UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 1, 2023
Glaukos Corporation
(Exact name of registrant as specified in its charter)
Delaware |
|
001-37463 |
|
33-0945406 |
(State or other jurisdiction |
|
(Commission |
|
(I.R.S. Employer |
of incorporation) |
|
File Number) |
|
Identification No.) |
One Glaukos Way |
|
|
Aliso Viejo |
|
|
California |
|
92656 |
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (949) 367-9600
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Title of each class: |
|
Trading Symbol |
|
Name of each exchange on which registered: |
Common Stock |
|
GKOS |
|
New York Stock Exchange |
Indicate by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02. Results of Operations and Financial Condition.
On November 1, 2023, Glaukos Corporation (the “Company”) issued a press release announcing its financial results for the third quarter ended September 30, 2023. A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated by reference herein.
The information contained in this Item 2.02 and in the accompanying Exhibit 99.1 shall not be deemed filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or incorporated by reference in any filing under the Exchange Act or the Securities Act of 1933, as amended (the “Securities Act”), except as shall be expressly set forth by specific reference in such filing.
Item 7.01. Regulation FD Disclosure.
A Quarterly Summary containing supplemental business and financial information for the Company’s third quarter ended September 30, 2023 is furnished as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated by reference herein. A copy of the Quarterly Summary is also available in the “Financials & Filings” section of the Company’s investor relations website at https://investors.glaukos.com.
The information contained in this Item 7.01 and in the accompanying Exhibit 99.2 shall not be deemed filed for purposes of Section 18 of the Exchange Act, or incorporated by reference in any filing under the Exchange Act or the Securities Act, except as shall be expressly set forth by specific reference in such filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
Exhibit No. |
|
Description |
99.1 |
|
Press Release of Glaukos Corporation, dated November 1, 2023 |
|
|
|
99.2 |
|
Quarterly Summary of Glaukos Corporation for the third quarter ended September 30, 2023 |
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
GLAUKOS CORPORATION |
||
|
|
||
|
By: |
/s/ Alex R. Thurman |
|
|
|
Name: |
Alex R. Thurman |
|
|
Title: |
Senior Vice President & Chief Financial Officer |
Date: November 1, 2023
Exhibit 99.1

FOR IMMEDIATE RELEASE
Contact:
Chris Lewis
Vice President, Investor Relations & Corporate Affairs
(949) 481-0510
clewis@glaukos.com
Glaukos Announces Third Quarter 2023 Financial Results
Aliso Viejo, CA – November 1, 2023 – Glaukos Corporation (NYSE: GKOS), an ophthalmic medical technology and pharmaceutical company focused on novel therapies for the treatment of glaucoma, corneal disorders and retinal diseases, today announced financial results for the third quarter ended September 30, 2023. Key highlights include:
· |
Net sales of $78.0 million in Q3 2023 increased 10% year-over-year on a reported basis and 9% year-over-year on a constant currency basis. |
· |
Glaucoma net sales of $58.3 million in Q3 2023 increased 9% year-over-year. |
· |
Corneal Health net sales of $19.7 million in Q3 2023 increased 12% year-over-year. |
· |
Gross margin of approximately 76% and non-GAAP gross margin of approximately 83% in Q3 2023. |
· |
Raised 2023 net sales guidance to $307 million to $310 million, compared to $304 million to $308 million previously. |
“Our solid third quarter results reflect successful execution of our global commercial strategies,” said Thomas Burns, Glaukos chairman and chief executive officer. “We continue to successfully advance our robust pipeline of novel, dropless platform technologies designed to meaningfully advance the standard of care and improve outcomes for patients suffering from chronic eye diseases.”
Third Quarter 2023 Financial Results
Net sales in the third quarter of 2023 of $78.0 million increased 10% on a reported basis, or 9% on a constant currency basis, compared to $71.3 million in the same period in 2022.
Gross margin for the third quarter of 2023 was approximately 76%, compared to approximately 76% in the same period in 2022. Non-GAAP gross margin for the third quarter of 2023 was approximately 83%, compared to approximately 84% in the same period in 2022.
Selling, general and administrative (SG&A) expenses for the third quarter of 2023 increased 15% to $54.2 million, compared to $47.1 million in the same period in 2022. Non-GAAP SG&A expenses for the third quarter of 2023 increased 15% to $53.5 million, compared to $46.4 million in the same period in 2022.
GAAP and non-GAAP research and development (R&D) expenses for the third quarter of 2023 increased 15% to $33.3 million, compared to $28.9 million in the same period in 2022.
Loss from operations in the third quarter of 2023 was $28.0 million, compared to operating loss of $21.6 million in the third quarter of 2022. Non-GAAP loss from operations in the third quarter of 2023 was $21.8 million, compared to non-GAAP operating loss of $15.3 million in the third quarter of 2022.
1

