on country of destination.
Our customer payment terms generally range from 30 to 120 days globally and do not include any significant financing components. Payment terms vary based on industry and business practices within the regions in which we operate. Our average worldwide collection period for accounts receivable is approximately 60 days after the revenue is recognized.
Additional Information
|
|
|
|
|
|
|
|
|
|
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Interest expense, net |
|
|
|
|
|
|
|
|
|
2021 Credit Facilities |
|
$ |
20,646 |
|
$ |
17,302 |
|
$ |
11,918 |
2022 Term loan |
|
|
862 |
|
|
589 |
|
|
— |
Amortization of debt issuance costs |
|
|
1,040 |
|
|
727 |
|
|
590 |
Other |
|
|
462 |
|
|
58 |
|
|
183 |
Interest expense |
|
|
23,010 |
|
|
18,676 |
|
|
12,691 |
Interest income |
|
|
(4,474) |
|
|
(3,355) |
|
|
(816) |
|
|
$ |
18,536 |
|
$ |
15,321 |
|
$ |
11,875 |
|
|
|
|
|
|
|
|
|
|
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Depreciation and amortization |
|
|
|
|
|
|
|
|
|
Depreciation of property, plant and equipment |
|
$ |
26,517 |
|
$ |
24,316 |
|
$ |
23,781 |
Amortization of intangible assets |
|
|
9,661 |
|
|
9,696 |
|
|
8,924 |
|
|
$ |
36,178 |
|
$ |
34,012 |
|
$ |
32,705 |
Depreciation of property, plant and equipment includes amortization of capitalized software costs of $1,436, $1,455 and $1,047 during 2024, 2023 and 2022, respectively.
Future amortization of intangible assets as of June 30, 2024 is expected to be:
For the Years Ending June 30 |
|
|
|
2025 |
|
$ |
7,918 |
2026 |
|
|
6,969 |
2027 |
|
|
6,591 |
2028 |
|
|
6,385 |
2029 |
|
|
6,385 |
Thereafter |
|
|
10,785 |
Total |
|
$ |
45,033 |
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Research and development expense |
|
$ |
29,194 |
|
$ |
24,395 |
|
$ |
20,832 |
93
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
5. Balance Sheets—Additional Information
As of June 30 |
|
2024 |
|
2023 |
||
Accounts receivable, net |
|
|
|
|
|
|
Trade accounts receivable |
|
$ |
170,913 |
|
$ |
165,069 |
Reserve for credit losses |
|
|
(1,461) |
|
|
(1,590) |
|
|
$ |
169,452 |
|
$ |
163,479 |
As of June 30 |
|
2024 |
|
2023 |
||
Reserve for credit losses |
|
|
|
|
|
|
Balance at beginning of period |
|
$ |
1,590 |
|
$ |
3,510 |
Provision for estimated credit losses |
|
|
1,538 |
|
|
943 |
Effect of changes in exchange rates |
|
|
(71) |
|
|
(61) |
Credit losses realized |
|
|
(1,596) |
|
|
(2,802) |
Balance at end of period |
|
$ |
1,461 |
|
$ |
1,590 |
|
|
|
|
|
||
As of June 30 |
|
2024 |
|
2023 |
||
Inventories, net |
|
|
|
|
|
|
Raw materials |
|
$ |
72,799 |
|
$ |
84,328 |
Work-in-process |
|
|
23,550 |
|
|
22,350 |
Finished goods |
|
|
169,562 |
|
|
170,892 |
|
|
$ |
265,911 |
|
$ |
277,570 |
As of June 30 |
|
2024 |
|
2023 |
||
Property, plant and equipment, net |
|
|
|
|
|
|
Land |
|
$ |
30,624 |
|
$ |
27,813 |
Buildings and improvements |
|
|
120,173 |
|
|
105,184 |
Machinery and equipment |
|
|
273,965 |
|
|
291,454 |
Construction in progress |
|
|
23,892 |
|
|
34,743 |
|
|
|
448,654 |
|
|
459,194 |
Accumulated depreciation |
|
|
(245,354) |
|
|
(263,626) |
|
|
$ |
203,300 |
|
$ |
195,568 |
Certain of our facilities in Israel are on leased land. These leases expire in calendar years 2039, 2045 and 2062.
Property, plant and equipment, net includes internal-use software costs, net of accumulated amortization, of $3,123 and $3,426 at June 30, 2024 and 2023, respectively.
94
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
|
|
Weighted- |
|
|
|
|
|
|
|
|
Average |
|
|
|
|
|
|
|
|
Useful Life |
|
|
|
|
|
|
As of June 30 |
|
(Years) |
|
2024 |
|
2023 |
||
Intangibles, net |
|
|
|
|
|
|
|
|
Cost |
|
|
|
|
|
|
|
|
Technology |
|
12 |
|
$ |
94,259 |
|
$ |
95,576 |
Product registrations, marketing and distribution rights |
|
9 |
|
|
18,117 |
|
|
18,557 |
Customer relationships |
|
12 |
|
|
30,418 |
|
|
30,235 |
Trade names, trademarks and other |
|
6 |
|
|
5,213 |
|
|
5,605 |
|
|
|
|
|
148,007 |
|
|
149,973 |
Accumulated amortization |
|
|
|
|
|
|
|
|
Technology |
|
|
|
|
(62,119) |
|
|
(55,396) |
Product registrations, marketing and distribution rights |
|
|
|
|
(17,326) |
|
|
(18,553) |
Customer relationships |
|
|
|
|
(19,001) |
|
|
(16,884) |
Trade names, trademarks and other |
|
|
|
|
(4,528) |
|
|
(4,153) |
|
|
|
|
|
(102,974) |
|
|
(94,986) |
|
|
|
|
$ |
45,033 |
|
$ |
54,987 |
As of June 30 |
|
2024 |
|
2023 |
||
Goodwill |
|
|
|
|
|
|
Balance at beginning of period |
|
$ |
53,274 |
|
$ |
53,226 |
Acquisition |
|
|
1,397 |
|
|
— |
Effect of changes in exchange rates |
|
|
(114) |
|
|
48 |
Balance at end of period |
|
$ |
54,557 |
|
$ |
53,274 |
|
|
|
|
|
||
As of June 30 |
|
2024 |
|
2023 |
||
Other assets |
|
|
|
|
|
|
ROU operating lease assets |
|
$ |
37,604 |
|
$ |
35,759 |
Deferred income taxes |
|
|
19,371 |
|
|
8,711 |
Deposits |
|
|
1,646 |
|
|
6,617 |
Insurance investments |
|
|
6,305 |
|
|
6,067 |
Equity method investments |
|
|
5,183 |
|
|
5,027 |
Derivative instruments |
|
|
— |
|
|
10,225 |
Debt issuance costs |
|
|
911 |
|
|
1,408 |
Other |
|
|
7,277 |
|
|
8,031 |
|
|
$ |
78,297 |
|
$ |
81,845 |
|
|
|
|
|
||
As of June 30 |
|
2024 |
|
2023 |
||
Accrued expenses and other current liabilities |
|
|
|
|
|
|
Employee related |
|
$ |
37,612 |
|
$ |
29,359 |
Current operating lease liabilities |
|
|
7,460 |
|
|
6,053 |
Commissions and rebates |
|
|
7,875 |
|
|
5,833 |
Professional fees |
|
|
8,918 |
|
|
5,032 |
Income and other taxes |
|
|
2,931 |
|
|
8,663 |
Insurance-related |
|
|
1,265 |
|
|
1,284 |
Insurance premium financing |
|
|
5,185 |
|
|
4,769 |
Other |
|
|
17,540 |
|
|
18,859 |
|
|
$ |
88,786 |
|
$ |
79,852 |
The insurance premium financing has a fixed interest rate of 6.95% and monthly payments of $648.
95
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
|
|
|
|
|
||
As of June 30 |
|
2024 |
|
2023 |
||
Other liabilities |
|
|
|
|
|
|
Long-term operating lease liabilities |
|
$ |
29,915 |
|
$ |
29,077 |
Long-term and deferred income taxes |
|
|
14,218 |
|
|
12,146 |
Supplemental retirement benefits, deferred compensation and other |
|
|
6,678 |
|
|
6,552 |
U.S. pension plan |
|
|
2,237 |
|
|
2,286 |
International retirement plans |
|
|
3,212 |
|
|
4,210 |
Other long-term liabilities |
|
|
6,846 |
|
|
6,076 |
|
|
$ |
63,106 |
|
$ |
60,347 |
|
|
|
|
|
||
As of June 30 |
|
2024 |
|
2023 |
||
Accumulated other comprehensive loss |
|
|
|
|
|
|
Derivative instruments |
|
$ |
13,104 |
|
$ |
24,589 |
Foreign currency translation adjustment |
|
|
(124,004) |
|
|
(115,062) |
Unrecognized net pension losses |
|
|
(13,012) |
|
|
(23,996) |
Provision for income taxes on derivative instruments |
|
|
(3,304) |
|
|
(6,207) |
Benefit for income taxes on long-term intercompany investments |
|
|
8,166 |
|
|
8,166 |
Provision for income taxes on net pension losses |
|
|
(4,477) |
|
|
(1,700) |
|
|
$ |
(123,527) |
|
$ |
(114,210) |
6. Debt
Subsequent Event – 2024 Credit Agreement
In July 2024, we entered into a Credit Agreement, (the “2024 Credit Agreement”) with a group of lenders, to refinance all our outstanding debt, to pay fees and expenses of the transaction, to finance the purchase price of the Proposed Acquisition upon completion and for ongoing working capital requirements and general corporate purposes. See “Note 17 — Subsequent Event — 2024 Credit Agreement.”
Term Loans and Revolving Credit Facilities
In April 2021, we entered into an amended and restated credit agreement (the “2021 Credit Agreement”) under which we had a term A loan in an aggregate initial principal amount of $300,000 (the “2021 Term A Loan”) and a revolving credit facility under which we could borrow up to $250,000, subject to the terms of the agreement (the “2021 Revolver”). In November 2022, we amended the 2021 Credit Facilities to increase the revolving commitments under the 2021 Revolver to an aggregate amount of $310,000 and to adopt the SOFR as the reference for the fluctuating rate of interest on the 2021 Credit Facilities, replacing the LIBOR reference rate. In June 2023, we obtained an additional incremental Term loan (the “2023 Incremental Term Loan”) in the amount of $50,000 (the 2021 Revolver, the 2021 Term A Loan, and the 2023 Incremental Term Loan are collectively referred to as the “2021 Credit Facilities”).
Borrowings under the 2021 Credit Facilities bear interest at rates based on the ratio of the Company and its subsidiaries’ net consolidated first lien indebtedness to the Company and its subsidiaries’ consolidated EBITDA (the “First Lien Net Leverage Ratio”). The interest rate per annum applicable to the 2021 Revolver and the 2021 Term A Loan is based on a fluctuating rate of interest plus an applicable rate equal to 1.50%, 1.75%, 2.00% or 2.25% in the case of adjusted SOFR rate loans and 0.50%, 0.75%, 1.00% or 1.25% in the case of base rate loans. The interest rate per annum applicable to the 2023 Incremental Term Loan is based on a fluctuating rate of interest plus an applicable rate equal to 2.00%, 2.25%, 2.50% or 2.75% in the case of adjusted SOFR rate loans and 1.00%, 1.25%, 1.50% or 1.75% in the case of base rate loans. The applicable rates are based on the First Lien Net Leverage Ratio (as defined in the 2021 Credit Agreement, as amended).
Pursuant to the terms of the 2021 Credit Agreement, the 2021 Credit Facilities are subject to various covenants that, among other things and subject to the permitted exceptions described therein, restrict us and our subsidiaries with respect to: (i) incurring additional debt; (ii) making certain restricted payments or making optional redemptions of other indebtedness; (iii) making investments or acquiring assets; (iv) disposing of assets (other than in the ordinary course of business); (v) creating any liens on our assets; (vi) entering into transactions with affiliates; (vii) entering into merger or consolidation transactions; and (viii) creating guarantee obligations; provided, however, that we are permitted to pay distributions to stockholders out of available cash subject to certain annual limitations and so long as no default or event of default under the 2021 Credit Facilities shall have occurred and be continuing at the time such distribution is declared.
96
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Indebtedness under the 2021 Credit Facilities is collateralized by a first priority lien on substantially all assets of Phibro and certain of our domestic subsidiaries. The 2021 Credit Facilities mature in April 2026.
The 2021 Credit Agreement requires, among other things, compliance with financial covenants that permit: (i) a maximum First Lien Net Leverage Ratio of 4.00:1.00 (or, specifically with respect to the Test Periods ended June 30, 2024, a maximum of 4.25:1.00); and (ii) a minimum interest coverage ratio of 3.00:1.00, each calculated on a trailing four-quarter basis. The 2021 Credit Agreement contains an acceleration clause should an event of default (as defined in the 2021 Credit Agreement) occur. As of June 30, 2024, we were in compliance with the financial covenants.
As of June 30, 2024, we had $176,000 in borrowings drawn under the 2021 Revolver and had outstanding letters of credit of $2,294, leaving $131,706 available for further borrowings and letters of credit under the 2021 Revolver, subject to restrictions in our 2021 Credit Facilities. We obtain letters of credit in connection with certain regulatory and insurance obligations, inventory purchases and other contractual obligations. The terms of these letters of credit are all less than one year.
Other Long-Term Debt
In September 2022, we entered into a credit agreement (the “2022 Term Loan”) in the amount of $12,000, collateralized by certain facilities. The 2022 Term Loan matures in September 2027. The interest rate per annum applicable to the 2022 Term Loan is based on a fluctuating rate of interest, at the Company’s election from time to time, equal to either (i) one-month adjusted SOFR plus 2.00%; or (ii) a base rate determined by reference to the greater of (a) the Prime Rate and (b) the Federal Funds Effective Rate plus 0.50%. The 2022 Term Loan is repayable in monthly installments of $35, with the balance payable at maturity.
Interest Rates
Interest rates as of the balance sheet dates and the weighted-average rates for the years presented were:
|
|
|
|
|
|
|
|
|||||
|
|
June 30 |
|
Years Ended June 30 |
||||||||
|
|
2024 |
2023 |
|
2024 |
|
2023 |
|
2022 |
|
||
2021 Revolver |
|
6.00 |
% |
6.09 |
% |
|
6.14 |
% |
5.42 |
% |
2.08 |
% |
2021 Term A Loan |
|
2.36 |
% |
2.36 |
% |
|
2.36 |
% |
2.37 |
% |
2.99 |
% |
2023 Incremental Term Loan |
|
7.68 |
% |
7.44 |
% |
|
7.64 |
% |
7.40 |
% |
— |
% |
2022 Term Loan |
|
7.43 |
% |
7.25 |
% |
|
7.41 |
% |
6.43 |
% |
— |
% |
Interest rates as of the balance sheet dates are based on rates in effect as of those dates, including SOFR fluctuating rates of interest, applicable rates and the interest rate swap agreement.
We are a party to an interest rate swap agreement on $300,000 of notional principal that effectively converts the floating SOFR portion of our interest obligation on that amount of debt to a fixed rate of 0.61% through June 2025. We have designated the interest rate swap as a highly effective cash flow hedge. For additional details, see “Note 14 — Derivatives.”
97
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Debt Maturities
|
|
|
|
|
||
As of June 30 |
|
2024 |
|
2023 |
||
2021 Term A Loan due April 2026 |
|
$ |
256,875 |
|
$ |
273,750 |
2023 Incremental Term Loan due April 2026 |
|
|
45,000 |
|
|
50,000 |
2022 Term Loan due September 2027 |
|
|
11,265 |
|
|
11,685 |
|
|
|
313,140 |
|
|
335,435 |
Unamortized debt issuance costs |
|
|
(1,056) |
|
|
(1,599) |
|
|
|
312,084 |
|
|
333,836 |
Less: current maturities of long-term debt and other |
|
|
(29,795) |
|
|
(22,295) |
Long-term debt |
|
$ |
282,289 |
|
$ |
311,541 |
Interest rates in the table are based on rates in effect as of the balance sheet dates, including fluctuating rates of interest, applicable rates and the interest rate swap agreement.
Aggregate Maturities of Long-Term Debt and Revolver
For the Years Ending June 30 |
|
|
Annual Maturities |
|
|
Interest Payments |
2025 |
|
$ |
29,795 |
|
$ |
19,785 |
2026 |
|
|
272,920 |
|
|
26,598 |
2027 |
|
|
420 |
|
|
755 |
2028 |
|
|
10,005 |
|
|
184 |
Total |
|
$ |
313,140 |
|
$ |
47,322 |
For purposes of estimating future interest payments until maturity, we assume long-term debt decreases in accordance with the scheduled amortization and the 2021 Revolver continues unchanged at the June 30, 2024. We assume the March 2020 interest rate swap agreement remains in place through its June 2025 maturity and future interest rates and applicable rates are the same as the rates at June 30, 2024.
7. Leases
Our lease portfolio consists of real estate, vehicles and equipment ROU assets, classified as operating leases. The remaining non-cancelable lease terms, inclusive of renewal options reasonably certain of exercise, range from one to 23 years.
The following table summarizes the ROU assets and the related lease liabilities recorded on the consolidated balance sheet:
As of June 30 |
|
2024 |
|
2023 |
|
Balance Sheet Classification |
||
Assets: |
|
|
|
|
|
|
|
|
Operating lease ROU assets |
|
$ |
37,604 |
|
$ |
35,759 |
|
Other Assets |
|
|
|
|
|
|
|
|
|
Liabilities: |
|
|
|
|
|
|
|
|
Current portion |
|
|
7,460 |
|
|
6,053 |
|
Accrued expenses and |
Non-current portion |
|
|
29,915 |
|
|
29,077 |
|
Other liabilities |
Total operating lease liabilities |
|
$ |
37,375 |
|
$ |
35,130 |
|
|
98
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The following table summarizes the composition of net lease expense:
For the Year Ended June 30 |
|
|
2024 |
|
|
2023 |
|
2022 |
|
Operating lease expense |
|
$ |
8,888 |
|
$ |
8,363 |
|
$ |
8,461 |
Variable lease expense |
|
|
1,150 |
|
|
1,139 |
|
|
1,376 |
Short-term lease expense |
|
|
1,397 |
|
|
1,522 |
|
|
1,214 |
Total lease expense |
|
$ |
11,435 |
|
$ |
11,024 |
|
$ |
11,051 |
The following tables include other supplemental information:
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Operating cash flows used for ROU operating leases |
|
$ |
8,231 |
|
$ |
7,798 |
|
$ |
8,642 |
Non-cash changes to ROU operating assets and lease liabilities |
|
$ |
9,283 |
|
$ |
5,114 |
|
$ |
11,930 |
As of June 30 |
|
2024 |
|
2023 |
|
Weighted average remaining lease term (in years) - operating leases |
|
9.6 |
|
10.6 |
|
Weighted average discount rate - operating leases |
|
4.73 |
% |
3.89 |
% |
At June 30, 2024 maturities of future lease liabilities were:
For the Years Ending June 30 |
|
|
|
2025 |
|
$ |
8,800 |
2026 |
|
|
7,085 |
2027 |
|
|
5,212 |
2028 |
|
|
3,920 |
2029 |
|
|
3,048 |
2030 and thereafter |
|
|
17,200 |
Total lease payments |
|
|
45,265 |
Less: interest |
|
|
7,890 |
Total operating lease liabilities |
|
$ |
37,375 |
There were no significant future payment obligations related to executed lease agreements for which the related lease had not yet commenced as of June 30, 2024. Our lease agreements do not contain any material restrictive covenants or residual value guarantee provisions.
8. Common Stock, Preferred Stock and Dividends
Preferred stock and common stock at June 30, 2024 and 2023 were:
As of June 30 |
|
2024 |
|
2023 |
|
|
|
|
2024 |
|
2023 |
|
|
Authorized Shares |
|
Par value |
|
Issued and outstanding shares |
|||||
Preferred stock |
|
16,000,000 |
|
16,000,000 |
|
$ |
0.0001 |
|
— |
|
— |
Common stock – Class A |
|
300,000,000 |
|
300,000,000 |
|
$ |
0.0001 |
|
20,337,574 |
|
20,337,574 |
Common stock – Class B |
|
30,000,000 |
|
30,000,000 |
|
$ |
0.0001 |
|
20,166,034 |
|
20,166,034 |
Holders of our Class B common stock converted zero shares of Class B common stock to Class A common stock in 2024 and 2023.
99
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Common Stock
General
Except as otherwise provided by our amended and restated certificate of incorporation or applicable law, the holders of our Class A common stock and Class B common stock shall vote together as a single class. There are no cumulative voting rights.
Holders of our Class A common stock and Class B common stock are entitled to receive dividends when and if declared by our Board of Directors out of funds legally available therefore, subject to any statutory or contractual restrictions on the payment of dividends and to any restrictions on the payment of dividends imposed by the terms of any outstanding preferred stock.
Upon our dissolution or liquidation or the sale of all or substantially all of our assets, after payment in full of all amounts required to be paid to creditors and to the holders of preferred stock having liquidation preferences, if any, the holders of our Class A common stock and Class B common stock will be entitled to receive our remaining assets available for distribution.
Class A Common Stock
Holders of our Class A common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders.
Holders of our Class A common stock do not have preemptive, subscription or conversion rights. Our Class A common stock is not convertible and there are no redemption or sinking fund provisions applicable to our Class A common stock. Unless our Board of Directors determines otherwise, we will issue all of our capital stock in uncertificated form.
Class B Common Stock
Holders of our Class B common stock are entitled to 10 votes for each share held of record on all matters submitted to a vote of stockholders. BFI holds all of our outstanding Class B common stock.
Holders of our Class B common stock do not have preemptive or subscription rights. There are no redemption or sinking fund provisions applicable to our Class B common stock.
Each share of Class B common stock is convertible at any time at the option of the holder into one share of Class A common stock. In addition, each share of Class B common stock will convert automatically into one share of Class A common stock upon any transfer, whether or not for value, except for certain transfers by and among BFI, its affiliates and certain Bendheim family members, as described in the amended and restated certificate of incorporation. Once transferred and converted into Class A common stock, the Class B common stock will not be reissued. In addition, all shares of Class B common stock will automatically convert to shares of Class A common stock when the outstanding shares of Class B common stock and Class A common stock held by BFI, its affiliates and certain Bendheim family members, together, is less than 15% of the total outstanding shares of Class A common stock and Class B common stock, taken as a single class.
Holders of our Class B common stock have the right to require us to register the sales of their shares under the Securities Act, under the terms of an agreement between us and the holders.
Preferred Stock
We do not have any preferred stock outstanding. Our Board of Directors has the authority to issue shares of preferred stock from time to time on terms it may determine, to divide shares of preferred stock into one or more series and to fix the designations, preferences, privileges, and restrictions of preferred stock, including dividend rights, conversion rights, voting rights, terms of redemption, liquidation preference, sinking fund terms, and the number of shares constituting any series or the designation of any series to the fullest extent permitted by the General Corporation Law of the State of Delaware.
100
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Dividends
We declared and paid quarterly cash dividends totaling $19,442 for the years ended June 30, 2024, 2023 and 2022, to holders of our Class A common stock and Class B common stock.
9. Stock Incentive Plan
In March 2008, our Board of Directors and stockholders adopted the 2008 Incentive Plan (the “Incentive Plan”). The Incentive Plan provides directors, officers, employees and consultants to the Company with opportunities to purchase common stock pursuant to options that may be granted and receive grants of restricted stock and other stock-based awards granted, from time to time by the Board of Directors or a committee approved by the Board. The Incentive Plan provides for grants of stock options, stock awards and other incentives for up to 6,630,000 shares. There were 4,481,620 Class A shares available for grant pursuant to the Incentive Plan as of June 30, 2024.
Restricted Stock Units
Our Board of Directors has approved grants of 600,000 restricted stock units (“RSUs”) to certain officers of the Company, pursuant to the Company's Incentive Plan and the RSU award agreements. Each RSU represents the right to receive a share of our common stock upon vesting. Certain RSUs are subject to time-based vesting, and certain RSUs are subject to performance-based vesting. The time-based RSUs vest in five equal annual amounts on each anniversary of the February 2024 grant date. The performance-based RSUs vest on the fourth anniversary of the July 2023 grant date and on the fifth anniversary of the February 2024 grant date, subject to continuation of employment on such dates, in increments of 10% (but no less than 20%) (with linear interpolation between increments) based upon the arithmetic average of the Company’s closing stock price per share for each trading day in the 90-calendar day period ending on the vesting date (the “90-Day Average”). None of the RSUs will vest if the 90-Day Average is below $20, and 100% of the RSUs will vest if the 90-Day Average is $60 or above. In the event of a change in control of the Company, following which either (i) 100% of the shares of stock cease to be traded on a nationally recognized stock exchange and the Company is no longer listed on any such exchange or (ii) a Qualifying Termination occurs within 12 months, all unvested RSUs will immediately vest in full. All RSUs were unvested as of June 30, 2024.
We used Monte Carlo simulation models to determine the grant date fair values of the performance-based RSUs. Assumptions used by the models were based on information as of the grant date and included a risk-free rate of return, expected volatility and an expected dividend yield. The risk-free rate of return is based on U.S. treasury yields for bonds with similar maturities. Expected volatility is based on the historical volatility of the Company’s common stock. The expected dividend yield considers estimated annual dividends and the grant date share price of the underlying common stock.
The fair value of the time-based RSUs is equal to the closing market price of the underlying common stock on the grant date, less the present value of expected dividends over the vesting period.
The weighted-average grant date fair value of the RSUs granted in 2024 was $5.44 per share. We recognize stock-based compensation expense for the RSUs on a straight-line basis over the vesting periods. Stock-based compensation expense related to RSUs was $475, $0 and $0 for the years ended June 30, 2024, 2023 and 2022, respectively. At June 30, 2024, there was $2,788 of unrecognized compensation expense related to the RSUs, which will be recognized over a weighted-average period of 4.1 years.
Stock Options
There was no stock-based compensation expense related to employee stock options in the periods included in the consolidated financial statements, and there was no stock option activity during 2024.
10. Related Party Transactions
Certain relatives of Jack C. Bendheim, our Chairman, President and Chief Executive Officer, provided services to the Company as employees or consultants and received aggregate compensation and benefits of $1,590, $1,924 and $2,203 during 2024, 2023 and 2022, respectively.
101
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Mr. Bendheim has sole authority to vote shares of our stock owned by BFI Co., LLC, an investment vehicle of the Bendheim family.
11. Employee Benefit Plans
Domestic Pension Plan
We maintain a noncontributory defined benefit pension plan for all domestic nonunion employees employed on or prior to December 31, 2013, who meet certain requirements of age, length of service and hours worked per year. We amended the plan to eliminate credit for future service and compensation increases, effective September 2016. Plan benefits are based upon years of service and average compensation, as defined. The measurement dates for the plan were as of June 30, 2024 and 2023.
In July 2023, we entered into an annuity purchase agreement to irrevocably transfer a portion of the pension benefit obligation to a third-party insurance company. The annuity purchase price was $26,381 and was approximately equal to the benefit obligation transferred. The annuity purchase was funded from pension assets. We recognized a partial settlement of the pension plan, resulting from the recognition of net pension losses previously included in Accumulated other comprehensive loss. We recorded $10,674 of expense in selling, general and administrative expenses in our consolidated statement of operations during the year ended June 30, 2024.
Changes in the projected benefit obligation were:
For the Year Ended June 30 |
|
2024 |
|
2023 |
||
Change in projected benefit obligation |
|
|
|
|
|
|
Projected benefit obligation at beginning of year |
|
$ |
60,673 |
|
$ |
63,079 |
Interest cost |
|
|
1,775 |
|
|
2,608 |
Benefits paid |
|
|
(954) |
|
|
(2,865) |
Actuarial gain |
|
|
(1,852) |
|
|
(2,149) |
Settlement payment |
|
|
(26,381) |
|
|
— |
Projected benefit obligation at end of year |
|
$ |
33,261 |
|
$ |
60,673 |
The discount rate used for the projected benefit obligation at June 30, 2024 and 2023, was 5.4% and 5.0%, respectively.
The projected benefit obligation for the year ended June 30, 2024 decreased due to the agreement to irrevocably transfer a portion of the pension benefit obligation to a third-party insurance company, as well as actuarial gains at the annual remeasurement period due to a higher discount rate, partially offset by losses derived from other actuarial assumptions. The discount rate used each period is determined with reference to current long-term bond market rates. The projected benefit obligation also increases each year by the interest cost due to the passage of time and decreases each year by the benefits paid to plan participants.
Changes in the plan assets and funded status of the plan were:
For the Year Ended June 30 |
|
2024 |
|
2023 |
||
Change in plan assets |
|
|
|
|
|
|
Fair value of plan assets at beginning of year |
|
$ |
58,387 |
|
$ |
61,286 |
Actual return on plan assets |
|
|
(28) |
|
|
(34) |
Benefits paid |
|
|
(954) |
|
|
(2,865) |
Settlement payment |
|
|
(26,381) |
|
|
— |
Fair value of plan assets at end of year |
|
$ |
31,024 |
|
$ |
58,387 |
Liability funded status at end of year |
|
$ |
(2,237) |
|
$ |
(2,286) |
The actual return on plan assets for the year ended June 30, 2024 was lower than expected due to a reduction in the market value of fixed income securities. Benefits paid decreased compared with the prior year as the pension settlement reduced the number of participants receiving benefits. Our investment strategy is to hold a significant portion of our plan assets in fixed income securities with maturities and amounts approximately matching projected future benefit payments.
102
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The funded status is included in other liabilities in the consolidated balance sheets at June 30, 2024 and 2023, respectively. We seek to maintain an asset balance that meets the long-term funding requirements identified by actuarial projections while also satisfying ERISA fiduciary responsibilities. We do not expect to contribute to the domestic pension plan during 2025.
Accumulated other comprehensive loss related to the plan was:
For the Year Ended June 30 |
|
2024 |
|
2023 |
||
Accumulated other comprehensive loss related to pension plan |
|
|
|
|
|
|
Balance at beginning of period |
|
$ |
(23,996) |
|
$ |
(24,208) |
Amortization of net actuarial loss |
|
|
370 |
|
|
721 |
Current period net actuarial loss |
|
|
(60) |
|
|
(509) |
Settlement expense recognized |
|
|
10,674 |
|
|
— |
Net change |
|
|
10,984 |
|
|
212 |
Balance at end of period |
|
$ |
(13,012) |
|
$ |
(23,996) |
Net periodic pension expense was:
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Interest cost on benefit obligation |
|
$ |
1,775 |
|
$ |
2,608 |
|
$ |
1,675 |
Expected return on plan assets |
|
|
(1,884) |
|
|
(2,624) |
|
|
(3,413) |
Amortization of net actuarial loss and prior service costs |
|
|
370 |
|
|
721 |
|
|
480 |
Settlement expense |
|
|
10,674 |
|
|
— |
|
|
— |
Net periodic pension expense (income) |
|
$ |
10,935 |
|
$ |
705 |
|
$ |
(1,258) |
Significant actuarial assumptions used for the net periodic pension expense for the plan were:
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
Discount rate for interest cost |
|
5.0 |
% |
4.3 |
% |
2.2 |
% |
Expected rate of return on plan assets |
|
5.8 |
% |
4.4 |
% |
4.4 |
% |
Discount rate for benefit obligation |
|
5.1 |
% |
4.6 |
% |
2.9 |
% |
The plan used the Aon AA Bond Universe as a benchmark for its discount rate as of June 30, 2024, 2023 and 2022. The discount rate is determined by matching the plan’s timing and amount of expected cash outflows to a bond yield curve constructed from a population of AA-rated corporate bond issues that are generally non-callable and have at least $250 million par value outstanding. From this, the discount rate that results in the same present value is calculated.
103
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Estimated future benefit payments, based on the benefit obligation as of June 30, 2024 are:
|
|
|
|
|
|
|
|
For the Years Ending June 30 |
|
|
|
2025 |
|
$ |
1,388 |
2026 |
|
|
1,600 |
2027 |
|
|
1,809 |
2028 |
|
|
1,955 |
2029 |
|
|
2,113 |
2030 – 2034 |
|
|
11,486 |
The plan’s target asset allocation for 2025 and the weighted-average asset allocation of plan assets as of June 30, 2024 and 2023 are:
|
|
Target |
|
|
|
|
|
|
Allocation |
|
Percentage of Plan Assets |
||
For the Year Ended June 30 |
|
2025 |
|
2024 |
|
2023 |
Debt securities |
|
65% - 85% |
|
79% |
|
75% |
Equity securities |
|
10% - 30% |
|
18% |
|
12% |
Global asset allocation/risk parity (1) |
|
0% - 15% |
|
2% |
|
3% |
Other |
|
0% - 10% |
|
1% |
|
10% |
| (1) | The global asset allocation/risk parity category consists of a variety of asset classes including, but not limited to, global bonds, global equities, real estate and commodities. |
The expected long-term rate of return for the plan’s total assets is generally based on the plan’s asset mix. In determining the rate to use, we consider the expected long-term real returns on asset categories, expectations for inflation, estimates of the effect of active management and actual historical returns.
The investment policy and strategy is to earn a long-term investment return sufficient to meet the obligations of the plan, while assuming a moderate amount of risk in order to maximize investment return. In order to achieve this goal, assets are invested in a diversified portfolio consisting of debt securities, equity securities and other investments in a manner consistent with ERISA’s fiduciary requirements.
The fair values of the plan assets by asset category were:
|
|
Fair Value Measurements Using |
||||||||||
As of June 30, 2024 |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
||||
Cash and cash equivalents |
|
$ |
277 |
|
|
— |
|
|
— |
|
$ |
277 |
Common-collective funds |
|
|
|
|
|
|
|
|
|
|
|
|
Global large cap equities |
|
|
— |
|
|
5,077 |
|
|
524 |
|
|
5,601 |
Fixed income securities |
|
|
— |
|
|
24,650 |
|
|
— |
|
|
24,650 |
Mutual funds |
|
|
|
|
|
|
|
|
|
|
|
|
Global asset allocations/risk parity |
|
|
496 |
|
|
— |
|
|
— |
|
|
496 |
|
|
$ |
773 |
|
$ |
29,727 |
|
$ |
524 |
|
$ |
31,024 |
|
|
Fair Value Measurements Using |
||||||||||
As of June 30, 2023 |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
||||
Cash and cash equivalents |
|
$ |
6,063 |
|
$ |
— |
|
$ |
— |
|
$ |
6,063 |
Common-collective funds |
|
|
|
|
|
|
|
|
|
|
|
|
Global large cap equities |
|
|
— |
|
|
5,552 |
|
|
1,519 |
|
|
7,071 |
Fixed income securities |
|
|
— |
|
|
43,794 |
|
|
— |
|
|
43,794 |
Mutual funds |
|
|
|
|
|
|
|
|
|
|
|
|
Global asset allocations/risk parity |
|
|
1,426 |
|
|
— |
|
|
— |
|
|
1,426 |
Other |
|
|
— |
|
|
— |
|
|
33 |
|
|
33 |
|
|
$ |
7,489 |
|
$ |
49,346 |
|
$ |
1,552 |
|
$ |
58,387 |
104
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The table below provides a summary of the changes in the fair value of Level 3 assets:
Change in Fair Value Level 3 assets |
|
2024 |
|
2023 |
||
Balance at beginning of period |
|
$ |
1,552 |
|
$ |
1,962 |
Redemptions |
|
|
(1,316) |
|
|
(603) |
Purchases |
|
|
200 |
|
|
— |
Change in fair value |
|
|
88 |
|
|
193 |
Balance at end of period |
|
$ |
524 |
|
$ |
1,552 |
The following outlines the valuation methodologies used to estimate the fair value of plan assets:
| ● | Cash and cash equivalents are valued at $1 per unit; |
| ● | Common-collective funds are determined based on current market values of the underlying assets of the fund; |
| ● | Mutual funds are valued using quoted market prices in active markets; and |
| ● | For Level 3 managed assets, business appraisers use a combination of valuations and appraisal methodologies, as well as a number of assumptions to create a price that brokers evaluate. For Level 3 non-managed assets, pricing is provided by various sources, such as issuer or investment manager. |
Other employee benefit plans
We provide a 401(k) retirement savings plan, under which United States employees may make pre-tax and post-tax contributions. The Company contributes: (i) a matching contribution equal to 100% of the first 6.0% of an employee’s contribution; and (ii) an additional discretionary contribution of up to 4.5% of compensation, depending on the employee’s age and years of service, provided that such contributions comply with ERISA non-discrimination requirements. Employee and Company contributions are subject to certain ERISA limitations. Employees are immediately vested in Company contributions. Our contribution expense was $5,395, $6,214 and $6,341 in 2024, 2023 and 2022, respectively.
Our consolidated balance sheets include other employee-related liabilities of $9,990 and $10,862 as of June 30, 2024 and 2023, respectively, including international retirement plans, supplemental retirement benefits and long-term incentive arrangements. Expense under these plans was $4,189, $4,067 and $3,788 in 2024, 2023 and 2022, respectively.
12. Income Taxes
The components of income before income taxes consisted of the following:
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Domestic |
|
$ |
(22,820) |
|
$ |
14,776 |
|
$ |
27,695 |
Foreign |
|
|
33,736 |
|
|
39,295 |
|
|
44,632 |
Income before income taxes |
|
$ |
10,916 |
|
$ |
54,071 |
|
$ |
72,327 |
105
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Components of the provision for income taxes were:
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Current provision: |
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
3,037 |
|
$ |
9,801 |
|
$ |
4,874 |
State and local |
|
|
1,718 |
|
|
1,810 |
|
|
1,468 |
Foreign |
|
|
15,740 |
|
|
12,750 |
|
|
17,613 |
Total current provision |
|
|
20,495 |
|
|
24,361 |
|
|
23,955 |
Deferred provision (benefit): |
|
|
|
|
|
|
|
|
|
Federal |
|
|
(4,755) |
|
|
(6,151) |
|
|
(75) |
State and local |
|
|
(1,523) |
|
|
(266) |
|
|
251 |
Foreign |
|
|
(4,468) |
|
|
3,424 |
|
|
23 |
Change in foreign valuation allowances |
|
|
(1,249) |
|
|
97 |
|
|
(1,002) |
Total deferred benefit |
|
|
(11,995) |
|
|
(2,896) |
|
|
(803) |
|
|
|
|
|
|
|
|
|
|
Provision for income taxes |
|
$ |
8,500 |
|
$ |
21,465 |
|
$ |
23,152 |
Reconciliations of the federal statutory rate to the Company’s effective tax rate were:
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|
U.S. federal statutory income tax rate |
|
21.0 |
% |
21.0 |
% |
21.0 |
% |
State and local taxes, net of federal benefit |
|
(1.1) |
|
2.0 |
|
2.0 |
|
Higher taxes on non-U.S. income |
|
9.9 |
|
8.9 |
|
4.8 |
|
Changes in uncertain tax positions |
|
30.7 |
|
5.1 |
|
4.4 |
|
Global Intangible Low-Taxed Income |
|
18.3 |
|
3.3 |
|
0.3 |
|
Recognition of federal and foreign tax credits |
|
(10.6) |
|
(3.1) |
|
(0.9) |
|
Change in valuation allowance |
|
(11.4) |
|
0.2 |
|
(1.4) |
|
Foreign derived intangible income |
|
(3.8) |
|
(3.7) |
|
(2.1) |
|
Non-U.S. withholding and related taxes, net, on planned repatriation |
|
28.4 |
|
— |
|
— |
|
Impact of foreign tax credit regulations and related changes |
|
(20.0) |
|
1.9 |
|
— |
|
Non-deductible operating expenses |
|
11.3 |
|
0.9 |
|
0.8 |
|
Non-deductible acquisition costs |
|
4.3 |
|
— |
|
— |
|
Other |
|
0.9 |
|
3.2 |
|
3.1 |
|
Effective income tax rate |
|
77.9 |
% |
39.7 |
% |
32.0 |
% |
We record the Global Intangible Low-Taxed Income (“GILTI”) aspects of comprehensive U.S. income tax legislation as a period expense. The provision for income taxes for the years ended June 30, 2024, 2023 and 2022, included $2,003, $1,775 and $207 of federal tax expense from the effects of GILTI, respectively.
The Company benefits from certain tax holidays in Israel; the impact of which are included within Higher taxes on non-U.S. income.
106
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The tax effects of significant temporary differences that comprise deferred tax assets and liabilities were:
As of June 30 |
|
2024 |
|
2023 |
||
Deferred tax assets: |
|
|
|
|
|
|
Employee-related accruals |
|
$ |
6,620 |
|
$ |
5,461 |
Inventory |
|
|
1,521 |
|
|
2,864 |
Environmental remediation |
|
|
767 |
|
|
1,733 |
Net operating loss carry forwards–domestic |
|
|
777 |
|
|
839 |
Net operating loss carry forwards–foreign |
|
|
3,813 |
|
|
4,389 |
Operating lease liabilities |
|
|
6,788 |
|
|
6,521 |
R&D cost capitalization |
|
|
7,227 |
|
|
4,283 |
Unrealized foreign exchange |
|
|
1,470 |
|
|
— |
Carried foreign interest expense |
|
|
4,518 |
|
|
— |
Other |
|
|
7,224 |
|
|
(1,311) |
|
|
|
40,725 |
|
|
24,779 |
Valuation allowance |
|
|
(1,288) |
|
|
(2,598) |
|
|
|
39,437 |
|
|
22,181 |
Deferred tax liabilities: |
|
|
|
|
|
|
Property, plant and equipment and intangible assets |
|
|
(5,282) |
|
|
(6,286) |
Operating lease ROU assets |
|
|
(6,441) |
|
|
(6,280) |
Unrealized foreign exchange |
|
|
— |
|
|
(1,906) |
Non-U.S. withholding and related taxes, net, on planned repatriation |
|
|
(2,828) |
|
|
— |
Other |
|
|
(6,182) |
|
|
(712) |
|
|
|
(20,733) |
|
|
(15,184) |
|
|
|
|
|
|
|
Net deferred tax asset |
|
$ |
18,704 |
|
$ |
6,997 |
Deferred taxes are included in the consolidated balance sheets as follows:
As of June 30 |
|
2024 |
|
2023 |
||
Other assets |
|
$ |
19,371 |
|
$ |
8,711 |
Other liabilities |
|
|
(667) |
|
|
(1,714) |
|
|
$ |
18,704 |
|
$ |
6,997 |
The valuation allowance established against deferred tax assets was:
As of June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Balance at beginning of period |
|
$ |
2,598 |
|
$ |
2,618 |
|
$ |
3,709 |
(Benefit) provision for income taxes |
|
|
(1,310) |
|
|
(20) |
|
|
(1,091) |
Balance at end of period |
|
$ |
1,288 |
|
$ |
2,598 |
|
$ |
2,618 |
The Company records valuation allowances against certain foreign and state deferred tax assets when, after considering all of the available evidence, it is more likely than not that these assets will not be realized.
The Company has $17,250 of state net operating loss carry forwards. $9,320 that will expire in 2024 through 2043, and $7,930 that do not expire, and $16,208 of foreign net operating loss carry forwards of which most are in jurisdictions that have no expiration.
If amounts are repatriated from certain of our foreign subsidiaries, we could be subject to additional non-U.S. income and withholding taxes. We expect to repatriate approximately $80,000 of non-U.S. earnings, which will be subject to applicable non-U.S. withholding and related taxes, net of reductions in U.S. income taxes. As of June 30, 2024 we recorded a liability of $2,828 related to the planned repatriation of international earnings. We consider all other undistributed earnings of such foreign subsidiaries to be indefinitely reinvested. At June 30, 2024, our cash and cash equivalents and short-term investments included $111,890 held by our international subsidiaries. We do not provide income taxes for foreign currency translation adjustments relating to investments in international subsidiaries that will be held indefinitely.
107
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
As tax law is complex and often subject to varied interpretations, it is uncertain whether some of our tax positions will be sustained upon examination. Tax liabilities associated with uncertain tax positions represent unrecognized tax benefits, which arise when the estimated benefit recorded in our financial statements differs from the amounts taken or expected to be taken in a tax return because of the uncertainties described above. Substantially all of these unrecognized tax benefits, if recognized, would reduce our effective income tax rate.
Reconciliations of the beginning and ending amounts of gross unrecognized tax benefits are as follows:
As of June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Unrecognized tax benefits–beginning of period |
|
$ |
9,449 |
|
$ |
7,832 |
|
$ |
5,311 |
Tax position changes–current period |
|
|
2,066 |
|
|
2,181 |
|
|
5,333 |
Tax position changes–prior periods, including settlements with tax authorities |
|
|
615 |
|
|
193 |
|
|
(1,175) |
Lapse of statute of limitations |
|
|
(58) |
|
|
(194) |
|
|
(1,071) |
Effect of changes in exchange rates |
|
|
(211) |
|
|
(563) |
|
|
(566) |
Unrecognized tax benefits–end of period |
|
|
11,861 |
|
|
9,449 |
|
|
7,832 |
Interest and penalties–end of period |
|
|
1,689 |
|
|
981 |
|
|
427 |
Total liabilities related to uncertain tax positions |
|
$ |
13,550 |
|
$ |
10,430 |
|
$ |
8,259 |
We recognize interest and penalties associated with uncertain tax positions as a component of the provision for income taxes. We recognized and recorded interest and penalties expense of $740, $589 and $74 for 2024, 2023 and 2022, respectively.
Income tax returns for the following periods are no longer subject to examination by the relevant tax authorities:
| ● | U.S. federal and significant states, through June 30, 2019; |
| ● | Brazil, through December 31, 2018; and |
| ● | Israel, through June 30, 2020, for certain subsidiaries and through June 30, 2021, for certain subsidiaries. |
13. Commitments and Contingencies
Environmental
Our operations and properties are subject to extensive federal, state, local and foreign laws and regulations, including those governing pollution; protection of the environment; the use, management, and release of hazardous materials, substances and wastes; air emissions; greenhouse gas emissions; water use, supply and discharges; the investigation and remediation of contamination; the manufacture, distribution, and sale of regulated materials, including pesticides; the importing, exporting and transportation of products; and the health and safety of our employees (collectively, “Environmental Laws”). As such, the nature of our current and former operations exposes us to the risk of claims with respect to such matters, including fines, penalties, and remediation obligations that may be imposed by regulatory authorities. Under certain circumstances, we might be required to curtail operations until a particular problem is remedied. Known costs and expenses under Environmental Laws incidental to ongoing operations, including the cost of litigation proceedings relating to environmental matters, are included within operating results. Potential costs and expenses may also be incurred in connection with the repair or upgrade of facilities to meet existing or new requirements under Environmental Laws or to investigate or remediate potential or actual contamination, and from time to time we establish reserves for such contemplated investigation and remediation costs. In many instances, the ultimate costs under Environmental Laws and the period during which such costs are likely to be incurred are difficult to predict.
While we believe that our operations are currently in material compliance with Environmental Laws, we have, from time to time, received notices of violation from governmental authorities, and have been involved in civil or criminal action for such violations. Additionally, at various sites, our subsidiaries are engaged in continuing investigation, remediation and/or monitoring efforts to address contamination associated with historic operations of the sites. We devote considerable resources to complying with Environmental Laws and managing environmental liabilities.
108
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
We have developed programs to identify requirements under, and maintain compliance with Environmental Laws; however, we cannot predict with certainty the effect of increased and more stringent regulation on our operations, future capital expenditure requirements, or the cost of compliance.
The nature of our current and former operations exposes us to the risk of claims with respect to environmental matters and we cannot assure we will not incur material costs and liabilities in connection with such claims. Based on our experience, we believe that the future cost of compliance with existing Environmental Laws, and liabilities for known environmental claims pursuant to such Environmental Laws, will not have a material adverse effect on our financial position, results of operations, cash flows or liquidity.
The United States Environmental Protection Agency (the “EPA”) oversees remediation of contaminated groundwater that has migrated from the Omega Chemical Corporation Superfund Site (“Omega Chemical Site”), which is upgradient of the Santa Fe Springs, California facility of our subsidiary, Phibro-Tech, Inc. (“Phibro-Tech”). The EPA entered into a settlement agreement and court-approved consent decree (the “Consent Decree”) with a group of companies, including Phibro-Tech and certain other subsidiaries of PAHC due to groundwater contamination from Phibro-Tech’s Santa Fe Springs facility that has allegedly commingled with contaminated groundwater from the Omega Chemical Site.
In February 2023, Phibro-Tech signed a definitive settlement agreement that provided for a “cash-out” settlement, with contribution protection, for Phibro-Tech and its affiliates releasing Phibro-Tech and its affiliates from liability for contamination of the groundwater plume affected by the Omega Chemical Site (with certain exceptions). The settlement agreement does not constitute an admission of liability on the part of Phibro-Tech or its affiliates. As part of the settlement, Phibro-Tech also resolved all claims for indemnification and contribution between Phibro-Tech and the successor to the prior owner of the Phibro-Tech site. The EPA, the Department of Justice and the district court have approved the definitive settlement agreement and the Consent Decree. The district court’s order can be appealed within 60 days, which period will expire on September 10, 2024. As of June 30, 2024, Phibro-Tech and one of its affiliates have made settlement payments totaling $5,019, which represents all cash payments required by the definitive settlement agreement.
Based upon information available, to the extent such costs can be estimated with reasonable certainty, we estimated the cost for further investigation and remediation of identified soil and groundwater problems at operating sites, closed sites and third-party sites, and closure costs for closed sites to be approximately $4,282 and $8,505 at June 30, 2024 and 2023, respectively, which is included in current and long-term liabilities on the consolidated balance sheets. However, future events, such as new information, changes in existing Environmental Laws or their interpretation, and more vigorous enforcement policies of regulatory agencies, may give rise to additional expenditures or liabilities that could be material. For all purposes of the discussion under this caption and elsewhere in this report, it should be noted that we take and have taken the position that neither PAHC nor any of our subsidiaries are liable for environmental or other claims made against one or more of our other subsidiaries or for which any of such other subsidiaries may ultimately be responsible.
Claims and Litigation
PAHC and its subsidiaries are party to various claims and lawsuits arising out of the normal course of business including product liabilities, payment disputes and governmental regulation. Certain of these actions seek damages in various amounts. In many cases, such claims are covered by insurance. We believe that none of the claims or pending lawsuits, either individually or in the aggregate, will have a material adverse effect on our financial position, results of operations, cash flows or liquidity.
Employment and Severance Agreements
We have entered into employment agreements with certain executive management and other employees that specify severance benefits of up to 15 months of the employee’s compensation.
109
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Purchase Commitments
As of June 30, 2024, we have agreements to purchase goods and services totaling approximately $2,000 that are enforceable and legally binding and include amounts for future purchases. Payments for these obligations are expected to be approximately $2,000 in 2025.
14. Derivatives
We monitor our exposure to foreign currency exchange rates and interest rates and from time-to-time use derivatives to manage certain of these risks. We designate derivatives as a hedge of a forecasted transaction or of the variability of the cash flows to be received or paid in the future related to a recognized asset or liability (cash flow hedge). All changes in the fair value of a highly effective cash flow hedge are recorded in accumulated other comprehensive income (loss).
We routinely assess whether the derivatives used to hedge transactions are effective. If we determine that a derivative ceases to be an effective hedge, we discontinue hedge accounting in the period of the assessment for that derivative, and immediately recognize any unrealized gains or losses related to the fair value of that derivative in the consolidated statements of operations.
We record derivatives at fair value in the consolidated balance sheets. For additional details regarding fair value, see “Note 15— Fair Value Measurements.”
We are a party to an interest rate swap agreement on $300,000 of notional principal that effectively converts the floating portion of our interest obligation on that amount of debt to a fixed interest rate of 0.61% through June 2025. We designated the interest rate swap as a highly effective cash flow hedge.
We are a party to foreign currency option contracts used to hedge cash flows related to monthly inventory purchases. The individual option contracts mature monthly through December 2024. The forecasted inventory purchases are probable of occurring and the individual option contracts were designated as highly effective cash flow hedges.
The consolidated balance sheet includes the net fair values of our outstanding foreign currency option contracts within the respective line items, based on the net financial position and maturity date of the individual contracts. The consolidated balance sheet includes the net fair values of our outstanding interest rate swap within the respective balance sheet line items, based on the expected timing of the cash flows. The consolidated balance sheet includes assets and liabilities for the fair values of outstanding derivatives that are designated and effective as cash flow hedges as follows:
|
|
|
|
|
||
As of June 30 |
|
2024 |
|
2023 |
||
Other current assets |
|
|
|
|
|
|
Foreign currency option contracts, net |
|
$ |
39 |
|
$ |
333 |
Interest rate swap |
|
|
13,151 |
|
|
14,031 |
Other assets |
|
|
|
|
|
|
Foreign currency option contracts, net |
|
|
— |
|
|
— |
Interest rate swap |
|
|
— |
|
|
10,225 |
Accrued expense and other current liabilities |
|
|
|
|
|
|
Foreign currency option contracts, net |
|
|
(41) |
|
|
— |
Interest rate swaps |
|
|
— |
|
|
— |
Total Fair Value |
|
|
|
|
|
|
Foreign currency option contracts, net |
|
|
(2) |
|
|
333 |
Interest rate swap |
|
|
13,151 |
|
|
24,256 |
110
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Notional amounts of the derivatives as of the balance sheet date were:
|
|
|
|
As of June 30 |
|
2024 |
|
Interest rate swap |
|
$ |
300,000 |
Brazil Real-USD call options |
|
R$ |
36,000 |
Brazil Real-USD put options |
|
R$ |
(36,000) |
USD-Israel shekel call options |
|
$ |
(3,043) |
USD-Israel shekel put options |
|
$ |
3,043 |
The consolidated statements of operations and statements of other comprehensive income (“OCI”) for the years ended June 30, 2024 and 2023 included the effects of derivatives as follows:
|
|
|
|
|
|
|
For the Year Ended June 30 |
|
2024 |
|
2023 |
||
Foreign currency option contracts, net |
|
|
|
|
|
|
(Income) expense recorded in consolidated statements of operations |
|
$ |
(1,126) |
|
$ |
1,237 |
Consolidated statement of operations - total cost of goods sold |
|
$ |
704,587 |
|
$ |
679,652 |
Consolidated statement of operations - total selling, general and administrative expenses |
|
$ |
259,777 |
|
$ |
226,390 |
(Income) expense recorded in comprehensive income |
|
$ |
380 |
|
$ |
270 |
Interest rate swap |
|
|
|
|
|
|
(Income) expense recorded in consolidated statements of operations |
|
$ |
(14,503) |
|
$ |
(9,870) |
Consolidated statement of operations - total interest expense, net |
|
$ |
18,536 |
|
$ |
15,321 |
(Income) expense recorded in comprehensive income |
|
$ |
11,105 |
|
$ |
(3,968) |
We recognize gains and losses related to certain foreign currency derivatives as a component of cost of goods sold at the time the hedged item is sold. Inventory as of June 30, 2024 included realized net gains of $1,142 related to matured contracts. We anticipate the net gains included in inventory will be recognized in cost of goods sold within the next 12 months.
15. Fair Value Measurements
Fair value is defined as the exit price that would be received to sell an asset or paid to transfer a liability. Fair value is a market-based measurement that should be determined using assumptions that market participants would use in pricing an asset or liability. Financial assets and liabilities are measured at fair value using the three-level valuation hierarchy for disclosure of fair value measurements. The determination of the applicable level within the hierarchy of a particular asset or liability depends on the inputs used in the valuation as of the measurement date, notably the extent to which the inputs are market-based (observable) or internally derived (unobservable). Observable inputs are inputs that market participants would use in pricing the asset or liability developed based on market data obtained from independent sources. Unobservable inputs are inputs based on a company’s own assumptions about market participant assumptions developed based on the best information available in the circumstances. The hierarchy is broken down into three levels based on the reliability of inputs as follows:
Level 1 — Quoted prices in active markets for identical assets or liabilities.
Level 2 — Significant observable inputs, other than quoted prices included within Level 1, that are observable for the asset or liability, either directly or indirectly through corroboration with observable market data.
Level 3 — Unobservable inputs for which there is little or no market data available, and that are significant to the overall fair value measurement, are employed that require the reporting entity to develop its own assumptions.
In assessing the fair value of financial instruments at June 30, 2024 and 2023, we used a variety of methods and assumptions that were based on estimates of market conditions and risks existing at the time.
111
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Short-Term Investments
Our short-term investments consist of cash deposits held at financial institutions. We consider the carrying amounts of these short-term investments to be representative of their fair value.
Current Assets and Liabilities
We consider the carrying amounts of current assets and current liabilities to be representative of their fair value because of the current nature of these items.
Debt
We record debt, including term loans and revolver balances, at amortized cost in our consolidated financial statements. We believe the carrying value of the debt is approximately equal to its fair value, due to the variable nature of the instruments and our evaluation of estimated market prices.
Derivatives
We determine the fair value of derivative instruments based upon pricing models using observable market inputs for these types of financial instruments, such as spot and forward currency translation rates.
Non-Financial Assets
Our non-financial assets, which primarily consist of goodwill, other intangible assets, property and equipment, and lease-related ROU assets, are not required to be measured at fair value on a recurring basis, and instead are reported at carrying value in the consolidated balance sheet. Assets and liabilities may be required to be measured at fair value on a non-recurring basis, either upon initial recognition or for subsequent accounting or reporting, including the initial recognition of net assets acquired in a business combination. These fair value measurements involve unobservable inputs that reflect estimates and assumptions that represent Level 3 inputs.
Fair Value of Assets (Liabilities)
As of |
|
June 30, 2024 |
|
June 30, 2023 |
||||||||||||||
|
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Level 1 |
|
Level 2 |
|
Level 3 |
||||||
Short-term investments |
|
$ |
44,000 |
|
$ |
— |
|
$ |
— |
|
$ |
40,000 |
|
$ |
— |
|
$ |
— |
Foreign currency derivatives |
|
$ |
— |
|
$ |
(2) |
|
$ |
— |
|
$ |
— |
|
$ |
333 |
|
$ |
— |
Interest rate swap |
|
$ |
— |
|
$ |
13,151 |
|
$ |
— |
|
$ |
— |
|
$ |
24,256 |
|
$ |
— |
There were no transfers between levels during the periods presented. There were no changes in the fair value of the Level 3 liabilities.
For a detailed discussion on the fair value of our pension plan assets, see “Note 11 — Employee Benefit Plans.”
16. Business Segments
We evaluate performance and allocate resources based on the Animal Health, Mineral Nutrition and Performance Products segments. Certain of our costs and assets are not directly attributable to a segment or segments and we refer to these items as Corporate. We do not allocate Corporate costs or assets to the other segments because they are not used to evaluate the segments’ operating results or financial position. Corporate costs include certain costs related to executive management, information technology, legal, finance, human resources and business development. The accounting policies of our segments are the same as those described in the summary of significant accounting policies included herein.
We evaluate performance of our segments based on Adjusted EBITDA. We calculate Adjusted EBITDA as net income plus (a) interest expense, net, (b) provision for income taxes or less benefit for income taxes, (c) depreciation and amortization, (d) other expense or less other income, as separately reported on our consolidated statements of operations, including foreign currency (gains) losses, net and (e) certain items that we consider to be unusual, non-operational or non-recurring.
112
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
|
|
|
|
|
|
|
|
|
|
For the Year Ended June 30 |
|
2024 |
|
2023 |
|
2022 |
|||
Net sales |
|
|
|
|
|
|
|
|
|
Animal Health |
|
$ |
706,482 |
|
$ |
659,851 |
|
$ |
607,055 |
Mineral Nutrition |
|
|
243,663 |
|
|
242,656 |
|
|
259,512 |
Performance Products |
|
|
67,534 |
|
|
75,382 |
|
|
75,694 |
Total segments |
|
$ |
1,017,679 |
|
$ |
977,889 |
|
$ |
942,261 |
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
|
|
|
|
|
|
|
Animal Health |
|
$ |
30,194 |
|
$ |
27,714 |
|
$ |
26,759 |
Mineral Nutrition |
|
|
2,427 |
|
|
2,638 |
|
|
2,616 |
Performance Products |
|
|
1,688 |
|
|
1,780 |
|
|
1,717 |
Total segments |
|
$ |
34,309 |
|
$ |
32,132 |
|
$ |
31,092 |
Adjusted EBITDA |
|
|
|
|
|
|
|
|
|
Animal Health |
|
$ |
145,606 |
|
$ |
136,139 |
|
$ |
124,106 |
Mineral Nutrition |
|
|
16,449 |
|
|
17,417 |
|
|
24,038 |
Performance Products |
|
|
7,662 |
|
|
9,346 |
|
|
8,706 |
Total segments |
|
$ |
169,717 |
|
$ |
162,902 |
|
$ |
156,850 |
Reconciliation of income before income taxes to Adjusted EBITDA |
|
|
|
|
|
|
|
|
|
Income before income taxes |
|
$ |
10,916 |
|
$ |
54,071 |
|
$ |
72,327 |
Interest expense, net |
|
|
18,536 |
|
|
15,321 |
|
|
11,875 |
Depreciation and amortization – Total segments |
|
|
34,309 |
|
|
32,132 |
|
|
31,092 |
Depreciation and amortization – Corporate |
|
|
1,869 |
|
|
1,880 |
|
|
1,613 |
Corporate costs |
|
|
58,480 |
|
|
50,149 |
|
|
45,767 |
Acquisition-related cost of goods sold |
|
|
521 |
|
|
— |
|
|
316 |
Acquisition-related transaction costs |
|
|
6,405 |
|
|
— |
|
|
279 |
Pension settlement cost |
|
|
10,674 |
|
|
— |
|
|
— |
Brazil employment taxes |
|
|
4,202 |
|
|
— |
|
|
— |
Stock-based compensation |
|
|
475 |
|
|
— |
|
|
— |
Phibro Forward income growth initiatives implementation costs |
|
|
366 |
|
|
— |
|
|
— |
Insurance proceeds |
|
|
(899) |
|
|
— |
|
|
— |
Environmental remediation costs |
|
|
— |
|
|
6,894 |
|
|
— |
Gain on sale of investment |
|
|
— |
|
|
— |
|
|
(1,203) |
Foreign currency (gains) losses, net |
|
|
23,863 |
|
|
2,455 |
|
|
(5,216) |
Adjusted EBITDA – Total segments |
|
$ |
169,717 |
|
$ |
162,902 |
|
$ |
156,850 |
|
|
|
|
|
||
As of June 30 |
|
2024 |
|
2023 |
||
Identifiable assets |
|
|
|
|
|
|
Animal Health |
|
$ |
684,407 |
|
$ |
698,522 |
Mineral Nutrition |
|
|
67,088 |
|
|
75,814 |
Performance Products |
|
|
50,862 |
|
|
49,678 |
Total segments |
|
|
802,357 |
|
|
824,014 |
Corporate |
|
|
179,827 |
|
|
147,383 |
Total |
|
$ |
982,184 |
|
$ |
971,397 |
The Animal Health segment includes all goodwill of the Company. Corporate assets include cash and cash equivalents, short-term investments, debt issuance costs, income tax related assets and certain other assets.
113
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The geographic location of property, plant and equipment, net was:
As of June 30 |
|
2024 |
|
2023 |
||
Property, plant and equipment, net |
|
|
|
|
|
|
United States |
|
$ |
87,526 |
|
$ |
79,404 |
Israel |
|
|
60,395 |
|
|
61,304 |
Brazil |
|
|
30,348 |
|
|
30,359 |
Ireland |
|
|
19,038 |
|
|
18,900 |
Other |
|
|
5,993 |
|
|
5,601 |
|
|
$ |
203,300 |
|
$ |
195,568 |
17. Subsequent Event – 2024 Credit Agreement
In July 2024, we entered into the 2024 Credit Agreement with a group of lenders. Initial borrowings were used to refinance all our outstanding debt, to pay fees and expenses of the transaction, and for ongoing working capital requirements and general corporate purposes. Borrowings under the Delayed Draw Term A-1 and A-2 Loans will be used to finance the purchase price of the Proposed Acquisition upon completion of the acquisition (the “Closing Date”).
Under the 2024 Credit Agreement, there are (i) Initial Term A-1 Loans in an initial aggregate principal amount of $162,000 (the “Initial Term A-1 Loans”), (ii) Delayed Draw Term A-1 Loans in an initial aggregate principal amount of $189,000 (the “Delayed Draw Term A-1 Loans” and, together with the Initial Term A-1 Loans, the “Term A-1 Loans”) (iii) Initial Term A-2 Loans in an initial aggregate principal amount of $138,000 (the “Initial Term A-2 Loans”), (iv) Delayed Draw Term A-2 Loans in an initial aggregate principal amount of $161,000 (the “Delayed Draw Term A-2 Loans” and, together with the Initial Term A-2 Loans, the “Term A-2 Loans”), and (v) Revolving Credit Commitments in an initial aggregate principal amount of $310,000 (the “Revolving Credit Commitments” and, together with the Term A-1 Loans and Term A-2 Loans, the “2024 Credit Facilities”). The 2024 Credit Facilities mature in July 2029 in the case of the Term A-1 Loans and the Revolving Credit Commitments and in July 2031 in the case of the Term A-2 Loans.
Borrowings under the 2024 Credit Facilities bear interest at rates based on the ratio of the Company and its subsidiaries’ net consolidated indebtedness to the Company and its subsidiaries’ consolidated EBITDA (the “Net Leverage Ratio”). The interest rates per annum for loans under the 2024 Credit Facilities are based on a fluctuating rate of interest as selected by the Company plus an applicable rate as set forth in the table below:
|
|
Revolving Credit and |
|
Term A-2 Loans |
||||||
Net Leverage Ratio |
|
Base rate |
SOFR |
|
Base rate |
SOFR |
||||
≥ 4.00:1.00 |
|
1.75 |
% |
2.75 |
% |
|
2.25 |
% |
3.25 |
% |
≥ 3.50:1.00 and < 4.00:1.00 |
|
1.50 |
% |
2.50 |
% |
|
2.00 |
% |
3.00 |
% |
≥ 2.25:1.00 and < 3.50:1.00 |
|
1.25 |
% |
2.25 |
% |
|
1.75 |
% |
2.75 |
% |
< 2.25:1.00 |
|
1.00 |
% |
2.00 |
% |
|
1.50 |
% |
2.50 |
% |
The Company may receive patronage from the lenders providing the Term A-2 Loans, to the extent eligible under such lender’s patronage program, as determined by such lender in its sole discretion.
Pursuant to the terms of the 2024 Credit Agreement, the 2024 Credit Facilities are subject to various covenants that, among other things and subject to the permitted exceptions described therein, restrict us and our subsidiaries with respect to: (i) incurring additional debt; (ii) making certain restricted payments or making optional redemptions of other indebtedness; (iii) making investments or acquiring assets; (iv) disposing of assets (other than in the ordinary course of business); (v) creating any liens on our assets; (vi) entering into transactions with affiliates; (vii) entering into merger or consolidation transactions; and (viii) creating guarantee obligations; provided, however, that we are permitted to pay distributions to stockholders out of available cash subject to certain annual limitations and a quarterly maximum Net Leverage Ratio of 4.0x and so long as no default or event of default under the 2024 Credit Facilities shall have occurred and be continuing at the time such distribution is declared. Indebtedness under the 2024 Credit Facilities is collateralized by a first priority lien on substantially all assets of Phibro and certain of our domestic subsidiaries.
114
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The 2024 Credit Agreement contains an acceleration clause should an event of default (as defined) occur.
The 2024 Credit Agreement requires, among other things, compliance with financial covenants that permit: (i) a maximum Net Leverage Ratio and (ii) a minimum interest coverage ratio, each calculated on a trailing four-quarter basis, as follows:
Period |
maximum |
|
minimum |
Prior to the Closing Date |
4.00:1.00 |
|
3.00:1.00 |
First fiscal quarter ending after the Closing Date through the eighteen-month anniversary of the Closing Date |
4.75:1.00 |
|
2.50:1.00 |
Eighteen-month anniversary of the Closing Date to the thirty-month anniversary of the Closing Date |
4.50:1.00 |
|
2.75:1.00 |
Thirty-month anniversary of Closing Date to the forty-two month anniversary of the Closing Date |
4.25:1.00 |
|
3.00:1.00 |
After the forty-two month anniversary of the Closing Date |
4.00:1.00 |
|
3.00:1.00 |
We are a party to an interest rate swap agreement on $300,000 of notional principal that effectively converts the floating portion of our interest obligation on that amount of debt to a fixed rate of 0.61% through June 2025. We have designated the interest rate swap as a highly effective cash flow hedge. For additional details, see “Note 14 — Derivatives.”
115
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to provide reasonable assurance that information required to be disclosed in our periodic reports that we file or submit under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) are recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), as appropriate, to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As required by Rules 13a-15(b) and 15d-15(b) of the Exchange Act, we carried out an evaluation, under the supervision and with the participation of our management, including our CEO and CFO, of the effectiveness of our disclosure controls and procedures as of June 30, 2024. Based on this evaluation, our CEO and CFO concluded that our disclosure controls and procedures were effective as of June 30, 2024 to provide the reasonable assurance described above.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act. Under the supervision and with the participation of our management, including the CEO and the CFO, we carried out an evaluation of the effectiveness of our internal control over financial reporting as of June 30, 2024 using the criteria established in “Internal Control-Integrated Framework” (2013), issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on that evaluation, management concluded that our internal control over financial reporting was effective as of June 30, 2024.
The effectiveness of our internal control over financial reporting as of June 30, 2024 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which is included under “Item 8 — Financial Statements and Supplementary Data.”
Changes in Internal Control Over Financial Reporting
There have been no changes to our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
None.
116
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this item is incorporated by reference to our 2024 Proxy Statement to be filed with the SEC within 120 days of the year ended June 30, 2024.
Our Board of Directors has adopted a Code of Business Conduct and Ethics applicable to all officers, directors and employees, which is available on our website (investors.pahc.com) under “Corporate Governance.”
Item 11. Executive Compensation
The information required by this item is incorporated by reference to our 2024 Proxy Statement to be filed with the SEC within 120 days of the year ended June 30, 2024.
Item 12. Security Ownership of Certain Beneficial Owners and Management Related Stockholder Matters
The information required by this item is incorporated by reference to our 2024 Proxy Statement to be filed with the SEC within 120 days of the year ended June 30, 2024.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item is incorporated by reference to our 2024 Proxy Statement to be filed with the SEC within 120 days of the year ended June 30, 2024.
Item 14. Principal Accountant Fees and Services
The information required by this item is incorporated by reference to our 2024 Proxy Statement to be filed with the SEC within 120 days of the year ended June 30, 2024.
117
PART IV
Item 15. Exhibits, Financial Statement Schedules
| (a) | We have filed the following documents as part of this Annual Report on Form 10-K: |
| (1) | Consolidated Financial Statements: |
Report of Independent Registered Public Accounting Firm
Consolidated Statements of Operations for the fiscal years ended June 30, 2024, 2023 and 2022
Consolidated Statements of Comprehensive Income for the fiscal years ended June 30, 2024, 2023 and 2022
Consolidated Balance Sheets at June 30, 2024 and 2023
Consolidated Statements of Cash Flows for the fiscal years ended June 30, 2024, 2023 and 2022
Consolidated Statements of Changes in Stockholders’ Equity for the fiscal years ended June 30, 2024, 2023 and 2022
Notes to Consolidated Financial Statements
| (2) | Schedules: None |
| (3) | The exhibits filed are listed in the Index to Exhibits immediately preceding the signature page of this Annual Report on Form 10-K. |
118
| (b) | Exhibits |
Exhibit Number |
Exhibit Description |
|
|
Exhibit 2.1 |
|
|
|
Exhibit 3.1 |
|
|
|
Exhibit 3.2 |
|
|
|
Exhibit 4.1 |
|
|
|
Exhibit 4.2 |
|
|
|
Exhibit 10.1 |
|
|
|
Exhibit 10.2 |
|
|
|
Exhibit 10.3 |
|
|
|
Exhibit 10.4 |
|
|
|
Exhibit 10.5 |
|
|
|
119
Exhibit 10.6 |
|
|
|
Exhibit 10.7 |
|
|
|
Exhibit 10.8 |
|
|
|
Exhibit 10.9 |
|
|
|
Exhibit 10.10 |
|
|
|
Exhibit 10.11 |
|
|
|
Exhibit 10.12 |
|
|
|
Exhibit 10.13 |
|
|
|
Exhibit 10.14 |
|
|
|
Exhibit 10.15* |
|
|
|
Exhibit 10.16* |
|
|
|
Exhibit 10.17 |
|
|
|
120
Exhibit 10.18 |
|
|
|
Exhibit 10.19 |
|
|
|
Exhibit 10.20 |
|
|
|
Exhibit 10.21 |
|
|
|
Exhibit 10.22 |
|
|
|
Exhibit 10.23 |
|
|
|
Exhibit 10.24 |
|
|
|
Exhibit 10.25 |
|
|
|
Exhibit 19.1 |
Phibro Animal Health Corporation Insider Trading and Disclosure of Confidential Information Policy. |
|
|
Exhibit 21.1 |
|
|
|
Exhibit 23.1 |
|
|
|
Exhibit 31.1 |
Chief Executive Officer-Certification pursuant to Sarbanes-Oxley Act of 2002 Section 302. |
|
|
Exhibit 31.2 |
Chief Financial Officer-Certification pursuant to Sarbanes-Oxley Act of 2002 Section 302. |
|
|
Exhibit 32.1** |
Chief Executive Officer-Certification pursuant to Sarbanes-Oxley Act of 2002 Section 906. |
|
|
Exhibit 32.2** |
Chief Financial Officer-Certification pursuant to Sarbanes-Oxley Act of 2002 Section 906. |
|
|
121
Exhibit 97.1 |
|
|
|
Exhibit 101.INS |
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
|
|
Exhibit 101.SCH |
Inline XBRL Taxonomy Extension Schema Document. |
|
|
Exhibit 101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
|
|
Exhibit 101.DEF |
Inline XBRL Taxonomy Extension Definition Linkbase Document. |
|
|
Exhibit 101.LAB |
Inline XBRL Taxonomy Extension Label Linkbase Document. |
|
|
Exhibit 101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
Exhibit 104 |
Cover Page Interactive Data File (embedded within the inline XBRL and contained in Exhibit 101) |
* |
Confidential treatment of certain provisions of this exhibit has been requested with the Securities and Exchange Commission. Omitted material for which confidential treatment has been requested has been filed separately with the Securities and Exchange Commission. |
** |
This certification is deemed not filed for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act. |
Item 16. Form 10-K Summary
Not applicable.
122
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned thereunto duly authorized.
|
Phibro Animal Health Corporation |
|
|
|
|
August 28, 2024 |
By: |
/s/ Jack C. Bendheim |
|
|
Jack C. Bendheim |
|
|
Chairman, President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Phibro Animal Health Corporation |
|
|
|
|
August 28, 2024 |
By: |
/s/ Jack C. Bendheim |
|
|
Jack C. Bendheim |
|
|
Chairman, President and Chief Executive Officer |
|
|
|
August 28, 2024 |
By: |
/s/ Glenn C. David |
|
|
Glenn C. David |
|
|
Chief Financial Officer |
|
|
|
August 28, 2024 |
By: |
/s/ Daniel M. Bendheim |
|
|
Daniel M. Bendheim |
|
|
Director and Executive Vice President, |
|
|
Corporate Strategy |
|
|
|
August 28, 2024 |
By: |
/s/ Jonathan Bendheim |
|
|
Jonathan Bendheim |
|
|
Director and President, MACIE Region and |
|
|
General Manager of Israel Operations |
|
|
|
August 28, 2024 |
By: |
/s/ Alejandro Bernal |
|
|
Alejandro Bernal |
|
|
Director |
|
|
|
August 28, 2024 |
By: |
/s/ E. Thomas Corcoran |
|
|
E. Thomas Corcoran |
|
|
Director |
|
|
|
August 28, 2024 |
By: |
/s/ Sam Gejdenson |
|
|
Sam Gejdenson |
|
|
Director |
|
|
|
August 28, 2024 |
By: |
/s/ Mary Lou Malanoski |
|
|
Mary Lou Malanoski |
|
|
Director |
|
|
|
August 28, 2024 |
By: |
/s/ Carol A. Wrenn |
|
|
Carol A. Wrenn |
|
|
Director |
123
Exhibit 2.1
EXECUTION VERSION
PURCHASE AND SALE AGREEMENT
BY AND AMONG
PHIBRO ANIMAL HEALTH CORPORATION,
PHIBRO ANIMAL HEALTH S.A.
AND
ZOETIS INC.
Dated as of April 28, 2024
TABLE OF CONTENTS
|
Page |
|
ARTICLE I DEFINITIONS |
1 |
|
Section 1.1 |
Definitions |
1 |
Section 1.2 |
Other Defined Terms |
15 |
|
|
|
ARTICLE II PURCHASE AND SALE; CLOSING |
19 |
|
Section 2.1 |
Purchase and Sale |
19 |
Section 2.2 |
Purchase Price |
19 |
Section 2.3 |
Closing Date |
19 |
Section 2.4 |
Purchased Assets |
19 |
Section 2.5 |
Excluded Assets |
22 |
Section 2.6 |
Assumed Liabilities |
24 |
Section 2.7 |
Retained Liabilities |
26 |
Section 2.8 |
Closing Deliveries |
27 |
Section 2.9 |
Adjustment to Base Purchase Price |
29 |
Section 2.10 |
Purchase Price Allocation |
32 |
Section 2.11 |
Transferred Marketing Authorizations |
34 |
Section 2.12 |
Bulk Sales |
36 |
Section 2.13 |
Withholding |
36 |
|
|
|
ARTICLE III REPRESENTATIONS AND WARRANTIES OF SELLER |
36 |
|
Section 3.1 |
Organization and Standing |
36 |
Section 3.2 |
Purchased Entities; Capital Structure |
36 |
Section 3.3 |
Authority; Enforceability |
37 |
Section 3.4 |
No Conflicts; Consents |
38 |
Section 3.5 |
Governmental Authorizations |
38 |
Section 3.6 |
Proceedings |
39 |
Section 3.7 |
Financial Statements; Absence of Undisclosed Liabilities |
39 |
Section 3.8 |
Absence of Changes or Events |
40 |
Section 3.9 |
Title to Assets; Sufficiency of Assets |
40 |
Section 3.10 |
Intellectual Property |
41 |
Section 3.11 |
Information Technology; Data Protection |
42 |
Section 3.12 |
Real Property |
43 |
Section 3.13 |
Contracts |
44 |
Section 3.14 |
Significant Customers; Significant Suppliers |
46 |
Section 3.15 |
Transferred Marketing Authorizations; Recalls |
46 |
Section 3.16 |
Compliance with Applicable Laws; Permits |
47 |
Section 3.17 |
Environmental Matters |
48 |
Section 3.18 |
Taxes |
49 |
Section 3.19 |
Benefit Plans |
50 |
Section 3.20 |
Labor Matters |
52 |
Section 3.21 |
Intercompany Arrangements |
53 |
Section 3.22 |
Insurance |
53 |
Section 3.23 |
Inventory; Accounts Payable; Accounts Receivable |
53 |
Section 3.24 |
Brokers |
54 |
-i-
Section 3.25 |
International Trade |
54 |
Section 3.26 |
No Other Representations or Warranties |
54 |
|
|
|
ARTICLE IV REPRESENTATIONS AND WARRANTIES OF PURCHASERS |
55 |
|
Section 4.1 |
Organization and Standing |
55 |
Section 4.2 |
Authority; Enforceability |
55 |
Section 4.3 |
No Conflicts; Consents |
56 |
Section 4.4 |
Governmental Authorizations |
56 |
Section 4.5 |
[Reserved] |
56 |
Section 4.6 |
Financing |
56 |
Section 4.7 |
Proceedings |
58 |
Section 4.8 |
Brokers |
58 |
Section 4.9 |
Solvency |
58 |
Section 4.10 |
Securities Laws |
59 |
Section 4.11 |
Acknowledgment of No Other Representations or Warranties |
59 |
|
|
|
ARTICLE V COVENANTS |
60 |
|
Section 5.1 |
Efforts |
60 |
Section 5.2 |
Covenants Relating to Conduct of Business |
63 |
Section 5.3 |
Confidentiality |
67 |
Section 5.4 |
Access to Information |
69 |
Section 5.5 |
Publicity |
70 |
Section 5.6 |
Intercompany Accounts and Intercompany Arrangements |
71 |
Section 5.7 |
Financing |
72 |
Section 5.8 |
Financing Cooperation |
74 |
Section 5.9 |
Financial Obligations |
76 |
Section 5.10 |
Trademark Matters |
77 |
Section 5.11 |
Global Separation Sublicenses |
79 |
Section 5.12 |
Background IP License |
79 |
Section 5.13 |
Insurance |
80 |
Section 5.14 |
Litigation Support |
80 |
Section 5.15 |
Third-Party Consents |
81 |
Section 5.16 |
Non-Solicitation of Employees; Non-Competition |
81 |
Section 5.17 |
Payments; Misallocated Assets |
84 |
Section 5.18 |
R&W Insurance Policy |
85 |
Section 5.19 |
Delivery of Financial Statements |
85 |
Section 5.20 |
Exclusivity |
87 |
Section 5.21 |
Certain Matters |
87 |
Section 5.22 |
Resignations |
87 |
Section 5.23 |
Restricted Cash |
87 |
Section 5.24 |
Additional Business Products. |
88 |
Section 5.25 |
Additional Business Product Marks |
89 |
Section 5.26 |
Expired Marks |
89 |
Section 5.27 |
Omitted Marks |
90 |
Section 5.28 |
Intellectual Property Chain of Title Corrections |
90 |
Section 5.29 |
Assignment of Business Registered IP |
90 |
Section 5.30 |
Transition Services |
91 |
-ii-
ARTICLE VI EMPLOYEE MATTERS |
91 |
|
Section 6.1 |
Transfer of Business Employees |
91 |
Section 6.2 |
Compensation and Employee Benefits |
92 |
Section 6.3 |
Seller Benefit Plans |
94 |
Section 6.4 |
Purchased Entity Benefit Plans |
95 |
Section 6.5 |
U.S. Defined Contribution Plans |
95 |
Section 6.6 |
Short-Term Incentive Compensation |
96 |
Section 6.7 |
Labor Matters |
96 |
Section 6.8 |
Workers’ Compensation |
97 |
Section 6.9 |
Immigration Compliance |
97 |
Section 6.10 |
Assignment and Transfer of Business Contingent Worker |
97 |
Section 6.11 |
Communications |
98 |
Section 6.12 |
Employee Restrictive Covenants |
98 |
Section 6.13 |
Third-Party Beneficiary Rights |
98 |
|
|
|
ARTICLE VII CERTAIN TAX MATTERS |
98 |
|
Section 7.1 |
Cooperation and Exchange of Information |
98 |
Section 7.2 |
Tax Sharing Agreements |
99 |
Section 7.3 |
Tax Treatment of Payments |
99 |
Section 7.4 |
Elections |
99 |
Section 7.5 |
Transfer Taxes and VAT/GST |
99 |
Section 7.6 |
Italian VAT Receivable |
100 |
Section 7.7 |
Straddle Period |
100 |
Section 7.8 |
Certain Tax Refunds |
100 |
|
|
|
ARTICLE VIII CONDITIONS PRECEDENT |
101 |
|
Section 8.1 |
Conditions to Each Party’s Obligations to Close |
101 |
Section 8.2 |
Conditions to Obligations of Purchasers to Close |
101 |
Section 8.3 |
Conditions to Obligations of Seller to Close |
102 |
|
|
|
ARTICLE IX TERMINATION; EFFECT OF TERMINATION |
103 |
|
Section 9.1 |
Termination |
103 |
Section 9.2 |
Effect of Termination |
104 |
Section 9.3 |
Notice of Termination |
104 |
|
|
|
ARTICLE X INDEMNIFICATION |
104 |
|
Section 10.1 |
Survival |
104 |
Section 10.2 |
Indemnification by Seller |
105 |
Section 10.3 |
Indemnification by Purchasers |
105 |
Section 10.4 |
Procedures |
106 |
Section 10.5 |
Exclusive Remedy and Release |
107 |
Section 10.6 |
Additional Indemnification Provisions |
108 |
Section 10.7 |
Mitigation |
108 |
|
|
|
ARTICLE XI GENERAL PROVISIONS |
109 |
|
Section 11.1 |
Entire Agreement |
109 |
Section 11.2 |
Disclosure Schedules |
109 |
Section 11.3 |
Assignment |
109 |
Section 11.4 |
Amendments and Waivers |
110 |
-iii-
Section 11.5 |
No Third-Party Beneficiaries |
110 |
Section 11.6 |
Notices |
110 |
Section 11.7 |
Specific Performance |
111 |
Section 11.8 |
Governing Law and Jurisdiction |
111 |
Section 11.9 |
Waiver of Jury Trial |
112 |
Section 11.10 |
Severability |
112 |
Section 11.11 |
Counterparts |
112 |
Section 11.12 |
Expenses |
113 |
Section 11.13 |
Interpretation; Absence of Presumption |
113 |
Section 11.14 |
Waiver of Conflicts Regarding Representation; Nonassertion of Attorney-Client Privilege |
114 |
Section 11.15 |
Financing Provisions |
115 |
EXHIBITS
Exhibit A |
Form of Transition Services Agreement |
Exhibit B |
Form of Assignment Agreement and Bill of Sale |
Exhibit C |
Allocation Schedule |
-iv-
PURCHASE AND SALE AGREEMENT
This PURCHASE AND SALE AGREEMENT, dated as of April 28, 2024 (this “Agreement”), is by and among Zoetis Inc., a Delaware corporation (“Seller”), Phibro Animal Health Corporation, a Delaware corporation (“Purchaser 1”), and Phibro Animal Health S.A., a Belgian company (“Purchaser 2”, and each of Purchaser 1 and Purchaser 2, a “Purchaser”, and collectively, the “Purchasers”, and together with Seller, the “Parties” and each, a “Party”).
WHEREAS, Seller and certain of its Subsidiaries are engaged in, among other things, the Business; and
WHEREAS, on the terms and subject to the conditions set forth herein, the Seller Entities shall sell, assign, transfer and convey to the Purchasers, and the Purchasers shall purchase, acquire and accept from the Seller Entities, all of their right, title and interest in and to the Purchased Assets, and the Purchasers shall assume the Assumed Liabilities (the “Transaction”).
NOW, THEREFORE, in consideration of the representations, warranties, covenants and agreements contained in this Agreement, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, on the terms and subject to the conditions of this Agreement, the Parties hereby agree as follows:
ARTICLE I
DEFINITIONS
Section 1.1Definitions. As used herein, the following terms have the meanings set forth below:
“Acquisition Proposal” means, other than the transactions contemplated hereby, any inquiry, proposal or offer for (a) any direct or indirect sale, lease, transfer or other disposition, in one or a series of related transactions, of all or more than 50% of the Purchased Assets or the Business, taken as a whole, by any Person or group of related Persons, or (b) any acquisition by any Person or group of related Persons, in one or a series of related transactions, of more than 50% of the total equity securities (determined by either total voting power or fair market value) of any of the Purchased Entities.
“Additional Business Products” means collectively or individually the medicated feed products in the U.S. or any foreign jurisdiction (including water soluble and solid feed additives) that (i) are owned by Seller or its Subsidiaries (including the Purchased Entities), (ii) were maintained, manufactured, distributed or sold by Seller or its Subsidiaries (including the Purchased Entities) within the 5 year period prior to the Closing, (iii) have the same active pharmaceutical ingredients as the Business Products and (iv) were distributed or sold under the same trademark as the Business Products; provided, that Additional Business Products shall in no event include any Business Products or ALBAC®.
“Affiliate” means, with respect to any Person, any other Person that directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with, such Person.
For purposes of this definition, “control” (including, with correlative meanings, the terms “controlled by” and “under common control with”), as used with respect to any Person means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of such Person, whether through the ownership of voting securities, by Contract or otherwise; provided that, from and after the Closing, (a) none of the Purchased Entities shall be considered an Affiliate of Seller or any of its Affiliates, and (b) none of Seller or its Affiliates shall be considered an Affiliate of any Purchased Entity.
“Ancillary Agreements” means the Transition Services Agreement, the Assignment Agreement and Bill of Sale, the Distribution Agreements and each other agreement, instrument or certificate entered into in connection with the transactions contemplated hereby, including all exhibits, schedules, annexes, supplements and amendments to any of the foregoing.
“Business” means, subject to the following sentence, the business, operations, products, services and activities related to manufacturing, distribution, marketing and selling of the Business Products, or otherwise exclusively relating to the medicated feed additive business (including liquid and solid feed additives) as carried on by Seller (directly or indirectly through its Subsidiaries, including the business, operations, products, services and activities of the Purchased Entities), in each case as conducted immediately prior to the Closing by Seller and its Subsidiaries. Seller and each Purchaser agree that the “Business” shall not include (a) the businesses, operations, products, services and activities set forth on Section 1.1(a) of the Seller Disclosure Schedules or (b) any other businesses, operations, products, services or activities of Seller or its Subsidiaries (clauses (a) and (b) collectively, the “Retained Business”).
“Business Contingent Worker” means each individual who is an independent contractor, consultant, any other non-employee worker or third-party agency worker who is actively providing services to a member of the Seller Group or a Purchased Entity immediately prior to the Closing and set forth on Section 1.1(b) of the Seller Disclosure Schedules, each of whom primarily dedicates his or her working time to the Business, which schedule may be updated by Seller from time to time prior to the Closing, with the prior written approval of the Purchasers if such approval is otherwise required under Section 5.2(b).
“Business Day” means any day, other than a Saturday, Sunday or any other day on which commercial banks are required or authorized to be closed in New York, New York.
“Business Employee” means each employee of any member of the Seller Group or a Purchased Entity who is either (a) set forth on Section 3.20(a)(i) of the Seller Disclosure Schedules, each of whom primarily dedicates his or her working time to the Business or (b) set forth on Section 3.20(a)(ii) of the Seller Disclosure Schedules, either of which schedule in clauses (a) and (b) may be updated by Seller from time to time prior to the Closing with the prior written approval of the Purchasers if such approval is otherwise required under Section 5.2(b), and in each case, may include, for the avoidance of doubt, an individual who is not actively working as of the Closing as a result of an illness, injury or leave of absence approved by the applicable human resources department or otherwise taken in accordance with applicable Law (including long-term disability).
-2-
“Business Material Adverse Effect” means any event, change, state of facts, occurrence, circumstance, development or effect that has had or would reasonably be expected to have a material adverse effect on the business, financial condition or results of operations of the Business taken as a whole; provided, however, that no such event, change, state of facts, occurrence, circumstance, development or effect resulting or arising from or in connection with any of the following matters shall be deemed, either alone or in combination, to constitute or contribute to, or be taken into account in determining whether there has been, a Business Material Adverse Effect: (a) general conditions or trends in the industries in which the Business operates; (b) general political, economic, business, monetary, financial or capital or credit market conditions (including interest rates, exchange rates, tariffs, trade wars and credit markets), including with respect to government spending, budgets and related matters; (c) any (i) geopolitical conditions, military conflict or actions, outbreak of hostilities, acts of war (whether or not declared), acts of foreign or domestic terrorism, rebellion or insurrection, acts of espionage or (ii) acts of cyberterrorism or any escalation or general worsening of any of the foregoing specified in clauses (i) and (ii), or any action taken by any Governmental Entity in response to any of the foregoing specified in clauses (i) and (ii); (d) earthquakes, hurricanes, tsunamis, typhoons, lightning, hail storms, blizzards, tornadoes, droughts, floods, cyclones, arctic frosts, mudslides, wildfires, epidemics, pandemics (including any escalation, general worsening, easing, or other change in the state of Covid-19), disease outbreaks or acts of God, or any other conditions resulting from natural or manmade disasters or weather developments; (e) the failure of the financial or operating performance of the Seller Group, the Purchased Entities or the Business to meet internal or external (including Purchasers or analyst) projections, forecasts or budgets for any period (provided that this clause (e) shall not be construed as implying that Seller is making any representation or warranty herein with respect to any such projections, forecasts or budgets and no such representations or warranties are being made; provided, further, that the exception in this clause (e) shall not prevent or otherwise affect a determination that any underlying change that is the cause of such failure has resulted in a Business Material Adverse Effect); (f) any matter set forth on Section 1.1(c) of the Seller Disclosure Schedules; (g) any action taken or omitted to be taken by Seller or its Subsidiaries solely to the extent such action or omission was at Purchasers’ express written request in accordance with and subject to the terms of this Agreement; (h) compliance with the covenants and other express terms of this Agreement (including the impact on or any loss of Business Employees, customers, suppliers, relationships with Governmental Entities or other business relationships resulting from any of the foregoing); (i) the execution, announcement, pendency or consummation of this Agreement, the Transaction or the other transactions contemplated hereby solely as a result of the identity of either Purchaser or their Affiliates (including the impact on or any loss of customers, suppliers, relationships with Governmental Entities or other business relationships resulting from any of the foregoing) (provided that this clause (i) shall not apply with respect to the representations and warranties in Section 3.4 or Section 3.5 or any condition to any Party’s obligation to consummate the Transaction relating to such representations and warranties); (j) changes in any Law (including any proposed Law) or GAAP or other applicable accounting principles or standard or any interpretations of any of the foregoing, in each case, following the date hereof; or (k) any Excluded Assets or Retained Liabilities; provided that any adverse events, changes, state of facts, occurrences, circumstances, developments or effects resulting from the matters described in clauses (a), (b), (c) and (d) may be taken into account in determining whether there has been a Business Material Adverse Effect to the extent, and only to the extent, that they have a materially disproportionate adverse effect on the Purchased Entities and the Business, taken as a whole, relative to businesses that operate in the industries or geographic areas in which the Business operates (in which case only such incremental materially disproportionate adverse effect may be taken into account in determining whether there has been a Business Material Adverse Effect).
-3-
“Business Products” means, collectively or individually, the products (including all raw materials and ingredients) that are or were manufactured, distributed or sold by Seller or its Subsidiaries (including the Purchased Entities) set forth on Section 1.1(d) of the Seller Disclosure Schedules.
“Business Technology Deliverables” means Technology that (a) are exclusively related to, exclusively used in or exclusively held for use in the Business, (b) are located at the Transferred Real Property and used in, and necessary for, the operation of the Business as of the Closing, or (c) constitute the know-how of the Business Employees to the extent related to the Business; provided that Business Technology Deliverables does not include Information Technology and any books and records.
“Cash” means an amount equal to the sum of, without duplication, (a) all cash and cash equivalents, bank and other depositary accounts and safe deposit boxes, demand accounts, certificates of deposit, time deposits, marketable securities, short-term deposits, securities and brokerage accounts (including, in each case, any accrued interest thereon), in each case of the Purchased Entities, plus (b) deposits in transfer, wires, drafts and checks in transit issued to or received by the Purchased Entities but not yet posted (net of outstanding but uncleared wires, drafts and checks written or issued by the Purchased Entities), in each case, such amounts calculated in a manner consistent with the Transaction Accounting Principles.
“Chinese Purchased Entity” means Zoetis Suzhou Manufacturing Co., Ltd.
“Closing Cash” means the Cash of the Purchased Entities as of the Closing; provided, that “Closing Cash ” shall not include any Cash held by the Chinese Purchased Entity as of the Closing in excess of the Chinese Operating Cash Amount (such excess amount, the “Excess Restricted Cash”).
“Closing Gross Profit” means the Gross Profit of the Business for the fiscal year ended December 31, 2023, derived from the Audited Financial Statements for the fiscal year ended December 31, 2023, delivered by Seller pursuant to Section 5.19(a).
“Closing Indebtedness” means the Indebtedness of the Purchased Entities as of the Closing.
“Closing Purchase Price” means (a) the Base Purchase Price, plus (b) the Estimated Closing Cash, plus (c) the Estimated WC Adjustment Amount (which may be a positive or negative number, or zero), minus (d) the Estimated Closing Indebtedness, plus (e) the Estimated Gross Profit Adjustment Amount (which may be a negative number or zero, but not a positive number).
“Closing Working Capital” means the Working Capital of the Business as of the Closing.
“Code” means the U.S. Internal Revenue Code of 1986, as amended.
-4-
“Collective Bargaining Agreement” means any collective bargaining agreement or other Contract with any labor organization, union, or associations, works council or other agency or employee representative body.
“Combined Tax Return” means any combined, consolidated, affiliated or unitary Tax Return that includes any member of the Seller Group, on the one hand, and any of the Purchased Entities, on the other hand.
“Completed Phase I Reports” means Phase I Environmental Site Assessments satisfying the All Appropriate Inquiry standard as defined in 40 C.F.R. § 312.20 for the following properties: (a) 601 Beam Street, Salisbury, Maryland; (b) 1020 Northwest 10th Street, Eagle Grove, Iowa; and (c) 400 State Street, Chicago Heights, Illinois.
“Contamination” means the emission, discharge or release of any Hazardous Material to, on, onto or into the environment.
“Contract” means any written or oral legally binding contract, agreement, sales order, purchase order, tender, lease, license, commitment, loan or credit agreement, indenture or agreement, or any other legally binding contractual obligation, commitment or understanding, other than any Seller Benefit Plan, any Collective Bargaining Agreement or any Permit.
“Covered Losses” means losses, Liabilities, claims, fines, deficiencies, damages, payments (including those arising out of any settlement or Judgment relating to any Proceeding), Taxes, penalties and reasonable attorneys’ and accountants’ fees and disbursements, in each case that are due and payable; provided that Covered Losses shall not include any consequential, special or punitive damages except (a) to the extent actually awarded against an Indemnified Party in connection with a Third-Party Claim or (b) solely in respect of consequential or special damages, to the extent reasonably foreseeable.
“Covid-19” means SARS-CoV-2 or Covid-19, and any evolutions thereof or related or associated epidemics, pandemics or disease outbreaks.
“Data Protection Laws” means in each case to the extent relating to the protection, use, transmission, or processing of Personal Data, data privacy or cybersecurity in any relevant jurisdiction: (a) all applicable Laws; and (b) Contracts to which the Purchased Entities (or with respect to the Business, Seller and its Subsidiaries) have entered into or are otherwise bound.
“Environmental Laws” means, collectively, any and all Laws and Judgments relating to Contamination, Hazardous Materials, public or worker health or safety, pollution or protection of the environment.
“ERISA” means the Employee Retirement Income Security Act of 1974, as amended, and the regulations promulgated thereunder.
“ERISA Affiliate” means, with respect to any entity, trade or business, any other entity, trade or business that is, or was at any relevant time, a member of a group described in Section 414(b), (c), (m) or (o) of the Code or Section 4001(b)(1) of ERISA that includes or included the first entity, trade or business, or that is, or at any relevant time, a member of the same “controlled group” as the first entity, trade or business pursuant to Section 4001(a)(14) of ERISA.
-5-
“Excluded Information” means any (a) description of all or any portion of the Financing, including any “description of notes”, “plan of distribution” and information customarily provided by investment banks or their counsel or advisors in the preparation of an offering memorandum for private placements of non-convertible bonds pursuant to Rule 144A under the Securities Act, (b) risk factors relating to, or any description of, all or any component of the financing contemplated thereby, (c) historical financial statements or other information required by Rule 3-05, Rule 3-09, Rule 3-10, Rule 3-16, Rule 13-01 or Rule 13-02 of Regulation S-X under the Securities Act; any compensation discussion and analysis or other information required by Item 10, Item 402 and Item 601 of Regulation S-K under the Securities Act or XBRL exhibits; or any information regarding executive compensation or related persons related to SEC Release Nos. 33-8732A, 34-54302A and IC-27444A, (d) other information customarily excluded from an offering memorandum for private placements of non-convertible high-yield bonds pursuant to Rule 144A under the Securities Act in a “Rule 144A-for-life” offering, (e) financial statements or other financial data (including selected financial data) for any period earlier than the year ended December 31, 2022, (f) financial information that Seller or its Subsidiaries do not maintain in the ordinary course of business, (g) information not reasonably available to Seller or its Subsidiaries under their respective current reporting systems, or (h) (x) footnote disclosures or (y) pro forma financial information or pro forma financial statements (other than, in the case of clauses (c), (f), (g) and (h)(x), to the extent specifically enumerated in and required by Section 5.19).
“Excluded Taxes” means any Taxes (a) imposed with respect to the Excluded Assets, the Retained Liabilities or any Combined Tax Return or (b) imposed on or payable by any member of the Seller Group (other than any Transfer Taxes).
“Ex-Im Laws” means all U.S. and non-U.S. Laws relating to export, reexport, transfer, and import controls, including the Export Administration Regulations, the customs and import Laws administered by U.S. Customs and Border Protection, and the EU Dual Use Regulation.
“FDA” means the United States Food and Drug Administration.
“FDA Laws” means all Laws applicable to the operation of the Business related to the research, investigation, development, production, marketing, distribution, storage, shipping, transport, advertising, labeling, promotion, sale, export, import, use handling and control, safety, efficacy, reliability or manufacturing of animal drugs and medicated feeds including, without limitation the FDCA and the rules and regulations promulgated and enforced by FDA thereunder and all comparable state, federal or foreign Laws relating to any of the foregoing.
“FDCA” means the U.S. Federal Food, Drug, and Cosmetic Act.
“Filings” means any registrations, applications, declarations, reports, submissions or other filings with, or any notices to, any Person (including any third party or Governmental Entity).
-6-
“Financing Information” means the Business Financial Information; provided, that notwithstanding anything to the contrary in this definition or otherwise, nothing herein shall require Seller or its Subsidiaries to provide (or be deemed to require Seller or its Subsidiaries to prepare) any reviewed or audited financial statements or other financial information other than as expressly set forth in Section 3.7(a) or Section 5.19, nor any Excluded Information whatsoever. If Seller shall in good faith believe that the Financing Information has been delivered to the Purchasers, Seller may deliver to the Purchasers a written notice to that effect (stating when it believes the delivery of the Financing Information to the Purchasers was completed), in which case Seller shall be deemed to have complied with such obligation to furnish the Financing Information and the Purchasers shall be deemed to have received the Financing Information, unless the Purchasers in good faith reasonably believes that Seller has not completed delivery of the Financing Information and not later than 5:00 p.m. (New York City time) two (2) Business Days after the delivery of such notice by Seller, delivers a written notice to Seller to that effect (stating with specificity which such Financing Information Seller has not delivered); provided, that notwithstanding the foregoing, the delivery of the Financing Information shall be satisfied at any time which (and so long as) the Purchasers shall have actually received the Financing Information, regardless of whether or when any such notice is delivered by Seller. Seller’s or its Subsidiaries’ filing with the SEC pursuant to the Securities Act, the Exchange Act and the rules and regulations of the SEC promulgated thereunder of any required audited financial statements with respect to it that is publicly available on Form 10-K or required unaudited financial statements with respect to it that is publicly available on Form 10-Q, in each case, will satisfy the requirements of this definition.
“Financing Parties” means each debt provider (including each agent and arranger) that commits to provide a Purchaser or any of its Subsidiaries Financing pursuant to the Commitment Letter (the “Financing Entities”), and their respective Representatives; provided, that neither Purchaser nor any Affiliate thereof shall be a Financing Party.
“Former Employee” means any individual whose employment with the Seller Group or a Purchased Entity (to the extent such individual employed by a Purchased Entity was not primarily dedicated to the Business) is terminated prior to the Closing.
“GAAP” means U.S. generally accepted accounting principles, as in effect from time to time.
“Governmental Entity” means any national, state, provincial, local, regional, supranational or foreign government or any court of competent jurisdiction, administrative agency or commission or other national, state, provincial, local, supranational or foreign governmental authority or instrumentality, or any arbitral body (public or private), or any other entity or body with administrative, executive, judicial, regulatory, taxing or similar authority of any nature.
“Gross Profit” means an amount equal to (a) revenue of the Business minus (b) cost of sales of the Business, calculated in a manner consistent with the illustrative example set forth in the Sample Closing Statement.
-7-
“Gross Profit Adjustment Amount” means (a) the Closing Gross Profit minus (b) the Target Gross Profit; provided that the Gross Profit Adjustment Amount shall be deemed zero if the Closing Gross Profit is greater than or equal to $109,678,500 (the “Lower Bound”); provided, further, that if the Closing Gross Profit is less than the Lower Bound, the Gross Profit Adjustment Amount shall equal (i) the incremental amount by which the Closing Gross Profit is less than the Lower Bound, (ii) multiplied by two (2). For illustrative purposes, if the Closing Gross Profit is equal to: (y) $115,000,000 or $109,678,500, the Gross Profit Adjustment Amount shall be zero; and (z) $108,000,000, the Gross Profit Adjustment Amount shall be -$3,357,000.
“Hazardous Material” means any substance, pollutant, contaminant, material or waste for which Liability or standards of conduct may be imposed under Environmental Law or that is otherwise classified in any applicable Environmental Law as “hazardous,” “toxic,” “dangerous,” a “pollutant,” a “contaminant” or words of similar meaning, including asbestos, asbestos-containing materials, polychlorinated biphenyls, petroleum or petroleum products, radioactive materials, per- or polyfluoroalkyl substances, lead, mold, noise, odor and radon gas.
“HSR Act” means the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, and the rules and regulations thereunder.
“Income Tax” means any Tax imposed on or measured by reference (in whole or in part) to gross or net income, profits, or capital gains (however denominated).
“Indebtedness” means, as of any time, an amount equal to the sum of, without duplication, the following obligations of a Person, in each case calculated in a manner consistent with the Transaction Accounting Principles: (a) the outstanding indebtedness for borrowed money (other than trade payables arising in the ordinary course of business); (b) all obligations evidenced by bonds (other than letters of credit, surety bonds or bank guarantees), debentures or notes; (c) all outstanding obligations in respect of letters of credit, surety bonds or bank guarantees, in each case solely to the extent funds have been drawn and are payable thereunder; (d) all guarantees by such Person of indebtedness of other Persons for borrowed money or evidenced by bonds, debentures, notes or similar instruments, in each case solely to the extent such guarantees are actually called upon and funds are payable thereunder; (e) the Separate Company Accrued Tax Amount; (f) [reserved]; (g) leases recorded as capital or finance leases under GAAP or recorded as such in the Business Financial Information (other than lease obligations classified as operating leases in the Business Financial Information); and (h) with respect to the foregoing clauses (a) through (c), all accrued but unpaid interest thereon and any termination fees, prepayment penalties, “breakage” cost or reimbursements payments associated with the repayments of such Indebtedness, in each case, solely to the extent due and payable on the Closing or as a result of the Transaction; provided, however, that in no event shall Indebtedness include (i) any Retained Liabilities, (ii) any Indebtedness to the extent owing from any of the Purchased Entities to any of the other Purchased Entities to the extent reconciled or eliminated in the consolidated financial statements of the Business at or prior to Closing or (iii) any Liabilities to the extent repaid or extinguished in connection with the Closing (including any Liabilities in respect of intercompany balances or accounts to be settled pursuant to Section 5.6).
“Information Technology” means any computer systems (including computers, screens, servers, workstations, routers, hubs, switches, networks, data communications lines and hardware), all software, data and databases embedded therein, and all peripheral equipment, and telecommunications systems, in each case, that are used by or for, or otherwise owned by the Seller Group or a Purchased Entity as of the Closing.
-8-
“Intellectual Property Rights” means any and all intellectual property, common law or statutory rights anywhere in the world arising under or associated with the following, whether registered or unregistered: (a) patents, patent applications, statutory invention registrations, registered designs, utility models, and similar or equivalent rights in inventions and designs and extensions, divisionals, continuations, continuations-in-part, reexaminations, and reissues thereof (“Patents”), (b) trademarks, service marks, trade dress, trade names, slogans, logos, corporate names and other designations of origin (including all goodwill associated with the foregoing) (“Marks”), (c) rights in domain names and uniform resource locators, social media identifiers and accounts, and other names and locators associated with Internet addresses and sites, (d) copyrights, copyright registrations and applications, and any other equivalent rights in works of authorship (including rights in software as a work of authorship) and any other related rights of authors (“Copyrights”), (e) trade secrets, industrial secret rights and rights in know-how, or confidential information (including in formulas, inventions, protocols, processes, methods, techniques, research and development information), in each case, that derive independent economic value from not being generally known (“Trade Secrets”), (f) other similar or equivalent intellectual property rights, and (g) applications and registrations of any of the foregoing clauses (a) through (f).
“International Business Employee” means each Business Employee who provides services primarily outside the United States.
“Italian Purchased Entity” means Zoetis Medolla Manufacturing S.r.l., an Italian società a responsabilità limitata.
“Italian VAT Receivable” means the value added tax receivable reflected on the books and records of the Italian Purchased Entity as of the date hereof.
“Judgment” means any judgment, injunction, writ, order, ruling, directive or decree of any Governmental Entity.
“Knowledge” means, with respect to Seller, the actual knowledge of any Person listed in Section 1.1(e) of the Seller Disclosure Schedules, and, with respect to the Purchasers, the actual knowledge of any Person listed in Section 1.1(a) of the Purchaser Disclosure Schedules.
“Law” means FDA Laws and any national, state, provincial, local, supranational or foreign law, statute, code, act, common law, Judgment, ordinance, rule, regulation or treaty (including any Tax treaty) or other requirements of any kind, in each case enacted, issued, enforced, entered or promulgated by a Governmental Entity.
“Liabilities” means all debts, liabilities, guarantees, assurances, commitments and obligations of any kind, whether fixed, contingent or absolute, asserted or unasserted, matured or unmatured, liquidated or unliquidated, accrued or not accrued, known or unknown, due or to become due, whenever or however arising (including whether arising out of any Contract or tort based on negligence or strict liability).
-9-
“Lien” means any mortgage, lien, pledge, right of first offer or first refusal, security interest, charge, easement, or similar encumbrance of any kind.
“Multiemployer Plan” means any “multiemployer plan” within the meaning of Section 4001(a)(3) of ERISA for the benefit of U.S. Business Employees.
“Non-Commercialized Business Product” means collectively or individually the antibiotic and coccidiostat medicated feed products in the U.S. or any foreign jurisdiction (including water soluble and solid feed additives) that (i) are owned by Seller or its Subsidiaries (including the Purchased Entities) and (ii) were maintained, manufactured, distributed or sold by Seller or its Subsidiaries (including the Purchased Entities) prior to the Closing.
“Permits” means permits, approvals, authorizations, consents, licenses, registrations, filings or certificates issued or granted by any Governmental Entity.
“Permitted Liens” means the following Liens: (a) Liens for Taxes, assessments or other governmental charges or levies that are not yet due or payable or that are being contested by appropriate Proceedings for which an adequate reserve has been established and reflected in the Business Financial Information; (b) statutory Liens of landlords and Liens of carriers, warehousemen, mechanics, materialmen, workmen, repairmen and other Liens imposed by Law in the ordinary course of business; (c) Liens incurred or deposits made in the ordinary course of business in connection with workers’ compensation, unemployment insurance or other types of social security; (d) Liens incurred in the ordinary course of business securing Liabilities that do not materially impair the use of or materially detract from the value of a Purchased Asset; (e) with respect to real property, (i) defects or imperfections of title, (ii) easements, declarations, covenants, rights-of-way, restrictions and other charges, instruments or encumbrances of record affecting title, (iii) zoning ordinances, variances, conditional use permits and similar regulations, permits, approvals and conditions regulating the use or occupancy of such real property or the activities conducted thereon which are imposed by any Governmental Entity having jurisdiction over such real property or (iv) Liens not created by Seller or any of its Subsidiaries that affect the underlying fee interest of any leased real property, including master leases or ground leases; provided, however, that with respect to this clause (e), any such item does not materially interfere with the ordinary conduct of the Business or materially impair the continued use and operation of such real property for the purpose for which it is used as of immediately prior to the Closing; (f) Liens set forth on Section 1.1(f) the Seller Disclosure Schedules; (g) with respect to any securities of any Person, any Liens created under securities Laws or expressly set forth in the governing documents of such Person; (h) Liens in the Purchasers’ favor deemed to be created by any of the Transaction Documents; or (i) non-exclusive licenses of Transferred Intellectual Property granted in the ordinary course of business.
“Person” means any individual, firm, corporation, partnership, limited liability company, trust, Governmental Entity or other entity.
“Personal Data” means any information about an identifiable natural person that alone or in combination with other information identifies, or could be used to identify, directly or indirectly, a natural person or household, and includes information that is defined as “personal data,” “personally identifiable information,” “individually identifiable health information,”
-10-
“protected health information” or “personal information” or a similar term under any applicable Law.
“Pre-Closing Tax Period” means any taxable period ending on or before the Closing Date and the portion through the end of the Closing Date for any Straddle Period.
“Proceeding” means any judicial, administrative or arbitral action, suit, litigation, charge, complaint, audit, inquiry, demand, investigation, claim, petition, or proceeding by or before any Governmental Entity or other body of competent jurisdiction.
“Purchased Entity Benefit Plan” means each Seller Benefit Plan that is (a) solely sponsored or maintained by a Purchased Entity, (b) maintained or contributed to by Seller or any of its Subsidiaries solely for the benefit of the Business Employees or (c) identified as a Purchased Entity Benefit Plan on Section 3.19(a) of the Seller Disclosure Schedules.
“Purchaser Disclosure Schedules” means those certain Purchaser Disclosure Schedules, dated as of the date of this Agreement, provided by the Purchasers to Seller.
“Purchaser Material Adverse Effect” means any event, change or effect that, individually or in the aggregate, materially impairs, hinders or delays the ability of the Purchasers and their respective Subsidiaries to perform their respective obligations under this Agreement and the other Transaction Documents or to consummate the transactions contemplated hereby and thereby.
“Registered IP” means all U.S., international or foreign (a) issued Patents and Patent applications, (b) registered Marks and applications to register Marks, (c) registered Copyrights and applications for Copyright registration and (d) registrations for domain names.
“Regulatory Approvals” means all approvals, rulings, consents, exemptions, clearances, written confirmations of no intention to initiate a Proceeding and waiting period terminations or expirations from Governmental Entities that are required under applicable Law (including pursuant to any Regulatory Law or FDA Law) (i) to permit the consummation of the Transaction and the other transactions contemplated by this Agreement or (ii) for the Purchasers to manufacture, sell or distribute the Business Products.
“Regulatory Laws” means statutes, rules, regulations, orders, decrees, administrative and judicial doctrines and other Laws of any jurisdiction that are designed or intended to (a) prohibit, restrict or regulate actions that may have the purpose or effect of creating a monopoly, lessening competition or restraining trade, or (b) prohibit, restrict or regulate foreign investments.
“Representatives” of a Person means such Person’s Affiliates and any officer, director or employee of such Person or its Affiliates or any investment banker, attorney, accountant or other advisor or representative of such Person or its Affiliates.
“Retained Claim” means any claim, cause of action, defense, right of offset or counterclaim, or settlement agreement (in any manner arising or existing, whether choate or inchoate, known or unknown, contingent or non-contingent) (a) to the extent related to the Excluded Assets, the Retained Liabilities or the Retained Business or (b) set forth on Section 1.1(g) of the Seller Disclosure Schedules.
-11-
“Sale Process” means all matters relating to the sale or separation of the Business and the review of strategic alternatives with respect to the Business and all activities in connection therewith, including matters relating to (a) the solicitation of proposals from and negotiations with third parties in connection with the sale of the Business or (b) the drafting, negotiation or interpretation of any of the provisions of this Agreement or the other Transaction Documents, or the determination of the allocation of any assets or Liabilities pursuant to the foregoing agreements or the transactions contemplated thereby.
“Sanctioned Country” means any country or region or government thereof that is, or has been in the last five years, the subject or target of a comprehensive embargo under Trade Controls (including Cuba, Iran, North Korea, Syria, Venezuela, the Crimea region of Ukraine, the so-called “Donetsk People’s Republic,” and the so-called “Luhansk People’s Republic”).
“Sanctioned Person” means any Person that is the subject or target of sanctions or restrictions under Trade Controls including: (i) any Person listed on any U.S. or non-U.S. sanctions- or export-related restricted party list, including the U.S. Department of the Treasury Office of Foreign Assets Control’s (“OFAC”) List of Specially Designated Nationals and Blocked Persons, or any other OFAC, U.S. Department of Commerce Bureau of Industry and Security, or U.S. Department of State sanctions- or export-related restricted party list; (ii) any Person located, organized, or resident in a Sanctioned Country; or (iii) any Person that is, in the aggregate, 50 percent or greater owned, directly or indirectly, or otherwise controlled by a Person or Persons described in clauses (i)-(ii).
“Sanctions” means all U.S. and non-U.S. Laws relating to economic or trade sanctions, including the Laws administered or enforced by the United States (including by OFAC or the U.S. Department of State) and the United Nations Security Council.
“SEC” means the U.S. Securities and Exchange Commission.
“Securities Act” means the U.S. Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Seller Benefit Plan” means any employee benefit plan (within the meaning of Section 3(3) of ERISA, whether or not subject to ERISA) and any bonus, stock option, stock purchase, restricted stock, incentive, deferred compensation, retiree health or life insurance, retirement, pension, superannuation, gratuity, jubilee, provident fund, employment, severance, retention, termination, change in control, welfare, post-employment, profit-sharing, disability, health, vacation, sick leave benefits, fringe benefits or other benefit plan, program, agreement or arrangement, that is sponsored, maintained, contributed to or required to be maintained or contributed to by Seller or any of its Subsidiaries (but excluding any such plan, program or arrangement mandated by or maintained solely pursuant to applicable Law or any Multiemployer Plan solely for purposes of Section 3.19), in each case, providing benefits to any Business Employee.
-12-
“Seller Disclosure Schedules” means those certain Seller Disclosure Schedules dated as of the date of this Agreement, provided by Seller to the Purchasers.
“Seller Employee” means each employee of the Purchased Entities who does not meet the definition of a Business Employee.
“Seller Entities” means Seller and all of its Subsidiaries that transfer Purchased Assets (including Purchased Entity Shares) or Assumed Liabilities to the Purchasers or any of their respective Subsidiaries pursuant to this Agreement.
“Seller Fundamental Representations” means the representations and warranties made by Seller contained in Section 3.1(a) (Organization and Standing), Section 3.2 (Purchased Entities; Capital Structure), Section 3.3 (Authority; Enforceability), and Section 3.24 (Brokers).
“Seller Group” means Seller and its Subsidiaries (other than the Purchased Entities).
“Separate Company Accrued Tax Amount” means an amount (which shall not be less than zero) equal to the aggregate unpaid Income Taxes of the Purchased Entities (and any unpaid Taxes required to be withheld from payments made or deemed made or transactions between a Purchased Entity and Seller or any of its Subsidiaries (including pursuant to Section 5.6)) for any Pre-Closing Tax Period for which the applicable annual Tax Return first becomes due after the date hereof, which amount shall (a) not include any Income Taxes with respect to a Combined Tax Return for which the Purchased Entities are liable solely as a result of being a member of the applicable group filing such Combined Tax Return, (b) not be expressed as a negative number in the aggregate or be computed by reference to a negative number in respect of any Tax Return, (c) exclude any deferred Tax liabilities or deferred Tax assets and any accruals for contingent Tax liabilities (in each case, taken into account for financial accounting purposes), (d) be computed in accordance with the past practices of the Purchased Entities except to the extent prohibited by applicable Law, (e) take into account any estimated Income Tax payments and overpayments of Income Taxes with respect to any Pre-Closing Tax Period as reductions in the amount of unpaid Income Taxes, but only to the extent that such payments have the effect of reducing (not below zero) the particular unpaid Income Taxes in respect of which such payments were made, (f) exclude any Income Taxes attributable to any transaction entered into by the Purchasers or any of their Affiliates (including, after the Closing, the Purchased Entities) after the Closing (limited, in the case of actions taken on the Closing Date after the Closing, to actions taken outside the ordinary course of business), (g) in the case of any Straddle Period, be determined in accordance with Section 7.7 and (h) treat as taxable income in a Pre-Closing Tax Period any taxable income shifted from a Pre-Closing Tax Period to a taxable period (or portion thereof) that begins after the Closing Date by reason of any change in method of accounting made or adopted after December 31, 2023 and prior to the Closing.
“Shared Contract” means any Contract pursuant to which Seller or its Subsidiaries is bound to sell any Business Products in combination with one or more products which itself is not a Business Product.
“Straddle Period” means any Tax period that begins on or before the Closing Date and ends after the Closing Date.
-13-
“Subsidiary” means, with respect to any Person, any corporation, partnership, limited liability company or other entity, whether incorporated or unincorporated, of which such first Person directly or indirectly owns or controls at least a majority of the securities or other interests having by their terms ordinary voting power to elect a majority of the board of directors or others performing similar functions; provided that, from and after the Closing, no Purchased Entity shall be deemed to be a Subsidiary of any Seller Entity.
“Tangible Personal Property” means machinery, office equipment, operating supplies, equipment, hardware, furniture, fixtures, tools and all other tangible personal property, it being understood that Tangible Personal Property shall not include any Intellectual Property Rights.
“Target Gross Profit” means $121,865,000.
“Target Working Capital” means $198,625,000.
“Tax” means any U.S. federal, state, local or non-U.S. tax or similar charge imposed by any Taxing Authority, including any income, gross receipts, property, sales, use, capital stock, social security, value added, goods and services, profits, license, withholding, payroll, employment, excise, premium, property, capital gains, transfer, stamp, environmental, customs, duties, alternative or add-on minimum, estimated, capital, capital gains, franchise and occupation tax, together with any interest and penalties imposed by any Taxing Authority with respect to such amounts.
“Tax Proceeding” means any audit, examination, contest, litigation or other Proceeding with or against any Taxing Authority.
“Tax Return” means any return, declaration, report, claim for refund or information return or statement required to be filed with any Taxing Authority with respect to Taxes, including any amendment thereof.
“Taxing Authority” means any Governmental Entity responsible for the administration or the imposition of any Tax.
“Technology” means embodiments of Intellectual Property Rights, including in the form of documentation, materials, product specifications, hardware, prototypes, data, databases and software; provided that Technology does not include any Intellectual Property Rights in such Technology.
“Territory” means the countries set forth on Section 1.1(h) of the Seller Disclosure Schedules.
“Transaction Documents” means this Agreement and the Ancillary Agreements.
“Transfer Taxes” means any federal, state, local, non-U.S. and other transfer (including real property transfer), registration, documentary, stamp, stamp duty, land, recording, conveyance or similar Taxes (but, for the avoidance of doubt, excluding any Income Taxes and Taxes imposed directly or indirectly on gain and withholding Taxes in respect of the foregoing) imposed on the sale and transfer, or recording of transfer, of the Purchased Assets (including, for the avoidance of doubt, the Purchased Entity Shares) and the assumption of the Assumed Liabilities contemplated by this Agreement; provided that “Transfer Taxes” shall not include any VAT/GST.
-14-
“U.S. Business Employee” means each Business Employee who provides services primarily inside the United States.
“U.S. Purchased Entity Benefit Plan” means each Purchased Entity Benefit Plan in which U.S. Business Employees are eligible to participate or receive benefits.
“VAT/GST” means any (i) Tax imposed in accordance with Directive 2006/112/EC and any permitted derogations therefrom, as well as any equivalent or similar Tax imposed under the laws of any jurisdiction that is not a member state of the European Union, or any goods and services or similar Taxes, and (ii) any sales or use Tax imposed by a state or local Taxing Authority in the United States, in each case, imposed on the sale and transfer, or recording of transfer, of the Purchased Assets and the assumption of the Assumed Liabilities. For the avoidance of doubt, VAT/GST shall exclude any Income Taxes and Taxes imposed directly or indirectly on gain and withholding Taxes in respect of the foregoing.
“WARN Act” means the Worker Adjustment and Retraining Notification Act of 1988, as amended, or any similar Laws.
“WC Adjustment Amount” means (a) the Closing Working Capital minus (b) the Target Working Capital.
“Working Capital” means, as of any time, (a) the current assets of the Business that are included in the specific line items of current assets specifically identified in the Sample Closing Statement reduced by (b) the current liabilities of the Business that are included in the specific line items of current liabilities specifically identified in the Sample Closing Statement, in each case, without duplication and without giving effect to the Transaction, and calculated in accordance with the Transaction Accounting Principles; provided, however, that in no event shall Working Capital include (i) any amount included within the definition of Cash or Indebtedness, (ii) any Excluded Assets or Retained Liabilities, (iii) any Liabilities to be repaid or extinguished pursuant to this Agreement in connection with the Closing (including any Liabilities in respect of intercompany balances or accounts to be settled pursuant to Section 5.6) or (iv) any amounts with respect to Income Tax assets or Income Tax Liabilities or deferred Tax assets or deferred Tax liabilities.