Net loss in the third quarter of 2023 was $30.4 million, or ($0.63) per diluted share, compared to net loss of $27.6 million, or ($0.58) per diluted share, in the third quarter of 2022. Non-GAAP net loss in the third quarter of 2023 was $24.2 million, or ($0.50) per diluted share, compared to non-GAAP net loss of $21.3 million, or ($0.45) per diluted share, in the third quarter of 2022.
The company ended the third quarter of 2023 with approximately $307 million in cash and cash equivalents, short-term investments and restricted cash.
2023 Revenue Guidance
The company expects 2023 net sales to be in the range of $307 million to $310 million based on the latest foreign currency exchange rates.
Webcast & Conference Call
The company will host a conference call and simultaneous webcast today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results and provide additional information about the company’s financial outlook. A link to the webcast is available on the company’s website at http://investors.glaukos.com. To participate in the conference call, please dial 888-210-2212 (U.S.) or 646-960-0390 (international) and enter Conference ID 7935742. A replay of the webcast will be archived on the company’s website following completion of the call.
Quarterly Summary Document
The company has posted a document on its Investor Relations website under the “Financials & Filings – Quarterly Results” section titled “Quarterly Summary.” This Quarterly Summary document is designed to provide the investment community with a summarized and easily accessible reference document that details the key facts associated with the quarter, the state of the company’s business objectives and strategies and any forward statements or guidance the company may make. This document is provided alongside the company’s earnings press release and is designed to be read by investors before the regularly scheduled quarterly conference call. As such, today’s conference call will be in a format primarily consisting of a questions and answers session, during which Glaukos will address any queries investors have regarding the company’s results. It is the company’s goal that this format will make its quarterly earnings process more efficient and impactful for the investment community going forward.
About Glaukos
Glaukos (www.glaukos.com) is an ophthalmic medical technology and pharmaceutical company focused on developing and commercializing novel therapies for the treatment of glaucoma, corneal disorders and retinal diseases. Glaukos first developed Micro-Invasive Glaucoma Surgery (MIGS) as an alternative to the traditional glaucoma treatment paradigm, launching its first MIGS device commercially in 2012, and continues to develop a portfolio of technologically distinct and leverageable platforms to support ongoing pharmaceutical and medical device innovations. Products or product candidates for each of these platforms are designed to advance the standard of care through better treatment options across the areas of glaucoma, corneal disorders and retinal diseases.
Forward-Looking Statements
This communication contains “forward-looking statements” within the meaning of federal securities laws. All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements. These statements are based on management’s current expectations, assumptions, estimates and beliefs.
2

Although we believe that we have a reasonable basis for forward-looking statements contained herein, we caution you that they are based on current expectations about future events affecting us and are subject to risks, uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control, that may cause our actual results to differ materially from those expressed or implied by forward-looking statements in this press release. These potential risks and uncertainties that could cause actual results to differ materially from those described in forward-looking statements include, without limitation, uncertainties regarding the impact of the COVID-19 pandemic or other future public health crises on our business; the impact of general macroeconomic conditions including foreign currency fluctuations; the reduced physician fee and ASC facility fee reimbursement rate finalized by CMS for 2022 and 2023 for procedures utilizing the Company’s iStent family of products and its impact on our U.S. combo-cataract glaucoma revenue; our ability to continue to generate sales of our commercialized products and develop and commercialize additional products; our dependence on a limited number of third-party suppliers, some of which are single-source, for components of our products; the occurrence of a crippling accident, natural disaster, or other disruption at our primary facility, which may materially affect our manufacturing capacity and operations; securing or maintaining adequate coverage or reimbursement by third-party payors for procedures using the iStent, the iStent inject, the iStent inject W, iAccess, iPRIME, iStent infinite, our corneal cross-linking products or other products in development; our ability to properly train, and gain acceptance and trust from ophthalmic surgeons in the use of our products; our ability to compete effectively in the medical device industry and against current and future technologies (including MIGS technologies); our compliance with federal, state and foreign laws and regulations for the approval and sale and marketing of our products and of our manufacturing processes; the lengthy and expensive clinical trial process and the uncertainty of timing and outcomes from any particular clinical trial or regulatory approval processes; the risk of recalls or serious safety issues with our products and the uncertainty of patient outcomes; our ability to protect, and the expense and time-consuming nature of protecting our intellectual property against third parties and competitors and the impact of any claims against us for infringement or misappropriation of third party intellectual property rights and any related litigation; and our ability to service our indebtedness. These and other known risks, uncertainties and factors are described in detail under the caption “Risk Factors” and elsewhere in our filings with the Securities and Exchange Commission (SEC), including in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, which is expected to be filed with the SEC by November 9, 2023. Our filings with the SEC are available in the Investor Section of our website at www.glaukos.com or at www.sec.gov. In addition, information about the risks and benefits of our products is available on our website at www.glaukos.com. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on the forward-looking statements in this press release, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise, except as may be required under applicable securities law.
Statement Regarding Use of Non-GAAP Financial Measures
To supplement the consolidated financial results prepared in accordance with Generally Accepted Accounting Principles (“GAAP”), the Company uses certain non-GAAP historical financial measures. Management makes adjustments to the GAAP measures for items (both charges and gains) that (a) do not reflect the core operational activities of the Company, (b) are commonly adjusted within the Company’s industry to enhance comparability of the Company’s financial results with those of its peer group, or (c) are inconsistent in amount or frequency between periods (albeit such items are monitored and controlled with equal diligence relative to core operations).
3

The Company uses the term “Non-GAAP” to exclude external acquisition-related costs incurred to effect a business combination; amortization of intangible assets acquired in a business combination, asset purchase transaction or other contractual relationship; impairment of goodwill and intangible assets; certain in-process R&D charges; fair value adjustments to contingent consideration liabilities and pre-acquisition contingencies arising from a business combination; integration and transition costs related to business combinations; fair market value adjustments to inventories acquired in a business combination or asset purchase transaction; restructuring charges, duplicative operating expenses, or asset write-offs (or reversals) associated with exiting or significantly downsizing a business; gain or loss from the sale of a business; gain or loss on the mark-to-market adjustment, impairment, or sale of long-term investments; mark-to-market adjustments on derivative instruments that hedge income or expense exposures in a future period; significant legal litigation costs and/or settlement expenses or proceeds legal and other associated expenses that are both unusual and significant related to governmental or internal inquiries; and significant discrete income and other tax adjustments related to transactions as well as changes in estimated acquisition-date tax effects associated with business combinations, and the impact from implementation of tax law changes and settlements. See “GAAP to Non-GAAP Reconciliations” for a reconciliation of each non-GAAP measure presented to the comparable GAAP financial measure.
In addition, in order to remove the impact of fluctuations in foreign currency exchange rates, the Company also presents certain net sales information on a constant currency basis, which represents the outcome that would have resulted had exchange rates in the current period been the same as the average exchange rates in effect in the comparable prior period. See “Reported Sales vs. Prior Periods” for a presentation of certain net sales information on a reported, GAAP and a constant currency basis.
4