Section 1.2Other Defined Terms. In addition, the following terms shall have the meanings ascribed to them in the corresponding section of this Agreement:
Term |
|
Section |
Additional Business Product Marks |
|
5.25 |
Agreement |
|
Preamble |
Allocation |
|
2.10(c) |
Allocation Schedule |
|
2.10(a) |
Alternative Financing |
|
5.7(c) |
-15-
Anti-Corruption Laws |
|
3.16(b) |
Approval Products |
|
5.10(c)(i) |
Assignment Agreement and Bill of Sale |
|
2.8(a)(vi) |
Assumed Liabilities |
|
2.6 |
Audited Financial Statements |
|
5.19(a) |
Balance Sheet Date |
|
3.7(d) |
Base Purchase Price |
|
2.2 |
Business Contingent Worker Contracts |
|
6.10 |
Business Financial Information |
|
3.7(a) |
Business Registered IP |
|
3.10(a) |
Cash Incentive Compensation |
|
6.6 |
Chinese Operating Cash Amount |
|
5.23 |
Closing |
|
2.3 |
Closing Date |
|
2.3 |
Closing Statement |
|
2.9(b) |
Commitment Letter |
|
4.6(a) |
Comparability Period |
|
6.2(a) |
Competing Business |
|
5.16(c) |
Confidential Business Information |
|
5.3(b) |
Confidential Retained Business Information |
|
5.3(c) |
Confidentiality Agreement |
|
5.3(a) |
Copyrights |
|
See definition of Intellectual Property Rights, 1.1 |
Covered Intellectual Property |
|
5.28 |
Current Representation |
|
11.14(a) |
Deferred Closing |
|
2.11(a) |
Deferred Closing Date |
|
2.11(a) |
Deferred Marketing Authorization |
|
2.11(a) |
Deferred Marketing Authorization Liability |
|
2.11(a) |
Definitive Agreements |
|
5.7(a) |
Designated Person |
|
11.14(a) |
Dispute Notice |
|
2.9(e) |
Dispute Resolution Period |
|
2.9(e) |
Distributed Excess Restricted Cash |
|
5.23 |
Distributed Excess Restricted Cash Transfer |
|
5.23 |
Distribution Agreement |
|
2.8(a)(iv) |
Estimated Closing Cash |
|
2.9(b) |
Estimated Closing Indebtedness |
|
2.9(b) |
Estimated Gross Profit Adjustment Amount |
|
2.9(b) |
Estimated WC Adjustment Amount |
|
2.9(b) |
Excess Restricted Cash |
|
See definition of Closing Cash, 1.1 |
Excess Restricted Cash Distribution |
|
5.23 |
Exchange Act |
|
3.5 |
Excluded Assets |
|
2.5 |
Expired Marks |
|
5.26 |
Fair Value |
|
4.9 |
Final Purchase Price |
|
2.9(g) |
-16-
Financial Statements |
|
5.19(a) |
Financing |
|
4.6(a) |
Financing Amounts |
|
4.6(d) |
Financing Entities |
|
See definition of Financing Parties, 1.1 |
fraud |
|
9.2 |
Global Separation Licenses |
|
5.11 |
Guarantees |
|
5.9 |
Indemnified Party |
|
10.4(a) |
Indemnifying Party |
|
10.4(a) |
Independent Accounting Firm |
|
2.9(e) |
Initial Allocation |
|
2.10(b) |
Inside Date |
|
2.3 |
International Transferred Employee |
|
6.1(d) |
Inventory |
|
2.4(n) |
IRS |
|
2.10(c) |
Leased Real Property |
|
2.4(g) |
Lenders |
|
4.6(a) |
Litigating Party |
|
5.14(a) |
Lower Bound |
|
See definition of Gross Profit Adjustment Amount, 1.1 |
Marks |
|
See definition of Intellectual Property Rights, 1.1 |
Material Contracts |
|
3.13(a) |
New Plans |
|
6.2(c) |
Non-U.S. Purchased Entity Benefit Plan |
|
3.19(k) |
OFAC |
|
See definition of Sanctioned Person, 1.1 |
Offer Employees |
|
6.1(b) |
Old Plans |
|
6.2(c) |
Original Seller Group |
|
5.16(d) |
Outside Date |
|
9.1(d) |
Owned Real Property |
|
2.4(g) |
Parties |
|
Preamble |
Party |
|
Preamble |
Patents |
|
See definition of Intellectual Property Rights, 1.1 |
Post-Closing Representation |
|
11.14(a) |
Post-Closing Statement |
|
2.9(d) |
Pre-Closing Restricted Cash Distribution |
|
5.23 |
Product Claims |
|
2.6(c) |
Prohibited Modification |
|
5.7(b) |
Proposed Initial Allocation |
|
2.10(b) |
Purchased Assets |
|
2.4 |
Purchased Entities |
|
2.4(a) |
Purchased Entity |
|
2.4(a) |
Purchased Entity Shares |
|
2.4(a) |
Purchaser |
|
Preamble |
Purchaser 1 |
|
Preamble |
Purchaser 2 |
|
Preamble |
Purchaser DC Plans |
|
6.5(a) |
-17-
Purchaser FSA Plan |
|
6.2(e) |
Purchaser Indemnified Parties |
|
10.2(a) |
Purchasers |
|
Preamble |
Purchasers’ Allocation Notice |
|
2.10(c) |
Purchasers’ Initial Allocation Notice |
|
2.10(b) |
R&W Insurance Policy |
|
5.18 |
Remedy Actions |
|
5.1(d) |
Required Approvals |
|
8.1(a) |
Retained Business |
|
See definition of Business, 1.1 |
Retained Liabilities |
|
2.7 |
Retained Marks |
|
5.10(a) |
Retained Seller Benefit Plans |
|
6.3 |
Sample Closing Statement |
|
2.9(a) |
Seller |
|
Preamble |
Seller Change of Control |
|
5.16(d) |
Seller Covered Person |
|
5.16(a) |
Seller DC Plans |
|
6.5(a) |
Seller FSA Plan |
|
6.2(e) |
Seller Indemnified Parties |
|
10.3 |
Seller’s Allocation |
|
2.10(c) |
Severance Cap |
|
6.2(c) |
Significant Customers |
|
3.13(a)(i) |
Significant Suppliers |
|
3.13(a)(ii) |
Specified Business Contracts |
|
2.4(f) |
Specified Date |
|
9.1(d) |
Suballocation |
|
2.10(d) |
Surviving Obligations |
|
9.2 |
Tax Benefit |
|
10.6 |
Third-Party Claim |
|
10.4(a) |
Third-Party Consents |
|
5.15 |
Trade Controls |
|
3.25(a) |
Trade Secrets |
|
See definition of Intellectual Property Rights, 1.1 |
Transaction |
|
Recitals |
Transaction Accounting Principles |
|
2.9(a) |
Transferred Employees |
|
6.1(d) |
Transferred FSA Balances |
|
6.2(e) |
Transferred Information Technology |
|
2.4(j) |
Transferred Intellectual Property |
|
2.4(h) |
Transferred Leases |
|
2.4(g) |
Transferred Marketing Authorizations |
|
3.15(a) |
Transferred Packaging |
|
5.10(c)(i) |
Transferred Permits |
|
2.4(o) |
Transferred Real Property |
|
2.4(g) |
Transferred Tangible Personal Property |
|
2.4(k) |
Transition Services Agreement |
|
2.8(a)(iii) |
U.S. DC Employees |
|
6.5(a) |
-18-
U.S. LTD Employee |
|
6.1(c) |
U.S. Transferred Employee |
|
6.1(d) |
Waiver of Subrogation Provision |
|
5.18 |
willful and material breach |
|
9.2 |
Workers’ Compensation Event |
|
6.8 |
ARTICLE II
PURCHASE AND SALE; CLOSING
Section 2.1Purchase and Sale. Subject to the terms and conditions of this Agreement, at the Closing, (a) Seller shall, and shall cause the other Seller Entities to, sell, assign, transfer and convey to Purchaser 1 and Purchaser 2, as applicable, (b) Purchaser 1 shall purchase and acquire from Seller and the other Seller Entities, all of Seller’s and the other Seller Entities’ right, title and interest as of the Closing in and to the Purchased Assets (other than the Purchased Assets that are the subject of Section 2.4(a) – 2.4(e)), free and clear of all Liens, other than Permitted Liens, and (c) Purchaser 2 shall purchase and acquire from Seller and the other Seller Entities, all of Seller’s and the other Seller Entities’ right, title and interest as of the Closing in and to the Purchased Assets that are the subject of Section 2.4(a) – 2.4(e), free and clear of all Liens, other than (i) in the case of the Purchased Entity Shares, Liens arising under applicable securities Laws, this Agreement or the governing documents of the Purchased Entities and (ii) in the case of all other Purchased Assets, Permitted Liens.
Section 2.2Purchase Price. In consideration for the Purchased Assets and the other obligations of Seller pursuant to this Agreement, at the Closing, the Purchasers shall (a) pay (or cause the applicable portion to be paid by Purchaser 1 or Purchaser 2, as applicable) to Seller (or one or more of Seller’s Subsidiaries designated by Seller in accordance with Section 2.8(a)(i)) three hundred and fifty million U.S. dollars ($350,000,000) in cash (the “Base Purchase Price”) as adjusted in accordance with Section 2.9 and paid in the manner set forth in Section 2.8 and Section 2.9; and (b) assume the Assumed Liabilities.
Section 2.3Closing Date. The closing of the Transaction (excluding any transactions to be consummated at any Deferred Closing) (the “Closing”) shall take place by remote exchange of signatures and documentation, at 8:00 a.m. (New York City time) on the fourth (4th) Business Day following the date on which the last of the conditions set forth in Article VIII (other than those conditions that by their nature are to be satisfied at the Closing, but subject to the satisfaction or waiver of such conditions at the Closing) have been satisfied (or, to the extent permitted, waived by the Party entitled to the benefits thereof) or at such other place, time and date as may be agreed in writing between Seller and the Purchasers; provided that, notwithstanding anything set forth herein to the contrary, the Closing shall not occur prior to the fourth (4th) Business Day following the date that is seventy five (75) days following the date of this Agreement without the prior, express written consent of each of Seller and the Purchasers (the “Inside Date”); provided, further, that, upon Seller’s prior written request, the Inside Date shall be extended by an additional fourteen (14) Business Days. The date on which the Closing occurs is referred to in this Agreement as the “Closing Date.”
Section 2.4Purchased Assets. Subject to the terms and conditions of this Agreement, on the Closing Date and at the Closing, Seller shall, and shall cause the other Seller Entities to, sell, assign, transfer and convey to the applicable Purchaser, and each such Purchaser shall purchase, acquire and accept from the Seller Entities, all of the Seller Entities’ right, title and interest as of the Closing in the following (the “Purchased Assets”):
-19-
(a)All of the equity interests (the “Purchased Entity Shares”) in the entities listed on Section 2.4(a) of the Seller Disclosure Schedules (each, a “Purchased Entity,” and, collectively, the “Purchased Entities”);
(b)Any and all accounts receivable of the Purchased Entities as of the Closing, in each case to the extent included in the calculation of the Closing Working Capital;
(c)All Cash of the Purchased Entities as of the Closing to the extent included in the calculation of Closing Cash (which, for the avoidance of doubt, shall exclude the Excess Restricted Cash);
(d)Except as set forth in Article VI, any and all assets of the Purchased Entity Benefit Plans (other than any assets that are controlled by a third party or Governmental Entity);
(e)All rights of the Purchased Entities under the Transaction Documents;
(f)(i) Each Contract to which Seller or any Seller Entity or any Subsidiary thereof is a party that exclusively relates to, is exclusively held for use by or is exclusively used in connection with the Business and (ii) the Business Contingent Worker Contracts (collectively, the “Specified Business Contracts”);
(g)(i) The owned real property listed on Section 2.4(g)(i) of the Seller Disclosure Schedules, together with all improvements, fixtures and appurtenances thereto (the “Owned Real Property”) and (ii) the leases with respect to the leased real property listed on Section 2.4(g)(ii) of the Seller Disclosure Schedules (such leases, including all amendments, extensions, renewals, guaranties and other agreements with respect thereto, the “Transferred Leases,” and such leased real property, the “Leased Real Property,” and together with the Owned Real Property, the “Transferred Real Property”);
(h)(i) The Registered IP listed in Section 2.4(h)(i) of the Seller Disclosure Schedules, including the goodwill appurtenant to the Marks included in the foregoing and (ii) the Intellectual Property Rights (other than Registered IP) owned by the Seller Group (or any of the Purchased Entities) as of the Closing and exclusively used, or held exclusively for use, in the operation of the Business (collectively, the “Transferred Intellectual Property”), including the right to damages for the past or future infringement or violation of the Transferred Intellectual Property;
(i)The Business Technology Deliverables;
(j)Any and all Information Technology (including all software loaded thereon but only to the extent such software is transferable to the Purchasers pursuant to the terms of this Agreement or the Purchasers independently have their own license for such software), in each case exclusively used, or held exclusively for use, in the operation of the Business (the “Transferred Information Technology”);
-20-
(k)Any and all Tangible Personal Property owned or leased by the Seller Entities and exclusively related to, exclusively used, or held exclusively for use, in the operation of the Business (the “Transferred Tangible Personal Property”);
(l)Any and all other current assets of the Business as of the Closing, in each case to the extent included in the calculation of the Closing Working Capital;
(m)Any and all credits, prepaid assets, prepaid expenses, deposits (including security deposits) and other amounts deposited with third parties in each case of the Business and to the extent included in the calculation of the Closing Working Capital;
(n)Any and all Business Products, raw materials, bulk substances, ingredients, work-in-process, finished goods, goods in transit, supplies and other inventories and related packaging materials or similar items, in each case exclusively used, or held exclusively for use, by the Business, and in each case to the extent included in the calculation of the Closing Working Capital (collectively, the “Inventory”);
(o)All Permits that are exclusively related to, exclusively held for use or exclusively used in connection with the Business, including those listed in Section 2.4(o) of the Seller Disclosure Schedules, other than marketing authorizations (the “Transferred Permits”);
(p)The Transferred Marketing Authorizations;
(q)Any and all goodwill of the Business;
(r)Any and all claims, causes of action, defenses, rights of offset or counterclaims, or settlement agreements (in any manner arising or existing, whether choate or inchoate, known or unknown, contingent or non-contingent), in each case, exclusively related to the Business, other than any Retained Claims;
(s)Copies of any and all (x) documents, instruments, papers, books, records, books of account, files and data (including customer, distributor and supplier lists, pricing lists, and repair and performance records, testing and clinical data, market research reports, marketing plans and other marketing-related information and materials, quality control, pharmacovigilance and regulatory records), catalogs, brochures, sales literature, promotional materials, certificates and other documents, in each case, exclusively related to the Business and in the possession of the Seller Entities or any of their Subsidiaries and (y) personnel and employment records of Transferred Employees and any other employees of the Purchased Entities, other than (i) any books, records or other materials that the Seller Entities are required by Law to retain (copies of which, to the extent permitted by Law, will be made available to the Purchasers upon the Purchasers’ reasonable request), (ii) personnel and employment records for employees and Former Employees who are not Transferred Employees or employees of a Purchased Entity and for Transferred Employees if prohibited by Law, (iii) any books, records or other materials that may be located in a facility of the Business (including the Transferred Real Property) to the extent not exclusively related to the Business, (iv) Tax Returns and other books and records related to Taxes and (v) records prepared in connection with the Transaction, including bids received from other Persons and analyses relating to the Business; provided that, with respect to any such books, records or other materials that are Purchased Assets pursuant to this clause (s), the Seller Entities shall be permitted to keep (A) copies of such books, records or other materials to the extent required to demonstrate compliance with applicable Law or pursuant to internal compliance procedures, (B) copies of such books, records or other materials to the extent they are relevant to any Excluded Assets or the Retained Business and (C) such books, records or other materials in the form of so-called “back-up” electronic tapes in the ordinary course of business; provided, further, that the obligation to sell, assign, transfer and convey any such books, records or other materials that are Purchased Assets pursuant to this clause (s) is only to the extent permitted by Data Protection Laws and the foregoing shall not include any materials that constitute Technology;
-21-
(t)The assets set forth on Section 2.4(u) of the Seller Disclosure Schedules;
(u)Additional Business Products in accordance with Section 5.24; and
(v)All other assets, Contracts, rights and properties not addressed by clauses (a) to (u), whether tangible or intangible, personal or mixed, wherever located and whether or not reflected on the books and records of any Seller Entities, exclusively related to, exclusively held for use or exclusively used in the Business; provided, that, for the avoidance of doubt, if any categories of assets, Contracts, rights and properties are specifically addressed by clauses (a) to (u), the terms of clauses (a) to (u) shall govern.
The transfer of any assets of the Purchased Entities that constitute Purchased Assets shall be effected solely by virtue of the transfer of the Seller Entities’ right, title and interest in the Purchased Entity Shares and not through the direct transfer of such assets, and Seller and its Subsidiaries shall not be required to transfer any such assets of the Purchased Entities other than through the transfer of the Seller Entities’ right, title and interest in the Purchased Entity Shares. Subject to Section 2.11, the Parties acknowledge and agree that no member of the Seller Group will retain after the Closing any direct or indirect right, title and interest in any Purchased Asset.
The Parties acknowledge and agree that a single asset may fall within more than one of clauses (a) through (v) in this Section 2.4; such fact does not imply that (i) such asset shall be transferred more than once or (ii) any duplication of such asset is required.
Section 2.5Excluded Assets. Notwithstanding anything in this Agreement to the contrary, the Purchasers expressly understand and agree that the following assets, Contracts, rights and properties of the Seller Entities and their Subsidiaries (the “Excluded Assets”) shall be retained by the Seller Group, shall be excluded from the Purchased Assets and may be transferred out of the Purchased Entities prior to the Closing, notwithstanding any other provision of this Agreement:
-22-
(a)Any and all legal and beneficial interest in the share capital or equity interest of any Person, other than the Purchased Entity Shares;
(b)Any and all Contracts, other than the Specified Business Contracts and the Transferred Leases;
(c)Any and all owned and leased real property and other interests in real property, other than the Transferred Real Property;
(d)Any and all Intellectual Property Rights, other than the Transferred Intellectual Property;
(e)Any and all Technology, other than the Business Technology Deliverables in the form transferred;
(f)Any and all Information Technology, other than the Transferred Information Technology;
(g)Any and all Tangible Personal Property, other than the Transferred Tangible Personal Property;
(h)Any and all raw materials, work-in-process, finished goods, supplies and other inventories, other than the Inventory;
(i)Any and all Permits, other than the Transferred Permits;
(j)Any and all marketing authorizations, other than the Transferred Marketing Authorizations;
(k)Any Retained Claim;
(l)Any and all documents, instruments, papers, books, records, books of account, files and data, catalogs, brochures, sales literature, promotional materials, certificates and other documents not specifically identified as Purchased Assets in Section 2.4;
(m)Except as set forth in Article VI, any and all assets of the Seller Benefit Plans (other than the assets of the Purchased Entity Benefit Plans);
(n)Any and all loans and advances if any, by the Seller Entities to any of their Affiliates or otherwise to the Business;
(o)The Italian VAT Receivable;
(p)Shared Contracts;
(q)Any and all prepaid Taxes, and any and all refunds, credits, overpayments or similar items or recoveries, of or against Excluded Taxes; (r)Tax Returns and other books and records related to Taxes paid or payable by Seller, the Seller Entities, or any of their respective Affiliates with respect to a Combined Tax Return or with respect to Excluded Taxes;
-23-
(s)Any and all Cash, (i) excluding any Cash of the Purchased Entities as of the Closing to the extent included in the calculation of the Closing Cash and (ii) including the Excess Restricted Cash;
(t)Any and all accounts receivable (other than those of the Purchased Entities as of the Closing to the extent included in the calculation of the Closing Working Capital) and any and all trade receivables, other current assets, prepaid expenses and security deposits (in each case, other than those of the Business as of the Closing to the extent included in the calculation of the Closing Working Capital);
(u)Any and all insurance policies and binders and interests in insurance pools and programs and self-insurance arrangements, whether or not related to the Business, for all periods before, through and after the Closing, including any and all refunds and credits due or to become due thereunder and any and all claims, rights to make claims and rights to proceeds on any such insurance policies, binders and interests for all periods before, through and after the Closing;
(v)All rights to receive payments of Seller or any of its Affiliates pursuant to a hedging or other currency exchange agreement existing before, on or after the Closing Date;
(w)All intercompany assets between members of the Seller Entities and their Subsidiaries (other than the Purchased Entities), on the one hand, and any other members of the Seller Entities and their Subsidiaries (other than the Purchased Entities), on the other hand;
(x)The assets set forth on Section 2.5(x) of the Seller Disclosure Schedules; and
(y)Except for those assets expressly identified as Purchased Assets in clauses (a) through (v) of Section 2.4, any and all assets, business lines, properties, rights, Contracts and claims of Seller or any of its Subsidiaries not exclusively used, or held exclusively for use, in the operation of the Business (including all assets, business lines, properties, rights, Contracts and claims constituting ownership interests in, or that are used or held for use in or related to, the Retained Business), wherever located, whether tangible or intangible, real, personal or mixed.
The Parties acknowledge and agree that neither Purchaser nor any of their respective Subsidiaries will acquire, and no Purchased Entity may retain after the Closing, any direct or indirect right, title and interest in any Excluded Assets.
Section 2.6Assumed Liabilities. Subject to the terms and conditions of this Agreement, at the Closing, each Purchaser shall, as applicable, (x) assume and hereby agrees to pay, satisfy, discharge and perform all of the Liabilities of the Seller Entities solely to the extent related to or arising out of the Purchased Assets and (y) cause the Purchased Entities to pay, satisfy, discharge and perform all of their respective Liabilities (the “Assumed Liabilities”), in each case, whether accruing or arising prior to, on or after the Closing, including the following (but excluding the Liabilities identified as Retained Liabilities in clauses (a) through (l) of Section 2.7):
-24-
(a)Any and all Liabilities to the extent relating to or arising out of the Specified Business Contracts or the Transferred Leases;
(b)Any and all Liabilities arising under Environmental Laws to the extent arising out of or relating to in any way any past, current or future businesses, operations, products or properties of or associated with the Purchased Assets or the Business;
(c)Any and all Liabilities and obligations to the extent arising from or relating to (i) any return, repair, warranty or similar obligations relating to products and services of the Business that were designed, manufactured or sold on, or prior to or after the Closing Date or that were held in the Inventory as of the Closing Date, (ii) infringement, dilution, misappropriation or other violation of any rights in respect of Transferred Intellectual Property, (iii) alleged or actual hazard or defect in design, manufacture, materials or workmanship, including any failure to warn or alleged or actual breach of express or implied warranty or representation or (iv) lawsuits or other claims, regardless of when commenced or made and irrespective of the legal theory asserted, with respect to the design, manufacture, testing, advertising, marketing, distribution or sale of the products and services of the Business, whether prior to or after the Closing (collectively, “Product Claims”);
(d)Any and all Liabilities relating to or arising out of the ownership, use or conduct of, or associated with the realization of the benefits of the Business, the Purchased Assets or the Purchased Entities, whether accruing or arising before, on or after the Closing, whether known or unknown, fixed or contingent, asserted or unasserted, and not satisfied or extinguished as of the Closing, including any and all Liabilities in respect of any Proceedings related thereto, other than the Liabilities identified as Retained Liabilities in clauses (a) through (l) of Section 2.7;
(e)Any and all Liabilities in respect of or relating to Business Employees, other than those expressly retained by Seller pursuant to Article VI;
(f)Any and all Liabilities of the Purchased Entities, other than with respect to Seller Employees and Former Employees;
(g)Any and all Liabilities to the extent relating to or arising out of (i) the Seller Benefit Plans (other than Purchased Entity Benefit Plans) with respect to or relating to Business Employees but only to the extent expressly assumed by a Purchaser pursuant to Article VI or (ii) the Purchased Entity Benefit Plans;
(h)Any and all Liabilities for which the Purchasers or their Subsidiaries expressly have responsibility pursuant to this Agreement, including pursuant to Section 5.7; (i)Any and all Liabilities to the extent included in the calculation of Closing Indebtedness; and
-25-
(j)Any and all accounts payable of the Purchased Entities and other Liabilities of the Business to the extent included in the calculation of the Closing Working Capital.
With respect to Assumed Liabilities that are Liabilities of the Purchased Entities, the applicable Purchaser shall cause the Purchased Entities to pay, satisfy, discharge and perform all of such Liabilities.
The Parties acknowledge and agree that a single Liability may fall within more than one of clauses (a) through (j) in this Section 2.6; such fact does not imply that (i) such Liability shall be transferred more than once or (ii) any duplication of such Liability is required.
Section 2.7Retained Liabilities. The Seller Entities shall retain, and the Purchasers shall not assume, the following Liabilities (the “Retained Liabilities”):
(a)Except as set forth in Section 2.6(f) and Section 2.6(i), any Indebtedness of the Seller Entities and their Subsidiaries;
(b)Liabilities for which any Seller Entity (other than the Purchased Entities) expressly has responsibility pursuant to this Agreement;
(c)Liabilities to the extent arising out of or related to the Excluded Assets (other than any Liabilities for which the Purchasers or any of their Subsidiaries expressly have responsibility pursuant to the terms of this Agreement or any Transaction Document);
(d)Except as expressly assumed by a Purchaser as set forth in Article VI, Liabilities to the extent relating to or arising out of any Seller Benefit Plan (other than a Purchased Entity Benefit Plan);
(e)Any and all accounts payable (other than those of the Purchased Entities as of the Closing to the extent included in the calculation of the Closing Working Capital);
(f)Liabilities for Excluded Taxes;
(g)Fees and expenses of brokers, finders, outside counsel, financial advisors, accountants, consultants and other professional advisors incurred by Seller or any of its Subsidiaries prior to the Closing in connection with the negotiation, execution and performance of this Agreement and the other Transaction Documents and the transactions contemplated hereby and thereby;
-26-
(h)Any and all Liabilities in respect of (i) any transaction, retention or change in control bonuses or nonqualified deferred compensation obligations payable to any Business Employee or other individual service provider of the Business or the Purchased Entities or of Seller and any of its Subsidiaries upon and solely by reason of the consummation of the transactions contemplated hereby, together with the employer portion of any payroll, social security, unemployment or similar Taxes related thereto, and (ii) any Taxes related to any equity or equity-based payments or benefits (including, any cancellation or accelerated vesting of any equity or equity-based arrangements) due or payable to any Person in connection with the consummation of the transactions contemplated hereby;
(i)Any and all Liabilities in respect of or to the extent relating to Former Employees or Seller Employees regardless of when they arise;
(j)Subject to Section 5.21 (with respect to Shared Contracts only), any and all Liabilities (including rebates) related to Shared Contracts (other than Liabilities with respect to Business Products sold by Purchasers or their Affiliates following the Closing under such Shared Contracts, which shall be covered by Section 2.6(c));
(k)All Liabilities prior to the Closing associated with workers compensation claims to the extent retained by the Seller Entities pursuant to Section 6.8; and
(l)All intercompany Liabilities or intercompany payables between members of the Seller Entities and their Subsidiaries (other than the Purchased Entities), on the one hand, and any other members of the Seller Entities and their Subsidiaries (other than the Purchased Entities), on the other hand.
Section 2.8Closing Deliveries.
(a)At the Closing, the Purchasers shall deliver, or cause to be delivered, to Seller (or one or more other Seller Entities designated by Seller) the following:
(i)payment, by wire transfer(s) to one or more bank accounts designated in writing by Seller (such designation to be made by Seller at least two (2) Business Days prior to the Closing Date), an amount in immediately available funds equal to the Closing Purchase Price;
(ii)the certificate to be delivered pursuant to Section 8.3(c);
(iii)a counterpart of the Transition Services Agreement, in substantially the form attached as Exhibit A hereto (the “Transition Services Agreement”), duly executed by the Purchasers;
(iv)a counterpart of one or more distribution agreements entered into pursuant to Section 2.11 (if any), in customary form for a transaction similar to this Transaction and on terms mutually agreed between the Parties in good faith (which terms shall generally be consistent with those applicable to the relevant services under the Transition Services Agreement) (each, a “Distribution Agreement”), duly executed by the applicable Purchaser(s);
(v)a fully executed and binding copy of the R&W Insurance Policy;
-27-
(vi)with respect to any Purchased Asset (other than the Purchased Entity Shares) or Assumed Liability that is not held by a Purchased Entity, a counterpart of an Assignment and Assumption Agreement and Bill of Sale providing for the transfer of the Seller Entities’ right, title and interest as of the Closing in and to the Purchased Assets (other than the Purchased Entity Shares) and the assumption by the applicable Purchaser of the Assumed Liabilities in accordance with and subject to this Agreement, by and between the applicable Seller Entities and Purchasers, in substantially the form attached hereto as Exhibit B (the “Assignment Agreement and Bill of Sale”), duly executed by the Purchasers; and
(vii)any other instruments necessary and appropriate to evidence the applicable Purchaser’s assumption of the Assumed Liabilities pursuant to and in accordance with this Agreement, in each case duly executed by such Purchaser, to the extent applicable.
(b)At the Closing, Seller shall deliver, or cause to be delivered, to the Purchasers the following:
(i)the certificate to be delivered pursuant to Section 8.2(c);
(ii)a counterpart of the Transition Services Agreement, duly executed by the Seller Entity named as a party thereto;
(iii)a counterpart of each Distribution Agreement (if any), duly executed by the Seller Entity named as a party thereto;
(iv)certificates evidencing the Purchased Entity Shares, to the extent that such Purchased Entity Shares are in certificated form, duly endorsed in blank or with stock powers duly executed in proper form for transfer, and, to the extent that such Purchased Entity Shares are not in certificated form, other evidence of ownership or assignment in form and substance reasonably satisfactory to the Purchasers;
(v)a completed and duly executed IRS Form W-9 of Seller;
(vi)with respect to any Purchased Asset (other than the Purchased Entity Shares) or Assumed Liability that is not held by a Purchased Entity, a counterpart of the Assignment Agreement and Bill of Sale duly executed by each Seller Entity named as a party thereto;
(vii)customary documentation (x) evidencing the release or termination of any Lien on any Purchased Asset securing indebtedness for borrowed money and (y) and the termination of any UCC financing statements in respect of any such Lien;
(viii)the Completed Phase I Reports and corresponding reliance letters or other documentation sufficient to allow Purchaser to establish the Phase I reports for the benefit of Purchasers, in each case prepared at the Purchasers’ sole cost and expense (provided, that the Parties hereby agree that Seller’s obligation to provide such Completed Phase I Reports and corresponding reliance letters or other documentation under this clause (viii) is conditioned on the Purchasers’ prompt reimbursement of any costs and expense); and
-28-
(ix)any other instruments of transfer necessary and appropriate to evidence the transfer of the Seller Entities’ right, title and interest in the Purchased Assets pursuant to and in accordance with this Agreement duly executed by each Seller Entity named as a party thereto, to the extent applicable.
Section 2.9Adjustment to Base Purchase Price.
(a)Section 2.9(a) of the Seller Disclosure Schedules sets forth an illustrative calculation (the “Sample Closing Statement”) of (i) the Working Capital of the Business and the Cash and Indebtedness of the Purchased Entities, in each case, as of December 31, 2023, including the current asset and current liability line items included in the calculation of Working Capital, in each case prepared in accordance with the accounting principles, practices, procedures, methodologies and policies set forth on Section 2.9(a) of the Seller Disclosure Schedules (collectively, the “Transaction Accounting Principles”) and (ii) the Gross Profit of the Business for the fiscal year ended December 31, 2023.
(b)At least four (4) Business Days prior to the Closing Date, Seller shall cause to be prepared and delivered to the Purchasers a closing statement (the “Closing Statement”) setting forth a good-faith estimate of (i) the WC Adjustment Amount (such estimate, the “Estimated WC Adjustment Amount”), (ii) the Closing Cash (such estimate, the “Estimated Closing Cash”), (iii) the Closing Indebtedness (such estimate, the “Estimated Closing Indebtedness”), and (iv) the Gross Profit Adjustment Amount (such estimate, the “Estimated Gross Profit Adjustment Amount”), together with reasonable supporting calculations and documentation used by Seller in determining the amounts set forth therein. The Closing Statement shall set forth the calculations of such amounts in a format consistent with the Sample Closing Statement and, in the case of the Estimated WC Adjustment Amount, the Estimated Closing Cash and the Estimated Closing Indebtedness, be prepared in accordance with the Transaction Accounting Principles, including the use of the same line item categories (and specific line items within those categories) set forth on and used in the preparation of the Sample Closing Statement.
(c)The Estimated WC Adjustment Amount, the Estimated Closing Cash, the Estimated Closing Indebtedness and the Estimated Gross Profit Adjustment Amount shall be used to calculate the Closing Purchase Price to be paid by the Purchasers to Seller at the Closing. The Purchasers agree that, following the Closing through the date that the Post-Closing Statement becomes final and binding in accordance with this Section 2.9, they will not take any actions with respect to any accounting books, records, policies or procedures on which the Sample Closing Statement or the Closing Statement is based, or on which the Post-Closing Statement is to be based, that would impede or prevent the final determination of the Post-Closing Statement. The Purchasers, their Subsidiaries and their respective Representatives shall have the right to review and comment on the Closing Statement, and Seller shall (and shall cause its Subsidiaries and its and their respective Representatives to) reasonably cooperate in connection with such review and Seller shall consider any comments proposed by or on behalf of the Purchasers in good faith (provided that, for the avoidance of doubt, no failure by the Purchasers to object to, or comment on, any item set forth in the Closing Statement shall prejudice the Purchasers with respect to any post-Closing adjustments pursuant to Section 2.9(d) or the resolution thereof).
-29-
Seller shall promptly provide, or cause to be provided, to the Purchasers and their Representatives with reasonable access to, upon advance written request, during normal business hours, the relevant personnel involved in the preparation of the Closing Statement, and to the books, records, documents, work papers and other information in the possession or control of Seller Group or its Representatives (subject to customary access letters and confidentiality undertakings) for purposes of assisting the Purchasers and their Representatives in their review of the Closing Statement.
(d)Following the Closing Date, Seller shall provide to the Purchasers reasonable access (subject to customary access letters), upon advance written request, during normal business hours, to the accounting advisors and other personnel involved in the preparation of the Closing Statement and to the books and records of the Seller Entities and any other document or information reasonably requested by the Purchasers or their Representatives in order to allow the Purchasers and their Representatives to prepare the Post-Closing Statement. Within one hundred (100) days after the Closing Date, the Purchasers shall prepare or cause to be prepared, and will provide to Seller, a written statement (the “Post-Closing Statement”), setting forth a good-faith calculation of the WC Adjustment Amount, the Closing Cash, the Closing Indebtedness and the Gross Profit Adjustment Amount, together with reasonable supporting calculations and documentation used by the Purchasers in determining the amounts set forth therein. The Post-Closing Statement shall set forth in reasonable detail the Purchasers’ calculations of such amounts in a format consistent with the Sample Closing Statement and, in the case of the WC Adjustment Amount, the Closing Cash and the Closing Indebtedness, shall be prepared in accordance with the Transaction Accounting Principles, including the use of the same line item categories (and specific line items within those categories) set forth on and used in the preparation of the Sample Closing Statement.
(e)Within forty-five (45) days following receipt by Seller of the Post-Closing Statement, Seller shall deliver written notice to the Purchasers of any dispute Seller has with respect to the calculation, preparation or content of the Post-Closing Statement (the “Dispute Notice”); provided, however, that if Seller does not deliver any Dispute Notice to the Purchasers within such forty-five (45)-day period, the Post-Closing Statement will be final, conclusive and binding on the Parties. The Dispute Notice shall set forth in reasonable detail (i) any item on the Post-Closing Statement that Seller disputes and (ii) the amount of such item that Seller believes is correct. Upon receipt by the Purchasers of a Dispute Notice, the Purchasers and Seller shall negotiate in good faith to resolve any dispute set forth therein. If the Purchasers and Seller, such good-faith effort notwithstanding, fail to resolve any such dispute within thirty (30) days after delivery of the Dispute Notice (the “Dispute Resolution Period”), then the Purchasers and Seller jointly shall engage, within ten (10) Business Days following the expiration of the Dispute Resolution Period, Deloitte or, if Deloitte is unavailable or conflicted, another nationally recognized independent accounting firm selected jointly by Seller and the Purchasers (the “Independent Accounting Firm”) to resolve any such dispute; provided that, if Deloitte is unavailable or conflicted and Seller and the Purchasers are unable to agree on the Independent Accounting Firm, then each of Seller and the Purchasers shall select a nationally recognized independent accounting firm, and require that the two (2) firms mutually select a third (3rd) nationally recognized independent accounting firm to serve as the Independent Accounting Firm.
-30-
As promptly as practicable, and in any event not more than fifteen (15) days following the engagement of the Independent Accounting Firm, the Purchasers and Seller shall each prepare and submit a presentation detailing such Party’s complete statement of proposed resolution of each issue still in dispute to the Independent Accounting Firm (and such presentation, and all other communications with the Independent Accounting Firm, will be simultaneously made or delivered to the other Party). The Purchasers and Seller shall instruct the Independent Accounting Firm to, as soon as practicable after the submission of the presentations described in the immediately preceding sentence and in any event not more than twenty (20) days following such presentations, make a final determination in writing setting forth such calculation along with its analysis in reasonable detail of the basis and quantification of the appropriate amount of each of the line items that remain in dispute as indicated in the Dispute Notice (and that have not been thereafter resolved by written agreement of the Parties). With respect to each disputed line item, such determination, if not in accordance with the position of either Seller or the Purchasers, shall not be in excess of the higher, nor less than the lower, of the amounts advocated by Seller or the Purchasers, as applicable, in the Dispute Notice and the Post-Closing Statement, respectively. The scope of the disputes to be resolved by the Independent Accounting Firm shall be limited to those line items that remain in dispute as indicated in the Dispute Notice (and that have not been thereafter resolved by written agreement of the Parties) and whether any disputed determinations of the WC Adjustment Amount, the Closing Cash and the Closing Indebtedness were properly calculated in accordance with the Transaction Accounting Principles and the provisions of this Agreement, and not based on independent review beyond the aforementioned scope. The Independent Accounting Firm shall act as an expert and not as an arbitrator. Neither the Purchasers, Seller nor any of their respective Subsidiaries or Representatives shall have any ex parte conversations or meetings with the Independent Accounting Firm in connection with any dispute submitted by the Purchasers or Seller to the Independent Accounting Firm pursuant to this Section 2.9(e) without the prior consent of the other Party. All fees and expenses relating to the work, if any, to be performed by the Independent Accounting Firm shall be borne by Seller and the Purchasers in proportion to the allocation of the dollar value of the amounts in dispute between Seller and the Purchasers resolved by the Independent Accounting Firm, such that the party prevailing on the greatest dollar value of such disputes pays the lesser proportion of the fees. For example, if Seller submits a Dispute Notice to the Independent Accounting Firm for $1,000, and if the Purchasers contest to the Independent Accounting Firm only $500 of the amount claimed by Seller, and if the Independent Accounting Firm ultimately resolves the dispute by awarding Seller $300 of the $500 contested, then the costs and expenses of the Independent Accounting Firm will be allocated 60% (i.e., 300/500) to the Purchasers and 40% (i.e., 200/500) to Seller. All determinations made by the Independent Accounting Firm, and the Post-Closing Statement, as modified by the Independent Accounting Firm and to reflect any items resolved by written agreement of the Parties, will be final, conclusive and binding on the Parties absent manifest error.
(f)For purposes of complying with the terms set forth in this Section 2.9, from and after delivery of the Post-Closing Statement until the earlier of the delivery of a Dispute Notice and the finalization of the Final Purchase Price in accordance with the terms hereof, each of Seller and the Purchasers shall reasonably cooperate with and make available to each other, the Independent Accounting Firm and each of their respective Representatives all information, records, data and working papers, in each case to the extent related to the Purchased Assets, Assumed Liabilities, Business, or Purchased Entities, and shall permit access to its and their facilities and personnel, as may be reasonably required in connection with the preparation, analysis and review of the Post-Closing Statement and the resolution of any disputes thereunder.
-31-
(g)The “Final Purchase Price” means the Base Purchase Price, plus (i) the Closing Cash, plus (ii) the WC Adjustment Amount (which may be a positive or negative number, or zero), minus (iii) the Closing Indebtedness, plus (iv) the Gross Profit Adjustment Amount (which may be a negative number or zero, but not a positive number), in the case of each of clauses (i), (ii), (iii) and (iv), as finally determined pursuant to Section 2.9(e).
(h)If the Closing Purchase Price shall exceed the Final Purchase Price, then Seller shall pay or cause to be paid an amount in cash equal to such excess to the Purchasers by wire transfer of immediately available funds to an account or accounts designated in writing by the Purchasers to Seller; or if the Final Purchase Price shall exceed the Closing Purchase Price, then Purchasers shall pay an amount in cash equal to such excess to Seller by wire transfer of immediately available funds to an account or accounts designated in writing by Seller to the Purchasers. Any such payment is to be made within five (5) Business Days of the date on which the WC Adjustment Amount, the Closing Cash, the Closing Indebtedness and the Gross Profit Adjustment Amount are finally determined pursuant to this Section 2.9.
(i)The process set forth in this Section 2.9 (and in Section 2.10) shall be the sole and exclusive remedy of any of the Parties and their respective Affiliates for any disputes related to the Closing Working Capital, the WC Adjustment Amount, the Closing Cash, the Closing Indebtedness, the Gross Profit Adjustment Amount, the Closing Purchase Price, the Final Purchase Price and the calculations and amounts on which they are based or set forth in the related statements and notices delivered in connection therewith; provided, that, for the avoidance of doubt, the foregoing shall not limit the Purchasers’ rights of recovery under the R&W Insurance Policy.
Section 2.10Purchase Price Allocation.
(a)Seller and the Purchasers agree to allocate and, as applicable, to cause their relevant Subsidiaries to allocate, the Final Purchase Price (as finally determined pursuant to Section 2.9) any other items that are treated as additional consideration for U.S. federal income Tax purposes among the Purchased Assets (and any other assets that, for U.S. federal income Tax purposes, are treated as assets purchased by the applicable Purchaser (or its relevant Subsidiaries) pursuant to this Agreement and any other Transaction Document) in accordance with Exhibit C attached hereto (the “Allocation Schedule”).
(b)To the extent necessary to prepare bills of sale, transfer agreements, or to otherwise timely comply with the requirements of applicable Law in respect of the sale of any of the Purchased Assets (or other assets), no later than ten (10) days prior to the Closing, Seller shall deliver a draft of the allocation of the Base Purchase Price and any other items that are treated as additional consideration for U.S. federal income Tax purposes among each applicable Seller Entity (or other Subsidiary of Seller) that sells, transfers or assigns any relevant Purchased Assets (or other assets) determined in a manner consistent with the Allocation Schedule (the “Proposed Initial Allocation”). If the Purchasers reasonably disagree with any item on the Proposed Initial Allocation, the Purchasers may, within five (5) days after delivery of the Proposed Initial Allocation, deliver a written notice (the “Purchasers’ Initial Allocation Notice”) to Seller to such effect, specifying those items as to which the Purchasers disagree and setting forth the Purchasers’ proposed allocation of such items.
-32-
During the three (3) days following the Purchasers’ delivery of a Purchasers’ Initial Allocation Notice, the Purchasers and Seller negotiate in good faith to resolve such dispute. The Proposed Initial Allocation, as prepared by Seller if no Purchasers’ Initial Allocation Notice has been given or as agreed to by Seller and the Purchasers following delivery of Purchasers’ Initial Allocation Notice (the “Initial Allocation”), shall be conclusive and binding on the Parties. The Initial Allocation or, in the event that the Initial Allocation has not become final pursuant to this Section 2.10 by the Closing, the Proposed Initial Allocation, shall be used for the purpose of determining the amount of any payments made on the Closing Date to the applicable Seller Entities.
(c)No later than ninety (90) days after the date on which the Final Purchase Price is finally determined pursuant to Section 2.9, Seller shall deliver to the Purchasers a proposed allocation of the Final Purchase Price and any other items that are treated as additional consideration for U.S. federal income Tax purposes among the Purchased Assets (including among the Purchased Entity Shares and among any other assets that, for U.S. federal income Tax purposes, are treated as assets purchased by the applicable Purchaser (or its relevant Subsidiaries) pursuant to this Agreement and any other Transaction Document), as of the Closing Date, determined in a manner that incorporates, reflects and is consistent with the Allocation Schedule, the Initial Allocation, and Sections 338 and 1060 of the Code and the Treasury Regulations promulgated thereunder (“Seller’s Allocation”). If the Purchasers disagree with Seller’s Allocation (other than with respect to any Initial Allocation reflected in Seller’s Allocation), the Purchasers may, within thirty (30) days after delivery of Seller’s Allocation, deliver a written notice (the “Purchasers’ Allocation Notice”) to Seller to such effect, specifying those items as to which the Purchasers disagree and setting forth the Purchasers’ proposed allocation of such items. If the Purchasers deliver a Purchasers’ Allocation Notice, Seller and the Purchasers shall, during the thirty (30) days following such delivery, negotiate in good faith to resolve such dispute within thirty (30) days after delivery of the Purchasers’ Allocation Notice. If Seller and the Purchasers are unable to reach such agreement, they shall promptly thereafter cause the Independent Accounting Firm (who shall be promptly engaged if not previously engaged pursuant to Section 2.9 in accordance with the procedures described in Section 2.9) to resolve any remaining disputes. The Purchasers and Seller shall each request that the Independent Accounting Firm make a final determination as to the disputed items, in a manner that is consistent with the Allocation Schedule and the Initial Allocation, within thirty (30) days after such engagement. All fees and expenses relating to the work, if any, to be performed by the Independent Accounting Firm shall be borne equally by Seller and the Purchasers. The allocation, as prepared by Seller if no Purchasers’ Allocation Notice has been duly and timely delivered, as adjusted pursuant to any agreement between Seller and the Purchasers or as determined by the Independent Accounting Firm in accordance with this Section 2.10(c) (the “Allocation”), shall be final, conclusive and binding on the Parties absent manifest error. The Allocation shall be adjusted (other than with respect to amounts allocated to the Purchased Entity Shares pursuant to Exhibit C), as necessary, to reflect any adjustments to the purchase price under this Agreement in a manner consistent with the Allocation Schedule. Each of Seller and the Purchasers shall (and shall cause their respective Subsidiaries to) prepare and file its Tax Returns (including Internal Revenue Service (“IRS”) Form 8594) on a basis consistent with the Allocation and shall (and shall cause its respective Subsidiaries to) take no position inconsistent with the Allocation on any Tax Return (including IRS Form 8594) or in any Tax Proceeding or otherwise, in each case, except to the extent otherwise required pursuant to a “determination” within the meaning of Section 1313(a) of the Code (or any analogous provision of state, local or non-U.S.
-33-
Law). In the event that the Allocation is disputed by any Taxing Authority, the Party receiving notice of the dispute shall promptly notify the other Party hereto, and both Seller and the Purchasers agree to use their commercially reasonable efforts to defend such Allocation in any Tax Proceeding.
(d)If, in connection with the applicable Deferred Closing, an allocation of the relevant portion of the purchase price among the assets and liabilities transferred in such Deferred Closing is required by applicable Law, and the Allocation has not become final pursuant to this Section 2.10 at the time of such Deferred Closing, the Parties shall agree on an allocation of the relevant portion of the purchase price and any other items that are treated as additional consideration for U.S. federal income Tax purposes among the applicable Deferred Marketing Authorizations and Deferred Marketing Authorization Liabilities (each, a “Suballocation”). Any such Suballocation shall be consistent with the Initial Allocation. If Seller and the Purchasers are unable to mutually agree on any such Suballocation, such disagreement shall be referred to the Independent Accounting Firm promptly for review and resolution (in accordance with the procedures set forth in Section 2.9).
Section 2.11Transferred Marketing Authorizations.
(a)Notwithstanding anything to the contrary herein, if, the sale, assignment, transfer, conveyance or delivery of any of the Transferred Marketing Authorizations or the purchase, acquisition, acceptance and assumption of any Assumed Liabilities relating thereto as contemplated by this Article II shall not have been consummated as of the Closing because a Regulatory Approval (other than a Required Approval) has not been received (each, a “Deferred Marketing Authorization” or a “Deferred Marketing Authorization Liability,” as applicable), then, notwithstanding anything to the contrary in this Agreement, such Deferred Marketing Authorizations and Deferred Marketing Authorization Liabilities shall not be transferred to the Purchasers at the Closing (but the Closing shall otherwise occur with respect to the Purchased Assets (other than any Deferred Marketing Authorizations) and Assumed Liabilities (other than any Deferred Marketing Authorization Liabilities)). Such Deferred Marketing Authorizations and Deferred Marketing Authorization Liabilities shall be transferred to the Purchasers on the second (2nd) Business Day following the receipt, satisfaction or waiver of such Regulatory Approval (each, a “Deferred Closing” and such date, a “Deferred Closing Date”) with respect to such Deferred Marketing Authorizations and Deferred Marketing Authorization Liabilities, as applicable; provided that the receipt, satisfaction or waiver of such Regulatory Approval allows the Purchasers or any of their Subsidiaries to manufacture, distribute and market the applicable Business Products in the applicable jurisdictions in substantially the same manner as, and consistent with, the practice of the Business prior to Closing. If the Purchasers or any of their Subsidiaries obtain a new marketing authorization (including any re-registration) that enables the Purchasers or any of their Subsidiaries to manufacture, distribute and market the applicable Business Products in the applicable jurisdictions in substantially the same manner as, and consistent with, the practice of the Business prior to Closing with respect to each such Deferred Marketing Authorizations (in which case Seller and its Subsidiaries shall, subject to prior consultation with and receipt of written confirmation from the Purchasers, be entitled to withdraw or terminate such Deferred Marketing Authorizations), or if the Purchasers or any of their Subsidiaries obtain a Deferred Marketing Authorization from an authorized third party (e.g., an importer, manufacturer or distributor) that holds such Deferred Marketing Authorization and confirms the same in writing to Seller, then Seller and its Subsidiaries shall be released of their obligations hereunder to transfer such Deferred Marketing Authorizations to the Purchasers.
-34-
In no event shall the Closing Purchase Price payable by the Purchasers at the Closing or the Final Purchase Price be reduced or deferred in respect of any Deferred Marketing Authorizations or Deferred Marketing Authorization Liabilities and the Cash, Indebtedness, Working Capital and Gross Profit of or relating to any Deferred Marketing Authorizations and Deferred Marketing Authorization Liabilities will be included in determining the Closing Cash, the WC Adjustment Amount, the Closing Indebtedness, the Closing Gross Profit and the Gross Profit Adjustment Amount pursuant to Section 2.9.
(b)With respect to any Deferred Marketing Authorizations or Deferred Marketing Authorization Liabilities, from the Closing Date until the earliest of (i) the applicable Deferred Closing Date, (ii) the date on which the Purchasers or any of their Subsidiaries obtain a new marketing authorization (including any re-registration) that enables the Purchasers or any of their Subsidiaries to manufacture, distribute and market the applicable Business Products in the applicable jurisdictions in substantially the same manner as, and consistent with, the practice of the Business prior to Closing with respect to each such Deferred Marketing Authorizations (in which case Seller and its Subsidiaries shall, subject to prior consultation with the Purchasers, be entitled to withdraw or terminate such Deferred Marketing Authorizations and be released of their obligations hereunder to transfer such Deferred Marketing Authorizations) and (iii) the date the Purchasers or any of their Subsidiaries obtain a Deferred Marketing Authorization from an authorized third party (e.g., an importer, manufacturer or distributor) that holds such Deferred Marketing Authorization (in which case Seller and its Subsidiaries shall be released of their obligations hereunder to transfer such Deferred Marketing Authorizations), (w) the Purchasers shall, at their sole cost, use reasonable best efforts to obtain, or cause to be obtained the applicable Regulatory Approval or a new marketing authorization (including any re-registration) that enables the Purchasers or their Subsidiaries to manufacture, distribute and market the applicable Business Products in the applicable jurisdictions in substantially the same manner as, and consistent with, the practice of the Business prior to Closing, (x) Seller shall use commercially reasonable efforts to assist the Purchasers in obtaining such Regulatory Approvals, (y) Seller (or its designated Subsidiary) and the Purchasers (or their designated Subsidiary) shall enter into a distribution agreement in substantially the form of the Distribution Agreement, and (z) the Parties shall treat the applicable Purchaser as the owner of any such Deferred Marketing Authorizations and Deferred Marketing Authorization Liabilities for Tax purposes as of the Closing Date. For so long as Seller or any member of the Seller Group holds any Deferred Marketing Authorizations or assumes any Deferred Marketing Authorization Liabilities following the Closing and provides the applicable Purchaser or its Subsidiaries any claims, rights and benefits of any such Deferred Marketing Authorizations or Deferred Marketing Authorization Liabilities pursuant to an arrangement described in this Section 2.11, the Purchasers shall indemnify and hold Seller and its Subsidiaries harmless from and against all Covered Losses incurred or asserted as a result of post-Closing direct or indirect ownership, management or operation of any such Deferred Marketing Authorizations or Deferred Marketing Authorization Liabilities by Seller or any member of the Seller Group.
-35-
Section 2.12Bulk Sales. Each Purchaser on behalf of itself and its Subsidiaries acknowledges that Seller and its Subsidiaries have not taken, and do not intend to take, any actions required to comply with any applicable “bulk transfer law” or “bulk sales law” (or any similar Law) of any jurisdiction. Each Purchaser on behalf of itself and its Subsidiaries waives compliance by Seller and its Subsidiaries with the provisions of any “bulk transfer law” or “bulk sales law” (or any similar law) of any jurisdiction in connection with the Transaction and the other transactions contemplated by this Agreement.
Section 2.13Withholding. The Purchasers and Seller shall be entitled to deduct and withhold (or cause to be deducted and withheld) from any amount payable pursuant to this Agreement such amounts as are required to be deducted and withheld under applicable Law; provided, that the Purchasers and Seller shall each use commercially reasonable efforts to provide the other Party with written notice of the Purchasers’ or Seller’s (as applicable) intention to withhold at least five (5) Business Days prior to any such withholding and the Purchasers and Seller shall cooperate in good faith to minimize any such Taxes. Amounts withheld pursuant to this Section 2.13 and paid over to the appropriate Taxing Authority shall be treated for all purposes of this Agreement as having been paid to the Person in respect of which such deduction and withholding was made.
ARTICLE III
REPRESENTATIONS AND WARRANTIES OF SELLER
Except as disclosed in, or qualified by any matter set forth in, the Seller Disclosure Schedules, Seller hereby represents and warrants to the Purchasers as follows:
Section 3.1Organization and Standing.
(a)Seller and each other Seller Entity is a corporation, partnership or other legal entity duly organized, validly existing and, where applicable, in good standing (or other local equivalent) under the Laws of the jurisdiction of its organization, except where the failure to be so organized, existing or in good standing would not have, individually or in the aggregate, a Business Material Adverse Effect or materially impair, hinder or delay the ability of Seller and its Subsidiaries to perform their respective obligations under this Agreement and the other Transaction Documents or to consummate the transactions contemplated hereby and thereby.
(b)Seller has made available to the Purchasers correct and complete copies of the governing documents of each Purchased Entity, which are in full force and effect, and no Purchased Entity is in breach or in violation of any such governing documents.
Section 3.2Purchased Entities; Capital Structure.
(a)Each of the Purchased Entities is a corporation, partnership or other legal entity duly organized and validly existing, with corporate or other similar applicable power and authority to own, lease and operate its properties and assets and to carry on its respective business, as currently conducted. Each of the Purchased Entities is duly qualified to do business and, where applicable, in good standing (or other local equivalent) in each jurisdiction where the nature of its business or properties makes such qualification necessary, except where the failure to be so qualified or in good standing would not be or reasonably expected to be, individually or in the aggregate, material to the Business and the Purchased Entities, taken as a whole.
-36-
No vote of any stockholders or other equity holders of a Purchased Entity or any of its Affiliates is required for the execution of this Agreement or any other Transaction Document or the consummation of the Transaction or the other transactions contemplated hereby and thereby.
(b)All of the outstanding equity interests of each of the Purchased Entities are validly issued, fully paid and, in the case of any Purchased Entity which is a corporation, non-assessable. There are no outstanding warrants, options, agreements, subscriptions, convertible or exchangeable securities or other commitments pursuant to which any of the Purchased Entities is or may become obligated to issue, sell, purchase, return, redeem or otherwise acquire any equity interests of the Purchased Entities. The Seller Entities own of record or beneficially all of the Purchased Entity Shares free and clear of all Liens, except for restrictions arising under applicable securities Laws, those that are expressly set forth in the governing documents of the Purchased Entities or those that will be discharged and released at or prior to the Closing. None of the issued and outstanding equity interests of the Purchased Entities were issued in violation of any preemptive rights, rights of first refusal, transfer restrictions or similar rights or any applicable Laws or are subject to any Liens (other than restrictions arising under applicable securities Laws, those that are expressly set forth in the governing documents of the Purchased Entities or those that will be discharged and released at or prior to the Closing). None of the Purchased Entities is party to, nor are the issued and outstanding equity securities of the Purchased Entities subject to, any equityholders, stockholders, voting trust or similar agreement or any other Contract related to the voting, transfer, redemption or repurchase of, or that otherwise provides for any rights in respect of, any such equity securities, except for the organizational documents of the Purchased Entities. None of the Purchased Entities owns any equity securities in any other Person or is subject to any obligation or requirement to make any investment (in the form of a loan, capital contribution or otherwise) in any other Person.
(c)Section 3.2(c) of the Seller Disclosure Schedules sets forth (as may be updated prior to the Closing pursuant to clause (iii)), as of the date hereof, (i) the name and the jurisdiction of organization of each of the Purchased Entities, (ii) the record owners of the outstanding equity interests of each of the Purchased Entities, (iii) the number and type of authorized, issued and outstanding equity interests of the Purchased Entities (provided, that, Seller may update Section 3.2(c)(iii) of the Seller Disclosure Schedules from time to time prior to the Closing) and (iv) the percentage interest held by each Seller Entity. None of the Purchased Entities has any Subsidiaries.
Section 3.3Authority; Enforceability.
(a)Seller has all requisite corporate power and authority to execute and deliver this Agreement and each other Transaction Document to which it is or will be a party and to perform its obligations hereunder and thereunder. The execution and delivery by Seller of this Agreement and each such Transaction Document, and the performance by Seller of its obligations hereunder and thereunder, have been, or will have been as of the Closing, duly authorized by all requisite corporate action. Each other Seller Entity has, or will have as of the Closing, all requisite corporate or other similar applicable power and authority to execute and deliver each Transaction Document to which it will be a party and to perform its obligations thereunder. The execution and delivery by each other Seller Entity of each Transaction Document to which it will be a party, if applicable, and the performance by it of its obligations thereunder, have been, or will have been as of the Closing, duly authorized by all requisite corporate or other similar applicable action.
-37-
(b)Seller and each other Seller Entity has, or will have as of the Closing, all requisite corporate or other similar applicable power and authority to carry on its respective business as it pertains to the Business as currently conducted and to own, lease and operate its properties and assets related to the Business, except where the failure to have such power and authority would not materially impair, hinder or delay the ability of Seller and its Subsidiaries to perform their respective obligations under this Agreement and the other Transaction Documents or to consummate the transactions contemplated hereby and thereby.
(c)This Agreement has been duly executed and delivered by Seller and, assuming this Agreement has been duly executed and delivered by the Purchasers, constitutes a valid and binding obligation of Seller, and each other Transaction Document will be as of the Closing duly executed and delivered by each Seller Entity that will be a party thereto and will, assuming such Transaction Document has been duly executed and delivered by each other party thereto, constitute a valid and binding obligation of such Seller Entity, in each case enforceable against such Seller Entity in accordance with its terms, except as enforcement may be limited by bankruptcy, insolvency, reorganization, fraudulent conveyance, moratorium or similar Laws affecting creditors’ rights generally or by general principles of equity (regardless of whether enforcement is sought in a proceeding in equity or law).
Section 3.4No Conflicts; Consents. The execution, delivery and performance of this Agreement and the other Transaction Documents to which Seller or any other Seller Entity will be a party by Seller or such other Seller Entity and the consummation of the transactions contemplated hereby and thereby by Seller or such other Seller Entity do not and will not (a) violate any provision of the certificate of incorporation, bylaws or other organizational documents of Seller, any of the other Seller Entities or any of the Purchased Entities, (b) subject to obtaining the consents set forth in Section 3.4(b) of the Seller Disclosure Schedules, conflict with, constitute a default under, or result in the breach or termination, cancellation or acceleration (whether after the giving of notice or the lapse of time or both) of any right or obligation of the Seller Entities or the Purchased Entities under, or to a loss of any benefit of the Business to which any of the Seller Entities or the Purchased Entities is entitled under any Material Contract or any other Contract that constitutes a Purchased Asset and (c) assuming compliance with the matters set forth in Section 3.5 and Section 4.4, violate or result in a breach of or constitute a default under any Law or other restriction of any Governmental Entity to which any Seller Entity or Purchased Entity is subject; except, with respect to clauses (b) and (c), as would not, individually or in the aggregate, be or reasonably be expected to be material to the Purchased Entities and the Business, taken as a whole.
Section 3.5Governmental Authorizations. The execution, delivery and performance of this Agreement by Seller does not require any Regulatory Approval of, or Filing with, any Governmental Entity, except for (a) compliance with (i) the applicable requirements of the HSR Act and (ii) any other applicable Regulatory Law, (b) compliance with the applicable requirements of the Securities Act and the U.S.
-38-
Securities Exchange Act of 1934, as amended (together with the rules and regulations promulgated thereunder, the “Exchange Act”), (c) compliance with the rules and regulations of the New York Stock Exchange, (d) compliance with any applicable foreign or state securities or blue sky Laws and (e) Regulatory Approvals and Filings which if not obtained or made would not, individually or in the aggregate, be or reasonably expected to be material to the Purchased Entities and the Business, taken as a whole.
Section 3.6Proceedings.
(a)There is no, and during the past three (3) years there has not been any, Proceeding pending or, to the Knowledge of Seller, threatened against a Purchased Entity, Seller or any of its Subsidiaries relating to the Business or any properties or rights of a Purchased Entity, other than Proceedings which would not, individually or in the aggregate, be material to the Purchased Entities and the Business, taken as a whole or which would not, individually or in the aggregate, have a Business Material Adverse Effect.
(b)As of the date of this Agreement, none of the Purchased Entities or the Seller Entities (relating to the Business) is subject to any outstanding Judgment other than those which would not, individually or in the aggregate, be material to the Purchased Entities and the Business, taken as a whole or which would, individually or in the aggregate, have a Business Material Adverse Effect.
Section 3.7Financial Statements; Absence of Undisclosed Liabilities.
(a)Section 3.7(a) of the Seller Disclosure Schedules sets forth correct and complete copies of the unaudited statement of assets and liabilities of the Business and related income statement as of and for the fiscal year ended December 31, 2023 (collectively, the “Business Financial Information”).
(b)The Business Financial Information (i) has been derived from the financial statements of Seller and its Subsidiaries, which have been prepared in accordance with GAAP, consistently applied during the periods involved, and (ii) fairly presents in all material respects (A) the financial condition, assets and liabilities of the Business as of the dates therein specified and (B) the results of operations of the Business for the periods indicated therein; provided, that the Business Financial Information does not include footnote disclosures (which if presented would not materially change the financial position and results of operations of the Business), is unaudited, may not include all adjustments that an audit may require and may include certain normalized adjustments that audited or unaudited financial statements may not require as set forth on Section 3.7(b) of the Seller Disclosure Schedules; provided, further, that the Business Financial Information and the foregoing representations and warranties are qualified by the fact that the Business has not operated as a separate standalone entity and has received certain allocated charges and credits which do not necessarily reflect amounts that would have resulted from arm’s-length transactions or that the Business would incur on a standalone basis.
(c)Seller and its Subsidiaries maintain systems of internal accounting controls designed to provide reasonable assurances (i) regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP in all material respects, (ii) the receipts and expenditures of the Business are being made in accordance with appropriate authorizations of management of the Seller Group and (iii) regarding prevention or timely detection of unauthorized acquisition, use or disposition of the assets of the Business that could have a material effect on the financial statements of the Business, it being understood that Seller’s policies and procedures are designed and implemented giving effect to Seller’s entire business and therefore levels of materiality and other determinations made with respect to Seller may not be the same as if the Business were operated on a standalone basis.