GLAUKOS CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
(in thousands, except per share amounts)
|
|
Three Months Ended |
|
Nine Months Ended |
||||||||
|
|
September 30, |
|
September 30, |
||||||||
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
||||
Net sales |
|
$ |
78,048 |
|
$ |
71,269 |
|
$ |
232,346 |
|
$ |
211,635 |
Cost of sales |
|
|
18,510 |
|
|
16,861 |
|
|
56,684 |
|
|
51,757 |
Gross profit |
|
|
59,538 |
|
|
54,408 |
|
|
175,662 |
|
|
159,878 |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
|
54,247 |
|
|
47,149 |
|
|
161,034 |
|
|
140,998 |
Research and development |
|
|
33,301 |
|
|
28,870 |
|
|
101,706 |
|
|
87,459 |
Acquired in-process research and development |
|
|
— |
|
|
— |
|
|
3,000 |
|
|
10,000 |
Litigation-related settlement |
|
|
— |
|
|
— |
|
|
— |
|
|
(30,000) |
Total operating expenses |
|
|
87,548 |
|
|
76,019 |
|
|
265,740 |
|
|
208,457 |
Loss from operations |
|
|
(28,010) |
|
|
(21,611) |
|
|
(90,078) |
|
|
(48,579) |
Non-operating expense: |
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
2,710 |
|
|
744 |
|
|
6,252 |
|
|
1,415 |
Interest expense |
|
|
(3,398) |
|
|
(3,481) |
|
|
(10,205) |
|
|
(10,311) |
Other expense, net |
|
|
(1,709) |
|
|
(2,981) |
|
|
(2,978) |
|
|
(9,792) |
Total non-operating expense |
|
|
(2,397) |
|
|
(5,718) |
|
|
(6,931) |
|
|
(18,688) |
Loss before taxes |
|
|
(30,407) |
|
|
(27,329) |
|
|
(97,009) |
|
|
(67,267) |
Income tax provision |
|
|
37 |
|
|
247 |
|
|
873 |
|
|
468 |
Net loss |
|
$ |
(30,444) |
|
$ |
(27,576) |
|
$ |
(97,882) |
|
$ |
(67,735) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share |
|
$ |
(0.63) |
|
$ |
(0.58) |
|
$ |
(2.03) |
|
$ |
(1.43) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares used to compute basic and diluted net loss per share |
|
|
48,675 |
|
|
47,614 |
|
|
48,284 |
|
|
47,346 |
5

GLAUKOS CORPORATION
CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands, except par values)
|
|
September 30, |
|
December 31, |
||
|
|
2023 |
|
2022 |
||
|
|
(unaudited) |
|
|
|
|
Assets |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
108,929 |
|
$ |
119,525 |
Short-term investments |
|
|
191,928 |
|
|
233,170 |
Accounts receivable, net |
|
|
39,326 |
|
|
36,073 |
Inventory |
|
|
39,781 |
|
|
37,841 |
Prepaid expenses and other current assets |
|
|
19,565 |
|
|
17,250 |
Total current assets |
|
|
399,529 |
|
|
443,859 |
Restricted cash |
|
|
5,856 |
|
|
7,078 |
Property and equipment, net |
|
|
103,075 |
|
|
94,403 |
Operating lease right-of-use assets |
|
|
27,273 |
|
|
25,826 |
Finance lease right-of-use asset |
|
|
44,786 |
|
|
46,601 |
Intangible assets, net |
|
|
289,184 |
|
|
307,869 |
Goodwill |
|
|
66,134 |
|
|
66,134 |
Deposits and other assets |
|
|
12,797 |
|
|
10,613 |
Total assets |
|
$ |
948,634 |
|
$ |
1,002,383 |
|
|
|
|
|
|
|
Liabilities and stockholders’ equity |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
10,413 |
|
$ |
14,403 |
Accrued liabilities |
|
|
56,734 |
|
|
57,956 |
Total current liabilities |
|
|
67,147 |
|
|
72,359 |
Convertible senior notes |
|
|
282,430 |
|
|
281,400 |
Operating lease liability |
|
|
30,572 |
|
|
28,905 |
Finance lease liability |
|
|
70,784 |
|
|
72,172 |
Deferred tax liability, net |
|
|
7,249 |
|
|
7,264 |
Other liabilities |
|
|
12,793 |
|
|
10,278 |
Total liabilities |
|
|
470,975 |
|
|
472,378 |
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value; 5,000 shares authorized; no shares issued or outstanding |
|
|
— |
|
|
— |
Common stock, $0.001 par value; 150,000 shares authorized; 48,748 and 47,782 shares issued and 48,720 and 47,754 shares outstanding at September 30, 2023 and December 31, 2022, respectively |
|
|
49 |
|
|
48 |
Additional paid-in capital |
|
|
1,039,266 |
|
|
997,470 |
Accumulated other comprehensive income (loss) |
|
|
764 |
|
|
(2,975) |
Accumulated deficit |
|
|
(562,288) |
|
|
(464,406) |
Less treasury stock (28 shares as of September 30, 2023 and December 31, 2022) |
|
|
(132) |
|
|
(132) |
Total stockholders’ equity |
|
|
477,659 |
|
|
530,005 |
Total liabilities and stockholders’ equity |
|
$ |
948,634 |
|
$ |
1,002,383 |
6

GLAUKOS CORPORATION
GAAP to Non-GAAP Reconciliations
(in thousands, except per share amounts and percentage data)
(unaudited)
|
|
Q3 2023 |
|
Q3 2022 |
|
||||||||||||||||
|
|
GAAP |
|
Adjustments |
|
|
Non-GAAP |
|
GAAP |
|
Adjustments |
|
|
Non-GAAP |
|
||||||
Cost of sales |
|
$ |
18,510 |
|
$ |
(5,523) |
|
(a) |
$ |
12,987 |
|
$ |
16,861 |
|
$ |
(5,536) |
|
(a) |
$ |
11,325 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross Margin |
|
|
76.3 |
% |
|
7.1 |
% |
|
|
83.4 |
% |
|
76.3 |
% |
|
7.8 |
% |
|
|
84.1 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
$ |
54,247 |
|
$ |
(705) |
|
(b) |
$ |
53,542 |
|
$ |
47,149 |
|
$ |
(787) |
|
(b) |
$ |
46,362 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
$ |
(28,010) |
|
$ |
6,228 |
|
|
$ |
(21,782) |
|
$ |
(21,611) |
|
$ |
6,323 |
|
|
$ |
(15,288) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(30,444) |
|
$ |
6,228 |
|
(c) |
$ |
(24,216) |
|
$ |
(27,576) |
|
$ |
6,323 |
|
(c) |
$ |
(21,253) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share |
|
$ |
(0.63) |
|
$ |
0.13 |
|
|
$ |
(0.50) |
|
$ |
(0.58) |
|
$ |
0.13 |
|
|
$ |
(0.45) |
|
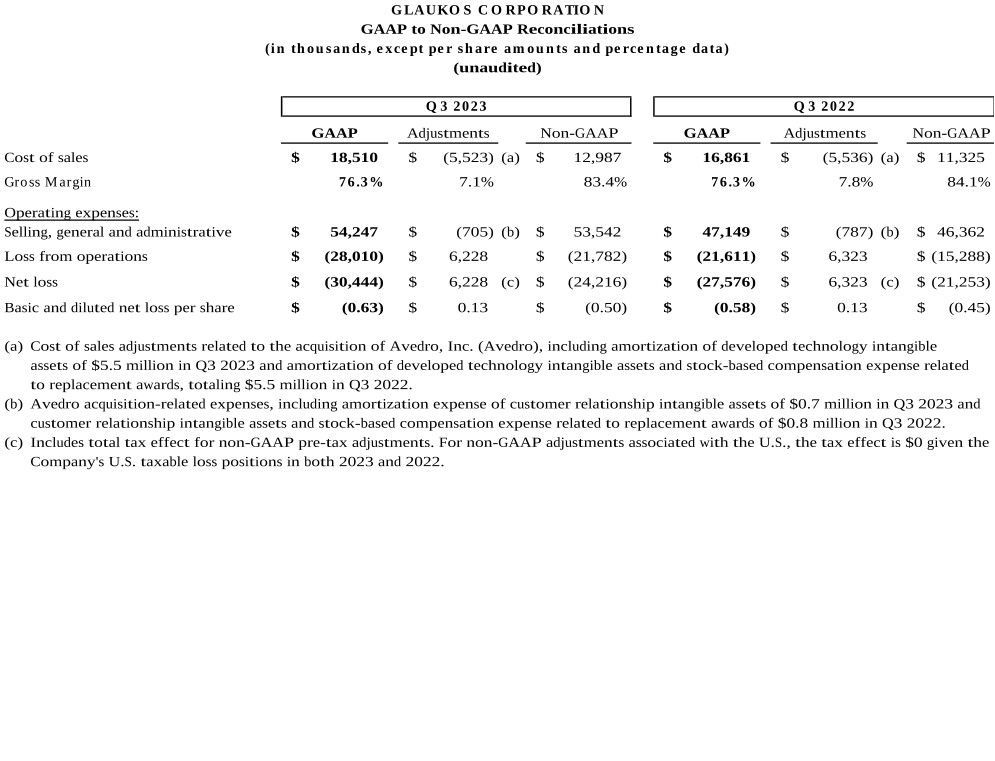
(a) |
Cost of sales adjustments related to the acquisition of Avedro, Inc. (Avedro), including amortization of developed technology intangible assets of $5.5 million in Q3 2023 and amortization of developed technology intangible assets and stock-based compensation expense related to replacement awards, totaling $5.5 million in Q3 2022. |
(b) |
Avedro acquisition-related expenses, including amortization expense of customer relationship intangible assets of $0.7 million in Q3 2023 and customer relationship intangible assets and stock-based compensation expense related to replacement awards of $0.8 million in Q3 2022. |
(c) |
Includes total tax effect for non-GAAP pre-tax adjustments. For non-GAAP adjustments associated with the U.S., the tax effect is $0 given the Company’s U.S. taxable loss positions in both 2023 and 2022. |
7