-39-
(d)Except as set forth on Section 3.7(d) of the Seller Disclosure Schedule, as of the date hereof, the Business does not have any Liabilities that would be required by GAAP to be reflected on a balance sheet of the Business, other than Liabilities that: (i) are specifically reflected and adequately reserved against in the most recent balance sheet and included in the Business Financial Information (“Balance Sheet Date”), (ii) were incurred since the Balance Sheet Date in the ordinary course of business (none of which is a Liability resulting from breach of contract, breach of warranty, tort, infringement or misappropriation or violation of Law), (iii) are Retained Liabilities, (iv) are disclosed in Section 3.7(d) of the Seller Disclosure Schedules, or (v) would not, individually or in the aggregate, be material to the Purchased Entities and the Business, taken as a whole.
(e)As of the date hereof, the Business meets the requirements for abbreviated financial statements set forth in Rule 3-05(e)(1)(i), (ii), (iii) and (iv) of Regulation S-X under the Securities Act.
Section 3.8Absence of Changes or Events. (a) Since the Balance Sheet Date through the date of this Agreement, the Business has been conducted in all material respects in the ordinary course of business, (b) since the Balance Sheet Date, there has not been any Business Material Adverse Effect and (c) since Balance Sheet Date through the date of this Agreement, no Seller Entity or Purchased Entity has, in relation to the Purchased Entities or the conduct of the Business, taken any action set forth in clauses (ii), (iii), (iv), (v), (vi), (viii), (ix), (x), (xii), (xiii), (xiv), (xix) or (xxiii) (to the extent clause (xxiii) relates to any of the foregoing clauses of Section 5.2(b)) of Section 5.2(b), that, if taken following the execution of this Agreement until the Closing, would require the consent of the Purchasers.
Section 3.9Title to Assets; Sufficiency of Assets.
(a)Except as would not, individually or in the aggregate, be material to the Purchased Entities and the Business, taken as a whole, the Seller Entities or the Purchased Entities will (assuming all Regulatory Approvals as may be required in connection with the consummation of the Transaction and the other transactions contemplated by this Agreement have been obtained) have as of the Closing good title to, or other legal rights to possess, use, and transfer, all of the Purchased Assets (other than any Deferred Marketing Authorizations) as conducted immediately prior to the Closing, free and clear of all Liens other than Permitted Liens.
(b)Except as set forth on Section 3.9(b) of the Seller Disclosure Schedules, the Purchased Assets (assuming all Regulatory Approvals as may be required in connection with the consummation of the Transaction and the other transactions contemplated by this Agreement have been obtained and assuming that any Deferred Marketing Authorizations are transferred to the Purchasers at the Closing), together with the services, rights and benefits to be provided pursuant to the Transaction Documents, shall, in the aggregate, constitute all of the rights, materials, goods, services, properties and assets (tangible or intangible) in all material respects necessary for the Purchasers and their Subsidiaries (including the Purchased Entities) to conduct the Business immediately following the Closing in substantially the same manner as conducted by Seller and its Subsidiaries as of the date of this Agreement.
-40-
Section 3.10Intellectual Property.
(a)Section 3.10(a) of the Seller Disclosure Schedules lists all Registered IP included in the Transferred Intellectual Property, as of the date of this Agreement (the “Business Registered IP”).
(b)Except as would not, individually or in the aggregate, reasonably be expected to be material to the Purchased Entities and the Business, taken as a whole: (i) the applicable Seller Entities or the Purchased Entities are current in the payment of all registration, maintenance and renewal fees with respect to the Business Registered IP, except in each case as Seller or any of its Subsidiaries has elected in its reasonable business judgment to abandon or permit to lapse a registration or application for any Intellectual Property Rights, (ii) the Business Registered IP and all Transferred Intellectual Property are solely owned by the Seller Entities or the Purchased Entities free and clear of all Liens, other than Permitted Liens, (iii) the Business Registered IP is subsisting, valid and, to the Knowledge of Seller, not unenforceable and (iv) as of the Closing, Purchasers or their Subsidiaries (including the Purchased Entities) will be the sole and exclusive owner of all Transferred Intellectual Property and, taking into account the services, rights and benefits to be provided pursuant to the Transaction Documents, will have the right or license to use all other Intellectual Property Rights necessary for the Purchasers and their Subsidiaries (including the Purchased Entities) to conduct the Business immediately following the Closing in substantially the same manner as conducted by Seller and its Subsidiaries as of the date of this Agreement.
(c)Except as would not reasonably be expected to be material to the Purchased Entities and the Business, taken as a whole: (i) none of the Transferred Intellectual Property is subject to any Judgment adversely affecting the use thereof or rights thereto by Seller and its Subsidiaries or the Purchased Entities; (ii) there is no opposition or cancellation Proceeding pending against Seller and its Subsidiaries or the Purchased Entities as of the date of this Agreement concerning the ownership, validity or enforceability of any Business Registered IP; (iii) as of the date of this Agreement, none of Seller, its Subsidiaries or the Purchased Entities, has received any written notice within the three (3)-year period prior to the date of this Agreement alleging that the conduct of the Business infringes, misappropriates, or violates the Intellectual Property Rights of any other Person; (iv) none of Seller, its Subsidiaries, the Purchased Entities as of the date hereof or within the three (3)-year period prior to the date of this Agreement has alleged (including by initiating any claim or action) that a third Person is infringing, misappropriating, or violating the Transferred Intellectual Property; and (v) since the date that is three (3) years prior to the date hereof, the conduct of the Business has not infringed, misappropriated, or violated the Intellectual Property Rights of any other Person.
-41-
(d)Except as would not reasonably be expected to be material to the Purchased Entities and the Business, taken as a whole, (i) Seller or its Subsidiaries, and the Purchased Entities, as applicable, have taken commercially reasonable measures to protect and maintain the confidentiality of Trade Secrets included in the Transferred Intellectual Property, (ii) there have been no material unauthorized uses or disclosures of any such Trade Secrets within the three (3)-year period prior to the date of this Agreement, except in the ordinary course of business pursuant to a written confidentiality, non-use, and non-disclosure agreement with the Purchased Entities, and (iii) to the Knowledge of Seller, there have been no breaches of any such agreements.
(e)Except as would not reasonably be expected to be material to the Purchased Entities and the Business, taken as a whole, all current and former officers and employees of, and consultants and independent contractors to, Seller and its Subsidiaries, or the Purchased Entities, as applicable, that were involved in the conception, creation, authoring, reduction to practice, or development of Transferred Intellectual Property have agreed to, or are subject to policies, restricting the disclosure and use of any confidential information (except for use for the benefit of Seller and its Subsidiaries) and have assigned either by operation of law, or by granting an assignment, to Seller or any of its Subsidiaries, or the applicable Purchased Entity, of all rights in and to any Transferred Intellectual Property conceived, created, authored, reduced to practice, or developed pursuant to services performed for the Business by such Persons for the Business.
Section 3.11Information Technology; Data Protection.
(a)Except as would not reasonably be expected to be material to the Purchased Entities and the Business, taken as a whole, during the three (3)-year period immediately prior to the date of this Agreement, there have not been any material vulnerabilities or defects that have resulted in any security breaches or unauthorized access or other security access incidents involving the unauthorized processing, access, deletion, exfiltration, use, loss, destruction, compromise, transfer, or disclosure of any Personal Data or Trade Secrets collected or received by the Purchased Entities, or adversely affecting, including resulting in a partial or complete loss of control of, the Transferred Information Technology.
(b)Except as would not reasonably be expected to be material to the Purchased Entities and the Business, taken as a whole, (i) Seller, its Subsidiaries and the Purchased Entities are and have been during the three (3) year period immediately prior to the date of this Agreement in compliance in all material respects with all applicable Data Protection Laws with respect to the Business, as well as their own rules, policies and procedures, relating to privacy, data protection and the collection, retention, protection, transfer, use and processing of Personal Data with respect to the Business; (ii) none of Seller, its Subsidiaries or the Purchased Entities is party to any pending or threatened Proceedings, or has, during the three (3) year period immediately prior to the date of this Agreement, received any written notice from any applicable Governmental Entity or been party to any Proceeding, in each case, alleging a violation of any Data Protection Laws with respect to the Business or otherwise in connection with the processing of Personal Data; (iii) the Purchased Entities (and the Seller Entities with respect to Personal Data collected or used in connection with the Business) have taken appropriate actions (including implementing reasonable technical, physical or administrative safeguards) to protect the Transferred Information Technology, Trade Secrets, and all Personal Data in their possession or under their control against any unauthorized use, access or disclosure; and (iv) during the three (3) year period immediately prior to the date of this Agreement, there have been no successful phishing incidents, ransomware or malware attacks or any unauthorized use, loss, access, destruction, processing, acquisition, compromise, transfer, or disclosure of any Personal Data or Trade Secrets collected or received by the Purchased Entities, or other security incident of or involving Personal Data collected by or used in connection with the Business.
-42-
(c)Except as would not reasonably be expected to be material to the Purchased Entities and the Business, taken as a whole, (i) the Purchased Entities own, lease, license, or otherwise have the legal right to use all Transferred Information Technology, and (ii) all Transferred Information Technology (A) is free from any material defect, bug, virus or programming, design or documentation error or corruptant that permit, or are designed to permit, unauthorized access to any Transferred Information Technology, (B) operates and runs in a reasonable and efficient business manner, and (C) is sufficient for the current and currently contemplated needs of the Business.
Section 3.12Real Property.
(a)Except as would not be or reasonably be expected to be, individually or in the aggregate, material to the Purchased Entities and the Business, taken as a whole:
(i)Seller, a Seller Entity or a Purchased Entity has good and valid fee simple title to the applicable Owned Real Property, free and clear of any Liens, other than Permitted Liens. Except as set forth in Section 3.12(a)(i) of the Seller Disclosure Schedules, as of the date hereof, (A) none of Seller, a Seller Entity or a Purchased Entity has leased or otherwise granted to any Person the right to use or occupy such Owned Real Property or any portion thereof and (B) the Purchased Entities are not a party to any agreement or option to purchase any real property or interest therein. None of the Owned Real Property is subject to any first refusal, purchase option, right to purchase or other similar right.
(ii)Seller, a Seller Entity or a Purchased Entity has a legal, valid, binding, enforceable and in full force and effect leasehold estate (as lessee) in all Leased Real Property as lessee or sublessee, in each case free and clear of all Liens, other than Permitted Liens. Seller has delivered to the Purchasers a true and complete copy of each such Transferred Lease as in effect as of the date hereof. Except as set forth in Section 3.12(a)(ii) of the Seller Disclosure Schedules, with respect to each of the Transferred Leases, as of the date hereof: (A) none of Seller, a Seller Entity or a Purchased Entity has subleased, licensed or otherwise granted any Person the right to use or occupy such Leased Real Property or any portion thereof, and (B) none of Seller, a Seller Entity or a Purchased Entity has collaterally assigned or granted any other security interest in such Transferred Lease or any interest therein.
(b)Except as would not have, individually or in the aggregate, a Business Material Adverse Effect, (i) all improvements located on the Owned Real Property have received all necessary Regulatory Approvals of Governmental Entities (including licenses and permits)
-43-
required in connection with the use thereof being made as of the date of this Agreement, (ii) there are no judicial or administrative Proceedings pending or, to the Knowledge of Seller, threatened in writing under any condemnation, environmental, zoning, eminent domain, land-use or other Law applicable to the Owned Real Property which, if adversely decided, would interfere with the present use in the Business of the Owned Real Property, (iii) there are no outstanding unpaid assessment notices against any of the Owned Real Property and (iv) all buildings, structures, improvements, fixtures, building systems and equipment, and all components thereof, included in the Owned Real Property are in good condition and repair and sufficient for the operation of the Business. The Owned Real Property and the Leased Real Property comprise all of the real property exclusively used in the Business.
Section 3.13Contracts.
(a)Section 3.13(a) of the Seller Disclosure Schedules sets forth the following Contracts to which any Purchased Entity or any Seller Entity (with respect to the Business) is a party to or bound by as of the date hereof (other than sales or purchase orders, statements of work, standard terms and conditions and similar instruments entered into or used in the ordinary course of business) (the “Material Contracts”):
(i)the Contracts with the ten (10) largest customers of the Business (measured by aggregate dollar amounts received by the Business in the applicable region in the fiscal year ended December 31, 2023) (such counterparties, the “Significant Customers”) for the provision of Business Products;
(ii)the Contracts with the ten (10) largest suppliers of the Business (measured by dollar amounts spent by the Business in the fiscal year ended December 31, 2023) (such counterparties, the “Significant Suppliers”) for the supply of services, products or materials for use in the Business;
(iii)each Contract providing for (A) the acquisition or disposition by the Business of any business or assets (whether by merger, sale of stock, sale of assets or otherwise), excluding ordinary course commercial Contracts, in the last three (3) years and (B) continuing earn-out, indemnification, deferred or contingent purchase price or other similar obligations following the date of this Agreement;
(iv)each Contract providing for a joint venture or other joint development agreement involving future payments or capital commitments or similar partnership agreement or arrangement with a third party;
(v)each Contract providing for indebtedness for borrowed money in excess of $250,000 owed to a Person that is currently outstanding, or otherwise granting a Lien to secure indebtedness for borrowed money, in each case, other than (A) any such indebtedness to the extent owing from any of the Purchased Entities to any of the other Purchased Entities to the extent reconciled or eliminated in the consolidated financial statements of the Business at or prior to Closing or (B) any such indebtedness to be repaid or extinguished at or prior to the Closing;
-44-
(vi)each Contract requiring future capital expenditure obligations of the Business in excess of $250,000;
(vii)each Contract relating to (A) the licensing of Intellectual Property Rights that are material to the Purchased Entities and the Business, taken as a whole, including any license granted (x) on an exclusive basis to any third party with respect to any Transferred Intellectual Property, or (y) to any of the Purchased Entities or Seller Entities with respect to Intellectual Property Rights of third parties, or (B) the Business’s ability to own, enforce, license or disclose any material Transferred Intellectual Property or relating to the acquisition, divestiture or development of any material Transferred Intellectual Property; excluding, in each case, licenses for commercially-available software or other Technology that involved aggregate annual payments less than $250,000 in the fiscal year ended December 31, 2023;
(viii)each Transferred Lease;
(ix)each Contract that by its express terms materially limits or materially impairs the ability of the Seller Entities (with respect to the Business) or the Purchased Entities to compete in any line of business or with any Person or in any geographic area (including through non-compete or exclusivity provisions), other than customary exclusive distribution agreements for the Business Products entered into in the ordinary course of business consistent with past practice;
(x)(A) each material Contract that contains any “most favored nation” or similar provision or (B) each Contract that contains minimum order or purchase requirements in excess of $250,000;
(xi)any Contract of lease of any personal property requiring future payments in excess of $250,000 for any successive calendar year; and
(xii)each Contract that is a settlement, conciliation or similar agreement with (A) any Governmental Entity, (B) entered into in the last three (3) years or (C) pursuant to which the Business or the Purchased Entities will have any material outstanding obligation after the date of this Agreement.
(b)(i) Each Material Contract is valid and binding on the Seller Entity or Purchased Entity that is a party thereto and, to the Knowledge of Seller, each other party thereto, and is in full force and effect, except as enforcement may be limited by bankruptcy, insolvency, reorganization, fraudulent conveyance, moratorium or similar Laws affecting creditors’ rights generally or by general principles of equity (regardless of whether enforcement is sought in a proceeding in equity or law), and (ii) no Seller Entity or Purchased Entity or, to the Knowledge of Seller, any other party thereto, is in breach of, or default under, any such Material Contract. No Seller Entity or Purchased Entity has received any written, or to the Knowledge of Seller, other claim or notice of any other party’s intention to terminate, modify or allow to expire, any such Material Contract, and to the Knowledge of Seller, there is not, and in the past three (3) years has not been, any dispute with respect to any Material Contract, in each case, which would be or reasonably expected to be material to the Business and the Purchased Entities, taken as a whole.
-45-
Except for any Shared Contracts, correct and complete copies of each Material Contract in effect as of the date hereof have been made available to the Purchasers.
Section 3.14Significant Customers; Significant Suppliers.
(a)Section 3.14(a) of the Seller Disclosure Schedules lists the names of each of the Significant Customers. To the Knowledge of Seller, none of the Significant Customers has modified, allowed to expire or terminated its relationship with the applicable Seller Entity or Purchased Entity. No member of the Seller Group or Purchased Entity has received any written notice that any Significant Customer has ceased or will cease to be a customer of the Business or that such Significant Customer intends to terminate, allow to expire or materially modify any existing Material Contract with any Seller Entity or Purchased Entity, as applicable. No Seller Entity or Purchased Entity has been engaged in the past three (3) years, or is currently engaging, in a material dispute with any Significant Customer.
(b)Section 3.14(b) of the Seller Disclosure Schedules lists the names of each of the Significant Suppliers. To the Knowledge of Seller, none of the Significant Suppliers has modified, allowed to expire or terminated its relationship with the applicable Seller Entity or Purchased Entity. No member of the Seller Group or Purchased Entity has received any written notice that any Significant Supplier has ceased or will cease to act as a supplier of the Business or that such Significant Supplier intends to terminate, allow to expire or materially modify any existing Material Contract with any Seller Entity or Purchased Entity, as applicable. No member of the Seller Group or Purchased Entity has been engaged in the past three (3) years, or is currently engaging, in a material dispute with any Significant Supplier.
Section 3.15Transferred Marketing Authorizations; Recalls.
(a)Section 3.15(a) of the Seller Disclosure Schedules sets forth, as of the date of this Agreement, a list of (i) the marketing applications, regulatory or marketing approvals, clearances or other authorizations used to sell, distribute or market the Business Products and granted by any Governmental Entity, (ii) any Investigational New Animal Drug (INAD) files, data, information or applications that are part of the Business or related to Business Products and (iii) any Veterinary Master File (VMF) related to the Business or Business Products (“Transferred Marketing Authorizations”).
(b)Except as would not, individually or in the aggregate, be or reasonably expected to be material to the Purchased Entities and the Business, taken as a whole, Seller or its Subsidiaries have exclusive ownership, or have obtained beneficial use, of all Transferred Marketing Authorizations, and all such Transferred Marketing Authorizations are and, to the extent freely transferrable by Seller and its Subsidiaries, will be immediately after the Closing, valid and in full force and effect. No Proceedings with respect to Transferred Marketing Authorizations are (i) pending against any member of the Seller Group or, to the Knowledge of Seller, against any third-party holding a Transferred Marketing Authorization, or (ii) to the Knowledge of Seller, threatened in writing to revoke, suspend, or modify any Transferred Marketing Authorization; (c)For the past twelve (12) months, none of Seller or any other member of the Seller Group has distributed, used, sold, marketed, manufactured, licensed, labeled, packaged, authorized any use of, offered a right of reference to, or letter of authorization for, any Additional Business Product corresponding to sales in excess of $2,000,000 during such period;
-46-
(d)For the past three (3) years, there has not been conducted or requested in writing, nor, to the Knowledge of Seller, is there any current consideration by Seller or any of its Subsidiaries or any Governmental Entity of, any recall in respect of any Business Product; and
(e)Except for ordinary course inquiries by Governmental Entities, there are not pending, or, to the Knowledge of Seller, threatened in writing, any Product Claims with respect to which liability is reasonably anticipated to exceed $500,000.
Section 3.16Compliance with Applicable Laws; Permits.
(a)None of the Seller Entities or the Purchased Entities is in violation of any Law applicable to the conduct of the Business as presently conducted, except for violations that would not, individually or in the aggregate, be material to the Purchased Entities and the Business, taken as a whole.
(b)(i) Within five (5) years prior to the date of this Agreement, none of the Seller Entities or the Purchased Entities or any of their respective officers, employees, or directors, nor, to the Knowledge of Seller, any of their agents or third party representatives, in each case, with respect to the Business, has made, paid, authorized, promised, received, or accepted any gift, bribe, payoff or kickback to or from any person in violation of any applicable Law relating to anti-bribery, anti-money laundering, or anti-corruption, including the U.S. Foreign Corrupt Practices Act of 1977, as amended (collectively, “Anti-Corruption Laws”) or otherwise been in violation of Anti-Corruption Laws and (ii) to the Knowledge of Seller, none of the Seller Entities or the Purchased Entities, in each case with respect to the Business, is under Governmental Entity investigation for, or has received any written notice from a Governmental Entity regarding, any violation of any Anti-Corruption Laws.
(c)The Seller Entities and/or the Purchased Entities hold all material Permits necessary for the conduct of the Business as presently conducted. The Seller Entities and the Purchased Entities, in each case with respect to the Business, are in compliance in all material respects with such Permits. The Seller Entities and/or the Purchased Entities hold all Permits required by all applicable FDA Laws and state animal feed regulations or animal remedies regulations, which Permits are in full force and effect, and no material suspension, cancellation, withdrawal, or revocation thereof is pending or, to the Knowledge of Seller, threatened.
(d)Each Seller Entity and Purchased Entity has complied in the past three (3) years in all material respects with all FDA Laws applicable to the Business Products and all other U.S. federal and state statutes and regulations governing the research, development manufacturing, packaging, labeling, storage, distribution, marketing and/or advertising of the Business Products. To the Knowledge of Seller, each of the Seller Entities and/or Purchased Entities has not in the past three (3) years introduced or caused the introduction into commerce of any Business Products, and does not hold any inventory, which is/are or was/were in any material respect “adulterated,” “misbranded,” an article which may not be introduced into interstate commerce under any provision of the FDCA, or otherwise violative within the meaning of the FDCA and/or under any other applicable legal requirement that would reasonably be expected to result in any material liability to the Business and the Purchased Entities, taken as a whole, under the FDA Laws applicable to the Business Products.
-47-
(e)Within the past three (3) years, there have been no pending or, to the Knowledge of Seller, threatened recalls, market withdrawals, stock recovery or public notifications, field notifications, food safety alerts, or similar actions with respect to any Business Products or other similar federal, state, or private actions with respect to such Business Products and, to the Knowledge of Seller, no facts or circumstances exist that would reasonably be expected to (i) result in the recall, market withdrawal or stock recovery of any Business Product, (ii) cause, as a result of any regulatory action by any Governmental Entity, (x) a material change in the research or development status or labeling of any Business Product or (y) a termination or suspension of the research, development, marketing, labeling or distribution of any Business Product, or (iii) result in any material liability to the Business and the Purchased Entities, taken as a whole, under the FDA Laws applicable to the Business Products.
(f)Except as set forth on Section 3.16(f) of the Seller Disclosure Schedules, in the past three (3) years, none of the Seller Entities and/or Purchased Entities has received any FDA Form 483, FDA Warning Letter, Federal Trade Commission Letter, or any written notice of an action brought by the United States under FDA Laws or the U.S. Federal Trade Commission Act with respect to any Business Products, nor has any Seller Entity or Purchased Entity received any comparable written warnings or notices from any other Governmental Entity and to the Knowledge of Seller, there are no such pending or threatened actions, in each case with respect to any Business Products.
(g)Each Seller Entity and Purchased Entity possesses, in all material respects, any required certification, and adequate substantiation, for any claims, promises, and affirmations of fact made with respect to any Business Product, including on the product labeling and in promotional materials.
Section 3.17Environmental Matters.
(a)(i) The Seller Entities and the Purchased Entities are, and for the past three (3) years have been, in compliance in all material respects with all Environmental Laws applicable to the conduct of the Business or the occupation of the Business’s properties or facilities, (ii) the Seller Entities and the Purchased Entities have obtained, maintained and are, and for the past three (3) years have been, in compliance in all material respects with all Permits pursuant to Environmental Laws required for the operation of the Business or the occupation of the Business’s properties or facilities, (iii) as of the Closing Date and for the past three (3) years (or earlier to the extent unresolved), the Seller Entities and the Purchased Entities have not received any written notice, report or other information and there are and have been no Proceedings pending, or to the Knowledge of Seller threatened, against the Seller Entities or the Purchased Entities, in each case alleging a material violation of, or material Liability under, Environmental Laws with respect to the Business or the Business’s properties or facilities, and (iv) there has been no storage, handling, treatment, transportation, disposal, arranging for or permitting the disposal, or release of, exposure of any Person to, or ownership or operation of any property or facility contaminated by, any Hazardous Material (or products or other items containing any Hazardous Material), in each case that would give rise to material Liability of the Seller Entities or Purchased Entities with respect to the Business and the Purchased Assets, taken as a whole, under Environmental Laws.
-48-
(b)Seller has made available to the Purchasers copies of all material environmental, health or safety audits, assessments and reports and other material environmental, health or safety documents for the past five (5) years relating to the current or former properties, facilities or operations of the Business, Purchased Assets or Purchased Entities, which are in its possession or under its reasonable control.
Section 3.18Taxes.
(a)Except with respect to (1) Taxes of any member of the Seller Group or (2) any Tax Return of any consolidated, combined, affiliated or unitary group that includes any member of the Seller Group, including any Combined Tax Return, (i) all material Tax Returns required to be filed by the Purchased Entities have been timely filed (taking into account extensions) and all such Tax Returns are correct and complete in all material respects; (ii) all material amounts of Taxes shown to be due on such Tax Returns have been paid; (iii) there is no pending Tax Proceeding by any Taxing Authority with respect to any material Taxes of the Purchased Entities; (iv) each of the Purchased Entities has complied in all material respects with all applicable Laws relating to the withholding of Taxes; and (v) there are no Liens for Taxes upon any of the Purchased Assets or any assets of a Purchased Entity other than Permitted Liens.
(b)The Purchased Entities have not been a member of an affiliated group within the meaning of Section 1504(a) of the Code (or any similar group defined under a similar provision of state, local, or foreign Law) filing a consolidated, affiliated or unitary income Tax Return, and the Purchased Entities do not have any liability for the Taxes of any Person under Treasury Regulations Section 1.1502-6 or any similar provision of Law, by contract (other than agreements not primarily related to Taxes), or as a transferee or successor.
(c)No closing agreements, private letter rulings, technical advice memoranda or similar agreements or rulings have been entered into with or issued by any Taxing Authority with respect to the Purchased Entities.
(d)The Purchased Entities will not be required to include any material item of income in, or exclude any material item of deduction from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting made prior to the Closing; (ii) use of an improper method of accounting prior to the Closing; (iii) “closing agreement” as described in Code Section 7121 (or similar or corresponding provision of Law) executed prior to the Closing; (iv) installment sale made prior to the Closing; or (v) prepaid amount received prior to the Closing outside the ordinary course of business.
(e)Each Purchased Entity is classified as an entity disregarded as separate from its owner for U.S. federal income Tax purposes.
-49-
(f)For the past three (3) years, the Purchased Entity Shares have been owned by a “controlled foreign corporation” as defined in Section 957 of the Code.
Section 3.19Benefit Plans.
(a)Section 3.19(a) of the Seller Disclosure Schedules sets forth a list, as of the date of this Agreement, of each material Seller Benefit Plan and identifies each such plan that is a Purchased Entity Benefit Plan.
(b)With respect to each material Purchased Entity Benefit Plan, Seller has made available to the Purchasers, to the extent applicable (i) the summary plan description, and the written document evidencing each Purchased Entity Benefit Plan or, with respect to any such plan that is not in writing, a written description of the material terms thereof, and all material amendments or material supplements to any Purchased Entity Benefit Plan, (ii) for any U.S. Purchased Entity Benefit Plan, the annual report (Form 5500), if any, filed with the IRS for the last plan year, and the most recently received IRS determination letter or prototype opinion letter, (iii) any related trust agreements, insurance contracts or documents of any other funding arrangements relating to a Purchased Entity Benefit Plan, (iv) the applicable portions of the most recently prepared actuarial report or financial statement relating to a U.S. Purchased Entity Benefit Plan, and (v) all material nonroutine correspondence with a Governmental Entity with respect to any U.S. Purchased Entity Benefit Plans within the past three (3) years. With respect to each Seller Benefit Plan that is not a Purchased Entity Benefit Plan, Seller has made available to the Purchasers the most recent summary plan description or a written description of the plan’s material terms.
(c)No Purchased Entity Benefit Plan is subject to Title IV of ERISA and with respect to each Seller Benefit Plan, (i) there does not exist any failure to meet the “minimum funding standard” of Section 412 of the Code or 302 of ERISA (whether or not waived), (ii) such plan is not in “at-risk” status for purposes of Section 430 of the Code and (iii) no reportable event within the meaning of Section 4043(c) of ERISA has occurred.
(d)Except as would not, individually or in the aggregate result in a material Liability to the Purchased Entities and the Business, taken as a whole, each Purchased Entity Benefit Plan that is intended to be qualified within the meaning of Section 401(a) of the Code has received a favorable determination letter as to its qualification or is covered by a prototype plan opinion letter, and nothing has occurred that would reasonably be expected to adversely affect the qualification of such U.S. Purchased Entity Benefit Plan.
(e)Each Purchased Entity Benefit Plan has been operated in all material respects in compliance with its terms and applicable Law and no event has occurred and no condition exists with respect to such Purchased Entity Benefit Plan, that has subjected, or would reasonably be expected to subject, any Purchased Entity to any material Liability imposed by ERISA, the Code or any other applicable Law.
(f)No Purchased Entity Benefit Plan is a Multiemployer Plan and, in the six (6) years prior to the date hereof, no Purchased Entity or an ERISA Affiliate of the Purchased Entities has withdrawn from a Multiemployer Plan to which the Purchased Entities or their ERISA Affiliates contribute in respect of Business Employees in a “complete withdrawal” or a “partial withdrawal” as defined in Sections 4203 and 4205 of ERISA, respectively, so as to result in a Liability of the Purchased Entities or their ERISA Affiliates that has not been fully paid.
-50-
(g)Except as would not, individually or in the aggregate, be material to the Purchased Entities and the Business, taken as a whole, there is no pending or, to the Knowledge of Seller, any threatened claim, action, suit, audit, Proceeding, investigation, litigation, inquiry, or other disputes on behalf or relating to each Purchased Entity Benefit Plan (other than routine claims for benefits thereunder), and no Purchased Entity Benefit Plan has within the three (3) years prior to the date hereof been the subject of an examination, investigation or audit by a Governmental Entity.
(h)Except as required by applicable Law, neither the execution of this Agreement nor the consummation of the Transaction will (either alone or in combination with any other event) (i) result in any material payment becoming due to any Business Employee or other individual service provider of the Business or the Purchased Entities under any Purchased Entity Benefit Plan or Seller Benefit Plan, (ii) materially increase the amount or value of, any compensation or benefits payable to any Business Employee or other individual service provider of the Business or the Purchased Entities under any Purchased Entity Benefit Plan, (iii) result in the acceleration of the time of payment, funding or vesting of any compensation or benefits of any Business Employee or other individual service provider of the Business or the Purchased Entities under any Purchased Entity Benefit Plan or Seller Benefit Plan, or (iv) result in any “parachute payment” (as such term is defined in Section 280G of the Code).
(i)Each Purchased Entity Benefit Plan that constitutes in any part a “nonqualified deferred compensation plan” (within the meaning of Section 409A of the Code) has been established, operated and maintained at all times in all material respects in operational and documentary compliance with Section 409A of the Code and applicable guidance thereunder and no amount under any such Purchased Entity Benefit Plan has been, is or could reasonably be expected to be, subject to material interest or additional Tax under Section 409A of the Code.
(j)Neither the Seller Entities nor the Purchased Entities maintain any obligations to indemnify, gross-up or reimburse any Business Employee or other individual service provider of the Business or the Purchased Entities for any Taxes or related interest or penalties incurred by such individual under Sections 409A or 4999 of the Code.
(k)With respect to each Purchased Entity Benefit Plan that is subject to the Laws of a jurisdiction other than the United States (whether or not United States law also applies) (a “Non-U.S. Purchased Entity Benefit Plan”): (i) all material employer and employee contributions to each Non-U.S. Purchased Entity Benefit Plan required by law or by the terms of such Purchased Entity Benefit Non-U.S. Plan have been timely made, or, if applicable, accrued in accordance with normal accounting practices, (ii) each Purchased Entity Benefit Non-U.S. Plan required to be registered has been registered and has been maintained in good standing with applicable regulatory authorities, except where any failure to comply would not, individually or in the aggregate, be reasonably expected to result in a material Liability to the Purchased Entities and the Business, taken as a whole, (iii) no Non-U.S. Purchased Entity Benefit Plan is a defined benefit plan (as defined in ERISA, whether or not subject to ERISA), and (iv) there are no material unfunded or underfunded material Liabilities with respect to any Non-U.S.
-51-
Purchased Entity Benefit Plan.
Section 3.20Labor Matters.
(a)Section 3.20(a) of the Seller Disclosure Schedules sets forth each Business Employee, and for each: (i) employee identification number; (ii) job title; (iii) primary work location (including country and state); (iv) hourly wage or base salary (as applicable); (v) target annual incentive compensation opportunity; (vi) exempt or non-exempt status (for U.S. Business Employees); (vii) active or inactive status (and as applicable, type of leave and anticipated return date); (viii) full-time or part-time status; (ix) visa status (as applicable); (x) union or non-union status; (xi) date of hire; (xii) employing entity; and (xiii) accrued unused paid time off. The Business Employees are sufficient in number and skill to operate the Business in substantially the same manner as conducted by the Purchased Entities and Seller Group prior to the Closing. Other than the Business Employees, there are no employees of the Seller Group that primarily provide services to the Business.
(b)Section 3.20(b) of the Seller Disclosure Schedules sets forth a list of each Collective Bargaining Agreement to which any Purchased Entity or Seller Entity (with respect to the Business) is a party or bound. Seller has made available to the Purchasers a copy of each such Collective Bargaining Agreement, including any national or industry contractor arrangement. Other than the Collective Bargaining Agreements set forth on Section 3.20(b) of the Seller Disclosure Schedules, there are no Collective Bargaining Agreements that pertain to any Business Employees, and none are currently being negotiated; and no Business Employees are represented by any labor union, labor organization, works council, employee representative or group of employees. There are no pre-signing legal or contractual requirements to provide notice or information to, bargain with, enter into any consultation procedure with, or obtain consent from, any labor union, works council, labor organization or employee representative, which is representing any Business Employees, or any applicable labor tribunal, in connection with the execution of this Agreement.
(c)To the Knowledge of Seller, in the past three (3) years, (i) there has been no organizational effort made or threatened by, or on behalf of, any Business Employees, labor organization, union, or association, works council, or other employee representative body with respect to any Business Employees, and (ii) no application, request or demand for recognition or certification of any Business Employees has been made by, or on behalf of, any Business Employees, labor organization, union, or association, works council, or other employee representative body.
(d)Except as would not, individually or in the aggregate, be material to the Purchased Entities and the Business, taken as a whole, (i) in the past three (3) years, there have been no actual or, to the Knowledge of Seller, threatened, unfair labor practice charges, labor grievances, labor arbitrations, strikes, work stoppages, slowdowns, picketing, hand billing, lockouts, or other labor disputes involving Business Employees or against or affecting the Purchased Entities or the Business and (ii) in the past three (3) years, there have been no unfair labor practice charges, strikes, or lockouts impacting the Business Employees.
-52-
(e)Each Purchased Entity and Seller Entity (with respect to the Business) has promptly, thoroughly, and impartially investigated all sexual harassment, or other harassment, discrimination, or retaliation allegations made during the past three (3) years relating to employees with an annual base compensation in excess of $150,000, officers or directors of such entities and of which the Seller has Knowledge. With respect to each such allegation with potential merit, the applicable Purchased Entity or Seller Entity has taken prompt corrective action reasonably calculated to prevent further improper action. No Purchased Entity or Seller Entity (with respect to the Business) (i) reasonably expects to incur any material Liability with respect to any such allegation or (ii) is aware of any such allegations that would indicate a breach of fiduciary duty or that, if known to the public, would bring the Business or any Purchased Entity into material disrepute.
Section 3.21Intercompany Arrangements. Except for (a) any Contracts that will be terminated at or prior to the Closing, (b) the Transaction Documents and (c) the organizational documents of the Purchased Entities, there are no Contracts in effect as of the date of this Agreement that are solely between or among the Seller Group, on the one hand, and the Purchased Entities, on the other hand.
Section 3.22Insurance. Except as would not reasonably be expected to be, individually or in the aggregate, material to the Purchased Entities and the Business, taken as a whole, (a) the Seller Entities and the Purchased Entities collectively own or hold policies of insurance, or are self-insured, in amounts providing reasonably adequate coverage against all risks customarily insured against by companies in similar lines of business as the Business and (b) as of the date hereof, all such insurance policies are in full force and effect, except for any expiration thereof in accordance with the terms thereof, no written notice of cancellation or modification has been received other than in connection with ordinary renewals, and there is no existing default or event (or failure to take action) which, with the giving of notice or lapse of time or both, would constitute a breach or default, by any insured thereunder, or otherwise would permit termination or modification of any of the insurance policies. As of the date hereof, no written notice of cancellation or termination has been received by any member of the Seller Group or a Purchased Entity with respect to any of the insurance policies applicable to the Business, and there are no material claims pending under any of such policies for which coverage has been denied or disputed by the applicable insurance carrier (other than a customary reservation of rights notice).
Section 3.23Inventory; Accounts Payable; Accounts Receivable. Except as would not be or reasonably be expected to be, individually or in the aggregate, material to the Purchased Entities and the Business, taken as a whole, (a) all Inventory, whether or not reflected in the Business Financial Information, consists of a quality and quantity usable and salable in the ordinary course, except for obsolete or slow-moving items that have been written off or written down to fair market value or for which adequate reserves have been established, in each case, in accordance with GAAP, and is fit for its intended use in all respects, (b) all Inventory is owned by a member of the Seller Group or a Purchased Entity, free and clear of all Liens (other than Permitted Liens), and no Inventory is held on a consignment basis, (c) all accounts payable of the Purchased Entities that are reflected in the Business Financial Information (except as specifically set forth therein) and all accounts payable of the Purchased Entities arising since the Balance Sheet Date are valid obligations and have arisen only from bona fide arm’s length transactions in the ordinary course of business, and all such accounts payable have either been paid, are not yet due and payable in the ordinary course of business, are being contested by the Purchased Entities in good faith (and appropriate reserves established therefor), and if not paid, have been properly recorded, (d) all accounts receivables of the Purchased Entities that are reflected in the Business Financial Information (except as specifically set forth therein) and all accounts receivables of the Purchased Entities arising since the Balance Sheet Date are valid obligations and have arisen only from bona fide arm’s length transactions in the ordinary course of business and are not subject to defenses, credits, setoffs or counterclaims, (e) no reasonable basis exists for the assertion of any claim of the repayment of any payments heretofore received by the Purchased Entities on account of any accounts receivable and (f) all of the outstanding receivables deemed uncollectible have been reserved against in the Business Financial Information in accordance with GAAP.
-53-
Section 3.24Brokers. No broker, investment banker, or financial advisor is entitled to any broker’s, finder’s, financial advisor’s or other similar fee or commission in connection with the Transaction and the other transactions contemplated by this Agreement based upon arrangements made by or on behalf of Seller or its Subsidiaries for which the Purchasers or any of their Subsidiaries (including, following the Closing, the Purchased Entities) would have any Liability.
Section 3.25International Trade.
(a)None of the Purchased Entities nor any of their respective officers, directors or employees, nor to the Knowledge of Seller, any agent or other third party representative acting at the direction of any of the Purchased Entities, is currently, or has in the last five (5) years: (i) been a Sanctioned Person; (ii) been engaging in any dealings or transactions with or for the benefit of any Sanctioned Person or in any Sanctioned Country; or (iii) otherwise been in violation of Sanctions, Ex-Im Laws, or U.S. anti-boycott Laws (collectively, “Trade Controls”).
(b)In the past five (5) years, none of the Purchased Entities has: (i) received from any Governmental Entity or any Person any written or, to the Knowledge of Seller, oral notice, inquiry, or internal or external allegation; (ii) made any voluntary or involuntary disclosure to a Governmental Entity; or (iii) conducted any internal investigation or audit concerning any actual or potential violation or wrongdoing, in each case, related to Trade Controls or Anti-Corruption Laws.
Section 3.26No Other Representations or Warranties. Except for the representations and warranties contained in this Article III and in any certificate delivered in connection with this Agreement, neither Seller nor any of its Affiliates, Representatives or any other Person makes any express or implied representation or warranty with respect to Seller, the other Seller Entities, the Purchased Entities or any of their respective Affiliates, the Purchased Assets, the Business or with respect to any other information provided, or made available, to the Purchasers or any of their Affiliates or Representatives in connection with the Transaction or the other transactions contemplated by this Agreement. Neither Seller nor any of its Affiliates, Representatives or any other Person has made any express or implied representation or warranty with respect to the prospects of the Business or its profitability for the Purchasers, or with respect to any forecasts, projections or business plans or other forward looking information delivered to the Purchasers or any of their Affiliates or Representatives in connection with the Purchasers’ review of the Business and the negotiation and execution of this Agreement, including as to the accuracy or completeness thereof or the reasonableness of any assumptions underlying any such forecasts, projections or business plans or other information.
-54-
Neither Seller nor any of its Affiliates, Representatives or any other Person will have, or be subject to, any Liability or other obligation to the Purchasers or any of their Affiliates or Representatives or any other Person resulting from the use by the Purchasers or any of their Affiliates or Representatives of, any information, including information, documents, projections, forecasts, business plans or other material (including any Evaluation Material (as defined in the Confidentiality Agreement)) made available to the Purchasers, their Affiliates or Representatives in any virtual data room, confidential information memorandum, management presentations, offering materials, site tours or visits, diligence calls or meetings or any documents prepared by, or on behalf of, Seller or any of its Affiliates or Representatives, or the Purchasers or their Affiliates or Representatives or any of the Purchasers’ potential financing sources (including the Financing Parties) in connection with the Purchasers’ financing activities with respect to the transactions contemplated by this Agreement. Each of Seller and its Affiliates disclaims any and all representations and warranties, whether express or implied, except for the representations and warranties contained in this Article III and in any certificate delivered in connection with this Agreement. Notwithstanding anything in this Agreement to the contrary, neither Seller nor any of its Affiliates makes any express or implied representation or warranty with respect to the Excluded Assets or the Retained Liabilities.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF PURCHASERS
Except as set forth in the Purchaser Disclosure Schedules, each Purchaser hereby represents and warrants, jointly and severally, to Seller as follows:
Section 4.1Organization and Standing. Each Purchaser is a corporation duly organized, validly existing and in good standing under the Laws of its jurisdiction of incorporation, except where the failure to be so organized, existing or in good standing would not have, individually or in the aggregate, a Purchaser Material Adverse Effect.
Section 4.2Authority; Enforceability.
(a)Each Purchaser has all requisite corporate power and authority to execute and deliver this Agreement and each such Transaction Document to which it is or will be a party, and to perform its obligations hereunder and thereunder. The execution and delivery by each Purchaser of this Agreement and each other Transaction Document to which it is or will be a party, and the performance by each Purchaser of its obligations hereunder and thereunder, have been, or, with respect to Transaction Documents to which it will be a party, will have been as of the Closing, duly authorized by all requisite corporate or other similar applicable action. No vote of any stockholders or other equity holders of either Purchaser or any of its Subsidiaries is required for the execution of this Agreement or any other Transaction Document or the consummation of the Transaction or the other transactions contemplated hereby and thereby.
-55-
(b)Each Purchaser has all requisite corporate or other similar applicable power and authority to carry on its business as it is now being conducted and to own, lease and operate its properties and assets, except where the failure to have such power and authority would not have, individually or in the aggregate, a Purchaser Material Adverse Effect.
(c)This Agreement has been duly executed and delivered by each Purchaser and, assuming this Agreement has been duly executed and delivered by Seller, constitutes a valid and binding obligation of each Purchaser, and each other Transaction Document will be duly executed and delivered by each Purchaser and will, assuming such Transaction Document has been duly executed and delivered by each Seller Entity that will be a party thereto, constitute a valid and binding obligation of each Purchaser, in each case enforceable against each Purchaser in accordance with its terms, except as enforcement may be limited by bankruptcy, insolvency, reorganization, fraudulent conveyance, moratorium or similar Laws affecting creditors’ rights generally or by general principles of equity (regardless of whether enforcement is sought in a proceeding in equity or law).
Section 4.3No Conflicts; Consents. The execution, delivery and performance by each Purchaser of this Agreement and the other Transaction Documents to which such Purchaser will be a party, and the consummation by each Purchaser of the transactions contemplated hereby and thereby, do not and will not (a) violate any provision of the certificate of incorporation, bylaws or other organizational documents of such Purchaser or any of its Affiliates, (b) conflict with, constitute a default under, or result in the breach or termination, cancellation or acceleration (whether after the giving of notice or the lapse of time or both) of any right or obligation of such Purchaser or any of its Affiliates under any Contract to which such Purchaser or any of its Affiliates is a party or is subject or (c) assuming compliance with the matters set forth in Section 3.5 and Section 4.4, violate or result in a breach of or constitute a default under any Law or other restriction of any Governmental Entity to which such Purchaser or any of its Affiliates is subject, except, with respect to clauses (b) and (c), as would not have, individually or in the aggregate, a Purchaser Material Adverse Effect.
Section 4.4Governmental Authorizations. The execution, delivery and performance of this Agreement by each Purchaser does not require any Regulatory Approval of, or Filing with, any Governmental Entity, except for (a) compliance with (i) the applicable requirements of the HSR Act and (ii) any other applicable Regulatory Law, (b) the Regulatory Approvals and Filings set forth in Section 4.4 of the Purchaser Disclosure Schedules and (c) the Regulatory Approvals and Filings which if not obtained or made would not have, individually or in the aggregate, a Purchaser Material Adverse Effect.
Section 4.5[Reserved].
Section 4.6Financing.
(a)Purchaser 1 is a party to that certain Debt Commitment Letter, dated as of the date hereof (together with all exhibits, schedules, annexes and amendments thereto, together with the fee letter referred to therein, the “Commitment Letter”), pursuant to which the lenders party thereto (collectively, the “Lenders”) have agreed, subject to the terms and conditions thereof, to provide debt financing in the amounts set forth therein. The debt financing committed pursuant to the Commitment Letter is collectively referred to in this Agreement as the “Financing.”
-56-
(b)The applicable Purchaser has delivered to Seller a true, complete and correct copy of the executed Commitment Letter and any fee letters related thereto, subject, in the case of the fee letter, to redaction solely of fee, “market flex”, other economic provisions or other commercially sensitive information that are customarily redacted in connection with transactions of this type, in each case, so long as no such redaction adversely affects any term in respect of the conditionality, enforceability, availability, termination or aggregate principal amount of the Financing (other than through operation of additional original issue discount).
(c)Except as expressly set forth in the Commitment Letter, there are no conditions precedent to the obligations of the Lenders to provide the Financing or any contingencies that would permit the Lenders to reduce the aggregate principal amount of the Financing below the Financing Amounts, including any condition or other contingency relating to the amount or availability of the Financing pursuant to any “flex” provision. As of the date hereof, neither Purchaser has any reason to believe that it will be unable to satisfy on a timely basis all terms and conditions to be satisfied by it in the Commitment Letter on or prior to the Closing Date, nor does either Purchaser have Knowledge that any Lender will not perform its obligations thereunder. As of the date hereof, there are no side letters, understandings or other agreements, contracts or arrangements of any kind relating to the Commitment Letter or the Financing that could reasonably be expected to affect the conditionality, enforceability, availability, termination or amount of the Financing, other than as expressly contained in the Commitment Letter.
(d)The Financing, when funded in accordance with the Commitment Letter and giving effect to any “flex” provision in the fee letter or related to the Commitment Letter (including with respect to fees and original issue discount), together with the Purchasers’ other available sources of funds, shall provide the Purchasers with cash proceeds on the Closing Date sufficient to provide the Purchasers with the funds necessary for it to fund all the Closing Purchase Price, the Final Purchase Price and any fees and expenses of or payable by each Purchaser or its Affiliates and all other amounts earned, due and payable pursuant to this Agreement on the Closing Date (such amounts, collectively, the “Financing Amounts”).
(e)As of the date hereof, the Commitment Letter constitutes the legal, valid, binding and enforceable obligations of Purchaser 1 and, to each Purchaser’s Knowledge, all the other parties thereto and is in full force and effect, in each case, except as enforcement may be limited by bankruptcy, insolvency, reorganization, fraudulent conveyance, moratorium or similar Laws affecting creditors’ rights generally or by general principles of equity (regardless of whether enforcement is sought in a proceeding in equity or law). As of the date hereof, no event has occurred which (with or without notice, lapse of time or both) constitutes, or would constitute, a default, breach or failure to satisfy a condition by the Purchasers under the terms and conditions of the Commitment Letter. As of the date hereof, the Purchasers do not have any reason to believe that any of the conditions to the Financing will not be satisfied by the Purchasers on a timely basis or that the Financing will not be available to the Purchasers on the Closing Date. Purchaser 1 has paid in full any and all commitment fees or other fees required to be paid pursuant to the terms of the Commitment Letter on or before the date of this Agreement and will pay in full any such amounts due on or before the Closing Date as and when due, in each case, pursuant to the terms of the Commitment Letter.
-57-
The Commitment Letter will not be amended, modified or altered at any time through the Closing, except as permitted by Section 5.7(b) (with any such modification, amendment or alteration promptly notified in writing to Seller), and none of the respective commitments under the Commitment Letter has been terminated, reduced, withdrawn or rescinded in any respect, and, to the Knowledge of the Purchasers, no termination, reduction, withdrawal, modification, amendment, alteration or rescission thereof is contemplated.
(f)In no event shall the receipt or availability of any funds or financing (including the Financing) by the Purchasers or any of their Affiliates or any other financing or other transactions be a condition to any of the Purchasers’ obligations under this Agreement.
Section 4.7Proceedings. There is no, and in the past three (3) years there has not been, any Proceeding pending or, to the Knowledge of the Purchasers, threatened against either Purchaser or any of their Subsidiaries, other than Proceedings that would not have a Purchaser Material Adverse Effect. As of the date of this Agreement, neither Purchaser nor any of their Subsidiaries is subject to any outstanding Judgment that would have a Purchaser Material Adverse Effect.
Section 4.8Brokers. No broker, investment banker or financial advisor is entitled to any broker’s, finder’s, financial advisor’s or other similar fee or commission in connection with the Transaction and the other transactions contemplated by this Agreement based upon arrangements made by or on behalf of any Purchaser or its Subsidiaries for which Seller or any of its Subsidiaries would have any Liability.
Section 4.9Solvency. No transfer of property is being made, and no obligation is being incurred in connection with the transactions contemplated by this Agreement or the other Transaction Documents with the intent to hinder, delay or defraud either present or future creditors of any Purchaser, any Seller Entity or any Subsidiary thereof (including any Purchased Entity). Immediately after giving effect to the consummation of the transactions contemplated by this Agreement (including the Financing and any other financings being entered into in connection therewith), in each case, assuming the satisfaction of the conditions to Closing set forth in Section 8.1 and Section 8.2:
(a)the Fair Value of the assets of the Purchasers and their Subsidiaries, taken as a whole, shall be greater than the total amount of the Purchasers’ and their Subsidiaries’ liabilities (including all liabilities, whether or not reflected in a balance sheet prepared in accordance with GAAP, and whether direct or indirect, fixed or contingent, secured or unsecured, disputed or undisputed), taken as a whole;
(b)the Purchasers and their Subsidiaries, taken as a whole, shall be able to pay their debts and obligations in the ordinary course of business as they become due; and
(c)the Purchasers and their Subsidiaries, taken as a whole, shall have adequate capital to carry on their businesses and all businesses in which they engage.
-58-
For the purposes of this Section 4.9, “Fair Value” means the amount at which the assets (both tangible and intangible), in their entirety, of the Purchasers and their Subsidiaries would change hands between a willing buyer and a willing seller, within a commercially reasonable period of time, each having reasonable knowledge of the relevant facts, with neither being under any compulsion to act.
Section 4.10Securities Laws. The Purchasers are acquiring the Purchased Entity Shares solely for the purpose of investment and not with a view to, or for sale in connection with, any distribution thereof in violation of the Securities Act or any other applicable state or foreign securities Laws. The Purchasers acknowledge that the Purchased Entity Shares are not registered under the Securities Act, any applicable state securities Laws or any applicable foreign securities Laws, and that such Purchased Entity Shares may not be transferred or sold, except pursuant to the registration provisions of the Securities Act and applicable state and foreign securities Laws or pursuant to an applicable exemption therefrom. The Purchasers have sufficient knowledge and experience in financial and business matters so as to be capable of evaluating the merits and risks of their investment in the Purchased Entity Shares and are capable of bearing the economic risks of such investment.
Section 4.11Acknowledgment of No Other Representations or Warranties.
(a)Except for the representations and warranties contained in this Article IV and in any certificate delivered in connection with this Agreement, neither Purchaser nor any of their Affiliates, Representatives or any other Person makes any express or implied representation or warranty with respect to either Purchaser or any of their respective Affiliates, or with respect to any other information provided, or made available, to Seller or any of its Affiliates or Representatives in connection with the Transaction or the other transactions contemplated by this Agreement. Any such other representation or warranty is hereby expressly disclaimed by each Purchaser on behalf of itself and its Affiliates.
(b)Each Purchaser acknowledges and agrees that, except for the representations and warranties contained in Article III and in any certificate delivered in connection with this Agreement, neither Seller nor any of its Affiliates, Representatives or any other Person makes any express or implied representation or warranty with respect to Seller, the other Seller Entities, the Purchased Entities or any of their respective Affiliates, the Purchased Assets, the Business or with respect to any other information provided, or made available, to the Purchasers or any of their Affiliates or Representatives in connection with the transactions contemplated hereby. Each Purchaser acknowledges and agrees that neither Seller nor any of its Affiliates, Representatives or any other Person has made any express or implied representation or warranty with respect to the prospects of the Business or its profitability for the Purchasers, or with respect to any forecasts, projections or business plans or other forward looking information delivered to the Purchasers or any of their Affiliates or Representatives in connection with the Purchasers’ review of the Business and the negotiation and execution of this Agreement, including as to the accuracy or completeness thereof or the reasonableness of any assumptions underlying any such forecasts, projections or business plans or other information.
-59-
Each Purchaser acknowledges and agrees that neither Seller nor any of its Affiliates, Representatives or any other Person will have, or be subject to, any Liability or other obligation to any Purchaser, its Affiliates or Representatives or any other Person resulting from the sale and purchase of the Purchased Assets or the Business to the Purchasers or their Affiliates or either Purchaser’s use of, or the use by any of its Affiliates or Representatives, of any information, including information, documents, projections, forecasts, business plans or other material (including any Evaluation Material (as defined in the Confidentiality Agreement)) made available to the Purchasers, their Affiliates or Representatives in any virtual data room, confidential information memorandum, management presentations, offering materials, site tours or visits, diligence calls or meetings or any documents prepared by, or on behalf of, Seller or any of its Affiliates or Representatives, or either Purchaser or its Affiliates or Representatives or any of such Purchaser’s potential financing sources (including the Financing Parties) in connection with such Purchaser’s financing activities with respect to the transactions contemplated by this Agreement. Each Purchaser acknowledges and agrees that it is not relying on any representation or warranty of Seller or any of its Affiliates or Representatives or any other Person, other than those representations and warranties specifically set forth in Article III and in any certificate delivered in connection with this Agreement. Each Purchaser acknowledges and agrees that each of Seller and its Affiliates disclaims any and all representations and warranties, whether express or implied, except for the representations and warranties contained in Article III and in any certificate delivered in connection with this Agreement.
(c)Each Purchaser acknowledges that it has conducted to its satisfaction an independent investigation of the financial condition, results of operations and projected operations of the Business and the nature and condition of its properties, assets, liabilities and businesses and, in making the determination to proceed with the transactions contemplated hereby, has relied solely on the results of its own independent investigation and the representations and warranties set forth in Article III and in any certificate delivered in connection with this Agreement.
ARTICLE V
COVENANTS
Section 5.1Efforts.
(a)From and after the date hereof, the Purchasers and Seller shall, and shall cause their respective Affiliates to, use their respective reasonable best efforts to take, or cause to be taken, all actions, and to do, or cause to be done, and shall cooperate with each other to take and do, all things necessary, proper or advisable under any applicable Law to consummate and make effective as promptly as practicable the Transaction and the other transactions contemplated by this Agreement, including (i) preparing and filing of all forms, registrations, Filings and notices required to be filed to satisfy the conditions precedent to this Agreement and to consummate the Transaction and the other transactions contemplated by this Agreement as soon as practicable, (ii) defending through litigation on the merits any claim asserted in any Proceedings by any Governmental Entity to avoid entry of, or to have vacated or terminated, any decree, order or judgment (whether temporary, preliminary or permanent) that would prevent the Closing from occurring prior to the Outside Date and (iii) the executing and delivering of any additional instruments necessary to consummate the Transaction and the other transactions contemplated by this Agreement and to fully carry out the purposes of this Agreement. Without limiting the foregoing, the Purchasers and Seller shall, and shall cause their respective Affiliates to, use reasonable best efforts to take all actions necessary to obtain (and shall cooperate with each other in obtaining) any Regulatory Approvals (which actions shall include furnishing all information required in connection with such Regulatory Approvals) required to be obtained or made by the Purchasers, Seller, the other Seller Entities or the Purchased Entities or any of their Affiliates in connection with the Transaction or the other transactions contemplated by this Agreement.
-60-
Additionally, the Purchasers and Seller shall not, and shall cause their respective Affiliates not to, acquire or agree to acquire, by merger, consolidation, stock or asset purchase or otherwise, any business or corporation, partnership or other business organization or division thereof, or merge or consolidate with any other Person, if such transaction would reasonably be expected to materially impair or materially delay the obtaining of, or result in not obtaining, any Regulatory Approval necessary to be obtained prior to the Closing.
(b)Prior to the Closing, the Purchasers and Seller shall each keep the other apprised of the status of matters relating to the completion of the Transaction and the other transactions contemplated by this Agreement and work cooperatively in connection with obtaining all required Regulatory Approvals. In that regard, prior to the Closing, subject to the Confidentiality Agreement and Section 5.3, each Party shall promptly consult with the other Party to provide any necessary information with respect to (and, in the case of correspondence, provide the other Party (or its counsel) copies of (other than Filings made pursuant to the HSR Act)) all substantive Filings made by such Party or any of its Affiliates with any Governmental Entity or any other information supplied by such Party or any of its Affiliates to, or correspondence with, a Governmental Entity in connection with this Agreement, the Transaction and the other transactions contemplated by this Agreement. Subject to the Confidentiality Agreement and Section 5.3, each Party shall promptly inform the other Party and, if in writing, furnish the other Party with copies of (or, in the case of oral communications, advise the other Party orally of) any communication received by such Party or any of its Affiliates or Representatives from any Governmental Entity regarding the Transaction and the other transactions contemplated by this Agreement, and permit the other Party to review and discuss in advance, and consider in good faith the views of the other Party in connection with, any proposed substantive communication with any such Governmental Entity regarding the Transaction and the other transactions contemplated by this Agreement. If either Party or any Affiliate or Representative of such Party receives a request for additional information or documentary material from any Governmental Entity with respect to the Transaction or the other transactions contemplated by this Agreement, then such Party will make, or cause to be made, promptly and after consultation with the other Party, an appropriate response to such request. No Party to this Agreement shall participate in any substantive meeting with any Governmental Entity in connection with this Agreement and the Transaction or the other transactions contemplated by this Agreement (or make substantive oral submissions at meetings or in telephone or other conversations), unless it consults with the other Party in advance and, to the extent not prohibited by such Governmental Entity, gives that Party the opportunity to attend and participate thereat. Subject to the Confidentiality Agreement and Section 5.3, each Party shall furnish the other Party with copies of all substantive correspondence and Filings (other than Filings made pursuant to the HSR Act) between it or any of its Affiliates or Representatives, on the one hand, and any Governmental Entity, on the other hand, with respect to this Agreement and the Transaction or the other transactions contemplated by this Agreement, and furnish the other Party with such necessary information and reasonable assistance as the other Party may reasonably request in connection with its preparation of Filings to any such Governmental Entity.
-61-
the Purchasers and Seller may, as each deems advisable and necessary, reasonably designate any competitively sensitive material provided to the other under this Agreement as “outside counsel only.” Such materials and the information contained therein shall be given only to the outside legal counsel and will not be disclosed by such outside counsel to employees, officers or directors of the recipient unless express permission is obtained in advance from the source of the materials (a Purchaser or Seller, as the case may be) or its legal counsel; provided, however, that materials provided pursuant to this Agreement may be redacted (i) to remove references concerning the valuation of or future plans for the Business or the Sale Process, (ii) as necessary to comply with contractual obligations or applicable Law and (iii) as necessary to address reasonable privilege concerns.
(c)Without limiting the foregoing, the Purchasers and Seller shall, and shall cause their respective Affiliates to, file, as promptly as practicable, but in any event no later than ten (10) Business Days after the date of this Agreement, notifications under the HSR Act, provided that there are no changes in the applicable regulations under the HSR Act between the date hereof and the date of filing pursuant to the HSR Act, in which instance Purchasers and Seller shall use reasonable best efforts to file notifications under the HSR Act as promptly as commercially practicable thereafter, and the Purchasers and Seller shall, and shall cause their respective Affiliates to, file as promptly as practicable any other Filings under applicable Regulatory Laws listed on Section 8.1(a) of the Seller Disclosure Schedules, but in any event, any such other Filings or initial drafts thereof shall be submitted no later than twenty (20) Business Days after the date of this Agreement.
(d)In furtherance of the foregoing, and notwithstanding anything in this Agreement to the contrary, the Purchasers shall, and shall cause their Affiliates to, take all such actions as may be necessary, proper or advisable to avoid or eliminate each and every impediment under any applicable Law with respect to the Transaction or the other transactions contemplated hereby and to resolve such objections, if any, as any Governmental Entity or any other Person may assert under any applicable Law with respect to the Transaction or the other transactions contemplated hereby, so as to enable the Closing to occur as soon as reasonably possible (and in any event prior to the Outside Date). In furtherance of the foregoing, the Purchasers shall, and shall cause their Affiliates to, and shall permit the Seller Entities (provided, that, the Parties shall consult and cooperate in all respects with one another, and consider in good faith the views of one another regarding the appropriate course of action), proffer, negotiate and agree to, by consent decree, hold separate order, or otherwise, (i) sell, divest, lease, license, transfer, dispose of or otherwise encumber or hold separate any assets, licenses, operations, rights, product lines, businesses or interests therein of the Business or of the Purchasers or their Subsidiaries; (ii) make any changes (including through a licensing arrangement) to or create any restrictions on, or other impairment of any Purchaser’s or its Subsidiaries’ ability to own, retain or operate, any such assets, licenses, product lines, businesses or interests of the Business or of the Purchasers or their Subsidiaries; (iii) create, terminate, unwind, divest or assign, subcontract or otherwise secure substitute parties for relationships, ventures, and contractual or commercial rights or obligations of the Business or of the Purchasers or their Subsidiaries; and (iv) make any changes to or create any restrictions on Purchaser’s or its Subsidiaries’ ability to acquire any assets or businesses in the future (the actions referred to in clauses (i), (ii), (iii) and (iv), “Remedy Actions”); provided, however, notwithstanding anything to contrary this Agreement, that the Purchasers shall not be required to take any Remedy Actions if any such Remedy Actions, individually or in the aggregate, would require the divestiture, holding separate (or any other remedy) of or with respect to any assets of Purchasers or their Subsidiaries or the Business representing in the aggregate more than fifteen (15%) of annual gross revenue generated by the Business between January 1, 2023 and December 31, 2023.
-62-
Notwithstanding anything in this Agreement to the contrary, Seller and its Affiliates shall not be obligated to take or agree or commit to take any action that is not conditioned on the occurrence of the Closing or to the extent related to any Excluded Assets or the Retained Business; and in no event shall Seller or any of its Affiliates be required to be the licensing, selling, divesting, leasing, transferring, disposing or encumbering party under any such agreements unless required by the relevant Governmental Entity or applicable Law, and, in any case, Seller and its Affiliates shall have no direct or indirect obligation or Liability in respect of any such agreements, transactions or relationships, including any indemnification obligations, for which Seller and its Affiliates are not fully indemnified by the Purchasers.
(e)The Purchasers shall be responsible for filing fees to any Governmental Entity in connection with Filings made pursuant to the HSR Act and any other required Regulatory Approvals pursuant to this Agreement.
Section 5.2Covenants Relating to Conduct of Business.
(a)Except (A) as set forth in Section 5.2(a) of the Seller Disclosure Schedules, (B) as required by applicable Law, (C) as otherwise expressly permitted by the terms of this Agreement, (D) to the extent primarily related to the Excluded Assets, the Retained Liabilities or the Retained Business and does not have a materially adverse and disproportionate or material effect on the Business, Business Products or Purchased Entities relative to the business of the Seller Entities, taken as a whole, or (E) as otherwise consented to by the Purchasers (such consent not to be unreasonably withheld, conditioned or delayed), from the date of this Agreement to the Closing, Seller shall, and shall cause each of its Subsidiaries, including each Purchased Entity to, use commercially reasonable efforts to (i) maintain and preserve intact the current organization, material assets, material Permits, relationships and goodwill of employees, customers, suppliers, regulators and others having material business relationships with the Business and (ii) conduct the Business in all material respects in the ordinary course; provided, however, that no action by Seller or its Subsidiaries with respect to matters specifically addressed by Section 5.2 shall be deemed a breach of this Section 5.2(a) unless such action would constitute a breach of such other provision.
(b)Except (A) as set forth in Section 5.2(b) of the Seller Disclosure Schedules, (B) as required by applicable Law, (C) as otherwise expressly permitted by the terms of this Agreement, (D) to the extent primarily related to the Excluded Assets, the Retained Liabilities or the Retained Business and does not have a materially adverse and disproportionate or material effect on the Business, Business Products or Purchased Entities relative to the business of the Seller Entities, taken as a whole, or (E) as otherwise consented to by the Purchasers (such consent not to be unreasonably withheld, conditioned or delayed), from the date of this Agreement to the Closing, Seller shall not, and shall cause each of its Subsidiaries, including each Purchased Entity, not to, in each case with respect to the Business, do any of the following without the prior consent of the Purchasers (such consent not to be unreasonably withheld, conditioned or delayed):
-63-
(i)except (A) as may be required by any Seller Benefit Plan, including any Purchased Entity Benefit Plan, or any Collective Bargaining Agreement, or (B) for any grant or award to any Business Employee or other individual service provider of the Business or the Purchased Entities with annual compensation of $150,000 or less for which the Seller or any of its Subsidiaries (including the Purchased Entities) shall be solely obligated to pay, (I) grant or promise to grant to any Business Employee or other individual service provider of the Business or the Purchased Entities with an annual base compensation in excess of $150,000 any increase in compensation or benefits under any Purchased Entity Benefit Plan, (II) grant or promise to grant any incentive awards, bonuses, change in control payments, deferred compensation, severance, retention or equity or equity-based rights to any Business Employee or other individual service provider of the Business or the Purchased Entities, (III) take any action to accelerate the vesting, funding or payment of any compensation payable or benefit provided to any Business Employee or other individual service provider of the Business or the Purchased Entities, (IV) hire, terminate (other than for cause), furlough or temporarily lay off any Business Employee (or individual who would be a Business Employee upon hire) or other individual service provider of the Business or the Purchased Entities with annual base compensation in excess of $150,000, or (V) adopt, enter into, modify, terminate or amend any Purchased Entity Benefit Plan (or any plan, program, policy, arrangement, agreement, or Contract that would be a Purchased Entity Benefit Plan if it were in existence as of the date of this Agreement);
(ii)adopt or effect any amendment to, or change, the organizational documents of any Purchased Entity, or form a Subsidiary of any Purchased Entity;
(iii)(A) split, combine or reclassify the outstanding equity interests of any of the Purchased Entities or (B) declare, set aside or pay any non-cash dividend or non-cash distribution to any Person;
(iv)issue, sell or grant, pledge, dispose or transfer or propose to issue, sell or grant, pledge, dispose or transfer any equity interests of any of the Purchased Entities, or securities convertible into, or exchangeable or exercisable for, or options with respect to, or warrants to purchase, or rights to subscribe for, equity interests of any of the Purchased Entities, in each case other than the granting of Permitted Liens;
(v)incur, create or assume (A) any indebtedness for borrowed money, or issue or sell any debt securities or warrants or other rights to acquire any debt security of the Purchased Entities, in each case, in excess of $250,000 in the aggregate, in each case, other than (x) between or among any of the Purchased Entities to the extent reconciled or eliminated in the consolidated financial statements of the Business at or prior to Closing or (y) that will be settled, repaid or extinguished at or prior to Closing, or (B) any Lien, other than Permitted Liens, with respect to any material asset of the Business, other than any Lien that will be released or discharged at or prior to the Closing;
-64-
(vi)acquire any assets or dispose of any assets of the Business with a value in excess of $500,000 in the aggregate, in each case, outside of a sale of inventory or products in the ordinary course of business;
(vii)(A) amend in any material respect, or waive any material right under, or voluntarily terminate (other than upon expiration in accordance with its terms), any Material Contract, or (B) enter into any Contract that, if in effect on the date hereof, would be a Material Contract, other than, in each case of clauses (A) and (B), (x) in the ordinary course of business, or (y) for the automatic renewal or extension of any Material Contract pursuant to its terms; provided, that no Selling Entity or Purchased Entity may, without the prior written consent of the Purchasers, enter into a Contract that would be required to be disclosed pursuant to Section 3.13(a)(ix) or Section 3.13(a)(x) if entered into before the date hereof; provided, further, that a Selling Entity or Purchased Entity may, without the prior written consent of the Purchasers, amend or renew a Contract that would be required to be disclosed pursuant to Section 3.13(a)(x) if such Contract was effective on or before the date hereof and included such a provision;
(viii)settle or compromise any Proceeding, or enter into any consent decree or settlement agreement with any Governmental Entity, against or pertaining to the Business or any Purchased Entity, other than settlements or compromises of any Proceeding where the amount paid in settlement or compromise does not exceed $500,000 individually or $2,500,000 in the aggregate (excluding any amounts covered by insurance or amounts Seller or another member of the Seller Group agrees to pay) and that do not impose any equitable relief, fines or criminal penalties on the Purchased Entities or the Business following the Closing;
(ix)with respect to any Purchased Entity, (A) make (except in the ordinary course of business), change or revoke any material Tax election, (B) change or adopt any annual accounting period or Tax accounting or transfer pricing policy or practice, (C) change or adopt any material method of accounting for Tax purposes, or (D) settle or compromise any claim or assessment in respect of Taxes or enter into any Tax closing agreement, (E) file an amended Tax Return, (F) surrender or abandon any right to claim a Tax refund, offset or other reduction in liability or (G) consent to any extension or waiver of the limitations period applicable to any Tax claim or assessment, in each case, except for any such action that would not result in more than a de minimis increase in the Tax liability of the Purchasers and their Subsidiaries (including, after the Closing, the Purchased Entities) after the Closing;
(x)(A) make any material change in any method of financial accounting or accounting practice or auditing practice or policy applicable to the Business, other than such changes as are required by GAAP or applicable Law or otherwise apply on an enterprise-wide basis to Seller and its Subsidiaries, or (B) make any material change to any of the practices, policies, procedures or timing of the collection of accounts receivable, billing of customers, payment terms, cash collections, cash payments, pricing or similar terms applicable to the Business, other than such changes as are required by GAAP or applicable Law, in the ordinary course of business or otherwise apply on an enterprise-wide basis to Seller and its Subsidiaries;
-65-
(xi)except as set forth in the capital budget of the Business made available to the Purchasers, commit or authorize any commitment to make any capital expenditures in excess of $100,000 individually or $500,000 in the aggregate (other than any such commitments which will be satisfied at or prior to Closing);
(xii)dissolve, or authorize, recommend, propose or announce an intention to adopt, or otherwise effect, a plan of complete or partial liquidation, dissolution or restructuring any Purchased Entity, or merge or consolidate any Purchased Entity with any other Person;
(xiii)(A) materially modify, extend, terminate or enter into any Collective Bargaining Agreement or (B) recognize or certify any labor union, labor organization, works council or group of employees as the bargaining representative for any Business Employees;
(xiv)implement or announce any employee layoffs, furloughs, reductions in force, plant closings, reductions in compensation or other similar actions that would trigger notice obligations under the WARN Act;
(xv)waive or release any noncompetition, nonsolicitation, nondisclosure or other restrictive covenant obligation of any current or former employee or independent contractor of the Purchased Entities or the Business, including any Business Employee;
(xvi)transfer, modify or reassign the duties of (A) a Business Employee such that he or she no longer meets the definition of a Business Employee or (B) any other employee of any Seller Entity such that he or she would become a Business Employee;
(xvii)transfer, modify or reassign the duties of (A) a Business Contingent Worker such that he or she no longer meets the definition of a Business Contingent Worker or (B) any other individual service provider of any Seller Entity such that he or she would become a Business Contingent Worker;
(xviii)transfer any employee or individual service providers into or out of a Purchased Entity other than as contemplated by Section 6.1(a) or Section 6.10;
(xix)designate any individual as a Business Employee who does not primarily provide services to the Business;
(xx)amend, modify, extend, renew or terminate any Transferred Lease and shall not enter into any new lease, sublease, license or other agreement for the use or occupancy of any real property other than in the ordinary course of business;
(xxi)enter into, amend, modify, extent, renew or terminate any Shared Contract, other than any amendments, modifications, extensions or renewals in the ordinary course of business;
-66-
(xxii)(A) sell, transfer, assign, exclusively license, or otherwise dispose of any material Transferred Intellectual Property, other than nonexclusive licenses granted by any of the Purchased Entities to customers or vendors in the ordinary course of business, or (B) disclose any material Trade Secrets to any Person except pursuant to valid and appropriate written non-disclosure agreements in the ordinary course of business; or
(xxiii)authorize any of, or commit or agree to take, whether in writing or otherwise, or do any of, the foregoing actions.
(c)Nothing contained in this Agreement shall be construed to give to the Purchasers, directly or indirectly, rights to control or direct the Business’s operations prior to the Closing. Prior to the Closing, Seller and its Subsidiaries shall exercise, consistent with the terms and conditions of this Agreement, complete control and supervision of the operations of the Business. Notwithstanding anything in this Agreement to the contrary, the Parties acknowledge and agree that nothing in this Section 5.2 or elsewhere in this Agreement shall limit the transfer (whether to or from Seller or any of its Subsidiaries, or otherwise) of any Excluded Asset or any Retained Liability; provided, that, other than in the case of the Italian VAT Receivable and the Pre-Closing Restricted Cash Distribution, which are governed by Section 7.6 and Section 5.23, respectively, (i) reasonable advance notice of any such transfer must be provided to the Purchasers, (ii) the Purchasers shall have the right to review and comment on any documentation in connection with any such transfer; provided, that such period of review shall in no event be longer than five (5) Business Days, and (iii) Seller shall reasonably cooperate in connection with such review and Seller shall consider any comments proposed by or on behalf of Purchasers in good faith.
Section 5.3Confidentiality.
(a)Each Purchaser acknowledges that the information being provided to it in connection with the Transaction and the other transactions contemplated hereby is subject to the terms of that certain confidentiality agreement between Phibro Animal Health Corporation and Zoetis Services LLC, dated as of November 15, 2023 (the “Confidentiality Agreement”), the terms of which are incorporated herein by reference in their entirety and shall survive the Closing. Effective upon, and only upon, the Closing, the Confidentiality Agreement shall terminate solely with respect to information relating to the Business; provided, however, that such Purchaser acknowledges that its obligations of confidentiality and non-disclosure with respect to any and all other information provided to it by or on behalf of Seller, the other Seller Entities, the Purchased Entities or any of their respective Affiliates or Representatives, concerning the Retained Business, Seller, the other Seller Entities or any of their respective Affiliates (other than solely with respect to the Business) shall continue to remain subject to the terms and conditions of the Confidentiality Agreement for the term thereof. The Parties expressly agree that, notwithstanding any provision of the Confidentiality Agreement to the contrary, including with respect to termination thereof, if, for any reason, the Closing does not occur and this Agreement is terminated, and the remaining term of the Confidentiality Agreement is less than twenty-four (24) months, the Confidentiality Agreement shall continue in full force and effect for a period of twenty-four (24) months following termination of this Agreement and otherwise in accordance with its terms, and this Agreement shall constitute the requisite consent of the Parties and their Affiliates, if applicable, to amend the Confidentiality Agreement accordingly.
-67-
(b)Seller hereby agrees with the Purchasers that it shall not, and shall not permit its Subsidiaries or their respective Representatives to, for a period of four (4) years after the Closing Date, without the prior written consent of the Purchasers, use or disclose to any third party (other than Seller’s Affiliates and its and their respective Representatives) any nonpublic information that is proprietary or competitively sensitive included in the Purchased Assets or relating to the Business (“Confidential Business Information”); provided, however, that the term “Confidential Business Information” will not include any information that (i) becomes available to Seller or its Affiliates or their respective Representatives from and after the Closing, from a third-party source that is not known by Seller to be under any obligations of confidentiality in respect of such information, (ii) is or becomes generally available to, or known by, the public (other than as a result of disclosure in violation hereof), (iii) is or was derived independently by Seller, its Affiliates or any of their respective Representatives without use of Confidential Business Information or (iv) constitutes Excluded Assets. Notwithstanding the foregoing, the foregoing shall not prohibit Seller, its Affiliates or any of their respective Representatives (A) from disclosing Confidential Business Information for the sole purpose of complying with the terms of this Agreement or any of the Transaction Documents or (B) from disclosing Confidential Business Information that Seller, any of its Affiliates or its or their Representatives are required by Law (by oral questions, interrogatories, requests for information, subpoena, civil investigative demand, or similar process) or requested by any Governmental Entity with jurisdiction over such Person to disclose (provided that Seller will, to the extent not legally prohibited, provide the Purchasers with prompt written notice of such request so that the Purchasers may seek, at their sole expense, an appropriate protective order or waive compliance with this Section 5.3(b)). Furthermore, the provisions of this Section 5.3(b) will not prohibit any retention of copies of records or any disclosure in connection with the preparation and filing of financial statements or Tax Returns of Seller or its Affiliates or any disclosure made in connection with the enforcement of any right or remedy relating to this Agreement, the other Transaction Documents or the transactions contemplated hereby and thereby.
(c)Each Purchaser hereby agrees with Seller that it shall not, and shall not permit its Affiliates (including the Purchased Entities following the Closing) or their respective Representatives to, for a period of three (3) years after the Closing Date, without the prior written consent of Seller, use or disclose to any third party (other than such Purchaser’s Affiliates and its and their respective Representatives) any nonpublic information that is proprietary or competitively sensitive to the extent relating to the Retained Business (“Confidential Retained Business Information”); provided, however, that the term “Confidential Retained Business Information” will not include any information that (i) becomes available to a Purchaser or its Affiliates or their respective Representatives from and after the Closing, from a third-party source that is not known by such applicable Purchaser to be under any obligations of confidentiality in respect of such information, (ii) is or becomes generally available to, or known by, the public (other than as a result of disclosure in violation hereof), (iii) is or was derived independently by a Purchaser, its Affiliates or any of their respective Representatives without use of Confidential Retained Business Information or (iv) constitutes Purchased Assets.
-68-
Notwithstanding the foregoing, the foregoing shall not prohibit any Purchaser, its Affiliates or any of their respective Representatives (A) from disclosing Confidential Retained Business Information for the sole purpose of complying with the terms of this Agreement or any of the Transaction Documents or (B) from disclosing Confidential Business Information that such Purchaser, any of its Affiliates or its or their Representatives are required by Law (by oral questions, interrogatories, requests for information, subpoena, civil investigative demand, or similar process) or requested by any Governmental Entity with jurisdiction over such Person to disclose (provided that such Purchaser will, to the extent not legally prohibited, provide Seller with prompt written notice of such request so that Seller may seek, at its sole expense, an appropriate protective order or waive compliance with this Section 5.3(c)).
Section 5.4Access to Information.
(a)Seller shall, and shall cause its Affiliates to, afford to each Purchaser, its Affiliates and their respective Representatives reasonable access, upon reasonable notice during normal business hours, so long as consistent with applicable Law and in accordance with the procedures established by Seller, during the period prior to the Closing, and only for purposes of integration planning and in furtherance of the Transaction and the other transactions contemplated by this Agreement, to the Business Employees and the facilities, properties, books, Contracts and records of Seller and its Subsidiaries to the extent related to the Business and the Purchased Assets; provided, however, that (i) neither Seller nor any of its Affiliates shall be required to violate any obligation of confidentiality to which it or any of its Affiliates may be subject in discharging their obligations pursuant to this Section 5.4 (it being agreed that, in the event that the restrictions set forth in this clause (i) apply, Seller shall inform such Purchaser as to the general nature of what is being withheld and shall cooperate in good faith to attempt to design and implement alternative disclosure arrangements to enable such Purchaser to evaluate any such information without violating an obligation of confidentiality to any third party); (ii) Seller shall not be required to make available, or cause its Subsidiaries to make available, Business Employee personnel files prior to the Closing and, with respect to any Business Employees, only if and when such Purchaser provides Seller with notice that the applicable Business Employees have provided such Purchaser with a release permitting transfer of those files (provided that Seller shall not be required to make, or cause to be made, available medical records, workers compensation records or the results of any drug testing; and that such Purchaser shall indemnify and hold Seller and its Affiliates (including the other Seller Entities) harmless from any Liabilities arising out of or relating to the access to and/or transfer of such personnel files); and (iii) prior to the Closing Date, the Purchasers shall not conduct any Phase II Environmental Site Assessment or conduct any invasive testing or any sampling of soil, sediment, surface water, ground water or building material at, on, under or within any facility on the Transferred Real Property, or any other property of Seller, the other Seller Entities, the Purchased Entities or any of their respective Affiliates without the prior written consent of Seller.
(b)Each Purchaser agrees that any investigation undertaken pursuant to the access granted under Section 5.4(a) shall be conducted in such a manner as not to unreasonably interfere with the operation of the Business, and neither Purchaser or any of their Affiliates or Representatives shall communicate with any of the Business Employees without the prior written consent of Seller, which consent shall not be unreasonably withheld, conditioned or delayed.
-69-
Notwithstanding anything in this Agreement to the contrary, neither Seller nor any of its Affiliates shall be required to provide access to or disclose information where, such access or disclosure would (i) reasonably be expected to jeopardize attorney-client or other applicable privilege or protection, or contravene any applicable Laws or contractual obligations (it being agreed that, in the event that the restrictions set forth in this clause (i) apply, Seller shall inform the Purchasers as to the general nature of what is being withheld and shall cooperate in good faith to attempt to design and implement alternative disclosure arrangements to enable the Purchasers to evaluate any such information without violating an obligation of confidentiality to any third party, jeopardizing the attorney-client or other applicable privilege or protection or contravening any Laws or contractual obligations), or (ii) such information concerns the valuation of the Business or the Sale Process. In addition, no access hereunder shall be permitted for a purpose relating to a dispute or potential dispute between or among the Parties or their respective Affiliates.
(c)At and after the Closing for a period of ten (10) years (except to the extent relating to Tax matters (access, cooperation and procedures with respect to which are governed exclusively by Article VII)), each Purchaser shall, and shall cause its Affiliates to, afford Seller, its Affiliates and their respective Representatives, during normal business hours, upon reasonable notice, reasonable access to the facilities, properties, books, Contracts, records and employees of the Business and the Purchased Entities to the extent that such access may be reasonably requested by Seller, including in connection with financial statements, taxes, reporting obligations and compliance with applicable Laws; provided, however, that nothing in this Agreement shall limit any of Seller’s or any of its Affiliates’ rights of discovery. Notwithstanding anything in this Agreement to the contrary, neither Purchaser nor any of their Affiliates shall be required to provide access to or disclose information where such access or disclosure would reasonably be expected to jeopardize attorney-client or other applicable privilege or protection, or contravene any applicable Laws or contractual obligations (it being agreed that, in the event that the restrictions set forth in this sentence apply, such Purchaser shall inform Seller as to the general nature of what is being withheld and shall cooperate in good faith to attempt to design and implement alternative disclosure arrangements to enable Seller to evaluate any such information without violating an obligation of confidentiality to any third party, jeopardizing the attorney-client or other applicable privilege or protection or contravening any Laws or contractual obligations). In addition, no access hereunder shall be permitted for a purpose relating to a dispute or potential dispute between or among the Parties or their respective Affiliates.
(d)Except for Tax Returns and other documents governed by Section 7.1, each Purchaser agrees to hold all the books and records of the Business existing on the Closing Date and not to destroy or dispose of any thereof for a period of ten (10) years from the Closing Date or such longer time as may be required by Law, and thereafter, if it desires to destroy or dispose of such books and records, to offer first in writing at least sixty (60) days prior to such destruction or disposition to surrender them to Seller. Seller agrees to hold all the books and records of the Business existing on the Closing Date but not transferred to the Purchasers and not to destroy or dispose of any thereof for a period of ten (10) years from the Closing Date or such longer time as may be required by Law, and thereafter, if it desires to destroy or dispose of such books and records, to offer first in writing at least sixty (60) days prior to such destruction or disposition to surrender them to the Purchasers.
Section 5.5Publicity. The initial press release with respect to the Transaction shall be a joint press release agreed upon by Seller and the Purchasers.
-70-
Other than such joint press release, neither Party nor any Affiliate or Representative of such Party shall issue or cause the publication of any press release or public announcement in respect of this Agreement, the Transaction or the other transactions contemplated by this Agreement without the prior written consent of the other Party (which consent shall not be unreasonably withheld, conditioned or delayed), except as may be required by applicable Law or stock exchange rules, in which case the Party required to publish such press release or public announcement shall use reasonable efforts to provide the other Party a reasonable opportunity to comment on such press release or public announcement in advance of such publication; provided that the foregoing shall not apply to any press release or public announcement so long as any statements contained therein concerning the Transaction or the other transactions contemplated by this Agreement are consistent in all material respects with previous releases or announcements made by the applicable Party with respect to which such Party has complied with the provisions of this Section 5.5.
Section 5.6Intercompany Accounts and Intercompany Arrangements.
(a)At or prior to the Closing, Seller shall cause, and shall cause each member of the Seller Group to cause, all intercompany balances and accounts between members of the Seller Group, on the one hand, and the Purchased Entities, on the other hand, to be settled or otherwise eliminated in such a manner as Seller shall determine in its sole discretion (including, if so determined by Seller, by the Seller Entities or any of their Affiliates removing from any Purchased Entity any or all cash, cash equivalents or funds from cash pools by means of dividends, distributions, contribution, the creation or repayment or refinancing of intercompany debt, increasing or decreasing of cash pool balances or otherwise). Intercompany balances and accounts solely among any of the Purchased Entities shall not be affected by the above provisions of this Section 5.6. Immediately prior to the Closing, except for the Transaction Documents, all arrangements, understandings or Contracts, including all obligations to provide goods, services or other benefits, between the members of the Seller Group, on the one hand, and the Purchased Entities, on the other hand, shall automatically be terminated without further payment or performance and cease to have any further force and effect, such that no party thereto shall have any further obligations or Liabilities therefor or thereunder.
(b)Except for any claim for fraud or to the extent provided to the contrary in this Section 5.6 or pursuant to Article X, effective as of the Closing, each Purchaser, on behalf of itself and its Affiliates, including the Purchased Entities, hereby (i) agrees not to, and agrees to cause its Affiliates not to, assert any claim against Seller or its Affiliates, and (ii) releases Seller and each of its Affiliates (and their respective officers, directors and employees, acting in their capacities as such) from any Liability, obligation or responsibility to any of them for any and all past actions or failures to take action prior to the Closing directly or indirectly relating to or arising out of the Business, the Retained Business, or the operations of the Purchased Entities prior to the Closing, or relating to or arising out of Seller’s or its Affiliate’s ownership of the Purchased Assets, in each case of clause (i) and (ii), except for any obligation pursuant to the provisions of this Agreement or the other Transaction Documents.
-71-
(c)Except for any claim for fraud or to the extent provided to the contrary in this Section 5.6 or pursuant to Article X, effective as of the Closing, Seller, on behalf of itself and its Affiliates, hereby (i) agrees not to, and agrees to cause its Affiliates not to, assert any claim against Purchasers or their Affiliates, and (ii) releases the Purchased Entities (and their respective officers, directors and employees, acting in their capacities as such) from any Liability, obligation or responsibility to any of them for any and all past actions or failures to take action prior to the Closing directly or indirectly relating to or arising out of the Business, the Retained Business, or the operations of the Purchased Entities prior to the Closing, or relating to or arising out of the Purchased Entities’ ownership of the Purchased Assets, in each case of clause (i) and (ii), except for any obligation pursuant to the provisions of this Agreement or the other Transaction Documents.
Section 5.7Financing.
(a)Purchaser 1 shall use its reasonable best efforts, and shall cause each of its Subsidiaries to use reasonable best efforts to, take, or cause to be taken, all actions, and do, or cause to be done, all things necessary, proper or advisable to (x) obtain funds sufficient to fund the Financing Amounts on or prior to the date on which the Transaction is required to be consummated pursuant to the terms hereof and (y) extend the Expiration Date (as defined in the Commitment Letter) in the Commitment Letter to 11:59 p.m. (New York City time) on a date no earlier than July 28, 2025 or obtain replacement financing that does not include any Prohibited Modification the term of which runs through 11:59 p.m. (New York City time) on a date no earlier than July 28, 2025; provided that, under no circumstances shall such Purchaser’s requirement to pursue an extension under this clause (y) require such Purchaser to (i) pay a materially higher amount (taken as a whole) of interest rate or fees (it being agreed that accruing ticking fees shall not be “fees that are higher”) relative to the interest rate and fees set forth in the Commitment Letter and fee letter as of the date hereof or (ii) instate a more onerous financial covenant than the financial covenant set forth in the Commitment Letter as of the date hereof. In furtherance and not in limitation of the foregoing, Purchaser 1 shall use its reasonable best efforts to take, or cause to be taken, all actions and do, or cause to be done, all things necessary, proper or advisable to obtain the proceeds of the Financing on the terms and subject only to the conditions described in the Commitment Letter prior to the date on which the Transaction is required to be consummated pursuant to the terms hereof, including by (i) maintaining in effect the Commitment Letter, subject to permitted amendments and/or replacements as set forth herein, (ii) negotiating and entering into definitive agreements with respect to the Financing (the “Definitive Agreements”) consistent with the terms and conditions contained therein (including, as necessary, any “flex” provisions contained in the Commitment Letter or any related fee letter) or on other terms agreed to in writing by the Seller, and without any Prohibited Modification and (iii) satisfying on a timely basis (or obtaining a waiver of) no later than the Closing all conditions applicable to it in the Commitment Letter and the Definitive Agreements and complying with its obligations thereunder, subject to any amendments and/or replacements permitted hereunder.
-72-
(b)Neither Purchaser 1 nor any of its Subsidiaries shall, without the prior written consent of Seller: (i) permit, consent to or agree to any amendment, replacement, supplement, termination, modification, or waiver of any provision or remedy under the Commitment Letter or the Definitive Agreements if such amendment, replacement, supplement, termination, modification, waiver or remedy (A) adds new (or adversely modifies any existing) conditions to the consummation of all or any portion of the Financing, (B) reduces the aggregate principal amount of the Financing such that the aggregate amount of Financing at the Closing is less than the aggregate amount of the Financing Amounts, (C) adversely affects the ability of such Purchaser to enforce its rights against other parties to the Commitment Letter or the Definitive Agreements as so amended, replaced, supplemented or otherwise modified or (D) would otherwise reasonably be expected to prevent or delay the Closing (the effects described in clauses (A) through (D), collectively, the “Prohibited Modifications”); or (ii) terminate or cause the termination of the Commitment Letter or any Definitive Agreement; in each case, other than (A) any amendment to the Commitment Letter solely to join lenders, lead arrangers, bookrunners, syndication agents or similar entities who have not executed the Commitment Letter as of the date of this Agreement and who have voted to or are required by the terms of such amendment to vote to amend the Purchasers’ credit agreement in the manner contemplated by the Commitment Letter or (B) any replacement and related termination of the Commitment Letter with a committed financing (including as a committed delayed draw term loan under the Purchasers’ credit facilities) and the definitive documentation of such replacement financing, solely to the extent that such amendment or replacement or the addition of such additional parties would not constitute a Prohibited Modification. Purchaser 1 shall promptly deliver to Seller copies of any amendment, replacement, supplement, termination, modification or waiver to the Commitment Letter and/or the Definitive Agreements.
(c)In the event that any portion of the Financing becomes unavailable on the terms contemplated in the Commitment Letter, regardless of the reason therefor, Purchaser 1 shall (i) promptly notify Seller in writing of such unavailability and the reason therefor and (ii) after giving effect to any other funds of such Purchaser available for application towards the Financing Amounts, use reasonable best efforts, and cause each of its Subsidiaries to use their reasonable best efforts, to arrange and obtain, as promptly as practicable following the occurrence of such event, alternative financing for any such unavailable portion from the same or alternative sources (the “Alternative Financing”) in an amount sufficient, when taken together with the available portion of the Financing, to consummate the Transaction and the other transactions contemplated by this Agreement and to pay the Financing Amounts and, without limiting the foregoing, shall use reasonable best efforts to cause such Alternative Financing to include terms and conditions that are not materially less favorable, taken as a whole, to such Purchaser than the terms and conditions set forth in the Commitment Letter; provided that, under no circumstances shall such Purchaser’s requirement to pursue Alternative Financing require such Purchaser to (i) pay an interest rate or fees that are higher by more than a material amount, taken as a whole, relative to the interest rate and fees set forth in the Commitment Letter and fee letter as of the date hereof or (ii) instate a more onerous financial covenant than the financial covenant set forth in the Commitment Letter as of the date hereof. Purchaser 1 shall provide Seller with prompt oral and written notice of any actual or threatened (in writing) breach, default, cancellation, termination or repudiation by any party to the Commitment Letter and a copy of any written notice or other written communication from any Lender or other financing source with respect to any actual breach, default, cancellation, termination or repudiation by any party to the Commitment Letter of any provision thereof. Purchaser 1 shall keep Seller reasonably informed on a current basis of the status of its efforts to consummate the Financing, including any Alternative Financing.
(d)The foregoing notwithstanding, compliance by the Purchasers with this Section 5.7 shall not relieve any Purchaser of its obligations to consummate the transactions contemplated by this Agreement whether or not the Financing or any Alternative Financing is available.
-73-
To the extent a Purchaser obtains Alternative Financing or amends, replaces, supplements, terminates, modifies or waives any of the Financing, in each case pursuant to this Section 5.7 and without any Prohibited Modification, references to the “Financing,” “Financing Parties,” “Commitment Letter” and “Definitive Agreements” (and other like terms in this Agreement) shall be deemed to refer to such Alternative Financing, the commitments thereunder and the agreements with respect thereto, or the Financing as so amended, replaced, supplemented, terminated, modified or waived.
(e)At all times prior to the Closing, the Purchasers shall maintain no less than $25,000,000 in unrestricted cash to be used to pay amounts due hereunder.
Section 5.8Financing Cooperation.
(a)Seller shall use its commercially reasonable efforts to provide, and shall cause the Seller Entities and Purchased Entities to use commercially reasonable efforts to provide, and each of them shall use their commercially reasonable efforts to cause their respective Representatives to use their commercially reasonable efforts to provide, customary cooperation, to the extent reasonably requested by the Purchasers in writing, necessary for the arrangement of the Financing (provided that such requested cooperation does not unreasonably interfere with the ongoing operations of Seller or any of its Affiliates), including using commercially reasonable efforts to:
(i)participate in a reasonable number of meetings, presentations, due diligence sessions and sessions with rating agencies, at reasonable times and with reasonable advance notice, and in each case which shall be virtual unless otherwise agreed to by Seller;
(ii)to the extent required by the Financing, facilitate the pledging of collateral of the Purchased Entities effective no earlier than the Closing;
(iii)provide reasonable and customary assistance to the Purchasers and the Lenders in the preparation of customary lender presentations, syndication memoranda, ratings agency presentations and other bank marketing materials for the Financing;
(iv)cooperate with the Lenders’ due diligence, to the extent customary and reasonable; and
(v)assist in the preparation of, and in the execution and delivery at Closing of, any pledge and security documents, other definitive financing documents (including credit agreements) and schedules and certificates (including a perfection certificate), in each case, as may be reasonably requested in writing by the Purchasers.
-74-
(b)The foregoing notwithstanding, neither Seller nor any of its Affiliates shall be required to take or permit the taking of any action pursuant to this Section 5.8 that would: (i) require Seller or its Affiliates or any Persons who are officers or directors of such entities to pass resolutions or consents to approve or authorize the execution of the Financing or enter into, execute or deliver any certificate, document, instrument or agreement or agree to any change or modification of any existing certificate, document, instrument or agreement (other than, with respect to the Purchased Entities only, resolutions that are contingent on the Closing), (ii) cause any representation or warranty in this Agreement to be breached by Seller or any of its Affiliates, (iii) require Seller or any of its Affiliates to pay any commitment or other similar fee or incur any other expense, liability or obligation in connection with the Financing or otherwise incur any obligation under any agreement, certificate, document or instrument, (iv) reasonably be expected to cause any director, officer, employee or stockholder of Seller or any of its Affiliates to incur any personal liability, (v) reasonably be expected to conflict with the organizational documents of Seller or any of its Affiliates or any Laws, (vi) reasonably be expected to result in a material violation or breach of, or a default (with or without notice, lapse of time, or both) under, any Contract to which Seller or any of its Affiliates is a party, (vii) provide access to or disclose information that Seller or any of its Affiliates determines would jeopardize any attorney-client privilege or other applicable privilege or protection of Seller or any of its Affiliates (provided that, upon a Purchaser’s reasonable written request, Seller shall provide written notice to such Purchaser that information is being withheld on this basis), (viii) require the delivery of any opinion of counsel, (ix) require Seller to prepare any financial statements or information that are not available to it and prepared in the ordinary course of its financial reporting practices or (x) require Seller to prepare or deliver any Excluded Information. Nothing contained in this Section 5.8 or otherwise in this Agreement shall require (A) Seller or any of its Affiliates (other than the Purchased Entities) to be an issuer or other obligor with respect to the Financing or (B) the Purchased Entities, prior to Closing, to be an issuer or other obligor with respect to the Financing. The Purchasers shall, promptly on request by Seller, reimburse Seller and each of its Affiliates for all reasonable out-of-pocket costs incurred by them or their respective Representatives in connection with such cooperation (other than the preparation of financial statements in the ordinary course of business of the Seller and/or the Purchased Entities) and shall reimburse, indemnify and hold harmless Seller and its Affiliates and their respective Representatives from and against any and all losses suffered or incurred by them in connection with the arrangement of the Financing, any action taken by them at the request of the Purchasers or their Representatives pursuant to this Section 5.8 and any information used in connection therewith, other than to the extent such losses are a direct result of the Seller acting in bad faith through the knowing, intentional and material breach of the Seller’s respective obligations under this Section 5.8 or through the knowing and intentional delivery of information under this Section 5.8 that is, when taken as a whole, materially inaccurate.
(c)The Parties acknowledge and agree that the provisions contained in this Section 5.8 represent the sole obligation of Seller and its Affiliates with respect to cooperation in connection with the arrangement of any financing (including the Financing) to be obtained by the Purchasers with respect to the transactions contemplated by this Agreement and the Commitment Letter, and no other provision of this Agreement (including the Exhibits and Schedules hereto) or the Commitment Letter shall be deemed to expand or modify such obligations. In no event shall the receipt or availability of any funds or financing (including the Financing) by the Purchasers or any of their Affiliates or any other financing or other transactions be a condition to any of the Purchasers’ obligations under this Agreement. Notwithstanding anything to the contrary in this Agreement, the Seller’s breach of any of the covenants required to be performed by it under this Section 5.8 shall not be considered in determining the satisfaction of the condition set forth in Section 8.2(b), unless (i) Purchaser provides written notice of a breach of this Section 5.8 to the Seller (which notice specifies such breach in reasonable detail), (ii) Seller fails to materially cure such breach within thirty (30) days of such notice and (iii) the failure to obtain the Financing was primarily caused by such breach.
-75-
(d)Prior to the Closing, if requested in writing by the Purchasers, Seller shall (i) provide to the Purchasers (A) the Financing Information and (B) the information necessary or proper for the applicable Purchaser to prepare the pro forma financial statements required by paragraph 3 of Exhibit C to the Commitment Letter, (ii) use commercially reasonable efforts to provide to the Purchasers executed, customary authorization letters authorizing the distribution of information to prospective lenders, and (iii) if requested in writing by the Purchasers at least ten (10) Business Days prior to the Closing Date, to furnish to the Purchasers, at least four (4) Business Days prior to the Closing Date, a certification regarding beneficial ownership and information regarding the Purchased Entities that is required by U.S. regulatory authorities under applicable “know your customer” and anti-money laundering rules and regulations, including the USA PATRIOT Act and 31 C.F.R. § 1010.230. All non-public or otherwise confidential information regarding Seller or any of its Affiliates obtained by the Purchasers or their Representatives pursuant to this Section 5.8 shall be kept confidential in accordance with the Confidentiality Agreement.
Section 5.9Financial Obligations. At or prior to the Closing, the Purchasers shall at their sole expense arrange for substitute letters of credit, surety bonds, Purchaser guarantees, advance payment guarantees, and other obligations to replace the outstanding letters of credit, surety bonds, guarantees, advance payment guarantees and other contractual obligations entered into by or on behalf of Seller or any of its Affiliates (other than the Purchased Entities) in connection with or relating to the Business, the Purchased Assets or the Assumed Liabilities set forth on Section 5.9 of the Seller Disclosure Schedules (together, the “Guarantees”), and assume all obligations under each Guarantee, obtaining from the creditor or other counterparty a full and irrevocable release of Seller and its Affiliates that are liable, directly or indirectly, for reimbursement to the creditor or fulfillment of other Liabilities to a counterparty in connection with amounts drawn under the Guarantees. Each Purchaser further agrees that to the extent Seller or any of its Affiliates incurs any cost or expense, or is required to make any payment, or is subject to any claim or Proceeding, in connection with such Guarantees on or after the Closing, such Purchaser shall, jointly and severally, indemnify and hold harmless Seller and its Affiliates against, and reimburse Seller and its Affiliates for, any and all Liabilities incurred or amounts paid, including costs or expenses in connection with such Guarantees, including Seller’s and any of its Affiliates’ expenses in maintaining such Guarantees, whether or not any such Guarantee is drawn upon or required to be performed, and shall in any event promptly reimburse, upon written demand therefor from Seller, Seller and any of its Affiliates to the extent that any Guarantee is called upon and Seller or any of its Affiliates makes any payment or incurs any Liability in respect of any such Guarantee; provided, that in no event shall Purchasers’ obligation to indemnify or reimburse Seller pursuant to this sentence exceed $50,000, individually or in the aggregate. For any Guarantees for which a Purchaser or any of its Affiliates, as applicable, is not substituted in all respects for Seller and its Affiliates (or for which Seller and its Affiliates are not fully released) effective as of the Closing and that cannot otherwise be terminated effective as of the Closing (with Seller and its Affiliates to be fully released in respect thereof), such Purchaser shall and shall cause its Affiliates to continue to use their commercially reasonable efforts to effect such substitution or termination and release after the Closing. Without limiting the foregoing, neither Purchaser nor any of their respective Affiliates shall extend or renew any Contract containing or underlying a Guarantee unless, prior to or concurrently with such extension or renewal, such Purchaser or its Affiliates are substituted in all respects for Seller and its Affiliates, and Seller and its Affiliates are fully released, in respect of all Liabilities under such Guarantees.
-76-
Section 5.10Trademark Matters.
(a)No Purchaser nor any of their Affiliates (including the Purchased Entities) shall acquire any rights in, or use, or have the right to use, the “Zoetis” names or any confusingly similar variations or derivatives thereof or any Marks of Seller or any of its Affiliates that are not included in the Purchased Assets (collectively, the “Retained Marks”) or any name or Mark that is confusingly similar to or derivative of the Retained Marks, except for the limited right to use such Retained Marks as expressly set forth in Section 5.10(c)(i).
(b)Within ten (10) Business Days of Closing, the Purchasers shall make all filings necessary to change the legal names, corporate names and business names of the Purchased Entities to remove any reference to the Retained Marks, and shall promptly take any and all follow-up actions that may be required or requested by any Governmental Entity to effect such changes as soon as practicable.
(c)Subject to compliance with the quality control provisions and other terms of this Section 5.10, the Business may continue to temporarily use the Retained Marks following the Closing, solely to the extent and in the same manner as used immediately prior to the Closing, so long as the Purchasers shall, and shall cause their Affiliates (including the Business), to (i) immediately after the Closing cease to hold itself out as having any affiliation with Seller or any of its Affiliates and (ii) use commercially reasonable efforts to minimize and eliminate use of the Retained Marks by the Business from and after the Closing; provided, that:
(i)the Business shall be permitted to use such Retained Marks for a period of up to two (2) years following the Closing (A) with respect to any finished goods Inventory in the Purchasers’ possession as of the Closing, until the expiration of the applicable shelf lives of such Inventory or the supply is exhausted, (B) with respect to any packaging included in Working Capital (“Transferred Packaging”), until the supply of such Transferred Packaging is exhausted, and (C) with respect to any Business Products for which such Retained Marks are required to be used under a Transferred Marketing Authorization or any other Regulatory Approval or Permit related to the Business Products (such products, the “Approval Products”), the use of such Retained Marks is no longer required under such Transferred Marketing Authorization and the use of the Retained Marks is no longer required under any Regulatory Approval or Permit; provided that the Purchasers shall coordinate with Seller and take all steps reasonably necessary to obtain or change the applicable Transferred Marketing Authorization, Regulatory Approval or Permit with respect to such Approval Products to eliminate any required use of such Retained Marks as soon as practicable after the Closing Date; provided, further, that if any Transferred Packaging has not been exhausted by the expiration of the two (2)-year period, or if the Purchasers will not be able to eliminate required use of such Retained Marks with respect to such Approval Products by the expiration of the two (2)-year period despite Purchasers’ diligent efforts to obtain or change the applicable Transferred Marketing Authorization, Regulatory Approval or Permit and due to circumstances outside the Purchaser’s reasonable control, then the Purchasers shall notify Seller in writing that the Transferred Packaging has not been exhausted, and of the specific jurisdictions for which the Retained Marks are still required to be used under any Transferred Marketing Authorization, Regulatory Approval or Permit (as applicable), and such period shall automatically be extended for up to two (2) additional periods of six (6) months each so long as the Purchasers remain diligent with respect to such transition during such extension. Notwithstanding the forgoing, with respect to the specific Retained Marks, the specific Approval Products and specific jurisdictions set forth on Section 5.10 of the Seller Disclosure Schedules, the Business shall be permitted to use such specific Retained Marks (including to use any Transferred Packaging in such specific jurisdictions) until the earlier of (x) the time period set forth set forth next to the specific Approval Products and specific jurisdictions on Section 5.10 of the Seller Disclosure Schedules and (y) such time that the Transferred Packaging is exhausted and the use of such Retained Marks is no longer required under such Transferred Marketing Authorization and the use of the Retained Marks is no longer required under any Regulatory Approval or Permit; provided that the Purchasers shall coordinate with Seller and take all steps reasonably necessary to obtain or change the applicable Transferred Marketing Authorization, Regulatory Approval or Permit with respect to such Approval Products to eliminate any required use of such Retained Marks as soon as practicable after the Closing Date.
-77-
(ii)for all other uses of the Retained Marks (subject to Section 5.10(b)), as soon as practicable after the Closing Date (and in any event within sixty (60) days thereafter), the Purchasers shall, and shall cause their Affiliates (including the Purchased Entities and the Business), to cease and discontinue use of all Retained Marks and complete the removal of the Retained Marks from all products, signage, vehicles, properties, technical information and promotional or other marketing materials and other assets.
(d)Notwithstanding the foregoing, the Purchasers and their Subsidiaries (including the Purchased Entities) may use the Retained Marks after the Closing (i) in a manner that constitutes fair use, or as permitted or required by Law, and (ii) for purposes of regulatory filings or describing the past ownership or affiliation of the Business; provided, that such use does not give rise to a likelihood of confusion as to the source or origin of any goods or services or imply any endorsement by, or ongoing association with, Seller or any of its Subsidiaries.
(e)To comply with Seller’s quality control standards, the Purchasers and their Affiliates shall: (i) use the Retained Marks in the same form and manner, and at least the same high level of quality, in effect for the Retained Marks as of the Closing, (ii) comply with all applicable Laws and regulations pertaining to any Business Products bearing the Retained Marks; (iii) adhere to Seller’s reasonable instructions regarding use and appearance of the Retained Marks (which, to the extent applicable, shall be consistent with Seller’s generally applicable instructions for other licensees of the Retained Marks); and (iv) not use the Retained Marks in any manner that would damage the goodwill associated therewith or the reputation of Seller or any of its Affiliates. Seller may terminate the rights granted under Section 5.10(c)(i) if the Purchasers or any of their Affiliates fail to comply with the foregoing quality control provisions or otherwise materially breach the terms of this Section 5.10 and such failure or material breach is not cured to Seller’s reasonable satisfaction within thirty (30) days of receiving written notice of such failure.
-78-
(f)The Purchasers, jointly and severally, shall indemnify and hold harmless Seller and its Affiliates for Third Party Claims arising from or relating to any use of the Retained Marks by such Purchaser or its Affiliates. All goodwill arising from the use of the Retained Marks as described in this Section shall inure to the sole and exclusive benefit of Seller and Seller’s Subsidiaries.
Section 5.11Global Separation Sublicenses. Seller and the Purchasers shall cooperate with each other and use their respective commercially reasonable efforts to obtain consent from the counterparty to certain Contracts listed in Section 5.11 of the Seller Disclosure Schedules (collectively, the “Global Separation Licenses”) to enter into a sublicense or new Contract that extends the rights to a Purchaser, and allows such Purchaser to assume the obligations, under such Global Separation Licenses to the extent related to the operations or conduct of the Business. Neither Seller nor any of its Affiliates shall have (a) any obligation under this Section 5.11 with respect to such consents that are not obtained on or prior to one hundred twenty (120) days following the Closing Date; and (b) any obligation to make any payments or other concession, or incur any other Liability, or commence or participate in any Proceeding to obtain any such consents, and the failure to receive any such consents shall not be taken into account in determining whether any condition to the Closing set forth in Article VIII shall have been satisfied. All reasonable and documented third-party legal and administrative fees, and other similar costs and expenses, payable in connection with obtaining any such consents shall be borne by Seller.
Section 5.12Background IP License.
(a)License to the Purchasers. Effective as of the Closing, and subject to the provisions hereof, Seller and its Subsidiaries hereby grant, and agree to grant, to the Purchasers and their Subsidiaries, including the Purchased Entities, a worldwide, irrevocable, non-exclusive, fully paid, royalty-free, non-sublicenseable, non-transferable (except as provided in Section 5.12(c)) license under: (i) Patents owned by the Seller Group as of the Closing to the extent practiced by, or absent a license thereto or ownership thereof, would be infringed by, the Business immediately prior to the Closing, to make, have made, import, use, offer to sell, sell, and otherwise provide any product or service of the Business and any natural evolutions and extensions thereof; and (ii) Intellectual Property Rights (other than Registered IP) owned by the Seller Group as of the Closing to the extent used in and related to the Business immediately prior to the Closing, to use, reproduce, distribute, disclose, make, modify, improve, display and perform, create derivative works of and otherwise exploit the Purchased Assets, in each case, solely with respect to the conduct of the Business and any natural evolutions and extensions thereof; it being understood that the foregoing license does not and shall not require the delivery or disclosure to the Purchasers of any tangible or intangible assets that are not Purchased Assets.
(b)Term. The licenses granted under this Section 5.12 will continue until the expiration of the last-to-expire of the Intellectual Property Rights licensed therein.
-79-
(c)Transfer of License. Except as expressly set forth herein, no Purchaser may assign or transfer the license granted in Section 5.12(a) directly or indirectly, in whole or in part, whether voluntarily or involuntarily or by operation of Law or otherwise, without Seller’s prior written consent. Notwithstanding the foregoing, a Purchaser may assign such license to a third party, or permit a third party to assume such license, in connection with the acquisition of such Purchaser (whether by equity or asset sale or merger or otherwise) or the sale of all or substantially all of the assets of any such Purchaser to which this Agreement relates; provided that the resulting, surviving or transferee Person or acquirer of such Purchaser assumes all of the applicable obligations of such Purchaser by operation of Law or by express assignment, as the case may be. Any assignment in violation of this Section 5.12(c) shall be null and void ab initio.
(d)Confidentiality. The Purchasers agree on behalf of themselves and their Subsidiaries that (i) they (and each of their Subsidiaries) shall treat the Trade Secrets and confidential information licensed under Section 5.12(a) with at least the same degree of care as they treat their own similar Trade Secrets and confidential information, but in no event with less than reasonable care, and (ii) neither they (nor any of their Subsidiaries) may use or disclose such Trade Secrets or confidential information, as applicable, except in accordance with the license granted in Section 5.12(a).
Section 5.13Insurance. Each Purchaser acknowledges and agrees that, from and after the Closing, the Business, the Purchased Entities, the Purchased Assets, the Assumed Liabilities, and the operations and assets and Liabilities in respect thereof, shall cease to be insured by Seller’s or its Affiliates’ insurance policies or by any of their self-insured programs, and neither Purchaser nor any of their Affiliates (including the Business and the Purchased Entities) shall have any access, right, title or interest to or in any such insurance policies (including to all claims and rights to make claims and all rights to proceeds) to cover the Business, the Purchased Entities, the Purchased Assets, the Assumed Liabilities, or the operations or assets or Liabilities in respect thereof. Seller or its Affiliates may amend any insurance policies in the manner it deems appropriate to give effect to this Section 5.13. From and after the Closing, the Purchasers shall be responsible for securing all insurance they consider appropriate for the Business, the Purchased Entities, the Purchased Assets, the Assumed Liabilities, and the operations and assets and Liabilities in respect thereof. From and after the date hereof and for the first six (6) months following the Closing, Seller and its Subsidiaries shall use their respective commercially reasonable efforts to cooperate with the Purchasers, upon a Purchaser’s reasonable request, in seeking assistance to secure such insurance. Each Purchaser further covenants and agrees not to seek to assert or to exercise any rights or claims of, or in respect of, the Business, the Purchased Entities, the Purchased Assets, the Assumed Liabilities, and the operations and assets and Liabilities in respect thereof, under or in respect of any past or current insurance policy under which any of the foregoing is a named insured.
Section 5.14Litigation Support.
-80-
(a)From and after the Closing, in the event that and for so long as a Party or any of its Affiliates (in such capacity, a “Litigating Party”) is prosecuting, contesting or defending any Proceeding, investigation, charge, claim or demand by or against a third party or otherwise addressing, negotiating, disputing, investigating, complying with, mitigating, discharging or otherwise performing or managing any obligation, Liability or loss in connection with (i) the Transaction or any of the other transactions contemplated under this Agreement, or (ii) any fact, situation, circumstance, status, condition, activity, practice, plan, occurrence, event, incident, action, failure to act, or transaction relating to, in connection with or arising from the Business, the Purchased Entities, the Purchased Assets or the Assumed Liabilities, the other Party shall, and shall cause its Affiliates (and its and their officers, employees and other Representatives) to, reasonably cooperate with the Litigating Party and its counsel in such prosecution, contest, defense or other action, including making available its personnel, participating in meetings and providing such testimony and access to its books and records and taking such other actions as shall be reasonably necessary in connection with such prosecution, contest, defense or other action. Notwithstanding anything in this Agreement to the contrary, Seller shall retain full control of prosecuting, contesting, defending, compromising, settling or taking any other action related to or in connection with any Proceeding, investigation, charge, claim or demand by or against a third party related to the Retained Business, any Excluded Asset or Retained Liability, whether arising before or after the Closing, and neither Purchaser nor their Affiliates shall have any rights in connection therewith, other than the right to indemnification for Retained Liabilities pursuant to Article X.
(b)The Purchasers agree to the matters set forth on Section 5.14(b) of the Seller Disclosure Schedules.
Section 5.15Third-Party Consents. Seller and the Purchasers shall, and shall cause their respective Subsidiaries to, cooperate and use their commercially reasonable efforts to obtain any consents from third parties under the Material Contracts required in connection with the consummation of the Transactions (the consents of third parties referred to in this Section 5.15, collectively, the “Third-Party Consents”). Notwithstanding anything to the contrary contained herein, (a) Seller and its Affiliates shall not have any further obligation under this Section 5.15 with respect to any consents of third parties (including the Third-Party Consents) that are not obtained on or prior to one hundred twenty (120) days following the Closing Date; and (b) neither Seller nor any of its Affiliates shall have any obligation to make any payments or other concession, or incur any other Liability, or commence or participate in any Proceeding to obtain any consents of third parties (including Third-Party Consents) or effect any of the transfers or arrangements contemplated by this Section 5.15, and the failure to receive any such consents or to effect any such transfers or arrangements shall not be taken into account in determining whether any condition to the Closing set forth in Article VIII shall have been satisfied. Notwithstanding anything in this Agreement to the contrary, none of Seller, the other Seller Entities or any of their respective Affiliates shall under any circumstance be required to pay or commit to pay any amount or incur any obligation in favor of or offer or grant any accommodation (financial or otherwise, regardless of any provision to the contrary in the underlying Contract, including any requirements for the securing or posting of any bonds, letters of credit or similar instruments, or the furnishing of any guarantees) to any Person to obtain any approval.
Section 5.16Non-Solicitation of Employees; Non-Competition.
-81-
(a)For a period of three (3) years from the Closing Date, without the prior written consent of the Purchasers, as to any Business Employee as of immediately prior to the Closing who shall have become employed by the Purchasers or their Subsidiaries as of immediately following the Closing (a “Seller Covered Person”), Seller agrees that none of Seller or any of its Subsidiaries will solicit for employment any Seller Covered Person; provided that Seller and its Subsidiaries shall not be precluded from soliciting, or taking any other action with respect to, any such individual (i) whose employment with the Purchasers or any of their Subsidiaries ceased prior to commencement of employment discussions between Seller or its Subsidiaries and such individual, (ii) who responds to solicitation not specifically targeted at employees of the Purchasers or any of their Subsidiaries or (iii) who initiates discussions regarding such employment without any solicitation by Seller or its Subsidiaries in violation of this Agreement; and provided, further, that Seller and its Subsidiaries shall not be restricted from engaging in general solicitations or advertising not specifically targeted at any such Persons described above.
(b)For a period of three (3) years from the Closing Date, without the prior written consent of Seller, each Purchaser agrees that neither such Purchaser nor any of its Subsidiaries will solicit for employment any employee of Seller or its Subsidiaries with whom the Purchasers or any of their Subsidiaries had contact during their evaluation of the transactions contemplated by this Agreement or in connection with the services provided pursuant to the Transition Services Agreement or the Distribution Agreements; provided that such Purchaser and its Subsidiaries shall not be precluded from soliciting, or taking any other action with respect to, any such individual (i) whose employment with Seller or any of its Subsidiaries ceased prior to commencement of employment discussions between such Purchaser or its Subsidiaries and such individual, (ii) who responds to solicitation not specifically targeted at employees of Seller or any of its Subsidiaries or (iii) who initiates discussions regarding such employment without any solicitation by such Purchaser or its Subsidiaries in violation of this Agreement; and provided, further, that such Purchaser and its Subsidiaries shall not be restricted from engaging in general solicitations or advertising not specifically targeted at any such Persons described above.
(c)For a period of three (3) years from the Closing Date, without the prior written consent of the Purchasers, Seller agrees not to, and agrees to cause its Subsidiaries not to, (i) acquire, finance, own any interest in, manage, control, participate in, consult with, render services for, operate or engage in, directly or indirectly, any business that manufactures, distributes, markets and sells medicated products added in feed or drinking water that compete with the Business Products in the Territory (a “Competing Business”), or (ii) intentionally solicit or intentionally entice, or attempt to solicit or entice, any Person that, to the knowledge of Seller, was a supplier of the Business as of immediately prior to the Closing to cease doing business with the Business or intentionally interfere in a materially adverse manner with the relationship between the Business and any Person that, to the knowledge of Seller, was a supplier of the Business as of immediately prior to the Closing; provided, however, that nothing herein shall preclude Seller or its Subsidiaries from:
(i)owning seven and a half percent (7.5%) or less of the outstanding stock or other securities of any Person;
(ii)acquiring and, after such acquisition, owning an interest in any Person (or its successor) that is engaged in a Competing Business and operating such Competing Business if such Competing Business generated less than seven and a half percent (7.5%) of actual revenue received by such Person in the last completed fiscal year of such Person;
-82-
(iii)acquiring and, after such acquisition, owning an interest in any Person (or its successor) that is engaged in a Competing Business and operating such Competing Business if (A) such Competing Business generated seven and a half percent (7.5%) or more of actual revenue received by such Person in the last completed fiscal year of such Person and (B) Seller or its applicable Subsidiary, within eighteen (18) months after the consummation of such acquisition, causes the divestiture of a sufficient portion of the Competing Business of such Person such that the restrictions set forth in this Section 5.16(c) would not have operated to restrict such ownership assuming the completion of such divestiture had occurred prior to such acquisition;
(iv)exercising its rights, performing or complying with its obligations under this Agreement or any of the other Transaction Documents;
(v)engaging in the Retained Business; or
(vi)engaging in the activities set forth on Section 5.16(c) of the Seller Disclosure Schedules.
(d)If the final judgment of a court of competent jurisdiction declares that any term or provision set forth in this Section 5.16 is invalid or unenforceable, the Parties agree that the court making the determination of invalidity or unenforceability shall have the power to reduce the scope, duration or area of the term or provision, to delete specific words or phrases, or to replace any invalid or unenforceable term or provision with a term or provision that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this Section 5.16 shall be enforceable as so modified after the expiration of the time within which the judgment may be appealed. The Parties intend that the agreements, covenants and obligations set forth in this Section 5.16 shall be deemed to be a series of separate covenants, one for each county or province of each and every state, commonwealth, territory or jurisdiction of each county or province anywhere in the world and one for each month of the applicable period of time (if any), as applicable. In the event of a breach or violation of any of the covenants, agreements or obligations in this Section 5.16, the period applicable to such breached or violated covenants, agreements or obligations shall be tolled until such breach or violation has been duly cured, to the extent such breach or violation is curable. Notwithstanding the foregoing, this Section 5.16 (x) shall not restrain or prohibit the consummation of any transaction or series of related transactions that results in a change of control of Seller, or involves the sale or transfer of a majority of the assets of the Seller Group or the equity of Seller, including any transaction following which the equity holders of Seller immediately prior to such transaction, in their capacity as such (and excluding any cross-ownership they may have in the equity of any counterparty to such transaction), cease to own a majority of the equity or voting power of Seller or the surviving entity in such transaction or its ultimate parent company immediately following such transaction (the “Seller Change of Control”); provided, that, following the consummation of any Seller Change of Control, Seller and its Subsidiaries that were bound by this Section 5.16 prior to the Seller Change of Control (the “Original Seller Group”) shall continue to be bound by the terms of this Section 5.16 in accordance with the terms hereof; provided, however, that, for the avoidance of doubt, Seller’s Affiliates (other than the Original Seller Group) shall in no event be bound by this Section 5.16; and (y) shall not restrain or prohibit any activities, actions or conduct of any Person that Seller or its Subsidiaries have an equity interest in as of the date hereof but is not directly or indirectly controlled by Seller or its Subsidiaries, including any such joint ventures, partnerships or co-investment vehicles that neither Seller nor any of its direct or indirect Subsidiaries controls.
-83-
Section 5.17Payments; Misallocated Assets.
(a)Seller shall, or shall cause its applicable Subsidiary to, promptly pay or deliver to the Purchasers (or their designated Subsidiaries) any monies or checks that have been sent to Seller or any of its Subsidiaries after the Closing by customers, suppliers or other contracting parties of the Business to the extent that they constitute a Purchased Asset. The Purchasers shall, or shall cause their applicable Subsidiary (including the Purchased Entities) to, promptly pay or deliver to Seller (or its designated Subsidiaries) any monies or checks that have been sent to a Purchaser or any of its Subsidiaries (including the Purchased Entities) after the Closing by customers, suppliers or other contracting parties of the Retained Business to the extent that they constitute an Excluded Asset.
(b)Subject to Section 5.13, if, at any time after the Closing, any asset held by a Purchaser or any of its Subsidiaries (including the Purchased Entities) is ultimately determined to be an Excluded Asset or such Purchaser or any of its Subsidiaries is found to be subject to a Retained Liability, (i) such Purchaser shall return or transfer and convey (at such Purchaser’s sole cost and expense (other than Transfer Taxes which shall be borne pursuant to Section 7.5) and without further consideration) to Seller or the appropriate Subsidiary of Seller such Excluded Asset or Retained Liability; (ii) Seller shall, or shall cause its appropriate Subsidiary to, assume (without further consideration) such Retained Liability; and (iii) Seller and such Purchaser shall, and shall cause their appropriate Subsidiaries to, execute such documents or instruments of conveyance or assumption and take such further acts as are reasonably necessary or desirable to effect the transfer of such Excluded Asset or Retained Liability back to Seller or its appropriate Subsidiary, in each case such that each Party is put into the same economic position as if such action had been taken on or prior to the Closing Date.