GLAUKOS CORPORATION
GAAP to Non-GAAP Reconciliations
(in thousands, except per share amounts and percentage data)
(unaudited)
|
|
Year-to-Date Q3 2023 |
|
Year-to-Date Q3 2022 |
|
||||||||||||||||
|
|
GAAP |
|
Adjustments |
|
|
Non-GAAP |
|
GAAP |
|
Adjustments |
|
|
Non-GAAP |
|
||||||
Cost of sales |
|
$ |
56,684 |
|
$ |
(16,569) |
|
(a) |
$ |
40,115 |
|
$ |
51,757 |
|
$ |
(16,633) |
|
(a) |
$ |
35,124 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross Margin |
|
|
75.6 |
% |
|
7.1 |
% |
|
|
82.7 |
% |
|
75.5 |
% |
|
7.9 |
% |
|
|
83.4 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
$ |
161,034 |
|
$ |
(2,115) |
|
(b) |
$ |
158,919 |
|
$ |
140,998 |
|
$ |
(2,533) |
|
(b) |
$ |
138,465 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
101,706 |
|
$ |
— |
|
|
$ |
101,706 |
|
$ |
87,459 |
|
$ |
(127) |
|
(c) |
$ |
87,332 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Litigation-related settlement |
|
$ |
— |
|
$ |
— |
|
|
$ |
— |
|
$ |
(30,000) |
|
$ |
30,000 |
|
(d) |
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
$ |
(90,078) |
|
$ |
18,684 |
|
|
$ |
(71,394) |
|
$ |
(48,579) |
|
$ |
(10,707) |
|
|
$ |
(59,286) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(97,882) |
|
$ |
18,684 |
|
(e) |
$ |
(79,198) |
|
$ |
(67,735) |
|
$ |
(10,707) |
|
(e) |
$ |
(78,442) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share |
|
$ |
(2.03) |
|
$ |
0.39 |
|
|
$ |
(1.64) |
|
$ |
(1.43) |
|
$ |
(0.23) |
|
|
$ |
(1.66) |
|
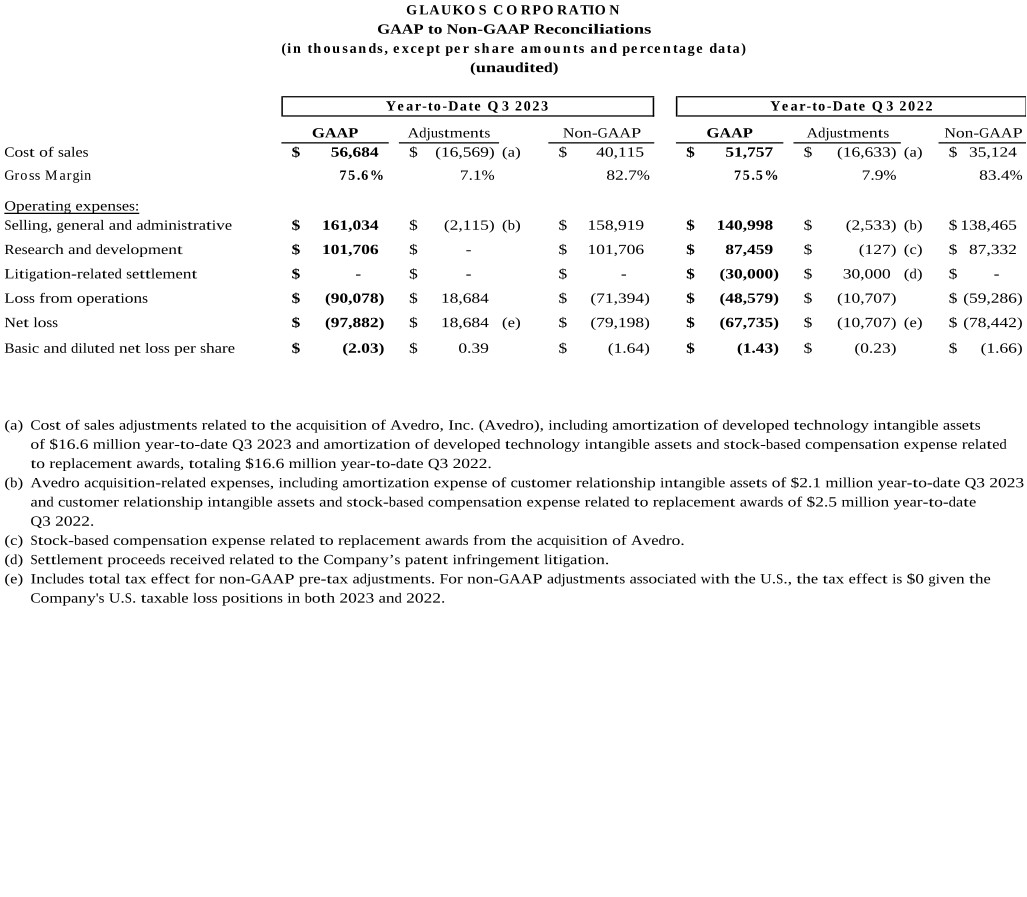
(a) |
Cost of sales adjustments related to the acquisition of Avedro, Inc. (Avedro), including amortization of developed technology intangible assets of $16.6 million year-to-date Q3 2023 and amortization of developed technology intangible assets and stock-based compensation expense related to replacement awards, totaling $16.6 million year-to-date Q3 2022. |
(b) |
Avedro acquisition-related expenses, including amortization expense of customer relationship intangible assets of $2.1 million year-to-date Q3 2023 and customer relationship intangible assets and stock-based compensation expense related to replacement awards of $2.5 million year-to-date Q3 2022. |
(c) |
Stock-based compensation expense related to replacement awards from the acquisition of Avedro. |
(d) |
Settlement proceeds received related to the Company’s patent infringement litigation. |
(e) |
Includes total tax effect for non-GAAP pre-tax adjustments. For non-GAAP adjustments associated with the U.S., the tax effect is $0 given the Company’s U.S. taxable loss positions in both 2023 and 2022. |
8

Reported Sales vs. Prior Periods (in thousands) |
|
|||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year-over-Year Percent Change |
|
|
Quarter-over-Quarter Percent Change |
|
||||||||||||
|
|
3Q 2023 |
|
|
3Q 2022 |
|
|
2Q 2023 |
|
|
Reported |
|
|
Operations (1) |
|
|
Currency (2) |
|
|
Reported |
|
|
Operations (1) |
|
|
Currency (2) |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
International Glaucoma |
|
$ |
20,280 |
|
|
$ |
16,532 |
|
|
$ |
22,305 |
|
|
22.7 |
% |
|
20.2 |
% |
|
2.5 |
% |
|
(9.1) |
% |
|
(8.0) |
% |
|
(1.1) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Net Sales |
|
$ |
78,048 |
|
|
$ |
71,269 |
|
|
$ |
80,399 |
|
|
9.5 |
% |
|
8.9 |
% |
|
0.6 |
% |
|
(2.9) |
% |
|
(2.6) |
% |
|
(0.3) |
% |
(1) Operational growth excludes the effect of translational currency
(2) Calculated by converting the current period numbers using the prior period’s average foreign exchange rates
9
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
GLAUKOS CORPORATION (NYSE: GKOS)
THIRD QUARTER 2023 IN REVIEW
Important Information
This document is intended to be read by investors in advance of regularly scheduled quarterly conference calls and was designed to provide a review of Glaukos Corporation’s recent financial and operational performance and general business outlook.
Please see “Forward-Looking Statements” and “Statement Regarding Use of Non-GAAP Financial Measures” in the “Additional Information” section of this document.
Conference Call Information
Date: November 1, 2023
Time: 4:30 p.m. ET / 1:30 p.m. PT
Dial-in numbers: 1-888-210-2212 (U.S.), 1-646-960-0390 (International)
Confirmation ID: 7935742
Live webcast: |
Events page at the Glaukos Investor Relations website at http://investors.glaukos.com or at this link. |
Webcast replay: |
A replay of the webcast will be archived on the Glaukos Investor Relations website following completion of the call. |

1
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
THIRD QUARTER 2023 FINANCIAL RESULTS SUMMARY
Business Description |
Ophthalmic medical technology and pharmaceutical company focused on developing and commercializing novel, dropless platform therapies designed to disrupt the conventional standard of care and improve outcomes for patients suffering from chronic eye diseases |
Disease Categories |
Glaucoma Corneal Health Retinal Disease |
Revenue (Growth) |
3Q 2023 $78.0 million (+10% reported, +9% constant currency vs. 3Q 2022) |
Gross Margin (Non-GAAP) |
3Q 2023 83.4% (versus 84.1% in 3Q 2022) |
Cash & Cash Equivalents, Short-Term Investments, and Restricted Cash |
$306.7 million as of September 30, 2023 (versus $309.7 million as of June 30, 2023) |
FY2023 Sales Guidance |
FY 2023 global consolidated revenues of $307 - $310 million expected (versus $304 - $308 million previously) |
See “Statement Regarding Use of Non-GAAP Financial Measures” and the Non-GAAP reconciliations included within the Additional Information section of this document. Reconciliations for each of constant currency revenue growth, Non-GAAP Gross Margin, and the other non-GAAP financial measures disclosed in this document to the most directly comparable GAAP financial measure are provided.
2
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Revenue Performance & Commercial Overview
Global Consolidated Revenue Performance
Glaukos reported third quarter net revenues of $78.0 million that were up 10% on a reported basis or up 9% on a constant currency basis versus 3Q 2022. Our third quarter performance reflected continued solid execution across our global Glaucoma and Corneal Health franchises.

Franchise Revenue Performance

3
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
U.S. Glaucoma
Our third quarter U.S. Glaucoma net revenues of $38.1 million increased 2% year-over-year versus 3Q 2022 as we experienced more pronounced seasonality headwinds in August offset by continued strength in July and September.
We continue to advance iStent infinite® ahead of establishing formal Medicare Administrative Contractor (MAC) coverage and payment. On that front, just last week, WPS, one of the seven MACs, published an updated MIGS LCD with a future effective date of December 24, 2023, that provides coverage for iStent infinite consistent with FDA approval and our reconsideration request. We are pleased with this final outcome as it relates to iStent infinite, but were disappointed at other aspects of the LCD that severely restricts clinical decision discretion for surgeons fighting a sight-threatening disease. The anticipated net impact to our business from the WPS final LCD, along with potentially additional final LCDs to be issued by other MACs in the future, remains unclear as we continue to evaluate the commercial implications across our entire product portfolio.
Looking ahead, the remaining six MACs have all taken preliminary steps to assess iStent infinite coverage, including four through proposed LCDs, and two with local coverage articles. We will continue to monitor the various MAC processes and policies as they advance and are ultimately finalized in the future as we remain supportive of expanding broad access to interventional glaucoma tools for physicians and patients.
While we await the release of CMS’s 2024 Final Rules, we remain encouraged that, as part of CMS’s 2024 Proposed Rules, the CPT code used to cover iStent infinite in standalone procedures, 0671T, was lifted to APC 5492, and the APC assignment for combined cataract plus trabecular bypass procedures, 66989 and 66991, was proposed to move to a newly restructured APC 5493. If finalized as proposed, we do believe these changes, while positive for our customers and our procedures, may create some transient disruptions to ordering patterns in late 2023 ahead of becoming effective on January 1, 2024.
As we focus on near-term execution, we are also accelerating efforts to support one of our founding missions at Glaukos, which is to advance glaucoma care by driving intervention of therapies earlier in the treatment paradigm for glaucoma disease, and in turn pioneering a new standalone market over time. We continue to lead and work closely with surgeons and thought leaders globally to organically drive this broader evolution in the standard of care, including through numerous events at the ESCRS annual meeting in Vienna in September and the Interventional Glaucoma Consortium in Salt Lake City in October. These efforts will once again be on full display at the upcoming AAO annual meeting being held in San Francisco this weekend.
International Glaucoma
Our third quarter International Glaucoma net revenues were approximately $20.3 million, representing year-over-year reported growth of 23%, or 20% on a constant currency basis, versus 3Q 2022. The strong growth internationally during the third quarter was broad-based as we continue to scale our international infrastructure, we are increasingly driving MIGS forward as the standard of care in each region and every major market in the world.
Corneal Health
Our record third quarter Corneal Health net revenues were approximately $19.7 million, representing year-over-year growth of 12% versus 3Q 2022, including U.S. Photrexa® record sales of $17.0 million, which increased 18% year-over-year as our key strategic initiatives continue to take hold in support of this important business.
4
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Similar to our U.S. Glaucoma franchise, our U.S. Corneal Health business experienced more pronounced seasonality headwinds in August, offset by strength throughout the remainder of the third quarter.
We continue to focus on expanding access for keratoconus patients suffering from this rare disease.
5
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Additional Commercial Updates & Commentary
We have had several additional positive commercial updates worth highlighting here:
| ✓ | Advanced initial commercial launch activities in the U.S. for iStent infinite in the third quarter of 2023 |
| o | On October 26, 2023, WPS, one of the seven MACs, published an updated MIGS LCD with a future effective date of December 24, 2023, that provides coverage for iStent infinite consistent with FDA approval and our reconsideration request. We are pleased with this final outcome as it relates to iStent infinite. |
| o | Looking ahead, the remaining six MACs have all taken preliminary steps to assess iStent infinite coverage, including four through proposed LCDs, and two with local coverage articles. |
| ✓ | While we are pleased with the WPS final LCD outcome as it relates to iStent infinite, we were disappointed at other aspects of the LCD that severely restricts clinical decision discretion for surgeons fighting a sight-threatening disease. |
| o | The anticipated net impact to our business from the WPS final LCD, along with potentially additional final LCDs to be issued by other MACs in the future, remains unclear as we continue to evaluate the commercial implications across our entire product portfolio. |
| ✓ | Our teams continue to make encouraging progress with the preparation and planning of the iDose® TR commercial launch targeted for early 2024 |
| ✓ | CMS’s Proposed Rules for Calendar Year 2024 – Hospital Outpatient Prospective Payment System (OPPS) and Ambulatory Surgical Center (ASC) Facility Fee Schedule updates: |
| o | APC assignment for standalone trabecular bypass procedure, CPT 0671T, proposed to move up to APC 5492 (versus APC 5491 in CY 2023) |
| o | APC assignment for combined cataract plus trabecular bypass procedures, CPT 66989 and CPT 66991, proposed to move up to a newly restructured APC 5493 (versus new technology code APC 1563 in CY 2023) |
| o | If finalized, these rules will go into effect on January 1, 2024 |
6
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
2023 Revenue Guidance Raised to Reflect Continued Momentum
Glaukos now expects full-year 2023 global consolidated net sales of $307 - $310 million, up from its previous guidance of $304 - $308 million. This upwardly revised guidance attempts to take into consideration:
| ● | Potential ordering headwinds within our U.S. Glaucoma franchise during in the fourth quarter 2023 due to (a) CMS’s Final 2024 Rule and associated reimbursement updates taking effect on January 1, 2024, and (b) potential impacts associated with the WPS final LCD, along with potentially additional final LCDs to be issued by other MACs in the future |
| ● | Potential growing contributions from iStent infinite |
| ● | Potential ordering disruptions within our U.S. Corneal Health franchise during the fourth quarter 2023 |
| ● | Incremental co-pay assistance for patients in our Corneal Health franchise domestically |
| ● | The potential normalization of ophthalmic procedure volumes in 2H 2023 as improved staffing dynamics allowed accounts to address their backlog of procedures, which may have been a driver for strong underlying procedure growth in 1H 2023 |
| ● | The continued estimated impact on U.S. Glaucoma volumes related to professional fee reimbursement for combination-cataract trabecular bypass surgery versus other more invasive alternatives |
| ● | The latest foreign currency exchange spot rates as of our 3Q23 earnings call on November 1, 2023 |
| ● | Combo-cataract MIGS competition globally |
7
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Research & Development / Pipeline Overview
Pipeline Summary
Our five key dropless technology therapy platforms designed to disrupt traditional treatment paradigms and generate cascades of future innovation are as follows:
|
iStent® micro-scale surgical devices |
|
iDose® sustained-release pharmaceuticals |
|
iLution™ transdermal pharmaceuticals |
|
iLink® bio-activated pharmaceuticals |
|
Retina XR bio-erodible sustained-release pharmaceuticals |