(c)Subject to Section 5.13, if, at any time after the Closing, any asset held by any of the Seller Entities or any of their Subsidiaries is ultimately determined to be a Purchased Asset or any of the Seller Entities or any of their Subsidiaries is found to be subject to an Assumed Liability, (i) Seller shall transfer and convey (at Seller’s sole cost and expense (other than Transfer Taxes which shall be borne pursuant to Section 7.5) and without further consideration) to the applicable Purchaser such Purchased Asset or Assumed Liability; (ii) such Purchaser shall assume (without further consideration) such Assumed Liability; and (iii) Seller and such Purchaser shall, and shall cause their appropriate Subsidiaries to, execute such documents or instruments of conveyance or assumption and take such further acts as are reasonably necessary or desirable to effect the transfer of such Purchased Asset or Assumed Liability to such Purchaser, in each case such that each Party is put into the same economic position as if such action had been taken on or prior to the Closing Date.
-84-
Section 5.18R&W Insurance Policy. The Purchasers shall use reasonable best efforts to take all actions necessary to complete the applicable conditions in the conditional binder (other than the condition that Closing has occurred, to which this sentence does not apply) to the representations and warranties insurance policy with respect to the transactions contemplated hereby (the “R&W Insurance Policy”) within the times set forth therein. Upon its final issuance, the Purchasers shall deliver a copy of the R&W Insurance Policy to Seller. The R&W Insurance Policy shall include a provision whereby the insurer expressly waives, and irrevocably agrees not to pursue, directly or indirectly, any subrogation rights in connection with this Agreement, the other Transaction Documents, and the transactions contemplated hereby and thereby (other than in respect of fraud) against Seller, its Subsidiaries or any of their respective direct or indirect past or present shareholder, partner, stockholder, manager, member, director, officer or employee (the “Waiver of Subrogation Provision”) and such Persons shall be express third-party beneficiaries of such provision. In addition, the Purchasers agree not to amend or waive the Waiver of Subrogation Provision in any manner that is adverse to Seller, its Subsidiaries or any of their respective direct or indirect past or present shareholder, partner, stockholder, manager, member, director, officer or employee without Seller’s prior written consent (which consent shall be in the sole and absolute discretion of Seller). Seller shall, and shall use commercially reasonable efforts to cause its Representatives to, reasonably cooperate with the Purchasers and use commercially reasonable efforts with respect to any reasonable requests made by the Purchasers or their Subsidiaries or Representatives in connection with the Purchasers’ procurement of the R&W Insurance Policy, including responding to and facilitating any additional reasonable diligence investigation and providing to the Purchasers and their Representatives, as well as the applicable insurers, any additional diligence information reasonably requested in connection with binding such policy or removing or limiting any exclusions under the R&W Insurance Policy.
Section 5.19Delivery of Financial Statements.
(a)Seller shall prepare and deliver as soon as reasonably practicable following the date hereof (and in any event no later than five (5) Business Days prior to the Closing) (x) audited financial statements of the Business, consisting of the audited statements of assets acquired and liabilities assumed as of December 31, 2023 and 2022, and the related audited statements of revenues and direct expenses for the twelve (12)-month periods then ended (the “Audited Financial Statements”), and (y) an unaudited statement of assets acquired and liabilities assumed of the Business and related statement of revenues and direct expenses for the three (3) month-period ended March 31, 2024, together with the comparative period for the prior year, reviewed in accordance with SAS 100 review procedures by independent accountants for the Business, in each case together with the notes thereto and prepared in accordance with the requirements for abbreviated financial statements set forth in Rule 3-05(e) of Regulation S-X under the Securities Act.
-85-
Seller shall prepare and deliver to the Purchasers as soon as reasonably practicable and not later than ninety (90) days or, with respect to annual results, one hundred and five (105) days, following the end of any fiscal quarter or fiscal year, as applicable, ending between the date hereof and the Closing Date (even if such delivery date occurs after the Closing Date), (i) as of the end of such quarterly period, an unaudited statement of assets acquired and liabilities assumed of the Business and related statement of revenues and direct expenses for the applicable year-to-date period, in each case, reviewed in accordance with SAS 100 review procedures by independent accountants for the Business and (ii) as of the end of such annual period, an audited statement of assets acquired and liabilities assumed of the Business and related statement of revenues and direct expenses for the applicable period, in each case together with the comparative period for the prior year and the notes thereto and prepared in accordance with the requirements for abbreviated financial statements set forth in Rule 3-05(e) of Regulation S-X under the Securities Act, and with respect to each such annual period (including with respect to the Audited Financial Statements), audited pursuant to the standards set forth by the American Institute of Certified Public Accountants and accompanied by an independent auditors’ report for the Business, and in each case (A) solely to the extent such additional financial statements are required by Item 9.01 of Form 8-K with respect to financial statements of the business acquired if included on a Form 8-K (or amendment thereto) filed by Purchaser 1 pursuant to the requirements of the Exchange Act and (B) which would satisfy the requirements of Item 9.01 of Form 8-K with respect to financial statements of the business acquired if included on a Form 8-K (or amendment thereto) filed by Purchaser 1 pursuant to the requirements of the Exchange Act (collectively with the Audited Financial Statements, the “Financial Statements”). For the avoidance of doubt, Seller shall not be required to deliver any financial statements of the Business for any fiscal quarter or fiscal year, as applicable, ending between the date hereof and the Closing Date if any previously delivered financial statements are not required to be filed on a Form 8-K pursuant to the prior two sentences (provided that Seller shall provide such cooperation as set forth in clause (b) below). The annual and quarterly Financial Statements shall be prepared from and in accordance with the books of account and financial records of the Business and in accordance with GAAP, consistently applied throughout the periods indicated, and present fairly in all material respects the assets acquired, liabilities assumed, revenues and direct expenses of the Business as of the times and for the periods referred to therein.
(b)Following the Closing, upon the Purchasers’ request, (i) solely to the extent required to be included by Purchaser 1 in a filing with the SEC, Seller will use its commercially reasonable efforts to cause the independent accountants for the Business to provide their written consent for the filing of the Audited Financial Statements with the SEC (provided that such Purchaser shall be required to provide the independent accountants for the Business with customary representations and letters in connection therewith as requested by such independent accountants), and (ii) if the Closing Date does not coincide with the last day of a fiscal quarter for which Seller delivered financial statements to the Purchasers pursuant to Section 5.19(a), Seller shall prepare and deliver, as soon as reasonably practicable and within sixty (60) days after the Closing Date (provided that in no event will such information be delivered less than fifteen (15) days before the due date for Purchaser 1’s quarterly report (or annual report in the case of Purchaser 1’s Form 10-K) initially required to be filed by Purchaser 1 following the Closing Date under applicable SEC rules), such financial information (which will not be reviewed or audited by the independent accountants) for the Business for the period subsequent to the end of the fiscal quarter for which financial statements were delivered to the Purchasers pursuant to Section 5.19(a) up to the Closing Date that is required by Purchaser 1 to establish an opening balance sheet for the Business as of the close of business on the Closing Date or that is reasonably requested for purposes of preparing (i) financial statements of the Business for any quarter(s) ending after the most recent quarter for which financial statements were delivered pursuant to Section 5.19(a) through the Closing Date and (ii) pro forma financial statements (which financial information need not be reviewed as set forth above); provided, however, that in no event shall Seller be obligated to deliver any such financial information relating to the Business that would be in excess of the information required to prepare abbreviated financial statements for the portion of such fiscal year in accordance with the requirements set forth in Rule 3-05(e)(2) of Regulation S-X (as if financial statements of the Business for such portion of the fiscal year were required to be filed); provided, further, that in no event shall Seller be obligated to deliver any such financial information relating to the Business for any period after the Closing Date.
-86-
(c)The Purchasers shall reimburse Seller for all documented out-of-pocket costs incurred by Seller or its Affiliates in connection with fulfilling its obligations pursuant to this Section 5.19; provided that such costs shall not exceed (i) $2,000,000 in the aggregate for the Audited Financial Statements and the unaudited financial statements corresponding to the fiscal quarter ended March 31, 2024 and (ii) $350,000 for each additional quarter required to be prepared and provided by Seller pursuant to this Section 5.19.
Section 5.20Exclusivity. During the period from the date of this Agreement until the earlier to occur of (a) the Closing and (b) the termination of this Agreement in accordance with its terms, Seller shall not, and shall not permit any of its Subsidiaries (including the Purchased Entities) or their respective Representatives to, directly or indirectly, (i) solicit, knowingly encourage or initiate any Acquisition Proposal, or (ii) institute, pursue or engage in any discussions or negotiations with, or enter into any Contract with, or provide any information relating to the Business, the Purchased Assets, the Assumed Liabilities, or any of the Purchased Entities, to, any Person of group of Persons (other than the Purchasers and their Representatives) in furtherance of an Acquisition Proposal. For the avoidance of doubt, Seller, its Subsidiaries or their respective Representatives shall not be restricted in any way with respect to or in connection with any disposition, merger, sale, change of control or other transaction (i) involving all or a majority of the assets or equity of Seller and its Subsidiaries (other than with respect to the Business or the Purchased Entities) on a consolidated basis, (ii) involving any business of Seller other than the Business or (iii) not prohibiting the consummation of the Transaction.
Section 5.21Certain Matters. The Parties agree to the matters set forth on Section 5.21 of the Seller Disclosure Schedules.
Section 5.22Resignations. On the Closing Date, Seller shall use commercially reasonable efforts to cause to be delivered to the Purchasers duly signed resignations, effective immediately after the Closing, of all of the directors and officers of the Purchased Entities in office as of the Closing Date identified by the Purchasers in writing at least five (5) Business Days prior to the Closing, or shall take such other action as is necessary to cause such persons to no longer be directors or officers of the Purchased Entities as the case may be, immediately after the Closing.
Section 5.23Restricted Cash. On or prior to the Closing, Seller shall, and shall cause the Chinese Purchased Entity to, use reasonable best efforts to distribute or otherwise transfer all Cash in excess of five million dollars ($5,000,000) (the “Chinese Operating Cash Amount”) from the Chinese Purchased Entity to a member of the Seller Group (the “Pre-Closing Restricted Cash Distribution”).
-87-
To the extent that, notwithstanding the foregoing, the Chinese Purchased Entity holds any Excess Restricted Cash following the Closing, then following the Closing, the Purchasers shall (i) use reasonable best efforts to cause the Chinese Purchased Entity to distribute or otherwise transfer any such portion of the Excess Restricted Cash to its direct owner (or, to the extent the Chinese Purchased Entity and its direct owner are organized in the same jurisdiction, to its first indirect owner organized in another jurisdiction) (any such distribution or other transfer, an “Excess Restricted Cash Distribution” and any Excess Restricted Cash so distributed or otherwise transferred, the “Distributed Excess Restricted Cash”), (ii) transfer to Seller or its designated Subsidiaries such Distributed Excess Restricted Cash, net of any Taxes or other reasonable and documented third-party costs borne by the Purchasers and their Affiliates in connection with such transfer imposed on the Excess Restricted Cash Distribution (each such transfer, a “Distributed Excess Restricted Cash Transfer”), and (iii) reasonably cooperate with the Seller Group to structure and effect any Excess Restricted Cash Distribution and Distributed Excess Restricted Cash Transfer in a manner that seeks to minimize any Taxes imposed on such distribution or transfer, if such structure does not adversely affect Purchasers or any of their Affiliates in a more than de minimis manner. For the avoidance of doubt, any such Excess Restricted Cash Distribution or Distributed Excess Restricted Cash Transfer shall be effected without any corresponding payment, distribution or transfer to the Purchasers or any other party.
Section 5.24Additional Business Products.
(a)Notwithstanding anything in this Agreement to the contrary, for a period of two (2) years following the Closing Date, each Purchaser shall have an option to provide Seller notice of its desire to acquire any Additional Business Products (as well as associated marketing approvals, product development approvals, applications, clearances or other authorizations used to sell, distribute, research or develop or market the Additional Business Product) identified with reasonable specificity by such Purchaser. As soon as reasonably practicable following such notice, the Seller or its Affiliates shall use their commercially reasonable efforts to contribute, grant, assign, transfer, convey or deliver, as applicable, such Additional Business Product to the applicable Purchaser (or, in the sole discretion of the Purchasers, any Affiliate thereof) on the terms set forth in this Agreement for no additional consideration, except the Purchasers shall reimburse Seller for all reasonable third-party costs associated with the transfer of any Additional Business Product. The Purchasers shall, and shall cause their Affiliates to, reasonably cooperate with Seller in connection with the foregoing.
(b)Following the date hereof, unless this Agreement is validly terminated pursuant to Article IX, Seller and its Affiliates shall not at any time (i) use, sell, divest, assign, distribute, manufacture, produce the Additional Business Products or Non-Commercialized Business Products; (ii) withdraw any drug master files or animal drug applications made with any regulatory or government agency related to the Additional Business Products or Non-Commercialized Business Products; and (iii) grant, authorize or permit any other Person the right to use, sell, distribute, manufacture, produce, or have a right to reference the Additional Business Products or Non-Commercialized Business Products, or exploit any Intellectual Property Rights that are exclusively used by or exclusively embedded in the Additional Business Products or Non-Commercialized Business Products in the exercise of the forgoing.
(c)Following the Closing, (i) Purchaser and its Affiliates shall have the right to reference any drug master file or animal drug application made with any regulatory or government agency for any Additional Business Product, and (ii) until the second (2nd) anniversary of the Closing Date Seller shall use commercially reasonable efforts to provide any such documentation needed for a right to reference, on behalf of or directly to Purchaser and its Affiliates.
-88-
(d)The Purchasers shall, and shall cause their Affiliates to, reasonably cooperate with Seller in connection with the foregoing and the Purchasers shall reimburse Seller for any Transfer Taxes, VAT/GST and all reasonable and documented third-party costs incurred by Seller or its Affiliates in connection with fulfilling its obligations pursuant to this Section 5.24.
Section 5.25Additional Business Product Marks. Subject to the terms and conditions of this Agreement, effective only upon the Closing, Seller, on behalf of itself and the other Seller Entities, hereby quitclaim, sell, assign, transfer and convey to the Purchasers all of Seller’s and the other Seller Entities’ right, title and interest as of the Closing in, to and under the Marks listed in Section 5.25 of the Seller Disclosure Schedules (which may be updated prior to the Closing pursuant to Section 5.28) (collectively, “Additional Business Product Marks”), together with all of the goodwill appurtenant thereto and including the right to damages for the past or future infringement, misappropriation or violation of the Additional Business Product Marks, and to recover or collect royalties, damages, proceeds, and profits in connection therewith. If any renewal, maintenance, or other fees relating to the registration or application for any Additional Business Product Marks become due and payable prior to Closing, Seller shall, at the Purchasers’ reasonable request, make any such payment at the Purchasers’ cost (or shall notify, authorize and assist the Purchasers to make any such payment directly with the relevant intellectual property office). Notwithstanding anything in this Agreement to the contrary, the Additional Business Product Marks are transferred to the Purchasers solely on an “as is, where is” basis and no representations or warranties whatsoever, whether express, implied or statutory, are made with respect to any of the Additional Business Product Marks. Without limiting the foregoing, the Additional Business Product Marks shall not be considered within the definition of Transferred Intellectual Property.
Section 5.26Expired Marks. Subject to the terms and conditions of this Agreement, effective only upon the Closing, Seller, on behalf of itself and the other Seller Entities, hereby quitclaim, sell, assign, transfer and convey to the Purchasers all of Seller’s and the other Seller Entities’ right, title and interest (and only to the extent Seller and the other Seller Entities have any right, title and interest) as of the Closing in, to and under the Expired Marks set forth in Section 5.26 of the Seller Disclosure Schedules (which may be updated prior to the Closing pursuant to Section 5.28) (collectively, the “Expired Marks”), together with the goodwill appurtenant thereto and including the right to damages for the past or future infringement, misappropriation or violation of the Expired Marks, and to recover or collect royalties, damages, proceeds, and profits in connection therewith; provided, that Seller makes no, and disclaims any, representation or warranty of any kind with respect to the Expired Marks, including whether such Expired Marks are entitled to protection as Marks, are distinctive, are capable of being registered with the United States Patent and Trademark Office or any other Governmental Entity, are owned by Seller or any of its Affiliates or may be used without infringing the rights of others. Without limiting the foregoing, the Expired Marks shall not be considered within the definition of Transferred Intellectual Property.
-89-
Section 5.27Omitted Marks. If, prior to the Closing, the Purchasers notify Seller of a Mark omitted from Sections 2.4(h)(i), Section 5.25 or Section 5.26 of the Seller Disclosure Schedules, which the Purchasers reasonably believe is (a) owned by the Seller Entities as of the date hereof, and (b) exclusively used, or held exclusively for use, in the operation of the Business, then the Parties shall promptly discuss in good faith whether such omitted Mark should be added to the Additional Business Product Marks or the Expired Marks and, if agreed, Section 5.25 or Section 5.26 of the Seller Disclosure Schedules shall be updated accordingly prior to the Closing. Notwithstanding the requirement of good faith discussions in the foregoing, if such omitted Mark is a foreign counterpart to a Mark listed in Sections 2.4(h)(i), Section 5.25 or Section 5.26 of the Seller Disclosure Schedules as of the date hereof, then such omitted Mark shall be presumed to be entitled to be added, as the case may be, to the Additional Business Product Marks or the Expired Marks.
Section 5.28Intellectual Property Chain of Title Corrections. Prior to the Closing, Seller shall use commercially reasonable efforts to cooperate with the Purchasers’ reasonable request to file any required name change documentation at the United States Patent and Trademark Office and intellectual property offices or agencies in any other jurisdictions as may be necessary and required to correct the named owner in the public record of any Patents set forth on Section 5.28 of the Seller Disclosure Schedules or other Transferred Intellectual Property where the aggregate sales of Business Products branded under such Transferred Intellectual Property in the jurisdiction where such Transferred Intellectual Property is registered meet or exceed $500,000 on an annual basis (each Transferred Intellectual Property meeting this criteria, a “Covered Intellectual Property”) to the extent the title records at the applicable intellectual property offices and agencies do not reflect ownership of such Covered Intellectual Property in the name of a Purchased Entity or a current Seller Entity as of the date hereof. Seller shall provide, upon the Purchasers’ reasonable request, the documentation and filings made in connection with the foregoing prior to the Closing. In the event that any such filing to update the title records at the applicable intellectual property offices to name a Purchased Entity (or a current Seller Entity, as applicable) as the owner of record of a Covered Intellectual Property has not been filed at or prior to the Closing, Seller shall use commercially reasonable efforts to execute and deliver such further instruments and documents (but excluding making any filings, which shall be the responsibility of Purchaser) as Purchaser may reasonably request, at the sole cost and expense of Seller, as necessary for Purchaser to complete such name change or correction following Closing. Notwithstanding anything to the contrary contained herein, if Seller’s commercially reasonable efforts prior to the Closing were unsuccessful in correcting the named owner of any Covered Intellectual Property, such failure shall not be taken into account in determining whether any condition to the Closing set forth in Article VIII shall have been satisfied and as of and following the Closing, Seller shall have no responsibility to take any further action.
Section 5.29Assignment of Business Registered IP. Promptly following the Closing, Seller or the applicable Seller Entity, as assignor, will execute and deliver to the applicable Purchaser, as assignee, a short-form recordable deed of assignment as may be required, and that is sufficient, to evidence the transfer, sale, assignment, and conveyance of the Seller Entities’ right, title and interest in the Business Registered IP pursuant to and in accordance with this Agreement.
-90-
Without limiting the foregoing, Seller shall use reasonable best efforts following the Closing to take such further action (including the execution and delivery of such other instruments of conveyance) as the Purchasers may reasonably request and provide any additional information that may be reasonably requested by Purchasers and required to effectuate the transfer of the Business Registered IP. Purchaser shall be solely responsible for, and shall bear all costs related to, any filings or recordings to effectuate such transfer. For the avoidance of doubt, as of and following the Closing, Seller will have no responsibility to take any action to maintain any of the Business Registered IP, or further prosecute or seek issuance of any applications included in the foregoing, including payment of fees, responses to any office action or other inquiries from agents of Governmental Entities or registrars, or otherwise.
Section 5.30Transition Services. Seller and the Purchasers agree, as soon as reasonably practicable following the date hereof and prior to the Closing, to amend and finalize in good faith the terms of the schedules to the Transition Services Agreement consistent with the general terms set forth on in the schedules attached to the Transition Service Agreement as of the date hereof (including, for the avoidance of doubt, the schedule of services and the costing methodology set forth in the Transition Services Agreement).
ARTICLE VI
EMPLOYEE MATTERS
Section 6.1Transfer of Business Employees.
(a)Transfer of Business Employees Generally. Except as otherwise provided in this Section 6.1, the applicable member of the Seller Group shall transfer the employment, employment Contracts and, subject to applicable Law and any required employee consents, accrued unused vacation of each Business Employee based in a jurisdiction where there is a Purchased Entity to a Purchased Entity no later than immediately prior to the Closing. The applicable member of the Seller Group shall transfer the employment and employment Contracts of each Seller Employee to a member of the Seller Group no later than as of immediately prior to the Closing. To the extent required by Law, Seller Group shall timely pay out any accrued unused vacation for such Seller Employees in connection with such transfer, or shall obtain any required employee consents to roll over such amounts to the Seller Group. For the avoidance of doubt, the Seller Group shall assume and retain all Liabilities with respect to any Seller Employees.
(b)Offers to Certain Business Employees. No later than thirty (30) days prior to the Closing Date, the Purchasers shall, or shall cause one of their Subsidiaries to, offer employment, (i) on terms and conditions consistent with the requirements of this Article VI and applicable Law and (ii) in a position that is substantially comparable to such individual’s position immediately prior to the time of offer including level of responsibility, authority and geographical location (i.e., same city or within thirty (30) miles of the current primary office location), to each Business Employee not employed by a Purchased Entity (the “Offer Employees”) other than any U.S. LTD Employee. Each such offer shall be subject to the prior review and reasonable comment of Seller. Other than the U.S. LTD Employees, the Seller Entities shall terminate the employment of each Offer Employee as of the Closing, provided that the Purchasers shall be responsible for any associated severance or other liability. The Seller Entities shall not discourage the Offer Employees from accepting such Purchaser’s or its Subsidiary’s offers of employment and shall cooperate with respect to the Business Employees’ transition to such Purchaser and its Subsidiaries.
-91-
(c)U.S. LTD Employees. If any U.S. Business Employee who is receiving long-term disability benefits as of the Closing Date (each, a “U.S. LTD Employee”) is, within twelve (12) months following the Closing Date or such longer time required by applicable Law, able to return to work, Purchaser 1 shall offer employment to such employee on terms consistent with those applicable to Business Employees generally under this Article VI. Notwithstanding the foregoing, Purchaser 1 shall return to work any inactive employee of the Business who is subject to a Collective Bargaining Agreement and who is receiving long-term disability benefits as of the Closing Date, but who subsequently becomes able to return to work within the period provided in the Collective Bargaining Agreement that applied to him or her immediately prior to the Closing Date. Unless otherwise specified in this Agreement, for any U.S. LTD Employee, references in this Agreement to the “Closing” and “Closing Date” shall be treated as references to the first day and time at which the applicable U.S. LTD Employee is released to return to work but shall not otherwise extend the Comparability Period under Section 6.2(a).
(d)Definitions. For purposes of this Agreement, (i) any U.S. Business Employee whose employment transfers (including any Offer Employee who commences employment with Purchaser 1 or its Subsidiaries) pursuant to this Section 6.1 shall be referred to as a “U.S. Transferred Employee,” and (ii) any International Business Employee whose employment transfers (including any Offer Employee who commences employment with Purchaser 2 or its Subsidiaries) pursuant to this Section 6.1 shall be referred to as an “International Transferred Employee” (collectively, the “Transferred Employees”).
Section 6.2Compensation and Employee Benefits.
(a)Compensation and Benefits Comparability. For a period of one (1) year following the Closing, or such shorter period a Transferred Employee is employed (the “Comparability Period”), the Purchasers shall, or shall cause their Subsidiaries (including the Purchased Entities) to, provide to each Transferred Employee during his or her employment with such Purchaser and its Subsidiaries (including the Purchased Entities) (i) annual base salary or wage rates (as applicable) that are not less than those in effect for each such Transferred Employee immediately prior to the Closing, (ii) annual or short-term cash incentive compensation (including cash bonus opportunities) opportunities that, in each case, are no less favorable than those in effect for each such Transferred Employee immediately prior to the Closing, (iii) severance benefits (including termination indemnity, redundancy or similar termination payments or benefits) that are no less favorable than the severance benefits (including termination indemnity, redundancy or similar termination payments or benefits) that would have been payable to each such Transferred Employee under the Seller Benefit Plan in which such Transferred Employee participated, or was eligible for, immediately prior to the Closing, and (iv) other employee benefits (excluding nonqualified deferred compensation) that are substantially comparable to those employee benefits provided to similarly situated employees of the Purchasers. In addition, Purchasers shall, or shall cause their Subsidiaries (including the Purchased Entities) to, provide to each Transferred Employee who immediately prior to Closing was otherwise eligible to receive equity compensation from Seller during the Comparability Period with a cash retention award with a value equal to the target value of the portion of such Transferred Employee’s Seller equity incentive compensation opportunity that would have been subject to vesting within 12 months from the grant date of such award, which retention award shall be subject to such terms, including vesting, as reasonably determined by the Purchaser (provided that the vesting period shall not exceed 12 months).
-92-
(b)Severance or Other Termination Liabilities. The Purchasers and their Subsidiaries shall be solely responsible for any severance, termination indemnity, redundancy or similar termination payments or benefits that may become payable to any Business Employee arising out of or in connection with the transactions contemplated by this Agreement, including any amounts paid or payable to any Business Employee who does not become an employee of the Purchasers or their Subsidiaries because such Business Employee rejects or does not accept an offer of employment or transfer of employment or refuses to transfer employment or otherwise challenges such transfer of employment pursuant to Section 6.1, provided that the Purchasers’ and their Subsidiaries’ obligation pursuant to this sentence shall be limited to an aggregate amount specified in Section 6.2(b) of the Seller Disclosure Schedule (the “Severance Cap”); provided, however, that the Severance Cap shall not apply to the extent a Liability arises out of or in connection with the Purchasers’ or their Subsidiaries’ noncompliance with applicable Law or the terms of this Agreement. In addition, to the extent that Seller or its Affiliates (other than the Purchased Entities) become liable for, or are legally required to make, severance, redundancy, termination indemnity or other termination payments, contributions or benefits to any Business Employee as a result of the transactions contemplated by this Agreement, the Purchasers shall, or shall cause their Affiliates to, reimburse Seller, as soon as practicable but in any event within thirty (30) days of receipt from Seller of an invoice, for all payments, costs and expenses for which the Purchasers or their Subsidiaries are liable pursuant to the immediately preceding sentence that are actually paid by Seller or its Affiliates (other than the Purchased Entities), including any employment, social security or other Taxes or any employer contributions, as required by applicable Law, Contract, Collective Bargaining Agreement or Seller Benefit Plan.
(c)Service Credit. For (i) all employment purposes including the calculation of all service-related entitlements under applicable Law (such as the calculation of severance (including termination, indemnity, redundancy or similar termination payments or benefits) pay and benefits eligibility) and (ii) purposes of determining vesting, eligibility to participate and, with respect to paid time off, vacation and severance, the level of benefits under the employee benefit plans of the Purchasers and their Subsidiaries (other than plans providing defined benefit pension or post-employment health or welfare benefits or perquisites) under which any Business Employees may be eligible to participate after the Closing (the “New Plans”), each Business Employee shall be credited with his or her years of service with Seller and its Subsidiaries and their respective predecessors prior to the Closing, to the same extent and for the same purpose as such Business Employee was entitled, prior to the Closing, to credit for such service under any similar Seller Benefit Plan in which such Business Employee participated or was eligible to participate immediately prior to the Closing; provided that the foregoing shall not apply for purposes of benefit accrual under any defined benefit pension plan, retiree medical plan (except with respect to any Purchased Entity Benefit Plan or as otherwise required by applicable Law) or to the extent that its application would result in a duplication of benefits for the same period of service. In addition, and without limiting the generality of the foregoing, the Purchasers shall allow each Business Employee to be immediately eligible to participate, without any waiting time, in any and all New Plans to the extent coverage under such New Plan is comparable to a Seller Benefit Plan in which such Business Employee participated immediately before the Closing (such plans, collectively, the “Old Plans”).
-93-
(d)Welfare Plans. For purposes of each New Plan providing medical, dental, pharmaceutical and/or vision benefits to any Business Employee, the Purchasers shall use commercially reasonable efforts to cause all pre-existing condition exclusions and actively-at-work requirements of such New Plan to be waived for such employee and his or her covered dependents, unless such conditions would not have been waived under the comparable Seller Benefit Plans in which such employee participated immediately prior to the Closing, and the Purchasers shall cause any eligible expenses incurred by such employee and his or her covered dependents during the portion of the plan year of the Old Plans ending on the date such employee’s participation in the corresponding New Plan begins to be taken into account under such New Plan for purposes of satisfying all deductible, coinsurance and maximum out-of-pocket requirements applicable to such employee and his or her covered dependents for the applicable plan year as if such amounts had been paid in accordance with such New Plan.
(e)Flexible Spending Accounts. Seller and the Purchasers shall take all actions necessary or appropriate so that, effective as of the Closing Date, (i) the account balances (whether positive or negative) (the “Transferred FSA Balances”) under the applicable flexible spending plan of Seller or its Subsidiaries (collectively, the “Seller FSA Plan”) of the Transferred Employees who are participants in the Seller FSA Plan shall be transferred to one or more comparable plans of the Purchasers (collectively, the “Purchaser FSA Plan”); (ii) the elections, contribution levels and coverage levels of such Transferred Employees shall apply under the Purchaser FSA Plan in the same manner as under the Seller FSA Plan; and (iii) such Transferred Employees shall be reimbursed from the Purchaser FSA Plan for claims incurred at any time during the plan year of the Seller FSA Plan in which the Closing Date occurs that are submitted to the Purchaser FSA Plan from and after the Closing Date on the same basis and the same terms and conditions as under the Seller FSA Plan. As soon as practicable after the Closing Date, and in any event within ten (10) Business Days after the amount of the Transferred FSA Balances is determined, Seller shall pay the applicable Purchaser the net aggregate amount of the Transferred FSA Balances, if such amount is positive, and the applicable Purchaser shall pay Seller the net aggregate amount of the Transferred FSA Balances, if such amount is negative.
(f)Accrued PTO. Seller shall timely make any payments to any Business Employees in respect of earned but unused paid time off that become due under applicable Law as a result of any transfer or termination of employment contemplated by Section 6.1. Subject to applicable law and any required employee consents, the applicable Purchaser will recognize and/or assume (as applicable and to the extent reflected in Working Capital) all other accrued but unused paid time off for Transferred Employees as of the Closing.
Section 6.3Seller Benefit Plans. From and after the Closing, the Transferred Employees shall cease to be active participants in the Seller Benefit Plans that are not Purchased Entity Benefit Plans (collectively, the “Retained Seller Benefit Plans”). Except as set forth in this Article VI, Seller shall indemnify and hold harmless the Purchasers and their Affiliates and their officers, directors, employees, and agents from and against any and all costs, damages, losses, expenses, or other Liabilities arising out of or related to the Retained Seller Benefit Plans.
-94-
Notwithstanding anything in this Article VI to the contrary, Seller and the Purchasers hereby agree that Seller and its Subsidiaries shall retain any obligation to provide long-term disability benefits to any Business Employee who (i) as of the Closing Date is receiving or entitled to receive short-term disability benefits and who subsequently becomes eligible to receive long-term disability benefits, or (ii) as of the Closing Date is receiving or entitled to receive long-term disability benefits. At least thirty (30) days prior to the Closing Date, Seller shall provide the Purchasers or their Subsidiaries, with any information requested by the Purchasers or their Subsidiaries necessary to effectuate the enrollment of the Business Employees in the New Plans effective as of the Closing Date, to the extent allowed under applicable Law.
Section 6.4Purchased Entity Benefit Plans. The applicable Purchaser and its Subsidiaries shall assume all Liabilities related to all Purchased Entity Benefit Plans. Each such Purchaser shall, severally and jointly, indemnify and hold harmless Seller and its Affiliates and their officers, directors, employees, and agents from and against any and all costs, damages, losses, expenses, or other Liabilities arising out of or related to the Purchased Entity Benefit Plans.
Section 6.5U.S. Defined Contribution Plans.
(a)Effective as of the Closing, the applicable Purchaser shall create or designate defined contribution plans (collectively, the “Purchaser DC Plans”) for the benefit of the U.S. Transferred Employees who participated in one or more of the defined contribution plans maintained by Seller or its Subsidiaries (other than the Purchased Entities) that are intended to be qualified under Section 401(a) of the Code immediately prior to the Closing (collectively, the “Seller DC Plans”). Such U.S. Transferred Employees are referred to hereinafter as the “U.S. DC Employees.” The U.S. DC Employees shall be given credit under the respective Purchaser DC Plan for all service with and compensation from Seller and its Subsidiaries and their respective predecessors as if it were service with and compensation from such Purchaser for purposes of determining eligibility, vesting and the amount of any benefits or benefit accruals under each respective Purchaser DC Plan. Effective as of the Closing Date, Seller shall take all actions necessary to 100% fully vest each U.S. DC Employee in his or her account balances under the Seller DC Plans.
(b)As of the Closing Date, the Seller Group shall retain, and no Purchased Entity shall assume or retain sponsorship of, or any assets or Liabilities with respect to, the Seller DC Plans, other than with respect to the rollover of account balances described in Section 6.5(c).
(c)Each Purchaser DC Plan will provide for the receipt from the U.S. DC Employees of “eligible rollover distributions” (as such term is defined under Section 402 of the Code), including notes corresponding to loans. The Purchasers and Seller will work together in order to facilitate any such distribution or rollover and to effect an eligible rollover distribution for those U.S. DC Employees who elect to roll over their account balances, including notes, directly into a Purchaser DC Plan. At least thirty (30) days prior to the Closing Date, Seller shall provide the Purchasers or their Subsidiaries, with any information regarding any plan loans associated with any U.S. DC Employees under the Seller DC Plans.
-95-
Section 6.6Short-Term Incentive Compensation. The Purchasers shall, or shall cause their Subsidiaries to, pay to each Transferred Employee any cash incentive compensation payable under the incentive compensation plan or arrangement in which such Transferred Employee participates in respect of the calendar year in which the Closing occurs (or any portion thereof) (the “Cash Incentive Compensation”), and Seller and its Subsidiaries shall not have any Liability for the Cash Incentive Compensation. All Cash Incentive Compensation shall be governed by plans, programs or arrangements maintained by the Purchasers and their Subsidiaries (including the Purchased Entities) on terms and conditions no less favorable than those that applied to each such Transferred Employee prior to the Closing Date, including with respect to target incentive opportunities, applicable performance metrics and service requirements; provided that the amount of Cash Incentive Compensation actually paid by the Purchasers and their Subsidiaries (including the Purchased Entities) to Transferred Employees shall be not less than the accrued amount of Cash Incentive Compensation included in Closing Working Capital.
Section 6.7Labor Matters.
(a)Seller shall, and shall cause its Subsidiaries to take all steps, on a timely basis, as are required under applicable Law or any Collective Bargaining Agreement to notify, provide information to, consult or bargain with, or negotiate the effect, impact, terms or timing of the transactions contemplated by this Agreement with each works council, union, labor organization, labor board, employee group or representative, or Governmental Entity (with respect to labor and similar matters) where so required under applicable Law and the Purchasers shall reasonably cooperate with the foregoing. Seller shall regularly review with the Purchasers the progress of the notifications, consultations and negotiations with each works council, union, labor organization, labor board, employee group or representative and such Governmental Entity regarding the effect, impact or timing of the transactions contemplated by this Agreement. Prior to the Closing, Seller shall, and shall cause its Subsidiaries to, timely comply with all applicable Laws, directives and regulations relating to the Business Employees and any Collective Bargaining Agreements, and the Purchasers shall reasonably cooperate with respect to the foregoing. The Purchasers or their applicable Subsidiaries shall, subject to bargaining with the unions to the extent required by applicable Law, assume the Collective Bargaining Agreements set forth on Section 3.20(b) of the Seller Disclosure Schedules effective as of the Closing, and shall, or shall cause their Subsidiaries to, be responsible for all Liabilities arising thereunder. The Purchasers shall, or shall cause their Subsidiaries to, be responsible for all Liabilities arising under and otherwise honor any Collective Bargaining Agreements which any Purchased Entity is party to, until its expiration, modification or termination in accordance with its terms or applicable Law. The Purchasers shall, or shall cause its Subsidiaries to, join any industrial, employer or similar association or federation if membership is required for the applicable Collective Bargaining Agreement to continue to apply. Seller shall use commercially reasonable efforts to negotiate a one-year extension with each applicable union with respect to each Collective Bargaining Agreement set forth on Section 3.20(b) of the Seller Disclosure Schedules. Notwithstanding anything in this Agreement to the contrary, the terms and conditions of employment for any Transferred Employee covered by a Collective Bargaining Agreement will be governed by the applicable Collective Bargaining Agreement until its expiration, modification or termination in accordance with its terms or applicable Law.
-96-
(b)On the Closing, Seller shall provide the Purchasers with a list setting forth termination date and work location, the job title of each employee or former employee of the Seller Entities who have suffered an “employment loss” under the WARN Act at any site of employment where a Business Employee is located within the ninety (90) days immediately preceding the Closing Date.
Section 6.8Workers’ Compensation. Seller and its Subsidiaries (other than the Purchased Entities) shall be responsible for all workers’ compensation benefits payable to or on behalf of the Transferred Employees based on a Workers’ Compensation Event that occurs prior to the Closing Date. The Purchasers or their Subsidiaries (including, after the Closing Date, the Purchased Entities) shall be responsible for all workers’ compensation benefits payable to or on behalf of the U.S. Business Employees based on a Workers Compensation Event that occurs on or after the Closing Date. Claim for workers’ compensation benefits shall be deemed to be incurred when the event giving rise to the claim occurs (a “Workers’ Compensation Event”). Notwithstanding the foregoing or anything to the contrary in this Agreement, with respect to any facts, events, circumstances or occurrences, known or unknown, related to or arising out of the Business Employees or Business Contingent Workers that occurred (in whole or in part) at or prior to the Closing that are covered by or insured under any employment practices liability insurance policies of Seller or any of its Subsidiaries, the Purchasers and their Subsidiaries (including, for the avoidance of doubt, from and after the Closing, the Purchased Entities) may make claims, to the extent such claims relate to or arise out of facts, events, circumstances or occurrences occurring at or prior to the Closing, under any such policies and programs, and Seller shall, and shall cause its Subsidiaries to, (i) take such actions as may be requested by a Purchaser in connection with the tendering of such claims to the applicable insurers under such policy or program, the pursuit of such claims or the collection of any losses, to the extent available under such policy or program, and (ii) provide the Purchasers or an Subsidiary thereof designated in writing by the Purchasers with the proceeds it realizes with respect to such claims.
Section 6.9Immigration Compliance. The applicable Purchaser and Seller shall, or shall cause their respective Subsidiaries to, cooperate to ensure that any foreign national who requires a visa in order to work for Seller or its Subsidiaries in his or her current position may continue to work in such position as a Transferred Employee on and following the Closing Date. To the extent any such visas cannot be transferred as of Closing, the applicable Purchaser and Seller will enter into an employee leasing arrangement such that such Purchaser may benefit from the services of any such Business Employee until their visa is transferred to a Purchaser or one of its Subsidiaries.
Section 6.10Assignment and Transfer of Business Contingent Worker. Effective as of no later than the Closing Date, the Parties shall use commercially reasonable efforts to take such actions as are necessary to ensure that the Contract of services of each Business Contingent Worker located in a jurisdiction where there is a Purchased Entity is transferred to a Purchased Entity no later than immediately prior to the Closing Date. Each of the Parties agrees to execute, and to seek to have the applicable Business Contingent Worker execute, such documentation, if any, as may be necessary to reflect such assignment and/or transfer. Each Contract of services of each Business Contingent Worker located in a jurisdiction where there is not a Purchased Entity (collectively, the “Business Contingent Worker Contracts”) shall constitute a Specified Business Contract and be transferred in accordance with Section 2.4.
-97-
Section 6.11Communications. Prior to the Closing, any employee notices or communication materials (including website postings) from a Purchaser or its Subsidiaries to the Business Employees, including notices or communication materials with respect to employment, compensation or benefits matters addressed in this Agreement or related, directly or indirectly, to the transactions contemplated by this Agreement or employment thereafter, shall be subject to the prior review of Seller.
Section 6.12Employee Restrictive Covenants. Seller agrees that, notwithstanding the terms of any noncompetition, customer non-solicit or other restrictive covenant obligation between Seller or its Subsidiaries and a Transferred Employee, such Transferred Employee shall be permitted to provide services to the Purchasers and their Subsidiaries following the Closing, and Seller and its Subsidiaries will not seek to enforce the terms of any such restrictive covenant following the Closing with respect to such Transferred Employee’s services to the Purchasers and their Subsidiaries, in each case solely with respect to the Business.
Section 6.13Third-Party Beneficiary Rights. This Article VI is included for the sole benefit of the parties to this Agreement and their respective transferees and permitted assigns and does not and shall not create any right in any Person, including any current or former employee of Seller or any of its Subsidiaries, any Business Employee, any Former Employee or any Transferred Employee, who is not a party to this Agreement. Nothing contained in this Agreement (express or implied) is intended to confer upon any individual any right to employment for any period of time, or any right to a particular term or condition of employment. No current or former employee of Seller or any of its Subsidiaries, nor any Business Employee, Former Employee or Transferred Employee, including any beneficiary or dependent thereof, or any other Person not a party to this Agreement, shall be entitled to assert any claim against any Purchaser, Seller or any of their respective Subsidiaries under this Article VI.
ARTICLE VII
CERTAIN TAX MATTERS
Section 7.1Cooperation and Exchange of Information.
(a)From and after the Closing, each Party shall, and shall cause its Subsidiaries to, provide to the other Party such cooperation, documentation and information relating to the Purchased Entities or the Purchased Assets as either of them may reasonably request in (i) filing any Tax Return, amended Tax Return or claim for refund, (ii) determining a liability for Taxes or a right to refund of Taxes, or (iii) conducting any Tax Proceeding. Such cooperation and information shall include providing copies of all relevant portions of relevant Tax Returns, together with all relevant portions of relevant accompanying schedules and relevant work papers, relevant documents relating to rulings or other determinations by Taxing Authorities and relevant records concerning the ownership and Tax basis of property and other information, which any such Party may possess. Notwithstanding anything to the contrary in this Agreement, such cooperation shall not include disclosure of, and neither the Purchasers nor any of their Subsidiaries shall be entitled to, any Tax Return (or copy thereof) of Seller, any Seller Entity or any of their respective Subsidiaries (other than a Purchased Entity) or the Seller Group. Each Party shall make its employees reasonably available on a mutually convenient basis at its cost to provide an explanation of any documents or information so provided.
-98-
(b)Each Party shall retain all Tax Returns, schedules and work papers, and all material records and other documents relating to Tax matters, of the relevant entities for their respective Pre-Closing Tax Periods until the later of (i) the expiration of the statute of limitations for the Tax periods to which the Tax Returns and other documents relate, or (ii) eight (8) years following the due date (without extension) for such Tax Returns. Thereafter, the Party holding such Tax Returns or other documents may dispose of them after offering the other Party reasonable notice and opportunity to take possession of such Tax Returns and other documents at such other Party’s own expense.
Section 7.2Tax Sharing Agreements. Seller shall terminate or cause to be terminated, on or before the Closing Date, the rights and obligations of the Purchased Entities pursuant to all Tax sharing agreements or arrangements (other than this Agreement), if any, to which any of the Purchased Entities, on the one hand, and Seller or any of its Subsidiaries (other than the Purchased Entities), on the other hand, are parties such that neither Seller nor any of its Subsidiaries (other than the Purchased Entities), on the one hand, nor any of the Purchased Entities, on the other hand, shall have any rights or obligations to each other after the Closing in respect of such agreements or arrangements.
Section 7.3Tax Treatment of Payments. Except to the extent otherwise required pursuant to a “determination” (within the meaning of Section 1313(a) of the Code or any similar provision of state, local or non-U.S. Law), Seller, the Purchasers, the Purchased Entities and their respective Subsidiaries shall treat any and all payments under Section 2.9 and Article X (and any other indemnity payments under this Agreement) as an adjustment to the purchase price for Tax purposes.
Section 7.4Elections. Notwithstanding anything herein to the contrary, the Purchasers shall not and shall cause their Subsidiaries (including, after the Closing, the Purchased Entities) not to (a) make any election with respect to any Purchased Entity (including any entity classification election pursuant to Treasury Regulations Section 301.7701-3), or change any method of Tax accounting or any Tax accounting period of any Purchased Entity, which election or change would be effective on or prior to the Closing Date or (b) take any other action or engage in any transaction that would reasonably be expected to increase Taxes included as a liability in the Separate Company Accrued Tax Amount or the WC Adjustment Amount.
Section 7.5Transfer Taxes and VAT/GST. Notwithstanding anything in this Agreement to the contrary, (a) each of Seller, on the one hand, and the Purchasers, on the other hand, shall be responsible for fifty percent (50%) of any Transfer Taxes and (b) Purchasers shall be responsible for any VAT/GST. The Party responsible under applicable Law for filing the Tax Returns with respect to any such Transfer Taxes (or paying any such Transfer Taxes) shall prepare and timely file such Tax Returns (and pay such Transfer Taxes) and promptly provide a copy of such Tax Return to the other Party and such other Party shall reimburse the filing Party for its share of any such Transfer Taxes paid by the filing Party that are required to be borne by the other Party pursuant to clause (a) of the preceding sentence. Purchasers shall pay to Seller (or its relevant Affiliates), in addition to the consideration otherwise payable, any VAT/GST upon receipt from Seller of a proper invoice. Seller and the Purchasers shall, and shall cause their respective Subsidiaries to, cooperate (y) to timely prepare and file any Tax Returns or other filings relating to Transfer Taxes or VAT/GST imposed on any transaction or supply contemplated by this Agreement, including any available claim for exemption or exclusion from the application or imposition of any Transfer Taxes or any such VAT/GST, and (z) to structure any transaction or supply contemplated by this Agreement so as to minimize Transfer Taxes and VAT/GST.
-99-
Section 7.6Italian VAT Receivable. Seller shall have the right to control any claim for refund and any Tax Proceeding, in each case, relating to the Italian VAT Receivable, and the Purchasers shall provide Seller with any information and cooperation reasonably required in connection with any such claim for refund or Tax Proceeding, including by causing the Italian Purchased Entity to provide Seller with a power of attorney to represent the Italian Purchased Entity in any such Tax Proceeding. In connection with any such Tax Proceeding, Seller shall timely provide the Purchasers with copies of all written communications and documents received, provide the Purchasers with a reasonable advance opportunity to comment on any written submissions, and allow the Purchasers to participate in any such Tax Proceeding at the Purchasers’ sole cost and expense. Any Taxes incurred by Purchasers or any of their Affiliates (including the Purchased Entities) in connection with obtaining or transferring the benefit of the Italian VAT Receivable to Seller shall be the responsibility of Seller.
Section 7.7Straddle Period. In the case of any Straddle Period of a Purchased Entity, (i) the amount of any Income Taxes which relate to the Pre-Closing Tax Period will be determined based on an interim closing of the books as of the close of business on the Closing Date and (ii) the amount of Taxes other than Income Taxes which relate to the Pre-Closing Tax Period will be deemed to be the amount of such Tax for the Straddle Period multiplied by a fraction, the numerator of which is the number of days in the portion of the Straddle Period ending on the Closing Date and the denominator of which is the total number of days in such Straddle Period.
Section 7.8Certain Tax Refunds. If a Purchaser or a Purchased Entity receives after the Closing Date a refund in cash (or a credit against Income Taxes otherwise payable, in lieu of a refund in cash) from a Taxing Authority that is specifically identified on Section 7.8 of the Seller Disclosure Schedule(which Seller shall be permitted to update prior to the Closing), in each case, such Purchaser shall (or shall cause the applicable Purchased Entity) pay to the applicable Seller Entity no later than thirty (30) days after receipt the amount of such refund that it actually receives in cash (or applies such credit to reduce Income Taxes otherwise payable), in each case, net of any third-party out-of-pocket costs or expenses (including Tax costs) incurred in connection with obtaining, claiming or paying over such amount. If any such refund or credit paid over to a Seller Entity is subsequently disallowed by a Taxing Authority within the two (2) year period ending after the payment of an associated amount to a Seller Entity pursuant to this paragraph, the Seller Entities shall return such amount to the applicable Purchaser or the applicable Purchased Entity within thirty (30) days of notification of such disallowance. Notwithstanding the forgoing, no Seller Entity shall be entitled to any refund to the extent that it (i) is attributable to the carryback of any Tax attribute generated in a taxable period (or portion thereof) beginning after the Closing Date, (ii) arises from an adjustment that results in an increase in Taxes imposed on Purchaser and its Subsidiaries, or a reduction of Tax benefits, in each case, in any Tax period or portion thereof beginning after the Closing or (iii) is received by a Purchaser or a Purchased Entity following the second (2nd) anniversary of the Closing Date.
-100-
For the avoidance of doubt, this Section 7.8 shall not apply to the Italian VAT Receivable or Tax refunds that are Excluded Assets.
ARTICLE VIII
CONDITIONS PRECEDENT
Section 8.1Conditions to Each Party’s Obligations to Close. The respective obligations of Seller and Purchasers to effect the Closing are subject to the satisfaction or (to the extent permitted by Law) waiver by Seller and Purchasers at or prior to the Closing of the following conditions:
(a)Regulatory Approvals. Any waiting period under the HSR Act applicable to the consummation of the Transaction or the other transactions contemplated by this Agreement shall have expired or been terminated, and any other Regulatory Approvals required to be obtained prior to the Closing under the Regulatory Laws of the jurisdictions listed on Section 8.1(a) of the Seller Disclosure Schedules shall have been obtained (or any applicable waiting period thereunder shall have expired or been terminated) (collectively, the “Required Approvals”).
(b)No Injunctions or Restraints. No Law or Judgment issued by any Governmental Entity of competent jurisdiction shall have been promulgated or entered and remain in effect which prevents, prohibits or restrains the consummation of the Closing.
Section 8.2Conditions to Obligations of Purchasers to Close. The obligation of Purchasers to effect the Closing is subject to the satisfaction (or waiver by Purchasers) at or prior to the Closing of the following additional conditions:
(a)Representations and Warranties.
(i)The representations and warranties made by Seller contained in Section 3.2(b) and Section 3.2(c) (Purchased Entities; Capital Structure) shall be true and correct in all respects other than de minimis inaccuracies as of the Closing Date as if made on and as of the Closing Date (or, in the case of representations and warranties that are made as of a specific date, as of such date).
(ii)(A) The Seller Fundamental Representations (other than those contained in Section 3.2(b) and Section 3.2(c) (Purchased Entities; Capital Structure)) that are qualified by “materiality” or “Material Adverse Effect” limitations shall be true and correct in all respects as of the Closing Date as if made on and as of the Closing Date (or, in the case of representations and warranties that are made as of a specific date, as of such date) and (B) the Seller Fundamental Representations that are not qualified by any “materiality” or “Material Adverse Effect” limitations shall be true and correct in all material respects as of the Closing Date as if made on and as of the Closing Date (or, in the case of representations and warranties that are made as of a specific date, as of such date).
(iii)The representations and warranties of Seller contained in Article III (other than the Seller Fundamental Representations and other than as set forth
-101-
in the following sentence) shall be true and correct (without giving effect to any “materiality” or “Business Material Adverse Effect” limitations set forth therein) as of the Closing Date as if made on and as of the Closing Date (or, in the case of representations and warranties that are made as of a specific date, as of such date), except where the failure of such representations and warranties to be true and correct would not have, individually or in the aggregate, a Business Material Adverse Effect. The representation and warranty of Seller set forth in Section 3.8(b) shall be true and correct in all respects as of the Closing Date as if made on and as of the Closing Date.
(b)Performance of Obligations of Seller. The covenants and agreements of Seller to be performed or complied with on or before the Closing in accordance with this Agreement shall have been performed and complied with in all material respects.
(c)Officer’s Certificate. The Purchasers shall have received a certificate, dated as of the Closing Date and signed on behalf of Seller by an executive officer of Seller, stating that the conditions specified in Section 8.2(a), Section 8.2(b) and Section 8.2(d) have been satisfied.
(d)No Business Material Adverse Effect. Since the date of this Agreement, there shall not have been a Business Material Adverse Effect that is continuing as of the Closing Date.
Section 8.3Conditions to Obligations of Seller to Close. The obligation of Seller to effect the Closing is subject to the satisfaction (or waiver by Seller) at or prior to the Closing of the following additional conditions:
(a)Representations and Warranties. The representations and warranties of the Purchasers contained in Article IV (other than as set forth in the following sentence) shall be true and correct as of the Closing Date as if made on and as of the Closing Date (or, in the case of representations and warranties that are made as of a specific date, as of such date), except where the failure of such representations and warranties to be true and correct would not have, individually or in the aggregate, a Purchaser Material Adverse Effect. The representations and warranties of the Purchasers set forth in Section 4.1, Section 4.2 and Section 4.8 shall be true and correct in all material respects as of the Closing Date as if made on and as of the Closing Date (or, in the case of representations and warranties that are made as of a specific date, as of such date).
(b)Performance of Obligations of Purchasers. The covenants and agreements of the Purchasers to be performed or complied with on or before the Closing in accordance with this Agreement shall have been performed and complied with in all material respects.
(c)Officer’s Certificate. Seller shall have received a certificate, dated as of the Closing Date and signed on behalf of the Purchasers by an executive officer of the Purchasers, stating that the conditions specified in Section 8.3(a) and Section 8.3(b) have been satisfied.
-102-
ARTICLE IX
TERMINATION; EFFECT OF TERMINATION
Section 9.1Termination. Notwithstanding anything to the contrary in this Agreement, this Agreement may be terminated and the Transaction and the other transactions contemplated by this Agreement abandoned at any time prior to the Closing:
(a)by mutual written consent of Seller and the Purchasers;
(b)by Seller, if the Purchasers shall have materially breached any of its representations, warranties, covenants or agreements contained in this Agreement, and such breach would give rise to the failure of a condition set forth in Section 8.3(a) or Section 8.3(b) and has not been cured by the earlier of (i) the date that is thirty (30) days after the date that Seller has notified the Purchasers of such breach stating Seller’s intention to terminate this Agreement pursuant to this Section 9.1(b) and the basis for such termination and (ii) the Outside Date; provided that Seller shall not be permitted to terminate this Agreement pursuant to this Section 9.1(b) if Seller has breached any of its representations, warranties, covenants or agreements contained in this Agreement, in each case, such that any condition set forth in Section 8.2(a) or Section 8.2(b) would not be satisfied;
(c)by the Purchasers, if Seller shall have materially breached any of its representations, warranties, covenants or agreements contained in this Agreement, and such breach would give rise to the failure of a condition set forth in Section 8.2(a) or Section 8.2(b) and has not been cured by the earlier of (i) the date that is thirty (30) days after the date that the Purchasers have notified Seller of such breach stating the Purchasers’ intention to terminate this Agreement pursuant to this Section 9.1(c) and the basis for such termination and (ii) the Outside Date; provided that the Purchasers shall not be permitted to terminate this Agreement pursuant to this Section 9.1(c) if the Purchasers have breached any of its representations, warranties, covenants or agreements contained in this Agreement, in each case such that any condition set forth in Section 8.3(a) or Section 8.3(b) would not be satisfied;
(d)by Seller or by the Purchasers, subject to Section 11.7, if the Closing shall not have occurred on or prior to 11:59 p.m. (New York City time) on January 28, 2025 (the “Outside Date”); provided that if all of the conditions set forth in Article VIII, other than the conditions set forth in Section 8.1(a) or Section 8.1(b) (solely if the Law or Judgment relates to any Regulatory Laws), shall have been satisfied or waived or shall be capable of being satisfied on such date if Closing were to take place on such date, then the Outside Date shall automatically be extended to 11:59 p.m. (New York City time) on April 28, 2025, which date shall thereafter be deemed to be the Outside Date; provided, that, the Purchasers and Seller shall amend the Outside Date to a date no earlier than July 28, 2025 if the Purchasers are able to obtain the extension under the Commitment Letter pursuant to Section 5.7(a)(y); provided, further, that (x) if all of the conditions set forth in Article VIII shall have been satisfied or waived (or in the case of conditions that by their nature are to be satisfied at the Closing, are then capable of being satisfied if the Closing were to take place on such date) on a date that occurs on or prior to the Outside Date but (y) the Closing would thereafter occur in accordance with Section 2.3 on a date (the “Specified Date”) that occurs after such Outside Date, then the Outside Date shall automatically be extended to such Specified Date (which, for the avoidance of doubt, will not be greater than four (4) Business Days after Closing pursuant to Section 2.3) and the Specified Date shall become the Outside Date for purposes of this Agreement; provided, further, that the right to terminate this Agreement pursuant to this Section 9.1(d) shall not be available to any Party whose failure to perform any covenant or agreement under this Agreement has been the primary cause of, or resulted in, the failure of the Closing to occur on or before such date; or
-103-
(e)by Seller or by the Purchasers, if a Judgment issued by a Governmental Entity of competent jurisdiction shall have become final and nonappealable, permanently enjoining or otherwise permanently preventing the consummation of the Transaction; provided that the right to terminate this Agreement pursuant to this Section 9.1(e) shall not be available to any Party whose failure to perform any covenant or agreement under this Agreement has been the primary cause of, or resulted in, the issuance of such Judgment.
Section 9.2Effect of Termination. If this Agreement is terminated as described in Section 9.1, this Agreement shall become null and void and of no further force and effect, without any Liability or obligation on the part of any Party or its Affiliates; provided that the last sentence of Section 5.8(d) and the provisions of Section 5.1(e), Section 5.3(a), Section 5.8(b), Section 5.19(c), this Section 9.2, Section 9.3 and Article XI shall remain in full force and effect (the “Surviving Obligations”); and provided, further, that nothing in this Section 9.2 shall release or relieve any Party from any Liability for any fraud or willful and material breach by such Party occurring prior to the termination of this Agreement. For the purposes of this Agreement, “willful and material breach” means a willful and material breach of this Agreement that is the consequence of an intentional act or intentional failure to act by a Party with the actual knowledge that the taking of such act or failure to take such action would be a material breach of this Agreement. For the purposes of this Agreement, “fraud” means an actual and intentional common law fraud (and not constructive fraud or negligent or reckless misrepresentation or a similar theory) under Delaware law with respect to the representations and warranties set forth in Article III or Article IV of this Agreement and in any certificate delivered in connection with this Agreement.
Section 9.3Notice of Termination. In the event of termination by Seller or the Purchasers pursuant to Section 9.1, written notice of such termination shall be given by the terminating Party to the other Party.
ARTICLE X
INDEMNIFICATION
Section 10.1Survival.
(a)The representations and warranties of Seller contained in this Agreement and in any certificate delivered hereunder (and any rights arising out of any breach of such representations and warranties) shall not survive the Closing and shall terminate at the Closing, and, following the Closing, neither Seller nor any of its Affiliates or any other Person shall have any Liability with respect to any of such representations or warranties.
(b)The representations and warranties of the Purchasers contained in this Agreement and in any certificate delivered hereunder (and any rights arising out of any breach of such representations and warranties) shall not survive the Closing and shall terminate at the Closing, and, following the Closing, neither the Purchasers nor any of their Affiliates or any other Person shall have any Liability with respect to any of such representations or warranties.
-104-
(c)The covenants and agreements contained in this Agreement that require performance prior to the Closing (and any rights arising out of any breach of such covenants and agreements) shall not survive the Closing and shall terminate at the Closing, and, following the Closing, neither Party nor any of its respective Affiliates or any other Person shall have any Liability with respect to any breach of any such covenants and agreements. The covenants and agreements in this Agreement that by their terms apply or are to be performed, in whole or in part, after the Closing (and any rights arising out of any breach of such covenants and agreements), in each case, shall survive the Closing for the period provided in such covenants and agreements, if any, or until fully performed. The obligation of Seller to retain, and indemnify and hold harmless the Purchaser Indemnified Parties for, any Retained Liabilities, and the obligation of the Purchasers to assume, and indemnify and hold harmless the Seller Indemnified Parties for, any Assumed Liabilities, as well as any covenants and agreements of the Parties that by their terms provide for indemnification or reimbursement or allocate fees, payments, costs or expenses as between the Parties, shall survive the Closing indefinitely.
(d)No Person shall be entitled to indemnification, and no Proceeding seeking to recover Covered Losses or other relief shall be commenced or maintained, after the end of the relevant survival period set forth herein, unless a claim for indemnification with respect thereto has previously been made in accordance with this Agreement. Notwithstanding anything to the contrary set forth in this Agreement or in any Ancillary Agreement, nothing in this Agreement or in any Ancillary Agreement shall limit or prevent any claims in respect of fraud.
Section 10.2Indemnification by Seller.
(a)Subject to the provisions of this Article X, effective as of and after the Closing, Seller shall indemnify and hold harmless the Purchasers and their Affiliates and their respective Representatives (collectively, the “Purchaser Indemnified Parties”), from and against any and all Covered Losses incurred or suffered by any of the Purchaser Indemnified Parties to the extent resulting from (i) any breach of any covenant or agreement of Seller contained in this Agreement that survives the Closing, for the period it survives or (ii) any Retained Liabilities.
(b)Seller shall not be required to indemnify or hold harmless any Purchaser Indemnified Party against, or reimburse any Purchaser Indemnified Party for, any Covered Losses to the extent that such Covered Losses are reflected, reserved, accrued, recorded or included in the Closing Working Capital or the Closing Indebtedness as finally determined pursuant to this Agreement.
Section 10.3Indemnification by Purchasers. Subject to the provisions of this Article X, effective as of and after the Closing, the Purchasers shall, severally and jointly, indemnify and hold harmless Seller and its Affiliates and their respective Representatives (collectively, the “Seller Indemnified Parties”), from and against any and all Covered Losses incurred or suffered by any of the Seller Indemnified Parties to the extent resulting from (i) any breach of any covenant or agreement of a Purchaser contained in this Agreement that survives the Closing, for the period it survives or (ii) any Assumed Liabilities.
-105-
Section 10.4Procedures.
(a)Any Person entitled to be indemnified under this Article X (the “Indemnified Party”) shall promptly give written notice to the Party from whom indemnification may be sought (the “Indemnifying Party”) of any pending or threatened Proceeding against the Indemnified Party that has given or would reasonably be expected to give rise to such right of indemnification with respect to such Proceeding (a “Third-Party Claim”), indicating, with reasonable specificity, the nature of such Third-Party Claim, the basis therefor, a copy of any documentation received from the third party, the amount and calculation of the Covered Losses for which the Indemnified Party is entitled to indemnification under this Article X (and a good-faith estimate of any such future Covered Losses relating thereto), and the provision(s) of this Agreement in respect of which such Covered Losses shall have occurred, and the Indemnified Party shall promptly deliver to the Indemnifying Party any information or documentation related to the foregoing reasonably requested by the Indemnifying Party. A failure by the Indemnified Party to give notice and to tender the defense of the Proceeding in a timely manner pursuant to this Section 10.4(a) shall not limit the obligations of the Indemnifying Party under this Article X, except to the extent such Indemnifying Party is materially prejudiced thereby.