8
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Key R&D and Pipeline Updates
We are continuing to prudently invest in and advance our fulsome pipeline of core novel platforms, supported by more than $500 million of investment into our R&D programs since 2018 alone. Recent updates in our pipeline include:
| ✓ | Announced FDA acceptance of NDA submission for iDose TR (May 2023) |
| o | Advancing towards FDA PDUFA date of December 22, 2023 |
| o | Completed pre-approval inspection (PAI) with no 483 observations (August 2023) |
| o | Completed mid-cycle review debrief meeting with the FDA (August 2023) |
| ✓ | Advancing patient follow-up in second Phase 3 confirmatory pivotal trial for Epioxa™ (Epi-on) |
| o | Phase 3 confirmatory trial results together with already-completed first Phase 3 trial expected to support targeted NDA submission for Epioxa by the end of 2024 |
| ✓ | Commenced PMA pivotal trial for iStent infinite in mild-to-moderate glaucoma patients (label expansion) |
| ✓ | Open Investigation New Drug (IND) for iLution™ Travoprost – preparing to commence Phase 2a clinical trial by end of 2023 |
| ✓ | Preparing to commence first-in-human Retina XR clinical development program for GLK-401 by end of 2023 |
| ✓ | PRESERFLO MicroShunt |
| o | U.S. Investigation Device Excemption (IDE) application submitted – targeting clinical study commencement in 1H 2024 |
| o | Ongoing regulatory submissions and approvals in Latin America |
9
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Other Financial Performance Overview
As a reminder, we discuss our financial performance on a non-GAAP basis and summarize our GAAP performance. We encourage investors to review our GAAP to non-GAAP reconciliation which can be found in our earnings press release, the Additional Information section contained herein, as well as the Investor Relations section of our website.
Third quarter 2023 financial performance summary:
|
|
3Q 2023: 83% 3Q 2022: 84% YoY ∆: (70 bps) |
●
Please note that our non-GAAP adjustments to cost of goods sold include substantial amounts related to Avedro acquisition accounting
●
YoY decrease reflects manufacturing system enhancements and impact of new product launches
|
|
|
3Q 2023: $53.5M 3Q 2022: $46.4M YoY ∆: +15% |
●
2% sequential increase vs $52.4M in 2Q 2023
●
YoY increase reflects commercial and G&A investments globally and new product launch activities
|
|
|
3Q 2023: $33.3M 3Q 2022: $28.9M YoY ∆: +15% |
●
Roughly flat sequentially vs $33.2M in 2Q 2023
●
YoY increase reflects continued investment in and advancement of R&D programs
|
|
|
3Q 2023: $86.8M 3Q 2022: $75.2M YoY ∆: +15% |
●
1% sequential increase vs $85.7M in 2Q 2023, reflecting some of the adjustments we’ve made in our earlier-stage pipeline programs as we continue to prioritize resources ahead of the anticipated iDose TR launch
|
|
|
Op Loss (Non-GAAP) 3Q 2023: ($21.8M) 3Q 2022: ($15.3M) Net Loss (Non-GAAP) 3Q 2023: ($24.2M) 3Q 2022: ($21.3M) Diluted EPS (Non-GAAP) 3Q 2023: ($0.50) 3Q 2022: ($0.45) |
|
|
|
3Q 2023: $3.4M 3Q 2022: $6.0M YoY ∆: (-$2.6M) |
●
Capital expenditures moderating to levels more consistent with historical norms, a trend expected to continue into 2024
●
YoY decrease reflects the substantial completion of Aliso Viejo, CA and Burlington, MA facilities, partially offset by continued investments in our San Clemente, CA manufacturing facility
|
|
|
3Q 2023: ~$307M 2Q 2023: ~$310M QoQ ∆: ($3M) |
●
Operating expenses and capital investments
|
10
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Additional Information

11
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Forward-Looking Statements
This communication contains “forward-looking statements” within the meaning of federal securities laws. All statements other than statements of historical facts included in this presentation that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements. These statements are based on management’s current expectations, assumptions, estimates and beliefs. Although we believe that we have a reasonable basis for forward-looking statements contained herein, we caution you that they are based on current expectations about future events affecting us and are subject to risks, uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control, that may cause our actual results to differ materially from those expressed or implied by forward-looking statements in this presentation. These potential risks and uncertainties that could cause actual results to differ materially from those described in forward-looking statements include, without limitation, uncertainties regarding the impact of the COVID-19 pandemic or other public health crises on our business; the impact of general macroeconomic conditions including foreign currency fluctuations; the reduced physician fee and ASC facility fee reimbursement rate finalized by CMS for 2022 and 2023 for procedures utilizing the Company’s iStent family of products and its impact on our U.S. combo-cataract glaucoma revenue; our ability to continue to generate sales of our commercialized products and develop and commercialize additional products; our dependence on a limited number of third-party suppliers, some of which are single-source, for components of our products; the occurrence of a crippling accident, natural disaster, or other disruption at our primary facility, which may materially affect our manufacturing capacity and operations; securing or maintaining adequate coverage or reimbursement by third-party payors for procedures using the iStent, the iStent inject, the iStent inject W, iAccess, iPRIME, iStent infinite, our corneal cross-linking products or other products in development; our ability to properly train, and gain acceptance and trust from ophthalmic surgeons in the use of our products; our ability to compete effectively in the medical device industry and against current and future technologies (including MIGS technologies); our compliance with federal, state and foreign laws and regulations for the approval and sale and marketing of our products and of our manufacturing processes; the lengthy and expensive clinical trial process and the uncertainty of timing and outcomes from any particular clinical trial or regulatory approval processes; the risk of recalls or serious safety issues with our products and the uncertainty of patient outcomes; our ability to protect, and the expense and time-consuming nature of protecting our intellectual property against third parties and competitors and the impact of any claims against us for infringement or misappropriation of third party intellectual property rights and any related litigation; and our ability to service our indebtedness. These and other known risks, uncertainties and factors are described in detail under the caption “Risk Factors” and elsewhere in our filings with the Securities and Exchange Commission (SEC), including our Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, which we expect to file on or before November 9, 2023. Our filings with the SEC are available in the Investor Section of our website at www.glaukos.com or at www.sec.gov. In addition, information about the risks and benefits of our products is available on our website at www.glaukos.com. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on the forward-looking statements in this press release, which speak only as of the date hereof. We do not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise, except as may be required under applicable securities law.
12
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Statement Regarding Use of Non-GAAP Financial Measures
To supplement the consolidated financial results prepared in accordance with Generally Accepted Accounting Principles ("GAAP"), the Company uses certain non-GAAP historical financial measures. Management makes adjustments to the GAAP measures for items (both charges and gains) that (a) do not reflect the core operational activities of the Company, (b) are commonly adjusted within the Company's industry to enhance comparability of the Company's financial results with those of its peer group, or (c) are inconsistent in amount or frequency between periods (albeit such items are monitored and controlled with equal diligence relative to core operations). The Company uses the term "Non-GAAP" to exclude external acquisition-related costs incurred to effect a business combination; amortization of intangible assets acquired in a business combination, asset purchase transaction or other contractual relationship; impairment of goodwill and intangible assets; certain in-process R&D charges; fair value adjustments to contingent consideration liabilities and pre-acquisition contingencies arising from a business combination; integration and transition costs related to business combinations; fair market value adjustments to inventories acquired in a business combination or asset purchase transaction; restructuring charges, duplicative operating expenses, or asset write-offs (or reversals) associated with exiting or significantly downsizing a business; gain or loss from the sale of a business; gain or loss on the mark-to-market adjustment, impairment, or sale of long-term investments; mark-to-market adjustments on derivative instruments that hedge income or expense exposures in a future period; significant legal litigation costs and/or settlement expenses or proceeds; legal and other associated expenses that are both unusual and significant related to governmental or internal inquiries; and significant discrete income and other tax adjustments related to transactions as well as changes in estimated acquisition-date tax effects associated with business combinations, and the impact from implementation of tax law changes and settlements. See “Primary GAAP to Non-GAAP Reconciliations” for a reconciliation of each non-GAAP measure presented to the comparable GAAP financial measure. Beginning in the second quarter of 2022, we no longer exclude certain upfront and contingent milestone payments in connection with collaborative and licensing arrangements and certain in-process R&D charges for non-GAAP reporting and disclosure purposes.
In addition, in order to remove the impact of fluctuations in foreign currency exchange rates, the Company also presents certain net sales information on a constant currency basis, which represents the outcome that would have resulted had exchange rates in the current period been the same as the average exchange rates in effect in the comparable prior period. See “Additional GAAP to Non-GAAP Reconciliations” for a presentation of certain net sales information on a reported, GAAP and a constant currency basis.
13
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
GAAP Income Statement

14
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
GAAP Balance Sheet

15
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Primary GAAP to Non-GAAP Reconciliations
16
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Primary GAAP to Non-GAAP Reconciliations
17
Exhibit 99.2
 NOVEMBER 1, 2023
NOVEMBER 1, 2023
Additional GAAP to Non-GAAP Reconciliations

For Non-GAAP disclosures associated with the company’s past quarterly results, included with respect to the sequential comparisons included herein, please see reconciliations here.
18