(b)With respect to any Third-Party Claim, the Indemnifying Party under this Article X shall have the right, but not the obligation, to assume the control and defense, at its own expense and by counsel of its own choosing, of such Third-Party Claim and any Third-Party Claims related to the same or a substantially similar set of facts; provided that the Indemnifying Party shall not be entitled to assume the control and defense of such Third-Party Claim if such Third-Party Claim seeks (i) an order, injunction or other equitable relief or relief other than monetary damages against the Indemnified Party that the Indemnified Party reasonably determines, after conferring with its outside counsel, cannot be separated from any related claim for monetary damages, or (ii) a finding or admission of violation of Law (including any Third-Party Claim seeking to impose criminal fines, penalties or sanctions) or any Judgment of a Governmental Entity against the Indemnified Party. If the Indemnifying Party so undertakes to control and defend any such Third-Party Claim, it shall notify the Indemnified Party of its intention to do so, and the Indemnified Party shall cooperate fully with the Indemnifying Party and its counsel in the defense against, and settlement of, any such Third-Party Claim; provided, however, that the Indemnifying Party shall not settle any such Third-Party Claim without the written consent of the Indemnified Party (not to be unreasonably withheld, conditioned or delayed) unless such settlement does not involve any injunctive relief against or any finding or admission of any violation of Law or wrongdoing by the Indemnified Party, and any money damages are borne solely by the Indemnifying Party. Subject to the foregoing, the Indemnified Party shall have the right to employ separate legal counsel and to participate in but not control the defense of such Proceeding at its own cost and expense; provided that, subject to the provisions of this Article X, the Indemnifying Party shall bear the reasonable fees of one firm of legal counsel (and one additional firm of legal counsel in each jurisdiction implicated in such Proceeding) representing all Indemnified Parties in such Proceeding and all related Proceedings, if, but only if, the defendants in such Proceeding include both an Indemnified Party and the Indemnifying Party, and such Indemnified Party shall have reasonably concluded, based on the advice of legal counsel, that there is a material conflict of interest between the Indemnifying Party and the Indemnified Party with respect to such Proceeding.
-106-
In any event, the Indemnified Party shall cause its legal counsel to reasonably cooperate with the Indemnifying Party and its legal counsel and shall not assert any position in any Proceeding inconsistent with that asserted by the Indemnifying Party. No Indemnified Party may settle any Third-Party Claim without the written consent of the Indemnifying Party (not to be unreasonably withheld, conditioned or delayed). If the Indemnifying Party does not assume the control and defense of a Third-Party Claim, it shall nevertheless be entitled to participate in the defense of such Proceeding at its own cost and expense, and the Indemnified Party shall cooperate fully with the Indemnifying Party and its counsel in the defense against, and settlement of, any such Third-Party Claim. Anything herein to the contrary notwithstanding, Seller shall have the exclusive right to control any Proceeding relating to Excluded Taxes.
(c)In the event that any Indemnified Party has or may have an indemnification claim against any Indemnifying Party under this Article X that does not involve a Third-Party Claim, the Indemnified Party shall promptly give written notice thereof to the Indemnifying Party indicating, with reasonable specificity, the nature of such claim, the basis therefor, the amount and calculation of the Covered Losses for which the Indemnified Party is entitled to indemnification under this Article X (and a good-faith estimate of any such future Covered Losses relating thereto), and the provision(s) of this Agreement in respect of which such Covered Losses shall have occurred, and the Indemnified Party shall promptly deliver to the Indemnifying Party any information or documentation related to the foregoing reasonably requested by the Indemnifying Party. A failure by the Indemnified Party to give notice in a timely manner pursuant to this Section 10.4(c) shall not limit the obligations of the Indemnifying Party under this Article X, except to the extent such Indemnifying Party is materially prejudiced thereby. If the Indemnifying Party disputes its liability with respect to such claim, the Indemnifying Party and the Indemnified Party shall proceed in good faith to negotiate a resolution of such dispute and, if not resolved through negotiations, such dispute shall be resolved by litigation in the appropriate court of competent jurisdiction set forth in Section 11.8.
Section 10.5Exclusive Remedy and Release. The Purchasers and Seller acknowledge and agree that, except with respect to claims under any Ancillary Agreement (which shall be governed exclusively by such Ancillary Agreement), claims seeking specific performance or other equitable relief with respect to covenants or agreements to be performed after the Closing, and claims for fraud, following the Closing, the indemnification provisions of Section 10.2 and Section 10.3 shall be the sole and exclusive remedies of the Purchasers and Seller, respectively, and any of their respective Affiliates, for any Liabilities (including in respect of any claims for breach of contract (including for breach of any representation, warranty, covenant or agreement), warranty, tortious conduct (including negligence), under Law or otherwise and whether predicated on common law, statute, strict liability, or otherwise) that each Party may at any time suffer or incur, or become subject to, as a result of or in connection with this Agreement, the Transaction or the other transactions contemplated by this Agreement, including any breach of any representation or warranty in this Agreement by any Party, or any breach of or failure by any Party to perform or comply with any covenant or agreement in this Agreement and the other Transaction Documents. Other than claims in respect of fraud, the Purchasers acknowledge and agree that they are relying exclusively on, and their sole recourse for any actual or alleged breach of any representation or warranty will be, the R&W Insurance Policy.
-107-
In furtherance of the foregoing, and subject to the indemnification provisions of this Article X or with respect to fraud, from and after the Closing, the Parties hereby waive, on behalf of themselves and their Affiliates, to the fullest extent permitted by applicable Law, any and all other rights, claims and causes of action (including rights of contribution, if any) known or unknown, foreseen or unforeseen, which exist or may arise in the future, that they may have against Seller or any of its Affiliates, or the Purchasers or any of their Affiliates, as the case may be, as a result of or in connection with this Agreement, the Transaction or the other transactions contemplated by this Agreement, whether arising under or based upon breach of contract (including for breach of any representation, warranty, covenant or agreement), warranty, tortious conduct (including negligence), under Law or otherwise and whether predicated on common law, statute, strict liability, or otherwise. Without limiting the generality of the foregoing, the Parties hereby irrevocably waive any right of rescission they may otherwise have or to which they may become entitled.
Section 10.6Additional Indemnification Provisions. With respect to each indemnification obligation contained in this Agreement, all Covered Losses shall be net of any (a) net cash Tax benefit actually recognized by a Purchased Entity in the country of its organization during the taxable year of the Covered Loss or the immediately succeeding taxable year (a “Tax Benefit”) and (b) third-party insurance or indemnity, contribution or similar proceeds that have been recovered (net of the deductible for such insurance policy, costs of enforcement and any Taxes or other expenses incurred in connection with such recovery) by the Indemnified Party or its Affiliates in connection with such Covered Loss (it being agreed that if third-party insurance or indemnification, contribution or similar proceeds in respect of such Covered Loss are received subsequent to the Indemnifying Party’s making of an indemnification payment in satisfaction of its applicable indemnification obligation, such proceeds shall be promptly remitted to the Indemnifying Party to the extent of the indemnification payment made), and the Indemnified Party shall use, and causes its Affiliates to use, reasonable best efforts to seek full recovery under all insurance and indemnity, contribution or similar provisions covering such Covered Loss to the same extent as it would if such Covered Loss were not subject to indemnification hereunder. To the extent any indemnification payment made to any Indemnified Party does not take into account any Tax Benefit that is subsequently realized by such Indemnified Party, such Indemnified Party shall pay the amount of such Tax Benefit to the Indemnifying Party. Upon making any payment to the Indemnified Party for any indemnification claim pursuant to this Article X, the Indemnifying Party shall be subrogated, to the extent of such payment, to any rights which the Indemnified Party may have against any third parties with respect to the subject matter underlying such indemnification claim, and the Indemnified Party shall assign any such rights to the Indemnifying Party and otherwise cooperate with the Indemnifying Party in seeking recovery thereunder.
Section 10.7Mitigation. Each of the Parties agrees to use, and to cause its Affiliates to use, its commercially reasonable efforts to mitigate its respective Covered Losses upon and after becoming aware of any event or condition that would reasonably be expected to give rise to any Covered Losses that are indemnifiable hereunder (provided, however, that the foregoing shall not require any party to initiate or pursue any Proceeding against third parties, other than taking the relevant administrative steps to file for or make ordinary course claims under insurance policies, in respect of such Covered Losses).
-108-
ARTICLE XI
GENERAL PROVISIONS
Section 11.1Entire Agreement. This Agreement and the other Transaction Documents, and the Schedules and Exhibits hereto and thereto, and the Confidentiality Agreement, along with the Seller Disclosure Schedules and Purchaser Disclosure Schedules, constitute the entire agreement and understanding between the Parties with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings, whether written or oral, relating to such subject matter. In the event of a conflict between the terms of this Agreement and the terms of any Transaction Document, the terms of this Agreement shall control.
Section 11.2Disclosure Schedules. The Seller Disclosure Schedules and the Purchaser Disclosure Schedules, and all schedules attached thereto, and all Exhibits attached to this Agreement shall be construed with and as an integral part of this Agreement to the same extent as if the same had been set forth verbatim herein. Any capitalized terms used in any Exhibit or in the Seller Disclosure Schedules or the Purchaser Disclosure Schedules but not otherwise defined therein shall be defined as set forth in this Agreement. Any information, item or other disclosure set forth in any Section of the Seller Disclosure Schedules or the Purchaser Disclosure Schedules, as the case may be, shall be deemed to be disclosed with respect to any other Section of this Agreement (or to have been set forth in any other Section of the Seller Disclosure Schedules or the Purchaser Disclosure Schedules, as the case may be), if the relevance of such disclosure to such other Section is reasonably apparent on its face, notwithstanding the omission of a reference or a cross-reference with respect thereto and notwithstanding any reference to a Section of the Seller Disclosure Schedules or the Purchaser Disclosure Schedules, as applicable, in such Section of this Agreement.
Section 11.3Assignment. Neither this Agreement nor any of the rights and obligations hereunder may be assigned or transferred by either Party (whether by operation of Law or otherwise) without the prior written consent of the other Party. Any attempted assignment in violation of this Section 11.3 shall be void. Notwithstanding anything to the contrary contained herein, (a) either Party may assign its rights and obligations (in whole or in part) under this Agreement to any of its Subsidiaries (and for the avoidance of doubt, in the case of each Purchaser, to any Subsidiary of Purchaser 1) or, following the Closing, to any third party who purchases directly or indirectly a majority of the equity interests or assets of Purchasers or the Purchased Entities without prior written consent, and (b) each Purchaser may make a collateral assignment of all or any part of its rights and obligations under this Agreement to any of its Subsidiaries, financing sources (or agent therefor) without prior written consent (provided that any assignment of obligations pursuant to this sentence shall only release the assigning party from such obligations to the extent actually performed and shall not impede or delay the consummation of the Transaction or the other transactions contemplated hereby). In the event any such assignment by a Purchaser results in any incremental withholding of Taxes from the consideration payable to Seller, Purchasers shall pay such additional amounts as are necessary to ensure that Seller receives the amounts it would have received absent such incremental withholding. Any attempted assignment in violation of this Section 11.3 will be void. Notwithstanding anything to the contrary contained herein, the Purchasers’ potential financing sources will be express third-party beneficiaries of, and will be entitled to rely on and may enforce, the provisions of this Section 11.3. Subject to the preceding sentences, this Agreement shall be binding upon and shall inure to the benefit of the Parties and their respective successors and assigns.
-109-
Section 11.4Amendments and Waivers. This Agreement may not be amended except by an instrument in writing signed on behalf of each of the Parties. By an instrument in writing, Purchasers, on the one hand, or Seller, on the other hand, may waive compliance by the other with any term or provision of this Agreement that the other Party was or is obligated to comply with or perform. Such waiver or failure to insist on strict compliance with such term or provision shall not operate as a waiver of, or estoppel with respect to, any subsequent or other failure of compliance.
Section 11.5No Third-Party Beneficiaries. Except for Section 5.7, Section 10.2 Section 10.3 and Section 11.15, which are intended to benefit, and to be enforceable by, the Persons specified therein, this Agreement, together with the other Transaction Documents and the Exhibits and Schedules hereto and thereto are not intended to confer in or on behalf of any Person not a party to this Agreement (and their successors and assigns) any rights, benefits, causes of action or remedies with respect to the subject matter or any provision hereof.
Section 11.6Notices. All notices and other communications to be given to any Party hereunder shall be sufficiently given for all purposes hereunder if in writing and delivered by hand, courier or overnight delivery service, or five (5) days after being mailed by certified or registered mail, return receipt requested, with appropriate postage prepaid, or when given in the form of an email transmission (unless the sender receives a non-delivery message), and shall be directed to the address set forth below (or at such other address or email address as such Party shall designate by like notice):
(a)if to Purchasers:
Phibro Animal Health Corporation
300 Frank W. Burr Blvd., Suite 21
Teaneck, NJ 07666
Attention: Daniel Bendheim, Judith Weinstein Zoetis Inc.
Email: Daniel.Bendheim@pahc.com
Judith.Weinstein@pahc.com
with a copy (which shall not constitute notice) to:
Kirkland & Ellis LLP
601 Lexington Avenue
New York, NY 10022
Attention: Daniel Wolf, P.C.
Keri Schick Norton, P.C.
Email: Daniel.Wolf@kirkland.com
Keri.SchickNorton@kirkland.com
-110-
(b)if to Seller:
10 Sylvan Way
Parsippany, New Jersey 07054
Attention: General Counsel
E-mail: LegalNotices@zoetis.com
with a copy (which shall not constitute notice) to:
Wachtell, Lipton, Rosen & Katz
51 West 52nd Street
New York, New York 10019
Attention: Igor Kirman, Esq.
Meng Lu, Esq.
Email: IKirman@wlrk.com
MLu@wlrk.com
Section 11.7Specific Performance. The Parties agree that irreparable damage, for which monetary damages (even if available) would not be an adequate remedy, would occur in the event that the Parties do not perform any provision of this Agreement in accordance with its specified terms or otherwise breach such provisions. Accordingly, the Parties acknowledge and agree that each of the Parties shall be entitled to an injunction, specific performance and other equitable relief to prevent breaches or threatened breaches of this Agreement and to enforce specifically the terms and provisions hereof, in addition to any other remedy to which such Party is entitled in Law or in equity. Each of the Parties agrees that it will not oppose the granting of an injunction, specific performance and other equitable relief on the basis that the other Party has an adequate remedy at Law or that any such award is not an appropriate remedy for any reason at Law or in equity. Any Party seeking an injunction or injunctions to prevent breaches or threatened breaches of this Agreement or to enforce specifically the terms and provisions of this Agreement shall not be required to provide any bond or other security in connection with such remedy. The foregoing is in addition to any other remedy to which any Party is entitled at law, in equity or otherwise. The Parties further agree that nothing set forth in this Section 11.7 shall require any Party hereto to institute any Proceeding for (or limit any Party’s right to institute any Proceeding for) specific performance under this Section 11.7 prior to or as a condition to exercising any termination right under Article IX (and pursuing damages after such termination).
Section 11.8Governing Law and Jurisdiction. This Agreement shall be governed by, and construed and enforced in accordance with, the laws of the State of Delaware, without regard to any choice or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of the laws of any jurisdiction other than the State of Delaware.
-111-
In addition, each of the Parties (a) in the event that any dispute (whether in contract, tort or otherwise) arises out of this Agreement or the Transaction or the other transactions contemplated hereby, submits to the exclusive personal jurisdiction of the Court of Chancery of the State of Delaware and any state appellate court therefrom within the State of Delaware, or in the event (but only in the event) that such court does not have subject matter jurisdiction over the applicable Proceeding, any state or federal court within the State of Delaware; (b) agrees that it will not attempt to deny or defeat such personal jurisdiction by motion or other request for leave from any such court; (c) agrees that it will not bring any Proceeding relating to this Agreement or the Transaction or the other transactions contemplated hereby in any court other than the above-named courts; and (d) agrees that it will not seek to assert by way of motion, as a defense or otherwise, that any such Proceeding (i) is brought in an inconvenient forum, (ii) should be transferred or removed to any court other than the above-named courts, (iii) should be stayed by reason of the pendency of some other proceeding in any court other than the above-named courts or (iv) that this Agreement or the subject matter hereof may not be enforced in or by the above-named courts. Each Party agrees that service of process upon such Party in any such Proceeding shall be effective if notice is given in accordance with Section 11.6.
Section 11.9Waiver of Jury Trial. EACH PARTY TO THIS AGREEMENT WAIVES TRIAL BY JURY IN ANY ACTION, PROCEEDING OR COUNTERCLAIM BROUGHT BY ANY OF THEM AGAINST THE OTHER ARISING OUT OF OR IN ANY WAY CONNECTED WITH THIS AGREEMENT, OR ANY OTHER AGREEMENTS EXECUTED IN CONNECTION HEREWITH OR THE ADMINISTRATION THEREOF OR THE TRANSACTION OR ANY OF THE OTHER TRANSACTIONS CONTEMPLATED HEREIN OR THEREIN. NO PARTY TO THIS AGREEMENT SHALL SEEK A JURY TRIAL IN ANY LAWSUIT, PROCEEDING, COUNTERCLAIM OR ANY OTHER LITIGATION PROCEDURE BASED UPON, OR ARISING OUT OF, THIS AGREEMENT OR ANY RELATED INSTRUMENTS. NO PARTY WILL SEEK TO CONSOLIDATE ANY SUCH ACTION IN WHICH A JURY TRIAL HAS BEEN WAIVED WITH ANY OTHER ACTION IN WHICH A JURY TRIAL CANNOT BE OR HAS NOT BEEN WAIVED. EACH PARTY TO THIS AGREEMENT CERTIFIES THAT IT HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT OR INSTRUMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS SET FORTH ABOVE IN THIS SECTION 11.9. NO PARTY HAS IN ANY WAY AGREED WITH OR REPRESENTED TO ANY OTHER PARTY THAT THE PROVISIONS OF THIS SECTION 11.9 WILL NOT BE FULLY ENFORCED IN ALL INSTANCES.
Section 11.10Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction or other competent authority to be invalid, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions of this Agreement shall remain in full force and effect and shall in no way be affected, impaired or invalidated. Upon such a determination, the Parties shall negotiate in good faith to modify this Agreement so as to effect the original intent of the Parties as closely as possible in a mutually acceptable manner in order that the Transaction and the other transactions contemplated hereby be consummated as originally contemplated to the fullest extent possible.
Section 11.11Counterparts. This Agreement may be executed in two (2) or more counterparts, all of which shall be considered an original, with the same effect as if the signatures thereto and hereto were upon the same instrument, and shall become effective when one (1) or more such counterparts have been signed by each Party and delivered (by facsimile, email, or otherwise) to the other Party. Signatures to this Agreement transmitted by facsimile transmission, by electronic mail in “portable document format” from, or by any other electronic means intended to preserve the original graphic and pictorial appearance of a document, will have the same effect as physical delivery of the paper document bearing the original signatures.
-112-
This Agreement has been executed in the English language. If this Agreement is translated into another language, the English language text shall in any event prevail.
Section 11.12Expenses. Except as otherwise provided in this Agreement, whether or not the Closing takes place, all costs and expenses incurred in connection with this Agreement, the Transaction and the other transactions contemplated hereby shall be paid by the Party incurring such expense.
Section 11.13Interpretation; Absence of Presumption. It is understood and agreed that the specification of any dollar amount in the representations and warranties or covenants and agreements contained in this Agreement or the inclusion of any specific item in the Seller Disclosure Schedules or Purchaser Disclosure Schedules is not intended to imply that such amounts or higher or lower amounts, or the items so included or other items, are or are not material, and no Party shall use the fact of the setting of such amounts or the fact of the inclusion of any such item in the Seller Disclosure Schedules or Purchaser Disclosure Schedules in any dispute or controversy between the Parties as to whether any obligation, item or matter not described in this Agreement or included or not included in the Seller Disclosure Schedules or Purchaser Disclosure Schedules is or is not material for purposes of this Agreement. Nothing herein (including the Seller Disclosure Schedules and the Purchaser Disclosure Schedules) shall be deemed an admission by either Party or any of its Affiliates, in any Proceeding or action, that such Party or any such Affiliate, or any third party, is or is not in breach or violation of, or in default in, the performance or observance of any term or provisions of any Contract or any Law. For the purposes of this Agreement, (a) words in the singular shall be held to include the plural and vice versa, and words of one gender shall be held to include the other gender as the context requires; (b) references to the terms Article, Section, paragraph, Exhibit and Schedule are references to the Articles, Sections, paragraphs, Exhibits and Schedules to this Agreement unless otherwise specified; (c) the terms “hereof,” “herein,” “hereby,” “hereto,” and derivative or similar words refer to this entire Agreement, including the Schedules and Exhibits hereto and the words “date hereof” refer to the date of this Agreement; (d) references to “Dollars” or “$” shall mean U.S.
-113-
dollars; (e) the word “including” and words of similar import when used in this Agreement and the Transaction Documents shall mean “including, without limitation,” unless otherwise specified; (f) the word “or” shall not be exclusive; (g) references to “written” or “in writing” include in electronic form; (h) provisions shall apply, when appropriate, to successive events and transactions; (i) the headings contained in this Agreement and the other Transaction Documents are for reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement and the other Transaction Documents; (j) Seller and Purchasers have each participated in the negotiation and drafting of this Agreement and the other Transaction Documents and if an ambiguity or question of interpretation should arise, this Agreement and the other Transaction Documents shall be construed as if drafted jointly by the Parties or the parties thereto, as applicable, and no presumption or burden of proof shall arise favoring or burdening any party by virtue of the authorship of any of the provisions in this Agreement or the other Transaction Documents; (k) a reference to any Person includes such Person’s successors and permitted assigns; (l) any reference to “days” means calendar days unless Business Days are expressly specified; (m) when calculating the period of time before which, within which or following which any act is to be done or step taken pursuant to this Agreement, the date that is the reference date in calculating such period shall be excluded and if the last day of such period is not a Business Day, the period shall end on the next succeeding Business Day; (n) any Law defined or referred to in this Agreement or in any agreement or instrument that is referred to herein means such Law as from time to time amended, modified or supplemented, including (in the case of statutes) by succession of comparable successor Laws and the related regulations thereunder and published interpretations thereof, and references to any Contract or instrument are to that Contract or instrument as from time to time amended, modified or supplemented; provided that, for purposes of any representations and warranties contained in this Agreement that are made as of a specific date or dates, references to any Law shall be deemed to refer to such Law, as amended, and the related regulations thereunder and published interpretations thereof, in each case, as of such date; (o) to the extent that this Agreement or any other Transaction Document requires a Subsidiary or an Affiliate of any Party to take or omit to take any action, such covenant or agreement includes the obligation of such Party to cause such Subsidiary or Affiliate to take or omit to take such action; (p) the words “made available to Purchasers” or “delivered to Purchasers” or words of similar import refer to materials posted to the virtual data room hosted by Intralinks located at intralinks.com (Project Delta) at least one (1) Business Day prior to the date of this Agreement; (q) the word “extent” in the phrase “to the extent” shall mean only the degree to which a subject or other thing extends, and such phrase shall not mean simply “if”; (r) any reference to “fiscal year” or “year ended December 31” with respect to the Business or the Purchased Entities shall be to the twelve (12) months ended December 31 in the case of U.S. entities and November 30 in the case of non-U.S. entities; (s) any reference to Purchasers shall mean any or all of the Purchasers unless the context otherwise requires; and (t) whenever the words “ordinary course of business” are used in this Agreement, they shall be deemed to be followed by the words “consistent with past practice”.
Section 11.14Waiver of Conflicts Regarding Representation; Nonassertion of Attorney-Client Privilege.
(a)Each Purchaser waives and will not assert, and agrees to cause its Affiliates, including, following the Closing, the Purchased Entities, to waive and not assert, any conflict of interest arising out of or relating to the representation, after the Closing (the “Post-Closing Representation”), of Seller or any of its Affiliates, or any shareholder, officer, employee or director of Seller or any of its Affiliates (any such Person, a “Designated Person”) in any matter involving the negotiation, documentation and consummation of this Agreement, the other Transaction Documents or any other agreements or transactions contemplated hereby or thereby, by any legal counsel currently representing any Designated Person in connection with the negotiation, documentation and consummation of this Agreement, the other Transaction Documents or any other agreements or transactions contemplated hereby or thereby, including Wachtell, Lipton, Rosen & Katz (any such representation, the “Current Representation”).
(b)Each Purchaser waives and will not assert, and agrees to cause its Affiliates, including, following the Closing, the Purchased Entities, to waive and not assert, any attorney-client or other applicable legal privilege or protection with respect to any communication between any legal counsel and any Designated Person occurring during the Current Representation or in connection with any Post-Closing Representation, including in connection with a dispute with such Purchaser or its Affiliates (including, following the Closing, any Purchased Entity), including in respect of any claim for indemnification by a Purchaser Indemnified Party, it being the intention of the Parties that all such rights to such attorney-client and other applicable legal privilege or protection and to control such attorney-client and other applicable legal privilege or protection shall be retained by Seller and its Affiliates and that Seller, and not any Purchaser or its Affiliates or the Purchased Entities, shall have the sole right to decide whether or not to waive any attorney-client or other applicable legal privilege or protection.
-114-
Accordingly, from and after the Closing, none of Purchasers or their Affiliates, including the Purchased Entities, shall have any access to any such communications or to the files of the Current Representation, all of which shall be and remain the property of Seller and not of any Purchaser or its Affiliates (including the Purchased Entities), and none of Purchasers nor their Affiliates, including, following the Closing, the Purchased Entities, or any Person acting or purporting to act on their behalf shall seek to obtain the same by any process on the grounds that the privilege and protection attaching to such communications and files belongs to either Purchaser or their Affiliates, including, following the Closing, the Purchased Entities, or does not belong to Seller. Notwithstanding the foregoing, in the event that a dispute arises between a Purchaser or its Affiliates, including, following the Closing, the Purchased Entities, on the one hand, and a third party other than Seller or its Affiliates, on the other hand, such Purchaser or its Affiliates, including, following the Closing, the Purchased Entities, may assert the attorney-client privilege, the expectation of client confidence and all other rights to any evidentiary privilege to prevent disclosure of confidential communications by the Current Representation.
Section 11.15Financing Provisions. Notwithstanding anything in this Agreement to the contrary, Seller, on behalf of itself, its Subsidiaries and each of its controlled Affiliates, hereby: (a) agrees that any legal action, whether in law or in equity, whether in contract or in tort or otherwise, involving the Financing Parties, arising out of or relating to, this Agreement or the Financing, shall be subject to the exclusive jurisdiction of any federal or state court in the Borough of Manhattan, New York, New York, so long as such forum is and remains available, and any appellate court thereof and each party hereto irrevocably submits itself and its property with respect to any such legal action to the exclusive jurisdiction of such court, (b) agrees that any such legal action shall be governed by the laws of the State of New York (without giving effect to any conflicts of law principles that would result in the application of the laws of another state), except as otherwise provided in any agreement relating to the Financing and except to the extent relating to the interpretation of any provisions in this Agreement (including any provision in the Commitment Letter or in any definitive documentation related to the Financing that expressly specifies that the interpretation of such provisions shall be governed by and construed in accordance with the law of the State of Delaware), (c) knowingly, intentionally and voluntarily waives to the fullest extent permitted by applicable law trial by jury in any such legal action brought against the Financing Parties in any way arising out of or relating to, this Agreement or the Financing, (d) agrees that none of the Financing Parties shall have any liability to Seller or any of its Subsidiaries or any of their respective controlled Affiliates or representatives relating to or arising out of this Agreement or the Financing, and (e) agrees that the Financing Parties are express third party beneficiaries of, and may enforce, any of the provisions of this Section 11.15 and that this Section 11.15 may not be amended in a manner materially adverse to the Financing Parties without the written consent of the Financing Entities (such consent not to be unreasonably withheld, conditioned or delayed). Notwithstanding the foregoing, nothing in this Section 11.15 shall in any way limit or modify the rights and obligations of any Purchaser under this Agreement, or any Financing Party’s obligations to the Purchasers under the Commitment Letter.
[Remainder of page intentionally left blank]
-115-
IN WITNESS WHEREOF, Seller and the Purchasers have duly executed this Agreement as of the date first written above.
|
ZOETIS INC. |
|
|
|
|
|
By: |
/s/ Kristin Peck |
|
Name: |
Kristin C. Peck |
|
Title: |
Chief Executive Officer and Director |
[Signature Page to Purchase and Sale Agreement]
|
PHIBRO ANIMAL HEALTH CORPORATION |
|
|
|
|
|
By: |
/s/ Jack Bendheim |
|
Name: |
Jack Bendheim |
|
Title: |
President & Chief Executive Officer |
|
PHIBRO ANIMAL HEALTH S.A. |
|
|
|
|
|
By: |
/s/ Jack Bendheim |
|
Name: |
Jack Bendheim |
|
Title: |
Director |
[Signature Page to Purchase and Sale Agreement]
Exhibit 10.1
Execution Version
STRICTLY CONFIDENTIAL
|
COÖPERATIEVE RABOBANK U.A., NEW YORK BRANCH 245 Park Avenue New York, NY 10167 |
COMPEER FINANCIAL, PCA 2600 Jenny Wren Trail PO Box 810 Sun Prairie, Wisconsin 53590 |
|
|
|
CITIBANK, N.A. 388 Greenwich Street New York, New York 10013 | |
CONFIDENTIAL
April 28, 2024
Phibro Animal Health Corporation
Glenpointe Centre East, 3rd Floor
300 Frank W. Burr Boulevard, Suite 21
Teaneck, New Jersey 07666-6712
Attention:Glenn David
Chief Financial Officer
Project Delta
Commitment Letter
Ladies and Gentlemen:
Reference is hereby made to the Amended and Restated Credit Agreement, dated as of April 22, 2021 (as amended by Amendment No. 1, dated as of November 8, 2022, Amendment No. 2, dated as of June 16, 2023 and as further amended, restated, amended and restated, supplemented or otherwise modified from time to time, the “Existing Credit Agreement”), among Phibro Animal Health Corporation, a Delaware corporation (the “Borrower” or “you”), Bank of America, N.A. (“Bank of America”), as Administrative Agent, Collateral Agent and L/C Issuer and each lender from time to time party thereto.
You have advised Coöperatieve Rabobank U.A., New York Branch (“Rabobank”), Compeer Financial, PCA (“Compeer”), Citibank, N.A. (“Citibank”, together with Rabobank and Compeer, “we”, “us”, or the “Commitment Parties”), Wells Fargo Bank, National Association (“Wells Fargo”) and Bank of America that you intend to (i) consummate the Transactions described in the Transaction Description attached hereto as Exhibit A (the “Transaction Description”) and (ii) seek certain amendments to the Existing Credit Agreement as set out more particularly on Annex I-A to Exhibit B hereto (the “Proposed Amendments”).
Capitalized terms used but not defined herein shall have the meanings assigned to them in the Existing Credit Agreement, the Transaction Description, the Summary of Principal Terms and Conditions attached hereto as Exhibit B (the “Term Sheet”) and the Summary of Additional Conditions attached hereto as Exhibit C; this commitment letter, the Transaction Description, the Term Sheet and the Summary of Additional Conditions attached hereto as Exhibit C (collectively, this “Commitment Letter”).
1. |
Commitments. |
In connection with the Transactions, each of Rabobank, Compeer and Citibank, severally but not jointly (each in such capacity, an “Incremental Lender” and collectively, the “Incremental Lenders”), is pleased to advise you of its respective commitment to provide the percentage set forth opposite such Incremental Lender’s name on Schedule 1 hereto of the aggregate principal amount of the Incremental Term Facility (as defined in Exhibit B), subject only to the applicable Closing Conditions (as defined below).
Each of the Incremental Lenders, Bank of America and Wells Fargo, on behalf of themselves and their affiliates and their respective affiliates’, managed funds and accounts that are Lenders under the Existing Credit Agreement on the date hereof (or that become Lenders under the Existing Credit Agreement after the date hereof and prior to the date of effectiveness of, and initial funding under, the Incremental Term Facility (the “Incremental Effective Date”)) (in such capacity referred to herein as the “Consenting Lenders”), which collectively constitute the Required Lenders as defined in and under the Existing Credit Agreement as of the date hereof, is pleased to advise you of its commitment to irrevocably consent to the Proposed Amendments.
Each of the Consenting Lenders party hereto hereby agrees that, if at any time prior to the Incremental Effective Date, such Consenting Lender assigns or sells any participation of any portion of their Commitments and/or Loans under the Existing Credit Agreement, such assignment and/or participation shall be subject to the relevant Assignee or Participant, as applicable, agreeing to the Proposed Amendments.
2. |
Titles and Roles. |
It is agreed that: (i) each of Rabobank, Compeer and Citibank will act as a joint lead arranger for the Incremental Term Facility (each in such capacity, a “Lead Arranger” and collectively, the “Lead Arrangers”), (ii) each of Rabobank, Compeer and Citibank will act as a joint bookrunner for the Incremental Term Facility (each in such capacity, a “Bookrunner” and collectively, the “Bookrunners”) and (iii) in connection with the Incremental Term Facility, Bank of America will continue to act as administrative agent and collateral agent under the Existing Credit Agreement and will also act as administrative agent and collateral agent with respect to the Incremental Term Facility.
You agree that no other agents, co-agents, arrangers, bookrunners, managers or co-managers will be appointed, no other titles will be awarded and no compensation (other than compensation expressly contemplated by this Commitment Letter and the Arranger Fee Letter (as defined below) referred to below) will be paid to any Lender (as defined below) by you or any of your affiliates in order to obtain its commitment to participate in the Incremental Term Facility unless you and the Lead Arrangers shall so agree.
3. |
[Reserved]. |
4. |
Information. |
2
You hereby represent and warrant that (in the case of Information and Projections regarding the Target Companies and their subsidiaries and their and their respective businesses provided prior to the date of the consummation of the Acquisition, to your knowledge), (a) all written information and written data (such information and data, other than (i) the financial estimates, forecasts and other projections delivered to the Commitment Parties by you (the “Projections”) and (ii) information of a general economic or industry specific nature, the “Information”) that has been or will be made available to the Commitment Parties directly or indirectly by, or at the request of, you or by any of your representatives, in each case, on your behalf in connection with the transactions contemplated hereby, when taken as a whole, is or will be, when furnished, correct in all material respects and does not or will not, when furnished and when taken as a whole, contain any untrue statement of a material fact or omit to state a material fact necessary in order to make the statements contained therein not materially misleading in light of the circumstances under which such statements are made (after giving effect to all supplements and updates thereto through the date of the consummation of the Acquisition) and (b) the Projections have been or will be prepared in good faith based upon assumptions that are believed by you to be reasonable at the time such Projections are so furnished to the Commitment Parties; it being understood that the Projections are as to future events and are not to be viewed as facts, the Projections are subject to significant uncertainties and contingencies, many of which are beyond your control, that no assurance can be given that any particular Projections will be realized and that actual results during the period or periods covered by any such Projections may differ significantly from the projected results and such differences may be material. You agree that, if at any time prior to the Incremental Effective Date, you become aware that any of the representations and warranties in the preceding sentence would be incorrect in any material respect if the Information and the Projections made available to the Commitment Parties directly or indirectly by, or at the request of, you or any of your representatives, and such representations and warranties were being made, at such time, then you will promptly inform the Commitment Parties thereof and will (or, with respect to the Information and such Projections relating to the Target Companies and their subsidiaries, will use commercially reasonable efforts to) promptly supplement the Information and such Projections such that such representations and warranties are correct in all material respects under those circumstances (or, in the case of the Information and Projections relating to the Target Companies and their subsidiaries and its and their respective businesses, to your knowledge, such representations and warranties are correct in all material respects under those circumstances). The Commitment Parties (i) will be entitled to use and rely on the Information and the Projections without responsibility for independent verification thereof and (ii) assume no responsibility for the accuracy or completeness of the Information or the Projections.
5. |
Fees. |
As consideration for (i) the commitments of the Incremental Lenders hereunder and (ii) the agreements of the Lead Arrangers and the Bookrunners to perform the services described herein, you agree to pay (or cause to be paid) the fees set forth in the (x) Term Sheet and (y) the Fee Letter dated the date hereof and delivered herewith with respect to the Incremental Term Facility (the “Arranger Fee Letter”), if, when and to the extent due and payable. Once paid, such fees shall not be refundable, except as expressly set forth therein or as otherwise separately agreed to in writing by you and the Commitment Parties.
6. |
Conditions. |
The agreements of the Consenting Lenders hereunder with respect to the Proposed Amendments are subject only to entry into definitive documents related thereto consistent with Exhibit B and subject to the 6th paragraph of Section 10 hereof.
The commitments of the Incremental Lenders hereunder to fund the Incremental Term Facility on the Incremental Effective Date and the agreements of the Lead Arrangers and the Bookrunners to perform the services described herein are subject solely to the conditions set forth in Exhibit C hereto (such conditions, the “Closing Conditions”), and upon satisfaction (or waiver by all Incremental Lenders) of such conditions, the initial funding of the Incremental Term Facility shall occur; it being understood and agreed that there are no other conditions (implied or otherwise) to the commitments hereunder, including compliance with the terms of this Commitment Letter, the Arranger Fee Letter and the documentation for the Incremental Term Facility (the “Incremental Term Facility Documentation”).
3
Notwithstanding anything to the contrary in this Commitment Letter (including each of the exhibits attached hereto), the Arranger Fee Letter, the Incremental Term Facility Documentation or any other letter agreement or other undertaking concerning the Transactions or the financing of the Transactions to the contrary, (i) the only representations and warranties the making and accuracy of which shall be a condition to the availability and funding of any of the Incremental Term Facility on the Incremental Effective Date shall be (A) such of the representations and warranties made by, or with respect to, the Target Companies and their subsidiaries in the Purchase Agreement as are material to the interests of the Incremental Lenders, but only to the extent that you (or your affiliate) have the right (taking into account any applicable cure provisions) to terminate your (or its) obligations under the Purchase Agreement or decline to consummate the Acquisition (in each case, in accordance with the terms thereof) as a result of a breach of any such representations and warranties in the Purchase Agreement, (to such extent, the “Specified Purchase Agreement Representations”) and (B) the Specified Representations (as defined below) made in the Incremental Term Facility Documentation and (ii) the terms of the Incremental Term Facility Documentation and the Closing Deliverables (as defined in Exhibit C) shall be in a form such that they do not impair the availability or funding of the Incremental Term Facility on the Incremental Effective Date if the applicable Closing Conditions are satisfied (or waived by all Incremental Lenders) (provided that, any guarantee by the Target Companies or their subsidiaries and any security interest in any Collateral (as defined in the Existing Credit Agreement) relating to the assets of the Target Companies or their subsidiaries (including creation or perfection thereof) shall be delivered and/or perfected after the Incremental Effective Date in accordance with Section 6.11 of the Existing Credit Agreement). For purposes hereof, “Specified Representations” means the representations and warranties of the Loan Parties, after giving effect to the Transactions, to be set forth in the Incremental Term Facility Documentation relating to organizational existence; power and authority, due authorization, execution, delivery, in each case only as they relate to the entering into and performance of the Incremental Term Facility Documentation; the enforceability of the Incremental Term Facility Documentation; solvency as of the Incremental Effective Date (after giving effect to the Transactions) of the Borrower and its subsidiaries on a consolidated basis; Federal Reserve margin regulations; the use of loan proceeds not violating the USA Patriot Act, the OFAC regulations and other applicable sanctions laws; and the United States Foreign Corrupt Practices Act (“FCPA”) and other applicable anti-corruption laws; the Investment Company Act; no conflicts between the Incremental Term Facility Documentation and the organizational documents of the Loan Parties (in each case, as they relate to the entering into and performance of the Incremental Term Facility Documentation); and subject to permitted liens and the limitations set forth in the prior sentence creation, validity, and perfection of security interests in the Collateral provided on the Incremental Effective Date. This paragraph, and the provisions herein, shall be referred to as the “Limited Conditionality Provisions”.
7. |
Indemnity. |
To induce the Commitment Parties and the Consenting Lenders to enter into this Commitment Letter and the Arranger Fee Letter, as applicable, and to proceed with the Incremental Term Facility Documentation, you agree to indemnify and hold harmless the Commitment Parties, the Consenting Lenders and each of their affiliates and their respective officers, directors, employees, agents, advisors, controlling persons and other representatives and successors (each an “Indemnified Party”) from and against (and will reimburse each Indemnified Party within thirty (30) days after demand therefor as the same are incurred) any and all claims, damages, losses, liabilities and reasonable and documented or invoiced out-of-pocket expenses (including, without limitation, the reasonable fees, disbursements and other charges of one counsel for all Indemnified Parties and, if necessary, one firm of local counsel in each appropriate jurisdiction (which may include a single special counsel acting in multiple jurisdictions) for all Indemnified Parties) that may be incurred by or asserted or awarded against any Indemnified Party, in each case arising out of or in connection with or by reason of (including, without limitation, in connection with any investigation, litigation or proceeding or preparation of a defense in connection therewith) any aspect of the Transactions, or any use made or proposed to be made with the proceeds of the Incremental Term Facility, except to the extent such claim, damage, loss, liability or expense (i) is found in a final, nonappealable judgment by a court of competent jurisdiction to have resulted from such Indemnified Party’s (or any of its affiliate's) gross negligence, bad faith or willful misconduct, (ii) is found in a final, nonappealable judgment by a court of competent jurisdiction to have resulted from a material breach of the definitive documents with respect to the Incremental Term Facility by such Indemnified Party or one of its Affiliates or (iii) arises from any dispute to the extent such dispute does not arise from any act or omission of the Borrower or any of its affiliates and that is brought by an Indemnified Party against any other Indemnified Party (other than claims against a Commitment Party or a Consenting Lender in its capacity as an administrative agent, arranger or similar role for the Incremental Term Facility).
4
In the case of an investigation, litigation or proceeding to which the indemnity in this paragraph applies, such indemnity shall be effective whether or not such investigation, litigation or other proceeding is brought by you, your equity holders or creditors or an Indemnified Party, whether or not an Indemnified Party is otherwise a party thereto and whether or not any aspect of the Transactions is consummated. You also agree that no Indemnified Party shall have any liability (whether direct or indirect, in contract or tort or otherwise) to you or your subsidiaries or affiliates or to your or their respective equity holders, directors, partners, or creditors arising out of, related to or in connection with any aspect of the Transactions, except to the extent of your direct, as opposed to special, indirect, consequential or punitive, damages determined in a final nonappealable judgment by a court of competent jurisdiction. Notwithstanding any other provision of this letter agreement, no Indemnified Party shall be liable for any damages arising from the use by others of information or other materials obtained through electronic telecommunications or other information transmission systems.
If the foregoing indemnification is for any reason unavailable or insufficient to hold any Indemnified Party harmless other than by virtue of the exceptions set forth in clauses (i), (ii) and (iii) of the prior paragraph, the Borrower shall contribute to the amount paid or payable by such Indemnified Party as a result of such loss, claim or damage in such proportion as is appropriate to reflect the relative benefits received by the Borrower on the one hand and each Indemnified Party on the other arising out of the matters contemplated by this letter agreement.
By executing this letter agreement, you agree to reimburse the Commitment Parties, the Consenting Lenders and their respective affiliates from time to time on demand for all reasonable and documented out-of-pocket fees and expenses (including but not limited to out-of-pocket expenses of the Commitment Parties’ and the Consenting Lenders’ due diligence investigation, travel expenses, but limited, in the case of legal fees and expenses, to the reasonable fees, disbursements and other charges of Davis Polk & Wardwell LLP, as counsel to the Commitment Parties and the Consenting Lenders and, if necessary, of a single local counsel to the Commitment Parties and the Consenting Lenders in each relevant jurisdiction) incurred in connection with the Transactions, the Incremental Term Facility and the preparation of the Incremental Term Facility Documentation. Upon Borrower’s request, the Commitment Parties and the Consenting Lenders will present the documentation and invoice(s) supporting the costs, expenses and charges in the foregoing sentence.
8. |
Sharing of Information, Absence of Fiduciary Relationships, Affiliate Activities. |
You acknowledge that the Commitment Parties, the Consenting Lenders and their respective affiliates may be providing debt financing, equity capital or other services (including, without limitation, financial advisory services) to other persons in respect of which you, the Target Companies, the Seller and your and their respective subsidiaries and affiliates may have conflicting interests regarding the transactions described herein and otherwise. In addition, you acknowledge that the Commitment Parties and the Consenting Lenders may be arranging or providing (or contemplating arranging or providing) a committed form of acquisition financing to other potential purchasers of the Target Companies and that, in such capacity, such Commitment Parties and Consenting Lenders may acquire information about the Target Companies and their subsidiaries, the sale thereof, and such other potential purchasers and their strategies and proposals, but such Commitment Parties and Consenting Lenders shall have no obligation to disclose to you the substance of such information or the fact that such Commitment Parties or Consenting Lenders are in possession thereof.
5
The Commitment Parties, the Consenting Lenders and their respective affiliates will not use or disclose confidential information obtained from you, the Target, the Investors, the Seller or any of your or their respective subsidiaries or affiliates by virtue of the transactions contemplated by this Commitment Letter or their other relationships with you, the Borrower, the Target or any of your or their respective subsidiaries or affiliates in connection with the performance by them or their affiliates of services for other persons, and the Commitment Parties, the Consenting Lenders and their respective affiliates will not furnish any such information to other persons, except to the extent permitted below. You also acknowledge that the Commitment Parties, the Consenting Lenders and their respective affiliates do not have any obligation to use in connection with the transactions contemplated by this Commitment Letter, or to furnish to you, confidential information obtained by them from other persons.
As you know, the Commitment Parties, the Consenting Lenders and their respective affiliates may be full service securities firms engaged, either directly or through their affiliates, in various activities, which may include securities trading, commodities trading, investment management, financing and brokerage activities and financial planning and benefits counseling for both companies and individuals. In the ordinary course of these activities, the Commitment Parties, the Consenting Lenders and their respective affiliates may actively engage in commodities trading or trade the debt and equity securities (or related derivative securities) and financial instruments (including bank loans and other obligations) of you, the Borrower, the Target Companies and their subsidiaries, the Target Companies’ customers or competitors and other companies which may be the subject of the arrangements contemplated by this Commitment Letter for their own account and for the accounts of their customers and may at any time hold long and short positions in such securities. The Commitment Parties, the Consenting Lenders and their respective affiliates may also co-invest with, make direct investments in, and invest or co-invest client monies in or with funds or other investment vehicles managed by other parties, and such funds or other investment vehicles may trade or make investments in securities of you, the Borrower, the Target Companies, the Target Companies’ subsidiaries or other companies which may be the subject of the arrangements contemplated by this Commitment Letter or engage in commodities or other trading with any thereof.
In connection with all aspects of the Transactions, you acknowledge and agree, and acknowledge your affiliates’ understanding, that: (i) in connection with the process leading to the Transactions, each Lead Arranger is and has been acting solely as a principal and is not the financial advisor, agent or fiduciary, for you or any of your affiliates, stockholders, creditors or employees or any other party; (ii) the Lead Arrangers have not assumed nor will assume an advisory, agency or fiduciary responsibility in your or your affiliates’ favor with respect to the Transactions or the process leading thereto (irrespective of whether such Lead Arranger has advised or is currently advising you or your affiliates on other matters) and the Lead Arrangers have no obligation to you or your affiliates with respect to the Transactions except those obligations expressly set forth in this Commitment Letter; (iii) The Lead Arrangers and their respective affiliates may be engaged in a broad range of transactions that involve interests that differ from yours, the Borrower’s, the Target Companies’ and your affiliates and the Lead Arrangers have no obligation to disclose any of such interests by virtue of any advisory, agency or fiduciary relationship; and (iv) the Lead Arrangers have not provided any legal, accounting, regulatory or tax advice with respect to the Transactions and you have consulted your own legal, accounting, regulatory and tax advisors to the extent you have deemed appropriate. You hereby waive and release, to the fullest extent permitted by law, any claims that you may have against the Lead Arrangers with respect to any breach or alleged breach of agency or fiduciary duty in connection with the arranging and other services described herein.
9. |
Confidentiality. |
6
You agree that you will not disclose, directly or indirectly, the Arranger Fee Letter or the contents thereof or this Commitment Letter, the Term Sheet, the other exhibits and attachments hereto or the contents of each thereof, or the activities of any Commitment Party or Consenting Lender pursuant hereto or thereto, to any person or entity without prior written approval of the Commitment Parties and the Consenting Lenders (such approval not to be unreasonably withheld or delayed), except (a) to your affiliates and your and your affiliates’ officers, directors, employees, agents, attorneys, accountants, advisors, controlling persons and equity holders and to actual and potential investors who are informed of the confidential nature thereof, in each case, on a confidential and need-to-know basis, (b) if the Commitment Parties and the Consenting Lenders consent in writing (such consent not to be unreasonably withheld or delayed) to such proposed disclosure, (c) pursuant to the order of any court or administrative agency or in any pending legal, judicial or administrative proceeding, or otherwise as required by applicable law, rule or regulation or compulsory legal process or to the extent requested or required by governmental and/or regulatory authorities, in each case based on the reasonable advice of your legal counsel (in which case you agree, to the extent practicable and not prohibited by applicable law, rule or regulation, to inform the Commitment Parties and the Consenting Lenders promptly thereof prior to disclosure) or (d) as required, in connection with the enforcement of our rights hereunder or under the Arranger Fee Letter, based on the reasonable advice of counsel; provided that (i) you may disclose this Commitment Letter and the contents hereof and the Arranger Fee Letter redacted in a customary manner to the Seller and its affiliates, the Target Companies and their subsidiaries and its and their respective officers, directors, employees, agents, attorneys, accountants, advisors, controlling persons and equity holders, in each case, on a confidential and need-to-know basis, (ii) you may disclose the Commitment Letter and its contents (including the Term Sheet and other exhibits and attachments hereto) (but not the Arranger Fee Letter or the contents thereof) in connection with any public or regulatory filing requirement relating to the Transactions, (iii) you may disclose the Term Sheet and the other exhibits and attachments to the Commitment Letter, and the contents thereof, to potential Lenders and to rating agencies, (iv) you may disclose the aggregate fee amount contained in the Arranger Fee Letter as part of Projections, pro forma information or a generic disclosure of aggregate sources and uses related to fee amounts related to the Transactions to the extent customary or required in marketing materials for the Incremental Term Facility or in any public or regulatory filing relating to the Transactions (and then only to the extent aggregated with all other fees and expenses of the Transactions and not presented as an individual line item unless required by applicable law, rule or regulation) and (v) if the fee amounts payable pursuant to the Arranger Fee Letter have been redacted (including the portions thereof addressing fees payable to the Commitment Parties and/or the Incremental Lenders) in a customary manner reasonably acceptable to the Lead Arrangers, you may disclose the Arranger Fee Letter and the contents thereof to the Target Companies and their subsidiaries and their and their respective officers, directors, employees, agents, attorneys, accountants, advisors, controlling persons and equity holders, in each case, on a confidential and need-to-know basis.
Each Commitment Party, Consenting Lender and each of their respective affiliates will use all non-public information provided to any of them or such affiliates by or on behalf of you hereunder or in connection with the Acquisition and the related Transactions solely for the purpose of providing the services and/or commitments, as the case may be, that are the subject of this Commitment Letter and negotiating, evaluating and contemplating the transactions contemplated hereby and shall treat confidentially all such information received by it in accordance with Section 10.08 of the Existing Credit Agreement, the provisions of which are hereby incorporated herein, mutatis mutandis; it being understood and agreed that, for purposes hereof, (i) references to “Information” in such section shall be construed to include the Information and Projections and (ii) each Commitment Party, Consenting Lenders and each of their respective affiliates shall be permitted to disclose such non-public information to (x) their respective investment committee members on a confidential basis and (y) to current and potential providers of leverage to or investors in a Commitment Party or a Consenting Lender on a confidential basis. For the avoidance of doubt, the Arranger Fee Letter may not be disclosed to any party to this Commitment Letter that is not a Lead Arranger, Bookrunner or the Borrower.
7
10. |
Miscellaneous. |
This Commitment Letter and the commitments hereunder shall not be assignable by any party hereto without the prior written consent of each other party hereto (such consent not to be unreasonably withheld or delayed) (and any attempted assignment without such consent shall be null and void). This Commitment Letter and the commitments hereunder are intended to be solely for the benefit of the parties hereto (and Indemnified Persons to the extent expressly set forth herein) and do not and are not intended to confer any benefits upon, or create any rights in favor of, any person other than the parties hereto (and Indemnified Persons to the extent expressly set forth herein). Each Commitment Party reserves the right to employ the services of its respective affiliates or branches (other than a Disqualified Lender) in providing services contemplated hereby and to allocate, in whole or in part, to their affiliates or branches certain fees payable to such Commitment Party in such manner as such Commitment Party and its respective affiliates or branches may agree in their sole discretion and, to the extent so employed, such affiliates and branches shall be entitled to the benefits and protections afforded to, and subject to the provisions governing the conduct of, such Commitment Party hereunder. This Commitment Letter may not be amended or any provision hereof waived or modified except by an instrument in writing signed by each of the Commitment Parties, the Consenting Lenders (to the extent that the Proposed Amendments are affected) and you.
This Commitment Letter shall become effective as of the date hereof. This Commitment Letter contains the entire understanding of the parties relating to the matters contemplated hereby, superseding all prior agreements or understandings with respect thereto. This Commitment Letter may be executed in counterparts, each of which shall be an original, but all of which taken together shall constitute the same instrument. Delivery of an executed counterpart of this Commitment Letter by facsimile or other electronic transmission shall constitute an original for purposes hereof. For the avoidance of doubt, the authorization under this paragraph may include, without limitation, use or acceptance by the parties hereto of a manually signed paper document which has been converted into electronic form (such as scanned into PDF format), or an electronically signed document converted into another format, for transmission, delivery and/or retention. For purposes hereof, “Electronic Record” and “Electronic Signature” shall have the meanings assigned to them, respectively, by 15 USC §7006, as it may be amended from time to time.
EACH OF THE PARTIES HERETO IRREVOCABLY WAIVES THE RIGHT TO TRIAL BY JURY IN ANY ACTION, PROCEEDING, CLAIM OR COUNTERCLAIM BROUGHT BY OR ON BEHALF OF ANY PARTY RELATED TO OR ARISING OUT OF THIS COMMITMENT LETTER OR THE ARRANGER FEE LETTER OR THE PERFORMANCE OF SERVICES HEREUNDER OR THEREUNDER.
This Commitment Letter shall be governed by, and construed in accordance with, the laws of the State of New York; provided that, notwithstanding the foregoing, it is understood and agreed that (a) the interpretation of the definition of Business Material Adverse Effect (as defined in the Purchase Agreement) (and whether or not a Business Material Adverse Effect has occurred), (b) the determination of the accuracy of any Specified Purchase Agreement Representations and whether as a result of any inaccuracy thereof you (or your affiliate) have the right (taking into account any applicable cure provisions) to terminate your (or its) obligations under the Purchase Agreement or decline to consummate the Acquisition and (c) the determination of whether the Acquisition has been consummated in accordance with the terms of the Purchase Agreement, in each case shall be governed by, and construed and enforced in accordance with, the laws of the State of Delaware, without regard to any choice or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of the laws of any jurisdiction other than the State of Delaware. Each of the parties hereto hereby irrevocably and unconditionally submits to the exclusive jurisdiction of any New York State court or Federal court of the United States of America sitting in the Borough of Manhattan in New York City in respect of any suit, action or proceeding arising out of or relating to the provisions of this Commitment Letter, the Transactions and the other transactions contemplated hereby and thereby and irrevocably agrees that all claims in respect of any such suit, action or proceeding may be heard and determined in any such court.
8
Each of the parties hereto waives, to the fullest extent permitted by applicable law, any objection that it may now or hereafter have to the laying of the venue of any such suit, action or proceedings brought in any such court, and any claim that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum.
Any Lead Arranger or Bookrunner may, with your approval, place customary advertisements in financial and other newspapers and periodicals or on a home page or similar place for dissemination of customary information on the Internet or worldwide web as it may choose, and circulate similar promotional materials, in each case, after the Incremental Effective Date, in the form of “tombstone” or otherwise describing the name of the Borrower and the amount, type and Incremental Effective Date of the Transactions, all at the expense of such Lead Arranger or Bookrunner.
Each of the parties hereto agrees that (i) this Commitment Letter is a binding and enforceable agreement with respect to the subject matter contained herein, including an agreement of each party to negotiate in good faith the Incremental Term Facility Documentation and documentation related to the Proposed Amendments in a manner consistent with this Commitment Letter, it being acknowledged and agreed that the commitments and agreements provided hereunder are subject only to conditions precedent as expressly provided herein, and (ii) the Arranger Fee Letter is a legally valid and binding agreement of the parties thereto with respect to the subject matter set forth therein.
The Lead Arrangers hereby notify you that pursuant to the requirements of the USA PATRIOT Act, Title III of Pub. L. 107-56 (signed into law October 26, 2001) (the “Act”) and the requirements of 31 C.F.R. § 1010.230 (the “Beneficial Ownership Regulation”), the Lead Arrangers subject to the Act and Beneficial Ownership Regulation are required to (i) obtain, verify and record information that identifies you, which information includes your name and address and other information that will allow the Lead Arrangers, as applicable, to identify you in accordance with the Act and (ii) obtain a certification regarding beneficial ownership from you under the Incremental Term Facility in compliance with the Beneficial Ownership Regulation.
The indemnification, reimbursement, jurisdiction, governing law, venue, waiver of jury trial, and confidentiality provisions contained herein and in the Arranger Fee Letter and the provisions of Section 8 of this Commitment Letter shall remain in full force and effect regardless of whether the Incremental Term Facility Documentation shall be executed and delivered and notwithstanding the termination or expiration of this Commitment Letter or each Incremental Lender’s commitments hereunder; provided that your obligations under this Commitment Letter (and other than your obligations with respect to the confidentiality of the Arranger Fee Letter and the contents thereof) shall automatically terminate and be superseded, in each case to the extent covered thereby, by the provisions of the Incremental Term Facility Documentation upon the initial funding thereunder, and you shall automatically be released from all liability in connection therewith at such time. You may terminate this Commitment Letter and/or the Incremental Lenders’ commitments with respect to all or a portion of any of the Incremental Term Facility hereunder (on a pro rata basis with respect to the commitments of the applicable Incremental Lenders hereunder) at any time subject to the provisions of the preceding sentence. In addition, in the event that a lesser amount of indebtedness is required to fund the Transactions for any reason, you may reduce the Incremental Lenders’ commitments with respect to the Incremental Term Facility (on a pro rata basis amongst the Incremental Lenders) in a manner consistent with the allocation of purchase price reduction described under paragraph 2 of Exhibit C.
Section headings used herein are for convenience of reference only and are not to affect the construction of, or to be taken into consideration in interpreting, this Commitment Letter.
9
If the foregoing correctly sets forth our agreement, please indicate your acceptance of the terms of this Commitment Letter and of the Arranger Fee Letter by returning to the Commitment Parties (or their legal counsel) and the Consenting Lenders (or their legal counsel) on behalf of the Commitment Parties and the Consenting Lenders, as applicable, executed counterparts hereof and of the Arranger Fee Letter not later than 11:59 p.m., New York City time, on April 28, 2024 (such date of your acceptance of this Commitment Letter and the Arranger Fee Letter, the “Signing Date”). The Incremental Lenders’ respective commitments and the obligations of the Commitment Parties and the Consenting Lenders hereunder will expire at such time in the event that Commitment Parties (or their legal counsel) and the Consenting Lenders (or their legal counsel) have not received such executed counterparts in accordance with the immediately preceding sentence. If you do so execute and deliver to the Commitment Parties and the Consenting Lenders, as applicable, this Commitment Letter and the Arranger Fee Letter at or prior to such time, the Commitment Parties and the Consenting Lenders agree to hold our commitments and all agreements and undertakings hereunder until the earliest of (i) after execution of the Purchase Agreement and prior to the time of the consummation of the Acquisition, the valid termination of the Purchase Agreement in accordance with its terms (other than with respect to provisions therein that expressly survive termination) in the event that the Acquisition is not consummated, (ii) the consummation of the Acquisition with or without the initial funding of the Incremental Term Facility and (iii) five business days after the Outside Date (as defined in the Purchase Agreement as in effect on the date hereof) (but in any event no later than 11:59 p.m., New York City time, April 28, 2025), without giving effect to any extension to a date later than April 28, 2025 set forth in the Purchase Agreement (regardless of whether any party thereto pursues a claim of specific performance)) (such earliest time, the “Expiration Date”). Upon the occurrence of any of the events referred to in the preceding sentence, this Commitment Letter and the commitments of the Commitment Parties and the Consenting Lenders hereunder and the agreement of the Commitment Parties and the Consenting Lenders to provide the services described herein shall automatically terminate unless the Commitment Parties and the Consenting Lenders shall, in their sole discretion, agree to an extension in writing.
[Remainder of this page intentionally left blank]
10
The Commitment Parties are pleased to have been given the opportunity to assist you in connection with the financing for the Transactions.
|
COÖPERATIEVE RABOBANK U.A., NEW YORK BRANCH |
|
|
|
|
|
By: |
/s/ Eric Rogowski |
|
Name: |
Eric Rogowski |
|
Title: |
Managing Director |
|
|
|
|
By: |
/s/ Joost Le Blansch |
|
Name: |
Joost Le Blansch |
|
Title: |
Vice President |
[Signature Page to Incremental Commitment Letter]
|
COMPEER FINANCIAL, PCA |
|
|
|
|
|
By: |
/s/ Corey J. Waldinger |
|
Name: |
Corey J. Waldinger |
|
Title: |
Managing Director, Capital Markets |
[Signature Page to Incremental Commitment Letter]
|
CITIBANK, N.A. |
|
|
|
|
|
By: |
/s/ Matthew Marzicola |
|
Name: |
Matthew Marzicola |
|
Title: |
SVP & Authorized Signor |
[Signature Page to Incremental Commitment Letter]
|
WELLS FARGO BANK, NATIONAL ASSOCIATION, as Consenting Lender |
|
|
|
|
|
By: |
/s/ Jonathan Antonio |
|
Name: |
Jonathan Antonio |
|
Title: |
Portfolio Manager |
[Signature Page to Incremental Commitment Letter]
|
BANK OF AMERICA, N.A., as Consenting Lender |
|
|
|
|
|
By: |
/s/ Dilcia P. Hill |
|
Name: |
Dilcia P. Hill |
|
Title: |
Senior Vice President |
[Signature Page to Incremental Commitment Letter]
Accepted and agreed to as of |
|
|||
the date first above written: |
|
|||
|
|
|||
PHIBRO ANIMAL HEALTH CORPORATION |
|
|||
|
|
|||
By: |
/s/ Jack Bendheim |
|
||
|
Name: |
Jack Bendheim |
|
|
|
Title: |
President & Chief Executive Officer |
|
|
[Signature Page to Incremental Commitment Letter]
EXHIBIT A
Incremental Term Facility
Transaction Description
Capitalized terms used but not defined in this Exhibit A shall have the meanings set forth in the other Exhibits to the Commitment Letter to which this Exhibit A is attached (the “Commitment Letter”) or in the Commitment Letter. In the case of any such capitalized term that is subject to multiple and differing definitions, the appropriate meaning thereof in this Exhibit A shall be determined by reference to the context in which it is used.
Phibro Animal Health Corporation, a Delaware corporation (“Purchaser 1”), and Phibro Animal Health S.A., a Belgian company (“Purchaser 2”, and together with Purchaser 1, “Purchasers”), intend to acquire (the “Acquisition”) all of the Purchased Assets of the entities previously identified to the Lead Arrangers as “Delta” (the “Target Companies”), from Zoetis Inc., a Delaware corporation (the “Seller”). Borrower intends to consummate the Acquisition pursuant to that certain Purchase and Sale Agreement, dated as of the Signing Date (together with all exhibits and schedules attached thereto, collectively, as modified, amended, supplemented, consented to or waived, the “Purchase Agreement”) by and between Purchasers and the Seller pursuant to which the Seller will receive cash in exchange for the Acquisition (collectively, the “Purchase Consideration”).
Substantially contemporaneously with the consummation of the Acquisition, the Borrower will, pursuant to Section 2.14 of the Existing Credit Agreement, obtain a senior secured incremental first lien term loan facility described in Exhibit B to the Commitment Letter in an aggregate principal amount of $325 million (the “Incremental Term Facility”).
In connection with the Incremental Term Facility, the Borrower will also obtain the Proposed Amendments to the Existing Credit Agreement.
The proceeds of the Incremental Term Facility and cash on hand at Borrower on the Incremental Effective Date will be applied (i) as described above to pay the Purchase Consideration and (ii) to pay the fees and expenses incurred in connection with the Transactions (such fees and expenses, the “Transaction Costs”) (the amounts set forth in clauses (i) and (ii) above, collectively, the “Acquisition Funds”)
The transactions described above (including the payment of Transaction Costs) are collectively referred to herein as the “Transactions”.
A-1
EXHIBIT B
Amendment No. 3
Incremental Term Facility
Summary of Principal Terms and Conditions1
See attached.
1 |
All capitalized terms used but not defined herein shall have the meaning given them in the Commitment Letter to which this Term Sheet is attached, including Exhibit A and Exhibit C thereto. |
B-1
Project Delta
$325 Million Incremental Term Loan A Facility
Summary of Principal Terms and Conditions
Set forth below is a summary of the principal terms and conditions for the Incremental Term Loans (as defined below). All capitalized terms used but not otherwise defined herein shall have the meaning assigned to such terms in the Existing Credit Agreement (as defined below).
Borrower: |
Phibro Animal Health Corporation (the “Borrower”). |
|
|
Guarantors: |
Same as the Existing Credit Agreement and including, after consummation of the Acquisition (as defined in Exhibit A to the Commitment Letter), the Target Companies that meet the criteria to be guarantors. |
|
|
Transactions: |
As set forth in Exhibit A to the Commitment Letter. |
|
|
Administrative Agent and |
Bank of America, N.A. will act as Administrative Agent and Collateral Agent (in such capacity, the “Administrative Agent”) for the banks, financial institutions and other entities reasonably acceptable to the Borrower (excluding Disqualified Lenders) with respect to the Incremental Term Loans and will perform the duties customarily associated with such roles. |
|
|
Lead Arrangers and Bookrunners: |
Cöooperatieve Rabobank, U.A., New York Branch, Compeer Financial, PCA and Citibank, N.A. will act as joint lead arrangers and joint bookrunners for the Incremental Term Loans (each, a “Lead Arranger” and together, the “Lead Arrangers”), and will perform the duties customarily associated with such roles. |
|
|
Incremental Term Loan Facility: |
A senior secured incremental term loan A facility denominated in dollars (the “Incremental Term Loan Facility”) in an aggregate principal amount of $325 million (the loans thereunder, the “Incremental Term Loans”). |
|
|
Purpose: |
The proceeds of borrowings under the Incremental Term Loan Facility will be used by the Borrower on the date of the initial funding of the Incremental Term Loan Facility (the “Incremental Effective Date”) to finance all or a portion of (a) the Purchase Consideration (as defined in Exhibit A to the Commitment Letter) and (b) the Transaction Costs (as defined in Exhibit A to the Commitment Letter). |
|
|
Availability: |
The Incremental Term Loan Facility will be available in a single drawing on the Incremental Effective Date. Amounts borrowed under the Incremental Term Loan Facility that are repaid or prepaid may not be reborrowed. |
|
|
Incremental Facilities: |
Substantially consistent with the Existing Credit Agreement, subject to the modifications set forth in Annex I-A hereto. |
1
Interest Rates: |
As set forth in Annex I hereto. |
|
|
Default Rate: |
Same as the Existing Credit Agreement. |
|
|
Final Maturity and Amortization: |
The Incremental Term Loan Facility will mature, and lending commitments thereunder will terminate on the same Maturity Date as the Term A Loans under the Existing Credit Agreement. The Incremental Term Loan Facility will amortize in quarterly installments equal to 2.500% of the initial aggregate principal amount of the Incremental Term Loans made on the Incremental Effective Date; provided that the aggregate principal amount of all Incremental Term Loans outstanding on the Maturity Date of the Incremental Term Loan Facility shall be repaid on such date. |
|
|
Guarantees: |
Same as the Existing Credit Agreement and, after consummation of the Acquisition, guarantees from the Target Companies that meet the criteria to be guarantors. |
|
|
Security: |
Same as the Existing Credit Agreement and, after consummation of the Acquisition, assets of the Target Companies that meet the criteria to be collateral. |
|
|
Mandatory Prepayments: |
Same as the Existing Credit Agreement, to be shared on a pro rata basis with the Term A Loans. |
|
|
Voluntary Prepayments and |
Same as the Existing Credit Agreement, to be shared on a pro rata basis with the Term A Loans. |
|
|
Documentation Principles: |
The definitive documentation for the Incremental Term Loan Facility will be in the form of an incremental facility amendment (the “Incremental Facility Documentation”) to that certain Amended and Restated Credit Agreement, dated as of April 22, 2021 (the “Original Closing Date”) among the Borrower (as defined below), the other loan parties from time to time party thereto, the lenders from time to time party thereto and Bank of America, N.A., as the Administrative Agent (as amended by that certain Amendment No. 1, dated November 8, 2022, that certain Amendment No. 2, dated as of June 16, 2023 and as further amended, restated, supplemented and/or otherwise modified from time to time prior to the date hereof, the “Existing Credit Agreement”) and will contain only those terms, conditions to borrowing, mandatory prepayments and other terms and provisions expressly set forth herein (including the modifications to the Existing Credit Agreement set forth in Annex I-A hereto). |
|
|
Representations and Warranties: |
Same as the Existing Credit Agreement, but subject to the Limited Conditionality Provisions (as defined in the Commitment Letter). |
|
|
Conditions to Borrowing: |
The availability of the Borrowing under the Incremental Term Loan Facility on the Incremental Effective Date will be subject |
2
|
solely to the satisfaction or waiver of the conditions set forth in Exhibit C to the Commitment Letter. |
|
|
Affirmative Covenants: |
Same as the Existing Credit Agreement. |
|
|
Negative Covenants: |
Same as the Existing Credit Agreement. |
|
|
Financial Maintenance Covenant: |
The Incremental Facility Documentation will contain the following financial covenants with regard to the Borrower and its restricted subsidiaries on a consolidated basis, which covenants shall be tested on a trailing four quarter basis as of the last day of any fiscal quarter of the Borrower for which financial statements have been delivered, commencing with the first full fiscal quarter following the Incremental Effective Date: |
|
|
|
(i) A maximum First Lien Net Leverage Ratio not to exceed 4.50 to 1.00; provided that, on and after the Incremental Effective Date, no step-ups in the maximum permitted First Lien Net Leverage Ratio shall be permitted in connection with an acquisition. |
|
|
|
(ii) A minimum Consolidated Interest Coverage Ratio of not less than 2.50 to 1.00. |
|
|
Unrestricted Subsidiaries: |
Same as the Existing Credit Agreement. |
|
|
Events of Default: |
Same as the Existing Credit Agreement. |
|
|
Voting: |
Same as the Existing Credit Agreement; provided, that the Incremental Term Loan Facility shall be deemed to be a separate Class from the Term A Loans and the 2023 Incremental Term Loans for purposes of the class voting rights set forth in the Existing Credit Agreement. |
|
|
Cost and Yield Protection: |
Same as the Existing Credit Agreement. |
|
|
Assignments and Participations: |
Same as the Existing Credit Agreement. |
|
|
Expenses and Indemnification: |
Same as the Existing Credit Agreement. |
|
|
Governing Law and Forum: |
New York. |
|
|
Counsel to the Administrative |
Davis Polk & Wardwell LLP. |
3
Annex I
Interest Rates: |
The interest rate under the Incremental Term Loan Facility will be, at the option of the Borrower, Term SOFR (as defined in the Existing Credit Agreement), Base Rate (as defined in the Existing Credit Agreement) or Daily SOFR (as defined in the Existing Credit Agreement), in each case, plus a margin determined in accordance with the table set forth below: |
|
First Lien |
Base Rate |
Term SOFR |
Daily SOFR |
|
≥ 4.00:1.00 |
2.25% |
3.25% |
3.25% |
|
≥ 3.50:1.00 and < 4.00:1.00 |
2.00% |
3.00% |
3.00% |
|
≥ 2.25:1.00 and < 3.50:1.00 |
1.75% |
2.75% |
2.75% |
|
< 2.25:1.00 |
1.50% |
2.50% |
2.50% |
|
Calculation of interest shall be as per the Existing Credit Agreement. |
|
|
|
Interest shall be payable as per the Existing Credit Agreement. |
1
ANNEX I-A TO EXHIBIT B
|
Term |
Agreed Modification to Existing Credit Agreement |
|---|---|---|
1. |
Definition of “Consolidated EBITDA” |
Include add-back for other add-backs and adjustments with respect to the Transactions reflected in the model agreed with the Lead Arrangers on April 16, 2024 (the “Model”). |
2. |
Definition of “Consolidated First Lien Net Debt” |
Replace the reference to “obligations in respect of Capitalized Leases and Indebtedness incurred pursuant to Section 7.03(f)” with “obligations in respect of Capitalized Leases and Indebtedness of the type described in Section 7.03(f)” |
3. |
Definition of “Incremental Facilities Cap”; Section 2.14(a) |
Increase the ratio incurrence tests in the unlimited prong to (i) 3.70x First Lien Net Leverage Ratio or, solely in the case of Incremental Facilities incurred to finance a Permitted Acquisition, (ii) 4.50x |
4. |
Section 2.14 |
The incurrence of the Incremental Term Loan Facility and the funding of the Incremental Term Loans shall be deemed permitted under Section 2.14 |
5. |
Section 4.02 |
The incurrence of the Incremental Term Loan Facility and the funding of the Incremental Term Loans shall not be subject to the requirements set forth in Section 4.02(a) (other than with respect to the Specified Representations (as defined in the Commitment Letter)) and (b) |
6. |
Section 7.02(j) |
The Acquisition shall be deemed to be a “Permitted Acquisition” for all purposes of the Existing Credit Agreement and shall not be subject to the provisions of Section 7.02(j) |
1
EXHIBIT C
Amendment No. 3
Incremental Term Facility
Summary of Additional Conditions2
Subject in all respects to the Limited Conditionality Provisions, the initial borrowings under the Incremental Term Facility shall be subject solely to the satisfaction or waiver by all Commitment Parties of the following conditions:
1.Since the date of the Purchase Agreement, there shall not have been a Business Material Adverse Effect (as defined in the Purchase Agreement (as in effect on the date hereof)) that is continuing as of the Closing Date (as defined in the Purchase Agreement).
2.The Acquisition shall have been consummated, or substantially simultaneously with the initial borrowings under the Incremental Term Facility, shall be consummated, in all material respects in accordance with the terms of the Purchase Agreement, without any amendment, modification or waiver to the Purchase Agreement or any consent thereunder that is materially adverse to the Commitment Parties in their capacities as such without the prior written consent of the Commitment Parties (such consent not to be unreasonably withheld, delayed or conditioned); provided, that, with respect to any amendment, modification or waiver to, or consent under, the Purchase Agreement requiring the consent of the Commitment Parties, such consent shall be deemed given if the Commitment Parties have not responded to such proposed amendment, modification or waiver to, or consent under, the Purchase Agreement within five (5) Business Days (as defined in the Purchase Agreement) of being provided a copy of such amendment, modification, waiver or consent by the Borrower or its counsel (it being understood that any modification, amendment, supplement, consent, waiver or request by you (or your affiliate) to the definition of Business Material Adverse Effect shall be deemed to be materially adverse to the interests of the Commitment Parties); provided, that, without limiting any other rights and/or obligations of this Exhibit C, including rights under paragraph 1 above, any reduction in the Base Purchase Price (as defined in the Purchase Agreement) (other than as set forth in the Purchase Agreement as of the date hereof) shall not, in and of itself, be deemed to be materially adverse to the interests of the Commitment Parties so long as any such reduction above $25,000,000 shall be applied to reduce the amount of commitments in respect of the Incremental Term Facility on a dollar for dollar basis.
3.The Lead Arranger shall have received the Financial Statements (as defined in the Purchase Agreement).
4.The Administrative Agent and the Lead Arrangers shall have received, at least three business days prior to the Incremental Effective Date, all documentation and other information about the Loan Parties and the Target Companies that shall have been reasonably requested by the Administrative Agent and the Lead Arrangers in writing at least 10 business days prior to the Incremental Effective Date and that the Administrative Agent and each Lead Arranger reasonably determines is required by regulatory authorities under applicable “know your customer” and anti-money laundering rules and regulations, including without limitation the PATRIOT Act (including, for the avoidance of doubt, a certification regarding beneficial ownership as required by the Beneficial Ownership Regulation).
2 |
Capitalized terms used in this Exhibit C shall have the meanings set forth in the other Exhibits attached to the Commitment Letter to which this Exhibit C is attached (the “Commitment Letter”). In the case of any such capitalized term that is subject to multiple and differing definitions, the appropriate meaning thereof in this Exhibit C shall be determined by reference to the context in which it is used. |
C-1
5.(x) The execution and delivery by the Borrower of the Incremental Term Facility Documentation, which shall be in accordance with the terms of the Commitment Letter and the Term Sheet (y) delivery to the Administrative Agent of the following (the “Closing Deliverables”): (i) customary legal opinions, customary officer’s closing certificates (which shall not require certification of no default or bringdown of representations and warranties), organizational documents, customary evidence of authorization and good standing certificates in jurisdictions of formation/organization (except, in the case of any Loan Parties other than the Borrower, where the failure of such Loan Parties to be in good standing could not reasonably be expected to have a Material Adverse Effect (as defined in the Existing Credit Agreement)), in each case with respect to the Loan Parties (to the extent applicable) (as such terms are defined in Exhibit B), (ii) a customary borrowing request and (iii) a certificate attesting to the Solvency (as defined in the Existing Credit Agreement) of the Borrower and its Subsidiaries (on a consolidated basis) dated as of the Incremental Effective Date and after giving effect to the Transactions, substantially in the form of Annex I attached to this Exhibit C, of a senior authorized financial executive or officer with equivalent duties of the Borrower (or, at the option of the Borrower, a third party opinion as to the solvency of the Borrower and its subsidiaries on a consolidated basis by a nationally recognized firm).
6.All fees required to be paid on the Incremental Effective Date pursuant to the Arranger Fee Letter and reasonable out-of-pocket expenses required to be paid on the Incremental Effective Date pursuant to the Commitment Letter, and with respect to expenses, to the extent invoiced at least three business days prior to the Incremental Effective Date (except as otherwise reasonably agreed by the Borrower), shall, upon the initial borrowings under the Incremental Term Facility, have been, or will be substantially simultaneously, paid (which amounts may be offset against the proceeds of the Incremental Term Facility).
7.The Specified Purchase Agreement Representations and the Specified Representations shall be true and correct in all material respects on and as of the Incremental Effective Date.
8.The Incremental Effective Date shall not occur prior to July 12, 2024.
C-2
ANNEX 1 TO EXHIBIT C
FORM OF SOLVENCY CERTIFICATE
[____][__], 20[__]
This Solvency Certificate (this “Certificate”) is being delivered pursuant to Section [__] of that certain Amendment No. [●] (the “Amendment”).
I, [●], solely in my capacity as a [●] of the Borrower, do hereby certify on behalf of the Borrower that, as of the date hereof, before and after giving effect to the consummation of the transactions on the Amendment No. [●] Effective Date:
7. |
the fair value of the property (for the avoidance of doubt, calculated to include goodwill and other intangibles) of the Borrower and its Subsidiaries is greater than the total amount of liabilities, including contingent liabilities, of the Borrower and its Subsidiaries; |
8. |
the present fair salable value of the assets of the Borrower and its Subsidiaries is not less than the amount that will be required to pay the probable liability of the Borrower and its Subsidiaries on its debts as they become absolute and matured; |
9. |
the Borrower and its Subsidiaries do not intend to, and do not believe that they will, incur debts or liabilities beyond the Borrower and its Subsidiaries’ ability to pay such debts and liabilities as they mature; and |
10. |
the Borrower and its Subsidiaries are not engaged in business or a transaction, and are not about to engage in business or a transaction, for which the Borrower and its Subsidiaries’ property would constitute an unreasonably small capital. |
For purposes of this Certificate, the amount of contingent liabilities at any time has been computed as the amount that, in the light of all the facts and circumstances existing at such time, represents the amount that can reasonably be expected to become an actual or matured liability.
[Remainder of this page intentionally left blank]
C-1
IN WITNESS WHEREOF, I have executed this Solvency Certificate on the date first written above.
|
[BORROWER] |
|
|
|
|
|
By: |
|
|
Name: |
|
|
Title: |
|
[Signature page to Solvency Certificate]
Exhibit 10.25

October 10, 2023
Glenn David
3 Spring Hill Ln.
Morristown, NJ 07960
Email: gcd1_98@msn.com
Dear Glenn,
I am pleased to present to you an offer of employment with Phibro Animal Health Corporation (the “Company”) as its Chief Financial Officer (CFO), reporting directly to me.
Our offer to you is as follows:
Start Date
Your anticipated start date with the Company will be agreed upon by you and the Company but will be no later than February 12, 2024, (“Start Date”)1 subject to you having satisfactorily cleared the pre-employment background check and substance abuse test referred to below.
Location
You will be based out of our corporate headquarters office located in Teaneck, New Jersey.
Compensation
You will be compensated at the semi-monthly base salary rate of $27,084 (equivalent to $650,000 annually) less applicable deductions as required by law. Your compensation is subject to periodic review per Company policy.
You will be eligible to participate in the Phibro Animal Health Corporation Management Incentive Plan (“MIP”). Your target bonus under the MIP will be 50% of your base salary (the MIP currently provides for a maximum payout of 75% of your base salary). Notwithstanding the foregoing, for fiscal year 2024 (beginning on July 1, 2023): (a) if your Start Date is on or before February 12, 2024, you will be only be eligible for a prorated bonus for the period of time between your start date and January 31, 2024 divided by 365 days or (b) if your Start Date is February 13, 2024 or later, you will not be eligible for any annual bonus for fiscal year 2024. Bonuses under the MIP are subject to corporate performance, contingent on satisfactory individual performance and subject to the approval of the Company’s Compensation Committee and the Company’s Board of Directors. In order to receive any bonus under the MIP, you must be employed by the Company on the date the applicable bonus is paid; provided, however, that if your employment with the Company has been involuntarily terminated without “Cause” or you have resigned for “Good Reason” (each defined below) prior to such payment date, you will remain eligible to receive a pro-rated MIP bonus, payable approximately 3 months following the end of the fiscal year to which the bonus relates.
Restricted Stock Units (RSUs): Subject to approval by the Board of Directors, on or as soon as reasonably practicable following your Start Date, the Company will grant to you an aggregate of 300,000 restricted stock units (“the RSU Grant”) that will vest as follows: (i) 150,000 RSUs will be subject to time vesting (collectively, the
1Employee and Company will determine whether the Employee will start as CFO, or with another title, dependent upon the fiscal reporting cycle for the most recent quarter of FY2024.
HEALTHY ANIMALS. HEALTHY FOOD. HEALTHY WORLD.®
Glenpointe Centre East / 3rd Floor / 300 Frank W. Burr Blvd., Ste. 21 / Teaneck, NJ 07666-6712
Direct: 201-329-7300 / Fax: 201-329-7399
|
October 10, 2023 |
|
Page 2 |
“Time Vesting RSUs”) and (ii) 150,000 RSUs will be subject to performance vesting (collectively, the “Performance Vesting RSUs”); provided, however that you must be employed on the date of such vesting unless your employment has been involuntarily terminated without “Cause” or you have resigned for “Good Reason.” The RSU Grant shall be subject to the terms and conditions of the Phibro Animal Health Corporation 2008 Incentive Plan (the “Plan”) and the Restricted Stock Unit Award Agreement (attached hereto).
Additional Compensation:
You will have the option of either being provided with a Company-leased vehicle for your business and personal use pursuant to the Company’s Fleet Policy or you will be provided with a car allowance of up to $800/month toward a vehicle (including gas reimbursement) to be used for your business and personal use.
You will receive a signing bonus in the gross amount of $135,000, less applicable deductions as required by law, payable on or about September 15, 2024; provided you are still employed by the Company on the applicable payment date. If your employment with the Company has been involuntarily terminated without “Cause” or you have resigned for “Good Reason” (each defined below) prior to payment of the signing bonus, you will receive payment of the signing bonus on or before September 15, 2024.
Separation Pay
In the event the Company terminates your employment without “Cause” or you terminate for “Good Reason,” as these terms are defined below, and the Company wishes to hold you to some or all of the one year non-compete restrictions set forth in Section 1(a) of the your NonCompetition and NonSolicitation Agreement, the Company will continue to pay your then Base Salary and the cost of your medical benefits under COBRA for the period of time the Company wishes you not to compete up to the maximum of one (1) year.
Definitions:
The Term “Cause” shall mean: (A) any willful or repeated failure by you to substantially perform your material duties hereunder, other than a failure resulting from your complete or partial incapacity due to physical or mental illness or impairment; (B) a material and willful violation of a federal or state law or regulation applicable to the business of the Company or that adversely affects the image of the Company; (C) commission of a willful act by you which constitutes gross misconduct and is injurious to the Company; (D) material breach or material violation of any company policy including but not limited to the Company’s Code of Business Conduct and Ethics; or (E) your material violation of any provision of any agreement(s) between the you and the Company and/or its Affiliates relating to the Company’s standard forms of Confidentiality and NonDisclosure, Employee Invention Agreements or NonCompetition and NonSolicitation Agreement.
The term “Good Reason” shall mean: (A) a material adverse change in your duties, responsibilities or authority or compensation (defined as base salary plus target bonus) from those in effect on the date of this Agreement without your written consent; provided that before you assert a claim for Good Reason to resign, you must provide the Company with written notice that identifies the manner in which you believe the Company has created a material adverse change in your duties, responsibilities or authority and a 30-day period for the Company to respond to such claims; or (B) a relocation of your principal place of employment more than 50 miles from Teaneck, New Jersey, without your consent. The acquisition of the assets or capital stock of the Company by another or any other Change in Controls (as defined in the Plan) shall not, by itself, constitute “Good Reason.”
Benefit Plan
HEALTHY ANIMALS. HEALTHY FOOD. HEALTHY WORLD.®
|
October 10, 2023 |
|
Page 3 |
You will be eligible to participate in the Company’s benefit plans, which includes health, dental, life and disability insurance after a 30-day waiting period, and the Company’s 401(k) Retirement and Savings Plan. Participation in these plans is subject to the terms and conditions of the plans, and they are subject to change at any time at the sole discretion of the Company. Please see the Summary of Insurance and Benefits for more details.
Vacation
You are eligible for 20 vacation days per year and will begin to accrue that time on a monthly basis with your Start Date. Your first-year entitlement will be prorated according to your Start Date.
Holidays
Currently the Company provides employees with 8 holidays and 3 floating holidays each year, for which you are eligible as of your start date.
Contingencies:
This offer is contingent upon:
· |
A satisfactory result on a pre-employment background check and substance abuse test. |
· |
Your signed acceptance of, and agreement to be bound by, the Company’s standard forms of Confidentiality and Nondisclosure, Noncompetition and Nonsolicitation, and Employee Invention agreements, and any other agreement between you and the Company or any of its affiliates related to restrictive covenants. |
· |
Your signed agreement to abide by the Company’s Code of Business Conduct and Ethics. |
· |
The approval by the Board of your appointment as the Chief Financial Officer and the terms of your compensation. |
Prior Employment Agreements
You agree that you have fully disclosed to the Company any post-termination obligations you may have with your current and prior employers, that your employment with the Company will not violate any such obligations, and that as a condition of your employment you will strictly comply with any such obligations, including any obligation to maintain the confidentiality of your current and prior employers’ confidential information.
Employment-At-Will
Your employment status with the Company will be that of an at-will employee. Nothing in this offer of employment at-will shall be deemed to create a contract of employment. This offer of employment is not for a fixed duration and may be terminated at any time by either you or the Company with or without cause.
This offer expires October 17, 2023.
Glenn, I am excited about your future at the Company and the potential of your leadership, and I look forward to working with you. If you agree with the above terms, please sign and date and return to me at your earliest convenience a copy of this letter; the Confidentiality and Nondisclosure, Noncompetition and Nonsolicitation, and Employee Invention agreements; and your agreement and written acknowledgement to abide by the Company’s Code of Business Conduct and Ethics.
HEALTHY ANIMALS. HEALTHY FOOD. HEALTHY WORLD.®
|
October 10, 2023 |
|
Page 4 |
If you have any questions regarding your employment with Phibro, please feel free to call me at 201-707-8272.
Sincerely,
/s/ Jack C. Bendheim
Jack C. Bendheim
Chief Executive Officer
Offer Accepted: |
/s/ Glenn David |
|
Glenn David |
|
|
|
10/12/2023 |
|
Date |
HEALTHY ANIMALS. HEALTHY FOOD. HEALTHY WORLD.®

PHIBRO ANIMAL HEALTH CORPORATION
NONCOMPETITION AND NONSOLICITATION AGREEMENT
In consideration of my employment by PHIBRO ANIMAL HEALTH CORPORATION, or any of its subsidiaries or affiliates (collectively “PAHC”), my access to and provision with PAHC’s confidential information and trade secrets under the terms and conditions of my Confidentiality and Nondisclosure Agreement with PAHC, and for other good and sufficient consideration, I hereby acknowledge and agree:
1. |
During my employment with PAHC and for a period of one year after my separation of employment regardless of the reason, I shall not: |
a. |
Directly or indirectly (i) own, (ii) be employed by or (iii) be engaged to perform work in a capacity similar to the position(s) I held with PAHC on behalf of, any firm engaged in any business: (A) similar to, or competitive with, PAHC’s business in those geographic regions or territories in which PAHC marketed its products or had sales during the twelve-month period prior to the separation of my employment at PAHC, or (B) which PAHC has plans to enter during the twelve-month period following the separation of my employment with PAHC of which I was aware during the term of my employment with PAHC. |
b. |
Directly or indirectly, or in any capacity, on my own behalf or on behalf of another, undertake or assist in the servicing or solicitation of any customer or prospective customer for the purpose of selling products or services of the type for which I had (i) responsibility, or (ii) access to confidential information and trade secrets, while employed by PAHC. This restriction shall apply only to those customers or prospective customers of PAHC with whom I came into contact during the 12-month period prior to the date of my separation of employment with PAHC. For the purposes of this section, the term “contact” means interaction between the customer and me which takes place to further the business relationship, or making sales to or performing services for the customer on behalf of PAHC. |
c. |
Directly or indirectly solicit any employee of PAHC to leave the employ of PAHC or to violate the terms of his or her employment arrangement with PAHC. This restriction shall apply only to those employees of PAHC with whom I came into contact during the 24-month period prior to the date of my separation of employment with PAHC. |
The restrictions in (a) above shall not apply (other than for termination of my employment for cause), except to the extent that PAHC makes any severance or other payments to me or on my behalf in connection with the termination of my employment (other
Revised Noncompete tied to severance (G. David)(Oct. 2023) |
|
than base salary and bonus actually earned through the date of termination) equivalent to my base salary for some or all of the period of one year after my termination of employment. The portion of the one-year period in which this restriction shall continue to apply shall be proportionate to the portion of my annual base salary represented by such payments made by PAHC. The Term “Cause” shall mean: (A) any willful or repeated failure by you to substantially perform your material duties, other than a failure resulting from your complete or partial incapacity due to physical or mental illness or impairment; (B) a material and willful violation of a federal or state law or regulation applicable to the business of the Company or that adversely affects the image of the Company; (C) commission of a willful act by you which constitutes gross misconduct and is injurious to the Company; (D) material breach or material violation of any company policy including but not limited to the Company’s Code of Business Conduct and Ethics; or (E) your material violation of any provision of any agreement(s) between the you and the Company and/or its Affiliates relating to the Company’s standard forms of Confidentiality and NonDisclosure, Employee Invention Agreements or this NonCompetition and NonSolicitation Agreement.
2. |
For the purposes of this Agreement, I understand that, as of October, 2023, PAHC is engaged in businesses which include but are not limited to manufacturing and/or marketing of pharmaceutical and nutritional products for animals (including but not limited to medicated and non-medicated feed additives and vaccines), plant nutrition products, manufacturing and/or marketing specialty chemicals including products used in ethanol-production, surface finishing and coating materials, and personal care ingredients and manufacture and/or marketing and sale of microbial products and bioproducts for environmental, industrial and agricultural applications. I further understand that, for the purposes of this Agreement, from time to time the businesses engaged in by PAHC may change from this description. |
3. |
For a period of one year following the separation of my employment from PAHC, regardless of reason, I shall notify any future or prospective employer of mine of the existence of this Agreement, and I further agree that PAHC may inform any future or prospective employer of mine of the existence of this Agreement. |
4. |
The unenforceability of any provision or portion of this Agreement shall not impair or affect the enforceability of any other provision or portion of this Agreement. If any provision or portion of this Agreement is declared illegal or unenforceable by any court of competent jurisdiction, that provision or portion shall be deemed modified so as to render it enforceable. |
5. |
THIS AGREEMENT WILL BE GOVERNED BY THE LAWS OF THE STATE OF NEW JERSEY WITHOUT REGARD FOR CONFLICTS OF LAWS PRINCIPLES. ANY ACTION OR PROCEEDING WITH RESPECT TO THIS AGREEMENT AND MY EMPLOYMENT SHALL BE BROUGHT EXCLUSIVELY IN THE STATE OR FEDERAL COURTS OF NEW JERSEY. I EXPRESSLY CONSENT TO VENUE IN, AND THE PERSONAL JURISDICTION OF, THE STATE AND FEDERAL COURTS LOCATED IN NEW JERSEY FOR ANY LAWSUIT ARISING FROM OR RELATING TO THIS AGREEMENT. |
6. |
This Agreement does not alter the status of my employment as an at-will employee of PAHC. |
7. |
I understand PAHC is engaged in a highly competitive business and that its competitive position depends upon its ability to maintain the confidentiality of its confidential information, proprietary information, and trade secrets, which were developed, compiled and acquired by PAHC at its great effort and expense. I further acknowledge and agree that compliance with the provisions of this Agreement and PAHC’s Confidentiality and Nondisclosure Agreement is necessary to protect the confidential information, proprietary information, and trade secrets, |
Revised Noncompete tied to severance (G. David)(Oct. 2023) |
- 2 - |
business and goodwill of PAHC, and that any breach of this Agreement will result in irreparable and continuing harm to PAHC, for which money damages may not provide adequate relief. Accordingly, in the event of a breach or threatened breach of this Agreement,
PAHC shall have full rights to injunctive relief, in addition to any other existing rights and remedies, without requirement of posting bond.
8. |
The terms of this Agreement shall survive my separation of employment with PAHC. |
9. |
This Agreement shall inure to the benefit of PAHC, its successors and assigns. |
10. |
This Agreement constitutes the entire agreement between PAHC and me with respect to the subject of this Agreement and supersedes all prior agreements between us relating to the same subject matter, except the Confidentiality and Nondisclosure Agreement between PAHC and me, which is incorporated herein by reference. Any waiver of a breach of any provision of this Agreement by PAHC shall not be construed as a waiver of any other breach of this Agreement, and no failure or delay by PAHC in exercising any right under this Agreement shall operate as a waiver of any breach by me. This Agreement cannot be changed except by written agreement of PAHC and me, wherein specific reference is made to this Agreement. |
I have read, understand and consent to the above Agreement.
By: /s/ Glenn David |
|
|
|
Name: Glenn David |
|
|
|
Date: January 31, 2024 |
|
Revised Noncompete tied to severance (G. David)(Oct. 2023) |
- 3 - |
Exhibit 19.1

INSIDER TRADING AND DISCLOSURE OF CONFIDENTIAL INFORMATION POLICY
This Policy (the “Insider Trading Policy”) has been adopted by the Board of Directors of Phibro Animal Health Corporation, a Delaware corporation (the “Company”). In adopting this Insider Trading Policy, the Board is mindful that the Company has responsibilities to several constituencies and has various objectives and that the manner in which the Company’s directors and employees trade in the Company’s securities can affect those responsibilities and objectives. Consequently, while all Company personnel are required to comply with applicable law, this Insider Trading Policy is broader than mere compliance with applicable securities laws and may prohibit conduct that is permitted by applicable law. Compliance with this Insider Trading Policy is required of all directors, officers and employees of the Company and its subsidiaries and includes all members of the “immediate family and household” of the foregoing and entities controlled by a person covered by this Insider Trading Policy. As used in this Insider Trading Policy, the term “immediate family and household” means a spouse, children, stepchildren, grandchildren, parents, stepparents, grandparents, siblings and in-laws, and anyone else who lives in your household, and any family members who do not live in your household but whose transactions in the Company’s securities are directed by you or are subject to your influence or control, such as parents or children who consult with you before they trade in the Company’s securities.
This Insider Trading Policy should not be interpreted to modify any agreements the Company and Company personnel may have entered into regarding the disclosure of confidential information.
1.Prohibition Against Trading and Tipping While Aware of Material, Non-Public Information.
It is a violation of Company policy for any person to engage in transactions in securities of the Company if he or she is aware of material, non-public information concerning the Company or its securities. It also violates this Insider Trading Policy for any director or employee in possession of material, non-public information to recommend that another person buy or sell the Company’s securities or to otherwise improperly disclose such information to others outside the Company who may trade on, or make recommendations to others to trade on, the basis of that information. In that case, both the “tippee” and the “tipper” may be liable for a violation of insider trading laws.
Information is material if a reasonable investor would consider that information important in making a decision to buy, sell or hold securities. There is no bright-line standard for assessing materiality; rather, materiality is based on an assessment of all of the facts and circumstances. Although it is not possible to list all types of information that might be deemed material under particular circumstances, information concerning the following subjects is often found material:
|
Insider Trading and Disclosure of Confidential Information Policy Rev. August 1, 2024 |
|
|
(i) internal forecasts or budgets; (ii) significant acquisitions or dispositions (including mergers, tender offers and asset purchase or sale transactions); (iii) major product changes or introductions; (iv) special dividends or changes in dividend policy; (v) changes in debt ratings; (vi) significant write-downs of assets or additions to reserves for bad debts or contingent liabilities; (vii) liquidity problems; (viii) extraordinary management developments; (ix) significant financing transactions; (x) major price or marketing changes; (xi) labor negotiations; (xii) significant cybersecurity incidents; and (xiii) significant litigation or investigations by governmental bodies. Information about a company generally is not material if its public dissemination would not have any impact on the price of the Company’s securities. It should be noted that either positive or adverse information may be material.
It should also be noted that materiality may depend on the type of securities involved in the analysis. Materiality can frequently be uncertain and, since your actions will be judged with hindsight, caution should be exercised. If you have any questions in this area, you should contact the Company’s General Counsel or Chief Financial Officer at +1 (201) 329-7300.
Information is non-public if it has not been disclosed to the public and, even after disclosure has been made (e.g., SEC filings, press releases or publicly accessible conference calls), until a reasonable time has passed after it has been disclosed by means likely to result in widespread public awareness. By contrast, information would likely not be considered widely disseminated if it is available only to the Company’s employees, or if it is only available to a select group of analysts, brokers and institutional investors. These prohibitions against trading while in possession of material, non-public information also apply to material, non-public information about any other company with which the Company does business, including a customer or supplier of the Company, or that is involved in a potential transaction or business relationship with the Company that has been obtained in the course of a person’s work for the Company.
This Insider Trading Policy continues to apply to your transactions in Company securities even after you have terminated employment or other services to the Company or a subsidiary. If you are aware of material, non-public information when your employment or service relationship terminates, you may not trade in Company securities until that information becomes public or is no longer material.
2.Restrictions on Selective Disclosure of Material, Non-Public Information.
It is a violation of Company policy to disclose in any manner any material, non-public information to any person who may trade on, or make recommendations to others to trade on, the basis of that information except as follows: (i) disclosure to a person who has signed an appropriate agreement to hold such information in confidence; (ii) disclosure to Senior Personnel (as defined below) of the Company; (iii) disclosure to personnel who need the information to carry out their services to the Company and who agree to hold the information in confidence; (iv) disclosure to the Company’s legal counsel, accountants or advisors if the information disclosed is related to a matter on which they are involved; or (v) as approved by the Chief Executive Officer, Chief Operating Officer, Chief Financial Officer or the General Counsel of the Company. All communications with investors, investor representatives, securities analysts and securities professionals shall be made solely by the Company’s Chief Executive Officer, Chief Financial Officer or a person specifically designated by either of them. All requests for
|
Insider Trading and Disclosure of Confidential Information Policy Rev. August 1, 2024 |
-2- |
|
information about the Company from stockholders, the financial press, investment analysts and others in the media or financial communities, whether or not involving confidential or non-public information, should be directed to the Company’s Chief Financial Officer at +1 (201) 329-7300, or a person designated by him or her from time to time. If any person subject to this Insider Trading Policy should inadvertently selectively disclose any material, non-public information to any person not covered by the exceptions above, this Insider Trading Policy requires that such inadvertent disclosure be reported as soon as possible to the Chief Executive Officer, Chief Operating Officer, General Counsel, or Chief Financial Officer of the Company. Such inadvertent disclosure may arise because of a mistaken belief about the materiality or non-public nature of the disclosed information, the identity of the recipient of such disclosure, the applicability of a confidentiality agreement or numerous other reasons. Applicable law (Regulation FD, in particular) generally requires that the Company publicly and promptly disclose the information that had been inadvertently disclosed.
3.Window Periods and Pre-Clearance Procedures.
It is not permissible for any member of the board of directors, officers, any employees with the title of vice president or higher, any finance employees who assist with SEC filings, legal department employees, any employees on the Company’s disclosure committee, and any other persons designated by the General Counsel as being subject to these procedures, as well as the immediate family and household, and controlled entities of such persons (“Senior Personnel”) to engage in any transaction in the Company’s securities (including the cashless exercise of any stock option that involves a market sale to generate cash to pay the exercise price or tax liability of such stock option, to the extent permitted, but excluding the exercise of a stock option and cash payment to the Company or the exercise of withholding by the Company of the exercise price or tax liability of a stock option) without first obtaining pre-clearance of the transaction from the General Counsel or Chief Financial Officer, or, if the transaction involves the Chief Financial Officer, from the General Counsel or if the transaction involves the General Counsel, from the Chief Financial Officer. The officer providing such pre-clearance is referred to herein as the “Pre-Clearance Officer.” A request for pre-clearance should be submitted to the Pre-Clearance Officer at least two business days in advance of the proposed transaction. Normally, the Pre-Clearance Officer will clear, to the extent consistent with Company policy, any transaction that complies with this Insider Trading Policy and applicable securities law and occurs inside a period in which transactions are permitted (a “Window Period”). When a request for pre-clearance is made, the requestor should carefully consider whether he or she may be aware of any material nonpublic information about the Company. The requestor, if subject to Section 16 of the Securities Exchange Act of 1934, as amended, should also indicate whether he or she has effected any non-exempt “opposite-way” transactions within the past six months. The requestor should also be prepared to comply with Section 16 of the Exchange Act and file Form 4 or Form 5 and SEC Rule 144 and file Form 144, if applicable, at the time of any sale.
However, the Pre-Clearance Officer is under no obligation to approve, and may determine not to permit, any transaction submitted for pre-clearance, even if the transaction falls inside a “Window Period”. If pre-clearance is denied, such denial must be kept confidential by the person requesting pre-clearance. Unless otherwise provided, pre-clearance of a transaction is valid for three business days. If the transaction is not executed within that time, the person requesting pre-clearance must request pre-clearance again.
|
Insider Trading and Disclosure of Confidential Information Policy Rev. August 1, 2024 |
-3- |
|
Quarterly Permitted Period. The “Window Period” shall include the period beginning two full business days following the release of the Company’s quarterly or annual financial results for the immediately preceding fiscal quarter or year and ending immediately preceding the 15th calendar day before the end of the then-current fiscal quarter. The release of quarterly or annual financial results invariably has the potential to have a material effect on the market for the Company’s securities. As such, a quarterly blackout period is imposed to avoid even the appearance of insider trading.
Event-specific Blackout Period. From time to time, an event may occur that is material to the Company and is known by certain parties. So long as the event remains material and non-public, directors, officers, and such other persons as are designated by the Pre-Clearance Officer may not trade in the Company’s securities. The existence of an event-specific blackout will not be announced. If, however, a person whose trades are subject to pre-clearance requests permission to trade in the Company’s securities during an event-specific blackout, the Pre-Clearance Officer will inform the requester of the existence of a blackout period, without disclosing the reason for the blackout. Any person made aware of the existence of an event-specific blackout should not disclose the existence of the blackout to any other person. The failure of the Pre-Clearance Officer to designate a person as being subject to an event-specific blackout will not relieve that person of the obligation not to trade while aware of material non-public information. A person in possession of material, non-public information about the Company may not engage in any transaction involving the Company’s securities either outside or inside the Window Period.
The foregoing procedures do not apply to the purchase or sale of securities in a “blind” trust, mutual fund, “wrap” account or similar arrangement, provided that there are no discussions with the trustee, money manager or other investment advisor who has discretion over the funds. Senior Personnel should consider asking their advisors to refrain from trading in Company securities to prevent any future misunderstanding or embarrassment. Further, the requirement for pre-clearance, the quarterly trading restrictions and event-driven trading restrictions do not apply to transactions conducted pursuant to approved Rule 10b5-1 plans, described under the heading “Prearranged Trading Plans.”
Employee Benefit Plan Blackout Periods. Section 306 of the Sarbanes-Oxley Act of 2002 and Regulation BTR prohibit executive officers and directors of a public company from directly or indirectly acquiring or disposing of any equity securities of a public company received in connection with such person’s service or employment as a director or executive officer during an individual account plan “blackout period.” “Individual account plans” include 401(k) plans, profit sharing plans, stock bonus plans and money purchase pension plans sponsored by the Company. An individual account plan “blackout period” exists whenever the Company or any plan fiduciary temporarily suspends for more than three consecutive business days the ability of 50% or more of the plan participants or beneficiaries under all individual account plans maintained by the Company to acquire or dispose of any of the Company’s equity securities held in the plans. This Insider Trading Policy extends this prohibition to all Senior Personnel.
4.Prearranged Trading Plans.
Rule 10b5-1 under the Exchange Act provides an affirmative defense to insider trading allegations under federal law. In order to be eligible to rely on this defense, a person subject to this
|
Insider Trading and Disclosure of Confidential Information Policy Rev. August 1, 2024 |
-4- |
|
Insider Trading Policy must enter into a Rule 10b5-1 plan for transactions in Company securities that meets the conditions specified in the Rule (a “Rule 10b5-1 Plan”). If the plan meets the requirements of Rule 10b5-1, Company securities may be purchased or sold without regard to certain insider trading restrictions described in this Insider Trading Policy.
To comply with the Policy, the adoption, modification or early termination of a Rule 10b5-1 Plan must be approved by the Pre-Clearance Officer, and all Rule 10b5-1 Plans must meet the requirements of Rule 10b5-1. Any Rule 10b5-1 Plan must be submitted for approval five business days prior to the entry into the Rule 10b5-1 Plan, and any proposed modifications or terminations thereof must be submitted for approval at least three business days prior to the consummation of such actions (or in each case such shorter period as shall be agreed by the Pre-Clearance Officer). No further pre-approval of transactions conducted pursuant to the Rule 10b5-1 Plan will be required.
In addition, a Rule 10b5-1 Plan may be entered into or modified in any material respect only (i) at a time when the person entering into or modifying the plan is not aware of material nonpublic information about the Company or Company securities and (ii) in the case of Covered Senior Persons, during an open “Window Period.” Once the plan is adopted, the person must not exercise any influence over the amount of securities to be traded, the price at which they are to be traded or the date of the trade. The plan must either specify the amount, pricing and timing of transactions in advance or delegate discretion on these matters to an independent third party.
Once a Rule 10b5-1 Plan is pre-cleared and is adopted or modified, it is subject to a “cooling-off” period before execution of the first trade. The “cooling-off” period for directors and officers subject to Section 16 of the Exchange Act ends on the later of: (1) 90 days following the Rule 10b5-1 Plan adoption or modification or (2) two business days following the disclosure in Form 10-Q or Form 10-K of the Company’s financial results for the fiscal quarter in which the Rule 10b5-1 Plan was adopted or modified (however, the cooling-off period will not exceed 120 days following plan adoption or modification). For all other individuals, a 30-day cooling-off period is required.
A person may not enter into overlapping Rule 10b5-1 Plans (subject to certain exceptions) and may only enter into one single-trade Rule 10b5-1 Plan during any 12-month period (subject to certain exceptions). Directors and officers subject to Section 16 of the Exchange Act must include a representation in their Rule 10b5-1 Plan certifying that: (i) they are not aware of any material nonpublic information and (ii) they are adopting the Rule 10b5-1 Plan in good faith and not as part of a plan or scheme to evade the prohibitions in Rule 10b-5.
All persons entering into a Rule 10b5-1 Plan must act in good faith with respect to that plan.
5.Prohibition Against Short Selling.
It violates this Insider Trading Policy for any person subject to this Insider Trading Policy to sell any equity security of the Company if such person either (a) does not own the security sold or (b) does not deliver the security against such sale within twenty days thereafter or does not
|
Insider Trading and Disclosure of Confidential Information Policy Rev. August 1, 2024 |
-5- |
|
within five days after such sale deposit the security in the mails or other usual channels of transportation.
6.Prohibition Against Trading in Derivatives.
It violates this Insider Trading Policy for any Senior Personnel to purchase, sell or engage in any other transaction involving any derivative securities related to any equity securities of the Company. A “derivative security” includes any option, warrant, convertible security, stock appreciation right or similar security with an exercise or conversion price or other value related to the value of any equity security of the Company. This prohibition does not, however, apply to any derivative security received pursuant to a Company compensatory or benefit plan, contract or arrangement.
7.Annual Certification.
All Senior Personnel of the Company are required to execute and deliver an annual statement to the Chief Financial Officer or General Counsel of the Company, certifying that such person has complied with this Insider Trading Policy at all times from the date hereof (or such lesser time as such person has been covered hereby).
8.Implementation.
The Board of Directors may adopt such reasonable procedures as it deems necessary or desirable in order to implement this Insider Trading Policy.
Where appropriate for this Insider Trading Policy, references to the General Counsel may include an individual designated by the General Counsel to aid the General Counsel in the performance of his or her duties.
* * * *
If you have any doubt as to your responsibilities under these guidelines, please seek clarification and guidance from the Chief Financial Officer or the General Counsel of the Company at +1 (201) 329-7300 before you act. Do not try to resolve uncertainties on your own.
The Company expects strict compliance with the foregoing policies by all persons subject to this Insider Trading Policy. Any failure to observe these guidelines may result in serious legal difficulties for you and the Company. Furthermore, any failure to follow this Insider Trading Policy will be considered a matter of extreme seriousness and may serve as a basis for termination of employment or service.
|
Insider Trading and Disclosure of Confidential Information Policy Rev. August 1, 2024 |
-6- |
|
EXHIBIT 21.1
PHIBRO ANIMAL HEALTH CORPORATION LIST OF SUBSIDIARIES
SUBSIDIARY |
JURISDICTION |
||
8861 Dice Road LLC |
|
California |
|
First Dice Road Company, A California Limited Partnership |
California |
||
Western Magnesium Corp. |
California |
||
Phibro Animal Health Holdings, Inc. |
Delaware |
||
Prince Agri Products, Inc. |
Delaware |
||
Phibro-Tech, Inc. |
Delaware |
||
PhibroWood, LLC |
|
Delaware |
|
PMC Quincy, Inc. |
|
Illinois |
|
C P Chemicals, Inc. |
New Jersey |
||
Phibrochem, Inc. |
New Jersey |
||
OmniGen Research, LLC |
Oregon |
||
Phibro Animal Health de Argentina SRL |
Argentina |
||
Phibro Animal Health PTY Limited |
Australia |
||
Phibro Animal Health S.A. |
Belgium |
||
Phibro Saude Animal Internacional Ltda. |
Brazil |
||
Phibro Saude e Nutricao Animal Ltda.(1) |
Brazil |
||
Quimica Real Ltda. |
|
Brazil |
|
Phibro Animal Health Ltd. |
Canada |
||
Phibro Animal Health Holdings, Inc. Chile Limitada |
Chile |
||
Phibro Animal Health (Shanghai) Co., Ltd. |
|
China |
|
Phibro Animal Health Colombia S.A.S. |
Colombia |
||
Phibro Animal Health de Republica Dominicana, SRL |
Dominican Republic |
||
Phibro Animal Health (Egypt) LLC |
Egypt |
||
Phibro Animal Health For Trading (Egypt) S.A.E. |
|
Egypt |
|
Phibro Corporation Limited |
Hong Kong |
||
Phibro Animal Health Limited |
Ireland |
||
Abic Biological Laboratories Ltd. |
Israel |
||
Abic Veterinary Products Ltd. |
Israel |
||
Phibro Animal Health Ltd.(2) |
Israel |
||
Phibro Animal Nutrition Ltd.(3) |
Israel |
||
Phibro Israel Holdings Ltd. |
|
Israel |
|
pHi-Tech Animal Health Technologies Ltd.(4) |
Israel |
||
Phibro Corporation (M) Sdn. Bhd. |
Malaysia |
||
PB Animal Health de Mexico S. de R.L. de C.V. |
Mexico |
||
PBAH Peruana S.A.C. |
Peru |
||
Phibro Animal Health (Philippines), Inc. |
Philippines |
||
Phibro Animal Health (Poland) sp. z.o.o. |
Poland |
||
Phibro Animal Health (Proprietary) Limited |
South Africa |
||
Phibro Animal Health (Thailand) Limited |
Thailand |
||
Phibro Hayvan Sagligi Urunleri Sanayi ve Ticaret A.S |
Turkey |
||
Ferro Metal and Chemical Corporation Ltd. |
United Kingdom |
||
Phibro Animal Health SRL |
|
Uruguay |
|
Phibro Animal Health de Venezuela, C.A. |
Venezuela |
||
California Water Technologies LLC(5) |
Michigan |
||
North Field Extension, LLC(5) |
New Jersey |
||
Marion Bio-Tech, LLC(5) |
Delaware |
||
Hannibal Bio-Tech, LLC(5) |
Delaware |
| (1) | Formerly known as Planalquimica Industrial Ltda. |
| (2) | Formerly known as Koffolk (1949) Ltd. |
| (3) | Formerly known as Agrozan Ltd. |
| (4) | Formerly known as Target Point-Technologies Ltd. |
| (5) | We directly or indirectly own 50% of the entity. |
EXHIBIT 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in the Registration Statement on Form S-8 (No. 333-198809) of Phibro Animal Health Corporation of our report dated August 28, 2024 relating to the financial statements and the effectiveness of internal control over financial reporting, which appears in this Form 10-K.
/s/ PricewaterhouseCoopers LLP
Florham Park, New Jersey
August 28, 2024
EXHIBIT 31.1
CERTIFICATIONS
I, Jack C. Bendheim, certify that:
Dated: August 28, 2024 |
/s/ Jack C. Bendheim |
|||
|
|
|
Jack C. Bendheim |
|
|
|
|
Chairman, President and Chief Executive Officer |
|
EXHIBIT 31.2
CERTIFICATIONS
I, Glenn C. David, certify that:
Dated: August 28, 2024 |
/s/ Glenn C. David |
|||
|
|
|
Glenn C. David |
|
|
|
|
Chief Financial Officer |
|
EXHIBIT 32.1
CERTIFICATION UNDER SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, the undersigned certifies that this periodic report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and that information contained in this periodic report fairly presents, in all material respects, the financial condition and results of operations of the issuer.
|
Dated: August 28, 2024 |
|
/s/ Jack C. Bendheim |
|
|
|
|
Jack C. Bendheim |
|
|
|
|
Chairman, President and Chief Executive Officer |
|
EXHIBIT 32.2
CERTIFICATION UNDER SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, the undersigned certifies that this periodic report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 and that information contained in this periodic report fairly presents, in all material respects, the financial condition and results of operations of the issuer.
|
Dated: August 28, 2024 |
|
/s/ Glenn C. David |
|
|
|
|
Glenn C. David |
|
|
|
|
Chief Financial Officer |
|
Exhibit 97.1

CLAWBACK POLICY
PHIBRO ANIMAL HEALTH CORPORATION
PURPOSE
Phibro Animal Health Corporation (the “Company”) believes that it is in the best interests of the Company and its shareholders to create and maintain a culture that emphasizes integrity and accountability and that reinforces the Company’s pay-for-performance compensation philosophy. The Company’s Board of Directors (the “Board”) has therefore adopted this policy, which provides for the recoupment of certain executive compensation in the event that the Company is required to prepare an accounting restatement of its financial statements due to material noncompliance with any financial reporting requirement under the federal securities laws (this “Policy”). This Policy is designed to comply with Section 10D of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), the rules promulgated thereunder, and the listing standards of the national securities exchange on which the Company’s securities are listed.
ADMINISTRATION
This Policy shall be administered by the Compensation Committee of the Board (the “Compensation Committee”). Any determinations made by the Compensation Committee shall be final and binding on all affected individuals.
COVERED EXECUTIVES
This Policy applies to the Company’s current and former executive officers (as determined by the Compensation Committee in accordance with Section 10D of the Exchange Act, the rules promulgated thereunder, and the listing standards of the national securities exchange on which the Company’s securities are listed) and such other senior executives or employees who may from time to time be deemed subject to this Policy by the Compensation Committee (collectively, the “Covered Executives”). This Policy shall be binding and enforceable against all Covered Executives.
RECOUPMENT; ACCOUNTING RESTATEMENT
In the event that the Company is required to prepare an accounting restatement of its financial statements due to the Company’s material noncompliance with any financial reporting requirement under the securities laws, including any required accounting restatement (i) to correct
Clawback Policy |
|
Last Revised: November 6, 2023 |
|
| |
an error in previously issued financial statements that is material to the previously issued financial statements, or (ii) that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period (each an “Accounting Restatement”), the Compensation Committee will reasonably promptly require reimbursement or forfeiture of the Overpayment (as defined below) received by any Covered Executive (x) after beginning service as a Covered Executive, (y) who served as a Covered Executive at any time during the performance period for the applicable Incentive-Based Compensation (as defined below), and (z) during the three (3) completed fiscal years immediately preceding the date on which the Company is required to prepare an Accounting Restatement and any transition period (that results from a change in the Company’s fiscal year) within or immediately following those three (3) completed fiscal years.
INCENTIVE-BASED COMPENSATION
For purposes of this Policy, “Incentive-Based Compensation” means any compensation that is granted, earned, or vested based wholly or in part upon the attainment of a financial reporting measure, including, but not limited to: (i) non-equity incentive plan awards that are earned solely or in part by satisfying a financial reporting measure performance goal; (ii) bonuses paid from a bonus pool, where the size of the pool is determined solely or in part by satisfying a financial reporting measure performance goal; (iii) other cash awards based on satisfaction of a financial reporting measure performance goal; (iv) restricted stock, restricted stock units, stock options, stock appreciation rights, and performance share units that are granted or vest solely or in part based on satisfaction of a financial reporting measure performance goal; and (v) proceeds from the sale of shares acquired through an incentive plan that were granted or vested solely or in part based on satisfaction of a financial reporting measure performance goal.
Compensation that would not be considered Incentive-Based Compensation includes, but is not limited to: (a) salaries; (b) bonuses paid solely based on satisfaction of subjective standards, such as demonstrating leadership, and/or completion of a specified employment period; (c) non-equity incentive plan awards earned solely based on satisfaction of strategic or operational measures; (d) wholly time-based equity awards; and (e) discretionary bonuses or other compensation that is not paid from a bonus pool that is determined by satisfying a financial reporting measure performance goal.
A financial reporting measure is: (i) any measure that is determined and presented in accordance with the accounting principles used in preparing financial statements, or any measure derived wholly or in part from such measure, such as revenues, EBITDA, Adjusted EBITDA or net income or (ii) stock price and total shareholder return. Financial reporting measures include, but are not limited to: revenues; net income; operating income; profitability of one or more reportable segments; financial ratios (e.g., accounts receivable turnover and inventory turnover rates); net assets or net asset value per share; earnings before interest, taxes, depreciation and amortization; funds from operations and adjusted funds from operations; liquidity measures (e.g., working capital, operating cash flow); return measures (e.g., return on invested capital, return on assets); earnings measures (e.g., earnings per share); sales per square foot or same store sales, where sales is subject to an accounting restatement; revenue per user, or average revenue per user, where revenue is subject to an accounting restatement; cost per employee, where cost is subject to an accounting restatement; any of such financial reporting measures relative to a peer group, where
Clawback Policy |
|
Last Revised: November 6, 2023 |
|
|
|
2 | |
the Company’s financial reporting measure is subject to an accounting restatement; and tax basis income.
OVERPAYMENT: AMOUNT SUBJECT TO RECOVERY
The amount to be recovered will be the amount of Incentive-Based Compensation received that exceeds the amount of Incentive-Based Compensation that otherwise would have been received had it been determined based on the restated amounts, and must be computed without regard to any taxes paid (the “Overpayment”). Incentive-Based Compensation is deemed received in the Company’s fiscal period during which the financial reporting measure specified in the incentive-based compensation award is attained, even if the vesting, payment or grant of the incentive-based compensation occurs after the end of that period.
For Incentive-Based Compensation based on stock price or total shareholder return, where the amount of erroneously awarded compensation is not subject to mathematical recalculation directly from the information in the Accounting Restatement, the amount must be based on a reasonable estimate of the effect of the Accounting Restatement on the stock price or total shareholder return upon which the Incentive-Based Compensation was received, and the Company must maintain documentation of the determination of that reasonable estimate and provide such documentation to the exchange on which the Company’s securities are listed.
METHOD OF RECOUPMENT
The Compensation Committee will determine, in its sole discretion, the method or methods for recouping any Overpayment hereunder which may include, without limitation:
| ● | requiring reimbursement of cash Incentive-Based Compensation previously paid; |
| ● | seeking recovery of any gain realized on the vesting, exercise, settlement, sale, transfer, or other disposition of any equity-based awards granted as Incentive-Based Compensation; |
| ● | offsetting any or all of the Overpayment from any compensation otherwise owed by the Company to the Covered Executive; |
| ● | cancelling outstanding vested or unvested equity awards; and/or |
| ● | taking any other remedial or recovery action permitted by law, as determined by the Compensation Committee. |
LIMITATION ON RECOVERY; NO ADDITIONAL PAYMENTS
The right to recovery will be limited to Overpayments received during the three (3) completed fiscal years prior to the date on which the Company is required to prepare an Accounting Restatement and any transition period (that results from a change in the Company’s fiscal year) within or immediately following those three (3) completed fiscal years. In no event shall the Company be required to award Covered Executives an additional payment if the restated or accurate financial results would have resulted in a higher Incentive-Based Compensation payment.
Clawback Policy |
|
Last Revised: November 6, 2023 |
|
|
|
3 | |
NO INDEMNIFICATION
The Company shall not indemnify any Covered Executives against the loss of any incorrectly awarded Incentive-Based Compensation.
INTERPRETATION
The Compensation Committee is authorized to interpret and construe this Policy and to make all determinations necessary, appropriate, or advisable for the administration of this Policy. It is intended that this Policy be interpreted in a manner that is consistent with the requirements of Section 10D of the Exchange Act and the applicable rules or standards adopted by the Securities and Exchange Commission or any national securities exchange on which the Company’s securities are listed.
EFFECTIVE DATE
This Policy shall be effective as of the date it is adopted by the Board (the “Effective Date”) and shall apply to Incentive-Based Compensation (including Incentive-Based Compensation granted pursuant to arrangements existing prior to the Effective Date). Notwithstanding the foregoing, this Policy shall only apply to Incentive-Based Compensation received (as determined pursuant to this Policy) on or after the effective date of NASDAQ Listing Rule 5608 (October 2, 2023).
AMENDMENT; TERMINATION
The Board may amend this Policy from time to time in its discretion. The Board may terminate this Policy at any time.
OTHER RECOUPMENT RIGHTS
The Board intends that this Policy will be applied to the fullest extent of the law. The Compensation Committee may require that any employment or service agreement, cash-based bonus plan or program, equity award agreement, or similar agreement entered into on or after the adoption of this Policy shall, as a condition to the grant of any benefit thereunder, require a Covered Executive to agree to abide by the terms of this Policy. Any right of recoupment under this Policy is in addition to, and not in lieu of, any other remedies or rights of recoupment that may be available to the Company pursuant to the terms of any similar policy in any employment agreement, equity award agreement, cash-based bonus plan or program, or similar agreement and any other legal remedies available to the Company.
IMPRACTICABILITY
The Compensation Committee shall recover any Overpayment in accordance with this Policy except to the extent that the Compensation Committee determines such recovery would be impracticable because:
Clawback Policy |
|
Last Revised: November 6, 2023 |
|
|
|
4 | |
(A) The direct expense paid to a third party to assist in enforcing this Policy would exceed the amount to be recovered;
(B) Recovery would violate home country law of the Company where that law was adopted prior to November 28, 2022; or
(C) Recovery would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of the Company, to fail to meet the requirements of 26 U.S.C. 401(a)(13) or 26 U.S.C. 411(a) and regulations thereunder.
SUCCESSORS
This Policy shall be binding and enforceable against all Covered Executives and their beneficiaries, heirs, executors, administrators or other legal representatives.
Clawback Policy |
|
Last Revised: November 6, 2023 |
|
|
|
5 | |